User login
Ponatinib approved in Australia
The Therapeutic Goods Administration (TGA) has approved marketing of ponatinib (Iclusig) in Australia.
The drug is now approved to treat adults with chronic myeloid leukemia (CML) who could not tolerate or were resistant to at least 2 prior tyrosine kinase inhibitors (TKIs), or those patients with a T315I mutation.
Ponatinib is also approved to treat certain adults with Philadelphia-chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).
These patients must be resistant to or intolerant of dasatinib and ineligible for treatment with imatinib, or they must have a T315I mutation.
Ponatinib is the only TKI approved in Australia for an indication that includes CML and Ph+ ALL patients with the T315I mutation.
The drug’s developers, Ariad Pharmaceuticals, Inc., and Specialized Therapeutics Australia Pty. Ltd., expect to launch ponatinib in early 2015.
The PACE trial
The TGA’s decision to approve ponatinib was based on results from the phase 2 PACE trial, which included patients with CML or Ph+ ALL who were resistant to or intolerant of prior TKI therapy, or who had the T315I mutation.
The median follow-up times were 15.3 months in chronic-phase CML patients, 15.8 months in accelerated-phase CML patients, and 6.2 months in patients with blast-phase CML or Ph+ ALL.
In chronic-phase CML, the primary endpoint was major cytogenetic response, and it occurred in 56% of patients. Among chronic-phase patients with the T315I mutation, 70% achieved a major cytogenetic response. Among patients who had failed treatment with dasatinib or nilotinib, 51% achieved a major cytogenetic response.
In accelerated-phase CML, the primary endpoint was major hematologic response. This occurred in 57% of all patients in this group, 50% of patients with the T315I mutation, and 58% of patients who had failed treatment with dasatinib or nilotinib.
The primary endpoint was major hematologic response in blast-phase CML/Ph+ ALL as well. Thirty-four percent of all patients in this group met this endpoint, as did 33% of patients with the T315I mutation and 35% of patients who had failed treatment with dasatinib or nilotinib.
Common non-hematologic adverse events included rash (38%), abdominal pain (38%), headache (35%), dry skin (35%), constipation (34%), fatigue (27%), pyrexia (27%), nausea (26%), arthralgia (25%), hypertension (21%), increased lipase (19%), and increased amylase (7%).
Hematologic events of any grade included thrombocytopenia (42%), neutropenia (24%), and anemia (20%). And serious adverse events of arterial thromboembolism, including arterial stenosis, occurred in patients with cardiovascular risk factors.
Safety concerns
Ponatinib is also approved to treat CML and Ph+ ALL in the European Union and the US. However, the drug was pulled from the US market for a little over 2 months, and ponatinib trials were placed on partial hold, after extended follow-up from the PACE trial showed an increase in thrombotic events.
The drug went back on the market last January, with new safety measures in place.
Ponatinib was not pulled from the market in the European Union, but the European Medicine’s Agency released recommendations for safer use of the drug. The Committee for Medicinal Products for Human Use reviewed data on ponatinib and decided the drug’s benefits outweigh its risks. ![]()

The Therapeutic Goods Administration (TGA) has approved marketing of ponatinib (Iclusig) in Australia.
The drug is now approved to treat adults with chronic myeloid leukemia (CML) who could not tolerate or were resistant to at least 2 prior tyrosine kinase inhibitors (TKIs), or those patients with a T315I mutation.
Ponatinib is also approved to treat certain adults with Philadelphia-chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).
These patients must be resistant to or intolerant of dasatinib and ineligible for treatment with imatinib, or they must have a T315I mutation.
Ponatinib is the only TKI approved in Australia for an indication that includes CML and Ph+ ALL patients with the T315I mutation.
The drug’s developers, Ariad Pharmaceuticals, Inc., and Specialized Therapeutics Australia Pty. Ltd., expect to launch ponatinib in early 2015.
The PACE trial
The TGA’s decision to approve ponatinib was based on results from the phase 2 PACE trial, which included patients with CML or Ph+ ALL who were resistant to or intolerant of prior TKI therapy, or who had the T315I mutation.
The median follow-up times were 15.3 months in chronic-phase CML patients, 15.8 months in accelerated-phase CML patients, and 6.2 months in patients with blast-phase CML or Ph+ ALL.
In chronic-phase CML, the primary endpoint was major cytogenetic response, and it occurred in 56% of patients. Among chronic-phase patients with the T315I mutation, 70% achieved a major cytogenetic response. Among patients who had failed treatment with dasatinib or nilotinib, 51% achieved a major cytogenetic response.
In accelerated-phase CML, the primary endpoint was major hematologic response. This occurred in 57% of all patients in this group, 50% of patients with the T315I mutation, and 58% of patients who had failed treatment with dasatinib or nilotinib.
The primary endpoint was major hematologic response in blast-phase CML/Ph+ ALL as well. Thirty-four percent of all patients in this group met this endpoint, as did 33% of patients with the T315I mutation and 35% of patients who had failed treatment with dasatinib or nilotinib.
Common non-hematologic adverse events included rash (38%), abdominal pain (38%), headache (35%), dry skin (35%), constipation (34%), fatigue (27%), pyrexia (27%), nausea (26%), arthralgia (25%), hypertension (21%), increased lipase (19%), and increased amylase (7%).
Hematologic events of any grade included thrombocytopenia (42%), neutropenia (24%), and anemia (20%). And serious adverse events of arterial thromboembolism, including arterial stenosis, occurred in patients with cardiovascular risk factors.
Safety concerns
Ponatinib is also approved to treat CML and Ph+ ALL in the European Union and the US. However, the drug was pulled from the US market for a little over 2 months, and ponatinib trials were placed on partial hold, after extended follow-up from the PACE trial showed an increase in thrombotic events.
The drug went back on the market last January, with new safety measures in place.
Ponatinib was not pulled from the market in the European Union, but the European Medicine’s Agency released recommendations for safer use of the drug. The Committee for Medicinal Products for Human Use reviewed data on ponatinib and decided the drug’s benefits outweigh its risks. ![]()

The Therapeutic Goods Administration (TGA) has approved marketing of ponatinib (Iclusig) in Australia.
The drug is now approved to treat adults with chronic myeloid leukemia (CML) who could not tolerate or were resistant to at least 2 prior tyrosine kinase inhibitors (TKIs), or those patients with a T315I mutation.
Ponatinib is also approved to treat certain adults with Philadelphia-chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).
These patients must be resistant to or intolerant of dasatinib and ineligible for treatment with imatinib, or they must have a T315I mutation.
Ponatinib is the only TKI approved in Australia for an indication that includes CML and Ph+ ALL patients with the T315I mutation.
The drug’s developers, Ariad Pharmaceuticals, Inc., and Specialized Therapeutics Australia Pty. Ltd., expect to launch ponatinib in early 2015.
The PACE trial
The TGA’s decision to approve ponatinib was based on results from the phase 2 PACE trial, which included patients with CML or Ph+ ALL who were resistant to or intolerant of prior TKI therapy, or who had the T315I mutation.
The median follow-up times were 15.3 months in chronic-phase CML patients, 15.8 months in accelerated-phase CML patients, and 6.2 months in patients with blast-phase CML or Ph+ ALL.
In chronic-phase CML, the primary endpoint was major cytogenetic response, and it occurred in 56% of patients. Among chronic-phase patients with the T315I mutation, 70% achieved a major cytogenetic response. Among patients who had failed treatment with dasatinib or nilotinib, 51% achieved a major cytogenetic response.
In accelerated-phase CML, the primary endpoint was major hematologic response. This occurred in 57% of all patients in this group, 50% of patients with the T315I mutation, and 58% of patients who had failed treatment with dasatinib or nilotinib.
The primary endpoint was major hematologic response in blast-phase CML/Ph+ ALL as well. Thirty-four percent of all patients in this group met this endpoint, as did 33% of patients with the T315I mutation and 35% of patients who had failed treatment with dasatinib or nilotinib.
Common non-hematologic adverse events included rash (38%), abdominal pain (38%), headache (35%), dry skin (35%), constipation (34%), fatigue (27%), pyrexia (27%), nausea (26%), arthralgia (25%), hypertension (21%), increased lipase (19%), and increased amylase (7%).
Hematologic events of any grade included thrombocytopenia (42%), neutropenia (24%), and anemia (20%). And serious adverse events of arterial thromboembolism, including arterial stenosis, occurred in patients with cardiovascular risk factors.
Safety concerns
Ponatinib is also approved to treat CML and Ph+ ALL in the European Union and the US. However, the drug was pulled from the US market for a little over 2 months, and ponatinib trials were placed on partial hold, after extended follow-up from the PACE trial showed an increase in thrombotic events.
The drug went back on the market last January, with new safety measures in place.
Ponatinib was not pulled from the market in the European Union, but the European Medicine’s Agency released recommendations for safer use of the drug. The Committee for Medicinal Products for Human Use reviewed data on ponatinib and decided the drug’s benefits outweigh its risks. ![]()
Protein inhibits HSC engraftment

in the bone marrow
Researchers say they have identified a protein that plays a key role in regulating hematopoietic stem cell (HSC) engraftment.
Experiments revealed that protein tyrosine phosphatase-sigma (PTP-sigma) suppresses normal HSC engraftment capacity, but targeted inhibition of
PTP-sigma can substantially improve mouse and human HSC engraftment.
The researchers described these findings in The Journal of Clinical Investigation.
Mamle Quarmyne, a graduate student at the University of California, Los Angeles, and her colleagues first found that PTP-sigma is expressed on a high percentage of mouse and human HSCs.
When the team deleted PTP-sigma in mice, they observed a marked increase in HSCs’ ability to engraft.
When they selected human HSCs that did not express PTP-sigma and transplanted these cells into immune-deficient mice, the researchers observed a 15-fold increase in HSC engraftment.
The team also discovered that PTP-sigma regulates HSC function by suppressing a protein called RAC1, which is known to promote HSC engraftment.
“These findings have tremendous therapeutic potential, since we have identified a new receptor on HSCs, PTP-sigma, which can be specifically targeted as a means to potently increase the engraftment of transplanted HSCs in patients,” said John P. Chute, MD, of the University of California, Los Angeles.
“This approach can also potentially accelerate hematologic recovery in cancer patients receiving chemotherapy and/or radiation, which also suppress the blood and immune systems.”
Now, the researchers are testing small molecules for their ability to inhibit PTP-sigma on HSCs. If these studies are successful, the team aims to translate these findings into clinical trials in the near future. ![]()

in the bone marrow
Researchers say they have identified a protein that plays a key role in regulating hematopoietic stem cell (HSC) engraftment.
Experiments revealed that protein tyrosine phosphatase-sigma (PTP-sigma) suppresses normal HSC engraftment capacity, but targeted inhibition of
PTP-sigma can substantially improve mouse and human HSC engraftment.
The researchers described these findings in The Journal of Clinical Investigation.
Mamle Quarmyne, a graduate student at the University of California, Los Angeles, and her colleagues first found that PTP-sigma is expressed on a high percentage of mouse and human HSCs.
When the team deleted PTP-sigma in mice, they observed a marked increase in HSCs’ ability to engraft.
When they selected human HSCs that did not express PTP-sigma and transplanted these cells into immune-deficient mice, the researchers observed a 15-fold increase in HSC engraftment.
The team also discovered that PTP-sigma regulates HSC function by suppressing a protein called RAC1, which is known to promote HSC engraftment.
“These findings have tremendous therapeutic potential, since we have identified a new receptor on HSCs, PTP-sigma, which can be specifically targeted as a means to potently increase the engraftment of transplanted HSCs in patients,” said John P. Chute, MD, of the University of California, Los Angeles.
“This approach can also potentially accelerate hematologic recovery in cancer patients receiving chemotherapy and/or radiation, which also suppress the blood and immune systems.”
Now, the researchers are testing small molecules for their ability to inhibit PTP-sigma on HSCs. If these studies are successful, the team aims to translate these findings into clinical trials in the near future. ![]()

in the bone marrow
Researchers say they have identified a protein that plays a key role in regulating hematopoietic stem cell (HSC) engraftment.
Experiments revealed that protein tyrosine phosphatase-sigma (PTP-sigma) suppresses normal HSC engraftment capacity, but targeted inhibition of
PTP-sigma can substantially improve mouse and human HSC engraftment.
The researchers described these findings in The Journal of Clinical Investigation.
Mamle Quarmyne, a graduate student at the University of California, Los Angeles, and her colleagues first found that PTP-sigma is expressed on a high percentage of mouse and human HSCs.
When the team deleted PTP-sigma in mice, they observed a marked increase in HSCs’ ability to engraft.
When they selected human HSCs that did not express PTP-sigma and transplanted these cells into immune-deficient mice, the researchers observed a 15-fold increase in HSC engraftment.
The team also discovered that PTP-sigma regulates HSC function by suppressing a protein called RAC1, which is known to promote HSC engraftment.
“These findings have tremendous therapeutic potential, since we have identified a new receptor on HSCs, PTP-sigma, which can be specifically targeted as a means to potently increase the engraftment of transplanted HSCs in patients,” said John P. Chute, MD, of the University of California, Los Angeles.
“This approach can also potentially accelerate hematologic recovery in cancer patients receiving chemotherapy and/or radiation, which also suppress the blood and immune systems.”
Now, the researchers are testing small molecules for their ability to inhibit PTP-sigma on HSCs. If these studies are successful, the team aims to translate these findings into clinical trials in the near future. ![]()
CAR T-cell therapy gets breakthrough designation

Credit: Charles Haymond
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for JCAR015, a chimeric antigen receptor (CAR) T-cell therapy, to treat patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
Breakthrough designation is designed to help accelerate the development and review of new drugs for serious or life-threatening conditions.
The designation comes with potential benefits, including intensive FDA guidance and eligibility for priority review. It is granted to applicants when preliminary clinical evidence indicates the drug may demonstrate substantial improvement over existing therapies on one or more clinical endpoints.
The FDA recently granted JCAR015 orphan designation to treat ALL as well.
JCAR015 consists of autologous T cells expressing a CD19-specific, CD28/CD3z CAR. The treatment has shown promise in an ongoing phase 1 trial of patients with B-cell ALL.
Initial results from this study were published in Science Translational Medicine last year and in February. Updated results were presented at the AACR Annual Meeting in April.
At that point, the researchers had enrolled 22 adult patients with relapsed or refractory B-ALL who were minimal residual disease-positive or were in first complete remission at enrollment. Patients in complete remission were monitored and only received JCAR015 if they relapsed.
The remaining patients received re-induction chemotherapy (physician’s choice), followed by an infusion of JCAR015. After treatment, patients could receive allogeneic transplant, a different salvage therapy, or monitoring.
Eighty-two percent of patients achieved a complete response to JCAR015. The average time to complete response was about 24.5 days.
Twelve of the responders were eligible for transplant. Of the 8 patients who ultimately underwent transplant and survived, 1 relapsed, but the rest remained in remission.
Ten patients had died at the time of the AACR presentation. Six deaths were a result of disease relapse or progression, and 2 patients died of complications from stem cell transplant.
The 2 remaining deaths prompted a temporary suspension of enrollment in this trial. Those deaths were related to complications from cytokine release syndrome. One patient died of cardiovascular disease, and the other died following persistent seizure activity.
So researchers at the Memorial Sloan-Kettering Cancer Center, where the trial is being conducted, reviewed these cases. The results prompted them to amend trial enrollment criteria and dosing recommendations. Now, patients with cardiac disease are ineligible to receive JCAR015.
And the T-cell dose a patient receives will depend on the extent of his or her disease. The hope is that this will reduce the risk of cytokine release syndrome and any resulting seizures. The researchers also noted that the monoclonal antibody tocilizumab has proven effective in treating cytokine release syndrome.
In addition to this trial, JCAR015 is under investigation at Memorial Sloan-Kettering Cancer Center in a phase 1 trial of patients with relapsed and refractory non-Hodgkin lymphoma.
Two other CAR T-cell product candidates under development by the same company, Juno Therapeutics, Inc., are being tested in clinical trials as well. JCAR017 is under investigation at Seattle Children’s Hospital for relapsed/refractory CD19-positive pediatric leukemia.
JCAR014 is being tested for refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, and ALL at the Fred Hutchinson Cancer Research Center. Data on these programs are set to be presented at the 56th ASH Annual Meeting next week in San Francisco. ![]()

Credit: Charles Haymond
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for JCAR015, a chimeric antigen receptor (CAR) T-cell therapy, to treat patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
Breakthrough designation is designed to help accelerate the development and review of new drugs for serious or life-threatening conditions.
The designation comes with potential benefits, including intensive FDA guidance and eligibility for priority review. It is granted to applicants when preliminary clinical evidence indicates the drug may demonstrate substantial improvement over existing therapies on one or more clinical endpoints.
The FDA recently granted JCAR015 orphan designation to treat ALL as well.
JCAR015 consists of autologous T cells expressing a CD19-specific, CD28/CD3z CAR. The treatment has shown promise in an ongoing phase 1 trial of patients with B-cell ALL.
Initial results from this study were published in Science Translational Medicine last year and in February. Updated results were presented at the AACR Annual Meeting in April.
At that point, the researchers had enrolled 22 adult patients with relapsed or refractory B-ALL who were minimal residual disease-positive or were in first complete remission at enrollment. Patients in complete remission were monitored and only received JCAR015 if they relapsed.
The remaining patients received re-induction chemotherapy (physician’s choice), followed by an infusion of JCAR015. After treatment, patients could receive allogeneic transplant, a different salvage therapy, or monitoring.
Eighty-two percent of patients achieved a complete response to JCAR015. The average time to complete response was about 24.5 days.
Twelve of the responders were eligible for transplant. Of the 8 patients who ultimately underwent transplant and survived, 1 relapsed, but the rest remained in remission.
Ten patients had died at the time of the AACR presentation. Six deaths were a result of disease relapse or progression, and 2 patients died of complications from stem cell transplant.
The 2 remaining deaths prompted a temporary suspension of enrollment in this trial. Those deaths were related to complications from cytokine release syndrome. One patient died of cardiovascular disease, and the other died following persistent seizure activity.
So researchers at the Memorial Sloan-Kettering Cancer Center, where the trial is being conducted, reviewed these cases. The results prompted them to amend trial enrollment criteria and dosing recommendations. Now, patients with cardiac disease are ineligible to receive JCAR015.
And the T-cell dose a patient receives will depend on the extent of his or her disease. The hope is that this will reduce the risk of cytokine release syndrome and any resulting seizures. The researchers also noted that the monoclonal antibody tocilizumab has proven effective in treating cytokine release syndrome.
In addition to this trial, JCAR015 is under investigation at Memorial Sloan-Kettering Cancer Center in a phase 1 trial of patients with relapsed and refractory non-Hodgkin lymphoma.
Two other CAR T-cell product candidates under development by the same company, Juno Therapeutics, Inc., are being tested in clinical trials as well. JCAR017 is under investigation at Seattle Children’s Hospital for relapsed/refractory CD19-positive pediatric leukemia.
JCAR014 is being tested for refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, and ALL at the Fred Hutchinson Cancer Research Center. Data on these programs are set to be presented at the 56th ASH Annual Meeting next week in San Francisco. ![]()

Credit: Charles Haymond
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for JCAR015, a chimeric antigen receptor (CAR) T-cell therapy, to treat patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
Breakthrough designation is designed to help accelerate the development and review of new drugs for serious or life-threatening conditions.
The designation comes with potential benefits, including intensive FDA guidance and eligibility for priority review. It is granted to applicants when preliminary clinical evidence indicates the drug may demonstrate substantial improvement over existing therapies on one or more clinical endpoints.
The FDA recently granted JCAR015 orphan designation to treat ALL as well.
JCAR015 consists of autologous T cells expressing a CD19-specific, CD28/CD3z CAR. The treatment has shown promise in an ongoing phase 1 trial of patients with B-cell ALL.
Initial results from this study were published in Science Translational Medicine last year and in February. Updated results were presented at the AACR Annual Meeting in April.
At that point, the researchers had enrolled 22 adult patients with relapsed or refractory B-ALL who were minimal residual disease-positive or were in first complete remission at enrollment. Patients in complete remission were monitored and only received JCAR015 if they relapsed.
The remaining patients received re-induction chemotherapy (physician’s choice), followed by an infusion of JCAR015. After treatment, patients could receive allogeneic transplant, a different salvage therapy, or monitoring.
Eighty-two percent of patients achieved a complete response to JCAR015. The average time to complete response was about 24.5 days.
Twelve of the responders were eligible for transplant. Of the 8 patients who ultimately underwent transplant and survived, 1 relapsed, but the rest remained in remission.
Ten patients had died at the time of the AACR presentation. Six deaths were a result of disease relapse or progression, and 2 patients died of complications from stem cell transplant.
The 2 remaining deaths prompted a temporary suspension of enrollment in this trial. Those deaths were related to complications from cytokine release syndrome. One patient died of cardiovascular disease, and the other died following persistent seizure activity.
So researchers at the Memorial Sloan-Kettering Cancer Center, where the trial is being conducted, reviewed these cases. The results prompted them to amend trial enrollment criteria and dosing recommendations. Now, patients with cardiac disease are ineligible to receive JCAR015.
And the T-cell dose a patient receives will depend on the extent of his or her disease. The hope is that this will reduce the risk of cytokine release syndrome and any resulting seizures. The researchers also noted that the monoclonal antibody tocilizumab has proven effective in treating cytokine release syndrome.
In addition to this trial, JCAR015 is under investigation at Memorial Sloan-Kettering Cancer Center in a phase 1 trial of patients with relapsed and refractory non-Hodgkin lymphoma.
Two other CAR T-cell product candidates under development by the same company, Juno Therapeutics, Inc., are being tested in clinical trials as well. JCAR017 is under investigation at Seattle Children’s Hospital for relapsed/refractory CD19-positive pediatric leukemia.
JCAR014 is being tested for refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, and ALL at the Fred Hutchinson Cancer Research Center. Data on these programs are set to be presented at the 56th ASH Annual Meeting next week in San Francisco. ![]()
What Matters: Salt substitutes to reduce blood pressure
Most of my counseling to patients about lowering blood pressure through self management is heavy on weight loss and light on dietary causes of high blood pressure – other than the obvious ones that make people gain weight.
Denial of the effects of dietary salt on blood pressure is not the issue. Rather, it is a time-hewn lack of confidence in the ability of patients to significantly and consistently modify their diet. Part of this may also relate to our inability to provide useful lifestyle tips on how to do so, other than the obvious referral to a dietitian. Telling them not to eat out does not solve this problem, because they can just as easily add salt at home.
However, a recent meta-analysis evaluating the effect of salt substitutes on blood pressure has reinvigorated my desire to counsel my patients on blood pressure self-management.
In this study, the investigators sought randomized, controlled trials with interventions lasting at least 6 months in duration (Am. J. Clin. Nutr. 2014;100:1448-54). Six cohorts were identified in the literature involving a total of 1,974 participants. Included studies took place in China and the Netherlands. Three studies used 65% NaCl/25% KCl/10% MgSO2, one used 41% NaCl/41% KCl/17% magnesium salt/trace minerals, and one used 65% NaCl/30% KCl/5% calcium salt and folic acid.
Salt substitutes had significant effects on systolic blood pressure with a mean difference of –4.9 mm Hg and on diastolic blood pressure with a mean difference of –1.5 mm Hg.
Overall, one may not be impressed with a 5–mm Hg change in systolic blood pressure. But this is the mean difference – and we presume population heterogeneity in response to salt substitution, such that some patients will respond to a higher degree than this and some to a lower degree. But we do not know which ones are which.
Population interventions aimed at reducing salt intake have been shown to be effective. The Finnish government, for example, collaborated with private industry and was able to achieve reductions in sodium content of food. That initiative resulted in a 33% reduction in the population’s average salt intake, a greater than 10–mm Hg decrease in the population average of both systolic BP and diastolic BP, and a 75%-80% decrease in both stroke and coronary artery disease mortality. Australia, Japan, and the United Kingdom were able to do similar things.
Because we are unlikely to ever see such organized political will exercised in the United States, it seems reasonable to recommend salt substitutes to our patients on an individual level. Adverse effects have been noted with salt substitutes in patients with kidney disease, however, which will have to be considered in that specific population of patients.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
Most of my counseling to patients about lowering blood pressure through self management is heavy on weight loss and light on dietary causes of high blood pressure – other than the obvious ones that make people gain weight.
Denial of the effects of dietary salt on blood pressure is not the issue. Rather, it is a time-hewn lack of confidence in the ability of patients to significantly and consistently modify their diet. Part of this may also relate to our inability to provide useful lifestyle tips on how to do so, other than the obvious referral to a dietitian. Telling them not to eat out does not solve this problem, because they can just as easily add salt at home.
However, a recent meta-analysis evaluating the effect of salt substitutes on blood pressure has reinvigorated my desire to counsel my patients on blood pressure self-management.
In this study, the investigators sought randomized, controlled trials with interventions lasting at least 6 months in duration (Am. J. Clin. Nutr. 2014;100:1448-54). Six cohorts were identified in the literature involving a total of 1,974 participants. Included studies took place in China and the Netherlands. Three studies used 65% NaCl/25% KCl/10% MgSO2, one used 41% NaCl/41% KCl/17% magnesium salt/trace minerals, and one used 65% NaCl/30% KCl/5% calcium salt and folic acid.
Salt substitutes had significant effects on systolic blood pressure with a mean difference of –4.9 mm Hg and on diastolic blood pressure with a mean difference of –1.5 mm Hg.
Overall, one may not be impressed with a 5–mm Hg change in systolic blood pressure. But this is the mean difference – and we presume population heterogeneity in response to salt substitution, such that some patients will respond to a higher degree than this and some to a lower degree. But we do not know which ones are which.
Population interventions aimed at reducing salt intake have been shown to be effective. The Finnish government, for example, collaborated with private industry and was able to achieve reductions in sodium content of food. That initiative resulted in a 33% reduction in the population’s average salt intake, a greater than 10–mm Hg decrease in the population average of both systolic BP and diastolic BP, and a 75%-80% decrease in both stroke and coronary artery disease mortality. Australia, Japan, and the United Kingdom were able to do similar things.
Because we are unlikely to ever see such organized political will exercised in the United States, it seems reasonable to recommend salt substitutes to our patients on an individual level. Adverse effects have been noted with salt substitutes in patients with kidney disease, however, which will have to be considered in that specific population of patients.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
Most of my counseling to patients about lowering blood pressure through self management is heavy on weight loss and light on dietary causes of high blood pressure – other than the obvious ones that make people gain weight.
Denial of the effects of dietary salt on blood pressure is not the issue. Rather, it is a time-hewn lack of confidence in the ability of patients to significantly and consistently modify their diet. Part of this may also relate to our inability to provide useful lifestyle tips on how to do so, other than the obvious referral to a dietitian. Telling them not to eat out does not solve this problem, because they can just as easily add salt at home.
However, a recent meta-analysis evaluating the effect of salt substitutes on blood pressure has reinvigorated my desire to counsel my patients on blood pressure self-management.
In this study, the investigators sought randomized, controlled trials with interventions lasting at least 6 months in duration (Am. J. Clin. Nutr. 2014;100:1448-54). Six cohorts were identified in the literature involving a total of 1,974 participants. Included studies took place in China and the Netherlands. Three studies used 65% NaCl/25% KCl/10% MgSO2, one used 41% NaCl/41% KCl/17% magnesium salt/trace minerals, and one used 65% NaCl/30% KCl/5% calcium salt and folic acid.
Salt substitutes had significant effects on systolic blood pressure with a mean difference of –4.9 mm Hg and on diastolic blood pressure with a mean difference of –1.5 mm Hg.
Overall, one may not be impressed with a 5–mm Hg change in systolic blood pressure. But this is the mean difference – and we presume population heterogeneity in response to salt substitution, such that some patients will respond to a higher degree than this and some to a lower degree. But we do not know which ones are which.
Population interventions aimed at reducing salt intake have been shown to be effective. The Finnish government, for example, collaborated with private industry and was able to achieve reductions in sodium content of food. That initiative resulted in a 33% reduction in the population’s average salt intake, a greater than 10–mm Hg decrease in the population average of both systolic BP and diastolic BP, and a 75%-80% decrease in both stroke and coronary artery disease mortality. Australia, Japan, and the United Kingdom were able to do similar things.
Because we are unlikely to ever see such organized political will exercised in the United States, it seems reasonable to recommend salt substitutes to our patients on an individual level. Adverse effects have been noted with salt substitutes in patients with kidney disease, however, which will have to be considered in that specific population of patients.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
You can’t get there from here
Readers of this column may recall that we have been following the fate of a small 14 primary care physician–owned accountable care organization bordering the Rio Grande River in Texas, the Rio Grande Valley Health Alliance. These physicians in 12 practices started with no infrastructure, no common electronic health records, or capital, and nonetheless took the plunge to become a Medicare Shared Savings Program accountable care organization beginning Jan. 1, 2013. It is time for an update on them.
Admittedly, I have been dragging my feet about an update, not because the results were poor, but because they were so great. With barely the minimum 5,000 beneficiaries, they saved more than $6 million in their first year. They are in the no-downside-risk plan, and thus got 50% of those savings. They have had time in 2014 to crunch the data even more to identify the 10% of patients driving more than 50% of costs and begin implementing complex high-risk patient management. For these reasons, I wager that they will do even better in 2014 through increased efficiencies.
How about quality? In the first year in the Medicare Shared Savings Program (MSSP), an ACO need only show the ability to report; they are not graded on their quality performance. But the Rio Grande Valley Health Alliance kept track internally, and the ACO regularly appears to be hitting the 90th percentile on the bulk of the 33 quality metrics. Their model tracked the elements for success outlined in previous columns.
So, why have I been I hesitant to report this?
Well, so many of you readers have called or written me to say that, while this type of physician-driven community or rural ACO with a primary care core makes sense, there is no way that you can get the money to organize and build the infrastructure necessary to succeed like RGVHA has. You would have to create a legal entity and apply to a program such as the MSSP, create infrastructure, track savings over a calendar year, then wait 6 or 8 months to get the results and the shared savings payment.
In sum, it’s a great idea. You are in the best position to drive high-value health improvement. You are located where the historic lack of access and medical infrastructure has resulted in high avoidable costs.
But the cruel irony is that, thanks to the fee-for-service system, those in the best position to drive value – primary care physicians – are in the worst position to front the necessary capital costs.
RGVHA was able to go forward because they were eligible for the now-gone Advance Payment Model program that advanced them the necessary operational costs. Their exciting success would ring hollow as a message to you if you couldn’t get this type of developmental financial support. Deferred shared savings and improved quality for your Medicare patients is a great concept – but this is a proverbial “you can’t get there from here” dilemma.
The CMS ACO investment model
The Centers for Medicare and Medicaid Services also saw this disconnect. So, CMS announced a new upfront infrastructure support program specifically to promote new small nonhospital* or managed care ACOs, rural ACOs, ACOs where there is low ACO penetration, and existing ACOs wanting to move toward taking financial risk. This prepaid shared savings builds on the Advance Payment Model program.
ACOs that plan to apply for the program in the next cycle and start in 2016 must have a preliminary prospective beneficiary assignment of 10,000 or less. CMS will give preference to new ACOs in rural or low-penetration areas, or in areas with exceptional need, or to ACOs with compelling proposals on how they would invest their funds and the CMS funds.
Each dollar given by CMS is a prepayment against the ACO’s shared savings distribution. If there are not enough shared savings, there is no further repayment obligation unless the ACO leaves the program before the 3-year program period.
Applications will be accepted during the summer of 2015, which is roughly the same time as the MSSP application period.
In my mind, this is the single best investment in improving health delivery and reining in runaway health care costs that CMS could have made. It will empower those in the best position to generate the highest quality at the lowest cost: readers like you.
This could be a game changer for primary care and rural care. But it won’t happen without physician leaders like those at RGVHA. The summer of 2015 seems a long way off, but the time to begin preparing your fully financed ACO is now!
* Exceptions to the nonhospital condition exist for critical access hospitals or inpatient prospective payment hospitals with 100 or fewer beds.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
Readers of this column may recall that we have been following the fate of a small 14 primary care physician–owned accountable care organization bordering the Rio Grande River in Texas, the Rio Grande Valley Health Alliance. These physicians in 12 practices started with no infrastructure, no common electronic health records, or capital, and nonetheless took the plunge to become a Medicare Shared Savings Program accountable care organization beginning Jan. 1, 2013. It is time for an update on them.
Admittedly, I have been dragging my feet about an update, not because the results were poor, but because they were so great. With barely the minimum 5,000 beneficiaries, they saved more than $6 million in their first year. They are in the no-downside-risk plan, and thus got 50% of those savings. They have had time in 2014 to crunch the data even more to identify the 10% of patients driving more than 50% of costs and begin implementing complex high-risk patient management. For these reasons, I wager that they will do even better in 2014 through increased efficiencies.
How about quality? In the first year in the Medicare Shared Savings Program (MSSP), an ACO need only show the ability to report; they are not graded on their quality performance. But the Rio Grande Valley Health Alliance kept track internally, and the ACO regularly appears to be hitting the 90th percentile on the bulk of the 33 quality metrics. Their model tracked the elements for success outlined in previous columns.
So, why have I been I hesitant to report this?
Well, so many of you readers have called or written me to say that, while this type of physician-driven community or rural ACO with a primary care core makes sense, there is no way that you can get the money to organize and build the infrastructure necessary to succeed like RGVHA has. You would have to create a legal entity and apply to a program such as the MSSP, create infrastructure, track savings over a calendar year, then wait 6 or 8 months to get the results and the shared savings payment.
In sum, it’s a great idea. You are in the best position to drive high-value health improvement. You are located where the historic lack of access and medical infrastructure has resulted in high avoidable costs.
But the cruel irony is that, thanks to the fee-for-service system, those in the best position to drive value – primary care physicians – are in the worst position to front the necessary capital costs.
RGVHA was able to go forward because they were eligible for the now-gone Advance Payment Model program that advanced them the necessary operational costs. Their exciting success would ring hollow as a message to you if you couldn’t get this type of developmental financial support. Deferred shared savings and improved quality for your Medicare patients is a great concept – but this is a proverbial “you can’t get there from here” dilemma.
The CMS ACO investment model
The Centers for Medicare and Medicaid Services also saw this disconnect. So, CMS announced a new upfront infrastructure support program specifically to promote new small nonhospital* or managed care ACOs, rural ACOs, ACOs where there is low ACO penetration, and existing ACOs wanting to move toward taking financial risk. This prepaid shared savings builds on the Advance Payment Model program.
ACOs that plan to apply for the program in the next cycle and start in 2016 must have a preliminary prospective beneficiary assignment of 10,000 or less. CMS will give preference to new ACOs in rural or low-penetration areas, or in areas with exceptional need, or to ACOs with compelling proposals on how they would invest their funds and the CMS funds.
Each dollar given by CMS is a prepayment against the ACO’s shared savings distribution. If there are not enough shared savings, there is no further repayment obligation unless the ACO leaves the program before the 3-year program period.
Applications will be accepted during the summer of 2015, which is roughly the same time as the MSSP application period.
In my mind, this is the single best investment in improving health delivery and reining in runaway health care costs that CMS could have made. It will empower those in the best position to generate the highest quality at the lowest cost: readers like you.
This could be a game changer for primary care and rural care. But it won’t happen without physician leaders like those at RGVHA. The summer of 2015 seems a long way off, but the time to begin preparing your fully financed ACO is now!
* Exceptions to the nonhospital condition exist for critical access hospitals or inpatient prospective payment hospitals with 100 or fewer beds.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
Readers of this column may recall that we have been following the fate of a small 14 primary care physician–owned accountable care organization bordering the Rio Grande River in Texas, the Rio Grande Valley Health Alliance. These physicians in 12 practices started with no infrastructure, no common electronic health records, or capital, and nonetheless took the plunge to become a Medicare Shared Savings Program accountable care organization beginning Jan. 1, 2013. It is time for an update on them.
Admittedly, I have been dragging my feet about an update, not because the results were poor, but because they were so great. With barely the minimum 5,000 beneficiaries, they saved more than $6 million in their first year. They are in the no-downside-risk plan, and thus got 50% of those savings. They have had time in 2014 to crunch the data even more to identify the 10% of patients driving more than 50% of costs and begin implementing complex high-risk patient management. For these reasons, I wager that they will do even better in 2014 through increased efficiencies.
How about quality? In the first year in the Medicare Shared Savings Program (MSSP), an ACO need only show the ability to report; they are not graded on their quality performance. But the Rio Grande Valley Health Alliance kept track internally, and the ACO regularly appears to be hitting the 90th percentile on the bulk of the 33 quality metrics. Their model tracked the elements for success outlined in previous columns.
So, why have I been I hesitant to report this?
Well, so many of you readers have called or written me to say that, while this type of physician-driven community or rural ACO with a primary care core makes sense, there is no way that you can get the money to organize and build the infrastructure necessary to succeed like RGVHA has. You would have to create a legal entity and apply to a program such as the MSSP, create infrastructure, track savings over a calendar year, then wait 6 or 8 months to get the results and the shared savings payment.
In sum, it’s a great idea. You are in the best position to drive high-value health improvement. You are located where the historic lack of access and medical infrastructure has resulted in high avoidable costs.
But the cruel irony is that, thanks to the fee-for-service system, those in the best position to drive value – primary care physicians – are in the worst position to front the necessary capital costs.
RGVHA was able to go forward because they were eligible for the now-gone Advance Payment Model program that advanced them the necessary operational costs. Their exciting success would ring hollow as a message to you if you couldn’t get this type of developmental financial support. Deferred shared savings and improved quality for your Medicare patients is a great concept – but this is a proverbial “you can’t get there from here” dilemma.
The CMS ACO investment model
The Centers for Medicare and Medicaid Services also saw this disconnect. So, CMS announced a new upfront infrastructure support program specifically to promote new small nonhospital* or managed care ACOs, rural ACOs, ACOs where there is low ACO penetration, and existing ACOs wanting to move toward taking financial risk. This prepaid shared savings builds on the Advance Payment Model program.
ACOs that plan to apply for the program in the next cycle and start in 2016 must have a preliminary prospective beneficiary assignment of 10,000 or less. CMS will give preference to new ACOs in rural or low-penetration areas, or in areas with exceptional need, or to ACOs with compelling proposals on how they would invest their funds and the CMS funds.
Each dollar given by CMS is a prepayment against the ACO’s shared savings distribution. If there are not enough shared savings, there is no further repayment obligation unless the ACO leaves the program before the 3-year program period.
Applications will be accepted during the summer of 2015, which is roughly the same time as the MSSP application period.
In my mind, this is the single best investment in improving health delivery and reining in runaway health care costs that CMS could have made. It will empower those in the best position to generate the highest quality at the lowest cost: readers like you.
This could be a game changer for primary care and rural care. But it won’t happen without physician leaders like those at RGVHA. The summer of 2015 seems a long way off, but the time to begin preparing your fully financed ACO is now!
* Exceptions to the nonhospital condition exist for critical access hospitals or inpatient prospective payment hospitals with 100 or fewer beds.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
Irritated and Downright Painful
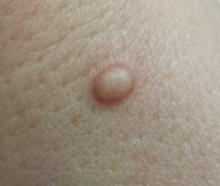
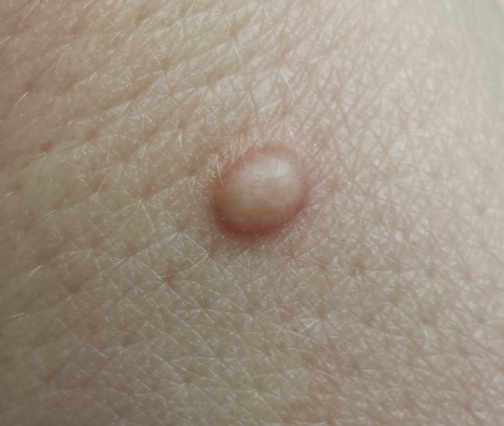
A 33-year-old woman presents with a lesion on the dorsum of her hand. Though it manifested several years ago, it was not problematic until recently, when it became irritated and downright painful. It has not changed in size and has never been red or swollen.
The patient denies ever having similar lesions elsewhere. She also denies any other serious health problems, specifically ophthalmologic problems.
EXAMINATION
The lesion, a 5-mm ovoid tan-orange papule, is located on the dorsum of her left hand. The surface is smooth, with a very firm feel. No skin changes are noted on the surrounding skin, and there are no palpable nodes in the arm or adjacent axilla.
In light of the patient’s concern, the lesion is excised, using an elliptical incision and minimal margins. The incision is carried down into superficial adipose tissue to ensure complete removal. Bleeding is controlled and the defect closed with simple interrupted sutures.
The pathology report shows numerous densely distributed polyhedral histiocytes. Many contain a large amount of cytoplasm.
What is the diagnosis?
DISCUSSION
The clinical presentation and pathology report were both consistent with an entity called juvenile xanthogranuloma (JXG). It usually presents, as in this case, as a solitary, firm, tan to orange papule or nodule. It is more commonly seen on the head and neck of young children, although it is by no means limited to that population (despite its name, which represents anachronistic derm terminology).
Based on the pathologic picture (typified by the biopsy report in this case), JXG may represent a disordered macrophage response to an unknown tissue injury that eventuates in a granulomatous reaction.
JXG is one of the more common members of a spectrum of histiocytic disorders that includes Langerhans cell histiocytosis. In addition to its more common cutaneous distribution, JXG has been seen in almost every internal organ as well. It is rarely associated with systemic manifestations.
The most significant extracutaneous manifestation of JXG is in the eye, where it is the most common cause of spontaneous hyphema in children (usually those younger than 2). This, in turn, can lead to a secondary glaucoma and eventual blindness. This is the main caveat with solitary cutaneous JXGs: They can be associated with ocular involvement, which would manifest as changes in the color of the iris or in the size of the globe itself. Fortunately, this is a rare complication.
Beyond the potential for ocular involvement, since the vast majority of cutaneous JXG lesions are easy to diagnose and self-limiting, there is no reason to remove or otherwise treat them.
The differential for cutaneous JXG includes Spitz tumor, molluscum, and intradermal nevus.
TAKE-HOME LEARNING POINTS
• Juvenile xanthogranuloma (JXG) lesions usually resolve on their own, without treatment.
• In rare instances, cutaneous JXG can be associated with ocular involvement, which manifests with either changes in iris color or in the size of the globe itself.
• JXG lesions are commonly seen on the head and neck of young children, usually as a solitary tan to orange firm papule.
• The differential for JXG includes molluscum, Spitz tumor, and nevus. Removal of JXG lesions is usually not necessary, but pathologic examination shows pathognomic changes that effectively distinguish it from its lookalikes.
A 33-year-old woman presents with a lesion on the dorsum of her hand. Though it manifested several years ago, it was not problematic until recently, when it became irritated and downright painful. It has not changed in size and has never been red or swollen.
The patient denies ever having similar lesions elsewhere. She also denies any other serious health problems, specifically ophthalmologic problems.
EXAMINATION
The lesion, a 5-mm ovoid tan-orange papule, is located on the dorsum of her left hand. The surface is smooth, with a very firm feel. No skin changes are noted on the surrounding skin, and there are no palpable nodes in the arm or adjacent axilla.
In light of the patient’s concern, the lesion is excised, using an elliptical incision and minimal margins. The incision is carried down into superficial adipose tissue to ensure complete removal. Bleeding is controlled and the defect closed with simple interrupted sutures.
The pathology report shows numerous densely distributed polyhedral histiocytes. Many contain a large amount of cytoplasm.
What is the diagnosis?
DISCUSSION
The clinical presentation and pathology report were both consistent with an entity called juvenile xanthogranuloma (JXG). It usually presents, as in this case, as a solitary, firm, tan to orange papule or nodule. It is more commonly seen on the head and neck of young children, although it is by no means limited to that population (despite its name, which represents anachronistic derm terminology).
Based on the pathologic picture (typified by the biopsy report in this case), JXG may represent a disordered macrophage response to an unknown tissue injury that eventuates in a granulomatous reaction.
JXG is one of the more common members of a spectrum of histiocytic disorders that includes Langerhans cell histiocytosis. In addition to its more common cutaneous distribution, JXG has been seen in almost every internal organ as well. It is rarely associated with systemic manifestations.
The most significant extracutaneous manifestation of JXG is in the eye, where it is the most common cause of spontaneous hyphema in children (usually those younger than 2). This, in turn, can lead to a secondary glaucoma and eventual blindness. This is the main caveat with solitary cutaneous JXGs: They can be associated with ocular involvement, which would manifest as changes in the color of the iris or in the size of the globe itself. Fortunately, this is a rare complication.
Beyond the potential for ocular involvement, since the vast majority of cutaneous JXG lesions are easy to diagnose and self-limiting, there is no reason to remove or otherwise treat them.
The differential for cutaneous JXG includes Spitz tumor, molluscum, and intradermal nevus.
TAKE-HOME LEARNING POINTS
• Juvenile xanthogranuloma (JXG) lesions usually resolve on their own, without treatment.
• In rare instances, cutaneous JXG can be associated with ocular involvement, which manifests with either changes in iris color or in the size of the globe itself.
• JXG lesions are commonly seen on the head and neck of young children, usually as a solitary tan to orange firm papule.
• The differential for JXG includes molluscum, Spitz tumor, and nevus. Removal of JXG lesions is usually not necessary, but pathologic examination shows pathognomic changes that effectively distinguish it from its lookalikes.
A 33-year-old woman presents with a lesion on the dorsum of her hand. Though it manifested several years ago, it was not problematic until recently, when it became irritated and downright painful. It has not changed in size and has never been red or swollen.
The patient denies ever having similar lesions elsewhere. She also denies any other serious health problems, specifically ophthalmologic problems.
EXAMINATION
The lesion, a 5-mm ovoid tan-orange papule, is located on the dorsum of her left hand. The surface is smooth, with a very firm feel. No skin changes are noted on the surrounding skin, and there are no palpable nodes in the arm or adjacent axilla.
In light of the patient’s concern, the lesion is excised, using an elliptical incision and minimal margins. The incision is carried down into superficial adipose tissue to ensure complete removal. Bleeding is controlled and the defect closed with simple interrupted sutures.
The pathology report shows numerous densely distributed polyhedral histiocytes. Many contain a large amount of cytoplasm.
What is the diagnosis?
DISCUSSION
The clinical presentation and pathology report were both consistent with an entity called juvenile xanthogranuloma (JXG). It usually presents, as in this case, as a solitary, firm, tan to orange papule or nodule. It is more commonly seen on the head and neck of young children, although it is by no means limited to that population (despite its name, which represents anachronistic derm terminology).
Based on the pathologic picture (typified by the biopsy report in this case), JXG may represent a disordered macrophage response to an unknown tissue injury that eventuates in a granulomatous reaction.
JXG is one of the more common members of a spectrum of histiocytic disorders that includes Langerhans cell histiocytosis. In addition to its more common cutaneous distribution, JXG has been seen in almost every internal organ as well. It is rarely associated with systemic manifestations.
The most significant extracutaneous manifestation of JXG is in the eye, where it is the most common cause of spontaneous hyphema in children (usually those younger than 2). This, in turn, can lead to a secondary glaucoma and eventual blindness. This is the main caveat with solitary cutaneous JXGs: They can be associated with ocular involvement, which would manifest as changes in the color of the iris or in the size of the globe itself. Fortunately, this is a rare complication.
Beyond the potential for ocular involvement, since the vast majority of cutaneous JXG lesions are easy to diagnose and self-limiting, there is no reason to remove or otherwise treat them.
The differential for cutaneous JXG includes Spitz tumor, molluscum, and intradermal nevus.
TAKE-HOME LEARNING POINTS
• Juvenile xanthogranuloma (JXG) lesions usually resolve on their own, without treatment.
• In rare instances, cutaneous JXG can be associated with ocular involvement, which manifests with either changes in iris color or in the size of the globe itself.
• JXG lesions are commonly seen on the head and neck of young children, usually as a solitary tan to orange firm papule.
• The differential for JXG includes molluscum, Spitz tumor, and nevus. Removal of JXG lesions is usually not necessary, but pathologic examination shows pathognomic changes that effectively distinguish it from its lookalikes.
Shoulder morbidity common after thyroid cancer surgery
CORONADO, CALIF. – More than 50% of patients who underwent surgery for differentiated thyroid cancer experienced shoulder morbidity up to 10 years after the procedure, results from a Dutch study showed.
“What’s causing the pain?” Dr. Romana T. Netea-Maier asked in an interview at the annual meeting of the American Thyroid Association. “It may be that the spinal accessory nerve or other nerves have been injured during the surgery. We don’t know.”
In what she said is the first study of its kind, Dr. Netea-Maier and her associates compared the prevalence of shoulder morbidity and its relation to clinical characteristics and quality of life in 109 patients who underwent surgery for differentiated thyroid cancer at Radboud University Medical Center, Nijmegen, the Netherlands, with a group of 81 healthy controls and a group of 59 patients who underwent surgery for benign thyroid pathology. Main outcome measures were the prevalence of shoulder complaints based on results of the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire-C-30 (EORTC QLQ-C30).
Dr. Netea-Maier, of the department of medicine at the university, reported that the mean age of patients in the two surgery groups was 46 years, and 73% were women. During an average of 10 years following surgery, 59% of patients in the thyroid cancer group and 49% of patients in the benign thyroid pathology group reported shoulder morbidity, compared with 14% of controls (P < .01). The chief complaints among patients in the thyroid cancer group were pain (25%), muscle weakness (8%), and tingling (8%), while the main complaints among those with benign thyroid pathology were pain (38%), and tingling (7%).
Compared with healthy controls, patients in the thyroid cancer group scored worse on all subscales of the DASH and the EORTC QLQ-C30. On bivariate analysis, level V neck dissection, spinal accessory nerve damage, and employment status were associated with the prevalence of shoulder complaints and DASH scores, while the prevalence of shoulder complaints and DASH scores correlated significantly with EORTC QLQ-C30 scores.
The researchers found that only 12% of patients in the thyroid cancer group received preoperative information on the potential for shoulder morbidity and 35% received additional care for postoperative shoulder complaints.
“The take-home message would be to inform your patients of the potential for shoulder comorbidity, because what we have shown here is that patients do not recall being informed about this possible complication before the surgery,” Dr. Netea-Maier said. “If they have complaints, start with physiotherapy early on.”
Dr. Netea-Maier reported having no financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – More than 50% of patients who underwent surgery for differentiated thyroid cancer experienced shoulder morbidity up to 10 years after the procedure, results from a Dutch study showed.
“What’s causing the pain?” Dr. Romana T. Netea-Maier asked in an interview at the annual meeting of the American Thyroid Association. “It may be that the spinal accessory nerve or other nerves have been injured during the surgery. We don’t know.”
In what she said is the first study of its kind, Dr. Netea-Maier and her associates compared the prevalence of shoulder morbidity and its relation to clinical characteristics and quality of life in 109 patients who underwent surgery for differentiated thyroid cancer at Radboud University Medical Center, Nijmegen, the Netherlands, with a group of 81 healthy controls and a group of 59 patients who underwent surgery for benign thyroid pathology. Main outcome measures were the prevalence of shoulder complaints based on results of the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire-C-30 (EORTC QLQ-C30).
Dr. Netea-Maier, of the department of medicine at the university, reported that the mean age of patients in the two surgery groups was 46 years, and 73% were women. During an average of 10 years following surgery, 59% of patients in the thyroid cancer group and 49% of patients in the benign thyroid pathology group reported shoulder morbidity, compared with 14% of controls (P < .01). The chief complaints among patients in the thyroid cancer group were pain (25%), muscle weakness (8%), and tingling (8%), while the main complaints among those with benign thyroid pathology were pain (38%), and tingling (7%).
Compared with healthy controls, patients in the thyroid cancer group scored worse on all subscales of the DASH and the EORTC QLQ-C30. On bivariate analysis, level V neck dissection, spinal accessory nerve damage, and employment status were associated with the prevalence of shoulder complaints and DASH scores, while the prevalence of shoulder complaints and DASH scores correlated significantly with EORTC QLQ-C30 scores.
The researchers found that only 12% of patients in the thyroid cancer group received preoperative information on the potential for shoulder morbidity and 35% received additional care for postoperative shoulder complaints.
“The take-home message would be to inform your patients of the potential for shoulder comorbidity, because what we have shown here is that patients do not recall being informed about this possible complication before the surgery,” Dr. Netea-Maier said. “If they have complaints, start with physiotherapy early on.”
Dr. Netea-Maier reported having no financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – More than 50% of patients who underwent surgery for differentiated thyroid cancer experienced shoulder morbidity up to 10 years after the procedure, results from a Dutch study showed.
“What’s causing the pain?” Dr. Romana T. Netea-Maier asked in an interview at the annual meeting of the American Thyroid Association. “It may be that the spinal accessory nerve or other nerves have been injured during the surgery. We don’t know.”
In what she said is the first study of its kind, Dr. Netea-Maier and her associates compared the prevalence of shoulder morbidity and its relation to clinical characteristics and quality of life in 109 patients who underwent surgery for differentiated thyroid cancer at Radboud University Medical Center, Nijmegen, the Netherlands, with a group of 81 healthy controls and a group of 59 patients who underwent surgery for benign thyroid pathology. Main outcome measures were the prevalence of shoulder complaints based on results of the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire and the European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire-C-30 (EORTC QLQ-C30).
Dr. Netea-Maier, of the department of medicine at the university, reported that the mean age of patients in the two surgery groups was 46 years, and 73% were women. During an average of 10 years following surgery, 59% of patients in the thyroid cancer group and 49% of patients in the benign thyroid pathology group reported shoulder morbidity, compared with 14% of controls (P < .01). The chief complaints among patients in the thyroid cancer group were pain (25%), muscle weakness (8%), and tingling (8%), while the main complaints among those with benign thyroid pathology were pain (38%), and tingling (7%).
Compared with healthy controls, patients in the thyroid cancer group scored worse on all subscales of the DASH and the EORTC QLQ-C30. On bivariate analysis, level V neck dissection, spinal accessory nerve damage, and employment status were associated with the prevalence of shoulder complaints and DASH scores, while the prevalence of shoulder complaints and DASH scores correlated significantly with EORTC QLQ-C30 scores.
The researchers found that only 12% of patients in the thyroid cancer group received preoperative information on the potential for shoulder morbidity and 35% received additional care for postoperative shoulder complaints.
“The take-home message would be to inform your patients of the potential for shoulder comorbidity, because what we have shown here is that patients do not recall being informed about this possible complication before the surgery,” Dr. Netea-Maier said. “If they have complaints, start with physiotherapy early on.”
Dr. Netea-Maier reported having no financial disclosures.
On Twitter @dougbrunk
AT THE ATA ANNUAL MEETING
Key clinical point: Postoperative shoulder morbidity is highly prevalent in patients who undergo surgery for thyroid cancer.
Major finding: During an average of 10 years following surgery, 59% of patients in the thyroid cancer group and 49% of patients in the benign thyroid pathology group reported shoulder morbidity, compared with 14% of controls (P < .01).
Data source: A Dutch study of 109 patients who underwent surgery for differentiated thyroid cancer, compared with 81 healthy controls and 59 patients who underwent surgery for benign thyroid pathology.
Disclosures: Dr. Netea-Maier reported having no financial disclosures.
A new treatment option for elderly AML patients?

Credit: NIH
In a phase 2 study, the anticancer quinolone derivative vosaroxin produced responses in poor-risk, elderly patients with previously untreated acute myeloid leukemia (AML), but most patients ultimately died.
Twenty-nine percent of patients achieved a complete response (CR) following treatment with vosaroxin.
However, 84% of patients discontinued treatment, most due to treatment failure. And 91% of patients died, most from disease progression.
Still, study investigators said single-agent vosaroxin shows promise as a treatment option for this group of patients, who are unlikely to benefit from standard induction chemotherapy.
“There remains an acute unmet medical need for new treatment options in AML, including patients 60 years of age and older who are unlikely to benefit from standard induction chemotherapy,” said Farhad Ravandi, MD, of the University of Texas MD Anderson Cancer Center.
“Vosaroxin is active and well-tolerated in this population, both as a single agent, as seen in [this] study, and in combination with decitabine, as seen in an ongoing MD Anderson Cancer Center-sponsored study.”
Dr Ravandi and his colleagues reported results of the phase 2 trial, called REVEAL-1, in the British Journal of Haematology. The study was sponsored by Sunesis Pharmaceuticals, the company developing vosaroxin.
The investigators evaluated vosaroxin in 113 patients aged 60 and older who had previously untreated AML with an unfavorable prognosis.
The patients’ median age was 75 years, and most (82%) had 2 or more risk factors, which included being 70 or older, having antecedent hematologic disease, having an ECOG performance status of 2, and having intermediate/unfavorable cytogenetics.
Patients received vosaroxin in sequential cohorts. In group A, they received 72 mg/m2 on days 1, 8, and 15. In group B, they received 72 mg/m2 on days 1 and 8. And in group C, they received 72 mg/m2 or 90 mg/m2 on days 1 and 4.
The primary efficacy endpoint was the combined CR rate and the rate of CR with incomplete platelet recovery (CRp). CR and CR/CRp rates were 29% and 32%, respectively. Responses occurred in all categories of risk factors, including in patients with 2 or more risk factors.
Ninety-five patients (84%) discontinued treatment due to treatment failure (n=50), death (n=21), unacceptable adverse events (n=6), relapse (n=5), their physician’s decision (n=5) or other reasons (n=8).
The all-cause mortality rate was 12% at 30 days and 31% at 60 days. The median overall survival (OS) was 7.0 months, and the 1-year OS rate was 34%.
Common grade 3/4 hematologic adverse events (occurring in 20% of patients or more) included thrombocytopenia (59%), febrile neutropenia (50%), anemia (49%), and neutropenia (29%).
Common grade 3/4 nonhematologic adverse events included sepsis (39%), pneumonia (30%), hypokalemia (25%), and oral mucositis/stomatitis (22%).
Ninety-one patients (81%) had one or more serious adverse event. The most common were pneumonia (24%), febrile neutropenia (21%), and oral mucositis/stomatitis (10%).
Of the 113 patients treated, 103 died. Most deaths (78%) were due to progressive disease.
Patients in group C (72 mg/m2) had the most favorable balance of safety and efficacy. They had faster hematologic recovery (a median of 27 days) than the other groups and the lowest incidence of aggregate sepsis (24%) and 30-day (7%) and 60-day (17%) all-cause mortality.
At this dose and schedule, CR and CR/CRp rates were 31% and 35%, respectively. The median OS was 7.7 months, and the 1-year OS rate was 38%.
“Publication of these data in the British Journal of Haematology further support our goal of establishing vosaroxin as a new standard of care in AML,” said Adam Craig, chief medical officer of Sunesis.
“Given ongoing demographic shifts in the US and other major territories, the challenge of treating AML in older adults will continue to grow, underscoring a need for new treatment options. We look forward to building on these data through further investigator-sponsored studies and, with the outcome of VALOR in relapsed or refractory AML, progressing towards initial regulatory approval.”
Based on results of the phase 3 VALOR trial, which were recently announced, Sunesis has filed a marketing authorization application with the European Medicines Agency and plans to meet with the US Food and Drug Administration to determine the appropriate regulatory path forward for vosaroxin in the treatment of relapsed or refractory AML. ![]()

Credit: NIH
In a phase 2 study, the anticancer quinolone derivative vosaroxin produced responses in poor-risk, elderly patients with previously untreated acute myeloid leukemia (AML), but most patients ultimately died.
Twenty-nine percent of patients achieved a complete response (CR) following treatment with vosaroxin.
However, 84% of patients discontinued treatment, most due to treatment failure. And 91% of patients died, most from disease progression.
Still, study investigators said single-agent vosaroxin shows promise as a treatment option for this group of patients, who are unlikely to benefit from standard induction chemotherapy.
“There remains an acute unmet medical need for new treatment options in AML, including patients 60 years of age and older who are unlikely to benefit from standard induction chemotherapy,” said Farhad Ravandi, MD, of the University of Texas MD Anderson Cancer Center.
“Vosaroxin is active and well-tolerated in this population, both as a single agent, as seen in [this] study, and in combination with decitabine, as seen in an ongoing MD Anderson Cancer Center-sponsored study.”
Dr Ravandi and his colleagues reported results of the phase 2 trial, called REVEAL-1, in the British Journal of Haematology. The study was sponsored by Sunesis Pharmaceuticals, the company developing vosaroxin.
The investigators evaluated vosaroxin in 113 patients aged 60 and older who had previously untreated AML with an unfavorable prognosis.
The patients’ median age was 75 years, and most (82%) had 2 or more risk factors, which included being 70 or older, having antecedent hematologic disease, having an ECOG performance status of 2, and having intermediate/unfavorable cytogenetics.
Patients received vosaroxin in sequential cohorts. In group A, they received 72 mg/m2 on days 1, 8, and 15. In group B, they received 72 mg/m2 on days 1 and 8. And in group C, they received 72 mg/m2 or 90 mg/m2 on days 1 and 4.
The primary efficacy endpoint was the combined CR rate and the rate of CR with incomplete platelet recovery (CRp). CR and CR/CRp rates were 29% and 32%, respectively. Responses occurred in all categories of risk factors, including in patients with 2 or more risk factors.
Ninety-five patients (84%) discontinued treatment due to treatment failure (n=50), death (n=21), unacceptable adverse events (n=6), relapse (n=5), their physician’s decision (n=5) or other reasons (n=8).
The all-cause mortality rate was 12% at 30 days and 31% at 60 days. The median overall survival (OS) was 7.0 months, and the 1-year OS rate was 34%.
Common grade 3/4 hematologic adverse events (occurring in 20% of patients or more) included thrombocytopenia (59%), febrile neutropenia (50%), anemia (49%), and neutropenia (29%).
Common grade 3/4 nonhematologic adverse events included sepsis (39%), pneumonia (30%), hypokalemia (25%), and oral mucositis/stomatitis (22%).
Ninety-one patients (81%) had one or more serious adverse event. The most common were pneumonia (24%), febrile neutropenia (21%), and oral mucositis/stomatitis (10%).
Of the 113 patients treated, 103 died. Most deaths (78%) were due to progressive disease.
Patients in group C (72 mg/m2) had the most favorable balance of safety and efficacy. They had faster hematologic recovery (a median of 27 days) than the other groups and the lowest incidence of aggregate sepsis (24%) and 30-day (7%) and 60-day (17%) all-cause mortality.
At this dose and schedule, CR and CR/CRp rates were 31% and 35%, respectively. The median OS was 7.7 months, and the 1-year OS rate was 38%.
“Publication of these data in the British Journal of Haematology further support our goal of establishing vosaroxin as a new standard of care in AML,” said Adam Craig, chief medical officer of Sunesis.
“Given ongoing demographic shifts in the US and other major territories, the challenge of treating AML in older adults will continue to grow, underscoring a need for new treatment options. We look forward to building on these data through further investigator-sponsored studies and, with the outcome of VALOR in relapsed or refractory AML, progressing towards initial regulatory approval.”
Based on results of the phase 3 VALOR trial, which were recently announced, Sunesis has filed a marketing authorization application with the European Medicines Agency and plans to meet with the US Food and Drug Administration to determine the appropriate regulatory path forward for vosaroxin in the treatment of relapsed or refractory AML. ![]()

Credit: NIH
In a phase 2 study, the anticancer quinolone derivative vosaroxin produced responses in poor-risk, elderly patients with previously untreated acute myeloid leukemia (AML), but most patients ultimately died.
Twenty-nine percent of patients achieved a complete response (CR) following treatment with vosaroxin.
However, 84% of patients discontinued treatment, most due to treatment failure. And 91% of patients died, most from disease progression.
Still, study investigators said single-agent vosaroxin shows promise as a treatment option for this group of patients, who are unlikely to benefit from standard induction chemotherapy.
“There remains an acute unmet medical need for new treatment options in AML, including patients 60 years of age and older who are unlikely to benefit from standard induction chemotherapy,” said Farhad Ravandi, MD, of the University of Texas MD Anderson Cancer Center.
“Vosaroxin is active and well-tolerated in this population, both as a single agent, as seen in [this] study, and in combination with decitabine, as seen in an ongoing MD Anderson Cancer Center-sponsored study.”
Dr Ravandi and his colleagues reported results of the phase 2 trial, called REVEAL-1, in the British Journal of Haematology. The study was sponsored by Sunesis Pharmaceuticals, the company developing vosaroxin.
The investigators evaluated vosaroxin in 113 patients aged 60 and older who had previously untreated AML with an unfavorable prognosis.
The patients’ median age was 75 years, and most (82%) had 2 or more risk factors, which included being 70 or older, having antecedent hematologic disease, having an ECOG performance status of 2, and having intermediate/unfavorable cytogenetics.
Patients received vosaroxin in sequential cohorts. In group A, they received 72 mg/m2 on days 1, 8, and 15. In group B, they received 72 mg/m2 on days 1 and 8. And in group C, they received 72 mg/m2 or 90 mg/m2 on days 1 and 4.
The primary efficacy endpoint was the combined CR rate and the rate of CR with incomplete platelet recovery (CRp). CR and CR/CRp rates were 29% and 32%, respectively. Responses occurred in all categories of risk factors, including in patients with 2 or more risk factors.
Ninety-five patients (84%) discontinued treatment due to treatment failure (n=50), death (n=21), unacceptable adverse events (n=6), relapse (n=5), their physician’s decision (n=5) or other reasons (n=8).
The all-cause mortality rate was 12% at 30 days and 31% at 60 days. The median overall survival (OS) was 7.0 months, and the 1-year OS rate was 34%.
Common grade 3/4 hematologic adverse events (occurring in 20% of patients or more) included thrombocytopenia (59%), febrile neutropenia (50%), anemia (49%), and neutropenia (29%).
Common grade 3/4 nonhematologic adverse events included sepsis (39%), pneumonia (30%), hypokalemia (25%), and oral mucositis/stomatitis (22%).
Ninety-one patients (81%) had one or more serious adverse event. The most common were pneumonia (24%), febrile neutropenia (21%), and oral mucositis/stomatitis (10%).
Of the 113 patients treated, 103 died. Most deaths (78%) were due to progressive disease.
Patients in group C (72 mg/m2) had the most favorable balance of safety and efficacy. They had faster hematologic recovery (a median of 27 days) than the other groups and the lowest incidence of aggregate sepsis (24%) and 30-day (7%) and 60-day (17%) all-cause mortality.
At this dose and schedule, CR and CR/CRp rates were 31% and 35%, respectively. The median OS was 7.7 months, and the 1-year OS rate was 38%.
“Publication of these data in the British Journal of Haematology further support our goal of establishing vosaroxin as a new standard of care in AML,” said Adam Craig, chief medical officer of Sunesis.
“Given ongoing demographic shifts in the US and other major territories, the challenge of treating AML in older adults will continue to grow, underscoring a need for new treatment options. We look forward to building on these data through further investigator-sponsored studies and, with the outcome of VALOR in relapsed or refractory AML, progressing towards initial regulatory approval.”
Based on results of the phase 3 VALOR trial, which were recently announced, Sunesis has filed a marketing authorization application with the European Medicines Agency and plans to meet with the US Food and Drug Administration to determine the appropriate regulatory path forward for vosaroxin in the treatment of relapsed or refractory AML. ![]()
NSAIDs Linked to Poor Pneumonia Outcomes
Nonsteroidal anti-inflammatory drugs (NSAIDs) given in the early stages of lower respiratory tract infection could be helping send younger adults to the intensive care unit (ICU) with serious pneumonia, say researchers from Hôpital Louis Mourier, Colombes, and Université Paris Diderot, both in France. Their concerns were triggered in part by witnessing several cases of unexpectedly severe forms of Streptococcus pneumoniae (S pneumoniae) community-acquired pneumonia (CAP) in healthy adults.
They analyzed data on 106 patients admitted with pneumococcal pneumonia or S pneumoniae and pneumonia as the discharge diagnosis. Twenty patients had received NSAIDs within 4 days prior to admission. The patients given NSAIDs were younger than those who were not prescribed NSAIDs (aged 43 years on average vs aged 62 years on average), usually working, and less likely to have comorbidities. The mean duration of NSAID treatment was 4 days. The time to the first medical consultation after pneumonia-related symptoms appeared was the same in both groups, but the patients on NSAIDs were prescribed antibiotics significantly later than those not taking NSAIDs (4.5 days vs 2 days, P = .001). They were also admitted to the ICU later.
A “noticeable and significant difference” was that more patients in the NSAID group had pleural effusion (P < .0006). New onset of pleuropulmonary complications during the ICU stay was significantly greater in the group who had received NSAIDs than in the no-NSAID group (P = .0008).
The researchers say their findings “highlight the overlooked risk of taking NSAIDs to treat physical symptoms at an early stage of CAP.” They hypothesize that patient age and comorbidity status led physicians to not diagnose CAP, and thus withhold antibiotics. NSAIDs may blunt general signs and symptoms and mask the severity of the infectious process, the researchers caution. Thus, they recommend ensuring appropriate antibiotic coverage along with NSAIDs.
In a survey of French general practitioners’ prescriptions, NSAIDs were prescribed for almost half of all patients seen for lower respiratory tract infection, “despite the fact that this prescription never appears in any national or international guideline,” the researchers say. That underscores the need to better inform general practitioners about the risks of NSAIDs, they say.
Source
Messika J, Sztrymf B, Bertrand F, et al. J Crit Care. 2014;29(5):733-738.
doi: 10.1016/j.jcrc.2014.05.021.
Nonsteroidal anti-inflammatory drugs (NSAIDs) given in the early stages of lower respiratory tract infection could be helping send younger adults to the intensive care unit (ICU) with serious pneumonia, say researchers from Hôpital Louis Mourier, Colombes, and Université Paris Diderot, both in France. Their concerns were triggered in part by witnessing several cases of unexpectedly severe forms of Streptococcus pneumoniae (S pneumoniae) community-acquired pneumonia (CAP) in healthy adults.
They analyzed data on 106 patients admitted with pneumococcal pneumonia or S pneumoniae and pneumonia as the discharge diagnosis. Twenty patients had received NSAIDs within 4 days prior to admission. The patients given NSAIDs were younger than those who were not prescribed NSAIDs (aged 43 years on average vs aged 62 years on average), usually working, and less likely to have comorbidities. The mean duration of NSAID treatment was 4 days. The time to the first medical consultation after pneumonia-related symptoms appeared was the same in both groups, but the patients on NSAIDs were prescribed antibiotics significantly later than those not taking NSAIDs (4.5 days vs 2 days, P = .001). They were also admitted to the ICU later.
A “noticeable and significant difference” was that more patients in the NSAID group had pleural effusion (P < .0006). New onset of pleuropulmonary complications during the ICU stay was significantly greater in the group who had received NSAIDs than in the no-NSAID group (P = .0008).
The researchers say their findings “highlight the overlooked risk of taking NSAIDs to treat physical symptoms at an early stage of CAP.” They hypothesize that patient age and comorbidity status led physicians to not diagnose CAP, and thus withhold antibiotics. NSAIDs may blunt general signs and symptoms and mask the severity of the infectious process, the researchers caution. Thus, they recommend ensuring appropriate antibiotic coverage along with NSAIDs.
In a survey of French general practitioners’ prescriptions, NSAIDs were prescribed for almost half of all patients seen for lower respiratory tract infection, “despite the fact that this prescription never appears in any national or international guideline,” the researchers say. That underscores the need to better inform general practitioners about the risks of NSAIDs, they say.
Source
Messika J, Sztrymf B, Bertrand F, et al. J Crit Care. 2014;29(5):733-738.
doi: 10.1016/j.jcrc.2014.05.021.
Nonsteroidal anti-inflammatory drugs (NSAIDs) given in the early stages of lower respiratory tract infection could be helping send younger adults to the intensive care unit (ICU) with serious pneumonia, say researchers from Hôpital Louis Mourier, Colombes, and Université Paris Diderot, both in France. Their concerns were triggered in part by witnessing several cases of unexpectedly severe forms of Streptococcus pneumoniae (S pneumoniae) community-acquired pneumonia (CAP) in healthy adults.
They analyzed data on 106 patients admitted with pneumococcal pneumonia or S pneumoniae and pneumonia as the discharge diagnosis. Twenty patients had received NSAIDs within 4 days prior to admission. The patients given NSAIDs were younger than those who were not prescribed NSAIDs (aged 43 years on average vs aged 62 years on average), usually working, and less likely to have comorbidities. The mean duration of NSAID treatment was 4 days. The time to the first medical consultation after pneumonia-related symptoms appeared was the same in both groups, but the patients on NSAIDs were prescribed antibiotics significantly later than those not taking NSAIDs (4.5 days vs 2 days, P = .001). They were also admitted to the ICU later.
A “noticeable and significant difference” was that more patients in the NSAID group had pleural effusion (P < .0006). New onset of pleuropulmonary complications during the ICU stay was significantly greater in the group who had received NSAIDs than in the no-NSAID group (P = .0008).
The researchers say their findings “highlight the overlooked risk of taking NSAIDs to treat physical symptoms at an early stage of CAP.” They hypothesize that patient age and comorbidity status led physicians to not diagnose CAP, and thus withhold antibiotics. NSAIDs may blunt general signs and symptoms and mask the severity of the infectious process, the researchers caution. Thus, they recommend ensuring appropriate antibiotic coverage along with NSAIDs.
In a survey of French general practitioners’ prescriptions, NSAIDs were prescribed for almost half of all patients seen for lower respiratory tract infection, “despite the fact that this prescription never appears in any national or international guideline,” the researchers say. That underscores the need to better inform general practitioners about the risks of NSAIDs, they say.
Source
Messika J, Sztrymf B, Bertrand F, et al. J Crit Care. 2014;29(5):733-738.
doi: 10.1016/j.jcrc.2014.05.021.
More education on SCD needed in sub-Saharan Africa

and a normal one
Credit: Betty Pace
Aggressive public health campaigns are needed to educate people in sub-Saharan Africa about sickle cell disease (SCD), according to a professor of health studies.
William Ebomoyi, PhD, of Chicago State University in Illinois, investigated the prevalence of SCD in sub-Saharan Africa, assessed the physical and emotional ramifications of the disease, evaluated ethical and legal issues related to SCD, and assessed the socio-cultural implications of the disease.
He reported his findings in the International Journal of Medical Engineering and Informatics.
Dr Ebomoyi explained that SCD occurs from a change in valine to glutamine substitution in the sixth amino acid position of the beta globin chain. If one of the two beta globin genes is affected, a person simply has sickle cell trait (SCT), but if both genes are involved, the person has SCD.
If two people with SCT decide to have a child, there is a 50% chance that child will have SCT, a 25% chance the child will have SCD, and a 25% chance the child will have neither condition. If one parent has SCT, there is a 50% chance the child will have SCT.
In some communities in sub-Saharan Africa, up to 2% of all children are born with SCD. And the prevalence of SCT ranges from 10% to 40% across equatorial Africa.
Dr Ebomoyi said early screening to identify infants with SCD is needed, as life-threatening complications can occur within the first 3 years of life. Unfortunately, the method of choice for SCD screening—cellulose acetate accompanied by citrate agar electrophoresis—is only available in two sub-Saharan African nations—South Africa and Ghana.
Similarly, innovative SCD treatment techniques have not been introduced in sub-Saharan African nations. There are not enough well-trained physicians, Dr Ebomoyi said. In fact, many SCD patients are treated improperly by traditional African healers.
Furthermore, aside from Senegal and Liberia, sub-Saharan African nations spend less than 10% of their gross domestic product on healthcare. And inadequate funding plays a major role in the high prevalence of SCD in these nations.
For all these reasons, it is important to raise awareness in sub-Saharan Africa about SCD, according to Dr Ebomoyi. He said members of the public should be aware of how they can pass SCD down to their children and inform their partners if they have SCT prior to conceiving a child.
He added that sickle cell education programs should be integrated into the curriculum of elementary, secondary, and tertiary academic institutions.
The abstract of this article, “Ethical, legal, social, and financial implications of neonatal screening for sickle cell anaemia in Sub-Sahara Africa in the age of genomic science,” can be found on the Inderscience Publishers website. ![]()

and a normal one
Credit: Betty Pace
Aggressive public health campaigns are needed to educate people in sub-Saharan Africa about sickle cell disease (SCD), according to a professor of health studies.
William Ebomoyi, PhD, of Chicago State University in Illinois, investigated the prevalence of SCD in sub-Saharan Africa, assessed the physical and emotional ramifications of the disease, evaluated ethical and legal issues related to SCD, and assessed the socio-cultural implications of the disease.
He reported his findings in the International Journal of Medical Engineering and Informatics.
Dr Ebomoyi explained that SCD occurs from a change in valine to glutamine substitution in the sixth amino acid position of the beta globin chain. If one of the two beta globin genes is affected, a person simply has sickle cell trait (SCT), but if both genes are involved, the person has SCD.
If two people with SCT decide to have a child, there is a 50% chance that child will have SCT, a 25% chance the child will have SCD, and a 25% chance the child will have neither condition. If one parent has SCT, there is a 50% chance the child will have SCT.
In some communities in sub-Saharan Africa, up to 2% of all children are born with SCD. And the prevalence of SCT ranges from 10% to 40% across equatorial Africa.
Dr Ebomoyi said early screening to identify infants with SCD is needed, as life-threatening complications can occur within the first 3 years of life. Unfortunately, the method of choice for SCD screening—cellulose acetate accompanied by citrate agar electrophoresis—is only available in two sub-Saharan African nations—South Africa and Ghana.
Similarly, innovative SCD treatment techniques have not been introduced in sub-Saharan African nations. There are not enough well-trained physicians, Dr Ebomoyi said. In fact, many SCD patients are treated improperly by traditional African healers.
Furthermore, aside from Senegal and Liberia, sub-Saharan African nations spend less than 10% of their gross domestic product on healthcare. And inadequate funding plays a major role in the high prevalence of SCD in these nations.
For all these reasons, it is important to raise awareness in sub-Saharan Africa about SCD, according to Dr Ebomoyi. He said members of the public should be aware of how they can pass SCD down to their children and inform their partners if they have SCT prior to conceiving a child.
He added that sickle cell education programs should be integrated into the curriculum of elementary, secondary, and tertiary academic institutions.
The abstract of this article, “Ethical, legal, social, and financial implications of neonatal screening for sickle cell anaemia in Sub-Sahara Africa in the age of genomic science,” can be found on the Inderscience Publishers website. ![]()

and a normal one
Credit: Betty Pace
Aggressive public health campaigns are needed to educate people in sub-Saharan Africa about sickle cell disease (SCD), according to a professor of health studies.
William Ebomoyi, PhD, of Chicago State University in Illinois, investigated the prevalence of SCD in sub-Saharan Africa, assessed the physical and emotional ramifications of the disease, evaluated ethical and legal issues related to SCD, and assessed the socio-cultural implications of the disease.
He reported his findings in the International Journal of Medical Engineering and Informatics.
Dr Ebomoyi explained that SCD occurs from a change in valine to glutamine substitution in the sixth amino acid position of the beta globin chain. If one of the two beta globin genes is affected, a person simply has sickle cell trait (SCT), but if both genes are involved, the person has SCD.
If two people with SCT decide to have a child, there is a 50% chance that child will have SCT, a 25% chance the child will have SCD, and a 25% chance the child will have neither condition. If one parent has SCT, there is a 50% chance the child will have SCT.
In some communities in sub-Saharan Africa, up to 2% of all children are born with SCD. And the prevalence of SCT ranges from 10% to 40% across equatorial Africa.
Dr Ebomoyi said early screening to identify infants with SCD is needed, as life-threatening complications can occur within the first 3 years of life. Unfortunately, the method of choice for SCD screening—cellulose acetate accompanied by citrate agar electrophoresis—is only available in two sub-Saharan African nations—South Africa and Ghana.
Similarly, innovative SCD treatment techniques have not been introduced in sub-Saharan African nations. There are not enough well-trained physicians, Dr Ebomoyi said. In fact, many SCD patients are treated improperly by traditional African healers.
Furthermore, aside from Senegal and Liberia, sub-Saharan African nations spend less than 10% of their gross domestic product on healthcare. And inadequate funding plays a major role in the high prevalence of SCD in these nations.
For all these reasons, it is important to raise awareness in sub-Saharan Africa about SCD, according to Dr Ebomoyi. He said members of the public should be aware of how they can pass SCD down to their children and inform their partners if they have SCT prior to conceiving a child.
He added that sickle cell education programs should be integrated into the curriculum of elementary, secondary, and tertiary academic institutions.
The abstract of this article, “Ethical, legal, social, and financial implications of neonatal screening for sickle cell anaemia in Sub-Sahara Africa in the age of genomic science,” can be found on the Inderscience Publishers website. ![]()