User login
Clomiphene better than letrozole to treat women with unexplained infertility
When the cause of infertility is unexplained, what is the best first-choice treatment option? Could letrozole, an oral nonsteroidal aromatase inhibitor, result in fewer multiple gestations than current standard therapy—gonadotropins or selective estrogen receptor modulators (clomiphene citrate)—without worsening the live birth rate?
Researchers from the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) trial investigated this question and presented data at the recent annual meeting of the American Society of Reproductive Medicine. The research was supported by the National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Study details
This prospective, randomized, multicenter clinical trial involved 900 women aged 18 to 40 with at least one patent fallopian tube and regular menses. Patients underwent ovarian stimulation for up to four cycles with an injectable gonadotropin (Gn; n = 300), clomiphene citrate (n = 301), or letrozole (n = 299), followed by intrauterine insemination (IUI).
Birth rate. Conception occurred in 46.8%, 35.7%, and 28.4% of women receiving Gn, clomiphene, and letrozole, respectively, with a live birth occurring in 32.2%, 23.3%, and 18.7% of respective cases. Pregnancy rates with letrozole were significantly less than with Gn (P<.001) and less than with clomiphene (P<.015).
Multiple gestations. The rate of multiple gestations was highest among women treated with Gn (10.3%). But the multiple gestation rate for letrozole was higher than that for clomiphene (2.7% vs 1.3%, respectively). All multiples treated with letrozole and clomiphene were twins; in the Gn group, there were 24 twin and 10 triplet gestations.
No significant difference was found in the rates of infants with congenital anomalies or other fetal or neonatal complications.1
Clomiphene plus IUI remains first-line therapy for unexplained infertility
Although ovarian stimulation with letrozole was safe overall, the number of live births was reduced when treatment with letrozole was compared with clomiphene or Gn, and the multiple pregnancy rate for letrozole fell between clomiphene and Gn.
“CC [clomiphene citrate] /IUI remains first-line therapy for women with unexplained infertility,” concludes Michael P. Diamond, Chair and Professor of the Department of Obstetrics and Gynecology at Georgia Regents University in Augusta, Georgia.
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
Reference
Diamond MP. Outcomes of the NICHD’s comparative effectiveness assessment of multiple intrauterine gestations from ovarian stimulation (AMIGOS) trial. The NICHD cooperative reproductive medicine network. Fertil Steril. 2014;102(3):e39. http://www.fertstert.org/article/S0015-0282%2814%2900767-5/fulltext. Accessed October 31, 2014.
When the cause of infertility is unexplained, what is the best first-choice treatment option? Could letrozole, an oral nonsteroidal aromatase inhibitor, result in fewer multiple gestations than current standard therapy—gonadotropins or selective estrogen receptor modulators (clomiphene citrate)—without worsening the live birth rate?
Researchers from the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) trial investigated this question and presented data at the recent annual meeting of the American Society of Reproductive Medicine. The research was supported by the National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Study details
This prospective, randomized, multicenter clinical trial involved 900 women aged 18 to 40 with at least one patent fallopian tube and regular menses. Patients underwent ovarian stimulation for up to four cycles with an injectable gonadotropin (Gn; n = 300), clomiphene citrate (n = 301), or letrozole (n = 299), followed by intrauterine insemination (IUI).
Birth rate. Conception occurred in 46.8%, 35.7%, and 28.4% of women receiving Gn, clomiphene, and letrozole, respectively, with a live birth occurring in 32.2%, 23.3%, and 18.7% of respective cases. Pregnancy rates with letrozole were significantly less than with Gn (P<.001) and less than with clomiphene (P<.015).
Multiple gestations. The rate of multiple gestations was highest among women treated with Gn (10.3%). But the multiple gestation rate for letrozole was higher than that for clomiphene (2.7% vs 1.3%, respectively). All multiples treated with letrozole and clomiphene were twins; in the Gn group, there were 24 twin and 10 triplet gestations.
No significant difference was found in the rates of infants with congenital anomalies or other fetal or neonatal complications.1
Clomiphene plus IUI remains first-line therapy for unexplained infertility
Although ovarian stimulation with letrozole was safe overall, the number of live births was reduced when treatment with letrozole was compared with clomiphene or Gn, and the multiple pregnancy rate for letrozole fell between clomiphene and Gn.
“CC [clomiphene citrate] /IUI remains first-line therapy for women with unexplained infertility,” concludes Michael P. Diamond, Chair and Professor of the Department of Obstetrics and Gynecology at Georgia Regents University in Augusta, Georgia.
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
When the cause of infertility is unexplained, what is the best first-choice treatment option? Could letrozole, an oral nonsteroidal aromatase inhibitor, result in fewer multiple gestations than current standard therapy—gonadotropins or selective estrogen receptor modulators (clomiphene citrate)—without worsening the live birth rate?
Researchers from the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) trial investigated this question and presented data at the recent annual meeting of the American Society of Reproductive Medicine. The research was supported by the National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Study details
This prospective, randomized, multicenter clinical trial involved 900 women aged 18 to 40 with at least one patent fallopian tube and regular menses. Patients underwent ovarian stimulation for up to four cycles with an injectable gonadotropin (Gn; n = 300), clomiphene citrate (n = 301), or letrozole (n = 299), followed by intrauterine insemination (IUI).
Birth rate. Conception occurred in 46.8%, 35.7%, and 28.4% of women receiving Gn, clomiphene, and letrozole, respectively, with a live birth occurring in 32.2%, 23.3%, and 18.7% of respective cases. Pregnancy rates with letrozole were significantly less than with Gn (P<.001) and less than with clomiphene (P<.015).
Multiple gestations. The rate of multiple gestations was highest among women treated with Gn (10.3%). But the multiple gestation rate for letrozole was higher than that for clomiphene (2.7% vs 1.3%, respectively). All multiples treated with letrozole and clomiphene were twins; in the Gn group, there were 24 twin and 10 triplet gestations.
No significant difference was found in the rates of infants with congenital anomalies or other fetal or neonatal complications.1
Clomiphene plus IUI remains first-line therapy for unexplained infertility
Although ovarian stimulation with letrozole was safe overall, the number of live births was reduced when treatment with letrozole was compared with clomiphene or Gn, and the multiple pregnancy rate for letrozole fell between clomiphene and Gn.
“CC [clomiphene citrate] /IUI remains first-line therapy for women with unexplained infertility,” concludes Michael P. Diamond, Chair and Professor of the Department of Obstetrics and Gynecology at Georgia Regents University in Augusta, Georgia.
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
Reference
Diamond MP. Outcomes of the NICHD’s comparative effectiveness assessment of multiple intrauterine gestations from ovarian stimulation (AMIGOS) trial. The NICHD cooperative reproductive medicine network. Fertil Steril. 2014;102(3):e39. http://www.fertstert.org/article/S0015-0282%2814%2900767-5/fulltext. Accessed October 31, 2014.
Reference
Diamond MP. Outcomes of the NICHD’s comparative effectiveness assessment of multiple intrauterine gestations from ovarian stimulation (AMIGOS) trial. The NICHD cooperative reproductive medicine network. Fertil Steril. 2014;102(3):e39. http://www.fertstert.org/article/S0015-0282%2814%2900767-5/fulltext. Accessed October 31, 2014.
Bob Wachter Says Cost Equation Is Shifting in Ever-Changing Healthcare Paradigm
HM pioneer says hospitalists who have flown under radar soon will be counted on to produce cost, waste reduction.
HM pioneer says hospitalists who have flown under radar soon will be counted on to produce cost, waste reduction.
HM pioneer says hospitalists who have flown under radar soon will be counted on to produce cost, waste reduction.
Hospitalists Flock to Annual Meeting's Bedside Procedures Pre-Courses
From early-career hospitalists looking to gain hands-on experience with intraosseous lines to family-medicine trained physicians brushing up on ultrasound usage, the procedures' pre-courses at SHM annual meetings receive rave reviews.
From early-career hospitalists looking to gain hands-on experience with intraosseous lines to family-medicine trained physicians brushing up on ultrasound usage, the procedures' pre-courses at SHM annual meetings receive rave reviews.
From early-career hospitalists looking to gain hands-on experience with intraosseous lines to family-medicine trained physicians brushing up on ultrasound usage, the procedures' pre-courses at SHM annual meetings receive rave reviews.
Congenital uterine anomalies: A resource of diagnostic images, Part 1
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of discussing the various uterine malformations as well as characterizing their appearance on 3D transvaginal ultrasound.
Unfortunately, many women are still subjected to the cost, inconvenience, and time involvement of magnetic resonance imaging (MRI) in cases of suspected uterine malformations. The exquisite visualization of 3D transvaginal ultrasound, so nicely depicted in this installment of Images in GYN Ultrasound, allow the observer to see the endometrial contours in the same plane as the serosal surface. This view is not available in traditional 2D ultrasound images. Thus, it is akin to doing laparoscopy and hysteroscopy simultaneously in order to arrive at the proper diagnosis. Although not mandatory, when such 3D ultrasound is performed late in the cycle, the thickened endometrium acts as a nice sonic backdrop to better delineate these structures. Alternatively, 3D saline infusion sonohysterography can be performed.
As more and more ultrasound equipment becomes available with 3D capability as a standard feature, clinicians who do perform ultrasonography will find that obtaining this “z-plane” is relatively simple and extremely informative, and can and should be done in cases of suspected uterine malformations in lieu of ordering MRI.
Congenital uterine anomalies: A resource of diagnostic images, Part 1
Michelle L. Stalnaker Ozcan, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
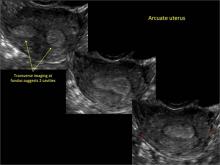
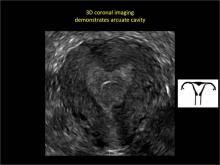
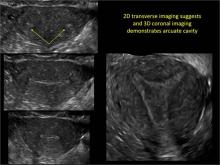
Uterine malformations make up a diverse group of congenital anomalies that can result from various alterations in the normal development of the Müllerian ducts, including underdevelopment of one or both Müllerian ducts, disorders in Müllerian duct fusion, and alterations in septum reabsorption. How common are such anomalies, how are they classified, and what is the best approach for optimal visualization? Here, we explore these questions and offer an atlas of diagnostic images as an ongoing reference for your practice. Many of the images we offer will be found only online at obgmanagement.com.
How common are congenital uterine anomalies?
The reported prevalence of uterine malformations varies among publications due to heterogeneous population samples, differences in diagnostic techniques, and variations in nomenclature. In general, they are estimated to occur in 0.4% (0.1% to 3.0%) of the population at large, 4% of infertile women, and between 3% and 38% of women with repetitive spontaneous miscarriage.1
Classical classification
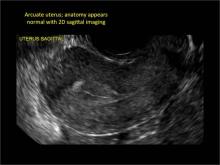
A classification of the Müllerian anomalies was introduced in 1980 and, with few modifications, was adopted by the American Fertility Society (currently, ASRM). The Society identified seven basic groups according to Müllerian development and their relationship to fertility: agenesis and hypoplasias, unicornuate uteri (unilateral hypoplasia), didelphys uteri (complete nonfusion), bicornuate uteri (incomplete fusion), septate uteri (nonreabsorption of septum), arcuate uteri (almost complete reabsorption of septum), and anomalies related to fetal DES exposure.2
Anomalies also can be categorized in terms of progression along the developmental continuum, taking into account that many cases result from partial failure of fusion and reabsorption: agenesis (Types I and II), lack of fusion (Types III and IV), lack of reabsorption(Types V and VI), and lack of posterior development (Type VII) (FIGURE 1).3
| FIGURE 1. Classification of müllerian anomalies |
|---|

|
| Source: The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988. 49(6):944-955. |
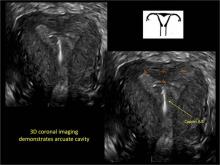
3D ultrasonography offers accurate, cost-efficient diagnosis
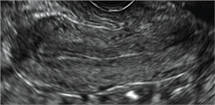
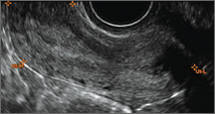
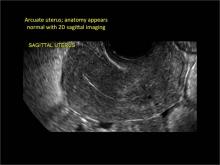
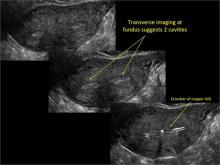
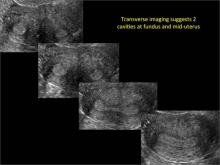
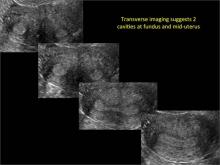
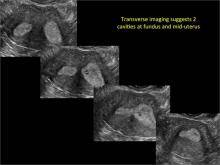
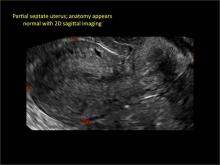
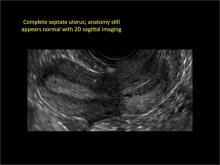
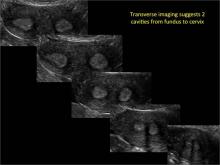
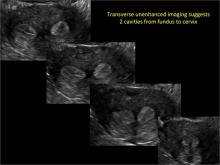
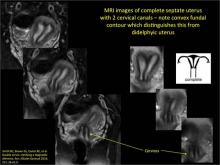
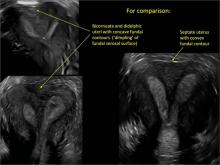
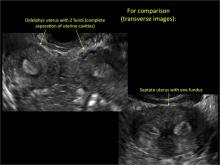
Using only 2D imaging, neither an unenhanced sonogram nor a sonohysterogram can provide definitive information regarding the possibility of a uterine anomaly. The fundal contour cannot be evaluated with 2D imaging; likewise, details regarding the configuration of the uterine cavity (or cavities) may not be appreciated with the use of 2D imaging (FIGURE 2).
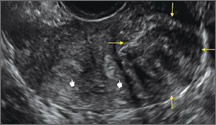
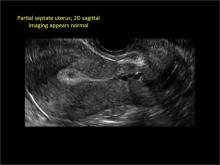
Figure 2: Normal appearance, but abnormal uteri
In sagittal view, a uterus with a congenital anomaly can appear normal. 2D sagittal views of a normal uterus (top), a didelphic uterus (middle), and a sonohysterogram of a septate uterus (bottom). |
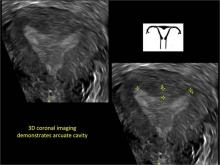
To fully evaluate the uterine fundal contour and determine the type of uterine anomaly, it previously was necessary to obtain magnetic resonance imaging (MRI) or perform laparoscopy. Today, however, 3D coronal ultrasonography (US) can allow for accurate evaluation of fundal contour and diagnosis of uterine anomalies with lower cost and greater patient convenience. Several studies have confirmed the high accuracy of 3D US compared with MRI and surgical findings in the diagnosis of uterine anomalies (with 3D US showing 98% to 100% sensitivity and specificity).4-6
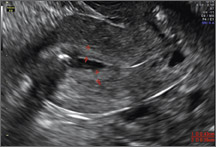
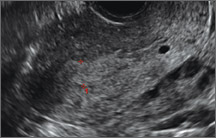
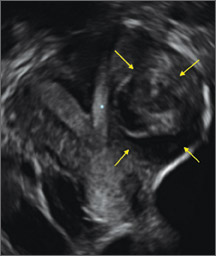
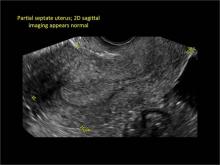
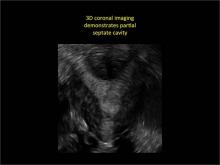
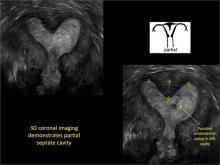
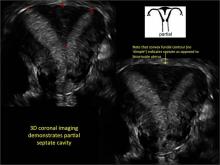
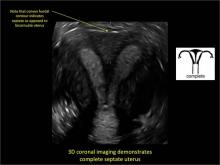
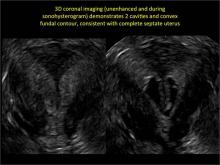
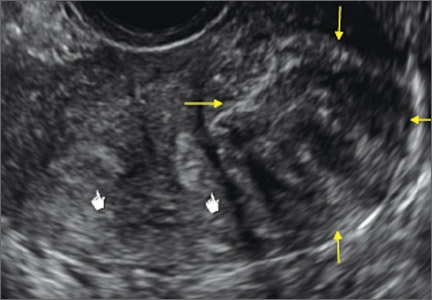
Case: Partial septate uterus
|
ADDITIONAL IMAGES
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bermejo C, Martinez Ten P, Cantarero R, et al. Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;35(5):593–601.
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–955.
- Acien P, Acien M. Updated classification of malformations. Hum Reprod. 2010;25(suppl 1):i81–i82.
- Deutch T, Bocca S, Oehninger S, Stadtmauer L, Abuhamad AZ. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of Müllerian anomalies [abstract P-465]. Fertil Steril. 2006;86(suppl):S308.
- Wu MH, Hsu CC, Huang KE. Detection of congenital Müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound. 1997;25(9):487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of Müllerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of discussing the various uterine malformations as well as characterizing their appearance on 3D transvaginal ultrasound.
Unfortunately, many women are still subjected to the cost, inconvenience, and time involvement of magnetic resonance imaging (MRI) in cases of suspected uterine malformations. The exquisite visualization of 3D transvaginal ultrasound, so nicely depicted in this installment of Images in GYN Ultrasound, allow the observer to see the endometrial contours in the same plane as the serosal surface. This view is not available in traditional 2D ultrasound images. Thus, it is akin to doing laparoscopy and hysteroscopy simultaneously in order to arrive at the proper diagnosis. Although not mandatory, when such 3D ultrasound is performed late in the cycle, the thickened endometrium acts as a nice sonic backdrop to better delineate these structures. Alternatively, 3D saline infusion sonohysterography can be performed.
As more and more ultrasound equipment becomes available with 3D capability as a standard feature, clinicians who do perform ultrasonography will find that obtaining this “z-plane” is relatively simple and extremely informative, and can and should be done in cases of suspected uterine malformations in lieu of ordering MRI.
Congenital uterine anomalies: A resource of diagnostic images, Part 1
Michelle L. Stalnaker Ozcan, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine malformations make up a diverse group of congenital anomalies that can result from various alterations in the normal development of the Müllerian ducts, including underdevelopment of one or both Müllerian ducts, disorders in Müllerian duct fusion, and alterations in septum reabsorption. How common are such anomalies, how are they classified, and what is the best approach for optimal visualization? Here, we explore these questions and offer an atlas of diagnostic images as an ongoing reference for your practice. Many of the images we offer will be found only online at obgmanagement.com.
How common are congenital uterine anomalies?
The reported prevalence of uterine malformations varies among publications due to heterogeneous population samples, differences in diagnostic techniques, and variations in nomenclature. In general, they are estimated to occur in 0.4% (0.1% to 3.0%) of the population at large, 4% of infertile women, and between 3% and 38% of women with repetitive spontaneous miscarriage.1
Classical classification
A classification of the Müllerian anomalies was introduced in 1980 and, with few modifications, was adopted by the American Fertility Society (currently, ASRM). The Society identified seven basic groups according to Müllerian development and their relationship to fertility: agenesis and hypoplasias, unicornuate uteri (unilateral hypoplasia), didelphys uteri (complete nonfusion), bicornuate uteri (incomplete fusion), septate uteri (nonreabsorption of septum), arcuate uteri (almost complete reabsorption of septum), and anomalies related to fetal DES exposure.2
Anomalies also can be categorized in terms of progression along the developmental continuum, taking into account that many cases result from partial failure of fusion and reabsorption: agenesis (Types I and II), lack of fusion (Types III and IV), lack of reabsorption(Types V and VI), and lack of posterior development (Type VII) (FIGURE 1).3
| FIGURE 1. Classification of müllerian anomalies |
|---|

|
| Source: The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988. 49(6):944-955. |
3D ultrasonography offers accurate, cost-efficient diagnosis
Using only 2D imaging, neither an unenhanced sonogram nor a sonohysterogram can provide definitive information regarding the possibility of a uterine anomaly. The fundal contour cannot be evaluated with 2D imaging; likewise, details regarding the configuration of the uterine cavity (or cavities) may not be appreciated with the use of 2D imaging (FIGURE 2).
Figure 2: Normal appearance, but abnormal uteri
In sagittal view, a uterus with a congenital anomaly can appear normal. 2D sagittal views of a normal uterus (top), a didelphic uterus (middle), and a sonohysterogram of a septate uterus (bottom). |
To fully evaluate the uterine fundal contour and determine the type of uterine anomaly, it previously was necessary to obtain magnetic resonance imaging (MRI) or perform laparoscopy. Today, however, 3D coronal ultrasonography (US) can allow for accurate evaluation of fundal contour and diagnosis of uterine anomalies with lower cost and greater patient convenience. Several studies have confirmed the high accuracy of 3D US compared with MRI and surgical findings in the diagnosis of uterine anomalies (with 3D US showing 98% to 100% sensitivity and specificity).4-6
Case: Partial septate uterus
|
ADDITIONAL IMAGES
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
INTRODUCTION
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
In this month’s Images in GYN Ultrasound, Drs. Stalnaker and Kaunitz have done an excellent job of discussing the various uterine malformations as well as characterizing their appearance on 3D transvaginal ultrasound.
Unfortunately, many women are still subjected to the cost, inconvenience, and time involvement of magnetic resonance imaging (MRI) in cases of suspected uterine malformations. The exquisite visualization of 3D transvaginal ultrasound, so nicely depicted in this installment of Images in GYN Ultrasound, allow the observer to see the endometrial contours in the same plane as the serosal surface. This view is not available in traditional 2D ultrasound images. Thus, it is akin to doing laparoscopy and hysteroscopy simultaneously in order to arrive at the proper diagnosis. Although not mandatory, when such 3D ultrasound is performed late in the cycle, the thickened endometrium acts as a nice sonic backdrop to better delineate these structures. Alternatively, 3D saline infusion sonohysterography can be performed.
As more and more ultrasound equipment becomes available with 3D capability as a standard feature, clinicians who do perform ultrasonography will find that obtaining this “z-plane” is relatively simple and extremely informative, and can and should be done in cases of suspected uterine malformations in lieu of ordering MRI.
Congenital uterine anomalies: A resource of diagnostic images, Part 1
Michelle L. Stalnaker Ozcan, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Uterine malformations make up a diverse group of congenital anomalies that can result from various alterations in the normal development of the Müllerian ducts, including underdevelopment of one or both Müllerian ducts, disorders in Müllerian duct fusion, and alterations in septum reabsorption. How common are such anomalies, how are they classified, and what is the best approach for optimal visualization? Here, we explore these questions and offer an atlas of diagnostic images as an ongoing reference for your practice. Many of the images we offer will be found only online at obgmanagement.com.
How common are congenital uterine anomalies?
The reported prevalence of uterine malformations varies among publications due to heterogeneous population samples, differences in diagnostic techniques, and variations in nomenclature. In general, they are estimated to occur in 0.4% (0.1% to 3.0%) of the population at large, 4% of infertile women, and between 3% and 38% of women with repetitive spontaneous miscarriage.1
Classical classification
A classification of the Müllerian anomalies was introduced in 1980 and, with few modifications, was adopted by the American Fertility Society (currently, ASRM). The Society identified seven basic groups according to Müllerian development and their relationship to fertility: agenesis and hypoplasias, unicornuate uteri (unilateral hypoplasia), didelphys uteri (complete nonfusion), bicornuate uteri (incomplete fusion), septate uteri (nonreabsorption of septum), arcuate uteri (almost complete reabsorption of septum), and anomalies related to fetal DES exposure.2
Anomalies also can be categorized in terms of progression along the developmental continuum, taking into account that many cases result from partial failure of fusion and reabsorption: agenesis (Types I and II), lack of fusion (Types III and IV), lack of reabsorption(Types V and VI), and lack of posterior development (Type VII) (FIGURE 1).3
| FIGURE 1. Classification of müllerian anomalies |
|---|

|
| Source: The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988. 49(6):944-955. |
3D ultrasonography offers accurate, cost-efficient diagnosis
Using only 2D imaging, neither an unenhanced sonogram nor a sonohysterogram can provide definitive information regarding the possibility of a uterine anomaly. The fundal contour cannot be evaluated with 2D imaging; likewise, details regarding the configuration of the uterine cavity (or cavities) may not be appreciated with the use of 2D imaging (FIGURE 2).
Figure 2: Normal appearance, but abnormal uteri
In sagittal view, a uterus with a congenital anomaly can appear normal. 2D sagittal views of a normal uterus (top), a didelphic uterus (middle), and a sonohysterogram of a septate uterus (bottom). |
To fully evaluate the uterine fundal contour and determine the type of uterine anomaly, it previously was necessary to obtain magnetic resonance imaging (MRI) or perform laparoscopy. Today, however, 3D coronal ultrasonography (US) can allow for accurate evaluation of fundal contour and diagnosis of uterine anomalies with lower cost and greater patient convenience. Several studies have confirmed the high accuracy of 3D US compared with MRI and surgical findings in the diagnosis of uterine anomalies (with 3D US showing 98% to 100% sensitivity and specificity).4-6
Case: Partial septate uterus
|
ADDITIONAL IMAGES
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bermejo C, Martinez Ten P, Cantarero R, et al. Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;35(5):593–601.
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–955.
- Acien P, Acien M. Updated classification of malformations. Hum Reprod. 2010;25(suppl 1):i81–i82.
- Deutch T, Bocca S, Oehninger S, Stadtmauer L, Abuhamad AZ. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of Müllerian anomalies [abstract P-465]. Fertil Steril. 2006;86(suppl):S308.
- Wu MH, Hsu CC, Huang KE. Detection of congenital Müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound. 1997;25(9):487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of Müllerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
- Bermejo C, Martinez Ten P, Cantarero R, et al. Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol. 2010;35(5):593–601.
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–955.
- Acien P, Acien M. Updated classification of malformations. Hum Reprod. 2010;25(suppl 1):i81–i82.
- Deutch T, Bocca S, Oehninger S, Stadtmauer L, Abuhamad AZ. Magnetic resonance imaging versus three-dimensional transvaginal ultrasound for the diagnosis of Müllerian anomalies [abstract P-465]. Fertil Steril. 2006;86(suppl):S308.
- Wu MH, Hsu CC, Huang KE. Detection of congenital Müllerian duct anomalies using three-dimensional ultrasound. J Clin Ultrasound. 1997;25(9):487–492.
- Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of Müllerian duct anomalies. J Ultrasound Med. 2008;27(3):413–423.
Hair Loss in a 12-Year-Old
A mother brings her 12-year-old son to dermatology following a referral from the boy’s pediatrician. Several months ago, she noticed her son’s hair loss. The change had been preceded by a stressful period in which she and her husband divorced and one of the boy’s grandparents died unexpectedly.
Both the mother and other relatives and friends had observed the boy reaching for his scalp frequently and twirling his hair “absentmindedly.” When asked if the area in question bothers him, the boy always answers in the negative. Although he knows he should leave his scalp and hair alone, he says he finds it difficult to do so—even though he acknowledges the social liability of his hair loss. According to the mother, the more his family discourages his behavior, the more it persists.
EXAMINATION
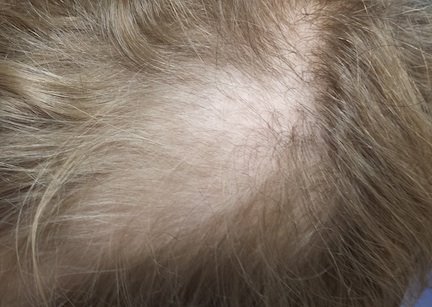
Distinct but incomplete hair loss is noted in an 8 x 10–cm area of his scalp crown. There is neither redness nor any disturbance to the skin there. On palpation, there is no tenderness or increased warmth. No nodes are felt in the adjacent head or neck. Hair pull test is negative.
Closer examination shows hairs of varying lengths in the affected area: many quite short, others of normal length, and many of intermediate length.
Blood work done by the referring pediatrician—including complete blood count, chemistry panel, antinuclear antibody test, and thyroid testing—yielded no abnormal results.
What is the diagnosis?
DISCUSSION
Hair loss, collectively termed alopecia, is a disturbing development, especially in a child. In this case, we had localized hair loss most likely caused by behavior that was not only witnessed by the boy’s parents but also admitted to by the patient. (We’re not always so fortunate.) Thus, it was fairly straightforward to diagnosis trichotillomania, also known as trichotillosis or hair-pulling disorder. This condition can mimic alopecia areata and tinea capitis.
In this case, the lack of epidermal change (scaling, redness, edema) and palpable adenopathy spoke loudly against fungal infection. The hair loss in alopecia areata (AA) is usually sharply defined and complete, which our patient’s hair loss was not. And the blood work that was done effectively ruled out systemic disease (an unlikely cause of localized hair loss in any case).
The jury is still out as to how exactly to classify trichotillomania (TTM). The new DSM-V lists it as an anxiety disorder, in part because it often appears in that context. What we do know is that girls are twice as likely as boys to be affected. And children ages 4 to 13 are seven times more likely than adults to develop TTM.
TTM can involve hair anywhere on the body, though children almost always confine their behavior to their scalp. Actual hair-pulling is not necessarily seen. Manipulation, such as the twirling in this case, is enough to weaken hair follicles, causing hair to fall out. In cases involving hair-pulling, a small percentage of patients actually ingest the hairs they’ve plucked out (trichophagia). Being indigestible, the hairs can accumulate in hairballs (trichobezoars).
Even though TTM is most likely a psychiatric disorder lying somewhere in the obsessive-compulsive spectrum, it is seen more often in primary care and dermatology offices. Scalp biopsy would certainly settle the matter, but a better alternative is simply shaving a dime-sized area of scalp and watching it for normal hair growth.
Most cases eventually resolve with time and persistent but gentle reminders, but a few will require psychiatric intervention. This typically includes habit reversal therapy or cognitive behavioral therapy, plus or minus combinations of psychoactive medications. (The latter decision depends on whether there psychiatric comorbidities.) Despite all these efforts, severe cases of TTM can persist for years or even a lifetime.
It remains to be seen how this particular patient responds to his parents’ efforts. It was an immense relief for them to know the cause of their son’s hair loss and that the condition is likely self-limiting.
TAKE-HOME LEARNING POINTS
• Trichotillomania (TTM) is an unusual form of localized hair loss, usually involving children’s scalps.
• TTM affects children ages 4 to 13 and at least twice as many girls as boys.
• TTM does not always involve actual plucking of hairs. Repetitive manipulation, such as twirling, can weaken the hairs enough to cause hair loss.
• Unlike alopecia areata (the main item in the alopecia differential for children), TTM is more likely to cause incomplete, poorly defined hair loss in an area where hairs of varying length can be seen.
• Usually self-limiting, TTM can require psychiatric attention, for which a variety of habit training techniques can be used.
A mother brings her 12-year-old son to dermatology following a referral from the boy’s pediatrician. Several months ago, she noticed her son’s hair loss. The change had been preceded by a stressful period in which she and her husband divorced and one of the boy’s grandparents died unexpectedly.
Both the mother and other relatives and friends had observed the boy reaching for his scalp frequently and twirling his hair “absentmindedly.” When asked if the area in question bothers him, the boy always answers in the negative. Although he knows he should leave his scalp and hair alone, he says he finds it difficult to do so—even though he acknowledges the social liability of his hair loss. According to the mother, the more his family discourages his behavior, the more it persists.
EXAMINATION
Distinct but incomplete hair loss is noted in an 8 x 10–cm area of his scalp crown. There is neither redness nor any disturbance to the skin there. On palpation, there is no tenderness or increased warmth. No nodes are felt in the adjacent head or neck. Hair pull test is negative.
Closer examination shows hairs of varying lengths in the affected area: many quite short, others of normal length, and many of intermediate length.
Blood work done by the referring pediatrician—including complete blood count, chemistry panel, antinuclear antibody test, and thyroid testing—yielded no abnormal results.
What is the diagnosis?
DISCUSSION
Hair loss, collectively termed alopecia, is a disturbing development, especially in a child. In this case, we had localized hair loss most likely caused by behavior that was not only witnessed by the boy’s parents but also admitted to by the patient. (We’re not always so fortunate.) Thus, it was fairly straightforward to diagnosis trichotillomania, also known as trichotillosis or hair-pulling disorder. This condition can mimic alopecia areata and tinea capitis.
In this case, the lack of epidermal change (scaling, redness, edema) and palpable adenopathy spoke loudly against fungal infection. The hair loss in alopecia areata (AA) is usually sharply defined and complete, which our patient’s hair loss was not. And the blood work that was done effectively ruled out systemic disease (an unlikely cause of localized hair loss in any case).
The jury is still out as to how exactly to classify trichotillomania (TTM). The new DSM-V lists it as an anxiety disorder, in part because it often appears in that context. What we do know is that girls are twice as likely as boys to be affected. And children ages 4 to 13 are seven times more likely than adults to develop TTM.
TTM can involve hair anywhere on the body, though children almost always confine their behavior to their scalp. Actual hair-pulling is not necessarily seen. Manipulation, such as the twirling in this case, is enough to weaken hair follicles, causing hair to fall out. In cases involving hair-pulling, a small percentage of patients actually ingest the hairs they’ve plucked out (trichophagia). Being indigestible, the hairs can accumulate in hairballs (trichobezoars).
Even though TTM is most likely a psychiatric disorder lying somewhere in the obsessive-compulsive spectrum, it is seen more often in primary care and dermatology offices. Scalp biopsy would certainly settle the matter, but a better alternative is simply shaving a dime-sized area of scalp and watching it for normal hair growth.
Most cases eventually resolve with time and persistent but gentle reminders, but a few will require psychiatric intervention. This typically includes habit reversal therapy or cognitive behavioral therapy, plus or minus combinations of psychoactive medications. (The latter decision depends on whether there psychiatric comorbidities.) Despite all these efforts, severe cases of TTM can persist for years or even a lifetime.
It remains to be seen how this particular patient responds to his parents’ efforts. It was an immense relief for them to know the cause of their son’s hair loss and that the condition is likely self-limiting.
TAKE-HOME LEARNING POINTS
• Trichotillomania (TTM) is an unusual form of localized hair loss, usually involving children’s scalps.
• TTM affects children ages 4 to 13 and at least twice as many girls as boys.
• TTM does not always involve actual plucking of hairs. Repetitive manipulation, such as twirling, can weaken the hairs enough to cause hair loss.
• Unlike alopecia areata (the main item in the alopecia differential for children), TTM is more likely to cause incomplete, poorly defined hair loss in an area where hairs of varying length can be seen.
• Usually self-limiting, TTM can require psychiatric attention, for which a variety of habit training techniques can be used.
A mother brings her 12-year-old son to dermatology following a referral from the boy’s pediatrician. Several months ago, she noticed her son’s hair loss. The change had been preceded by a stressful period in which she and her husband divorced and one of the boy’s grandparents died unexpectedly.
Both the mother and other relatives and friends had observed the boy reaching for his scalp frequently and twirling his hair “absentmindedly.” When asked if the area in question bothers him, the boy always answers in the negative. Although he knows he should leave his scalp and hair alone, he says he finds it difficult to do so—even though he acknowledges the social liability of his hair loss. According to the mother, the more his family discourages his behavior, the more it persists.
EXAMINATION
Distinct but incomplete hair loss is noted in an 8 x 10–cm area of his scalp crown. There is neither redness nor any disturbance to the skin there. On palpation, there is no tenderness or increased warmth. No nodes are felt in the adjacent head or neck. Hair pull test is negative.
Closer examination shows hairs of varying lengths in the affected area: many quite short, others of normal length, and many of intermediate length.
Blood work done by the referring pediatrician—including complete blood count, chemistry panel, antinuclear antibody test, and thyroid testing—yielded no abnormal results.
What is the diagnosis?
DISCUSSION
Hair loss, collectively termed alopecia, is a disturbing development, especially in a child. In this case, we had localized hair loss most likely caused by behavior that was not only witnessed by the boy’s parents but also admitted to by the patient. (We’re not always so fortunate.) Thus, it was fairly straightforward to diagnosis trichotillomania, also known as trichotillosis or hair-pulling disorder. This condition can mimic alopecia areata and tinea capitis.
In this case, the lack of epidermal change (scaling, redness, edema) and palpable adenopathy spoke loudly against fungal infection. The hair loss in alopecia areata (AA) is usually sharply defined and complete, which our patient’s hair loss was not. And the blood work that was done effectively ruled out systemic disease (an unlikely cause of localized hair loss in any case).
The jury is still out as to how exactly to classify trichotillomania (TTM). The new DSM-V lists it as an anxiety disorder, in part because it often appears in that context. What we do know is that girls are twice as likely as boys to be affected. And children ages 4 to 13 are seven times more likely than adults to develop TTM.
TTM can involve hair anywhere on the body, though children almost always confine their behavior to their scalp. Actual hair-pulling is not necessarily seen. Manipulation, such as the twirling in this case, is enough to weaken hair follicles, causing hair to fall out. In cases involving hair-pulling, a small percentage of patients actually ingest the hairs they’ve plucked out (trichophagia). Being indigestible, the hairs can accumulate in hairballs (trichobezoars).
Even though TTM is most likely a psychiatric disorder lying somewhere in the obsessive-compulsive spectrum, it is seen more often in primary care and dermatology offices. Scalp biopsy would certainly settle the matter, but a better alternative is simply shaving a dime-sized area of scalp and watching it for normal hair growth.
Most cases eventually resolve with time and persistent but gentle reminders, but a few will require psychiatric intervention. This typically includes habit reversal therapy or cognitive behavioral therapy, plus or minus combinations of psychoactive medications. (The latter decision depends on whether there psychiatric comorbidities.) Despite all these efforts, severe cases of TTM can persist for years or even a lifetime.
It remains to be seen how this particular patient responds to his parents’ efforts. It was an immense relief for them to know the cause of their son’s hair loss and that the condition is likely self-limiting.
TAKE-HOME LEARNING POINTS
• Trichotillomania (TTM) is an unusual form of localized hair loss, usually involving children’s scalps.
• TTM affects children ages 4 to 13 and at least twice as many girls as boys.
• TTM does not always involve actual plucking of hairs. Repetitive manipulation, such as twirling, can weaken the hairs enough to cause hair loss.
• Unlike alopecia areata (the main item in the alopecia differential for children), TTM is more likely to cause incomplete, poorly defined hair loss in an area where hairs of varying length can be seen.
• Usually self-limiting, TTM can require psychiatric attention, for which a variety of habit training techniques can be used.
FDA lifts clinical hold on imetelstat
The US Food and Drug Administration (FDA) has removed the full clinical hold placed on the investigational new drug application for the telomerase inhibitor imetelstat.
The hold, which was placed in March, suspended a phase 2 study of imetelstat in patients with essential thrombocythemia (ET) or polycythemia vera (PV), as well as a phase 2 study of the drug in patients with multiple myeloma (MM).
The hold also delayed a planned phase 2 trial in patients with myelofibrosis (MF).
And it temporarily suspended an investigator-sponsored trial of imetelstat in MF. The FDA lifted the hold on the investigator-sponsored trial in June.
The FDA halted these trials due to reports of persistent, low-grade liver function test (LFT) abnormalities observed in the phase 2 study of ET/PV patients and the potential risk of chronic liver injury following long-term exposure to imetelstat. The FDA expressed concern about whether these LFT abnormalities are reversible.
Now, data provided by the Geron Corporation, the company developing imetelstat, has convinced the FDA to lift the hold on all trials.
The FDA said the proposed clinical development plan for imetelstat, which is focused on high-risk myeloid disorders such as MF, is acceptable. Geron Corporation has said it does not intend to conduct further studies with, or develop imetelstat for, patients with ET or PV.
To address the clinical hold, the FDA required Geron to provide follow-up information from imetelstat-treated patients who experienced LFT abnormalities until such abnormalities resolved to normal or baseline.
Geron obtained follow-up information from patients in the previously ongoing company-sponsored phase 2 trials in ET/PV and MM. These data were submitted to the FDA as part of the company’s complete response.
The company’s analysis of these data showed that, in the ET/PV trial, LFT abnormalities resolved to normal or baseline in 14 of 18 follow-up patients. For the remaining 4 patients, at the time of the data cut-off, 3 patients showed improvement in LFT abnormalities, and 1 patient had unresolved LFT abnormalities. Two of the remaining 4 patients continue in follow-up.
In the MM trial, LFT abnormalities resolved to normal or baseline in all 9 follow-up patients. In addition, no emergent hepatic adverse events were reported during follow-up for either study.
The FDA also requested information regarding the reversibility of liver toxicity after chronic imetelstat administration in animals. Geron submitted data from its non-clinical toxicology studies, which included a 6-month study in mice and a 9-month study in cynomolgus monkeys.
In these studies, no clinical pathology changes indicative of hepatocellular injury were observed, and no clear signal of LFT abnormalities were identified.
With the clinical hold lifted, a multicenter phase 2 trial in MF is projected to begin in the first half of 2015. ![]()
The US Food and Drug Administration (FDA) has removed the full clinical hold placed on the investigational new drug application for the telomerase inhibitor imetelstat.
The hold, which was placed in March, suspended a phase 2 study of imetelstat in patients with essential thrombocythemia (ET) or polycythemia vera (PV), as well as a phase 2 study of the drug in patients with multiple myeloma (MM).
The hold also delayed a planned phase 2 trial in patients with myelofibrosis (MF).
And it temporarily suspended an investigator-sponsored trial of imetelstat in MF. The FDA lifted the hold on the investigator-sponsored trial in June.
The FDA halted these trials due to reports of persistent, low-grade liver function test (LFT) abnormalities observed in the phase 2 study of ET/PV patients and the potential risk of chronic liver injury following long-term exposure to imetelstat. The FDA expressed concern about whether these LFT abnormalities are reversible.
Now, data provided by the Geron Corporation, the company developing imetelstat, has convinced the FDA to lift the hold on all trials.
The FDA said the proposed clinical development plan for imetelstat, which is focused on high-risk myeloid disorders such as MF, is acceptable. Geron Corporation has said it does not intend to conduct further studies with, or develop imetelstat for, patients with ET or PV.
To address the clinical hold, the FDA required Geron to provide follow-up information from imetelstat-treated patients who experienced LFT abnormalities until such abnormalities resolved to normal or baseline.
Geron obtained follow-up information from patients in the previously ongoing company-sponsored phase 2 trials in ET/PV and MM. These data were submitted to the FDA as part of the company’s complete response.
The company’s analysis of these data showed that, in the ET/PV trial, LFT abnormalities resolved to normal or baseline in 14 of 18 follow-up patients. For the remaining 4 patients, at the time of the data cut-off, 3 patients showed improvement in LFT abnormalities, and 1 patient had unresolved LFT abnormalities. Two of the remaining 4 patients continue in follow-up.
In the MM trial, LFT abnormalities resolved to normal or baseline in all 9 follow-up patients. In addition, no emergent hepatic adverse events were reported during follow-up for either study.
The FDA also requested information regarding the reversibility of liver toxicity after chronic imetelstat administration in animals. Geron submitted data from its non-clinical toxicology studies, which included a 6-month study in mice and a 9-month study in cynomolgus monkeys.
In these studies, no clinical pathology changes indicative of hepatocellular injury were observed, and no clear signal of LFT abnormalities were identified.
With the clinical hold lifted, a multicenter phase 2 trial in MF is projected to begin in the first half of 2015. ![]()
The US Food and Drug Administration (FDA) has removed the full clinical hold placed on the investigational new drug application for the telomerase inhibitor imetelstat.
The hold, which was placed in March, suspended a phase 2 study of imetelstat in patients with essential thrombocythemia (ET) or polycythemia vera (PV), as well as a phase 2 study of the drug in patients with multiple myeloma (MM).
The hold also delayed a planned phase 2 trial in patients with myelofibrosis (MF).
And it temporarily suspended an investigator-sponsored trial of imetelstat in MF. The FDA lifted the hold on the investigator-sponsored trial in June.
The FDA halted these trials due to reports of persistent, low-grade liver function test (LFT) abnormalities observed in the phase 2 study of ET/PV patients and the potential risk of chronic liver injury following long-term exposure to imetelstat. The FDA expressed concern about whether these LFT abnormalities are reversible.
Now, data provided by the Geron Corporation, the company developing imetelstat, has convinced the FDA to lift the hold on all trials.
The FDA said the proposed clinical development plan for imetelstat, which is focused on high-risk myeloid disorders such as MF, is acceptable. Geron Corporation has said it does not intend to conduct further studies with, or develop imetelstat for, patients with ET or PV.
To address the clinical hold, the FDA required Geron to provide follow-up information from imetelstat-treated patients who experienced LFT abnormalities until such abnormalities resolved to normal or baseline.
Geron obtained follow-up information from patients in the previously ongoing company-sponsored phase 2 trials in ET/PV and MM. These data were submitted to the FDA as part of the company’s complete response.
The company’s analysis of these data showed that, in the ET/PV trial, LFT abnormalities resolved to normal or baseline in 14 of 18 follow-up patients. For the remaining 4 patients, at the time of the data cut-off, 3 patients showed improvement in LFT abnormalities, and 1 patient had unresolved LFT abnormalities. Two of the remaining 4 patients continue in follow-up.
In the MM trial, LFT abnormalities resolved to normal or baseline in all 9 follow-up patients. In addition, no emergent hepatic adverse events were reported during follow-up for either study.
The FDA also requested information regarding the reversibility of liver toxicity after chronic imetelstat administration in animals. Geron submitted data from its non-clinical toxicology studies, which included a 6-month study in mice and a 9-month study in cynomolgus monkeys.
In these studies, no clinical pathology changes indicative of hepatocellular injury were observed, and no clear signal of LFT abnormalities were identified.
With the clinical hold lifted, a multicenter phase 2 trial in MF is projected to begin in the first half of 2015. ![]()
Brentuximab tops chemo in HL, doc says

NEW YORK—Brentuximab vendotin—not conventional chemotherapy—is the second-line regimen of choice for recurrent Hodgkin lymphoma (HL) patients prior to stem cell transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Catherine Diefenbach, MD, of New York University’s Langone Medical Center in New York, argued that conventional chemotherapy, the existing paradigm for first salvage therapy, does not maximize a cure or minimize toxicity, is inconvenient, and is not cost-effective.
She noted that a quarter of HL patients relapse or have a primary refractory diagnosis.
“These are young patients,” Dr Diefenbach said. “Autologous stem cell transplant [ASCT] cures only half of them. There is no other established curative salvage therapy.”
She noted that the median time to progression with relapse after transplant is 3.8 months in patients treated with subsequent therapy, with a median survival of 26 months.
After ASCT, the median progression-free survival with relapse is 1.3 years. And about three-quarters of relapses occur within the first year.
“Achieving a complete response [CR] before ASCT is the most important factor in determining long-term disease-free survival and to maximizing transplant-related benefit,” Dr Diefenbach said. “High overall response rates [ORRs] do not equal high CR. Only one-third of patients achieve a CR [with chemotherapy].”
She said studies have shown that chemotherapy with ICE (ifosfamide, carboplatin, and etoposide) leads to a 3-year event-free survival rate of 22%. Post-ASCT, event-free survival increases to more than 52%.
The overall survival post-ASCT is 44%. The median survival of patients who do not get therapy is 3.7 months. Myelosuppression and deaths are common.
“Conventional chemotherapy fails,” Dr Diefenbach continued. “There is inadequate disease control, unacceptable toxicity, it’s not cost-effective, requires patients to be hospitalized, and there is no clear standard of care.”
On the other hand, brentuximab as first salvage is highly active with minimal adverse events in relapsed HL.
“Rash is the only grade 3-4 toxicity,” Dr Diefenbach said. “There are no significant cytopenias [and] no febrile neutropenia. Growth factor support is not required, and it’s administered outpatient.”
Using brentuximab as second-line therapy results in an ORR of 85.7% and a CR of 50%, she added. And ASCT after brentuximab shows similar successes and toxicities.
Brentuximab followed by ICE leads to high rates of PET normalization, allows successful transplantation of virtually all evaluable patients, and poses no issues with stem cell collection.
Furthermore, studies show a 92% disease-free survival, with minimal toxicity at 10 months of follow-up.
In conclusion, Dr Diefenbach said, “Maximizing disease control prior to ASCT will maximize cure from ASCT. Brentuximab vedotin is a novel agent that leads to a high ORR and high CR rate with low toxicity and outpatient administration. In contrast, conventional chemotherapy fails to provide a high CR rate, has unacceptable toxicity, and there is no single standard of care.”
For an opposing opinion on salvage in HL, see “Speaker adovcates chemo-based salvage in HL.” ![]()

NEW YORK—Brentuximab vendotin—not conventional chemotherapy—is the second-line regimen of choice for recurrent Hodgkin lymphoma (HL) patients prior to stem cell transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Catherine Diefenbach, MD, of New York University’s Langone Medical Center in New York, argued that conventional chemotherapy, the existing paradigm for first salvage therapy, does not maximize a cure or minimize toxicity, is inconvenient, and is not cost-effective.
She noted that a quarter of HL patients relapse or have a primary refractory diagnosis.
“These are young patients,” Dr Diefenbach said. “Autologous stem cell transplant [ASCT] cures only half of them. There is no other established curative salvage therapy.”
She noted that the median time to progression with relapse after transplant is 3.8 months in patients treated with subsequent therapy, with a median survival of 26 months.
After ASCT, the median progression-free survival with relapse is 1.3 years. And about three-quarters of relapses occur within the first year.
“Achieving a complete response [CR] before ASCT is the most important factor in determining long-term disease-free survival and to maximizing transplant-related benefit,” Dr Diefenbach said. “High overall response rates [ORRs] do not equal high CR. Only one-third of patients achieve a CR [with chemotherapy].”
She said studies have shown that chemotherapy with ICE (ifosfamide, carboplatin, and etoposide) leads to a 3-year event-free survival rate of 22%. Post-ASCT, event-free survival increases to more than 52%.
The overall survival post-ASCT is 44%. The median survival of patients who do not get therapy is 3.7 months. Myelosuppression and deaths are common.
“Conventional chemotherapy fails,” Dr Diefenbach continued. “There is inadequate disease control, unacceptable toxicity, it’s not cost-effective, requires patients to be hospitalized, and there is no clear standard of care.”
On the other hand, brentuximab as first salvage is highly active with minimal adverse events in relapsed HL.
“Rash is the only grade 3-4 toxicity,” Dr Diefenbach said. “There are no significant cytopenias [and] no febrile neutropenia. Growth factor support is not required, and it’s administered outpatient.”
Using brentuximab as second-line therapy results in an ORR of 85.7% and a CR of 50%, she added. And ASCT after brentuximab shows similar successes and toxicities.
Brentuximab followed by ICE leads to high rates of PET normalization, allows successful transplantation of virtually all evaluable patients, and poses no issues with stem cell collection.
Furthermore, studies show a 92% disease-free survival, with minimal toxicity at 10 months of follow-up.
In conclusion, Dr Diefenbach said, “Maximizing disease control prior to ASCT will maximize cure from ASCT. Brentuximab vedotin is a novel agent that leads to a high ORR and high CR rate with low toxicity and outpatient administration. In contrast, conventional chemotherapy fails to provide a high CR rate, has unacceptable toxicity, and there is no single standard of care.”
For an opposing opinion on salvage in HL, see “Speaker adovcates chemo-based salvage in HL.” ![]()

NEW YORK—Brentuximab vendotin—not conventional chemotherapy—is the second-line regimen of choice for recurrent Hodgkin lymphoma (HL) patients prior to stem cell transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Catherine Diefenbach, MD, of New York University’s Langone Medical Center in New York, argued that conventional chemotherapy, the existing paradigm for first salvage therapy, does not maximize a cure or minimize toxicity, is inconvenient, and is not cost-effective.
She noted that a quarter of HL patients relapse or have a primary refractory diagnosis.
“These are young patients,” Dr Diefenbach said. “Autologous stem cell transplant [ASCT] cures only half of them. There is no other established curative salvage therapy.”
She noted that the median time to progression with relapse after transplant is 3.8 months in patients treated with subsequent therapy, with a median survival of 26 months.
After ASCT, the median progression-free survival with relapse is 1.3 years. And about three-quarters of relapses occur within the first year.
“Achieving a complete response [CR] before ASCT is the most important factor in determining long-term disease-free survival and to maximizing transplant-related benefit,” Dr Diefenbach said. “High overall response rates [ORRs] do not equal high CR. Only one-third of patients achieve a CR [with chemotherapy].”
She said studies have shown that chemotherapy with ICE (ifosfamide, carboplatin, and etoposide) leads to a 3-year event-free survival rate of 22%. Post-ASCT, event-free survival increases to more than 52%.
The overall survival post-ASCT is 44%. The median survival of patients who do not get therapy is 3.7 months. Myelosuppression and deaths are common.
“Conventional chemotherapy fails,” Dr Diefenbach continued. “There is inadequate disease control, unacceptable toxicity, it’s not cost-effective, requires patients to be hospitalized, and there is no clear standard of care.”
On the other hand, brentuximab as first salvage is highly active with minimal adverse events in relapsed HL.
“Rash is the only grade 3-4 toxicity,” Dr Diefenbach said. “There are no significant cytopenias [and] no febrile neutropenia. Growth factor support is not required, and it’s administered outpatient.”
Using brentuximab as second-line therapy results in an ORR of 85.7% and a CR of 50%, she added. And ASCT after brentuximab shows similar successes and toxicities.
Brentuximab followed by ICE leads to high rates of PET normalization, allows successful transplantation of virtually all evaluable patients, and poses no issues with stem cell collection.
Furthermore, studies show a 92% disease-free survival, with minimal toxicity at 10 months of follow-up.
In conclusion, Dr Diefenbach said, “Maximizing disease control prior to ASCT will maximize cure from ASCT. Brentuximab vedotin is a novel agent that leads to a high ORR and high CR rate with low toxicity and outpatient administration. In contrast, conventional chemotherapy fails to provide a high CR rate, has unacceptable toxicity, and there is no single standard of care.”
For an opposing opinion on salvage in HL, see “Speaker adovcates chemo-based salvage in HL.” ![]()
Transfusions benefit adults with sickle cell disease
PHILADELPHIA—Blood transfusions can provide pain relief in adults with sickle cell disease (SCD) who have failed treatment with hydroxyurea, a pilot study suggests.
Patients had fewer visits to the emergency department (ED) and fewer hospital admissions for pain control after they received chronic transfusions for pain prophylaxis than they did prior to receiving transfusions.
Matthew S. Karafin, MD, of the Blood Center of Wisconsin in Milwaukee, presented these results at the AABB Annual Meeting 2014 (abstract S42-030G).
“Pain in adults with sickle cell disease is probably one of the most important things that we deal with in our clinics,” he began. “It is the leading cause of morbidity in this population.”
Dr Karafin also noted that adults with SCD seem to experience pain differently from children, reporting more of a constant pain, as opposed to the episodic pain observed in kids. And although previous studies have suggested that transfusions do provide pain relief in SCD, most of those studies have focused on children.
So Dr Karafin and his colleagues set out to determine the impact of prophylactic transfusions on the rate of serious pain episodes in adults with SCD. The team retrospectively analyzed a cohort of patients who received chronic transfusions at 3- to 8-week intervals from January 2009 to October 2013.
The researchers defined chronic transfusions as receiving blood—either simple transfusions or red cell exchanges—in an outpatient setting 3 days a week with the goal of controlling hemoglobin (Hb) S percentage, maintaining it at less than 30%.
Patients had to have at least 1 ED or hospital visit for severe pain per month prior to starting transfusions, they were required to have failed hydroxyurea therapy, and they had to have at least 3 months both on and off chronic transfusions. The patients could have no other reason for receiving chronic transfusions (ie, no previous stroke).
So the study included 17 patients, 12 of whom were female. Fifteen (88.1%) had Hb SS disease, and 2 had Hb SC disease. Their median age was 26 (range, 20-54).
“We were able to record 541 total ED admissions over the study period and 404 total hospital admissions,” Dr Karafin said. “The median study evaluation period pre-transfusion was about 3.5 years, and we were able to study [patients for] a median of more than a year for the post-transfusion protocol period.”
Dr Karafin also noted that most of the patients were not transfusion-naïve, but they received significantly more units after being placed on the transfusion protocol.
The median number of red cell units received per 100 days was 1.2 (range, 0-7.2) pre-transfusion and 10.2 (range, 6.7-24.3) post-transfusion (P=0.0003). Nine of the patients received simple transfusions, and 8 received red cell exchanges.
There was a significant difference in the median Hb S pre- and post-transfusion—79% (range, 26.5%-89.6%) and 30.2% (range, 10.9%-57.4%), respectively (P=0.0003).
But there was no significant difference in median ferritin levels—1128.2 ng/mL (range, 65.4-11,130) and 2632.8 ng/mL (range, 16.7-8023.6), respectively (P=0.18). Dr Karafin said this could be explained by the fact that patients were not transfusion-naïve prior to starting the protocol.
Similarly, the median new alloantibody rate per 100 units was 0 both pre- and post-transfusion. This may be due to the fact that all patients received C-, E-, and KEL-matched blood, as well as the freshest available units, Dr Karafin said.
He and his colleagues also found that the median ED admission rate was significantly lower post-transfusion compared to pre-transfusion—0.79 (range, 0-6.6) and 2 (range, 0.4-11) visits every 100 days, respectively (P=0.04).
Thirteen patients (76.5%) had a reduced ED visit rate after chronic transfusion, and there was a 60.5% reduction in the ED visit rate overall.
Likewise, the median hospital admission rate decreased from 1.7 per 100 days (range, 0.05-5.8) pre-transfusion to 1.3 per 100 days (range, 0.2-3.2) post-transfusion (P=0.004).
Fifteen patients (88.2%) had reduced hospital admissions after chronic transfusion, and there was a 20.3% reduction in hospital admissions overall.
Dr Karafin noted that this study had a number of limitations, including a small number of patients, its retrospective nature, and the fact that it was conducted at a comprehensive SCD clinic.
“However, limitations aside, we found significant evidence to support that the findings observed in children seem to be similar in the adult population,” he said.
Namely, chronic transfusions can prevent serious pain episodes in adults with SCD who have failed treatment with hydroxyurea. ![]()
PHILADELPHIA—Blood transfusions can provide pain relief in adults with sickle cell disease (SCD) who have failed treatment with hydroxyurea, a pilot study suggests.
Patients had fewer visits to the emergency department (ED) and fewer hospital admissions for pain control after they received chronic transfusions for pain prophylaxis than they did prior to receiving transfusions.
Matthew S. Karafin, MD, of the Blood Center of Wisconsin in Milwaukee, presented these results at the AABB Annual Meeting 2014 (abstract S42-030G).
“Pain in adults with sickle cell disease is probably one of the most important things that we deal with in our clinics,” he began. “It is the leading cause of morbidity in this population.”
Dr Karafin also noted that adults with SCD seem to experience pain differently from children, reporting more of a constant pain, as opposed to the episodic pain observed in kids. And although previous studies have suggested that transfusions do provide pain relief in SCD, most of those studies have focused on children.
So Dr Karafin and his colleagues set out to determine the impact of prophylactic transfusions on the rate of serious pain episodes in adults with SCD. The team retrospectively analyzed a cohort of patients who received chronic transfusions at 3- to 8-week intervals from January 2009 to October 2013.
The researchers defined chronic transfusions as receiving blood—either simple transfusions or red cell exchanges—in an outpatient setting 3 days a week with the goal of controlling hemoglobin (Hb) S percentage, maintaining it at less than 30%.
Patients had to have at least 1 ED or hospital visit for severe pain per month prior to starting transfusions, they were required to have failed hydroxyurea therapy, and they had to have at least 3 months both on and off chronic transfusions. The patients could have no other reason for receiving chronic transfusions (ie, no previous stroke).
So the study included 17 patients, 12 of whom were female. Fifteen (88.1%) had Hb SS disease, and 2 had Hb SC disease. Their median age was 26 (range, 20-54).
“We were able to record 541 total ED admissions over the study period and 404 total hospital admissions,” Dr Karafin said. “The median study evaluation period pre-transfusion was about 3.5 years, and we were able to study [patients for] a median of more than a year for the post-transfusion protocol period.”
Dr Karafin also noted that most of the patients were not transfusion-naïve, but they received significantly more units after being placed on the transfusion protocol.
The median number of red cell units received per 100 days was 1.2 (range, 0-7.2) pre-transfusion and 10.2 (range, 6.7-24.3) post-transfusion (P=0.0003). Nine of the patients received simple transfusions, and 8 received red cell exchanges.
There was a significant difference in the median Hb S pre- and post-transfusion—79% (range, 26.5%-89.6%) and 30.2% (range, 10.9%-57.4%), respectively (P=0.0003).
But there was no significant difference in median ferritin levels—1128.2 ng/mL (range, 65.4-11,130) and 2632.8 ng/mL (range, 16.7-8023.6), respectively (P=0.18). Dr Karafin said this could be explained by the fact that patients were not transfusion-naïve prior to starting the protocol.
Similarly, the median new alloantibody rate per 100 units was 0 both pre- and post-transfusion. This may be due to the fact that all patients received C-, E-, and KEL-matched blood, as well as the freshest available units, Dr Karafin said.
He and his colleagues also found that the median ED admission rate was significantly lower post-transfusion compared to pre-transfusion—0.79 (range, 0-6.6) and 2 (range, 0.4-11) visits every 100 days, respectively (P=0.04).
Thirteen patients (76.5%) had a reduced ED visit rate after chronic transfusion, and there was a 60.5% reduction in the ED visit rate overall.
Likewise, the median hospital admission rate decreased from 1.7 per 100 days (range, 0.05-5.8) pre-transfusion to 1.3 per 100 days (range, 0.2-3.2) post-transfusion (P=0.004).
Fifteen patients (88.2%) had reduced hospital admissions after chronic transfusion, and there was a 20.3% reduction in hospital admissions overall.
Dr Karafin noted that this study had a number of limitations, including a small number of patients, its retrospective nature, and the fact that it was conducted at a comprehensive SCD clinic.
“However, limitations aside, we found significant evidence to support that the findings observed in children seem to be similar in the adult population,” he said.
Namely, chronic transfusions can prevent serious pain episodes in adults with SCD who have failed treatment with hydroxyurea. ![]()
PHILADELPHIA—Blood transfusions can provide pain relief in adults with sickle cell disease (SCD) who have failed treatment with hydroxyurea, a pilot study suggests.
Patients had fewer visits to the emergency department (ED) and fewer hospital admissions for pain control after they received chronic transfusions for pain prophylaxis than they did prior to receiving transfusions.
Matthew S. Karafin, MD, of the Blood Center of Wisconsin in Milwaukee, presented these results at the AABB Annual Meeting 2014 (abstract S42-030G).
“Pain in adults with sickle cell disease is probably one of the most important things that we deal with in our clinics,” he began. “It is the leading cause of morbidity in this population.”
Dr Karafin also noted that adults with SCD seem to experience pain differently from children, reporting more of a constant pain, as opposed to the episodic pain observed in kids. And although previous studies have suggested that transfusions do provide pain relief in SCD, most of those studies have focused on children.
So Dr Karafin and his colleagues set out to determine the impact of prophylactic transfusions on the rate of serious pain episodes in adults with SCD. The team retrospectively analyzed a cohort of patients who received chronic transfusions at 3- to 8-week intervals from January 2009 to October 2013.
The researchers defined chronic transfusions as receiving blood—either simple transfusions or red cell exchanges—in an outpatient setting 3 days a week with the goal of controlling hemoglobin (Hb) S percentage, maintaining it at less than 30%.
Patients had to have at least 1 ED or hospital visit for severe pain per month prior to starting transfusions, they were required to have failed hydroxyurea therapy, and they had to have at least 3 months both on and off chronic transfusions. The patients could have no other reason for receiving chronic transfusions (ie, no previous stroke).
So the study included 17 patients, 12 of whom were female. Fifteen (88.1%) had Hb SS disease, and 2 had Hb SC disease. Their median age was 26 (range, 20-54).
“We were able to record 541 total ED admissions over the study period and 404 total hospital admissions,” Dr Karafin said. “The median study evaluation period pre-transfusion was about 3.5 years, and we were able to study [patients for] a median of more than a year for the post-transfusion protocol period.”
Dr Karafin also noted that most of the patients were not transfusion-naïve, but they received significantly more units after being placed on the transfusion protocol.
The median number of red cell units received per 100 days was 1.2 (range, 0-7.2) pre-transfusion and 10.2 (range, 6.7-24.3) post-transfusion (P=0.0003). Nine of the patients received simple transfusions, and 8 received red cell exchanges.
There was a significant difference in the median Hb S pre- and post-transfusion—79% (range, 26.5%-89.6%) and 30.2% (range, 10.9%-57.4%), respectively (P=0.0003).
But there was no significant difference in median ferritin levels—1128.2 ng/mL (range, 65.4-11,130) and 2632.8 ng/mL (range, 16.7-8023.6), respectively (P=0.18). Dr Karafin said this could be explained by the fact that patients were not transfusion-naïve prior to starting the protocol.
Similarly, the median new alloantibody rate per 100 units was 0 both pre- and post-transfusion. This may be due to the fact that all patients received C-, E-, and KEL-matched blood, as well as the freshest available units, Dr Karafin said.
He and his colleagues also found that the median ED admission rate was significantly lower post-transfusion compared to pre-transfusion—0.79 (range, 0-6.6) and 2 (range, 0.4-11) visits every 100 days, respectively (P=0.04).
Thirteen patients (76.5%) had a reduced ED visit rate after chronic transfusion, and there was a 60.5% reduction in the ED visit rate overall.
Likewise, the median hospital admission rate decreased from 1.7 per 100 days (range, 0.05-5.8) pre-transfusion to 1.3 per 100 days (range, 0.2-3.2) post-transfusion (P=0.004).
Fifteen patients (88.2%) had reduced hospital admissions after chronic transfusion, and there was a 20.3% reduction in hospital admissions overall.
Dr Karafin noted that this study had a number of limitations, including a small number of patients, its retrospective nature, and the fact that it was conducted at a comprehensive SCD clinic.
“However, limitations aside, we found significant evidence to support that the findings observed in children seem to be similar in the adult population,” he said.
Namely, chronic transfusions can prevent serious pain episodes in adults with SCD who have failed treatment with hydroxyurea. ![]()
Speaker advocates chemo-based salvage in HL

Credit: Rhoda Baer
NEW YORK—Physicians should use chemotherapy-based approaches—not brentuximab vedotin—as second-line therapy for recurrent
Hodgkin lymphoma (HL) prior to transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Nina Wagner-Johnston, MD, of Washington University in St Louis, Missouri, provided the meeting’s audience with a handful of arguments to support chemotherapy-based salvage regimens and a number of reasons why brentuximab is not ideal as salvage therapy in HL.
First, there is data supporting the use of chemotherapy-based salvage regimens in these patients, but the brentuximab data is lacking.
“Other issues include chemosensitivity, stem cell collection, unknown progressive multifocal leukoencephalopathy (PML) risk with brentuximab pre-ASCT [autologous stem cell transplant], cost, and alternative roles for brentuximab to enhance cure upfront post-ASCT maintenance,” Dr Wagner-Johnston said.
She also noted that chemotherapy-based salvage regimens yield high response rates with adequate stem cell collections.
“The vast majority of patients proceed to ASCT, 86% with ICE chemotherapy [ifosfamide, carboplatin, and etoposide],” she said. “Many are cured with a chemotherapy-based approach, with a 5-year PFS [progression-free survival] of 50% to 60%, with a low incidence of febrile neutropenia.”
Dr Wagner-Johnston also raised the possibility that giving patients brentuximab prior to ASCT might increase the risk of PML. The risk is already increased with lymphoma and ASCT. And in cases of PML in which patients are treated with brentuximab, the onset is rapid, with a median of about 2 months after initiation.
Furthermore, the cost of therapy clearly favors chemotherapy over brentuximab, she said. Three cycles of brentuximab at 1.8 mg/kg cost more than $58,000, and 2 cycles of brentuximab (3 weeks on, 1 week off) at 1.2 mg/kg cost more than $78,000.
In comparison, 3 cycles of ICE cost less than $38,000 and 3 cycles of ESHAP (etoposide, methylprednisolone, high-dose cytarabine, and cisplatin) cost $17,000. These costs do not account for the increased risk of febrile neutropenia.
Dr Wagner-Johnston expressed other concerns about brentuximab as well.
“Brentuximab is probably not effective to pursue as a single agent,” she said. “The addition of brentuximab to chemotherapy increases toxicity and cost. Continuous progressions years after ASCT call into question a 2-year benchmark and further highlight the importance of a maintenance approach.”
There are several unanswered pivotal questions about brentuximab, Dr Wagner-Johnston continued.
“Is brentuximab sensitivity equivalent to chemosensitivity?” she asked. “Does improved CR [complete response] rate with brentuximab-based treatment equate to better outcomes? Is the likelihood of cure higher with brentuximab-based approaches?”
She said brentuximab may be best in the upfront setting. There are more upfront cures in the highest-risk group with this novel agent, and it may allow a greater number of patients to avoid the toxicity of ASCT.
Brentuximab has demonstrated safety in phase 1/2 studies, Dr Wagner-Johnston said, adding “we await data from an ongoing phase 3 trial to determine a PFS benefit with brentuximab.”
Furthermore, post-ASCT maintenance with brentuximab may be a better alternative than salvage brentuximab pre-ASCT. In the relapsed setting, the median duration of response for brentuximab is short (6.7 months in a phase 2 study) after a median of 9 cycles.
In the phase 3 placebo-controlled trial of maintenance brentuximab following ASCT, “an interim analysis based on safety and utility recommends continuation,” Dr Wagner-Johnston said.
In conclusion, she said, “Before letting brentuximab take over, we need to confirm its safety, efficacy, and determine the best positioning of brentuximab to enhance outcomes. In the meantime, stick with what we know works.”
For the dissenting opinion on salvage in HL, see “Brentuximab tops chemo in HL, doc says.” ![]()

Credit: Rhoda Baer
NEW YORK—Physicians should use chemotherapy-based approaches—not brentuximab vedotin—as second-line therapy for recurrent
Hodgkin lymphoma (HL) prior to transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Nina Wagner-Johnston, MD, of Washington University in St Louis, Missouri, provided the meeting’s audience with a handful of arguments to support chemotherapy-based salvage regimens and a number of reasons why brentuximab is not ideal as salvage therapy in HL.
First, there is data supporting the use of chemotherapy-based salvage regimens in these patients, but the brentuximab data is lacking.
“Other issues include chemosensitivity, stem cell collection, unknown progressive multifocal leukoencephalopathy (PML) risk with brentuximab pre-ASCT [autologous stem cell transplant], cost, and alternative roles for brentuximab to enhance cure upfront post-ASCT maintenance,” Dr Wagner-Johnston said.
She also noted that chemotherapy-based salvage regimens yield high response rates with adequate stem cell collections.
“The vast majority of patients proceed to ASCT, 86% with ICE chemotherapy [ifosfamide, carboplatin, and etoposide],” she said. “Many are cured with a chemotherapy-based approach, with a 5-year PFS [progression-free survival] of 50% to 60%, with a low incidence of febrile neutropenia.”
Dr Wagner-Johnston also raised the possibility that giving patients brentuximab prior to ASCT might increase the risk of PML. The risk is already increased with lymphoma and ASCT. And in cases of PML in which patients are treated with brentuximab, the onset is rapid, with a median of about 2 months after initiation.
Furthermore, the cost of therapy clearly favors chemotherapy over brentuximab, she said. Three cycles of brentuximab at 1.8 mg/kg cost more than $58,000, and 2 cycles of brentuximab (3 weeks on, 1 week off) at 1.2 mg/kg cost more than $78,000.
In comparison, 3 cycles of ICE cost less than $38,000 and 3 cycles of ESHAP (etoposide, methylprednisolone, high-dose cytarabine, and cisplatin) cost $17,000. These costs do not account for the increased risk of febrile neutropenia.
Dr Wagner-Johnston expressed other concerns about brentuximab as well.
“Brentuximab is probably not effective to pursue as a single agent,” she said. “The addition of brentuximab to chemotherapy increases toxicity and cost. Continuous progressions years after ASCT call into question a 2-year benchmark and further highlight the importance of a maintenance approach.”
There are several unanswered pivotal questions about brentuximab, Dr Wagner-Johnston continued.
“Is brentuximab sensitivity equivalent to chemosensitivity?” she asked. “Does improved CR [complete response] rate with brentuximab-based treatment equate to better outcomes? Is the likelihood of cure higher with brentuximab-based approaches?”
She said brentuximab may be best in the upfront setting. There are more upfront cures in the highest-risk group with this novel agent, and it may allow a greater number of patients to avoid the toxicity of ASCT.
Brentuximab has demonstrated safety in phase 1/2 studies, Dr Wagner-Johnston said, adding “we await data from an ongoing phase 3 trial to determine a PFS benefit with brentuximab.”
Furthermore, post-ASCT maintenance with brentuximab may be a better alternative than salvage brentuximab pre-ASCT. In the relapsed setting, the median duration of response for brentuximab is short (6.7 months in a phase 2 study) after a median of 9 cycles.
In the phase 3 placebo-controlled trial of maintenance brentuximab following ASCT, “an interim analysis based on safety and utility recommends continuation,” Dr Wagner-Johnston said.
In conclusion, she said, “Before letting brentuximab take over, we need to confirm its safety, efficacy, and determine the best positioning of brentuximab to enhance outcomes. In the meantime, stick with what we know works.”
For the dissenting opinion on salvage in HL, see “Brentuximab tops chemo in HL, doc says.” ![]()

Credit: Rhoda Baer
NEW YORK—Physicians should use chemotherapy-based approaches—not brentuximab vedotin—as second-line therapy for recurrent
Hodgkin lymphoma (HL) prior to transplant, according to a speaker at the Lymphoma & Myeloma 2014 congress.
Nina Wagner-Johnston, MD, of Washington University in St Louis, Missouri, provided the meeting’s audience with a handful of arguments to support chemotherapy-based salvage regimens and a number of reasons why brentuximab is not ideal as salvage therapy in HL.
First, there is data supporting the use of chemotherapy-based salvage regimens in these patients, but the brentuximab data is lacking.
“Other issues include chemosensitivity, stem cell collection, unknown progressive multifocal leukoencephalopathy (PML) risk with brentuximab pre-ASCT [autologous stem cell transplant], cost, and alternative roles for brentuximab to enhance cure upfront post-ASCT maintenance,” Dr Wagner-Johnston said.
She also noted that chemotherapy-based salvage regimens yield high response rates with adequate stem cell collections.
“The vast majority of patients proceed to ASCT, 86% with ICE chemotherapy [ifosfamide, carboplatin, and etoposide],” she said. “Many are cured with a chemotherapy-based approach, with a 5-year PFS [progression-free survival] of 50% to 60%, with a low incidence of febrile neutropenia.”
Dr Wagner-Johnston also raised the possibility that giving patients brentuximab prior to ASCT might increase the risk of PML. The risk is already increased with lymphoma and ASCT. And in cases of PML in which patients are treated with brentuximab, the onset is rapid, with a median of about 2 months after initiation.
Furthermore, the cost of therapy clearly favors chemotherapy over brentuximab, she said. Three cycles of brentuximab at 1.8 mg/kg cost more than $58,000, and 2 cycles of brentuximab (3 weeks on, 1 week off) at 1.2 mg/kg cost more than $78,000.
In comparison, 3 cycles of ICE cost less than $38,000 and 3 cycles of ESHAP (etoposide, methylprednisolone, high-dose cytarabine, and cisplatin) cost $17,000. These costs do not account for the increased risk of febrile neutropenia.
Dr Wagner-Johnston expressed other concerns about brentuximab as well.
“Brentuximab is probably not effective to pursue as a single agent,” she said. “The addition of brentuximab to chemotherapy increases toxicity and cost. Continuous progressions years after ASCT call into question a 2-year benchmark and further highlight the importance of a maintenance approach.”
There are several unanswered pivotal questions about brentuximab, Dr Wagner-Johnston continued.
“Is brentuximab sensitivity equivalent to chemosensitivity?” she asked. “Does improved CR [complete response] rate with brentuximab-based treatment equate to better outcomes? Is the likelihood of cure higher with brentuximab-based approaches?”
She said brentuximab may be best in the upfront setting. There are more upfront cures in the highest-risk group with this novel agent, and it may allow a greater number of patients to avoid the toxicity of ASCT.
Brentuximab has demonstrated safety in phase 1/2 studies, Dr Wagner-Johnston said, adding “we await data from an ongoing phase 3 trial to determine a PFS benefit with brentuximab.”
Furthermore, post-ASCT maintenance with brentuximab may be a better alternative than salvage brentuximab pre-ASCT. In the relapsed setting, the median duration of response for brentuximab is short (6.7 months in a phase 2 study) after a median of 9 cycles.
In the phase 3 placebo-controlled trial of maintenance brentuximab following ASCT, “an interim analysis based on safety and utility recommends continuation,” Dr Wagner-Johnston said.
In conclusion, she said, “Before letting brentuximab take over, we need to confirm its safety, efficacy, and determine the best positioning of brentuximab to enhance outcomes. In the meantime, stick with what we know works.”
For the dissenting opinion on salvage in HL, see “Brentuximab tops chemo in HL, doc says.” ![]()
The economics of gynecologic surgery: 13 coding tips to ensure fair payment
The payment structure for physicians is changing. Our government, the American public, purchasers, and employers are unhappy with the fee-for-service system as it currently exists, and are pushing to drive the system into what is called “value-based purchasing.”
But what is value?
One way to define it is quality divided by cost—but how do we measure quality?
At present, insurers are measuring your quality based on some nebulous definition created at United Healthcare or Blue Cross Blue Shield—looking specifically at your “efficiency,” based on the costs attributed to you, as revealed in the codes you and others submit to payers.
Let’s say you perform minimally invasive surgery, and the referring physician ordered a lot of tests before sending the patient to you. Are you aware that all of those costs may be attributed to you in an administrative system?
ACOG is working hard to establish clinical systems rather than administrative ones to determine the true cost of care. We may want to think of obstetrics and gynecology as primary care and take advantage of advanced payment models and the opportunities afforded to accountable care organizations, but the truth is, insurers frequently do not consider us primary care. Although some of us may develop medical homes for women’s health care, we are unlikely to collect a per-patient, per-month income like primary care physicians do. That means that we need to be more assertive in negotiating contracts with insurers.
In this article, I offer recommendations for such negotiations and explain how to determine what you can and cannot accept in terms of payment.
You are the responsible party
Some of us do our own coding and some of us do not. However, if that coding is inaccurate, it is the physician who goes to jail, not the coder. You are personally responsible and liable for the coding submitted under your provider number.
Clearly, we need to do a better job of advocating for ourselves. We need to lobby. Legislators and bureaucrats are less likely to target people who have strong lobbyists working consistently on their behalf.
Accountable care organizations may have some leverage in negotiating lower prices, and some market forces may come into play in large systems. It remains to be seen which models will succeed as new payment structures develop. The overarching question: What can we do today to optimize our payments, given the system that we have? Here are 13 tactics that can enhance your bottom line.
1. Know the rules
To play the game, you must know the rules. You need to know what systems payers are using to determine your reimbursement—and you have to understand those systems as well as, or better than, the payers do. Then you’ll be able to use them to your advantage.
Payers are well aware that we don’t like to focus on this end of practice, that what we really want to do is spend the day practicing medicine. However, we need to learn these details because we’re leaving money on the table every single day.
2. Educate yourself
With the change to the International Classification of Diseases (ICD) scheduled to take effect on October 1, 2015, many of us are worried that payers are going to reject our claims because of our lack of familiarity with ICD-10.
Rest assured. There are crosswalks from ICD-9 to ICD-10. ACOG has published an information sheet for both obstetrics and gynecology that pairs typically used ICD-9 codes with their ICD-10 counterparts. And because it is published by ACOG, payers will find it hard to claim that it’s inaccurate.
ACOG also offers half-day courses on ICD-10 coding for both physicians and staff.
3. Record your decision-making process
When I audit medical charts, I often discover that this process has been neglected. Instead, the coder has relied on documentation from the electronic health record and a basic description of the treatment plan. But a plan is just that—what someone intends to do. It doesn’t convey the decision-making that underlies it. What was the differential diagnosis? What did you discuss with the patient? These details are critical for appropriate coding of the level of service—whether it’s high, intermediate, or low.
4. Refine your approach to coding
Recognize that the system is currently set up to pay physicians for the services we provide—and that service must be justified by the appropriate diagnosis code. Tougher cases, or high-risk patients, tend to have longer surgeries and hospital stays, and their outcomes often are not as good as those of more typical patients. They may have more complications because they’re obese or have severe diabetes, for example. If so, it is critical that these other conditions—obesity and severe diabetes—be included with the principal diagnosis code so that risk stratification is possible. Otherwise, we will be held to the same standard as someone treating a routine, low-risk case.
Risk stratification is being performed according to algorithms in the payers’ software—and payers are unlikely to share the details with us. However, the only real data payers have to run through these algorithms come from diagnosis coding. Even though you’re not required to code for variables such as obesity and diabetes in order to get paid for what you do, you do need to use those additional codes to make risk stratification possible—so that you don’t get inappropriately placed into a group of low-risk providers when you are treating a higher-risk cohort.
5. Develop an understanding of RVUs
Another variable that changes regularly is relative value units (RVUs) under Medicare rules. ACOG’s Committee on Health Economics and Coding—which enjoys the participation of AAGL, the American Urogynecologic Society (AUGS), the Society of Gynecologic Surgeons (SGS), and the Society of Gynecologic Oncology (SGO), as well as other organizations—tries to maintain the RVUs as up to date and appropriate as possible relative to other services in the fee schedule.
For example, about 10 years ago many urogynecologic procedures were getting bundled together when they were performed at the same time. We had only one or two ICD-9 codes to describe prolapse, with no separate codes to describe whether it affected the anterior, apical, or posterior compartment, even though we performed different procedures in the individual compartments. Payers were mapping all prolapse procedures to the same diagnosis code. So ACOG went to the National Center for Health Statistics, where ICD-9 coding was done—and developed a series of about 10 codes to describe the different areas that prolapse could affect.
That kind of nuanced coding is continuing today. In fact, we have a long list of areas to go forward with now that ICD-10 is scheduled to take effect. A good example involves new Pap smear guidelines, which recommend testing every 3 or 5 years except for patients who have undergone hysterectomy for benign disease. How do you code for a patient who has had a hysterectomy? There was no code for a woman with an absent cervix, so we created a “V-code,” a code classification for factors that influence health status, so that it is possible to explain why a Pap smear was not performed.
As we go forward into a value-based system, specialists like us likely will be negotiating contracts according to RVU-based payments. That’s why it’s important for you to understand the resource-based relative value scale (RBRVS). It has three components: a work component, which makes up about 52% of the total RVUs; a practice expense, which makes up more than 45% of total RVUS; and, finally, a malpractice component, a small percentage. There also is a geographic adjustment and a uniform conversion factor.
When you hear about the sustainable growth rate (SGR) fix, and the fact that we’re going to see a 20% or 24% reduction in payment, that talk is referring to a reduction in the conversion factor. Each component of the RVU is adjusted for geography and then multiplied by the dollar conversion factor to calculate the total RVUs. The work, practice, and malpractice components vary by where the service is provided.
Let’s use placement of Essure inserts as an example. If you perform the procedure in the hospital, then the hospital buys the equipment, including the hysteroscope and light source. The hospital also pays for the room and staff and manages equipment sterilization. If, on the other hand, you perform the procedure in your office, all those responsibilities are yours. If it’s done in your office, you get paid more but it also costs you more.
The Relative Value Update Committee, or RUC, plays a major role in determining RVUs. This committee is composed of 31 clinicians, including nonphysician providers, psychologists, and nurses who deliver services under the Medicare fee schedule. The RUC makes recommendations to the Centers for Medicare and Medicaid Services (CMS), but it is the Secretary of Health and Human Services who determines the final rule on RVUs.
Approximately 75% to 95% of the recommendations of the RUC are accepted by the Secretary and become law. So it’s not the RUC or the American Medical Association (AMA) that determines RVUs; in the long run, it is CMS and the Secretary of Health and Human Services. We are fortunate that, when CMS assigns RVUs we’re not happy with, we have an opportunity to appeal.
Under Medicare, all physician payments are based on the same conversion factor, regardless of specialty. That’s not necessarily true for other payers, who may, essentially, do whatever they wish. These other payers frequently will contract at higher or lower rates, depending on how prevalent a specialist is in the community. Sometimes they use a higher conversion factor for surgical specialists than they use for primary care.
6. Find out which RVUs the payer is using
When you negotiate contracts with payers, and you are in private practice or part of a medical practice, it’s important to know what year’s RVUs the payer is using, as RVUs vary from year to year. For example, if the payer is using the RBRVS from 2002, it is paying you less than you should be getting. So when you look at a contract, you should determine not only whether the payer is anchoring your payment to the RBRVS but also whether it is keeping up with current RVUs as well. What dollar conversion factor is the payer using? What global periods—the same as CMS, or something different?
7. Determine what global period is in play
Some private payers use 6 postoperative weeks as the global period for a surgical procedure, whereas Medicare uses 90 days. You need to know which period is in play so that you don’t leave money on the table if you see the patient within 90 days but more than 6 weeks postoperatively.
Current Procedural Terminology (CPT) has global surgical packages that include a 10-day or 90-day period. But those periods do not include services provided more than 24 hours before the procedure. They don’t include the administration of anesthesia or conscious sedation. And they don’t include management of complications, exacerbations, or recurrences. Nor do they include additional services that might be necessary due to the presence of another disease or injury.
Under Medicare, the rules are different. Medicare preoperative services begin 1 day before surgery. However, any preoperative intervention is included whether it’s performed 1 day or 1 week before surgery. If it’s simply a preoperative physical examination for the patient and you aren’t performing significant evaluation and management, it’s included in the global package, along with all the intraoperative work. In addition, under Medicare, you don’t get paid for the management of complications unless a return to the operating room is required.
8. Learn to use modifiers
As ObGyns, we often see patients for multiple conditions or problem reports, so you need to be aware that if a patient is within a global period and you do not submit a bill with a modifier to indicate special circumstances, the intervention will be bundled into the global and you will not get paid for it. Modifiers are two-digit codes that describe these separate services. They provide critical information to payers so that their computer programs separate these services out for payment.
Major surgical procedures don’t include unrelated procedures that are performed at the same time of surgery. Nor do they include visits that take place during the global period that are unrelated to the original surgery. For example, if a patient presents with a breast lump after you performed a hysterectomy, and you do a work-up, you deserve full payment for that evaluation and management service. If you don’t use a modifier, however, you won’t get that payment.
9. Don’t be passive when payers won’t pay
Let’s say you contract with HMOs or independent practice associations (IPAs), and they’re not compensating you for the extra things you’re doing and are failing to recognize surgical modifiers. What can you do about it?
You need to develop a profile of your typical patient. Because these organizations are individualizing it—they are saying that, in a typical scenario, this is the type of work you do. So these organizations offer a different kind of contract. Nevertheless, you can use your coding to help you determine what a fair payment should be, by going through your billing to determine what you’ve spent.
10. Analyze payer bundling
Medicare put in place a correct coding initiative (CCI) that lists services typically provided by the same person on the same day of service. The aim: to prevent separate payment for these services. These are “bundled” services. The CCI bundles are revised every quarter. They are listed on the ACOG Web site under “practice management.”
On October 1, 2014, the CCI inappropriately bundled pelvic organ prolapse repair procedures into the vaginal hysterectomy codes. ACOG, AUGS, SGS, and AAGL are arguing vehemently as this article is going to press to ensure that these damaging bundles are rescinded.
Private payers can bundle anything, and it may or may not make sense or be fair. One ACOG resource is the book Ob/Gyn Coding Manual: Components of Correct Procedural Coding, which is revised every year. It has a tear-out page for every procedure code and will help you determine whether or not a bundle is appropriate.
You need to know about bundling and dispute resolution. Why? Because it is possible to insert clauses into your contract that give you some rights. Insurers have all the clout and you have nothing unless you fight for it.
You may see clauses such as “the company reserves the right to re-bundle to the primary procedure....” You shouldn’t tolerate that. Rather, you want to say, “the company will use CCI bundled rules” so that you at least know what the rules are.
11. Don’t be afraid to revise a contract
If we have to hold a payer harmless, the payer should hold us harmless as well. If we consult an insurer’s Web site to confirm that a patient is covered, and we take her to surgery because we have evidence she has insurance, the insurer shouldn’t be able to rescind payment 6 months later because the patient didn’t pay for her insurance that month. That’s not fair. The company told you she was covered, and you deserve to get paid for that surgery because you are relying on information from the company itself. So when you sign a contract, you need to ensure that you are being held harmless as well as the insurer.
12. Calculate your own RVUs
Use your claims software for data. Consult the Federal Register or ACOG to determine the total number of RVUs for a given CPT code. Multiply the RVUs by the quantity for each code. Let’s say it’s an evaluation and management visit, code 99213, and you’ve done 50 this month. That’s 50 multiplied by 1.3 RVUs. Add all the codes together, then use your monthly profit and loss statement to determine what your expenses are. Divide your total expenses by the total number of RVUs to determine your practice cost per RVU. You then can decide on a conversion factor you can tolerate, and you can use this information when contracting with IPAs, HMOs, and other insurers.
13. Spend money to make money
There are many coding resources available to you. Coding is well worth what you spend on it because you can get it back in a heartbeat.
This information may not be easy to master, but it’s critically important for your economic survival—to get what’s rightfully yours and get paid fairly for what you do.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The payment structure for physicians is changing. Our government, the American public, purchasers, and employers are unhappy with the fee-for-service system as it currently exists, and are pushing to drive the system into what is called “value-based purchasing.”
But what is value?
One way to define it is quality divided by cost—but how do we measure quality?
At present, insurers are measuring your quality based on some nebulous definition created at United Healthcare or Blue Cross Blue Shield—looking specifically at your “efficiency,” based on the costs attributed to you, as revealed in the codes you and others submit to payers.
Let’s say you perform minimally invasive surgery, and the referring physician ordered a lot of tests before sending the patient to you. Are you aware that all of those costs may be attributed to you in an administrative system?
ACOG is working hard to establish clinical systems rather than administrative ones to determine the true cost of care. We may want to think of obstetrics and gynecology as primary care and take advantage of advanced payment models and the opportunities afforded to accountable care organizations, but the truth is, insurers frequently do not consider us primary care. Although some of us may develop medical homes for women’s health care, we are unlikely to collect a per-patient, per-month income like primary care physicians do. That means that we need to be more assertive in negotiating contracts with insurers.
In this article, I offer recommendations for such negotiations and explain how to determine what you can and cannot accept in terms of payment.
You are the responsible party
Some of us do our own coding and some of us do not. However, if that coding is inaccurate, it is the physician who goes to jail, not the coder. You are personally responsible and liable for the coding submitted under your provider number.
Clearly, we need to do a better job of advocating for ourselves. We need to lobby. Legislators and bureaucrats are less likely to target people who have strong lobbyists working consistently on their behalf.
Accountable care organizations may have some leverage in negotiating lower prices, and some market forces may come into play in large systems. It remains to be seen which models will succeed as new payment structures develop. The overarching question: What can we do today to optimize our payments, given the system that we have? Here are 13 tactics that can enhance your bottom line.
1. Know the rules
To play the game, you must know the rules. You need to know what systems payers are using to determine your reimbursement—and you have to understand those systems as well as, or better than, the payers do. Then you’ll be able to use them to your advantage.
Payers are well aware that we don’t like to focus on this end of practice, that what we really want to do is spend the day practicing medicine. However, we need to learn these details because we’re leaving money on the table every single day.
2. Educate yourself
With the change to the International Classification of Diseases (ICD) scheduled to take effect on October 1, 2015, many of us are worried that payers are going to reject our claims because of our lack of familiarity with ICD-10.
Rest assured. There are crosswalks from ICD-9 to ICD-10. ACOG has published an information sheet for both obstetrics and gynecology that pairs typically used ICD-9 codes with their ICD-10 counterparts. And because it is published by ACOG, payers will find it hard to claim that it’s inaccurate.
ACOG also offers half-day courses on ICD-10 coding for both physicians and staff.
3. Record your decision-making process
When I audit medical charts, I often discover that this process has been neglected. Instead, the coder has relied on documentation from the electronic health record and a basic description of the treatment plan. But a plan is just that—what someone intends to do. It doesn’t convey the decision-making that underlies it. What was the differential diagnosis? What did you discuss with the patient? These details are critical for appropriate coding of the level of service—whether it’s high, intermediate, or low.
4. Refine your approach to coding
Recognize that the system is currently set up to pay physicians for the services we provide—and that service must be justified by the appropriate diagnosis code. Tougher cases, or high-risk patients, tend to have longer surgeries and hospital stays, and their outcomes often are not as good as those of more typical patients. They may have more complications because they’re obese or have severe diabetes, for example. If so, it is critical that these other conditions—obesity and severe diabetes—be included with the principal diagnosis code so that risk stratification is possible. Otherwise, we will be held to the same standard as someone treating a routine, low-risk case.
Risk stratification is being performed according to algorithms in the payers’ software—and payers are unlikely to share the details with us. However, the only real data payers have to run through these algorithms come from diagnosis coding. Even though you’re not required to code for variables such as obesity and diabetes in order to get paid for what you do, you do need to use those additional codes to make risk stratification possible—so that you don’t get inappropriately placed into a group of low-risk providers when you are treating a higher-risk cohort.
5. Develop an understanding of RVUs
Another variable that changes regularly is relative value units (RVUs) under Medicare rules. ACOG’s Committee on Health Economics and Coding—which enjoys the participation of AAGL, the American Urogynecologic Society (AUGS), the Society of Gynecologic Surgeons (SGS), and the Society of Gynecologic Oncology (SGO), as well as other organizations—tries to maintain the RVUs as up to date and appropriate as possible relative to other services in the fee schedule.
For example, about 10 years ago many urogynecologic procedures were getting bundled together when they were performed at the same time. We had only one or two ICD-9 codes to describe prolapse, with no separate codes to describe whether it affected the anterior, apical, or posterior compartment, even though we performed different procedures in the individual compartments. Payers were mapping all prolapse procedures to the same diagnosis code. So ACOG went to the National Center for Health Statistics, where ICD-9 coding was done—and developed a series of about 10 codes to describe the different areas that prolapse could affect.
That kind of nuanced coding is continuing today. In fact, we have a long list of areas to go forward with now that ICD-10 is scheduled to take effect. A good example involves new Pap smear guidelines, which recommend testing every 3 or 5 years except for patients who have undergone hysterectomy for benign disease. How do you code for a patient who has had a hysterectomy? There was no code for a woman with an absent cervix, so we created a “V-code,” a code classification for factors that influence health status, so that it is possible to explain why a Pap smear was not performed.
As we go forward into a value-based system, specialists like us likely will be negotiating contracts according to RVU-based payments. That’s why it’s important for you to understand the resource-based relative value scale (RBRVS). It has three components: a work component, which makes up about 52% of the total RVUs; a practice expense, which makes up more than 45% of total RVUS; and, finally, a malpractice component, a small percentage. There also is a geographic adjustment and a uniform conversion factor.
When you hear about the sustainable growth rate (SGR) fix, and the fact that we’re going to see a 20% or 24% reduction in payment, that talk is referring to a reduction in the conversion factor. Each component of the RVU is adjusted for geography and then multiplied by the dollar conversion factor to calculate the total RVUs. The work, practice, and malpractice components vary by where the service is provided.
Let’s use placement of Essure inserts as an example. If you perform the procedure in the hospital, then the hospital buys the equipment, including the hysteroscope and light source. The hospital also pays for the room and staff and manages equipment sterilization. If, on the other hand, you perform the procedure in your office, all those responsibilities are yours. If it’s done in your office, you get paid more but it also costs you more.
The Relative Value Update Committee, or RUC, plays a major role in determining RVUs. This committee is composed of 31 clinicians, including nonphysician providers, psychologists, and nurses who deliver services under the Medicare fee schedule. The RUC makes recommendations to the Centers for Medicare and Medicaid Services (CMS), but it is the Secretary of Health and Human Services who determines the final rule on RVUs.
Approximately 75% to 95% of the recommendations of the RUC are accepted by the Secretary and become law. So it’s not the RUC or the American Medical Association (AMA) that determines RVUs; in the long run, it is CMS and the Secretary of Health and Human Services. We are fortunate that, when CMS assigns RVUs we’re not happy with, we have an opportunity to appeal.
Under Medicare, all physician payments are based on the same conversion factor, regardless of specialty. That’s not necessarily true for other payers, who may, essentially, do whatever they wish. These other payers frequently will contract at higher or lower rates, depending on how prevalent a specialist is in the community. Sometimes they use a higher conversion factor for surgical specialists than they use for primary care.
6. Find out which RVUs the payer is using
When you negotiate contracts with payers, and you are in private practice or part of a medical practice, it’s important to know what year’s RVUs the payer is using, as RVUs vary from year to year. For example, if the payer is using the RBRVS from 2002, it is paying you less than you should be getting. So when you look at a contract, you should determine not only whether the payer is anchoring your payment to the RBRVS but also whether it is keeping up with current RVUs as well. What dollar conversion factor is the payer using? What global periods—the same as CMS, or something different?
7. Determine what global period is in play
Some private payers use 6 postoperative weeks as the global period for a surgical procedure, whereas Medicare uses 90 days. You need to know which period is in play so that you don’t leave money on the table if you see the patient within 90 days but more than 6 weeks postoperatively.
Current Procedural Terminology (CPT) has global surgical packages that include a 10-day or 90-day period. But those periods do not include services provided more than 24 hours before the procedure. They don’t include the administration of anesthesia or conscious sedation. And they don’t include management of complications, exacerbations, or recurrences. Nor do they include additional services that might be necessary due to the presence of another disease or injury.
Under Medicare, the rules are different. Medicare preoperative services begin 1 day before surgery. However, any preoperative intervention is included whether it’s performed 1 day or 1 week before surgery. If it’s simply a preoperative physical examination for the patient and you aren’t performing significant evaluation and management, it’s included in the global package, along with all the intraoperative work. In addition, under Medicare, you don’t get paid for the management of complications unless a return to the operating room is required.
8. Learn to use modifiers
As ObGyns, we often see patients for multiple conditions or problem reports, so you need to be aware that if a patient is within a global period and you do not submit a bill with a modifier to indicate special circumstances, the intervention will be bundled into the global and you will not get paid for it. Modifiers are two-digit codes that describe these separate services. They provide critical information to payers so that their computer programs separate these services out for payment.
Major surgical procedures don’t include unrelated procedures that are performed at the same time of surgery. Nor do they include visits that take place during the global period that are unrelated to the original surgery. For example, if a patient presents with a breast lump after you performed a hysterectomy, and you do a work-up, you deserve full payment for that evaluation and management service. If you don’t use a modifier, however, you won’t get that payment.
9. Don’t be passive when payers won’t pay
Let’s say you contract with HMOs or independent practice associations (IPAs), and they’re not compensating you for the extra things you’re doing and are failing to recognize surgical modifiers. What can you do about it?
You need to develop a profile of your typical patient. Because these organizations are individualizing it—they are saying that, in a typical scenario, this is the type of work you do. So these organizations offer a different kind of contract. Nevertheless, you can use your coding to help you determine what a fair payment should be, by going through your billing to determine what you’ve spent.
10. Analyze payer bundling
Medicare put in place a correct coding initiative (CCI) that lists services typically provided by the same person on the same day of service. The aim: to prevent separate payment for these services. These are “bundled” services. The CCI bundles are revised every quarter. They are listed on the ACOG Web site under “practice management.”
On October 1, 2014, the CCI inappropriately bundled pelvic organ prolapse repair procedures into the vaginal hysterectomy codes. ACOG, AUGS, SGS, and AAGL are arguing vehemently as this article is going to press to ensure that these damaging bundles are rescinded.
Private payers can bundle anything, and it may or may not make sense or be fair. One ACOG resource is the book Ob/Gyn Coding Manual: Components of Correct Procedural Coding, which is revised every year. It has a tear-out page for every procedure code and will help you determine whether or not a bundle is appropriate.
You need to know about bundling and dispute resolution. Why? Because it is possible to insert clauses into your contract that give you some rights. Insurers have all the clout and you have nothing unless you fight for it.
You may see clauses such as “the company reserves the right to re-bundle to the primary procedure....” You shouldn’t tolerate that. Rather, you want to say, “the company will use CCI bundled rules” so that you at least know what the rules are.
11. Don’t be afraid to revise a contract
If we have to hold a payer harmless, the payer should hold us harmless as well. If we consult an insurer’s Web site to confirm that a patient is covered, and we take her to surgery because we have evidence she has insurance, the insurer shouldn’t be able to rescind payment 6 months later because the patient didn’t pay for her insurance that month. That’s not fair. The company told you she was covered, and you deserve to get paid for that surgery because you are relying on information from the company itself. So when you sign a contract, you need to ensure that you are being held harmless as well as the insurer.
12. Calculate your own RVUs
Use your claims software for data. Consult the Federal Register or ACOG to determine the total number of RVUs for a given CPT code. Multiply the RVUs by the quantity for each code. Let’s say it’s an evaluation and management visit, code 99213, and you’ve done 50 this month. That’s 50 multiplied by 1.3 RVUs. Add all the codes together, then use your monthly profit and loss statement to determine what your expenses are. Divide your total expenses by the total number of RVUs to determine your practice cost per RVU. You then can decide on a conversion factor you can tolerate, and you can use this information when contracting with IPAs, HMOs, and other insurers.
13. Spend money to make money
There are many coding resources available to you. Coding is well worth what you spend on it because you can get it back in a heartbeat.
This information may not be easy to master, but it’s critically important for your economic survival—to get what’s rightfully yours and get paid fairly for what you do.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The payment structure for physicians is changing. Our government, the American public, purchasers, and employers are unhappy with the fee-for-service system as it currently exists, and are pushing to drive the system into what is called “value-based purchasing.”
But what is value?
One way to define it is quality divided by cost—but how do we measure quality?
At present, insurers are measuring your quality based on some nebulous definition created at United Healthcare or Blue Cross Blue Shield—looking specifically at your “efficiency,” based on the costs attributed to you, as revealed in the codes you and others submit to payers.
Let’s say you perform minimally invasive surgery, and the referring physician ordered a lot of tests before sending the patient to you. Are you aware that all of those costs may be attributed to you in an administrative system?
ACOG is working hard to establish clinical systems rather than administrative ones to determine the true cost of care. We may want to think of obstetrics and gynecology as primary care and take advantage of advanced payment models and the opportunities afforded to accountable care organizations, but the truth is, insurers frequently do not consider us primary care. Although some of us may develop medical homes for women’s health care, we are unlikely to collect a per-patient, per-month income like primary care physicians do. That means that we need to be more assertive in negotiating contracts with insurers.
In this article, I offer recommendations for such negotiations and explain how to determine what you can and cannot accept in terms of payment.
You are the responsible party
Some of us do our own coding and some of us do not. However, if that coding is inaccurate, it is the physician who goes to jail, not the coder. You are personally responsible and liable for the coding submitted under your provider number.
Clearly, we need to do a better job of advocating for ourselves. We need to lobby. Legislators and bureaucrats are less likely to target people who have strong lobbyists working consistently on their behalf.
Accountable care organizations may have some leverage in negotiating lower prices, and some market forces may come into play in large systems. It remains to be seen which models will succeed as new payment structures develop. The overarching question: What can we do today to optimize our payments, given the system that we have? Here are 13 tactics that can enhance your bottom line.
1. Know the rules
To play the game, you must know the rules. You need to know what systems payers are using to determine your reimbursement—and you have to understand those systems as well as, or better than, the payers do. Then you’ll be able to use them to your advantage.
Payers are well aware that we don’t like to focus on this end of practice, that what we really want to do is spend the day practicing medicine. However, we need to learn these details because we’re leaving money on the table every single day.
2. Educate yourself
With the change to the International Classification of Diseases (ICD) scheduled to take effect on October 1, 2015, many of us are worried that payers are going to reject our claims because of our lack of familiarity with ICD-10.
Rest assured. There are crosswalks from ICD-9 to ICD-10. ACOG has published an information sheet for both obstetrics and gynecology that pairs typically used ICD-9 codes with their ICD-10 counterparts. And because it is published by ACOG, payers will find it hard to claim that it’s inaccurate.
ACOG also offers half-day courses on ICD-10 coding for both physicians and staff.
3. Record your decision-making process
When I audit medical charts, I often discover that this process has been neglected. Instead, the coder has relied on documentation from the electronic health record and a basic description of the treatment plan. But a plan is just that—what someone intends to do. It doesn’t convey the decision-making that underlies it. What was the differential diagnosis? What did you discuss with the patient? These details are critical for appropriate coding of the level of service—whether it’s high, intermediate, or low.
4. Refine your approach to coding
Recognize that the system is currently set up to pay physicians for the services we provide—and that service must be justified by the appropriate diagnosis code. Tougher cases, or high-risk patients, tend to have longer surgeries and hospital stays, and their outcomes often are not as good as those of more typical patients. They may have more complications because they’re obese or have severe diabetes, for example. If so, it is critical that these other conditions—obesity and severe diabetes—be included with the principal diagnosis code so that risk stratification is possible. Otherwise, we will be held to the same standard as someone treating a routine, low-risk case.
Risk stratification is being performed according to algorithms in the payers’ software—and payers are unlikely to share the details with us. However, the only real data payers have to run through these algorithms come from diagnosis coding. Even though you’re not required to code for variables such as obesity and diabetes in order to get paid for what you do, you do need to use those additional codes to make risk stratification possible—so that you don’t get inappropriately placed into a group of low-risk providers when you are treating a higher-risk cohort.
5. Develop an understanding of RVUs
Another variable that changes regularly is relative value units (RVUs) under Medicare rules. ACOG’s Committee on Health Economics and Coding—which enjoys the participation of AAGL, the American Urogynecologic Society (AUGS), the Society of Gynecologic Surgeons (SGS), and the Society of Gynecologic Oncology (SGO), as well as other organizations—tries to maintain the RVUs as up to date and appropriate as possible relative to other services in the fee schedule.
For example, about 10 years ago many urogynecologic procedures were getting bundled together when they were performed at the same time. We had only one or two ICD-9 codes to describe prolapse, with no separate codes to describe whether it affected the anterior, apical, or posterior compartment, even though we performed different procedures in the individual compartments. Payers were mapping all prolapse procedures to the same diagnosis code. So ACOG went to the National Center for Health Statistics, where ICD-9 coding was done—and developed a series of about 10 codes to describe the different areas that prolapse could affect.
That kind of nuanced coding is continuing today. In fact, we have a long list of areas to go forward with now that ICD-10 is scheduled to take effect. A good example involves new Pap smear guidelines, which recommend testing every 3 or 5 years except for patients who have undergone hysterectomy for benign disease. How do you code for a patient who has had a hysterectomy? There was no code for a woman with an absent cervix, so we created a “V-code,” a code classification for factors that influence health status, so that it is possible to explain why a Pap smear was not performed.
As we go forward into a value-based system, specialists like us likely will be negotiating contracts according to RVU-based payments. That’s why it’s important for you to understand the resource-based relative value scale (RBRVS). It has three components: a work component, which makes up about 52% of the total RVUs; a practice expense, which makes up more than 45% of total RVUS; and, finally, a malpractice component, a small percentage. There also is a geographic adjustment and a uniform conversion factor.
When you hear about the sustainable growth rate (SGR) fix, and the fact that we’re going to see a 20% or 24% reduction in payment, that talk is referring to a reduction in the conversion factor. Each component of the RVU is adjusted for geography and then multiplied by the dollar conversion factor to calculate the total RVUs. The work, practice, and malpractice components vary by where the service is provided.
Let’s use placement of Essure inserts as an example. If you perform the procedure in the hospital, then the hospital buys the equipment, including the hysteroscope and light source. The hospital also pays for the room and staff and manages equipment sterilization. If, on the other hand, you perform the procedure in your office, all those responsibilities are yours. If it’s done in your office, you get paid more but it also costs you more.
The Relative Value Update Committee, or RUC, plays a major role in determining RVUs. This committee is composed of 31 clinicians, including nonphysician providers, psychologists, and nurses who deliver services under the Medicare fee schedule. The RUC makes recommendations to the Centers for Medicare and Medicaid Services (CMS), but it is the Secretary of Health and Human Services who determines the final rule on RVUs.
Approximately 75% to 95% of the recommendations of the RUC are accepted by the Secretary and become law. So it’s not the RUC or the American Medical Association (AMA) that determines RVUs; in the long run, it is CMS and the Secretary of Health and Human Services. We are fortunate that, when CMS assigns RVUs we’re not happy with, we have an opportunity to appeal.
Under Medicare, all physician payments are based on the same conversion factor, regardless of specialty. That’s not necessarily true for other payers, who may, essentially, do whatever they wish. These other payers frequently will contract at higher or lower rates, depending on how prevalent a specialist is in the community. Sometimes they use a higher conversion factor for surgical specialists than they use for primary care.
6. Find out which RVUs the payer is using
When you negotiate contracts with payers, and you are in private practice or part of a medical practice, it’s important to know what year’s RVUs the payer is using, as RVUs vary from year to year. For example, if the payer is using the RBRVS from 2002, it is paying you less than you should be getting. So when you look at a contract, you should determine not only whether the payer is anchoring your payment to the RBRVS but also whether it is keeping up with current RVUs as well. What dollar conversion factor is the payer using? What global periods—the same as CMS, or something different?
7. Determine what global period is in play
Some private payers use 6 postoperative weeks as the global period for a surgical procedure, whereas Medicare uses 90 days. You need to know which period is in play so that you don’t leave money on the table if you see the patient within 90 days but more than 6 weeks postoperatively.
Current Procedural Terminology (CPT) has global surgical packages that include a 10-day or 90-day period. But those periods do not include services provided more than 24 hours before the procedure. They don’t include the administration of anesthesia or conscious sedation. And they don’t include management of complications, exacerbations, or recurrences. Nor do they include additional services that might be necessary due to the presence of another disease or injury.
Under Medicare, the rules are different. Medicare preoperative services begin 1 day before surgery. However, any preoperative intervention is included whether it’s performed 1 day or 1 week before surgery. If it’s simply a preoperative physical examination for the patient and you aren’t performing significant evaluation and management, it’s included in the global package, along with all the intraoperative work. In addition, under Medicare, you don’t get paid for the management of complications unless a return to the operating room is required.
8. Learn to use modifiers
As ObGyns, we often see patients for multiple conditions or problem reports, so you need to be aware that if a patient is within a global period and you do not submit a bill with a modifier to indicate special circumstances, the intervention will be bundled into the global and you will not get paid for it. Modifiers are two-digit codes that describe these separate services. They provide critical information to payers so that their computer programs separate these services out for payment.
Major surgical procedures don’t include unrelated procedures that are performed at the same time of surgery. Nor do they include visits that take place during the global period that are unrelated to the original surgery. For example, if a patient presents with a breast lump after you performed a hysterectomy, and you do a work-up, you deserve full payment for that evaluation and management service. If you don’t use a modifier, however, you won’t get that payment.
9. Don’t be passive when payers won’t pay
Let’s say you contract with HMOs or independent practice associations (IPAs), and they’re not compensating you for the extra things you’re doing and are failing to recognize surgical modifiers. What can you do about it?
You need to develop a profile of your typical patient. Because these organizations are individualizing it—they are saying that, in a typical scenario, this is the type of work you do. So these organizations offer a different kind of contract. Nevertheless, you can use your coding to help you determine what a fair payment should be, by going through your billing to determine what you’ve spent.
10. Analyze payer bundling
Medicare put in place a correct coding initiative (CCI) that lists services typically provided by the same person on the same day of service. The aim: to prevent separate payment for these services. These are “bundled” services. The CCI bundles are revised every quarter. They are listed on the ACOG Web site under “practice management.”
On October 1, 2014, the CCI inappropriately bundled pelvic organ prolapse repair procedures into the vaginal hysterectomy codes. ACOG, AUGS, SGS, and AAGL are arguing vehemently as this article is going to press to ensure that these damaging bundles are rescinded.
Private payers can bundle anything, and it may or may not make sense or be fair. One ACOG resource is the book Ob/Gyn Coding Manual: Components of Correct Procedural Coding, which is revised every year. It has a tear-out page for every procedure code and will help you determine whether or not a bundle is appropriate.
You need to know about bundling and dispute resolution. Why? Because it is possible to insert clauses into your contract that give you some rights. Insurers have all the clout and you have nothing unless you fight for it.
You may see clauses such as “the company reserves the right to re-bundle to the primary procedure....” You shouldn’t tolerate that. Rather, you want to say, “the company will use CCI bundled rules” so that you at least know what the rules are.
11. Don’t be afraid to revise a contract
If we have to hold a payer harmless, the payer should hold us harmless as well. If we consult an insurer’s Web site to confirm that a patient is covered, and we take her to surgery because we have evidence she has insurance, the insurer shouldn’t be able to rescind payment 6 months later because the patient didn’t pay for her insurance that month. That’s not fair. The company told you she was covered, and you deserve to get paid for that surgery because you are relying on information from the company itself. So when you sign a contract, you need to ensure that you are being held harmless as well as the insurer.
12. Calculate your own RVUs
Use your claims software for data. Consult the Federal Register or ACOG to determine the total number of RVUs for a given CPT code. Multiply the RVUs by the quantity for each code. Let’s say it’s an evaluation and management visit, code 99213, and you’ve done 50 this month. That’s 50 multiplied by 1.3 RVUs. Add all the codes together, then use your monthly profit and loss statement to determine what your expenses are. Divide your total expenses by the total number of RVUs to determine your practice cost per RVU. You then can decide on a conversion factor you can tolerate, and you can use this information when contracting with IPAs, HMOs, and other insurers.
13. Spend money to make money
There are many coding resources available to you. Coding is well worth what you spend on it because you can get it back in a heartbeat.
This information may not be easy to master, but it’s critically important for your economic survival—to get what’s rightfully yours and get paid fairly for what you do.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.