User login
Molecular monitoring and minimal residual disease in the management of chronic myelogenous leukemia
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) in 2001 for treatment of chronic myelogenous leukemia (CML) marked a paradigm shift in management of the disease. With that advance, CML has been largely managed as a chronic condition, with daily medication and frequent monitoring. Optimizing monitoring methods and identifying factors associated with response and long-term outcomes has thus been a major clinical research focus. Given the improved understanding of surveillance techniques in CML and the advent of several recently approved second- and third-generation TKIs, there have been recent updates to clinical practice guidelines.
Click on the PDF icon at the top of this introduction to read the full article.
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) in 2001 for treatment of chronic myelogenous leukemia (CML) marked a paradigm shift in management of the disease. With that advance, CML has been largely managed as a chronic condition, with daily medication and frequent monitoring. Optimizing monitoring methods and identifying factors associated with response and long-term outcomes has thus been a major clinical research focus. Given the improved understanding of surveillance techniques in CML and the advent of several recently approved second- and third-generation TKIs, there have been recent updates to clinical practice guidelines.
Click on the PDF icon at the top of this introduction to read the full article.
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) in 2001 for treatment of chronic myelogenous leukemia (CML) marked a paradigm shift in management of the disease. With that advance, CML has been largely managed as a chronic condition, with daily medication and frequent monitoring. Optimizing monitoring methods and identifying factors associated with response and long-term outcomes has thus been a major clinical research focus. Given the improved understanding of surveillance techniques in CML and the advent of several recently approved second- and third-generation TKIs, there have been recent updates to clinical practice guidelines.
Click on the PDF icon at the top of this introduction to read the full article.
Distinguishing the killers among us
When I was in high school, one of my friends told me he intended to kill another one of my friends. It was a different day and age back then, and with more than 4,000 students, my high school was rather large and impersonal – it was easy to get lost in the crowd.
"Juan" (not his real name) confided in me that he wanted to kill "Joe" (also not his real name), which I found a bit odd. Joe was well liked but not did really stand out. Juan was perhaps quieter and more studious, but he also did not stand out. Both boys had friends, did well in school, and were not obviously troubled. When I asked Juan why he wanted to kill Joe, he responded, "Because he’s the all-American boy."
I worried about this. I told my mother, and I told a teacher I trusted. No one knew what to do, and Juan’s plan did not sound feasible. He was going to bring a knife to school in a folder, and when Joe was walking in a crowded hallway, Juan would walk behind him and thrust out the folder to sent the knife flying. The physics of this just didn’t make sense to me, and I didn’t see how the knife would gain enough momentum to travel very far, much less penetrate a person through their clothes. Feasible, or not, however, the equation changed on a day that Juan and I were sitting together in the back row of Latin class. Juan opened his calculus book and showed me the butcher knife.
I excused myself from class, went to a pay phone, and called my mother. She called the school, the principal called the police, and Juan was quietly removed from school when the period ended. It would be hard to imagine that a teenager who plotted the murder of another student, by illogical means for an illogical reason, wasn’t emotionally disturbed. I don’t know what happens to someone like Juan in 2014 – I’m thinking nothing good – but let me tell you what happened to Juan in the late 1970s.
Juan was out of high school for 6 weeks. I heard he had psychological testing, but aside from that, I have no idea what transpired. One of his friends told me it was a joke. He returned to high school, never said another word to me, graduated, and attended an Ivy League university – one of several prestigious schools to which he gained admission. A Google search reveals that Juan later earned a graduate degree and works as an executive making a six-figure salary. (Isn’t Google great?) He’s an adjunct professor at a college where he gets glowing reviews on RateMyProfessors.com. He was elected to serve on a local government committee. He’s married and has children. Compliments of Facebook, I know that he continues to have contact with high school friends.
From what I can tell, Juan’s brief period of being a disturbed adolescent did not progress to a life defined by chronic mental illness, and he did not go on to become a violent felon. I have no idea if I did Juan a favor, prevented a murder, got him much-needed treatment, or simply added an embarrassing diversion to his life. I don’t know if he ever really would have harmed Joe, or if it was in fact a joke designed to yank my chain.
Apparently, my threshold is on the low side, and not long ago I called a friend late one night and insisted she check on her teenager when a Facebook post struck me as alarming and a bit too final. The response I got was, "They were lyrics from a song and you’re overreacting." So be it. I called the mother and not the National Guard, and if the teen had become a statistic, I would have regretted not reacting.
I did wonder, while writing this article, how it would be received if I were to contact Juan and ask his thoughts on those events decades ago. Somehow, it seemed best not to uncover old wounds.
The media talk about the dangerously mentally ill as though they are a separate breed of humans, ones who can be easily distinguished by simply assessing who stands on which side of a well-marked "us-versus-them" line. Human beings, especially young human beings, may go through phases, and they may struggle with controlling their emotions, behaviors, and impulses and with finding their identity at a time in life when fitting in has undue importance. Some of them, no doubt, have these crises in the throes of an acute episode of mental distress – illness, if you’d prefer – and others do so at the genesis of what will prove to be a chronic and persistent psychiatric disorder. It may not be clear at any given moment who is going through a phase, who is suffering from an acute episode of mental illness, and who is beginning their course of a severe and persistent disorder.
Still, there are those who want to neatly categorize those with "serious" mental illness so that we can focus more of our resources on their needs and not on those who they deem to be the "worried well." Juan, it appears, did not fall into that category of chronically or persistently mentally ill. Similarly, all of us know chaotic, misbehaving, and even suicidal teenagers who go on to live productive lives. Does that designation matter if those not-so-seriously (or not-so-obviously) mentally ill individuals kill someone or die during their brief moments of emotional turbulence?
It seems to me that the goal is to keep people safe and help usher them through difficult periods in their lives, regardless of any diagnostic certainty or severity. I would contend that the mentally healthy among us also are subject to impulsive, distressed, and dangerous moments and that the world does not always neatly divide people into proper categorical entities.
Dr. Miller is a coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011).
When I was in high school, one of my friends told me he intended to kill another one of my friends. It was a different day and age back then, and with more than 4,000 students, my high school was rather large and impersonal – it was easy to get lost in the crowd.
"Juan" (not his real name) confided in me that he wanted to kill "Joe" (also not his real name), which I found a bit odd. Joe was well liked but not did really stand out. Juan was perhaps quieter and more studious, but he also did not stand out. Both boys had friends, did well in school, and were not obviously troubled. When I asked Juan why he wanted to kill Joe, he responded, "Because he’s the all-American boy."
I worried about this. I told my mother, and I told a teacher I trusted. No one knew what to do, and Juan’s plan did not sound feasible. He was going to bring a knife to school in a folder, and when Joe was walking in a crowded hallway, Juan would walk behind him and thrust out the folder to sent the knife flying. The physics of this just didn’t make sense to me, and I didn’t see how the knife would gain enough momentum to travel very far, much less penetrate a person through their clothes. Feasible, or not, however, the equation changed on a day that Juan and I were sitting together in the back row of Latin class. Juan opened his calculus book and showed me the butcher knife.
I excused myself from class, went to a pay phone, and called my mother. She called the school, the principal called the police, and Juan was quietly removed from school when the period ended. It would be hard to imagine that a teenager who plotted the murder of another student, by illogical means for an illogical reason, wasn’t emotionally disturbed. I don’t know what happens to someone like Juan in 2014 – I’m thinking nothing good – but let me tell you what happened to Juan in the late 1970s.
Juan was out of high school for 6 weeks. I heard he had psychological testing, but aside from that, I have no idea what transpired. One of his friends told me it was a joke. He returned to high school, never said another word to me, graduated, and attended an Ivy League university – one of several prestigious schools to which he gained admission. A Google search reveals that Juan later earned a graduate degree and works as an executive making a six-figure salary. (Isn’t Google great?) He’s an adjunct professor at a college where he gets glowing reviews on RateMyProfessors.com. He was elected to serve on a local government committee. He’s married and has children. Compliments of Facebook, I know that he continues to have contact with high school friends.
From what I can tell, Juan’s brief period of being a disturbed adolescent did not progress to a life defined by chronic mental illness, and he did not go on to become a violent felon. I have no idea if I did Juan a favor, prevented a murder, got him much-needed treatment, or simply added an embarrassing diversion to his life. I don’t know if he ever really would have harmed Joe, or if it was in fact a joke designed to yank my chain.
Apparently, my threshold is on the low side, and not long ago I called a friend late one night and insisted she check on her teenager when a Facebook post struck me as alarming and a bit too final. The response I got was, "They were lyrics from a song and you’re overreacting." So be it. I called the mother and not the National Guard, and if the teen had become a statistic, I would have regretted not reacting.
I did wonder, while writing this article, how it would be received if I were to contact Juan and ask his thoughts on those events decades ago. Somehow, it seemed best not to uncover old wounds.
The media talk about the dangerously mentally ill as though they are a separate breed of humans, ones who can be easily distinguished by simply assessing who stands on which side of a well-marked "us-versus-them" line. Human beings, especially young human beings, may go through phases, and they may struggle with controlling their emotions, behaviors, and impulses and with finding their identity at a time in life when fitting in has undue importance. Some of them, no doubt, have these crises in the throes of an acute episode of mental distress – illness, if you’d prefer – and others do so at the genesis of what will prove to be a chronic and persistent psychiatric disorder. It may not be clear at any given moment who is going through a phase, who is suffering from an acute episode of mental illness, and who is beginning their course of a severe and persistent disorder.
Still, there are those who want to neatly categorize those with "serious" mental illness so that we can focus more of our resources on their needs and not on those who they deem to be the "worried well." Juan, it appears, did not fall into that category of chronically or persistently mentally ill. Similarly, all of us know chaotic, misbehaving, and even suicidal teenagers who go on to live productive lives. Does that designation matter if those not-so-seriously (or not-so-obviously) mentally ill individuals kill someone or die during their brief moments of emotional turbulence?
It seems to me that the goal is to keep people safe and help usher them through difficult periods in their lives, regardless of any diagnostic certainty or severity. I would contend that the mentally healthy among us also are subject to impulsive, distressed, and dangerous moments and that the world does not always neatly divide people into proper categorical entities.
Dr. Miller is a coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011).
When I was in high school, one of my friends told me he intended to kill another one of my friends. It was a different day and age back then, and with more than 4,000 students, my high school was rather large and impersonal – it was easy to get lost in the crowd.
"Juan" (not his real name) confided in me that he wanted to kill "Joe" (also not his real name), which I found a bit odd. Joe was well liked but not did really stand out. Juan was perhaps quieter and more studious, but he also did not stand out. Both boys had friends, did well in school, and were not obviously troubled. When I asked Juan why he wanted to kill Joe, he responded, "Because he’s the all-American boy."
I worried about this. I told my mother, and I told a teacher I trusted. No one knew what to do, and Juan’s plan did not sound feasible. He was going to bring a knife to school in a folder, and when Joe was walking in a crowded hallway, Juan would walk behind him and thrust out the folder to sent the knife flying. The physics of this just didn’t make sense to me, and I didn’t see how the knife would gain enough momentum to travel very far, much less penetrate a person through their clothes. Feasible, or not, however, the equation changed on a day that Juan and I were sitting together in the back row of Latin class. Juan opened his calculus book and showed me the butcher knife.
I excused myself from class, went to a pay phone, and called my mother. She called the school, the principal called the police, and Juan was quietly removed from school when the period ended. It would be hard to imagine that a teenager who plotted the murder of another student, by illogical means for an illogical reason, wasn’t emotionally disturbed. I don’t know what happens to someone like Juan in 2014 – I’m thinking nothing good – but let me tell you what happened to Juan in the late 1970s.
Juan was out of high school for 6 weeks. I heard he had psychological testing, but aside from that, I have no idea what transpired. One of his friends told me it was a joke. He returned to high school, never said another word to me, graduated, and attended an Ivy League university – one of several prestigious schools to which he gained admission. A Google search reveals that Juan later earned a graduate degree and works as an executive making a six-figure salary. (Isn’t Google great?) He’s an adjunct professor at a college where he gets glowing reviews on RateMyProfessors.com. He was elected to serve on a local government committee. He’s married and has children. Compliments of Facebook, I know that he continues to have contact with high school friends.
From what I can tell, Juan’s brief period of being a disturbed adolescent did not progress to a life defined by chronic mental illness, and he did not go on to become a violent felon. I have no idea if I did Juan a favor, prevented a murder, got him much-needed treatment, or simply added an embarrassing diversion to his life. I don’t know if he ever really would have harmed Joe, or if it was in fact a joke designed to yank my chain.
Apparently, my threshold is on the low side, and not long ago I called a friend late one night and insisted she check on her teenager when a Facebook post struck me as alarming and a bit too final. The response I got was, "They were lyrics from a song and you’re overreacting." So be it. I called the mother and not the National Guard, and if the teen had become a statistic, I would have regretted not reacting.
I did wonder, while writing this article, how it would be received if I were to contact Juan and ask his thoughts on those events decades ago. Somehow, it seemed best not to uncover old wounds.
The media talk about the dangerously mentally ill as though they are a separate breed of humans, ones who can be easily distinguished by simply assessing who stands on which side of a well-marked "us-versus-them" line. Human beings, especially young human beings, may go through phases, and they may struggle with controlling their emotions, behaviors, and impulses and with finding their identity at a time in life when fitting in has undue importance. Some of them, no doubt, have these crises in the throes of an acute episode of mental distress – illness, if you’d prefer – and others do so at the genesis of what will prove to be a chronic and persistent psychiatric disorder. It may not be clear at any given moment who is going through a phase, who is suffering from an acute episode of mental illness, and who is beginning their course of a severe and persistent disorder.
Still, there are those who want to neatly categorize those with "serious" mental illness so that we can focus more of our resources on their needs and not on those who they deem to be the "worried well." Juan, it appears, did not fall into that category of chronically or persistently mentally ill. Similarly, all of us know chaotic, misbehaving, and even suicidal teenagers who go on to live productive lives. Does that designation matter if those not-so-seriously (or not-so-obviously) mentally ill individuals kill someone or die during their brief moments of emotional turbulence?
It seems to me that the goal is to keep people safe and help usher them through difficult periods in their lives, regardless of any diagnostic certainty or severity. I would contend that the mentally healthy among us also are subject to impulsive, distressed, and dangerous moments and that the world does not always neatly divide people into proper categorical entities.
Dr. Miller is a coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011).
New and Noteworthy Information—June 2014
Older patients with migraine may be more likely to have silent brain injury than older patients without migraine, according to research published online ahead of print May 15 in Stroke. Researchers analyzed data from the Northern Manhattan Study, which quantified subclinical brain infarctions and white matter hyperintensity volumes among participants with migraine. Of the 546 participants analyzed, 41% were men, 65% were Hispanic, and mean age at MRI was 71. Patients with migraine had double the odds of subclinical brain infarction, compared with those reporting no migraine, after the investigators adjusted for sociodemographics and vascular risk factors. No association was observed between migraine with or without aura and white matter hyperintensity volume. Patients with migraine should not worry, because their risk of ischemic stroke is small, said the authors.
People who are exposed to paint, glue, or degreaser fumes at work may experience memory and thinking problems in retirement, according to a study published May 13 in Neurology. Researchers examined data for 2,143 retired utility workers who underwent cognitive testing in 2010. The authors assessed workers’ lifetime exposure to chlorinated solvents, petroleum solvents, and benzene using a job exposure matrix. Approximately 33% of participants were exposed to chlorinated solvents, 26% to benzene, and 25% to petroleum solvents. Workers highly exposed to chlorinated solvents were at risk of impairment on the Mini-Mental State Examination, the Digit Symbol Substitution Test, semantic fluency test, and the Trail Making Test B. Retirees at greatest risk for deficits had high lifetime exposure to solvents and were last exposed 12 to 30 years before testing.
Females susceptible to multiple sclerosis (MS) produce higher levels of the blood vessel receptor protein S1PR2 than males, according to data published online ahead of print May 8 in the Journal of Clinical Investigation. S1PR2 is present at high levels in the brain areas that MS typically damages. Investigators studied a mouse model of MS and found increased activity of S1PR2, which opens up the blood–brain barrier. When the researchers tested brain tissue samples obtained from 20 human patients after death, they found more S1PR2 in patients with MS than in those without the disorder. Brain tissue from females also had higher levels of S1PR2, compared with male brain tissue. These findings may help explain why more women than men get the disease, said the authors.
The FDA has required the manufacturer of the sleep drug Lunesta (eszopiclone) to lower the recommended starting dose from 2 mg to 1 mg for men and women. Data show that eszopiclone levels in some patients may be high enough on the morning after treatment to impair activities that require alertness, including driving. The 1-mg dose, taken at bedtime, can be increased to 2 mg or 3 mg if needed, but the higher doses are more likely to result in next-day impairment. Using lower doses ensures that less drug will remain in the body during the morning hours. Patients currently taking the 2-mg and 3-mg doses of Lunesta should contact their health care professional to ask for instructions, according to the FDA.
The rate of visits to an emergency department (ED) for traumatic brain injury (TBI) increased by approximately 30% between 2006 and 2010, according to research published in the May 14 issue of JAMA. The increase may be attributable to various factors, including increased awareness and diagnoses, said the authors. The investigators examined data from the Nationwide Emergency Department Sample database to determine national trends in ED visits for TBI from 2006 through 2010. An estimated 2.5 million ED visits for TBI occurred in 2010, representing a 29% increase in the rate of visits for TBI during the study period. By comparison, total ED visits increased by 3.6%. Children younger than 3 and adults older than 60 had the largest increase in TBI rates.
The pathophysiologic biomarkers and the topographic markers of Alzheimer’s disease should be revised, according to a position paper by the International Working Group published in the June issue of Lancet Neurology. The group proposed that biomarkers of Alzheimer’s pathology be restricted to those indicating the presence of tau pathology (ie, CSF or PET tau) and amyloid pathology (ie, CSF or PET amyloid). These biomarkers are specific enough to diagnose Alzheimer’s disease at any point on the disease continuum, said the authors. Downstream topographic markers of brain regional structural and metabolic changes have insufficient pathologic specificity and should not be used in diagnosis, according to the researchers. Instead, these markers can be used to measure disease progression. The group also provided diagnostic criteria for atypical, mixed, and preclinical Alzheimer’s disease.
Prenatal supplementation with docosahexaenoic acid (DHA) does not result in improved cognitive, problem-solving, or language abilities for children at age 4, according to the results of a trial published in the May 7 issue of JAMA. Investigators conducted longer-term follow-up from a previous study in which pregnant women received 800 mg/day of DHA or placebo. In the initial study, the researchers found that average cognitive, language, and motor scores did not differ between children at 18 months of age. Approximately 92% of eligible families participated in the follow-up study. The DHA group included 313 participants, and the control group included 333 participants. The investigators found that measures of cognition, the ability to perform complex mental processing, language, and executive functioning (eg, memory, reasoning, and problem solving) did not differ significantly between groups at age 4.
The FDA has informed Acorda Therapeutics that it has completed its review of the company’s new drug application for Plumiaz (diazepam) nasal spray and that the application cannot be approved in its present form. The drug was developed for the treatment of people with epilepsy who experience cluster seizures. Acorda Therapeutics is developing a response to address the items outlined in the letter. Based on the requirements for approval outlined in the letter, the company does not expect Plumiaz to receive FDA approval in 2014. Plumiaz previously received orphan drug designation for the treatment of cluster seizures. [For related news, see page 9.]
Older people with memory and thinking problems who do not have dementia may have a lower risk of dying from cancer than people who have no memory and thinking problems, according to a study published April 22 in Neurology. Researchers studied 2,627 people age 65 and older who did not have dementia at baseline. Participants underwent tests of memory and thinking skills at baseline and at three years. Follow-up lasted for an average of approximately 13 years. During the study, 1,003 participants died. About 34% of deaths occurred among patients with the fastest decline in thinking skills. Approximately 21% of participants in the group with the fastest decline in thinking skills died of cancer, compared with 29% of participants in the other two groups.
A new technique may predict with 95% accuracy which patients with stroke will benefit from IV t-PA and which will have potentially lethal bleeding in the brain, according to a study published online ahead of print May 15 in Stroke. Researchers used a computer program that shows physicians the amount of gadolinium, injected during an MRI scan, that has leaked into the brain tissue from surrounding blood vessels. By quantifying this damage in 75 patients with stroke, the researchers identified a threshold for determining how much leakage was dangerous. They applied this threshold to the records for the 75 patients to determine how well it would predict who had had a brain hemorrhage and who had not. The new test correctly predicted the outcome with 95% accuracy.
Freezing of gait in patients with Parkinson’s disease may correlate with poor quality of life, disease severity, apathy, and exposure to antimuscarinics, according to a study published online ahead of print May 19 in JAMA Neurology. Investigators performed a cross-sectional survey of 672 patients with idiopathic Parkinson’s disease. Patients with freezing of gait were identified as those with a score of 1 or greater on item 14 of the Unified Parkinson’s Disease Rating Scale (UPDRS) in the on condition. Approximately 38% of patients reported freezing of gait during the on state, which was significantly related to lower quality of life scores. Freezing of gait was also correlated with longer disease duration, higher UPDRS parts II and III scores, apathy, and a higher levodopa equivalent daily dose.
Among college football players, concussion and years of football played may have a significant inverse relationship with hippocampal volume, according to research published May 14 in JAMA. Years of football experience also may correlate with slower reaction time. Investigators conducted a cross-sectional study of 25 college football players with a history of clinician-diagnosed concussion, 25 college football players without a history of concussion, and 25 nonfootball-playing, age-, sex-, and education-matched healthy controls. Players with and without a history of concussion had smaller hippocampal volumes, compared with healthy controls. Players with a history of concussion had smaller hippocampal volumes than players without concussion. In both athlete groups, investigators found a statistically significant inverse relationship between left hippocampal volume and number of years of football played.
Deficiencies in hyaluronan can lead to spontaneous epileptic seizures, according to research published April 30 in the Journal of Neuroscience. In a multicenter study, investigators examined the role of hyaluronan using mutant mice deficient in each of the three hyaluronan synthase genes (ie, Has1, Has2, Has3). The mutant mice were prone to epileptic seizures. In Has3(-/-) mice, this phenotype likely results from a reduction in the size of the brain extracellular space (ECS), said the researchers. Among the three Has knockout models, seizures were most prevalent in Has3(-/-) mice, which also had the greatest hyaluronan reduction in the hippocampus. The results provide the first direct evidence for the physiologic role of hyaluronan in the regulation of ECS volume, according to the investigators.
—Erik Greb
Older patients with migraine may be more likely to have silent brain injury than older patients without migraine, according to research published online ahead of print May 15 in Stroke. Researchers analyzed data from the Northern Manhattan Study, which quantified subclinical brain infarctions and white matter hyperintensity volumes among participants with migraine. Of the 546 participants analyzed, 41% were men, 65% were Hispanic, and mean age at MRI was 71. Patients with migraine had double the odds of subclinical brain infarction, compared with those reporting no migraine, after the investigators adjusted for sociodemographics and vascular risk factors. No association was observed between migraine with or without aura and white matter hyperintensity volume. Patients with migraine should not worry, because their risk of ischemic stroke is small, said the authors.
People who are exposed to paint, glue, or degreaser fumes at work may experience memory and thinking problems in retirement, according to a study published May 13 in Neurology. Researchers examined data for 2,143 retired utility workers who underwent cognitive testing in 2010. The authors assessed workers’ lifetime exposure to chlorinated solvents, petroleum solvents, and benzene using a job exposure matrix. Approximately 33% of participants were exposed to chlorinated solvents, 26% to benzene, and 25% to petroleum solvents. Workers highly exposed to chlorinated solvents were at risk of impairment on the Mini-Mental State Examination, the Digit Symbol Substitution Test, semantic fluency test, and the Trail Making Test B. Retirees at greatest risk for deficits had high lifetime exposure to solvents and were last exposed 12 to 30 years before testing.
Females susceptible to multiple sclerosis (MS) produce higher levels of the blood vessel receptor protein S1PR2 than males, according to data published online ahead of print May 8 in the Journal of Clinical Investigation. S1PR2 is present at high levels in the brain areas that MS typically damages. Investigators studied a mouse model of MS and found increased activity of S1PR2, which opens up the blood–brain barrier. When the researchers tested brain tissue samples obtained from 20 human patients after death, they found more S1PR2 in patients with MS than in those without the disorder. Brain tissue from females also had higher levels of S1PR2, compared with male brain tissue. These findings may help explain why more women than men get the disease, said the authors.
The FDA has required the manufacturer of the sleep drug Lunesta (eszopiclone) to lower the recommended starting dose from 2 mg to 1 mg for men and women. Data show that eszopiclone levels in some patients may be high enough on the morning after treatment to impair activities that require alertness, including driving. The 1-mg dose, taken at bedtime, can be increased to 2 mg or 3 mg if needed, but the higher doses are more likely to result in next-day impairment. Using lower doses ensures that less drug will remain in the body during the morning hours. Patients currently taking the 2-mg and 3-mg doses of Lunesta should contact their health care professional to ask for instructions, according to the FDA.
The rate of visits to an emergency department (ED) for traumatic brain injury (TBI) increased by approximately 30% between 2006 and 2010, according to research published in the May 14 issue of JAMA. The increase may be attributable to various factors, including increased awareness and diagnoses, said the authors. The investigators examined data from the Nationwide Emergency Department Sample database to determine national trends in ED visits for TBI from 2006 through 2010. An estimated 2.5 million ED visits for TBI occurred in 2010, representing a 29% increase in the rate of visits for TBI during the study period. By comparison, total ED visits increased by 3.6%. Children younger than 3 and adults older than 60 had the largest increase in TBI rates.
The pathophysiologic biomarkers and the topographic markers of Alzheimer’s disease should be revised, according to a position paper by the International Working Group published in the June issue of Lancet Neurology. The group proposed that biomarkers of Alzheimer’s pathology be restricted to those indicating the presence of tau pathology (ie, CSF or PET tau) and amyloid pathology (ie, CSF or PET amyloid). These biomarkers are specific enough to diagnose Alzheimer’s disease at any point on the disease continuum, said the authors. Downstream topographic markers of brain regional structural and metabolic changes have insufficient pathologic specificity and should not be used in diagnosis, according to the researchers. Instead, these markers can be used to measure disease progression. The group also provided diagnostic criteria for atypical, mixed, and preclinical Alzheimer’s disease.
Prenatal supplementation with docosahexaenoic acid (DHA) does not result in improved cognitive, problem-solving, or language abilities for children at age 4, according to the results of a trial published in the May 7 issue of JAMA. Investigators conducted longer-term follow-up from a previous study in which pregnant women received 800 mg/day of DHA or placebo. In the initial study, the researchers found that average cognitive, language, and motor scores did not differ between children at 18 months of age. Approximately 92% of eligible families participated in the follow-up study. The DHA group included 313 participants, and the control group included 333 participants. The investigators found that measures of cognition, the ability to perform complex mental processing, language, and executive functioning (eg, memory, reasoning, and problem solving) did not differ significantly between groups at age 4.
The FDA has informed Acorda Therapeutics that it has completed its review of the company’s new drug application for Plumiaz (diazepam) nasal spray and that the application cannot be approved in its present form. The drug was developed for the treatment of people with epilepsy who experience cluster seizures. Acorda Therapeutics is developing a response to address the items outlined in the letter. Based on the requirements for approval outlined in the letter, the company does not expect Plumiaz to receive FDA approval in 2014. Plumiaz previously received orphan drug designation for the treatment of cluster seizures. [For related news, see page 9.]
Older people with memory and thinking problems who do not have dementia may have a lower risk of dying from cancer than people who have no memory and thinking problems, according to a study published April 22 in Neurology. Researchers studied 2,627 people age 65 and older who did not have dementia at baseline. Participants underwent tests of memory and thinking skills at baseline and at three years. Follow-up lasted for an average of approximately 13 years. During the study, 1,003 participants died. About 34% of deaths occurred among patients with the fastest decline in thinking skills. Approximately 21% of participants in the group with the fastest decline in thinking skills died of cancer, compared with 29% of participants in the other two groups.
A new technique may predict with 95% accuracy which patients with stroke will benefit from IV t-PA and which will have potentially lethal bleeding in the brain, according to a study published online ahead of print May 15 in Stroke. Researchers used a computer program that shows physicians the amount of gadolinium, injected during an MRI scan, that has leaked into the brain tissue from surrounding blood vessels. By quantifying this damage in 75 patients with stroke, the researchers identified a threshold for determining how much leakage was dangerous. They applied this threshold to the records for the 75 patients to determine how well it would predict who had had a brain hemorrhage and who had not. The new test correctly predicted the outcome with 95% accuracy.
Freezing of gait in patients with Parkinson’s disease may correlate with poor quality of life, disease severity, apathy, and exposure to antimuscarinics, according to a study published online ahead of print May 19 in JAMA Neurology. Investigators performed a cross-sectional survey of 672 patients with idiopathic Parkinson’s disease. Patients with freezing of gait were identified as those with a score of 1 or greater on item 14 of the Unified Parkinson’s Disease Rating Scale (UPDRS) in the on condition. Approximately 38% of patients reported freezing of gait during the on state, which was significantly related to lower quality of life scores. Freezing of gait was also correlated with longer disease duration, higher UPDRS parts II and III scores, apathy, and a higher levodopa equivalent daily dose.
Among college football players, concussion and years of football played may have a significant inverse relationship with hippocampal volume, according to research published May 14 in JAMA. Years of football experience also may correlate with slower reaction time. Investigators conducted a cross-sectional study of 25 college football players with a history of clinician-diagnosed concussion, 25 college football players without a history of concussion, and 25 nonfootball-playing, age-, sex-, and education-matched healthy controls. Players with and without a history of concussion had smaller hippocampal volumes, compared with healthy controls. Players with a history of concussion had smaller hippocampal volumes than players without concussion. In both athlete groups, investigators found a statistically significant inverse relationship between left hippocampal volume and number of years of football played.
Deficiencies in hyaluronan can lead to spontaneous epileptic seizures, according to research published April 30 in the Journal of Neuroscience. In a multicenter study, investigators examined the role of hyaluronan using mutant mice deficient in each of the three hyaluronan synthase genes (ie, Has1, Has2, Has3). The mutant mice were prone to epileptic seizures. In Has3(-/-) mice, this phenotype likely results from a reduction in the size of the brain extracellular space (ECS), said the researchers. Among the three Has knockout models, seizures were most prevalent in Has3(-/-) mice, which also had the greatest hyaluronan reduction in the hippocampus. The results provide the first direct evidence for the physiologic role of hyaluronan in the regulation of ECS volume, according to the investigators.
—Erik Greb
Older patients with migraine may be more likely to have silent brain injury than older patients without migraine, according to research published online ahead of print May 15 in Stroke. Researchers analyzed data from the Northern Manhattan Study, which quantified subclinical brain infarctions and white matter hyperintensity volumes among participants with migraine. Of the 546 participants analyzed, 41% were men, 65% were Hispanic, and mean age at MRI was 71. Patients with migraine had double the odds of subclinical brain infarction, compared with those reporting no migraine, after the investigators adjusted for sociodemographics and vascular risk factors. No association was observed between migraine with or without aura and white matter hyperintensity volume. Patients with migraine should not worry, because their risk of ischemic stroke is small, said the authors.
People who are exposed to paint, glue, or degreaser fumes at work may experience memory and thinking problems in retirement, according to a study published May 13 in Neurology. Researchers examined data for 2,143 retired utility workers who underwent cognitive testing in 2010. The authors assessed workers’ lifetime exposure to chlorinated solvents, petroleum solvents, and benzene using a job exposure matrix. Approximately 33% of participants were exposed to chlorinated solvents, 26% to benzene, and 25% to petroleum solvents. Workers highly exposed to chlorinated solvents were at risk of impairment on the Mini-Mental State Examination, the Digit Symbol Substitution Test, semantic fluency test, and the Trail Making Test B. Retirees at greatest risk for deficits had high lifetime exposure to solvents and were last exposed 12 to 30 years before testing.
Females susceptible to multiple sclerosis (MS) produce higher levels of the blood vessel receptor protein S1PR2 than males, according to data published online ahead of print May 8 in the Journal of Clinical Investigation. S1PR2 is present at high levels in the brain areas that MS typically damages. Investigators studied a mouse model of MS and found increased activity of S1PR2, which opens up the blood–brain barrier. When the researchers tested brain tissue samples obtained from 20 human patients after death, they found more S1PR2 in patients with MS than in those without the disorder. Brain tissue from females also had higher levels of S1PR2, compared with male brain tissue. These findings may help explain why more women than men get the disease, said the authors.
The FDA has required the manufacturer of the sleep drug Lunesta (eszopiclone) to lower the recommended starting dose from 2 mg to 1 mg for men and women. Data show that eszopiclone levels in some patients may be high enough on the morning after treatment to impair activities that require alertness, including driving. The 1-mg dose, taken at bedtime, can be increased to 2 mg or 3 mg if needed, but the higher doses are more likely to result in next-day impairment. Using lower doses ensures that less drug will remain in the body during the morning hours. Patients currently taking the 2-mg and 3-mg doses of Lunesta should contact their health care professional to ask for instructions, according to the FDA.
The rate of visits to an emergency department (ED) for traumatic brain injury (TBI) increased by approximately 30% between 2006 and 2010, according to research published in the May 14 issue of JAMA. The increase may be attributable to various factors, including increased awareness and diagnoses, said the authors. The investigators examined data from the Nationwide Emergency Department Sample database to determine national trends in ED visits for TBI from 2006 through 2010. An estimated 2.5 million ED visits for TBI occurred in 2010, representing a 29% increase in the rate of visits for TBI during the study period. By comparison, total ED visits increased by 3.6%. Children younger than 3 and adults older than 60 had the largest increase in TBI rates.
The pathophysiologic biomarkers and the topographic markers of Alzheimer’s disease should be revised, according to a position paper by the International Working Group published in the June issue of Lancet Neurology. The group proposed that biomarkers of Alzheimer’s pathology be restricted to those indicating the presence of tau pathology (ie, CSF or PET tau) and amyloid pathology (ie, CSF or PET amyloid). These biomarkers are specific enough to diagnose Alzheimer’s disease at any point on the disease continuum, said the authors. Downstream topographic markers of brain regional structural and metabolic changes have insufficient pathologic specificity and should not be used in diagnosis, according to the researchers. Instead, these markers can be used to measure disease progression. The group also provided diagnostic criteria for atypical, mixed, and preclinical Alzheimer’s disease.
Prenatal supplementation with docosahexaenoic acid (DHA) does not result in improved cognitive, problem-solving, or language abilities for children at age 4, according to the results of a trial published in the May 7 issue of JAMA. Investigators conducted longer-term follow-up from a previous study in which pregnant women received 800 mg/day of DHA or placebo. In the initial study, the researchers found that average cognitive, language, and motor scores did not differ between children at 18 months of age. Approximately 92% of eligible families participated in the follow-up study. The DHA group included 313 participants, and the control group included 333 participants. The investigators found that measures of cognition, the ability to perform complex mental processing, language, and executive functioning (eg, memory, reasoning, and problem solving) did not differ significantly between groups at age 4.
The FDA has informed Acorda Therapeutics that it has completed its review of the company’s new drug application for Plumiaz (diazepam) nasal spray and that the application cannot be approved in its present form. The drug was developed for the treatment of people with epilepsy who experience cluster seizures. Acorda Therapeutics is developing a response to address the items outlined in the letter. Based on the requirements for approval outlined in the letter, the company does not expect Plumiaz to receive FDA approval in 2014. Plumiaz previously received orphan drug designation for the treatment of cluster seizures. [For related news, see page 9.]
Older people with memory and thinking problems who do not have dementia may have a lower risk of dying from cancer than people who have no memory and thinking problems, according to a study published April 22 in Neurology. Researchers studied 2,627 people age 65 and older who did not have dementia at baseline. Participants underwent tests of memory and thinking skills at baseline and at three years. Follow-up lasted for an average of approximately 13 years. During the study, 1,003 participants died. About 34% of deaths occurred among patients with the fastest decline in thinking skills. Approximately 21% of participants in the group with the fastest decline in thinking skills died of cancer, compared with 29% of participants in the other two groups.
A new technique may predict with 95% accuracy which patients with stroke will benefit from IV t-PA and which will have potentially lethal bleeding in the brain, according to a study published online ahead of print May 15 in Stroke. Researchers used a computer program that shows physicians the amount of gadolinium, injected during an MRI scan, that has leaked into the brain tissue from surrounding blood vessels. By quantifying this damage in 75 patients with stroke, the researchers identified a threshold for determining how much leakage was dangerous. They applied this threshold to the records for the 75 patients to determine how well it would predict who had had a brain hemorrhage and who had not. The new test correctly predicted the outcome with 95% accuracy.
Freezing of gait in patients with Parkinson’s disease may correlate with poor quality of life, disease severity, apathy, and exposure to antimuscarinics, according to a study published online ahead of print May 19 in JAMA Neurology. Investigators performed a cross-sectional survey of 672 patients with idiopathic Parkinson’s disease. Patients with freezing of gait were identified as those with a score of 1 or greater on item 14 of the Unified Parkinson’s Disease Rating Scale (UPDRS) in the on condition. Approximately 38% of patients reported freezing of gait during the on state, which was significantly related to lower quality of life scores. Freezing of gait was also correlated with longer disease duration, higher UPDRS parts II and III scores, apathy, and a higher levodopa equivalent daily dose.
Among college football players, concussion and years of football played may have a significant inverse relationship with hippocampal volume, according to research published May 14 in JAMA. Years of football experience also may correlate with slower reaction time. Investigators conducted a cross-sectional study of 25 college football players with a history of clinician-diagnosed concussion, 25 college football players without a history of concussion, and 25 nonfootball-playing, age-, sex-, and education-matched healthy controls. Players with and without a history of concussion had smaller hippocampal volumes, compared with healthy controls. Players with a history of concussion had smaller hippocampal volumes than players without concussion. In both athlete groups, investigators found a statistically significant inverse relationship between left hippocampal volume and number of years of football played.
Deficiencies in hyaluronan can lead to spontaneous epileptic seizures, according to research published April 30 in the Journal of Neuroscience. In a multicenter study, investigators examined the role of hyaluronan using mutant mice deficient in each of the three hyaluronan synthase genes (ie, Has1, Has2, Has3). The mutant mice were prone to epileptic seizures. In Has3(-/-) mice, this phenotype likely results from a reduction in the size of the brain extracellular space (ECS), said the researchers. Among the three Has knockout models, seizures were most prevalent in Has3(-/-) mice, which also had the greatest hyaluronan reduction in the hippocampus. The results provide the first direct evidence for the physiologic role of hyaluronan in the regulation of ECS volume, according to the investigators.
—Erik Greb
Hepatitis B screening recommended for high-risk patients
Physicians should screen all asymptomatic but high-risk adolescents and adults for hepatitis B virus infection, according to an updated recommendation by the U.S. Preventive Services Task Force that was published online May 27 in Annals of Internal Medicine.
Since the last USPSTF recommendation on HBV screening in 2004, which focused on the general population and didn’t advocate screening of this subset of patients, research has documented that antiviral treatment improves both intermediate outcomes such as virologic and histologic responses and long-term outcomes such as prevention of hepatocellular carcinoma, cirrhosis, and end-stage liver disease.
Given this effectiveness, along with the 98% sensitivity and specificity of HBV screening tests, the group has now issued a level B recommendation that high-risk patients be screened, said Dr. Michael L. LeFevre, chair of the USPSTF and professor of family and community medicine at the University of Missouri, Columbia, and his associates.
High-risk patients include the following:
• People born in regions where the prevalence of HBV infection is 2% or greater, such as sub-Saharan Africa, central and southeast Asia, China, the Pacific Islands, and parts of Latin America. People born in these areas account for 47%-95% of the chronic HBV infection in the United States.
• American-born children of parents from these regions, who may not have been vaccinated in infancy.
• HIV-positive persons.
• IV-drug users.
• Household contacts of people with HBV infection.
• Men who have sex with men.
The updated USPSTF recommendations are in line with those of the Centers for Disease Control and Prevention, the American Association for the Study of Liver Diseases, the Institute of Medicine, and the American Academy of Family Physicians. The CDC additionally recommends HBV screening for blood, organ, or tissue donors; people with occupational or other exposure to infectious blood or body fluids; and patients receiving hemodialysis, cytotoxic therapy, or immunosuppressive therapy.
The USPSTF still does not recommend HBV screening for the general population. The prevalence of the infection is low in the U.S. general population, and most members of the general population who are infected with HBV do not develop the chronic form of the infection and do not develop complications like hepatocellular carcinoma or cirrhosis. The potential harms of general screening, then, probably exceed the potential benefits, Dr. LeFevre and his associates noted (Ann Intern. Med. 2014 May 27 [doi:10.7326/M14-1018]).
The USPSTF has separate recommendations regarding hepatitis B in pregnant women. These, along with the updated recommendations for high-risk patients, are available at www.uspreventiveservicestaskforce.org.
The USPSTF is a voluntary group funded by the Agency for Healthcare Research and Quality but otherwise independent of the federal government. Dr. LeFevre and his associates reported no potential financial conflicts of interest.
These "long overdue" recommendations are "a dramatic and welcome upgrade from the 2004 USPSTF guidelines, which issued a grade D recommendation against screening asymptomatic persons for HBV infection," said Dr. Ruma Rajbhandari and Dr. Raymond T. Chung.
"Many would argue that the USPSTF should have endorsed screening for HBV infection in high-risk populations a decade ago," they wrote. The group lagged far behind the American Association for the Study of Liver Diseases’ recommendations in 2001 and the CDC’s recommendations in 2005. "We may have thus missed an opportunity to screen many high-risk persons in the United States," Dr. Rajbhandari and Dr. Chung said.
The USPSTF update "would be more useful if they provided a clearer definition of the high-risk patient. ... We worry that busy generalist clinicians do not have the time to estimate their patients’ risks for HBV infection." Physicians may find it more helpful to look up the CDC’s table listing all the factors that render a patient high risk, they added.
Dr. Rajbhandari and Dr. Chung are with the liver center and gastrointestinal division at Massachusetts General Hospital, Boston. They reported no relevant conflicts of interest. These remarks were taken from their editorial accompanying Dr. Lefevre’s report (Ann. Intern. Med. 2014 May 27 [doi:10.7326/M14-1153]).
These "long overdue" recommendations are "a dramatic and welcome upgrade from the 2004 USPSTF guidelines, which issued a grade D recommendation against screening asymptomatic persons for HBV infection," said Dr. Ruma Rajbhandari and Dr. Raymond T. Chung.
"Many would argue that the USPSTF should have endorsed screening for HBV infection in high-risk populations a decade ago," they wrote. The group lagged far behind the American Association for the Study of Liver Diseases’ recommendations in 2001 and the CDC’s recommendations in 2005. "We may have thus missed an opportunity to screen many high-risk persons in the United States," Dr. Rajbhandari and Dr. Chung said.
The USPSTF update "would be more useful if they provided a clearer definition of the high-risk patient. ... We worry that busy generalist clinicians do not have the time to estimate their patients’ risks for HBV infection." Physicians may find it more helpful to look up the CDC’s table listing all the factors that render a patient high risk, they added.
Dr. Rajbhandari and Dr. Chung are with the liver center and gastrointestinal division at Massachusetts General Hospital, Boston. They reported no relevant conflicts of interest. These remarks were taken from their editorial accompanying Dr. Lefevre’s report (Ann. Intern. Med. 2014 May 27 [doi:10.7326/M14-1153]).
These "long overdue" recommendations are "a dramatic and welcome upgrade from the 2004 USPSTF guidelines, which issued a grade D recommendation against screening asymptomatic persons for HBV infection," said Dr. Ruma Rajbhandari and Dr. Raymond T. Chung.
"Many would argue that the USPSTF should have endorsed screening for HBV infection in high-risk populations a decade ago," they wrote. The group lagged far behind the American Association for the Study of Liver Diseases’ recommendations in 2001 and the CDC’s recommendations in 2005. "We may have thus missed an opportunity to screen many high-risk persons in the United States," Dr. Rajbhandari and Dr. Chung said.
The USPSTF update "would be more useful if they provided a clearer definition of the high-risk patient. ... We worry that busy generalist clinicians do not have the time to estimate their patients’ risks for HBV infection." Physicians may find it more helpful to look up the CDC’s table listing all the factors that render a patient high risk, they added.
Dr. Rajbhandari and Dr. Chung are with the liver center and gastrointestinal division at Massachusetts General Hospital, Boston. They reported no relevant conflicts of interest. These remarks were taken from their editorial accompanying Dr. Lefevre’s report (Ann. Intern. Med. 2014 May 27 [doi:10.7326/M14-1153]).
Physicians should screen all asymptomatic but high-risk adolescents and adults for hepatitis B virus infection, according to an updated recommendation by the U.S. Preventive Services Task Force that was published online May 27 in Annals of Internal Medicine.
Since the last USPSTF recommendation on HBV screening in 2004, which focused on the general population and didn’t advocate screening of this subset of patients, research has documented that antiviral treatment improves both intermediate outcomes such as virologic and histologic responses and long-term outcomes such as prevention of hepatocellular carcinoma, cirrhosis, and end-stage liver disease.
Given this effectiveness, along with the 98% sensitivity and specificity of HBV screening tests, the group has now issued a level B recommendation that high-risk patients be screened, said Dr. Michael L. LeFevre, chair of the USPSTF and professor of family and community medicine at the University of Missouri, Columbia, and his associates.
High-risk patients include the following:
• People born in regions where the prevalence of HBV infection is 2% or greater, such as sub-Saharan Africa, central and southeast Asia, China, the Pacific Islands, and parts of Latin America. People born in these areas account for 47%-95% of the chronic HBV infection in the United States.
• American-born children of parents from these regions, who may not have been vaccinated in infancy.
• HIV-positive persons.
• IV-drug users.
• Household contacts of people with HBV infection.
• Men who have sex with men.
The updated USPSTF recommendations are in line with those of the Centers for Disease Control and Prevention, the American Association for the Study of Liver Diseases, the Institute of Medicine, and the American Academy of Family Physicians. The CDC additionally recommends HBV screening for blood, organ, or tissue donors; people with occupational or other exposure to infectious blood or body fluids; and patients receiving hemodialysis, cytotoxic therapy, or immunosuppressive therapy.
The USPSTF still does not recommend HBV screening for the general population. The prevalence of the infection is low in the U.S. general population, and most members of the general population who are infected with HBV do not develop the chronic form of the infection and do not develop complications like hepatocellular carcinoma or cirrhosis. The potential harms of general screening, then, probably exceed the potential benefits, Dr. LeFevre and his associates noted (Ann Intern. Med. 2014 May 27 [doi:10.7326/M14-1018]).
The USPSTF has separate recommendations regarding hepatitis B in pregnant women. These, along with the updated recommendations for high-risk patients, are available at www.uspreventiveservicestaskforce.org.
The USPSTF is a voluntary group funded by the Agency for Healthcare Research and Quality but otherwise independent of the federal government. Dr. LeFevre and his associates reported no potential financial conflicts of interest.
Physicians should screen all asymptomatic but high-risk adolescents and adults for hepatitis B virus infection, according to an updated recommendation by the U.S. Preventive Services Task Force that was published online May 27 in Annals of Internal Medicine.
Since the last USPSTF recommendation on HBV screening in 2004, which focused on the general population and didn’t advocate screening of this subset of patients, research has documented that antiviral treatment improves both intermediate outcomes such as virologic and histologic responses and long-term outcomes such as prevention of hepatocellular carcinoma, cirrhosis, and end-stage liver disease.
Given this effectiveness, along with the 98% sensitivity and specificity of HBV screening tests, the group has now issued a level B recommendation that high-risk patients be screened, said Dr. Michael L. LeFevre, chair of the USPSTF and professor of family and community medicine at the University of Missouri, Columbia, and his associates.
High-risk patients include the following:
• People born in regions where the prevalence of HBV infection is 2% or greater, such as sub-Saharan Africa, central and southeast Asia, China, the Pacific Islands, and parts of Latin America. People born in these areas account for 47%-95% of the chronic HBV infection in the United States.
• American-born children of parents from these regions, who may not have been vaccinated in infancy.
• HIV-positive persons.
• IV-drug users.
• Household contacts of people with HBV infection.
• Men who have sex with men.
The updated USPSTF recommendations are in line with those of the Centers for Disease Control and Prevention, the American Association for the Study of Liver Diseases, the Institute of Medicine, and the American Academy of Family Physicians. The CDC additionally recommends HBV screening for blood, organ, or tissue donors; people with occupational or other exposure to infectious blood or body fluids; and patients receiving hemodialysis, cytotoxic therapy, or immunosuppressive therapy.
The USPSTF still does not recommend HBV screening for the general population. The prevalence of the infection is low in the U.S. general population, and most members of the general population who are infected with HBV do not develop the chronic form of the infection and do not develop complications like hepatocellular carcinoma or cirrhosis. The potential harms of general screening, then, probably exceed the potential benefits, Dr. LeFevre and his associates noted (Ann Intern. Med. 2014 May 27 [doi:10.7326/M14-1018]).
The USPSTF has separate recommendations regarding hepatitis B in pregnant women. These, along with the updated recommendations for high-risk patients, are available at www.uspreventiveservicestaskforce.org.
The USPSTF is a voluntary group funded by the Agency for Healthcare Research and Quality but otherwise independent of the federal government. Dr. LeFevre and his associates reported no potential financial conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: HBV screening is appropriate in all at-risk populations.
Major finding: Physicians should screen all adolescents and adults at high risk for HBV infection, including those born in regions where the virus is endemic, American-born children of such parents, household contacts of people with HBV, people with HIV, IV-drug users, and men who have sex with men.
Data source: A comprehensive review of the literature since 2004 regarding the benefits and harms of screening high-risk patients for HBV infection, and a compilation of recommendations for screening high-risk patients.
Disclosures: The USPSTF is a voluntary group funded by the Agency for Healthcare Research and Quality but otherwise independent of the federal government. Dr. LeFevre and his associates reported no potential financial conflicts of interest.
Refugees struggle to access cancer treatment
Credit: UK Department
for International Development
A new study published in The Lancet Oncology reveals a high demand for costly cancer treatment among refugees from the recent conflicts in Iraq and Syria, with host countries struggling to find the money and medicine to treat their new patients.
The findings prompted a call from study author Paul Spiegel, MD, the United Nations High Commissioner for Refugees (UNHCR) Chief Medical Expert, for schemes to improve access to affordable cancer care for refugees.
In the first study of its kind, Dr Spiegel and his colleagues examined data from funding applications made to the UNHCR Exceptional Care Committee (ECC) from refugees in Jordan and Syria whose cancer treatment costs were likely to exceed US$2000 a year.
The results indicate that cancer is an important public health problem in refugee settings, the researchers said. The study also highlights the challenges and costs national health systems and humanitarian organizations face when overwhelmed by massive influxes of refugees.
For example, in Jordan, the ECC assessed 1989 applications for treatment between 2010 and 2012. Roughly a quarter of these (511) were for cancer, with breast cancer and colorectal cancer being the most common. Around half (48%) of these cases were approved and funded.
Funding was often denied because the patient had a poor prognosis (43% of cases in 2011 and 31% in 2012) or the treatment was too costly (25% in 2011). The average amount requested from the ECC for cancer treatment was $11,540 in 2011 and $5151 in 2012. However, the amounts approved were substantially lower—$4626 in 2011 and $3501 in 2012.
“The countries in the Middle East have welcomed millions of refugees, first from Iraq and then Syria,” Dr Spiegel said. “This massive influx has strained health systems at all levels. Despite help from international organizations and donors to expand health facilities and pay for additional personnel and drugs, it has been insufficient.”
“The burden has fallen disproportionately on the host countries to absorb the costs. For example, the Jordanian Ministry of Health footed an estimated $53 million bill for medical care for refugees in the first 4 months of 2013.”
Dr Spiegel and his colleagues are therefore calling for improved cancer prevention and treatment in refugee settings through the use of innovative financing schemes; better primary care, including screening for common cancers (eg, colonoscopies and mammograms); and the development of web-based cancer registries to prevent the interruption of treatment.
“Until now, the responses to humanitarian crises have been primarily based on experiences from refugee camps in sub-Saharan Africa where infectious diseases and malnutrition have been the priority,” Dr Spiegel said. “In the 21st century, refugee situations are substantially longer and increasingly occur in middle-income countries where the levels of chronic diseases, including cancer, are higher.”
“Cancer diagnosis and care in humanitarian emergencies typifies a growing trend towards more costly chronic disease care, something that seems to have been overlooked but is of increasing importance because the number of refugees is growing.” ![]()

Credit: UK Department
for International Development
A new study published in The Lancet Oncology reveals a high demand for costly cancer treatment among refugees from the recent conflicts in Iraq and Syria, with host countries struggling to find the money and medicine to treat their new patients.
The findings prompted a call from study author Paul Spiegel, MD, the United Nations High Commissioner for Refugees (UNHCR) Chief Medical Expert, for schemes to improve access to affordable cancer care for refugees.
In the first study of its kind, Dr Spiegel and his colleagues examined data from funding applications made to the UNHCR Exceptional Care Committee (ECC) from refugees in Jordan and Syria whose cancer treatment costs were likely to exceed US$2000 a year.
The results indicate that cancer is an important public health problem in refugee settings, the researchers said. The study also highlights the challenges and costs national health systems and humanitarian organizations face when overwhelmed by massive influxes of refugees.
For example, in Jordan, the ECC assessed 1989 applications for treatment between 2010 and 2012. Roughly a quarter of these (511) were for cancer, with breast cancer and colorectal cancer being the most common. Around half (48%) of these cases were approved and funded.
Funding was often denied because the patient had a poor prognosis (43% of cases in 2011 and 31% in 2012) or the treatment was too costly (25% in 2011). The average amount requested from the ECC for cancer treatment was $11,540 in 2011 and $5151 in 2012. However, the amounts approved were substantially lower—$4626 in 2011 and $3501 in 2012.
“The countries in the Middle East have welcomed millions of refugees, first from Iraq and then Syria,” Dr Spiegel said. “This massive influx has strained health systems at all levels. Despite help from international organizations and donors to expand health facilities and pay for additional personnel and drugs, it has been insufficient.”
“The burden has fallen disproportionately on the host countries to absorb the costs. For example, the Jordanian Ministry of Health footed an estimated $53 million bill for medical care for refugees in the first 4 months of 2013.”
Dr Spiegel and his colleagues are therefore calling for improved cancer prevention and treatment in refugee settings through the use of innovative financing schemes; better primary care, including screening for common cancers (eg, colonoscopies and mammograms); and the development of web-based cancer registries to prevent the interruption of treatment.
“Until now, the responses to humanitarian crises have been primarily based on experiences from refugee camps in sub-Saharan Africa where infectious diseases and malnutrition have been the priority,” Dr Spiegel said. “In the 21st century, refugee situations are substantially longer and increasingly occur in middle-income countries where the levels of chronic diseases, including cancer, are higher.”
“Cancer diagnosis and care in humanitarian emergencies typifies a growing trend towards more costly chronic disease care, something that seems to have been overlooked but is of increasing importance because the number of refugees is growing.” ![]()

Credit: UK Department
for International Development
A new study published in The Lancet Oncology reveals a high demand for costly cancer treatment among refugees from the recent conflicts in Iraq and Syria, with host countries struggling to find the money and medicine to treat their new patients.
The findings prompted a call from study author Paul Spiegel, MD, the United Nations High Commissioner for Refugees (UNHCR) Chief Medical Expert, for schemes to improve access to affordable cancer care for refugees.
In the first study of its kind, Dr Spiegel and his colleagues examined data from funding applications made to the UNHCR Exceptional Care Committee (ECC) from refugees in Jordan and Syria whose cancer treatment costs were likely to exceed US$2000 a year.
The results indicate that cancer is an important public health problem in refugee settings, the researchers said. The study also highlights the challenges and costs national health systems and humanitarian organizations face when overwhelmed by massive influxes of refugees.
For example, in Jordan, the ECC assessed 1989 applications for treatment between 2010 and 2012. Roughly a quarter of these (511) were for cancer, with breast cancer and colorectal cancer being the most common. Around half (48%) of these cases were approved and funded.
Funding was often denied because the patient had a poor prognosis (43% of cases in 2011 and 31% in 2012) or the treatment was too costly (25% in 2011). The average amount requested from the ECC for cancer treatment was $11,540 in 2011 and $5151 in 2012. However, the amounts approved were substantially lower—$4626 in 2011 and $3501 in 2012.
“The countries in the Middle East have welcomed millions of refugees, first from Iraq and then Syria,” Dr Spiegel said. “This massive influx has strained health systems at all levels. Despite help from international organizations and donors to expand health facilities and pay for additional personnel and drugs, it has been insufficient.”
“The burden has fallen disproportionately on the host countries to absorb the costs. For example, the Jordanian Ministry of Health footed an estimated $53 million bill for medical care for refugees in the first 4 months of 2013.”
Dr Spiegel and his colleagues are therefore calling for improved cancer prevention and treatment in refugee settings through the use of innovative financing schemes; better primary care, including screening for common cancers (eg, colonoscopies and mammograms); and the development of web-based cancer registries to prevent the interruption of treatment.
“Until now, the responses to humanitarian crises have been primarily based on experiences from refugee camps in sub-Saharan Africa where infectious diseases and malnutrition have been the priority,” Dr Spiegel said. “In the 21st century, refugee situations are substantially longer and increasingly occur in middle-income countries where the levels of chronic diseases, including cancer, are higher.”
“Cancer diagnosis and care in humanitarian emergencies typifies a growing trend towards more costly chronic disease care, something that seems to have been overlooked but is of increasing importance because the number of refugees is growing.” ![]()
Study explains why pneumococcal vaccines fall short in SCD

Credit: St Jude Children’s
Research Hospital
A new study reveals differences in the pneumococcal genome that explain why current vaccines do not sufficiently protect children with sickle cell disease (SCD) from pneumococcal infections.
Researchers performed whole-genome sequencing of hundreds of pneumococcal bacteria collected from healthy subjects and patients with SCD.
The team found that disease-causing strains of the bacteria differed between the 2 groups.
And the pneumococcal strains from the SCD patients differed from the 13 pneumococcal strains included in the current vaccine recommended for children age 5 and younger.
“The results help explain why current vaccines haven’t been as successful at protecting children with sickle cell disease from pneumococcal infections as they have in protecting other children,” said Joshua Wolf, MD, of St Jude Children’s Research Hospital.
Dr Wolf and his colleagues detailed these results in Cell Host & Microbe.
The researchers had compared the genomes of 322 pneumococcal bacteria collected from SCD patients between 1994 and 2011 to DNA from 327 strains obtained from individuals without SCD.
The analysis revealed that, over time, the genomes of bacteria isolated from SCD patients shrank, as genes and the corresponding DNA were discarded or combined. The changes reflected bacterial adaptation to the SCD host and contributed to the bacteria’s ability to persist despite advances in preventive care.
The researchers then used transposon sequencing to compare the bacterial fitness of pneumococcal genes in mice with and without SCD. This revealed 60 genes whose transposon disruption resulted in fitness differences between the 2 types of mice.
So the bacteria faced different conditions in animals with and without SCD. The bloodstream of normal mice was a more hostile environment for pneumococcal bacteria than the bloodstream of mice with SCD.
When the researchers evaluated the aforementioned 60 genes in bacteria isolated from SCD patients, they found 6 that were missing or altered in a significant percentage of samples (P<0.05). This included SP0511, SP0946, SP1032, SP1449, SP1483, and SP1835.
These genes are involved in transporting iron into bacteria, bacterial metabolism, and other processes that are likely altered in patients with SCD.
“We demonstrated that genes necessary to cause disease in the general public are expendable in patients with sickle cell disease,” said study author Jason Rosch, PhD, also of St Jude.
The researchers believe these findings will aid efforts to improve vaccine effectiveness and inform research into new ways to protect young SCD patients from life-threatening pneumococcal infections that can lead to pneumonia, meningitis, bloodstream infections, and other problems. ![]()

Credit: St Jude Children’s
Research Hospital
A new study reveals differences in the pneumococcal genome that explain why current vaccines do not sufficiently protect children with sickle cell disease (SCD) from pneumococcal infections.
Researchers performed whole-genome sequencing of hundreds of pneumococcal bacteria collected from healthy subjects and patients with SCD.
The team found that disease-causing strains of the bacteria differed between the 2 groups.
And the pneumococcal strains from the SCD patients differed from the 13 pneumococcal strains included in the current vaccine recommended for children age 5 and younger.
“The results help explain why current vaccines haven’t been as successful at protecting children with sickle cell disease from pneumococcal infections as they have in protecting other children,” said Joshua Wolf, MD, of St Jude Children’s Research Hospital.
Dr Wolf and his colleagues detailed these results in Cell Host & Microbe.
The researchers had compared the genomes of 322 pneumococcal bacteria collected from SCD patients between 1994 and 2011 to DNA from 327 strains obtained from individuals without SCD.
The analysis revealed that, over time, the genomes of bacteria isolated from SCD patients shrank, as genes and the corresponding DNA were discarded or combined. The changes reflected bacterial adaptation to the SCD host and contributed to the bacteria’s ability to persist despite advances in preventive care.
The researchers then used transposon sequencing to compare the bacterial fitness of pneumococcal genes in mice with and without SCD. This revealed 60 genes whose transposon disruption resulted in fitness differences between the 2 types of mice.
So the bacteria faced different conditions in animals with and without SCD. The bloodstream of normal mice was a more hostile environment for pneumococcal bacteria than the bloodstream of mice with SCD.
When the researchers evaluated the aforementioned 60 genes in bacteria isolated from SCD patients, they found 6 that were missing or altered in a significant percentage of samples (P<0.05). This included SP0511, SP0946, SP1032, SP1449, SP1483, and SP1835.
These genes are involved in transporting iron into bacteria, bacterial metabolism, and other processes that are likely altered in patients with SCD.
“We demonstrated that genes necessary to cause disease in the general public are expendable in patients with sickle cell disease,” said study author Jason Rosch, PhD, also of St Jude.
The researchers believe these findings will aid efforts to improve vaccine effectiveness and inform research into new ways to protect young SCD patients from life-threatening pneumococcal infections that can lead to pneumonia, meningitis, bloodstream infections, and other problems. ![]()

Credit: St Jude Children’s
Research Hospital
A new study reveals differences in the pneumococcal genome that explain why current vaccines do not sufficiently protect children with sickle cell disease (SCD) from pneumococcal infections.
Researchers performed whole-genome sequencing of hundreds of pneumococcal bacteria collected from healthy subjects and patients with SCD.
The team found that disease-causing strains of the bacteria differed between the 2 groups.
And the pneumococcal strains from the SCD patients differed from the 13 pneumococcal strains included in the current vaccine recommended for children age 5 and younger.
“The results help explain why current vaccines haven’t been as successful at protecting children with sickle cell disease from pneumococcal infections as they have in protecting other children,” said Joshua Wolf, MD, of St Jude Children’s Research Hospital.
Dr Wolf and his colleagues detailed these results in Cell Host & Microbe.
The researchers had compared the genomes of 322 pneumococcal bacteria collected from SCD patients between 1994 and 2011 to DNA from 327 strains obtained from individuals without SCD.
The analysis revealed that, over time, the genomes of bacteria isolated from SCD patients shrank, as genes and the corresponding DNA were discarded or combined. The changes reflected bacterial adaptation to the SCD host and contributed to the bacteria’s ability to persist despite advances in preventive care.
The researchers then used transposon sequencing to compare the bacterial fitness of pneumococcal genes in mice with and without SCD. This revealed 60 genes whose transposon disruption resulted in fitness differences between the 2 types of mice.
So the bacteria faced different conditions in animals with and without SCD. The bloodstream of normal mice was a more hostile environment for pneumococcal bacteria than the bloodstream of mice with SCD.
When the researchers evaluated the aforementioned 60 genes in bacteria isolated from SCD patients, they found 6 that were missing or altered in a significant percentage of samples (P<0.05). This included SP0511, SP0946, SP1032, SP1449, SP1483, and SP1835.
These genes are involved in transporting iron into bacteria, bacterial metabolism, and other processes that are likely altered in patients with SCD.
“We demonstrated that genes necessary to cause disease in the general public are expendable in patients with sickle cell disease,” said study author Jason Rosch, PhD, also of St Jude.
The researchers believe these findings will aid efforts to improve vaccine effectiveness and inform research into new ways to protect young SCD patients from life-threatening pneumococcal infections that can lead to pneumonia, meningitis, bloodstream infections, and other problems. ![]()
South Africa changes blood donation policy

Photo courtesy of UAB Hospital
The South African National Blood Service (SANBS) has lessened restrictions on blood donation for men who have sex with men (MSM) but placed a new restriction on heterosexual donors.
Now, all South Africans are prohibited from donating blood unless they have been celibate or in a monogamous sexual relationship for at least 6 months.
So any individual with a new sexual partner or multiple partners will not be allowed to donate blood, regardless of sexual orientation.
South Africa’s former policy was that MSM could only donate blood if they had been celibate for 6 months or longer. But heterosexual individuals who engaged in risky or casual sex were still allowed to donate.
This was because MSM were considered at high risk of contracting HIV. However, the South African HIV epidemic is primarily a heterosexual one, so the SANBS’s policy was thought by many to be discriminatory.
With the new policy, the question intended to identify MSM has been removed from the blood donor questionnaire. The new questionnaire addresses sexual risk in general, and any sexual act or contact with a new partner or partners during the preceding 6 months will be deemed a risk to the safety of the blood supply.
“It took us a while [to make this decision] because we didn’t have local facts that warranted changing our policy, although we knew South Africa was different from other countries in terms of risk of HIV,” said Vanessa Raju, SANBS Communications Manager.
“The policy wasn’t meant to be discriminatory, but it was seen as such. We then worked closely with the Department of Health and other organizations to reassess the situation.”
Blood donation policies perceived as discriminatory against MSM are in place in many countries.
In the US and Northern Ireland, for example, MSM are banned from donating blood for life. Canada lifted its lifetime ban last year but still requires that MSM be celibate for 5 years before donating.
Other countries have recently adopted similar policies. In England, Scotland, and Wales, MSM must be celibate for 12 months prior to donating blood. ![]()

Photo courtesy of UAB Hospital
The South African National Blood Service (SANBS) has lessened restrictions on blood donation for men who have sex with men (MSM) but placed a new restriction on heterosexual donors.
Now, all South Africans are prohibited from donating blood unless they have been celibate or in a monogamous sexual relationship for at least 6 months.
So any individual with a new sexual partner or multiple partners will not be allowed to donate blood, regardless of sexual orientation.
South Africa’s former policy was that MSM could only donate blood if they had been celibate for 6 months or longer. But heterosexual individuals who engaged in risky or casual sex were still allowed to donate.
This was because MSM were considered at high risk of contracting HIV. However, the South African HIV epidemic is primarily a heterosexual one, so the SANBS’s policy was thought by many to be discriminatory.
With the new policy, the question intended to identify MSM has been removed from the blood donor questionnaire. The new questionnaire addresses sexual risk in general, and any sexual act or contact with a new partner or partners during the preceding 6 months will be deemed a risk to the safety of the blood supply.
“It took us a while [to make this decision] because we didn’t have local facts that warranted changing our policy, although we knew South Africa was different from other countries in terms of risk of HIV,” said Vanessa Raju, SANBS Communications Manager.
“The policy wasn’t meant to be discriminatory, but it was seen as such. We then worked closely with the Department of Health and other organizations to reassess the situation.”
Blood donation policies perceived as discriminatory against MSM are in place in many countries.
In the US and Northern Ireland, for example, MSM are banned from donating blood for life. Canada lifted its lifetime ban last year but still requires that MSM be celibate for 5 years before donating.
Other countries have recently adopted similar policies. In England, Scotland, and Wales, MSM must be celibate for 12 months prior to donating blood. ![]()

Photo courtesy of UAB Hospital
The South African National Blood Service (SANBS) has lessened restrictions on blood donation for men who have sex with men (MSM) but placed a new restriction on heterosexual donors.
Now, all South Africans are prohibited from donating blood unless they have been celibate or in a monogamous sexual relationship for at least 6 months.
So any individual with a new sexual partner or multiple partners will not be allowed to donate blood, regardless of sexual orientation.
South Africa’s former policy was that MSM could only donate blood if they had been celibate for 6 months or longer. But heterosexual individuals who engaged in risky or casual sex were still allowed to donate.
This was because MSM were considered at high risk of contracting HIV. However, the South African HIV epidemic is primarily a heterosexual one, so the SANBS’s policy was thought by many to be discriminatory.
With the new policy, the question intended to identify MSM has been removed from the blood donor questionnaire. The new questionnaire addresses sexual risk in general, and any sexual act or contact with a new partner or partners during the preceding 6 months will be deemed a risk to the safety of the blood supply.
“It took us a while [to make this decision] because we didn’t have local facts that warranted changing our policy, although we knew South Africa was different from other countries in terms of risk of HIV,” said Vanessa Raju, SANBS Communications Manager.
“The policy wasn’t meant to be discriminatory, but it was seen as such. We then worked closely with the Department of Health and other organizations to reassess the situation.”
Blood donation policies perceived as discriminatory against MSM are in place in many countries.
In the US and Northern Ireland, for example, MSM are banned from donating blood for life. Canada lifted its lifetime ban last year but still requires that MSM be celibate for 5 years before donating.
Other countries have recently adopted similar policies. In England, Scotland, and Wales, MSM must be celibate for 12 months prior to donating blood. ![]()
Drug gets orphan designation for CLL/SLL

The US Food and Drug Administration (FDA) has granted orphan designation for MOR208, an anti-CD19 monoclonal antibody, for the treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL).
The European Medicines Agency (EMA) also announced a positive opinion of the orphan medicinal product application for MOR208 to treat patients with CLL/SLL.
Researchers said MOR208 showed promise in a phase 1 study of CLL/SLL patients.
Orphan drug and orphan medicinal product status are granted by the FDA and EMA to promote the development of promising therapeutics for the treatment of rare diseases affecting fewer than 200,000 people in the US annually and no more than 5 in 10,000 people in the European Union (EU).
Orphan drug designation includes benefits such as a 7-year period of marketing exclusivity in the US and 10 years of market exclusivity in the EU after approval. Other potential advantages come in the form of protocol assistance, the ability to apply for research funding, tax credits for certain research expenses, and fee waivers for the regulatory procedures.
MOR208 development, results
MOR208 is a humanized monoclonal antibody that targets the antigen CD19 for treatment of B-cell malignancies and autoimmune diseases. The development program for MOR208 is currently in phase 2 development in CLL, B-cell acute lymphoblastic leukemia, and non-Hodgkin lymphoma.
The drug is being developed by MorphoSys AG. The development program was in-licensed from Xencor in 2010.
Researchers evaluated MOR208 (formerly known as XmAb5574) in a phase 1 study of patients with CLL/SLL and reported their results at the 2012 ASH Annual Meeting (abstract 2894). The study was sponsored by Xencor.
The study included 27 patients with relapsed or refractory CLL/SLL. The median patient age was 66 years (range, 40-84), and patients were generally high-risk. The median number of prior therapies was 4 (range, 1-14).
Patients received MOR208 at a range of doses—0.3 mg/kg, 1 mg/kg, 3 mg/kg, 6 mg/kg, 9 mg/kg, and 12 mg/kg. MOR208 was administered as an intravenous infusion on days 1, 4, 8, 15, and 22 of cycle 1, and on days 1, 8, 15, and 22 of cycle 2.
Dose escalation continued without dose-limiting toxicities until the highest dose level. At this dose, 1 patient experienced grade 4 neutropenia associated with febrile neutropenia, and the patient was taken off treatment.
All 27 patients experienced adverse events, but the majority of them were grade 1-2. The most common events were infusion reactions. These occurred in 67% (n=18) of patients and were all grade 1 or 2.
Nineteen percent of patients (n=5) experienced grade 3-4 treatment-related adverse events, including neutropenia (n=3), thrombocytopenia (n=2), increased aspartate aminotransferase (n=1), febrile neutropenia (n=1), and tumor lysis syndrome (n=1). Four of these patients were on the 12 mg/kg dose, but 1 patient who experienced neutropenia was receiving 1 mg/kg.
Eleven percent of patients experienced a partial response according to IWCLL 2008 criteria. Responses occurred at the 6 mg/kg, 9 mg/kg, and 12 mg/kg dose levels.
All objective responses occurred in patients categorized as CLL as opposed to SLL, and none of the patients with lymph nodes greater than 5 cm responded. Two patients had progressed at the 8-week evaluation point. ![]()

The US Food and Drug Administration (FDA) has granted orphan designation for MOR208, an anti-CD19 monoclonal antibody, for the treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL).
The European Medicines Agency (EMA) also announced a positive opinion of the orphan medicinal product application for MOR208 to treat patients with CLL/SLL.
Researchers said MOR208 showed promise in a phase 1 study of CLL/SLL patients.
Orphan drug and orphan medicinal product status are granted by the FDA and EMA to promote the development of promising therapeutics for the treatment of rare diseases affecting fewer than 200,000 people in the US annually and no more than 5 in 10,000 people in the European Union (EU).
Orphan drug designation includes benefits such as a 7-year period of marketing exclusivity in the US and 10 years of market exclusivity in the EU after approval. Other potential advantages come in the form of protocol assistance, the ability to apply for research funding, tax credits for certain research expenses, and fee waivers for the regulatory procedures.
MOR208 development, results
MOR208 is a humanized monoclonal antibody that targets the antigen CD19 for treatment of B-cell malignancies and autoimmune diseases. The development program for MOR208 is currently in phase 2 development in CLL, B-cell acute lymphoblastic leukemia, and non-Hodgkin lymphoma.
The drug is being developed by MorphoSys AG. The development program was in-licensed from Xencor in 2010.
Researchers evaluated MOR208 (formerly known as XmAb5574) in a phase 1 study of patients with CLL/SLL and reported their results at the 2012 ASH Annual Meeting (abstract 2894). The study was sponsored by Xencor.
The study included 27 patients with relapsed or refractory CLL/SLL. The median patient age was 66 years (range, 40-84), and patients were generally high-risk. The median number of prior therapies was 4 (range, 1-14).
Patients received MOR208 at a range of doses—0.3 mg/kg, 1 mg/kg, 3 mg/kg, 6 mg/kg, 9 mg/kg, and 12 mg/kg. MOR208 was administered as an intravenous infusion on days 1, 4, 8, 15, and 22 of cycle 1, and on days 1, 8, 15, and 22 of cycle 2.
Dose escalation continued without dose-limiting toxicities until the highest dose level. At this dose, 1 patient experienced grade 4 neutropenia associated with febrile neutropenia, and the patient was taken off treatment.
All 27 patients experienced adverse events, but the majority of them were grade 1-2. The most common events were infusion reactions. These occurred in 67% (n=18) of patients and were all grade 1 or 2.
Nineteen percent of patients (n=5) experienced grade 3-4 treatment-related adverse events, including neutropenia (n=3), thrombocytopenia (n=2), increased aspartate aminotransferase (n=1), febrile neutropenia (n=1), and tumor lysis syndrome (n=1). Four of these patients were on the 12 mg/kg dose, but 1 patient who experienced neutropenia was receiving 1 mg/kg.
Eleven percent of patients experienced a partial response according to IWCLL 2008 criteria. Responses occurred at the 6 mg/kg, 9 mg/kg, and 12 mg/kg dose levels.
All objective responses occurred in patients categorized as CLL as opposed to SLL, and none of the patients with lymph nodes greater than 5 cm responded. Two patients had progressed at the 8-week evaluation point. ![]()

The US Food and Drug Administration (FDA) has granted orphan designation for MOR208, an anti-CD19 monoclonal antibody, for the treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL).
The European Medicines Agency (EMA) also announced a positive opinion of the orphan medicinal product application for MOR208 to treat patients with CLL/SLL.
Researchers said MOR208 showed promise in a phase 1 study of CLL/SLL patients.
Orphan drug and orphan medicinal product status are granted by the FDA and EMA to promote the development of promising therapeutics for the treatment of rare diseases affecting fewer than 200,000 people in the US annually and no more than 5 in 10,000 people in the European Union (EU).
Orphan drug designation includes benefits such as a 7-year period of marketing exclusivity in the US and 10 years of market exclusivity in the EU after approval. Other potential advantages come in the form of protocol assistance, the ability to apply for research funding, tax credits for certain research expenses, and fee waivers for the regulatory procedures.
MOR208 development, results
MOR208 is a humanized monoclonal antibody that targets the antigen CD19 for treatment of B-cell malignancies and autoimmune diseases. The development program for MOR208 is currently in phase 2 development in CLL, B-cell acute lymphoblastic leukemia, and non-Hodgkin lymphoma.
The drug is being developed by MorphoSys AG. The development program was in-licensed from Xencor in 2010.
Researchers evaluated MOR208 (formerly known as XmAb5574) in a phase 1 study of patients with CLL/SLL and reported their results at the 2012 ASH Annual Meeting (abstract 2894). The study was sponsored by Xencor.
The study included 27 patients with relapsed or refractory CLL/SLL. The median patient age was 66 years (range, 40-84), and patients were generally high-risk. The median number of prior therapies was 4 (range, 1-14).
Patients received MOR208 at a range of doses—0.3 mg/kg, 1 mg/kg, 3 mg/kg, 6 mg/kg, 9 mg/kg, and 12 mg/kg. MOR208 was administered as an intravenous infusion on days 1, 4, 8, 15, and 22 of cycle 1, and on days 1, 8, 15, and 22 of cycle 2.
Dose escalation continued without dose-limiting toxicities until the highest dose level. At this dose, 1 patient experienced grade 4 neutropenia associated with febrile neutropenia, and the patient was taken off treatment.
All 27 patients experienced adverse events, but the majority of them were grade 1-2. The most common events were infusion reactions. These occurred in 67% (n=18) of patients and were all grade 1 or 2.
Nineteen percent of patients (n=5) experienced grade 3-4 treatment-related adverse events, including neutropenia (n=3), thrombocytopenia (n=2), increased aspartate aminotransferase (n=1), febrile neutropenia (n=1), and tumor lysis syndrome (n=1). Four of these patients were on the 12 mg/kg dose, but 1 patient who experienced neutropenia was receiving 1 mg/kg.
Eleven percent of patients experienced a partial response according to IWCLL 2008 criteria. Responses occurred at the 6 mg/kg, 9 mg/kg, and 12 mg/kg dose levels.
All objective responses occurred in patients categorized as CLL as opposed to SLL, and none of the patients with lymph nodes greater than 5 cm responded. Two patients had progressed at the 8-week evaluation point. ![]()
An Inpatient Clinical Decision Algorithm
Decision‐making capacity is a dynamic, integrative cognitive function necessary for informed consent. Capacity is assessed relative to a specific choice about medical care (eg, Does this patient with mild Alzheimer's disease have the capacity to decide whether to undergo valvuloplasty for severe aortic stenosis?), Capacity may be impaired by acute illnesses (eg, toxidromes and withdrawal states, medical illness‐related delirium, decompensated psychiatric episodes), as well as chronic conditions (eg, dementia, developmental disability, traumatic brain injuries, central nervous system (CNS) degenerative disorders). Given the proper training, clinicians from any specialty can assess a patient's decision‐making capacity.[1] A patient must satisfy 4 principles to have the capacity for a given decision: understanding of the condition, ability to communicate a choice, conception of the risks and benefits of the decision, and a rational approach to decision making.[2, 3, 4] Management of incapacitated persons may require consideration of the individual's stated or demonstrated preferences, medical ethics principles (eg, to consider the balance between autonomy, beneficence, and nonmaleficence during shared decision making), and institutional and situational norms and standards. Management may include immediate or long‐term medical and safety planning, and the selection of a surrogate decision maker or public guardian.[1, 2, 3, 4, 5, 6, 7, 8] A related term, competency, describes a legal judgment regarding a person's ability to make decisions, and persons deemed incompetent require an appointed guardian to make 1 or more types of decision (eg, medical, financial, and long‐term care planning).[1, 8]
Over one‐quarter of general medical inpatients display impaired decision‐making capacity based on a recent review of multiple studies.[2] Nursing home residents, persons with Alzheimer's dementia, and persons with developmental disabilitygroups commonly encountered in the inpatient settingdemonstrate impaired capacity in greater than 40% to 60% of cases.[2] Capacity impairment is present in three‐quarters of inpatients with life‐threatening illnesses.[5] The frequency of capacity impairment is complicated by the fact that physicians fail to recognize impaired capacity in as much as 60% of cases.[1, 2] Misunderstanding of the laws and medical and ethical principles related to capacity is common, even among specialists who commonly care for incapacitated patients, such as consult liaison psychiatrists, geriatricians, and psychologists.[1]
Loss of decision‐making capacity may be associated with negative consequences to the patient and to the provider‐patient dyad. Patients with capacity impairment have been shown to have an increased risk of mortality in a community setting.[6] Potential ethical pitfalls between provider and incapacitated patient have been described.[5] The high cost of long‐term management of subsets of incapacitated patients has also been noted.[7]
Improved identification and management of incapacitated patients has potential benefit to medical outcomes, patient safety, and cost containment.[6, 7, 9] The importance of education in this regard, especially to early career clinicians and to providers in specialties other than mental health, has been noted.[9] This article describes a clinical quality improvement project at San Francisco General Hospital and Trauma Center (SFGH) to improve provider identification and management of patients with impaired decision‐making capacity via a clinical decision algorithm.
METHODS
In 2012, the Department of Risk Management at SFGH created a multidisciplinary workgroup, including attending physicians, nurses, administrators, and hospital safety officers to improve the institutional process for identification and management of inpatients with impaired decision‐making capacity. The workgroup reviewed prior experience with incapacitated patients and data from multiple sources, including unusual occurrence reports, hospital root cause analyses, and hospital policies regarding patients with cognitive impairment. Expert opinion was solicited from attending psychiatry and neuropsychology providers.
SFGHan urban, academic, safety‐net hospitalcares for a diverse, underserved, and medically vulnerable patient population with high rates of cognitive and capacity impairment. A publication currently under review from SFGH shows that among a cohort of roughly 700 general medical inpatients 50 years and older, greater than 54% have mild or greater degrees of cognitive impairment based on the Telephone Interview for Cognitive Status test (unpublished data).[10] Among SFGH medical inpatients with extended lengths of stay, roughly one‐third have impaired capacity, require a family surrogate decision maker, or have an established public guardian (unpublished data). Among incapacitated patients, a particularly challenging subset have impaired decision making but significant physical capacity, creating risk of harm to self or others (eg, during the 18 months preintervention, an average of 9 incapacitated but physically capable inpatients per month attempted to leave SFGH prior to discharge) (unpublished data).
The majority of incapacitated patients at SFGH are cared for by 5 inpatient medical services staffed by resident and attending physicians from the University of California San Francisco: cardiology, family medicine, internal medicine, neurology, and psychiatry (unpublished data). Despite the commonality of capacity impairment on these services, education about capacity impairment and management was consistently reviewed only in the Department of Psychiatry.
Challenges common to prior experience with incapacitated patients were considered, including inefficient navigation of a complex, multistep identification and management process; difficulty addressing the high‐risk subset of incapacitated, able‐bodied patients who may pose an immediate safety risk; and incomplete understanding of the timing and indications for consultants (including psychiatry, neuropsychology, and medical ethics). To improve clinical outcome and patient safety through clinician identification and management, the workgroup created a clinical decision algorithm in a visual process map format for ease of use at the point of care.
Using MEDLINE and PubMed, the workgroup conducted a brief review of existing tools for incapacitated patients with relevant search terms and Medical Subjects Headings, including capacity, inpatient, shared decision making, mental competency, guideline, and algorithm. Publications reviewed included tools for capacity assessment (Addenbrooke's Cognitive Examination, MacArthur Competence Assessment Tool for Treatment)[2, 3, 4, 11] delineation of the basic process of capacity evaluation and subsequent management,[12, 13, 14, 15, 16] and explanation of the role of specialty consultation.[3, 9, 17] Specific attention was given to finding published visual algorithms; here, search results tended to focus on specialty consultation (eg, neuropsychology testing),[17] highly specific clinical situations (eg, sexual assault),[18] or to systems outside the United States.[19, 20, 21, 22] Byatt et al.'s work (2006) contains a useful visual algorithm about management of incapacitated patients, but it operates from the perspective of consult liaison psychiatrists, and the algorithm does not include principles of capacity assessment.[23] Derse ([16]) provides a text‐based algorithm relevant to primary inpatient providers, but does not have a visual illustration.[16] In our review, we were unable to find a visual algorithm that consolidates the process of identification, evaluation, and management of hospital inpatients with impaired decision‐making capacity.
Based on the described needs assessment, the workgroup created a draft algorithm for review by the SFGH medical executive committee, nursing quality council, and ethics committee.
RESULTS
The Clinical Decision Algorithm for Hospital Inpatients With Impaired Decision‐Making Capacity (adapted version, Figure 1) consolidates identification and management into a 1‐page visual process map, emphasizes safety planning for high‐risk patients, and explains indication and timing for multidisciplinary consultation, thereby addressing the 3 most prominent challenges based on our data and case review. Following hospital executive approval, the algorithm and a set of illustrative cases were disseminated to clinicians via email from service leadership, laminated copies were posted in housestaff workrooms, an electronic copy was posted on the website of the SFGH Department of Risk Management, and the algorithm was incorporated into hospital policy. Workgroup members conducted trainings with housestaff from the services identified as most frequently caring for incapacitated inpatients.
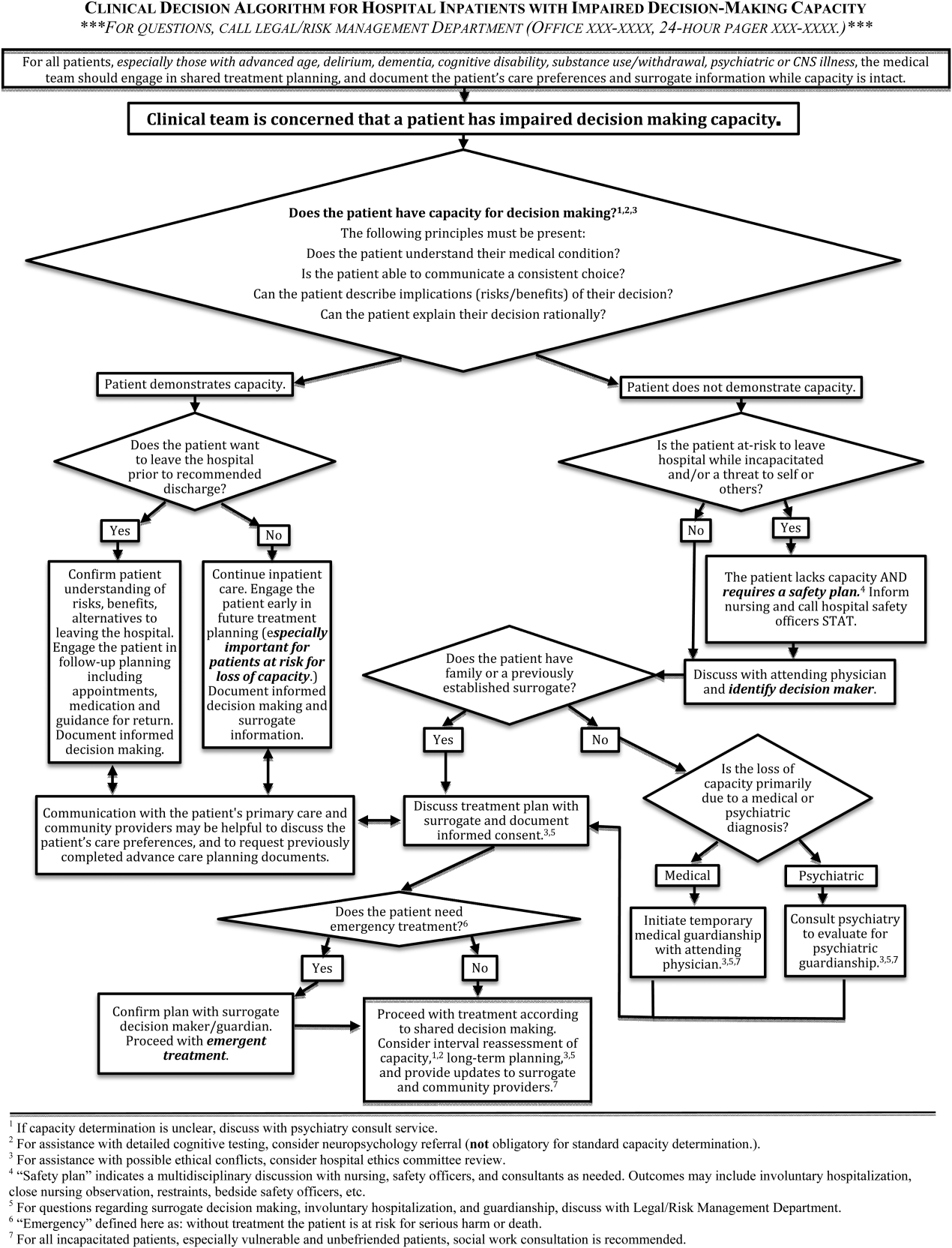
During trainings, housestaff participants expressed an improved sense of understanding and decreased anxiety about identification and management of incapacitated patients. During subsequent discussions, inpatient housestaff noted improvement in teamwork with safety officers, including cases involving agitated or threatening patients prior to capacity assessment.
An unexpected benefit of the algorithm was recognition of the need for associated resources, including a surrogate decision‐maker documentation form, off‐hours attending physician oversight for medical inpatients with capacity‐related emergencies, and a formal agreement with hospital safety officers regarding the care of high‐risk incapacitated patients not previously on a legal hold or surrogate guardianship. These were created in parallel with the algorithm and have become an integral part of management of incapacitated patients.
CLINICAL DECISION ALGORITHM APPLICATION TO PATIENT SCENARIOS
The following 3 scenarios exemplify common challenges in caring for inpatients with compromised decision‐making capacity. Assessment and multidisciplinary management are explained in relation to the clinical decision algorithm (Figure 1.)
Case 1
An 87‐year‐old woman with mild cognitive impairment presents to the emergency department with community‐acquired pneumonia. The patient is widowed, lives alone in a senior community, and has an established relationship with a primary care physician in the area. On initial examination, the patient is febrile and dyspneic, but still alert and able to give a coherent history. She is able to close the loop and teach‐back regarding the diagnosis of pneumonia and agrees with the treatment plan as explained. Should consideration be given to this patient's decision‐making capacity at this time? What capacity‐related information would be helpful to review with the patient and to document in the record?
Inpatient teams should prospectively identify patients at‐risk for loss of capacity and create a shared treatment plan with the patient while capacity is intact (as noted in the top box in Figure 1). When the inpatient team first meets this patient, she retains decision‐making capacity with regard to hospitalization for pneumonia (left branch after first diamond, Figure 1); however she is at risk for delirium based on her age, mild cognitive impairment, and pneumonia.25 She is willing to stay in the hospital for treatment (right branch after second diamond, Figure 1). For this patient at risk for loss of capacity, it is especially important that the inpatient team explore the patient's care preferences regarding predictable crisis points in the care plan (eg, need for invasive respiratory support or intensive care unit admission.) Her surrogate decision maker's name and contact information should be confirmed. Communication with the patient's primary care provider is advised to review knowledge about the patient's care preferences and request previously completed advance‐care planning documents.
Case 2
A 37‐year‐old man is admitted to the hospital for alcohol withdrawal. On hospital day 1, he develops hyperactive delirium and attempts to leave the hospital. The patient becomes agitated and physically aggressive when the nurse and physician inform him that it is not safe to leave the hospital. He denies having any health problems, he is unable to explain potential risks if his alcohol withdrawal is left untreated, and he cannot articulate a plan to care for himself. The patient attempts to strike a staff member and runs out of the inpatient unit. The patient's family members live in the area, and they can be reached by phone. What are the next appropriate management steps?
This patient has alcohol withdrawal delirium, an emergent medical condition requiring inpatient treatment. The patient demonstrates impaired decision‐making capacity related to treatment because he does not understand his medical condition, he is unable to describe the consequences of the proposed action to leave the hospital, and he is not explaining his decision in rational terms (right hand branch of the algorithm after first diamond, Figure 1). The situation is made more urgent by the patient's aggressive behavior and flight from the inpatient unit, and he poses a risk of harm to self, to staff, and the public (right branch after second diamond, Figure 1). This patient requires a safety plan, and hospital safety officers should be notified immediately. The attending physician and surrogate decision maker should be contacted to create a safe management plan. In this case, a family member is available (left branch after third diamond, Figure 1). The patient requires emergent treatment of his alcohol withdrawal (left branch after fourth diamond, Figure 1). The team should proceed with this emergent treatment with documentation of the assessment, plan, and informed consent of the surrogate. As the patient recovers from acute alcohol withdrawal, the team should reassess his decision‐making capacity and continue to involve the surrogate decision maker until the patient regains capacity to make his own decisions.
Case 3
A 74‐year‐old woman is brought to the hospital by ambulance after being found by her neighbors wandering the hallways of her apartment building. She is disoriented, and her neighbors report a progressive functional decline over the past several months with worsening forgetfulness and occasional falls. She recently started a small fire in her toaster, which a neighbor extinguished after hearing the fire alarm. She is admitted and ultimately diagnosed her with Alzheimer's dementia (Functional Assessment Staging Test (FAST) Tool stage 6a). She is chronically disoriented, happy to be cared for by the hospital staff, and unable to get out of bed independently. She is deemed unsafe to be discharged to home, but she declines to be transferred to a location other than her apartment and declines in‐home care. She has no family or friends. What is the most appropriate course of action to establish a safe long‐term plan for the patient? What medicolegal principles inform the team's responsibility and authority? What consultations may be helpful to the primary medical team?
This patient is incapacitated with regard to long‐term care planning due to dementia. She does not understand her medical condition and cannot articulate the risks and benefits of returning to her apartment (right branch of algorithm after first diamond, Figure 1). The patient is physically unable leave the hospital and does not pose an immediate threat to self or others, thus safety officer assistance is not immediately indicated (left branch at second diamond, Figure 1). Without an available surrogate, this patient might be classified as unbefriended or unrepresented.[7] She will likely require a physician to assist with immediate medical decisions (bottom right corner of algorithm, Figure 1). Emergent treatment is not needed (right branch after fourth diamond,) but long term planning for this vulnerable patient should begin early in the hospital course. Discussion between inpatient and community‐based providers, especially primary care, is recommended to understand the patient's prior care preferences and investigate if she has completed advance care planning documents (two‐headed arrow connecting to square at left side of algorithm.) Involvement of the hospital risk management/legal department may assist with the legal proceedings needed to establish long‐term guardianship (algorithm footnote 5, Figure 1). Ethics consultation may be helpful to consider the balance between the patient's demonstrated values, her autonomy, and the role of substituted judgment in long‐term care planning[7] (algorithm footnote 3, Figure 1). Psychiatric or neuropsychology consultation during her inpatient admission may be useful in preparation for a competency hearing (algorithm footnotes 1 and 2, Figure 1). Social work consultation to provide advocacy for this vulnerable patient would be advisable (algorithm footnote 7).
DISCUSSION
Impaired decision‐making capacity is a common and challenging condition among hospitalized patients, including at our institution. Prior studies show that physicians frequently fail to recognize capacity impairment, and also demonstrate common misunderstandings about the medicolegal framework that governs capacity determination and subsequent care. Patients with impaired decision‐making capacity are vulnerable to adverse outcomes, and there is potential for negative effects on healthcare systems. The management of patients with impaired capacity may involve multiple disciplines and a complex intersection of medical, legal, ethical, and neuropsychological principles.
To promote safety of this vulnerable population at SFGH, our workgroup created a visual algorithm to guide clinicians. The algorithm may improve on existing tools by consolidating the steps from identification through management into a 1‐page visual tool, by emphasizing safety planning for high‐risk incapacitated patients and by elucidating roles and timing for other members of the multidisciplinary management team. Creation of the algorithm facilitated intervention for other practical issues, including institutional and departmental agreements and documentation regarding surrogate decision makers for incapacitated patients.
Although based on a multispecialty institutional review and previously published tools, there are potential limitations to this tool. It seems reasonable to assume that a tool to organize a complex process, such as identification and management of incapacitated patients, should improve patient care versus a non‐standardized process. Although the algorithm is posted in resident workrooms, on the hospital's risk management website, and incorporated as part of hospital policy, we have not yet had the opportunity to study the frequency of its use and impact in patient care. Patient safety and clinical outcome of patients managed with this algorithm could be assessed; however, the impact of the algorithm at SFGH may be confounded by a separate intervention addressing nursing and safety officers that was initiated shortly after the algorithm was produced.
To assess health‐system effects of incapacitated patients, future studies might compare patients with capacity impairment versus those with intact decision making relative to demographic background and payer mix, rates of adverse events during inpatient stay (eg, hospital‐acquired injury), rates of morbidity and mortality, rate of provider identification and documentation of surrogates, patient and surrogate satisfaction data, length of stay and cost of hospitalization, and rates of successful discharge to a community‐based setting. We present this algorithm as an example for diverse settings to address the common challenge of caring for acutely ill patients with impaired decision‐making capacity.
Acknowledgements
The authors thank Lee Rawitscher, MD, for his contribution of capacity assessment handout and review of this manuscript, and to Jeff Critchfield, MD; Robyn Schanzenbach, JD; and Troy Williams, RN, MSN for review of this manuscript. The San Francisco General Hospital Workgroup on Patient Capacity and Medical Decision Making includes Richard Brooks, MD; Beth Brumell, RN; Andy Brunner, JD; Jack Chase, MD; Jeff Critchfield, MD; Leslie Dubbin, RN, MSN, PhD(c); Larry Haber, MD; Lee Rawitscher, MD; and Troy Williams, RN, MSN.
Disclosures
Nothing to report.
- Ten myths about decision making capacity: a report by the National Ethics Committee of the Veterans Health Administration. Department of Veterans Affairs; September 2002. Available at: http://www.ethics.va.gov/docs/necrpts/nec_report_20020201_ten_myths_about_dmc.pdf. Accessed August 13, 2013.
- , , . Does this patient have decision making capacity? JAMA. 2011;306(4):420–427.
- , . Assessing patients' capacities to consent for treatment. N Engl J Med. 1988;319:1635–1638.
- . Assessment of patients' competence to consent to treatment. N Engl J Med. 2007;357:1834–1840.
- , . Can we improve treatment decision‐making for incapacitated patients? Hastings Cent Rep. 2010;40(5):36–45.
- , , , , . Poor decision making is associated with an increased risk of mortality among community‐dwelling older persons without dementia. Neuroepidemiology. 2013;40(4):247–252.
- . Making medical decisions for patients without surrogates. N Engl J Med. 2013;369:1976–1978.
- American Bar Association Commission on Law and Aging and American Psychological Association. Assessment of Older Adults With Diminished Capacity: A Handbook for Lawyers. Washington, DC: American Bar Association and American Psychological Association; 2005.
- , , . Psychiatric evaluation of mental capacity in the general hospital: a significant teaching opportunity. Psychosomatics. 2009;50:468–473.
- , , , , , . Telephone‐based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol. 2011;68(5):607–614.
- , , , et al. Assessment of patient capacity to consent to treatment. J Gen Intern Med. 1999;14(1):27–34.
- . Competency and the capacity to make treatment decisions: a primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 1999;1(5):131–141.
- . Can the patient decide? Evaluating patient capacity in practice. Am Fam Physician. 2001;64(2):299–308.
- , . Capacity decisions in the general hospital: when can you refuse to follow a person's wishes? Prim Care Companion J Clin Psychiatry. 2003;5(4):177–181.
- , . Assessing capacity. Emerg Med Clin North Am. 2000;18(2):233–242, viii.
- . What part of “no” don't you understand? Patient refusal of recommended treatment in the emergency department. Mt Sinai J Med. 2005;72(4):221–227.
- , , . Neuropsychological evaluation in primary care. Am Fam Physician. 2010;82(5):495–502.
- , , . Determining competency in the sexually assaulted patient: a decision algorithm. J Forensic Leg Med. 2010;17:275–279.
- , . A practical guide to capacity assessment and patient consent in Hong Kong. Hong Kong Med J. 2003;9:284–289.
- Alberta (Canada) Health Services. Algorithm range of capacity and decision making options. Available at: http://www.albertahealthservices.ca/hp/if‐hp‐phys‐consent‐capacity‐decision‐algorithm.pdf. Accessed August 13, 2013.
- , . The Mental Capacity Act 2007 and capacity assessments: a guide for the non‐psychiatrist. Clin Med. 2008;8(1):65–69.
- NICE clinical guideline 16: self harm. The short‐term physical and psychological management and secondary prevention of self‐harm in primary and secondary care. London, UK: National Institute for Clinical Excellence (NICE); July 2004. Available at: http://guidance.nice.org.uk/CG16, accessed on August 13, 2013.
- , , . Involuntary hospitalization of medical patients who lack decisional capacity: an unresolved issue. Psychosomatics. 2006;47(5):443–448.
- , , , et al. The AWOL tool: derivation and validation of a delirium prediction rule. J Hosp Med. 2013;8:493–499.
Decision‐making capacity is a dynamic, integrative cognitive function necessary for informed consent. Capacity is assessed relative to a specific choice about medical care (eg, Does this patient with mild Alzheimer's disease have the capacity to decide whether to undergo valvuloplasty for severe aortic stenosis?), Capacity may be impaired by acute illnesses (eg, toxidromes and withdrawal states, medical illness‐related delirium, decompensated psychiatric episodes), as well as chronic conditions (eg, dementia, developmental disability, traumatic brain injuries, central nervous system (CNS) degenerative disorders). Given the proper training, clinicians from any specialty can assess a patient's decision‐making capacity.[1] A patient must satisfy 4 principles to have the capacity for a given decision: understanding of the condition, ability to communicate a choice, conception of the risks and benefits of the decision, and a rational approach to decision making.[2, 3, 4] Management of incapacitated persons may require consideration of the individual's stated or demonstrated preferences, medical ethics principles (eg, to consider the balance between autonomy, beneficence, and nonmaleficence during shared decision making), and institutional and situational norms and standards. Management may include immediate or long‐term medical and safety planning, and the selection of a surrogate decision maker or public guardian.[1, 2, 3, 4, 5, 6, 7, 8] A related term, competency, describes a legal judgment regarding a person's ability to make decisions, and persons deemed incompetent require an appointed guardian to make 1 or more types of decision (eg, medical, financial, and long‐term care planning).[1, 8]
Over one‐quarter of general medical inpatients display impaired decision‐making capacity based on a recent review of multiple studies.[2] Nursing home residents, persons with Alzheimer's dementia, and persons with developmental disabilitygroups commonly encountered in the inpatient settingdemonstrate impaired capacity in greater than 40% to 60% of cases.[2] Capacity impairment is present in three‐quarters of inpatients with life‐threatening illnesses.[5] The frequency of capacity impairment is complicated by the fact that physicians fail to recognize impaired capacity in as much as 60% of cases.[1, 2] Misunderstanding of the laws and medical and ethical principles related to capacity is common, even among specialists who commonly care for incapacitated patients, such as consult liaison psychiatrists, geriatricians, and psychologists.[1]
Loss of decision‐making capacity may be associated with negative consequences to the patient and to the provider‐patient dyad. Patients with capacity impairment have been shown to have an increased risk of mortality in a community setting.[6] Potential ethical pitfalls between provider and incapacitated patient have been described.[5] The high cost of long‐term management of subsets of incapacitated patients has also been noted.[7]
Improved identification and management of incapacitated patients has potential benefit to medical outcomes, patient safety, and cost containment.[6, 7, 9] The importance of education in this regard, especially to early career clinicians and to providers in specialties other than mental health, has been noted.[9] This article describes a clinical quality improvement project at San Francisco General Hospital and Trauma Center (SFGH) to improve provider identification and management of patients with impaired decision‐making capacity via a clinical decision algorithm.
METHODS
In 2012, the Department of Risk Management at SFGH created a multidisciplinary workgroup, including attending physicians, nurses, administrators, and hospital safety officers to improve the institutional process for identification and management of inpatients with impaired decision‐making capacity. The workgroup reviewed prior experience with incapacitated patients and data from multiple sources, including unusual occurrence reports, hospital root cause analyses, and hospital policies regarding patients with cognitive impairment. Expert opinion was solicited from attending psychiatry and neuropsychology providers.
SFGHan urban, academic, safety‐net hospitalcares for a diverse, underserved, and medically vulnerable patient population with high rates of cognitive and capacity impairment. A publication currently under review from SFGH shows that among a cohort of roughly 700 general medical inpatients 50 years and older, greater than 54% have mild or greater degrees of cognitive impairment based on the Telephone Interview for Cognitive Status test (unpublished data).[10] Among SFGH medical inpatients with extended lengths of stay, roughly one‐third have impaired capacity, require a family surrogate decision maker, or have an established public guardian (unpublished data). Among incapacitated patients, a particularly challenging subset have impaired decision making but significant physical capacity, creating risk of harm to self or others (eg, during the 18 months preintervention, an average of 9 incapacitated but physically capable inpatients per month attempted to leave SFGH prior to discharge) (unpublished data).
The majority of incapacitated patients at SFGH are cared for by 5 inpatient medical services staffed by resident and attending physicians from the University of California San Francisco: cardiology, family medicine, internal medicine, neurology, and psychiatry (unpublished data). Despite the commonality of capacity impairment on these services, education about capacity impairment and management was consistently reviewed only in the Department of Psychiatry.
Challenges common to prior experience with incapacitated patients were considered, including inefficient navigation of a complex, multistep identification and management process; difficulty addressing the high‐risk subset of incapacitated, able‐bodied patients who may pose an immediate safety risk; and incomplete understanding of the timing and indications for consultants (including psychiatry, neuropsychology, and medical ethics). To improve clinical outcome and patient safety through clinician identification and management, the workgroup created a clinical decision algorithm in a visual process map format for ease of use at the point of care.
Using MEDLINE and PubMed, the workgroup conducted a brief review of existing tools for incapacitated patients with relevant search terms and Medical Subjects Headings, including capacity, inpatient, shared decision making, mental competency, guideline, and algorithm. Publications reviewed included tools for capacity assessment (Addenbrooke's Cognitive Examination, MacArthur Competence Assessment Tool for Treatment)[2, 3, 4, 11] delineation of the basic process of capacity evaluation and subsequent management,[12, 13, 14, 15, 16] and explanation of the role of specialty consultation.[3, 9, 17] Specific attention was given to finding published visual algorithms; here, search results tended to focus on specialty consultation (eg, neuropsychology testing),[17] highly specific clinical situations (eg, sexual assault),[18] or to systems outside the United States.[19, 20, 21, 22] Byatt et al.'s work (2006) contains a useful visual algorithm about management of incapacitated patients, but it operates from the perspective of consult liaison psychiatrists, and the algorithm does not include principles of capacity assessment.[23] Derse ([16]) provides a text‐based algorithm relevant to primary inpatient providers, but does not have a visual illustration.[16] In our review, we were unable to find a visual algorithm that consolidates the process of identification, evaluation, and management of hospital inpatients with impaired decision‐making capacity.
Based on the described needs assessment, the workgroup created a draft algorithm for review by the SFGH medical executive committee, nursing quality council, and ethics committee.
RESULTS
The Clinical Decision Algorithm for Hospital Inpatients With Impaired Decision‐Making Capacity (adapted version, Figure 1) consolidates identification and management into a 1‐page visual process map, emphasizes safety planning for high‐risk patients, and explains indication and timing for multidisciplinary consultation, thereby addressing the 3 most prominent challenges based on our data and case review. Following hospital executive approval, the algorithm and a set of illustrative cases were disseminated to clinicians via email from service leadership, laminated copies were posted in housestaff workrooms, an electronic copy was posted on the website of the SFGH Department of Risk Management, and the algorithm was incorporated into hospital policy. Workgroup members conducted trainings with housestaff from the services identified as most frequently caring for incapacitated inpatients.

During trainings, housestaff participants expressed an improved sense of understanding and decreased anxiety about identification and management of incapacitated patients. During subsequent discussions, inpatient housestaff noted improvement in teamwork with safety officers, including cases involving agitated or threatening patients prior to capacity assessment.
An unexpected benefit of the algorithm was recognition of the need for associated resources, including a surrogate decision‐maker documentation form, off‐hours attending physician oversight for medical inpatients with capacity‐related emergencies, and a formal agreement with hospital safety officers regarding the care of high‐risk incapacitated patients not previously on a legal hold or surrogate guardianship. These were created in parallel with the algorithm and have become an integral part of management of incapacitated patients.
CLINICAL DECISION ALGORITHM APPLICATION TO PATIENT SCENARIOS
The following 3 scenarios exemplify common challenges in caring for inpatients with compromised decision‐making capacity. Assessment and multidisciplinary management are explained in relation to the clinical decision algorithm (Figure 1.)
Case 1
An 87‐year‐old woman with mild cognitive impairment presents to the emergency department with community‐acquired pneumonia. The patient is widowed, lives alone in a senior community, and has an established relationship with a primary care physician in the area. On initial examination, the patient is febrile and dyspneic, but still alert and able to give a coherent history. She is able to close the loop and teach‐back regarding the diagnosis of pneumonia and agrees with the treatment plan as explained. Should consideration be given to this patient's decision‐making capacity at this time? What capacity‐related information would be helpful to review with the patient and to document in the record?
Inpatient teams should prospectively identify patients at‐risk for loss of capacity and create a shared treatment plan with the patient while capacity is intact (as noted in the top box in Figure 1). When the inpatient team first meets this patient, she retains decision‐making capacity with regard to hospitalization for pneumonia (left branch after first diamond, Figure 1); however she is at risk for delirium based on her age, mild cognitive impairment, and pneumonia.25 She is willing to stay in the hospital for treatment (right branch after second diamond, Figure 1). For this patient at risk for loss of capacity, it is especially important that the inpatient team explore the patient's care preferences regarding predictable crisis points in the care plan (eg, need for invasive respiratory support or intensive care unit admission.) Her surrogate decision maker's name and contact information should be confirmed. Communication with the patient's primary care provider is advised to review knowledge about the patient's care preferences and request previously completed advance‐care planning documents.
Case 2
A 37‐year‐old man is admitted to the hospital for alcohol withdrawal. On hospital day 1, he develops hyperactive delirium and attempts to leave the hospital. The patient becomes agitated and physically aggressive when the nurse and physician inform him that it is not safe to leave the hospital. He denies having any health problems, he is unable to explain potential risks if his alcohol withdrawal is left untreated, and he cannot articulate a plan to care for himself. The patient attempts to strike a staff member and runs out of the inpatient unit. The patient's family members live in the area, and they can be reached by phone. What are the next appropriate management steps?
This patient has alcohol withdrawal delirium, an emergent medical condition requiring inpatient treatment. The patient demonstrates impaired decision‐making capacity related to treatment because he does not understand his medical condition, he is unable to describe the consequences of the proposed action to leave the hospital, and he is not explaining his decision in rational terms (right hand branch of the algorithm after first diamond, Figure 1). The situation is made more urgent by the patient's aggressive behavior and flight from the inpatient unit, and he poses a risk of harm to self, to staff, and the public (right branch after second diamond, Figure 1). This patient requires a safety plan, and hospital safety officers should be notified immediately. The attending physician and surrogate decision maker should be contacted to create a safe management plan. In this case, a family member is available (left branch after third diamond, Figure 1). The patient requires emergent treatment of his alcohol withdrawal (left branch after fourth diamond, Figure 1). The team should proceed with this emergent treatment with documentation of the assessment, plan, and informed consent of the surrogate. As the patient recovers from acute alcohol withdrawal, the team should reassess his decision‐making capacity and continue to involve the surrogate decision maker until the patient regains capacity to make his own decisions.
Case 3
A 74‐year‐old woman is brought to the hospital by ambulance after being found by her neighbors wandering the hallways of her apartment building. She is disoriented, and her neighbors report a progressive functional decline over the past several months with worsening forgetfulness and occasional falls. She recently started a small fire in her toaster, which a neighbor extinguished after hearing the fire alarm. She is admitted and ultimately diagnosed her with Alzheimer's dementia (Functional Assessment Staging Test (FAST) Tool stage 6a). She is chronically disoriented, happy to be cared for by the hospital staff, and unable to get out of bed independently. She is deemed unsafe to be discharged to home, but she declines to be transferred to a location other than her apartment and declines in‐home care. She has no family or friends. What is the most appropriate course of action to establish a safe long‐term plan for the patient? What medicolegal principles inform the team's responsibility and authority? What consultations may be helpful to the primary medical team?
This patient is incapacitated with regard to long‐term care planning due to dementia. She does not understand her medical condition and cannot articulate the risks and benefits of returning to her apartment (right branch of algorithm after first diamond, Figure 1). The patient is physically unable leave the hospital and does not pose an immediate threat to self or others, thus safety officer assistance is not immediately indicated (left branch at second diamond, Figure 1). Without an available surrogate, this patient might be classified as unbefriended or unrepresented.[7] She will likely require a physician to assist with immediate medical decisions (bottom right corner of algorithm, Figure 1). Emergent treatment is not needed (right branch after fourth diamond,) but long term planning for this vulnerable patient should begin early in the hospital course. Discussion between inpatient and community‐based providers, especially primary care, is recommended to understand the patient's prior care preferences and investigate if she has completed advance care planning documents (two‐headed arrow connecting to square at left side of algorithm.) Involvement of the hospital risk management/legal department may assist with the legal proceedings needed to establish long‐term guardianship (algorithm footnote 5, Figure 1). Ethics consultation may be helpful to consider the balance between the patient's demonstrated values, her autonomy, and the role of substituted judgment in long‐term care planning[7] (algorithm footnote 3, Figure 1). Psychiatric or neuropsychology consultation during her inpatient admission may be useful in preparation for a competency hearing (algorithm footnotes 1 and 2, Figure 1). Social work consultation to provide advocacy for this vulnerable patient would be advisable (algorithm footnote 7).
DISCUSSION
Impaired decision‐making capacity is a common and challenging condition among hospitalized patients, including at our institution. Prior studies show that physicians frequently fail to recognize capacity impairment, and also demonstrate common misunderstandings about the medicolegal framework that governs capacity determination and subsequent care. Patients with impaired decision‐making capacity are vulnerable to adverse outcomes, and there is potential for negative effects on healthcare systems. The management of patients with impaired capacity may involve multiple disciplines and a complex intersection of medical, legal, ethical, and neuropsychological principles.
To promote safety of this vulnerable population at SFGH, our workgroup created a visual algorithm to guide clinicians. The algorithm may improve on existing tools by consolidating the steps from identification through management into a 1‐page visual tool, by emphasizing safety planning for high‐risk incapacitated patients and by elucidating roles and timing for other members of the multidisciplinary management team. Creation of the algorithm facilitated intervention for other practical issues, including institutional and departmental agreements and documentation regarding surrogate decision makers for incapacitated patients.
Although based on a multispecialty institutional review and previously published tools, there are potential limitations to this tool. It seems reasonable to assume that a tool to organize a complex process, such as identification and management of incapacitated patients, should improve patient care versus a non‐standardized process. Although the algorithm is posted in resident workrooms, on the hospital's risk management website, and incorporated as part of hospital policy, we have not yet had the opportunity to study the frequency of its use and impact in patient care. Patient safety and clinical outcome of patients managed with this algorithm could be assessed; however, the impact of the algorithm at SFGH may be confounded by a separate intervention addressing nursing and safety officers that was initiated shortly after the algorithm was produced.
To assess health‐system effects of incapacitated patients, future studies might compare patients with capacity impairment versus those with intact decision making relative to demographic background and payer mix, rates of adverse events during inpatient stay (eg, hospital‐acquired injury), rates of morbidity and mortality, rate of provider identification and documentation of surrogates, patient and surrogate satisfaction data, length of stay and cost of hospitalization, and rates of successful discharge to a community‐based setting. We present this algorithm as an example for diverse settings to address the common challenge of caring for acutely ill patients with impaired decision‐making capacity.
Acknowledgements
The authors thank Lee Rawitscher, MD, for his contribution of capacity assessment handout and review of this manuscript, and to Jeff Critchfield, MD; Robyn Schanzenbach, JD; and Troy Williams, RN, MSN for review of this manuscript. The San Francisco General Hospital Workgroup on Patient Capacity and Medical Decision Making includes Richard Brooks, MD; Beth Brumell, RN; Andy Brunner, JD; Jack Chase, MD; Jeff Critchfield, MD; Leslie Dubbin, RN, MSN, PhD(c); Larry Haber, MD; Lee Rawitscher, MD; and Troy Williams, RN, MSN.
Disclosures
Nothing to report.
Decision‐making capacity is a dynamic, integrative cognitive function necessary for informed consent. Capacity is assessed relative to a specific choice about medical care (eg, Does this patient with mild Alzheimer's disease have the capacity to decide whether to undergo valvuloplasty for severe aortic stenosis?), Capacity may be impaired by acute illnesses (eg, toxidromes and withdrawal states, medical illness‐related delirium, decompensated psychiatric episodes), as well as chronic conditions (eg, dementia, developmental disability, traumatic brain injuries, central nervous system (CNS) degenerative disorders). Given the proper training, clinicians from any specialty can assess a patient's decision‐making capacity.[1] A patient must satisfy 4 principles to have the capacity for a given decision: understanding of the condition, ability to communicate a choice, conception of the risks and benefits of the decision, and a rational approach to decision making.[2, 3, 4] Management of incapacitated persons may require consideration of the individual's stated or demonstrated preferences, medical ethics principles (eg, to consider the balance between autonomy, beneficence, and nonmaleficence during shared decision making), and institutional and situational norms and standards. Management may include immediate or long‐term medical and safety planning, and the selection of a surrogate decision maker or public guardian.[1, 2, 3, 4, 5, 6, 7, 8] A related term, competency, describes a legal judgment regarding a person's ability to make decisions, and persons deemed incompetent require an appointed guardian to make 1 or more types of decision (eg, medical, financial, and long‐term care planning).[1, 8]
Over one‐quarter of general medical inpatients display impaired decision‐making capacity based on a recent review of multiple studies.[2] Nursing home residents, persons with Alzheimer's dementia, and persons with developmental disabilitygroups commonly encountered in the inpatient settingdemonstrate impaired capacity in greater than 40% to 60% of cases.[2] Capacity impairment is present in three‐quarters of inpatients with life‐threatening illnesses.[5] The frequency of capacity impairment is complicated by the fact that physicians fail to recognize impaired capacity in as much as 60% of cases.[1, 2] Misunderstanding of the laws and medical and ethical principles related to capacity is common, even among specialists who commonly care for incapacitated patients, such as consult liaison psychiatrists, geriatricians, and psychologists.[1]
Loss of decision‐making capacity may be associated with negative consequences to the patient and to the provider‐patient dyad. Patients with capacity impairment have been shown to have an increased risk of mortality in a community setting.[6] Potential ethical pitfalls between provider and incapacitated patient have been described.[5] The high cost of long‐term management of subsets of incapacitated patients has also been noted.[7]
Improved identification and management of incapacitated patients has potential benefit to medical outcomes, patient safety, and cost containment.[6, 7, 9] The importance of education in this regard, especially to early career clinicians and to providers in specialties other than mental health, has been noted.[9] This article describes a clinical quality improvement project at San Francisco General Hospital and Trauma Center (SFGH) to improve provider identification and management of patients with impaired decision‐making capacity via a clinical decision algorithm.
METHODS
In 2012, the Department of Risk Management at SFGH created a multidisciplinary workgroup, including attending physicians, nurses, administrators, and hospital safety officers to improve the institutional process for identification and management of inpatients with impaired decision‐making capacity. The workgroup reviewed prior experience with incapacitated patients and data from multiple sources, including unusual occurrence reports, hospital root cause analyses, and hospital policies regarding patients with cognitive impairment. Expert opinion was solicited from attending psychiatry and neuropsychology providers.
SFGHan urban, academic, safety‐net hospitalcares for a diverse, underserved, and medically vulnerable patient population with high rates of cognitive and capacity impairment. A publication currently under review from SFGH shows that among a cohort of roughly 700 general medical inpatients 50 years and older, greater than 54% have mild or greater degrees of cognitive impairment based on the Telephone Interview for Cognitive Status test (unpublished data).[10] Among SFGH medical inpatients with extended lengths of stay, roughly one‐third have impaired capacity, require a family surrogate decision maker, or have an established public guardian (unpublished data). Among incapacitated patients, a particularly challenging subset have impaired decision making but significant physical capacity, creating risk of harm to self or others (eg, during the 18 months preintervention, an average of 9 incapacitated but physically capable inpatients per month attempted to leave SFGH prior to discharge) (unpublished data).
The majority of incapacitated patients at SFGH are cared for by 5 inpatient medical services staffed by resident and attending physicians from the University of California San Francisco: cardiology, family medicine, internal medicine, neurology, and psychiatry (unpublished data). Despite the commonality of capacity impairment on these services, education about capacity impairment and management was consistently reviewed only in the Department of Psychiatry.
Challenges common to prior experience with incapacitated patients were considered, including inefficient navigation of a complex, multistep identification and management process; difficulty addressing the high‐risk subset of incapacitated, able‐bodied patients who may pose an immediate safety risk; and incomplete understanding of the timing and indications for consultants (including psychiatry, neuropsychology, and medical ethics). To improve clinical outcome and patient safety through clinician identification and management, the workgroup created a clinical decision algorithm in a visual process map format for ease of use at the point of care.
Using MEDLINE and PubMed, the workgroup conducted a brief review of existing tools for incapacitated patients with relevant search terms and Medical Subjects Headings, including capacity, inpatient, shared decision making, mental competency, guideline, and algorithm. Publications reviewed included tools for capacity assessment (Addenbrooke's Cognitive Examination, MacArthur Competence Assessment Tool for Treatment)[2, 3, 4, 11] delineation of the basic process of capacity evaluation and subsequent management,[12, 13, 14, 15, 16] and explanation of the role of specialty consultation.[3, 9, 17] Specific attention was given to finding published visual algorithms; here, search results tended to focus on specialty consultation (eg, neuropsychology testing),[17] highly specific clinical situations (eg, sexual assault),[18] or to systems outside the United States.[19, 20, 21, 22] Byatt et al.'s work (2006) contains a useful visual algorithm about management of incapacitated patients, but it operates from the perspective of consult liaison psychiatrists, and the algorithm does not include principles of capacity assessment.[23] Derse ([16]) provides a text‐based algorithm relevant to primary inpatient providers, but does not have a visual illustration.[16] In our review, we were unable to find a visual algorithm that consolidates the process of identification, evaluation, and management of hospital inpatients with impaired decision‐making capacity.
Based on the described needs assessment, the workgroup created a draft algorithm for review by the SFGH medical executive committee, nursing quality council, and ethics committee.
RESULTS
The Clinical Decision Algorithm for Hospital Inpatients With Impaired Decision‐Making Capacity (adapted version, Figure 1) consolidates identification and management into a 1‐page visual process map, emphasizes safety planning for high‐risk patients, and explains indication and timing for multidisciplinary consultation, thereby addressing the 3 most prominent challenges based on our data and case review. Following hospital executive approval, the algorithm and a set of illustrative cases were disseminated to clinicians via email from service leadership, laminated copies were posted in housestaff workrooms, an electronic copy was posted on the website of the SFGH Department of Risk Management, and the algorithm was incorporated into hospital policy. Workgroup members conducted trainings with housestaff from the services identified as most frequently caring for incapacitated inpatients.

During trainings, housestaff participants expressed an improved sense of understanding and decreased anxiety about identification and management of incapacitated patients. During subsequent discussions, inpatient housestaff noted improvement in teamwork with safety officers, including cases involving agitated or threatening patients prior to capacity assessment.
An unexpected benefit of the algorithm was recognition of the need for associated resources, including a surrogate decision‐maker documentation form, off‐hours attending physician oversight for medical inpatients with capacity‐related emergencies, and a formal agreement with hospital safety officers regarding the care of high‐risk incapacitated patients not previously on a legal hold or surrogate guardianship. These were created in parallel with the algorithm and have become an integral part of management of incapacitated patients.
CLINICAL DECISION ALGORITHM APPLICATION TO PATIENT SCENARIOS
The following 3 scenarios exemplify common challenges in caring for inpatients with compromised decision‐making capacity. Assessment and multidisciplinary management are explained in relation to the clinical decision algorithm (Figure 1.)
Case 1
An 87‐year‐old woman with mild cognitive impairment presents to the emergency department with community‐acquired pneumonia. The patient is widowed, lives alone in a senior community, and has an established relationship with a primary care physician in the area. On initial examination, the patient is febrile and dyspneic, but still alert and able to give a coherent history. She is able to close the loop and teach‐back regarding the diagnosis of pneumonia and agrees with the treatment plan as explained. Should consideration be given to this patient's decision‐making capacity at this time? What capacity‐related information would be helpful to review with the patient and to document in the record?
Inpatient teams should prospectively identify patients at‐risk for loss of capacity and create a shared treatment plan with the patient while capacity is intact (as noted in the top box in Figure 1). When the inpatient team first meets this patient, she retains decision‐making capacity with regard to hospitalization for pneumonia (left branch after first diamond, Figure 1); however she is at risk for delirium based on her age, mild cognitive impairment, and pneumonia.25 She is willing to stay in the hospital for treatment (right branch after second diamond, Figure 1). For this patient at risk for loss of capacity, it is especially important that the inpatient team explore the patient's care preferences regarding predictable crisis points in the care plan (eg, need for invasive respiratory support or intensive care unit admission.) Her surrogate decision maker's name and contact information should be confirmed. Communication with the patient's primary care provider is advised to review knowledge about the patient's care preferences and request previously completed advance‐care planning documents.
Case 2
A 37‐year‐old man is admitted to the hospital for alcohol withdrawal. On hospital day 1, he develops hyperactive delirium and attempts to leave the hospital. The patient becomes agitated and physically aggressive when the nurse and physician inform him that it is not safe to leave the hospital. He denies having any health problems, he is unable to explain potential risks if his alcohol withdrawal is left untreated, and he cannot articulate a plan to care for himself. The patient attempts to strike a staff member and runs out of the inpatient unit. The patient's family members live in the area, and they can be reached by phone. What are the next appropriate management steps?
This patient has alcohol withdrawal delirium, an emergent medical condition requiring inpatient treatment. The patient demonstrates impaired decision‐making capacity related to treatment because he does not understand his medical condition, he is unable to describe the consequences of the proposed action to leave the hospital, and he is not explaining his decision in rational terms (right hand branch of the algorithm after first diamond, Figure 1). The situation is made more urgent by the patient's aggressive behavior and flight from the inpatient unit, and he poses a risk of harm to self, to staff, and the public (right branch after second diamond, Figure 1). This patient requires a safety plan, and hospital safety officers should be notified immediately. The attending physician and surrogate decision maker should be contacted to create a safe management plan. In this case, a family member is available (left branch after third diamond, Figure 1). The patient requires emergent treatment of his alcohol withdrawal (left branch after fourth diamond, Figure 1). The team should proceed with this emergent treatment with documentation of the assessment, plan, and informed consent of the surrogate. As the patient recovers from acute alcohol withdrawal, the team should reassess his decision‐making capacity and continue to involve the surrogate decision maker until the patient regains capacity to make his own decisions.
Case 3
A 74‐year‐old woman is brought to the hospital by ambulance after being found by her neighbors wandering the hallways of her apartment building. She is disoriented, and her neighbors report a progressive functional decline over the past several months with worsening forgetfulness and occasional falls. She recently started a small fire in her toaster, which a neighbor extinguished after hearing the fire alarm. She is admitted and ultimately diagnosed her with Alzheimer's dementia (Functional Assessment Staging Test (FAST) Tool stage 6a). She is chronically disoriented, happy to be cared for by the hospital staff, and unable to get out of bed independently. She is deemed unsafe to be discharged to home, but she declines to be transferred to a location other than her apartment and declines in‐home care. She has no family or friends. What is the most appropriate course of action to establish a safe long‐term plan for the patient? What medicolegal principles inform the team's responsibility and authority? What consultations may be helpful to the primary medical team?
This patient is incapacitated with regard to long‐term care planning due to dementia. She does not understand her medical condition and cannot articulate the risks and benefits of returning to her apartment (right branch of algorithm after first diamond, Figure 1). The patient is physically unable leave the hospital and does not pose an immediate threat to self or others, thus safety officer assistance is not immediately indicated (left branch at second diamond, Figure 1). Without an available surrogate, this patient might be classified as unbefriended or unrepresented.[7] She will likely require a physician to assist with immediate medical decisions (bottom right corner of algorithm, Figure 1). Emergent treatment is not needed (right branch after fourth diamond,) but long term planning for this vulnerable patient should begin early in the hospital course. Discussion between inpatient and community‐based providers, especially primary care, is recommended to understand the patient's prior care preferences and investigate if she has completed advance care planning documents (two‐headed arrow connecting to square at left side of algorithm.) Involvement of the hospital risk management/legal department may assist with the legal proceedings needed to establish long‐term guardianship (algorithm footnote 5, Figure 1). Ethics consultation may be helpful to consider the balance between the patient's demonstrated values, her autonomy, and the role of substituted judgment in long‐term care planning[7] (algorithm footnote 3, Figure 1). Psychiatric or neuropsychology consultation during her inpatient admission may be useful in preparation for a competency hearing (algorithm footnotes 1 and 2, Figure 1). Social work consultation to provide advocacy for this vulnerable patient would be advisable (algorithm footnote 7).
DISCUSSION
Impaired decision‐making capacity is a common and challenging condition among hospitalized patients, including at our institution. Prior studies show that physicians frequently fail to recognize capacity impairment, and also demonstrate common misunderstandings about the medicolegal framework that governs capacity determination and subsequent care. Patients with impaired decision‐making capacity are vulnerable to adverse outcomes, and there is potential for negative effects on healthcare systems. The management of patients with impaired capacity may involve multiple disciplines and a complex intersection of medical, legal, ethical, and neuropsychological principles.
To promote safety of this vulnerable population at SFGH, our workgroup created a visual algorithm to guide clinicians. The algorithm may improve on existing tools by consolidating the steps from identification through management into a 1‐page visual tool, by emphasizing safety planning for high‐risk incapacitated patients and by elucidating roles and timing for other members of the multidisciplinary management team. Creation of the algorithm facilitated intervention for other practical issues, including institutional and departmental agreements and documentation regarding surrogate decision makers for incapacitated patients.
Although based on a multispecialty institutional review and previously published tools, there are potential limitations to this tool. It seems reasonable to assume that a tool to organize a complex process, such as identification and management of incapacitated patients, should improve patient care versus a non‐standardized process. Although the algorithm is posted in resident workrooms, on the hospital's risk management website, and incorporated as part of hospital policy, we have not yet had the opportunity to study the frequency of its use and impact in patient care. Patient safety and clinical outcome of patients managed with this algorithm could be assessed; however, the impact of the algorithm at SFGH may be confounded by a separate intervention addressing nursing and safety officers that was initiated shortly after the algorithm was produced.
To assess health‐system effects of incapacitated patients, future studies might compare patients with capacity impairment versus those with intact decision making relative to demographic background and payer mix, rates of adverse events during inpatient stay (eg, hospital‐acquired injury), rates of morbidity and mortality, rate of provider identification and documentation of surrogates, patient and surrogate satisfaction data, length of stay and cost of hospitalization, and rates of successful discharge to a community‐based setting. We present this algorithm as an example for diverse settings to address the common challenge of caring for acutely ill patients with impaired decision‐making capacity.
Acknowledgements
The authors thank Lee Rawitscher, MD, for his contribution of capacity assessment handout and review of this manuscript, and to Jeff Critchfield, MD; Robyn Schanzenbach, JD; and Troy Williams, RN, MSN for review of this manuscript. The San Francisco General Hospital Workgroup on Patient Capacity and Medical Decision Making includes Richard Brooks, MD; Beth Brumell, RN; Andy Brunner, JD; Jack Chase, MD; Jeff Critchfield, MD; Leslie Dubbin, RN, MSN, PhD(c); Larry Haber, MD; Lee Rawitscher, MD; and Troy Williams, RN, MSN.
Disclosures
Nothing to report.
- Ten myths about decision making capacity: a report by the National Ethics Committee of the Veterans Health Administration. Department of Veterans Affairs; September 2002. Available at: http://www.ethics.va.gov/docs/necrpts/nec_report_20020201_ten_myths_about_dmc.pdf. Accessed August 13, 2013.
- , , . Does this patient have decision making capacity? JAMA. 2011;306(4):420–427.
- , . Assessing patients' capacities to consent for treatment. N Engl J Med. 1988;319:1635–1638.
- . Assessment of patients' competence to consent to treatment. N Engl J Med. 2007;357:1834–1840.
- , . Can we improve treatment decision‐making for incapacitated patients? Hastings Cent Rep. 2010;40(5):36–45.
- , , , , . Poor decision making is associated with an increased risk of mortality among community‐dwelling older persons without dementia. Neuroepidemiology. 2013;40(4):247–252.
- . Making medical decisions for patients without surrogates. N Engl J Med. 2013;369:1976–1978.
- American Bar Association Commission on Law and Aging and American Psychological Association. Assessment of Older Adults With Diminished Capacity: A Handbook for Lawyers. Washington, DC: American Bar Association and American Psychological Association; 2005.
- , , . Psychiatric evaluation of mental capacity in the general hospital: a significant teaching opportunity. Psychosomatics. 2009;50:468–473.
- , , , , , . Telephone‐based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol. 2011;68(5):607–614.
- , , , et al. Assessment of patient capacity to consent to treatment. J Gen Intern Med. 1999;14(1):27–34.
- . Competency and the capacity to make treatment decisions: a primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 1999;1(5):131–141.
- . Can the patient decide? Evaluating patient capacity in practice. Am Fam Physician. 2001;64(2):299–308.
- , . Capacity decisions in the general hospital: when can you refuse to follow a person's wishes? Prim Care Companion J Clin Psychiatry. 2003;5(4):177–181.
- , . Assessing capacity. Emerg Med Clin North Am. 2000;18(2):233–242, viii.
- . What part of “no” don't you understand? Patient refusal of recommended treatment in the emergency department. Mt Sinai J Med. 2005;72(4):221–227.
- , , . Neuropsychological evaluation in primary care. Am Fam Physician. 2010;82(5):495–502.
- , , . Determining competency in the sexually assaulted patient: a decision algorithm. J Forensic Leg Med. 2010;17:275–279.
- , . A practical guide to capacity assessment and patient consent in Hong Kong. Hong Kong Med J. 2003;9:284–289.
- Alberta (Canada) Health Services. Algorithm range of capacity and decision making options. Available at: http://www.albertahealthservices.ca/hp/if‐hp‐phys‐consent‐capacity‐decision‐algorithm.pdf. Accessed August 13, 2013.
- , . The Mental Capacity Act 2007 and capacity assessments: a guide for the non‐psychiatrist. Clin Med. 2008;8(1):65–69.
- NICE clinical guideline 16: self harm. The short‐term physical and psychological management and secondary prevention of self‐harm in primary and secondary care. London, UK: National Institute for Clinical Excellence (NICE); July 2004. Available at: http://guidance.nice.org.uk/CG16, accessed on August 13, 2013.
- , , . Involuntary hospitalization of medical patients who lack decisional capacity: an unresolved issue. Psychosomatics. 2006;47(5):443–448.
- , , , et al. The AWOL tool: derivation and validation of a delirium prediction rule. J Hosp Med. 2013;8:493–499.
- Ten myths about decision making capacity: a report by the National Ethics Committee of the Veterans Health Administration. Department of Veterans Affairs; September 2002. Available at: http://www.ethics.va.gov/docs/necrpts/nec_report_20020201_ten_myths_about_dmc.pdf. Accessed August 13, 2013.
- , , . Does this patient have decision making capacity? JAMA. 2011;306(4):420–427.
- , . Assessing patients' capacities to consent for treatment. N Engl J Med. 1988;319:1635–1638.
- . Assessment of patients' competence to consent to treatment. N Engl J Med. 2007;357:1834–1840.
- , . Can we improve treatment decision‐making for incapacitated patients? Hastings Cent Rep. 2010;40(5):36–45.
- , , , , . Poor decision making is associated with an increased risk of mortality among community‐dwelling older persons without dementia. Neuroepidemiology. 2013;40(4):247–252.
- . Making medical decisions for patients without surrogates. N Engl J Med. 2013;369:1976–1978.
- American Bar Association Commission on Law and Aging and American Psychological Association. Assessment of Older Adults With Diminished Capacity: A Handbook for Lawyers. Washington, DC: American Bar Association and American Psychological Association; 2005.
- , , . Psychiatric evaluation of mental capacity in the general hospital: a significant teaching opportunity. Psychosomatics. 2009;50:468–473.
- , , , , , . Telephone‐based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol. 2011;68(5):607–614.
- , , , et al. Assessment of patient capacity to consent to treatment. J Gen Intern Med. 1999;14(1):27–34.
- . Competency and the capacity to make treatment decisions: a primer for primary care physicians. Prim Care Companion J Clin Psychiatry. 1999;1(5):131–141.
- . Can the patient decide? Evaluating patient capacity in practice. Am Fam Physician. 2001;64(2):299–308.
- , . Capacity decisions in the general hospital: when can you refuse to follow a person's wishes? Prim Care Companion J Clin Psychiatry. 2003;5(4):177–181.
- , . Assessing capacity. Emerg Med Clin North Am. 2000;18(2):233–242, viii.
- . What part of “no” don't you understand? Patient refusal of recommended treatment in the emergency department. Mt Sinai J Med. 2005;72(4):221–227.
- , , . Neuropsychological evaluation in primary care. Am Fam Physician. 2010;82(5):495–502.
- , , . Determining competency in the sexually assaulted patient: a decision algorithm. J Forensic Leg Med. 2010;17:275–279.
- , . A practical guide to capacity assessment and patient consent in Hong Kong. Hong Kong Med J. 2003;9:284–289.
- Alberta (Canada) Health Services. Algorithm range of capacity and decision making options. Available at: http://www.albertahealthservices.ca/hp/if‐hp‐phys‐consent‐capacity‐decision‐algorithm.pdf. Accessed August 13, 2013.
- , . The Mental Capacity Act 2007 and capacity assessments: a guide for the non‐psychiatrist. Clin Med. 2008;8(1):65–69.
- NICE clinical guideline 16: self harm. The short‐term physical and psychological management and secondary prevention of self‐harm in primary and secondary care. London, UK: National Institute for Clinical Excellence (NICE); July 2004. Available at: http://guidance.nice.org.uk/CG16, accessed on August 13, 2013.
- , , . Involuntary hospitalization of medical patients who lack decisional capacity: an unresolved issue. Psychosomatics. 2006;47(5):443–448.
- , , , et al. The AWOL tool: derivation and validation of a delirium prediction rule. J Hosp Med. 2013;8:493–499.
Gynecologic cancer patients treated at high-volume hospitals live longer
Women with gynecologic cancers live significantly longer if they’re cared for at hospitals that frequently treat such patients rather than at centers that infrequently care for them. This is according to results from a study of more than 860,000 women.1 The research, which used the National Cancer Database to identify gynecologic cancer cases, was presented at the 2014 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer.
Results of the study
Overall, the median survival for women with all types of gynecologic cancer was 122.7 months at centers with the highest volume (nearly 300 such cases per year) compared with 110 months at the lowest-volume hospitals (<20 per year)—a difference of more than a year. The difference was even greater for cancers that are rare or require particularly complex treatment.
Patients with vaginal cancer, for example, had a median survival of 72.2 months at the highest-volume centers, compared with 38.1 months at the lowest-volume facilities—a difference of close to 3 years. For women with ovarian cancer, the difference in median survival was nearly 17 months (49.4 months for highest-volume versus 32.5 for lowest-volume).
The researchers analyzed data on 863,156 women treated for cervical, ovarian, uterine, vaginal, or vulvar cancer from 1998 to 2011. The patients received treatment at 1666 medical centers, which the researchers divided into quartiles based on the number of reproductive cancer cases treated annually.
Over the 13-year study period, the number of women with gynecologic cancer who received treatment at high-volume facilities rose steadily, said Jeff F. Lin, MD, lead author of the study and a physician in gynecologic oncology at Magee-Womens Hospital at the University of Pittsburgh Medical Center. This rise in treatment at high-volume centers did not benefit elderly patients and those with more advanced cancers, however, as these patients were more likely to receive treatment at lower-volume facilities.
Refer women to high-volume centers, say the authors
The research results did not explain why women treated at high-volume centers live longer, nor the reason more patients with gynecologic cancer are being treated at these facilities. Better coordination of care, better access to cutting-edge clinical trials, and greater likelihood of receiving care from gynecologic oncologists are possible reasons women treated at high-volume centers have better outcomes, said Dr. Lin. He added that, as gynecologic cancer care becomes more complex, physicians may feel more comfortable referring patients to high-volume centers and specialists. “Based on this and other studies, we should be trying to steer even more patients to high-volume hospitals,” he said.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. We will consider publishing your letter in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Reference
1. Women with gynecologic cancers may live longer when treated at high-volume medical centers [press release]. Chicago: Society of Gynecologic Oncology. March 24, 2014. https://www.sgo.org/newsroom/news-releases/women-with-gynecologic-cancers-may-live-longer-when-treated-at-high-volume-medical-centers/. Accessed May 19, 2014.
Women with gynecologic cancers live significantly longer if they’re cared for at hospitals that frequently treat such patients rather than at centers that infrequently care for them. This is according to results from a study of more than 860,000 women.1 The research, which used the National Cancer Database to identify gynecologic cancer cases, was presented at the 2014 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer.
Results of the study
Overall, the median survival for women with all types of gynecologic cancer was 122.7 months at centers with the highest volume (nearly 300 such cases per year) compared with 110 months at the lowest-volume hospitals (<20 per year)—a difference of more than a year. The difference was even greater for cancers that are rare or require particularly complex treatment.
Patients with vaginal cancer, for example, had a median survival of 72.2 months at the highest-volume centers, compared with 38.1 months at the lowest-volume facilities—a difference of close to 3 years. For women with ovarian cancer, the difference in median survival was nearly 17 months (49.4 months for highest-volume versus 32.5 for lowest-volume).
The researchers analyzed data on 863,156 women treated for cervical, ovarian, uterine, vaginal, or vulvar cancer from 1998 to 2011. The patients received treatment at 1666 medical centers, which the researchers divided into quartiles based on the number of reproductive cancer cases treated annually.
Over the 13-year study period, the number of women with gynecologic cancer who received treatment at high-volume facilities rose steadily, said Jeff F. Lin, MD, lead author of the study and a physician in gynecologic oncology at Magee-Womens Hospital at the University of Pittsburgh Medical Center. This rise in treatment at high-volume centers did not benefit elderly patients and those with more advanced cancers, however, as these patients were more likely to receive treatment at lower-volume facilities.
Refer women to high-volume centers, say the authors
The research results did not explain why women treated at high-volume centers live longer, nor the reason more patients with gynecologic cancer are being treated at these facilities. Better coordination of care, better access to cutting-edge clinical trials, and greater likelihood of receiving care from gynecologic oncologists are possible reasons women treated at high-volume centers have better outcomes, said Dr. Lin. He added that, as gynecologic cancer care becomes more complex, physicians may feel more comfortable referring patients to high-volume centers and specialists. “Based on this and other studies, we should be trying to steer even more patients to high-volume hospitals,” he said.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. We will consider publishing your letter in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Women with gynecologic cancers live significantly longer if they’re cared for at hospitals that frequently treat such patients rather than at centers that infrequently care for them. This is according to results from a study of more than 860,000 women.1 The research, which used the National Cancer Database to identify gynecologic cancer cases, was presented at the 2014 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer.
Results of the study
Overall, the median survival for women with all types of gynecologic cancer was 122.7 months at centers with the highest volume (nearly 300 such cases per year) compared with 110 months at the lowest-volume hospitals (<20 per year)—a difference of more than a year. The difference was even greater for cancers that are rare or require particularly complex treatment.
Patients with vaginal cancer, for example, had a median survival of 72.2 months at the highest-volume centers, compared with 38.1 months at the lowest-volume facilities—a difference of close to 3 years. For women with ovarian cancer, the difference in median survival was nearly 17 months (49.4 months for highest-volume versus 32.5 for lowest-volume).
The researchers analyzed data on 863,156 women treated for cervical, ovarian, uterine, vaginal, or vulvar cancer from 1998 to 2011. The patients received treatment at 1666 medical centers, which the researchers divided into quartiles based on the number of reproductive cancer cases treated annually.
Over the 13-year study period, the number of women with gynecologic cancer who received treatment at high-volume facilities rose steadily, said Jeff F. Lin, MD, lead author of the study and a physician in gynecologic oncology at Magee-Womens Hospital at the University of Pittsburgh Medical Center. This rise in treatment at high-volume centers did not benefit elderly patients and those with more advanced cancers, however, as these patients were more likely to receive treatment at lower-volume facilities.
Refer women to high-volume centers, say the authors
The research results did not explain why women treated at high-volume centers live longer, nor the reason more patients with gynecologic cancer are being treated at these facilities. Better coordination of care, better access to cutting-edge clinical trials, and greater likelihood of receiving care from gynecologic oncologists are possible reasons women treated at high-volume centers have better outcomes, said Dr. Lin. He added that, as gynecologic cancer care becomes more complex, physicians may feel more comfortable referring patients to high-volume centers and specialists. “Based on this and other studies, we should be trying to steer even more patients to high-volume hospitals,” he said.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. We will consider publishing your letter in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Reference
1. Women with gynecologic cancers may live longer when treated at high-volume medical centers [press release]. Chicago: Society of Gynecologic Oncology. March 24, 2014. https://www.sgo.org/newsroom/news-releases/women-with-gynecologic-cancers-may-live-longer-when-treated-at-high-volume-medical-centers/. Accessed May 19, 2014.
Reference
1. Women with gynecologic cancers may live longer when treated at high-volume medical centers [press release]. Chicago: Society of Gynecologic Oncology. March 24, 2014. https://www.sgo.org/newsroom/news-releases/women-with-gynecologic-cancers-may-live-longer-when-treated-at-high-volume-medical-centers/. Accessed May 19, 2014.