User login
Computer Navigation Systems in Unicompartmental Knee Arthroplasty: A Systematic Review
Could ‘Rx: Pet therapy’ come back to bite you?
Dear Dr. Mossman,
My patient, Ms. A, asked me to write a letter to her landlord (who has a “no pets” policy) stating that she needed to keep her dog in her apartment for “therapeutic” purposes—to provide comfort and reduce her posttraumatic stress (PTSD) and anxiety. I hesitated. Could my written statement make me liable if her dog bit someone?
Submitted by “Dr. B”
Studies showing that animals can help outpatients manage psychiatric conditions have received a lot of publicity lately. As a result, more patients are asking physicians to provide documentation to support having pets in their apartments or letting their pets accompany them on planes and buses and at restaurants and shopping malls.
But sometimes, animals hurt people. The Centers for Disease Control and Prevention reports that dogs bite 4.5 million Americans each year and that one-fifth of dog bites cause injury that requires medical attention; in 2012, more than 27,000 dog-bite victims needed reconstructive surgery.1 If Dr. B writes a letter to support letting Ms. A keep a dog in her apartment, how likely is Dr. B to incur professional liability?
To answer this question, let’s examine:
• the history and background of “pet therapy”
• types of assistance animals
• potential liability for owners, landlords, and clinicians.
History and background
Using animals to improve hospitalized patients’ mental well-being dates back to the 18th century.2 In the late 1980s, medical publications began to document systematically how service dogs whose primary role was to help physically disabled individuals to navigate independently also provided social and emotional benefits.3-7 Since the 1990s, accessibility mandates in Title III of the Americans with Disabilities Act (ADA) (Table 18) have led to the gradual acceptance of service animals in public places where their presence was previously frowned upon or prohibited.9,10
If service dogs help people with physical problems feel better, it only makes sense that dogs and other animals might lessen emotional ailments, too.11-13 Florence Nightingale and Sigmund Freud both recognized that involving pets in treatment reduced patients’ depression and anxiety,14 but credit for formally introducing animals into therapy usually goes to psychologist Boris Levinson, whose 1969 book described how his dog Jingles helped troubled children communicate.15 Over the past decade, using animals— trained and untrained—for psychological assistance has become an increasingly popular therapeutic maneuver for diverse mental disorders, including autism, anxiety, schizophrenia, and PTSD.16-19
Terminology
Because animals can provide many types of assistance and support, a variety of terms are used to refer to them: service animals, companion animals, therapy pets, and so on. In certain situations (including the one described by Dr. B), carefully delineating animals’ roles and functions can reduce confusion and misinterpretation by patients, health care professionals, policy makers, and regulators.
Parenti et al20 have proposed a “taxonomy” for assistance dogs based on variables that include:
• performing task related to a disability
• the skill level required of the dog
• who uses the dog
• applicable training standards
• legal protections for the dog and its handler.
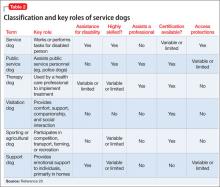
Table 220 summarizes this classification system and key variables that differentiate types of assistance dogs.
Certification
Health care facilities often require that visiting dogs have some form of “certification” that they are well behaved, and the ADA and many state statutes require that service dogs and some other animals be “certified” to perform their roles. Yet no federal or state statutes lay out explicit training standards or requirements for certification. Therapy Dogs International21 and Pet Partners22 are 2 organizations that provide certifications accepted by many agencies and organizations.
Assistance Dogs International, an assistance animal advocacy group, has proposed “minimum standards” for training and deployment of service dogs. These include responding to basic obedience commands from the client in public and at home, being able to perform at least 3 tasks to mitigate the client’s disability, teaching the client about dog training and health care, and scheduled follow-ups for skill maintenance. Dogs also should be spayed or neutered, properly vaccinated, nonaggressive, clean, and continent in public places.23
Liability laws
Most U.S. jurisdictions make owners liable for animal-caused injuries, including injuries caused by service dogs.24 In many states (eg, Minnesota25), an owner can be liable for dog-bite injury even if the owner did nothing wrong and had no reason to suspect from prior behavior that the dog might bite someone. Other jurisdictions require evidence of owner negligence, or they allow liability only when bites occur off the owner’s premises26 or if the owner let the dog run loose.27 Many homeowners’ insurance policies include liability coverage for dog bites, and a few companies offer a special canine liability policy.
Landlords often try to bar tenants from having a dog, partly to avoid liability for dog bites. Most states have case law stating that, if a tenant’s apparently friendly dog bites someone, the landlord is not liable for the injury28,29; landlords can be liable only if they know about a dangerous dog and do nothing about it.30 In a recent decision, however, the Kentucky Supreme Court made landlords statutory owners with potential liability for dog bites if they give tenants permission to have dogs “on or about” the rental premises.31
Clinicians and liability
Asking tenants to provide documentation about their need for therapeutic pets has become standard operating procedure for landlords in many states, so Ms. A’s request to Dr. B sounds reasonable. But could Dr. B’s written statement lead to liability if Ms. A’s dog bit and injured someone else?
The best answer is, “It’s conceivable, but really unlikely.” Donna Vanderpool, MBA, JD, an author and attorney who develops and implements risk management services for psychiatrists, has not seen any claims or case reports on litigation blaming mental health clinicians for injury caused by emotional support pets after the clinicians had written a letter for housing purposes (oral and written communications, April 7-13, 2014).
Dr. B might wonder whether writing a letter for Ms. A would imply that he had evaluated the dog and Ms. A’s ability to control it. Psychiatrists don’t usually discuss—let alone evaluate—the temperament or behavior of their patients’ pets; even if they did they aren’t experts on pet training. Recognizing this, Dr. B’s letter could include a statement to the effect that he was not vouching for the dog’s behavior, but only for how the dog would help Ms. A.
Dr. B also might talk with Ms. A about her need for the dog and whether she had obtained appropriate certification, as discussed above. The ADA provisions pertaining to use and presence of service animals do not apply to dogs that are merely patients’ pets, notwithstanding the genuine emotional benefits that a dog’s companionship might provide. Stating that a patient needs an animal to treat an illness might be fraud if the doctor knew the pet was just a buddy.
Bottom Line
Psychiatrists can expect that more and more patients will ask them for letters to support having pets accompany them at home or in public. Although liability seems unlikely, cautious psychiatrists can state in such letters that they have not evaluated the animal in question, only the potential benefits that the patient might derive from it.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing articles.
1. Dog Bites. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/homeandrecreationalsafety/ dog-bites/index.html. Updated October 25, 2013. Accessed April 22, 2014.
2. Serpell JA. Animal-assisted interventions in historical perspective. In Fine AH, ed. Handbook on animal-assisted therapy: theoretical foundations and guidelines for practice. 3rd ed. Burlington, MA: Academic Press; 2010:17-32.
3. Eddy J, Hart LA, Boltz RP. The effects of service dogs on social acknowledgments of people in wheelchairs. J Psychol. 1988;122(1):39-45.
4. Mader B, Hart LA, Bergin B. Social acknowledgments for children with disabilities: effects of service dogs. Child Dev. 1989;60(6):1529-1534.
5. Allen K, Blascovich J. The value of service dogs for people with severe ambulatory disabilities. A randomized controlled trial. JAMA. 1996;275(13):1001-1006.
6. Camp MM. The use of service dogs as an adaptive strategy: a qualitative study. Am J Occup Ther. 2001;55(5):509-517.
7. Allen K, Shykoff BE, Izzo JL Jr. Pet ownership, but not ace inhibitor therapy, blunts home blood pressure responses to mental stress. Hypertension. 2001;38(4):815-820.
8. ADA requirements: service animals. United States Department of Justice Civil Rights Division, Disability Rights Section Web site. http://www.ada.gov/service_ animals_2010.htm. Published September 15, 2010. Accessed April 22, 2014.
9. Eames E, Eames T. Interpreting legal mandates. Assistance dogs in medical facilities. Nurs Manage. 1997;28(6):49-51.
10. Houghtalen RP, Doody J. After the ADA: service dogs on inpatient psychiatric units. Bull Am Acad Psychiatry Law. 1995;23(2):211-217.
11. Wenthold N, Savage TA. Ethical issues with service animals. Top Stroke Rehabil. 2007;14(2):68-74.
12. DiSalvo H, Haiduven D, Johnson N, et al. Who let the dogs out? Infection control did: utility of dogs in health care settings and infection control aspects. Am J Infect Control. 2006;34:301-307.
13. Collins DM, Fitzgerald SG, Sachs-Ericsson N, et al. Psychosocial well-being and community participation of service dog partners. Disabil Rehabil Assist Technol. 2006;1(1-2):41-48.
14. Coren S. Foreward. In: Fine AH, ed. Handbook on animal-assisted therapy: theoretical foundations and guidelines for practice. 3rd ed. Burlington, MA: Academic Press; 2010: xv-xviii.
15. Levinson BM, Mallon GP. Pet-oriented child psychotherapy. 2nd ed. Springfield IL: Charles C Thomas Publisher, Ltd; 1997.
16. Esnayra J. Help from man’s best friend. Psychiatric service dogs are helping consumers deal with the symptoms of mental illness. Behav Healthc. 2007;27(7):30-32.
17. Barak Y, Savorai O, Mavashev S, et al. Animal-assisted therapy for elderly schizophrenic patients: a one year controlled trial. Am J Geriatr Psychiatry. 2001;9(4):439-442.
18. Burrows KE, Adams CL, Millman ST. Factors affecting behavior and welfare of service dogs for children with autism spectrum disorder. J Appl Anim Welf Sci. 2008;11(1):42-62.
19. Yount RA, Olmert MD, Lee MR. Service dog training program for treatment of posttraumatic stress in service members. US Army Med Dep J. 2012:63-69.
20. Parenti L, Foreman A, Meade BJ, et al. A revised taxonomy of assistance animals. J Rehabil Res Dev. 2013;50(6):745-756.
21. Testing Requirements. Therapy Dogs International. http:// www.tdi-dog.org/images/TestingBrochure.pdf. Accessed April 22, 2014.
22. How to become a registered therapy animal team. Pet Partners. http://www.petpartners.org/TAPinfo. Accessed April 22, 2014.
23. ADI Guide to Assistance Dog Laws. Assistance Dogs International. http://www.assistancedogsinternational. org/access-and-laws/adi-guide-to-assistance-dog-laws. Accessed April 22, 2014.
24. Id Stat §56-704.
25. Seim v Garavalia, 306 NW2d 806 (Minn 1981).
26. ME Rev Stat title 7, §3961.
27. Chadbourne v Kappaz, 2001 779 A2d 293 (DC App).
28. Stokes v Lyddy, 2002 75 (Conn App 252).
29. Georgianna v Gizzy, 483 NYS2d 892 (NY 1984).
30. Linebaugh v Hyndman, 516 A2d 638 (NJ 1986).
31. Benningfield v Zinsmeister, 367 SW3d 561 (Ky 2012).
Dear Dr. Mossman,
My patient, Ms. A, asked me to write a letter to her landlord (who has a “no pets” policy) stating that she needed to keep her dog in her apartment for “therapeutic” purposes—to provide comfort and reduce her posttraumatic stress (PTSD) and anxiety. I hesitated. Could my written statement make me liable if her dog bit someone?
Submitted by “Dr. B”
Studies showing that animals can help outpatients manage psychiatric conditions have received a lot of publicity lately. As a result, more patients are asking physicians to provide documentation to support having pets in their apartments or letting their pets accompany them on planes and buses and at restaurants and shopping malls.
But sometimes, animals hurt people. The Centers for Disease Control and Prevention reports that dogs bite 4.5 million Americans each year and that one-fifth of dog bites cause injury that requires medical attention; in 2012, more than 27,000 dog-bite victims needed reconstructive surgery.1 If Dr. B writes a letter to support letting Ms. A keep a dog in her apartment, how likely is Dr. B to incur professional liability?
To answer this question, let’s examine:
• the history and background of “pet therapy”
• types of assistance animals
• potential liability for owners, landlords, and clinicians.
History and background
Using animals to improve hospitalized patients’ mental well-being dates back to the 18th century.2 In the late 1980s, medical publications began to document systematically how service dogs whose primary role was to help physically disabled individuals to navigate independently also provided social and emotional benefits.3-7 Since the 1990s, accessibility mandates in Title III of the Americans with Disabilities Act (ADA) (Table 18) have led to the gradual acceptance of service animals in public places where their presence was previously frowned upon or prohibited.9,10
If service dogs help people with physical problems feel better, it only makes sense that dogs and other animals might lessen emotional ailments, too.11-13 Florence Nightingale and Sigmund Freud both recognized that involving pets in treatment reduced patients’ depression and anxiety,14 but credit for formally introducing animals into therapy usually goes to psychologist Boris Levinson, whose 1969 book described how his dog Jingles helped troubled children communicate.15 Over the past decade, using animals— trained and untrained—for psychological assistance has become an increasingly popular therapeutic maneuver for diverse mental disorders, including autism, anxiety, schizophrenia, and PTSD.16-19
Terminology
Because animals can provide many types of assistance and support, a variety of terms are used to refer to them: service animals, companion animals, therapy pets, and so on. In certain situations (including the one described by Dr. B), carefully delineating animals’ roles and functions can reduce confusion and misinterpretation by patients, health care professionals, policy makers, and regulators.
Parenti et al20 have proposed a “taxonomy” for assistance dogs based on variables that include:
• performing task related to a disability
• the skill level required of the dog
• who uses the dog
• applicable training standards
• legal protections for the dog and its handler.
Table 220 summarizes this classification system and key variables that differentiate types of assistance dogs.
Certification
Health care facilities often require that visiting dogs have some form of “certification” that they are well behaved, and the ADA and many state statutes require that service dogs and some other animals be “certified” to perform their roles. Yet no federal or state statutes lay out explicit training standards or requirements for certification. Therapy Dogs International21 and Pet Partners22 are 2 organizations that provide certifications accepted by many agencies and organizations.
Assistance Dogs International, an assistance animal advocacy group, has proposed “minimum standards” for training and deployment of service dogs. These include responding to basic obedience commands from the client in public and at home, being able to perform at least 3 tasks to mitigate the client’s disability, teaching the client about dog training and health care, and scheduled follow-ups for skill maintenance. Dogs also should be spayed or neutered, properly vaccinated, nonaggressive, clean, and continent in public places.23
Liability laws
Most U.S. jurisdictions make owners liable for animal-caused injuries, including injuries caused by service dogs.24 In many states (eg, Minnesota25), an owner can be liable for dog-bite injury even if the owner did nothing wrong and had no reason to suspect from prior behavior that the dog might bite someone. Other jurisdictions require evidence of owner negligence, or they allow liability only when bites occur off the owner’s premises26 or if the owner let the dog run loose.27 Many homeowners’ insurance policies include liability coverage for dog bites, and a few companies offer a special canine liability policy.
Landlords often try to bar tenants from having a dog, partly to avoid liability for dog bites. Most states have case law stating that, if a tenant’s apparently friendly dog bites someone, the landlord is not liable for the injury28,29; landlords can be liable only if they know about a dangerous dog and do nothing about it.30 In a recent decision, however, the Kentucky Supreme Court made landlords statutory owners with potential liability for dog bites if they give tenants permission to have dogs “on or about” the rental premises.31
Clinicians and liability
Asking tenants to provide documentation about their need for therapeutic pets has become standard operating procedure for landlords in many states, so Ms. A’s request to Dr. B sounds reasonable. But could Dr. B’s written statement lead to liability if Ms. A’s dog bit and injured someone else?
The best answer is, “It’s conceivable, but really unlikely.” Donna Vanderpool, MBA, JD, an author and attorney who develops and implements risk management services for psychiatrists, has not seen any claims or case reports on litigation blaming mental health clinicians for injury caused by emotional support pets after the clinicians had written a letter for housing purposes (oral and written communications, April 7-13, 2014).
Dr. B might wonder whether writing a letter for Ms. A would imply that he had evaluated the dog and Ms. A’s ability to control it. Psychiatrists don’t usually discuss—let alone evaluate—the temperament or behavior of their patients’ pets; even if they did they aren’t experts on pet training. Recognizing this, Dr. B’s letter could include a statement to the effect that he was not vouching for the dog’s behavior, but only for how the dog would help Ms. A.
Dr. B also might talk with Ms. A about her need for the dog and whether she had obtained appropriate certification, as discussed above. The ADA provisions pertaining to use and presence of service animals do not apply to dogs that are merely patients’ pets, notwithstanding the genuine emotional benefits that a dog’s companionship might provide. Stating that a patient needs an animal to treat an illness might be fraud if the doctor knew the pet was just a buddy.
Bottom Line
Psychiatrists can expect that more and more patients will ask them for letters to support having pets accompany them at home or in public. Although liability seems unlikely, cautious psychiatrists can state in such letters that they have not evaluated the animal in question, only the potential benefits that the patient might derive from it.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing articles.
Dear Dr. Mossman,
My patient, Ms. A, asked me to write a letter to her landlord (who has a “no pets” policy) stating that she needed to keep her dog in her apartment for “therapeutic” purposes—to provide comfort and reduce her posttraumatic stress (PTSD) and anxiety. I hesitated. Could my written statement make me liable if her dog bit someone?
Submitted by “Dr. B”
Studies showing that animals can help outpatients manage psychiatric conditions have received a lot of publicity lately. As a result, more patients are asking physicians to provide documentation to support having pets in their apartments or letting their pets accompany them on planes and buses and at restaurants and shopping malls.
But sometimes, animals hurt people. The Centers for Disease Control and Prevention reports that dogs bite 4.5 million Americans each year and that one-fifth of dog bites cause injury that requires medical attention; in 2012, more than 27,000 dog-bite victims needed reconstructive surgery.1 If Dr. B writes a letter to support letting Ms. A keep a dog in her apartment, how likely is Dr. B to incur professional liability?
To answer this question, let’s examine:
• the history and background of “pet therapy”
• types of assistance animals
• potential liability for owners, landlords, and clinicians.
History and background
Using animals to improve hospitalized patients’ mental well-being dates back to the 18th century.2 In the late 1980s, medical publications began to document systematically how service dogs whose primary role was to help physically disabled individuals to navigate independently also provided social and emotional benefits.3-7 Since the 1990s, accessibility mandates in Title III of the Americans with Disabilities Act (ADA) (Table 18) have led to the gradual acceptance of service animals in public places where their presence was previously frowned upon or prohibited.9,10
If service dogs help people with physical problems feel better, it only makes sense that dogs and other animals might lessen emotional ailments, too.11-13 Florence Nightingale and Sigmund Freud both recognized that involving pets in treatment reduced patients’ depression and anxiety,14 but credit for formally introducing animals into therapy usually goes to psychologist Boris Levinson, whose 1969 book described how his dog Jingles helped troubled children communicate.15 Over the past decade, using animals— trained and untrained—for psychological assistance has become an increasingly popular therapeutic maneuver for diverse mental disorders, including autism, anxiety, schizophrenia, and PTSD.16-19
Terminology
Because animals can provide many types of assistance and support, a variety of terms are used to refer to them: service animals, companion animals, therapy pets, and so on. In certain situations (including the one described by Dr. B), carefully delineating animals’ roles and functions can reduce confusion and misinterpretation by patients, health care professionals, policy makers, and regulators.
Parenti et al20 have proposed a “taxonomy” for assistance dogs based on variables that include:
• performing task related to a disability
• the skill level required of the dog
• who uses the dog
• applicable training standards
• legal protections for the dog and its handler.
Table 220 summarizes this classification system and key variables that differentiate types of assistance dogs.
Certification
Health care facilities often require that visiting dogs have some form of “certification” that they are well behaved, and the ADA and many state statutes require that service dogs and some other animals be “certified” to perform their roles. Yet no federal or state statutes lay out explicit training standards or requirements for certification. Therapy Dogs International21 and Pet Partners22 are 2 organizations that provide certifications accepted by many agencies and organizations.
Assistance Dogs International, an assistance animal advocacy group, has proposed “minimum standards” for training and deployment of service dogs. These include responding to basic obedience commands from the client in public and at home, being able to perform at least 3 tasks to mitigate the client’s disability, teaching the client about dog training and health care, and scheduled follow-ups for skill maintenance. Dogs also should be spayed or neutered, properly vaccinated, nonaggressive, clean, and continent in public places.23
Liability laws
Most U.S. jurisdictions make owners liable for animal-caused injuries, including injuries caused by service dogs.24 In many states (eg, Minnesota25), an owner can be liable for dog-bite injury even if the owner did nothing wrong and had no reason to suspect from prior behavior that the dog might bite someone. Other jurisdictions require evidence of owner negligence, or they allow liability only when bites occur off the owner’s premises26 or if the owner let the dog run loose.27 Many homeowners’ insurance policies include liability coverage for dog bites, and a few companies offer a special canine liability policy.
Landlords often try to bar tenants from having a dog, partly to avoid liability for dog bites. Most states have case law stating that, if a tenant’s apparently friendly dog bites someone, the landlord is not liable for the injury28,29; landlords can be liable only if they know about a dangerous dog and do nothing about it.30 In a recent decision, however, the Kentucky Supreme Court made landlords statutory owners with potential liability for dog bites if they give tenants permission to have dogs “on or about” the rental premises.31
Clinicians and liability
Asking tenants to provide documentation about their need for therapeutic pets has become standard operating procedure for landlords in many states, so Ms. A’s request to Dr. B sounds reasonable. But could Dr. B’s written statement lead to liability if Ms. A’s dog bit and injured someone else?
The best answer is, “It’s conceivable, but really unlikely.” Donna Vanderpool, MBA, JD, an author and attorney who develops and implements risk management services for psychiatrists, has not seen any claims or case reports on litigation blaming mental health clinicians for injury caused by emotional support pets after the clinicians had written a letter for housing purposes (oral and written communications, April 7-13, 2014).
Dr. B might wonder whether writing a letter for Ms. A would imply that he had evaluated the dog and Ms. A’s ability to control it. Psychiatrists don’t usually discuss—let alone evaluate—the temperament or behavior of their patients’ pets; even if they did they aren’t experts on pet training. Recognizing this, Dr. B’s letter could include a statement to the effect that he was not vouching for the dog’s behavior, but only for how the dog would help Ms. A.
Dr. B also might talk with Ms. A about her need for the dog and whether she had obtained appropriate certification, as discussed above. The ADA provisions pertaining to use and presence of service animals do not apply to dogs that are merely patients’ pets, notwithstanding the genuine emotional benefits that a dog’s companionship might provide. Stating that a patient needs an animal to treat an illness might be fraud if the doctor knew the pet was just a buddy.
Bottom Line
Psychiatrists can expect that more and more patients will ask them for letters to support having pets accompany them at home or in public. Although liability seems unlikely, cautious psychiatrists can state in such letters that they have not evaluated the animal in question, only the potential benefits that the patient might derive from it.
Disclosure
Dr. Mossman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing articles.
1. Dog Bites. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/homeandrecreationalsafety/ dog-bites/index.html. Updated October 25, 2013. Accessed April 22, 2014.
2. Serpell JA. Animal-assisted interventions in historical perspective. In Fine AH, ed. Handbook on animal-assisted therapy: theoretical foundations and guidelines for practice. 3rd ed. Burlington, MA: Academic Press; 2010:17-32.
3. Eddy J, Hart LA, Boltz RP. The effects of service dogs on social acknowledgments of people in wheelchairs. J Psychol. 1988;122(1):39-45.
4. Mader B, Hart LA, Bergin B. Social acknowledgments for children with disabilities: effects of service dogs. Child Dev. 1989;60(6):1529-1534.
5. Allen K, Blascovich J. The value of service dogs for people with severe ambulatory disabilities. A randomized controlled trial. JAMA. 1996;275(13):1001-1006.
6. Camp MM. The use of service dogs as an adaptive strategy: a qualitative study. Am J Occup Ther. 2001;55(5):509-517.
7. Allen K, Shykoff BE, Izzo JL Jr. Pet ownership, but not ace inhibitor therapy, blunts home blood pressure responses to mental stress. Hypertension. 2001;38(4):815-820.
8. ADA requirements: service animals. United States Department of Justice Civil Rights Division, Disability Rights Section Web site. http://www.ada.gov/service_ animals_2010.htm. Published September 15, 2010. Accessed April 22, 2014.
9. Eames E, Eames T. Interpreting legal mandates. Assistance dogs in medical facilities. Nurs Manage. 1997;28(6):49-51.
10. Houghtalen RP, Doody J. After the ADA: service dogs on inpatient psychiatric units. Bull Am Acad Psychiatry Law. 1995;23(2):211-217.
11. Wenthold N, Savage TA. Ethical issues with service animals. Top Stroke Rehabil. 2007;14(2):68-74.
12. DiSalvo H, Haiduven D, Johnson N, et al. Who let the dogs out? Infection control did: utility of dogs in health care settings and infection control aspects. Am J Infect Control. 2006;34:301-307.
13. Collins DM, Fitzgerald SG, Sachs-Ericsson N, et al. Psychosocial well-being and community participation of service dog partners. Disabil Rehabil Assist Technol. 2006;1(1-2):41-48.
14. Coren S. Foreward. In: Fine AH, ed. Handbook on animal-assisted therapy: theoretical foundations and guidelines for practice. 3rd ed. Burlington, MA: Academic Press; 2010: xv-xviii.
15. Levinson BM, Mallon GP. Pet-oriented child psychotherapy. 2nd ed. Springfield IL: Charles C Thomas Publisher, Ltd; 1997.
16. Esnayra J. Help from man’s best friend. Psychiatric service dogs are helping consumers deal with the symptoms of mental illness. Behav Healthc. 2007;27(7):30-32.
17. Barak Y, Savorai O, Mavashev S, et al. Animal-assisted therapy for elderly schizophrenic patients: a one year controlled trial. Am J Geriatr Psychiatry. 2001;9(4):439-442.
18. Burrows KE, Adams CL, Millman ST. Factors affecting behavior and welfare of service dogs for children with autism spectrum disorder. J Appl Anim Welf Sci. 2008;11(1):42-62.
19. Yount RA, Olmert MD, Lee MR. Service dog training program for treatment of posttraumatic stress in service members. US Army Med Dep J. 2012:63-69.
20. Parenti L, Foreman A, Meade BJ, et al. A revised taxonomy of assistance animals. J Rehabil Res Dev. 2013;50(6):745-756.
21. Testing Requirements. Therapy Dogs International. http:// www.tdi-dog.org/images/TestingBrochure.pdf. Accessed April 22, 2014.
22. How to become a registered therapy animal team. Pet Partners. http://www.petpartners.org/TAPinfo. Accessed April 22, 2014.
23. ADI Guide to Assistance Dog Laws. Assistance Dogs International. http://www.assistancedogsinternational. org/access-and-laws/adi-guide-to-assistance-dog-laws. Accessed April 22, 2014.
24. Id Stat §56-704.
25. Seim v Garavalia, 306 NW2d 806 (Minn 1981).
26. ME Rev Stat title 7, §3961.
27. Chadbourne v Kappaz, 2001 779 A2d 293 (DC App).
28. Stokes v Lyddy, 2002 75 (Conn App 252).
29. Georgianna v Gizzy, 483 NYS2d 892 (NY 1984).
30. Linebaugh v Hyndman, 516 A2d 638 (NJ 1986).
31. Benningfield v Zinsmeister, 367 SW3d 561 (Ky 2012).
1. Dog Bites. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/homeandrecreationalsafety/ dog-bites/index.html. Updated October 25, 2013. Accessed April 22, 2014.
2. Serpell JA. Animal-assisted interventions in historical perspective. In Fine AH, ed. Handbook on animal-assisted therapy: theoretical foundations and guidelines for practice. 3rd ed. Burlington, MA: Academic Press; 2010:17-32.
3. Eddy J, Hart LA, Boltz RP. The effects of service dogs on social acknowledgments of people in wheelchairs. J Psychol. 1988;122(1):39-45.
4. Mader B, Hart LA, Bergin B. Social acknowledgments for children with disabilities: effects of service dogs. Child Dev. 1989;60(6):1529-1534.
5. Allen K, Blascovich J. The value of service dogs for people with severe ambulatory disabilities. A randomized controlled trial. JAMA. 1996;275(13):1001-1006.
6. Camp MM. The use of service dogs as an adaptive strategy: a qualitative study. Am J Occup Ther. 2001;55(5):509-517.
7. Allen K, Shykoff BE, Izzo JL Jr. Pet ownership, but not ace inhibitor therapy, blunts home blood pressure responses to mental stress. Hypertension. 2001;38(4):815-820.
8. ADA requirements: service animals. United States Department of Justice Civil Rights Division, Disability Rights Section Web site. http://www.ada.gov/service_ animals_2010.htm. Published September 15, 2010. Accessed April 22, 2014.
9. Eames E, Eames T. Interpreting legal mandates. Assistance dogs in medical facilities. Nurs Manage. 1997;28(6):49-51.
10. Houghtalen RP, Doody J. After the ADA: service dogs on inpatient psychiatric units. Bull Am Acad Psychiatry Law. 1995;23(2):211-217.
11. Wenthold N, Savage TA. Ethical issues with service animals. Top Stroke Rehabil. 2007;14(2):68-74.
12. DiSalvo H, Haiduven D, Johnson N, et al. Who let the dogs out? Infection control did: utility of dogs in health care settings and infection control aspects. Am J Infect Control. 2006;34:301-307.
13. Collins DM, Fitzgerald SG, Sachs-Ericsson N, et al. Psychosocial well-being and community participation of service dog partners. Disabil Rehabil Assist Technol. 2006;1(1-2):41-48.
14. Coren S. Foreward. In: Fine AH, ed. Handbook on animal-assisted therapy: theoretical foundations and guidelines for practice. 3rd ed. Burlington, MA: Academic Press; 2010: xv-xviii.
15. Levinson BM, Mallon GP. Pet-oriented child psychotherapy. 2nd ed. Springfield IL: Charles C Thomas Publisher, Ltd; 1997.
16. Esnayra J. Help from man’s best friend. Psychiatric service dogs are helping consumers deal with the symptoms of mental illness. Behav Healthc. 2007;27(7):30-32.
17. Barak Y, Savorai O, Mavashev S, et al. Animal-assisted therapy for elderly schizophrenic patients: a one year controlled trial. Am J Geriatr Psychiatry. 2001;9(4):439-442.
18. Burrows KE, Adams CL, Millman ST. Factors affecting behavior and welfare of service dogs for children with autism spectrum disorder. J Appl Anim Welf Sci. 2008;11(1):42-62.
19. Yount RA, Olmert MD, Lee MR. Service dog training program for treatment of posttraumatic stress in service members. US Army Med Dep J. 2012:63-69.
20. Parenti L, Foreman A, Meade BJ, et al. A revised taxonomy of assistance animals. J Rehabil Res Dev. 2013;50(6):745-756.
21. Testing Requirements. Therapy Dogs International. http:// www.tdi-dog.org/images/TestingBrochure.pdf. Accessed April 22, 2014.
22. How to become a registered therapy animal team. Pet Partners. http://www.petpartners.org/TAPinfo. Accessed April 22, 2014.
23. ADI Guide to Assistance Dog Laws. Assistance Dogs International. http://www.assistancedogsinternational. org/access-and-laws/adi-guide-to-assistance-dog-laws. Accessed April 22, 2014.
24. Id Stat §56-704.
25. Seim v Garavalia, 306 NW2d 806 (Minn 1981).
26. ME Rev Stat title 7, §3961.
27. Chadbourne v Kappaz, 2001 779 A2d 293 (DC App).
28. Stokes v Lyddy, 2002 75 (Conn App 252).
29. Georgianna v Gizzy, 483 NYS2d 892 (NY 1984).
30. Linebaugh v Hyndman, 516 A2d 638 (NJ 1986).
31. Benningfield v Zinsmeister, 367 SW3d 561 (Ky 2012).
Delaying clopidogrel can increase risk of MI, death

Credit: CDC
Patients who delay filling a prescription of clopidogrel after coronary stenting may increase their risk of recurrent myocardial infarction (MI) and death, according to a study published in the Journal of the American Heart Association.
Researchers analyzed records of more than 15,000 patients who received drug-eluting or bare metal stents.
Roughly 30% of patients in each group failed to fill their prescription of the anticoagulant clopidogrel within 3 days of hospital discharge.
And this roughly doubled the patients’ risk of death and recurrent MI, regardless of their stent type.
“It is very important that patients take clopidogrel after having a coronary stent implanted to prevent blood clots forming within the stent,” said study author Nicholas Cruden, MBChB, PhD, of the Royal Infirmary of Edinburgh in the UK.
He and his colleagues analyzed hospital administrative, community pharmacy, and cardiac revascularization data from all patients who received a coronary stent in British Columbia between 2004 and 2006, with follow-up out to 2 years.
Of the 15,629 patients, 3599 had received at least 1 drug-eluting stent (DES), and 12,030 had received a bare metal stent (BMS). Thirty percent (n=1064) of patients in the DES group, and 31% (n=3758) of patients in the BMS group failed to fill their prescription for clopidogrel within 3 days of hospital discharge.
And a delay of more than 3 days was predictive of mortality and recurrent MI, regardless of the stent type. The hazard ratios (HRs) for mortality were 2.4 for the DES group and 2.2 for the BMS group. The HRs for recurrent MI were 2.0 and 1.8, respectively.
The excess risk associated with a delay in filling the prescription was greatest in the immediate period after hospital discharge—up to 30 days. In all patients, the HRs were 5.5 for mortality and 3.1 for recurrent MI.
Delaying filling the prescription for more than 3 days remained an independent predictor of death and MI beyond 30 days from hospital discharge. The HRs were 2.1 and 2.0, respectively, for patients in the DES group and 2.0 and 1.8, respectively, for patients in the BMS group.
“This study highlights the importance of ensuring patients have access to medications as soon as they leave the hospital,” Dr Cruden said. “Even a delay of a day or 2 was associated with worse outcomes.”
Discharging patients from the hospital with enough medicine for the highest-risk period (the first month or so) could help, he added. ![]()

Credit: CDC
Patients who delay filling a prescription of clopidogrel after coronary stenting may increase their risk of recurrent myocardial infarction (MI) and death, according to a study published in the Journal of the American Heart Association.
Researchers analyzed records of more than 15,000 patients who received drug-eluting or bare metal stents.
Roughly 30% of patients in each group failed to fill their prescription of the anticoagulant clopidogrel within 3 days of hospital discharge.
And this roughly doubled the patients’ risk of death and recurrent MI, regardless of their stent type.
“It is very important that patients take clopidogrel after having a coronary stent implanted to prevent blood clots forming within the stent,” said study author Nicholas Cruden, MBChB, PhD, of the Royal Infirmary of Edinburgh in the UK.
He and his colleagues analyzed hospital administrative, community pharmacy, and cardiac revascularization data from all patients who received a coronary stent in British Columbia between 2004 and 2006, with follow-up out to 2 years.
Of the 15,629 patients, 3599 had received at least 1 drug-eluting stent (DES), and 12,030 had received a bare metal stent (BMS). Thirty percent (n=1064) of patients in the DES group, and 31% (n=3758) of patients in the BMS group failed to fill their prescription for clopidogrel within 3 days of hospital discharge.
And a delay of more than 3 days was predictive of mortality and recurrent MI, regardless of the stent type. The hazard ratios (HRs) for mortality were 2.4 for the DES group and 2.2 for the BMS group. The HRs for recurrent MI were 2.0 and 1.8, respectively.
The excess risk associated with a delay in filling the prescription was greatest in the immediate period after hospital discharge—up to 30 days. In all patients, the HRs were 5.5 for mortality and 3.1 for recurrent MI.
Delaying filling the prescription for more than 3 days remained an independent predictor of death and MI beyond 30 days from hospital discharge. The HRs were 2.1 and 2.0, respectively, for patients in the DES group and 2.0 and 1.8, respectively, for patients in the BMS group.
“This study highlights the importance of ensuring patients have access to medications as soon as they leave the hospital,” Dr Cruden said. “Even a delay of a day or 2 was associated with worse outcomes.”
Discharging patients from the hospital with enough medicine for the highest-risk period (the first month or so) could help, he added. ![]()

Credit: CDC
Patients who delay filling a prescription of clopidogrel after coronary stenting may increase their risk of recurrent myocardial infarction (MI) and death, according to a study published in the Journal of the American Heart Association.
Researchers analyzed records of more than 15,000 patients who received drug-eluting or bare metal stents.
Roughly 30% of patients in each group failed to fill their prescription of the anticoagulant clopidogrel within 3 days of hospital discharge.
And this roughly doubled the patients’ risk of death and recurrent MI, regardless of their stent type.
“It is very important that patients take clopidogrel after having a coronary stent implanted to prevent blood clots forming within the stent,” said study author Nicholas Cruden, MBChB, PhD, of the Royal Infirmary of Edinburgh in the UK.
He and his colleagues analyzed hospital administrative, community pharmacy, and cardiac revascularization data from all patients who received a coronary stent in British Columbia between 2004 and 2006, with follow-up out to 2 years.
Of the 15,629 patients, 3599 had received at least 1 drug-eluting stent (DES), and 12,030 had received a bare metal stent (BMS). Thirty percent (n=1064) of patients in the DES group, and 31% (n=3758) of patients in the BMS group failed to fill their prescription for clopidogrel within 3 days of hospital discharge.
And a delay of more than 3 days was predictive of mortality and recurrent MI, regardless of the stent type. The hazard ratios (HRs) for mortality were 2.4 for the DES group and 2.2 for the BMS group. The HRs for recurrent MI were 2.0 and 1.8, respectively.
The excess risk associated with a delay in filling the prescription was greatest in the immediate period after hospital discharge—up to 30 days. In all patients, the HRs were 5.5 for mortality and 3.1 for recurrent MI.
Delaying filling the prescription for more than 3 days remained an independent predictor of death and MI beyond 30 days from hospital discharge. The HRs were 2.1 and 2.0, respectively, for patients in the DES group and 2.0 and 1.8, respectively, for patients in the BMS group.
“This study highlights the importance of ensuring patients have access to medications as soon as they leave the hospital,” Dr Cruden said. “Even a delay of a day or 2 was associated with worse outcomes.”
Discharging patients from the hospital with enough medicine for the highest-risk period (the first month or so) could help, he added. ![]()
Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”?
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery

John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery

John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery

John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
Have you read Dr. Errol R. Norwitz's Update on Operative Vaginal Delivery? Click here to access.
Woman loses both legs after salpingectomy: $64.3M award
Woman loses both legs after salpingectomy: $64.3M award
Due to an ectopic pregnancy, a 29-year-old woman underwent laparoscopic salpingectomy in October 2009. A resident supervised by Dr. A (gynecologist) performed the surgery. Although the patient reported abdominal pain and was febrile, Dr. B (gynecologist) discharged her on postsurgical day 2.
The next day, she returned to the emergency department (ED) with abdominal swelling and pain. Dr. C (ED physician), Dr. D (gynecologist), and Dr. E (general surgeon) examined her. Dr. D began conservative treatment for bowel obstruction. Two days later she was in septic shock. Dr. E repaired a 5-mm injury to the sigmoid colon and created a colostomy. The patient was placed in a medically induced coma for 3 weeks. She experienced cardiac arrest 3 times during her 73-day ICU stay. She underwent skin grafts, and suffered hearing loss as a result of antibiotic treatment. Due to gangrene, both legs were amputated below the knee.
At the trial’s conclusion in January 2014, the colostomy had not been reversed. She has difficulty caring for her daughter and has not worked since the initial operation.
PATIENT’S CLAIM The resident, who injured the colon and did not detect the injury during surgery, was improperly supervised by Dr. A. Hospital staff did not communicate the patient’s problem reports to the physicians. Dr. B should not have discharged her after surgery; based on her reported symptoms, additional testing was warranted. Drs. C, D, and E did not react to the patient’s pain reports in a timely manner, nor treat the resulting sepsis aggressively enough, leading to gangrene.
DEFENDANTS’ DEFENSE The patient’s colon injury was diagnosed and treated in a timely manner, but her condition deteriorated rapidly. The physicians acted responsibly based on the available information; a computed tomography scan did not show the colon injury. The injury likely occurred after the procedure due to an underlying bowel condition and is a known risk of the procedure. The colostomy can be reversed. Their efforts saved her life.
VERDICT The patient and Dr. E negotiated a $2.3 million settlement. A $62 million New York verdict was returned. The jury found the hospital 40% liable; Dr. A 30% liable; Dr. B 20% liable; and Dr. D 10% liable. Claims were dropped against the resident and Dr. C.
Related article: Oophorectomy or salpingectomy—which makes more sense? William H. Parker, MD (March 2014)
PARENTS REQUESTED EARLIER CESAREAN: CHILD HAS CP
A woman was in labor for 2 full days before her ObGyn performed a cesarean delivery. The child was born with abnormal Apgar scores and had seizures. Imaging studies revealed brain damage. She received a diagnosis of cerebral palsy.
PARENTS’ CLAIM The parents first requested cesarean delivery early on the second day, but the ObGyn allowed labor to progress. When the fetal heart-rate monitor showed signs of fetal distress 3 hours later, the parents made a second request; the ObGyn continued with vaginal delivery. The child was ultimately born by cesarean delivery. Her brain damage was caused by lack of oxygen from failure to perform an earlier cesarean delivery.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $4.25 million Massachusetts settlement was reached.
BLADDER INJURED DURING CESAREAN DELIVERY
A 33-year-old woman gave birth via cesarean delivery performed by her ObGyn. During the procedure, the patient’s bladder was lacerated and the injury was immediately repaired. The patient reports occasional urinary incontinence and pain.
PATIENT’S CLAIM The ObGyn should have anticipated that the bladder would be shifted because of the patient’s previous cesarean delivery.
PHYSICIAN’S DEFENSE The injury is a known risk of the procedure. The patient had developed adhesions that caused the bladder to become displaced. She does not suffer permanent residual effects from the injury.
VERDICT A $125,000 New York verdict was returned.
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery. Baha M. Sibai (March 2012)
PARENTS REQUESTED SPECIFIC GENETIC TESTING, BUT CHILD IS BORN WITH RARE CHROMOSOMAL CONDITION: $50M VERDICT
Parents sought prenatal genetic testing to determine if their fetus had a specific genetic condition because the father carries a rare chromosomal abnormality called an unbalanced chromosome translocation. This defect can only be identified if the laboratory is told precisely where to look for the specific translocation; it is not detected on routine prenatal genetic testing. After testing, the parents were told that the fetus did not have the chromosomal abnormality.
The child was born with the condition for which testing was sought, resulting in severe physical and cognitive impairments and multiple physical abnormalities. He will require 24-hour care for life.
PARENTS’ CLAIM Testing failed to identify the condition; the couple had decided to terminate the pregnancy if the child was affected. Due to budget cuts in the maternal-fetal medicine clinic, the medical center borrowed a genetic counselor from another hospital one day a week. The parents told the genetic counselor of the family’s history of the defect and explained that the laboratory’s procedures require the referring center to obtain and share the necessary detailed information with the lab. The lab was apparently notified that the couple had a family history of the defect, but the genetic counselor did not transmit specific information to the lab, and lab personnel did not appropriately follow-up.
DEFENDANTS’ DEFENSE The medical center blamed the laboratory: the lab’s standard procedures state that the lab should call the referring center to obtain the necessary detailed information if it was not provided; the lab employee who handled the specimen did not do so. The lab claimed that the genetic counselor did not transmit the specific information to the lab.
The laboratory disputed the child’s need for 24/7 care, maintaining that he could live in a group home with only occasional nursing care.
VERDICT A $50 million Washington verdict was returned against the medical center and laboratory; each defendant will pay $25 million.
Related article: Noninvasive prenatal testing: Where we are and where we’re going. Lee P. Shulman, MD (Commentary; May 2014)
NECROTIZING FASCIITIS AFTER SURGERY
A 57-year-old woman underwent surgery to repair vaginal vault prolapse, rectocele, and enterocele, performed by her gynecologist. Several days after discharge, the patient returned to the hospital with an infection in her leg that had evolved into necrotizing fasciitis. She underwent five fasciotomies and was hospitalized for 3 weeks.
PATIENT’S CLAIM The gynecologist should have administered prophylactic antibiotics before, during, and after surgery. The patient has massive scarring of her leg.
PHYSICIAN’S DEFENSE The infection was not a result of failing to administer antibiotics. The patient failed to seek timely treatment of symptoms that developed after surgery.
VERDICT A $400,000 New York verdict was returned but reduced because the jury found the patient 49% at fault.
OXYTOCIN BLAMED FOR CHILD’S CP
A mother had bariatric surgery 12 months before becoming pregnant, and she smoked during pregnancy. She developed placental insufficiency and labor was induced shortly after she reached 37 weeks’ gestation.
During delivery, the mother was given oxytocin to increase the frequency and strength of contractions. Nurses repeatedly stopped the oxytocin in response to decelerations in the fetal heart rate, but physicians ordered the oxytocin resumed, even after fetal heart-rate monitoring showed fetal distress.
Three days after birth, the child was transferred to another hospital, and was found to have cerebral palsy and other injuries. At age 5, the child is nonverbal, cannot walk, and requires a feeding tube.
PARENTS’ CLAIM Oxytocin should have been stopped and a cesarean delivery performed when fetal distress was first noted.
DEFENDANTS’ DEFENSE There was no need for cesarean delivery. Apgar scores, blood gases, and fetal presentation indicated that the injury occurred prior to labor.
VERDICT A $6 million Texas settlement was reached during the trial.
Related article: Q: Following cesarean delivery, what is the optimal oxytocin infusion duration to prevent postpartum bleeding? Robert L. Barbieri, MD (Editorial; April 2014)
MOTHER DISCHARGED DESPITE SEVERE ABDOMINAL PAIN
A woman had prenatal care at different locations. Her history included two cesarean deliveries.
Reporting severe abdominal pain, she was taken from a homeless shelter to an ED by ambulance. The mother was uncertain of the fetus’ gestational age; a 4th-year obstetric resident determined by physical examination that the pregnancy was at 36.5 weeks. The resident discussed the case with the attending ObGyn, who said to discharge the mother if her pain was gone. After 11 hours, the mother was returned to the shelter.
The mother returned to the ED 12 hours later. Thirty-five minutes after fetal distress was identified, an emergency cesarean delivery was performed. At birth, the child was found to be at 38 to 39 weeks’ gestation. He received a diagnosis of severe hypoxic ischemic encephalopathy and was transferred to a children’s hospital for brain cooling.
The child lives in a long-term care facility and is dependent on a ventilator and gastronomy tube.
PARENT’S CLAIM The mother should not have been discharged after the first visit. A cesarean delivery should have been performed at that time. The attending ObGyn never saw the mother.
DEFENDANTS’ DEFENSE The mother should have given her correct due date, which was in her prenatal records based on previous ultrasonograpy. The first discharge was proper, as the pain had improved. The homeless shelter should have called an ambulance earlier for the second admission.
VERDICT A $7.5 million California settlement was reached, plus payment of medical expenses exceeding $300,000.
Timing of child’s injury disputed
Vaginal birth after cesarean (VBAC) had been planned. After reporting to her ObGyn that she was in labor, a mother went to the ED.
During the next few hours, hospital staff called the ObGyn twice to report that fetal monitor strips indicated tachycardia. The ObGyn then spoke to the mother by phone and told her that cesarean delivery was necessary but could wait for him to get to the hospital. After the ObGyn arrived, he removed the fetal heart-rate monitor to prepare the mother’s abdomen; cesarean delivery occurred 15 minutes later.
The child has spastic dystonic quadriplegia and requires 24-hour care.
PARENT’S CLAIM The ObGyn should have come to the hospital and performed cesarean delivery when he was first notified that the fetus was tachycardic. The baby suffered an hypoxic ischemic event in the 15-minute period between when the monitor was removed and birth, causing hypoxic ischemic encephalopathy.
PHYSICIAN’S DEFENSE There was no indication of a need for earlier delivery. The brain injury occurred prior to labor and delivery.
VERDICT The hospital settled for a confidential amount before the trial. An Illinois defense verdict was returned for the ObGyn.
Were mammograms properly interpreted?
After reporting a lump in her breast, a 39-year-old woman underwent mammography in 2008 and 2009. Two different radiologists reported their findings as negative for cancer.
In 2010, the patient was found to have breast cancer. She underwent a mastectomy, chemotherapy, and radiation therapy, and was given a 75%–80% chance of 5-year survival.
PATIENT’S CLAIM The ObGyn failed to follow-up on the patient’s reports of a breast lump. The radiologists did not correctly interpret the 2008 and 2009 mammograms. If cancer had been detected earlier, treatment would have been less extreme.
PHYSICIANS’ DEFENSE The ObGyn claimed that he would have felt a lump if it was present. The first radiologist claimed that the 2008 mammography report was correct, noting that the patient’s cancer was a lobular carcinoma that does not always show on mammography or in patients with dense breasts, which this patient has.
VERDICT A directed verdict was granted to the radiologist who interpreted the 2009 mammography, as the results were lost. An Ohio defense verdict was returned for the ObGyn and the other radiologist.
Related article: Does screening mammography save lives? Janelle Yates, Senior Editor (April 2014)
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
TELL US WHAT YOU THINK! Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice.
Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
Stay in touch! Your feedback is important to us!
Woman loses both legs after salpingectomy: $64.3M award
Due to an ectopic pregnancy, a 29-year-old woman underwent laparoscopic salpingectomy in October 2009. A resident supervised by Dr. A (gynecologist) performed the surgery. Although the patient reported abdominal pain and was febrile, Dr. B (gynecologist) discharged her on postsurgical day 2.
The next day, she returned to the emergency department (ED) with abdominal swelling and pain. Dr. C (ED physician), Dr. D (gynecologist), and Dr. E (general surgeon) examined her. Dr. D began conservative treatment for bowel obstruction. Two days later she was in septic shock. Dr. E repaired a 5-mm injury to the sigmoid colon and created a colostomy. The patient was placed in a medically induced coma for 3 weeks. She experienced cardiac arrest 3 times during her 73-day ICU stay. She underwent skin grafts, and suffered hearing loss as a result of antibiotic treatment. Due to gangrene, both legs were amputated below the knee.
At the trial’s conclusion in January 2014, the colostomy had not been reversed. She has difficulty caring for her daughter and has not worked since the initial operation.
PATIENT’S CLAIM The resident, who injured the colon and did not detect the injury during surgery, was improperly supervised by Dr. A. Hospital staff did not communicate the patient’s problem reports to the physicians. Dr. B should not have discharged her after surgery; based on her reported symptoms, additional testing was warranted. Drs. C, D, and E did not react to the patient’s pain reports in a timely manner, nor treat the resulting sepsis aggressively enough, leading to gangrene.
DEFENDANTS’ DEFENSE The patient’s colon injury was diagnosed and treated in a timely manner, but her condition deteriorated rapidly. The physicians acted responsibly based on the available information; a computed tomography scan did not show the colon injury. The injury likely occurred after the procedure due to an underlying bowel condition and is a known risk of the procedure. The colostomy can be reversed. Their efforts saved her life.
VERDICT The patient and Dr. E negotiated a $2.3 million settlement. A $62 million New York verdict was returned. The jury found the hospital 40% liable; Dr. A 30% liable; Dr. B 20% liable; and Dr. D 10% liable. Claims were dropped against the resident and Dr. C.
Related article: Oophorectomy or salpingectomy—which makes more sense? William H. Parker, MD (March 2014)
PARENTS REQUESTED EARLIER CESAREAN: CHILD HAS CP
A woman was in labor for 2 full days before her ObGyn performed a cesarean delivery. The child was born with abnormal Apgar scores and had seizures. Imaging studies revealed brain damage. She received a diagnosis of cerebral palsy.
PARENTS’ CLAIM The parents first requested cesarean delivery early on the second day, but the ObGyn allowed labor to progress. When the fetal heart-rate monitor showed signs of fetal distress 3 hours later, the parents made a second request; the ObGyn continued with vaginal delivery. The child was ultimately born by cesarean delivery. Her brain damage was caused by lack of oxygen from failure to perform an earlier cesarean delivery.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $4.25 million Massachusetts settlement was reached.
BLADDER INJURED DURING CESAREAN DELIVERY
A 33-year-old woman gave birth via cesarean delivery performed by her ObGyn. During the procedure, the patient’s bladder was lacerated and the injury was immediately repaired. The patient reports occasional urinary incontinence and pain.
PATIENT’S CLAIM The ObGyn should have anticipated that the bladder would be shifted because of the patient’s previous cesarean delivery.
PHYSICIAN’S DEFENSE The injury is a known risk of the procedure. The patient had developed adhesions that caused the bladder to become displaced. She does not suffer permanent residual effects from the injury.
VERDICT A $125,000 New York verdict was returned.
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery. Baha M. Sibai (March 2012)
PARENTS REQUESTED SPECIFIC GENETIC TESTING, BUT CHILD IS BORN WITH RARE CHROMOSOMAL CONDITION: $50M VERDICT
Parents sought prenatal genetic testing to determine if their fetus had a specific genetic condition because the father carries a rare chromosomal abnormality called an unbalanced chromosome translocation. This defect can only be identified if the laboratory is told precisely where to look for the specific translocation; it is not detected on routine prenatal genetic testing. After testing, the parents were told that the fetus did not have the chromosomal abnormality.
The child was born with the condition for which testing was sought, resulting in severe physical and cognitive impairments and multiple physical abnormalities. He will require 24-hour care for life.
PARENTS’ CLAIM Testing failed to identify the condition; the couple had decided to terminate the pregnancy if the child was affected. Due to budget cuts in the maternal-fetal medicine clinic, the medical center borrowed a genetic counselor from another hospital one day a week. The parents told the genetic counselor of the family’s history of the defect and explained that the laboratory’s procedures require the referring center to obtain and share the necessary detailed information with the lab. The lab was apparently notified that the couple had a family history of the defect, but the genetic counselor did not transmit specific information to the lab, and lab personnel did not appropriately follow-up.
DEFENDANTS’ DEFENSE The medical center blamed the laboratory: the lab’s standard procedures state that the lab should call the referring center to obtain the necessary detailed information if it was not provided; the lab employee who handled the specimen did not do so. The lab claimed that the genetic counselor did not transmit the specific information to the lab.
The laboratory disputed the child’s need for 24/7 care, maintaining that he could live in a group home with only occasional nursing care.
VERDICT A $50 million Washington verdict was returned against the medical center and laboratory; each defendant will pay $25 million.
Related article: Noninvasive prenatal testing: Where we are and where we’re going. Lee P. Shulman, MD (Commentary; May 2014)
NECROTIZING FASCIITIS AFTER SURGERY
A 57-year-old woman underwent surgery to repair vaginal vault prolapse, rectocele, and enterocele, performed by her gynecologist. Several days after discharge, the patient returned to the hospital with an infection in her leg that had evolved into necrotizing fasciitis. She underwent five fasciotomies and was hospitalized for 3 weeks.
PATIENT’S CLAIM The gynecologist should have administered prophylactic antibiotics before, during, and after surgery. The patient has massive scarring of her leg.
PHYSICIAN’S DEFENSE The infection was not a result of failing to administer antibiotics. The patient failed to seek timely treatment of symptoms that developed after surgery.
VERDICT A $400,000 New York verdict was returned but reduced because the jury found the patient 49% at fault.
OXYTOCIN BLAMED FOR CHILD’S CP
A mother had bariatric surgery 12 months before becoming pregnant, and she smoked during pregnancy. She developed placental insufficiency and labor was induced shortly after she reached 37 weeks’ gestation.
During delivery, the mother was given oxytocin to increase the frequency and strength of contractions. Nurses repeatedly stopped the oxytocin in response to decelerations in the fetal heart rate, but physicians ordered the oxytocin resumed, even after fetal heart-rate monitoring showed fetal distress.
Three days after birth, the child was transferred to another hospital, and was found to have cerebral palsy and other injuries. At age 5, the child is nonverbal, cannot walk, and requires a feeding tube.
PARENTS’ CLAIM Oxytocin should have been stopped and a cesarean delivery performed when fetal distress was first noted.
DEFENDANTS’ DEFENSE There was no need for cesarean delivery. Apgar scores, blood gases, and fetal presentation indicated that the injury occurred prior to labor.
VERDICT A $6 million Texas settlement was reached during the trial.
Related article: Q: Following cesarean delivery, what is the optimal oxytocin infusion duration to prevent postpartum bleeding? Robert L. Barbieri, MD (Editorial; April 2014)
MOTHER DISCHARGED DESPITE SEVERE ABDOMINAL PAIN
A woman had prenatal care at different locations. Her history included two cesarean deliveries.
Reporting severe abdominal pain, she was taken from a homeless shelter to an ED by ambulance. The mother was uncertain of the fetus’ gestational age; a 4th-year obstetric resident determined by physical examination that the pregnancy was at 36.5 weeks. The resident discussed the case with the attending ObGyn, who said to discharge the mother if her pain was gone. After 11 hours, the mother was returned to the shelter.
The mother returned to the ED 12 hours later. Thirty-five minutes after fetal distress was identified, an emergency cesarean delivery was performed. At birth, the child was found to be at 38 to 39 weeks’ gestation. He received a diagnosis of severe hypoxic ischemic encephalopathy and was transferred to a children’s hospital for brain cooling.
The child lives in a long-term care facility and is dependent on a ventilator and gastronomy tube.
PARENT’S CLAIM The mother should not have been discharged after the first visit. A cesarean delivery should have been performed at that time. The attending ObGyn never saw the mother.
DEFENDANTS’ DEFENSE The mother should have given her correct due date, which was in her prenatal records based on previous ultrasonograpy. The first discharge was proper, as the pain had improved. The homeless shelter should have called an ambulance earlier for the second admission.
VERDICT A $7.5 million California settlement was reached, plus payment of medical expenses exceeding $300,000.
Timing of child’s injury disputed
Vaginal birth after cesarean (VBAC) had been planned. After reporting to her ObGyn that she was in labor, a mother went to the ED.
During the next few hours, hospital staff called the ObGyn twice to report that fetal monitor strips indicated tachycardia. The ObGyn then spoke to the mother by phone and told her that cesarean delivery was necessary but could wait for him to get to the hospital. After the ObGyn arrived, he removed the fetal heart-rate monitor to prepare the mother’s abdomen; cesarean delivery occurred 15 minutes later.
The child has spastic dystonic quadriplegia and requires 24-hour care.
PARENT’S CLAIM The ObGyn should have come to the hospital and performed cesarean delivery when he was first notified that the fetus was tachycardic. The baby suffered an hypoxic ischemic event in the 15-minute period between when the monitor was removed and birth, causing hypoxic ischemic encephalopathy.
PHYSICIAN’S DEFENSE There was no indication of a need for earlier delivery. The brain injury occurred prior to labor and delivery.
VERDICT The hospital settled for a confidential amount before the trial. An Illinois defense verdict was returned for the ObGyn.
Were mammograms properly interpreted?
After reporting a lump in her breast, a 39-year-old woman underwent mammography in 2008 and 2009. Two different radiologists reported their findings as negative for cancer.
In 2010, the patient was found to have breast cancer. She underwent a mastectomy, chemotherapy, and radiation therapy, and was given a 75%–80% chance of 5-year survival.
PATIENT’S CLAIM The ObGyn failed to follow-up on the patient’s reports of a breast lump. The radiologists did not correctly interpret the 2008 and 2009 mammograms. If cancer had been detected earlier, treatment would have been less extreme.
PHYSICIANS’ DEFENSE The ObGyn claimed that he would have felt a lump if it was present. The first radiologist claimed that the 2008 mammography report was correct, noting that the patient’s cancer was a lobular carcinoma that does not always show on mammography or in patients with dense breasts, which this patient has.
VERDICT A directed verdict was granted to the radiologist who interpreted the 2009 mammography, as the results were lost. An Ohio defense verdict was returned for the ObGyn and the other radiologist.
Related article: Does screening mammography save lives? Janelle Yates, Senior Editor (April 2014)
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
TELL US WHAT YOU THINK! Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice.
Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
Stay in touch! Your feedback is important to us!
Woman loses both legs after salpingectomy: $64.3M award
Due to an ectopic pregnancy, a 29-year-old woman underwent laparoscopic salpingectomy in October 2009. A resident supervised by Dr. A (gynecologist) performed the surgery. Although the patient reported abdominal pain and was febrile, Dr. B (gynecologist) discharged her on postsurgical day 2.
The next day, she returned to the emergency department (ED) with abdominal swelling and pain. Dr. C (ED physician), Dr. D (gynecologist), and Dr. E (general surgeon) examined her. Dr. D began conservative treatment for bowel obstruction. Two days later she was in septic shock. Dr. E repaired a 5-mm injury to the sigmoid colon and created a colostomy. The patient was placed in a medically induced coma for 3 weeks. She experienced cardiac arrest 3 times during her 73-day ICU stay. She underwent skin grafts, and suffered hearing loss as a result of antibiotic treatment. Due to gangrene, both legs were amputated below the knee.
At the trial’s conclusion in January 2014, the colostomy had not been reversed. She has difficulty caring for her daughter and has not worked since the initial operation.
PATIENT’S CLAIM The resident, who injured the colon and did not detect the injury during surgery, was improperly supervised by Dr. A. Hospital staff did not communicate the patient’s problem reports to the physicians. Dr. B should not have discharged her after surgery; based on her reported symptoms, additional testing was warranted. Drs. C, D, and E did not react to the patient’s pain reports in a timely manner, nor treat the resulting sepsis aggressively enough, leading to gangrene.
DEFENDANTS’ DEFENSE The patient’s colon injury was diagnosed and treated in a timely manner, but her condition deteriorated rapidly. The physicians acted responsibly based on the available information; a computed tomography scan did not show the colon injury. The injury likely occurred after the procedure due to an underlying bowel condition and is a known risk of the procedure. The colostomy can be reversed. Their efforts saved her life.
VERDICT The patient and Dr. E negotiated a $2.3 million settlement. A $62 million New York verdict was returned. The jury found the hospital 40% liable; Dr. A 30% liable; Dr. B 20% liable; and Dr. D 10% liable. Claims were dropped against the resident and Dr. C.
Related article: Oophorectomy or salpingectomy—which makes more sense? William H. Parker, MD (March 2014)
PARENTS REQUESTED EARLIER CESAREAN: CHILD HAS CP
A woman was in labor for 2 full days before her ObGyn performed a cesarean delivery. The child was born with abnormal Apgar scores and had seizures. Imaging studies revealed brain damage. She received a diagnosis of cerebral palsy.
PARENTS’ CLAIM The parents first requested cesarean delivery early on the second day, but the ObGyn allowed labor to progress. When the fetal heart-rate monitor showed signs of fetal distress 3 hours later, the parents made a second request; the ObGyn continued with vaginal delivery. The child was ultimately born by cesarean delivery. Her brain damage was caused by lack of oxygen from failure to perform an earlier cesarean delivery.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $4.25 million Massachusetts settlement was reached.
BLADDER INJURED DURING CESAREAN DELIVERY
A 33-year-old woman gave birth via cesarean delivery performed by her ObGyn. During the procedure, the patient’s bladder was lacerated and the injury was immediately repaired. The patient reports occasional urinary incontinence and pain.
PATIENT’S CLAIM The ObGyn should have anticipated that the bladder would be shifted because of the patient’s previous cesarean delivery.
PHYSICIAN’S DEFENSE The injury is a known risk of the procedure. The patient had developed adhesions that caused the bladder to become displaced. She does not suffer permanent residual effects from the injury.
VERDICT A $125,000 New York verdict was returned.
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery. Baha M. Sibai (March 2012)
PARENTS REQUESTED SPECIFIC GENETIC TESTING, BUT CHILD IS BORN WITH RARE CHROMOSOMAL CONDITION: $50M VERDICT
Parents sought prenatal genetic testing to determine if their fetus had a specific genetic condition because the father carries a rare chromosomal abnormality called an unbalanced chromosome translocation. This defect can only be identified if the laboratory is told precisely where to look for the specific translocation; it is not detected on routine prenatal genetic testing. After testing, the parents were told that the fetus did not have the chromosomal abnormality.
The child was born with the condition for which testing was sought, resulting in severe physical and cognitive impairments and multiple physical abnormalities. He will require 24-hour care for life.
PARENTS’ CLAIM Testing failed to identify the condition; the couple had decided to terminate the pregnancy if the child was affected. Due to budget cuts in the maternal-fetal medicine clinic, the medical center borrowed a genetic counselor from another hospital one day a week. The parents told the genetic counselor of the family’s history of the defect and explained that the laboratory’s procedures require the referring center to obtain and share the necessary detailed information with the lab. The lab was apparently notified that the couple had a family history of the defect, but the genetic counselor did not transmit specific information to the lab, and lab personnel did not appropriately follow-up.
DEFENDANTS’ DEFENSE The medical center blamed the laboratory: the lab’s standard procedures state that the lab should call the referring center to obtain the necessary detailed information if it was not provided; the lab employee who handled the specimen did not do so. The lab claimed that the genetic counselor did not transmit the specific information to the lab.
The laboratory disputed the child’s need for 24/7 care, maintaining that he could live in a group home with only occasional nursing care.
VERDICT A $50 million Washington verdict was returned against the medical center and laboratory; each defendant will pay $25 million.
Related article: Noninvasive prenatal testing: Where we are and where we’re going. Lee P. Shulman, MD (Commentary; May 2014)
NECROTIZING FASCIITIS AFTER SURGERY
A 57-year-old woman underwent surgery to repair vaginal vault prolapse, rectocele, and enterocele, performed by her gynecologist. Several days after discharge, the patient returned to the hospital with an infection in her leg that had evolved into necrotizing fasciitis. She underwent five fasciotomies and was hospitalized for 3 weeks.
PATIENT’S CLAIM The gynecologist should have administered prophylactic antibiotics before, during, and after surgery. The patient has massive scarring of her leg.
PHYSICIAN’S DEFENSE The infection was not a result of failing to administer antibiotics. The patient failed to seek timely treatment of symptoms that developed after surgery.
VERDICT A $400,000 New York verdict was returned but reduced because the jury found the patient 49% at fault.
OXYTOCIN BLAMED FOR CHILD’S CP
A mother had bariatric surgery 12 months before becoming pregnant, and she smoked during pregnancy. She developed placental insufficiency and labor was induced shortly after she reached 37 weeks’ gestation.
During delivery, the mother was given oxytocin to increase the frequency and strength of contractions. Nurses repeatedly stopped the oxytocin in response to decelerations in the fetal heart rate, but physicians ordered the oxytocin resumed, even after fetal heart-rate monitoring showed fetal distress.
Three days after birth, the child was transferred to another hospital, and was found to have cerebral palsy and other injuries. At age 5, the child is nonverbal, cannot walk, and requires a feeding tube.
PARENTS’ CLAIM Oxytocin should have been stopped and a cesarean delivery performed when fetal distress was first noted.
DEFENDANTS’ DEFENSE There was no need for cesarean delivery. Apgar scores, blood gases, and fetal presentation indicated that the injury occurred prior to labor.
VERDICT A $6 million Texas settlement was reached during the trial.
Related article: Q: Following cesarean delivery, what is the optimal oxytocin infusion duration to prevent postpartum bleeding? Robert L. Barbieri, MD (Editorial; April 2014)
MOTHER DISCHARGED DESPITE SEVERE ABDOMINAL PAIN
A woman had prenatal care at different locations. Her history included two cesarean deliveries.
Reporting severe abdominal pain, she was taken from a homeless shelter to an ED by ambulance. The mother was uncertain of the fetus’ gestational age; a 4th-year obstetric resident determined by physical examination that the pregnancy was at 36.5 weeks. The resident discussed the case with the attending ObGyn, who said to discharge the mother if her pain was gone. After 11 hours, the mother was returned to the shelter.
The mother returned to the ED 12 hours later. Thirty-five minutes after fetal distress was identified, an emergency cesarean delivery was performed. At birth, the child was found to be at 38 to 39 weeks’ gestation. He received a diagnosis of severe hypoxic ischemic encephalopathy and was transferred to a children’s hospital for brain cooling.
The child lives in a long-term care facility and is dependent on a ventilator and gastronomy tube.
PARENT’S CLAIM The mother should not have been discharged after the first visit. A cesarean delivery should have been performed at that time. The attending ObGyn never saw the mother.
DEFENDANTS’ DEFENSE The mother should have given her correct due date, which was in her prenatal records based on previous ultrasonograpy. The first discharge was proper, as the pain had improved. The homeless shelter should have called an ambulance earlier for the second admission.
VERDICT A $7.5 million California settlement was reached, plus payment of medical expenses exceeding $300,000.
Timing of child’s injury disputed
Vaginal birth after cesarean (VBAC) had been planned. After reporting to her ObGyn that she was in labor, a mother went to the ED.
During the next few hours, hospital staff called the ObGyn twice to report that fetal monitor strips indicated tachycardia. The ObGyn then spoke to the mother by phone and told her that cesarean delivery was necessary but could wait for him to get to the hospital. After the ObGyn arrived, he removed the fetal heart-rate monitor to prepare the mother’s abdomen; cesarean delivery occurred 15 minutes later.
The child has spastic dystonic quadriplegia and requires 24-hour care.
PARENT’S CLAIM The ObGyn should have come to the hospital and performed cesarean delivery when he was first notified that the fetus was tachycardic. The baby suffered an hypoxic ischemic event in the 15-minute period between when the monitor was removed and birth, causing hypoxic ischemic encephalopathy.
PHYSICIAN’S DEFENSE There was no indication of a need for earlier delivery. The brain injury occurred prior to labor and delivery.
VERDICT The hospital settled for a confidential amount before the trial. An Illinois defense verdict was returned for the ObGyn.
Were mammograms properly interpreted?
After reporting a lump in her breast, a 39-year-old woman underwent mammography in 2008 and 2009. Two different radiologists reported their findings as negative for cancer.
In 2010, the patient was found to have breast cancer. She underwent a mastectomy, chemotherapy, and radiation therapy, and was given a 75%–80% chance of 5-year survival.
PATIENT’S CLAIM The ObGyn failed to follow-up on the patient’s reports of a breast lump. The radiologists did not correctly interpret the 2008 and 2009 mammograms. If cancer had been detected earlier, treatment would have been less extreme.
PHYSICIANS’ DEFENSE The ObGyn claimed that he would have felt a lump if it was present. The first radiologist claimed that the 2008 mammography report was correct, noting that the patient’s cancer was a lobular carcinoma that does not always show on mammography or in patients with dense breasts, which this patient has.
VERDICT A directed verdict was granted to the radiologist who interpreted the 2009 mammography, as the results were lost. An Ohio defense verdict was returned for the ObGyn and the other radiologist.
Related article: Does screening mammography save lives? Janelle Yates, Senior Editor (April 2014)
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
TELL US WHAT YOU THINK! Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice.
Tell us what you think by emailing us at: [email protected] Please include your name, city and state.
Stay in touch! Your feedback is important to us!
Wealth appears to affect distribution of cancer types

Credit: Rhoda Baer
Results of a large study suggest certain malignanices are more concentrated in areas with high levels of poverty, while other cancer types arise more often in wealthy regions.
The research, conducted using data from nearly 3 million US cancer cases, also indicated that areas with higher poverty levels tended to have a lower cancer incidence but higher mortality rate than areas with lower poverty levels.
Researchers reported these findings in the journal Cancer.
Francis Boscoe, PhD, of the New York State Cancer Registry in Albany, and his colleagues conducted this research to evaluate the role of socioeconomics in cancer incidence and mortality.
So the team compared individuals living in areas with the highest poverty levels to those living in areas with the lowest poverty levels.
The researchers collected information on nearly 3 million cancer cases diagnosed between 2005 and 2009 in16 states, plus Los Angeles (an area covering 42% of the US population). Cases were divided into 1 of 4 groupings based on the poverty rate of the residential census tract at the time of diagnosis.
For all malignancies combined, there was a negligible association between cancer incidence and poverty. However, 32 of 39 cancer types showed a significant association with poverty.
Fourteen cancers were positively associated with poverty, and 18 were negatively associated. Myeloma, Hodgkin lymphoma, and non-Hodgkin lymphoma (NHL) were among the negatively associated malignancies.
The cancers most significantly associated with higher poverty levels were Kaposi sarcoma and cancers of the larynx, cervix, penis, and liver. The cancers most significantly associated with lower poverty levels were melanoma, thyroid cancer, other non-epithelial skin cancers, and testis cancer.
Overall, male malignancy rates were more sensitive to poverty level than female rates. And, in general, the race-specific cancer incidence rates differed, but the poverty gradients were similar.
For example, there was a roughly 20-fold difference in rates of melanoma between white and black individuals, but the relationship between poverty and incidence remained regardless of race.
This was not true for NHL, however. Among black individuals, the incidence of NHL increased as the poverty level increased. But among white individuals, the incidence of NHL decreased as the poverty level increased.
The researchers pointed out that—for all cancer sites, races, and sexes combined—the difference in risk between the greatest and lowest poverty category was less than 2%.
“At first glance, the effects seem to cancel one another out,” Dr Boscoe said. “But the cancers more associated with poverty have lower incidence and higher mortality, and those associated with wealth have higher incidence and lower mortality. When it comes to cancer, the poor are more likely to die of the disease, while the affluent are more likely to die with the disease.”
Dr Boscoe noted that recent gains in technology have made it much easier to link patient addresses with neighborhood characteristics, therefore making it possible to incorporate socioeconomic status into cancer surveillance.
“Our hope is that our paper will illustrate the value and necessity of doing this routinely in the future,” he concluded. ![]()

Credit: Rhoda Baer
Results of a large study suggest certain malignanices are more concentrated in areas with high levels of poverty, while other cancer types arise more often in wealthy regions.
The research, conducted using data from nearly 3 million US cancer cases, also indicated that areas with higher poverty levels tended to have a lower cancer incidence but higher mortality rate than areas with lower poverty levels.
Researchers reported these findings in the journal Cancer.
Francis Boscoe, PhD, of the New York State Cancer Registry in Albany, and his colleagues conducted this research to evaluate the role of socioeconomics in cancer incidence and mortality.
So the team compared individuals living in areas with the highest poverty levels to those living in areas with the lowest poverty levels.
The researchers collected information on nearly 3 million cancer cases diagnosed between 2005 and 2009 in16 states, plus Los Angeles (an area covering 42% of the US population). Cases were divided into 1 of 4 groupings based on the poverty rate of the residential census tract at the time of diagnosis.
For all malignancies combined, there was a negligible association between cancer incidence and poverty. However, 32 of 39 cancer types showed a significant association with poverty.
Fourteen cancers were positively associated with poverty, and 18 were negatively associated. Myeloma, Hodgkin lymphoma, and non-Hodgkin lymphoma (NHL) were among the negatively associated malignancies.
The cancers most significantly associated with higher poverty levels were Kaposi sarcoma and cancers of the larynx, cervix, penis, and liver. The cancers most significantly associated with lower poverty levels were melanoma, thyroid cancer, other non-epithelial skin cancers, and testis cancer.
Overall, male malignancy rates were more sensitive to poverty level than female rates. And, in general, the race-specific cancer incidence rates differed, but the poverty gradients were similar.
For example, there was a roughly 20-fold difference in rates of melanoma between white and black individuals, but the relationship between poverty and incidence remained regardless of race.
This was not true for NHL, however. Among black individuals, the incidence of NHL increased as the poverty level increased. But among white individuals, the incidence of NHL decreased as the poverty level increased.
The researchers pointed out that—for all cancer sites, races, and sexes combined—the difference in risk between the greatest and lowest poverty category was less than 2%.
“At first glance, the effects seem to cancel one another out,” Dr Boscoe said. “But the cancers more associated with poverty have lower incidence and higher mortality, and those associated with wealth have higher incidence and lower mortality. When it comes to cancer, the poor are more likely to die of the disease, while the affluent are more likely to die with the disease.”
Dr Boscoe noted that recent gains in technology have made it much easier to link patient addresses with neighborhood characteristics, therefore making it possible to incorporate socioeconomic status into cancer surveillance.
“Our hope is that our paper will illustrate the value and necessity of doing this routinely in the future,” he concluded. ![]()

Credit: Rhoda Baer
Results of a large study suggest certain malignanices are more concentrated in areas with high levels of poverty, while other cancer types arise more often in wealthy regions.
The research, conducted using data from nearly 3 million US cancer cases, also indicated that areas with higher poverty levels tended to have a lower cancer incidence but higher mortality rate than areas with lower poverty levels.
Researchers reported these findings in the journal Cancer.
Francis Boscoe, PhD, of the New York State Cancer Registry in Albany, and his colleagues conducted this research to evaluate the role of socioeconomics in cancer incidence and mortality.
So the team compared individuals living in areas with the highest poverty levels to those living in areas with the lowest poverty levels.
The researchers collected information on nearly 3 million cancer cases diagnosed between 2005 and 2009 in16 states, plus Los Angeles (an area covering 42% of the US population). Cases were divided into 1 of 4 groupings based on the poverty rate of the residential census tract at the time of diagnosis.
For all malignancies combined, there was a negligible association between cancer incidence and poverty. However, 32 of 39 cancer types showed a significant association with poverty.
Fourteen cancers were positively associated with poverty, and 18 were negatively associated. Myeloma, Hodgkin lymphoma, and non-Hodgkin lymphoma (NHL) were among the negatively associated malignancies.
The cancers most significantly associated with higher poverty levels were Kaposi sarcoma and cancers of the larynx, cervix, penis, and liver. The cancers most significantly associated with lower poverty levels were melanoma, thyroid cancer, other non-epithelial skin cancers, and testis cancer.
Overall, male malignancy rates were more sensitive to poverty level than female rates. And, in general, the race-specific cancer incidence rates differed, but the poverty gradients were similar.
For example, there was a roughly 20-fold difference in rates of melanoma between white and black individuals, but the relationship between poverty and incidence remained regardless of race.
This was not true for NHL, however. Among black individuals, the incidence of NHL increased as the poverty level increased. But among white individuals, the incidence of NHL decreased as the poverty level increased.
The researchers pointed out that—for all cancer sites, races, and sexes combined—the difference in risk between the greatest and lowest poverty category was less than 2%.
“At first glance, the effects seem to cancel one another out,” Dr Boscoe said. “But the cancers more associated with poverty have lower incidence and higher mortality, and those associated with wealth have higher incidence and lower mortality. When it comes to cancer, the poor are more likely to die of the disease, while the affluent are more likely to die with the disease.”
Dr Boscoe noted that recent gains in technology have made it much easier to link patient addresses with neighborhood characteristics, therefore making it possible to incorporate socioeconomic status into cancer surveillance.
“Our hope is that our paper will illustrate the value and necessity of doing this routinely in the future,” he concluded. ![]()
CHMP recommends ofatumumab for CLL

Credit: Linda Bartlett
The European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion for ofatumumab (Arzerra), a monoclonal antibody targeting CD20.
The CHMP is recommending that ofatumumab receive conditional approval for use in combination with chlorambucil or bendamustine to treat patients with chronic lymphocytic leukemia (CLL) who have not received prior therapy and are not eligible for fludarabine-based therapy.
The European Commission (EC) will take the CHMP’s opinion into account when deciding whether to approve ofatumumab for this indication.
Ofatumumab already has conditional approval in the Europe Union to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab received conditional approval because the drug’s benefits appear to outweigh the risks it poses. The drug will not receive full
approval until the companies developing ofatumumab, GlaxoSmithKline and Genmab, submit results of additional research to the EC.
The CHMP’s recommendation for expanded approval is based on results from 2 trials in patients with CLL who were ineligible for fludarabine-based treatment.
The first is a phase 2 study (OMB115991) in which researchers evaluated the efficacy of ofatumumab in combination with bendamustine. The second is the phase 3 COMPLEMENT 1 study (OMB110911), a randomized trial in which researchers compared ofatumumab and chlorambucil in combination to chlorambucil alone.
Phase 2 study
Results from this single-arm study were presented at the 2013 International Workshop on CLL. Researchers enrolled 97 patients with CLL, 44 of whom were previously untreated and 53 who had relapsed. The median ages were 62.5 and 68 years, respectively.
Treatment began with acetaminophen, an antihistamine, and a glucocorticoid. Patients then received ofatumumab at 300 mg on day 1 and 1000 mg on day 8 of cycle 1. For cycles 2 through 6, they received 1000 mg on day 1 every 28 days.
Patients received bendamustine on days 1 and 2, every 28 days for up to 6 cycles. The initial dose was 90 mg/m2 for the untreated patients and 70 mg/m2 for relapsed patients.
However, patients required a dose reduction due to toxicity. The previously untreated patients were reduced to 60 mg/m2, and the relapsed patients were reduced to 50 mg/m2.
The overall response rate was 95% in the previously untreated group and 74% in the relapsed group. Complete responses occurred in 43% and 11%, respectively.
CT results showed the overall response rate was 82% in the previously untreated group and 70% in the relapsed group. Complete responses occurred in 27% and 9%, respectively. The median time to response was 0.95 months for both groups.
Grade 3 or higher adverse events occurred in 25% of patients in the previously untreated group and 38% of those in the relapsed group.
This included infusion reactions (11% and 8%, respectively), neutropenia (16% and 29%, respectively), infections (11% and 15%, respectively), rash (2% of previously untreated patients), febrile neutropenia (4% of relapsed patients), and thrombocytopenia (4% of relapsed patients).
There were no deaths in the previously untreated group, but 4 patients died in the relapsed group. Two of these deaths may have been related to study treatment. One patient died of pneumonia and hemolytic anemia 15 days after the last dose of treatment, and 1 patient died of sepsis 4 days after the last dose of treatment.
Phase 3 study
Data from the COMPLEMENT 1 study were presented at ASH 2013. Researchers compared ofatumumab plus chlorambucil to chlorambucil alone in 447 previously untreated patients with CLL who were ineligible for fludarabine-based therapy.
Patients had a median age of 69 years (range, 35 to 92). Seventy-two percent had 2 or more comorbidities, and 48% had a creatinine clearance of less than 70 mL/min.
Patients in the ofatumumab arm received the drug at 300 mg in cycle 1 on day 1, 1000 mg in cycle 1 on day 8, and 1000 mg administered on day 1 of all subsequent 28-day cycles.
In both arms, patients received chlorambucil at a dose of 10 mg/m2 orally on days 1 to 7, every 28 days. Prior to each infusion of ofatumumab, patients received acetaminophen, an antihistamine, and a glucocorticoid.
The overall response rate was 82% in the ofatumumab-chlorambucil group and 69% in the chlorambucil-alone group (P=0.001). Complete response rates were 12% and 1%, respectively.
At a median follow-up of 29 months, the median overall survival was not reached for either treatment arm. However, ofatumumab-treated patients had longer progression-free survival than patients who received chlorambucil alone—22.4 months and 13.1 months, respectively (P<0.001).
Grade 3 or higher adverse events occurred in 50% of ofatumumab-treated patients and 43% of patients who received chlorambucil alone. The most common event was neutropenia, which occurred in 26% and 14% of patients, respectively.
Grade 3 or higher infections occurred in 15% of ofatumumab-treated patients and 14% of patients who received chlorambucil alone. The most common was pneumonia, which occurred in 4% and 3%, respectively.
In the entire cohort, grade 3 or higher infusion-related events occurred in 10% of patients, but there were no fatal infusion reactions. Two percent of subjects in both arms died during treatment. ![]()

Credit: Linda Bartlett
The European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion for ofatumumab (Arzerra), a monoclonal antibody targeting CD20.
The CHMP is recommending that ofatumumab receive conditional approval for use in combination with chlorambucil or bendamustine to treat patients with chronic lymphocytic leukemia (CLL) who have not received prior therapy and are not eligible for fludarabine-based therapy.
The European Commission (EC) will take the CHMP’s opinion into account when deciding whether to approve ofatumumab for this indication.
Ofatumumab already has conditional approval in the Europe Union to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab received conditional approval because the drug’s benefits appear to outweigh the risks it poses. The drug will not receive full
approval until the companies developing ofatumumab, GlaxoSmithKline and Genmab, submit results of additional research to the EC.
The CHMP’s recommendation for expanded approval is based on results from 2 trials in patients with CLL who were ineligible for fludarabine-based treatment.
The first is a phase 2 study (OMB115991) in which researchers evaluated the efficacy of ofatumumab in combination with bendamustine. The second is the phase 3 COMPLEMENT 1 study (OMB110911), a randomized trial in which researchers compared ofatumumab and chlorambucil in combination to chlorambucil alone.
Phase 2 study
Results from this single-arm study were presented at the 2013 International Workshop on CLL. Researchers enrolled 97 patients with CLL, 44 of whom were previously untreated and 53 who had relapsed. The median ages were 62.5 and 68 years, respectively.
Treatment began with acetaminophen, an antihistamine, and a glucocorticoid. Patients then received ofatumumab at 300 mg on day 1 and 1000 mg on day 8 of cycle 1. For cycles 2 through 6, they received 1000 mg on day 1 every 28 days.
Patients received bendamustine on days 1 and 2, every 28 days for up to 6 cycles. The initial dose was 90 mg/m2 for the untreated patients and 70 mg/m2 for relapsed patients.
However, patients required a dose reduction due to toxicity. The previously untreated patients were reduced to 60 mg/m2, and the relapsed patients were reduced to 50 mg/m2.
The overall response rate was 95% in the previously untreated group and 74% in the relapsed group. Complete responses occurred in 43% and 11%, respectively.
CT results showed the overall response rate was 82% in the previously untreated group and 70% in the relapsed group. Complete responses occurred in 27% and 9%, respectively. The median time to response was 0.95 months for both groups.
Grade 3 or higher adverse events occurred in 25% of patients in the previously untreated group and 38% of those in the relapsed group.
This included infusion reactions (11% and 8%, respectively), neutropenia (16% and 29%, respectively), infections (11% and 15%, respectively), rash (2% of previously untreated patients), febrile neutropenia (4% of relapsed patients), and thrombocytopenia (4% of relapsed patients).
There were no deaths in the previously untreated group, but 4 patients died in the relapsed group. Two of these deaths may have been related to study treatment. One patient died of pneumonia and hemolytic anemia 15 days after the last dose of treatment, and 1 patient died of sepsis 4 days after the last dose of treatment.
Phase 3 study
Data from the COMPLEMENT 1 study were presented at ASH 2013. Researchers compared ofatumumab plus chlorambucil to chlorambucil alone in 447 previously untreated patients with CLL who were ineligible for fludarabine-based therapy.
Patients had a median age of 69 years (range, 35 to 92). Seventy-two percent had 2 or more comorbidities, and 48% had a creatinine clearance of less than 70 mL/min.
Patients in the ofatumumab arm received the drug at 300 mg in cycle 1 on day 1, 1000 mg in cycle 1 on day 8, and 1000 mg administered on day 1 of all subsequent 28-day cycles.
In both arms, patients received chlorambucil at a dose of 10 mg/m2 orally on days 1 to 7, every 28 days. Prior to each infusion of ofatumumab, patients received acetaminophen, an antihistamine, and a glucocorticoid.
The overall response rate was 82% in the ofatumumab-chlorambucil group and 69% in the chlorambucil-alone group (P=0.001). Complete response rates were 12% and 1%, respectively.
At a median follow-up of 29 months, the median overall survival was not reached for either treatment arm. However, ofatumumab-treated patients had longer progression-free survival than patients who received chlorambucil alone—22.4 months and 13.1 months, respectively (P<0.001).
Grade 3 or higher adverse events occurred in 50% of ofatumumab-treated patients and 43% of patients who received chlorambucil alone. The most common event was neutropenia, which occurred in 26% and 14% of patients, respectively.
Grade 3 or higher infections occurred in 15% of ofatumumab-treated patients and 14% of patients who received chlorambucil alone. The most common was pneumonia, which occurred in 4% and 3%, respectively.
In the entire cohort, grade 3 or higher infusion-related events occurred in 10% of patients, but there were no fatal infusion reactions. Two percent of subjects in both arms died during treatment. ![]()

Credit: Linda Bartlett
The European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion for ofatumumab (Arzerra), a monoclonal antibody targeting CD20.
The CHMP is recommending that ofatumumab receive conditional approval for use in combination with chlorambucil or bendamustine to treat patients with chronic lymphocytic leukemia (CLL) who have not received prior therapy and are not eligible for fludarabine-based therapy.
The European Commission (EC) will take the CHMP’s opinion into account when deciding whether to approve ofatumumab for this indication.
Ofatumumab already has conditional approval in the Europe Union to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Ofatumumab received conditional approval because the drug’s benefits appear to outweigh the risks it poses. The drug will not receive full
approval until the companies developing ofatumumab, GlaxoSmithKline and Genmab, submit results of additional research to the EC.
The CHMP’s recommendation for expanded approval is based on results from 2 trials in patients with CLL who were ineligible for fludarabine-based treatment.
The first is a phase 2 study (OMB115991) in which researchers evaluated the efficacy of ofatumumab in combination with bendamustine. The second is the phase 3 COMPLEMENT 1 study (OMB110911), a randomized trial in which researchers compared ofatumumab and chlorambucil in combination to chlorambucil alone.
Phase 2 study
Results from this single-arm study were presented at the 2013 International Workshop on CLL. Researchers enrolled 97 patients with CLL, 44 of whom were previously untreated and 53 who had relapsed. The median ages were 62.5 and 68 years, respectively.
Treatment began with acetaminophen, an antihistamine, and a glucocorticoid. Patients then received ofatumumab at 300 mg on day 1 and 1000 mg on day 8 of cycle 1. For cycles 2 through 6, they received 1000 mg on day 1 every 28 days.
Patients received bendamustine on days 1 and 2, every 28 days for up to 6 cycles. The initial dose was 90 mg/m2 for the untreated patients and 70 mg/m2 for relapsed patients.
However, patients required a dose reduction due to toxicity. The previously untreated patients were reduced to 60 mg/m2, and the relapsed patients were reduced to 50 mg/m2.
The overall response rate was 95% in the previously untreated group and 74% in the relapsed group. Complete responses occurred in 43% and 11%, respectively.
CT results showed the overall response rate was 82% in the previously untreated group and 70% in the relapsed group. Complete responses occurred in 27% and 9%, respectively. The median time to response was 0.95 months for both groups.
Grade 3 or higher adverse events occurred in 25% of patients in the previously untreated group and 38% of those in the relapsed group.
This included infusion reactions (11% and 8%, respectively), neutropenia (16% and 29%, respectively), infections (11% and 15%, respectively), rash (2% of previously untreated patients), febrile neutropenia (4% of relapsed patients), and thrombocytopenia (4% of relapsed patients).
There were no deaths in the previously untreated group, but 4 patients died in the relapsed group. Two of these deaths may have been related to study treatment. One patient died of pneumonia and hemolytic anemia 15 days after the last dose of treatment, and 1 patient died of sepsis 4 days after the last dose of treatment.
Phase 3 study
Data from the COMPLEMENT 1 study were presented at ASH 2013. Researchers compared ofatumumab plus chlorambucil to chlorambucil alone in 447 previously untreated patients with CLL who were ineligible for fludarabine-based therapy.
Patients had a median age of 69 years (range, 35 to 92). Seventy-two percent had 2 or more comorbidities, and 48% had a creatinine clearance of less than 70 mL/min.
Patients in the ofatumumab arm received the drug at 300 mg in cycle 1 on day 1, 1000 mg in cycle 1 on day 8, and 1000 mg administered on day 1 of all subsequent 28-day cycles.
In both arms, patients received chlorambucil at a dose of 10 mg/m2 orally on days 1 to 7, every 28 days. Prior to each infusion of ofatumumab, patients received acetaminophen, an antihistamine, and a glucocorticoid.
The overall response rate was 82% in the ofatumumab-chlorambucil group and 69% in the chlorambucil-alone group (P=0.001). Complete response rates were 12% and 1%, respectively.
At a median follow-up of 29 months, the median overall survival was not reached for either treatment arm. However, ofatumumab-treated patients had longer progression-free survival than patients who received chlorambucil alone—22.4 months and 13.1 months, respectively (P<0.001).
Grade 3 or higher adverse events occurred in 50% of ofatumumab-treated patients and 43% of patients who received chlorambucil alone. The most common event was neutropenia, which occurred in 26% and 14% of patients, respectively.
Grade 3 or higher infections occurred in 15% of ofatumumab-treated patients and 14% of patients who received chlorambucil alone. The most common was pneumonia, which occurred in 4% and 3%, respectively.
In the entire cohort, grade 3 or higher infusion-related events occurred in 10% of patients, but there were no fatal infusion reactions. Two percent of subjects in both arms died during treatment. ![]()
Development and Validation of TAISCH
Patient satisfaction scores are being reported publicly and will affect hospital reimbursement rates under Hospital Value Based Purchasing.[1] Patient satisfaction scores are currently obtained through metrics such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS)[2] and Press Ganey (PG)[3] surveys. Such surveys are mailed to a variable proportion of patients following their discharge from the hospital, and ask patients about the quality of care they received during their admission. Domains assessed regarding the patients' inpatient experiences range from room cleanliness to the amount of time the physician spent with them.
The Society of Hospital Medicine (SHM), the largest professional medical society representing hospitalists, encourages the use of patient satisfaction surveys to measure hospitalist providers' quality of patient care.[4] Ideally, accurate information would be delivered as feedback to individual providers in a timely manner in hopes of improving performance; however, the current methodology has shortcomings that limit its usefulness. First, several hospitalists and consultants may be involved in the care of 1 patient during the hospital stay, but the score can only be tied to a single physician. Current survey methods attribute all responses to that particular doctor, usually the attending of record, although patients may very well be thinking of other physicians when responding to questions. Second, only a few questions on the surveys ask about doctors' performance. Aforementioned surveys have 3 to 8 questions about doctors' care, which limits the ability to assess physician performance comprehensively. Finally, the surveys are mailed approximately 1 week after the patient's discharge, usually without a name or photograph of the physician to facilitate patient/caregiver recall. This time lag and lack of information to prompt patient recall likely lead to impreciseness in assessment. In addition, the response rates to these surveys are typically low, around 25% (personal oral communication with our division's service excellence stakeholder Dr. L.P. in September 2013). These deficiencies limit the usefulness of such data in coaching individual providers about their performance because they cannot be delivered in a timely fashion, and the reliability of the attribution is suspect.
With these considerations in mind, we developed and validated a new survey metric, the Tool to Assess Inpatient Satisfaction with Care from Hospitalists (TAISCH). We hypothesized that the results would be different from those collected using conventional methodologies.
PATIENTS AND METHODS
Study Design and Subjects
Our cross‐sectional study surveyed inpatients under the care of hospitalist physicians working without the support of trainees or allied health professionals (such as nurse practitioners or physician assistants). The subjects were hospitalized at a 560‐bed academic medical center on a general medical floor between September 2012 and December 2012. All participating hospitalist physicians were members of a division of hospital medicine.
TAISCH Development
Several steps were taken to establish content validity evidence.[5] We developed TAISCH by building upon the theoretical underpinnings of the quality of care measures that are endorsed by the SHM Membership Committee Guidelines for Hospitalists Patient Satisfaction.[4] This directive recommends that patient satisfaction with hospitalist care should be assessed across 6 domains: physician availability, physician concern for patients, physician communication skills, physician courteousness, physician clinical skills, and physician involvement of patients' families. Other existing validated measures tied to the quality of patient care were reviewed, and items related to the physician's care were considered for inclusion to further substantiate content validity.[6, 7, 8, 9, 10, 11, 12] Input from colleagues with expertise in clinical excellence and service excellence was also solicited. This included the director of Hopkins' Miller Coulson Academy of Clinical Excellence and the grant review committee members of the Johns Hopkins Osler Center for Clinical Excellence (who funded this study).[13, 14]
The preliminary instrument contained 17 items, including 2 conditional questions, and was first pilot tested on 5 hospitalized patients. We assessed the time it took to administer the surveys as well as patients' comments and questions about each survey item. This resulted in minor wording changes for clarification and changes in the order of the questions. We then pursued a second phase of piloting using the revised survey, which was administered to >20 patients. There were no further adjustments as patients reported that TAISCH was clear and concise.
From interviews with patients after pilot testing, it became clear that respondents were carefully reflecting on the quality of care and performance of their treating physician, thereby generating response process validity evidence.[5]
Data Collection
To ensure that patients had perspective upon which to base their assessment, they were only asked to appraise physicians after being cared for by the same hospitalist provider for at least 2 consecutive days. Patients who were on isolation, those who were non‐English speaking, and those with impaired decision‐making capacity (such as mental status change or dementia) were excluded. Patients were enrolled only if they could correctly name their doctor or at least identify a photograph of their hospitalist provider on a page that included pictures of all division members. Those patients who were able to name the provider or correctly select the provider from the page of photographs were considered to have correctly identified their provider. In order to ensure the confidentiality of the patients and their responses, all data collections were performed by a trained research assistant who had no patient‐care responsibilities. The survey was confidential, did not include any patient identifiers, and patients were assured that providers would never see their individual responses. The patients were given options to complete TAISCH either by verbally responding to the research assistant's questions, filling out the paper survey, or completing the survey online using an iPad at the bedside. TAISCH specifically asked the patients to rate their hospitalist provider's performance along several domains: communication skills, clinical skills, availability, empathy, courteousness, and discharge planning; 5‐point Likert scales were used exclusively.
In addition to the TAISCH questions, we asked patients (1) an overall satisfaction question, I would recommend Dr. X to my loved ones should he or she need hospitalization in the future (response options: strongly disagree, disagree, neutral, agree, strongly agree), (2) their pain level using the Wong‐Baker pain scale,[15] and (3) the Jefferson Scale of Patient's Perceptions of Physician Empathy (JSPPPE).[16, 17] Associations between TAISCH and these variables (as well as PG data) would be examined to ascertain relations to other variables validity evidence.[5] Specifically, we sought to ascertain discriminant and convergent validity where the TAISCH is associated positively with constructs where we expect positive associations (convergent) and negatively with those we expect negative associations (discriminant).[18] The Wong‐Baker pain scale is a recommended pain‐assessment tool by the Joint Commission on Accreditation of Healthcare Organizations, and is widely used in hospitals and various healthcare settings.[19] The scale has a range from 0 to 10 (0 for no pain and 10 indicating the worst pain). The hypothesis was that the patients' pain levels would adversely affect their perception of the physician's performance (discriminant validity). JSPPPE is a 5‐item validated scale developed to measure patients' perceptions of their physicians' empathic engagement. It has significant correlations with the American Board of Internal Medicine's patient rating surveys, and it is used in standardized patient examinations for medical students.[20] The hypothesis was that patient perception about the quality of physician care would correlate positively with their assessment of the physician's empathy (convergent validity).
Although all of the hospitalist providers in the division consented to participate in this study, only hospitalist providers for whom at least 4 patient surveys were collected were included in the analysis. The study was approved by our institutional review board.
Data Analysis
All data were analyzed using Stata 11 (StataCorp, College Station, TX). Data were analyzed to determine the potential for a single comprehensive assessment of physician performance with confirmatory factor analysis (CFA) using maximum likelihood extraction. Additional factor analyses examined the potential for a multiple factor solution using exploratory factor analysis (EFA) with principle component factor analysis and varimax rotation. Examination of scree plots, factor loadings for individual items greater than 0.40, eigenvalues greater than 1.0, and substantive meaning of the factors were all taken into consideration when determining the number of factors to retain from factor analytic models.[21] Cronbach's s were calculated for each factor to assess reliability. These data provided internal structure validity evidence (demonstrated by acceptable reliability and factor structure) to TAISCH.[5]
After arriving at the final TAISCH scale, composite TAISCH scores were computed. Associations between composite TAISCH scores with the Wong‐Baker pain scale, the JSPPPE, and the overall satisfaction question were assessed using linear regression with the svy command in Stata to account for the nested design of having each patient report on a single hospitalist provider. Correlation between composite TAISCH score and PG physician care scores (comprised of 5 questions: time physician spent with you, physician concern with questions/worries, physician kept you informed, friendliness/courtesy of physician, and skill of physician) were assessed at the provider level when both data were available.
RESULTS
A total of 330 patients were considered to be eligible through medical record screening. Of those patients, 73 (22%) were already discharged by the time the research assistant attempted to enroll them after 2 days of care by a single physician. Of 257 inpatients approached, 30 patients (12%) refused to participate. Among the 227 consented patients, 24 (9%) were excluded as they were unable to correctly identify their hospitalist provider. A total of 203 patients were enrolled, and each patient rated a single hospitalist; a total of 29 unique hospitalists were assessed by these patients. The patients' mean age was 60 years, 114 (56%) were female, and 61 (30%) were of nonwhite race (Table 1). The hospitalist physicians' demographic information is also shown in Table 1. Two hospitalists with fewer than 4 surveys collected were excluded from the analysis. Thus, final analysis included 200 unique patients assessing 1 of the 27 hospitalists (mean=7.4 surveys per hospitalist).
| Characteristics | Value |
|---|---|
| |
| Patients, N=203 | |
| Age, y, mean (SD) | 60.0 (17.2) |
| Female, n (%) | 114 (56.1) |
| Nonwhite race, n (%) | 61 (30.5) |
| Observation stay, n (%) | 45 (22.1) |
| How are you feeling today? n (%) | |
| Very poor | 11 (5.5) |
| Poor | 14 (7.0) |
| Fair | 67 (33.5) |
| Good | 71 (35.5) |
| Very good | 33 (16.5) |
| Excellent | 4 (2.0) |
| Hospitalists, N=29 | |
| Age, n (%) | |
| 2630 years | 7 (24.1) |
| 3135 years | 8 (27.6) |
| 3640 years | 12 (41.4) |
| 4145 years | 2 (6.9) |
| Female, n (%) | 11 (37.9) |
| International medical graduate, n (%) | 18 (62.1) |
| Years in current practice, n (%) | |
| <1 | 9 (31.0) |
| 12 | 7 (24.1) |
| 34 | 6 (20.7) |
| 56 | 5 (17.2) |
| 7 or more | 2 (6.9) |
| Race, n (%) | |
| Caucasian | 4 (13.8) |
| Asian | 19 (65.5) |
| African/African American | 5 (17.2) |
| Other | 1 (3.4) |
| Academic rank, n (%) | |
| Assistant professor | 9 (31.0) |
| Clinical instructor | 10 (34.5) |
| Clinical associate/nonfaculty | 10 (34.5) |
| Percentage of clinical effort, n (%) | |
| >70% | 6 (20.7) |
| 50%70% | 19 (65.5) |
| <50% | 4 (13.8) |
Validation of TAISCH
On the 17‐item TAISCH administered, the 2 conditional questions (When I asked to see Dr. X, s/he came within a reasonable amount of time. and If Dr. X interacted with your family, how well did s/he deal with them?) were applicable to fewer than 40% of patients. As such, they were not included in the analysis.
Internal Structure Validity Evidence
Results from factor analyses are shown in Table 2. The CFA modeling of a single factor solution with 15 items explained 42% of the total variance. The 27 hospitalists' average 15‐item TAISCH score ranged from 3.25 to 4.28 (mean [standard deviation]=3.82 [0.24]; possible score range: 15). Reliability of the 15‐item TAISCH was appropriate (Cronbach's =0.88).
| TAISCH (Cronbach's =0.88) | Factor Loading |
|---|---|
| |
| Compared to all other physicians that you know, how do you rate Dr. X's compassion, empathy, and concern for you?* | 0.91 |
| Compared to all other physicians that you know, how do you rate Dr. X's ability to communicate with you?* | 0.88 |
| Compared to all other physicians that you know, how do you rate Dr. X's skill in diagnosing and treating your medical conditions?* | 0.88 |
| Compared to all other physicians that you know, how do you rate Dr. X's fund of knowledge?* | 0.80 |
| How much confidence do you have in Dr. X's plan for your care? | 0.71 |
| Dr. X kept me informed of the plans for my care. | 0.69 |
| Effectively preparing patients for discharge is an important part of what doctors in the hospital do. How well has Dr. X done in getting you ready to be discharged from the hospital? | 0.67 |
| Dr. X let me talk without interrupting. | 0.60 |
| Dr. X encouraged me to ask questions. | 0.59 |
| Dr. X checks to be sure I understood everything. | 0.55 |
| I sensed Dr. X was in a rush when s/he was with me. (reverse coded) | 0.55 |
| Dr. X showed interest in my views and opinions about my health. | 0.54 |
| Dr. X discusses options with me and involves me in decision making. | 0.47 |
| Dr. X asked permission to enter the room and waited for an answer. | 0.25 |
| Dr. X sat down when s/he visited my bedside. | 0.14 |
As shown in Table 2, 2 variables had factor loadings below the minimum threshold of 0.40 in the CFA for the 15‐item TAISCH when modeling a single factor solution. Both items were related to physician etiquette: Dr. X asked permission to enter the room and waited for an answer. and Dr. X sat down when he/she visited my bedside.
When CFA was executed again, as a single factor omitting the 2 items that demonstrated lower factor loadings, the 13‐item single factor solution explained 47% of the total variance, and the Cronbach's was 0.92.
EFA models were also explored for potential alternate solutions. These analyses resulted in lesser reliability (low Cronbach's ), weak construct operationalization, and poor face validity (as judged by the research team).
Both the 13‐ and 15‐item single factor solutions were examined further to determine whether associations with criterion variables (pain, empathy) differed substantively. Given that results were similar across both solutions, subsequent analyses were completed with the 15‐item single factor solution, which included the etiquette‐related variables.
Relationship to Other Variables Validity Evidence
The association between the 15‐item TAISCH and JSPPPE was significantly positive (=12.2, P<0.001). Additionally, there was a positive and significant association between TAISCH and the overall satisfaction question: I would recommend Dr. X to my loved ones should they need hospitalization in the future. (=11.2, P<0.001). This overall satisfaction question was also associated positively with JSPPPE (=13.2, P<0.001). There was a statistically significant negative association between TAISCH and Wong‐Baker pain scale (=2.42, P<0.05).
The PG data from the same period were available for 24 out of 27 hospitalists. The number of PG surveys collected per provider ranged from 5 to 30 (mean=14). At the provider level, there was not a statistically significant correlation between PG and the 15‐item TAISCH (P=0.51). Of note, PG was also not significantly correlated with the overall satisfaction question, JSPPPE, or the Wong‐Baker pain scale (all P>0.10).
DISCUSSION
Our new metric, TAISCH, was found to be a reliable and valid measurement tool to assess patient satisfaction with the hospitalist physician's care. Because we only surveyed patients who could correctly identify their hospitalist physicians after interacting for at least 2 consecutive days, the attribution of the data to the individual hospitalist is almost certainly correct. The high participation rate indicates that the patients were not hesitant about rating their hospitalist provider's quality of care, even when asked while they were still in the hospital.
The majority of the patients approached were able to correctly identify their hospitalist provider. This rate (91%) was much higher than the rate previously reported in the literature where a picture card was used to improve provider recognition.[22] It is also likely that 1 physician, rather than a team of physicians, taking care of patients make it easier for patients to recall the name and recognize the face of their inpatient provider.
The CFA of TAISCH showed good fit but suggests that 2 variables, both from Kahn's etiquette‐based medicine (EtBM) checklist,[9] may not load in the same way as the other items. Tackett and colleagues reported that hospitalists who performed more EtBM behaviors scored higher on PG evaluations.[23] Such results, along with the comparable explanation of variance and reliability, convinced us to retain these 2 items in the final 15‐item TAISCH as dictated by the CFA. Although the literature supports the fact that physician etiquette is related to perception of high‐quality care, it is possible that these 2 questions were answered differently (and thereby failed to load the same way), because environmental limitations may be preventing physicians' ability to perform them consistently. We prefer the 15‐item version of TAISCH and future studies may provide additional information about its performance as compared to the 13‐item adaptation.
The significantly negative association between the Wong‐Baker pain scale and TAISCH stresses the importance of adequately addressing and treating the patient's pain. Hanna et al. showed that the patients' perceptions of pain control was associated with their overall satisfaction score measured by HCAHPS.[24] The association seen in our study was not unexpected, because TAISCH is administered while the patients are acutely ill in the hospital, when pain is likely more prevalent and severe than it is during the postdischarge settings (when the HCAHPS or PG surveys are administered). Interestingly, Hanna et al. discovered that the team's attention to controlling pain was more strongly correlated with overall satisfaction than was the actual pain control.[24] These data, now confirmed by our study, should serve to remind us that a hospitalist's concern and effort to relieve pain may augment patient satisfaction with the quality of care, even when eliminating the pain may be difficult or impossible.
TAISCH was found not to be correlated with PG scores. Several explanations for this deserve consideration. First, the postdischarge PG survey that is used for our institution does not list the name of the specific hospitalist providers for the patients to evaluate. Because patients encounter multiple physicians during their hospital stay (eg, emergency department physicians, hospitalist providers, consultants), it is possible that patients are not reflecting on the named doctor when assessing the the attending of record on the PG mailed questionnaire. Second, the representation of patients who responded to TAISCH and PG were different; almost all patients completed TAISCH as opposed to a small minority who decide to respond to the PG survey. Third, TAISCH measures the physicians' performance more comprehensively with a larger number of variables. Last, it is possible that we were underpowered to detect significant correlation, because there were only 24 providers who had data from both TAISCH and PG. However, our results endorse using caution in interpreting PG scores for individual hospitalist's performance, particularly for high‐stakes consequences (including the provision of incentives to high performer and the insistence on remediation for low performers).
Several limitations of this study should be considered. First, only hospitalist providers from a single division were assessed. This may limit the generalizability of our findings. Second, although patients were assured about confidentiality of their responses, they might have provided more favorable answers, because they may have felt uncomfortable rating their physician poorly. One review article of the measurement of healthcare satisfaction indicated that impersonal (mailed) methods result in more criticism and lower satisfaction than assessments made in person using interviews. As the trade‐off, the mailed surveys yield lower response rates that may introduce other forms of bias.[25] Even on the HCHAPS survey report for the same period from our institution, 78% of patients gave top box ratings for our doctors' communication skills, which is at the state average.[26] Similarly, a study that used postdischarge telephone interviews to collect patients' satisfaction with hospitalists' care quality reported an average score of 4.20 out of 5.[27] These findings confirm that highly skewed ratings are common for these types of surveys, irrespective of how or when the data are collected.
Despite the aforementioned limitations, TAISCH use need not be limited to hospitalist physicians. It may also be used to assess allied health professionals or trainees performance, which cannot be assessed by HCHAPS or PG. Applying TAISCH in different hospital settings (eg, emergency department or critical care units), assessing hospitalists' reactions to TAISCH, learning whether TAISCH leads to hospitalists' behavior changes or appraising whether performance can improve in response to coaching interventions for those performing poorly are all research questions that merit additional consideration.
CONCLUSION
TAISCH allows for obtaining patient satisfaction data that are highly attributable to specific hospitalist providers. The data collection method also permits high response rates so that input comes from almost all patients. The timeliness of the TAISCH assessments also makes it possible for real‐time service recovery, which is impossible with other commonly used metrics assessing patient satisfaction. Our next step will include testing the most effective way to provide feedback to providers and to coach these individuals so as to improve performance.
Acknowledgements
The authors would like to thank Po‐Han Chen at the BEAD Core for his statistical analysis support.
Disclosures: This study was supported by the Johns Hopkins Osler Center for Clinical Excellence. Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. The authors report no conflicts of interest.
- , . Hospital value‐based purchasing. J Hosp Med. 2013;8:271–277.
- HCAHPS survey. Hospital Consumer Assessment of Healthcare Providers and Systems website. Available at: http://www.hcahpsonline.org/home.aspx. Accessed August 27, 2011.
- Press Ganey survey. Press Ganey website. Available at: http://www.pressganey.com/index.aspx. Accessed February 12, 2013.
- Society of Hospital Medicine. Membership Committee Guidelines for Hospitalists Patient Satisfaction Surveys. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources119:166.e7–e16.
- , , . Measuring patient views of physician communication skills: development and testing of the Communication Assessment Tool. Patient Educ Couns. 2007;67:333–342.
- , , . The Picker Patient Experience Questionnaire: development and validation using data from in‐patient surveys in five countries. Int J Qual Health Care. 2002;14:353–358.
- The Patient Satisfaction Questionnaire from RAND Health. RAND Health website. Available at: http://www.rand.org/health/surveys_tools/psq.html. Accessed December 30, 2011.
- . Etiquette‐based medicine. N Engl J Med. 2008;358:1988–1989.
- , , , . Defining clinical excellence in academic medicine: a qualitative study of the master clinicians. Mayo Clin Proc. 2008;83:989–994.
- , , , , . Creating an academy of clinical excellence at Johns Hopkins Bayview Medical Center: a 3‐year experience. Acad Med. 2010;85:1833–1839.
- , , , , . Patients' perspectives on ideal physician behaviors. Mayo Clin Proc. 2006;81(3):338–344.
- The Miller‐Coulson Academy of Clinical Excellence at Johns Hopkins. Available at: http://www.hopkinsmedicine.org/innovative/signature_programs/academy_of_clinical_excellence/. Accessed April 25, 2014.
- Osler Center for Clinical Excellence at Johns Hopkins. Available at: http://www.hopkinsmedicine.org/johns_hopkins_bayview/education_training/continuing_education/osler_center_for_clinical_excellence. Accessed April 25, 2014.
- Wong‐Baker FACES Foundation. Available at: http://www.wongbakerfaces.org. Accessed July 8, 2013.
- , , , , . Jefferson Scale of Patient's Perceptions of Physician Empathy: preliminary psychometric data. Croat Med J. 2007;48:81–86.
- , , , , . Relationships between scores on the Jefferson Scale of Physician Empathy, patient perceptions of physician empathy, and humanistic approaches to patient care: a validity study. Med Sci Monit. 2007;13(7):CR291–CR294.
- , . Convergent and discriminant validation by the multitrait‐multimethod matrix. Psychol Bul. 1959;56(2):81–105.
- The Joint Commission. Facts about pain management. Available at: http://www.jointcommission.org/pain_management. Accessed April 25, 2014.
- , , , et al. Medical students' self‐reported empathy and simulated patients' assessments of student empathy: an analysis by gender and ethnicity. Acad Med. 2011;86(8):984–988.
- . Factor Analysis. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Pateint Saf. 1009;35(12):613–619.
- , , et al. Appraising the practice of etiquette‐based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908–913.
- , , , et al. Does patient perception of pain control affect patient satisfaction across surgical units in a tertiary teaching hospital? Am J Med Qual. 2012;27:411–416.
- , , , et al. The measurement of satisfaction with health care: implications for practice from a systematic review of the literature. Health Technol Assess. 2002;6(32):1–244.
- Centers for Medicare 7(2):131–136.
Patient satisfaction scores are being reported publicly and will affect hospital reimbursement rates under Hospital Value Based Purchasing.[1] Patient satisfaction scores are currently obtained through metrics such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS)[2] and Press Ganey (PG)[3] surveys. Such surveys are mailed to a variable proportion of patients following their discharge from the hospital, and ask patients about the quality of care they received during their admission. Domains assessed regarding the patients' inpatient experiences range from room cleanliness to the amount of time the physician spent with them.
The Society of Hospital Medicine (SHM), the largest professional medical society representing hospitalists, encourages the use of patient satisfaction surveys to measure hospitalist providers' quality of patient care.[4] Ideally, accurate information would be delivered as feedback to individual providers in a timely manner in hopes of improving performance; however, the current methodology has shortcomings that limit its usefulness. First, several hospitalists and consultants may be involved in the care of 1 patient during the hospital stay, but the score can only be tied to a single physician. Current survey methods attribute all responses to that particular doctor, usually the attending of record, although patients may very well be thinking of other physicians when responding to questions. Second, only a few questions on the surveys ask about doctors' performance. Aforementioned surveys have 3 to 8 questions about doctors' care, which limits the ability to assess physician performance comprehensively. Finally, the surveys are mailed approximately 1 week after the patient's discharge, usually without a name or photograph of the physician to facilitate patient/caregiver recall. This time lag and lack of information to prompt patient recall likely lead to impreciseness in assessment. In addition, the response rates to these surveys are typically low, around 25% (personal oral communication with our division's service excellence stakeholder Dr. L.P. in September 2013). These deficiencies limit the usefulness of such data in coaching individual providers about their performance because they cannot be delivered in a timely fashion, and the reliability of the attribution is suspect.
With these considerations in mind, we developed and validated a new survey metric, the Tool to Assess Inpatient Satisfaction with Care from Hospitalists (TAISCH). We hypothesized that the results would be different from those collected using conventional methodologies.
PATIENTS AND METHODS
Study Design and Subjects
Our cross‐sectional study surveyed inpatients under the care of hospitalist physicians working without the support of trainees or allied health professionals (such as nurse practitioners or physician assistants). The subjects were hospitalized at a 560‐bed academic medical center on a general medical floor between September 2012 and December 2012. All participating hospitalist physicians were members of a division of hospital medicine.
TAISCH Development
Several steps were taken to establish content validity evidence.[5] We developed TAISCH by building upon the theoretical underpinnings of the quality of care measures that are endorsed by the SHM Membership Committee Guidelines for Hospitalists Patient Satisfaction.[4] This directive recommends that patient satisfaction with hospitalist care should be assessed across 6 domains: physician availability, physician concern for patients, physician communication skills, physician courteousness, physician clinical skills, and physician involvement of patients' families. Other existing validated measures tied to the quality of patient care were reviewed, and items related to the physician's care were considered for inclusion to further substantiate content validity.[6, 7, 8, 9, 10, 11, 12] Input from colleagues with expertise in clinical excellence and service excellence was also solicited. This included the director of Hopkins' Miller Coulson Academy of Clinical Excellence and the grant review committee members of the Johns Hopkins Osler Center for Clinical Excellence (who funded this study).[13, 14]
The preliminary instrument contained 17 items, including 2 conditional questions, and was first pilot tested on 5 hospitalized patients. We assessed the time it took to administer the surveys as well as patients' comments and questions about each survey item. This resulted in minor wording changes for clarification and changes in the order of the questions. We then pursued a second phase of piloting using the revised survey, which was administered to >20 patients. There were no further adjustments as patients reported that TAISCH was clear and concise.
From interviews with patients after pilot testing, it became clear that respondents were carefully reflecting on the quality of care and performance of their treating physician, thereby generating response process validity evidence.[5]
Data Collection
To ensure that patients had perspective upon which to base their assessment, they were only asked to appraise physicians after being cared for by the same hospitalist provider for at least 2 consecutive days. Patients who were on isolation, those who were non‐English speaking, and those with impaired decision‐making capacity (such as mental status change or dementia) were excluded. Patients were enrolled only if they could correctly name their doctor or at least identify a photograph of their hospitalist provider on a page that included pictures of all division members. Those patients who were able to name the provider or correctly select the provider from the page of photographs were considered to have correctly identified their provider. In order to ensure the confidentiality of the patients and their responses, all data collections were performed by a trained research assistant who had no patient‐care responsibilities. The survey was confidential, did not include any patient identifiers, and patients were assured that providers would never see their individual responses. The patients were given options to complete TAISCH either by verbally responding to the research assistant's questions, filling out the paper survey, or completing the survey online using an iPad at the bedside. TAISCH specifically asked the patients to rate their hospitalist provider's performance along several domains: communication skills, clinical skills, availability, empathy, courteousness, and discharge planning; 5‐point Likert scales were used exclusively.
In addition to the TAISCH questions, we asked patients (1) an overall satisfaction question, I would recommend Dr. X to my loved ones should he or she need hospitalization in the future (response options: strongly disagree, disagree, neutral, agree, strongly agree), (2) their pain level using the Wong‐Baker pain scale,[15] and (3) the Jefferson Scale of Patient's Perceptions of Physician Empathy (JSPPPE).[16, 17] Associations between TAISCH and these variables (as well as PG data) would be examined to ascertain relations to other variables validity evidence.[5] Specifically, we sought to ascertain discriminant and convergent validity where the TAISCH is associated positively with constructs where we expect positive associations (convergent) and negatively with those we expect negative associations (discriminant).[18] The Wong‐Baker pain scale is a recommended pain‐assessment tool by the Joint Commission on Accreditation of Healthcare Organizations, and is widely used in hospitals and various healthcare settings.[19] The scale has a range from 0 to 10 (0 for no pain and 10 indicating the worst pain). The hypothesis was that the patients' pain levels would adversely affect their perception of the physician's performance (discriminant validity). JSPPPE is a 5‐item validated scale developed to measure patients' perceptions of their physicians' empathic engagement. It has significant correlations with the American Board of Internal Medicine's patient rating surveys, and it is used in standardized patient examinations for medical students.[20] The hypothesis was that patient perception about the quality of physician care would correlate positively with their assessment of the physician's empathy (convergent validity).
Although all of the hospitalist providers in the division consented to participate in this study, only hospitalist providers for whom at least 4 patient surveys were collected were included in the analysis. The study was approved by our institutional review board.
Data Analysis
All data were analyzed using Stata 11 (StataCorp, College Station, TX). Data were analyzed to determine the potential for a single comprehensive assessment of physician performance with confirmatory factor analysis (CFA) using maximum likelihood extraction. Additional factor analyses examined the potential for a multiple factor solution using exploratory factor analysis (EFA) with principle component factor analysis and varimax rotation. Examination of scree plots, factor loadings for individual items greater than 0.40, eigenvalues greater than 1.0, and substantive meaning of the factors were all taken into consideration when determining the number of factors to retain from factor analytic models.[21] Cronbach's s were calculated for each factor to assess reliability. These data provided internal structure validity evidence (demonstrated by acceptable reliability and factor structure) to TAISCH.[5]
After arriving at the final TAISCH scale, composite TAISCH scores were computed. Associations between composite TAISCH scores with the Wong‐Baker pain scale, the JSPPPE, and the overall satisfaction question were assessed using linear regression with the svy command in Stata to account for the nested design of having each patient report on a single hospitalist provider. Correlation between composite TAISCH score and PG physician care scores (comprised of 5 questions: time physician spent with you, physician concern with questions/worries, physician kept you informed, friendliness/courtesy of physician, and skill of physician) were assessed at the provider level when both data were available.
RESULTS
A total of 330 patients were considered to be eligible through medical record screening. Of those patients, 73 (22%) were already discharged by the time the research assistant attempted to enroll them after 2 days of care by a single physician. Of 257 inpatients approached, 30 patients (12%) refused to participate. Among the 227 consented patients, 24 (9%) were excluded as they were unable to correctly identify their hospitalist provider. A total of 203 patients were enrolled, and each patient rated a single hospitalist; a total of 29 unique hospitalists were assessed by these patients. The patients' mean age was 60 years, 114 (56%) were female, and 61 (30%) were of nonwhite race (Table 1). The hospitalist physicians' demographic information is also shown in Table 1. Two hospitalists with fewer than 4 surveys collected were excluded from the analysis. Thus, final analysis included 200 unique patients assessing 1 of the 27 hospitalists (mean=7.4 surveys per hospitalist).
| Characteristics | Value |
|---|---|
| |
| Patients, N=203 | |
| Age, y, mean (SD) | 60.0 (17.2) |
| Female, n (%) | 114 (56.1) |
| Nonwhite race, n (%) | 61 (30.5) |
| Observation stay, n (%) | 45 (22.1) |
| How are you feeling today? n (%) | |
| Very poor | 11 (5.5) |
| Poor | 14 (7.0) |
| Fair | 67 (33.5) |
| Good | 71 (35.5) |
| Very good | 33 (16.5) |
| Excellent | 4 (2.0) |
| Hospitalists, N=29 | |
| Age, n (%) | |
| 2630 years | 7 (24.1) |
| 3135 years | 8 (27.6) |
| 3640 years | 12 (41.4) |
| 4145 years | 2 (6.9) |
| Female, n (%) | 11 (37.9) |
| International medical graduate, n (%) | 18 (62.1) |
| Years in current practice, n (%) | |
| <1 | 9 (31.0) |
| 12 | 7 (24.1) |
| 34 | 6 (20.7) |
| 56 | 5 (17.2) |
| 7 or more | 2 (6.9) |
| Race, n (%) | |
| Caucasian | 4 (13.8) |
| Asian | 19 (65.5) |
| African/African American | 5 (17.2) |
| Other | 1 (3.4) |
| Academic rank, n (%) | |
| Assistant professor | 9 (31.0) |
| Clinical instructor | 10 (34.5) |
| Clinical associate/nonfaculty | 10 (34.5) |
| Percentage of clinical effort, n (%) | |
| >70% | 6 (20.7) |
| 50%70% | 19 (65.5) |
| <50% | 4 (13.8) |
Validation of TAISCH
On the 17‐item TAISCH administered, the 2 conditional questions (When I asked to see Dr. X, s/he came within a reasonable amount of time. and If Dr. X interacted with your family, how well did s/he deal with them?) were applicable to fewer than 40% of patients. As such, they were not included in the analysis.
Internal Structure Validity Evidence
Results from factor analyses are shown in Table 2. The CFA modeling of a single factor solution with 15 items explained 42% of the total variance. The 27 hospitalists' average 15‐item TAISCH score ranged from 3.25 to 4.28 (mean [standard deviation]=3.82 [0.24]; possible score range: 15). Reliability of the 15‐item TAISCH was appropriate (Cronbach's =0.88).
| TAISCH (Cronbach's =0.88) | Factor Loading |
|---|---|
| |
| Compared to all other physicians that you know, how do you rate Dr. X's compassion, empathy, and concern for you?* | 0.91 |
| Compared to all other physicians that you know, how do you rate Dr. X's ability to communicate with you?* | 0.88 |
| Compared to all other physicians that you know, how do you rate Dr. X's skill in diagnosing and treating your medical conditions?* | 0.88 |
| Compared to all other physicians that you know, how do you rate Dr. X's fund of knowledge?* | 0.80 |
| How much confidence do you have in Dr. X's plan for your care? | 0.71 |
| Dr. X kept me informed of the plans for my care. | 0.69 |
| Effectively preparing patients for discharge is an important part of what doctors in the hospital do. How well has Dr. X done in getting you ready to be discharged from the hospital? | 0.67 |
| Dr. X let me talk without interrupting. | 0.60 |
| Dr. X encouraged me to ask questions. | 0.59 |
| Dr. X checks to be sure I understood everything. | 0.55 |
| I sensed Dr. X was in a rush when s/he was with me. (reverse coded) | 0.55 |
| Dr. X showed interest in my views and opinions about my health. | 0.54 |
| Dr. X discusses options with me and involves me in decision making. | 0.47 |
| Dr. X asked permission to enter the room and waited for an answer. | 0.25 |
| Dr. X sat down when s/he visited my bedside. | 0.14 |
As shown in Table 2, 2 variables had factor loadings below the minimum threshold of 0.40 in the CFA for the 15‐item TAISCH when modeling a single factor solution. Both items were related to physician etiquette: Dr. X asked permission to enter the room and waited for an answer. and Dr. X sat down when he/she visited my bedside.
When CFA was executed again, as a single factor omitting the 2 items that demonstrated lower factor loadings, the 13‐item single factor solution explained 47% of the total variance, and the Cronbach's was 0.92.
EFA models were also explored for potential alternate solutions. These analyses resulted in lesser reliability (low Cronbach's ), weak construct operationalization, and poor face validity (as judged by the research team).
Both the 13‐ and 15‐item single factor solutions were examined further to determine whether associations with criterion variables (pain, empathy) differed substantively. Given that results were similar across both solutions, subsequent analyses were completed with the 15‐item single factor solution, which included the etiquette‐related variables.
Relationship to Other Variables Validity Evidence
The association between the 15‐item TAISCH and JSPPPE was significantly positive (=12.2, P<0.001). Additionally, there was a positive and significant association between TAISCH and the overall satisfaction question: I would recommend Dr. X to my loved ones should they need hospitalization in the future. (=11.2, P<0.001). This overall satisfaction question was also associated positively with JSPPPE (=13.2, P<0.001). There was a statistically significant negative association between TAISCH and Wong‐Baker pain scale (=2.42, P<0.05).
The PG data from the same period were available for 24 out of 27 hospitalists. The number of PG surveys collected per provider ranged from 5 to 30 (mean=14). At the provider level, there was not a statistically significant correlation between PG and the 15‐item TAISCH (P=0.51). Of note, PG was also not significantly correlated with the overall satisfaction question, JSPPPE, or the Wong‐Baker pain scale (all P>0.10).
DISCUSSION
Our new metric, TAISCH, was found to be a reliable and valid measurement tool to assess patient satisfaction with the hospitalist physician's care. Because we only surveyed patients who could correctly identify their hospitalist physicians after interacting for at least 2 consecutive days, the attribution of the data to the individual hospitalist is almost certainly correct. The high participation rate indicates that the patients were not hesitant about rating their hospitalist provider's quality of care, even when asked while they were still in the hospital.
The majority of the patients approached were able to correctly identify their hospitalist provider. This rate (91%) was much higher than the rate previously reported in the literature where a picture card was used to improve provider recognition.[22] It is also likely that 1 physician, rather than a team of physicians, taking care of patients make it easier for patients to recall the name and recognize the face of their inpatient provider.
The CFA of TAISCH showed good fit but suggests that 2 variables, both from Kahn's etiquette‐based medicine (EtBM) checklist,[9] may not load in the same way as the other items. Tackett and colleagues reported that hospitalists who performed more EtBM behaviors scored higher on PG evaluations.[23] Such results, along with the comparable explanation of variance and reliability, convinced us to retain these 2 items in the final 15‐item TAISCH as dictated by the CFA. Although the literature supports the fact that physician etiquette is related to perception of high‐quality care, it is possible that these 2 questions were answered differently (and thereby failed to load the same way), because environmental limitations may be preventing physicians' ability to perform them consistently. We prefer the 15‐item version of TAISCH and future studies may provide additional information about its performance as compared to the 13‐item adaptation.
The significantly negative association between the Wong‐Baker pain scale and TAISCH stresses the importance of adequately addressing and treating the patient's pain. Hanna et al. showed that the patients' perceptions of pain control was associated with their overall satisfaction score measured by HCAHPS.[24] The association seen in our study was not unexpected, because TAISCH is administered while the patients are acutely ill in the hospital, when pain is likely more prevalent and severe than it is during the postdischarge settings (when the HCAHPS or PG surveys are administered). Interestingly, Hanna et al. discovered that the team's attention to controlling pain was more strongly correlated with overall satisfaction than was the actual pain control.[24] These data, now confirmed by our study, should serve to remind us that a hospitalist's concern and effort to relieve pain may augment patient satisfaction with the quality of care, even when eliminating the pain may be difficult or impossible.
TAISCH was found not to be correlated with PG scores. Several explanations for this deserve consideration. First, the postdischarge PG survey that is used for our institution does not list the name of the specific hospitalist providers for the patients to evaluate. Because patients encounter multiple physicians during their hospital stay (eg, emergency department physicians, hospitalist providers, consultants), it is possible that patients are not reflecting on the named doctor when assessing the the attending of record on the PG mailed questionnaire. Second, the representation of patients who responded to TAISCH and PG were different; almost all patients completed TAISCH as opposed to a small minority who decide to respond to the PG survey. Third, TAISCH measures the physicians' performance more comprehensively with a larger number of variables. Last, it is possible that we were underpowered to detect significant correlation, because there were only 24 providers who had data from both TAISCH and PG. However, our results endorse using caution in interpreting PG scores for individual hospitalist's performance, particularly for high‐stakes consequences (including the provision of incentives to high performer and the insistence on remediation for low performers).
Several limitations of this study should be considered. First, only hospitalist providers from a single division were assessed. This may limit the generalizability of our findings. Second, although patients were assured about confidentiality of their responses, they might have provided more favorable answers, because they may have felt uncomfortable rating their physician poorly. One review article of the measurement of healthcare satisfaction indicated that impersonal (mailed) methods result in more criticism and lower satisfaction than assessments made in person using interviews. As the trade‐off, the mailed surveys yield lower response rates that may introduce other forms of bias.[25] Even on the HCHAPS survey report for the same period from our institution, 78% of patients gave top box ratings for our doctors' communication skills, which is at the state average.[26] Similarly, a study that used postdischarge telephone interviews to collect patients' satisfaction with hospitalists' care quality reported an average score of 4.20 out of 5.[27] These findings confirm that highly skewed ratings are common for these types of surveys, irrespective of how or when the data are collected.
Despite the aforementioned limitations, TAISCH use need not be limited to hospitalist physicians. It may also be used to assess allied health professionals or trainees performance, which cannot be assessed by HCHAPS or PG. Applying TAISCH in different hospital settings (eg, emergency department or critical care units), assessing hospitalists' reactions to TAISCH, learning whether TAISCH leads to hospitalists' behavior changes or appraising whether performance can improve in response to coaching interventions for those performing poorly are all research questions that merit additional consideration.
CONCLUSION
TAISCH allows for obtaining patient satisfaction data that are highly attributable to specific hospitalist providers. The data collection method also permits high response rates so that input comes from almost all patients. The timeliness of the TAISCH assessments also makes it possible for real‐time service recovery, which is impossible with other commonly used metrics assessing patient satisfaction. Our next step will include testing the most effective way to provide feedback to providers and to coach these individuals so as to improve performance.
Acknowledgements
The authors would like to thank Po‐Han Chen at the BEAD Core for his statistical analysis support.
Disclosures: This study was supported by the Johns Hopkins Osler Center for Clinical Excellence. Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. The authors report no conflicts of interest.
Patient satisfaction scores are being reported publicly and will affect hospital reimbursement rates under Hospital Value Based Purchasing.[1] Patient satisfaction scores are currently obtained through metrics such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS)[2] and Press Ganey (PG)[3] surveys. Such surveys are mailed to a variable proportion of patients following their discharge from the hospital, and ask patients about the quality of care they received during their admission. Domains assessed regarding the patients' inpatient experiences range from room cleanliness to the amount of time the physician spent with them.
The Society of Hospital Medicine (SHM), the largest professional medical society representing hospitalists, encourages the use of patient satisfaction surveys to measure hospitalist providers' quality of patient care.[4] Ideally, accurate information would be delivered as feedback to individual providers in a timely manner in hopes of improving performance; however, the current methodology has shortcomings that limit its usefulness. First, several hospitalists and consultants may be involved in the care of 1 patient during the hospital stay, but the score can only be tied to a single physician. Current survey methods attribute all responses to that particular doctor, usually the attending of record, although patients may very well be thinking of other physicians when responding to questions. Second, only a few questions on the surveys ask about doctors' performance. Aforementioned surveys have 3 to 8 questions about doctors' care, which limits the ability to assess physician performance comprehensively. Finally, the surveys are mailed approximately 1 week after the patient's discharge, usually without a name or photograph of the physician to facilitate patient/caregiver recall. This time lag and lack of information to prompt patient recall likely lead to impreciseness in assessment. In addition, the response rates to these surveys are typically low, around 25% (personal oral communication with our division's service excellence stakeholder Dr. L.P. in September 2013). These deficiencies limit the usefulness of such data in coaching individual providers about their performance because they cannot be delivered in a timely fashion, and the reliability of the attribution is suspect.
With these considerations in mind, we developed and validated a new survey metric, the Tool to Assess Inpatient Satisfaction with Care from Hospitalists (TAISCH). We hypothesized that the results would be different from those collected using conventional methodologies.
PATIENTS AND METHODS
Study Design and Subjects
Our cross‐sectional study surveyed inpatients under the care of hospitalist physicians working without the support of trainees or allied health professionals (such as nurse practitioners or physician assistants). The subjects were hospitalized at a 560‐bed academic medical center on a general medical floor between September 2012 and December 2012. All participating hospitalist physicians were members of a division of hospital medicine.
TAISCH Development
Several steps were taken to establish content validity evidence.[5] We developed TAISCH by building upon the theoretical underpinnings of the quality of care measures that are endorsed by the SHM Membership Committee Guidelines for Hospitalists Patient Satisfaction.[4] This directive recommends that patient satisfaction with hospitalist care should be assessed across 6 domains: physician availability, physician concern for patients, physician communication skills, physician courteousness, physician clinical skills, and physician involvement of patients' families. Other existing validated measures tied to the quality of patient care were reviewed, and items related to the physician's care were considered for inclusion to further substantiate content validity.[6, 7, 8, 9, 10, 11, 12] Input from colleagues with expertise in clinical excellence and service excellence was also solicited. This included the director of Hopkins' Miller Coulson Academy of Clinical Excellence and the grant review committee members of the Johns Hopkins Osler Center for Clinical Excellence (who funded this study).[13, 14]
The preliminary instrument contained 17 items, including 2 conditional questions, and was first pilot tested on 5 hospitalized patients. We assessed the time it took to administer the surveys as well as patients' comments and questions about each survey item. This resulted in minor wording changes for clarification and changes in the order of the questions. We then pursued a second phase of piloting using the revised survey, which was administered to >20 patients. There were no further adjustments as patients reported that TAISCH was clear and concise.
From interviews with patients after pilot testing, it became clear that respondents were carefully reflecting on the quality of care and performance of their treating physician, thereby generating response process validity evidence.[5]
Data Collection
To ensure that patients had perspective upon which to base their assessment, they were only asked to appraise physicians after being cared for by the same hospitalist provider for at least 2 consecutive days. Patients who were on isolation, those who were non‐English speaking, and those with impaired decision‐making capacity (such as mental status change or dementia) were excluded. Patients were enrolled only if they could correctly name their doctor or at least identify a photograph of their hospitalist provider on a page that included pictures of all division members. Those patients who were able to name the provider or correctly select the provider from the page of photographs were considered to have correctly identified their provider. In order to ensure the confidentiality of the patients and their responses, all data collections were performed by a trained research assistant who had no patient‐care responsibilities. The survey was confidential, did not include any patient identifiers, and patients were assured that providers would never see their individual responses. The patients were given options to complete TAISCH either by verbally responding to the research assistant's questions, filling out the paper survey, or completing the survey online using an iPad at the bedside. TAISCH specifically asked the patients to rate their hospitalist provider's performance along several domains: communication skills, clinical skills, availability, empathy, courteousness, and discharge planning; 5‐point Likert scales were used exclusively.
In addition to the TAISCH questions, we asked patients (1) an overall satisfaction question, I would recommend Dr. X to my loved ones should he or she need hospitalization in the future (response options: strongly disagree, disagree, neutral, agree, strongly agree), (2) their pain level using the Wong‐Baker pain scale,[15] and (3) the Jefferson Scale of Patient's Perceptions of Physician Empathy (JSPPPE).[16, 17] Associations between TAISCH and these variables (as well as PG data) would be examined to ascertain relations to other variables validity evidence.[5] Specifically, we sought to ascertain discriminant and convergent validity where the TAISCH is associated positively with constructs where we expect positive associations (convergent) and negatively with those we expect negative associations (discriminant).[18] The Wong‐Baker pain scale is a recommended pain‐assessment tool by the Joint Commission on Accreditation of Healthcare Organizations, and is widely used in hospitals and various healthcare settings.[19] The scale has a range from 0 to 10 (0 for no pain and 10 indicating the worst pain). The hypothesis was that the patients' pain levels would adversely affect their perception of the physician's performance (discriminant validity). JSPPPE is a 5‐item validated scale developed to measure patients' perceptions of their physicians' empathic engagement. It has significant correlations with the American Board of Internal Medicine's patient rating surveys, and it is used in standardized patient examinations for medical students.[20] The hypothesis was that patient perception about the quality of physician care would correlate positively with their assessment of the physician's empathy (convergent validity).
Although all of the hospitalist providers in the division consented to participate in this study, only hospitalist providers for whom at least 4 patient surveys were collected were included in the analysis. The study was approved by our institutional review board.
Data Analysis
All data were analyzed using Stata 11 (StataCorp, College Station, TX). Data were analyzed to determine the potential for a single comprehensive assessment of physician performance with confirmatory factor analysis (CFA) using maximum likelihood extraction. Additional factor analyses examined the potential for a multiple factor solution using exploratory factor analysis (EFA) with principle component factor analysis and varimax rotation. Examination of scree plots, factor loadings for individual items greater than 0.40, eigenvalues greater than 1.0, and substantive meaning of the factors were all taken into consideration when determining the number of factors to retain from factor analytic models.[21] Cronbach's s were calculated for each factor to assess reliability. These data provided internal structure validity evidence (demonstrated by acceptable reliability and factor structure) to TAISCH.[5]
After arriving at the final TAISCH scale, composite TAISCH scores were computed. Associations between composite TAISCH scores with the Wong‐Baker pain scale, the JSPPPE, and the overall satisfaction question were assessed using linear regression with the svy command in Stata to account for the nested design of having each patient report on a single hospitalist provider. Correlation between composite TAISCH score and PG physician care scores (comprised of 5 questions: time physician spent with you, physician concern with questions/worries, physician kept you informed, friendliness/courtesy of physician, and skill of physician) were assessed at the provider level when both data were available.
RESULTS
A total of 330 patients were considered to be eligible through medical record screening. Of those patients, 73 (22%) were already discharged by the time the research assistant attempted to enroll them after 2 days of care by a single physician. Of 257 inpatients approached, 30 patients (12%) refused to participate. Among the 227 consented patients, 24 (9%) were excluded as they were unable to correctly identify their hospitalist provider. A total of 203 patients were enrolled, and each patient rated a single hospitalist; a total of 29 unique hospitalists were assessed by these patients. The patients' mean age was 60 years, 114 (56%) were female, and 61 (30%) were of nonwhite race (Table 1). The hospitalist physicians' demographic information is also shown in Table 1. Two hospitalists with fewer than 4 surveys collected were excluded from the analysis. Thus, final analysis included 200 unique patients assessing 1 of the 27 hospitalists (mean=7.4 surveys per hospitalist).
| Characteristics | Value |
|---|---|
| |
| Patients, N=203 | |
| Age, y, mean (SD) | 60.0 (17.2) |
| Female, n (%) | 114 (56.1) |
| Nonwhite race, n (%) | 61 (30.5) |
| Observation stay, n (%) | 45 (22.1) |
| How are you feeling today? n (%) | |
| Very poor | 11 (5.5) |
| Poor | 14 (7.0) |
| Fair | 67 (33.5) |
| Good | 71 (35.5) |
| Very good | 33 (16.5) |
| Excellent | 4 (2.0) |
| Hospitalists, N=29 | |
| Age, n (%) | |
| 2630 years | 7 (24.1) |
| 3135 years | 8 (27.6) |
| 3640 years | 12 (41.4) |
| 4145 years | 2 (6.9) |
| Female, n (%) | 11 (37.9) |
| International medical graduate, n (%) | 18 (62.1) |
| Years in current practice, n (%) | |
| <1 | 9 (31.0) |
| 12 | 7 (24.1) |
| 34 | 6 (20.7) |
| 56 | 5 (17.2) |
| 7 or more | 2 (6.9) |
| Race, n (%) | |
| Caucasian | 4 (13.8) |
| Asian | 19 (65.5) |
| African/African American | 5 (17.2) |
| Other | 1 (3.4) |
| Academic rank, n (%) | |
| Assistant professor | 9 (31.0) |
| Clinical instructor | 10 (34.5) |
| Clinical associate/nonfaculty | 10 (34.5) |
| Percentage of clinical effort, n (%) | |
| >70% | 6 (20.7) |
| 50%70% | 19 (65.5) |
| <50% | 4 (13.8) |
Validation of TAISCH
On the 17‐item TAISCH administered, the 2 conditional questions (When I asked to see Dr. X, s/he came within a reasonable amount of time. and If Dr. X interacted with your family, how well did s/he deal with them?) were applicable to fewer than 40% of patients. As such, they were not included in the analysis.
Internal Structure Validity Evidence
Results from factor analyses are shown in Table 2. The CFA modeling of a single factor solution with 15 items explained 42% of the total variance. The 27 hospitalists' average 15‐item TAISCH score ranged from 3.25 to 4.28 (mean [standard deviation]=3.82 [0.24]; possible score range: 15). Reliability of the 15‐item TAISCH was appropriate (Cronbach's =0.88).
| TAISCH (Cronbach's =0.88) | Factor Loading |
|---|---|
| |
| Compared to all other physicians that you know, how do you rate Dr. X's compassion, empathy, and concern for you?* | 0.91 |
| Compared to all other physicians that you know, how do you rate Dr. X's ability to communicate with you?* | 0.88 |
| Compared to all other physicians that you know, how do you rate Dr. X's skill in diagnosing and treating your medical conditions?* | 0.88 |
| Compared to all other physicians that you know, how do you rate Dr. X's fund of knowledge?* | 0.80 |
| How much confidence do you have in Dr. X's plan for your care? | 0.71 |
| Dr. X kept me informed of the plans for my care. | 0.69 |
| Effectively preparing patients for discharge is an important part of what doctors in the hospital do. How well has Dr. X done in getting you ready to be discharged from the hospital? | 0.67 |
| Dr. X let me talk without interrupting. | 0.60 |
| Dr. X encouraged me to ask questions. | 0.59 |
| Dr. X checks to be sure I understood everything. | 0.55 |
| I sensed Dr. X was in a rush when s/he was with me. (reverse coded) | 0.55 |
| Dr. X showed interest in my views and opinions about my health. | 0.54 |
| Dr. X discusses options with me and involves me in decision making. | 0.47 |
| Dr. X asked permission to enter the room and waited for an answer. | 0.25 |
| Dr. X sat down when s/he visited my bedside. | 0.14 |
As shown in Table 2, 2 variables had factor loadings below the minimum threshold of 0.40 in the CFA for the 15‐item TAISCH when modeling a single factor solution. Both items were related to physician etiquette: Dr. X asked permission to enter the room and waited for an answer. and Dr. X sat down when he/she visited my bedside.
When CFA was executed again, as a single factor omitting the 2 items that demonstrated lower factor loadings, the 13‐item single factor solution explained 47% of the total variance, and the Cronbach's was 0.92.
EFA models were also explored for potential alternate solutions. These analyses resulted in lesser reliability (low Cronbach's ), weak construct operationalization, and poor face validity (as judged by the research team).
Both the 13‐ and 15‐item single factor solutions were examined further to determine whether associations with criterion variables (pain, empathy) differed substantively. Given that results were similar across both solutions, subsequent analyses were completed with the 15‐item single factor solution, which included the etiquette‐related variables.
Relationship to Other Variables Validity Evidence
The association between the 15‐item TAISCH and JSPPPE was significantly positive (=12.2, P<0.001). Additionally, there was a positive and significant association between TAISCH and the overall satisfaction question: I would recommend Dr. X to my loved ones should they need hospitalization in the future. (=11.2, P<0.001). This overall satisfaction question was also associated positively with JSPPPE (=13.2, P<0.001). There was a statistically significant negative association between TAISCH and Wong‐Baker pain scale (=2.42, P<0.05).
The PG data from the same period were available for 24 out of 27 hospitalists. The number of PG surveys collected per provider ranged from 5 to 30 (mean=14). At the provider level, there was not a statistically significant correlation between PG and the 15‐item TAISCH (P=0.51). Of note, PG was also not significantly correlated with the overall satisfaction question, JSPPPE, or the Wong‐Baker pain scale (all P>0.10).
DISCUSSION
Our new metric, TAISCH, was found to be a reliable and valid measurement tool to assess patient satisfaction with the hospitalist physician's care. Because we only surveyed patients who could correctly identify their hospitalist physicians after interacting for at least 2 consecutive days, the attribution of the data to the individual hospitalist is almost certainly correct. The high participation rate indicates that the patients were not hesitant about rating their hospitalist provider's quality of care, even when asked while they were still in the hospital.
The majority of the patients approached were able to correctly identify their hospitalist provider. This rate (91%) was much higher than the rate previously reported in the literature where a picture card was used to improve provider recognition.[22] It is also likely that 1 physician, rather than a team of physicians, taking care of patients make it easier for patients to recall the name and recognize the face of their inpatient provider.
The CFA of TAISCH showed good fit but suggests that 2 variables, both from Kahn's etiquette‐based medicine (EtBM) checklist,[9] may not load in the same way as the other items. Tackett and colleagues reported that hospitalists who performed more EtBM behaviors scored higher on PG evaluations.[23] Such results, along with the comparable explanation of variance and reliability, convinced us to retain these 2 items in the final 15‐item TAISCH as dictated by the CFA. Although the literature supports the fact that physician etiquette is related to perception of high‐quality care, it is possible that these 2 questions were answered differently (and thereby failed to load the same way), because environmental limitations may be preventing physicians' ability to perform them consistently. We prefer the 15‐item version of TAISCH and future studies may provide additional information about its performance as compared to the 13‐item adaptation.
The significantly negative association between the Wong‐Baker pain scale and TAISCH stresses the importance of adequately addressing and treating the patient's pain. Hanna et al. showed that the patients' perceptions of pain control was associated with their overall satisfaction score measured by HCAHPS.[24] The association seen in our study was not unexpected, because TAISCH is administered while the patients are acutely ill in the hospital, when pain is likely more prevalent and severe than it is during the postdischarge settings (when the HCAHPS or PG surveys are administered). Interestingly, Hanna et al. discovered that the team's attention to controlling pain was more strongly correlated with overall satisfaction than was the actual pain control.[24] These data, now confirmed by our study, should serve to remind us that a hospitalist's concern and effort to relieve pain may augment patient satisfaction with the quality of care, even when eliminating the pain may be difficult or impossible.
TAISCH was found not to be correlated with PG scores. Several explanations for this deserve consideration. First, the postdischarge PG survey that is used for our institution does not list the name of the specific hospitalist providers for the patients to evaluate. Because patients encounter multiple physicians during their hospital stay (eg, emergency department physicians, hospitalist providers, consultants), it is possible that patients are not reflecting on the named doctor when assessing the the attending of record on the PG mailed questionnaire. Second, the representation of patients who responded to TAISCH and PG were different; almost all patients completed TAISCH as opposed to a small minority who decide to respond to the PG survey. Third, TAISCH measures the physicians' performance more comprehensively with a larger number of variables. Last, it is possible that we were underpowered to detect significant correlation, because there were only 24 providers who had data from both TAISCH and PG. However, our results endorse using caution in interpreting PG scores for individual hospitalist's performance, particularly for high‐stakes consequences (including the provision of incentives to high performer and the insistence on remediation for low performers).
Several limitations of this study should be considered. First, only hospitalist providers from a single division were assessed. This may limit the generalizability of our findings. Second, although patients were assured about confidentiality of their responses, they might have provided more favorable answers, because they may have felt uncomfortable rating their physician poorly. One review article of the measurement of healthcare satisfaction indicated that impersonal (mailed) methods result in more criticism and lower satisfaction than assessments made in person using interviews. As the trade‐off, the mailed surveys yield lower response rates that may introduce other forms of bias.[25] Even on the HCHAPS survey report for the same period from our institution, 78% of patients gave top box ratings for our doctors' communication skills, which is at the state average.[26] Similarly, a study that used postdischarge telephone interviews to collect patients' satisfaction with hospitalists' care quality reported an average score of 4.20 out of 5.[27] These findings confirm that highly skewed ratings are common for these types of surveys, irrespective of how or when the data are collected.
Despite the aforementioned limitations, TAISCH use need not be limited to hospitalist physicians. It may also be used to assess allied health professionals or trainees performance, which cannot be assessed by HCHAPS or PG. Applying TAISCH in different hospital settings (eg, emergency department or critical care units), assessing hospitalists' reactions to TAISCH, learning whether TAISCH leads to hospitalists' behavior changes or appraising whether performance can improve in response to coaching interventions for those performing poorly are all research questions that merit additional consideration.
CONCLUSION
TAISCH allows for obtaining patient satisfaction data that are highly attributable to specific hospitalist providers. The data collection method also permits high response rates so that input comes from almost all patients. The timeliness of the TAISCH assessments also makes it possible for real‐time service recovery, which is impossible with other commonly used metrics assessing patient satisfaction. Our next step will include testing the most effective way to provide feedback to providers and to coach these individuals so as to improve performance.
Acknowledgements
The authors would like to thank Po‐Han Chen at the BEAD Core for his statistical analysis support.
Disclosures: This study was supported by the Johns Hopkins Osler Center for Clinical Excellence. Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. The authors report no conflicts of interest.
- , . Hospital value‐based purchasing. J Hosp Med. 2013;8:271–277.
- HCAHPS survey. Hospital Consumer Assessment of Healthcare Providers and Systems website. Available at: http://www.hcahpsonline.org/home.aspx. Accessed August 27, 2011.
- Press Ganey survey. Press Ganey website. Available at: http://www.pressganey.com/index.aspx. Accessed February 12, 2013.
- Society of Hospital Medicine. Membership Committee Guidelines for Hospitalists Patient Satisfaction Surveys. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources119:166.e7–e16.
- , , . Measuring patient views of physician communication skills: development and testing of the Communication Assessment Tool. Patient Educ Couns. 2007;67:333–342.
- , , . The Picker Patient Experience Questionnaire: development and validation using data from in‐patient surveys in five countries. Int J Qual Health Care. 2002;14:353–358.
- The Patient Satisfaction Questionnaire from RAND Health. RAND Health website. Available at: http://www.rand.org/health/surveys_tools/psq.html. Accessed December 30, 2011.
- . Etiquette‐based medicine. N Engl J Med. 2008;358:1988–1989.
- , , , . Defining clinical excellence in academic medicine: a qualitative study of the master clinicians. Mayo Clin Proc. 2008;83:989–994.
- , , , , . Creating an academy of clinical excellence at Johns Hopkins Bayview Medical Center: a 3‐year experience. Acad Med. 2010;85:1833–1839.
- , , , , . Patients' perspectives on ideal physician behaviors. Mayo Clin Proc. 2006;81(3):338–344.
- The Miller‐Coulson Academy of Clinical Excellence at Johns Hopkins. Available at: http://www.hopkinsmedicine.org/innovative/signature_programs/academy_of_clinical_excellence/. Accessed April 25, 2014.
- Osler Center for Clinical Excellence at Johns Hopkins. Available at: http://www.hopkinsmedicine.org/johns_hopkins_bayview/education_training/continuing_education/osler_center_for_clinical_excellence. Accessed April 25, 2014.
- Wong‐Baker FACES Foundation. Available at: http://www.wongbakerfaces.org. Accessed July 8, 2013.
- , , , , . Jefferson Scale of Patient's Perceptions of Physician Empathy: preliminary psychometric data. Croat Med J. 2007;48:81–86.
- , , , , . Relationships between scores on the Jefferson Scale of Physician Empathy, patient perceptions of physician empathy, and humanistic approaches to patient care: a validity study. Med Sci Monit. 2007;13(7):CR291–CR294.
- , . Convergent and discriminant validation by the multitrait‐multimethod matrix. Psychol Bul. 1959;56(2):81–105.
- The Joint Commission. Facts about pain management. Available at: http://www.jointcommission.org/pain_management. Accessed April 25, 2014.
- , , , et al. Medical students' self‐reported empathy and simulated patients' assessments of student empathy: an analysis by gender and ethnicity. Acad Med. 2011;86(8):984–988.
- . Factor Analysis. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Pateint Saf. 1009;35(12):613–619.
- , , et al. Appraising the practice of etiquette‐based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908–913.
- , , , et al. Does patient perception of pain control affect patient satisfaction across surgical units in a tertiary teaching hospital? Am J Med Qual. 2012;27:411–416.
- , , , et al. The measurement of satisfaction with health care: implications for practice from a systematic review of the literature. Health Technol Assess. 2002;6(32):1–244.
- Centers for Medicare 7(2):131–136.
- , . Hospital value‐based purchasing. J Hosp Med. 2013;8:271–277.
- HCAHPS survey. Hospital Consumer Assessment of Healthcare Providers and Systems website. Available at: http://www.hcahpsonline.org/home.aspx. Accessed August 27, 2011.
- Press Ganey survey. Press Ganey website. Available at: http://www.pressganey.com/index.aspx. Accessed February 12, 2013.
- Society of Hospital Medicine. Membership Committee Guidelines for Hospitalists Patient Satisfaction Surveys. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources119:166.e7–e16.
- , , . Measuring patient views of physician communication skills: development and testing of the Communication Assessment Tool. Patient Educ Couns. 2007;67:333–342.
- , , . The Picker Patient Experience Questionnaire: development and validation using data from in‐patient surveys in five countries. Int J Qual Health Care. 2002;14:353–358.
- The Patient Satisfaction Questionnaire from RAND Health. RAND Health website. Available at: http://www.rand.org/health/surveys_tools/psq.html. Accessed December 30, 2011.
- . Etiquette‐based medicine. N Engl J Med. 2008;358:1988–1989.
- , , , . Defining clinical excellence in academic medicine: a qualitative study of the master clinicians. Mayo Clin Proc. 2008;83:989–994.
- , , , , . Creating an academy of clinical excellence at Johns Hopkins Bayview Medical Center: a 3‐year experience. Acad Med. 2010;85:1833–1839.
- , , , , . Patients' perspectives on ideal physician behaviors. Mayo Clin Proc. 2006;81(3):338–344.
- The Miller‐Coulson Academy of Clinical Excellence at Johns Hopkins. Available at: http://www.hopkinsmedicine.org/innovative/signature_programs/academy_of_clinical_excellence/. Accessed April 25, 2014.
- Osler Center for Clinical Excellence at Johns Hopkins. Available at: http://www.hopkinsmedicine.org/johns_hopkins_bayview/education_training/continuing_education/osler_center_for_clinical_excellence. Accessed April 25, 2014.
- Wong‐Baker FACES Foundation. Available at: http://www.wongbakerfaces.org. Accessed July 8, 2013.
- , , , , . Jefferson Scale of Patient's Perceptions of Physician Empathy: preliminary psychometric data. Croat Med J. 2007;48:81–86.
- , , , , . Relationships between scores on the Jefferson Scale of Physician Empathy, patient perceptions of physician empathy, and humanistic approaches to patient care: a validity study. Med Sci Monit. 2007;13(7):CR291–CR294.
- , . Convergent and discriminant validation by the multitrait‐multimethod matrix. Psychol Bul. 1959;56(2):81–105.
- The Joint Commission. Facts about pain management. Available at: http://www.jointcommission.org/pain_management. Accessed April 25, 2014.
- , , , et al. Medical students' self‐reported empathy and simulated patients' assessments of student empathy: an analysis by gender and ethnicity. Acad Med. 2011;86(8):984–988.
- . Factor Analysis. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Pateint Saf. 1009;35(12):613–619.
- , , et al. Appraising the practice of etiquette‐based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908–913.
- , , , et al. Does patient perception of pain control affect patient satisfaction across surgical units in a tertiary teaching hospital? Am J Med Qual. 2012;27:411–416.
- , , , et al. The measurement of satisfaction with health care: implications for practice from a systematic review of the literature. Health Technol Assess. 2002;6(32):1–244.
- Centers for Medicare 7(2):131–136.
© 2014 Society of Hospital Medicine
Guideline updates for non-Hodgkin lymphomas
At the 2014 National Comprehensive Cancer Network annual conference in Hollywood, Florida, Dr Andrew D Zelenetz, chair of the NCCN Non-Hodgkin Lymphomas Guidelines panel, presented guideline updates for non-Hodgkin lymphomas.
Click on the PDF icon at the top of this introduction to read the full article.
At the 2014 National Comprehensive Cancer Network annual conference in Hollywood, Florida, Dr Andrew D Zelenetz, chair of the NCCN Non-Hodgkin Lymphomas Guidelines panel, presented guideline updates for non-Hodgkin lymphomas.
Click on the PDF icon at the top of this introduction to read the full article.
At the 2014 National Comprehensive Cancer Network annual conference in Hollywood, Florida, Dr Andrew D Zelenetz, chair of the NCCN Non-Hodgkin Lymphomas Guidelines panel, presented guideline updates for non-Hodgkin lymphomas.
Click on the PDF icon at the top of this introduction to read the full article.
The importance of hematologic, cytogenetic, and molecular testing and mutational analysis in chronic myeloid leukemia
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) for treatment of chronic myeloid leukemia (CML) has made it possible for this cancer to be controlled in many patients for long periods with chronic medication and regular monitoring of disease status. Hematologic and cytogenetic testing, molecular monitoring, and BCR-ABL1 mutational analysis have become integral to the routine management of CML. The information that each type of test provides is essential to confirm a diagnosis, determine the disease stage, assess response to treatment, and monitor for signals of disease progression – all of which can be used to identify patients who might require further evaluation, closer follow-up, and additional intervention, and to guide clinical decisions.
Click on the PDF icon at the top of this introduction to read the full article.
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) for treatment of chronic myeloid leukemia (CML) has made it possible for this cancer to be controlled in many patients for long periods with chronic medication and regular monitoring of disease status. Hematologic and cytogenetic testing, molecular monitoring, and BCR-ABL1 mutational analysis have become integral to the routine management of CML. The information that each type of test provides is essential to confirm a diagnosis, determine the disease stage, assess response to treatment, and monitor for signals of disease progression – all of which can be used to identify patients who might require further evaluation, closer follow-up, and additional intervention, and to guide clinical decisions.
Click on the PDF icon at the top of this introduction to read the full article.
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) for treatment of chronic myeloid leukemia (CML) has made it possible for this cancer to be controlled in many patients for long periods with chronic medication and regular monitoring of disease status. Hematologic and cytogenetic testing, molecular monitoring, and BCR-ABL1 mutational analysis have become integral to the routine management of CML. The information that each type of test provides is essential to confirm a diagnosis, determine the disease stage, assess response to treatment, and monitor for signals of disease progression – all of which can be used to identify patients who might require further evaluation, closer follow-up, and additional intervention, and to guide clinical decisions.
Click on the PDF icon at the top of this introduction to read the full article.