User login
Hemorrhagic ovarian cysts: One entity with many appearances
FOREWARD
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
This is the inaugural offering in a new series, titled Images in Gyn Ultrasound. It is interesting and important that Dr. Michelle Stalnaker and Dr. Andrew Kaunitz have chosen hemorrhagic ovarian cysts as their debut topic.
Realize that since the vaginal probe was introduced in the 1980s, our entire specialty has had to undergo a learning curve--just as individuals will have a learning curve. In the early days of transvaginal ultrasound, an imager often provided a differential for such masses, along the lines of “compatible with hemorrhagic cyst, endometrioma, dermoid…cannot rule out neoplasia.” Today, however, with better understanding, and especially with the addition of color flow Doppler, very often a definitive diagnosis can be made.
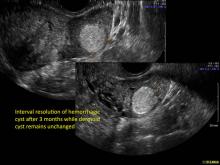
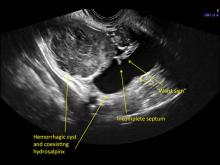
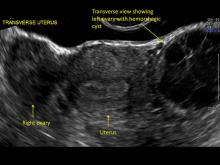
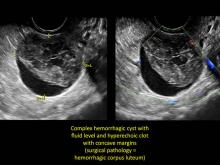
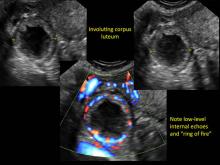
These “hemorrhagic cysts” are nothing more than bleeding into a corpus luteum at the time of ovulation−the more blood that collects before tamponade or clot stops its accumulation, the larger the “cyst” can become. As the cyst goes through a “maturation” process and undergoes clot retraction and clot lysis, the variable internal echo patterns presented in the following images are possible, but there will ALWAYS only be peripheral blood flow as evidenced by the morphologic appearance of the vascular distribution. See video.
Study these images carefully as they are very representative of the many faces of the hemorrhagic corpus luteum.
Hemorrhagic ovarian cysts: One entity with many appearances
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
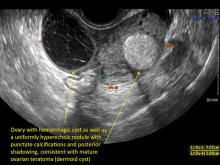
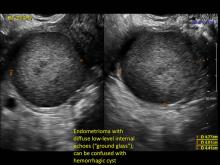
Hemorrhagic cysts are normal in ovulatory women, usually resolving within 8 weeks. They can be quite variable in appearance, however, and can be confused with ovarian endometriomae. Presenting characteristics can include:
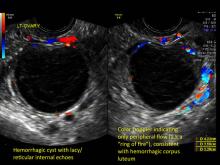
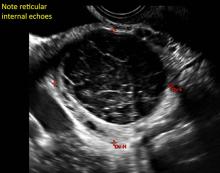
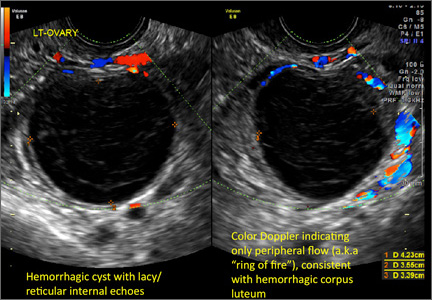
- reticular (lacy, cobweb, fishnet) internal echoes due to fibrin strands
- a solid-appearing area with concave margins
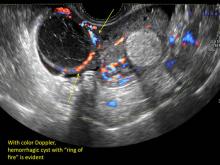
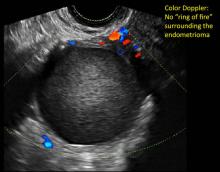
- on Color Doppler: circumferential peripheral vascular flow (“ring of fire”), with no internal flow
Management. With respect to hemorrhagic cysts, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement indicates:
- For premenopausal women:
- No follow-up imaging needed unless there’s an uncertain diagnosis or if the cyst is larger than 5 cm
- Cyst size > 5 cm; short-interval follow-up ultrasound is indicated (6-12 weeks)
- For recently menopausal women:
- Follow-up ultrasound in 6 to 12 weeks to ensure resolution of the initial findings
- For later postmenopausal women:
- Cyst possibly neoplastic; consider surgical removal
FOREWARD
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
This is the inaugural offering in a new series, titled Images in Gyn Ultrasound. It is interesting and important that Dr. Michelle Stalnaker and Dr. Andrew Kaunitz have chosen hemorrhagic ovarian cysts as their debut topic.
Realize that since the vaginal probe was introduced in the 1980s, our entire specialty has had to undergo a learning curve--just as individuals will have a learning curve. In the early days of transvaginal ultrasound, an imager often provided a differential for such masses, along the lines of “compatible with hemorrhagic cyst, endometrioma, dermoid…cannot rule out neoplasia.” Today, however, with better understanding, and especially with the addition of color flow Doppler, very often a definitive diagnosis can be made.
These “hemorrhagic cysts” are nothing more than bleeding into a corpus luteum at the time of ovulation−the more blood that collects before tamponade or clot stops its accumulation, the larger the “cyst” can become. As the cyst goes through a “maturation” process and undergoes clot retraction and clot lysis, the variable internal echo patterns presented in the following images are possible, but there will ALWAYS only be peripheral blood flow as evidenced by the morphologic appearance of the vascular distribution. See video.
Study these images carefully as they are very representative of the many faces of the hemorrhagic corpus luteum.
Hemorrhagic ovarian cysts: One entity with many appearances
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Hemorrhagic cysts are normal in ovulatory women, usually resolving within 8 weeks. They can be quite variable in appearance, however, and can be confused with ovarian endometriomae. Presenting characteristics can include:
- reticular (lacy, cobweb, fishnet) internal echoes due to fibrin strands
- a solid-appearing area with concave margins
- on Color Doppler: circumferential peripheral vascular flow (“ring of fire”), with no internal flow
Management. With respect to hemorrhagic cysts, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement indicates:
- For premenopausal women:
- No follow-up imaging needed unless there’s an uncertain diagnosis or if the cyst is larger than 5 cm
- Cyst size > 5 cm; short-interval follow-up ultrasound is indicated (6-12 weeks)
- For recently menopausal women:
- Follow-up ultrasound in 6 to 12 weeks to ensure resolution of the initial findings
- For later postmenopausal women:
- Cyst possibly neoplastic; consider surgical removal
FOREWARD
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
This is the inaugural offering in a new series, titled Images in Gyn Ultrasound. It is interesting and important that Dr. Michelle Stalnaker and Dr. Andrew Kaunitz have chosen hemorrhagic ovarian cysts as their debut topic.
Realize that since the vaginal probe was introduced in the 1980s, our entire specialty has had to undergo a learning curve--just as individuals will have a learning curve. In the early days of transvaginal ultrasound, an imager often provided a differential for such masses, along the lines of “compatible with hemorrhagic cyst, endometrioma, dermoid…cannot rule out neoplasia.” Today, however, with better understanding, and especially with the addition of color flow Doppler, very often a definitive diagnosis can be made.
These “hemorrhagic cysts” are nothing more than bleeding into a corpus luteum at the time of ovulation−the more blood that collects before tamponade or clot stops its accumulation, the larger the “cyst” can become. As the cyst goes through a “maturation” process and undergoes clot retraction and clot lysis, the variable internal echo patterns presented in the following images are possible, but there will ALWAYS only be peripheral blood flow as evidenced by the morphologic appearance of the vascular distribution. See video.
Study these images carefully as they are very representative of the many faces of the hemorrhagic corpus luteum.
Hemorrhagic ovarian cysts: One entity with many appearances
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Hemorrhagic cysts are normal in ovulatory women, usually resolving within 8 weeks. They can be quite variable in appearance, however, and can be confused with ovarian endometriomae. Presenting characteristics can include:
- reticular (lacy, cobweb, fishnet) internal echoes due to fibrin strands
- a solid-appearing area with concave margins
- on Color Doppler: circumferential peripheral vascular flow (“ring of fire”), with no internal flow
Management. With respect to hemorrhagic cysts, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement indicates:
- For premenopausal women:
- No follow-up imaging needed unless there’s an uncertain diagnosis or if the cyst is larger than 5 cm
- Cyst size > 5 cm; short-interval follow-up ultrasound is indicated (6-12 weeks)
- For recently menopausal women:
- Follow-up ultrasound in 6 to 12 weeks to ensure resolution of the initial findings
- For later postmenopausal women:
- Cyst possibly neoplastic; consider surgical removal
FDA approves first molecular test for blood typing

Credit: Juan D. Alfonso
The US Food and Drug Administration (FDA) has approved the first molecular assay for determining blood compatibility prior to transfusion.
The Immucor PreciseType Human Erythrocyte Antigen (HEA) Molecular BeadChip Test can be used to determine donor and patient non-ABO/non-RhD red blood cell types.
The test provides an alternative to serological typing and may enhance patient care in certain situations, according to Karen Midthun, MD, director of the FDA’s Center for Biologics Evaluation and Research.
The Immucor PreciseType HEA Molecular BeadChip Test works by detecting genes that govern the expression of 36 antigens that can appear on the surface of red blood cells.
The test uses thousands of coded beads that bind with the genes coding for non-ABO red blood cell antigens that are present in a blood sample.
A light signal is generated from each bead that has captured a specific gene. Accompanying computer software decodes the light signals and reports which antigens are predicted to be present on the red cells, based on the genes detected.
Researchers conducted a study to compare the typing results of the PreciseType HEA Molecular BeadChip Test with licensed serological reagents and DNA sequencing. And the results demonstrated comparable performance between the methods.
The product was brought before the FDA’s Blood Products Advisory Committee on March 18, 2014. After reviewing the relevant information, the committee said the data provided reasonable assurance that the Immucor PreciseType HEA Molecular BeadChip Test is safe and effective for its intended use.
The test is manufactured by BioArray Solutions Ltd. of Warren, New Jersey. ![]()

Credit: Juan D. Alfonso
The US Food and Drug Administration (FDA) has approved the first molecular assay for determining blood compatibility prior to transfusion.
The Immucor PreciseType Human Erythrocyte Antigen (HEA) Molecular BeadChip Test can be used to determine donor and patient non-ABO/non-RhD red blood cell types.
The test provides an alternative to serological typing and may enhance patient care in certain situations, according to Karen Midthun, MD, director of the FDA’s Center for Biologics Evaluation and Research.
The Immucor PreciseType HEA Molecular BeadChip Test works by detecting genes that govern the expression of 36 antigens that can appear on the surface of red blood cells.
The test uses thousands of coded beads that bind with the genes coding for non-ABO red blood cell antigens that are present in a blood sample.
A light signal is generated from each bead that has captured a specific gene. Accompanying computer software decodes the light signals and reports which antigens are predicted to be present on the red cells, based on the genes detected.
Researchers conducted a study to compare the typing results of the PreciseType HEA Molecular BeadChip Test with licensed serological reagents and DNA sequencing. And the results demonstrated comparable performance between the methods.
The product was brought before the FDA’s Blood Products Advisory Committee on March 18, 2014. After reviewing the relevant information, the committee said the data provided reasonable assurance that the Immucor PreciseType HEA Molecular BeadChip Test is safe and effective for its intended use.
The test is manufactured by BioArray Solutions Ltd. of Warren, New Jersey. ![]()

Credit: Juan D. Alfonso
The US Food and Drug Administration (FDA) has approved the first molecular assay for determining blood compatibility prior to transfusion.
The Immucor PreciseType Human Erythrocyte Antigen (HEA) Molecular BeadChip Test can be used to determine donor and patient non-ABO/non-RhD red blood cell types.
The test provides an alternative to serological typing and may enhance patient care in certain situations, according to Karen Midthun, MD, director of the FDA’s Center for Biologics Evaluation and Research.
The Immucor PreciseType HEA Molecular BeadChip Test works by detecting genes that govern the expression of 36 antigens that can appear on the surface of red blood cells.
The test uses thousands of coded beads that bind with the genes coding for non-ABO red blood cell antigens that are present in a blood sample.
A light signal is generated from each bead that has captured a specific gene. Accompanying computer software decodes the light signals and reports which antigens are predicted to be present on the red cells, based on the genes detected.
Researchers conducted a study to compare the typing results of the PreciseType HEA Molecular BeadChip Test with licensed serological reagents and DNA sequencing. And the results demonstrated comparable performance between the methods.
The product was brought before the FDA’s Blood Products Advisory Committee on March 18, 2014. After reviewing the relevant information, the committee said the data provided reasonable assurance that the Immucor PreciseType HEA Molecular BeadChip Test is safe and effective for its intended use.
The test is manufactured by BioArray Solutions Ltd. of Warren, New Jersey. ![]()
Trapping parasites to fight malaria

Credit: St Jude Children’s
Research Hospital
Investigators have identified antibodies that prevent malaria-causing parasites at the schizont stage from rupturing and spilling into the bloodstream.
These antibodies reduced loads of the parasite significantly in mice and humans, and they might one day be exploited to create a malaria vaccine, according to the researchers.
Jonathan Kurtis, MD, PhD, of Rhode Island Hospital in Providence, and his colleagues described the antibodies and their effects in Science.
The investigators studied the plasma of malaria-resistant 2-year-olds in Tanzania, where the disease is endemic. The team thought the naturally acquired immunity in these chronically exposed individuals provided a good model through which to identify vaccine antigens.
The analysis revealed that a particular Plasmodium falciparum antigen, known as P falciparum schizont egress antigen-1 (PfSEA-1), triggered antibodies in the children that, in turn, blocked replication of the parasite.
When the researchers vaccinated malaria-infected mice with the antigen or passively transferred PfSEA-1 antibodies to the rodents, they observed a 4-fold reduction of malaria parasites in the animals’ blood.
“When my post-doctoral fellow, Dipak Raj, discovered that antibodies to this protein, PfSEA-1, effectively trapped the malaria-causing parasite within the red blood cells, it was truly a moment of discovery,” Dr Kurtis said.
“Many researchers are trying to find ways to develop a malaria vaccine by preventing the parasite from entering the red blood cell, and, here, we found a way to block it from leaving the cell once it has entered. If it’s trapped in the red blood cell, it can’t go anywhere. It can’t do any further damage.”
The presence of PfSEA-1 antibodies also appeared to protect the Tanzanian study participants from severe cases of malaria. The investigators measured antibodies to PfSEA-1 in the entire cohort of 785 children and found that, among those with antibodies to PfSEA-1, there were no cases of severe malaria.
To generalize their results, the researchers then went back to serum samples they had collected from 140 children in Kenya in 1997. Analyses revealed that individuals with antibodies to PfSEA-1 had 50% lower parasitemia than individuals without these antibodies during a high-transmission season.
The investigators believe these findings could bring researchers a step closer to an effective malaria vaccine that targets parasites at multiple life stages.
“We still have additional trials ahead of us, first in another animal model, but we hope to begin phase 1 trials in humans very soon,” Dr Kurtis said.
“Our findings support PfSEA-1 as a potential vaccine candidate. And we are confident that, by partnering with our colleagues at the National Institutes of Health and other researchers focused on vaccines to prevent the parasites from entering red blood cells, we can approach the parasite from all angles, which could help us develop a truly effective vaccine to prevent this infectious disease that kills millions of children every year.” ![]()

Credit: St Jude Children’s
Research Hospital
Investigators have identified antibodies that prevent malaria-causing parasites at the schizont stage from rupturing and spilling into the bloodstream.
These antibodies reduced loads of the parasite significantly in mice and humans, and they might one day be exploited to create a malaria vaccine, according to the researchers.
Jonathan Kurtis, MD, PhD, of Rhode Island Hospital in Providence, and his colleagues described the antibodies and their effects in Science.
The investigators studied the plasma of malaria-resistant 2-year-olds in Tanzania, where the disease is endemic. The team thought the naturally acquired immunity in these chronically exposed individuals provided a good model through which to identify vaccine antigens.
The analysis revealed that a particular Plasmodium falciparum antigen, known as P falciparum schizont egress antigen-1 (PfSEA-1), triggered antibodies in the children that, in turn, blocked replication of the parasite.
When the researchers vaccinated malaria-infected mice with the antigen or passively transferred PfSEA-1 antibodies to the rodents, they observed a 4-fold reduction of malaria parasites in the animals’ blood.
“When my post-doctoral fellow, Dipak Raj, discovered that antibodies to this protein, PfSEA-1, effectively trapped the malaria-causing parasite within the red blood cells, it was truly a moment of discovery,” Dr Kurtis said.
“Many researchers are trying to find ways to develop a malaria vaccine by preventing the parasite from entering the red blood cell, and, here, we found a way to block it from leaving the cell once it has entered. If it’s trapped in the red blood cell, it can’t go anywhere. It can’t do any further damage.”
The presence of PfSEA-1 antibodies also appeared to protect the Tanzanian study participants from severe cases of malaria. The investigators measured antibodies to PfSEA-1 in the entire cohort of 785 children and found that, among those with antibodies to PfSEA-1, there were no cases of severe malaria.
To generalize their results, the researchers then went back to serum samples they had collected from 140 children in Kenya in 1997. Analyses revealed that individuals with antibodies to PfSEA-1 had 50% lower parasitemia than individuals without these antibodies during a high-transmission season.
The investigators believe these findings could bring researchers a step closer to an effective malaria vaccine that targets parasites at multiple life stages.
“We still have additional trials ahead of us, first in another animal model, but we hope to begin phase 1 trials in humans very soon,” Dr Kurtis said.
“Our findings support PfSEA-1 as a potential vaccine candidate. And we are confident that, by partnering with our colleagues at the National Institutes of Health and other researchers focused on vaccines to prevent the parasites from entering red blood cells, we can approach the parasite from all angles, which could help us develop a truly effective vaccine to prevent this infectious disease that kills millions of children every year.” ![]()

Credit: St Jude Children’s
Research Hospital
Investigators have identified antibodies that prevent malaria-causing parasites at the schizont stage from rupturing and spilling into the bloodstream.
These antibodies reduced loads of the parasite significantly in mice and humans, and they might one day be exploited to create a malaria vaccine, according to the researchers.
Jonathan Kurtis, MD, PhD, of Rhode Island Hospital in Providence, and his colleagues described the antibodies and their effects in Science.
The investigators studied the plasma of malaria-resistant 2-year-olds in Tanzania, where the disease is endemic. The team thought the naturally acquired immunity in these chronically exposed individuals provided a good model through which to identify vaccine antigens.
The analysis revealed that a particular Plasmodium falciparum antigen, known as P falciparum schizont egress antigen-1 (PfSEA-1), triggered antibodies in the children that, in turn, blocked replication of the parasite.
When the researchers vaccinated malaria-infected mice with the antigen or passively transferred PfSEA-1 antibodies to the rodents, they observed a 4-fold reduction of malaria parasites in the animals’ blood.
“When my post-doctoral fellow, Dipak Raj, discovered that antibodies to this protein, PfSEA-1, effectively trapped the malaria-causing parasite within the red blood cells, it was truly a moment of discovery,” Dr Kurtis said.
“Many researchers are trying to find ways to develop a malaria vaccine by preventing the parasite from entering the red blood cell, and, here, we found a way to block it from leaving the cell once it has entered. If it’s trapped in the red blood cell, it can’t go anywhere. It can’t do any further damage.”
The presence of PfSEA-1 antibodies also appeared to protect the Tanzanian study participants from severe cases of malaria. The investigators measured antibodies to PfSEA-1 in the entire cohort of 785 children and found that, among those with antibodies to PfSEA-1, there were no cases of severe malaria.
To generalize their results, the researchers then went back to serum samples they had collected from 140 children in Kenya in 1997. Analyses revealed that individuals with antibodies to PfSEA-1 had 50% lower parasitemia than individuals without these antibodies during a high-transmission season.
The investigators believe these findings could bring researchers a step closer to an effective malaria vaccine that targets parasites at multiple life stages.
“We still have additional trials ahead of us, first in another animal model, but we hope to begin phase 1 trials in humans very soon,” Dr Kurtis said.
“Our findings support PfSEA-1 as a potential vaccine candidate. And we are confident that, by partnering with our colleagues at the National Institutes of Health and other researchers focused on vaccines to prevent the parasites from entering red blood cells, we can approach the parasite from all angles, which could help us develop a truly effective vaccine to prevent this infectious disease that kills millions of children every year.” ![]()
Large-volume infusion pump recalled

Credit: CDC
The medical technology company CareFusion has announced a Class I recall of its Alaris Pump model 8100, software version 9.1.18.
This large-volume infusion pump is used for the delivery of fluids, medicines, blood, and blood products.
Version 9.1.18 of the Alaris Pump model 8100 is being recalled due to the possibility of a software failure in which the pump module will not properly delay an infusion when the “Delay Until” option or “Multidose” feature is used.
There have been no reports of adverse events or deaths related to this malfunction, but it does pose risks. CareFusion has received 1 report where the device malfunctioned when the “Delay Until” option was selected.
The software failure also prevents the pump from properly delivering a multidose infusion under the following conditions:
- When the first dose is programmed to infuse when the system time is earlier than 7 pm and a subsequent dose is intended to infuse between 7 pm and 11:59 pm
- When the first dose is programmed to infuse when the system time is between 7 pm and 11:59 pm and a subsequent dose is intended to infuse between 12 am and 6:59 pm the next day.
If the infusion starts earlier or later than intended and is not immediately detected and stopped, serious injury or death could result. Therefore, healthcare professionals should not use the Alaris Pump module “Delay Until” option or the “Multidose” option.
However, CareFusion said it has identified the root cause of the issue and recommends that the previous Alaris Pump module software version 9.1.17 be installed to address this recall. The company said it will contact all affected customers to schedule the installation of software version 9.1.17.
As an interim guidance, customers may update their dataset to disable both “Delay” options (“Delay Until” and “Delay For”) and/or the “Multidose” option across all profiles to prevent the use of these features. These are shared configurations with the Alaris Syringe module and, if disabled, would prevent use of these features with the Alaris Syringe module as well.
For more information on this recall, see CareFusion’s recall notice, or contact the CareFusion Support Center at 888-562-6018 or [email protected].
To report adverse reactions or quality problems associated with this product, visit the Food and Drug Administration’s MedWatch website. ![]()

Credit: CDC
The medical technology company CareFusion has announced a Class I recall of its Alaris Pump model 8100, software version 9.1.18.
This large-volume infusion pump is used for the delivery of fluids, medicines, blood, and blood products.
Version 9.1.18 of the Alaris Pump model 8100 is being recalled due to the possibility of a software failure in which the pump module will not properly delay an infusion when the “Delay Until” option or “Multidose” feature is used.
There have been no reports of adverse events or deaths related to this malfunction, but it does pose risks. CareFusion has received 1 report where the device malfunctioned when the “Delay Until” option was selected.
The software failure also prevents the pump from properly delivering a multidose infusion under the following conditions:
- When the first dose is programmed to infuse when the system time is earlier than 7 pm and a subsequent dose is intended to infuse between 7 pm and 11:59 pm
- When the first dose is programmed to infuse when the system time is between 7 pm and 11:59 pm and a subsequent dose is intended to infuse between 12 am and 6:59 pm the next day.
If the infusion starts earlier or later than intended and is not immediately detected and stopped, serious injury or death could result. Therefore, healthcare professionals should not use the Alaris Pump module “Delay Until” option or the “Multidose” option.
However, CareFusion said it has identified the root cause of the issue and recommends that the previous Alaris Pump module software version 9.1.17 be installed to address this recall. The company said it will contact all affected customers to schedule the installation of software version 9.1.17.
As an interim guidance, customers may update their dataset to disable both “Delay” options (“Delay Until” and “Delay For”) and/or the “Multidose” option across all profiles to prevent the use of these features. These are shared configurations with the Alaris Syringe module and, if disabled, would prevent use of these features with the Alaris Syringe module as well.
For more information on this recall, see CareFusion’s recall notice, or contact the CareFusion Support Center at 888-562-6018 or [email protected].
To report adverse reactions or quality problems associated with this product, visit the Food and Drug Administration’s MedWatch website. ![]()

Credit: CDC
The medical technology company CareFusion has announced a Class I recall of its Alaris Pump model 8100, software version 9.1.18.
This large-volume infusion pump is used for the delivery of fluids, medicines, blood, and blood products.
Version 9.1.18 of the Alaris Pump model 8100 is being recalled due to the possibility of a software failure in which the pump module will not properly delay an infusion when the “Delay Until” option or “Multidose” feature is used.
There have been no reports of adverse events or deaths related to this malfunction, but it does pose risks. CareFusion has received 1 report where the device malfunctioned when the “Delay Until” option was selected.
The software failure also prevents the pump from properly delivering a multidose infusion under the following conditions:
- When the first dose is programmed to infuse when the system time is earlier than 7 pm and a subsequent dose is intended to infuse between 7 pm and 11:59 pm
- When the first dose is programmed to infuse when the system time is between 7 pm and 11:59 pm and a subsequent dose is intended to infuse between 12 am and 6:59 pm the next day.
If the infusion starts earlier or later than intended and is not immediately detected and stopped, serious injury or death could result. Therefore, healthcare professionals should not use the Alaris Pump module “Delay Until” option or the “Multidose” option.
However, CareFusion said it has identified the root cause of the issue and recommends that the previous Alaris Pump module software version 9.1.17 be installed to address this recall. The company said it will contact all affected customers to schedule the installation of software version 9.1.17.
As an interim guidance, customers may update their dataset to disable both “Delay” options (“Delay Until” and “Delay For”) and/or the “Multidose” option across all profiles to prevent the use of these features. These are shared configurations with the Alaris Syringe module and, if disabled, would prevent use of these features with the Alaris Syringe module as well.
For more information on this recall, see CareFusion’s recall notice, or contact the CareFusion Support Center at 888-562-6018 or [email protected].
To report adverse reactions or quality problems associated with this product, visit the Food and Drug Administration’s MedWatch website. ![]()
TPN calculation software recalled
The US Food and Drug Administration (FDA) has announced a Class I recall of Baxter Corporation Englewood’s ABACUS Total Parenteral Nutrition (TPN) Calculation Software, versions 3.1, 3.0, 2.1, and 2.0.
Baxter has received 2 reports of malfunctioning software and said errors with this software may cause adverse effects.
ABACUS TPN Calculation Software is a Windows-based software application used by pharmacists to calculate or order TPN formulas.
The errors explained
Due to software failures, the following errors may occur:
- ABACUS v3.1 may calculate quantities of electrolytes that are double the expected values during the creation of TPN orders.
- ABACUS v3.1 may automatically add additional sterile water to a formula equal to the volume of a premix, resulting in an over-dilution.
- All software versions of ABACUS software display the calcium phosphate curve points for Premasol incorrectly.
- All software versions of ABACUS may display an inaccurate estimation for calcium and phosphate precipitation in certain circumstances where multiple ingredients provide calcium.
If any of these failures occur, patients may be at risk of developing overdose symptoms. The symptoms are varied and depend on the type of software failure and composition of the fluid being compounded.
Symptoms may be non-specific and include nausea, vomiting, dizziness, or fatigue. Some more severe symptoms include cardiac arrhythmia, pulmonary edema, congestive heart failure, and seizures. A fatal outcome is possible, especially in the high-risk population.
Actions to take
Baxter is recommending that customers contact the company to ensure the ABACUS software is configured correctly.
Customers with a software version earlier than 3.1 will have software version 3.1 installed, which addresses the issues that prompted the recall. In addition, Baxter Support Services will schedule upgrades and assist customers with establishing the proper ABACUS configuration in the customers’ facilities.
Baxter has also requested that customers follow safe compounding practices. Namely, use the “Summary” button to verify the order against the calculated amounts prior to completing the order.
In addition, verify that the ordered ingredients and quantities displayed in the software and printed on the Bag label and the Solution Formula label match the PN prescription prior to preparation. And use a filter for administration of a PN bag.
For more information on the recall, see the FDA’s recall notice, or contact Baxter at 303-617-2242. For technical support, call 1-800-678-2292 or email [email protected].
To report adverse reactions or quality problems related to this product, visit the FDA’s MedWatch website. ![]()

The US Food and Drug Administration (FDA) has announced a Class I recall of Baxter Corporation Englewood’s ABACUS Total Parenteral Nutrition (TPN) Calculation Software, versions 3.1, 3.0, 2.1, and 2.0.
Baxter has received 2 reports of malfunctioning software and said errors with this software may cause adverse effects.
ABACUS TPN Calculation Software is a Windows-based software application used by pharmacists to calculate or order TPN formulas.
The errors explained
Due to software failures, the following errors may occur:
- ABACUS v3.1 may calculate quantities of electrolytes that are double the expected values during the creation of TPN orders.
- ABACUS v3.1 may automatically add additional sterile water to a formula equal to the volume of a premix, resulting in an over-dilution.
- All software versions of ABACUS software display the calcium phosphate curve points for Premasol incorrectly.
- All software versions of ABACUS may display an inaccurate estimation for calcium and phosphate precipitation in certain circumstances where multiple ingredients provide calcium.
If any of these failures occur, patients may be at risk of developing overdose symptoms. The symptoms are varied and depend on the type of software failure and composition of the fluid being compounded.
Symptoms may be non-specific and include nausea, vomiting, dizziness, or fatigue. Some more severe symptoms include cardiac arrhythmia, pulmonary edema, congestive heart failure, and seizures. A fatal outcome is possible, especially in the high-risk population.
Actions to take
Baxter is recommending that customers contact the company to ensure the ABACUS software is configured correctly.
Customers with a software version earlier than 3.1 will have software version 3.1 installed, which addresses the issues that prompted the recall. In addition, Baxter Support Services will schedule upgrades and assist customers with establishing the proper ABACUS configuration in the customers’ facilities.
Baxter has also requested that customers follow safe compounding practices. Namely, use the “Summary” button to verify the order against the calculated amounts prior to completing the order.
In addition, verify that the ordered ingredients and quantities displayed in the software and printed on the Bag label and the Solution Formula label match the PN prescription prior to preparation. And use a filter for administration of a PN bag.
For more information on the recall, see the FDA’s recall notice, or contact Baxter at 303-617-2242. For technical support, call 1-800-678-2292 or email [email protected].
To report adverse reactions or quality problems related to this product, visit the FDA’s MedWatch website. ![]()

The US Food and Drug Administration (FDA) has announced a Class I recall of Baxter Corporation Englewood’s ABACUS Total Parenteral Nutrition (TPN) Calculation Software, versions 3.1, 3.0, 2.1, and 2.0.
Baxter has received 2 reports of malfunctioning software and said errors with this software may cause adverse effects.
ABACUS TPN Calculation Software is a Windows-based software application used by pharmacists to calculate or order TPN formulas.
The errors explained
Due to software failures, the following errors may occur:
- ABACUS v3.1 may calculate quantities of electrolytes that are double the expected values during the creation of TPN orders.
- ABACUS v3.1 may automatically add additional sterile water to a formula equal to the volume of a premix, resulting in an over-dilution.
- All software versions of ABACUS software display the calcium phosphate curve points for Premasol incorrectly.
- All software versions of ABACUS may display an inaccurate estimation for calcium and phosphate precipitation in certain circumstances where multiple ingredients provide calcium.
If any of these failures occur, patients may be at risk of developing overdose symptoms. The symptoms are varied and depend on the type of software failure and composition of the fluid being compounded.
Symptoms may be non-specific and include nausea, vomiting, dizziness, or fatigue. Some more severe symptoms include cardiac arrhythmia, pulmonary edema, congestive heart failure, and seizures. A fatal outcome is possible, especially in the high-risk population.
Actions to take
Baxter is recommending that customers contact the company to ensure the ABACUS software is configured correctly.
Customers with a software version earlier than 3.1 will have software version 3.1 installed, which addresses the issues that prompted the recall. In addition, Baxter Support Services will schedule upgrades and assist customers with establishing the proper ABACUS configuration in the customers’ facilities.
Baxter has also requested that customers follow safe compounding practices. Namely, use the “Summary” button to verify the order against the calculated amounts prior to completing the order.
In addition, verify that the ordered ingredients and quantities displayed in the software and printed on the Bag label and the Solution Formula label match the PN prescription prior to preparation. And use a filter for administration of a PN bag.
For more information on the recall, see the FDA’s recall notice, or contact Baxter at 303-617-2242. For technical support, call 1-800-678-2292 or email [email protected].
To report adverse reactions or quality problems related to this product, visit the FDA’s MedWatch website. ![]()
How to save a life in 15 minutes or less
It is important to recognize that as pediatricians we have the unique opportunity to see to the lives of a very vulnerable group of people known as teenagers.
We can all relate to the discomfort of the stone-faced teenager with one-word answers and one foot out the door. There is usually a parent present who is answering all of the questions, and if you are lucky, the patient may put the cell phone down long enough to get an eye exam in, but, we must realize that the 15 minutes of captive audience could be the most important 15 minutes of the teen’s life.
Before we start our exam, we should have a plan in place for what topics we should be addressing. Every thorough physical should include a screen on drugs and alcohol, depression, sexual activity, and violence. In a busy practice, it seems impossible to address these issues in a time-conservative manner, but if we plan ahead, we can be thorough, casual, and informative.
First, you must analyze your own style. If having these discussions is uncomfortable for you, then attempting them without a plan will be disastrous. Many pediatricians just choose to avoid the entire discussion and hope that the parent is parenting and will address the major issues. But fewer than half of all parents talk to their children about the issues that they are faced with daily, and a great majority are ill-informed, or driven by their own beliefs.
First, pediatricians must make a list of hot topics to be discussed. Review the most current data and how they are affecting the teens in your area. Next, whether your talking style is comfortable or not, having a questionnaire that introduces each topic is always helpful (Am. J. Psychiatry 1995;152:1601-7
Lastly, have teenagers come in by themselves. Parents cannot help themselves and will always speak for their children, and most teens will not ask questions that they don’t think their parent will approve of or that relate to private family issues. So, you must set the stage for a comfortable talking environment. By having the questionnaire available, you can use it as a guide to see what issues are affecting the patient.
Knowing current information is also imperative to a good wellness exam. Know what the latest drugs are being used by the teens in the area, and know the street names of drugs (drugabuse.gov/drugs-abuse). Where do the local teens hang out? Major issues happening at the local high schools can help guide your conversations and build trust as patients begin to see you as an active and involved leader in the community.
Depression affects 8% of teens every year. Therefore, there is a guarantee that at least a handful will present in your office every year. Asking the right questions is key to getting helpful answers. Be direct, ask, "Have you ever, or are you now having suicidal ideation?" Over 90% of children and adolescents who commit suicide have a mental disorder (J. Clin. Psychiatry 1999;60 (Suppl. 2):70-4). There is a Web site supported by the American Academy of Pediatrics that has questionnaires to assist in identifying symptoms of depression (brightfutures.aap.org). Knowing the family history of psychiatric disorders can be very helpful in guiding the physician of what questions to ask. Many teens are fearful that they may be having symptoms of a psychiatric disorder, but are too afraid to ask, given the stigma that goes along with it.
Address issues of self-image. If patients are overweight, give tips on healthy eating and exercise. Develop a nutritional plan and track a patient’s progress by having her follow up. Allow her to discuss what make her feel sad or uncomfortable. How is she interacting with her peers, does she fit in or is she often alone?
A wellness exam is not complete without addressing sex and sexuality. No matter how you slice it, talking about sex with a complete stranger will never be easy. Using the questionnaire to bring up the topic helps. Start with generalizations about the risks of unprotected sex and general statistics of sexually transmitted infections in teenagers. Next, a general statement about abstinence is important so that teens realize it is an option. Review the common birth control methods and their risks. Encourage him to have at least one adult that he can trust to discuss delicate issues with and to return to your office if other issues arise.
Teenagers also are under the belief that they are invincible and that bad things only happen to other people. Discuss the leading cause of death in teenagers so they understand the reality of risk taking. Talk about date rape and physical abuse amongst teen couples. In a study done in California, 35% of teens questioned had experienced some form of violence with-in their relationships (Social Work 1986;31:465-8)
Knowing the laws that govern what advice can be given and what information can remain confidential is imperative. A great resource in understanding the basic laws that protect the physician and the patient’s rights is guttmacher.org/statecenter/spibs/spib_OMCL.pdf. Most states provide an online version of their laws governing teens and medical practice.
Establishing a rapport with your teenage patients can be very rewarding. Many teenagers are in search of a listening ear and need guidance in this new and critical era of their life. With a little planning and practice, you will provide with ease the information to help them make good decisions. It is important that we are equipped and ready because you may just save a life!
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected]. Go to pediatricnews.com to view similar columns.
It is important to recognize that as pediatricians we have the unique opportunity to see to the lives of a very vulnerable group of people known as teenagers.
We can all relate to the discomfort of the stone-faced teenager with one-word answers and one foot out the door. There is usually a parent present who is answering all of the questions, and if you are lucky, the patient may put the cell phone down long enough to get an eye exam in, but, we must realize that the 15 minutes of captive audience could be the most important 15 minutes of the teen’s life.
Before we start our exam, we should have a plan in place for what topics we should be addressing. Every thorough physical should include a screen on drugs and alcohol, depression, sexual activity, and violence. In a busy practice, it seems impossible to address these issues in a time-conservative manner, but if we plan ahead, we can be thorough, casual, and informative.
First, you must analyze your own style. If having these discussions is uncomfortable for you, then attempting them without a plan will be disastrous. Many pediatricians just choose to avoid the entire discussion and hope that the parent is parenting and will address the major issues. But fewer than half of all parents talk to their children about the issues that they are faced with daily, and a great majority are ill-informed, or driven by their own beliefs.
First, pediatricians must make a list of hot topics to be discussed. Review the most current data and how they are affecting the teens in your area. Next, whether your talking style is comfortable or not, having a questionnaire that introduces each topic is always helpful (Am. J. Psychiatry 1995;152:1601-7
Lastly, have teenagers come in by themselves. Parents cannot help themselves and will always speak for their children, and most teens will not ask questions that they don’t think their parent will approve of or that relate to private family issues. So, you must set the stage for a comfortable talking environment. By having the questionnaire available, you can use it as a guide to see what issues are affecting the patient.
Knowing current information is also imperative to a good wellness exam. Know what the latest drugs are being used by the teens in the area, and know the street names of drugs (drugabuse.gov/drugs-abuse). Where do the local teens hang out? Major issues happening at the local high schools can help guide your conversations and build trust as patients begin to see you as an active and involved leader in the community.
Depression affects 8% of teens every year. Therefore, there is a guarantee that at least a handful will present in your office every year. Asking the right questions is key to getting helpful answers. Be direct, ask, "Have you ever, or are you now having suicidal ideation?" Over 90% of children and adolescents who commit suicide have a mental disorder (J. Clin. Psychiatry 1999;60 (Suppl. 2):70-4). There is a Web site supported by the American Academy of Pediatrics that has questionnaires to assist in identifying symptoms of depression (brightfutures.aap.org). Knowing the family history of psychiatric disorders can be very helpful in guiding the physician of what questions to ask. Many teens are fearful that they may be having symptoms of a psychiatric disorder, but are too afraid to ask, given the stigma that goes along with it.
Address issues of self-image. If patients are overweight, give tips on healthy eating and exercise. Develop a nutritional plan and track a patient’s progress by having her follow up. Allow her to discuss what make her feel sad or uncomfortable. How is she interacting with her peers, does she fit in or is she often alone?
A wellness exam is not complete without addressing sex and sexuality. No matter how you slice it, talking about sex with a complete stranger will never be easy. Using the questionnaire to bring up the topic helps. Start with generalizations about the risks of unprotected sex and general statistics of sexually transmitted infections in teenagers. Next, a general statement about abstinence is important so that teens realize it is an option. Review the common birth control methods and their risks. Encourage him to have at least one adult that he can trust to discuss delicate issues with and to return to your office if other issues arise.
Teenagers also are under the belief that they are invincible and that bad things only happen to other people. Discuss the leading cause of death in teenagers so they understand the reality of risk taking. Talk about date rape and physical abuse amongst teen couples. In a study done in California, 35% of teens questioned had experienced some form of violence with-in their relationships (Social Work 1986;31:465-8)
Knowing the laws that govern what advice can be given and what information can remain confidential is imperative. A great resource in understanding the basic laws that protect the physician and the patient’s rights is guttmacher.org/statecenter/spibs/spib_OMCL.pdf. Most states provide an online version of their laws governing teens and medical practice.
Establishing a rapport with your teenage patients can be very rewarding. Many teenagers are in search of a listening ear and need guidance in this new and critical era of their life. With a little planning and practice, you will provide with ease the information to help them make good decisions. It is important that we are equipped and ready because you may just save a life!
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected]. Go to pediatricnews.com to view similar columns.
It is important to recognize that as pediatricians we have the unique opportunity to see to the lives of a very vulnerable group of people known as teenagers.
We can all relate to the discomfort of the stone-faced teenager with one-word answers and one foot out the door. There is usually a parent present who is answering all of the questions, and if you are lucky, the patient may put the cell phone down long enough to get an eye exam in, but, we must realize that the 15 minutes of captive audience could be the most important 15 minutes of the teen’s life.
Before we start our exam, we should have a plan in place for what topics we should be addressing. Every thorough physical should include a screen on drugs and alcohol, depression, sexual activity, and violence. In a busy practice, it seems impossible to address these issues in a time-conservative manner, but if we plan ahead, we can be thorough, casual, and informative.
First, you must analyze your own style. If having these discussions is uncomfortable for you, then attempting them without a plan will be disastrous. Many pediatricians just choose to avoid the entire discussion and hope that the parent is parenting and will address the major issues. But fewer than half of all parents talk to their children about the issues that they are faced with daily, and a great majority are ill-informed, or driven by their own beliefs.
First, pediatricians must make a list of hot topics to be discussed. Review the most current data and how they are affecting the teens in your area. Next, whether your talking style is comfortable or not, having a questionnaire that introduces each topic is always helpful (Am. J. Psychiatry 1995;152:1601-7
Lastly, have teenagers come in by themselves. Parents cannot help themselves and will always speak for their children, and most teens will not ask questions that they don’t think their parent will approve of or that relate to private family issues. So, you must set the stage for a comfortable talking environment. By having the questionnaire available, you can use it as a guide to see what issues are affecting the patient.
Knowing current information is also imperative to a good wellness exam. Know what the latest drugs are being used by the teens in the area, and know the street names of drugs (drugabuse.gov/drugs-abuse). Where do the local teens hang out? Major issues happening at the local high schools can help guide your conversations and build trust as patients begin to see you as an active and involved leader in the community.
Depression affects 8% of teens every year. Therefore, there is a guarantee that at least a handful will present in your office every year. Asking the right questions is key to getting helpful answers. Be direct, ask, "Have you ever, or are you now having suicidal ideation?" Over 90% of children and adolescents who commit suicide have a mental disorder (J. Clin. Psychiatry 1999;60 (Suppl. 2):70-4). There is a Web site supported by the American Academy of Pediatrics that has questionnaires to assist in identifying symptoms of depression (brightfutures.aap.org). Knowing the family history of psychiatric disorders can be very helpful in guiding the physician of what questions to ask. Many teens are fearful that they may be having symptoms of a psychiatric disorder, but are too afraid to ask, given the stigma that goes along with it.
Address issues of self-image. If patients are overweight, give tips on healthy eating and exercise. Develop a nutritional plan and track a patient’s progress by having her follow up. Allow her to discuss what make her feel sad or uncomfortable. How is she interacting with her peers, does she fit in or is she often alone?
A wellness exam is not complete without addressing sex and sexuality. No matter how you slice it, talking about sex with a complete stranger will never be easy. Using the questionnaire to bring up the topic helps. Start with generalizations about the risks of unprotected sex and general statistics of sexually transmitted infections in teenagers. Next, a general statement about abstinence is important so that teens realize it is an option. Review the common birth control methods and their risks. Encourage him to have at least one adult that he can trust to discuss delicate issues with and to return to your office if other issues arise.
Teenagers also are under the belief that they are invincible and that bad things only happen to other people. Discuss the leading cause of death in teenagers so they understand the reality of risk taking. Talk about date rape and physical abuse amongst teen couples. In a study done in California, 35% of teens questioned had experienced some form of violence with-in their relationships (Social Work 1986;31:465-8)
Knowing the laws that govern what advice can be given and what information can remain confidential is imperative. A great resource in understanding the basic laws that protect the physician and the patient’s rights is guttmacher.org/statecenter/spibs/spib_OMCL.pdf. Most states provide an online version of their laws governing teens and medical practice.
Establishing a rapport with your teenage patients can be very rewarding. Many teenagers are in search of a listening ear and need guidance in this new and critical era of their life. With a little planning and practice, you will provide with ease the information to help them make good decisions. It is important that we are equipped and ready because you may just save a life!
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected]. Go to pediatricnews.com to view similar columns.
Obesity Intervention With Follow‐up
Obesity‐related medical care remains a substantial driver in escalating healthcare costs. Not surprisingly, healthcare costs for obese patients are 40% higher annually than those for normal‐weight individuals.[1] In 2002, the morbidity attributable to obesity was calculated to equal, if not exceed, that associated with smoking.[2] Though inpatient outcomes appear similar for obese individuals, nearly all obesity‐related comorbidities can lead to hospitalization, and obesity has been linked to early mortality.[3, 4, 5] As obesity‐related costs continue to grow, so does the need to intervene in this at‐risk patient population.[3, 4, 5] Though significant efforts have focused on obesity interventions in the outpatient setting, a paucity of data exists on how best to address obesity during inpatient hospitalization.
Hospitalization itself has often been described as a teachable moment, a time during which a life event leads to increased receptivity to behavior change.[6, 7, 8] The positive effects of inpatient smoking cessation efforts are well recognized. Such initiatives typically include an inpatient counseling session, followed by supportive contact postdischarge.[9, 10] Features common to successful outpatient weight loss interventions include ongoing patient contact of variable duration, frequent self‐weighing, diet modifications, and increased activity.[11, 12, 13, 14, 15] To date, little is known about the effectiveness of such programs in the inpatient setting, though research has shown that obese inpatients are receptive to weight loss initiatives.[16] Accomplishing even modest weight reductions in such patients has the potential to lead to significant health and cost benefits.[1, 17, 18, 19]
In this study we sought to determine whether inpatient weight loss counseling with post discharge phone follow‐up would result in significant weight loss at 6 months when compared to controls. Secondary end points included weight change from baseline and changes in waist‐to‐hip ratios (WHRs). To our knowledge, this is the first randomized trial designed to evaluate the effect of an inpatient obesity intervention with postdischarge follow‐up in a general medicine population.
METHODS
Setting/Participants
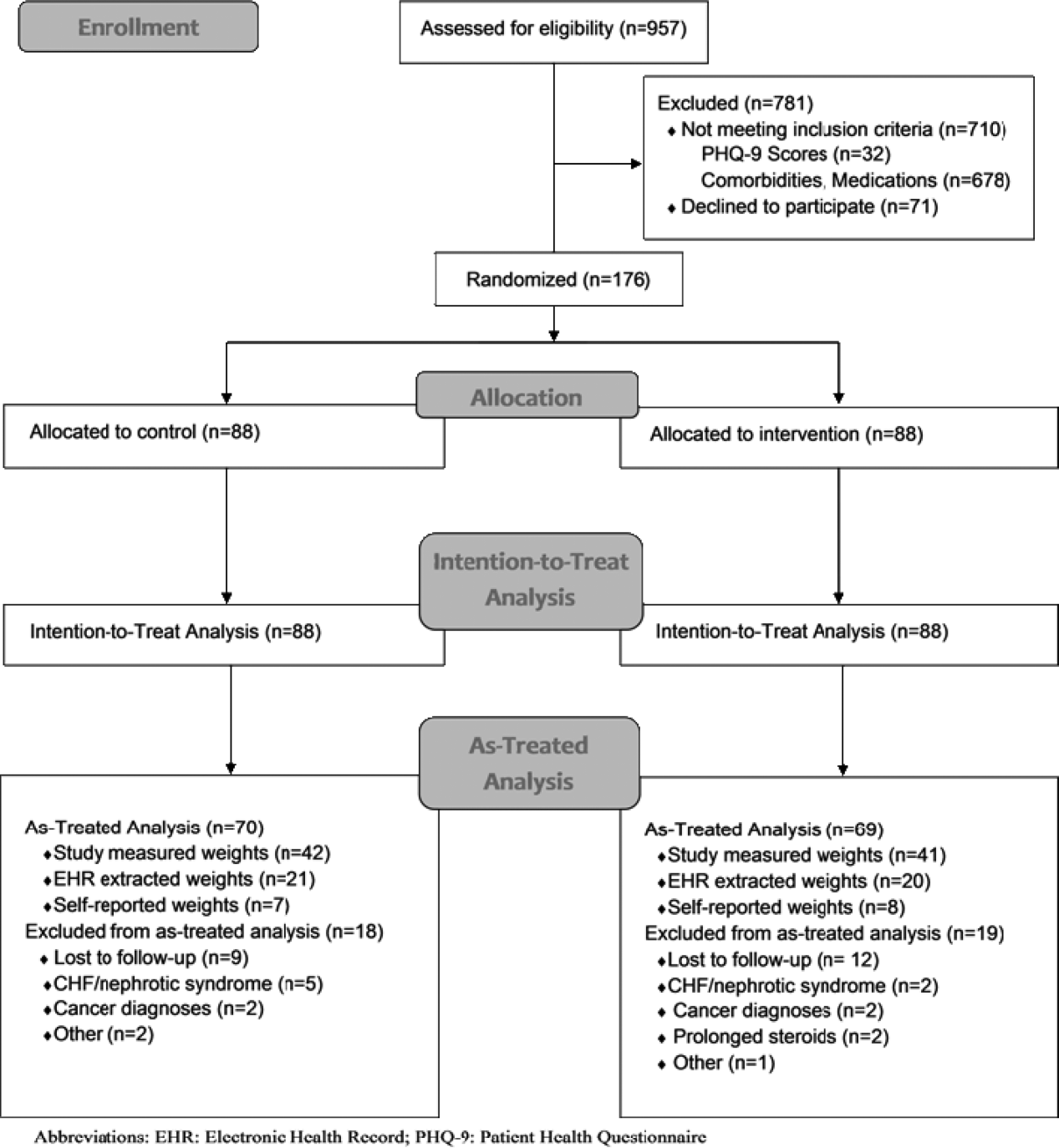
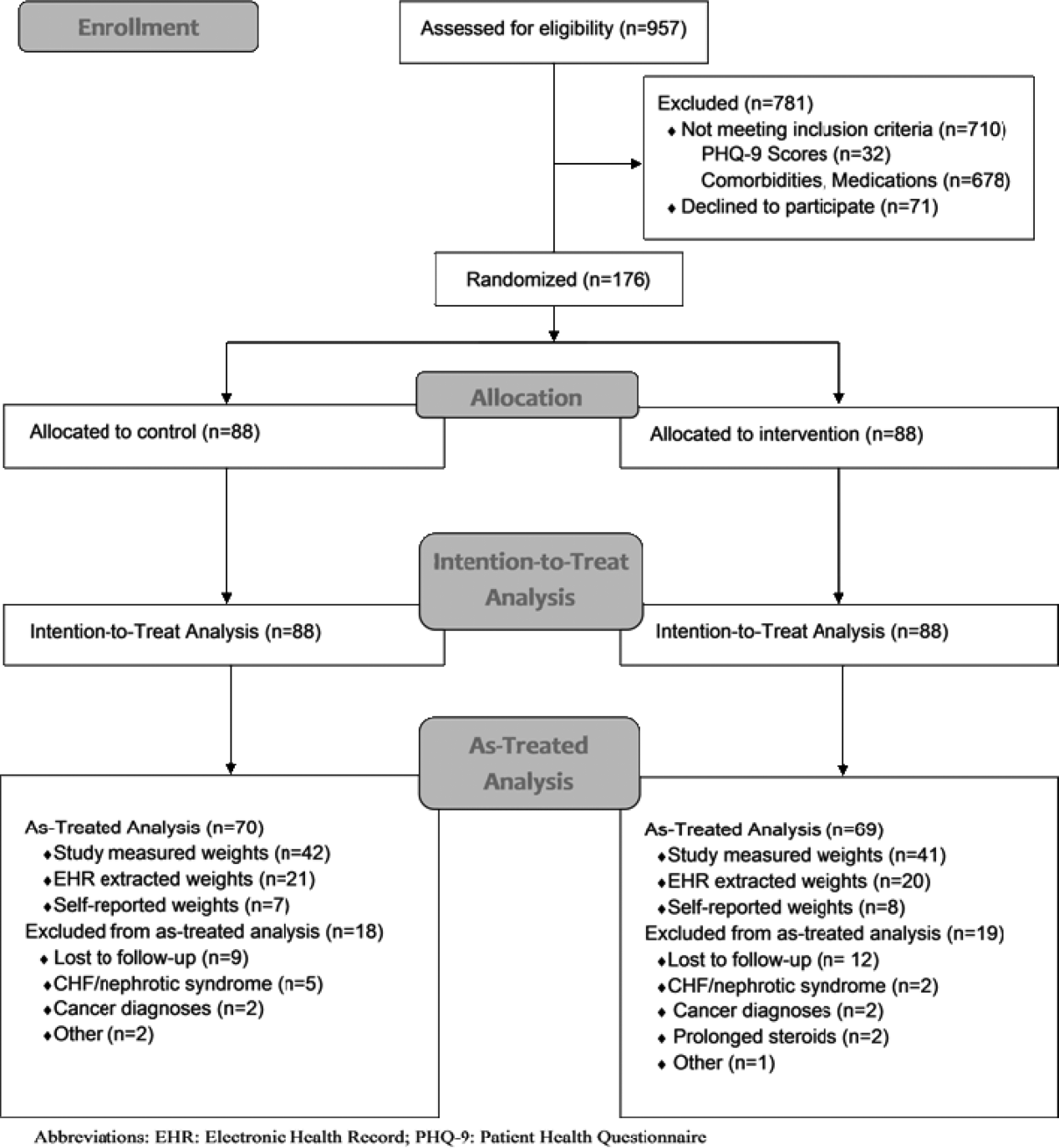
We conducted a prospective, randomized controlled trial from January 2011 to May 2012 at a single, large (854‐bed), academic medical center in Chicago, Illinois. Eligible subjects were those with a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) between 30 and 50 kg/m2, ages 18 to 65 years old, admitted to an internal medicine service. Exclusion criteria included the presence of acute medical conditions known to affect weight, Charlson comorbidity index >3, moderate to severe major depression, prolonged steroid use (>2 weeks), initiation of medications known to affect weight (eg, diuretics), non‐English speaking, and precontemplation stage of change. Upon enrollment, subjects were randomly assigned to either the control or intervention group. A computer‐generated block randomization scheme was used to generate group assignments. Study research assistants sequentially assigned enrolled patients according to the computer‐generated randomization scheme. Group assignment was only revealed to each study participant after enrollment was complete. Figure 1 summarizes subject recruitment, randomization, and follow‐up. Informed written consent was obtained from all participants. Study participants, physicians, and investigators were unblinded. Study subjects were informed that they were participating in an obesity study as outlined on the study consent form. Study protocols and procedures were approved by the institutional review board at Northwestern University.
Interventions
After enrollment, all subjects had body weight measured on a calibrated study scale in light clothing or hospital gown without shoes. Waist circumference (narrowest circumference between the ribs and iliac crest) and hip circumference (maximum circumference of the hips) were measured to the nearest 0.1 cm. Measurements were taken in triplicate and averaged. WHR was calculated as waist circumference divided by hip circumference. All participants completed a demographic questionnaire and rated their level of agreement with 6 statements relating to weight perceptions and weight loss using a Likert scale from 1 (strongly disagree) to 10 (strongly agree).
Participants in the control group were not provided with any specific instructions regarding weight loss, diet, or exercise prior to discharge. Intervention group subjects were asked to view a 13‐minute weight loss education video (addressed specific caloric intake goals for weight loss, portion sizes), undergo a 25‐minute personalized counseling session with a certified health educator or study physician, and to set 3 specific lifestyle goals prior to discharge (weight loss, dietary, and fitness). A personal weight loss goal of 10% baseline body weight was set for intervention subjects based on obesity treatment guidelines suggesting subjects could safely lose 1 to 2 lb per week over the course of the study.[20] Clinically significant weight loss was defined as weight loss of 5% or more from baseline body weight based on literature illustrating health benefits with this amount of weight loss.[17, 18, 19]
All study subjects received a phone call schedule and weight‐tracking sheet prior to discharge, with calls scheduled at weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24. Phone calls for both groups were used to obtain weight and identify changes in medications or health condition and were conducted by a certified health educator or study physician. No problem solving, motivational support, or other specific instruction was provided to the control group, whereas phone calls for intervention subjects utilized motivational interviewing and problem‐solving techniques.
Study subjects were asked to return for an in‐person follow‐up visit at 6 months. Weight was reassessed with subjects in light clothing and without shoes on the same calibrated study scale by a certified health educator. Follow‐up WHRs were also collected.
Outcomes
The primary outcome of the study was the difference in mean weight change (change in kilograms from baseline) between control and intervention groups at 6 months. Secondary outcome measures included intragroup weight change from baseline and changes in WHR.
Measured weights were obtained for subjects who returned for 6‐month follow‐up. For those unable or unwilling to return at 6 months, measured weights were obtained from the electronic health record (EHR) and self‐reported weights requested for use in imputed weight calculations. Imputation weights for missing weight values were prioritized as follows: (1) in‐person 6‐month follow‐up weight used if available, (2) inpatient or outpatient EHR obtained weight used if in‐person weight unavailable, and (3) if neither an in‐person or EHR weight was available, a self‐reported weight was used.[21] For intention‐to‐treat analysis, baseline weight was carried forward for subjects lacking follow‐up data after enrollment, historically considered a conservative strategy in weight loss trials.[22, 23]
Statistical Analysis
Baseline patient characteristics were compared using 2 tests for categorical variables and 2‐sample t tests for continuous variables. The primary study outcome of weight change over time for each group was assessed for all study participants using an intention‐to‐treat analysis. Separate as‐treated analyses were also performed utilizing imputed weights for those who failed to follow‐up at 6 months and for study completers who had a measured study weight documented at 6 months.
Three analyzable datasets were computed: intention‐to‐treat (using all participants randomized to the study), as‐treated analysis with imputed weights, and as treated analysis with measured 6‐month study weights only. Intent‐to‐treat analysis provides the unbiased comparisons among the treatment groups. To avoid dilution of treatment effect, as‐treated analyses with imputed weights (including measured weights at 6‐month follow‐up obtained from other sources [eg, clinic visit]) and with measured study weights (completers only) were performed.
Weight change over time was analyzed with a longitudinal covariance pattern model, using an unstructured variance‐covariance matrix. Specifically, weight was modeled at all time points (baseline and weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24) using a priori contrasts and treating baseline as the reference cell to assess weight change, relative to baseline, at the 4 postbaseline time points.[24] Group effects on these a priori time contrasts were included to test for weight change differences between groups, and we specifically tested whether the group effect on weight change was equal or varied across the postbaseline time points.
We aimed to obtain a sample size of 176 subjects (88 in each group) in order to achieve 80% power to detect a 5‐kg weight loss in the intervention group after 6 months (at most standard deviation [SD]=15) and a 5‐kg difference in weight loss between groups (SD=10), assuming an of 0.05 using 2‐tailed testing and an attrition rate of 20%.
RESULTS
Over a period of 18 months we were able to recruit 176 subjects. We found no significant differences in baseline characteristics between groups (Table 1). Sixteen subjects developed exclusionary conditions after enrollment and were subsequently excluded from as‐treated data analyses. Follow‐up weight data for as‐treated analysis were available for 139 study subjects through the use of in‐person (n=83), EHR (n=41), and self‐reported (n=15) weights.
| Intervention, N=88 | Control, N=88 | |
|---|---|---|
| ||
| Age, y, mean (SD) | 48.9 (10.5) | 48.7 (10.3) |
| Female, % | 67.1 | 62.5 |
| Race/ethnicity, % | ||
| African American | 50.0 | 41.4 |
| Caucasian | 36.4 | 46.5 |
| Other | 13.6 | 11.6 |
| Education level, % | ||
| High school | 11.4 | 11.5 |
| College | 68.2 | 64.4 |
| Graduate level | 20.5 | 24.1 |
| Annual income, % | ||
| <$50,000 | 43.0 | 45.2 |
| $50,000$100,000 | 45.4 | 33.3 |
| >$100,000 | 11.6 | 21.4 |
| BMI, mean (SD), kg/m2 | 38.0 (5.1) | 37.5 (4.9) |
| BMI category, % | ||
| 3034.9 | 34.1 | 34.1 |
| 3539.9 | 28.4 | 37.5 |
| 40 | 37.5 | 28.4 |
| Waist‐hip ratio, mean (SD)a | 0.95 (0.08) | 0.96 (0.08) |
| Length of stay, d, median (interquartile range) | 2.0 (1.13.0) | 2.2 (1.33.3) |
| Diabetes, % | 27.3 | 25.0 |
| Admit diagnosis, % | ||
| Cardiovascular | 34.1 | 25.0 |
| Gastrointestinal | 15.9 | 18.2 |
| Pulmonary | 10.2 | 5.7 |
| Infectious | 11.4 | 13.6 |
| Endocrine | 3.4 | 2.3 |
| Other | 25.0 | 35.2 |
Change in Weight Loss and WHR
For the 176 participants included in the intent‐to‐treat analysis, mean weight loss for the intervention group and control groups was 1.08 kg (SD=4.33) and 1.35 kg (SD=3.64) at 6 months, respectively. We found no significant difference in weight loss between groups at 6 months (P=0.26), though there was statistically significant weight loss from baseline noted in both groups (P=0.02 and P=0.0008, respectively) (Table 2).
| Characteristic | Intervention Group | Control Group | P Valuea |
|---|---|---|---|
| |||
| Intent‐to‐treat analysis (all participants), kg (SD) | |||
| No. | 88 | 88 | |
| Baseline | 107.7 (16.7) | 105.1 (17.4) | 0.23 |
| 6‐month follow‐up | 106.6 (16.1) | 103.8 (17.1) | 0.16 |
| Weight change | 1.08 (4.33) | 1.35 (3.64) | 0.26 |
| As treated analysis with imputed weights, kg (SD) | |||
| No. | 69 | 70 | |
| Baseline | 108.9 (16.7) | 104.0 (16.2) | 0.08 |
| 6‐month follow‐up | 106.1 (17.2) | 102.4 (15.9) | 0.18 |
| Weight change | 2.88 (5.77) | 1.69 (5.09) | 0.12 |
| As treated analysis with measured 6‐month weights (completers), kg (SD) | |||
| No. | 41 | 42 | |
| Baseline | 109.8 (16.2) | 107.0 (18.0) | 0.47 |
| 6‐month follow‐up | 107.4 (15.0) | 104.2 (17.7) | 0.37 |
| Weight change | 2.32 (6.16) | 2.83 (4.88) | 0.68 |
Of 139 participants in the as‐treated analysis utilizing imputed weights, weight loss for the intervention group and control groups was 2.88 kg (SD=5.77) and 1.69 kg (SD=5.09). There was statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.006, P=0.004, respectively). However, there were neither statistically nor clinically significant differences between the 2 groups (1.19 kg, P=0.12). Finally, for the 83 completers in the as‐treated analysis with measured study weights only, weight loss for the intervention group and control group was 2.32 kg (SD=6.16) and 2.83 kg (SD=4.88), respectively. Though we again noted statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.02, P=0.0005, respectively), we found neither statistically nor clinically significant differences in weight loss between the 2 groups (0.51 kg, P=0.68). Figure 2 illustrates weight change over time for the intervention and control subjects who returned for in‐person follow‐up at 6 months.
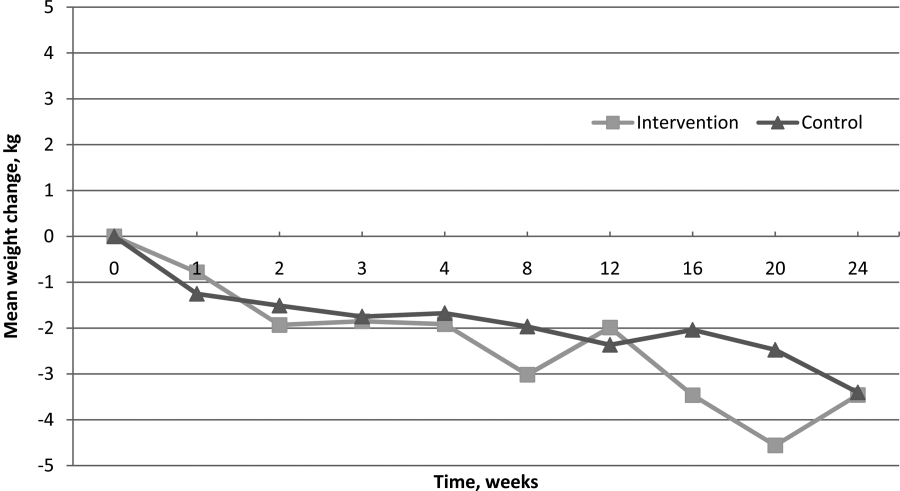
For WHRs, we found no difference in WHR change between groups at 6 months (0.04 vs 0.04, P=0.59). However, among those who completed the study, there was a statistically significant decrease in WHR from baseline within both groups, decreasing 0.040.06 (P=0.006) in the intervention group and 0.040.04 (P<0.001) among controls.
Weight Perceptions
Only 34% of participants accurately perceived their weight and correctly identified themselves as either obese or morbidly obese. Nearly half of the study participants (47%) classified themselves as overweight rather than obese, though all met criteria for obesity. We found weight perception was most accurate among Caucasians (48%) and least accurate among African Americans (24%) and morbidly obese individuals (26%). Nearly all subjects felt weight loss was important (99%), and most assumed weight had contributed to their hospitalization (64%).
DISCUSSION
We hypothesized that intervention group subjects would lose more weight than those assigned to control given that they received weight loss interventions previously shown to be effective.[13, 25, 26, 27] However, intention‐to‐treat analysis showed no difference in weight loss between intervention and control subjects at 6 months. Interestingly, as‐treated analyses did suggest that subjects in both study arms lost a modest amount of weight over the duration of the study. Though modest weight reductions have been shown to give rise to health benefits, neither group met our prespecified goal for clinically significant weight loss (5% of baseline body weight).[18, 19] There were also no differences in WHRs noted between the intervention and control groups. The modest reductions in WHRs from baseline in both groups are of uncertain clinical significance but of interest given the well‐established graded relationship between WHR and risk of cardiovascular disease.[28, 29, 30, 31]
Though the control group subjects received no specific instruction regarding weight loss, we suspect that the influences of study enrollment, discussion of obesity while an inpatient, regular phone contacts, and weight tracking may have been sufficient to affect weight behaviors. Certainly, this exceeds usual care for hospitalized patients suffering from obesity. Though it is possible that all of obese patients lose weight over the 6‐month period following hospitalization, we feel this is unlikely. The exclusion of subjects with an elevated Charlson comorbidity index lessened the likelihood of weight loss due to chronic disease, and without intervention, obese individuals tend to gain rather than lose weight over time.[32] Nonetheless, the lack of significant weight loss between groups suggests that the specific weight loss instruction provided to the intervention group did not promote more weight loss than the general education and regular phone calls provided to controls.
Our findings related to weight perception were similar to those established in prior studies. Individuals frequently misperceive their weight and weight perceptions are least accurate among severely obese individuals and nonwhites.[16, 33, 34] Contrary to prior studies, we found that the majority of participants felt their weight negatively impacted their health, and most thought their hospitalization was weight‐related.[35] Interestingly, research suggests that weight‐related perception of health risk correlates with the likelihood of making a weight loss attempt, another factor that may have influenced the behavior of study participants.[35]
This study has several limitations. It was conducted and based on practices at a single institution, thus limiting generalizability. Additionally, the percentage of subjects who returned for 6‐month follow‐up was lower than desired at 50%. However, high attrition rates commonly plague obesity trials, and we are unaware of any existing studies documenting expected attrition rates among obese inpatients.[23, 36, 37, 38] To help address this, we used imputed weights in our as‐treated analysis to obtain follow‐up weight values on 79% of subjects. Further, the intentional exclusion of subjects in the precontemplation stage of change likely resulted in selection of a more motivated patient population. However, this was done assuming that most inpatient obesity interventions would primarily target patients interested in losing weight. Finally, the lack of a usual care group that more accurately reflects the experience of most hospitalized obese patientsno regular postdischarge interactionsdoes limit interpretation of the modest weight loss noted in both study groups.
In conclusion, an inpatient obesity intervention with post‐discharge follow‐up did not result in intervention subjects losing more weight than controls over a 6‐month period. However, the finding of modest weight loss among both groups is of interest and may warrant further investigation. It remains unclear whether this is a naturally occurring phenomenon or whether other factors influence behavior change in this patient population. Additional studies will be needed to clarify the impact of hospitalization, obesity recognition, perception of health risk, weight tracking, and follow‐up on weight behaviors. Given the proven benefits of even modest weight reductions, encouraging any amount of weight loss in these at‐risk individuals would appear to be a step in the right direction. We have yet to determine whether inpatient obesity interventions represent a lost opportunity.
- , , , . The impact of weight loss among seniors on Medicare spending. Health Econ Rev. 2013;3(1):7.
- . The effects of obesity, smoking, and drinking on medical problems and costs. Health Aff (Millwood). 2002;21(2):245–253.
- , , , , , . The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88.
- , , , , , . The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103(10):1895–1901.
- , , , . Assessment of excess mortality in obesity. Am J Epidemiol. 1998;147(1):42–48.
- , , . Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure‐a review. Am J Cardiol. 2004;94(9):1155–1160.
- . In‐hospital initiation of statins: taking advantage of the “teachable moment”. Cleve Clin J Med. 2003;70(6):502, 504–506.
- , , . Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170.
- , , . Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008;168(18):1950–1960.
- , . Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial. CMAJ. 2009;180(13):1297–1303.
- , , , , , . Phone and e‐mail counselling are effective for weight management in an overweight working population: a randomized controlled trial. BMC Public Health. 2009;9:6.
- , . Weighing the evidence: benefits of regular weight monitoring for weight control. J Nutr Educ Behav. 2005;37(6):319–322.
- , , , et al. Comparative effectiveness of weight‐loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968.
- , , , et al. Weight‐loss outcomes: a systematic review and meta‐analysis of weight‐loss clinical trials with a minimum 1‐year follow‐up. J Am Diet Assoc. 2007;107(10):1755–1767.
- , , . Self‐monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102.
- , , , . Willingness for weight loss intervention among overweight and obese inpatients. South Med J. 2011;104(6):397–400.
- , , , et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486.
- , , . The beneficial effects of modest weight loss on cardiovascular risk factors. Int J Obes Relat Metab Disord. 1997;21(suppl 1):S5–S9.
- . Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16(6):397–415.
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(suppl 2):51S–209S.
- , , , et al. Validity of clinical body weight measures as substitutes for missing data in a randomized trial. Obes Res Clin Pract. 2008;2(4):277–281.
- , , . Advances in analysis of longitudinal data. Ann Rev Clin Psychol. 2010;6:79–107.
- , , , et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PloS One. 2009;4(8):e6624.
- , . Longitudinal Data Analysis. Hoboken, NJ: Wiley‐Interscience; 2006.
- , , , . Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150(4):255–262.
- . Clinical practice. Nonsurgical management of obesity in adults. N Engl J Med. 2008;358(18):1941–1950.
- , , . A meta‐analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord. 1997;21(10):941–947.
- , , , et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population‐based prospective study. Circulation. 2007;116(25):2933–2943.
- , , , et al. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50(8):752–759.
- , , , et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case‐control study. Lancet. 2005;366(9497):1640–1649.
- , , , . Waist circumference and waist‐to‐hip ratio as predictors of cardiovascular events: meta‐regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–856.
- , , , . Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855.
- , . Self‐perception of weight appropriateness in the United States. Am J Prev Med. 2003;24(4):332–339.
- , , , et al. Differences in weight perception among blacks and whites. J Womens Health (Larchmt). 2011;20(12):1805–1811.
- , , , , . Perceived health risk of excess body weight among overweight and obese men and women: differences by sex. Prev Med. 2008;47(1):46–52.
- , , . Obesity research—limitations of methods, measurements, and medications. JAMA. 2006;295(7):826–828.
- . Interpreting incomplete data in studies of diet and weight loss. N Engl J Med. 2003;348(21):2136–2137.
- , , , . Predictors of attrition in a large clinic‐based weight‐loss program. Obes Res. 2003;11(7):888–894.
Obesity‐related medical care remains a substantial driver in escalating healthcare costs. Not surprisingly, healthcare costs for obese patients are 40% higher annually than those for normal‐weight individuals.[1] In 2002, the morbidity attributable to obesity was calculated to equal, if not exceed, that associated with smoking.[2] Though inpatient outcomes appear similar for obese individuals, nearly all obesity‐related comorbidities can lead to hospitalization, and obesity has been linked to early mortality.[3, 4, 5] As obesity‐related costs continue to grow, so does the need to intervene in this at‐risk patient population.[3, 4, 5] Though significant efforts have focused on obesity interventions in the outpatient setting, a paucity of data exists on how best to address obesity during inpatient hospitalization.
Hospitalization itself has often been described as a teachable moment, a time during which a life event leads to increased receptivity to behavior change.[6, 7, 8] The positive effects of inpatient smoking cessation efforts are well recognized. Such initiatives typically include an inpatient counseling session, followed by supportive contact postdischarge.[9, 10] Features common to successful outpatient weight loss interventions include ongoing patient contact of variable duration, frequent self‐weighing, diet modifications, and increased activity.[11, 12, 13, 14, 15] To date, little is known about the effectiveness of such programs in the inpatient setting, though research has shown that obese inpatients are receptive to weight loss initiatives.[16] Accomplishing even modest weight reductions in such patients has the potential to lead to significant health and cost benefits.[1, 17, 18, 19]
In this study we sought to determine whether inpatient weight loss counseling with post discharge phone follow‐up would result in significant weight loss at 6 months when compared to controls. Secondary end points included weight change from baseline and changes in waist‐to‐hip ratios (WHRs). To our knowledge, this is the first randomized trial designed to evaluate the effect of an inpatient obesity intervention with postdischarge follow‐up in a general medicine population.
METHODS
Setting/Participants
We conducted a prospective, randomized controlled trial from January 2011 to May 2012 at a single, large (854‐bed), academic medical center in Chicago, Illinois. Eligible subjects were those with a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) between 30 and 50 kg/m2, ages 18 to 65 years old, admitted to an internal medicine service. Exclusion criteria included the presence of acute medical conditions known to affect weight, Charlson comorbidity index >3, moderate to severe major depression, prolonged steroid use (>2 weeks), initiation of medications known to affect weight (eg, diuretics), non‐English speaking, and precontemplation stage of change. Upon enrollment, subjects were randomly assigned to either the control or intervention group. A computer‐generated block randomization scheme was used to generate group assignments. Study research assistants sequentially assigned enrolled patients according to the computer‐generated randomization scheme. Group assignment was only revealed to each study participant after enrollment was complete. Figure 1 summarizes subject recruitment, randomization, and follow‐up. Informed written consent was obtained from all participants. Study participants, physicians, and investigators were unblinded. Study subjects were informed that they were participating in an obesity study as outlined on the study consent form. Study protocols and procedures were approved by the institutional review board at Northwestern University.

Interventions
After enrollment, all subjects had body weight measured on a calibrated study scale in light clothing or hospital gown without shoes. Waist circumference (narrowest circumference between the ribs and iliac crest) and hip circumference (maximum circumference of the hips) were measured to the nearest 0.1 cm. Measurements were taken in triplicate and averaged. WHR was calculated as waist circumference divided by hip circumference. All participants completed a demographic questionnaire and rated their level of agreement with 6 statements relating to weight perceptions and weight loss using a Likert scale from 1 (strongly disagree) to 10 (strongly agree).
Participants in the control group were not provided with any specific instructions regarding weight loss, diet, or exercise prior to discharge. Intervention group subjects were asked to view a 13‐minute weight loss education video (addressed specific caloric intake goals for weight loss, portion sizes), undergo a 25‐minute personalized counseling session with a certified health educator or study physician, and to set 3 specific lifestyle goals prior to discharge (weight loss, dietary, and fitness). A personal weight loss goal of 10% baseline body weight was set for intervention subjects based on obesity treatment guidelines suggesting subjects could safely lose 1 to 2 lb per week over the course of the study.[20] Clinically significant weight loss was defined as weight loss of 5% or more from baseline body weight based on literature illustrating health benefits with this amount of weight loss.[17, 18, 19]
All study subjects received a phone call schedule and weight‐tracking sheet prior to discharge, with calls scheduled at weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24. Phone calls for both groups were used to obtain weight and identify changes in medications or health condition and were conducted by a certified health educator or study physician. No problem solving, motivational support, or other specific instruction was provided to the control group, whereas phone calls for intervention subjects utilized motivational interviewing and problem‐solving techniques.
Study subjects were asked to return for an in‐person follow‐up visit at 6 months. Weight was reassessed with subjects in light clothing and without shoes on the same calibrated study scale by a certified health educator. Follow‐up WHRs were also collected.
Outcomes
The primary outcome of the study was the difference in mean weight change (change in kilograms from baseline) between control and intervention groups at 6 months. Secondary outcome measures included intragroup weight change from baseline and changes in WHR.
Measured weights were obtained for subjects who returned for 6‐month follow‐up. For those unable or unwilling to return at 6 months, measured weights were obtained from the electronic health record (EHR) and self‐reported weights requested for use in imputed weight calculations. Imputation weights for missing weight values were prioritized as follows: (1) in‐person 6‐month follow‐up weight used if available, (2) inpatient or outpatient EHR obtained weight used if in‐person weight unavailable, and (3) if neither an in‐person or EHR weight was available, a self‐reported weight was used.[21] For intention‐to‐treat analysis, baseline weight was carried forward for subjects lacking follow‐up data after enrollment, historically considered a conservative strategy in weight loss trials.[22, 23]
Statistical Analysis
Baseline patient characteristics were compared using 2 tests for categorical variables and 2‐sample t tests for continuous variables. The primary study outcome of weight change over time for each group was assessed for all study participants using an intention‐to‐treat analysis. Separate as‐treated analyses were also performed utilizing imputed weights for those who failed to follow‐up at 6 months and for study completers who had a measured study weight documented at 6 months.
Three analyzable datasets were computed: intention‐to‐treat (using all participants randomized to the study), as‐treated analysis with imputed weights, and as treated analysis with measured 6‐month study weights only. Intent‐to‐treat analysis provides the unbiased comparisons among the treatment groups. To avoid dilution of treatment effect, as‐treated analyses with imputed weights (including measured weights at 6‐month follow‐up obtained from other sources [eg, clinic visit]) and with measured study weights (completers only) were performed.
Weight change over time was analyzed with a longitudinal covariance pattern model, using an unstructured variance‐covariance matrix. Specifically, weight was modeled at all time points (baseline and weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24) using a priori contrasts and treating baseline as the reference cell to assess weight change, relative to baseline, at the 4 postbaseline time points.[24] Group effects on these a priori time contrasts were included to test for weight change differences between groups, and we specifically tested whether the group effect on weight change was equal or varied across the postbaseline time points.
We aimed to obtain a sample size of 176 subjects (88 in each group) in order to achieve 80% power to detect a 5‐kg weight loss in the intervention group after 6 months (at most standard deviation [SD]=15) and a 5‐kg difference in weight loss between groups (SD=10), assuming an of 0.05 using 2‐tailed testing and an attrition rate of 20%.
RESULTS
Over a period of 18 months we were able to recruit 176 subjects. We found no significant differences in baseline characteristics between groups (Table 1). Sixteen subjects developed exclusionary conditions after enrollment and were subsequently excluded from as‐treated data analyses. Follow‐up weight data for as‐treated analysis were available for 139 study subjects through the use of in‐person (n=83), EHR (n=41), and self‐reported (n=15) weights.
| Intervention, N=88 | Control, N=88 | |
|---|---|---|
| ||
| Age, y, mean (SD) | 48.9 (10.5) | 48.7 (10.3) |
| Female, % | 67.1 | 62.5 |
| Race/ethnicity, % | ||
| African American | 50.0 | 41.4 |
| Caucasian | 36.4 | 46.5 |
| Other | 13.6 | 11.6 |
| Education level, % | ||
| High school | 11.4 | 11.5 |
| College | 68.2 | 64.4 |
| Graduate level | 20.5 | 24.1 |
| Annual income, % | ||
| <$50,000 | 43.0 | 45.2 |
| $50,000$100,000 | 45.4 | 33.3 |
| >$100,000 | 11.6 | 21.4 |
| BMI, mean (SD), kg/m2 | 38.0 (5.1) | 37.5 (4.9) |
| BMI category, % | ||
| 3034.9 | 34.1 | 34.1 |
| 3539.9 | 28.4 | 37.5 |
| 40 | 37.5 | 28.4 |
| Waist‐hip ratio, mean (SD)a | 0.95 (0.08) | 0.96 (0.08) |
| Length of stay, d, median (interquartile range) | 2.0 (1.13.0) | 2.2 (1.33.3) |
| Diabetes, % | 27.3 | 25.0 |
| Admit diagnosis, % | ||
| Cardiovascular | 34.1 | 25.0 |
| Gastrointestinal | 15.9 | 18.2 |
| Pulmonary | 10.2 | 5.7 |
| Infectious | 11.4 | 13.6 |
| Endocrine | 3.4 | 2.3 |
| Other | 25.0 | 35.2 |
Change in Weight Loss and WHR
For the 176 participants included in the intent‐to‐treat analysis, mean weight loss for the intervention group and control groups was 1.08 kg (SD=4.33) and 1.35 kg (SD=3.64) at 6 months, respectively. We found no significant difference in weight loss between groups at 6 months (P=0.26), though there was statistically significant weight loss from baseline noted in both groups (P=0.02 and P=0.0008, respectively) (Table 2).
| Characteristic | Intervention Group | Control Group | P Valuea |
|---|---|---|---|
| |||
| Intent‐to‐treat analysis (all participants), kg (SD) | |||
| No. | 88 | 88 | |
| Baseline | 107.7 (16.7) | 105.1 (17.4) | 0.23 |
| 6‐month follow‐up | 106.6 (16.1) | 103.8 (17.1) | 0.16 |
| Weight change | 1.08 (4.33) | 1.35 (3.64) | 0.26 |
| As treated analysis with imputed weights, kg (SD) | |||
| No. | 69 | 70 | |
| Baseline | 108.9 (16.7) | 104.0 (16.2) | 0.08 |
| 6‐month follow‐up | 106.1 (17.2) | 102.4 (15.9) | 0.18 |
| Weight change | 2.88 (5.77) | 1.69 (5.09) | 0.12 |
| As treated analysis with measured 6‐month weights (completers), kg (SD) | |||
| No. | 41 | 42 | |
| Baseline | 109.8 (16.2) | 107.0 (18.0) | 0.47 |
| 6‐month follow‐up | 107.4 (15.0) | 104.2 (17.7) | 0.37 |
| Weight change | 2.32 (6.16) | 2.83 (4.88) | 0.68 |
Of 139 participants in the as‐treated analysis utilizing imputed weights, weight loss for the intervention group and control groups was 2.88 kg (SD=5.77) and 1.69 kg (SD=5.09). There was statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.006, P=0.004, respectively). However, there were neither statistically nor clinically significant differences between the 2 groups (1.19 kg, P=0.12). Finally, for the 83 completers in the as‐treated analysis with measured study weights only, weight loss for the intervention group and control group was 2.32 kg (SD=6.16) and 2.83 kg (SD=4.88), respectively. Though we again noted statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.02, P=0.0005, respectively), we found neither statistically nor clinically significant differences in weight loss between the 2 groups (0.51 kg, P=0.68). Figure 2 illustrates weight change over time for the intervention and control subjects who returned for in‐person follow‐up at 6 months.

For WHRs, we found no difference in WHR change between groups at 6 months (0.04 vs 0.04, P=0.59). However, among those who completed the study, there was a statistically significant decrease in WHR from baseline within both groups, decreasing 0.040.06 (P=0.006) in the intervention group and 0.040.04 (P<0.001) among controls.
Weight Perceptions
Only 34% of participants accurately perceived their weight and correctly identified themselves as either obese or morbidly obese. Nearly half of the study participants (47%) classified themselves as overweight rather than obese, though all met criteria for obesity. We found weight perception was most accurate among Caucasians (48%) and least accurate among African Americans (24%) and morbidly obese individuals (26%). Nearly all subjects felt weight loss was important (99%), and most assumed weight had contributed to their hospitalization (64%).
DISCUSSION
We hypothesized that intervention group subjects would lose more weight than those assigned to control given that they received weight loss interventions previously shown to be effective.[13, 25, 26, 27] However, intention‐to‐treat analysis showed no difference in weight loss between intervention and control subjects at 6 months. Interestingly, as‐treated analyses did suggest that subjects in both study arms lost a modest amount of weight over the duration of the study. Though modest weight reductions have been shown to give rise to health benefits, neither group met our prespecified goal for clinically significant weight loss (5% of baseline body weight).[18, 19] There were also no differences in WHRs noted between the intervention and control groups. The modest reductions in WHRs from baseline in both groups are of uncertain clinical significance but of interest given the well‐established graded relationship between WHR and risk of cardiovascular disease.[28, 29, 30, 31]
Though the control group subjects received no specific instruction regarding weight loss, we suspect that the influences of study enrollment, discussion of obesity while an inpatient, regular phone contacts, and weight tracking may have been sufficient to affect weight behaviors. Certainly, this exceeds usual care for hospitalized patients suffering from obesity. Though it is possible that all of obese patients lose weight over the 6‐month period following hospitalization, we feel this is unlikely. The exclusion of subjects with an elevated Charlson comorbidity index lessened the likelihood of weight loss due to chronic disease, and without intervention, obese individuals tend to gain rather than lose weight over time.[32] Nonetheless, the lack of significant weight loss between groups suggests that the specific weight loss instruction provided to the intervention group did not promote more weight loss than the general education and regular phone calls provided to controls.
Our findings related to weight perception were similar to those established in prior studies. Individuals frequently misperceive their weight and weight perceptions are least accurate among severely obese individuals and nonwhites.[16, 33, 34] Contrary to prior studies, we found that the majority of participants felt their weight negatively impacted their health, and most thought their hospitalization was weight‐related.[35] Interestingly, research suggests that weight‐related perception of health risk correlates with the likelihood of making a weight loss attempt, another factor that may have influenced the behavior of study participants.[35]
This study has several limitations. It was conducted and based on practices at a single institution, thus limiting generalizability. Additionally, the percentage of subjects who returned for 6‐month follow‐up was lower than desired at 50%. However, high attrition rates commonly plague obesity trials, and we are unaware of any existing studies documenting expected attrition rates among obese inpatients.[23, 36, 37, 38] To help address this, we used imputed weights in our as‐treated analysis to obtain follow‐up weight values on 79% of subjects. Further, the intentional exclusion of subjects in the precontemplation stage of change likely resulted in selection of a more motivated patient population. However, this was done assuming that most inpatient obesity interventions would primarily target patients interested in losing weight. Finally, the lack of a usual care group that more accurately reflects the experience of most hospitalized obese patientsno regular postdischarge interactionsdoes limit interpretation of the modest weight loss noted in both study groups.
In conclusion, an inpatient obesity intervention with post‐discharge follow‐up did not result in intervention subjects losing more weight than controls over a 6‐month period. However, the finding of modest weight loss among both groups is of interest and may warrant further investigation. It remains unclear whether this is a naturally occurring phenomenon or whether other factors influence behavior change in this patient population. Additional studies will be needed to clarify the impact of hospitalization, obesity recognition, perception of health risk, weight tracking, and follow‐up on weight behaviors. Given the proven benefits of even modest weight reductions, encouraging any amount of weight loss in these at‐risk individuals would appear to be a step in the right direction. We have yet to determine whether inpatient obesity interventions represent a lost opportunity.
Obesity‐related medical care remains a substantial driver in escalating healthcare costs. Not surprisingly, healthcare costs for obese patients are 40% higher annually than those for normal‐weight individuals.[1] In 2002, the morbidity attributable to obesity was calculated to equal, if not exceed, that associated with smoking.[2] Though inpatient outcomes appear similar for obese individuals, nearly all obesity‐related comorbidities can lead to hospitalization, and obesity has been linked to early mortality.[3, 4, 5] As obesity‐related costs continue to grow, so does the need to intervene in this at‐risk patient population.[3, 4, 5] Though significant efforts have focused on obesity interventions in the outpatient setting, a paucity of data exists on how best to address obesity during inpatient hospitalization.
Hospitalization itself has often been described as a teachable moment, a time during which a life event leads to increased receptivity to behavior change.[6, 7, 8] The positive effects of inpatient smoking cessation efforts are well recognized. Such initiatives typically include an inpatient counseling session, followed by supportive contact postdischarge.[9, 10] Features common to successful outpatient weight loss interventions include ongoing patient contact of variable duration, frequent self‐weighing, diet modifications, and increased activity.[11, 12, 13, 14, 15] To date, little is known about the effectiveness of such programs in the inpatient setting, though research has shown that obese inpatients are receptive to weight loss initiatives.[16] Accomplishing even modest weight reductions in such patients has the potential to lead to significant health and cost benefits.[1, 17, 18, 19]
In this study we sought to determine whether inpatient weight loss counseling with post discharge phone follow‐up would result in significant weight loss at 6 months when compared to controls. Secondary end points included weight change from baseline and changes in waist‐to‐hip ratios (WHRs). To our knowledge, this is the first randomized trial designed to evaluate the effect of an inpatient obesity intervention with postdischarge follow‐up in a general medicine population.
METHODS
Setting/Participants
We conducted a prospective, randomized controlled trial from January 2011 to May 2012 at a single, large (854‐bed), academic medical center in Chicago, Illinois. Eligible subjects were those with a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) between 30 and 50 kg/m2, ages 18 to 65 years old, admitted to an internal medicine service. Exclusion criteria included the presence of acute medical conditions known to affect weight, Charlson comorbidity index >3, moderate to severe major depression, prolonged steroid use (>2 weeks), initiation of medications known to affect weight (eg, diuretics), non‐English speaking, and precontemplation stage of change. Upon enrollment, subjects were randomly assigned to either the control or intervention group. A computer‐generated block randomization scheme was used to generate group assignments. Study research assistants sequentially assigned enrolled patients according to the computer‐generated randomization scheme. Group assignment was only revealed to each study participant after enrollment was complete. Figure 1 summarizes subject recruitment, randomization, and follow‐up. Informed written consent was obtained from all participants. Study participants, physicians, and investigators were unblinded. Study subjects were informed that they were participating in an obesity study as outlined on the study consent form. Study protocols and procedures were approved by the institutional review board at Northwestern University.

Interventions
After enrollment, all subjects had body weight measured on a calibrated study scale in light clothing or hospital gown without shoes. Waist circumference (narrowest circumference between the ribs and iliac crest) and hip circumference (maximum circumference of the hips) were measured to the nearest 0.1 cm. Measurements were taken in triplicate and averaged. WHR was calculated as waist circumference divided by hip circumference. All participants completed a demographic questionnaire and rated their level of agreement with 6 statements relating to weight perceptions and weight loss using a Likert scale from 1 (strongly disagree) to 10 (strongly agree).
Participants in the control group were not provided with any specific instructions regarding weight loss, diet, or exercise prior to discharge. Intervention group subjects were asked to view a 13‐minute weight loss education video (addressed specific caloric intake goals for weight loss, portion sizes), undergo a 25‐minute personalized counseling session with a certified health educator or study physician, and to set 3 specific lifestyle goals prior to discharge (weight loss, dietary, and fitness). A personal weight loss goal of 10% baseline body weight was set for intervention subjects based on obesity treatment guidelines suggesting subjects could safely lose 1 to 2 lb per week over the course of the study.[20] Clinically significant weight loss was defined as weight loss of 5% or more from baseline body weight based on literature illustrating health benefits with this amount of weight loss.[17, 18, 19]
All study subjects received a phone call schedule and weight‐tracking sheet prior to discharge, with calls scheduled at weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24. Phone calls for both groups were used to obtain weight and identify changes in medications or health condition and were conducted by a certified health educator or study physician. No problem solving, motivational support, or other specific instruction was provided to the control group, whereas phone calls for intervention subjects utilized motivational interviewing and problem‐solving techniques.
Study subjects were asked to return for an in‐person follow‐up visit at 6 months. Weight was reassessed with subjects in light clothing and without shoes on the same calibrated study scale by a certified health educator. Follow‐up WHRs were also collected.
Outcomes
The primary outcome of the study was the difference in mean weight change (change in kilograms from baseline) between control and intervention groups at 6 months. Secondary outcome measures included intragroup weight change from baseline and changes in WHR.
Measured weights were obtained for subjects who returned for 6‐month follow‐up. For those unable or unwilling to return at 6 months, measured weights were obtained from the electronic health record (EHR) and self‐reported weights requested for use in imputed weight calculations. Imputation weights for missing weight values were prioritized as follows: (1) in‐person 6‐month follow‐up weight used if available, (2) inpatient or outpatient EHR obtained weight used if in‐person weight unavailable, and (3) if neither an in‐person or EHR weight was available, a self‐reported weight was used.[21] For intention‐to‐treat analysis, baseline weight was carried forward for subjects lacking follow‐up data after enrollment, historically considered a conservative strategy in weight loss trials.[22, 23]
Statistical Analysis
Baseline patient characteristics were compared using 2 tests for categorical variables and 2‐sample t tests for continuous variables. The primary study outcome of weight change over time for each group was assessed for all study participants using an intention‐to‐treat analysis. Separate as‐treated analyses were also performed utilizing imputed weights for those who failed to follow‐up at 6 months and for study completers who had a measured study weight documented at 6 months.
Three analyzable datasets were computed: intention‐to‐treat (using all participants randomized to the study), as‐treated analysis with imputed weights, and as treated analysis with measured 6‐month study weights only. Intent‐to‐treat analysis provides the unbiased comparisons among the treatment groups. To avoid dilution of treatment effect, as‐treated analyses with imputed weights (including measured weights at 6‐month follow‐up obtained from other sources [eg, clinic visit]) and with measured study weights (completers only) were performed.
Weight change over time was analyzed with a longitudinal covariance pattern model, using an unstructured variance‐covariance matrix. Specifically, weight was modeled at all time points (baseline and weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24) using a priori contrasts and treating baseline as the reference cell to assess weight change, relative to baseline, at the 4 postbaseline time points.[24] Group effects on these a priori time contrasts were included to test for weight change differences between groups, and we specifically tested whether the group effect on weight change was equal or varied across the postbaseline time points.
We aimed to obtain a sample size of 176 subjects (88 in each group) in order to achieve 80% power to detect a 5‐kg weight loss in the intervention group after 6 months (at most standard deviation [SD]=15) and a 5‐kg difference in weight loss between groups (SD=10), assuming an of 0.05 using 2‐tailed testing and an attrition rate of 20%.
RESULTS
Over a period of 18 months we were able to recruit 176 subjects. We found no significant differences in baseline characteristics between groups (Table 1). Sixteen subjects developed exclusionary conditions after enrollment and were subsequently excluded from as‐treated data analyses. Follow‐up weight data for as‐treated analysis were available for 139 study subjects through the use of in‐person (n=83), EHR (n=41), and self‐reported (n=15) weights.
| Intervention, N=88 | Control, N=88 | |
|---|---|---|
| ||
| Age, y, mean (SD) | 48.9 (10.5) | 48.7 (10.3) |
| Female, % | 67.1 | 62.5 |
| Race/ethnicity, % | ||
| African American | 50.0 | 41.4 |
| Caucasian | 36.4 | 46.5 |
| Other | 13.6 | 11.6 |
| Education level, % | ||
| High school | 11.4 | 11.5 |
| College | 68.2 | 64.4 |
| Graduate level | 20.5 | 24.1 |
| Annual income, % | ||
| <$50,000 | 43.0 | 45.2 |
| $50,000$100,000 | 45.4 | 33.3 |
| >$100,000 | 11.6 | 21.4 |
| BMI, mean (SD), kg/m2 | 38.0 (5.1) | 37.5 (4.9) |
| BMI category, % | ||
| 3034.9 | 34.1 | 34.1 |
| 3539.9 | 28.4 | 37.5 |
| 40 | 37.5 | 28.4 |
| Waist‐hip ratio, mean (SD)a | 0.95 (0.08) | 0.96 (0.08) |
| Length of stay, d, median (interquartile range) | 2.0 (1.13.0) | 2.2 (1.33.3) |
| Diabetes, % | 27.3 | 25.0 |
| Admit diagnosis, % | ||
| Cardiovascular | 34.1 | 25.0 |
| Gastrointestinal | 15.9 | 18.2 |
| Pulmonary | 10.2 | 5.7 |
| Infectious | 11.4 | 13.6 |
| Endocrine | 3.4 | 2.3 |
| Other | 25.0 | 35.2 |
Change in Weight Loss and WHR
For the 176 participants included in the intent‐to‐treat analysis, mean weight loss for the intervention group and control groups was 1.08 kg (SD=4.33) and 1.35 kg (SD=3.64) at 6 months, respectively. We found no significant difference in weight loss between groups at 6 months (P=0.26), though there was statistically significant weight loss from baseline noted in both groups (P=0.02 and P=0.0008, respectively) (Table 2).
| Characteristic | Intervention Group | Control Group | P Valuea |
|---|---|---|---|
| |||
| Intent‐to‐treat analysis (all participants), kg (SD) | |||
| No. | 88 | 88 | |
| Baseline | 107.7 (16.7) | 105.1 (17.4) | 0.23 |
| 6‐month follow‐up | 106.6 (16.1) | 103.8 (17.1) | 0.16 |
| Weight change | 1.08 (4.33) | 1.35 (3.64) | 0.26 |
| As treated analysis with imputed weights, kg (SD) | |||
| No. | 69 | 70 | |
| Baseline | 108.9 (16.7) | 104.0 (16.2) | 0.08 |
| 6‐month follow‐up | 106.1 (17.2) | 102.4 (15.9) | 0.18 |
| Weight change | 2.88 (5.77) | 1.69 (5.09) | 0.12 |
| As treated analysis with measured 6‐month weights (completers), kg (SD) | |||
| No. | 41 | 42 | |
| Baseline | 109.8 (16.2) | 107.0 (18.0) | 0.47 |
| 6‐month follow‐up | 107.4 (15.0) | 104.2 (17.7) | 0.37 |
| Weight change | 2.32 (6.16) | 2.83 (4.88) | 0.68 |
Of 139 participants in the as‐treated analysis utilizing imputed weights, weight loss for the intervention group and control groups was 2.88 kg (SD=5.77) and 1.69 kg (SD=5.09). There was statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.006, P=0.004, respectively). However, there were neither statistically nor clinically significant differences between the 2 groups (1.19 kg, P=0.12). Finally, for the 83 completers in the as‐treated analysis with measured study weights only, weight loss for the intervention group and control group was 2.32 kg (SD=6.16) and 2.83 kg (SD=4.88), respectively. Though we again noted statistically significant weight loss at the 6‐month follow‐up from baseline in both groups (P=0.02, P=0.0005, respectively), we found neither statistically nor clinically significant differences in weight loss between the 2 groups (0.51 kg, P=0.68). Figure 2 illustrates weight change over time for the intervention and control subjects who returned for in‐person follow‐up at 6 months.

For WHRs, we found no difference in WHR change between groups at 6 months (0.04 vs 0.04, P=0.59). However, among those who completed the study, there was a statistically significant decrease in WHR from baseline within both groups, decreasing 0.040.06 (P=0.006) in the intervention group and 0.040.04 (P<0.001) among controls.
Weight Perceptions
Only 34% of participants accurately perceived their weight and correctly identified themselves as either obese or morbidly obese. Nearly half of the study participants (47%) classified themselves as overweight rather than obese, though all met criteria for obesity. We found weight perception was most accurate among Caucasians (48%) and least accurate among African Americans (24%) and morbidly obese individuals (26%). Nearly all subjects felt weight loss was important (99%), and most assumed weight had contributed to their hospitalization (64%).
DISCUSSION
We hypothesized that intervention group subjects would lose more weight than those assigned to control given that they received weight loss interventions previously shown to be effective.[13, 25, 26, 27] However, intention‐to‐treat analysis showed no difference in weight loss between intervention and control subjects at 6 months. Interestingly, as‐treated analyses did suggest that subjects in both study arms lost a modest amount of weight over the duration of the study. Though modest weight reductions have been shown to give rise to health benefits, neither group met our prespecified goal for clinically significant weight loss (5% of baseline body weight).[18, 19] There were also no differences in WHRs noted between the intervention and control groups. The modest reductions in WHRs from baseline in both groups are of uncertain clinical significance but of interest given the well‐established graded relationship between WHR and risk of cardiovascular disease.[28, 29, 30, 31]
Though the control group subjects received no specific instruction regarding weight loss, we suspect that the influences of study enrollment, discussion of obesity while an inpatient, regular phone contacts, and weight tracking may have been sufficient to affect weight behaviors. Certainly, this exceeds usual care for hospitalized patients suffering from obesity. Though it is possible that all of obese patients lose weight over the 6‐month period following hospitalization, we feel this is unlikely. The exclusion of subjects with an elevated Charlson comorbidity index lessened the likelihood of weight loss due to chronic disease, and without intervention, obese individuals tend to gain rather than lose weight over time.[32] Nonetheless, the lack of significant weight loss between groups suggests that the specific weight loss instruction provided to the intervention group did not promote more weight loss than the general education and regular phone calls provided to controls.
Our findings related to weight perception were similar to those established in prior studies. Individuals frequently misperceive their weight and weight perceptions are least accurate among severely obese individuals and nonwhites.[16, 33, 34] Contrary to prior studies, we found that the majority of participants felt their weight negatively impacted their health, and most thought their hospitalization was weight‐related.[35] Interestingly, research suggests that weight‐related perception of health risk correlates with the likelihood of making a weight loss attempt, another factor that may have influenced the behavior of study participants.[35]
This study has several limitations. It was conducted and based on practices at a single institution, thus limiting generalizability. Additionally, the percentage of subjects who returned for 6‐month follow‐up was lower than desired at 50%. However, high attrition rates commonly plague obesity trials, and we are unaware of any existing studies documenting expected attrition rates among obese inpatients.[23, 36, 37, 38] To help address this, we used imputed weights in our as‐treated analysis to obtain follow‐up weight values on 79% of subjects. Further, the intentional exclusion of subjects in the precontemplation stage of change likely resulted in selection of a more motivated patient population. However, this was done assuming that most inpatient obesity interventions would primarily target patients interested in losing weight. Finally, the lack of a usual care group that more accurately reflects the experience of most hospitalized obese patientsno regular postdischarge interactionsdoes limit interpretation of the modest weight loss noted in both study groups.
In conclusion, an inpatient obesity intervention with post‐discharge follow‐up did not result in intervention subjects losing more weight than controls over a 6‐month period. However, the finding of modest weight loss among both groups is of interest and may warrant further investigation. It remains unclear whether this is a naturally occurring phenomenon or whether other factors influence behavior change in this patient population. Additional studies will be needed to clarify the impact of hospitalization, obesity recognition, perception of health risk, weight tracking, and follow‐up on weight behaviors. Given the proven benefits of even modest weight reductions, encouraging any amount of weight loss in these at‐risk individuals would appear to be a step in the right direction. We have yet to determine whether inpatient obesity interventions represent a lost opportunity.
- , , , . The impact of weight loss among seniors on Medicare spending. Health Econ Rev. 2013;3(1):7.
- . The effects of obesity, smoking, and drinking on medical problems and costs. Health Aff (Millwood). 2002;21(2):245–253.
- , , , , , . The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88.
- , , , , , . The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103(10):1895–1901.
- , , , . Assessment of excess mortality in obesity. Am J Epidemiol. 1998;147(1):42–48.
- , , . Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure‐a review. Am J Cardiol. 2004;94(9):1155–1160.
- . In‐hospital initiation of statins: taking advantage of the “teachable moment”. Cleve Clin J Med. 2003;70(6):502, 504–506.
- , , . Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170.
- , , . Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008;168(18):1950–1960.
- , . Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial. CMAJ. 2009;180(13):1297–1303.
- , , , , , . Phone and e‐mail counselling are effective for weight management in an overweight working population: a randomized controlled trial. BMC Public Health. 2009;9:6.
- , . Weighing the evidence: benefits of regular weight monitoring for weight control. J Nutr Educ Behav. 2005;37(6):319–322.
- , , , et al. Comparative effectiveness of weight‐loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968.
- , , , et al. Weight‐loss outcomes: a systematic review and meta‐analysis of weight‐loss clinical trials with a minimum 1‐year follow‐up. J Am Diet Assoc. 2007;107(10):1755–1767.
- , , . Self‐monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102.
- , , , . Willingness for weight loss intervention among overweight and obese inpatients. South Med J. 2011;104(6):397–400.
- , , , et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486.
- , , . The beneficial effects of modest weight loss on cardiovascular risk factors. Int J Obes Relat Metab Disord. 1997;21(suppl 1):S5–S9.
- . Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16(6):397–415.
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(suppl 2):51S–209S.
- , , , et al. Validity of clinical body weight measures as substitutes for missing data in a randomized trial. Obes Res Clin Pract. 2008;2(4):277–281.
- , , . Advances in analysis of longitudinal data. Ann Rev Clin Psychol. 2010;6:79–107.
- , , , et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PloS One. 2009;4(8):e6624.
- , . Longitudinal Data Analysis. Hoboken, NJ: Wiley‐Interscience; 2006.
- , , , . Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150(4):255–262.
- . Clinical practice. Nonsurgical management of obesity in adults. N Engl J Med. 2008;358(18):1941–1950.
- , , . A meta‐analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord. 1997;21(10):941–947.
- , , , et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population‐based prospective study. Circulation. 2007;116(25):2933–2943.
- , , , et al. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50(8):752–759.
- , , , et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case‐control study. Lancet. 2005;366(9497):1640–1649.
- , , , . Waist circumference and waist‐to‐hip ratio as predictors of cardiovascular events: meta‐regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–856.
- , , , . Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855.
- , . Self‐perception of weight appropriateness in the United States. Am J Prev Med. 2003;24(4):332–339.
- , , , et al. Differences in weight perception among blacks and whites. J Womens Health (Larchmt). 2011;20(12):1805–1811.
- , , , , . Perceived health risk of excess body weight among overweight and obese men and women: differences by sex. Prev Med. 2008;47(1):46–52.
- , , . Obesity research—limitations of methods, measurements, and medications. JAMA. 2006;295(7):826–828.
- . Interpreting incomplete data in studies of diet and weight loss. N Engl J Med. 2003;348(21):2136–2137.
- , , , . Predictors of attrition in a large clinic‐based weight‐loss program. Obes Res. 2003;11(7):888–894.
- , , , . The impact of weight loss among seniors on Medicare spending. Health Econ Rev. 2013;3(1):7.
- . The effects of obesity, smoking, and drinking on medical problems and costs. Health Aff (Millwood). 2002;21(2):245–253.
- , , , , , . The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88.
- , , , , , . The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103(10):1895–1901.
- , , , . Assessment of excess mortality in obesity. Am J Epidemiol. 1998;147(1):42–48.
- , , . Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure‐a review. Am J Cardiol. 2004;94(9):1155–1160.
- . In‐hospital initiation of statins: taking advantage of the “teachable moment”. Cleve Clin J Med. 2003;70(6):502, 504–506.
- , , . Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170.
- , , . Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008;168(18):1950–1960.
- , . Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial. CMAJ. 2009;180(13):1297–1303.
- , , , , , . Phone and e‐mail counselling are effective for weight management in an overweight working population: a randomized controlled trial. BMC Public Health. 2009;9:6.
- , . Weighing the evidence: benefits of regular weight monitoring for weight control. J Nutr Educ Behav. 2005;37(6):319–322.
- , , , et al. Comparative effectiveness of weight‐loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968.
- , , , et al. Weight‐loss outcomes: a systematic review and meta‐analysis of weight‐loss clinical trials with a minimum 1‐year follow‐up. J Am Diet Assoc. 2007;107(10):1755–1767.
- , , . Self‐monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102.
- , , , . Willingness for weight loss intervention among overweight and obese inpatients. South Med J. 2011;104(6):397–400.
- , , , et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486.
- , , . The beneficial effects of modest weight loss on cardiovascular risk factors. Int J Obes Relat Metab Disord. 1997;21(suppl 1):S5–S9.
- . Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16(6):397–415.
- Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(suppl 2):51S–209S.
- , , , et al. Validity of clinical body weight measures as substitutes for missing data in a randomized trial. Obes Res Clin Pract. 2008;2(4):277–281.
- , , . Advances in analysis of longitudinal data. Ann Rev Clin Psychol. 2010;6:79–107.
- , , , et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PloS One. 2009;4(8):e6624.
- , . Longitudinal Data Analysis. Hoboken, NJ: Wiley‐Interscience; 2006.
- , , , . Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150(4):255–262.
- . Clinical practice. Nonsurgical management of obesity in adults. N Engl J Med. 2008;358(18):1941–1950.
- , , . A meta‐analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord. 1997;21(10):941–947.
- , , , et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population‐based prospective study. Circulation. 2007;116(25):2933–2943.
- , , , et al. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50(8):752–759.
- , , , et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case‐control study. Lancet. 2005;366(9497):1640–1649.
- , , , . Waist circumference and waist‐to‐hip ratio as predictors of cardiovascular events: meta‐regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–856.
- , , , . Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855.
- , . Self‐perception of weight appropriateness in the United States. Am J Prev Med. 2003;24(4):332–339.
- , , , et al. Differences in weight perception among blacks and whites. J Womens Health (Larchmt). 2011;20(12):1805–1811.
- , , , , . Perceived health risk of excess body weight among overweight and obese men and women: differences by sex. Prev Med. 2008;47(1):46–52.
- , , . Obesity research—limitations of methods, measurements, and medications. JAMA. 2006;295(7):826–828.
- . Interpreting incomplete data in studies of diet and weight loss. N Engl J Med. 2003;348(21):2136–2137.
- , , , . Predictors of attrition in a large clinic‐based weight‐loss program. Obes Res. 2003;11(7):888–894.
© 2014 Society of Hospital Medicine
Much to like on the stroke guidelines menu
The devastation of an acute stroke is something relatively few of us have experienced personally, but professionally we see it very regularly. An estimated 690,000-plus adults in the United States suffer an ischemic stroke annually, and an additional 240,000 experience a transient ischemic attack.
The good news is that the current estimated annual rate of future stroke in this patient population (3%-4%) is historically low, thanks to preventive measures, according to the new "Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals," which was published online in Stroke in May (Stroke 2014 May 1 [doi: 10.1161/STR.0000000000000024]). This updated guideline gives evidence-based recommendations on secondary stroke prevention as well as primary prevention in those who have suffered a transient ischemic attack (TIA).
This very extensive guide from the American Heart Association and the American Stroke Association addresses a wide variety of scenarios, ranging from general risk factor modification to specific circumstances, such as myocardial infarction and thrombus, cardiomyopathy, pregnancy, arterial dissection, and aortic arch atherosclerosis.
I welcome the recommendation to consider adding clopidogrel 75 mg/day to aspirin for 90 days in patients with a recent (within 30 days) stroke or TIA attributable to high-grade stenosis (70%-99%) of a major intracranial artery. I used to feel rather helpless to improve the long-term outcome in these patients, but now there seems to be something more we can do, other than just using statins and single antiplatelet therapy.
Other new recommendations stress nutrition. One item suggests performing a nutritional assessment for patients with a history of ischemic stroke or TIA. While many patients may never get around to seeing a nutritionist as an outpatient, no matter how often their primary care physician stresses the importance, when they are in the hospital we have a captive audience. So why not order a nutrition consult, along with the consult for physical, occupational, and speech therapy?
After having experienced an acute neurologic event, many patients and their families are highly motivated to make whatever changes are necessary to prevent a future, potentially catastrophic stroke. Reduction of sodium from 3.3 g/day to 2.5 g/day or less is reasonable, according to the guidelines, though lowering intake to less than 1.5 g/day will lower blood pressure even further. A nutritionist’s input into how to attain these levels without eating a diet that tastes like cardboard can be invaluable. The new guidelines also suggest counseling patients to follow a Mediterranean-type diet – emphasizing whole grains, fruits, vegetables, nuts, olive oil, legumes, fish, poultry, and even low-fat dairy products – instead of the traditional low fat diet.
These new recommendations are only the tip of the iceberg, and this document is highly worthwhile for all practicing clinicians.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
The devastation of an acute stroke is something relatively few of us have experienced personally, but professionally we see it very regularly. An estimated 690,000-plus adults in the United States suffer an ischemic stroke annually, and an additional 240,000 experience a transient ischemic attack.
The good news is that the current estimated annual rate of future stroke in this patient population (3%-4%) is historically low, thanks to preventive measures, according to the new "Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals," which was published online in Stroke in May (Stroke 2014 May 1 [doi: 10.1161/STR.0000000000000024]). This updated guideline gives evidence-based recommendations on secondary stroke prevention as well as primary prevention in those who have suffered a transient ischemic attack (TIA).
This very extensive guide from the American Heart Association and the American Stroke Association addresses a wide variety of scenarios, ranging from general risk factor modification to specific circumstances, such as myocardial infarction and thrombus, cardiomyopathy, pregnancy, arterial dissection, and aortic arch atherosclerosis.
I welcome the recommendation to consider adding clopidogrel 75 mg/day to aspirin for 90 days in patients with a recent (within 30 days) stroke or TIA attributable to high-grade stenosis (70%-99%) of a major intracranial artery. I used to feel rather helpless to improve the long-term outcome in these patients, but now there seems to be something more we can do, other than just using statins and single antiplatelet therapy.
Other new recommendations stress nutrition. One item suggests performing a nutritional assessment for patients with a history of ischemic stroke or TIA. While many patients may never get around to seeing a nutritionist as an outpatient, no matter how often their primary care physician stresses the importance, when they are in the hospital we have a captive audience. So why not order a nutrition consult, along with the consult for physical, occupational, and speech therapy?
After having experienced an acute neurologic event, many patients and their families are highly motivated to make whatever changes are necessary to prevent a future, potentially catastrophic stroke. Reduction of sodium from 3.3 g/day to 2.5 g/day or less is reasonable, according to the guidelines, though lowering intake to less than 1.5 g/day will lower blood pressure even further. A nutritionist’s input into how to attain these levels without eating a diet that tastes like cardboard can be invaluable. The new guidelines also suggest counseling patients to follow a Mediterranean-type diet – emphasizing whole grains, fruits, vegetables, nuts, olive oil, legumes, fish, poultry, and even low-fat dairy products – instead of the traditional low fat diet.
These new recommendations are only the tip of the iceberg, and this document is highly worthwhile for all practicing clinicians.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
The devastation of an acute stroke is something relatively few of us have experienced personally, but professionally we see it very regularly. An estimated 690,000-plus adults in the United States suffer an ischemic stroke annually, and an additional 240,000 experience a transient ischemic attack.
The good news is that the current estimated annual rate of future stroke in this patient population (3%-4%) is historically low, thanks to preventive measures, according to the new "Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals," which was published online in Stroke in May (Stroke 2014 May 1 [doi: 10.1161/STR.0000000000000024]). This updated guideline gives evidence-based recommendations on secondary stroke prevention as well as primary prevention in those who have suffered a transient ischemic attack (TIA).
This very extensive guide from the American Heart Association and the American Stroke Association addresses a wide variety of scenarios, ranging from general risk factor modification to specific circumstances, such as myocardial infarction and thrombus, cardiomyopathy, pregnancy, arterial dissection, and aortic arch atherosclerosis.
I welcome the recommendation to consider adding clopidogrel 75 mg/day to aspirin for 90 days in patients with a recent (within 30 days) stroke or TIA attributable to high-grade stenosis (70%-99%) of a major intracranial artery. I used to feel rather helpless to improve the long-term outcome in these patients, but now there seems to be something more we can do, other than just using statins and single antiplatelet therapy.
Other new recommendations stress nutrition. One item suggests performing a nutritional assessment for patients with a history of ischemic stroke or TIA. While many patients may never get around to seeing a nutritionist as an outpatient, no matter how often their primary care physician stresses the importance, when they are in the hospital we have a captive audience. So why not order a nutrition consult, along with the consult for physical, occupational, and speech therapy?
After having experienced an acute neurologic event, many patients and their families are highly motivated to make whatever changes are necessary to prevent a future, potentially catastrophic stroke. Reduction of sodium from 3.3 g/day to 2.5 g/day or less is reasonable, according to the guidelines, though lowering intake to less than 1.5 g/day will lower blood pressure even further. A nutritionist’s input into how to attain these levels without eating a diet that tastes like cardboard can be invaluable. The new guidelines also suggest counseling patients to follow a Mediterranean-type diet – emphasizing whole grains, fruits, vegetables, nuts, olive oil, legumes, fish, poultry, and even low-fat dairy products – instead of the traditional low fat diet.
These new recommendations are only the tip of the iceberg, and this document is highly worthwhile for all practicing clinicians.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
NIDA releases updated tools to help parents talk to teens about marijuana
The National Institute on Drug Abuse has released an updated set of resources to help parents "sort out marijuana myths from science based facts," according to a statement released May 20 by the National Institutes of Health.
Two updated booklets are being released in conjunction with the Substance Abuse and Mental Health Services Administration’s National Prevention Week 2014, which takes place May 18-24.
"Marijuana Facts for Teens" covers the topics of marijuana’s health consequences in teens, its effect on the developing brain, addiction risk, and updated information about its potential medical benefits. "Marijuana: Facts Parents Need to Know" contains updated guidelines for parents on how to tell if their child is using marijuana and how to discuss the topic with their children.
Both publications have been updated to include new sections on the dangers of synthetic marijuana, the effect of marijuana use on teens’ IQ, and potential therapeutic uses of the drug.
According to the 2013 Monitoring the Future survey, 45.5% of teens will have tried marijuana at least once by the time they graduate high school, and more than 6% of high school seniors report smoking daily.
For more information, visit http://teens.drugabuse.gov/.
The National Institute on Drug Abuse has released an updated set of resources to help parents "sort out marijuana myths from science based facts," according to a statement released May 20 by the National Institutes of Health.
Two updated booklets are being released in conjunction with the Substance Abuse and Mental Health Services Administration’s National Prevention Week 2014, which takes place May 18-24.
"Marijuana Facts for Teens" covers the topics of marijuana’s health consequences in teens, its effect on the developing brain, addiction risk, and updated information about its potential medical benefits. "Marijuana: Facts Parents Need to Know" contains updated guidelines for parents on how to tell if their child is using marijuana and how to discuss the topic with their children.
Both publications have been updated to include new sections on the dangers of synthetic marijuana, the effect of marijuana use on teens’ IQ, and potential therapeutic uses of the drug.
According to the 2013 Monitoring the Future survey, 45.5% of teens will have tried marijuana at least once by the time they graduate high school, and more than 6% of high school seniors report smoking daily.
For more information, visit http://teens.drugabuse.gov/.
The National Institute on Drug Abuse has released an updated set of resources to help parents "sort out marijuana myths from science based facts," according to a statement released May 20 by the National Institutes of Health.
Two updated booklets are being released in conjunction with the Substance Abuse and Mental Health Services Administration’s National Prevention Week 2014, which takes place May 18-24.
"Marijuana Facts for Teens" covers the topics of marijuana’s health consequences in teens, its effect on the developing brain, addiction risk, and updated information about its potential medical benefits. "Marijuana: Facts Parents Need to Know" contains updated guidelines for parents on how to tell if their child is using marijuana and how to discuss the topic with their children.
Both publications have been updated to include new sections on the dangers of synthetic marijuana, the effect of marijuana use on teens’ IQ, and potential therapeutic uses of the drug.
According to the 2013 Monitoring the Future survey, 45.5% of teens will have tried marijuana at least once by the time they graduate high school, and more than 6% of high school seniors report smoking daily.
For more information, visit http://teens.drugabuse.gov/.
What Matters: Anxiolytics, hypnotics and eternal sleep
Data from the Centers for Disease Control and Prevention’s National Ambulatory Medical Center Survey reveal benzodiazepine prescriptions grew by 12.5% per year between 2002 and 2009. Data from the National Health and Nutrition Examination Survey suggest that prescriptions for sleep aids (sedatives and hypnotics) tripled between 1998 and 2006. Four percent of U.S. adults age 20 years or older and 7% of adults age 80 years or older report using a prescription sleep aid in the past month.
Aside from the addictive potential and their limited long-term effectiveness, they may be associated with an increased risk of death.
Dr. Scott Weich and his colleagues at University of Warwick, Coventry, England, analyzed data from a retrospective matched cohort study involving 34,727 patients aged at least 16 years who received prescriptions for anxiolytics or hypnotics and 69,418 patients who did not (BMJ 2014;348:g1996). To reduce the likelihood that patients received a prescription that they did not fill, only patients receiving at least two prescriptions were included. The average follow-up period was 7.6 years. The most commonly prescribed drugs were diazepam (48%), temazepam (35%), zopiclone (34%), and zolpidem (8%).
Significantly higher ratios for mortality were observed with the use of these drugs. Adjusting for potential confounders, the hazard ratio for mortality during the whole follow-up period was significantly elevated for the group receiving any sedative or hypnotic in the first year of recruitment (hazard ratio, 3.32; 95% confidence interval: 3.19-3.45).
Dose responses were observed for study drugs. For example, the HR for patients receiving more than 90 doses during the first year was 4.51 (95% CI: 4.22-4.82). Patients who did not receive study drugs beyond 1 year were less likely to die than those who continued to take them. The authors point out that these data translate into four excess deaths linked to use of these drugs per 100 people over 7.6 years after the initial prescription.
The biggest challenge will be to figure out how best to incorporate this information into our counseling of patients without sounding like we are "fear-mongering." Fear-mongering doesn’t work – it just makes our patients more anxious, when what we really need to do is calm them down.
Cognitive-behavioral therapy works for insomnia, but patients report not having the time. I always start the discussion by telling patients to read the book "No More Sleepless Nights" and to start a sleep log. Amazing what we can learn from this. This as least gets the ball rolling.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. Dr. Ebbert does not receive royalties from the sale of "No More Sleepless Nights."
Data from the Centers for Disease Control and Prevention’s National Ambulatory Medical Center Survey reveal benzodiazepine prescriptions grew by 12.5% per year between 2002 and 2009. Data from the National Health and Nutrition Examination Survey suggest that prescriptions for sleep aids (sedatives and hypnotics) tripled between 1998 and 2006. Four percent of U.S. adults age 20 years or older and 7% of adults age 80 years or older report using a prescription sleep aid in the past month.
Aside from the addictive potential and their limited long-term effectiveness, they may be associated with an increased risk of death.
Dr. Scott Weich and his colleagues at University of Warwick, Coventry, England, analyzed data from a retrospective matched cohort study involving 34,727 patients aged at least 16 years who received prescriptions for anxiolytics or hypnotics and 69,418 patients who did not (BMJ 2014;348:g1996). To reduce the likelihood that patients received a prescription that they did not fill, only patients receiving at least two prescriptions were included. The average follow-up period was 7.6 years. The most commonly prescribed drugs were diazepam (48%), temazepam (35%), zopiclone (34%), and zolpidem (8%).
Significantly higher ratios for mortality were observed with the use of these drugs. Adjusting for potential confounders, the hazard ratio for mortality during the whole follow-up period was significantly elevated for the group receiving any sedative or hypnotic in the first year of recruitment (hazard ratio, 3.32; 95% confidence interval: 3.19-3.45).
Dose responses were observed for study drugs. For example, the HR for patients receiving more than 90 doses during the first year was 4.51 (95% CI: 4.22-4.82). Patients who did not receive study drugs beyond 1 year were less likely to die than those who continued to take them. The authors point out that these data translate into four excess deaths linked to use of these drugs per 100 people over 7.6 years after the initial prescription.
The biggest challenge will be to figure out how best to incorporate this information into our counseling of patients without sounding like we are "fear-mongering." Fear-mongering doesn’t work – it just makes our patients more anxious, when what we really need to do is calm them down.
Cognitive-behavioral therapy works for insomnia, but patients report not having the time. I always start the discussion by telling patients to read the book "No More Sleepless Nights" and to start a sleep log. Amazing what we can learn from this. This as least gets the ball rolling.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. Dr. Ebbert does not receive royalties from the sale of "No More Sleepless Nights."
Data from the Centers for Disease Control and Prevention’s National Ambulatory Medical Center Survey reveal benzodiazepine prescriptions grew by 12.5% per year between 2002 and 2009. Data from the National Health and Nutrition Examination Survey suggest that prescriptions for sleep aids (sedatives and hypnotics) tripled between 1998 and 2006. Four percent of U.S. adults age 20 years or older and 7% of adults age 80 years or older report using a prescription sleep aid in the past month.
Aside from the addictive potential and their limited long-term effectiveness, they may be associated with an increased risk of death.
Dr. Scott Weich and his colleagues at University of Warwick, Coventry, England, analyzed data from a retrospective matched cohort study involving 34,727 patients aged at least 16 years who received prescriptions for anxiolytics or hypnotics and 69,418 patients who did not (BMJ 2014;348:g1996). To reduce the likelihood that patients received a prescription that they did not fill, only patients receiving at least two prescriptions were included. The average follow-up period was 7.6 years. The most commonly prescribed drugs were diazepam (48%), temazepam (35%), zopiclone (34%), and zolpidem (8%).
Significantly higher ratios for mortality were observed with the use of these drugs. Adjusting for potential confounders, the hazard ratio for mortality during the whole follow-up period was significantly elevated for the group receiving any sedative or hypnotic in the first year of recruitment (hazard ratio, 3.32; 95% confidence interval: 3.19-3.45).
Dose responses were observed for study drugs. For example, the HR for patients receiving more than 90 doses during the first year was 4.51 (95% CI: 4.22-4.82). Patients who did not receive study drugs beyond 1 year were less likely to die than those who continued to take them. The authors point out that these data translate into four excess deaths linked to use of these drugs per 100 people over 7.6 years after the initial prescription.
The biggest challenge will be to figure out how best to incorporate this information into our counseling of patients without sounding like we are "fear-mongering." Fear-mongering doesn’t work – it just makes our patients more anxious, when what we really need to do is calm them down.
Cognitive-behavioral therapy works for insomnia, but patients report not having the time. I always start the discussion by telling patients to read the book "No More Sleepless Nights" and to start a sleep log. Amazing what we can learn from this. This as least gets the ball rolling.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. Dr. Ebbert does not receive royalties from the sale of "No More Sleepless Nights."