User login
Joining forces
Tough economic times and the unpredictable consequences of health care reform are making a growing number of solo practitioners and small private groups very nervous. I’ve received many inquiries about protective options, such as joining a multispecialty group, or merging two or more small practices into larger entities.
If becoming an employee of a large corporation does not appeal to you, a merger can offer significant advantages in stabilization of income and expenses; but careful planning, and a written agreement, are essential.
If you are considering this option, here are some things to think about:
What is the compensation formula? Will everyone be paid only for what they do individually, or will revenue be shared equally? I favor a combination; productivity is rewarded, but your income doesn’t drop to zero when you take time off.
Who will be in charge, and what percentage vote will be needed to approve important decisions? Typically, the majority rules, but you may wish to create a list of pivotal moves that will require unanimous approval, such as purchasing expensive equipment, borrowing money, or adding new partners.
Will you keep your retirement plans separate, or combine them? If the latter, you will have to agree on the terms of the new plan, which can be the same or different from any of the existing plans. You’ll probably need some legal guidance to ensure that assets from existing plans can be transferred into a new plan without tax issues.
Since most private practices are incorporated, there are two basic options for combining them: Corporation A can simply absorb corporation B; the latter ceases to exist, and corporation A, the so-called “surviving entity,” assumes all assets and liabilities of both old corporations. Corporation B shareholders exchange shares of its stock for shares of corporation A, with adjustments for any inequalities in stock value.
The second option is to start a completely new corporation. Both separate entities dissolve and distribute their equipment and charts to their shareholders, who then transfer the assets to the new corporation.
Option 2 is popular, but I am not a fan. It is billed as an opportunity to start fresh, shielding everyone from exposure to malpractice suits and other liabilities. However, the reality is that anyone looking to sue either old corporation will simply sue the new entity as the so-called “successor” corporation, on the grounds that it has assumed responsibility for its predecessors’ liabilities. You also will need new provider numbers, which may impede cash flow for months. Plus, the IRS treats corporate liquidations, even for merger purposes, as sales of assets, and taxes them.
In general, most experts that I’ve talked with favor outright merger of the corporations. This option is tax neutral, and while it may theoretically be less satisfactory liability-wise, you can minimize risk by examining financial and legal records, and by identifying any glaring flaws in charting or coding. Your lawyers can add “hold harmless” clauses to the merger agreement, indemnifying each party against the others’ liabilities. This area in particular is where you need experienced, competent legal advice.
Another common sticking point is known as “equalization.” Ideally, each party brings an equal amount of assets to the table, but in the real world that is rarely the case. One party may contribute more equipment, for example, and the others are often asked to make up the difference (“equalize”) with something else, usually cash.
An alternative is to agree that any inequalities will be compensated at the other end, in the form of buyout value; that is, physicians contributing more assets will receive larger buyouts when they leave or retire than those contributing less.
Non-compete provisions are always a difficult issue, mostly because they are so hard (and expensive) to enforce. An increasingly popular alternative is, once again, to deal with it at the other end, with a buyout penalty. An unhappy partner can leave, and compete, but at the cost of a substantially reduced buyout. This permits competition, but discourages it; and it compensates the remaining partners.
These are only some of the pivotal business and legal issues that must be settled in advance. A little planning and negotiation can prevent a lot of grief, regret, and legal expenses in the future. I’ll mention some other, more complicated merger options in a future column.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Skin & Allergy News. Additional columns are available online at edermatologynews.com.
Tough economic times and the unpredictable consequences of health care reform are making a growing number of solo practitioners and small private groups very nervous. I’ve received many inquiries about protective options, such as joining a multispecialty group, or merging two or more small practices into larger entities.
If becoming an employee of a large corporation does not appeal to you, a merger can offer significant advantages in stabilization of income and expenses; but careful planning, and a written agreement, are essential.
If you are considering this option, here are some things to think about:
What is the compensation formula? Will everyone be paid only for what they do individually, or will revenue be shared equally? I favor a combination; productivity is rewarded, but your income doesn’t drop to zero when you take time off.
Who will be in charge, and what percentage vote will be needed to approve important decisions? Typically, the majority rules, but you may wish to create a list of pivotal moves that will require unanimous approval, such as purchasing expensive equipment, borrowing money, or adding new partners.
Will you keep your retirement plans separate, or combine them? If the latter, you will have to agree on the terms of the new plan, which can be the same or different from any of the existing plans. You’ll probably need some legal guidance to ensure that assets from existing plans can be transferred into a new plan without tax issues.
Since most private practices are incorporated, there are two basic options for combining them: Corporation A can simply absorb corporation B; the latter ceases to exist, and corporation A, the so-called “surviving entity,” assumes all assets and liabilities of both old corporations. Corporation B shareholders exchange shares of its stock for shares of corporation A, with adjustments for any inequalities in stock value.
The second option is to start a completely new corporation. Both separate entities dissolve and distribute their equipment and charts to their shareholders, who then transfer the assets to the new corporation.
Option 2 is popular, but I am not a fan. It is billed as an opportunity to start fresh, shielding everyone from exposure to malpractice suits and other liabilities. However, the reality is that anyone looking to sue either old corporation will simply sue the new entity as the so-called “successor” corporation, on the grounds that it has assumed responsibility for its predecessors’ liabilities. You also will need new provider numbers, which may impede cash flow for months. Plus, the IRS treats corporate liquidations, even for merger purposes, as sales of assets, and taxes them.
In general, most experts that I’ve talked with favor outright merger of the corporations. This option is tax neutral, and while it may theoretically be less satisfactory liability-wise, you can minimize risk by examining financial and legal records, and by identifying any glaring flaws in charting or coding. Your lawyers can add “hold harmless” clauses to the merger agreement, indemnifying each party against the others’ liabilities. This area in particular is where you need experienced, competent legal advice.
Another common sticking point is known as “equalization.” Ideally, each party brings an equal amount of assets to the table, but in the real world that is rarely the case. One party may contribute more equipment, for example, and the others are often asked to make up the difference (“equalize”) with something else, usually cash.
An alternative is to agree that any inequalities will be compensated at the other end, in the form of buyout value; that is, physicians contributing more assets will receive larger buyouts when they leave or retire than those contributing less.
Non-compete provisions are always a difficult issue, mostly because they are so hard (and expensive) to enforce. An increasingly popular alternative is, once again, to deal with it at the other end, with a buyout penalty. An unhappy partner can leave, and compete, but at the cost of a substantially reduced buyout. This permits competition, but discourages it; and it compensates the remaining partners.
These are only some of the pivotal business and legal issues that must be settled in advance. A little planning and negotiation can prevent a lot of grief, regret, and legal expenses in the future. I’ll mention some other, more complicated merger options in a future column.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Skin & Allergy News. Additional columns are available online at edermatologynews.com.
Tough economic times and the unpredictable consequences of health care reform are making a growing number of solo practitioners and small private groups very nervous. I’ve received many inquiries about protective options, such as joining a multispecialty group, or merging two or more small practices into larger entities.
If becoming an employee of a large corporation does not appeal to you, a merger can offer significant advantages in stabilization of income and expenses; but careful planning, and a written agreement, are essential.
If you are considering this option, here are some things to think about:
What is the compensation formula? Will everyone be paid only for what they do individually, or will revenue be shared equally? I favor a combination; productivity is rewarded, but your income doesn’t drop to zero when you take time off.
Who will be in charge, and what percentage vote will be needed to approve important decisions? Typically, the majority rules, but you may wish to create a list of pivotal moves that will require unanimous approval, such as purchasing expensive equipment, borrowing money, or adding new partners.
Will you keep your retirement plans separate, or combine them? If the latter, you will have to agree on the terms of the new plan, which can be the same or different from any of the existing plans. You’ll probably need some legal guidance to ensure that assets from existing plans can be transferred into a new plan without tax issues.
Since most private practices are incorporated, there are two basic options for combining them: Corporation A can simply absorb corporation B; the latter ceases to exist, and corporation A, the so-called “surviving entity,” assumes all assets and liabilities of both old corporations. Corporation B shareholders exchange shares of its stock for shares of corporation A, with adjustments for any inequalities in stock value.
The second option is to start a completely new corporation. Both separate entities dissolve and distribute their equipment and charts to their shareholders, who then transfer the assets to the new corporation.
Option 2 is popular, but I am not a fan. It is billed as an opportunity to start fresh, shielding everyone from exposure to malpractice suits and other liabilities. However, the reality is that anyone looking to sue either old corporation will simply sue the new entity as the so-called “successor” corporation, on the grounds that it has assumed responsibility for its predecessors’ liabilities. You also will need new provider numbers, which may impede cash flow for months. Plus, the IRS treats corporate liquidations, even for merger purposes, as sales of assets, and taxes them.
In general, most experts that I’ve talked with favor outright merger of the corporations. This option is tax neutral, and while it may theoretically be less satisfactory liability-wise, you can minimize risk by examining financial and legal records, and by identifying any glaring flaws in charting or coding. Your lawyers can add “hold harmless” clauses to the merger agreement, indemnifying each party against the others’ liabilities. This area in particular is where you need experienced, competent legal advice.
Another common sticking point is known as “equalization.” Ideally, each party brings an equal amount of assets to the table, but in the real world that is rarely the case. One party may contribute more equipment, for example, and the others are often asked to make up the difference (“equalize”) with something else, usually cash.
An alternative is to agree that any inequalities will be compensated at the other end, in the form of buyout value; that is, physicians contributing more assets will receive larger buyouts when they leave or retire than those contributing less.
Non-compete provisions are always a difficult issue, mostly because they are so hard (and expensive) to enforce. An increasingly popular alternative is, once again, to deal with it at the other end, with a buyout penalty. An unhappy partner can leave, and compete, but at the cost of a substantially reduced buyout. This permits competition, but discourages it; and it compensates the remaining partners.
These are only some of the pivotal business and legal issues that must be settled in advance. A little planning and negotiation can prevent a lot of grief, regret, and legal expenses in the future. I’ll mention some other, more complicated merger options in a future column.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Skin & Allergy News. Additional columns are available online at edermatologynews.com.
Study shows long-term survival improvements in blood cancers

Credit: Rhoda Baer
A new study suggests that half of all people recently diagnosed with cancer in England and Wales should survive their disease for at least 10 years, whereas, 40 years ago, only about a quarter of cancer patients could expect the same.
The research, published in The Lancet, showed significant improvements in long-term cancer survival rates from 1971 to 2011, particularly among patients with hematologic malignancies.
Unfortunately, the outlook for some malignancies remained extremely poor over the period studied.
“Although survival for some cancers has improved dramatically over the past 40 years, others are lagging far behind,” said Manuela Quaresma, of the London School of Hygiene & Tropical Medicine in the UK.
“More investment is urgently needed to improve early diagnosis and provide the best treatment, including more specialist surgeons for poor-prognosis cancers like lung cancer, which have shown little or no evidence of improvement in long-term survival (5 and 10 years after diagnosis) over the past 40 years.”
Quaresma and her colleagues analyzed survival trends for more than 7 million adults (aged 15 to 99 years) diagnosed with one of 21 common cancers in England and Wales between 1971 and 2011, and followed up to the end of 2012.
The researchers observed an increase in 10-year survival for all cancers combined. Twenty-four percent of patients diagnosed in 1971-1972 survived at least 10 years. And 49.8% of patients diagnosed in 2010-2011 are expected to survive at least 10 years.
The data revealed substantial improvements for patients with hematologic malignancies as well. For Hodgkin lymphoma, the 10-year survival rate rose from 47.7% for patients diagnosed in 1971-1972 to 80% for those diagnosed in 2010-2011.
For non-Hodgkin lymphoma, the 10-year survival rate increased from 22% to 63.1%. For multiple myeloma, it rose from 6.2% to 32.6%. And for leukemia, it rose from 6.9% to 46.1%.
The researchers noted that the most recent 10-year survival estimates are above 70% for cancers of the breast, prostate, testis, and uterus, as well as for melanoma and Hodgkin lymphoma. Furthermore, improvements in survival are greatest for these cancers.
Unfortunately, several other malignancies continue to have poor long-term survival. For cancers of the brain, stomach, lung, esophagus, and pancreas, 10-year survival after diagnosis is still below 15% for patients diagnosed in 2010-2011.
“These 5 cancers impose a huge public health burden, both because they are common and because they are often diagnosed at a late stage, when they are much harder to treat,” said study author Bernard Rachet, MD, PhD, of the London School of Hygiene & Tropical Medicine.
Dr Rachet also noted that this research confirms a persistent “age gap” in survival between younger and older patients for all cancers.
“Even after we have adjusted for the fact that older people have much higher death rates from other diseases than younger people, elderly cancer patients are doing worse for all cancers,” he said.
“This problem is particularly marked in the UK. In other countries, the age gap in cancer survival has become much narrower over the last 15 to 20 years than in England and Wales.” ![]()

Credit: Rhoda Baer
A new study suggests that half of all people recently diagnosed with cancer in England and Wales should survive their disease for at least 10 years, whereas, 40 years ago, only about a quarter of cancer patients could expect the same.
The research, published in The Lancet, showed significant improvements in long-term cancer survival rates from 1971 to 2011, particularly among patients with hematologic malignancies.
Unfortunately, the outlook for some malignancies remained extremely poor over the period studied.
“Although survival for some cancers has improved dramatically over the past 40 years, others are lagging far behind,” said Manuela Quaresma, of the London School of Hygiene & Tropical Medicine in the UK.
“More investment is urgently needed to improve early diagnosis and provide the best treatment, including more specialist surgeons for poor-prognosis cancers like lung cancer, which have shown little or no evidence of improvement in long-term survival (5 and 10 years after diagnosis) over the past 40 years.”
Quaresma and her colleagues analyzed survival trends for more than 7 million adults (aged 15 to 99 years) diagnosed with one of 21 common cancers in England and Wales between 1971 and 2011, and followed up to the end of 2012.
The researchers observed an increase in 10-year survival for all cancers combined. Twenty-four percent of patients diagnosed in 1971-1972 survived at least 10 years. And 49.8% of patients diagnosed in 2010-2011 are expected to survive at least 10 years.
The data revealed substantial improvements for patients with hematologic malignancies as well. For Hodgkin lymphoma, the 10-year survival rate rose from 47.7% for patients diagnosed in 1971-1972 to 80% for those diagnosed in 2010-2011.
For non-Hodgkin lymphoma, the 10-year survival rate increased from 22% to 63.1%. For multiple myeloma, it rose from 6.2% to 32.6%. And for leukemia, it rose from 6.9% to 46.1%.
The researchers noted that the most recent 10-year survival estimates are above 70% for cancers of the breast, prostate, testis, and uterus, as well as for melanoma and Hodgkin lymphoma. Furthermore, improvements in survival are greatest for these cancers.
Unfortunately, several other malignancies continue to have poor long-term survival. For cancers of the brain, stomach, lung, esophagus, and pancreas, 10-year survival after diagnosis is still below 15% for patients diagnosed in 2010-2011.
“These 5 cancers impose a huge public health burden, both because they are common and because they are often diagnosed at a late stage, when they are much harder to treat,” said study author Bernard Rachet, MD, PhD, of the London School of Hygiene & Tropical Medicine.
Dr Rachet also noted that this research confirms a persistent “age gap” in survival between younger and older patients for all cancers.
“Even after we have adjusted for the fact that older people have much higher death rates from other diseases than younger people, elderly cancer patients are doing worse for all cancers,” he said.
“This problem is particularly marked in the UK. In other countries, the age gap in cancer survival has become much narrower over the last 15 to 20 years than in England and Wales.” ![]()

Credit: Rhoda Baer
A new study suggests that half of all people recently diagnosed with cancer in England and Wales should survive their disease for at least 10 years, whereas, 40 years ago, only about a quarter of cancer patients could expect the same.
The research, published in The Lancet, showed significant improvements in long-term cancer survival rates from 1971 to 2011, particularly among patients with hematologic malignancies.
Unfortunately, the outlook for some malignancies remained extremely poor over the period studied.
“Although survival for some cancers has improved dramatically over the past 40 years, others are lagging far behind,” said Manuela Quaresma, of the London School of Hygiene & Tropical Medicine in the UK.
“More investment is urgently needed to improve early diagnosis and provide the best treatment, including more specialist surgeons for poor-prognosis cancers like lung cancer, which have shown little or no evidence of improvement in long-term survival (5 and 10 years after diagnosis) over the past 40 years.”
Quaresma and her colleagues analyzed survival trends for more than 7 million adults (aged 15 to 99 years) diagnosed with one of 21 common cancers in England and Wales between 1971 and 2011, and followed up to the end of 2012.
The researchers observed an increase in 10-year survival for all cancers combined. Twenty-four percent of patients diagnosed in 1971-1972 survived at least 10 years. And 49.8% of patients diagnosed in 2010-2011 are expected to survive at least 10 years.
The data revealed substantial improvements for patients with hematologic malignancies as well. For Hodgkin lymphoma, the 10-year survival rate rose from 47.7% for patients diagnosed in 1971-1972 to 80% for those diagnosed in 2010-2011.
For non-Hodgkin lymphoma, the 10-year survival rate increased from 22% to 63.1%. For multiple myeloma, it rose from 6.2% to 32.6%. And for leukemia, it rose from 6.9% to 46.1%.
The researchers noted that the most recent 10-year survival estimates are above 70% for cancers of the breast, prostate, testis, and uterus, as well as for melanoma and Hodgkin lymphoma. Furthermore, improvements in survival are greatest for these cancers.
Unfortunately, several other malignancies continue to have poor long-term survival. For cancers of the brain, stomach, lung, esophagus, and pancreas, 10-year survival after diagnosis is still below 15% for patients diagnosed in 2010-2011.
“These 5 cancers impose a huge public health burden, both because they are common and because they are often diagnosed at a late stage, when they are much harder to treat,” said study author Bernard Rachet, MD, PhD, of the London School of Hygiene & Tropical Medicine.
Dr Rachet also noted that this research confirms a persistent “age gap” in survival between younger and older patients for all cancers.
“Even after we have adjusted for the fact that older people have much higher death rates from other diseases than younger people, elderly cancer patients are doing worse for all cancers,” he said.
“This problem is particularly marked in the UK. In other countries, the age gap in cancer survival has become much narrower over the last 15 to 20 years than in England and Wales.” ![]()
Drug gets orphan designation for MM & CLL/SLL

The European Medicines Agency (EMA) has granted orphan drug designation for selinexor (KPT-330) to treat multiple myeloma (MM) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), including Richter’s transformation.
Selinexor previously received orphan designation from both the EMA and the US Food and Drug Administration to treat patients with acute myeloid leukemia and those with diffuse large B-cell lymphoma.
Orphan designation is granted to promote the development of drugs that target rare, life-threatening or debilitating conditions and are expected to provide a significant therapeutic advantage over existing treatments.
Orphan designation qualifies a company—in this case, Karyopharm Therapeutics Inc.—for benefits that include targeted scientific advice from the EMA regarding drug development and 10 years of market exclusivity following the drug’s approval.
About selinexor
Selinexor (KPT-330) is a first-in-class, oral selective inhibitor of nuclear export (SINE) compound. Selinexor functions by inhibiting the nuclear export protein XPO1 (also called CRM1).
This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which subsequently reinitiates and amplifies their tumor suppressor function. This is thought to prompt apoptosis in cancer cells while largely sparing normal cells.
Selinexor has shown promise in an ongoing phase 1 study of patients with a range of hematologic malignancies. Results of this trial were presented at the 2014 ASCO Annual Meeting.
At that point, the study included 51 patients who had received selinexor across 8 dose levels, ranging from 3 mg/m2 to 60 mg/m2.
Among the 43 patients evaluable for response, the overall response rate was 28%, and the complete response rate was 5%.
Most adverse events were gastrointestinal in nature, and most of them were grade 1 or 2. The most common adverse events were nausea, anorexia, and fatigue.
There were 3 dose-limiting toxicities, including 1 MM patient with grade 4 thrombocytopenia, 1 follicular lymphoma patient with grade 4 thrombocytopenia, and 1 CLL patient with grade 2 fatigue. ![]()

The European Medicines Agency (EMA) has granted orphan drug designation for selinexor (KPT-330) to treat multiple myeloma (MM) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), including Richter’s transformation.
Selinexor previously received orphan designation from both the EMA and the US Food and Drug Administration to treat patients with acute myeloid leukemia and those with diffuse large B-cell lymphoma.
Orphan designation is granted to promote the development of drugs that target rare, life-threatening or debilitating conditions and are expected to provide a significant therapeutic advantage over existing treatments.
Orphan designation qualifies a company—in this case, Karyopharm Therapeutics Inc.—for benefits that include targeted scientific advice from the EMA regarding drug development and 10 years of market exclusivity following the drug’s approval.
About selinexor
Selinexor (KPT-330) is a first-in-class, oral selective inhibitor of nuclear export (SINE) compound. Selinexor functions by inhibiting the nuclear export protein XPO1 (also called CRM1).
This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which subsequently reinitiates and amplifies their tumor suppressor function. This is thought to prompt apoptosis in cancer cells while largely sparing normal cells.
Selinexor has shown promise in an ongoing phase 1 study of patients with a range of hematologic malignancies. Results of this trial were presented at the 2014 ASCO Annual Meeting.
At that point, the study included 51 patients who had received selinexor across 8 dose levels, ranging from 3 mg/m2 to 60 mg/m2.
Among the 43 patients evaluable for response, the overall response rate was 28%, and the complete response rate was 5%.
Most adverse events were gastrointestinal in nature, and most of them were grade 1 or 2. The most common adverse events were nausea, anorexia, and fatigue.
There were 3 dose-limiting toxicities, including 1 MM patient with grade 4 thrombocytopenia, 1 follicular lymphoma patient with grade 4 thrombocytopenia, and 1 CLL patient with grade 2 fatigue. ![]()

The European Medicines Agency (EMA) has granted orphan drug designation for selinexor (KPT-330) to treat multiple myeloma (MM) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), including Richter’s transformation.
Selinexor previously received orphan designation from both the EMA and the US Food and Drug Administration to treat patients with acute myeloid leukemia and those with diffuse large B-cell lymphoma.
Orphan designation is granted to promote the development of drugs that target rare, life-threatening or debilitating conditions and are expected to provide a significant therapeutic advantage over existing treatments.
Orphan designation qualifies a company—in this case, Karyopharm Therapeutics Inc.—for benefits that include targeted scientific advice from the EMA regarding drug development and 10 years of market exclusivity following the drug’s approval.
About selinexor
Selinexor (KPT-330) is a first-in-class, oral selective inhibitor of nuclear export (SINE) compound. Selinexor functions by inhibiting the nuclear export protein XPO1 (also called CRM1).
This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which subsequently reinitiates and amplifies their tumor suppressor function. This is thought to prompt apoptosis in cancer cells while largely sparing normal cells.
Selinexor has shown promise in an ongoing phase 1 study of patients with a range of hematologic malignancies. Results of this trial were presented at the 2014 ASCO Annual Meeting.
At that point, the study included 51 patients who had received selinexor across 8 dose levels, ranging from 3 mg/m2 to 60 mg/m2.
Among the 43 patients evaluable for response, the overall response rate was 28%, and the complete response rate was 5%.
Most adverse events were gastrointestinal in nature, and most of them were grade 1 or 2. The most common adverse events were nausea, anorexia, and fatigue.
There were 3 dose-limiting toxicities, including 1 MM patient with grade 4 thrombocytopenia, 1 follicular lymphoma patient with grade 4 thrombocytopenia, and 1 CLL patient with grade 2 fatigue. ![]()
NICE offers conditional support for eculizumab

Credit: Globovision
The UK’s National Institute for Health and Care Excellence (NICE) has issued a final draft guidance recommending eculizumab (Soliris) for funding to treat atypical hemolytic uremic syndrome (aHUS).
However, the agency has a few requirements. Eculizumab use must be coordinated through an expert center.
And monitoring systems must record the number of people with aHUS, the number who receive eculizumab, and the dose and duration of treatment.
NICE is also requiring a national protocol for starting and stopping eculizumab for clinical reasons and a research program with robust methods to evaluate when stopping treatment or dose adjustment might occur.
“[A NICE advisory] committee accepted that eculizumab is a step change in the management of aHUS and can be considered a significant innovation for a disease with a high unmet clinical need,” said NICE Chief Executive Sir Andrew Dillon.
“Eculizumab offers people with aHUS the possibility of avoiding end-stage renal failure, dialysis, and kidney transplantation, as well as other organ damage. The drug is, however, very expensive. The committee felt that the budget impact of eculizumab would be lower if the potential for dose adjustment and stopping treatment was explored.”
“This is reflected in the draft guidance, which recommends eculizumab should be funded only if important conditions are met, including the development of rules for starting and stopping treatment for clinical reasons. In the meantime, NHS England and the company [developing the drug, Alexion Pharmaceuticals] should consider what opportunities might exist to reduce the cost of eculizumab to the NHS.”
Eculizumab: Dosing, cost, and benefit
Eculizumab is given intravenously in adults as initial treatment at a dose of 900 mg for 4 weeks, then as maintenance treatment at a dose of 1200 mg on week 5, and then every 12 to 16 days. The summary of product characteristics for eculizumab states that “treatment is recommended to continue for a patient’s lifetime, unless discontinuation of treatment is clinically indicated.”
Eculizumab costs £3150 per 30 ml vial (excluding value-added tax). The net budget impact of eculizumab based on the company’s predicted rate of uptake over a 5-year period is confidential.
However, to allow consultees and commentators to properly engage in the consultation process, NICE has prepared an illustration of the possible budget impact of eculizumab for aHUS, using information available in the public domain.
NICE’s estimate is based on a treatment cost of £340,200 per adult patient in the first year (based on the acquisition cost of the drug and the recommended dosing for an adult), and assumes a patient cohort of 170, as estimated by NHS England in its interim commissioning policy.
If it is assumed that all of these adult patients with aHUS are treated with eculizumab, the budget impact for the first year would be £57.8 million.
If an additional 20 new patients are treated the following year (based on a worldwide incidence of 0.4 per million), the budget impact will rise to £62.5 million in year 2, assuming all new patients are treated and all existing patients continue to be treated at the maintenance cost of £327,600 per year.
Using the same assumptions, the budget impact will rise to £69 million in year 3 (190 existing and 20 new patients), £75 million in year 4 (210 existing and 20 new patients), and £82 million in year 5 (230 existing and 20 new patients).
NHS England has indicated that the amount of the budget allocated for highly specialized services in 2013/2014 was £544 million, and the spending on high-cost drugs was £156 million.
The advisory committee acknowledged that the company’s estimate of the incremental cost of eculizumab compared with standard care was considerable and that incremental costs estimated by the evidence review group were higher still (results are confidential).
The company estimated that eculizumab produced 25.22 additional quality-adjusted life-years (QALYs) per patient compared with standard care. Although the QALYs estimated in the evidence review group’s analysis were markedly lower than those calculated by the company, both analyses produced substantial QALY gains of a magnitude that is rarely seen for any new drug treatment.
NICE has not yet issued final guidance on eculizumab in aHUS. The draft guidance is now with consultees, including the company, healthcare professionals, and patient/carer organizations, who have the opportunity to appeal against the draft recommendations.
Final guidance on the use of eculizumab to treat aHUS is expected in January 2015. ![]()

Credit: Globovision
The UK’s National Institute for Health and Care Excellence (NICE) has issued a final draft guidance recommending eculizumab (Soliris) for funding to treat atypical hemolytic uremic syndrome (aHUS).
However, the agency has a few requirements. Eculizumab use must be coordinated through an expert center.
And monitoring systems must record the number of people with aHUS, the number who receive eculizumab, and the dose and duration of treatment.
NICE is also requiring a national protocol for starting and stopping eculizumab for clinical reasons and a research program with robust methods to evaluate when stopping treatment or dose adjustment might occur.
“[A NICE advisory] committee accepted that eculizumab is a step change in the management of aHUS and can be considered a significant innovation for a disease with a high unmet clinical need,” said NICE Chief Executive Sir Andrew Dillon.
“Eculizumab offers people with aHUS the possibility of avoiding end-stage renal failure, dialysis, and kidney transplantation, as well as other organ damage. The drug is, however, very expensive. The committee felt that the budget impact of eculizumab would be lower if the potential for dose adjustment and stopping treatment was explored.”
“This is reflected in the draft guidance, which recommends eculizumab should be funded only if important conditions are met, including the development of rules for starting and stopping treatment for clinical reasons. In the meantime, NHS England and the company [developing the drug, Alexion Pharmaceuticals] should consider what opportunities might exist to reduce the cost of eculizumab to the NHS.”
Eculizumab: Dosing, cost, and benefit
Eculizumab is given intravenously in adults as initial treatment at a dose of 900 mg for 4 weeks, then as maintenance treatment at a dose of 1200 mg on week 5, and then every 12 to 16 days. The summary of product characteristics for eculizumab states that “treatment is recommended to continue for a patient’s lifetime, unless discontinuation of treatment is clinically indicated.”
Eculizumab costs £3150 per 30 ml vial (excluding value-added tax). The net budget impact of eculizumab based on the company’s predicted rate of uptake over a 5-year period is confidential.
However, to allow consultees and commentators to properly engage in the consultation process, NICE has prepared an illustration of the possible budget impact of eculizumab for aHUS, using information available in the public domain.
NICE’s estimate is based on a treatment cost of £340,200 per adult patient in the first year (based on the acquisition cost of the drug and the recommended dosing for an adult), and assumes a patient cohort of 170, as estimated by NHS England in its interim commissioning policy.
If it is assumed that all of these adult patients with aHUS are treated with eculizumab, the budget impact for the first year would be £57.8 million.
If an additional 20 new patients are treated the following year (based on a worldwide incidence of 0.4 per million), the budget impact will rise to £62.5 million in year 2, assuming all new patients are treated and all existing patients continue to be treated at the maintenance cost of £327,600 per year.
Using the same assumptions, the budget impact will rise to £69 million in year 3 (190 existing and 20 new patients), £75 million in year 4 (210 existing and 20 new patients), and £82 million in year 5 (230 existing and 20 new patients).
NHS England has indicated that the amount of the budget allocated for highly specialized services in 2013/2014 was £544 million, and the spending on high-cost drugs was £156 million.
The advisory committee acknowledged that the company’s estimate of the incremental cost of eculizumab compared with standard care was considerable and that incremental costs estimated by the evidence review group were higher still (results are confidential).
The company estimated that eculizumab produced 25.22 additional quality-adjusted life-years (QALYs) per patient compared with standard care. Although the QALYs estimated in the evidence review group’s analysis were markedly lower than those calculated by the company, both analyses produced substantial QALY gains of a magnitude that is rarely seen for any new drug treatment.
NICE has not yet issued final guidance on eculizumab in aHUS. The draft guidance is now with consultees, including the company, healthcare professionals, and patient/carer organizations, who have the opportunity to appeal against the draft recommendations.
Final guidance on the use of eculizumab to treat aHUS is expected in January 2015. ![]()

Credit: Globovision
The UK’s National Institute for Health and Care Excellence (NICE) has issued a final draft guidance recommending eculizumab (Soliris) for funding to treat atypical hemolytic uremic syndrome (aHUS).
However, the agency has a few requirements. Eculizumab use must be coordinated through an expert center.
And monitoring systems must record the number of people with aHUS, the number who receive eculizumab, and the dose and duration of treatment.
NICE is also requiring a national protocol for starting and stopping eculizumab for clinical reasons and a research program with robust methods to evaluate when stopping treatment or dose adjustment might occur.
“[A NICE advisory] committee accepted that eculizumab is a step change in the management of aHUS and can be considered a significant innovation for a disease with a high unmet clinical need,” said NICE Chief Executive Sir Andrew Dillon.
“Eculizumab offers people with aHUS the possibility of avoiding end-stage renal failure, dialysis, and kidney transplantation, as well as other organ damage. The drug is, however, very expensive. The committee felt that the budget impact of eculizumab would be lower if the potential for dose adjustment and stopping treatment was explored.”
“This is reflected in the draft guidance, which recommends eculizumab should be funded only if important conditions are met, including the development of rules for starting and stopping treatment for clinical reasons. In the meantime, NHS England and the company [developing the drug, Alexion Pharmaceuticals] should consider what opportunities might exist to reduce the cost of eculizumab to the NHS.”
Eculizumab: Dosing, cost, and benefit
Eculizumab is given intravenously in adults as initial treatment at a dose of 900 mg for 4 weeks, then as maintenance treatment at a dose of 1200 mg on week 5, and then every 12 to 16 days. The summary of product characteristics for eculizumab states that “treatment is recommended to continue for a patient’s lifetime, unless discontinuation of treatment is clinically indicated.”
Eculizumab costs £3150 per 30 ml vial (excluding value-added tax). The net budget impact of eculizumab based on the company’s predicted rate of uptake over a 5-year period is confidential.
However, to allow consultees and commentators to properly engage in the consultation process, NICE has prepared an illustration of the possible budget impact of eculizumab for aHUS, using information available in the public domain.
NICE’s estimate is based on a treatment cost of £340,200 per adult patient in the first year (based on the acquisition cost of the drug and the recommended dosing for an adult), and assumes a patient cohort of 170, as estimated by NHS England in its interim commissioning policy.
If it is assumed that all of these adult patients with aHUS are treated with eculizumab, the budget impact for the first year would be £57.8 million.
If an additional 20 new patients are treated the following year (based on a worldwide incidence of 0.4 per million), the budget impact will rise to £62.5 million in year 2, assuming all new patients are treated and all existing patients continue to be treated at the maintenance cost of £327,600 per year.
Using the same assumptions, the budget impact will rise to £69 million in year 3 (190 existing and 20 new patients), £75 million in year 4 (210 existing and 20 new patients), and £82 million in year 5 (230 existing and 20 new patients).
NHS England has indicated that the amount of the budget allocated for highly specialized services in 2013/2014 was £544 million, and the spending on high-cost drugs was £156 million.
The advisory committee acknowledged that the company’s estimate of the incremental cost of eculizumab compared with standard care was considerable and that incremental costs estimated by the evidence review group were higher still (results are confidential).
The company estimated that eculizumab produced 25.22 additional quality-adjusted life-years (QALYs) per patient compared with standard care. Although the QALYs estimated in the evidence review group’s analysis were markedly lower than those calculated by the company, both analyses produced substantial QALY gains of a magnitude that is rarely seen for any new drug treatment.
NICE has not yet issued final guidance on eculizumab in aHUS. The draft guidance is now with consultees, including the company, healthcare professionals, and patient/carer organizations, who have the opportunity to appeal against the draft recommendations.
Final guidance on the use of eculizumab to treat aHUS is expected in January 2015. ![]()
FDA grants drug orphan designation for AML
The US Food and Drug Administration (FDA) has granted orphan drug designation for Actimab-A, an alpha radiolabeled antibody, to treat patients over the age of 60 who are newly diagnosed with acute myeloid leukemia (AML).
Actimab-A consists of the CD33 antibody lintuzumab linked to the actinium-225 payload. The product is currently under investigation in a multicenter, phase 1/2 trial of elderly AML patients.
The company developing Actimab-A, Actinium Pharmaceuticals, Inc., recently announced positive interim data from this trial.
Nine patients were evaluable. They had a median age of 76 (range, 73-81) and intermediate- or poor-risk cytogenetics.
The median overall survival was 5.4 months (range, 2.2-24 months), but survival was better for the 7 patients who had secondary AML. These patients had a median overall survival of 9.1 months from study entry (range, 2.3-24 months).
Two secondary AML patients lived longer than 12 months, and the longest surviving patient lived more than 24 months.
Two dosing levels of Actimab-A have been evaluated to date (0.5 or 1.0 μCi/kg/fraction), and the study is ongoing at higher doses until the maximum tolerated dose is reached.
Actinium expects additional data from this trial to be available in 2015.
“The FDA’s decision to grant orphan drug status for Actimab-A is a significant milestone for the company and recognizes the need for innovative new approaches to treat AML,” said Kaushik J. Dave, PhD, President and CEO of Actinium.
“The designation will provide Actinium access to various development benefits and financial incentives from the agency, including an exemption from prescription drug user fees for Actimab-A for this indication and, if the drug receives marketing approval, it will enjoy 7 years of market exclusivity in the United States.” ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for Actimab-A, an alpha radiolabeled antibody, to treat patients over the age of 60 who are newly diagnosed with acute myeloid leukemia (AML).
Actimab-A consists of the CD33 antibody lintuzumab linked to the actinium-225 payload. The product is currently under investigation in a multicenter, phase 1/2 trial of elderly AML patients.
The company developing Actimab-A, Actinium Pharmaceuticals, Inc., recently announced positive interim data from this trial.
Nine patients were evaluable. They had a median age of 76 (range, 73-81) and intermediate- or poor-risk cytogenetics.
The median overall survival was 5.4 months (range, 2.2-24 months), but survival was better for the 7 patients who had secondary AML. These patients had a median overall survival of 9.1 months from study entry (range, 2.3-24 months).
Two secondary AML patients lived longer than 12 months, and the longest surviving patient lived more than 24 months.
Two dosing levels of Actimab-A have been evaluated to date (0.5 or 1.0 μCi/kg/fraction), and the study is ongoing at higher doses until the maximum tolerated dose is reached.
Actinium expects additional data from this trial to be available in 2015.
“The FDA’s decision to grant orphan drug status for Actimab-A is a significant milestone for the company and recognizes the need for innovative new approaches to treat AML,” said Kaushik J. Dave, PhD, President and CEO of Actinium.
“The designation will provide Actinium access to various development benefits and financial incentives from the agency, including an exemption from prescription drug user fees for Actimab-A for this indication and, if the drug receives marketing approval, it will enjoy 7 years of market exclusivity in the United States.” ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for Actimab-A, an alpha radiolabeled antibody, to treat patients over the age of 60 who are newly diagnosed with acute myeloid leukemia (AML).
Actimab-A consists of the CD33 antibody lintuzumab linked to the actinium-225 payload. The product is currently under investigation in a multicenter, phase 1/2 trial of elderly AML patients.
The company developing Actimab-A, Actinium Pharmaceuticals, Inc., recently announced positive interim data from this trial.
Nine patients were evaluable. They had a median age of 76 (range, 73-81) and intermediate- or poor-risk cytogenetics.
The median overall survival was 5.4 months (range, 2.2-24 months), but survival was better for the 7 patients who had secondary AML. These patients had a median overall survival of 9.1 months from study entry (range, 2.3-24 months).
Two secondary AML patients lived longer than 12 months, and the longest surviving patient lived more than 24 months.
Two dosing levels of Actimab-A have been evaluated to date (0.5 or 1.0 μCi/kg/fraction), and the study is ongoing at higher doses until the maximum tolerated dose is reached.
Actinium expects additional data from this trial to be available in 2015.
“The FDA’s decision to grant orphan drug status for Actimab-A is a significant milestone for the company and recognizes the need for innovative new approaches to treat AML,” said Kaushik J. Dave, PhD, President and CEO of Actinium.
“The designation will provide Actinium access to various development benefits and financial incentives from the agency, including an exemption from prescription drug user fees for Actimab-A for this indication and, if the drug receives marketing approval, it will enjoy 7 years of market exclusivity in the United States.” ![]()
Science and Scholarship
In 2015, the Journal of Hospital Medicine will be publishing its 10th volume, marking an important milestone for hospital medicine. The journal has survived an early period where the need for and viability of an academic hospital medicine journal was not at all clear. More to the point, the Journal of Hospital Medicine has prospered and grown.
The journal continues to evolve as hospital medicine grows and changes. When the Journal of Hospital Medicine began, there was no particular focus on readmissions, ‐blockers were important to reduce perioperative risk, and tight glucose control was thought to be a critical goal for hospitalized patients. Evidence for hospitalists' effectiveness was also changing; initial evidence supporting uniform improvements in length of stay and possible improvement in outcomes were soon tempered by larger‐scale studies.
Clearly, things have changed in medicine writ large, and hospital medicine has changed and caused things to be changed as well. We now exist in a world of value‐based purchasing, accountable care, long‐overdue focus on patient‐centered care (and research), and all in a healthcare setting that is increasingly electronic in nature. Although healthcare delivery has changed, hospital medicine has retained critical core values, including our specialty's focus on interdisciplinary care, quality improvement, and safety.
The Journal of Hospital Medicine has been active in reflecting the field of hospital medicine since the journal began. Due in no small part to the vision of the field's leaders and the exertion and vision of the Journal of Hospital Medicine's founding editor, Mark Williams, the journal is a vigorous and important contributor to academic internal medicine. It has gone from 6 issues per year to 12 issues, has rapidly expanded the number of downloads and citations, and its impact in the field of medicine continues to grow.
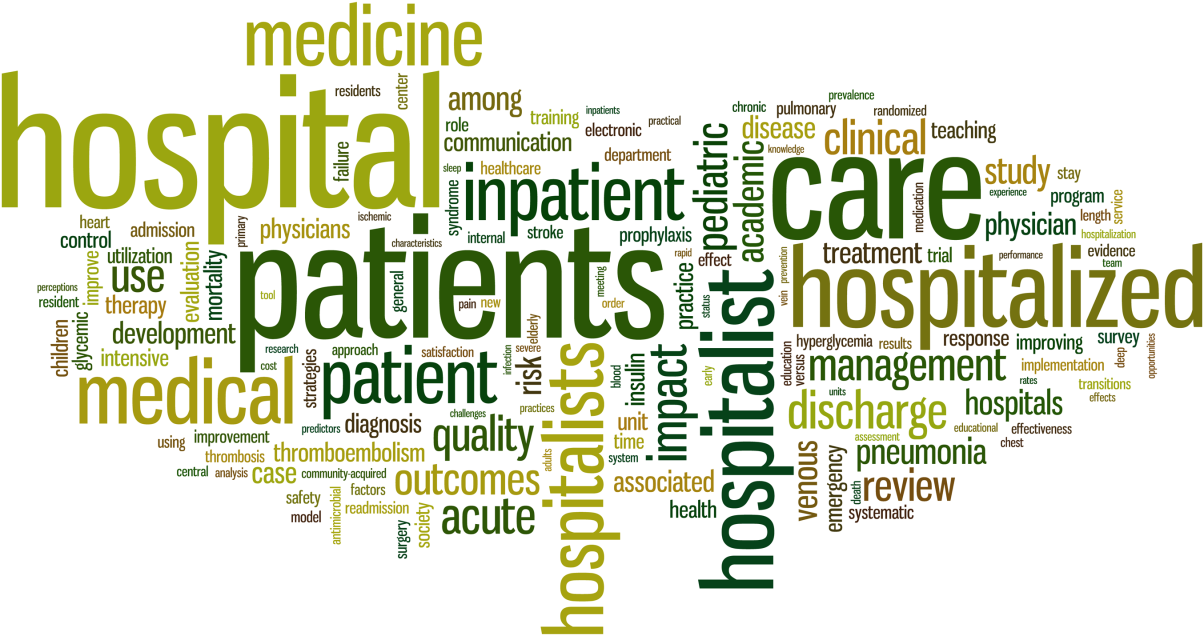
When reflecting on the words most often used in the Journal of Hospital Medicine's article titles (Figure 1) over the last 9 volumes, we were not surprised to see that the words hospital and hospitalists figured prominently. However, we were more pleased to see that the words patient and care were a large part of the universe of the Journal of Hospital Medicine's article titles.
The Journal of Hospital Medicine's articles are also being read and cited more and more often. The top 10 most downloaded articles are ones that represent a wide range of topics of clinical and operational importance to the field (Table 1), and the 10 most cited articles (Table 2) represent how the scholarship being produced by the field of hospital medicine is being used to advance other scientific and policy initiatives. Only 1 article[1] was listed in both places, demonstrating the difference between what the Journal of Hospital Medicine publishes as a way to help hospitalists provide better care on a day‐to‐day basis and what the journal publishes to advance the field.
| Article Title | Total Downloads |
|---|---|
| 1. Making inpatient medication reconciliation patient centered, clinically relevant, and implementable: a consensus statement on key principles and necessary first steps[3] | 9,766 |
| 2. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists[1] | 4,243 |
| 3. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists[4] | 3,722 |
| 4. Observation and inpatient status: clinical impact of the 2‐midnight rule[5] | 2,587 |
| 5. Measuring the modified early warning score and the Rothman Index: advantages of utilizing the electronic medical record in an early warning system[6] | 1,923 |
| 6. Acute coronary syndrome update for the hospitalist[7] | 1,514 |
| 7. Hospital performance trends on national quality measures and the association with Joint Commission accreditation[8] | 1,505 |
| 8. The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine[9] | 1,218 |
| 9. Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis[10] | 1,135 |
| 10. Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: a systematic review and meta‐analysis[11] | 1,069 |
| Title | Total Citations |
|---|---|
| 1. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists[1] | 152 |
| 2. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out[12] | 110 |
| 3, Reduction of 30‐day postdischarge hospital readmission or emergency department (ED) visit rates in high‐risk elderly medical patients through delivery of a targeted care bundle[13] | 69 |
| 4. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice[14] | 61 |
| 5. Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit[15] | 60 |
| 6. Transition of care for hospitalized elderly patientsdevelopment of a discharge checklist for hospitalists[16] | 59 |
| 7. Hospitalist handoffs: a systematic review and task force recommendations[17] | 54 |
| 8. Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital[18] | 53 |
| 9. Transitions of care consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine[19] | 50 |
| 10. Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum[20] | 49 |
Our 10th volume will continue the work of promoting scholarship and inquiry in hospital medicine by retaining a clear focus on the science that represents the best of an intellectual agenda[2] for our field. To achieve this important goal, the Journal of Hospital Medicine and the field will need to develop durable evidence for not only how hospitalists deliver care, but also evidence for how care can be improved by providing better treatments. Stated differently, hospitalists and the Journal of Hospital Medicine will need to be focused not only on improving the healthcare system, but also on evaluating new technologies, drugs, and devices that can be used in a value‐focused health system.
In 2015, the Journal of Hospital Medicine will also be launching a new series focusing on improving healthcare value. Titled Choosing Wisely: Next Steps in Improving Healthcare Value, this series of invited reviews will cover important topics needed to frame approaches to reducing healthcare costs while improving care quality and safety. This American Board of Internal Medicine‐sponsored series will be accompanied by a parallel series titled Things We Do for No Reason. Things We Do will provide a series of case studies of tests, medications, or procedures hospitalists encounter every day. We hope these topics, along with the Society of Hospital Medicine's Choosing Wisely focus areas, will continue to outline useful opportunities to improve healthcare value at the bedside or at least frame what we expect will be a lively debate.
We are confident that the combination of highest‐quality peer review and new article offerings will only enhance our ability to share the highest‐quality research, perspectives, reviews, and clinical cases and conundrums with Journal of Hospital Medicine readers. The journal's editors and I look forward to this new year, the Journal of Hospital Medicine's 10th volume, and to many volumes of the Journal of Hospital Medicine to come.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- . An intellectual agenda for hospitalists: lessons from bloodletting. J Hosp Med. 2013;8(7):418–419.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123–128.
- , , , et al. Observation and inpatient status: clinical impact of the 2‐midnight rule. J Hosp Med. 2014;9(4):203–209.
- , , . Measuring the modified early warning score and the Rothman Index: advantages of utilizing the electronic medical record in an early warning system. J Hosp Med. 2014;9(2):116–119.
- . Acute coronary syndrome update for hospitalists. J Hosp Med. 2010;5(suppl 4):S15–S21.
- , , , , . Hospital performance trends on national quality measures and the association with Joint Commission accreditation. J Hosp Med. 2011;6(8):454–461.
- The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1(suppl 1):2–95.
- , , , . Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis. J Hosp Med. 2010;5(7):E12–E13.
- , , , , , . Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: a systematic review and meta‐analysis. J Hosp Med. 2014;9(9):579–585.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , et al. Reduction of 30‐day postdischarge hospital readmission or emergency department (ED) visit rates in high‐risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211–218.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48–54.
- , , , , . Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354–360.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , , . Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital. J Hosp Med. 2006;1(3):145–150.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , et al. Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum. J Hosp Med. 2007;2(4):203–211.
In 2015, the Journal of Hospital Medicine will be publishing its 10th volume, marking an important milestone for hospital medicine. The journal has survived an early period where the need for and viability of an academic hospital medicine journal was not at all clear. More to the point, the Journal of Hospital Medicine has prospered and grown.
The journal continues to evolve as hospital medicine grows and changes. When the Journal of Hospital Medicine began, there was no particular focus on readmissions, ‐blockers were important to reduce perioperative risk, and tight glucose control was thought to be a critical goal for hospitalized patients. Evidence for hospitalists' effectiveness was also changing; initial evidence supporting uniform improvements in length of stay and possible improvement in outcomes were soon tempered by larger‐scale studies.
Clearly, things have changed in medicine writ large, and hospital medicine has changed and caused things to be changed as well. We now exist in a world of value‐based purchasing, accountable care, long‐overdue focus on patient‐centered care (and research), and all in a healthcare setting that is increasingly electronic in nature. Although healthcare delivery has changed, hospital medicine has retained critical core values, including our specialty's focus on interdisciplinary care, quality improvement, and safety.
The Journal of Hospital Medicine has been active in reflecting the field of hospital medicine since the journal began. Due in no small part to the vision of the field's leaders and the exertion and vision of the Journal of Hospital Medicine's founding editor, Mark Williams, the journal is a vigorous and important contributor to academic internal medicine. It has gone from 6 issues per year to 12 issues, has rapidly expanded the number of downloads and citations, and its impact in the field of medicine continues to grow.
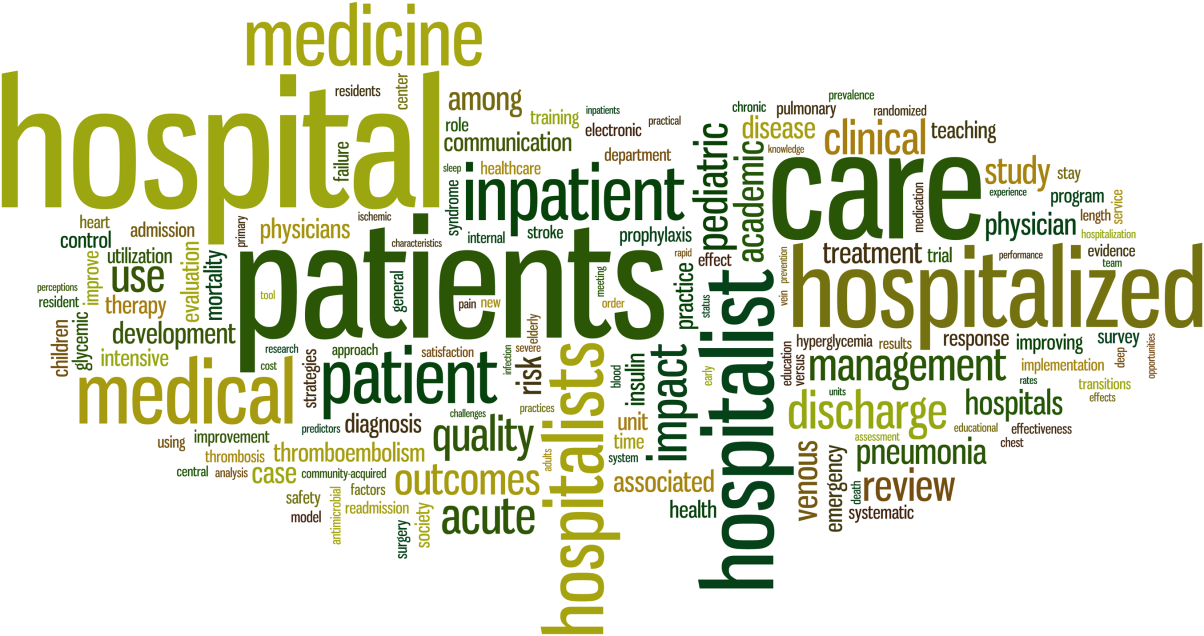
When reflecting on the words most often used in the Journal of Hospital Medicine's article titles (Figure 1) over the last 9 volumes, we were not surprised to see that the words hospital and hospitalists figured prominently. However, we were more pleased to see that the words patient and care were a large part of the universe of the Journal of Hospital Medicine's article titles.

The Journal of Hospital Medicine's articles are also being read and cited more and more often. The top 10 most downloaded articles are ones that represent a wide range of topics of clinical and operational importance to the field (Table 1), and the 10 most cited articles (Table 2) represent how the scholarship being produced by the field of hospital medicine is being used to advance other scientific and policy initiatives. Only 1 article[1] was listed in both places, demonstrating the difference between what the Journal of Hospital Medicine publishes as a way to help hospitalists provide better care on a day‐to‐day basis and what the journal publishes to advance the field.
| Article Title | Total Downloads |
|---|---|
| 1. Making inpatient medication reconciliation patient centered, clinically relevant, and implementable: a consensus statement on key principles and necessary first steps[3] | 9,766 |
| 2. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists[1] | 4,243 |
| 3. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists[4] | 3,722 |
| 4. Observation and inpatient status: clinical impact of the 2‐midnight rule[5] | 2,587 |
| 5. Measuring the modified early warning score and the Rothman Index: advantages of utilizing the electronic medical record in an early warning system[6] | 1,923 |
| 6. Acute coronary syndrome update for the hospitalist[7] | 1,514 |
| 7. Hospital performance trends on national quality measures and the association with Joint Commission accreditation[8] | 1,505 |
| 8. The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine[9] | 1,218 |
| 9. Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis[10] | 1,135 |
| 10. Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: a systematic review and meta‐analysis[11] | 1,069 |
| Title | Total Citations |
|---|---|
| 1. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists[1] | 152 |
| 2. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out[12] | 110 |
| 3, Reduction of 30‐day postdischarge hospital readmission or emergency department (ED) visit rates in high‐risk elderly medical patients through delivery of a targeted care bundle[13] | 69 |
| 4. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice[14] | 61 |
| 5. Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit[15] | 60 |
| 6. Transition of care for hospitalized elderly patientsdevelopment of a discharge checklist for hospitalists[16] | 59 |
| 7. Hospitalist handoffs: a systematic review and task force recommendations[17] | 54 |
| 8. Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital[18] | 53 |
| 9. Transitions of care consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine[19] | 50 |
| 10. Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum[20] | 49 |
Our 10th volume will continue the work of promoting scholarship and inquiry in hospital medicine by retaining a clear focus on the science that represents the best of an intellectual agenda[2] for our field. To achieve this important goal, the Journal of Hospital Medicine and the field will need to develop durable evidence for not only how hospitalists deliver care, but also evidence for how care can be improved by providing better treatments. Stated differently, hospitalists and the Journal of Hospital Medicine will need to be focused not only on improving the healthcare system, but also on evaluating new technologies, drugs, and devices that can be used in a value‐focused health system.
In 2015, the Journal of Hospital Medicine will also be launching a new series focusing on improving healthcare value. Titled Choosing Wisely: Next Steps in Improving Healthcare Value, this series of invited reviews will cover important topics needed to frame approaches to reducing healthcare costs while improving care quality and safety. This American Board of Internal Medicine‐sponsored series will be accompanied by a parallel series titled Things We Do for No Reason. Things We Do will provide a series of case studies of tests, medications, or procedures hospitalists encounter every day. We hope these topics, along with the Society of Hospital Medicine's Choosing Wisely focus areas, will continue to outline useful opportunities to improve healthcare value at the bedside or at least frame what we expect will be a lively debate.
We are confident that the combination of highest‐quality peer review and new article offerings will only enhance our ability to share the highest‐quality research, perspectives, reviews, and clinical cases and conundrums with Journal of Hospital Medicine readers. The journal's editors and I look forward to this new year, the Journal of Hospital Medicine's 10th volume, and to many volumes of the Journal of Hospital Medicine to come.
In 2015, the Journal of Hospital Medicine will be publishing its 10th volume, marking an important milestone for hospital medicine. The journal has survived an early period where the need for and viability of an academic hospital medicine journal was not at all clear. More to the point, the Journal of Hospital Medicine has prospered and grown.
The journal continues to evolve as hospital medicine grows and changes. When the Journal of Hospital Medicine began, there was no particular focus on readmissions, ‐blockers were important to reduce perioperative risk, and tight glucose control was thought to be a critical goal for hospitalized patients. Evidence for hospitalists' effectiveness was also changing; initial evidence supporting uniform improvements in length of stay and possible improvement in outcomes were soon tempered by larger‐scale studies.
Clearly, things have changed in medicine writ large, and hospital medicine has changed and caused things to be changed as well. We now exist in a world of value‐based purchasing, accountable care, long‐overdue focus on patient‐centered care (and research), and all in a healthcare setting that is increasingly electronic in nature. Although healthcare delivery has changed, hospital medicine has retained critical core values, including our specialty's focus on interdisciplinary care, quality improvement, and safety.
The Journal of Hospital Medicine has been active in reflecting the field of hospital medicine since the journal began. Due in no small part to the vision of the field's leaders and the exertion and vision of the Journal of Hospital Medicine's founding editor, Mark Williams, the journal is a vigorous and important contributor to academic internal medicine. It has gone from 6 issues per year to 12 issues, has rapidly expanded the number of downloads and citations, and its impact in the field of medicine continues to grow.
When reflecting on the words most often used in the Journal of Hospital Medicine's article titles (Figure 1) over the last 9 volumes, we were not surprised to see that the words hospital and hospitalists figured prominently. However, we were more pleased to see that the words patient and care were a large part of the universe of the Journal of Hospital Medicine's article titles.

The Journal of Hospital Medicine's articles are also being read and cited more and more often. The top 10 most downloaded articles are ones that represent a wide range of topics of clinical and operational importance to the field (Table 1), and the 10 most cited articles (Table 2) represent how the scholarship being produced by the field of hospital medicine is being used to advance other scientific and policy initiatives. Only 1 article[1] was listed in both places, demonstrating the difference between what the Journal of Hospital Medicine publishes as a way to help hospitalists provide better care on a day‐to‐day basis and what the journal publishes to advance the field.
| Article Title | Total Downloads |
|---|---|
| 1. Making inpatient medication reconciliation patient centered, clinically relevant, and implementable: a consensus statement on key principles and necessary first steps[3] | 9,766 |
| 2. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists[1] | 4,243 |
| 3. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists[4] | 3,722 |
| 4. Observation and inpatient status: clinical impact of the 2‐midnight rule[5] | 2,587 |
| 5. Measuring the modified early warning score and the Rothman Index: advantages of utilizing the electronic medical record in an early warning system[6] | 1,923 |
| 6. Acute coronary syndrome update for the hospitalist[7] | 1,514 |
| 7. Hospital performance trends on national quality measures and the association with Joint Commission accreditation[8] | 1,505 |
| 8. The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine[9] | 1,218 |
| 9. Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis[10] | 1,135 |
| 10. Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: a systematic review and meta‐analysis[11] | 1,069 |
| Title | Total Citations |
|---|---|
| 1. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists[1] | 152 |
| 2. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out[12] | 110 |
| 3, Reduction of 30‐day postdischarge hospital readmission or emergency department (ED) visit rates in high‐risk elderly medical patients through delivery of a targeted care bundle[13] | 69 |
| 4. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice[14] | 61 |
| 5. Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit[15] | 60 |
| 6. Transition of care for hospitalized elderly patientsdevelopment of a discharge checklist for hospitalists[16] | 59 |
| 7. Hospitalist handoffs: a systematic review and task force recommendations[17] | 54 |
| 8. Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital[18] | 53 |
| 9. Transitions of care consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine[19] | 50 |
| 10. Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum[20] | 49 |
Our 10th volume will continue the work of promoting scholarship and inquiry in hospital medicine by retaining a clear focus on the science that represents the best of an intellectual agenda[2] for our field. To achieve this important goal, the Journal of Hospital Medicine and the field will need to develop durable evidence for not only how hospitalists deliver care, but also evidence for how care can be improved by providing better treatments. Stated differently, hospitalists and the Journal of Hospital Medicine will need to be focused not only on improving the healthcare system, but also on evaluating new technologies, drugs, and devices that can be used in a value‐focused health system.
In 2015, the Journal of Hospital Medicine will also be launching a new series focusing on improving healthcare value. Titled Choosing Wisely: Next Steps in Improving Healthcare Value, this series of invited reviews will cover important topics needed to frame approaches to reducing healthcare costs while improving care quality and safety. This American Board of Internal Medicine‐sponsored series will be accompanied by a parallel series titled Things We Do for No Reason. Things We Do will provide a series of case studies of tests, medications, or procedures hospitalists encounter every day. We hope these topics, along with the Society of Hospital Medicine's Choosing Wisely focus areas, will continue to outline useful opportunities to improve healthcare value at the bedside or at least frame what we expect will be a lively debate.
We are confident that the combination of highest‐quality peer review and new article offerings will only enhance our ability to share the highest‐quality research, perspectives, reviews, and clinical cases and conundrums with Journal of Hospital Medicine readers. The journal's editors and I look forward to this new year, the Journal of Hospital Medicine's 10th volume, and to many volumes of the Journal of Hospital Medicine to come.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- . An intellectual agenda for hospitalists: lessons from bloodletting. J Hosp Med. 2013;8(7):418–419.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123–128.
- , , , et al. Observation and inpatient status: clinical impact of the 2‐midnight rule. J Hosp Med. 2014;9(4):203–209.
- , , . Measuring the modified early warning score and the Rothman Index: advantages of utilizing the electronic medical record in an early warning system. J Hosp Med. 2014;9(2):116–119.
- . Acute coronary syndrome update for hospitalists. J Hosp Med. 2010;5(suppl 4):S15–S21.
- , , , , . Hospital performance trends on national quality measures and the association with Joint Commission accreditation. J Hosp Med. 2011;6(8):454–461.
- The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1(suppl 1):2–95.
- , , , . Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis. J Hosp Med. 2010;5(7):E12–E13.
- , , , , , . Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: a systematic review and meta‐analysis. J Hosp Med. 2014;9(9):579–585.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , et al. Reduction of 30‐day postdischarge hospital readmission or emergency department (ED) visit rates in high‐risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211–218.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48–54.
- , , , , . Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354–360.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , , . Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital. J Hosp Med. 2006;1(3):145–150.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , et al. Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum. J Hosp Med. 2007;2(4):203–211.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- . An intellectual agenda for hospitalists: lessons from bloodletting. J Hosp Med. 2013;8(7):418–419.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , , et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123–128.
- , , , et al. Observation and inpatient status: clinical impact of the 2‐midnight rule. J Hosp Med. 2014;9(4):203–209.
- , , . Measuring the modified early warning score and the Rothman Index: advantages of utilizing the electronic medical record in an early warning system. J Hosp Med. 2014;9(2):116–119.
- . Acute coronary syndrome update for hospitalists. J Hosp Med. 2010;5(suppl 4):S15–S21.
- , , , , . Hospital performance trends on national quality measures and the association with Joint Commission accreditation. J Hosp Med. 2011;6(8):454–461.
- The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1(suppl 1):2–95.
- , , , . Iliac vein compression syndrome: an underdiagnosed cause of lower extremity deep venous thrombosis. J Hosp Med. 2010;5(7):E12–E13.
- , , , , , . Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: a systematic review and meta‐analysis. J Hosp Med. 2014;9(9):579–585.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , et al. Reduction of 30‐day postdischarge hospital readmission or emergency department (ED) visit rates in high‐risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211–218.
- , , , , . Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48–54.
- , , , , . Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397–403.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354–360.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , , . Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital. J Hosp Med. 2006;1(3):145–150.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4(6):364–370.
- , , , et al. Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum. J Hosp Med. 2007;2(4):203–211.
COPD Readmission Penalties Hurt Hospitals Serving Low-Income Patients
Government penalties meant to reduce COPD readmissions will unfairly impact hospitals that care for vulnerable patients, according to a report from the University of Michigan.
Beginning in January 2015, the Centers for Medicare & Medicaid Services will add COPD to its list of medical conditions for which it penalizes hospitals for excessive readmissions and fines them up to 3% of their total Medicare reimbursement for COPD readmissions.
Researchers Michael W. Sjoding, MD, and Colin R. Cooke, MD, MSc, MS, both of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor, evaluated three years of data on 3,018 hospitals and found that COPD readmission rates ranged from 17% to 28% across all hospitals. Hospitals designated as major teaching hospitals, those with a high percentage of patients with low socioeconomic status, and those with a high volume of COPD patients were associated with higher COPD readmission rates (P<0.001 for all).
The findings were published last month in the American Journal of Respiratory and Critical Care Medicine.
"It has been shown that there is a correlation between patients' social structures and support at home and COPD readmissions," Dr. Sjoding says. "Economic resources and education level can also drive readmissions, situations that are beyond hospital control."
Policies that measure hospital quality, Dr. Sjoding says, are important to ensure that patients have access to quality care across the country. However, when creating policies aimed at reducing readmission rates, CMS should level the playing field, he says. For example, academic hospitals caring for complex patients should be compared against their peers.
"It's important that physicians speak up to make sure that policies do the right thing," he says.
Visit our website for more information about managing patients with COPD.
Government penalties meant to reduce COPD readmissions will unfairly impact hospitals that care for vulnerable patients, according to a report from the University of Michigan.
Beginning in January 2015, the Centers for Medicare & Medicaid Services will add COPD to its list of medical conditions for which it penalizes hospitals for excessive readmissions and fines them up to 3% of their total Medicare reimbursement for COPD readmissions.
Researchers Michael W. Sjoding, MD, and Colin R. Cooke, MD, MSc, MS, both of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor, evaluated three years of data on 3,018 hospitals and found that COPD readmission rates ranged from 17% to 28% across all hospitals. Hospitals designated as major teaching hospitals, those with a high percentage of patients with low socioeconomic status, and those with a high volume of COPD patients were associated with higher COPD readmission rates (P<0.001 for all).
The findings were published last month in the American Journal of Respiratory and Critical Care Medicine.
"It has been shown that there is a correlation between patients' social structures and support at home and COPD readmissions," Dr. Sjoding says. "Economic resources and education level can also drive readmissions, situations that are beyond hospital control."
Policies that measure hospital quality, Dr. Sjoding says, are important to ensure that patients have access to quality care across the country. However, when creating policies aimed at reducing readmission rates, CMS should level the playing field, he says. For example, academic hospitals caring for complex patients should be compared against their peers.
"It's important that physicians speak up to make sure that policies do the right thing," he says.
Visit our website for more information about managing patients with COPD.
Government penalties meant to reduce COPD readmissions will unfairly impact hospitals that care for vulnerable patients, according to a report from the University of Michigan.
Beginning in January 2015, the Centers for Medicare & Medicaid Services will add COPD to its list of medical conditions for which it penalizes hospitals for excessive readmissions and fines them up to 3% of their total Medicare reimbursement for COPD readmissions.
Researchers Michael W. Sjoding, MD, and Colin R. Cooke, MD, MSc, MS, both of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor, evaluated three years of data on 3,018 hospitals and found that COPD readmission rates ranged from 17% to 28% across all hospitals. Hospitals designated as major teaching hospitals, those with a high percentage of patients with low socioeconomic status, and those with a high volume of COPD patients were associated with higher COPD readmission rates (P<0.001 for all).
The findings were published last month in the American Journal of Respiratory and Critical Care Medicine.
"It has been shown that there is a correlation between patients' social structures and support at home and COPD readmissions," Dr. Sjoding says. "Economic resources and education level can also drive readmissions, situations that are beyond hospital control."
Policies that measure hospital quality, Dr. Sjoding says, are important to ensure that patients have access to quality care across the country. However, when creating policies aimed at reducing readmission rates, CMS should level the playing field, he says. For example, academic hospitals caring for complex patients should be compared against their peers.
"It's important that physicians speak up to make sure that policies do the right thing," he says.
Visit our website for more information about managing patients with COPD.
Hospitalists Unionize to Avoid Outsourced Management Model
A group of hospitalists in Oregon have formed what is believed to be the first hospitalist union in the country—but it may not be the last.
Hospitalists at PeaceHealth Sacred Heart Medical Center locations in Springfield and Eugene, Ore., voted to form the union, dubbed Pacific Northwest Hospital Medicine Association, to have more say in patient care and working conditions there. Talk of unionizing started after hospitalists objected to a recommendation by a PeaceHealth consultant that their group's employment model be outsourced and run by a national management firm rather than remain hospital-owned.
"We really didn't have much of a say other than all quitting, which we didn't want to do because we like where we work," says hospitalist and union spokesperson David Schwartz, MD. "We started talking about unionizing."
The union is under the umbrella of the American Federation of Teachers, and likely is the first of its kind in the nation. The group started with 38 members, but 12 physicians who did not want to be managed under a national firm have left.
Now, the union is trying to persuade PeaceHealth to keep the group's management in-house. If not, the union will look to negotiate a contract with a national management firm chosen by its hospital administration.
A union of hospital physicians is uncommon. Healthcare workers often unionize but not individual specialists. Dr. Schwartz says he is curious to see whether other hospitalists who feel they want more of a say in their practice management will follow suit.
"The fact that we unionized seemed to galvanize a lot of the staff at the hospital," he says. "This might be a wave of the future. … Now people have a choice, which is interesting to watch."
Visit our website for more information on managing hospitalist groups.
A group of hospitalists in Oregon have formed what is believed to be the first hospitalist union in the country—but it may not be the last.
Hospitalists at PeaceHealth Sacred Heart Medical Center locations in Springfield and Eugene, Ore., voted to form the union, dubbed Pacific Northwest Hospital Medicine Association, to have more say in patient care and working conditions there. Talk of unionizing started after hospitalists objected to a recommendation by a PeaceHealth consultant that their group's employment model be outsourced and run by a national management firm rather than remain hospital-owned.
"We really didn't have much of a say other than all quitting, which we didn't want to do because we like where we work," says hospitalist and union spokesperson David Schwartz, MD. "We started talking about unionizing."
The union is under the umbrella of the American Federation of Teachers, and likely is the first of its kind in the nation. The group started with 38 members, but 12 physicians who did not want to be managed under a national firm have left.
Now, the union is trying to persuade PeaceHealth to keep the group's management in-house. If not, the union will look to negotiate a contract with a national management firm chosen by its hospital administration.
A union of hospital physicians is uncommon. Healthcare workers often unionize but not individual specialists. Dr. Schwartz says he is curious to see whether other hospitalists who feel they want more of a say in their practice management will follow suit.
"The fact that we unionized seemed to galvanize a lot of the staff at the hospital," he says. "This might be a wave of the future. … Now people have a choice, which is interesting to watch."
Visit our website for more information on managing hospitalist groups.
A group of hospitalists in Oregon have formed what is believed to be the first hospitalist union in the country—but it may not be the last.
Hospitalists at PeaceHealth Sacred Heart Medical Center locations in Springfield and Eugene, Ore., voted to form the union, dubbed Pacific Northwest Hospital Medicine Association, to have more say in patient care and working conditions there. Talk of unionizing started after hospitalists objected to a recommendation by a PeaceHealth consultant that their group's employment model be outsourced and run by a national management firm rather than remain hospital-owned.
"We really didn't have much of a say other than all quitting, which we didn't want to do because we like where we work," says hospitalist and union spokesperson David Schwartz, MD. "We started talking about unionizing."
The union is under the umbrella of the American Federation of Teachers, and likely is the first of its kind in the nation. The group started with 38 members, but 12 physicians who did not want to be managed under a national firm have left.
Now, the union is trying to persuade PeaceHealth to keep the group's management in-house. If not, the union will look to negotiate a contract with a national management firm chosen by its hospital administration.
A union of hospital physicians is uncommon. Healthcare workers often unionize but not individual specialists. Dr. Schwartz says he is curious to see whether other hospitalists who feel they want more of a say in their practice management will follow suit.
"The fact that we unionized seemed to galvanize a lot of the staff at the hospital," he says. "This might be a wave of the future. … Now people have a choice, which is interesting to watch."
Visit our website for more information on managing hospitalist groups.
Men on androgen deprivation therapy not getting bisphosphonates
The rate of prescribing bisphosphonates to protect the bone health of prostate cancer patients taking androgen deprivation therapy is extremely low in Ontario, even among those at high risk of fracture, according to a research letter published online Dec. 2 in JAMA.
Canadian guidelines have recommended bisphosphonates for men at risk for bone fracture since 2002, and specifically for men taking androgen deprivation therapy (ADT) since 2006. However, prescribing patterns for these agents are relatively unknown, said Dr. Husayn Gulamhusein of University Health Network, Toronto, and his associates.
The investigators assessed rates of these prescriptions over time by analyzing data in an Ontario cancer registry regarding 35,487 men aged 66 years and older who initiated ADT for prostate cancer between 1995 and 2012. They found that bisphosphonate prescriptions were filled for only 0.35/100 men in the earliest years of the study period, a rate that rose to only 3.40/100 men in the final years. “Even among those with prior osteoporosis or fragility fracture, rates remained low,” the researchers said (JAMA 2014;312:2285-6).Their findings indicate “limited awareness among clinicians regarding optimal bone health management,” they added.
There was a decrease in bisphosphonate prescriptions after 2009, which “may be partly due to negative media regarding the association of bisphosphonates with rare osteonecrosis of the jaw and atypical femoral fractures. This is appropriate for groups at low risk for fractures, but the decrease in use for high-risk patients is concerning,” Dr. Gulamhusein and his associates noted.
The rate of prescribing bisphosphonates to protect the bone health of prostate cancer patients taking androgen deprivation therapy is extremely low in Ontario, even among those at high risk of fracture, according to a research letter published online Dec. 2 in JAMA.
Canadian guidelines have recommended bisphosphonates for men at risk for bone fracture since 2002, and specifically for men taking androgen deprivation therapy (ADT) since 2006. However, prescribing patterns for these agents are relatively unknown, said Dr. Husayn Gulamhusein of University Health Network, Toronto, and his associates.
The investigators assessed rates of these prescriptions over time by analyzing data in an Ontario cancer registry regarding 35,487 men aged 66 years and older who initiated ADT for prostate cancer between 1995 and 2012. They found that bisphosphonate prescriptions were filled for only 0.35/100 men in the earliest years of the study period, a rate that rose to only 3.40/100 men in the final years. “Even among those with prior osteoporosis or fragility fracture, rates remained low,” the researchers said (JAMA 2014;312:2285-6).Their findings indicate “limited awareness among clinicians regarding optimal bone health management,” they added.
There was a decrease in bisphosphonate prescriptions after 2009, which “may be partly due to negative media regarding the association of bisphosphonates with rare osteonecrosis of the jaw and atypical femoral fractures. This is appropriate for groups at low risk for fractures, but the decrease in use for high-risk patients is concerning,” Dr. Gulamhusein and his associates noted.
The rate of prescribing bisphosphonates to protect the bone health of prostate cancer patients taking androgen deprivation therapy is extremely low in Ontario, even among those at high risk of fracture, according to a research letter published online Dec. 2 in JAMA.
Canadian guidelines have recommended bisphosphonates for men at risk for bone fracture since 2002, and specifically for men taking androgen deprivation therapy (ADT) since 2006. However, prescribing patterns for these agents are relatively unknown, said Dr. Husayn Gulamhusein of University Health Network, Toronto, and his associates.
The investigators assessed rates of these prescriptions over time by analyzing data in an Ontario cancer registry regarding 35,487 men aged 66 years and older who initiated ADT for prostate cancer between 1995 and 2012. They found that bisphosphonate prescriptions were filled for only 0.35/100 men in the earliest years of the study period, a rate that rose to only 3.40/100 men in the final years. “Even among those with prior osteoporosis or fragility fracture, rates remained low,” the researchers said (JAMA 2014;312:2285-6).Their findings indicate “limited awareness among clinicians regarding optimal bone health management,” they added.
There was a decrease in bisphosphonate prescriptions after 2009, which “may be partly due to negative media regarding the association of bisphosphonates with rare osteonecrosis of the jaw and atypical femoral fractures. This is appropriate for groups at low risk for fractures, but the decrease in use for high-risk patients is concerning,” Dr. Gulamhusein and his associates noted.
Key clinical point: Men taking androgen deprivation therapy for prostate cancer aren’t being prescribed bisphosphonates to protect their bone health.
Major finding: Bisphosphonate prescriptions were filled for only 0.35/100 men in the earliest years of the study period, a rate that rose to only 3.40/100 men in the final years.
Data source: A retrospective cohort study involving 35,487 prostate cancer patients in Ontario taking androgen deprivation therapy during a 17-year period.
Disclosures: This study was supported in part by Toronto General Hospital, the Toronto Western Hospital Research Foundation, the Canadian Cancer Society, and the Canadian Institutes of Health Research. Dr. Gulamhusein reported having no financial disclosures; one of his associates reported receiving honoraria from Merck.
LISTEN NOW: Highlights of the December 2014 issue of The Hospitalist newsmagazine
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2014/12/2014-December-Hospitalist-Highlights.mp3"][/audio]
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2014/12/2014-December-Hospitalist-Highlights.mp3"][/audio]
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2014/12/2014-December-Hospitalist-Highlights.mp3"][/audio]