User login
Ebola Post on The Hospitalist Leader Blog Widely Viewed
Brett Hendel-Paterson, MD, hospitalist and assistant professor of internal medicine and global health at the University of Minnesota, reminded clinicians on “The Hospital Leader” blog about the need to respond to news about Ebola reasonably and with compassion.
“It is okay if you do not know everything about Ebola, and it is even okay to be scared of it,” Dr. Hendel-Paterson wrote. “But learn about it, learn how to prevent spread, and find out what your hospital would do if you have a patient with it.”
The post has been popular with hospitalists and others.
In less than a week, it was read more than 6,000 times and shared on Medspace.com and KevinMD.com.
Check out more hospitalist insights at www.shmblog.org. If you’d like to contribute your own ideas and experiences to “The Hospital Leader,” e-mail [email protected].
Brett Hendel-Paterson, MD, hospitalist and assistant professor of internal medicine and global health at the University of Minnesota, reminded clinicians on “The Hospital Leader” blog about the need to respond to news about Ebola reasonably and with compassion.
“It is okay if you do not know everything about Ebola, and it is even okay to be scared of it,” Dr. Hendel-Paterson wrote. “But learn about it, learn how to prevent spread, and find out what your hospital would do if you have a patient with it.”
The post has been popular with hospitalists and others.
In less than a week, it was read more than 6,000 times and shared on Medspace.com and KevinMD.com.
Check out more hospitalist insights at www.shmblog.org. If you’d like to contribute your own ideas and experiences to “The Hospital Leader,” e-mail [email protected].
Brett Hendel-Paterson, MD, hospitalist and assistant professor of internal medicine and global health at the University of Minnesota, reminded clinicians on “The Hospital Leader” blog about the need to respond to news about Ebola reasonably and with compassion.
“It is okay if you do not know everything about Ebola, and it is even okay to be scared of it,” Dr. Hendel-Paterson wrote. “But learn about it, learn how to prevent spread, and find out what your hospital would do if you have a patient with it.”
The post has been popular with hospitalists and others.
In less than a week, it was read more than 6,000 times and shared on Medspace.com and KevinMD.com.
Check out more hospitalist insights at www.shmblog.org. If you’d like to contribute your own ideas and experiences to “The Hospital Leader,” e-mail [email protected].
Society of Hospital Medicine Plans Events to Link Hospitalists-in-Training, HM Leaders
Whether it’s the acuity of patient conditions, interest in quality improvement, or work-life balance, more medical students and residents than ever are interested in exploring careers in hospital medicine. That’s why SHM is organizing more events to link up hospitalists-in-training to leaders in the specialty.
After successful meetings this year in Chicago, Denver, and Philadelphia, SHM is planning 2015 events in Philadelphia, Chicago, Los Angeles, and possibly other cities. Dates and other details will be announced in the next few weeks.
For more information, visit the “Member” section of www.hospitalmedicine.org.
Whether it’s the acuity of patient conditions, interest in quality improvement, or work-life balance, more medical students and residents than ever are interested in exploring careers in hospital medicine. That’s why SHM is organizing more events to link up hospitalists-in-training to leaders in the specialty.
After successful meetings this year in Chicago, Denver, and Philadelphia, SHM is planning 2015 events in Philadelphia, Chicago, Los Angeles, and possibly other cities. Dates and other details will be announced in the next few weeks.
For more information, visit the “Member” section of www.hospitalmedicine.org.
Whether it’s the acuity of patient conditions, interest in quality improvement, or work-life balance, more medical students and residents than ever are interested in exploring careers in hospital medicine. That’s why SHM is organizing more events to link up hospitalists-in-training to leaders in the specialty.
After successful meetings this year in Chicago, Denver, and Philadelphia, SHM is planning 2015 events in Philadelphia, Chicago, Los Angeles, and possibly other cities. Dates and other details will be announced in the next few weeks.
For more information, visit the “Member” section of www.hospitalmedicine.org.
Society of Hospital Medicine Event Dates, Deadlines
December 17, 2015
Masters Deadline for Nominations
Do you know someone who has earned a place in the “Hall of Fame” for hospital medicine? Nominations for the Master in Hospital Medicine are due next month.
December 31, 2014
Membership Ambassadors
All active SHM members can earn 2015-2016 dues credits and special recognition for recruiting new physician, physician assistant, nurse practitioner, pharmacist, or affiliate members.
January 9, 2015
SFHM and FHM Submission Deadline
Don’t wait until the last minute to submit your application for the Fellow or Senior Fellow in Hospital Medicine. Start now and submit ahead of time.
February 2, 2015
Early registration discount deadline for Hospital Medicine 2015.
March 29-April 1, 2015
Hospital Medicine 2015
May 7-9, 2015
Quality and Safety Educators Academy
QI and patient safety are no longer just electives for trainees; they are part of the core education. That’s why educators everywhere need to learn from SHM’s Quality and Safety Educators Academy.
December 17, 2015
Masters Deadline for Nominations
Do you know someone who has earned a place in the “Hall of Fame” for hospital medicine? Nominations for the Master in Hospital Medicine are due next month.
December 31, 2014
Membership Ambassadors
All active SHM members can earn 2015-2016 dues credits and special recognition for recruiting new physician, physician assistant, nurse practitioner, pharmacist, or affiliate members.
January 9, 2015
SFHM and FHM Submission Deadline
Don’t wait until the last minute to submit your application for the Fellow or Senior Fellow in Hospital Medicine. Start now and submit ahead of time.
February 2, 2015
Early registration discount deadline for Hospital Medicine 2015.
March 29-April 1, 2015
Hospital Medicine 2015
May 7-9, 2015
Quality and Safety Educators Academy
QI and patient safety are no longer just electives for trainees; they are part of the core education. That’s why educators everywhere need to learn from SHM’s Quality and Safety Educators Academy.
December 17, 2015
Masters Deadline for Nominations
Do you know someone who has earned a place in the “Hall of Fame” for hospital medicine? Nominations for the Master in Hospital Medicine are due next month.
December 31, 2014
Membership Ambassadors
All active SHM members can earn 2015-2016 dues credits and special recognition for recruiting new physician, physician assistant, nurse practitioner, pharmacist, or affiliate members.
January 9, 2015
SFHM and FHM Submission Deadline
Don’t wait until the last minute to submit your application for the Fellow or Senior Fellow in Hospital Medicine. Start now and submit ahead of time.
February 2, 2015
Early registration discount deadline for Hospital Medicine 2015.
March 29-April 1, 2015
Hospital Medicine 2015
May 7-9, 2015
Quality and Safety Educators Academy
QI and patient safety are no longer just electives for trainees; they are part of the core education. That’s why educators everywhere need to learn from SHM’s Quality and Safety Educators Academy.
Hospitalists’ Potential Impact in Accountable Care Organizations
The rise of Accountable Care Organizations (ACOs), a group of healthcare providers who are answerable for the overall care of patients assigned to them, inclusive of quality and cost, accelerated as a result of the Affordable Care Act. In September CMS announced quality and financial performance results for the 18-month reporting period showing that 53 of 204 Medicare Share Savings Program ACOs saved $12 million on average while improving inpatient care, proving that ACOs are an innovative way to reduce costs and enhance quality. The savings of $636 million will be split 50/50 between CMS and the ACOs that reached their targets.
Respondents to SHM’s 2014 State of Hospital Medicine report indicate that nearly 36% of respondent HM groups (HMGs) nationwide worked at a hospital that was either already involved in or was contemplating involvement in an ACO. Academic HMGs were more likely to be involved (55.8%) than nonacademic groups (30.3%). The driving force behind everything we as hospitalists do is consistent with the principles of an ACO, which are to provide high quality, cost-effective healthcare that improves the health and well-being of our patients and communities.
With that said, there is a need for hospitalists to embrace innovative processes and relationships both within the hospital and throughout the continuum of care. This includes taking a fresh look at mechanisms to improve quality and customer satisfaction with a positive impact on cost. At WellStar, we have focused on implementing a number of tactical approaches aimed at transforming care delivery in multiple arenas. Key initiatives our hospitalists have been involved in include:
- Keen focus on management optimization of observation units;
- Establishment of an accountable care unit, where HM patients are co-located, and real-time, multidisciplinary, team-based bedside care is delivered;
- Concerted efforts to improve transitions of care to home and post-acute care settings by providing not only follow-up appointments but also an avenue for patients to ask questions and get answers post-discharge;
- Developing post-acute network partnerships that allow for insight into the care delivery models used at area nursing homes, long-term acute care facilities, and hospice; and
- Creating meaningful participation in the co-management of surgical patients through participation in the pre-admission testing process.
These innovations clearly demonstrate the value of hospital medicine in the new environment of accountable care. And, while there is a great deal of effort behind each of these initiatives, the payoff has a strong impact on the organization and the community.
Dr. Akopov is vice president and chief of hospital medicine operations at WellStar Health System in Atlanta, Ga., and serves as chair of the WellStar Health Network ACO. Ms. Papetti is assistant vice president of WellStar Medical Group in Atlanta and a member of SHM’s Practice Analysis Committee.
The rise of Accountable Care Organizations (ACOs), a group of healthcare providers who are answerable for the overall care of patients assigned to them, inclusive of quality and cost, accelerated as a result of the Affordable Care Act. In September CMS announced quality and financial performance results for the 18-month reporting period showing that 53 of 204 Medicare Share Savings Program ACOs saved $12 million on average while improving inpatient care, proving that ACOs are an innovative way to reduce costs and enhance quality. The savings of $636 million will be split 50/50 between CMS and the ACOs that reached their targets.
Respondents to SHM’s 2014 State of Hospital Medicine report indicate that nearly 36% of respondent HM groups (HMGs) nationwide worked at a hospital that was either already involved in or was contemplating involvement in an ACO. Academic HMGs were more likely to be involved (55.8%) than nonacademic groups (30.3%). The driving force behind everything we as hospitalists do is consistent with the principles of an ACO, which are to provide high quality, cost-effective healthcare that improves the health and well-being of our patients and communities.
With that said, there is a need for hospitalists to embrace innovative processes and relationships both within the hospital and throughout the continuum of care. This includes taking a fresh look at mechanisms to improve quality and customer satisfaction with a positive impact on cost. At WellStar, we have focused on implementing a number of tactical approaches aimed at transforming care delivery in multiple arenas. Key initiatives our hospitalists have been involved in include:
- Keen focus on management optimization of observation units;
- Establishment of an accountable care unit, where HM patients are co-located, and real-time, multidisciplinary, team-based bedside care is delivered;
- Concerted efforts to improve transitions of care to home and post-acute care settings by providing not only follow-up appointments but also an avenue for patients to ask questions and get answers post-discharge;
- Developing post-acute network partnerships that allow for insight into the care delivery models used at area nursing homes, long-term acute care facilities, and hospice; and
- Creating meaningful participation in the co-management of surgical patients through participation in the pre-admission testing process.
These innovations clearly demonstrate the value of hospital medicine in the new environment of accountable care. And, while there is a great deal of effort behind each of these initiatives, the payoff has a strong impact on the organization and the community.
Dr. Akopov is vice president and chief of hospital medicine operations at WellStar Health System in Atlanta, Ga., and serves as chair of the WellStar Health Network ACO. Ms. Papetti is assistant vice president of WellStar Medical Group in Atlanta and a member of SHM’s Practice Analysis Committee.
The rise of Accountable Care Organizations (ACOs), a group of healthcare providers who are answerable for the overall care of patients assigned to them, inclusive of quality and cost, accelerated as a result of the Affordable Care Act. In September CMS announced quality and financial performance results for the 18-month reporting period showing that 53 of 204 Medicare Share Savings Program ACOs saved $12 million on average while improving inpatient care, proving that ACOs are an innovative way to reduce costs and enhance quality. The savings of $636 million will be split 50/50 between CMS and the ACOs that reached their targets.
Respondents to SHM’s 2014 State of Hospital Medicine report indicate that nearly 36% of respondent HM groups (HMGs) nationwide worked at a hospital that was either already involved in or was contemplating involvement in an ACO. Academic HMGs were more likely to be involved (55.8%) than nonacademic groups (30.3%). The driving force behind everything we as hospitalists do is consistent with the principles of an ACO, which are to provide high quality, cost-effective healthcare that improves the health and well-being of our patients and communities.
With that said, there is a need for hospitalists to embrace innovative processes and relationships both within the hospital and throughout the continuum of care. This includes taking a fresh look at mechanisms to improve quality and customer satisfaction with a positive impact on cost. At WellStar, we have focused on implementing a number of tactical approaches aimed at transforming care delivery in multiple arenas. Key initiatives our hospitalists have been involved in include:
- Keen focus on management optimization of observation units;
- Establishment of an accountable care unit, where HM patients are co-located, and real-time, multidisciplinary, team-based bedside care is delivered;
- Concerted efforts to improve transitions of care to home and post-acute care settings by providing not only follow-up appointments but also an avenue for patients to ask questions and get answers post-discharge;
- Developing post-acute network partnerships that allow for insight into the care delivery models used at area nursing homes, long-term acute care facilities, and hospice; and
- Creating meaningful participation in the co-management of surgical patients through participation in the pre-admission testing process.
These innovations clearly demonstrate the value of hospital medicine in the new environment of accountable care. And, while there is a great deal of effort behind each of these initiatives, the payoff has a strong impact on the organization and the community.
Dr. Akopov is vice president and chief of hospital medicine operations at WellStar Health System in Atlanta, Ga., and serves as chair of the WellStar Health Network ACO. Ms. Papetti is assistant vice president of WellStar Medical Group in Atlanta and a member of SHM’s Practice Analysis Committee.
Society of Hospital Medicine's Advocacy Efforts, Then and Now
Another year older, another year wiser. SHM’s advocacy efforts have grown immensely in the last year thanks to proactive members who understand the importance of being involved and taking action. Because of member engagement—through the online Legislative Action Center and in-person meetings with members of Congress—hospitalists are widely recognized on Capitol Hill as thoughtful resources on policy issues who prioritize the improvement of patient care and the healthcare system.
This year alone, we have had a demonstrable impact on three key health policy issues: the sustainable growth rate (SGR), meaningful use, and observation status.
SGR repeal has been a consistent source of frustration and a regular feature of SHM’s advocacy efforts, including messages from hundreds of hospitalists to Congress, numerous requests for input from SHM as Congress worked on developing repeal legislation, and meetings with lawmakers to further shape legislation and to ask for the prioritization of a permanent, reliable solution. Although temporary patches have been the norm, SHM has not given up on repeal efforts and continues to push Congress to move past this failed formula.
Awareness of the precarious position of hospitalists in meaningful use has broadened significantly, and SHM worked with CMS to secure an exception from penalties for most hospitalists for 2015. The exception is only temporary, however, and SHM members will be essential in the effort to convince both CMS and Congress to enact a sensible permanent solution.
Observation status and the two-midnight rule saw significant coverage and interest throughout the year. Hospitalist and SHM Public Policy Committee member Ann Sheehy, MD, MS, FHM, testified about the impacts of observation status on hospitals and on patients in front of both the House Ways and Means Subcommittee on Health and the Senate Special Committee on Aging. The SHM Public Policy Committee also published a widely read white paper explaining SHM’s position on the issue.
Here is a key passage that perfectly reflects SHM’s position on observation status:
“Any policy change should be rooted in common sense, reflective of clinical reality, and designed to ensure that patients and providers are incentivized to work together to improve health. Patients should be able to get the care that they need, when they need it, including access to SNF [skilled nursing facility] care. Medicare policies should not be unnecessary impediments to physician judgment and workflow and should be geared toward reducing administrative burden and complexity.”1
Advocacy needs the active voices of those who understand the daily impacts of health policies. SHM would not be as successful a resource without our hospitalist members taking steps to better the healthcare system overall.
Building relationships with policymakers is the key to SHM remaining a prominent resource that is trusted and respected in Washington, D.C. At the 2015 annual meeting in National Harbor, Md., SHM will be sponsoring another “Hospitalists on the Hill” event, where we plan to build on recent successes. In 2013, more than 100 hospitalists took to the halls of Congress to advocate for pressing policy issues. Be sure to plan accordingly, as Hill Day will be an all-day event on April 1, the final day of the meeting.
Ellen Boyer is SHM’s government relations project coordinator.
Reference
Another year older, another year wiser. SHM’s advocacy efforts have grown immensely in the last year thanks to proactive members who understand the importance of being involved and taking action. Because of member engagement—through the online Legislative Action Center and in-person meetings with members of Congress—hospitalists are widely recognized on Capitol Hill as thoughtful resources on policy issues who prioritize the improvement of patient care and the healthcare system.
This year alone, we have had a demonstrable impact on three key health policy issues: the sustainable growth rate (SGR), meaningful use, and observation status.
SGR repeal has been a consistent source of frustration and a regular feature of SHM’s advocacy efforts, including messages from hundreds of hospitalists to Congress, numerous requests for input from SHM as Congress worked on developing repeal legislation, and meetings with lawmakers to further shape legislation and to ask for the prioritization of a permanent, reliable solution. Although temporary patches have been the norm, SHM has not given up on repeal efforts and continues to push Congress to move past this failed formula.
Awareness of the precarious position of hospitalists in meaningful use has broadened significantly, and SHM worked with CMS to secure an exception from penalties for most hospitalists for 2015. The exception is only temporary, however, and SHM members will be essential in the effort to convince both CMS and Congress to enact a sensible permanent solution.
Observation status and the two-midnight rule saw significant coverage and interest throughout the year. Hospitalist and SHM Public Policy Committee member Ann Sheehy, MD, MS, FHM, testified about the impacts of observation status on hospitals and on patients in front of both the House Ways and Means Subcommittee on Health and the Senate Special Committee on Aging. The SHM Public Policy Committee also published a widely read white paper explaining SHM’s position on the issue.
Here is a key passage that perfectly reflects SHM’s position on observation status:
“Any policy change should be rooted in common sense, reflective of clinical reality, and designed to ensure that patients and providers are incentivized to work together to improve health. Patients should be able to get the care that they need, when they need it, including access to SNF [skilled nursing facility] care. Medicare policies should not be unnecessary impediments to physician judgment and workflow and should be geared toward reducing administrative burden and complexity.”1
Advocacy needs the active voices of those who understand the daily impacts of health policies. SHM would not be as successful a resource without our hospitalist members taking steps to better the healthcare system overall.
Building relationships with policymakers is the key to SHM remaining a prominent resource that is trusted and respected in Washington, D.C. At the 2015 annual meeting in National Harbor, Md., SHM will be sponsoring another “Hospitalists on the Hill” event, where we plan to build on recent successes. In 2013, more than 100 hospitalists took to the halls of Congress to advocate for pressing policy issues. Be sure to plan accordingly, as Hill Day will be an all-day event on April 1, the final day of the meeting.
Ellen Boyer is SHM’s government relations project coordinator.
Reference
Another year older, another year wiser. SHM’s advocacy efforts have grown immensely in the last year thanks to proactive members who understand the importance of being involved and taking action. Because of member engagement—through the online Legislative Action Center and in-person meetings with members of Congress—hospitalists are widely recognized on Capitol Hill as thoughtful resources on policy issues who prioritize the improvement of patient care and the healthcare system.
This year alone, we have had a demonstrable impact on three key health policy issues: the sustainable growth rate (SGR), meaningful use, and observation status.
SGR repeal has been a consistent source of frustration and a regular feature of SHM’s advocacy efforts, including messages from hundreds of hospitalists to Congress, numerous requests for input from SHM as Congress worked on developing repeal legislation, and meetings with lawmakers to further shape legislation and to ask for the prioritization of a permanent, reliable solution. Although temporary patches have been the norm, SHM has not given up on repeal efforts and continues to push Congress to move past this failed formula.
Awareness of the precarious position of hospitalists in meaningful use has broadened significantly, and SHM worked with CMS to secure an exception from penalties for most hospitalists for 2015. The exception is only temporary, however, and SHM members will be essential in the effort to convince both CMS and Congress to enact a sensible permanent solution.
Observation status and the two-midnight rule saw significant coverage and interest throughout the year. Hospitalist and SHM Public Policy Committee member Ann Sheehy, MD, MS, FHM, testified about the impacts of observation status on hospitals and on patients in front of both the House Ways and Means Subcommittee on Health and the Senate Special Committee on Aging. The SHM Public Policy Committee also published a widely read white paper explaining SHM’s position on the issue.
Here is a key passage that perfectly reflects SHM’s position on observation status:
“Any policy change should be rooted in common sense, reflective of clinical reality, and designed to ensure that patients and providers are incentivized to work together to improve health. Patients should be able to get the care that they need, when they need it, including access to SNF [skilled nursing facility] care. Medicare policies should not be unnecessary impediments to physician judgment and workflow and should be geared toward reducing administrative burden and complexity.”1
Advocacy needs the active voices of those who understand the daily impacts of health policies. SHM would not be as successful a resource without our hospitalist members taking steps to better the healthcare system overall.
Building relationships with policymakers is the key to SHM remaining a prominent resource that is trusted and respected in Washington, D.C. At the 2015 annual meeting in National Harbor, Md., SHM will be sponsoring another “Hospitalists on the Hill” event, where we plan to build on recent successes. In 2013, more than 100 hospitalists took to the halls of Congress to advocate for pressing policy issues. Be sure to plan accordingly, as Hill Day will be an all-day event on April 1, the final day of the meeting.
Ellen Boyer is SHM’s government relations project coordinator.
Reference
Should Patients with an Unprovoked VTE Be Screened for Malignancy or a Hypercoagulable State?
Case
A 56-year-old woman with hypertension and diabetes presents to the hospital with acute onset of painful swelling in her right calf. She has had no recent surgeries, trauma, or travel, and takes lisinopril and metformin. An ultrasound of her right lower extremity demonstrates a venous thromboembolism (VTE). The patient’s last mammogram was three years ago, and she’s never undergone a screening colonoscopy. On lab workup, she is noted to have a microcytic anemia.
Should this patient be screened for an underlying hypercoagulable state or malignancy?
Background
An estimated 550,000 hospitalized adults are diagnosed with VTE each year.1 VTE can occur in the absence of known precipitants (unprovoked) or can be temporally associated with a known major risk factor (provoked). This practical division has implications for both treatment duration and risk of recurrence. A VTE is considered provoked if it occurs in the setting of surgery, leg trauma, fracture, pregnancy within the previous three months, estrogen therapy, immobility from an acute illness for more than one week, travel lasting more than six hours, or active malignancy.2 If none of these provoking factors is present, the VTE is considered unprovoked.2
Nearly 20% of first-time VTE events can be attributed to malignancy.3 Additionally, patients presenting with an unprovoked VTE possess a higher risk of being diagnosed with a cancer, raising the question of whether unprovoked VTEs should compel aggressive malignancy screening.4
Before the discovery of antithrombin deficiency in 1965, most unprovoked VTE events remained unexplained. Since then, numerous inherited coagulation abnormalities have been identified. It is now estimated that coagulation abnormalities can be found in up to half of patients with unprovoked thrombi.5
The increase in availability of molecular and genetic assays for hypercoagulability has been accompanied by a dramatic rise in the rate of testing for these disorders.6 Despite increased testing available for inherited thrombophilias, disagreement exists over the utility of this workup.6
Review of the Data
Hypercoagulability leading to venous thrombosis can be broadly divided into two groups: acquired and hereditary (see Table 1). First, let’s examine acquired hypercoagulable states.
Malignancy: Armand Trousseau first suggested an association between thrombotic events and malignancy in 1865. Malignancy causes a hypercoagulable state; additionally, tumors can cause thromboemboli by other mechanisms, such as vascular invasion or external compression of vasculature.7
Multiple studies demonstrate that malignancy increases the chance of developing a VTE. A Danish cohort study of nearly 60,000 cancer patients compared with over 280,000 controls over nine years offered twice the incidence of VTE in patients with cancer.8 Other studies reveal that VTE rates peak in the first year after a cancer diagnosis; moreover, VTE events are associated with more advanced disease and worse prognosis.9 Approximately 11% of cancer patients will develop a clinically evident VTE during the course of their disease.10,11
The majority of cancers associated with VTE events are clinically evident; however, some patients with thrombi have an occult malignancy. During the two years following an unprovoked VTE, the rate of discovering a previously undiagnosed malignancy was three times higher when compared with provoked VTE.6
This potential to diagnose occult malignancy in patients with idiopathic thromboembolic events stimulates debate around the usefulness of extensive cancer screening for these patients. One large systematic review compared routine and extensive cancer screening strategies following an unprovoked VTE. An extensive screening strategy consisting of CT scans of the abdomen and pelvis significantly increased the proportion of previously undiagnosed cancers; however, the authors did not determine complication rates, cost effectiveness, or difference in morbidity and mortality associated with extensive screening strategies.7
Other studies have demonstrated that extensive screening with CT, endoscopy, and tumor markers finds more previously undetected cancers; however, up to half of these malignancies could have been identified without resorting to such expensive and invasive workups.12 Additionally, no prospective data demonstrate improved outcomes or increased survival from these diagnoses. Likewise, no cost-effectiveness data exist to support this expensive and aggressive screening approach.7
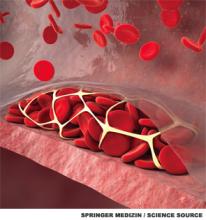
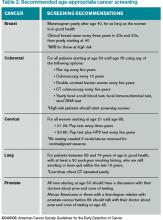
All patients with an idiopathic VTE should undergo a complete history and physical examination with attention to common areas of malignancy. Patients should have basic lab work and be recommended for age-appropriate cancer screening (see Table 2). Any abnormalities uncovered on this initial workup should be aggressively investigated.13 If overt cancer is detected, then low molecular weight heparin would be preferred over oral anticoagulation as treatment for the VTE.14 Extensive malignancy evaluation in all patients with unprovoked VTE is not warranted, however, given the lack of data regarding efficacy of extensive screening, the potential for increased harms, and the costs associated with this approach.
Antiphospholipid syndrome: Antiphospholipid syndrome is the most common acquired cause of thrombophilia.15 Characterized by the presence of antiphospholipid antibodies (e.g. lupus anticoagulant antibodies or anticardiolipin antibodies), this syndrome is usually secondary to cancer or an autoimmune disease.
Antiphospholipid antibody syndrome is a thrombophilic disorder in which both venous and arterial thrombosis may occur. Patients with this disorder are considered at high risk for thrombotic events. Data suggest that antiphospholipid antibody syndrome also increases the risk of VTE recurrence. In one retrospective study, cessation of warfarin therapy in patients with antiphospholipid antibodies after a VTE resulted in 69% of patients having recurrent thrombosis in the first year.16 Given this substantial risk, antiphospholipid antibody testing is recommended in those with a suggestive history, including patients with 1) recurrent fetal loss, 2) fetal loss after 10 weeks, or 3) known collagen vascular disease.16 Lifelong anticoagulation is recommended for these patients.
Inherited hypercoagulable states: The most frequent causes of an inherited hypercoagulable state are the factor V Leiden mutation and the prothrombin gene mutation, accounting for 50% to 60% of hereditary thrombophilias. Protein S, protein C, and antithrombin defects account for most of the remaining cases of inherited thrombophilias.15
Currently, there is no consensus regarding who should be tested for inherited thrombophilia. Testing for an inherited thrombophilia would be indicated if the results added prognostic information or changed management. Arguments against testing hinge on the fact that neither prognosis nor management is affected by the presence of an inherited thrombophilia.
The presence of a thrombophilia also does not change the method or intensity of anticoagulation.17 The risk of recurrence after discontinuing anticoagulation therapy is not affected.17,18 The strongest predictor of VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia.15 Recurrent VTE is nearly twice as frequent in patients with idiopathic VTE compared to those with provoked VTE.15
The American College of Chest Physicians (ACCP) recommends treating a provoked VTE for three months.19 According to the same guidelines, an unprovoked VTE should be treated for a minimum of three months, and lifelong anticoagulation should be considered.19
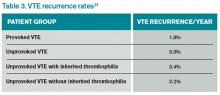
Overall, the rate of recurrence after a first VTE is considerable after completion of anticoagulation, especially for an unprovoked thrombotic event. Studies show a 7%-15% recurrence rate during the two years following the index VTE (see Table 3).17,20,21 Currently, no data suggest that a hereditary thrombophilia substantially changes this baseline high recurrent risk. ACCP recommendations state that the presence of hereditary thrombophilia should not be used as a major factor to guide duration of anticoagulation.19
Back to the Case
Our patient presented with an unprovoked VTE. She should be started on anticoagulation therapy with low molecular weight heparin and transitioned to oral anticoagulation.
Her highest risk for VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia. Since the presence of an inherited thrombophilia will not change duration or intensity of management, our patient should not be tested.
There are no prospective trials showing improved outcomes from aggressive workup for occult malignancy. Given this information, an extensive workup for occult malignancy should not be undertaken; however, this patient has an idiopathic VTE and should undergo a complete history, physical examination, and basic lab work, with attention to common areas of malignancy. Any abnormalities uncovered on this initial workup should be investigated more aggressively. Screening with mammography and Pap smear should be arranged in outpatient follow-up and communicated to the primary care physician, because she is not up to date with these age-appropriate screening tests.
Based on new evidence, a low-dose chest CT would be a consideration if she had a smoking history of at least 30 pack-years.22 Her microcytic anemia uncovered on routine lab work should be investigated further for a possible underlying gastrointestinal malignancy.
Bottom Line
An initial diagnosis of unprovoked VTE remains the strongest risk factor for recurrent thromboembolic events. The presence of an inherited thrombophilia does not significantly alter management. Aggressive workup for occult malignancy has not prospectively improved outcomes, but age-appropriate malignancy screening should be recommended.
Drs. Czernik and Anderson are hospitalists and instructors of medicine at the University of Colorado Denver (UCD). Dr. Wolfe is a hospitalist and assistant professor of medicine at UCD. Dr. Cumbler is a hospitalist and associate professor of medicine at UCD.
References
- Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61(22);401-404.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220.
- Heit, JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245-1248.
- Iodice S, Gandini S, Löhr M, Lowenfels AB, Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost. 2008;6(5):781-788.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5(9):1979-1981.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149(5):323-333.
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947-953.
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464.
- Lee JL, Lee JH, Kim MK, et al. A case of bone marrow necrosis with thrombotic thrombocytopenic purpura as a manifestation of occult colon cancer. Jpn J Clin Oncol. 2004;34(8):476-480.
- Sack GH Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977;56(1):1-37.
- Prins MH, Hettiarachchi RJ, Lensing AW, Hirsh J. Newly diagnosed malignancy in patients with venous thromboembolism. Search or wait and see? Thromb Haemost. 1997;78(1):121-125.
- Cornuz J, Pearson SD, Creager MA, Cook EF, Goldman L. Importance of findings on the initial evaluation for cancer in patients with symptomatic idiopathic deep venous thrombosis. Ann Intern Med. 1996;125(10):785-793.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121(6):458-463.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425-1434.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med. 2006;119(1):50-53.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Douketis, James, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361.
- American Cancer Society Guidelines for the Early Detection of Cancer. Available at: http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed November 15, 2014.
Case
A 56-year-old woman with hypertension and diabetes presents to the hospital with acute onset of painful swelling in her right calf. She has had no recent surgeries, trauma, or travel, and takes lisinopril and metformin. An ultrasound of her right lower extremity demonstrates a venous thromboembolism (VTE). The patient’s last mammogram was three years ago, and she’s never undergone a screening colonoscopy. On lab workup, she is noted to have a microcytic anemia.
Should this patient be screened for an underlying hypercoagulable state or malignancy?
Background
An estimated 550,000 hospitalized adults are diagnosed with VTE each year.1 VTE can occur in the absence of known precipitants (unprovoked) or can be temporally associated with a known major risk factor (provoked). This practical division has implications for both treatment duration and risk of recurrence. A VTE is considered provoked if it occurs in the setting of surgery, leg trauma, fracture, pregnancy within the previous three months, estrogen therapy, immobility from an acute illness for more than one week, travel lasting more than six hours, or active malignancy.2 If none of these provoking factors is present, the VTE is considered unprovoked.2
Nearly 20% of first-time VTE events can be attributed to malignancy.3 Additionally, patients presenting with an unprovoked VTE possess a higher risk of being diagnosed with a cancer, raising the question of whether unprovoked VTEs should compel aggressive malignancy screening.4
Before the discovery of antithrombin deficiency in 1965, most unprovoked VTE events remained unexplained. Since then, numerous inherited coagulation abnormalities have been identified. It is now estimated that coagulation abnormalities can be found in up to half of patients with unprovoked thrombi.5
The increase in availability of molecular and genetic assays for hypercoagulability has been accompanied by a dramatic rise in the rate of testing for these disorders.6 Despite increased testing available for inherited thrombophilias, disagreement exists over the utility of this workup.6
Review of the Data
Hypercoagulability leading to venous thrombosis can be broadly divided into two groups: acquired and hereditary (see Table 1). First, let’s examine acquired hypercoagulable states.
Malignancy: Armand Trousseau first suggested an association between thrombotic events and malignancy in 1865. Malignancy causes a hypercoagulable state; additionally, tumors can cause thromboemboli by other mechanisms, such as vascular invasion or external compression of vasculature.7
Multiple studies demonstrate that malignancy increases the chance of developing a VTE. A Danish cohort study of nearly 60,000 cancer patients compared with over 280,000 controls over nine years offered twice the incidence of VTE in patients with cancer.8 Other studies reveal that VTE rates peak in the first year after a cancer diagnosis; moreover, VTE events are associated with more advanced disease and worse prognosis.9 Approximately 11% of cancer patients will develop a clinically evident VTE during the course of their disease.10,11
The majority of cancers associated with VTE events are clinically evident; however, some patients with thrombi have an occult malignancy. During the two years following an unprovoked VTE, the rate of discovering a previously undiagnosed malignancy was three times higher when compared with provoked VTE.6
This potential to diagnose occult malignancy in patients with idiopathic thromboembolic events stimulates debate around the usefulness of extensive cancer screening for these patients. One large systematic review compared routine and extensive cancer screening strategies following an unprovoked VTE. An extensive screening strategy consisting of CT scans of the abdomen and pelvis significantly increased the proportion of previously undiagnosed cancers; however, the authors did not determine complication rates, cost effectiveness, or difference in morbidity and mortality associated with extensive screening strategies.7
Other studies have demonstrated that extensive screening with CT, endoscopy, and tumor markers finds more previously undetected cancers; however, up to half of these malignancies could have been identified without resorting to such expensive and invasive workups.12 Additionally, no prospective data demonstrate improved outcomes or increased survival from these diagnoses. Likewise, no cost-effectiveness data exist to support this expensive and aggressive screening approach.7
All patients with an idiopathic VTE should undergo a complete history and physical examination with attention to common areas of malignancy. Patients should have basic lab work and be recommended for age-appropriate cancer screening (see Table 2). Any abnormalities uncovered on this initial workup should be aggressively investigated.13 If overt cancer is detected, then low molecular weight heparin would be preferred over oral anticoagulation as treatment for the VTE.14 Extensive malignancy evaluation in all patients with unprovoked VTE is not warranted, however, given the lack of data regarding efficacy of extensive screening, the potential for increased harms, and the costs associated with this approach.
Antiphospholipid syndrome: Antiphospholipid syndrome is the most common acquired cause of thrombophilia.15 Characterized by the presence of antiphospholipid antibodies (e.g. lupus anticoagulant antibodies or anticardiolipin antibodies), this syndrome is usually secondary to cancer or an autoimmune disease.
Antiphospholipid antibody syndrome is a thrombophilic disorder in which both venous and arterial thrombosis may occur. Patients with this disorder are considered at high risk for thrombotic events. Data suggest that antiphospholipid antibody syndrome also increases the risk of VTE recurrence. In one retrospective study, cessation of warfarin therapy in patients with antiphospholipid antibodies after a VTE resulted in 69% of patients having recurrent thrombosis in the first year.16 Given this substantial risk, antiphospholipid antibody testing is recommended in those with a suggestive history, including patients with 1) recurrent fetal loss, 2) fetal loss after 10 weeks, or 3) known collagen vascular disease.16 Lifelong anticoagulation is recommended for these patients.
Inherited hypercoagulable states: The most frequent causes of an inherited hypercoagulable state are the factor V Leiden mutation and the prothrombin gene mutation, accounting for 50% to 60% of hereditary thrombophilias. Protein S, protein C, and antithrombin defects account for most of the remaining cases of inherited thrombophilias.15
Currently, there is no consensus regarding who should be tested for inherited thrombophilia. Testing for an inherited thrombophilia would be indicated if the results added prognostic information or changed management. Arguments against testing hinge on the fact that neither prognosis nor management is affected by the presence of an inherited thrombophilia.
The presence of a thrombophilia also does not change the method or intensity of anticoagulation.17 The risk of recurrence after discontinuing anticoagulation therapy is not affected.17,18 The strongest predictor of VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia.15 Recurrent VTE is nearly twice as frequent in patients with idiopathic VTE compared to those with provoked VTE.15
The American College of Chest Physicians (ACCP) recommends treating a provoked VTE for three months.19 According to the same guidelines, an unprovoked VTE should be treated for a minimum of three months, and lifelong anticoagulation should be considered.19
Overall, the rate of recurrence after a first VTE is considerable after completion of anticoagulation, especially for an unprovoked thrombotic event. Studies show a 7%-15% recurrence rate during the two years following the index VTE (see Table 3).17,20,21 Currently, no data suggest that a hereditary thrombophilia substantially changes this baseline high recurrent risk. ACCP recommendations state that the presence of hereditary thrombophilia should not be used as a major factor to guide duration of anticoagulation.19
Back to the Case
Our patient presented with an unprovoked VTE. She should be started on anticoagulation therapy with low molecular weight heparin and transitioned to oral anticoagulation.
Her highest risk for VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia. Since the presence of an inherited thrombophilia will not change duration or intensity of management, our patient should not be tested.
There are no prospective trials showing improved outcomes from aggressive workup for occult malignancy. Given this information, an extensive workup for occult malignancy should not be undertaken; however, this patient has an idiopathic VTE and should undergo a complete history, physical examination, and basic lab work, with attention to common areas of malignancy. Any abnormalities uncovered on this initial workup should be investigated more aggressively. Screening with mammography and Pap smear should be arranged in outpatient follow-up and communicated to the primary care physician, because she is not up to date with these age-appropriate screening tests.
Based on new evidence, a low-dose chest CT would be a consideration if she had a smoking history of at least 30 pack-years.22 Her microcytic anemia uncovered on routine lab work should be investigated further for a possible underlying gastrointestinal malignancy.
Bottom Line
An initial diagnosis of unprovoked VTE remains the strongest risk factor for recurrent thromboembolic events. The presence of an inherited thrombophilia does not significantly alter management. Aggressive workup for occult malignancy has not prospectively improved outcomes, but age-appropriate malignancy screening should be recommended.
Drs. Czernik and Anderson are hospitalists and instructors of medicine at the University of Colorado Denver (UCD). Dr. Wolfe is a hospitalist and assistant professor of medicine at UCD. Dr. Cumbler is a hospitalist and associate professor of medicine at UCD.
References
- Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61(22);401-404.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220.
- Heit, JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245-1248.
- Iodice S, Gandini S, Löhr M, Lowenfels AB, Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost. 2008;6(5):781-788.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5(9):1979-1981.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149(5):323-333.
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947-953.
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464.
- Lee JL, Lee JH, Kim MK, et al. A case of bone marrow necrosis with thrombotic thrombocytopenic purpura as a manifestation of occult colon cancer. Jpn J Clin Oncol. 2004;34(8):476-480.
- Sack GH Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977;56(1):1-37.
- Prins MH, Hettiarachchi RJ, Lensing AW, Hirsh J. Newly diagnosed malignancy in patients with venous thromboembolism. Search or wait and see? Thromb Haemost. 1997;78(1):121-125.
- Cornuz J, Pearson SD, Creager MA, Cook EF, Goldman L. Importance of findings on the initial evaluation for cancer in patients with symptomatic idiopathic deep venous thrombosis. Ann Intern Med. 1996;125(10):785-793.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121(6):458-463.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425-1434.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med. 2006;119(1):50-53.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Douketis, James, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361.
- American Cancer Society Guidelines for the Early Detection of Cancer. Available at: http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed November 15, 2014.
Case
A 56-year-old woman with hypertension and diabetes presents to the hospital with acute onset of painful swelling in her right calf. She has had no recent surgeries, trauma, or travel, and takes lisinopril and metformin. An ultrasound of her right lower extremity demonstrates a venous thromboembolism (VTE). The patient’s last mammogram was three years ago, and she’s never undergone a screening colonoscopy. On lab workup, she is noted to have a microcytic anemia.
Should this patient be screened for an underlying hypercoagulable state or malignancy?
Background
An estimated 550,000 hospitalized adults are diagnosed with VTE each year.1 VTE can occur in the absence of known precipitants (unprovoked) or can be temporally associated with a known major risk factor (provoked). This practical division has implications for both treatment duration and risk of recurrence. A VTE is considered provoked if it occurs in the setting of surgery, leg trauma, fracture, pregnancy within the previous three months, estrogen therapy, immobility from an acute illness for more than one week, travel lasting more than six hours, or active malignancy.2 If none of these provoking factors is present, the VTE is considered unprovoked.2
Nearly 20% of first-time VTE events can be attributed to malignancy.3 Additionally, patients presenting with an unprovoked VTE possess a higher risk of being diagnosed with a cancer, raising the question of whether unprovoked VTEs should compel aggressive malignancy screening.4
Before the discovery of antithrombin deficiency in 1965, most unprovoked VTE events remained unexplained. Since then, numerous inherited coagulation abnormalities have been identified. It is now estimated that coagulation abnormalities can be found in up to half of patients with unprovoked thrombi.5
The increase in availability of molecular and genetic assays for hypercoagulability has been accompanied by a dramatic rise in the rate of testing for these disorders.6 Despite increased testing available for inherited thrombophilias, disagreement exists over the utility of this workup.6
Review of the Data
Hypercoagulability leading to venous thrombosis can be broadly divided into two groups: acquired and hereditary (see Table 1). First, let’s examine acquired hypercoagulable states.
Malignancy: Armand Trousseau first suggested an association between thrombotic events and malignancy in 1865. Malignancy causes a hypercoagulable state; additionally, tumors can cause thromboemboli by other mechanisms, such as vascular invasion or external compression of vasculature.7
Multiple studies demonstrate that malignancy increases the chance of developing a VTE. A Danish cohort study of nearly 60,000 cancer patients compared with over 280,000 controls over nine years offered twice the incidence of VTE in patients with cancer.8 Other studies reveal that VTE rates peak in the first year after a cancer diagnosis; moreover, VTE events are associated with more advanced disease and worse prognosis.9 Approximately 11% of cancer patients will develop a clinically evident VTE during the course of their disease.10,11
The majority of cancers associated with VTE events are clinically evident; however, some patients with thrombi have an occult malignancy. During the two years following an unprovoked VTE, the rate of discovering a previously undiagnosed malignancy was three times higher when compared with provoked VTE.6
This potential to diagnose occult malignancy in patients with idiopathic thromboembolic events stimulates debate around the usefulness of extensive cancer screening for these patients. One large systematic review compared routine and extensive cancer screening strategies following an unprovoked VTE. An extensive screening strategy consisting of CT scans of the abdomen and pelvis significantly increased the proportion of previously undiagnosed cancers; however, the authors did not determine complication rates, cost effectiveness, or difference in morbidity and mortality associated with extensive screening strategies.7
Other studies have demonstrated that extensive screening with CT, endoscopy, and tumor markers finds more previously undetected cancers; however, up to half of these malignancies could have been identified without resorting to such expensive and invasive workups.12 Additionally, no prospective data demonstrate improved outcomes or increased survival from these diagnoses. Likewise, no cost-effectiveness data exist to support this expensive and aggressive screening approach.7
All patients with an idiopathic VTE should undergo a complete history and physical examination with attention to common areas of malignancy. Patients should have basic lab work and be recommended for age-appropriate cancer screening (see Table 2). Any abnormalities uncovered on this initial workup should be aggressively investigated.13 If overt cancer is detected, then low molecular weight heparin would be preferred over oral anticoagulation as treatment for the VTE.14 Extensive malignancy evaluation in all patients with unprovoked VTE is not warranted, however, given the lack of data regarding efficacy of extensive screening, the potential for increased harms, and the costs associated with this approach.
Antiphospholipid syndrome: Antiphospholipid syndrome is the most common acquired cause of thrombophilia.15 Characterized by the presence of antiphospholipid antibodies (e.g. lupus anticoagulant antibodies or anticardiolipin antibodies), this syndrome is usually secondary to cancer or an autoimmune disease.
Antiphospholipid antibody syndrome is a thrombophilic disorder in which both venous and arterial thrombosis may occur. Patients with this disorder are considered at high risk for thrombotic events. Data suggest that antiphospholipid antibody syndrome also increases the risk of VTE recurrence. In one retrospective study, cessation of warfarin therapy in patients with antiphospholipid antibodies after a VTE resulted in 69% of patients having recurrent thrombosis in the first year.16 Given this substantial risk, antiphospholipid antibody testing is recommended in those with a suggestive history, including patients with 1) recurrent fetal loss, 2) fetal loss after 10 weeks, or 3) known collagen vascular disease.16 Lifelong anticoagulation is recommended for these patients.
Inherited hypercoagulable states: The most frequent causes of an inherited hypercoagulable state are the factor V Leiden mutation and the prothrombin gene mutation, accounting for 50% to 60% of hereditary thrombophilias. Protein S, protein C, and antithrombin defects account for most of the remaining cases of inherited thrombophilias.15
Currently, there is no consensus regarding who should be tested for inherited thrombophilia. Testing for an inherited thrombophilia would be indicated if the results added prognostic information or changed management. Arguments against testing hinge on the fact that neither prognosis nor management is affected by the presence of an inherited thrombophilia.
The presence of a thrombophilia also does not change the method or intensity of anticoagulation.17 The risk of recurrence after discontinuing anticoagulation therapy is not affected.17,18 The strongest predictor of VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia.15 Recurrent VTE is nearly twice as frequent in patients with idiopathic VTE compared to those with provoked VTE.15
The American College of Chest Physicians (ACCP) recommends treating a provoked VTE for three months.19 According to the same guidelines, an unprovoked VTE should be treated for a minimum of three months, and lifelong anticoagulation should be considered.19
Overall, the rate of recurrence after a first VTE is considerable after completion of anticoagulation, especially for an unprovoked thrombotic event. Studies show a 7%-15% recurrence rate during the two years following the index VTE (see Table 3).17,20,21 Currently, no data suggest that a hereditary thrombophilia substantially changes this baseline high recurrent risk. ACCP recommendations state that the presence of hereditary thrombophilia should not be used as a major factor to guide duration of anticoagulation.19
Back to the Case
Our patient presented with an unprovoked VTE. She should be started on anticoagulation therapy with low molecular weight heparin and transitioned to oral anticoagulation.
Her highest risk for VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia. Since the presence of an inherited thrombophilia will not change duration or intensity of management, our patient should not be tested.
There are no prospective trials showing improved outcomes from aggressive workup for occult malignancy. Given this information, an extensive workup for occult malignancy should not be undertaken; however, this patient has an idiopathic VTE and should undergo a complete history, physical examination, and basic lab work, with attention to common areas of malignancy. Any abnormalities uncovered on this initial workup should be investigated more aggressively. Screening with mammography and Pap smear should be arranged in outpatient follow-up and communicated to the primary care physician, because she is not up to date with these age-appropriate screening tests.
Based on new evidence, a low-dose chest CT would be a consideration if she had a smoking history of at least 30 pack-years.22 Her microcytic anemia uncovered on routine lab work should be investigated further for a possible underlying gastrointestinal malignancy.
Bottom Line
An initial diagnosis of unprovoked VTE remains the strongest risk factor for recurrent thromboembolic events. The presence of an inherited thrombophilia does not significantly alter management. Aggressive workup for occult malignancy has not prospectively improved outcomes, but age-appropriate malignancy screening should be recommended.
Drs. Czernik and Anderson are hospitalists and instructors of medicine at the University of Colorado Denver (UCD). Dr. Wolfe is a hospitalist and assistant professor of medicine at UCD. Dr. Cumbler is a hospitalist and associate professor of medicine at UCD.
References
- Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61(22);401-404.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220.
- Heit, JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245-1248.
- Iodice S, Gandini S, Löhr M, Lowenfels AB, Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost. 2008;6(5):781-788.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5(9):1979-1981.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149(5):323-333.
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947-953.
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464.
- Lee JL, Lee JH, Kim MK, et al. A case of bone marrow necrosis with thrombotic thrombocytopenic purpura as a manifestation of occult colon cancer. Jpn J Clin Oncol. 2004;34(8):476-480.
- Sack GH Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977;56(1):1-37.
- Prins MH, Hettiarachchi RJ, Lensing AW, Hirsh J. Newly diagnosed malignancy in patients with venous thromboembolism. Search or wait and see? Thromb Haemost. 1997;78(1):121-125.
- Cornuz J, Pearson SD, Creager MA, Cook EF, Goldman L. Importance of findings on the initial evaluation for cancer in patients with symptomatic idiopathic deep venous thrombosis. Ann Intern Med. 1996;125(10):785-793.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121(6):458-463.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425-1434.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med. 2006;119(1):50-53.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Douketis, James, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361.
- American Cancer Society Guidelines for the Early Detection of Cancer. Available at: http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed November 15, 2014.
Pediatric Hospitalist David Pressel, MD, Hooked on Hospital Medicine
Sometimes a physician’s choice of specialty is borne of one patient, one mentor, or one experience. And then sometimes there’s just a good feeling.
Put David Pressel, MD, PhD, FHM, in the latter category.
He simultaneously earned his medical degree and a doctorate in neuroscience from Washington University in Saint Louis in 1993. He did his internship and residency in pediatrics at St. Louis Children’s Hospital.
He hasn’t left HM since.
It was the “first temporary job out of training that I really liked and continued,” Dr. Pressel says.
Fast forward nearly 20 years and Dr. Pressel is one of six new members of Team Hospitalist, the volunteer editorial advisory board of The Hospitalist. Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del. He has served as an associate professor of pediatrics at Jefferson Medical College in Philadelphia since 2008 and was an assistant professor at Temple University School of Medicine before that.
–Dr. Pressel
He was Nemours/Alfred I. duPont Hospital physician of the year in 2008 and recently won the Marcum Innovator of the Year Award for development of a hospital program to improve the care of patients experiencing a behavior emergency. He also has been an executive board member of SHM’s Philadelphia chapter for two years.
He admits to having a lot of trepidation and uncertainty as a nascent physician but happily notes that those emotions subsided as he “became more competent and independent.”
Of course, the stresses of the job are still there today, but he can’t imagine a better job than being a pediatric hospitalist. Now, Dr. Pressel enjoys the variety of experiences that HM provides: interacting with patients and colleagues across the hospital, taking satisfaction from mentoring others, and networking at national meetings. In fact, he got his current job by chatting up a colleague at an SHM convention.
Question: Why did you choose a career in medicine?
Answer: Had an unbelievably positive experience with a physician during a personal illness. My degree of emotional concern was disproportionate to my physical issues—the doc I saw was perceptive and discussed them with me. It was an epiphany, and I decided to become a physician. Unfortunately, I don’t know her name and have no way of letting her know what a profound impact she had on my life.
Q: Did you have a mentor during training or early career? If so, who was the mentor and what were the most important lessons you learned from him/her?
A: Several. How to integrate work-life balance. Some folks I worked with seemed to be consumed by their career to the detriment of home life. I learned from one mentor to try to integrate one’s interests. I have tried to involve my family in my teaching—my wife and kids have role-played as model patients. My son is a co-author on a presentation regarding violent patients in which he plays a violent patient that workshop participants need to control and care for.
Q: What’s the biggest change you’ve seen in HM in your career?
A: Development as a full career rather than a temporary choice. I started as a hospitalist before the term was coined and initially was thinking I’d need to do a fellowship to have a successful career in academic medicine. This has not been the case.
Q: What’s the biggest change you would like to see in HM?
A: Board certification with salary increase.
Q: As a group leader, why is it important for you to continue seeing patients?
A: A boss who is disconnected from the front line is potentially dangerous.
Q: As a hospitalist, seeing most of your patients for the very first time, what aspect of patient care is most challenging?
A: The uncertainty of whether I’m making a mistake. Dealing with families with mental illness.
Q: What aspect of patient care is most rewarding?
A: Getting thanked.
Q: What aspect of teaching in the 21st century is most difficult? And, what is most enjoyable?
A: Same as 20th century except mobile technology, and the students are better. I am a late adopter of technology, having become reasonably successful and happy without these tools.
Q: Outside of patient care, tell me about your career interests.
A: Lots, including comanagement and violent patients. As above, agitated patients who become violent are encountered in hospital medicine. I’ve been bitten twice; other staff members have been injured by patients. There is limited training for staff in this area. By necessity, I have become expert and am expanding my skill and research interests in this area.
Richard Quinn is a freelance writer in New Jersey.
Sometimes a physician’s choice of specialty is borne of one patient, one mentor, or one experience. And then sometimes there’s just a good feeling.
Put David Pressel, MD, PhD, FHM, in the latter category.
He simultaneously earned his medical degree and a doctorate in neuroscience from Washington University in Saint Louis in 1993. He did his internship and residency in pediatrics at St. Louis Children’s Hospital.
He hasn’t left HM since.
It was the “first temporary job out of training that I really liked and continued,” Dr. Pressel says.
Fast forward nearly 20 years and Dr. Pressel is one of six new members of Team Hospitalist, the volunteer editorial advisory board of The Hospitalist. Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del. He has served as an associate professor of pediatrics at Jefferson Medical College in Philadelphia since 2008 and was an assistant professor at Temple University School of Medicine before that.
–Dr. Pressel
He was Nemours/Alfred I. duPont Hospital physician of the year in 2008 and recently won the Marcum Innovator of the Year Award for development of a hospital program to improve the care of patients experiencing a behavior emergency. He also has been an executive board member of SHM’s Philadelphia chapter for two years.
He admits to having a lot of trepidation and uncertainty as a nascent physician but happily notes that those emotions subsided as he “became more competent and independent.”
Of course, the stresses of the job are still there today, but he can’t imagine a better job than being a pediatric hospitalist. Now, Dr. Pressel enjoys the variety of experiences that HM provides: interacting with patients and colleagues across the hospital, taking satisfaction from mentoring others, and networking at national meetings. In fact, he got his current job by chatting up a colleague at an SHM convention.
Question: Why did you choose a career in medicine?
Answer: Had an unbelievably positive experience with a physician during a personal illness. My degree of emotional concern was disproportionate to my physical issues—the doc I saw was perceptive and discussed them with me. It was an epiphany, and I decided to become a physician. Unfortunately, I don’t know her name and have no way of letting her know what a profound impact she had on my life.
Q: Did you have a mentor during training or early career? If so, who was the mentor and what were the most important lessons you learned from him/her?
A: Several. How to integrate work-life balance. Some folks I worked with seemed to be consumed by their career to the detriment of home life. I learned from one mentor to try to integrate one’s interests. I have tried to involve my family in my teaching—my wife and kids have role-played as model patients. My son is a co-author on a presentation regarding violent patients in which he plays a violent patient that workshop participants need to control and care for.
Q: What’s the biggest change you’ve seen in HM in your career?
A: Development as a full career rather than a temporary choice. I started as a hospitalist before the term was coined and initially was thinking I’d need to do a fellowship to have a successful career in academic medicine. This has not been the case.
Q: What’s the biggest change you would like to see in HM?
A: Board certification with salary increase.
Q: As a group leader, why is it important for you to continue seeing patients?
A: A boss who is disconnected from the front line is potentially dangerous.
Q: As a hospitalist, seeing most of your patients for the very first time, what aspect of patient care is most challenging?
A: The uncertainty of whether I’m making a mistake. Dealing with families with mental illness.
Q: What aspect of patient care is most rewarding?
A: Getting thanked.
Q: What aspect of teaching in the 21st century is most difficult? And, what is most enjoyable?
A: Same as 20th century except mobile technology, and the students are better. I am a late adopter of technology, having become reasonably successful and happy without these tools.
Q: Outside of patient care, tell me about your career interests.
A: Lots, including comanagement and violent patients. As above, agitated patients who become violent are encountered in hospital medicine. I’ve been bitten twice; other staff members have been injured by patients. There is limited training for staff in this area. By necessity, I have become expert and am expanding my skill and research interests in this area.
Richard Quinn is a freelance writer in New Jersey.
Sometimes a physician’s choice of specialty is borne of one patient, one mentor, or one experience. And then sometimes there’s just a good feeling.
Put David Pressel, MD, PhD, FHM, in the latter category.
He simultaneously earned his medical degree and a doctorate in neuroscience from Washington University in Saint Louis in 1993. He did his internship and residency in pediatrics at St. Louis Children’s Hospital.
He hasn’t left HM since.
It was the “first temporary job out of training that I really liked and continued,” Dr. Pressel says.
Fast forward nearly 20 years and Dr. Pressel is one of six new members of Team Hospitalist, the volunteer editorial advisory board of The Hospitalist. Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del. He has served as an associate professor of pediatrics at Jefferson Medical College in Philadelphia since 2008 and was an assistant professor at Temple University School of Medicine before that.
–Dr. Pressel
He was Nemours/Alfred I. duPont Hospital physician of the year in 2008 and recently won the Marcum Innovator of the Year Award for development of a hospital program to improve the care of patients experiencing a behavior emergency. He also has been an executive board member of SHM’s Philadelphia chapter for two years.
He admits to having a lot of trepidation and uncertainty as a nascent physician but happily notes that those emotions subsided as he “became more competent and independent.”
Of course, the stresses of the job are still there today, but he can’t imagine a better job than being a pediatric hospitalist. Now, Dr. Pressel enjoys the variety of experiences that HM provides: interacting with patients and colleagues across the hospital, taking satisfaction from mentoring others, and networking at national meetings. In fact, he got his current job by chatting up a colleague at an SHM convention.
Question: Why did you choose a career in medicine?
Answer: Had an unbelievably positive experience with a physician during a personal illness. My degree of emotional concern was disproportionate to my physical issues—the doc I saw was perceptive and discussed them with me. It was an epiphany, and I decided to become a physician. Unfortunately, I don’t know her name and have no way of letting her know what a profound impact she had on my life.
Q: Did you have a mentor during training or early career? If so, who was the mentor and what were the most important lessons you learned from him/her?
A: Several. How to integrate work-life balance. Some folks I worked with seemed to be consumed by their career to the detriment of home life. I learned from one mentor to try to integrate one’s interests. I have tried to involve my family in my teaching—my wife and kids have role-played as model patients. My son is a co-author on a presentation regarding violent patients in which he plays a violent patient that workshop participants need to control and care for.
Q: What’s the biggest change you’ve seen in HM in your career?
A: Development as a full career rather than a temporary choice. I started as a hospitalist before the term was coined and initially was thinking I’d need to do a fellowship to have a successful career in academic medicine. This has not been the case.
Q: What’s the biggest change you would like to see in HM?
A: Board certification with salary increase.
Q: As a group leader, why is it important for you to continue seeing patients?
A: A boss who is disconnected from the front line is potentially dangerous.
Q: As a hospitalist, seeing most of your patients for the very first time, what aspect of patient care is most challenging?
A: The uncertainty of whether I’m making a mistake. Dealing with families with mental illness.
Q: What aspect of patient care is most rewarding?
A: Getting thanked.
Q: What aspect of teaching in the 21st century is most difficult? And, what is most enjoyable?
A: Same as 20th century except mobile technology, and the students are better. I am a late adopter of technology, having become reasonably successful and happy without these tools.
Q: Outside of patient care, tell me about your career interests.
A: Lots, including comanagement and violent patients. As above, agitated patients who become violent are encountered in hospital medicine. I’ve been bitten twice; other staff members have been injured by patients. There is limited training for staff in this area. By necessity, I have become expert and am expanding my skill and research interests in this area.
Richard Quinn is a freelance writer in New Jersey.
Inpatient Strokes Average 4.5 Hours From Recognition to Computed Tomography
Time, in hours, from recognition of stroke symptoms to computed tomography for hospitalized patients, compared with 1.3 hours for stroke patients brought to the ED, according to a study recently presented at the Canadian Stroke Congress.
The report estimates 17% of strokes occur among inpatients, but those patients wait longer for both neuroimaging and thrombolysis. Inpatients who experienced strokes also had longer hospital stays and higher mortality rates than patients brought to the ED. Researchers suggest that signs of stroke may be overlooked in patients who were admitted to the hospital for another reason, which is the main focus for their hospital caregivers, compared with stroke symptoms that occur in the community, where they may stand out more starkly.
Time, in hours, from recognition of stroke symptoms to computed tomography for hospitalized patients, compared with 1.3 hours for stroke patients brought to the ED, according to a study recently presented at the Canadian Stroke Congress.
The report estimates 17% of strokes occur among inpatients, but those patients wait longer for both neuroimaging and thrombolysis. Inpatients who experienced strokes also had longer hospital stays and higher mortality rates than patients brought to the ED. Researchers suggest that signs of stroke may be overlooked in patients who were admitted to the hospital for another reason, which is the main focus for their hospital caregivers, compared with stroke symptoms that occur in the community, where they may stand out more starkly.
Time, in hours, from recognition of stroke symptoms to computed tomography for hospitalized patients, compared with 1.3 hours for stroke patients brought to the ED, according to a study recently presented at the Canadian Stroke Congress.
The report estimates 17% of strokes occur among inpatients, but those patients wait longer for both neuroimaging and thrombolysis. Inpatients who experienced strokes also had longer hospital stays and higher mortality rates than patients brought to the ED. Researchers suggest that signs of stroke may be overlooked in patients who were admitted to the hospital for another reason, which is the main focus for their hospital caregivers, compared with stroke symptoms that occur in the community, where they may stand out more starkly.
In-Room Computer Tablets Help Hospital Patients Learn, Communicate
New York’s Mount Sinai Hospital is among a growing number of healthcare systems nationwide providing patients with tablet computing devices.
Loaded with an HIPAA-compliant “patient itinerary” application designed by the hospital’s information technology (IT) department, the tablets give patients access to customized educational materials about their hospital stay. The program has been shown to ease communication between patients and caregivers, help patients to feel more engaged with their care, reduce patient stress, and improve staff workflow, according to a report in MHealthNews.
Mount Sinai’s inpatient care model redesign team—comprising both clinical and IT managers—deployed 100 tablets in the project’s pilot phase.
Meanwhile, a study from the United Kingdom reports that most patients don’t bring their own tablets to a hospital stay because “they are still unlikely to be able to connect to wifi when they get there,” according to a report in TheInformationDaily.com. Most of the country’s 2,300 hospitals fail to offer wireless access, and the access they do offer—bedside terminals with hotel-like charges for telephone, TV, video, and internet—mostly goes unused.
New York’s Mount Sinai Hospital is among a growing number of healthcare systems nationwide providing patients with tablet computing devices.
Loaded with an HIPAA-compliant “patient itinerary” application designed by the hospital’s information technology (IT) department, the tablets give patients access to customized educational materials about their hospital stay. The program has been shown to ease communication between patients and caregivers, help patients to feel more engaged with their care, reduce patient stress, and improve staff workflow, according to a report in MHealthNews.
Mount Sinai’s inpatient care model redesign team—comprising both clinical and IT managers—deployed 100 tablets in the project’s pilot phase.
Meanwhile, a study from the United Kingdom reports that most patients don’t bring their own tablets to a hospital stay because “they are still unlikely to be able to connect to wifi when they get there,” according to a report in TheInformationDaily.com. Most of the country’s 2,300 hospitals fail to offer wireless access, and the access they do offer—bedside terminals with hotel-like charges for telephone, TV, video, and internet—mostly goes unused.
New York’s Mount Sinai Hospital is among a growing number of healthcare systems nationwide providing patients with tablet computing devices.
Loaded with an HIPAA-compliant “patient itinerary” application designed by the hospital’s information technology (IT) department, the tablets give patients access to customized educational materials about their hospital stay. The program has been shown to ease communication between patients and caregivers, help patients to feel more engaged with their care, reduce patient stress, and improve staff workflow, according to a report in MHealthNews.
Mount Sinai’s inpatient care model redesign team—comprising both clinical and IT managers—deployed 100 tablets in the project’s pilot phase.
Meanwhile, a study from the United Kingdom reports that most patients don’t bring their own tablets to a hospital stay because “they are still unlikely to be able to connect to wifi when they get there,” according to a report in TheInformationDaily.com. Most of the country’s 2,300 hospitals fail to offer wireless access, and the access they do offer—bedside terminals with hotel-like charges for telephone, TV, video, and internet—mostly goes unused.
Medicare Readmissions Penalties Expected to Reach $428 Million
CMS started the third year of its Hospital Readmissions Reduction Program on October 1, with 2,610 U.S. hospitals—slightly more than in previous years—on the hook for penalties of up to 3% of their Medicare diagnosis-related grouping payments based on 30-day readmissions rates for diagnoses of myocardial infarction, heart failure, pneumonia, COPD, and elective total hip and total knee arthroplasty posted between July 2010 and June 2013.
According to analysis by Kaiser Health News, 39 hospitals will incur the maximum penalty, and hospitals collectively will pay an estimated $428 million in penalties in the current fiscal year for readmission rates deemed higher than expected by CMS formulas.
Medicare’s overall readmission rate in 2013 was 18%, which was down slightly from previous years but still amounted to two million patients. CMS estimates that these readmissions cost $26 billion, 65% of which was attributed to avoidable readmissions. CMS’ fiscal year 2015 final rule for reimbursement under the Hospital Inpatient Prospective Payment System, first published in the Federal Register, spells out fiscal year 2015 penalties and readmissions payment adjustment factors.
CMS started the third year of its Hospital Readmissions Reduction Program on October 1, with 2,610 U.S. hospitals—slightly more than in previous years—on the hook for penalties of up to 3% of their Medicare diagnosis-related grouping payments based on 30-day readmissions rates for diagnoses of myocardial infarction, heart failure, pneumonia, COPD, and elective total hip and total knee arthroplasty posted between July 2010 and June 2013.
According to analysis by Kaiser Health News, 39 hospitals will incur the maximum penalty, and hospitals collectively will pay an estimated $428 million in penalties in the current fiscal year for readmission rates deemed higher than expected by CMS formulas.
Medicare’s overall readmission rate in 2013 was 18%, which was down slightly from previous years but still amounted to two million patients. CMS estimates that these readmissions cost $26 billion, 65% of which was attributed to avoidable readmissions. CMS’ fiscal year 2015 final rule for reimbursement under the Hospital Inpatient Prospective Payment System, first published in the Federal Register, spells out fiscal year 2015 penalties and readmissions payment adjustment factors.
CMS started the third year of its Hospital Readmissions Reduction Program on October 1, with 2,610 U.S. hospitals—slightly more than in previous years—on the hook for penalties of up to 3% of their Medicare diagnosis-related grouping payments based on 30-day readmissions rates for diagnoses of myocardial infarction, heart failure, pneumonia, COPD, and elective total hip and total knee arthroplasty posted between July 2010 and June 2013.
According to analysis by Kaiser Health News, 39 hospitals will incur the maximum penalty, and hospitals collectively will pay an estimated $428 million in penalties in the current fiscal year for readmission rates deemed higher than expected by CMS formulas.
Medicare’s overall readmission rate in 2013 was 18%, which was down slightly from previous years but still amounted to two million patients. CMS estimates that these readmissions cost $26 billion, 65% of which was attributed to avoidable readmissions. CMS’ fiscal year 2015 final rule for reimbursement under the Hospital Inpatient Prospective Payment System, first published in the Federal Register, spells out fiscal year 2015 penalties and readmissions payment adjustment factors.