User login
Can medication reduce crime?
Lately, I've been busy wading through a heavily publicized study that was published this month in the Lancet. In their paper, "Antipsychotics, mood stabilisers and risk of violent crime," Dr. Seena Fazel and his associates linked Swedish national registers to compare rates of violent crime among 82,647 male and female psychiatric patients to assess the effect of medication on this outcome.
The study made quite a splash in the news, because the outcome was almost too good to be true. There was a 64% reduction in violent crime among patients who had been prescribed any antipsychotic or mood stabilizer, compared with those taking other psychotropics. The reduction in violence for those taking neuroleptics and mood stabilizers was 45% and 24%, respectively. Selective serotonin reuptake inhibitors (SSRIs) had no apparent effect on crime (Lancet 2014 [doi:10.1016/S0140-6736(14)60379-2]).
Given our American anxiety over spree shooters and other high-profile crimes allegedly committed by untreated psychiatric patients, this study clearly deserves some scrutiny to thoroughly understand the findings, limitations, and other factors that could limit generalizability to the United States.
The authors compared mental health treatment registries with the national criminal history database. They looked at the rate and types of crimes committed by psychiatric patients when they were in and out of treatment. The "in-treatment" time interval was defined as the time between two or more prescriptions, as long as the prescriptions were no more than 4 months apart. Individuals who had only been given one script [prescription] were excluded. The outcome measure was any criminal conviction. The conviction outcome was based upon the date the offense took place, not the date of conviction. Individuals were excluded if the offense date was unknown.
A within-individual analysis showed significant reduction in all crimes, including violent crime, drug-related crime, and less severe crimes, during times when patients were prescribed medication, compared with medication-free intervals. When medicated, the rate of violent crime did not differ between patients with and without a history of violent offenses when diagnosis was not considered. When the analysis was limited to people with schizophrenia, bipolar disorder, or other psychotic disorders, the prescription of neuroleptics significantly reduced violent crime for both men and women.
For bipolar disorder, mood stabilizing medication reduced violent crime for men but not for women. The SSRI-medicated group was used as a control, to account for the general effect of contact with the mental health system and non-medication interventions related to this, and there was no effect on violent crime with this class of medication.
Now on to the limitations. Medication adherence was not assessed and could not be verified apart from patients given depot neuroleptics. The overall rate of violent crime was low, as would be expected. Only 6% of men and 1% of the women committed a violent crime. The numbers were so low that the study could not statistically assess the impact of violent crime history among patients diagnosed with psychosis. This is a small but crucial finding that did not make the traditional media coverage of this study.
Also, only 40% of those patients taking antipsychotics and mood stabilizers had a diagnosis of schizophrenia, other psychosis, or bipolar disorder, suggesting that, in Sweden, these medications might be prescribed for other indications such as characterologic low frustration tolerance or irritability. The analysis did not look at impact on violent crime by personality disorder diagnosis.
The authors acknowledged that their research could not prove a causal link between psychiatric illness and violence, another important conclusion that was not mentioned in traditional media coverage. In Sweden, mental illness cannot be used to prevent or mitigate a criminal conviction, so any connection between psychiatric symptoms and crime in this population can't be determined. The study also did not consider which subjects, if any, were taking medication or in treatment under court-mandated conditions.
As legislators and advocacy groups push to strengthen involuntary treatment laws, there is a risk that "bottom line" media coverage of research like this may inappropriately sway public opinion. Psychiatrists should be prepared to respond to proposed policies based on inaccurate interpretation of research.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011). The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson's employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
Lately, I've been busy wading through a heavily publicized study that was published this month in the Lancet. In their paper, "Antipsychotics, mood stabilisers and risk of violent crime," Dr. Seena Fazel and his associates linked Swedish national registers to compare rates of violent crime among 82,647 male and female psychiatric patients to assess the effect of medication on this outcome.
The study made quite a splash in the news, because the outcome was almost too good to be true. There was a 64% reduction in violent crime among patients who had been prescribed any antipsychotic or mood stabilizer, compared with those taking other psychotropics. The reduction in violence for those taking neuroleptics and mood stabilizers was 45% and 24%, respectively. Selective serotonin reuptake inhibitors (SSRIs) had no apparent effect on crime (Lancet 2014 [doi:10.1016/S0140-6736(14)60379-2]).
Given our American anxiety over spree shooters and other high-profile crimes allegedly committed by untreated psychiatric patients, this study clearly deserves some scrutiny to thoroughly understand the findings, limitations, and other factors that could limit generalizability to the United States.
The authors compared mental health treatment registries with the national criminal history database. They looked at the rate and types of crimes committed by psychiatric patients when they were in and out of treatment. The "in-treatment" time interval was defined as the time between two or more prescriptions, as long as the prescriptions were no more than 4 months apart. Individuals who had only been given one script [prescription] were excluded. The outcome measure was any criminal conviction. The conviction outcome was based upon the date the offense took place, not the date of conviction. Individuals were excluded if the offense date was unknown.
A within-individual analysis showed significant reduction in all crimes, including violent crime, drug-related crime, and less severe crimes, during times when patients were prescribed medication, compared with medication-free intervals. When medicated, the rate of violent crime did not differ between patients with and without a history of violent offenses when diagnosis was not considered. When the analysis was limited to people with schizophrenia, bipolar disorder, or other psychotic disorders, the prescription of neuroleptics significantly reduced violent crime for both men and women.
For bipolar disorder, mood stabilizing medication reduced violent crime for men but not for women. The SSRI-medicated group was used as a control, to account for the general effect of contact with the mental health system and non-medication interventions related to this, and there was no effect on violent crime with this class of medication.
Now on to the limitations. Medication adherence was not assessed and could not be verified apart from patients given depot neuroleptics. The overall rate of violent crime was low, as would be expected. Only 6% of men and 1% of the women committed a violent crime. The numbers were so low that the study could not statistically assess the impact of violent crime history among patients diagnosed with psychosis. This is a small but crucial finding that did not make the traditional media coverage of this study.
Also, only 40% of those patients taking antipsychotics and mood stabilizers had a diagnosis of schizophrenia, other psychosis, or bipolar disorder, suggesting that, in Sweden, these medications might be prescribed for other indications such as characterologic low frustration tolerance or irritability. The analysis did not look at impact on violent crime by personality disorder diagnosis.
The authors acknowledged that their research could not prove a causal link between psychiatric illness and violence, another important conclusion that was not mentioned in traditional media coverage. In Sweden, mental illness cannot be used to prevent or mitigate a criminal conviction, so any connection between psychiatric symptoms and crime in this population can't be determined. The study also did not consider which subjects, if any, were taking medication or in treatment under court-mandated conditions.
As legislators and advocacy groups push to strengthen involuntary treatment laws, there is a risk that "bottom line" media coverage of research like this may inappropriately sway public opinion. Psychiatrists should be prepared to respond to proposed policies based on inaccurate interpretation of research.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011). The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson's employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
Lately, I've been busy wading through a heavily publicized study that was published this month in the Lancet. In their paper, "Antipsychotics, mood stabilisers and risk of violent crime," Dr. Seena Fazel and his associates linked Swedish national registers to compare rates of violent crime among 82,647 male and female psychiatric patients to assess the effect of medication on this outcome.
The study made quite a splash in the news, because the outcome was almost too good to be true. There was a 64% reduction in violent crime among patients who had been prescribed any antipsychotic or mood stabilizer, compared with those taking other psychotropics. The reduction in violence for those taking neuroleptics and mood stabilizers was 45% and 24%, respectively. Selective serotonin reuptake inhibitors (SSRIs) had no apparent effect on crime (Lancet 2014 [doi:10.1016/S0140-6736(14)60379-2]).
Given our American anxiety over spree shooters and other high-profile crimes allegedly committed by untreated psychiatric patients, this study clearly deserves some scrutiny to thoroughly understand the findings, limitations, and other factors that could limit generalizability to the United States.
The authors compared mental health treatment registries with the national criminal history database. They looked at the rate and types of crimes committed by psychiatric patients when they were in and out of treatment. The "in-treatment" time interval was defined as the time between two or more prescriptions, as long as the prescriptions were no more than 4 months apart. Individuals who had only been given one script [prescription] were excluded. The outcome measure was any criminal conviction. The conviction outcome was based upon the date the offense took place, not the date of conviction. Individuals were excluded if the offense date was unknown.
A within-individual analysis showed significant reduction in all crimes, including violent crime, drug-related crime, and less severe crimes, during times when patients were prescribed medication, compared with medication-free intervals. When medicated, the rate of violent crime did not differ between patients with and without a history of violent offenses when diagnosis was not considered. When the analysis was limited to people with schizophrenia, bipolar disorder, or other psychotic disorders, the prescription of neuroleptics significantly reduced violent crime for both men and women.
For bipolar disorder, mood stabilizing medication reduced violent crime for men but not for women. The SSRI-medicated group was used as a control, to account for the general effect of contact with the mental health system and non-medication interventions related to this, and there was no effect on violent crime with this class of medication.
Now on to the limitations. Medication adherence was not assessed and could not be verified apart from patients given depot neuroleptics. The overall rate of violent crime was low, as would be expected. Only 6% of men and 1% of the women committed a violent crime. The numbers were so low that the study could not statistically assess the impact of violent crime history among patients diagnosed with psychosis. This is a small but crucial finding that did not make the traditional media coverage of this study.
Also, only 40% of those patients taking antipsychotics and mood stabilizers had a diagnosis of schizophrenia, other psychosis, or bipolar disorder, suggesting that, in Sweden, these medications might be prescribed for other indications such as characterologic low frustration tolerance or irritability. The analysis did not look at impact on violent crime by personality disorder diagnosis.
The authors acknowledged that their research could not prove a causal link between psychiatric illness and violence, another important conclusion that was not mentioned in traditional media coverage. In Sweden, mental illness cannot be used to prevent or mitigate a criminal conviction, so any connection between psychiatric symptoms and crime in this population can't be determined. The study also did not consider which subjects, if any, were taking medication or in treatment under court-mandated conditions.
As legislators and advocacy groups push to strengthen involuntary treatment laws, there is a risk that "bottom line" media coverage of research like this may inappropriately sway public opinion. Psychiatrists should be prepared to respond to proposed policies based on inaccurate interpretation of research.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011). The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson's employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
RealSelf
If you have patients who express interest in cosmetic procedures, and especially if you are a cosmetic dermatologist or a plastic surgeon, you might want to familiarize yourself with RealSelf.com. Founded in 2006, RealSelf is an online community for learning and sharing information about cosmetic surgery, dermatology, dentistry, and other elective treatments. In 2013, the site had 36 million unique visitors, and it is expected to grow.
Why might RealSelf be relevant for you? Simply put, it’s another channel to market you and your practice. It works by allowing physicians to answer users’ questions about cosmetic procedures ranging from rhinoplasty and liposuction to tattoo removal and Botox. Over time, your participation can lead to new consultations at your practice.
To ensure credibility, physicians must be board-certified in order to join RealSelf’s physician community. There is an element of game mechanics: The more active the physician, the more exposure his or her profile and practice receives. Similarly, paid subscriptions lead to more exposure than free subscriptions (more on this later.) Although this model does not appeal to some physicians, many of them do like the platform, and see it as a way to build a reputation as an expert and to market their practices.
Unlike doctor review sites that focus on the physician, RealSelf focuses on the procedure. For each procedure, users will find actual patient reviews and before and after photos, as well as Q&A’s with board-certified physicians. Users will also find licensed physicians in their area as well as the average cost for the procedure. RealSelf believes that patients value transparency, and including prices creates transparency.
Since most patients genuinely want to help other patients make informed medical decisions, the reviews tend to be thoughtful and thorough, and many of them contain multiple before-and-after photos. As a physician perusing the patient reviews, you’ll start to notice that most of them are reasonable. For example, customer satisfaction with laser treatment for melasma was 51%, whereas satisfaction for laser treatment for rosacea was 80%.
Patients and prospective patients are flocking to the site because it allows them to share their experiences, interact with other patients, and gain access to physician experts in the field. Many patients have difficulty making decisions about cosmetic procedures; RealSelf aims to alleviate their fears and help them "make confident health and beauty decisions." If a prospective patient wants to see a video of tattoo removal or Botox injections, he or she can. If a patient wants to ask physicians their opinions, he or she can. According to RealSelf, physicians have answered over 500,000 questions on the site.
Of course, all this isn’t free for physicians. RealSelf is a business. They have a tiered membership – free, pro, and spotlight. To obtain free membership, you simply visit the site and follow the prompts to "claim your profile." Once your profile is completed, you will have access to a "doctor advisor" who can help you "optimize your visibility on the site." Both "pro" and "spotlight" offer additional benefits, such as integrating patient reviews on your practice website, promotions on Facebook and Twitter, extended directory listings, and exposure in your local area. RealSelf does not discuss costs of membership until you have claimed your profile.
Only you can determine if RealSelf is beneficial to you and your practice. If, for example, you’re not looking for new patients, then you might find it unnecessary. But at the very least, you’ll know what RealSelf is the next time a fellow cosmetic physician brings it up at a conference. And it’s never a bad idea to be familiar with current social technologies that may affect your livelihood.
If you’ve used RealSelf, let us know what you think. For more information, visit RealSelf.com.
Disclaimer: I have no financial interest in RealSelf and am not an active member.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @Dermdoc on Twitter.
If you have patients who express interest in cosmetic procedures, and especially if you are a cosmetic dermatologist or a plastic surgeon, you might want to familiarize yourself with RealSelf.com. Founded in 2006, RealSelf is an online community for learning and sharing information about cosmetic surgery, dermatology, dentistry, and other elective treatments. In 2013, the site had 36 million unique visitors, and it is expected to grow.
Why might RealSelf be relevant for you? Simply put, it’s another channel to market you and your practice. It works by allowing physicians to answer users’ questions about cosmetic procedures ranging from rhinoplasty and liposuction to tattoo removal and Botox. Over time, your participation can lead to new consultations at your practice.
To ensure credibility, physicians must be board-certified in order to join RealSelf’s physician community. There is an element of game mechanics: The more active the physician, the more exposure his or her profile and practice receives. Similarly, paid subscriptions lead to more exposure than free subscriptions (more on this later.) Although this model does not appeal to some physicians, many of them do like the platform, and see it as a way to build a reputation as an expert and to market their practices.
Unlike doctor review sites that focus on the physician, RealSelf focuses on the procedure. For each procedure, users will find actual patient reviews and before and after photos, as well as Q&A’s with board-certified physicians. Users will also find licensed physicians in their area as well as the average cost for the procedure. RealSelf believes that patients value transparency, and including prices creates transparency.
Since most patients genuinely want to help other patients make informed medical decisions, the reviews tend to be thoughtful and thorough, and many of them contain multiple before-and-after photos. As a physician perusing the patient reviews, you’ll start to notice that most of them are reasonable. For example, customer satisfaction with laser treatment for melasma was 51%, whereas satisfaction for laser treatment for rosacea was 80%.
Patients and prospective patients are flocking to the site because it allows them to share their experiences, interact with other patients, and gain access to physician experts in the field. Many patients have difficulty making decisions about cosmetic procedures; RealSelf aims to alleviate their fears and help them "make confident health and beauty decisions." If a prospective patient wants to see a video of tattoo removal or Botox injections, he or she can. If a patient wants to ask physicians their opinions, he or she can. According to RealSelf, physicians have answered over 500,000 questions on the site.
Of course, all this isn’t free for physicians. RealSelf is a business. They have a tiered membership – free, pro, and spotlight. To obtain free membership, you simply visit the site and follow the prompts to "claim your profile." Once your profile is completed, you will have access to a "doctor advisor" who can help you "optimize your visibility on the site." Both "pro" and "spotlight" offer additional benefits, such as integrating patient reviews on your practice website, promotions on Facebook and Twitter, extended directory listings, and exposure in your local area. RealSelf does not discuss costs of membership until you have claimed your profile.
Only you can determine if RealSelf is beneficial to you and your practice. If, for example, you’re not looking for new patients, then you might find it unnecessary. But at the very least, you’ll know what RealSelf is the next time a fellow cosmetic physician brings it up at a conference. And it’s never a bad idea to be familiar with current social technologies that may affect your livelihood.
If you’ve used RealSelf, let us know what you think. For more information, visit RealSelf.com.
Disclaimer: I have no financial interest in RealSelf and am not an active member.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @Dermdoc on Twitter.
If you have patients who express interest in cosmetic procedures, and especially if you are a cosmetic dermatologist or a plastic surgeon, you might want to familiarize yourself with RealSelf.com. Founded in 2006, RealSelf is an online community for learning and sharing information about cosmetic surgery, dermatology, dentistry, and other elective treatments. In 2013, the site had 36 million unique visitors, and it is expected to grow.
Why might RealSelf be relevant for you? Simply put, it’s another channel to market you and your practice. It works by allowing physicians to answer users’ questions about cosmetic procedures ranging from rhinoplasty and liposuction to tattoo removal and Botox. Over time, your participation can lead to new consultations at your practice.
To ensure credibility, physicians must be board-certified in order to join RealSelf’s physician community. There is an element of game mechanics: The more active the physician, the more exposure his or her profile and practice receives. Similarly, paid subscriptions lead to more exposure than free subscriptions (more on this later.) Although this model does not appeal to some physicians, many of them do like the platform, and see it as a way to build a reputation as an expert and to market their practices.
Unlike doctor review sites that focus on the physician, RealSelf focuses on the procedure. For each procedure, users will find actual patient reviews and before and after photos, as well as Q&A’s with board-certified physicians. Users will also find licensed physicians in their area as well as the average cost for the procedure. RealSelf believes that patients value transparency, and including prices creates transparency.
Since most patients genuinely want to help other patients make informed medical decisions, the reviews tend to be thoughtful and thorough, and many of them contain multiple before-and-after photos. As a physician perusing the patient reviews, you’ll start to notice that most of them are reasonable. For example, customer satisfaction with laser treatment for melasma was 51%, whereas satisfaction for laser treatment for rosacea was 80%.
Patients and prospective patients are flocking to the site because it allows them to share their experiences, interact with other patients, and gain access to physician experts in the field. Many patients have difficulty making decisions about cosmetic procedures; RealSelf aims to alleviate their fears and help them "make confident health and beauty decisions." If a prospective patient wants to see a video of tattoo removal or Botox injections, he or she can. If a patient wants to ask physicians their opinions, he or she can. According to RealSelf, physicians have answered over 500,000 questions on the site.
Of course, all this isn’t free for physicians. RealSelf is a business. They have a tiered membership – free, pro, and spotlight. To obtain free membership, you simply visit the site and follow the prompts to "claim your profile." Once your profile is completed, you will have access to a "doctor advisor" who can help you "optimize your visibility on the site." Both "pro" and "spotlight" offer additional benefits, such as integrating patient reviews on your practice website, promotions on Facebook and Twitter, extended directory listings, and exposure in your local area. RealSelf does not discuss costs of membership until you have claimed your profile.
Only you can determine if RealSelf is beneficial to you and your practice. If, for example, you’re not looking for new patients, then you might find it unnecessary. But at the very least, you’ll know what RealSelf is the next time a fellow cosmetic physician brings it up at a conference. And it’s never a bad idea to be familiar with current social technologies that may affect your livelihood.
If you’ve used RealSelf, let us know what you think. For more information, visit RealSelf.com.
Disclaimer: I have no financial interest in RealSelf and am not an active member.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @Dermdoc on Twitter.
Cosmeceutical Critique: Benzoyl peroxide
Benzoyl peroxide (BPO) has been used for more than 45 years for the treatment of acne, and has recently been enjoying renewed popularity, thanks to its performance in recent studies of both prescription and over-the-counter formulations (J. Drugs Dermatol. 2013;12:180-5). In fact, BPO is one of the two most common ingredients in OTC acne products (Semin. Cutan. Med. Surg. 2008;27:170-6). The prescription form is used alone or in combination with tretinoin, adapalene, or clindamycin. BPO, originally sourced from the coal tar component chlorhydroxyquinoline, is now typically prepared by treating hydrogen peroxide with benzoyl chloride (Dermatol. Clin. 2009;27:17-24). Because it can generate reactive oxygen species and commonly leads to skin irritation, its use is somewhat limited.
Antibacterial uses
BPO imparts bactericidal activity by releasing highly reactive oxygen (free radicals) that can oxidize proteins in bacterial cell membranes. It also exhibits antibacterial action against Propionibacterium acnes and Corynebacterium acnes, the bacteria implicated in the pathophysiology of acne (Dermatol. Ther. 2012;25:6-11), as well as Staphylococcus capitis, S. epidermis, S. hominis, P. avidum, P. granulosum, and the yeast Pityrosporum ovale (J. Appl. Bacteriol. 1983;54:379-82).
Acne
Many studies over the years have shown that topically applied BPO effectively treats acne (Expert Opin. Pharmacother. 2009;10:2555-62). These ameliorative results, which include enhancing the benefits of other topical antimicrobials, are thought to arise because BPO, a highly lipophilic molecule, penetrates through the sebum and into the pilosebaceous unit, and exerts bactericidal, keratolytic, and anti-inflammatory activity (Skin Pharmacol. Physiol. 2006;19:283-9). BPO may contribute to the antiacne efficacy of other antimicrobials by preventing bacterial resistance and promoting penetration into the sebum, keratin, and polysaccharides to reach the target bacteria. Specifically, the oxidative activity of BPO helps eliminate the biofilm polysaccharides secreted by P. acnes, thus expediting the delivery of other agents to the bacteria (Int. J. Dermatol. 2006;45:872; Int. J. Dermatol. 2003;42:925-7).
Not surprisingly, several studies have shown that the antiacne efficacy of a combination of BPO with other antimicrobials, such as clindamycin, is greater than that of either agent used alone. Simpson et al. demonstrated that the use of clindamycin and BPO together led to a 61% decline in inflammatory lesions after 3 months, as compared with 39% and 35%, respectively, when the agents were used alone (J. Am. Acad. Dermatol. 1997;37:590-5). BPO is frequently paired with salicylic acid to treat acne (Clin. Exp. Dermatol. 2011;36:840-3).
Acne often improves more rapidly with BPO treatment than with retinoids and other acne therapies, and data suggest that the faster clearing of acne lesions and comedones is most likely because of its keratolytic activity (Dermatol. Clin. 2009;27:17-24; J. Dermatolog. Treat. 2003;14:166-71). However, the dryness and irritation associated with BPO usage may undermine patient compliance. Several studies have suggested that BPO is effective in cleanser formulations, which seem to reduce irritation (Clin. Exp. Dermatol. 2011;36:840-3).
Photocarcinogenicity
Reports that BPO predisposed mice to skin cancer, particularly when they were exposed to ultraviolet radiation, prompted the Food and Drug Administration to form an advisory committee in 1992 to review the safety of BPO. The committee called for additional photocarcinogenicity studies while suggesting that BPO products include animal safety data on the labels. BPO-containing acne products were kept on the market. In the ensuing two decades, newer safety studies have led the FDA to change the classification of BPO to category I, deeming the OTC topical treatment of acne to be generally recognized as safe and effective (GRASE) (Fed. Regist. 2010;75:9767-77).
Photoaging
When BPO breaks down into benzoic acid in the skin, benzoyloxy, a free radical, forms as an intermediate (Prog. Clin. Biol. Res. 1995;391:245). Benzoyloxy can decarboxylate into a phenyl radical. These free radicals produce oxidative stress, which may cause DNA strand breaks in keratinocytes or may harm proteins or lipids. In addition to becoming a free radical, BPO depletes membrane and cytosolic antioxidants (Toxicology 2001;165:225-34). No retrospective trials looking at the effects of long-term use of BP on photoaging have been performed, so the role of BPO in photoaging is not clear. One study in mice found that topical BP has some of the same effects on skin as UVB (J. Invest. Dermatol. 1999;112:933-38).
Other safety issues
Acne is not uncommon among pregnant women. Although safety studies of BPO use by pregnant women have not been performed, various authors suggest that only about 5% of topically applied BPO is absorbed systemically, implying that topical BPO can be safely used during pregnancy (Int. J. Dermatol. 2002;41:197-203; Can. Fam. Physician 2011;57:665-7; Drugs 2013;73:779-87; Dermatol. Ther. 2013;26:302-11).
In approximately 1% of patients, topical BPO causes contact or irritant dermatitis (Contact Dermatitis 1999;41:233; Contact Dermatitis 1996;34:68-9). The use of barrier repair moisturizers may reduce the incidence of irritation, though this has not been proven.
Usage considerations
BPO use for acne is linked to a reduction in antibiotic resistance (J. Drugs Dermatol. 2013;12:s73-6). Because BPO, a potent oxidizer, eliminates bacteria by generating reactive oxygen species in the sebaceous follicle, it is important to consider the chemical compatibility of BPO with other agents (J. Am. Acad. Dermatol. 1981;4:31-7). Martin et al. showed that BPO tends to degrade tretinoin to about 80% of initial content, an effect that is markedly enhanced by indoor light. However, even in the presence of light, adapalene is not degraded by BPO (Br. J. Dermatol. 1998;139 Suppl 52:8-11). But the order in which products are applied is important, given that BPO can inactivate other ingredients.
Studies have demonstrated that the use of BPO in body washes leads to greater efficacy when the product is left on for 5 minutes before rinsing (J. Drugs Dermatol. 2010;9:622-5; J. Clin. Aesthet. Dermatol. 2010;3:26-9). Notably, the efficacy of BPO in cleansing products is comparable to that observed in leave-on products, but BPO provokes less irritation than leave-on formulations (J. Drugs Dermatol. 2009;8:657-61; Skinmed. 2005;4:370).
Conclusion
BPO remains quite effective in acne therapy, and it is one of the few acne medications available both over the counter and by prescription in the United States. BPO helps prevent antibiotic resistance to erythromycin and clindamycin, which makes it an important ingredient in many acne skin care regimens. However, it is pro-oxidant, and clinicians and patients should take into account the risk of BPO contributing to skin aging because of the free radicals it produces.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Benzoyl peroxide (BPO) has been used for more than 45 years for the treatment of acne, and has recently been enjoying renewed popularity, thanks to its performance in recent studies of both prescription and over-the-counter formulations (J. Drugs Dermatol. 2013;12:180-5). In fact, BPO is one of the two most common ingredients in OTC acne products (Semin. Cutan. Med. Surg. 2008;27:170-6). The prescription form is used alone or in combination with tretinoin, adapalene, or clindamycin. BPO, originally sourced from the coal tar component chlorhydroxyquinoline, is now typically prepared by treating hydrogen peroxide with benzoyl chloride (Dermatol. Clin. 2009;27:17-24). Because it can generate reactive oxygen species and commonly leads to skin irritation, its use is somewhat limited.
Antibacterial uses
BPO imparts bactericidal activity by releasing highly reactive oxygen (free radicals) that can oxidize proteins in bacterial cell membranes. It also exhibits antibacterial action against Propionibacterium acnes and Corynebacterium acnes, the bacteria implicated in the pathophysiology of acne (Dermatol. Ther. 2012;25:6-11), as well as Staphylococcus capitis, S. epidermis, S. hominis, P. avidum, P. granulosum, and the yeast Pityrosporum ovale (J. Appl. Bacteriol. 1983;54:379-82).
Acne
Many studies over the years have shown that topically applied BPO effectively treats acne (Expert Opin. Pharmacother. 2009;10:2555-62). These ameliorative results, which include enhancing the benefits of other topical antimicrobials, are thought to arise because BPO, a highly lipophilic molecule, penetrates through the sebum and into the pilosebaceous unit, and exerts bactericidal, keratolytic, and anti-inflammatory activity (Skin Pharmacol. Physiol. 2006;19:283-9). BPO may contribute to the antiacne efficacy of other antimicrobials by preventing bacterial resistance and promoting penetration into the sebum, keratin, and polysaccharides to reach the target bacteria. Specifically, the oxidative activity of BPO helps eliminate the biofilm polysaccharides secreted by P. acnes, thus expediting the delivery of other agents to the bacteria (Int. J. Dermatol. 2006;45:872; Int. J. Dermatol. 2003;42:925-7).
Not surprisingly, several studies have shown that the antiacne efficacy of a combination of BPO with other antimicrobials, such as clindamycin, is greater than that of either agent used alone. Simpson et al. demonstrated that the use of clindamycin and BPO together led to a 61% decline in inflammatory lesions after 3 months, as compared with 39% and 35%, respectively, when the agents were used alone (J. Am. Acad. Dermatol. 1997;37:590-5). BPO is frequently paired with salicylic acid to treat acne (Clin. Exp. Dermatol. 2011;36:840-3).
Acne often improves more rapidly with BPO treatment than with retinoids and other acne therapies, and data suggest that the faster clearing of acne lesions and comedones is most likely because of its keratolytic activity (Dermatol. Clin. 2009;27:17-24; J. Dermatolog. Treat. 2003;14:166-71). However, the dryness and irritation associated with BPO usage may undermine patient compliance. Several studies have suggested that BPO is effective in cleanser formulations, which seem to reduce irritation (Clin. Exp. Dermatol. 2011;36:840-3).
Photocarcinogenicity
Reports that BPO predisposed mice to skin cancer, particularly when they were exposed to ultraviolet radiation, prompted the Food and Drug Administration to form an advisory committee in 1992 to review the safety of BPO. The committee called for additional photocarcinogenicity studies while suggesting that BPO products include animal safety data on the labels. BPO-containing acne products were kept on the market. In the ensuing two decades, newer safety studies have led the FDA to change the classification of BPO to category I, deeming the OTC topical treatment of acne to be generally recognized as safe and effective (GRASE) (Fed. Regist. 2010;75:9767-77).
Photoaging
When BPO breaks down into benzoic acid in the skin, benzoyloxy, a free radical, forms as an intermediate (Prog. Clin. Biol. Res. 1995;391:245). Benzoyloxy can decarboxylate into a phenyl radical. These free radicals produce oxidative stress, which may cause DNA strand breaks in keratinocytes or may harm proteins or lipids. In addition to becoming a free radical, BPO depletes membrane and cytosolic antioxidants (Toxicology 2001;165:225-34). No retrospective trials looking at the effects of long-term use of BP on photoaging have been performed, so the role of BPO in photoaging is not clear. One study in mice found that topical BP has some of the same effects on skin as UVB (J. Invest. Dermatol. 1999;112:933-38).
Other safety issues
Acne is not uncommon among pregnant women. Although safety studies of BPO use by pregnant women have not been performed, various authors suggest that only about 5% of topically applied BPO is absorbed systemically, implying that topical BPO can be safely used during pregnancy (Int. J. Dermatol. 2002;41:197-203; Can. Fam. Physician 2011;57:665-7; Drugs 2013;73:779-87; Dermatol. Ther. 2013;26:302-11).
In approximately 1% of patients, topical BPO causes contact or irritant dermatitis (Contact Dermatitis 1999;41:233; Contact Dermatitis 1996;34:68-9). The use of barrier repair moisturizers may reduce the incidence of irritation, though this has not been proven.
Usage considerations
BPO use for acne is linked to a reduction in antibiotic resistance (J. Drugs Dermatol. 2013;12:s73-6). Because BPO, a potent oxidizer, eliminates bacteria by generating reactive oxygen species in the sebaceous follicle, it is important to consider the chemical compatibility of BPO with other agents (J. Am. Acad. Dermatol. 1981;4:31-7). Martin et al. showed that BPO tends to degrade tretinoin to about 80% of initial content, an effect that is markedly enhanced by indoor light. However, even in the presence of light, adapalene is not degraded by BPO (Br. J. Dermatol. 1998;139 Suppl 52:8-11). But the order in which products are applied is important, given that BPO can inactivate other ingredients.
Studies have demonstrated that the use of BPO in body washes leads to greater efficacy when the product is left on for 5 minutes before rinsing (J. Drugs Dermatol. 2010;9:622-5; J. Clin. Aesthet. Dermatol. 2010;3:26-9). Notably, the efficacy of BPO in cleansing products is comparable to that observed in leave-on products, but BPO provokes less irritation than leave-on formulations (J. Drugs Dermatol. 2009;8:657-61; Skinmed. 2005;4:370).
Conclusion
BPO remains quite effective in acne therapy, and it is one of the few acne medications available both over the counter and by prescription in the United States. BPO helps prevent antibiotic resistance to erythromycin and clindamycin, which makes it an important ingredient in many acne skin care regimens. However, it is pro-oxidant, and clinicians and patients should take into account the risk of BPO contributing to skin aging because of the free radicals it produces.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Benzoyl peroxide (BPO) has been used for more than 45 years for the treatment of acne, and has recently been enjoying renewed popularity, thanks to its performance in recent studies of both prescription and over-the-counter formulations (J. Drugs Dermatol. 2013;12:180-5). In fact, BPO is one of the two most common ingredients in OTC acne products (Semin. Cutan. Med. Surg. 2008;27:170-6). The prescription form is used alone or in combination with tretinoin, adapalene, or clindamycin. BPO, originally sourced from the coal tar component chlorhydroxyquinoline, is now typically prepared by treating hydrogen peroxide with benzoyl chloride (Dermatol. Clin. 2009;27:17-24). Because it can generate reactive oxygen species and commonly leads to skin irritation, its use is somewhat limited.
Antibacterial uses
BPO imparts bactericidal activity by releasing highly reactive oxygen (free radicals) that can oxidize proteins in bacterial cell membranes. It also exhibits antibacterial action against Propionibacterium acnes and Corynebacterium acnes, the bacteria implicated in the pathophysiology of acne (Dermatol. Ther. 2012;25:6-11), as well as Staphylococcus capitis, S. epidermis, S. hominis, P. avidum, P. granulosum, and the yeast Pityrosporum ovale (J. Appl. Bacteriol. 1983;54:379-82).
Acne
Many studies over the years have shown that topically applied BPO effectively treats acne (Expert Opin. Pharmacother. 2009;10:2555-62). These ameliorative results, which include enhancing the benefits of other topical antimicrobials, are thought to arise because BPO, a highly lipophilic molecule, penetrates through the sebum and into the pilosebaceous unit, and exerts bactericidal, keratolytic, and anti-inflammatory activity (Skin Pharmacol. Physiol. 2006;19:283-9). BPO may contribute to the antiacne efficacy of other antimicrobials by preventing bacterial resistance and promoting penetration into the sebum, keratin, and polysaccharides to reach the target bacteria. Specifically, the oxidative activity of BPO helps eliminate the biofilm polysaccharides secreted by P. acnes, thus expediting the delivery of other agents to the bacteria (Int. J. Dermatol. 2006;45:872; Int. J. Dermatol. 2003;42:925-7).
Not surprisingly, several studies have shown that the antiacne efficacy of a combination of BPO with other antimicrobials, such as clindamycin, is greater than that of either agent used alone. Simpson et al. demonstrated that the use of clindamycin and BPO together led to a 61% decline in inflammatory lesions after 3 months, as compared with 39% and 35%, respectively, when the agents were used alone (J. Am. Acad. Dermatol. 1997;37:590-5). BPO is frequently paired with salicylic acid to treat acne (Clin. Exp. Dermatol. 2011;36:840-3).
Acne often improves more rapidly with BPO treatment than with retinoids and other acne therapies, and data suggest that the faster clearing of acne lesions and comedones is most likely because of its keratolytic activity (Dermatol. Clin. 2009;27:17-24; J. Dermatolog. Treat. 2003;14:166-71). However, the dryness and irritation associated with BPO usage may undermine patient compliance. Several studies have suggested that BPO is effective in cleanser formulations, which seem to reduce irritation (Clin. Exp. Dermatol. 2011;36:840-3).
Photocarcinogenicity
Reports that BPO predisposed mice to skin cancer, particularly when they were exposed to ultraviolet radiation, prompted the Food and Drug Administration to form an advisory committee in 1992 to review the safety of BPO. The committee called for additional photocarcinogenicity studies while suggesting that BPO products include animal safety data on the labels. BPO-containing acne products were kept on the market. In the ensuing two decades, newer safety studies have led the FDA to change the classification of BPO to category I, deeming the OTC topical treatment of acne to be generally recognized as safe and effective (GRASE) (Fed. Regist. 2010;75:9767-77).
Photoaging
When BPO breaks down into benzoic acid in the skin, benzoyloxy, a free radical, forms as an intermediate (Prog. Clin. Biol. Res. 1995;391:245). Benzoyloxy can decarboxylate into a phenyl radical. These free radicals produce oxidative stress, which may cause DNA strand breaks in keratinocytes or may harm proteins or lipids. In addition to becoming a free radical, BPO depletes membrane and cytosolic antioxidants (Toxicology 2001;165:225-34). No retrospective trials looking at the effects of long-term use of BP on photoaging have been performed, so the role of BPO in photoaging is not clear. One study in mice found that topical BP has some of the same effects on skin as UVB (J. Invest. Dermatol. 1999;112:933-38).
Other safety issues
Acne is not uncommon among pregnant women. Although safety studies of BPO use by pregnant women have not been performed, various authors suggest that only about 5% of topically applied BPO is absorbed systemically, implying that topical BPO can be safely used during pregnancy (Int. J. Dermatol. 2002;41:197-203; Can. Fam. Physician 2011;57:665-7; Drugs 2013;73:779-87; Dermatol. Ther. 2013;26:302-11).
In approximately 1% of patients, topical BPO causes contact or irritant dermatitis (Contact Dermatitis 1999;41:233; Contact Dermatitis 1996;34:68-9). The use of barrier repair moisturizers may reduce the incidence of irritation, though this has not been proven.
Usage considerations
BPO use for acne is linked to a reduction in antibiotic resistance (J. Drugs Dermatol. 2013;12:s73-6). Because BPO, a potent oxidizer, eliminates bacteria by generating reactive oxygen species in the sebaceous follicle, it is important to consider the chemical compatibility of BPO with other agents (J. Am. Acad. Dermatol. 1981;4:31-7). Martin et al. showed that BPO tends to degrade tretinoin to about 80% of initial content, an effect that is markedly enhanced by indoor light. However, even in the presence of light, adapalene is not degraded by BPO (Br. J. Dermatol. 1998;139 Suppl 52:8-11). But the order in which products are applied is important, given that BPO can inactivate other ingredients.
Studies have demonstrated that the use of BPO in body washes leads to greater efficacy when the product is left on for 5 minutes before rinsing (J. Drugs Dermatol. 2010;9:622-5; J. Clin. Aesthet. Dermatol. 2010;3:26-9). Notably, the efficacy of BPO in cleansing products is comparable to that observed in leave-on products, but BPO provokes less irritation than leave-on formulations (J. Drugs Dermatol. 2009;8:657-61; Skinmed. 2005;4:370).
Conclusion
BPO remains quite effective in acne therapy, and it is one of the few acne medications available both over the counter and by prescription in the United States. BPO helps prevent antibiotic resistance to erythromycin and clindamycin, which makes it an important ingredient in many acne skin care regimens. However, it is pro-oxidant, and clinicians and patients should take into account the risk of BPO contributing to skin aging because of the free radicals it produces.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
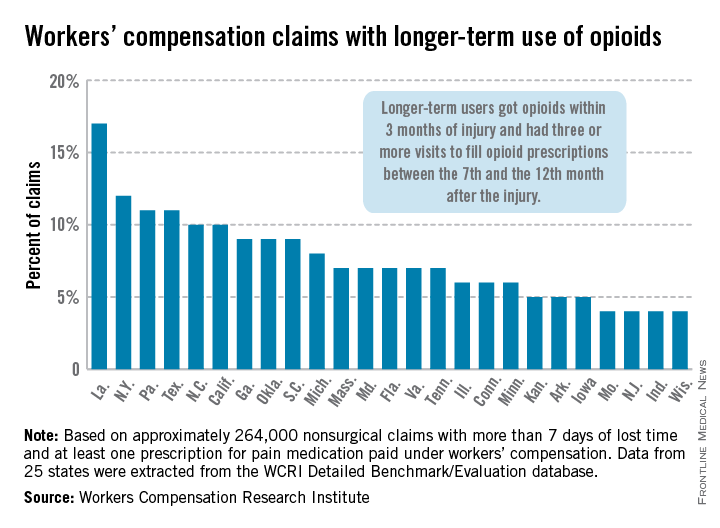
Longer-term opioid use in workers’ comp cases highest in Louisiana
In Louisiana, opioid use lasted more than 6 months in 17% of nonsurgical workers’ compensation claims involving employees who received at least one prescription for pain medication, the Workers Compensation Research Institute reported.
In cases with more than 7 days of lost time, that was the highest rate seen among the 25 states in the study, with New York second at 12% and Pennsylvania and Texas tied for third at 11%. There were four states tied for the lowest rate, at 4%: Missouri, New Jersey, Indiana, and Wisconsin, according to the WCRI report.
Overall, use of narcotics for pain relief by injured workers in such cases ranged from 60% in New Jersey to 88% in Arkansas (median, 76%), while use of any pain medication ranged from 85% in Minnesota to 95% in Florida, Georgia, Tennessee, and Texas (median, 94%), the report showed.
The study involved claims with injuries that occurred from Oct. 1, 2009, through Sept. 30, 2010, with prescriptions filled through March 31, 2012. Longer-term users received a prescription for opioids within 3 months of their injury and had three or more visits to fill opioid prescriptions between the 7th and the 12th month after the injury.
The 25 states in the study "represent more than 70% of the workers’ compensation benefits paid in the United States," the WCRI noted.
The study was based on approximately 264,000 nonsurgical claims and more than 1.5 million prescriptions for pain medications. Data were extracted from the WCRI Detailed Benchmark/Evaluation database and consisted of detailed prescription transactions "collected from workers’ compensation payers and their medical bill review and pharmacy benefit management vendors," the report noted.
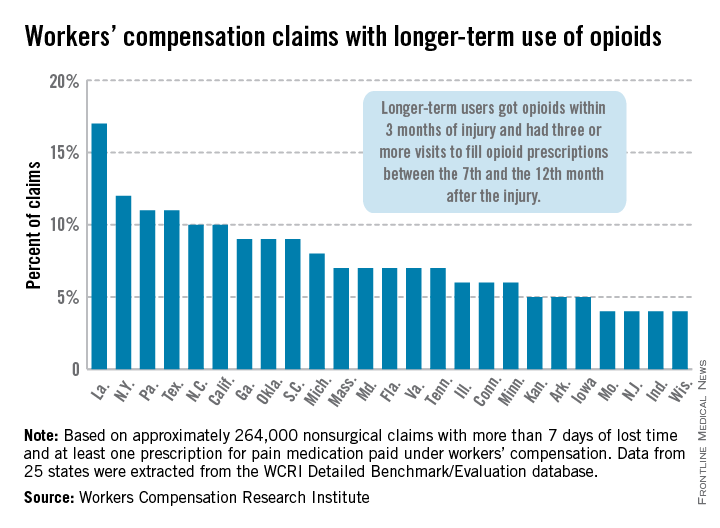
In Louisiana, opioid use lasted more than 6 months in 17% of nonsurgical workers’ compensation claims involving employees who received at least one prescription for pain medication, the Workers Compensation Research Institute reported.
In cases with more than 7 days of lost time, that was the highest rate seen among the 25 states in the study, with New York second at 12% and Pennsylvania and Texas tied for third at 11%. There were four states tied for the lowest rate, at 4%: Missouri, New Jersey, Indiana, and Wisconsin, according to the WCRI report.
Overall, use of narcotics for pain relief by injured workers in such cases ranged from 60% in New Jersey to 88% in Arkansas (median, 76%), while use of any pain medication ranged from 85% in Minnesota to 95% in Florida, Georgia, Tennessee, and Texas (median, 94%), the report showed.
The study involved claims with injuries that occurred from Oct. 1, 2009, through Sept. 30, 2010, with prescriptions filled through March 31, 2012. Longer-term users received a prescription for opioids within 3 months of their injury and had three or more visits to fill opioid prescriptions between the 7th and the 12th month after the injury.
The 25 states in the study "represent more than 70% of the workers’ compensation benefits paid in the United States," the WCRI noted.
The study was based on approximately 264,000 nonsurgical claims and more than 1.5 million prescriptions for pain medications. Data were extracted from the WCRI Detailed Benchmark/Evaluation database and consisted of detailed prescription transactions "collected from workers’ compensation payers and their medical bill review and pharmacy benefit management vendors," the report noted.

In Louisiana, opioid use lasted more than 6 months in 17% of nonsurgical workers’ compensation claims involving employees who received at least one prescription for pain medication, the Workers Compensation Research Institute reported.
In cases with more than 7 days of lost time, that was the highest rate seen among the 25 states in the study, with New York second at 12% and Pennsylvania and Texas tied for third at 11%. There were four states tied for the lowest rate, at 4%: Missouri, New Jersey, Indiana, and Wisconsin, according to the WCRI report.
Overall, use of narcotics for pain relief by injured workers in such cases ranged from 60% in New Jersey to 88% in Arkansas (median, 76%), while use of any pain medication ranged from 85% in Minnesota to 95% in Florida, Georgia, Tennessee, and Texas (median, 94%), the report showed.
The study involved claims with injuries that occurred from Oct. 1, 2009, through Sept. 30, 2010, with prescriptions filled through March 31, 2012. Longer-term users received a prescription for opioids within 3 months of their injury and had three or more visits to fill opioid prescriptions between the 7th and the 12th month after the injury.
The 25 states in the study "represent more than 70% of the workers’ compensation benefits paid in the United States," the WCRI noted.
The study was based on approximately 264,000 nonsurgical claims and more than 1.5 million prescriptions for pain medications. Data were extracted from the WCRI Detailed Benchmark/Evaluation database and consisted of detailed prescription transactions "collected from workers’ compensation payers and their medical bill review and pharmacy benefit management vendors," the report noted.

Management of Papillary Thyroid Cancer: An Overview for the Primary Care Physician
From the Yale School of Medicine, New Haven, CT.
ABSTRACT
• Objective: To review management of papillary thyroid cancer.
• Methods: Review of the literature.
• Results: Papillary thyroid cancer is the most common endocrine malignancy. The standard treatment for papillary thyroid cancer is thyroidectomy. Adjuvant therapy includes lifelong thyroid-stimulating hormone suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event.
• Conclusion: The incidence of papillary thyroid cancer is rapidly increasing. Surgery remains the cornerstone of treatment.
Papillary thyroid cancer is the most common endocrine malignancy and accounts for the majority of cancers of the thyroid. The incidence of papillary thyroid cancer is rapidly increasing [1]. Although increasing detection has been proposed as a possible factor [2], some studies reject this hypothesis, reporting increase in the incidence of larger tumors [3]. Papillary thyroid cancer is characterized by a low mortality but a high recurrence rate [1], posing challenges not only to the endocrinologist and oncologist but also to the general practitioner.
The most frequent presentation of papillary thyroid cancer is a palpable thyroid nodule, cervical lymphadenopathy, or incidental detection on imaging. Locally advanced disease can present with hoarseness or voice alteration. Common risks factors include history of radiation exposure during childhood (the most important risk factor), thyroid cancer in a first-degree relative, family history of a thyroid cancer syndrome (such as Werner syndrome, Cowden syndrome, Carney complex, or familial polyposis), and female sex (2.5:1). Thyroid nodules in the context of an autoimmune thyroiditis may have a higher risk of malignancy [4].
CASE STUDY
Initial Presentation
A 49-year-old man with no significant past medical history presents with a painless mass in the anterior part of his neck.
History, Physical Examination, and Initial Investigations
He has no other symptoms, no weight changes, no history of radiation exposure to the neck, and no family history of malignancy. Physical exam shows a mass in the left thyroid lobe. There is no evidence of cardiac arrhythmias, tremors, or ophthalmologic abnormalities. Thyroid-stimulating hormone (TSH) level is 2.8 mIU/L (normal range, 0.4–4.5 mIU/L) and free thyroxine (T4) level is 1.1 ng/dL (normal range, 0.8–1.5 ng/dL). An ultrasound scan of the neck shows enlargement of the left lobe of thyroid gland, containing multiple complex lesions, the largest measuring 2 x 3 cm, with calcification as well as 3 enlarged lymph nodes in the left level IV. Fine-needle aspiration of the thyroid mass is positive for papillary carcinoma.
• What is the approach to the initial evaluation of a thyroid nodule?
Initial diagnostic evaluation includes history, physical examination, and TSH measurement; nonfunctioning nodules, associated with normal or high values of TSH, carry a higher risk of malignancy [5]. Cervical ultrasound should be performed in all patients with nodules. Fine-needle aspiration (FNA) should be used to evaluate nonfunctioning nodules > 1 cm or subcentimeter nodules with suspicious ultrasound features or if the patient has major risk factors (history of ionizing radiation exposure, external beam radiation exposure, family or personal history of papillary thyroid cancer, or FDG-PET [fluorinated glucose positron emission tomography]–positive thyroid nodules). Scintigraphy can be used to evaluate the need for ultrasound and FNA in patients with low TSH values [6,7]; hyperfunctioning nodules are at low risk for malignancy and do not require biopsy.
• What is initial treatment of papillary thyroid cancer?
Surgery is the primary treatment for papillary thyroid cancer. Unlike for many cancers, surgical removal of the primary tumor is indicated even in the presence of metastatic disease [8]. Total or near-total thyroidectomy is used to treat patients with tumors > 1 cm or with tumors < 1 cm and associated risk factors (eg, contralateral nodules, affected lymph nodes, metastasis, history of radiation, first-degree family history of papillary thyroid cancer, or age > 45 years) [6]. There is a lower risk of recurrence in patients treated with total thyroidectomy versus lobectomy in papillary thyroid cancer [9,10]. Thyroid lobectomy may be used in small (< 1 cm) unifocal tumors without the presence of the associated risk factors listed above.
Patients with central or lateral neck lymph node involvement should also undergo central-compartment (level VI) neck dissection. Therapeutic lateral neck compartmental lymph node dissection is recommended in patients with biopsy-proven metastatic lateral cervical adenopathy [6,7]. The role of unilateral or bilateral prophylactic central-compartment nodal dissection (PCND), that is, lymph node dissection in the level VI compartment of neck in patients without evidence of lymphadenopathy, is controversial. The data for the possible benefit of PCND are inconclusive [11] although the ATA recommends the procedure for locally invasive T3 and T4 tumors [6].
The American Thyroid Association (ATA) and National Comprehensive Cancer Network (NCCN) guidelines [6,7] recommend a preoperative cervical ultrasound in patients with biopsy-proven papillary thyroid cancer to evaluate the presence of disease in the cervical lymph nodes, especially in the lateral and central compartments, and in the contralateral thyroid lobe. If suspicious lymph nodes are found, FNA confirmation is necessary only if this would change management. Systematic use of other preoperative imaging studies, such as CT or MRI, is not recommended [6,7].
Surgical Treatment
The patient underwent a total thyroidectomy with bilateral central neck dissection and selective supraclavicular left-sided lateral neck dissection. Lymph nodes on both sides of the neck (paratracheal nodes) as well as the left supraclavicular nodes were removed. Pathology showed multifocal papillary cancer with extracapsular extension to the paratracheal soft tissue, 14/14 lymph nodes affected, stage IVA T4N1bM0.
• How is papillary thyroid cancer staged?
• How should this patient be treated after surgery? Is any adjuvant therapy indicated?
TSH Suppression
In an effort to reduce risk of recurrence, patients should receive lifelong suppression of TSH using supraphysiologic doses of levothyroxine after total thyroidectomy. This is based upon the hypothesis that TSH is a growth factor for thyroid cancer cells [12,13]. Although a meta-analysis [14] supports the efficacy of TSH suppression therapy, some authors have questioned its widespread use, especially in light of the adverse effects of its use over the long term [15]. Many support its use only in high-risk patients [16], arguing that there is no evidence of benefit for low-risk patients [17]. This view is reflected in the ATA guidelines, which recommend TSH suppression below 0.1 mU/L for high-risk and intermediate-risk patients, while normal or slightly below normal TSH levels are recommended for low-risk patients [6].
Adverse effects of TSH suppression therapy are derived from the induced mild thyrotoxicosis, including cardiovascular and skeletal manifestations. Notably, elderly patients have a higher risk of cardiovascular side effects [18] such as atrial fibrillation, diastolic dysfunction, tachyarrhythmias, increased heart rate or increased left ventricular mass. Likewise, postmenopausal women are most susceptible for skeletal effects such as decreased mineral bone density and fractures [19].
Radioiodine Ablative Therapy
Radioactive iodine (RAI or radioiodine) therapy is based on the capacity of thyroid tissue to take up and retain iodine, specifically, radioiodine. This capacity is present but reduced in papillary and follicular cancer cells.
Radioiodine remnant ablation is performed after surgery, acting as adjuvant therapy by destroying remnant pathological or normal thyroid tissue. The destruction of normal thyroid tissue is useful as it increases the reliability of thyroglobulin testing and radioiodine scanning in the detection of recurrent or metastatic disease. Moreover, remnant ablation has been shown to prevent new thyroid neoplasias in high-risk patients (ie, those with history of radiation exposure). Radioiodine ablative therapy has been shown to reduce recurrence and cause-specific mortality [20] in certain subgroups; however, patients with low mortality risk do not seem to benefit from this therapy [21,22]. Its use is recommended in patients with distant metastases, tumors > 4 cm, or with extrathyroidal extension. It is also recommended for selected patients with tumors 1–4 cm who have high-risk features (such as lymph node involvement, history of radiation, or others previously mentioned) when there is an intermediate to high risk of recurrence or death from thyroid cancer [6]. Lymph node involvement can occur in up to 50% of cases [39] and normally responds to radioiodine therapy.
Since TSH increases radioiodine uptake by normal or pathological thyroid cells, TSH stimulation is required for radioiodine therapy. This can be done by endogenous TSH elevation or by recombinant human TSH (rhTSH). The former can be achieved by either stopping thyroxine 2 to 3 weeks prior to the remnant ablation, or by withdrawing thyroxine and switching to liothyronine for 2 to 3 weeks followed by a discontinuation of liothyronine for 2 weeks. Both approaches seem to produce the same incidence of hypothyroid symptoms [23]. Thyroxine therapy can be resumed 2 to 3 days after radioiodine ablative therapy. Recombinant human TSH can be used with equal efficacy in place of thyroxine withdrawal [24], with the advantage of not producing transitory hypothyroidism. It is especially recommended for patients who are unable to tolerate hypothyroidism or who cannot achieve an adequate TSH level. Short-term recurrence rates are similar in patients treated with rhTSH or thyroxine withdrawal [25].
In addition, a low-iodine diet for 1 or 2 weeks is recommended for patients undergoing radioiodine remnant ablation. The rationale is that a high-iodine diet or iodine exposure (ie, amiodarone treatment or intravenous contrast) can decrease radioiodine uptake by papillary cancer cells due to further dilution of radioactive iodine in an expanded endogenous non-radioactive iodine pool. Patients with suspected high iodine levels can be screened using spot urinary levels [26].
Commonly, a diagnostic scan using low activities of iodine-131 is performed prior to radioablation to avoid the controversial “stunning effect” [27] from any exposure to sublethal radiation in a diagnostic dose. In stunning, the diagnostic RAI dose decreases uptake of a subsequent therapeutic dose. Alternatively, we use [I-123] radioiodine at very low dose (1.4 mCi) in pre-ablation patients. Uptake in the thyroid bed occurs in 75% to 100% of patients, commonly due to remnant normal thyroid tissue [28].
The typical activity used for RAI ablative therapy is 30–100 mCi. The administration of high activities (150–200 mCi) of [I-131] radioiodine has been used to treat recurrent or metastatic disease. This treatment can be very effective, especially in young patients [29].
Side Effects and Contraindications
Common side effects of radioiodine treatment include sialadenitis, radiation thyroiditis, tumor hemorrhage or edema, nausea, transient oligospermia or amenorrhea and nasolacrimal duct obstruction. Moreover, patients treated with radioiodine have a modest increased risk of developing other malignancies [30].
[I-131]Radioiodine must be avoided in pregnancy and in breastfeeding [31]. Indeed, breast tissue has a strong tendency to uptake iodine so breastfeeding should be stopped 5 to 8 weeks before radioiodine treatment, otherwise it can lead to a false-positive radioiodine scan in the chest [32], or worse, deliver radioiodine to the baby with detrimental effects and potential ablation to the baby’s thyroid gland.
Patients treated with radioiodine are advised to drink abundant water after the treatment in order to increase its renal elimination. If no stool elimination occurs in 14 to 24 hours, laxatives may be indicated to eliminate radioiodine from the gastrointestinal track. In addition, patients are advised to avoid sexual contact, avoid sharing bed, utensils, towels, toothbrushes, razors, and avoid public transportation and public places among other measures to avoid exposing the population to radiation [33]. The duration of this restriction depends on the dose administered.
Adjuvant Treatment in this Patient
As the patient was at high risk for recurrence, he received TSH suppression therapy to levels < 0.1 mIU/L. He was referred to nuclear medicine for I-131 treatment. However, at 3 months following thyroidectomy, thyroglobulin measurement showed an elevation (40.5 ng/mL). Ultrasound showed enlarged lymph nodes at level II at the right and at level II at the left. A FNA of left neck node was positive for papillary thyroid cancer.
• How should the patient be treated now?
Treatment of Locoregional Metastatic Disease
The best treatment for residual disease or local recurrences is surgery. ATA guidelines recommend compartmental lateral and/or central neck dissection for patients with persistent or recurrent disease confined to the neck [6]. Radioiodine can be an alternative when recurrent disease is not visible on imaging. Other treatments that can be used for local recurrences or isolated metastases when surgery is not possible are radiofrequency ablation [34], chemo-embolization [35], or ethanol ablation [36]. External beam radiotherapy, which is discussed later, could also be used in selected cases.
Further Treatment
The patient underwent a bilateral modified radical neck dissection followed by adjunctive radioiodine therapy. His initial radioiodine scan showed mild uptake in the neck at the site of his prior surgery. He received treatment with 215 mCi, then 6 months later he was treated with 250 mCi, as his scan showed continued mild uptake. Eleven months later his radioiodine scan showed no uptake and thyroglobulin levels remained stable at 14.4 ng/mL.
One year later, in a follow-up blood analysis he was found to have an elevated thyroglobulin level (90.4 ng/mL). A PET/CT scan showed multiple bone metastases. A neck ultrasound revealed enlarged lymph nodes in the right thyroid bed.
• How common is radioiodine-refractory thyroid cancer?
Radioiodine-refractory thyroid cancer in patients with progression of disease despite radioiodine therapy, or with non-radioiodine-avid lesions [37], is uncommon. It has a poor prognosis with a median survival of 3 to 6 years after diagnosis. It is more frequent in older patients. These lesions are often hypermetabolic and hence [F-18]FDG-avid [38], with a worse prognosis. In one study of patients with metastatic differentiated thyroid cancer, the 10-year overall survival rate was 56% in patients with radioiodine-avid lesions but only 10% in patients with non-radioiodine-avid lesions [38].
• Is the bone a common place for metastasis? Where else should we expect to find a lesion?
Metastatic Pattern
The most common sites for distant metastasis of papillary thyroid cancer are the lungs and the bone. The 10-year survival rate of papillary thyroid cancer patients with lung metastases is between 30% and 50% [38,39]; the prog-nosis is better in patients < 45 years and with radiodine uptake [40]; indeed, patients with pulmonary metastasis seen only in 131-I scans and not on CT or chest x-ray have a longer survival [41]. Pulmonary metastasis can be treated with radioiodine if they are radioiodine-avid. With this treatment complete remission is possible, although it is extremely difficult to achieve in macronodular metastasis.
Bones are the second most common place for distant metastases. Bone metastases seem to have a worse response to treatment with an unfavorable prognosis [42]. Pamidronate (a biphosphonate) and denosumab (a RANK ligand inhibitor) have been used to prevent skeletal related events, including pathologic fractures and cord compression, in bone metastases from other cancers such as breast and prostate, and may also be useful in thyroid cancer, although this has not yet been studied [43,44]. Moreover, surgical resection of isolated bone metastasis seems to improve survival [45].
Skin, liver, and brain metastasis, although uncommon, can also occur. There are also reported rare cases of metastasis in the breast, parotid, larynx, pharynx, adrenal glands, pituitary, kidney, liver, orbit, the sphenoid sinus, choroid plexus, pancreas, and skeletal muscles [46].
• Which treatments can we offer to a patient with metastatic disease refractory to radioiodine?
Chemotherapy and Treatment of Radioiodine-Resistant Disease
Therapeutic options for patients with metastatic papillary thyroid cancer resistant to radioiodine and TSH suppression are limited. Cytotoxic drugs do not play a major role in the treatment of refractory metastatic papillary thyroid cancer, and new research is mainly focused on tyrosine kinase inhibitors (TKIs) with a considerable number of clinical trials either completed or ongoing.
Tyrosine kinases are enzymes that transfer phosphate groups from adenosine triphosphate to proteins. In tumor cells their signaling paths promote proliferation, avoidance of apoptosis, invasion, angiogenesis, and metastasis. TKIs are small molecules that are able to inhibit tyrosine kinase function even at very low intracellullar concentrations. Some of them inhibit various tyrosine kinases and are known as multi-kinase inhibitors (MKIs).
Sorafenib
Sorafenib (400 mg twice daily) is an oral MKI that targets RAF, platelet-derived growth factor receptor, vascular endothelial growth factor receptors 2 and 3, RET and c-Kit [47]. It was approved in November 2013 for patients with radioiodine-refractory differentiated thyroid cancer [48]. Three phase II studies had previously evaluated sorafenib in papillary thyroid cancer, showing a partial response in 15% to 31% of patients and a progression-free survival up to 79 weeks [49–51]. Common adverse effects included weight loss, fatigue, rash, hypertension and the main dose-limiting toxicity—a hand-foot syndrome consisting of swelling, reddening, numbness, and desquamation on palms and soles [52].
Approval of the drug was based on the DECISION trial [52]. A total of 417 patients were randomized (207 to sorafenib and 210 to placebo), of which 57% had papillary thyroid cancer. The primary endpoint of progression-free survival (PFS) was significantly higher in the sorafenib arm, (median, 10.8 months) compared with placebo (median, 5.8 months) (hazard ratio [HR] 0.58, 95% confidence interval [CI] 0.45–0.75, P < 0.001). Median overall survival had not been reached in either arm [52]. The PFS of 5.8 months in the placebo arm confirmed that the group of patients in this study had a rapidly progressing disease, unlike the majority of patients with RAI-sensitive disease.
Selumetinib
Radioiodine re-sensitization was addressed in a study using selumitinib, an inhibitor of mitogen-activated protein kinase kinase (MAPK kinase or MEK). Preclinical models had shown that radioiodine-refractory tumors exposed to inhibitors of this enzyme were able to uptake radioiodine again. Twenty patients with radioiodine-refractory thyroid cancers were treated with selumetinib for 4 weeks and 12 showed increased radioiodine uptake following the treatment. Furthermore, 8 of these patients went on to show responses clinically to retreatment with radioiodine [53].Further studies with this agent will be needed to determine its place in treating patients with differentiated thyroid cancer.
External Beam Radiotherapy and Local Treatment for Metastases
The role of external beam radiotherapy in papillary thyroid cancer is mainly for symptom management. Local radiation can be used in patients with refractory metastatic disease or in lesions that do not uptake radioiodine. Examples include painful bone metastasis or brain metastasis that cannot be treated with surgery. In addition, radiofrequency ablation, chemo-embolization, or ethanol ablation can be used in certain patients.
Sequence of Treatments
In the setting of symptomatic metastatic, radioiodine-resistant disease, we prefer to use a TKI, normally sorafenib, as a first-line treatment. For second-line treatments, enrollment in a clinical trial is an option. Over 70% of patients with metastatic papillary thyroid cancer have mutations of the enzyme BRAF kinase. Vemurafenib is an inhibitor of this enzyme and appears to have some activity in patients with RAI-refractory thyroid cancer in early clinical trials [54–58]. Other TKIs such as sunitinib can also be used. Doxorubicin is only used in cases when a patient is not eligible for a trial and the off-label use of another TKI is contraindicated.
Further Treatment in this Patient
The patient received a trial of sorafenib. He showed disease stabilization that lasted 5 months. The treatment was stopped due to adverse effects (loss of weight and vomiting) and progression of the disease. He was then enrolled in a trial of vemurafenib. He stopped treatment because of adverse events related to the medication and currently has stable disease.
Summary
Papillary thyroid cancer is the most common endocrine malignancy. It is characterized by low mortality but high recurrence rate and can have a considerable impact on quality of life. Any anterior neck nodule, especially in a patient with a history of neck irradiation, should raise concern for this disease. Surgery remains the cornerstone of treatment. Adjuvant therapy includes lifelong TSH suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event. Thyroid cancer has a tendency to metastasize to the bones and lungs. Metastatic radioiodine-resistant disease is often treated with TKIs such as sorafenib. Enrollment in clinical trials is recommended as second-line therapy in radioiodine-resistant metastatic disease.
Corresponding author: Hari A. Deshpande, MD, Yale Cancer Center, FMP 124, 333 Cedar St., New Haven, CT 06520, [email protected]
Financial disclosures: Dr. Deshpande reports that he is on the advisory board of Bayer/Onyx.
Author contributions: conception and design, PT, EHH, GGC, HAD; drafting of article, PT, EHH, GGC, HAD; critical revision of the article, EHH, GGC, HAD.
REFERENCES
1. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2010.
2. Katoh R, Sasaki J, Kurihara H, et al. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma: a clinicopathologic study of 105 consecutive patients. Cancer 1992;70:1585–90.
3. Morris LG, Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 2010;200:454–61.
4. Fiore E, Rago T, Latrofa F, et al. Hashimoto’s thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with Lthyroxine. Endocr Relat Cancer 2011;18:429–37.
5. Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 2008;93:809–14.
6. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214.
7. National Comprehensive Cancer Network guidelines. Available at www.nccn.org/professionals/physician_gls/pdf/thyroid. pdf.
8. Stephenson BM, Wheeler MH, Clark OH. The role of total thyroidectomy in the management of differentiated thyroid cancer. Curr Opin Gen Surg 1994 53–9.
9. Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 2007;246:375–81.
10. Hay ID, Grant CS, Bergstralh EJ, et al. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery 1998;124:958–64.
11. McLeod DS, Sawka AM, Cooper DS. Controversies in primarytreatment of low-risk papillary thyroid cancer. Lancet 2013;381:1046–57.
12. Brabant G. 2008 Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J Clin Endocrinol Metab 2008;93:1167–9.
13. Kim HK, Yoon JH, Kim SJ, Cho JS. Higher TSH level is a risk factor for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2013;78:472–7.
14. McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med 2002;34:554–64.
15. Zafón C. TSH-suppressive treatment in differentiated thyroid cancer. A dogma under review. Endocrin Nutr 2012;59:125–30.
16. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998;8:737-44.
17. Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006;16:1229–42.
18. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 1994;33:1249–52.
19. Kung AW, Yeung SS. Prevention of bone loss induced by thyroxine suppressive therapy in postmenopausal women: the effect of calcium and calcitonin. J Clin Endocrinol Metab 1996;81:1232–36.
20. Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab 1992;75:714–20.
21. Sugitani I, Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J 1999;46:209–16.
22. Kim S, Wei JP, Braveman JM, Brams DM. Predictingoutcome and directing therapy for papillary thyroid carcinoma. Arch Surg 2004;139:390–4.
23. Leboeuf R, Perron P, Carpentier AC, et al. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clin Endocrinol (Oxf ) 2007;67:839–44.
24. Pacini F, Ladenson PW, Schlumberger M, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab 2006;91:926–32.
25. Tuttle RM, Brokhin M, Omry G, et al. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal. J Nucl Med 2008;49:764–70.
26. Pluijmen MJ, Eustatia-Rutten C, Goslings BM, et al. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf ) 2003;58:428–35.
27. Park HM. Stunned thyroid after high-dose I-131 imaging. Clin Nucl Med 1992; 17:501–2.
28. Salvatori M, Raffaelli M, Castaldi P, et al. Evaluation of the surgical completeness after total thyroidectomy for differentiated thyroid carcinoma. Eur J Surg Oncol 2007;33:648–54.
29. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–9.
30. Lang BH, Wong IO, Wong KP, et al. Risk of second primary malignancy in differentiated thyroid carcinoma treated with radioactive iodine therapy. Surgery 2012;151:844–50.
31. Rubow S, Klopper J. Excretion of radioiodine in human milk following a therapeutic dose of I-131. Eur J Nucl Med 1988;14:632–3.
32. Bakheet SM, Hammami MM. Patterns of radioiodine uptake by the lactating breast. Eur J Nucl Med 1994;21:604–8.
33. American Thyroid Association Taskforce on Radioiodine Safety, Sisson JC, Freitas J, et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: practice recommendations of the American Thyroid Association. Thyroid 2011;21:335–46.
34. Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from welldifferentiated thyroid malignancy. Surgery 2001;130:971–7.
35. Eustatia-Rutten CF, Romijn JA, Guijt MJ, et al. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J Clin Endocrinol Metab 2003;88:3184–9.
36. Lewis BD, Hay ID, Charboneau JW, et al. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. Am J Roentgenol 2002;178:699–704.
37. Xing MM, Haugen B, Schlumberger M. Progress in molecular based management of differentiated thyroid cancer. Lancet 2013;381:1058–69.
38. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–99.
39. Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome Clin Endoc 2005;63:87–93.
40. Ronga G, Filesi M, Montesano T, et al. Lung metastases from differentiated thyroid carcinoma. A 40 years’ experience. Q J Nucl Med Mol Imaging 2004;48:12–19.
41. Samaan NA, Schultz PN, Haynie TP, Ordonez NG. Pulmonary metastasis of differentiated thyroid carcinoma: treatment results in 101 patients. J Clin Endocrinol Metab 1985;60:376–80.
42. Lang BH, Wong KP, Cheung CY, et al. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 2013;20:1329–35.
43. Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med 1996;335:1785–92.
44. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebocontrolled trial. Lancet 379:39–46.
45. Zettinig G, Fueger BJ, Passler C, et al. Long-term follow-up of patients with bone metastases from differentiated thyroid carcinoma—surgery or conventional therapy? Clin Endocrinol (Oxf ) 2002;56:377–82.
46. Song H-J, Xue Y-L, Xu Y-H, et al. Rare metastases of differentiated thyroid carcinoma: pictorial review Endocr Relat Cancer 2011;18:R165–R174.
47. Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–109.
48. www.fda.gov/NewsEvents/Newsroom/PresAnnouncements/ucm376443.htm.
49. Kloos RT, Ringel MD, Knopp MV et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 2009;27:1675–84.
50. Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 2008;26:4714–9.
51. Schneider TC, Abdulrahman RM, Corssmit EP, et al. Longterm analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol 2012;167:643–50.
52. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine refractory differentiated thyroid cancer: The phase III DECISION trial. J Clin Oncol 2013;31(Suppl, abstr 4).
53. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 2013;368:623–32.
54. Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res 2009;15:485–91.
55. Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364–8.
56. Elisei R, Ugolini C, Viola D, et al. BRAF (V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 2008;93:3943–9.
57. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAFV600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2103;309:1493–501.
58. Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring
From the Yale School of Medicine, New Haven, CT.
ABSTRACT
• Objective: To review management of papillary thyroid cancer.
• Methods: Review of the literature.
• Results: Papillary thyroid cancer is the most common endocrine malignancy. The standard treatment for papillary thyroid cancer is thyroidectomy. Adjuvant therapy includes lifelong thyroid-stimulating hormone suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event.
• Conclusion: The incidence of papillary thyroid cancer is rapidly increasing. Surgery remains the cornerstone of treatment.
Papillary thyroid cancer is the most common endocrine malignancy and accounts for the majority of cancers of the thyroid. The incidence of papillary thyroid cancer is rapidly increasing [1]. Although increasing detection has been proposed as a possible factor [2], some studies reject this hypothesis, reporting increase in the incidence of larger tumors [3]. Papillary thyroid cancer is characterized by a low mortality but a high recurrence rate [1], posing challenges not only to the endocrinologist and oncologist but also to the general practitioner.
The most frequent presentation of papillary thyroid cancer is a palpable thyroid nodule, cervical lymphadenopathy, or incidental detection on imaging. Locally advanced disease can present with hoarseness or voice alteration. Common risks factors include history of radiation exposure during childhood (the most important risk factor), thyroid cancer in a first-degree relative, family history of a thyroid cancer syndrome (such as Werner syndrome, Cowden syndrome, Carney complex, or familial polyposis), and female sex (2.5:1). Thyroid nodules in the context of an autoimmune thyroiditis may have a higher risk of malignancy [4].
CASE STUDY
Initial Presentation
A 49-year-old man with no significant past medical history presents with a painless mass in the anterior part of his neck.
History, Physical Examination, and Initial Investigations
He has no other symptoms, no weight changes, no history of radiation exposure to the neck, and no family history of malignancy. Physical exam shows a mass in the left thyroid lobe. There is no evidence of cardiac arrhythmias, tremors, or ophthalmologic abnormalities. Thyroid-stimulating hormone (TSH) level is 2.8 mIU/L (normal range, 0.4–4.5 mIU/L) and free thyroxine (T4) level is 1.1 ng/dL (normal range, 0.8–1.5 ng/dL). An ultrasound scan of the neck shows enlargement of the left lobe of thyroid gland, containing multiple complex lesions, the largest measuring 2 x 3 cm, with calcification as well as 3 enlarged lymph nodes in the left level IV. Fine-needle aspiration of the thyroid mass is positive for papillary carcinoma.
• What is the approach to the initial evaluation of a thyroid nodule?
Initial diagnostic evaluation includes history, physical examination, and TSH measurement; nonfunctioning nodules, associated with normal or high values of TSH, carry a higher risk of malignancy [5]. Cervical ultrasound should be performed in all patients with nodules. Fine-needle aspiration (FNA) should be used to evaluate nonfunctioning nodules > 1 cm or subcentimeter nodules with suspicious ultrasound features or if the patient has major risk factors (history of ionizing radiation exposure, external beam radiation exposure, family or personal history of papillary thyroid cancer, or FDG-PET [fluorinated glucose positron emission tomography]–positive thyroid nodules). Scintigraphy can be used to evaluate the need for ultrasound and FNA in patients with low TSH values [6,7]; hyperfunctioning nodules are at low risk for malignancy and do not require biopsy.
• What is initial treatment of papillary thyroid cancer?
Surgery is the primary treatment for papillary thyroid cancer. Unlike for many cancers, surgical removal of the primary tumor is indicated even in the presence of metastatic disease [8]. Total or near-total thyroidectomy is used to treat patients with tumors > 1 cm or with tumors < 1 cm and associated risk factors (eg, contralateral nodules, affected lymph nodes, metastasis, history of radiation, first-degree family history of papillary thyroid cancer, or age > 45 years) [6]. There is a lower risk of recurrence in patients treated with total thyroidectomy versus lobectomy in papillary thyroid cancer [9,10]. Thyroid lobectomy may be used in small (< 1 cm) unifocal tumors without the presence of the associated risk factors listed above.
Patients with central or lateral neck lymph node involvement should also undergo central-compartment (level VI) neck dissection. Therapeutic lateral neck compartmental lymph node dissection is recommended in patients with biopsy-proven metastatic lateral cervical adenopathy [6,7]. The role of unilateral or bilateral prophylactic central-compartment nodal dissection (PCND), that is, lymph node dissection in the level VI compartment of neck in patients without evidence of lymphadenopathy, is controversial. The data for the possible benefit of PCND are inconclusive [11] although the ATA recommends the procedure for locally invasive T3 and T4 tumors [6].
The American Thyroid Association (ATA) and National Comprehensive Cancer Network (NCCN) guidelines [6,7] recommend a preoperative cervical ultrasound in patients with biopsy-proven papillary thyroid cancer to evaluate the presence of disease in the cervical lymph nodes, especially in the lateral and central compartments, and in the contralateral thyroid lobe. If suspicious lymph nodes are found, FNA confirmation is necessary only if this would change management. Systematic use of other preoperative imaging studies, such as CT or MRI, is not recommended [6,7].
Surgical Treatment
The patient underwent a total thyroidectomy with bilateral central neck dissection and selective supraclavicular left-sided lateral neck dissection. Lymph nodes on both sides of the neck (paratracheal nodes) as well as the left supraclavicular nodes were removed. Pathology showed multifocal papillary cancer with extracapsular extension to the paratracheal soft tissue, 14/14 lymph nodes affected, stage IVA T4N1bM0.
• How is papillary thyroid cancer staged?
• How should this patient be treated after surgery? Is any adjuvant therapy indicated?
TSH Suppression
In an effort to reduce risk of recurrence, patients should receive lifelong suppression of TSH using supraphysiologic doses of levothyroxine after total thyroidectomy. This is based upon the hypothesis that TSH is a growth factor for thyroid cancer cells [12,13]. Although a meta-analysis [14] supports the efficacy of TSH suppression therapy, some authors have questioned its widespread use, especially in light of the adverse effects of its use over the long term [15]. Many support its use only in high-risk patients [16], arguing that there is no evidence of benefit for low-risk patients [17]. This view is reflected in the ATA guidelines, which recommend TSH suppression below 0.1 mU/L for high-risk and intermediate-risk patients, while normal or slightly below normal TSH levels are recommended for low-risk patients [6].
Adverse effects of TSH suppression therapy are derived from the induced mild thyrotoxicosis, including cardiovascular and skeletal manifestations. Notably, elderly patients have a higher risk of cardiovascular side effects [18] such as atrial fibrillation, diastolic dysfunction, tachyarrhythmias, increased heart rate or increased left ventricular mass. Likewise, postmenopausal women are most susceptible for skeletal effects such as decreased mineral bone density and fractures [19].
Radioiodine Ablative Therapy
Radioactive iodine (RAI or radioiodine) therapy is based on the capacity of thyroid tissue to take up and retain iodine, specifically, radioiodine. This capacity is present but reduced in papillary and follicular cancer cells.
Radioiodine remnant ablation is performed after surgery, acting as adjuvant therapy by destroying remnant pathological or normal thyroid tissue. The destruction of normal thyroid tissue is useful as it increases the reliability of thyroglobulin testing and radioiodine scanning in the detection of recurrent or metastatic disease. Moreover, remnant ablation has been shown to prevent new thyroid neoplasias in high-risk patients (ie, those with history of radiation exposure). Radioiodine ablative therapy has been shown to reduce recurrence and cause-specific mortality [20] in certain subgroups; however, patients with low mortality risk do not seem to benefit from this therapy [21,22]. Its use is recommended in patients with distant metastases, tumors > 4 cm, or with extrathyroidal extension. It is also recommended for selected patients with tumors 1–4 cm who have high-risk features (such as lymph node involvement, history of radiation, or others previously mentioned) when there is an intermediate to high risk of recurrence or death from thyroid cancer [6]. Lymph node involvement can occur in up to 50% of cases [39] and normally responds to radioiodine therapy.
Since TSH increases radioiodine uptake by normal or pathological thyroid cells, TSH stimulation is required for radioiodine therapy. This can be done by endogenous TSH elevation or by recombinant human TSH (rhTSH). The former can be achieved by either stopping thyroxine 2 to 3 weeks prior to the remnant ablation, or by withdrawing thyroxine and switching to liothyronine for 2 to 3 weeks followed by a discontinuation of liothyronine for 2 weeks. Both approaches seem to produce the same incidence of hypothyroid symptoms [23]. Thyroxine therapy can be resumed 2 to 3 days after radioiodine ablative therapy. Recombinant human TSH can be used with equal efficacy in place of thyroxine withdrawal [24], with the advantage of not producing transitory hypothyroidism. It is especially recommended for patients who are unable to tolerate hypothyroidism or who cannot achieve an adequate TSH level. Short-term recurrence rates are similar in patients treated with rhTSH or thyroxine withdrawal [25].
In addition, a low-iodine diet for 1 or 2 weeks is recommended for patients undergoing radioiodine remnant ablation. The rationale is that a high-iodine diet or iodine exposure (ie, amiodarone treatment or intravenous contrast) can decrease radioiodine uptake by papillary cancer cells due to further dilution of radioactive iodine in an expanded endogenous non-radioactive iodine pool. Patients with suspected high iodine levels can be screened using spot urinary levels [26].
Commonly, a diagnostic scan using low activities of iodine-131 is performed prior to radioablation to avoid the controversial “stunning effect” [27] from any exposure to sublethal radiation in a diagnostic dose. In stunning, the diagnostic RAI dose decreases uptake of a subsequent therapeutic dose. Alternatively, we use [I-123] radioiodine at very low dose (1.4 mCi) in pre-ablation patients. Uptake in the thyroid bed occurs in 75% to 100% of patients, commonly due to remnant normal thyroid tissue [28].
The typical activity used for RAI ablative therapy is 30–100 mCi. The administration of high activities (150–200 mCi) of [I-131] radioiodine has been used to treat recurrent or metastatic disease. This treatment can be very effective, especially in young patients [29].
Side Effects and Contraindications
Common side effects of radioiodine treatment include sialadenitis, radiation thyroiditis, tumor hemorrhage or edema, nausea, transient oligospermia or amenorrhea and nasolacrimal duct obstruction. Moreover, patients treated with radioiodine have a modest increased risk of developing other malignancies [30].
[I-131]Radioiodine must be avoided in pregnancy and in breastfeeding [31]. Indeed, breast tissue has a strong tendency to uptake iodine so breastfeeding should be stopped 5 to 8 weeks before radioiodine treatment, otherwise it can lead to a false-positive radioiodine scan in the chest [32], or worse, deliver radioiodine to the baby with detrimental effects and potential ablation to the baby’s thyroid gland.
Patients treated with radioiodine are advised to drink abundant water after the treatment in order to increase its renal elimination. If no stool elimination occurs in 14 to 24 hours, laxatives may be indicated to eliminate radioiodine from the gastrointestinal track. In addition, patients are advised to avoid sexual contact, avoid sharing bed, utensils, towels, toothbrushes, razors, and avoid public transportation and public places among other measures to avoid exposing the population to radiation [33]. The duration of this restriction depends on the dose administered.
Adjuvant Treatment in this Patient
As the patient was at high risk for recurrence, he received TSH suppression therapy to levels < 0.1 mIU/L. He was referred to nuclear medicine for I-131 treatment. However, at 3 months following thyroidectomy, thyroglobulin measurement showed an elevation (40.5 ng/mL). Ultrasound showed enlarged lymph nodes at level II at the right and at level II at the left. A FNA of left neck node was positive for papillary thyroid cancer.
• How should the patient be treated now?
Treatment of Locoregional Metastatic Disease
The best treatment for residual disease or local recurrences is surgery. ATA guidelines recommend compartmental lateral and/or central neck dissection for patients with persistent or recurrent disease confined to the neck [6]. Radioiodine can be an alternative when recurrent disease is not visible on imaging. Other treatments that can be used for local recurrences or isolated metastases when surgery is not possible are radiofrequency ablation [34], chemo-embolization [35], or ethanol ablation [36]. External beam radiotherapy, which is discussed later, could also be used in selected cases.
Further Treatment
The patient underwent a bilateral modified radical neck dissection followed by adjunctive radioiodine therapy. His initial radioiodine scan showed mild uptake in the neck at the site of his prior surgery. He received treatment with 215 mCi, then 6 months later he was treated with 250 mCi, as his scan showed continued mild uptake. Eleven months later his radioiodine scan showed no uptake and thyroglobulin levels remained stable at 14.4 ng/mL.
One year later, in a follow-up blood analysis he was found to have an elevated thyroglobulin level (90.4 ng/mL). A PET/CT scan showed multiple bone metastases. A neck ultrasound revealed enlarged lymph nodes in the right thyroid bed.
• How common is radioiodine-refractory thyroid cancer?
Radioiodine-refractory thyroid cancer in patients with progression of disease despite radioiodine therapy, or with non-radioiodine-avid lesions [37], is uncommon. It has a poor prognosis with a median survival of 3 to 6 years after diagnosis. It is more frequent in older patients. These lesions are often hypermetabolic and hence [F-18]FDG-avid [38], with a worse prognosis. In one study of patients with metastatic differentiated thyroid cancer, the 10-year overall survival rate was 56% in patients with radioiodine-avid lesions but only 10% in patients with non-radioiodine-avid lesions [38].
• Is the bone a common place for metastasis? Where else should we expect to find a lesion?
Metastatic Pattern
The most common sites for distant metastasis of papillary thyroid cancer are the lungs and the bone. The 10-year survival rate of papillary thyroid cancer patients with lung metastases is between 30% and 50% [38,39]; the prog-nosis is better in patients < 45 years and with radiodine uptake [40]; indeed, patients with pulmonary metastasis seen only in 131-I scans and not on CT or chest x-ray have a longer survival [41]. Pulmonary metastasis can be treated with radioiodine if they are radioiodine-avid. With this treatment complete remission is possible, although it is extremely difficult to achieve in macronodular metastasis.
Bones are the second most common place for distant metastases. Bone metastases seem to have a worse response to treatment with an unfavorable prognosis [42]. Pamidronate (a biphosphonate) and denosumab (a RANK ligand inhibitor) have been used to prevent skeletal related events, including pathologic fractures and cord compression, in bone metastases from other cancers such as breast and prostate, and may also be useful in thyroid cancer, although this has not yet been studied [43,44]. Moreover, surgical resection of isolated bone metastasis seems to improve survival [45].
Skin, liver, and brain metastasis, although uncommon, can also occur. There are also reported rare cases of metastasis in the breast, parotid, larynx, pharynx, adrenal glands, pituitary, kidney, liver, orbit, the sphenoid sinus, choroid plexus, pancreas, and skeletal muscles [46].
• Which treatments can we offer to a patient with metastatic disease refractory to radioiodine?
Chemotherapy and Treatment of Radioiodine-Resistant Disease
Therapeutic options for patients with metastatic papillary thyroid cancer resistant to radioiodine and TSH suppression are limited. Cytotoxic drugs do not play a major role in the treatment of refractory metastatic papillary thyroid cancer, and new research is mainly focused on tyrosine kinase inhibitors (TKIs) with a considerable number of clinical trials either completed or ongoing.
Tyrosine kinases are enzymes that transfer phosphate groups from adenosine triphosphate to proteins. In tumor cells their signaling paths promote proliferation, avoidance of apoptosis, invasion, angiogenesis, and metastasis. TKIs are small molecules that are able to inhibit tyrosine kinase function even at very low intracellullar concentrations. Some of them inhibit various tyrosine kinases and are known as multi-kinase inhibitors (MKIs).
Sorafenib
Sorafenib (400 mg twice daily) is an oral MKI that targets RAF, platelet-derived growth factor receptor, vascular endothelial growth factor receptors 2 and 3, RET and c-Kit [47]. It was approved in November 2013 for patients with radioiodine-refractory differentiated thyroid cancer [48]. Three phase II studies had previously evaluated sorafenib in papillary thyroid cancer, showing a partial response in 15% to 31% of patients and a progression-free survival up to 79 weeks [49–51]. Common adverse effects included weight loss, fatigue, rash, hypertension and the main dose-limiting toxicity—a hand-foot syndrome consisting of swelling, reddening, numbness, and desquamation on palms and soles [52].
Approval of the drug was based on the DECISION trial [52]. A total of 417 patients were randomized (207 to sorafenib and 210 to placebo), of which 57% had papillary thyroid cancer. The primary endpoint of progression-free survival (PFS) was significantly higher in the sorafenib arm, (median, 10.8 months) compared with placebo (median, 5.8 months) (hazard ratio [HR] 0.58, 95% confidence interval [CI] 0.45–0.75, P < 0.001). Median overall survival had not been reached in either arm [52]. The PFS of 5.8 months in the placebo arm confirmed that the group of patients in this study had a rapidly progressing disease, unlike the majority of patients with RAI-sensitive disease.
Selumetinib
Radioiodine re-sensitization was addressed in a study using selumitinib, an inhibitor of mitogen-activated protein kinase kinase (MAPK kinase or MEK). Preclinical models had shown that radioiodine-refractory tumors exposed to inhibitors of this enzyme were able to uptake radioiodine again. Twenty patients with radioiodine-refractory thyroid cancers were treated with selumetinib for 4 weeks and 12 showed increased radioiodine uptake following the treatment. Furthermore, 8 of these patients went on to show responses clinically to retreatment with radioiodine [53].Further studies with this agent will be needed to determine its place in treating patients with differentiated thyroid cancer.
External Beam Radiotherapy and Local Treatment for Metastases
The role of external beam radiotherapy in papillary thyroid cancer is mainly for symptom management. Local radiation can be used in patients with refractory metastatic disease or in lesions that do not uptake radioiodine. Examples include painful bone metastasis or brain metastasis that cannot be treated with surgery. In addition, radiofrequency ablation, chemo-embolization, or ethanol ablation can be used in certain patients.
Sequence of Treatments
In the setting of symptomatic metastatic, radioiodine-resistant disease, we prefer to use a TKI, normally sorafenib, as a first-line treatment. For second-line treatments, enrollment in a clinical trial is an option. Over 70% of patients with metastatic papillary thyroid cancer have mutations of the enzyme BRAF kinase. Vemurafenib is an inhibitor of this enzyme and appears to have some activity in patients with RAI-refractory thyroid cancer in early clinical trials [54–58]. Other TKIs such as sunitinib can also be used. Doxorubicin is only used in cases when a patient is not eligible for a trial and the off-label use of another TKI is contraindicated.
Further Treatment in this Patient
The patient received a trial of sorafenib. He showed disease stabilization that lasted 5 months. The treatment was stopped due to adverse effects (loss of weight and vomiting) and progression of the disease. He was then enrolled in a trial of vemurafenib. He stopped treatment because of adverse events related to the medication and currently has stable disease.
Summary
Papillary thyroid cancer is the most common endocrine malignancy. It is characterized by low mortality but high recurrence rate and can have a considerable impact on quality of life. Any anterior neck nodule, especially in a patient with a history of neck irradiation, should raise concern for this disease. Surgery remains the cornerstone of treatment. Adjuvant therapy includes lifelong TSH suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event. Thyroid cancer has a tendency to metastasize to the bones and lungs. Metastatic radioiodine-resistant disease is often treated with TKIs such as sorafenib. Enrollment in clinical trials is recommended as second-line therapy in radioiodine-resistant metastatic disease.
Corresponding author: Hari A. Deshpande, MD, Yale Cancer Center, FMP 124, 333 Cedar St., New Haven, CT 06520, [email protected]
Financial disclosures: Dr. Deshpande reports that he is on the advisory board of Bayer/Onyx.
Author contributions: conception and design, PT, EHH, GGC, HAD; drafting of article, PT, EHH, GGC, HAD; critical revision of the article, EHH, GGC, HAD.
REFERENCES
1. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2010.
2. Katoh R, Sasaki J, Kurihara H, et al. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma: a clinicopathologic study of 105 consecutive patients. Cancer 1992;70:1585–90.
3. Morris LG, Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 2010;200:454–61.
4. Fiore E, Rago T, Latrofa F, et al. Hashimoto’s thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with Lthyroxine. Endocr Relat Cancer 2011;18:429–37.
5. Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 2008;93:809–14.
6. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214.
7. National Comprehensive Cancer Network guidelines. Available at www.nccn.org/professionals/physician_gls/pdf/thyroid. pdf.
8. Stephenson BM, Wheeler MH, Clark OH. The role of total thyroidectomy in the management of differentiated thyroid cancer. Curr Opin Gen Surg 1994 53–9.
9. Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 2007;246:375–81.
10. Hay ID, Grant CS, Bergstralh EJ, et al. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery 1998;124:958–64.
11. McLeod DS, Sawka AM, Cooper DS. Controversies in primarytreatment of low-risk papillary thyroid cancer. Lancet 2013;381:1046–57.
12. Brabant G. 2008 Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J Clin Endocrinol Metab 2008;93:1167–9.
13. Kim HK, Yoon JH, Kim SJ, Cho JS. Higher TSH level is a risk factor for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2013;78:472–7.
14. McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med 2002;34:554–64.
15. Zafón C. TSH-suppressive treatment in differentiated thyroid cancer. A dogma under review. Endocrin Nutr 2012;59:125–30.
16. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998;8:737-44.
17. Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006;16:1229–42.
18. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 1994;33:1249–52.
19. Kung AW, Yeung SS. Prevention of bone loss induced by thyroxine suppressive therapy in postmenopausal women: the effect of calcium and calcitonin. J Clin Endocrinol Metab 1996;81:1232–36.
20. Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab 1992;75:714–20.
21. Sugitani I, Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J 1999;46:209–16.
22. Kim S, Wei JP, Braveman JM, Brams DM. Predictingoutcome and directing therapy for papillary thyroid carcinoma. Arch Surg 2004;139:390–4.
23. Leboeuf R, Perron P, Carpentier AC, et al. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clin Endocrinol (Oxf ) 2007;67:839–44.
24. Pacini F, Ladenson PW, Schlumberger M, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab 2006;91:926–32.
25. Tuttle RM, Brokhin M, Omry G, et al. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal. J Nucl Med 2008;49:764–70.
26. Pluijmen MJ, Eustatia-Rutten C, Goslings BM, et al. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf ) 2003;58:428–35.
27. Park HM. Stunned thyroid after high-dose I-131 imaging. Clin Nucl Med 1992; 17:501–2.
28. Salvatori M, Raffaelli M, Castaldi P, et al. Evaluation of the surgical completeness after total thyroidectomy for differentiated thyroid carcinoma. Eur J Surg Oncol 2007;33:648–54.
29. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–9.
30. Lang BH, Wong IO, Wong KP, et al. Risk of second primary malignancy in differentiated thyroid carcinoma treated with radioactive iodine therapy. Surgery 2012;151:844–50.
31. Rubow S, Klopper J. Excretion of radioiodine in human milk following a therapeutic dose of I-131. Eur J Nucl Med 1988;14:632–3.
32. Bakheet SM, Hammami MM. Patterns of radioiodine uptake by the lactating breast. Eur J Nucl Med 1994;21:604–8.
33. American Thyroid Association Taskforce on Radioiodine Safety, Sisson JC, Freitas J, et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: practice recommendations of the American Thyroid Association. Thyroid 2011;21:335–46.
34. Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from welldifferentiated thyroid malignancy. Surgery 2001;130:971–7.
35. Eustatia-Rutten CF, Romijn JA, Guijt MJ, et al. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J Clin Endocrinol Metab 2003;88:3184–9.
36. Lewis BD, Hay ID, Charboneau JW, et al. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. Am J Roentgenol 2002;178:699–704.
37. Xing MM, Haugen B, Schlumberger M. Progress in molecular based management of differentiated thyroid cancer. Lancet 2013;381:1058–69.
38. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–99.
39. Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome Clin Endoc 2005;63:87–93.
40. Ronga G, Filesi M, Montesano T, et al. Lung metastases from differentiated thyroid carcinoma. A 40 years’ experience. Q J Nucl Med Mol Imaging 2004;48:12–19.
41. Samaan NA, Schultz PN, Haynie TP, Ordonez NG. Pulmonary metastasis of differentiated thyroid carcinoma: treatment results in 101 patients. J Clin Endocrinol Metab 1985;60:376–80.
42. Lang BH, Wong KP, Cheung CY, et al. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 2013;20:1329–35.
43. Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med 1996;335:1785–92.
44. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebocontrolled trial. Lancet 379:39–46.
45. Zettinig G, Fueger BJ, Passler C, et al. Long-term follow-up of patients with bone metastases from differentiated thyroid carcinoma—surgery or conventional therapy? Clin Endocrinol (Oxf ) 2002;56:377–82.
46. Song H-J, Xue Y-L, Xu Y-H, et al. Rare metastases of differentiated thyroid carcinoma: pictorial review Endocr Relat Cancer 2011;18:R165–R174.
47. Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–109.
48. www.fda.gov/NewsEvents/Newsroom/PresAnnouncements/ucm376443.htm.
49. Kloos RT, Ringel MD, Knopp MV et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 2009;27:1675–84.
50. Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 2008;26:4714–9.
51. Schneider TC, Abdulrahman RM, Corssmit EP, et al. Longterm analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol 2012;167:643–50.
52. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine refractory differentiated thyroid cancer: The phase III DECISION trial. J Clin Oncol 2013;31(Suppl, abstr 4).
53. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 2013;368:623–32.
54. Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res 2009;15:485–91.
55. Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364–8.
56. Elisei R, Ugolini C, Viola D, et al. BRAF (V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 2008;93:3943–9.
57. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAFV600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2103;309:1493–501.
58. Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring
From the Yale School of Medicine, New Haven, CT.
ABSTRACT
• Objective: To review management of papillary thyroid cancer.
• Methods: Review of the literature.
• Results: Papillary thyroid cancer is the most common endocrine malignancy. The standard treatment for papillary thyroid cancer is thyroidectomy. Adjuvant therapy includes lifelong thyroid-stimulating hormone suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event.
• Conclusion: The incidence of papillary thyroid cancer is rapidly increasing. Surgery remains the cornerstone of treatment.
Papillary thyroid cancer is the most common endocrine malignancy and accounts for the majority of cancers of the thyroid. The incidence of papillary thyroid cancer is rapidly increasing [1]. Although increasing detection has been proposed as a possible factor [2], some studies reject this hypothesis, reporting increase in the incidence of larger tumors [3]. Papillary thyroid cancer is characterized by a low mortality but a high recurrence rate [1], posing challenges not only to the endocrinologist and oncologist but also to the general practitioner.
The most frequent presentation of papillary thyroid cancer is a palpable thyroid nodule, cervical lymphadenopathy, or incidental detection on imaging. Locally advanced disease can present with hoarseness or voice alteration. Common risks factors include history of radiation exposure during childhood (the most important risk factor), thyroid cancer in a first-degree relative, family history of a thyroid cancer syndrome (such as Werner syndrome, Cowden syndrome, Carney complex, or familial polyposis), and female sex (2.5:1). Thyroid nodules in the context of an autoimmune thyroiditis may have a higher risk of malignancy [4].
CASE STUDY
Initial Presentation
A 49-year-old man with no significant past medical history presents with a painless mass in the anterior part of his neck.
History, Physical Examination, and Initial Investigations
He has no other symptoms, no weight changes, no history of radiation exposure to the neck, and no family history of malignancy. Physical exam shows a mass in the left thyroid lobe. There is no evidence of cardiac arrhythmias, tremors, or ophthalmologic abnormalities. Thyroid-stimulating hormone (TSH) level is 2.8 mIU/L (normal range, 0.4–4.5 mIU/L) and free thyroxine (T4) level is 1.1 ng/dL (normal range, 0.8–1.5 ng/dL). An ultrasound scan of the neck shows enlargement of the left lobe of thyroid gland, containing multiple complex lesions, the largest measuring 2 x 3 cm, with calcification as well as 3 enlarged lymph nodes in the left level IV. Fine-needle aspiration of the thyroid mass is positive for papillary carcinoma.
• What is the approach to the initial evaluation of a thyroid nodule?
Initial diagnostic evaluation includes history, physical examination, and TSH measurement; nonfunctioning nodules, associated with normal or high values of TSH, carry a higher risk of malignancy [5]. Cervical ultrasound should be performed in all patients with nodules. Fine-needle aspiration (FNA) should be used to evaluate nonfunctioning nodules > 1 cm or subcentimeter nodules with suspicious ultrasound features or if the patient has major risk factors (history of ionizing radiation exposure, external beam radiation exposure, family or personal history of papillary thyroid cancer, or FDG-PET [fluorinated glucose positron emission tomography]–positive thyroid nodules). Scintigraphy can be used to evaluate the need for ultrasound and FNA in patients with low TSH values [6,7]; hyperfunctioning nodules are at low risk for malignancy and do not require biopsy.
• What is initial treatment of papillary thyroid cancer?
Surgery is the primary treatment for papillary thyroid cancer. Unlike for many cancers, surgical removal of the primary tumor is indicated even in the presence of metastatic disease [8]. Total or near-total thyroidectomy is used to treat patients with tumors > 1 cm or with tumors < 1 cm and associated risk factors (eg, contralateral nodules, affected lymph nodes, metastasis, history of radiation, first-degree family history of papillary thyroid cancer, or age > 45 years) [6]. There is a lower risk of recurrence in patients treated with total thyroidectomy versus lobectomy in papillary thyroid cancer [9,10]. Thyroid lobectomy may be used in small (< 1 cm) unifocal tumors without the presence of the associated risk factors listed above.
Patients with central or lateral neck lymph node involvement should also undergo central-compartment (level VI) neck dissection. Therapeutic lateral neck compartmental lymph node dissection is recommended in patients with biopsy-proven metastatic lateral cervical adenopathy [6,7]. The role of unilateral or bilateral prophylactic central-compartment nodal dissection (PCND), that is, lymph node dissection in the level VI compartment of neck in patients without evidence of lymphadenopathy, is controversial. The data for the possible benefit of PCND are inconclusive [11] although the ATA recommends the procedure for locally invasive T3 and T4 tumors [6].
The American Thyroid Association (ATA) and National Comprehensive Cancer Network (NCCN) guidelines [6,7] recommend a preoperative cervical ultrasound in patients with biopsy-proven papillary thyroid cancer to evaluate the presence of disease in the cervical lymph nodes, especially in the lateral and central compartments, and in the contralateral thyroid lobe. If suspicious lymph nodes are found, FNA confirmation is necessary only if this would change management. Systematic use of other preoperative imaging studies, such as CT or MRI, is not recommended [6,7].
Surgical Treatment
The patient underwent a total thyroidectomy with bilateral central neck dissection and selective supraclavicular left-sided lateral neck dissection. Lymph nodes on both sides of the neck (paratracheal nodes) as well as the left supraclavicular nodes were removed. Pathology showed multifocal papillary cancer with extracapsular extension to the paratracheal soft tissue, 14/14 lymph nodes affected, stage IVA T4N1bM0.
• How is papillary thyroid cancer staged?
• How should this patient be treated after surgery? Is any adjuvant therapy indicated?
TSH Suppression
In an effort to reduce risk of recurrence, patients should receive lifelong suppression of TSH using supraphysiologic doses of levothyroxine after total thyroidectomy. This is based upon the hypothesis that TSH is a growth factor for thyroid cancer cells [12,13]. Although a meta-analysis [14] supports the efficacy of TSH suppression therapy, some authors have questioned its widespread use, especially in light of the adverse effects of its use over the long term [15]. Many support its use only in high-risk patients [16], arguing that there is no evidence of benefit for low-risk patients [17]. This view is reflected in the ATA guidelines, which recommend TSH suppression below 0.1 mU/L for high-risk and intermediate-risk patients, while normal or slightly below normal TSH levels are recommended for low-risk patients [6].
Adverse effects of TSH suppression therapy are derived from the induced mild thyrotoxicosis, including cardiovascular and skeletal manifestations. Notably, elderly patients have a higher risk of cardiovascular side effects [18] such as atrial fibrillation, diastolic dysfunction, tachyarrhythmias, increased heart rate or increased left ventricular mass. Likewise, postmenopausal women are most susceptible for skeletal effects such as decreased mineral bone density and fractures [19].
Radioiodine Ablative Therapy
Radioactive iodine (RAI or radioiodine) therapy is based on the capacity of thyroid tissue to take up and retain iodine, specifically, radioiodine. This capacity is present but reduced in papillary and follicular cancer cells.
Radioiodine remnant ablation is performed after surgery, acting as adjuvant therapy by destroying remnant pathological or normal thyroid tissue. The destruction of normal thyroid tissue is useful as it increases the reliability of thyroglobulin testing and radioiodine scanning in the detection of recurrent or metastatic disease. Moreover, remnant ablation has been shown to prevent new thyroid neoplasias in high-risk patients (ie, those with history of radiation exposure). Radioiodine ablative therapy has been shown to reduce recurrence and cause-specific mortality [20] in certain subgroups; however, patients with low mortality risk do not seem to benefit from this therapy [21,22]. Its use is recommended in patients with distant metastases, tumors > 4 cm, or with extrathyroidal extension. It is also recommended for selected patients with tumors 1–4 cm who have high-risk features (such as lymph node involvement, history of radiation, or others previously mentioned) when there is an intermediate to high risk of recurrence or death from thyroid cancer [6]. Lymph node involvement can occur in up to 50% of cases [39] and normally responds to radioiodine therapy.
Since TSH increases radioiodine uptake by normal or pathological thyroid cells, TSH stimulation is required for radioiodine therapy. This can be done by endogenous TSH elevation or by recombinant human TSH (rhTSH). The former can be achieved by either stopping thyroxine 2 to 3 weeks prior to the remnant ablation, or by withdrawing thyroxine and switching to liothyronine for 2 to 3 weeks followed by a discontinuation of liothyronine for 2 weeks. Both approaches seem to produce the same incidence of hypothyroid symptoms [23]. Thyroxine therapy can be resumed 2 to 3 days after radioiodine ablative therapy. Recombinant human TSH can be used with equal efficacy in place of thyroxine withdrawal [24], with the advantage of not producing transitory hypothyroidism. It is especially recommended for patients who are unable to tolerate hypothyroidism or who cannot achieve an adequate TSH level. Short-term recurrence rates are similar in patients treated with rhTSH or thyroxine withdrawal [25].
In addition, a low-iodine diet for 1 or 2 weeks is recommended for patients undergoing radioiodine remnant ablation. The rationale is that a high-iodine diet or iodine exposure (ie, amiodarone treatment or intravenous contrast) can decrease radioiodine uptake by papillary cancer cells due to further dilution of radioactive iodine in an expanded endogenous non-radioactive iodine pool. Patients with suspected high iodine levels can be screened using spot urinary levels [26].
Commonly, a diagnostic scan using low activities of iodine-131 is performed prior to radioablation to avoid the controversial “stunning effect” [27] from any exposure to sublethal radiation in a diagnostic dose. In stunning, the diagnostic RAI dose decreases uptake of a subsequent therapeutic dose. Alternatively, we use [I-123] radioiodine at very low dose (1.4 mCi) in pre-ablation patients. Uptake in the thyroid bed occurs in 75% to 100% of patients, commonly due to remnant normal thyroid tissue [28].
The typical activity used for RAI ablative therapy is 30–100 mCi. The administration of high activities (150–200 mCi) of [I-131] radioiodine has been used to treat recurrent or metastatic disease. This treatment can be very effective, especially in young patients [29].
Side Effects and Contraindications
Common side effects of radioiodine treatment include sialadenitis, radiation thyroiditis, tumor hemorrhage or edema, nausea, transient oligospermia or amenorrhea and nasolacrimal duct obstruction. Moreover, patients treated with radioiodine have a modest increased risk of developing other malignancies [30].
[I-131]Radioiodine must be avoided in pregnancy and in breastfeeding [31]. Indeed, breast tissue has a strong tendency to uptake iodine so breastfeeding should be stopped 5 to 8 weeks before radioiodine treatment, otherwise it can lead to a false-positive radioiodine scan in the chest [32], or worse, deliver radioiodine to the baby with detrimental effects and potential ablation to the baby’s thyroid gland.
Patients treated with radioiodine are advised to drink abundant water after the treatment in order to increase its renal elimination. If no stool elimination occurs in 14 to 24 hours, laxatives may be indicated to eliminate radioiodine from the gastrointestinal track. In addition, patients are advised to avoid sexual contact, avoid sharing bed, utensils, towels, toothbrushes, razors, and avoid public transportation and public places among other measures to avoid exposing the population to radiation [33]. The duration of this restriction depends on the dose administered.
Adjuvant Treatment in this Patient
As the patient was at high risk for recurrence, he received TSH suppression therapy to levels < 0.1 mIU/L. He was referred to nuclear medicine for I-131 treatment. However, at 3 months following thyroidectomy, thyroglobulin measurement showed an elevation (40.5 ng/mL). Ultrasound showed enlarged lymph nodes at level II at the right and at level II at the left. A FNA of left neck node was positive for papillary thyroid cancer.
• How should the patient be treated now?
Treatment of Locoregional Metastatic Disease
The best treatment for residual disease or local recurrences is surgery. ATA guidelines recommend compartmental lateral and/or central neck dissection for patients with persistent or recurrent disease confined to the neck [6]. Radioiodine can be an alternative when recurrent disease is not visible on imaging. Other treatments that can be used for local recurrences or isolated metastases when surgery is not possible are radiofrequency ablation [34], chemo-embolization [35], or ethanol ablation [36]. External beam radiotherapy, which is discussed later, could also be used in selected cases.
Further Treatment
The patient underwent a bilateral modified radical neck dissection followed by adjunctive radioiodine therapy. His initial radioiodine scan showed mild uptake in the neck at the site of his prior surgery. He received treatment with 215 mCi, then 6 months later he was treated with 250 mCi, as his scan showed continued mild uptake. Eleven months later his radioiodine scan showed no uptake and thyroglobulin levels remained stable at 14.4 ng/mL.
One year later, in a follow-up blood analysis he was found to have an elevated thyroglobulin level (90.4 ng/mL). A PET/CT scan showed multiple bone metastases. A neck ultrasound revealed enlarged lymph nodes in the right thyroid bed.
• How common is radioiodine-refractory thyroid cancer?
Radioiodine-refractory thyroid cancer in patients with progression of disease despite radioiodine therapy, or with non-radioiodine-avid lesions [37], is uncommon. It has a poor prognosis with a median survival of 3 to 6 years after diagnosis. It is more frequent in older patients. These lesions are often hypermetabolic and hence [F-18]FDG-avid [38], with a worse prognosis. In one study of patients with metastatic differentiated thyroid cancer, the 10-year overall survival rate was 56% in patients with radioiodine-avid lesions but only 10% in patients with non-radioiodine-avid lesions [38].
• Is the bone a common place for metastasis? Where else should we expect to find a lesion?
Metastatic Pattern
The most common sites for distant metastasis of papillary thyroid cancer are the lungs and the bone. The 10-year survival rate of papillary thyroid cancer patients with lung metastases is between 30% and 50% [38,39]; the prog-nosis is better in patients < 45 years and with radiodine uptake [40]; indeed, patients with pulmonary metastasis seen only in 131-I scans and not on CT or chest x-ray have a longer survival [41]. Pulmonary metastasis can be treated with radioiodine if they are radioiodine-avid. With this treatment complete remission is possible, although it is extremely difficult to achieve in macronodular metastasis.
Bones are the second most common place for distant metastases. Bone metastases seem to have a worse response to treatment with an unfavorable prognosis [42]. Pamidronate (a biphosphonate) and denosumab (a RANK ligand inhibitor) have been used to prevent skeletal related events, including pathologic fractures and cord compression, in bone metastases from other cancers such as breast and prostate, and may also be useful in thyroid cancer, although this has not yet been studied [43,44]. Moreover, surgical resection of isolated bone metastasis seems to improve survival [45].
Skin, liver, and brain metastasis, although uncommon, can also occur. There are also reported rare cases of metastasis in the breast, parotid, larynx, pharynx, adrenal glands, pituitary, kidney, liver, orbit, the sphenoid sinus, choroid plexus, pancreas, and skeletal muscles [46].
• Which treatments can we offer to a patient with metastatic disease refractory to radioiodine?
Chemotherapy and Treatment of Radioiodine-Resistant Disease
Therapeutic options for patients with metastatic papillary thyroid cancer resistant to radioiodine and TSH suppression are limited. Cytotoxic drugs do not play a major role in the treatment of refractory metastatic papillary thyroid cancer, and new research is mainly focused on tyrosine kinase inhibitors (TKIs) with a considerable number of clinical trials either completed or ongoing.
Tyrosine kinases are enzymes that transfer phosphate groups from adenosine triphosphate to proteins. In tumor cells their signaling paths promote proliferation, avoidance of apoptosis, invasion, angiogenesis, and metastasis. TKIs are small molecules that are able to inhibit tyrosine kinase function even at very low intracellullar concentrations. Some of them inhibit various tyrosine kinases and are known as multi-kinase inhibitors (MKIs).
Sorafenib
Sorafenib (400 mg twice daily) is an oral MKI that targets RAF, platelet-derived growth factor receptor, vascular endothelial growth factor receptors 2 and 3, RET and c-Kit [47]. It was approved in November 2013 for patients with radioiodine-refractory differentiated thyroid cancer [48]. Three phase II studies had previously evaluated sorafenib in papillary thyroid cancer, showing a partial response in 15% to 31% of patients and a progression-free survival up to 79 weeks [49–51]. Common adverse effects included weight loss, fatigue, rash, hypertension and the main dose-limiting toxicity—a hand-foot syndrome consisting of swelling, reddening, numbness, and desquamation on palms and soles [52].
Approval of the drug was based on the DECISION trial [52]. A total of 417 patients were randomized (207 to sorafenib and 210 to placebo), of which 57% had papillary thyroid cancer. The primary endpoint of progression-free survival (PFS) was significantly higher in the sorafenib arm, (median, 10.8 months) compared with placebo (median, 5.8 months) (hazard ratio [HR] 0.58, 95% confidence interval [CI] 0.45–0.75, P < 0.001). Median overall survival had not been reached in either arm [52]. The PFS of 5.8 months in the placebo arm confirmed that the group of patients in this study had a rapidly progressing disease, unlike the majority of patients with RAI-sensitive disease.
Selumetinib
Radioiodine re-sensitization was addressed in a study using selumitinib, an inhibitor of mitogen-activated protein kinase kinase (MAPK kinase or MEK). Preclinical models had shown that radioiodine-refractory tumors exposed to inhibitors of this enzyme were able to uptake radioiodine again. Twenty patients with radioiodine-refractory thyroid cancers were treated with selumetinib for 4 weeks and 12 showed increased radioiodine uptake following the treatment. Furthermore, 8 of these patients went on to show responses clinically to retreatment with radioiodine [53].Further studies with this agent will be needed to determine its place in treating patients with differentiated thyroid cancer.
External Beam Radiotherapy and Local Treatment for Metastases
The role of external beam radiotherapy in papillary thyroid cancer is mainly for symptom management. Local radiation can be used in patients with refractory metastatic disease or in lesions that do not uptake radioiodine. Examples include painful bone metastasis or brain metastasis that cannot be treated with surgery. In addition, radiofrequency ablation, chemo-embolization, or ethanol ablation can be used in certain patients.
Sequence of Treatments
In the setting of symptomatic metastatic, radioiodine-resistant disease, we prefer to use a TKI, normally sorafenib, as a first-line treatment. For second-line treatments, enrollment in a clinical trial is an option. Over 70% of patients with metastatic papillary thyroid cancer have mutations of the enzyme BRAF kinase. Vemurafenib is an inhibitor of this enzyme and appears to have some activity in patients with RAI-refractory thyroid cancer in early clinical trials [54–58]. Other TKIs such as sunitinib can also be used. Doxorubicin is only used in cases when a patient is not eligible for a trial and the off-label use of another TKI is contraindicated.
Further Treatment in this Patient
The patient received a trial of sorafenib. He showed disease stabilization that lasted 5 months. The treatment was stopped due to adverse effects (loss of weight and vomiting) and progression of the disease. He was then enrolled in a trial of vemurafenib. He stopped treatment because of adverse events related to the medication and currently has stable disease.
Summary
Papillary thyroid cancer is the most common endocrine malignancy. It is characterized by low mortality but high recurrence rate and can have a considerable impact on quality of life. Any anterior neck nodule, especially in a patient with a history of neck irradiation, should raise concern for this disease. Surgery remains the cornerstone of treatment. Adjuvant therapy includes lifelong TSH suppression and radioiodine therapy. Local recurrence is common and is normally treated with surgery and/or radioiodine. Metastatic radioiodine-resistant disease is a more infrequent event. Thyroid cancer has a tendency to metastasize to the bones and lungs. Metastatic radioiodine-resistant disease is often treated with TKIs such as sorafenib. Enrollment in clinical trials is recommended as second-line therapy in radioiodine-resistant metastatic disease.
Corresponding author: Hari A. Deshpande, MD, Yale Cancer Center, FMP 124, 333 Cedar St., New Haven, CT 06520, [email protected]
Financial disclosures: Dr. Deshpande reports that he is on the advisory board of Bayer/Onyx.
Author contributions: conception and design, PT, EHH, GGC, HAD; drafting of article, PT, EHH, GGC, HAD; critical revision of the article, EHH, GGC, HAD.
REFERENCES
1. Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2010.
2. Katoh R, Sasaki J, Kurihara H, et al. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma: a clinicopathologic study of 105 consecutive patients. Cancer 1992;70:1585–90.
3. Morris LG, Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 2010;200:454–61.
4. Fiore E, Rago T, Latrofa F, et al. Hashimoto’s thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with Lthyroxine. Endocr Relat Cancer 2011;18:429–37.
5. Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 2008;93:809–14.
6. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214.
7. National Comprehensive Cancer Network guidelines. Available at www.nccn.org/professionals/physician_gls/pdf/thyroid. pdf.
8. Stephenson BM, Wheeler MH, Clark OH. The role of total thyroidectomy in the management of differentiated thyroid cancer. Curr Opin Gen Surg 1994 53–9.
9. Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg 2007;246:375–81.
10. Hay ID, Grant CS, Bergstralh EJ, et al. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery 1998;124:958–64.
11. McLeod DS, Sawka AM, Cooper DS. Controversies in primarytreatment of low-risk papillary thyroid cancer. Lancet 2013;381:1046–57.
12. Brabant G. 2008 Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J Clin Endocrinol Metab 2008;93:1167–9.
13. Kim HK, Yoon JH, Kim SJ, Cho JS. Higher TSH level is a risk factor for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2013;78:472–7.
14. McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med 2002;34:554–64.
15. Zafón C. TSH-suppressive treatment in differentiated thyroid cancer. A dogma under review. Endocrin Nutr 2012;59:125–30.
16. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998;8:737-44.
17. Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006;16:1229–42.
18. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 1994;33:1249–52.
19. Kung AW, Yeung SS. Prevention of bone loss induced by thyroxine suppressive therapy in postmenopausal women: the effect of calcium and calcitonin. J Clin Endocrinol Metab 1996;81:1232–36.
20. Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab 1992;75:714–20.
21. Sugitani I, Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J 1999;46:209–16.
22. Kim S, Wei JP, Braveman JM, Brams DM. Predictingoutcome and directing therapy for papillary thyroid carcinoma. Arch Surg 2004;139:390–4.
23. Leboeuf R, Perron P, Carpentier AC, et al. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clin Endocrinol (Oxf ) 2007;67:839–44.
24. Pacini F, Ladenson PW, Schlumberger M, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab 2006;91:926–32.
25. Tuttle RM, Brokhin M, Omry G, et al. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal. J Nucl Med 2008;49:764–70.
26. Pluijmen MJ, Eustatia-Rutten C, Goslings BM, et al. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf ) 2003;58:428–35.
27. Park HM. Stunned thyroid after high-dose I-131 imaging. Clin Nucl Med 1992; 17:501–2.
28. Salvatori M, Raffaelli M, Castaldi P, et al. Evaluation of the surgical completeness after total thyroidectomy for differentiated thyroid carcinoma. Eur J Surg Oncol 2007;33:648–54.
29. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–9.
30. Lang BH, Wong IO, Wong KP, et al. Risk of second primary malignancy in differentiated thyroid carcinoma treated with radioactive iodine therapy. Surgery 2012;151:844–50.
31. Rubow S, Klopper J. Excretion of radioiodine in human milk following a therapeutic dose of I-131. Eur J Nucl Med 1988;14:632–3.
32. Bakheet SM, Hammami MM. Patterns of radioiodine uptake by the lactating breast. Eur J Nucl Med 1994;21:604–8.
33. American Thyroid Association Taskforce on Radioiodine Safety, Sisson JC, Freitas J, et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: practice recommendations of the American Thyroid Association. Thyroid 2011;21:335–46.
34. Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from welldifferentiated thyroid malignancy. Surgery 2001;130:971–7.
35. Eustatia-Rutten CF, Romijn JA, Guijt MJ, et al. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J Clin Endocrinol Metab 2003;88:3184–9.
36. Lewis BD, Hay ID, Charboneau JW, et al. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. Am J Roentgenol 2002;178:699–704.
37. Xing MM, Haugen B, Schlumberger M. Progress in molecular based management of differentiated thyroid cancer. Lancet 2013;381:1058–69.
38. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–99.
39. Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome Clin Endoc 2005;63:87–93.
40. Ronga G, Filesi M, Montesano T, et al. Lung metastases from differentiated thyroid carcinoma. A 40 years’ experience. Q J Nucl Med Mol Imaging 2004;48:12–19.
41. Samaan NA, Schultz PN, Haynie TP, Ordonez NG. Pulmonary metastasis of differentiated thyroid carcinoma: treatment results in 101 patients. J Clin Endocrinol Metab 1985;60:376–80.
42. Lang BH, Wong KP, Cheung CY, et al. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 2013;20:1329–35.
43. Hortobagyi GN, Theriault RL, Porter L, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med 1996;335:1785–92.
44. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebocontrolled trial. Lancet 379:39–46.
45. Zettinig G, Fueger BJ, Passler C, et al. Long-term follow-up of patients with bone metastases from differentiated thyroid carcinoma—surgery or conventional therapy? Clin Endocrinol (Oxf ) 2002;56:377–82.
46. Song H-J, Xue Y-L, Xu Y-H, et al. Rare metastases of differentiated thyroid carcinoma: pictorial review Endocr Relat Cancer 2011;18:R165–R174.
47. Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–109.
48. www.fda.gov/NewsEvents/Newsroom/PresAnnouncements/ucm376443.htm.
49. Kloos RT, Ringel MD, Knopp MV et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 2009;27:1675–84.
50. Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 2008;26:4714–9.
51. Schneider TC, Abdulrahman RM, Corssmit EP, et al. Longterm analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol 2012;167:643–50.
52. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine refractory differentiated thyroid cancer: The phase III DECISION trial. J Clin Oncol 2013;31(Suppl, abstr 4).
53. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 2013;368:623–32.
54. Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res 2009;15:485–91.
55. Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364–8.
56. Elisei R, Ugolini C, Viola D, et al. BRAF (V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 2008;93:3943–9.
57. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAFV600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2103;309:1493–501.
58. Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring
Another Win for Veggies
Study Overview
Objective. To determine the association between a vegetarian diet and blood pressure (BP).
Design. Systematic review and meta-analysis of controlled clinical trials and observational studies.
Setting and participants. MEDLINE and Web of Science were respectively searched for English articles published between 1946 to October 2013 and 1900 to November 2013. Inclusion criteria were age > 20 years and vegetarian diet. This included a vegan diet (omitting all animal products), ovo/lacto/pesco vegetarian diet (including eggs/dairy/fish), or semi-vegetarian diet (meat or fish rarely). Exclusion criteria included studies of twins, a multipronged intervention, describing only categorical BP, and reliance on a case series. A total of 258 records were identified. Seven clinical trials and 32 observational studies met inclusion criteria. The 7 clinical trials encompassed 311 participants (median, 38; range, 11–113) with a mean age of 44.5 years (range, 38.0–54.3). All were open-label, 6 were randomized, and 6 provided food to participants. The 32 observational studies included 21,604 participants (median, 152; range, 20–9242) with a mean age of 46.6 years (range, 28.8–68.4 years). Fifteen of these studies included mixed diet types (vegan, lacto, ovolacto, pesca, and/or semivegetarian).
Main outcome measures. The primary outcome was BP. Differences in systolic BP (SBP) and diastolic BP (DBP) between groups consuming vegetarian or comparison diets were pooled using a random effects model. The study compared clinical trials and observational studies separately. Funnel plots, the Egger test, and the trim-and-fill method were all used to assess and correct for publication bias.
Results. Vegetarian diets in clinical trials were associated with a mean SBP reduction of −4.8 mm Hg (95% confidence interval [CI], −6.6 to −3.1; P < 0.001; I2 = 0; P = 0.45 for heterogeneity) and DBP reduction of −2.2 mm Hg (95% CI, −3.5 to −1.0; P < 0.001; I2 = 0; P= 0.43 for heterogeneity) when compared with omnivorous diets. Observational studies had larger reductions but significant heterogeneity: SBP −6.9 mm Hg (95% CI, −9.1 to −4.7; P < 0.001; I2 = 91.4; P < 0.001 for heterogeneity) and DBP −4.7 mm Hg (95% CI, −6.3 to −3.1; P < 0.001; I2 = 92.6; P < 0.001 for heterogeneity). This heterogeneity was best explained by proportion of men (β −0.03; P < 0.001), baseline SBP (β −0.13; P = 0.003), baseline DBP (β −0.30; P < 0.001), sample size (β 0.001; P< 0.001), and BMI (β −0.46; P = 0.02). This suggests that vegetarian diets and lower BP are more strongly associated in men and those with higher baseline BP and BMI.
Subgroup analysis included stratification by age, gender, BMI, diet type, sample size, diet duration, BP medication use, baseline BP, and geographic region. In subgroup analysis of clinical trials, no statistically significant difference between group variation or heterogeneity existed. In comparison, subgroup analysis of observational studies reduced heterogeneity and often effect size. For example, lower SBP was evident in the majority male subgroups (mean SBP/DBP: –18.5 mm Hg/–10.1 mm Hg).
Publication bias existed for both clinical trials and observational studies. According to trim-and-fill methodology, 3 clinical trials of smaller size and larger BP reduction likely were missing (Egger P = 0.04). Their addition shifted mean SBP reduction from −4.8 mm Hg (−6.6 to −3.1) to −5.2 mm Hg (−6.9 to −3.5). Observational studies lacked medium sized negative trials and were overrepresented by larger positive trials (Egger P < 0.001), although this was not confirmed by trim-and-fill (yet this method performs less well under heterogeneous conditions) [1].
Conclusion. Vegetarian diets, when compared with omnivorous diets, are associated with reductions in BP.
Commentary
Several studies show that dietary modifications are effective in preventing and managing hypertension [2,3]. Landmark randomized trials, including the DASH diet [4], DASH-sodium diet [5], and OmniHeart diets [6], all of which emphasize fruit and vegetable intake but are not vegetarian, have led to SBP and DBP reductions ranging from 5.5 to 9.5 mm Hg and 3.0 to 5.2, respectively. However, the impact of a vegetarian diet still remains debated, particularly given disparate findings among randomized controlled trials (RCTs). For example, findings in the early- and mid-1980s of small RCTs with ovolactovegetarians (a vegetarian who consumes eggs and dairy products but not animal flesh) suggested reductions similar to the pooled SBP reduction of –4.8 mm Hg Yokoyama et al report [7,8]. In contrast, one RCT comparing ovolactovegetarian with lean meat diets failed to show a BP benefit [9]. Striking is the dearth of RCTs in the last 20 years to assist in better estimating this impact, particularly given its continual recommendation in the scientific [10] and lay communities [11]. To the authors’ credit, this is the first meta-analysis and second systematic review of this important relationship [12].
A vegetarian diet likely supports BP reductions through a variety of mechanisms, most notably via an abundance of potassium [13]. Potassium likely promotes vasodilation, which facilitates glomerular filtration, allowing decreased renal sodium reabsorption and decreased platelet aggregation. Other more controversial hypotheses include decreased energy density leading to reduced BMI [14], decreased sodium intake [15], reduced blood viscosity [16], and high polyunsaturated with low saturated fat content [17].
Strengths of this analysis include the large observational sample size, the separate analysis of clinical trials and observational studies, the lengthy search time-frame, the subgroup analyses, and the adjustment for publication bias. Although the overall association was robust throughout, we agree with the authors that large heterogeneity among observational studies, small clinical trial sample sizes, and the variation in what “vegetarian” represents throughout the world and in individual studies all represent limitations. The participants in many of the observational studies could have technically eaten meat with unclear and undefined frequency, and this may have explained the heterogeneity of these studies. Correspondingly, the lack of heterogeneity observed in the clinical trials may be due to the fact that participants were provided meals in 6 out of 7 studies.
It was surprising that only 7 clinical trials were found. The authors utilized 2 databases, but perhaps searching additional databases such as EMBASE or CINAHL would have yielded other pertinent studies. The authors also did not use a bias assessment tool such as that proposed by the Cochrane bias methods group, which could have better discriminated high- from low-quality trials and made for useful subgroup analyses [1]. Similarly, reporting on both attrition and adherence could have assisted in decreasing heterogeneity during subgroup analyses and determining high-quality from low-quality studies. For example, adherence in Ferdowsian et al (vegan diet) was determined by unannounced dietician phone calls and found that only 57% of participants abstained from animal products. This may have been secondary to the study’s design, in that “providing meals” included simply making them an available option at the company cafeteria instead of requiring consumption of a study-specific vegetarian meal [18].
Applications for Clinical Practice
In this meta-analysis, vegetarian diets were associated with –4.8 mm Hg SBP and −2.2 mm Hg DBP reductions, indicating that providers can recommend a vegetarian diet as on par with other lifestyle changes, including low-sodium diet, weight loss, and exercise. A vegetarian diet may be comparable to pharmacologic therapy in magnitude of BP change. Short- and long-term pharmacologic therapy is associated with respective SBP/DBP reductions of –8.3/–3.8 and –5.4/–2.3, not altogether different from reductions seen with vegetarian or vegetable-heavy diets [19].
Although there are barriers to a vegetarian diet, including provider attitudes [20], cost [21], poor culinary skill [22], palatability, and adherence, pharmacologic BP treatment also presents barriers: adherence to BP medications is estimated to be 50% to 70% [23], and harm due to side effects can preclude use. Thus, providers can present a vegetarian diet as a potentially effective option, depending on patient preference and ability to adhere.
—David M. Levine, MD, MA, New York University
School of Medicine, and Melanie Jay, MD, MS
References
1. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at http://handbook.cochrane.org/chapter_10/10_4_4_2_trim_and_fill.htm.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013 Nov 12. [Epub ahead of print]
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20.
4. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117–24.
5. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 2001;344:3–10.
6. Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids. JAMA 2005;294:2455–64.
7. Rouse IL, Beilin LJ, Armstrong BK, Vandongen R. Blood-pressure–lowering effect of a vegetarian diet: controlled trial in normotensive subjects. Lancet 1983;321:5–10.
8. Margetts BM, Beilin LJ, Vandongen R, Armstrong BK. Vegetarian diet in mild hypertension: a randomised controlled trial. BMJ 1986;293:1468–71.
9. Kestin M, Rouse IL, Correll RA, Nestel PJ. Cardiovascular disease risk factors in free-living men: comparison of two prudent diets, one based on lactoovovegetarianism and the other allowing lean meat. Am J Clin Nutr 1989;50:280–7.
10. Alpert JS. Nutritional advice for the patient with heart disease: what diet should we recommend for our patients? Circulation 2011;124:e258–e260.
11. Gordinier J. Making vegan a new normal. New York Times. 26 Sept 2012. Page D1.
12. Berkow SE, Barnard ND. Blood pressure regulation and vegetarian diets. Nutr Rev 2005;63:1–8.
13. Aburto NJ, Hanson S, Gutierrez H, et al. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 2013;346:f1378.
14. Berkow SE, Barnard N. Vegetarian diets and weight status. Nutr Rev 2006;64:175–88.
15. Larsson CL, Johansson GK. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr 2002;76:100–6.
16. Ernst E, Pietsch L, Matrai A, Eisenberg J. Blood rheology in vegetarians. Br J Nutr 1986;56:555–60.
17. Iacono JM, Dougherty RM. Effects of polyunsaturated fats on blood pressure. Annu Rev Nutr 1993;13:243–60.
18. Ferdowsian HR, Barnard ND, Hoover VJ, et al. A multicomponent intervention reduces body weight and cardiovascular risk at a GEICO corporate site. Am J Health Promot 2010;24:384–7.
19. Brugts JJ, Ninomiya T, Boersma E, et al. The consistency of the treatment effect of an ACE-inhibitor based treatment regimen in patients with vascular disease or high risk of vascular disease: a combined analysis of individual data of ADVANCE, EUROPA, and PROGRESS trials. Eur Heart J 2009;30:1385–94.
20. Berman BM, Singh BB, Hartnoll SM, et al. Primary care physicians and complementary-alternative medicine: training, attitudes, and practice patterns. Am Board Fam Pract 1998;11:272–81.
21. Drewnowski A, Darmon N. The economics of obesity: dietary energy density and energy cost. Am J Clin Nutr 2005;82(1 Suppl):265S-273S.
22. Lea EJ, Crawford D, Worsley A. Public views of the benefits and barriers to the consumption of a plant-based diet. Eur J Clin Nutr 2006;60:828–37.
23. Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure–lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med 2004;164:722–32.
Study Overview
Objective. To determine the association between a vegetarian diet and blood pressure (BP).
Design. Systematic review and meta-analysis of controlled clinical trials and observational studies.
Setting and participants. MEDLINE and Web of Science were respectively searched for English articles published between 1946 to October 2013 and 1900 to November 2013. Inclusion criteria were age > 20 years and vegetarian diet. This included a vegan diet (omitting all animal products), ovo/lacto/pesco vegetarian diet (including eggs/dairy/fish), or semi-vegetarian diet (meat or fish rarely). Exclusion criteria included studies of twins, a multipronged intervention, describing only categorical BP, and reliance on a case series. A total of 258 records were identified. Seven clinical trials and 32 observational studies met inclusion criteria. The 7 clinical trials encompassed 311 participants (median, 38; range, 11–113) with a mean age of 44.5 years (range, 38.0–54.3). All were open-label, 6 were randomized, and 6 provided food to participants. The 32 observational studies included 21,604 participants (median, 152; range, 20–9242) with a mean age of 46.6 years (range, 28.8–68.4 years). Fifteen of these studies included mixed diet types (vegan, lacto, ovolacto, pesca, and/or semivegetarian).
Main outcome measures. The primary outcome was BP. Differences in systolic BP (SBP) and diastolic BP (DBP) between groups consuming vegetarian or comparison diets were pooled using a random effects model. The study compared clinical trials and observational studies separately. Funnel plots, the Egger test, and the trim-and-fill method were all used to assess and correct for publication bias.
Results. Vegetarian diets in clinical trials were associated with a mean SBP reduction of −4.8 mm Hg (95% confidence interval [CI], −6.6 to −3.1; P < 0.001; I2 = 0; P = 0.45 for heterogeneity) and DBP reduction of −2.2 mm Hg (95% CI, −3.5 to −1.0; P < 0.001; I2 = 0; P= 0.43 for heterogeneity) when compared with omnivorous diets. Observational studies had larger reductions but significant heterogeneity: SBP −6.9 mm Hg (95% CI, −9.1 to −4.7; P < 0.001; I2 = 91.4; P < 0.001 for heterogeneity) and DBP −4.7 mm Hg (95% CI, −6.3 to −3.1; P < 0.001; I2 = 92.6; P < 0.001 for heterogeneity). This heterogeneity was best explained by proportion of men (β −0.03; P < 0.001), baseline SBP (β −0.13; P = 0.003), baseline DBP (β −0.30; P < 0.001), sample size (β 0.001; P< 0.001), and BMI (β −0.46; P = 0.02). This suggests that vegetarian diets and lower BP are more strongly associated in men and those with higher baseline BP and BMI.
Subgroup analysis included stratification by age, gender, BMI, diet type, sample size, diet duration, BP medication use, baseline BP, and geographic region. In subgroup analysis of clinical trials, no statistically significant difference between group variation or heterogeneity existed. In comparison, subgroup analysis of observational studies reduced heterogeneity and often effect size. For example, lower SBP was evident in the majority male subgroups (mean SBP/DBP: –18.5 mm Hg/–10.1 mm Hg).
Publication bias existed for both clinical trials and observational studies. According to trim-and-fill methodology, 3 clinical trials of smaller size and larger BP reduction likely were missing (Egger P = 0.04). Their addition shifted mean SBP reduction from −4.8 mm Hg (−6.6 to −3.1) to −5.2 mm Hg (−6.9 to −3.5). Observational studies lacked medium sized negative trials and were overrepresented by larger positive trials (Egger P < 0.001), although this was not confirmed by trim-and-fill (yet this method performs less well under heterogeneous conditions) [1].
Conclusion. Vegetarian diets, when compared with omnivorous diets, are associated with reductions in BP.
Commentary
Several studies show that dietary modifications are effective in preventing and managing hypertension [2,3]. Landmark randomized trials, including the DASH diet [4], DASH-sodium diet [5], and OmniHeart diets [6], all of which emphasize fruit and vegetable intake but are not vegetarian, have led to SBP and DBP reductions ranging from 5.5 to 9.5 mm Hg and 3.0 to 5.2, respectively. However, the impact of a vegetarian diet still remains debated, particularly given disparate findings among randomized controlled trials (RCTs). For example, findings in the early- and mid-1980s of small RCTs with ovolactovegetarians (a vegetarian who consumes eggs and dairy products but not animal flesh) suggested reductions similar to the pooled SBP reduction of –4.8 mm Hg Yokoyama et al report [7,8]. In contrast, one RCT comparing ovolactovegetarian with lean meat diets failed to show a BP benefit [9]. Striking is the dearth of RCTs in the last 20 years to assist in better estimating this impact, particularly given its continual recommendation in the scientific [10] and lay communities [11]. To the authors’ credit, this is the first meta-analysis and second systematic review of this important relationship [12].
A vegetarian diet likely supports BP reductions through a variety of mechanisms, most notably via an abundance of potassium [13]. Potassium likely promotes vasodilation, which facilitates glomerular filtration, allowing decreased renal sodium reabsorption and decreased platelet aggregation. Other more controversial hypotheses include decreased energy density leading to reduced BMI [14], decreased sodium intake [15], reduced blood viscosity [16], and high polyunsaturated with low saturated fat content [17].
Strengths of this analysis include the large observational sample size, the separate analysis of clinical trials and observational studies, the lengthy search time-frame, the subgroup analyses, and the adjustment for publication bias. Although the overall association was robust throughout, we agree with the authors that large heterogeneity among observational studies, small clinical trial sample sizes, and the variation in what “vegetarian” represents throughout the world and in individual studies all represent limitations. The participants in many of the observational studies could have technically eaten meat with unclear and undefined frequency, and this may have explained the heterogeneity of these studies. Correspondingly, the lack of heterogeneity observed in the clinical trials may be due to the fact that participants were provided meals in 6 out of 7 studies.
It was surprising that only 7 clinical trials were found. The authors utilized 2 databases, but perhaps searching additional databases such as EMBASE or CINAHL would have yielded other pertinent studies. The authors also did not use a bias assessment tool such as that proposed by the Cochrane bias methods group, which could have better discriminated high- from low-quality trials and made for useful subgroup analyses [1]. Similarly, reporting on both attrition and adherence could have assisted in decreasing heterogeneity during subgroup analyses and determining high-quality from low-quality studies. For example, adherence in Ferdowsian et al (vegan diet) was determined by unannounced dietician phone calls and found that only 57% of participants abstained from animal products. This may have been secondary to the study’s design, in that “providing meals” included simply making them an available option at the company cafeteria instead of requiring consumption of a study-specific vegetarian meal [18].
Applications for Clinical Practice
In this meta-analysis, vegetarian diets were associated with –4.8 mm Hg SBP and −2.2 mm Hg DBP reductions, indicating that providers can recommend a vegetarian diet as on par with other lifestyle changes, including low-sodium diet, weight loss, and exercise. A vegetarian diet may be comparable to pharmacologic therapy in magnitude of BP change. Short- and long-term pharmacologic therapy is associated with respective SBP/DBP reductions of –8.3/–3.8 and –5.4/–2.3, not altogether different from reductions seen with vegetarian or vegetable-heavy diets [19].
Although there are barriers to a vegetarian diet, including provider attitudes [20], cost [21], poor culinary skill [22], palatability, and adherence, pharmacologic BP treatment also presents barriers: adherence to BP medications is estimated to be 50% to 70% [23], and harm due to side effects can preclude use. Thus, providers can present a vegetarian diet as a potentially effective option, depending on patient preference and ability to adhere.
—David M. Levine, MD, MA, New York University
School of Medicine, and Melanie Jay, MD, MS
References
1. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at http://handbook.cochrane.org/chapter_10/10_4_4_2_trim_and_fill.htm.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013 Nov 12. [Epub ahead of print]
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20.
4. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117–24.
5. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 2001;344:3–10.
6. Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids. JAMA 2005;294:2455–64.
7. Rouse IL, Beilin LJ, Armstrong BK, Vandongen R. Blood-pressure–lowering effect of a vegetarian diet: controlled trial in normotensive subjects. Lancet 1983;321:5–10.
8. Margetts BM, Beilin LJ, Vandongen R, Armstrong BK. Vegetarian diet in mild hypertension: a randomised controlled trial. BMJ 1986;293:1468–71.
9. Kestin M, Rouse IL, Correll RA, Nestel PJ. Cardiovascular disease risk factors in free-living men: comparison of two prudent diets, one based on lactoovovegetarianism and the other allowing lean meat. Am J Clin Nutr 1989;50:280–7.
10. Alpert JS. Nutritional advice for the patient with heart disease: what diet should we recommend for our patients? Circulation 2011;124:e258–e260.
11. Gordinier J. Making vegan a new normal. New York Times. 26 Sept 2012. Page D1.
12. Berkow SE, Barnard ND. Blood pressure regulation and vegetarian diets. Nutr Rev 2005;63:1–8.
13. Aburto NJ, Hanson S, Gutierrez H, et al. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 2013;346:f1378.
14. Berkow SE, Barnard N. Vegetarian diets and weight status. Nutr Rev 2006;64:175–88.
15. Larsson CL, Johansson GK. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr 2002;76:100–6.
16. Ernst E, Pietsch L, Matrai A, Eisenberg J. Blood rheology in vegetarians. Br J Nutr 1986;56:555–60.
17. Iacono JM, Dougherty RM. Effects of polyunsaturated fats on blood pressure. Annu Rev Nutr 1993;13:243–60.
18. Ferdowsian HR, Barnard ND, Hoover VJ, et al. A multicomponent intervention reduces body weight and cardiovascular risk at a GEICO corporate site. Am J Health Promot 2010;24:384–7.
19. Brugts JJ, Ninomiya T, Boersma E, et al. The consistency of the treatment effect of an ACE-inhibitor based treatment regimen in patients with vascular disease or high risk of vascular disease: a combined analysis of individual data of ADVANCE, EUROPA, and PROGRESS trials. Eur Heart J 2009;30:1385–94.
20. Berman BM, Singh BB, Hartnoll SM, et al. Primary care physicians and complementary-alternative medicine: training, attitudes, and practice patterns. Am Board Fam Pract 1998;11:272–81.
21. Drewnowski A, Darmon N. The economics of obesity: dietary energy density and energy cost. Am J Clin Nutr 2005;82(1 Suppl):265S-273S.
22. Lea EJ, Crawford D, Worsley A. Public views of the benefits and barriers to the consumption of a plant-based diet. Eur J Clin Nutr 2006;60:828–37.
23. Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure–lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med 2004;164:722–32.
Study Overview
Objective. To determine the association between a vegetarian diet and blood pressure (BP).
Design. Systematic review and meta-analysis of controlled clinical trials and observational studies.
Setting and participants. MEDLINE and Web of Science were respectively searched for English articles published between 1946 to October 2013 and 1900 to November 2013. Inclusion criteria were age > 20 years and vegetarian diet. This included a vegan diet (omitting all animal products), ovo/lacto/pesco vegetarian diet (including eggs/dairy/fish), or semi-vegetarian diet (meat or fish rarely). Exclusion criteria included studies of twins, a multipronged intervention, describing only categorical BP, and reliance on a case series. A total of 258 records were identified. Seven clinical trials and 32 observational studies met inclusion criteria. The 7 clinical trials encompassed 311 participants (median, 38; range, 11–113) with a mean age of 44.5 years (range, 38.0–54.3). All were open-label, 6 were randomized, and 6 provided food to participants. The 32 observational studies included 21,604 participants (median, 152; range, 20–9242) with a mean age of 46.6 years (range, 28.8–68.4 years). Fifteen of these studies included mixed diet types (vegan, lacto, ovolacto, pesca, and/or semivegetarian).
Main outcome measures. The primary outcome was BP. Differences in systolic BP (SBP) and diastolic BP (DBP) between groups consuming vegetarian or comparison diets were pooled using a random effects model. The study compared clinical trials and observational studies separately. Funnel plots, the Egger test, and the trim-and-fill method were all used to assess and correct for publication bias.
Results. Vegetarian diets in clinical trials were associated with a mean SBP reduction of −4.8 mm Hg (95% confidence interval [CI], −6.6 to −3.1; P < 0.001; I2 = 0; P = 0.45 for heterogeneity) and DBP reduction of −2.2 mm Hg (95% CI, −3.5 to −1.0; P < 0.001; I2 = 0; P= 0.43 for heterogeneity) when compared with omnivorous diets. Observational studies had larger reductions but significant heterogeneity: SBP −6.9 mm Hg (95% CI, −9.1 to −4.7; P < 0.001; I2 = 91.4; P < 0.001 for heterogeneity) and DBP −4.7 mm Hg (95% CI, −6.3 to −3.1; P < 0.001; I2 = 92.6; P < 0.001 for heterogeneity). This heterogeneity was best explained by proportion of men (β −0.03; P < 0.001), baseline SBP (β −0.13; P = 0.003), baseline DBP (β −0.30; P < 0.001), sample size (β 0.001; P< 0.001), and BMI (β −0.46; P = 0.02). This suggests that vegetarian diets and lower BP are more strongly associated in men and those with higher baseline BP and BMI.
Subgroup analysis included stratification by age, gender, BMI, diet type, sample size, diet duration, BP medication use, baseline BP, and geographic region. In subgroup analysis of clinical trials, no statistically significant difference between group variation or heterogeneity existed. In comparison, subgroup analysis of observational studies reduced heterogeneity and often effect size. For example, lower SBP was evident in the majority male subgroups (mean SBP/DBP: –18.5 mm Hg/–10.1 mm Hg).
Publication bias existed for both clinical trials and observational studies. According to trim-and-fill methodology, 3 clinical trials of smaller size and larger BP reduction likely were missing (Egger P = 0.04). Their addition shifted mean SBP reduction from −4.8 mm Hg (−6.6 to −3.1) to −5.2 mm Hg (−6.9 to −3.5). Observational studies lacked medium sized negative trials and were overrepresented by larger positive trials (Egger P < 0.001), although this was not confirmed by trim-and-fill (yet this method performs less well under heterogeneous conditions) [1].
Conclusion. Vegetarian diets, when compared with omnivorous diets, are associated with reductions in BP.
Commentary
Several studies show that dietary modifications are effective in preventing and managing hypertension [2,3]. Landmark randomized trials, including the DASH diet [4], DASH-sodium diet [5], and OmniHeart diets [6], all of which emphasize fruit and vegetable intake but are not vegetarian, have led to SBP and DBP reductions ranging from 5.5 to 9.5 mm Hg and 3.0 to 5.2, respectively. However, the impact of a vegetarian diet still remains debated, particularly given disparate findings among randomized controlled trials (RCTs). For example, findings in the early- and mid-1980s of small RCTs with ovolactovegetarians (a vegetarian who consumes eggs and dairy products but not animal flesh) suggested reductions similar to the pooled SBP reduction of –4.8 mm Hg Yokoyama et al report [7,8]. In contrast, one RCT comparing ovolactovegetarian with lean meat diets failed to show a BP benefit [9]. Striking is the dearth of RCTs in the last 20 years to assist in better estimating this impact, particularly given its continual recommendation in the scientific [10] and lay communities [11]. To the authors’ credit, this is the first meta-analysis and second systematic review of this important relationship [12].
A vegetarian diet likely supports BP reductions through a variety of mechanisms, most notably via an abundance of potassium [13]. Potassium likely promotes vasodilation, which facilitates glomerular filtration, allowing decreased renal sodium reabsorption and decreased platelet aggregation. Other more controversial hypotheses include decreased energy density leading to reduced BMI [14], decreased sodium intake [15], reduced blood viscosity [16], and high polyunsaturated with low saturated fat content [17].
Strengths of this analysis include the large observational sample size, the separate analysis of clinical trials and observational studies, the lengthy search time-frame, the subgroup analyses, and the adjustment for publication bias. Although the overall association was robust throughout, we agree with the authors that large heterogeneity among observational studies, small clinical trial sample sizes, and the variation in what “vegetarian” represents throughout the world and in individual studies all represent limitations. The participants in many of the observational studies could have technically eaten meat with unclear and undefined frequency, and this may have explained the heterogeneity of these studies. Correspondingly, the lack of heterogeneity observed in the clinical trials may be due to the fact that participants were provided meals in 6 out of 7 studies.
It was surprising that only 7 clinical trials were found. The authors utilized 2 databases, but perhaps searching additional databases such as EMBASE or CINAHL would have yielded other pertinent studies. The authors also did not use a bias assessment tool such as that proposed by the Cochrane bias methods group, which could have better discriminated high- from low-quality trials and made for useful subgroup analyses [1]. Similarly, reporting on both attrition and adherence could have assisted in decreasing heterogeneity during subgroup analyses and determining high-quality from low-quality studies. For example, adherence in Ferdowsian et al (vegan diet) was determined by unannounced dietician phone calls and found that only 57% of participants abstained from animal products. This may have been secondary to the study’s design, in that “providing meals” included simply making them an available option at the company cafeteria instead of requiring consumption of a study-specific vegetarian meal [18].
Applications for Clinical Practice
In this meta-analysis, vegetarian diets were associated with –4.8 mm Hg SBP and −2.2 mm Hg DBP reductions, indicating that providers can recommend a vegetarian diet as on par with other lifestyle changes, including low-sodium diet, weight loss, and exercise. A vegetarian diet may be comparable to pharmacologic therapy in magnitude of BP change. Short- and long-term pharmacologic therapy is associated with respective SBP/DBP reductions of –8.3/–3.8 and –5.4/–2.3, not altogether different from reductions seen with vegetarian or vegetable-heavy diets [19].
Although there are barriers to a vegetarian diet, including provider attitudes [20], cost [21], poor culinary skill [22], palatability, and adherence, pharmacologic BP treatment also presents barriers: adherence to BP medications is estimated to be 50% to 70% [23], and harm due to side effects can preclude use. Thus, providers can present a vegetarian diet as a potentially effective option, depending on patient preference and ability to adhere.
—David M. Levine, MD, MA, New York University
School of Medicine, and Melanie Jay, MD, MS
References
1. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at http://handbook.cochrane.org/chapter_10/10_4_4_2_trim_and_fill.htm.
2. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013 Nov 12. [Epub ahead of print]
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20.
4. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117–24.
5. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 2001;344:3–10.
6. Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids. JAMA 2005;294:2455–64.
7. Rouse IL, Beilin LJ, Armstrong BK, Vandongen R. Blood-pressure–lowering effect of a vegetarian diet: controlled trial in normotensive subjects. Lancet 1983;321:5–10.
8. Margetts BM, Beilin LJ, Vandongen R, Armstrong BK. Vegetarian diet in mild hypertension: a randomised controlled trial. BMJ 1986;293:1468–71.
9. Kestin M, Rouse IL, Correll RA, Nestel PJ. Cardiovascular disease risk factors in free-living men: comparison of two prudent diets, one based on lactoovovegetarianism and the other allowing lean meat. Am J Clin Nutr 1989;50:280–7.
10. Alpert JS. Nutritional advice for the patient with heart disease: what diet should we recommend for our patients? Circulation 2011;124:e258–e260.
11. Gordinier J. Making vegan a new normal. New York Times. 26 Sept 2012. Page D1.
12. Berkow SE, Barnard ND. Blood pressure regulation and vegetarian diets. Nutr Rev 2005;63:1–8.
13. Aburto NJ, Hanson S, Gutierrez H, et al. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 2013;346:f1378.
14. Berkow SE, Barnard N. Vegetarian diets and weight status. Nutr Rev 2006;64:175–88.
15. Larsson CL, Johansson GK. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr 2002;76:100–6.
16. Ernst E, Pietsch L, Matrai A, Eisenberg J. Blood rheology in vegetarians. Br J Nutr 1986;56:555–60.
17. Iacono JM, Dougherty RM. Effects of polyunsaturated fats on blood pressure. Annu Rev Nutr 1993;13:243–60.
18. Ferdowsian HR, Barnard ND, Hoover VJ, et al. A multicomponent intervention reduces body weight and cardiovascular risk at a GEICO corporate site. Am J Health Promot 2010;24:384–7.
19. Brugts JJ, Ninomiya T, Boersma E, et al. The consistency of the treatment effect of an ACE-inhibitor based treatment regimen in patients with vascular disease or high risk of vascular disease: a combined analysis of individual data of ADVANCE, EUROPA, and PROGRESS trials. Eur Heart J 2009;30:1385–94.
20. Berman BM, Singh BB, Hartnoll SM, et al. Primary care physicians and complementary-alternative medicine: training, attitudes, and practice patterns. Am Board Fam Pract 1998;11:272–81.
21. Drewnowski A, Darmon N. The economics of obesity: dietary energy density and energy cost. Am J Clin Nutr 2005;82(1 Suppl):265S-273S.
22. Lea EJ, Crawford D, Worsley A. Public views of the benefits and barriers to the consumption of a plant-based diet. Eur J Clin Nutr 2006;60:828–37.
23. Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure–lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med 2004;164:722–32.
New Cholesterol Guidelines Would Significantly Increase Statin Use If Implemented
Pencina MJ, Navar-Boggan AM, D’Agostino RB, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014;370:1422–31.
Study Overview
Objective. To quantify how many people would qualify for statin treatment under the 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines [1].
Design. Descriptive, repeated cross-sectional study examining data from the 2005–2010 National Health and Nutrition Examination Surveys (NHANES). Data on the medical diagnoses and risk factors for cardiovascular disease for NHANES participants aged 40–75 years (n = 3773) were used to extrapolate to 115.4 million US adults in the same age-range. Exclusions were for triglyceride levels > 400 mg/dL (100 participants) and missing LDL cholesterol measurement (36 participants).
Main outcome measure. Percentage of the US adult population that would be recommended statin therapy according to the 2013 ACC/AHA guidelines as compared with the 2004 guideline produced by the Third Adult Treatment Panel (ATP III) of the National Cholesterol Education Program [2,3].
Main results. Of the NHANES participants, 49% were male, 13% had cardiovascular disease, 46% had hypertension, 21% had diabetes, 21% were smokers, and 41% had obesity. Median age was 56 years (interquartile range [IQR], 41–73), median total cholesterol was 199 mg/dL (IQR, 138–272), median LDL cholesterol was 118 mg/dL (IQR, 64–182), and HDL cholesterol was 52 mg/dL (IQR, 33–86).
Overall, 2135 participants (57%) qualified for statin treatment according to the ACC/AHA guidelines as compared with 1583 (42%) under the ATP III guidelines. Additional participants qualifying under the ACC/AHA guideline were more likely to be male, older in age, have a lower LDL cholesterol, and without known cardiovascular disease, diabetes, obesity, or hypertension. Extrapolated to the US population, 56 million people (49% of the US population age 40 to 75 years, 95% CI, 46–51) would be recommended for statin treatment under the ACC/AHA guidelines compared with 43.2 million (37.5%, 95% CI, 35.3–39.7) under ATP III.
Most new candidates for statins meet criteria for primary prevention of a cardiovascular event: 2.2 million persons with diabetes and 8.2 million considered at high risk for an event in 10 years based on the new ACC/AHA risk calculator [4]. Age also was an important predictor of newly eligible statin candidates. According to ATP III, 48% of 60- to 75-year-olds would qualify for treatment, but 78% would qualify based on ACC/AHA. According to extrapolated NHANES data, 25.2 million people were taking statins from 2005 to 2010; the ACC/AHA guidelines would more than double this number.
Conclusion. The 2013 ACC/AHA cholesterol treatment guidelines would substantially increase the number of patients recommended for statin therapy.
Commentary
In November 2013, the long-awaited cholesterol treatment guidelines from the ACC/AHA hit like an earthquake [5]. The guidelines called for abandoning the traditional treat-to-target approach, in which clinicians treat patients to specific levels of LDL cholesterol [1] and instead called for statin treatment based on cardiovascular risk profile. The guideline authors made this change because of the lack of evidence supporting a treat-to-target approach; nearly all randomized controlled trials with statins used fixed doses of statins rather than trying to achieve specific LDL levels. This study by Pencina and colleagues demonstrates how implementation of the new guideline could dramatically change practice. If fully implemented, the guideline would lead to treatment for more than 12 million more patients and double the number of currently treated patients. Nearly all of the newly treated patients would receive treatment for primary prevention.
The guideline defines 4 categories of patients to be considered for treatment: (1) patients with known cardiovascular disease, (2) patients with LDL cholesterol ≥ 190 mg/dL, (3) patients with diabetes aged 40 to 75 years and LDL cholesterol ≥ 70 mg/dL, and (4) patients aged 40 to 75 years with LDL cholesterol ≥ 70 mg/dL and an estimated 10-year risk of a cardiovascular event of ≥ 7.5%. The guidelines call for patients in groups 1 and 2 to receive high-intensity statins (rosuvastatin 20 to 40 mg, atorvastatin 40 to 80 mg), although patients with known cardiovascular disease > 75 years of age can receive moderate-intensity statins. Group 3 should receive high-intensity statins if their 10-year risk is ≥ 7.5%; if otherwise, they can receive a moderate-intensity statin. Group 4 should receive a moderate-to high-intensity statin. As with most guidelines, the guidelines offer the caveat that physicians should take an informed consent approach regarding treatment and make decisions in consultation with their patients.
The publicity surrounding the new guidelines was heightened by the controversy that emerged regarding the new Pooled Cohort Risk Equation developed by the guideline committee [4] for determining 10-year risk. Using data from 5 well-known cohort studies (over 24,000 participants), they created the new risk calculator because of what they viewed as limitations of existing risk calculators: (1) the lack of racial diversity in samples used to derive them, (2) the lack of use of stroke as a cardiovascular outcome, and (3) the use of some subjective outcomes, such as coronary revascularization, angina, and congestive heart failure. Critics have suggested that the new risk calculator is poorly calibrated to more recent cohorts and that the threshold for treatment (≥ 7.5% 10-year risk) is too low and should be 10% or higher [6,7].
Physicians have long used risk calculators to help guide treatment. As an example, the Framingham Heart Study risk score was endorsed by the ATP III guideline. However, all risk scores have limitations, as clearly articulated by the developers of the ACC/AHA risk calculator:
This process is admittedly imperfect; no one has 10% or 20% of a heart attack during a 10-year period. Individuals with the same estimated risk will either have or not have the event of interest, and only those patients who are destined to have an event can have their event prevented by therapy. The criticism of the risk estimation approach to treatment-decision making also applies to the alternative, and much less efficient approach, of checking the patient’s characteristics against numerous and complex inclusion and exclusion criteria for a potentially large number of pertinent trials [4].
No matter how well calibrated or thoughtful, all calculators will be flawed. But guidelines are meant to be just that—guides rather than a prescription for treatment.
Applications for Clinical Practice
The ACC and AHA have promised a 2014 update to their guideline, which may come with adjustments to the risk calculator. Perhaps calibration of the calculator in newer cohorts will improve and the threshold for treatment will change. In the meantime, the guidelines and the accompanying calculator have an important role in helping physicians decide whom to treat for primary prevention of cardiovascular disease. Physicians should consider applying the new guidelines, while having an informed consent discussion with their patients about the risks and benefits of treatment.
—Jason P. Block, MD, MPH
References
1. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013 Nov 7 [Epub ahead of print].
2. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. J Am Coll Cardiol 2004;44:720–32.
3. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421.
4. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013 Nov 7 [Epub ahead of print].
5. Kolata G. Risk calculator for cholesterol appears flawed. New York Times. 17 November 2013. Accessed at www.nytimes.com/2013/11/18/health/risk-calculator-for-cholesterol-appears-flawed.html?_r_0 on 10 April 2014.
6. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013;382: 1762–5.
7. Downs J, Good C. New cholesterol guidelines: has Godot finally arrived? Ann Intern Med 2014;160:354–5.
Pencina MJ, Navar-Boggan AM, D’Agostino RB, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014;370:1422–31.
Study Overview
Objective. To quantify how many people would qualify for statin treatment under the 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines [1].
Design. Descriptive, repeated cross-sectional study examining data from the 2005–2010 National Health and Nutrition Examination Surveys (NHANES). Data on the medical diagnoses and risk factors for cardiovascular disease for NHANES participants aged 40–75 years (n = 3773) were used to extrapolate to 115.4 million US adults in the same age-range. Exclusions were for triglyceride levels > 400 mg/dL (100 participants) and missing LDL cholesterol measurement (36 participants).
Main outcome measure. Percentage of the US adult population that would be recommended statin therapy according to the 2013 ACC/AHA guidelines as compared with the 2004 guideline produced by the Third Adult Treatment Panel (ATP III) of the National Cholesterol Education Program [2,3].
Main results. Of the NHANES participants, 49% were male, 13% had cardiovascular disease, 46% had hypertension, 21% had diabetes, 21% were smokers, and 41% had obesity. Median age was 56 years (interquartile range [IQR], 41–73), median total cholesterol was 199 mg/dL (IQR, 138–272), median LDL cholesterol was 118 mg/dL (IQR, 64–182), and HDL cholesterol was 52 mg/dL (IQR, 33–86).
Overall, 2135 participants (57%) qualified for statin treatment according to the ACC/AHA guidelines as compared with 1583 (42%) under the ATP III guidelines. Additional participants qualifying under the ACC/AHA guideline were more likely to be male, older in age, have a lower LDL cholesterol, and without known cardiovascular disease, diabetes, obesity, or hypertension. Extrapolated to the US population, 56 million people (49% of the US population age 40 to 75 years, 95% CI, 46–51) would be recommended for statin treatment under the ACC/AHA guidelines compared with 43.2 million (37.5%, 95% CI, 35.3–39.7) under ATP III.
Most new candidates for statins meet criteria for primary prevention of a cardiovascular event: 2.2 million persons with diabetes and 8.2 million considered at high risk for an event in 10 years based on the new ACC/AHA risk calculator [4]. Age also was an important predictor of newly eligible statin candidates. According to ATP III, 48% of 60- to 75-year-olds would qualify for treatment, but 78% would qualify based on ACC/AHA. According to extrapolated NHANES data, 25.2 million people were taking statins from 2005 to 2010; the ACC/AHA guidelines would more than double this number.
Conclusion. The 2013 ACC/AHA cholesterol treatment guidelines would substantially increase the number of patients recommended for statin therapy.
Commentary
In November 2013, the long-awaited cholesterol treatment guidelines from the ACC/AHA hit like an earthquake [5]. The guidelines called for abandoning the traditional treat-to-target approach, in which clinicians treat patients to specific levels of LDL cholesterol [1] and instead called for statin treatment based on cardiovascular risk profile. The guideline authors made this change because of the lack of evidence supporting a treat-to-target approach; nearly all randomized controlled trials with statins used fixed doses of statins rather than trying to achieve specific LDL levels. This study by Pencina and colleagues demonstrates how implementation of the new guideline could dramatically change practice. If fully implemented, the guideline would lead to treatment for more than 12 million more patients and double the number of currently treated patients. Nearly all of the newly treated patients would receive treatment for primary prevention.
The guideline defines 4 categories of patients to be considered for treatment: (1) patients with known cardiovascular disease, (2) patients with LDL cholesterol ≥ 190 mg/dL, (3) patients with diabetes aged 40 to 75 years and LDL cholesterol ≥ 70 mg/dL, and (4) patients aged 40 to 75 years with LDL cholesterol ≥ 70 mg/dL and an estimated 10-year risk of a cardiovascular event of ≥ 7.5%. The guidelines call for patients in groups 1 and 2 to receive high-intensity statins (rosuvastatin 20 to 40 mg, atorvastatin 40 to 80 mg), although patients with known cardiovascular disease > 75 years of age can receive moderate-intensity statins. Group 3 should receive high-intensity statins if their 10-year risk is ≥ 7.5%; if otherwise, they can receive a moderate-intensity statin. Group 4 should receive a moderate-to high-intensity statin. As with most guidelines, the guidelines offer the caveat that physicians should take an informed consent approach regarding treatment and make decisions in consultation with their patients.
The publicity surrounding the new guidelines was heightened by the controversy that emerged regarding the new Pooled Cohort Risk Equation developed by the guideline committee [4] for determining 10-year risk. Using data from 5 well-known cohort studies (over 24,000 participants), they created the new risk calculator because of what they viewed as limitations of existing risk calculators: (1) the lack of racial diversity in samples used to derive them, (2) the lack of use of stroke as a cardiovascular outcome, and (3) the use of some subjective outcomes, such as coronary revascularization, angina, and congestive heart failure. Critics have suggested that the new risk calculator is poorly calibrated to more recent cohorts and that the threshold for treatment (≥ 7.5% 10-year risk) is too low and should be 10% or higher [6,7].
Physicians have long used risk calculators to help guide treatment. As an example, the Framingham Heart Study risk score was endorsed by the ATP III guideline. However, all risk scores have limitations, as clearly articulated by the developers of the ACC/AHA risk calculator:
This process is admittedly imperfect; no one has 10% or 20% of a heart attack during a 10-year period. Individuals with the same estimated risk will either have or not have the event of interest, and only those patients who are destined to have an event can have their event prevented by therapy. The criticism of the risk estimation approach to treatment-decision making also applies to the alternative, and much less efficient approach, of checking the patient’s characteristics against numerous and complex inclusion and exclusion criteria for a potentially large number of pertinent trials [4].
No matter how well calibrated or thoughtful, all calculators will be flawed. But guidelines are meant to be just that—guides rather than a prescription for treatment.
Applications for Clinical Practice
The ACC and AHA have promised a 2014 update to their guideline, which may come with adjustments to the risk calculator. Perhaps calibration of the calculator in newer cohorts will improve and the threshold for treatment will change. In the meantime, the guidelines and the accompanying calculator have an important role in helping physicians decide whom to treat for primary prevention of cardiovascular disease. Physicians should consider applying the new guidelines, while having an informed consent discussion with their patients about the risks and benefits of treatment.
—Jason P. Block, MD, MPH
References
1. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013 Nov 7 [Epub ahead of print].
2. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. J Am Coll Cardiol 2004;44:720–32.
3. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421.
4. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013 Nov 7 [Epub ahead of print].
5. Kolata G. Risk calculator for cholesterol appears flawed. New York Times. 17 November 2013. Accessed at www.nytimes.com/2013/11/18/health/risk-calculator-for-cholesterol-appears-flawed.html?_r_0 on 10 April 2014.
6. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013;382: 1762–5.
7. Downs J, Good C. New cholesterol guidelines: has Godot finally arrived? Ann Intern Med 2014;160:354–5.
Pencina MJ, Navar-Boggan AM, D’Agostino RB, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014;370:1422–31.
Study Overview
Objective. To quantify how many people would qualify for statin treatment under the 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines [1].
Design. Descriptive, repeated cross-sectional study examining data from the 2005–2010 National Health and Nutrition Examination Surveys (NHANES). Data on the medical diagnoses and risk factors for cardiovascular disease for NHANES participants aged 40–75 years (n = 3773) were used to extrapolate to 115.4 million US adults in the same age-range. Exclusions were for triglyceride levels > 400 mg/dL (100 participants) and missing LDL cholesterol measurement (36 participants).
Main outcome measure. Percentage of the US adult population that would be recommended statin therapy according to the 2013 ACC/AHA guidelines as compared with the 2004 guideline produced by the Third Adult Treatment Panel (ATP III) of the National Cholesterol Education Program [2,3].
Main results. Of the NHANES participants, 49% were male, 13% had cardiovascular disease, 46% had hypertension, 21% had diabetes, 21% were smokers, and 41% had obesity. Median age was 56 years (interquartile range [IQR], 41–73), median total cholesterol was 199 mg/dL (IQR, 138–272), median LDL cholesterol was 118 mg/dL (IQR, 64–182), and HDL cholesterol was 52 mg/dL (IQR, 33–86).
Overall, 2135 participants (57%) qualified for statin treatment according to the ACC/AHA guidelines as compared with 1583 (42%) under the ATP III guidelines. Additional participants qualifying under the ACC/AHA guideline were more likely to be male, older in age, have a lower LDL cholesterol, and without known cardiovascular disease, diabetes, obesity, or hypertension. Extrapolated to the US population, 56 million people (49% of the US population age 40 to 75 years, 95% CI, 46–51) would be recommended for statin treatment under the ACC/AHA guidelines compared with 43.2 million (37.5%, 95% CI, 35.3–39.7) under ATP III.
Most new candidates for statins meet criteria for primary prevention of a cardiovascular event: 2.2 million persons with diabetes and 8.2 million considered at high risk for an event in 10 years based on the new ACC/AHA risk calculator [4]. Age also was an important predictor of newly eligible statin candidates. According to ATP III, 48% of 60- to 75-year-olds would qualify for treatment, but 78% would qualify based on ACC/AHA. According to extrapolated NHANES data, 25.2 million people were taking statins from 2005 to 2010; the ACC/AHA guidelines would more than double this number.
Conclusion. The 2013 ACC/AHA cholesterol treatment guidelines would substantially increase the number of patients recommended for statin therapy.
Commentary
In November 2013, the long-awaited cholesterol treatment guidelines from the ACC/AHA hit like an earthquake [5]. The guidelines called for abandoning the traditional treat-to-target approach, in which clinicians treat patients to specific levels of LDL cholesterol [1] and instead called for statin treatment based on cardiovascular risk profile. The guideline authors made this change because of the lack of evidence supporting a treat-to-target approach; nearly all randomized controlled trials with statins used fixed doses of statins rather than trying to achieve specific LDL levels. This study by Pencina and colleagues demonstrates how implementation of the new guideline could dramatically change practice. If fully implemented, the guideline would lead to treatment for more than 12 million more patients and double the number of currently treated patients. Nearly all of the newly treated patients would receive treatment for primary prevention.
The guideline defines 4 categories of patients to be considered for treatment: (1) patients with known cardiovascular disease, (2) patients with LDL cholesterol ≥ 190 mg/dL, (3) patients with diabetes aged 40 to 75 years and LDL cholesterol ≥ 70 mg/dL, and (4) patients aged 40 to 75 years with LDL cholesterol ≥ 70 mg/dL and an estimated 10-year risk of a cardiovascular event of ≥ 7.5%. The guidelines call for patients in groups 1 and 2 to receive high-intensity statins (rosuvastatin 20 to 40 mg, atorvastatin 40 to 80 mg), although patients with known cardiovascular disease > 75 years of age can receive moderate-intensity statins. Group 3 should receive high-intensity statins if their 10-year risk is ≥ 7.5%; if otherwise, they can receive a moderate-intensity statin. Group 4 should receive a moderate-to high-intensity statin. As with most guidelines, the guidelines offer the caveat that physicians should take an informed consent approach regarding treatment and make decisions in consultation with their patients.
The publicity surrounding the new guidelines was heightened by the controversy that emerged regarding the new Pooled Cohort Risk Equation developed by the guideline committee [4] for determining 10-year risk. Using data from 5 well-known cohort studies (over 24,000 participants), they created the new risk calculator because of what they viewed as limitations of existing risk calculators: (1) the lack of racial diversity in samples used to derive them, (2) the lack of use of stroke as a cardiovascular outcome, and (3) the use of some subjective outcomes, such as coronary revascularization, angina, and congestive heart failure. Critics have suggested that the new risk calculator is poorly calibrated to more recent cohorts and that the threshold for treatment (≥ 7.5% 10-year risk) is too low and should be 10% or higher [6,7].
Physicians have long used risk calculators to help guide treatment. As an example, the Framingham Heart Study risk score was endorsed by the ATP III guideline. However, all risk scores have limitations, as clearly articulated by the developers of the ACC/AHA risk calculator:
This process is admittedly imperfect; no one has 10% or 20% of a heart attack during a 10-year period. Individuals with the same estimated risk will either have or not have the event of interest, and only those patients who are destined to have an event can have their event prevented by therapy. The criticism of the risk estimation approach to treatment-decision making also applies to the alternative, and much less efficient approach, of checking the patient’s characteristics against numerous and complex inclusion and exclusion criteria for a potentially large number of pertinent trials [4].
No matter how well calibrated or thoughtful, all calculators will be flawed. But guidelines are meant to be just that—guides rather than a prescription for treatment.
Applications for Clinical Practice
The ACC and AHA have promised a 2014 update to their guideline, which may come with adjustments to the risk calculator. Perhaps calibration of the calculator in newer cohorts will improve and the threshold for treatment will change. In the meantime, the guidelines and the accompanying calculator have an important role in helping physicians decide whom to treat for primary prevention of cardiovascular disease. Physicians should consider applying the new guidelines, while having an informed consent discussion with their patients about the risks and benefits of treatment.
—Jason P. Block, MD, MPH
References
1. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013 Nov 7 [Epub ahead of print].
2. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. J Am Coll Cardiol 2004;44:720–32.
3. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421.
4. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013 Nov 7 [Epub ahead of print].
5. Kolata G. Risk calculator for cholesterol appears flawed. New York Times. 17 November 2013. Accessed at www.nytimes.com/2013/11/18/health/risk-calculator-for-cholesterol-appears-flawed.html?_r_0 on 10 April 2014.
6. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013;382: 1762–5.
7. Downs J, Good C. New cholesterol guidelines: has Godot finally arrived? Ann Intern Med 2014;160:354–5.
Using Quality Improvement Methods to Implement an Individualized Home Pain Management Plan for Children with Sickle Cell Disease
From the Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
This article is the third in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
ABSTRACT
• Objective: To develop and implement individualized home pain management plans that included pharmacologic as well as nonpharmacologic strategies for children with sickle cell disease (SCD).
• Methods: A multidisciplinary quality improvement team developed a questionnaire to assess the frequency, location, and severity of a patient’s pain during a routine comprehensive visit in order to help the patient and family develop an effective home pain management plan. Using plan-do-study-act cycles, the team was able to build this process into the daily workflow for all SCD patients age 5 years to 21 years of age. Patients with comprehensive visits scheduled from January 2012 to May 2013 were included (n = 188) in the intervention.
• Results: By May of 2013, 88% of eligible patients had an individualized home plan in place. There was a concomitant reduction in the percentage of SCD patients seen in the ED for uncomplicated SCD pain (6.9% vs. 1.1%).
• Conclusions: Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. This intervention has the potential to improve patient outcomes by decreasing avoidable ED visits as well as reducing overall health care costs.
Sickle cell disease (SCD) is one of the most common genetic disorders in the United States, affecting approximately 1 in 500 African-American infants each year [1]. The genetic mutation that causes SCD results in the production of an abnormal hemoglobin molecule (HbS) in the red blood cells (RBC). Under low oxygen conditions, the HbS polymerizes and causes the RBCs to elongate into a sickle form (crescent shape) and decreases the life span of the RBC. Additionally, RBCs with HbS are more “sticky,” adhering to vessel walls and limiting blood flow and oxygen delivery to many tissues and organs in the body. The resultant tissue ischemia causes progressive organ injury as well as episodes of pain (vaso-occlusive crisis).
Recurrent pain episodes are the hallmark of this disease, accounting for the majority of emergency department (ED) visits as well as hospitalizations. High-quality outpatient care can reduce acute care and ED visits as well as hospitalization rates in patients with SCD [2]. Additionally, ensuring that patients have a home pain management plan and understand how to assess and reassess their pain may improve outcomes [3]. Data from our population of children with SCD indicate that 40% to 50% of ED visits in 2011 were for uncomplicated pain episodes (no concomitant medical issues such as fever, increased respiratory rate, wheezing, worsening pallor). If these pain episodes had been effectively managed at home, the ED visits might have been avoided.
In an effort to reduce these potentially preventable ED visits and subsequent hospitalizations, the Comprehensive Sickle Cell Center at Cincinnati Children’s Hospital Medical Center assembled a quality improvement (QI) team to partner with patients and their families to develop individualized home pain management plans (HPMP) that incorporated both pharmacologic and nonpharmacologic pain management strategies. We also sought to identify and remove barriers to the successful use of a home pain management plan, such as not having enough analgesics at home or not allowing enough time for analgesics to work before presenting to the ED. We documented the plan in a standard location and format in the electronic medical record (EMR), making it available to all medical center providers. This paper describes the development, refinement, and testing of an individualized HPMP intervention and related outcomes.
METHODS
Setting
Cincinnati Children’s Hospital Medical Center is a nonprofit pediatric medical center with 587 inpatient beds in Ohio providing acute and chronic care for children in Southern Ohio, Northern Kentucky, and Southeastern Indiana. The center’s Comprehensive Sickle Cell Center provides comprehensive care to approximately 280 children with SCD in the region from birth to 21 years of age. The medical center is the only major pediatric inpatient facility in the tri-state area. Greater than 75% of the SCD patients at our center live within a 15-mile radius, therefore, essentially all ED visits and hospitalizations for our patients occur at our center.
Participants
Improvement Team
The core QI team consisted of multidisciplinary health care providers with experience caring for patients with SCD, including 3 SCD nurse care managers, 2 physicians, 2 PhD psychologists, 4 nurse practitioners, a QI consultant, and a data analyst. Additional support and suggestions were received from other SCD team members (eg, social workers, school interventionists). The core QI multidisciplinary team met weekly to design and test the intervention and implementation process.
Intervention
The intervention consisted of the following elements: (1) pre-visit review to identify eligible patients needing a new or updated home pain management plan; (2) family completion of a pain assessment tool; (3) review of pain assessment tool by SCD team; (4) development of collaborative home plan with family and the medical team; (4) integration of nonpharmacological strategies into the home plan (developed with the psychologist); (5) printed copy of the plan for family to take home; (6) documentation of HPMP in the EMR (Table 2); and, (7) a follow-up phone call for eligible patients with ED or urgent care visits for uncomplicated SCD pain by the nurse care manager.
Implementation
Each week the data analyst generated a list of eligible patients with ICD-9 diagnostic codes for SCD using SQL (structured query language) to extract the data from the EMR (Table 1). The SCD nurse care managers reviewed the list and notified the team of those patients needing a pain assessment and updated HPMP during the daily pre-clinic patient review rounds each morning.
The provider seeing the patient that day facilitated the patient and family’s completion of the pain assessment tool. The pain assessment tool consisted of 13 items and measured recent illnesses or transfusions, patient’s pain location, intensity, associated symptoms, potential triggers, and the impact of the pain on quality of life (missed days of school/work). In addition, the patient’s current pain management strategies, perceived effectiveness of those strategies, and analgesics available at home was recorded.
After discussing the results with the team, a medical provider reviewed the findings with the patient and family and developed a plan for pharmacologic pain management at home utilizing a stepwise approach based on the World Health Organization (WHO) analgesic ladder for selecting pain-relief drugs [4,5] and the American Pain Society guidelines for management of acute and chronic sickle cell pain [6]. The medication’s method of action, side effects, risks, and benefits were reviewed and prescriptions were provided as needed.
During the same visit, patients who reported acute or chronic pain within the last month met with the team psychology provider. The psychology provider educated the patient and family about pain, the mind-body connection, and nonpharmacologic approaches to pain management that could be incorporated in the home plan. Following the education, the psychology provider taught the patient at least one relaxation strategy (eg, diaphragmatic breathing, guided imagery, progressive muscle relaxation) and provided written materials to take home to encourage practice. At the time of discharge from the clinic, patients and families received a copy of the comprehensive home pain plan and any needed prescriptions for analgesics. Families were encouraged to access a copy of their plan at home by logging on to MyChart (Epic Systems), a limited version of the child’s EMR designed for patients and families.
After each ED or urgent care visit for uncomplicated SCD pain, the nurse care manager attempted to call the family within 3 business days to ask whether the home pain management plan had been used and determine if it needed to be revised. Medication refills were confirmed via phone follow-up by the nurse care manager at this time. Laminated pocket guides for the care managers facilitated and standardized the follow-up questions. A maximum of 3 attempts were made to contact the family. Information from the telephone encounter was documented in the patient’s EMR in a standard format and location. This information was then communicated to the SCD provider (nurse practitioner or physician) who modified the plan as needed. If the patient did not have any ED or acute care outpatient visits, the HPMP was reviewed every 6 months at a routinely scheduled comprehensive visit.
The team used multiple plan-do-study-act cycles (PDSAs) to refine the intervention and implementation process. One PDSA involved a focus group consisting of 3 young adult patients and 1 parent. Participants were asked if they knew what we were referring to when we used the term “home pain management plan,” what they remembered about their plan, and if they thought we should keep or change the name. All 4 participants reported that they were familiar with the term and were able to describe aspects of their or their child’s home pain management plan. Although 1 participant suggested shortening the name, the SCD team had worked to develop a high level of familiarity with the name, so it was retained. Another PDSA was conducted to assess whether the pediatric hematology fellows (post-graduate trainees) were aware of the HPMP and how to access it in the EMR. Eight of the 10 fellows responded, and the majority indicated that they were aware of the HPMP; however, only 1 fellow knew where to locate it in the EMR. This resulted in PDSAs to increase fellows’ awareness and use of the HPMP.
The QI team also completed a failure mode and effects analysis (FMEA) to identify potential failures in the clinic flow process. The FMEA helped to identify low-hanging fruit “quick fixes,” PDSAs, and develop process maps. Weekly data guided our PDSAs and allowed us to continuously improve our processes, and team members were accountable for specific weekly action items.
Measurement/Analysis
The home pain management implementation process was monitored and tracked using 2 weekly run charts: one that displayed the percentage of eligible SCD patients who needed a HPMP each week that actually received one and one that showed the overall number of eligible SCD patients with a HPMP (population metric). Run charts provide a graphic display of process performance over time and allowed the team to track and monitor process outcomes. The goal was that at least 85% of eligible patients would receive the HPMP intervention by November 2012.
Outcomes were evaluated using a monthly p-chart showing the percentage of SCD patients seen in the ED for uncomplicated SCD pain. For the current project, a p-chart was used because ED visits were categorized (see below) and the sample size varied by month. We conducted a retrospective chart review of each ED visit to extract the initial complaint and the final assessment from the ED providers’ notes. ED visits were categorized as follows: (1) fever (with or without other symptoms such as pain), (2) uncomplicated SCD pain only, and (3) other (eg, trauma, asthma). The goal was to monitor ED visits for uncomplicated SCD pain only to determine if the rate of this type of ED visit decreased after the implementation of the HPMP. Based on the chart review of the 12 months prior to the implementation of the HPMP, the majority of SCD patients seen in the ED had 0–3 ED visits for uncomplicated SCD pain. Only 7 patients had more than 3 ED visits: two had 4 ED visits, two had 5 ED visits, one had 6 visits, one had 7 visits, and one had 13 visits to the ED. Because the patient with 13 visits has complex psychosocial issues that greatly impact the use of the ED and inpatient medical services, this data was excluded from our analyses.
The Children’s Hospital Medical Center Institutional Review Board exempted this study from review because it was deemed to be a QI project with the intent to improve care locally and not to develop generalizable
knowledge.
RESULTS
DISCUSSION
Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. The QI team provided critical guidance, organization, and resources for refining the HPMP intervention and implementing it into a very busy outpatient clinical setting. QI methods such as the PDSAs, FMEA, and process maps allowed us to continuously improve the intervention and develop an effective implementation process. As a result, we were able to reach our goal of ensuring that 100% of eligible patients received a HPMP during their clinic visit.
Several studies have shown cognitive-behavioral therapies, such as relaxation, imagery, and self-hypnosis, to improve outcomes in children and adults with SCD [7–10]. We believe that having psychology providers on our team who could train families in nonpharmacological strategies was critical to the project’s success. Most SCD patients are taught to increase fluid intake and use warm compresses, but few are trained in adjunctive nonpharmacologic strategies while awaiting the effects of oral analgesics. Thus, our multidisciplinary protocol is innovative; future studies may show it to to be more effective than interventions using pharmacologic or nonpharmacologic strategies alone.
Implementing a comprehensive home pain management intervention in a very busy clinical setting was challenging; it required a substantial coordination and communication among the clinical team. Although each member of the team had a well-defined role, we found that our nurse care managers were the drivers of the process during the clinic visit. They ensured the documentation of the HPMP and reconciliation of medications were completed in the EMR, that prescriptions for analgesics were written and educated families to execute the HPMP.
We were able to exceed our goal of ensuring that at least 85% of eligible patients in our population had a home plan in place. This is clinically significant as most SCD pain episodes occur at home [11]. Typically, the pain management strategies used by patients and families at home are inconsistent, and several studies indicate that parents may be reluctant to use analgesics for their children, use a dose that is too small, or do not give the medicine often enough [12–14]. Developing an home pain plan with a patient and family allowed for education about distinguishing different types of pain and the appropriate use of medications for specific types of pain.
Challenges to implementation of the home plan protocol included limited time during clinics visit to integrate the plan given competing clinical issues. Some families felt the visit lasted too long and were eager to leave the clinic without further delays. Additionally, the fixed design of the EMR posed some limitations related to documentation, medication reconciliation, and updating of the home plan because different team members could not simultaneously access some parts of the EMR. We also initially overlooked the need to educate other providers in our division about the home plan, such as fellows who take calls about patients after hours. This has subsequently been addressed via ongoing PDSAs to test processes for making fellows aware of the home pain plan and to ensure they use it consistently to coordinate care.
Following implementation of the protocol, the percentage of ED visits for SCD uncomplicated pain decreased by 84%. These results build on the previous literature which has focused primarily on standardized pain management protocols in the ED [15–17]. However, it makes a unique contribution in that the focus was on systematically teaching families strategies to use at home with the goal of minimizing the need for ED or urgent care intervention. We also learned more about the reasons for some ED visits: there were patients who presented to the ED with presumed acute SCD pain that actually had acute exacerbations of chronic back pain (8 patients), headaches (5 patients), or abdominal pain due to constipation (12 patients). Each of these is managed differently than acute SCD pain, and the HPMP was not designed for these conditions. In addition, we discovered that a few patients (3 patients) used opiate analgesics for difficulties with sleeping rather than pain, further supporting the need for ongoing patient/family education about pain management in pediatric SCD.
We conclude that the home pain plan intervention served to empower patients with SCD and their families by providing them with the tools to manage uncomplicated pain events at home thereby reduce utilization of the ED. Hence, the home plan intervention has the potential to improve patient outcomes by decreasing avoidable ED visits and reducing overall health care costs. It is hoped that other clinics or hospitals could use QI methods to implement home pain plans that would allow achievement of similar outcomes. Finally, this paper contributes to the limited literature on both QI and home management in pediatric SCD and addresses a critical gap in the literature: a clinical approach to reducing potentially preventable ED visits and subsequent hospitalizations for youth with SCD. It also serves as the basis for future innovative research examining the relationship between a home pain management, health care utilization, and health care costs.
Corresponding author: Kenya Simmons, MBA, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., Cincinnati, OH 45229.
Funding/support: This project was funded in part by HRSA grant #U38MC22218 and NIH grant #K07HL108720-03.
References
1. Armstrong FD. Acute and long-term neurodevelopmental outcomes in children following bone marrow transplantation. Front Biosc 2001;6:G6–G12.
2. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
3. Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: rates and risk factors. N Engl J Med 1991;325:11–16.
4. Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician 2010;56:514–7.
5. Ventafridda V, Stjernsward J. Pain control and the World Health Organization analgesic ladder. JAMA 1996;275:835–6.
6. Rees DC, Olujohungbe AD, Parker NE, et al. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematol 2003:120:744–52.
7. Dampier C, Ely E, Eggleston B, et al. Physical and cognitive behavioral activities used in the home management of sickle pain: A daily diary study in children and adolescents. Ped Blood cancer 2004;43:674–8.
8. Dinges DF, Whitehouse WG, Orne EC, et al. Self-hypnosis training as an adjunctive treatment in the management of pain associated with sickle cell disease. Int J Clin Exp Hypnosis 1997;45:417–32.
9. Thomas VN, Wilson Barnett J, Goodhart F. The role of cognitive behavioural therapy in the management of pain in patients with sickle cell disease. J Adv Nurs 1998;27:1002–9.
10. Gil KM, Anthony KK, Carson JW, et al. Daily coping practice predicts treatment effects in children with sickle cell disease. J Ped Psychol 2001;26:163–73.
11. Dampier C, Ely E, Brodecki D, O’Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Ped hematol oncol 2002;24:643–7.
12. Ferrell BR. Pain management: a moral imperative. Communique (Wash DC) 1996;5:4–5.
13. Finley GA, McGrath PJ, Forward SP, et al. Parents’ management of children’s pain following ‘minor’ surgery. Pain 1996;64:83–7.
14. Forward SP, Brown TL, McGrath PJ. Mothers’ attitudes and behavior toward medicating children’s pain. Pain 1996;67:469–74.
15. Givens M, Rutherford C, Joshi G, Delaney K. Impact of an emergency department pain management protocol on the pattern of visits by patients with sickle cell disease. J Emerg Med 2007;32:239–43.
16. Powers RD. Management protocol for sickle-cell disease patients with acute pain: impact on emergency department and narcotic use. Am J Emerg Med 1986;4:267–8.
17. Silbergleit R, Jancis MOS, McNamara RM. Management of sickle cell pain crisis in the emergency department at teaching hospitals. J Emerg Med 1999:17:625–30.
From the Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
This article is the third in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
ABSTRACT
• Objective: To develop and implement individualized home pain management plans that included pharmacologic as well as nonpharmacologic strategies for children with sickle cell disease (SCD).
• Methods: A multidisciplinary quality improvement team developed a questionnaire to assess the frequency, location, and severity of a patient’s pain during a routine comprehensive visit in order to help the patient and family develop an effective home pain management plan. Using plan-do-study-act cycles, the team was able to build this process into the daily workflow for all SCD patients age 5 years to 21 years of age. Patients with comprehensive visits scheduled from January 2012 to May 2013 were included (n = 188) in the intervention.
• Results: By May of 2013, 88% of eligible patients had an individualized home plan in place. There was a concomitant reduction in the percentage of SCD patients seen in the ED for uncomplicated SCD pain (6.9% vs. 1.1%).
• Conclusions: Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. This intervention has the potential to improve patient outcomes by decreasing avoidable ED visits as well as reducing overall health care costs.
Sickle cell disease (SCD) is one of the most common genetic disorders in the United States, affecting approximately 1 in 500 African-American infants each year [1]. The genetic mutation that causes SCD results in the production of an abnormal hemoglobin molecule (HbS) in the red blood cells (RBC). Under low oxygen conditions, the HbS polymerizes and causes the RBCs to elongate into a sickle form (crescent shape) and decreases the life span of the RBC. Additionally, RBCs with HbS are more “sticky,” adhering to vessel walls and limiting blood flow and oxygen delivery to many tissues and organs in the body. The resultant tissue ischemia causes progressive organ injury as well as episodes of pain (vaso-occlusive crisis).
Recurrent pain episodes are the hallmark of this disease, accounting for the majority of emergency department (ED) visits as well as hospitalizations. High-quality outpatient care can reduce acute care and ED visits as well as hospitalization rates in patients with SCD [2]. Additionally, ensuring that patients have a home pain management plan and understand how to assess and reassess their pain may improve outcomes [3]. Data from our population of children with SCD indicate that 40% to 50% of ED visits in 2011 were for uncomplicated pain episodes (no concomitant medical issues such as fever, increased respiratory rate, wheezing, worsening pallor). If these pain episodes had been effectively managed at home, the ED visits might have been avoided.
In an effort to reduce these potentially preventable ED visits and subsequent hospitalizations, the Comprehensive Sickle Cell Center at Cincinnati Children’s Hospital Medical Center assembled a quality improvement (QI) team to partner with patients and their families to develop individualized home pain management plans (HPMP) that incorporated both pharmacologic and nonpharmacologic pain management strategies. We also sought to identify and remove barriers to the successful use of a home pain management plan, such as not having enough analgesics at home or not allowing enough time for analgesics to work before presenting to the ED. We documented the plan in a standard location and format in the electronic medical record (EMR), making it available to all medical center providers. This paper describes the development, refinement, and testing of an individualized HPMP intervention and related outcomes.
METHODS
Setting
Cincinnati Children’s Hospital Medical Center is a nonprofit pediatric medical center with 587 inpatient beds in Ohio providing acute and chronic care for children in Southern Ohio, Northern Kentucky, and Southeastern Indiana. The center’s Comprehensive Sickle Cell Center provides comprehensive care to approximately 280 children with SCD in the region from birth to 21 years of age. The medical center is the only major pediatric inpatient facility in the tri-state area. Greater than 75% of the SCD patients at our center live within a 15-mile radius, therefore, essentially all ED visits and hospitalizations for our patients occur at our center.
Participants
Improvement Team
The core QI team consisted of multidisciplinary health care providers with experience caring for patients with SCD, including 3 SCD nurse care managers, 2 physicians, 2 PhD psychologists, 4 nurse practitioners, a QI consultant, and a data analyst. Additional support and suggestions were received from other SCD team members (eg, social workers, school interventionists). The core QI multidisciplinary team met weekly to design and test the intervention and implementation process.
Intervention
The intervention consisted of the following elements: (1) pre-visit review to identify eligible patients needing a new or updated home pain management plan; (2) family completion of a pain assessment tool; (3) review of pain assessment tool by SCD team; (4) development of collaborative home plan with family and the medical team; (4) integration of nonpharmacological strategies into the home plan (developed with the psychologist); (5) printed copy of the plan for family to take home; (6) documentation of HPMP in the EMR (Table 2); and, (7) a follow-up phone call for eligible patients with ED or urgent care visits for uncomplicated SCD pain by the nurse care manager.
Implementation
Each week the data analyst generated a list of eligible patients with ICD-9 diagnostic codes for SCD using SQL (structured query language) to extract the data from the EMR (Table 1). The SCD nurse care managers reviewed the list and notified the team of those patients needing a pain assessment and updated HPMP during the daily pre-clinic patient review rounds each morning.
The provider seeing the patient that day facilitated the patient and family’s completion of the pain assessment tool. The pain assessment tool consisted of 13 items and measured recent illnesses or transfusions, patient’s pain location, intensity, associated symptoms, potential triggers, and the impact of the pain on quality of life (missed days of school/work). In addition, the patient’s current pain management strategies, perceived effectiveness of those strategies, and analgesics available at home was recorded.
After discussing the results with the team, a medical provider reviewed the findings with the patient and family and developed a plan for pharmacologic pain management at home utilizing a stepwise approach based on the World Health Organization (WHO) analgesic ladder for selecting pain-relief drugs [4,5] and the American Pain Society guidelines for management of acute and chronic sickle cell pain [6]. The medication’s method of action, side effects, risks, and benefits were reviewed and prescriptions were provided as needed.
During the same visit, patients who reported acute or chronic pain within the last month met with the team psychology provider. The psychology provider educated the patient and family about pain, the mind-body connection, and nonpharmacologic approaches to pain management that could be incorporated in the home plan. Following the education, the psychology provider taught the patient at least one relaxation strategy (eg, diaphragmatic breathing, guided imagery, progressive muscle relaxation) and provided written materials to take home to encourage practice. At the time of discharge from the clinic, patients and families received a copy of the comprehensive home pain plan and any needed prescriptions for analgesics. Families were encouraged to access a copy of their plan at home by logging on to MyChart (Epic Systems), a limited version of the child’s EMR designed for patients and families.
After each ED or urgent care visit for uncomplicated SCD pain, the nurse care manager attempted to call the family within 3 business days to ask whether the home pain management plan had been used and determine if it needed to be revised. Medication refills were confirmed via phone follow-up by the nurse care manager at this time. Laminated pocket guides for the care managers facilitated and standardized the follow-up questions. A maximum of 3 attempts were made to contact the family. Information from the telephone encounter was documented in the patient’s EMR in a standard format and location. This information was then communicated to the SCD provider (nurse practitioner or physician) who modified the plan as needed. If the patient did not have any ED or acute care outpatient visits, the HPMP was reviewed every 6 months at a routinely scheduled comprehensive visit.
The team used multiple plan-do-study-act cycles (PDSAs) to refine the intervention and implementation process. One PDSA involved a focus group consisting of 3 young adult patients and 1 parent. Participants were asked if they knew what we were referring to when we used the term “home pain management plan,” what they remembered about their plan, and if they thought we should keep or change the name. All 4 participants reported that they were familiar with the term and were able to describe aspects of their or their child’s home pain management plan. Although 1 participant suggested shortening the name, the SCD team had worked to develop a high level of familiarity with the name, so it was retained. Another PDSA was conducted to assess whether the pediatric hematology fellows (post-graduate trainees) were aware of the HPMP and how to access it in the EMR. Eight of the 10 fellows responded, and the majority indicated that they were aware of the HPMP; however, only 1 fellow knew where to locate it in the EMR. This resulted in PDSAs to increase fellows’ awareness and use of the HPMP.
The QI team also completed a failure mode and effects analysis (FMEA) to identify potential failures in the clinic flow process. The FMEA helped to identify low-hanging fruit “quick fixes,” PDSAs, and develop process maps. Weekly data guided our PDSAs and allowed us to continuously improve our processes, and team members were accountable for specific weekly action items.
Measurement/Analysis
The home pain management implementation process was monitored and tracked using 2 weekly run charts: one that displayed the percentage of eligible SCD patients who needed a HPMP each week that actually received one and one that showed the overall number of eligible SCD patients with a HPMP (population metric). Run charts provide a graphic display of process performance over time and allowed the team to track and monitor process outcomes. The goal was that at least 85% of eligible patients would receive the HPMP intervention by November 2012.
Outcomes were evaluated using a monthly p-chart showing the percentage of SCD patients seen in the ED for uncomplicated SCD pain. For the current project, a p-chart was used because ED visits were categorized (see below) and the sample size varied by month. We conducted a retrospective chart review of each ED visit to extract the initial complaint and the final assessment from the ED providers’ notes. ED visits were categorized as follows: (1) fever (with or without other symptoms such as pain), (2) uncomplicated SCD pain only, and (3) other (eg, trauma, asthma). The goal was to monitor ED visits for uncomplicated SCD pain only to determine if the rate of this type of ED visit decreased after the implementation of the HPMP. Based on the chart review of the 12 months prior to the implementation of the HPMP, the majority of SCD patients seen in the ED had 0–3 ED visits for uncomplicated SCD pain. Only 7 patients had more than 3 ED visits: two had 4 ED visits, two had 5 ED visits, one had 6 visits, one had 7 visits, and one had 13 visits to the ED. Because the patient with 13 visits has complex psychosocial issues that greatly impact the use of the ED and inpatient medical services, this data was excluded from our analyses.
The Children’s Hospital Medical Center Institutional Review Board exempted this study from review because it was deemed to be a QI project with the intent to improve care locally and not to develop generalizable
knowledge.
RESULTS
DISCUSSION
Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. The QI team provided critical guidance, organization, and resources for refining the HPMP intervention and implementing it into a very busy outpatient clinical setting. QI methods such as the PDSAs, FMEA, and process maps allowed us to continuously improve the intervention and develop an effective implementation process. As a result, we were able to reach our goal of ensuring that 100% of eligible patients received a HPMP during their clinic visit.
Several studies have shown cognitive-behavioral therapies, such as relaxation, imagery, and self-hypnosis, to improve outcomes in children and adults with SCD [7–10]. We believe that having psychology providers on our team who could train families in nonpharmacological strategies was critical to the project’s success. Most SCD patients are taught to increase fluid intake and use warm compresses, but few are trained in adjunctive nonpharmacologic strategies while awaiting the effects of oral analgesics. Thus, our multidisciplinary protocol is innovative; future studies may show it to to be more effective than interventions using pharmacologic or nonpharmacologic strategies alone.
Implementing a comprehensive home pain management intervention in a very busy clinical setting was challenging; it required a substantial coordination and communication among the clinical team. Although each member of the team had a well-defined role, we found that our nurse care managers were the drivers of the process during the clinic visit. They ensured the documentation of the HPMP and reconciliation of medications were completed in the EMR, that prescriptions for analgesics were written and educated families to execute the HPMP.
We were able to exceed our goal of ensuring that at least 85% of eligible patients in our population had a home plan in place. This is clinically significant as most SCD pain episodes occur at home [11]. Typically, the pain management strategies used by patients and families at home are inconsistent, and several studies indicate that parents may be reluctant to use analgesics for their children, use a dose that is too small, or do not give the medicine often enough [12–14]. Developing an home pain plan with a patient and family allowed for education about distinguishing different types of pain and the appropriate use of medications for specific types of pain.
Challenges to implementation of the home plan protocol included limited time during clinics visit to integrate the plan given competing clinical issues. Some families felt the visit lasted too long and were eager to leave the clinic without further delays. Additionally, the fixed design of the EMR posed some limitations related to documentation, medication reconciliation, and updating of the home plan because different team members could not simultaneously access some parts of the EMR. We also initially overlooked the need to educate other providers in our division about the home plan, such as fellows who take calls about patients after hours. This has subsequently been addressed via ongoing PDSAs to test processes for making fellows aware of the home pain plan and to ensure they use it consistently to coordinate care.
Following implementation of the protocol, the percentage of ED visits for SCD uncomplicated pain decreased by 84%. These results build on the previous literature which has focused primarily on standardized pain management protocols in the ED [15–17]. However, it makes a unique contribution in that the focus was on systematically teaching families strategies to use at home with the goal of minimizing the need for ED or urgent care intervention. We also learned more about the reasons for some ED visits: there were patients who presented to the ED with presumed acute SCD pain that actually had acute exacerbations of chronic back pain (8 patients), headaches (5 patients), or abdominal pain due to constipation (12 patients). Each of these is managed differently than acute SCD pain, and the HPMP was not designed for these conditions. In addition, we discovered that a few patients (3 patients) used opiate analgesics for difficulties with sleeping rather than pain, further supporting the need for ongoing patient/family education about pain management in pediatric SCD.
We conclude that the home pain plan intervention served to empower patients with SCD and their families by providing them with the tools to manage uncomplicated pain events at home thereby reduce utilization of the ED. Hence, the home plan intervention has the potential to improve patient outcomes by decreasing avoidable ED visits and reducing overall health care costs. It is hoped that other clinics or hospitals could use QI methods to implement home pain plans that would allow achievement of similar outcomes. Finally, this paper contributes to the limited literature on both QI and home management in pediatric SCD and addresses a critical gap in the literature: a clinical approach to reducing potentially preventable ED visits and subsequent hospitalizations for youth with SCD. It also serves as the basis for future innovative research examining the relationship between a home pain management, health care utilization, and health care costs.
Corresponding author: Kenya Simmons, MBA, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., Cincinnati, OH 45229.
Funding/support: This project was funded in part by HRSA grant #U38MC22218 and NIH grant #K07HL108720-03.
References
1. Armstrong FD. Acute and long-term neurodevelopmental outcomes in children following bone marrow transplantation. Front Biosc 2001;6:G6–G12.
2. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
3. Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: rates and risk factors. N Engl J Med 1991;325:11–16.
4. Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician 2010;56:514–7.
5. Ventafridda V, Stjernsward J. Pain control and the World Health Organization analgesic ladder. JAMA 1996;275:835–6.
6. Rees DC, Olujohungbe AD, Parker NE, et al. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematol 2003:120:744–52.
7. Dampier C, Ely E, Eggleston B, et al. Physical and cognitive behavioral activities used in the home management of sickle pain: A daily diary study in children and adolescents. Ped Blood cancer 2004;43:674–8.
8. Dinges DF, Whitehouse WG, Orne EC, et al. Self-hypnosis training as an adjunctive treatment in the management of pain associated with sickle cell disease. Int J Clin Exp Hypnosis 1997;45:417–32.
9. Thomas VN, Wilson Barnett J, Goodhart F. The role of cognitive behavioural therapy in the management of pain in patients with sickle cell disease. J Adv Nurs 1998;27:1002–9.
10. Gil KM, Anthony KK, Carson JW, et al. Daily coping practice predicts treatment effects in children with sickle cell disease. J Ped Psychol 2001;26:163–73.
11. Dampier C, Ely E, Brodecki D, O’Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Ped hematol oncol 2002;24:643–7.
12. Ferrell BR. Pain management: a moral imperative. Communique (Wash DC) 1996;5:4–5.
13. Finley GA, McGrath PJ, Forward SP, et al. Parents’ management of children’s pain following ‘minor’ surgery. Pain 1996;64:83–7.
14. Forward SP, Brown TL, McGrath PJ. Mothers’ attitudes and behavior toward medicating children’s pain. Pain 1996;67:469–74.
15. Givens M, Rutherford C, Joshi G, Delaney K. Impact of an emergency department pain management protocol on the pattern of visits by patients with sickle cell disease. J Emerg Med 2007;32:239–43.
16. Powers RD. Management protocol for sickle-cell disease patients with acute pain: impact on emergency department and narcotic use. Am J Emerg Med 1986;4:267–8.
17. Silbergleit R, Jancis MOS, McNamara RM. Management of sickle cell pain crisis in the emergency department at teaching hospitals. J Emerg Med 1999:17:625–30.
From the Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
This article is the third in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
ABSTRACT
• Objective: To develop and implement individualized home pain management plans that included pharmacologic as well as nonpharmacologic strategies for children with sickle cell disease (SCD).
• Methods: A multidisciplinary quality improvement team developed a questionnaire to assess the frequency, location, and severity of a patient’s pain during a routine comprehensive visit in order to help the patient and family develop an effective home pain management plan. Using plan-do-study-act cycles, the team was able to build this process into the daily workflow for all SCD patients age 5 years to 21 years of age. Patients with comprehensive visits scheduled from January 2012 to May 2013 were included (n = 188) in the intervention.
• Results: By May of 2013, 88% of eligible patients had an individualized home plan in place. There was a concomitant reduction in the percentage of SCD patients seen in the ED for uncomplicated SCD pain (6.9% vs. 1.1%).
• Conclusions: Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. This intervention has the potential to improve patient outcomes by decreasing avoidable ED visits as well as reducing overall health care costs.
Sickle cell disease (SCD) is one of the most common genetic disorders in the United States, affecting approximately 1 in 500 African-American infants each year [1]. The genetic mutation that causes SCD results in the production of an abnormal hemoglobin molecule (HbS) in the red blood cells (RBC). Under low oxygen conditions, the HbS polymerizes and causes the RBCs to elongate into a sickle form (crescent shape) and decreases the life span of the RBC. Additionally, RBCs with HbS are more “sticky,” adhering to vessel walls and limiting blood flow and oxygen delivery to many tissues and organs in the body. The resultant tissue ischemia causes progressive organ injury as well as episodes of pain (vaso-occlusive crisis).
Recurrent pain episodes are the hallmark of this disease, accounting for the majority of emergency department (ED) visits as well as hospitalizations. High-quality outpatient care can reduce acute care and ED visits as well as hospitalization rates in patients with SCD [2]. Additionally, ensuring that patients have a home pain management plan and understand how to assess and reassess their pain may improve outcomes [3]. Data from our population of children with SCD indicate that 40% to 50% of ED visits in 2011 were for uncomplicated pain episodes (no concomitant medical issues such as fever, increased respiratory rate, wheezing, worsening pallor). If these pain episodes had been effectively managed at home, the ED visits might have been avoided.
In an effort to reduce these potentially preventable ED visits and subsequent hospitalizations, the Comprehensive Sickle Cell Center at Cincinnati Children’s Hospital Medical Center assembled a quality improvement (QI) team to partner with patients and their families to develop individualized home pain management plans (HPMP) that incorporated both pharmacologic and nonpharmacologic pain management strategies. We also sought to identify and remove barriers to the successful use of a home pain management plan, such as not having enough analgesics at home or not allowing enough time for analgesics to work before presenting to the ED. We documented the plan in a standard location and format in the electronic medical record (EMR), making it available to all medical center providers. This paper describes the development, refinement, and testing of an individualized HPMP intervention and related outcomes.
METHODS
Setting
Cincinnati Children’s Hospital Medical Center is a nonprofit pediatric medical center with 587 inpatient beds in Ohio providing acute and chronic care for children in Southern Ohio, Northern Kentucky, and Southeastern Indiana. The center’s Comprehensive Sickle Cell Center provides comprehensive care to approximately 280 children with SCD in the region from birth to 21 years of age. The medical center is the only major pediatric inpatient facility in the tri-state area. Greater than 75% of the SCD patients at our center live within a 15-mile radius, therefore, essentially all ED visits and hospitalizations for our patients occur at our center.
Participants
Improvement Team
The core QI team consisted of multidisciplinary health care providers with experience caring for patients with SCD, including 3 SCD nurse care managers, 2 physicians, 2 PhD psychologists, 4 nurse practitioners, a QI consultant, and a data analyst. Additional support and suggestions were received from other SCD team members (eg, social workers, school interventionists). The core QI multidisciplinary team met weekly to design and test the intervention and implementation process.
Intervention
The intervention consisted of the following elements: (1) pre-visit review to identify eligible patients needing a new or updated home pain management plan; (2) family completion of a pain assessment tool; (3) review of pain assessment tool by SCD team; (4) development of collaborative home plan with family and the medical team; (4) integration of nonpharmacological strategies into the home plan (developed with the psychologist); (5) printed copy of the plan for family to take home; (6) documentation of HPMP in the EMR (Table 2); and, (7) a follow-up phone call for eligible patients with ED or urgent care visits for uncomplicated SCD pain by the nurse care manager.
Implementation
Each week the data analyst generated a list of eligible patients with ICD-9 diagnostic codes for SCD using SQL (structured query language) to extract the data from the EMR (Table 1). The SCD nurse care managers reviewed the list and notified the team of those patients needing a pain assessment and updated HPMP during the daily pre-clinic patient review rounds each morning.
The provider seeing the patient that day facilitated the patient and family’s completion of the pain assessment tool. The pain assessment tool consisted of 13 items and measured recent illnesses or transfusions, patient’s pain location, intensity, associated symptoms, potential triggers, and the impact of the pain on quality of life (missed days of school/work). In addition, the patient’s current pain management strategies, perceived effectiveness of those strategies, and analgesics available at home was recorded.
After discussing the results with the team, a medical provider reviewed the findings with the patient and family and developed a plan for pharmacologic pain management at home utilizing a stepwise approach based on the World Health Organization (WHO) analgesic ladder for selecting pain-relief drugs [4,5] and the American Pain Society guidelines for management of acute and chronic sickle cell pain [6]. The medication’s method of action, side effects, risks, and benefits were reviewed and prescriptions were provided as needed.
During the same visit, patients who reported acute or chronic pain within the last month met with the team psychology provider. The psychology provider educated the patient and family about pain, the mind-body connection, and nonpharmacologic approaches to pain management that could be incorporated in the home plan. Following the education, the psychology provider taught the patient at least one relaxation strategy (eg, diaphragmatic breathing, guided imagery, progressive muscle relaxation) and provided written materials to take home to encourage practice. At the time of discharge from the clinic, patients and families received a copy of the comprehensive home pain plan and any needed prescriptions for analgesics. Families were encouraged to access a copy of their plan at home by logging on to MyChart (Epic Systems), a limited version of the child’s EMR designed for patients and families.
After each ED or urgent care visit for uncomplicated SCD pain, the nurse care manager attempted to call the family within 3 business days to ask whether the home pain management plan had been used and determine if it needed to be revised. Medication refills were confirmed via phone follow-up by the nurse care manager at this time. Laminated pocket guides for the care managers facilitated and standardized the follow-up questions. A maximum of 3 attempts were made to contact the family. Information from the telephone encounter was documented in the patient’s EMR in a standard format and location. This information was then communicated to the SCD provider (nurse practitioner or physician) who modified the plan as needed. If the patient did not have any ED or acute care outpatient visits, the HPMP was reviewed every 6 months at a routinely scheduled comprehensive visit.
The team used multiple plan-do-study-act cycles (PDSAs) to refine the intervention and implementation process. One PDSA involved a focus group consisting of 3 young adult patients and 1 parent. Participants were asked if they knew what we were referring to when we used the term “home pain management plan,” what they remembered about their plan, and if they thought we should keep or change the name. All 4 participants reported that they were familiar with the term and were able to describe aspects of their or their child’s home pain management plan. Although 1 participant suggested shortening the name, the SCD team had worked to develop a high level of familiarity with the name, so it was retained. Another PDSA was conducted to assess whether the pediatric hematology fellows (post-graduate trainees) were aware of the HPMP and how to access it in the EMR. Eight of the 10 fellows responded, and the majority indicated that they were aware of the HPMP; however, only 1 fellow knew where to locate it in the EMR. This resulted in PDSAs to increase fellows’ awareness and use of the HPMP.
The QI team also completed a failure mode and effects analysis (FMEA) to identify potential failures in the clinic flow process. The FMEA helped to identify low-hanging fruit “quick fixes,” PDSAs, and develop process maps. Weekly data guided our PDSAs and allowed us to continuously improve our processes, and team members were accountable for specific weekly action items.
Measurement/Analysis
The home pain management implementation process was monitored and tracked using 2 weekly run charts: one that displayed the percentage of eligible SCD patients who needed a HPMP each week that actually received one and one that showed the overall number of eligible SCD patients with a HPMP (population metric). Run charts provide a graphic display of process performance over time and allowed the team to track and monitor process outcomes. The goal was that at least 85% of eligible patients would receive the HPMP intervention by November 2012.
Outcomes were evaluated using a monthly p-chart showing the percentage of SCD patients seen in the ED for uncomplicated SCD pain. For the current project, a p-chart was used because ED visits were categorized (see below) and the sample size varied by month. We conducted a retrospective chart review of each ED visit to extract the initial complaint and the final assessment from the ED providers’ notes. ED visits were categorized as follows: (1) fever (with or without other symptoms such as pain), (2) uncomplicated SCD pain only, and (3) other (eg, trauma, asthma). The goal was to monitor ED visits for uncomplicated SCD pain only to determine if the rate of this type of ED visit decreased after the implementation of the HPMP. Based on the chart review of the 12 months prior to the implementation of the HPMP, the majority of SCD patients seen in the ED had 0–3 ED visits for uncomplicated SCD pain. Only 7 patients had more than 3 ED visits: two had 4 ED visits, two had 5 ED visits, one had 6 visits, one had 7 visits, and one had 13 visits to the ED. Because the patient with 13 visits has complex psychosocial issues that greatly impact the use of the ED and inpatient medical services, this data was excluded from our analyses.
The Children’s Hospital Medical Center Institutional Review Board exempted this study from review because it was deemed to be a QI project with the intent to improve care locally and not to develop generalizable
knowledge.
RESULTS
DISCUSSION
Using quality improvement methods, an individualized home pain management intervention was incorporated successfully into the daily workflow of a busy outpatient SCD clinic. The QI team provided critical guidance, organization, and resources for refining the HPMP intervention and implementing it into a very busy outpatient clinical setting. QI methods such as the PDSAs, FMEA, and process maps allowed us to continuously improve the intervention and develop an effective implementation process. As a result, we were able to reach our goal of ensuring that 100% of eligible patients received a HPMP during their clinic visit.
Several studies have shown cognitive-behavioral therapies, such as relaxation, imagery, and self-hypnosis, to improve outcomes in children and adults with SCD [7–10]. We believe that having psychology providers on our team who could train families in nonpharmacological strategies was critical to the project’s success. Most SCD patients are taught to increase fluid intake and use warm compresses, but few are trained in adjunctive nonpharmacologic strategies while awaiting the effects of oral analgesics. Thus, our multidisciplinary protocol is innovative; future studies may show it to to be more effective than interventions using pharmacologic or nonpharmacologic strategies alone.
Implementing a comprehensive home pain management intervention in a very busy clinical setting was challenging; it required a substantial coordination and communication among the clinical team. Although each member of the team had a well-defined role, we found that our nurse care managers were the drivers of the process during the clinic visit. They ensured the documentation of the HPMP and reconciliation of medications were completed in the EMR, that prescriptions for analgesics were written and educated families to execute the HPMP.
We were able to exceed our goal of ensuring that at least 85% of eligible patients in our population had a home plan in place. This is clinically significant as most SCD pain episodes occur at home [11]. Typically, the pain management strategies used by patients and families at home are inconsistent, and several studies indicate that parents may be reluctant to use analgesics for their children, use a dose that is too small, or do not give the medicine often enough [12–14]. Developing an home pain plan with a patient and family allowed for education about distinguishing different types of pain and the appropriate use of medications for specific types of pain.
Challenges to implementation of the home plan protocol included limited time during clinics visit to integrate the plan given competing clinical issues. Some families felt the visit lasted too long and were eager to leave the clinic without further delays. Additionally, the fixed design of the EMR posed some limitations related to documentation, medication reconciliation, and updating of the home plan because different team members could not simultaneously access some parts of the EMR. We also initially overlooked the need to educate other providers in our division about the home plan, such as fellows who take calls about patients after hours. This has subsequently been addressed via ongoing PDSAs to test processes for making fellows aware of the home pain plan and to ensure they use it consistently to coordinate care.
Following implementation of the protocol, the percentage of ED visits for SCD uncomplicated pain decreased by 84%. These results build on the previous literature which has focused primarily on standardized pain management protocols in the ED [15–17]. However, it makes a unique contribution in that the focus was on systematically teaching families strategies to use at home with the goal of minimizing the need for ED or urgent care intervention. We also learned more about the reasons for some ED visits: there were patients who presented to the ED with presumed acute SCD pain that actually had acute exacerbations of chronic back pain (8 patients), headaches (5 patients), or abdominal pain due to constipation (12 patients). Each of these is managed differently than acute SCD pain, and the HPMP was not designed for these conditions. In addition, we discovered that a few patients (3 patients) used opiate analgesics for difficulties with sleeping rather than pain, further supporting the need for ongoing patient/family education about pain management in pediatric SCD.
We conclude that the home pain plan intervention served to empower patients with SCD and their families by providing them with the tools to manage uncomplicated pain events at home thereby reduce utilization of the ED. Hence, the home plan intervention has the potential to improve patient outcomes by decreasing avoidable ED visits and reducing overall health care costs. It is hoped that other clinics or hospitals could use QI methods to implement home pain plans that would allow achievement of similar outcomes. Finally, this paper contributes to the limited literature on both QI and home management in pediatric SCD and addresses a critical gap in the literature: a clinical approach to reducing potentially preventable ED visits and subsequent hospitalizations for youth with SCD. It also serves as the basis for future innovative research examining the relationship between a home pain management, health care utilization, and health care costs.
Corresponding author: Kenya Simmons, MBA, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., Cincinnati, OH 45229.
Funding/support: This project was funded in part by HRSA grant #U38MC22218 and NIH grant #K07HL108720-03.
References
1. Armstrong FD. Acute and long-term neurodevelopmental outcomes in children following bone marrow transplantation. Front Biosc 2001;6:G6–G12.
2. Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94.
3. Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: rates and risk factors. N Engl J Med 1991;325:11–16.
4. Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can Fam Physician 2010;56:514–7.
5. Ventafridda V, Stjernsward J. Pain control and the World Health Organization analgesic ladder. JAMA 1996;275:835–6.
6. Rees DC, Olujohungbe AD, Parker NE, et al. Guidelines for the management of the acute painful crisis in sickle cell disease. Br J Haematol 2003:120:744–52.
7. Dampier C, Ely E, Eggleston B, et al. Physical and cognitive behavioral activities used in the home management of sickle pain: A daily diary study in children and adolescents. Ped Blood cancer 2004;43:674–8.
8. Dinges DF, Whitehouse WG, Orne EC, et al. Self-hypnosis training as an adjunctive treatment in the management of pain associated with sickle cell disease. Int J Clin Exp Hypnosis 1997;45:417–32.
9. Thomas VN, Wilson Barnett J, Goodhart F. The role of cognitive behavioural therapy in the management of pain in patients with sickle cell disease. J Adv Nurs 1998;27:1002–9.
10. Gil KM, Anthony KK, Carson JW, et al. Daily coping practice predicts treatment effects in children with sickle cell disease. J Ped Psychol 2001;26:163–73.
11. Dampier C, Ely E, Brodecki D, O’Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Ped hematol oncol 2002;24:643–7.
12. Ferrell BR. Pain management: a moral imperative. Communique (Wash DC) 1996;5:4–5.
13. Finley GA, McGrath PJ, Forward SP, et al. Parents’ management of children’s pain following ‘minor’ surgery. Pain 1996;64:83–7.
14. Forward SP, Brown TL, McGrath PJ. Mothers’ attitudes and behavior toward medicating children’s pain. Pain 1996;67:469–74.
15. Givens M, Rutherford C, Joshi G, Delaney K. Impact of an emergency department pain management protocol on the pattern of visits by patients with sickle cell disease. J Emerg Med 2007;32:239–43.
16. Powers RD. Management protocol for sickle-cell disease patients with acute pain: impact on emergency department and narcotic use. Am J Emerg Med 1986;4:267–8.
17. Silbergleit R, Jancis MOS, McNamara RM. Management of sickle cell pain crisis in the emergency department at teaching hospitals. J Emerg Med 1999:17:625–30.
Reducing Transmission of Methicillin-Resistant <em>Staphylococcus aureus</em> and Vancomycin-Resistant <em>Enterococcus</em> in the ICU—An Update on Prevention and Infection Control Practices
From the Department of Medicine, Infectious Disease Practice and Innovations, The Medical City, Pasig City, Philippines (Dr. Abad), the Division of Emergency Medicine, University of Wisconsin Medical School, Madison, WI (Dr. Pulia), University of Wisconsin Hospital and Clinics, Madison, WI (Ms. Krupp), and the Willam S. Middleton Memorial Veterans Affairs Hospital, Madison, WI (Dr. Safdar).
Patients in intensive care units (ICUs) are at greatly increased risk of developing health care-associated infections (HAIs) [1]. More than 70% of the bacteria that cause HAIs are resistant to at least one of the antimicrobials commonly used to treat these infections [2]. Two such pathogens, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are responsible for a considerable proportion of ICU infections that are associated with increased morbidity, mortality, and costs [3–5]. In this review, we discuss the epidemiology of colonization and infection by MRSA and VRE and provide an update on practices for prevention of transmission and infection by MRSA and VRE in the ICU.
EPIDEMIOLOGY AND MECHANISMS OF RESISTANCE
MRSA is the major cause of HAIs worldwide [6]. Among ICUs in the United States, the proportion of methicillin resistance among S. aureus isolates increased from 35.9% in 1992 to 64.4% in 2003 [4]. Approximately 8% of patients are colonized with MRSA upon admission, and an average of 5% will acquire MRSA colonization in the ICU [7,8]. A comparison study of academic tertiary care facilities found medical ICUs had higher MRSA admission prevalence rates than surgical ICUs, whereas surgical ICUs had a higher incidence rate [7]. Enteroccoccus is the third most common pathogen associated with HAIs, with 33% resistant to vancomycin [9]. VRE infection is associated with increased ICU cost and increased length of stay [10]. Incidence of ICU-acquired VRE varies among regions and countries. For example, in the United States, Warren et al [11] reported a VRE incidence of 27 cases per 1000 patient ICU days, whereas Kohlenberg et al [12] reported a mean incidence of 0.29 cases per 1000 patient ICU days in Germany.
Understanding the mechanisms that allow development of resistant strains of S. aureus and Enterococcus species is important to devise preventive strategies. Methicillin resistance in MRSA is determined by the staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that carries the mecA gene. The mecA gene codes for an additional penicillin-binding protein (PBP) that has a reduced affinity towards methicillin (PBP2a/PBP2'). This results in a reduced ability to bind to the bacterial cell wall and inhibit synthesis [13,14]. Study of molecular epidemiology has identified MRSA as originating from 8 major variants of the mecA gene [15]. The majority of MRSA infections are caused by strains belonging to a few internationally disseminated clones [14]. The first identified strains were associated with infections in hospitalized patients (hospital-associated MRSA), but community-associated MRSA strains have since emerged and have become established globally, including in health care institutions [16].
Community-acquired MRSA can cause severe infections in health hosts [17]; possible explanations include increased CA-MRSA virulence due to the acquisition of mobile genetic elements, namely those containing Panton-Valentine leukocidin (PVL) [18] or increased expression of core genome-encoded virulence genes, such as phenol-soluble modulin (PSM) cytolysins, α-toxin, and other virulence determinants [19].
Enterococcus is intrinsically resistant to several antimicrobial drugs, with resistance to vancomycin encoded by several clusters of genes known as vancomycin resistance gene clusters (eg, vanA, vanB). The gene clusters generate resistance through multiple pathways which encode enzymes to determine the structure of peptidoglycan precursors [20,21]. Genetically diverse, hospital-associated VRE outbreaks have been associated with single clones, multiple clones, and changing molecular epidemiology over time [21]. While up to 25% of the VRE genome includes acquired elements, the majority of hospital-associated infections are traced to a few clonal complexes, which differ from community-associated VRE strains [22].
The evolution of these efficient mechanisms that promote drug resistance has made it extremely challenging to eradicate organisms such as MRSA and VRE. However, advances in recent years have furthered our understanding of the epidemiology, pathogenesis, and methods of prevention and containment.
RISK FACTORS FOR COLONIZATION AND INFECTION
MRSA
The risk factors underlying MRSA colonization and infection in the ICU setting can be categorized as either patient/host or environmental factors. A wide range of patient-level factors is associated with MRSA colonization upon admission. General principles regarding the transmission of MRSA in the community include close contact with colonized or infected individuals, breaks in the skin, crowded living conditions and poor hygiene. These factors, alone or in combination, are thought to underlie observed outbreaks among sports teams, military personnel, correction facilities, American Indian communities, and daycare centers [23–34].
Two recently published systematic reviews have summarized important patient-level factors associated with MRSA colonization at the time of hospital admission. Forster et al [35] examined 27 studies and identified previous admission to hospital, transfer from nursing home or long-term care facility, and previous antibiotic use as the top 3 factors associated with MRSA colonization. A similar review conducted by McKinnell and colleagues [36] found that prior hospitalization, nursing home contact, recent antibiotic use, and exposure to health care-associated pathogens (MRSA carriage, VRE carriage, or Clostridium difficile infection) were found to portend the highest risk. Specific comorbid conditions also conveyed an increased risk, including congestive heart failure, chronic wounds/bedsores, diabetes mellitus, pulmonary disease, immunosuppression, urinary catheter, and renal failure/dialysis. It is clear that health care contacts, especially recent hospitalization, residence in a long-term care facility, and antibiotic use, are significant risk factors for MRSA colonization [37–39].
 In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
Regardless of whether MRSA colonization precedes admission or occurs due to nosocomial spread, it is associated with increased risk of developing a HAI [46–49]. In 2 large prospective observational cohort studies, the hazard ratios of MRSA colonization developing into S. aureus infections during the ICU stay were 3.84 and 4.70, respectively [50,51]. High levels of concordance between MRSA colonization strains and HAI strains have also been reported [52]. Nasal colonization with S. aureus has also been identified as an independent risk factor for developing ventilator-associated pneumonia (VAP) and bacteremia [53,54]. A case series of ICU patients with S. aureus nasal colonization who developed lower respiratory tract infections demonstrated genetically identical nasal and bronchial strains in 15/16 cases [55]. This finding strongly suggests that nasopharyngeal colonization with S. aureus contaminates oral secretions that are aspirated by critically ill patients, resulting in subsequent pneumonia. In a long-term outcomes study among a matched cohort of veterans, MRSA colonization was associated with an increased risk of infection-related readmission and mortality [56]. These findings reflect the critically important nature of measures designed to curb nosocomial transmission and acquisition of MRSA, especially among the vulnerable ICU population.
VRE
As with MRSA, risk factors associated with VRE colonization include both patient-level and ICU-level (or environmental) factors [57]. Examples of patient-level factors include previous antimicrobial exposure [58–62], underlying medical illnesses such as chronic renal failure requiring hemodialysis [11,63], length of hospital or ICU stay [11,59,64,65], and recent exposure to health care facilities. ICU-level factors of relevance are the prevalence of VRE in the unit, with high levels of endemicity leading to higher risk of colonization and transmission.
Antibiotic use is a major risk factor for VRE acquisition, although the type and class of antibiotic varies considerably across studies; the most frequently identified antibiotics are broad-spectrum cephalosporins, vancomycin, and anti-anaerobic agents [58,62,64]. Patients with chronic liver disease and post-transplantation are at exceedingly high risk for VRE acquisition [59]. In a recent study by Pan [66], for example, the authors found that the incidence of newly acquired VRE was 21.9 per 1000 patient-days in an ICU setting. On multivariate analysis, the authors found that, similar to other reports [11,59,67], length of stay in the ICU was associated with increased risk of VRE acquisition, with each additional day of stay increasing risk of VRE by 1.03 times. Warren et al undertook a prospective cohort study involving 519 patients admitted to the ICU for more than 48 hours [11]. Seventy-four (21%) of 352 patients were subsequently colonized with VRE. The median time to development of a positive VRE culture after ICU admission was 6 days. Increased mean APACHE II score on ICU admission (P = 0.002), sucralfate use (P = 0.003), vasopressor use (P = 0.01), tracheostomy in the ICU (P = 0.02), and C. difficile diarrhea (P = 0.002) appeared to be associated with VRE acquisition.
 It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
REDUCING TRANSMISSION—MRSA AND VRE PREVENTION STRATEGIES
 Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Antimicrobial Stewardship
Antibiotic use is a major driver of antibiotic resistance. A meta-analysis by de Bruin and Riley [71] studied the effect of vancomycin usage on VRE colonization and infection. A total of 12 articles describing 13 studies were included; none were randomized controlled trials. All studies were quasi-experimental and lacked control groups. Among all studies, less than half (46%) implemented vancomycin reduction measures as the sole type of intervention [72–76]. The remaining studies implemented other infection control modalities and or restricted the use of other antimicrobials [77–83]. Although all studies that implemented vancomycin restriction alone as a single strategy showed a decline in vancomycin usage, only 2 of these [74,75] showed a relative risk reduction in VRE acquisition post-intervention. Also, studies that restricted vancomycin alone revealed a trend towards lower efficacy in reducing VRE colonization and infection (33%) when compared with those that used additional measures (71%). While judicious antibiotic use should always be practiced, the evidence for vancomycin restriction as a sole intervention to control VRE is scant. It may be that other antibiotics are as big or bigger drivers of resistance in enterococci than vancomycin. For example, a growing body of literature supports antibiotic restriction, especially fluoroquinolones, for reducing MRSA. In several time-series quasi-experimental studies, restriction of fluorquinolones was associated with reduced trends in MRSA infections in the acute care setting, and consideration should be given to monitor and optimize fluoroquinolone use in the ICU setting [84,85].
Antimicrobial stewardship programs are fundamental to optimizing antibiotic use in the ICU and the authors strongly recommend that all ICUs should have such a program in place.
Educational Interventions
Infection control and multidrug-resistant organism (MDRO)–specific education programs for health care workers is a core principle of the CDC’s prevention guidelines. The HICPAC VRE guideline also explicitly states “continuing education programs for hospital staff (including attending and consulting physicians, medical residents, and students; pharmacy, nursing, and laboratory personnel; and other direct patient-care providers) should include information concerning the epidemiology of VRE and the potential impact of this pathogen on the cost and outcome of patient care [70].” A systematic review published in 2008 [86] that included 26 studies showed that such interventions to prevent HCAIs are usually successful; in this review, 20 of 26 studies showed a statistically significant decrease in infection rates, with risk ratios ranging from 0 to 1.6. Education was usually part of a broader array of infection control interventions. While clearly essential, education alone is unlikely to have a sustained impact on reducing MRSA and VRE infections.
Infection Control Measures
Major infection control interventions include hand hygiene, the use of personal protective equipment (PPE), and cohorting. These measures can be grouped into “horizontal” (or global) vs. “vertical” (or targeted) strategies. Although not mutually exclusive, horizontal approaches are designed to have an impact on multiple pathogens (pathogen nonspecific), whereas vertical approaches are designed to reduce the impact of specific pathogens (such as VRE). For the purposes of this review, we will discuss both strategies for containment of MRSA and VRE. Horizontal strategies include hand hygiene, universal gloving and/or gowning, environmental cleaning, and daily bathing with chlorhexidine. Vertical strategies include screening for either MRSA or VRE followed by placement in contact precautions and decolonization with mupirocin.
Hand Hygiene
Hand washing is fundamental to reducing transmission of MDROs in health care institutions; however, optimal compliance is hard to achieve and sustain. Barriers to adherence may include unavailability of sinks or hand hygiene materials (eg, alcohol-based gels, gloves) time constraints, forgetfulness, or lack of knowledge [87–95]. Several monitoring strategies have been evaluated to increase compliance with hand hygiene. Most involve direct observation followed by performance assessment and feedback.
Trials examining the impact of improvements in hand hygiene compliance on HAIs in the ICU setting have largely found benefit, although not all studies showed a decline in HAI. In a prospective crossover trial, Rupp et al [96] found dramatic improvements in compliance with hand gel availability, but this did not translate to decreased nosocomial MRSA infections. Venkatesh et al [97] carried out a before-and-after interventional prospective study in a hematology unit in a tertiary level hospital to evaluate the use of an electronic method of surveillance to determine compliance with hand hygiene. The authors also used rates of horizontal transmission of VRE as a secondary end-point. Results of the study showed that hand hygiene compliance improved from 36.3% at baseline to 70.1%. This represented an OR of 4.1 (95% confidence interval, 3.7–4.5), which the authors attributed to the use of automated alerts. VRE transmission rates before and during intervention were not statistically different, but the rates of infection were lower at 1.0 per month in comparison with 4.7 infections per month in the preceding 6 months (P = 0.096).
While improved hand hygiene may result in significant reductions in HAIs [40], research indicates hand hygiene alone influences about 40% of infections in the ICU setting [98]. As such, hand hygiene should be viewed as a necessary component of a comprehensive infection control program [99]. Despite the success of hand hygiene in reducing HAIs in the ICU, effective strategies to improve compliance remain elusive even under study conditions and further research is needed in this area [100].
Personal Protective Equipment
Tenorio et al [101] conducted a study to assess the effectiveness of gloving in the prevention of hand carriage of VRE by health care workers. The study showed that among 50 health care workers who had contact with patients colonized with VRE, 6 carried a similar patient strain even prior to known contact, and 17 of 44 (69%) had a patient-related VRE strain on their gloves after contact. This suggests a relatively high rate of colonization after usual patient-care contact. Factors associated with acquisition of VRE on gloves included duration of contact, contact with a patient’s body fluids, presence of diarrhea in a patient, mean VRE colony counts on a patient’s skin, and number of body sites colonized with VRE. Although gloves reduced the risk of VRE acquisition of VRE by 71% (ie, 12/17 did not have VRE on their hands after de-gloving) the protection afforded by gloves was incomplete. As such, hand hygiene after glove removal is recommended.
Slaughter et al [102] compared the use of personal protective equipment in the acquisition of VRE. During this study, 93 patients in glove-and-gown rooms and 88 patients in glove-only rooms had similar rates of VRE at baseline entry into the ICU and after the intervention. Mean times to colonization among the patients who became colonized were 8.0 days in the glove-and-gown group and 7.1 days in the glove-only group. None of these comparisons were statistically significant and the authors concluded that the universal use of gown and gloves was no better than the use of gloves alone in preventing VRE colonization.
A recent cluster randomized trial compared the effect of universal PPE (ie, gowning and gloving) with usual care for reducing acquisition of MRSA or VRE as a composite outcome [103]. The study did not find that universal gowning and gloving reduced VRE or MRSA acquisition but found a 40% decline in MRSA acquisition in the intervention ICUs compared with baseline rates of MRSA. No major adverse effects of universal gowning and gloving were noted in this study. A thoughtful editorial commenting on this article proposes that several aspects of the study deserve consideration, including the possibility of false-negative screening tests for VRE, which may have partially accounted for the negative primary outcome [69].
Based on these studies, it appears that the use of barrier precautions may be of value more for MRSA than VRE but further studies are needed to examine its impact on other types of pathogens, including new and emerging MDROs. Until further evidence becomes available, routine gowning and gloving may be of value in units with a high prevalence of MRSA.
Environmental Cleaning
Accumulating data suggests that the environment may play a major role in transmission of pathogens. MRSA has the ability to survive for days to weeks on inanimate objects [104–107]. Environmental contamination results in contamination of staff clothing and gloves [107,108] and is highly correlated with colonization strains among inpatients [109]. Although some studies of enhanced cleaning techniques and increased environmental services staff time have demonstrated reductions in MRSA outbreaks [110–112], the results are not universally favorable [113,114] and further studies are needed to examine the impact of environmental cleaning on rates of MRSA colonization or infection.
Several studies have implicated contaminated equipment as vectors for transmission of VRE during outbreaks [115–117], but the direction of fomite transfer from patient to environment has been difficult to ascertain. VRE have been found frequently on a variety of inanimate objects and surfaces in different health care environments [118–123], including gloved or ungloved hands of health care workers [101,124,125]. Hayden et al [126] determined the effect of improved environmental cleaning on VRE acquisition rates. This study was a pre-and-post intervention study carried out in a 21-bed medical intensive care unit (MICU) in a tertiary hospital over several phases. The intervention included the creation of a unique and improved cleaning program, as well as in-services to housekeeper services, education of the MICU staff, and a hand hygiene campaign. The results of the study showed decreased acquisition of VRE from 33.47 cases per 1000 patient days at risk in period 1 to 10.40 cases per 1000 patient-days at risk by period 4 of the study. Increased environmental cleaning was also associated with reduced growth of VRE from environmental cultures. At baseline, weekly contamination rates were 0.15 and 0.1 for samples obtained before and after cleaning, respectively. Culture positivity decreased to 0.07 and 0.04 for before and after cleaning in period 2 and then remained at low levels during the remainder of the study. It is important to note that the method for disinfecting used in this study was the “bucket method” as promoted by Byers [127]. This study provides further support for the importance of an environmental reservoir and of environmental decontamination to prevent endemic cross-transmission of VRE [126].
Goodman et al [128] used similar interventions but added a feedback tool using a black-light monitoring system (ie, use of an invisible, nontoxic marker to delineate areas that are adequately or inadequately cleaned) to reduce the likelihood of isolating either MRSA or VRE from an ICU environment. This study also showed favorable results, and notably, the use of the black-light monitoring system identified specific areas that were typically inadequately disinfected. Results showed that flat, horizontal surfaces (eg, countertops, bedside tray tables, and hamper tops) were adequately cleaned more often than small, vertical surfaces (eg, doorknobs, toilet handles, light switches, and electronics).
Part of the controversy surrounding the impact of environmental cleaning is the difficulty in determining its individual value as part of an overall infection control bundle [129]. A proposed area of demonstrable impact for environmental cleaning are frequently touched sites which are more likely to be contaminated with pathogens. Focusing on these “hot-bed” areas of the care environment may offer a useful adjunct to other infection control measures [129].
Active Surveillance
Active surveillance refers to periodic screening for asymp-tomatic carriers followed by placement of colonized patients in contact isolation. This practice is highly variable across institutions, as the evidence supporting this practice is conflicting and there are concerns about the cost of implementing this approach without solid evidence [70,130,131]. Despite lack of randomized controlled trials to guide this practice for MRSA prevention, many hospitals utilize MRSA surveillance and it is mandated by law in 9 states [132,133].
A prospective, interventional cohort study of universal MRSA screening on admission to surgical wards failed to reduce nosocomial MRSA infections [134]. Most recently, a pragmatic, cluster-randomized ICU trial reported that universal decolonization with chlorhexidine wipes and mupirocin use was more effective than screening and isolation in reducing rates of MRSA clinical isolates [65]. However, concerns regarding the risk of mupirocin resistance have been expressed [135,136]. The only randomized trial that compared active surveillance for MRSA and VRE followed by contact precautions to usual care did not find a benefit to active surveillance.
Huskins et al [137], in a large, cluster-randomized trial of 19 ICUs from different hospitals, determined the utility of using a culture-based active surveillance and contact isolation, compared with usual care (contact isolation for patients colonized with MRSA or VRE) as identified by existing hospital protocols, to reduce the incidence of colonization or infection with MRSA or VRE. In this trial, which spanned 6 months and involved 3488 participants, the authors found no significant difference between the intervention and control ICUs in terms of MRSA and VRE colonization or infection rates.
Conflicting with these findings is an observational study comparing MRSA infection rates before and after institution of a universal screening protocol, which demonstrated a 69.6% (CI, –89.2% to –19.6%]; P = 0.03) reduction in hospital wide MRSA prevalence density with screening [138]. The “MRSA bundle” implemented in 2007 at VA hospitals nationwide, which included universal screening, produced a 62% (P < 0.001) reduction in MRSA ICU infections; the relative contribution of the various bundle components is uncertain [139,140].
A proposed cost-saving alternative to universal screening is selective screening based on risk factor assessment [141]. The effectiveness of this type of program depends on creating a clinical decision-making tool capable of accurately identifying high-risk individuals while also accounting for the different risk factor profiles between HA-MRSA and CA-MRSA [142]. It has been proposed that targeted screening protocols may be more cost-effective in settings with < 5% prevalence of MRSA colonization on admission [143].
Many studies [61,144–149] have shown that active surveillance against VRE is cost-effective. For example, Calfee et al [144] showed that an established active surveillance program results in control of endemic VRE in high-risk patients. The infection control program was established in response to a hospital-wide VRE outbreak, and was sustained after the outbreak was controlled. The study by Calfee et al spanned 5 years and was performed at a tertiary-level university hospital, where cultures from perirectal areas were used to identify high-risk patients who were asymptomatically colonized with VRE. During the latter 2 years, 768 new cases of VRE colonization were detected among 69,672 admissions (1.1% of admissions), of which 730 (95.1%) were identified by active surveillance methods. This implies that routine clinical cultures would probably have missed the majority of colonized patients. During this period, the incidence of VRE infection was likewise extremely low at 0.12/1000 patient days (ie, 90 nosocomial VRE infections were identified in 83 patients during 743,956 days of patient care). Sixty-nine of the 83 patients (83%) who developed nosocomial VRE infections were found to be colonized with VRE by surveillance culture before the onset of infection.
Patient Decolonization
Chlorhexidine gluconate has been used in several settings to control outbreaks and infections related to MRSA and VRE due to its broad-spectrum activity against these pathogens. Chlorhexidine-based solutions reduce the density of skin colonization with pathogens such as MRSA and VRE (skin asepsis), thus lowering the risk for horizontal transmission between health care workers and patients.
Decolonization with chlorhexidine as an MRSA infection reduction technique has demonstrated benefit in the ICU setting [150]. The previously mentioned large, cluster-randomized ICU trial by Huang and colleagues found universal decolonization with twice-daily intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the entire ICU stay was superior to targeted decolonization of known MRSA carriers in preventing overall MRSA isolates. However, universal decolonization failed to show a reduction in MRSA bacteremia [151], and concerns about mupirocin resistance may limit the applicability of this approach.
There are now several studies [152–154] that show decreased acquisition of VRE with use of daily chlorhexidine bathing. In a study including 1787 ICU patients, Vernon et al found [154] that the reducing microbial density of VRE on patient’s skin by using chlorhexidine led to decreased transmission. In another study by Climo et al [153] that involved 6 ICUs at 4 academic centers and measured the incidence of MRSA and VRE colonization and blood stream infections (BSI) during a period of bathing with routine soap for 6 months compared with a 6-month period where all admitted patients received daily bathing with a chlorhexidine solution, results found decreased acquisition of VRE by 50% (4.35 vs. 2.19 cases/1000 patient days, P < 0.008) following the introduction of daily chlorhexidine bathing. Furthermore, compared with 16 of 270 patients colonized with VRE who subsequently developed VRE bacteremia at baseline, only 4 of 226 VRE-colonized patients bathed with chlorhexidine in the intervention period developed a BSI, translating into a relative risk reduction of 3.35 (95% CI, 1.13–9.87; P < 0.035). Patients colonized with VRE were 3 times less likely to develop VRE bacteremia when bathed with chlorhexidine compared with regular bathing. Despite the success of this protocol for VRE, when analyzed by individual organism no significant reductions in MRSA acquisition or BSI were reported. This finding is similarly corroborated by a trial conducted in the pediatric ICU setting which found an overall reduction in bacteremia with daily chlorhexidine washes but no significant decrease in cases due to S. aureus [155].
The results of these studies suggest that daily bathing with chlorhexidine should be part of routine practice in health care, especially in ICUs where endemic MRSA or VRE rates are high. Whether there is benefit in other settings needs to be studied.
In addition to chlorhexidine washes, other decolonization techniques have been proposed to reduce colonization and the spread of HAIs in the ICU setting. A randomized controlled trial of daily 5% tea tree oil body washes for the prevention of MRSA colonization failed to significantly reduce rates compared to standard soap body washes [156]. Another proposed decolonization intervention that has not been widely adopted in the United States due to concerns related to development of resistant organisms is selective digestive decontamination (SDD) or selective oropharyngeal decontamination (SOD) with antimicrobial agents [157,158]. In terms of clinical benefit, SDD/SOD have been found to decrease MDRO infection rate [159] and mortality [160].
Cohorting
There is insufficient evidence to conclude that cohorting isolated patients is of benefit for routine use in the endemic ICU setting. A few studies, mainly in the outbreak setting, have examined this approach and the results are conflicting [161,162]. Pending further studies in this area, it is reasonable to cohort patients colonized with the same microorganisms, especially if patients cannot be placed in single rooms.
CONCLUSION
The emergence of MRSA and VRE has led to a resurgence of interest and emphasis on infection control practices and prevention. CDC guidelines to help health care practitioners manage these MDROs in the hospital and ICU-setting exist; however, many questions remain regarding best practice.
Prevention of MRSA and VRE needs to be a 2-pronged approach—antimicrobial stewardship [163] and infection control. A robust antimicrobial stewardship program to optimize and minimize inappropriate antibiotic use is necessary in every institution. From the infection prevention standpoint, it is unclear if systematic identification of MRSA and VRE colonization followed by contact precautions is useful in reducing transmission. It is clear that a strong institutional climate of promoting patient safety and a culture of infection prevention will help in reducing MRSA and VRE facility-wide. It also appears that universal gowning and gloving may be useful for reducing MRSA, but not VRE, transmission. While universal decolonization with mupirocin is efficacious in reducing MRSA, this strategy is not recommended because of promoting mupirocin resistance. However, the use of daily bathing with chlorhexidine represents a relatively low-cost, high-yield intervention that should be adopted. Pending further data, patients known to be colonized or infected with MRSA should be placed in contact precuations as is current practice in most institutions. Finally, in this era of MDROs, hand hygiene remains our best defense against the spread of pathogens in the health care environment.
Note: This article does not represent the views of the Department of Veterans Affairs.
Corresponding author: Nasia Safdar, MD, Willam S. Middleton Memorial Veterans Affairs Hospital, 2500 Overlook Terrace, Madison, WI 53705, [email protected].
Funding/support: This work is funded by a MERIT award from the Department of Veterans Affairs to Nasia Safdar.
Financial disclosures: None.
REFERENCES
1. Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 2009;301:727–36.
2. LeDell K, Muto CA, Jarvis WR, Farr BM. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 2003;24:639–41.
3. Giske CG, Monnet DL, Cars O, Carmeli Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 2008;52:813–21.
4. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Pub Health Rep 2007;122:160–6.
5. Schwaber MJ, Carmeli Y. The effect of antimicrobial resistance on patient outcomes: importance of proper evaluation of appropriate therapy. Crit Care 2009;13:106.
6. Grundmann H, Hori S, Winter B, et al. Risk factors for the transmission of methicillin-resistant Staphylococcus aureus in an adult intensive care unit: fitting a model to the data. J Inf Dis 2002;185:481–8.
7. Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis 2007;195:330–8.
8. Muder RR, Cunningham C, McCray E, et al. Implementation of an industrial systems-engineering approach to reduce the incidence of methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2008;29:702–8.
9. Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011.
10. Pelz RK, Lipsett PA, Swoboda SM, et al. Vancomycin-sensitive and vancomycin-resistant enterococcal infections in the ICU: attributable costs and outcomes. Intensive Care Med 2002;28:692–7.
11. Warren DK, Kollef MH, Seiler SM, et al. The epidemiology of vancomycin-resistant Enterococcus colonization in a medical intensive care unit. Infect Control Hosp Epidemiol 2003;24:257–63.
12. Kohlenberg A, Schwab F, Meyer E, et al. Regional trends in multidrug-resistant infections in German intensive care units: a real-time model for epidemiological monitoring and analysis. J Hosp Infect 2009;73:239–45.
13. Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol 2008;8:747–63.
14. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008;46 Suppl 5:S350–9.
15. Zhang K, McClure JA, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009;53:531–40.
16. Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol 2013;5:205–17.
17. Otto M. MRSA virulence and spread. Cell Microbiol 2012;14:1513–21.
18. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978–84.
19. Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 2009;106:5883–8.
20. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 2006;42 Suppl 1:S25–34.
21. Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 2001;33:210–9.
22. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–78.
23. Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis 1998;178:577–80.
24. Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 2004;113:e347–52.
25. Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 2001;286:1201–5.
26. Hewlett AL, Falk PS, Hughes KS, Mayhall CG. Epidemiology of methicillin-resistant Staphylococcus aureus in a university medical center day care facility. Infect Control Hosp Epidemiol 2009;30:985–92.
27. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468–75.
28. Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA 2012;308:50–9.
29. Lindenmayer JM, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med 1998;158:895–9.
30. Malcolm B. The rise of methicillin-resistant staphylococcus aureus in U.S. correctional populations. J Correct Health Care 2011;17:254–65.
31. Nerby JM, Gorwitz R, Lesher L, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2011;30:927–32.
32. Stemper ME, Shukla SK, Reed KD. Emergence and spread of community-associated methicillin-resistant Staphylococcus aureus in rural Wisconsin, 1989 to 1999. J Clin Microbiol 2004;42:5673–80.
33. Turabelidze G, Lin M, Wolkoff B, et al. Personal hygiene and methicillin-resistant Staphylococcus aureus infection. Emerg Infect Dis 2006;12:422–7.
34. Ellis MW, Hospenthal DR, Dooley DP, et al. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004;39:971–9.
35. Forster AJ, Oake N, Roth V, et al. Patient-level factors associated with methicillin-resistant Staphylococcus aureus carriage at hospital admission: a systematic review. Am J Infect Control 2013;41:214–20.
36. McKinnell JA, Miller LG, Eells SJ, et al. A systematic literature review and meta-analysis of factors associated with methicillin-resistant staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Contol Hosp Epidemiol 2013;34:1077–86.
37. Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med 2006;166:580–5.
38. Jernigan JA, Pullen AL, Flowers L, et al. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol 2003;24:409–14.
39. Horner C, Parnell P, Hall D, Kearns A, Heritage J, Wilcox M. Meticillin-resistant Staphylococcus aureus in elderly residents of care homes: colonization rates and molecular epidemiology. J Hosp Infect 2013;83:212–8.
40. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009;73:305–15.
41. Boyce JM. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect Dis Clin North Am 1989;3:901–13.
42. Jernigan JA. Methicillin-resistant Staphylococcus aureus colonization among health care personnel in the emergency department: what does it tell us? Ann Emerg Med 2008;52:534–6.
43. Carnicer-Pont D, Bailey KA, Mason BW, Walker AM, Evans MR, Salmon RL. Risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a case-control study. Epidemiol Infect 2006;134:1167–73.
44. Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 2002;49:999–1005.
45. Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med 1982;97:309–17.
46. Davis KA, Stewart JJ, Crouch HK,et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004;39:776–82.
47. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997;10:505–20.
48. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med 2008;121:310–5.
49. Wertheim HFL, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
50. Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 2001;22:687–92.
51. Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol;31:584–91.
52. von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001;344:11–6.
53. Pujol M, Pea C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med 1996;100:509–16.
54. Rocha LA, Marques Ribas R, da Costa Darini AL, Gontijo Filho PP. Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am J Infect Control 2013;41:236–40.
55. Corne P, Marchandin Hln, Jonquet O, Campos J, Bauls A-L. Molecular evidence that nasal carriage of Staphylococcus aureus plays a role in respiratory tract infections of critically ill patients. J Clin Microbiol 2005;43:3491–3.
56. Quezada Joaquin NM, Diekema DJ, Perencevich EN, et al. Long-term risk for readmission, methicillin-resistant Staphylococcus aureus (MRSA) infection, and death among MRSA-colonized veterans. Antimicrob Agents Chemother 2013;57:1169–72.
57. Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: recognition and prevention in intensive care units. Crit Care Med 2010;38:S335–44.
58. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002;8:802–7.
59. Ostrowsky BE, Venkataraman L, D’Agata EM, et al. Vancomycin-resistant enterococci in intensive care units: high frequency of stool carriage during a non-outbreak period. Arch Intern Med 1999;159:1467–72.
60. Bonten MJ, Hayden MK, Nathan C, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet 1996;348:1615–9.
61. Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med 2001;344:1427–33.
62. Padiglione AA, Wolfe R, Grabsch EA, et al. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother 2003;47:2492–8.
63. Batistao DW, Gontijo-Filho PP, Conceicao N, et al. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz 2012;107:57–63.
64. Furtado GH, Martins ST, Coutinho AP, et al. Prevalence and factors associated with rectal vancomycin-resistant enterococci colonization in two intensive care units in Sao Paulo, Brazil. Braz J Infect Dis 2005;9:64–9.
65. Huang SS, Datta R, Rifas-Shiman S, et al. Colonization with antibiotic-susceptible strains protects against methicillin-resistant Staphylococcus aureus but not vancomycin-resistant enterococci acquisition: a nested case-control study. Crit Care 2011;15:R210.
66. Pan SC, Wang JT, Chen YC, et al. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One 2012;7:e47297.
67. Se YB, Chun HJ, Yi HJ, et al. Incidence and risk factors of infection caused by vancomycin-resistant enterococcus colonization in neurosurgical intensive care unit patients. J Korean Neurosurg Soc 2009;46:123–9.
68. Healthcare Infection Control Practices Advisory Committee (HICPAC). Management of multidrug-resistant organisms in healthcare settings, 2006. Accessed 11 Oct 2013 at www.cdc.gov/hicpac/mdro/mdro_toc.html.
69. Malani PN. Preventing infections in the ICU: one size does not fit all. JAMA 2013;310:1567–8.
70. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 1995;44:1–13.
71. de Bruin MA, Riley LW. Does vancomycin prescribing intervention affect vancomycin-resistant enterococcus infection and colonization in hospitals? A systematic review. BMC Infect Dis 2007;7:24.
72. Adachi W, Bolding F, Armstrong R. Experience with vancomycin education and order sheet to limit vancomycin use. Hosp Pharm 1997:1370–3.
73. Fridkin SK, Lawton R, Edwards JR, et al. Monitoring antimicrobial use and resistance: comparison with a national benchmark on reducing vancomycin use and vancomycin-resistant enterococci. Emerg Infect Dis 2002;8:702–7.
74. Guglielmo BJ, Dudas V, Maewal I, et al. Impact of a series of interventions in vancomycin prescribing on use and prevalence of vancomycin-resistant enterococci. Jt Comm J Qual Patient Saf 2005;31:469–75.
75. Lautenbach E, LaRosa LA, Marr AM, et al. Changes in the prevalence of vancomycin-resistant enterococci in response to antimicrobial formulary interventions: impact of progressive restrictions on use of vancomycin and third-generation cephalosporins. Clin Infect Dis 2003;36:440–6.
76. Morgan AS, Brennan PJ, Fishman NO. Impact of a vancomycin restriction policy on use and cost of vancomycin and incidence of vancomycin-resistant Enterococcus. Ann Pharmacother 1997;31:970–3.
77. Anglim AM, Klym B, Byers KE, et al. Effect of a vancomycin restriction policy on ordering practices during an outbreak of vancomycin-resistant Enterococcus faecium. Arch Intern Med 1997;157:1132–6.
78. Montecalvo MA, Jarvis WR, Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med 1999;131:269–72.
79. Morris JG Jr, Shay DK, Hebden JN, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med 1995;123:250–9.
80. Quale J, Landman D, Saurina G, et al. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis 1996;23:1020-5.
81. Rubin LG, Tucci V, Cercenado E, et al. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol 1992;13:700–5.
82. Lai KK, Kelley AL, Melvin ZS, et al. Failure to eradicate vancomycin-resistant enterococci in a university hospital and the cost of barrier precautions. Infect Control Hosp Epidemiol 1998;19:647–52.
83. Shaikh ZH, Osting CA, Hanna HA, et al. Effectiveness of a multifaceted infection control policy in reducing vancomycin usage and vancomycin-resistant enterococci at a tertiary care cancer centre. J Hosp Infect 2002;51:52–8.
84. Lafaurie M, Porcher R, Donay JL, et al. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother 2012;67:1010–5.
85. Parienti JJ, Cattoir V, Thibon P, et al. Hospital-wide modification of fluoroquinolone policy and meticillin-resistant Staphylococcus aureus rates: a 10-year interrupted time-series analysis. J Hosp Infect 2011;78:118–22.
86. Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: a systematic review. Crit Care Med 2008;36:933–40.
87. Boyce JM. It is time for action: improving hand hygiene in hospitals. Ann Intern Med 1999;130:153–5.
88. Jackson M, Chiarello LA, Gaynes RP, Gerberding JL. Nurse staffing and healthcare-associated infections: proceedings from a working group meeting. J Nurs Adm 2002;32:314–22.
89. Kuzu N, Ozer F, Aydemir S, et al. Compliance with hand hygiene and glove use in a university-affiliated hospital. Infect Control Hosp Epidemiol 2005;26:312–5.
90. Larson E, Killien M. Factors influencing handwashing behavior of patient care personnel. Am J Infect Control 1982;10:93–9.
91. Larson E, Kretzer EK. Compliance with handwashing and barrier precautions. J Hosp Infect 1995;30 Suppl:88–106.
92. Naikoba S, Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers - a systematic review. J Hosp Infect 2001;47:173–80.
93. Pittet D, Simon A, Hugonnet S, et al. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med 2004;141:1–8.
94. Trick WE, Vernon MO, Welbel SF, et al. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infect Control Hosp Epidemiol 2007;28:42–9.
95. Wisniewski MF, Kim S, Trick WE, et al. Effect of education on hand hygiene beliefs and practices: a 5-year program. Infect Control Hosp Epidemiol 2007;28:88–91.
96. Rupp ME, Fitzgerald T, Puumala S, et al. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol 2008;29:8–15.
97. Venkatesh AK, Lankford MG, Rooney DM, et al. Use of electronic alerts to enhance hand hygiene compliance and decrease transmission of vancomycin-resistant Enterococcus in a hematology unit. Am J Infect Control 2008;36:199–205.
98. Silvestri L, Petros AJ, Sarginson RE, et al. Handwashing in the intensive care unit: a big measure with modest effects. J Hosp Infect 2005;59:172–9.
99. Akyol A, Ulusoy H, Ozen I. Handwashing: a simple, economical and effective method for preventing nosocomial infections in intensive care units. J Hosp Infect 2006;62:395–405.
100. Simmons B, Bryant J, Neiman K, et al. The role of handwashing in prevention of endemic intensive care unit infections. Infect Control Hosp Epidemiol 1990;11:589–94.
101. Tenorio AR, Badri SM, Sahgal NB, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis 2001;32:826–9.
102. Slaughter S, Hayden MK, Nathan C, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med 1996;125:448–56.
103. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013;310:1571–80.
104. Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect 2001;49:255–61.
105. Hardy KJ, Oppenheim BA, Gossain S, et al. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect Control Hosp Epidemiol 2006;27:127–32.
106. Jawad A, Heritage J, Snelling AM, et al. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol 1996;34:2881–7.
107. Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol 2007;28:1142–7.
108. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
109. Sexton T, Clarke P, O’Neill E, et al. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect 2006;62:187–94.
110. Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet infect dis 2008;8:101–13.
111. Dancer SJ, White LF, Lamb J, et al. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC med 2009;7.
112. Rampling A, Wiseman S, Davis L, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 2001;49:109–16.
113. Wilson APR, Smyth D, Moore G, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 2011;39:651–8.
114. Hess AS, Shardell M, Johnson JK, et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infection Control Hosp Epidemiol 2013;34:487–93.
115. Falk PS, Winnike J, Woodmansee C, et al. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol 2000;21:575–82.
116. Livornese LL Jr, Dias S, Samel C, et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med 1992;117:112–6.
117. Porwancher R, Sheth A, Remphrey S, et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol 1997;18:771–3.
118. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000;343:1925–32.
119. Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol 2000;38:724–6.
120. Noskin GA, Bednarz P, Suriano T, et al. Persistent contamination of fabric-covered furniture by vancomycin-resistant enterococci: implications for upholstery selection in hospitals. Am J Infect Control 2000;28:311–3.
121. Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol 1995;16:577–81.
122. Smith TL, Iwen PC, Olson SB, Rupp ME. Environmental contamination with vancomycin-resistant enterococci in an outpatient setting. Infect Control Hosp Epidemiol 1998;19:515–8.
123. Wendt C, Wiesenthal B, Dietz E, Ruden H. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol 1998;36:3734–6.
124. Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol 2004;25:164–7.
125. Ray AJ, Hoyen CK, Taub TF, et al. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA 2002;287:1400–1.
126. Hayden MK, Bonten MJ, Blom DW, et al. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 2006;42:1552–60.
127. Byers KE, Durbin LJ, Simonton BM, et al. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol 1998;19:261–4.
128. Goodman ER, Platt R, Bass R, et al. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol 2008;29:593–9.
129. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
130. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA) infections. Accessed 11 Oct 2013 at www.cdc.gov/mrsa/index.html.
131. Edmond MB, Wenzel RP. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1471.
132. Lai KK, Fontecchio S, Melvin Z, Baker SP. Impact of alcohol-based, waterless hand antiseptic on the incidence of infection and colonization with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Infect Control Hosp Epidemiol 2006;27:1018–24.
133. Ostrowsky B, Steinberg JT, Farr B, et al. Reality check: should we try to detect and isolate vancomycin-resistant enterococci patients? Infect Control Hosp Epidemiol 2001;22:116–9.
134. Harbarth S, Sax H, Uckay I, et al. A predictive model for identifying surgical patients at risk of methicillin-resistant Staphylococcus aureus carriage on admission. J Am Coll Surg 2008;207:683–9.
135. Jarvis WR. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1469.
136. Krause R, Honigl M, Zollner-Schwetz I. Targeted decolonization to prevent ICU infections. N Engl J Med;369:1469–70.
137. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med;364:1407–18.
138. Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148:409–18.
139. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
140. Gurieva T, Bootsma MCJ, Bonten MJM. Successful Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections revisited. Clin Infect Dis 2012:54:1618–20.
141. Gavalda L, Masuet C, Beltran J, et al. Comparative cost of selective screening to prevent transmission of methicillin-resistant Staphylococcus aureus (MRSA), compared with the attributable costs of MRSA infection. Infection control and hospital epidemiology 2006;27:1264–6.
142. Otter JA, Herdman MT, Williams B, et al. Low prevalence of methicillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect 2013;83:114–21.
143. Harbarth S, Hawkey PM, Tenover F, et al. Update on screening and clinical diagnosis of methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 2011;37:110–7.
144. Calfee DP, Giannetta ET, Durbin LJ, et al. Control of endemic vancomycin-resistant Enterococcus among inpatients at a university hospital. Clin Infect Dis 2003;37:326–32.
145. Hendrix CW, Hammond JM, Swoboda SM, et al. Surveillance strategies and impact of vancomycin-resistant enterococcal colonization and infection in critically ill patients. Ann Surg 2001;233:259–65.
146. Muto CA, Giannetta ET, Durbin LJ, et al. Cost-effectiveness of perirectal surveillance cultures for controlling vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2002;23:429–35.
147. Price CS, Paule S, Noskin GA, Peterson LR. Active surveillance reduces the incidence of vancomycin-resistant enterococcal bacteremia. Clin Infect Dis 2003;37:921–8.
148. Shadel BN, Puzniak LA, Gillespie KN, et al. Surveillance for vancomycin-resistant enterococci: type, rates, costs, and implications. Infect Control Hosp Epidemiol 2006;27:1068–75.
149. Siddiqui AH, Harris AD, Hebden J, et al. The effect of active surveillance for vancomycin-resistant enterococci in high-risk units on vancomycin-resistant enterococci incidence hospital-wide. Am J Infect Control 2002;30:40–3.
150. Sandri AM, Dalarosa MG, Ruschel de Alcantara L, et al. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol 2006;27:185–7.
151. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65.
152. Bleasdale SC, Trick WE, Gonzalez IM, et al. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–9.
153. Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858–65.
154. Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306–12.
155. Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013;381:1099–106.
156. Blackwood B, Thompson G, McMullan R, et al. Tea tree oil (5%) body wash versus standard care (Johnson’s Baby Softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: a randomized controlled trial. J Antimicrob Chemother 2013;68:1193–9.
157. Daneman N, Sarwar S, Fowler RA, et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:328–41.
158. Verwaest C, Verhaegen J, Ferdinande P, et al. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 1997;25:63–71.
159. de Smet AMGA, Kluytmans JAJW, Blok HEM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis 2011;11:372–80.
160. de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003;362:1011–6.
161. Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet 2005;365:295–304.
162. Dhaliwal J, McGeer A. Does isolation prevent the spread of methicillin-resistant Staphylococcus aureus? CMAJ 2005;172:875.
163. Kollef MH, Micek ST. Antimicrobial stewardship programs: mandatory for all ICUs. Crit Care 2012;16:179.
164. McKinnell JA, Huang SS, Eells SJ, et al. Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 2013;34:161–70.
165. Denkinger CM, Grant AD, Denkinger M, et al. Increased multi-drug resistance among the elderly on admission to the hospital—a 12-year surveillance study. Arch Gerontol Geriatr 2013;56:227–30.
166. Boisseau D, Alfandari S, Gauzit R, et al. Staphylococcus aureus nasal carriage during the infectious diseases national congress in France. Med Mal Infect 2012;42:435–9.
167. Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med 2012;166:551–7.
168. Rafee Y, Abdel-Haq N, Asmar B, et al. Increased prevalence of methicillin-resistant Staphylococcus aureus nasal colonization in household contacts of children with community acquired disease. BMC Infect Dis 2012;12:45.
169. Schechter-Perkins EM, Mitchell PM, Murray KA, et al. Prevalence and predictors of nasal and extranasal staphylococcal colonization in patients presenting to the emergency department. Ann Emerg Med 2011;57:492–9.
170. Bisaga A, Paquette K, Sabatini L, Lovell E. A prevalence study of methicillin-resistant staphylococcus aureus colonization in emergency department health care workers. Ann Emerg Med 2008;52:525–8.
171. Suffoletto B, Cannon E, Ilkhanipour K, Yealy D. Prevalence of Staphylococcus aureus nasal colonization in emergency department personnel. Ann Emerg Med 2008;52:529–33.
172. Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg 2004;139:947-51; discussion 51–3.
173. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 2003;36:131–9.
From the Department of Medicine, Infectious Disease Practice and Innovations, The Medical City, Pasig City, Philippines (Dr. Abad), the Division of Emergency Medicine, University of Wisconsin Medical School, Madison, WI (Dr. Pulia), University of Wisconsin Hospital and Clinics, Madison, WI (Ms. Krupp), and the Willam S. Middleton Memorial Veterans Affairs Hospital, Madison, WI (Dr. Safdar).
Patients in intensive care units (ICUs) are at greatly increased risk of developing health care-associated infections (HAIs) [1]. More than 70% of the bacteria that cause HAIs are resistant to at least one of the antimicrobials commonly used to treat these infections [2]. Two such pathogens, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are responsible for a considerable proportion of ICU infections that are associated with increased morbidity, mortality, and costs [3–5]. In this review, we discuss the epidemiology of colonization and infection by MRSA and VRE and provide an update on practices for prevention of transmission and infection by MRSA and VRE in the ICU.
EPIDEMIOLOGY AND MECHANISMS OF RESISTANCE
MRSA is the major cause of HAIs worldwide [6]. Among ICUs in the United States, the proportion of methicillin resistance among S. aureus isolates increased from 35.9% in 1992 to 64.4% in 2003 [4]. Approximately 8% of patients are colonized with MRSA upon admission, and an average of 5% will acquire MRSA colonization in the ICU [7,8]. A comparison study of academic tertiary care facilities found medical ICUs had higher MRSA admission prevalence rates than surgical ICUs, whereas surgical ICUs had a higher incidence rate [7]. Enteroccoccus is the third most common pathogen associated with HAIs, with 33% resistant to vancomycin [9]. VRE infection is associated with increased ICU cost and increased length of stay [10]. Incidence of ICU-acquired VRE varies among regions and countries. For example, in the United States, Warren et al [11] reported a VRE incidence of 27 cases per 1000 patient ICU days, whereas Kohlenberg et al [12] reported a mean incidence of 0.29 cases per 1000 patient ICU days in Germany.
Understanding the mechanisms that allow development of resistant strains of S. aureus and Enterococcus species is important to devise preventive strategies. Methicillin resistance in MRSA is determined by the staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that carries the mecA gene. The mecA gene codes for an additional penicillin-binding protein (PBP) that has a reduced affinity towards methicillin (PBP2a/PBP2'). This results in a reduced ability to bind to the bacterial cell wall and inhibit synthesis [13,14]. Study of molecular epidemiology has identified MRSA as originating from 8 major variants of the mecA gene [15]. The majority of MRSA infections are caused by strains belonging to a few internationally disseminated clones [14]. The first identified strains were associated with infections in hospitalized patients (hospital-associated MRSA), but community-associated MRSA strains have since emerged and have become established globally, including in health care institutions [16].
Community-acquired MRSA can cause severe infections in health hosts [17]; possible explanations include increased CA-MRSA virulence due to the acquisition of mobile genetic elements, namely those containing Panton-Valentine leukocidin (PVL) [18] or increased expression of core genome-encoded virulence genes, such as phenol-soluble modulin (PSM) cytolysins, α-toxin, and other virulence determinants [19].
Enterococcus is intrinsically resistant to several antimicrobial drugs, with resistance to vancomycin encoded by several clusters of genes known as vancomycin resistance gene clusters (eg, vanA, vanB). The gene clusters generate resistance through multiple pathways which encode enzymes to determine the structure of peptidoglycan precursors [20,21]. Genetically diverse, hospital-associated VRE outbreaks have been associated with single clones, multiple clones, and changing molecular epidemiology over time [21]. While up to 25% of the VRE genome includes acquired elements, the majority of hospital-associated infections are traced to a few clonal complexes, which differ from community-associated VRE strains [22].
The evolution of these efficient mechanisms that promote drug resistance has made it extremely challenging to eradicate organisms such as MRSA and VRE. However, advances in recent years have furthered our understanding of the epidemiology, pathogenesis, and methods of prevention and containment.
RISK FACTORS FOR COLONIZATION AND INFECTION
MRSA
The risk factors underlying MRSA colonization and infection in the ICU setting can be categorized as either patient/host or environmental factors. A wide range of patient-level factors is associated with MRSA colonization upon admission. General principles regarding the transmission of MRSA in the community include close contact with colonized or infected individuals, breaks in the skin, crowded living conditions and poor hygiene. These factors, alone or in combination, are thought to underlie observed outbreaks among sports teams, military personnel, correction facilities, American Indian communities, and daycare centers [23–34].
Two recently published systematic reviews have summarized important patient-level factors associated with MRSA colonization at the time of hospital admission. Forster et al [35] examined 27 studies and identified previous admission to hospital, transfer from nursing home or long-term care facility, and previous antibiotic use as the top 3 factors associated with MRSA colonization. A similar review conducted by McKinnell and colleagues [36] found that prior hospitalization, nursing home contact, recent antibiotic use, and exposure to health care-associated pathogens (MRSA carriage, VRE carriage, or Clostridium difficile infection) were found to portend the highest risk. Specific comorbid conditions also conveyed an increased risk, including congestive heart failure, chronic wounds/bedsores, diabetes mellitus, pulmonary disease, immunosuppression, urinary catheter, and renal failure/dialysis. It is clear that health care contacts, especially recent hospitalization, residence in a long-term care facility, and antibiotic use, are significant risk factors for MRSA colonization [37–39].
 In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
Regardless of whether MRSA colonization precedes admission or occurs due to nosocomial spread, it is associated with increased risk of developing a HAI [46–49]. In 2 large prospective observational cohort studies, the hazard ratios of MRSA colonization developing into S. aureus infections during the ICU stay were 3.84 and 4.70, respectively [50,51]. High levels of concordance between MRSA colonization strains and HAI strains have also been reported [52]. Nasal colonization with S. aureus has also been identified as an independent risk factor for developing ventilator-associated pneumonia (VAP) and bacteremia [53,54]. A case series of ICU patients with S. aureus nasal colonization who developed lower respiratory tract infections demonstrated genetically identical nasal and bronchial strains in 15/16 cases [55]. This finding strongly suggests that nasopharyngeal colonization with S. aureus contaminates oral secretions that are aspirated by critically ill patients, resulting in subsequent pneumonia. In a long-term outcomes study among a matched cohort of veterans, MRSA colonization was associated with an increased risk of infection-related readmission and mortality [56]. These findings reflect the critically important nature of measures designed to curb nosocomial transmission and acquisition of MRSA, especially among the vulnerable ICU population.
VRE
As with MRSA, risk factors associated with VRE colonization include both patient-level and ICU-level (or environmental) factors [57]. Examples of patient-level factors include previous antimicrobial exposure [58–62], underlying medical illnesses such as chronic renal failure requiring hemodialysis [11,63], length of hospital or ICU stay [11,59,64,65], and recent exposure to health care facilities. ICU-level factors of relevance are the prevalence of VRE in the unit, with high levels of endemicity leading to higher risk of colonization and transmission.
Antibiotic use is a major risk factor for VRE acquisition, although the type and class of antibiotic varies considerably across studies; the most frequently identified antibiotics are broad-spectrum cephalosporins, vancomycin, and anti-anaerobic agents [58,62,64]. Patients with chronic liver disease and post-transplantation are at exceedingly high risk for VRE acquisition [59]. In a recent study by Pan [66], for example, the authors found that the incidence of newly acquired VRE was 21.9 per 1000 patient-days in an ICU setting. On multivariate analysis, the authors found that, similar to other reports [11,59,67], length of stay in the ICU was associated with increased risk of VRE acquisition, with each additional day of stay increasing risk of VRE by 1.03 times. Warren et al undertook a prospective cohort study involving 519 patients admitted to the ICU for more than 48 hours [11]. Seventy-four (21%) of 352 patients were subsequently colonized with VRE. The median time to development of a positive VRE culture after ICU admission was 6 days. Increased mean APACHE II score on ICU admission (P = 0.002), sucralfate use (P = 0.003), vasopressor use (P = 0.01), tracheostomy in the ICU (P = 0.02), and C. difficile diarrhea (P = 0.002) appeared to be associated with VRE acquisition.
 It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
REDUCING TRANSMISSION—MRSA AND VRE PREVENTION STRATEGIES
 Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Antimicrobial Stewardship
Antibiotic use is a major driver of antibiotic resistance. A meta-analysis by de Bruin and Riley [71] studied the effect of vancomycin usage on VRE colonization and infection. A total of 12 articles describing 13 studies were included; none were randomized controlled trials. All studies were quasi-experimental and lacked control groups. Among all studies, less than half (46%) implemented vancomycin reduction measures as the sole type of intervention [72–76]. The remaining studies implemented other infection control modalities and or restricted the use of other antimicrobials [77–83]. Although all studies that implemented vancomycin restriction alone as a single strategy showed a decline in vancomycin usage, only 2 of these [74,75] showed a relative risk reduction in VRE acquisition post-intervention. Also, studies that restricted vancomycin alone revealed a trend towards lower efficacy in reducing VRE colonization and infection (33%) when compared with those that used additional measures (71%). While judicious antibiotic use should always be practiced, the evidence for vancomycin restriction as a sole intervention to control VRE is scant. It may be that other antibiotics are as big or bigger drivers of resistance in enterococci than vancomycin. For example, a growing body of literature supports antibiotic restriction, especially fluoroquinolones, for reducing MRSA. In several time-series quasi-experimental studies, restriction of fluorquinolones was associated with reduced trends in MRSA infections in the acute care setting, and consideration should be given to monitor and optimize fluoroquinolone use in the ICU setting [84,85].
Antimicrobial stewardship programs are fundamental to optimizing antibiotic use in the ICU and the authors strongly recommend that all ICUs should have such a program in place.
Educational Interventions
Infection control and multidrug-resistant organism (MDRO)–specific education programs for health care workers is a core principle of the CDC’s prevention guidelines. The HICPAC VRE guideline also explicitly states “continuing education programs for hospital staff (including attending and consulting physicians, medical residents, and students; pharmacy, nursing, and laboratory personnel; and other direct patient-care providers) should include information concerning the epidemiology of VRE and the potential impact of this pathogen on the cost and outcome of patient care [70].” A systematic review published in 2008 [86] that included 26 studies showed that such interventions to prevent HCAIs are usually successful; in this review, 20 of 26 studies showed a statistically significant decrease in infection rates, with risk ratios ranging from 0 to 1.6. Education was usually part of a broader array of infection control interventions. While clearly essential, education alone is unlikely to have a sustained impact on reducing MRSA and VRE infections.
Infection Control Measures
Major infection control interventions include hand hygiene, the use of personal protective equipment (PPE), and cohorting. These measures can be grouped into “horizontal” (or global) vs. “vertical” (or targeted) strategies. Although not mutually exclusive, horizontal approaches are designed to have an impact on multiple pathogens (pathogen nonspecific), whereas vertical approaches are designed to reduce the impact of specific pathogens (such as VRE). For the purposes of this review, we will discuss both strategies for containment of MRSA and VRE. Horizontal strategies include hand hygiene, universal gloving and/or gowning, environmental cleaning, and daily bathing with chlorhexidine. Vertical strategies include screening for either MRSA or VRE followed by placement in contact precautions and decolonization with mupirocin.
Hand Hygiene
Hand washing is fundamental to reducing transmission of MDROs in health care institutions; however, optimal compliance is hard to achieve and sustain. Barriers to adherence may include unavailability of sinks or hand hygiene materials (eg, alcohol-based gels, gloves) time constraints, forgetfulness, or lack of knowledge [87–95]. Several monitoring strategies have been evaluated to increase compliance with hand hygiene. Most involve direct observation followed by performance assessment and feedback.
Trials examining the impact of improvements in hand hygiene compliance on HAIs in the ICU setting have largely found benefit, although not all studies showed a decline in HAI. In a prospective crossover trial, Rupp et al [96] found dramatic improvements in compliance with hand gel availability, but this did not translate to decreased nosocomial MRSA infections. Venkatesh et al [97] carried out a before-and-after interventional prospective study in a hematology unit in a tertiary level hospital to evaluate the use of an electronic method of surveillance to determine compliance with hand hygiene. The authors also used rates of horizontal transmission of VRE as a secondary end-point. Results of the study showed that hand hygiene compliance improved from 36.3% at baseline to 70.1%. This represented an OR of 4.1 (95% confidence interval, 3.7–4.5), which the authors attributed to the use of automated alerts. VRE transmission rates before and during intervention were not statistically different, but the rates of infection were lower at 1.0 per month in comparison with 4.7 infections per month in the preceding 6 months (P = 0.096).
While improved hand hygiene may result in significant reductions in HAIs [40], research indicates hand hygiene alone influences about 40% of infections in the ICU setting [98]. As such, hand hygiene should be viewed as a necessary component of a comprehensive infection control program [99]. Despite the success of hand hygiene in reducing HAIs in the ICU, effective strategies to improve compliance remain elusive even under study conditions and further research is needed in this area [100].
Personal Protective Equipment
Tenorio et al [101] conducted a study to assess the effectiveness of gloving in the prevention of hand carriage of VRE by health care workers. The study showed that among 50 health care workers who had contact with patients colonized with VRE, 6 carried a similar patient strain even prior to known contact, and 17 of 44 (69%) had a patient-related VRE strain on their gloves after contact. This suggests a relatively high rate of colonization after usual patient-care contact. Factors associated with acquisition of VRE on gloves included duration of contact, contact with a patient’s body fluids, presence of diarrhea in a patient, mean VRE colony counts on a patient’s skin, and number of body sites colonized with VRE. Although gloves reduced the risk of VRE acquisition of VRE by 71% (ie, 12/17 did not have VRE on their hands after de-gloving) the protection afforded by gloves was incomplete. As such, hand hygiene after glove removal is recommended.
Slaughter et al [102] compared the use of personal protective equipment in the acquisition of VRE. During this study, 93 patients in glove-and-gown rooms and 88 patients in glove-only rooms had similar rates of VRE at baseline entry into the ICU and after the intervention. Mean times to colonization among the patients who became colonized were 8.0 days in the glove-and-gown group and 7.1 days in the glove-only group. None of these comparisons were statistically significant and the authors concluded that the universal use of gown and gloves was no better than the use of gloves alone in preventing VRE colonization.
A recent cluster randomized trial compared the effect of universal PPE (ie, gowning and gloving) with usual care for reducing acquisition of MRSA or VRE as a composite outcome [103]. The study did not find that universal gowning and gloving reduced VRE or MRSA acquisition but found a 40% decline in MRSA acquisition in the intervention ICUs compared with baseline rates of MRSA. No major adverse effects of universal gowning and gloving were noted in this study. A thoughtful editorial commenting on this article proposes that several aspects of the study deserve consideration, including the possibility of false-negative screening tests for VRE, which may have partially accounted for the negative primary outcome [69].
Based on these studies, it appears that the use of barrier precautions may be of value more for MRSA than VRE but further studies are needed to examine its impact on other types of pathogens, including new and emerging MDROs. Until further evidence becomes available, routine gowning and gloving may be of value in units with a high prevalence of MRSA.
Environmental Cleaning
Accumulating data suggests that the environment may play a major role in transmission of pathogens. MRSA has the ability to survive for days to weeks on inanimate objects [104–107]. Environmental contamination results in contamination of staff clothing and gloves [107,108] and is highly correlated with colonization strains among inpatients [109]. Although some studies of enhanced cleaning techniques and increased environmental services staff time have demonstrated reductions in MRSA outbreaks [110–112], the results are not universally favorable [113,114] and further studies are needed to examine the impact of environmental cleaning on rates of MRSA colonization or infection.
Several studies have implicated contaminated equipment as vectors for transmission of VRE during outbreaks [115–117], but the direction of fomite transfer from patient to environment has been difficult to ascertain. VRE have been found frequently on a variety of inanimate objects and surfaces in different health care environments [118–123], including gloved or ungloved hands of health care workers [101,124,125]. Hayden et al [126] determined the effect of improved environmental cleaning on VRE acquisition rates. This study was a pre-and-post intervention study carried out in a 21-bed medical intensive care unit (MICU) in a tertiary hospital over several phases. The intervention included the creation of a unique and improved cleaning program, as well as in-services to housekeeper services, education of the MICU staff, and a hand hygiene campaign. The results of the study showed decreased acquisition of VRE from 33.47 cases per 1000 patient days at risk in period 1 to 10.40 cases per 1000 patient-days at risk by period 4 of the study. Increased environmental cleaning was also associated with reduced growth of VRE from environmental cultures. At baseline, weekly contamination rates were 0.15 and 0.1 for samples obtained before and after cleaning, respectively. Culture positivity decreased to 0.07 and 0.04 for before and after cleaning in period 2 and then remained at low levels during the remainder of the study. It is important to note that the method for disinfecting used in this study was the “bucket method” as promoted by Byers [127]. This study provides further support for the importance of an environmental reservoir and of environmental decontamination to prevent endemic cross-transmission of VRE [126].
Goodman et al [128] used similar interventions but added a feedback tool using a black-light monitoring system (ie, use of an invisible, nontoxic marker to delineate areas that are adequately or inadequately cleaned) to reduce the likelihood of isolating either MRSA or VRE from an ICU environment. This study also showed favorable results, and notably, the use of the black-light monitoring system identified specific areas that were typically inadequately disinfected. Results showed that flat, horizontal surfaces (eg, countertops, bedside tray tables, and hamper tops) were adequately cleaned more often than small, vertical surfaces (eg, doorknobs, toilet handles, light switches, and electronics).
Part of the controversy surrounding the impact of environmental cleaning is the difficulty in determining its individual value as part of an overall infection control bundle [129]. A proposed area of demonstrable impact for environmental cleaning are frequently touched sites which are more likely to be contaminated with pathogens. Focusing on these “hot-bed” areas of the care environment may offer a useful adjunct to other infection control measures [129].
Active Surveillance
Active surveillance refers to periodic screening for asymp-tomatic carriers followed by placement of colonized patients in contact isolation. This practice is highly variable across institutions, as the evidence supporting this practice is conflicting and there are concerns about the cost of implementing this approach without solid evidence [70,130,131]. Despite lack of randomized controlled trials to guide this practice for MRSA prevention, many hospitals utilize MRSA surveillance and it is mandated by law in 9 states [132,133].
A prospective, interventional cohort study of universal MRSA screening on admission to surgical wards failed to reduce nosocomial MRSA infections [134]. Most recently, a pragmatic, cluster-randomized ICU trial reported that universal decolonization with chlorhexidine wipes and mupirocin use was more effective than screening and isolation in reducing rates of MRSA clinical isolates [65]. However, concerns regarding the risk of mupirocin resistance have been expressed [135,136]. The only randomized trial that compared active surveillance for MRSA and VRE followed by contact precautions to usual care did not find a benefit to active surveillance.
Huskins et al [137], in a large, cluster-randomized trial of 19 ICUs from different hospitals, determined the utility of using a culture-based active surveillance and contact isolation, compared with usual care (contact isolation for patients colonized with MRSA or VRE) as identified by existing hospital protocols, to reduce the incidence of colonization or infection with MRSA or VRE. In this trial, which spanned 6 months and involved 3488 participants, the authors found no significant difference between the intervention and control ICUs in terms of MRSA and VRE colonization or infection rates.
Conflicting with these findings is an observational study comparing MRSA infection rates before and after institution of a universal screening protocol, which demonstrated a 69.6% (CI, –89.2% to –19.6%]; P = 0.03) reduction in hospital wide MRSA prevalence density with screening [138]. The “MRSA bundle” implemented in 2007 at VA hospitals nationwide, which included universal screening, produced a 62% (P < 0.001) reduction in MRSA ICU infections; the relative contribution of the various bundle components is uncertain [139,140].
A proposed cost-saving alternative to universal screening is selective screening based on risk factor assessment [141]. The effectiveness of this type of program depends on creating a clinical decision-making tool capable of accurately identifying high-risk individuals while also accounting for the different risk factor profiles between HA-MRSA and CA-MRSA [142]. It has been proposed that targeted screening protocols may be more cost-effective in settings with < 5% prevalence of MRSA colonization on admission [143].
Many studies [61,144–149] have shown that active surveillance against VRE is cost-effective. For example, Calfee et al [144] showed that an established active surveillance program results in control of endemic VRE in high-risk patients. The infection control program was established in response to a hospital-wide VRE outbreak, and was sustained after the outbreak was controlled. The study by Calfee et al spanned 5 years and was performed at a tertiary-level university hospital, where cultures from perirectal areas were used to identify high-risk patients who were asymptomatically colonized with VRE. During the latter 2 years, 768 new cases of VRE colonization were detected among 69,672 admissions (1.1% of admissions), of which 730 (95.1%) were identified by active surveillance methods. This implies that routine clinical cultures would probably have missed the majority of colonized patients. During this period, the incidence of VRE infection was likewise extremely low at 0.12/1000 patient days (ie, 90 nosocomial VRE infections were identified in 83 patients during 743,956 days of patient care). Sixty-nine of the 83 patients (83%) who developed nosocomial VRE infections were found to be colonized with VRE by surveillance culture before the onset of infection.
Patient Decolonization
Chlorhexidine gluconate has been used in several settings to control outbreaks and infections related to MRSA and VRE due to its broad-spectrum activity against these pathogens. Chlorhexidine-based solutions reduce the density of skin colonization with pathogens such as MRSA and VRE (skin asepsis), thus lowering the risk for horizontal transmission between health care workers and patients.
Decolonization with chlorhexidine as an MRSA infection reduction technique has demonstrated benefit in the ICU setting [150]. The previously mentioned large, cluster-randomized ICU trial by Huang and colleagues found universal decolonization with twice-daily intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the entire ICU stay was superior to targeted decolonization of known MRSA carriers in preventing overall MRSA isolates. However, universal decolonization failed to show a reduction in MRSA bacteremia [151], and concerns about mupirocin resistance may limit the applicability of this approach.
There are now several studies [152–154] that show decreased acquisition of VRE with use of daily chlorhexidine bathing. In a study including 1787 ICU patients, Vernon et al found [154] that the reducing microbial density of VRE on patient’s skin by using chlorhexidine led to decreased transmission. In another study by Climo et al [153] that involved 6 ICUs at 4 academic centers and measured the incidence of MRSA and VRE colonization and blood stream infections (BSI) during a period of bathing with routine soap for 6 months compared with a 6-month period where all admitted patients received daily bathing with a chlorhexidine solution, results found decreased acquisition of VRE by 50% (4.35 vs. 2.19 cases/1000 patient days, P < 0.008) following the introduction of daily chlorhexidine bathing. Furthermore, compared with 16 of 270 patients colonized with VRE who subsequently developed VRE bacteremia at baseline, only 4 of 226 VRE-colonized patients bathed with chlorhexidine in the intervention period developed a BSI, translating into a relative risk reduction of 3.35 (95% CI, 1.13–9.87; P < 0.035). Patients colonized with VRE were 3 times less likely to develop VRE bacteremia when bathed with chlorhexidine compared with regular bathing. Despite the success of this protocol for VRE, when analyzed by individual organism no significant reductions in MRSA acquisition or BSI were reported. This finding is similarly corroborated by a trial conducted in the pediatric ICU setting which found an overall reduction in bacteremia with daily chlorhexidine washes but no significant decrease in cases due to S. aureus [155].
The results of these studies suggest that daily bathing with chlorhexidine should be part of routine practice in health care, especially in ICUs where endemic MRSA or VRE rates are high. Whether there is benefit in other settings needs to be studied.
In addition to chlorhexidine washes, other decolonization techniques have been proposed to reduce colonization and the spread of HAIs in the ICU setting. A randomized controlled trial of daily 5% tea tree oil body washes for the prevention of MRSA colonization failed to significantly reduce rates compared to standard soap body washes [156]. Another proposed decolonization intervention that has not been widely adopted in the United States due to concerns related to development of resistant organisms is selective digestive decontamination (SDD) or selective oropharyngeal decontamination (SOD) with antimicrobial agents [157,158]. In terms of clinical benefit, SDD/SOD have been found to decrease MDRO infection rate [159] and mortality [160].
Cohorting
There is insufficient evidence to conclude that cohorting isolated patients is of benefit for routine use in the endemic ICU setting. A few studies, mainly in the outbreak setting, have examined this approach and the results are conflicting [161,162]. Pending further studies in this area, it is reasonable to cohort patients colonized with the same microorganisms, especially if patients cannot be placed in single rooms.
CONCLUSION
The emergence of MRSA and VRE has led to a resurgence of interest and emphasis on infection control practices and prevention. CDC guidelines to help health care practitioners manage these MDROs in the hospital and ICU-setting exist; however, many questions remain regarding best practice.
Prevention of MRSA and VRE needs to be a 2-pronged approach—antimicrobial stewardship [163] and infection control. A robust antimicrobial stewardship program to optimize and minimize inappropriate antibiotic use is necessary in every institution. From the infection prevention standpoint, it is unclear if systematic identification of MRSA and VRE colonization followed by contact precautions is useful in reducing transmission. It is clear that a strong institutional climate of promoting patient safety and a culture of infection prevention will help in reducing MRSA and VRE facility-wide. It also appears that universal gowning and gloving may be useful for reducing MRSA, but not VRE, transmission. While universal decolonization with mupirocin is efficacious in reducing MRSA, this strategy is not recommended because of promoting mupirocin resistance. However, the use of daily bathing with chlorhexidine represents a relatively low-cost, high-yield intervention that should be adopted. Pending further data, patients known to be colonized or infected with MRSA should be placed in contact precuations as is current practice in most institutions. Finally, in this era of MDROs, hand hygiene remains our best defense against the spread of pathogens in the health care environment.
Note: This article does not represent the views of the Department of Veterans Affairs.
Corresponding author: Nasia Safdar, MD, Willam S. Middleton Memorial Veterans Affairs Hospital, 2500 Overlook Terrace, Madison, WI 53705, [email protected].
Funding/support: This work is funded by a MERIT award from the Department of Veterans Affairs to Nasia Safdar.
Financial disclosures: None.
REFERENCES
1. Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 2009;301:727–36.
2. LeDell K, Muto CA, Jarvis WR, Farr BM. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 2003;24:639–41.
3. Giske CG, Monnet DL, Cars O, Carmeli Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 2008;52:813–21.
4. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Pub Health Rep 2007;122:160–6.
5. Schwaber MJ, Carmeli Y. The effect of antimicrobial resistance on patient outcomes: importance of proper evaluation of appropriate therapy. Crit Care 2009;13:106.
6. Grundmann H, Hori S, Winter B, et al. Risk factors for the transmission of methicillin-resistant Staphylococcus aureus in an adult intensive care unit: fitting a model to the data. J Inf Dis 2002;185:481–8.
7. Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis 2007;195:330–8.
8. Muder RR, Cunningham C, McCray E, et al. Implementation of an industrial systems-engineering approach to reduce the incidence of methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2008;29:702–8.
9. Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011.
10. Pelz RK, Lipsett PA, Swoboda SM, et al. Vancomycin-sensitive and vancomycin-resistant enterococcal infections in the ICU: attributable costs and outcomes. Intensive Care Med 2002;28:692–7.
11. Warren DK, Kollef MH, Seiler SM, et al. The epidemiology of vancomycin-resistant Enterococcus colonization in a medical intensive care unit. Infect Control Hosp Epidemiol 2003;24:257–63.
12. Kohlenberg A, Schwab F, Meyer E, et al. Regional trends in multidrug-resistant infections in German intensive care units: a real-time model for epidemiological monitoring and analysis. J Hosp Infect 2009;73:239–45.
13. Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol 2008;8:747–63.
14. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008;46 Suppl 5:S350–9.
15. Zhang K, McClure JA, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009;53:531–40.
16. Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol 2013;5:205–17.
17. Otto M. MRSA virulence and spread. Cell Microbiol 2012;14:1513–21.
18. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978–84.
19. Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 2009;106:5883–8.
20. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 2006;42 Suppl 1:S25–34.
21. Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 2001;33:210–9.
22. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–78.
23. Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis 1998;178:577–80.
24. Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 2004;113:e347–52.
25. Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 2001;286:1201–5.
26. Hewlett AL, Falk PS, Hughes KS, Mayhall CG. Epidemiology of methicillin-resistant Staphylococcus aureus in a university medical center day care facility. Infect Control Hosp Epidemiol 2009;30:985–92.
27. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468–75.
28. Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA 2012;308:50–9.
29. Lindenmayer JM, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med 1998;158:895–9.
30. Malcolm B. The rise of methicillin-resistant staphylococcus aureus in U.S. correctional populations. J Correct Health Care 2011;17:254–65.
31. Nerby JM, Gorwitz R, Lesher L, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2011;30:927–32.
32. Stemper ME, Shukla SK, Reed KD. Emergence and spread of community-associated methicillin-resistant Staphylococcus aureus in rural Wisconsin, 1989 to 1999. J Clin Microbiol 2004;42:5673–80.
33. Turabelidze G, Lin M, Wolkoff B, et al. Personal hygiene and methicillin-resistant Staphylococcus aureus infection. Emerg Infect Dis 2006;12:422–7.
34. Ellis MW, Hospenthal DR, Dooley DP, et al. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004;39:971–9.
35. Forster AJ, Oake N, Roth V, et al. Patient-level factors associated with methicillin-resistant Staphylococcus aureus carriage at hospital admission: a systematic review. Am J Infect Control 2013;41:214–20.
36. McKinnell JA, Miller LG, Eells SJ, et al. A systematic literature review and meta-analysis of factors associated with methicillin-resistant staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Contol Hosp Epidemiol 2013;34:1077–86.
37. Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med 2006;166:580–5.
38. Jernigan JA, Pullen AL, Flowers L, et al. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol 2003;24:409–14.
39. Horner C, Parnell P, Hall D, Kearns A, Heritage J, Wilcox M. Meticillin-resistant Staphylococcus aureus in elderly residents of care homes: colonization rates and molecular epidemiology. J Hosp Infect 2013;83:212–8.
40. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009;73:305–15.
41. Boyce JM. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect Dis Clin North Am 1989;3:901–13.
42. Jernigan JA. Methicillin-resistant Staphylococcus aureus colonization among health care personnel in the emergency department: what does it tell us? Ann Emerg Med 2008;52:534–6.
43. Carnicer-Pont D, Bailey KA, Mason BW, Walker AM, Evans MR, Salmon RL. Risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a case-control study. Epidemiol Infect 2006;134:1167–73.
44. Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 2002;49:999–1005.
45. Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med 1982;97:309–17.
46. Davis KA, Stewart JJ, Crouch HK,et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004;39:776–82.
47. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997;10:505–20.
48. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med 2008;121:310–5.
49. Wertheim HFL, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
50. Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 2001;22:687–92.
51. Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol;31:584–91.
52. von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001;344:11–6.
53. Pujol M, Pea C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med 1996;100:509–16.
54. Rocha LA, Marques Ribas R, da Costa Darini AL, Gontijo Filho PP. Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am J Infect Control 2013;41:236–40.
55. Corne P, Marchandin Hln, Jonquet O, Campos J, Bauls A-L. Molecular evidence that nasal carriage of Staphylococcus aureus plays a role in respiratory tract infections of critically ill patients. J Clin Microbiol 2005;43:3491–3.
56. Quezada Joaquin NM, Diekema DJ, Perencevich EN, et al. Long-term risk for readmission, methicillin-resistant Staphylococcus aureus (MRSA) infection, and death among MRSA-colonized veterans. Antimicrob Agents Chemother 2013;57:1169–72.
57. Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: recognition and prevention in intensive care units. Crit Care Med 2010;38:S335–44.
58. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002;8:802–7.
59. Ostrowsky BE, Venkataraman L, D’Agata EM, et al. Vancomycin-resistant enterococci in intensive care units: high frequency of stool carriage during a non-outbreak period. Arch Intern Med 1999;159:1467–72.
60. Bonten MJ, Hayden MK, Nathan C, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet 1996;348:1615–9.
61. Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med 2001;344:1427–33.
62. Padiglione AA, Wolfe R, Grabsch EA, et al. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother 2003;47:2492–8.
63. Batistao DW, Gontijo-Filho PP, Conceicao N, et al. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz 2012;107:57–63.
64. Furtado GH, Martins ST, Coutinho AP, et al. Prevalence and factors associated with rectal vancomycin-resistant enterococci colonization in two intensive care units in Sao Paulo, Brazil. Braz J Infect Dis 2005;9:64–9.
65. Huang SS, Datta R, Rifas-Shiman S, et al. Colonization with antibiotic-susceptible strains protects against methicillin-resistant Staphylococcus aureus but not vancomycin-resistant enterococci acquisition: a nested case-control study. Crit Care 2011;15:R210.
66. Pan SC, Wang JT, Chen YC, et al. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One 2012;7:e47297.
67. Se YB, Chun HJ, Yi HJ, et al. Incidence and risk factors of infection caused by vancomycin-resistant enterococcus colonization in neurosurgical intensive care unit patients. J Korean Neurosurg Soc 2009;46:123–9.
68. Healthcare Infection Control Practices Advisory Committee (HICPAC). Management of multidrug-resistant organisms in healthcare settings, 2006. Accessed 11 Oct 2013 at www.cdc.gov/hicpac/mdro/mdro_toc.html.
69. Malani PN. Preventing infections in the ICU: one size does not fit all. JAMA 2013;310:1567–8.
70. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 1995;44:1–13.
71. de Bruin MA, Riley LW. Does vancomycin prescribing intervention affect vancomycin-resistant enterococcus infection and colonization in hospitals? A systematic review. BMC Infect Dis 2007;7:24.
72. Adachi W, Bolding F, Armstrong R. Experience with vancomycin education and order sheet to limit vancomycin use. Hosp Pharm 1997:1370–3.
73. Fridkin SK, Lawton R, Edwards JR, et al. Monitoring antimicrobial use and resistance: comparison with a national benchmark on reducing vancomycin use and vancomycin-resistant enterococci. Emerg Infect Dis 2002;8:702–7.
74. Guglielmo BJ, Dudas V, Maewal I, et al. Impact of a series of interventions in vancomycin prescribing on use and prevalence of vancomycin-resistant enterococci. Jt Comm J Qual Patient Saf 2005;31:469–75.
75. Lautenbach E, LaRosa LA, Marr AM, et al. Changes in the prevalence of vancomycin-resistant enterococci in response to antimicrobial formulary interventions: impact of progressive restrictions on use of vancomycin and third-generation cephalosporins. Clin Infect Dis 2003;36:440–6.
76. Morgan AS, Brennan PJ, Fishman NO. Impact of a vancomycin restriction policy on use and cost of vancomycin and incidence of vancomycin-resistant Enterococcus. Ann Pharmacother 1997;31:970–3.
77. Anglim AM, Klym B, Byers KE, et al. Effect of a vancomycin restriction policy on ordering practices during an outbreak of vancomycin-resistant Enterococcus faecium. Arch Intern Med 1997;157:1132–6.
78. Montecalvo MA, Jarvis WR, Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med 1999;131:269–72.
79. Morris JG Jr, Shay DK, Hebden JN, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med 1995;123:250–9.
80. Quale J, Landman D, Saurina G, et al. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis 1996;23:1020-5.
81. Rubin LG, Tucci V, Cercenado E, et al. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol 1992;13:700–5.
82. Lai KK, Kelley AL, Melvin ZS, et al. Failure to eradicate vancomycin-resistant enterococci in a university hospital and the cost of barrier precautions. Infect Control Hosp Epidemiol 1998;19:647–52.
83. Shaikh ZH, Osting CA, Hanna HA, et al. Effectiveness of a multifaceted infection control policy in reducing vancomycin usage and vancomycin-resistant enterococci at a tertiary care cancer centre. J Hosp Infect 2002;51:52–8.
84. Lafaurie M, Porcher R, Donay JL, et al. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother 2012;67:1010–5.
85. Parienti JJ, Cattoir V, Thibon P, et al. Hospital-wide modification of fluoroquinolone policy and meticillin-resistant Staphylococcus aureus rates: a 10-year interrupted time-series analysis. J Hosp Infect 2011;78:118–22.
86. Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: a systematic review. Crit Care Med 2008;36:933–40.
87. Boyce JM. It is time for action: improving hand hygiene in hospitals. Ann Intern Med 1999;130:153–5.
88. Jackson M, Chiarello LA, Gaynes RP, Gerberding JL. Nurse staffing and healthcare-associated infections: proceedings from a working group meeting. J Nurs Adm 2002;32:314–22.
89. Kuzu N, Ozer F, Aydemir S, et al. Compliance with hand hygiene and glove use in a university-affiliated hospital. Infect Control Hosp Epidemiol 2005;26:312–5.
90. Larson E, Killien M. Factors influencing handwashing behavior of patient care personnel. Am J Infect Control 1982;10:93–9.
91. Larson E, Kretzer EK. Compliance with handwashing and barrier precautions. J Hosp Infect 1995;30 Suppl:88–106.
92. Naikoba S, Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers - a systematic review. J Hosp Infect 2001;47:173–80.
93. Pittet D, Simon A, Hugonnet S, et al. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med 2004;141:1–8.
94. Trick WE, Vernon MO, Welbel SF, et al. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infect Control Hosp Epidemiol 2007;28:42–9.
95. Wisniewski MF, Kim S, Trick WE, et al. Effect of education on hand hygiene beliefs and practices: a 5-year program. Infect Control Hosp Epidemiol 2007;28:88–91.
96. Rupp ME, Fitzgerald T, Puumala S, et al. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol 2008;29:8–15.
97. Venkatesh AK, Lankford MG, Rooney DM, et al. Use of electronic alerts to enhance hand hygiene compliance and decrease transmission of vancomycin-resistant Enterococcus in a hematology unit. Am J Infect Control 2008;36:199–205.
98. Silvestri L, Petros AJ, Sarginson RE, et al. Handwashing in the intensive care unit: a big measure with modest effects. J Hosp Infect 2005;59:172–9.
99. Akyol A, Ulusoy H, Ozen I. Handwashing: a simple, economical and effective method for preventing nosocomial infections in intensive care units. J Hosp Infect 2006;62:395–405.
100. Simmons B, Bryant J, Neiman K, et al. The role of handwashing in prevention of endemic intensive care unit infections. Infect Control Hosp Epidemiol 1990;11:589–94.
101. Tenorio AR, Badri SM, Sahgal NB, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis 2001;32:826–9.
102. Slaughter S, Hayden MK, Nathan C, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med 1996;125:448–56.
103. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013;310:1571–80.
104. Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect 2001;49:255–61.
105. Hardy KJ, Oppenheim BA, Gossain S, et al. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect Control Hosp Epidemiol 2006;27:127–32.
106. Jawad A, Heritage J, Snelling AM, et al. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol 1996;34:2881–7.
107. Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol 2007;28:1142–7.
108. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
109. Sexton T, Clarke P, O’Neill E, et al. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect 2006;62:187–94.
110. Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet infect dis 2008;8:101–13.
111. Dancer SJ, White LF, Lamb J, et al. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC med 2009;7.
112. Rampling A, Wiseman S, Davis L, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 2001;49:109–16.
113. Wilson APR, Smyth D, Moore G, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 2011;39:651–8.
114. Hess AS, Shardell M, Johnson JK, et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infection Control Hosp Epidemiol 2013;34:487–93.
115. Falk PS, Winnike J, Woodmansee C, et al. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol 2000;21:575–82.
116. Livornese LL Jr, Dias S, Samel C, et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med 1992;117:112–6.
117. Porwancher R, Sheth A, Remphrey S, et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol 1997;18:771–3.
118. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000;343:1925–32.
119. Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol 2000;38:724–6.
120. Noskin GA, Bednarz P, Suriano T, et al. Persistent contamination of fabric-covered furniture by vancomycin-resistant enterococci: implications for upholstery selection in hospitals. Am J Infect Control 2000;28:311–3.
121. Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol 1995;16:577–81.
122. Smith TL, Iwen PC, Olson SB, Rupp ME. Environmental contamination with vancomycin-resistant enterococci in an outpatient setting. Infect Control Hosp Epidemiol 1998;19:515–8.
123. Wendt C, Wiesenthal B, Dietz E, Ruden H. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol 1998;36:3734–6.
124. Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol 2004;25:164–7.
125. Ray AJ, Hoyen CK, Taub TF, et al. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA 2002;287:1400–1.
126. Hayden MK, Bonten MJ, Blom DW, et al. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 2006;42:1552–60.
127. Byers KE, Durbin LJ, Simonton BM, et al. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol 1998;19:261–4.
128. Goodman ER, Platt R, Bass R, et al. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol 2008;29:593–9.
129. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
130. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA) infections. Accessed 11 Oct 2013 at www.cdc.gov/mrsa/index.html.
131. Edmond MB, Wenzel RP. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1471.
132. Lai KK, Fontecchio S, Melvin Z, Baker SP. Impact of alcohol-based, waterless hand antiseptic on the incidence of infection and colonization with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Infect Control Hosp Epidemiol 2006;27:1018–24.
133. Ostrowsky B, Steinberg JT, Farr B, et al. Reality check: should we try to detect and isolate vancomycin-resistant enterococci patients? Infect Control Hosp Epidemiol 2001;22:116–9.
134. Harbarth S, Sax H, Uckay I, et al. A predictive model for identifying surgical patients at risk of methicillin-resistant Staphylococcus aureus carriage on admission. J Am Coll Surg 2008;207:683–9.
135. Jarvis WR. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1469.
136. Krause R, Honigl M, Zollner-Schwetz I. Targeted decolonization to prevent ICU infections. N Engl J Med;369:1469–70.
137. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med;364:1407–18.
138. Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148:409–18.
139. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
140. Gurieva T, Bootsma MCJ, Bonten MJM. Successful Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections revisited. Clin Infect Dis 2012:54:1618–20.
141. Gavalda L, Masuet C, Beltran J, et al. Comparative cost of selective screening to prevent transmission of methicillin-resistant Staphylococcus aureus (MRSA), compared with the attributable costs of MRSA infection. Infection control and hospital epidemiology 2006;27:1264–6.
142. Otter JA, Herdman MT, Williams B, et al. Low prevalence of methicillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect 2013;83:114–21.
143. Harbarth S, Hawkey PM, Tenover F, et al. Update on screening and clinical diagnosis of methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 2011;37:110–7.
144. Calfee DP, Giannetta ET, Durbin LJ, et al. Control of endemic vancomycin-resistant Enterococcus among inpatients at a university hospital. Clin Infect Dis 2003;37:326–32.
145. Hendrix CW, Hammond JM, Swoboda SM, et al. Surveillance strategies and impact of vancomycin-resistant enterococcal colonization and infection in critically ill patients. Ann Surg 2001;233:259–65.
146. Muto CA, Giannetta ET, Durbin LJ, et al. Cost-effectiveness of perirectal surveillance cultures for controlling vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2002;23:429–35.
147. Price CS, Paule S, Noskin GA, Peterson LR. Active surveillance reduces the incidence of vancomycin-resistant enterococcal bacteremia. Clin Infect Dis 2003;37:921–8.
148. Shadel BN, Puzniak LA, Gillespie KN, et al. Surveillance for vancomycin-resistant enterococci: type, rates, costs, and implications. Infect Control Hosp Epidemiol 2006;27:1068–75.
149. Siddiqui AH, Harris AD, Hebden J, et al. The effect of active surveillance for vancomycin-resistant enterococci in high-risk units on vancomycin-resistant enterococci incidence hospital-wide. Am J Infect Control 2002;30:40–3.
150. Sandri AM, Dalarosa MG, Ruschel de Alcantara L, et al. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol 2006;27:185–7.
151. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65.
152. Bleasdale SC, Trick WE, Gonzalez IM, et al. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–9.
153. Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858–65.
154. Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306–12.
155. Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013;381:1099–106.
156. Blackwood B, Thompson G, McMullan R, et al. Tea tree oil (5%) body wash versus standard care (Johnson’s Baby Softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: a randomized controlled trial. J Antimicrob Chemother 2013;68:1193–9.
157. Daneman N, Sarwar S, Fowler RA, et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:328–41.
158. Verwaest C, Verhaegen J, Ferdinande P, et al. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 1997;25:63–71.
159. de Smet AMGA, Kluytmans JAJW, Blok HEM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis 2011;11:372–80.
160. de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003;362:1011–6.
161. Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet 2005;365:295–304.
162. Dhaliwal J, McGeer A. Does isolation prevent the spread of methicillin-resistant Staphylococcus aureus? CMAJ 2005;172:875.
163. Kollef MH, Micek ST. Antimicrobial stewardship programs: mandatory for all ICUs. Crit Care 2012;16:179.
164. McKinnell JA, Huang SS, Eells SJ, et al. Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 2013;34:161–70.
165. Denkinger CM, Grant AD, Denkinger M, et al. Increased multi-drug resistance among the elderly on admission to the hospital—a 12-year surveillance study. Arch Gerontol Geriatr 2013;56:227–30.
166. Boisseau D, Alfandari S, Gauzit R, et al. Staphylococcus aureus nasal carriage during the infectious diseases national congress in France. Med Mal Infect 2012;42:435–9.
167. Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med 2012;166:551–7.
168. Rafee Y, Abdel-Haq N, Asmar B, et al. Increased prevalence of methicillin-resistant Staphylococcus aureus nasal colonization in household contacts of children with community acquired disease. BMC Infect Dis 2012;12:45.
169. Schechter-Perkins EM, Mitchell PM, Murray KA, et al. Prevalence and predictors of nasal and extranasal staphylococcal colonization in patients presenting to the emergency department. Ann Emerg Med 2011;57:492–9.
170. Bisaga A, Paquette K, Sabatini L, Lovell E. A prevalence study of methicillin-resistant staphylococcus aureus colonization in emergency department health care workers. Ann Emerg Med 2008;52:525–8.
171. Suffoletto B, Cannon E, Ilkhanipour K, Yealy D. Prevalence of Staphylococcus aureus nasal colonization in emergency department personnel. Ann Emerg Med 2008;52:529–33.
172. Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg 2004;139:947-51; discussion 51–3.
173. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 2003;36:131–9.
From the Department of Medicine, Infectious Disease Practice and Innovations, The Medical City, Pasig City, Philippines (Dr. Abad), the Division of Emergency Medicine, University of Wisconsin Medical School, Madison, WI (Dr. Pulia), University of Wisconsin Hospital and Clinics, Madison, WI (Ms. Krupp), and the Willam S. Middleton Memorial Veterans Affairs Hospital, Madison, WI (Dr. Safdar).
Patients in intensive care units (ICUs) are at greatly increased risk of developing health care-associated infections (HAIs) [1]. More than 70% of the bacteria that cause HAIs are resistant to at least one of the antimicrobials commonly used to treat these infections [2]. Two such pathogens, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) are responsible for a considerable proportion of ICU infections that are associated with increased morbidity, mortality, and costs [3–5]. In this review, we discuss the epidemiology of colonization and infection by MRSA and VRE and provide an update on practices for prevention of transmission and infection by MRSA and VRE in the ICU.
EPIDEMIOLOGY AND MECHANISMS OF RESISTANCE
MRSA is the major cause of HAIs worldwide [6]. Among ICUs in the United States, the proportion of methicillin resistance among S. aureus isolates increased from 35.9% in 1992 to 64.4% in 2003 [4]. Approximately 8% of patients are colonized with MRSA upon admission, and an average of 5% will acquire MRSA colonization in the ICU [7,8]. A comparison study of academic tertiary care facilities found medical ICUs had higher MRSA admission prevalence rates than surgical ICUs, whereas surgical ICUs had a higher incidence rate [7]. Enteroccoccus is the third most common pathogen associated with HAIs, with 33% resistant to vancomycin [9]. VRE infection is associated with increased ICU cost and increased length of stay [10]. Incidence of ICU-acquired VRE varies among regions and countries. For example, in the United States, Warren et al [11] reported a VRE incidence of 27 cases per 1000 patient ICU days, whereas Kohlenberg et al [12] reported a mean incidence of 0.29 cases per 1000 patient ICU days in Germany.
Understanding the mechanisms that allow development of resistant strains of S. aureus and Enterococcus species is important to devise preventive strategies. Methicillin resistance in MRSA is determined by the staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that carries the mecA gene. The mecA gene codes for an additional penicillin-binding protein (PBP) that has a reduced affinity towards methicillin (PBP2a/PBP2'). This results in a reduced ability to bind to the bacterial cell wall and inhibit synthesis [13,14]. Study of molecular epidemiology has identified MRSA as originating from 8 major variants of the mecA gene [15]. The majority of MRSA infections are caused by strains belonging to a few internationally disseminated clones [14]. The first identified strains were associated with infections in hospitalized patients (hospital-associated MRSA), but community-associated MRSA strains have since emerged and have become established globally, including in health care institutions [16].
Community-acquired MRSA can cause severe infections in health hosts [17]; possible explanations include increased CA-MRSA virulence due to the acquisition of mobile genetic elements, namely those containing Panton-Valentine leukocidin (PVL) [18] or increased expression of core genome-encoded virulence genes, such as phenol-soluble modulin (PSM) cytolysins, α-toxin, and other virulence determinants [19].
Enterococcus is intrinsically resistant to several antimicrobial drugs, with resistance to vancomycin encoded by several clusters of genes known as vancomycin resistance gene clusters (eg, vanA, vanB). The gene clusters generate resistance through multiple pathways which encode enzymes to determine the structure of peptidoglycan precursors [20,21]. Genetically diverse, hospital-associated VRE outbreaks have been associated with single clones, multiple clones, and changing molecular epidemiology over time [21]. While up to 25% of the VRE genome includes acquired elements, the majority of hospital-associated infections are traced to a few clonal complexes, which differ from community-associated VRE strains [22].
The evolution of these efficient mechanisms that promote drug resistance has made it extremely challenging to eradicate organisms such as MRSA and VRE. However, advances in recent years have furthered our understanding of the epidemiology, pathogenesis, and methods of prevention and containment.
RISK FACTORS FOR COLONIZATION AND INFECTION
MRSA
The risk factors underlying MRSA colonization and infection in the ICU setting can be categorized as either patient/host or environmental factors. A wide range of patient-level factors is associated with MRSA colonization upon admission. General principles regarding the transmission of MRSA in the community include close contact with colonized or infected individuals, breaks in the skin, crowded living conditions and poor hygiene. These factors, alone or in combination, are thought to underlie observed outbreaks among sports teams, military personnel, correction facilities, American Indian communities, and daycare centers [23–34].
Two recently published systematic reviews have summarized important patient-level factors associated with MRSA colonization at the time of hospital admission. Forster et al [35] examined 27 studies and identified previous admission to hospital, transfer from nursing home or long-term care facility, and previous antibiotic use as the top 3 factors associated with MRSA colonization. A similar review conducted by McKinnell and colleagues [36] found that prior hospitalization, nursing home contact, recent antibiotic use, and exposure to health care-associated pathogens (MRSA carriage, VRE carriage, or Clostridium difficile infection) were found to portend the highest risk. Specific comorbid conditions also conveyed an increased risk, including congestive heart failure, chronic wounds/bedsores, diabetes mellitus, pulmonary disease, immunosuppression, urinary catheter, and renal failure/dialysis. It is clear that health care contacts, especially recent hospitalization, residence in a long-term care facility, and antibiotic use, are significant risk factors for MRSA colonization [37–39].
 In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
In contrast to those already colonized with MRSA, some patients acquire MRSA during hospitalization. In these cases, transmission via hands of health care workers is likely the most common mechanism for spread of MRSA [6,40–42]. An understaffed ICU has also been cited as a potential risk factor for ICU MRSA transmission, perhaps due to sacrifices in hand hygiene practices by overextended staff [6]. Additional factors associated with increased risk of nosocomial MRSA acquisition include duration of antibiotic therapy, exposure to quinolone or macrolide antibiotics, length of hospital stay, enteral feeding, post-surgical status, insertion of central line or urinary catheter during admission, ICU admission, and proximity to another patient with MRSA infection or colonization [43–45]. A summary of risk factors for MRSA acquisition is shown in Table 1.
Regardless of whether MRSA colonization precedes admission or occurs due to nosocomial spread, it is associated with increased risk of developing a HAI [46–49]. In 2 large prospective observational cohort studies, the hazard ratios of MRSA colonization developing into S. aureus infections during the ICU stay were 3.84 and 4.70, respectively [50,51]. High levels of concordance between MRSA colonization strains and HAI strains have also been reported [52]. Nasal colonization with S. aureus has also been identified as an independent risk factor for developing ventilator-associated pneumonia (VAP) and bacteremia [53,54]. A case series of ICU patients with S. aureus nasal colonization who developed lower respiratory tract infections demonstrated genetically identical nasal and bronchial strains in 15/16 cases [55]. This finding strongly suggests that nasopharyngeal colonization with S. aureus contaminates oral secretions that are aspirated by critically ill patients, resulting in subsequent pneumonia. In a long-term outcomes study among a matched cohort of veterans, MRSA colonization was associated with an increased risk of infection-related readmission and mortality [56]. These findings reflect the critically important nature of measures designed to curb nosocomial transmission and acquisition of MRSA, especially among the vulnerable ICU population.
VRE
As with MRSA, risk factors associated with VRE colonization include both patient-level and ICU-level (or environmental) factors [57]. Examples of patient-level factors include previous antimicrobial exposure [58–62], underlying medical illnesses such as chronic renal failure requiring hemodialysis [11,63], length of hospital or ICU stay [11,59,64,65], and recent exposure to health care facilities. ICU-level factors of relevance are the prevalence of VRE in the unit, with high levels of endemicity leading to higher risk of colonization and transmission.
Antibiotic use is a major risk factor for VRE acquisition, although the type and class of antibiotic varies considerably across studies; the most frequently identified antibiotics are broad-spectrum cephalosporins, vancomycin, and anti-anaerobic agents [58,62,64]. Patients with chronic liver disease and post-transplantation are at exceedingly high risk for VRE acquisition [59]. In a recent study by Pan [66], for example, the authors found that the incidence of newly acquired VRE was 21.9 per 1000 patient-days in an ICU setting. On multivariate analysis, the authors found that, similar to other reports [11,59,67], length of stay in the ICU was associated with increased risk of VRE acquisition, with each additional day of stay increasing risk of VRE by 1.03 times. Warren et al undertook a prospective cohort study involving 519 patients admitted to the ICU for more than 48 hours [11]. Seventy-four (21%) of 352 patients were subsequently colonized with VRE. The median time to development of a positive VRE culture after ICU admission was 6 days. Increased mean APACHE II score on ICU admission (P = 0.002), sucralfate use (P = 0.003), vasopressor use (P = 0.01), tracheostomy in the ICU (P = 0.02), and C. difficile diarrhea (P = 0.002) appeared to be associated with VRE acquisition.
 It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
It appears that VRE acquisition is often associated with the sick subgroup of patients, and risk factors generally associated with VRE colonization and infection co-relate with disease chronicity and severity of illness. Length of hospitalization, ICU stay, hemodialysis, or transplantation may all be markers of disease severity. A summary of risk factors for VRE acquisition is shown in Table 2.
REDUCING TRANSMISSION—MRSA AND VRE PREVENTION STRATEGIES
 Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Evidence-based guidelines developed by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee (HICPAC) for prevention of MRSA and VRE are available [68]. Several recently conducted well-designed clinical trials also provide additional insight that may be particularly helpful in the ICU setting [69]. A summary of the MRSA prevention guidelines issued by the CDC and included in its “MRSA toolkit” is provided in Table 3. A similar guideline on prevention of VRE [70], published more than a decade ago, has similar elements. Table 3 shows a side-by-side comparison of these elements. Unfortunately, despite these guidelines and extensive research regarding prevention and control, considerable controversy exists as to the most effective approaches. As such, these recommendations should be tailored to meet the needs of the specific ICU setting.
Antimicrobial Stewardship
Antibiotic use is a major driver of antibiotic resistance. A meta-analysis by de Bruin and Riley [71] studied the effect of vancomycin usage on VRE colonization and infection. A total of 12 articles describing 13 studies were included; none were randomized controlled trials. All studies were quasi-experimental and lacked control groups. Among all studies, less than half (46%) implemented vancomycin reduction measures as the sole type of intervention [72–76]. The remaining studies implemented other infection control modalities and or restricted the use of other antimicrobials [77–83]. Although all studies that implemented vancomycin restriction alone as a single strategy showed a decline in vancomycin usage, only 2 of these [74,75] showed a relative risk reduction in VRE acquisition post-intervention. Also, studies that restricted vancomycin alone revealed a trend towards lower efficacy in reducing VRE colonization and infection (33%) when compared with those that used additional measures (71%). While judicious antibiotic use should always be practiced, the evidence for vancomycin restriction as a sole intervention to control VRE is scant. It may be that other antibiotics are as big or bigger drivers of resistance in enterococci than vancomycin. For example, a growing body of literature supports antibiotic restriction, especially fluoroquinolones, for reducing MRSA. In several time-series quasi-experimental studies, restriction of fluorquinolones was associated with reduced trends in MRSA infections in the acute care setting, and consideration should be given to monitor and optimize fluoroquinolone use in the ICU setting [84,85].
Antimicrobial stewardship programs are fundamental to optimizing antibiotic use in the ICU and the authors strongly recommend that all ICUs should have such a program in place.
Educational Interventions
Infection control and multidrug-resistant organism (MDRO)–specific education programs for health care workers is a core principle of the CDC’s prevention guidelines. The HICPAC VRE guideline also explicitly states “continuing education programs for hospital staff (including attending and consulting physicians, medical residents, and students; pharmacy, nursing, and laboratory personnel; and other direct patient-care providers) should include information concerning the epidemiology of VRE and the potential impact of this pathogen on the cost and outcome of patient care [70].” A systematic review published in 2008 [86] that included 26 studies showed that such interventions to prevent HCAIs are usually successful; in this review, 20 of 26 studies showed a statistically significant decrease in infection rates, with risk ratios ranging from 0 to 1.6. Education was usually part of a broader array of infection control interventions. While clearly essential, education alone is unlikely to have a sustained impact on reducing MRSA and VRE infections.
Infection Control Measures
Major infection control interventions include hand hygiene, the use of personal protective equipment (PPE), and cohorting. These measures can be grouped into “horizontal” (or global) vs. “vertical” (or targeted) strategies. Although not mutually exclusive, horizontal approaches are designed to have an impact on multiple pathogens (pathogen nonspecific), whereas vertical approaches are designed to reduce the impact of specific pathogens (such as VRE). For the purposes of this review, we will discuss both strategies for containment of MRSA and VRE. Horizontal strategies include hand hygiene, universal gloving and/or gowning, environmental cleaning, and daily bathing with chlorhexidine. Vertical strategies include screening for either MRSA or VRE followed by placement in contact precautions and decolonization with mupirocin.
Hand Hygiene
Hand washing is fundamental to reducing transmission of MDROs in health care institutions; however, optimal compliance is hard to achieve and sustain. Barriers to adherence may include unavailability of sinks or hand hygiene materials (eg, alcohol-based gels, gloves) time constraints, forgetfulness, or lack of knowledge [87–95]. Several monitoring strategies have been evaluated to increase compliance with hand hygiene. Most involve direct observation followed by performance assessment and feedback.
Trials examining the impact of improvements in hand hygiene compliance on HAIs in the ICU setting have largely found benefit, although not all studies showed a decline in HAI. In a prospective crossover trial, Rupp et al [96] found dramatic improvements in compliance with hand gel availability, but this did not translate to decreased nosocomial MRSA infections. Venkatesh et al [97] carried out a before-and-after interventional prospective study in a hematology unit in a tertiary level hospital to evaluate the use of an electronic method of surveillance to determine compliance with hand hygiene. The authors also used rates of horizontal transmission of VRE as a secondary end-point. Results of the study showed that hand hygiene compliance improved from 36.3% at baseline to 70.1%. This represented an OR of 4.1 (95% confidence interval, 3.7–4.5), which the authors attributed to the use of automated alerts. VRE transmission rates before and during intervention were not statistically different, but the rates of infection were lower at 1.0 per month in comparison with 4.7 infections per month in the preceding 6 months (P = 0.096).
While improved hand hygiene may result in significant reductions in HAIs [40], research indicates hand hygiene alone influences about 40% of infections in the ICU setting [98]. As such, hand hygiene should be viewed as a necessary component of a comprehensive infection control program [99]. Despite the success of hand hygiene in reducing HAIs in the ICU, effective strategies to improve compliance remain elusive even under study conditions and further research is needed in this area [100].
Personal Protective Equipment
Tenorio et al [101] conducted a study to assess the effectiveness of gloving in the prevention of hand carriage of VRE by health care workers. The study showed that among 50 health care workers who had contact with patients colonized with VRE, 6 carried a similar patient strain even prior to known contact, and 17 of 44 (69%) had a patient-related VRE strain on their gloves after contact. This suggests a relatively high rate of colonization after usual patient-care contact. Factors associated with acquisition of VRE on gloves included duration of contact, contact with a patient’s body fluids, presence of diarrhea in a patient, mean VRE colony counts on a patient’s skin, and number of body sites colonized with VRE. Although gloves reduced the risk of VRE acquisition of VRE by 71% (ie, 12/17 did not have VRE on their hands after de-gloving) the protection afforded by gloves was incomplete. As such, hand hygiene after glove removal is recommended.
Slaughter et al [102] compared the use of personal protective equipment in the acquisition of VRE. During this study, 93 patients in glove-and-gown rooms and 88 patients in glove-only rooms had similar rates of VRE at baseline entry into the ICU and after the intervention. Mean times to colonization among the patients who became colonized were 8.0 days in the glove-and-gown group and 7.1 days in the glove-only group. None of these comparisons were statistically significant and the authors concluded that the universal use of gown and gloves was no better than the use of gloves alone in preventing VRE colonization.
A recent cluster randomized trial compared the effect of universal PPE (ie, gowning and gloving) with usual care for reducing acquisition of MRSA or VRE as a composite outcome [103]. The study did not find that universal gowning and gloving reduced VRE or MRSA acquisition but found a 40% decline in MRSA acquisition in the intervention ICUs compared with baseline rates of MRSA. No major adverse effects of universal gowning and gloving were noted in this study. A thoughtful editorial commenting on this article proposes that several aspects of the study deserve consideration, including the possibility of false-negative screening tests for VRE, which may have partially accounted for the negative primary outcome [69].
Based on these studies, it appears that the use of barrier precautions may be of value more for MRSA than VRE but further studies are needed to examine its impact on other types of pathogens, including new and emerging MDROs. Until further evidence becomes available, routine gowning and gloving may be of value in units with a high prevalence of MRSA.
Environmental Cleaning
Accumulating data suggests that the environment may play a major role in transmission of pathogens. MRSA has the ability to survive for days to weeks on inanimate objects [104–107]. Environmental contamination results in contamination of staff clothing and gloves [107,108] and is highly correlated with colonization strains among inpatients [109]. Although some studies of enhanced cleaning techniques and increased environmental services staff time have demonstrated reductions in MRSA outbreaks [110–112], the results are not universally favorable [113,114] and further studies are needed to examine the impact of environmental cleaning on rates of MRSA colonization or infection.
Several studies have implicated contaminated equipment as vectors for transmission of VRE during outbreaks [115–117], but the direction of fomite transfer from patient to environment has been difficult to ascertain. VRE have been found frequently on a variety of inanimate objects and surfaces in different health care environments [118–123], including gloved or ungloved hands of health care workers [101,124,125]. Hayden et al [126] determined the effect of improved environmental cleaning on VRE acquisition rates. This study was a pre-and-post intervention study carried out in a 21-bed medical intensive care unit (MICU) in a tertiary hospital over several phases. The intervention included the creation of a unique and improved cleaning program, as well as in-services to housekeeper services, education of the MICU staff, and a hand hygiene campaign. The results of the study showed decreased acquisition of VRE from 33.47 cases per 1000 patient days at risk in period 1 to 10.40 cases per 1000 patient-days at risk by period 4 of the study. Increased environmental cleaning was also associated with reduced growth of VRE from environmental cultures. At baseline, weekly contamination rates were 0.15 and 0.1 for samples obtained before and after cleaning, respectively. Culture positivity decreased to 0.07 and 0.04 for before and after cleaning in period 2 and then remained at low levels during the remainder of the study. It is important to note that the method for disinfecting used in this study was the “bucket method” as promoted by Byers [127]. This study provides further support for the importance of an environmental reservoir and of environmental decontamination to prevent endemic cross-transmission of VRE [126].
Goodman et al [128] used similar interventions but added a feedback tool using a black-light monitoring system (ie, use of an invisible, nontoxic marker to delineate areas that are adequately or inadequately cleaned) to reduce the likelihood of isolating either MRSA or VRE from an ICU environment. This study also showed favorable results, and notably, the use of the black-light monitoring system identified specific areas that were typically inadequately disinfected. Results showed that flat, horizontal surfaces (eg, countertops, bedside tray tables, and hamper tops) were adequately cleaned more often than small, vertical surfaces (eg, doorknobs, toilet handles, light switches, and electronics).
Part of the controversy surrounding the impact of environmental cleaning is the difficulty in determining its individual value as part of an overall infection control bundle [129]. A proposed area of demonstrable impact for environmental cleaning are frequently touched sites which are more likely to be contaminated with pathogens. Focusing on these “hot-bed” areas of the care environment may offer a useful adjunct to other infection control measures [129].
Active Surveillance
Active surveillance refers to periodic screening for asymp-tomatic carriers followed by placement of colonized patients in contact isolation. This practice is highly variable across institutions, as the evidence supporting this practice is conflicting and there are concerns about the cost of implementing this approach without solid evidence [70,130,131]. Despite lack of randomized controlled trials to guide this practice for MRSA prevention, many hospitals utilize MRSA surveillance and it is mandated by law in 9 states [132,133].
A prospective, interventional cohort study of universal MRSA screening on admission to surgical wards failed to reduce nosocomial MRSA infections [134]. Most recently, a pragmatic, cluster-randomized ICU trial reported that universal decolonization with chlorhexidine wipes and mupirocin use was more effective than screening and isolation in reducing rates of MRSA clinical isolates [65]. However, concerns regarding the risk of mupirocin resistance have been expressed [135,136]. The only randomized trial that compared active surveillance for MRSA and VRE followed by contact precautions to usual care did not find a benefit to active surveillance.
Huskins et al [137], in a large, cluster-randomized trial of 19 ICUs from different hospitals, determined the utility of using a culture-based active surveillance and contact isolation, compared with usual care (contact isolation for patients colonized with MRSA or VRE) as identified by existing hospital protocols, to reduce the incidence of colonization or infection with MRSA or VRE. In this trial, which spanned 6 months and involved 3488 participants, the authors found no significant difference between the intervention and control ICUs in terms of MRSA and VRE colonization or infection rates.
Conflicting with these findings is an observational study comparing MRSA infection rates before and after institution of a universal screening protocol, which demonstrated a 69.6% (CI, –89.2% to –19.6%]; P = 0.03) reduction in hospital wide MRSA prevalence density with screening [138]. The “MRSA bundle” implemented in 2007 at VA hospitals nationwide, which included universal screening, produced a 62% (P < 0.001) reduction in MRSA ICU infections; the relative contribution of the various bundle components is uncertain [139,140].
A proposed cost-saving alternative to universal screening is selective screening based on risk factor assessment [141]. The effectiveness of this type of program depends on creating a clinical decision-making tool capable of accurately identifying high-risk individuals while also accounting for the different risk factor profiles between HA-MRSA and CA-MRSA [142]. It has been proposed that targeted screening protocols may be more cost-effective in settings with < 5% prevalence of MRSA colonization on admission [143].
Many studies [61,144–149] have shown that active surveillance against VRE is cost-effective. For example, Calfee et al [144] showed that an established active surveillance program results in control of endemic VRE in high-risk patients. The infection control program was established in response to a hospital-wide VRE outbreak, and was sustained after the outbreak was controlled. The study by Calfee et al spanned 5 years and was performed at a tertiary-level university hospital, where cultures from perirectal areas were used to identify high-risk patients who were asymptomatically colonized with VRE. During the latter 2 years, 768 new cases of VRE colonization were detected among 69,672 admissions (1.1% of admissions), of which 730 (95.1%) were identified by active surveillance methods. This implies that routine clinical cultures would probably have missed the majority of colonized patients. During this period, the incidence of VRE infection was likewise extremely low at 0.12/1000 patient days (ie, 90 nosocomial VRE infections were identified in 83 patients during 743,956 days of patient care). Sixty-nine of the 83 patients (83%) who developed nosocomial VRE infections were found to be colonized with VRE by surveillance culture before the onset of infection.
Patient Decolonization
Chlorhexidine gluconate has been used in several settings to control outbreaks and infections related to MRSA and VRE due to its broad-spectrum activity against these pathogens. Chlorhexidine-based solutions reduce the density of skin colonization with pathogens such as MRSA and VRE (skin asepsis), thus lowering the risk for horizontal transmission between health care workers and patients.
Decolonization with chlorhexidine as an MRSA infection reduction technique has demonstrated benefit in the ICU setting [150]. The previously mentioned large, cluster-randomized ICU trial by Huang and colleagues found universal decolonization with twice-daily intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the entire ICU stay was superior to targeted decolonization of known MRSA carriers in preventing overall MRSA isolates. However, universal decolonization failed to show a reduction in MRSA bacteremia [151], and concerns about mupirocin resistance may limit the applicability of this approach.
There are now several studies [152–154] that show decreased acquisition of VRE with use of daily chlorhexidine bathing. In a study including 1787 ICU patients, Vernon et al found [154] that the reducing microbial density of VRE on patient’s skin by using chlorhexidine led to decreased transmission. In another study by Climo et al [153] that involved 6 ICUs at 4 academic centers and measured the incidence of MRSA and VRE colonization and blood stream infections (BSI) during a period of bathing with routine soap for 6 months compared with a 6-month period where all admitted patients received daily bathing with a chlorhexidine solution, results found decreased acquisition of VRE by 50% (4.35 vs. 2.19 cases/1000 patient days, P < 0.008) following the introduction of daily chlorhexidine bathing. Furthermore, compared with 16 of 270 patients colonized with VRE who subsequently developed VRE bacteremia at baseline, only 4 of 226 VRE-colonized patients bathed with chlorhexidine in the intervention period developed a BSI, translating into a relative risk reduction of 3.35 (95% CI, 1.13–9.87; P < 0.035). Patients colonized with VRE were 3 times less likely to develop VRE bacteremia when bathed with chlorhexidine compared with regular bathing. Despite the success of this protocol for VRE, when analyzed by individual organism no significant reductions in MRSA acquisition or BSI were reported. This finding is similarly corroborated by a trial conducted in the pediatric ICU setting which found an overall reduction in bacteremia with daily chlorhexidine washes but no significant decrease in cases due to S. aureus [155].
The results of these studies suggest that daily bathing with chlorhexidine should be part of routine practice in health care, especially in ICUs where endemic MRSA or VRE rates are high. Whether there is benefit in other settings needs to be studied.
In addition to chlorhexidine washes, other decolonization techniques have been proposed to reduce colonization and the spread of HAIs in the ICU setting. A randomized controlled trial of daily 5% tea tree oil body washes for the prevention of MRSA colonization failed to significantly reduce rates compared to standard soap body washes [156]. Another proposed decolonization intervention that has not been widely adopted in the United States due to concerns related to development of resistant organisms is selective digestive decontamination (SDD) or selective oropharyngeal decontamination (SOD) with antimicrobial agents [157,158]. In terms of clinical benefit, SDD/SOD have been found to decrease MDRO infection rate [159] and mortality [160].
Cohorting
There is insufficient evidence to conclude that cohorting isolated patients is of benefit for routine use in the endemic ICU setting. A few studies, mainly in the outbreak setting, have examined this approach and the results are conflicting [161,162]. Pending further studies in this area, it is reasonable to cohort patients colonized with the same microorganisms, especially if patients cannot be placed in single rooms.
CONCLUSION
The emergence of MRSA and VRE has led to a resurgence of interest and emphasis on infection control practices and prevention. CDC guidelines to help health care practitioners manage these MDROs in the hospital and ICU-setting exist; however, many questions remain regarding best practice.
Prevention of MRSA and VRE needs to be a 2-pronged approach—antimicrobial stewardship [163] and infection control. A robust antimicrobial stewardship program to optimize and minimize inappropriate antibiotic use is necessary in every institution. From the infection prevention standpoint, it is unclear if systematic identification of MRSA and VRE colonization followed by contact precautions is useful in reducing transmission. It is clear that a strong institutional climate of promoting patient safety and a culture of infection prevention will help in reducing MRSA and VRE facility-wide. It also appears that universal gowning and gloving may be useful for reducing MRSA, but not VRE, transmission. While universal decolonization with mupirocin is efficacious in reducing MRSA, this strategy is not recommended because of promoting mupirocin resistance. However, the use of daily bathing with chlorhexidine represents a relatively low-cost, high-yield intervention that should be adopted. Pending further data, patients known to be colonized or infected with MRSA should be placed in contact precuations as is current practice in most institutions. Finally, in this era of MDROs, hand hygiene remains our best defense against the spread of pathogens in the health care environment.
Note: This article does not represent the views of the Department of Veterans Affairs.
Corresponding author: Nasia Safdar, MD, Willam S. Middleton Memorial Veterans Affairs Hospital, 2500 Overlook Terrace, Madison, WI 53705, [email protected].
Funding/support: This work is funded by a MERIT award from the Department of Veterans Affairs to Nasia Safdar.
Financial disclosures: None.
REFERENCES
1. Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 2009;301:727–36.
2. LeDell K, Muto CA, Jarvis WR, Farr BM. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 2003;24:639–41.
3. Giske CG, Monnet DL, Cars O, Carmeli Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 2008;52:813–21.
4. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Pub Health Rep 2007;122:160–6.
5. Schwaber MJ, Carmeli Y. The effect of antimicrobial resistance on patient outcomes: importance of proper evaluation of appropriate therapy. Crit Care 2009;13:106.
6. Grundmann H, Hori S, Winter B, et al. Risk factors for the transmission of methicillin-resistant Staphylococcus aureus in an adult intensive care unit: fitting a model to the data. J Inf Dis 2002;185:481–8.
7. Huang SS, Rifas-Shiman SL, Warren DK, et al. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J Infect Dis 2007;195:330–8.
8. Muder RR, Cunningham C, McCray E, et al. Implementation of an industrial systems-engineering approach to reduce the incidence of methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2008;29:702–8.
9. Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011.
10. Pelz RK, Lipsett PA, Swoboda SM, et al. Vancomycin-sensitive and vancomycin-resistant enterococcal infections in the ICU: attributable costs and outcomes. Intensive Care Med 2002;28:692–7.
11. Warren DK, Kollef MH, Seiler SM, et al. The epidemiology of vancomycin-resistant Enterococcus colonization in a medical intensive care unit. Infect Control Hosp Epidemiol 2003;24:257–63.
12. Kohlenberg A, Schwab F, Meyer E, et al. Regional trends in multidrug-resistant infections in German intensive care units: a real-time model for epidemiological monitoring and analysis. J Hosp Infect 2009;73:239–45.
13. Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol 2008;8:747–63.
14. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008;46 Suppl 5:S350–9.
15. Zhang K, McClure JA, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009;53:531–40.
16. Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol 2013;5:205–17.
17. Otto M. MRSA virulence and spread. Cell Microbiol 2012;14:1513–21.
18. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978–84.
19. Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 2009;106:5883–8.
20. Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 2006;42 Suppl 1:S25–34.
21. Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 2001;33:210–9.
22. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012;10:266–78.
23. Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis 1998;178:577–80.
24. Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 2004;113:e347–52.
25. Groom AV, Wolsey DH, Naimi TS, et al. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 2001;286:1201–5.
26. Hewlett AL, Falk PS, Hughes KS, Mayhall CG. Epidemiology of methicillin-resistant Staphylococcus aureus in a university medical center day care facility. Infect Control Hosp Epidemiol 2009;30:985–92.
27. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468–75.
28. Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA 2012;308:50–9.
29. Lindenmayer JM, Schoenfeld S, O’Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med 1998;158:895–9.
30. Malcolm B. The rise of methicillin-resistant staphylococcus aureus in U.S. correctional populations. J Correct Health Care 2011;17:254–65.
31. Nerby JM, Gorwitz R, Lesher L, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2011;30:927–32.
32. Stemper ME, Shukla SK, Reed KD. Emergence and spread of community-associated methicillin-resistant Staphylococcus aureus in rural Wisconsin, 1989 to 1999. J Clin Microbiol 2004;42:5673–80.
33. Turabelidze G, Lin M, Wolkoff B, et al. Personal hygiene and methicillin-resistant Staphylococcus aureus infection. Emerg Infect Dis 2006;12:422–7.
34. Ellis MW, Hospenthal DR, Dooley DP, et al. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004;39:971–9.
35. Forster AJ, Oake N, Roth V, et al. Patient-level factors associated with methicillin-resistant Staphylococcus aureus carriage at hospital admission: a systematic review. Am J Infect Control 2013;41:214–20.
36. McKinnell JA, Miller LG, Eells SJ, et al. A systematic literature review and meta-analysis of factors associated with methicillin-resistant staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Contol Hosp Epidemiol 2013;34:1077–86.
37. Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med 2006;166:580–5.
38. Jernigan JA, Pullen AL, Flowers L, et al. Prevalence of and risk factors for colonization with methicillin-resistant Staphylococcus aureus at the time of hospital admission. Infect Control Hosp Epidemiol 2003;24:409–14.
39. Horner C, Parnell P, Hall D, Kearns A, Heritage J, Wilcox M. Meticillin-resistant Staphylococcus aureus in elderly residents of care homes: colonization rates and molecular epidemiology. J Hosp Infect 2013;83:212–8.
40. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009;73:305–15.
41. Boyce JM. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect Dis Clin North Am 1989;3:901–13.
42. Jernigan JA. Methicillin-resistant Staphylococcus aureus colonization among health care personnel in the emergency department: what does it tell us? Ann Emerg Med 2008;52:534–6.
43. Carnicer-Pont D, Bailey KA, Mason BW, Walker AM, Evans MR, Salmon RL. Risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a case-control study. Epidemiol Infect 2006;134:1167–73.
44. Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 2002;49:999–1005.
45. Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med 1982;97:309–17.
46. Davis KA, Stewart JJ, Crouch HK,et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004;39:776–82.
47. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997;10:505–20.
48. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med 2008;121:310–5.
49. Wertheim HFL, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
50. Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 2001;22:687–92.
51. Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol;31:584–91.
52. von Eiff C, Becker K, Machka K, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001;344:11–6.
53. Pujol M, Pea C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med 1996;100:509–16.
54. Rocha LA, Marques Ribas R, da Costa Darini AL, Gontijo Filho PP. Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am J Infect Control 2013;41:236–40.
55. Corne P, Marchandin Hln, Jonquet O, Campos J, Bauls A-L. Molecular evidence that nasal carriage of Staphylococcus aureus plays a role in respiratory tract infections of critically ill patients. J Clin Microbiol 2005;43:3491–3.
56. Quezada Joaquin NM, Diekema DJ, Perencevich EN, et al. Long-term risk for readmission, methicillin-resistant Staphylococcus aureus (MRSA) infection, and death among MRSA-colonized veterans. Antimicrob Agents Chemother 2013;57:1169–72.
57. Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococcus: recognition and prevention in intensive care units. Crit Care Med 2010;38:S335–44.
58. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002;8:802–7.
59. Ostrowsky BE, Venkataraman L, D’Agata EM, et al. Vancomycin-resistant enterococci in intensive care units: high frequency of stool carriage during a non-outbreak period. Arch Intern Med 1999;159:1467–72.
60. Bonten MJ, Hayden MK, Nathan C, et al. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet 1996;348:1615–9.
61. Ostrowsky BE, Trick WE, Sohn AH, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med 2001;344:1427–33.
62. Padiglione AA, Wolfe R, Grabsch EA, et al. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob Agents Chemother 2003;47:2492–8.
63. Batistao DW, Gontijo-Filho PP, Conceicao N, et al. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem Inst Oswaldo Cruz 2012;107:57–63.
64. Furtado GH, Martins ST, Coutinho AP, et al. Prevalence and factors associated with rectal vancomycin-resistant enterococci colonization in two intensive care units in Sao Paulo, Brazil. Braz J Infect Dis 2005;9:64–9.
65. Huang SS, Datta R, Rifas-Shiman S, et al. Colonization with antibiotic-susceptible strains protects against methicillin-resistant Staphylococcus aureus but not vancomycin-resistant enterococci acquisition: a nested case-control study. Crit Care 2011;15:R210.
66. Pan SC, Wang JT, Chen YC, et al. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One 2012;7:e47297.
67. Se YB, Chun HJ, Yi HJ, et al. Incidence and risk factors of infection caused by vancomycin-resistant enterococcus colonization in neurosurgical intensive care unit patients. J Korean Neurosurg Soc 2009;46:123–9.
68. Healthcare Infection Control Practices Advisory Committee (HICPAC). Management of multidrug-resistant organisms in healthcare settings, 2006. Accessed 11 Oct 2013 at www.cdc.gov/hicpac/mdro/mdro_toc.html.
69. Malani PN. Preventing infections in the ICU: one size does not fit all. JAMA 2013;310:1567–8.
70. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 1995;44:1–13.
71. de Bruin MA, Riley LW. Does vancomycin prescribing intervention affect vancomycin-resistant enterococcus infection and colonization in hospitals? A systematic review. BMC Infect Dis 2007;7:24.
72. Adachi W, Bolding F, Armstrong R. Experience with vancomycin education and order sheet to limit vancomycin use. Hosp Pharm 1997:1370–3.
73. Fridkin SK, Lawton R, Edwards JR, et al. Monitoring antimicrobial use and resistance: comparison with a national benchmark on reducing vancomycin use and vancomycin-resistant enterococci. Emerg Infect Dis 2002;8:702–7.
74. Guglielmo BJ, Dudas V, Maewal I, et al. Impact of a series of interventions in vancomycin prescribing on use and prevalence of vancomycin-resistant enterococci. Jt Comm J Qual Patient Saf 2005;31:469–75.
75. Lautenbach E, LaRosa LA, Marr AM, et al. Changes in the prevalence of vancomycin-resistant enterococci in response to antimicrobial formulary interventions: impact of progressive restrictions on use of vancomycin and third-generation cephalosporins. Clin Infect Dis 2003;36:440–6.
76. Morgan AS, Brennan PJ, Fishman NO. Impact of a vancomycin restriction policy on use and cost of vancomycin and incidence of vancomycin-resistant Enterococcus. Ann Pharmacother 1997;31:970–3.
77. Anglim AM, Klym B, Byers KE, et al. Effect of a vancomycin restriction policy on ordering practices during an outbreak of vancomycin-resistant Enterococcus faecium. Arch Intern Med 1997;157:1132–6.
78. Montecalvo MA, Jarvis WR, Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med 1999;131:269–72.
79. Morris JG Jr, Shay DK, Hebden JN, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med 1995;123:250–9.
80. Quale J, Landman D, Saurina G, et al. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis 1996;23:1020-5.
81. Rubin LG, Tucci V, Cercenado E, et al. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol 1992;13:700–5.
82. Lai KK, Kelley AL, Melvin ZS, et al. Failure to eradicate vancomycin-resistant enterococci in a university hospital and the cost of barrier precautions. Infect Control Hosp Epidemiol 1998;19:647–52.
83. Shaikh ZH, Osting CA, Hanna HA, et al. Effectiveness of a multifaceted infection control policy in reducing vancomycin usage and vancomycin-resistant enterococci at a tertiary care cancer centre. J Hosp Infect 2002;51:52–8.
84. Lafaurie M, Porcher R, Donay JL, et al. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother 2012;67:1010–5.
85. Parienti JJ, Cattoir V, Thibon P, et al. Hospital-wide modification of fluoroquinolone policy and meticillin-resistant Staphylococcus aureus rates: a 10-year interrupted time-series analysis. J Hosp Infect 2011;78:118–22.
86. Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: a systematic review. Crit Care Med 2008;36:933–40.
87. Boyce JM. It is time for action: improving hand hygiene in hospitals. Ann Intern Med 1999;130:153–5.
88. Jackson M, Chiarello LA, Gaynes RP, Gerberding JL. Nurse staffing and healthcare-associated infections: proceedings from a working group meeting. J Nurs Adm 2002;32:314–22.
89. Kuzu N, Ozer F, Aydemir S, et al. Compliance with hand hygiene and glove use in a university-affiliated hospital. Infect Control Hosp Epidemiol 2005;26:312–5.
90. Larson E, Killien M. Factors influencing handwashing behavior of patient care personnel. Am J Infect Control 1982;10:93–9.
91. Larson E, Kretzer EK. Compliance with handwashing and barrier precautions. J Hosp Infect 1995;30 Suppl:88–106.
92. Naikoba S, Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers - a systematic review. J Hosp Infect 2001;47:173–80.
93. Pittet D, Simon A, Hugonnet S, et al. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med 2004;141:1–8.
94. Trick WE, Vernon MO, Welbel SF, et al. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infect Control Hosp Epidemiol 2007;28:42–9.
95. Wisniewski MF, Kim S, Trick WE, et al. Effect of education on hand hygiene beliefs and practices: a 5-year program. Infect Control Hosp Epidemiol 2007;28:88–91.
96. Rupp ME, Fitzgerald T, Puumala S, et al. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol 2008;29:8–15.
97. Venkatesh AK, Lankford MG, Rooney DM, et al. Use of electronic alerts to enhance hand hygiene compliance and decrease transmission of vancomycin-resistant Enterococcus in a hematology unit. Am J Infect Control 2008;36:199–205.
98. Silvestri L, Petros AJ, Sarginson RE, et al. Handwashing in the intensive care unit: a big measure with modest effects. J Hosp Infect 2005;59:172–9.
99. Akyol A, Ulusoy H, Ozen I. Handwashing: a simple, economical and effective method for preventing nosocomial infections in intensive care units. J Hosp Infect 2006;62:395–405.
100. Simmons B, Bryant J, Neiman K, et al. The role of handwashing in prevention of endemic intensive care unit infections. Infect Control Hosp Epidemiol 1990;11:589–94.
101. Tenorio AR, Badri SM, Sahgal NB, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis 2001;32:826–9.
102. Slaughter S, Hayden MK, Nathan C, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med 1996;125:448–56.
103. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 2013;310:1571–80.
104. Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect 2001;49:255–61.
105. Hardy KJ, Oppenheim BA, Gossain S, et al. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect Control Hosp Epidemiol 2006;27:127–32.
106. Jawad A, Heritage J, Snelling AM, et al. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol 1996;34:2881–7.
107. Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol 2007;28:1142–7.
108. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
109. Sexton T, Clarke P, O’Neill E, et al. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect 2006;62:187–94.
110. Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet infect dis 2008;8:101–13.
111. Dancer SJ, White LF, Lamb J, et al. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC med 2009;7.
112. Rampling A, Wiseman S, Davis L, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 2001;49:109–16.
113. Wilson APR, Smyth D, Moore G, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med 2011;39:651–8.
114. Hess AS, Shardell M, Johnson JK, et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infection Control Hosp Epidemiol 2013;34:487–93.
115. Falk PS, Winnike J, Woodmansee C, et al. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol 2000;21:575–82.
116. Livornese LL Jr, Dias S, Samel C, et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med 1992;117:112–6.
117. Porwancher R, Sheth A, Remphrey S, et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol 1997;18:771–3.
118. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000;343:1925–32.
119. Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol 2000;38:724–6.
120. Noskin GA, Bednarz P, Suriano T, et al. Persistent contamination of fabric-covered furniture by vancomycin-resistant enterococci: implications for upholstery selection in hospitals. Am J Infect Control 2000;28:311–3.
121. Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol 1995;16:577–81.
122. Smith TL, Iwen PC, Olson SB, Rupp ME. Environmental contamination with vancomycin-resistant enterococci in an outpatient setting. Infect Control Hosp Epidemiol 1998;19:515–8.
123. Wendt C, Wiesenthal B, Dietz E, Ruden H. Survival of vancomycin-resistant and vancomycin-susceptible enterococci on dry surfaces. J Clin Microbiol 1998;36:3734–6.
124. Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol 2004;25:164–7.
125. Ray AJ, Hoyen CK, Taub TF, et al. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA 2002;287:1400–1.
126. Hayden MK, Bonten MJ, Blom DW, et al. Reduction in acquisition of vancomycin-resistant enterococcus after enforcement of routine environmental cleaning measures. Clin Infect Dis 2006;42:1552–60.
127. Byers KE, Durbin LJ, Simonton BM, et al. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol 1998;19:261–4.
128. Goodman ER, Platt R, Bass R, et al. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care unit rooms. Infect Control Hosp Epidemiol 2008;29:593–9.
129. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
130. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA) infections. Accessed 11 Oct 2013 at www.cdc.gov/mrsa/index.html.
131. Edmond MB, Wenzel RP. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1471.
132. Lai KK, Fontecchio S, Melvin Z, Baker SP. Impact of alcohol-based, waterless hand antiseptic on the incidence of infection and colonization with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Infect Control Hosp Epidemiol 2006;27:1018–24.
133. Ostrowsky B, Steinberg JT, Farr B, et al. Reality check: should we try to detect and isolate vancomycin-resistant enterococci patients? Infect Control Hosp Epidemiol 2001;22:116–9.
134. Harbarth S, Sax H, Uckay I, et al. A predictive model for identifying surgical patients at risk of methicillin-resistant Staphylococcus aureus carriage on admission. J Am Coll Surg 2008;207:683–9.
135. Jarvis WR. Targeted decolonization to prevent ICU infections. N Engl J Med 2013;369:1469.
136. Krause R, Honigl M, Zollner-Schwetz I. Targeted decolonization to prevent ICU infections. N Engl J Med;369:1469–70.
137. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med;364:1407–18.
138. Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148:409–18.
139. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
140. Gurieva T, Bootsma MCJ, Bonten MJM. Successful Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections revisited. Clin Infect Dis 2012:54:1618–20.
141. Gavalda L, Masuet C, Beltran J, et al. Comparative cost of selective screening to prevent transmission of methicillin-resistant Staphylococcus aureus (MRSA), compared with the attributable costs of MRSA infection. Infection control and hospital epidemiology 2006;27:1264–6.
142. Otter JA, Herdman MT, Williams B, et al. Low prevalence of methicillin-resistant Staphylococcus aureus carriage at hospital admission: implications for risk-factor-based vs universal screening. J Hosp Infect 2013;83:114–21.
143. Harbarth S, Hawkey PM, Tenover F, et al. Update on screening and clinical diagnosis of methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 2011;37:110–7.
144. Calfee DP, Giannetta ET, Durbin LJ, et al. Control of endemic vancomycin-resistant Enterococcus among inpatients at a university hospital. Clin Infect Dis 2003;37:326–32.
145. Hendrix CW, Hammond JM, Swoboda SM, et al. Surveillance strategies and impact of vancomycin-resistant enterococcal colonization and infection in critically ill patients. Ann Surg 2001;233:259–65.
146. Muto CA, Giannetta ET, Durbin LJ, et al. Cost-effectiveness of perirectal surveillance cultures for controlling vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2002;23:429–35.
147. Price CS, Paule S, Noskin GA, Peterson LR. Active surveillance reduces the incidence of vancomycin-resistant enterococcal bacteremia. Clin Infect Dis 2003;37:921–8.
148. Shadel BN, Puzniak LA, Gillespie KN, et al. Surveillance for vancomycin-resistant enterococci: type, rates, costs, and implications. Infect Control Hosp Epidemiol 2006;27:1068–75.
149. Siddiqui AH, Harris AD, Hebden J, et al. The effect of active surveillance for vancomycin-resistant enterococci in high-risk units on vancomycin-resistant enterococci incidence hospital-wide. Am J Infect Control 2002;30:40–3.
150. Sandri AM, Dalarosa MG, Ruschel de Alcantara L, et al. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol 2006;27:185–7.
151. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65.
152. Bleasdale SC, Trick WE, Gonzalez IM, et al. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–9.
153. Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009;37:1858–65.
154. Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med 2006;166:306–12.
155. Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013;381:1099–106.
156. Blackwood B, Thompson G, McMullan R, et al. Tea tree oil (5%) body wash versus standard care (Johnson’s Baby Softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: a randomized controlled trial. J Antimicrob Chemother 2013;68:1193–9.
157. Daneman N, Sarwar S, Fowler RA, et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:328–41.
158. Verwaest C, Verhaegen J, Ferdinande P, et al. Randomized, controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multidisciplinary intensive care unit. Crit Care Med 1997;25:63–71.
159. de Smet AMGA, Kluytmans JAJW, Blok HEM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis 2011;11:372–80.
160. de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003;362:1011–6.
161. Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet 2005;365:295–304.
162. Dhaliwal J, McGeer A. Does isolation prevent the spread of methicillin-resistant Staphylococcus aureus? CMAJ 2005;172:875.
163. Kollef MH, Micek ST. Antimicrobial stewardship programs: mandatory for all ICUs. Crit Care 2012;16:179.
164. McKinnell JA, Huang SS, Eells SJ, et al. Quantifying the impact of extranasal testing of body sites for methicillin-resistant Staphylococcus aureus colonization at the time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 2013;34:161–70.
165. Denkinger CM, Grant AD, Denkinger M, et al. Increased multi-drug resistance among the elderly on admission to the hospital—a 12-year surveillance study. Arch Gerontol Geriatr 2013;56:227–30.
166. Boisseau D, Alfandari S, Gauzit R, et al. Staphylococcus aureus nasal carriage during the infectious diseases national congress in France. Med Mal Infect 2012;42:435–9.
167. Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med 2012;166:551–7.
168. Rafee Y, Abdel-Haq N, Asmar B, et al. Increased prevalence of methicillin-resistant Staphylococcus aureus nasal colonization in household contacts of children with community acquired disease. BMC Infect Dis 2012;12:45.
169. Schechter-Perkins EM, Mitchell PM, Murray KA, et al. Prevalence and predictors of nasal and extranasal staphylococcal colonization in patients presenting to the emergency department. Ann Emerg Med 2011;57:492–9.
170. Bisaga A, Paquette K, Sabatini L, Lovell E. A prevalence study of methicillin-resistant staphylococcus aureus colonization in emergency department health care workers. Ann Emerg Med 2008;52:525–8.
171. Suffoletto B, Cannon E, Ilkhanipour K, Yealy D. Prevalence of Staphylococcus aureus nasal colonization in emergency department personnel. Ann Emerg Med 2008;52:529–33.
172. Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg 2004;139:947-51; discussion 51–3.
173. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 2003;36:131–9.
Empathic Disclosure of Adverse Events to Patients
In 1987, the chief of staff of the Lexington VAMC and the staff attorney for the VA Regional Counsel Office in Lexington, Kentucky, discovered that a recent patient death was due to a mistake made in the medical care provided at their facility. They decided to disclose what happened to the family who had no knowledge of this mistake in care because “it was the right thing to do.”
The Lexington Model for disclosure, as it became known worldwide, continued to flourish under the leadership of Kraman and Hamm.1,2 The VA National Center for Ethics in Health Care adopted these principles of disclosure in drafting a national VHA policy directive in 2008, which was updated in 2012.3 However, despite the ethical and professional imperatives, disclosing adverse events (AEs) to patients and family members has continued to be one of the most difficult challenges in the practice of medicine.
VHA policy has made a distinction between clinical disclosure, conducted by a clinician with a patient as a routine professional practice, and institutional disclosure, conducted by institutional leadership for an AE rising above a threshold of serious patient harm. According to VHA Director of Risk Management Yuri Walker in a 2013 personal communication, the frequency of institutional disclosure reports from VAMCs since 2011 have reflected significant variation in disclosure practice among facilities of similar size and complexity.
In this report, the authors share their experience developing and delivering a simulation-based disclosure training program in the VHA intended to close the gap between policy expectations and practical challenges for providers and institutions when facing the task of disclosing an AE to patients and families.
Medical Error Disclosure
It is not difficult to understand why health care providers (HCPs) are uncomfortable about disclosing AEs to patients. The study by Delbanco and Bell describes physicians experiencing guilt, shame, and fear of retribution after a patient experiences an AE. The resulting silence and avoidance of the patient only compounds patient harm.4 Many HCPs believe disclosure will lead to tort claims, provide evidence against their defense, encourage reporting to the National Practitioners’ Databank, and damage their reputations with a potentially negative impact on their careers.5-7
In a 2009 survey of 1,891 practicing physicians in the U.S., one-third did not agree with disclosing serious medical errors to patients.8 Another survey of physicians reported wide variations in responses about whether they would offer an apology after making a medical mistake.9 Therefore, a gap between patient expectations and HCP communication when a medical mistake occurs should be expected.10
Few HCPs receive training in empathic communication skills for effective disclosure of AEs to patients and families.11 In a survey of 3,171 physicians in the U.S. and Canada, Waterman and colleagues found that only 10% of physicians believed they had adequate support from their health care organizations (HCOs) after an AE occurred, even though 86% expressed significant interest in receiving training on the disclosure of AEs.12 Despite this gap, some medical educators, such as Katie Watson at Northwestern University, are successfully demonstrating the power of teaching medical students improvisational acting skills to enhance professionalism and communication in future physician–patient interaction.13
Disclosure Training Program
In 2010, the Lexington VAMC was awarded a 3-year VA Systems Improvement Capability Grant, which funded the development of a Disclosure Training Program (DTP). A team of investigators designed a 2-day workshop based on principles of experiential learning. Each workshop incorporated interactive teaching techniques using filmed clinical vignettes to provide a context for facilitated small-group disclosure simulations with professional actors.14 A total of 14 workshops were conducted for 346 participants from December 2011 to September 2012.
The DTP workshop integrates focused didactic sessions with interactive audience-workshop facilitator discussion, debriefing of teaching films, and disclosure simulations, with the majority of time spent the conducting and debriefing of simulations. Core content addressed during workshop activities included the following:
1. Historical origins of disclosure policy at the VHA
2. Ethical obligation, professional duty, and legal mandates for disclosure
3. Empathic communication–cognitive and emotive
4. VHA Handbook 1004.08, Disclosure of Adverse Events to Patient
5. Institutional and Clinical Disclosure of AEs
6. Psychological and physical needs of patients after an AE
7. Disclosure linking risk management to patient safety in a health care system
8. Legal implications for disclosure
9. State apology laws
10. Implementing disclosure programs in health care facilities
11. Facility support for providers after a patient AE
The principles of empathic communication and the core elements of AE disclosure to patients are reinforced during small-group simulations with actors portraying patients or family members. Each small-group simulation typically involves 3 to 4 workshop participants and 1 to 2 actors. Participants are given the task of conducting a clinical or institutional disclosure.
A facilitator manages each simulation, based on a scripted scenario or teaching film viewed by workshop participants. In the simulations attendees assume the roles of hospital staff that might be realistically involved in disclosure conversations, including executive leaders, physicians, nurses, risk managers, pharmacists, chaplains, and social workers.
Simulations average 5 to 7 minutes and are followed by a debriefing, including simulation participants, workshop facilitators, and the professional actor, who remain in character. By the end of each 2-day workshop, all attendees have participated in multiple small-group simulations of both clinical and institutional disclosures. Pre- and postworkshop knowledge questions and program evaluation data are collected with immediate-response polling technology used throughout the workshop.
Between 20 and 40 HCPs attended each workshop, which was designed for clinical and administrative leaders as well as others supporting the disclosure process, such as nurse managers, patient safety managers, social workers, chaplains, and pharmacists. The facility director, chief of staff, risk manager, and lawyers from the Regional Counsel office all play an important role in institutional disclosures and all were strongly encouraged to attend. The DTP facilitators observed the importance of senior executive leadership—participation, which enhanced dialogue in the large group sessions and small-group simulation-based learning.
DTP Workshop Results
Fourteen workshops were conducted for 346 employees from 26 VAMCs in 2012. Audience response technology was used to elicit participant feedback regarding workshop quality and effectiveness. Additional questions were asked as a pre/post-test of subject matter knowledge. Following the workshop, the participants showed a 30% overall improvement over preworkshop tests (Table), and 95% of participants favorably rated the workshop for quality and effectiveness.
There was a positive association between workshops with facility directors and actively engaged chiefs of staff in attendance and higher improvement scores in the test of knowledge. Among the top 7 performers on this test, 6 were individual facilities hosting the workshops and 1 VISN hosting for several facility representatives. Eleven of the 14 workshops with these characteristics (3 of which included VISN directors) evidenced more than 20% improvement on the test knowledge. These findings confirmed the original program design intended for individual facilities with leadership in attendance.
Iterative improvements were made to the program throughout 2012 based on feedback from workshop attendees, the National Office of Risk Management, the National Center for Ethics in Health Care and participating VA facilities and VISNs.
Despite these encouraging results, the DTP has some significant limitations: It is expensive, labor intensive, and dependent on faculty with expertise in clinical medicine, bioethics, and the law. Considering tight federal budgets, justifying the expenses to host a training program is difficult for a VAMC compared with that of other spending priorities. The actual and opportunity costs of travel to host sites for several facilitators and a group of professional actors to conduct a 2-day workshop for busy HCPs is not trivial.
Another limitation is the use of immediate response technology for data collection. Although this method maximizes response rates and seems to keep attendees engaged in presentations and discussions, technical failures could result in dropped responses, and ultimately the choice to respond is dependent on participant willingness to use the device.
Conclusion
Encouraging results suggest a bright future for the DTP, which has relevance for any health care organization, including the VA, academic affiliates, or those in the private sector. Wherever health care is delivered, providers will have the difficult task of disclosing AEs to meet their duty of care when patients experience harm. Learning empathic communication skills and successful strategies for disclosure will enhance this interaction and contribute to the maintenance of trust that is critical to the provider–patient relationship.
The DTP workshop has a flexible design and can be packaged to accommodate host medical centers for workshops of 1 to 2 days’ duration. The didactic presentations are constant, whereas the number of simulations will vary, depending on the length of the workshop (2-3 simulations for 1 day and 5-7 for two days). Participants from every workshop consistently cite that the simulations with professional actors are a powerful learning experience of significant personal value.
The DTP was developed as a unique, simulation-based program for clinicians, administrators, and allied health care personnel to enhance the effective disclosure of AEs to patients. Feedback from participants in 14 workshops in 2012 cited the value of the program with a high favorability rating. In a test of knowledge, participants also demonstrated an increase in learning. This feedback from the health care professionals who have attended the workshops has validated the pedagogic design of the program, which leverages adult learning principles of learning through experience. This approach was described by Aristotle in his best-known work on ethics, Nicomachean Ethics, “For the things we have to learn before we can do them, we learn by doing them.”15
Acknowledgements
For their significant contributions to the development and implementation of the VHA Disclosure Training Program, the authors thank Aaliyah Eaves-Leanos, Mary Duke, Lindsay Hall, and Uzair Munis. We thank the Institute for Healthcare Communication for their assistance in the program development. We express our utmost appreciation to Lee Taft for his many invaluable contributions to this program, including the critical role he continues to assume as a faculty member in the workshops. And, we are grateful for the continued contributions from our talented professional actors of Heyman Talent in Louisville, KY.
We express our sincere gratitude for their continuous feedback and important technical advice informing iterative improvements in the DTP workshops throughout 2012 from Virginia Ashby Sharpe (VA National Center for Ethics in Health Care); Yuri Walker (director of the Risk Management Program); and Barbara Rose (data analyst in the Risk Management Program), all at the VA central office in Washington, DC. And finally, we thank Heather Woodward-Hagg, Director of the VA Center for Applied Systems Engineering in Indianapolis, IN for her continued support in making DTP workshops available to VA Medical Centers throughout the country upon request.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Kraman SS, Hamm G. Risk management: Extreme honesty may be the best policy. Ann Intern Med. 1999;131(12):963-967.
2. Hamm GM, Kraman SS. New standards, new dilemmas: Reflections on managing medical mistakes. Bioethics Forum. 2001;17(2):19-25.
3. Veterans Health Administration. Disclosure of adverse events to patients. Handbook 100408. United States Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2800. Corrected copy October 12, 2012. Accessed April 3, 2014.
4. Delbanco T, Bell SK. Guilty, afraid, and alone—struggling with medical error. N Engl J Med. 2007;357(17):1682-1683.
5. Taft L. Apology and medical mistake: Opportunity or foil? Ann Health L. 2005;14:55-94.
6. Gallagher TH, Garbutt JM, Waterman AD, et al. Choosing your words carefully: How physicians would disclose harmful medical error to patients. Arch Intern Med. 2006;166(15):1585-1593.
7. Banja J. Medical Errors and Medical Narcissism. Sudbury, MD: Jones and Bartlett; 2005.
8. Iezzoni LI, Rao SR, DesRoches CM, Vogeli C, Campbell EG. Survey shows that at least some physicians are not always open or honest with patients. Health Aff (Millwood). 2012;31(2):383-391.
9. Gallagher TW, Waterman AD, Garbutt JM, et al. US and Canadian physicians’ attitudes and experiences regarding disclosure errors to patients. Arch Intern Med. 2006;166(15):1605-1611.
10. Robbennolt JK. Apologies and medical error. Clin Orthop Relat Res. 2009;467(2):376-382 White AA, Bell SK, Krauss MJ, et al. How trainees would disclose medical errors: Educational implications for training programmes. Med Educ. 201;45(4):372-380.
11. White AA, Bell SK, Krauss MJ, et al. How trainees would disclose medical errors: Educational implications for training programmes. Med Educ. 201;45(4):372-380.
12. Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467-476.
13. Watson K. Serious play: Teaching medical skills with improvisational theater techniques. Acad Med. 2011;86(10):1260-1265.
14. Eaves-Leanos A, Dunn EJ. Open disclosure of adverse events: Transparency and safety in healthcare. Surg Clin North Am. 2012;92(1):163-177.
15. Aristotle. Nichomachean Ethics (edited by Roger Crisp). Cambridge University Press, New York, NY (2012): Book II, Chapter 1 (1103b), p. 23.
In 1987, the chief of staff of the Lexington VAMC and the staff attorney for the VA Regional Counsel Office in Lexington, Kentucky, discovered that a recent patient death was due to a mistake made in the medical care provided at their facility. They decided to disclose what happened to the family who had no knowledge of this mistake in care because “it was the right thing to do.”
The Lexington Model for disclosure, as it became known worldwide, continued to flourish under the leadership of Kraman and Hamm.1,2 The VA National Center for Ethics in Health Care adopted these principles of disclosure in drafting a national VHA policy directive in 2008, which was updated in 2012.3 However, despite the ethical and professional imperatives, disclosing adverse events (AEs) to patients and family members has continued to be one of the most difficult challenges in the practice of medicine.
VHA policy has made a distinction between clinical disclosure, conducted by a clinician with a patient as a routine professional practice, and institutional disclosure, conducted by institutional leadership for an AE rising above a threshold of serious patient harm. According to VHA Director of Risk Management Yuri Walker in a 2013 personal communication, the frequency of institutional disclosure reports from VAMCs since 2011 have reflected significant variation in disclosure practice among facilities of similar size and complexity.
In this report, the authors share their experience developing and delivering a simulation-based disclosure training program in the VHA intended to close the gap between policy expectations and practical challenges for providers and institutions when facing the task of disclosing an AE to patients and families.
Medical Error Disclosure
It is not difficult to understand why health care providers (HCPs) are uncomfortable about disclosing AEs to patients. The study by Delbanco and Bell describes physicians experiencing guilt, shame, and fear of retribution after a patient experiences an AE. The resulting silence and avoidance of the patient only compounds patient harm.4 Many HCPs believe disclosure will lead to tort claims, provide evidence against their defense, encourage reporting to the National Practitioners’ Databank, and damage their reputations with a potentially negative impact on their careers.5-7
In a 2009 survey of 1,891 practicing physicians in the U.S., one-third did not agree with disclosing serious medical errors to patients.8 Another survey of physicians reported wide variations in responses about whether they would offer an apology after making a medical mistake.9 Therefore, a gap between patient expectations and HCP communication when a medical mistake occurs should be expected.10
Few HCPs receive training in empathic communication skills for effective disclosure of AEs to patients and families.11 In a survey of 3,171 physicians in the U.S. and Canada, Waterman and colleagues found that only 10% of physicians believed they had adequate support from their health care organizations (HCOs) after an AE occurred, even though 86% expressed significant interest in receiving training on the disclosure of AEs.12 Despite this gap, some medical educators, such as Katie Watson at Northwestern University, are successfully demonstrating the power of teaching medical students improvisational acting skills to enhance professionalism and communication in future physician–patient interaction.13
Disclosure Training Program
In 2010, the Lexington VAMC was awarded a 3-year VA Systems Improvement Capability Grant, which funded the development of a Disclosure Training Program (DTP). A team of investigators designed a 2-day workshop based on principles of experiential learning. Each workshop incorporated interactive teaching techniques using filmed clinical vignettes to provide a context for facilitated small-group disclosure simulations with professional actors.14 A total of 14 workshops were conducted for 346 participants from December 2011 to September 2012.
The DTP workshop integrates focused didactic sessions with interactive audience-workshop facilitator discussion, debriefing of teaching films, and disclosure simulations, with the majority of time spent the conducting and debriefing of simulations. Core content addressed during workshop activities included the following:
1. Historical origins of disclosure policy at the VHA
2. Ethical obligation, professional duty, and legal mandates for disclosure
3. Empathic communication–cognitive and emotive
4. VHA Handbook 1004.08, Disclosure of Adverse Events to Patient
5. Institutional and Clinical Disclosure of AEs
6. Psychological and physical needs of patients after an AE
7. Disclosure linking risk management to patient safety in a health care system
8. Legal implications for disclosure
9. State apology laws
10. Implementing disclosure programs in health care facilities
11. Facility support for providers after a patient AE
The principles of empathic communication and the core elements of AE disclosure to patients are reinforced during small-group simulations with actors portraying patients or family members. Each small-group simulation typically involves 3 to 4 workshop participants and 1 to 2 actors. Participants are given the task of conducting a clinical or institutional disclosure.
A facilitator manages each simulation, based on a scripted scenario or teaching film viewed by workshop participants. In the simulations attendees assume the roles of hospital staff that might be realistically involved in disclosure conversations, including executive leaders, physicians, nurses, risk managers, pharmacists, chaplains, and social workers.
Simulations average 5 to 7 minutes and are followed by a debriefing, including simulation participants, workshop facilitators, and the professional actor, who remain in character. By the end of each 2-day workshop, all attendees have participated in multiple small-group simulations of both clinical and institutional disclosures. Pre- and postworkshop knowledge questions and program evaluation data are collected with immediate-response polling technology used throughout the workshop.
Between 20 and 40 HCPs attended each workshop, which was designed for clinical and administrative leaders as well as others supporting the disclosure process, such as nurse managers, patient safety managers, social workers, chaplains, and pharmacists. The facility director, chief of staff, risk manager, and lawyers from the Regional Counsel office all play an important role in institutional disclosures and all were strongly encouraged to attend. The DTP facilitators observed the importance of senior executive leadership—participation, which enhanced dialogue in the large group sessions and small-group simulation-based learning.
DTP Workshop Results
Fourteen workshops were conducted for 346 employees from 26 VAMCs in 2012. Audience response technology was used to elicit participant feedback regarding workshop quality and effectiveness. Additional questions were asked as a pre/post-test of subject matter knowledge. Following the workshop, the participants showed a 30% overall improvement over preworkshop tests (Table), and 95% of participants favorably rated the workshop for quality and effectiveness.
There was a positive association between workshops with facility directors and actively engaged chiefs of staff in attendance and higher improvement scores in the test of knowledge. Among the top 7 performers on this test, 6 were individual facilities hosting the workshops and 1 VISN hosting for several facility representatives. Eleven of the 14 workshops with these characteristics (3 of which included VISN directors) evidenced more than 20% improvement on the test knowledge. These findings confirmed the original program design intended for individual facilities with leadership in attendance.
Iterative improvements were made to the program throughout 2012 based on feedback from workshop attendees, the National Office of Risk Management, the National Center for Ethics in Health Care and participating VA facilities and VISNs.
Despite these encouraging results, the DTP has some significant limitations: It is expensive, labor intensive, and dependent on faculty with expertise in clinical medicine, bioethics, and the law. Considering tight federal budgets, justifying the expenses to host a training program is difficult for a VAMC compared with that of other spending priorities. The actual and opportunity costs of travel to host sites for several facilitators and a group of professional actors to conduct a 2-day workshop for busy HCPs is not trivial.
Another limitation is the use of immediate response technology for data collection. Although this method maximizes response rates and seems to keep attendees engaged in presentations and discussions, technical failures could result in dropped responses, and ultimately the choice to respond is dependent on participant willingness to use the device.
Conclusion
Encouraging results suggest a bright future for the DTP, which has relevance for any health care organization, including the VA, academic affiliates, or those in the private sector. Wherever health care is delivered, providers will have the difficult task of disclosing AEs to meet their duty of care when patients experience harm. Learning empathic communication skills and successful strategies for disclosure will enhance this interaction and contribute to the maintenance of trust that is critical to the provider–patient relationship.
The DTP workshop has a flexible design and can be packaged to accommodate host medical centers for workshops of 1 to 2 days’ duration. The didactic presentations are constant, whereas the number of simulations will vary, depending on the length of the workshop (2-3 simulations for 1 day and 5-7 for two days). Participants from every workshop consistently cite that the simulations with professional actors are a powerful learning experience of significant personal value.
The DTP was developed as a unique, simulation-based program for clinicians, administrators, and allied health care personnel to enhance the effective disclosure of AEs to patients. Feedback from participants in 14 workshops in 2012 cited the value of the program with a high favorability rating. In a test of knowledge, participants also demonstrated an increase in learning. This feedback from the health care professionals who have attended the workshops has validated the pedagogic design of the program, which leverages adult learning principles of learning through experience. This approach was described by Aristotle in his best-known work on ethics, Nicomachean Ethics, “For the things we have to learn before we can do them, we learn by doing them.”15
Acknowledgements
For their significant contributions to the development and implementation of the VHA Disclosure Training Program, the authors thank Aaliyah Eaves-Leanos, Mary Duke, Lindsay Hall, and Uzair Munis. We thank the Institute for Healthcare Communication for their assistance in the program development. We express our utmost appreciation to Lee Taft for his many invaluable contributions to this program, including the critical role he continues to assume as a faculty member in the workshops. And, we are grateful for the continued contributions from our talented professional actors of Heyman Talent in Louisville, KY.
We express our sincere gratitude for their continuous feedback and important technical advice informing iterative improvements in the DTP workshops throughout 2012 from Virginia Ashby Sharpe (VA National Center for Ethics in Health Care); Yuri Walker (director of the Risk Management Program); and Barbara Rose (data analyst in the Risk Management Program), all at the VA central office in Washington, DC. And finally, we thank Heather Woodward-Hagg, Director of the VA Center for Applied Systems Engineering in Indianapolis, IN for her continued support in making DTP workshops available to VA Medical Centers throughout the country upon request.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
In 1987, the chief of staff of the Lexington VAMC and the staff attorney for the VA Regional Counsel Office in Lexington, Kentucky, discovered that a recent patient death was due to a mistake made in the medical care provided at their facility. They decided to disclose what happened to the family who had no knowledge of this mistake in care because “it was the right thing to do.”
The Lexington Model for disclosure, as it became known worldwide, continued to flourish under the leadership of Kraman and Hamm.1,2 The VA National Center for Ethics in Health Care adopted these principles of disclosure in drafting a national VHA policy directive in 2008, which was updated in 2012.3 However, despite the ethical and professional imperatives, disclosing adverse events (AEs) to patients and family members has continued to be one of the most difficult challenges in the practice of medicine.
VHA policy has made a distinction between clinical disclosure, conducted by a clinician with a patient as a routine professional practice, and institutional disclosure, conducted by institutional leadership for an AE rising above a threshold of serious patient harm. According to VHA Director of Risk Management Yuri Walker in a 2013 personal communication, the frequency of institutional disclosure reports from VAMCs since 2011 have reflected significant variation in disclosure practice among facilities of similar size and complexity.
In this report, the authors share their experience developing and delivering a simulation-based disclosure training program in the VHA intended to close the gap between policy expectations and practical challenges for providers and institutions when facing the task of disclosing an AE to patients and families.
Medical Error Disclosure
It is not difficult to understand why health care providers (HCPs) are uncomfortable about disclosing AEs to patients. The study by Delbanco and Bell describes physicians experiencing guilt, shame, and fear of retribution after a patient experiences an AE. The resulting silence and avoidance of the patient only compounds patient harm.4 Many HCPs believe disclosure will lead to tort claims, provide evidence against their defense, encourage reporting to the National Practitioners’ Databank, and damage their reputations with a potentially negative impact on their careers.5-7
In a 2009 survey of 1,891 practicing physicians in the U.S., one-third did not agree with disclosing serious medical errors to patients.8 Another survey of physicians reported wide variations in responses about whether they would offer an apology after making a medical mistake.9 Therefore, a gap between patient expectations and HCP communication when a medical mistake occurs should be expected.10
Few HCPs receive training in empathic communication skills for effective disclosure of AEs to patients and families.11 In a survey of 3,171 physicians in the U.S. and Canada, Waterman and colleagues found that only 10% of physicians believed they had adequate support from their health care organizations (HCOs) after an AE occurred, even though 86% expressed significant interest in receiving training on the disclosure of AEs.12 Despite this gap, some medical educators, such as Katie Watson at Northwestern University, are successfully demonstrating the power of teaching medical students improvisational acting skills to enhance professionalism and communication in future physician–patient interaction.13
Disclosure Training Program
In 2010, the Lexington VAMC was awarded a 3-year VA Systems Improvement Capability Grant, which funded the development of a Disclosure Training Program (DTP). A team of investigators designed a 2-day workshop based on principles of experiential learning. Each workshop incorporated interactive teaching techniques using filmed clinical vignettes to provide a context for facilitated small-group disclosure simulations with professional actors.14 A total of 14 workshops were conducted for 346 participants from December 2011 to September 2012.
The DTP workshop integrates focused didactic sessions with interactive audience-workshop facilitator discussion, debriefing of teaching films, and disclosure simulations, with the majority of time spent the conducting and debriefing of simulations. Core content addressed during workshop activities included the following:
1. Historical origins of disclosure policy at the VHA
2. Ethical obligation, professional duty, and legal mandates for disclosure
3. Empathic communication–cognitive and emotive
4. VHA Handbook 1004.08, Disclosure of Adverse Events to Patient
5. Institutional and Clinical Disclosure of AEs
6. Psychological and physical needs of patients after an AE
7. Disclosure linking risk management to patient safety in a health care system
8. Legal implications for disclosure
9. State apology laws
10. Implementing disclosure programs in health care facilities
11. Facility support for providers after a patient AE
The principles of empathic communication and the core elements of AE disclosure to patients are reinforced during small-group simulations with actors portraying patients or family members. Each small-group simulation typically involves 3 to 4 workshop participants and 1 to 2 actors. Participants are given the task of conducting a clinical or institutional disclosure.
A facilitator manages each simulation, based on a scripted scenario or teaching film viewed by workshop participants. In the simulations attendees assume the roles of hospital staff that might be realistically involved in disclosure conversations, including executive leaders, physicians, nurses, risk managers, pharmacists, chaplains, and social workers.
Simulations average 5 to 7 minutes and are followed by a debriefing, including simulation participants, workshop facilitators, and the professional actor, who remain in character. By the end of each 2-day workshop, all attendees have participated in multiple small-group simulations of both clinical and institutional disclosures. Pre- and postworkshop knowledge questions and program evaluation data are collected with immediate-response polling technology used throughout the workshop.
Between 20 and 40 HCPs attended each workshop, which was designed for clinical and administrative leaders as well as others supporting the disclosure process, such as nurse managers, patient safety managers, social workers, chaplains, and pharmacists. The facility director, chief of staff, risk manager, and lawyers from the Regional Counsel office all play an important role in institutional disclosures and all were strongly encouraged to attend. The DTP facilitators observed the importance of senior executive leadership—participation, which enhanced dialogue in the large group sessions and small-group simulation-based learning.
DTP Workshop Results
Fourteen workshops were conducted for 346 employees from 26 VAMCs in 2012. Audience response technology was used to elicit participant feedback regarding workshop quality and effectiveness. Additional questions were asked as a pre/post-test of subject matter knowledge. Following the workshop, the participants showed a 30% overall improvement over preworkshop tests (Table), and 95% of participants favorably rated the workshop for quality and effectiveness.
There was a positive association between workshops with facility directors and actively engaged chiefs of staff in attendance and higher improvement scores in the test of knowledge. Among the top 7 performers on this test, 6 were individual facilities hosting the workshops and 1 VISN hosting for several facility representatives. Eleven of the 14 workshops with these characteristics (3 of which included VISN directors) evidenced more than 20% improvement on the test knowledge. These findings confirmed the original program design intended for individual facilities with leadership in attendance.
Iterative improvements were made to the program throughout 2012 based on feedback from workshop attendees, the National Office of Risk Management, the National Center for Ethics in Health Care and participating VA facilities and VISNs.
Despite these encouraging results, the DTP has some significant limitations: It is expensive, labor intensive, and dependent on faculty with expertise in clinical medicine, bioethics, and the law. Considering tight federal budgets, justifying the expenses to host a training program is difficult for a VAMC compared with that of other spending priorities. The actual and opportunity costs of travel to host sites for several facilitators and a group of professional actors to conduct a 2-day workshop for busy HCPs is not trivial.
Another limitation is the use of immediate response technology for data collection. Although this method maximizes response rates and seems to keep attendees engaged in presentations and discussions, technical failures could result in dropped responses, and ultimately the choice to respond is dependent on participant willingness to use the device.
Conclusion
Encouraging results suggest a bright future for the DTP, which has relevance for any health care organization, including the VA, academic affiliates, or those in the private sector. Wherever health care is delivered, providers will have the difficult task of disclosing AEs to meet their duty of care when patients experience harm. Learning empathic communication skills and successful strategies for disclosure will enhance this interaction and contribute to the maintenance of trust that is critical to the provider–patient relationship.
The DTP workshop has a flexible design and can be packaged to accommodate host medical centers for workshops of 1 to 2 days’ duration. The didactic presentations are constant, whereas the number of simulations will vary, depending on the length of the workshop (2-3 simulations for 1 day and 5-7 for two days). Participants from every workshop consistently cite that the simulations with professional actors are a powerful learning experience of significant personal value.
The DTP was developed as a unique, simulation-based program for clinicians, administrators, and allied health care personnel to enhance the effective disclosure of AEs to patients. Feedback from participants in 14 workshops in 2012 cited the value of the program with a high favorability rating. In a test of knowledge, participants also demonstrated an increase in learning. This feedback from the health care professionals who have attended the workshops has validated the pedagogic design of the program, which leverages adult learning principles of learning through experience. This approach was described by Aristotle in his best-known work on ethics, Nicomachean Ethics, “For the things we have to learn before we can do them, we learn by doing them.”15
Acknowledgements
For their significant contributions to the development and implementation of the VHA Disclosure Training Program, the authors thank Aaliyah Eaves-Leanos, Mary Duke, Lindsay Hall, and Uzair Munis. We thank the Institute for Healthcare Communication for their assistance in the program development. We express our utmost appreciation to Lee Taft for his many invaluable contributions to this program, including the critical role he continues to assume as a faculty member in the workshops. And, we are grateful for the continued contributions from our talented professional actors of Heyman Talent in Louisville, KY.
We express our sincere gratitude for their continuous feedback and important technical advice informing iterative improvements in the DTP workshops throughout 2012 from Virginia Ashby Sharpe (VA National Center for Ethics in Health Care); Yuri Walker (director of the Risk Management Program); and Barbara Rose (data analyst in the Risk Management Program), all at the VA central office in Washington, DC. And finally, we thank Heather Woodward-Hagg, Director of the VA Center for Applied Systems Engineering in Indianapolis, IN for her continued support in making DTP workshops available to VA Medical Centers throughout the country upon request.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Kraman SS, Hamm G. Risk management: Extreme honesty may be the best policy. Ann Intern Med. 1999;131(12):963-967.
2. Hamm GM, Kraman SS. New standards, new dilemmas: Reflections on managing medical mistakes. Bioethics Forum. 2001;17(2):19-25.
3. Veterans Health Administration. Disclosure of adverse events to patients. Handbook 100408. United States Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2800. Corrected copy October 12, 2012. Accessed April 3, 2014.
4. Delbanco T, Bell SK. Guilty, afraid, and alone—struggling with medical error. N Engl J Med. 2007;357(17):1682-1683.
5. Taft L. Apology and medical mistake: Opportunity or foil? Ann Health L. 2005;14:55-94.
6. Gallagher TH, Garbutt JM, Waterman AD, et al. Choosing your words carefully: How physicians would disclose harmful medical error to patients. Arch Intern Med. 2006;166(15):1585-1593.
7. Banja J. Medical Errors and Medical Narcissism. Sudbury, MD: Jones and Bartlett; 2005.
8. Iezzoni LI, Rao SR, DesRoches CM, Vogeli C, Campbell EG. Survey shows that at least some physicians are not always open or honest with patients. Health Aff (Millwood). 2012;31(2):383-391.
9. Gallagher TW, Waterman AD, Garbutt JM, et al. US and Canadian physicians’ attitudes and experiences regarding disclosure errors to patients. Arch Intern Med. 2006;166(15):1605-1611.
10. Robbennolt JK. Apologies and medical error. Clin Orthop Relat Res. 2009;467(2):376-382 White AA, Bell SK, Krauss MJ, et al. How trainees would disclose medical errors: Educational implications for training programmes. Med Educ. 201;45(4):372-380.
11. White AA, Bell SK, Krauss MJ, et al. How trainees would disclose medical errors: Educational implications for training programmes. Med Educ. 201;45(4):372-380.
12. Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467-476.
13. Watson K. Serious play: Teaching medical skills with improvisational theater techniques. Acad Med. 2011;86(10):1260-1265.
14. Eaves-Leanos A, Dunn EJ. Open disclosure of adverse events: Transparency and safety in healthcare. Surg Clin North Am. 2012;92(1):163-177.
15. Aristotle. Nichomachean Ethics (edited by Roger Crisp). Cambridge University Press, New York, NY (2012): Book II, Chapter 1 (1103b), p. 23.
1. Kraman SS, Hamm G. Risk management: Extreme honesty may be the best policy. Ann Intern Med. 1999;131(12):963-967.
2. Hamm GM, Kraman SS. New standards, new dilemmas: Reflections on managing medical mistakes. Bioethics Forum. 2001;17(2):19-25.
3. Veterans Health Administration. Disclosure of adverse events to patients. Handbook 100408. United States Department of Veterans Affairs Website. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2800. Corrected copy October 12, 2012. Accessed April 3, 2014.
4. Delbanco T, Bell SK. Guilty, afraid, and alone—struggling with medical error. N Engl J Med. 2007;357(17):1682-1683.
5. Taft L. Apology and medical mistake: Opportunity or foil? Ann Health L. 2005;14:55-94.
6. Gallagher TH, Garbutt JM, Waterman AD, et al. Choosing your words carefully: How physicians would disclose harmful medical error to patients. Arch Intern Med. 2006;166(15):1585-1593.
7. Banja J. Medical Errors and Medical Narcissism. Sudbury, MD: Jones and Bartlett; 2005.
8. Iezzoni LI, Rao SR, DesRoches CM, Vogeli C, Campbell EG. Survey shows that at least some physicians are not always open or honest with patients. Health Aff (Millwood). 2012;31(2):383-391.
9. Gallagher TW, Waterman AD, Garbutt JM, et al. US and Canadian physicians’ attitudes and experiences regarding disclosure errors to patients. Arch Intern Med. 2006;166(15):1605-1611.
10. Robbennolt JK. Apologies and medical error. Clin Orthop Relat Res. 2009;467(2):376-382 White AA, Bell SK, Krauss MJ, et al. How trainees would disclose medical errors: Educational implications for training programmes. Med Educ. 201;45(4):372-380.
11. White AA, Bell SK, Krauss MJ, et al. How trainees would disclose medical errors: Educational implications for training programmes. Med Educ. 201;45(4):372-380.
12. Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467-476.
13. Watson K. Serious play: Teaching medical skills with improvisational theater techniques. Acad Med. 2011;86(10):1260-1265.
14. Eaves-Leanos A, Dunn EJ. Open disclosure of adverse events: Transparency and safety in healthcare. Surg Clin North Am. 2012;92(1):163-177.
15. Aristotle. Nichomachean Ethics (edited by Roger Crisp). Cambridge University Press, New York, NY (2012): Book II, Chapter 1 (1103b), p. 23.