User login
Man With Diverticulitis Undergoes Precolonoscopy Evaluation
ANSWER
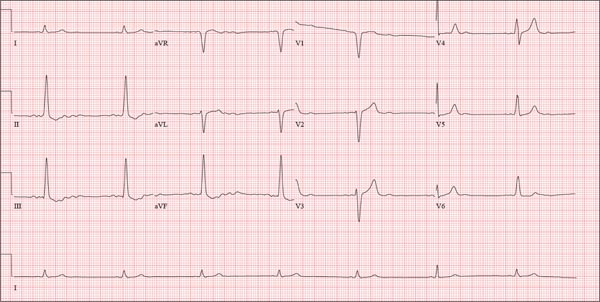
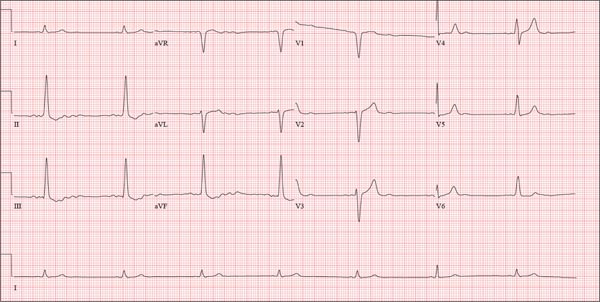
This ECG shows probable ectopic rhythm with second-degree atrioventricular (AV) block and a nonspecific intraventricular conduction block.
The P-wave morphology is unusual; rather than being upright and positive in leads II and aVF, the P waves are biphasic and prolonged, suggesting they originate from an atrial source other than the sinus node.
The rhythm strip of lead I in this ECG isn’t of much help in determining the atrial rhythm, as the P waves are small. However, if you look at the strip beginning with either lead II or III and keep in mind that the strip is continuous even though the leads change (eg, lead III becomes lead aVF which becomes V3, etc), you can see an atrial complex immediately following the T wave that is very similar to the P wave prior to the QRS complex. The rate of the P waves is 84 beats/min, which is twice that of the QRS complex (42 beats/min) and therefore consistent with a 2:1 heart block.
A nonspecific AV conduction block is evidenced by a QRS duration > 120 ms that does not have the appearance of a right or left bundle branch block.
Finally, while the QT interval of 514 ms is worrisome for long QT interval, it is normal when corrected for rate.
ANSWER
This ECG shows probable ectopic rhythm with second-degree atrioventricular (AV) block and a nonspecific intraventricular conduction block.
The P-wave morphology is unusual; rather than being upright and positive in leads II and aVF, the P waves are biphasic and prolonged, suggesting they originate from an atrial source other than the sinus node.
The rhythm strip of lead I in this ECG isn’t of much help in determining the atrial rhythm, as the P waves are small. However, if you look at the strip beginning with either lead II or III and keep in mind that the strip is continuous even though the leads change (eg, lead III becomes lead aVF which becomes V3, etc), you can see an atrial complex immediately following the T wave that is very similar to the P wave prior to the QRS complex. The rate of the P waves is 84 beats/min, which is twice that of the QRS complex (42 beats/min) and therefore consistent with a 2:1 heart block.
A nonspecific AV conduction block is evidenced by a QRS duration > 120 ms that does not have the appearance of a right or left bundle branch block.
Finally, while the QT interval of 514 ms is worrisome for long QT interval, it is normal when corrected for rate.
ANSWER
This ECG shows probable ectopic rhythm with second-degree atrioventricular (AV) block and a nonspecific intraventricular conduction block.
The P-wave morphology is unusual; rather than being upright and positive in leads II and aVF, the P waves are biphasic and prolonged, suggesting they originate from an atrial source other than the sinus node.
The rhythm strip of lead I in this ECG isn’t of much help in determining the atrial rhythm, as the P waves are small. However, if you look at the strip beginning with either lead II or III and keep in mind that the strip is continuous even though the leads change (eg, lead III becomes lead aVF which becomes V3, etc), you can see an atrial complex immediately following the T wave that is very similar to the P wave prior to the QRS complex. The rate of the P waves is 84 beats/min, which is twice that of the QRS complex (42 beats/min) and therefore consistent with a 2:1 heart block.
A nonspecific AV conduction block is evidenced by a QRS duration > 120 ms that does not have the appearance of a right or left bundle branch block.
Finally, while the QT interval of 514 ms is worrisome for long QT interval, it is normal when corrected for rate.
A 74-year-old man with recurring episodes of melena presents for a preoperative evaluation prior to colonoscopy. He has had three such procedures in the past five years, all of which indicated diverticulitis. The current episode began about a month ago, but the patient delayed seeking care until last week due to work obligations. The patient reports feeling more lethargic and becoming more easily tired than he has with previous episodes, which concerns him. He doesn’t think he has lost more blood than before but admits he’s been “too busy” to notice. He denies chest pain, shortness of breath, palpitations, peripheral extremity swelling, or recent weight change (gain or loss). He has not experienced loss of appetite or abdominal pain. Medical history is remarkable for hypertension, cholecystitis, and diverticulitis. There is no history of coronary artery disease, diabetes, or chronic obstructive pulmonary disease. Surgical history is remarkable for cholecystectomy and surgical repair of a high fracture of the left ankle. The patient owns a 475-acre farm, where he has lived his entire life. He is a widower who relies on his four sons to help with chores, although he insists on driving the combine himself during harvest (which is why he delayed seeking care this time). He does not smoke or drink. His current medications include hydrochlorothiazide and naproxen as needed for musculoskeletal discomfort. The review of systems is remarkable for fatigue and “the usual aches and pains of working on a farm.” The remainder of the review is noncontributory. The physical exam reveals a thin, weather-worn male in no distress. His height is 76 in and his weight, 172 lb. Both are unchanged from his previous clinic visit (six months ago). Vital signs include a blood pressure of 138/78 mm Hg; pulse, 46 beats/min; O2 saturation, 96%; and temperature, 98.2°F. Pertinent findings include normal breath sounds, a regular (albeit slow at 46 beats/min) rhythm, an early grade II/VI systolic murmur heard at the left upper sternal border, and a soft, nontender abdomen. There is no peripheral edema and no femoral or carotid bruits. The neurologic exam is intact. While the patient is undergoing preoperative laboratory tests and ECG, you review his medical record. Of note, the bradycardia found during today’s physical was not present six months ago. Laboratory data include a normal chemistry panel and a hematocrit of 38%. The ECG reveals a ventricular rate of 42 beats/min; PR interval, not reported; QRS duration, 130 ms; QT/QTc interval, 514/429 ms; P axis, 83°; R axis, 84°; and T axis, –43°. What is your interpretation of this ECG?
Man Unresponsive After Being Struck by Car
ANSWER
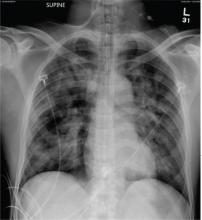
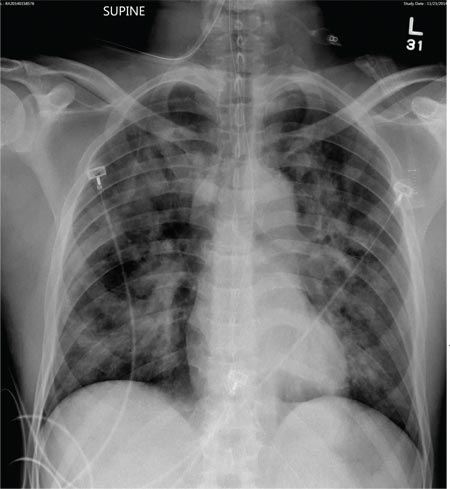
The radiograph demonstrates bilateral patchy, fluffy infiltrates as well as what is sometimes referred to as ground-glass opacities. In the setting of trauma and respiratory compromise, these areas are most suggestive of pulmonary contusions and early acute respiratory distress syndrome. Other possibilities in the differential diagnosis include pulmonary edema, atypical pneumonia, and pulmonary metastases.
ANSWER
The radiograph demonstrates bilateral patchy, fluffy infiltrates as well as what is sometimes referred to as ground-glass opacities. In the setting of trauma and respiratory compromise, these areas are most suggestive of pulmonary contusions and early acute respiratory distress syndrome. Other possibilities in the differential diagnosis include pulmonary edema, atypical pneumonia, and pulmonary metastases.
ANSWER
The radiograph demonstrates bilateral patchy, fluffy infiltrates as well as what is sometimes referred to as ground-glass opacities. In the setting of trauma and respiratory compromise, these areas are most suggestive of pulmonary contusions and early acute respiratory distress syndrome. Other possibilities in the differential diagnosis include pulmonary edema, atypical pneumonia, and pulmonary metastases.
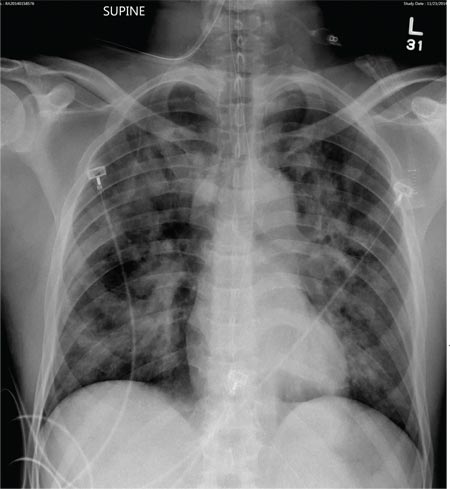
A 50-year-old man is transferred to your facility from an outlying community hospital. He is purportedly a pedestrian who was struck by a car. EMS personnel reported him to be unresponsive at the scene. He was intubated for airway protection and stabilized at the outside facility prior to transfer. Upon arrival at your facility, he is still intubated and unresponsive, and his Glasgow Coma Scale score is 3T. His heart rate is 150 beats/min and his blood pressure, 105/56 mm Hg. No additional history is available. Primary survey reveals a large scalp laceration with currently controlled bleeding. His pupils are nonreactive bilaterally. The patient is tachycardic with bilateral crackles. He also has a laceration and deformity of his right lower extremity. No imaging was provided in the transfer, so you obtain a portable chest radiograph. What is your impression?
Approach can cure even high-risk FL, study suggests
SAN FRANCISCO—Follicular lymphoma (FL) patients who receive high-dose therapy with autologous stem cell transplant (HDT/ASCT) after they’ve responded to chemotherapy can achieve long-term cancer-free survival, new research suggests.
The study showed that many patients transplanted in complete remission (CR) did not relapse and could be considered cured.
Patients transplanted in their first CR fared the best, as median progression-free survival (PFS) and overall survival (OS) times were not reached.
But even patients transplanted in their second/third CR or in their first partial remission (PR) survived a median of 15 years or more, although their PFS times were shorter, at about 14 years and 3 years, respectively.
Carlos Grande García, MD, of Hospital Universitario 12 de Octubre in Madrid, Spain, presented these results at the 2014 ASH Annual Meeting (abstract 675.)*
“In follicular lymphoma patients, intensification with high-dose therapy and autologous stem cell support offers an advantage in terms of progression-free survival in comparison with conventional chemo,” he said. “But, so far, no randomized studies have yet shown any overall survival advantage.”
“Follicular lymphoma has a long natural course, and most patients have received different salvage therapies. Probably, this is why the available phase 3 studies have had insufficient time to confirm the impact on OS.”
To investigate the impact of HDT/ASCT on OS, Dr Grande García and his colleagues conducted a retrospective study of 655 FL patients who received HDT/ASCT from 1989 to 2007. Patients with histological transformation, those undergoing a second transplant, and those with a follow-up of less than 7 years were excluded.
Patient characteristics
The median follow-up was 12 years from HDT/ASCT and 14.4 years from diagnosis. At diagnosis, the median patient age was 47, 49.6% of patients were male, and 90% had stage III/IV disease.
According to FLIPI, 33% of patients were good risk, 36% were intermediate risk, and 31% were poor risk. According to FLIPI-2, the percentages were 22%, 38%, and 40%, respectively. Thirty percent of patients had received rituximab prior to HDT/ASCT.
Thirty-one percent of patients (n=203) were in their first CR at the time of transplant, 43% of whom required more than one line of therapy to reach first CR.
Thirty-one percent of patients (n=202) were in second or third CR, 21.5% (n=149) were in first PR, 12.5% (n=81) were in sensitive relapse (defined as a response other than CR or first PR), and 5% (n=29) had overt disease (which included untreated relapsed disease, first refractory disease, and second refractory disease).
Patients received a variety of conditioning regimens, including total-body irradiation plus cyclophosphamide, BEAM (carmustine, etoposide, cytarabine, and melphalan), BEAC (carmustine, etoposide, cytarabine, and cyclophosphamide), and other regimens. They received stem cells from peripheral blood (81%), bone marrow (14%), or both sources (5%).
There were 4 graft failures and 17 early toxic deaths. Thirty-one percent of patients experienced grade 3/4 hematologic toxicities.
PFS and OS
In all patients, the median PFS was 9.25 years, and the median OS was 19.5 years.
When the researchers looked at outcomes according to patients’ status at transplant, they found the median OS and PFS were not reached among patients in first CR. At a median follow-up of 12.75 years, the OS rate was 72%, and the PFS rate was 68%.
“Beginning at 10 years from transplantation, only 6 patients have died,” Dr Grande García noted, “one from disease progression, 3 from second malignancy, [and] 2 from unrelated causes.”
For patients in second or third CR, the median OS was not reached, and the median PFS was 13.9 years. For those in first PR, the median OS was 15 years, and the median PFS was 2.6 years.
For patients with sensitive disease, the median OS was 5.1 years, and the median PFS was 2 years. For those with overt disease, the median OS was 4.4 years, and the median PFS was 0.5 years.
In multivariate analysis, the following characteristics were significant predictors of OS: being older than 47 years of age (hazard ratio [HR]=1.74, P=0.0001), female sex (HR=0.58, P=0.00004), status at HDT/ASCT (HR=2.06, P<10-5), and receipt of rituximab prior to HDT/ASCT (HR=0.61, P=0.004).
Significant predictors of PFS included age (HR=1.34, P=0.01), sex (HR=0.64, P<10-5), status at HDT/ASCT (HR=2.15, P<10-5), and rituximab use (HR=0.67, P=0.003).
For patients transplanted in first CR, only sex was a significant predictor of PFS (HR=0.48, P=0.008) and OS (HR=0.43, P=0.007).
Secondary malignancies
Overall, 13% of patients developed secondary malignancies, of which 46% were solid neoplasias, 44% were myelodysplastic syndromes/acute myeloid leukemias, and 10% were other malignancies.
The incidence of secondary malignancies at 10 years was 3.5%, and the median time from HDT/ASCT to diagnosis was 16 years. There were no significant differences in the rate of secondary malignancy according to a patient’s status at HDT/ASCT or according to the use of rituximab.
“The incidence of second malignancies is not higher than that reported in other series without transplantation,” Dr Grande García noted.
“[HDT/ASCT] is highly effective, even for patients with poor initial features. A significant number of patients transplanted in CR never relapse and may be considered cured.” ![]()
*Information in the abstract differs from that presented at the meeting.
SAN FRANCISCO—Follicular lymphoma (FL) patients who receive high-dose therapy with autologous stem cell transplant (HDT/ASCT) after they’ve responded to chemotherapy can achieve long-term cancer-free survival, new research suggests.
The study showed that many patients transplanted in complete remission (CR) did not relapse and could be considered cured.
Patients transplanted in their first CR fared the best, as median progression-free survival (PFS) and overall survival (OS) times were not reached.
But even patients transplanted in their second/third CR or in their first partial remission (PR) survived a median of 15 years or more, although their PFS times were shorter, at about 14 years and 3 years, respectively.
Carlos Grande García, MD, of Hospital Universitario 12 de Octubre in Madrid, Spain, presented these results at the 2014 ASH Annual Meeting (abstract 675.)*
“In follicular lymphoma patients, intensification with high-dose therapy and autologous stem cell support offers an advantage in terms of progression-free survival in comparison with conventional chemo,” he said. “But, so far, no randomized studies have yet shown any overall survival advantage.”
“Follicular lymphoma has a long natural course, and most patients have received different salvage therapies. Probably, this is why the available phase 3 studies have had insufficient time to confirm the impact on OS.”
To investigate the impact of HDT/ASCT on OS, Dr Grande García and his colleagues conducted a retrospective study of 655 FL patients who received HDT/ASCT from 1989 to 2007. Patients with histological transformation, those undergoing a second transplant, and those with a follow-up of less than 7 years were excluded.
Patient characteristics
The median follow-up was 12 years from HDT/ASCT and 14.4 years from diagnosis. At diagnosis, the median patient age was 47, 49.6% of patients were male, and 90% had stage III/IV disease.
According to FLIPI, 33% of patients were good risk, 36% were intermediate risk, and 31% were poor risk. According to FLIPI-2, the percentages were 22%, 38%, and 40%, respectively. Thirty percent of patients had received rituximab prior to HDT/ASCT.
Thirty-one percent of patients (n=203) were in their first CR at the time of transplant, 43% of whom required more than one line of therapy to reach first CR.
Thirty-one percent of patients (n=202) were in second or third CR, 21.5% (n=149) were in first PR, 12.5% (n=81) were in sensitive relapse (defined as a response other than CR or first PR), and 5% (n=29) had overt disease (which included untreated relapsed disease, first refractory disease, and second refractory disease).
Patients received a variety of conditioning regimens, including total-body irradiation plus cyclophosphamide, BEAM (carmustine, etoposide, cytarabine, and melphalan), BEAC (carmustine, etoposide, cytarabine, and cyclophosphamide), and other regimens. They received stem cells from peripheral blood (81%), bone marrow (14%), or both sources (5%).
There were 4 graft failures and 17 early toxic deaths. Thirty-one percent of patients experienced grade 3/4 hematologic toxicities.
PFS and OS
In all patients, the median PFS was 9.25 years, and the median OS was 19.5 years.
When the researchers looked at outcomes according to patients’ status at transplant, they found the median OS and PFS were not reached among patients in first CR. At a median follow-up of 12.75 years, the OS rate was 72%, and the PFS rate was 68%.
“Beginning at 10 years from transplantation, only 6 patients have died,” Dr Grande García noted, “one from disease progression, 3 from second malignancy, [and] 2 from unrelated causes.”
For patients in second or third CR, the median OS was not reached, and the median PFS was 13.9 years. For those in first PR, the median OS was 15 years, and the median PFS was 2.6 years.
For patients with sensitive disease, the median OS was 5.1 years, and the median PFS was 2 years. For those with overt disease, the median OS was 4.4 years, and the median PFS was 0.5 years.
In multivariate analysis, the following characteristics were significant predictors of OS: being older than 47 years of age (hazard ratio [HR]=1.74, P=0.0001), female sex (HR=0.58, P=0.00004), status at HDT/ASCT (HR=2.06, P<10-5), and receipt of rituximab prior to HDT/ASCT (HR=0.61, P=0.004).
Significant predictors of PFS included age (HR=1.34, P=0.01), sex (HR=0.64, P<10-5), status at HDT/ASCT (HR=2.15, P<10-5), and rituximab use (HR=0.67, P=0.003).
For patients transplanted in first CR, only sex was a significant predictor of PFS (HR=0.48, P=0.008) and OS (HR=0.43, P=0.007).
Secondary malignancies
Overall, 13% of patients developed secondary malignancies, of which 46% were solid neoplasias, 44% were myelodysplastic syndromes/acute myeloid leukemias, and 10% were other malignancies.
The incidence of secondary malignancies at 10 years was 3.5%, and the median time from HDT/ASCT to diagnosis was 16 years. There were no significant differences in the rate of secondary malignancy according to a patient’s status at HDT/ASCT or according to the use of rituximab.
“The incidence of second malignancies is not higher than that reported in other series without transplantation,” Dr Grande García noted.
“[HDT/ASCT] is highly effective, even for patients with poor initial features. A significant number of patients transplanted in CR never relapse and may be considered cured.” ![]()
*Information in the abstract differs from that presented at the meeting.
SAN FRANCISCO—Follicular lymphoma (FL) patients who receive high-dose therapy with autologous stem cell transplant (HDT/ASCT) after they’ve responded to chemotherapy can achieve long-term cancer-free survival, new research suggests.
The study showed that many patients transplanted in complete remission (CR) did not relapse and could be considered cured.
Patients transplanted in their first CR fared the best, as median progression-free survival (PFS) and overall survival (OS) times were not reached.
But even patients transplanted in their second/third CR or in their first partial remission (PR) survived a median of 15 years or more, although their PFS times were shorter, at about 14 years and 3 years, respectively.
Carlos Grande García, MD, of Hospital Universitario 12 de Octubre in Madrid, Spain, presented these results at the 2014 ASH Annual Meeting (abstract 675.)*
“In follicular lymphoma patients, intensification with high-dose therapy and autologous stem cell support offers an advantage in terms of progression-free survival in comparison with conventional chemo,” he said. “But, so far, no randomized studies have yet shown any overall survival advantage.”
“Follicular lymphoma has a long natural course, and most patients have received different salvage therapies. Probably, this is why the available phase 3 studies have had insufficient time to confirm the impact on OS.”
To investigate the impact of HDT/ASCT on OS, Dr Grande García and his colleagues conducted a retrospective study of 655 FL patients who received HDT/ASCT from 1989 to 2007. Patients with histological transformation, those undergoing a second transplant, and those with a follow-up of less than 7 years were excluded.
Patient characteristics
The median follow-up was 12 years from HDT/ASCT and 14.4 years from diagnosis. At diagnosis, the median patient age was 47, 49.6% of patients were male, and 90% had stage III/IV disease.
According to FLIPI, 33% of patients were good risk, 36% were intermediate risk, and 31% were poor risk. According to FLIPI-2, the percentages were 22%, 38%, and 40%, respectively. Thirty percent of patients had received rituximab prior to HDT/ASCT.
Thirty-one percent of patients (n=203) were in their first CR at the time of transplant, 43% of whom required more than one line of therapy to reach first CR.
Thirty-one percent of patients (n=202) were in second or third CR, 21.5% (n=149) were in first PR, 12.5% (n=81) were in sensitive relapse (defined as a response other than CR or first PR), and 5% (n=29) had overt disease (which included untreated relapsed disease, first refractory disease, and second refractory disease).
Patients received a variety of conditioning regimens, including total-body irradiation plus cyclophosphamide, BEAM (carmustine, etoposide, cytarabine, and melphalan), BEAC (carmustine, etoposide, cytarabine, and cyclophosphamide), and other regimens. They received stem cells from peripheral blood (81%), bone marrow (14%), or both sources (5%).
There were 4 graft failures and 17 early toxic deaths. Thirty-one percent of patients experienced grade 3/4 hematologic toxicities.
PFS and OS
In all patients, the median PFS was 9.25 years, and the median OS was 19.5 years.
When the researchers looked at outcomes according to patients’ status at transplant, they found the median OS and PFS were not reached among patients in first CR. At a median follow-up of 12.75 years, the OS rate was 72%, and the PFS rate was 68%.
“Beginning at 10 years from transplantation, only 6 patients have died,” Dr Grande García noted, “one from disease progression, 3 from second malignancy, [and] 2 from unrelated causes.”
For patients in second or third CR, the median OS was not reached, and the median PFS was 13.9 years. For those in first PR, the median OS was 15 years, and the median PFS was 2.6 years.
For patients with sensitive disease, the median OS was 5.1 years, and the median PFS was 2 years. For those with overt disease, the median OS was 4.4 years, and the median PFS was 0.5 years.
In multivariate analysis, the following characteristics were significant predictors of OS: being older than 47 years of age (hazard ratio [HR]=1.74, P=0.0001), female sex (HR=0.58, P=0.00004), status at HDT/ASCT (HR=2.06, P<10-5), and receipt of rituximab prior to HDT/ASCT (HR=0.61, P=0.004).
Significant predictors of PFS included age (HR=1.34, P=0.01), sex (HR=0.64, P<10-5), status at HDT/ASCT (HR=2.15, P<10-5), and rituximab use (HR=0.67, P=0.003).
For patients transplanted in first CR, only sex was a significant predictor of PFS (HR=0.48, P=0.008) and OS (HR=0.43, P=0.007).
Secondary malignancies
Overall, 13% of patients developed secondary malignancies, of which 46% were solid neoplasias, 44% were myelodysplastic syndromes/acute myeloid leukemias, and 10% were other malignancies.
The incidence of secondary malignancies at 10 years was 3.5%, and the median time from HDT/ASCT to diagnosis was 16 years. There were no significant differences in the rate of secondary malignancy according to a patient’s status at HDT/ASCT or according to the use of rituximab.
“The incidence of second malignancies is not higher than that reported in other series without transplantation,” Dr Grande García noted.
“[HDT/ASCT] is highly effective, even for patients with poor initial features. A significant number of patients transplanted in CR never relapse and may be considered cured.” ![]()
*Information in the abstract differs from that presented at the meeting.
Age-adjusted D-dimer is ‘probably safe,’ team says

Credit: Medical College
of Georgia
A new study shows that, although age-adjusted D-dimer testing produces fewer false-positive results than conventional D-dimer testing, some cases of pulmonary embolism (PE) slip through the cracks.
Researchers compared the two testing methods in patients older than 50 and found that using an age-adjusted D-dimer threshold reduced the need for additional imaging.
Unfortunately, it also had a false-negative rate of 1.5%, failing to catch PE in 4 patients.
Scott Woller, MD, of Intermountain Medical Center in Salt Lake City, Utah, and his colleagues reported these findings in CHEST.
The team conducted this study with the goal of eliminating false-positive D-dimer results and reducing the need for additional imaging, which can be detrimental to older patients.
“A CT scan is most often used to ultimately rule out a pulmonary embolism,” Dr Woller said. “However, it delivers radiation to the patient and contrast dye.”
“Elderly patients are at greater risk for inadvertent harm related to the CT scan, and the contrast dye may also impact kidney function. Plus, the scan adds to the cost of the patient’s care. If we can safely and accurately diagnose the patient’s risk of a pulmonary embolism using [age-adjusted D-dimer], we can eliminate the need for additional imaging tests.”
With this in mind, the researchers evaluated 923 patients older than 50 years of age who presented to the emergency department at Intermountain Medical Center with a suspected PE, a calculated Revised Geneva Score (RGS), and a D-dimer test.
All of the patients underwent CT pulmonary angiography (CTPA), and the researchers compared the false-negative rate of a conventional D-dimer threshold with an age-adjusted D-dimer threshold.
The team found that age-adjusted D-dimer reduced the need for CTPA by 18.3% (95% CI, 15.9%-21.0%), compared to conventional D-dimer.
However, in the 273 patients with a negative age-adjusted D-dimer result and an RGS of 10 or greater, 4 PEs occurred within 90 days. This translates to a false-negative result rate of 1.5% (95% CI, 0.4%-3.7%).
In comparison, the false-negative rate for conventional D-dimer was 0% (95% CI, 0%-2.8%). Among the 104 patients who had a negative test result and an RGS of 10 or greater, there were no PEs within 90 days.
These results suggest an age-adjusted D-dimer threshold does reduce the need for imaging in patients older than 50, the researchers said. They added that this method is probably safe for these patients, but a prospective trial is needed to more thoroughly investigate safety. ![]()

Credit: Medical College
of Georgia
A new study shows that, although age-adjusted D-dimer testing produces fewer false-positive results than conventional D-dimer testing, some cases of pulmonary embolism (PE) slip through the cracks.
Researchers compared the two testing methods in patients older than 50 and found that using an age-adjusted D-dimer threshold reduced the need for additional imaging.
Unfortunately, it also had a false-negative rate of 1.5%, failing to catch PE in 4 patients.
Scott Woller, MD, of Intermountain Medical Center in Salt Lake City, Utah, and his colleagues reported these findings in CHEST.
The team conducted this study with the goal of eliminating false-positive D-dimer results and reducing the need for additional imaging, which can be detrimental to older patients.
“A CT scan is most often used to ultimately rule out a pulmonary embolism,” Dr Woller said. “However, it delivers radiation to the patient and contrast dye.”
“Elderly patients are at greater risk for inadvertent harm related to the CT scan, and the contrast dye may also impact kidney function. Plus, the scan adds to the cost of the patient’s care. If we can safely and accurately diagnose the patient’s risk of a pulmonary embolism using [age-adjusted D-dimer], we can eliminate the need for additional imaging tests.”
With this in mind, the researchers evaluated 923 patients older than 50 years of age who presented to the emergency department at Intermountain Medical Center with a suspected PE, a calculated Revised Geneva Score (RGS), and a D-dimer test.
All of the patients underwent CT pulmonary angiography (CTPA), and the researchers compared the false-negative rate of a conventional D-dimer threshold with an age-adjusted D-dimer threshold.
The team found that age-adjusted D-dimer reduced the need for CTPA by 18.3% (95% CI, 15.9%-21.0%), compared to conventional D-dimer.
However, in the 273 patients with a negative age-adjusted D-dimer result and an RGS of 10 or greater, 4 PEs occurred within 90 days. This translates to a false-negative result rate of 1.5% (95% CI, 0.4%-3.7%).
In comparison, the false-negative rate for conventional D-dimer was 0% (95% CI, 0%-2.8%). Among the 104 patients who had a negative test result and an RGS of 10 or greater, there were no PEs within 90 days.
These results suggest an age-adjusted D-dimer threshold does reduce the need for imaging in patients older than 50, the researchers said. They added that this method is probably safe for these patients, but a prospective trial is needed to more thoroughly investigate safety. ![]()

Credit: Medical College
of Georgia
A new study shows that, although age-adjusted D-dimer testing produces fewer false-positive results than conventional D-dimer testing, some cases of pulmonary embolism (PE) slip through the cracks.
Researchers compared the two testing methods in patients older than 50 and found that using an age-adjusted D-dimer threshold reduced the need for additional imaging.
Unfortunately, it also had a false-negative rate of 1.5%, failing to catch PE in 4 patients.
Scott Woller, MD, of Intermountain Medical Center in Salt Lake City, Utah, and his colleagues reported these findings in CHEST.
The team conducted this study with the goal of eliminating false-positive D-dimer results and reducing the need for additional imaging, which can be detrimental to older patients.
“A CT scan is most often used to ultimately rule out a pulmonary embolism,” Dr Woller said. “However, it delivers radiation to the patient and contrast dye.”
“Elderly patients are at greater risk for inadvertent harm related to the CT scan, and the contrast dye may also impact kidney function. Plus, the scan adds to the cost of the patient’s care. If we can safely and accurately diagnose the patient’s risk of a pulmonary embolism using [age-adjusted D-dimer], we can eliminate the need for additional imaging tests.”
With this in mind, the researchers evaluated 923 patients older than 50 years of age who presented to the emergency department at Intermountain Medical Center with a suspected PE, a calculated Revised Geneva Score (RGS), and a D-dimer test.
All of the patients underwent CT pulmonary angiography (CTPA), and the researchers compared the false-negative rate of a conventional D-dimer threshold with an age-adjusted D-dimer threshold.
The team found that age-adjusted D-dimer reduced the need for CTPA by 18.3% (95% CI, 15.9%-21.0%), compared to conventional D-dimer.
However, in the 273 patients with a negative age-adjusted D-dimer result and an RGS of 10 or greater, 4 PEs occurred within 90 days. This translates to a false-negative result rate of 1.5% (95% CI, 0.4%-3.7%).
In comparison, the false-negative rate for conventional D-dimer was 0% (95% CI, 0%-2.8%). Among the 104 patients who had a negative test result and an RGS of 10 or greater, there were no PEs within 90 days.
These results suggest an age-adjusted D-dimer threshold does reduce the need for imaging in patients older than 50, the researchers said. They added that this method is probably safe for these patients, but a prospective trial is needed to more thoroughly investigate safety. ![]()
FDA approves new formulation of drug for ALL

The US Food and Drug Administration (FDA) has approved the intravenous administration of asparaginase Erwinia chrysanthemi (Erwinaze).
The product is indicated as a component of a multi-agent chemotherapy regimen to treat patients with acute lymphoblastic leukemia (ALL) who have developed hypersensitivity to E coli-derived asparaginase.
Previously, the only FDA-approved route of administration for asparaginase Erwinia chrysanthemi was through intramuscular injection.
The FDA’s decision to expand the drug’s use was based on a pharmacokinetic study (published in Blood in 2013) of intravenous asparaginase Erwinia chrysanthemi.
The trial included 30 patients with ALL or lymphoblastic lymphoma who developed hypersensitivity (grade ≥ 2) to E coli–derived asparaginase. The patients’ median age was 6.5 years (range, 1-17), 63% were male, and 83% were Caucasian.
Patients received intravenous asparaginase Erwinia chrysanthemi at 25,000 IU/m2/dose, on a Monday/Wednesday/Friday schedule for 2 consecutive weeks (6 doses=1 cycle) for each dose of pegaspargase remaining in their original treatment plan. All other chemotherapy was continued per the original treatment plan.
Before the first dose of intravenous asparaginase Erwinia chrysanthemi, nadir serum asparaginase activity (NSAA) levels were below the limit of quantification (defined as 0.0129 IU/mL) for 91% of patients.
The study’s primary endpoint was the proportion of patients who achieved NSAA ≥ 0.1 IU/mL, which has been associated with complete asparagine depletion, at 48 hours after dose 5 in cycle 1. Nineteen of the 23 evaluable patients (83%) achieved this endpoint.
A secondary objective of the study was to determine the proportion of patients who achieved NSAA ≥ 0.1 IU/mL at 72 hours after dose 6 in cycle 1. Nine patients (45%) achieved this endpoint.
In all 30 patients, the most common asparaginase-related toxicities reported during cycle 1 were hypersensitivity (23%), vomiting (20%), nausea (20%), and hyperglycemia (13%). Pancreatitis and thrombosis each occurred in 3% of patients. One patient experienced a transient ischemic attack.
The most common grade 3 or 4 adverse event was febrile neutropenia (7%). Four patients discontinued treatment before completing cycle 1—3 of them due to hypersensitivity and 1 due to pancreatitis. There were no deaths.
This study was funded by Jazz Pharmaceuticals, the company developing asparaginase Erwinia chrysanthemi. ![]()

The US Food and Drug Administration (FDA) has approved the intravenous administration of asparaginase Erwinia chrysanthemi (Erwinaze).
The product is indicated as a component of a multi-agent chemotherapy regimen to treat patients with acute lymphoblastic leukemia (ALL) who have developed hypersensitivity to E coli-derived asparaginase.
Previously, the only FDA-approved route of administration for asparaginase Erwinia chrysanthemi was through intramuscular injection.
The FDA’s decision to expand the drug’s use was based on a pharmacokinetic study (published in Blood in 2013) of intravenous asparaginase Erwinia chrysanthemi.
The trial included 30 patients with ALL or lymphoblastic lymphoma who developed hypersensitivity (grade ≥ 2) to E coli–derived asparaginase. The patients’ median age was 6.5 years (range, 1-17), 63% were male, and 83% were Caucasian.
Patients received intravenous asparaginase Erwinia chrysanthemi at 25,000 IU/m2/dose, on a Monday/Wednesday/Friday schedule for 2 consecutive weeks (6 doses=1 cycle) for each dose of pegaspargase remaining in their original treatment plan. All other chemotherapy was continued per the original treatment plan.
Before the first dose of intravenous asparaginase Erwinia chrysanthemi, nadir serum asparaginase activity (NSAA) levels were below the limit of quantification (defined as 0.0129 IU/mL) for 91% of patients.
The study’s primary endpoint was the proportion of patients who achieved NSAA ≥ 0.1 IU/mL, which has been associated with complete asparagine depletion, at 48 hours after dose 5 in cycle 1. Nineteen of the 23 evaluable patients (83%) achieved this endpoint.
A secondary objective of the study was to determine the proportion of patients who achieved NSAA ≥ 0.1 IU/mL at 72 hours after dose 6 in cycle 1. Nine patients (45%) achieved this endpoint.
In all 30 patients, the most common asparaginase-related toxicities reported during cycle 1 were hypersensitivity (23%), vomiting (20%), nausea (20%), and hyperglycemia (13%). Pancreatitis and thrombosis each occurred in 3% of patients. One patient experienced a transient ischemic attack.
The most common grade 3 or 4 adverse event was febrile neutropenia (7%). Four patients discontinued treatment before completing cycle 1—3 of them due to hypersensitivity and 1 due to pancreatitis. There were no deaths.
This study was funded by Jazz Pharmaceuticals, the company developing asparaginase Erwinia chrysanthemi. ![]()

The US Food and Drug Administration (FDA) has approved the intravenous administration of asparaginase Erwinia chrysanthemi (Erwinaze).
The product is indicated as a component of a multi-agent chemotherapy regimen to treat patients with acute lymphoblastic leukemia (ALL) who have developed hypersensitivity to E coli-derived asparaginase.
Previously, the only FDA-approved route of administration for asparaginase Erwinia chrysanthemi was through intramuscular injection.
The FDA’s decision to expand the drug’s use was based on a pharmacokinetic study (published in Blood in 2013) of intravenous asparaginase Erwinia chrysanthemi.
The trial included 30 patients with ALL or lymphoblastic lymphoma who developed hypersensitivity (grade ≥ 2) to E coli–derived asparaginase. The patients’ median age was 6.5 years (range, 1-17), 63% were male, and 83% were Caucasian.
Patients received intravenous asparaginase Erwinia chrysanthemi at 25,000 IU/m2/dose, on a Monday/Wednesday/Friday schedule for 2 consecutive weeks (6 doses=1 cycle) for each dose of pegaspargase remaining in their original treatment plan. All other chemotherapy was continued per the original treatment plan.
Before the first dose of intravenous asparaginase Erwinia chrysanthemi, nadir serum asparaginase activity (NSAA) levels were below the limit of quantification (defined as 0.0129 IU/mL) for 91% of patients.
The study’s primary endpoint was the proportion of patients who achieved NSAA ≥ 0.1 IU/mL, which has been associated with complete asparagine depletion, at 48 hours after dose 5 in cycle 1. Nineteen of the 23 evaluable patients (83%) achieved this endpoint.
A secondary objective of the study was to determine the proportion of patients who achieved NSAA ≥ 0.1 IU/mL at 72 hours after dose 6 in cycle 1. Nine patients (45%) achieved this endpoint.
In all 30 patients, the most common asparaginase-related toxicities reported during cycle 1 were hypersensitivity (23%), vomiting (20%), nausea (20%), and hyperglycemia (13%). Pancreatitis and thrombosis each occurred in 3% of patients. One patient experienced a transient ischemic attack.
The most common grade 3 or 4 adverse event was febrile neutropenia (7%). Four patients discontinued treatment before completing cycle 1—3 of them due to hypersensitivity and 1 due to pancreatitis. There were no deaths.
This study was funded by Jazz Pharmaceuticals, the company developing asparaginase Erwinia chrysanthemi. ![]()
USPSTF: Use ambulatory BP screening before diagnosing hypertension
Physicians should use ambulatory blood pressure screening to confirm elevated office measurements before diagnosing hypertension, according to a draft recommendation from the U.S. Preventive Services Task Force.
Because high blood pressure affects nearly a third of U.S. adults, the USPSTF recommends screening all adults for high blood pressure, based on good evidence that screening and treatment reduce cardiovascular events with few major harms.
However, blood pressure fluctuates with emotion, stress, pain, physical activity, medications, and even the presence of health care providers. So, the USPSTF issued a draft, A-level recommendation to use ambulatory or home blood pressure monitoring following an initial elevated screening to confirm a diagnosis of hypertension, except when initiating therapy immediately is medically necessary.
Patients with blood pressure at or above 180/110 mm Hg or evidence of end-organ damage should begin drug therapy immediately. In addition, patients diagnosed with secondary hypertension do not need ambulatory monitoring confirmation.
The USPSTF recommendations are based on a meta-analysis published Dec. 22 (Ann. Intern. Med. 2014: [doi10.7326/M14-1539]. Although the evidence for ambulatory screening confirmation was of good quality, the evidence base is less robust for home monitoring, the task force noted.
“Our evidence review shows that overdiagnosis of hypertension from unconfirmed office-based screening could result in unnecessary treatment in a substantial number of persons,” reported Margaret A. Piper, Ph.D., of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates in the study. “Ambulatory BP monitoring provides multiple measurements over time in a nonmedical setting, which potentially avoids measurement error, regression to the mean, and misdiagnosis of isolated clinic hypertension, and is best correlated with long-term outcomes.”
Dr. Piper’s team searched for good- and fair-quality studies in MEDLINE, PubMed, the Cochrane Central Register of Controlled Trials and CINAHL through August 2014, yielding 1 trial for the benefits of screening, 7 studies on the diagnostic accuracy of office blood pressure measurement, 11 studies on the diagnostic accuracy of ambulatory blood pressure measurement, 27 studies on using ambulatory screenings to confirm hypertension, 4 studies on harms of screening, and 40 studies on rescreening intervals and hypertension incidence in those with normal blood pressure.
The meta-analysis showed that 5%-65% of patients were not diagnosed with hypertension following ambulatory blood pressure monitoring after an initially elevated office screening measurement.
The USPSTF draft recommendation also noted past epidemiological evidence that 15%-30% of those diagnosed with hypertension may actually have lower blood pressure when not in a medical setting.
The meta-analysis also found that the risk of fatal and nonfatal stroke and cardiovascular events was “consistently and significantly associated with” elevated systolic ambulatory blood pressure, regardless of the measurements in an office.
The primary harms of screening identified in the study were greater absenteeism from work and greater illness episodes after diagnosis, as well as sleep disturbances, discomfort, and daily activity restrictions because of the ambulatory device.
On the basis of the evidence from the meta-analysis, the USPSTF recommended annual screenings for adults age 40 years and older and those at high risk for hypertension, including African Americans, those who are overweight or obese, and those with a normally high blood pressure (130-139/85-89 mm Hg). Screenings should occur every 3-5 years for those age 18-39 years with no risk factors and a normal blood pressure.
Target blood pressure should remain below 140/90 mm Hg for adults younger than 60 years, and below 150/90 mm Hg for adults 60 years or older with neither diabetes nor chronic kidney disease, according to guidelines from the Eighth Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure.
The U.S. Agency for Healthcare Research and Quality funded the meta-analysis. The authors had no relevant disclosures.
Physicians should use ambulatory blood pressure screening to confirm elevated office measurements before diagnosing hypertension, according to a draft recommendation from the U.S. Preventive Services Task Force.
Because high blood pressure affects nearly a third of U.S. adults, the USPSTF recommends screening all adults for high blood pressure, based on good evidence that screening and treatment reduce cardiovascular events with few major harms.
However, blood pressure fluctuates with emotion, stress, pain, physical activity, medications, and even the presence of health care providers. So, the USPSTF issued a draft, A-level recommendation to use ambulatory or home blood pressure monitoring following an initial elevated screening to confirm a diagnosis of hypertension, except when initiating therapy immediately is medically necessary.
Patients with blood pressure at or above 180/110 mm Hg or evidence of end-organ damage should begin drug therapy immediately. In addition, patients diagnosed with secondary hypertension do not need ambulatory monitoring confirmation.
The USPSTF recommendations are based on a meta-analysis published Dec. 22 (Ann. Intern. Med. 2014: [doi10.7326/M14-1539]. Although the evidence for ambulatory screening confirmation was of good quality, the evidence base is less robust for home monitoring, the task force noted.
“Our evidence review shows that overdiagnosis of hypertension from unconfirmed office-based screening could result in unnecessary treatment in a substantial number of persons,” reported Margaret A. Piper, Ph.D., of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates in the study. “Ambulatory BP monitoring provides multiple measurements over time in a nonmedical setting, which potentially avoids measurement error, regression to the mean, and misdiagnosis of isolated clinic hypertension, and is best correlated with long-term outcomes.”
Dr. Piper’s team searched for good- and fair-quality studies in MEDLINE, PubMed, the Cochrane Central Register of Controlled Trials and CINAHL through August 2014, yielding 1 trial for the benefits of screening, 7 studies on the diagnostic accuracy of office blood pressure measurement, 11 studies on the diagnostic accuracy of ambulatory blood pressure measurement, 27 studies on using ambulatory screenings to confirm hypertension, 4 studies on harms of screening, and 40 studies on rescreening intervals and hypertension incidence in those with normal blood pressure.
The meta-analysis showed that 5%-65% of patients were not diagnosed with hypertension following ambulatory blood pressure monitoring after an initially elevated office screening measurement.
The USPSTF draft recommendation also noted past epidemiological evidence that 15%-30% of those diagnosed with hypertension may actually have lower blood pressure when not in a medical setting.
The meta-analysis also found that the risk of fatal and nonfatal stroke and cardiovascular events was “consistently and significantly associated with” elevated systolic ambulatory blood pressure, regardless of the measurements in an office.
The primary harms of screening identified in the study were greater absenteeism from work and greater illness episodes after diagnosis, as well as sleep disturbances, discomfort, and daily activity restrictions because of the ambulatory device.
On the basis of the evidence from the meta-analysis, the USPSTF recommended annual screenings for adults age 40 years and older and those at high risk for hypertension, including African Americans, those who are overweight or obese, and those with a normally high blood pressure (130-139/85-89 mm Hg). Screenings should occur every 3-5 years for those age 18-39 years with no risk factors and a normal blood pressure.
Target blood pressure should remain below 140/90 mm Hg for adults younger than 60 years, and below 150/90 mm Hg for adults 60 years or older with neither diabetes nor chronic kidney disease, according to guidelines from the Eighth Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure.
The U.S. Agency for Healthcare Research and Quality funded the meta-analysis. The authors had no relevant disclosures.
Physicians should use ambulatory blood pressure screening to confirm elevated office measurements before diagnosing hypertension, according to a draft recommendation from the U.S. Preventive Services Task Force.
Because high blood pressure affects nearly a third of U.S. adults, the USPSTF recommends screening all adults for high blood pressure, based on good evidence that screening and treatment reduce cardiovascular events with few major harms.
However, blood pressure fluctuates with emotion, stress, pain, physical activity, medications, and even the presence of health care providers. So, the USPSTF issued a draft, A-level recommendation to use ambulatory or home blood pressure monitoring following an initial elevated screening to confirm a diagnosis of hypertension, except when initiating therapy immediately is medically necessary.
Patients with blood pressure at or above 180/110 mm Hg or evidence of end-organ damage should begin drug therapy immediately. In addition, patients diagnosed with secondary hypertension do not need ambulatory monitoring confirmation.
The USPSTF recommendations are based on a meta-analysis published Dec. 22 (Ann. Intern. Med. 2014: [doi10.7326/M14-1539]. Although the evidence for ambulatory screening confirmation was of good quality, the evidence base is less robust for home monitoring, the task force noted.
“Our evidence review shows that overdiagnosis of hypertension from unconfirmed office-based screening could result in unnecessary treatment in a substantial number of persons,” reported Margaret A. Piper, Ph.D., of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates in the study. “Ambulatory BP monitoring provides multiple measurements over time in a nonmedical setting, which potentially avoids measurement error, regression to the mean, and misdiagnosis of isolated clinic hypertension, and is best correlated with long-term outcomes.”
Dr. Piper’s team searched for good- and fair-quality studies in MEDLINE, PubMed, the Cochrane Central Register of Controlled Trials and CINAHL through August 2014, yielding 1 trial for the benefits of screening, 7 studies on the diagnostic accuracy of office blood pressure measurement, 11 studies on the diagnostic accuracy of ambulatory blood pressure measurement, 27 studies on using ambulatory screenings to confirm hypertension, 4 studies on harms of screening, and 40 studies on rescreening intervals and hypertension incidence in those with normal blood pressure.
The meta-analysis showed that 5%-65% of patients were not diagnosed with hypertension following ambulatory blood pressure monitoring after an initially elevated office screening measurement.
The USPSTF draft recommendation also noted past epidemiological evidence that 15%-30% of those diagnosed with hypertension may actually have lower blood pressure when not in a medical setting.
The meta-analysis also found that the risk of fatal and nonfatal stroke and cardiovascular events was “consistently and significantly associated with” elevated systolic ambulatory blood pressure, regardless of the measurements in an office.
The primary harms of screening identified in the study were greater absenteeism from work and greater illness episodes after diagnosis, as well as sleep disturbances, discomfort, and daily activity restrictions because of the ambulatory device.
On the basis of the evidence from the meta-analysis, the USPSTF recommended annual screenings for adults age 40 years and older and those at high risk for hypertension, including African Americans, those who are overweight or obese, and those with a normally high blood pressure (130-139/85-89 mm Hg). Screenings should occur every 3-5 years for those age 18-39 years with no risk factors and a normal blood pressure.
Target blood pressure should remain below 140/90 mm Hg for adults younger than 60 years, and below 150/90 mm Hg for adults 60 years or older with neither diabetes nor chronic kidney disease, according to guidelines from the Eighth Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure.
The U.S. Agency for Healthcare Research and Quality funded the meta-analysis. The authors had no relevant disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Ambulatory blood pressure screening should be used to confirm elevated office measurements before diagnosing hypertension.
Major finding: 5%-65% of patients with elevated office blood pressure readings were later not diagnosed with hypertension following ambulatory blood pressure monitoring.
Data source: A meta-analysis of studies on blood pressure screenings published through August 2014.
Disclosures: The study was funded by the U.S. Agency for Healthcare Research and Quality. The authors had no relevant disclosures.
Seven Years of Pain Between the Toes
ANSWER
The correct answer is soft corn (choice “c”). They are caused by bony friction and almost always found between the fourth and fifth toes.
Soft corns are often mistaken for warts (choice “a”). But warts don’t present as painful, macerated lesions between the toes.
Morton neuroma (choice “b”) is actually a neurofibroma, not a virtual tumor. It is usually found on the plantar forefoot between the second and third toes.
Interdigital fungal infections (choice “d”) often develop between the fourth and fifth toes and are often macerated. However, they do not take the form of lesions and do not hurt.
DISCUSSION
Soft corns are known in podiatric circles as heloma molle but are sometimes called kissing corns because they’re caused by friction between bony prominences on the fourth and fifth phalanges, which rub together with every step. Normally, these toes are hourglass shaped, but in patients prone to develop soft corns, the proximal bases of the toes are too wide. The type of shoe the patient wears can be an important factor as well, especially when high heels and/or narrow toe boxes are involved.
The treatment of soft corns can be nonsurgical—sometimes as simple as separating the toes with a tuft of lambswool. However, surgical intervention is often required. In such cases, the head of the proximal phalanx is cut and removed to make the adjacent bones more parallel. Occasionally, the skin is so damaged that it too must be removed and the toes sewn together.
Removing corns with chemicals, shaving, or excision provides no lasting relief, since these methods do not address the underlying structural issues.
Hard corns, also known as heloma durum, tend to develop on the dorsal aspect of the fifth toe secondary to pressure from shoes. Changing the type of shoe worn is one solution, but often, as with soft corns, the underlying bony prominence must be addressed.
There is a third type of corn, the periungual corn, which develops on or near the edge of a nail. These corns are often erroneously called warts.
This patient was referred to a podiatrist, who will likely solve the problem. There is no topical product that can help, and nonsurgical approaches will provide temporary relief at best.
ANSWER
The correct answer is soft corn (choice “c”). They are caused by bony friction and almost always found between the fourth and fifth toes.
Soft corns are often mistaken for warts (choice “a”). But warts don’t present as painful, macerated lesions between the toes.
Morton neuroma (choice “b”) is actually a neurofibroma, not a virtual tumor. It is usually found on the plantar forefoot between the second and third toes.
Interdigital fungal infections (choice “d”) often develop between the fourth and fifth toes and are often macerated. However, they do not take the form of lesions and do not hurt.
DISCUSSION
Soft corns are known in podiatric circles as heloma molle but are sometimes called kissing corns because they’re caused by friction between bony prominences on the fourth and fifth phalanges, which rub together with every step. Normally, these toes are hourglass shaped, but in patients prone to develop soft corns, the proximal bases of the toes are too wide. The type of shoe the patient wears can be an important factor as well, especially when high heels and/or narrow toe boxes are involved.
The treatment of soft corns can be nonsurgical—sometimes as simple as separating the toes with a tuft of lambswool. However, surgical intervention is often required. In such cases, the head of the proximal phalanx is cut and removed to make the adjacent bones more parallel. Occasionally, the skin is so damaged that it too must be removed and the toes sewn together.
Removing corns with chemicals, shaving, or excision provides no lasting relief, since these methods do not address the underlying structural issues.
Hard corns, also known as heloma durum, tend to develop on the dorsal aspect of the fifth toe secondary to pressure from shoes. Changing the type of shoe worn is one solution, but often, as with soft corns, the underlying bony prominence must be addressed.
There is a third type of corn, the periungual corn, which develops on or near the edge of a nail. These corns are often erroneously called warts.
This patient was referred to a podiatrist, who will likely solve the problem. There is no topical product that can help, and nonsurgical approaches will provide temporary relief at best.
ANSWER
The correct answer is soft corn (choice “c”). They are caused by bony friction and almost always found between the fourth and fifth toes.
Soft corns are often mistaken for warts (choice “a”). But warts don’t present as painful, macerated lesions between the toes.
Morton neuroma (choice “b”) is actually a neurofibroma, not a virtual tumor. It is usually found on the plantar forefoot between the second and third toes.
Interdigital fungal infections (choice “d”) often develop between the fourth and fifth toes and are often macerated. However, they do not take the form of lesions and do not hurt.
DISCUSSION
Soft corns are known in podiatric circles as heloma molle but are sometimes called kissing corns because they’re caused by friction between bony prominences on the fourth and fifth phalanges, which rub together with every step. Normally, these toes are hourglass shaped, but in patients prone to develop soft corns, the proximal bases of the toes are too wide. The type of shoe the patient wears can be an important factor as well, especially when high heels and/or narrow toe boxes are involved.
The treatment of soft corns can be nonsurgical—sometimes as simple as separating the toes with a tuft of lambswool. However, surgical intervention is often required. In such cases, the head of the proximal phalanx is cut and removed to make the adjacent bones more parallel. Occasionally, the skin is so damaged that it too must be removed and the toes sewn together.
Removing corns with chemicals, shaving, or excision provides no lasting relief, since these methods do not address the underlying structural issues.
Hard corns, also known as heloma durum, tend to develop on the dorsal aspect of the fifth toe secondary to pressure from shoes. Changing the type of shoe worn is one solution, but often, as with soft corns, the underlying bony prominence must be addressed.
There is a third type of corn, the periungual corn, which develops on or near the edge of a nail. These corns are often erroneously called warts.
This patient was referred to a podiatrist, who will likely solve the problem. There is no topical product that can help, and nonsurgical approaches will provide temporary relief at best.
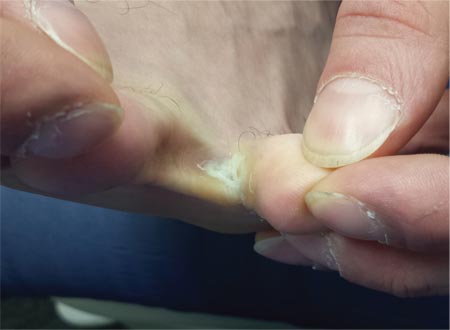
For at least seven years, this 40-year-old man has had pain in the area between the fourth and fifth toes on his left foot. During that time, he has consulted clinicians in a number of settings—including urgent care centers and the emergency department—and received “at least 30” prescriptions for oral antibiotics. Given his persistent pain, none of these treatment attempts has helped. He spends a great deal of time on his feet at work, which worsens the pain. The only relief he experiences is when he goes home at night and removes his socks and shoes. Walking barefoot, he reports, results in relatively little discomfort. The patient claims to be in good health otherwise, specifically denying diabetes. He takes no medications regularly. The skin in the lowest point of the webspace between his fourth and fifth toes is focally thickened, white, and macerated, but there is no redness. The area is exquisitely tender to touch. Examination of the rest of his foot is unremarkable.
State concussion laws boost health care use in children
Health care utilization for concussion among children has increased significantly, partly because of increased awareness but also because of the introduction of health care legislation mandating medical intervention before return to play, new data suggest.
Analysis of health insurance claims for insured children aged 12-18 years from the MarketScan database showed a 92% increase in concussion-related health care utilization between 2008-2009 and 2011-2012 in states with concussion legislation, compared with a 75% overall increase in states without the legislation, according Teresa B. Gibson, Ph.D., of the department of health care policy at Harvard Medical School, Boston, and her colleagues (JAMA Pediatrics 2014 Dec. 22 [doi:10.1001/jamapediatrics.2014.2320]).
After 2009, when the first state concussion laws were passed, states without those laws still demonstrated a 20.9% annual increase in health care utilization for concussion, while states with those laws showed, on average, an additional 13.1% increase, they noted.
At the end of the 2011-2012 school year, 35 states (70%) plus the District of Columbia had laws about sports-related concussion in children.
“We estimate that slightly more than half (60%) the increase in states without laws in effect resulted from the continued trend of increasing health care utilization established before the first law was passed,” the investigators wrote. Although the sources leading to the remaining 40% increase in utilization were not studied, it is likely that increased awareness of concussion brought by local and national media attention played a role.
The study was partly supported by Truven Health from the National Institute of Child Health and Human Development. No other conflicts of interest were declared.
Health care utilization for concussion among children has increased significantly, partly because of increased awareness but also because of the introduction of health care legislation mandating medical intervention before return to play, new data suggest.
Analysis of health insurance claims for insured children aged 12-18 years from the MarketScan database showed a 92% increase in concussion-related health care utilization between 2008-2009 and 2011-2012 in states with concussion legislation, compared with a 75% overall increase in states without the legislation, according Teresa B. Gibson, Ph.D., of the department of health care policy at Harvard Medical School, Boston, and her colleagues (JAMA Pediatrics 2014 Dec. 22 [doi:10.1001/jamapediatrics.2014.2320]).
After 2009, when the first state concussion laws were passed, states without those laws still demonstrated a 20.9% annual increase in health care utilization for concussion, while states with those laws showed, on average, an additional 13.1% increase, they noted.
At the end of the 2011-2012 school year, 35 states (70%) plus the District of Columbia had laws about sports-related concussion in children.
“We estimate that slightly more than half (60%) the increase in states without laws in effect resulted from the continued trend of increasing health care utilization established before the first law was passed,” the investigators wrote. Although the sources leading to the remaining 40% increase in utilization were not studied, it is likely that increased awareness of concussion brought by local and national media attention played a role.
The study was partly supported by Truven Health from the National Institute of Child Health and Human Development. No other conflicts of interest were declared.
Health care utilization for concussion among children has increased significantly, partly because of increased awareness but also because of the introduction of health care legislation mandating medical intervention before return to play, new data suggest.
Analysis of health insurance claims for insured children aged 12-18 years from the MarketScan database showed a 92% increase in concussion-related health care utilization between 2008-2009 and 2011-2012 in states with concussion legislation, compared with a 75% overall increase in states without the legislation, according Teresa B. Gibson, Ph.D., of the department of health care policy at Harvard Medical School, Boston, and her colleagues (JAMA Pediatrics 2014 Dec. 22 [doi:10.1001/jamapediatrics.2014.2320]).
After 2009, when the first state concussion laws were passed, states without those laws still demonstrated a 20.9% annual increase in health care utilization for concussion, while states with those laws showed, on average, an additional 13.1% increase, they noted.
At the end of the 2011-2012 school year, 35 states (70%) plus the District of Columbia had laws about sports-related concussion in children.
“We estimate that slightly more than half (60%) the increase in states without laws in effect resulted from the continued trend of increasing health care utilization established before the first law was passed,” the investigators wrote. Although the sources leading to the remaining 40% increase in utilization were not studied, it is likely that increased awareness of concussion brought by local and national media attention played a role.
The study was partly supported by Truven Health from the National Institute of Child Health and Human Development. No other conflicts of interest were declared.
FROM JAMA PEDIATRICS
Key clinical point: The introduction of state-based health care legislation mandating medical intervention for concussion has contributed to an increase in health care use.
Major finding: Health care utilization for concussion increased 92% between 2008-2009 and 2011-2012 in states with concussion legislation, compared with a 75% overall increase in states without the legislation
Data source: Analysis of health care utilization data for insured children from the MarketScan database.
Disclosures: The study was partly supported by Truven Health from the National Institute of Child Health and Human Development. There were no other conflicts of interest declared.
Unusual Form and Location of a Tumor: Multiosseous Ewing Sarcoma in the Foot
Ewing sarcomas are characterized as primitive malignant round cell tumors.1 These tumors are diagnosed by neuroectodermal differentiation and by their common histologic and immunohistochemical properties.2 Ewing sarcoma is the second most common malignant bone tumor in adolescents and young adults. It is the fourth most common primary malignant tumor, accounting for about 9% of all malignant tumors of bone. The most common primary bone tumors are multiple myeloma, osteosarcoma, and chondrosarcoma.3
The diaphyses of long bones (eg, femur, tibia, humerus) and flat bones (eg, pelvis, scapula) are the most commonly involved sites. Involvement of bones in the hands and feet is uncommon (3%-5% of reported cases).4 The foot bones most commonly involved include the calcaneus and the metatarsals, in the series by Casadei and colleagues.5
About 90% of Ewing sarcoma cases present before age 20 years (mean age, 13 years).6 Typical presentation is that of localized pain at the involved site. Some patients have systemic symptoms, such as fever, malaise, weight loss, leukocytosis, and increased erythrocyte sedimentation rate (ESR) mimicking infection. Radiographically, Ewing sarcoma appears as a permeative destructive bone lesion with a moth-eaten appearance (almost 76% of cases).7 This is usually associated with lamellated periosteal new bone formation or an “onion skin” appearance. Less commonly, a sunburst configuration with an associated soft-tissue mass can be seen. Computed tomography (CT) and magnetic resonance imaging (MRI) show the osseous extent of the tumor and the presence or absence of the soft-tissue component of the tumor. Radionuclide bone scans show increased technetium-99m methylene diphosphonate accumulation and are typically hot.6
Histopathologically, the tumor is composed of small, uniformly sized cells characterized by an almost clear eosinophilic cytoplasm and very little intercellular matrix. There are lobules and strands divided by prominent septa. Macroscopically, appearance can range from a soft, fleshy solid mass to an almost liquid form, as the lesion does not produce any matrix. At time of surgery, the tumor may have a liquefied component and the appearance of pus.6 Prognostic factors are tumor site in foot and treatment according to the series by Casadei and colleagues.5 Patients with large central tumors, especially in the pelvis, have worse outcomes than patients with distal tumors.8
In this article, we report a case of multifocal Ewing sarcoma involving multiple bones in the foot. Given the multifocal nature of the disease confined to the foot, the initial impression was that of osteomyelitis. We describe the histologic, radiologic, and diagnostic features of the tumor and outline treatment and prognosis. To our knowledge, this is the first report of multifocal Ewing sarcoma involving multiple bones in the foot. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old man visited our clinic with the chief complaints of left foot pain and swelling. The pain started 10 months earlier and was followed by swelling. Complaints started after a minor local trauma. The man sought outside medical attention 8 months after pain onset. At his first visit at another institution, an initial radiograph was reported as normal, and all laboratory measures, including complete blood cell count (CBC) differential, ESR, and C-reactive protein (CRP) level, were within normal limits. Under the erroneous diagnosis of infection, the patient was treated with cloxacillin 500 mg 4 times a day for 4 weeks.
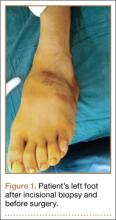
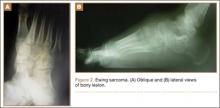
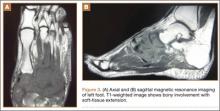
The patient’s pain had started 10 months before presentation (2 months after antibiotic therapy was initiated) (Figure 1). Physical examination at our institution revealed a palpable mass on the dorsum of the left foot. Anteroposterior and lateral plain radiographs showed a permeative lytic lesion with cortical destruction in the left calcaneus, navicular, cuboid, and cuneiform bones and in all metatarsal bones except the first (Figure 2). A soft-tissue mass around the involved bones was noted as well. The talus was not involved (Figure 3).
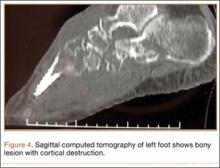
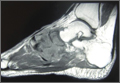
CT showed permeative destruction of left foot bones, including the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first. Invasion through the overlying cortex of the involved bones indicated aggressive biological activity of the tumor (Figure 4). MRI showed a destructive bony lesion of the mentioned bones associated with the soft-tissue mass (Figure 3).
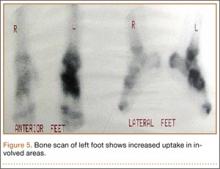
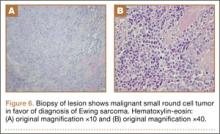
Bone scan showed increased uptake in the involved areas (Figure 5). Chest plain radiographs and CT showed no distant metastasis. An incisional biopsy was performed, and histopathology showed a malignant small round cell tumor, identified as Ewing sarcoma (Figure 6). An immunohistochemistry study demonstrated positive CD99 and negative cytokeratin, leukocyte common antigen, desmin, and synaptophysin.
The patient was started on 4 cycles of adjuvant chemotherapy. Cycles 1 and 3 involved cyclophosphamide 2 g, vincristine 2 g, and doxorubicin 50 mg; cycles 2 and 4 involved ifosfamide 3.5 g and etoposide 200 mg. Tumor shrinkage occurred after chemotherapy. Clinical response to preoperative chemotherapy was documented by a decrease in tumor size at follow-ups. The patient underwent below-knee amputation.
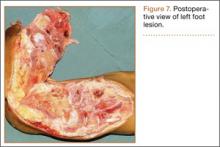
Postoperative histopathology confirmed the diagnosis of Ewing sarcoma of the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first (Figure 7). At 2-year follow-up, the patient had no evidence of local recurrence or distant metastasis based on chest CT and clinical examination of the affected site.
Discussion
Ewing sarcoma is the prototype of round small cell malignancies that arise from the long bones and the flat bones. It seldom involves the hands or feet. To our knowledge, this is the first report of Ewing sarcoma of the foot with multiple-bone involvement. Our literature review found a case of Ewing sarcoma of the first phalanx of the third toe, the second metatarsal bone, the cuneiform, the cuboid, and the talus, with lesser soft-tissue extension compared with our patient’s case.9
As this foot tumor is rare, there are few reports on its clinical aspects, appropriate treatment, and long-term outcome. For treatment of nonmetastatic Ewing sarcoma, limb-salvage surgery is advised if response has been good and there is a reasonable expectation of negative margins and good functional results.
Radiation and surgery may be part of the overall treatment plan. Radiation without surgery has a unique role in pelvic Ewing sarcoma, in contrast to extremity Ewing sarcoma. In our opinion, margins and histologic necrosis in the resection specimen are examined after surgery, and, if the margins are widely negative and histologic response is good, no further local control is advised. If the margin is positive, postoperative radiation therapy is recommended.1 Amputation has gradually become a (rare) choice in the treatment of extremity sarcomas.9 In our patient’s case, surgery was preferred over radiation therapy after chemotherapy because of the low risk of local side effects and the expected high efficacy. In addition, radiation at such high doses for Ewing sarcoma in the foot causes functional impairment. Because of the multiple-bone involvement, a salvage procedure was not possible for our patient. Given the calcaneal involvement, however, below-knee amputation was considered safer than ankle disarticulation.
Multiple-bone involvement occurs in the advanced stage of Ewing sarcoma, usually after visceral and pulmonary metastases are detected.9 The case reported by Rammal and colleagues9 had both multiple-bone involvement in the foot and pulmonary metastasis. The authors indicated that hematogenous spread of the tumor was discerned because the lesions were noncontiguous.9 Our patient had no distant metastases. We think his tumor originated in a tarsal or midtarsal bone and extended to adjacent bones. Therefore, it probably spread through its capsular and ligamentous attachment among tarsal and midtarsal bones, as the involvement was contiguous rather than distinct.
Average delay from symptom onset to diagnosis was reported to be 34 weeks.3 Average physician delay from initial visit to correct diagnosis was reported to be 19 weeks.3 Patients may have erythema, fever, and swelling, suggestive of osteomyelitis.3 Laboratory results may show increased white blood cell count and elevated ESR and CRP level.3 In addition, needle biopsy of the tumor may reveal an appearance grossly similar to that of pus.3 Therefore, physicians may send all the tissue out for microbiological analysis (according to the erroneous diagnosis of infection) and none out for pathologic analysis. The situation can be further complicated when Ewing sarcoma occurs in the foot, an uncommon site. In this special case, multiple-bone involvement can present a misleading clinical picture of infection.10 In other words, infection is one of the best choices in the differential diagnosis.7 Also to be considered are multicentric giant cell tumor, fibrosarcoma,11 and osteosarcoma.12
1. Herring JA. Malignant tumors of bone. In: Herring JA, ed. Tachdjian’s Pediatric Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2008:2324-2327.
2. Cavazzana AO, Miser JS, Jefferson J, Triche TJ. Experimental evidence for a neural origin of Ewing’s sarcoma of bone. Am J Pathol. 1987;127(3):507-518.
3. Canale ST, Beaty JH. Malignant tumors of bone. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Philadelphia, PA: Mosby Elsevier; 2008:910-913.
4. Unni KK. Ewing sarcoma. In: Unni KK, ed. Dahlin’s Bone Tumor: General Aspects and Data on 11087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:121-142.
5. Casadei R, Magnani M, Biagini R, Mercuri M. Prognostic factors in Ewing’s sarcoma of the foot. Clin Orthop. 2004;(420):230-238.
6. Greenspan A, Jundt G, Remagen W. Bone-forming (osteogenic) lesions. In: Greenspan A, Jundt G, Remagen W, eds. Differential Diagnosis in Orthopaedic Oncology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:114.
7. Metcalfe JE, Grimer RJ. Ewing’s sarcoma of the foot masquerading as osteomyelitis. Foot Ankle Surg. 2004;10(1):29-33.
8. Hoffmann C, Ahrens S, Dunst J, et al. Pelvis Ewing sarcoma: a retrospective analysis of 241 cases. Cancer. 1999;85(4):869-877.
9. Rammal H, Ghanem I, Torbey PH, Dagher F, Kharrat K. Multifocal Ewing sarcoma of the foot. J Pediatr Hematol Oncol. 2008;30(4):298-300.
10. Ledermann HP, Morrison WB, Schweitzer ME. MR image analysis of pedal osteomyelitis: distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223(3):747-755.
11. Dhillon MS, Prabhudev Prasad AP, Virk MS, Aggarwal S. Multicentric giant cell tumor involving the same foot: a case report and review of literature. Indian J Orthop. 2007;41(2):154-157.
12. Baraga JJ, Amarami KK, Swee RG, Wold L, Unni KK. Radiographic features of Ewing’s sarcoma of the bones of the hand and feet. Skeletal Radiol. 2001;30(3):121-126.
Ewing sarcomas are characterized as primitive malignant round cell tumors.1 These tumors are diagnosed by neuroectodermal differentiation and by their common histologic and immunohistochemical properties.2 Ewing sarcoma is the second most common malignant bone tumor in adolescents and young adults. It is the fourth most common primary malignant tumor, accounting for about 9% of all malignant tumors of bone. The most common primary bone tumors are multiple myeloma, osteosarcoma, and chondrosarcoma.3
The diaphyses of long bones (eg, femur, tibia, humerus) and flat bones (eg, pelvis, scapula) are the most commonly involved sites. Involvement of bones in the hands and feet is uncommon (3%-5% of reported cases).4 The foot bones most commonly involved include the calcaneus and the metatarsals, in the series by Casadei and colleagues.5
About 90% of Ewing sarcoma cases present before age 20 years (mean age, 13 years).6 Typical presentation is that of localized pain at the involved site. Some patients have systemic symptoms, such as fever, malaise, weight loss, leukocytosis, and increased erythrocyte sedimentation rate (ESR) mimicking infection. Radiographically, Ewing sarcoma appears as a permeative destructive bone lesion with a moth-eaten appearance (almost 76% of cases).7 This is usually associated with lamellated periosteal new bone formation or an “onion skin” appearance. Less commonly, a sunburst configuration with an associated soft-tissue mass can be seen. Computed tomography (CT) and magnetic resonance imaging (MRI) show the osseous extent of the tumor and the presence or absence of the soft-tissue component of the tumor. Radionuclide bone scans show increased technetium-99m methylene diphosphonate accumulation and are typically hot.6
Histopathologically, the tumor is composed of small, uniformly sized cells characterized by an almost clear eosinophilic cytoplasm and very little intercellular matrix. There are lobules and strands divided by prominent septa. Macroscopically, appearance can range from a soft, fleshy solid mass to an almost liquid form, as the lesion does not produce any matrix. At time of surgery, the tumor may have a liquefied component and the appearance of pus.6 Prognostic factors are tumor site in foot and treatment according to the series by Casadei and colleagues.5 Patients with large central tumors, especially in the pelvis, have worse outcomes than patients with distal tumors.8
In this article, we report a case of multifocal Ewing sarcoma involving multiple bones in the foot. Given the multifocal nature of the disease confined to the foot, the initial impression was that of osteomyelitis. We describe the histologic, radiologic, and diagnostic features of the tumor and outline treatment and prognosis. To our knowledge, this is the first report of multifocal Ewing sarcoma involving multiple bones in the foot. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old man visited our clinic with the chief complaints of left foot pain and swelling. The pain started 10 months earlier and was followed by swelling. Complaints started after a minor local trauma. The man sought outside medical attention 8 months after pain onset. At his first visit at another institution, an initial radiograph was reported as normal, and all laboratory measures, including complete blood cell count (CBC) differential, ESR, and C-reactive protein (CRP) level, were within normal limits. Under the erroneous diagnosis of infection, the patient was treated with cloxacillin 500 mg 4 times a day for 4 weeks.
The patient’s pain had started 10 months before presentation (2 months after antibiotic therapy was initiated) (Figure 1). Physical examination at our institution revealed a palpable mass on the dorsum of the left foot. Anteroposterior and lateral plain radiographs showed a permeative lytic lesion with cortical destruction in the left calcaneus, navicular, cuboid, and cuneiform bones and in all metatarsal bones except the first (Figure 2). A soft-tissue mass around the involved bones was noted as well. The talus was not involved (Figure 3).
CT showed permeative destruction of left foot bones, including the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first. Invasion through the overlying cortex of the involved bones indicated aggressive biological activity of the tumor (Figure 4). MRI showed a destructive bony lesion of the mentioned bones associated with the soft-tissue mass (Figure 3).
Bone scan showed increased uptake in the involved areas (Figure 5). Chest plain radiographs and CT showed no distant metastasis. An incisional biopsy was performed, and histopathology showed a malignant small round cell tumor, identified as Ewing sarcoma (Figure 6). An immunohistochemistry study demonstrated positive CD99 and negative cytokeratin, leukocyte common antigen, desmin, and synaptophysin.
The patient was started on 4 cycles of adjuvant chemotherapy. Cycles 1 and 3 involved cyclophosphamide 2 g, vincristine 2 g, and doxorubicin 50 mg; cycles 2 and 4 involved ifosfamide 3.5 g and etoposide 200 mg. Tumor shrinkage occurred after chemotherapy. Clinical response to preoperative chemotherapy was documented by a decrease in tumor size at follow-ups. The patient underwent below-knee amputation.
Postoperative histopathology confirmed the diagnosis of Ewing sarcoma of the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first (Figure 7). At 2-year follow-up, the patient had no evidence of local recurrence or distant metastasis based on chest CT and clinical examination of the affected site.
Discussion
Ewing sarcoma is the prototype of round small cell malignancies that arise from the long bones and the flat bones. It seldom involves the hands or feet. To our knowledge, this is the first report of Ewing sarcoma of the foot with multiple-bone involvement. Our literature review found a case of Ewing sarcoma of the first phalanx of the third toe, the second metatarsal bone, the cuneiform, the cuboid, and the talus, with lesser soft-tissue extension compared with our patient’s case.9
As this foot tumor is rare, there are few reports on its clinical aspects, appropriate treatment, and long-term outcome. For treatment of nonmetastatic Ewing sarcoma, limb-salvage surgery is advised if response has been good and there is a reasonable expectation of negative margins and good functional results.
Radiation and surgery may be part of the overall treatment plan. Radiation without surgery has a unique role in pelvic Ewing sarcoma, in contrast to extremity Ewing sarcoma. In our opinion, margins and histologic necrosis in the resection specimen are examined after surgery, and, if the margins are widely negative and histologic response is good, no further local control is advised. If the margin is positive, postoperative radiation therapy is recommended.1 Amputation has gradually become a (rare) choice in the treatment of extremity sarcomas.9 In our patient’s case, surgery was preferred over radiation therapy after chemotherapy because of the low risk of local side effects and the expected high efficacy. In addition, radiation at such high doses for Ewing sarcoma in the foot causes functional impairment. Because of the multiple-bone involvement, a salvage procedure was not possible for our patient. Given the calcaneal involvement, however, below-knee amputation was considered safer than ankle disarticulation.
Multiple-bone involvement occurs in the advanced stage of Ewing sarcoma, usually after visceral and pulmonary metastases are detected.9 The case reported by Rammal and colleagues9 had both multiple-bone involvement in the foot and pulmonary metastasis. The authors indicated that hematogenous spread of the tumor was discerned because the lesions were noncontiguous.9 Our patient had no distant metastases. We think his tumor originated in a tarsal or midtarsal bone and extended to adjacent bones. Therefore, it probably spread through its capsular and ligamentous attachment among tarsal and midtarsal bones, as the involvement was contiguous rather than distinct.
Average delay from symptom onset to diagnosis was reported to be 34 weeks.3 Average physician delay from initial visit to correct diagnosis was reported to be 19 weeks.3 Patients may have erythema, fever, and swelling, suggestive of osteomyelitis.3 Laboratory results may show increased white blood cell count and elevated ESR and CRP level.3 In addition, needle biopsy of the tumor may reveal an appearance grossly similar to that of pus.3 Therefore, physicians may send all the tissue out for microbiological analysis (according to the erroneous diagnosis of infection) and none out for pathologic analysis. The situation can be further complicated when Ewing sarcoma occurs in the foot, an uncommon site. In this special case, multiple-bone involvement can present a misleading clinical picture of infection.10 In other words, infection is one of the best choices in the differential diagnosis.7 Also to be considered are multicentric giant cell tumor, fibrosarcoma,11 and osteosarcoma.12
Ewing sarcomas are characterized as primitive malignant round cell tumors.1 These tumors are diagnosed by neuroectodermal differentiation and by their common histologic and immunohistochemical properties.2 Ewing sarcoma is the second most common malignant bone tumor in adolescents and young adults. It is the fourth most common primary malignant tumor, accounting for about 9% of all malignant tumors of bone. The most common primary bone tumors are multiple myeloma, osteosarcoma, and chondrosarcoma.3
The diaphyses of long bones (eg, femur, tibia, humerus) and flat bones (eg, pelvis, scapula) are the most commonly involved sites. Involvement of bones in the hands and feet is uncommon (3%-5% of reported cases).4 The foot bones most commonly involved include the calcaneus and the metatarsals, in the series by Casadei and colleagues.5
About 90% of Ewing sarcoma cases present before age 20 years (mean age, 13 years).6 Typical presentation is that of localized pain at the involved site. Some patients have systemic symptoms, such as fever, malaise, weight loss, leukocytosis, and increased erythrocyte sedimentation rate (ESR) mimicking infection. Radiographically, Ewing sarcoma appears as a permeative destructive bone lesion with a moth-eaten appearance (almost 76% of cases).7 This is usually associated with lamellated periosteal new bone formation or an “onion skin” appearance. Less commonly, a sunburst configuration with an associated soft-tissue mass can be seen. Computed tomography (CT) and magnetic resonance imaging (MRI) show the osseous extent of the tumor and the presence or absence of the soft-tissue component of the tumor. Radionuclide bone scans show increased technetium-99m methylene diphosphonate accumulation and are typically hot.6
Histopathologically, the tumor is composed of small, uniformly sized cells characterized by an almost clear eosinophilic cytoplasm and very little intercellular matrix. There are lobules and strands divided by prominent septa. Macroscopically, appearance can range from a soft, fleshy solid mass to an almost liquid form, as the lesion does not produce any matrix. At time of surgery, the tumor may have a liquefied component and the appearance of pus.6 Prognostic factors are tumor site in foot and treatment according to the series by Casadei and colleagues.5 Patients with large central tumors, especially in the pelvis, have worse outcomes than patients with distal tumors.8
In this article, we report a case of multifocal Ewing sarcoma involving multiple bones in the foot. Given the multifocal nature of the disease confined to the foot, the initial impression was that of osteomyelitis. We describe the histologic, radiologic, and diagnostic features of the tumor and outline treatment and prognosis. To our knowledge, this is the first report of multifocal Ewing sarcoma involving multiple bones in the foot. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old man visited our clinic with the chief complaints of left foot pain and swelling. The pain started 10 months earlier and was followed by swelling. Complaints started after a minor local trauma. The man sought outside medical attention 8 months after pain onset. At his first visit at another institution, an initial radiograph was reported as normal, and all laboratory measures, including complete blood cell count (CBC) differential, ESR, and C-reactive protein (CRP) level, were within normal limits. Under the erroneous diagnosis of infection, the patient was treated with cloxacillin 500 mg 4 times a day for 4 weeks.
The patient’s pain had started 10 months before presentation (2 months after antibiotic therapy was initiated) (Figure 1). Physical examination at our institution revealed a palpable mass on the dorsum of the left foot. Anteroposterior and lateral plain radiographs showed a permeative lytic lesion with cortical destruction in the left calcaneus, navicular, cuboid, and cuneiform bones and in all metatarsal bones except the first (Figure 2). A soft-tissue mass around the involved bones was noted as well. The talus was not involved (Figure 3).
CT showed permeative destruction of left foot bones, including the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first. Invasion through the overlying cortex of the involved bones indicated aggressive biological activity of the tumor (Figure 4). MRI showed a destructive bony lesion of the mentioned bones associated with the soft-tissue mass (Figure 3).
Bone scan showed increased uptake in the involved areas (Figure 5). Chest plain radiographs and CT showed no distant metastasis. An incisional biopsy was performed, and histopathology showed a malignant small round cell tumor, identified as Ewing sarcoma (Figure 6). An immunohistochemistry study demonstrated positive CD99 and negative cytokeratin, leukocyte common antigen, desmin, and synaptophysin.
The patient was started on 4 cycles of adjuvant chemotherapy. Cycles 1 and 3 involved cyclophosphamide 2 g, vincristine 2 g, and doxorubicin 50 mg; cycles 2 and 4 involved ifosfamide 3.5 g and etoposide 200 mg. Tumor shrinkage occurred after chemotherapy. Clinical response to preoperative chemotherapy was documented by a decrease in tumor size at follow-ups. The patient underwent below-knee amputation.
Postoperative histopathology confirmed the diagnosis of Ewing sarcoma of the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first (Figure 7). At 2-year follow-up, the patient had no evidence of local recurrence or distant metastasis based on chest CT and clinical examination of the affected site.
Discussion
Ewing sarcoma is the prototype of round small cell malignancies that arise from the long bones and the flat bones. It seldom involves the hands or feet. To our knowledge, this is the first report of Ewing sarcoma of the foot with multiple-bone involvement. Our literature review found a case of Ewing sarcoma of the first phalanx of the third toe, the second metatarsal bone, the cuneiform, the cuboid, and the talus, with lesser soft-tissue extension compared with our patient’s case.9
As this foot tumor is rare, there are few reports on its clinical aspects, appropriate treatment, and long-term outcome. For treatment of nonmetastatic Ewing sarcoma, limb-salvage surgery is advised if response has been good and there is a reasonable expectation of negative margins and good functional results.
Radiation and surgery may be part of the overall treatment plan. Radiation without surgery has a unique role in pelvic Ewing sarcoma, in contrast to extremity Ewing sarcoma. In our opinion, margins and histologic necrosis in the resection specimen are examined after surgery, and, if the margins are widely negative and histologic response is good, no further local control is advised. If the margin is positive, postoperative radiation therapy is recommended.1 Amputation has gradually become a (rare) choice in the treatment of extremity sarcomas.9 In our patient’s case, surgery was preferred over radiation therapy after chemotherapy because of the low risk of local side effects and the expected high efficacy. In addition, radiation at such high doses for Ewing sarcoma in the foot causes functional impairment. Because of the multiple-bone involvement, a salvage procedure was not possible for our patient. Given the calcaneal involvement, however, below-knee amputation was considered safer than ankle disarticulation.
Multiple-bone involvement occurs in the advanced stage of Ewing sarcoma, usually after visceral and pulmonary metastases are detected.9 The case reported by Rammal and colleagues9 had both multiple-bone involvement in the foot and pulmonary metastasis. The authors indicated that hematogenous spread of the tumor was discerned because the lesions were noncontiguous.9 Our patient had no distant metastases. We think his tumor originated in a tarsal or midtarsal bone and extended to adjacent bones. Therefore, it probably spread through its capsular and ligamentous attachment among tarsal and midtarsal bones, as the involvement was contiguous rather than distinct.
Average delay from symptom onset to diagnosis was reported to be 34 weeks.3 Average physician delay from initial visit to correct diagnosis was reported to be 19 weeks.3 Patients may have erythema, fever, and swelling, suggestive of osteomyelitis.3 Laboratory results may show increased white blood cell count and elevated ESR and CRP level.3 In addition, needle biopsy of the tumor may reveal an appearance grossly similar to that of pus.3 Therefore, physicians may send all the tissue out for microbiological analysis (according to the erroneous diagnosis of infection) and none out for pathologic analysis. The situation can be further complicated when Ewing sarcoma occurs in the foot, an uncommon site. In this special case, multiple-bone involvement can present a misleading clinical picture of infection.10 In other words, infection is one of the best choices in the differential diagnosis.7 Also to be considered are multicentric giant cell tumor, fibrosarcoma,11 and osteosarcoma.12
1. Herring JA. Malignant tumors of bone. In: Herring JA, ed. Tachdjian’s Pediatric Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2008:2324-2327.
2. Cavazzana AO, Miser JS, Jefferson J, Triche TJ. Experimental evidence for a neural origin of Ewing’s sarcoma of bone. Am J Pathol. 1987;127(3):507-518.
3. Canale ST, Beaty JH. Malignant tumors of bone. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Philadelphia, PA: Mosby Elsevier; 2008:910-913.
4. Unni KK. Ewing sarcoma. In: Unni KK, ed. Dahlin’s Bone Tumor: General Aspects and Data on 11087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:121-142.
5. Casadei R, Magnani M, Biagini R, Mercuri M. Prognostic factors in Ewing’s sarcoma of the foot. Clin Orthop. 2004;(420):230-238.
6. Greenspan A, Jundt G, Remagen W. Bone-forming (osteogenic) lesions. In: Greenspan A, Jundt G, Remagen W, eds. Differential Diagnosis in Orthopaedic Oncology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:114.
7. Metcalfe JE, Grimer RJ. Ewing’s sarcoma of the foot masquerading as osteomyelitis. Foot Ankle Surg. 2004;10(1):29-33.
8. Hoffmann C, Ahrens S, Dunst J, et al. Pelvis Ewing sarcoma: a retrospective analysis of 241 cases. Cancer. 1999;85(4):869-877.
9. Rammal H, Ghanem I, Torbey PH, Dagher F, Kharrat K. Multifocal Ewing sarcoma of the foot. J Pediatr Hematol Oncol. 2008;30(4):298-300.
10. Ledermann HP, Morrison WB, Schweitzer ME. MR image analysis of pedal osteomyelitis: distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223(3):747-755.
11. Dhillon MS, Prabhudev Prasad AP, Virk MS, Aggarwal S. Multicentric giant cell tumor involving the same foot: a case report and review of literature. Indian J Orthop. 2007;41(2):154-157.
12. Baraga JJ, Amarami KK, Swee RG, Wold L, Unni KK. Radiographic features of Ewing’s sarcoma of the bones of the hand and feet. Skeletal Radiol. 2001;30(3):121-126.
1. Herring JA. Malignant tumors of bone. In: Herring JA, ed. Tachdjian’s Pediatric Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2008:2324-2327.
2. Cavazzana AO, Miser JS, Jefferson J, Triche TJ. Experimental evidence for a neural origin of Ewing’s sarcoma of bone. Am J Pathol. 1987;127(3):507-518.
3. Canale ST, Beaty JH. Malignant tumors of bone. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Philadelphia, PA: Mosby Elsevier; 2008:910-913.
4. Unni KK. Ewing sarcoma. In: Unni KK, ed. Dahlin’s Bone Tumor: General Aspects and Data on 11087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:121-142.
5. Casadei R, Magnani M, Biagini R, Mercuri M. Prognostic factors in Ewing’s sarcoma of the foot. Clin Orthop. 2004;(420):230-238.
6. Greenspan A, Jundt G, Remagen W. Bone-forming (osteogenic) lesions. In: Greenspan A, Jundt G, Remagen W, eds. Differential Diagnosis in Orthopaedic Oncology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:114.
7. Metcalfe JE, Grimer RJ. Ewing’s sarcoma of the foot masquerading as osteomyelitis. Foot Ankle Surg. 2004;10(1):29-33.
8. Hoffmann C, Ahrens S, Dunst J, et al. Pelvis Ewing sarcoma: a retrospective analysis of 241 cases. Cancer. 1999;85(4):869-877.
9. Rammal H, Ghanem I, Torbey PH, Dagher F, Kharrat K. Multifocal Ewing sarcoma of the foot. J Pediatr Hematol Oncol. 2008;30(4):298-300.
10. Ledermann HP, Morrison WB, Schweitzer ME. MR image analysis of pedal osteomyelitis: distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223(3):747-755.
11. Dhillon MS, Prabhudev Prasad AP, Virk MS, Aggarwal S. Multicentric giant cell tumor involving the same foot: a case report and review of literature. Indian J Orthop. 2007;41(2):154-157.
12. Baraga JJ, Amarami KK, Swee RG, Wold L, Unni KK. Radiographic features of Ewing’s sarcoma of the bones of the hand and feet. Skeletal Radiol. 2001;30(3):121-126.
Early Intervention Could Lead to Reduction in CKD Cases
Q) When I see a patient for an annual physical or gynecologic exam (or even just to give a flu shot), I try to encourage healthy living. Are there any CKD statistics I can use to “encourage” my hypertensive or overweight patients to follow a better plan of care?
According to a recent study by McMahon et al, risk factors for chronic kidney disease (CKD)—including hypertension, dyslipidemia, and diabetes—may be present up to 30 years prior to diagnosis of CKD.1 Since these risk factors are modifiable, the researchers concluded that early intervention could lead to a reduction in new CKD cases.1
Using data from the Framingham Offspring Study, the researchers identified 441 patients with incident CKD and then matched them with a control group of 882 patients who did not develop CKD during the 30-year study period. Subjects who eventually developed CKD were more likely than their counterparts to have hypertension (odds ratio [OR], 1.76), obesity (OR, 1.71), and elevated triglyceride levels (OR, 1.43) 30 years prior to CKD diagnosis. Having diabetes nearly tripled a patient’s likelihood of developing CKD within 20 years (OR, 2.90).1
Early identification of these risk factors and treatment of affected patients is imperative to help prevent kidney disease. Regular screening of young and middle-aged adult patients, as well as early intervention when risk factors are identified, should slow not only the progression of these detrimental conditions but also the development of CKD.
Joanne Hindlet, ACNP, CNN-NP
Houston Nephrology Group
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Q) When I see a patient for an annual physical or gynecologic exam (or even just to give a flu shot), I try to encourage healthy living. Are there any CKD statistics I can use to “encourage” my hypertensive or overweight patients to follow a better plan of care?
According to a recent study by McMahon et al, risk factors for chronic kidney disease (CKD)—including hypertension, dyslipidemia, and diabetes—may be present up to 30 years prior to diagnosis of CKD.1 Since these risk factors are modifiable, the researchers concluded that early intervention could lead to a reduction in new CKD cases.1
Using data from the Framingham Offspring Study, the researchers identified 441 patients with incident CKD and then matched them with a control group of 882 patients who did not develop CKD during the 30-year study period. Subjects who eventually developed CKD were more likely than their counterparts to have hypertension (odds ratio [OR], 1.76), obesity (OR, 1.71), and elevated triglyceride levels (OR, 1.43) 30 years prior to CKD diagnosis. Having diabetes nearly tripled a patient’s likelihood of developing CKD within 20 years (OR, 2.90).1
Early identification of these risk factors and treatment of affected patients is imperative to help prevent kidney disease. Regular screening of young and middle-aged adult patients, as well as early intervention when risk factors are identified, should slow not only the progression of these detrimental conditions but also the development of CKD.
Joanne Hindlet, ACNP, CNN-NP
Houston Nephrology Group
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Q) When I see a patient for an annual physical or gynecologic exam (or even just to give a flu shot), I try to encourage healthy living. Are there any CKD statistics I can use to “encourage” my hypertensive or overweight patients to follow a better plan of care?
According to a recent study by McMahon et al, risk factors for chronic kidney disease (CKD)—including hypertension, dyslipidemia, and diabetes—may be present up to 30 years prior to diagnosis of CKD.1 Since these risk factors are modifiable, the researchers concluded that early intervention could lead to a reduction in new CKD cases.1
Using data from the Framingham Offspring Study, the researchers identified 441 patients with incident CKD and then matched them with a control group of 882 patients who did not develop CKD during the 30-year study period. Subjects who eventually developed CKD were more likely than their counterparts to have hypertension (odds ratio [OR], 1.76), obesity (OR, 1.71), and elevated triglyceride levels (OR, 1.43) 30 years prior to CKD diagnosis. Having diabetes nearly tripled a patient’s likelihood of developing CKD within 20 years (OR, 2.90).1
Early identification of these risk factors and treatment of affected patients is imperative to help prevent kidney disease. Regular screening of young and middle-aged adult patients, as well as early intervention when risk factors are identified, should slow not only the progression of these detrimental conditions but also the development of CKD.
Joanne Hindlet, ACNP, CNN-NP
Houston Nephrology Group
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.