User login
Children don’t have to belong to the clean plate club
It’s a scene you’re all too familiar with – you’ve gone to a lot of trouble to give your children a well-prepared and nutritious meal, but a lot of it never leaves the plate. It’s frustrating, and you worry about their health, but according to the Cornell Food and Brand Lab, this is not unusual behavior.
When an adult serves him or herself food, it almost all gets eaten, according to a study from Brian Wansink, Ph.D., director of the Cornell Food and Brand Lab in Ithaca, N.Y., with the average adult eating 92% of the food served. However, the same study also found that children eat only about 60% of the food they serve to themselves, indicating a broad difference in how adults and children approach food.
Adults know what they like, and if they try something new, they might only take a little bit in case they don’t like it. Children tend not to fully understand their limits and may take a lot of something they’ve never had before because it looks appetizing, try it, realize they don’t like it, and not finish it. “It’s natural, for them to make some mistakes and take a food they don’t like or to serve too much,” Dr. Wansink said.
Dr. Wansink noted that the children in the study were not eating with their parents, and perhaps they would have eaten more if their parents had been there, but not enough to account for the vast discrepancy between the two groups. Other possible factors in children eating less include uncertainty toward how much will make them full, whether or not they are eating with utensils, the presence of friends, and whether they are introverted or extroverted.
So for all the frustrated parents out there“who want his/her noncooperating children to be vegetable-eating members of the clean plate club,” there is good news in these results. “They show that children who only eat half to two-thirds of the food they serve themselves aren’t being wasteful, belligerent, or disrespectful,” Dr. Wansink concluded. It’s a matter of kids just being kids.
It’s a scene you’re all too familiar with – you’ve gone to a lot of trouble to give your children a well-prepared and nutritious meal, but a lot of it never leaves the plate. It’s frustrating, and you worry about their health, but according to the Cornell Food and Brand Lab, this is not unusual behavior.
When an adult serves him or herself food, it almost all gets eaten, according to a study from Brian Wansink, Ph.D., director of the Cornell Food and Brand Lab in Ithaca, N.Y., with the average adult eating 92% of the food served. However, the same study also found that children eat only about 60% of the food they serve to themselves, indicating a broad difference in how adults and children approach food.
Adults know what they like, and if they try something new, they might only take a little bit in case they don’t like it. Children tend not to fully understand their limits and may take a lot of something they’ve never had before because it looks appetizing, try it, realize they don’t like it, and not finish it. “It’s natural, for them to make some mistakes and take a food they don’t like or to serve too much,” Dr. Wansink said.
Dr. Wansink noted that the children in the study were not eating with their parents, and perhaps they would have eaten more if their parents had been there, but not enough to account for the vast discrepancy between the two groups. Other possible factors in children eating less include uncertainty toward how much will make them full, whether or not they are eating with utensils, the presence of friends, and whether they are introverted or extroverted.
So for all the frustrated parents out there“who want his/her noncooperating children to be vegetable-eating members of the clean plate club,” there is good news in these results. “They show that children who only eat half to two-thirds of the food they serve themselves aren’t being wasteful, belligerent, or disrespectful,” Dr. Wansink concluded. It’s a matter of kids just being kids.
It’s a scene you’re all too familiar with – you’ve gone to a lot of trouble to give your children a well-prepared and nutritious meal, but a lot of it never leaves the plate. It’s frustrating, and you worry about their health, but according to the Cornell Food and Brand Lab, this is not unusual behavior.
When an adult serves him or herself food, it almost all gets eaten, according to a study from Brian Wansink, Ph.D., director of the Cornell Food and Brand Lab in Ithaca, N.Y., with the average adult eating 92% of the food served. However, the same study also found that children eat only about 60% of the food they serve to themselves, indicating a broad difference in how adults and children approach food.
Adults know what they like, and if they try something new, they might only take a little bit in case they don’t like it. Children tend not to fully understand their limits and may take a lot of something they’ve never had before because it looks appetizing, try it, realize they don’t like it, and not finish it. “It’s natural, for them to make some mistakes and take a food they don’t like or to serve too much,” Dr. Wansink said.
Dr. Wansink noted that the children in the study were not eating with their parents, and perhaps they would have eaten more if their parents had been there, but not enough to account for the vast discrepancy between the two groups. Other possible factors in children eating less include uncertainty toward how much will make them full, whether or not they are eating with utensils, the presence of friends, and whether they are introverted or extroverted.
So for all the frustrated parents out there“who want his/her noncooperating children to be vegetable-eating members of the clean plate club,” there is good news in these results. “They show that children who only eat half to two-thirds of the food they serve themselves aren’t being wasteful, belligerent, or disrespectful,” Dr. Wansink concluded. It’s a matter of kids just being kids.
PD-1 checkpoint inhibitors show mettle against relapsed Hodgkin’s lymphoma
SAN FRANCISCO– PD-1 checkpoint inhibitors, which have shown remarkable efficacy against advanced malignant melanoma, appear to hold similar promise in the treatment of relapsed or refractory Hodgkin’s lymphoma, results from two early studies suggest.
In a phase I study, the PD-1 blocking antibody nivolumab produced an 87% response rate in 23 heavily pre-treated patients with relapsed Hodgkin’s lymphoma (HL). In a separate phase Ib study, pembrolizumab, which blocks the PD-1 and PD-2 ligands, produced a 66% overall response rate, 21% complete remission rate, and 86% clinical benefit rate among 29 patients with HL for whom therapy with brentuximab vedotin (Adcetris) had failed.
The studies were presented at a media briefing prior to the presentation of data in oral sessions at the annual meeting of the American Society of Hematology.
“Classical Hodgkin lymphoma appears to be a tumor with genetically determined vulnerability to PD-1 blockade. We hope that PD-1 blockade in the future can become an important part of the treatment of patients with Hodgkin lymphoma,” said Dr. Phillipe Armand from the Dana-Farber Cancer Institute in Boston, an investigator for the nivolumab study.
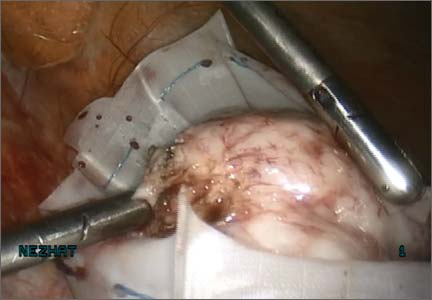
Evidence from preclinical studies suggests that the Reed-Sternberg malignant cells characteristic of HL may use the PD-1 (programmed death 1) pathway to evade detection by immune cells, as suggested by pathologic studies showing the cells surrounded by an extensive but ineffective infiltrate of inflammatory cells.
“We’ve wondered for a long time how Hodgkin lymphoma could attract such a brisk immune response and yet have this immune response fail to kill the tumor,” he said.
Genetic Achilles heel
Genetic analyses had shown that HL frequently has a mutation that results in amplification of a region on chromosome 9 (9p24.1) which leads to increased expression of PD-1 ligands 1 and 2, and leads to a downregulation or weakening of the immune response. The mutation appears to work through the Janus kinase (JAK)-signal transducer and activator transcription (STAT) signalling. These findings suggested to researchers that classical HL has a genetically driven and, ideally, targetable dependence on the PD-1 pathway for survival, Dr. Armand explained.
To test this idea, he and colleagues studied 23 patients with relapsed or refractory HL that had been heavily pre-treated who were part of an independent expansion cohort of a study of nivolumab in hematologic malignancies. Of these patients, 78% were enrolled after a relapse following autologous stem cell transplantation, and 22% after treatment with brentuximab vedotin had failed.
The patients received nivolumab 3 mg/kg every 2 weeks until they had either a complete response, tumor progression, or excessive side effects. In all, 20 of the 23 patients (87%) had an objective response to the single-agent therapy, including 4 (17%) complete responses and 16 (70%) partial responses. The remaining three patients (13%) had stable disease.
The longest time on study at the data cutoff point was 72 weeks. Among all responders, 60% had a response by 8 weeks of therapy, 48% are ongoing, and 43% of patients are still on treatment.
Drug-related adverse events were reported in 18 patients, most commonly rash and decreased platelet count. Five patients had grade 3 events. There were no drug-related grade 4 events or deaths.
In an editorial accompanying the study, which was also published online in The New England Journal of Medicine, Dr. Mario Sznoll and Dr. Dan L. Longo from the Yale University School of Medicine in New Haven, Connecticut write that “with recent data showing impressive clinical activity of PD-1 or PD-L1 antagonists in subgroups of patients with a variety of different cancers, the critical and foundational role of immune interventions in cancer treatment is no longer deniable,” (NEJM, Dec. 6, 2014 [DOI: 10.1056/NEJMoa1411087]).
Pembrolizumab trial
Dr. Craig H. Moskowitz from Memorial Sloan-Kettering Cancer Center in New York City discussed results of the second study, dubbed KEYNOTE-013 (A Phase Ib Multi-Cohort Trial of MK-3475 in Subjects With Hematologic Malignancies).
In this study, patients with HL who were not transplant eligible or for whom transplant had failed and who either had a relapse or were refractory to therapy with brentuximab vedotin received 19 mg/kg IV infusion of pembrolizumab every 2 weeks until complete response, partial response/stable disease, or disease progression.
Of the 31 patients enrolled, 29 were available for the analysis. As of the data cutoff in November 2014, 6 patients (21%) had achieved a complete remission, and 13 (45%) had a partial response, for an overall response rate of 66%. The median time to response was 12 weeks, and as of the data cutoff 17 of 19 patients had ongoing responses. The median response duration has not yet been reached. An additional 6 patients (21%) had stable disease, leading to an overall clinical benefit rate (responses plus stable disease) of 86%.
The patients generally tolerated the drug well. There were 4 treatment-related adverse events in 3 patients, including axillary pain, hypoxia, joint swelling, and pneumonitis. There were no grade 4 treatment-related events or deaths.
Of the tumor samples evaluable, all expressed PD-L1, supporting the rationale for PD-1 blockade in this population, Dr. Moskowitz said.
The results of both his and Dr. Armand’s study support the continued development of PD-1 inhibitors in various subsets of patients with classical Hodgkin’s lymphoma, he said.
SAN FRANCISCO– PD-1 checkpoint inhibitors, which have shown remarkable efficacy against advanced malignant melanoma, appear to hold similar promise in the treatment of relapsed or refractory Hodgkin’s lymphoma, results from two early studies suggest.
In a phase I study, the PD-1 blocking antibody nivolumab produced an 87% response rate in 23 heavily pre-treated patients with relapsed Hodgkin’s lymphoma (HL). In a separate phase Ib study, pembrolizumab, which blocks the PD-1 and PD-2 ligands, produced a 66% overall response rate, 21% complete remission rate, and 86% clinical benefit rate among 29 patients with HL for whom therapy with brentuximab vedotin (Adcetris) had failed.
The studies were presented at a media briefing prior to the presentation of data in oral sessions at the annual meeting of the American Society of Hematology.
“Classical Hodgkin lymphoma appears to be a tumor with genetically determined vulnerability to PD-1 blockade. We hope that PD-1 blockade in the future can become an important part of the treatment of patients with Hodgkin lymphoma,” said Dr. Phillipe Armand from the Dana-Farber Cancer Institute in Boston, an investigator for the nivolumab study.
Evidence from preclinical studies suggests that the Reed-Sternberg malignant cells characteristic of HL may use the PD-1 (programmed death 1) pathway to evade detection by immune cells, as suggested by pathologic studies showing the cells surrounded by an extensive but ineffective infiltrate of inflammatory cells.
“We’ve wondered for a long time how Hodgkin lymphoma could attract such a brisk immune response and yet have this immune response fail to kill the tumor,” he said.
Genetic Achilles heel
Genetic analyses had shown that HL frequently has a mutation that results in amplification of a region on chromosome 9 (9p24.1) which leads to increased expression of PD-1 ligands 1 and 2, and leads to a downregulation or weakening of the immune response. The mutation appears to work through the Janus kinase (JAK)-signal transducer and activator transcription (STAT) signalling. These findings suggested to researchers that classical HL has a genetically driven and, ideally, targetable dependence on the PD-1 pathway for survival, Dr. Armand explained.
To test this idea, he and colleagues studied 23 patients with relapsed or refractory HL that had been heavily pre-treated who were part of an independent expansion cohort of a study of nivolumab in hematologic malignancies. Of these patients, 78% were enrolled after a relapse following autologous stem cell transplantation, and 22% after treatment with brentuximab vedotin had failed.
The patients received nivolumab 3 mg/kg every 2 weeks until they had either a complete response, tumor progression, or excessive side effects. In all, 20 of the 23 patients (87%) had an objective response to the single-agent therapy, including 4 (17%) complete responses and 16 (70%) partial responses. The remaining three patients (13%) had stable disease.
The longest time on study at the data cutoff point was 72 weeks. Among all responders, 60% had a response by 8 weeks of therapy, 48% are ongoing, and 43% of patients are still on treatment.
Drug-related adverse events were reported in 18 patients, most commonly rash and decreased platelet count. Five patients had grade 3 events. There were no drug-related grade 4 events or deaths.
In an editorial accompanying the study, which was also published online in The New England Journal of Medicine, Dr. Mario Sznoll and Dr. Dan L. Longo from the Yale University School of Medicine in New Haven, Connecticut write that “with recent data showing impressive clinical activity of PD-1 or PD-L1 antagonists in subgroups of patients with a variety of different cancers, the critical and foundational role of immune interventions in cancer treatment is no longer deniable,” (NEJM, Dec. 6, 2014 [DOI: 10.1056/NEJMoa1411087]).
Pembrolizumab trial
Dr. Craig H. Moskowitz from Memorial Sloan-Kettering Cancer Center in New York City discussed results of the second study, dubbed KEYNOTE-013 (A Phase Ib Multi-Cohort Trial of MK-3475 in Subjects With Hematologic Malignancies).
In this study, patients with HL who were not transplant eligible or for whom transplant had failed and who either had a relapse or were refractory to therapy with brentuximab vedotin received 19 mg/kg IV infusion of pembrolizumab every 2 weeks until complete response, partial response/stable disease, or disease progression.
Of the 31 patients enrolled, 29 were available for the analysis. As of the data cutoff in November 2014, 6 patients (21%) had achieved a complete remission, and 13 (45%) had a partial response, for an overall response rate of 66%. The median time to response was 12 weeks, and as of the data cutoff 17 of 19 patients had ongoing responses. The median response duration has not yet been reached. An additional 6 patients (21%) had stable disease, leading to an overall clinical benefit rate (responses plus stable disease) of 86%.
The patients generally tolerated the drug well. There were 4 treatment-related adverse events in 3 patients, including axillary pain, hypoxia, joint swelling, and pneumonitis. There were no grade 4 treatment-related events or deaths.
Of the tumor samples evaluable, all expressed PD-L1, supporting the rationale for PD-1 blockade in this population, Dr. Moskowitz said.
The results of both his and Dr. Armand’s study support the continued development of PD-1 inhibitors in various subsets of patients with classical Hodgkin’s lymphoma, he said.
SAN FRANCISCO– PD-1 checkpoint inhibitors, which have shown remarkable efficacy against advanced malignant melanoma, appear to hold similar promise in the treatment of relapsed or refractory Hodgkin’s lymphoma, results from two early studies suggest.
In a phase I study, the PD-1 blocking antibody nivolumab produced an 87% response rate in 23 heavily pre-treated patients with relapsed Hodgkin’s lymphoma (HL). In a separate phase Ib study, pembrolizumab, which blocks the PD-1 and PD-2 ligands, produced a 66% overall response rate, 21% complete remission rate, and 86% clinical benefit rate among 29 patients with HL for whom therapy with brentuximab vedotin (Adcetris) had failed.
The studies were presented at a media briefing prior to the presentation of data in oral sessions at the annual meeting of the American Society of Hematology.
“Classical Hodgkin lymphoma appears to be a tumor with genetically determined vulnerability to PD-1 blockade. We hope that PD-1 blockade in the future can become an important part of the treatment of patients with Hodgkin lymphoma,” said Dr. Phillipe Armand from the Dana-Farber Cancer Institute in Boston, an investigator for the nivolumab study.
Evidence from preclinical studies suggests that the Reed-Sternberg malignant cells characteristic of HL may use the PD-1 (programmed death 1) pathway to evade detection by immune cells, as suggested by pathologic studies showing the cells surrounded by an extensive but ineffective infiltrate of inflammatory cells.
“We’ve wondered for a long time how Hodgkin lymphoma could attract such a brisk immune response and yet have this immune response fail to kill the tumor,” he said.
Genetic Achilles heel
Genetic analyses had shown that HL frequently has a mutation that results in amplification of a region on chromosome 9 (9p24.1) which leads to increased expression of PD-1 ligands 1 and 2, and leads to a downregulation or weakening of the immune response. The mutation appears to work through the Janus kinase (JAK)-signal transducer and activator transcription (STAT) signalling. These findings suggested to researchers that classical HL has a genetically driven and, ideally, targetable dependence on the PD-1 pathway for survival, Dr. Armand explained.
To test this idea, he and colleagues studied 23 patients with relapsed or refractory HL that had been heavily pre-treated who were part of an independent expansion cohort of a study of nivolumab in hematologic malignancies. Of these patients, 78% were enrolled after a relapse following autologous stem cell transplantation, and 22% after treatment with brentuximab vedotin had failed.
The patients received nivolumab 3 mg/kg every 2 weeks until they had either a complete response, tumor progression, or excessive side effects. In all, 20 of the 23 patients (87%) had an objective response to the single-agent therapy, including 4 (17%) complete responses and 16 (70%) partial responses. The remaining three patients (13%) had stable disease.
The longest time on study at the data cutoff point was 72 weeks. Among all responders, 60% had a response by 8 weeks of therapy, 48% are ongoing, and 43% of patients are still on treatment.
Drug-related adverse events were reported in 18 patients, most commonly rash and decreased platelet count. Five patients had grade 3 events. There were no drug-related grade 4 events or deaths.
In an editorial accompanying the study, which was also published online in The New England Journal of Medicine, Dr. Mario Sznoll and Dr. Dan L. Longo from the Yale University School of Medicine in New Haven, Connecticut write that “with recent data showing impressive clinical activity of PD-1 or PD-L1 antagonists in subgroups of patients with a variety of different cancers, the critical and foundational role of immune interventions in cancer treatment is no longer deniable,” (NEJM, Dec. 6, 2014 [DOI: 10.1056/NEJMoa1411087]).
Pembrolizumab trial
Dr. Craig H. Moskowitz from Memorial Sloan-Kettering Cancer Center in New York City discussed results of the second study, dubbed KEYNOTE-013 (A Phase Ib Multi-Cohort Trial of MK-3475 in Subjects With Hematologic Malignancies).
In this study, patients with HL who were not transplant eligible or for whom transplant had failed and who either had a relapse or were refractory to therapy with brentuximab vedotin received 19 mg/kg IV infusion of pembrolizumab every 2 weeks until complete response, partial response/stable disease, or disease progression.
Of the 31 patients enrolled, 29 were available for the analysis. As of the data cutoff in November 2014, 6 patients (21%) had achieved a complete remission, and 13 (45%) had a partial response, for an overall response rate of 66%. The median time to response was 12 weeks, and as of the data cutoff 17 of 19 patients had ongoing responses. The median response duration has not yet been reached. An additional 6 patients (21%) had stable disease, leading to an overall clinical benefit rate (responses plus stable disease) of 86%.
The patients generally tolerated the drug well. There were 4 treatment-related adverse events in 3 patients, including axillary pain, hypoxia, joint swelling, and pneumonitis. There were no grade 4 treatment-related events or deaths.
Of the tumor samples evaluable, all expressed PD-L1, supporting the rationale for PD-1 blockade in this population, Dr. Moskowitz said.
The results of both his and Dr. Armand’s study support the continued development of PD-1 inhibitors in various subsets of patients with classical Hodgkin’s lymphoma, he said.
Key clinical point: PD-1 checkpoint inhibition appears to be an effective strategy against treatment-refractory Hodgkin’s lymphoma.
Major finding: Nivolumab produced an 87% objective response rate and pembrolizumab a 66% response rate in patients with heavily pre-treated Hodgkin’s lymphoma.
Data source: A phase I study with 23 patients and a phase Ib study with 29 patients with relapsed or refractory Hodgkin’s lymphoma.
Disclosures: Dr. Armand’s study is supported by Bristol-Myers Squibb. He reported grant support from Bristol-Myers Squibb during the conduct of the study and personal fees from Merck outside the study. Dr. Moskowitz’ study is supported by Merck. He reported receiving research funding from the company. Dr. Sznol reported personal fees from Bristol-Myers Squibb, Dr. Longo reported no relevant disclosures.
Health Canada approves ibrutinib for CLL
Health Canada recently approved the Bruton tyrosine kinase inhibitor ibrutinib (Imbruvica) for the treatment of chronic lymphocytic leukemia (CLL).
The drug can now be used to treat CLL patients, including those with 17p deletion, who have received at least one prior therapy. It can also be used as frontline treatment in CLL patients with 17p deletion.
Health Canada’s approval of ibrutinib is based on results of the phase 3 RESONATE trial, which were presented at this year’s ASCO and EHA meetings.
The trial included 391 previously treated patients, 127 of whom had 17p deletion. Patients were randomized to receive ibrutinib or the anti-CD20 monoclonal antibody ofatumumab until disease progression or unacceptable toxicity.
The trial was stopped early after a pre-planned interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
At the time of interim analysis, the patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm.
Ibrutinib significantly prolonged progression-free and overall survival. The median progression-free survival was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Of the 127 patients with 17p deletion, those treated with ibrutinib experienced a 75% reduction in the risk of disease progression or death.
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
Ibrutinib is being developed by Cilag GmbH International (a member of the Janssen Pharmaceutical Companies) and Pharmacyclics, Inc. Janssen will commercialize the drug in Canada, and Janssen affiliates will commercialize it around the world, except in the US, where Pharmacyclics and Janssen Biotech, Inc. co-market it. ![]()

Health Canada recently approved the Bruton tyrosine kinase inhibitor ibrutinib (Imbruvica) for the treatment of chronic lymphocytic leukemia (CLL).
The drug can now be used to treat CLL patients, including those with 17p deletion, who have received at least one prior therapy. It can also be used as frontline treatment in CLL patients with 17p deletion.
Health Canada’s approval of ibrutinib is based on results of the phase 3 RESONATE trial, which were presented at this year’s ASCO and EHA meetings.
The trial included 391 previously treated patients, 127 of whom had 17p deletion. Patients were randomized to receive ibrutinib or the anti-CD20 monoclonal antibody ofatumumab until disease progression or unacceptable toxicity.
The trial was stopped early after a pre-planned interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
At the time of interim analysis, the patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm.
Ibrutinib significantly prolonged progression-free and overall survival. The median progression-free survival was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Of the 127 patients with 17p deletion, those treated with ibrutinib experienced a 75% reduction in the risk of disease progression or death.
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
Ibrutinib is being developed by Cilag GmbH International (a member of the Janssen Pharmaceutical Companies) and Pharmacyclics, Inc. Janssen will commercialize the drug in Canada, and Janssen affiliates will commercialize it around the world, except in the US, where Pharmacyclics and Janssen Biotech, Inc. co-market it. ![]()

Health Canada recently approved the Bruton tyrosine kinase inhibitor ibrutinib (Imbruvica) for the treatment of chronic lymphocytic leukemia (CLL).
The drug can now be used to treat CLL patients, including those with 17p deletion, who have received at least one prior therapy. It can also be used as frontline treatment in CLL patients with 17p deletion.
Health Canada’s approval of ibrutinib is based on results of the phase 3 RESONATE trial, which were presented at this year’s ASCO and EHA meetings.
The trial included 391 previously treated patients, 127 of whom had 17p deletion. Patients were randomized to receive ibrutinib or the anti-CD20 monoclonal antibody ofatumumab until disease progression or unacceptable toxicity.
The trial was stopped early after a pre-planned interim analysis showed that ibrutinib-treated patients experienced a 78% reduction in the risk of disease progression or death.
At the time of interim analysis, the patients’ median time on study was 9.4 months. The best overall response among evaluable patients was 78% in the ibrutinib arm and 11% in the ofatumumab arm.
Ibrutinib significantly prolonged progression-free and overall survival. The median progression-free survival was 8.1 months in the ofatumumab arm and was not reached in the ibrutinib arm (P<0.0001). The median overall survival was not reached in either arm, but the hazard ratio was 0.434 (P=0.0049).
Of the 127 patients with 17p deletion, those treated with ibrutinib experienced a 75% reduction in the risk of disease progression or death.
Adverse events occurred in 99% of patients in the ibrutinib arm and 98% of those in the ofatumumab arm. Grade 3/4 events occurred in 51% and 39%, respectively.
Atrial fibrillation, bleeding-related events, diarrhea, and arthralgia were more common in the ibrutinib arm. Infusion-related reactions, peripheral sensory neuropathy, urticaria, night sweats, and pruritus were more common in the ofatumumab arm.
Ibrutinib is being developed by Cilag GmbH International (a member of the Janssen Pharmaceutical Companies) and Pharmacyclics, Inc. Janssen will commercialize the drug in Canada, and Janssen affiliates will commercialize it around the world, except in the US, where Pharmacyclics and Janssen Biotech, Inc. co-market it. ![]()
People often dismiss cancer symptoms, survey suggests

Credit: NIH
People could be putting their lives at risk by dismissing potential warning signs of cancer as less serious symptoms, according to a study published in PLOS ONE.
In a survey of about 1700 people, more than half of respondents said they had experienced at least
one red-flag cancer “alarm” symptom—such as persistent, unexplained pain or an unexplained lump—during the previous 3 months, but
only 2% of them thought cancer was a possible cause.
The survey had been sent to people aged 50 and older who were registered with 3 London general practices. The questionnaire listed 17 symptoms, including 10 widely publicized potential cancer warning signs, such as an unexplained cough, bleeding, and a persistent change in bowel or bladder habits.
Cancer was not mentioned, but the survey asked which of the symptoms subjects had experienced, what they thought caused them, if they were concerned that symptoms were serious, and whether they had consulted their doctor.
Of the 1724 subjects who responded, 53% had experienced at least one cancer “alarm” symptom in the previous 3 months.
This included unexplained cough or hoarseness; persistent change in bowel habits; persistent, unexplained pain; persistent change in bladder habits; unexplained lump; a change in the appearance of a mole; a sore that does not heal; unexplained bleeding; unexplained weight loss; and persistent difficulty swallowing.
Persistent cough (20%) and persistent change in bowel habits (18%) were the most common symptoms. Difficulty swallowing and unexplained weight loss (both 4%) were least common.
Overall, subjects appraised the cancer warning “alarm” symptoms as more serious than “non-alarm” symptoms, such as sore throat and feeling tired. Fifty-nine percent of respondents said they contacted a doctor about their “alarm” symptoms.
However, subjects rarely attributed potential signs of cancer to the disease, putting them down to other reasons, such as age, infection, arthritis, piles, and cysts.
“Most people with potential warning symptoms don’t have cancer, but some will, and others may have other diseases that would benefit from early attention,” said study author Katriina Whitaker, PhD, of University College London in the UK.
“That’s why it’s important that these symptoms are checked out, especially if they don’t go away. But people could delay seeing a doctor if they don’t acknowledge cancer as a possible cause. It’s worrying that even the more obvious warning symptoms, such as unexplained lumps or changes to the appearance of a mole, were rarely attributed to cancer, although they are often well recognized in surveys that assess the public’s knowledge of the disease.”
“Even when people thought warning symptoms might be serious, cancer didn’t tend to spring to mind. This might be because people were frightened and reluctant to mention cancer, thought cancer wouldn’t happen to them, or believed other causes were more likely.” ![]()

Credit: NIH
People could be putting their lives at risk by dismissing potential warning signs of cancer as less serious symptoms, according to a study published in PLOS ONE.
In a survey of about 1700 people, more than half of respondents said they had experienced at least
one red-flag cancer “alarm” symptom—such as persistent, unexplained pain or an unexplained lump—during the previous 3 months, but
only 2% of them thought cancer was a possible cause.
The survey had been sent to people aged 50 and older who were registered with 3 London general practices. The questionnaire listed 17 symptoms, including 10 widely publicized potential cancer warning signs, such as an unexplained cough, bleeding, and a persistent change in bowel or bladder habits.
Cancer was not mentioned, but the survey asked which of the symptoms subjects had experienced, what they thought caused them, if they were concerned that symptoms were serious, and whether they had consulted their doctor.
Of the 1724 subjects who responded, 53% had experienced at least one cancer “alarm” symptom in the previous 3 months.
This included unexplained cough or hoarseness; persistent change in bowel habits; persistent, unexplained pain; persistent change in bladder habits; unexplained lump; a change in the appearance of a mole; a sore that does not heal; unexplained bleeding; unexplained weight loss; and persistent difficulty swallowing.
Persistent cough (20%) and persistent change in bowel habits (18%) were the most common symptoms. Difficulty swallowing and unexplained weight loss (both 4%) were least common.
Overall, subjects appraised the cancer warning “alarm” symptoms as more serious than “non-alarm” symptoms, such as sore throat and feeling tired. Fifty-nine percent of respondents said they contacted a doctor about their “alarm” symptoms.
However, subjects rarely attributed potential signs of cancer to the disease, putting them down to other reasons, such as age, infection, arthritis, piles, and cysts.
“Most people with potential warning symptoms don’t have cancer, but some will, and others may have other diseases that would benefit from early attention,” said study author Katriina Whitaker, PhD, of University College London in the UK.
“That’s why it’s important that these symptoms are checked out, especially if they don’t go away. But people could delay seeing a doctor if they don’t acknowledge cancer as a possible cause. It’s worrying that even the more obvious warning symptoms, such as unexplained lumps or changes to the appearance of a mole, were rarely attributed to cancer, although they are often well recognized in surveys that assess the public’s knowledge of the disease.”
“Even when people thought warning symptoms might be serious, cancer didn’t tend to spring to mind. This might be because people were frightened and reluctant to mention cancer, thought cancer wouldn’t happen to them, or believed other causes were more likely.” ![]()

Credit: NIH
People could be putting their lives at risk by dismissing potential warning signs of cancer as less serious symptoms, according to a study published in PLOS ONE.
In a survey of about 1700 people, more than half of respondents said they had experienced at least
one red-flag cancer “alarm” symptom—such as persistent, unexplained pain or an unexplained lump—during the previous 3 months, but
only 2% of them thought cancer was a possible cause.
The survey had been sent to people aged 50 and older who were registered with 3 London general practices. The questionnaire listed 17 symptoms, including 10 widely publicized potential cancer warning signs, such as an unexplained cough, bleeding, and a persistent change in bowel or bladder habits.
Cancer was not mentioned, but the survey asked which of the symptoms subjects had experienced, what they thought caused them, if they were concerned that symptoms were serious, and whether they had consulted their doctor.
Of the 1724 subjects who responded, 53% had experienced at least one cancer “alarm” symptom in the previous 3 months.
This included unexplained cough or hoarseness; persistent change in bowel habits; persistent, unexplained pain; persistent change in bladder habits; unexplained lump; a change in the appearance of a mole; a sore that does not heal; unexplained bleeding; unexplained weight loss; and persistent difficulty swallowing.
Persistent cough (20%) and persistent change in bowel habits (18%) were the most common symptoms. Difficulty swallowing and unexplained weight loss (both 4%) were least common.
Overall, subjects appraised the cancer warning “alarm” symptoms as more serious than “non-alarm” symptoms, such as sore throat and feeling tired. Fifty-nine percent of respondents said they contacted a doctor about their “alarm” symptoms.
However, subjects rarely attributed potential signs of cancer to the disease, putting them down to other reasons, such as age, infection, arthritis, piles, and cysts.
“Most people with potential warning symptoms don’t have cancer, but some will, and others may have other diseases that would benefit from early attention,” said study author Katriina Whitaker, PhD, of University College London in the UK.
“That’s why it’s important that these symptoms are checked out, especially if they don’t go away. But people could delay seeing a doctor if they don’t acknowledge cancer as a possible cause. It’s worrying that even the more obvious warning symptoms, such as unexplained lumps or changes to the appearance of a mole, were rarely attributed to cancer, although they are often well recognized in surveys that assess the public’s knowledge of the disease.”
“Even when people thought warning symptoms might be serious, cancer didn’t tend to spring to mind. This might be because people were frightened and reluctant to mention cancer, thought cancer wouldn’t happen to them, or believed other causes were more likely.” ![]()
Rivaroxaban matches warfarin’s total Afib costs
CHICAGO – Patients with atrial fibrillation who start treatment with a new oral anticoagulant may spend more on their medication than if they were prescribed generic warfarin, but their overall health care costs may wind up being about the same, based on an analysis of health care expense records for more than 4,500 U.S. patients.
“Despite higher anticoagulant costs, total all-cause and atrial fibrillation–related costs remain comparable” between patients prescribed warfarin and those who received the new oral anticoagulant rivaroxaban (Xarelto), said Concetta Crivera, Pharm.D., at the American Heart Association Scientific Sessions. Higher drug costs for daily treatment with rivaroxaban were offset by reduced hospital lengths of stays and hence reduced hospitalization costs, said Dr. Crivera, director of cardiovascular health economics and outcomes research at Janssen in Raritan, N.J., the company that markets rivaroxaban along with Bayer.
“I can believe that hospitalized days would be reduced because patients treated with rivaroxaban or any of the other new oral anticoagulants don’t need to remain in the hospital while you wait for their international normalized ratio to enter the therapeutic range,” which is what happens with patients treated with warfarin, commented Dr. Jeffrey Weitz, professor of medicine and director of the Juravinski Hospital and Cancer Centre of McMaster University in Hamilton, Ont.
The new findings by Dr. Crivera “are not definitive on their own, but they add to a growing body of data that indicate that all the new oral anticoagulants are cost effective,” said Dr. Weitz, who specializes in thrombosis and anticoagulants. “The worst time for a patient on warfarin is when they start treatment. You do a disservice to patients with new-onset atrial fibrillation by using warfarin, because some patients will get into the therapeutic range but others never will. That’s why the new oral anticoagulants are better, because everybody gets into therapeutic range,” he said in an interview.
Dr. Crivera and her associates conducted a retrospective study of health records for 2,253 patients with nonvalvular atrial fibrillation (AF) who started anticoagulation treatment with rivaroxaban during the period of November 2011 (when the drug received U.S. marketing approval) through December 2012. The data came from the patient records of Humana, a U.S. HMO and insurer that covers both commercially insured patients and those covered by Medicare. The researchers matched each of the patients initiating rivaroxaban treatment with a similar patient from the Humana database with nonvalvular AF who started on warfarin during the same period. The average age of patients in the study was about 74 years, and patients in the two groups were closely matched for their demographic and clinical characteristics, including comorbidities. Data on health care use were available for patients for an average of about 4 months following their start of anticoagulant treatment.
The analysis showed that during the first months on treatment, patients prescribed rivaroxaban averaged 2.11 days of hospitalization for an AF-related episode, compared with 3.02 days for those prescribed warfarin, a statistically significant difference. Hospitalizations for any cause averaged a total of 2.71 days in the rivaroxaban group and 3.87 days in the patients on warfarin, also a significant difference, Dr. Crivera reported.
This difference in days hospitalized translated into reduced hospitalization costs, a roughly $2,000 average difference per patient in actual hospitalization costs in favor of the rivaroxaban patients for all-cause hospitalizations, and an average $1,300 difference per patient for hospitalization costs directly related to AF.
Although the rivaroxaban patients spent an average of $2,700 more per patient on pharmaceuticals for all causes, and an average $2,200 more for AF-related drugs, the total average all-cause and AF-related costs for drugs, hospitalizations, outpatient visits, and emergency department visits were similar in the two subgroups: an average total of $17,590 per patient for the rivaroxaban patients and $18,676 for warfarin patients for all causes, and an average of $7,394 for the rivaroxaban patients and $7,319 for those on warfarin for AF-related care. The between-group differences for both sets of total costs were not statistically significant.
The study was sponsored by Janssen, which along with Bayer markets rivaroxaban (Xarelto). Dr. Crivera is a Janssen employee. Dr. Weitz has been an adviser or a consultant to Janssen and Bayer and to Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, Pfizer, and Portola.
On Twitter @mitchelzoler
CHICAGO – Patients with atrial fibrillation who start treatment with a new oral anticoagulant may spend more on their medication than if they were prescribed generic warfarin, but their overall health care costs may wind up being about the same, based on an analysis of health care expense records for more than 4,500 U.S. patients.
“Despite higher anticoagulant costs, total all-cause and atrial fibrillation–related costs remain comparable” between patients prescribed warfarin and those who received the new oral anticoagulant rivaroxaban (Xarelto), said Concetta Crivera, Pharm.D., at the American Heart Association Scientific Sessions. Higher drug costs for daily treatment with rivaroxaban were offset by reduced hospital lengths of stays and hence reduced hospitalization costs, said Dr. Crivera, director of cardiovascular health economics and outcomes research at Janssen in Raritan, N.J., the company that markets rivaroxaban along with Bayer.
“I can believe that hospitalized days would be reduced because patients treated with rivaroxaban or any of the other new oral anticoagulants don’t need to remain in the hospital while you wait for their international normalized ratio to enter the therapeutic range,” which is what happens with patients treated with warfarin, commented Dr. Jeffrey Weitz, professor of medicine and director of the Juravinski Hospital and Cancer Centre of McMaster University in Hamilton, Ont.
The new findings by Dr. Crivera “are not definitive on their own, but they add to a growing body of data that indicate that all the new oral anticoagulants are cost effective,” said Dr. Weitz, who specializes in thrombosis and anticoagulants. “The worst time for a patient on warfarin is when they start treatment. You do a disservice to patients with new-onset atrial fibrillation by using warfarin, because some patients will get into the therapeutic range but others never will. That’s why the new oral anticoagulants are better, because everybody gets into therapeutic range,” he said in an interview.
Dr. Crivera and her associates conducted a retrospective study of health records for 2,253 patients with nonvalvular atrial fibrillation (AF) who started anticoagulation treatment with rivaroxaban during the period of November 2011 (when the drug received U.S. marketing approval) through December 2012. The data came from the patient records of Humana, a U.S. HMO and insurer that covers both commercially insured patients and those covered by Medicare. The researchers matched each of the patients initiating rivaroxaban treatment with a similar patient from the Humana database with nonvalvular AF who started on warfarin during the same period. The average age of patients in the study was about 74 years, and patients in the two groups were closely matched for their demographic and clinical characteristics, including comorbidities. Data on health care use were available for patients for an average of about 4 months following their start of anticoagulant treatment.
The analysis showed that during the first months on treatment, patients prescribed rivaroxaban averaged 2.11 days of hospitalization for an AF-related episode, compared with 3.02 days for those prescribed warfarin, a statistically significant difference. Hospitalizations for any cause averaged a total of 2.71 days in the rivaroxaban group and 3.87 days in the patients on warfarin, also a significant difference, Dr. Crivera reported.
This difference in days hospitalized translated into reduced hospitalization costs, a roughly $2,000 average difference per patient in actual hospitalization costs in favor of the rivaroxaban patients for all-cause hospitalizations, and an average $1,300 difference per patient for hospitalization costs directly related to AF.
Although the rivaroxaban patients spent an average of $2,700 more per patient on pharmaceuticals for all causes, and an average $2,200 more for AF-related drugs, the total average all-cause and AF-related costs for drugs, hospitalizations, outpatient visits, and emergency department visits were similar in the two subgroups: an average total of $17,590 per patient for the rivaroxaban patients and $18,676 for warfarin patients for all causes, and an average of $7,394 for the rivaroxaban patients and $7,319 for those on warfarin for AF-related care. The between-group differences for both sets of total costs were not statistically significant.
The study was sponsored by Janssen, which along with Bayer markets rivaroxaban (Xarelto). Dr. Crivera is a Janssen employee. Dr. Weitz has been an adviser or a consultant to Janssen and Bayer and to Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, Pfizer, and Portola.
On Twitter @mitchelzoler
CHICAGO – Patients with atrial fibrillation who start treatment with a new oral anticoagulant may spend more on their medication than if they were prescribed generic warfarin, but their overall health care costs may wind up being about the same, based on an analysis of health care expense records for more than 4,500 U.S. patients.
“Despite higher anticoagulant costs, total all-cause and atrial fibrillation–related costs remain comparable” between patients prescribed warfarin and those who received the new oral anticoagulant rivaroxaban (Xarelto), said Concetta Crivera, Pharm.D., at the American Heart Association Scientific Sessions. Higher drug costs for daily treatment with rivaroxaban were offset by reduced hospital lengths of stays and hence reduced hospitalization costs, said Dr. Crivera, director of cardiovascular health economics and outcomes research at Janssen in Raritan, N.J., the company that markets rivaroxaban along with Bayer.
“I can believe that hospitalized days would be reduced because patients treated with rivaroxaban or any of the other new oral anticoagulants don’t need to remain in the hospital while you wait for their international normalized ratio to enter the therapeutic range,” which is what happens with patients treated with warfarin, commented Dr. Jeffrey Weitz, professor of medicine and director of the Juravinski Hospital and Cancer Centre of McMaster University in Hamilton, Ont.
The new findings by Dr. Crivera “are not definitive on their own, but they add to a growing body of data that indicate that all the new oral anticoagulants are cost effective,” said Dr. Weitz, who specializes in thrombosis and anticoagulants. “The worst time for a patient on warfarin is when they start treatment. You do a disservice to patients with new-onset atrial fibrillation by using warfarin, because some patients will get into the therapeutic range but others never will. That’s why the new oral anticoagulants are better, because everybody gets into therapeutic range,” he said in an interview.
Dr. Crivera and her associates conducted a retrospective study of health records for 2,253 patients with nonvalvular atrial fibrillation (AF) who started anticoagulation treatment with rivaroxaban during the period of November 2011 (when the drug received U.S. marketing approval) through December 2012. The data came from the patient records of Humana, a U.S. HMO and insurer that covers both commercially insured patients and those covered by Medicare. The researchers matched each of the patients initiating rivaroxaban treatment with a similar patient from the Humana database with nonvalvular AF who started on warfarin during the same period. The average age of patients in the study was about 74 years, and patients in the two groups were closely matched for their demographic and clinical characteristics, including comorbidities. Data on health care use were available for patients for an average of about 4 months following their start of anticoagulant treatment.
The analysis showed that during the first months on treatment, patients prescribed rivaroxaban averaged 2.11 days of hospitalization for an AF-related episode, compared with 3.02 days for those prescribed warfarin, a statistically significant difference. Hospitalizations for any cause averaged a total of 2.71 days in the rivaroxaban group and 3.87 days in the patients on warfarin, also a significant difference, Dr. Crivera reported.
This difference in days hospitalized translated into reduced hospitalization costs, a roughly $2,000 average difference per patient in actual hospitalization costs in favor of the rivaroxaban patients for all-cause hospitalizations, and an average $1,300 difference per patient for hospitalization costs directly related to AF.
Although the rivaroxaban patients spent an average of $2,700 more per patient on pharmaceuticals for all causes, and an average $2,200 more for AF-related drugs, the total average all-cause and AF-related costs for drugs, hospitalizations, outpatient visits, and emergency department visits were similar in the two subgroups: an average total of $17,590 per patient for the rivaroxaban patients and $18,676 for warfarin patients for all causes, and an average of $7,394 for the rivaroxaban patients and $7,319 for those on warfarin for AF-related care. The between-group differences for both sets of total costs were not statistically significant.
The study was sponsored by Janssen, which along with Bayer markets rivaroxaban (Xarelto). Dr. Crivera is a Janssen employee. Dr. Weitz has been an adviser or a consultant to Janssen and Bayer and to Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, Pfizer, and Portola.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Patients with atrial fibrillation who started on rivaroxaban treatment had total hospitalization and drug costs that were similar to those of patients begun on warfarin.
Major finding: Rivaroxaban-treated patients averaged $17,590 in total all-cause hospitalization and drug costs, compared with $18,676 for warfarin-treated patients.
Data source: A retrospective, matched-cohort study of 4,506 U.S. patients with atrial fibrillation and newly initiated anticoagulant treatment using health records maintained by Humana.
Disclosures: The study was sponsored by Janssen, which along with Bayer markets rivaroxaban (Xarelto). Dr. Crivera is a Janssen employee. Dr. Weitz has been an adviser or a consultant to Janssen and Bayer and to Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, Pfizer, and Portola.
Breast cancer drug could treat MPNs, AML

Credit: CDC
Tamoxifen, a drug used to treat breast cancer, may be effective against myeloproliferative neoplasms (MPNs) and acute myeloid leukemia (AML) as well, according to research published in Cell Stem Cell.
The study showed that estrogens regulate the survival, proliferation, and self-renewal of stem cells that give rise to MPNs and AML.
Tamoxifen, which targets estrogen receptors, prevented JAK2V617F-induced MPNs in mice and enhanced the effects of chemotherapy against MLL-AF9-induced AML.
“In this study, we demonstrate that tamoxifen has specific effects on certain cells in the bone marrow: the hematopoietic stem cells and their immediate descendants, known as multipotent progenitors,” explained study author Abel Sánchez-Aguilera, PhD, of Centro Nacional de Investigaciones Cardiovasculares (CNIC) in Madrid.
The researchers found that, unlike in breast cancer, where tamoxifen blocks the action of estrogens, in blood cells, the drug acts by imitating the function of the hormone.
Tamoxifen induced apoptosis in short-term hematopoietic stem cells (HSCs) and multipotent progenitors. But in quiescent, long-term HSCs, the drug prompted proliferation and partial loss of function, which was reversible.
Tamoxifen also prevented polycythemia vera-like MPN from developing in mice with HSCs expressing JAK2V617F. The drug prevented MPN-associated neutrophilia and thrombocytosis; alleviated the early increase in red cell counts, hemoglobin, and hematocrit; decreased bone marrow megakaryocytes; and reduced MPN-associated splenomegaly.
Tamoxifen worked by restoring normal levels of apoptosis in mutant cells. It also prompted apoptosis of human JAK2V617F+ hematopoietic stem and progenitor cells (HSPCs) in a xenograft model.
In these models, tamoxifen caused little alteration in the rest of the blood cells, which were maintained at normal levels even after prolonged treatment with the drug.
“Our results suggest that tamoxifen, at a similar dose used for the treatment of other diseases, might be useful to treat myeloproliferative neoplasms at various stages, without being toxic to normal blood cells,” said study author Simón Méndez-Ferrer, PhD, also of CNIC.
In addition, tamoxifen enhanced the effects of conventional chemotherapy on cancerous cells in mice with MLL-AF9-induced AML.
Tamoxifen- and vehicle-treated mice had similar numbers of MLL-AF9+ cells and HSPCs shortly after chemotherapy. But tamoxifen delayed the reappearance of circulating leukemic cells after chemotherapy.
Tamoxifen-treated mice had fewer leukemic cells in the bone marrow, spleen, and blood, although they ultimately died of their disease.
Nevertheless, the researchers concluded that tamoxifen could be a feasible treatment option for patients with MPNs or AML. And the fact that tamoxifen is already approved for clinical use increases the chances of these results leading to a clinical trial. ![]()

Credit: CDC
Tamoxifen, a drug used to treat breast cancer, may be effective against myeloproliferative neoplasms (MPNs) and acute myeloid leukemia (AML) as well, according to research published in Cell Stem Cell.
The study showed that estrogens regulate the survival, proliferation, and self-renewal of stem cells that give rise to MPNs and AML.
Tamoxifen, which targets estrogen receptors, prevented JAK2V617F-induced MPNs in mice and enhanced the effects of chemotherapy against MLL-AF9-induced AML.
“In this study, we demonstrate that tamoxifen has specific effects on certain cells in the bone marrow: the hematopoietic stem cells and their immediate descendants, known as multipotent progenitors,” explained study author Abel Sánchez-Aguilera, PhD, of Centro Nacional de Investigaciones Cardiovasculares (CNIC) in Madrid.
The researchers found that, unlike in breast cancer, where tamoxifen blocks the action of estrogens, in blood cells, the drug acts by imitating the function of the hormone.
Tamoxifen induced apoptosis in short-term hematopoietic stem cells (HSCs) and multipotent progenitors. But in quiescent, long-term HSCs, the drug prompted proliferation and partial loss of function, which was reversible.
Tamoxifen also prevented polycythemia vera-like MPN from developing in mice with HSCs expressing JAK2V617F. The drug prevented MPN-associated neutrophilia and thrombocytosis; alleviated the early increase in red cell counts, hemoglobin, and hematocrit; decreased bone marrow megakaryocytes; and reduced MPN-associated splenomegaly.
Tamoxifen worked by restoring normal levels of apoptosis in mutant cells. It also prompted apoptosis of human JAK2V617F+ hematopoietic stem and progenitor cells (HSPCs) in a xenograft model.
In these models, tamoxifen caused little alteration in the rest of the blood cells, which were maintained at normal levels even after prolonged treatment with the drug.
“Our results suggest that tamoxifen, at a similar dose used for the treatment of other diseases, might be useful to treat myeloproliferative neoplasms at various stages, without being toxic to normal blood cells,” said study author Simón Méndez-Ferrer, PhD, also of CNIC.
In addition, tamoxifen enhanced the effects of conventional chemotherapy on cancerous cells in mice with MLL-AF9-induced AML.
Tamoxifen- and vehicle-treated mice had similar numbers of MLL-AF9+ cells and HSPCs shortly after chemotherapy. But tamoxifen delayed the reappearance of circulating leukemic cells after chemotherapy.
Tamoxifen-treated mice had fewer leukemic cells in the bone marrow, spleen, and blood, although they ultimately died of their disease.
Nevertheless, the researchers concluded that tamoxifen could be a feasible treatment option for patients with MPNs or AML. And the fact that tamoxifen is already approved for clinical use increases the chances of these results leading to a clinical trial. ![]()

Credit: CDC
Tamoxifen, a drug used to treat breast cancer, may be effective against myeloproliferative neoplasms (MPNs) and acute myeloid leukemia (AML) as well, according to research published in Cell Stem Cell.
The study showed that estrogens regulate the survival, proliferation, and self-renewal of stem cells that give rise to MPNs and AML.
Tamoxifen, which targets estrogen receptors, prevented JAK2V617F-induced MPNs in mice and enhanced the effects of chemotherapy against MLL-AF9-induced AML.
“In this study, we demonstrate that tamoxifen has specific effects on certain cells in the bone marrow: the hematopoietic stem cells and their immediate descendants, known as multipotent progenitors,” explained study author Abel Sánchez-Aguilera, PhD, of Centro Nacional de Investigaciones Cardiovasculares (CNIC) in Madrid.
The researchers found that, unlike in breast cancer, where tamoxifen blocks the action of estrogens, in blood cells, the drug acts by imitating the function of the hormone.
Tamoxifen induced apoptosis in short-term hematopoietic stem cells (HSCs) and multipotent progenitors. But in quiescent, long-term HSCs, the drug prompted proliferation and partial loss of function, which was reversible.
Tamoxifen also prevented polycythemia vera-like MPN from developing in mice with HSCs expressing JAK2V617F. The drug prevented MPN-associated neutrophilia and thrombocytosis; alleviated the early increase in red cell counts, hemoglobin, and hematocrit; decreased bone marrow megakaryocytes; and reduced MPN-associated splenomegaly.
Tamoxifen worked by restoring normal levels of apoptosis in mutant cells. It also prompted apoptosis of human JAK2V617F+ hematopoietic stem and progenitor cells (HSPCs) in a xenograft model.
In these models, tamoxifen caused little alteration in the rest of the blood cells, which were maintained at normal levels even after prolonged treatment with the drug.
“Our results suggest that tamoxifen, at a similar dose used for the treatment of other diseases, might be useful to treat myeloproliferative neoplasms at various stages, without being toxic to normal blood cells,” said study author Simón Méndez-Ferrer, PhD, also of CNIC.
In addition, tamoxifen enhanced the effects of conventional chemotherapy on cancerous cells in mice with MLL-AF9-induced AML.
Tamoxifen- and vehicle-treated mice had similar numbers of MLL-AF9+ cells and HSPCs shortly after chemotherapy. But tamoxifen delayed the reappearance of circulating leukemic cells after chemotherapy.
Tamoxifen-treated mice had fewer leukemic cells in the bone marrow, spleen, and blood, although they ultimately died of their disease.
Nevertheless, the researchers concluded that tamoxifen could be a feasible treatment option for patients with MPNs or AML. And the fact that tamoxifen is already approved for clinical use increases the chances of these results leading to a clinical trial. ![]()
NICE reconsiders obinutuzumab for CLL

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a draft guidance that recommends obinutuzumab, marketed by Roche as Gazyvaro, for certain patients with untreated chronic lymphocytic leukemia (CLL).
In an earlier preliminary guidance, NICE said it could not recommend the drug due to uncertainties in the company’s submission.
In response, Roche submitted revised cost-effectiveness analyses and a patient access scheme.
This prompted NICE to recommend obinutuzumab in combination with chlorambucil as an option for adults with untreated CLL who have comorbidities that make them ineligible for full-dose fludarabine-based therapy.
But NICE is only recommending this as an option if bendamustine-based therapy has been deemed unsuitable and if Roche provides obinutuzumab with the discount agreed in the patient access scheme.
“We are pleased that Roche responded to our consultation and provided further analyses to allow us to propose recommending obinutuzumab as a treatment option for untreated chronic lymphocytic leukemia,” said Carole Longson, director of the Centre for Health Technology Evaluation at NICE.
“Half of the people who need treatment for their condition are not able to use the standard first-line treatment of fludarabine combination therapy. NICE recommends alternative treatment with bendamustine, but there are some patients for whom this is also unsuitable. Obinutuzumab is a clinically effective treatment which is associated with fewer adverse events and provides another option to help prevent people’s disease from progressing.”
NICE recommends obinutuzumab on the basis that Roche provides the treatment to the National Health Service (NHS) at a reduced price. The company has agreed with the Department of Health that the size of the discount is to be confidential.
The list price of obinutuzumab is £3312 per 1000 mg vial (excluding value-added tax). According to Roche, a course of treatment costs £26,496 (£9936 for cycle 1 and £3312 for cycles 2 to 6, excluding tax).
Consultees, including the company, healthcare professionals, and members of the public, have until Tuesday, January 6, 2015, to comment on the preliminary recommendations via the NICE website.
NICE has not yet issued the final guidance to the NHS. Until then, NHS bodies should make decisions locally on the funding of specific treatments. ![]()

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a draft guidance that recommends obinutuzumab, marketed by Roche as Gazyvaro, for certain patients with untreated chronic lymphocytic leukemia (CLL).
In an earlier preliminary guidance, NICE said it could not recommend the drug due to uncertainties in the company’s submission.
In response, Roche submitted revised cost-effectiveness analyses and a patient access scheme.
This prompted NICE to recommend obinutuzumab in combination with chlorambucil as an option for adults with untreated CLL who have comorbidities that make them ineligible for full-dose fludarabine-based therapy.
But NICE is only recommending this as an option if bendamustine-based therapy has been deemed unsuitable and if Roche provides obinutuzumab with the discount agreed in the patient access scheme.
“We are pleased that Roche responded to our consultation and provided further analyses to allow us to propose recommending obinutuzumab as a treatment option for untreated chronic lymphocytic leukemia,” said Carole Longson, director of the Centre for Health Technology Evaluation at NICE.
“Half of the people who need treatment for their condition are not able to use the standard first-line treatment of fludarabine combination therapy. NICE recommends alternative treatment with bendamustine, but there are some patients for whom this is also unsuitable. Obinutuzumab is a clinically effective treatment which is associated with fewer adverse events and provides another option to help prevent people’s disease from progressing.”
NICE recommends obinutuzumab on the basis that Roche provides the treatment to the National Health Service (NHS) at a reduced price. The company has agreed with the Department of Health that the size of the discount is to be confidential.
The list price of obinutuzumab is £3312 per 1000 mg vial (excluding value-added tax). According to Roche, a course of treatment costs £26,496 (£9936 for cycle 1 and £3312 for cycles 2 to 6, excluding tax).
Consultees, including the company, healthcare professionals, and members of the public, have until Tuesday, January 6, 2015, to comment on the preliminary recommendations via the NICE website.
NICE has not yet issued the final guidance to the NHS. Until then, NHS bodies should make decisions locally on the funding of specific treatments. ![]()

Credit: Linda Bartlett
The UK’s National Institute for Health and Care Excellence (NICE) has issued a draft guidance that recommends obinutuzumab, marketed by Roche as Gazyvaro, for certain patients with untreated chronic lymphocytic leukemia (CLL).
In an earlier preliminary guidance, NICE said it could not recommend the drug due to uncertainties in the company’s submission.
In response, Roche submitted revised cost-effectiveness analyses and a patient access scheme.
This prompted NICE to recommend obinutuzumab in combination with chlorambucil as an option for adults with untreated CLL who have comorbidities that make them ineligible for full-dose fludarabine-based therapy.
But NICE is only recommending this as an option if bendamustine-based therapy has been deemed unsuitable and if Roche provides obinutuzumab with the discount agreed in the patient access scheme.
“We are pleased that Roche responded to our consultation and provided further analyses to allow us to propose recommending obinutuzumab as a treatment option for untreated chronic lymphocytic leukemia,” said Carole Longson, director of the Centre for Health Technology Evaluation at NICE.
“Half of the people who need treatment for their condition are not able to use the standard first-line treatment of fludarabine combination therapy. NICE recommends alternative treatment with bendamustine, but there are some patients for whom this is also unsuitable. Obinutuzumab is a clinically effective treatment which is associated with fewer adverse events and provides another option to help prevent people’s disease from progressing.”
NICE recommends obinutuzumab on the basis that Roche provides the treatment to the National Health Service (NHS) at a reduced price. The company has agreed with the Department of Health that the size of the discount is to be confidential.
The list price of obinutuzumab is £3312 per 1000 mg vial (excluding value-added tax). According to Roche, a course of treatment costs £26,496 (£9936 for cycle 1 and £3312 for cycles 2 to 6, excluding tax).
Consultees, including the company, healthcare professionals, and members of the public, have until Tuesday, January 6, 2015, to comment on the preliminary recommendations via the NICE website.
NICE has not yet issued the final guidance to the NHS. Until then, NHS bodies should make decisions locally on the funding of specific treatments. ![]()
FDA approves first drug for polycythemia vera

Credit: AFIP
The US Food and Drug Administration (FDA) has expanded the approved use of ruxolitinib (Jakafi) to include treatment of patients with polycythemia vera (PV).
This is the first drug approved by the FDA for this condition.
Ruxolitinib can now be used to treat PV patients who have an inadequate response to hydroxyurea or cannot tolerate the drug.
The FDA said the approval of ruxolitinib for PV patients will help decrease splenomegaly and the need for phlebotomy.
“The approval of Jakafi for polycythemia vera underscores the importance of developing drugs matched to our increasing knowledge of the mechanisms of diseases,” said Richard Pazdur, MD, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
“The trial used to evaluate Jakafi confirmed clinically meaningful reductions in spleen size and the need for phlebotomies to control the disease.”
Results from that study, the phase 3 RESPONSE trial, were presented at the 2014 ASCO Annual Meeting. RESPONSE was funded by Incyte Corporation, the company developing ruxolitinib.
The trial included 222 patients who had PV for at least 24 weeks. All patients had an inadequate response to or could not tolerate hydroxyurea, had undergone a phlebotomy procedure, and exhibited an enlarged spleen.
They were randomized to receive ruxolitinib at a starting dose of 10 mg twice daily or best available therapy (BAT) as determined by the investigator on a participant-by-participant basis. The ruxolitinib dose was adjusted as needed throughout the study.
The study was designed to measure the reduced need for phlebotomy beginning at week 8 and continuing through week 32, in addition to at least a 35% reduction in spleen volume at week 32.
Twenty-one percent of ruxolitinib-treated patients met this endpoint, compared to 1% of patients who received BAT. At week 32, 77% of patients on ruxolitinib and 20% on BAT achieved hematocrit control or spleen reduction.
Ruxolitinib was generally well-tolerated, but 3.6% of patients discontinued treatment due to adverse events, compared to 1.8% of patients on BAT.
The most common events associated with ruxolitinib were anemia and thrombocytopenia. The most common non-hematologic events were headache, diarrhea, fatigue, dizziness, constipation, and shingles.
The FDA reviewed ruxolitinib’s use for PV under the agency’s priority review program because, at the time the application was submitted, the drug demonstrated the potential to be a significant improvement over available therapy. Ruxolitinib also received orphan product designation.
Ruxolitinib is currently approved in more than 60 countries for patients with myelofibrosis (MF). In 2011, the FDA approved the drug to treat patients with intermediate or high-risk MF, including primary MF, post-PV MF, and post-essential thrombocythemia MF. ![]()

Credit: AFIP
The US Food and Drug Administration (FDA) has expanded the approved use of ruxolitinib (Jakafi) to include treatment of patients with polycythemia vera (PV).
This is the first drug approved by the FDA for this condition.
Ruxolitinib can now be used to treat PV patients who have an inadequate response to hydroxyurea or cannot tolerate the drug.
The FDA said the approval of ruxolitinib for PV patients will help decrease splenomegaly and the need for phlebotomy.
“The approval of Jakafi for polycythemia vera underscores the importance of developing drugs matched to our increasing knowledge of the mechanisms of diseases,” said Richard Pazdur, MD, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
“The trial used to evaluate Jakafi confirmed clinically meaningful reductions in spleen size and the need for phlebotomies to control the disease.”
Results from that study, the phase 3 RESPONSE trial, were presented at the 2014 ASCO Annual Meeting. RESPONSE was funded by Incyte Corporation, the company developing ruxolitinib.
The trial included 222 patients who had PV for at least 24 weeks. All patients had an inadequate response to or could not tolerate hydroxyurea, had undergone a phlebotomy procedure, and exhibited an enlarged spleen.
They were randomized to receive ruxolitinib at a starting dose of 10 mg twice daily or best available therapy (BAT) as determined by the investigator on a participant-by-participant basis. The ruxolitinib dose was adjusted as needed throughout the study.
The study was designed to measure the reduced need for phlebotomy beginning at week 8 and continuing through week 32, in addition to at least a 35% reduction in spleen volume at week 32.
Twenty-one percent of ruxolitinib-treated patients met this endpoint, compared to 1% of patients who received BAT. At week 32, 77% of patients on ruxolitinib and 20% on BAT achieved hematocrit control or spleen reduction.
Ruxolitinib was generally well-tolerated, but 3.6% of patients discontinued treatment due to adverse events, compared to 1.8% of patients on BAT.
The most common events associated with ruxolitinib were anemia and thrombocytopenia. The most common non-hematologic events were headache, diarrhea, fatigue, dizziness, constipation, and shingles.
The FDA reviewed ruxolitinib’s use for PV under the agency’s priority review program because, at the time the application was submitted, the drug demonstrated the potential to be a significant improvement over available therapy. Ruxolitinib also received orphan product designation.
Ruxolitinib is currently approved in more than 60 countries for patients with myelofibrosis (MF). In 2011, the FDA approved the drug to treat patients with intermediate or high-risk MF, including primary MF, post-PV MF, and post-essential thrombocythemia MF. ![]()

Credit: AFIP
The US Food and Drug Administration (FDA) has expanded the approved use of ruxolitinib (Jakafi) to include treatment of patients with polycythemia vera (PV).
This is the first drug approved by the FDA for this condition.
Ruxolitinib can now be used to treat PV patients who have an inadequate response to hydroxyurea or cannot tolerate the drug.
The FDA said the approval of ruxolitinib for PV patients will help decrease splenomegaly and the need for phlebotomy.
“The approval of Jakafi for polycythemia vera underscores the importance of developing drugs matched to our increasing knowledge of the mechanisms of diseases,” said Richard Pazdur, MD, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research.
“The trial used to evaluate Jakafi confirmed clinically meaningful reductions in spleen size and the need for phlebotomies to control the disease.”
Results from that study, the phase 3 RESPONSE trial, were presented at the 2014 ASCO Annual Meeting. RESPONSE was funded by Incyte Corporation, the company developing ruxolitinib.
The trial included 222 patients who had PV for at least 24 weeks. All patients had an inadequate response to or could not tolerate hydroxyurea, had undergone a phlebotomy procedure, and exhibited an enlarged spleen.
They were randomized to receive ruxolitinib at a starting dose of 10 mg twice daily or best available therapy (BAT) as determined by the investigator on a participant-by-participant basis. The ruxolitinib dose was adjusted as needed throughout the study.
The study was designed to measure the reduced need for phlebotomy beginning at week 8 and continuing through week 32, in addition to at least a 35% reduction in spleen volume at week 32.
Twenty-one percent of ruxolitinib-treated patients met this endpoint, compared to 1% of patients who received BAT. At week 32, 77% of patients on ruxolitinib and 20% on BAT achieved hematocrit control or spleen reduction.
Ruxolitinib was generally well-tolerated, but 3.6% of patients discontinued treatment due to adverse events, compared to 1.8% of patients on BAT.
The most common events associated with ruxolitinib were anemia and thrombocytopenia. The most common non-hematologic events were headache, diarrhea, fatigue, dizziness, constipation, and shingles.
The FDA reviewed ruxolitinib’s use for PV under the agency’s priority review program because, at the time the application was submitted, the drug demonstrated the potential to be a significant improvement over available therapy. Ruxolitinib also received orphan product designation.
Ruxolitinib is currently approved in more than 60 countries for patients with myelofibrosis (MF). In 2011, the FDA approved the drug to treat patients with intermediate or high-risk MF, including primary MF, post-PV MF, and post-essential thrombocythemia MF. ![]()
The posterior colpotomy: An alternative approach to tissue extraction
CASE: Patient opts for myomectomy
A 41-year-old woman, G0, with symptomatic myomas wishes to preserve her reproductive organs rather than undergo hysterectomy. She chooses laparoscopic myomectomy.
Preoperative imaging with transvaginal ultrasound reveals a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma. Preoperative videohysteroscopy reveals external compression of the anterior intramural myoma without intracavitary extension. Both tubal ostia appear normal.
During a multipuncture technique with a 5-mm laparoscope and 5-mm accessory ports,1 the abdomen and pelvis are evaluated. The 4-cm pedunculated myoma is visualized posteriorly and to the left of midline. The 5-cm intramural myoma enlarges the contour of the uterine fundus.
How would you proceed?
With intracorporeal electromechanical “power” morcellation under scrutiny due to the potential dissemination of benign and malignant tissue, many surgeons are seeking alternatives that will allow them to continue offering minimally invasive surgical options.2–4
Intracorporeal power morcellation is used during minimally invasive gynecologic procedures, including total hysterectomy, supracervical hysterectomy, and myomectomy. Two current alternatives—laparoscopic-assisted minilaparotomy and tissue extraction through a posterior colpotomy—show promise in minimizing the risks of tissue dissemination.5–7 Regardless of the route selected for tissue extraction, the use of endoscopic specimen bags and surgical retractors may ease tissue removal and limit dissemination.
In this article, we describe contained transvaginal tissue extraction through a posterior colpotomy in the setting of laparoscopic myomectomy, describing an actual case. A video of our technique is available at obgmanagement.com.
Technique, tips, and tricks
Posterior colpotomy allows the removal of fibroids during laparoscopic myomectomy without the need to enlarge the abdominal incisions and without the use of intracorporeal power morcellation. Instead, tissue is extracted transvaginally. The incision is hidden in a natural orifice, the vagina.
Equipment consists of a:
- 5-mm laparoscope and 5-mm accessory ports
- LapSac specimen-retrieval bag (Cook Medical; various sizes available)
- AirSeal Access Port (SurgiQuest), 12 mm in diameter and 150 mm in length (FIGURE 1).
Figure 1: Equipment 

The AirSeal Access Port (Top) and LapSac specimen-retrieval bag (Bottom). |
Preparatory steps Place a manipulator in the uterus and elevate it anteriorly. Position the AirSeal Access Port transvaginally, with the sharp tip below the cervix in the posterior fornix. Take care not to injure the rectum.
Confirm proper placement of the Access Port and visualize the posterior cul-de-sac laparoscopically.
Insert the 12-mm Access Port for pneumoperitoneum and the introduction and removal of suture, curved needles, and the specimen-retrieval bag.
The Access Port also provides excellent smoke evacuation and optimal visualization during the myomectomy. It is a new-concept laparoscopic port without any mechanical seal. The technology assists in maintaining pneumoperitoneum at a constant pressure despite the size of the opening.
Amputating the myomas
Choose a specimen-retrieval bag just slightly larger than the largest myoma. In this case, the larger of the two myomas is approximately 5 cm. Therefore, a 5 × 8 cm LapSac is appropriate. We roll up the LapSac and place it through the Access Port using smooth forceps, situating the bag in the abdomen prior to the start of the myomectomy, with the opening toward the uterus, so that the myomas can be collected as they are removed (FIGURE 2).
We then inject dilute vasopressin (one 20-unit ampule in 60 cc normal saline) near the base of the pedunculated myoma stalk and use monopolar electrosurgery to amputate the myoma. We place the myoma in the specimen-retrieval bag (FIGURE 3).
Next, we inject dilute vasopressin into the serosa overlying the intramural myoma and use electrosurgery to incise the serosa and myometrium. We enucleate the second myoma and place it in the bag. We then close the uterine incision using a combination of interrupted Vicryl and running V-Loc sutures on a curved CT-2 needle introduced through the Access Port (FIGURE 4).
Figure 2: Introduce the bag 
Introduce the LapSac through the Access Port. Figure 3: Contain the specimen 
| Figure 4: Close the uterine incision 
In preparation for closure, insert a curved CT-2 needle and suture material through the Access Port. Figure 5: Cinch the sac 
|
Tissue extraction
We place a blunt-tipped grasper transvaginally through the 12-mm Access Port to retrieve the blue polypropylene drawstring of the specimen bag (FIGURE 5). We then deactivate the Access Port and AirSeal system.
The bag containing the myomas is too large to fit through the port and the posterior colpotomy, so it is necessary to remove the Access Port from the vagina without losing the drawstrings of the specimen bag (FIGURE 6).
We vaginally exteriorize the opening of the bag (FIGURE 7), reorient the pedunculated myoma, which is oblong in shape, using forceps, and remove it without morcellation.
Manual morcellation will be necessary for the second, larger myoma. We perform that morcellation sharply using a scalpel within the specimen retrieval bag, taking care not to puncture the bag (FIGURE 8). When the myoma pieces are small enough, we remove them, along with the bag, through the posterior colpotomy. We then close the colpotomy laparoscopically using two interrupted 0 Vicryl sutures, and we copiously irrigate the pelvis (FIGURE 9).
Figure 6: Remove the Access Port 
Prior to tissue extraction, remove the Access Port from the vagina. Figure 7: Exteriorize the bag 
| Figure 8: Contain the morcellation 
Manually morcellate the specimen within the bag and remove it transvaginally. Figure 9: Close the colpotomy 
|
Benefits of this approach
The greatest benefit of this technique is the safe removal of specimens when performing fertility-sparing surgery. The 5-mm incisions are cosmetically inconspicuous. Moreover, the risk of port-site hernia is lower with 5-mm incisions, as opposed to extended incisions to remove specimens transabdominally.
The posterior colpotomy is associated with reduced pain and does not increase the rate of dyspareunia or infection; it also helps prevent pelvic adhesions.8–11
In 1993, we reported the results of second-look laparoscopy in 22 women who had undergone laparoscopic posterior colpotomy for tissue extraction. None had obliterative adhesions in the posterior cul-de-sac.11 This advantage is especially important in fertility-sparing surgery.
We have used this approach for specimen removal after several different procedures, including laparoscopic cystectomy and appendectomy.12,13 For laparoscopic cystectomy, once the cyst is drained, we enucleate it and place the cyst capsule into a specimen bag that has been inserted transvaginally through a posterior colpotomy.12 Laparoscopic appendectomy can be performed using a 12-mm stapler introduced via the colpotomy. We simply remove the specimen in its entirety through the posterior colpotomy.13
The bottom line: Gynecologic surgeons need to continue performing minimally invasive surgery for the benefit of patients. Moving forward and innovating to develop alternatives to intracorporeal power morcellation, when possible, should be our aim rather than falling back on surgeries through large abdominal incisions.
CASE: Resolved
At her 1-week postoperative visit, the patient’s 5-mm incisions are healing well and she has minimal pain.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. King LP, Nezhat C, Nezhat F, et al. Laparoscopic access. In: Nezhat C, Nezhat F, Nezhat CH, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:41–53.
2. Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014;311(9):905–906.
3. Kho KA, Anderson TL, Nezhat CH. Intracorporeal electromechanical tissue morcellation: a critical review and recommendations for clinical practice. Obstet Gynecol. 2014;124(4):787–793.
4. Kho K, Nezhat CH. Parasitic myomas. Obstet Gynecol. 2009;114(3):611–615.
5. Nezhat C, Nezhat F, Bess O, Nezhat CH, Mashiach R. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil. 1994;39(1):39–44.
6. Seidman DS, Nezhat CH, Nezhat F, Nezhat C. The role of laparoscopic-assisted myomectomy (LAM). JSLS. 2001;5(4):299–303.
7. Kho KA, Shin JH, Nezhat C. Vaginal extraction of large uteri with the Alexis retractor. JMIG. 2009;16(5):616–617.
8. Ghezzi F, Cromi A, Uccella S, Bogani G, Serati M, Bolis P. Transumbilical versus transvaginal retrieval of surgical specimens at laparoscopy: a randomized trial. Am J Obstet Gynecol. 2012;207(2):112.e1–e6.
9. Ghezzi F, Raio L, Mueller MD, Gyr T, Buttarelli M, Franchi M. Vaginal extraction of pelvic masses following operative laparoscopy. Surg Endosc. 2002;16(12):1691–1696.
10. Guarner-Argente C, Beltrán M, Martínez-Pallí G, et al. Infection during natural orifice transluminal endoscopic surgery peritoneoscopy: a randomized comparative study in a survival porcine model. J Minim Invasive Gynecol. 2011;18(6):741–746.
11. Nezhat F, Brill AI, Nezhat CH, Nezhat C. Adhesion formation after endoscopic posterior colpotomy. J Reprod Med. 1993;38(7):534–536.
12. Nezhat CH. Laparoscopic large ovarian cystectomy and removal through a natural orifice in a 16-year-old female. Video presented at: 21st Annual Meeting of the Society of Laparoscopic Surgeons; September 5–8, 2012; Boston, Massachusetts.
13. Nezhat CH, Datta MS, DeFazio A, Nezhat F, Nezhat C. Natural orifice-assisted laparoscopic appendectomy. JSLS. 2009;13(1):14–18.
CASE: Patient opts for myomectomy
A 41-year-old woman, G0, with symptomatic myomas wishes to preserve her reproductive organs rather than undergo hysterectomy. She chooses laparoscopic myomectomy.
Preoperative imaging with transvaginal ultrasound reveals a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma. Preoperative videohysteroscopy reveals external compression of the anterior intramural myoma without intracavitary extension. Both tubal ostia appear normal.
During a multipuncture technique with a 5-mm laparoscope and 5-mm accessory ports,1 the abdomen and pelvis are evaluated. The 4-cm pedunculated myoma is visualized posteriorly and to the left of midline. The 5-cm intramural myoma enlarges the contour of the uterine fundus.
How would you proceed?
With intracorporeal electromechanical “power” morcellation under scrutiny due to the potential dissemination of benign and malignant tissue, many surgeons are seeking alternatives that will allow them to continue offering minimally invasive surgical options.2–4
Intracorporeal power morcellation is used during minimally invasive gynecologic procedures, including total hysterectomy, supracervical hysterectomy, and myomectomy. Two current alternatives—laparoscopic-assisted minilaparotomy and tissue extraction through a posterior colpotomy—show promise in minimizing the risks of tissue dissemination.5–7 Regardless of the route selected for tissue extraction, the use of endoscopic specimen bags and surgical retractors may ease tissue removal and limit dissemination.
In this article, we describe contained transvaginal tissue extraction through a posterior colpotomy in the setting of laparoscopic myomectomy, describing an actual case. A video of our technique is available at obgmanagement.com.
Technique, tips, and tricks
Posterior colpotomy allows the removal of fibroids during laparoscopic myomectomy without the need to enlarge the abdominal incisions and without the use of intracorporeal power morcellation. Instead, tissue is extracted transvaginally. The incision is hidden in a natural orifice, the vagina.
Equipment consists of a:
- 5-mm laparoscope and 5-mm accessory ports
- LapSac specimen-retrieval bag (Cook Medical; various sizes available)
- AirSeal Access Port (SurgiQuest), 12 mm in diameter and 150 mm in length (FIGURE 1).
Figure 1: Equipment 

The AirSeal Access Port (Top) and LapSac specimen-retrieval bag (Bottom). |
Preparatory steps Place a manipulator in the uterus and elevate it anteriorly. Position the AirSeal Access Port transvaginally, with the sharp tip below the cervix in the posterior fornix. Take care not to injure the rectum.
Confirm proper placement of the Access Port and visualize the posterior cul-de-sac laparoscopically.
Insert the 12-mm Access Port for pneumoperitoneum and the introduction and removal of suture, curved needles, and the specimen-retrieval bag.
The Access Port also provides excellent smoke evacuation and optimal visualization during the myomectomy. It is a new-concept laparoscopic port without any mechanical seal. The technology assists in maintaining pneumoperitoneum at a constant pressure despite the size of the opening.
Amputating the myomas
Choose a specimen-retrieval bag just slightly larger than the largest myoma. In this case, the larger of the two myomas is approximately 5 cm. Therefore, a 5 × 8 cm LapSac is appropriate. We roll up the LapSac and place it through the Access Port using smooth forceps, situating the bag in the abdomen prior to the start of the myomectomy, with the opening toward the uterus, so that the myomas can be collected as they are removed (FIGURE 2).
We then inject dilute vasopressin (one 20-unit ampule in 60 cc normal saline) near the base of the pedunculated myoma stalk and use monopolar electrosurgery to amputate the myoma. We place the myoma in the specimen-retrieval bag (FIGURE 3).
Next, we inject dilute vasopressin into the serosa overlying the intramural myoma and use electrosurgery to incise the serosa and myometrium. We enucleate the second myoma and place it in the bag. We then close the uterine incision using a combination of interrupted Vicryl and running V-Loc sutures on a curved CT-2 needle introduced through the Access Port (FIGURE 4).
Figure 2: Introduce the bag 
Introduce the LapSac through the Access Port. Figure 3: Contain the specimen 
| Figure 4: Close the uterine incision 
In preparation for closure, insert a curved CT-2 needle and suture material through the Access Port. Figure 5: Cinch the sac 
|
Tissue extraction
We place a blunt-tipped grasper transvaginally through the 12-mm Access Port to retrieve the blue polypropylene drawstring of the specimen bag (FIGURE 5). We then deactivate the Access Port and AirSeal system.
The bag containing the myomas is too large to fit through the port and the posterior colpotomy, so it is necessary to remove the Access Port from the vagina without losing the drawstrings of the specimen bag (FIGURE 6).
We vaginally exteriorize the opening of the bag (FIGURE 7), reorient the pedunculated myoma, which is oblong in shape, using forceps, and remove it without morcellation.
Manual morcellation will be necessary for the second, larger myoma. We perform that morcellation sharply using a scalpel within the specimen retrieval bag, taking care not to puncture the bag (FIGURE 8). When the myoma pieces are small enough, we remove them, along with the bag, through the posterior colpotomy. We then close the colpotomy laparoscopically using two interrupted 0 Vicryl sutures, and we copiously irrigate the pelvis (FIGURE 9).
Figure 6: Remove the Access Port 
Prior to tissue extraction, remove the Access Port from the vagina. Figure 7: Exteriorize the bag 
| Figure 8: Contain the morcellation 
Manually morcellate the specimen within the bag and remove it transvaginally. Figure 9: Close the colpotomy 
|
Benefits of this approach
The greatest benefit of this technique is the safe removal of specimens when performing fertility-sparing surgery. The 5-mm incisions are cosmetically inconspicuous. Moreover, the risk of port-site hernia is lower with 5-mm incisions, as opposed to extended incisions to remove specimens transabdominally.
The posterior colpotomy is associated with reduced pain and does not increase the rate of dyspareunia or infection; it also helps prevent pelvic adhesions.8–11
In 1993, we reported the results of second-look laparoscopy in 22 women who had undergone laparoscopic posterior colpotomy for tissue extraction. None had obliterative adhesions in the posterior cul-de-sac.11 This advantage is especially important in fertility-sparing surgery.
We have used this approach for specimen removal after several different procedures, including laparoscopic cystectomy and appendectomy.12,13 For laparoscopic cystectomy, once the cyst is drained, we enucleate it and place the cyst capsule into a specimen bag that has been inserted transvaginally through a posterior colpotomy.12 Laparoscopic appendectomy can be performed using a 12-mm stapler introduced via the colpotomy. We simply remove the specimen in its entirety through the posterior colpotomy.13
The bottom line: Gynecologic surgeons need to continue performing minimally invasive surgery for the benefit of patients. Moving forward and innovating to develop alternatives to intracorporeal power morcellation, when possible, should be our aim rather than falling back on surgeries through large abdominal incisions.
CASE: Resolved
At her 1-week postoperative visit, the patient’s 5-mm incisions are healing well and she has minimal pain.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Patient opts for myomectomy
A 41-year-old woman, G0, with symptomatic myomas wishes to preserve her reproductive organs rather than undergo hysterectomy. She chooses laparoscopic myomectomy.
Preoperative imaging with transvaginal ultrasound reveals a 4-cm posterior pedunculated myoma and a 5-cm fundal intramural myoma. Preoperative videohysteroscopy reveals external compression of the anterior intramural myoma without intracavitary extension. Both tubal ostia appear normal.
During a multipuncture technique with a 5-mm laparoscope and 5-mm accessory ports,1 the abdomen and pelvis are evaluated. The 4-cm pedunculated myoma is visualized posteriorly and to the left of midline. The 5-cm intramural myoma enlarges the contour of the uterine fundus.
How would you proceed?
With intracorporeal electromechanical “power” morcellation under scrutiny due to the potential dissemination of benign and malignant tissue, many surgeons are seeking alternatives that will allow them to continue offering minimally invasive surgical options.2–4
Intracorporeal power morcellation is used during minimally invasive gynecologic procedures, including total hysterectomy, supracervical hysterectomy, and myomectomy. Two current alternatives—laparoscopic-assisted minilaparotomy and tissue extraction through a posterior colpotomy—show promise in minimizing the risks of tissue dissemination.5–7 Regardless of the route selected for tissue extraction, the use of endoscopic specimen bags and surgical retractors may ease tissue removal and limit dissemination.
In this article, we describe contained transvaginal tissue extraction through a posterior colpotomy in the setting of laparoscopic myomectomy, describing an actual case. A video of our technique is available at obgmanagement.com.
Technique, tips, and tricks
Posterior colpotomy allows the removal of fibroids during laparoscopic myomectomy without the need to enlarge the abdominal incisions and without the use of intracorporeal power morcellation. Instead, tissue is extracted transvaginally. The incision is hidden in a natural orifice, the vagina.
Equipment consists of a:
- 5-mm laparoscope and 5-mm accessory ports
- LapSac specimen-retrieval bag (Cook Medical; various sizes available)
- AirSeal Access Port (SurgiQuest), 12 mm in diameter and 150 mm in length (FIGURE 1).
Figure 1: Equipment 

The AirSeal Access Port (Top) and LapSac specimen-retrieval bag (Bottom). |
Preparatory steps Place a manipulator in the uterus and elevate it anteriorly. Position the AirSeal Access Port transvaginally, with the sharp tip below the cervix in the posterior fornix. Take care not to injure the rectum.
Confirm proper placement of the Access Port and visualize the posterior cul-de-sac laparoscopically.
Insert the 12-mm Access Port for pneumoperitoneum and the introduction and removal of suture, curved needles, and the specimen-retrieval bag.
The Access Port also provides excellent smoke evacuation and optimal visualization during the myomectomy. It is a new-concept laparoscopic port without any mechanical seal. The technology assists in maintaining pneumoperitoneum at a constant pressure despite the size of the opening.
Amputating the myomas
Choose a specimen-retrieval bag just slightly larger than the largest myoma. In this case, the larger of the two myomas is approximately 5 cm. Therefore, a 5 × 8 cm LapSac is appropriate. We roll up the LapSac and place it through the Access Port using smooth forceps, situating the bag in the abdomen prior to the start of the myomectomy, with the opening toward the uterus, so that the myomas can be collected as they are removed (FIGURE 2).
We then inject dilute vasopressin (one 20-unit ampule in 60 cc normal saline) near the base of the pedunculated myoma stalk and use monopolar electrosurgery to amputate the myoma. We place the myoma in the specimen-retrieval bag (FIGURE 3).
Next, we inject dilute vasopressin into the serosa overlying the intramural myoma and use electrosurgery to incise the serosa and myometrium. We enucleate the second myoma and place it in the bag. We then close the uterine incision using a combination of interrupted Vicryl and running V-Loc sutures on a curved CT-2 needle introduced through the Access Port (FIGURE 4).
Figure 2: Introduce the bag 
Introduce the LapSac through the Access Port. Figure 3: Contain the specimen 
| Figure 4: Close the uterine incision 
In preparation for closure, insert a curved CT-2 needle and suture material through the Access Port. Figure 5: Cinch the sac 
|
Tissue extraction
We place a blunt-tipped grasper transvaginally through the 12-mm Access Port to retrieve the blue polypropylene drawstring of the specimen bag (FIGURE 5). We then deactivate the Access Port and AirSeal system.
The bag containing the myomas is too large to fit through the port and the posterior colpotomy, so it is necessary to remove the Access Port from the vagina without losing the drawstrings of the specimen bag (FIGURE 6).
We vaginally exteriorize the opening of the bag (FIGURE 7), reorient the pedunculated myoma, which is oblong in shape, using forceps, and remove it without morcellation.
Manual morcellation will be necessary for the second, larger myoma. We perform that morcellation sharply using a scalpel within the specimen retrieval bag, taking care not to puncture the bag (FIGURE 8). When the myoma pieces are small enough, we remove them, along with the bag, through the posterior colpotomy. We then close the colpotomy laparoscopically using two interrupted 0 Vicryl sutures, and we copiously irrigate the pelvis (FIGURE 9).
Figure 6: Remove the Access Port 
Prior to tissue extraction, remove the Access Port from the vagina. Figure 7: Exteriorize the bag 
| Figure 8: Contain the morcellation 
Manually morcellate the specimen within the bag and remove it transvaginally. Figure 9: Close the colpotomy 
|
Benefits of this approach
The greatest benefit of this technique is the safe removal of specimens when performing fertility-sparing surgery. The 5-mm incisions are cosmetically inconspicuous. Moreover, the risk of port-site hernia is lower with 5-mm incisions, as opposed to extended incisions to remove specimens transabdominally.
The posterior colpotomy is associated with reduced pain and does not increase the rate of dyspareunia or infection; it also helps prevent pelvic adhesions.8–11
In 1993, we reported the results of second-look laparoscopy in 22 women who had undergone laparoscopic posterior colpotomy for tissue extraction. None had obliterative adhesions in the posterior cul-de-sac.11 This advantage is especially important in fertility-sparing surgery.
We have used this approach for specimen removal after several different procedures, including laparoscopic cystectomy and appendectomy.12,13 For laparoscopic cystectomy, once the cyst is drained, we enucleate it and place the cyst capsule into a specimen bag that has been inserted transvaginally through a posterior colpotomy.12 Laparoscopic appendectomy can be performed using a 12-mm stapler introduced via the colpotomy. We simply remove the specimen in its entirety through the posterior colpotomy.13
The bottom line: Gynecologic surgeons need to continue performing minimally invasive surgery for the benefit of patients. Moving forward and innovating to develop alternatives to intracorporeal power morcellation, when possible, should be our aim rather than falling back on surgeries through large abdominal incisions.
CASE: Resolved
At her 1-week postoperative visit, the patient’s 5-mm incisions are healing well and she has minimal pain.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. King LP, Nezhat C, Nezhat F, et al. Laparoscopic access. In: Nezhat C, Nezhat F, Nezhat CH, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:41–53.
2. Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014;311(9):905–906.
3. Kho KA, Anderson TL, Nezhat CH. Intracorporeal electromechanical tissue morcellation: a critical review and recommendations for clinical practice. Obstet Gynecol. 2014;124(4):787–793.
4. Kho K, Nezhat CH. Parasitic myomas. Obstet Gynecol. 2009;114(3):611–615.
5. Nezhat C, Nezhat F, Bess O, Nezhat CH, Mashiach R. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil. 1994;39(1):39–44.
6. Seidman DS, Nezhat CH, Nezhat F, Nezhat C. The role of laparoscopic-assisted myomectomy (LAM). JSLS. 2001;5(4):299–303.
7. Kho KA, Shin JH, Nezhat C. Vaginal extraction of large uteri with the Alexis retractor. JMIG. 2009;16(5):616–617.
8. Ghezzi F, Cromi A, Uccella S, Bogani G, Serati M, Bolis P. Transumbilical versus transvaginal retrieval of surgical specimens at laparoscopy: a randomized trial. Am J Obstet Gynecol. 2012;207(2):112.e1–e6.
9. Ghezzi F, Raio L, Mueller MD, Gyr T, Buttarelli M, Franchi M. Vaginal extraction of pelvic masses following operative laparoscopy. Surg Endosc. 2002;16(12):1691–1696.
10. Guarner-Argente C, Beltrán M, Martínez-Pallí G, et al. Infection during natural orifice transluminal endoscopic surgery peritoneoscopy: a randomized comparative study in a survival porcine model. J Minim Invasive Gynecol. 2011;18(6):741–746.
11. Nezhat F, Brill AI, Nezhat CH, Nezhat C. Adhesion formation after endoscopic posterior colpotomy. J Reprod Med. 1993;38(7):534–536.
12. Nezhat CH. Laparoscopic large ovarian cystectomy and removal through a natural orifice in a 16-year-old female. Video presented at: 21st Annual Meeting of the Society of Laparoscopic Surgeons; September 5–8, 2012; Boston, Massachusetts.
13. Nezhat CH, Datta MS, DeFazio A, Nezhat F, Nezhat C. Natural orifice-assisted laparoscopic appendectomy. JSLS. 2009;13(1):14–18.
1. King LP, Nezhat C, Nezhat F, et al. Laparoscopic access. In: Nezhat C, Nezhat F, Nezhat CH, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:41–53.
2. Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014;311(9):905–906.
3. Kho KA, Anderson TL, Nezhat CH. Intracorporeal electromechanical tissue morcellation: a critical review and recommendations for clinical practice. Obstet Gynecol. 2014;124(4):787–793.
4. Kho K, Nezhat CH. Parasitic myomas. Obstet Gynecol. 2009;114(3):611–615.
5. Nezhat C, Nezhat F, Bess O, Nezhat CH, Mashiach R. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil. 1994;39(1):39–44.
6. Seidman DS, Nezhat CH, Nezhat F, Nezhat C. The role of laparoscopic-assisted myomectomy (LAM). JSLS. 2001;5(4):299–303.
7. Kho KA, Shin JH, Nezhat C. Vaginal extraction of large uteri with the Alexis retractor. JMIG. 2009;16(5):616–617.
8. Ghezzi F, Cromi A, Uccella S, Bogani G, Serati M, Bolis P. Transumbilical versus transvaginal retrieval of surgical specimens at laparoscopy: a randomized trial. Am J Obstet Gynecol. 2012;207(2):112.e1–e6.
9. Ghezzi F, Raio L, Mueller MD, Gyr T, Buttarelli M, Franchi M. Vaginal extraction of pelvic masses following operative laparoscopy. Surg Endosc. 2002;16(12):1691–1696.
10. Guarner-Argente C, Beltrán M, Martínez-Pallí G, et al. Infection during natural orifice transluminal endoscopic surgery peritoneoscopy: a randomized comparative study in a survival porcine model. J Minim Invasive Gynecol. 2011;18(6):741–746.
11. Nezhat F, Brill AI, Nezhat CH, Nezhat C. Adhesion formation after endoscopic posterior colpotomy. J Reprod Med. 1993;38(7):534–536.
12. Nezhat CH. Laparoscopic large ovarian cystectomy and removal through a natural orifice in a 16-year-old female. Video presented at: 21st Annual Meeting of the Society of Laparoscopic Surgeons; September 5–8, 2012; Boston, Massachusetts.
13. Nezhat CH, Datta MS, DeFazio A, Nezhat F, Nezhat C. Natural orifice-assisted laparoscopic appendectomy. JSLS. 2009;13(1):14–18.
Using the Internet in your practice. Part 4: Reputation management—how to gather kudos and combat negative online reviews
“It takes 20 years to build a reputation and 5 minutes to ruin it. If you think about that, you’ll do things differently.”
—Warren Buffet
CASE: Decline in new patients
A well-respected physician—one of the best in his field—notices that the number of new patients in his practice has fallen off drastically over the past year. Baffled, he hires a consultant, who discovers that the doctor’s online reputation has plummeted, thanks to four negative reviews and no positive ones.
What can the physician do to remedy the situation and restore his reputation?
The problem can be fixed, but it takes time—like major surgery. Rather than wait until negative reviews are posted, we recommend that you become proactive and take steps as soon as possible to secure your online reputation. That way, you won’t get caught by surprise when one or two unhappy patients try to smear your good name. In this article, we step you through a number of remedies and proactive strategies for boosting positive online reviews and combating negative ones.
The Internet: A one-stop source of information
The Internet has become everyone’s go-to source for pretty much any kind of data, including details on products, services, and people. Anyone can access all kinds of information simply by asking.
Today, people research medical conditions on the Web, often using Google. If you have done your search engine optimization, your Web site will come up in the first page of search results, making it possible for prospective patients to click through to your homepage. (For the scoop on search engine optimization, see Part 3 of this series, “Maximizing your online reach through SEO and pay-per-click,” which appeared in the September 2014 issue of OBG Management.)
If visitors like what they see at your site, they may make an appointment. But they are more likely to visit three or four other sites before making a decision. And in all likelihood, they will research each physician to find out what patients have to say about her or him. It’s no different than looking at the reviews of hotels or products you are considering.
You are an open book on the Internet. Only a few short years ago, your peers and patients knew your reputation primarily through word of mouth, which traveled at the speed of molasses. For the most part, that information was favorable. Today your exposure is much greater, and negative comments about you can be viewed by thousands of potential patients. The speed of information has increased, as well. What is posted on the Internet can become readily available to hundreds, thousands, and even millions of Web users in a nanosecond.
The Internet provides a forum for people to say whatever they want about their experiences, both positive and negative. Regrettably, the positive experiences do not find their way online nearly as often as the negative ones!
The bottom line? In today’s Internet-savvy world, you need to pay regular attention to your online reputation. You need to take steps to ensure that your name and practice look their best and to negate any complaints that may appear.
What patients share about their experience with you
Many online review sites provide an opportunity for your patients to describe their experience with you and your practice. To name a few: RateMDs.com, Vitals.com, ZocDoc.com, healthgrades.com, UcompareHealth.com, Citysearch.com, yelp.com, and, of course, Google Plus reviews.
And when patients post comments on the Internet, you likely will be rated on:
- the patient’s wait time
- how your staff treated the patient
- the diagnosis
- your attitude
- the level of trust in your decisions
- treatment and outcome.
The online surfer searching for a reputable physician is likely to believe whatever he or she finds on the leading review sites.
The good news: Most physicians have a very favorable rating, averaging 9.3 out of 10 on a scale of 1 to 10. In fact, 70% of doctors have perfect scores!1
The bad news: Someone who is unhappy with her treatment or outcome will go out of her way to find every online review site possible and proclaim your faults to the cyber-world, using the Internet as a forum, whether her facts are straight or not. Patients who are pleased and satisfied rarely bother to place a positive review.
How you can control your online reputation
It is incumbent upon you to keep an eye on your online reputation at all times. Here are some tips for taking charge:
- If someone posts a negative review, respond to them directly in the review site. Doing so does not violate privacy laws as long as you do not mention the patient’s name or give other identifying details. Explain your side of the story without confirming or denying that the reviewer is or was a patient. Do not mention the specifics of any patient’s condition.
- If you feel that a negative review is completely unjustified, file a dispute with the review site. Many review sites will remove the unfavorable content if you can convince them that the patient is merely ranting.
- To protect your reputation over the long term, use your name or practice name to set up an alert with Google Alerts by visiting the site Google.com/alerts.
- Do a Google search of your name and the name of your practice at least once a month and check out all the review sites that come up. Read the comments!
Develop a proactive system
You have a lot of control when it comes to protecting your online reputation, provided you are willing to take the time to set up a system to regularly request feedback or testimonials from your patients.
Regrettably, this is where most medical practices fall short, by failing to establish a system to solicit positive reviews.
The process need not be complicated. Such a system can be set in motion by scheduling a quick meeting with your staff to announce your plans to solicit testimonials from patients. Often there will be a flurry of activity for a couple of weeks before the task is forgotten. To keep your system from falling through the cracks, make a checklist and decide who on your staff is responsible for each step in the process. Go over the results in your staff meetings on a regular basis—ie, at least monthly.
You want to solicit positive reviews for use in two places:
- your Web site
- the review sites we mentioned earlier.
Posting testimonials on your Web site
Your site is the place prospective patients visit when they are looking for information about you and your services. Here are a few tips on gathering and posting testimonials:
- The best time to solicit feedback from the patient is after the follow-up appointment, when her needs have been met and she has had at least two experiences with your practice. If she is happy with her outcome, she is likely to be receptive to the idea of providing a testimonial while the details are fresh in her mind.
- Post testimonials on your homepage and every other page at your site. They should be visible when each page loads without the need to scroll down. A testimonial is worthless if it can’t be easily seen.
- Post testimonials in italics, with quotation marks around the comments to distinguish them from other elements on the page.
- Give each testimonial a headline in bold italics. Use key words likely to resonate with the reader. For example, if the patient reports: “I had a surgical procedure and it was a game changer. You turned my life around! Thank you!” the headline might be: “You turned my life around.”
- Create a Web page just for testimonials and order the comments and headlines so that they will appeal to a diversity of prospective patients. The visitor may not read every testimonial, but she will at least read and scroll through the headlines.
Gathering feedback: Your options
- One option for automating the gathering of feedback is to include a patient feedback survey on your Web site. It’s a convenient way to ask for comments. When the patient is in the office, you or your staff can simply ask her to visit the survey page on your site and answer the questions. The problem with this approach is that many patients will agree to complete the survey but few will actually follow through.
- A far more effective way to get patients to complete a survey while they are still in your office is to have the receptionist hand the patient an iPad after her appointment and ask her to take a couple of minutes to complete the survey. You can then transcribe her comments and post them on your site.
- Asking patients to post positive comments on review sites such as healthgrades.com is another option—but, again, patients are unlikely to follow through unless you make it as easy and fast as possible. The best way to do this is to provide your patient with a blueprint for how to proceed. We offer a “patient feedback” form that contains four or five questions (FIGURE). The answers to these questions will provide a great testimonial for the doctor and the practice. Providing your patients with the right questions to elicit an emotional response will help them describe their experiences more fully. If you let the patient create a testimonial on her own, you’ll probably just receive comments such as, “I’m very happy with my results” or “She is a great doctor.”
- Also provide patients with a step-by-step process for entering their feedback on the desired review sites. This can be a daunting task for your patient, so your instructions should be clear and simple. Better yet, have someone on your staff sit with the patient at a computer or iPad to help her through the process.
- Another way to control your online reputation is to capture positive comments at the point of service. In our practice, we have a testimonial poster in every exam room as well as the reception area. It contains a quick response (QR) code that can be scanned to allow the patient to submit a testimonial about her experience with the practice. With this system, we are able to collect three to five positive reviews every day.
FIGURE: Patient follow-up satisfaction survey
| It is our intention to provide our patients with the absolute best medical care available to produce optimal results. Your feedback about your procedure and patient care is an important measure of our performance. Please take the time to let us know how you feel about your results:
Your name: _______________________________ Date: ________ Thank you for telling us about the results of your procedure. How you feel about your experience helps us better understand the physical and emotional needs of our patients. We would like to share your experience with others who might be struggling with the same issues. By signing this form, you agree to let us share this information on our Web site and informational material to help other patients understand the benefits of having these types of procedures performed. |
CASE: Resolved
The physician institutes a process in his practice to gather testimonials and positive feedback, and his staff takes time to help willing patients post their reviews online. He also disputes the negative comments that have already been posted online, offering an objective response to the complaints and asking the Web sites to take down the reviews that are merely ranting. In addition, he posts selected testimonials on the homepage of his Web site and adds a page that is just for testimonials.
Within a few weeks, the number of new patients scheduling appointments with him begins to increase until he once again enjoys a bustling practice.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Schwartz SK. Online patient feedback: what to do. Physicianspractice.com. http://www.physicianspractice.com/health-it/online-patient-feedback-what-do. Published December 27, 2012. Accessed November 15, 2014.
“It takes 20 years to build a reputation and 5 minutes to ruin it. If you think about that, you’ll do things differently.”
—Warren Buffet
CASE: Decline in new patients
A well-respected physician—one of the best in his field—notices that the number of new patients in his practice has fallen off drastically over the past year. Baffled, he hires a consultant, who discovers that the doctor’s online reputation has plummeted, thanks to four negative reviews and no positive ones.
What can the physician do to remedy the situation and restore his reputation?
The problem can be fixed, but it takes time—like major surgery. Rather than wait until negative reviews are posted, we recommend that you become proactive and take steps as soon as possible to secure your online reputation. That way, you won’t get caught by surprise when one or two unhappy patients try to smear your good name. In this article, we step you through a number of remedies and proactive strategies for boosting positive online reviews and combating negative ones.
The Internet: A one-stop source of information
The Internet has become everyone’s go-to source for pretty much any kind of data, including details on products, services, and people. Anyone can access all kinds of information simply by asking.
Today, people research medical conditions on the Web, often using Google. If you have done your search engine optimization, your Web site will come up in the first page of search results, making it possible for prospective patients to click through to your homepage. (For the scoop on search engine optimization, see Part 3 of this series, “Maximizing your online reach through SEO and pay-per-click,” which appeared in the September 2014 issue of OBG Management.)
If visitors like what they see at your site, they may make an appointment. But they are more likely to visit three or four other sites before making a decision. And in all likelihood, they will research each physician to find out what patients have to say about her or him. It’s no different than looking at the reviews of hotels or products you are considering.
You are an open book on the Internet. Only a few short years ago, your peers and patients knew your reputation primarily through word of mouth, which traveled at the speed of molasses. For the most part, that information was favorable. Today your exposure is much greater, and negative comments about you can be viewed by thousands of potential patients. The speed of information has increased, as well. What is posted on the Internet can become readily available to hundreds, thousands, and even millions of Web users in a nanosecond.
The Internet provides a forum for people to say whatever they want about their experiences, both positive and negative. Regrettably, the positive experiences do not find their way online nearly as often as the negative ones!
The bottom line? In today’s Internet-savvy world, you need to pay regular attention to your online reputation. You need to take steps to ensure that your name and practice look their best and to negate any complaints that may appear.
What patients share about their experience with you
Many online review sites provide an opportunity for your patients to describe their experience with you and your practice. To name a few: RateMDs.com, Vitals.com, ZocDoc.com, healthgrades.com, UcompareHealth.com, Citysearch.com, yelp.com, and, of course, Google Plus reviews.
And when patients post comments on the Internet, you likely will be rated on:
- the patient’s wait time
- how your staff treated the patient
- the diagnosis
- your attitude
- the level of trust in your decisions
- treatment and outcome.
The online surfer searching for a reputable physician is likely to believe whatever he or she finds on the leading review sites.
The good news: Most physicians have a very favorable rating, averaging 9.3 out of 10 on a scale of 1 to 10. In fact, 70% of doctors have perfect scores!1
The bad news: Someone who is unhappy with her treatment or outcome will go out of her way to find every online review site possible and proclaim your faults to the cyber-world, using the Internet as a forum, whether her facts are straight or not. Patients who are pleased and satisfied rarely bother to place a positive review.
How you can control your online reputation
It is incumbent upon you to keep an eye on your online reputation at all times. Here are some tips for taking charge:
- If someone posts a negative review, respond to them directly in the review site. Doing so does not violate privacy laws as long as you do not mention the patient’s name or give other identifying details. Explain your side of the story without confirming or denying that the reviewer is or was a patient. Do not mention the specifics of any patient’s condition.
- If you feel that a negative review is completely unjustified, file a dispute with the review site. Many review sites will remove the unfavorable content if you can convince them that the patient is merely ranting.
- To protect your reputation over the long term, use your name or practice name to set up an alert with Google Alerts by visiting the site Google.com/alerts.
- Do a Google search of your name and the name of your practice at least once a month and check out all the review sites that come up. Read the comments!
Develop a proactive system
You have a lot of control when it comes to protecting your online reputation, provided you are willing to take the time to set up a system to regularly request feedback or testimonials from your patients.
Regrettably, this is where most medical practices fall short, by failing to establish a system to solicit positive reviews.
The process need not be complicated. Such a system can be set in motion by scheduling a quick meeting with your staff to announce your plans to solicit testimonials from patients. Often there will be a flurry of activity for a couple of weeks before the task is forgotten. To keep your system from falling through the cracks, make a checklist and decide who on your staff is responsible for each step in the process. Go over the results in your staff meetings on a regular basis—ie, at least monthly.
You want to solicit positive reviews for use in two places:
- your Web site
- the review sites we mentioned earlier.
Posting testimonials on your Web site
Your site is the place prospective patients visit when they are looking for information about you and your services. Here are a few tips on gathering and posting testimonials:
- The best time to solicit feedback from the patient is after the follow-up appointment, when her needs have been met and she has had at least two experiences with your practice. If she is happy with her outcome, she is likely to be receptive to the idea of providing a testimonial while the details are fresh in her mind.
- Post testimonials on your homepage and every other page at your site. They should be visible when each page loads without the need to scroll down. A testimonial is worthless if it can’t be easily seen.
- Post testimonials in italics, with quotation marks around the comments to distinguish them from other elements on the page.
- Give each testimonial a headline in bold italics. Use key words likely to resonate with the reader. For example, if the patient reports: “I had a surgical procedure and it was a game changer. You turned my life around! Thank you!” the headline might be: “You turned my life around.”
- Create a Web page just for testimonials and order the comments and headlines so that they will appeal to a diversity of prospective patients. The visitor may not read every testimonial, but she will at least read and scroll through the headlines.
Gathering feedback: Your options
- One option for automating the gathering of feedback is to include a patient feedback survey on your Web site. It’s a convenient way to ask for comments. When the patient is in the office, you or your staff can simply ask her to visit the survey page on your site and answer the questions. The problem with this approach is that many patients will agree to complete the survey but few will actually follow through.
- A far more effective way to get patients to complete a survey while they are still in your office is to have the receptionist hand the patient an iPad after her appointment and ask her to take a couple of minutes to complete the survey. You can then transcribe her comments and post them on your site.
- Asking patients to post positive comments on review sites such as healthgrades.com is another option—but, again, patients are unlikely to follow through unless you make it as easy and fast as possible. The best way to do this is to provide your patient with a blueprint for how to proceed. We offer a “patient feedback” form that contains four or five questions (FIGURE). The answers to these questions will provide a great testimonial for the doctor and the practice. Providing your patients with the right questions to elicit an emotional response will help them describe their experiences more fully. If you let the patient create a testimonial on her own, you’ll probably just receive comments such as, “I’m very happy with my results” or “She is a great doctor.”
- Also provide patients with a step-by-step process for entering their feedback on the desired review sites. This can be a daunting task for your patient, so your instructions should be clear and simple. Better yet, have someone on your staff sit with the patient at a computer or iPad to help her through the process.
- Another way to control your online reputation is to capture positive comments at the point of service. In our practice, we have a testimonial poster in every exam room as well as the reception area. It contains a quick response (QR) code that can be scanned to allow the patient to submit a testimonial about her experience with the practice. With this system, we are able to collect three to five positive reviews every day.
FIGURE: Patient follow-up satisfaction survey
| It is our intention to provide our patients with the absolute best medical care available to produce optimal results. Your feedback about your procedure and patient care is an important measure of our performance. Please take the time to let us know how you feel about your results:
Your name: _______________________________ Date: ________ Thank you for telling us about the results of your procedure. How you feel about your experience helps us better understand the physical and emotional needs of our patients. We would like to share your experience with others who might be struggling with the same issues. By signing this form, you agree to let us share this information on our Web site and informational material to help other patients understand the benefits of having these types of procedures performed. |
CASE: Resolved
The physician institutes a process in his practice to gather testimonials and positive feedback, and his staff takes time to help willing patients post their reviews online. He also disputes the negative comments that have already been posted online, offering an objective response to the complaints and asking the Web sites to take down the reviews that are merely ranting. In addition, he posts selected testimonials on the homepage of his Web site and adds a page that is just for testimonials.
Within a few weeks, the number of new patients scheduling appointments with him begins to increase until he once again enjoys a bustling practice.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“It takes 20 years to build a reputation and 5 minutes to ruin it. If you think about that, you’ll do things differently.”
—Warren Buffet
CASE: Decline in new patients
A well-respected physician—one of the best in his field—notices that the number of new patients in his practice has fallen off drastically over the past year. Baffled, he hires a consultant, who discovers that the doctor’s online reputation has plummeted, thanks to four negative reviews and no positive ones.
What can the physician do to remedy the situation and restore his reputation?
The problem can be fixed, but it takes time—like major surgery. Rather than wait until negative reviews are posted, we recommend that you become proactive and take steps as soon as possible to secure your online reputation. That way, you won’t get caught by surprise when one or two unhappy patients try to smear your good name. In this article, we step you through a number of remedies and proactive strategies for boosting positive online reviews and combating negative ones.
The Internet: A one-stop source of information
The Internet has become everyone’s go-to source for pretty much any kind of data, including details on products, services, and people. Anyone can access all kinds of information simply by asking.
Today, people research medical conditions on the Web, often using Google. If you have done your search engine optimization, your Web site will come up in the first page of search results, making it possible for prospective patients to click through to your homepage. (For the scoop on search engine optimization, see Part 3 of this series, “Maximizing your online reach through SEO and pay-per-click,” which appeared in the September 2014 issue of OBG Management.)
If visitors like what they see at your site, they may make an appointment. But they are more likely to visit three or four other sites before making a decision. And in all likelihood, they will research each physician to find out what patients have to say about her or him. It’s no different than looking at the reviews of hotels or products you are considering.
You are an open book on the Internet. Only a few short years ago, your peers and patients knew your reputation primarily through word of mouth, which traveled at the speed of molasses. For the most part, that information was favorable. Today your exposure is much greater, and negative comments about you can be viewed by thousands of potential patients. The speed of information has increased, as well. What is posted on the Internet can become readily available to hundreds, thousands, and even millions of Web users in a nanosecond.
The Internet provides a forum for people to say whatever they want about their experiences, both positive and negative. Regrettably, the positive experiences do not find their way online nearly as often as the negative ones!
The bottom line? In today’s Internet-savvy world, you need to pay regular attention to your online reputation. You need to take steps to ensure that your name and practice look their best and to negate any complaints that may appear.
What patients share about their experience with you
Many online review sites provide an opportunity for your patients to describe their experience with you and your practice. To name a few: RateMDs.com, Vitals.com, ZocDoc.com, healthgrades.com, UcompareHealth.com, Citysearch.com, yelp.com, and, of course, Google Plus reviews.
And when patients post comments on the Internet, you likely will be rated on:
- the patient’s wait time
- how your staff treated the patient
- the diagnosis
- your attitude
- the level of trust in your decisions
- treatment and outcome.
The online surfer searching for a reputable physician is likely to believe whatever he or she finds on the leading review sites.
The good news: Most physicians have a very favorable rating, averaging 9.3 out of 10 on a scale of 1 to 10. In fact, 70% of doctors have perfect scores!1
The bad news: Someone who is unhappy with her treatment or outcome will go out of her way to find every online review site possible and proclaim your faults to the cyber-world, using the Internet as a forum, whether her facts are straight or not. Patients who are pleased and satisfied rarely bother to place a positive review.
How you can control your online reputation
It is incumbent upon you to keep an eye on your online reputation at all times. Here are some tips for taking charge:
- If someone posts a negative review, respond to them directly in the review site. Doing so does not violate privacy laws as long as you do not mention the patient’s name or give other identifying details. Explain your side of the story without confirming or denying that the reviewer is or was a patient. Do not mention the specifics of any patient’s condition.
- If you feel that a negative review is completely unjustified, file a dispute with the review site. Many review sites will remove the unfavorable content if you can convince them that the patient is merely ranting.
- To protect your reputation over the long term, use your name or practice name to set up an alert with Google Alerts by visiting the site Google.com/alerts.
- Do a Google search of your name and the name of your practice at least once a month and check out all the review sites that come up. Read the comments!
Develop a proactive system
You have a lot of control when it comes to protecting your online reputation, provided you are willing to take the time to set up a system to regularly request feedback or testimonials from your patients.
Regrettably, this is where most medical practices fall short, by failing to establish a system to solicit positive reviews.
The process need not be complicated. Such a system can be set in motion by scheduling a quick meeting with your staff to announce your plans to solicit testimonials from patients. Often there will be a flurry of activity for a couple of weeks before the task is forgotten. To keep your system from falling through the cracks, make a checklist and decide who on your staff is responsible for each step in the process. Go over the results in your staff meetings on a regular basis—ie, at least monthly.
You want to solicit positive reviews for use in two places:
- your Web site
- the review sites we mentioned earlier.
Posting testimonials on your Web site
Your site is the place prospective patients visit when they are looking for information about you and your services. Here are a few tips on gathering and posting testimonials:
- The best time to solicit feedback from the patient is after the follow-up appointment, when her needs have been met and she has had at least two experiences with your practice. If she is happy with her outcome, she is likely to be receptive to the idea of providing a testimonial while the details are fresh in her mind.
- Post testimonials on your homepage and every other page at your site. They should be visible when each page loads without the need to scroll down. A testimonial is worthless if it can’t be easily seen.
- Post testimonials in italics, with quotation marks around the comments to distinguish them from other elements on the page.
- Give each testimonial a headline in bold italics. Use key words likely to resonate with the reader. For example, if the patient reports: “I had a surgical procedure and it was a game changer. You turned my life around! Thank you!” the headline might be: “You turned my life around.”
- Create a Web page just for testimonials and order the comments and headlines so that they will appeal to a diversity of prospective patients. The visitor may not read every testimonial, but she will at least read and scroll through the headlines.
Gathering feedback: Your options
- One option for automating the gathering of feedback is to include a patient feedback survey on your Web site. It’s a convenient way to ask for comments. When the patient is in the office, you or your staff can simply ask her to visit the survey page on your site and answer the questions. The problem with this approach is that many patients will agree to complete the survey but few will actually follow through.
- A far more effective way to get patients to complete a survey while they are still in your office is to have the receptionist hand the patient an iPad after her appointment and ask her to take a couple of minutes to complete the survey. You can then transcribe her comments and post them on your site.
- Asking patients to post positive comments on review sites such as healthgrades.com is another option—but, again, patients are unlikely to follow through unless you make it as easy and fast as possible. The best way to do this is to provide your patient with a blueprint for how to proceed. We offer a “patient feedback” form that contains four or five questions (FIGURE). The answers to these questions will provide a great testimonial for the doctor and the practice. Providing your patients with the right questions to elicit an emotional response will help them describe their experiences more fully. If you let the patient create a testimonial on her own, you’ll probably just receive comments such as, “I’m very happy with my results” or “She is a great doctor.”
- Also provide patients with a step-by-step process for entering their feedback on the desired review sites. This can be a daunting task for your patient, so your instructions should be clear and simple. Better yet, have someone on your staff sit with the patient at a computer or iPad to help her through the process.
- Another way to control your online reputation is to capture positive comments at the point of service. In our practice, we have a testimonial poster in every exam room as well as the reception area. It contains a quick response (QR) code that can be scanned to allow the patient to submit a testimonial about her experience with the practice. With this system, we are able to collect three to five positive reviews every day.
FIGURE: Patient follow-up satisfaction survey
| It is our intention to provide our patients with the absolute best medical care available to produce optimal results. Your feedback about your procedure and patient care is an important measure of our performance. Please take the time to let us know how you feel about your results:
Your name: _______________________________ Date: ________ Thank you for telling us about the results of your procedure. How you feel about your experience helps us better understand the physical and emotional needs of our patients. We would like to share your experience with others who might be struggling with the same issues. By signing this form, you agree to let us share this information on our Web site and informational material to help other patients understand the benefits of having these types of procedures performed. |
CASE: Resolved
The physician institutes a process in his practice to gather testimonials and positive feedback, and his staff takes time to help willing patients post their reviews online. He also disputes the negative comments that have already been posted online, offering an objective response to the complaints and asking the Web sites to take down the reviews that are merely ranting. In addition, he posts selected testimonials on the homepage of his Web site and adds a page that is just for testimonials.
Within a few weeks, the number of new patients scheduling appointments with him begins to increase until he once again enjoys a bustling practice.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Schwartz SK. Online patient feedback: what to do. Physicianspractice.com. http://www.physicianspractice.com/health-it/online-patient-feedback-what-do. Published December 27, 2012. Accessed November 15, 2014.
Reference
- Schwartz SK. Online patient feedback: what to do. Physicianspractice.com. http://www.physicianspractice.com/health-it/online-patient-feedback-what-do. Published December 27, 2012. Accessed November 15, 2014.