User login
Bronchogenic Squamous Cell Carcinoma With Soft-Tissue Metastasis to the Hand: An Unusual Case Presentation and Review of the Literature
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
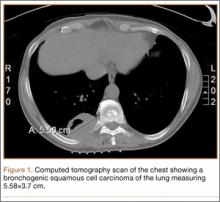
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
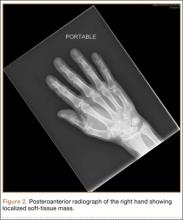
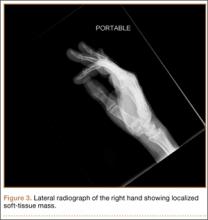
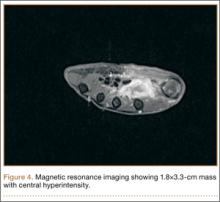
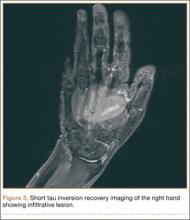
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
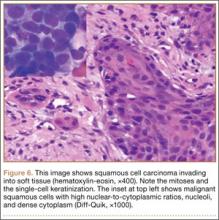
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
Anterior Hip Capsuloligamentous Reconstruction for Recurrent Instability After Hip Arthroscopy
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
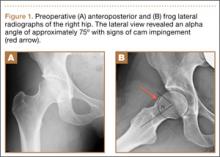
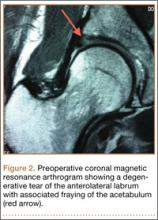
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
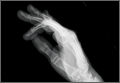
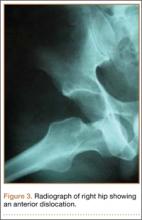
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
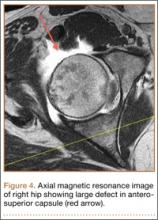
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
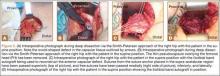
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
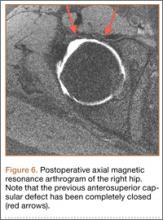
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
Young adults with ALL have better survival with pediatric regimens
SAN FRANCISCO– Teens and young adults don’t like being treated like children, but they should make an exception when it comes to acute lymphoblastic leukemia therapy, because pediatric ALL regimens are associated with significantly better event-free and overall survival among patients with ALL from the ages of 16 to 40 years.
Those findings come from a clinical trial 14 years in the making, in which 296 adolescents and young adults (AYA) with ALL were treated with an intensive pediatric chemotherapy combination regiment rather than a less-intensive adult regimen. At 2-year follow-up, the rate of overall survival (OS) was 78%, with the median overall survival not yet reached, and the event-free survival (EFS) rate was 66%, reported Dr. Wendy Stock from the University of Chicago Medical Center.
In contrast, EFS rates among AYA treated with adult regimens have historically ranged from 35-40%, Dr. Stock said at a press briefing at the annual meeting of the American Society of Hematology.
“These data really started 14 years ago at this ASH meeting when we presented data showing that young adults ages 16 to 20 who were treated on adult cooperative group studies in the United States fared much worse than the same age group who were treated on pediatric studies,” she said.
In 2008, Dr. Stock and her colleagues published a study (Blood 2008;112:1646-54) showing that AYAs treated under Children’s Cancer Group (CCG) protocols had an overall survival rate at 7 years of 67% and an EFS rate of 63%. In contrast, AYAs treated under Cancer and Leukemia Group B (CALGB) protocols had an OS of 46% and EFS of only 34%. The risk for worse outcomes was approximately twofold among adolescents treated with adult regimens, compared with those treated with pediatric regimens. The findings were similar in studies from France, the United Kingdom, and the Netherlands, Dr. Stock noted.
The investigators determined at that time that, under the CCG regimens, the teen and young adult patients received earlier and more intensive central nervous system prophylaxis and higher doses of nonmyelosuppressive drugs, especially glucocorticoids, vincristine, and pegylated asparignase, while those on the CALGB regimens received higher doses of myelosuppressive agents such as anthracyclines.
Because their original findings came from a retrospective study, the investigators decided to launch a prospective study,US Intergroup trial C10403, designed to evaluate outcomes among patients with ALL from the ages of 16-40 years when they were treated with a pediatric regimen by adult hematologists/oncologists in the cooperative group setting.
A total of 296 eligible patients with a median age of 25 years were enrolled. The patients had newly diagnosed ALL of T-cell or B-cell lineage; patients with Burkitt’s type ALL or ALL positive for the Philadelphia chromosome were excluded. The patients were treated with a regimen identical to the Capizzi methotrexate arm of the Children’s Oncology Group AALL0232 study. The regimen consisted of four intensive courses: remission induction, remission consolidation, interim maintenance and delayed intensification, and prolonged maintenance therapy. Patients who had an M2 marrow response after remission induction (more than 5% but less than 25% lymphoblasts) received an extended remission induction course of therapy.
As noted before, the EFS rate was 66% and the median EFS duration was 59 months. The 2-year overall survival rate was 79%. EFS rates were similar between patients with B-cell lineage (65%) and T-cell lineage (68%) ALL, and there were no significant differences in EFS or OS by sex or by age.
There were five (2%) treatment-related deaths during protocol therapy, including two cases of liver failure, both occurring during induction; two infections (one in the induction phase and one in the consolidation phase); and one ventricular arrhythmia (during induction). Treatment toxicities in general were similar to those seen in the standard therapy of the COG AALL0232 trial, although patients in the current study had an increase in risk for thrombosis and early hyperbilirubinemia.
“Our outcomes are similar to other prospective international studies which apply pediatric regimens to the young adult population of acute lymphoblastic leukemia,” Dr. Stock said.
In analyses of biological factors that affect outcome, the investigators found that white blood cell counts above 30,000/uL were associated with worse EFS and OS, and that the presence of a BCR-ABL1-like signature and overexpression of the gene CRLF2 were common and associated with significantly worse survival.
The investigators plan to use the study as a basis for future studies incorporating target antibodies and kinase inhibitors in an attempt to improve survival further by eradicating minimal residual disease, Dr. Stock said.
SAN FRANCISCO– Teens and young adults don’t like being treated like children, but they should make an exception when it comes to acute lymphoblastic leukemia therapy, because pediatric ALL regimens are associated with significantly better event-free and overall survival among patients with ALL from the ages of 16 to 40 years.
Those findings come from a clinical trial 14 years in the making, in which 296 adolescents and young adults (AYA) with ALL were treated with an intensive pediatric chemotherapy combination regiment rather than a less-intensive adult regimen. At 2-year follow-up, the rate of overall survival (OS) was 78%, with the median overall survival not yet reached, and the event-free survival (EFS) rate was 66%, reported Dr. Wendy Stock from the University of Chicago Medical Center.
In contrast, EFS rates among AYA treated with adult regimens have historically ranged from 35-40%, Dr. Stock said at a press briefing at the annual meeting of the American Society of Hematology.
“These data really started 14 years ago at this ASH meeting when we presented data showing that young adults ages 16 to 20 who were treated on adult cooperative group studies in the United States fared much worse than the same age group who were treated on pediatric studies,” she said.
In 2008, Dr. Stock and her colleagues published a study (Blood 2008;112:1646-54) showing that AYAs treated under Children’s Cancer Group (CCG) protocols had an overall survival rate at 7 years of 67% and an EFS rate of 63%. In contrast, AYAs treated under Cancer and Leukemia Group B (CALGB) protocols had an OS of 46% and EFS of only 34%. The risk for worse outcomes was approximately twofold among adolescents treated with adult regimens, compared with those treated with pediatric regimens. The findings were similar in studies from France, the United Kingdom, and the Netherlands, Dr. Stock noted.
The investigators determined at that time that, under the CCG regimens, the teen and young adult patients received earlier and more intensive central nervous system prophylaxis and higher doses of nonmyelosuppressive drugs, especially glucocorticoids, vincristine, and pegylated asparignase, while those on the CALGB regimens received higher doses of myelosuppressive agents such as anthracyclines.
Because their original findings came from a retrospective study, the investigators decided to launch a prospective study,US Intergroup trial C10403, designed to evaluate outcomes among patients with ALL from the ages of 16-40 years when they were treated with a pediatric regimen by adult hematologists/oncologists in the cooperative group setting.
A total of 296 eligible patients with a median age of 25 years were enrolled. The patients had newly diagnosed ALL of T-cell or B-cell lineage; patients with Burkitt’s type ALL or ALL positive for the Philadelphia chromosome were excluded. The patients were treated with a regimen identical to the Capizzi methotrexate arm of the Children’s Oncology Group AALL0232 study. The regimen consisted of four intensive courses: remission induction, remission consolidation, interim maintenance and delayed intensification, and prolonged maintenance therapy. Patients who had an M2 marrow response after remission induction (more than 5% but less than 25% lymphoblasts) received an extended remission induction course of therapy.
As noted before, the EFS rate was 66% and the median EFS duration was 59 months. The 2-year overall survival rate was 79%. EFS rates were similar between patients with B-cell lineage (65%) and T-cell lineage (68%) ALL, and there were no significant differences in EFS or OS by sex or by age.
There were five (2%) treatment-related deaths during protocol therapy, including two cases of liver failure, both occurring during induction; two infections (one in the induction phase and one in the consolidation phase); and one ventricular arrhythmia (during induction). Treatment toxicities in general were similar to those seen in the standard therapy of the COG AALL0232 trial, although patients in the current study had an increase in risk for thrombosis and early hyperbilirubinemia.
“Our outcomes are similar to other prospective international studies which apply pediatric regimens to the young adult population of acute lymphoblastic leukemia,” Dr. Stock said.
In analyses of biological factors that affect outcome, the investigators found that white blood cell counts above 30,000/uL were associated with worse EFS and OS, and that the presence of a BCR-ABL1-like signature and overexpression of the gene CRLF2 were common and associated with significantly worse survival.
The investigators plan to use the study as a basis for future studies incorporating target antibodies and kinase inhibitors in an attempt to improve survival further by eradicating minimal residual disease, Dr. Stock said.
SAN FRANCISCO– Teens and young adults don’t like being treated like children, but they should make an exception when it comes to acute lymphoblastic leukemia therapy, because pediatric ALL regimens are associated with significantly better event-free and overall survival among patients with ALL from the ages of 16 to 40 years.
Those findings come from a clinical trial 14 years in the making, in which 296 adolescents and young adults (AYA) with ALL were treated with an intensive pediatric chemotherapy combination regiment rather than a less-intensive adult regimen. At 2-year follow-up, the rate of overall survival (OS) was 78%, with the median overall survival not yet reached, and the event-free survival (EFS) rate was 66%, reported Dr. Wendy Stock from the University of Chicago Medical Center.
In contrast, EFS rates among AYA treated with adult regimens have historically ranged from 35-40%, Dr. Stock said at a press briefing at the annual meeting of the American Society of Hematology.
“These data really started 14 years ago at this ASH meeting when we presented data showing that young adults ages 16 to 20 who were treated on adult cooperative group studies in the United States fared much worse than the same age group who were treated on pediatric studies,” she said.
In 2008, Dr. Stock and her colleagues published a study (Blood 2008;112:1646-54) showing that AYAs treated under Children’s Cancer Group (CCG) protocols had an overall survival rate at 7 years of 67% and an EFS rate of 63%. In contrast, AYAs treated under Cancer and Leukemia Group B (CALGB) protocols had an OS of 46% and EFS of only 34%. The risk for worse outcomes was approximately twofold among adolescents treated with adult regimens, compared with those treated with pediatric regimens. The findings were similar in studies from France, the United Kingdom, and the Netherlands, Dr. Stock noted.
The investigators determined at that time that, under the CCG regimens, the teen and young adult patients received earlier and more intensive central nervous system prophylaxis and higher doses of nonmyelosuppressive drugs, especially glucocorticoids, vincristine, and pegylated asparignase, while those on the CALGB regimens received higher doses of myelosuppressive agents such as anthracyclines.
Because their original findings came from a retrospective study, the investigators decided to launch a prospective study,US Intergroup trial C10403, designed to evaluate outcomes among patients with ALL from the ages of 16-40 years when they were treated with a pediatric regimen by adult hematologists/oncologists in the cooperative group setting.
A total of 296 eligible patients with a median age of 25 years were enrolled. The patients had newly diagnosed ALL of T-cell or B-cell lineage; patients with Burkitt’s type ALL or ALL positive for the Philadelphia chromosome were excluded. The patients were treated with a regimen identical to the Capizzi methotrexate arm of the Children’s Oncology Group AALL0232 study. The regimen consisted of four intensive courses: remission induction, remission consolidation, interim maintenance and delayed intensification, and prolonged maintenance therapy. Patients who had an M2 marrow response after remission induction (more than 5% but less than 25% lymphoblasts) received an extended remission induction course of therapy.
As noted before, the EFS rate was 66% and the median EFS duration was 59 months. The 2-year overall survival rate was 79%. EFS rates were similar between patients with B-cell lineage (65%) and T-cell lineage (68%) ALL, and there were no significant differences in EFS or OS by sex or by age.
There were five (2%) treatment-related deaths during protocol therapy, including two cases of liver failure, both occurring during induction; two infections (one in the induction phase and one in the consolidation phase); and one ventricular arrhythmia (during induction). Treatment toxicities in general were similar to those seen in the standard therapy of the COG AALL0232 trial, although patients in the current study had an increase in risk for thrombosis and early hyperbilirubinemia.
“Our outcomes are similar to other prospective international studies which apply pediatric regimens to the young adult population of acute lymphoblastic leukemia,” Dr. Stock said.
In analyses of biological factors that affect outcome, the investigators found that white blood cell counts above 30,000/uL were associated with worse EFS and OS, and that the presence of a BCR-ABL1-like signature and overexpression of the gene CRLF2 were common and associated with significantly worse survival.
The investigators plan to use the study as a basis for future studies incorporating target antibodies and kinase inhibitors in an attempt to improve survival further by eradicating minimal residual disease, Dr. Stock said.
Key clinical point: Adolescents and young adults with acute lymphoblastic leukemia should be treated with a pediatric rather than an adult ALL regimen.
Major finding: Overall survival was 78% and event-free survival rate was 66%, compared with 46% and 34% for young adults treated with adult-style regimens in the past.
Data source: Prospective trial with 296 patients from the ages of 16-40 years with ALL.
Disclosures: The study is supported by the National Institutes of Health. Dr. Stock disclosed serving as an advisor and receiving research funding from Sigma-Tau Pharmaceuticals.
Improved Function and Joint Kinematics After Correction of Tibial Malalignment
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
The tibia is the most commonly fractured long bone in adults, and tibial malunions occur in up to 60% of these patients.1,2 Persistent tibial malalignment, particularly varus alignment, negatively alters gait and joint kinematics, leading to altered weight-bearing forces across the knee and ankle joints. These altered forces may lead to osteoarthritis.3-8
Several studies have identified a relationship between extent of tibial malalignment and changes in joint reaction forces.3,5-7,9-13 Puno and colleagues14 developed a mathematical model to better define the changes in neighboring joints relative to the pattern of the tibia malalignment. Not surprisingly, their work showed that, with distal tibial malunions, altered stress concentrations were realized at the ankle joint, and more proximal tibial deformities led to larger alterations in the joint stresses at the knee. More recently, van der Schoot and colleagues8 found a high prevalence of ipsilateral ankle osteoarthritis with tibial malalignment of 5° or more, and Greenwood and colleagues15 showed a higher incidence of knee pain, lower limb osteoarthritis, and disability in patients with previous tibia fractures. Given these findings, it would seem that correction of tibial malalignment would lead to normative lower extremity joint kinematic values, joint reaction forces, and overall quality of life (QOL).
The ability to ambulate has been recognized as an important milestone in functional recovery after lower extremity injury.2,16,17 Gait analysis, assessment of joint kinematics, and QOL and health status questionnaires can provide information to evaluate rehabilitation protocols, treatment algorithms, and surgical outcomes. Recently, these measures have been used to assess patients recovering from acetabular fractures, femoral shaft fractures, and calcaneal fractures.4,11,17-24 However, no study has used these measures to assess the benefits of surgical correction of malaligned tibias.
We conducted a study to determine improvement in gait, joint kinematics, and patients’ perceptions of overall well-being after surgical correction of tibial malunions. The null hypothesis was that correction of tibial malunion would have no effect on gait, joint kinematics, or patients’ perceptions of function and QOL.
Materials and Methods
This prospective double-time-point study, which was approved by the Institutional Review Board of Washington University/Barnes-Jewish Hospital, evaluated 11 consecutive patients with a varus tibial malunion treated by a single surgeon between September 2003 and January 2006. All patients were treated using a technique that included oblique osteotomy and open reduction and internal fixation (ORIF) or osteotomy and intramedullary nailing. Study inclusion criteria were age 18 years or older; symptomatic varus malunion of the tibia of 10º or more; absence of a developmental or pathologic process leading to the fracture and subsequent deformity; no neurologic deficit of either lower extremity or contralateral lower extremity deformity; and ability to ambulate 9 meters with or without use of an assistive device.
The 11 patients (6 men, 5 women) who met these criteria enrolled in the study. Mean age was 53 years (range, 43-68 years). Eight malunions involved the left tibia. The mechanisms of injury were motor vehicle crash (6 patients), fall from a great height (3), being struck by a motor vehicle (1), and gunshot (1). Mean time from injury to corrective surgery was 16.9 years (range, 1-34 years). Before surgery, each patient had a thorough physical examination, with plain radiographs, including anteroposterior (AP), lateral, and oblique views, obtained to assess degree of limb malalignment. Patients completed the Short Form-36 (SF-36) and the Musculoskeletal Function Assessment (MFA) and underwent joint kinematics and gait analysis. Five malunions were located in the mid-diaphysis of the tibia, 3 in the proximal third, and 2 in the distal third of the tibial shaft. One patient had posttraumatic deformity involving the proximal and the mid-diaphysis (Table 1). After surgery, each patient was followed at regular intervals in the surgeon’s private office. Minimum follow-up was 7 months (mean, 11 months; range 7-17 months). At follow-up, radiographs were obtained, and each patient completed the SF-36 and the MFA and underwent joint kinematics and gait analysis.
We obtained preoperative AP and lateral radiographs of the malaligned and contralateral normal tibias for each patient. Angular deformity was determined in the sagittal and coronal planes to determine location and magnitude of the deformity. Specifically, on each AP and lateral radiograph, a line was drawn the length of the tibia proximal and distal to the area of the deformity. The angle generated by the intersection of these lines on the AP and lateral radiographs was then plotted on a grid to determine the precise plane and magnitude of the deformity (Table 2).1,12 Clinically, relevant rotational deformity of the involved limb was assessed by physical examination, and the results were compared with those of the contralateral limb. Owing to the lack of considerable rotational deformity in any of these 11 patients, we did not obtain computed tomography scans for further assessment of rotation.
Perioperative intravenous antibiotics were administered: 2 g cefazolin 30 minutes before incision and 1 g every 8 hours for 24 hours after surgery. A pneumatic tourniquet was placed on the proximal thigh, and the entire leg was prepared and draped in a sterile fashion. The limb was elevated and exsanguinated with an Esmark bandage and the tourniquet raised to 250 mm Hg. With fluoroscopy, the site of the tibial deformity was identified. Generally, an incision was made centered over the apex of the deformity and one fingerbreadth lateral to the palpable tibial crest. In most cases, the anterolateral aspect of the tibia was exposed while minimizing soft-tissue and periosteal stripping. The plane of the maximum deformity was identified with both direct visualization and fluoroscopy. The osteotomy was performed with an oscillating saw, and in each case a fibular osteotomy was also performed. Malalignment was corrected using a combination of manual manipulation and femoral distractor.25,26 Intraoperative biplanar radiographs were compared with our preoperative plan and with reversed images of the contralateral tibia to assess correction of the deformity. If lengthening was required, in addition to the tibial osteotomy, a corticotomy was created, and a circular external fixator applied and distraction osteogenesis performed.
We maintained the limbs in a short-leg splint for about 10 days after surgery and then initiated active-assisted range of motion of neighboring joints. Patients were maintained on toe-touch weight-bearing for the initial 6 weeks and were then advanced to partial weight-bearing (23 kg). Physical therapy for lower extremity strengthening and gait training was started 6 weeks after surgery. Three months after surgery, patients were advanced to weight-bearing as tolerated and were allowed to return to their activities of daily living without restrictions if radiographs and clinical examination were consistent with healing of the osteotomy.
Each patient was examined and radiographs obtained at regular intervals (2, 6, and 12 weeks and then about every 3 months) after surgery until healing. Bone union was determined by history and physical examination with pain-free weight-bearing without use of assistive devices and by return of functional use of the extremity. Radiographic union was considered to have occurred when bridging trabeculae were present across the osteotomy and there was no loosening or failure of the implants. Occasionally, if there were questions regarding healing, a musculoskeletal radiologist was consulted. Acceptable tibia alignment was defined as alignment of less than 5° varus or less than 10° valgus in the coronal plane and less than 15° procurvatum or recurvatum in the sagittal plane. Immediate postoperative radiographs and most recent radiographs were used to determine the final amount of angular correction.27
Two patients subsequently required secondary operative procedures. One had varus collapse through the regenerate, and the other developed a nonunion of the osteotomy site and required exchange intramedullary nailing. In each case, the final assessment was done after the patient had healed after the second surgery and had fully recovered.
Perceived Functional Assessment
The MFA is a 100-item self-administered QOL questionnaire designed to assess self-perception of physical, psychological, and social well-being in patients with a musculoskeletal injury or condition. The MFA provides a summary score and separate score for each of 10 functional domains. The lower the score, the better the patient’s perception of function. Validated and published norms are available.20,28-30
Perceived Health Status
The Short Form-36 is a 36-item multipurpose self-administered health survey questionnaire. The SF-36, which assesses overall health status, provides a Physical Component Score (PCS) and a Mental Component Score (MCS). The higher the score, the better the patient’s perception of function. Validated and published norms are available.31
Gait Analysis
Video data from a 6-camera high-resolution system (Motion Analysis, Santa Rosa, California) were used to assess gait. A set of 3 reflective surface markers was placed on each of 4 areas: trunk, thighs, legs, and feet.18,19 The patient walked barefoot along a 9-meter walkway, and video data were collected during the middle 2 meters. For each patient, data from 4 to 7 trials were collected. Computerized software produced data describing the averaged joint angle as a function of the gait cycle for each of the 3 principal planes of the body. Specific points in the gait cycle were analyzed. Variables included maximum knee varus in stance phase; maximum knee valgus in swing; maximum knee flexion in stance and swing; minimum knee flexion in stance; maximum ankle inversion in terminal stance; maximum ankle eversion in stance; maximum ankle dorsiflexion in stance and swing; and maximum ankle plantarflexion at takeoff. In addition to the lower extremity joint kinematics, angular measurements, basic gait measurements of step length, stride length, cadence, and speed were also recorded.
Statistical Analysis
Paired t tests were used to determine if significant changes occurred as a consequence of the surgery for the outcome variables (P < .05). Normative gait data were used to assess the quality of any changes that occurred in the variables, but no statistical analysis was performed to determine significant differences.18
Results
All 11 patients had clinical and radiographic evidence of healing and deformity correction at most recent follow-up. Nine patients (82%) healed after the index procedure. Mean total angular correction in the coronal plane was 21° (range, 14° varus to 7° valgus), and mean total angular correction in the sagittal plane was 9° (range, 21° recurvatum to 15° procurvatum) (Table 2).
For the group, mean preoperative MFA score was 39 (SD, 18; range, 10-69), and mean postoperative MFA score was 28 (SD, 14; range, 8-53). Patients reported the most improvement in 2 domains: In Leisure, mean (SD) preoperative score was 8 (2), and mean postoperative score was 5 (2); in Emotional, mean preoperative score was 5 (2), and mean postoperative score was 4 (1). The other domains were not significantly different between the 2 assessments.
On the SF-36, mean (SD) PCS significantly (P < .05) improved from 32 (8) to 43 (9). Mean (SD) MCS showed little change: preoperative, 46 (16); postoperative, 48 (13). The PCS subcategories that showed the most improvement were Physical Function, mean (SD) preoperative, 26 (20), to postoperative, 52 (26); Role of Physical Health, preoperative, 18 (24), to postoperative, 60 (41); and Bodily Pain, preoperative, 39 (27), to 58 (18).
The results from the preoperative and postoperative gait analysis showed no significant differences for the ankle, knee, and hip variables during swing phase (Table 3). In an analysis of the changes in joint kinematics during stance, maximum hip adduction (increased) and maximum knee varus (decreased) on the operative side were significantly improved toward normative values as a consequence of the surgery (Table 3). The other kinematic stance variables were not significantly different. No significant changes were observed in stance time, step length, stride length, cadence, or speed as a consequence of the surgery (Table 4).
Discussion
Correction of malaligned tibias leads to improved limb alignment and patients’ perceptions of functional abilities and health but had a limited effect on joint kinematics and gait. In a group of like patients, we used common techniques to realign malunited tibias and validated instruments to measure functional outcome, health status, joint kinematics, and gait. The goals of this study were to evaluate changes in perceived function and health status and changes in joint kinematics and gait as a result of correction of a posttraumatic limb deformity.
Other investigators have reported outcomes of treating symptomatic malunions,32 nonunions,24 and leg-length discrepancies.33 In these reports, correction of deformity improved patient satisfaction and function, though objective means of assessment were infrequently used. Good results were reported with use of a dome-shaped supramalleolar osteotomy for the correction of tibial malunion.32 In this study, supramalleolar osteotomy was performed on 8 patients for correction of a malunited tibia. Postoperative assessment included subjective assessment of pain, limp, appearance, instability, and activity. Of these 8 patients, 7 reported overall symptomatic improvement after healing, and the 1 who lost the deformity correction remained symptomatic. Significant improvement in overall health has been reported after successful treatment of tibia nonunions.24 The investigators used the SF-36 to assess patients who underwent treatment for a tibial nonunion. Analysis of these patients’ results showed a significant improvement in physical and mental functioning after healing. In addition, improved gait symmetry was reported in patients successfully treated for leg-length discrepancies.33 Unfortunately, how improvement in gait related to overall patient function was not assessed. In the present study, we used stringent objective and subjective validated instruments to assess changes in joint gait kinematics and functional outcome before and after treatment of a tibial malunion. In general, our results are consistent with published results and indicate that realignment of tibial malunions improves patients’ perceptions of function. Our results also indicate improvements toward normative values in maximal hip adduction and knee varus, thus confirming the efficacy of the surgery from a functional perspective. Unfortunately, we did not show significant improvements in the remaining joint kinematics measurements or temporal gait parameters.
It is not entirely clear whether tibial malalignment leads to degenerative changes of the ipsilateral knee and/or ankle and what role this might play in functioning. In a retrospective analysis of 92 patients, angular deformity within 15° of normal alignment did not lead to ankle arthrosis.9 Milner and colleagues4 found that, though varus malunion of the tibia may lead to arthrosis of the medial compartment of the knee, other factors were more important in causing arthrosis of the ankle.
Wu and colleagues34 used tibial osteotomies in New Zealand white rabbits to investigate cartilage and bone changes of the knee after creation of varus or valgus tibial deformities. Thirty-four weeks after osteotomy, rabbits with up to 30° of deformity had severe cartilage changes with osteophytes, fibrillation, derangement of cell columns, and associated increased subchondral bone density of the knees. Cadaveric studies have also shown increased contact pressures within the knees and ankles with ever increasing amounts of tibial deformity.6,10 In each cadaveric study, malalignment in the distal third of the tibia caused the largest changes in the ankle, and changes in the alignment in the proximal third caused the largest changes in the knee.
Consistent with these animal and cadaveric studies are several retrospective clinical studies that have correlated tibial malalignment (particularly varus) with development of knee and ankle arthrosis.3,5,8 Kettelkamp and colleagues3 found a direct correlation between magnitude of deformity and length of time with development of knee arthrosis. These findings have led many to recommend that surgeons try to restore tibial alignment to as near normal as possible to reduce the likelihood of arthrosis after tibia fracture. We found significant improvement toward normative values for maximum hip adduction (increased) and tibial varus (decreased) after surgery. These improvements would shift the weight-bearing forces back to the central part of the knee and therefore more uniformly distribute weight-bearing forces.
Posttraumatic arthrosis that develops after fracture is thought to result from increased joint pressures and possibly factors related to the injury. Although surgical correction of tibial alignment is unlikely to reverse these cartilage changes, it may restore joint pressure symmetry and “offload” compromised compartments. Offloading of already degenerative compartments may explain our patients’ improved perceptions of function and overall health status.
There were several limitations to our study. First, plain radiographs of malaligned and uninjured tibia and fibula were used, and these do not allow complete assessment of the weight-bearing access of the limb. Our patients, however, had isolated tibia fractures, which involved a normal limb before injury, so any alterations in joint kinematics, gait, or function would likely be the result of the fracture. Another limitation of our study is its nonrandomized design. However, the patients reflect the typical heterogeneous trauma patient population, who typically develop tibial malunions and seek correction. Another limitation was the lack of a treatment protocol regarding exact surgical technique and implants used to stabilize the osteotomies. In general, the patients were treated similarly, and their preoperative and postoperative assessments were exactly the same, as was their state-of-the-art joint kinematics and gait analysis, combined with the use of previously validated outcome measures. In addition, the lack of improvement in gait could have resulted from postoperative physical therapy that focused on joint mobilization and muscle strengthening and not on correction of abnormal gait parameters noted on preoperative gait analysis. Despite the potential limitations of the study, surgical correction of these symptomatic tibial malunions resulted in significant improvement in functional outcome and improved joint kinematics on the operative side.
Conclusion
Significant effort should be made to restore and maintain near-anatomical tibial alignment until a tibia fracture is healed. In patients who develop a symptomatic tibial malunion, surgical correction should be undertaken with the intent to restore normal limb alignment and improve joint kinematics, function, and overall health status.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
1. Probe RA. Lower extremity angular malunion: evaluation and surgical correction. J Am Acad Orthop Surg. 2003;11(5):302-311.
2. van der Linden W, Larsson K. Plate fixation versus conservative treatment of tibial shaft fractures. A randomized trial. J Bone Joint Surg Am. 1979;61(6):873-878.
3. Kettelkamp DB, Hillberry BM, Murrish DE, Heck DA. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop. 1988;(234):159-169.
4. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
5. Puno RM, Vaughan JJ, Stetten ML, Johnson JR. Long-term effects of tibial angular malunion on the knee and ankle joints. J Orthop Trauma. 1991;5(3):247-254.
6. Tarr RR, Resnick CT, Wagner KS, Sarmiento A. Changes in tibiotalar joint contact areas following experimentally induced tibial angular deformities. Clin Orthop. 1985;(199):72-80.
7. Ting AJ, Tarr RR, Sarmiento A, Wagner K, Resnick C. The role of subtalar motion and ankle contact pressure changes from angular deformities of the tibia. Foot Ankle. 1987;7(5):290-299.
8. van der Schoot DK, Den Outer AJ, Bode PJ, Obermann WR, van Vugt AB. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients. J Bone Joint Surg Br. 1996;78(5):722-725.
9. Kristensen KD, Kiaer T, Blicher J. No arthrosis of the ankle 20 years after malaligned tibial-shaft fracture. Acta Orthop Scand. 1989;60(2):208-209.
10. McKellop HA, Sigholm G, Redfern FC, Doyle B, Sarmiento A, Luck JV Sr. The effect of simulated fracture-angulations of the tibia on cartilage pressures in the knee joint. J Bone Joint Surg Am. 1991;73(9):1382-1391.
11. Merchant TC, Dietz FR. Long-term follow-up after fractures of the tibial and fibular shafts. J Bone Joint Surg Am. 1989;71(4):599-606.
12. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
13. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992.
14. Puno RM, Vaughan JJ, von Fraunhofer JA, Stetten ML, Johnson JR. A method of determining the angular malalignments of the knee and ankle joints resulting from a tibial malunion. Clin Orthop. 1987;(223):213-219.
15. Greenwood DC, Muir KR, Doherty M, Milner SA, Stevens M, Davis TR. Conservatively managed tibial shaft fractures in Nottingham, UK: are pain, osteoarthritis, and disability long-term complications? J Epidemiol Community Health. 1997;51(6):701-704.
16. Dehne E, Deffer PA, Hall RM, Brown PW, Johnson EV. The natural history of the fractured tibia. Surg Clin North Am. 1961;41(6):1495-1513.
17. Kitaoka HB, Schaap EJ, Chao EY, An KN. Displaced intra-articular fractures of the calcaneus treated non-operatively. Clinical results and analysis of motion and ground-reaction and temporal forces. J Bone Joint Surg Am. 1994;76(10):1531-1540.
18. Borrelli J Jr, Goldfarb C, Ricci W, Wagner JM, Engsberg JR. Functional outcome after isolated acetabular fractures. J Orthop Trauma. 2002;16(2):73-81.
19. Borrelli J Jr, Ricci WM, Anglen JO, Gregush R, Engsberg J. Muscle strength recovery and its effects on outcome after open reduction and internal fixation of acetabular fractures. J Orthop Trauma. 2006;20(6):388-395.
20. Jaglal S, Lakhani Z, Schatzker J. Reliability, validity, and responsiveness of the lower extremity measure for patients with a hip fracture. J Bone Joint Surg Am. 2000;82(7):955-962.
21. Madsen MS, Ritter MA, Morris HH, et al. The effect of total hip arthroplasty surgical approach on gait. J Orthop Res. 2004;22(1):44-50.
22. Mittlmeier T, Morlock MM, Hertlein H, et al. Analysis of morphology and gait function after intraarticular calcaneal fracture. J Orthop Trauma. 1993;7(4):303-310.
23. Song KM, Halliday SE, Little DG. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am. 1997;79(11):1690-1698.
24. Zlowodzki M, Obremskey WT, Thomison JB, Kregor PJ. Functional outcome after treatment of lower-extremity nonunions. J Trauma. 2005;58(2):312-317.
25. Sanders R, Anglen JO, Mark JB. Oblique osteotomy for the correction of tibial malunion. J Bone Joint Surg Am. 1995;77(2):240-246.
26. Sangeorzan BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically directed single-cut osteotomy for correction of tibial malunion. J Orthop Trauma. 1989;3(4):267-275.
27. Borrelli J Jr, Leduc S, Gregush R, Ricci WM. Tricortical bone grafts for treatment of malaligned tibias and fibulas. Clin Orthop. 2009;467(4):1056-1063.
28. Engelberg R, Martin DP, Agel J, Obremsky W, Coronado G, Swiontkowski MF. Musculoskeletal Function Assessment instrument: criterion and construct validity. J Orthop Res. 1996;14(2):182-192.
29. Engelberg R, Martin DP, Agel J, Swiontkowski MF. Musculoskeletal Function Assessment: reference values for patient and non-patient samples. J Orthop Res. 1999;17(1):101-109.
30. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short Musculoskeletal Function Assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-1260.
31. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
32. Graehl PM, Hersh MR, Heckman JD. Supramalleolar osteotomy for the treatment of symptomatic tibial malunion. J Orthop Trauma. 1987;1(4):281-292.
33. Bhave A, Paley D, Herzenberg JE. Improvement in gait parameters after lengthening for the treatment of limb-length discrepancy. J Bone Joint Surg Am. 1999;81(4):529-534.
34. Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990;8(4):572-585.
A new standard of care for relapsed MM?
SAN FRANCISCO—Trial results suggest a 3-drug regimen could represent a new standard of care for relapsed multiple myeloma (MM), according to a speaker at the 2014 ASH Annual Meeting.
In the phase 3 ASPIRE trial, patients who received combination carfilzomib, lenalidomide, and dexamethasone (KRd) had superior progression-free survival compared to patients who received only lenalidomide and dexamethasone (Rd).
There was a trend toward improved overall survival with KRd as well.
Patients who received KRd did experience more adverse events (AEs), but fewer patients discontinued treatment due to AEs in the KRd arm than in the Rd arm.
Keith Stewart, MBChB, of the Mayo Clinic in Arizona, presented these results at the meeting as abstract 79. The data were published in The New England Journal of Medicine as well. The trial was sponsored by Onyx Pharmaceuticals, Inc., the company developing carfilzomib.
ASPIRE included 792 patients with relapsed MM who had received 1 to 3 prior treatment regimens. Patients were randomized to treatment with KRd (n=396) or Rd (n=396), and baseline characteristics were similar between the arms.
“There was a slight preponderance of patients over the age of 65 in the Rd arm of the trial,” Dr Stewart noted. “Conversely, there were more patients on the Rd arm of the trial who had lower-risk cytogenetics.”
“Patients were also well-balanced for baseline exposure to prior therapies. Prior therapies included transplant in 55% of patients, bortezomib in two-thirds of patients, and lenalidomide in 20% of patients—again, equal in both arms of the trial.”
All patients received a standard dosing schedule of lenalidomide (25 mg on days 1-21) and low-dose dexamethasone (40 mg on days 1, 8, 15, and 22).
Patients in the KRd arm also received carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1 and 27 mg/m2 thereafter). They received a 10-minute infusion of the drug on days 1, 2, 8, 9, 15, and 16. Carfilzomib was not given on days 8 and 9 in cycles 13 to 18 and not administered beyond 18 cycles.
‘Unprecedented’ results
The study’s primary endpoint was progression-free survival. And results showed progression-free survival was significantly longer in the KRd arm than in the Rd arm—26.3 months and 17.6 months, respectively (hazard ratio=0.69; P<0.0001).
“Progression-free survival was significantly improved by 8.7 months with KRd,” Dr Stewart noted. “In a phase 3 clinical trial setting, this is unprecedented.”
“In all prespecified subgroups, the advantage of KRd in progression-free survival was maintained. That includes age, international staging system, and prior exposure to either bortezomib or lenalidomide, or both drugs.”
The secondary endpoints of the trial were overall survival, overall response rate, duration of response, health-related quality of life, and safety.
The data for median overall survival are not yet mature based on the prespecified statistical boundary at the interim analysis (P=0.005). However, there was a trend in favor of KRd (hazard ratio, 0.79; P=0.018).
The overall response rate was 87.1% with KRd and 66.7% with Rd (P<0.0001), and the complete response rates were 14.1% and 4.3%, respectively (P<0.001). The median duration of response was 28.6 months and 21.2 months, respectively.
In addition, KRd improved global health-related quality of life compared with Rd over 18 cycles of treatment (P=0.0001).
‘Reassuring’ toxicity data
“In the discussion of adverse events,” Dr Stewart said, “it’s important to note that the median treatment duration was 88 weeks with KRd and 57 weeks with Rd.”
Most patients in each arm experienced at least one AE—96.9% in the KRd arm and 97.2% in the RD arm.
In the KRd arm, 7.7% of patients died while still on treatment or within 30 days of receiving their last dose of treatment, as did 8.5% of patients in the Rd arm. The percentage of deaths attributable to AEs was 6.9% in both arms.
The rates of treatment discontinuation were 69.9% in the KRd arm and 77.9% in the Rd arm. More patients discontinued treatment due to disease progression—39.8% in the KRd arm and 50.1% in the Rd arm—than to AEs—15.3% in the KRd arm and 17.7% in the Rd arm.
The most common grade 3 or higher hematologic treatment-emergent AEs (in the KRd and Rd arms, respectively) were neutropenia (29.6% vs 26.5%), anemia (17.9% vs 17.2%), and thrombocytopenia (16.6% vs 12.3%).
The most common grade 3 or higher nonhematologic treatment-emergent AEs (in the KRd and Rd arms, respectively) were hypokalemia (9.4% vs 4.9%), fatigue (7.7% vs 6.4%), and diarrhea (3.8% vs 4.1%).
Other treatment-emergent AEs of any grade (in the KRd and Rd arms, respectively) included dyspnea (19.4% vs 14.9%), hypertension (14.3% vs 6.9%), acute renal failure (8.4% vs 7.2%), cardiac failure (6.4% vs 4.1%), ischemic heart disease (5.9% vs 4.6%), and peripheral neuropathy (17.1% vs 17.0%).
“The results [are] very reassuring with respect to cardiac and renal events, which were reported at rates consistent with, or even lower than, those reported in prior studies of single-agent carfilzomib or more heavily pretreated patients,” Dr Stewart said.
“Based on the results of this phase 3 trial, I think it’s fair to say that KRd could represent a new standard of care in relapsed multiple myeloma.” ![]()
SAN FRANCISCO—Trial results suggest a 3-drug regimen could represent a new standard of care for relapsed multiple myeloma (MM), according to a speaker at the 2014 ASH Annual Meeting.
In the phase 3 ASPIRE trial, patients who received combination carfilzomib, lenalidomide, and dexamethasone (KRd) had superior progression-free survival compared to patients who received only lenalidomide and dexamethasone (Rd).
There was a trend toward improved overall survival with KRd as well.
Patients who received KRd did experience more adverse events (AEs), but fewer patients discontinued treatment due to AEs in the KRd arm than in the Rd arm.
Keith Stewart, MBChB, of the Mayo Clinic in Arizona, presented these results at the meeting as abstract 79. The data were published in The New England Journal of Medicine as well. The trial was sponsored by Onyx Pharmaceuticals, Inc., the company developing carfilzomib.
ASPIRE included 792 patients with relapsed MM who had received 1 to 3 prior treatment regimens. Patients were randomized to treatment with KRd (n=396) or Rd (n=396), and baseline characteristics were similar between the arms.
“There was a slight preponderance of patients over the age of 65 in the Rd arm of the trial,” Dr Stewart noted. “Conversely, there were more patients on the Rd arm of the trial who had lower-risk cytogenetics.”
“Patients were also well-balanced for baseline exposure to prior therapies. Prior therapies included transplant in 55% of patients, bortezomib in two-thirds of patients, and lenalidomide in 20% of patients—again, equal in both arms of the trial.”
All patients received a standard dosing schedule of lenalidomide (25 mg on days 1-21) and low-dose dexamethasone (40 mg on days 1, 8, 15, and 22).
Patients in the KRd arm also received carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1 and 27 mg/m2 thereafter). They received a 10-minute infusion of the drug on days 1, 2, 8, 9, 15, and 16. Carfilzomib was not given on days 8 and 9 in cycles 13 to 18 and not administered beyond 18 cycles.
‘Unprecedented’ results
The study’s primary endpoint was progression-free survival. And results showed progression-free survival was significantly longer in the KRd arm than in the Rd arm—26.3 months and 17.6 months, respectively (hazard ratio=0.69; P<0.0001).
“Progression-free survival was significantly improved by 8.7 months with KRd,” Dr Stewart noted. “In a phase 3 clinical trial setting, this is unprecedented.”
“In all prespecified subgroups, the advantage of KRd in progression-free survival was maintained. That includes age, international staging system, and prior exposure to either bortezomib or lenalidomide, or both drugs.”
The secondary endpoints of the trial were overall survival, overall response rate, duration of response, health-related quality of life, and safety.
The data for median overall survival are not yet mature based on the prespecified statistical boundary at the interim analysis (P=0.005). However, there was a trend in favor of KRd (hazard ratio, 0.79; P=0.018).
The overall response rate was 87.1% with KRd and 66.7% with Rd (P<0.0001), and the complete response rates were 14.1% and 4.3%, respectively (P<0.001). The median duration of response was 28.6 months and 21.2 months, respectively.
In addition, KRd improved global health-related quality of life compared with Rd over 18 cycles of treatment (P=0.0001).
‘Reassuring’ toxicity data
“In the discussion of adverse events,” Dr Stewart said, “it’s important to note that the median treatment duration was 88 weeks with KRd and 57 weeks with Rd.”
Most patients in each arm experienced at least one AE—96.9% in the KRd arm and 97.2% in the RD arm.
In the KRd arm, 7.7% of patients died while still on treatment or within 30 days of receiving their last dose of treatment, as did 8.5% of patients in the Rd arm. The percentage of deaths attributable to AEs was 6.9% in both arms.
The rates of treatment discontinuation were 69.9% in the KRd arm and 77.9% in the Rd arm. More patients discontinued treatment due to disease progression—39.8% in the KRd arm and 50.1% in the Rd arm—than to AEs—15.3% in the KRd arm and 17.7% in the Rd arm.
The most common grade 3 or higher hematologic treatment-emergent AEs (in the KRd and Rd arms, respectively) were neutropenia (29.6% vs 26.5%), anemia (17.9% vs 17.2%), and thrombocytopenia (16.6% vs 12.3%).
The most common grade 3 or higher nonhematologic treatment-emergent AEs (in the KRd and Rd arms, respectively) were hypokalemia (9.4% vs 4.9%), fatigue (7.7% vs 6.4%), and diarrhea (3.8% vs 4.1%).
Other treatment-emergent AEs of any grade (in the KRd and Rd arms, respectively) included dyspnea (19.4% vs 14.9%), hypertension (14.3% vs 6.9%), acute renal failure (8.4% vs 7.2%), cardiac failure (6.4% vs 4.1%), ischemic heart disease (5.9% vs 4.6%), and peripheral neuropathy (17.1% vs 17.0%).
“The results [are] very reassuring with respect to cardiac and renal events, which were reported at rates consistent with, or even lower than, those reported in prior studies of single-agent carfilzomib or more heavily pretreated patients,” Dr Stewart said.
“Based on the results of this phase 3 trial, I think it’s fair to say that KRd could represent a new standard of care in relapsed multiple myeloma.” ![]()
SAN FRANCISCO—Trial results suggest a 3-drug regimen could represent a new standard of care for relapsed multiple myeloma (MM), according to a speaker at the 2014 ASH Annual Meeting.
In the phase 3 ASPIRE trial, patients who received combination carfilzomib, lenalidomide, and dexamethasone (KRd) had superior progression-free survival compared to patients who received only lenalidomide and dexamethasone (Rd).
There was a trend toward improved overall survival with KRd as well.
Patients who received KRd did experience more adverse events (AEs), but fewer patients discontinued treatment due to AEs in the KRd arm than in the Rd arm.
Keith Stewart, MBChB, of the Mayo Clinic in Arizona, presented these results at the meeting as abstract 79. The data were published in The New England Journal of Medicine as well. The trial was sponsored by Onyx Pharmaceuticals, Inc., the company developing carfilzomib.
ASPIRE included 792 patients with relapsed MM who had received 1 to 3 prior treatment regimens. Patients were randomized to treatment with KRd (n=396) or Rd (n=396), and baseline characteristics were similar between the arms.
“There was a slight preponderance of patients over the age of 65 in the Rd arm of the trial,” Dr Stewart noted. “Conversely, there were more patients on the Rd arm of the trial who had lower-risk cytogenetics.”
“Patients were also well-balanced for baseline exposure to prior therapies. Prior therapies included transplant in 55% of patients, bortezomib in two-thirds of patients, and lenalidomide in 20% of patients—again, equal in both arms of the trial.”
All patients received a standard dosing schedule of lenalidomide (25 mg on days 1-21) and low-dose dexamethasone (40 mg on days 1, 8, 15, and 22).
Patients in the KRd arm also received carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1 and 27 mg/m2 thereafter). They received a 10-minute infusion of the drug on days 1, 2, 8, 9, 15, and 16. Carfilzomib was not given on days 8 and 9 in cycles 13 to 18 and not administered beyond 18 cycles.
‘Unprecedented’ results
The study’s primary endpoint was progression-free survival. And results showed progression-free survival was significantly longer in the KRd arm than in the Rd arm—26.3 months and 17.6 months, respectively (hazard ratio=0.69; P<0.0001).
“Progression-free survival was significantly improved by 8.7 months with KRd,” Dr Stewart noted. “In a phase 3 clinical trial setting, this is unprecedented.”
“In all prespecified subgroups, the advantage of KRd in progression-free survival was maintained. That includes age, international staging system, and prior exposure to either bortezomib or lenalidomide, or both drugs.”
The secondary endpoints of the trial were overall survival, overall response rate, duration of response, health-related quality of life, and safety.
The data for median overall survival are not yet mature based on the prespecified statistical boundary at the interim analysis (P=0.005). However, there was a trend in favor of KRd (hazard ratio, 0.79; P=0.018).
The overall response rate was 87.1% with KRd and 66.7% with Rd (P<0.0001), and the complete response rates were 14.1% and 4.3%, respectively (P<0.001). The median duration of response was 28.6 months and 21.2 months, respectively.
In addition, KRd improved global health-related quality of life compared with Rd over 18 cycles of treatment (P=0.0001).
‘Reassuring’ toxicity data
“In the discussion of adverse events,” Dr Stewart said, “it’s important to note that the median treatment duration was 88 weeks with KRd and 57 weeks with Rd.”
Most patients in each arm experienced at least one AE—96.9% in the KRd arm and 97.2% in the RD arm.
In the KRd arm, 7.7% of patients died while still on treatment or within 30 days of receiving their last dose of treatment, as did 8.5% of patients in the Rd arm. The percentage of deaths attributable to AEs was 6.9% in both arms.
The rates of treatment discontinuation were 69.9% in the KRd arm and 77.9% in the Rd arm. More patients discontinued treatment due to disease progression—39.8% in the KRd arm and 50.1% in the Rd arm—than to AEs—15.3% in the KRd arm and 17.7% in the Rd arm.
The most common grade 3 or higher hematologic treatment-emergent AEs (in the KRd and Rd arms, respectively) were neutropenia (29.6% vs 26.5%), anemia (17.9% vs 17.2%), and thrombocytopenia (16.6% vs 12.3%).
The most common grade 3 or higher nonhematologic treatment-emergent AEs (in the KRd and Rd arms, respectively) were hypokalemia (9.4% vs 4.9%), fatigue (7.7% vs 6.4%), and diarrhea (3.8% vs 4.1%).
Other treatment-emergent AEs of any grade (in the KRd and Rd arms, respectively) included dyspnea (19.4% vs 14.9%), hypertension (14.3% vs 6.9%), acute renal failure (8.4% vs 7.2%), cardiac failure (6.4% vs 4.1%), ischemic heart disease (5.9% vs 4.6%), and peripheral neuropathy (17.1% vs 17.0%).
“The results [are] very reassuring with respect to cardiac and renal events, which were reported at rates consistent with, or even lower than, those reported in prior studies of single-agent carfilzomib or more heavily pretreated patients,” Dr Stewart said.
“Based on the results of this phase 3 trial, I think it’s fair to say that KRd could represent a new standard of care in relapsed multiple myeloma.” ![]()
Mouse model reveals insight into CLL relapse

Researchers say they have discovered why patients with chronic lymphocytic leukemia (CLL) often relapse.
Using a mouse model, the team demonstrated that crosstalk between leukemic cells and a group of stromal cells in the spleen is crucial for tumor growth.
The group also found a way to prevent leukemic cell proliferation and stop the cells from entering the spleen, thereby identifying new targets for future therapies in CLL.
Kristina Heinig, of Max Delbrück Center for Molecular Medicine in Berlin, Germany, and her colleagues reported these findings in Cancer Discovery.
The team theorized that the processes that normally regulate the migration of B lymphocytes into the B-cell follicle are also the reason for the migration of leukemia cells into the lymphoid organs. Hence, within the B-cell follicle, the survival and growth of malignant B cells may depend on the contact of leukemia cells with follicular dendritic cells (FDCs).
The researchers validated this theory with their mouse model. They found the chemokine CXCL13 and its receptor, CXCR5, on the surface of the leukemia cells are needed to ensure that leukemia cells can reach the spleen. With the aid of this homing receptor, the cancer cells are lured into the B-cell follicle of the spleen, where the FDCs secrete CXCL13.
When the researchers blocked CXCR5 in the mice, the leukemia cells could no longer migrate into the stromal cell niche and proliferated much more slowly.
In a second step, the group studied the consequences of the interaction between malignant B cells and the FDCs in the B-cell follicle. They found the close contact between the leukemia cells and the FDC network stimulates the cancer cells to increasingly produce another signaling substance, lymphotoxin.
The lymphotoxin binds to the lymphotoxin-beta receptor on the FDCs, which then increasingly secrete CXCL13. This creates a positive feedback loop because CXCL13 plays a major role in the recruitment of leukemia cells in the B-cell follicles.
The FDCs also provide growth factors that promote the proliferation of leukemia cells in the stromal niche.
When the researchers inhibited the binding of the lymphotoxin to the lymphotoxin-beta receptor on the FDCs with an immunologically active substance, they were able to end this ping-pong match between leukemia cells and the FDCs and dramatically reduce tumor growth.
The team thus identified two different targets that may complement the chemotherapy currently used to treat CLL. The first is blocking the chemokine/homing receptor CXCR5 on the leukemia cells, which prevents the cancer cells from lodging in the B-cell follicle.
The second is blocking the lymphotoxin-beta receptor on the FDCs so the reciprocal crosstalk between the leukemia cells and the FDCs is interrupted and tumor development is reduced.
From the results of their study, the researchers infer that chemotherapies already in clinical use combined with immune therapies that interrupt the crosstalk between leukemia cells and the FDCs may be beneficial.
This combination could prevent the residual leukemia cells that have escaped chemotherapy or radiation therapy from recovering in the stromal cell niche and from triggering a relapse. ![]()

Researchers say they have discovered why patients with chronic lymphocytic leukemia (CLL) often relapse.
Using a mouse model, the team demonstrated that crosstalk between leukemic cells and a group of stromal cells in the spleen is crucial for tumor growth.
The group also found a way to prevent leukemic cell proliferation and stop the cells from entering the spleen, thereby identifying new targets for future therapies in CLL.
Kristina Heinig, of Max Delbrück Center for Molecular Medicine in Berlin, Germany, and her colleagues reported these findings in Cancer Discovery.
The team theorized that the processes that normally regulate the migration of B lymphocytes into the B-cell follicle are also the reason for the migration of leukemia cells into the lymphoid organs. Hence, within the B-cell follicle, the survival and growth of malignant B cells may depend on the contact of leukemia cells with follicular dendritic cells (FDCs).
The researchers validated this theory with their mouse model. They found the chemokine CXCL13 and its receptor, CXCR5, on the surface of the leukemia cells are needed to ensure that leukemia cells can reach the spleen. With the aid of this homing receptor, the cancer cells are lured into the B-cell follicle of the spleen, where the FDCs secrete CXCL13.
When the researchers blocked CXCR5 in the mice, the leukemia cells could no longer migrate into the stromal cell niche and proliferated much more slowly.
In a second step, the group studied the consequences of the interaction between malignant B cells and the FDCs in the B-cell follicle. They found the close contact between the leukemia cells and the FDC network stimulates the cancer cells to increasingly produce another signaling substance, lymphotoxin.
The lymphotoxin binds to the lymphotoxin-beta receptor on the FDCs, which then increasingly secrete CXCL13. This creates a positive feedback loop because CXCL13 plays a major role in the recruitment of leukemia cells in the B-cell follicles.
The FDCs also provide growth factors that promote the proliferation of leukemia cells in the stromal niche.
When the researchers inhibited the binding of the lymphotoxin to the lymphotoxin-beta receptor on the FDCs with an immunologically active substance, they were able to end this ping-pong match between leukemia cells and the FDCs and dramatically reduce tumor growth.
The team thus identified two different targets that may complement the chemotherapy currently used to treat CLL. The first is blocking the chemokine/homing receptor CXCR5 on the leukemia cells, which prevents the cancer cells from lodging in the B-cell follicle.
The second is blocking the lymphotoxin-beta receptor on the FDCs so the reciprocal crosstalk between the leukemia cells and the FDCs is interrupted and tumor development is reduced.
From the results of their study, the researchers infer that chemotherapies already in clinical use combined with immune therapies that interrupt the crosstalk between leukemia cells and the FDCs may be beneficial.
This combination could prevent the residual leukemia cells that have escaped chemotherapy or radiation therapy from recovering in the stromal cell niche and from triggering a relapse. ![]()

Researchers say they have discovered why patients with chronic lymphocytic leukemia (CLL) often relapse.
Using a mouse model, the team demonstrated that crosstalk between leukemic cells and a group of stromal cells in the spleen is crucial for tumor growth.
The group also found a way to prevent leukemic cell proliferation and stop the cells from entering the spleen, thereby identifying new targets for future therapies in CLL.
Kristina Heinig, of Max Delbrück Center for Molecular Medicine in Berlin, Germany, and her colleagues reported these findings in Cancer Discovery.
The team theorized that the processes that normally regulate the migration of B lymphocytes into the B-cell follicle are also the reason for the migration of leukemia cells into the lymphoid organs. Hence, within the B-cell follicle, the survival and growth of malignant B cells may depend on the contact of leukemia cells with follicular dendritic cells (FDCs).
The researchers validated this theory with their mouse model. They found the chemokine CXCL13 and its receptor, CXCR5, on the surface of the leukemia cells are needed to ensure that leukemia cells can reach the spleen. With the aid of this homing receptor, the cancer cells are lured into the B-cell follicle of the spleen, where the FDCs secrete CXCL13.
When the researchers blocked CXCR5 in the mice, the leukemia cells could no longer migrate into the stromal cell niche and proliferated much more slowly.
In a second step, the group studied the consequences of the interaction between malignant B cells and the FDCs in the B-cell follicle. They found the close contact between the leukemia cells and the FDC network stimulates the cancer cells to increasingly produce another signaling substance, lymphotoxin.
The lymphotoxin binds to the lymphotoxin-beta receptor on the FDCs, which then increasingly secrete CXCL13. This creates a positive feedback loop because CXCL13 plays a major role in the recruitment of leukemia cells in the B-cell follicles.
The FDCs also provide growth factors that promote the proliferation of leukemia cells in the stromal niche.
When the researchers inhibited the binding of the lymphotoxin to the lymphotoxin-beta receptor on the FDCs with an immunologically active substance, they were able to end this ping-pong match between leukemia cells and the FDCs and dramatically reduce tumor growth.
The team thus identified two different targets that may complement the chemotherapy currently used to treat CLL. The first is blocking the chemokine/homing receptor CXCR5 on the leukemia cells, which prevents the cancer cells from lodging in the B-cell follicle.
The second is blocking the lymphotoxin-beta receptor on the FDCs so the reciprocal crosstalk between the leukemia cells and the FDCs is interrupted and tumor development is reduced.
From the results of their study, the researchers infer that chemotherapies already in clinical use combined with immune therapies that interrupt the crosstalk between leukemia cells and the FDCs may be beneficial.
This combination could prevent the residual leukemia cells that have escaped chemotherapy or radiation therapy from recovering in the stromal cell niche and from triggering a relapse. ![]()
Inpatient Pediatric Service Redesign
Given its positive effects on improving effectiveness and efficiency, Lean Six Sigma (LSS) is a business approach that is receiving a great deal of attention in the healthcare industry.[1, 2, 3, 4, 5, 6, 7] Although there are differences between Lean and Six Sigma, at their core they are both customer‐centered, quality methodologies designed to improve process efficiency and product quality through waste elimination, creating standardized work and reducing variation.[8]
Six Sigma is a rigorous problem‐focused process improvement method that focuses on defect removal, variation reduction, and customer satisfaction that relies heavily on statistical analysis. It includes 5 steps: define, measure, analyze, improvement, and control.[7, 8] Six Sigma assumes through variation reduction, defect removal, and meeting customer specifications, the performance of the organization can be improved and also meet the requirements of the customer.[8]
Lean is more process‐focused. It places emphasis on creating flow by removing waste and getting the steps of any given process in the right sequence.[8] In Lean terms, waste is defined as anything that the customer does not value and anything that is not done right the first time.[9] This category of waste is termed nonvalue adding and unnecessary. It is estimated that 30% to 50% of all steps of hospital processes are nonvalue adding and unnecessary, and therefore can be defined as waste.[10] Lean identifies 8 different types of nonvalue adding and unnecessary wastes. They are defects and rework, overproduction, waiting, nonutilization of resources, transport, inventory, motion, and extra processing. Waste creates delays that negatively impact patient care and reduce healthcare productivity.[10] Therefore, it makes sense to apply Lean concepts of waste identification and elimination to improve process efficiency. For example, when a facility is at or exceeds its bed capacity, any delay in discharge creates throughput delays throughout the hospital.[5] Discharge delays often result in emergency department (ED) overcrowding, and also affects a hospital's ability to accommodate internal downgrades and outside referrals in a timely fashion.[11, 12] However, because the sequence of steps of the discharge process is variable and not standardized, the goal to achieve early discharges remains elusive.[13]
There are emerging data to support that current rounding censuses exceed most hospitalist's abilities to deliver safe and efficient care.[12, 14, 15, 16] It is unclear what that threshold should be, but the current industry standard has nonacademic hospitalists seeing 15 patients per day. Therefore, high patient censuses could be contributing to delays in patient discharge times that effect hospital throughput. We speculated that by implementing a lean, quick‐strike approach[17] designed to improve the sequencing of housestaff, attending, and nursing work by eliminating the wastes of rework, waiting, extra processing, and nonutilization of physician resources by restaffing, we could improve patient discharge times. We augmented the intervention by creating standardized workflow expectations, a discharge checklist, and implemented daily interdisciplinary discharge planning huddles.
We hypothesized these interventions would improve the median time of discharge order entry and time of patient discharge. Primary outcome measures were: (1) the change in time of discharge order and discharge time and (2) the proportion of patients discharged before noon and 2 pm. Secondary outcomes that were used as balance measures were length of stay (LOS) and 7‐day, 14‐day, and 30‐day readmission rates.
METHODS
Study Design
This was a prospective quality improvement intervention with concurrent controls aimed to determine if discharge efficiency could be improved by load‐balancing our service line with existing faculty and residents, creating daily standard work using a discharge checklist and interdisciplinary huddles (see Supporting Figure 1 through Supporting Figure 3 in the online version of this article). All discharge data were collected as part of our medical center's Department of Logistics standard data collection procedures using solutions from TeleTracking Technologies, Inc. (Pittsburgh, PA). All patients discharged Monday through Friday from the pediatric hospitalist service prior to the 6‐month high‐census period (before intervention) and the 6‐month high‐census period (intervention period) were included in the study. To serve as our control, we collected the same discharge data during the same time periods for the remaining services of the children's hospital. This study was approved by Penn State Hershey Medical Center's institutional review board.
Study Setting
The study was conducted at the Penn State Hershey Children's Hospital (PSHCH), which is a physically free‐standing 133‐bed university‐based tertiary care hospital located in central Pennsylvania. The hospital has 36 pediatric medical/surgical beds located in 2 units (1 general and 1 intermediate care). PSHCH performs approximately 4100 admissions per year, of which approximately 1100 are performed by the Division of Pediatric Hospital Medicine. Our division is composed of 8 academic hospitalists with 1 to 20+ years' experience. Historically, the months of October through April are months when our service‐line has average daily censuses (ADC) that routinely exceed 12 patients per hospitalist. During these months, the median times patient discharge orders are placed and patient discharges occur historically approach 2 pm and 4 pm, respectively, and exceed our internal benchmark by 2 hours. Discharges from the remaining medical and surgical service lines at PSHCH that occurred Monday through Friday during the concurrent pre‐ and postintervention time periods served as the control group.
Needs Assessment and LSS
Traditionally, morning patient rounds are allotted approximately 180 minutes. Therefore, a rounding team can only be expected to spend 13 minutes or less per patient when the census exceeds 12 patients. The cycle time to perform 1 discharge using our electronic medical record is approximately 20 minutes, which is almost 10 minutes longer than the allotted time per patient. During high‐census months, our service averages 4 to 5 discharges per day. To accommodate performing discharges during rounds would require spending 80 to 100 minutes of the 180 available minutes. This would leave only 80 to 100 minutes to see the remaining 8 to 10 patients. As a result of these constraints, discharges are typically completed by the residents in unsupervised batches each afternoon following the noon conference (Figure 1A).
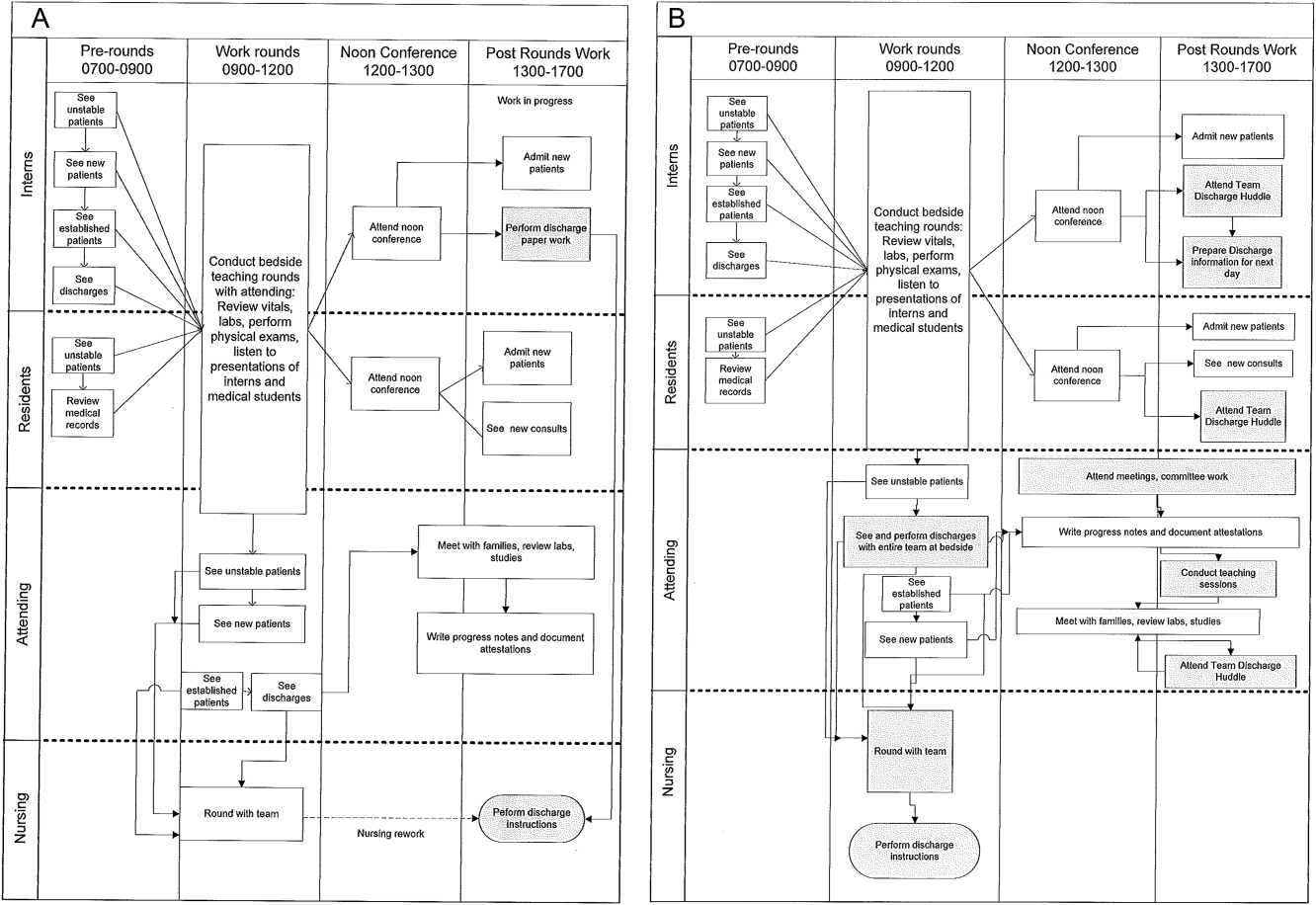
Because LSS focuses on eliminating nonvalue adding and unnecessary waste by load balancing processes and minimizing batching tasks,[8] this approach should lead one to question whether the current rounding model that requires 1 attending to see >12 high‐acuity patients with a maximum of 13 minutes per patient is system design flaw that leads to errors and inefficiency,[16] Theoretically, having an additional attending present would allow teams to resequence the work on smaller batches of patients and double the time to spend on each patient. This could create the opportunity to do value‐added work at the bedside in the presence of the family and nurse and eliminate the amount of nursing rework and time spent as work in progress on dischargeable patients (Figure 1).
Additionally, improving discharge efficiency creates virtual beds. Virtual beds permit hospitals to accommodate additional admissions despite operating with a fixed‐bed capacity. A way to calculate virtual beds is to calculate the reduction in LOS, and multiply that by the number of admissions per year divided by 36518 (see Supporting Figure 4 in the online version of this article). Our study was intended to determine the impact of discharge efficiency on this metric.
Intervention
We re‐structured our service line in a way that would balance both physician workload needs and patient expectations. To accomplish this, off service attendings were reallocated to round with a smaller resident team on fewer patients for the duration of the 6‐month study. Each member of the division agreed to work an average of 3 more weeks per year. One work day was estimated to be approximately 10 hours and 1 work week equaled 5 days, which asked for 150 hours of additional work per year. Because there increases in functional FTEs, the 2 teams consolidated into 1 team each weekend, to meet the group requirement that this model not result in additional weekend coverage. A balanced workload also theoretically allows the physician to spend more time at the bedside in direct patient care and resident education activities/observations.
In addition to reallocating physician and resident resources, our model created standard work expectations to reduce the variations in physician work sequences that can account for delayed discharge orders and delayed discharges, which is also an LSS principle. The intervention consisted of 3 changes: (1) fundamentally altering the composition of the rounding teams to optimize the provider: patient ratio; (2) defining rounding standards to expedite discharges; and (3) establishing a daily predischarge planning process.
The preintervention team typically had 1 attending, 1 to 2 senior residents, and 2 to 3 interns. The intervention period required creating 2 independently functioning teams, each composed of 1 hospitalist attending, and a minimum of 1 senior and 1 intern. The intervention occurred November through April, when the censuses predictably exceed 12 patients for the rounding attending. Because both teams functioned independently, all of the patients were divided equally between the 2 teams. Each team carried a panel of patients that included new, established, and dischargeable patients (Figure 1). We did not compare the number of provider handoffs before and during the intervention or time spent per patient.
Because the intervention required increasing the number of weeks on‐service by 2 to 3weeks per physician to reduce clinical work time, it meant redeploying previously off‐service attendings to coincide with peak demands. This aspect of the intervention made group buy‐in mandatory. The group agreed to distribute the predictably heavy workload that usually falls on 1 attending by adding a second attending for the busiest 6 months of the year. Our division voted unanimously to adopt this model despite the increase in service time, as long as weekend coverage was not increased.
As part of the intervention, we created standard work expectations within our division to (1) start rounds on dischargeable patients who were identified the prior evening during the (2) interdisciplinary huddle, and (3) have the entire departure process completed at the bedside using a discharge checklist (see Supporting Figure 1 through Figure 3 in the online version of this article). The expectations included a standard script for beginning rounds, selecting patients who could be discharged first, and completing all necessary discharge computer work at the bedside, before proceeding to the next patient. The daily predischarge huddle was instituted each afternoon to prepare discharges that were expected to occur the following day. The huddles were attended by care coordinators, social workers, and both medical teams. During the huddle, the team discussed anticipated discharges, scheduled follow‐up appointments and testing, faxed necessary prescriptions, and arranged any needed home services.
Inclusion and Exclusion Criteria for Patients
All patients discharged from the pediatric hospitalist inpatient service between Monday and Friday from April 8, 2013 to October 25, 2013 (preimplementation cohort) and October 28, 2013 to April 18, 2014 (postimplementation cohort) were eligible for inclusion. This included admitted patients and observation status patients. Patients discharged from the remaining PSHCH medical and surgical service lines were included in the control group analysis using the above criteria.
OUTCOME MEASURES
Throughput and Patient‐Level Outcomes
Primary outcomes included (1) time of electronic discharge order placement, (2) actual patient discharge time, (3) proportion of patients discharged before noon and 2 pm, (4) 7‐day, 14‐day, and 30‐day readmission rates, (5) length of stay (LOS), and (6) average daily census (ADC).
Statistical Analysis
The null hypothesis was that there would be no difference in discharge order time, discharge time, LOS, readmission rates, and daily discharges in the preintervention group compared to the intervention group. For time of order placement and actual patient discharge, the significance was assessed using Wilcoxon rank sum test and expressed as median time among the groups. Patient discharge before noon/2 pm was assessed by a logistic regression model. The predictor being the intervention group with the results expressed as odds ratios of discharge before noon/2 pm comparing the intervention group to the preintervention group. Readmission rates were assessed using a [2] test to see if there was a significant difference from what would be expected. Last, LOS and ADC were assessed by a Student t test and expressed as the means. The data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
For our division's service line, both the ADC and number of patients discharged per day were significantly higher during the intervention months (Table 1). By comparison, the control group had a significantly lower ADC and lower average of discharges per day in the intervention time period. The new model permitted the teams to enter discharge orders earlier in the day, which ultimately lead to earlier patient discharges. The additive effect of the 3 interventions had a statistically significant effect on process efficiency metrics (Table 1). The median discharge order entry time decreased by 200 minutes from 14:05 to 10:45, and the median time of patient discharge decreased by 93 minutes from 15:48 to 14:15. By comparison, the median time of discharge order entry decreased 13:13 to 12:56 pm, but the median time of discharge increased 5 minutes 14:45 versus 14:50 in the control group. A significantly higher proportion of patients were discharged by noon (27% vs 14%; P<0.0001; odds ratio [OR]:2.2; 95% confidence interval [CI]: 1.6‐3.1) and by 2 pm during the intervention period (47% vs 30%; P<0.0001; OR: 2.1; 95% CI: 1.6‐2.7). There was no observed difference in the proportion of patients who were discharged by noon or 2 pm in the control group. Finally, in the intervention group, approximately 50% of patients had discharge orders entered before noon compared to 23% in the control group (Figure 2). The intervention demonstrated statistical significance in shifting the time of discharge order entry and the time of patient discharge when compared to the relatively less burdened PSHCH control group (Figures 2 and 3). As seen in Figure 4, the results were sustained for the duration of the study and appeared to improve throughout intervention. Finally, readmission rates at 7, 14, and 30 days postdischarge and LOS were not negatively affected (Table 1) in either the intervention or control group.
| Outcomes | Experimental Model | Control Group | ||||
|---|---|---|---|---|---|---|
| Preintervention, n=421 | Intervention, n=552 | P Value | Preintervention, n=1,390 | Intervention, n=1,146 | P Value | |
| Average daily census | 9.7 | 12.4 | <0.0001 | 45.9 | 43.4 | 0.002 |
| Discharges per day | 3.1 | 4.5 | <0.00001 | 9.5 | 9.2 | 0.419 |
| Average length of stay | 3.1 | 3.0 | 0.864 | 6.3 | 5.9 | 0.714 |
| Discharge order time, median | 14:05 | 10:45 | <0.0001 | 13:13 | 12:56 | 0.053 |
| Discharge from hospital, median | 15:48 | 14:15 | <0.0001 | 14:45 | 14:50 | 0.113 |
| Patients discharged before noon | 59 (14%) | 147 (27%) | <0.0001 | 176 (13%) | 170 (15%) | 0.138 |
| Patients discharged before 2 pm | 128 (30%) | 261 (47%) | <0.0001 | 519 (38%) | 447 (39%) | 0.512 |
| 7‐day readmission rates | 3.1% | 3.5% | 0.965 | 6.7% | 6.8% | 0.970 |
| 14‐day readmission rates | 5.8% | 5.8% | 0.981 | 12.0% | 13.5% | 0.301 |
| 30‐day readmission rates | 9.4% | 9.1% | 0.703 | 20.0% | 20.6% | 0.705 |
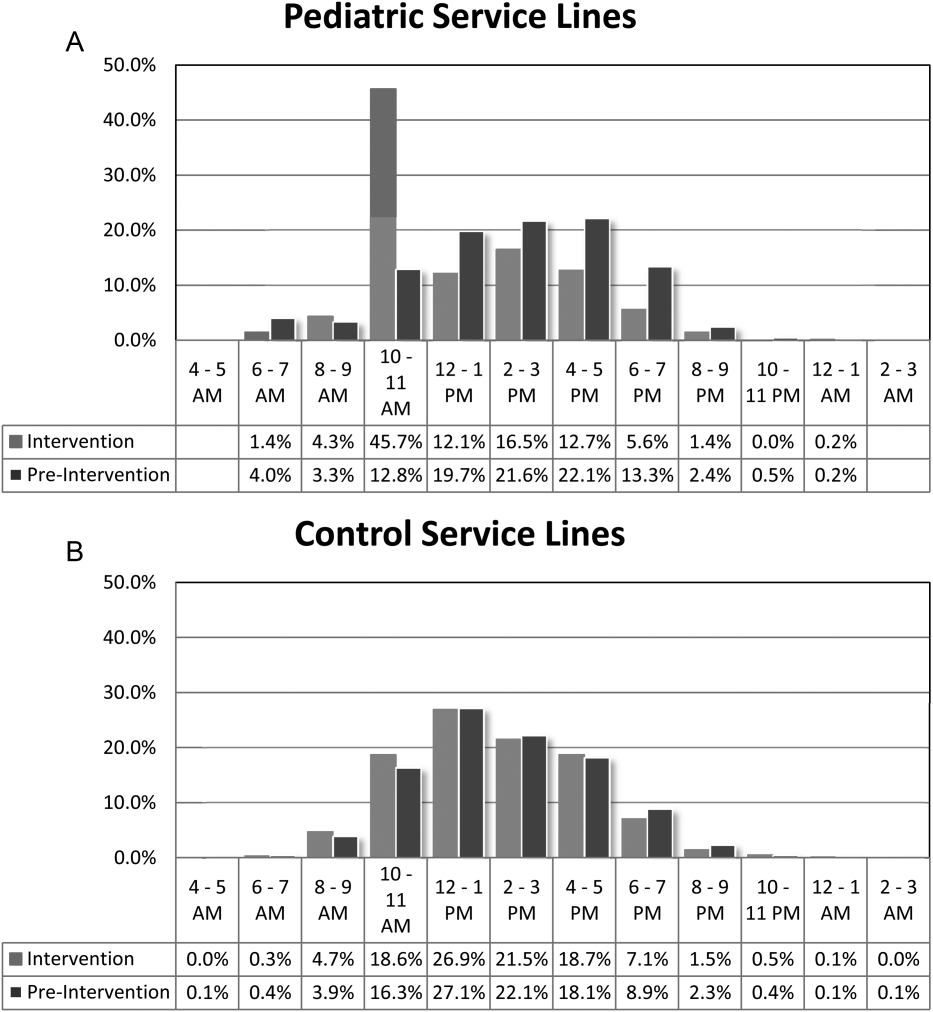
DISCUSSION
We demonstrated a statistically significant and what appears to be a sustainable improvement in median discharge order times, discharge times, and proportion of discharges by noon and 2 pm. Ours was the only service line in our medical center to achieve a median time discharge before our institution's internal metric of 2 pm and maintain it for 3 consecutive months. Additionally, the process demonstrated consistent performance independent of the varying styles and experience of the rounding attending during the busiest months of the year without incurring a negative impact on LOS or readmission rates.
Although our intervention demonstrated statistical significance in shifting the discharge distribution curves by almost 2 hours, more relevant is its potential clinical and financial impact. First, it puts our hospital in compliance with the Joint Commission's recommendations standard LD.04.03.1, stipulating that hospitals measure and set goals for mitigating and managing the flow of patients though the hospital. Second, our findings confirm the results of earlier studies suggesting that shifting discharge times could likely be achieved without the additional staff, but with alterations in staff shift scheduling.[11] Third, by doing required discharge work at the bedside and making it available earlier in the day, every day, we consistently reduced patient waiting along the entire supply chain.
Advancing the discharge time creates virtual beds that allow our facility to theoretically accommodate new patients. Using the calculation in the Methods section (see Supporting Figure 4 in the online version of this article) on how to calculate virtual beds, we determined that our intervention created between 0.30 and 0.38 virtual beds in a hospital with only 72 beds. We calculated that this would create 6.8 more open bed hours per day, 74 additional patient days per year, and assuming patients were waiting for the beds and rapid bed turnover, our intervention theoretically created the capacity to accommodate approximately 25 additional admissions per year (see Supporting Figure 4 in the online version of this article). As the only children's hospital in the region, this intervention will enable our organization to provide timelier access and possibly reduce time sensitive medical errors.
Timelier evaluations also have revenue potential by eliminating lost referrals, thus turning waste into value. When comparing the previous year's high‐census monthsOctober through Marchthere were 20 lost referrals due to lack of bed capacity, as compared to zero lost referrals during our intervention period. By accommodating these 20 additional admissions, we estimated this generated between $275,000 and $412,000 dollars in additional revenue without additional resources but simply staffing to demand.
Finally, when we looked at patient satisfaction metrics obtained through Press Ganey (PG), comparing the time periods we observed that overall satisfaction increased from the 91 percentile to the 94 percentile, trust in doctor increased from the 20 percentile to the 70 percentile, and would recommend this hospital to others increased from the 53 percentile to the 75 percentile. Interestingly, despite being a study that improved discharge efficiency, none of the discharge metrics gathered by PG improved. It is possible that this is a limitation of the PG survey, or could reflect the possibility that our new process exposed that our discharge order entry and discharge processes are misaligned.
When we surveyed the nursing staff and members of the division regarding whether or not to continue the intervention rounding model, 75% and 100%, respectively, voted in favor of continuing with the intervention model. Unfortunately, housestaff satisfaction was not measured for this study.
Despite more weeks in the hospital, but because there was better process sequencing, our providers indicated that because the workload of the primary attending was reduced and the workload for the additional attending was light, there was ample time to engage in afternoon nonclinical activities (Figure 1B). In fact, several division members assumed departmental and educational leadership positions, and others volunteered to facilitate highly valued, but unsubsidized, afternoon medical student and resident teaching sessions that occurred solely as a result of the resequenced and redistributed clinical load.
There are limitations to this study. First, because 3 interventions were implemented simultaneously, it is difficult to identify which component of the intervention was the primary driver for the measured differences. It is conceivable the proactive discharge planning that occurred during the afternoon predischarge planning huddles allowed the teams to complete discharge requirements the night before anticipated discharge therefore expediting the next morning's discharge. A second limitation was not simultaneously comparing the traditional rounding structure with the experimental model. One could argue that the improved efficiency we observed was not due to any of the interventions and represents secular trends that all residents' teams experience through the course of the year as they get more adept at performing patient discharges. However, when we compare our performance to the control group performance, this efficiency trend was not present. Additionally, it was possible that attendings were so result focused that they delayed discharges if the 2 pm discharge goal was missed for that day and planned for early discharges the following morning. If this behavior occurred, this would likely have been reflected as increases in our LOS data, however this was not observed. Third, because our preintervention data reflected discharge behaviors during a low‐census period, it is possible that there was less urgency to discharge patients when bed capacity issues did not exist. Comparing the intervention period to a period when censuses are similar would better address this issue. Finally, although we assert that attending workload is a fundamental waste‐producing constraint in the discharge process, this study did not determine what the optimal patient census should be.
Most hospitalists struggle with finding a balance of meeting patient quality and administrative productivity demands.[16] Hospitalists at academic medical centers have the added demand of maintaining their educational mission. Since 2001, the Institute of Medicine[19] has advocated process re‐engineering using more patient‐centered approaches. A recent study found that when hospitals reach capacity, the excess workload placed on internal medicine hospitalists reduces efficiency and increases costs.[16] Interestingly, in a study conducted by McMahon et al., they found that reducing team censuses by 50%, resident educational outcomes can be improved.[20] Similar to this study, our study reduced attending workload by 50% with the goal to assess the impact on discharge efficiency rather than educational outcomes. Also similar to that study, we radically altered the operational model in which physicians historically had functioned.[14, 20] Because the rounding structures in both studies reduced patient:provider ratios we believe that our model will successfully balance education, patient quality, and productivity.
LSS, when thoughtfully applied to the problems we face, could be part of the solution. It delivers quick results without large capital investments, by identifying and implementing high‐leverage changes that value a creative solution before a capital investment. One of the strengths of this model is that it does not require substantial financial investment to produce these outcomes. Because the morning clinical loads were more evenly distributed during the busiest months of the year, our division members were able to engage in nonclinical duties and teaching sessions, both of which often required afternoon commitments, but permitted us to balance work and professional achievement (Figure 1B). Finally, as part of any new process, one must consider the factors that influence its sustainability: provider level satisfaction, impact of the process change, and remuneration. Because the intervention reduced lost referrals, the departmental and institutional leadership agreed to financially incentivize the value‐generating potential this intervention had on increasing patient access by facilitating organizational throughput. Therefore, having met the three aforementioned elements, we believe this model is sustainable.
Although many studies remain results focused with aims at documenting how hospital processes fail when overburdened, our study takes a novel process‐focused approach to look at how processes can excel during periods of high demands, simply by reallocating existing resources.
Medicine is in the midst of multiple paradigm shifts involving resident work hour reduction, public safety reporting, reimbursement constraints, and value‐driven care, to name a few. Whether we take a resident or patient‐centered approach, it seems highly unlikely that the current approaches will meet these demands without making significant changes in how we deliver care. Next steps should include construction of a value stream map (VSM), with the input of all of the process stakeholders, that diagrams the entire discharge process. The VSM should highlight all nonvalue adding steps and eliminate them. They are likely a contributing cause to the disproportionate reduction in time of discharge order entry (200 minutes) versus actual discharge (93 minutes) seen in our study. Future work needs to establish the generalizability and sustainability of this model across other hospital service lines. Future studies should establish if this model has sustained impact on patient, provider, and resident satisfaction and overall system efficiency (ED boarding), with aims to quantify the revenue generating potential that occurs through waste elimination.
We close with the following thought: [T]o ask people to make different decisions without fundamentally changing the equation presented to them is wrong. If we wish to change the types of decisions our people make, we owe it to them to design and build processes that will put them in a position to succeed.[21]
Acknowledgements
The authors recognize the contributions made by members of the Division of Pediatric Hospital Medicine at PSHCH, without which this project would not have been possible. They are: Drs. Marta Biderman, Anika Kumar, Margaret Mikula, Chris O'Hara, Brandon Smith, and Ron Williams, and Heidi Wolf, and Lyndsay Gardener, CRNP. This project would not have been possible without the help from Brenda Ruhl, Manjula Narasimhan, and Heather Boyle from the Department of Logistics. Finally, the authors would like to thank the following for their thoughtful reviews of this manuscript: Drs. Lisa Scalzi, Barbara Ostrov, Jed Gonzalo, Chris Sciamanna, and Matt Wain.
Disclosures: Nothing to report.
- . Theory of constraints—a review of the philosophy and its applications. Int J Oper Prod Man. 1998;18(4):336–355.
- , , , et al. Use of lean and six sigma methodology to improve operating room efficiency in a high‐volume tertiary‐care academic medical center. J Am Coll Surg. 2011;213(1):83–92; discussion 93–84.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , . Assessing the evidence of Six Sigma and Lean in the health care industry. Qual Manag Health Care. 2010;19(3):211–225.
- , , , et al. Redesigning care at the Flinders Medical Centre: clinical process redesign using “lean thinking”. Med J Aust. 2008;188(6 suppl):S27–S31.
- , , , , . Impact of 5 years of lean six sigma in a University Medical Center. Qual Manag Health Care. 2012;21(4):262–268.
- , , , et al. A Lean Six Sigma quality improvement project to increase discharge paperwork completeness for admission to a comprehensive integrated inpatient rehabilitation program. Am J Med Qual. 2013;28(4):301–307.
- . How to compare Six Sigma, lean and the theory of constraints—a framework for choosing what's best for your organization. Qual Prog. 2002;35(3):73–78.
- , , , , . Application of lean manufacturing techniques in the Emergency Department. J Emerg Med. 2009;37(2):177–182.
- . Lean Hospitals: Improving Quality, Patient Safety, and Employee Engagement. 2nd ed. New York, NY: Productivity Press/Taylor 2012.
- , , , , , . The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186–196.
- , , , . Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Aust. 2012;24(5):510–517.
- . Need to speed up discharges? The pros and cons of putting discharges on the clock. Today's Hospitalist. 2013. Accessed November 2014. Available at: http://www.todayshospitalist.com/index.php?b=articles_read3(2):144–150.
- , . Hospitalist staffing requirements. Eff Clin Pract. 1999;2(3):126–130.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- . Accelerating Lean Six Sigma Results: How to Achieve Improvement Excellence in the New Economy. Plantation, FL: J. Ross Publishing; 2011.
- . “Virtual bed capacity” may offer revenue boost for hospitals. Healthcare Finance. Accessed October 2014. Available at: http://www.healthcarefinancenews.com/blog/virtual‐bed‐capacity‐may‐offer‐revenue‐boost‐hospitals. Published November 30, 2011.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , , . Evaluation of a redesign initiative in an internal‐medicine residency. N Engl J Med. 2010;362(14):1304–1311.
- John Kim and Associates website. Available at: http://www.johnkimconsulting.com. Accessed June 2014.
Given its positive effects on improving effectiveness and efficiency, Lean Six Sigma (LSS) is a business approach that is receiving a great deal of attention in the healthcare industry.[1, 2, 3, 4, 5, 6, 7] Although there are differences between Lean and Six Sigma, at their core they are both customer‐centered, quality methodologies designed to improve process efficiency and product quality through waste elimination, creating standardized work and reducing variation.[8]
Six Sigma is a rigorous problem‐focused process improvement method that focuses on defect removal, variation reduction, and customer satisfaction that relies heavily on statistical analysis. It includes 5 steps: define, measure, analyze, improvement, and control.[7, 8] Six Sigma assumes through variation reduction, defect removal, and meeting customer specifications, the performance of the organization can be improved and also meet the requirements of the customer.[8]
Lean is more process‐focused. It places emphasis on creating flow by removing waste and getting the steps of any given process in the right sequence.[8] In Lean terms, waste is defined as anything that the customer does not value and anything that is not done right the first time.[9] This category of waste is termed nonvalue adding and unnecessary. It is estimated that 30% to 50% of all steps of hospital processes are nonvalue adding and unnecessary, and therefore can be defined as waste.[10] Lean identifies 8 different types of nonvalue adding and unnecessary wastes. They are defects and rework, overproduction, waiting, nonutilization of resources, transport, inventory, motion, and extra processing. Waste creates delays that negatively impact patient care and reduce healthcare productivity.[10] Therefore, it makes sense to apply Lean concepts of waste identification and elimination to improve process efficiency. For example, when a facility is at or exceeds its bed capacity, any delay in discharge creates throughput delays throughout the hospital.[5] Discharge delays often result in emergency department (ED) overcrowding, and also affects a hospital's ability to accommodate internal downgrades and outside referrals in a timely fashion.[11, 12] However, because the sequence of steps of the discharge process is variable and not standardized, the goal to achieve early discharges remains elusive.[13]
There are emerging data to support that current rounding censuses exceed most hospitalist's abilities to deliver safe and efficient care.[12, 14, 15, 16] It is unclear what that threshold should be, but the current industry standard has nonacademic hospitalists seeing 15 patients per day. Therefore, high patient censuses could be contributing to delays in patient discharge times that effect hospital throughput. We speculated that by implementing a lean, quick‐strike approach[17] designed to improve the sequencing of housestaff, attending, and nursing work by eliminating the wastes of rework, waiting, extra processing, and nonutilization of physician resources by restaffing, we could improve patient discharge times. We augmented the intervention by creating standardized workflow expectations, a discharge checklist, and implemented daily interdisciplinary discharge planning huddles.
We hypothesized these interventions would improve the median time of discharge order entry and time of patient discharge. Primary outcome measures were: (1) the change in time of discharge order and discharge time and (2) the proportion of patients discharged before noon and 2 pm. Secondary outcomes that were used as balance measures were length of stay (LOS) and 7‐day, 14‐day, and 30‐day readmission rates.
METHODS
Study Design
This was a prospective quality improvement intervention with concurrent controls aimed to determine if discharge efficiency could be improved by load‐balancing our service line with existing faculty and residents, creating daily standard work using a discharge checklist and interdisciplinary huddles (see Supporting Figure 1 through Supporting Figure 3 in the online version of this article). All discharge data were collected as part of our medical center's Department of Logistics standard data collection procedures using solutions from TeleTracking Technologies, Inc. (Pittsburgh, PA). All patients discharged Monday through Friday from the pediatric hospitalist service prior to the 6‐month high‐census period (before intervention) and the 6‐month high‐census period (intervention period) were included in the study. To serve as our control, we collected the same discharge data during the same time periods for the remaining services of the children's hospital. This study was approved by Penn State Hershey Medical Center's institutional review board.
Study Setting
The study was conducted at the Penn State Hershey Children's Hospital (PSHCH), which is a physically free‐standing 133‐bed university‐based tertiary care hospital located in central Pennsylvania. The hospital has 36 pediatric medical/surgical beds located in 2 units (1 general and 1 intermediate care). PSHCH performs approximately 4100 admissions per year, of which approximately 1100 are performed by the Division of Pediatric Hospital Medicine. Our division is composed of 8 academic hospitalists with 1 to 20+ years' experience. Historically, the months of October through April are months when our service‐line has average daily censuses (ADC) that routinely exceed 12 patients per hospitalist. During these months, the median times patient discharge orders are placed and patient discharges occur historically approach 2 pm and 4 pm, respectively, and exceed our internal benchmark by 2 hours. Discharges from the remaining medical and surgical service lines at PSHCH that occurred Monday through Friday during the concurrent pre‐ and postintervention time periods served as the control group.
Needs Assessment and LSS
Traditionally, morning patient rounds are allotted approximately 180 minutes. Therefore, a rounding team can only be expected to spend 13 minutes or less per patient when the census exceeds 12 patients. The cycle time to perform 1 discharge using our electronic medical record is approximately 20 minutes, which is almost 10 minutes longer than the allotted time per patient. During high‐census months, our service averages 4 to 5 discharges per day. To accommodate performing discharges during rounds would require spending 80 to 100 minutes of the 180 available minutes. This would leave only 80 to 100 minutes to see the remaining 8 to 10 patients. As a result of these constraints, discharges are typically completed by the residents in unsupervised batches each afternoon following the noon conference (Figure 1A).

Because LSS focuses on eliminating nonvalue adding and unnecessary waste by load balancing processes and minimizing batching tasks,[8] this approach should lead one to question whether the current rounding model that requires 1 attending to see >12 high‐acuity patients with a maximum of 13 minutes per patient is system design flaw that leads to errors and inefficiency,[16] Theoretically, having an additional attending present would allow teams to resequence the work on smaller batches of patients and double the time to spend on each patient. This could create the opportunity to do value‐added work at the bedside in the presence of the family and nurse and eliminate the amount of nursing rework and time spent as work in progress on dischargeable patients (Figure 1).
Additionally, improving discharge efficiency creates virtual beds. Virtual beds permit hospitals to accommodate additional admissions despite operating with a fixed‐bed capacity. A way to calculate virtual beds is to calculate the reduction in LOS, and multiply that by the number of admissions per year divided by 36518 (see Supporting Figure 4 in the online version of this article). Our study was intended to determine the impact of discharge efficiency on this metric.
Intervention
We re‐structured our service line in a way that would balance both physician workload needs and patient expectations. To accomplish this, off service attendings were reallocated to round with a smaller resident team on fewer patients for the duration of the 6‐month study. Each member of the division agreed to work an average of 3 more weeks per year. One work day was estimated to be approximately 10 hours and 1 work week equaled 5 days, which asked for 150 hours of additional work per year. Because there increases in functional FTEs, the 2 teams consolidated into 1 team each weekend, to meet the group requirement that this model not result in additional weekend coverage. A balanced workload also theoretically allows the physician to spend more time at the bedside in direct patient care and resident education activities/observations.
In addition to reallocating physician and resident resources, our model created standard work expectations to reduce the variations in physician work sequences that can account for delayed discharge orders and delayed discharges, which is also an LSS principle. The intervention consisted of 3 changes: (1) fundamentally altering the composition of the rounding teams to optimize the provider: patient ratio; (2) defining rounding standards to expedite discharges; and (3) establishing a daily predischarge planning process.
The preintervention team typically had 1 attending, 1 to 2 senior residents, and 2 to 3 interns. The intervention period required creating 2 independently functioning teams, each composed of 1 hospitalist attending, and a minimum of 1 senior and 1 intern. The intervention occurred November through April, when the censuses predictably exceed 12 patients for the rounding attending. Because both teams functioned independently, all of the patients were divided equally between the 2 teams. Each team carried a panel of patients that included new, established, and dischargeable patients (Figure 1). We did not compare the number of provider handoffs before and during the intervention or time spent per patient.
Because the intervention required increasing the number of weeks on‐service by 2 to 3weeks per physician to reduce clinical work time, it meant redeploying previously off‐service attendings to coincide with peak demands. This aspect of the intervention made group buy‐in mandatory. The group agreed to distribute the predictably heavy workload that usually falls on 1 attending by adding a second attending for the busiest 6 months of the year. Our division voted unanimously to adopt this model despite the increase in service time, as long as weekend coverage was not increased.
As part of the intervention, we created standard work expectations within our division to (1) start rounds on dischargeable patients who were identified the prior evening during the (2) interdisciplinary huddle, and (3) have the entire departure process completed at the bedside using a discharge checklist (see Supporting Figure 1 through Figure 3 in the online version of this article). The expectations included a standard script for beginning rounds, selecting patients who could be discharged first, and completing all necessary discharge computer work at the bedside, before proceeding to the next patient. The daily predischarge huddle was instituted each afternoon to prepare discharges that were expected to occur the following day. The huddles were attended by care coordinators, social workers, and both medical teams. During the huddle, the team discussed anticipated discharges, scheduled follow‐up appointments and testing, faxed necessary prescriptions, and arranged any needed home services.
Inclusion and Exclusion Criteria for Patients
All patients discharged from the pediatric hospitalist inpatient service between Monday and Friday from April 8, 2013 to October 25, 2013 (preimplementation cohort) and October 28, 2013 to April 18, 2014 (postimplementation cohort) were eligible for inclusion. This included admitted patients and observation status patients. Patients discharged from the remaining PSHCH medical and surgical service lines were included in the control group analysis using the above criteria.
OUTCOME MEASURES
Throughput and Patient‐Level Outcomes
Primary outcomes included (1) time of electronic discharge order placement, (2) actual patient discharge time, (3) proportion of patients discharged before noon and 2 pm, (4) 7‐day, 14‐day, and 30‐day readmission rates, (5) length of stay (LOS), and (6) average daily census (ADC).
Statistical Analysis
The null hypothesis was that there would be no difference in discharge order time, discharge time, LOS, readmission rates, and daily discharges in the preintervention group compared to the intervention group. For time of order placement and actual patient discharge, the significance was assessed using Wilcoxon rank sum test and expressed as median time among the groups. Patient discharge before noon/2 pm was assessed by a logistic regression model. The predictor being the intervention group with the results expressed as odds ratios of discharge before noon/2 pm comparing the intervention group to the preintervention group. Readmission rates were assessed using a [2] test to see if there was a significant difference from what would be expected. Last, LOS and ADC were assessed by a Student t test and expressed as the means. The data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
For our division's service line, both the ADC and number of patients discharged per day were significantly higher during the intervention months (Table 1). By comparison, the control group had a significantly lower ADC and lower average of discharges per day in the intervention time period. The new model permitted the teams to enter discharge orders earlier in the day, which ultimately lead to earlier patient discharges. The additive effect of the 3 interventions had a statistically significant effect on process efficiency metrics (Table 1). The median discharge order entry time decreased by 200 minutes from 14:05 to 10:45, and the median time of patient discharge decreased by 93 minutes from 15:48 to 14:15. By comparison, the median time of discharge order entry decreased 13:13 to 12:56 pm, but the median time of discharge increased 5 minutes 14:45 versus 14:50 in the control group. A significantly higher proportion of patients were discharged by noon (27% vs 14%; P<0.0001; odds ratio [OR]:2.2; 95% confidence interval [CI]: 1.6‐3.1) and by 2 pm during the intervention period (47% vs 30%; P<0.0001; OR: 2.1; 95% CI: 1.6‐2.7). There was no observed difference in the proportion of patients who were discharged by noon or 2 pm in the control group. Finally, in the intervention group, approximately 50% of patients had discharge orders entered before noon compared to 23% in the control group (Figure 2). The intervention demonstrated statistical significance in shifting the time of discharge order entry and the time of patient discharge when compared to the relatively less burdened PSHCH control group (Figures 2 and 3). As seen in Figure 4, the results were sustained for the duration of the study and appeared to improve throughout intervention. Finally, readmission rates at 7, 14, and 30 days postdischarge and LOS were not negatively affected (Table 1) in either the intervention or control group.
| Outcomes | Experimental Model | Control Group | ||||
|---|---|---|---|---|---|---|
| Preintervention, n=421 | Intervention, n=552 | P Value | Preintervention, n=1,390 | Intervention, n=1,146 | P Value | |
| Average daily census | 9.7 | 12.4 | <0.0001 | 45.9 | 43.4 | 0.002 |
| Discharges per day | 3.1 | 4.5 | <0.00001 | 9.5 | 9.2 | 0.419 |
| Average length of stay | 3.1 | 3.0 | 0.864 | 6.3 | 5.9 | 0.714 |
| Discharge order time, median | 14:05 | 10:45 | <0.0001 | 13:13 | 12:56 | 0.053 |
| Discharge from hospital, median | 15:48 | 14:15 | <0.0001 | 14:45 | 14:50 | 0.113 |
| Patients discharged before noon | 59 (14%) | 147 (27%) | <0.0001 | 176 (13%) | 170 (15%) | 0.138 |
| Patients discharged before 2 pm | 128 (30%) | 261 (47%) | <0.0001 | 519 (38%) | 447 (39%) | 0.512 |
| 7‐day readmission rates | 3.1% | 3.5% | 0.965 | 6.7% | 6.8% | 0.970 |
| 14‐day readmission rates | 5.8% | 5.8% | 0.981 | 12.0% | 13.5% | 0.301 |
| 30‐day readmission rates | 9.4% | 9.1% | 0.703 | 20.0% | 20.6% | 0.705 |



DISCUSSION
We demonstrated a statistically significant and what appears to be a sustainable improvement in median discharge order times, discharge times, and proportion of discharges by noon and 2 pm. Ours was the only service line in our medical center to achieve a median time discharge before our institution's internal metric of 2 pm and maintain it for 3 consecutive months. Additionally, the process demonstrated consistent performance independent of the varying styles and experience of the rounding attending during the busiest months of the year without incurring a negative impact on LOS or readmission rates.
Although our intervention demonstrated statistical significance in shifting the discharge distribution curves by almost 2 hours, more relevant is its potential clinical and financial impact. First, it puts our hospital in compliance with the Joint Commission's recommendations standard LD.04.03.1, stipulating that hospitals measure and set goals for mitigating and managing the flow of patients though the hospital. Second, our findings confirm the results of earlier studies suggesting that shifting discharge times could likely be achieved without the additional staff, but with alterations in staff shift scheduling.[11] Third, by doing required discharge work at the bedside and making it available earlier in the day, every day, we consistently reduced patient waiting along the entire supply chain.
Advancing the discharge time creates virtual beds that allow our facility to theoretically accommodate new patients. Using the calculation in the Methods section (see Supporting Figure 4 in the online version of this article) on how to calculate virtual beds, we determined that our intervention created between 0.30 and 0.38 virtual beds in a hospital with only 72 beds. We calculated that this would create 6.8 more open bed hours per day, 74 additional patient days per year, and assuming patients were waiting for the beds and rapid bed turnover, our intervention theoretically created the capacity to accommodate approximately 25 additional admissions per year (see Supporting Figure 4 in the online version of this article). As the only children's hospital in the region, this intervention will enable our organization to provide timelier access and possibly reduce time sensitive medical errors.
Timelier evaluations also have revenue potential by eliminating lost referrals, thus turning waste into value. When comparing the previous year's high‐census monthsOctober through Marchthere were 20 lost referrals due to lack of bed capacity, as compared to zero lost referrals during our intervention period. By accommodating these 20 additional admissions, we estimated this generated between $275,000 and $412,000 dollars in additional revenue without additional resources but simply staffing to demand.
Finally, when we looked at patient satisfaction metrics obtained through Press Ganey (PG), comparing the time periods we observed that overall satisfaction increased from the 91 percentile to the 94 percentile, trust in doctor increased from the 20 percentile to the 70 percentile, and would recommend this hospital to others increased from the 53 percentile to the 75 percentile. Interestingly, despite being a study that improved discharge efficiency, none of the discharge metrics gathered by PG improved. It is possible that this is a limitation of the PG survey, or could reflect the possibility that our new process exposed that our discharge order entry and discharge processes are misaligned.
When we surveyed the nursing staff and members of the division regarding whether or not to continue the intervention rounding model, 75% and 100%, respectively, voted in favor of continuing with the intervention model. Unfortunately, housestaff satisfaction was not measured for this study.
Despite more weeks in the hospital, but because there was better process sequencing, our providers indicated that because the workload of the primary attending was reduced and the workload for the additional attending was light, there was ample time to engage in afternoon nonclinical activities (Figure 1B). In fact, several division members assumed departmental and educational leadership positions, and others volunteered to facilitate highly valued, but unsubsidized, afternoon medical student and resident teaching sessions that occurred solely as a result of the resequenced and redistributed clinical load.
There are limitations to this study. First, because 3 interventions were implemented simultaneously, it is difficult to identify which component of the intervention was the primary driver for the measured differences. It is conceivable the proactive discharge planning that occurred during the afternoon predischarge planning huddles allowed the teams to complete discharge requirements the night before anticipated discharge therefore expediting the next morning's discharge. A second limitation was not simultaneously comparing the traditional rounding structure with the experimental model. One could argue that the improved efficiency we observed was not due to any of the interventions and represents secular trends that all residents' teams experience through the course of the year as they get more adept at performing patient discharges. However, when we compare our performance to the control group performance, this efficiency trend was not present. Additionally, it was possible that attendings were so result focused that they delayed discharges if the 2 pm discharge goal was missed for that day and planned for early discharges the following morning. If this behavior occurred, this would likely have been reflected as increases in our LOS data, however this was not observed. Third, because our preintervention data reflected discharge behaviors during a low‐census period, it is possible that there was less urgency to discharge patients when bed capacity issues did not exist. Comparing the intervention period to a period when censuses are similar would better address this issue. Finally, although we assert that attending workload is a fundamental waste‐producing constraint in the discharge process, this study did not determine what the optimal patient census should be.
Most hospitalists struggle with finding a balance of meeting patient quality and administrative productivity demands.[16] Hospitalists at academic medical centers have the added demand of maintaining their educational mission. Since 2001, the Institute of Medicine[19] has advocated process re‐engineering using more patient‐centered approaches. A recent study found that when hospitals reach capacity, the excess workload placed on internal medicine hospitalists reduces efficiency and increases costs.[16] Interestingly, in a study conducted by McMahon et al., they found that reducing team censuses by 50%, resident educational outcomes can be improved.[20] Similar to this study, our study reduced attending workload by 50% with the goal to assess the impact on discharge efficiency rather than educational outcomes. Also similar to that study, we radically altered the operational model in which physicians historically had functioned.[14, 20] Because the rounding structures in both studies reduced patient:provider ratios we believe that our model will successfully balance education, patient quality, and productivity.
LSS, when thoughtfully applied to the problems we face, could be part of the solution. It delivers quick results without large capital investments, by identifying and implementing high‐leverage changes that value a creative solution before a capital investment. One of the strengths of this model is that it does not require substantial financial investment to produce these outcomes. Because the morning clinical loads were more evenly distributed during the busiest months of the year, our division members were able to engage in nonclinical duties and teaching sessions, both of which often required afternoon commitments, but permitted us to balance work and professional achievement (Figure 1B). Finally, as part of any new process, one must consider the factors that influence its sustainability: provider level satisfaction, impact of the process change, and remuneration. Because the intervention reduced lost referrals, the departmental and institutional leadership agreed to financially incentivize the value‐generating potential this intervention had on increasing patient access by facilitating organizational throughput. Therefore, having met the three aforementioned elements, we believe this model is sustainable.
Although many studies remain results focused with aims at documenting how hospital processes fail when overburdened, our study takes a novel process‐focused approach to look at how processes can excel during periods of high demands, simply by reallocating existing resources.
Medicine is in the midst of multiple paradigm shifts involving resident work hour reduction, public safety reporting, reimbursement constraints, and value‐driven care, to name a few. Whether we take a resident or patient‐centered approach, it seems highly unlikely that the current approaches will meet these demands without making significant changes in how we deliver care. Next steps should include construction of a value stream map (VSM), with the input of all of the process stakeholders, that diagrams the entire discharge process. The VSM should highlight all nonvalue adding steps and eliminate them. They are likely a contributing cause to the disproportionate reduction in time of discharge order entry (200 minutes) versus actual discharge (93 minutes) seen in our study. Future work needs to establish the generalizability and sustainability of this model across other hospital service lines. Future studies should establish if this model has sustained impact on patient, provider, and resident satisfaction and overall system efficiency (ED boarding), with aims to quantify the revenue generating potential that occurs through waste elimination.
We close with the following thought: [T]o ask people to make different decisions without fundamentally changing the equation presented to them is wrong. If we wish to change the types of decisions our people make, we owe it to them to design and build processes that will put them in a position to succeed.[21]
Acknowledgements
The authors recognize the contributions made by members of the Division of Pediatric Hospital Medicine at PSHCH, without which this project would not have been possible. They are: Drs. Marta Biderman, Anika Kumar, Margaret Mikula, Chris O'Hara, Brandon Smith, and Ron Williams, and Heidi Wolf, and Lyndsay Gardener, CRNP. This project would not have been possible without the help from Brenda Ruhl, Manjula Narasimhan, and Heather Boyle from the Department of Logistics. Finally, the authors would like to thank the following for their thoughtful reviews of this manuscript: Drs. Lisa Scalzi, Barbara Ostrov, Jed Gonzalo, Chris Sciamanna, and Matt Wain.
Disclosures: Nothing to report.
Given its positive effects on improving effectiveness and efficiency, Lean Six Sigma (LSS) is a business approach that is receiving a great deal of attention in the healthcare industry.[1, 2, 3, 4, 5, 6, 7] Although there are differences between Lean and Six Sigma, at their core they are both customer‐centered, quality methodologies designed to improve process efficiency and product quality through waste elimination, creating standardized work and reducing variation.[8]
Six Sigma is a rigorous problem‐focused process improvement method that focuses on defect removal, variation reduction, and customer satisfaction that relies heavily on statistical analysis. It includes 5 steps: define, measure, analyze, improvement, and control.[7, 8] Six Sigma assumes through variation reduction, defect removal, and meeting customer specifications, the performance of the organization can be improved and also meet the requirements of the customer.[8]
Lean is more process‐focused. It places emphasis on creating flow by removing waste and getting the steps of any given process in the right sequence.[8] In Lean terms, waste is defined as anything that the customer does not value and anything that is not done right the first time.[9] This category of waste is termed nonvalue adding and unnecessary. It is estimated that 30% to 50% of all steps of hospital processes are nonvalue adding and unnecessary, and therefore can be defined as waste.[10] Lean identifies 8 different types of nonvalue adding and unnecessary wastes. They are defects and rework, overproduction, waiting, nonutilization of resources, transport, inventory, motion, and extra processing. Waste creates delays that negatively impact patient care and reduce healthcare productivity.[10] Therefore, it makes sense to apply Lean concepts of waste identification and elimination to improve process efficiency. For example, when a facility is at or exceeds its bed capacity, any delay in discharge creates throughput delays throughout the hospital.[5] Discharge delays often result in emergency department (ED) overcrowding, and also affects a hospital's ability to accommodate internal downgrades and outside referrals in a timely fashion.[11, 12] However, because the sequence of steps of the discharge process is variable and not standardized, the goal to achieve early discharges remains elusive.[13]
There are emerging data to support that current rounding censuses exceed most hospitalist's abilities to deliver safe and efficient care.[12, 14, 15, 16] It is unclear what that threshold should be, but the current industry standard has nonacademic hospitalists seeing 15 patients per day. Therefore, high patient censuses could be contributing to delays in patient discharge times that effect hospital throughput. We speculated that by implementing a lean, quick‐strike approach[17] designed to improve the sequencing of housestaff, attending, and nursing work by eliminating the wastes of rework, waiting, extra processing, and nonutilization of physician resources by restaffing, we could improve patient discharge times. We augmented the intervention by creating standardized workflow expectations, a discharge checklist, and implemented daily interdisciplinary discharge planning huddles.
We hypothesized these interventions would improve the median time of discharge order entry and time of patient discharge. Primary outcome measures were: (1) the change in time of discharge order and discharge time and (2) the proportion of patients discharged before noon and 2 pm. Secondary outcomes that were used as balance measures were length of stay (LOS) and 7‐day, 14‐day, and 30‐day readmission rates.
METHODS
Study Design
This was a prospective quality improvement intervention with concurrent controls aimed to determine if discharge efficiency could be improved by load‐balancing our service line with existing faculty and residents, creating daily standard work using a discharge checklist and interdisciplinary huddles (see Supporting Figure 1 through Supporting Figure 3 in the online version of this article). All discharge data were collected as part of our medical center's Department of Logistics standard data collection procedures using solutions from TeleTracking Technologies, Inc. (Pittsburgh, PA). All patients discharged Monday through Friday from the pediatric hospitalist service prior to the 6‐month high‐census period (before intervention) and the 6‐month high‐census period (intervention period) were included in the study. To serve as our control, we collected the same discharge data during the same time periods for the remaining services of the children's hospital. This study was approved by Penn State Hershey Medical Center's institutional review board.
Study Setting
The study was conducted at the Penn State Hershey Children's Hospital (PSHCH), which is a physically free‐standing 133‐bed university‐based tertiary care hospital located in central Pennsylvania. The hospital has 36 pediatric medical/surgical beds located in 2 units (1 general and 1 intermediate care). PSHCH performs approximately 4100 admissions per year, of which approximately 1100 are performed by the Division of Pediatric Hospital Medicine. Our division is composed of 8 academic hospitalists with 1 to 20+ years' experience. Historically, the months of October through April are months when our service‐line has average daily censuses (ADC) that routinely exceed 12 patients per hospitalist. During these months, the median times patient discharge orders are placed and patient discharges occur historically approach 2 pm and 4 pm, respectively, and exceed our internal benchmark by 2 hours. Discharges from the remaining medical and surgical service lines at PSHCH that occurred Monday through Friday during the concurrent pre‐ and postintervention time periods served as the control group.
Needs Assessment and LSS
Traditionally, morning patient rounds are allotted approximately 180 minutes. Therefore, a rounding team can only be expected to spend 13 minutes or less per patient when the census exceeds 12 patients. The cycle time to perform 1 discharge using our electronic medical record is approximately 20 minutes, which is almost 10 minutes longer than the allotted time per patient. During high‐census months, our service averages 4 to 5 discharges per day. To accommodate performing discharges during rounds would require spending 80 to 100 minutes of the 180 available minutes. This would leave only 80 to 100 minutes to see the remaining 8 to 10 patients. As a result of these constraints, discharges are typically completed by the residents in unsupervised batches each afternoon following the noon conference (Figure 1A).

Because LSS focuses on eliminating nonvalue adding and unnecessary waste by load balancing processes and minimizing batching tasks,[8] this approach should lead one to question whether the current rounding model that requires 1 attending to see >12 high‐acuity patients with a maximum of 13 minutes per patient is system design flaw that leads to errors and inefficiency,[16] Theoretically, having an additional attending present would allow teams to resequence the work on smaller batches of patients and double the time to spend on each patient. This could create the opportunity to do value‐added work at the bedside in the presence of the family and nurse and eliminate the amount of nursing rework and time spent as work in progress on dischargeable patients (Figure 1).
Additionally, improving discharge efficiency creates virtual beds. Virtual beds permit hospitals to accommodate additional admissions despite operating with a fixed‐bed capacity. A way to calculate virtual beds is to calculate the reduction in LOS, and multiply that by the number of admissions per year divided by 36518 (see Supporting Figure 4 in the online version of this article). Our study was intended to determine the impact of discharge efficiency on this metric.
Intervention
We re‐structured our service line in a way that would balance both physician workload needs and patient expectations. To accomplish this, off service attendings were reallocated to round with a smaller resident team on fewer patients for the duration of the 6‐month study. Each member of the division agreed to work an average of 3 more weeks per year. One work day was estimated to be approximately 10 hours and 1 work week equaled 5 days, which asked for 150 hours of additional work per year. Because there increases in functional FTEs, the 2 teams consolidated into 1 team each weekend, to meet the group requirement that this model not result in additional weekend coverage. A balanced workload also theoretically allows the physician to spend more time at the bedside in direct patient care and resident education activities/observations.
In addition to reallocating physician and resident resources, our model created standard work expectations to reduce the variations in physician work sequences that can account for delayed discharge orders and delayed discharges, which is also an LSS principle. The intervention consisted of 3 changes: (1) fundamentally altering the composition of the rounding teams to optimize the provider: patient ratio; (2) defining rounding standards to expedite discharges; and (3) establishing a daily predischarge planning process.
The preintervention team typically had 1 attending, 1 to 2 senior residents, and 2 to 3 interns. The intervention period required creating 2 independently functioning teams, each composed of 1 hospitalist attending, and a minimum of 1 senior and 1 intern. The intervention occurred November through April, when the censuses predictably exceed 12 patients for the rounding attending. Because both teams functioned independently, all of the patients were divided equally between the 2 teams. Each team carried a panel of patients that included new, established, and dischargeable patients (Figure 1). We did not compare the number of provider handoffs before and during the intervention or time spent per patient.
Because the intervention required increasing the number of weeks on‐service by 2 to 3weeks per physician to reduce clinical work time, it meant redeploying previously off‐service attendings to coincide with peak demands. This aspect of the intervention made group buy‐in mandatory. The group agreed to distribute the predictably heavy workload that usually falls on 1 attending by adding a second attending for the busiest 6 months of the year. Our division voted unanimously to adopt this model despite the increase in service time, as long as weekend coverage was not increased.
As part of the intervention, we created standard work expectations within our division to (1) start rounds on dischargeable patients who were identified the prior evening during the (2) interdisciplinary huddle, and (3) have the entire departure process completed at the bedside using a discharge checklist (see Supporting Figure 1 through Figure 3 in the online version of this article). The expectations included a standard script for beginning rounds, selecting patients who could be discharged first, and completing all necessary discharge computer work at the bedside, before proceeding to the next patient. The daily predischarge huddle was instituted each afternoon to prepare discharges that were expected to occur the following day. The huddles were attended by care coordinators, social workers, and both medical teams. During the huddle, the team discussed anticipated discharges, scheduled follow‐up appointments and testing, faxed necessary prescriptions, and arranged any needed home services.
Inclusion and Exclusion Criteria for Patients
All patients discharged from the pediatric hospitalist inpatient service between Monday and Friday from April 8, 2013 to October 25, 2013 (preimplementation cohort) and October 28, 2013 to April 18, 2014 (postimplementation cohort) were eligible for inclusion. This included admitted patients and observation status patients. Patients discharged from the remaining PSHCH medical and surgical service lines were included in the control group analysis using the above criteria.
OUTCOME MEASURES
Throughput and Patient‐Level Outcomes
Primary outcomes included (1) time of electronic discharge order placement, (2) actual patient discharge time, (3) proportion of patients discharged before noon and 2 pm, (4) 7‐day, 14‐day, and 30‐day readmission rates, (5) length of stay (LOS), and (6) average daily census (ADC).
Statistical Analysis
The null hypothesis was that there would be no difference in discharge order time, discharge time, LOS, readmission rates, and daily discharges in the preintervention group compared to the intervention group. For time of order placement and actual patient discharge, the significance was assessed using Wilcoxon rank sum test and expressed as median time among the groups. Patient discharge before noon/2 pm was assessed by a logistic regression model. The predictor being the intervention group with the results expressed as odds ratios of discharge before noon/2 pm comparing the intervention group to the preintervention group. Readmission rates were assessed using a [2] test to see if there was a significant difference from what would be expected. Last, LOS and ADC were assessed by a Student t test and expressed as the means. The data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
For our division's service line, both the ADC and number of patients discharged per day were significantly higher during the intervention months (Table 1). By comparison, the control group had a significantly lower ADC and lower average of discharges per day in the intervention time period. The new model permitted the teams to enter discharge orders earlier in the day, which ultimately lead to earlier patient discharges. The additive effect of the 3 interventions had a statistically significant effect on process efficiency metrics (Table 1). The median discharge order entry time decreased by 200 minutes from 14:05 to 10:45, and the median time of patient discharge decreased by 93 minutes from 15:48 to 14:15. By comparison, the median time of discharge order entry decreased 13:13 to 12:56 pm, but the median time of discharge increased 5 minutes 14:45 versus 14:50 in the control group. A significantly higher proportion of patients were discharged by noon (27% vs 14%; P<0.0001; odds ratio [OR]:2.2; 95% confidence interval [CI]: 1.6‐3.1) and by 2 pm during the intervention period (47% vs 30%; P<0.0001; OR: 2.1; 95% CI: 1.6‐2.7). There was no observed difference in the proportion of patients who were discharged by noon or 2 pm in the control group. Finally, in the intervention group, approximately 50% of patients had discharge orders entered before noon compared to 23% in the control group (Figure 2). The intervention demonstrated statistical significance in shifting the time of discharge order entry and the time of patient discharge when compared to the relatively less burdened PSHCH control group (Figures 2 and 3). As seen in Figure 4, the results were sustained for the duration of the study and appeared to improve throughout intervention. Finally, readmission rates at 7, 14, and 30 days postdischarge and LOS were not negatively affected (Table 1) in either the intervention or control group.
| Outcomes | Experimental Model | Control Group | ||||
|---|---|---|---|---|---|---|
| Preintervention, n=421 | Intervention, n=552 | P Value | Preintervention, n=1,390 | Intervention, n=1,146 | P Value | |
| Average daily census | 9.7 | 12.4 | <0.0001 | 45.9 | 43.4 | 0.002 |
| Discharges per day | 3.1 | 4.5 | <0.00001 | 9.5 | 9.2 | 0.419 |
| Average length of stay | 3.1 | 3.0 | 0.864 | 6.3 | 5.9 | 0.714 |
| Discharge order time, median | 14:05 | 10:45 | <0.0001 | 13:13 | 12:56 | 0.053 |
| Discharge from hospital, median | 15:48 | 14:15 | <0.0001 | 14:45 | 14:50 | 0.113 |
| Patients discharged before noon | 59 (14%) | 147 (27%) | <0.0001 | 176 (13%) | 170 (15%) | 0.138 |
| Patients discharged before 2 pm | 128 (30%) | 261 (47%) | <0.0001 | 519 (38%) | 447 (39%) | 0.512 |
| 7‐day readmission rates | 3.1% | 3.5% | 0.965 | 6.7% | 6.8% | 0.970 |
| 14‐day readmission rates | 5.8% | 5.8% | 0.981 | 12.0% | 13.5% | 0.301 |
| 30‐day readmission rates | 9.4% | 9.1% | 0.703 | 20.0% | 20.6% | 0.705 |



DISCUSSION
We demonstrated a statistically significant and what appears to be a sustainable improvement in median discharge order times, discharge times, and proportion of discharges by noon and 2 pm. Ours was the only service line in our medical center to achieve a median time discharge before our institution's internal metric of 2 pm and maintain it for 3 consecutive months. Additionally, the process demonstrated consistent performance independent of the varying styles and experience of the rounding attending during the busiest months of the year without incurring a negative impact on LOS or readmission rates.
Although our intervention demonstrated statistical significance in shifting the discharge distribution curves by almost 2 hours, more relevant is its potential clinical and financial impact. First, it puts our hospital in compliance with the Joint Commission's recommendations standard LD.04.03.1, stipulating that hospitals measure and set goals for mitigating and managing the flow of patients though the hospital. Second, our findings confirm the results of earlier studies suggesting that shifting discharge times could likely be achieved without the additional staff, but with alterations in staff shift scheduling.[11] Third, by doing required discharge work at the bedside and making it available earlier in the day, every day, we consistently reduced patient waiting along the entire supply chain.
Advancing the discharge time creates virtual beds that allow our facility to theoretically accommodate new patients. Using the calculation in the Methods section (see Supporting Figure 4 in the online version of this article) on how to calculate virtual beds, we determined that our intervention created between 0.30 and 0.38 virtual beds in a hospital with only 72 beds. We calculated that this would create 6.8 more open bed hours per day, 74 additional patient days per year, and assuming patients were waiting for the beds and rapid bed turnover, our intervention theoretically created the capacity to accommodate approximately 25 additional admissions per year (see Supporting Figure 4 in the online version of this article). As the only children's hospital in the region, this intervention will enable our organization to provide timelier access and possibly reduce time sensitive medical errors.
Timelier evaluations also have revenue potential by eliminating lost referrals, thus turning waste into value. When comparing the previous year's high‐census monthsOctober through Marchthere were 20 lost referrals due to lack of bed capacity, as compared to zero lost referrals during our intervention period. By accommodating these 20 additional admissions, we estimated this generated between $275,000 and $412,000 dollars in additional revenue without additional resources but simply staffing to demand.
Finally, when we looked at patient satisfaction metrics obtained through Press Ganey (PG), comparing the time periods we observed that overall satisfaction increased from the 91 percentile to the 94 percentile, trust in doctor increased from the 20 percentile to the 70 percentile, and would recommend this hospital to others increased from the 53 percentile to the 75 percentile. Interestingly, despite being a study that improved discharge efficiency, none of the discharge metrics gathered by PG improved. It is possible that this is a limitation of the PG survey, or could reflect the possibility that our new process exposed that our discharge order entry and discharge processes are misaligned.
When we surveyed the nursing staff and members of the division regarding whether or not to continue the intervention rounding model, 75% and 100%, respectively, voted in favor of continuing with the intervention model. Unfortunately, housestaff satisfaction was not measured for this study.
Despite more weeks in the hospital, but because there was better process sequencing, our providers indicated that because the workload of the primary attending was reduced and the workload for the additional attending was light, there was ample time to engage in afternoon nonclinical activities (Figure 1B). In fact, several division members assumed departmental and educational leadership positions, and others volunteered to facilitate highly valued, but unsubsidized, afternoon medical student and resident teaching sessions that occurred solely as a result of the resequenced and redistributed clinical load.
There are limitations to this study. First, because 3 interventions were implemented simultaneously, it is difficult to identify which component of the intervention was the primary driver for the measured differences. It is conceivable the proactive discharge planning that occurred during the afternoon predischarge planning huddles allowed the teams to complete discharge requirements the night before anticipated discharge therefore expediting the next morning's discharge. A second limitation was not simultaneously comparing the traditional rounding structure with the experimental model. One could argue that the improved efficiency we observed was not due to any of the interventions and represents secular trends that all residents' teams experience through the course of the year as they get more adept at performing patient discharges. However, when we compare our performance to the control group performance, this efficiency trend was not present. Additionally, it was possible that attendings were so result focused that they delayed discharges if the 2 pm discharge goal was missed for that day and planned for early discharges the following morning. If this behavior occurred, this would likely have been reflected as increases in our LOS data, however this was not observed. Third, because our preintervention data reflected discharge behaviors during a low‐census period, it is possible that there was less urgency to discharge patients when bed capacity issues did not exist. Comparing the intervention period to a period when censuses are similar would better address this issue. Finally, although we assert that attending workload is a fundamental waste‐producing constraint in the discharge process, this study did not determine what the optimal patient census should be.
Most hospitalists struggle with finding a balance of meeting patient quality and administrative productivity demands.[16] Hospitalists at academic medical centers have the added demand of maintaining their educational mission. Since 2001, the Institute of Medicine[19] has advocated process re‐engineering using more patient‐centered approaches. A recent study found that when hospitals reach capacity, the excess workload placed on internal medicine hospitalists reduces efficiency and increases costs.[16] Interestingly, in a study conducted by McMahon et al., they found that reducing team censuses by 50%, resident educational outcomes can be improved.[20] Similar to this study, our study reduced attending workload by 50% with the goal to assess the impact on discharge efficiency rather than educational outcomes. Also similar to that study, we radically altered the operational model in which physicians historically had functioned.[14, 20] Because the rounding structures in both studies reduced patient:provider ratios we believe that our model will successfully balance education, patient quality, and productivity.
LSS, when thoughtfully applied to the problems we face, could be part of the solution. It delivers quick results without large capital investments, by identifying and implementing high‐leverage changes that value a creative solution before a capital investment. One of the strengths of this model is that it does not require substantial financial investment to produce these outcomes. Because the morning clinical loads were more evenly distributed during the busiest months of the year, our division members were able to engage in nonclinical duties and teaching sessions, both of which often required afternoon commitments, but permitted us to balance work and professional achievement (Figure 1B). Finally, as part of any new process, one must consider the factors that influence its sustainability: provider level satisfaction, impact of the process change, and remuneration. Because the intervention reduced lost referrals, the departmental and institutional leadership agreed to financially incentivize the value‐generating potential this intervention had on increasing patient access by facilitating organizational throughput. Therefore, having met the three aforementioned elements, we believe this model is sustainable.
Although many studies remain results focused with aims at documenting how hospital processes fail when overburdened, our study takes a novel process‐focused approach to look at how processes can excel during periods of high demands, simply by reallocating existing resources.
Medicine is in the midst of multiple paradigm shifts involving resident work hour reduction, public safety reporting, reimbursement constraints, and value‐driven care, to name a few. Whether we take a resident or patient‐centered approach, it seems highly unlikely that the current approaches will meet these demands without making significant changes in how we deliver care. Next steps should include construction of a value stream map (VSM), with the input of all of the process stakeholders, that diagrams the entire discharge process. The VSM should highlight all nonvalue adding steps and eliminate them. They are likely a contributing cause to the disproportionate reduction in time of discharge order entry (200 minutes) versus actual discharge (93 minutes) seen in our study. Future work needs to establish the generalizability and sustainability of this model across other hospital service lines. Future studies should establish if this model has sustained impact on patient, provider, and resident satisfaction and overall system efficiency (ED boarding), with aims to quantify the revenue generating potential that occurs through waste elimination.
We close with the following thought: [T]o ask people to make different decisions without fundamentally changing the equation presented to them is wrong. If we wish to change the types of decisions our people make, we owe it to them to design and build processes that will put them in a position to succeed.[21]
Acknowledgements
The authors recognize the contributions made by members of the Division of Pediatric Hospital Medicine at PSHCH, without which this project would not have been possible. They are: Drs. Marta Biderman, Anika Kumar, Margaret Mikula, Chris O'Hara, Brandon Smith, and Ron Williams, and Heidi Wolf, and Lyndsay Gardener, CRNP. This project would not have been possible without the help from Brenda Ruhl, Manjula Narasimhan, and Heather Boyle from the Department of Logistics. Finally, the authors would like to thank the following for their thoughtful reviews of this manuscript: Drs. Lisa Scalzi, Barbara Ostrov, Jed Gonzalo, Chris Sciamanna, and Matt Wain.
Disclosures: Nothing to report.
- . Theory of constraints—a review of the philosophy and its applications. Int J Oper Prod Man. 1998;18(4):336–355.
- , , , et al. Use of lean and six sigma methodology to improve operating room efficiency in a high‐volume tertiary‐care academic medical center. J Am Coll Surg. 2011;213(1):83–92; discussion 93–84.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , . Assessing the evidence of Six Sigma and Lean in the health care industry. Qual Manag Health Care. 2010;19(3):211–225.
- , , , et al. Redesigning care at the Flinders Medical Centre: clinical process redesign using “lean thinking”. Med J Aust. 2008;188(6 suppl):S27–S31.
- , , , , . Impact of 5 years of lean six sigma in a University Medical Center. Qual Manag Health Care. 2012;21(4):262–268.
- , , , et al. A Lean Six Sigma quality improvement project to increase discharge paperwork completeness for admission to a comprehensive integrated inpatient rehabilitation program. Am J Med Qual. 2013;28(4):301–307.
- . How to compare Six Sigma, lean and the theory of constraints—a framework for choosing what's best for your organization. Qual Prog. 2002;35(3):73–78.
- , , , , . Application of lean manufacturing techniques in the Emergency Department. J Emerg Med. 2009;37(2):177–182.
- . Lean Hospitals: Improving Quality, Patient Safety, and Employee Engagement. 2nd ed. New York, NY: Productivity Press/Taylor 2012.
- , , , , , . The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186–196.
- , , , . Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Aust. 2012;24(5):510–517.
- . Need to speed up discharges? The pros and cons of putting discharges on the clock. Today's Hospitalist. 2013. Accessed November 2014. Available at: http://www.todayshospitalist.com/index.php?b=articles_read3(2):144–150.
- , . Hospitalist staffing requirements. Eff Clin Pract. 1999;2(3):126–130.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- . Accelerating Lean Six Sigma Results: How to Achieve Improvement Excellence in the New Economy. Plantation, FL: J. Ross Publishing; 2011.
- . “Virtual bed capacity” may offer revenue boost for hospitals. Healthcare Finance. Accessed October 2014. Available at: http://www.healthcarefinancenews.com/blog/virtual‐bed‐capacity‐may‐offer‐revenue‐boost‐hospitals. Published November 30, 2011.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , , . Evaluation of a redesign initiative in an internal‐medicine residency. N Engl J Med. 2010;362(14):1304–1311.
- John Kim and Associates website. Available at: http://www.johnkimconsulting.com. Accessed June 2014.
- . Theory of constraints—a review of the philosophy and its applications. Int J Oper Prod Man. 1998;18(4):336–355.
- , , , et al. Use of lean and six sigma methodology to improve operating room efficiency in a high‐volume tertiary‐care academic medical center. J Am Coll Surg. 2011;213(1):83–92; discussion 93–84.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , . Assessing the evidence of Six Sigma and Lean in the health care industry. Qual Manag Health Care. 2010;19(3):211–225.
- , , , et al. Redesigning care at the Flinders Medical Centre: clinical process redesign using “lean thinking”. Med J Aust. 2008;188(6 suppl):S27–S31.
- , , , , . Impact of 5 years of lean six sigma in a University Medical Center. Qual Manag Health Care. 2012;21(4):262–268.
- , , , et al. A Lean Six Sigma quality improvement project to increase discharge paperwork completeness for admission to a comprehensive integrated inpatient rehabilitation program. Am J Med Qual. 2013;28(4):301–307.
- . How to compare Six Sigma, lean and the theory of constraints—a framework for choosing what's best for your organization. Qual Prog. 2002;35(3):73–78.
- , , , , . Application of lean manufacturing techniques in the Emergency Department. J Emerg Med. 2009;37(2):177–182.
- . Lean Hospitals: Improving Quality, Patient Safety, and Employee Engagement. 2nd ed. New York, NY: Productivity Press/Taylor 2012.
- , , , , , . The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186–196.
- , , , . Unravelling relationships: Hospital occupancy levels, discharge timing and emergency department access block. Emerg Med Aust. 2012;24(5):510–517.
- . Need to speed up discharges? The pros and cons of putting discharges on the clock. Today's Hospitalist. 2013. Accessed November 2014. Available at: http://www.todayshospitalist.com/index.php?b=articles_read3(2):144–150.
- , . Hospitalist staffing requirements. Eff Clin Pract. 1999;2(3):126–130.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- . Accelerating Lean Six Sigma Results: How to Achieve Improvement Excellence in the New Economy. Plantation, FL: J. Ross Publishing; 2011.
- . “Virtual bed capacity” may offer revenue boost for hospitals. Healthcare Finance. Accessed October 2014. Available at: http://www.healthcarefinancenews.com/blog/virtual‐bed‐capacity‐may‐offer‐revenue‐boost‐hospitals. Published November 30, 2011.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , , . Evaluation of a redesign initiative in an internal‐medicine residency. N Engl J Med. 2010;362(14):1304–1311.
- John Kim and Associates website. Available at: http://www.johnkimconsulting.com. Accessed June 2014.
© 2014 Society of Hospital Medicine
Ofatumumab maintenance halves risk of progression in relapsed CLL
SAN FRANCISCO – Ofatumumab maintenance therapy nearly doubled progression-free survival in patients with relapsed CLL, according to a preplanned interim analysis of the phase III PROLONG study.
At a median follow-up of 19.1 months, progression-free survival was 15.2 months with the standard approach of observation alone and 29.4 months with maintenance ofatumumab (Hazard ratio, 0.50; P < .0001).
Ofatumumab (Arzerra) also significantly increased the median time to next treatment from 31.1 months to 38 months (HR, 0.66; P = .0108), Dr. Marinus van Oers reported at the annual meeting of the American Society of Hematology.
The benefit in progression-free survival (PFS) with maintenance was “statistically significant and clinical relevant” and was present in all subgroups, he said. It was independent of age, gender, number and type of prior treatment, minimal residual disease status at study entry, and “response at study entry, although we have the impression that it’s more effective in patients on PR [partial response] than in patients on CR [complete response],” he added.
The rationale for the trial lies in the fact that despite recent advances, there is still no curative treatment for chronic lymphocytic leukemia (CLL). Ofatumumab, a type 1 CD20 monoclonal antibody, has a role as maintenance in follicular lymphoma (FL), which shares similarities in biological behavior with CLL. This role is debated, but a recent meta-analysis shows ofatumumab maintenance prolongs PFS and tends to prolong overall survival in relapsed patients with FL, Dr. van Oers of the Academic Medical Center in Amsterdam, The Netherlands, observed.
PROLONG randomized 474 patients with relapsed CLL to observation or ofatumumab 300 mg in week 1 and 1,000 mg in week 2, and every 8 weeks for 2 years. All patients were in second or third remission and within 3 months of response assessment after the last reinduction treatment. Patients with refractory disease or prior maintenance therapy or stem cell transplantation were excluded.
At baseline, the median age was about 65 years, 70% had at least two prior treatments, 80% were in partial remission from their last CLL treatment, and less than 10% had poor-risk cytogenetics 11p or 17p deletions. At the time of the analysis, 25% of patients had received all 13 cycles of ofatumumab.
Adverse events of any grade were increased with the addition of ofatumumab versus placebo (86% vs. 72%; P < .0001). Sixty percent were related to study treatment, but none resulted in study withdrawal, Dr. van Oers said. In all, 17 patients on the experimental arm dropped out due to physician decision or patient wish.
Among grade 3 events, neutropenia was significantly increased with maintenance therapy versus placebo (24% vs. 10%; P < .0001) and there was a non-significant increase in infections (13% vs. 8%). Five deaths occurred in the observation arm and two in the ofatumumab arm, one due to sepsis two months after the end of treatment and the other due to unrelated GI obstruction.
Median overall survival has not been reached for either arm (HR, 0.85; P = .487), he reported on behalf of HOVON and the NORDIC CLL group, co-developers of the study.
SAN FRANCISCO – Ofatumumab maintenance therapy nearly doubled progression-free survival in patients with relapsed CLL, according to a preplanned interim analysis of the phase III PROLONG study.
At a median follow-up of 19.1 months, progression-free survival was 15.2 months with the standard approach of observation alone and 29.4 months with maintenance ofatumumab (Hazard ratio, 0.50; P < .0001).
Ofatumumab (Arzerra) also significantly increased the median time to next treatment from 31.1 months to 38 months (HR, 0.66; P = .0108), Dr. Marinus van Oers reported at the annual meeting of the American Society of Hematology.
The benefit in progression-free survival (PFS) with maintenance was “statistically significant and clinical relevant” and was present in all subgroups, he said. It was independent of age, gender, number and type of prior treatment, minimal residual disease status at study entry, and “response at study entry, although we have the impression that it’s more effective in patients on PR [partial response] than in patients on CR [complete response],” he added.
The rationale for the trial lies in the fact that despite recent advances, there is still no curative treatment for chronic lymphocytic leukemia (CLL). Ofatumumab, a type 1 CD20 monoclonal antibody, has a role as maintenance in follicular lymphoma (FL), which shares similarities in biological behavior with CLL. This role is debated, but a recent meta-analysis shows ofatumumab maintenance prolongs PFS and tends to prolong overall survival in relapsed patients with FL, Dr. van Oers of the Academic Medical Center in Amsterdam, The Netherlands, observed.
PROLONG randomized 474 patients with relapsed CLL to observation or ofatumumab 300 mg in week 1 and 1,000 mg in week 2, and every 8 weeks for 2 years. All patients were in second or third remission and within 3 months of response assessment after the last reinduction treatment. Patients with refractory disease or prior maintenance therapy or stem cell transplantation were excluded.
At baseline, the median age was about 65 years, 70% had at least two prior treatments, 80% were in partial remission from their last CLL treatment, and less than 10% had poor-risk cytogenetics 11p or 17p deletions. At the time of the analysis, 25% of patients had received all 13 cycles of ofatumumab.
Adverse events of any grade were increased with the addition of ofatumumab versus placebo (86% vs. 72%; P < .0001). Sixty percent were related to study treatment, but none resulted in study withdrawal, Dr. van Oers said. In all, 17 patients on the experimental arm dropped out due to physician decision or patient wish.
Among grade 3 events, neutropenia was significantly increased with maintenance therapy versus placebo (24% vs. 10%; P < .0001) and there was a non-significant increase in infections (13% vs. 8%). Five deaths occurred in the observation arm and two in the ofatumumab arm, one due to sepsis two months after the end of treatment and the other due to unrelated GI obstruction.
Median overall survival has not been reached for either arm (HR, 0.85; P = .487), he reported on behalf of HOVON and the NORDIC CLL group, co-developers of the study.
SAN FRANCISCO – Ofatumumab maintenance therapy nearly doubled progression-free survival in patients with relapsed CLL, according to a preplanned interim analysis of the phase III PROLONG study.
At a median follow-up of 19.1 months, progression-free survival was 15.2 months with the standard approach of observation alone and 29.4 months with maintenance ofatumumab (Hazard ratio, 0.50; P < .0001).
Ofatumumab (Arzerra) also significantly increased the median time to next treatment from 31.1 months to 38 months (HR, 0.66; P = .0108), Dr. Marinus van Oers reported at the annual meeting of the American Society of Hematology.
The benefit in progression-free survival (PFS) with maintenance was “statistically significant and clinical relevant” and was present in all subgroups, he said. It was independent of age, gender, number and type of prior treatment, minimal residual disease status at study entry, and “response at study entry, although we have the impression that it’s more effective in patients on PR [partial response] than in patients on CR [complete response],” he added.
The rationale for the trial lies in the fact that despite recent advances, there is still no curative treatment for chronic lymphocytic leukemia (CLL). Ofatumumab, a type 1 CD20 monoclonal antibody, has a role as maintenance in follicular lymphoma (FL), which shares similarities in biological behavior with CLL. This role is debated, but a recent meta-analysis shows ofatumumab maintenance prolongs PFS and tends to prolong overall survival in relapsed patients with FL, Dr. van Oers of the Academic Medical Center in Amsterdam, The Netherlands, observed.
PROLONG randomized 474 patients with relapsed CLL to observation or ofatumumab 300 mg in week 1 and 1,000 mg in week 2, and every 8 weeks for 2 years. All patients were in second or third remission and within 3 months of response assessment after the last reinduction treatment. Patients with refractory disease or prior maintenance therapy or stem cell transplantation were excluded.
At baseline, the median age was about 65 years, 70% had at least two prior treatments, 80% were in partial remission from their last CLL treatment, and less than 10% had poor-risk cytogenetics 11p or 17p deletions. At the time of the analysis, 25% of patients had received all 13 cycles of ofatumumab.
Adverse events of any grade were increased with the addition of ofatumumab versus placebo (86% vs. 72%; P < .0001). Sixty percent were related to study treatment, but none resulted in study withdrawal, Dr. van Oers said. In all, 17 patients on the experimental arm dropped out due to physician decision or patient wish.
Among grade 3 events, neutropenia was significantly increased with maintenance therapy versus placebo (24% vs. 10%; P < .0001) and there was a non-significant increase in infections (13% vs. 8%). Five deaths occurred in the observation arm and two in the ofatumumab arm, one due to sepsis two months after the end of treatment and the other due to unrelated GI obstruction.
Median overall survival has not been reached for either arm (HR, 0.85; P = .487), he reported on behalf of HOVON and the NORDIC CLL group, co-developers of the study.
AT ASH 2014
Key clinical point: Maintenance ofatumumab cuts the risk of progression in half among patients with relapsed CLL.
Major finding: Progression-free survival was 15.2 months with observation alone and 29.4 months with maintenance ofatumumab (Hazard ratio, 0.50; P < .0001).
Data source: Randomized phase III trial in 474 patients with relapsed CLL.
Disclosures: GlaxoSmithKline sponsored the study. Dr. van Oers reported having no financial disclosures.
Brentuximab changes landscape for post-transplant Hodgkin’s lymphoma patients
SAN FRANCISCO – Early post-transplant brentuximab vedotin dramatically slows Hodgkin’s lymphoma progression in patients at high risk for relapse or progression, the phase III AETHERA study shows.
After a median follow-up of about 28 months, the primary end point of progression-free survival (PFS) per independent review increased from a median of 24 months with placebo and best supportive care (BSC) to 43 months with brentuximab and BSC (Hazard ratio, 0.57; P = .001).
Per investigator assessment, median PFS was 16 months with placebo and had not been reached with brentuximab (HR, 0.50),Dr. Craig Moskowitz reported at the annual meeting of the American Society of Hematology.
The benefit of brentuximab maintenance was consistent across every subgroup. Historically, roughly half of patients who undergo an autologous stem cell transplant will relapse.
“Once this study is published, in patients who met eligibility criteria to be on this study - and once again I’ll remind you that’s remission duration less than one year, disease outside the lymph node system, or primary refractory disease - in my opinion, this will be the standard of care,” Dr. Moskowitz said during a press briefing.
Brentuximab, an anti-CD30 antibody conjugate, is already approved in the U.S. for the management of Hodgkin’s lymphoma (HL) after failure of autologous stem cell transplantation (ASCT) or at least two prior lines of multi-agent chemotherapy in patients ineligible for ASCT.
Brentuximab is also indicated for systemic anaplastic large cell lymphoma after failure of at least one multi-agent chemotherapy regimen.
In September it was announced that AETHERA met its primary end point, but this was the first full look at the absolute survival rates and safety data.
The 2-year PFS rate for the brentuximab and placebo groups is now 63% vs. 51% per independent review and 65% vs. 45% per investigator.
“The bottom line is there’s a 20% difference in progression-free survival at 2 years upon investigator review. This has never been seen in patients with relapsed, refractory lymphoma, let alone Hodgkin lymphoma,” Dr. Moskowitz, clinical director of hematology oncology at Memorial Sloan-Kettering Cancer Center in New York City, said.
Overall survival data are immature, but was the same in both groups at 2 years (P = .62). The likelihood of showing a survival difference was not expected because 85% of patients who relapsed on the placebo arm crossed over to brentuximab, as allowed per protocol, and it’s known that brentuximab alone in auto-transplant failures improves outcomes by at least a year, he said. Also, twice as many patients who failed placebo received a second transplant.
Dr. Moskowitz stressed that nearly every patient in the trial had at least three risk factors that would place them at high risk of treatment failure and that studies have shown that for patients with this many risk factors, the chance of being cured by an auto-transplant is about 25%. The risk factors are: relapsed less than 12 months or refractory to frontline therapy, best response of partial remission or stable disease to most recent salvage therapy, extranodal disease at pre-ASCT relapse, B symptoms at pre-ASCT relapse, or two or more prior salvage therapies.
Press briefing moderator Dr. Brad Kahl of the University of Wisconsin-Madison, said Aethera is the first study to show a significant benefit for a post-transplant strategy.
“The biggest question in my mind is whether the application of maintenance brentuximab vedotin is just delaying the inevitable relapse, so the patients will still relapse, just later, or has the brentuximab taken patients who are destined to relapse and turned them into a cured patient,” Dr. Kahl said. “We don’t know the answer to that question. That will become apparent with more time.”
Dr. Moscowitz observed that relapses almost never happen after two year, adding, “So if you’re in remission at two years after stem cell transplantation for Hodgkin lymphoma, you are likely to be cured.”
AETHERA enrolled 329 patients with Hodgkin’s lymphoma and randomly assigned them after ASCT to brentuximab vedotin 1.8 mg/kg or placebo given every 3 weeks for up to 16 cycles, plus BSC. All patients were required to have achieved a complete response, partial remission, or stable disease to salvage therapy prior to ASCT. Their median age was 32 years and 53% were male.
Roughly 60% were refractory to upfront therapy, 43% in the brentuximab arm and 48% of controls had received 2 or more prior systemic therapies, and a third in each arm had extranodal involvement.
Consolidation therapy with brentuximab was generally well tolerated, Dr. Moskowitz said. Peripheral sensory neuropathy was the most common side effect, experienced at any grade in 67% on brentuximab vs. 19% of controls and at grade 3 in 13% vs. 1%. There were no grade 4 events and 85% of patients had resolution or improvement with dose reductions or stopping the drug.
Other adverse events in the brentuximab and control groups were neutropenia (35% vs. 12%), upper respiratory tract infections (26% vs. 23%), and fatigue (24% vs. 18%). Two patients died within 40 days of dosing with brentuximab, one from treatment-related acute respiratory distress syndrome associated with pneumonitis and one following an episode of treatment-related acute pancreatitis that had resolved at the time of death, he reported.
Based on the results, study sponsor Seattle Genetics is expected to seek approval for brentuximab in this consolidation setting in the first half of 2015, according to a statement from the company. The ongoing phase III ECHELON-1 and ECHELON 2 trials in HL and mature T-cell lymphomas are looking at the use of brentuximab in frontline disease.
SAN FRANCISCO – Early post-transplant brentuximab vedotin dramatically slows Hodgkin’s lymphoma progression in patients at high risk for relapse or progression, the phase III AETHERA study shows.
After a median follow-up of about 28 months, the primary end point of progression-free survival (PFS) per independent review increased from a median of 24 months with placebo and best supportive care (BSC) to 43 months with brentuximab and BSC (Hazard ratio, 0.57; P = .001).
Per investigator assessment, median PFS was 16 months with placebo and had not been reached with brentuximab (HR, 0.50),Dr. Craig Moskowitz reported at the annual meeting of the American Society of Hematology.
The benefit of brentuximab maintenance was consistent across every subgroup. Historically, roughly half of patients who undergo an autologous stem cell transplant will relapse.
“Once this study is published, in patients who met eligibility criteria to be on this study - and once again I’ll remind you that’s remission duration less than one year, disease outside the lymph node system, or primary refractory disease - in my opinion, this will be the standard of care,” Dr. Moskowitz said during a press briefing.
Brentuximab, an anti-CD30 antibody conjugate, is already approved in the U.S. for the management of Hodgkin’s lymphoma (HL) after failure of autologous stem cell transplantation (ASCT) or at least two prior lines of multi-agent chemotherapy in patients ineligible for ASCT.
Brentuximab is also indicated for systemic anaplastic large cell lymphoma after failure of at least one multi-agent chemotherapy regimen.
In September it was announced that AETHERA met its primary end point, but this was the first full look at the absolute survival rates and safety data.
The 2-year PFS rate for the brentuximab and placebo groups is now 63% vs. 51% per independent review and 65% vs. 45% per investigator.
“The bottom line is there’s a 20% difference in progression-free survival at 2 years upon investigator review. This has never been seen in patients with relapsed, refractory lymphoma, let alone Hodgkin lymphoma,” Dr. Moskowitz, clinical director of hematology oncology at Memorial Sloan-Kettering Cancer Center in New York City, said.
Overall survival data are immature, but was the same in both groups at 2 years (P = .62). The likelihood of showing a survival difference was not expected because 85% of patients who relapsed on the placebo arm crossed over to brentuximab, as allowed per protocol, and it’s known that brentuximab alone in auto-transplant failures improves outcomes by at least a year, he said. Also, twice as many patients who failed placebo received a second transplant.
Dr. Moskowitz stressed that nearly every patient in the trial had at least three risk factors that would place them at high risk of treatment failure and that studies have shown that for patients with this many risk factors, the chance of being cured by an auto-transplant is about 25%. The risk factors are: relapsed less than 12 months or refractory to frontline therapy, best response of partial remission or stable disease to most recent salvage therapy, extranodal disease at pre-ASCT relapse, B symptoms at pre-ASCT relapse, or two or more prior salvage therapies.
Press briefing moderator Dr. Brad Kahl of the University of Wisconsin-Madison, said Aethera is the first study to show a significant benefit for a post-transplant strategy.
“The biggest question in my mind is whether the application of maintenance brentuximab vedotin is just delaying the inevitable relapse, so the patients will still relapse, just later, or has the brentuximab taken patients who are destined to relapse and turned them into a cured patient,” Dr. Kahl said. “We don’t know the answer to that question. That will become apparent with more time.”
Dr. Moscowitz observed that relapses almost never happen after two year, adding, “So if you’re in remission at two years after stem cell transplantation for Hodgkin lymphoma, you are likely to be cured.”
AETHERA enrolled 329 patients with Hodgkin’s lymphoma and randomly assigned them after ASCT to brentuximab vedotin 1.8 mg/kg or placebo given every 3 weeks for up to 16 cycles, plus BSC. All patients were required to have achieved a complete response, partial remission, or stable disease to salvage therapy prior to ASCT. Their median age was 32 years and 53% were male.
Roughly 60% were refractory to upfront therapy, 43% in the brentuximab arm and 48% of controls had received 2 or more prior systemic therapies, and a third in each arm had extranodal involvement.
Consolidation therapy with brentuximab was generally well tolerated, Dr. Moskowitz said. Peripheral sensory neuropathy was the most common side effect, experienced at any grade in 67% on brentuximab vs. 19% of controls and at grade 3 in 13% vs. 1%. There were no grade 4 events and 85% of patients had resolution or improvement with dose reductions or stopping the drug.
Other adverse events in the brentuximab and control groups were neutropenia (35% vs. 12%), upper respiratory tract infections (26% vs. 23%), and fatigue (24% vs. 18%). Two patients died within 40 days of dosing with brentuximab, one from treatment-related acute respiratory distress syndrome associated with pneumonitis and one following an episode of treatment-related acute pancreatitis that had resolved at the time of death, he reported.
Based on the results, study sponsor Seattle Genetics is expected to seek approval for brentuximab in this consolidation setting in the first half of 2015, according to a statement from the company. The ongoing phase III ECHELON-1 and ECHELON 2 trials in HL and mature T-cell lymphomas are looking at the use of brentuximab in frontline disease.
SAN FRANCISCO – Early post-transplant brentuximab vedotin dramatically slows Hodgkin’s lymphoma progression in patients at high risk for relapse or progression, the phase III AETHERA study shows.
After a median follow-up of about 28 months, the primary end point of progression-free survival (PFS) per independent review increased from a median of 24 months with placebo and best supportive care (BSC) to 43 months with brentuximab and BSC (Hazard ratio, 0.57; P = .001).
Per investigator assessment, median PFS was 16 months with placebo and had not been reached with brentuximab (HR, 0.50),Dr. Craig Moskowitz reported at the annual meeting of the American Society of Hematology.
The benefit of brentuximab maintenance was consistent across every subgroup. Historically, roughly half of patients who undergo an autologous stem cell transplant will relapse.
“Once this study is published, in patients who met eligibility criteria to be on this study - and once again I’ll remind you that’s remission duration less than one year, disease outside the lymph node system, or primary refractory disease - in my opinion, this will be the standard of care,” Dr. Moskowitz said during a press briefing.
Brentuximab, an anti-CD30 antibody conjugate, is already approved in the U.S. for the management of Hodgkin’s lymphoma (HL) after failure of autologous stem cell transplantation (ASCT) or at least two prior lines of multi-agent chemotherapy in patients ineligible for ASCT.
Brentuximab is also indicated for systemic anaplastic large cell lymphoma after failure of at least one multi-agent chemotherapy regimen.
In September it was announced that AETHERA met its primary end point, but this was the first full look at the absolute survival rates and safety data.
The 2-year PFS rate for the brentuximab and placebo groups is now 63% vs. 51% per independent review and 65% vs. 45% per investigator.
“The bottom line is there’s a 20% difference in progression-free survival at 2 years upon investigator review. This has never been seen in patients with relapsed, refractory lymphoma, let alone Hodgkin lymphoma,” Dr. Moskowitz, clinical director of hematology oncology at Memorial Sloan-Kettering Cancer Center in New York City, said.
Overall survival data are immature, but was the same in both groups at 2 years (P = .62). The likelihood of showing a survival difference was not expected because 85% of patients who relapsed on the placebo arm crossed over to brentuximab, as allowed per protocol, and it’s known that brentuximab alone in auto-transplant failures improves outcomes by at least a year, he said. Also, twice as many patients who failed placebo received a second transplant.
Dr. Moskowitz stressed that nearly every patient in the trial had at least three risk factors that would place them at high risk of treatment failure and that studies have shown that for patients with this many risk factors, the chance of being cured by an auto-transplant is about 25%. The risk factors are: relapsed less than 12 months or refractory to frontline therapy, best response of partial remission or stable disease to most recent salvage therapy, extranodal disease at pre-ASCT relapse, B symptoms at pre-ASCT relapse, or two or more prior salvage therapies.
Press briefing moderator Dr. Brad Kahl of the University of Wisconsin-Madison, said Aethera is the first study to show a significant benefit for a post-transplant strategy.
“The biggest question in my mind is whether the application of maintenance brentuximab vedotin is just delaying the inevitable relapse, so the patients will still relapse, just later, or has the brentuximab taken patients who are destined to relapse and turned them into a cured patient,” Dr. Kahl said. “We don’t know the answer to that question. That will become apparent with more time.”
Dr. Moscowitz observed that relapses almost never happen after two year, adding, “So if you’re in remission at two years after stem cell transplantation for Hodgkin lymphoma, you are likely to be cured.”
AETHERA enrolled 329 patients with Hodgkin’s lymphoma and randomly assigned them after ASCT to brentuximab vedotin 1.8 mg/kg or placebo given every 3 weeks for up to 16 cycles, plus BSC. All patients were required to have achieved a complete response, partial remission, or stable disease to salvage therapy prior to ASCT. Their median age was 32 years and 53% were male.
Roughly 60% were refractory to upfront therapy, 43% in the brentuximab arm and 48% of controls had received 2 or more prior systemic therapies, and a third in each arm had extranodal involvement.
Consolidation therapy with brentuximab was generally well tolerated, Dr. Moskowitz said. Peripheral sensory neuropathy was the most common side effect, experienced at any grade in 67% on brentuximab vs. 19% of controls and at grade 3 in 13% vs. 1%. There were no grade 4 events and 85% of patients had resolution or improvement with dose reductions or stopping the drug.
Other adverse events in the brentuximab and control groups were neutropenia (35% vs. 12%), upper respiratory tract infections (26% vs. 23%), and fatigue (24% vs. 18%). Two patients died within 40 days of dosing with brentuximab, one from treatment-related acute respiratory distress syndrome associated with pneumonitis and one following an episode of treatment-related acute pancreatitis that had resolved at the time of death, he reported.
Based on the results, study sponsor Seattle Genetics is expected to seek approval for brentuximab in this consolidation setting in the first half of 2015, according to a statement from the company. The ongoing phase III ECHELON-1 and ECHELON 2 trials in HL and mature T-cell lymphomas are looking at the use of brentuximab in frontline disease.
AT ASH 2014
Key clinical point: Brentuximab vedotin given immediately post-transplant significantly improves progression-free survival in patients with Hodgkin’s lymphoma at high risk for progression.
Major finding: Median progression-free survival per independent review was 24 months with placebo vs. 43 months with brentuximab (HR, 0.57; P = .001).
Data source: Randomized, double-blind, phase III study in 329 patients with Hodgkin’s lymphoma.
Disclosures: Seattle Genetics sponsored the study. Dr. Moskowitz reported research funding from Genentech and Merck, and research funding from and consultancy for Seattle Genetics. Several co-authors reported financial ties with industry, including employment with or equity ownership in Seattle Genetics.
Ultrasound bests elastography for specificity of thyroid cancer diagnosis
CORONADO, CALIF.– Compared with elastography, ultrasound predictors of malignancy were more specific for presurgical diagnosis and in differentiating between benign and malignant thyroid nodules, results from a pooled analysis showed.
“Elastography is controversial,” Dr. Parisha Bhatia said in an interview during the annual meeting of the American Thyroid Association. “Some studies have reported that it has better sensitivity and specificity, compared with conventional ultrasound, but others have found it not to be helpful.”
In an effort to compare the efficacy of elastography and ultrasound in determining benign and malignant thyroid nodules, and to determine if elastography has a complementary role to fine needle aspiration (FNA), Dr. Bhatia and her associates searched Embase and PubMed databases for articles involving more than 50 nodules using specimen histology as the reference standard. They discovered 14 prospective studies and organized them into one of two groups. Group 1 included nodules with FNA cytology–proven “benign/malignant” result. Group 2 included nodules with FNA cytology as “intermediate.” The elasticity score was compared with ultrasound features such as taller than wide, irregular margins, internal vascularity, calcification, and absence of halo to determine validity measures and likelihood ratios.
Dr. Bhatia, an endocrine surgeon at Tulane University, New Orleans, reported on findings from 2,732 nodules in the pooled analysis. Of these, 64% were benign, 25% were malignant, and 11% were indeterminate.
In group 1, elastography showed a sensitivity of 75%, a specificity of 80%, a positive predictive value (PPV) of 61%, a negative predictive value (NPV) of 89%, and a likelihood ratio of 5.7, with a higher predictive value for nodules smaller than 1 cm in diameter (PPV of 79% and NPV of 83%). The strongest ultrasound-related predictor of malignancies was “taller than wide” (a specificity of 92%, a NPV of 51%, yet a sensitivity of only 24%), followed by irregular margins (a specificity of 91%, sensitivity of 48%, and a PPV of 64%). Combination of elastography and ultrasound had the highest sensitivity (96%) and NPV (96%), yet lower specificity (46%) and PPV (46%).
In group 2, elastography yielded a higher sensitivity (90%) and NPV (92%), while ultrasound features were highly specific, with the highest values for “taller than wide” shape (85%) in these thyroid nodules.
“Ultrasound predictors of malignancy prove to be more specific for presurgical diagnosis and differentiation of benign and malignant thyroid nodules,” the researchers wrote in their abstract. “When used as an adjunct, the higher sensitivity and NPV of combination of both the techniques can lead to better selection of candidates for FNA.”
Dr. Bhatia acknowledged certain limitations of the study, including the fact that the pooled data contained little information on indeterminate thyroid nodules. She reported having no financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF.– Compared with elastography, ultrasound predictors of malignancy were more specific for presurgical diagnosis and in differentiating between benign and malignant thyroid nodules, results from a pooled analysis showed.
“Elastography is controversial,” Dr. Parisha Bhatia said in an interview during the annual meeting of the American Thyroid Association. “Some studies have reported that it has better sensitivity and specificity, compared with conventional ultrasound, but others have found it not to be helpful.”
In an effort to compare the efficacy of elastography and ultrasound in determining benign and malignant thyroid nodules, and to determine if elastography has a complementary role to fine needle aspiration (FNA), Dr. Bhatia and her associates searched Embase and PubMed databases for articles involving more than 50 nodules using specimen histology as the reference standard. They discovered 14 prospective studies and organized them into one of two groups. Group 1 included nodules with FNA cytology–proven “benign/malignant” result. Group 2 included nodules with FNA cytology as “intermediate.” The elasticity score was compared with ultrasound features such as taller than wide, irregular margins, internal vascularity, calcification, and absence of halo to determine validity measures and likelihood ratios.
Dr. Bhatia, an endocrine surgeon at Tulane University, New Orleans, reported on findings from 2,732 nodules in the pooled analysis. Of these, 64% were benign, 25% were malignant, and 11% were indeterminate.
In group 1, elastography showed a sensitivity of 75%, a specificity of 80%, a positive predictive value (PPV) of 61%, a negative predictive value (NPV) of 89%, and a likelihood ratio of 5.7, with a higher predictive value for nodules smaller than 1 cm in diameter (PPV of 79% and NPV of 83%). The strongest ultrasound-related predictor of malignancies was “taller than wide” (a specificity of 92%, a NPV of 51%, yet a sensitivity of only 24%), followed by irregular margins (a specificity of 91%, sensitivity of 48%, and a PPV of 64%). Combination of elastography and ultrasound had the highest sensitivity (96%) and NPV (96%), yet lower specificity (46%) and PPV (46%).
In group 2, elastography yielded a higher sensitivity (90%) and NPV (92%), while ultrasound features were highly specific, with the highest values for “taller than wide” shape (85%) in these thyroid nodules.
“Ultrasound predictors of malignancy prove to be more specific for presurgical diagnosis and differentiation of benign and malignant thyroid nodules,” the researchers wrote in their abstract. “When used as an adjunct, the higher sensitivity and NPV of combination of both the techniques can lead to better selection of candidates for FNA.”
Dr. Bhatia acknowledged certain limitations of the study, including the fact that the pooled data contained little information on indeterminate thyroid nodules. She reported having no financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF.– Compared with elastography, ultrasound predictors of malignancy were more specific for presurgical diagnosis and in differentiating between benign and malignant thyroid nodules, results from a pooled analysis showed.
“Elastography is controversial,” Dr. Parisha Bhatia said in an interview during the annual meeting of the American Thyroid Association. “Some studies have reported that it has better sensitivity and specificity, compared with conventional ultrasound, but others have found it not to be helpful.”
In an effort to compare the efficacy of elastography and ultrasound in determining benign and malignant thyroid nodules, and to determine if elastography has a complementary role to fine needle aspiration (FNA), Dr. Bhatia and her associates searched Embase and PubMed databases for articles involving more than 50 nodules using specimen histology as the reference standard. They discovered 14 prospective studies and organized them into one of two groups. Group 1 included nodules with FNA cytology–proven “benign/malignant” result. Group 2 included nodules with FNA cytology as “intermediate.” The elasticity score was compared with ultrasound features such as taller than wide, irregular margins, internal vascularity, calcification, and absence of halo to determine validity measures and likelihood ratios.
Dr. Bhatia, an endocrine surgeon at Tulane University, New Orleans, reported on findings from 2,732 nodules in the pooled analysis. Of these, 64% were benign, 25% were malignant, and 11% were indeterminate.
In group 1, elastography showed a sensitivity of 75%, a specificity of 80%, a positive predictive value (PPV) of 61%, a negative predictive value (NPV) of 89%, and a likelihood ratio of 5.7, with a higher predictive value for nodules smaller than 1 cm in diameter (PPV of 79% and NPV of 83%). The strongest ultrasound-related predictor of malignancies was “taller than wide” (a specificity of 92%, a NPV of 51%, yet a sensitivity of only 24%), followed by irregular margins (a specificity of 91%, sensitivity of 48%, and a PPV of 64%). Combination of elastography and ultrasound had the highest sensitivity (96%) and NPV (96%), yet lower specificity (46%) and PPV (46%).
In group 2, elastography yielded a higher sensitivity (90%) and NPV (92%), while ultrasound features were highly specific, with the highest values for “taller than wide” shape (85%) in these thyroid nodules.
“Ultrasound predictors of malignancy prove to be more specific for presurgical diagnosis and differentiation of benign and malignant thyroid nodules,” the researchers wrote in their abstract. “When used as an adjunct, the higher sensitivity and NPV of combination of both the techniques can lead to better selection of candidates for FNA.”
Dr. Bhatia acknowledged certain limitations of the study, including the fact that the pooled data contained little information on indeterminate thyroid nodules. She reported having no financial disclosures.
On Twitter @dougbrunk
AT THE ATA ANNUAL MEETING
Key clinical point: Ultrasound is more specific than elastography in helping clinicians make a presurgical diagnosis of thyroid cancer.
Major finding: Compared with elastography, ultrasound was more specific in presurgical diagnosis and in differentiating between benign and malignant thyroid nodules (specificity of 92% vs. 80%, respectively).
Data source: A pooled analysis of 14 prospective studies involving findings from 2,732 thyroid nodules.
Disclosures: Dr. Bhatia reported having no financial disclosures.