User login
Perioperative medicine: Combining the science and the art
In this issue of the Cleveland Clinic Journal of Medicine,1 Dr. Steven L. Cohn provides a succinct review of the recently published guidelines by the American College of Cardiology and American Heart Association (ACC/AHA) on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery.2 Although no drastic changes have been made in these guidelines, several significant modifications have been implemented and are highlighted in his review.
A BREACH OF SCIENTIFIC INTEGRITY
First, I am pleased Dr. Cohn described how the writing committee of the new guidelines handled the well-publicized breaches of scientific integrity by Dr. Don Poldermans, a prolific perioperative-medicine researcher at Erasmus University in the Netherlands who has contributed an abundance of literature that influenced clinical practice. Although some of his key publications were excluded by the ACC/AHA committee in its overall analysis, it remains unclear to me if simply ignoring some of his work is truly possible. For better or for worse, his publications have significantly shaped clinical practice in addition to guiding subsequent research in this field.
ASSESSING RISK
Along with continuing to endorse the Revised Cardiac Risk Index (RCRI),3 the guidelines now include another option for objective preoperative cardiovascular risk assessment. Dr. Cohn nicely outlines the pros and cons of the surgical risk calculator (often referred to as the “Gupta calculator”) derived from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database.4
Although the RCRI is not perfect, I agree with Dr. Cohn that the ACS NSQIP tool has limitations, including a cumbersome calculation (requiring a smartphone application or online calculator), lack of external validation, and use of the American Society of Anesthesiologists Physical Status Classification System, which has been notoriously confusing for generalists and has demonstrated poor inter-rater reliability among anesthesiologists.5,6
Of note, a patient may have very different risk-prediction scores depending on which tool is used. For example, a 66-year-old man with a history of ischemic heart disease, diabetes on insulin therapy, hypertension, and chronic kidney disease with a serum creatinine level greater than 2.0 mg/dL who is scheduled to undergo total hip arthroplasty would have a risk of a perioperative cardiovascular event of about 10% according to the RCRI, but only 1.1% according to the ACS NSQIP calculator. How widely this newer risk-stratification tool will be adopted in clinical practice will be interesting to observe.
In what appears to be an effort to simplify the guidelines, the ACC/AHA now recommends combining the patient’s clinical and surgical risks into estimating an overall perioperative risk for developing major adverse cardiac events. This estimate is now whittled down to only two categories: “low risk” and “elevated risk.” I am concerned that the new guidelines may have become too streamlined and lack the direction to assist providers in making important clinical decisions. Most notably, and as Dr. Cohn appropriately suggests, many patients will be in a gray zone with respect to whether cardiac stress testing should be obtained before surgery.
STRESS TESTING
Significant background knowledge is required to answer the important question in the ACC/AHA algorithm, ie, whether further testing will have an impact on decision-making or perioperative care.2 Dr. Cohn provides some of this information by noting the abysmal positive predictive value of preoperative noninvasive cardiac testing (with studies ranging from 0% to 37%) and by correctly stating that no benefit has been observed with preoperative cardiac revascularization.
If this is not widely known, I share Dr. Cohn’s fear that the new guidelines may stimulate increased ordering of preoperative stress tests. We observed this trend with the highly scripted 2002 ACC/AHA perioperative guidelines7 and subsequently learned that stress testing before surgery very seldom changes patient management.
A preoperative stress test should be reserved for patients with symptoms suggestive of ischemic heart disease. As a diagnostic study, the value of stress testing is excellent. This is not true when it is used as a screening test for asymptomatic patients, where its ability to predict perioperative cardiovascular events is extremely poor. The only other indication for preoperative stress testing is the rare occasion when further risk stratification is desired for exceptionally high-risk patients. In this scenario, test results may influence the decision to proceed with surgery vs seeking nonoperative approaches or palliative care.
MANAGING MEDICATIONS
Dr. Cohn discusses pertinent issues in the perioperative management of patients’ medications, an important component of the preoperative evaluation.
Despite the inconsistent clinical trial results on perioperative beta-blockers, his assessment of their risks and benefits is clinically accurate and practical. Furthermore, I fully agree with Dr. Cohn’s thoughtful approach regarding perioperative statins, despite the limited data available from randomized controlled trials.
With respect to perioperative aspirin use, I have concerns with Dr. Cohn’s statement that it may be reasonable to continue aspirin perioperatively if the risk of potential cardiac events outweighs the risk of bleeding. Given the result of the recently published second Perioperative Ischemic Evaluation (POISE-2) trial8 that showed a significantly higher risk of major perioperative bleeding in patients randomized to low-dose aspirin, it is difficult to advocate continuing aspirin when no cardiovascular protection was found in this very large trial. I agree with Dr. Cohn that this applies only to patients with no history of coronary artery stent placement, as patients with a stent should remain on low-dose aspirin throughout the entire perioperative period.
Controversy also surrounds angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Dr. Cohn agrees with the ACC/AHA guidelines to continue these agents before surgery; however, I favor holding them on the day of surgery. Although the risk of hypotension-induced cardiac events has not been clearly demonstrated, a recent retrospective study involving more than 1,100 patients showed significantly more acute kidney injury (even after adjusting for hypotension) as well as an increased length of hospital stay in the patients exposed to these agents before surgery.9 Given these findings, in addition to the postinduction hypotension (which can be profound) commonly observed by our anesthesiology colleagues, I recommend holding angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the day of surgery, with very few exceptions.
THE SCIENCE AND ART OF MEDICINE
Dr. Cohn acknowledges that we lack scientific data to answer many questions that arise when caring for the perioperative patient and thus we rely on the ACC/AHA guidelines to provide a framework. These scientific knowledge gaps emphasize the importance of the art of medicine in the perioperative arena. Although we may desire “cookbook” guidelines, the significant gaps in the perioperative medicine evidence base reinforce the necessity to provide individual patient-level care in a multidisciplinary environment with our surgery and anesthesiology colleagues. Without the proper balance of science and art in perioperative medicine, we sacrifice our ability to deliver optimal care for this high-risk patient population.
- Cohn SL. Updated guidelines on cardiovascular evaluation before noncardiac surgery: a view from the trenches. Cleve Clin J Med 2014; 81:742–751.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of Anesthesiologists Physical Status Classification Scale. AANA J 2003; 71:265–274.
- Mak PH, Campbell RC, Irwin MG; American Society of Anesthesiologists. The ASA Physical Status Classification: inter-observer consistency. American Society of Anesthesiologists. Anaesth Intensive Care 2002; 30:633–640.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2002; 39:542–553.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9:283–288.
In this issue of the Cleveland Clinic Journal of Medicine,1 Dr. Steven L. Cohn provides a succinct review of the recently published guidelines by the American College of Cardiology and American Heart Association (ACC/AHA) on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery.2 Although no drastic changes have been made in these guidelines, several significant modifications have been implemented and are highlighted in his review.
A BREACH OF SCIENTIFIC INTEGRITY
First, I am pleased Dr. Cohn described how the writing committee of the new guidelines handled the well-publicized breaches of scientific integrity by Dr. Don Poldermans, a prolific perioperative-medicine researcher at Erasmus University in the Netherlands who has contributed an abundance of literature that influenced clinical practice. Although some of his key publications were excluded by the ACC/AHA committee in its overall analysis, it remains unclear to me if simply ignoring some of his work is truly possible. For better or for worse, his publications have significantly shaped clinical practice in addition to guiding subsequent research in this field.
ASSESSING RISK
Along with continuing to endorse the Revised Cardiac Risk Index (RCRI),3 the guidelines now include another option for objective preoperative cardiovascular risk assessment. Dr. Cohn nicely outlines the pros and cons of the surgical risk calculator (often referred to as the “Gupta calculator”) derived from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database.4
Although the RCRI is not perfect, I agree with Dr. Cohn that the ACS NSQIP tool has limitations, including a cumbersome calculation (requiring a smartphone application or online calculator), lack of external validation, and use of the American Society of Anesthesiologists Physical Status Classification System, which has been notoriously confusing for generalists and has demonstrated poor inter-rater reliability among anesthesiologists.5,6
Of note, a patient may have very different risk-prediction scores depending on which tool is used. For example, a 66-year-old man with a history of ischemic heart disease, diabetes on insulin therapy, hypertension, and chronic kidney disease with a serum creatinine level greater than 2.0 mg/dL who is scheduled to undergo total hip arthroplasty would have a risk of a perioperative cardiovascular event of about 10% according to the RCRI, but only 1.1% according to the ACS NSQIP calculator. How widely this newer risk-stratification tool will be adopted in clinical practice will be interesting to observe.
In what appears to be an effort to simplify the guidelines, the ACC/AHA now recommends combining the patient’s clinical and surgical risks into estimating an overall perioperative risk for developing major adverse cardiac events. This estimate is now whittled down to only two categories: “low risk” and “elevated risk.” I am concerned that the new guidelines may have become too streamlined and lack the direction to assist providers in making important clinical decisions. Most notably, and as Dr. Cohn appropriately suggests, many patients will be in a gray zone with respect to whether cardiac stress testing should be obtained before surgery.
STRESS TESTING
Significant background knowledge is required to answer the important question in the ACC/AHA algorithm, ie, whether further testing will have an impact on decision-making or perioperative care.2 Dr. Cohn provides some of this information by noting the abysmal positive predictive value of preoperative noninvasive cardiac testing (with studies ranging from 0% to 37%) and by correctly stating that no benefit has been observed with preoperative cardiac revascularization.
If this is not widely known, I share Dr. Cohn’s fear that the new guidelines may stimulate increased ordering of preoperative stress tests. We observed this trend with the highly scripted 2002 ACC/AHA perioperative guidelines7 and subsequently learned that stress testing before surgery very seldom changes patient management.
A preoperative stress test should be reserved for patients with symptoms suggestive of ischemic heart disease. As a diagnostic study, the value of stress testing is excellent. This is not true when it is used as a screening test for asymptomatic patients, where its ability to predict perioperative cardiovascular events is extremely poor. The only other indication for preoperative stress testing is the rare occasion when further risk stratification is desired for exceptionally high-risk patients. In this scenario, test results may influence the decision to proceed with surgery vs seeking nonoperative approaches or palliative care.
MANAGING MEDICATIONS
Dr. Cohn discusses pertinent issues in the perioperative management of patients’ medications, an important component of the preoperative evaluation.
Despite the inconsistent clinical trial results on perioperative beta-blockers, his assessment of their risks and benefits is clinically accurate and practical. Furthermore, I fully agree with Dr. Cohn’s thoughtful approach regarding perioperative statins, despite the limited data available from randomized controlled trials.
With respect to perioperative aspirin use, I have concerns with Dr. Cohn’s statement that it may be reasonable to continue aspirin perioperatively if the risk of potential cardiac events outweighs the risk of bleeding. Given the result of the recently published second Perioperative Ischemic Evaluation (POISE-2) trial8 that showed a significantly higher risk of major perioperative bleeding in patients randomized to low-dose aspirin, it is difficult to advocate continuing aspirin when no cardiovascular protection was found in this very large trial. I agree with Dr. Cohn that this applies only to patients with no history of coronary artery stent placement, as patients with a stent should remain on low-dose aspirin throughout the entire perioperative period.
Controversy also surrounds angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Dr. Cohn agrees with the ACC/AHA guidelines to continue these agents before surgery; however, I favor holding them on the day of surgery. Although the risk of hypotension-induced cardiac events has not been clearly demonstrated, a recent retrospective study involving more than 1,100 patients showed significantly more acute kidney injury (even after adjusting for hypotension) as well as an increased length of hospital stay in the patients exposed to these agents before surgery.9 Given these findings, in addition to the postinduction hypotension (which can be profound) commonly observed by our anesthesiology colleagues, I recommend holding angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the day of surgery, with very few exceptions.
THE SCIENCE AND ART OF MEDICINE
Dr. Cohn acknowledges that we lack scientific data to answer many questions that arise when caring for the perioperative patient and thus we rely on the ACC/AHA guidelines to provide a framework. These scientific knowledge gaps emphasize the importance of the art of medicine in the perioperative arena. Although we may desire “cookbook” guidelines, the significant gaps in the perioperative medicine evidence base reinforce the necessity to provide individual patient-level care in a multidisciplinary environment with our surgery and anesthesiology colleagues. Without the proper balance of science and art in perioperative medicine, we sacrifice our ability to deliver optimal care for this high-risk patient population.
In this issue of the Cleveland Clinic Journal of Medicine,1 Dr. Steven L. Cohn provides a succinct review of the recently published guidelines by the American College of Cardiology and American Heart Association (ACC/AHA) on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery.2 Although no drastic changes have been made in these guidelines, several significant modifications have been implemented and are highlighted in his review.
A BREACH OF SCIENTIFIC INTEGRITY
First, I am pleased Dr. Cohn described how the writing committee of the new guidelines handled the well-publicized breaches of scientific integrity by Dr. Don Poldermans, a prolific perioperative-medicine researcher at Erasmus University in the Netherlands who has contributed an abundance of literature that influenced clinical practice. Although some of his key publications were excluded by the ACC/AHA committee in its overall analysis, it remains unclear to me if simply ignoring some of his work is truly possible. For better or for worse, his publications have significantly shaped clinical practice in addition to guiding subsequent research in this field.
ASSESSING RISK
Along with continuing to endorse the Revised Cardiac Risk Index (RCRI),3 the guidelines now include another option for objective preoperative cardiovascular risk assessment. Dr. Cohn nicely outlines the pros and cons of the surgical risk calculator (often referred to as the “Gupta calculator”) derived from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database.4
Although the RCRI is not perfect, I agree with Dr. Cohn that the ACS NSQIP tool has limitations, including a cumbersome calculation (requiring a smartphone application or online calculator), lack of external validation, and use of the American Society of Anesthesiologists Physical Status Classification System, which has been notoriously confusing for generalists and has demonstrated poor inter-rater reliability among anesthesiologists.5,6
Of note, a patient may have very different risk-prediction scores depending on which tool is used. For example, a 66-year-old man with a history of ischemic heart disease, diabetes on insulin therapy, hypertension, and chronic kidney disease with a serum creatinine level greater than 2.0 mg/dL who is scheduled to undergo total hip arthroplasty would have a risk of a perioperative cardiovascular event of about 10% according to the RCRI, but only 1.1% according to the ACS NSQIP calculator. How widely this newer risk-stratification tool will be adopted in clinical practice will be interesting to observe.
In what appears to be an effort to simplify the guidelines, the ACC/AHA now recommends combining the patient’s clinical and surgical risks into estimating an overall perioperative risk for developing major adverse cardiac events. This estimate is now whittled down to only two categories: “low risk” and “elevated risk.” I am concerned that the new guidelines may have become too streamlined and lack the direction to assist providers in making important clinical decisions. Most notably, and as Dr. Cohn appropriately suggests, many patients will be in a gray zone with respect to whether cardiac stress testing should be obtained before surgery.
STRESS TESTING
Significant background knowledge is required to answer the important question in the ACC/AHA algorithm, ie, whether further testing will have an impact on decision-making or perioperative care.2 Dr. Cohn provides some of this information by noting the abysmal positive predictive value of preoperative noninvasive cardiac testing (with studies ranging from 0% to 37%) and by correctly stating that no benefit has been observed with preoperative cardiac revascularization.
If this is not widely known, I share Dr. Cohn’s fear that the new guidelines may stimulate increased ordering of preoperative stress tests. We observed this trend with the highly scripted 2002 ACC/AHA perioperative guidelines7 and subsequently learned that stress testing before surgery very seldom changes patient management.
A preoperative stress test should be reserved for patients with symptoms suggestive of ischemic heart disease. As a diagnostic study, the value of stress testing is excellent. This is not true when it is used as a screening test for asymptomatic patients, where its ability to predict perioperative cardiovascular events is extremely poor. The only other indication for preoperative stress testing is the rare occasion when further risk stratification is desired for exceptionally high-risk patients. In this scenario, test results may influence the decision to proceed with surgery vs seeking nonoperative approaches or palliative care.
MANAGING MEDICATIONS
Dr. Cohn discusses pertinent issues in the perioperative management of patients’ medications, an important component of the preoperative evaluation.
Despite the inconsistent clinical trial results on perioperative beta-blockers, his assessment of their risks and benefits is clinically accurate and practical. Furthermore, I fully agree with Dr. Cohn’s thoughtful approach regarding perioperative statins, despite the limited data available from randomized controlled trials.
With respect to perioperative aspirin use, I have concerns with Dr. Cohn’s statement that it may be reasonable to continue aspirin perioperatively if the risk of potential cardiac events outweighs the risk of bleeding. Given the result of the recently published second Perioperative Ischemic Evaluation (POISE-2) trial8 that showed a significantly higher risk of major perioperative bleeding in patients randomized to low-dose aspirin, it is difficult to advocate continuing aspirin when no cardiovascular protection was found in this very large trial. I agree with Dr. Cohn that this applies only to patients with no history of coronary artery stent placement, as patients with a stent should remain on low-dose aspirin throughout the entire perioperative period.
Controversy also surrounds angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Dr. Cohn agrees with the ACC/AHA guidelines to continue these agents before surgery; however, I favor holding them on the day of surgery. Although the risk of hypotension-induced cardiac events has not been clearly demonstrated, a recent retrospective study involving more than 1,100 patients showed significantly more acute kidney injury (even after adjusting for hypotension) as well as an increased length of hospital stay in the patients exposed to these agents before surgery.9 Given these findings, in addition to the postinduction hypotension (which can be profound) commonly observed by our anesthesiology colleagues, I recommend holding angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the day of surgery, with very few exceptions.
THE SCIENCE AND ART OF MEDICINE
Dr. Cohn acknowledges that we lack scientific data to answer many questions that arise when caring for the perioperative patient and thus we rely on the ACC/AHA guidelines to provide a framework. These scientific knowledge gaps emphasize the importance of the art of medicine in the perioperative arena. Although we may desire “cookbook” guidelines, the significant gaps in the perioperative medicine evidence base reinforce the necessity to provide individual patient-level care in a multidisciplinary environment with our surgery and anesthesiology colleagues. Without the proper balance of science and art in perioperative medicine, we sacrifice our ability to deliver optimal care for this high-risk patient population.
- Cohn SL. Updated guidelines on cardiovascular evaluation before noncardiac surgery: a view from the trenches. Cleve Clin J Med 2014; 81:742–751.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of Anesthesiologists Physical Status Classification Scale. AANA J 2003; 71:265–274.
- Mak PH, Campbell RC, Irwin MG; American Society of Anesthesiologists. The ASA Physical Status Classification: inter-observer consistency. American Society of Anesthesiologists. Anaesth Intensive Care 2002; 30:633–640.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2002; 39:542–553.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9:283–288.
- Cohn SL. Updated guidelines on cardiovascular evaluation before noncardiac surgery: a view from the trenches. Cleve Clin J Med 2014; 81:742–751.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of Anesthesiologists Physical Status Classification Scale. AANA J 2003; 71:265–274.
- Mak PH, Campbell RC, Irwin MG; American Society of Anesthesiologists. The ASA Physical Status Classification: inter-observer consistency. American Society of Anesthesiologists. Anaesth Intensive Care 2002; 30:633–640.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2002; 39:542–553.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9:283–288.
Identifying statin-associated autoimmune necrotizing myopathy
Statins are among the most widely prescribed drugs, as they reduce cardiovascular risk very effectively. They work by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), a key enzyme in cholesterol biosynthesis. Although most patients tolerate statins well, muscle-related toxicity can limit the use of these drugs.
Recently, progressive necrotizing myopathy leading to profound weakness has been directly linked to statin therapy. Proper recognition of this ominous complication is important to prevent further damage from statin use.
STATIN-ASSOCIATED MUSCLE EFFECTS: A SPECTRUM
Muscle symptoms are among the best known and most important side effects of statins, ranging from asymptomatic, mild elevation of creatine kinase and benign myalgias to life-threatening rhabdomyolysis. As the various terms are often used inconsistently in the literature, we will briefly review each entity to put immune-necrotizing myopathy in its proper context on the spectrum of statin-associated muscle symptoms.
Myalgia
Myalgia, ie, muscle pain without elevation of muscle enzymes, is the most common side effect of statins. The incidence is about 10% in observational studies1,2; however, the incidence in the real world of clinical practice seems much higher. Myalgias can present as widespread pain or as localized pain, usually in the lower extremities. Muscle cramps and tendonitis-related pain are commonly reported.
Several clinical predictors of increased risk of statin-associated muscle pain were noted in the Prediction of Muscular Risk in Observational Conditions (PRIMO) study,2 assessing mild to moderate muscular symptoms in patients on high-dose statins. These included a history of muscle pain with another lipid-lowering agent, a history of cramps, a history of elevated creatine kinase levels, a personal or family history of muscle symptoms, untreated hypothyroidism, and a background of fibromyalgia-like symptoms. The incidence of muscle symptoms increased with the level of physical activity, and the median time of symptom onset was 1 month after starting statin therapy or titrating to a high dose.
In patients with myalgia alone, symptoms often improve when the statin is stopped.
Myopathy, myositis
The general terms myopathy and myositis have been used to refer to elevated muscle enzymes together with the muscle symptoms of pain, cramps, soreness, or weakness. An analysis of 21 clinical trials of statin therapy found that myopathy (creatine kinase level more than 10 times the upper limit of normal) occurred in 5 patients per 100,000 person-years.3 The incidence of myopathy increases when statins are used in high doses.
In another cohort of patients seen for statin intolerance,1 conventional risk factors for overt myositis included renal disease, diabetes, and thyroid disease. Electrolyte abnormalities did not differ between statin-tolerant and statin-intolerant patients.1
The search for genetic indicators of risk
In 2008, the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group conducted a genome-wide association study to identify genetic variants associated with myopathy with high-dose statin therapy.4 Eighty-five patients who had developed definite myopathy (muscle symptoms with enzyme levels more than 10 times the upper limit of normal) or incipient myopathy (asymptomatic or symptomatic, but enzyme levels at least three times the upper limit of normal) while taking simvastatin 80 mg were compared with 90 controls taking the same daily dose. The study reported variants in the SLCO1B1 gene as “strongly associated with an increased risk of statin-associated myopathy.”4
SLCO1B1 encodes the peptide responsible for hepatic uptake of statins, thus affecting the blood level of these drugs. Patients in the study who had the C variant, which predisposes to higher blood levels of statins, were at higher risk of myopathy than those with the T variant. The odds ratio for myopathy increased from 4.4 in heterozygotes for the C allele to 17.4 for homozygotes. This effect was similar even with lower doses of statins.4
We believe that these findings provide a strong basis for genetic testing of patients who may be at risk of statin-associated myopathy.
Rechallenging with a different statin
Often, patients with myopathy can be rechallenged with a different statin agent. In a study done in a lipid clinic, patients identified as having simvastatin-associated myopathy were given another statin.5 Between 15% and 42% tolerated the second statin, with no statistically significant difference between the tolerability rates with the different agents used (atorvastatin, rosuvastatin, pravastatin, and fluvastatin).
Rhabdomyolysis
Rhabdomyolysis, the most devastating complication of statin use, is marked by a creatine kinase level more than 10 times the upper limit of normal or greater than 10,000 IU/L, resulting from acute and massive destruction of muscle fibers and release of their contents into the bloodstream.
But rhabdomyolysis is a clinical syndrome, not solely an alarming increase in muscle enzyme levels. It can include renal failure and death. The rate of occurrence is low (1/100,000), but it can occur at any point in treatment.6 An analysis of Canadian and US case reports of statin-associated rhabdomyolysis showed an average of 824 cases each year.7 A dose-response relationship was observed with higher statin doses.
But statin-associated myopathy may not stop when the drug is stopped
The types of muscle toxicity discussed above stem from direct myotoxic effects of statins and are thought to be related to the blood concentration of the statin.6,8 They may be limited by genetic susceptibility. Mechanisms may include a change in muscle membrane excitability caused by modulation in membrane cholesterol levels, impaired mitochondrial function and calcium signaling, induction of apoptosis, and increased lipid peroxidation. Stopping the offending drug can often halt these downstream effects—hence the dictum that statin myopathy is self-limiting and should resolve with cessation of the drug.
But as we discuss in the following sections, some patients on statin therapy develop an immune-mediated myopathy that does not resolve with discontinuation of the statin and that may only resolve with immunosuppressive therapy.
STATINS AND AUTOIMMUNE NECROTIZING MYOPATHY
At the Johns Hopkins Myositis Center, a group of patients was identified who had necrotizing myopathy on biopsy but no known underlying condition or associated autoantibodies. In an attempt to establish an autoimmune basis for their disease, the sera from 26 patients were screened for novel antibodies.9 Sera from 16 of the patients immunoprecipitated a pair of proteins (sizes 100 kd and 200 kd), indicating these patients had an antibody to these proteins. This finding was highly specific for necrotizing myopathy when compared with controls, ie, other patients with myositis. Patients who had this finding displayed proximal muscle weakness, elevated muscle enzyme levels, and myopathic findings on electromyography; 63% had been exposed to statins before the onset of weakness, and when only patients over age 50 were included, the number rose to 83%.
This association of statins with necrotizing myopathy had been previously noted by two other groups. Needham et al10 described eight patients who, while on statins, developed myopathy that continued to worsen despite cessation of the drugs. An analysis of their muscle pathology revealed myofiber necrosis with little inflammatory infiltrate, as well as widespread up-regulation of expression of major histocompatibility complex class 1. These patients required immunosuppressive treatment (prednisone and methotrexate) to control their disease.
Grable-Esposito et al11 corroborated this finding by identifying 25 additional patients who developed a similar necrotizing myopathy while on statins.11 They also noted a significantly higher frequency of statin use in patients with necrotizing myopathy than in age-matched controls with polymyositis or inclusion-body myositis.
The researchers at the Johns Hopkins Myositis Center noted the similarity between these two patient groups and their own group of patients with necrotizing myopathy. Thus, a follow-up study was done to identify the 100-kg and 200-kd autoantigens observed in their earlier study. Exposure to a statin was found to up-regulate the expression of the two molecules.12 HMGCR was hypothesized as being the 100-kd antigen, because of its 97-kd molecular weight, and also because statin treatment had already been shown to up-regulate the expression of HMGCR.13 The researchers concluded that HMGCR was indeed the 100-kd antigen, with no distinctive antibodies recognizing the 200-kd protein. Although the 200-kd protein was once postulated to be a dimer of the 100-kd protein, its identity remains unknown.
The anti-HMGCR antibody was then screened for in a cohort of 750 myositis patients. The 16 patients previously found to have anti-200/100 were all positive for anti-HMGCR antibody. An additional 45 patients from the cohort (6%) were anti-HMGCR-positive by enzyme-linked immunosorbent assay, and all had necrotizing myopathy. Patients with other types of myopathy, including inflammatory myopathy, do not possess this antibody.12
The HMGCR antibody was quite specific for immune-mediated necrotizing myopathy, and this suggested that statins were capable of triggering an immune-mediated myopathy that is then perpetuated even if the drug is discontinued. As it was also demonstrated that statins increase the expression of HMGCR in muscle as well as in regenerating cells, the process may be sustained through persistently increased HMGCR expression associated with muscle repair.12
The C allele of the SLCO1B1 gene, which has been associated with statin-associated myopathy, was not increased in this population of patients positive for anti-HMGCR. Follow-up studies of the prevalence of anti-HMGCR in statin users in the Atherosclerosis Risk in Communities (ARIC) cohort, including those with self-limited statin myotoxicity, have also shown the absence of this antibody.14 This shows that anti-HMGCR is not found in the majority of statin-exposed patients and is highly specific for autoimmune myopathy. This also suggests that statin-associated autoimmune myopathy represents a pathologic process that is distinct from self-limited statin intolerance.
HOW THE CONDITION PRESENTS
Immune-mediated statin myopathy presents similarly to other idiopathic inflammatory myopathies such as polymyositis (Table 1). Symptoms often develop in a subacute to chronic course and can occur at any time with statin treatment. In one study,10 the average duration of statin use before the onset of weakness was 3 years (range 2 months to 10 years). In some patients whose statin had been stopped because of abnormal creatine kinase levels, weakness developed later, at a range of 0.5 to 20 months. Even low doses of statins (such as 10 mg of simvastatin) have been found to trigger this condition.10
Patients uniformly develop symmetric proximal arm and leg weakness, and distal weakness can also occur.11 Other features have included dysphagia, arthralgias, myalgias, and Raynaud phenomenon.9 Men and women are represented in roughly equal numbers.
The muscle enzymes are strikingly elevated in this disease, with a mean creatine kinase value of 10,333 IU/L at initial presentation.9 Although the creatine kinase level may be very elevated, patients often do not present with weakness until a certain threshold value is reached, in contrast with patients with anti-signal recognition particle necrotizing myopathy, who can present with profound weakness at a lower level. Hence, by the time patients are clinically symptomatic, the process may have been going on for some time. Despite the seemingly massive leak in muscle enzymes, the patients do not develop rhabdomyolysis. Inflammatory markers need not be elevated, and an association with other antibodies such as antinuclear antibody is not often seen.
Magnetic resonance imaging of the thigh has shown muscle edema in all patients.9 In decreasing order of frequency, other findings are atrophy, fatty replacement, and fascial edema.
Electromyography of involved muscle has shown irritable myopathy in most patients (88%) and nonirritable myopathy in a few.
Muscle biopsy studies have shown prominent necrotic and regenerating fibers without significant inflammatory infiltrate.11 There is also myophagocytosis of necrotic fibers and diffuse or focal up-regulation of major histocompatability complex class I expression.10
PATIENTS WITH ANTI-HMGCR WHO HAVE AND WHO HAVE NEVER TAKEN STATINS
When the anti-HMGCR antibody was tested for in the Johns Hopkins cohort,12 33% of patients with a necrotizing myopathy associated with this antibody had never taken a statin. The two groups were clinically indistinguishable, save for a few aspects. Compared with patients who had taken a statin, those who had never taken a statin were younger (mean age 37 vs 59), had higher levels of creatine kinase (13,392 vs 7,881 IU/L), and had a different race distribution (46.7% vs 86.7% white). Initial HMGCR antibody levels were also noted to correlate with creatine kinase levels and strength in statin-exposed patients but not in those who had never taken a statin.15
We hypothesize that in patients who have never taken a statin, other genetic or environmental factors may be the cause of the increased HMGCR expression, which then triggers the autoimmune response. Until further data are gathered, we should probably treat these patients as we treat those who develop this disease after taking a statin, and avoid giving them statins altogether.
MANAGEMENT OF STATIN-ASSOCIATED AUTOIMMUNE NECROTIZING MYOPATHY
Treatment of statin-associated autoimmune necrotizing myopathy can be challenging and requires immunosuppressive drugs.
Statin therapy should be stopped once this condition is suspected. Patients who continue to have elevated muscle enzymes or weakness should undergo further testing with electromyography, magnetic resonance imaging, and muscle biopsy. Electromyography detects myopathy and shows chronicity, distribution, and degree of severity. Although not necessary for diagnosis, magnetic resonance imaging helps to evaluate the extent of muscle involvement and damage and provides guidance when choosing a site for muscle biopsy. Muscle biopsy is necessary to determine the actual pathology and to exclude mimics such as dystrophy or metabolic myopathies.
When an immune-mediated myopathy is confirmed, prompt referral to a rheumatologist or a neuromuscular specialist is recommended.
Steroids are usually the first-line treatment for this disease. Other immunosuppressives, such as methotrexate, azathioprine, mycophenolate mofetil, and rituximab have been used with varying levels of success. In our experience, intravenous immunoglobulin has been particularly beneficial for refractory cases. With treatment, muscle enzyme levels and weakness improve, but relapses can occur. The ideal choice of immunosuppressive therapy and the duration of therapy are currently under investigation.
Rechallenge with another statin
At this time, the issue of rechallenging the patient with another statin has not been clarified. Given the autoimmune nature of the disease, we would avoid exposing the patient to a known trigger. However, this may be a difficult decision in patients with cardiovascular risk factors who require statins for primary or secondary prevention. We suggest using alternative cholesterol-lowering agents first and using them in combination if needed.
We have had some success in maintaining a handful of patients on a statin while treating them concurrently with immunosuppression. This is not ideal because they are constantly being exposed to the likely trigger for their disease, but it may be unavoidable if statins are deemed absolutely necessary. We have also had a patient with known statin-associated immune-mediated necrotizing myopathy who later became profoundly weak after another physician started her on a newer-generation agent, pitavastatin. This suggests to us that rechallenging patients, even with a different statin, can have deleterious effects.
IMPLICATIONS FOR CLINICAL PRACTICE
The true prevalence of statin-associated autoimmune myopathy in practice is unknown. In the Johns Hopkins Myositis Center cohort of patients with suspected myopathy, anti-HMGCR was found in 6% of the patients and was the second most frequent antibody found after anti-Jo1.12
Given the frequency of muscle-related complaints in patients on statins, we recommend obtaining baseline muscle enzyme measurements before starting statin therapy. As recommended by the National Lipid Association Statin Safety Assessment Task Force, the creatine kinase level should be measured when a patient develops muscular complaints, to help gauge the severity of the disease and to help decide whether to continue therapy.8 Random testing of the creatine kinase level in asymptomatic patients is not recommended.
At present, the diagnosis of statin-associated autoimmune necrotizing myopathy is based on a combination of findings—elevated muscle enzyme levels, muscle weakness, irritable findings on electromyography, and necrotizing myopathy on biopsy in a patient on a statin. The finding of the HMGCR antibody confirms the diagnosis. A test for this antibody is now commercially available in the United States. We suggest testing for the antibody in the following scenarios:
- A persistently elevated or rising creatine kinase, aspartate aminotransferase, alanine aminotransferase, or aldolase level after the statin is stopped; although no fixed creatine kinase level has been determined, a level above 1,000 U/L would be a reasonable cutoff at which to test
- Muscle symptoms (proximal or distal weakness) that persist 12 weeks after statin cessation regardless of the creatine kinase level, especially if the patient has dysphagia
- The finding of muscle irritability on electromyography or diffuse muscle edema on magnetic resonance imaging when testing for other myositis-specific antibodies is negative
- Muscle biopsy showing necrotizing myopathy with little or no inflammation.
In addition, since necrotizing myopathy is known to be associated with malignancy and since necrotizing myopathy is more common in older people, who are also more likely to be taking a statin, an age-appropriate malignancy evaluation is warranted as well.
- Harris LJ, Thapa R, Brown M, et al. Clinical and laboratory phenotype of patients experiencing statin intolerance attributable to myalgia. J Clin Lipidol 2011; 5:299–307.
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol 2006; 97:52C–60C.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Fung EC, Crook MA. Statin myopathy: a lipid clinic experience on the tolerability of statin rechallenge. Cardiovasc Ther 2012; 30:e212–e218.
- Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol 2008; 8:333–338.
- Holbrook A, Wright M, Sung M, Ribic C, Baker S. Statin-associated rhabdomyolysis: is there a dose-response relationship? Can J Cardiol 2011; 27:146–151.
- McKenney JM, Davidson MH, Jacobson TA, Guyton JR; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–94C.
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62:2757–2766.
- Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord 2007; 17:194–200.
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41:185–190.
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63:713–721.
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990; 343:425–430.
- Mammen AL, Pak K, Williams EK, et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res (Hoboken) 2012; 64:269–272.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
Statins are among the most widely prescribed drugs, as they reduce cardiovascular risk very effectively. They work by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), a key enzyme in cholesterol biosynthesis. Although most patients tolerate statins well, muscle-related toxicity can limit the use of these drugs.
Recently, progressive necrotizing myopathy leading to profound weakness has been directly linked to statin therapy. Proper recognition of this ominous complication is important to prevent further damage from statin use.
STATIN-ASSOCIATED MUSCLE EFFECTS: A SPECTRUM
Muscle symptoms are among the best known and most important side effects of statins, ranging from asymptomatic, mild elevation of creatine kinase and benign myalgias to life-threatening rhabdomyolysis. As the various terms are often used inconsistently in the literature, we will briefly review each entity to put immune-necrotizing myopathy in its proper context on the spectrum of statin-associated muscle symptoms.
Myalgia
Myalgia, ie, muscle pain without elevation of muscle enzymes, is the most common side effect of statins. The incidence is about 10% in observational studies1,2; however, the incidence in the real world of clinical practice seems much higher. Myalgias can present as widespread pain or as localized pain, usually in the lower extremities. Muscle cramps and tendonitis-related pain are commonly reported.
Several clinical predictors of increased risk of statin-associated muscle pain were noted in the Prediction of Muscular Risk in Observational Conditions (PRIMO) study,2 assessing mild to moderate muscular symptoms in patients on high-dose statins. These included a history of muscle pain with another lipid-lowering agent, a history of cramps, a history of elevated creatine kinase levels, a personal or family history of muscle symptoms, untreated hypothyroidism, and a background of fibromyalgia-like symptoms. The incidence of muscle symptoms increased with the level of physical activity, and the median time of symptom onset was 1 month after starting statin therapy or titrating to a high dose.
In patients with myalgia alone, symptoms often improve when the statin is stopped.
Myopathy, myositis
The general terms myopathy and myositis have been used to refer to elevated muscle enzymes together with the muscle symptoms of pain, cramps, soreness, or weakness. An analysis of 21 clinical trials of statin therapy found that myopathy (creatine kinase level more than 10 times the upper limit of normal) occurred in 5 patients per 100,000 person-years.3 The incidence of myopathy increases when statins are used in high doses.
In another cohort of patients seen for statin intolerance,1 conventional risk factors for overt myositis included renal disease, diabetes, and thyroid disease. Electrolyte abnormalities did not differ between statin-tolerant and statin-intolerant patients.1
The search for genetic indicators of risk
In 2008, the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group conducted a genome-wide association study to identify genetic variants associated with myopathy with high-dose statin therapy.4 Eighty-five patients who had developed definite myopathy (muscle symptoms with enzyme levels more than 10 times the upper limit of normal) or incipient myopathy (asymptomatic or symptomatic, but enzyme levels at least three times the upper limit of normal) while taking simvastatin 80 mg were compared with 90 controls taking the same daily dose. The study reported variants in the SLCO1B1 gene as “strongly associated with an increased risk of statin-associated myopathy.”4
SLCO1B1 encodes the peptide responsible for hepatic uptake of statins, thus affecting the blood level of these drugs. Patients in the study who had the C variant, which predisposes to higher blood levels of statins, were at higher risk of myopathy than those with the T variant. The odds ratio for myopathy increased from 4.4 in heterozygotes for the C allele to 17.4 for homozygotes. This effect was similar even with lower doses of statins.4
We believe that these findings provide a strong basis for genetic testing of patients who may be at risk of statin-associated myopathy.
Rechallenging with a different statin
Often, patients with myopathy can be rechallenged with a different statin agent. In a study done in a lipid clinic, patients identified as having simvastatin-associated myopathy were given another statin.5 Between 15% and 42% tolerated the second statin, with no statistically significant difference between the tolerability rates with the different agents used (atorvastatin, rosuvastatin, pravastatin, and fluvastatin).
Rhabdomyolysis
Rhabdomyolysis, the most devastating complication of statin use, is marked by a creatine kinase level more than 10 times the upper limit of normal or greater than 10,000 IU/L, resulting from acute and massive destruction of muscle fibers and release of their contents into the bloodstream.
But rhabdomyolysis is a clinical syndrome, not solely an alarming increase in muscle enzyme levels. It can include renal failure and death. The rate of occurrence is low (1/100,000), but it can occur at any point in treatment.6 An analysis of Canadian and US case reports of statin-associated rhabdomyolysis showed an average of 824 cases each year.7 A dose-response relationship was observed with higher statin doses.
But statin-associated myopathy may not stop when the drug is stopped
The types of muscle toxicity discussed above stem from direct myotoxic effects of statins and are thought to be related to the blood concentration of the statin.6,8 They may be limited by genetic susceptibility. Mechanisms may include a change in muscle membrane excitability caused by modulation in membrane cholesterol levels, impaired mitochondrial function and calcium signaling, induction of apoptosis, and increased lipid peroxidation. Stopping the offending drug can often halt these downstream effects—hence the dictum that statin myopathy is self-limiting and should resolve with cessation of the drug.
But as we discuss in the following sections, some patients on statin therapy develop an immune-mediated myopathy that does not resolve with discontinuation of the statin and that may only resolve with immunosuppressive therapy.
STATINS AND AUTOIMMUNE NECROTIZING MYOPATHY
At the Johns Hopkins Myositis Center, a group of patients was identified who had necrotizing myopathy on biopsy but no known underlying condition or associated autoantibodies. In an attempt to establish an autoimmune basis for their disease, the sera from 26 patients were screened for novel antibodies.9 Sera from 16 of the patients immunoprecipitated a pair of proteins (sizes 100 kd and 200 kd), indicating these patients had an antibody to these proteins. This finding was highly specific for necrotizing myopathy when compared with controls, ie, other patients with myositis. Patients who had this finding displayed proximal muscle weakness, elevated muscle enzyme levels, and myopathic findings on electromyography; 63% had been exposed to statins before the onset of weakness, and when only patients over age 50 were included, the number rose to 83%.
This association of statins with necrotizing myopathy had been previously noted by two other groups. Needham et al10 described eight patients who, while on statins, developed myopathy that continued to worsen despite cessation of the drugs. An analysis of their muscle pathology revealed myofiber necrosis with little inflammatory infiltrate, as well as widespread up-regulation of expression of major histocompatibility complex class 1. These patients required immunosuppressive treatment (prednisone and methotrexate) to control their disease.
Grable-Esposito et al11 corroborated this finding by identifying 25 additional patients who developed a similar necrotizing myopathy while on statins.11 They also noted a significantly higher frequency of statin use in patients with necrotizing myopathy than in age-matched controls with polymyositis or inclusion-body myositis.
The researchers at the Johns Hopkins Myositis Center noted the similarity between these two patient groups and their own group of patients with necrotizing myopathy. Thus, a follow-up study was done to identify the 100-kg and 200-kd autoantigens observed in their earlier study. Exposure to a statin was found to up-regulate the expression of the two molecules.12 HMGCR was hypothesized as being the 100-kd antigen, because of its 97-kd molecular weight, and also because statin treatment had already been shown to up-regulate the expression of HMGCR.13 The researchers concluded that HMGCR was indeed the 100-kd antigen, with no distinctive antibodies recognizing the 200-kd protein. Although the 200-kd protein was once postulated to be a dimer of the 100-kd protein, its identity remains unknown.
The anti-HMGCR antibody was then screened for in a cohort of 750 myositis patients. The 16 patients previously found to have anti-200/100 were all positive for anti-HMGCR antibody. An additional 45 patients from the cohort (6%) were anti-HMGCR-positive by enzyme-linked immunosorbent assay, and all had necrotizing myopathy. Patients with other types of myopathy, including inflammatory myopathy, do not possess this antibody.12
The HMGCR antibody was quite specific for immune-mediated necrotizing myopathy, and this suggested that statins were capable of triggering an immune-mediated myopathy that is then perpetuated even if the drug is discontinued. As it was also demonstrated that statins increase the expression of HMGCR in muscle as well as in regenerating cells, the process may be sustained through persistently increased HMGCR expression associated with muscle repair.12
The C allele of the SLCO1B1 gene, which has been associated with statin-associated myopathy, was not increased in this population of patients positive for anti-HMGCR. Follow-up studies of the prevalence of anti-HMGCR in statin users in the Atherosclerosis Risk in Communities (ARIC) cohort, including those with self-limited statin myotoxicity, have also shown the absence of this antibody.14 This shows that anti-HMGCR is not found in the majority of statin-exposed patients and is highly specific for autoimmune myopathy. This also suggests that statin-associated autoimmune myopathy represents a pathologic process that is distinct from self-limited statin intolerance.
HOW THE CONDITION PRESENTS
Immune-mediated statin myopathy presents similarly to other idiopathic inflammatory myopathies such as polymyositis (Table 1). Symptoms often develop in a subacute to chronic course and can occur at any time with statin treatment. In one study,10 the average duration of statin use before the onset of weakness was 3 years (range 2 months to 10 years). In some patients whose statin had been stopped because of abnormal creatine kinase levels, weakness developed later, at a range of 0.5 to 20 months. Even low doses of statins (such as 10 mg of simvastatin) have been found to trigger this condition.10
Patients uniformly develop symmetric proximal arm and leg weakness, and distal weakness can also occur.11 Other features have included dysphagia, arthralgias, myalgias, and Raynaud phenomenon.9 Men and women are represented in roughly equal numbers.
The muscle enzymes are strikingly elevated in this disease, with a mean creatine kinase value of 10,333 IU/L at initial presentation.9 Although the creatine kinase level may be very elevated, patients often do not present with weakness until a certain threshold value is reached, in contrast with patients with anti-signal recognition particle necrotizing myopathy, who can present with profound weakness at a lower level. Hence, by the time patients are clinically symptomatic, the process may have been going on for some time. Despite the seemingly massive leak in muscle enzymes, the patients do not develop rhabdomyolysis. Inflammatory markers need not be elevated, and an association with other antibodies such as antinuclear antibody is not often seen.
Magnetic resonance imaging of the thigh has shown muscle edema in all patients.9 In decreasing order of frequency, other findings are atrophy, fatty replacement, and fascial edema.
Electromyography of involved muscle has shown irritable myopathy in most patients (88%) and nonirritable myopathy in a few.
Muscle biopsy studies have shown prominent necrotic and regenerating fibers without significant inflammatory infiltrate.11 There is also myophagocytosis of necrotic fibers and diffuse or focal up-regulation of major histocompatability complex class I expression.10
PATIENTS WITH ANTI-HMGCR WHO HAVE AND WHO HAVE NEVER TAKEN STATINS
When the anti-HMGCR antibody was tested for in the Johns Hopkins cohort,12 33% of patients with a necrotizing myopathy associated with this antibody had never taken a statin. The two groups were clinically indistinguishable, save for a few aspects. Compared with patients who had taken a statin, those who had never taken a statin were younger (mean age 37 vs 59), had higher levels of creatine kinase (13,392 vs 7,881 IU/L), and had a different race distribution (46.7% vs 86.7% white). Initial HMGCR antibody levels were also noted to correlate with creatine kinase levels and strength in statin-exposed patients but not in those who had never taken a statin.15
We hypothesize that in patients who have never taken a statin, other genetic or environmental factors may be the cause of the increased HMGCR expression, which then triggers the autoimmune response. Until further data are gathered, we should probably treat these patients as we treat those who develop this disease after taking a statin, and avoid giving them statins altogether.
MANAGEMENT OF STATIN-ASSOCIATED AUTOIMMUNE NECROTIZING MYOPATHY
Treatment of statin-associated autoimmune necrotizing myopathy can be challenging and requires immunosuppressive drugs.
Statin therapy should be stopped once this condition is suspected. Patients who continue to have elevated muscle enzymes or weakness should undergo further testing with electromyography, magnetic resonance imaging, and muscle biopsy. Electromyography detects myopathy and shows chronicity, distribution, and degree of severity. Although not necessary for diagnosis, magnetic resonance imaging helps to evaluate the extent of muscle involvement and damage and provides guidance when choosing a site for muscle biopsy. Muscle biopsy is necessary to determine the actual pathology and to exclude mimics such as dystrophy or metabolic myopathies.
When an immune-mediated myopathy is confirmed, prompt referral to a rheumatologist or a neuromuscular specialist is recommended.
Steroids are usually the first-line treatment for this disease. Other immunosuppressives, such as methotrexate, azathioprine, mycophenolate mofetil, and rituximab have been used with varying levels of success. In our experience, intravenous immunoglobulin has been particularly beneficial for refractory cases. With treatment, muscle enzyme levels and weakness improve, but relapses can occur. The ideal choice of immunosuppressive therapy and the duration of therapy are currently under investigation.
Rechallenge with another statin
At this time, the issue of rechallenging the patient with another statin has not been clarified. Given the autoimmune nature of the disease, we would avoid exposing the patient to a known trigger. However, this may be a difficult decision in patients with cardiovascular risk factors who require statins for primary or secondary prevention. We suggest using alternative cholesterol-lowering agents first and using them in combination if needed.
We have had some success in maintaining a handful of patients on a statin while treating them concurrently with immunosuppression. This is not ideal because they are constantly being exposed to the likely trigger for their disease, but it may be unavoidable if statins are deemed absolutely necessary. We have also had a patient with known statin-associated immune-mediated necrotizing myopathy who later became profoundly weak after another physician started her on a newer-generation agent, pitavastatin. This suggests to us that rechallenging patients, even with a different statin, can have deleterious effects.
IMPLICATIONS FOR CLINICAL PRACTICE
The true prevalence of statin-associated autoimmune myopathy in practice is unknown. In the Johns Hopkins Myositis Center cohort of patients with suspected myopathy, anti-HMGCR was found in 6% of the patients and was the second most frequent antibody found after anti-Jo1.12
Given the frequency of muscle-related complaints in patients on statins, we recommend obtaining baseline muscle enzyme measurements before starting statin therapy. As recommended by the National Lipid Association Statin Safety Assessment Task Force, the creatine kinase level should be measured when a patient develops muscular complaints, to help gauge the severity of the disease and to help decide whether to continue therapy.8 Random testing of the creatine kinase level in asymptomatic patients is not recommended.
At present, the diagnosis of statin-associated autoimmune necrotizing myopathy is based on a combination of findings—elevated muscle enzyme levels, muscle weakness, irritable findings on electromyography, and necrotizing myopathy on biopsy in a patient on a statin. The finding of the HMGCR antibody confirms the diagnosis. A test for this antibody is now commercially available in the United States. We suggest testing for the antibody in the following scenarios:
- A persistently elevated or rising creatine kinase, aspartate aminotransferase, alanine aminotransferase, or aldolase level after the statin is stopped; although no fixed creatine kinase level has been determined, a level above 1,000 U/L would be a reasonable cutoff at which to test
- Muscle symptoms (proximal or distal weakness) that persist 12 weeks after statin cessation regardless of the creatine kinase level, especially if the patient has dysphagia
- The finding of muscle irritability on electromyography or diffuse muscle edema on magnetic resonance imaging when testing for other myositis-specific antibodies is negative
- Muscle biopsy showing necrotizing myopathy with little or no inflammation.
In addition, since necrotizing myopathy is known to be associated with malignancy and since necrotizing myopathy is more common in older people, who are also more likely to be taking a statin, an age-appropriate malignancy evaluation is warranted as well.
Statins are among the most widely prescribed drugs, as they reduce cardiovascular risk very effectively. They work by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), a key enzyme in cholesterol biosynthesis. Although most patients tolerate statins well, muscle-related toxicity can limit the use of these drugs.
Recently, progressive necrotizing myopathy leading to profound weakness has been directly linked to statin therapy. Proper recognition of this ominous complication is important to prevent further damage from statin use.
STATIN-ASSOCIATED MUSCLE EFFECTS: A SPECTRUM
Muscle symptoms are among the best known and most important side effects of statins, ranging from asymptomatic, mild elevation of creatine kinase and benign myalgias to life-threatening rhabdomyolysis. As the various terms are often used inconsistently in the literature, we will briefly review each entity to put immune-necrotizing myopathy in its proper context on the spectrum of statin-associated muscle symptoms.
Myalgia
Myalgia, ie, muscle pain without elevation of muscle enzymes, is the most common side effect of statins. The incidence is about 10% in observational studies1,2; however, the incidence in the real world of clinical practice seems much higher. Myalgias can present as widespread pain or as localized pain, usually in the lower extremities. Muscle cramps and tendonitis-related pain are commonly reported.
Several clinical predictors of increased risk of statin-associated muscle pain were noted in the Prediction of Muscular Risk in Observational Conditions (PRIMO) study,2 assessing mild to moderate muscular symptoms in patients on high-dose statins. These included a history of muscle pain with another lipid-lowering agent, a history of cramps, a history of elevated creatine kinase levels, a personal or family history of muscle symptoms, untreated hypothyroidism, and a background of fibromyalgia-like symptoms. The incidence of muscle symptoms increased with the level of physical activity, and the median time of symptom onset was 1 month after starting statin therapy or titrating to a high dose.
In patients with myalgia alone, symptoms often improve when the statin is stopped.
Myopathy, myositis
The general terms myopathy and myositis have been used to refer to elevated muscle enzymes together with the muscle symptoms of pain, cramps, soreness, or weakness. An analysis of 21 clinical trials of statin therapy found that myopathy (creatine kinase level more than 10 times the upper limit of normal) occurred in 5 patients per 100,000 person-years.3 The incidence of myopathy increases when statins are used in high doses.
In another cohort of patients seen for statin intolerance,1 conventional risk factors for overt myositis included renal disease, diabetes, and thyroid disease. Electrolyte abnormalities did not differ between statin-tolerant and statin-intolerant patients.1
The search for genetic indicators of risk
In 2008, the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group conducted a genome-wide association study to identify genetic variants associated with myopathy with high-dose statin therapy.4 Eighty-five patients who had developed definite myopathy (muscle symptoms with enzyme levels more than 10 times the upper limit of normal) or incipient myopathy (asymptomatic or symptomatic, but enzyme levels at least three times the upper limit of normal) while taking simvastatin 80 mg were compared with 90 controls taking the same daily dose. The study reported variants in the SLCO1B1 gene as “strongly associated with an increased risk of statin-associated myopathy.”4
SLCO1B1 encodes the peptide responsible for hepatic uptake of statins, thus affecting the blood level of these drugs. Patients in the study who had the C variant, which predisposes to higher blood levels of statins, were at higher risk of myopathy than those with the T variant. The odds ratio for myopathy increased from 4.4 in heterozygotes for the C allele to 17.4 for homozygotes. This effect was similar even with lower doses of statins.4
We believe that these findings provide a strong basis for genetic testing of patients who may be at risk of statin-associated myopathy.
Rechallenging with a different statin
Often, patients with myopathy can be rechallenged with a different statin agent. In a study done in a lipid clinic, patients identified as having simvastatin-associated myopathy were given another statin.5 Between 15% and 42% tolerated the second statin, with no statistically significant difference between the tolerability rates with the different agents used (atorvastatin, rosuvastatin, pravastatin, and fluvastatin).
Rhabdomyolysis
Rhabdomyolysis, the most devastating complication of statin use, is marked by a creatine kinase level more than 10 times the upper limit of normal or greater than 10,000 IU/L, resulting from acute and massive destruction of muscle fibers and release of their contents into the bloodstream.
But rhabdomyolysis is a clinical syndrome, not solely an alarming increase in muscle enzyme levels. It can include renal failure and death. The rate of occurrence is low (1/100,000), but it can occur at any point in treatment.6 An analysis of Canadian and US case reports of statin-associated rhabdomyolysis showed an average of 824 cases each year.7 A dose-response relationship was observed with higher statin doses.
But statin-associated myopathy may not stop when the drug is stopped
The types of muscle toxicity discussed above stem from direct myotoxic effects of statins and are thought to be related to the blood concentration of the statin.6,8 They may be limited by genetic susceptibility. Mechanisms may include a change in muscle membrane excitability caused by modulation in membrane cholesterol levels, impaired mitochondrial function and calcium signaling, induction of apoptosis, and increased lipid peroxidation. Stopping the offending drug can often halt these downstream effects—hence the dictum that statin myopathy is self-limiting and should resolve with cessation of the drug.
But as we discuss in the following sections, some patients on statin therapy develop an immune-mediated myopathy that does not resolve with discontinuation of the statin and that may only resolve with immunosuppressive therapy.
STATINS AND AUTOIMMUNE NECROTIZING MYOPATHY
At the Johns Hopkins Myositis Center, a group of patients was identified who had necrotizing myopathy on biopsy but no known underlying condition or associated autoantibodies. In an attempt to establish an autoimmune basis for their disease, the sera from 26 patients were screened for novel antibodies.9 Sera from 16 of the patients immunoprecipitated a pair of proteins (sizes 100 kd and 200 kd), indicating these patients had an antibody to these proteins. This finding was highly specific for necrotizing myopathy when compared with controls, ie, other patients with myositis. Patients who had this finding displayed proximal muscle weakness, elevated muscle enzyme levels, and myopathic findings on electromyography; 63% had been exposed to statins before the onset of weakness, and when only patients over age 50 were included, the number rose to 83%.
This association of statins with necrotizing myopathy had been previously noted by two other groups. Needham et al10 described eight patients who, while on statins, developed myopathy that continued to worsen despite cessation of the drugs. An analysis of their muscle pathology revealed myofiber necrosis with little inflammatory infiltrate, as well as widespread up-regulation of expression of major histocompatibility complex class 1. These patients required immunosuppressive treatment (prednisone and methotrexate) to control their disease.
Grable-Esposito et al11 corroborated this finding by identifying 25 additional patients who developed a similar necrotizing myopathy while on statins.11 They also noted a significantly higher frequency of statin use in patients with necrotizing myopathy than in age-matched controls with polymyositis or inclusion-body myositis.
The researchers at the Johns Hopkins Myositis Center noted the similarity between these two patient groups and their own group of patients with necrotizing myopathy. Thus, a follow-up study was done to identify the 100-kg and 200-kd autoantigens observed in their earlier study. Exposure to a statin was found to up-regulate the expression of the two molecules.12 HMGCR was hypothesized as being the 100-kd antigen, because of its 97-kd molecular weight, and also because statin treatment had already been shown to up-regulate the expression of HMGCR.13 The researchers concluded that HMGCR was indeed the 100-kd antigen, with no distinctive antibodies recognizing the 200-kd protein. Although the 200-kd protein was once postulated to be a dimer of the 100-kd protein, its identity remains unknown.
The anti-HMGCR antibody was then screened for in a cohort of 750 myositis patients. The 16 patients previously found to have anti-200/100 were all positive for anti-HMGCR antibody. An additional 45 patients from the cohort (6%) were anti-HMGCR-positive by enzyme-linked immunosorbent assay, and all had necrotizing myopathy. Patients with other types of myopathy, including inflammatory myopathy, do not possess this antibody.12
The HMGCR antibody was quite specific for immune-mediated necrotizing myopathy, and this suggested that statins were capable of triggering an immune-mediated myopathy that is then perpetuated even if the drug is discontinued. As it was also demonstrated that statins increase the expression of HMGCR in muscle as well as in regenerating cells, the process may be sustained through persistently increased HMGCR expression associated with muscle repair.12
The C allele of the SLCO1B1 gene, which has been associated with statin-associated myopathy, was not increased in this population of patients positive for anti-HMGCR. Follow-up studies of the prevalence of anti-HMGCR in statin users in the Atherosclerosis Risk in Communities (ARIC) cohort, including those with self-limited statin myotoxicity, have also shown the absence of this antibody.14 This shows that anti-HMGCR is not found in the majority of statin-exposed patients and is highly specific for autoimmune myopathy. This also suggests that statin-associated autoimmune myopathy represents a pathologic process that is distinct from self-limited statin intolerance.
HOW THE CONDITION PRESENTS
Immune-mediated statin myopathy presents similarly to other idiopathic inflammatory myopathies such as polymyositis (Table 1). Symptoms often develop in a subacute to chronic course and can occur at any time with statin treatment. In one study,10 the average duration of statin use before the onset of weakness was 3 years (range 2 months to 10 years). In some patients whose statin had been stopped because of abnormal creatine kinase levels, weakness developed later, at a range of 0.5 to 20 months. Even low doses of statins (such as 10 mg of simvastatin) have been found to trigger this condition.10
Patients uniformly develop symmetric proximal arm and leg weakness, and distal weakness can also occur.11 Other features have included dysphagia, arthralgias, myalgias, and Raynaud phenomenon.9 Men and women are represented in roughly equal numbers.
The muscle enzymes are strikingly elevated in this disease, with a mean creatine kinase value of 10,333 IU/L at initial presentation.9 Although the creatine kinase level may be very elevated, patients often do not present with weakness until a certain threshold value is reached, in contrast with patients with anti-signal recognition particle necrotizing myopathy, who can present with profound weakness at a lower level. Hence, by the time patients are clinically symptomatic, the process may have been going on for some time. Despite the seemingly massive leak in muscle enzymes, the patients do not develop rhabdomyolysis. Inflammatory markers need not be elevated, and an association with other antibodies such as antinuclear antibody is not often seen.
Magnetic resonance imaging of the thigh has shown muscle edema in all patients.9 In decreasing order of frequency, other findings are atrophy, fatty replacement, and fascial edema.
Electromyography of involved muscle has shown irritable myopathy in most patients (88%) and nonirritable myopathy in a few.
Muscle biopsy studies have shown prominent necrotic and regenerating fibers without significant inflammatory infiltrate.11 There is also myophagocytosis of necrotic fibers and diffuse or focal up-regulation of major histocompatability complex class I expression.10
PATIENTS WITH ANTI-HMGCR WHO HAVE AND WHO HAVE NEVER TAKEN STATINS
When the anti-HMGCR antibody was tested for in the Johns Hopkins cohort,12 33% of patients with a necrotizing myopathy associated with this antibody had never taken a statin. The two groups were clinically indistinguishable, save for a few aspects. Compared with patients who had taken a statin, those who had never taken a statin were younger (mean age 37 vs 59), had higher levels of creatine kinase (13,392 vs 7,881 IU/L), and had a different race distribution (46.7% vs 86.7% white). Initial HMGCR antibody levels were also noted to correlate with creatine kinase levels and strength in statin-exposed patients but not in those who had never taken a statin.15
We hypothesize that in patients who have never taken a statin, other genetic or environmental factors may be the cause of the increased HMGCR expression, which then triggers the autoimmune response. Until further data are gathered, we should probably treat these patients as we treat those who develop this disease after taking a statin, and avoid giving them statins altogether.
MANAGEMENT OF STATIN-ASSOCIATED AUTOIMMUNE NECROTIZING MYOPATHY
Treatment of statin-associated autoimmune necrotizing myopathy can be challenging and requires immunosuppressive drugs.
Statin therapy should be stopped once this condition is suspected. Patients who continue to have elevated muscle enzymes or weakness should undergo further testing with electromyography, magnetic resonance imaging, and muscle biopsy. Electromyography detects myopathy and shows chronicity, distribution, and degree of severity. Although not necessary for diagnosis, magnetic resonance imaging helps to evaluate the extent of muscle involvement and damage and provides guidance when choosing a site for muscle biopsy. Muscle biopsy is necessary to determine the actual pathology and to exclude mimics such as dystrophy or metabolic myopathies.
When an immune-mediated myopathy is confirmed, prompt referral to a rheumatologist or a neuromuscular specialist is recommended.
Steroids are usually the first-line treatment for this disease. Other immunosuppressives, such as methotrexate, azathioprine, mycophenolate mofetil, and rituximab have been used with varying levels of success. In our experience, intravenous immunoglobulin has been particularly beneficial for refractory cases. With treatment, muscle enzyme levels and weakness improve, but relapses can occur. The ideal choice of immunosuppressive therapy and the duration of therapy are currently under investigation.
Rechallenge with another statin
At this time, the issue of rechallenging the patient with another statin has not been clarified. Given the autoimmune nature of the disease, we would avoid exposing the patient to a known trigger. However, this may be a difficult decision in patients with cardiovascular risk factors who require statins for primary or secondary prevention. We suggest using alternative cholesterol-lowering agents first and using them in combination if needed.
We have had some success in maintaining a handful of patients on a statin while treating them concurrently with immunosuppression. This is not ideal because they are constantly being exposed to the likely trigger for their disease, but it may be unavoidable if statins are deemed absolutely necessary. We have also had a patient with known statin-associated immune-mediated necrotizing myopathy who later became profoundly weak after another physician started her on a newer-generation agent, pitavastatin. This suggests to us that rechallenging patients, even with a different statin, can have deleterious effects.
IMPLICATIONS FOR CLINICAL PRACTICE
The true prevalence of statin-associated autoimmune myopathy in practice is unknown. In the Johns Hopkins Myositis Center cohort of patients with suspected myopathy, anti-HMGCR was found in 6% of the patients and was the second most frequent antibody found after anti-Jo1.12
Given the frequency of muscle-related complaints in patients on statins, we recommend obtaining baseline muscle enzyme measurements before starting statin therapy. As recommended by the National Lipid Association Statin Safety Assessment Task Force, the creatine kinase level should be measured when a patient develops muscular complaints, to help gauge the severity of the disease and to help decide whether to continue therapy.8 Random testing of the creatine kinase level in asymptomatic patients is not recommended.
At present, the diagnosis of statin-associated autoimmune necrotizing myopathy is based on a combination of findings—elevated muscle enzyme levels, muscle weakness, irritable findings on electromyography, and necrotizing myopathy on biopsy in a patient on a statin. The finding of the HMGCR antibody confirms the diagnosis. A test for this antibody is now commercially available in the United States. We suggest testing for the antibody in the following scenarios:
- A persistently elevated or rising creatine kinase, aspartate aminotransferase, alanine aminotransferase, or aldolase level after the statin is stopped; although no fixed creatine kinase level has been determined, a level above 1,000 U/L would be a reasonable cutoff at which to test
- Muscle symptoms (proximal or distal weakness) that persist 12 weeks after statin cessation regardless of the creatine kinase level, especially if the patient has dysphagia
- The finding of muscle irritability on electromyography or diffuse muscle edema on magnetic resonance imaging when testing for other myositis-specific antibodies is negative
- Muscle biopsy showing necrotizing myopathy with little or no inflammation.
In addition, since necrotizing myopathy is known to be associated with malignancy and since necrotizing myopathy is more common in older people, who are also more likely to be taking a statin, an age-appropriate malignancy evaluation is warranted as well.
- Harris LJ, Thapa R, Brown M, et al. Clinical and laboratory phenotype of patients experiencing statin intolerance attributable to myalgia. J Clin Lipidol 2011; 5:299–307.
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol 2006; 97:52C–60C.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Fung EC, Crook MA. Statin myopathy: a lipid clinic experience on the tolerability of statin rechallenge. Cardiovasc Ther 2012; 30:e212–e218.
- Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol 2008; 8:333–338.
- Holbrook A, Wright M, Sung M, Ribic C, Baker S. Statin-associated rhabdomyolysis: is there a dose-response relationship? Can J Cardiol 2011; 27:146–151.
- McKenney JM, Davidson MH, Jacobson TA, Guyton JR; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–94C.
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62:2757–2766.
- Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord 2007; 17:194–200.
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41:185–190.
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63:713–721.
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990; 343:425–430.
- Mammen AL, Pak K, Williams EK, et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res (Hoboken) 2012; 64:269–272.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
- Harris LJ, Thapa R, Brown M, et al. Clinical and laboratory phenotype of patients experiencing statin intolerance attributable to myalgia. J Clin Lipidol 2011; 5:299–307.
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol 2006; 97:52C–60C.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Fung EC, Crook MA. Statin myopathy: a lipid clinic experience on the tolerability of statin rechallenge. Cardiovasc Ther 2012; 30:e212–e218.
- Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol 2008; 8:333–338.
- Holbrook A, Wright M, Sung M, Ribic C, Baker S. Statin-associated rhabdomyolysis: is there a dose-response relationship? Can J Cardiol 2011; 27:146–151.
- McKenney JM, Davidson MH, Jacobson TA, Guyton JR; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–94C.
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62:2757–2766.
- Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord 2007; 17:194–200.
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41:185–190.
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63:713–721.
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990; 343:425–430.
- Mammen AL, Pak K, Williams EK, et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res (Hoboken) 2012; 64:269–272.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
KEY POINTS
- Most cases of muscle symptoms associated with statin use are a direct effect of the statin on the muscle and resolve after the statin is discontinued.
- In contrast to simple myalgia or myositis, statin-associated autoimmune necrotizing myopathy can persist or even arise de novo after the statin is stopped.
- This condition presents with symmetric proximal arm and leg weakness and striking elevations of muscle enzymes such as creatine kinase.
- Treatment can be challenging and requires immunosuppressive drugs; referral to a specialist is recommended.
- Statin therapy should be discontinued once this condition is suspected. Patients who continue to have elevated muscle enzymes or weakness should undergo further testing with electromyography, magnetic resonance imaging, and muscle biopsy.
A 41-year-old man with abdominal pain
A 41-year-old man presented with pain in the left upper quadrant for 4 days. The pain was constant, was worse on inspiration, and did not radiate. He denied fevers, night sweats, nausea, vomiting, diarrhea, and urinary symptoms. He had been diagnosed with multiple sclerosis a few years earlier, and he had undergone aortofemoral bypass surgery on the left side 2 years ago. He denied smoking or using illicit drugs and described himself as a social drinker.
In the emergency room, he appeared comfortable. He was afebrile, blood pressure 136/69 mm Hg, pulse rate 98 per minute, and respiratory rate 16. All pulses were palpable and equal, the jugular venous pressure was not elevated, and no cardiac murmurs were heard. The abdomen was tender in the left upper quadrant, with no guarding or rigidity. Examination of the nervous, musculoskeletal, and respiratory systems was unremarkable. Skin examination revealed only scars from previous surgery.
LABORATORY AND IMAGING RESULTS
- White blood cell count 7.2 × 109/L (reference range 4.0–10.0) with a normal differential
- Hemoglobin 134 g/dL (140–180)
- Platelet count 167 × 109/L (150–400)
- Renal and liver panels were normal
- Erythrocyte sedimentation rate 30 mm/hour
- C-reactive protein level 14.3 mg/L
- D-dimer level 2,670 ng/mL (< 500)
- International normalized ratio (INR) 1.0 (0.9–1.3)
- Activated partial thromboplastin time (aPTT) 44 seconds (25–38)
- Fibrinogen level 3.0 g/L (1.8–3.5)
- Urinalysis negative for leukocytes and casts.
Computed tomography of the abdomen showed a wedge-shaped area of hypodensity along the inferolateral aspect of the spleen measuring 6 × 3.8 cm, consistent with a recent infarct (Figure 1). There was also evidence of a previous infarct in the posterolateral aspect of the spleen. Splenic, celiac, superior mesenteric, and inferior mesenteric arteries were patent.
1. Given these findings, which of the following diagnoses should be considered?
- Subacute infective endocarditis
- Inherited thrombophilia
- Antiphospholipid syndrome
All three diagnoses should be considered in this case.
Endocarditis
Embolism from a source in the heart caused by subacute bacterial endocarditis is more common than the other two conditions listed here and must be excluded.
Our patient lacks key features of this condition: he has no predisposing factors (artificial valve, cyanotic congenital heart disease, previous endocarditis, intravenous drug abuse); no constitutional symptoms of fever, night sweats, and weight loss; no findings on examination of skin and cardiovascular systems; and a normal white blood cell count. Nevertheless, even though the absence of these features makes bacterial endocarditis unlikely, it does not exclude it. Blood cultures and transesophageal echocardiography are indicated to rule out bacterial endocarditis.
We obtained serial blood cultures, which were negative, and transesophageal echocardiography showed normal valves and no evidence of thrombus or vegetation, thus excluding a cardiac source of emboli.
Thrombophilia
Our patient has a history of recurrent thromboembolic episodes, and this warrants testing to rule out an inherited thrombophilia. A family history of thromboembolic disease should also be sought.1
In our patient, tests for prothrombotic activity including protein C chromogen, activated protein C ratio, free protein S, functional protein S, antithrombin factor V Leiden, and the prothrombin 20210G>A mutation were either negative or within the reference range. A negative family history of thromboembolic disease and the negative laboratory tests make inherited thrombophilia unlikely in our patient.
Sickle cell disease, polycythemia vera, and essential thrombocythemia may also cause splenic infarction but can be ruled out in this patient on the basis of history and initial blood tests.
Antiphospholipid syndrome
A history of vascular disease (aortofemoral bypass surgery), a recent splenic infarct, and an elevated aPTT makes antiphospholipid syndrome the likeliest diagnosis in this patient.
Appropriate tests are for lupus anticoagulant, immunoglobulin G (IgG) or IgM cardiolipin antibody, and beta-2 glycoprotein 1 (beta-2 GP1) antibody, as well as the dilute Russell viper venom time (dRVVT) and the dRVVT ratio. The IgG and IgM cardiolipin antibody and beta-2 GP1 antibody tests have the same diagnostic value, and only medium to high titers should be considered positive.
Our patient’s IgG cardiolipin antibody level was in the normal range at 15 IgG phospholipid units (reference range 0–22); his IgM cardiolipin antibody level was high at 41 IgM phospholipid units (0–10). The dRVVT was 57 seconds (24–42), and the dRVVT ratio was 2.0 (0.0–1.3).
2. What further investigations are indicated before starting treatment?
- No further investigations required
- Repeat testing for phospholipid antibodies in 12 weeks
- Test for antinuclear antibodies
Antiphospholipid antibodies may appear transiently in certain infections, such as syphilis, Lyme disease, Epstein-Barr virus, cytomegalovirus, hepatitis C, and human immunodeficiency virus. Therefore, the presence of antiphospholipid antibodies must be confirmed over time, with two positive results at least 12 weeks apart.2
When repeated 12 weeks later, our patient’s IgG anticardiolipin antibody level was 14 GPL units, and the IgM anticardiolipin antibody level was 30 MPL units; the dRVVT was 55 seconds, and the dRVVT ratio was 1.8. These results, along with a history of recurrent arterial thrombosis, confirmed antiphospholipid syndrome.
The 2009 update of the International Society of Thrombosis and Haemostasis guidelines recommend two tests, the dRVVT and the aPTT, since no single test is 100% sensitive for lupus anticoagulant.3 The dRVVT has a high specificity for lupus anticoagulant in patients at high risk of thrombosis.
A SYNDROME WITH A WIDE RANGE OF EFFECTS AND COMPLICATIONS
Antiphospholipid syndrome is a systemic autoimmune disease that manifests as arterial and venous thrombosis and as obstetric complications. Thrombosis tends to be recurrent and may involve any site. For example, it can cause blurred vision in one or both eyes; amaurosis fugax; visual field defects; central or branch retinal artery or vein occlusion; deep vein thrombosis; pulmonary embolism; myocardial infarction; transient ischemic attack and stroke; cerebral vein thrombosis; and portal, renal, and mesenteric infarction involving veins or arteries.4 Pulmonary capillaritis may cause diffuse alveolar hemorrhage. Livedo reticularis, digital gangrene, cutaneous necrosis, splinter hemorrhages, chorea, and transverse myelopathy may also occur.
Obstetric complications of antiphospholipid syndrome include recurrent miscarriage and pregnancy loss at or after 10 weeks of gestation, eclampsia, preeclampsia, and placental insufficiency.5 The syndrome also has a potentially lethal variant characterized by multiorgan thrombosis affecting mainly small vessels.
The diagnosis of antiphospholipid syndrome requires relevant clinical features and symptoms and the presence of at least one of the antiphospholipid antibodies. Because the rate of false-positive tests for antiphospholipid antibodies ranges from 3% to 20% in the general population, asymptomatic patients should not be tested.6
Antiphospholipid syndrome may occur in the setting of other autoimmune diseases, most commonly systemic lupus erythematosus, when it is termed “secondary” antiphospholipid syndrome. Although only 40% of patients with lupus have antiphospholipid antibodies and less than 40% will have a thrombotic event, thrombotic antiphospholipid syndrome is a major adverse prognostic factor in these patients.7,8 Therefore, it is prudent to consider systemic lupus erythematosus and to do appropriate tests if the patient has other features suggestive of lupus, such as renal, skin, or musculoskeletal lesions.
In our patient, antinuclear antibody testing was positive, with a titer of 1:320, and showed a finely speckled staining pattern. Tests for antibodies to Sjögren syndrome A and B antigens were negative. The complement C3 level was 1.28 g/L (reference range 0.74–1.85) and the C4 level was 0.24 g/L (0.16–0.44). Although the speckled staining pattern can be seen in lupus, it is more common in Sjögren syndrome, mixed connective tissue disease, scleroderma, and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia).9 Moreover, normal levels of complement C3 and C4, in the absence of clinical features, make lupus unlikely. Similarly, our patient had no clinical features of other connective tissue disorders. Therefore, he had primary antiphospholipid syndrome.
3. How should this patient be managed?
- Antiplatelet therapy
- Warfarin to maintain an INR between 2.0 and 3.0
- Warfarin to maintain an INR above 3.0
The risk of recurrent thrombosis is high in patients who test positive for lupus anticoagulant, and the risk is highest in patients who are also positive for anticardiolipin and anti-beta-2 GP1 antibodies: the incidence of thrombosis is 12.2% at 1 year, 26.1% at 5 years, 44.2% at 10 years.10
Since our patient is positive for lupus anticoagulant (prolonged aPTT and elevated dRVVT, both indicating lupus anticoagulant positivity) and for anticardiolipin antibodies (anti-beta-2 GP1 not tested), his risk of recurrent thrombosis is high, and he requires lifelong anticoagulation therapy.
The intensity of anticoagulation in different subgroups of patients is controversial. Based on retrospective trials, indefinite anticoagulation at an INR of 2.0 to 3.0 has been suggested for patients with antiphospholipid syndrome presenting with venous thrombosis, and more intense anticoagulation with an INR above 3.0 in patients with recurrent or arterial thrombosis.11 The combination of warfarin with an INR between 2.0 and 3.0 and aspirin 100 mg daily has also been proposed for patients with arterial thrombosis.12
Modifiable risk factors such as smoking, obesity, and use of estrogens should be addressed in all patients with antiphospholipid syndrome.
In pregnant women with complications such as preeclampsia, low-dose aspirin can be used, and in women with a history of miscarriage, the combination of low-dose aspirin and heparin is recommended throughout the prenatal period.4
In patients who have recurrent thrombosis despite adequate anticoagulation, an expert committee12 has proposed that alternative regimens could include long-term low-molecular-weight heparin instead of warfarin, the combination of warfarin and aspirin, or warfarin and hydroxychloroquine. Adding a statin can also be considered.
Treatment of catastrophic antiphospholipid syndrome is based on expert opinion. A combination of anticoagulation, corticosteroids, plasma exchange, intravenous immunoglobulins, and rituximab has been tried, but the mortality rate remains high.13
OUR PATIENT'S COURSE
Our patient was started on warfarin, with a target INR above 3.0, and was doing well at 6 months of follow-up.
- De Stefano V, Rossi E. Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost 2013; 110:697–705.
- Galli M. Interpretation and recommended testing for antiphospholipid antibodies. Semin Thromb Hemost 2012; 38:348–352.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012; 157:47–58.
- Misita CP, Moll S. Antiphospholipid antibodies. Circulation 2005; 112:e39–e44.
- Rand JH, Wolgast LR. Do’s and don’t’s in diagnosing antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program 2012; 2012:455–459.
- Mok CC, Tang SS, To CH, Petri M. Incidence and risk factors of thromboembolism in systemic lupus erythematosus: a comparison of three ethnic groups. Arthritis Rheum 2005; 52:2774–2782.
- Ruiz-Irastorza G, Egurbide MV, Ugalde J, Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch Intern Med 2004; 164:77–82.
- Locht H, Pelck R, Manthorpe R. Clinical manifestations correlated to the prevalence of autoantibodies in a large (n = 321) cohort of patients with primary Sjögren’s syndrome: a comparison of patients initially diagnosed according to the Copenhagen classification criteria with the American-European consensus criteria. Autoimmun Rev 2005; 4:276–281.
- Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010; 8:237–242.
- Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet 2010; 376:1498–1509.
- Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th International Congress on antiphospholipid antibodies. Lupus 2011; 20:206–218.
- Cervera R. Update on the diagnosis, treatment, and prognosis of the catastrophic antiphospholipid syndrome. Curr Rheumatol Rep 2010; 12:70–76.
A 41-year-old man presented with pain in the left upper quadrant for 4 days. The pain was constant, was worse on inspiration, and did not radiate. He denied fevers, night sweats, nausea, vomiting, diarrhea, and urinary symptoms. He had been diagnosed with multiple sclerosis a few years earlier, and he had undergone aortofemoral bypass surgery on the left side 2 years ago. He denied smoking or using illicit drugs and described himself as a social drinker.
In the emergency room, he appeared comfortable. He was afebrile, blood pressure 136/69 mm Hg, pulse rate 98 per minute, and respiratory rate 16. All pulses were palpable and equal, the jugular venous pressure was not elevated, and no cardiac murmurs were heard. The abdomen was tender in the left upper quadrant, with no guarding or rigidity. Examination of the nervous, musculoskeletal, and respiratory systems was unremarkable. Skin examination revealed only scars from previous surgery.
LABORATORY AND IMAGING RESULTS
- White blood cell count 7.2 × 109/L (reference range 4.0–10.0) with a normal differential
- Hemoglobin 134 g/dL (140–180)
- Platelet count 167 × 109/L (150–400)
- Renal and liver panels were normal
- Erythrocyte sedimentation rate 30 mm/hour
- C-reactive protein level 14.3 mg/L
- D-dimer level 2,670 ng/mL (< 500)
- International normalized ratio (INR) 1.0 (0.9–1.3)
- Activated partial thromboplastin time (aPTT) 44 seconds (25–38)
- Fibrinogen level 3.0 g/L (1.8–3.5)
- Urinalysis negative for leukocytes and casts.
Computed tomography of the abdomen showed a wedge-shaped area of hypodensity along the inferolateral aspect of the spleen measuring 6 × 3.8 cm, consistent with a recent infarct (Figure 1). There was also evidence of a previous infarct in the posterolateral aspect of the spleen. Splenic, celiac, superior mesenteric, and inferior mesenteric arteries were patent.
1. Given these findings, which of the following diagnoses should be considered?
- Subacute infective endocarditis
- Inherited thrombophilia
- Antiphospholipid syndrome
All three diagnoses should be considered in this case.
Endocarditis
Embolism from a source in the heart caused by subacute bacterial endocarditis is more common than the other two conditions listed here and must be excluded.
Our patient lacks key features of this condition: he has no predisposing factors (artificial valve, cyanotic congenital heart disease, previous endocarditis, intravenous drug abuse); no constitutional symptoms of fever, night sweats, and weight loss; no findings on examination of skin and cardiovascular systems; and a normal white blood cell count. Nevertheless, even though the absence of these features makes bacterial endocarditis unlikely, it does not exclude it. Blood cultures and transesophageal echocardiography are indicated to rule out bacterial endocarditis.
We obtained serial blood cultures, which were negative, and transesophageal echocardiography showed normal valves and no evidence of thrombus or vegetation, thus excluding a cardiac source of emboli.
Thrombophilia
Our patient has a history of recurrent thromboembolic episodes, and this warrants testing to rule out an inherited thrombophilia. A family history of thromboembolic disease should also be sought.1
In our patient, tests for prothrombotic activity including protein C chromogen, activated protein C ratio, free protein S, functional protein S, antithrombin factor V Leiden, and the prothrombin 20210G>A mutation were either negative or within the reference range. A negative family history of thromboembolic disease and the negative laboratory tests make inherited thrombophilia unlikely in our patient.
Sickle cell disease, polycythemia vera, and essential thrombocythemia may also cause splenic infarction but can be ruled out in this patient on the basis of history and initial blood tests.
Antiphospholipid syndrome
A history of vascular disease (aortofemoral bypass surgery), a recent splenic infarct, and an elevated aPTT makes antiphospholipid syndrome the likeliest diagnosis in this patient.
Appropriate tests are for lupus anticoagulant, immunoglobulin G (IgG) or IgM cardiolipin antibody, and beta-2 glycoprotein 1 (beta-2 GP1) antibody, as well as the dilute Russell viper venom time (dRVVT) and the dRVVT ratio. The IgG and IgM cardiolipin antibody and beta-2 GP1 antibody tests have the same diagnostic value, and only medium to high titers should be considered positive.
Our patient’s IgG cardiolipin antibody level was in the normal range at 15 IgG phospholipid units (reference range 0–22); his IgM cardiolipin antibody level was high at 41 IgM phospholipid units (0–10). The dRVVT was 57 seconds (24–42), and the dRVVT ratio was 2.0 (0.0–1.3).
2. What further investigations are indicated before starting treatment?
- No further investigations required
- Repeat testing for phospholipid antibodies in 12 weeks
- Test for antinuclear antibodies
Antiphospholipid antibodies may appear transiently in certain infections, such as syphilis, Lyme disease, Epstein-Barr virus, cytomegalovirus, hepatitis C, and human immunodeficiency virus. Therefore, the presence of antiphospholipid antibodies must be confirmed over time, with two positive results at least 12 weeks apart.2
When repeated 12 weeks later, our patient’s IgG anticardiolipin antibody level was 14 GPL units, and the IgM anticardiolipin antibody level was 30 MPL units; the dRVVT was 55 seconds, and the dRVVT ratio was 1.8. These results, along with a history of recurrent arterial thrombosis, confirmed antiphospholipid syndrome.
The 2009 update of the International Society of Thrombosis and Haemostasis guidelines recommend two tests, the dRVVT and the aPTT, since no single test is 100% sensitive for lupus anticoagulant.3 The dRVVT has a high specificity for lupus anticoagulant in patients at high risk of thrombosis.
A SYNDROME WITH A WIDE RANGE OF EFFECTS AND COMPLICATIONS
Antiphospholipid syndrome is a systemic autoimmune disease that manifests as arterial and venous thrombosis and as obstetric complications. Thrombosis tends to be recurrent and may involve any site. For example, it can cause blurred vision in one or both eyes; amaurosis fugax; visual field defects; central or branch retinal artery or vein occlusion; deep vein thrombosis; pulmonary embolism; myocardial infarction; transient ischemic attack and stroke; cerebral vein thrombosis; and portal, renal, and mesenteric infarction involving veins or arteries.4 Pulmonary capillaritis may cause diffuse alveolar hemorrhage. Livedo reticularis, digital gangrene, cutaneous necrosis, splinter hemorrhages, chorea, and transverse myelopathy may also occur.
Obstetric complications of antiphospholipid syndrome include recurrent miscarriage and pregnancy loss at or after 10 weeks of gestation, eclampsia, preeclampsia, and placental insufficiency.5 The syndrome also has a potentially lethal variant characterized by multiorgan thrombosis affecting mainly small vessels.
The diagnosis of antiphospholipid syndrome requires relevant clinical features and symptoms and the presence of at least one of the antiphospholipid antibodies. Because the rate of false-positive tests for antiphospholipid antibodies ranges from 3% to 20% in the general population, asymptomatic patients should not be tested.6
Antiphospholipid syndrome may occur in the setting of other autoimmune diseases, most commonly systemic lupus erythematosus, when it is termed “secondary” antiphospholipid syndrome. Although only 40% of patients with lupus have antiphospholipid antibodies and less than 40% will have a thrombotic event, thrombotic antiphospholipid syndrome is a major adverse prognostic factor in these patients.7,8 Therefore, it is prudent to consider systemic lupus erythematosus and to do appropriate tests if the patient has other features suggestive of lupus, such as renal, skin, or musculoskeletal lesions.
In our patient, antinuclear antibody testing was positive, with a titer of 1:320, and showed a finely speckled staining pattern. Tests for antibodies to Sjögren syndrome A and B antigens were negative. The complement C3 level was 1.28 g/L (reference range 0.74–1.85) and the C4 level was 0.24 g/L (0.16–0.44). Although the speckled staining pattern can be seen in lupus, it is more common in Sjögren syndrome, mixed connective tissue disease, scleroderma, and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia).9 Moreover, normal levels of complement C3 and C4, in the absence of clinical features, make lupus unlikely. Similarly, our patient had no clinical features of other connective tissue disorders. Therefore, he had primary antiphospholipid syndrome.
3. How should this patient be managed?
- Antiplatelet therapy
- Warfarin to maintain an INR between 2.0 and 3.0
- Warfarin to maintain an INR above 3.0
The risk of recurrent thrombosis is high in patients who test positive for lupus anticoagulant, and the risk is highest in patients who are also positive for anticardiolipin and anti-beta-2 GP1 antibodies: the incidence of thrombosis is 12.2% at 1 year, 26.1% at 5 years, 44.2% at 10 years.10
Since our patient is positive for lupus anticoagulant (prolonged aPTT and elevated dRVVT, both indicating lupus anticoagulant positivity) and for anticardiolipin antibodies (anti-beta-2 GP1 not tested), his risk of recurrent thrombosis is high, and he requires lifelong anticoagulation therapy.
The intensity of anticoagulation in different subgroups of patients is controversial. Based on retrospective trials, indefinite anticoagulation at an INR of 2.0 to 3.0 has been suggested for patients with antiphospholipid syndrome presenting with venous thrombosis, and more intense anticoagulation with an INR above 3.0 in patients with recurrent or arterial thrombosis.11 The combination of warfarin with an INR between 2.0 and 3.0 and aspirin 100 mg daily has also been proposed for patients with arterial thrombosis.12
Modifiable risk factors such as smoking, obesity, and use of estrogens should be addressed in all patients with antiphospholipid syndrome.
In pregnant women with complications such as preeclampsia, low-dose aspirin can be used, and in women with a history of miscarriage, the combination of low-dose aspirin and heparin is recommended throughout the prenatal period.4
In patients who have recurrent thrombosis despite adequate anticoagulation, an expert committee12 has proposed that alternative regimens could include long-term low-molecular-weight heparin instead of warfarin, the combination of warfarin and aspirin, or warfarin and hydroxychloroquine. Adding a statin can also be considered.
Treatment of catastrophic antiphospholipid syndrome is based on expert opinion. A combination of anticoagulation, corticosteroids, plasma exchange, intravenous immunoglobulins, and rituximab has been tried, but the mortality rate remains high.13
OUR PATIENT'S COURSE
Our patient was started on warfarin, with a target INR above 3.0, and was doing well at 6 months of follow-up.
A 41-year-old man presented with pain in the left upper quadrant for 4 days. The pain was constant, was worse on inspiration, and did not radiate. He denied fevers, night sweats, nausea, vomiting, diarrhea, and urinary symptoms. He had been diagnosed with multiple sclerosis a few years earlier, and he had undergone aortofemoral bypass surgery on the left side 2 years ago. He denied smoking or using illicit drugs and described himself as a social drinker.
In the emergency room, he appeared comfortable. He was afebrile, blood pressure 136/69 mm Hg, pulse rate 98 per minute, and respiratory rate 16. All pulses were palpable and equal, the jugular venous pressure was not elevated, and no cardiac murmurs were heard. The abdomen was tender in the left upper quadrant, with no guarding or rigidity. Examination of the nervous, musculoskeletal, and respiratory systems was unremarkable. Skin examination revealed only scars from previous surgery.
LABORATORY AND IMAGING RESULTS
- White blood cell count 7.2 × 109/L (reference range 4.0–10.0) with a normal differential
- Hemoglobin 134 g/dL (140–180)
- Platelet count 167 × 109/L (150–400)
- Renal and liver panels were normal
- Erythrocyte sedimentation rate 30 mm/hour
- C-reactive protein level 14.3 mg/L
- D-dimer level 2,670 ng/mL (< 500)
- International normalized ratio (INR) 1.0 (0.9–1.3)
- Activated partial thromboplastin time (aPTT) 44 seconds (25–38)
- Fibrinogen level 3.0 g/L (1.8–3.5)
- Urinalysis negative for leukocytes and casts.
Computed tomography of the abdomen showed a wedge-shaped area of hypodensity along the inferolateral aspect of the spleen measuring 6 × 3.8 cm, consistent with a recent infarct (Figure 1). There was also evidence of a previous infarct in the posterolateral aspect of the spleen. Splenic, celiac, superior mesenteric, and inferior mesenteric arteries were patent.
1. Given these findings, which of the following diagnoses should be considered?
- Subacute infective endocarditis
- Inherited thrombophilia
- Antiphospholipid syndrome
All three diagnoses should be considered in this case.
Endocarditis
Embolism from a source in the heart caused by subacute bacterial endocarditis is more common than the other two conditions listed here and must be excluded.
Our patient lacks key features of this condition: he has no predisposing factors (artificial valve, cyanotic congenital heart disease, previous endocarditis, intravenous drug abuse); no constitutional symptoms of fever, night sweats, and weight loss; no findings on examination of skin and cardiovascular systems; and a normal white blood cell count. Nevertheless, even though the absence of these features makes bacterial endocarditis unlikely, it does not exclude it. Blood cultures and transesophageal echocardiography are indicated to rule out bacterial endocarditis.
We obtained serial blood cultures, which were negative, and transesophageal echocardiography showed normal valves and no evidence of thrombus or vegetation, thus excluding a cardiac source of emboli.
Thrombophilia
Our patient has a history of recurrent thromboembolic episodes, and this warrants testing to rule out an inherited thrombophilia. A family history of thromboembolic disease should also be sought.1
In our patient, tests for prothrombotic activity including protein C chromogen, activated protein C ratio, free protein S, functional protein S, antithrombin factor V Leiden, and the prothrombin 20210G>A mutation were either negative or within the reference range. A negative family history of thromboembolic disease and the negative laboratory tests make inherited thrombophilia unlikely in our patient.
Sickle cell disease, polycythemia vera, and essential thrombocythemia may also cause splenic infarction but can be ruled out in this patient on the basis of history and initial blood tests.
Antiphospholipid syndrome
A history of vascular disease (aortofemoral bypass surgery), a recent splenic infarct, and an elevated aPTT makes antiphospholipid syndrome the likeliest diagnosis in this patient.
Appropriate tests are for lupus anticoagulant, immunoglobulin G (IgG) or IgM cardiolipin antibody, and beta-2 glycoprotein 1 (beta-2 GP1) antibody, as well as the dilute Russell viper venom time (dRVVT) and the dRVVT ratio. The IgG and IgM cardiolipin antibody and beta-2 GP1 antibody tests have the same diagnostic value, and only medium to high titers should be considered positive.
Our patient’s IgG cardiolipin antibody level was in the normal range at 15 IgG phospholipid units (reference range 0–22); his IgM cardiolipin antibody level was high at 41 IgM phospholipid units (0–10). The dRVVT was 57 seconds (24–42), and the dRVVT ratio was 2.0 (0.0–1.3).
2. What further investigations are indicated before starting treatment?
- No further investigations required
- Repeat testing for phospholipid antibodies in 12 weeks
- Test for antinuclear antibodies
Antiphospholipid antibodies may appear transiently in certain infections, such as syphilis, Lyme disease, Epstein-Barr virus, cytomegalovirus, hepatitis C, and human immunodeficiency virus. Therefore, the presence of antiphospholipid antibodies must be confirmed over time, with two positive results at least 12 weeks apart.2
When repeated 12 weeks later, our patient’s IgG anticardiolipin antibody level was 14 GPL units, and the IgM anticardiolipin antibody level was 30 MPL units; the dRVVT was 55 seconds, and the dRVVT ratio was 1.8. These results, along with a history of recurrent arterial thrombosis, confirmed antiphospholipid syndrome.
The 2009 update of the International Society of Thrombosis and Haemostasis guidelines recommend two tests, the dRVVT and the aPTT, since no single test is 100% sensitive for lupus anticoagulant.3 The dRVVT has a high specificity for lupus anticoagulant in patients at high risk of thrombosis.
A SYNDROME WITH A WIDE RANGE OF EFFECTS AND COMPLICATIONS
Antiphospholipid syndrome is a systemic autoimmune disease that manifests as arterial and venous thrombosis and as obstetric complications. Thrombosis tends to be recurrent and may involve any site. For example, it can cause blurred vision in one or both eyes; amaurosis fugax; visual field defects; central or branch retinal artery or vein occlusion; deep vein thrombosis; pulmonary embolism; myocardial infarction; transient ischemic attack and stroke; cerebral vein thrombosis; and portal, renal, and mesenteric infarction involving veins or arteries.4 Pulmonary capillaritis may cause diffuse alveolar hemorrhage. Livedo reticularis, digital gangrene, cutaneous necrosis, splinter hemorrhages, chorea, and transverse myelopathy may also occur.
Obstetric complications of antiphospholipid syndrome include recurrent miscarriage and pregnancy loss at or after 10 weeks of gestation, eclampsia, preeclampsia, and placental insufficiency.5 The syndrome also has a potentially lethal variant characterized by multiorgan thrombosis affecting mainly small vessels.
The diagnosis of antiphospholipid syndrome requires relevant clinical features and symptoms and the presence of at least one of the antiphospholipid antibodies. Because the rate of false-positive tests for antiphospholipid antibodies ranges from 3% to 20% in the general population, asymptomatic patients should not be tested.6
Antiphospholipid syndrome may occur in the setting of other autoimmune diseases, most commonly systemic lupus erythematosus, when it is termed “secondary” antiphospholipid syndrome. Although only 40% of patients with lupus have antiphospholipid antibodies and less than 40% will have a thrombotic event, thrombotic antiphospholipid syndrome is a major adverse prognostic factor in these patients.7,8 Therefore, it is prudent to consider systemic lupus erythematosus and to do appropriate tests if the patient has other features suggestive of lupus, such as renal, skin, or musculoskeletal lesions.
In our patient, antinuclear antibody testing was positive, with a titer of 1:320, and showed a finely speckled staining pattern. Tests for antibodies to Sjögren syndrome A and B antigens were negative. The complement C3 level was 1.28 g/L (reference range 0.74–1.85) and the C4 level was 0.24 g/L (0.16–0.44). Although the speckled staining pattern can be seen in lupus, it is more common in Sjögren syndrome, mixed connective tissue disease, scleroderma, and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia).9 Moreover, normal levels of complement C3 and C4, in the absence of clinical features, make lupus unlikely. Similarly, our patient had no clinical features of other connective tissue disorders. Therefore, he had primary antiphospholipid syndrome.
3. How should this patient be managed?
- Antiplatelet therapy
- Warfarin to maintain an INR between 2.0 and 3.0
- Warfarin to maintain an INR above 3.0
The risk of recurrent thrombosis is high in patients who test positive for lupus anticoagulant, and the risk is highest in patients who are also positive for anticardiolipin and anti-beta-2 GP1 antibodies: the incidence of thrombosis is 12.2% at 1 year, 26.1% at 5 years, 44.2% at 10 years.10
Since our patient is positive for lupus anticoagulant (prolonged aPTT and elevated dRVVT, both indicating lupus anticoagulant positivity) and for anticardiolipin antibodies (anti-beta-2 GP1 not tested), his risk of recurrent thrombosis is high, and he requires lifelong anticoagulation therapy.
The intensity of anticoagulation in different subgroups of patients is controversial. Based on retrospective trials, indefinite anticoagulation at an INR of 2.0 to 3.0 has been suggested for patients with antiphospholipid syndrome presenting with venous thrombosis, and more intense anticoagulation with an INR above 3.0 in patients with recurrent or arterial thrombosis.11 The combination of warfarin with an INR between 2.0 and 3.0 and aspirin 100 mg daily has also been proposed for patients with arterial thrombosis.12
Modifiable risk factors such as smoking, obesity, and use of estrogens should be addressed in all patients with antiphospholipid syndrome.
In pregnant women with complications such as preeclampsia, low-dose aspirin can be used, and in women with a history of miscarriage, the combination of low-dose aspirin and heparin is recommended throughout the prenatal period.4
In patients who have recurrent thrombosis despite adequate anticoagulation, an expert committee12 has proposed that alternative regimens could include long-term low-molecular-weight heparin instead of warfarin, the combination of warfarin and aspirin, or warfarin and hydroxychloroquine. Adding a statin can also be considered.
Treatment of catastrophic antiphospholipid syndrome is based on expert opinion. A combination of anticoagulation, corticosteroids, plasma exchange, intravenous immunoglobulins, and rituximab has been tried, but the mortality rate remains high.13
OUR PATIENT'S COURSE
Our patient was started on warfarin, with a target INR above 3.0, and was doing well at 6 months of follow-up.
- De Stefano V, Rossi E. Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost 2013; 110:697–705.
- Galli M. Interpretation and recommended testing for antiphospholipid antibodies. Semin Thromb Hemost 2012; 38:348–352.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012; 157:47–58.
- Misita CP, Moll S. Antiphospholipid antibodies. Circulation 2005; 112:e39–e44.
- Rand JH, Wolgast LR. Do’s and don’t’s in diagnosing antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program 2012; 2012:455–459.
- Mok CC, Tang SS, To CH, Petri M. Incidence and risk factors of thromboembolism in systemic lupus erythematosus: a comparison of three ethnic groups. Arthritis Rheum 2005; 52:2774–2782.
- Ruiz-Irastorza G, Egurbide MV, Ugalde J, Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch Intern Med 2004; 164:77–82.
- Locht H, Pelck R, Manthorpe R. Clinical manifestations correlated to the prevalence of autoantibodies in a large (n = 321) cohort of patients with primary Sjögren’s syndrome: a comparison of patients initially diagnosed according to the Copenhagen classification criteria with the American-European consensus criteria. Autoimmun Rev 2005; 4:276–281.
- Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010; 8:237–242.
- Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet 2010; 376:1498–1509.
- Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th International Congress on antiphospholipid antibodies. Lupus 2011; 20:206–218.
- Cervera R. Update on the diagnosis, treatment, and prognosis of the catastrophic antiphospholipid syndrome. Curr Rheumatol Rep 2010; 12:70–76.
- De Stefano V, Rossi E. Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost 2013; 110:697–705.
- Galli M. Interpretation and recommended testing for antiphospholipid antibodies. Semin Thromb Hemost 2012; 38:348–352.
- Pengo V, Tripodi A, Reber G, et al; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740.
- Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012; 157:47–58.
- Misita CP, Moll S. Antiphospholipid antibodies. Circulation 2005; 112:e39–e44.
- Rand JH, Wolgast LR. Do’s and don’t’s in diagnosing antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program 2012; 2012:455–459.
- Mok CC, Tang SS, To CH, Petri M. Incidence and risk factors of thromboembolism in systemic lupus erythematosus: a comparison of three ethnic groups. Arthritis Rheum 2005; 52:2774–2782.
- Ruiz-Irastorza G, Egurbide MV, Ugalde J, Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch Intern Med 2004; 164:77–82.
- Locht H, Pelck R, Manthorpe R. Clinical manifestations correlated to the prevalence of autoantibodies in a large (n = 321) cohort of patients with primary Sjögren’s syndrome: a comparison of patients initially diagnosed according to the Copenhagen classification criteria with the American-European consensus criteria. Autoimmun Rev 2005; 4:276–281.
- Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010; 8:237–242.
- Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet 2010; 376:1498–1509.
- Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th International Congress on antiphospholipid antibodies. Lupus 2011; 20:206–218.
- Cervera R. Update on the diagnosis, treatment, and prognosis of the catastrophic antiphospholipid syndrome. Curr Rheumatol Rep 2010; 12:70–76.
Is antibiotic treatment indicated in a patient with a positive urine culture but no symptoms?
The 2005 Infectious Diseases Society of America (IDSA) guidelines1 recommend screening pregnant women and patients who will undergo an invasive urologic procedure with a urine culture and treating them with antibiotics if bacteriuria is significant. The IDSA recommends against screening for or treating asymptomatic bacteriuria in other populations.
WHAT IS ASYMPTOMATIC BACTERIURIA?
A positive urine culture can represent three different conditions:
- Symptomatic urinary tract infection
- Contamination of the sample by organisms that are present distal to the bladder and that enter the urine at the time the specimen is collected
- Asymptomatic bacteriuria, defined as the isolation of a specified quantitative count of a single uropathogen in an appropriately collected urine specimen obtained from someone without symptoms or signs attributable to a urinary tract infection (Table 1). It represents the true presence of bacteria in the bladder and may be thought of as a state of colonization.
HOW COMMON IS ASYMPTOMATIC BACTERIURIA?
Rates vary depending on the age (higher in older persons), sex (higher in women), and presence of genitourinary abnormalities of the population studied. Prevalence rates are estimated to be 1% to 5% in healthy premenopausal women, 2% to 9% in pregnant women, 9% to 27% in diabetic women, 15% to 50% in elderly men and women in long-term care facilities, and 28% in patients undergoing hemodialysis.2–6 In patients with an indwelling urinary catheter, the rate goes up by 3% to 8% per day, and bacteriuria is nearly universal at 30 days.7,8 Asymptomatic bacteriuria can be transient, as commonly occurs in healthy young women, or it may be more prolonged, as commonly occurs in elderly patients or those with a chronic indwelling urinary catheter.
WHOM SHOULD WE SCREEN?
Screening for asymptomatic bacteriuria and treating it are strongly recommended (grade A-I recommendation) in pregnant women and in men who will undergo transurethral resection of the prostate.
Pregnant women have a risk of pyelonephritis 20 to 30 times higher if they have asymptomatic bacteriuria.9 Cohort studies and randomized clinical trials have consistently reported significant reductions in rates of pyelonephritis and low birth weight when antibiotic therapy is given for asymptomatic bacteriuria during pregnancy.
The ideal time to screen for this in pregnancy is between the 9th and 16th weeks of gestation. The appropriate screening test is a urine culture, since screening for pyuria has a low sensitivity and specificity. The choice of antibiotic is based on the results of culture. Antibiotics that have been safely used in these patients include nitrofurantoin, cephalexin, amoxicillin, and fosfomycin.10 The recommended treatment duration is between 3 and 7 days. Periodic screening for recurrent bacteriuria should be performed during the remainder of the pregnancy.
Men about to undergo transurethral resection of the prostate1 who have asymptomatic bacteriuria before the procedure have a 60% rate of bacteremia and a 6% to 10% rate of sepsis after the procedure if they do not receive antibiotic therapy. Clinical trials have documented significant reductions in these complications when antimicrobial therapy is given before the procedure.
The optimal time for obtaining the urine culture, the optimal time for starting antimicrobial therapy, and the optimal duration of antimicrobial therapy are not well defined, although some data support giving antibiotics the night before or just before the procedure.
The recommendation has been extrapolated to include not only men undergoing transurethral resection of the prostate but also any patient undergoing a urologic procedure associated with significant mucosal bleeding.
Women with catheter-acquired asymptomatic bacteriuria. If the bacteriuria persists 48 hours after catheter removal, the IDSA guidelines state that antibiotic therapy may be considered (grade B-I recommendation). However, there are no recommendations to screen women 48 hours after catheter removal.
WHAT IS THE EVIDENCE FOR NO TREATMENT?
Asymptomatic bacteriuria should not be screened for or treated in:
- Premenopausal women who are not pregnant (grade A-I recommendation)
- Diabetic women (A-I)
- Older persons residing in the community (A-II)
- Elderly residents of long-term care facilities (A-I)
- Patients with spinal cord injury (A-I)
- Patients with an indwelling urethral catheter (A-I).
Randomized controlled trials comparing antibiotic therapy with no therapy in these groups showed no benefit of antibiotic treatment in reducing the frequency of symptomatic urinary tract infection11–16 and no decrease in rates of fever or reinfection in patients with a long-term catheter.17 Moreover, in a number of trials,12,14,17 antibiotic therapy for asymptomatic bacteriuria was associated with an increase in adverse antimicrobial effects and reinfection with resistant organisms.
In transplant recipients. Because of lack of evidence, the 2005 IDSA guidelines could not make a recommendation for or against screening for or treatment of asymptomatic bacteriuria in renal transplant or other solid-organ transplant recipients (C-III). A more recent review18 noted a lack of consensus as to whether asymptomatic bacteriuria should be treated in renal transplant recipients. Based on available data, the authors recommended limiting routine screening for it to the first 1 to 3 months after renal transplantation and limiting treatment to 5 to 7 days, using the narrowest-spectrum antibiotic available.18
In prosthetic joint recipients. The 2005 IDSA guidelines recommended further research to determine if screening and treatment before surgical procedures with prosthetic implantation have clinical benefit.
Since then, two studies19,20 have suggested no benefit of screening or treatment before prosthetic joint implantation. Rates of prosthetic joint infection were not different in patients with asymptomatic bacteriuria before hip arthroplasty randomized to receive no antibiotic therapy vs those receiving antibiotic therapy specific for organisms cultured from the urine.19 Asymptomatic bacteriuria was found to be an independent risk factor for prosthetic joint infection.20 However, rates of joint infection were not different in those treated with antibiotics than in those not treated, and in no case were the microorganisms isolated in the prosthetic joint infection the same as in their preoperative urine culture.20
The authors concluded that asymptomatic bacteriuria may be a surrogate marker for increased risk of infection, but that preoperative antibiotic treatment was not beneficial.20
WHAT DOES 'ASYMPTOMATIC' MEAN?
According to the definition, asymptomatic refers to patients who do not have symptoms or signs attributable to a urinary tract infection. Thus, in patients who have symptoms or signs clearly attributable to another condition, screening with urine culture testing and treatment are not indicated. In nursing home residents, nonspecific symptoms such as a change in mental status, fever, and leukocytosis should not automatically be attributed to a positive urine culture without a careful evaluation for another cause, given the high prevalence of asymptomatic bacteriuria in this population.21 Screening with urine culture testing in this population is also not recommended for isolated foul-smelling or cloudy urine, after every urethral catheter change, upon admission, or after treatment to document cure.22
Finally, pyuria (defined as the presence of at least 5 to 10 white blood cells per high-power field) is not by itself a reason to perform a urine culture or to treat a positive urine culture, since pyuria is common in asymptomatic bacteriuria, as well as in other conditions associated with inflammation in the genitourinary system.1
TAKE-HOME POINTS
- Screening for and treating asymptomatic bacteriuria is recommended for pregnant women and for patients about to undergo an invasive urologic procedure associated with significant mucosal injury
- Screening and treatment are not recommended for premenopausal nonpregnant women, diabetic women, older persons residing in the community, elderly residents of long-term care facilities, patients with spinal cord injury, or patients with an indwelling urethral catheter.
- A urine culture should not be ordered, but if it is ordered, a positive culture should not be treated in a patient whose symptoms are attributable to another cause.
- Pyuria is not helpful in distinguishing symptomatic from asymptomatic bacteriuria.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Hooton TM, Scholes D, Stapleton AE, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med 2000; 343:992–997.
- Whalley P. Bacteriuria of pregnancy. Am J Obstet Gynecol 1967; 97:723–738.
- Zhanel GG, Nicolle LE, Harding GK. Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus. The Manitoba Diabetic Urinary Infection Study Group. Clin Infect Dis 1995; 21:316–322.
- Nicolle LE. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am 1997; 11:647–662.
- Chaudhry A, Stone WJ, Breyer JA. Occurrence of pyuria and bacteriuria in asymptomatic hemodialysis patients. Am J Kidney Dis 1993; 21:180–183.
- Garibaldi RA, Burke JP, Dickman ML, Smith CB. Factors predisposing to bacteriuria during indwelling urethral catheterization. N Engl J Med 1974; 291:215–219.
- Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis 1982; 146:719–723.
- Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2007; 2:CD000490.
- Guinto VT, De Guia B, Festin MR, Dowswell T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2010; 9:CD007855.
- Asscher AW, Sussman M, Waters WE, et al. Asymptomatic significant bacteriuria in the non-pregnant woman. II. Response to treatment and follow-up. Br Med J 1969; 1:804–806.
- Harding GK, Zhanel GG, Nicolle LE, Cheang M; Manitoba Diabetes Urinary Tract Infection Study Group. Antimicrobial treatment in diabetic women with asymptomatic bacteriuria. N Engl J Med 2002; 347:1576–1583.
- Boscia JA, Kobasa WD, Knight RA, Abrutyn E, Levison ME, Kaye D. Therapy vs no therapy for bacteriuria in elderly ambulatory nonhospitalized women. JAMA 1987; 257:1067–1071.
- Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med 1987; 83:27–33.
- Nicolle LE, Bjornson J, Harding GK, MacDonell JA. Bacteriuria in elderly institutionalized men. N Engl J Med 1983; 309:1420–1425
- Mohler JL, Cowen DL, Flanigan RC. Suppression and treatment of urinary tract infection in patients with an intermittently catheterized neurogenic bladder. J Urol 1987; 138:336–340.
- Warren JW, Anthony WC, Hoopes JM, Muncie HL Jr. Cephalexin for susceptible bacteriuria in afebrile, long-term catheterized patients. JAMA 1982; 248:454–458.
- Parasuraman R, Julian K; AST Infectious Diseases Community of Practice. Urinary tract infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):327–336.
- Cordero-Ampuero J, González-Fernández E, Martínez-Vélez D, Esteban J. Are antibiotics necessary in hip arthroplasty with asymptomatic bacteriuria? Seeding risk with/without treatment. Clin Orthop Relat Res 2013; 471:3822–3829.
- Sousa R, Muñoz-Mahamud E, Quayle J, et al. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis 2014: ciu235. Epub ahead of print.
- Orr PH, Nicolle LE, Duckworth H, et al. Febrile urinary infection in the institutionalized elderly. Am J Med 1996; 100:71–77.
- Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long-term care facility through an educational intervention. Am J Infect Control 2008; 36:476–480.
The 2005 Infectious Diseases Society of America (IDSA) guidelines1 recommend screening pregnant women and patients who will undergo an invasive urologic procedure with a urine culture and treating them with antibiotics if bacteriuria is significant. The IDSA recommends against screening for or treating asymptomatic bacteriuria in other populations.
WHAT IS ASYMPTOMATIC BACTERIURIA?
A positive urine culture can represent three different conditions:
- Symptomatic urinary tract infection
- Contamination of the sample by organisms that are present distal to the bladder and that enter the urine at the time the specimen is collected
- Asymptomatic bacteriuria, defined as the isolation of a specified quantitative count of a single uropathogen in an appropriately collected urine specimen obtained from someone without symptoms or signs attributable to a urinary tract infection (Table 1). It represents the true presence of bacteria in the bladder and may be thought of as a state of colonization.
HOW COMMON IS ASYMPTOMATIC BACTERIURIA?
Rates vary depending on the age (higher in older persons), sex (higher in women), and presence of genitourinary abnormalities of the population studied. Prevalence rates are estimated to be 1% to 5% in healthy premenopausal women, 2% to 9% in pregnant women, 9% to 27% in diabetic women, 15% to 50% in elderly men and women in long-term care facilities, and 28% in patients undergoing hemodialysis.2–6 In patients with an indwelling urinary catheter, the rate goes up by 3% to 8% per day, and bacteriuria is nearly universal at 30 days.7,8 Asymptomatic bacteriuria can be transient, as commonly occurs in healthy young women, or it may be more prolonged, as commonly occurs in elderly patients or those with a chronic indwelling urinary catheter.
WHOM SHOULD WE SCREEN?
Screening for asymptomatic bacteriuria and treating it are strongly recommended (grade A-I recommendation) in pregnant women and in men who will undergo transurethral resection of the prostate.
Pregnant women have a risk of pyelonephritis 20 to 30 times higher if they have asymptomatic bacteriuria.9 Cohort studies and randomized clinical trials have consistently reported significant reductions in rates of pyelonephritis and low birth weight when antibiotic therapy is given for asymptomatic bacteriuria during pregnancy.
The ideal time to screen for this in pregnancy is between the 9th and 16th weeks of gestation. The appropriate screening test is a urine culture, since screening for pyuria has a low sensitivity and specificity. The choice of antibiotic is based on the results of culture. Antibiotics that have been safely used in these patients include nitrofurantoin, cephalexin, amoxicillin, and fosfomycin.10 The recommended treatment duration is between 3 and 7 days. Periodic screening for recurrent bacteriuria should be performed during the remainder of the pregnancy.
Men about to undergo transurethral resection of the prostate1 who have asymptomatic bacteriuria before the procedure have a 60% rate of bacteremia and a 6% to 10% rate of sepsis after the procedure if they do not receive antibiotic therapy. Clinical trials have documented significant reductions in these complications when antimicrobial therapy is given before the procedure.
The optimal time for obtaining the urine culture, the optimal time for starting antimicrobial therapy, and the optimal duration of antimicrobial therapy are not well defined, although some data support giving antibiotics the night before or just before the procedure.
The recommendation has been extrapolated to include not only men undergoing transurethral resection of the prostate but also any patient undergoing a urologic procedure associated with significant mucosal bleeding.
Women with catheter-acquired asymptomatic bacteriuria. If the bacteriuria persists 48 hours after catheter removal, the IDSA guidelines state that antibiotic therapy may be considered (grade B-I recommendation). However, there are no recommendations to screen women 48 hours after catheter removal.
WHAT IS THE EVIDENCE FOR NO TREATMENT?
Asymptomatic bacteriuria should not be screened for or treated in:
- Premenopausal women who are not pregnant (grade A-I recommendation)
- Diabetic women (A-I)
- Older persons residing in the community (A-II)
- Elderly residents of long-term care facilities (A-I)
- Patients with spinal cord injury (A-I)
- Patients with an indwelling urethral catheter (A-I).
Randomized controlled trials comparing antibiotic therapy with no therapy in these groups showed no benefit of antibiotic treatment in reducing the frequency of symptomatic urinary tract infection11–16 and no decrease in rates of fever or reinfection in patients with a long-term catheter.17 Moreover, in a number of trials,12,14,17 antibiotic therapy for asymptomatic bacteriuria was associated with an increase in adverse antimicrobial effects and reinfection with resistant organisms.
In transplant recipients. Because of lack of evidence, the 2005 IDSA guidelines could not make a recommendation for or against screening for or treatment of asymptomatic bacteriuria in renal transplant or other solid-organ transplant recipients (C-III). A more recent review18 noted a lack of consensus as to whether asymptomatic bacteriuria should be treated in renal transplant recipients. Based on available data, the authors recommended limiting routine screening for it to the first 1 to 3 months after renal transplantation and limiting treatment to 5 to 7 days, using the narrowest-spectrum antibiotic available.18
In prosthetic joint recipients. The 2005 IDSA guidelines recommended further research to determine if screening and treatment before surgical procedures with prosthetic implantation have clinical benefit.
Since then, two studies19,20 have suggested no benefit of screening or treatment before prosthetic joint implantation. Rates of prosthetic joint infection were not different in patients with asymptomatic bacteriuria before hip arthroplasty randomized to receive no antibiotic therapy vs those receiving antibiotic therapy specific for organisms cultured from the urine.19 Asymptomatic bacteriuria was found to be an independent risk factor for prosthetic joint infection.20 However, rates of joint infection were not different in those treated with antibiotics than in those not treated, and in no case were the microorganisms isolated in the prosthetic joint infection the same as in their preoperative urine culture.20
The authors concluded that asymptomatic bacteriuria may be a surrogate marker for increased risk of infection, but that preoperative antibiotic treatment was not beneficial.20
WHAT DOES 'ASYMPTOMATIC' MEAN?
According to the definition, asymptomatic refers to patients who do not have symptoms or signs attributable to a urinary tract infection. Thus, in patients who have symptoms or signs clearly attributable to another condition, screening with urine culture testing and treatment are not indicated. In nursing home residents, nonspecific symptoms such as a change in mental status, fever, and leukocytosis should not automatically be attributed to a positive urine culture without a careful evaluation for another cause, given the high prevalence of asymptomatic bacteriuria in this population.21 Screening with urine culture testing in this population is also not recommended for isolated foul-smelling or cloudy urine, after every urethral catheter change, upon admission, or after treatment to document cure.22
Finally, pyuria (defined as the presence of at least 5 to 10 white blood cells per high-power field) is not by itself a reason to perform a urine culture or to treat a positive urine culture, since pyuria is common in asymptomatic bacteriuria, as well as in other conditions associated with inflammation in the genitourinary system.1
TAKE-HOME POINTS
- Screening for and treating asymptomatic bacteriuria is recommended for pregnant women and for patients about to undergo an invasive urologic procedure associated with significant mucosal injury
- Screening and treatment are not recommended for premenopausal nonpregnant women, diabetic women, older persons residing in the community, elderly residents of long-term care facilities, patients with spinal cord injury, or patients with an indwelling urethral catheter.
- A urine culture should not be ordered, but if it is ordered, a positive culture should not be treated in a patient whose symptoms are attributable to another cause.
- Pyuria is not helpful in distinguishing symptomatic from asymptomatic bacteriuria.
The 2005 Infectious Diseases Society of America (IDSA) guidelines1 recommend screening pregnant women and patients who will undergo an invasive urologic procedure with a urine culture and treating them with antibiotics if bacteriuria is significant. The IDSA recommends against screening for or treating asymptomatic bacteriuria in other populations.
WHAT IS ASYMPTOMATIC BACTERIURIA?
A positive urine culture can represent three different conditions:
- Symptomatic urinary tract infection
- Contamination of the sample by organisms that are present distal to the bladder and that enter the urine at the time the specimen is collected
- Asymptomatic bacteriuria, defined as the isolation of a specified quantitative count of a single uropathogen in an appropriately collected urine specimen obtained from someone without symptoms or signs attributable to a urinary tract infection (Table 1). It represents the true presence of bacteria in the bladder and may be thought of as a state of colonization.
HOW COMMON IS ASYMPTOMATIC BACTERIURIA?
Rates vary depending on the age (higher in older persons), sex (higher in women), and presence of genitourinary abnormalities of the population studied. Prevalence rates are estimated to be 1% to 5% in healthy premenopausal women, 2% to 9% in pregnant women, 9% to 27% in diabetic women, 15% to 50% in elderly men and women in long-term care facilities, and 28% in patients undergoing hemodialysis.2–6 In patients with an indwelling urinary catheter, the rate goes up by 3% to 8% per day, and bacteriuria is nearly universal at 30 days.7,8 Asymptomatic bacteriuria can be transient, as commonly occurs in healthy young women, or it may be more prolonged, as commonly occurs in elderly patients or those with a chronic indwelling urinary catheter.
WHOM SHOULD WE SCREEN?
Screening for asymptomatic bacteriuria and treating it are strongly recommended (grade A-I recommendation) in pregnant women and in men who will undergo transurethral resection of the prostate.
Pregnant women have a risk of pyelonephritis 20 to 30 times higher if they have asymptomatic bacteriuria.9 Cohort studies and randomized clinical trials have consistently reported significant reductions in rates of pyelonephritis and low birth weight when antibiotic therapy is given for asymptomatic bacteriuria during pregnancy.
The ideal time to screen for this in pregnancy is between the 9th and 16th weeks of gestation. The appropriate screening test is a urine culture, since screening for pyuria has a low sensitivity and specificity. The choice of antibiotic is based on the results of culture. Antibiotics that have been safely used in these patients include nitrofurantoin, cephalexin, amoxicillin, and fosfomycin.10 The recommended treatment duration is between 3 and 7 days. Periodic screening for recurrent bacteriuria should be performed during the remainder of the pregnancy.
Men about to undergo transurethral resection of the prostate1 who have asymptomatic bacteriuria before the procedure have a 60% rate of bacteremia and a 6% to 10% rate of sepsis after the procedure if they do not receive antibiotic therapy. Clinical trials have documented significant reductions in these complications when antimicrobial therapy is given before the procedure.
The optimal time for obtaining the urine culture, the optimal time for starting antimicrobial therapy, and the optimal duration of antimicrobial therapy are not well defined, although some data support giving antibiotics the night before or just before the procedure.
The recommendation has been extrapolated to include not only men undergoing transurethral resection of the prostate but also any patient undergoing a urologic procedure associated with significant mucosal bleeding.
Women with catheter-acquired asymptomatic bacteriuria. If the bacteriuria persists 48 hours after catheter removal, the IDSA guidelines state that antibiotic therapy may be considered (grade B-I recommendation). However, there are no recommendations to screen women 48 hours after catheter removal.
WHAT IS THE EVIDENCE FOR NO TREATMENT?
Asymptomatic bacteriuria should not be screened for or treated in:
- Premenopausal women who are not pregnant (grade A-I recommendation)
- Diabetic women (A-I)
- Older persons residing in the community (A-II)
- Elderly residents of long-term care facilities (A-I)
- Patients with spinal cord injury (A-I)
- Patients with an indwelling urethral catheter (A-I).
Randomized controlled trials comparing antibiotic therapy with no therapy in these groups showed no benefit of antibiotic treatment in reducing the frequency of symptomatic urinary tract infection11–16 and no decrease in rates of fever or reinfection in patients with a long-term catheter.17 Moreover, in a number of trials,12,14,17 antibiotic therapy for asymptomatic bacteriuria was associated with an increase in adverse antimicrobial effects and reinfection with resistant organisms.
In transplant recipients. Because of lack of evidence, the 2005 IDSA guidelines could not make a recommendation for or against screening for or treatment of asymptomatic bacteriuria in renal transplant or other solid-organ transplant recipients (C-III). A more recent review18 noted a lack of consensus as to whether asymptomatic bacteriuria should be treated in renal transplant recipients. Based on available data, the authors recommended limiting routine screening for it to the first 1 to 3 months after renal transplantation and limiting treatment to 5 to 7 days, using the narrowest-spectrum antibiotic available.18
In prosthetic joint recipients. The 2005 IDSA guidelines recommended further research to determine if screening and treatment before surgical procedures with prosthetic implantation have clinical benefit.
Since then, two studies19,20 have suggested no benefit of screening or treatment before prosthetic joint implantation. Rates of prosthetic joint infection were not different in patients with asymptomatic bacteriuria before hip arthroplasty randomized to receive no antibiotic therapy vs those receiving antibiotic therapy specific for organisms cultured from the urine.19 Asymptomatic bacteriuria was found to be an independent risk factor for prosthetic joint infection.20 However, rates of joint infection were not different in those treated with antibiotics than in those not treated, and in no case were the microorganisms isolated in the prosthetic joint infection the same as in their preoperative urine culture.20
The authors concluded that asymptomatic bacteriuria may be a surrogate marker for increased risk of infection, but that preoperative antibiotic treatment was not beneficial.20
WHAT DOES 'ASYMPTOMATIC' MEAN?
According to the definition, asymptomatic refers to patients who do not have symptoms or signs attributable to a urinary tract infection. Thus, in patients who have symptoms or signs clearly attributable to another condition, screening with urine culture testing and treatment are not indicated. In nursing home residents, nonspecific symptoms such as a change in mental status, fever, and leukocytosis should not automatically be attributed to a positive urine culture without a careful evaluation for another cause, given the high prevalence of asymptomatic bacteriuria in this population.21 Screening with urine culture testing in this population is also not recommended for isolated foul-smelling or cloudy urine, after every urethral catheter change, upon admission, or after treatment to document cure.22
Finally, pyuria (defined as the presence of at least 5 to 10 white blood cells per high-power field) is not by itself a reason to perform a urine culture or to treat a positive urine culture, since pyuria is common in asymptomatic bacteriuria, as well as in other conditions associated with inflammation in the genitourinary system.1
TAKE-HOME POINTS
- Screening for and treating asymptomatic bacteriuria is recommended for pregnant women and for patients about to undergo an invasive urologic procedure associated with significant mucosal injury
- Screening and treatment are not recommended for premenopausal nonpregnant women, diabetic women, older persons residing in the community, elderly residents of long-term care facilities, patients with spinal cord injury, or patients with an indwelling urethral catheter.
- A urine culture should not be ordered, but if it is ordered, a positive culture should not be treated in a patient whose symptoms are attributable to another cause.
- Pyuria is not helpful in distinguishing symptomatic from asymptomatic bacteriuria.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Hooton TM, Scholes D, Stapleton AE, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med 2000; 343:992–997.
- Whalley P. Bacteriuria of pregnancy. Am J Obstet Gynecol 1967; 97:723–738.
- Zhanel GG, Nicolle LE, Harding GK. Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus. The Manitoba Diabetic Urinary Infection Study Group. Clin Infect Dis 1995; 21:316–322.
- Nicolle LE. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am 1997; 11:647–662.
- Chaudhry A, Stone WJ, Breyer JA. Occurrence of pyuria and bacteriuria in asymptomatic hemodialysis patients. Am J Kidney Dis 1993; 21:180–183.
- Garibaldi RA, Burke JP, Dickman ML, Smith CB. Factors predisposing to bacteriuria during indwelling urethral catheterization. N Engl J Med 1974; 291:215–219.
- Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis 1982; 146:719–723.
- Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2007; 2:CD000490.
- Guinto VT, De Guia B, Festin MR, Dowswell T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2010; 9:CD007855.
- Asscher AW, Sussman M, Waters WE, et al. Asymptomatic significant bacteriuria in the non-pregnant woman. II. Response to treatment and follow-up. Br Med J 1969; 1:804–806.
- Harding GK, Zhanel GG, Nicolle LE, Cheang M; Manitoba Diabetes Urinary Tract Infection Study Group. Antimicrobial treatment in diabetic women with asymptomatic bacteriuria. N Engl J Med 2002; 347:1576–1583.
- Boscia JA, Kobasa WD, Knight RA, Abrutyn E, Levison ME, Kaye D. Therapy vs no therapy for bacteriuria in elderly ambulatory nonhospitalized women. JAMA 1987; 257:1067–1071.
- Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med 1987; 83:27–33.
- Nicolle LE, Bjornson J, Harding GK, MacDonell JA. Bacteriuria in elderly institutionalized men. N Engl J Med 1983; 309:1420–1425
- Mohler JL, Cowen DL, Flanigan RC. Suppression and treatment of urinary tract infection in patients with an intermittently catheterized neurogenic bladder. J Urol 1987; 138:336–340.
- Warren JW, Anthony WC, Hoopes JM, Muncie HL Jr. Cephalexin for susceptible bacteriuria in afebrile, long-term catheterized patients. JAMA 1982; 248:454–458.
- Parasuraman R, Julian K; AST Infectious Diseases Community of Practice. Urinary tract infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):327–336.
- Cordero-Ampuero J, González-Fernández E, Martínez-Vélez D, Esteban J. Are antibiotics necessary in hip arthroplasty with asymptomatic bacteriuria? Seeding risk with/without treatment. Clin Orthop Relat Res 2013; 471:3822–3829.
- Sousa R, Muñoz-Mahamud E, Quayle J, et al. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis 2014: ciu235. Epub ahead of print.
- Orr PH, Nicolle LE, Duckworth H, et al. Febrile urinary infection in the institutionalized elderly. Am J Med 1996; 100:71–77.
- Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long-term care facility through an educational intervention. Am J Infect Control 2008; 36:476–480.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Hooton TM, Scholes D, Stapleton AE, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med 2000; 343:992–997.
- Whalley P. Bacteriuria of pregnancy. Am J Obstet Gynecol 1967; 97:723–738.
- Zhanel GG, Nicolle LE, Harding GK. Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus. The Manitoba Diabetic Urinary Infection Study Group. Clin Infect Dis 1995; 21:316–322.
- Nicolle LE. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am 1997; 11:647–662.
- Chaudhry A, Stone WJ, Breyer JA. Occurrence of pyuria and bacteriuria in asymptomatic hemodialysis patients. Am J Kidney Dis 1993; 21:180–183.
- Garibaldi RA, Burke JP, Dickman ML, Smith CB. Factors predisposing to bacteriuria during indwelling urethral catheterization. N Engl J Med 1974; 291:215–219.
- Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis 1982; 146:719–723.
- Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2007; 2:CD000490.
- Guinto VT, De Guia B, Festin MR, Dowswell T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2010; 9:CD007855.
- Asscher AW, Sussman M, Waters WE, et al. Asymptomatic significant bacteriuria in the non-pregnant woman. II. Response to treatment and follow-up. Br Med J 1969; 1:804–806.
- Harding GK, Zhanel GG, Nicolle LE, Cheang M; Manitoba Diabetes Urinary Tract Infection Study Group. Antimicrobial treatment in diabetic women with asymptomatic bacteriuria. N Engl J Med 2002; 347:1576–1583.
- Boscia JA, Kobasa WD, Knight RA, Abrutyn E, Levison ME, Kaye D. Therapy vs no therapy for bacteriuria in elderly ambulatory nonhospitalized women. JAMA 1987; 257:1067–1071.
- Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med 1987; 83:27–33.
- Nicolle LE, Bjornson J, Harding GK, MacDonell JA. Bacteriuria in elderly institutionalized men. N Engl J Med 1983; 309:1420–1425
- Mohler JL, Cowen DL, Flanigan RC. Suppression and treatment of urinary tract infection in patients with an intermittently catheterized neurogenic bladder. J Urol 1987; 138:336–340.
- Warren JW, Anthony WC, Hoopes JM, Muncie HL Jr. Cephalexin for susceptible bacteriuria in afebrile, long-term catheterized patients. JAMA 1982; 248:454–458.
- Parasuraman R, Julian K; AST Infectious Diseases Community of Practice. Urinary tract infections in solid organ transplantation. Am J Transplant 2013; 13(suppl 4):327–336.
- Cordero-Ampuero J, González-Fernández E, Martínez-Vélez D, Esteban J. Are antibiotics necessary in hip arthroplasty with asymptomatic bacteriuria? Seeding risk with/without treatment. Clin Orthop Relat Res 2013; 471:3822–3829.
- Sousa R, Muñoz-Mahamud E, Quayle J, et al. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis 2014: ciu235. Epub ahead of print.
- Orr PH, Nicolle LE, Duckworth H, et al. Febrile urinary infection in the institutionalized elderly. Am J Med 1996; 100:71–77.
- Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long-term care facility through an educational intervention. Am J Infect Control 2008; 36:476–480.
When does an adult with headaches need central nervous system imaging?
A 32-year-old woman presents to the clinic for evaluation of headaches, which she describes as pulsatile and throbbing, usually unilateral but involving different sides of the head at different times, and severe, causing her to miss work. They usually last between 12 and 24 hours and are associated with nausea but no vomiting and no changes in vision. They are worse around the time of her menses, have been occurring about twice a month for the past 6 months, and respond to ibuprofen. She thought they were caused by chronic seasonal allergies and sinusitis and has tried antihistamines and nasal irrigation without success. They are not affected by body position, they are not explosive, and they are not brought on by the Valsalva maneuver. She reports no other neurologic or systemic symptoms.
A detailed neurologic examination shows no deficits. However, the patient is concerned, as one of her friends was recently diagnosed with cancer. She requests imaging to “make sure there is no cancer.” Would it be appropriate to order imaging at this time?
No, it would not. Patients who have primary headache disorders without red-flag symptoms should not undergo imaging of the central nervous system (CNS) as part of their initial evaluation.1–4 (The list of potential red-flag symptoms is long but includes new onset after age 50, persistent neurologic changes, systemic symptoms or immunosuppression, sudden onset, progressive pain, positional nature, headaches precipitated by the Valsalva maneuver, and papilledema.)
CNS imaging may be appropriate for patients with features that increase the likelihood of structural diseases such as arteriovenous malformation, aneurysm, tumor, or subarachnoid hemorrhage. This patient, however, does not have worrisome signs or symptoms. Her symptoms are most consistent with migraine headache without aura. In patients with migraine headache without symptoms suggesting structural disease, CNS imaging is unwarranted and may be harmful.
DIAGNOSING MIGRAINE ACCURATELY
Diagnosing migraine headache can be a challenge, and up to half of all patients with migraine may be undiagnosed.5 The proper diagnosis of headache type is critical to the initial evaluation. In diagnosing migraine, one can use the mnemonic POUND4:
- Pulsatile
- One-day duration (4–72 hours)
- Unilateral
- Nausea or vomiting
- Disabling.
If four or five of these features are present, the likelihood ratio that the patient has migraine headache is 24, making it overwhelmingly likely that is the correct diagnosis.4 With three features the likelihood ratio is 3.5. If two or fewer features are present, migraine is much less likely, with a likelihood ratio of 0.41. Thus, patients with classic symptoms of migraine can be confidently and accurately diagnosed without the need for any imaging studies.
The patient in the vignette has all five POUND criteria. If we estimate her pretest probability of migraine headache at 50% (which is actually a conservative estimate—see Guidelines and Choosing Wisely, below), then, utilizing Bayes’ theorem, the likelihood ratio of 24 would result in a 95% probability that her headaches represent migraine.
GUIDELINES AND CHOOSING WISELY
High-quality reviews have found no benefit in performing imaging for primary headache disorders.1–3 This is due, in large part, to the rarity of secondary headache disorders in the primary care setting. In fact, most patients—90% in one study6—presenting to their primary care physicians with headaches meet the diagnostic criteria for migraine.
Significant abnormalities on imaging in patients with migraine headaches are also very rare. In patients with migraine headaches who undergo imaging, the rate of worrisome abnormalities that could lead to a change in management (0.2%) is less than that in the general population at the time of autopsy (0.8%).7
As part of the Choosing Wisely campaign, the American College of Radiology and the American Headache Society recommend against imaging for patients at low risk with migraine headaches. Because of the potential for harm from radiation exposure, the American Headache Society also recommends against computed tomography (CT) for evaluating headaches when magnetic resonance imaging (MRI) is available, except in emergencies.
Lists of tests and treatments that physicians and patients should question and discuss together to make wise decisions are available at www.choosingwisely.org.
HARMS ASSOCIATED WITH CNS IMAGING
Medical tests can be associated with significant harm. Potential harms of head imaging include radiation exposure from CT and false-positive findings. These false-positives, such as the finding of lesions that eventually prove to be benign, may require further testing and cause significant anxiety to the patient.
The effective radiation dose from a CT scan of the head is 2.0 mSv, equivalent to 250 days of background radiation exposure or 100 chest radiographs. Radiation exposure has been linked to increased risk of fatal cancer, and the risks increase with subsequent radiation doses.8
Incidental findings are common on head imaging and often lead to additional medical procedures and workup, without improvements in patient well-being. While the harms of false-positive testing and the finding of benign lesions are difficult to quantify, it is clear that downstream costs can accumulate and that these results cause significant undue worry to the patient.
CLINICAL BOTTOM LINE
Patients with migraine headache who do not have red-flag signs or symptoms are unlikely to benefit from CNS imaging and may experience harm. The rate of abnormalities in this population is not significantly different from that in the general population. A thorough history and physical examination should be done to find the proper diagnosis and to uncover any red-flag symptoms. For migraine headaches that are worsened by identified triggers, those triggers should be addressed before further evaluation is performed. When imaging is needed, physicians should consider minimizing radiation risk by ordering MRI instead of CT.
- Beithon J, Gallenberg M, Johnson K, et al; Institute for Clinical Systems Improvement. Diagnosis and treatment of headache. www.icsi.org/_asset/qwrznq/headache.pdf. Accessed September 5, 2014.
- Frishberg BM, Rosenberg JH, Matchar DB, et al; US Headache Consortium. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. www.aan.com/professionals/practice/pdfs/gl0088.pdf. Accessed September 5, 2014.
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55:754–762.
- Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM. Does this patient with headache have a migraine or need neuroimaging? JAMA 2006; 296:1274–1283.
- Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: results from the American Migraine Study II. Headache 2001; 41:638–645.
- Dowson A, Dahlof C, Tepper S, Newman L. Prevalence and diagnosis of migraine in a primary care setting (abstract). Cephalalgia 2002; 22:590–591.
- Frishberg BM. The utility of neuroimaging in the evaluation of headache in patients with normal neurologic examinations. Neurology 1994; 44:1191–1197.
- Semelka RC, Armao DM, Elias J Jr, Huda W. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging 2007; 25:900–909.
A 32-year-old woman presents to the clinic for evaluation of headaches, which she describes as pulsatile and throbbing, usually unilateral but involving different sides of the head at different times, and severe, causing her to miss work. They usually last between 12 and 24 hours and are associated with nausea but no vomiting and no changes in vision. They are worse around the time of her menses, have been occurring about twice a month for the past 6 months, and respond to ibuprofen. She thought they were caused by chronic seasonal allergies and sinusitis and has tried antihistamines and nasal irrigation without success. They are not affected by body position, they are not explosive, and they are not brought on by the Valsalva maneuver. She reports no other neurologic or systemic symptoms.
A detailed neurologic examination shows no deficits. However, the patient is concerned, as one of her friends was recently diagnosed with cancer. She requests imaging to “make sure there is no cancer.” Would it be appropriate to order imaging at this time?
No, it would not. Patients who have primary headache disorders without red-flag symptoms should not undergo imaging of the central nervous system (CNS) as part of their initial evaluation.1–4 (The list of potential red-flag symptoms is long but includes new onset after age 50, persistent neurologic changes, systemic symptoms or immunosuppression, sudden onset, progressive pain, positional nature, headaches precipitated by the Valsalva maneuver, and papilledema.)
CNS imaging may be appropriate for patients with features that increase the likelihood of structural diseases such as arteriovenous malformation, aneurysm, tumor, or subarachnoid hemorrhage. This patient, however, does not have worrisome signs or symptoms. Her symptoms are most consistent with migraine headache without aura. In patients with migraine headache without symptoms suggesting structural disease, CNS imaging is unwarranted and may be harmful.
DIAGNOSING MIGRAINE ACCURATELY
Diagnosing migraine headache can be a challenge, and up to half of all patients with migraine may be undiagnosed.5 The proper diagnosis of headache type is critical to the initial evaluation. In diagnosing migraine, one can use the mnemonic POUND4:
- Pulsatile
- One-day duration (4–72 hours)
- Unilateral
- Nausea or vomiting
- Disabling.
If four or five of these features are present, the likelihood ratio that the patient has migraine headache is 24, making it overwhelmingly likely that is the correct diagnosis.4 With three features the likelihood ratio is 3.5. If two or fewer features are present, migraine is much less likely, with a likelihood ratio of 0.41. Thus, patients with classic symptoms of migraine can be confidently and accurately diagnosed without the need for any imaging studies.
The patient in the vignette has all five POUND criteria. If we estimate her pretest probability of migraine headache at 50% (which is actually a conservative estimate—see Guidelines and Choosing Wisely, below), then, utilizing Bayes’ theorem, the likelihood ratio of 24 would result in a 95% probability that her headaches represent migraine.
GUIDELINES AND CHOOSING WISELY
High-quality reviews have found no benefit in performing imaging for primary headache disorders.1–3 This is due, in large part, to the rarity of secondary headache disorders in the primary care setting. In fact, most patients—90% in one study6—presenting to their primary care physicians with headaches meet the diagnostic criteria for migraine.
Significant abnormalities on imaging in patients with migraine headaches are also very rare. In patients with migraine headaches who undergo imaging, the rate of worrisome abnormalities that could lead to a change in management (0.2%) is less than that in the general population at the time of autopsy (0.8%).7
As part of the Choosing Wisely campaign, the American College of Radiology and the American Headache Society recommend against imaging for patients at low risk with migraine headaches. Because of the potential for harm from radiation exposure, the American Headache Society also recommends against computed tomography (CT) for evaluating headaches when magnetic resonance imaging (MRI) is available, except in emergencies.
Lists of tests and treatments that physicians and patients should question and discuss together to make wise decisions are available at www.choosingwisely.org.
HARMS ASSOCIATED WITH CNS IMAGING
Medical tests can be associated with significant harm. Potential harms of head imaging include radiation exposure from CT and false-positive findings. These false-positives, such as the finding of lesions that eventually prove to be benign, may require further testing and cause significant anxiety to the patient.
The effective radiation dose from a CT scan of the head is 2.0 mSv, equivalent to 250 days of background radiation exposure or 100 chest radiographs. Radiation exposure has been linked to increased risk of fatal cancer, and the risks increase with subsequent radiation doses.8
Incidental findings are common on head imaging and often lead to additional medical procedures and workup, without improvements in patient well-being. While the harms of false-positive testing and the finding of benign lesions are difficult to quantify, it is clear that downstream costs can accumulate and that these results cause significant undue worry to the patient.
CLINICAL BOTTOM LINE
Patients with migraine headache who do not have red-flag signs or symptoms are unlikely to benefit from CNS imaging and may experience harm. The rate of abnormalities in this population is not significantly different from that in the general population. A thorough history and physical examination should be done to find the proper diagnosis and to uncover any red-flag symptoms. For migraine headaches that are worsened by identified triggers, those triggers should be addressed before further evaluation is performed. When imaging is needed, physicians should consider minimizing radiation risk by ordering MRI instead of CT.
A 32-year-old woman presents to the clinic for evaluation of headaches, which she describes as pulsatile and throbbing, usually unilateral but involving different sides of the head at different times, and severe, causing her to miss work. They usually last between 12 and 24 hours and are associated with nausea but no vomiting and no changes in vision. They are worse around the time of her menses, have been occurring about twice a month for the past 6 months, and respond to ibuprofen. She thought they were caused by chronic seasonal allergies and sinusitis and has tried antihistamines and nasal irrigation without success. They are not affected by body position, they are not explosive, and they are not brought on by the Valsalva maneuver. She reports no other neurologic or systemic symptoms.
A detailed neurologic examination shows no deficits. However, the patient is concerned, as one of her friends was recently diagnosed with cancer. She requests imaging to “make sure there is no cancer.” Would it be appropriate to order imaging at this time?
No, it would not. Patients who have primary headache disorders without red-flag symptoms should not undergo imaging of the central nervous system (CNS) as part of their initial evaluation.1–4 (The list of potential red-flag symptoms is long but includes new onset after age 50, persistent neurologic changes, systemic symptoms or immunosuppression, sudden onset, progressive pain, positional nature, headaches precipitated by the Valsalva maneuver, and papilledema.)
CNS imaging may be appropriate for patients with features that increase the likelihood of structural diseases such as arteriovenous malformation, aneurysm, tumor, or subarachnoid hemorrhage. This patient, however, does not have worrisome signs or symptoms. Her symptoms are most consistent with migraine headache without aura. In patients with migraine headache without symptoms suggesting structural disease, CNS imaging is unwarranted and may be harmful.
DIAGNOSING MIGRAINE ACCURATELY
Diagnosing migraine headache can be a challenge, and up to half of all patients with migraine may be undiagnosed.5 The proper diagnosis of headache type is critical to the initial evaluation. In diagnosing migraine, one can use the mnemonic POUND4:
- Pulsatile
- One-day duration (4–72 hours)
- Unilateral
- Nausea or vomiting
- Disabling.
If four or five of these features are present, the likelihood ratio that the patient has migraine headache is 24, making it overwhelmingly likely that is the correct diagnosis.4 With three features the likelihood ratio is 3.5. If two or fewer features are present, migraine is much less likely, with a likelihood ratio of 0.41. Thus, patients with classic symptoms of migraine can be confidently and accurately diagnosed without the need for any imaging studies.
The patient in the vignette has all five POUND criteria. If we estimate her pretest probability of migraine headache at 50% (which is actually a conservative estimate—see Guidelines and Choosing Wisely, below), then, utilizing Bayes’ theorem, the likelihood ratio of 24 would result in a 95% probability that her headaches represent migraine.
GUIDELINES AND CHOOSING WISELY
High-quality reviews have found no benefit in performing imaging for primary headache disorders.1–3 This is due, in large part, to the rarity of secondary headache disorders in the primary care setting. In fact, most patients—90% in one study6—presenting to their primary care physicians with headaches meet the diagnostic criteria for migraine.
Significant abnormalities on imaging in patients with migraine headaches are also very rare. In patients with migraine headaches who undergo imaging, the rate of worrisome abnormalities that could lead to a change in management (0.2%) is less than that in the general population at the time of autopsy (0.8%).7
As part of the Choosing Wisely campaign, the American College of Radiology and the American Headache Society recommend against imaging for patients at low risk with migraine headaches. Because of the potential for harm from radiation exposure, the American Headache Society also recommends against computed tomography (CT) for evaluating headaches when magnetic resonance imaging (MRI) is available, except in emergencies.
Lists of tests and treatments that physicians and patients should question and discuss together to make wise decisions are available at www.choosingwisely.org.
HARMS ASSOCIATED WITH CNS IMAGING
Medical tests can be associated with significant harm. Potential harms of head imaging include radiation exposure from CT and false-positive findings. These false-positives, such as the finding of lesions that eventually prove to be benign, may require further testing and cause significant anxiety to the patient.
The effective radiation dose from a CT scan of the head is 2.0 mSv, equivalent to 250 days of background radiation exposure or 100 chest radiographs. Radiation exposure has been linked to increased risk of fatal cancer, and the risks increase with subsequent radiation doses.8
Incidental findings are common on head imaging and often lead to additional medical procedures and workup, without improvements in patient well-being. While the harms of false-positive testing and the finding of benign lesions are difficult to quantify, it is clear that downstream costs can accumulate and that these results cause significant undue worry to the patient.
CLINICAL BOTTOM LINE
Patients with migraine headache who do not have red-flag signs or symptoms are unlikely to benefit from CNS imaging and may experience harm. The rate of abnormalities in this population is not significantly different from that in the general population. A thorough history and physical examination should be done to find the proper diagnosis and to uncover any red-flag symptoms. For migraine headaches that are worsened by identified triggers, those triggers should be addressed before further evaluation is performed. When imaging is needed, physicians should consider minimizing radiation risk by ordering MRI instead of CT.
- Beithon J, Gallenberg M, Johnson K, et al; Institute for Clinical Systems Improvement. Diagnosis and treatment of headache. www.icsi.org/_asset/qwrznq/headache.pdf. Accessed September 5, 2014.
- Frishberg BM, Rosenberg JH, Matchar DB, et al; US Headache Consortium. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. www.aan.com/professionals/practice/pdfs/gl0088.pdf. Accessed September 5, 2014.
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55:754–762.
- Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM. Does this patient with headache have a migraine or need neuroimaging? JAMA 2006; 296:1274–1283.
- Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: results from the American Migraine Study II. Headache 2001; 41:638–645.
- Dowson A, Dahlof C, Tepper S, Newman L. Prevalence and diagnosis of migraine in a primary care setting (abstract). Cephalalgia 2002; 22:590–591.
- Frishberg BM. The utility of neuroimaging in the evaluation of headache in patients with normal neurologic examinations. Neurology 1994; 44:1191–1197.
- Semelka RC, Armao DM, Elias J Jr, Huda W. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging 2007; 25:900–909.
- Beithon J, Gallenberg M, Johnson K, et al; Institute for Clinical Systems Improvement. Diagnosis and treatment of headache. www.icsi.org/_asset/qwrznq/headache.pdf. Accessed September 5, 2014.
- Frishberg BM, Rosenberg JH, Matchar DB, et al; US Headache Consortium. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. www.aan.com/professionals/practice/pdfs/gl0088.pdf. Accessed September 5, 2014.
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55:754–762.
- Detsky ME, McDonald DR, Baerlocher MO, Tomlinson GA, McCrory DC, Booth CM. Does this patient with headache have a migraine or need neuroimaging? JAMA 2006; 296:1274–1283.
- Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: results from the American Migraine Study II. Headache 2001; 41:638–645.
- Dowson A, Dahlof C, Tepper S, Newman L. Prevalence and diagnosis of migraine in a primary care setting (abstract). Cephalalgia 2002; 22:590–591.
- Frishberg BM. The utility of neuroimaging in the evaluation of headache in patients with normal neurologic examinations. Neurology 1994; 44:1191–1197.
- Semelka RC, Armao DM, Elias J Jr, Huda W. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging 2007; 25:900–909.
Sarcoidal infiltration of tattoos
A 42-year-old woman presented with painless lesions on her eyebrows that had been progressively growing for the past 3 months. Inspection and palpation revealed reddish papules and nodules on both eyebrows (Figure 1) and on the lips. The remainder of the physical examination was normal.
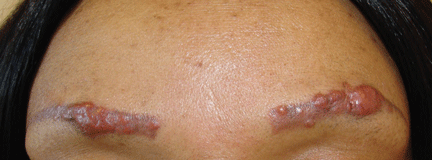
The patient said she had undergone cosmetic tattooing of her eyebrows and lips 10 years previously. She had no other significant medical history.
Her complete blood cell count and biochemistry and immunologic test panels were normal except for a high angiotensin-converting enzyme level.
Suspecting that the lesions represented sarcoidal infiltration of the tattoos, we obtained a biopsy. Histopathologic study showed multiple dermal noncaseating granulomas of epithelioid cells, together with polarizable foreign material and pigmented granules (Figure 2).
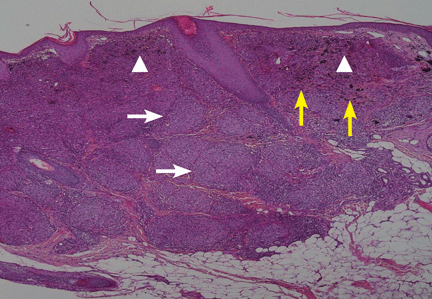
Thoracic multislice computed tomography revealed multiple areas of lymphadenopathy in the mediastinum and both lung hila, with a perilymphatic micronodular interstitial pattern and with thickened and nodular bronchial walls (Figure 3). The patient’s condition was diagnosed as systemic sarcoidosis presenting as sarcoidal infiltration of the tattooed areas.
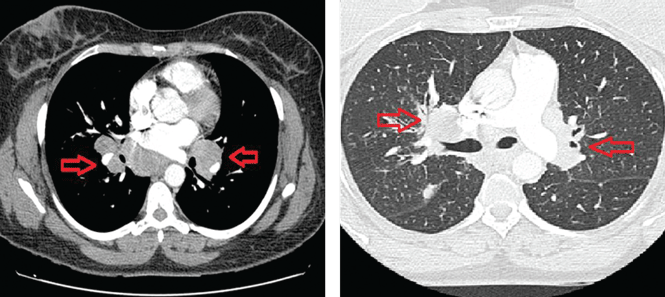
Treatment with oral prednisone 20 mg daily and monthly intralesional injections of triamcinolone 12 ng/mL in the eyebrows and lips resulted in clinical improvement after 3 months (Figure 4).
CUTANEOUS SARCOIDOSIS
Sarcoidosis is a multisystemic granulomatous disease characterized by hyperactivity of the cellular immune system. It usually appears between ages 25 and 35 and is more severe in African Americans.1 About one-third of patients with systemic sarcoidosis develop cutaneous lesions, and these may be the first sign of this disease.
Specific cutaneous lesions contain noncaseating granulomas. They manifest as reddish-brown papules, plaques, and macules and may appear over scars and tattoos.2,3 Other, nonspecific manifestations include erythema nodosum, erythema multiforme, nail clubbing, and Sweet syndrome.4
Diascopy and dermoscopy may be useful in diagnosing cutaneous sarcoidosis. The lesions typically have a characteristic yellowish-brown (“apple-jelly”) color.5
Sarcoidosis is a diagnosis of exclusion. It is suspected clinically, and histologic, radiologic, and analytical tests are indicated. Laboratory evaluation may show an elevated serum angiotensin-converting enzyme level, but this finding alone is not sensitive enough to be useful for diagnosis.6
The treatment and prognosis of skin sarcoidosis depend on the degree of systemic involvement. Localized cutaneous lesions can respond to intralesional corticosteroid injections and tacrolimus 0.1% ointment.7 However, if there is systemic involvement, low-dose prednisolone and weekly methotrexate should be started. This combination has few adverse effects.8 Recently, improvement of cutaneous and systemic sarcoidosis has been observed with agents that block tumor necrosis factor alpha.4
1. English JC 3rd, Patel PJ, Greer KE. Sarcoidosis. J Am Acad Dermatol 2001; 44:725–743.
2. Antonovich DD, Callen JP. Development of sarcoidosis in cosmetic tattoos. Arch Dermatol 2005; 141:869–872.
3. Anolik R, Mandal R, Franks AG Jr. Sarcoidal tattoo granuloma. Dermatol Online J 2010; 16:19.
4. Su Ö, Onsun N, Topukçu B, Özçelik HK, Çakıter AU, Büyükpınarbaşılı N. Disseminated scar sarcoidosis may predict pulmonary involvement in sarcoidosis. Acta Dermatovenerol Alp Pannonica Adriat 2013; 22:71–74.
5. Hunt RD, Gonzalez ME, Robinson M, Meehan SA, Franks AG Jr. Ulcerative sarcoidosis. Dermatol Online J 2012; 18:29.
6. Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med 2011; 183:573–581.
7. Landers MC, Skokan M, Law S, Storrs FJ. Cutaneous and pulmonary sarcoidosis in association with tattoos. Cutis 2005; 75:44–48.
8. Nagai S, Yokomatsu T, Tanizawa K, et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med 2014; 53:427–433.
A 42-year-old woman presented with painless lesions on her eyebrows that had been progressively growing for the past 3 months. Inspection and palpation revealed reddish papules and nodules on both eyebrows (Figure 1) and on the lips. The remainder of the physical examination was normal.

The patient said she had undergone cosmetic tattooing of her eyebrows and lips 10 years previously. She had no other significant medical history.
Her complete blood cell count and biochemistry and immunologic test panels were normal except for a high angiotensin-converting enzyme level.
Suspecting that the lesions represented sarcoidal infiltration of the tattoos, we obtained a biopsy. Histopathologic study showed multiple dermal noncaseating granulomas of epithelioid cells, together with polarizable foreign material and pigmented granules (Figure 2).

Thoracic multislice computed tomography revealed multiple areas of lymphadenopathy in the mediastinum and both lung hila, with a perilymphatic micronodular interstitial pattern and with thickened and nodular bronchial walls (Figure 3). The patient’s condition was diagnosed as systemic sarcoidosis presenting as sarcoidal infiltration of the tattooed areas.

Treatment with oral prednisone 20 mg daily and monthly intralesional injections of triamcinolone 12 ng/mL in the eyebrows and lips resulted in clinical improvement after 3 months (Figure 4).

CUTANEOUS SARCOIDOSIS
Sarcoidosis is a multisystemic granulomatous disease characterized by hyperactivity of the cellular immune system. It usually appears between ages 25 and 35 and is more severe in African Americans.1 About one-third of patients with systemic sarcoidosis develop cutaneous lesions, and these may be the first sign of this disease.
Specific cutaneous lesions contain noncaseating granulomas. They manifest as reddish-brown papules, plaques, and macules and may appear over scars and tattoos.2,3 Other, nonspecific manifestations include erythema nodosum, erythema multiforme, nail clubbing, and Sweet syndrome.4
Diascopy and dermoscopy may be useful in diagnosing cutaneous sarcoidosis. The lesions typically have a characteristic yellowish-brown (“apple-jelly”) color.5
Sarcoidosis is a diagnosis of exclusion. It is suspected clinically, and histologic, radiologic, and analytical tests are indicated. Laboratory evaluation may show an elevated serum angiotensin-converting enzyme level, but this finding alone is not sensitive enough to be useful for diagnosis.6
The treatment and prognosis of skin sarcoidosis depend on the degree of systemic involvement. Localized cutaneous lesions can respond to intralesional corticosteroid injections and tacrolimus 0.1% ointment.7 However, if there is systemic involvement, low-dose prednisolone and weekly methotrexate should be started. This combination has few adverse effects.8 Recently, improvement of cutaneous and systemic sarcoidosis has been observed with agents that block tumor necrosis factor alpha.4
A 42-year-old woman presented with painless lesions on her eyebrows that had been progressively growing for the past 3 months. Inspection and palpation revealed reddish papules and nodules on both eyebrows (Figure 1) and on the lips. The remainder of the physical examination was normal.

The patient said she had undergone cosmetic tattooing of her eyebrows and lips 10 years previously. She had no other significant medical history.
Her complete blood cell count and biochemistry and immunologic test panels were normal except for a high angiotensin-converting enzyme level.
Suspecting that the lesions represented sarcoidal infiltration of the tattoos, we obtained a biopsy. Histopathologic study showed multiple dermal noncaseating granulomas of epithelioid cells, together with polarizable foreign material and pigmented granules (Figure 2).

Thoracic multislice computed tomography revealed multiple areas of lymphadenopathy in the mediastinum and both lung hila, with a perilymphatic micronodular interstitial pattern and with thickened and nodular bronchial walls (Figure 3). The patient’s condition was diagnosed as systemic sarcoidosis presenting as sarcoidal infiltration of the tattooed areas.

Treatment with oral prednisone 20 mg daily and monthly intralesional injections of triamcinolone 12 ng/mL in the eyebrows and lips resulted in clinical improvement after 3 months (Figure 4).

CUTANEOUS SARCOIDOSIS
Sarcoidosis is a multisystemic granulomatous disease characterized by hyperactivity of the cellular immune system. It usually appears between ages 25 and 35 and is more severe in African Americans.1 About one-third of patients with systemic sarcoidosis develop cutaneous lesions, and these may be the first sign of this disease.
Specific cutaneous lesions contain noncaseating granulomas. They manifest as reddish-brown papules, plaques, and macules and may appear over scars and tattoos.2,3 Other, nonspecific manifestations include erythema nodosum, erythema multiforme, nail clubbing, and Sweet syndrome.4
Diascopy and dermoscopy may be useful in diagnosing cutaneous sarcoidosis. The lesions typically have a characteristic yellowish-brown (“apple-jelly”) color.5
Sarcoidosis is a diagnosis of exclusion. It is suspected clinically, and histologic, radiologic, and analytical tests are indicated. Laboratory evaluation may show an elevated serum angiotensin-converting enzyme level, but this finding alone is not sensitive enough to be useful for diagnosis.6
The treatment and prognosis of skin sarcoidosis depend on the degree of systemic involvement. Localized cutaneous lesions can respond to intralesional corticosteroid injections and tacrolimus 0.1% ointment.7 However, if there is systemic involvement, low-dose prednisolone and weekly methotrexate should be started. This combination has few adverse effects.8 Recently, improvement of cutaneous and systemic sarcoidosis has been observed with agents that block tumor necrosis factor alpha.4
1. English JC 3rd, Patel PJ, Greer KE. Sarcoidosis. J Am Acad Dermatol 2001; 44:725–743.
2. Antonovich DD, Callen JP. Development of sarcoidosis in cosmetic tattoos. Arch Dermatol 2005; 141:869–872.
3. Anolik R, Mandal R, Franks AG Jr. Sarcoidal tattoo granuloma. Dermatol Online J 2010; 16:19.
4. Su Ö, Onsun N, Topukçu B, Özçelik HK, Çakıter AU, Büyükpınarbaşılı N. Disseminated scar sarcoidosis may predict pulmonary involvement in sarcoidosis. Acta Dermatovenerol Alp Pannonica Adriat 2013; 22:71–74.
5. Hunt RD, Gonzalez ME, Robinson M, Meehan SA, Franks AG Jr. Ulcerative sarcoidosis. Dermatol Online J 2012; 18:29.
6. Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med 2011; 183:573–581.
7. Landers MC, Skokan M, Law S, Storrs FJ. Cutaneous and pulmonary sarcoidosis in association with tattoos. Cutis 2005; 75:44–48.
8. Nagai S, Yokomatsu T, Tanizawa K, et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med 2014; 53:427–433.
1. English JC 3rd, Patel PJ, Greer KE. Sarcoidosis. J Am Acad Dermatol 2001; 44:725–743.
2. Antonovich DD, Callen JP. Development of sarcoidosis in cosmetic tattoos. Arch Dermatol 2005; 141:869–872.
3. Anolik R, Mandal R, Franks AG Jr. Sarcoidal tattoo granuloma. Dermatol Online J 2010; 16:19.
4. Su Ö, Onsun N, Topukçu B, Özçelik HK, Çakıter AU, Büyükpınarbaşılı N. Disseminated scar sarcoidosis may predict pulmonary involvement in sarcoidosis. Acta Dermatovenerol Alp Pannonica Adriat 2013; 22:71–74.
5. Hunt RD, Gonzalez ME, Robinson M, Meehan SA, Franks AG Jr. Ulcerative sarcoidosis. Dermatol Online J 2012; 18:29.
6. Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med 2011; 183:573–581.
7. Landers MC, Skokan M, Law S, Storrs FJ. Cutaneous and pulmonary sarcoidosis in association with tattoos. Cutis 2005; 75:44–48.
8. Nagai S, Yokomatsu T, Tanizawa K, et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med 2014; 53:427–433.
Ebola—lessons still to be learned
In this issue of the Journal, Dr. Kyle Brizendine reviews the basics of the Ebola virus and its natural history, diagnosis, and management.
Like many of you, I have followed the Ebola story with disquietude. So far, the disease has barely touched our country, with fewer than 10 confirmed cases on US soil, but it has had a big impact on our health care system and our national psyche.
The creation of specialized containment and management units may deplete some hospitals and their communities of intensive care beds. Specially trained caregivers will need to be diverted to staff these units, and the public’s fear may dissuade patients from undergoing elective procedures at hospitals caring for patients with Ebola. All of these pose a financial challenge to the hospitals most capable of dealing with these patients.
We have yet to hear about management guidelines dealing with renal replacement therapy and ventilator support, which may extend life but also pose extra risks to caregivers. Do we understand the disease well enough to know when advanced supportive therapies might be futile? Many lessons were learned from the Liberian patient who died of Ebola in Dallas, but many more clinical questions remain. I had hoped that in our sophisticated ICUs patients treated relatively early with aggressive supportive care would likely survive. We do not yet know if that is true. One death does not make it false, but it does give one pause.
About a half dozen other Ebola patients have survived with treatment here, but they were not African. Does genetic background play a role in disease severity and survival? Were the survivors treated sooner or differently in ways that matter? How much of the end-organ damage from the virus is from direct organ infection that cannot be reversed or prevented by even the best supportive treatment? Does the ability of the virus to suppress the immune system doom patients to opportunistic infections during prolonged supportive therapy? Is the viral-associated immunosuppression enough to prevent some patients from mounting an effective innate (interferon-based) or acquired (viral-specific T-cell or humoral) antiviral response? And is transfusing blood from survivors, presumably conferring passive immunity, actually efficacious?
I was relieved there were no new Ebola cases among the staff caring for Mr. Duncan at his second emergency room visit in Dallas, since at that time he was clearly quite ill, viremic, and contagious. Universal safety precautions must have helped. But how did the other nurses become infected, even though they presumably wore better protection? Hopefully, we will gain further understanding of transmissibility and resistance. We need this knowledge to inform safe and manageable protocols of care, particularly if successful vaccine development is delayed.
In this issue of the Journal, Dr. Kyle Brizendine reviews the basics of the Ebola virus and its natural history, diagnosis, and management.
Like many of you, I have followed the Ebola story with disquietude. So far, the disease has barely touched our country, with fewer than 10 confirmed cases on US soil, but it has had a big impact on our health care system and our national psyche.
The creation of specialized containment and management units may deplete some hospitals and their communities of intensive care beds. Specially trained caregivers will need to be diverted to staff these units, and the public’s fear may dissuade patients from undergoing elective procedures at hospitals caring for patients with Ebola. All of these pose a financial challenge to the hospitals most capable of dealing with these patients.
We have yet to hear about management guidelines dealing with renal replacement therapy and ventilator support, which may extend life but also pose extra risks to caregivers. Do we understand the disease well enough to know when advanced supportive therapies might be futile? Many lessons were learned from the Liberian patient who died of Ebola in Dallas, but many more clinical questions remain. I had hoped that in our sophisticated ICUs patients treated relatively early with aggressive supportive care would likely survive. We do not yet know if that is true. One death does not make it false, but it does give one pause.
About a half dozen other Ebola patients have survived with treatment here, but they were not African. Does genetic background play a role in disease severity and survival? Were the survivors treated sooner or differently in ways that matter? How much of the end-organ damage from the virus is from direct organ infection that cannot be reversed or prevented by even the best supportive treatment? Does the ability of the virus to suppress the immune system doom patients to opportunistic infections during prolonged supportive therapy? Is the viral-associated immunosuppression enough to prevent some patients from mounting an effective innate (interferon-based) or acquired (viral-specific T-cell or humoral) antiviral response? And is transfusing blood from survivors, presumably conferring passive immunity, actually efficacious?
I was relieved there were no new Ebola cases among the staff caring for Mr. Duncan at his second emergency room visit in Dallas, since at that time he was clearly quite ill, viremic, and contagious. Universal safety precautions must have helped. But how did the other nurses become infected, even though they presumably wore better protection? Hopefully, we will gain further understanding of transmissibility and resistance. We need this knowledge to inform safe and manageable protocols of care, particularly if successful vaccine development is delayed.
In this issue of the Journal, Dr. Kyle Brizendine reviews the basics of the Ebola virus and its natural history, diagnosis, and management.
Like many of you, I have followed the Ebola story with disquietude. So far, the disease has barely touched our country, with fewer than 10 confirmed cases on US soil, but it has had a big impact on our health care system and our national psyche.
The creation of specialized containment and management units may deplete some hospitals and their communities of intensive care beds. Specially trained caregivers will need to be diverted to staff these units, and the public’s fear may dissuade patients from undergoing elective procedures at hospitals caring for patients with Ebola. All of these pose a financial challenge to the hospitals most capable of dealing with these patients.
We have yet to hear about management guidelines dealing with renal replacement therapy and ventilator support, which may extend life but also pose extra risks to caregivers. Do we understand the disease well enough to know when advanced supportive therapies might be futile? Many lessons were learned from the Liberian patient who died of Ebola in Dallas, but many more clinical questions remain. I had hoped that in our sophisticated ICUs patients treated relatively early with aggressive supportive care would likely survive. We do not yet know if that is true. One death does not make it false, but it does give one pause.
About a half dozen other Ebola patients have survived with treatment here, but they were not African. Does genetic background play a role in disease severity and survival? Were the survivors treated sooner or differently in ways that matter? How much of the end-organ damage from the virus is from direct organ infection that cannot be reversed or prevented by even the best supportive treatment? Does the ability of the virus to suppress the immune system doom patients to opportunistic infections during prolonged supportive therapy? Is the viral-associated immunosuppression enough to prevent some patients from mounting an effective innate (interferon-based) or acquired (viral-specific T-cell or humoral) antiviral response? And is transfusing blood from survivors, presumably conferring passive immunity, actually efficacious?
I was relieved there were no new Ebola cases among the staff caring for Mr. Duncan at his second emergency room visit in Dallas, since at that time he was clearly quite ill, viremic, and contagious. Universal safety precautions must have helped. But how did the other nurses become infected, even though they presumably wore better protection? Hopefully, we will gain further understanding of transmissibility and resistance. We need this knowledge to inform safe and manageable protocols of care, particularly if successful vaccine development is delayed.
Ebola virus: Questions, answers, and more questions
A 50-year-old man who returned from a business trip to Nigeria 24 days ago presents with complaints of the sudden onset of fever, diarrhea, myalgia, and headache. He reports 10 bowel movements per day and has seen bloody stools.
During his trip he flew in to Murtala Muhammed International Airport in Lagos, ate meals only in his hotel, and attended meetings in Lagos central business district. He had no exposure to animals, mosquitoes, ticks, or sick people, and no sexual activity. After returning home, he felt well for the first 3 weeks.
The patient has a history of hypertension. He does not smoke, drink alcohol, or use injection drugs. He is married, works with commercial banks and financial institutions, and lives in Cleveland, OH.
On physical examination his temperature is 100.0˚F (37.8˚C), pulse 98, respirations 15, blood pressure 105/70 mm Hg, and weight 78 kg (172 lb). He appears comfortable but is a little diaphoretic. His abdomen is tender to palpation in the epigastrium and slightly to the right; he has no signs of peritonitis. His skin is without rash, bleeding, or bruising. The remainder of the examination is normal.
His white blood cell count is 17 × 109/L, hemoglobin 15 g/dL, hematocrit 41%, and platelet count 172 × 109/L. His sodium level is 126 mmol/L, potassium 3.8 mmol/L, chloride 95 mmol/L, carbon dioxide 20 mmol/L, blood urea nitrogen 11 mg/dL, creatinine 0.7 mg/dL, and glucose 130 mg/dL. His aminotransferase and alkaline phosphatase levels are normal.
Could this patient have Ebola virus disease?
With Ebola virus disease on the rise in West Africa, physicians who encounter patients like this one need to include it in the differential diagnosis. Because the disease is new, many questions are raised for which we as yet have no answers. Here, I will review what we know and do not know in an effort to remove some of the fear and uncertainty.
A NEW DISEASE
Ebola virus disease is a severe hemorrhagic fever caused by negative-sense single-stranded RNA viruses classified by the International Committee on Taxonomy of Viruses as belonging to the genus Ebolavirus in the family Filoviridae. Filoviruses get their name from the Latin filum, or thread-like structure.
The family Filoviridae was discovered in 1967 after inadvertent importation of infected monkeys from Uganda into Yugoslavia and Marburg, Germany. Outbreaks of severe illness occurred in workers at a vaccine plant who came into direct contact with the animals by killing them, removing their kidneys, or preparing primary cell cultures for polio vaccine production.
Ebola virus was discovered in 1976 by Peter Piot, who was working at the Institute of Tropical Medicine in Antwerp, Belgium. The blood of a Belgian woman who had been working in what is now the Democratic Republic of the Congo (formerly Zaire) had been sent to the institute; she and Mabalo Lokela, a school headmaster and the first recorded victim of Ebola virus, had been working near Yambuku, about 96 km from the Ebola River.
Before the 2014 outbreak, all known outbreaks had caused fewer than 2,400 cases across a dozen African countries over 3 decades.
Five species of Ebola virus
The genus Ebolavirus contains five species, each associated with a consistent case-fatality rate and a more or less well-identified endemic area.1
Zaire ebolavirus was recognized in 1976; it has caused multiple outbreaks, with high case-fatality rates.
Sudan ebolavirus was seen first in the 1970s; it has a 50% case-fatality rate.
Tai Forest ebolavirus has been found in only one person, an ethologist working with deceased chimpanzees.
Bundibugyo ebolavirus emerged in 2007 and has a 30% case-fatality rate.
Reston ebolavirus is maintained in an animal reservoir in the Philippines and is not found in Africa. It caused an outbreak of lethal infection in macaques imported into the United States in 1989. There is evidence that Reston ebolavirus can cause asymptomatic infection in humans. None of the caretakers of the macaques became ill, nor did farmers working with infected pigs, although both groups seroconverted.
A reservoir in bats?
A reservoir in nonhuman primates was initially suspected. However, studies subsequently showed that monkeys are susceptible to rapidly lethal filoviral disease, precluding any role as a host for persistent viral infection. It is likely that Ebola virus is maintained in small animals that serve as a source of infection for both humans and wild primates. A prominent suspect is fruit bats, which are consumed in soup in West Africa.
Transmission is person-to-person or nosocomial
Ebola virus is transmitted by direct contact with body fluids such as blood, urine, sweat, vomitus, semen, and breast milk. Filoviruses can initiate infection via ingestion, inhalation (although probably not Ebola), or passage through breaks in the skin. Droplet inoculation into the mouth or eyes has been shown to result from inadvertent transfer of virus from contaminated hands. Patients transmit the virus while febrile and through later stages of disease, as well as postmortem through contact with the body during funeral preparations. The virus has been isolated in semen for as many as 61 days after illness onset.
Ebola virus can also be spread nosocomially. In 1976, a 44-year-old teacher sought care for fever at the Yambuku Mission Hospital. He was given parenteral chloroquine as empiric treatment for presumed malaria, which was routine for all febrile patients. However, he had unrecognized Ebola virus infection. Moreover, syringes were rinsed in the same pan of water and reused, which spread the infection to nearly 100 people, all of whom developed fulminant Ebola virus disease and died. Infection then spread to family caregivers, the hospital staff, and those who prepared the bodies for burial.
Nosocomial transmission was also responsible for an outbreak of Lake Victoria Marburg virus in Uige Province in northern Angola in 2005, with 374 putative cases and 329 deaths. When teams from Médecins Sans Frontières started setting up the Marburg ward, there were five patients with hemorrhagic fever in a makeshift isolation room in the hospital, together with corpses that the hospital staff had been too afraid to remove. Healers found in many rural African communities were administering injections in homes or in makeshift clinics with reused needles or syringes.2
There is no evidence that filoviruses are carried by mosquitoes or other biting arthropods. Also, the risk of transmission via fomites appears to be low when currently recommended infection-control guidelines for the viral hemorrhagic fevers are followed.3 One primary human case generates only one to three secondary cases on average.
EBOLA IS AN IMMUNODEFICIENCY VIRUS
The main targets of infection are endothelial cells, mononuclear phagocytes, and hepatocytes. Ebola virus replicates at an unusually high rate. Macrophages infected with Zaire ebolavirus produce tumor necrosis factor alpha, interleukin (IL) 1 beta, IL-6, macrophage chemotactic protein 1, and nitric oxide. Virus-infected macrophages synthesize cell-surface tissue factor, triggering the extrinsic coagulation pathway.
Ebola is an immunodeficiency virus. Dendritic cells, which initiate adaptive immune responses, are a major site of filoviral replication. Infected cells cannot present antigens to naïve lymphocytes. Patients who die of Ebola virus disease do not develop antibodies to the virus. Lymphocytes remain uninfected, but undergo “bystander” apoptosis induced by inflammatory mediators.
CLINICAL MANIFESTATIONS
The incubation period is generally 5 to 7 days (range 2 to 28 days), during which the patient is not infectious. Symptoms begin abruptly, with fever, chills, general malaise, weakness, severe headache, and myalgia. By the time of case detection in West Africa, most patients also had nausea, vomiting, diarrhea, and abdominal pain. Once symptoms arise, patients have high levels of the virus in their blood and fluids and are infectious. Hemorrhagic symptoms have apparently been uncommon in West Africa, occurring in 1.0% to 5.7%, but “unexplained bleeding” has been documented in 18% of cases.4 Among those in whom the disease enters its hemorrhagic terminal phase, there is characteristic internal and subcutaneous bleeding, vomiting of blood, and subconjunctival hemorrhage.4
Laboratory findings include lymphocytopenia (often with counts as low as 1.0 × 109/L), thrombocytopenia (with counts in the range of 50 to 100 × 109/L), elevated aminotransferase levels (including aspartate aminotransferase levels 7 to 12 times higher than alanine aminotransferase in fatal cases), low total protein (due to capillary leak), and disseminated intravascular coagulation. Those who survive begin to improve in the second week, during which viremia resolves in association with the appearance of virus-specific antibodies.4
DIAGNOSIS
In symptomatic patients, Ebola virus infection is diagnosed by detection in blood or body fluids of viral antigens by enzyme-linked immunosorbent assay, or RNA sequences by reverse transcriptase polymerase chain reaction. The diagnosis is confirmed with cell culture (in a BSL-4 containment laboratory) showing characteristic viral particles by electron microscopy.
CARING FOR PATIENTS
The most detailed descriptions of the care of patients with Ebola virus disease have come from Dr. Bruce Ribner, of Emory University Hospital, in an October 2014 report of his experience caring for Ebola-infected patients at Emory University Hospital in Atlanta, GA.5 He described fluid losses of 5 to 10 L/day, profound hyponatremia, hypokalemia, and hypocalcemia, which were associated with cardiac arrhythmias and the need for intravenous and oral electrolyte repletion and hemodialysis. Intensive one-to-one nursing was critical, as was the coordination of many medical subspecialties. The Emory team arranged point-of-care testing near the unit and generally kept laboratory testing to a minimum. The team was surprised to learn that commercial carriers refused to transport specimens even when they were licensed for category A agents. Difficulties with the local water authority and waste disposal contractor required the hospital to dedicate an autoclave to process all materials used in clinical care.
TREATMENT: SUPPORTIVE AND EXPERIMENTAL
Treatment is supportive to maintain circulatory function and blood pressure and to correct coagulopathy. However, a variety of vaccines, antibodies, small-molecule agents, and antiviral agents are undergoing testing, mostly in animals at this point.
Vaccines. A therapeutic vaccine that worked only slightly was a live-attenuated recombinant vesicular stomatitis virus expressing Ebola virus transmembrane glycoproteins, which was tested in mice, guinea pigs, and rhesus macaques who had been exposed to Ebola virus.6
A preventive vaccine worked better. Stanley et al7 evaluated a replication-defective chimpanzee adenovirus 3-vectored vaccine that also contained Ebola virus glycoprotein. They gave macaques a single injection of this vaccine, and then 5 weeks later gave them a lethal dose of Ebola virus. All the vaccinated animals survived the infection, and half (2 of 4) survived when challenged 10 months later. With a prime-boost strategy (modified vaccinia virus Ankara, a poxvirus), all survived when challenged 10 months later.
KZ52, a neutralizing antibody, did not work. Oswald et al8 gave a human IgG monoclonal antibody against Zaire Ebola virus, designated KZ52, to four rhesus macaques, challenged them with the virus 24 hours later, and administered a second shot of KZ52 on day 4. All of them died.
ZMAb is a combination of three murine monoclonal antibodies, designated 1H3, 2G4, and 4G7. Ad-IFN is a human adenovirus, serotype 5, that expresses human interferon alpha. Qui et al9 gave ZMAb and Ad-IFN to macaques in several experiments. In experiment 1, eight macaques were infected and then were given ZMAb and Ad-IFN 3 days later, and ZMAb again on days 6 and 9. Seven of the eight survived. In a second experiment, Ad-IFN was given first, when the viral load was still less than the limit of detection of known assays, and then ZMAb was given upon detection of viremia and fever. Two of four macaques survived. Control animals had undetectable levels of IgG, whereas Ebola virus GP–specific IgG levels were detected in all survivors. IFN-gamma ELISpots showed high EBOV-GP–specific T-cell response in all survivors.
ZMapp is another cocktail of monoclonal antibodies, containing two from ZMab (2G4 and 4G7), plus a third, c13C6. In experiments in rhesus macaques, three groups of six animals each received three doses of ZMapp at varying times after being infected with Ebola virus: at 3, 6, and 9 days; at 4, 7, and 10 days, and at 5, 8, and 11 days. All 18 macaques treated with ZMapp survived. Thus, Zmapp extended the treatment window to 5 days postexposure.10 One of the American health care workers who contracted Ebola virus in Liberia received this medication.
HSPA5-PMO. Endoplasmic reticulum chaperone heat shock 70 kDa protein 5 (HSPA5) is instrumental in the maturation of envelope proteins in hepatitis C and influenza A virus. It plays a role in viral entry for coxsackievirus A9 and dengue virus serotype 2, and it may be involved in Ebola viral budding. Phosphorodiamidate morpholino oligomers (PMOs) are a class of antisense DNA nucleotide analogs.
Reid et al11 reported that mice treated with HSPA5–PMO were completely protected from lethal Ebola challenge. Therefore, HSPA5 appears to be a promising target for the development of antifilovirus countermeasures.
Favipiravir, an antiviral agent also known as T-705, is a pyrazinecarboxamide derivative. Invented in 2002 by Toyama Chemicals as an inhibitor of influenza virus replication, it acts as a nucleotide analog, selectively inhibiting the viral RNA-dependent RNA polymerase, or causes lethal mutagenesis upon incorporation into the virus RNA. Favipiravir suppresses Ebola virus replication by 4 log10 units in cell culture.12
Mice were challenged with intranasal inoculation of 1,000 focus-forming units of Ebola virus diluted in phosphate-buffered saline. Until the first day of treatment (postinfection day 6), all mice in the T-705 group lost weight similarly to control mice, developed viremia, and showed elevated serum levels of aspartate aminotransferase and alanine aminotransferase. Within 4 days of T-705 treatment (post-infection day 10), the animals had cleared the virus from blood. Surviving mice developed Ebola virus-specific antibodies and CD8+ T cells specific for the viral nucleoprotein.12
The authors hypothesized that suppression of virus replication by T-705 allowed the host to mount a virus-specific adaptive immune response, and concluded that T-705 was 100% effective in the treatment of Zaire Ebola virus infection up to postinfection day 6 but was hardly beneficial at the terminal stage of disease.12 Of note, favipiravir is undergoing phase 2 and phase 3 trials as an anti-influenza agent in Japan.
THE CURRENT OUTBREAK
The current outbreak is with Zaire ebolavirus. It seems to have started in a 2-year-old child who died in Meliandou in Guéckédou Prefecture, Guinea, on December 6, 2013. On March 21, 2014, the Guinea Ministry of Health reported the outbreak of an illness characterized by fever, severe diarrhea, vomiting, and a high case-fatality rate (59%) in 49 persons. On May 25, 2014, Kenema Government Hospital confirmed the first case of Ebola virus disease in Sierra Leone, probably brought there by a traditional healer who had treated Ebola patients from Guinea. Tracing led to 13 additional cases—all women who attended the burial.13
The Center for Systems Biology at Harvard University and the Broad Institute of Massachusetts Institute of Technology generated 99 Ebola virus genome sequences from 78 patients with confirmed disease, representing more than 70% of the patients diagnosed with the disease in Sierra Leone from May to mid-June 2014. They found genetic similarity across the sequenced 2014 samples, suggesting a single transmission from the natural reservoir, followed by human-to-human transmission during the outbreak. Continued human-reservoir exposure is unlikely to have contributed to the growth of this epidemic.14
As of October 14, 2014, there were 8,914 suspected and confirmed cases of Ebola virus infection, and 4,477 deaths.15
But how did Zaire Ebola virus make the 2,000-mile trek from Central Africa to Guinea in West Africa? There are two possibilities: it has always been present in the region but we just never noticed, or it was recently introduced. Bayesian phylogenetic analyses and sequence divergence studies suggest the virus has been present in bat populations in Guinea without previously infecting humans.
Why Guinea and why Guéckédou? Guinea is one of the poorest countries in the world, ranking 178th of 187 countries on the Human Development Index of the United Nations Development Programme, just behind Liberia (174th) and Sierra Leone (177th). In Guinea, the life expectancy is 56 years and the gross national income per capita is $440. The region has been systematically plundered and the forest decimated by clear-cut logging, leaving the Guinea Forest Region largely deforested, resulting in increased contact between humans and the small animals that serve as the source of infection.1
LIMITED CAPACITY, EVEN IN THE UNITED STATES
A few hospitals in the United States have dedicated units to handle serious infectious diseases such as Ebola: Emory University Hospital; Nebraska Medicine in Omaha; Providence St. Patrick Hospital in Missoula, MT; and the National Institutes of Health in Bethesda, MD. However, in total they have only 19 beds.
QUESTIONS, ANSWERS—AND MORE QUESTIONS
(The following is from a question-and-answer discussion that followed Dr. Brizendine’s Grand Rounds presentation.)
Q: Are there any differences between survivors and those who die of the disease? A: We do not know. Patient survival depends on early recognition and supportive care. There are disparities in the care of patients. Schieffelin et al16 analyzed the characteristics of patients who died or who survived in Sierra Leone and found that the mortality rate was higher in older patients and those with a higher viral load on presentation.
Q: Does the virus block production or release of interferon early in infection? A: Yes, it has been shown17 that Ebola virus protein VP24 inhibits signaling downstream of both interferon alpha/beta and interferon gamma by indirectly impairing the transport of a transcription factor termed STAT1. VP24 is also able to bind STAT1 directly. The resulting suppression of host interferon very early on in the incubation phase is key to the virulence of the virus.
Q: Does infection with one of the viral species confer immunity from other species? A: No, there is no cross-immunity.
Q: How soon do patients test positive? A: About 5 days after exposure, when they develop a fever. At this time patients are highly viremic, which PCR can detect.
Q: Before the virus is detectable in the blood, where is it? A: The liver, endothelial cells, antigen-presenting cells, and adrenal glands.
Q: Do we really need to quarantine ill patients and health care workers returning from Africa, per CDC recommendations? A: We don’t know everything, and some people do make bad decisions, such as traveling while symptomatic. I support a period of observation, although confinement is not reasonable, as it may pose a disincentive to cooperation.
Q: What is the role of giving plasma from survivors? A: Dr. Kent Brantly (see American citizens infected with Ebola) received the blood of a 14-year-old who survived. We don’t know. It is not proved. It did not result in improvement in animal models.
Q: Is the bleeding caused by a mechanism similar to that in enterohemorrhagic Escherichia coli infection? A: No. That is a bacterial toxin, whereas this is more like disseminated intravascular coagulation, with an intrinsic pathway anticoagulation cascade.
Q: How long does the virus remain viable outside the body? A: In one study,18 Ebola virus could not be recovered from experimentally contaminated surfaces (plastic, metal or glass) at room temperature. In another in which it was dried onto a surface,19 Ebola virus survived in the dark for several hours between 20 and 25°C. When dried in tissue culture media onto glass and stored at 4°C, it has survived for over 50 days.
Q: How long does the virus remain in breast milk? A: We know it has been detected 15 days after disease onset and think possibly as late as 28 days from symptom onset.3
Q: How are people actually infected? A: I believe people get the virus on their hands and then touch their face, eyes, or mouth. If you are wearing personal protective equipment, it must occur while doffing the equipment.
Q: Could we increase the sensitivity of the test so that we could detect the virus before the onset of symptoms? A: In theory it may be possible. The virus is somewhere in the body during the incubation period. Perhaps we could sample the right compartment in an enriched mononuclear cell line.
Q: When can patients who recover resume their normal activities? A: After their viral load returns to 0, I would still advise abstaining from unprotected sex and from breastfeeding for a few months. but as for other activities, no special precautions are needed.
Q: Does the virus appear to be mutating at a high rate? A: Looking back to 2004, mutations are occurring, but there is no sign that any of these mutations has contributed to the size of the outbreak by changing the characteristics of the Ebola virus. Can it become aerosolized? It has been suggested that the virus that caused the outbreak separated from those that caused past Ebola outbreaks but does not seem to be affecting the spread or efficacy of experimental drugs and vaccines. So, even though it is an RNA virus and mutations are occurring, no serious changes have emerged.14
BACK TO OUR PATIENT
The differential diagnosis for the patient described at the beginning of this paper includes travelers’ diarrhea, malaria, typhoid fever, yellow fever, meningococcal disease … and Ebola virus disease, although this is much less likely in view of the epidemiology and incubation period of this disease. When his stool was tested by enzyme immunoassay and culture, it was found to be positive for Campylobacter. He recovered with oral rehydration.
- Bausch DG, Schwarz L. Outbreak of ebola virus disease in Guinea: where ecology meets economy. PLoS Negl Trop Dis 2014; 8:e3056.
- Roddy P, Thomas SL, Jeffs B, et al. Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige, Angola. J Infect Dis 2010; 201:1909–1918.
- Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196(suppl 2):S142–S147.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–1495.
- Ribner BS. Treating patients with Ebola virus infections in the US: lessons learned. Presented at IDWeek, October 8, 2014. Philadelphia PA.
- Feldman H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2.
- Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–1129.
- Oswald WB, Geisbert TW, Davis KJ, et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLos Pathog 2007; 3:e9.
- Qui X, Wong G, Fernando L, et al. mAbs and Ad-vectored IFN-a therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med 2013; 5:207ra143.
- Qui X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53.
- Reid SP, Shurtleff AC, Costantino JA, et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 2014; 109:171–174.
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 2014; 105:17–21.
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus dsease in Guinea. N Engl J Med 2014; 371:1418–1425.
- Gire SK, Goba A, Andersen KG, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014; 345:1369–1372.
- Chamary JV. 4000 deaths and counting: the Ebola epidemic in 4 charts. Forbes. http://www.forbes.com/sites/jvchamary/2014/10/13/ebola-trends. Accessed November 5, 2014.
- Schieffelin JS, Shaffer JG, Goba A, et al, for the KGH Lassa Fever Program, the Viral Hemorrhagic Fever Consortium, and the WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014 Oct 29 [Epub ahead of print]. DOI: 10.1056/NEJMoa1411680.
- Zhang AP, Bornholdt ZA, Liu T, et al. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog 2012; 8:e1002550.
- Piercy TJ, Smither SJ, Steward JA, Eastaugh L, Lever MS. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol 2010; 109:1531–1539.
- Sagripanti JL, Rom AM, Holland LE. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol 2010; 155:2035–2039.
A 50-year-old man who returned from a business trip to Nigeria 24 days ago presents with complaints of the sudden onset of fever, diarrhea, myalgia, and headache. He reports 10 bowel movements per day and has seen bloody stools.
During his trip he flew in to Murtala Muhammed International Airport in Lagos, ate meals only in his hotel, and attended meetings in Lagos central business district. He had no exposure to animals, mosquitoes, ticks, or sick people, and no sexual activity. After returning home, he felt well for the first 3 weeks.
The patient has a history of hypertension. He does not smoke, drink alcohol, or use injection drugs. He is married, works with commercial banks and financial institutions, and lives in Cleveland, OH.
On physical examination his temperature is 100.0˚F (37.8˚C), pulse 98, respirations 15, blood pressure 105/70 mm Hg, and weight 78 kg (172 lb). He appears comfortable but is a little diaphoretic. His abdomen is tender to palpation in the epigastrium and slightly to the right; he has no signs of peritonitis. His skin is without rash, bleeding, or bruising. The remainder of the examination is normal.
His white blood cell count is 17 × 109/L, hemoglobin 15 g/dL, hematocrit 41%, and platelet count 172 × 109/L. His sodium level is 126 mmol/L, potassium 3.8 mmol/L, chloride 95 mmol/L, carbon dioxide 20 mmol/L, blood urea nitrogen 11 mg/dL, creatinine 0.7 mg/dL, and glucose 130 mg/dL. His aminotransferase and alkaline phosphatase levels are normal.
Could this patient have Ebola virus disease?
With Ebola virus disease on the rise in West Africa, physicians who encounter patients like this one need to include it in the differential diagnosis. Because the disease is new, many questions are raised for which we as yet have no answers. Here, I will review what we know and do not know in an effort to remove some of the fear and uncertainty.
A NEW DISEASE
Ebola virus disease is a severe hemorrhagic fever caused by negative-sense single-stranded RNA viruses classified by the International Committee on Taxonomy of Viruses as belonging to the genus Ebolavirus in the family Filoviridae. Filoviruses get their name from the Latin filum, or thread-like structure.
The family Filoviridae was discovered in 1967 after inadvertent importation of infected monkeys from Uganda into Yugoslavia and Marburg, Germany. Outbreaks of severe illness occurred in workers at a vaccine plant who came into direct contact with the animals by killing them, removing their kidneys, or preparing primary cell cultures for polio vaccine production.
Ebola virus was discovered in 1976 by Peter Piot, who was working at the Institute of Tropical Medicine in Antwerp, Belgium. The blood of a Belgian woman who had been working in what is now the Democratic Republic of the Congo (formerly Zaire) had been sent to the institute; she and Mabalo Lokela, a school headmaster and the first recorded victim of Ebola virus, had been working near Yambuku, about 96 km from the Ebola River.
Before the 2014 outbreak, all known outbreaks had caused fewer than 2,400 cases across a dozen African countries over 3 decades.
Five species of Ebola virus
The genus Ebolavirus contains five species, each associated with a consistent case-fatality rate and a more or less well-identified endemic area.1
Zaire ebolavirus was recognized in 1976; it has caused multiple outbreaks, with high case-fatality rates.
Sudan ebolavirus was seen first in the 1970s; it has a 50% case-fatality rate.
Tai Forest ebolavirus has been found in only one person, an ethologist working with deceased chimpanzees.
Bundibugyo ebolavirus emerged in 2007 and has a 30% case-fatality rate.
Reston ebolavirus is maintained in an animal reservoir in the Philippines and is not found in Africa. It caused an outbreak of lethal infection in macaques imported into the United States in 1989. There is evidence that Reston ebolavirus can cause asymptomatic infection in humans. None of the caretakers of the macaques became ill, nor did farmers working with infected pigs, although both groups seroconverted.
A reservoir in bats?
A reservoir in nonhuman primates was initially suspected. However, studies subsequently showed that monkeys are susceptible to rapidly lethal filoviral disease, precluding any role as a host for persistent viral infection. It is likely that Ebola virus is maintained in small animals that serve as a source of infection for both humans and wild primates. A prominent suspect is fruit bats, which are consumed in soup in West Africa.
Transmission is person-to-person or nosocomial
Ebola virus is transmitted by direct contact with body fluids such as blood, urine, sweat, vomitus, semen, and breast milk. Filoviruses can initiate infection via ingestion, inhalation (although probably not Ebola), or passage through breaks in the skin. Droplet inoculation into the mouth or eyes has been shown to result from inadvertent transfer of virus from contaminated hands. Patients transmit the virus while febrile and through later stages of disease, as well as postmortem through contact with the body during funeral preparations. The virus has been isolated in semen for as many as 61 days after illness onset.
Ebola virus can also be spread nosocomially. In 1976, a 44-year-old teacher sought care for fever at the Yambuku Mission Hospital. He was given parenteral chloroquine as empiric treatment for presumed malaria, which was routine for all febrile patients. However, he had unrecognized Ebola virus infection. Moreover, syringes were rinsed in the same pan of water and reused, which spread the infection to nearly 100 people, all of whom developed fulminant Ebola virus disease and died. Infection then spread to family caregivers, the hospital staff, and those who prepared the bodies for burial.
Nosocomial transmission was also responsible for an outbreak of Lake Victoria Marburg virus in Uige Province in northern Angola in 2005, with 374 putative cases and 329 deaths. When teams from Médecins Sans Frontières started setting up the Marburg ward, there were five patients with hemorrhagic fever in a makeshift isolation room in the hospital, together with corpses that the hospital staff had been too afraid to remove. Healers found in many rural African communities were administering injections in homes or in makeshift clinics with reused needles or syringes.2
There is no evidence that filoviruses are carried by mosquitoes or other biting arthropods. Also, the risk of transmission via fomites appears to be low when currently recommended infection-control guidelines for the viral hemorrhagic fevers are followed.3 One primary human case generates only one to three secondary cases on average.
EBOLA IS AN IMMUNODEFICIENCY VIRUS
The main targets of infection are endothelial cells, mononuclear phagocytes, and hepatocytes. Ebola virus replicates at an unusually high rate. Macrophages infected with Zaire ebolavirus produce tumor necrosis factor alpha, interleukin (IL) 1 beta, IL-6, macrophage chemotactic protein 1, and nitric oxide. Virus-infected macrophages synthesize cell-surface tissue factor, triggering the extrinsic coagulation pathway.
Ebola is an immunodeficiency virus. Dendritic cells, which initiate adaptive immune responses, are a major site of filoviral replication. Infected cells cannot present antigens to naïve lymphocytes. Patients who die of Ebola virus disease do not develop antibodies to the virus. Lymphocytes remain uninfected, but undergo “bystander” apoptosis induced by inflammatory mediators.
CLINICAL MANIFESTATIONS
The incubation period is generally 5 to 7 days (range 2 to 28 days), during which the patient is not infectious. Symptoms begin abruptly, with fever, chills, general malaise, weakness, severe headache, and myalgia. By the time of case detection in West Africa, most patients also had nausea, vomiting, diarrhea, and abdominal pain. Once symptoms arise, patients have high levels of the virus in their blood and fluids and are infectious. Hemorrhagic symptoms have apparently been uncommon in West Africa, occurring in 1.0% to 5.7%, but “unexplained bleeding” has been documented in 18% of cases.4 Among those in whom the disease enters its hemorrhagic terminal phase, there is characteristic internal and subcutaneous bleeding, vomiting of blood, and subconjunctival hemorrhage.4
Laboratory findings include lymphocytopenia (often with counts as low as 1.0 × 109/L), thrombocytopenia (with counts in the range of 50 to 100 × 109/L), elevated aminotransferase levels (including aspartate aminotransferase levels 7 to 12 times higher than alanine aminotransferase in fatal cases), low total protein (due to capillary leak), and disseminated intravascular coagulation. Those who survive begin to improve in the second week, during which viremia resolves in association with the appearance of virus-specific antibodies.4
DIAGNOSIS
In symptomatic patients, Ebola virus infection is diagnosed by detection in blood or body fluids of viral antigens by enzyme-linked immunosorbent assay, or RNA sequences by reverse transcriptase polymerase chain reaction. The diagnosis is confirmed with cell culture (in a BSL-4 containment laboratory) showing characteristic viral particles by electron microscopy.
CARING FOR PATIENTS
The most detailed descriptions of the care of patients with Ebola virus disease have come from Dr. Bruce Ribner, of Emory University Hospital, in an October 2014 report of his experience caring for Ebola-infected patients at Emory University Hospital in Atlanta, GA.5 He described fluid losses of 5 to 10 L/day, profound hyponatremia, hypokalemia, and hypocalcemia, which were associated with cardiac arrhythmias and the need for intravenous and oral electrolyte repletion and hemodialysis. Intensive one-to-one nursing was critical, as was the coordination of many medical subspecialties. The Emory team arranged point-of-care testing near the unit and generally kept laboratory testing to a minimum. The team was surprised to learn that commercial carriers refused to transport specimens even when they were licensed for category A agents. Difficulties with the local water authority and waste disposal contractor required the hospital to dedicate an autoclave to process all materials used in clinical care.
TREATMENT: SUPPORTIVE AND EXPERIMENTAL
Treatment is supportive to maintain circulatory function and blood pressure and to correct coagulopathy. However, a variety of vaccines, antibodies, small-molecule agents, and antiviral agents are undergoing testing, mostly in animals at this point.
Vaccines. A therapeutic vaccine that worked only slightly was a live-attenuated recombinant vesicular stomatitis virus expressing Ebola virus transmembrane glycoproteins, which was tested in mice, guinea pigs, and rhesus macaques who had been exposed to Ebola virus.6
A preventive vaccine worked better. Stanley et al7 evaluated a replication-defective chimpanzee adenovirus 3-vectored vaccine that also contained Ebola virus glycoprotein. They gave macaques a single injection of this vaccine, and then 5 weeks later gave them a lethal dose of Ebola virus. All the vaccinated animals survived the infection, and half (2 of 4) survived when challenged 10 months later. With a prime-boost strategy (modified vaccinia virus Ankara, a poxvirus), all survived when challenged 10 months later.
KZ52, a neutralizing antibody, did not work. Oswald et al8 gave a human IgG monoclonal antibody against Zaire Ebola virus, designated KZ52, to four rhesus macaques, challenged them with the virus 24 hours later, and administered a second shot of KZ52 on day 4. All of them died.
ZMAb is a combination of three murine monoclonal antibodies, designated 1H3, 2G4, and 4G7. Ad-IFN is a human adenovirus, serotype 5, that expresses human interferon alpha. Qui et al9 gave ZMAb and Ad-IFN to macaques in several experiments. In experiment 1, eight macaques were infected and then were given ZMAb and Ad-IFN 3 days later, and ZMAb again on days 6 and 9. Seven of the eight survived. In a second experiment, Ad-IFN was given first, when the viral load was still less than the limit of detection of known assays, and then ZMAb was given upon detection of viremia and fever. Two of four macaques survived. Control animals had undetectable levels of IgG, whereas Ebola virus GP–specific IgG levels were detected in all survivors. IFN-gamma ELISpots showed high EBOV-GP–specific T-cell response in all survivors.
ZMapp is another cocktail of monoclonal antibodies, containing two from ZMab (2G4 and 4G7), plus a third, c13C6. In experiments in rhesus macaques, three groups of six animals each received three doses of ZMapp at varying times after being infected with Ebola virus: at 3, 6, and 9 days; at 4, 7, and 10 days, and at 5, 8, and 11 days. All 18 macaques treated with ZMapp survived. Thus, Zmapp extended the treatment window to 5 days postexposure.10 One of the American health care workers who contracted Ebola virus in Liberia received this medication.
HSPA5-PMO. Endoplasmic reticulum chaperone heat shock 70 kDa protein 5 (HSPA5) is instrumental in the maturation of envelope proteins in hepatitis C and influenza A virus. It plays a role in viral entry for coxsackievirus A9 and dengue virus serotype 2, and it may be involved in Ebola viral budding. Phosphorodiamidate morpholino oligomers (PMOs) are a class of antisense DNA nucleotide analogs.
Reid et al11 reported that mice treated with HSPA5–PMO were completely protected from lethal Ebola challenge. Therefore, HSPA5 appears to be a promising target for the development of antifilovirus countermeasures.
Favipiravir, an antiviral agent also known as T-705, is a pyrazinecarboxamide derivative. Invented in 2002 by Toyama Chemicals as an inhibitor of influenza virus replication, it acts as a nucleotide analog, selectively inhibiting the viral RNA-dependent RNA polymerase, or causes lethal mutagenesis upon incorporation into the virus RNA. Favipiravir suppresses Ebola virus replication by 4 log10 units in cell culture.12
Mice were challenged with intranasal inoculation of 1,000 focus-forming units of Ebola virus diluted in phosphate-buffered saline. Until the first day of treatment (postinfection day 6), all mice in the T-705 group lost weight similarly to control mice, developed viremia, and showed elevated serum levels of aspartate aminotransferase and alanine aminotransferase. Within 4 days of T-705 treatment (post-infection day 10), the animals had cleared the virus from blood. Surviving mice developed Ebola virus-specific antibodies and CD8+ T cells specific for the viral nucleoprotein.12
The authors hypothesized that suppression of virus replication by T-705 allowed the host to mount a virus-specific adaptive immune response, and concluded that T-705 was 100% effective in the treatment of Zaire Ebola virus infection up to postinfection day 6 but was hardly beneficial at the terminal stage of disease.12 Of note, favipiravir is undergoing phase 2 and phase 3 trials as an anti-influenza agent in Japan.
THE CURRENT OUTBREAK
The current outbreak is with Zaire ebolavirus. It seems to have started in a 2-year-old child who died in Meliandou in Guéckédou Prefecture, Guinea, on December 6, 2013. On March 21, 2014, the Guinea Ministry of Health reported the outbreak of an illness characterized by fever, severe diarrhea, vomiting, and a high case-fatality rate (59%) in 49 persons. On May 25, 2014, Kenema Government Hospital confirmed the first case of Ebola virus disease in Sierra Leone, probably brought there by a traditional healer who had treated Ebola patients from Guinea. Tracing led to 13 additional cases—all women who attended the burial.13
The Center for Systems Biology at Harvard University and the Broad Institute of Massachusetts Institute of Technology generated 99 Ebola virus genome sequences from 78 patients with confirmed disease, representing more than 70% of the patients diagnosed with the disease in Sierra Leone from May to mid-June 2014. They found genetic similarity across the sequenced 2014 samples, suggesting a single transmission from the natural reservoir, followed by human-to-human transmission during the outbreak. Continued human-reservoir exposure is unlikely to have contributed to the growth of this epidemic.14
As of October 14, 2014, there were 8,914 suspected and confirmed cases of Ebola virus infection, and 4,477 deaths.15
But how did Zaire Ebola virus make the 2,000-mile trek from Central Africa to Guinea in West Africa? There are two possibilities: it has always been present in the region but we just never noticed, or it was recently introduced. Bayesian phylogenetic analyses and sequence divergence studies suggest the virus has been present in bat populations in Guinea without previously infecting humans.
Why Guinea and why Guéckédou? Guinea is one of the poorest countries in the world, ranking 178th of 187 countries on the Human Development Index of the United Nations Development Programme, just behind Liberia (174th) and Sierra Leone (177th). In Guinea, the life expectancy is 56 years and the gross national income per capita is $440. The region has been systematically plundered and the forest decimated by clear-cut logging, leaving the Guinea Forest Region largely deforested, resulting in increased contact between humans and the small animals that serve as the source of infection.1
LIMITED CAPACITY, EVEN IN THE UNITED STATES
A few hospitals in the United States have dedicated units to handle serious infectious diseases such as Ebola: Emory University Hospital; Nebraska Medicine in Omaha; Providence St. Patrick Hospital in Missoula, MT; and the National Institutes of Health in Bethesda, MD. However, in total they have only 19 beds.
QUESTIONS, ANSWERS—AND MORE QUESTIONS
(The following is from a question-and-answer discussion that followed Dr. Brizendine’s Grand Rounds presentation.)
Q: Are there any differences between survivors and those who die of the disease? A: We do not know. Patient survival depends on early recognition and supportive care. There are disparities in the care of patients. Schieffelin et al16 analyzed the characteristics of patients who died or who survived in Sierra Leone and found that the mortality rate was higher in older patients and those with a higher viral load on presentation.
Q: Does the virus block production or release of interferon early in infection? A: Yes, it has been shown17 that Ebola virus protein VP24 inhibits signaling downstream of both interferon alpha/beta and interferon gamma by indirectly impairing the transport of a transcription factor termed STAT1. VP24 is also able to bind STAT1 directly. The resulting suppression of host interferon very early on in the incubation phase is key to the virulence of the virus.
Q: Does infection with one of the viral species confer immunity from other species? A: No, there is no cross-immunity.
Q: How soon do patients test positive? A: About 5 days after exposure, when they develop a fever. At this time patients are highly viremic, which PCR can detect.
Q: Before the virus is detectable in the blood, where is it? A: The liver, endothelial cells, antigen-presenting cells, and adrenal glands.
Q: Do we really need to quarantine ill patients and health care workers returning from Africa, per CDC recommendations? A: We don’t know everything, and some people do make bad decisions, such as traveling while symptomatic. I support a period of observation, although confinement is not reasonable, as it may pose a disincentive to cooperation.
Q: What is the role of giving plasma from survivors? A: Dr. Kent Brantly (see American citizens infected with Ebola) received the blood of a 14-year-old who survived. We don’t know. It is not proved. It did not result in improvement in animal models.
Q: Is the bleeding caused by a mechanism similar to that in enterohemorrhagic Escherichia coli infection? A: No. That is a bacterial toxin, whereas this is more like disseminated intravascular coagulation, with an intrinsic pathway anticoagulation cascade.
Q: How long does the virus remain viable outside the body? A: In one study,18 Ebola virus could not be recovered from experimentally contaminated surfaces (plastic, metal or glass) at room temperature. In another in which it was dried onto a surface,19 Ebola virus survived in the dark for several hours between 20 and 25°C. When dried in tissue culture media onto glass and stored at 4°C, it has survived for over 50 days.
Q: How long does the virus remain in breast milk? A: We know it has been detected 15 days after disease onset and think possibly as late as 28 days from symptom onset.3
Q: How are people actually infected? A: I believe people get the virus on their hands and then touch their face, eyes, or mouth. If you are wearing personal protective equipment, it must occur while doffing the equipment.
Q: Could we increase the sensitivity of the test so that we could detect the virus before the onset of symptoms? A: In theory it may be possible. The virus is somewhere in the body during the incubation period. Perhaps we could sample the right compartment in an enriched mononuclear cell line.
Q: When can patients who recover resume their normal activities? A: After their viral load returns to 0, I would still advise abstaining from unprotected sex and from breastfeeding for a few months. but as for other activities, no special precautions are needed.
Q: Does the virus appear to be mutating at a high rate? A: Looking back to 2004, mutations are occurring, but there is no sign that any of these mutations has contributed to the size of the outbreak by changing the characteristics of the Ebola virus. Can it become aerosolized? It has been suggested that the virus that caused the outbreak separated from those that caused past Ebola outbreaks but does not seem to be affecting the spread or efficacy of experimental drugs and vaccines. So, even though it is an RNA virus and mutations are occurring, no serious changes have emerged.14
BACK TO OUR PATIENT
The differential diagnosis for the patient described at the beginning of this paper includes travelers’ diarrhea, malaria, typhoid fever, yellow fever, meningococcal disease … and Ebola virus disease, although this is much less likely in view of the epidemiology and incubation period of this disease. When his stool was tested by enzyme immunoassay and culture, it was found to be positive for Campylobacter. He recovered with oral rehydration.
A 50-year-old man who returned from a business trip to Nigeria 24 days ago presents with complaints of the sudden onset of fever, diarrhea, myalgia, and headache. He reports 10 bowel movements per day and has seen bloody stools.
During his trip he flew in to Murtala Muhammed International Airport in Lagos, ate meals only in his hotel, and attended meetings in Lagos central business district. He had no exposure to animals, mosquitoes, ticks, or sick people, and no sexual activity. After returning home, he felt well for the first 3 weeks.
The patient has a history of hypertension. He does not smoke, drink alcohol, or use injection drugs. He is married, works with commercial banks and financial institutions, and lives in Cleveland, OH.
On physical examination his temperature is 100.0˚F (37.8˚C), pulse 98, respirations 15, blood pressure 105/70 mm Hg, and weight 78 kg (172 lb). He appears comfortable but is a little diaphoretic. His abdomen is tender to palpation in the epigastrium and slightly to the right; he has no signs of peritonitis. His skin is without rash, bleeding, or bruising. The remainder of the examination is normal.
His white blood cell count is 17 × 109/L, hemoglobin 15 g/dL, hematocrit 41%, and platelet count 172 × 109/L. His sodium level is 126 mmol/L, potassium 3.8 mmol/L, chloride 95 mmol/L, carbon dioxide 20 mmol/L, blood urea nitrogen 11 mg/dL, creatinine 0.7 mg/dL, and glucose 130 mg/dL. His aminotransferase and alkaline phosphatase levels are normal.
Could this patient have Ebola virus disease?
With Ebola virus disease on the rise in West Africa, physicians who encounter patients like this one need to include it in the differential diagnosis. Because the disease is new, many questions are raised for which we as yet have no answers. Here, I will review what we know and do not know in an effort to remove some of the fear and uncertainty.
A NEW DISEASE
Ebola virus disease is a severe hemorrhagic fever caused by negative-sense single-stranded RNA viruses classified by the International Committee on Taxonomy of Viruses as belonging to the genus Ebolavirus in the family Filoviridae. Filoviruses get their name from the Latin filum, or thread-like structure.
The family Filoviridae was discovered in 1967 after inadvertent importation of infected monkeys from Uganda into Yugoslavia and Marburg, Germany. Outbreaks of severe illness occurred in workers at a vaccine plant who came into direct contact with the animals by killing them, removing their kidneys, or preparing primary cell cultures for polio vaccine production.
Ebola virus was discovered in 1976 by Peter Piot, who was working at the Institute of Tropical Medicine in Antwerp, Belgium. The blood of a Belgian woman who had been working in what is now the Democratic Republic of the Congo (formerly Zaire) had been sent to the institute; she and Mabalo Lokela, a school headmaster and the first recorded victim of Ebola virus, had been working near Yambuku, about 96 km from the Ebola River.
Before the 2014 outbreak, all known outbreaks had caused fewer than 2,400 cases across a dozen African countries over 3 decades.
Five species of Ebola virus
The genus Ebolavirus contains five species, each associated with a consistent case-fatality rate and a more or less well-identified endemic area.1
Zaire ebolavirus was recognized in 1976; it has caused multiple outbreaks, with high case-fatality rates.
Sudan ebolavirus was seen first in the 1970s; it has a 50% case-fatality rate.
Tai Forest ebolavirus has been found in only one person, an ethologist working with deceased chimpanzees.
Bundibugyo ebolavirus emerged in 2007 and has a 30% case-fatality rate.
Reston ebolavirus is maintained in an animal reservoir in the Philippines and is not found in Africa. It caused an outbreak of lethal infection in macaques imported into the United States in 1989. There is evidence that Reston ebolavirus can cause asymptomatic infection in humans. None of the caretakers of the macaques became ill, nor did farmers working with infected pigs, although both groups seroconverted.
A reservoir in bats?
A reservoir in nonhuman primates was initially suspected. However, studies subsequently showed that monkeys are susceptible to rapidly lethal filoviral disease, precluding any role as a host for persistent viral infection. It is likely that Ebola virus is maintained in small animals that serve as a source of infection for both humans and wild primates. A prominent suspect is fruit bats, which are consumed in soup in West Africa.
Transmission is person-to-person or nosocomial
Ebola virus is transmitted by direct contact with body fluids such as blood, urine, sweat, vomitus, semen, and breast milk. Filoviruses can initiate infection via ingestion, inhalation (although probably not Ebola), or passage through breaks in the skin. Droplet inoculation into the mouth or eyes has been shown to result from inadvertent transfer of virus from contaminated hands. Patients transmit the virus while febrile and through later stages of disease, as well as postmortem through contact with the body during funeral preparations. The virus has been isolated in semen for as many as 61 days after illness onset.
Ebola virus can also be spread nosocomially. In 1976, a 44-year-old teacher sought care for fever at the Yambuku Mission Hospital. He was given parenteral chloroquine as empiric treatment for presumed malaria, which was routine for all febrile patients. However, he had unrecognized Ebola virus infection. Moreover, syringes were rinsed in the same pan of water and reused, which spread the infection to nearly 100 people, all of whom developed fulminant Ebola virus disease and died. Infection then spread to family caregivers, the hospital staff, and those who prepared the bodies for burial.
Nosocomial transmission was also responsible for an outbreak of Lake Victoria Marburg virus in Uige Province in northern Angola in 2005, with 374 putative cases and 329 deaths. When teams from Médecins Sans Frontières started setting up the Marburg ward, there were five patients with hemorrhagic fever in a makeshift isolation room in the hospital, together with corpses that the hospital staff had been too afraid to remove. Healers found in many rural African communities were administering injections in homes or in makeshift clinics with reused needles or syringes.2
There is no evidence that filoviruses are carried by mosquitoes or other biting arthropods. Also, the risk of transmission via fomites appears to be low when currently recommended infection-control guidelines for the viral hemorrhagic fevers are followed.3 One primary human case generates only one to three secondary cases on average.
EBOLA IS AN IMMUNODEFICIENCY VIRUS
The main targets of infection are endothelial cells, mononuclear phagocytes, and hepatocytes. Ebola virus replicates at an unusually high rate. Macrophages infected with Zaire ebolavirus produce tumor necrosis factor alpha, interleukin (IL) 1 beta, IL-6, macrophage chemotactic protein 1, and nitric oxide. Virus-infected macrophages synthesize cell-surface tissue factor, triggering the extrinsic coagulation pathway.
Ebola is an immunodeficiency virus. Dendritic cells, which initiate adaptive immune responses, are a major site of filoviral replication. Infected cells cannot present antigens to naïve lymphocytes. Patients who die of Ebola virus disease do not develop antibodies to the virus. Lymphocytes remain uninfected, but undergo “bystander” apoptosis induced by inflammatory mediators.
CLINICAL MANIFESTATIONS
The incubation period is generally 5 to 7 days (range 2 to 28 days), during which the patient is not infectious. Symptoms begin abruptly, with fever, chills, general malaise, weakness, severe headache, and myalgia. By the time of case detection in West Africa, most patients also had nausea, vomiting, diarrhea, and abdominal pain. Once symptoms arise, patients have high levels of the virus in their blood and fluids and are infectious. Hemorrhagic symptoms have apparently been uncommon in West Africa, occurring in 1.0% to 5.7%, but “unexplained bleeding” has been documented in 18% of cases.4 Among those in whom the disease enters its hemorrhagic terminal phase, there is characteristic internal and subcutaneous bleeding, vomiting of blood, and subconjunctival hemorrhage.4
Laboratory findings include lymphocytopenia (often with counts as low as 1.0 × 109/L), thrombocytopenia (with counts in the range of 50 to 100 × 109/L), elevated aminotransferase levels (including aspartate aminotransferase levels 7 to 12 times higher than alanine aminotransferase in fatal cases), low total protein (due to capillary leak), and disseminated intravascular coagulation. Those who survive begin to improve in the second week, during which viremia resolves in association with the appearance of virus-specific antibodies.4
DIAGNOSIS
In symptomatic patients, Ebola virus infection is diagnosed by detection in blood or body fluids of viral antigens by enzyme-linked immunosorbent assay, or RNA sequences by reverse transcriptase polymerase chain reaction. The diagnosis is confirmed with cell culture (in a BSL-4 containment laboratory) showing characteristic viral particles by electron microscopy.
CARING FOR PATIENTS
The most detailed descriptions of the care of patients with Ebola virus disease have come from Dr. Bruce Ribner, of Emory University Hospital, in an October 2014 report of his experience caring for Ebola-infected patients at Emory University Hospital in Atlanta, GA.5 He described fluid losses of 5 to 10 L/day, profound hyponatremia, hypokalemia, and hypocalcemia, which were associated with cardiac arrhythmias and the need for intravenous and oral electrolyte repletion and hemodialysis. Intensive one-to-one nursing was critical, as was the coordination of many medical subspecialties. The Emory team arranged point-of-care testing near the unit and generally kept laboratory testing to a minimum. The team was surprised to learn that commercial carriers refused to transport specimens even when they were licensed for category A agents. Difficulties with the local water authority and waste disposal contractor required the hospital to dedicate an autoclave to process all materials used in clinical care.
TREATMENT: SUPPORTIVE AND EXPERIMENTAL
Treatment is supportive to maintain circulatory function and blood pressure and to correct coagulopathy. However, a variety of vaccines, antibodies, small-molecule agents, and antiviral agents are undergoing testing, mostly in animals at this point.
Vaccines. A therapeutic vaccine that worked only slightly was a live-attenuated recombinant vesicular stomatitis virus expressing Ebola virus transmembrane glycoproteins, which was tested in mice, guinea pigs, and rhesus macaques who had been exposed to Ebola virus.6
A preventive vaccine worked better. Stanley et al7 evaluated a replication-defective chimpanzee adenovirus 3-vectored vaccine that also contained Ebola virus glycoprotein. They gave macaques a single injection of this vaccine, and then 5 weeks later gave them a lethal dose of Ebola virus. All the vaccinated animals survived the infection, and half (2 of 4) survived when challenged 10 months later. With a prime-boost strategy (modified vaccinia virus Ankara, a poxvirus), all survived when challenged 10 months later.
KZ52, a neutralizing antibody, did not work. Oswald et al8 gave a human IgG monoclonal antibody against Zaire Ebola virus, designated KZ52, to four rhesus macaques, challenged them with the virus 24 hours later, and administered a second shot of KZ52 on day 4. All of them died.
ZMAb is a combination of three murine monoclonal antibodies, designated 1H3, 2G4, and 4G7. Ad-IFN is a human adenovirus, serotype 5, that expresses human interferon alpha. Qui et al9 gave ZMAb and Ad-IFN to macaques in several experiments. In experiment 1, eight macaques were infected and then were given ZMAb and Ad-IFN 3 days later, and ZMAb again on days 6 and 9. Seven of the eight survived. In a second experiment, Ad-IFN was given first, when the viral load was still less than the limit of detection of known assays, and then ZMAb was given upon detection of viremia and fever. Two of four macaques survived. Control animals had undetectable levels of IgG, whereas Ebola virus GP–specific IgG levels were detected in all survivors. IFN-gamma ELISpots showed high EBOV-GP–specific T-cell response in all survivors.
ZMapp is another cocktail of monoclonal antibodies, containing two from ZMab (2G4 and 4G7), plus a third, c13C6. In experiments in rhesus macaques, three groups of six animals each received three doses of ZMapp at varying times after being infected with Ebola virus: at 3, 6, and 9 days; at 4, 7, and 10 days, and at 5, 8, and 11 days. All 18 macaques treated with ZMapp survived. Thus, Zmapp extended the treatment window to 5 days postexposure.10 One of the American health care workers who contracted Ebola virus in Liberia received this medication.
HSPA5-PMO. Endoplasmic reticulum chaperone heat shock 70 kDa protein 5 (HSPA5) is instrumental in the maturation of envelope proteins in hepatitis C and influenza A virus. It plays a role in viral entry for coxsackievirus A9 and dengue virus serotype 2, and it may be involved in Ebola viral budding. Phosphorodiamidate morpholino oligomers (PMOs) are a class of antisense DNA nucleotide analogs.
Reid et al11 reported that mice treated with HSPA5–PMO were completely protected from lethal Ebola challenge. Therefore, HSPA5 appears to be a promising target for the development of antifilovirus countermeasures.
Favipiravir, an antiviral agent also known as T-705, is a pyrazinecarboxamide derivative. Invented in 2002 by Toyama Chemicals as an inhibitor of influenza virus replication, it acts as a nucleotide analog, selectively inhibiting the viral RNA-dependent RNA polymerase, or causes lethal mutagenesis upon incorporation into the virus RNA. Favipiravir suppresses Ebola virus replication by 4 log10 units in cell culture.12
Mice were challenged with intranasal inoculation of 1,000 focus-forming units of Ebola virus diluted in phosphate-buffered saline. Until the first day of treatment (postinfection day 6), all mice in the T-705 group lost weight similarly to control mice, developed viremia, and showed elevated serum levels of aspartate aminotransferase and alanine aminotransferase. Within 4 days of T-705 treatment (post-infection day 10), the animals had cleared the virus from blood. Surviving mice developed Ebola virus-specific antibodies and CD8+ T cells specific for the viral nucleoprotein.12
The authors hypothesized that suppression of virus replication by T-705 allowed the host to mount a virus-specific adaptive immune response, and concluded that T-705 was 100% effective in the treatment of Zaire Ebola virus infection up to postinfection day 6 but was hardly beneficial at the terminal stage of disease.12 Of note, favipiravir is undergoing phase 2 and phase 3 trials as an anti-influenza agent in Japan.
THE CURRENT OUTBREAK
The current outbreak is with Zaire ebolavirus. It seems to have started in a 2-year-old child who died in Meliandou in Guéckédou Prefecture, Guinea, on December 6, 2013. On March 21, 2014, the Guinea Ministry of Health reported the outbreak of an illness characterized by fever, severe diarrhea, vomiting, and a high case-fatality rate (59%) in 49 persons. On May 25, 2014, Kenema Government Hospital confirmed the first case of Ebola virus disease in Sierra Leone, probably brought there by a traditional healer who had treated Ebola patients from Guinea. Tracing led to 13 additional cases—all women who attended the burial.13
The Center for Systems Biology at Harvard University and the Broad Institute of Massachusetts Institute of Technology generated 99 Ebola virus genome sequences from 78 patients with confirmed disease, representing more than 70% of the patients diagnosed with the disease in Sierra Leone from May to mid-June 2014. They found genetic similarity across the sequenced 2014 samples, suggesting a single transmission from the natural reservoir, followed by human-to-human transmission during the outbreak. Continued human-reservoir exposure is unlikely to have contributed to the growth of this epidemic.14
As of October 14, 2014, there were 8,914 suspected and confirmed cases of Ebola virus infection, and 4,477 deaths.15
But how did Zaire Ebola virus make the 2,000-mile trek from Central Africa to Guinea in West Africa? There are two possibilities: it has always been present in the region but we just never noticed, or it was recently introduced. Bayesian phylogenetic analyses and sequence divergence studies suggest the virus has been present in bat populations in Guinea without previously infecting humans.
Why Guinea and why Guéckédou? Guinea is one of the poorest countries in the world, ranking 178th of 187 countries on the Human Development Index of the United Nations Development Programme, just behind Liberia (174th) and Sierra Leone (177th). In Guinea, the life expectancy is 56 years and the gross national income per capita is $440. The region has been systematically plundered and the forest decimated by clear-cut logging, leaving the Guinea Forest Region largely deforested, resulting in increased contact between humans and the small animals that serve as the source of infection.1
LIMITED CAPACITY, EVEN IN THE UNITED STATES
A few hospitals in the United States have dedicated units to handle serious infectious diseases such as Ebola: Emory University Hospital; Nebraska Medicine in Omaha; Providence St. Patrick Hospital in Missoula, MT; and the National Institutes of Health in Bethesda, MD. However, in total they have only 19 beds.
QUESTIONS, ANSWERS—AND MORE QUESTIONS
(The following is from a question-and-answer discussion that followed Dr. Brizendine’s Grand Rounds presentation.)
Q: Are there any differences between survivors and those who die of the disease? A: We do not know. Patient survival depends on early recognition and supportive care. There are disparities in the care of patients. Schieffelin et al16 analyzed the characteristics of patients who died or who survived in Sierra Leone and found that the mortality rate was higher in older patients and those with a higher viral load on presentation.
Q: Does the virus block production or release of interferon early in infection? A: Yes, it has been shown17 that Ebola virus protein VP24 inhibits signaling downstream of both interferon alpha/beta and interferon gamma by indirectly impairing the transport of a transcription factor termed STAT1. VP24 is also able to bind STAT1 directly. The resulting suppression of host interferon very early on in the incubation phase is key to the virulence of the virus.
Q: Does infection with one of the viral species confer immunity from other species? A: No, there is no cross-immunity.
Q: How soon do patients test positive? A: About 5 days after exposure, when they develop a fever. At this time patients are highly viremic, which PCR can detect.
Q: Before the virus is detectable in the blood, where is it? A: The liver, endothelial cells, antigen-presenting cells, and adrenal glands.
Q: Do we really need to quarantine ill patients and health care workers returning from Africa, per CDC recommendations? A: We don’t know everything, and some people do make bad decisions, such as traveling while symptomatic. I support a period of observation, although confinement is not reasonable, as it may pose a disincentive to cooperation.
Q: What is the role of giving plasma from survivors? A: Dr. Kent Brantly (see American citizens infected with Ebola) received the blood of a 14-year-old who survived. We don’t know. It is not proved. It did not result in improvement in animal models.
Q: Is the bleeding caused by a mechanism similar to that in enterohemorrhagic Escherichia coli infection? A: No. That is a bacterial toxin, whereas this is more like disseminated intravascular coagulation, with an intrinsic pathway anticoagulation cascade.
Q: How long does the virus remain viable outside the body? A: In one study,18 Ebola virus could not be recovered from experimentally contaminated surfaces (plastic, metal or glass) at room temperature. In another in which it was dried onto a surface,19 Ebola virus survived in the dark for several hours between 20 and 25°C. When dried in tissue culture media onto glass and stored at 4°C, it has survived for over 50 days.
Q: How long does the virus remain in breast milk? A: We know it has been detected 15 days after disease onset and think possibly as late as 28 days from symptom onset.3
Q: How are people actually infected? A: I believe people get the virus on their hands and then touch their face, eyes, or mouth. If you are wearing personal protective equipment, it must occur while doffing the equipment.
Q: Could we increase the sensitivity of the test so that we could detect the virus before the onset of symptoms? A: In theory it may be possible. The virus is somewhere in the body during the incubation period. Perhaps we could sample the right compartment in an enriched mononuclear cell line.
Q: When can patients who recover resume their normal activities? A: After their viral load returns to 0, I would still advise abstaining from unprotected sex and from breastfeeding for a few months. but as for other activities, no special precautions are needed.
Q: Does the virus appear to be mutating at a high rate? A: Looking back to 2004, mutations are occurring, but there is no sign that any of these mutations has contributed to the size of the outbreak by changing the characteristics of the Ebola virus. Can it become aerosolized? It has been suggested that the virus that caused the outbreak separated from those that caused past Ebola outbreaks but does not seem to be affecting the spread or efficacy of experimental drugs and vaccines. So, even though it is an RNA virus and mutations are occurring, no serious changes have emerged.14
BACK TO OUR PATIENT
The differential diagnosis for the patient described at the beginning of this paper includes travelers’ diarrhea, malaria, typhoid fever, yellow fever, meningococcal disease … and Ebola virus disease, although this is much less likely in view of the epidemiology and incubation period of this disease. When his stool was tested by enzyme immunoassay and culture, it was found to be positive for Campylobacter. He recovered with oral rehydration.
- Bausch DG, Schwarz L. Outbreak of ebola virus disease in Guinea: where ecology meets economy. PLoS Negl Trop Dis 2014; 8:e3056.
- Roddy P, Thomas SL, Jeffs B, et al. Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige, Angola. J Infect Dis 2010; 201:1909–1918.
- Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196(suppl 2):S142–S147.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–1495.
- Ribner BS. Treating patients with Ebola virus infections in the US: lessons learned. Presented at IDWeek, October 8, 2014. Philadelphia PA.
- Feldman H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2.
- Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–1129.
- Oswald WB, Geisbert TW, Davis KJ, et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLos Pathog 2007; 3:e9.
- Qui X, Wong G, Fernando L, et al. mAbs and Ad-vectored IFN-a therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med 2013; 5:207ra143.
- Qui X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53.
- Reid SP, Shurtleff AC, Costantino JA, et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 2014; 109:171–174.
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 2014; 105:17–21.
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus dsease in Guinea. N Engl J Med 2014; 371:1418–1425.
- Gire SK, Goba A, Andersen KG, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014; 345:1369–1372.
- Chamary JV. 4000 deaths and counting: the Ebola epidemic in 4 charts. Forbes. http://www.forbes.com/sites/jvchamary/2014/10/13/ebola-trends. Accessed November 5, 2014.
- Schieffelin JS, Shaffer JG, Goba A, et al, for the KGH Lassa Fever Program, the Viral Hemorrhagic Fever Consortium, and the WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014 Oct 29 [Epub ahead of print]. DOI: 10.1056/NEJMoa1411680.
- Zhang AP, Bornholdt ZA, Liu T, et al. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog 2012; 8:e1002550.
- Piercy TJ, Smither SJ, Steward JA, Eastaugh L, Lever MS. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol 2010; 109:1531–1539.
- Sagripanti JL, Rom AM, Holland LE. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol 2010; 155:2035–2039.
- Bausch DG, Schwarz L. Outbreak of ebola virus disease in Guinea: where ecology meets economy. PLoS Negl Trop Dis 2014; 8:e3056.
- Roddy P, Thomas SL, Jeffs B, et al. Factors associated with Marburg hemorrhagic fever: analysis of patient data from Uige, Angola. J Infect Dis 2010; 201:1909–1918.
- Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196(suppl 2):S142–S147.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–1495.
- Ribner BS. Treating patients with Ebola virus infections in the US: lessons learned. Presented at IDWeek, October 8, 2014. Philadelphia PA.
- Feldman H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2.
- Stanley DA, Honko AN, Asiedu C, et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–1129.
- Oswald WB, Geisbert TW, Davis KJ, et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLos Pathog 2007; 3:e9.
- Qui X, Wong G, Fernando L, et al. mAbs and Ad-vectored IFN-a therapy rescue Ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med 2013; 5:207ra143.
- Qui X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53.
- Reid SP, Shurtleff AC, Costantino JA, et al. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 2014; 109:171–174.
- Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 2014; 105:17–21.
- Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus dsease in Guinea. N Engl J Med 2014; 371:1418–1425.
- Gire SK, Goba A, Andersen KG, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014; 345:1369–1372.
- Chamary JV. 4000 deaths and counting: the Ebola epidemic in 4 charts. Forbes. http://www.forbes.com/sites/jvchamary/2014/10/13/ebola-trends. Accessed November 5, 2014.
- Schieffelin JS, Shaffer JG, Goba A, et al, for the KGH Lassa Fever Program, the Viral Hemorrhagic Fever Consortium, and the WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014 Oct 29 [Epub ahead of print]. DOI: 10.1056/NEJMoa1411680.
- Zhang AP, Bornholdt ZA, Liu T, et al. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog 2012; 8:e1002550.
- Piercy TJ, Smither SJ, Steward JA, Eastaugh L, Lever MS. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol 2010; 109:1531–1539.
- Sagripanti JL, Rom AM, Holland LE. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch Virol 2010; 155:2035–2039.
KEY POINTS
- Ebola virus is spread by contact with body fluids, with no evidence to date that it is airborne.
- Ebola virus is likely maintained in a reservoir of small animals, possibly bats.
- The incubation period is about 5 to 7 days, during which the patient is not infectious.
- Symptoms begin abruptly, with fever, chills, and general malaise, which in some patients leads to weakness, severe headache, myalgia, nausea, vomiting, diarrhea, and abdominal pain.
- Once the disease is symptomatic, patients have high levels of virus in the blood and other body fluids and are therefore infectious.
- Survivors show improvement in the second week of illness, during which viremia resolves and virus-specific antibodies appear.
David Henry's JCSO podcast, November 2014
Among the items featured in Dr David Henry’s monthly podcast for The Journal of Community and Supportive Oncology, are reports on congestive heart failure during induction with anthracycline-based therapy in patients with acute promyelocytic leukemia and on the impact of aprepitant on emesis control, dose intensity, and recurrence-free survival in head and neck cancer patients on cisplatin chemotherapy. Two articles focus on patient quality of life: one examines peripheral neuropathy and its impact on QoL after chemotherapy and another looks at QoL and symptoms after stereotactic body radiotherapy in early-stage lung cancer. There’s also a Case Report about a patient with superior vena cava syndrome as an initial presentation of low-grade follicular lymphoma, a feature article on choice of anesthesia during cancer surgery and patient outcomes, and a comprehensive and informative round-up of ASCO’s 2013-2014 guideline releases, updates, and endorsements.
Among the items featured in Dr David Henry’s monthly podcast for The Journal of Community and Supportive Oncology, are reports on congestive heart failure during induction with anthracycline-based therapy in patients with acute promyelocytic leukemia and on the impact of aprepitant on emesis control, dose intensity, and recurrence-free survival in head and neck cancer patients on cisplatin chemotherapy. Two articles focus on patient quality of life: one examines peripheral neuropathy and its impact on QoL after chemotherapy and another looks at QoL and symptoms after stereotactic body radiotherapy in early-stage lung cancer. There’s also a Case Report about a patient with superior vena cava syndrome as an initial presentation of low-grade follicular lymphoma, a feature article on choice of anesthesia during cancer surgery and patient outcomes, and a comprehensive and informative round-up of ASCO’s 2013-2014 guideline releases, updates, and endorsements.
Among the items featured in Dr David Henry’s monthly podcast for The Journal of Community and Supportive Oncology, are reports on congestive heart failure during induction with anthracycline-based therapy in patients with acute promyelocytic leukemia and on the impact of aprepitant on emesis control, dose intensity, and recurrence-free survival in head and neck cancer patients on cisplatin chemotherapy. Two articles focus on patient quality of life: one examines peripheral neuropathy and its impact on QoL after chemotherapy and another looks at QoL and symptoms after stereotactic body radiotherapy in early-stage lung cancer. There’s also a Case Report about a patient with superior vena cava syndrome as an initial presentation of low-grade follicular lymphoma, a feature article on choice of anesthesia during cancer surgery and patient outcomes, and a comprehensive and informative round-up of ASCO’s 2013-2014 guideline releases, updates, and endorsements.
Health Canada expands indication for apixaban

Credit: Andre E.X. Brown
Health Canada has expanded the indication for the oral anticoagulant apixaban (Eliquis).
The direct factor Xa inhibitor can now be used to prevent venous thromboembolism (VTE) in adult patients who have undergone elective total hip surgery or knee replacement surgery.
The drug was already approved in Canada to treat and prevent recurrent VTE and for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
“The development of VTE or pulmonary embolism is an important risk for patients having major orthopedic surgery such as total knee or hip replacement,” said John Eikelboom, MBBS, of McMaster University in Hamilton, Ontario.
“The approval of apixaban gives Canadian surgeons a new option to help prevent VTE in these patients. As an oral option for patients in hospital and once they return home after surgery, where most clotting complications can take place after they are discharged from hospital, apixaban offers patients an alternative to an injected anticoagulant.”
Health Canada’s approval of apixaban is based on results of the ADVANCE clinical trials. In these trials, researchers randomized 11,659 patients and assessed the efficacy and safety of apixaban compared to enoxaparin.
The primary efficacy endpoint of the trials was the composite of asymptomatic and symptomatic deep vein thrombosis (DVT), nonfatal pulmonary embolism (PE), and death from any cause during study treatment. The principal safety measure was the composite of major and clinically relevant nonmajor bleeding.
Results of the first ADVANCE study suggested apixaban was roughly as effective as enoxaparin at preventing DVT and PE in patients who had undergone total knee replacement surgery. But apixaban posed a significantly lower risk of major and nonmajor bleeding.
The ADVANCE-2 study, on the other hand, indicated that apixaban was a more effective means of thromboprophylaxis than enoxaparin in this patient population. And there was no significant difference between the treatment arms in the frequency of major or clinically relevant bleeding.
The ADVANCE-3 study suggested apixaban was more effective than enoxaparin in preventing DVT and PE among patients undergoing hip replacement. And there was no significant difference between the groups with regard to major or clinically relevant bleeding.
Apixaban is under development in Canada by Bristol-Myers Squibb and Pfizer Canada Inc. For more details on the drug, see the product monograph. ![]()

Credit: Andre E.X. Brown
Health Canada has expanded the indication for the oral anticoagulant apixaban (Eliquis).
The direct factor Xa inhibitor can now be used to prevent venous thromboembolism (VTE) in adult patients who have undergone elective total hip surgery or knee replacement surgery.
The drug was already approved in Canada to treat and prevent recurrent VTE and for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
“The development of VTE or pulmonary embolism is an important risk for patients having major orthopedic surgery such as total knee or hip replacement,” said John Eikelboom, MBBS, of McMaster University in Hamilton, Ontario.
“The approval of apixaban gives Canadian surgeons a new option to help prevent VTE in these patients. As an oral option for patients in hospital and once they return home after surgery, where most clotting complications can take place after they are discharged from hospital, apixaban offers patients an alternative to an injected anticoagulant.”
Health Canada’s approval of apixaban is based on results of the ADVANCE clinical trials. In these trials, researchers randomized 11,659 patients and assessed the efficacy and safety of apixaban compared to enoxaparin.
The primary efficacy endpoint of the trials was the composite of asymptomatic and symptomatic deep vein thrombosis (DVT), nonfatal pulmonary embolism (PE), and death from any cause during study treatment. The principal safety measure was the composite of major and clinically relevant nonmajor bleeding.
Results of the first ADVANCE study suggested apixaban was roughly as effective as enoxaparin at preventing DVT and PE in patients who had undergone total knee replacement surgery. But apixaban posed a significantly lower risk of major and nonmajor bleeding.
The ADVANCE-2 study, on the other hand, indicated that apixaban was a more effective means of thromboprophylaxis than enoxaparin in this patient population. And there was no significant difference between the treatment arms in the frequency of major or clinically relevant bleeding.
The ADVANCE-3 study suggested apixaban was more effective than enoxaparin in preventing DVT and PE among patients undergoing hip replacement. And there was no significant difference between the groups with regard to major or clinically relevant bleeding.
Apixaban is under development in Canada by Bristol-Myers Squibb and Pfizer Canada Inc. For more details on the drug, see the product monograph. ![]()

Credit: Andre E.X. Brown
Health Canada has expanded the indication for the oral anticoagulant apixaban (Eliquis).
The direct factor Xa inhibitor can now be used to prevent venous thromboembolism (VTE) in adult patients who have undergone elective total hip surgery or knee replacement surgery.
The drug was already approved in Canada to treat and prevent recurrent VTE and for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
“The development of VTE or pulmonary embolism is an important risk for patients having major orthopedic surgery such as total knee or hip replacement,” said John Eikelboom, MBBS, of McMaster University in Hamilton, Ontario.
“The approval of apixaban gives Canadian surgeons a new option to help prevent VTE in these patients. As an oral option for patients in hospital and once they return home after surgery, where most clotting complications can take place after they are discharged from hospital, apixaban offers patients an alternative to an injected anticoagulant.”
Health Canada’s approval of apixaban is based on results of the ADVANCE clinical trials. In these trials, researchers randomized 11,659 patients and assessed the efficacy and safety of apixaban compared to enoxaparin.
The primary efficacy endpoint of the trials was the composite of asymptomatic and symptomatic deep vein thrombosis (DVT), nonfatal pulmonary embolism (PE), and death from any cause during study treatment. The principal safety measure was the composite of major and clinically relevant nonmajor bleeding.
Results of the first ADVANCE study suggested apixaban was roughly as effective as enoxaparin at preventing DVT and PE in patients who had undergone total knee replacement surgery. But apixaban posed a significantly lower risk of major and nonmajor bleeding.
The ADVANCE-2 study, on the other hand, indicated that apixaban was a more effective means of thromboprophylaxis than enoxaparin in this patient population. And there was no significant difference between the treatment arms in the frequency of major or clinically relevant bleeding.
The ADVANCE-3 study suggested apixaban was more effective than enoxaparin in preventing DVT and PE among patients undergoing hip replacement. And there was no significant difference between the groups with regard to major or clinically relevant bleeding.
Apixaban is under development in Canada by Bristol-Myers Squibb and Pfizer Canada Inc. For more details on the drug, see the product monograph. ![]()