User login
The Right Choice? Paternalism, Autonomy, and the Incidental Finding
The case had been straightforward. My patient had primary hyperparathyroidism and her localization studies had shown a single parathyroid adenoma. In the operating room, with her under general anesthesia, I had found and removed the abnormal parathyroid gland. The intraoperative parathyroid hormone levels were being run outside the OR door. I was getting ready to close with my fellow when I happened to palpate the thyroid isthmus. There was a firm nodule right in the center of the isthmus. The thyroid looked fine, but the nodule was unmistakable.
This was a surprise. The patient had undergone an ultrasound in radiology the week before, and the study was notable for there being no thyroid nodules. We had performed our own ultrasound in the OR. We had confirmed the location of the parathyroid adenoma and saw no thyroid nodules. I was faced with the initial question of what to do with this incidental finding. Although I could not see the nodule, certainly by feel, it was suspicious, but it was also very small – several millimeters at most. One option was to simply ignore the finding – certainly a bad choice. I knew that the patient had come to the hospital with her sister and a close friend. They were both in the waiting room expecting my update as soon as we were finished. I could have discussed this unexpected finding with the sister and friend, but I felt certain that no one would object to me removing a small piece of thyroid when this added little or no risk. It seemed unnecessary to seek permission to do this small additional procedure.
We proceeded to resect the nodule within the thyroid gland, taking enough adjacent thyroid tissue that I never actually saw the nodule. Once it was removed, I faced another question: Should I send it for frozen section? This seemed to be an easy one to answer. If I was suspicious enough to remove it, I should also know what it is.
The frozen section report was called in a short time later. It was a 4-mm papillary thyroid cancer (PTC) that was within normal thyroid tissue. I had been expecting this possible result. Now I had more choices. I could talk with the family/friend in the waiting room and seek advice on what to do. Alternatively, I could simply say that the presence of PTC was enough of a reason to just take out the thyroid gland since I had not expected this finding and the negative preoperative ultrasound had certainly missed this small tumor (and there might even be others). Finally, I could simply close the patient based on the fact that a 4-mm PTC is of no real clinical significance. Certainly, if this small PTC had been removed with a thyroid lobe for other reasons, we would never go back to take out the rest of the thyroid gland.
As I considered these options, it seemed clear to me that if I went to talk with the family/friend with an unexpected diagnosis of cancer, it was very likely that the patient would wind up with a bigger operation than might be necessary. Ten or fifteen years ago, most surgeons would have removed the thyroid gland for almost any diagnosis of PTC so that patients could go on to receive radioactive iodine. However, today many patients with small incidental PTCs found on lobectomy are simply followed with surveillance ultrasounds because the risks of recurrence or spread are very low. It was clear that I had no basis to take out the whole thyroid gland for a small PTC that was already out. It also seemed unwise to ask what to do, when I felt certain that I knew what was best for the patient. Of course, the suggestion that “I knew what was best for the patient” is a very paternalistic thing to say. It suggests that the medical issues trump all others. It is also quite contrary to the movement of medical ethics in the last several decades that has emphasized shared decision making yet doing what is best for the patient is what surgical patients expect of their surgeons.
I decided to close the patient and then explain what I did and why I did it. She might have been angry with me that I had found a cancer and had not taken out her thyroid gland. However, I felt that the medical evidence supported a less-aggressive surgical approach. In addition, I could always take out her thyroid if she was too worried by the concept of surveillance but I could never put it back if I had removed it!
The patient was understandably surprised and concerned when talked to her. Her first response was one of concern about recurrence. She wanted to know how I knew that there was no more cancer in her thyroid gland. I explained that I actually could not know that, but based on the ultrasound, there was no evidence of any clinically significant thyroid cancer. Fortunately, she was ultimately relieved that the thyroid cancer had been found even though it raised concerns for the future that she had never considered previously.
Whenever surgeons operate on patients under general anesthesia, we are faced with the potential need to make decisions for our patients without the patient’s input. Sometimes it is appropriate to seek input from family when there are multiple good options. However, surgery requires surgeons to make many decisions on their patient’s behalf with no input from the patient – that is, surgeons are expected to act paternalistically in the OR. Rather than being detrimental to the ethical care of patients, such limited paternalism is sometimes the best that we can offer our patients and critical to our role as surgeon.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
The case had been straightforward. My patient had primary hyperparathyroidism and her localization studies had shown a single parathyroid adenoma. In the operating room, with her under general anesthesia, I had found and removed the abnormal parathyroid gland. The intraoperative parathyroid hormone levels were being run outside the OR door. I was getting ready to close with my fellow when I happened to palpate the thyroid isthmus. There was a firm nodule right in the center of the isthmus. The thyroid looked fine, but the nodule was unmistakable.
This was a surprise. The patient had undergone an ultrasound in radiology the week before, and the study was notable for there being no thyroid nodules. We had performed our own ultrasound in the OR. We had confirmed the location of the parathyroid adenoma and saw no thyroid nodules. I was faced with the initial question of what to do with this incidental finding. Although I could not see the nodule, certainly by feel, it was suspicious, but it was also very small – several millimeters at most. One option was to simply ignore the finding – certainly a bad choice. I knew that the patient had come to the hospital with her sister and a close friend. They were both in the waiting room expecting my update as soon as we were finished. I could have discussed this unexpected finding with the sister and friend, but I felt certain that no one would object to me removing a small piece of thyroid when this added little or no risk. It seemed unnecessary to seek permission to do this small additional procedure.
We proceeded to resect the nodule within the thyroid gland, taking enough adjacent thyroid tissue that I never actually saw the nodule. Once it was removed, I faced another question: Should I send it for frozen section? This seemed to be an easy one to answer. If I was suspicious enough to remove it, I should also know what it is.
The frozen section report was called in a short time later. It was a 4-mm papillary thyroid cancer (PTC) that was within normal thyroid tissue. I had been expecting this possible result. Now I had more choices. I could talk with the family/friend in the waiting room and seek advice on what to do. Alternatively, I could simply say that the presence of PTC was enough of a reason to just take out the thyroid gland since I had not expected this finding and the negative preoperative ultrasound had certainly missed this small tumor (and there might even be others). Finally, I could simply close the patient based on the fact that a 4-mm PTC is of no real clinical significance. Certainly, if this small PTC had been removed with a thyroid lobe for other reasons, we would never go back to take out the rest of the thyroid gland.
As I considered these options, it seemed clear to me that if I went to talk with the family/friend with an unexpected diagnosis of cancer, it was very likely that the patient would wind up with a bigger operation than might be necessary. Ten or fifteen years ago, most surgeons would have removed the thyroid gland for almost any diagnosis of PTC so that patients could go on to receive radioactive iodine. However, today many patients with small incidental PTCs found on lobectomy are simply followed with surveillance ultrasounds because the risks of recurrence or spread are very low. It was clear that I had no basis to take out the whole thyroid gland for a small PTC that was already out. It also seemed unwise to ask what to do, when I felt certain that I knew what was best for the patient. Of course, the suggestion that “I knew what was best for the patient” is a very paternalistic thing to say. It suggests that the medical issues trump all others. It is also quite contrary to the movement of medical ethics in the last several decades that has emphasized shared decision making yet doing what is best for the patient is what surgical patients expect of their surgeons.
I decided to close the patient and then explain what I did and why I did it. She might have been angry with me that I had found a cancer and had not taken out her thyroid gland. However, I felt that the medical evidence supported a less-aggressive surgical approach. In addition, I could always take out her thyroid if she was too worried by the concept of surveillance but I could never put it back if I had removed it!
The patient was understandably surprised and concerned when talked to her. Her first response was one of concern about recurrence. She wanted to know how I knew that there was no more cancer in her thyroid gland. I explained that I actually could not know that, but based on the ultrasound, there was no evidence of any clinically significant thyroid cancer. Fortunately, she was ultimately relieved that the thyroid cancer had been found even though it raised concerns for the future that she had never considered previously.
Whenever surgeons operate on patients under general anesthesia, we are faced with the potential need to make decisions for our patients without the patient’s input. Sometimes it is appropriate to seek input from family when there are multiple good options. However, surgery requires surgeons to make many decisions on their patient’s behalf with no input from the patient – that is, surgeons are expected to act paternalistically in the OR. Rather than being detrimental to the ethical care of patients, such limited paternalism is sometimes the best that we can offer our patients and critical to our role as surgeon.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
The case had been straightforward. My patient had primary hyperparathyroidism and her localization studies had shown a single parathyroid adenoma. In the operating room, with her under general anesthesia, I had found and removed the abnormal parathyroid gland. The intraoperative parathyroid hormone levels were being run outside the OR door. I was getting ready to close with my fellow when I happened to palpate the thyroid isthmus. There was a firm nodule right in the center of the isthmus. The thyroid looked fine, but the nodule was unmistakable.
This was a surprise. The patient had undergone an ultrasound in radiology the week before, and the study was notable for there being no thyroid nodules. We had performed our own ultrasound in the OR. We had confirmed the location of the parathyroid adenoma and saw no thyroid nodules. I was faced with the initial question of what to do with this incidental finding. Although I could not see the nodule, certainly by feel, it was suspicious, but it was also very small – several millimeters at most. One option was to simply ignore the finding – certainly a bad choice. I knew that the patient had come to the hospital with her sister and a close friend. They were both in the waiting room expecting my update as soon as we were finished. I could have discussed this unexpected finding with the sister and friend, but I felt certain that no one would object to me removing a small piece of thyroid when this added little or no risk. It seemed unnecessary to seek permission to do this small additional procedure.
We proceeded to resect the nodule within the thyroid gland, taking enough adjacent thyroid tissue that I never actually saw the nodule. Once it was removed, I faced another question: Should I send it for frozen section? This seemed to be an easy one to answer. If I was suspicious enough to remove it, I should also know what it is.
The frozen section report was called in a short time later. It was a 4-mm papillary thyroid cancer (PTC) that was within normal thyroid tissue. I had been expecting this possible result. Now I had more choices. I could talk with the family/friend in the waiting room and seek advice on what to do. Alternatively, I could simply say that the presence of PTC was enough of a reason to just take out the thyroid gland since I had not expected this finding and the negative preoperative ultrasound had certainly missed this small tumor (and there might even be others). Finally, I could simply close the patient based on the fact that a 4-mm PTC is of no real clinical significance. Certainly, if this small PTC had been removed with a thyroid lobe for other reasons, we would never go back to take out the rest of the thyroid gland.
As I considered these options, it seemed clear to me that if I went to talk with the family/friend with an unexpected diagnosis of cancer, it was very likely that the patient would wind up with a bigger operation than might be necessary. Ten or fifteen years ago, most surgeons would have removed the thyroid gland for almost any diagnosis of PTC so that patients could go on to receive radioactive iodine. However, today many patients with small incidental PTCs found on lobectomy are simply followed with surveillance ultrasounds because the risks of recurrence or spread are very low. It was clear that I had no basis to take out the whole thyroid gland for a small PTC that was already out. It also seemed unwise to ask what to do, when I felt certain that I knew what was best for the patient. Of course, the suggestion that “I knew what was best for the patient” is a very paternalistic thing to say. It suggests that the medical issues trump all others. It is also quite contrary to the movement of medical ethics in the last several decades that has emphasized shared decision making yet doing what is best for the patient is what surgical patients expect of their surgeons.
I decided to close the patient and then explain what I did and why I did it. She might have been angry with me that I had found a cancer and had not taken out her thyroid gland. However, I felt that the medical evidence supported a less-aggressive surgical approach. In addition, I could always take out her thyroid if she was too worried by the concept of surveillance but I could never put it back if I had removed it!
The patient was understandably surprised and concerned when talked to her. Her first response was one of concern about recurrence. She wanted to know how I knew that there was no more cancer in her thyroid gland. I explained that I actually could not know that, but based on the ultrasound, there was no evidence of any clinically significant thyroid cancer. Fortunately, she was ultimately relieved that the thyroid cancer had been found even though it raised concerns for the future that she had never considered previously.
Whenever surgeons operate on patients under general anesthesia, we are faced with the potential need to make decisions for our patients without the patient’s input. Sometimes it is appropriate to seek input from family when there are multiple good options. However, surgery requires surgeons to make many decisions on their patient’s behalf with no input from the patient – that is, surgeons are expected to act paternalistically in the OR. Rather than being detrimental to the ethical care of patients, such limited paternalism is sometimes the best that we can offer our patients and critical to our role as surgeon.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
FDA approves simeprevir-sofosbuvir combo for hepatitis C
The Food and Drug Administration has approved the use of sofosbuvir in combination with simeprevir for treatment of patients with chronic hepatitis C virus. It is a ribavirin- and interferon-free regimen.
The approval is reflected in changes to the label of simeprevir (Olysio), an HCV NS3/4A protease inhibitor, which was approved in 2013 for chronic hepatitis C genotype 1 infection “as a component of a combination antiviral treatment regimen.”
The new label summarizes the results of COSMOS, an open label, randomized phase II trial of HCV genotype 1–infected prior null responders with a METAVIR fibrosis score of F0 F2 or treatment naive subjects and prior null responders with a METAVIR fibrosis score of F3 F4 and compensated liver disease. The sustained virologic response rates 12 weeks after planned end of treatment was 93% among those treated with the combination for 12 weeks, and 97% among those treated for 24 weeks. The study was published online (Lancet 2014 July [doi:10.1016/S0140-6736(14)61036-9]).
In COSMOS, the most common adverse reactions reported by more than 10% of treated patients during 12 weeks of combination treatment were fatigue in 25%, headache (21%), nausea (21%), insomnia (14%), pruritus (11%), rash (11%), and photosensitivity (7%). Among those treated for 24 weeks, dizziness (16%) and diarrhea (16%) were reported, according to the revised label.
When combined with sofosbuvir, treatment of treatment-naive and treatment-experienced patients is recommended for 12 weeks (for patients without cirrhosis) or 24 weeks (for patients with cirrhosis).
Simeprevir is marketed by Janssen Therapeutics. Sofosbuvir, an HCV nucleotide analog NS5B polymerase inhibitor approved in December 2013 for treatment of chronic hepatitis C as a component of a combination antiviral treatment regimen, is marketed as Sovaldi by Gilead Sciences. The combination was approved Nov. 5.
The Food and Drug Administration has approved the use of sofosbuvir in combination with simeprevir for treatment of patients with chronic hepatitis C virus. It is a ribavirin- and interferon-free regimen.
The approval is reflected in changes to the label of simeprevir (Olysio), an HCV NS3/4A protease inhibitor, which was approved in 2013 for chronic hepatitis C genotype 1 infection “as a component of a combination antiviral treatment regimen.”
The new label summarizes the results of COSMOS, an open label, randomized phase II trial of HCV genotype 1–infected prior null responders with a METAVIR fibrosis score of F0 F2 or treatment naive subjects and prior null responders with a METAVIR fibrosis score of F3 F4 and compensated liver disease. The sustained virologic response rates 12 weeks after planned end of treatment was 93% among those treated with the combination for 12 weeks, and 97% among those treated for 24 weeks. The study was published online (Lancet 2014 July [doi:10.1016/S0140-6736(14)61036-9]).
In COSMOS, the most common adverse reactions reported by more than 10% of treated patients during 12 weeks of combination treatment were fatigue in 25%, headache (21%), nausea (21%), insomnia (14%), pruritus (11%), rash (11%), and photosensitivity (7%). Among those treated for 24 weeks, dizziness (16%) and diarrhea (16%) were reported, according to the revised label.
When combined with sofosbuvir, treatment of treatment-naive and treatment-experienced patients is recommended for 12 weeks (for patients without cirrhosis) or 24 weeks (for patients with cirrhosis).
Simeprevir is marketed by Janssen Therapeutics. Sofosbuvir, an HCV nucleotide analog NS5B polymerase inhibitor approved in December 2013 for treatment of chronic hepatitis C as a component of a combination antiviral treatment regimen, is marketed as Sovaldi by Gilead Sciences. The combination was approved Nov. 5.
The Food and Drug Administration has approved the use of sofosbuvir in combination with simeprevir for treatment of patients with chronic hepatitis C virus. It is a ribavirin- and interferon-free regimen.
The approval is reflected in changes to the label of simeprevir (Olysio), an HCV NS3/4A protease inhibitor, which was approved in 2013 for chronic hepatitis C genotype 1 infection “as a component of a combination antiviral treatment regimen.”
The new label summarizes the results of COSMOS, an open label, randomized phase II trial of HCV genotype 1–infected prior null responders with a METAVIR fibrosis score of F0 F2 or treatment naive subjects and prior null responders with a METAVIR fibrosis score of F3 F4 and compensated liver disease. The sustained virologic response rates 12 weeks after planned end of treatment was 93% among those treated with the combination for 12 weeks, and 97% among those treated for 24 weeks. The study was published online (Lancet 2014 July [doi:10.1016/S0140-6736(14)61036-9]).
In COSMOS, the most common adverse reactions reported by more than 10% of treated patients during 12 weeks of combination treatment were fatigue in 25%, headache (21%), nausea (21%), insomnia (14%), pruritus (11%), rash (11%), and photosensitivity (7%). Among those treated for 24 weeks, dizziness (16%) and diarrhea (16%) were reported, according to the revised label.
When combined with sofosbuvir, treatment of treatment-naive and treatment-experienced patients is recommended for 12 weeks (for patients without cirrhosis) or 24 weeks (for patients with cirrhosis).
Simeprevir is marketed by Janssen Therapeutics. Sofosbuvir, an HCV nucleotide analog NS5B polymerase inhibitor approved in December 2013 for treatment of chronic hepatitis C as a component of a combination antiviral treatment regimen, is marketed as Sovaldi by Gilead Sciences. The combination was approved Nov. 5.
Results support transfusing with caution in TTP, HIT
PHILADELPHIA—Results of a large study support the recommendation that patients with platelet consumptive disorders only receive platelet transfusions if they exhibit severe or life-threatening bleeding that is refractory to other therapies.
The research indicated that platelet transfusions may increase the risk of arterial thrombosis and mortality among hospitalized patients with thrombotic thrombocytopenic purpura (TTP) and those with heparin-induced thrombocytopenia (HIT).
Platelet transfusions were also associated with a greater risk of acute myocardial infarction in TTP patients.
However, transfused patients with immune thrombocytopenia (ITP) did not have an increased risk of such complications.
The study did not establish a causal link between transfusions and complications, as it was retrospective and the researchers did not know the exact timing of events.
However, the complications and the transfusions did occur during the same hospital admission, noted Ruchika Goel, MD, of Johns Hopkins University in Baltimore, Maryland. She presented these findings at the AABB Annual Meeting 2014 (abstract S41-030G).
Dr Goel and her colleagues conducted this study to assess current platelet transfusion practices in the US in hospitalized patients with TTP, HIT, and ITP. The team wanted to explore any associations between transfusions and bleeding, venous and arterial thrombotic events, acute myocardial infarction, stroke, and in-hospital mortality in these patients.
“Currently, very little data are available on the risks and benefits associated with platelet transfusions in various platelet consumptive or disruptive disorders,” Dr Goel said. “Thus, evidence-based platelet transfusion guidelines in these disorders are either non-existent or they’re based on consensus statements, with not much supportive data.”
With this in mind, the researchers analyzed data from the Nationwide Inpatient Sample, a stratified probability sample of 20% of all discharges at community hospitals in the US, which covers more than 1100 hospitals across 47 states. The team looked at 5 years of data spanning the period from 2007 through 2011.
They included patients in whom TTP and ITP were the primary admitting diagnoses and patients in whom HIT was 1 of the top 3 diagnoses. Hospitalizations in which patients had a prior history of thrombosis were excluded, as were hospitalizations with any thrombosis/thromboembolism listed as the primary admitting diagnosis (implying that it was already present at admission).
So the analysis included 10,624 patients with TTP, 6332 with HIT, and 79,980 with ITP. The median ages were 47.4, 61.8, and 47.5, respectively. And platelet transfusions were given to 10.1%, 7.1%, and 25.8% of patients, respectively.
When the researchers adjusted their analysis for age and gender, they discovered a significantly increased risk of bleeding among all transfused patients. The odds ratios (ORs) were 2.3 for TTP, 5.5 for HIT, and 5.1 for ITP patients.
“The odds of platelet transfusion were significantly higher in patients who had bleeding, thus implying that . . . that was the indication for the transfusion—an actual bleeding complication,” Dr Goel said.
The results also showed that none of the transfused patients had a significantly increased risk of venous thrombosis or stroke. The ORs for venous thrombosis were 1.1 for TTP, 0.8 for HIT, and 1.3 for ITP patients. And the ORs for stroke were 1.6, 0.5, and 1.3, respectively.
However, both TTP patients and HIT patients had a significantly increased risk of arterial thrombosis. The ORs were 5.8 for TTP, 3.4 for HIT, and 0.3 for ITP patients.
TTP patients also had a significantly increased risk of acute myocardial infarction. The ORs were 2.0 for TTP, 1.9 for HIT, and 1.3 for ITP patients.
And patients with TTP and HIT had a significantly increased risk of in-hospital mortality. The ORs were 2.0 for TTP, 5.2 for HIT, and 1.1 for ITP patients.
Dr Goel noted that this study had several limitations. The temporality of events was not reported, there was no information on platelet thresholds for transfusion or disease severity and the effect on outcomes, and accuracy was limited by the precision of discharge coding.
Therefore, further studies are needed to assess whether platelet transfusions are directly responsible for complications or if they serve as a surrogate marker for the severity of illness.
“We propose that, until such studies or trials are indeed available, which are very hard [to conduct in] these rare disorders, platelets should continue to be considered relatively contraindicated and used only for severe or life-threatening bleeding which is refractory to other therapies,” Dr Goel concluded. ![]()
PHILADELPHIA—Results of a large study support the recommendation that patients with platelet consumptive disorders only receive platelet transfusions if they exhibit severe or life-threatening bleeding that is refractory to other therapies.
The research indicated that platelet transfusions may increase the risk of arterial thrombosis and mortality among hospitalized patients with thrombotic thrombocytopenic purpura (TTP) and those with heparin-induced thrombocytopenia (HIT).
Platelet transfusions were also associated with a greater risk of acute myocardial infarction in TTP patients.
However, transfused patients with immune thrombocytopenia (ITP) did not have an increased risk of such complications.
The study did not establish a causal link between transfusions and complications, as it was retrospective and the researchers did not know the exact timing of events.
However, the complications and the transfusions did occur during the same hospital admission, noted Ruchika Goel, MD, of Johns Hopkins University in Baltimore, Maryland. She presented these findings at the AABB Annual Meeting 2014 (abstract S41-030G).
Dr Goel and her colleagues conducted this study to assess current platelet transfusion practices in the US in hospitalized patients with TTP, HIT, and ITP. The team wanted to explore any associations between transfusions and bleeding, venous and arterial thrombotic events, acute myocardial infarction, stroke, and in-hospital mortality in these patients.
“Currently, very little data are available on the risks and benefits associated with platelet transfusions in various platelet consumptive or disruptive disorders,” Dr Goel said. “Thus, evidence-based platelet transfusion guidelines in these disorders are either non-existent or they’re based on consensus statements, with not much supportive data.”
With this in mind, the researchers analyzed data from the Nationwide Inpatient Sample, a stratified probability sample of 20% of all discharges at community hospitals in the US, which covers more than 1100 hospitals across 47 states. The team looked at 5 years of data spanning the period from 2007 through 2011.
They included patients in whom TTP and ITP were the primary admitting diagnoses and patients in whom HIT was 1 of the top 3 diagnoses. Hospitalizations in which patients had a prior history of thrombosis were excluded, as were hospitalizations with any thrombosis/thromboembolism listed as the primary admitting diagnosis (implying that it was already present at admission).
So the analysis included 10,624 patients with TTP, 6332 with HIT, and 79,980 with ITP. The median ages were 47.4, 61.8, and 47.5, respectively. And platelet transfusions were given to 10.1%, 7.1%, and 25.8% of patients, respectively.
When the researchers adjusted their analysis for age and gender, they discovered a significantly increased risk of bleeding among all transfused patients. The odds ratios (ORs) were 2.3 for TTP, 5.5 for HIT, and 5.1 for ITP patients.
“The odds of platelet transfusion were significantly higher in patients who had bleeding, thus implying that . . . that was the indication for the transfusion—an actual bleeding complication,” Dr Goel said.
The results also showed that none of the transfused patients had a significantly increased risk of venous thrombosis or stroke. The ORs for venous thrombosis were 1.1 for TTP, 0.8 for HIT, and 1.3 for ITP patients. And the ORs for stroke were 1.6, 0.5, and 1.3, respectively.
However, both TTP patients and HIT patients had a significantly increased risk of arterial thrombosis. The ORs were 5.8 for TTP, 3.4 for HIT, and 0.3 for ITP patients.
TTP patients also had a significantly increased risk of acute myocardial infarction. The ORs were 2.0 for TTP, 1.9 for HIT, and 1.3 for ITP patients.
And patients with TTP and HIT had a significantly increased risk of in-hospital mortality. The ORs were 2.0 for TTP, 5.2 for HIT, and 1.1 for ITP patients.
Dr Goel noted that this study had several limitations. The temporality of events was not reported, there was no information on platelet thresholds for transfusion or disease severity and the effect on outcomes, and accuracy was limited by the precision of discharge coding.
Therefore, further studies are needed to assess whether platelet transfusions are directly responsible for complications or if they serve as a surrogate marker for the severity of illness.
“We propose that, until such studies or trials are indeed available, which are very hard [to conduct in] these rare disorders, platelets should continue to be considered relatively contraindicated and used only for severe or life-threatening bleeding which is refractory to other therapies,” Dr Goel concluded. ![]()
PHILADELPHIA—Results of a large study support the recommendation that patients with platelet consumptive disorders only receive platelet transfusions if they exhibit severe or life-threatening bleeding that is refractory to other therapies.
The research indicated that platelet transfusions may increase the risk of arterial thrombosis and mortality among hospitalized patients with thrombotic thrombocytopenic purpura (TTP) and those with heparin-induced thrombocytopenia (HIT).
Platelet transfusions were also associated with a greater risk of acute myocardial infarction in TTP patients.
However, transfused patients with immune thrombocytopenia (ITP) did not have an increased risk of such complications.
The study did not establish a causal link between transfusions and complications, as it was retrospective and the researchers did not know the exact timing of events.
However, the complications and the transfusions did occur during the same hospital admission, noted Ruchika Goel, MD, of Johns Hopkins University in Baltimore, Maryland. She presented these findings at the AABB Annual Meeting 2014 (abstract S41-030G).
Dr Goel and her colleagues conducted this study to assess current platelet transfusion practices in the US in hospitalized patients with TTP, HIT, and ITP. The team wanted to explore any associations between transfusions and bleeding, venous and arterial thrombotic events, acute myocardial infarction, stroke, and in-hospital mortality in these patients.
“Currently, very little data are available on the risks and benefits associated with platelet transfusions in various platelet consumptive or disruptive disorders,” Dr Goel said. “Thus, evidence-based platelet transfusion guidelines in these disorders are either non-existent or they’re based on consensus statements, with not much supportive data.”
With this in mind, the researchers analyzed data from the Nationwide Inpatient Sample, a stratified probability sample of 20% of all discharges at community hospitals in the US, which covers more than 1100 hospitals across 47 states. The team looked at 5 years of data spanning the period from 2007 through 2011.
They included patients in whom TTP and ITP were the primary admitting diagnoses and patients in whom HIT was 1 of the top 3 diagnoses. Hospitalizations in which patients had a prior history of thrombosis were excluded, as were hospitalizations with any thrombosis/thromboembolism listed as the primary admitting diagnosis (implying that it was already present at admission).
So the analysis included 10,624 patients with TTP, 6332 with HIT, and 79,980 with ITP. The median ages were 47.4, 61.8, and 47.5, respectively. And platelet transfusions were given to 10.1%, 7.1%, and 25.8% of patients, respectively.
When the researchers adjusted their analysis for age and gender, they discovered a significantly increased risk of bleeding among all transfused patients. The odds ratios (ORs) were 2.3 for TTP, 5.5 for HIT, and 5.1 for ITP patients.
“The odds of platelet transfusion were significantly higher in patients who had bleeding, thus implying that . . . that was the indication for the transfusion—an actual bleeding complication,” Dr Goel said.
The results also showed that none of the transfused patients had a significantly increased risk of venous thrombosis or stroke. The ORs for venous thrombosis were 1.1 for TTP, 0.8 for HIT, and 1.3 for ITP patients. And the ORs for stroke were 1.6, 0.5, and 1.3, respectively.
However, both TTP patients and HIT patients had a significantly increased risk of arterial thrombosis. The ORs were 5.8 for TTP, 3.4 for HIT, and 0.3 for ITP patients.
TTP patients also had a significantly increased risk of acute myocardial infarction. The ORs were 2.0 for TTP, 1.9 for HIT, and 1.3 for ITP patients.
And patients with TTP and HIT had a significantly increased risk of in-hospital mortality. The ORs were 2.0 for TTP, 5.2 for HIT, and 1.1 for ITP patients.
Dr Goel noted that this study had several limitations. The temporality of events was not reported, there was no information on platelet thresholds for transfusion or disease severity and the effect on outcomes, and accuracy was limited by the precision of discharge coding.
Therefore, further studies are needed to assess whether platelet transfusions are directly responsible for complications or if they serve as a surrogate marker for the severity of illness.
“We propose that, until such studies or trials are indeed available, which are very hard [to conduct in] these rare disorders, platelets should continue to be considered relatively contraindicated and used only for severe or life-threatening bleeding which is refractory to other therapies,” Dr Goel concluded. ![]()
RNAi therapy may eliminate resistance in B-ALL
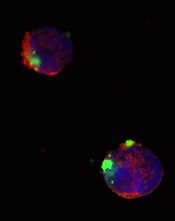
CD22ΔE12-siRNA nanoparticle
Credit: Fatih Uckun
Researchers have identified a molecular target for RNA interference (RNAi) therapy, and they believe that, by targeting this molecular lesion, they may be able to eliminate drug resistance in B-lineage acute lymphoblastic leukemia (ALL).
“We knew that we could kill chemotherapy-resistant leukemia cells if we only knew what made them so resistant,” said study author Fatih Uckun, MD, PhD, of the Children’s Hospital Los Angeles in California.
“Once we determined the mechanism, the next step was obvious—to rationally design a drug that would take out that specific target.”
The target is a defective gene that results in the production of abnormal CD22—CD22ΔE12. The researchers found that pediatric and adult B-lineage lymphoid malignancies are characterized by a high incidence of CD22ΔE12.
The group’s experiments revealed a causal link between CD22ΔE12 and the aggressive biology of B-ALL cells. When the researchers knocked down CD22ΔE12 in primary B-ALL cells using small interfering RNA (siRNA), they observed inhibition of clonogenicity.
The team therefore theorized that the siRNA-mediated depletion of CD22ΔE12 might be useful for treating B-ALL.
So they designed a nanoparticle formulation of CD22ΔE12-siRNA in which a polypeptide functions as the delivery system. The particle has a diameter of 100 nanometers.
Experiments showed that the nanoparticle could deliver its siRNA cargo into the cytosol of ALL-1 cells, thereby depleting CD22ΔE12 and inhibiting leukemic cell growth in vitro.
The researchers said that further development of this nanoparticle or other nanoformulation platforms for CD22ΔE12-siRNA could facilitate an effective therapeutic RNAi strategy to treat aggressive or chemotherapy-resistant B-lineage lymphoid malignancies.
“The goal is to translate our recent research discoveries in nanotechnology and biotherapy into effective patient-tailored treatment programs for the most common form of childhood cancer,” Dr Uckun said.
He and his colleagues described this research in EBioMedicine. ![]()

CD22ΔE12-siRNA nanoparticle
Credit: Fatih Uckun
Researchers have identified a molecular target for RNA interference (RNAi) therapy, and they believe that, by targeting this molecular lesion, they may be able to eliminate drug resistance in B-lineage acute lymphoblastic leukemia (ALL).
“We knew that we could kill chemotherapy-resistant leukemia cells if we only knew what made them so resistant,” said study author Fatih Uckun, MD, PhD, of the Children’s Hospital Los Angeles in California.
“Once we determined the mechanism, the next step was obvious—to rationally design a drug that would take out that specific target.”
The target is a defective gene that results in the production of abnormal CD22—CD22ΔE12. The researchers found that pediatric and adult B-lineage lymphoid malignancies are characterized by a high incidence of CD22ΔE12.
The group’s experiments revealed a causal link between CD22ΔE12 and the aggressive biology of B-ALL cells. When the researchers knocked down CD22ΔE12 in primary B-ALL cells using small interfering RNA (siRNA), they observed inhibition of clonogenicity.
The team therefore theorized that the siRNA-mediated depletion of CD22ΔE12 might be useful for treating B-ALL.
So they designed a nanoparticle formulation of CD22ΔE12-siRNA in which a polypeptide functions as the delivery system. The particle has a diameter of 100 nanometers.
Experiments showed that the nanoparticle could deliver its siRNA cargo into the cytosol of ALL-1 cells, thereby depleting CD22ΔE12 and inhibiting leukemic cell growth in vitro.
The researchers said that further development of this nanoparticle or other nanoformulation platforms for CD22ΔE12-siRNA could facilitate an effective therapeutic RNAi strategy to treat aggressive or chemotherapy-resistant B-lineage lymphoid malignancies.
“The goal is to translate our recent research discoveries in nanotechnology and biotherapy into effective patient-tailored treatment programs for the most common form of childhood cancer,” Dr Uckun said.
He and his colleagues described this research in EBioMedicine. ![]()

CD22ΔE12-siRNA nanoparticle
Credit: Fatih Uckun
Researchers have identified a molecular target for RNA interference (RNAi) therapy, and they believe that, by targeting this molecular lesion, they may be able to eliminate drug resistance in B-lineage acute lymphoblastic leukemia (ALL).
“We knew that we could kill chemotherapy-resistant leukemia cells if we only knew what made them so resistant,” said study author Fatih Uckun, MD, PhD, of the Children’s Hospital Los Angeles in California.
“Once we determined the mechanism, the next step was obvious—to rationally design a drug that would take out that specific target.”
The target is a defective gene that results in the production of abnormal CD22—CD22ΔE12. The researchers found that pediatric and adult B-lineage lymphoid malignancies are characterized by a high incidence of CD22ΔE12.
The group’s experiments revealed a causal link between CD22ΔE12 and the aggressive biology of B-ALL cells. When the researchers knocked down CD22ΔE12 in primary B-ALL cells using small interfering RNA (siRNA), they observed inhibition of clonogenicity.
The team therefore theorized that the siRNA-mediated depletion of CD22ΔE12 might be useful for treating B-ALL.
So they designed a nanoparticle formulation of CD22ΔE12-siRNA in which a polypeptide functions as the delivery system. The particle has a diameter of 100 nanometers.
Experiments showed that the nanoparticle could deliver its siRNA cargo into the cytosol of ALL-1 cells, thereby depleting CD22ΔE12 and inhibiting leukemic cell growth in vitro.
The researchers said that further development of this nanoparticle or other nanoformulation platforms for CD22ΔE12-siRNA could facilitate an effective therapeutic RNAi strategy to treat aggressive or chemotherapy-resistant B-lineage lymphoid malignancies.
“The goal is to translate our recent research discoveries in nanotechnology and biotherapy into effective patient-tailored treatment programs for the most common form of childhood cancer,” Dr Uckun said.
He and his colleagues described this research in EBioMedicine. ![]()
Dabigatran increases major, GI bleeding in AF

Dabigatran poses a greater risk of major bleeding and gastrointestinal (GI) bleeding compared to warfarin, according to a study of patients with atrial fibrillation.
The incidence of GI and major bleeding with dabigatran was particularly high in African Americans, patients with chronic kidney disease (CKD), patients age 75
and older, and those with 7 or more comorbidities.
On the other hand, the risk of intracranial hemorrhage was higher in most warfarin-treated patients. African Americans were the exception to this rule.
Researchers reported these results in JAMA Internal Medicine alongside a related editorial.
“Dabigatran was introduced in 2010 and, at the time of approval, it was the only available alternative to warfarin,” noted study author Yuting Zhang, PhD, of the University of Pittsburgh in Pennsylvania.
“Warfarin dosing can be tricky, and regular monitoring with blood tests is required, so doctors and patients were glad to have a drug that was easier to manage. But some recent studies suggest that dabigatran is associated with a higher risk of bleeding.”
To investigate that possibility, Dr Zhang and her colleagues reviewed pharmacy and medical claims data from 2010 and 2011 of a random national sample of Medicare beneficiaries. The team tracked 1302 dabigatran users and 8102 warfarin users to see whether they experienced bleeding episodes.
The researchers classified bleeding events as major, such as intracranial bleeding or GI bleeding requiring a hospital or emergency room stay, or minor, such as GI bleeding that was treated on an outpatient basis or nose bleeds.
The incidence of any bleeding was significantly higher in dabigatran-treated patients than in warfarin-treated patients (32.7% vs 26.5%, P<0.001). The same was true of major bleeding (9% vs 5.9%, P<0.001) and GI bleeding (17.4% vs 10%, P<0.001).
However, the rate of intracranial bleeding was higher with warfarin than with dabigatran (1.8% vs 0.6%, P<0.001), as was the rate of epistaxis (3.1% vs 2%, P=0.002) and not-otherwise-specified hemorrhage (5.9% vs 4.4%, P=0.003).
All other types of bleeding were more frequent in the dabigatran group than the warfarin group. This included hematuria (12% vs 8.8%, P<0.001), vaginal bleeding (0.7% vs 0.3%, P=0.003), hemarthrosis (0.5% vs 0.2%, P=0.007), and hemoptysis (2% vs 1.4%, P=0.03).
The researchers also looked at bleeding episodes in 4 high-risk subgroups: patients who were 75 years of age and older, African Americans, patients with CKD, and those with 7 or more co-existing medical problems.
The risk of major bleeding was higher among dabigatran-treated patients belonging to these subgroups. The hazard ratios (HRs) were 1.60 for patients 75 and older, 2.12 for African Americans, 2.07 for patients with CKD, and 1.88 for patients with 7 or more comorbidities.
Likewise, the risk of GI bleeding was higher among dabigatran-treated patients in the subgroups. The HRs were 1.85 for patients 75 and older, 2.38 for African Americans, 1.81 for patients with CKD, and 1.81 for patients with 7 or more comorbidities.
“These findings indicate that physicians should be cautious when prescribing dabigatran, particularly to African Americans and patients with kidney impairments,” said study author Inmaculada Hernandez, PharmD, of the University of Pittsburgh.
“Also, the incidence of gastrointestinal bleeding was high in all the subgroups, so we recommend doctors explain to patients how to detect it so that it can be treated promptly.”
The researchers also noted that the risk of intracranial bleeding varied among the subgroups. The HRs for dabigatran vs warfarin were 0.10 for patients 75 and older, 2.29 for African Americans, 0.71 for patients with CKD, and 0.59 for patients with 7 or more comorbidities.
“We plan to examine 2012 data to monitor the risk of stroke for patients on dabigatran, which is the primary indication for taking the blood thinner,” Dr Zhang said. “It’s possible that, for some patients, a greater reduction in the risk of stroke will outweigh the higher risk of bleeding with dabigatran compared to warfarin.” ![]()

Dabigatran poses a greater risk of major bleeding and gastrointestinal (GI) bleeding compared to warfarin, according to a study of patients with atrial fibrillation.
The incidence of GI and major bleeding with dabigatran was particularly high in African Americans, patients with chronic kidney disease (CKD), patients age 75
and older, and those with 7 or more comorbidities.
On the other hand, the risk of intracranial hemorrhage was higher in most warfarin-treated patients. African Americans were the exception to this rule.
Researchers reported these results in JAMA Internal Medicine alongside a related editorial.
“Dabigatran was introduced in 2010 and, at the time of approval, it was the only available alternative to warfarin,” noted study author Yuting Zhang, PhD, of the University of Pittsburgh in Pennsylvania.
“Warfarin dosing can be tricky, and regular monitoring with blood tests is required, so doctors and patients were glad to have a drug that was easier to manage. But some recent studies suggest that dabigatran is associated with a higher risk of bleeding.”
To investigate that possibility, Dr Zhang and her colleagues reviewed pharmacy and medical claims data from 2010 and 2011 of a random national sample of Medicare beneficiaries. The team tracked 1302 dabigatran users and 8102 warfarin users to see whether they experienced bleeding episodes.
The researchers classified bleeding events as major, such as intracranial bleeding or GI bleeding requiring a hospital or emergency room stay, or minor, such as GI bleeding that was treated on an outpatient basis or nose bleeds.
The incidence of any bleeding was significantly higher in dabigatran-treated patients than in warfarin-treated patients (32.7% vs 26.5%, P<0.001). The same was true of major bleeding (9% vs 5.9%, P<0.001) and GI bleeding (17.4% vs 10%, P<0.001).
However, the rate of intracranial bleeding was higher with warfarin than with dabigatran (1.8% vs 0.6%, P<0.001), as was the rate of epistaxis (3.1% vs 2%, P=0.002) and not-otherwise-specified hemorrhage (5.9% vs 4.4%, P=0.003).
All other types of bleeding were more frequent in the dabigatran group than the warfarin group. This included hematuria (12% vs 8.8%, P<0.001), vaginal bleeding (0.7% vs 0.3%, P=0.003), hemarthrosis (0.5% vs 0.2%, P=0.007), and hemoptysis (2% vs 1.4%, P=0.03).
The researchers also looked at bleeding episodes in 4 high-risk subgroups: patients who were 75 years of age and older, African Americans, patients with CKD, and those with 7 or more co-existing medical problems.
The risk of major bleeding was higher among dabigatran-treated patients belonging to these subgroups. The hazard ratios (HRs) were 1.60 for patients 75 and older, 2.12 for African Americans, 2.07 for patients with CKD, and 1.88 for patients with 7 or more comorbidities.
Likewise, the risk of GI bleeding was higher among dabigatran-treated patients in the subgroups. The HRs were 1.85 for patients 75 and older, 2.38 for African Americans, 1.81 for patients with CKD, and 1.81 for patients with 7 or more comorbidities.
“These findings indicate that physicians should be cautious when prescribing dabigatran, particularly to African Americans and patients with kidney impairments,” said study author Inmaculada Hernandez, PharmD, of the University of Pittsburgh.
“Also, the incidence of gastrointestinal bleeding was high in all the subgroups, so we recommend doctors explain to patients how to detect it so that it can be treated promptly.”
The researchers also noted that the risk of intracranial bleeding varied among the subgroups. The HRs for dabigatran vs warfarin were 0.10 for patients 75 and older, 2.29 for African Americans, 0.71 for patients with CKD, and 0.59 for patients with 7 or more comorbidities.
“We plan to examine 2012 data to monitor the risk of stroke for patients on dabigatran, which is the primary indication for taking the blood thinner,” Dr Zhang said. “It’s possible that, for some patients, a greater reduction in the risk of stroke will outweigh the higher risk of bleeding with dabigatran compared to warfarin.” ![]()

Dabigatran poses a greater risk of major bleeding and gastrointestinal (GI) bleeding compared to warfarin, according to a study of patients with atrial fibrillation.
The incidence of GI and major bleeding with dabigatran was particularly high in African Americans, patients with chronic kidney disease (CKD), patients age 75
and older, and those with 7 or more comorbidities.
On the other hand, the risk of intracranial hemorrhage was higher in most warfarin-treated patients. African Americans were the exception to this rule.
Researchers reported these results in JAMA Internal Medicine alongside a related editorial.
“Dabigatran was introduced in 2010 and, at the time of approval, it was the only available alternative to warfarin,” noted study author Yuting Zhang, PhD, of the University of Pittsburgh in Pennsylvania.
“Warfarin dosing can be tricky, and regular monitoring with blood tests is required, so doctors and patients were glad to have a drug that was easier to manage. But some recent studies suggest that dabigatran is associated with a higher risk of bleeding.”
To investigate that possibility, Dr Zhang and her colleagues reviewed pharmacy and medical claims data from 2010 and 2011 of a random national sample of Medicare beneficiaries. The team tracked 1302 dabigatran users and 8102 warfarin users to see whether they experienced bleeding episodes.
The researchers classified bleeding events as major, such as intracranial bleeding or GI bleeding requiring a hospital or emergency room stay, or minor, such as GI bleeding that was treated on an outpatient basis or nose bleeds.
The incidence of any bleeding was significantly higher in dabigatran-treated patients than in warfarin-treated patients (32.7% vs 26.5%, P<0.001). The same was true of major bleeding (9% vs 5.9%, P<0.001) and GI bleeding (17.4% vs 10%, P<0.001).
However, the rate of intracranial bleeding was higher with warfarin than with dabigatran (1.8% vs 0.6%, P<0.001), as was the rate of epistaxis (3.1% vs 2%, P=0.002) and not-otherwise-specified hemorrhage (5.9% vs 4.4%, P=0.003).
All other types of bleeding were more frequent in the dabigatran group than the warfarin group. This included hematuria (12% vs 8.8%, P<0.001), vaginal bleeding (0.7% vs 0.3%, P=0.003), hemarthrosis (0.5% vs 0.2%, P=0.007), and hemoptysis (2% vs 1.4%, P=0.03).
The researchers also looked at bleeding episodes in 4 high-risk subgroups: patients who were 75 years of age and older, African Americans, patients with CKD, and those with 7 or more co-existing medical problems.
The risk of major bleeding was higher among dabigatran-treated patients belonging to these subgroups. The hazard ratios (HRs) were 1.60 for patients 75 and older, 2.12 for African Americans, 2.07 for patients with CKD, and 1.88 for patients with 7 or more comorbidities.
Likewise, the risk of GI bleeding was higher among dabigatran-treated patients in the subgroups. The HRs were 1.85 for patients 75 and older, 2.38 for African Americans, 1.81 for patients with CKD, and 1.81 for patients with 7 or more comorbidities.
“These findings indicate that physicians should be cautious when prescribing dabigatran, particularly to African Americans and patients with kidney impairments,” said study author Inmaculada Hernandez, PharmD, of the University of Pittsburgh.
“Also, the incidence of gastrointestinal bleeding was high in all the subgroups, so we recommend doctors explain to patients how to detect it so that it can be treated promptly.”
The researchers also noted that the risk of intracranial bleeding varied among the subgroups. The HRs for dabigatran vs warfarin were 0.10 for patients 75 and older, 2.29 for African Americans, 0.71 for patients with CKD, and 0.59 for patients with 7 or more comorbidities.
“We plan to examine 2012 data to monitor the risk of stroke for patients on dabigatran, which is the primary indication for taking the blood thinner,” Dr Zhang said. “It’s possible that, for some patients, a greater reduction in the risk of stroke will outweigh the higher risk of bleeding with dabigatran compared to warfarin.” ![]()
NICE recommends ofatumumab in CLL

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a preliminary draft guidance recommending ofatumumab (Arzerra) for use in patients with chronic lymphocytic leukemia (CLL).
The agency is recommending the anti-CD20 monoclonal antibody in combination with chlorambucil for patients with untreated CLL who are ineligible for treatment with fludarabine combination therapy and for whom bendamustine is unsuitable.
NICE believes ofatumumab is a cost-effective use of National Health Service (NHS) resources for this patient population, as GlaxoSmithKline, the company developing ofatumumab, has agreed to provide the drug at a reduced price.
The company has agreed with the Department of Health that the size of the discount be confidential.
Clinical effectiveness
“The information provided by GlaxoSmithKline, who market the drug, showed that ofatumumab with chlorambucil is a clinically effective treatment option for those people unable to take fludarabine combination therapy or bendamustine,” said Sir Andrew Dillon, NICE chief executive.
In the phase 3 COMPLEMENT 1 trial, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
A NICE advisory committee also considered the use of ofatumumab in combination with bendamustine. But the committee said that, due to limited clinical evidence and the absence of cost-effectiveness estimates, it could not make a recommendation on this combination.
In a phase 2 trial known as OMB115991, ofatumumab plus bendamustine elicited an overall response rate of 95% and a complete response rate of 43%.
Cost-effectiveness
Ofatumumab’s list price is £182 for a 100 mg vial and £1820 for a 1000 mg vial. Assuming 6 cycles and no drug wastage, the mean cost of a treatment course for ofatumumab at its list price is £11,466 for 6300 mg.
GlaxoSmithKline has agreed to a patient access scheme with the Department of Health that makes ofatumumab available with a discount on the list price, though the exact amount is confidential.
The NICE advisory committee said the most plausible cost-effectiveness estimate for ofatumumab plus chlorambucil compared with chlorambucil alone using the
ofatumumab patient access scheme price was £26,000 per quality-adjusted life year gained.
Consultees, including GlaxoSmithKline, healthcare professionals, and members of the public, can now comment on NICE’s preliminary draft guidance. It will be available for public consultation until November 25, 2014.
Until the final guidance is issued, NHS bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a preliminary draft guidance recommending ofatumumab (Arzerra) for use in patients with chronic lymphocytic leukemia (CLL).
The agency is recommending the anti-CD20 monoclonal antibody in combination with chlorambucil for patients with untreated CLL who are ineligible for treatment with fludarabine combination therapy and for whom bendamustine is unsuitable.
NICE believes ofatumumab is a cost-effective use of National Health Service (NHS) resources for this patient population, as GlaxoSmithKline, the company developing ofatumumab, has agreed to provide the drug at a reduced price.
The company has agreed with the Department of Health that the size of the discount be confidential.
Clinical effectiveness
“The information provided by GlaxoSmithKline, who market the drug, showed that ofatumumab with chlorambucil is a clinically effective treatment option for those people unable to take fludarabine combination therapy or bendamustine,” said Sir Andrew Dillon, NICE chief executive.
In the phase 3 COMPLEMENT 1 trial, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
A NICE advisory committee also considered the use of ofatumumab in combination with bendamustine. But the committee said that, due to limited clinical evidence and the absence of cost-effectiveness estimates, it could not make a recommendation on this combination.
In a phase 2 trial known as OMB115991, ofatumumab plus bendamustine elicited an overall response rate of 95% and a complete response rate of 43%.
Cost-effectiveness
Ofatumumab’s list price is £182 for a 100 mg vial and £1820 for a 1000 mg vial. Assuming 6 cycles and no drug wastage, the mean cost of a treatment course for ofatumumab at its list price is £11,466 for 6300 mg.
GlaxoSmithKline has agreed to a patient access scheme with the Department of Health that makes ofatumumab available with a discount on the list price, though the exact amount is confidential.
The NICE advisory committee said the most plausible cost-effectiveness estimate for ofatumumab plus chlorambucil compared with chlorambucil alone using the
ofatumumab patient access scheme price was £26,000 per quality-adjusted life year gained.
Consultees, including GlaxoSmithKline, healthcare professionals, and members of the public, can now comment on NICE’s preliminary draft guidance. It will be available for public consultation until November 25, 2014.
Until the final guidance is issued, NHS bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a preliminary draft guidance recommending ofatumumab (Arzerra) for use in patients with chronic lymphocytic leukemia (CLL).
The agency is recommending the anti-CD20 monoclonal antibody in combination with chlorambucil for patients with untreated CLL who are ineligible for treatment with fludarabine combination therapy and for whom bendamustine is unsuitable.
NICE believes ofatumumab is a cost-effective use of National Health Service (NHS) resources for this patient population, as GlaxoSmithKline, the company developing ofatumumab, has agreed to provide the drug at a reduced price.
The company has agreed with the Department of Health that the size of the discount be confidential.
Clinical effectiveness
“The information provided by GlaxoSmithKline, who market the drug, showed that ofatumumab with chlorambucil is a clinically effective treatment option for those people unable to take fludarabine combination therapy or bendamustine,” said Sir Andrew Dillon, NICE chief executive.
In the phase 3 COMPLEMENT 1 trial, ofatumumab plus chlorambucil improved progression-free survival compared to chlorambucil alone. The median times were 22.4 months and 13.1 months, respectively, and the hazard ratio was 0.57 (P<0.001).
A NICE advisory committee also considered the use of ofatumumab in combination with bendamustine. But the committee said that, due to limited clinical evidence and the absence of cost-effectiveness estimates, it could not make a recommendation on this combination.
In a phase 2 trial known as OMB115991, ofatumumab plus bendamustine elicited an overall response rate of 95% and a complete response rate of 43%.
Cost-effectiveness
Ofatumumab’s list price is £182 for a 100 mg vial and £1820 for a 1000 mg vial. Assuming 6 cycles and no drug wastage, the mean cost of a treatment course for ofatumumab at its list price is £11,466 for 6300 mg.
GlaxoSmithKline has agreed to a patient access scheme with the Department of Health that makes ofatumumab available with a discount on the list price, though the exact amount is confidential.
The NICE advisory committee said the most plausible cost-effectiveness estimate for ofatumumab plus chlorambucil compared with chlorambucil alone using the
ofatumumab patient access scheme price was £26,000 per quality-adjusted life year gained.
Consultees, including GlaxoSmithKline, healthcare professionals, and members of the public, can now comment on NICE’s preliminary draft guidance. It will be available for public consultation until November 25, 2014.
Until the final guidance is issued, NHS bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()
Point/Counterpoint: Dual antiplatelet therapy for vascular patients: Yes, no, or sometimes?
Introduction
As evidenced by this month’s Point/Counterpoint article by Dr. William Jordan and Dr. Joseph Mills, there is still debate as to the benefit of antiplatelet agents in patients with peripheral artery disease. Currently, dual antiplatelet agents refer to aspirin and clopidogrel, but over the last year or two, ticagrelor and vorapaxar are also being prescribed for patients with peripheral atherosclerosis. The addition of these medications will probably only add to our confusion! Why don’t you weigh in on this discussion by voting on our online poll at www.vascularspecialistonline.com?
Dr. Russell Samson is the medical editor of Vascular Specialist.
Definitely, maybe.
William D. Jordan, M.D.
First, primary prevention must be considered, as few patients with no prior intervention require dual antiplatelet therapy. It seems that we only have some scant data on the prevention of first-time events in at-risk patients when they are treated with aspirin alone.
While lipid management seems to be the most recent focus for primary prevention, single antiplatelet therapy seems appropriate for many patients who have higher risk due to atherosclerotic disease. A recent study from the University of Alabama at Birmingham found that asymptomatic carotid artery stenosis patients treated with dual antiplatelet therapy actually had higher bleed rates, higher mortality, and lower neurologic event rates, compared with those treated with aspirin alone.
While this study examined only a select group of patients treated for carotid artery disease, these vascular patients had worse outcomes when treated with aspirin and clopidogrel. Thus, caution should be considered before adding too many medical therapies.
Now, consider the short-term outlook for patients – specifically those who undergo some type of vascular intervention. The very nature of vascular intervention is disruptive to the arterial endothelium. Of course, most of the arteries that we enter have some underlying pathology; thus the intimal layer is not normal. The pathologic process is already, at least, partly underway. The concept of antiplatelet therapy is focused on limiting the platelet adhesions that might exaggerate the response to injury that creates a hyperplastic reaction within the vessels. We abhor the excessive response to injury due to the potential failure of the arterial reconstruction. Paradoxically, the same platelet inhibition can also cause excessive bleeding that may complicate the vascular repair. In the current medical climate, most of us tolerate the aggravation of diminished platelet function during open reconstruction in order to protect the target repair site and to avoid the dreaded “troponin leak” that may get classified as a myocardial infarction.
Some of our nonvascular colleagues avoid intervention (e.g., axial anesthesia, endoscopic biopsy) if stronger antiplatelet medication is present.
Now, to borrow some insight from other specialties, we should consider the extensive cardiology literature that shows the value of dual platelet inhibition after percutaneous coronary intervention or a recent cardiac event. While this strategy has shown improvement in PCI results, probably due to the great risk of thrombosis when disrupting the endothelial layer of a 2- to 3-mm coronary artery, the bleeding complications, including access-site hematomas, pseudoaneurysms, or retroperitoneal bleeding, are not analyzed as extensively. The preponderance of literature supports aggressive inhibition, but the long term needs to be considered – both expense and bleeding risk.
Currently, we use short-term dual treatment when the arterial endothelium is intentionally disrupted such as after an endarterectomy, angioplasty, with or without a stent, or atherectomy. Specifically, we add short-term IV dextran to oral agents for patients undergoing a carotid endarterectomy, but most are discharged on a single oral agent unless there is another indication for dual therapy.
Patients undergoing lower-extremity, catheter-based interventions are given dual therapy for the first 30 days, which is then adjusted according to the clinical response, duplex findings and other medical conditions. Bypass graft patients (yes, we still do some) are usually given a single agent unless the graft or patient has exhibited some concern for early failure.
In summary, I suspect that dual therapy is overused in our “pill-driven” population, but there still seem to be areas for its application. On each encounter with patients, I would encourage all vascular specialists to review the indications for antiplatelet therapy to consider removing a medication, improving compliance, and limiting bleeding risk.
Dr. Jordan is the director of the division of vascular surgery at the University of Alabama Birmingham School of Medicine. He has no relevant conflicts.
Dueling over dual antiplatelet therapy
Joseph L. Mills Sr., M.D.
Amidst a background of constant clamoring for data-driven and evidence-based medical decision-making, the practice of vascular medicine and surgery remains mired in the anecdotal. If a little is good, more must be better, seems the rationale for dual antiplatelet therapy. There may be small, as yet unclearly defined subsets of patients for whom dual therapy is actually beneficial, but an unbiased review of the current literature leads to the inescapable conclusion that more patients would be harmed by injudicious application of the more-is-better principle as concerns antiplatelet therapy for patients with carotid artery and peripheral artery disease (PAD).
The best evidence for antiplatelet therapy in patients with carotid disease suggests that aspirin as a single agent in doses ranging from 75 to 150 mg daily is preferred, and at least two meta-analyses and systematic reviews were unable to support the use of dual antiplatelet therapy.1 At least in symptomatic patients, combination or dual antiplatelet therapy may increase the short-term risk of hemorrhagic complications in patients with acute ischemic stroke caused by large-artery disease.2 Long-term dual therapy for secondary stroke prevention with aspirin and clopidogrel has been associated with four times the bleeding risk of monotherapy.3 These bleeds are often intracranial or gastrointestinal and are serious.
With respect to asymptomatic patients, while the CHARISMA trial did demonstrate a modest reduction in subsequent thrombotic events in a subset analysis of patients with stable, preexisting vascular disease, the bottom line was that dual antiplatelet therapy was associated with a 4% risk of moderate to severe bleeding and that there were strong correlations between moderate bleeding and all cause mortality (hazard ratio, 2.55), myocardial infarction (HR, 2.92) and stroke (HR, 4.2).4 The bleeding risk of dual antiplatelet therapy thus seems to outweigh the benefits.
Other data muddy the situation, but only a little. While at least two small studies (one a randomized controlled trial – CARESS [Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis])5 have suggested that the combination of aspirin and clopidogrel may reduce the frequency of perioperative microembolic signals detected early after carotid endarterectomy compared with monotherapy, it is not established that this surrogate measure would translate into long-term clinical benefit and would outweigh the established bleeding risk of dual therapy.
At present, the vascular surgeon should urge his patients to stop smoking, prescribe a statin, control blood pressure (but not necessarily with a beta-blocker), control diabetes, and prescribe a single antiplatelet agent, most often baby aspirin, for most of his or her patients. More good than harm will result.
References
Dr. Mills is professor of surgery and chief, division of vascular and endovascular surgery, University of Arizona Health Sciences Center, Tucson, and an associate medical editor for Vascular Specialist. He has no relevant conflicts.
Introduction
As evidenced by this month’s Point/Counterpoint article by Dr. William Jordan and Dr. Joseph Mills, there is still debate as to the benefit of antiplatelet agents in patients with peripheral artery disease. Currently, dual antiplatelet agents refer to aspirin and clopidogrel, but over the last year or two, ticagrelor and vorapaxar are also being prescribed for patients with peripheral atherosclerosis. The addition of these medications will probably only add to our confusion! Why don’t you weigh in on this discussion by voting on our online poll at www.vascularspecialistonline.com?
Dr. Russell Samson is the medical editor of Vascular Specialist.
Definitely, maybe.
William D. Jordan, M.D.
First, primary prevention must be considered, as few patients with no prior intervention require dual antiplatelet therapy. It seems that we only have some scant data on the prevention of first-time events in at-risk patients when they are treated with aspirin alone.
While lipid management seems to be the most recent focus for primary prevention, single antiplatelet therapy seems appropriate for many patients who have higher risk due to atherosclerotic disease. A recent study from the University of Alabama at Birmingham found that asymptomatic carotid artery stenosis patients treated with dual antiplatelet therapy actually had higher bleed rates, higher mortality, and lower neurologic event rates, compared with those treated with aspirin alone.
While this study examined only a select group of patients treated for carotid artery disease, these vascular patients had worse outcomes when treated with aspirin and clopidogrel. Thus, caution should be considered before adding too many medical therapies.
Now, consider the short-term outlook for patients – specifically those who undergo some type of vascular intervention. The very nature of vascular intervention is disruptive to the arterial endothelium. Of course, most of the arteries that we enter have some underlying pathology; thus the intimal layer is not normal. The pathologic process is already, at least, partly underway. The concept of antiplatelet therapy is focused on limiting the platelet adhesions that might exaggerate the response to injury that creates a hyperplastic reaction within the vessels. We abhor the excessive response to injury due to the potential failure of the arterial reconstruction. Paradoxically, the same platelet inhibition can also cause excessive bleeding that may complicate the vascular repair. In the current medical climate, most of us tolerate the aggravation of diminished platelet function during open reconstruction in order to protect the target repair site and to avoid the dreaded “troponin leak” that may get classified as a myocardial infarction.
Some of our nonvascular colleagues avoid intervention (e.g., axial anesthesia, endoscopic biopsy) if stronger antiplatelet medication is present.
Now, to borrow some insight from other specialties, we should consider the extensive cardiology literature that shows the value of dual platelet inhibition after percutaneous coronary intervention or a recent cardiac event. While this strategy has shown improvement in PCI results, probably due to the great risk of thrombosis when disrupting the endothelial layer of a 2- to 3-mm coronary artery, the bleeding complications, including access-site hematomas, pseudoaneurysms, or retroperitoneal bleeding, are not analyzed as extensively. The preponderance of literature supports aggressive inhibition, but the long term needs to be considered – both expense and bleeding risk.
Currently, we use short-term dual treatment when the arterial endothelium is intentionally disrupted such as after an endarterectomy, angioplasty, with or without a stent, or atherectomy. Specifically, we add short-term IV dextran to oral agents for patients undergoing a carotid endarterectomy, but most are discharged on a single oral agent unless there is another indication for dual therapy.
Patients undergoing lower-extremity, catheter-based interventions are given dual therapy for the first 30 days, which is then adjusted according to the clinical response, duplex findings and other medical conditions. Bypass graft patients (yes, we still do some) are usually given a single agent unless the graft or patient has exhibited some concern for early failure.
In summary, I suspect that dual therapy is overused in our “pill-driven” population, but there still seem to be areas for its application. On each encounter with patients, I would encourage all vascular specialists to review the indications for antiplatelet therapy to consider removing a medication, improving compliance, and limiting bleeding risk.
Dr. Jordan is the director of the division of vascular surgery at the University of Alabama Birmingham School of Medicine. He has no relevant conflicts.
Dueling over dual antiplatelet therapy
Joseph L. Mills Sr., M.D.
Amidst a background of constant clamoring for data-driven and evidence-based medical decision-making, the practice of vascular medicine and surgery remains mired in the anecdotal. If a little is good, more must be better, seems the rationale for dual antiplatelet therapy. There may be small, as yet unclearly defined subsets of patients for whom dual therapy is actually beneficial, but an unbiased review of the current literature leads to the inescapable conclusion that more patients would be harmed by injudicious application of the more-is-better principle as concerns antiplatelet therapy for patients with carotid artery and peripheral artery disease (PAD).
The best evidence for antiplatelet therapy in patients with carotid disease suggests that aspirin as a single agent in doses ranging from 75 to 150 mg daily is preferred, and at least two meta-analyses and systematic reviews were unable to support the use of dual antiplatelet therapy.1 At least in symptomatic patients, combination or dual antiplatelet therapy may increase the short-term risk of hemorrhagic complications in patients with acute ischemic stroke caused by large-artery disease.2 Long-term dual therapy for secondary stroke prevention with aspirin and clopidogrel has been associated with four times the bleeding risk of monotherapy.3 These bleeds are often intracranial or gastrointestinal and are serious.
With respect to asymptomatic patients, while the CHARISMA trial did demonstrate a modest reduction in subsequent thrombotic events in a subset analysis of patients with stable, preexisting vascular disease, the bottom line was that dual antiplatelet therapy was associated with a 4% risk of moderate to severe bleeding and that there were strong correlations between moderate bleeding and all cause mortality (hazard ratio, 2.55), myocardial infarction (HR, 2.92) and stroke (HR, 4.2).4 The bleeding risk of dual antiplatelet therapy thus seems to outweigh the benefits.
Other data muddy the situation, but only a little. While at least two small studies (one a randomized controlled trial – CARESS [Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis])5 have suggested that the combination of aspirin and clopidogrel may reduce the frequency of perioperative microembolic signals detected early after carotid endarterectomy compared with monotherapy, it is not established that this surrogate measure would translate into long-term clinical benefit and would outweigh the established bleeding risk of dual therapy.
At present, the vascular surgeon should urge his patients to stop smoking, prescribe a statin, control blood pressure (but not necessarily with a beta-blocker), control diabetes, and prescribe a single antiplatelet agent, most often baby aspirin, for most of his or her patients. More good than harm will result.
References
Dr. Mills is professor of surgery and chief, division of vascular and endovascular surgery, University of Arizona Health Sciences Center, Tucson, and an associate medical editor for Vascular Specialist. He has no relevant conflicts.
Introduction
As evidenced by this month’s Point/Counterpoint article by Dr. William Jordan and Dr. Joseph Mills, there is still debate as to the benefit of antiplatelet agents in patients with peripheral artery disease. Currently, dual antiplatelet agents refer to aspirin and clopidogrel, but over the last year or two, ticagrelor and vorapaxar are also being prescribed for patients with peripheral atherosclerosis. The addition of these medications will probably only add to our confusion! Why don’t you weigh in on this discussion by voting on our online poll at www.vascularspecialistonline.com?
Dr. Russell Samson is the medical editor of Vascular Specialist.
Definitely, maybe.
William D. Jordan, M.D.
First, primary prevention must be considered, as few patients with no prior intervention require dual antiplatelet therapy. It seems that we only have some scant data on the prevention of first-time events in at-risk patients when they are treated with aspirin alone.
While lipid management seems to be the most recent focus for primary prevention, single antiplatelet therapy seems appropriate for many patients who have higher risk due to atherosclerotic disease. A recent study from the University of Alabama at Birmingham found that asymptomatic carotid artery stenosis patients treated with dual antiplatelet therapy actually had higher bleed rates, higher mortality, and lower neurologic event rates, compared with those treated with aspirin alone.
While this study examined only a select group of patients treated for carotid artery disease, these vascular patients had worse outcomes when treated with aspirin and clopidogrel. Thus, caution should be considered before adding too many medical therapies.
Now, consider the short-term outlook for patients – specifically those who undergo some type of vascular intervention. The very nature of vascular intervention is disruptive to the arterial endothelium. Of course, most of the arteries that we enter have some underlying pathology; thus the intimal layer is not normal. The pathologic process is already, at least, partly underway. The concept of antiplatelet therapy is focused on limiting the platelet adhesions that might exaggerate the response to injury that creates a hyperplastic reaction within the vessels. We abhor the excessive response to injury due to the potential failure of the arterial reconstruction. Paradoxically, the same platelet inhibition can also cause excessive bleeding that may complicate the vascular repair. In the current medical climate, most of us tolerate the aggravation of diminished platelet function during open reconstruction in order to protect the target repair site and to avoid the dreaded “troponin leak” that may get classified as a myocardial infarction.
Some of our nonvascular colleagues avoid intervention (e.g., axial anesthesia, endoscopic biopsy) if stronger antiplatelet medication is present.
Now, to borrow some insight from other specialties, we should consider the extensive cardiology literature that shows the value of dual platelet inhibition after percutaneous coronary intervention or a recent cardiac event. While this strategy has shown improvement in PCI results, probably due to the great risk of thrombosis when disrupting the endothelial layer of a 2- to 3-mm coronary artery, the bleeding complications, including access-site hematomas, pseudoaneurysms, or retroperitoneal bleeding, are not analyzed as extensively. The preponderance of literature supports aggressive inhibition, but the long term needs to be considered – both expense and bleeding risk.
Currently, we use short-term dual treatment when the arterial endothelium is intentionally disrupted such as after an endarterectomy, angioplasty, with or without a stent, or atherectomy. Specifically, we add short-term IV dextran to oral agents for patients undergoing a carotid endarterectomy, but most are discharged on a single oral agent unless there is another indication for dual therapy.
Patients undergoing lower-extremity, catheter-based interventions are given dual therapy for the first 30 days, which is then adjusted according to the clinical response, duplex findings and other medical conditions. Bypass graft patients (yes, we still do some) are usually given a single agent unless the graft or patient has exhibited some concern for early failure.
In summary, I suspect that dual therapy is overused in our “pill-driven” population, but there still seem to be areas for its application. On each encounter with patients, I would encourage all vascular specialists to review the indications for antiplatelet therapy to consider removing a medication, improving compliance, and limiting bleeding risk.
Dr. Jordan is the director of the division of vascular surgery at the University of Alabama Birmingham School of Medicine. He has no relevant conflicts.
Dueling over dual antiplatelet therapy
Joseph L. Mills Sr., M.D.
Amidst a background of constant clamoring for data-driven and evidence-based medical decision-making, the practice of vascular medicine and surgery remains mired in the anecdotal. If a little is good, more must be better, seems the rationale for dual antiplatelet therapy. There may be small, as yet unclearly defined subsets of patients for whom dual therapy is actually beneficial, but an unbiased review of the current literature leads to the inescapable conclusion that more patients would be harmed by injudicious application of the more-is-better principle as concerns antiplatelet therapy for patients with carotid artery and peripheral artery disease (PAD).
The best evidence for antiplatelet therapy in patients with carotid disease suggests that aspirin as a single agent in doses ranging from 75 to 150 mg daily is preferred, and at least two meta-analyses and systematic reviews were unable to support the use of dual antiplatelet therapy.1 At least in symptomatic patients, combination or dual antiplatelet therapy may increase the short-term risk of hemorrhagic complications in patients with acute ischemic stroke caused by large-artery disease.2 Long-term dual therapy for secondary stroke prevention with aspirin and clopidogrel has been associated with four times the bleeding risk of monotherapy.3 These bleeds are often intracranial or gastrointestinal and are serious.
With respect to asymptomatic patients, while the CHARISMA trial did demonstrate a modest reduction in subsequent thrombotic events in a subset analysis of patients with stable, preexisting vascular disease, the bottom line was that dual antiplatelet therapy was associated with a 4% risk of moderate to severe bleeding and that there were strong correlations between moderate bleeding and all cause mortality (hazard ratio, 2.55), myocardial infarction (HR, 2.92) and stroke (HR, 4.2).4 The bleeding risk of dual antiplatelet therapy thus seems to outweigh the benefits.
Other data muddy the situation, but only a little. While at least two small studies (one a randomized controlled trial – CARESS [Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis])5 have suggested that the combination of aspirin and clopidogrel may reduce the frequency of perioperative microembolic signals detected early after carotid endarterectomy compared with monotherapy, it is not established that this surrogate measure would translate into long-term clinical benefit and would outweigh the established bleeding risk of dual therapy.
At present, the vascular surgeon should urge his patients to stop smoking, prescribe a statin, control blood pressure (but not necessarily with a beta-blocker), control diabetes, and prescribe a single antiplatelet agent, most often baby aspirin, for most of his or her patients. More good than harm will result.
References
Dr. Mills is professor of surgery and chief, division of vascular and endovascular surgery, University of Arizona Health Sciences Center, Tucson, and an associate medical editor for Vascular Specialist. He has no relevant conflicts.
Guidelines: Urinate 2 liters daily to stop kidney stones’ return
Patients who have had kidney stones at least once should increase their fluid consumption to produce at least 2 L of urine per day to prevent the condition from recurring, according to new guidelines published by the American College of Physicians.
If increased fluid intake doesn’t prevent recurrent nephrolithiasis, the updated ACP guidelines recommend adding treatment with a thiazide diuretic, citrate, or allopurinol. “Increased fluid intake spread throughout the day can decrease stone recurrence by at least half with virtually no side effects,” said Dr. David A. Fleming, ACP president, in a statement. “However, people who already drink the recommended amount of liquids, or when increased fluid intake is contraindicated, should not increase their fluid intake.”
The guidelines are based on a review of nephrolithiasis studies, in which the ACP’s director of clinical policy, Dr. Amir Qaseem, and his associates examined baseline stone composition, blood and urine chemistries, and final health outcomes, among other factors. Only English-language trials were used, and research was collected through searches of MEDLINE, the Cochrane Database of Systematic Reviews, Google Scholar, ClinicalTrials.gov, and Web of Science (Ann. Intern. Med. 2014;161:659-67).
Thiazide diuretics, citrates, or allopurinol significantly reduced recurrence of calcium-based stones (the most common type of kidney stone) in patients who had nephrolithiasis at least twice before, according to the analysis.
The ACP guidelines also caution against consuming non-fruit–flavored sodas and carbonated beverages, because they are acidified by phosphoric acid, and dietary animal protein and purines.
However, the guidelines encourage consumption of dietary oxalate – commonly found in chocolate, beets, nuts, rhubarb, spinach, strawberries, tea, and wheat bran – and support maintaining normal dietary calcium. There was no evidence to suggest a risk reduction advantage in drinking tap water rather than mineral water.
The recommendations do not apply to “patients with suspected hyperparathyroidism or other rare cases,” the guidelines authors noted.
Financial support for the guidelines came from the ACP operating budget. The authors had no other relevant financial disclosures.
Patients who have had kidney stones at least once should increase their fluid consumption to produce at least 2 L of urine per day to prevent the condition from recurring, according to new guidelines published by the American College of Physicians.
If increased fluid intake doesn’t prevent recurrent nephrolithiasis, the updated ACP guidelines recommend adding treatment with a thiazide diuretic, citrate, or allopurinol. “Increased fluid intake spread throughout the day can decrease stone recurrence by at least half with virtually no side effects,” said Dr. David A. Fleming, ACP president, in a statement. “However, people who already drink the recommended amount of liquids, or when increased fluid intake is contraindicated, should not increase their fluid intake.”
The guidelines are based on a review of nephrolithiasis studies, in which the ACP’s director of clinical policy, Dr. Amir Qaseem, and his associates examined baseline stone composition, blood and urine chemistries, and final health outcomes, among other factors. Only English-language trials were used, and research was collected through searches of MEDLINE, the Cochrane Database of Systematic Reviews, Google Scholar, ClinicalTrials.gov, and Web of Science (Ann. Intern. Med. 2014;161:659-67).
Thiazide diuretics, citrates, or allopurinol significantly reduced recurrence of calcium-based stones (the most common type of kidney stone) in patients who had nephrolithiasis at least twice before, according to the analysis.
The ACP guidelines also caution against consuming non-fruit–flavored sodas and carbonated beverages, because they are acidified by phosphoric acid, and dietary animal protein and purines.
However, the guidelines encourage consumption of dietary oxalate – commonly found in chocolate, beets, nuts, rhubarb, spinach, strawberries, tea, and wheat bran – and support maintaining normal dietary calcium. There was no evidence to suggest a risk reduction advantage in drinking tap water rather than mineral water.
The recommendations do not apply to “patients with suspected hyperparathyroidism or other rare cases,” the guidelines authors noted.
Financial support for the guidelines came from the ACP operating budget. The authors had no other relevant financial disclosures.
Patients who have had kidney stones at least once should increase their fluid consumption to produce at least 2 L of urine per day to prevent the condition from recurring, according to new guidelines published by the American College of Physicians.
If increased fluid intake doesn’t prevent recurrent nephrolithiasis, the updated ACP guidelines recommend adding treatment with a thiazide diuretic, citrate, or allopurinol. “Increased fluid intake spread throughout the day can decrease stone recurrence by at least half with virtually no side effects,” said Dr. David A. Fleming, ACP president, in a statement. “However, people who already drink the recommended amount of liquids, or when increased fluid intake is contraindicated, should not increase their fluid intake.”
The guidelines are based on a review of nephrolithiasis studies, in which the ACP’s director of clinical policy, Dr. Amir Qaseem, and his associates examined baseline stone composition, blood and urine chemistries, and final health outcomes, among other factors. Only English-language trials were used, and research was collected through searches of MEDLINE, the Cochrane Database of Systematic Reviews, Google Scholar, ClinicalTrials.gov, and Web of Science (Ann. Intern. Med. 2014;161:659-67).
Thiazide diuretics, citrates, or allopurinol significantly reduced recurrence of calcium-based stones (the most common type of kidney stone) in patients who had nephrolithiasis at least twice before, according to the analysis.
The ACP guidelines also caution against consuming non-fruit–flavored sodas and carbonated beverages, because they are acidified by phosphoric acid, and dietary animal protein and purines.
However, the guidelines encourage consumption of dietary oxalate – commonly found in chocolate, beets, nuts, rhubarb, spinach, strawberries, tea, and wheat bran – and support maintaining normal dietary calcium. There was no evidence to suggest a risk reduction advantage in drinking tap water rather than mineral water.
The recommendations do not apply to “patients with suspected hyperparathyroidism or other rare cases,” the guidelines authors noted.
Financial support for the guidelines came from the ACP operating budget. The authors had no other relevant financial disclosures.
FROM THE ANNALS OF INTERNAL MEDICINE
Hospitalist Pioneer Bob Wachter Warns Waste Reduction Is New Quality Focus
Dr. Wachter closed SHM's 2013 annual meeting in National Harbor, MD, with a keynote address that identified cost and waste reduction as new planks of hospitalist's value proposition.
Dr. Wachter closed SHM's 2013 annual meeting in National Harbor, MD, with a keynote address that identified cost and waste reduction as new planks of hospitalist's value proposition.
Dr. Wachter closed SHM's 2013 annual meeting in National Harbor, MD, with a keynote address that identified cost and waste reduction as new planks of hospitalist's value proposition.
Evidence-Based Medicine Guru Implores Hospitalists to Join Cause
Gordon Guyatt, MD, who coined the term evidence-based medicine in a 1992 JAMA article, outlined EBM principles and challenged hospitalists to challenge the research.
Gordon Guyatt, MD, who coined the term evidence-based medicine in a 1992 JAMA article, outlined EBM principles and challenged hospitalists to challenge the research.
Gordon Guyatt, MD, who coined the term evidence-based medicine in a 1992 JAMA article, outlined EBM principles and challenged hospitalists to challenge the research.





