User login
Is immediate-release topiramate an effective treatment for adult obesity?
EVIDENCE-BASED ANSWER:
Yes. Topiramate (at daily doses of 64-400 mg) produces an average 5.34 kg of additional weight loss compared with placebo (95% confidence interval [CI], -6.12 to -4.56) in overweight to obese adults for periods of 16 to 60 weeks (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs]).
Topiramate increases the chances of losing 5% or more of baseline body weight (BBW) with a number needed to treat (NNT) of 3 (95% CI, 2-3) and 10% or more of BBW with an NNT of 4 (95% CI, 3-4). However, approximately 17% of patients discontinue the drug because of adverse effects, including paresthesia, hypoesthesia, taste perversion, and psychomotor impairment (SOR: A, meta-analyses of RCTs).
EVIDENCE SUMMARY
A meta-analysis of 10 well-done RCTs with a total of 3320 patients found that topiramate produced more weight loss than placebo.1 Studies included men and women ages 18 to 75 years, with a body mass index (BMI) of 27 to 50. Several studies included patients with hypertension, dyslipidemia, and diabetes mellitus; one study included patients with binge eating disorder. Investigators recruited subjects from sites in Europe, North America, Australia, and South Africa. The studies lasted 16 to 60 weeks and used variable doses of topiramate (64-400 mg daily). Most incorporated a structured lifestyle intervention program for both the treatment and control groups.
Patients taking topiramate lost 5.34 kg (95% CI, -6.12 to -4.56) more than subjects taking placebo. All studies showed significantly greater weight loss in the topiramate groups, regardless of dose and duration, although there was some heterogeneity among the results. The NNTs to achieve weight loss of 5% or more of BBW and 10% or more of BBW were 3 (95% CI, 2-3) and 4 (95% CI, 3-4), respectively.
No major adverse events, but some unpleasant effects
A safety analysis on 6620 subjects found no major adverse events.1 Subjects in the topiramate group were more likely to withdraw because of adverse effects (odds ratio=1.97; 95% CI, 1.64-2.29; number needed to harm=14; 95% CI, 11-18). The most common adverse effects were paresthesia, hypoesthesia, taste perversion, and psychomotor impairment, and these effects were most likely to lead to discontinuation at daily doses >96 mg.
Two formulas are effective in patients with diabetes
Investigators stopped 6 studies early because the sponsor wanted to pursue development of a controlled-release formulation of topiramate. The meta-analysis includes a single study of controlled-release topiramate, 175 mg daily in patients with diabetes, that showed equivalent efficacy and similar tolerability to immediate-release topiramate.2
Three other RCTs included in the meta-analysis specifically examined obese patients with type 2 diabetes, a population deemed more resistant to typical weight loss regimens, treated with immediate-release topiramate in dosages of 96 mg and 192 mg daily.3-5 These patients also experienced greater weight loss than patients taking placebo, comparable to what was seen in the overall meta-analysis.
FDA approval and cost of therapy
Topiramate monotherapy isn’t approved by the US Food and Drug Administration (FDA) for obesity treatment. In 2012, the FDA approved phentermine/topiramate extended-release (Qsymia) for long-term treatment of obesity; the monthly cost for a maintenance dose of 7.5 mg/46 mg daily is approximately $185.6 Topiramate immediate-release tablets cost approximately $25 per month for twice daily doses of 50 to 100 mg.7
RECOMMENDATIONS
The US Preventive Services Task Force recommends screening all adults for obesity by measuring BMI and referring patients with a BMI ≥30 for high-intensity, comprehensive behavioral interventions. It makes no recommendation for pharmacologic management.8
The Institute for Clinical Systems Improvement concludes that pharmacotherapy should be used only as part of a comprehensive obesity treatment plan. Pharmacotherapy should be considered if obese patients are unable to lose 1 pound per week with diet, physical activity, and behavior modification.9
1. Kramer CK, Leitão CB, Pinto LC, et al. Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials. Obes Rev. 2011;12:e338-e347.
2. Rosenstock J, Hollander P, Gadde KM, et al; OBD-202 Study Group. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30:1480-1486.
3. Stenlöf K, Rössner S, Vercruysse F, et al. Topiramate in the treatment of obese subjects with drug-naive type 2 diabetes. Diabetes Obes Metab. 2007;9:360-368.
4. Toplak H, Hamann A, Moore R, et al. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond). 2007;31:138-146.
5. Eliasson B, Gudbjörnsdottir S, Cederholm J, et al. Weight loss and metabolic effects of topiramate in overweight and obese type 2 diabetic patients: randomized double-blind placebo-controlled trial. Int J Obes (Lond). 2007;31: 1140-1147.
6. Drugs.com. Qsymia. Drugs.com Web site. Available at: www.drugs.com/pro/qsymia.html. Accessed September 26, 2014.
7. Drugs.com. Topirimate prices, coupons and patient assistance programs. Drugs.com Web site. Available at: www.drugs.com/price-guide/topiramate. Accessed September 26, 2014.
8. US Preventive Services Task Force. Obesity in Adults: Screening and management. US Preventive Services Task Force Web site. Available at: www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed September 30, 2014.
9. Fitch A, Everling L, Fox C, et al. Prevention and management of obesity for adults. Institute for Clinical Systems Improvement Web site. Available at: www.icsi.org/_asset/s935hy/Obesity-Adults.pdf. Accessed September 30, 2014.
EVIDENCE-BASED ANSWER:
Yes. Topiramate (at daily doses of 64-400 mg) produces an average 5.34 kg of additional weight loss compared with placebo (95% confidence interval [CI], -6.12 to -4.56) in overweight to obese adults for periods of 16 to 60 weeks (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs]).
Topiramate increases the chances of losing 5% or more of baseline body weight (BBW) with a number needed to treat (NNT) of 3 (95% CI, 2-3) and 10% or more of BBW with an NNT of 4 (95% CI, 3-4). However, approximately 17% of patients discontinue the drug because of adverse effects, including paresthesia, hypoesthesia, taste perversion, and psychomotor impairment (SOR: A, meta-analyses of RCTs).
EVIDENCE SUMMARY
A meta-analysis of 10 well-done RCTs with a total of 3320 patients found that topiramate produced more weight loss than placebo.1 Studies included men and women ages 18 to 75 years, with a body mass index (BMI) of 27 to 50. Several studies included patients with hypertension, dyslipidemia, and diabetes mellitus; one study included patients with binge eating disorder. Investigators recruited subjects from sites in Europe, North America, Australia, and South Africa. The studies lasted 16 to 60 weeks and used variable doses of topiramate (64-400 mg daily). Most incorporated a structured lifestyle intervention program for both the treatment and control groups.
Patients taking topiramate lost 5.34 kg (95% CI, -6.12 to -4.56) more than subjects taking placebo. All studies showed significantly greater weight loss in the topiramate groups, regardless of dose and duration, although there was some heterogeneity among the results. The NNTs to achieve weight loss of 5% or more of BBW and 10% or more of BBW were 3 (95% CI, 2-3) and 4 (95% CI, 3-4), respectively.
No major adverse events, but some unpleasant effects
A safety analysis on 6620 subjects found no major adverse events.1 Subjects in the topiramate group were more likely to withdraw because of adverse effects (odds ratio=1.97; 95% CI, 1.64-2.29; number needed to harm=14; 95% CI, 11-18). The most common adverse effects were paresthesia, hypoesthesia, taste perversion, and psychomotor impairment, and these effects were most likely to lead to discontinuation at daily doses >96 mg.
Two formulas are effective in patients with diabetes
Investigators stopped 6 studies early because the sponsor wanted to pursue development of a controlled-release formulation of topiramate. The meta-analysis includes a single study of controlled-release topiramate, 175 mg daily in patients with diabetes, that showed equivalent efficacy and similar tolerability to immediate-release topiramate.2
Three other RCTs included in the meta-analysis specifically examined obese patients with type 2 diabetes, a population deemed more resistant to typical weight loss regimens, treated with immediate-release topiramate in dosages of 96 mg and 192 mg daily.3-5 These patients also experienced greater weight loss than patients taking placebo, comparable to what was seen in the overall meta-analysis.
FDA approval and cost of therapy
Topiramate monotherapy isn’t approved by the US Food and Drug Administration (FDA) for obesity treatment. In 2012, the FDA approved phentermine/topiramate extended-release (Qsymia) for long-term treatment of obesity; the monthly cost for a maintenance dose of 7.5 mg/46 mg daily is approximately $185.6 Topiramate immediate-release tablets cost approximately $25 per month for twice daily doses of 50 to 100 mg.7
RECOMMENDATIONS
The US Preventive Services Task Force recommends screening all adults for obesity by measuring BMI and referring patients with a BMI ≥30 for high-intensity, comprehensive behavioral interventions. It makes no recommendation for pharmacologic management.8
The Institute for Clinical Systems Improvement concludes that pharmacotherapy should be used only as part of a comprehensive obesity treatment plan. Pharmacotherapy should be considered if obese patients are unable to lose 1 pound per week with diet, physical activity, and behavior modification.9
EVIDENCE-BASED ANSWER:
Yes. Topiramate (at daily doses of 64-400 mg) produces an average 5.34 kg of additional weight loss compared with placebo (95% confidence interval [CI], -6.12 to -4.56) in overweight to obese adults for periods of 16 to 60 weeks (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs]).
Topiramate increases the chances of losing 5% or more of baseline body weight (BBW) with a number needed to treat (NNT) of 3 (95% CI, 2-3) and 10% or more of BBW with an NNT of 4 (95% CI, 3-4). However, approximately 17% of patients discontinue the drug because of adverse effects, including paresthesia, hypoesthesia, taste perversion, and psychomotor impairment (SOR: A, meta-analyses of RCTs).
EVIDENCE SUMMARY
A meta-analysis of 10 well-done RCTs with a total of 3320 patients found that topiramate produced more weight loss than placebo.1 Studies included men and women ages 18 to 75 years, with a body mass index (BMI) of 27 to 50. Several studies included patients with hypertension, dyslipidemia, and diabetes mellitus; one study included patients with binge eating disorder. Investigators recruited subjects from sites in Europe, North America, Australia, and South Africa. The studies lasted 16 to 60 weeks and used variable doses of topiramate (64-400 mg daily). Most incorporated a structured lifestyle intervention program for both the treatment and control groups.
Patients taking topiramate lost 5.34 kg (95% CI, -6.12 to -4.56) more than subjects taking placebo. All studies showed significantly greater weight loss in the topiramate groups, regardless of dose and duration, although there was some heterogeneity among the results. The NNTs to achieve weight loss of 5% or more of BBW and 10% or more of BBW were 3 (95% CI, 2-3) and 4 (95% CI, 3-4), respectively.
No major adverse events, but some unpleasant effects
A safety analysis on 6620 subjects found no major adverse events.1 Subjects in the topiramate group were more likely to withdraw because of adverse effects (odds ratio=1.97; 95% CI, 1.64-2.29; number needed to harm=14; 95% CI, 11-18). The most common adverse effects were paresthesia, hypoesthesia, taste perversion, and psychomotor impairment, and these effects were most likely to lead to discontinuation at daily doses >96 mg.
Two formulas are effective in patients with diabetes
Investigators stopped 6 studies early because the sponsor wanted to pursue development of a controlled-release formulation of topiramate. The meta-analysis includes a single study of controlled-release topiramate, 175 mg daily in patients with diabetes, that showed equivalent efficacy and similar tolerability to immediate-release topiramate.2
Three other RCTs included in the meta-analysis specifically examined obese patients with type 2 diabetes, a population deemed more resistant to typical weight loss regimens, treated with immediate-release topiramate in dosages of 96 mg and 192 mg daily.3-5 These patients also experienced greater weight loss than patients taking placebo, comparable to what was seen in the overall meta-analysis.
FDA approval and cost of therapy
Topiramate monotherapy isn’t approved by the US Food and Drug Administration (FDA) for obesity treatment. In 2012, the FDA approved phentermine/topiramate extended-release (Qsymia) for long-term treatment of obesity; the monthly cost for a maintenance dose of 7.5 mg/46 mg daily is approximately $185.6 Topiramate immediate-release tablets cost approximately $25 per month for twice daily doses of 50 to 100 mg.7
RECOMMENDATIONS
The US Preventive Services Task Force recommends screening all adults for obesity by measuring BMI and referring patients with a BMI ≥30 for high-intensity, comprehensive behavioral interventions. It makes no recommendation for pharmacologic management.8
The Institute for Clinical Systems Improvement concludes that pharmacotherapy should be used only as part of a comprehensive obesity treatment plan. Pharmacotherapy should be considered if obese patients are unable to lose 1 pound per week with diet, physical activity, and behavior modification.9
1. Kramer CK, Leitão CB, Pinto LC, et al. Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials. Obes Rev. 2011;12:e338-e347.
2. Rosenstock J, Hollander P, Gadde KM, et al; OBD-202 Study Group. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30:1480-1486.
3. Stenlöf K, Rössner S, Vercruysse F, et al. Topiramate in the treatment of obese subjects with drug-naive type 2 diabetes. Diabetes Obes Metab. 2007;9:360-368.
4. Toplak H, Hamann A, Moore R, et al. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond). 2007;31:138-146.
5. Eliasson B, Gudbjörnsdottir S, Cederholm J, et al. Weight loss and metabolic effects of topiramate in overweight and obese type 2 diabetic patients: randomized double-blind placebo-controlled trial. Int J Obes (Lond). 2007;31: 1140-1147.
6. Drugs.com. Qsymia. Drugs.com Web site. Available at: www.drugs.com/pro/qsymia.html. Accessed September 26, 2014.
7. Drugs.com. Topirimate prices, coupons and patient assistance programs. Drugs.com Web site. Available at: www.drugs.com/price-guide/topiramate. Accessed September 26, 2014.
8. US Preventive Services Task Force. Obesity in Adults: Screening and management. US Preventive Services Task Force Web site. Available at: www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed September 30, 2014.
9. Fitch A, Everling L, Fox C, et al. Prevention and management of obesity for adults. Institute for Clinical Systems Improvement Web site. Available at: www.icsi.org/_asset/s935hy/Obesity-Adults.pdf. Accessed September 30, 2014.
1. Kramer CK, Leitão CB, Pinto LC, et al. Efficacy and safety of topiramate on weight loss: a meta-analysis of randomized controlled trials. Obes Rev. 2011;12:e338-e347.
2. Rosenstock J, Hollander P, Gadde KM, et al; OBD-202 Study Group. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30:1480-1486.
3. Stenlöf K, Rössner S, Vercruysse F, et al. Topiramate in the treatment of obese subjects with drug-naive type 2 diabetes. Diabetes Obes Metab. 2007;9:360-368.
4. Toplak H, Hamann A, Moore R, et al. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond). 2007;31:138-146.
5. Eliasson B, Gudbjörnsdottir S, Cederholm J, et al. Weight loss and metabolic effects of topiramate in overweight and obese type 2 diabetic patients: randomized double-blind placebo-controlled trial. Int J Obes (Lond). 2007;31: 1140-1147.
6. Drugs.com. Qsymia. Drugs.com Web site. Available at: www.drugs.com/pro/qsymia.html. Accessed September 26, 2014.
7. Drugs.com. Topirimate prices, coupons and patient assistance programs. Drugs.com Web site. Available at: www.drugs.com/price-guide/topiramate. Accessed September 26, 2014.
8. US Preventive Services Task Force. Obesity in Adults: Screening and management. US Preventive Services Task Force Web site. Available at: www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed September 30, 2014.
9. Fitch A, Everling L, Fox C, et al. Prevention and management of obesity for adults. Institute for Clinical Systems Improvement Web site. Available at: www.icsi.org/_asset/s935hy/Obesity-Adults.pdf. Accessed September 30, 2014.
Evidence-based answers from the Family Physicians Inquiries Network
High failure rate seen with limited parathyroidectomy in patients with MEN-1
SAN FRANCISCO – Patients with hyperparathyroidism due to multiple endocrine neoplasia type 1 (MEN-1) have a 4 in 10 chance of persistent hyperparathyroidism if they undergo surgery that leaves at least one gland in place, according to a retrospective cohort study presented at the annual clinical congress of the American College of Surgeons.
“Limited initial parathyroidectomy in patients with MEN-1–associated primary hyperparathyroidism results in a high failure rate. Additional enlarged contralateral parathyroid glands are frequently missed by preoperative localizing studies,” commented lead investigator Dr. Naris Nilubol, a staff clinician with the endocrine oncology branch of the Center for Cancer Research, National Cancer Institute, Bethesda, Md.
“We conclude that limited parathyroidectomy in MEN-1 guided by preoperative localizing studies is associated with high failure rates and therefore should not be performed,” he maintained.
In an interview, session comoderator Dr. Marybeth S. Hughes, a staff clinician with the thoracic and gastrointestinal oncology branch, Center for Cancer Research, National Cancer Institute, commented, “In general, I would say that the data presented just reiterates the standard of care, that MEN-1 patients should have bilateral neck exploration with [removal of] three and half glands, or four glands with autotransplantation. So it just basically solidifies what is being done standardly. I don’t think there is a compelling argument to change the standard.”
Dr. Nilubol and colleagues reviewed the charts of 99 patients with MEN-1 who underwent at least one parathyroidectomy at the National Institutes of Health (NIH).
Of the 64 patients who had initial surgery at NIH and had preoperative localizing studies done, 32 had only a single enlarged gland identified by the tests, suggesting they would be good candidates for limited surgery, according to Dr. Nilubol. Bilateral neck dissection at the time of parathyroidectomy showed that in 22 (69%) of these 32 patients, the studies had correctly identified the largest gland; however, in 19 (87%) of those 22, it missed another enlarged gland on the contralateral side. Furthermore, in 5 (16%) of the 32, the largest gland was found on the contralateral side.
With a median follow-up of 23 months, the risk of persistent hyperparathyroidism was 41% for patients who had limited parathyroidectomy (three or fewer glands removed) at initial surgery, significantly and sharply higher than the 6% seen in patients who had subtotal parathyroidectomy or more extensive surgery (at least three and a half glands removed).
Looking at the cumulative number of glands removed during initial and subsequent surgeries, 57% of patients having two or fewer glands removed and 45% of those having two and a half to three glands removed had persistent hyperparathyroidism – both significantly higher than the 5% of patients having at least three and a half glands removed.
Regarding complications, 10% of the patients who had their initial surgery at NIH developed permanent hypoparathyroidism, reported Dr. Nilubol, who disclosed that he had no relevant conflicts of interest.
Session attendees asked about the use of parathyroid hormone levels intraoperatively to guide surgery and what strategy surgeons follow at his institution in this patient population.
Previous research has suggested that intraoperative parathyroid hormone levels do not add any information that would change the operative plan, Dr. Nilubol replied. “Everybody at NIH has preop localizing studies as part of the clinical investigation, but it doesn’t change the way we approach it. Everybody gets a bilateral neck exploration and three and a half–gland removal,” provided all glands can be found, he said.
Session attendee Dr. Michael J. Campbell, a surgeon at the University California, Davis, commented, “A 10% permanent hypoparathyroidism rate in these patients – and they have a tendency to be young, most of them in their late teens, early 20s – that’s a major complication. So could you take your data and make exactly the opposite argument, that maybe you should be doing less to these patients to limit that fairly life-altering complication?” Permanent hypothyroidism at that age is “a significant medical problem,” Dr. Nilubol agreed. However, “at the NIH, we don’t operate on everybody just because they have primary hyperparathyroidism. They have to fulfill metabolic complications before we choose to operate on them. We want to delay the surgeries and [time] between the surgeries because if they live long enough, it will recur, so we want to operate when we can make the most difference, meaning [addressing] kidney stone, bone loss, etc. The most common reason for young patients is they have kidney stones, which leads to surgery.”
SAN FRANCISCO – Patients with hyperparathyroidism due to multiple endocrine neoplasia type 1 (MEN-1) have a 4 in 10 chance of persistent hyperparathyroidism if they undergo surgery that leaves at least one gland in place, according to a retrospective cohort study presented at the annual clinical congress of the American College of Surgeons.
“Limited initial parathyroidectomy in patients with MEN-1–associated primary hyperparathyroidism results in a high failure rate. Additional enlarged contralateral parathyroid glands are frequently missed by preoperative localizing studies,” commented lead investigator Dr. Naris Nilubol, a staff clinician with the endocrine oncology branch of the Center for Cancer Research, National Cancer Institute, Bethesda, Md.
“We conclude that limited parathyroidectomy in MEN-1 guided by preoperative localizing studies is associated with high failure rates and therefore should not be performed,” he maintained.
In an interview, session comoderator Dr. Marybeth S. Hughes, a staff clinician with the thoracic and gastrointestinal oncology branch, Center for Cancer Research, National Cancer Institute, commented, “In general, I would say that the data presented just reiterates the standard of care, that MEN-1 patients should have bilateral neck exploration with [removal of] three and half glands, or four glands with autotransplantation. So it just basically solidifies what is being done standardly. I don’t think there is a compelling argument to change the standard.”
Dr. Nilubol and colleagues reviewed the charts of 99 patients with MEN-1 who underwent at least one parathyroidectomy at the National Institutes of Health (NIH).
Of the 64 patients who had initial surgery at NIH and had preoperative localizing studies done, 32 had only a single enlarged gland identified by the tests, suggesting they would be good candidates for limited surgery, according to Dr. Nilubol. Bilateral neck dissection at the time of parathyroidectomy showed that in 22 (69%) of these 32 patients, the studies had correctly identified the largest gland; however, in 19 (87%) of those 22, it missed another enlarged gland on the contralateral side. Furthermore, in 5 (16%) of the 32, the largest gland was found on the contralateral side.
With a median follow-up of 23 months, the risk of persistent hyperparathyroidism was 41% for patients who had limited parathyroidectomy (three or fewer glands removed) at initial surgery, significantly and sharply higher than the 6% seen in patients who had subtotal parathyroidectomy or more extensive surgery (at least three and a half glands removed).
Looking at the cumulative number of glands removed during initial and subsequent surgeries, 57% of patients having two or fewer glands removed and 45% of those having two and a half to three glands removed had persistent hyperparathyroidism – both significantly higher than the 5% of patients having at least three and a half glands removed.
Regarding complications, 10% of the patients who had their initial surgery at NIH developed permanent hypoparathyroidism, reported Dr. Nilubol, who disclosed that he had no relevant conflicts of interest.
Session attendees asked about the use of parathyroid hormone levels intraoperatively to guide surgery and what strategy surgeons follow at his institution in this patient population.
Previous research has suggested that intraoperative parathyroid hormone levels do not add any information that would change the operative plan, Dr. Nilubol replied. “Everybody at NIH has preop localizing studies as part of the clinical investigation, but it doesn’t change the way we approach it. Everybody gets a bilateral neck exploration and three and a half–gland removal,” provided all glands can be found, he said.
Session attendee Dr. Michael J. Campbell, a surgeon at the University California, Davis, commented, “A 10% permanent hypoparathyroidism rate in these patients – and they have a tendency to be young, most of them in their late teens, early 20s – that’s a major complication. So could you take your data and make exactly the opposite argument, that maybe you should be doing less to these patients to limit that fairly life-altering complication?” Permanent hypothyroidism at that age is “a significant medical problem,” Dr. Nilubol agreed. However, “at the NIH, we don’t operate on everybody just because they have primary hyperparathyroidism. They have to fulfill metabolic complications before we choose to operate on them. We want to delay the surgeries and [time] between the surgeries because if they live long enough, it will recur, so we want to operate when we can make the most difference, meaning [addressing] kidney stone, bone loss, etc. The most common reason for young patients is they have kidney stones, which leads to surgery.”
SAN FRANCISCO – Patients with hyperparathyroidism due to multiple endocrine neoplasia type 1 (MEN-1) have a 4 in 10 chance of persistent hyperparathyroidism if they undergo surgery that leaves at least one gland in place, according to a retrospective cohort study presented at the annual clinical congress of the American College of Surgeons.
“Limited initial parathyroidectomy in patients with MEN-1–associated primary hyperparathyroidism results in a high failure rate. Additional enlarged contralateral parathyroid glands are frequently missed by preoperative localizing studies,” commented lead investigator Dr. Naris Nilubol, a staff clinician with the endocrine oncology branch of the Center for Cancer Research, National Cancer Institute, Bethesda, Md.
“We conclude that limited parathyroidectomy in MEN-1 guided by preoperative localizing studies is associated with high failure rates and therefore should not be performed,” he maintained.
In an interview, session comoderator Dr. Marybeth S. Hughes, a staff clinician with the thoracic and gastrointestinal oncology branch, Center for Cancer Research, National Cancer Institute, commented, “In general, I would say that the data presented just reiterates the standard of care, that MEN-1 patients should have bilateral neck exploration with [removal of] three and half glands, or four glands with autotransplantation. So it just basically solidifies what is being done standardly. I don’t think there is a compelling argument to change the standard.”
Dr. Nilubol and colleagues reviewed the charts of 99 patients with MEN-1 who underwent at least one parathyroidectomy at the National Institutes of Health (NIH).
Of the 64 patients who had initial surgery at NIH and had preoperative localizing studies done, 32 had only a single enlarged gland identified by the tests, suggesting they would be good candidates for limited surgery, according to Dr. Nilubol. Bilateral neck dissection at the time of parathyroidectomy showed that in 22 (69%) of these 32 patients, the studies had correctly identified the largest gland; however, in 19 (87%) of those 22, it missed another enlarged gland on the contralateral side. Furthermore, in 5 (16%) of the 32, the largest gland was found on the contralateral side.
With a median follow-up of 23 months, the risk of persistent hyperparathyroidism was 41% for patients who had limited parathyroidectomy (three or fewer glands removed) at initial surgery, significantly and sharply higher than the 6% seen in patients who had subtotal parathyroidectomy or more extensive surgery (at least three and a half glands removed).
Looking at the cumulative number of glands removed during initial and subsequent surgeries, 57% of patients having two or fewer glands removed and 45% of those having two and a half to three glands removed had persistent hyperparathyroidism – both significantly higher than the 5% of patients having at least three and a half glands removed.
Regarding complications, 10% of the patients who had their initial surgery at NIH developed permanent hypoparathyroidism, reported Dr. Nilubol, who disclosed that he had no relevant conflicts of interest.
Session attendees asked about the use of parathyroid hormone levels intraoperatively to guide surgery and what strategy surgeons follow at his institution in this patient population.
Previous research has suggested that intraoperative parathyroid hormone levels do not add any information that would change the operative plan, Dr. Nilubol replied. “Everybody at NIH has preop localizing studies as part of the clinical investigation, but it doesn’t change the way we approach it. Everybody gets a bilateral neck exploration and three and a half–gland removal,” provided all glands can be found, he said.
Session attendee Dr. Michael J. Campbell, a surgeon at the University California, Davis, commented, “A 10% permanent hypoparathyroidism rate in these patients – and they have a tendency to be young, most of them in their late teens, early 20s – that’s a major complication. So could you take your data and make exactly the opposite argument, that maybe you should be doing less to these patients to limit that fairly life-altering complication?” Permanent hypothyroidism at that age is “a significant medical problem,” Dr. Nilubol agreed. However, “at the NIH, we don’t operate on everybody just because they have primary hyperparathyroidism. They have to fulfill metabolic complications before we choose to operate on them. We want to delay the surgeries and [time] between the surgeries because if they live long enough, it will recur, so we want to operate when we can make the most difference, meaning [addressing] kidney stone, bone loss, etc. The most common reason for young patients is they have kidney stones, which leads to surgery.”
AT THE ACS CLINICAL CONGRESS
Key clinical point: Patients are more likely to have persistent hyperparathyroidism if a gland is left behind.
Major finding: The failure rate after initial parathyroidectomy was 41% with limited surgery versus 6% with subtotal or more extensive surgery.
Data source: A retrospective chart review of 99 patients with MEN-1–associated hyperparathyroidism.
Disclosures: Dr. Nilubol disclosed that he had no relevant conflicts of interest.
Diabetes therapy and cancer risk
To the Editor: I would like to add three points to the excellent review of diabetes therapy and cancer risk by Drs. Sun, Kashyap, and Nasr in the October 2014 issue of Cleveland Clinic Journal of Medicine.1
First, a recent 10-year prospective observational study of more than 190,000 patients showed no increase in bladder cancer with exposure to or long-term use of pioglitazone vs comparator when smoking status was controlled. Although publicly released, these 10-year data have not yet been published.
Second, a recent paper2 from the US Food and Drug Administration and European Medicine Agency reviewed the pancreatic safety of incretin-based therapies. They concluded that there is no evidence that these agents increase the risk of pancreatitis or of pancreatic cancer. So I believe that the authors’ comment that pancreatitis is a “potential side effect” of these agents is not quite accurate.
Lastly, the authors cite no substantial evidence that would support their statement to avoid using glucagon-like protein 1 (GLP-1) receptor agonists in those with a personal history of differentiated thyroid cancer. Indeed these patients, if adequately treated, should have no remnant thyroid tissue. The rodent data indicate an effect of GLP-1 agonists on rodent C cells, not thyroid follicular cells.3 In addition, the prescribing information for these agents does not advise such a limitation on their use.
- Ching Sun GE, Kashyap SR, Nasr C. Diabetes therapy and cancer risk: where do we stand when treating patients? Cleve Clin J Med 2014; 81:620–628.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
To the Editor: I would like to add three points to the excellent review of diabetes therapy and cancer risk by Drs. Sun, Kashyap, and Nasr in the October 2014 issue of Cleveland Clinic Journal of Medicine.1
First, a recent 10-year prospective observational study of more than 190,000 patients showed no increase in bladder cancer with exposure to or long-term use of pioglitazone vs comparator when smoking status was controlled. Although publicly released, these 10-year data have not yet been published.
Second, a recent paper2 from the US Food and Drug Administration and European Medicine Agency reviewed the pancreatic safety of incretin-based therapies. They concluded that there is no evidence that these agents increase the risk of pancreatitis or of pancreatic cancer. So I believe that the authors’ comment that pancreatitis is a “potential side effect” of these agents is not quite accurate.
Lastly, the authors cite no substantial evidence that would support their statement to avoid using glucagon-like protein 1 (GLP-1) receptor agonists in those with a personal history of differentiated thyroid cancer. Indeed these patients, if adequately treated, should have no remnant thyroid tissue. The rodent data indicate an effect of GLP-1 agonists on rodent C cells, not thyroid follicular cells.3 In addition, the prescribing information for these agents does not advise such a limitation on their use.
To the Editor: I would like to add three points to the excellent review of diabetes therapy and cancer risk by Drs. Sun, Kashyap, and Nasr in the October 2014 issue of Cleveland Clinic Journal of Medicine.1
First, a recent 10-year prospective observational study of more than 190,000 patients showed no increase in bladder cancer with exposure to or long-term use of pioglitazone vs comparator when smoking status was controlled. Although publicly released, these 10-year data have not yet been published.
Second, a recent paper2 from the US Food and Drug Administration and European Medicine Agency reviewed the pancreatic safety of incretin-based therapies. They concluded that there is no evidence that these agents increase the risk of pancreatitis or of pancreatic cancer. So I believe that the authors’ comment that pancreatitis is a “potential side effect” of these agents is not quite accurate.
Lastly, the authors cite no substantial evidence that would support their statement to avoid using glucagon-like protein 1 (GLP-1) receptor agonists in those with a personal history of differentiated thyroid cancer. Indeed these patients, if adequately treated, should have no remnant thyroid tissue. The rodent data indicate an effect of GLP-1 agonists on rodent C cells, not thyroid follicular cells.3 In addition, the prescribing information for these agents does not advise such a limitation on their use.
- Ching Sun GE, Kashyap SR, Nasr C. Diabetes therapy and cancer risk: where do we stand when treating patients? Cleve Clin J Med 2014; 81:620–628.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
- Ching Sun GE, Kashyap SR, Nasr C. Diabetes therapy and cancer risk: where do we stand when treating patients? Cleve Clin J Med 2014; 81:620–628.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010; 151:1473–1486.
In reply: Diabetes therapy and cancer risk
In Reply: In regard to Dr. Weiss’s first point, the Kaiser Permanente Northern California diabetes registry study aimed to assess the association between bladder cancer and pioglitazone in 193,099 patients. In their 2011 interim 5-year analysis, Lewis et al reported a modest but statistically significant increased risk of bladder cancer in patients with type 2 diabetes mellitus who used pioglitazone for 2 or more years.1
We appreciate Dr. Weiss’s comment on the 10-year study conclusion data. As Dr. Weiss has indicated, the recent Takeda news release2 showed that the primary analysis found no association between pioglitazone use and bladder cancer risk. Furthermore, no association was found between bladder cancer risk and duration of use, higher cumulative doses, or time since initiation of pioglitazone.2
Regarding Dr. Weiss’s second point, we agree that at this time the cumulative data are not supportive of pancreatitis as per Egan et al.3 Recent publication of the SAVOR-TIMI trial4 of saxagliptin documented no increased risk of pancreatitis or pancreatic cancer over 2.1 years of follow-up in more than 16,000 patients over the age of 40 with type 2 diabetes. However, since amylase and lipase levels were not routinely checked in study participants, subclinical and asymptomatic cases may not have been recognized.4 Therefore, we stand by our statement that pancreatitis is a potential side effect.
It is important to recognize that although the observational data reviewed by both agencies (the US Food and Drug Administration and European Medicine Agency) in the publication by Egan et al3 are reassuring, we cannot yet say with absolute certainty that there is no associated risk. In fact, the concluding statements of the publication are as follows: “Although the totality of the data that have been reviewed provides reassurance, pancreatitis will continue to be considered a risk associated with these drugs until more data are available; both agencies continue to investigate this safety signal.”3
On September 18, 2014, the newest approved GLP-1 receptor agonist, dulaglutide, was approved with a boxed warning that it causes thyroid C-cell tumors in rats, that whether it causes thyroid C-cell tumors including medullary thyroid carcinoma (MTC) in humans is unknown, and that since relevance to humans could not be determined from clinical or nonclinical studies, dulaglutide is contraindicated in patients with a personal or family history of MTC, as well as in patients with multiple endocrine neoplasia syndrome type 2.5
It is important to recognize that despite these controversies, which have not been well-supported to date, incretin-based therapies have numerous metabolic benefits, including favorable glycemic and weight effects.
In regard to Dr. Weiss’s last point, we would like to point out the study by Gier et al6 in which GLP-1 receptor expression was found in 3 of 17 cases of human papillary thyroid cancer. The implication is that abnormal thyroid tissue does not behave the same way as normal tissue.
Furthermore, Dr. Weiss brings up the point that patients with thyroid cancer, if it is adequately treated, should have no remnant thyroid tissue. Certainly, adequate treatment would be an easy call to make if a stimulated thyroglobulin level is below the assay’s detection limit and there is no imaging evidence of residual thyroid cancer. For example, in someone with a history of thyroid cancer diagnosed more than 10 years ago without biochemical or imaging evidence of disease, any potential concerns of GLP-1 receptor agonist use in regards to thyroid cancer would be nominal. But not everyone with thyroid cancer falls into this category.
We do not suggest that these potential risks preclude the use of these agents in all patients, but rather that a discussion should occur between physician and patient. Diabetes therapy, as in treatment of other medical conditions, should be tailored to the individual patient, and all potential risk and benefits should be disclosed and considered.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Takeda Pharmaceuticals. 2014. Takeda announces completion of the post-marketing commitment to submit data to the FDA, the EMA and the PMDA for pioglitazone containing medicines including ACTOS. [Press release]. Accessed 19 October 2014. www.takeda.us/newsroom/press_release_detail.aspx?year=2014&id=314. Accessed November 3, 2014.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Raz I, Bhatt DL, Hirshberg B, et al. Incidence of pancreatitis and pancreatic cancer in a randomized controlled multicenter trial (SAVOR-TIMI 53) of the dipeptidyl peptidase-4 inhibitor saxagliptin. Diabetes Care 2014; 37:2435–2441.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly & Company; 2014.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
In Reply: In regard to Dr. Weiss’s first point, the Kaiser Permanente Northern California diabetes registry study aimed to assess the association between bladder cancer and pioglitazone in 193,099 patients. In their 2011 interim 5-year analysis, Lewis et al reported a modest but statistically significant increased risk of bladder cancer in patients with type 2 diabetes mellitus who used pioglitazone for 2 or more years.1
We appreciate Dr. Weiss’s comment on the 10-year study conclusion data. As Dr. Weiss has indicated, the recent Takeda news release2 showed that the primary analysis found no association between pioglitazone use and bladder cancer risk. Furthermore, no association was found between bladder cancer risk and duration of use, higher cumulative doses, or time since initiation of pioglitazone.2
Regarding Dr. Weiss’s second point, we agree that at this time the cumulative data are not supportive of pancreatitis as per Egan et al.3 Recent publication of the SAVOR-TIMI trial4 of saxagliptin documented no increased risk of pancreatitis or pancreatic cancer over 2.1 years of follow-up in more than 16,000 patients over the age of 40 with type 2 diabetes. However, since amylase and lipase levels were not routinely checked in study participants, subclinical and asymptomatic cases may not have been recognized.4 Therefore, we stand by our statement that pancreatitis is a potential side effect.
It is important to recognize that although the observational data reviewed by both agencies (the US Food and Drug Administration and European Medicine Agency) in the publication by Egan et al3 are reassuring, we cannot yet say with absolute certainty that there is no associated risk. In fact, the concluding statements of the publication are as follows: “Although the totality of the data that have been reviewed provides reassurance, pancreatitis will continue to be considered a risk associated with these drugs until more data are available; both agencies continue to investigate this safety signal.”3
On September 18, 2014, the newest approved GLP-1 receptor agonist, dulaglutide, was approved with a boxed warning that it causes thyroid C-cell tumors in rats, that whether it causes thyroid C-cell tumors including medullary thyroid carcinoma (MTC) in humans is unknown, and that since relevance to humans could not be determined from clinical or nonclinical studies, dulaglutide is contraindicated in patients with a personal or family history of MTC, as well as in patients with multiple endocrine neoplasia syndrome type 2.5
It is important to recognize that despite these controversies, which have not been well-supported to date, incretin-based therapies have numerous metabolic benefits, including favorable glycemic and weight effects.
In regard to Dr. Weiss’s last point, we would like to point out the study by Gier et al6 in which GLP-1 receptor expression was found in 3 of 17 cases of human papillary thyroid cancer. The implication is that abnormal thyroid tissue does not behave the same way as normal tissue.
Furthermore, Dr. Weiss brings up the point that patients with thyroid cancer, if it is adequately treated, should have no remnant thyroid tissue. Certainly, adequate treatment would be an easy call to make if a stimulated thyroglobulin level is below the assay’s detection limit and there is no imaging evidence of residual thyroid cancer. For example, in someone with a history of thyroid cancer diagnosed more than 10 years ago without biochemical or imaging evidence of disease, any potential concerns of GLP-1 receptor agonist use in regards to thyroid cancer would be nominal. But not everyone with thyroid cancer falls into this category.
We do not suggest that these potential risks preclude the use of these agents in all patients, but rather that a discussion should occur between physician and patient. Diabetes therapy, as in treatment of other medical conditions, should be tailored to the individual patient, and all potential risk and benefits should be disclosed and considered.
In Reply: In regard to Dr. Weiss’s first point, the Kaiser Permanente Northern California diabetes registry study aimed to assess the association between bladder cancer and pioglitazone in 193,099 patients. In their 2011 interim 5-year analysis, Lewis et al reported a modest but statistically significant increased risk of bladder cancer in patients with type 2 diabetes mellitus who used pioglitazone for 2 or more years.1
We appreciate Dr. Weiss’s comment on the 10-year study conclusion data. As Dr. Weiss has indicated, the recent Takeda news release2 showed that the primary analysis found no association between pioglitazone use and bladder cancer risk. Furthermore, no association was found between bladder cancer risk and duration of use, higher cumulative doses, or time since initiation of pioglitazone.2
Regarding Dr. Weiss’s second point, we agree that at this time the cumulative data are not supportive of pancreatitis as per Egan et al.3 Recent publication of the SAVOR-TIMI trial4 of saxagliptin documented no increased risk of pancreatitis or pancreatic cancer over 2.1 years of follow-up in more than 16,000 patients over the age of 40 with type 2 diabetes. However, since amylase and lipase levels were not routinely checked in study participants, subclinical and asymptomatic cases may not have been recognized.4 Therefore, we stand by our statement that pancreatitis is a potential side effect.
It is important to recognize that although the observational data reviewed by both agencies (the US Food and Drug Administration and European Medicine Agency) in the publication by Egan et al3 are reassuring, we cannot yet say with absolute certainty that there is no associated risk. In fact, the concluding statements of the publication are as follows: “Although the totality of the data that have been reviewed provides reassurance, pancreatitis will continue to be considered a risk associated with these drugs until more data are available; both agencies continue to investigate this safety signal.”3
On September 18, 2014, the newest approved GLP-1 receptor agonist, dulaglutide, was approved with a boxed warning that it causes thyroid C-cell tumors in rats, that whether it causes thyroid C-cell tumors including medullary thyroid carcinoma (MTC) in humans is unknown, and that since relevance to humans could not be determined from clinical or nonclinical studies, dulaglutide is contraindicated in patients with a personal or family history of MTC, as well as in patients with multiple endocrine neoplasia syndrome type 2.5
It is important to recognize that despite these controversies, which have not been well-supported to date, incretin-based therapies have numerous metabolic benefits, including favorable glycemic and weight effects.
In regard to Dr. Weiss’s last point, we would like to point out the study by Gier et al6 in which GLP-1 receptor expression was found in 3 of 17 cases of human papillary thyroid cancer. The implication is that abnormal thyroid tissue does not behave the same way as normal tissue.
Furthermore, Dr. Weiss brings up the point that patients with thyroid cancer, if it is adequately treated, should have no remnant thyroid tissue. Certainly, adequate treatment would be an easy call to make if a stimulated thyroglobulin level is below the assay’s detection limit and there is no imaging evidence of residual thyroid cancer. For example, in someone with a history of thyroid cancer diagnosed more than 10 years ago without biochemical or imaging evidence of disease, any potential concerns of GLP-1 receptor agonist use in regards to thyroid cancer would be nominal. But not everyone with thyroid cancer falls into this category.
We do not suggest that these potential risks preclude the use of these agents in all patients, but rather that a discussion should occur between physician and patient. Diabetes therapy, as in treatment of other medical conditions, should be tailored to the individual patient, and all potential risk and benefits should be disclosed and considered.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Takeda Pharmaceuticals. 2014. Takeda announces completion of the post-marketing commitment to submit data to the FDA, the EMA and the PMDA for pioglitazone containing medicines including ACTOS. [Press release]. Accessed 19 October 2014. www.takeda.us/newsroom/press_release_detail.aspx?year=2014&id=314. Accessed November 3, 2014.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Raz I, Bhatt DL, Hirshberg B, et al. Incidence of pancreatitis and pancreatic cancer in a randomized controlled multicenter trial (SAVOR-TIMI 53) of the dipeptidyl peptidase-4 inhibitor saxagliptin. Diabetes Care 2014; 37:2435–2441.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly & Company; 2014.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
- Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011; 34:916–922.
- Takeda Pharmaceuticals. 2014. Takeda announces completion of the post-marketing commitment to submit data to the FDA, the EMA and the PMDA for pioglitazone containing medicines including ACTOS. [Press release]. Accessed 19 October 2014. www.takeda.us/newsroom/press_release_detail.aspx?year=2014&id=314. Accessed November 3, 2014.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med 2014; 370:794–797.
- Raz I, Bhatt DL, Hirshberg B, et al. Incidence of pancreatitis and pancreatic cancer in a randomized controlled multicenter trial (SAVOR-TIMI 53) of the dipeptidyl peptidase-4 inhibitor saxagliptin. Diabetes Care 2014; 37:2435–2441.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly & Company; 2014.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab 2012; 97:121–131.
Mutations indicate predisposition to blood cancers

Credit: Graham Colm
Two teams of researchers have identified somatic mutations that increase the likelihood a person will develop a hematologic malignancy.
This “pre-malignant” stage was detected simply by sequencing DNA from blood samples.
The researchers found that subjects carrying certain mutations had more than 10 times the risk of developing a hematologic malignancy than individuals without the mutations. And the risk increased with age.
Steven McCarroll, PhD, of Harvard Medical School in Boston, Massachusetts, and Benjamin Ebert, MD, PhD, also of Harvard Medical School, reported these findings in NEJM.
Both research teams looked at somatic mutations in DNA samples collected from the blood of subjects who had not been diagnosed with cancer or blood disorders.
Taking two very different approaches, the teams found that a surprising percentage of individuals had acquired a subset of the somatic mutations present in hematologic malignancies. And subjects with the mutations were more likely to develop these cancers.
This pre-malignant state was rare in individuals under the age of 40. But it appeared with increasing frequency with each decade of life, ultimately appearing in more than 10% of individuals over the age of 70.
The researchers believe these early mutations lie in wait for follow-on, cooperating mutations that, when they occur in the same cells as the earlier mutations, drive the cells toward cancer. The majority of mutations occurred in just 3 genes: DNMT3A, TET2, and ASXL1.
Dr Ebert’s group
Dr Ebert and his colleagues had hypothesized that, since hematologic malignancies increase with age, it might be possible to detect early somatic mutations that could be initiating the disease process, and these mutations might increase with age.
The researchers looked specifically at 160 genes known to be recurrently mutated in hematologic malignancies, using genetic data derived from approximately 17,000 blood samples originally obtained for studies on the genetics of type 2 diabetes.
The team found a roughly 11-fold increase in the risk of hematologic malignancy among subjects with the subset of somatic mutations linked to blood cancers. And there was a clear association between age and the frequency of these mutations.
Men were slightly more likely to have the mutations than women, and Hispanics were slightly less likely to have the mutations than other racial/ethnic groups.
The researchers also found an association between the presence of this pre-malignant state and the risk of overall mortality independent of malignancy. Individuals with the mutations had a higher risk of type 2 diabetes, coronary heart disease, and ischemic stroke as well.
However, additional research will be needed to determine the nature of these associations.
Dr McCarroll’s group
Dr McCarroll and his colleagues discovered the same phenomenon while trying to determine whether somatic mutations contribute to the risk of developing schizophrenia.
The team studied roughly 12,000 DNA samples from patients with schizophrenia and bipolar disorder, as well as healthy controls, searching across the whole genome at all of the protein-coding genes for patterns in somatic mutations.
The somatic mutations were concentrated in a handful of genes that turned out to be cancer genes.
So the researchers used electronic medical records to follow the patients’ medical histories, finding that subjects with these acquired mutations had a nearly 13-fold higher risk of developing a hematologic malignancy than subjects without the mutations.
The team conducted follow-up analyses on tumor samples from 2 patients who had progressed from this pre-malignant state to cancer. In both cases, the cancer developed from the same cells that had harbored the initiating mutations years earlier.
“The fact that both teams converged on strikingly similar findings, using very different approaches and looking at DNA from very different sets of patients, has given us great confidence in the results,” said study author Giulio Genovese, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts.
Next steps
The researchers emphasized that there is no clinical benefit today for testing for this pre-malignant state, as there are no treatments currently available that would address this condition in otherwise healthy people.
However, they said the results open the door to entirely new directions for research, toward early detection and even prevention of hematologic malignancies.
“The results demonstrate a way to identify high-risk cohorts—people who are at much higher than average risk of progressing to cancer—which could be a population for clinical trials of future prevention strategies,” Dr McCarroll said. “The abundance of these mutated cells could also serve as a biomarker—like LDL cholesterol is for cardiovascular disease—to test the effects of potential prevention therapies in clinical trials.”
Dr Ebert added, “A new focus of investigation will now be to develop interventions that might decrease the likelihood that individuals with these mutations will go on to develop overt malignancies, or therapeutic strategies to decrease mortality from other conditions that may be instigated by these mutations.”
This research is set to be presented on December 9 at the 56th ASH Annual Meeting in San Francisco. ![]()

Credit: Graham Colm
Two teams of researchers have identified somatic mutations that increase the likelihood a person will develop a hematologic malignancy.
This “pre-malignant” stage was detected simply by sequencing DNA from blood samples.
The researchers found that subjects carrying certain mutations had more than 10 times the risk of developing a hematologic malignancy than individuals without the mutations. And the risk increased with age.
Steven McCarroll, PhD, of Harvard Medical School in Boston, Massachusetts, and Benjamin Ebert, MD, PhD, also of Harvard Medical School, reported these findings in NEJM.
Both research teams looked at somatic mutations in DNA samples collected from the blood of subjects who had not been diagnosed with cancer or blood disorders.
Taking two very different approaches, the teams found that a surprising percentage of individuals had acquired a subset of the somatic mutations present in hematologic malignancies. And subjects with the mutations were more likely to develop these cancers.
This pre-malignant state was rare in individuals under the age of 40. But it appeared with increasing frequency with each decade of life, ultimately appearing in more than 10% of individuals over the age of 70.
The researchers believe these early mutations lie in wait for follow-on, cooperating mutations that, when they occur in the same cells as the earlier mutations, drive the cells toward cancer. The majority of mutations occurred in just 3 genes: DNMT3A, TET2, and ASXL1.
Dr Ebert’s group
Dr Ebert and his colleagues had hypothesized that, since hematologic malignancies increase with age, it might be possible to detect early somatic mutations that could be initiating the disease process, and these mutations might increase with age.
The researchers looked specifically at 160 genes known to be recurrently mutated in hematologic malignancies, using genetic data derived from approximately 17,000 blood samples originally obtained for studies on the genetics of type 2 diabetes.
The team found a roughly 11-fold increase in the risk of hematologic malignancy among subjects with the subset of somatic mutations linked to blood cancers. And there was a clear association between age and the frequency of these mutations.
Men were slightly more likely to have the mutations than women, and Hispanics were slightly less likely to have the mutations than other racial/ethnic groups.
The researchers also found an association between the presence of this pre-malignant state and the risk of overall mortality independent of malignancy. Individuals with the mutations had a higher risk of type 2 diabetes, coronary heart disease, and ischemic stroke as well.
However, additional research will be needed to determine the nature of these associations.
Dr McCarroll’s group
Dr McCarroll and his colleagues discovered the same phenomenon while trying to determine whether somatic mutations contribute to the risk of developing schizophrenia.
The team studied roughly 12,000 DNA samples from patients with schizophrenia and bipolar disorder, as well as healthy controls, searching across the whole genome at all of the protein-coding genes for patterns in somatic mutations.
The somatic mutations were concentrated in a handful of genes that turned out to be cancer genes.
So the researchers used electronic medical records to follow the patients’ medical histories, finding that subjects with these acquired mutations had a nearly 13-fold higher risk of developing a hematologic malignancy than subjects without the mutations.
The team conducted follow-up analyses on tumor samples from 2 patients who had progressed from this pre-malignant state to cancer. In both cases, the cancer developed from the same cells that had harbored the initiating mutations years earlier.
“The fact that both teams converged on strikingly similar findings, using very different approaches and looking at DNA from very different sets of patients, has given us great confidence in the results,” said study author Giulio Genovese, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts.
Next steps
The researchers emphasized that there is no clinical benefit today for testing for this pre-malignant state, as there are no treatments currently available that would address this condition in otherwise healthy people.
However, they said the results open the door to entirely new directions for research, toward early detection and even prevention of hematologic malignancies.
“The results demonstrate a way to identify high-risk cohorts—people who are at much higher than average risk of progressing to cancer—which could be a population for clinical trials of future prevention strategies,” Dr McCarroll said. “The abundance of these mutated cells could also serve as a biomarker—like LDL cholesterol is for cardiovascular disease—to test the effects of potential prevention therapies in clinical trials.”
Dr Ebert added, “A new focus of investigation will now be to develop interventions that might decrease the likelihood that individuals with these mutations will go on to develop overt malignancies, or therapeutic strategies to decrease mortality from other conditions that may be instigated by these mutations.”
This research is set to be presented on December 9 at the 56th ASH Annual Meeting in San Francisco. ![]()

Credit: Graham Colm
Two teams of researchers have identified somatic mutations that increase the likelihood a person will develop a hematologic malignancy.
This “pre-malignant” stage was detected simply by sequencing DNA from blood samples.
The researchers found that subjects carrying certain mutations had more than 10 times the risk of developing a hematologic malignancy than individuals without the mutations. And the risk increased with age.
Steven McCarroll, PhD, of Harvard Medical School in Boston, Massachusetts, and Benjamin Ebert, MD, PhD, also of Harvard Medical School, reported these findings in NEJM.
Both research teams looked at somatic mutations in DNA samples collected from the blood of subjects who had not been diagnosed with cancer or blood disorders.
Taking two very different approaches, the teams found that a surprising percentage of individuals had acquired a subset of the somatic mutations present in hematologic malignancies. And subjects with the mutations were more likely to develop these cancers.
This pre-malignant state was rare in individuals under the age of 40. But it appeared with increasing frequency with each decade of life, ultimately appearing in more than 10% of individuals over the age of 70.
The researchers believe these early mutations lie in wait for follow-on, cooperating mutations that, when they occur in the same cells as the earlier mutations, drive the cells toward cancer. The majority of mutations occurred in just 3 genes: DNMT3A, TET2, and ASXL1.
Dr Ebert’s group
Dr Ebert and his colleagues had hypothesized that, since hematologic malignancies increase with age, it might be possible to detect early somatic mutations that could be initiating the disease process, and these mutations might increase with age.
The researchers looked specifically at 160 genes known to be recurrently mutated in hematologic malignancies, using genetic data derived from approximately 17,000 blood samples originally obtained for studies on the genetics of type 2 diabetes.
The team found a roughly 11-fold increase in the risk of hematologic malignancy among subjects with the subset of somatic mutations linked to blood cancers. And there was a clear association between age and the frequency of these mutations.
Men were slightly more likely to have the mutations than women, and Hispanics were slightly less likely to have the mutations than other racial/ethnic groups.
The researchers also found an association between the presence of this pre-malignant state and the risk of overall mortality independent of malignancy. Individuals with the mutations had a higher risk of type 2 diabetes, coronary heart disease, and ischemic stroke as well.
However, additional research will be needed to determine the nature of these associations.
Dr McCarroll’s group
Dr McCarroll and his colleagues discovered the same phenomenon while trying to determine whether somatic mutations contribute to the risk of developing schizophrenia.
The team studied roughly 12,000 DNA samples from patients with schizophrenia and bipolar disorder, as well as healthy controls, searching across the whole genome at all of the protein-coding genes for patterns in somatic mutations.
The somatic mutations were concentrated in a handful of genes that turned out to be cancer genes.
So the researchers used electronic medical records to follow the patients’ medical histories, finding that subjects with these acquired mutations had a nearly 13-fold higher risk of developing a hematologic malignancy than subjects without the mutations.
The team conducted follow-up analyses on tumor samples from 2 patients who had progressed from this pre-malignant state to cancer. In both cases, the cancer developed from the same cells that had harbored the initiating mutations years earlier.
“The fact that both teams converged on strikingly similar findings, using very different approaches and looking at DNA from very different sets of patients, has given us great confidence in the results,” said study author Giulio Genovese, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts.
Next steps
The researchers emphasized that there is no clinical benefit today for testing for this pre-malignant state, as there are no treatments currently available that would address this condition in otherwise healthy people.
However, they said the results open the door to entirely new directions for research, toward early detection and even prevention of hematologic malignancies.
“The results demonstrate a way to identify high-risk cohorts—people who are at much higher than average risk of progressing to cancer—which could be a population for clinical trials of future prevention strategies,” Dr McCarroll said. “The abundance of these mutated cells could also serve as a biomarker—like LDL cholesterol is for cardiovascular disease—to test the effects of potential prevention therapies in clinical trials.”
Dr Ebert added, “A new focus of investigation will now be to develop interventions that might decrease the likelihood that individuals with these mutations will go on to develop overt malignancies, or therapeutic strategies to decrease mortality from other conditions that may be instigated by these mutations.”
This research is set to be presented on December 9 at the 56th ASH Annual Meeting in San Francisco. ![]()
Management of Bleeding Complications in Patients with Cancer
Patients with cancer can have many hematologic complications. One of the most serious is bleeding, which can range in severity from laboratory abnormalities to life-threatening hemorrhage. The bleeding can be due to complications of the cancer, its therapy, or treatment for complications of cancer such as thrombosis. This manual discusses an approach to the cancer patient with bleeding, with a specific focus on issues such as coagulation defects, thrombocytopenia, and platelet dysfunction. Bleeding complications of specific cancers and their treatment will be discussed as well.
To read the full article in PDF:
Patients with cancer can have many hematologic complications. One of the most serious is bleeding, which can range in severity from laboratory abnormalities to life-threatening hemorrhage. The bleeding can be due to complications of the cancer, its therapy, or treatment for complications of cancer such as thrombosis. This manual discusses an approach to the cancer patient with bleeding, with a specific focus on issues such as coagulation defects, thrombocytopenia, and platelet dysfunction. Bleeding complications of specific cancers and their treatment will be discussed as well.
To read the full article in PDF:
Patients with cancer can have many hematologic complications. One of the most serious is bleeding, which can range in severity from laboratory abnormalities to life-threatening hemorrhage. The bleeding can be due to complications of the cancer, its therapy, or treatment for complications of cancer such as thrombosis. This manual discusses an approach to the cancer patient with bleeding, with a specific focus on issues such as coagulation defects, thrombocytopenia, and platelet dysfunction. Bleeding complications of specific cancers and their treatment will be discussed as well.
To read the full article in PDF:
Metastatic Prostate Cancer: A Case Study
Prostate cancer remains the second leading cause of death in men in the United States as of 2012. It is estimated that prostate cancer affected more than 241,000 new men in 2012, with 15% of these patients presenting with advanced disease. As one would expect, compared to localized prostate cancer, metastatic disease remains the more challenging type to treat. In 1941 Huggins and Hodges demonstrated the dependence of prostatic tissues on androgens and from this work hormonal therapy was developed as the primary treatment for metastatic prostate cancer. Since then, significant progress has been made in the treatment of metastatic prostate cancer, including advances in androgen deprivation therapy and in the treatment of castrationresistant prostate cancer (CRPC), with many advances yet to come. CPRC has been an exciting topic for recent research and advancement, as our understanding of how prostate cancer utilizes very low levels of androgen has evolved considerably.
To read the full article in PDF:
Prostate cancer remains the second leading cause of death in men in the United States as of 2012. It is estimated that prostate cancer affected more than 241,000 new men in 2012, with 15% of these patients presenting with advanced disease. As one would expect, compared to localized prostate cancer, metastatic disease remains the more challenging type to treat. In 1941 Huggins and Hodges demonstrated the dependence of prostatic tissues on androgens and from this work hormonal therapy was developed as the primary treatment for metastatic prostate cancer. Since then, significant progress has been made in the treatment of metastatic prostate cancer, including advances in androgen deprivation therapy and in the treatment of castrationresistant prostate cancer (CRPC), with many advances yet to come. CPRC has been an exciting topic for recent research and advancement, as our understanding of how prostate cancer utilizes very low levels of androgen has evolved considerably.
To read the full article in PDF:
Prostate cancer remains the second leading cause of death in men in the United States as of 2012. It is estimated that prostate cancer affected more than 241,000 new men in 2012, with 15% of these patients presenting with advanced disease. As one would expect, compared to localized prostate cancer, metastatic disease remains the more challenging type to treat. In 1941 Huggins and Hodges demonstrated the dependence of prostatic tissues on androgens and from this work hormonal therapy was developed as the primary treatment for metastatic prostate cancer. Since then, significant progress has been made in the treatment of metastatic prostate cancer, including advances in androgen deprivation therapy and in the treatment of castrationresistant prostate cancer (CRPC), with many advances yet to come. CPRC has been an exciting topic for recent research and advancement, as our understanding of how prostate cancer utilizes very low levels of androgen has evolved considerably.
To read the full article in PDF:
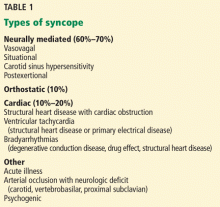
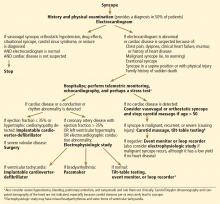
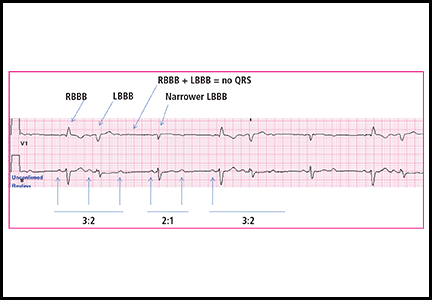
Syncope: Etiology and diagnostic approach
Syncope is a transient loss of consciousness and postural tone with spontaneous, complete recovery. There are three major types: neurally mediated, orthostatic, and cardiac (Table 1).
NEURALLY MEDIATED SYNCOPE
Neurally mediated (reflex) syncope is the most common type, accounting for two-thirds of cases.1–3 It results from autonomic reflexes that respond inappropriately, leading to vasodilation and bradycardia.
See related patient-education handout
Neurally mediated syncope is usually preceded by premonitory symptoms such as lightheadedness, diaphoresis, nausea, malaise, abdominal discomfort, and tunnel vision. However, this may not be the case in one-third of patients, especially in elderly patients, who may not recognize or remember the warning symptoms. Palpitations are frequently reported with neurally mediated syncope and do not necessarily imply that the syncope is due to an arrhythmia.4,5 Neurally mediated syncope does not usually occur in the supine position4,5 but can occur in the seated position.6
Subtypes of neurally mediated syncope are as follows:
Vasovagal syncope
Vasovagal syncope is usually triggered by sudden emotional stress, prolonged sitting or standing, dehydration, or a warm environment, but it can also occur without a trigger. It is the most common type of syncope in young patients (more so in females than in males), but contrary to a common misconception, it can also occur in the elderly.7 Usually, it is not only preceded by but also followed by nausea, malaise, fatigue, and diaphoresis4,5,8; full recovery may be slow. If the syncope lasts longer than 30 to 60 seconds, clonic movements and loss of bladder control are common.9
Mechanism. Vasovagal syncope is initiated by anything that leads to strong myocardial contractions in an "empty" heart. Emotional stress, reduced venous return (from dehydration or prolonged standing), or vasodilation (caused by a hot environment) stimulates the sympathetic nervous system and reduces the left ventricular cavity size, which leads to strong hyperdynamic contractions in a relatively empty heart. This hyperdynamic cavity obliteration activates myocardial mechanoreceptors, initiating a paradoxical vagal reflex with vasodilation and relative bradycardia.10 Vasodilation is usually the predominant mechanism (vasodepressor response), particularly in older patients, but severe bradycardia is also possible (cardioinhibitory response), particularly in younger patients.7 Diuretic and vasodilator therapies increase the predisposition to vasovagal syncope, particularly in the elderly.
On tilt-table testing, vasovagal syncope is characterized by hypotension and relative bradycardia, sometimes severe (see Note on Tilt-Table Testing).10–12
Situational syncope
Situational syncope is caused by a reflex triggered in specific circumstances such as micturition, defecation, coughing, weight-lifting, laughing, or deglutition. The reflex may be initiated by a receptor on the visceral wall (eg, the bladder wall) or by straining that reduces venous return.
Carotid sinus hypersensitivity
Carotid sinus hypersensitivity is an abnormal response to carotid massage, predominantly occurring in patients over the age of 50. In spontaneous carotid sinus syndrome, syncope clearly occurs in a situation that stimulates the carotid sinus, such as head rotation, head extension, shaving, or wearing a tight collar. It is a rare cause of syncope, responsible for about 1% of cases. Conversely, induced carotid sinus syndrome is much more common and represents carotid sinus hypersensitivity in a patient with unexplained syncope and without obvious triggers; the abnormal response is mainly induced during carotid massage rather than spontaneously. In the latter case, carotid sinus hypersensitivity is a marker of a diseased sinus node or atrioventricular node that cannot withstand any inhibition. This diseased node is the true cause of syncope rather than carotid sinus hypersensitivity per se, and carotid massage is a "stress test" that unveils conduction disease.
Thus, carotid massage is indicated in cases of unexplained syncope regardless of circumstantial triggers. This test consists of applying firm pressure over each carotid bifurcation (just below the angle of the jaw) consecutively for 10 seconds. It is performed at the bedside, and may be performed with the patient in both supine and erect positions during tilt-table testing; erect positioning of the patient increases the sensitivity of this test.
An abnormal response to carotid sinus massage is defined as any of the following13–15:
- Vasodepressor response: the systolic blood pressure decreases by at least 50 mm Hg
- Cardioinhibitory response: sinus or atrioventricular block causes the heartbeat to pause for 3 or more seconds
- Mixed vasodepressor and cardioinhibitory response.
Overall, a cardioinhibitory component is present in about two-thirds of cases of carotid sinus hypersensitivity.
Carotid sinus hypersensitivity is found in 25% to 50% of patients over age 50 who have had unexplained syncope or a fall, and it is seen almost equally in men and women.13
One study correlated carotid sinus hypersensitivity with the later occurrence of asystolic syncope during prolonged internal loop monitoring; subsequent pacemaker therapy reduced the burden of syncope.14 Another study, in patients over 50 years old with unexplained falls, found that 16% had cardioinhibitory carotid sinus hypersensitivity. Pacemaker placement reduced falls and syncope by 70% compared with no pacemaker therapy in these patients.15
On the other hand, carotid sinus hypersensitivity can be found in 39% of elderly patients who do not have a history of fainting or falling, so it is important to rule out other causes of syncope before attributing it to carotid sinus hypersensitivity.
Postexertional syncope
While syncope on exertion raises the worrisome possibility of a cardiac cause, postexertional syncope is usually a form of vasovagal syncope. When exercise ceases, venous blood stops getting pumped back to the heart by peripheral muscular contraction. Yet the heart is still exposed to the catecholamine surge induced by exercising, and it hypercontracts on an empty cavity. This triggers a vagal reflex.
Postexertional syncope may also be seen in hypertrophic obstructive cardiomyopathy or aortic stenosis, in which the small left ventricular cavity is less likely to tolerate the reduced preload after exercise and is more likely to obliterate.
ORTHOSTATIC HYPOTENSION
Orthostatic hypotension accounts for about 10% of cases of syncope.1–3
Normally, after the first few minutes of standing, about 25% to 30% of the blood pools in the veins of the pelvis and the lower extremities, strikingly reducing venous return and stroke volume. Upon more prolonged standing, more blood leaves the vascular space and collects in the extravascular space, further reducing venous return. This normally leads to a reflex increase in sympathetic tone, peripheral and splanchnic vasoconstriction, and an increase in heart rate of 10 to 15 beats per minute. Overall, cardiac output is reduced and vascular resistance is increased while blood pressure is maintained, blood pressure being equal to cardiac output times vascular resistance.
Orthostatic hypotension is characterized by autonomic failure, with a lack of compensatory increase in vascular resistance or heart rate upon orthostasis, or by significant hypovolemia that cannot be overcome by sympathetic mechanisms. It is defined as a drop in systolic blood pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more after 30 seconds to 5 minutes of upright posture. Blood pressure is checked immediately upon standing and at 3 and 5 minutes. This may be done at the bedside or during tilt-table testing.2,4
Some patients have an immediate drop in blood pressure of more than 40 mm Hg upon standing, with a quick return to normal within 30 seconds. This "initial orthostatic hypotension" may be common in elderly patients taking antihypertensive drugs and may elude detection during standard blood pressure measurement.2 Other patients with milder orthostatic hypotension may develop a more delayed hypotension 10 to 15 minutes later, as more blood pools in the periphery.16
Along with the drop in blood pressure, a failure of the heart rate to increase identifies autonomic dysfunction. On the other hand, an increase in the heart rate of more than 20 to 30 beats per minute may signify a hypovolemic state even if blood pressure is maintained, the lack of blood pressure drop being related to the excessive heart rate increase.
Orthostatic hypotension is the most common cause of syncope in the elderly and may be due to autonomic dysfunction (related to age, diabetes, uremia, or Parkinson disease), volume depletion, or drugs that block autonomic effects or cause hypovolemia, such as vasodilators, beta-blockers, diuretics, neuropsychiatric medications, and alcohol.
Since digestion leads to peripheral vasodilation and splanchnic blood pooling, syncope that occurs within 1 hour after eating has a mechanism similar to that of orthostatic syncope.
Supine hypertension with orthostatic hypotension. Some patients with severe autonomic dysfunction and the inability to regulate vascular tone have severe hypertension when supine and significant hypotension when upright.
Postural orthostatic tachycardia syndrome, another form of orthostatic failure, occurs most frequently in young women (under the age of 50). In this syndrome, autonomic dysfunction affects peripheral vascular resistance, which fails to increase in response to orthostatic stress. This autonomic dysfunction does not affect the heart, which manifests a striking compensatory increase in rate of more than 30 beats per minute within the first 10 minutes of orthostasis, or an absolute heart rate greater than 120 beats per minute. Unlike in orthostatic hypotension, blood pressure and cardiac output are maintained through this increase in heart rate, although the patient still develops symptoms of severe fatigue or near-syncope, possibly because of flow maldistribution and reduced cerebral flow.2
While postural orthostatic tachycardia syndrome per se does not induce syncope,2 it may be associated with a vasovagal form of syncope that occurs beyond the first 10 minutes of orthostasis in up to 38% of these patients.17
In a less common, hyperadrenergic form of postural orthostatic tachycardia syndrome, there is no autonomic failure but the sympathetic system is overly activated, with orthostasis leading to excessive tachycardia.10,18
CARDIAC SYNCOPE
Accounting for 10% to 20% of cases of syncope, a cardiac cause is the main concern in patients presenting with syncope, as cardiac syncope predicts an increased risk of death and may herald sudden cardiac death.1,2,8,19,20 It often occurs suddenly without any warning signs, in which case it is called malignant syncope. Unlike what occurs in neurally mediated syncope, the postrecovery period is not usually marked by lingering malaise.
There are three forms of cardiac syncope:
Syncope due to structural heart disease with cardiac obstruction
In cases of aortic stenosis, hypertrophic obstructive cardiomyopathy, or severe pulmonary arterial hypertension, peripheral vasodilation occurs during exercise, but cardiac output cannot increase because of the fixed or dynamic obstruction to the ventricular outflow. Since blood pressure is equal to cardiac output times peripheral vascular resistance, pressure drops with the reduction in peripheral vascular resistance. Exertional ventricular arrhythmias may also occur in these patients. Conversely, postexertional syncope is usually benign.
Syncope due to ventricular tachycardia
Ventricular tachycardia can be secondary to underlying structural heart disease, with or without reduced ejection fraction, such as coronary arterial disease, hypertrophic cardiomyopathy, hypertensive cardiomyopathy, or valvular disease. It can also be secondary to primary electrical disease (eg, long QT syndrome, Wolff-Parkinson-White syndrome, Brugada syndrome, arrhythmogenic right ventricular dysplasia, sarcoidosis).
Occasionally, fast supraventricular tachycardia causes syncope at its onset, before vascular compensation develops. This occurs in patients with underlying heart disease.2,8,19
Syncope from bradyarrhythmias
Bradyarrhythmias can occur with or without underlying structural heart disease. They are most often related to degeneration of the conduction system or to medications rather than to cardiomyopathy.
Caveats
When a patient with a history of heart failure presents with syncope, the top considerations are ventricular tachycardia and bradyarrhythmia. Nevertheless, about half of cases of syncope in patients with cardiac disease have a noncardiac cause,19 including the hypotensive or bradycardiac side effect of drugs.
As noted above, most cases of syncope are neurally mediated. However, long asystolic pauses due to sinus or atrioventricular nodal block are the most frequent mechanism of unexplained syncope and are seen in more than 50% of syncope cases on prolonged rhythm monitoring.1,21 These pauses may be related to intrinsic sinus or atrioventricular nodal disease or, more commonly, to extrinsic effects such as the vasovagal mechanism. Some experts favor classifying and treating syncope on the basis of the final mechanism rather than the initiating process, but this is not universally accepted.1,22
OTHER CAUSES OF SYNCOPE
Acute medical or cardiovascular illnesses can cause syncope and are looked for in the appropriate clinical context: severe hypovolemia or gastrointestinal bleeding, large pulmonary embolus with hemodynamic compromise, tamponade, aortic dissection, or hypoglycemia.
Bilateral critical carotid disease or severe vertebrobasilar disease very rarely cause syncope, and, when they do, they are associated with focal neurologic deficits.2 Vertebrobasilar disease may cause "drop attacks," ie, a loss of muscular tone with falling but without loss of consciousness.23
Severe proximal subclavian disease leads to reversal of the flow in the ipsilateral vertebral artery as blood is shunted toward the upper extremity. It manifests as dizziness and syncope during the ipsilateral upper extremity activity, usually with focal neurologic signs (subclavian steal syndrome).2
Psychogenic pseudosyncope is characterized by frequent attacks that typically last longer than true syncope and occur multiple times per day or week, sometimes with a loss of motor tone.2 It occurs in patients with anxiety or somatization disorders.
SEIZURE: A SYNCOPE MIMIC
Certain features differentiate seizure from syncope:
- In seizure, unconsciousness often lasts longer than 5 minutes
- After a seizure, the patient may experience postictal confusion or paralysis
- Seizure may include prolonged tonic-clonic movements; although these movements may be seen with any form of syncope lasting more than 30 seconds, the movements during syncope are more limited and brief, lasting less than 15 seconds
- Tongue biting strongly suggests seizure.
Urinary incontinence does not help distinguish the two, as it frequently occurs with syncope as well as seizure.
DIAGNOSTIC EVALUATION OF SYNCOPE
Table 2 lists clinical clues to the type of syncope.2–5,8
Underlying structural heart disease is the most important predictor of ventricular arrhythmia and death.20,24–26 Thus, the primary goal of the evaluation is to rule out structural heart disease by history, examination, electrocardiography, and echocardiography (Figure 1).
Initial strategy for finding the cause
The cause of syncope is diagnosed by history and physical examination alone in up to 50% of cases, mainly neurally mediated syncope, orthostatic syncope, or seizure.2,3,19
Always check blood pressure with the patient both standing and sitting and in both arms, and obtain an electrocardiogram.
Perform carotid massage in all patients over age 50 if syncope is not clearly vasovagal or orthostatic and if cardiac syncope is not likely. Carotid massage is contraindicated if the patient has a carotid bruit or a history of stroke.
Electrocardiography establishes or suggests a diagnosis in 10% of patients (Table 3, Figure 2).1,2,8,19 A normal electrocardiogram or a mild nonspecific ST-T abnormality suggests a low likelihood of cardiac syncope and is associated with an excellent prognosis. Abnormal electrocardiographic findings are seen in 90% of cases of cardiac syncope and in only 6% of cases of neurally mediated syncope.27 In one study of syncope patients with normal electrocardiograms and negative cardiac histories, none had an abnormal echocardiogram.28
If the heart is normal
If the history suggests neurally mediated syncope or orthostatic hypotension and the history, examination, and electrocardiogram do not suggest coronary artery disease or any other cardiac disease, the workup is stopped.
If the patient has signs or symptoms of heart disease
If the patient has signs or symptoms of heart disease (angina, exertional syncope, dyspnea, clinical signs of heart failure, murmur), a history of heart disease, or exertional, supine, or malignant features, heart disease should be looked for and the following performed:
- Echocardiography to assess left ventricular function, severe valvular disease, and left ventricular hypertrophy
- A stress test (possibly) in cases of exertional syncope or associated angina; however, the overall yield of stress testing in syncope is low (< 5%).29
If electrocardiography and echocardiography do not suggest heart disease
Often, in this situation, the workup can be stopped and syncope can be considered neurally mediated. The likelihood of cardiac syncope is very low in patients with normal findings on electrocardiography and echocardiography, and several studies have shown that patients with syncope who have no structural heart disease have normal long-term survival rates.20,26,30
The following workup may, however, be ordered if the presentation is atypical and syncope is malignant, recurrent, or associated with physical injury, or occurs in the supine position19:
Carotid sinus massage in patients over age 50, if not already performed. Up to 50% of these patients with unexplained syncope have carotid sinus hypersensitivity.13
24-hour Holter monitoring rarely detects significant arrhythmias, but if syncope or dizziness occurs without any arrhythmia, Holter monitoring rules out arrhythmia as the cause of the symptoms.31 The diagnostic yield of Holter monitoring is low (1% to 2%) in patients with infrequent symptoms1,2 and is not improved with 72-hour monitoring.30 The yield is higher in patients with very frequent daily symptoms, many of whom have psychogenic pseudosyncope.2
Tilt-table testing to diagnose vasovagal syncope. This test is positive for a vasovagal response in up to 66% of patients with unexplained syncope.1,19 Patients with heart disease taking vasodilators or beta-blockers may have abnormal baroreflexes. Therefore, a positive tilt test is less specific in these patients and does not necessarily indicate vasovagal syncope.
Event monitoring. If the etiology remains unclear or there are some concerns about arrhythmia, an event monitor (4 weeks of external rhythm monitoring) or an implantable loop recorder (implanted subcutaneously in the prepectoral area for 1 to 2 years) is placed. These monitors record the rhythm when the rate is lower or higher than predefined cutoffs or when the rhythm is irregular, regardless of symptoms. The patient or an observer can also activate the event monitor during or after an event, which freezes the recording of the 2 to 5 minutes preceding the activation and the 1 minute after it.
In a patient who has had syncope, a pacemaker is indicated for episodes of high-grade atrioventricular block, pauses longer than 3 seconds while awake, or bradycardia (< 40 beats per minute) while awake, and an implantable cardioverter-defibrillator is indicated for sustained ventricular tachycardia, even if syncope does not occur concomitantly with these findings. The finding of nonsustained ventricular tachycardia on monitoring increases the suspicion of ventricular tachycardia as the cause of syncope but does not prove it, nor does it necessarily dictate implantation of a cardioverter-defibrillator device.
An electrophysiologic study has a low yield in patients with normal electrocardiographic and echocardiographic studies. Bradycardia is detected in 10%.31
If heart disease or a rhythm abnormality is found
If heart disease is diagnosed by echocardiography or if significant electrocardiographic abnormalities are found, perform the following:
Pacemaker placement for the following electrocardiographic abnormalities1,2,19:
- Second-degree Mobitz II or third-degree atrioventricular block
- Sinus pause (> 3 seconds) or bradycardia (< 40 beats per minute) while awake
- Alternating left bundle branch block and right bundle branch block on the same electrocardiogram or separate ones.
Telemetric monitoring (inpatient).
An electrophysiologic study is valuable mainly for patients with structural heart disease, including an ejection fraction 36% to 49%, coronary artery disease, or left ventricular hypertrophy with a normal ejection fraction.32 Overall, in patients with structural heart disease and unexplained syncope, the yield is 55% (inducible ventricular tachycardia in 21%, abnormal indices of bradycardia in 34%).31
However, the yield of electrophysiologic testing is low in bradyarrhythmia and in patients with an ejection fraction of 35% or less.33 In the latter case, the syncope is often arrhythmia-related and the patient often has an indication for an implantable cardioverter-defibrillator regardless of electrophysiologic study results, especially if the low ejection fraction has persisted despite medical therapy.32
If the electrophysiologic study is negative
If the electrophysiologic study is negative, the differential diagnosis still includes arrhythmia, as the yield of electrophysiologic study is low for bradyarrhythmias and some ventricular tachycardias, and the differential diagnosis also includes, at this point, neurally mediated syncope.
The next step may be either prolonged rhythm monitoring or tilt-table testing. An event monitor or an implantable loop recorder can be placed for prolonged monitoring. The yield of the 30-day event monitor is highest in patients with frequently recurring syncope, in whom it reaches a yield of up to 40% (10% to 20% will have a positive diagnosis of arrhythmia, while 15% to 20% will have symptoms with a normal rhythm).31,34 The implantable recorder has a high overall diagnostic yield and is used in patients with infrequent syncopal episodes (yield up to 50%).1,35,36
In brief, there are two diagnostic approaches to unexplained syncope: the monitoring approach (loop recorder) and the testing approach (tilt-table testing). A combination of both strategies is frequently required in patients with unexplained syncope, and, according to some investigators, a loop recorder may be implanted early on.21
Heart disease with left ventricular dysfunction and low ejection fraction
In patients with heart disease with left ventricular dysfunction and an ejection fraction of 35% or less, an implantable cardioverter-defibrillator can be placed without the need for an electrophysiologic study. These patients need these devices anyway to prevent sudden death, even if the cause of syncope is not an arrhythmia. Patients with a low ejection fraction and a history of syncope are at a high risk of sudden cardiac death.32 Yet in some patients with newly diagnosed cardiomyopathy, left ventricular function may improve with medical therapy. Because the arrhythmic risk is essentially high during the period of ventricular dysfunction, a wearable external defibrillator may be placed while the decision about an implantable defibrillator is finalized within the ensuing months.
In patients with hypertrophic cardiomyopathy, place an implantable cardioverter-defibrillator after any unexplained syncopal episode.
Valvular heart disease needs surgical correction.
If ischemic heart disease is suspected, coronary angiography is indicated, with revascularization if appropriate. An implantable cardioverter-defibrillator should be placed if the ejection fraction is lower than 35%. Except in a large acute myocardial infarction, the substrate for ventricular tachycardia is not ameliorated with revascularization.32,37 Consider an electrophysiologic study when syncope occurs with coronary artery disease and a higher ejection fraction.
A note on left or right bundle branch block
Patients with left or right bundle branch block and unexplained syncope (not clearly vasovagal or orthostatic) likely have syncope related to intermittent high-grade atrioventricular block.38
One study monitored these patients with an implanted loop recorder and showed that about 40% had a recurrence of syncope within 48 days, often concomitantly with complete atrioventricular block. About 55% of these patients had a major event (syncope or high-grade atrioventricular block).39 Many of the patients had had a positive tilt test; thus, tilt testing is not specific for vasovagal syncope in these patients and should not be used to exclude a bradyarrhythmic syncope. Also, patients selected for this study had undergone carotid sinus massage and an electrophysiology study with a negative result.
In another analysis, an electrophysiologic study detected a proportion of the bradyarrhythmias but, more importantly, it induced ventricular tachycardia in 14% of patients with right or left bundle branch block. Although it is not sensitive enough for bradyarrhythmia, electrophysiologic study was highly specific and fairly sensitive for the occurrence of ventricular tachycardia on follow-up.38 Thus, unexplained syncope in a patient with right or left bundle branch block may warrant carotid sinus massage, then an electrophysiologic study to rule out ventricular tachycardia, followed by placement of a dual-chamber pacemaker if the study is negative for ventricular tachycardia, or at least placement of a loop recorder.
INDICATIONS FOR HOSPITALIZATION
Patients should be hospitalized if they have severe hypovolemia or bleeding, or if there is any suspicion of heart disease by history, examination, or electrocardiography, including:
- History of heart failure, low ejection fraction, or coronary artery disease
- An electrocardiogram suggestive of arrhythmia (Table 3)
- Family history of sudden death
- Lack of prodromes; occurrence of physical injury, exertional syncope, syncope in a supine position, or syncope associated with dyspnea or chest pain.2,40
In these situations, there is concern about arrhythmia, structural heart disease, or acute myocardial ischemia. The patient is admitted for immediate telemetric monitoring. Echocardiography and sometimes stress testing are performed. The patient is discharged if this initial workup does not suggest underlying heart disease. Alternatively, an electrophysiologic study is performed or a device is placed in patients found to have structural heart disease. Prolonged rhythm monitoring or tilt-table testing may be performed when syncope with underlying heart disease or worrisome features remains unexplained.
Several Web-based interactive algorithms have been used to determine the indication for hospitalization. They incorporate the above clinical, electrocardiographic, and sometimes echocardiographic features.2,24,25,40–42 A cardiology consultation is usually necessary in patients with the above features, as they frequently require specialized cardiac testing.
Among high-risk patients, the risk of sudden death, a major cardiovascular event, or significant arrhythmia is high in the first few days after the index syncopal episode, justifying the hospitalization and inpatient rhythm monitoring and workup in the presence of the above criteria.24,40,42
SYNCOPE AND DRIVING
A study has shown that the most common cause of syncope while driving is vasovagal syncope.6 In all patients, the risk of another episode of syncope was relatively higher during the first 6 months after the event, with a 12% recurrence rate during this period. However, recurrences were often also seen more than 6 months later (12% recurrence between 6 months and the following few years).6 Fortunately, those episodes rarely occurred while the patient was driving. In a study in survivors of ventricular arrhythmia, the risk of recurrence of arrhythmic events was highest during the first 6 to 12 months after the event.43
Thus, in general, patients with syncope should be prohibited from driving for at least the period of time (eg, 6 months) during which the risk of a recurrent episode of syncope is highest and during which serious cardiac disease or arrhythmia, if present, would emerge. Recurrence of syncope is more likely and more dangerous for commercial drivers who spend a significant proportion of their time driving; individualized decisions are made in these cases.
- Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol 2012; 59:1583–1591.
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS); Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009 Eur Heart J 2009; 30:2631–2671.
- Kapoor WN. Syncope. N Engl J Med 2000; 343:1856–1862.
- Graham LA, Kenny RA. Clinical characteristics of patients with vasovagal reactions presenting as unexplained syncope. Europace 2001; 3:141–146.
- Calkins H, Shyr Y, Frumin H, Schork A, Morady F. The value of the clinical history in the differentiation of syncope due to ventricular tachycardia, atrioventricular block, and neurocardiogenic syncope. Am J Med 1995; 98:365–373.
- Sorajja D, Nesbitt GC, Hodge DO, et al. Syncope while driving: clinical characteristics, causes, and prognosis. Circulation 2009; 120:928–934.
- Kochiadakis GE, Papadimitriou EA, Marketou ME, Chrysostomakis SI, Simantirakis EN, Vardas PE. Autonomic nervous system changes in vasovagal syncope: is there any difference between young and older patients? Pacing Clin Electrophysiol 2004; 27:1371–1377.
- Alboni P, Brignole M, Menozzi C, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol 2001; 37:1921–1928.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Task force on syncope, European Society of Cardiology. Part 1. The initial evaluation of patients with syncope. Europace 2001; 3:253–260.
- Grubb BP. Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation 2005; 111:2997–3006.
- Brignole M, Menozzi C, Del Rosso A, et al. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace 2000; 2:66–76.
- Grubb BP, Kosinski D. Tilt table testing: concepts and limitations. Pacing Clin Electrophysiol 1997; 20:781–787.
- Brignole M, Menozzi C, Gianfranchi L, Oddone D, Lolli G, Bertulla A. Carotid sinus massage, eyeball compression, and head-up tilt test in patients with syncope of uncertain origin and in healthy control subjects. Am Heart J 1991; 122:1644–1651.
- Maggi R, Menozzi C, Brignole M, et al. Cardioinhibitory carotid sinus hypersensitivity predicts an asystolic mechanism of spontaneous neurally mediated syncope. Europace 2007; 9:563–567.
- Kenny RA, Richardson DA, Steen N, Bexton RS, Shaw FE, Bond J. Carotid sinus syndrome: a modifiable risk factor for nonaccidental falls in older adults (SAFE PACE). J Am Coll Cardiol 2001; 38:1491–1496.
- Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 2006; 67:28–32.
- Ojha A, McNeeley K, Heller E, Alshekhlee A, Chelimsky G, Chelimsky TC. Orthostatic syndromes differ in syncope frequency. Am J Med 2010; 123:245–249.
- Kanjwal Y, Kosinski D, Grubb BP. The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management. Pacing Clin Electrophysiol 2003; 26:1747–1757.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J 2001; 22:1256–1306.
- Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med 2002; 347:878–885.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125:2566–2571.
- Kubak MJ, Millikan CH. Diagnosis, pathogenesis, and treatment of "drop attacks." Arch Neurol 1964; 11:107–113.
- Quinn J, McDermott D, Stiell I, Kohn M, Wells G. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Ann Emerg Med 2006; 47:448–454.
- Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M; OESIL (Osservatorio Epidemiologico sulla Sincope nel Lazio) Study Investigators. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur Heart J 2003; 24:811–819.
- Kapoor WN, Hanusa BH. Is syncope a risk factor for poor outcomes? Comparison of patients with and without syncope. Am J Med 1996; 100:646–655.
- Sarasin FP, Louis-Simonet M, Carballo D, et al. Prospective evaluation of patients with syncope: a population-based study. Am J Med 2001; 111:177–184.
- Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart 2002; 88:363–367.
- AlJaroudi WA, Alraies MC, Wazni O, Cerqueira MD, Jaber WA. Yield and diagnostic value of stress myocardial perfusion imaging in patients without known coronary artery disease presenting with syncope. Circ Cardiovasc Imaging 2013; 6:384–391.
- Ungar A, Del Rosso A, Giada F, et al; Evaluation of Guidelines in Syncope Study 2 Group. Early and late outcome of treated patients referred for syncope to emergency department: the EGSYS 2 follow-up study. Eur Heart J 2010; 31:2021–2026.
- Linzer M, Yang EH, Estes NA 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med 1997; 127:76–86.
- Strickberger SA, Benson DW, Biaggioni I, et al; American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke; Quality of Care and Outcomes Research Interdisciplinary Working Group; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF scientific statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation In Collaboration With the Heart Rhythm Society. J Am Coll Cardiol 2006; 47:473–484.
- Fujimura O, Yee R, Klein GJ, Sharma AD, Boahene KA. The diagnostic sensitivity of electrophysiologic testing in patients with syncope caused by transient bradycardia. N Engl J Med 1989; 321:1703–1707.
- Linzer M, Pritchett EL, Pontinen M, McCarthy E, Divine GW. Incremental diagnostic yield of loop electrocardiographic recorders in unexplained syncope. Am J Cardiol 1990; 66:214–219.
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13:262–269.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brugada J, Aguinaga L, Mont L, Betriu A, Mulet J, Sanz G. Coronary artery revascularization in patients with sustained ventricular arrhythmias in the chronic phase of a myocardial infarction: effects on the electrophysiologic substrate and outcome. J Am Coll Cardiol 2001; 37:529–533.
- Moya A, García-Civera R, Croci F, et al; Bradycardia detection in Bundle Branch Block (B4) study. Diagnosis, management, and outcomes of patients with syncope and bundle branch block. Eur Heart J 2011; 32:1535–1541.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology (ISSUE) Investigators. Mechanism of syncope in patients with bundle branch block and negative electrophysiological test. Circulation 2001; 104:2045–2050.
- Brignole M, Shen WK. Syncope management from emergency department to hospital. J Am Coll Cardiol 2008; 51:284–287.
- Daccarett M, Jetter TL, Wasmund SL, Brignole M, Hamdan MH. Syncope in the emergency department: comparison of standardized admission criteria with clinical practice. Europace 2011; 13:1632–1638.
- Costantino G, Perego F, Dipaola F, et al; STePS Investigators. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J Am Coll Cardiol 2008; 51:276–283.
- Larsen GC, Stupey MR, Walance CG, et al. Recurrent cardiac events in survivors of ventricular fibrillation or tachycardia. Implications for driving restrictions. JAMA 1994; 271:1335–1339.
Syncope is a transient loss of consciousness and postural tone with spontaneous, complete recovery. There are three major types: neurally mediated, orthostatic, and cardiac (Table 1).
NEURALLY MEDIATED SYNCOPE
Neurally mediated (reflex) syncope is the most common type, accounting for two-thirds of cases.1–3 It results from autonomic reflexes that respond inappropriately, leading to vasodilation and bradycardia.
See related patient-education handout
Neurally mediated syncope is usually preceded by premonitory symptoms such as lightheadedness, diaphoresis, nausea, malaise, abdominal discomfort, and tunnel vision. However, this may not be the case in one-third of patients, especially in elderly patients, who may not recognize or remember the warning symptoms. Palpitations are frequently reported with neurally mediated syncope and do not necessarily imply that the syncope is due to an arrhythmia.4,5 Neurally mediated syncope does not usually occur in the supine position4,5 but can occur in the seated position.6
Subtypes of neurally mediated syncope are as follows:
Vasovagal syncope
Vasovagal syncope is usually triggered by sudden emotional stress, prolonged sitting or standing, dehydration, or a warm environment, but it can also occur without a trigger. It is the most common type of syncope in young patients (more so in females than in males), but contrary to a common misconception, it can also occur in the elderly.7 Usually, it is not only preceded by but also followed by nausea, malaise, fatigue, and diaphoresis4,5,8; full recovery may be slow. If the syncope lasts longer than 30 to 60 seconds, clonic movements and loss of bladder control are common.9
Mechanism. Vasovagal syncope is initiated by anything that leads to strong myocardial contractions in an "empty" heart. Emotional stress, reduced venous return (from dehydration or prolonged standing), or vasodilation (caused by a hot environment) stimulates the sympathetic nervous system and reduces the left ventricular cavity size, which leads to strong hyperdynamic contractions in a relatively empty heart. This hyperdynamic cavity obliteration activates myocardial mechanoreceptors, initiating a paradoxical vagal reflex with vasodilation and relative bradycardia.10 Vasodilation is usually the predominant mechanism (vasodepressor response), particularly in older patients, but severe bradycardia is also possible (cardioinhibitory response), particularly in younger patients.7 Diuretic and vasodilator therapies increase the predisposition to vasovagal syncope, particularly in the elderly.
On tilt-table testing, vasovagal syncope is characterized by hypotension and relative bradycardia, sometimes severe (see Note on Tilt-Table Testing).10–12
Situational syncope
Situational syncope is caused by a reflex triggered in specific circumstances such as micturition, defecation, coughing, weight-lifting, laughing, or deglutition. The reflex may be initiated by a receptor on the visceral wall (eg, the bladder wall) or by straining that reduces venous return.
Carotid sinus hypersensitivity
Carotid sinus hypersensitivity is an abnormal response to carotid massage, predominantly occurring in patients over the age of 50. In spontaneous carotid sinus syndrome, syncope clearly occurs in a situation that stimulates the carotid sinus, such as head rotation, head extension, shaving, or wearing a tight collar. It is a rare cause of syncope, responsible for about 1% of cases. Conversely, induced carotid sinus syndrome is much more common and represents carotid sinus hypersensitivity in a patient with unexplained syncope and without obvious triggers; the abnormal response is mainly induced during carotid massage rather than spontaneously. In the latter case, carotid sinus hypersensitivity is a marker of a diseased sinus node or atrioventricular node that cannot withstand any inhibition. This diseased node is the true cause of syncope rather than carotid sinus hypersensitivity per se, and carotid massage is a "stress test" that unveils conduction disease.
Thus, carotid massage is indicated in cases of unexplained syncope regardless of circumstantial triggers. This test consists of applying firm pressure over each carotid bifurcation (just below the angle of the jaw) consecutively for 10 seconds. It is performed at the bedside, and may be performed with the patient in both supine and erect positions during tilt-table testing; erect positioning of the patient increases the sensitivity of this test.
An abnormal response to carotid sinus massage is defined as any of the following13–15:
- Vasodepressor response: the systolic blood pressure decreases by at least 50 mm Hg
- Cardioinhibitory response: sinus or atrioventricular block causes the heartbeat to pause for 3 or more seconds
- Mixed vasodepressor and cardioinhibitory response.
Overall, a cardioinhibitory component is present in about two-thirds of cases of carotid sinus hypersensitivity.
Carotid sinus hypersensitivity is found in 25% to 50% of patients over age 50 who have had unexplained syncope or a fall, and it is seen almost equally in men and women.13
One study correlated carotid sinus hypersensitivity with the later occurrence of asystolic syncope during prolonged internal loop monitoring; subsequent pacemaker therapy reduced the burden of syncope.14 Another study, in patients over 50 years old with unexplained falls, found that 16% had cardioinhibitory carotid sinus hypersensitivity. Pacemaker placement reduced falls and syncope by 70% compared with no pacemaker therapy in these patients.15
On the other hand, carotid sinus hypersensitivity can be found in 39% of elderly patients who do not have a history of fainting or falling, so it is important to rule out other causes of syncope before attributing it to carotid sinus hypersensitivity.
Postexertional syncope
While syncope on exertion raises the worrisome possibility of a cardiac cause, postexertional syncope is usually a form of vasovagal syncope. When exercise ceases, venous blood stops getting pumped back to the heart by peripheral muscular contraction. Yet the heart is still exposed to the catecholamine surge induced by exercising, and it hypercontracts on an empty cavity. This triggers a vagal reflex.
Postexertional syncope may also be seen in hypertrophic obstructive cardiomyopathy or aortic stenosis, in which the small left ventricular cavity is less likely to tolerate the reduced preload after exercise and is more likely to obliterate.
ORTHOSTATIC HYPOTENSION
Orthostatic hypotension accounts for about 10% of cases of syncope.1–3
Normally, after the first few minutes of standing, about 25% to 30% of the blood pools in the veins of the pelvis and the lower extremities, strikingly reducing venous return and stroke volume. Upon more prolonged standing, more blood leaves the vascular space and collects in the extravascular space, further reducing venous return. This normally leads to a reflex increase in sympathetic tone, peripheral and splanchnic vasoconstriction, and an increase in heart rate of 10 to 15 beats per minute. Overall, cardiac output is reduced and vascular resistance is increased while blood pressure is maintained, blood pressure being equal to cardiac output times vascular resistance.
Orthostatic hypotension is characterized by autonomic failure, with a lack of compensatory increase in vascular resistance or heart rate upon orthostasis, or by significant hypovolemia that cannot be overcome by sympathetic mechanisms. It is defined as a drop in systolic blood pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more after 30 seconds to 5 minutes of upright posture. Blood pressure is checked immediately upon standing and at 3 and 5 minutes. This may be done at the bedside or during tilt-table testing.2,4
Some patients have an immediate drop in blood pressure of more than 40 mm Hg upon standing, with a quick return to normal within 30 seconds. This "initial orthostatic hypotension" may be common in elderly patients taking antihypertensive drugs and may elude detection during standard blood pressure measurement.2 Other patients with milder orthostatic hypotension may develop a more delayed hypotension 10 to 15 minutes later, as more blood pools in the periphery.16
Along with the drop in blood pressure, a failure of the heart rate to increase identifies autonomic dysfunction. On the other hand, an increase in the heart rate of more than 20 to 30 beats per minute may signify a hypovolemic state even if blood pressure is maintained, the lack of blood pressure drop being related to the excessive heart rate increase.
Orthostatic hypotension is the most common cause of syncope in the elderly and may be due to autonomic dysfunction (related to age, diabetes, uremia, or Parkinson disease), volume depletion, or drugs that block autonomic effects or cause hypovolemia, such as vasodilators, beta-blockers, diuretics, neuropsychiatric medications, and alcohol.
Since digestion leads to peripheral vasodilation and splanchnic blood pooling, syncope that occurs within 1 hour after eating has a mechanism similar to that of orthostatic syncope.
Supine hypertension with orthostatic hypotension. Some patients with severe autonomic dysfunction and the inability to regulate vascular tone have severe hypertension when supine and significant hypotension when upright.
Postural orthostatic tachycardia syndrome, another form of orthostatic failure, occurs most frequently in young women (under the age of 50). In this syndrome, autonomic dysfunction affects peripheral vascular resistance, which fails to increase in response to orthostatic stress. This autonomic dysfunction does not affect the heart, which manifests a striking compensatory increase in rate of more than 30 beats per minute within the first 10 minutes of orthostasis, or an absolute heart rate greater than 120 beats per minute. Unlike in orthostatic hypotension, blood pressure and cardiac output are maintained through this increase in heart rate, although the patient still develops symptoms of severe fatigue or near-syncope, possibly because of flow maldistribution and reduced cerebral flow.2
While postural orthostatic tachycardia syndrome per se does not induce syncope,2 it may be associated with a vasovagal form of syncope that occurs beyond the first 10 minutes of orthostasis in up to 38% of these patients.17
In a less common, hyperadrenergic form of postural orthostatic tachycardia syndrome, there is no autonomic failure but the sympathetic system is overly activated, with orthostasis leading to excessive tachycardia.10,18
CARDIAC SYNCOPE
Accounting for 10% to 20% of cases of syncope, a cardiac cause is the main concern in patients presenting with syncope, as cardiac syncope predicts an increased risk of death and may herald sudden cardiac death.1,2,8,19,20 It often occurs suddenly without any warning signs, in which case it is called malignant syncope. Unlike what occurs in neurally mediated syncope, the postrecovery period is not usually marked by lingering malaise.
There are three forms of cardiac syncope:
Syncope due to structural heart disease with cardiac obstruction
In cases of aortic stenosis, hypertrophic obstructive cardiomyopathy, or severe pulmonary arterial hypertension, peripheral vasodilation occurs during exercise, but cardiac output cannot increase because of the fixed or dynamic obstruction to the ventricular outflow. Since blood pressure is equal to cardiac output times peripheral vascular resistance, pressure drops with the reduction in peripheral vascular resistance. Exertional ventricular arrhythmias may also occur in these patients. Conversely, postexertional syncope is usually benign.
Syncope due to ventricular tachycardia
Ventricular tachycardia can be secondary to underlying structural heart disease, with or without reduced ejection fraction, such as coronary arterial disease, hypertrophic cardiomyopathy, hypertensive cardiomyopathy, or valvular disease. It can also be secondary to primary electrical disease (eg, long QT syndrome, Wolff-Parkinson-White syndrome, Brugada syndrome, arrhythmogenic right ventricular dysplasia, sarcoidosis).
Occasionally, fast supraventricular tachycardia causes syncope at its onset, before vascular compensation develops. This occurs in patients with underlying heart disease.2,8,19
Syncope from bradyarrhythmias
Bradyarrhythmias can occur with or without underlying structural heart disease. They are most often related to degeneration of the conduction system or to medications rather than to cardiomyopathy.
Caveats
When a patient with a history of heart failure presents with syncope, the top considerations are ventricular tachycardia and bradyarrhythmia. Nevertheless, about half of cases of syncope in patients with cardiac disease have a noncardiac cause,19 including the hypotensive or bradycardiac side effect of drugs.
As noted above, most cases of syncope are neurally mediated. However, long asystolic pauses due to sinus or atrioventricular nodal block are the most frequent mechanism of unexplained syncope and are seen in more than 50% of syncope cases on prolonged rhythm monitoring.1,21 These pauses may be related to intrinsic sinus or atrioventricular nodal disease or, more commonly, to extrinsic effects such as the vasovagal mechanism. Some experts favor classifying and treating syncope on the basis of the final mechanism rather than the initiating process, but this is not universally accepted.1,22
OTHER CAUSES OF SYNCOPE
Acute medical or cardiovascular illnesses can cause syncope and are looked for in the appropriate clinical context: severe hypovolemia or gastrointestinal bleeding, large pulmonary embolus with hemodynamic compromise, tamponade, aortic dissection, or hypoglycemia.
Bilateral critical carotid disease or severe vertebrobasilar disease very rarely cause syncope, and, when they do, they are associated with focal neurologic deficits.2 Vertebrobasilar disease may cause "drop attacks," ie, a loss of muscular tone with falling but without loss of consciousness.23
Severe proximal subclavian disease leads to reversal of the flow in the ipsilateral vertebral artery as blood is shunted toward the upper extremity. It manifests as dizziness and syncope during the ipsilateral upper extremity activity, usually with focal neurologic signs (subclavian steal syndrome).2
Psychogenic pseudosyncope is characterized by frequent attacks that typically last longer than true syncope and occur multiple times per day or week, sometimes with a loss of motor tone.2 It occurs in patients with anxiety or somatization disorders.
SEIZURE: A SYNCOPE MIMIC
Certain features differentiate seizure from syncope:
- In seizure, unconsciousness often lasts longer than 5 minutes
- After a seizure, the patient may experience postictal confusion or paralysis
- Seizure may include prolonged tonic-clonic movements; although these movements may be seen with any form of syncope lasting more than 30 seconds, the movements during syncope are more limited and brief, lasting less than 15 seconds
- Tongue biting strongly suggests seizure.
Urinary incontinence does not help distinguish the two, as it frequently occurs with syncope as well as seizure.
DIAGNOSTIC EVALUATION OF SYNCOPE
Table 2 lists clinical clues to the type of syncope.2–5,8
Underlying structural heart disease is the most important predictor of ventricular arrhythmia and death.20,24–26 Thus, the primary goal of the evaluation is to rule out structural heart disease by history, examination, electrocardiography, and echocardiography (Figure 1).
Initial strategy for finding the cause
The cause of syncope is diagnosed by history and physical examination alone in up to 50% of cases, mainly neurally mediated syncope, orthostatic syncope, or seizure.2,3,19
Always check blood pressure with the patient both standing and sitting and in both arms, and obtain an electrocardiogram.
Perform carotid massage in all patients over age 50 if syncope is not clearly vasovagal or orthostatic and if cardiac syncope is not likely. Carotid massage is contraindicated if the patient has a carotid bruit or a history of stroke.
Electrocardiography establishes or suggests a diagnosis in 10% of patients (Table 3, Figure 2).1,2,8,19 A normal electrocardiogram or a mild nonspecific ST-T abnormality suggests a low likelihood of cardiac syncope and is associated with an excellent prognosis. Abnormal electrocardiographic findings are seen in 90% of cases of cardiac syncope and in only 6% of cases of neurally mediated syncope.27 In one study of syncope patients with normal electrocardiograms and negative cardiac histories, none had an abnormal echocardiogram.28
If the heart is normal
If the history suggests neurally mediated syncope or orthostatic hypotension and the history, examination, and electrocardiogram do not suggest coronary artery disease or any other cardiac disease, the workup is stopped.
If the patient has signs or symptoms of heart disease
If the patient has signs or symptoms of heart disease (angina, exertional syncope, dyspnea, clinical signs of heart failure, murmur), a history of heart disease, or exertional, supine, or malignant features, heart disease should be looked for and the following performed:
- Echocardiography to assess left ventricular function, severe valvular disease, and left ventricular hypertrophy
- A stress test (possibly) in cases of exertional syncope or associated angina; however, the overall yield of stress testing in syncope is low (< 5%).29
If electrocardiography and echocardiography do not suggest heart disease
Often, in this situation, the workup can be stopped and syncope can be considered neurally mediated. The likelihood of cardiac syncope is very low in patients with normal findings on electrocardiography and echocardiography, and several studies have shown that patients with syncope who have no structural heart disease have normal long-term survival rates.20,26,30
The following workup may, however, be ordered if the presentation is atypical and syncope is malignant, recurrent, or associated with physical injury, or occurs in the supine position19:
Carotid sinus massage in patients over age 50, if not already performed. Up to 50% of these patients with unexplained syncope have carotid sinus hypersensitivity.13
24-hour Holter monitoring rarely detects significant arrhythmias, but if syncope or dizziness occurs without any arrhythmia, Holter monitoring rules out arrhythmia as the cause of the symptoms.31 The diagnostic yield of Holter monitoring is low (1% to 2%) in patients with infrequent symptoms1,2 and is not improved with 72-hour monitoring.30 The yield is higher in patients with very frequent daily symptoms, many of whom have psychogenic pseudosyncope.2
Tilt-table testing to diagnose vasovagal syncope. This test is positive for a vasovagal response in up to 66% of patients with unexplained syncope.1,19 Patients with heart disease taking vasodilators or beta-blockers may have abnormal baroreflexes. Therefore, a positive tilt test is less specific in these patients and does not necessarily indicate vasovagal syncope.
Event monitoring. If the etiology remains unclear or there are some concerns about arrhythmia, an event monitor (4 weeks of external rhythm monitoring) or an implantable loop recorder (implanted subcutaneously in the prepectoral area for 1 to 2 years) is placed. These monitors record the rhythm when the rate is lower or higher than predefined cutoffs or when the rhythm is irregular, regardless of symptoms. The patient or an observer can also activate the event monitor during or after an event, which freezes the recording of the 2 to 5 minutes preceding the activation and the 1 minute after it.
In a patient who has had syncope, a pacemaker is indicated for episodes of high-grade atrioventricular block, pauses longer than 3 seconds while awake, or bradycardia (< 40 beats per minute) while awake, and an implantable cardioverter-defibrillator is indicated for sustained ventricular tachycardia, even if syncope does not occur concomitantly with these findings. The finding of nonsustained ventricular tachycardia on monitoring increases the suspicion of ventricular tachycardia as the cause of syncope but does not prove it, nor does it necessarily dictate implantation of a cardioverter-defibrillator device.
An electrophysiologic study has a low yield in patients with normal electrocardiographic and echocardiographic studies. Bradycardia is detected in 10%.31
If heart disease or a rhythm abnormality is found
If heart disease is diagnosed by echocardiography or if significant electrocardiographic abnormalities are found, perform the following:
Pacemaker placement for the following electrocardiographic abnormalities1,2,19:
- Second-degree Mobitz II or third-degree atrioventricular block
- Sinus pause (> 3 seconds) or bradycardia (< 40 beats per minute) while awake
- Alternating left bundle branch block and right bundle branch block on the same electrocardiogram or separate ones.
Telemetric monitoring (inpatient).
An electrophysiologic study is valuable mainly for patients with structural heart disease, including an ejection fraction 36% to 49%, coronary artery disease, or left ventricular hypertrophy with a normal ejection fraction.32 Overall, in patients with structural heart disease and unexplained syncope, the yield is 55% (inducible ventricular tachycardia in 21%, abnormal indices of bradycardia in 34%).31
However, the yield of electrophysiologic testing is low in bradyarrhythmia and in patients with an ejection fraction of 35% or less.33 In the latter case, the syncope is often arrhythmia-related and the patient often has an indication for an implantable cardioverter-defibrillator regardless of electrophysiologic study results, especially if the low ejection fraction has persisted despite medical therapy.32
If the electrophysiologic study is negative
If the electrophysiologic study is negative, the differential diagnosis still includes arrhythmia, as the yield of electrophysiologic study is low for bradyarrhythmias and some ventricular tachycardias, and the differential diagnosis also includes, at this point, neurally mediated syncope.
The next step may be either prolonged rhythm monitoring or tilt-table testing. An event monitor or an implantable loop recorder can be placed for prolonged monitoring. The yield of the 30-day event monitor is highest in patients with frequently recurring syncope, in whom it reaches a yield of up to 40% (10% to 20% will have a positive diagnosis of arrhythmia, while 15% to 20% will have symptoms with a normal rhythm).31,34 The implantable recorder has a high overall diagnostic yield and is used in patients with infrequent syncopal episodes (yield up to 50%).1,35,36
In brief, there are two diagnostic approaches to unexplained syncope: the monitoring approach (loop recorder) and the testing approach (tilt-table testing). A combination of both strategies is frequently required in patients with unexplained syncope, and, according to some investigators, a loop recorder may be implanted early on.21
Heart disease with left ventricular dysfunction and low ejection fraction
In patients with heart disease with left ventricular dysfunction and an ejection fraction of 35% or less, an implantable cardioverter-defibrillator can be placed without the need for an electrophysiologic study. These patients need these devices anyway to prevent sudden death, even if the cause of syncope is not an arrhythmia. Patients with a low ejection fraction and a history of syncope are at a high risk of sudden cardiac death.32 Yet in some patients with newly diagnosed cardiomyopathy, left ventricular function may improve with medical therapy. Because the arrhythmic risk is essentially high during the period of ventricular dysfunction, a wearable external defibrillator may be placed while the decision about an implantable defibrillator is finalized within the ensuing months.
In patients with hypertrophic cardiomyopathy, place an implantable cardioverter-defibrillator after any unexplained syncopal episode.
Valvular heart disease needs surgical correction.
If ischemic heart disease is suspected, coronary angiography is indicated, with revascularization if appropriate. An implantable cardioverter-defibrillator should be placed if the ejection fraction is lower than 35%. Except in a large acute myocardial infarction, the substrate for ventricular tachycardia is not ameliorated with revascularization.32,37 Consider an electrophysiologic study when syncope occurs with coronary artery disease and a higher ejection fraction.
A note on left or right bundle branch block
Patients with left or right bundle branch block and unexplained syncope (not clearly vasovagal or orthostatic) likely have syncope related to intermittent high-grade atrioventricular block.38
One study monitored these patients with an implanted loop recorder and showed that about 40% had a recurrence of syncope within 48 days, often concomitantly with complete atrioventricular block. About 55% of these patients had a major event (syncope or high-grade atrioventricular block).39 Many of the patients had had a positive tilt test; thus, tilt testing is not specific for vasovagal syncope in these patients and should not be used to exclude a bradyarrhythmic syncope. Also, patients selected for this study had undergone carotid sinus massage and an electrophysiology study with a negative result.
In another analysis, an electrophysiologic study detected a proportion of the bradyarrhythmias but, more importantly, it induced ventricular tachycardia in 14% of patients with right or left bundle branch block. Although it is not sensitive enough for bradyarrhythmia, electrophysiologic study was highly specific and fairly sensitive for the occurrence of ventricular tachycardia on follow-up.38 Thus, unexplained syncope in a patient with right or left bundle branch block may warrant carotid sinus massage, then an electrophysiologic study to rule out ventricular tachycardia, followed by placement of a dual-chamber pacemaker if the study is negative for ventricular tachycardia, or at least placement of a loop recorder.
INDICATIONS FOR HOSPITALIZATION
Patients should be hospitalized if they have severe hypovolemia or bleeding, or if there is any suspicion of heart disease by history, examination, or electrocardiography, including:
- History of heart failure, low ejection fraction, or coronary artery disease
- An electrocardiogram suggestive of arrhythmia (Table 3)
- Family history of sudden death
- Lack of prodromes; occurrence of physical injury, exertional syncope, syncope in a supine position, or syncope associated with dyspnea or chest pain.2,40
In these situations, there is concern about arrhythmia, structural heart disease, or acute myocardial ischemia. The patient is admitted for immediate telemetric monitoring. Echocardiography and sometimes stress testing are performed. The patient is discharged if this initial workup does not suggest underlying heart disease. Alternatively, an electrophysiologic study is performed or a device is placed in patients found to have structural heart disease. Prolonged rhythm monitoring or tilt-table testing may be performed when syncope with underlying heart disease or worrisome features remains unexplained.
Several Web-based interactive algorithms have been used to determine the indication for hospitalization. They incorporate the above clinical, electrocardiographic, and sometimes echocardiographic features.2,24,25,40–42 A cardiology consultation is usually necessary in patients with the above features, as they frequently require specialized cardiac testing.
Among high-risk patients, the risk of sudden death, a major cardiovascular event, or significant arrhythmia is high in the first few days after the index syncopal episode, justifying the hospitalization and inpatient rhythm monitoring and workup in the presence of the above criteria.24,40,42
SYNCOPE AND DRIVING
A study has shown that the most common cause of syncope while driving is vasovagal syncope.6 In all patients, the risk of another episode of syncope was relatively higher during the first 6 months after the event, with a 12% recurrence rate during this period. However, recurrences were often also seen more than 6 months later (12% recurrence between 6 months and the following few years).6 Fortunately, those episodes rarely occurred while the patient was driving. In a study in survivors of ventricular arrhythmia, the risk of recurrence of arrhythmic events was highest during the first 6 to 12 months after the event.43
Thus, in general, patients with syncope should be prohibited from driving for at least the period of time (eg, 6 months) during which the risk of a recurrent episode of syncope is highest and during which serious cardiac disease or arrhythmia, if present, would emerge. Recurrence of syncope is more likely and more dangerous for commercial drivers who spend a significant proportion of their time driving; individualized decisions are made in these cases.
Syncope is a transient loss of consciousness and postural tone with spontaneous, complete recovery. There are three major types: neurally mediated, orthostatic, and cardiac (Table 1).
NEURALLY MEDIATED SYNCOPE
Neurally mediated (reflex) syncope is the most common type, accounting for two-thirds of cases.1–3 It results from autonomic reflexes that respond inappropriately, leading to vasodilation and bradycardia.
See related patient-education handout
Neurally mediated syncope is usually preceded by premonitory symptoms such as lightheadedness, diaphoresis, nausea, malaise, abdominal discomfort, and tunnel vision. However, this may not be the case in one-third of patients, especially in elderly patients, who may not recognize or remember the warning symptoms. Palpitations are frequently reported with neurally mediated syncope and do not necessarily imply that the syncope is due to an arrhythmia.4,5 Neurally mediated syncope does not usually occur in the supine position4,5 but can occur in the seated position.6
Subtypes of neurally mediated syncope are as follows:
Vasovagal syncope
Vasovagal syncope is usually triggered by sudden emotional stress, prolonged sitting or standing, dehydration, or a warm environment, but it can also occur without a trigger. It is the most common type of syncope in young patients (more so in females than in males), but contrary to a common misconception, it can also occur in the elderly.7 Usually, it is not only preceded by but also followed by nausea, malaise, fatigue, and diaphoresis4,5,8; full recovery may be slow. If the syncope lasts longer than 30 to 60 seconds, clonic movements and loss of bladder control are common.9
Mechanism. Vasovagal syncope is initiated by anything that leads to strong myocardial contractions in an "empty" heart. Emotional stress, reduced venous return (from dehydration or prolonged standing), or vasodilation (caused by a hot environment) stimulates the sympathetic nervous system and reduces the left ventricular cavity size, which leads to strong hyperdynamic contractions in a relatively empty heart. This hyperdynamic cavity obliteration activates myocardial mechanoreceptors, initiating a paradoxical vagal reflex with vasodilation and relative bradycardia.10 Vasodilation is usually the predominant mechanism (vasodepressor response), particularly in older patients, but severe bradycardia is also possible (cardioinhibitory response), particularly in younger patients.7 Diuretic and vasodilator therapies increase the predisposition to vasovagal syncope, particularly in the elderly.
On tilt-table testing, vasovagal syncope is characterized by hypotension and relative bradycardia, sometimes severe (see Note on Tilt-Table Testing).10–12
Situational syncope
Situational syncope is caused by a reflex triggered in specific circumstances such as micturition, defecation, coughing, weight-lifting, laughing, or deglutition. The reflex may be initiated by a receptor on the visceral wall (eg, the bladder wall) or by straining that reduces venous return.
Carotid sinus hypersensitivity
Carotid sinus hypersensitivity is an abnormal response to carotid massage, predominantly occurring in patients over the age of 50. In spontaneous carotid sinus syndrome, syncope clearly occurs in a situation that stimulates the carotid sinus, such as head rotation, head extension, shaving, or wearing a tight collar. It is a rare cause of syncope, responsible for about 1% of cases. Conversely, induced carotid sinus syndrome is much more common and represents carotid sinus hypersensitivity in a patient with unexplained syncope and without obvious triggers; the abnormal response is mainly induced during carotid massage rather than spontaneously. In the latter case, carotid sinus hypersensitivity is a marker of a diseased sinus node or atrioventricular node that cannot withstand any inhibition. This diseased node is the true cause of syncope rather than carotid sinus hypersensitivity per se, and carotid massage is a "stress test" that unveils conduction disease.
Thus, carotid massage is indicated in cases of unexplained syncope regardless of circumstantial triggers. This test consists of applying firm pressure over each carotid bifurcation (just below the angle of the jaw) consecutively for 10 seconds. It is performed at the bedside, and may be performed with the patient in both supine and erect positions during tilt-table testing; erect positioning of the patient increases the sensitivity of this test.
An abnormal response to carotid sinus massage is defined as any of the following13–15:
- Vasodepressor response: the systolic blood pressure decreases by at least 50 mm Hg
- Cardioinhibitory response: sinus or atrioventricular block causes the heartbeat to pause for 3 or more seconds
- Mixed vasodepressor and cardioinhibitory response.
Overall, a cardioinhibitory component is present in about two-thirds of cases of carotid sinus hypersensitivity.
Carotid sinus hypersensitivity is found in 25% to 50% of patients over age 50 who have had unexplained syncope or a fall, and it is seen almost equally in men and women.13
One study correlated carotid sinus hypersensitivity with the later occurrence of asystolic syncope during prolonged internal loop monitoring; subsequent pacemaker therapy reduced the burden of syncope.14 Another study, in patients over 50 years old with unexplained falls, found that 16% had cardioinhibitory carotid sinus hypersensitivity. Pacemaker placement reduced falls and syncope by 70% compared with no pacemaker therapy in these patients.15
On the other hand, carotid sinus hypersensitivity can be found in 39% of elderly patients who do not have a history of fainting or falling, so it is important to rule out other causes of syncope before attributing it to carotid sinus hypersensitivity.
Postexertional syncope
While syncope on exertion raises the worrisome possibility of a cardiac cause, postexertional syncope is usually a form of vasovagal syncope. When exercise ceases, venous blood stops getting pumped back to the heart by peripheral muscular contraction. Yet the heart is still exposed to the catecholamine surge induced by exercising, and it hypercontracts on an empty cavity. This triggers a vagal reflex.
Postexertional syncope may also be seen in hypertrophic obstructive cardiomyopathy or aortic stenosis, in which the small left ventricular cavity is less likely to tolerate the reduced preload after exercise and is more likely to obliterate.
ORTHOSTATIC HYPOTENSION
Orthostatic hypotension accounts for about 10% of cases of syncope.1–3
Normally, after the first few minutes of standing, about 25% to 30% of the blood pools in the veins of the pelvis and the lower extremities, strikingly reducing venous return and stroke volume. Upon more prolonged standing, more blood leaves the vascular space and collects in the extravascular space, further reducing venous return. This normally leads to a reflex increase in sympathetic tone, peripheral and splanchnic vasoconstriction, and an increase in heart rate of 10 to 15 beats per minute. Overall, cardiac output is reduced and vascular resistance is increased while blood pressure is maintained, blood pressure being equal to cardiac output times vascular resistance.
Orthostatic hypotension is characterized by autonomic failure, with a lack of compensatory increase in vascular resistance or heart rate upon orthostasis, or by significant hypovolemia that cannot be overcome by sympathetic mechanisms. It is defined as a drop in systolic blood pressure of 20 mm Hg or more or a drop in diastolic pressure of 10 mm Hg or more after 30 seconds to 5 minutes of upright posture. Blood pressure is checked immediately upon standing and at 3 and 5 minutes. This may be done at the bedside or during tilt-table testing.2,4
Some patients have an immediate drop in blood pressure of more than 40 mm Hg upon standing, with a quick return to normal within 30 seconds. This "initial orthostatic hypotension" may be common in elderly patients taking antihypertensive drugs and may elude detection during standard blood pressure measurement.2 Other patients with milder orthostatic hypotension may develop a more delayed hypotension 10 to 15 minutes later, as more blood pools in the periphery.16
Along with the drop in blood pressure, a failure of the heart rate to increase identifies autonomic dysfunction. On the other hand, an increase in the heart rate of more than 20 to 30 beats per minute may signify a hypovolemic state even if blood pressure is maintained, the lack of blood pressure drop being related to the excessive heart rate increase.
Orthostatic hypotension is the most common cause of syncope in the elderly and may be due to autonomic dysfunction (related to age, diabetes, uremia, or Parkinson disease), volume depletion, or drugs that block autonomic effects or cause hypovolemia, such as vasodilators, beta-blockers, diuretics, neuropsychiatric medications, and alcohol.
Since digestion leads to peripheral vasodilation and splanchnic blood pooling, syncope that occurs within 1 hour after eating has a mechanism similar to that of orthostatic syncope.
Supine hypertension with orthostatic hypotension. Some patients with severe autonomic dysfunction and the inability to regulate vascular tone have severe hypertension when supine and significant hypotension when upright.
Postural orthostatic tachycardia syndrome, another form of orthostatic failure, occurs most frequently in young women (under the age of 50). In this syndrome, autonomic dysfunction affects peripheral vascular resistance, which fails to increase in response to orthostatic stress. This autonomic dysfunction does not affect the heart, which manifests a striking compensatory increase in rate of more than 30 beats per minute within the first 10 minutes of orthostasis, or an absolute heart rate greater than 120 beats per minute. Unlike in orthostatic hypotension, blood pressure and cardiac output are maintained through this increase in heart rate, although the patient still develops symptoms of severe fatigue or near-syncope, possibly because of flow maldistribution and reduced cerebral flow.2
While postural orthostatic tachycardia syndrome per se does not induce syncope,2 it may be associated with a vasovagal form of syncope that occurs beyond the first 10 minutes of orthostasis in up to 38% of these patients.17
In a less common, hyperadrenergic form of postural orthostatic tachycardia syndrome, there is no autonomic failure but the sympathetic system is overly activated, with orthostasis leading to excessive tachycardia.10,18
CARDIAC SYNCOPE
Accounting for 10% to 20% of cases of syncope, a cardiac cause is the main concern in patients presenting with syncope, as cardiac syncope predicts an increased risk of death and may herald sudden cardiac death.1,2,8,19,20 It often occurs suddenly without any warning signs, in which case it is called malignant syncope. Unlike what occurs in neurally mediated syncope, the postrecovery period is not usually marked by lingering malaise.
There are three forms of cardiac syncope:
Syncope due to structural heart disease with cardiac obstruction
In cases of aortic stenosis, hypertrophic obstructive cardiomyopathy, or severe pulmonary arterial hypertension, peripheral vasodilation occurs during exercise, but cardiac output cannot increase because of the fixed or dynamic obstruction to the ventricular outflow. Since blood pressure is equal to cardiac output times peripheral vascular resistance, pressure drops with the reduction in peripheral vascular resistance. Exertional ventricular arrhythmias may also occur in these patients. Conversely, postexertional syncope is usually benign.
Syncope due to ventricular tachycardia
Ventricular tachycardia can be secondary to underlying structural heart disease, with or without reduced ejection fraction, such as coronary arterial disease, hypertrophic cardiomyopathy, hypertensive cardiomyopathy, or valvular disease. It can also be secondary to primary electrical disease (eg, long QT syndrome, Wolff-Parkinson-White syndrome, Brugada syndrome, arrhythmogenic right ventricular dysplasia, sarcoidosis).
Occasionally, fast supraventricular tachycardia causes syncope at its onset, before vascular compensation develops. This occurs in patients with underlying heart disease.2,8,19
Syncope from bradyarrhythmias
Bradyarrhythmias can occur with or without underlying structural heart disease. They are most often related to degeneration of the conduction system or to medications rather than to cardiomyopathy.
Caveats
When a patient with a history of heart failure presents with syncope, the top considerations are ventricular tachycardia and bradyarrhythmia. Nevertheless, about half of cases of syncope in patients with cardiac disease have a noncardiac cause,19 including the hypotensive or bradycardiac side effect of drugs.
As noted above, most cases of syncope are neurally mediated. However, long asystolic pauses due to sinus or atrioventricular nodal block are the most frequent mechanism of unexplained syncope and are seen in more than 50% of syncope cases on prolonged rhythm monitoring.1,21 These pauses may be related to intrinsic sinus or atrioventricular nodal disease or, more commonly, to extrinsic effects such as the vasovagal mechanism. Some experts favor classifying and treating syncope on the basis of the final mechanism rather than the initiating process, but this is not universally accepted.1,22
OTHER CAUSES OF SYNCOPE
Acute medical or cardiovascular illnesses can cause syncope and are looked for in the appropriate clinical context: severe hypovolemia or gastrointestinal bleeding, large pulmonary embolus with hemodynamic compromise, tamponade, aortic dissection, or hypoglycemia.
Bilateral critical carotid disease or severe vertebrobasilar disease very rarely cause syncope, and, when they do, they are associated with focal neurologic deficits.2 Vertebrobasilar disease may cause "drop attacks," ie, a loss of muscular tone with falling but without loss of consciousness.23
Severe proximal subclavian disease leads to reversal of the flow in the ipsilateral vertebral artery as blood is shunted toward the upper extremity. It manifests as dizziness and syncope during the ipsilateral upper extremity activity, usually with focal neurologic signs (subclavian steal syndrome).2
Psychogenic pseudosyncope is characterized by frequent attacks that typically last longer than true syncope and occur multiple times per day or week, sometimes with a loss of motor tone.2 It occurs in patients with anxiety or somatization disorders.
SEIZURE: A SYNCOPE MIMIC
Certain features differentiate seizure from syncope:
- In seizure, unconsciousness often lasts longer than 5 minutes
- After a seizure, the patient may experience postictal confusion or paralysis
- Seizure may include prolonged tonic-clonic movements; although these movements may be seen with any form of syncope lasting more than 30 seconds, the movements during syncope are more limited and brief, lasting less than 15 seconds
- Tongue biting strongly suggests seizure.
Urinary incontinence does not help distinguish the two, as it frequently occurs with syncope as well as seizure.
DIAGNOSTIC EVALUATION OF SYNCOPE
Table 2 lists clinical clues to the type of syncope.2–5,8
Underlying structural heart disease is the most important predictor of ventricular arrhythmia and death.20,24–26 Thus, the primary goal of the evaluation is to rule out structural heart disease by history, examination, electrocardiography, and echocardiography (Figure 1).
Initial strategy for finding the cause
The cause of syncope is diagnosed by history and physical examination alone in up to 50% of cases, mainly neurally mediated syncope, orthostatic syncope, or seizure.2,3,19
Always check blood pressure with the patient both standing and sitting and in both arms, and obtain an electrocardiogram.
Perform carotid massage in all patients over age 50 if syncope is not clearly vasovagal or orthostatic and if cardiac syncope is not likely. Carotid massage is contraindicated if the patient has a carotid bruit or a history of stroke.
Electrocardiography establishes or suggests a diagnosis in 10% of patients (Table 3, Figure 2).1,2,8,19 A normal electrocardiogram or a mild nonspecific ST-T abnormality suggests a low likelihood of cardiac syncope and is associated with an excellent prognosis. Abnormal electrocardiographic findings are seen in 90% of cases of cardiac syncope and in only 6% of cases of neurally mediated syncope.27 In one study of syncope patients with normal electrocardiograms and negative cardiac histories, none had an abnormal echocardiogram.28
If the heart is normal
If the history suggests neurally mediated syncope or orthostatic hypotension and the history, examination, and electrocardiogram do not suggest coronary artery disease or any other cardiac disease, the workup is stopped.
If the patient has signs or symptoms of heart disease
If the patient has signs or symptoms of heart disease (angina, exertional syncope, dyspnea, clinical signs of heart failure, murmur), a history of heart disease, or exertional, supine, or malignant features, heart disease should be looked for and the following performed:
- Echocardiography to assess left ventricular function, severe valvular disease, and left ventricular hypertrophy
- A stress test (possibly) in cases of exertional syncope or associated angina; however, the overall yield of stress testing in syncope is low (< 5%).29
If electrocardiography and echocardiography do not suggest heart disease
Often, in this situation, the workup can be stopped and syncope can be considered neurally mediated. The likelihood of cardiac syncope is very low in patients with normal findings on electrocardiography and echocardiography, and several studies have shown that patients with syncope who have no structural heart disease have normal long-term survival rates.20,26,30
The following workup may, however, be ordered if the presentation is atypical and syncope is malignant, recurrent, or associated with physical injury, or occurs in the supine position19:
Carotid sinus massage in patients over age 50, if not already performed. Up to 50% of these patients with unexplained syncope have carotid sinus hypersensitivity.13
24-hour Holter monitoring rarely detects significant arrhythmias, but if syncope or dizziness occurs without any arrhythmia, Holter monitoring rules out arrhythmia as the cause of the symptoms.31 The diagnostic yield of Holter monitoring is low (1% to 2%) in patients with infrequent symptoms1,2 and is not improved with 72-hour monitoring.30 The yield is higher in patients with very frequent daily symptoms, many of whom have psychogenic pseudosyncope.2
Tilt-table testing to diagnose vasovagal syncope. This test is positive for a vasovagal response in up to 66% of patients with unexplained syncope.1,19 Patients with heart disease taking vasodilators or beta-blockers may have abnormal baroreflexes. Therefore, a positive tilt test is less specific in these patients and does not necessarily indicate vasovagal syncope.
Event monitoring. If the etiology remains unclear or there are some concerns about arrhythmia, an event monitor (4 weeks of external rhythm monitoring) or an implantable loop recorder (implanted subcutaneously in the prepectoral area for 1 to 2 years) is placed. These monitors record the rhythm when the rate is lower or higher than predefined cutoffs or when the rhythm is irregular, regardless of symptoms. The patient or an observer can also activate the event monitor during or after an event, which freezes the recording of the 2 to 5 minutes preceding the activation and the 1 minute after it.
In a patient who has had syncope, a pacemaker is indicated for episodes of high-grade atrioventricular block, pauses longer than 3 seconds while awake, or bradycardia (< 40 beats per minute) while awake, and an implantable cardioverter-defibrillator is indicated for sustained ventricular tachycardia, even if syncope does not occur concomitantly with these findings. The finding of nonsustained ventricular tachycardia on monitoring increases the suspicion of ventricular tachycardia as the cause of syncope but does not prove it, nor does it necessarily dictate implantation of a cardioverter-defibrillator device.
An electrophysiologic study has a low yield in patients with normal electrocardiographic and echocardiographic studies. Bradycardia is detected in 10%.31
If heart disease or a rhythm abnormality is found
If heart disease is diagnosed by echocardiography or if significant electrocardiographic abnormalities are found, perform the following:
Pacemaker placement for the following electrocardiographic abnormalities1,2,19:
- Second-degree Mobitz II or third-degree atrioventricular block
- Sinus pause (> 3 seconds) or bradycardia (< 40 beats per minute) while awake
- Alternating left bundle branch block and right bundle branch block on the same electrocardiogram or separate ones.
Telemetric monitoring (inpatient).
An electrophysiologic study is valuable mainly for patients with structural heart disease, including an ejection fraction 36% to 49%, coronary artery disease, or left ventricular hypertrophy with a normal ejection fraction.32 Overall, in patients with structural heart disease and unexplained syncope, the yield is 55% (inducible ventricular tachycardia in 21%, abnormal indices of bradycardia in 34%).31
However, the yield of electrophysiologic testing is low in bradyarrhythmia and in patients with an ejection fraction of 35% or less.33 In the latter case, the syncope is often arrhythmia-related and the patient often has an indication for an implantable cardioverter-defibrillator regardless of electrophysiologic study results, especially if the low ejection fraction has persisted despite medical therapy.32
If the electrophysiologic study is negative
If the electrophysiologic study is negative, the differential diagnosis still includes arrhythmia, as the yield of electrophysiologic study is low for bradyarrhythmias and some ventricular tachycardias, and the differential diagnosis also includes, at this point, neurally mediated syncope.
The next step may be either prolonged rhythm monitoring or tilt-table testing. An event monitor or an implantable loop recorder can be placed for prolonged monitoring. The yield of the 30-day event monitor is highest in patients with frequently recurring syncope, in whom it reaches a yield of up to 40% (10% to 20% will have a positive diagnosis of arrhythmia, while 15% to 20% will have symptoms with a normal rhythm).31,34 The implantable recorder has a high overall diagnostic yield and is used in patients with infrequent syncopal episodes (yield up to 50%).1,35,36
In brief, there are two diagnostic approaches to unexplained syncope: the monitoring approach (loop recorder) and the testing approach (tilt-table testing). A combination of both strategies is frequently required in patients with unexplained syncope, and, according to some investigators, a loop recorder may be implanted early on.21
Heart disease with left ventricular dysfunction and low ejection fraction
In patients with heart disease with left ventricular dysfunction and an ejection fraction of 35% or less, an implantable cardioverter-defibrillator can be placed without the need for an electrophysiologic study. These patients need these devices anyway to prevent sudden death, even if the cause of syncope is not an arrhythmia. Patients with a low ejection fraction and a history of syncope are at a high risk of sudden cardiac death.32 Yet in some patients with newly diagnosed cardiomyopathy, left ventricular function may improve with medical therapy. Because the arrhythmic risk is essentially high during the period of ventricular dysfunction, a wearable external defibrillator may be placed while the decision about an implantable defibrillator is finalized within the ensuing months.
In patients with hypertrophic cardiomyopathy, place an implantable cardioverter-defibrillator after any unexplained syncopal episode.
Valvular heart disease needs surgical correction.
If ischemic heart disease is suspected, coronary angiography is indicated, with revascularization if appropriate. An implantable cardioverter-defibrillator should be placed if the ejection fraction is lower than 35%. Except in a large acute myocardial infarction, the substrate for ventricular tachycardia is not ameliorated with revascularization.32,37 Consider an electrophysiologic study when syncope occurs with coronary artery disease and a higher ejection fraction.
A note on left or right bundle branch block
Patients with left or right bundle branch block and unexplained syncope (not clearly vasovagal or orthostatic) likely have syncope related to intermittent high-grade atrioventricular block.38
One study monitored these patients with an implanted loop recorder and showed that about 40% had a recurrence of syncope within 48 days, often concomitantly with complete atrioventricular block. About 55% of these patients had a major event (syncope or high-grade atrioventricular block).39 Many of the patients had had a positive tilt test; thus, tilt testing is not specific for vasovagal syncope in these patients and should not be used to exclude a bradyarrhythmic syncope. Also, patients selected for this study had undergone carotid sinus massage and an electrophysiology study with a negative result.
In another analysis, an electrophysiologic study detected a proportion of the bradyarrhythmias but, more importantly, it induced ventricular tachycardia in 14% of patients with right or left bundle branch block. Although it is not sensitive enough for bradyarrhythmia, electrophysiologic study was highly specific and fairly sensitive for the occurrence of ventricular tachycardia on follow-up.38 Thus, unexplained syncope in a patient with right or left bundle branch block may warrant carotid sinus massage, then an electrophysiologic study to rule out ventricular tachycardia, followed by placement of a dual-chamber pacemaker if the study is negative for ventricular tachycardia, or at least placement of a loop recorder.
INDICATIONS FOR HOSPITALIZATION
Patients should be hospitalized if they have severe hypovolemia or bleeding, or if there is any suspicion of heart disease by history, examination, or electrocardiography, including:
- History of heart failure, low ejection fraction, or coronary artery disease
- An electrocardiogram suggestive of arrhythmia (Table 3)
- Family history of sudden death
- Lack of prodromes; occurrence of physical injury, exertional syncope, syncope in a supine position, or syncope associated with dyspnea or chest pain.2,40
In these situations, there is concern about arrhythmia, structural heart disease, or acute myocardial ischemia. The patient is admitted for immediate telemetric monitoring. Echocardiography and sometimes stress testing are performed. The patient is discharged if this initial workup does not suggest underlying heart disease. Alternatively, an electrophysiologic study is performed or a device is placed in patients found to have structural heart disease. Prolonged rhythm monitoring or tilt-table testing may be performed when syncope with underlying heart disease or worrisome features remains unexplained.
Several Web-based interactive algorithms have been used to determine the indication for hospitalization. They incorporate the above clinical, electrocardiographic, and sometimes echocardiographic features.2,24,25,40–42 A cardiology consultation is usually necessary in patients with the above features, as they frequently require specialized cardiac testing.
Among high-risk patients, the risk of sudden death, a major cardiovascular event, or significant arrhythmia is high in the first few days after the index syncopal episode, justifying the hospitalization and inpatient rhythm monitoring and workup in the presence of the above criteria.24,40,42
SYNCOPE AND DRIVING
A study has shown that the most common cause of syncope while driving is vasovagal syncope.6 In all patients, the risk of another episode of syncope was relatively higher during the first 6 months after the event, with a 12% recurrence rate during this period. However, recurrences were often also seen more than 6 months later (12% recurrence between 6 months and the following few years).6 Fortunately, those episodes rarely occurred while the patient was driving. In a study in survivors of ventricular arrhythmia, the risk of recurrence of arrhythmic events was highest during the first 6 to 12 months after the event.43
Thus, in general, patients with syncope should be prohibited from driving for at least the period of time (eg, 6 months) during which the risk of a recurrent episode of syncope is highest and during which serious cardiac disease or arrhythmia, if present, would emerge. Recurrence of syncope is more likely and more dangerous for commercial drivers who spend a significant proportion of their time driving; individualized decisions are made in these cases.
- Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol 2012; 59:1583–1591.
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS); Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009 Eur Heart J 2009; 30:2631–2671.
- Kapoor WN. Syncope. N Engl J Med 2000; 343:1856–1862.
- Graham LA, Kenny RA. Clinical characteristics of patients with vasovagal reactions presenting as unexplained syncope. Europace 2001; 3:141–146.
- Calkins H, Shyr Y, Frumin H, Schork A, Morady F. The value of the clinical history in the differentiation of syncope due to ventricular tachycardia, atrioventricular block, and neurocardiogenic syncope. Am J Med 1995; 98:365–373.
- Sorajja D, Nesbitt GC, Hodge DO, et al. Syncope while driving: clinical characteristics, causes, and prognosis. Circulation 2009; 120:928–934.
- Kochiadakis GE, Papadimitriou EA, Marketou ME, Chrysostomakis SI, Simantirakis EN, Vardas PE. Autonomic nervous system changes in vasovagal syncope: is there any difference between young and older patients? Pacing Clin Electrophysiol 2004; 27:1371–1377.
- Alboni P, Brignole M, Menozzi C, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol 2001; 37:1921–1928.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Task force on syncope, European Society of Cardiology. Part 1. The initial evaluation of patients with syncope. Europace 2001; 3:253–260.
- Grubb BP. Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation 2005; 111:2997–3006.
- Brignole M, Menozzi C, Del Rosso A, et al. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace 2000; 2:66–76.
- Grubb BP, Kosinski D. Tilt table testing: concepts and limitations. Pacing Clin Electrophysiol 1997; 20:781–787.
- Brignole M, Menozzi C, Gianfranchi L, Oddone D, Lolli G, Bertulla A. Carotid sinus massage, eyeball compression, and head-up tilt test in patients with syncope of uncertain origin and in healthy control subjects. Am Heart J 1991; 122:1644–1651.
- Maggi R, Menozzi C, Brignole M, et al. Cardioinhibitory carotid sinus hypersensitivity predicts an asystolic mechanism of spontaneous neurally mediated syncope. Europace 2007; 9:563–567.
- Kenny RA, Richardson DA, Steen N, Bexton RS, Shaw FE, Bond J. Carotid sinus syndrome: a modifiable risk factor for nonaccidental falls in older adults (SAFE PACE). J Am Coll Cardiol 2001; 38:1491–1496.
- Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 2006; 67:28–32.
- Ojha A, McNeeley K, Heller E, Alshekhlee A, Chelimsky G, Chelimsky TC. Orthostatic syndromes differ in syncope frequency. Am J Med 2010; 123:245–249.
- Kanjwal Y, Kosinski D, Grubb BP. The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management. Pacing Clin Electrophysiol 2003; 26:1747–1757.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J 2001; 22:1256–1306.
- Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med 2002; 347:878–885.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125:2566–2571.
- Kubak MJ, Millikan CH. Diagnosis, pathogenesis, and treatment of "drop attacks." Arch Neurol 1964; 11:107–113.
- Quinn J, McDermott D, Stiell I, Kohn M, Wells G. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Ann Emerg Med 2006; 47:448–454.
- Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M; OESIL (Osservatorio Epidemiologico sulla Sincope nel Lazio) Study Investigators. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur Heart J 2003; 24:811–819.
- Kapoor WN, Hanusa BH. Is syncope a risk factor for poor outcomes? Comparison of patients with and without syncope. Am J Med 1996; 100:646–655.
- Sarasin FP, Louis-Simonet M, Carballo D, et al. Prospective evaluation of patients with syncope: a population-based study. Am J Med 2001; 111:177–184.
- Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart 2002; 88:363–367.
- AlJaroudi WA, Alraies MC, Wazni O, Cerqueira MD, Jaber WA. Yield and diagnostic value of stress myocardial perfusion imaging in patients without known coronary artery disease presenting with syncope. Circ Cardiovasc Imaging 2013; 6:384–391.
- Ungar A, Del Rosso A, Giada F, et al; Evaluation of Guidelines in Syncope Study 2 Group. Early and late outcome of treated patients referred for syncope to emergency department: the EGSYS 2 follow-up study. Eur Heart J 2010; 31:2021–2026.
- Linzer M, Yang EH, Estes NA 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med 1997; 127:76–86.
- Strickberger SA, Benson DW, Biaggioni I, et al; American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke; Quality of Care and Outcomes Research Interdisciplinary Working Group; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF scientific statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation In Collaboration With the Heart Rhythm Society. J Am Coll Cardiol 2006; 47:473–484.
- Fujimura O, Yee R, Klein GJ, Sharma AD, Boahene KA. The diagnostic sensitivity of electrophysiologic testing in patients with syncope caused by transient bradycardia. N Engl J Med 1989; 321:1703–1707.
- Linzer M, Pritchett EL, Pontinen M, McCarthy E, Divine GW. Incremental diagnostic yield of loop electrocardiographic recorders in unexplained syncope. Am J Cardiol 1990; 66:214–219.
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13:262–269.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brugada J, Aguinaga L, Mont L, Betriu A, Mulet J, Sanz G. Coronary artery revascularization in patients with sustained ventricular arrhythmias in the chronic phase of a myocardial infarction: effects on the electrophysiologic substrate and outcome. J Am Coll Cardiol 2001; 37:529–533.
- Moya A, García-Civera R, Croci F, et al; Bradycardia detection in Bundle Branch Block (B4) study. Diagnosis, management, and outcomes of patients with syncope and bundle branch block. Eur Heart J 2011; 32:1535–1541.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology (ISSUE) Investigators. Mechanism of syncope in patients with bundle branch block and negative electrophysiological test. Circulation 2001; 104:2045–2050.
- Brignole M, Shen WK. Syncope management from emergency department to hospital. J Am Coll Cardiol 2008; 51:284–287.
- Daccarett M, Jetter TL, Wasmund SL, Brignole M, Hamdan MH. Syncope in the emergency department: comparison of standardized admission criteria with clinical practice. Europace 2011; 13:1632–1638.
- Costantino G, Perego F, Dipaola F, et al; STePS Investigators. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J Am Coll Cardiol 2008; 51:276–283.
- Larsen GC, Stupey MR, Walance CG, et al. Recurrent cardiac events in survivors of ventricular fibrillation or tachycardia. Implications for driving restrictions. JAMA 1994; 271:1335–1339.
- Brignole M, Hamdan MH. New concepts in the assessment of syncope. J Am Coll Cardiol 2012; 59:1583–1591.
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS); Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009 Eur Heart J 2009; 30:2631–2671.
- Kapoor WN. Syncope. N Engl J Med 2000; 343:1856–1862.
- Graham LA, Kenny RA. Clinical characteristics of patients with vasovagal reactions presenting as unexplained syncope. Europace 2001; 3:141–146.
- Calkins H, Shyr Y, Frumin H, Schork A, Morady F. The value of the clinical history in the differentiation of syncope due to ventricular tachycardia, atrioventricular block, and neurocardiogenic syncope. Am J Med 1995; 98:365–373.
- Sorajja D, Nesbitt GC, Hodge DO, et al. Syncope while driving: clinical characteristics, causes, and prognosis. Circulation 2009; 120:928–934.
- Kochiadakis GE, Papadimitriou EA, Marketou ME, Chrysostomakis SI, Simantirakis EN, Vardas PE. Autonomic nervous system changes in vasovagal syncope: is there any difference between young and older patients? Pacing Clin Electrophysiol 2004; 27:1371–1377.
- Alboni P, Brignole M, Menozzi C, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol 2001; 37:1921–1928.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Task force on syncope, European Society of Cardiology. Part 1. The initial evaluation of patients with syncope. Europace 2001; 3:253–260.
- Grubb BP. Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation 2005; 111:2997–3006.
- Brignole M, Menozzi C, Del Rosso A, et al. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace 2000; 2:66–76.
- Grubb BP, Kosinski D. Tilt table testing: concepts and limitations. Pacing Clin Electrophysiol 1997; 20:781–787.
- Brignole M, Menozzi C, Gianfranchi L, Oddone D, Lolli G, Bertulla A. Carotid sinus massage, eyeball compression, and head-up tilt test in patients with syncope of uncertain origin and in healthy control subjects. Am Heart J 1991; 122:1644–1651.
- Maggi R, Menozzi C, Brignole M, et al. Cardioinhibitory carotid sinus hypersensitivity predicts an asystolic mechanism of spontaneous neurally mediated syncope. Europace 2007; 9:563–567.
- Kenny RA, Richardson DA, Steen N, Bexton RS, Shaw FE, Bond J. Carotid sinus syndrome: a modifiable risk factor for nonaccidental falls in older adults (SAFE PACE). J Am Coll Cardiol 2001; 38:1491–1496.
- Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology 2006; 67:28–32.
- Ojha A, McNeeley K, Heller E, Alshekhlee A, Chelimsky G, Chelimsky TC. Orthostatic syndromes differ in syncope frequency. Am J Med 2010; 123:245–249.
- Kanjwal Y, Kosinski D, Grubb BP. The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management. Pacing Clin Electrophysiol 2003; 26:1747–1757.
- Brignole M, Alboni P, Benditt D, et al; Task Force on Syncope; European Society of Cardiology. Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J 2001; 22:1256–1306.
- Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med 2002; 347:878–885.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125:2566–2571.
- Kubak MJ, Millikan CH. Diagnosis, pathogenesis, and treatment of "drop attacks." Arch Neurol 1964; 11:107–113.
- Quinn J, McDermott D, Stiell I, Kohn M, Wells G. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Ann Emerg Med 2006; 47:448–454.
- Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M; OESIL (Osservatorio Epidemiologico sulla Sincope nel Lazio) Study Investigators. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur Heart J 2003; 24:811–819.
- Kapoor WN, Hanusa BH. Is syncope a risk factor for poor outcomes? Comparison of patients with and without syncope. Am J Med 1996; 100:646–655.
- Sarasin FP, Louis-Simonet M, Carballo D, et al. Prospective evaluation of patients with syncope: a population-based study. Am J Med 2001; 111:177–184.
- Sarasin FP, Junod AF, Carballo D, Slama S, Unger PF, Louis-Simonet M. Role of echocardiography in the evaluation of syncope: a prospective study. Heart 2002; 88:363–367.
- AlJaroudi WA, Alraies MC, Wazni O, Cerqueira MD, Jaber WA. Yield and diagnostic value of stress myocardial perfusion imaging in patients without known coronary artery disease presenting with syncope. Circ Cardiovasc Imaging 2013; 6:384–391.
- Ungar A, Del Rosso A, Giada F, et al; Evaluation of Guidelines in Syncope Study 2 Group. Early and late outcome of treated patients referred for syncope to emergency department: the EGSYS 2 follow-up study. Eur Heart J 2010; 31:2021–2026.
- Linzer M, Yang EH, Estes NA 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Intern Med 1997; 127:76–86.
- Strickberger SA, Benson DW, Biaggioni I, et al; American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke; Quality of Care and Outcomes Research Interdisciplinary Working Group; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF scientific statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation In Collaboration With the Heart Rhythm Society. J Am Coll Cardiol 2006; 47:473–484.
- Fujimura O, Yee R, Klein GJ, Sharma AD, Boahene KA. The diagnostic sensitivity of electrophysiologic testing in patients with syncope caused by transient bradycardia. N Engl J Med 1989; 321:1703–1707.
- Linzer M, Pritchett EL, Pontinen M, McCarthy E, Divine GW. Incremental diagnostic yield of loop electrocardiographic recorders in unexplained syncope. Am J Cardiol 1990; 66:214–219.
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13:262–269.
- Brignole M, Sutton R, Menozzi C, et al; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27:1085–1092.
- Brugada J, Aguinaga L, Mont L, Betriu A, Mulet J, Sanz G. Coronary artery revascularization in patients with sustained ventricular arrhythmias in the chronic phase of a myocardial infarction: effects on the electrophysiologic substrate and outcome. J Am Coll Cardiol 2001; 37:529–533.
- Moya A, García-Civera R, Croci F, et al; Bradycardia detection in Bundle Branch Block (B4) study. Diagnosis, management, and outcomes of patients with syncope and bundle branch block. Eur Heart J 2011; 32:1535–1541.
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology (ISSUE) Investigators. Mechanism of syncope in patients with bundle branch block and negative electrophysiological test. Circulation 2001; 104:2045–2050.
- Brignole M, Shen WK. Syncope management from emergency department to hospital. J Am Coll Cardiol 2008; 51:284–287.
- Daccarett M, Jetter TL, Wasmund SL, Brignole M, Hamdan MH. Syncope in the emergency department: comparison of standardized admission criteria with clinical practice. Europace 2011; 13:1632–1638.
- Costantino G, Perego F, Dipaola F, et al; STePS Investigators. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J Am Coll Cardiol 2008; 51:276–283.
- Larsen GC, Stupey MR, Walance CG, et al. Recurrent cardiac events in survivors of ventricular fibrillation or tachycardia. Implications for driving restrictions. JAMA 1994; 271:1335–1339.
KEY POINTS
- Neurally mediated forms of syncope, such as vasovagal, result from autonomic reflexes that respond inappropriately, leading to vasodilation and relative bradycardia.
- Orthostatic hypotension is the most common cause of syncope in the elderly and may be due to autonomic dysfunction, volume depletion, or drugs that block autonomic effects or cause hypovolemia, such as vasodilators, beta-blockers, diuretics, neuropsychiatric medications, and alcohol.
- The likelihood of cardiac syncope is low in patients with normal electrocardiographic and echocardiographic findings.
- Hospitalization is indicated in patients with syncope who have or are suspected of having structural heart disease.
Tilt-table testing
Fainting, also called syncope (pronounced “SIN-ko-pea”), is caused by a temporary decrease in blood flow to the brain. If you have syncope, your doctor may order a tilt-table test to determine why the blood flow is decreased and how to treat it. The purpose of the test is not to make you faint, although some people do faint during the test.
What happens before the test?
An intravenous line will be placed in a vein in your arm or the back of your hand. It may be used to take blood samples and also to give medications (if you need them) during the test.
Blood pressure cuffs will be placed around both arms, and small, sticky patches called electrodes will be placed on your chest. The electrodes are connected to a monitor that shows your heart’s rate and rhythm.
What happens during the test?
You will lie on a motorized table with a metal footboard. For your safety, soft straps will be placed across your body to secure you when the table is tilted during the test. Your blood pressure and heart rate will be constantly monitored. You will be asked to remain as still and quiet as possible so that the results can be accurately recorded.
A nurse or technician will tilt the table to different angles during the test—30 degrees for 2 minutes, then 45 degrees for 2 minutes, and then 70 degrees for up to 45 minutes. You will always be “head up”; you will never be upside-down. When the table is at 30 and 45 degrees, you will feel as if you are lying on a steep hill. When it is at 70 degrees, you will be in an upright position and your feet will be supported by the footboard at the end of the table.
How long will the test last?
About 1½ hours—plan on being at the hospital for about 2 hours total. You will need a responsible adult to drive you home.
Should I take my medications?
Yes. You can take your prescription medications as you normally would, with water. However, do not take diuretics or laxatives before the test.
If you have questions or need help adjusting your medications, please call your physician. Do not discontinue any medication without talking to your physician first.
Can I eat before the test?
Eat a normal meal the evening before your procedure. Do not eat or drink anything except small amounts of water for 4 hours before the test. If you must take medications, please take them with small sips of water.
If you have diabetes, please request a 10:30 am appointment time for your test so you can eat a light breakfast before 7 am and also complete the test in time for lunch.
What do the test results mean?
A positive test means that you may have a condition that causes an abnormal change in blood pressure or heart rate when you stand up. A negative test means that you did not show signs of such a condition. In either case, additional tests may be needed to diagnose your condition.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Center for Consumer Health Information web site, www.clevelandclinic.org/health
Fainting, also called syncope (pronounced “SIN-ko-pea”), is caused by a temporary decrease in blood flow to the brain. If you have syncope, your doctor may order a tilt-table test to determine why the blood flow is decreased and how to treat it. The purpose of the test is not to make you faint, although some people do faint during the test.
What happens before the test?
An intravenous line will be placed in a vein in your arm or the back of your hand. It may be used to take blood samples and also to give medications (if you need them) during the test.
Blood pressure cuffs will be placed around both arms, and small, sticky patches called electrodes will be placed on your chest. The electrodes are connected to a monitor that shows your heart’s rate and rhythm.
What happens during the test?
You will lie on a motorized table with a metal footboard. For your safety, soft straps will be placed across your body to secure you when the table is tilted during the test. Your blood pressure and heart rate will be constantly monitored. You will be asked to remain as still and quiet as possible so that the results can be accurately recorded.
A nurse or technician will tilt the table to different angles during the test—30 degrees for 2 minutes, then 45 degrees for 2 minutes, and then 70 degrees for up to 45 minutes. You will always be “head up”; you will never be upside-down. When the table is at 30 and 45 degrees, you will feel as if you are lying on a steep hill. When it is at 70 degrees, you will be in an upright position and your feet will be supported by the footboard at the end of the table.
How long will the test last?
About 1½ hours—plan on being at the hospital for about 2 hours total. You will need a responsible adult to drive you home.
Should I take my medications?
Yes. You can take your prescription medications as you normally would, with water. However, do not take diuretics or laxatives before the test.
If you have questions or need help adjusting your medications, please call your physician. Do not discontinue any medication without talking to your physician first.
Can I eat before the test?
Eat a normal meal the evening before your procedure. Do not eat or drink anything except small amounts of water for 4 hours before the test. If you must take medications, please take them with small sips of water.
If you have diabetes, please request a 10:30 am appointment time for your test so you can eat a light breakfast before 7 am and also complete the test in time for lunch.
What do the test results mean?
A positive test means that you may have a condition that causes an abnormal change in blood pressure or heart rate when you stand up. A negative test means that you did not show signs of such a condition. In either case, additional tests may be needed to diagnose your condition.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Center for Consumer Health Information web site, www.clevelandclinic.org/health
Fainting, also called syncope (pronounced “SIN-ko-pea”), is caused by a temporary decrease in blood flow to the brain. If you have syncope, your doctor may order a tilt-table test to determine why the blood flow is decreased and how to treat it. The purpose of the test is not to make you faint, although some people do faint during the test.
What happens before the test?
An intravenous line will be placed in a vein in your arm or the back of your hand. It may be used to take blood samples and also to give medications (if you need them) during the test.
Blood pressure cuffs will be placed around both arms, and small, sticky patches called electrodes will be placed on your chest. The electrodes are connected to a monitor that shows your heart’s rate and rhythm.
What happens during the test?
You will lie on a motorized table with a metal footboard. For your safety, soft straps will be placed across your body to secure you when the table is tilted during the test. Your blood pressure and heart rate will be constantly monitored. You will be asked to remain as still and quiet as possible so that the results can be accurately recorded.
A nurse or technician will tilt the table to different angles during the test—30 degrees for 2 minutes, then 45 degrees for 2 minutes, and then 70 degrees for up to 45 minutes. You will always be “head up”; you will never be upside-down. When the table is at 30 and 45 degrees, you will feel as if you are lying on a steep hill. When it is at 70 degrees, you will be in an upright position and your feet will be supported by the footboard at the end of the table.
How long will the test last?
About 1½ hours—plan on being at the hospital for about 2 hours total. You will need a responsible adult to drive you home.
Should I take my medications?
Yes. You can take your prescription medications as you normally would, with water. However, do not take diuretics or laxatives before the test.
If you have questions or need help adjusting your medications, please call your physician. Do not discontinue any medication without talking to your physician first.
Can I eat before the test?
Eat a normal meal the evening before your procedure. Do not eat or drink anything except small amounts of water for 4 hours before the test. If you must take medications, please take them with small sips of water.
If you have diabetes, please request a 10:30 am appointment time for your test so you can eat a light breakfast before 7 am and also complete the test in time for lunch.
What do the test results mean?
A positive test means that you may have a condition that causes an abnormal change in blood pressure or heart rate when you stand up. A negative test means that you did not show signs of such a condition. In either case, additional tests may be needed to diagnose your condition.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Center for Consumer Health Information web site, www.clevelandclinic.org/health
Updated guidelines on cardiovascular evaluation before noncardiac surgery: A view from the trenches
Guidelines jointly issued by the American College of Cardiology and American Heart Association (ACC/AHA)1 provide a framework for evaluating and managing perioperative cardiac risk in noncardiac surgery. An overriding theme in successive documents from these organizations through the years has been that preoperative intervention, coronary artery bypass grafting, or percutaneous coronary intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
This article highlights some of the key recommendations in the 2014 updates to these guidelines,1–3 how they differ from previous guidelines,4 and the ongoing challenges and unresolved issues facing physicians involved in perioperative care.
Of note, while these guidelines were being updated, Erasmus University5 expressed concern about the scientific integrity of some of the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) trials. As a result, the evidence review committee included these trials in its analysis but not in a systematic review of beta-blockers.2 These trials were not included in the clinical practice guideline supplements and tables but were cited in the text if relevant.
The European Society of Cardiology and European Society of Anesthesiology6 revised their guidelines concurrently with but independently of the ACC/AHA, and although they discussed and aligned some recommendations, many differences remain between the two sets of guidelines. Readers should consult the full guidelines for more detailed information.1
THE ROLE OF THE PREOPERATIVE CARDIAC EVALUATION
The purpose of preoperative medical evaluation is not to "get medical clearance" but rather to evaluate the patient’s medical status and risk of complications. The process includes:
- Identifying risk factors and assessing their severity and stability
- Establishing a clinical risk profile for informed and shared decision-making
- Recommending needed changes in management, further testing, or specialty consultation.
The updated guidelines emphasize the importance of communication among the perioperative team and with the patient. They reiterate the focus on appropriateness of care and cost containment—one should order a test only if the result may change the patient’s management.
HOW URGENT IS SURGERY? HOW RISKY?
The new guidelines classify the urgency of surgery as follows:
- Emergency (necessary within 6 hours)
- Urgent (necessary within 6–24 hours)
- Time-sensitive (can delay 1–6 weeks)
- Elective (can delay up to 1 year).
Surgical risk is now classified as either low (< 1% risk of major adverse cardiac events) or elevated (≥ 1%) on the basis of surgical and patient characteristics. Previous schemas included an intermediate-risk category. Low-risk procedures include endoscopic procedures, superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Elevated-risk procedures include vascular surgery, intraperitoneal and intrathoracic surgery, head and neck surgery, orthopedic surgery, and prostate surgery.
Risk calculators and biomarkers
To estimate the perioperative risk of major adverse cardiac events, the guidelines suggest incorporating the Revised Cardiac Risk Index (RCRI)7 with an estimation of surgical risk or using a newer surgical risk calculator derived from a database of the American College of Surgeons’ National Surgical Quality Improvement Project (ACS NSQIP).
The RCRI is based on six risk factors, each worth 1 point:
- High-risk surgery
- Ischemic heart disease
- Heart failure
- Stroke or transient ischemic attack
- Diabetes requiring insulin
- Renal insufficiency (serum creatinine > 2.0 mg/dL).7
MICA. The Myocardial Infarction or Cardiac Arrest (MICA) calculator8 has a narrower focus and was validated in only one center.
ACS NSQIP. The recommended newer ACS NSQIP surgical risk calculator9 provides an estimate of procedure-specific risk based on Current Procedural Terminology code and includes 21 patient-specific variables to predict death, major adverse cardiac events, and eight other outcomes. While more comprehensive, this risk calculator has yet to be validated outside of the ACS NSQIP database.
Reconstructed RCRI. The RCRI has been externally validated, but it underestimates risk in major vascular surgery and was outperformed by the MICA calculator. Although not discussed in the new guidelines, a recently published "reconstructed RCRI,"10 in which a serum creatinine level greater than 2 mg/dL in the original RCRI is replaced by a glomerular filtration rate less than 30 mL/min and diabetes is eliminated, may outperform the standard RCRI. A patient with either an RCRI score or a reconstructed RCRI score of 0 or 1 would be considered to be at low risk, whereas patients with two or more risk factors would have an elevated risk.
Cardiac biomarkers, primarily B-type natriuretic peptide (BNP) and N-terminal (NT) proBNP, are independent predictors of cardiac risk, and their addition to preoperative risk indices may provide incremental predictive value. However, how to use these biomarkers and whether any treatment aimed at them will reduce risk is unclear, and the new guidelines did not recommend their routine use.
CLINICAL RISK FACTORS
Coronary artery disease
Ischemic symptoms, a history of myocardial infarction, and elevated cardiac biomarkers are individually associated with perioperative risk of morbidity and death. The risk is modified by how long ago the infarction occurred, whether the patient underwent coronary revascularization, and if so, what type (bypass grafting or percutaneous coronary intervention). A patient with acute coronary syndrome (currently or in the recent past) is at higher risk, and should have elective surgery delayed and be referred for cardiac evaluation and management according to guidelines.
Heart failure
In terms of posing a risk for major adverse cardiac events, heart failure is at least equal to coronary artery disease, and is possibly worse. Its impact depends on its stability, its symptoms, and the patient’s left ventricular function. Symptomatic decompensated heart failure and depressed left ventricular function (ejection fraction < 30% or 40%) confer higher risk than asymptomatic heart failure and preserved left ventricular function. However, evidence is limited with respect to asymptomatic left ventricular dysfunction and diastolic dysfunction. Patients with stable heart failure treated according to guidelines may have better perioperative outcomes.
Valvular heart disease
Significant valvular heart disease is associated with increased risk of postoperative cardiac complications. This risk depends on the type and severity of the valvular lesion and type of noncardiac surgery, but can be minimized by clinical and echocardiographic assessment, choosing appropriate anesthesia, and closer perioperative monitoring. Aortic and mitral stenosis are associated with greater risk of perioperative adverse cardiac events than regurgitant valvular disease.
Echocardiography is recommended in patients suspected of having moderate to severe stenotic or regurgitant lesions if it has not been done within the past year or if the patient’s clinical condition has worsened.
If indicated, valvular intervention can reduce perioperative risk in these patients. Even if the planned noncardiac surgery is high-risk, it may be reasonable to proceed with it (using appropriate perioperative hemodynamic monitoring, which is not specified but typically would be with an arterial line, central line, and possibly a pulmonary arterial catheter) in patients who have asymptomatic severe aortic or mitral regurgitation or aortic stenosis. Surgery may also be reasonable in patients with asymptomatic severe mitral stenosis who are not candidates for repair.
Arrhythmias
Cardiac arrhythmias and conduction defects are often seen in the perioperative period, but there is only limited evidence as to how they affect surgical risk. In addition to their hemodynamic effects, certain arrhythmias (atrial fibrillation, ventricular tachycardia) often indicate underlying structural heart disease, which requires further evaluation before surgery.
The new guidelines refer the reader to previously published clinical practice guidelines for atrial fibrillation,11 supraventricular arrhythmias,12 and device-based therapy.13
ALGORITHM FOR PREOPERATIVE CARDIAC ASSESSMENT
The new algorithm for evaluating a patient who is known to have coronary artery disease or risk factors for it has seven steps (Figure 1).1,11,12,14–17 It differs from the previous algorithm in several details:
- Instead of listing the four active cardiac conditions for which elective surgery should be delayed while the patient is being evaluated and treated (unstable coronary syndrome, decompensated heart failure, significant arrhythmias, severe valvular heart disease), the new version specifically asks about acute coronary syndrome and recommends cardiac evaluation and treatment according to guidelines. A footnote directs readers to other clinical practice guidelines for symptomatic heart failure,14 valvular heart disease,15 and arrhythmias.11,12
- Instead of asking if the procedure is low-risk, the guidelines recommend estimating risk of major adverse cardiac events on the basis of combined clinical and surgical risk and define only two categories: low or elevated. Patients at low risk proceed to surgery with no further testing, as in the earlier algorithm.
- "Excellent" exercise capacity (> 10 metabolic equivalents of task [METs]) is separated from "moderate/good" (4–10 METs), presumably to indicate a stronger recommendation, but patients in both categories proceed to surgery as before.
- If the patient cannot exercise to at least 4 METs, the new algorithm asks whether further testing will affect decision-making or perioperative care (an addition to the previous algorithm). This entails discussing with the patient and perioperative team whether the original surgery will be performed and whether the patient is willing to undergo revascularization if indicated. If so, pharmacologic stress testing is recommended. Previously, this decision also included the number of RCRI factors as well as the type of surgery (vascular or nonvascular).
- If testing will not affect the decision or if the stress test is normal, in addition to recommending proceeding to surgery according to guidelines the new algorithm also lists an option for alternative strategies, including palliation.
- If the stress test is abnormal, especially with left main disease, it recommends coronary revascularization according to the 2011 clinical practice guidelines.18,19
TESTING FOR LEFT VENTRICULAR DYSFUNCTION OR ISCHEMIA
In patients with dyspnea of unexplained cause or worsening dyspnea, assessment of left ventricular function is reasonable, but this is not part of a routine preoperative evaluation.
Pharmacologic stress testing is reasonable for patients at elevated risk with poor functional capacity if the results will change their management, but it is not useful for patients undergoing low-risk surgery. Although dobutamine stress echocardiography may be slightly superior to pharmacologic myocardial perfusion imaging, there are no head-to-head randomized controlled trials, and the guidelines suggest considering local expertise in deciding which test to use.
The presence of moderate to large areas of ischemia (reversible perfusion defects or new wall-motion abnormalities) is associated with risk of perioperative myocardial infarction or death, whereas evidence of an old infarction is associated with long-term but not short-term risk. The negative predictive value of these tests in predicting postoperative cardiac events is high (> 90%), but the positive predictive value is low.
CORONARY REVASCULARIZATION
Coronary artery bypass grafting and percutaneous coronary intervention
The guidelines recommend coronary revascularization before noncardiac surgery only when it is indicated anyway, on the basis of existing clinical practice guidelines.
Whether performing percutaneous coronary intervention before surgery will reduce perioperative cardiac complications is uncertain, and coronary revascularization should not be routinely performed solely to reduce perioperative cardiac events. The only two randomized controlled trials, Coronary Artery Revascularization Prophylaxis (CARP)20 and DECREASE V21 evaluating prophylactic coronary revascularization before noncardiac surgery found no difference in either short-term or long-term outcomes, although subgroup analysis found a survival benefit in patients with left main disease who underwent bypass grafting. Preoperative percutaneous coronary intervention should be limited to patients with left main disease in whom comorbidities preclude bypass surgery and those with unstable coronary disease who may benefit from early invasive management.
The urgency and timing of the noncardiac surgery needs to be taken into account if percutaneous coronary intervention is being considered because of the need for antiplatelet therapy after the procedure, and the potential risks of bleeding and stent thrombosis. If the planned surgery is deemed time-sensitive, then balloon angioplasty or bare-metal stenting is preferred over placement of a drug-eluting stent.
The new guidelines continue to recommend that elective noncardiac surgery be delayed at least 14 days after balloon angioplasty, 30 days after bare-metal stent implantation, and ideally 365 days after drug-eluting stent placement, and reiterate that it is potentially harmful to perform elective surgery within these time frames without any antiplatelet therapy. However, a new class IIb recommendation (benefit ≥ risk) states that "elective noncardiac surgery after [drug-eluting stent] implantation may be considered after 180 days if the risk of further delay is greater than the expected risks of ischemia and stent thrombosis."
This is an important addition to the guidelines because we are often faced with patients needing to undergo surgery in the 6 to 12 months after placement of a drug-eluting stent. Based on previous guidelines, whether it was safe to proceed in this setting created controversy among the perioperative team caring for the patient, and surgery was often delayed unnecessarily. Recent studies22,23 suggest that the newer drug-eluting stents may require a shorter duration of dual antiplatelet therapy, at least in the nonsurgical setting.
MEDICAL THERAPY
Antiplatelet therapy: Stop or continue?
The risk of perioperative bleeding if antiplatelet drugs are continued must be weighed against the risk of stent thrombosis and ischemia if they are stopped before the recommended duration of therapy. Ideally, some antiplatelet therapy should be continued perioperatively in these situations, but the guidelines recommend that a consensus decision among the treating physicians should be made regarding the relative risks of surgery and discontinuation or continuation of antiplatelet therapy. Whenever possible, aspirin should be continued in these patients.
Although the Perioperative Ischemic Evaluation (POISE)-2 trial24 found that perioperative aspirin use was not associated with lower rates of postoperative myocardial infarction or death, it increased bleeding. Patients with stents who had not completed the recommended duration of antiplatelet therapy were excluded from the trial. Additionally, only 5% of the study patients had undergone percutaneous coronary intervention.
According to the guidelines and package inserts, if antiplatelet agents need to be discontinued before surgery, aspirin can be stopped 3 to 7 days before, clopidogrel and ticagrelor 5 days before, and prasugrel 7 days before. In patients without stents, it may be reasonable to continue aspirin perioperatively if the risk of cardiac events outweighs the risk of bleeding, but starting aspirin is not beneficial for patients undergoing elective noncardiac noncarotid surgery unless the risk of ischemic events outweighs the risk of bleeding.
Beta-blockers
In view of the issue of scientific integrity of the DECREASE trials, a separately commissioned systematic review2 of perioperative beta-blocker therapy was performed. This review suggested that giving beta-blockers before surgery was associated with fewer postoperative cardiac events, primarily ischemia and nonfatal myocardial infarction, but few data supported their use to reduce postoperative mortality. Beta-blocker use was associated with adverse outcomes that included bradycardia and stroke. These findings were similar with the inclusion or exclusion of the DECREASE trials in question or of the POISE trial.25
In addition to recommending continuing beta-blockers in patients already on them (class I—the highest recommendation), the guidelines say that it may be reasonable to start them in patients with intermediate- or high-risk ischemia on stress tests as well as in patients with three or more RCRI risk factors (class IIb). In the absence of these indications, initiating beta-blockers preoperatively to reduce risk even in patients with long-term indications is of uncertain benefit. They also recommended starting beta-blockers more than 1 day preoperatively, preferably at least 2 to 7 days before, and note that it was harmful to start them on the day of surgery, particularly at high doses, and with long-acting formulations.
Additionally, there is evidence of differences in outcome within the class of beta-blockers, with the more cardioselective drugs bisoprolol and atenolol being associated with more favorable outcomes than metoprolol in observational studies.
Statins
Multiple observational trials have reported that statins are associated with decreased perioperative morbidity and mortality. Limited evidence from three randomized controlled trials (including two from the discredited DECREASE group) suggests that there is a benefit in patients undergoing vascular surgery, but it is unclear for nonvascular surgery.26–30
The ACC/AHA guidelines again give a class I recommendation to continue statin therapy perioperatively in patients already taking statins and undergoing noncardiac surgery, as there is some evidence that statin withdrawal is associated with increased risk. The guidelines comment that starting statin therapy perioperatively is reasonable for patients undergoing vascular surgery (class IIa) and may be considered in patients with other clinical guideline indications who are undergoing elevated-risk surgery (class IIb).
The mechanism of this benefit is unclear and may relate to the pleotropic as well as the lipid-lowering effects of the statins. Statins may also have beneficial effects in reducing the incidence of acute kidney injury and postoperative atrial fibrillation.
Whether a particular statin, dose, or time of initiation before surgery affects risk is also unknown at this time. The European guidelines6 recommend starting a longer-acting statin ideally at least 2 weeks before surgery for maximal plaque-stabilizing effects.
The risk of statin-induced myopathy, rhabdomyolysis, and hepatic injury appears to be minimal.
Other medications
Of note, the new guidelines do not recommend starting alpha-2 agonists for preventing cardiac events in patients undergoing noncardiac surgery. Despite previous evidence from smaller studies suggesting a benefit, the POISE-2 trial31 demonstrated that perioperative use of clonidine did not reduce cardiac events and was associated with a significant increase in hypotension and nonfatal cardiac arrest. However, clonidine should be continued in patients already taking it.
A somewhat surprising recommendation is that it is reasonable to continue angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and if they are held before surgery, to restart them as soon as possible postoperatively (class IIa). The guidelines note reports of increased hypotension associated with induction of anesthesia in patients taking these drugs but also note that there was no change in important postoperative cardiac and other outcomes. Although evidence of harm if these drugs are temporarily discontinued before surgery is sparse, the guidelines advocate continuing them in patients with heart failure or hypertension.
ANESTHESIA AND INTRAOPERATIVE MANAGEMENT
The classes of anesthesia include local, regional (nerve block or neuraxial), monitored anesthesia care (ie, intravenous sedation), and general (volatile agent, total intravenous, or a combination). The guideline committee found no evidence to support the use of neuraxial over general anesthesia, volatile over total intravenous anesthesia, or monitored anesthesia care over general anesthesia. Neuraxial anesthesia for postoperative pain relief in patients undergoing abdominal aortic surgery did reduce the incidence of myocardial infarction.
The guidelines do not recommend routinely using intraoperative transesophageal echocardiography during noncardiac surgery to screen for cardiac abnormalities or to monitor for myocardial ischemia in patients without risk factors or procedural risks for significant hemodynamic, pulmonary, or neurologic compromise. Only in emergency settings do they deem perioperative transesophageal echocardiography reasonable to determine the cause of hemodynamic instability when it persists despite attempted corrective therapy.
Maintenance of normothermia is reasonable, as studies evaluating hypothermia or use of warmed air did not find a lower rate of cardiac events.32,33
POSTOPERATIVE SURVEILLANCE
In observational studies, elevated troponin levels, and even detectable levels within the normal range, have been associated with adverse outcomes and predict mortality after noncardiac surgery—the higher the level, the higher the mortality rate.34 Elevated troponins have many potential causes, both cardiac and noncardiac.
An entity termed myocardial injury after noncardiac surgery (MINS)35 was described as prognostically relevant myocardial injury with a troponin T level higher than 0.03 ng/mL in the absence of a nonischemic etiology but not requiring the presence of ischemic features. Patients who had MINS had a higher 30-day mortality rate (9.8% vs 1.1%) and were also at higher risk of nonfatal cardiac arrest, heart failure, and stroke compared with patients who did not.
The guidelines recommend obtaining an electrocardiogram and troponin levels if there are signs or symptoms suggesting myocardial ischemia or infarction. However, despite the association between troponin and mortality, the guidelines state that "the usefulness of postoperative screening with troponin levels (and electrocardiograms) in patients at high risk for perioperative myocardial infarction, but without signs or symptoms suggestive of myocardial ischemia or infarction, is uncertain in the absence of established risks and benefits of a defined management strategy." They also recommend against routinely measuring postoperative troponins in unselected patients without signs or symptoms suggestive of myocardial ischemia or infarction, stating it is not useful for guiding perioperative management.
Although there was a suggestion that patients in the POISE trial36 who suffered postoperative myocardial infarction had better outcomes if they had received aspirin and statins, and another study37 showed that intensification of cardiac therapy in patients with elevated postoperative troponin levels after vascular surgery led to better 1-year outcomes, there are no randomized controlled trials at this time to support any specific plan or intervention.
IMPACT ON CLINICAL PRACTICE: A PERIOPERATIVE HOSPITALIST'S VIEW
Regarding testing
Although the updated guidelines provide some novel concepts in risk stratification, the new algorithm still leaves many patients in a gray zone with respect to noninvasive testing. Patients with heart failure, valvular heart disease, and arrhythmias appear to be somewhat disconnected from the algorithm in this version, and management according to clinical practice guidelines is recommended.
Patients with acute coronary syndrome remain embedded in the algorithm, with recommendations for cardiology evaluation and management according to standard guidelines before proceeding to elective surgery.
The concept of a combined risk based on clinical factors along with the surgical procedure is important, and an alternative to the RCRI factors is offered. However, while this new NSQIP surgical risk calculator is more comprehensive, it may be too time-consuming for routine clinical use and still needs to be externally validated.
The concept of shared decision-making and team communication is stressed, but the physician may still have difficulty deciding when further testing may influence management. The guidelines remain somewhat vague, and many physicians may be uncomfortable and will continue to look for further guidance in this area.
Without more specific recommendations, this uncertainty may result in more stress tests being ordered—often inappropriately, as they rarely change management. Future prospective studies using biomarkers in conjunction with risk calculators may shed some light on this decision.
The new perioperative guidelines incorporate other ACC/AHA guidelines for valvular heart disease15 and heart failure.14 Some of their recommendations, in my opinion, may lead to excessive testing (eg, repeat echocardiograms) that will not change perioperative management.
Regarding revascularization
The ACC/AHA guidelines continue to emphasize the important concept that coronary revascularization is rarely indicated just to get the patient through surgery.
The new guidelines give physicians some leeway in allowing patients with drug-eluting stents to undergo surgery after 6 rather than 12 months of dual antiplatelet therapy if they believe that delaying surgery would place the patient at more risk than that of stent thrombosis. There is evidence in the nonsurgical setting that the newer stents currently being used may require no more than 6 months of therapy. In my opinion it was never clear that there was a statistically significant benefit in delaying surgery more than 6 months after placement of a drug-eluting stent, so this is a welcome addition.
Regarding beta-blockers
The systematic review of beta-blockers reinforces the importance of continuing them preoperatively while downgrading recommendations for their prophylactic use in patients who are not at increased risk.
Although the debate continues, there is no doubt that beta-blockers are associated with a decrease in myocardial ischemia and infarction but an increase in bradycardia and hypotension. They probably are associated with some increased risk of stroke, although this may be related to the specific beta-blocker used as well as the time of initiation before surgery. Evidence of a possible effect on mortality depends on whether the DECREASE and POISE trials are included or excluded in the analysis.
In the absence of new large-scale randomized controlled trials, we are forced to rely on observational trials and expert opinion in the meantime. I think that if a beta-blocker is to be started preoperatively, it should be done at least 1 week before surgery, and a more cardioselective beta-blocker should be used.
Regarding other drugs and tests
I agree with the recommendation to continue ACE inhibitors and ARBs preoperatively in patients with heart failure and poorly controlled hypertension. Although somewhat contrary to current practice, continuance of these drugs has not been associated with an increase in myocardial infarction or death despite concern about intraoperative hypotension.
Data from randomized controlled trials of perioperative statins are limited, but the information from observational studies is favorable, and I see little downside to initiating statins preoperatively in patients who otherwise have indications for their use, particularly if undergoing vascular or other high-risk noncardiac surgery. It is not known whether the specific drug, dose, or timing of initiation of statins influences outcome.
Although multiple studies of biomarkers suggest that there is an association with outcome, there are no randomized controlled trials or specific interventions shown to improve outcome.
Some of the recommended interventions have included various cardiac medications, stress testing, possible coronary angiography, and revascularization, which are not without risk. In the absence of data and following the directive to "first do no harm," the ACC/AHA has been appropriately cautious in not recommending them for routine use at this time.
The updated guidelines have summarized the new evidence in perioperative cardiac evaluation and management. Many of their recommendations were reinforced by this information and remain essentially unchanged. Several new recommendations will lead to changes in management going forward. Unfortunately, we lack the evidence to answer many questions that arise in routine practice and are therefore forced to rely on expert opinion and our clinical judgment in these cases. The ACC/AHA guidelines do provide a framework for our evaluation and management and help keep clinicians up-to-date with the latest evidence.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Wijeysundera DN, Duncan D, Nkonde-Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05528-4. doi: 10.1016/j.jacc.2014.07.939. [Epub ahead of print].
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05537-5. doi: 10.1016/j.jacc.2014.07.945. [Epub ahead of print].
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- Erasmus MC Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity. September 30, 2012. http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed October 30, 2014.
- Anderson JL, Antman EM, Harold JG, et al. Clinical practice guidelines on perioperative cardiovascular evaluation: collaborative efforts among the ACC, AHA, and ESC. J Am Coll Cardiol 2014 Jul 29. pii: S0735-1097(14)05527-2. doi: 10.1016/j.jacc.2014.07.938. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842. e1-3.
- Davis C, Tait G, Carroll J, Wijeysundera DN, Beattie WS. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth 2013; 60:855–863.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; e-pub before print. doi:10.1016/j.jacc.2014.03.022.
- Aliot EM, Alpert JS, Calkins H, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-SVA-FT.pdf. Accessed October 30,2014.
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 2011; 8:1114–1154.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012; 60:645-681.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- McFalls EO, Ward HB, Krupski WC, et al. Prophylactic coronary artery revascularization for elective vascular surgery: study design. Veterans Affairs Cooperative Study Group on Coronary Artery Revascularization Prophylaxis for Elective Vascular Surgery. Control Clin Trials 1999; 20:297–308.
- Schouten O, van Kuijk JP, Flu WJ, et al. Long-term outcome of prophylactic coronary revascularization in cardiac high-risk patients undergoing major vascular surgery (from the randomized DECREASE-V Pilot Study). Am J Cardiol 2009; 103:897–901.
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126:1355-1362.
- Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310:1462–1472.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Lindenauer PK, Pekow P, Wang K, et al. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kennedy J, Quan H, Buchan AM, et al. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- Raju MG, Pachika A, Punnam SR, et al. Statin therapy in the reduction of cardiovascular events in patients undergoing intermediate-risk noncardiac, nonvascular surgery. Clin Cardiol 2013; 36:456–461.
- Desai H, Aronow WS, Ahn C, et al. Incidence of perioperative myocardial infarction and of 2-year mortality in 577 elderly patients undergoing noncardiac vascular surgery treated with and without statins. Arch Gerontol Geriatr 2010; 51:149–151.
- Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1504–1513.
- Nguyen HP, Zaroff JG, Bayman EO, et al. Perioperative hypothermia (33 degrees C) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology 2010; 113:327–342.
- Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277:1127–1134.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Foucrier A, Rodseth R, Aissaoui M, Ibanes C, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
Guidelines jointly issued by the American College of Cardiology and American Heart Association (ACC/AHA)1 provide a framework for evaluating and managing perioperative cardiac risk in noncardiac surgery. An overriding theme in successive documents from these organizations through the years has been that preoperative intervention, coronary artery bypass grafting, or percutaneous coronary intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
This article highlights some of the key recommendations in the 2014 updates to these guidelines,1–3 how they differ from previous guidelines,4 and the ongoing challenges and unresolved issues facing physicians involved in perioperative care.
Of note, while these guidelines were being updated, Erasmus University5 expressed concern about the scientific integrity of some of the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) trials. As a result, the evidence review committee included these trials in its analysis but not in a systematic review of beta-blockers.2 These trials were not included in the clinical practice guideline supplements and tables but were cited in the text if relevant.
The European Society of Cardiology and European Society of Anesthesiology6 revised their guidelines concurrently with but independently of the ACC/AHA, and although they discussed and aligned some recommendations, many differences remain between the two sets of guidelines. Readers should consult the full guidelines for more detailed information.1
THE ROLE OF THE PREOPERATIVE CARDIAC EVALUATION
The purpose of preoperative medical evaluation is not to "get medical clearance" but rather to evaluate the patient’s medical status and risk of complications. The process includes:
- Identifying risk factors and assessing their severity and stability
- Establishing a clinical risk profile for informed and shared decision-making
- Recommending needed changes in management, further testing, or specialty consultation.
The updated guidelines emphasize the importance of communication among the perioperative team and with the patient. They reiterate the focus on appropriateness of care and cost containment—one should order a test only if the result may change the patient’s management.
HOW URGENT IS SURGERY? HOW RISKY?
The new guidelines classify the urgency of surgery as follows:
- Emergency (necessary within 6 hours)
- Urgent (necessary within 6–24 hours)
- Time-sensitive (can delay 1–6 weeks)
- Elective (can delay up to 1 year).
Surgical risk is now classified as either low (< 1% risk of major adverse cardiac events) or elevated (≥ 1%) on the basis of surgical and patient characteristics. Previous schemas included an intermediate-risk category. Low-risk procedures include endoscopic procedures, superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Elevated-risk procedures include vascular surgery, intraperitoneal and intrathoracic surgery, head and neck surgery, orthopedic surgery, and prostate surgery.
Risk calculators and biomarkers
To estimate the perioperative risk of major adverse cardiac events, the guidelines suggest incorporating the Revised Cardiac Risk Index (RCRI)7 with an estimation of surgical risk or using a newer surgical risk calculator derived from a database of the American College of Surgeons’ National Surgical Quality Improvement Project (ACS NSQIP).
The RCRI is based on six risk factors, each worth 1 point:
- High-risk surgery
- Ischemic heart disease
- Heart failure
- Stroke or transient ischemic attack
- Diabetes requiring insulin
- Renal insufficiency (serum creatinine > 2.0 mg/dL).7
MICA. The Myocardial Infarction or Cardiac Arrest (MICA) calculator8 has a narrower focus and was validated in only one center.
ACS NSQIP. The recommended newer ACS NSQIP surgical risk calculator9 provides an estimate of procedure-specific risk based on Current Procedural Terminology code and includes 21 patient-specific variables to predict death, major adverse cardiac events, and eight other outcomes. While more comprehensive, this risk calculator has yet to be validated outside of the ACS NSQIP database.
Reconstructed RCRI. The RCRI has been externally validated, but it underestimates risk in major vascular surgery and was outperformed by the MICA calculator. Although not discussed in the new guidelines, a recently published "reconstructed RCRI,"10 in which a serum creatinine level greater than 2 mg/dL in the original RCRI is replaced by a glomerular filtration rate less than 30 mL/min and diabetes is eliminated, may outperform the standard RCRI. A patient with either an RCRI score or a reconstructed RCRI score of 0 or 1 would be considered to be at low risk, whereas patients with two or more risk factors would have an elevated risk.
Cardiac biomarkers, primarily B-type natriuretic peptide (BNP) and N-terminal (NT) proBNP, are independent predictors of cardiac risk, and their addition to preoperative risk indices may provide incremental predictive value. However, how to use these biomarkers and whether any treatment aimed at them will reduce risk is unclear, and the new guidelines did not recommend their routine use.
CLINICAL RISK FACTORS
Coronary artery disease
Ischemic symptoms, a history of myocardial infarction, and elevated cardiac biomarkers are individually associated with perioperative risk of morbidity and death. The risk is modified by how long ago the infarction occurred, whether the patient underwent coronary revascularization, and if so, what type (bypass grafting or percutaneous coronary intervention). A patient with acute coronary syndrome (currently or in the recent past) is at higher risk, and should have elective surgery delayed and be referred for cardiac evaluation and management according to guidelines.
Heart failure
In terms of posing a risk for major adverse cardiac events, heart failure is at least equal to coronary artery disease, and is possibly worse. Its impact depends on its stability, its symptoms, and the patient’s left ventricular function. Symptomatic decompensated heart failure and depressed left ventricular function (ejection fraction < 30% or 40%) confer higher risk than asymptomatic heart failure and preserved left ventricular function. However, evidence is limited with respect to asymptomatic left ventricular dysfunction and diastolic dysfunction. Patients with stable heart failure treated according to guidelines may have better perioperative outcomes.
Valvular heart disease
Significant valvular heart disease is associated with increased risk of postoperative cardiac complications. This risk depends on the type and severity of the valvular lesion and type of noncardiac surgery, but can be minimized by clinical and echocardiographic assessment, choosing appropriate anesthesia, and closer perioperative monitoring. Aortic and mitral stenosis are associated with greater risk of perioperative adverse cardiac events than regurgitant valvular disease.
Echocardiography is recommended in patients suspected of having moderate to severe stenotic or regurgitant lesions if it has not been done within the past year or if the patient’s clinical condition has worsened.
If indicated, valvular intervention can reduce perioperative risk in these patients. Even if the planned noncardiac surgery is high-risk, it may be reasonable to proceed with it (using appropriate perioperative hemodynamic monitoring, which is not specified but typically would be with an arterial line, central line, and possibly a pulmonary arterial catheter) in patients who have asymptomatic severe aortic or mitral regurgitation or aortic stenosis. Surgery may also be reasonable in patients with asymptomatic severe mitral stenosis who are not candidates for repair.
Arrhythmias
Cardiac arrhythmias and conduction defects are often seen in the perioperative period, but there is only limited evidence as to how they affect surgical risk. In addition to their hemodynamic effects, certain arrhythmias (atrial fibrillation, ventricular tachycardia) often indicate underlying structural heart disease, which requires further evaluation before surgery.
The new guidelines refer the reader to previously published clinical practice guidelines for atrial fibrillation,11 supraventricular arrhythmias,12 and device-based therapy.13
ALGORITHM FOR PREOPERATIVE CARDIAC ASSESSMENT
The new algorithm for evaluating a patient who is known to have coronary artery disease or risk factors for it has seven steps (Figure 1).1,11,12,14–17 It differs from the previous algorithm in several details:
- Instead of listing the four active cardiac conditions for which elective surgery should be delayed while the patient is being evaluated and treated (unstable coronary syndrome, decompensated heart failure, significant arrhythmias, severe valvular heart disease), the new version specifically asks about acute coronary syndrome and recommends cardiac evaluation and treatment according to guidelines. A footnote directs readers to other clinical practice guidelines for symptomatic heart failure,14 valvular heart disease,15 and arrhythmias.11,12
- Instead of asking if the procedure is low-risk, the guidelines recommend estimating risk of major adverse cardiac events on the basis of combined clinical and surgical risk and define only two categories: low or elevated. Patients at low risk proceed to surgery with no further testing, as in the earlier algorithm.
- "Excellent" exercise capacity (> 10 metabolic equivalents of task [METs]) is separated from "moderate/good" (4–10 METs), presumably to indicate a stronger recommendation, but patients in both categories proceed to surgery as before.
- If the patient cannot exercise to at least 4 METs, the new algorithm asks whether further testing will affect decision-making or perioperative care (an addition to the previous algorithm). This entails discussing with the patient and perioperative team whether the original surgery will be performed and whether the patient is willing to undergo revascularization if indicated. If so, pharmacologic stress testing is recommended. Previously, this decision also included the number of RCRI factors as well as the type of surgery (vascular or nonvascular).
- If testing will not affect the decision or if the stress test is normal, in addition to recommending proceeding to surgery according to guidelines the new algorithm also lists an option for alternative strategies, including palliation.
- If the stress test is abnormal, especially with left main disease, it recommends coronary revascularization according to the 2011 clinical practice guidelines.18,19
TESTING FOR LEFT VENTRICULAR DYSFUNCTION OR ISCHEMIA
In patients with dyspnea of unexplained cause or worsening dyspnea, assessment of left ventricular function is reasonable, but this is not part of a routine preoperative evaluation.
Pharmacologic stress testing is reasonable for patients at elevated risk with poor functional capacity if the results will change their management, but it is not useful for patients undergoing low-risk surgery. Although dobutamine stress echocardiography may be slightly superior to pharmacologic myocardial perfusion imaging, there are no head-to-head randomized controlled trials, and the guidelines suggest considering local expertise in deciding which test to use.
The presence of moderate to large areas of ischemia (reversible perfusion defects or new wall-motion abnormalities) is associated with risk of perioperative myocardial infarction or death, whereas evidence of an old infarction is associated with long-term but not short-term risk. The negative predictive value of these tests in predicting postoperative cardiac events is high (> 90%), but the positive predictive value is low.
CORONARY REVASCULARIZATION
Coronary artery bypass grafting and percutaneous coronary intervention
The guidelines recommend coronary revascularization before noncardiac surgery only when it is indicated anyway, on the basis of existing clinical practice guidelines.
Whether performing percutaneous coronary intervention before surgery will reduce perioperative cardiac complications is uncertain, and coronary revascularization should not be routinely performed solely to reduce perioperative cardiac events. The only two randomized controlled trials, Coronary Artery Revascularization Prophylaxis (CARP)20 and DECREASE V21 evaluating prophylactic coronary revascularization before noncardiac surgery found no difference in either short-term or long-term outcomes, although subgroup analysis found a survival benefit in patients with left main disease who underwent bypass grafting. Preoperative percutaneous coronary intervention should be limited to patients with left main disease in whom comorbidities preclude bypass surgery and those with unstable coronary disease who may benefit from early invasive management.
The urgency and timing of the noncardiac surgery needs to be taken into account if percutaneous coronary intervention is being considered because of the need for antiplatelet therapy after the procedure, and the potential risks of bleeding and stent thrombosis. If the planned surgery is deemed time-sensitive, then balloon angioplasty or bare-metal stenting is preferred over placement of a drug-eluting stent.
The new guidelines continue to recommend that elective noncardiac surgery be delayed at least 14 days after balloon angioplasty, 30 days after bare-metal stent implantation, and ideally 365 days after drug-eluting stent placement, and reiterate that it is potentially harmful to perform elective surgery within these time frames without any antiplatelet therapy. However, a new class IIb recommendation (benefit ≥ risk) states that "elective noncardiac surgery after [drug-eluting stent] implantation may be considered after 180 days if the risk of further delay is greater than the expected risks of ischemia and stent thrombosis."
This is an important addition to the guidelines because we are often faced with patients needing to undergo surgery in the 6 to 12 months after placement of a drug-eluting stent. Based on previous guidelines, whether it was safe to proceed in this setting created controversy among the perioperative team caring for the patient, and surgery was often delayed unnecessarily. Recent studies22,23 suggest that the newer drug-eluting stents may require a shorter duration of dual antiplatelet therapy, at least in the nonsurgical setting.
MEDICAL THERAPY
Antiplatelet therapy: Stop or continue?
The risk of perioperative bleeding if antiplatelet drugs are continued must be weighed against the risk of stent thrombosis and ischemia if they are stopped before the recommended duration of therapy. Ideally, some antiplatelet therapy should be continued perioperatively in these situations, but the guidelines recommend that a consensus decision among the treating physicians should be made regarding the relative risks of surgery and discontinuation or continuation of antiplatelet therapy. Whenever possible, aspirin should be continued in these patients.
Although the Perioperative Ischemic Evaluation (POISE)-2 trial24 found that perioperative aspirin use was not associated with lower rates of postoperative myocardial infarction or death, it increased bleeding. Patients with stents who had not completed the recommended duration of antiplatelet therapy were excluded from the trial. Additionally, only 5% of the study patients had undergone percutaneous coronary intervention.
According to the guidelines and package inserts, if antiplatelet agents need to be discontinued before surgery, aspirin can be stopped 3 to 7 days before, clopidogrel and ticagrelor 5 days before, and prasugrel 7 days before. In patients without stents, it may be reasonable to continue aspirin perioperatively if the risk of cardiac events outweighs the risk of bleeding, but starting aspirin is not beneficial for patients undergoing elective noncardiac noncarotid surgery unless the risk of ischemic events outweighs the risk of bleeding.
Beta-blockers
In view of the issue of scientific integrity of the DECREASE trials, a separately commissioned systematic review2 of perioperative beta-blocker therapy was performed. This review suggested that giving beta-blockers before surgery was associated with fewer postoperative cardiac events, primarily ischemia and nonfatal myocardial infarction, but few data supported their use to reduce postoperative mortality. Beta-blocker use was associated with adverse outcomes that included bradycardia and stroke. These findings were similar with the inclusion or exclusion of the DECREASE trials in question or of the POISE trial.25
In addition to recommending continuing beta-blockers in patients already on them (class I—the highest recommendation), the guidelines say that it may be reasonable to start them in patients with intermediate- or high-risk ischemia on stress tests as well as in patients with three or more RCRI risk factors (class IIb). In the absence of these indications, initiating beta-blockers preoperatively to reduce risk even in patients with long-term indications is of uncertain benefit. They also recommended starting beta-blockers more than 1 day preoperatively, preferably at least 2 to 7 days before, and note that it was harmful to start them on the day of surgery, particularly at high doses, and with long-acting formulations.
Additionally, there is evidence of differences in outcome within the class of beta-blockers, with the more cardioselective drugs bisoprolol and atenolol being associated with more favorable outcomes than metoprolol in observational studies.
Statins
Multiple observational trials have reported that statins are associated with decreased perioperative morbidity and mortality. Limited evidence from three randomized controlled trials (including two from the discredited DECREASE group) suggests that there is a benefit in patients undergoing vascular surgery, but it is unclear for nonvascular surgery.26–30
The ACC/AHA guidelines again give a class I recommendation to continue statin therapy perioperatively in patients already taking statins and undergoing noncardiac surgery, as there is some evidence that statin withdrawal is associated with increased risk. The guidelines comment that starting statin therapy perioperatively is reasonable for patients undergoing vascular surgery (class IIa) and may be considered in patients with other clinical guideline indications who are undergoing elevated-risk surgery (class IIb).
The mechanism of this benefit is unclear and may relate to the pleotropic as well as the lipid-lowering effects of the statins. Statins may also have beneficial effects in reducing the incidence of acute kidney injury and postoperative atrial fibrillation.
Whether a particular statin, dose, or time of initiation before surgery affects risk is also unknown at this time. The European guidelines6 recommend starting a longer-acting statin ideally at least 2 weeks before surgery for maximal plaque-stabilizing effects.
The risk of statin-induced myopathy, rhabdomyolysis, and hepatic injury appears to be minimal.
Other medications
Of note, the new guidelines do not recommend starting alpha-2 agonists for preventing cardiac events in patients undergoing noncardiac surgery. Despite previous evidence from smaller studies suggesting a benefit, the POISE-2 trial31 demonstrated that perioperative use of clonidine did not reduce cardiac events and was associated with a significant increase in hypotension and nonfatal cardiac arrest. However, clonidine should be continued in patients already taking it.
A somewhat surprising recommendation is that it is reasonable to continue angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and if they are held before surgery, to restart them as soon as possible postoperatively (class IIa). The guidelines note reports of increased hypotension associated with induction of anesthesia in patients taking these drugs but also note that there was no change in important postoperative cardiac and other outcomes. Although evidence of harm if these drugs are temporarily discontinued before surgery is sparse, the guidelines advocate continuing them in patients with heart failure or hypertension.
ANESTHESIA AND INTRAOPERATIVE MANAGEMENT
The classes of anesthesia include local, regional (nerve block or neuraxial), monitored anesthesia care (ie, intravenous sedation), and general (volatile agent, total intravenous, or a combination). The guideline committee found no evidence to support the use of neuraxial over general anesthesia, volatile over total intravenous anesthesia, or monitored anesthesia care over general anesthesia. Neuraxial anesthesia for postoperative pain relief in patients undergoing abdominal aortic surgery did reduce the incidence of myocardial infarction.
The guidelines do not recommend routinely using intraoperative transesophageal echocardiography during noncardiac surgery to screen for cardiac abnormalities or to monitor for myocardial ischemia in patients without risk factors or procedural risks for significant hemodynamic, pulmonary, or neurologic compromise. Only in emergency settings do they deem perioperative transesophageal echocardiography reasonable to determine the cause of hemodynamic instability when it persists despite attempted corrective therapy.
Maintenance of normothermia is reasonable, as studies evaluating hypothermia or use of warmed air did not find a lower rate of cardiac events.32,33
POSTOPERATIVE SURVEILLANCE
In observational studies, elevated troponin levels, and even detectable levels within the normal range, have been associated with adverse outcomes and predict mortality after noncardiac surgery—the higher the level, the higher the mortality rate.34 Elevated troponins have many potential causes, both cardiac and noncardiac.
An entity termed myocardial injury after noncardiac surgery (MINS)35 was described as prognostically relevant myocardial injury with a troponin T level higher than 0.03 ng/mL in the absence of a nonischemic etiology but not requiring the presence of ischemic features. Patients who had MINS had a higher 30-day mortality rate (9.8% vs 1.1%) and were also at higher risk of nonfatal cardiac arrest, heart failure, and stroke compared with patients who did not.
The guidelines recommend obtaining an electrocardiogram and troponin levels if there are signs or symptoms suggesting myocardial ischemia or infarction. However, despite the association between troponin and mortality, the guidelines state that "the usefulness of postoperative screening with troponin levels (and electrocardiograms) in patients at high risk for perioperative myocardial infarction, but without signs or symptoms suggestive of myocardial ischemia or infarction, is uncertain in the absence of established risks and benefits of a defined management strategy." They also recommend against routinely measuring postoperative troponins in unselected patients without signs or symptoms suggestive of myocardial ischemia or infarction, stating it is not useful for guiding perioperative management.
Although there was a suggestion that patients in the POISE trial36 who suffered postoperative myocardial infarction had better outcomes if they had received aspirin and statins, and another study37 showed that intensification of cardiac therapy in patients with elevated postoperative troponin levels after vascular surgery led to better 1-year outcomes, there are no randomized controlled trials at this time to support any specific plan or intervention.
IMPACT ON CLINICAL PRACTICE: A PERIOPERATIVE HOSPITALIST'S VIEW
Regarding testing
Although the updated guidelines provide some novel concepts in risk stratification, the new algorithm still leaves many patients in a gray zone with respect to noninvasive testing. Patients with heart failure, valvular heart disease, and arrhythmias appear to be somewhat disconnected from the algorithm in this version, and management according to clinical practice guidelines is recommended.
Patients with acute coronary syndrome remain embedded in the algorithm, with recommendations for cardiology evaluation and management according to standard guidelines before proceeding to elective surgery.
The concept of a combined risk based on clinical factors along with the surgical procedure is important, and an alternative to the RCRI factors is offered. However, while this new NSQIP surgical risk calculator is more comprehensive, it may be too time-consuming for routine clinical use and still needs to be externally validated.
The concept of shared decision-making and team communication is stressed, but the physician may still have difficulty deciding when further testing may influence management. The guidelines remain somewhat vague, and many physicians may be uncomfortable and will continue to look for further guidance in this area.
Without more specific recommendations, this uncertainty may result in more stress tests being ordered—often inappropriately, as they rarely change management. Future prospective studies using biomarkers in conjunction with risk calculators may shed some light on this decision.
The new perioperative guidelines incorporate other ACC/AHA guidelines for valvular heart disease15 and heart failure.14 Some of their recommendations, in my opinion, may lead to excessive testing (eg, repeat echocardiograms) that will not change perioperative management.
Regarding revascularization
The ACC/AHA guidelines continue to emphasize the important concept that coronary revascularization is rarely indicated just to get the patient through surgery.
The new guidelines give physicians some leeway in allowing patients with drug-eluting stents to undergo surgery after 6 rather than 12 months of dual antiplatelet therapy if they believe that delaying surgery would place the patient at more risk than that of stent thrombosis. There is evidence in the nonsurgical setting that the newer stents currently being used may require no more than 6 months of therapy. In my opinion it was never clear that there was a statistically significant benefit in delaying surgery more than 6 months after placement of a drug-eluting stent, so this is a welcome addition.
Regarding beta-blockers
The systematic review of beta-blockers reinforces the importance of continuing them preoperatively while downgrading recommendations for their prophylactic use in patients who are not at increased risk.
Although the debate continues, there is no doubt that beta-blockers are associated with a decrease in myocardial ischemia and infarction but an increase in bradycardia and hypotension. They probably are associated with some increased risk of stroke, although this may be related to the specific beta-blocker used as well as the time of initiation before surgery. Evidence of a possible effect on mortality depends on whether the DECREASE and POISE trials are included or excluded in the analysis.
In the absence of new large-scale randomized controlled trials, we are forced to rely on observational trials and expert opinion in the meantime. I think that if a beta-blocker is to be started preoperatively, it should be done at least 1 week before surgery, and a more cardioselective beta-blocker should be used.
Regarding other drugs and tests
I agree with the recommendation to continue ACE inhibitors and ARBs preoperatively in patients with heart failure and poorly controlled hypertension. Although somewhat contrary to current practice, continuance of these drugs has not been associated with an increase in myocardial infarction or death despite concern about intraoperative hypotension.
Data from randomized controlled trials of perioperative statins are limited, but the information from observational studies is favorable, and I see little downside to initiating statins preoperatively in patients who otherwise have indications for their use, particularly if undergoing vascular or other high-risk noncardiac surgery. It is not known whether the specific drug, dose, or timing of initiation of statins influences outcome.
Although multiple studies of biomarkers suggest that there is an association with outcome, there are no randomized controlled trials or specific interventions shown to improve outcome.
Some of the recommended interventions have included various cardiac medications, stress testing, possible coronary angiography, and revascularization, which are not without risk. In the absence of data and following the directive to "first do no harm," the ACC/AHA has been appropriately cautious in not recommending them for routine use at this time.
The updated guidelines have summarized the new evidence in perioperative cardiac evaluation and management. Many of their recommendations were reinforced by this information and remain essentially unchanged. Several new recommendations will lead to changes in management going forward. Unfortunately, we lack the evidence to answer many questions that arise in routine practice and are therefore forced to rely on expert opinion and our clinical judgment in these cases. The ACC/AHA guidelines do provide a framework for our evaluation and management and help keep clinicians up-to-date with the latest evidence.
Guidelines jointly issued by the American College of Cardiology and American Heart Association (ACC/AHA)1 provide a framework for evaluating and managing perioperative cardiac risk in noncardiac surgery. An overriding theme in successive documents from these organizations through the years has been that preoperative intervention, coronary artery bypass grafting, or percutaneous coronary intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
This article highlights some of the key recommendations in the 2014 updates to these guidelines,1–3 how they differ from previous guidelines,4 and the ongoing challenges and unresolved issues facing physicians involved in perioperative care.
Of note, while these guidelines were being updated, Erasmus University5 expressed concern about the scientific integrity of some of the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) trials. As a result, the evidence review committee included these trials in its analysis but not in a systematic review of beta-blockers.2 These trials were not included in the clinical practice guideline supplements and tables but were cited in the text if relevant.
The European Society of Cardiology and European Society of Anesthesiology6 revised their guidelines concurrently with but independently of the ACC/AHA, and although they discussed and aligned some recommendations, many differences remain between the two sets of guidelines. Readers should consult the full guidelines for more detailed information.1
THE ROLE OF THE PREOPERATIVE CARDIAC EVALUATION
The purpose of preoperative medical evaluation is not to "get medical clearance" but rather to evaluate the patient’s medical status and risk of complications. The process includes:
- Identifying risk factors and assessing their severity and stability
- Establishing a clinical risk profile for informed and shared decision-making
- Recommending needed changes in management, further testing, or specialty consultation.
The updated guidelines emphasize the importance of communication among the perioperative team and with the patient. They reiterate the focus on appropriateness of care and cost containment—one should order a test only if the result may change the patient’s management.
HOW URGENT IS SURGERY? HOW RISKY?
The new guidelines classify the urgency of surgery as follows:
- Emergency (necessary within 6 hours)
- Urgent (necessary within 6–24 hours)
- Time-sensitive (can delay 1–6 weeks)
- Elective (can delay up to 1 year).
Surgical risk is now classified as either low (< 1% risk of major adverse cardiac events) or elevated (≥ 1%) on the basis of surgical and patient characteristics. Previous schemas included an intermediate-risk category. Low-risk procedures include endoscopic procedures, superficial procedures, cataract surgery, breast surgery, and ambulatory surgery. Elevated-risk procedures include vascular surgery, intraperitoneal and intrathoracic surgery, head and neck surgery, orthopedic surgery, and prostate surgery.
Risk calculators and biomarkers
To estimate the perioperative risk of major adverse cardiac events, the guidelines suggest incorporating the Revised Cardiac Risk Index (RCRI)7 with an estimation of surgical risk or using a newer surgical risk calculator derived from a database of the American College of Surgeons’ National Surgical Quality Improvement Project (ACS NSQIP).
The RCRI is based on six risk factors, each worth 1 point:
- High-risk surgery
- Ischemic heart disease
- Heart failure
- Stroke or transient ischemic attack
- Diabetes requiring insulin
- Renal insufficiency (serum creatinine > 2.0 mg/dL).7
MICA. The Myocardial Infarction or Cardiac Arrest (MICA) calculator8 has a narrower focus and was validated in only one center.
ACS NSQIP. The recommended newer ACS NSQIP surgical risk calculator9 provides an estimate of procedure-specific risk based on Current Procedural Terminology code and includes 21 patient-specific variables to predict death, major adverse cardiac events, and eight other outcomes. While more comprehensive, this risk calculator has yet to be validated outside of the ACS NSQIP database.
Reconstructed RCRI. The RCRI has been externally validated, but it underestimates risk in major vascular surgery and was outperformed by the MICA calculator. Although not discussed in the new guidelines, a recently published "reconstructed RCRI,"10 in which a serum creatinine level greater than 2 mg/dL in the original RCRI is replaced by a glomerular filtration rate less than 30 mL/min and diabetes is eliminated, may outperform the standard RCRI. A patient with either an RCRI score or a reconstructed RCRI score of 0 or 1 would be considered to be at low risk, whereas patients with two or more risk factors would have an elevated risk.
Cardiac biomarkers, primarily B-type natriuretic peptide (BNP) and N-terminal (NT) proBNP, are independent predictors of cardiac risk, and their addition to preoperative risk indices may provide incremental predictive value. However, how to use these biomarkers and whether any treatment aimed at them will reduce risk is unclear, and the new guidelines did not recommend their routine use.
CLINICAL RISK FACTORS
Coronary artery disease
Ischemic symptoms, a history of myocardial infarction, and elevated cardiac biomarkers are individually associated with perioperative risk of morbidity and death. The risk is modified by how long ago the infarction occurred, whether the patient underwent coronary revascularization, and if so, what type (bypass grafting or percutaneous coronary intervention). A patient with acute coronary syndrome (currently or in the recent past) is at higher risk, and should have elective surgery delayed and be referred for cardiac evaluation and management according to guidelines.
Heart failure
In terms of posing a risk for major adverse cardiac events, heart failure is at least equal to coronary artery disease, and is possibly worse. Its impact depends on its stability, its symptoms, and the patient’s left ventricular function. Symptomatic decompensated heart failure and depressed left ventricular function (ejection fraction < 30% or 40%) confer higher risk than asymptomatic heart failure and preserved left ventricular function. However, evidence is limited with respect to asymptomatic left ventricular dysfunction and diastolic dysfunction. Patients with stable heart failure treated according to guidelines may have better perioperative outcomes.
Valvular heart disease
Significant valvular heart disease is associated with increased risk of postoperative cardiac complications. This risk depends on the type and severity of the valvular lesion and type of noncardiac surgery, but can be minimized by clinical and echocardiographic assessment, choosing appropriate anesthesia, and closer perioperative monitoring. Aortic and mitral stenosis are associated with greater risk of perioperative adverse cardiac events than regurgitant valvular disease.
Echocardiography is recommended in patients suspected of having moderate to severe stenotic or regurgitant lesions if it has not been done within the past year or if the patient’s clinical condition has worsened.
If indicated, valvular intervention can reduce perioperative risk in these patients. Even if the planned noncardiac surgery is high-risk, it may be reasonable to proceed with it (using appropriate perioperative hemodynamic monitoring, which is not specified but typically would be with an arterial line, central line, and possibly a pulmonary arterial catheter) in patients who have asymptomatic severe aortic or mitral regurgitation or aortic stenosis. Surgery may also be reasonable in patients with asymptomatic severe mitral stenosis who are not candidates for repair.
Arrhythmias
Cardiac arrhythmias and conduction defects are often seen in the perioperative period, but there is only limited evidence as to how they affect surgical risk. In addition to their hemodynamic effects, certain arrhythmias (atrial fibrillation, ventricular tachycardia) often indicate underlying structural heart disease, which requires further evaluation before surgery.
The new guidelines refer the reader to previously published clinical practice guidelines for atrial fibrillation,11 supraventricular arrhythmias,12 and device-based therapy.13
ALGORITHM FOR PREOPERATIVE CARDIAC ASSESSMENT
The new algorithm for evaluating a patient who is known to have coronary artery disease or risk factors for it has seven steps (Figure 1).1,11,12,14–17 It differs from the previous algorithm in several details:
- Instead of listing the four active cardiac conditions for which elective surgery should be delayed while the patient is being evaluated and treated (unstable coronary syndrome, decompensated heart failure, significant arrhythmias, severe valvular heart disease), the new version specifically asks about acute coronary syndrome and recommends cardiac evaluation and treatment according to guidelines. A footnote directs readers to other clinical practice guidelines for symptomatic heart failure,14 valvular heart disease,15 and arrhythmias.11,12
- Instead of asking if the procedure is low-risk, the guidelines recommend estimating risk of major adverse cardiac events on the basis of combined clinical and surgical risk and define only two categories: low or elevated. Patients at low risk proceed to surgery with no further testing, as in the earlier algorithm.
- "Excellent" exercise capacity (> 10 metabolic equivalents of task [METs]) is separated from "moderate/good" (4–10 METs), presumably to indicate a stronger recommendation, but patients in both categories proceed to surgery as before.
- If the patient cannot exercise to at least 4 METs, the new algorithm asks whether further testing will affect decision-making or perioperative care (an addition to the previous algorithm). This entails discussing with the patient and perioperative team whether the original surgery will be performed and whether the patient is willing to undergo revascularization if indicated. If so, pharmacologic stress testing is recommended. Previously, this decision also included the number of RCRI factors as well as the type of surgery (vascular or nonvascular).
- If testing will not affect the decision or if the stress test is normal, in addition to recommending proceeding to surgery according to guidelines the new algorithm also lists an option for alternative strategies, including palliation.
- If the stress test is abnormal, especially with left main disease, it recommends coronary revascularization according to the 2011 clinical practice guidelines.18,19
TESTING FOR LEFT VENTRICULAR DYSFUNCTION OR ISCHEMIA
In patients with dyspnea of unexplained cause or worsening dyspnea, assessment of left ventricular function is reasonable, but this is not part of a routine preoperative evaluation.
Pharmacologic stress testing is reasonable for patients at elevated risk with poor functional capacity if the results will change their management, but it is not useful for patients undergoing low-risk surgery. Although dobutamine stress echocardiography may be slightly superior to pharmacologic myocardial perfusion imaging, there are no head-to-head randomized controlled trials, and the guidelines suggest considering local expertise in deciding which test to use.
The presence of moderate to large areas of ischemia (reversible perfusion defects or new wall-motion abnormalities) is associated with risk of perioperative myocardial infarction or death, whereas evidence of an old infarction is associated with long-term but not short-term risk. The negative predictive value of these tests in predicting postoperative cardiac events is high (> 90%), but the positive predictive value is low.
CORONARY REVASCULARIZATION
Coronary artery bypass grafting and percutaneous coronary intervention
The guidelines recommend coronary revascularization before noncardiac surgery only when it is indicated anyway, on the basis of existing clinical practice guidelines.
Whether performing percutaneous coronary intervention before surgery will reduce perioperative cardiac complications is uncertain, and coronary revascularization should not be routinely performed solely to reduce perioperative cardiac events. The only two randomized controlled trials, Coronary Artery Revascularization Prophylaxis (CARP)20 and DECREASE V21 evaluating prophylactic coronary revascularization before noncardiac surgery found no difference in either short-term or long-term outcomes, although subgroup analysis found a survival benefit in patients with left main disease who underwent bypass grafting. Preoperative percutaneous coronary intervention should be limited to patients with left main disease in whom comorbidities preclude bypass surgery and those with unstable coronary disease who may benefit from early invasive management.
The urgency and timing of the noncardiac surgery needs to be taken into account if percutaneous coronary intervention is being considered because of the need for antiplatelet therapy after the procedure, and the potential risks of bleeding and stent thrombosis. If the planned surgery is deemed time-sensitive, then balloon angioplasty or bare-metal stenting is preferred over placement of a drug-eluting stent.
The new guidelines continue to recommend that elective noncardiac surgery be delayed at least 14 days after balloon angioplasty, 30 days after bare-metal stent implantation, and ideally 365 days after drug-eluting stent placement, and reiterate that it is potentially harmful to perform elective surgery within these time frames without any antiplatelet therapy. However, a new class IIb recommendation (benefit ≥ risk) states that "elective noncardiac surgery after [drug-eluting stent] implantation may be considered after 180 days if the risk of further delay is greater than the expected risks of ischemia and stent thrombosis."
This is an important addition to the guidelines because we are often faced with patients needing to undergo surgery in the 6 to 12 months after placement of a drug-eluting stent. Based on previous guidelines, whether it was safe to proceed in this setting created controversy among the perioperative team caring for the patient, and surgery was often delayed unnecessarily. Recent studies22,23 suggest that the newer drug-eluting stents may require a shorter duration of dual antiplatelet therapy, at least in the nonsurgical setting.
MEDICAL THERAPY
Antiplatelet therapy: Stop or continue?
The risk of perioperative bleeding if antiplatelet drugs are continued must be weighed against the risk of stent thrombosis and ischemia if they are stopped before the recommended duration of therapy. Ideally, some antiplatelet therapy should be continued perioperatively in these situations, but the guidelines recommend that a consensus decision among the treating physicians should be made regarding the relative risks of surgery and discontinuation or continuation of antiplatelet therapy. Whenever possible, aspirin should be continued in these patients.
Although the Perioperative Ischemic Evaluation (POISE)-2 trial24 found that perioperative aspirin use was not associated with lower rates of postoperative myocardial infarction or death, it increased bleeding. Patients with stents who had not completed the recommended duration of antiplatelet therapy were excluded from the trial. Additionally, only 5% of the study patients had undergone percutaneous coronary intervention.
According to the guidelines and package inserts, if antiplatelet agents need to be discontinued before surgery, aspirin can be stopped 3 to 7 days before, clopidogrel and ticagrelor 5 days before, and prasugrel 7 days before. In patients without stents, it may be reasonable to continue aspirin perioperatively if the risk of cardiac events outweighs the risk of bleeding, but starting aspirin is not beneficial for patients undergoing elective noncardiac noncarotid surgery unless the risk of ischemic events outweighs the risk of bleeding.
Beta-blockers
In view of the issue of scientific integrity of the DECREASE trials, a separately commissioned systematic review2 of perioperative beta-blocker therapy was performed. This review suggested that giving beta-blockers before surgery was associated with fewer postoperative cardiac events, primarily ischemia and nonfatal myocardial infarction, but few data supported their use to reduce postoperative mortality. Beta-blocker use was associated with adverse outcomes that included bradycardia and stroke. These findings were similar with the inclusion or exclusion of the DECREASE trials in question or of the POISE trial.25
In addition to recommending continuing beta-blockers in patients already on them (class I—the highest recommendation), the guidelines say that it may be reasonable to start them in patients with intermediate- or high-risk ischemia on stress tests as well as in patients with three or more RCRI risk factors (class IIb). In the absence of these indications, initiating beta-blockers preoperatively to reduce risk even in patients with long-term indications is of uncertain benefit. They also recommended starting beta-blockers more than 1 day preoperatively, preferably at least 2 to 7 days before, and note that it was harmful to start them on the day of surgery, particularly at high doses, and with long-acting formulations.
Additionally, there is evidence of differences in outcome within the class of beta-blockers, with the more cardioselective drugs bisoprolol and atenolol being associated with more favorable outcomes than metoprolol in observational studies.
Statins
Multiple observational trials have reported that statins are associated with decreased perioperative morbidity and mortality. Limited evidence from three randomized controlled trials (including two from the discredited DECREASE group) suggests that there is a benefit in patients undergoing vascular surgery, but it is unclear for nonvascular surgery.26–30
The ACC/AHA guidelines again give a class I recommendation to continue statin therapy perioperatively in patients already taking statins and undergoing noncardiac surgery, as there is some evidence that statin withdrawal is associated with increased risk. The guidelines comment that starting statin therapy perioperatively is reasonable for patients undergoing vascular surgery (class IIa) and may be considered in patients with other clinical guideline indications who are undergoing elevated-risk surgery (class IIb).
The mechanism of this benefit is unclear and may relate to the pleotropic as well as the lipid-lowering effects of the statins. Statins may also have beneficial effects in reducing the incidence of acute kidney injury and postoperative atrial fibrillation.
Whether a particular statin, dose, or time of initiation before surgery affects risk is also unknown at this time. The European guidelines6 recommend starting a longer-acting statin ideally at least 2 weeks before surgery for maximal plaque-stabilizing effects.
The risk of statin-induced myopathy, rhabdomyolysis, and hepatic injury appears to be minimal.
Other medications
Of note, the new guidelines do not recommend starting alpha-2 agonists for preventing cardiac events in patients undergoing noncardiac surgery. Despite previous evidence from smaller studies suggesting a benefit, the POISE-2 trial31 demonstrated that perioperative use of clonidine did not reduce cardiac events and was associated with a significant increase in hypotension and nonfatal cardiac arrest. However, clonidine should be continued in patients already taking it.
A somewhat surprising recommendation is that it is reasonable to continue angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and if they are held before surgery, to restart them as soon as possible postoperatively (class IIa). The guidelines note reports of increased hypotension associated with induction of anesthesia in patients taking these drugs but also note that there was no change in important postoperative cardiac and other outcomes. Although evidence of harm if these drugs are temporarily discontinued before surgery is sparse, the guidelines advocate continuing them in patients with heart failure or hypertension.
ANESTHESIA AND INTRAOPERATIVE MANAGEMENT
The classes of anesthesia include local, regional (nerve block or neuraxial), monitored anesthesia care (ie, intravenous sedation), and general (volatile agent, total intravenous, or a combination). The guideline committee found no evidence to support the use of neuraxial over general anesthesia, volatile over total intravenous anesthesia, or monitored anesthesia care over general anesthesia. Neuraxial anesthesia for postoperative pain relief in patients undergoing abdominal aortic surgery did reduce the incidence of myocardial infarction.
The guidelines do not recommend routinely using intraoperative transesophageal echocardiography during noncardiac surgery to screen for cardiac abnormalities or to monitor for myocardial ischemia in patients without risk factors or procedural risks for significant hemodynamic, pulmonary, or neurologic compromise. Only in emergency settings do they deem perioperative transesophageal echocardiography reasonable to determine the cause of hemodynamic instability when it persists despite attempted corrective therapy.
Maintenance of normothermia is reasonable, as studies evaluating hypothermia or use of warmed air did not find a lower rate of cardiac events.32,33
POSTOPERATIVE SURVEILLANCE
In observational studies, elevated troponin levels, and even detectable levels within the normal range, have been associated with adverse outcomes and predict mortality after noncardiac surgery—the higher the level, the higher the mortality rate.34 Elevated troponins have many potential causes, both cardiac and noncardiac.
An entity termed myocardial injury after noncardiac surgery (MINS)35 was described as prognostically relevant myocardial injury with a troponin T level higher than 0.03 ng/mL in the absence of a nonischemic etiology but not requiring the presence of ischemic features. Patients who had MINS had a higher 30-day mortality rate (9.8% vs 1.1%) and were also at higher risk of nonfatal cardiac arrest, heart failure, and stroke compared with patients who did not.
The guidelines recommend obtaining an electrocardiogram and troponin levels if there are signs or symptoms suggesting myocardial ischemia or infarction. However, despite the association between troponin and mortality, the guidelines state that "the usefulness of postoperative screening with troponin levels (and electrocardiograms) in patients at high risk for perioperative myocardial infarction, but without signs or symptoms suggestive of myocardial ischemia or infarction, is uncertain in the absence of established risks and benefits of a defined management strategy." They also recommend against routinely measuring postoperative troponins in unselected patients without signs or symptoms suggestive of myocardial ischemia or infarction, stating it is not useful for guiding perioperative management.
Although there was a suggestion that patients in the POISE trial36 who suffered postoperative myocardial infarction had better outcomes if they had received aspirin and statins, and another study37 showed that intensification of cardiac therapy in patients with elevated postoperative troponin levels after vascular surgery led to better 1-year outcomes, there are no randomized controlled trials at this time to support any specific plan or intervention.
IMPACT ON CLINICAL PRACTICE: A PERIOPERATIVE HOSPITALIST'S VIEW
Regarding testing
Although the updated guidelines provide some novel concepts in risk stratification, the new algorithm still leaves many patients in a gray zone with respect to noninvasive testing. Patients with heart failure, valvular heart disease, and arrhythmias appear to be somewhat disconnected from the algorithm in this version, and management according to clinical practice guidelines is recommended.
Patients with acute coronary syndrome remain embedded in the algorithm, with recommendations for cardiology evaluation and management according to standard guidelines before proceeding to elective surgery.
The concept of a combined risk based on clinical factors along with the surgical procedure is important, and an alternative to the RCRI factors is offered. However, while this new NSQIP surgical risk calculator is more comprehensive, it may be too time-consuming for routine clinical use and still needs to be externally validated.
The concept of shared decision-making and team communication is stressed, but the physician may still have difficulty deciding when further testing may influence management. The guidelines remain somewhat vague, and many physicians may be uncomfortable and will continue to look for further guidance in this area.
Without more specific recommendations, this uncertainty may result in more stress tests being ordered—often inappropriately, as they rarely change management. Future prospective studies using biomarkers in conjunction with risk calculators may shed some light on this decision.
The new perioperative guidelines incorporate other ACC/AHA guidelines for valvular heart disease15 and heart failure.14 Some of their recommendations, in my opinion, may lead to excessive testing (eg, repeat echocardiograms) that will not change perioperative management.
Regarding revascularization
The ACC/AHA guidelines continue to emphasize the important concept that coronary revascularization is rarely indicated just to get the patient through surgery.
The new guidelines give physicians some leeway in allowing patients with drug-eluting stents to undergo surgery after 6 rather than 12 months of dual antiplatelet therapy if they believe that delaying surgery would place the patient at more risk than that of stent thrombosis. There is evidence in the nonsurgical setting that the newer stents currently being used may require no more than 6 months of therapy. In my opinion it was never clear that there was a statistically significant benefit in delaying surgery more than 6 months after placement of a drug-eluting stent, so this is a welcome addition.
Regarding beta-blockers
The systematic review of beta-blockers reinforces the importance of continuing them preoperatively while downgrading recommendations for their prophylactic use in patients who are not at increased risk.
Although the debate continues, there is no doubt that beta-blockers are associated with a decrease in myocardial ischemia and infarction but an increase in bradycardia and hypotension. They probably are associated with some increased risk of stroke, although this may be related to the specific beta-blocker used as well as the time of initiation before surgery. Evidence of a possible effect on mortality depends on whether the DECREASE and POISE trials are included or excluded in the analysis.
In the absence of new large-scale randomized controlled trials, we are forced to rely on observational trials and expert opinion in the meantime. I think that if a beta-blocker is to be started preoperatively, it should be done at least 1 week before surgery, and a more cardioselective beta-blocker should be used.
Regarding other drugs and tests
I agree with the recommendation to continue ACE inhibitors and ARBs preoperatively in patients with heart failure and poorly controlled hypertension. Although somewhat contrary to current practice, continuance of these drugs has not been associated with an increase in myocardial infarction or death despite concern about intraoperative hypotension.
Data from randomized controlled trials of perioperative statins are limited, but the information from observational studies is favorable, and I see little downside to initiating statins preoperatively in patients who otherwise have indications for their use, particularly if undergoing vascular or other high-risk noncardiac surgery. It is not known whether the specific drug, dose, or timing of initiation of statins influences outcome.
Although multiple studies of biomarkers suggest that there is an association with outcome, there are no randomized controlled trials or specific interventions shown to improve outcome.
Some of the recommended interventions have included various cardiac medications, stress testing, possible coronary angiography, and revascularization, which are not without risk. In the absence of data and following the directive to "first do no harm," the ACC/AHA has been appropriately cautious in not recommending them for routine use at this time.
The updated guidelines have summarized the new evidence in perioperative cardiac evaluation and management. Many of their recommendations were reinforced by this information and remain essentially unchanged. Several new recommendations will lead to changes in management going forward. Unfortunately, we lack the evidence to answer many questions that arise in routine practice and are therefore forced to rely on expert opinion and our clinical judgment in these cases. The ACC/AHA guidelines do provide a framework for our evaluation and management and help keep clinicians up-to-date with the latest evidence.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Wijeysundera DN, Duncan D, Nkonde-Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05528-4. doi: 10.1016/j.jacc.2014.07.939. [Epub ahead of print].
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05537-5. doi: 10.1016/j.jacc.2014.07.945. [Epub ahead of print].
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- Erasmus MC Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity. September 30, 2012. http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed October 30, 2014.
- Anderson JL, Antman EM, Harold JG, et al. Clinical practice guidelines on perioperative cardiovascular evaluation: collaborative efforts among the ACC, AHA, and ESC. J Am Coll Cardiol 2014 Jul 29. pii: S0735-1097(14)05527-2. doi: 10.1016/j.jacc.2014.07.938. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842. e1-3.
- Davis C, Tait G, Carroll J, Wijeysundera DN, Beattie WS. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth 2013; 60:855–863.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; e-pub before print. doi:10.1016/j.jacc.2014.03.022.
- Aliot EM, Alpert JS, Calkins H, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-SVA-FT.pdf. Accessed October 30,2014.
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 2011; 8:1114–1154.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012; 60:645-681.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- McFalls EO, Ward HB, Krupski WC, et al. Prophylactic coronary artery revascularization for elective vascular surgery: study design. Veterans Affairs Cooperative Study Group on Coronary Artery Revascularization Prophylaxis for Elective Vascular Surgery. Control Clin Trials 1999; 20:297–308.
- Schouten O, van Kuijk JP, Flu WJ, et al. Long-term outcome of prophylactic coronary revascularization in cardiac high-risk patients undergoing major vascular surgery (from the randomized DECREASE-V Pilot Study). Am J Cardiol 2009; 103:897–901.
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126:1355-1362.
- Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310:1462–1472.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Lindenauer PK, Pekow P, Wang K, et al. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kennedy J, Quan H, Buchan AM, et al. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- Raju MG, Pachika A, Punnam SR, et al. Statin therapy in the reduction of cardiovascular events in patients undergoing intermediate-risk noncardiac, nonvascular surgery. Clin Cardiol 2013; 36:456–461.
- Desai H, Aronow WS, Ahn C, et al. Incidence of perioperative myocardial infarction and of 2-year mortality in 577 elderly patients undergoing noncardiac vascular surgery treated with and without statins. Arch Gerontol Geriatr 2010; 51:149–151.
- Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1504–1513.
- Nguyen HP, Zaroff JG, Bayman EO, et al. Perioperative hypothermia (33 degrees C) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology 2010; 113:327–342.
- Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277:1127–1134.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Foucrier A, Rodseth R, Aissaoui M, Ibanes C, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Wijeysundera DN, Duncan D, Nkonde-Price C, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05528-4. doi: 10.1016/j.jacc.2014.07.939. [Epub ahead of print].
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05537-5. doi: 10.1016/j.jacc.2014.07.945. [Epub ahead of print].
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50:e159–e242.
- Erasmus MC Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity. September 30, 2012. http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf. Accessed October 30, 2014.
- Anderson JL, Antman EM, Harold JG, et al. Clinical practice guidelines on perioperative cardiovascular evaluation: collaborative efforts among the ACC, AHA, and ESC. J Am Coll Cardiol 2014 Jul 29. pii: S0735-1097(14)05527-2. doi: 10.1016/j.jacc.2014.07.938. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842. e1-3.
- Davis C, Tait G, Carroll J, Wijeysundera DN, Beattie WS. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth 2013; 60:855–863.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; e-pub before print. doi:10.1016/j.jacc.2014.03.022.
- Aliot EM, Alpert JS, Calkins H, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-SVA-FT.pdf. Accessed October 30,2014.
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm 2011; 8:1114–1154.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012; 60:645-681.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- McFalls EO, Ward HB, Krupski WC, et al. Prophylactic coronary artery revascularization for elective vascular surgery: study design. Veterans Affairs Cooperative Study Group on Coronary Artery Revascularization Prophylaxis for Elective Vascular Surgery. Control Clin Trials 1999; 20:297–308.
- Schouten O, van Kuijk JP, Flu WJ, et al. Long-term outcome of prophylactic coronary revascularization in cardiac high-risk patients undergoing major vascular surgery (from the randomized DECREASE-V Pilot Study). Am J Cardiol 2009; 103:897–901.
- Wijeysundera DN, Wijeysundera HC, Yun L, et al. risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012; 126:1355-1362.
- Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310:1462–1472.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Lindenauer PK, Pekow P, Wang K, et al. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kennedy J, Quan H, Buchan AM, et al. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- Raju MG, Pachika A, Punnam SR, et al. Statin therapy in the reduction of cardiovascular events in patients undergoing intermediate-risk noncardiac, nonvascular surgery. Clin Cardiol 2013; 36:456–461.
- Desai H, Aronow WS, Ahn C, et al. Incidence of perioperative myocardial infarction and of 2-year mortality in 577 elderly patients undergoing noncardiac vascular surgery treated with and without statins. Arch Gerontol Geriatr 2010; 51:149–151.
- Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1504–1513.
- Nguyen HP, Zaroff JG, Bayman EO, et al. Perioperative hypothermia (33 degrees C) does not increase the occurrence of cardiovascular events in patients undergoing cerebral aneurysm surgery: findings from the Intraoperative Hypothermia for Aneurysm Surgery Trial. Anesthesiology 2010; 113:327–342.
- Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997; 277:1127–1134.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Foucrier A, Rodseth R, Aissaoui M, Ibanes C, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
KEY POINTS
- Like earlier guidelines, the update recommends preoperative cardiac testing only when the results may influence the patient’s management.
- Preoperative intervention is rarely necessary just to get the patient through surgery, unless it is otherwise indicated independent of the need for surgery.
- The update proposes a modified algorithm for preoperative risk assessment and management and suggests using a new calculator of surgical risk.
- The report also updates information on the timing of surgery after percutaneous coronary intervention, as well as on antiplatelet therapy, other medical therapy, and biomarkers.