User login
Cystic lung disease: Systematic, stepwise diagnosis
Air-filled pulmonary lesions commonly detected on chest computed tomography. Cystic lung lesions should be distinguished from other air-filled lesions to facilitate diagnosis. Primary care physicians play an integral role in the recognition of cystic lung disease.
The differential diagnosis of cystic lung disease is broad and includes isolated pulmonary, systemic, infectious, and congenital etiologies.
Here, we aim to provide a systematic, stepwise approach to help differentiate among the various cystic lung diseases and devise an algorithm for diagnosis. In doing so, we will discuss the clinical and radiographic features of many of these diseases:
- Lymphangioleiomyomatosis
- Birt-Hogg-Dubé syndrome
- Pulmonary Langerhans cell histiocytosis
- Interstitial pneumonia (desquamative interstitial pneumonia, lymphocytic interstitial pneumonia)
- Congenital cystic lung disease (congenital pulmonary airway malformation, pulmonary sequestration, bronchogenic cyst) Pulmonary infection
- Systemic disease (amyloidosis, light chain deposition disease, neurofibromatosis type 1).
STEP 1: RULE OUT CYST-MIMICS
A pulmonary cyst is a round, circumscribed space surrounded by an epithelial or fibrous wall of variable thickness.1 On chest radiography and computed tomography, a cyst appears as a round parenchymal lucency or low-attenuating area with a well-defined interface with normal lung.1 Cysts vary in wall thickness but usually have a thin wall (< 2 mm) and occur without associated pulmonary emphysema.1 They typically contain air but occasionally contain fluid or solid material.
A pulmonary cyst can be categorized as a bulla, bleb, or pneumatocele.
Bullae are larger than 1 cm in diameter, sharply demarcated by a thin wall, and usually accompanied by emphysematous changes in the adjacent lung.1
Blebs are no larger than 1 cm in diameter, are located within the visceral pleura or the subpleural space, and appear on computed tomography as thin-walled air spaces that are contiguous with the pleura.1 The distinction between a bleb and a bulla is of little clinical importance, and is often unnecessary.
Pneumatoceles are cysts that are frequently caused by acute pneumonia, trauma, or aspiration of hydrocarbon fluid, and are usually transient.1
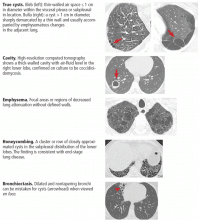
Mimics of pulmonary cysts include pulmonary cavities, emphysema, loculated pneumothoraces, honeycomb lung, and bronchiectasis (Figure 1).2
Pulmonary cavities differ from cysts in that their walls are typically thicker (usually > 4 mm).3
Emphysema differs from cystic lung disease as it typically leads to focal areas or regions of decreased lung attenuation that do not have defined walls.1
Honeycombing refers to a cluster or row of cysts, 1 to 3 mm in wall thickness and typically 3 to 10 mm in diameter, that are associated with end-stage lung fibrosis.1 They are typically subpleural in distribution and are accompanied by fibrotic features such as reticulation and traction bronchiectasis.1
Bronchiectasis is dilation and distortion of bronchi and bronchioles and can be mistaken for cysts when viewed en face.1
Loculated pneumothoraces can also mimic pulmonary cysts, but they typically fail to adhere to a defined anatomic unit and are subpleural in distribution.
STEP 2: CHARACTERIZE THE CLINICAL PRESENTATION
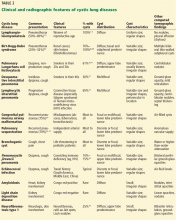
Clinical signs and symptoms of cystic lung disease play a key role in diagnosis (Table 1). For instance, spontaneous pneumothorax is commonly associated with diffuse cystic lung disease (lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome), while insidious dyspnea, with or without associated pneumothorax, is usually associated with the interstitial pneumonias (lymphocytic interstitial pneumonia and desquamative interstitial pneumonia).
In addition, congenital abnormalities of the lung can lead to cyst formation. These abnormalities, especially when associated with other congenital abnormalities, are often diagnosed in the prenatal and perinatal periods. However, some remain undetected until incidentally found later in adulthood or if superimposing infection develops.
Primary pulmonary infections can also cause parenchymal necrosis, which in turn cavitates or forms cysts.4
Lastly, cystic lung diseases can occur as part of a multiorgan or systemic illness in which the lung is one of the organs involved. Although usually diagnosed before the discovery of cysts or manifestations of pulmonary symptoms, they can present as a diagnostic challenge, especially when lung cysts are the initial presentation.bsence of amyloid fibrils.
In view of the features of the different types of cystic lung disease, adults with cystic lung disease can be grouped according to their typical clinical presentations (Table 2):
- Insidious dyspnea or spontaneous pneumothorax
- Incidentally found cysts or recurrent pneumonia
- Signs and symptoms of primary pulmonary infection
- Signs and symptoms that are primarily nonpulmonary.
Insidious dyspnea or spontaneous pneumothorax
Insidious dyspnea or spontaneous pneumothorax can be manifestations of lymphangioleiomyomatosis, Birt-Hogg-Dubé syndrome, pulmonary Langerhans cell histiocytosis, desquamative interstitial pneumonia, or lymphocytic interstitial pneumonia.
Lymphangioleiomyomatosis is characterized by abnormal cellular proliferation within the lung, kidney, lymphatic system, or any combination.5 The peak prevalence is in the third to fourth decades of life, and most patients are women of childbearing age.6 In addition to progressive dyspnea on exertion and pneumothorax, other signs and symptoms include hemoptysis, nonproductive cough, chylous pleural effusion, and ascites.7,8
Birt-Hogg-Dubé syndrome is caused by germline mutations in the folliculin (FLCN) gene.9 It is characterized by skin fibrofolliculomas, pulmonary cysts, spontaneous pneumothorax, and renal cancer.10
Pulmonary Langerhans cell histiocytosis is part of the spectrum of Langerhans cell histiocytosis that, in addition to the lungs, can also involve the bone, pituitary gland, thyroid, skin, lymph nodes, and liver.11 It occurs almost exclusively in smokers, affecting individuals in their 20s and 30s, with no gender predilection.12,13 In addition to nonproductive cough and dyspnea, patients can also present with fever, anorexia, and weight loss,13 but approximately 25% of patients are asymptomatic.14
Desquamative interstitial pneumonia is an idiopathic interstitial pneumonia that, like pulmonary Langerhans cell histiocytosis, is seen almost exclusively in current or former smokers, who account for about 90% of patients with this disease. It affects almost twice as many men as women.15,16 The mean age at onset is 42 to 46.15,16 In addition to insidious cough and dyspnea, digital clubbing develops in 26% to 40% of patients.16,17
Lymphocytic interstitial pneumonia is another rare idiopathic pneumonia, usually associated with connective tissue disease, Sjögren syndrome, immunodeficiencies, and viral infections.18–21 It is more common in women, presenting between the 4th and 7th decades of life, with a mean age at diagnosis of 50 to 56.18,22 In addition to progressive dyspnea and cough, other symptoms include weight loss, pleuritic pain, arthralgias, fatigue, night sweats, and fever.23
In summary, in this clinical group, lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome should be considered when patients present with spontaneous pneumothorax; those with Birt-Hogg-Dubé syndrome also present with skin lesions or renal cancer. In patients with progressive dyspnea and cough, lymphocytic interstitial pneumonia should be considered in those with a known history of connective tissue disease or immunodeficiency. Pulmonary Langerhans cell histiocytosis typically presents at a younger age (20 to 30 years old) than desquamative interstitial pneumonia (smokers in their 40s). Making the distinction, however, will likely require imaging with computed tomography.
Incidentally found cysts or recurrent pneumonia
Incidentally found cysts or recurrent pneumonia can be manifestations of congenital pulmonary airway malformation, pulmonary sequestration, or bronchogenic cyst.
Congenital pulmonary airway malformation, of which there are five types, is the most common pulmonary congenital abnormality. It accounts for up to 95% of cases of congenital cystic lung disease.24,25 About 85% of cases are detected in the prenatal or perinatal periods.26 Late-onset congenital pulmonary airway malformation (arising in childhood to adulthood) presents with recurrent pneumonia in about 75% of cases and can be misdiagnosed as lung abscess, pulmonary tuberculosis, or bronchiectasis.27
Pulmonary sequestration, the second most common pulmonary congenital abnormality, is characterized by a portion of lung that does not connect to the tracheobronchial tree and has its own systemic arterial supply.24 Intralobar sequestration, which shares the pleural investment with normal lung, accounts for about 80% of cases of pulmonary sequestration.28–30 In addition to signs or symptoms of pulmonary infection, patients with pulmonary sequestration can remain asymp-
tomatic (about 25% of cases), or can present with hemoptysis or hemothorax.28–30 In adults, the typical age at presentation is between 20 and 25.29,30
Bronchogenic cyst is usually life-threatening in children. In adults, it commonly causes cough and chest pain.31 Hemoptysis, dysphagia, hoarseness, and diaphragmatic paralysis can also occur.32,33 The mean age at diagnosis in adults is 35 to 40.31,32
In summary, most cases of recurrent pneumonia with cysts are due to congenital pulmonary airway malformation. Pulmonary sequestration is the second most common cause of cystic lung disease in this group. Bronchogenic cyst is usually fatal in fetal development; smaller cysts can go unnoticed during the earlier years and are later found incidentally as imaging abnormalities in adults.
Signs and symptoms of primary pulmonary infections
Signs and symptoms of primary pulmonary infections can be due to Pneumocystis jirovecii pneumonia or echinococcal infections.
P jirovecii pneumonia commonly develops in patients with human immunodeficiency virus infection and low CD4 counts, recipients of hematologic or solid-organ transplants, and those receiving immunosuppressive therapy (eg, glucocorticoids or chemotherapy).
Echinococcal infections (with Echinococcus granulosus or multilocularis species) are more common in less-developed countries such as those in South America or the Middle East, in China, or in patients who have traveled to endemic areas.34
In summary, cystic lung disease in patients with primary pulmonary infections can be diagnosed by the patient’s clinical history and risk factors for infections. Those with human immunodeficiency virus infection and other causes of immunodeficiency are predisposed to P jirovecii pneumonia. Echinococcal infections occur in those with a history of travel to an endemic area.
Primarily nonpulmonary signs and symptoms
If the patient has primarily nonpulmonary signs and symptoms, think about pulmonary amyloidosis, light chain deposition disease, and neurofibromatosis type 1.
Pulmonary amyloidosis has a variety of manifestations, including tracheobronchial disease, nodular parenchymal disease, diffuse or alveolar septal pattern, pleural disease, lymphadenopathy, and pulmonary cysts.4
Light chain deposition disease shares some clinical features with amyloidosis. However, the light chain fragments in this disease do not form amyloid fibrils and therefore do not stain positively with Congo red. The kidney is the most commonly involved organ.4
Neurofibromatosis type 1 is characterized by collections of neurofibromas, café-au-lait spots, and pigmented hamartomas in the iris (Lisch nodules).35
In summary, patients in this group typically present with complications related to systemic involvement. Those with neurofibromatosis type 1 present with ophthalmologic, dermatologic, and neurologic manifestations. Amyloidosis and light chain deposition disease most commonly involve the renal system; their distinction will likely require tissue biopsy and Congo-red staining.
STEP 3: CHARACTERIZE THE RADIOGRAPHIC FEATURES
Characterization of pulmonary cysts and their distribution plays a key role in the diagnosis. Radiographically, cystic lung diseases can be subclassified into two major categories according to their cystic distribution:
- Discrete (focal or multifocal)
- Diffuse (unilobular or panlobular).2,3
Discrete cystic lung diseases include congenital abnormalities, infectious diseases, and interstitial pneumonias.2,3
Diffuse, panlobular cystic lung diseases include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, Birt-Hogg-Dubé syndrome, amyloidosis, light chain deposition disease, and neurofibromatosis type 1.7,13,36–39
In addition, other associated radiographic findings play a major role in diagnosis.
Cysts in patients presenting with insidious dyspnea or spontaneous pneumothorax
Lymphangioleiomyomatosis. Cysts are seen in nearly all cases of advanced lymphangioleiomyomatosis, typically in a diffuse pattern, varying from 2 mm to 40 mm in diameter, and uniform in shape (Figure 2A).7,8,40–42
Other radiographic features include vessels located at the periphery of the cysts (in contrast to the centrilobular pattern seen with emphysema), and chylous pleural effusions (in about 22% of patients).40 Nodules are typically not seen with lymphangioleiomyomatosis, and if found represent type 2 pneumocyte hyperplasia.
Pulmonary Langerhans cell histiocytosis. Nodules measuring 1 to 10 mm in diameter and favoring a centrilobular location are often seen on computed tomography. Pulmonary cysts occur in about 61% of patients.13,43 Cysts are variable in size and shape (Figure 2B), in contrast to their uniform appearance in lymphangioleiomyomatosis. Most cysts are less than 10 mm in diameter; however, they can be up to 80 mm.13,43 Early in its course, nodules may predominate in the upper and middle lobes. Over time, diffuse cysts become more common and can be difficult to differentiate from advanced smoking-induced emphysema.44
Birt-Hogg-Dubé syndrome. Approximately 70% to 100% of patients with Birt-Hogg-Dubé syndrome will have multiple pulmonary cysts detected on computed tomography. These cysts are characteristically basal and subpleural in location, with varying sizes and irregular shapes in otherwise normal lung parenchyma (Figure 2C).36,45,46
Desquamative interstitial pneumonia. Pulmonary cysts are present on computed tomography in about 32% of patients.47 They are usually round and less than 20 mm in diameter.48 Ground-glass opacity is present in almost all cases of desquamative interstitial pneumonia, with a diffuse pattern in 25% to 44% of patients.16,17,47
Pulmonary cysts occur in up to two-thirds of those with lymphocytic interstitial pneumonia. Cysts are usually multifocal and perivascular in distribution and have varying sizes and shapes (Figure 2D).22 Ground-glass opacity and poorly defined centrilobular nodules are also frequently seen. Other computed tomographic findings include thickening of the bronchovascular bundles, focal consolidation, interseptal lobular thickening, pleural thickening, and lymph node enlargement.22
In summary, in this group of patients, diffuse panlobular cysts are due to lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, or Birt-Hogg-Dubé syndrome. Cysts due to lymphangioleiomyomatosis have a diffuse distribution, while those due to pulmonary Langerhans cell histiocytosis tend to be upper-lobe-predominant and in the early stages are associated with stellate centrilobular nodules. Cysts in Birt-Hogg-Dubé syndrome tend to be subpleural and those due to lymphocytic interstitial pneumonia are perivascular in distribution.
Cysts that are incidentally found or occur in patients with recurrent pneumonia
Congenital pulmonary airway malformation types 1, 2, and 4 (Figure 3A, 3B). Cysts are typically discrete and focal or multifocal in distribution, but cases of multilobar and bilateral distribution have also been reported.27,49 The lower lobes are more often involved.49 Cysts vary in size and shape and can contain air, fluid, or both.27,49 Up to 50% of cases can occur in conjunction with pulmonary sequestration.50
Pulmonary sequestration displays an anomalous arterial supply on computed tomography (Figure 3C). Other imaging findings include mass lesions (49%), cystic lesions (29%), cavitary lesions (12%), and bronchiectasis.30 Air trapping can be seen in the adjacent lung. Lower lobe involvement accounts for more than 95% of total cases of sequestration.30 The cysts are usually discrete or focal in distribution. Misdiagnosis of pulmonary sequestration is common, and can include pulmonary abscess, pneumonia, bronchiectasis, and lung cancer.30
Bronchogenic cyst. Cyst contents generally demonstrate water attenuation, or higher attenuation if filled with proteinaceous/mucoid material or calcium deposits; air-fluid levels are seen in infected cysts.32 Intrapulmonary cysts have a predilection for the lower lobes and are usually discrete or focal in distribution.31,32 Mediastinal cysts are usually homogeneous, solitary, and located in the middle mediastinum.32 Cysts vary in size from 20 to 90 mm, with a mean diameter of 40 mm.31
In summary, in this group of cystic lung diseases, characteristic computed tomographic findings will suggest the diagnosis—air-filled cysts of varying sizes for congenital pulmonary airway malformation and anomalous vascular supply for pulmonary sequestration. Bronchogenic cysts will tend to have water or higher-than-water attenuation due to proteinaceous-mucoid material or calcium deposits.
Cysts in patients with signs and symptoms of primary pulmonary infections
P jirovecii pneumonia. Between 10% and 15% of patients have cysts, and about 18% present with spontaneous pneumothorax.51 Cysts in P jirovecii pneumonia vary in size from 15 to 85 mm in diameter and tend to occur in the upper lobes (Figure 4A).51,52
Echinococcal infection. Echinococcal pulmonary cysts typically are single and located more often in the lower lobes (Figure 4B).53,54 Cysts can be complicated by air-fluid levels, hydropneumothorax, or pneumothorax, or they can turn into cavitary lesions.
The diagnoses of these pulmonary infections are usually made by clinical and computed tomographic findings and depend less on detecting and characterizing lung cysts. Patients with P jirovecii pneumonia tend to have bilateral perihilar ground-glass opacities, while air-fluid levels suggest echinococcal infections. Cysts in this group of patients tend to be discrete or focal or multifocal in distribution, and vary in size.
Cysts in patients with primarily nonpulmonary signs and symptoms
Amyloidosis. Cyst formation is rare in amyloidosis.4 When present, cysts can be diffuse and scattered in distribution, in varying sizes (usually < 30 mm in diameter) and irregular shapes (Figure 5).55,56
Pulmonary light chain deposition disease usually presents as linear opacities and small nodules on chest computed tomography. Numerous cysts that are diffuse in distribution and have no topographic predominance can also be present. They can progress in number and size and coalesce to form irregular shapes.57
Neurofibromatosis type 1. In neurofibromatosis type 1, the most common radiographic presentations are bibasilar reticular opacities (50%), bullae (50%), and ground glass opacities (37%).58 Well-formed cysts occur in up to 25% of patients and tend to be diffuse and smaller (2 to 18 mm in diameter), with upper lobe predominance.58,59
In summary, in this group of patients, bibasilar reticular and ground-glass opacities suggest neurofibromatosis type 1, while nodules and linear opacities suggest amyloidosis or light chain deposition disease. Cysts tend to be diffuse with varying sizes.
STEP 4: PUT IT ALL TOGETHER
Diagnosis in insidious dyspnea or spontaneous pneumothorax
For patients who present with insidious dyspnea or spontaneous pneumothorax, the diagnosis of cystic lung disease can be made by characterizing the distribution, size, and shape of the cysts (Table 3).
Diffuse, panlobular distribution. Cystic lung diseases with this pattern include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, and Birt-Hogg-Dubé syndrome. In this group, cysts that are uniform in size and regular in shape are invariably due to lymphangioleiomyomatosis. Those with variable size and irregular shapes can be due to pulmonary Langerhans cell histiocytosis or Birt-Hogg-Dubé syndrome. Patients with pulmonary Langerhans cell histiocytosis tend to be smokers and their cysts tend to be upper- lobe-predominant. Those with Birt-Hogg-Dubé syndrome will likely have renal cancer or skin lesions; their cysts tend to be basilar and subpleural in distribution.
Cysts that are focal or multifocal and unilobular are due to lymphocytic interstitial pneumonia or desquamative interstitial pneumonia. Patients with lymphocytic interstitial pneumonia tend to have underlying connective tissue disease; those with desquamative interstitial pneumonia are almost always smokers. The definitive diagnosis for lymphocytic interstitial pneumonia or desquamative interstitial pneumonia can require a tissue biopsy.
Diagnosis in patients with incidentally found cysts or recurrent pneumonia
In those who present with incidentally found cysts or recurrent pneumonia, suspicion for a congenital lung malformation should be raised. Patients with a type 1, 2, or 4 congenital pulmonary airway malformation typically have air-filled cysts in varying sizes; those with pulmonary sequestration have an anomalous arterial supply in addition to cysts that are usually located in the lower lobes. Bronchogenic cysts tend to be larger, with attenuation equal to or greater than that of water, and distinguishing them from congenital pulmonary airway malformation will likely require surgical examination.
Diagnosis in patients with signs and symptoms of pulmonary infections
Patients with signs and symptoms of pulmonary infections should be investigated according to clinical risk factors for P jirovecii pneumonia or echinococcal infections.
Diagnosis in patients with primarily nonpulmonary presentations
The distinction between amyloidosis and neurofibromatosis type 1 can be made by the history and the clinical examination. However, a definitive diagnosis of amyloidosis or light chain deposition disease requires tissue examination for the presence or absence of amyloid fibrils.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722.
- Cosgrove GP, Frankel SK, Brown KK. Challenges in pulmonary fibrosis. 3: cystic lung disease. Thorax 2007; 62:820–829.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc 2003; 78:744–752.
- Ryu JH, Tian X, Baqir M, Xu K. Diffuse cystic lung diseases. Front Med 2013; 7:316–327.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Johnson SR, Cordier JF, Lazor R, et al; Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010; 35:14–26.
- Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med 1990; 323:1254–1260.
- Chu SC, Horiba K, Usuki J. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest 1999; 115:1041–1052.
- Graham RB, Nolasco M, Peterlin B, Garcia CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med 2005; 172:39–44.
- Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977; 113:1674–1677.
- Sundar KM, Gosselin MV, Chung HL, Cahill BC. Pulmonary Langerhans cell histiocytosis: emerging concepts in pathobiology, radiology, and clinical evolution of disease. Chest 2003; 123:1673–1683.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med 2002; 346:484–490.
- Mendez JL, Nadrous HF, Vassallo R, Decker PA, Ryu JH. Pneumothorax in pulmonary Langerhans cell histiocytosis. Chest 2004; 125:1028–1032.
- Carrington CB, Gaensler EA, Coutu RE, FitzGerald MX, Gupta RG. Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med 1978; 298:801–809.
- Ryu JH, Myers JL, Capizzi SA, Douglas WW, Vassallo R, Decker PA. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005; 127:178–184.
- Lynch DA, Travis WD, Müller NL, et al. Idiopathic interstitial pneumonias: CT features. Radiology 2005; 236:10–21.
- Strimlan CV, Rosenow EC 3rd, Weiland LH, Brown LR. Lymphocytic interstitial pneumonitis. Review of 13 cases. Ann Intern Med 1978; 88:616–621.
- Arish N, Eldor R, Fellig Y, et al. Lymphocytic interstitial pneumonia associated with common variable immunodeficiency resolved with intravenous immunoglobulins. Thorax 2006; 61:1096–1097.
- Schooley RT, Carey RW, Miller G, et al. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis. Clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med 1986; 104:636–643.
- Kramer MR, Saldana MJ, Ramos M, Pitchenik AE. High titers of Epstein-Barr virus antibodies in adult patients with lymphocytic interstitial pneumonitis associated with AIDS. Respir Med 1992; 86:49–52.
- Johkoh T, Müller NL, Pickford HA, et al. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1999; 212:567–572.
- Swigris JJ, Berry GJ, Raffin TA, Kuschner WG. Lymphoid interstitial pneumonia: a narrative review. Chest 2002; 122:2150–2164.
- Biyyam DR, Chapman T, Ferguson MR, Deutsch G, Dighe MK. Congenital lung abnormalities: embryologic features, prenatal diagnosis, and postnatal radiologic-pathologic correlation. Radiographics 2010; 30:1721–1738.
- Cloutier MM, Schaeffer DA, Hight D. Congenital cystic adenomatoid malformation. Chest 1993; 103:761–764.
- Luján M, Bosque M, Mirapeix RM, Marco MT, Asensio O, Domingo C. Late-onset congenital cystic adenomatoid malformation of the lung. Embryology, clinical symptomatology, diagnostic procedures, therapeutic approach and clinical follow-up. Respiration 2002; 69:148–154.
- Oh BJ, Lee JS, Kim JS, Lim CM, Koh Y. Congenital cystic adenomatoid malformation of the lung in adults: clinical and CT evaluation of seven patients. Respirology 2006; 11:496–501.
- Tsolakis CC, Kollias VD, Panayotopoulos PP. Pulmonary sequestration. Experience with eight consecutive cases. Scand Cardiovasc J 1997; 31:229–232.
- Sauvanet A, Regnard JF, Calanducci F, Rojas-Miranda A, Dartevelle P, Levasseur P. Pulmonary sequestration. Surgical aspects based on 61 cases. Rev Pneumol Clin 1991; 47:126–132. Article in French.
- Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2,625 cases in China. Eur J Cardiothorac Surg 2011; 40:e39–e42.
- Patel SR, Meeker DP, Biscotti CV, Kirby TJ, Rice TW. Presentation and management of bronchogenic cysts in the adult. Chest 1994; 106:79–85.
- Limaïem F, Ayadi-Kaddour A, Djilani H, Kilani T, El Mezni F. Pulmonary and mediastinal bronchogenic cysts: a clinicopathologic study of 33 cases. Lung 2008; 186:55–61.
- Liu HS, Li SQ, Cao ZL, Zhang ZY, Ren H. Clinical features and treatment of bronchogenic cyst in adults. Chin Med Sci J 2009; 24:60–63.
- Jenkins DJ, Romig T, Thompson RC. Emergence/re-emergence of Echinococcus spp.—a global update. Int J Parasitol 2005; 35:1205–1219.
- Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J Med 1981; 305:1617–1627.
- Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med 2007; 175:1044–1053.
- Biko DM, Schwartz M, Anupindi SA, Altes TA. Subpleural lung cysts in Down syndrome: prevalence and association with coexisting diagnoses. Pediatr Radiol 2008; 38:280–284.
- Colombat M, Stern M, Groussard O, et al. Pulmonary cystic disorder related to light chain deposition disease. Am J Respir Crit Care Med 2006; 173:777–780.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Johnson SR, Tattersfield AE. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax 2000; 55:1052–1057.
- Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med 1995; 151:527–533.
- Urban T, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P). Medicine (Baltimore) 1999; 78:321–337.
- Schönfeld N, Frank W, Wenig S, et al. Clinical and radiologic features, lung function and therapeutic results in pulmonary histiocytosis X. Respiration 1993; 60:38–44.
- Lacronique J, Roth C, Battesti JP, Basset F, Chretien J. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax 1982; 37:104–109.
- Kluger N, Giraud S, Coupier I, et al. Birt-Hogg-Dubé syndrome: clinical and genetic studies of 10 French families. Br J Dermatol 2010; 162:527–537.
- Tobino K, Gunji Y, Kurihara M, et al. Characteristics of pulmonary cysts in Birt-Hogg-Dubé syndrome: thin-section CT findings of the chest in 12 patients. Eur J Radiol 2011; 77:403–409.
- Hartman TE, Primack SL, Swensen SJ, Hansell D, McGuinness G, Müller NL. Desquamative interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1993; 187:787–790.
- Koyama M, Johkoh T, Honda O, et al. Chronic cystic lung disease: diagnostic accuracy of high-resolution CT in 92 patients. AJR Am J Roentgenol 2003; 180:827–835.
- Patz EF Jr, Müller NL, Swensen SJ, Dodd LG. Congenital cystic adenomatoid malformation in adults: CT findings. J Comput Assist Tomogr 1995; 19:361–364.
- Conran RM, Stocker JT. Extralobar sequestration with frequently associated congenital cystic adenomatoid malformation, type 2: report of 50 cases. Pediatr Dev Pathol 1999; 2:454–463.
- Kennedy CA, Goetz MB. Atypical roentgenographic manifestations of Pneumocystis carinii pneumonia. Arch Intern Med 1992; 152:1390–1398.
- Sandhu JS, Goodman PC. Pulmonary cysts associated with Pneumocystis carinii pneumonia in patients with AIDS. Radiology 1989; 173:33–35.
- Doğan R, Yüksel M, Cetin G, et al. Surgical treatment of hydatid cysts of the lung: report on 1,055 patients. Thorax 1989; 44:192–199.
- Salih OK, Topcuoğlu MS, Celik SK, Ulus T, Tokcan A. Surgical treatment of hydatid cysts of the lung: analysis of 405 patients. Can J Surg 1998; 41:131–135.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Sakai M, Yamaoka M, Kawaguchi M, Hizawa N, Sato Y. Multiple cystic pulmonary amyloidosis. Ann Thorac Surg 2011; 92:e109.
- Colombat M, Caudroy S, Lagonotte E, et al. Pathomechanisms of cyst formation in pulmonary light chain deposition disease. Eur Respir J 2008; 32:1399–1403.
- Zamora AC, Collard HR, Wolters PJ, Webb WR, King TE. Neurofibromatosis-associated lung disease: a case series and literature review. Eur Respir J 2007; 29:210–214.
- Oikonomou A, Vadikolias K, Birbilis T, Bouros D, Prassopoulos P. HRCT findings in the lungs of non-smokers with neurofibromatosis. Eur J Radiol 2011; 80:e520–e523.
Air-filled pulmonary lesions commonly detected on chest computed tomography. Cystic lung lesions should be distinguished from other air-filled lesions to facilitate diagnosis. Primary care physicians play an integral role in the recognition of cystic lung disease.
The differential diagnosis of cystic lung disease is broad and includes isolated pulmonary, systemic, infectious, and congenital etiologies.
Here, we aim to provide a systematic, stepwise approach to help differentiate among the various cystic lung diseases and devise an algorithm for diagnosis. In doing so, we will discuss the clinical and radiographic features of many of these diseases:
- Lymphangioleiomyomatosis
- Birt-Hogg-Dubé syndrome
- Pulmonary Langerhans cell histiocytosis
- Interstitial pneumonia (desquamative interstitial pneumonia, lymphocytic interstitial pneumonia)
- Congenital cystic lung disease (congenital pulmonary airway malformation, pulmonary sequestration, bronchogenic cyst) Pulmonary infection
- Systemic disease (amyloidosis, light chain deposition disease, neurofibromatosis type 1).
STEP 1: RULE OUT CYST-MIMICS
A pulmonary cyst is a round, circumscribed space surrounded by an epithelial or fibrous wall of variable thickness.1 On chest radiography and computed tomography, a cyst appears as a round parenchymal lucency or low-attenuating area with a well-defined interface with normal lung.1 Cysts vary in wall thickness but usually have a thin wall (< 2 mm) and occur without associated pulmonary emphysema.1 They typically contain air but occasionally contain fluid or solid material.
A pulmonary cyst can be categorized as a bulla, bleb, or pneumatocele.
Bullae are larger than 1 cm in diameter, sharply demarcated by a thin wall, and usually accompanied by emphysematous changes in the adjacent lung.1
Blebs are no larger than 1 cm in diameter, are located within the visceral pleura or the subpleural space, and appear on computed tomography as thin-walled air spaces that are contiguous with the pleura.1 The distinction between a bleb and a bulla is of little clinical importance, and is often unnecessary.
Pneumatoceles are cysts that are frequently caused by acute pneumonia, trauma, or aspiration of hydrocarbon fluid, and are usually transient.1
Mimics of pulmonary cysts include pulmonary cavities, emphysema, loculated pneumothoraces, honeycomb lung, and bronchiectasis (Figure 1).2
Pulmonary cavities differ from cysts in that their walls are typically thicker (usually > 4 mm).3
Emphysema differs from cystic lung disease as it typically leads to focal areas or regions of decreased lung attenuation that do not have defined walls.1
Honeycombing refers to a cluster or row of cysts, 1 to 3 mm in wall thickness and typically 3 to 10 mm in diameter, that are associated with end-stage lung fibrosis.1 They are typically subpleural in distribution and are accompanied by fibrotic features such as reticulation and traction bronchiectasis.1
Bronchiectasis is dilation and distortion of bronchi and bronchioles and can be mistaken for cysts when viewed en face.1
Loculated pneumothoraces can also mimic pulmonary cysts, but they typically fail to adhere to a defined anatomic unit and are subpleural in distribution.
STEP 2: CHARACTERIZE THE CLINICAL PRESENTATION
Clinical signs and symptoms of cystic lung disease play a key role in diagnosis (Table 1). For instance, spontaneous pneumothorax is commonly associated with diffuse cystic lung disease (lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome), while insidious dyspnea, with or without associated pneumothorax, is usually associated with the interstitial pneumonias (lymphocytic interstitial pneumonia and desquamative interstitial pneumonia).
In addition, congenital abnormalities of the lung can lead to cyst formation. These abnormalities, especially when associated with other congenital abnormalities, are often diagnosed in the prenatal and perinatal periods. However, some remain undetected until incidentally found later in adulthood or if superimposing infection develops.
Primary pulmonary infections can also cause parenchymal necrosis, which in turn cavitates or forms cysts.4
Lastly, cystic lung diseases can occur as part of a multiorgan or systemic illness in which the lung is one of the organs involved. Although usually diagnosed before the discovery of cysts or manifestations of pulmonary symptoms, they can present as a diagnostic challenge, especially when lung cysts are the initial presentation.bsence of amyloid fibrils.
In view of the features of the different types of cystic lung disease, adults with cystic lung disease can be grouped according to their typical clinical presentations (Table 2):
- Insidious dyspnea or spontaneous pneumothorax
- Incidentally found cysts or recurrent pneumonia
- Signs and symptoms of primary pulmonary infection
- Signs and symptoms that are primarily nonpulmonary.
Insidious dyspnea or spontaneous pneumothorax
Insidious dyspnea or spontaneous pneumothorax can be manifestations of lymphangioleiomyomatosis, Birt-Hogg-Dubé syndrome, pulmonary Langerhans cell histiocytosis, desquamative interstitial pneumonia, or lymphocytic interstitial pneumonia.
Lymphangioleiomyomatosis is characterized by abnormal cellular proliferation within the lung, kidney, lymphatic system, or any combination.5 The peak prevalence is in the third to fourth decades of life, and most patients are women of childbearing age.6 In addition to progressive dyspnea on exertion and pneumothorax, other signs and symptoms include hemoptysis, nonproductive cough, chylous pleural effusion, and ascites.7,8
Birt-Hogg-Dubé syndrome is caused by germline mutations in the folliculin (FLCN) gene.9 It is characterized by skin fibrofolliculomas, pulmonary cysts, spontaneous pneumothorax, and renal cancer.10
Pulmonary Langerhans cell histiocytosis is part of the spectrum of Langerhans cell histiocytosis that, in addition to the lungs, can also involve the bone, pituitary gland, thyroid, skin, lymph nodes, and liver.11 It occurs almost exclusively in smokers, affecting individuals in their 20s and 30s, with no gender predilection.12,13 In addition to nonproductive cough and dyspnea, patients can also present with fever, anorexia, and weight loss,13 but approximately 25% of patients are asymptomatic.14
Desquamative interstitial pneumonia is an idiopathic interstitial pneumonia that, like pulmonary Langerhans cell histiocytosis, is seen almost exclusively in current or former smokers, who account for about 90% of patients with this disease. It affects almost twice as many men as women.15,16 The mean age at onset is 42 to 46.15,16 In addition to insidious cough and dyspnea, digital clubbing develops in 26% to 40% of patients.16,17
Lymphocytic interstitial pneumonia is another rare idiopathic pneumonia, usually associated with connective tissue disease, Sjögren syndrome, immunodeficiencies, and viral infections.18–21 It is more common in women, presenting between the 4th and 7th decades of life, with a mean age at diagnosis of 50 to 56.18,22 In addition to progressive dyspnea and cough, other symptoms include weight loss, pleuritic pain, arthralgias, fatigue, night sweats, and fever.23
In summary, in this clinical group, lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome should be considered when patients present with spontaneous pneumothorax; those with Birt-Hogg-Dubé syndrome also present with skin lesions or renal cancer. In patients with progressive dyspnea and cough, lymphocytic interstitial pneumonia should be considered in those with a known history of connective tissue disease or immunodeficiency. Pulmonary Langerhans cell histiocytosis typically presents at a younger age (20 to 30 years old) than desquamative interstitial pneumonia (smokers in their 40s). Making the distinction, however, will likely require imaging with computed tomography.
Incidentally found cysts or recurrent pneumonia
Incidentally found cysts or recurrent pneumonia can be manifestations of congenital pulmonary airway malformation, pulmonary sequestration, or bronchogenic cyst.
Congenital pulmonary airway malformation, of which there are five types, is the most common pulmonary congenital abnormality. It accounts for up to 95% of cases of congenital cystic lung disease.24,25 About 85% of cases are detected in the prenatal or perinatal periods.26 Late-onset congenital pulmonary airway malformation (arising in childhood to adulthood) presents with recurrent pneumonia in about 75% of cases and can be misdiagnosed as lung abscess, pulmonary tuberculosis, or bronchiectasis.27
Pulmonary sequestration, the second most common pulmonary congenital abnormality, is characterized by a portion of lung that does not connect to the tracheobronchial tree and has its own systemic arterial supply.24 Intralobar sequestration, which shares the pleural investment with normal lung, accounts for about 80% of cases of pulmonary sequestration.28–30 In addition to signs or symptoms of pulmonary infection, patients with pulmonary sequestration can remain asymp-
tomatic (about 25% of cases), or can present with hemoptysis or hemothorax.28–30 In adults, the typical age at presentation is between 20 and 25.29,30
Bronchogenic cyst is usually life-threatening in children. In adults, it commonly causes cough and chest pain.31 Hemoptysis, dysphagia, hoarseness, and diaphragmatic paralysis can also occur.32,33 The mean age at diagnosis in adults is 35 to 40.31,32
In summary, most cases of recurrent pneumonia with cysts are due to congenital pulmonary airway malformation. Pulmonary sequestration is the second most common cause of cystic lung disease in this group. Bronchogenic cyst is usually fatal in fetal development; smaller cysts can go unnoticed during the earlier years and are later found incidentally as imaging abnormalities in adults.
Signs and symptoms of primary pulmonary infections
Signs and symptoms of primary pulmonary infections can be due to Pneumocystis jirovecii pneumonia or echinococcal infections.
P jirovecii pneumonia commonly develops in patients with human immunodeficiency virus infection and low CD4 counts, recipients of hematologic or solid-organ transplants, and those receiving immunosuppressive therapy (eg, glucocorticoids or chemotherapy).
Echinococcal infections (with Echinococcus granulosus or multilocularis species) are more common in less-developed countries such as those in South America or the Middle East, in China, or in patients who have traveled to endemic areas.34
In summary, cystic lung disease in patients with primary pulmonary infections can be diagnosed by the patient’s clinical history and risk factors for infections. Those with human immunodeficiency virus infection and other causes of immunodeficiency are predisposed to P jirovecii pneumonia. Echinococcal infections occur in those with a history of travel to an endemic area.
Primarily nonpulmonary signs and symptoms
If the patient has primarily nonpulmonary signs and symptoms, think about pulmonary amyloidosis, light chain deposition disease, and neurofibromatosis type 1.
Pulmonary amyloidosis has a variety of manifestations, including tracheobronchial disease, nodular parenchymal disease, diffuse or alveolar septal pattern, pleural disease, lymphadenopathy, and pulmonary cysts.4
Light chain deposition disease shares some clinical features with amyloidosis. However, the light chain fragments in this disease do not form amyloid fibrils and therefore do not stain positively with Congo red. The kidney is the most commonly involved organ.4
Neurofibromatosis type 1 is characterized by collections of neurofibromas, café-au-lait spots, and pigmented hamartomas in the iris (Lisch nodules).35
In summary, patients in this group typically present with complications related to systemic involvement. Those with neurofibromatosis type 1 present with ophthalmologic, dermatologic, and neurologic manifestations. Amyloidosis and light chain deposition disease most commonly involve the renal system; their distinction will likely require tissue biopsy and Congo-red staining.
STEP 3: CHARACTERIZE THE RADIOGRAPHIC FEATURES
Characterization of pulmonary cysts and their distribution plays a key role in the diagnosis. Radiographically, cystic lung diseases can be subclassified into two major categories according to their cystic distribution:
- Discrete (focal or multifocal)
- Diffuse (unilobular or panlobular).2,3
Discrete cystic lung diseases include congenital abnormalities, infectious diseases, and interstitial pneumonias.2,3
Diffuse, panlobular cystic lung diseases include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, Birt-Hogg-Dubé syndrome, amyloidosis, light chain deposition disease, and neurofibromatosis type 1.7,13,36–39
In addition, other associated radiographic findings play a major role in diagnosis.
Cysts in patients presenting with insidious dyspnea or spontaneous pneumothorax
Lymphangioleiomyomatosis. Cysts are seen in nearly all cases of advanced lymphangioleiomyomatosis, typically in a diffuse pattern, varying from 2 mm to 40 mm in diameter, and uniform in shape (Figure 2A).7,8,40–42
Other radiographic features include vessels located at the periphery of the cysts (in contrast to the centrilobular pattern seen with emphysema), and chylous pleural effusions (in about 22% of patients).40 Nodules are typically not seen with lymphangioleiomyomatosis, and if found represent type 2 pneumocyte hyperplasia.
Pulmonary Langerhans cell histiocytosis. Nodules measuring 1 to 10 mm in diameter and favoring a centrilobular location are often seen on computed tomography. Pulmonary cysts occur in about 61% of patients.13,43 Cysts are variable in size and shape (Figure 2B), in contrast to their uniform appearance in lymphangioleiomyomatosis. Most cysts are less than 10 mm in diameter; however, they can be up to 80 mm.13,43 Early in its course, nodules may predominate in the upper and middle lobes. Over time, diffuse cysts become more common and can be difficult to differentiate from advanced smoking-induced emphysema.44
Birt-Hogg-Dubé syndrome. Approximately 70% to 100% of patients with Birt-Hogg-Dubé syndrome will have multiple pulmonary cysts detected on computed tomography. These cysts are characteristically basal and subpleural in location, with varying sizes and irregular shapes in otherwise normal lung parenchyma (Figure 2C).36,45,46
Desquamative interstitial pneumonia. Pulmonary cysts are present on computed tomography in about 32% of patients.47 They are usually round and less than 20 mm in diameter.48 Ground-glass opacity is present in almost all cases of desquamative interstitial pneumonia, with a diffuse pattern in 25% to 44% of patients.16,17,47
Pulmonary cysts occur in up to two-thirds of those with lymphocytic interstitial pneumonia. Cysts are usually multifocal and perivascular in distribution and have varying sizes and shapes (Figure 2D).22 Ground-glass opacity and poorly defined centrilobular nodules are also frequently seen. Other computed tomographic findings include thickening of the bronchovascular bundles, focal consolidation, interseptal lobular thickening, pleural thickening, and lymph node enlargement.22
In summary, in this group of patients, diffuse panlobular cysts are due to lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, or Birt-Hogg-Dubé syndrome. Cysts due to lymphangioleiomyomatosis have a diffuse distribution, while those due to pulmonary Langerhans cell histiocytosis tend to be upper-lobe-predominant and in the early stages are associated with stellate centrilobular nodules. Cysts in Birt-Hogg-Dubé syndrome tend to be subpleural and those due to lymphocytic interstitial pneumonia are perivascular in distribution.
Cysts that are incidentally found or occur in patients with recurrent pneumonia
Congenital pulmonary airway malformation types 1, 2, and 4 (Figure 3A, 3B). Cysts are typically discrete and focal or multifocal in distribution, but cases of multilobar and bilateral distribution have also been reported.27,49 The lower lobes are more often involved.49 Cysts vary in size and shape and can contain air, fluid, or both.27,49 Up to 50% of cases can occur in conjunction with pulmonary sequestration.50
Pulmonary sequestration displays an anomalous arterial supply on computed tomography (Figure 3C). Other imaging findings include mass lesions (49%), cystic lesions (29%), cavitary lesions (12%), and bronchiectasis.30 Air trapping can be seen in the adjacent lung. Lower lobe involvement accounts for more than 95% of total cases of sequestration.30 The cysts are usually discrete or focal in distribution. Misdiagnosis of pulmonary sequestration is common, and can include pulmonary abscess, pneumonia, bronchiectasis, and lung cancer.30
Bronchogenic cyst. Cyst contents generally demonstrate water attenuation, or higher attenuation if filled with proteinaceous/mucoid material or calcium deposits; air-fluid levels are seen in infected cysts.32 Intrapulmonary cysts have a predilection for the lower lobes and are usually discrete or focal in distribution.31,32 Mediastinal cysts are usually homogeneous, solitary, and located in the middle mediastinum.32 Cysts vary in size from 20 to 90 mm, with a mean diameter of 40 mm.31
In summary, in this group of cystic lung diseases, characteristic computed tomographic findings will suggest the diagnosis—air-filled cysts of varying sizes for congenital pulmonary airway malformation and anomalous vascular supply for pulmonary sequestration. Bronchogenic cysts will tend to have water or higher-than-water attenuation due to proteinaceous-mucoid material or calcium deposits.
Cysts in patients with signs and symptoms of primary pulmonary infections
P jirovecii pneumonia. Between 10% and 15% of patients have cysts, and about 18% present with spontaneous pneumothorax.51 Cysts in P jirovecii pneumonia vary in size from 15 to 85 mm in diameter and tend to occur in the upper lobes (Figure 4A).51,52
Echinococcal infection. Echinococcal pulmonary cysts typically are single and located more often in the lower lobes (Figure 4B).53,54 Cysts can be complicated by air-fluid levels, hydropneumothorax, or pneumothorax, or they can turn into cavitary lesions.
The diagnoses of these pulmonary infections are usually made by clinical and computed tomographic findings and depend less on detecting and characterizing lung cysts. Patients with P jirovecii pneumonia tend to have bilateral perihilar ground-glass opacities, while air-fluid levels suggest echinococcal infections. Cysts in this group of patients tend to be discrete or focal or multifocal in distribution, and vary in size.
Cysts in patients with primarily nonpulmonary signs and symptoms
Amyloidosis. Cyst formation is rare in amyloidosis.4 When present, cysts can be diffuse and scattered in distribution, in varying sizes (usually < 30 mm in diameter) and irregular shapes (Figure 5).55,56
Pulmonary light chain deposition disease usually presents as linear opacities and small nodules on chest computed tomography. Numerous cysts that are diffuse in distribution and have no topographic predominance can also be present. They can progress in number and size and coalesce to form irregular shapes.57
Neurofibromatosis type 1. In neurofibromatosis type 1, the most common radiographic presentations are bibasilar reticular opacities (50%), bullae (50%), and ground glass opacities (37%).58 Well-formed cysts occur in up to 25% of patients and tend to be diffuse and smaller (2 to 18 mm in diameter), with upper lobe predominance.58,59
In summary, in this group of patients, bibasilar reticular and ground-glass opacities suggest neurofibromatosis type 1, while nodules and linear opacities suggest amyloidosis or light chain deposition disease. Cysts tend to be diffuse with varying sizes.
STEP 4: PUT IT ALL TOGETHER
Diagnosis in insidious dyspnea or spontaneous pneumothorax
For patients who present with insidious dyspnea or spontaneous pneumothorax, the diagnosis of cystic lung disease can be made by characterizing the distribution, size, and shape of the cysts (Table 3).
Diffuse, panlobular distribution. Cystic lung diseases with this pattern include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, and Birt-Hogg-Dubé syndrome. In this group, cysts that are uniform in size and regular in shape are invariably due to lymphangioleiomyomatosis. Those with variable size and irregular shapes can be due to pulmonary Langerhans cell histiocytosis or Birt-Hogg-Dubé syndrome. Patients with pulmonary Langerhans cell histiocytosis tend to be smokers and their cysts tend to be upper- lobe-predominant. Those with Birt-Hogg-Dubé syndrome will likely have renal cancer or skin lesions; their cysts tend to be basilar and subpleural in distribution.
Cysts that are focal or multifocal and unilobular are due to lymphocytic interstitial pneumonia or desquamative interstitial pneumonia. Patients with lymphocytic interstitial pneumonia tend to have underlying connective tissue disease; those with desquamative interstitial pneumonia are almost always smokers. The definitive diagnosis for lymphocytic interstitial pneumonia or desquamative interstitial pneumonia can require a tissue biopsy.
Diagnosis in patients with incidentally found cysts or recurrent pneumonia
In those who present with incidentally found cysts or recurrent pneumonia, suspicion for a congenital lung malformation should be raised. Patients with a type 1, 2, or 4 congenital pulmonary airway malformation typically have air-filled cysts in varying sizes; those with pulmonary sequestration have an anomalous arterial supply in addition to cysts that are usually located in the lower lobes. Bronchogenic cysts tend to be larger, with attenuation equal to or greater than that of water, and distinguishing them from congenital pulmonary airway malformation will likely require surgical examination.
Diagnosis in patients with signs and symptoms of pulmonary infections
Patients with signs and symptoms of pulmonary infections should be investigated according to clinical risk factors for P jirovecii pneumonia or echinococcal infections.
Diagnosis in patients with primarily nonpulmonary presentations
The distinction between amyloidosis and neurofibromatosis type 1 can be made by the history and the clinical examination. However, a definitive diagnosis of amyloidosis or light chain deposition disease requires tissue examination for the presence or absence of amyloid fibrils.
Air-filled pulmonary lesions commonly detected on chest computed tomography. Cystic lung lesions should be distinguished from other air-filled lesions to facilitate diagnosis. Primary care physicians play an integral role in the recognition of cystic lung disease.
The differential diagnosis of cystic lung disease is broad and includes isolated pulmonary, systemic, infectious, and congenital etiologies.
Here, we aim to provide a systematic, stepwise approach to help differentiate among the various cystic lung diseases and devise an algorithm for diagnosis. In doing so, we will discuss the clinical and radiographic features of many of these diseases:
- Lymphangioleiomyomatosis
- Birt-Hogg-Dubé syndrome
- Pulmonary Langerhans cell histiocytosis
- Interstitial pneumonia (desquamative interstitial pneumonia, lymphocytic interstitial pneumonia)
- Congenital cystic lung disease (congenital pulmonary airway malformation, pulmonary sequestration, bronchogenic cyst) Pulmonary infection
- Systemic disease (amyloidosis, light chain deposition disease, neurofibromatosis type 1).
STEP 1: RULE OUT CYST-MIMICS
A pulmonary cyst is a round, circumscribed space surrounded by an epithelial or fibrous wall of variable thickness.1 On chest radiography and computed tomography, a cyst appears as a round parenchymal lucency or low-attenuating area with a well-defined interface with normal lung.1 Cysts vary in wall thickness but usually have a thin wall (< 2 mm) and occur without associated pulmonary emphysema.1 They typically contain air but occasionally contain fluid or solid material.
A pulmonary cyst can be categorized as a bulla, bleb, or pneumatocele.
Bullae are larger than 1 cm in diameter, sharply demarcated by a thin wall, and usually accompanied by emphysematous changes in the adjacent lung.1
Blebs are no larger than 1 cm in diameter, are located within the visceral pleura or the subpleural space, and appear on computed tomography as thin-walled air spaces that are contiguous with the pleura.1 The distinction between a bleb and a bulla is of little clinical importance, and is often unnecessary.
Pneumatoceles are cysts that are frequently caused by acute pneumonia, trauma, or aspiration of hydrocarbon fluid, and are usually transient.1
Mimics of pulmonary cysts include pulmonary cavities, emphysema, loculated pneumothoraces, honeycomb lung, and bronchiectasis (Figure 1).2
Pulmonary cavities differ from cysts in that their walls are typically thicker (usually > 4 mm).3
Emphysema differs from cystic lung disease as it typically leads to focal areas or regions of decreased lung attenuation that do not have defined walls.1
Honeycombing refers to a cluster or row of cysts, 1 to 3 mm in wall thickness and typically 3 to 10 mm in diameter, that are associated with end-stage lung fibrosis.1 They are typically subpleural in distribution and are accompanied by fibrotic features such as reticulation and traction bronchiectasis.1
Bronchiectasis is dilation and distortion of bronchi and bronchioles and can be mistaken for cysts when viewed en face.1
Loculated pneumothoraces can also mimic pulmonary cysts, but they typically fail to adhere to a defined anatomic unit and are subpleural in distribution.
STEP 2: CHARACTERIZE THE CLINICAL PRESENTATION
Clinical signs and symptoms of cystic lung disease play a key role in diagnosis (Table 1). For instance, spontaneous pneumothorax is commonly associated with diffuse cystic lung disease (lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome), while insidious dyspnea, with or without associated pneumothorax, is usually associated with the interstitial pneumonias (lymphocytic interstitial pneumonia and desquamative interstitial pneumonia).
In addition, congenital abnormalities of the lung can lead to cyst formation. These abnormalities, especially when associated with other congenital abnormalities, are often diagnosed in the prenatal and perinatal periods. However, some remain undetected until incidentally found later in adulthood or if superimposing infection develops.
Primary pulmonary infections can also cause parenchymal necrosis, which in turn cavitates or forms cysts.4
Lastly, cystic lung diseases can occur as part of a multiorgan or systemic illness in which the lung is one of the organs involved. Although usually diagnosed before the discovery of cysts or manifestations of pulmonary symptoms, they can present as a diagnostic challenge, especially when lung cysts are the initial presentation.bsence of amyloid fibrils.
In view of the features of the different types of cystic lung disease, adults with cystic lung disease can be grouped according to their typical clinical presentations (Table 2):
- Insidious dyspnea or spontaneous pneumothorax
- Incidentally found cysts or recurrent pneumonia
- Signs and symptoms of primary pulmonary infection
- Signs and symptoms that are primarily nonpulmonary.
Insidious dyspnea or spontaneous pneumothorax
Insidious dyspnea or spontaneous pneumothorax can be manifestations of lymphangioleiomyomatosis, Birt-Hogg-Dubé syndrome, pulmonary Langerhans cell histiocytosis, desquamative interstitial pneumonia, or lymphocytic interstitial pneumonia.
Lymphangioleiomyomatosis is characterized by abnormal cellular proliferation within the lung, kidney, lymphatic system, or any combination.5 The peak prevalence is in the third to fourth decades of life, and most patients are women of childbearing age.6 In addition to progressive dyspnea on exertion and pneumothorax, other signs and symptoms include hemoptysis, nonproductive cough, chylous pleural effusion, and ascites.7,8
Birt-Hogg-Dubé syndrome is caused by germline mutations in the folliculin (FLCN) gene.9 It is characterized by skin fibrofolliculomas, pulmonary cysts, spontaneous pneumothorax, and renal cancer.10
Pulmonary Langerhans cell histiocytosis is part of the spectrum of Langerhans cell histiocytosis that, in addition to the lungs, can also involve the bone, pituitary gland, thyroid, skin, lymph nodes, and liver.11 It occurs almost exclusively in smokers, affecting individuals in their 20s and 30s, with no gender predilection.12,13 In addition to nonproductive cough and dyspnea, patients can also present with fever, anorexia, and weight loss,13 but approximately 25% of patients are asymptomatic.14
Desquamative interstitial pneumonia is an idiopathic interstitial pneumonia that, like pulmonary Langerhans cell histiocytosis, is seen almost exclusively in current or former smokers, who account for about 90% of patients with this disease. It affects almost twice as many men as women.15,16 The mean age at onset is 42 to 46.15,16 In addition to insidious cough and dyspnea, digital clubbing develops in 26% to 40% of patients.16,17
Lymphocytic interstitial pneumonia is another rare idiopathic pneumonia, usually associated with connective tissue disease, Sjögren syndrome, immunodeficiencies, and viral infections.18–21 It is more common in women, presenting between the 4th and 7th decades of life, with a mean age at diagnosis of 50 to 56.18,22 In addition to progressive dyspnea and cough, other symptoms include weight loss, pleuritic pain, arthralgias, fatigue, night sweats, and fever.23
In summary, in this clinical group, lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome should be considered when patients present with spontaneous pneumothorax; those with Birt-Hogg-Dubé syndrome also present with skin lesions or renal cancer. In patients with progressive dyspnea and cough, lymphocytic interstitial pneumonia should be considered in those with a known history of connective tissue disease or immunodeficiency. Pulmonary Langerhans cell histiocytosis typically presents at a younger age (20 to 30 years old) than desquamative interstitial pneumonia (smokers in their 40s). Making the distinction, however, will likely require imaging with computed tomography.
Incidentally found cysts or recurrent pneumonia
Incidentally found cysts or recurrent pneumonia can be manifestations of congenital pulmonary airway malformation, pulmonary sequestration, or bronchogenic cyst.
Congenital pulmonary airway malformation, of which there are five types, is the most common pulmonary congenital abnormality. It accounts for up to 95% of cases of congenital cystic lung disease.24,25 About 85% of cases are detected in the prenatal or perinatal periods.26 Late-onset congenital pulmonary airway malformation (arising in childhood to adulthood) presents with recurrent pneumonia in about 75% of cases and can be misdiagnosed as lung abscess, pulmonary tuberculosis, or bronchiectasis.27
Pulmonary sequestration, the second most common pulmonary congenital abnormality, is characterized by a portion of lung that does not connect to the tracheobronchial tree and has its own systemic arterial supply.24 Intralobar sequestration, which shares the pleural investment with normal lung, accounts for about 80% of cases of pulmonary sequestration.28–30 In addition to signs or symptoms of pulmonary infection, patients with pulmonary sequestration can remain asymp-
tomatic (about 25% of cases), or can present with hemoptysis or hemothorax.28–30 In adults, the typical age at presentation is between 20 and 25.29,30
Bronchogenic cyst is usually life-threatening in children. In adults, it commonly causes cough and chest pain.31 Hemoptysis, dysphagia, hoarseness, and diaphragmatic paralysis can also occur.32,33 The mean age at diagnosis in adults is 35 to 40.31,32
In summary, most cases of recurrent pneumonia with cysts are due to congenital pulmonary airway malformation. Pulmonary sequestration is the second most common cause of cystic lung disease in this group. Bronchogenic cyst is usually fatal in fetal development; smaller cysts can go unnoticed during the earlier years and are later found incidentally as imaging abnormalities in adults.
Signs and symptoms of primary pulmonary infections
Signs and symptoms of primary pulmonary infections can be due to Pneumocystis jirovecii pneumonia or echinococcal infections.
P jirovecii pneumonia commonly develops in patients with human immunodeficiency virus infection and low CD4 counts, recipients of hematologic or solid-organ transplants, and those receiving immunosuppressive therapy (eg, glucocorticoids or chemotherapy).
Echinococcal infections (with Echinococcus granulosus or multilocularis species) are more common in less-developed countries such as those in South America or the Middle East, in China, or in patients who have traveled to endemic areas.34
In summary, cystic lung disease in patients with primary pulmonary infections can be diagnosed by the patient’s clinical history and risk factors for infections. Those with human immunodeficiency virus infection and other causes of immunodeficiency are predisposed to P jirovecii pneumonia. Echinococcal infections occur in those with a history of travel to an endemic area.
Primarily nonpulmonary signs and symptoms
If the patient has primarily nonpulmonary signs and symptoms, think about pulmonary amyloidosis, light chain deposition disease, and neurofibromatosis type 1.
Pulmonary amyloidosis has a variety of manifestations, including tracheobronchial disease, nodular parenchymal disease, diffuse or alveolar septal pattern, pleural disease, lymphadenopathy, and pulmonary cysts.4
Light chain deposition disease shares some clinical features with amyloidosis. However, the light chain fragments in this disease do not form amyloid fibrils and therefore do not stain positively with Congo red. The kidney is the most commonly involved organ.4
Neurofibromatosis type 1 is characterized by collections of neurofibromas, café-au-lait spots, and pigmented hamartomas in the iris (Lisch nodules).35
In summary, patients in this group typically present with complications related to systemic involvement. Those with neurofibromatosis type 1 present with ophthalmologic, dermatologic, and neurologic manifestations. Amyloidosis and light chain deposition disease most commonly involve the renal system; their distinction will likely require tissue biopsy and Congo-red staining.
STEP 3: CHARACTERIZE THE RADIOGRAPHIC FEATURES
Characterization of pulmonary cysts and their distribution plays a key role in the diagnosis. Radiographically, cystic lung diseases can be subclassified into two major categories according to their cystic distribution:
- Discrete (focal or multifocal)
- Diffuse (unilobular or panlobular).2,3
Discrete cystic lung diseases include congenital abnormalities, infectious diseases, and interstitial pneumonias.2,3
Diffuse, panlobular cystic lung diseases include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, Birt-Hogg-Dubé syndrome, amyloidosis, light chain deposition disease, and neurofibromatosis type 1.7,13,36–39
In addition, other associated radiographic findings play a major role in diagnosis.
Cysts in patients presenting with insidious dyspnea or spontaneous pneumothorax
Lymphangioleiomyomatosis. Cysts are seen in nearly all cases of advanced lymphangioleiomyomatosis, typically in a diffuse pattern, varying from 2 mm to 40 mm in diameter, and uniform in shape (Figure 2A).7,8,40–42
Other radiographic features include vessels located at the periphery of the cysts (in contrast to the centrilobular pattern seen with emphysema), and chylous pleural effusions (in about 22% of patients).40 Nodules are typically not seen with lymphangioleiomyomatosis, and if found represent type 2 pneumocyte hyperplasia.
Pulmonary Langerhans cell histiocytosis. Nodules measuring 1 to 10 mm in diameter and favoring a centrilobular location are often seen on computed tomography. Pulmonary cysts occur in about 61% of patients.13,43 Cysts are variable in size and shape (Figure 2B), in contrast to their uniform appearance in lymphangioleiomyomatosis. Most cysts are less than 10 mm in diameter; however, they can be up to 80 mm.13,43 Early in its course, nodules may predominate in the upper and middle lobes. Over time, diffuse cysts become more common and can be difficult to differentiate from advanced smoking-induced emphysema.44
Birt-Hogg-Dubé syndrome. Approximately 70% to 100% of patients with Birt-Hogg-Dubé syndrome will have multiple pulmonary cysts detected on computed tomography. These cysts are characteristically basal and subpleural in location, with varying sizes and irregular shapes in otherwise normal lung parenchyma (Figure 2C).36,45,46
Desquamative interstitial pneumonia. Pulmonary cysts are present on computed tomography in about 32% of patients.47 They are usually round and less than 20 mm in diameter.48 Ground-glass opacity is present in almost all cases of desquamative interstitial pneumonia, with a diffuse pattern in 25% to 44% of patients.16,17,47
Pulmonary cysts occur in up to two-thirds of those with lymphocytic interstitial pneumonia. Cysts are usually multifocal and perivascular in distribution and have varying sizes and shapes (Figure 2D).22 Ground-glass opacity and poorly defined centrilobular nodules are also frequently seen. Other computed tomographic findings include thickening of the bronchovascular bundles, focal consolidation, interseptal lobular thickening, pleural thickening, and lymph node enlargement.22
In summary, in this group of patients, diffuse panlobular cysts are due to lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, or Birt-Hogg-Dubé syndrome. Cysts due to lymphangioleiomyomatosis have a diffuse distribution, while those due to pulmonary Langerhans cell histiocytosis tend to be upper-lobe-predominant and in the early stages are associated with stellate centrilobular nodules. Cysts in Birt-Hogg-Dubé syndrome tend to be subpleural and those due to lymphocytic interstitial pneumonia are perivascular in distribution.
Cysts that are incidentally found or occur in patients with recurrent pneumonia
Congenital pulmonary airway malformation types 1, 2, and 4 (Figure 3A, 3B). Cysts are typically discrete and focal or multifocal in distribution, but cases of multilobar and bilateral distribution have also been reported.27,49 The lower lobes are more often involved.49 Cysts vary in size and shape and can contain air, fluid, or both.27,49 Up to 50% of cases can occur in conjunction with pulmonary sequestration.50
Pulmonary sequestration displays an anomalous arterial supply on computed tomography (Figure 3C). Other imaging findings include mass lesions (49%), cystic lesions (29%), cavitary lesions (12%), and bronchiectasis.30 Air trapping can be seen in the adjacent lung. Lower lobe involvement accounts for more than 95% of total cases of sequestration.30 The cysts are usually discrete or focal in distribution. Misdiagnosis of pulmonary sequestration is common, and can include pulmonary abscess, pneumonia, bronchiectasis, and lung cancer.30
Bronchogenic cyst. Cyst contents generally demonstrate water attenuation, or higher attenuation if filled with proteinaceous/mucoid material or calcium deposits; air-fluid levels are seen in infected cysts.32 Intrapulmonary cysts have a predilection for the lower lobes and are usually discrete or focal in distribution.31,32 Mediastinal cysts are usually homogeneous, solitary, and located in the middle mediastinum.32 Cysts vary in size from 20 to 90 mm, with a mean diameter of 40 mm.31
In summary, in this group of cystic lung diseases, characteristic computed tomographic findings will suggest the diagnosis—air-filled cysts of varying sizes for congenital pulmonary airway malformation and anomalous vascular supply for pulmonary sequestration. Bronchogenic cysts will tend to have water or higher-than-water attenuation due to proteinaceous-mucoid material or calcium deposits.
Cysts in patients with signs and symptoms of primary pulmonary infections
P jirovecii pneumonia. Between 10% and 15% of patients have cysts, and about 18% present with spontaneous pneumothorax.51 Cysts in P jirovecii pneumonia vary in size from 15 to 85 mm in diameter and tend to occur in the upper lobes (Figure 4A).51,52
Echinococcal infection. Echinococcal pulmonary cysts typically are single and located more often in the lower lobes (Figure 4B).53,54 Cysts can be complicated by air-fluid levels, hydropneumothorax, or pneumothorax, or they can turn into cavitary lesions.
The diagnoses of these pulmonary infections are usually made by clinical and computed tomographic findings and depend less on detecting and characterizing lung cysts. Patients with P jirovecii pneumonia tend to have bilateral perihilar ground-glass opacities, while air-fluid levels suggest echinococcal infections. Cysts in this group of patients tend to be discrete or focal or multifocal in distribution, and vary in size.
Cysts in patients with primarily nonpulmonary signs and symptoms
Amyloidosis. Cyst formation is rare in amyloidosis.4 When present, cysts can be diffuse and scattered in distribution, in varying sizes (usually < 30 mm in diameter) and irregular shapes (Figure 5).55,56
Pulmonary light chain deposition disease usually presents as linear opacities and small nodules on chest computed tomography. Numerous cysts that are diffuse in distribution and have no topographic predominance can also be present. They can progress in number and size and coalesce to form irregular shapes.57
Neurofibromatosis type 1. In neurofibromatosis type 1, the most common radiographic presentations are bibasilar reticular opacities (50%), bullae (50%), and ground glass opacities (37%).58 Well-formed cysts occur in up to 25% of patients and tend to be diffuse and smaller (2 to 18 mm in diameter), with upper lobe predominance.58,59
In summary, in this group of patients, bibasilar reticular and ground-glass opacities suggest neurofibromatosis type 1, while nodules and linear opacities suggest amyloidosis or light chain deposition disease. Cysts tend to be diffuse with varying sizes.
STEP 4: PUT IT ALL TOGETHER
Diagnosis in insidious dyspnea or spontaneous pneumothorax
For patients who present with insidious dyspnea or spontaneous pneumothorax, the diagnosis of cystic lung disease can be made by characterizing the distribution, size, and shape of the cysts (Table 3).
Diffuse, panlobular distribution. Cystic lung diseases with this pattern include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, and Birt-Hogg-Dubé syndrome. In this group, cysts that are uniform in size and regular in shape are invariably due to lymphangioleiomyomatosis. Those with variable size and irregular shapes can be due to pulmonary Langerhans cell histiocytosis or Birt-Hogg-Dubé syndrome. Patients with pulmonary Langerhans cell histiocytosis tend to be smokers and their cysts tend to be upper- lobe-predominant. Those with Birt-Hogg-Dubé syndrome will likely have renal cancer or skin lesions; their cysts tend to be basilar and subpleural in distribution.
Cysts that are focal or multifocal and unilobular are due to lymphocytic interstitial pneumonia or desquamative interstitial pneumonia. Patients with lymphocytic interstitial pneumonia tend to have underlying connective tissue disease; those with desquamative interstitial pneumonia are almost always smokers. The definitive diagnosis for lymphocytic interstitial pneumonia or desquamative interstitial pneumonia can require a tissue biopsy.
Diagnosis in patients with incidentally found cysts or recurrent pneumonia
In those who present with incidentally found cysts or recurrent pneumonia, suspicion for a congenital lung malformation should be raised. Patients with a type 1, 2, or 4 congenital pulmonary airway malformation typically have air-filled cysts in varying sizes; those with pulmonary sequestration have an anomalous arterial supply in addition to cysts that are usually located in the lower lobes. Bronchogenic cysts tend to be larger, with attenuation equal to or greater than that of water, and distinguishing them from congenital pulmonary airway malformation will likely require surgical examination.
Diagnosis in patients with signs and symptoms of pulmonary infections
Patients with signs and symptoms of pulmonary infections should be investigated according to clinical risk factors for P jirovecii pneumonia or echinococcal infections.
Diagnosis in patients with primarily nonpulmonary presentations
The distinction between amyloidosis and neurofibromatosis type 1 can be made by the history and the clinical examination. However, a definitive diagnosis of amyloidosis or light chain deposition disease requires tissue examination for the presence or absence of amyloid fibrils.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722.
- Cosgrove GP, Frankel SK, Brown KK. Challenges in pulmonary fibrosis. 3: cystic lung disease. Thorax 2007; 62:820–829.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc 2003; 78:744–752.
- Ryu JH, Tian X, Baqir M, Xu K. Diffuse cystic lung diseases. Front Med 2013; 7:316–327.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Johnson SR, Cordier JF, Lazor R, et al; Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010; 35:14–26.
- Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med 1990; 323:1254–1260.
- Chu SC, Horiba K, Usuki J. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest 1999; 115:1041–1052.
- Graham RB, Nolasco M, Peterlin B, Garcia CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med 2005; 172:39–44.
- Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977; 113:1674–1677.
- Sundar KM, Gosselin MV, Chung HL, Cahill BC. Pulmonary Langerhans cell histiocytosis: emerging concepts in pathobiology, radiology, and clinical evolution of disease. Chest 2003; 123:1673–1683.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med 2002; 346:484–490.
- Mendez JL, Nadrous HF, Vassallo R, Decker PA, Ryu JH. Pneumothorax in pulmonary Langerhans cell histiocytosis. Chest 2004; 125:1028–1032.
- Carrington CB, Gaensler EA, Coutu RE, FitzGerald MX, Gupta RG. Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med 1978; 298:801–809.
- Ryu JH, Myers JL, Capizzi SA, Douglas WW, Vassallo R, Decker PA. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005; 127:178–184.
- Lynch DA, Travis WD, Müller NL, et al. Idiopathic interstitial pneumonias: CT features. Radiology 2005; 236:10–21.
- Strimlan CV, Rosenow EC 3rd, Weiland LH, Brown LR. Lymphocytic interstitial pneumonitis. Review of 13 cases. Ann Intern Med 1978; 88:616–621.
- Arish N, Eldor R, Fellig Y, et al. Lymphocytic interstitial pneumonia associated with common variable immunodeficiency resolved with intravenous immunoglobulins. Thorax 2006; 61:1096–1097.
- Schooley RT, Carey RW, Miller G, et al. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis. Clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med 1986; 104:636–643.
- Kramer MR, Saldana MJ, Ramos M, Pitchenik AE. High titers of Epstein-Barr virus antibodies in adult patients with lymphocytic interstitial pneumonitis associated with AIDS. Respir Med 1992; 86:49–52.
- Johkoh T, Müller NL, Pickford HA, et al. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1999; 212:567–572.
- Swigris JJ, Berry GJ, Raffin TA, Kuschner WG. Lymphoid interstitial pneumonia: a narrative review. Chest 2002; 122:2150–2164.
- Biyyam DR, Chapman T, Ferguson MR, Deutsch G, Dighe MK. Congenital lung abnormalities: embryologic features, prenatal diagnosis, and postnatal radiologic-pathologic correlation. Radiographics 2010; 30:1721–1738.
- Cloutier MM, Schaeffer DA, Hight D. Congenital cystic adenomatoid malformation. Chest 1993; 103:761–764.
- Luján M, Bosque M, Mirapeix RM, Marco MT, Asensio O, Domingo C. Late-onset congenital cystic adenomatoid malformation of the lung. Embryology, clinical symptomatology, diagnostic procedures, therapeutic approach and clinical follow-up. Respiration 2002; 69:148–154.
- Oh BJ, Lee JS, Kim JS, Lim CM, Koh Y. Congenital cystic adenomatoid malformation of the lung in adults: clinical and CT evaluation of seven patients. Respirology 2006; 11:496–501.
- Tsolakis CC, Kollias VD, Panayotopoulos PP. Pulmonary sequestration. Experience with eight consecutive cases. Scand Cardiovasc J 1997; 31:229–232.
- Sauvanet A, Regnard JF, Calanducci F, Rojas-Miranda A, Dartevelle P, Levasseur P. Pulmonary sequestration. Surgical aspects based on 61 cases. Rev Pneumol Clin 1991; 47:126–132. Article in French.
- Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2,625 cases in China. Eur J Cardiothorac Surg 2011; 40:e39–e42.
- Patel SR, Meeker DP, Biscotti CV, Kirby TJ, Rice TW. Presentation and management of bronchogenic cysts in the adult. Chest 1994; 106:79–85.
- Limaïem F, Ayadi-Kaddour A, Djilani H, Kilani T, El Mezni F. Pulmonary and mediastinal bronchogenic cysts: a clinicopathologic study of 33 cases. Lung 2008; 186:55–61.
- Liu HS, Li SQ, Cao ZL, Zhang ZY, Ren H. Clinical features and treatment of bronchogenic cyst in adults. Chin Med Sci J 2009; 24:60–63.
- Jenkins DJ, Romig T, Thompson RC. Emergence/re-emergence of Echinococcus spp.—a global update. Int J Parasitol 2005; 35:1205–1219.
- Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J Med 1981; 305:1617–1627.
- Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med 2007; 175:1044–1053.
- Biko DM, Schwartz M, Anupindi SA, Altes TA. Subpleural lung cysts in Down syndrome: prevalence and association with coexisting diagnoses. Pediatr Radiol 2008; 38:280–284.
- Colombat M, Stern M, Groussard O, et al. Pulmonary cystic disorder related to light chain deposition disease. Am J Respir Crit Care Med 2006; 173:777–780.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Johnson SR, Tattersfield AE. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax 2000; 55:1052–1057.
- Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med 1995; 151:527–533.
- Urban T, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P). Medicine (Baltimore) 1999; 78:321–337.
- Schönfeld N, Frank W, Wenig S, et al. Clinical and radiologic features, lung function and therapeutic results in pulmonary histiocytosis X. Respiration 1993; 60:38–44.
- Lacronique J, Roth C, Battesti JP, Basset F, Chretien J. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax 1982; 37:104–109.
- Kluger N, Giraud S, Coupier I, et al. Birt-Hogg-Dubé syndrome: clinical and genetic studies of 10 French families. Br J Dermatol 2010; 162:527–537.
- Tobino K, Gunji Y, Kurihara M, et al. Characteristics of pulmonary cysts in Birt-Hogg-Dubé syndrome: thin-section CT findings of the chest in 12 patients. Eur J Radiol 2011; 77:403–409.
- Hartman TE, Primack SL, Swensen SJ, Hansell D, McGuinness G, Müller NL. Desquamative interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1993; 187:787–790.
- Koyama M, Johkoh T, Honda O, et al. Chronic cystic lung disease: diagnostic accuracy of high-resolution CT in 92 patients. AJR Am J Roentgenol 2003; 180:827–835.
- Patz EF Jr, Müller NL, Swensen SJ, Dodd LG. Congenital cystic adenomatoid malformation in adults: CT findings. J Comput Assist Tomogr 1995; 19:361–364.
- Conran RM, Stocker JT. Extralobar sequestration with frequently associated congenital cystic adenomatoid malformation, type 2: report of 50 cases. Pediatr Dev Pathol 1999; 2:454–463.
- Kennedy CA, Goetz MB. Atypical roentgenographic manifestations of Pneumocystis carinii pneumonia. Arch Intern Med 1992; 152:1390–1398.
- Sandhu JS, Goodman PC. Pulmonary cysts associated with Pneumocystis carinii pneumonia in patients with AIDS. Radiology 1989; 173:33–35.
- Doğan R, Yüksel M, Cetin G, et al. Surgical treatment of hydatid cysts of the lung: report on 1,055 patients. Thorax 1989; 44:192–199.
- Salih OK, Topcuoğlu MS, Celik SK, Ulus T, Tokcan A. Surgical treatment of hydatid cysts of the lung: analysis of 405 patients. Can J Surg 1998; 41:131–135.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Sakai M, Yamaoka M, Kawaguchi M, Hizawa N, Sato Y. Multiple cystic pulmonary amyloidosis. Ann Thorac Surg 2011; 92:e109.
- Colombat M, Caudroy S, Lagonotte E, et al. Pathomechanisms of cyst formation in pulmonary light chain deposition disease. Eur Respir J 2008; 32:1399–1403.
- Zamora AC, Collard HR, Wolters PJ, Webb WR, King TE. Neurofibromatosis-associated lung disease: a case series and literature review. Eur Respir J 2007; 29:210–214.
- Oikonomou A, Vadikolias K, Birbilis T, Bouros D, Prassopoulos P. HRCT findings in the lungs of non-smokers with neurofibromatosis. Eur J Radiol 2011; 80:e520–e523.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722.
- Cosgrove GP, Frankel SK, Brown KK. Challenges in pulmonary fibrosis. 3: cystic lung disease. Thorax 2007; 62:820–829.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc 2003; 78:744–752.
- Ryu JH, Tian X, Baqir M, Xu K. Diffuse cystic lung diseases. Front Med 2013; 7:316–327.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Johnson SR, Cordier JF, Lazor R, et al; Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010; 35:14–26.
- Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med 1990; 323:1254–1260.
- Chu SC, Horiba K, Usuki J. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest 1999; 115:1041–1052.
- Graham RB, Nolasco M, Peterlin B, Garcia CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med 2005; 172:39–44.
- Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977; 113:1674–1677.
- Sundar KM, Gosselin MV, Chung HL, Cahill BC. Pulmonary Langerhans cell histiocytosis: emerging concepts in pathobiology, radiology, and clinical evolution of disease. Chest 2003; 123:1673–1683.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med 2002; 346:484–490.
- Mendez JL, Nadrous HF, Vassallo R, Decker PA, Ryu JH. Pneumothorax in pulmonary Langerhans cell histiocytosis. Chest 2004; 125:1028–1032.
- Carrington CB, Gaensler EA, Coutu RE, FitzGerald MX, Gupta RG. Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med 1978; 298:801–809.
- Ryu JH, Myers JL, Capizzi SA, Douglas WW, Vassallo R, Decker PA. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005; 127:178–184.
- Lynch DA, Travis WD, Müller NL, et al. Idiopathic interstitial pneumonias: CT features. Radiology 2005; 236:10–21.
- Strimlan CV, Rosenow EC 3rd, Weiland LH, Brown LR. Lymphocytic interstitial pneumonitis. Review of 13 cases. Ann Intern Med 1978; 88:616–621.
- Arish N, Eldor R, Fellig Y, et al. Lymphocytic interstitial pneumonia associated with common variable immunodeficiency resolved with intravenous immunoglobulins. Thorax 2006; 61:1096–1097.
- Schooley RT, Carey RW, Miller G, et al. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis. Clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med 1986; 104:636–643.
- Kramer MR, Saldana MJ, Ramos M, Pitchenik AE. High titers of Epstein-Barr virus antibodies in adult patients with lymphocytic interstitial pneumonitis associated with AIDS. Respir Med 1992; 86:49–52.
- Johkoh T, Müller NL, Pickford HA, et al. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1999; 212:567–572.
- Swigris JJ, Berry GJ, Raffin TA, Kuschner WG. Lymphoid interstitial pneumonia: a narrative review. Chest 2002; 122:2150–2164.
- Biyyam DR, Chapman T, Ferguson MR, Deutsch G, Dighe MK. Congenital lung abnormalities: embryologic features, prenatal diagnosis, and postnatal radiologic-pathologic correlation. Radiographics 2010; 30:1721–1738.
- Cloutier MM, Schaeffer DA, Hight D. Congenital cystic adenomatoid malformation. Chest 1993; 103:761–764.
- Luján M, Bosque M, Mirapeix RM, Marco MT, Asensio O, Domingo C. Late-onset congenital cystic adenomatoid malformation of the lung. Embryology, clinical symptomatology, diagnostic procedures, therapeutic approach and clinical follow-up. Respiration 2002; 69:148–154.
- Oh BJ, Lee JS, Kim JS, Lim CM, Koh Y. Congenital cystic adenomatoid malformation of the lung in adults: clinical and CT evaluation of seven patients. Respirology 2006; 11:496–501.
- Tsolakis CC, Kollias VD, Panayotopoulos PP. Pulmonary sequestration. Experience with eight consecutive cases. Scand Cardiovasc J 1997; 31:229–232.
- Sauvanet A, Regnard JF, Calanducci F, Rojas-Miranda A, Dartevelle P, Levasseur P. Pulmonary sequestration. Surgical aspects based on 61 cases. Rev Pneumol Clin 1991; 47:126–132. Article in French.
- Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2,625 cases in China. Eur J Cardiothorac Surg 2011; 40:e39–e42.
- Patel SR, Meeker DP, Biscotti CV, Kirby TJ, Rice TW. Presentation and management of bronchogenic cysts in the adult. Chest 1994; 106:79–85.
- Limaïem F, Ayadi-Kaddour A, Djilani H, Kilani T, El Mezni F. Pulmonary and mediastinal bronchogenic cysts: a clinicopathologic study of 33 cases. Lung 2008; 186:55–61.
- Liu HS, Li SQ, Cao ZL, Zhang ZY, Ren H. Clinical features and treatment of bronchogenic cyst in adults. Chin Med Sci J 2009; 24:60–63.
- Jenkins DJ, Romig T, Thompson RC. Emergence/re-emergence of Echinococcus spp.—a global update. Int J Parasitol 2005; 35:1205–1219.
- Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J Med 1981; 305:1617–1627.
- Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med 2007; 175:1044–1053.
- Biko DM, Schwartz M, Anupindi SA, Altes TA. Subpleural lung cysts in Down syndrome: prevalence and association with coexisting diagnoses. Pediatr Radiol 2008; 38:280–284.
- Colombat M, Stern M, Groussard O, et al. Pulmonary cystic disorder related to light chain deposition disease. Am J Respir Crit Care Med 2006; 173:777–780.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Johnson SR, Tattersfield AE. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax 2000; 55:1052–1057.
- Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med 1995; 151:527–533.
- Urban T, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P). Medicine (Baltimore) 1999; 78:321–337.
- Schönfeld N, Frank W, Wenig S, et al. Clinical and radiologic features, lung function and therapeutic results in pulmonary histiocytosis X. Respiration 1993; 60:38–44.
- Lacronique J, Roth C, Battesti JP, Basset F, Chretien J. Chest radiological features of pulmonary histiocytosis X: a report based on 50 adult cases. Thorax 1982; 37:104–109.
- Kluger N, Giraud S, Coupier I, et al. Birt-Hogg-Dubé syndrome: clinical and genetic studies of 10 French families. Br J Dermatol 2010; 162:527–537.
- Tobino K, Gunji Y, Kurihara M, et al. Characteristics of pulmonary cysts in Birt-Hogg-Dubé syndrome: thin-section CT findings of the chest in 12 patients. Eur J Radiol 2011; 77:403–409.
- Hartman TE, Primack SL, Swensen SJ, Hansell D, McGuinness G, Müller NL. Desquamative interstitial pneumonia: thin-section CT findings in 22 patients. Radiology 1993; 187:787–790.
- Koyama M, Johkoh T, Honda O, et al. Chronic cystic lung disease: diagnostic accuracy of high-resolution CT in 92 patients. AJR Am J Roentgenol 2003; 180:827–835.
- Patz EF Jr, Müller NL, Swensen SJ, Dodd LG. Congenital cystic adenomatoid malformation in adults: CT findings. J Comput Assist Tomogr 1995; 19:361–364.
- Conran RM, Stocker JT. Extralobar sequestration with frequently associated congenital cystic adenomatoid malformation, type 2: report of 50 cases. Pediatr Dev Pathol 1999; 2:454–463.
- Kennedy CA, Goetz MB. Atypical roentgenographic manifestations of Pneumocystis carinii pneumonia. Arch Intern Med 1992; 152:1390–1398.
- Sandhu JS, Goodman PC. Pulmonary cysts associated with Pneumocystis carinii pneumonia in patients with AIDS. Radiology 1989; 173:33–35.
- Doğan R, Yüksel M, Cetin G, et al. Surgical treatment of hydatid cysts of the lung: report on 1,055 patients. Thorax 1989; 44:192–199.
- Salih OK, Topcuoğlu MS, Celik SK, Ulus T, Tokcan A. Surgical treatment of hydatid cysts of the lung: analysis of 405 patients. Can J Surg 1998; 41:131–135.
- Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J 1996; 9:1569–1571.
- Sakai M, Yamaoka M, Kawaguchi M, Hizawa N, Sato Y. Multiple cystic pulmonary amyloidosis. Ann Thorac Surg 2011; 92:e109.
- Colombat M, Caudroy S, Lagonotte E, et al. Pathomechanisms of cyst formation in pulmonary light chain deposition disease. Eur Respir J 2008; 32:1399–1403.
- Zamora AC, Collard HR, Wolters PJ, Webb WR, King TE. Neurofibromatosis-associated lung disease: a case series and literature review. Eur Respir J 2007; 29:210–214.
- Oikonomou A, Vadikolias K, Birbilis T, Bouros D, Prassopoulos P. HRCT findings in the lungs of non-smokers with neurofibromatosis. Eur J Radiol 2011; 80:e520–e523.
KEY POINTS
- Pulmonary cysts should be differentiated from cyst-mimics.
- Adults with cystic lung disease can be grouped by the clinical presentation: ie, insidious dyspnea or spontaneous pneumothorax; incidentally found cysts or recurrent pneumonia; signs and symptoms of primary pulmonary infection; or signs and symptoms that are primarily nonpulmonary.
- Characterization of pulmonary cysts and their distribution plays a key role in diagnosis. Radiographically, cystic lung disease can be subclassified into two major categories according to the distribution of cysts: discrete (focal or multifocal) and diffuse (unilobular or panlobular).
Denosumab: A novel antiresorptive drug for osteoporosis
A 68-year-old white woman presents with mid- thoracic back pain. Plain radiographs reveal a compression fracture of the 10th thoracic vertebra. She is diagnosed with osteoporosis on the basis of dual energy x-ray absorptiometry (DXA) scans that show T scores of –2.9 in her lumbar spine and –2.6 in her left femoral neck. Her 10-year probability of fracture is estimated as 23% for major osteoporotic fracture and 5.9% for hip fracture (based on the World Health Organization’s absolute fracture risk assessment tool, adapted for the United States, and available at www.shef.ac.uk/FRAX).
After excluding common secondary causes of osteoporosis, her physician recommends a bisphosphonate to reduce her risk of fracture, but she develops upper-gastrointestinal adverse effects with both alendronate and risedronate despite correctly following the instructions for oral administration.
What should her physician consider next?
OSTEOPOROSIS IS A MAJOR PROBLEM
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, predisposing to an increased risk of fragility fractures, particularly of the spine, hip, and wrist.
It is a major public health problem, affecting 200 million people throughout the world, with 9 million osteoporotic fractures reported in the year 2000.1 The incidence of hip fracture alone is predicted to rise to 2.6 million by the year 2025, and to 4.5 million by the year 2050.2 In the United States, the total burden was estimated to be about 2 million incident fractures in the year 2005, projected to rise by another 50% by the year 2025,3 primarily because of the aging of the population. Population studies have indeed suggested that about 40% of white women and 13% of white men over the age of 50 are at risk of sustaining an osteoporotic fracture during the remainder of their lifetime.4
The consequences of osteoporotic fractures can be devastating. Hip fractures are associated with a risk of death ranging from 8.4% to 36% during the first year after fracture.5 One-fifth of patients who sustain a hip fracture require long-term nursing home care, and more than half of the survivors do not regain their previous level of independence.
Patients with vertebral fractures are also at increased risk of death, although the results of some studies suggest that this could be the result of comorbid factors.6–9 Vertebral fractures can result in chronic back pain, loss of height from spinal deformity, reduced mobility, loss of self-esteem, and in severe cases, respiratory and digestive problems because of contact between the lower ribs and pelvis.
A person with one vertebral compression fracture is five times more likely to have another vertebral fracture,10 and a person with two or more compression fractures is 12 times more likely.11
The costs of treating osteoporotic fractures are greater than those of treating myocardial infarction or stroke12,13; they include not only direct costs incurred in treating the fracture, but also indirect societal costs owing to the long-term morbidity associated with the fracture. In the United States, the total cost of treating osteoporotic fractures was estimated at $19 billion in the year 2005.3 By 2025, the annual costs are projected to rise by almost 50%.3
A NEED FOR MORE OPTIONS
Until fairly recently, bisphosphonates were the only drugs of first choice, but adherence to oral bisphosphonate therapy is generally poor (< 50% at 1 year),14 most commonly because of dyspepsia,15 and poor adherence has been shown to be associated with increased fracture risk.16,17 Hence the need for additional therapeutic options.
In this review, we discuss denosumab, an antiresorptive drug approved by the US Food and Drug Administration (FDA) in 2010. First, we discuss its mechanism of action, efficacy, and safety, and then we offer recommendations for its use in clinical practice.
WHAT IS DENOSUMAB AND HOW DOES IT WORK?
Bone remodeling is a dynamic process involving a balance between bone resorption by osteoclasts on the one hand and new bone formation by osteoblasts on the other. A net gain in bone occurs when the activity of osteoblasts exceeds that of osteoclasts, and bone loss occurs when there is increased osteoclast activity or reduced osteoblast activity, or both. The activities of osteoblasts and osteoclasts are tightly coupled because of the opposing effects of two sets of proteins, namely, receptor activator of nuclear factor kappa b ligand (RANKL) and osteoprotegerin.
Both RANKL and osteoprotegerin are produced by osteoblasts. RANKL binds to its receptor (RANK) on preosteoclasts and osteoclasts and induces their differentiation and activation, respectively. Osteoprotegerin is the decoy receptor and natural antagonist for RANKL. By binding with RANKL, it blocks its interaction with RANK.18 In healthy individuals, a fine balance between RANKL and osteoprotegerin ensures that bone remodeling is regulated.
In postmenopausal women, estrogen deficiency leads to an imbalance between RANKL and osteoprotegerin (increased RANKL and reduced osteoprotegerin), resulting in net bone loss. This imbalance is also a feature of rheumatoid arthritis, myeloma bone disease, and osteolytic metastatic bone disease; it also occurs in those receiving androgen deprivation therapy for prostate cancer or aromatase inhibitors for breast cancer.
Denosumab is a fully human monoclonal antibody that targets RANKL.19 By binding to RANKL, this drug prevents the maturation and differentiation of preosteoclasts and promotes apoptosis of osteoclasts. Bone resorption is therefore slowed. It was parenteral osteoprotegerin that was initially developed by denosumab’s manufacturer,20 but this approach failed because neutralizing antibodies developed to osteoprotegerin, rendering it ineffective. Development of neutralizing antibodies has thus far not been a problem with denosumab.
Denosumab, with its property of RANKL inhibition, has also been used to prevent skeletal events in patients with bone metastases from solid tumors and to treat unresectable giant cell tumors of the bone (both FDA-approved indications) and hypercalcemia of malignancy. There is limited clinical experience in Paget disease of the bone as well.21–23 These other potential uses of denosumab are beyond the scope of this review.
HOW WELL DOES DENOSUMAB WORK FOR OSTEOPOROSIS?
Several phase 2 and phase 3 randomized controlled trials have evaluated the efficacy of denosumab, but only one, the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, included fracture reduction as the primary outcome measure. The rest evaluated changes in bone mineral density or in markers of bone turnover, or both.
FREEDOM was a double-blind, randomized controlled trial in 7,808 postmenopausal women with T scores between –2.5 and –4.0 at the lumbar spine or hip.24 Twenty-four percent of the patients had vertebral fractures at baseline. Patients were randomized to receive either denosumab 60 mg (n = 3,902) or placebo (n = 3,906) every 6 months for up to 36 months. All patients also received adequate calcium and vitamin D supplementation.
At 36 months, compared with those who were randomized to receive placebo, those who were randomized to denosumab had lower incidence rates of:
- New vertebral fracture
(2.3% vs 7.2%, risk ratio 0.32,
95% CI 0.26–0.41, P < .001) - Nonvertebral fracture
(6.5% vs 8.0%, risk ratio 0.80,
95% CI 0.67–0.95, P = .01) - Hip fracture
(0.7% vs 1.2%, risk ratio 0.60,
95% CI 0.37–0.97, P = .04).
Increases in bone mineral density at the lumbar spine and hip, and decreases in bone turnover markers were also significantly greater in the denosumab group. The number needed to treat to prevent one new fracture over 3 years was 21 for vertebral fracture, 67 for nonvertebral fracture, and 200 for hip fracture, reflecting the relatively low event rate in the study.
In an open-label extension of the FREEDOM trial, the fracture incidence rates among participants who continued to receive denosumab for an additional 5 years remained low, and still below those projected for a “virtual placebo cohort” (total duration of exposure of 8 years). The rates among participants who switched from placebo to denosumab were similar to those of the denosumab group from the parent trial.25,26
A subgroup analysis of the FREEDOM trial suggested that denosumab reduced the risk of new vertebral fractures irrespective of age, body mass index, femoral neck bone mineral density, prevalent vertebral fractures, or prior nonvertebral fractures (risk ratio 0.32; 95% CI 0.26–0.41, P < .001), whereas the risk of nonvertebral fractures was only reduced in those women with body mass indices less than 25 kg/m2, femoral neck bone mineral density T scores less than –2.5, and in those without a prevalent vertebral fracture.27
A post hoc analysis revealed that denosumab significantly reduced the risk of new vertebral and hip fractures even in subgroups of women at higher risk of fracture.28 At 10% fracture probability (as estimated by the FRAX risk calculator), denosumab reduced the fracture risk by 11% (P = .629), whereas at 30% probability (moderate to high risk), the reduction was 50% (P = .001).29
Other phase 2 and phase 3 trials, in postmenopausal women with low bone mineral density, demonstrated that compared with placebo, denosumab significantly increased bone mineral density at all skeletal sites, increased volumetric bone mineral density at the distal radius, improved hip structural analysis parameters, and reduced bone turnover markers.30–33 Increases in bone mineral density and reductions in bone turnover markers with denosumab have been shown in men as well.34
In a randomized controlled trial,35 improvement in bone mineral density was better in those who received the combination of denosumab and teriparatide than in those who received either drug on its own.
Denosumab has also been shown to reduce the incidence of new vertebral fractures and improve bone mineral density in men receiving androgen-deprivation therapy for nonmetastatic prostate cancer,36 and to improve bone mineral density in women with metastatic breast cancer and low bone mass who were receiving adjuvant aromatase inhibitor therapy.37
HOW DOES DENOSUMAB COMPARE WITH OTHER OSTEOPOROSIS DRUGS?
A double-blind randomized controlled trial in postmenopausal women with low bone mass demonstrated that denosumab was superior to alendronate in improving bone mineral density at all skeletal sites (3.5% vs 2.6% for total hip bone mineral density, P < .0001).38
Another double-blind trial demonstrated that in patients previously treated with alendronate, switching to denosumab resulted in significantly greater increases in bone mineral density at all skeletal sites compared with continuing with alendronate (P < .0001).39
Denosumab has also been shown to be superior to alendronate in improving cortical bone mineral density, as measured by quantitative computed tomography.40
No trial has directly compared the efficacy of denosumab with other osteoporosis drugs in reducing fracture risk, but a systematic literature review of multiple databases,41 comparing the antifracture efficacy of nine osteoporosis drugs, concluded that teriparatide, zoledronic acid, and denosumab had the highest probabilities of being most efficacious for nonvertebral and vertebral fractures, with the greatest effect sizes. Indirect comparisons of the relative risk of fracture with denosumab (based on the results of FREEDOM), alendronate, risedronate, raloxifene, and strontium (based on a meta-analysis of randomized controlled trials) are presented in Table 1.42
A 2-year randomized, open-label, crossover study43 randomized patients to receive either denosumab followed by alendronate or alendronate followed by denosumab over successive 12-month periods. The results suggested that postmenopausal women with osteoporosis were more adherent, compliant, and persistent with denosumab therapy (a subcutaneous injection every 6 months) than with alendronate therapy in the form of oral tablets, self-administered weekly (7.5% nonadherence vs 36.5% at the end of 2 years). After receiving both treatments, women reported greater satisfaction with denosumab, with 92.4% preferring it over oral alendronate. Bone mineral density remained stable when patients were switched from denosumab to alendronate, but improved further when they were switched from alendronate to denosumab.
HOW SAFE IS DENOSUMAB?
The most frequent adverse events with denosumab reported in the long-term extension of one phase 2 study were upper respiratory tract infections (13.5%), arthralgia (11.5%), and back pain (9.0%).30
Increased risk of infection, cancer, and dermatologic reactions has been a concern, as RANKL and RANK are expressed by a wide variety of cells, including T lymphocytes, B cells, and dendritic cells.44 However, there were no significant differences in the overall incidences of adverse events between patients who received denosumab and those who received placebo or alendronate in any of the phase 2, phase 3, or extension studies.
In the FREEDOM trial,24 there was no significant difference between the two groups in the overall incidence of infection (52.9% with denosumab vs 54.4% with placebo, P = .17), or serious infection (4.1% with denosumab vs 3.4% with placebo, P = .14), although the incidence of “serious” cellulitis requiring hospitalization was higher in the denosumab group (0.3% vs < 0.1%, P = .002). There were more serious infections involving the gastrointestinal system, urinary tract, and ear and cases of endocarditis in the denosumab group, but the number of events was small, and there was no relationship with the timing of administration or duration of exposure to denosumab.45 Eczema was more common in the denosumab than in the placebo group (3.0% vs 1.7%, P < .001), but the extension data from the first 3 years did not provide any evidence for an increased risk of cellulitis or eczema with denosumab.26
Although randomized controlled trials reported more cases of neoplasms in the denosumab than in the placebo groups, meta-analyses have failed to detect a statistically significant difference (risk ratio 1.11, 95% CI 0.91–1.36).46 The overall incidence of adverse and serious adverse events reported in the 8-year extension of FREEDOM were consistent with data reported in the previous extension studies.25
In the FREEDOM extension trial, four events in the long-term group (n = 2,343), and two in the crossover group (n = 2,207) were adjudicated as being consistent with osteonecrosis of the jaw.26 One mid-shaft fracture in the crossover group was adjudicated as an atypical femoral fracture. There were, however, no reports of osteonecrosis of the jaw or atypical femoral fracture in the long-term phase 2 trial after 8 years of follow-up.30 By September 2013, postmarketing safety surveillance data for denosumab (estimated exposure of 1.2 million patient-years) had recorded four cases of atypical femoral fracture. All four patients had previously been on bisphosphonates. There were also 32 reports of osteonecrosis of the jaw.47
Denosumab’s manufacturer aims to communicate the risks of treatment to health care professionals and patients. Information is available online at www.proliahcp.com/risk-evaluation-mitigation-strategy/.
WHAT ARE THE PRECAUTIONS?
Several precautions need to be taken when considering treatment with denosumab.
Antiresorptives can aggravate hypocalcemia by inhibiting bone turnover. Serum calcium should therefore be checked and preexisting hypocalcemia should be corrected before starting denosumab.48
Denosumab is contraindicated in women who are pregnant or are planning to become pregnant, as fetal loss and teratogenicity have been reported in animal experiments. (Denosumab is unlikely to be used in premenopausal women, as it is not approved for use in this group.)
There are no data on excretion of denosumab in human milk, so it should not be given to nursing mothers.
Renal impairment is not a contraindication, and no dose adjustment is necessary (even for patients on renal replacement therapy), as denosumab, being an antibody, is eliminated through the reticuloendothelial system.49,50 However, in practice, any antiresorptive agent should be used with caution in patients with severe renal impairment because of the possible presence of adynamic bone disease. Further reduction of bone turnover would be detrimental in such patients. Also, severe hypocalcemia has been reported in patients with a creatinine clearance rate less than 30 mL/min and in those receiving dialysis.51,52 Postmarketing surveillance data have reported eight cases of severe symptomatic hypocalcemia, of which seven were in patients with chronic kidney disease.47
The manufacturer suggests that patients receive a dental examination with appropriate preventive dentistry before starting denosumab to reduce the incidence of osteonecrosis of the jaw, despite the lack of evidence in support of this strategy. The American Dental Association recommends regular dental visits and maintenance of good oral hygiene for patients already established on antiresorptive therapy.53,54
SHOULD PATIENTS ON DENOSUMAB BE OFFERED A DRUG HOLIDAY?
A drug holiday (temporary discontinuation of the drug after a certain duration of treatment) has been proposed for patients receiving bisphosphonates because of the risk of atypical femoral fracture and osteonecrosis of the jaw (although small) consequent to long-term continuous suppression of bone turnover.55 The antifracture efficacy of bisphosphonates is likely to persist for an unknown length of time after discontinuation because of their long skeletal half-life, while the risks gradually diminish.
By contrast, denosumab targets RANKL in the extracellular fluid and does not become embedded within the bone tissue.56 Pharmacokinetic studies have shown that denosumab has a rapid offset of action, with a half-life of only 26 days and biological activity lasting only 6 months.57 The results of a phase 2 extension study suggest that bone mineral density starts to decline and bone turnover markers start to rise within 12 months of discontinuing denosumab.58
Although fracture risk did not increase in those who were randomized to stopping the treatment and bone mineral density increased further when treatment was restarted, a drug holiday cannot presently be recommended for patients receiving denosumab because of the lack of supportive data.
HOW COST-EFFECTIVE IS DENOSUMAB?
The wholesale acquisition cost is $825 per 60-mg prefilled syringe of denosumab, although this may vary depending on where the drug is obtained. This does not include physician-related service costs associated with administration of denosumab.
Cost-effectiveness analyses conducted in the United States, the United Kingdom, and Sweden have all concluded that denosumab would offer a cost-effective alternative to other osteoporosis medications for primary prevention and secondary prevention of fractures.59–61
The Swedish study also incorporated adherence in the cost-effectiveness model and showed that denosumab was a cost-effective alternative to oral bisphosphonates, particularly for patients who were not expected to adhere well to oral treatments.61
WHICH OSTEOPOROSIS PATIENTS ARE CANDIDATES FOR DENOSUMAB?
The FDA has approved denosumab for the treatment of postmenopausal women and men at high risk of fracture (defined as having a history of osteoporotic fracture or multiple risk factors for fracture), or in those who cannot tolerate other osteoporosis medications or for whom other medications have failed.
Denosumab is also approved for men at high risk of fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer, and for women at high risk of fracture receiving adjuvant aromatase inhibitor therapy for breast cancer.
WHAT DO THE GUIDELINES RECOMMEND?
The National Osteoporosis Foundation guidelines recommend pharmacologic treatment for patients with hip or vertebral fractures (clinical or asymptomatic); T scores lower than –2.5 at the femoral neck, total hip, or lumbar spine; and those with a 10-year probability of hip fracture of more than 3% or of a major osteoporotic fracture more than 20% based on the US-adapted FRAX calculator.62 The American College of Endocrinology guidelines have proposed similar thresholds for pharmacologic treatment, and they recommend alendronate, risedronate, zoledronate, and denosumab as first-line agents.63
The 2010 Osteoporosis Canada guidelines recommend denosumab, alendronate, risedronate, and zoledronate as first-line therapies for preventing hip, nonvertebral, and vertebral fractures in postmenopausal women (grade A recommendation).64 The National Institute of Health and Clinical Excellence in England and Wales, on the other hand, recommends denosumab only for patients who are unable to take a bisphosphonate.65
PRACTICAL PRESCRIBING TIPS
The patient described at the beginning of this article has already sustained a vertebral compression fracture, and her DXA scan shows T scores in the osteoporotic range. She is therefore at increased risk of another fragility fracture (with a fivefold higher risk of another vertebral fracture). Pharmacologic therapy should be considered. In addition, she should be encouraged to adhere to lifestyle measures such as a healthy diet and regular weight-bearing exercise, her risk of falling should be assessed, and adequate calcium and vitamin D supplementation should be given.
Secondary causes of osteoporosis are present in about 30% of women and 55% of men who have vertebral fractures.66 A complete blood count, erythrocyte sedimentation rate, bone biochemistry, 25-hydroxyvitamin D, thyroid-stimulating hormone, and renal and liver function tests should be requested in all patients. Further tests should be considered depending on the clinical evaluation and results of initial investigations.
Because this patient cannot tolerate oral bisphosphonates, she could be offered the option of annual intravenous zoledronic acid infusions or 6-monthly subcutaneous denosumab injections. In clinical trials, gastrointestinal adverse effects were noted with intravenous bisphosphonates as well, but the adverse effects reported were no different than those with placebo. The potential advantages with denosumab include better bone mineral density gains, adherence and patient satisfaction compared with oral bisphosphonates, convenient twice-yearly administration, safety in patients with renal impairment, and absence of gastrointestinal effects.
Raloxifene, a selective estrogen receptor modulator, has estrogen-like action on the bone and antiestrogen actions on the breast and uterus. Unlike standard hormone replacement therapy, raloxifene can therefore increase bone mineral density without increasing the risk of breast and endometrial cancers. However, it has only been shown to reduce the risk of vertebral fracture, not hip fracture. Hence, it would be a more appropriate choice for younger postmenopausal women. Moreover, it may cause troublesome menopausal symptoms.
Teriparatide, the recombinant parathyroid hormone, is an anabolic agent. It is very expensive, and because of this, guidelines in several countries restrict its use to women with severe osteoporosis and multiple fractures who fail to respond to standard treatments. It cannot be used for longer than 2 years because of its association with osteosarcoma in rats.
If our patient prefers denosumab, therapy should be initiated after appropriate counseling (see precautions above). The dose is 60 mg, given subcutaneously, once every 6 months.
Monitoring
There is no consensus regarding the optimal frequency for monitoring patients on treatment, owing to the lack of prospective trial data. The National Osteoporosis Foundation recommends repeating the bone mineral density measurements about 2 years after starting therapy, and about every 2 years thereafter.62 Some studies suggest that changes in bone mineral density correlate with reduction in fracture risk.67,68 A change in bone mineral density is considered significant when it is greater than the range of error of the densitometer (also known as the least significant change).69 If the bone mineral density is stable or improving, therapy could be continued, but if it is declining and the decline is greater than the least significant change, a change in therapy should be considered if no secondary causes for bone loss are evident (but see What are the areas of uncertainty? below).
The National Osteoporosis Foundation also recommends measuring a bone turnover marker at baseline and then 3 to 6 months later, as its suppression predicts greater bone mineral density responses and fracture risk reduction.70 If there is a decrease of more than 30% in serum carboxy-terminal collagen crosslinks (CTX) or more than 50% in urinary N-telopeptide (NTX),71 the patient can be reassured that the next bone mineral density measurement will be stable or improved. In patients on oral bisphosphonates, measurement of bone turnover markers also provides evidence of compliance.
Clinical trials suggest that a numerical increase in bone mineral density can be expected in most patients on treatment, though this depends on the measurement site and the length of time between examinations. In one phase 3 trial of denosumab in postmenopausal women, only 5% of the participants had unchanged or diminished bone mineral density at the lumbar spine, and 8% at the hip, after 36 months of treatment.72 However, the CTX levels fell to below the lower limit of the reference interval as early as 1 month after commencing treatment in all denosumab-treated patients.68
Hence, bone turnover markers may be a more sensitive indicator of treatment effect than bone mineral density, but this would ultimately need to be evaluated against fracture rates in a real-world setting.
WHAT ARE THE AREAS OF UNCERTAINTY?
There are currently no guidelines for long-term management of patients on denosumab, and also no data to suggest whether patients should be switched to a weaker antiresorptive drug after a certain number of years in order to reduce the possible risk of atypical femoral fracture or osteonecrosis of the jaw.
No head-to-head trials have directly compared the antifracture efficacy of denosumab with that of other standard osteoporosis therapies. The antifracture efficacy and safety of combination therapies involving denosumab are also uncertain. For adherent patients who have a suboptimal response, there is no evidence to guide the further course of action. The International Osteoporosis Foundation guidelines suggest replacing a stronger antiresorptive with an anabolic agent, but acknowledge that this is only based on expert opinion.71
The very-long-term effects (beyond 8 years) of continuous denosumab administration on increasing the risk of atypical femoral fracture, osteonecrosis of the jaw, malignancy, or infection or the duration after which risks would start to outweigh benefits is not known. However, postmarketing safety data continue to be collected through the voluntary Post-marketing Active Safety Surveillance Program (for prespecified adverse events) in addition to the FDA’s MedWatch program.
CASE PROGRESSION
The patient described in the vignette is presented with two options—zoledronate and denosumab. She chooses denosumab. Her renal function and serum calcium are checked and are found to be satisfactory. She undergoes a dental examination, which is also satisfactory. She is counseled about the possible increased risk of infection, and then she is started on 60 mg of denosumab subcutaneously, once every 6 months.
When reviewed after 2 years, she reports no further fractures. Her bone mineral density remains stable compared with the values obtained before starting treatment. She reports no adverse effects and is happy to continue with denosumab.
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17:1726–1733.
- Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int 1997; 7:407–413.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007; 22:465–475.
- Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res 1992; 7:1005–1010.
- Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009; 20:1633–1650.
- Jalava T, Sarna S, Pylkkänen L, et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 2003; 18:1254–1260.
- Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 1998; 8:291–297.
- Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 2000; 48:241–249.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999; 159:1215–1220.
- Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001; 285:320–323.
- Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991; 114:919–923.
- Piscitelli P, Iolascon G, Argentiero A, et al. Incidence and costs of hip fractures vs strokes and acute myocardial infarction in Italy: comparative analysis based on national hospitalization records. Clin Interv Aging 2012; 7:575–583.
- Johnell O, Kanis JA, Jonsson B, Oden A, Johansson H, De Laet C. The burden of hospitalised fractures in Sweden. Osteoporos Int 2005; 16:222–228.
- Confavreux CB, Canoui-Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat RD. Persistence at 1 year of oral antiosteoporotic drugs: a prospective study in a comprehensive health insurance database. Eur J Endocrinol 2012; 166:735–741.
- Biswas PN, Wilton LV, Shakir SA. Pharmacovigilance study of alendronate in England. Osteoporos Int 2003; 14:507–514.
- Landfeldt E, Ström O, Robbins S, Borgström F. Adherence to treatment of primary osteoporosis and its association to fractures—the Swedish Adherence Register Analysis (SARA). Osteoporos Int 2012; 23:433–443.
- Sampalis JS, Adachi JD, Rampakakis E, Vaillancourt J, Karellis A, Kindundu C. Long-term impact of adherence to oral bisphosphonates on osteoporotic fracture incidence. J Bone Miner Res 2012; 27:202–210.
- Schwarz EM, Ritchlin CT. Clinical development of anti-RANKL therapy. Arthritis Res Ther 2007; 9(suppl 1):S7.
- Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract 2012; 66:1139–1146.
- Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 2001; 16:348–360.
- Rizzoli R, Body JJ, Brandi ML, et al; International Osteoporosis Foundation Committee of Scientific Advisors Working Group on Cancer-Induced Bone Disease. Cancer-associated bone disease. Osteoporos Int 2013; 24:2929–2953.
- Schwarz P, Rasmussen AQ, Kvist TM, Andersen UB, Jørgensen NR. Paget’s disease of the bone after treatment with denosumab: a case report. Bone 2012; 50:1023–1025.
- Hu MI, Glezerman IG, Leboulleux S, et al. Denosumab for treatment of hypercalcemia of malignancy. J Clin Endocrinol Metab 2014; Jun 10 [Epub ahead of print].
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361:756–765.
- Papapoulos S, Lippuner K, Roux C, et al. Eight years of denosumab treatment in postmenopausal women with osteoporosis: results from the first five years of the FREEDOM extension [abstract]. Presented at the 2013 annual meeting of the American Society for Bone and Mineral Research, Baltimore, MD, October 4–7, 2013.
- Bone HG, Chapurlat R, Brandi ML, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 2013; 98:4483–4492.
- McClung MR, Boonen S, Törring O, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res 2012; 27:211–218.
- Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 2011; 96:1727–1736.
- McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 2012; 27:1480–1486.
- McClung MR, Lewiecki EM, Geller ML, et al. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int 2013; 24:227–235.
- McClung MR, Lewiecki EM, Cohen SB, et al; AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006; 354:821–831.
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 2008; 93:2149–2157.
- Genant HK, Engelke K, Hanley DA, et al. Denosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral density. Bone 2010; 47:131–139.
- Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab 2012; 97:3161–3169.
- Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013; 382:50–56.
- Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009; 361:745–755.
- Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 2008; 26:4875–4882.
- Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 2009; 24:153–161.
- Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 2010; 25:72–81.
- Seeman E, Delmas PD, Hanley DA, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 2010; 25:1886–1894.
- Hopkins RB, Goeree R, Pullenayegum E, et al. The relative efficacy of nine osteoporosis medications for reducing the rate of fractures in post-menopausal women. BMC Musculoskelet Disord 2011; 12:209.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal guidance: TA161. Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women (amended). http://publications.nice.org.uk/alendronate-etidronate-risedronate-raloxifene-strontium-ranelate-and-teriparatide-for-ta161. Accessed January 9, 2015.
- Freemantle N, Satram-Hoang S, Tang ET, et al; DAPS Investigators. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 2012; 23:317–326.
- Lewiecki EM. Safety and tolerability of denosumab for the treatment of postmenopausal osteoporosis. Drug Healthc Patient Saf 2011; 3:79–91.
- Watts NB, Roux C, Modlin JF, et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: coincidence or causal association? Osteoporos Int 2012; 23:327–337.
- von Keyserlingk C, Hopkins R, Anastasilakis A, et al. Clinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: a meta-analysis. Semin Arthritis Rheum 2011; 41:178–186.
- Geller M, Wagman RB, Ho PR, et al. Early findings from Prolia postmarketing safety surveillance for atypical femoral fracture, osteonecrosis of the jaw, severe symptomatic hypocalcemia, and anaphylaxis (abstract). Osteoporos Int 2014; 25(suppl 2). OC40; www.wco-iof-esceo.org/sites/ecceo14/docs/wco14-abstractbook.pdf. Accessed January 9, 2015.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 2011; 26:1829–1835.
- Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012; 27:1471–1479.
- Ungprasert P, Cheungpasitporn W, Srivali N, Kittanamongkolchai W, Bischof EF. Life-threatening hypocalcemia associated with denosumab in a patient with moderate renal insufficiency. Am J Emerg Med 2013; 31:756.e1–e2.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Rachner TD, Platzbecker U, Felsenberg D, Hofbauer LC. Osteonecrosis of the jaw after osteoporosis therapy with denosumab following long-term bisphosphonate therapy. Mayo Clin Proc 2013; 88:418–419.
- Epstein MS, Ephros HD, Epstein JB. Review of current literature and implications of RANKL inhibitors for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116:e437–e442.
- McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 2013; 126:13–20.
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011; 48:677–692.
- Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 2004; 19:1059–1066.
- Miller PD, Bolognese MA, Lewiecki EM, et al; Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008; 43:222–229.
- Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy 2013; 11:485–497.
- Scotland G, Waugh N, Royle P, McNamee P, Henderson R, Hollick R. Denosumab for the prevention of osteoporotic fractures in post-menopausal women: a NICE single technology appraisal. Pharmacoeconomics 2011; 29:951–961.
- Jönsson B, Ström O, Eisman JA, et al. Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 2011; 22:967–982.
- Clinician’s guide to prevention and treatment of osteoporosis. Washington DC: National Osteoporosis Foundation, 2013.
- Watts NB, Bilezikian JP, Camacho PM, et al; AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010;16(suppl 3):1–37.
- Papaioannou A, Morin S, Cheung AM, et al; Scientific Advisory Council of Osteoporosis Canada. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010; 182:1864–1673.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal: TA204. Denosumab for the prevention of osteoporotic fractures in postmenopausal women. http://guidance.nice.org.uk/TA204. Accessed January 9, 2015.
- Premaor MO, Compston JE. Testing for secondary causes of osteoporosis. BMJ 2010; 341:c6959.
- Hochberg MC, Ross PD, Black D, et al. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum 1999; 42:1246–1254.
- Eastell R, Vrijens B, Cahall DL, Ringe JD, Garnero P, Watts NB. Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J Bone Miner Res 2011; 26:1662–1669.
- Diez-Perez A, Adachi JD, Agnusdei D, et al; IOF CSA Inadequate Responders Working Group. Treatment failure in osteoporosis. Osteoporos Int 2012; 23:2769–2774.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 2003; 18:1051–1056.
- Rosen HN, Moses AC, Garber J, Ross DS, Lee SL, Greenspan SL. Utility of biochemical markers of bone turnover in the follow-up of patients treated with bisphosphonates. Calcif Tissue Int 1998; 63:363–368.
- Bolognese MA, Teglbjærg CS, Zanchetta JR, et al. Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom 2013; 16:147–153.
A 68-year-old white woman presents with mid- thoracic back pain. Plain radiographs reveal a compression fracture of the 10th thoracic vertebra. She is diagnosed with osteoporosis on the basis of dual energy x-ray absorptiometry (DXA) scans that show T scores of –2.9 in her lumbar spine and –2.6 in her left femoral neck. Her 10-year probability of fracture is estimated as 23% for major osteoporotic fracture and 5.9% for hip fracture (based on the World Health Organization’s absolute fracture risk assessment tool, adapted for the United States, and available at www.shef.ac.uk/FRAX).
After excluding common secondary causes of osteoporosis, her physician recommends a bisphosphonate to reduce her risk of fracture, but she develops upper-gastrointestinal adverse effects with both alendronate and risedronate despite correctly following the instructions for oral administration.
What should her physician consider next?
OSTEOPOROSIS IS A MAJOR PROBLEM
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, predisposing to an increased risk of fragility fractures, particularly of the spine, hip, and wrist.
It is a major public health problem, affecting 200 million people throughout the world, with 9 million osteoporotic fractures reported in the year 2000.1 The incidence of hip fracture alone is predicted to rise to 2.6 million by the year 2025, and to 4.5 million by the year 2050.2 In the United States, the total burden was estimated to be about 2 million incident fractures in the year 2005, projected to rise by another 50% by the year 2025,3 primarily because of the aging of the population. Population studies have indeed suggested that about 40% of white women and 13% of white men over the age of 50 are at risk of sustaining an osteoporotic fracture during the remainder of their lifetime.4
The consequences of osteoporotic fractures can be devastating. Hip fractures are associated with a risk of death ranging from 8.4% to 36% during the first year after fracture.5 One-fifth of patients who sustain a hip fracture require long-term nursing home care, and more than half of the survivors do not regain their previous level of independence.
Patients with vertebral fractures are also at increased risk of death, although the results of some studies suggest that this could be the result of comorbid factors.6–9 Vertebral fractures can result in chronic back pain, loss of height from spinal deformity, reduced mobility, loss of self-esteem, and in severe cases, respiratory and digestive problems because of contact between the lower ribs and pelvis.
A person with one vertebral compression fracture is five times more likely to have another vertebral fracture,10 and a person with two or more compression fractures is 12 times more likely.11
The costs of treating osteoporotic fractures are greater than those of treating myocardial infarction or stroke12,13; they include not only direct costs incurred in treating the fracture, but also indirect societal costs owing to the long-term morbidity associated with the fracture. In the United States, the total cost of treating osteoporotic fractures was estimated at $19 billion in the year 2005.3 By 2025, the annual costs are projected to rise by almost 50%.3
A NEED FOR MORE OPTIONS
Until fairly recently, bisphosphonates were the only drugs of first choice, but adherence to oral bisphosphonate therapy is generally poor (< 50% at 1 year),14 most commonly because of dyspepsia,15 and poor adherence has been shown to be associated with increased fracture risk.16,17 Hence the need for additional therapeutic options.
In this review, we discuss denosumab, an antiresorptive drug approved by the US Food and Drug Administration (FDA) in 2010. First, we discuss its mechanism of action, efficacy, and safety, and then we offer recommendations for its use in clinical practice.
WHAT IS DENOSUMAB AND HOW DOES IT WORK?
Bone remodeling is a dynamic process involving a balance between bone resorption by osteoclasts on the one hand and new bone formation by osteoblasts on the other. A net gain in bone occurs when the activity of osteoblasts exceeds that of osteoclasts, and bone loss occurs when there is increased osteoclast activity or reduced osteoblast activity, or both. The activities of osteoblasts and osteoclasts are tightly coupled because of the opposing effects of two sets of proteins, namely, receptor activator of nuclear factor kappa b ligand (RANKL) and osteoprotegerin.
Both RANKL and osteoprotegerin are produced by osteoblasts. RANKL binds to its receptor (RANK) on preosteoclasts and osteoclasts and induces their differentiation and activation, respectively. Osteoprotegerin is the decoy receptor and natural antagonist for RANKL. By binding with RANKL, it blocks its interaction with RANK.18 In healthy individuals, a fine balance between RANKL and osteoprotegerin ensures that bone remodeling is regulated.
In postmenopausal women, estrogen deficiency leads to an imbalance between RANKL and osteoprotegerin (increased RANKL and reduced osteoprotegerin), resulting in net bone loss. This imbalance is also a feature of rheumatoid arthritis, myeloma bone disease, and osteolytic metastatic bone disease; it also occurs in those receiving androgen deprivation therapy for prostate cancer or aromatase inhibitors for breast cancer.
Denosumab is a fully human monoclonal antibody that targets RANKL.19 By binding to RANKL, this drug prevents the maturation and differentiation of preosteoclasts and promotes apoptosis of osteoclasts. Bone resorption is therefore slowed. It was parenteral osteoprotegerin that was initially developed by denosumab’s manufacturer,20 but this approach failed because neutralizing antibodies developed to osteoprotegerin, rendering it ineffective. Development of neutralizing antibodies has thus far not been a problem with denosumab.
Denosumab, with its property of RANKL inhibition, has also been used to prevent skeletal events in patients with bone metastases from solid tumors and to treat unresectable giant cell tumors of the bone (both FDA-approved indications) and hypercalcemia of malignancy. There is limited clinical experience in Paget disease of the bone as well.21–23 These other potential uses of denosumab are beyond the scope of this review.
HOW WELL DOES DENOSUMAB WORK FOR OSTEOPOROSIS?
Several phase 2 and phase 3 randomized controlled trials have evaluated the efficacy of denosumab, but only one, the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, included fracture reduction as the primary outcome measure. The rest evaluated changes in bone mineral density or in markers of bone turnover, or both.
FREEDOM was a double-blind, randomized controlled trial in 7,808 postmenopausal women with T scores between –2.5 and –4.0 at the lumbar spine or hip.24 Twenty-four percent of the patients had vertebral fractures at baseline. Patients were randomized to receive either denosumab 60 mg (n = 3,902) or placebo (n = 3,906) every 6 months for up to 36 months. All patients also received adequate calcium and vitamin D supplementation.
At 36 months, compared with those who were randomized to receive placebo, those who were randomized to denosumab had lower incidence rates of:
- New vertebral fracture
(2.3% vs 7.2%, risk ratio 0.32,
95% CI 0.26–0.41, P < .001) - Nonvertebral fracture
(6.5% vs 8.0%, risk ratio 0.80,
95% CI 0.67–0.95, P = .01) - Hip fracture
(0.7% vs 1.2%, risk ratio 0.60,
95% CI 0.37–0.97, P = .04).
Increases in bone mineral density at the lumbar spine and hip, and decreases in bone turnover markers were also significantly greater in the denosumab group. The number needed to treat to prevent one new fracture over 3 years was 21 for vertebral fracture, 67 for nonvertebral fracture, and 200 for hip fracture, reflecting the relatively low event rate in the study.
In an open-label extension of the FREEDOM trial, the fracture incidence rates among participants who continued to receive denosumab for an additional 5 years remained low, and still below those projected for a “virtual placebo cohort” (total duration of exposure of 8 years). The rates among participants who switched from placebo to denosumab were similar to those of the denosumab group from the parent trial.25,26
A subgroup analysis of the FREEDOM trial suggested that denosumab reduced the risk of new vertebral fractures irrespective of age, body mass index, femoral neck bone mineral density, prevalent vertebral fractures, or prior nonvertebral fractures (risk ratio 0.32; 95% CI 0.26–0.41, P < .001), whereas the risk of nonvertebral fractures was only reduced in those women with body mass indices less than 25 kg/m2, femoral neck bone mineral density T scores less than –2.5, and in those without a prevalent vertebral fracture.27
A post hoc analysis revealed that denosumab significantly reduced the risk of new vertebral and hip fractures even in subgroups of women at higher risk of fracture.28 At 10% fracture probability (as estimated by the FRAX risk calculator), denosumab reduced the fracture risk by 11% (P = .629), whereas at 30% probability (moderate to high risk), the reduction was 50% (P = .001).29
Other phase 2 and phase 3 trials, in postmenopausal women with low bone mineral density, demonstrated that compared with placebo, denosumab significantly increased bone mineral density at all skeletal sites, increased volumetric bone mineral density at the distal radius, improved hip structural analysis parameters, and reduced bone turnover markers.30–33 Increases in bone mineral density and reductions in bone turnover markers with denosumab have been shown in men as well.34
In a randomized controlled trial,35 improvement in bone mineral density was better in those who received the combination of denosumab and teriparatide than in those who received either drug on its own.
Denosumab has also been shown to reduce the incidence of new vertebral fractures and improve bone mineral density in men receiving androgen-deprivation therapy for nonmetastatic prostate cancer,36 and to improve bone mineral density in women with metastatic breast cancer and low bone mass who were receiving adjuvant aromatase inhibitor therapy.37
HOW DOES DENOSUMAB COMPARE WITH OTHER OSTEOPOROSIS DRUGS?
A double-blind randomized controlled trial in postmenopausal women with low bone mass demonstrated that denosumab was superior to alendronate in improving bone mineral density at all skeletal sites (3.5% vs 2.6% for total hip bone mineral density, P < .0001).38
Another double-blind trial demonstrated that in patients previously treated with alendronate, switching to denosumab resulted in significantly greater increases in bone mineral density at all skeletal sites compared with continuing with alendronate (P < .0001).39
Denosumab has also been shown to be superior to alendronate in improving cortical bone mineral density, as measured by quantitative computed tomography.40
No trial has directly compared the efficacy of denosumab with other osteoporosis drugs in reducing fracture risk, but a systematic literature review of multiple databases,41 comparing the antifracture efficacy of nine osteoporosis drugs, concluded that teriparatide, zoledronic acid, and denosumab had the highest probabilities of being most efficacious for nonvertebral and vertebral fractures, with the greatest effect sizes. Indirect comparisons of the relative risk of fracture with denosumab (based on the results of FREEDOM), alendronate, risedronate, raloxifene, and strontium (based on a meta-analysis of randomized controlled trials) are presented in Table 1.42
A 2-year randomized, open-label, crossover study43 randomized patients to receive either denosumab followed by alendronate or alendronate followed by denosumab over successive 12-month periods. The results suggested that postmenopausal women with osteoporosis were more adherent, compliant, and persistent with denosumab therapy (a subcutaneous injection every 6 months) than with alendronate therapy in the form of oral tablets, self-administered weekly (7.5% nonadherence vs 36.5% at the end of 2 years). After receiving both treatments, women reported greater satisfaction with denosumab, with 92.4% preferring it over oral alendronate. Bone mineral density remained stable when patients were switched from denosumab to alendronate, but improved further when they were switched from alendronate to denosumab.
HOW SAFE IS DENOSUMAB?
The most frequent adverse events with denosumab reported in the long-term extension of one phase 2 study were upper respiratory tract infections (13.5%), arthralgia (11.5%), and back pain (9.0%).30
Increased risk of infection, cancer, and dermatologic reactions has been a concern, as RANKL and RANK are expressed by a wide variety of cells, including T lymphocytes, B cells, and dendritic cells.44 However, there were no significant differences in the overall incidences of adverse events between patients who received denosumab and those who received placebo or alendronate in any of the phase 2, phase 3, or extension studies.
In the FREEDOM trial,24 there was no significant difference between the two groups in the overall incidence of infection (52.9% with denosumab vs 54.4% with placebo, P = .17), or serious infection (4.1% with denosumab vs 3.4% with placebo, P = .14), although the incidence of “serious” cellulitis requiring hospitalization was higher in the denosumab group (0.3% vs < 0.1%, P = .002). There were more serious infections involving the gastrointestinal system, urinary tract, and ear and cases of endocarditis in the denosumab group, but the number of events was small, and there was no relationship with the timing of administration or duration of exposure to denosumab.45 Eczema was more common in the denosumab than in the placebo group (3.0% vs 1.7%, P < .001), but the extension data from the first 3 years did not provide any evidence for an increased risk of cellulitis or eczema with denosumab.26
Although randomized controlled trials reported more cases of neoplasms in the denosumab than in the placebo groups, meta-analyses have failed to detect a statistically significant difference (risk ratio 1.11, 95% CI 0.91–1.36).46 The overall incidence of adverse and serious adverse events reported in the 8-year extension of FREEDOM were consistent with data reported in the previous extension studies.25
In the FREEDOM extension trial, four events in the long-term group (n = 2,343), and two in the crossover group (n = 2,207) were adjudicated as being consistent with osteonecrosis of the jaw.26 One mid-shaft fracture in the crossover group was adjudicated as an atypical femoral fracture. There were, however, no reports of osteonecrosis of the jaw or atypical femoral fracture in the long-term phase 2 trial after 8 years of follow-up.30 By September 2013, postmarketing safety surveillance data for denosumab (estimated exposure of 1.2 million patient-years) had recorded four cases of atypical femoral fracture. All four patients had previously been on bisphosphonates. There were also 32 reports of osteonecrosis of the jaw.47
Denosumab’s manufacturer aims to communicate the risks of treatment to health care professionals and patients. Information is available online at www.proliahcp.com/risk-evaluation-mitigation-strategy/.
WHAT ARE THE PRECAUTIONS?
Several precautions need to be taken when considering treatment with denosumab.
Antiresorptives can aggravate hypocalcemia by inhibiting bone turnover. Serum calcium should therefore be checked and preexisting hypocalcemia should be corrected before starting denosumab.48
Denosumab is contraindicated in women who are pregnant or are planning to become pregnant, as fetal loss and teratogenicity have been reported in animal experiments. (Denosumab is unlikely to be used in premenopausal women, as it is not approved for use in this group.)
There are no data on excretion of denosumab in human milk, so it should not be given to nursing mothers.
Renal impairment is not a contraindication, and no dose adjustment is necessary (even for patients on renal replacement therapy), as denosumab, being an antibody, is eliminated through the reticuloendothelial system.49,50 However, in practice, any antiresorptive agent should be used with caution in patients with severe renal impairment because of the possible presence of adynamic bone disease. Further reduction of bone turnover would be detrimental in such patients. Also, severe hypocalcemia has been reported in patients with a creatinine clearance rate less than 30 mL/min and in those receiving dialysis.51,52 Postmarketing surveillance data have reported eight cases of severe symptomatic hypocalcemia, of which seven were in patients with chronic kidney disease.47
The manufacturer suggests that patients receive a dental examination with appropriate preventive dentistry before starting denosumab to reduce the incidence of osteonecrosis of the jaw, despite the lack of evidence in support of this strategy. The American Dental Association recommends regular dental visits and maintenance of good oral hygiene for patients already established on antiresorptive therapy.53,54
SHOULD PATIENTS ON DENOSUMAB BE OFFERED A DRUG HOLIDAY?
A drug holiday (temporary discontinuation of the drug after a certain duration of treatment) has been proposed for patients receiving bisphosphonates because of the risk of atypical femoral fracture and osteonecrosis of the jaw (although small) consequent to long-term continuous suppression of bone turnover.55 The antifracture efficacy of bisphosphonates is likely to persist for an unknown length of time after discontinuation because of their long skeletal half-life, while the risks gradually diminish.
By contrast, denosumab targets RANKL in the extracellular fluid and does not become embedded within the bone tissue.56 Pharmacokinetic studies have shown that denosumab has a rapid offset of action, with a half-life of only 26 days and biological activity lasting only 6 months.57 The results of a phase 2 extension study suggest that bone mineral density starts to decline and bone turnover markers start to rise within 12 months of discontinuing denosumab.58
Although fracture risk did not increase in those who were randomized to stopping the treatment and bone mineral density increased further when treatment was restarted, a drug holiday cannot presently be recommended for patients receiving denosumab because of the lack of supportive data.
HOW COST-EFFECTIVE IS DENOSUMAB?
The wholesale acquisition cost is $825 per 60-mg prefilled syringe of denosumab, although this may vary depending on where the drug is obtained. This does not include physician-related service costs associated with administration of denosumab.
Cost-effectiveness analyses conducted in the United States, the United Kingdom, and Sweden have all concluded that denosumab would offer a cost-effective alternative to other osteoporosis medications for primary prevention and secondary prevention of fractures.59–61
The Swedish study also incorporated adherence in the cost-effectiveness model and showed that denosumab was a cost-effective alternative to oral bisphosphonates, particularly for patients who were not expected to adhere well to oral treatments.61
WHICH OSTEOPOROSIS PATIENTS ARE CANDIDATES FOR DENOSUMAB?
The FDA has approved denosumab for the treatment of postmenopausal women and men at high risk of fracture (defined as having a history of osteoporotic fracture or multiple risk factors for fracture), or in those who cannot tolerate other osteoporosis medications or for whom other medications have failed.
Denosumab is also approved for men at high risk of fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer, and for women at high risk of fracture receiving adjuvant aromatase inhibitor therapy for breast cancer.
WHAT DO THE GUIDELINES RECOMMEND?
The National Osteoporosis Foundation guidelines recommend pharmacologic treatment for patients with hip or vertebral fractures (clinical or asymptomatic); T scores lower than –2.5 at the femoral neck, total hip, or lumbar spine; and those with a 10-year probability of hip fracture of more than 3% or of a major osteoporotic fracture more than 20% based on the US-adapted FRAX calculator.62 The American College of Endocrinology guidelines have proposed similar thresholds for pharmacologic treatment, and they recommend alendronate, risedronate, zoledronate, and denosumab as first-line agents.63
The 2010 Osteoporosis Canada guidelines recommend denosumab, alendronate, risedronate, and zoledronate as first-line therapies for preventing hip, nonvertebral, and vertebral fractures in postmenopausal women (grade A recommendation).64 The National Institute of Health and Clinical Excellence in England and Wales, on the other hand, recommends denosumab only for patients who are unable to take a bisphosphonate.65
PRACTICAL PRESCRIBING TIPS
The patient described at the beginning of this article has already sustained a vertebral compression fracture, and her DXA scan shows T scores in the osteoporotic range. She is therefore at increased risk of another fragility fracture (with a fivefold higher risk of another vertebral fracture). Pharmacologic therapy should be considered. In addition, she should be encouraged to adhere to lifestyle measures such as a healthy diet and regular weight-bearing exercise, her risk of falling should be assessed, and adequate calcium and vitamin D supplementation should be given.
Secondary causes of osteoporosis are present in about 30% of women and 55% of men who have vertebral fractures.66 A complete blood count, erythrocyte sedimentation rate, bone biochemistry, 25-hydroxyvitamin D, thyroid-stimulating hormone, and renal and liver function tests should be requested in all patients. Further tests should be considered depending on the clinical evaluation and results of initial investigations.
Because this patient cannot tolerate oral bisphosphonates, she could be offered the option of annual intravenous zoledronic acid infusions or 6-monthly subcutaneous denosumab injections. In clinical trials, gastrointestinal adverse effects were noted with intravenous bisphosphonates as well, but the adverse effects reported were no different than those with placebo. The potential advantages with denosumab include better bone mineral density gains, adherence and patient satisfaction compared with oral bisphosphonates, convenient twice-yearly administration, safety in patients with renal impairment, and absence of gastrointestinal effects.
Raloxifene, a selective estrogen receptor modulator, has estrogen-like action on the bone and antiestrogen actions on the breast and uterus. Unlike standard hormone replacement therapy, raloxifene can therefore increase bone mineral density without increasing the risk of breast and endometrial cancers. However, it has only been shown to reduce the risk of vertebral fracture, not hip fracture. Hence, it would be a more appropriate choice for younger postmenopausal women. Moreover, it may cause troublesome menopausal symptoms.
Teriparatide, the recombinant parathyroid hormone, is an anabolic agent. It is very expensive, and because of this, guidelines in several countries restrict its use to women with severe osteoporosis and multiple fractures who fail to respond to standard treatments. It cannot be used for longer than 2 years because of its association with osteosarcoma in rats.
If our patient prefers denosumab, therapy should be initiated after appropriate counseling (see precautions above). The dose is 60 mg, given subcutaneously, once every 6 months.
Monitoring
There is no consensus regarding the optimal frequency for monitoring patients on treatment, owing to the lack of prospective trial data. The National Osteoporosis Foundation recommends repeating the bone mineral density measurements about 2 years after starting therapy, and about every 2 years thereafter.62 Some studies suggest that changes in bone mineral density correlate with reduction in fracture risk.67,68 A change in bone mineral density is considered significant when it is greater than the range of error of the densitometer (also known as the least significant change).69 If the bone mineral density is stable or improving, therapy could be continued, but if it is declining and the decline is greater than the least significant change, a change in therapy should be considered if no secondary causes for bone loss are evident (but see What are the areas of uncertainty? below).
The National Osteoporosis Foundation also recommends measuring a bone turnover marker at baseline and then 3 to 6 months later, as its suppression predicts greater bone mineral density responses and fracture risk reduction.70 If there is a decrease of more than 30% in serum carboxy-terminal collagen crosslinks (CTX) or more than 50% in urinary N-telopeptide (NTX),71 the patient can be reassured that the next bone mineral density measurement will be stable or improved. In patients on oral bisphosphonates, measurement of bone turnover markers also provides evidence of compliance.
Clinical trials suggest that a numerical increase in bone mineral density can be expected in most patients on treatment, though this depends on the measurement site and the length of time between examinations. In one phase 3 trial of denosumab in postmenopausal women, only 5% of the participants had unchanged or diminished bone mineral density at the lumbar spine, and 8% at the hip, after 36 months of treatment.72 However, the CTX levels fell to below the lower limit of the reference interval as early as 1 month after commencing treatment in all denosumab-treated patients.68
Hence, bone turnover markers may be a more sensitive indicator of treatment effect than bone mineral density, but this would ultimately need to be evaluated against fracture rates in a real-world setting.
WHAT ARE THE AREAS OF UNCERTAINTY?
There are currently no guidelines for long-term management of patients on denosumab, and also no data to suggest whether patients should be switched to a weaker antiresorptive drug after a certain number of years in order to reduce the possible risk of atypical femoral fracture or osteonecrosis of the jaw.
No head-to-head trials have directly compared the antifracture efficacy of denosumab with that of other standard osteoporosis therapies. The antifracture efficacy and safety of combination therapies involving denosumab are also uncertain. For adherent patients who have a suboptimal response, there is no evidence to guide the further course of action. The International Osteoporosis Foundation guidelines suggest replacing a stronger antiresorptive with an anabolic agent, but acknowledge that this is only based on expert opinion.71
The very-long-term effects (beyond 8 years) of continuous denosumab administration on increasing the risk of atypical femoral fracture, osteonecrosis of the jaw, malignancy, or infection or the duration after which risks would start to outweigh benefits is not known. However, postmarketing safety data continue to be collected through the voluntary Post-marketing Active Safety Surveillance Program (for prespecified adverse events) in addition to the FDA’s MedWatch program.
CASE PROGRESSION
The patient described in the vignette is presented with two options—zoledronate and denosumab. She chooses denosumab. Her renal function and serum calcium are checked and are found to be satisfactory. She undergoes a dental examination, which is also satisfactory. She is counseled about the possible increased risk of infection, and then she is started on 60 mg of denosumab subcutaneously, once every 6 months.
When reviewed after 2 years, she reports no further fractures. Her bone mineral density remains stable compared with the values obtained before starting treatment. She reports no adverse effects and is happy to continue with denosumab.
A 68-year-old white woman presents with mid- thoracic back pain. Plain radiographs reveal a compression fracture of the 10th thoracic vertebra. She is diagnosed with osteoporosis on the basis of dual energy x-ray absorptiometry (DXA) scans that show T scores of –2.9 in her lumbar spine and –2.6 in her left femoral neck. Her 10-year probability of fracture is estimated as 23% for major osteoporotic fracture and 5.9% for hip fracture (based on the World Health Organization’s absolute fracture risk assessment tool, adapted for the United States, and available at www.shef.ac.uk/FRAX).
After excluding common secondary causes of osteoporosis, her physician recommends a bisphosphonate to reduce her risk of fracture, but she develops upper-gastrointestinal adverse effects with both alendronate and risedronate despite correctly following the instructions for oral administration.
What should her physician consider next?
OSTEOPOROSIS IS A MAJOR PROBLEM
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, predisposing to an increased risk of fragility fractures, particularly of the spine, hip, and wrist.
It is a major public health problem, affecting 200 million people throughout the world, with 9 million osteoporotic fractures reported in the year 2000.1 The incidence of hip fracture alone is predicted to rise to 2.6 million by the year 2025, and to 4.5 million by the year 2050.2 In the United States, the total burden was estimated to be about 2 million incident fractures in the year 2005, projected to rise by another 50% by the year 2025,3 primarily because of the aging of the population. Population studies have indeed suggested that about 40% of white women and 13% of white men over the age of 50 are at risk of sustaining an osteoporotic fracture during the remainder of their lifetime.4
The consequences of osteoporotic fractures can be devastating. Hip fractures are associated with a risk of death ranging from 8.4% to 36% during the first year after fracture.5 One-fifth of patients who sustain a hip fracture require long-term nursing home care, and more than half of the survivors do not regain their previous level of independence.
Patients with vertebral fractures are also at increased risk of death, although the results of some studies suggest that this could be the result of comorbid factors.6–9 Vertebral fractures can result in chronic back pain, loss of height from spinal deformity, reduced mobility, loss of self-esteem, and in severe cases, respiratory and digestive problems because of contact between the lower ribs and pelvis.
A person with one vertebral compression fracture is five times more likely to have another vertebral fracture,10 and a person with two or more compression fractures is 12 times more likely.11
The costs of treating osteoporotic fractures are greater than those of treating myocardial infarction or stroke12,13; they include not only direct costs incurred in treating the fracture, but also indirect societal costs owing to the long-term morbidity associated with the fracture. In the United States, the total cost of treating osteoporotic fractures was estimated at $19 billion in the year 2005.3 By 2025, the annual costs are projected to rise by almost 50%.3
A NEED FOR MORE OPTIONS
Until fairly recently, bisphosphonates were the only drugs of first choice, but adherence to oral bisphosphonate therapy is generally poor (< 50% at 1 year),14 most commonly because of dyspepsia,15 and poor adherence has been shown to be associated with increased fracture risk.16,17 Hence the need for additional therapeutic options.
In this review, we discuss denosumab, an antiresorptive drug approved by the US Food and Drug Administration (FDA) in 2010. First, we discuss its mechanism of action, efficacy, and safety, and then we offer recommendations for its use in clinical practice.
WHAT IS DENOSUMAB AND HOW DOES IT WORK?
Bone remodeling is a dynamic process involving a balance between bone resorption by osteoclasts on the one hand and new bone formation by osteoblasts on the other. A net gain in bone occurs when the activity of osteoblasts exceeds that of osteoclasts, and bone loss occurs when there is increased osteoclast activity or reduced osteoblast activity, or both. The activities of osteoblasts and osteoclasts are tightly coupled because of the opposing effects of two sets of proteins, namely, receptor activator of nuclear factor kappa b ligand (RANKL) and osteoprotegerin.
Both RANKL and osteoprotegerin are produced by osteoblasts. RANKL binds to its receptor (RANK) on preosteoclasts and osteoclasts and induces their differentiation and activation, respectively. Osteoprotegerin is the decoy receptor and natural antagonist for RANKL. By binding with RANKL, it blocks its interaction with RANK.18 In healthy individuals, a fine balance between RANKL and osteoprotegerin ensures that bone remodeling is regulated.
In postmenopausal women, estrogen deficiency leads to an imbalance between RANKL and osteoprotegerin (increased RANKL and reduced osteoprotegerin), resulting in net bone loss. This imbalance is also a feature of rheumatoid arthritis, myeloma bone disease, and osteolytic metastatic bone disease; it also occurs in those receiving androgen deprivation therapy for prostate cancer or aromatase inhibitors for breast cancer.
Denosumab is a fully human monoclonal antibody that targets RANKL.19 By binding to RANKL, this drug prevents the maturation and differentiation of preosteoclasts and promotes apoptosis of osteoclasts. Bone resorption is therefore slowed. It was parenteral osteoprotegerin that was initially developed by denosumab’s manufacturer,20 but this approach failed because neutralizing antibodies developed to osteoprotegerin, rendering it ineffective. Development of neutralizing antibodies has thus far not been a problem with denosumab.
Denosumab, with its property of RANKL inhibition, has also been used to prevent skeletal events in patients with bone metastases from solid tumors and to treat unresectable giant cell tumors of the bone (both FDA-approved indications) and hypercalcemia of malignancy. There is limited clinical experience in Paget disease of the bone as well.21–23 These other potential uses of denosumab are beyond the scope of this review.
HOW WELL DOES DENOSUMAB WORK FOR OSTEOPOROSIS?
Several phase 2 and phase 3 randomized controlled trials have evaluated the efficacy of denosumab, but only one, the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, included fracture reduction as the primary outcome measure. The rest evaluated changes in bone mineral density or in markers of bone turnover, or both.
FREEDOM was a double-blind, randomized controlled trial in 7,808 postmenopausal women with T scores between –2.5 and –4.0 at the lumbar spine or hip.24 Twenty-four percent of the patients had vertebral fractures at baseline. Patients were randomized to receive either denosumab 60 mg (n = 3,902) or placebo (n = 3,906) every 6 months for up to 36 months. All patients also received adequate calcium and vitamin D supplementation.
At 36 months, compared with those who were randomized to receive placebo, those who were randomized to denosumab had lower incidence rates of:
- New vertebral fracture
(2.3% vs 7.2%, risk ratio 0.32,
95% CI 0.26–0.41, P < .001) - Nonvertebral fracture
(6.5% vs 8.0%, risk ratio 0.80,
95% CI 0.67–0.95, P = .01) - Hip fracture
(0.7% vs 1.2%, risk ratio 0.60,
95% CI 0.37–0.97, P = .04).
Increases in bone mineral density at the lumbar spine and hip, and decreases in bone turnover markers were also significantly greater in the denosumab group. The number needed to treat to prevent one new fracture over 3 years was 21 for vertebral fracture, 67 for nonvertebral fracture, and 200 for hip fracture, reflecting the relatively low event rate in the study.
In an open-label extension of the FREEDOM trial, the fracture incidence rates among participants who continued to receive denosumab for an additional 5 years remained low, and still below those projected for a “virtual placebo cohort” (total duration of exposure of 8 years). The rates among participants who switched from placebo to denosumab were similar to those of the denosumab group from the parent trial.25,26
A subgroup analysis of the FREEDOM trial suggested that denosumab reduced the risk of new vertebral fractures irrespective of age, body mass index, femoral neck bone mineral density, prevalent vertebral fractures, or prior nonvertebral fractures (risk ratio 0.32; 95% CI 0.26–0.41, P < .001), whereas the risk of nonvertebral fractures was only reduced in those women with body mass indices less than 25 kg/m2, femoral neck bone mineral density T scores less than –2.5, and in those without a prevalent vertebral fracture.27
A post hoc analysis revealed that denosumab significantly reduced the risk of new vertebral and hip fractures even in subgroups of women at higher risk of fracture.28 At 10% fracture probability (as estimated by the FRAX risk calculator), denosumab reduced the fracture risk by 11% (P = .629), whereas at 30% probability (moderate to high risk), the reduction was 50% (P = .001).29
Other phase 2 and phase 3 trials, in postmenopausal women with low bone mineral density, demonstrated that compared with placebo, denosumab significantly increased bone mineral density at all skeletal sites, increased volumetric bone mineral density at the distal radius, improved hip structural analysis parameters, and reduced bone turnover markers.30–33 Increases in bone mineral density and reductions in bone turnover markers with denosumab have been shown in men as well.34
In a randomized controlled trial,35 improvement in bone mineral density was better in those who received the combination of denosumab and teriparatide than in those who received either drug on its own.
Denosumab has also been shown to reduce the incidence of new vertebral fractures and improve bone mineral density in men receiving androgen-deprivation therapy for nonmetastatic prostate cancer,36 and to improve bone mineral density in women with metastatic breast cancer and low bone mass who were receiving adjuvant aromatase inhibitor therapy.37
HOW DOES DENOSUMAB COMPARE WITH OTHER OSTEOPOROSIS DRUGS?
A double-blind randomized controlled trial in postmenopausal women with low bone mass demonstrated that denosumab was superior to alendronate in improving bone mineral density at all skeletal sites (3.5% vs 2.6% for total hip bone mineral density, P < .0001).38
Another double-blind trial demonstrated that in patients previously treated with alendronate, switching to denosumab resulted in significantly greater increases in bone mineral density at all skeletal sites compared with continuing with alendronate (P < .0001).39
Denosumab has also been shown to be superior to alendronate in improving cortical bone mineral density, as measured by quantitative computed tomography.40
No trial has directly compared the efficacy of denosumab with other osteoporosis drugs in reducing fracture risk, but a systematic literature review of multiple databases,41 comparing the antifracture efficacy of nine osteoporosis drugs, concluded that teriparatide, zoledronic acid, and denosumab had the highest probabilities of being most efficacious for nonvertebral and vertebral fractures, with the greatest effect sizes. Indirect comparisons of the relative risk of fracture with denosumab (based on the results of FREEDOM), alendronate, risedronate, raloxifene, and strontium (based on a meta-analysis of randomized controlled trials) are presented in Table 1.42
A 2-year randomized, open-label, crossover study43 randomized patients to receive either denosumab followed by alendronate or alendronate followed by denosumab over successive 12-month periods. The results suggested that postmenopausal women with osteoporosis were more adherent, compliant, and persistent with denosumab therapy (a subcutaneous injection every 6 months) than with alendronate therapy in the form of oral tablets, self-administered weekly (7.5% nonadherence vs 36.5% at the end of 2 years). After receiving both treatments, women reported greater satisfaction with denosumab, with 92.4% preferring it over oral alendronate. Bone mineral density remained stable when patients were switched from denosumab to alendronate, but improved further when they were switched from alendronate to denosumab.
HOW SAFE IS DENOSUMAB?
The most frequent adverse events with denosumab reported in the long-term extension of one phase 2 study were upper respiratory tract infections (13.5%), arthralgia (11.5%), and back pain (9.0%).30
Increased risk of infection, cancer, and dermatologic reactions has been a concern, as RANKL and RANK are expressed by a wide variety of cells, including T lymphocytes, B cells, and dendritic cells.44 However, there were no significant differences in the overall incidences of adverse events between patients who received denosumab and those who received placebo or alendronate in any of the phase 2, phase 3, or extension studies.
In the FREEDOM trial,24 there was no significant difference between the two groups in the overall incidence of infection (52.9% with denosumab vs 54.4% with placebo, P = .17), or serious infection (4.1% with denosumab vs 3.4% with placebo, P = .14), although the incidence of “serious” cellulitis requiring hospitalization was higher in the denosumab group (0.3% vs < 0.1%, P = .002). There were more serious infections involving the gastrointestinal system, urinary tract, and ear and cases of endocarditis in the denosumab group, but the number of events was small, and there was no relationship with the timing of administration or duration of exposure to denosumab.45 Eczema was more common in the denosumab than in the placebo group (3.0% vs 1.7%, P < .001), but the extension data from the first 3 years did not provide any evidence for an increased risk of cellulitis or eczema with denosumab.26
Although randomized controlled trials reported more cases of neoplasms in the denosumab than in the placebo groups, meta-analyses have failed to detect a statistically significant difference (risk ratio 1.11, 95% CI 0.91–1.36).46 The overall incidence of adverse and serious adverse events reported in the 8-year extension of FREEDOM were consistent with data reported in the previous extension studies.25
In the FREEDOM extension trial, four events in the long-term group (n = 2,343), and two in the crossover group (n = 2,207) were adjudicated as being consistent with osteonecrosis of the jaw.26 One mid-shaft fracture in the crossover group was adjudicated as an atypical femoral fracture. There were, however, no reports of osteonecrosis of the jaw or atypical femoral fracture in the long-term phase 2 trial after 8 years of follow-up.30 By September 2013, postmarketing safety surveillance data for denosumab (estimated exposure of 1.2 million patient-years) had recorded four cases of atypical femoral fracture. All four patients had previously been on bisphosphonates. There were also 32 reports of osteonecrosis of the jaw.47
Denosumab’s manufacturer aims to communicate the risks of treatment to health care professionals and patients. Information is available online at www.proliahcp.com/risk-evaluation-mitigation-strategy/.
WHAT ARE THE PRECAUTIONS?
Several precautions need to be taken when considering treatment with denosumab.
Antiresorptives can aggravate hypocalcemia by inhibiting bone turnover. Serum calcium should therefore be checked and preexisting hypocalcemia should be corrected before starting denosumab.48
Denosumab is contraindicated in women who are pregnant or are planning to become pregnant, as fetal loss and teratogenicity have been reported in animal experiments. (Denosumab is unlikely to be used in premenopausal women, as it is not approved for use in this group.)
There are no data on excretion of denosumab in human milk, so it should not be given to nursing mothers.
Renal impairment is not a contraindication, and no dose adjustment is necessary (even for patients on renal replacement therapy), as denosumab, being an antibody, is eliminated through the reticuloendothelial system.49,50 However, in practice, any antiresorptive agent should be used with caution in patients with severe renal impairment because of the possible presence of adynamic bone disease. Further reduction of bone turnover would be detrimental in such patients. Also, severe hypocalcemia has been reported in patients with a creatinine clearance rate less than 30 mL/min and in those receiving dialysis.51,52 Postmarketing surveillance data have reported eight cases of severe symptomatic hypocalcemia, of which seven were in patients with chronic kidney disease.47
The manufacturer suggests that patients receive a dental examination with appropriate preventive dentistry before starting denosumab to reduce the incidence of osteonecrosis of the jaw, despite the lack of evidence in support of this strategy. The American Dental Association recommends regular dental visits and maintenance of good oral hygiene for patients already established on antiresorptive therapy.53,54
SHOULD PATIENTS ON DENOSUMAB BE OFFERED A DRUG HOLIDAY?
A drug holiday (temporary discontinuation of the drug after a certain duration of treatment) has been proposed for patients receiving bisphosphonates because of the risk of atypical femoral fracture and osteonecrosis of the jaw (although small) consequent to long-term continuous suppression of bone turnover.55 The antifracture efficacy of bisphosphonates is likely to persist for an unknown length of time after discontinuation because of their long skeletal half-life, while the risks gradually diminish.
By contrast, denosumab targets RANKL in the extracellular fluid and does not become embedded within the bone tissue.56 Pharmacokinetic studies have shown that denosumab has a rapid offset of action, with a half-life of only 26 days and biological activity lasting only 6 months.57 The results of a phase 2 extension study suggest that bone mineral density starts to decline and bone turnover markers start to rise within 12 months of discontinuing denosumab.58
Although fracture risk did not increase in those who were randomized to stopping the treatment and bone mineral density increased further when treatment was restarted, a drug holiday cannot presently be recommended for patients receiving denosumab because of the lack of supportive data.
HOW COST-EFFECTIVE IS DENOSUMAB?
The wholesale acquisition cost is $825 per 60-mg prefilled syringe of denosumab, although this may vary depending on where the drug is obtained. This does not include physician-related service costs associated with administration of denosumab.
Cost-effectiveness analyses conducted in the United States, the United Kingdom, and Sweden have all concluded that denosumab would offer a cost-effective alternative to other osteoporosis medications for primary prevention and secondary prevention of fractures.59–61
The Swedish study also incorporated adherence in the cost-effectiveness model and showed that denosumab was a cost-effective alternative to oral bisphosphonates, particularly for patients who were not expected to adhere well to oral treatments.61
WHICH OSTEOPOROSIS PATIENTS ARE CANDIDATES FOR DENOSUMAB?
The FDA has approved denosumab for the treatment of postmenopausal women and men at high risk of fracture (defined as having a history of osteoporotic fracture or multiple risk factors for fracture), or in those who cannot tolerate other osteoporosis medications or for whom other medications have failed.
Denosumab is also approved for men at high risk of fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer, and for women at high risk of fracture receiving adjuvant aromatase inhibitor therapy for breast cancer.
WHAT DO THE GUIDELINES RECOMMEND?
The National Osteoporosis Foundation guidelines recommend pharmacologic treatment for patients with hip or vertebral fractures (clinical or asymptomatic); T scores lower than –2.5 at the femoral neck, total hip, or lumbar spine; and those with a 10-year probability of hip fracture of more than 3% or of a major osteoporotic fracture more than 20% based on the US-adapted FRAX calculator.62 The American College of Endocrinology guidelines have proposed similar thresholds for pharmacologic treatment, and they recommend alendronate, risedronate, zoledronate, and denosumab as first-line agents.63
The 2010 Osteoporosis Canada guidelines recommend denosumab, alendronate, risedronate, and zoledronate as first-line therapies for preventing hip, nonvertebral, and vertebral fractures in postmenopausal women (grade A recommendation).64 The National Institute of Health and Clinical Excellence in England and Wales, on the other hand, recommends denosumab only for patients who are unable to take a bisphosphonate.65
PRACTICAL PRESCRIBING TIPS
The patient described at the beginning of this article has already sustained a vertebral compression fracture, and her DXA scan shows T scores in the osteoporotic range. She is therefore at increased risk of another fragility fracture (with a fivefold higher risk of another vertebral fracture). Pharmacologic therapy should be considered. In addition, she should be encouraged to adhere to lifestyle measures such as a healthy diet and regular weight-bearing exercise, her risk of falling should be assessed, and adequate calcium and vitamin D supplementation should be given.
Secondary causes of osteoporosis are present in about 30% of women and 55% of men who have vertebral fractures.66 A complete blood count, erythrocyte sedimentation rate, bone biochemistry, 25-hydroxyvitamin D, thyroid-stimulating hormone, and renal and liver function tests should be requested in all patients. Further tests should be considered depending on the clinical evaluation and results of initial investigations.
Because this patient cannot tolerate oral bisphosphonates, she could be offered the option of annual intravenous zoledronic acid infusions or 6-monthly subcutaneous denosumab injections. In clinical trials, gastrointestinal adverse effects were noted with intravenous bisphosphonates as well, but the adverse effects reported were no different than those with placebo. The potential advantages with denosumab include better bone mineral density gains, adherence and patient satisfaction compared with oral bisphosphonates, convenient twice-yearly administration, safety in patients with renal impairment, and absence of gastrointestinal effects.
Raloxifene, a selective estrogen receptor modulator, has estrogen-like action on the bone and antiestrogen actions on the breast and uterus. Unlike standard hormone replacement therapy, raloxifene can therefore increase bone mineral density without increasing the risk of breast and endometrial cancers. However, it has only been shown to reduce the risk of vertebral fracture, not hip fracture. Hence, it would be a more appropriate choice for younger postmenopausal women. Moreover, it may cause troublesome menopausal symptoms.
Teriparatide, the recombinant parathyroid hormone, is an anabolic agent. It is very expensive, and because of this, guidelines in several countries restrict its use to women with severe osteoporosis and multiple fractures who fail to respond to standard treatments. It cannot be used for longer than 2 years because of its association with osteosarcoma in rats.
If our patient prefers denosumab, therapy should be initiated after appropriate counseling (see precautions above). The dose is 60 mg, given subcutaneously, once every 6 months.
Monitoring
There is no consensus regarding the optimal frequency for monitoring patients on treatment, owing to the lack of prospective trial data. The National Osteoporosis Foundation recommends repeating the bone mineral density measurements about 2 years after starting therapy, and about every 2 years thereafter.62 Some studies suggest that changes in bone mineral density correlate with reduction in fracture risk.67,68 A change in bone mineral density is considered significant when it is greater than the range of error of the densitometer (also known as the least significant change).69 If the bone mineral density is stable or improving, therapy could be continued, but if it is declining and the decline is greater than the least significant change, a change in therapy should be considered if no secondary causes for bone loss are evident (but see What are the areas of uncertainty? below).
The National Osteoporosis Foundation also recommends measuring a bone turnover marker at baseline and then 3 to 6 months later, as its suppression predicts greater bone mineral density responses and fracture risk reduction.70 If there is a decrease of more than 30% in serum carboxy-terminal collagen crosslinks (CTX) or more than 50% in urinary N-telopeptide (NTX),71 the patient can be reassured that the next bone mineral density measurement will be stable or improved. In patients on oral bisphosphonates, measurement of bone turnover markers also provides evidence of compliance.
Clinical trials suggest that a numerical increase in bone mineral density can be expected in most patients on treatment, though this depends on the measurement site and the length of time between examinations. In one phase 3 trial of denosumab in postmenopausal women, only 5% of the participants had unchanged or diminished bone mineral density at the lumbar spine, and 8% at the hip, after 36 months of treatment.72 However, the CTX levels fell to below the lower limit of the reference interval as early as 1 month after commencing treatment in all denosumab-treated patients.68
Hence, bone turnover markers may be a more sensitive indicator of treatment effect than bone mineral density, but this would ultimately need to be evaluated against fracture rates in a real-world setting.
WHAT ARE THE AREAS OF UNCERTAINTY?
There are currently no guidelines for long-term management of patients on denosumab, and also no data to suggest whether patients should be switched to a weaker antiresorptive drug after a certain number of years in order to reduce the possible risk of atypical femoral fracture or osteonecrosis of the jaw.
No head-to-head trials have directly compared the antifracture efficacy of denosumab with that of other standard osteoporosis therapies. The antifracture efficacy and safety of combination therapies involving denosumab are also uncertain. For adherent patients who have a suboptimal response, there is no evidence to guide the further course of action. The International Osteoporosis Foundation guidelines suggest replacing a stronger antiresorptive with an anabolic agent, but acknowledge that this is only based on expert opinion.71
The very-long-term effects (beyond 8 years) of continuous denosumab administration on increasing the risk of atypical femoral fracture, osteonecrosis of the jaw, malignancy, or infection or the duration after which risks would start to outweigh benefits is not known. However, postmarketing safety data continue to be collected through the voluntary Post-marketing Active Safety Surveillance Program (for prespecified adverse events) in addition to the FDA’s MedWatch program.
CASE PROGRESSION
The patient described in the vignette is presented with two options—zoledronate and denosumab. She chooses denosumab. Her renal function and serum calcium are checked and are found to be satisfactory. She undergoes a dental examination, which is also satisfactory. She is counseled about the possible increased risk of infection, and then she is started on 60 mg of denosumab subcutaneously, once every 6 months.
When reviewed after 2 years, she reports no further fractures. Her bone mineral density remains stable compared with the values obtained before starting treatment. She reports no adverse effects and is happy to continue with denosumab.
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17:1726–1733.
- Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int 1997; 7:407–413.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007; 22:465–475.
- Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res 1992; 7:1005–1010.
- Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009; 20:1633–1650.
- Jalava T, Sarna S, Pylkkänen L, et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 2003; 18:1254–1260.
- Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 1998; 8:291–297.
- Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 2000; 48:241–249.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999; 159:1215–1220.
- Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001; 285:320–323.
- Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991; 114:919–923.
- Piscitelli P, Iolascon G, Argentiero A, et al. Incidence and costs of hip fractures vs strokes and acute myocardial infarction in Italy: comparative analysis based on national hospitalization records. Clin Interv Aging 2012; 7:575–583.
- Johnell O, Kanis JA, Jonsson B, Oden A, Johansson H, De Laet C. The burden of hospitalised fractures in Sweden. Osteoporos Int 2005; 16:222–228.
- Confavreux CB, Canoui-Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat RD. Persistence at 1 year of oral antiosteoporotic drugs: a prospective study in a comprehensive health insurance database. Eur J Endocrinol 2012; 166:735–741.
- Biswas PN, Wilton LV, Shakir SA. Pharmacovigilance study of alendronate in England. Osteoporos Int 2003; 14:507–514.
- Landfeldt E, Ström O, Robbins S, Borgström F. Adherence to treatment of primary osteoporosis and its association to fractures—the Swedish Adherence Register Analysis (SARA). Osteoporos Int 2012; 23:433–443.
- Sampalis JS, Adachi JD, Rampakakis E, Vaillancourt J, Karellis A, Kindundu C. Long-term impact of adherence to oral bisphosphonates on osteoporotic fracture incidence. J Bone Miner Res 2012; 27:202–210.
- Schwarz EM, Ritchlin CT. Clinical development of anti-RANKL therapy. Arthritis Res Ther 2007; 9(suppl 1):S7.
- Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract 2012; 66:1139–1146.
- Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 2001; 16:348–360.
- Rizzoli R, Body JJ, Brandi ML, et al; International Osteoporosis Foundation Committee of Scientific Advisors Working Group on Cancer-Induced Bone Disease. Cancer-associated bone disease. Osteoporos Int 2013; 24:2929–2953.
- Schwarz P, Rasmussen AQ, Kvist TM, Andersen UB, Jørgensen NR. Paget’s disease of the bone after treatment with denosumab: a case report. Bone 2012; 50:1023–1025.
- Hu MI, Glezerman IG, Leboulleux S, et al. Denosumab for treatment of hypercalcemia of malignancy. J Clin Endocrinol Metab 2014; Jun 10 [Epub ahead of print].
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361:756–765.
- Papapoulos S, Lippuner K, Roux C, et al. Eight years of denosumab treatment in postmenopausal women with osteoporosis: results from the first five years of the FREEDOM extension [abstract]. Presented at the 2013 annual meeting of the American Society for Bone and Mineral Research, Baltimore, MD, October 4–7, 2013.
- Bone HG, Chapurlat R, Brandi ML, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 2013; 98:4483–4492.
- McClung MR, Boonen S, Törring O, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res 2012; 27:211–218.
- Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 2011; 96:1727–1736.
- McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 2012; 27:1480–1486.
- McClung MR, Lewiecki EM, Geller ML, et al. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int 2013; 24:227–235.
- McClung MR, Lewiecki EM, Cohen SB, et al; AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006; 354:821–831.
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 2008; 93:2149–2157.
- Genant HK, Engelke K, Hanley DA, et al. Denosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral density. Bone 2010; 47:131–139.
- Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab 2012; 97:3161–3169.
- Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013; 382:50–56.
- Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009; 361:745–755.
- Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 2008; 26:4875–4882.
- Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 2009; 24:153–161.
- Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 2010; 25:72–81.
- Seeman E, Delmas PD, Hanley DA, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 2010; 25:1886–1894.
- Hopkins RB, Goeree R, Pullenayegum E, et al. The relative efficacy of nine osteoporosis medications for reducing the rate of fractures in post-menopausal women. BMC Musculoskelet Disord 2011; 12:209.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal guidance: TA161. Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women (amended). http://publications.nice.org.uk/alendronate-etidronate-risedronate-raloxifene-strontium-ranelate-and-teriparatide-for-ta161. Accessed January 9, 2015.
- Freemantle N, Satram-Hoang S, Tang ET, et al; DAPS Investigators. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 2012; 23:317–326.
- Lewiecki EM. Safety and tolerability of denosumab for the treatment of postmenopausal osteoporosis. Drug Healthc Patient Saf 2011; 3:79–91.
- Watts NB, Roux C, Modlin JF, et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: coincidence or causal association? Osteoporos Int 2012; 23:327–337.
- von Keyserlingk C, Hopkins R, Anastasilakis A, et al. Clinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: a meta-analysis. Semin Arthritis Rheum 2011; 41:178–186.
- Geller M, Wagman RB, Ho PR, et al. Early findings from Prolia postmarketing safety surveillance for atypical femoral fracture, osteonecrosis of the jaw, severe symptomatic hypocalcemia, and anaphylaxis (abstract). Osteoporos Int 2014; 25(suppl 2). OC40; www.wco-iof-esceo.org/sites/ecceo14/docs/wco14-abstractbook.pdf. Accessed January 9, 2015.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 2011; 26:1829–1835.
- Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012; 27:1471–1479.
- Ungprasert P, Cheungpasitporn W, Srivali N, Kittanamongkolchai W, Bischof EF. Life-threatening hypocalcemia associated with denosumab in a patient with moderate renal insufficiency. Am J Emerg Med 2013; 31:756.e1–e2.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Rachner TD, Platzbecker U, Felsenberg D, Hofbauer LC. Osteonecrosis of the jaw after osteoporosis therapy with denosumab following long-term bisphosphonate therapy. Mayo Clin Proc 2013; 88:418–419.
- Epstein MS, Ephros HD, Epstein JB. Review of current literature and implications of RANKL inhibitors for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116:e437–e442.
- McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 2013; 126:13–20.
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011; 48:677–692.
- Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 2004; 19:1059–1066.
- Miller PD, Bolognese MA, Lewiecki EM, et al; Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008; 43:222–229.
- Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy 2013; 11:485–497.
- Scotland G, Waugh N, Royle P, McNamee P, Henderson R, Hollick R. Denosumab for the prevention of osteoporotic fractures in post-menopausal women: a NICE single technology appraisal. Pharmacoeconomics 2011; 29:951–961.
- Jönsson B, Ström O, Eisman JA, et al. Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 2011; 22:967–982.
- Clinician’s guide to prevention and treatment of osteoporosis. Washington DC: National Osteoporosis Foundation, 2013.
- Watts NB, Bilezikian JP, Camacho PM, et al; AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010;16(suppl 3):1–37.
- Papaioannou A, Morin S, Cheung AM, et al; Scientific Advisory Council of Osteoporosis Canada. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010; 182:1864–1673.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal: TA204. Denosumab for the prevention of osteoporotic fractures in postmenopausal women. http://guidance.nice.org.uk/TA204. Accessed January 9, 2015.
- Premaor MO, Compston JE. Testing for secondary causes of osteoporosis. BMJ 2010; 341:c6959.
- Hochberg MC, Ross PD, Black D, et al. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum 1999; 42:1246–1254.
- Eastell R, Vrijens B, Cahall DL, Ringe JD, Garnero P, Watts NB. Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J Bone Miner Res 2011; 26:1662–1669.
- Diez-Perez A, Adachi JD, Agnusdei D, et al; IOF CSA Inadequate Responders Working Group. Treatment failure in osteoporosis. Osteoporos Int 2012; 23:2769–2774.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 2003; 18:1051–1056.
- Rosen HN, Moses AC, Garber J, Ross DS, Lee SL, Greenspan SL. Utility of biochemical markers of bone turnover in the follow-up of patients treated with bisphosphonates. Calcif Tissue Int 1998; 63:363–368.
- Bolognese MA, Teglbjærg CS, Zanchetta JR, et al. Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom 2013; 16:147–153.
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17:1726–1733.
- Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int 1997; 7:407–413.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007; 22:465–475.
- Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res 1992; 7:1005–1010.
- Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009; 20:1633–1650.
- Jalava T, Sarna S, Pylkkänen L, et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 2003; 18:1254–1260.
- Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 1998; 8:291–297.
- Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 2000; 48:241–249.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999; 159:1215–1220.
- Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001; 285:320–323.
- Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991; 114:919–923.
- Piscitelli P, Iolascon G, Argentiero A, et al. Incidence and costs of hip fractures vs strokes and acute myocardial infarction in Italy: comparative analysis based on national hospitalization records. Clin Interv Aging 2012; 7:575–583.
- Johnell O, Kanis JA, Jonsson B, Oden A, Johansson H, De Laet C. The burden of hospitalised fractures in Sweden. Osteoporos Int 2005; 16:222–228.
- Confavreux CB, Canoui-Poitrine F, Schott AM, Ambrosi V, Tainturier V, Chapurlat RD. Persistence at 1 year of oral antiosteoporotic drugs: a prospective study in a comprehensive health insurance database. Eur J Endocrinol 2012; 166:735–741.
- Biswas PN, Wilton LV, Shakir SA. Pharmacovigilance study of alendronate in England. Osteoporos Int 2003; 14:507–514.
- Landfeldt E, Ström O, Robbins S, Borgström F. Adherence to treatment of primary osteoporosis and its association to fractures—the Swedish Adherence Register Analysis (SARA). Osteoporos Int 2012; 23:433–443.
- Sampalis JS, Adachi JD, Rampakakis E, Vaillancourt J, Karellis A, Kindundu C. Long-term impact of adherence to oral bisphosphonates on osteoporotic fracture incidence. J Bone Miner Res 2012; 27:202–210.
- Schwarz EM, Ritchlin CT. Clinical development of anti-RANKL therapy. Arthritis Res Ther 2007; 9(suppl 1):S7.
- Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract 2012; 66:1139–1146.
- Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 2001; 16:348–360.
- Rizzoli R, Body JJ, Brandi ML, et al; International Osteoporosis Foundation Committee of Scientific Advisors Working Group on Cancer-Induced Bone Disease. Cancer-associated bone disease. Osteoporos Int 2013; 24:2929–2953.
- Schwarz P, Rasmussen AQ, Kvist TM, Andersen UB, Jørgensen NR. Paget’s disease of the bone after treatment with denosumab: a case report. Bone 2012; 50:1023–1025.
- Hu MI, Glezerman IG, Leboulleux S, et al. Denosumab for treatment of hypercalcemia of malignancy. J Clin Endocrinol Metab 2014; Jun 10 [Epub ahead of print].
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361:756–765.
- Papapoulos S, Lippuner K, Roux C, et al. Eight years of denosumab treatment in postmenopausal women with osteoporosis: results from the first five years of the FREEDOM extension [abstract]. Presented at the 2013 annual meeting of the American Society for Bone and Mineral Research, Baltimore, MD, October 4–7, 2013.
- Bone HG, Chapurlat R, Brandi ML, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 2013; 98:4483–4492.
- McClung MR, Boonen S, Törring O, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res 2012; 27:211–218.
- Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 2011; 96:1727–1736.
- McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 2012; 27:1480–1486.
- McClung MR, Lewiecki EM, Geller ML, et al. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int 2013; 24:227–235.
- McClung MR, Lewiecki EM, Cohen SB, et al; AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006; 354:821–831.
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 2008; 93:2149–2157.
- Genant HK, Engelke K, Hanley DA, et al. Denosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral density. Bone 2010; 47:131–139.
- Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab 2012; 97:3161–3169.
- Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013; 382:50–56.
- Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009; 361:745–755.
- Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 2008; 26:4875–4882.
- Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 2009; 24:153–161.
- Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 2010; 25:72–81.
- Seeman E, Delmas PD, Hanley DA, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 2010; 25:1886–1894.
- Hopkins RB, Goeree R, Pullenayegum E, et al. The relative efficacy of nine osteoporosis medications for reducing the rate of fractures in post-menopausal women. BMC Musculoskelet Disord 2011; 12:209.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal guidance: TA161. Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women (amended). http://publications.nice.org.uk/alendronate-etidronate-risedronate-raloxifene-strontium-ranelate-and-teriparatide-for-ta161. Accessed January 9, 2015.
- Freemantle N, Satram-Hoang S, Tang ET, et al; DAPS Investigators. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 2012; 23:317–326.
- Lewiecki EM. Safety and tolerability of denosumab for the treatment of postmenopausal osteoporosis. Drug Healthc Patient Saf 2011; 3:79–91.
- Watts NB, Roux C, Modlin JF, et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: coincidence or causal association? Osteoporos Int 2012; 23:327–337.
- von Keyserlingk C, Hopkins R, Anastasilakis A, et al. Clinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: a meta-analysis. Semin Arthritis Rheum 2011; 41:178–186.
- Geller M, Wagman RB, Ho PR, et al. Early findings from Prolia postmarketing safety surveillance for atypical femoral fracture, osteonecrosis of the jaw, severe symptomatic hypocalcemia, and anaphylaxis (abstract). Osteoporos Int 2014; 25(suppl 2). OC40; www.wco-iof-esceo.org/sites/ecceo14/docs/wco14-abstractbook.pdf. Accessed January 9, 2015.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 2011; 26:1829–1835.
- Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012; 27:1471–1479.
- Ungprasert P, Cheungpasitporn W, Srivali N, Kittanamongkolchai W, Bischof EF. Life-threatening hypocalcemia associated with denosumab in a patient with moderate renal insufficiency. Am J Emerg Med 2013; 31:756.e1–e2.
- McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis 2012; 60:626–628.
- Rachner TD, Platzbecker U, Felsenberg D, Hofbauer LC. Osteonecrosis of the jaw after osteoporosis therapy with denosumab following long-term bisphosphonate therapy. Mayo Clin Proc 2013; 88:418–419.
- Epstein MS, Ephros HD, Epstein JB. Review of current literature and implications of RANKL inhibitors for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116:e437–e442.
- McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 2013; 126:13–20.
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011; 48:677–692.
- Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 2004; 19:1059–1066.
- Miller PD, Bolognese MA, Lewiecki EM, et al; Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008; 43:222–229.
- Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy 2013; 11:485–497.
- Scotland G, Waugh N, Royle P, McNamee P, Henderson R, Hollick R. Denosumab for the prevention of osteoporotic fractures in post-menopausal women: a NICE single technology appraisal. Pharmacoeconomics 2011; 29:951–961.
- Jönsson B, Ström O, Eisman JA, et al. Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int 2011; 22:967–982.
- Clinician’s guide to prevention and treatment of osteoporosis. Washington DC: National Osteoporosis Foundation, 2013.
- Watts NB, Bilezikian JP, Camacho PM, et al; AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010;16(suppl 3):1–37.
- Papaioannou A, Morin S, Cheung AM, et al; Scientific Advisory Council of Osteoporosis Canada. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010; 182:1864–1673.
- National Institute for Health and Care Excellence (NICE). NICE technology appraisal: TA204. Denosumab for the prevention of osteoporotic fractures in postmenopausal women. http://guidance.nice.org.uk/TA204. Accessed January 9, 2015.
- Premaor MO, Compston JE. Testing for secondary causes of osteoporosis. BMJ 2010; 341:c6959.
- Hochberg MC, Ross PD, Black D, et al. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum 1999; 42:1246–1254.
- Eastell R, Vrijens B, Cahall DL, Ringe JD, Garnero P, Watts NB. Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J Bone Miner Res 2011; 26:1662–1669.
- Diez-Perez A, Adachi JD, Agnusdei D, et al; IOF CSA Inadequate Responders Working Group. Treatment failure in osteoporosis. Osteoporos Int 2012; 23:2769–2774.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 2003; 18:1051–1056.
- Rosen HN, Moses AC, Garber J, Ross DS, Lee SL, Greenspan SL. Utility of biochemical markers of bone turnover in the follow-up of patients treated with bisphosphonates. Calcif Tissue Int 1998; 63:363–368.
- Bolognese MA, Teglbjærg CS, Zanchetta JR, et al. Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom 2013; 16:147–153.
KEY POINTS
- Denosumab is a fully human monoclonal antibody that targets the receptor activator of nuclear factor kappa b ligand, a key mediator of osteoclastic bone resorption.
- Commpared with placebo, denosumab has been shown to significantly reduce the risk of vertebral, nonvertebral, and hip fractures in postmenopausal women with osteoporosis.
- Patients taking denosumab are more adherent, compliant, and persistent with therapy than those taking alendronate. Denosumab is also superior to alendronate in improving bone mineral density at all skeletal sites.
- Denosumab is safe, with safety data now available for up to 8 years of exposure.
Genetics and hepatitis C: It’s good to be ‘CC’
What a difference a single nucleotide can make! The human genome contains more than 3 billion base pairs. Yet having a different nucleotide in only one pair can make a big difference in how we respond to a disease or its treatment.
Specifically, in hepatitis C virus infection, people born with the nucleotide cytosine (C) at location rs12979860 in both alleles of the gene that codes for interleukin 28B (the IL28B CC genotype) can count themselves luckier than those born with thymine (T) in this location in one of their alleles (the CT genotype) or both of their alleles (the TT genotype). Those with the CC genotype are more likely to clear the virus spontaneously, and even if the infection persists, it is less likely to progress to liver cancer and more likely to respond to treatment with interferon.
Here, we review the IL28B polymorphism and its implications in treating hepatitis C.
GENETIC POLYMORPHISM AND HUMAN DISEASE
Of the 3 billion base pairs of nucleotides, fewer than 1% differ between individuals, but this 1% is responsible for the diversity of human beings. Differences in genetic sequences among individuals are called genetic polymorphisms. A single-nucleotide polymorphism is a DNA sequence variation that occurs in a single nucleotide in the genome. For example, two sequenced DNA fragments from different individuals, AAGCCTA and AAGCTTA, contain a difference in a single nucleotide.
Genetic variations such as these underlie some of the differences in our susceptibility to disease, the severity of illness we develop, and our response to treatments. Therefore, identifying genetic polymorphisms may shed light on biologic pathways involved in diseases and may uncover new targets for therapy.1
Genome-wide association studies have looked at hundreds of thousands of single-nucleotide polymorphisms to try to identify most of the common genetic differences among people and relate them to common chronic diseases such as coronary artery disease,2 type 2 diabetes,3 stroke,4 breast cancer,5 rheumatoid arthritis,6 Alzheimer disease,7 and, more recently, hepatitis C virus infection.8
HEPATITIS C VIRUS: A MAJOR CAUSE OF LIVER DISEASE
Hepatitis C virus infection is a major cause of chronic liver disease and hepatocellular carcinoma and has become the most common indication for liver transplantation in the United States.9
This virus has six distinct genotypes throughout the world, with multiple subtypes in each genotype. (A genotype is a classification of a virus based on its RNA.9) In this review, we will focus on genotype 1; hence, “hepatitis C virus” will refer to hepatitis C virus genotype 1.
Our knowledge of the biology, pathogenesis, and treatment of hepatitis C has been advancing. Originally, fewer than 50% of patients responded to therapy with the combination of pegylated interferon and ribavirin,10,11 but since 2011 the response rate has increased to approximately 70% with the approval of the protease inhibitors telaprevir and boceprevir, used in combination with pegylated interferon and ribavirin.12–15
Unfortunately, interferon-based treatment is often complicated by side effects such as fatigue, influenza-like symptoms, hematologic abnormalities, and neuropsychiatric symptoms. An accurate way to predict response would help patients make informed decisions about antiviral treatment, taking into account the risk and possible benefit for individual patients.
GENETIC POLYMORPHISM AND HEPATITIS C VIRUS INFECTION
Genome-wide association studies have identified single-nucleotide polymorphisms in the IL28B gene that are associated with differences in response to hepatitis C treatment.8
Studying 565,759 polymorphisms in 1,137 patients, researchers at Duke University identified a single-nucleotide polymorphism at location rs12979860 in IL28B (Figure 1) that was strongly associated with response to combination therapy with pegylated interferon and ribavirin.8 The chance of cure with this standard treatment is twice as high in patients who are homozygous for cytosine in this location (the CC genotype) than in those who are heterozygous (CT) or homozygous for thymine in this location (the TT genotype) (Table 1).
Adding one of the new protease inhibitors, telaprevir or boceprevir, to the standard hepatitis C treatment substantially improves the cure rates in all three IL28B genotypes, but especially in people with CT or TT, in whom the response rate almost triples with the addition of one of these drugs. Those with the CC genotype (who are more likely to be cured with pegylated interferon and ribavirin alone) also achieve an increase (although minimal) in cure rates when a protease inhibitor is included in the regimen (TABLE 1).13–15 Thus, it remains unclear if adding a protease inhibitor to pegylated interferon plus ribavirin in patients with the IL28B CC genotype translates into added effectiveness worth the additional cost of the protease inhibitor in previously untreated patients.
Additionally, the effect of the IL28B genotype on telaprevir-based triple therapy has been disputed in more recent studies. In a subgroup analysis of the results of a trial that evaluated telaprevir in the treatment of hepatitis C, researchers found that sustained virologic response rates were significantly higher in the telaprevir group, and this was similar across the different IL28B polymorphisms.16
The favorable IL28B CC genotype is associated with higher rates of rapid virologic response to antiviral therapy.13–15 Of note, almost all patients who achieve a rapid virologic response do well, with a high rate of sustained virologic response even after a shorter duration of therapy (24 vs 48 weeks). Therefore, in addition to predicting response to interferon before starting treatment, the IL28B CC genotype may also identify patients who need only a shorter duration of therapy.
Interestingly, the C allele is much more frequent in white than in African American populations, an important observation that explains the racial difference in response to hepatitis C therapy.8
Two other research groups, from Asia and Australia, performed independent genome-wide association studies that identified different single-nucleotide polymorphisms (eg, rs8099917) in the same IL28B gene as predictors of response to treatment in patients with hepatitis C virus infection.17,18 These findings may be explained by linkage disequilibrium, which means that these single-nucleotide polymorphisms are found more frequently together in the same patient due to their proximity to each other. In this review, we will focus on the rs12979860 polymorphism; hence “IL28B genotype” will refer to the single-nucleotide polymorphism at rs12979860, unless otherwise specified.
The favorable CC genotype is less common in African Americans than in patients of other ethnicities.19 Moreover, although IL28B CC is associated with a better response rate to interferon-based antiviral therapy across all ethnicities, those of African American descent with the CC genotype are less likely to achieve a sustained virologic response than white or Hispanic Americans.8
BIOLOGIC ASSOCIATION: IL28B POLYMORPHISM AND HEPATITIS C
The interferon lambda family consists of three cytokines:
- Interleukin 29 (interferon lambda 1)
- Interleukin 28A (interferon lambda 2)
- Interleukin 28B (interferon lambda 3).
Production of these three molecules can be triggered by viral infection, and they induce antiviral activity through both innate and adaptive immune pathways. They signal through the IL10R-IL28R receptor complex.20–22 This receptor activates the JAK-STAT (Janus kinase-signal transducer and activator of transcription) pathway, which regulates a large number of interferon-stimulated genes, primarily through the interferon-stimulated response element (Figure 2).
A 2013 study found that interferon-stimulated gene expression levels in patients with normal livers were highest in those with the CC genotype, intermediate with CT, and lowest with TT. Interestingly, this pattern was reversed in those with hepatitis C virus infection, indicating a relationship between the IL28B genotype and gene expression before infection.23
The mechanism underlying the association between the IL28B polymorphism and response to hepatitis C treatment is not well understood. The unfavorable TT genotype seems to lead to continuous activation of a subset of interferon-stimulated genes in the presence of intracellular hepatitis C viral RNA. But this level of expression is not sufficient to eliminate the virus from the cells. Instead, it might lead to up-regulation of interferon-inhibitory molecules that suppress JAK-STAT signaling, thereby reducing sensitivity to interferon signaling. Therefore, the hepatocyte not only cannot clear the virus by itself, but also cannot induce strong interferon-stimulated gene expression when interferon is given during therapy.20–22
The recently identified ss469425590 polymorphism, which is located in close proximity to rs12979860 in the IL28B gene, is particularly interesting, as it suggests a possible molecular mechanism. The delta G frameshift variant creates a novel gene called IFNL4, which is transiently activated in response to hepatitis C virus infection.24IFNL4 stimulates STAT1 and STAT2 phosphorylation and induces the expression of interferon-stimulated genes. Increased interferon-stimulated gene expression has been shown to be associated with decreased response to pegylated interferon-ribavirin treatment. These observations suggest that the ss469425590 delta G allele is responsible for the increased activation of interferon-stimulated genes and the lower sustained virologic response rate observed in patients who receive pegylated interferon-ribavirin treatment. It is possible that the activation of interferon-stimulated genes in patients with the ss469425590 delta G/delta G genotype reduces interferon-stimulated gene responsiveness to interferon alpha, which normally activates interferon-stimulated genes and inhibits hepatitis C progression.24
IL28B POLYMORPHISM AND ACUTE HEPATITIS C VIRUS INFECTION
From 70% to 80% of acute hepatitis C virus infections persist and become chronic, while 20% to 30% spontaneously resolve. Epidemiologic, viral, and host factors have been associated with the differences in viral clearance or persistence, and studies have found that a strong host immune response against the virus favors viral clearance. Thus, variation in the genes involved in the immune response may contribute to one’s ability to clear the virus. Consistent with these observations, recent studies have shown that the polymorphism in the IL28 gene region encoding interferon lambda 3 strongly predicts spontaneous resolution of acute hepatitis C virus infection. People who have the IL28B CC genotype are three times more likely to spontaneously clear the virus than those with the CT or TT genotype (Figure 3).24
IL28B POLYMORPHISM AND THE NATURAL HISTORY OF HEPATITIS C
In people in whom hepatitis C virus infection persists, up to 20% develop progressive liver fibrosis and eventually cirrhosis over 10 to 20 years.19,25,26 The speed at which fibrosis develops in these patients is variable and unpredictable.25 The relationship between IL28B polymorphisms and hepatic fibrosis in patients with chronic hepatitis C virus infection has not been clearly established, although a study indicated that in patients with a known date of infection, the IL28B genotype is not associated with progression of hepatic fibrosis.27 Obstacles in this field of study are that it is difficult to determine accurately when the patient contracted the virus, and that serial liver biopsies are needed to investigate the progression of hepatic fibrosis.
Patients with chronic hepatitis C virus infection are also at higher risk of hepatocellular carcinoma compared with the general population.28 An analysis of explanted livers of patients with hepatitis C found that the prevalence of hepatocellular carcinoma in those with the unfavorable TT genotype was significantly higher than with the other genotypes.29 Similarly, an earlier study demonstrated that patients with hepatitis C-associated hepatocellular carcinoma carried the T allele more frequently.30 As with other aspects of IL28B associations with hepatitis C, these findings indicate that the C allele confers a certain degree of protection.
An important implication of these relationships is that they may eventually help identify patients at greater risk, who therefore need earlier intervention.
IL28B POLYMORPHISM AND LIVER TRANSPLANTATION
Hepatitis C virus infection always recurs after liver transplantation, with serious consequences that include cirrhosis and liver failure. Recurrent hepatitis C virus infection has become an important reason for repeat transplantation in the United States.
Results of treatment with pegylated interferon and ribavirin for recurrent hepatitis C after liver transplantation have been disappointing, with response rates lower than 30% and significant side effects.31 Identifying the factors that predict the response to therapy allows for better selection of treatment candidates.
Similar to the way the IL28B genotype predicts response to antiviral therapy in the nontransplant setting, the IL28B genotypes of both the recipient and the donor are strongly and independently associated with response to interferon-based treatment in patients with hepatitis C after liver transplantation. The IL28B CC genotype in either the recipient or the donor is associated with a higher rate of response to pegylated interferon and ribavirin combination therapy after liver transplantation.30,32 For example, the response rate to therapy after liver transplantation reaches 86% in CC-donor and CC-recipient livers, compared with 0% in TT-donor and TT-recipient livers.
Additionally, the IL28B genotype of the recipient may determine the severity of histologic recurrence of hepatitis C, as indicated by progressive hepatic fibrosis. A recipient IL28B TT genotype is associated with more severe histologic recurrence of hepatitis C.33
These data suggest that CC donor livers might be preferentially allocated to patients with hepatitis C virus infection.
IL28B AND OTHER FACTORS IN HEPATITIS C VIRUS INFECTION
Although it is tempting to think that the IL28B polymorphism is the sole predictor of response to antiviral therapy, it is but one of several known factors in the virus and the host.
While IL28B polymorphisms are the most important predictor of sustained virologic response with an interferon-based regimen, a rapid virologic response (undetectable viral load at 4 weeks) had superior predictive value and specificity in one study.34 In fact, for patients with chronic hepatitis C infection who achieved a rapid virologic response with pegylated interferon and ribavirin, the IL28B polymorphism had no effect on the rate of sustained virologic response. However, it did predict a sustained virologic response in the group who did not achieve rapid virologic response.
In a study of patients with acute hepatitis C infection,35 jaundice and the IL28 rs12979860 CC genotype both predicted spontaneous clearance. The best predictor of viral persistence was the combination of the CT or TT genotype plus the absence of jaundice, which had a predictive value of 98%.
IL28B AND THE FUTURE OF HEPATITIS C VIRUS THERAPY
New oral agents were recently approved for treating hepatitis C. As of November 2014, these included simeprevir, sofosbuvir, and ledipasvir.
Simeprevir is a second-generation NS3/4A protease inhibitor approved for use in combination with pegylated interferon and ribavirin. A recent phase 3 trial evaluating simeprevir in patients who had relapsed after prior therapy found sustained virologic response rates to be higher with simeprevir than with placebo, irrespective of IL28B status.36 This finding was similar to that of a trial of telaprevir.16
Sofosbuvir is a nucleotide analogue NS5B polymerase inhibitor that becomes incorporated into the growing RNA, inducing a chain termination event.37 In phase 3 trials,38,39 researchers found an initial rapid decrease in viral load for patients treated with this agent regardless of IL28B status.
In the NEUTRINO trial (Sofosbuvir With Peginterferon Alfa 2a and Ribavirin for 12 Weeks in Treatment-Naive Subjects With Chronic Genotype 1, 4, 5, or 6 HCV Infection),38 which used sofusbuvir in combination with interferon and ribavirin, the rate of sustained virologic response was higher in those with the favorable CC genotype (98%) than with a non-CC genotype (87%).
In COSMOS (A Study of TMC435 in Combination With PSI-7977 [GS7977] in Chronic Hepatitis C Genotype 1-Infected Prior Null Responders to Peginterferon/Ribavirin Therapy or HCV Treatment-Naive Patients),39 which used a combination of simeprevir, sofosbuvir, and ribavirin, the rate of sustained virologic response was higher in those with the CC genotype (100%) than with the TT genotype (83%; Table 1).
These new medications have radically changed the landscape of hepatitis C therapy and have also unlocked the potential for developing completely interferon-free regimens.
Other new interferon-free regimens such as ledipasvir, daclatasvir, and asunaprevir promise high rates of sustained virologic response, which makes the utility of testing for IL28B polymorphisms to predict sustained virologic response very much diminished (Table 1).40,41 However, these new drugs are expected to be expensive, and IL28B polymorphisms may be used to identify candidates who are more likely to respond to pegylated interferon and ribavirin, particularly in resource-poor settings and in developing countries. Additionally, patients who have contraindications to these newer therapies will still likely need an interferon-based regimen, and thus the IL28B polymorphism will still be important in predicting treatment response and prognosis.
IL28B WILL STILL BE RELEVANT IN THE INTERFERON-FREE AGE
The IL28B polymorphism is a strong predictor of spontaneous clearance of hepatitis C virus and responsiveness to interferon-based therapy, and testing for it has demonstrated a great potential to improve patient care. IL28B testing has become available for clinical use and may optimize the outcome of hepatitis C treatment by helping us to select the best treatment for individual patients and minimizing the duration of therapy and the side effects associated with interferon-based antiviral medications.
As newer therapies have shifted toward interferon-free regimens that offer very high sustained virologic response rates, the usefulness of IL28B polymorphism as a clinical test to predict the response rate to antiviral therapy is minimized substantially. It may remain clinically relevant in resource-poor settings and in developing countries, especially in light of the potentially prohibitive costs of the newer regimens, and for patients in whom these treatments are contraindicated. This does not minimize the lesson we learned from the discovery of the IL28B gene and the impact on our understanding of the pathogenesis of hepatitis C virus infection.
- Attia J, Ioannidis JP, Thakkinstian A, et al. How to use an article about genetic association: A: background concepts. JAMA 2009; 301:74–81.
- Samani NJ, Erdmann J, Hall AS, et al; WTCCC and the Cardiogenics Consortium. Genomewide association analysis of coronary artery disease. N Engl J Med 2007; 357:443–453.
- Zeggini E, Weedon MN, Lindgren CM, et al; Wellcome Trust Case Control Consortium (WTCCC). Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316:1336–1341.
- Matarín M, Brown WM, Scholz S, et al. A genome-wide genotyping study in patients with ischaemic stroke: initial analysis and data release. Lancet Neurol 2007; 6:414–420.
- Easton DF, Pooley KA, Dunning AM, et al; AOCS Management Group. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007; 447:1087–1093.
- Plenge RM, Seielstad M, Padyukov L, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N Engl J Med 2007; 357:1199–1209.
- Coon KD, Myers AJ, Craig DW, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J Clin Psychiatry 2007; 68:613–618.
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance (letter). Nature 2009; 461:399–401.
- Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahepatic manifestations. Cleve Clin J Med 2005; 72:1005-1019.
- Hanouneh IA, Feldstein AE, Lopez R, et al. Clinical significance of metabolic syndrome in the setting of chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 2008; 6:584–589.
- Elgouhari HM, Zein CO, Hanouneh I, Feldstein AE, Zein NN. Diabetes mellitus is associated with impaired response to antiviral therapy in chronic hepatitis C infection. Dig Dis Sci 2009; 54:2699–2705.
- Alkhouri N, Zein NN. Protease inhibitors: silver bullets for chronic hepatitis C infection? Cleve Clin J Med 2012; 79:213–222.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364:2405–2416.
- Jacobson IM, Catlett I, Marcellin P, et al. Telaprevir substantially improved SVR rates across all IL28B genotypes in the ADVANCE trial. J Hepatol 2011; 54(suppl 1):S542–S543.
- Pol S, Aerssens J, Zeuzem S, et al. Limited impact of IL28B genotype on response rates in telaprevir-treated patients with prior treatment failure. J Hepatol 2013; 58:883–889.
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009; 41:1100–1104.
- Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009; 41:1105–1109.
- Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009; 461:798–801.
- Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest 2009; 119:1745–1754.
- Marcello T, Grakoui A, Barba-Spaeth G, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 2006; 131:1887–1898.
- Doyle SE, Schreckhise H, Khuu-Duong K, et al. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 2006; 44:896–906.
- Raglow Z, Thoma-Perry C, Gilroy R, Wan YJ. IL28B genotype and the expression of ISGs in normal liver. Liver Int 2013; 33:991–998.
- Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet 2013; 45:164–171.
- Hanouneh IA, Zein NN, Askar M, Lopez R, John B. Interleukin-28B polymorphisms are associated with fibrosing cholestatic hepatitis in recurrent hepatitis C after liver transplantation. Clin Transplant 2012; 26:E335–E336.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825–832.
- Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000; 284:450–456.
- Bochud PY, Cai T, Overbeck K, et al; Swiss Hepatitis C Cohort Study Group. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol 2009; 51:655–666.
- Marabita F, Aghemo A, De Nicola S, et al. Genetic variation in the interleukin-28B gene is not associated with fibrosis progression in patients with chronic hepatitis C and known date of infection. Hepatology 2011; 54:1127–1134.
- Fabris C, Falleti E, Cussigh A, et al. IL-28B rs12979860 C/T allele distribution in patients with liver cirrhosis: role in the course of chronic viral hepatitis and the development of HCC. J Hepatol 2011; 54:716–722.
- Eurich D, Boas-Knoop S, Bahra M, et al. Role of IL28B polymorphism in the development of hepatitis C virus-induced hepatocellular carcinoma, graft fibrosis, and posttransplant antiviral therapy. Transplantation 2012; 93:644–649.
- Hanouneh IA, Miller C, Aucejo F, Lopez R, Quinn MK, Zein NN. Recurrent hepatitis C after liver transplantation: on-treatment prediction of response to peginterferon/ribavirin therapy. Liver Transpl 2008; 14:53–58.
- Charlton MR, Thompson A, Veldt BJ, et al. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology 2011; 53:317–324.
- Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 2010; 139:120–129.e18.
- Beinhardt S, Payer BA, Datz C, et al. A diagnostic score for the prediction of spontaneous resolution of acute hepatitis C virus infection. J Hepatol 2013; 59:972–977.
- Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology 2014; 146:1669–1679.e3.
- Sofia MJ, Bao D, Chang W, et al. Discovery of a ß-d-2’-deoxy-2’-ß-fluoro-2’-ß-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J Med Chem 2010; 53:7202–7218.
- Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–1887.
- Sulkowski MS, Jacobson IM, Ghalib R, et al. Once-daily simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in HCV genotype 1 prior null responders with metavir F0-2: COSMOS study subgroup analysis. 49th EASL, April 2014, London. Oral abstract O7. www.natap.org/2014/EASL/EASL_46.htm. Accesed January 9, 2015.
- Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 2012; 366:216–224.
- Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–1898.
What a difference a single nucleotide can make! The human genome contains more than 3 billion base pairs. Yet having a different nucleotide in only one pair can make a big difference in how we respond to a disease or its treatment.
Specifically, in hepatitis C virus infection, people born with the nucleotide cytosine (C) at location rs12979860 in both alleles of the gene that codes for interleukin 28B (the IL28B CC genotype) can count themselves luckier than those born with thymine (T) in this location in one of their alleles (the CT genotype) or both of their alleles (the TT genotype). Those with the CC genotype are more likely to clear the virus spontaneously, and even if the infection persists, it is less likely to progress to liver cancer and more likely to respond to treatment with interferon.
Here, we review the IL28B polymorphism and its implications in treating hepatitis C.
GENETIC POLYMORPHISM AND HUMAN DISEASE
Of the 3 billion base pairs of nucleotides, fewer than 1% differ between individuals, but this 1% is responsible for the diversity of human beings. Differences in genetic sequences among individuals are called genetic polymorphisms. A single-nucleotide polymorphism is a DNA sequence variation that occurs in a single nucleotide in the genome. For example, two sequenced DNA fragments from different individuals, AAGCCTA and AAGCTTA, contain a difference in a single nucleotide.
Genetic variations such as these underlie some of the differences in our susceptibility to disease, the severity of illness we develop, and our response to treatments. Therefore, identifying genetic polymorphisms may shed light on biologic pathways involved in diseases and may uncover new targets for therapy.1
Genome-wide association studies have looked at hundreds of thousands of single-nucleotide polymorphisms to try to identify most of the common genetic differences among people and relate them to common chronic diseases such as coronary artery disease,2 type 2 diabetes,3 stroke,4 breast cancer,5 rheumatoid arthritis,6 Alzheimer disease,7 and, more recently, hepatitis C virus infection.8
HEPATITIS C VIRUS: A MAJOR CAUSE OF LIVER DISEASE
Hepatitis C virus infection is a major cause of chronic liver disease and hepatocellular carcinoma and has become the most common indication for liver transplantation in the United States.9
This virus has six distinct genotypes throughout the world, with multiple subtypes in each genotype. (A genotype is a classification of a virus based on its RNA.9) In this review, we will focus on genotype 1; hence, “hepatitis C virus” will refer to hepatitis C virus genotype 1.
Our knowledge of the biology, pathogenesis, and treatment of hepatitis C has been advancing. Originally, fewer than 50% of patients responded to therapy with the combination of pegylated interferon and ribavirin,10,11 but since 2011 the response rate has increased to approximately 70% with the approval of the protease inhibitors telaprevir and boceprevir, used in combination with pegylated interferon and ribavirin.12–15
Unfortunately, interferon-based treatment is often complicated by side effects such as fatigue, influenza-like symptoms, hematologic abnormalities, and neuropsychiatric symptoms. An accurate way to predict response would help patients make informed decisions about antiviral treatment, taking into account the risk and possible benefit for individual patients.
GENETIC POLYMORPHISM AND HEPATITIS C VIRUS INFECTION
Genome-wide association studies have identified single-nucleotide polymorphisms in the IL28B gene that are associated with differences in response to hepatitis C treatment.8
Studying 565,759 polymorphisms in 1,137 patients, researchers at Duke University identified a single-nucleotide polymorphism at location rs12979860 in IL28B (Figure 1) that was strongly associated with response to combination therapy with pegylated interferon and ribavirin.8 The chance of cure with this standard treatment is twice as high in patients who are homozygous for cytosine in this location (the CC genotype) than in those who are heterozygous (CT) or homozygous for thymine in this location (the TT genotype) (Table 1).
Adding one of the new protease inhibitors, telaprevir or boceprevir, to the standard hepatitis C treatment substantially improves the cure rates in all three IL28B genotypes, but especially in people with CT or TT, in whom the response rate almost triples with the addition of one of these drugs. Those with the CC genotype (who are more likely to be cured with pegylated interferon and ribavirin alone) also achieve an increase (although minimal) in cure rates when a protease inhibitor is included in the regimen (TABLE 1).13–15 Thus, it remains unclear if adding a protease inhibitor to pegylated interferon plus ribavirin in patients with the IL28B CC genotype translates into added effectiveness worth the additional cost of the protease inhibitor in previously untreated patients.
Additionally, the effect of the IL28B genotype on telaprevir-based triple therapy has been disputed in more recent studies. In a subgroup analysis of the results of a trial that evaluated telaprevir in the treatment of hepatitis C, researchers found that sustained virologic response rates were significantly higher in the telaprevir group, and this was similar across the different IL28B polymorphisms.16
The favorable IL28B CC genotype is associated with higher rates of rapid virologic response to antiviral therapy.13–15 Of note, almost all patients who achieve a rapid virologic response do well, with a high rate of sustained virologic response even after a shorter duration of therapy (24 vs 48 weeks). Therefore, in addition to predicting response to interferon before starting treatment, the IL28B CC genotype may also identify patients who need only a shorter duration of therapy.
Interestingly, the C allele is much more frequent in white than in African American populations, an important observation that explains the racial difference in response to hepatitis C therapy.8
Two other research groups, from Asia and Australia, performed independent genome-wide association studies that identified different single-nucleotide polymorphisms (eg, rs8099917) in the same IL28B gene as predictors of response to treatment in patients with hepatitis C virus infection.17,18 These findings may be explained by linkage disequilibrium, which means that these single-nucleotide polymorphisms are found more frequently together in the same patient due to their proximity to each other. In this review, we will focus on the rs12979860 polymorphism; hence “IL28B genotype” will refer to the single-nucleotide polymorphism at rs12979860, unless otherwise specified.
The favorable CC genotype is less common in African Americans than in patients of other ethnicities.19 Moreover, although IL28B CC is associated with a better response rate to interferon-based antiviral therapy across all ethnicities, those of African American descent with the CC genotype are less likely to achieve a sustained virologic response than white or Hispanic Americans.8
BIOLOGIC ASSOCIATION: IL28B POLYMORPHISM AND HEPATITIS C
The interferon lambda family consists of three cytokines:
- Interleukin 29 (interferon lambda 1)
- Interleukin 28A (interferon lambda 2)
- Interleukin 28B (interferon lambda 3).
Production of these three molecules can be triggered by viral infection, and they induce antiviral activity through both innate and adaptive immune pathways. They signal through the IL10R-IL28R receptor complex.20–22 This receptor activates the JAK-STAT (Janus kinase-signal transducer and activator of transcription) pathway, which regulates a large number of interferon-stimulated genes, primarily through the interferon-stimulated response element (Figure 2).
A 2013 study found that interferon-stimulated gene expression levels in patients with normal livers were highest in those with the CC genotype, intermediate with CT, and lowest with TT. Interestingly, this pattern was reversed in those with hepatitis C virus infection, indicating a relationship between the IL28B genotype and gene expression before infection.23
The mechanism underlying the association between the IL28B polymorphism and response to hepatitis C treatment is not well understood. The unfavorable TT genotype seems to lead to continuous activation of a subset of interferon-stimulated genes in the presence of intracellular hepatitis C viral RNA. But this level of expression is not sufficient to eliminate the virus from the cells. Instead, it might lead to up-regulation of interferon-inhibitory molecules that suppress JAK-STAT signaling, thereby reducing sensitivity to interferon signaling. Therefore, the hepatocyte not only cannot clear the virus by itself, but also cannot induce strong interferon-stimulated gene expression when interferon is given during therapy.20–22
The recently identified ss469425590 polymorphism, which is located in close proximity to rs12979860 in the IL28B gene, is particularly interesting, as it suggests a possible molecular mechanism. The delta G frameshift variant creates a novel gene called IFNL4, which is transiently activated in response to hepatitis C virus infection.24IFNL4 stimulates STAT1 and STAT2 phosphorylation and induces the expression of interferon-stimulated genes. Increased interferon-stimulated gene expression has been shown to be associated with decreased response to pegylated interferon-ribavirin treatment. These observations suggest that the ss469425590 delta G allele is responsible for the increased activation of interferon-stimulated genes and the lower sustained virologic response rate observed in patients who receive pegylated interferon-ribavirin treatment. It is possible that the activation of interferon-stimulated genes in patients with the ss469425590 delta G/delta G genotype reduces interferon-stimulated gene responsiveness to interferon alpha, which normally activates interferon-stimulated genes and inhibits hepatitis C progression.24
IL28B POLYMORPHISM AND ACUTE HEPATITIS C VIRUS INFECTION
From 70% to 80% of acute hepatitis C virus infections persist and become chronic, while 20% to 30% spontaneously resolve. Epidemiologic, viral, and host factors have been associated with the differences in viral clearance or persistence, and studies have found that a strong host immune response against the virus favors viral clearance. Thus, variation in the genes involved in the immune response may contribute to one’s ability to clear the virus. Consistent with these observations, recent studies have shown that the polymorphism in the IL28 gene region encoding interferon lambda 3 strongly predicts spontaneous resolution of acute hepatitis C virus infection. People who have the IL28B CC genotype are three times more likely to spontaneously clear the virus than those with the CT or TT genotype (Figure 3).24
IL28B POLYMORPHISM AND THE NATURAL HISTORY OF HEPATITIS C
In people in whom hepatitis C virus infection persists, up to 20% develop progressive liver fibrosis and eventually cirrhosis over 10 to 20 years.19,25,26 The speed at which fibrosis develops in these patients is variable and unpredictable.25 The relationship between IL28B polymorphisms and hepatic fibrosis in patients with chronic hepatitis C virus infection has not been clearly established, although a study indicated that in patients with a known date of infection, the IL28B genotype is not associated with progression of hepatic fibrosis.27 Obstacles in this field of study are that it is difficult to determine accurately when the patient contracted the virus, and that serial liver biopsies are needed to investigate the progression of hepatic fibrosis.
Patients with chronic hepatitis C virus infection are also at higher risk of hepatocellular carcinoma compared with the general population.28 An analysis of explanted livers of patients with hepatitis C found that the prevalence of hepatocellular carcinoma in those with the unfavorable TT genotype was significantly higher than with the other genotypes.29 Similarly, an earlier study demonstrated that patients with hepatitis C-associated hepatocellular carcinoma carried the T allele more frequently.30 As with other aspects of IL28B associations with hepatitis C, these findings indicate that the C allele confers a certain degree of protection.
An important implication of these relationships is that they may eventually help identify patients at greater risk, who therefore need earlier intervention.
IL28B POLYMORPHISM AND LIVER TRANSPLANTATION
Hepatitis C virus infection always recurs after liver transplantation, with serious consequences that include cirrhosis and liver failure. Recurrent hepatitis C virus infection has become an important reason for repeat transplantation in the United States.
Results of treatment with pegylated interferon and ribavirin for recurrent hepatitis C after liver transplantation have been disappointing, with response rates lower than 30% and significant side effects.31 Identifying the factors that predict the response to therapy allows for better selection of treatment candidates.
Similar to the way the IL28B genotype predicts response to antiviral therapy in the nontransplant setting, the IL28B genotypes of both the recipient and the donor are strongly and independently associated with response to interferon-based treatment in patients with hepatitis C after liver transplantation. The IL28B CC genotype in either the recipient or the donor is associated with a higher rate of response to pegylated interferon and ribavirin combination therapy after liver transplantation.30,32 For example, the response rate to therapy after liver transplantation reaches 86% in CC-donor and CC-recipient livers, compared with 0% in TT-donor and TT-recipient livers.
Additionally, the IL28B genotype of the recipient may determine the severity of histologic recurrence of hepatitis C, as indicated by progressive hepatic fibrosis. A recipient IL28B TT genotype is associated with more severe histologic recurrence of hepatitis C.33
These data suggest that CC donor livers might be preferentially allocated to patients with hepatitis C virus infection.
IL28B AND OTHER FACTORS IN HEPATITIS C VIRUS INFECTION
Although it is tempting to think that the IL28B polymorphism is the sole predictor of response to antiviral therapy, it is but one of several known factors in the virus and the host.
While IL28B polymorphisms are the most important predictor of sustained virologic response with an interferon-based regimen, a rapid virologic response (undetectable viral load at 4 weeks) had superior predictive value and specificity in one study.34 In fact, for patients with chronic hepatitis C infection who achieved a rapid virologic response with pegylated interferon and ribavirin, the IL28B polymorphism had no effect on the rate of sustained virologic response. However, it did predict a sustained virologic response in the group who did not achieve rapid virologic response.
In a study of patients with acute hepatitis C infection,35 jaundice and the IL28 rs12979860 CC genotype both predicted spontaneous clearance. The best predictor of viral persistence was the combination of the CT or TT genotype plus the absence of jaundice, which had a predictive value of 98%.
IL28B AND THE FUTURE OF HEPATITIS C VIRUS THERAPY
New oral agents were recently approved for treating hepatitis C. As of November 2014, these included simeprevir, sofosbuvir, and ledipasvir.
Simeprevir is a second-generation NS3/4A protease inhibitor approved for use in combination with pegylated interferon and ribavirin. A recent phase 3 trial evaluating simeprevir in patients who had relapsed after prior therapy found sustained virologic response rates to be higher with simeprevir than with placebo, irrespective of IL28B status.36 This finding was similar to that of a trial of telaprevir.16
Sofosbuvir is a nucleotide analogue NS5B polymerase inhibitor that becomes incorporated into the growing RNA, inducing a chain termination event.37 In phase 3 trials,38,39 researchers found an initial rapid decrease in viral load for patients treated with this agent regardless of IL28B status.
In the NEUTRINO trial (Sofosbuvir With Peginterferon Alfa 2a and Ribavirin for 12 Weeks in Treatment-Naive Subjects With Chronic Genotype 1, 4, 5, or 6 HCV Infection),38 which used sofusbuvir in combination with interferon and ribavirin, the rate of sustained virologic response was higher in those with the favorable CC genotype (98%) than with a non-CC genotype (87%).
In COSMOS (A Study of TMC435 in Combination With PSI-7977 [GS7977] in Chronic Hepatitis C Genotype 1-Infected Prior Null Responders to Peginterferon/Ribavirin Therapy or HCV Treatment-Naive Patients),39 which used a combination of simeprevir, sofosbuvir, and ribavirin, the rate of sustained virologic response was higher in those with the CC genotype (100%) than with the TT genotype (83%; Table 1).
These new medications have radically changed the landscape of hepatitis C therapy and have also unlocked the potential for developing completely interferon-free regimens.
Other new interferon-free regimens such as ledipasvir, daclatasvir, and asunaprevir promise high rates of sustained virologic response, which makes the utility of testing for IL28B polymorphisms to predict sustained virologic response very much diminished (Table 1).40,41 However, these new drugs are expected to be expensive, and IL28B polymorphisms may be used to identify candidates who are more likely to respond to pegylated interferon and ribavirin, particularly in resource-poor settings and in developing countries. Additionally, patients who have contraindications to these newer therapies will still likely need an interferon-based regimen, and thus the IL28B polymorphism will still be important in predicting treatment response and prognosis.
IL28B WILL STILL BE RELEVANT IN THE INTERFERON-FREE AGE
The IL28B polymorphism is a strong predictor of spontaneous clearance of hepatitis C virus and responsiveness to interferon-based therapy, and testing for it has demonstrated a great potential to improve patient care. IL28B testing has become available for clinical use and may optimize the outcome of hepatitis C treatment by helping us to select the best treatment for individual patients and minimizing the duration of therapy and the side effects associated with interferon-based antiviral medications.
As newer therapies have shifted toward interferon-free regimens that offer very high sustained virologic response rates, the usefulness of IL28B polymorphism as a clinical test to predict the response rate to antiviral therapy is minimized substantially. It may remain clinically relevant in resource-poor settings and in developing countries, especially in light of the potentially prohibitive costs of the newer regimens, and for patients in whom these treatments are contraindicated. This does not minimize the lesson we learned from the discovery of the IL28B gene and the impact on our understanding of the pathogenesis of hepatitis C virus infection.
What a difference a single nucleotide can make! The human genome contains more than 3 billion base pairs. Yet having a different nucleotide in only one pair can make a big difference in how we respond to a disease or its treatment.
Specifically, in hepatitis C virus infection, people born with the nucleotide cytosine (C) at location rs12979860 in both alleles of the gene that codes for interleukin 28B (the IL28B CC genotype) can count themselves luckier than those born with thymine (T) in this location in one of their alleles (the CT genotype) or both of their alleles (the TT genotype). Those with the CC genotype are more likely to clear the virus spontaneously, and even if the infection persists, it is less likely to progress to liver cancer and more likely to respond to treatment with interferon.
Here, we review the IL28B polymorphism and its implications in treating hepatitis C.
GENETIC POLYMORPHISM AND HUMAN DISEASE
Of the 3 billion base pairs of nucleotides, fewer than 1% differ between individuals, but this 1% is responsible for the diversity of human beings. Differences in genetic sequences among individuals are called genetic polymorphisms. A single-nucleotide polymorphism is a DNA sequence variation that occurs in a single nucleotide in the genome. For example, two sequenced DNA fragments from different individuals, AAGCCTA and AAGCTTA, contain a difference in a single nucleotide.
Genetic variations such as these underlie some of the differences in our susceptibility to disease, the severity of illness we develop, and our response to treatments. Therefore, identifying genetic polymorphisms may shed light on biologic pathways involved in diseases and may uncover new targets for therapy.1
Genome-wide association studies have looked at hundreds of thousands of single-nucleotide polymorphisms to try to identify most of the common genetic differences among people and relate them to common chronic diseases such as coronary artery disease,2 type 2 diabetes,3 stroke,4 breast cancer,5 rheumatoid arthritis,6 Alzheimer disease,7 and, more recently, hepatitis C virus infection.8
HEPATITIS C VIRUS: A MAJOR CAUSE OF LIVER DISEASE
Hepatitis C virus infection is a major cause of chronic liver disease and hepatocellular carcinoma and has become the most common indication for liver transplantation in the United States.9
This virus has six distinct genotypes throughout the world, with multiple subtypes in each genotype. (A genotype is a classification of a virus based on its RNA.9) In this review, we will focus on genotype 1; hence, “hepatitis C virus” will refer to hepatitis C virus genotype 1.
Our knowledge of the biology, pathogenesis, and treatment of hepatitis C has been advancing. Originally, fewer than 50% of patients responded to therapy with the combination of pegylated interferon and ribavirin,10,11 but since 2011 the response rate has increased to approximately 70% with the approval of the protease inhibitors telaprevir and boceprevir, used in combination with pegylated interferon and ribavirin.12–15
Unfortunately, interferon-based treatment is often complicated by side effects such as fatigue, influenza-like symptoms, hematologic abnormalities, and neuropsychiatric symptoms. An accurate way to predict response would help patients make informed decisions about antiviral treatment, taking into account the risk and possible benefit for individual patients.
GENETIC POLYMORPHISM AND HEPATITIS C VIRUS INFECTION
Genome-wide association studies have identified single-nucleotide polymorphisms in the IL28B gene that are associated with differences in response to hepatitis C treatment.8
Studying 565,759 polymorphisms in 1,137 patients, researchers at Duke University identified a single-nucleotide polymorphism at location rs12979860 in IL28B (Figure 1) that was strongly associated with response to combination therapy with pegylated interferon and ribavirin.8 The chance of cure with this standard treatment is twice as high in patients who are homozygous for cytosine in this location (the CC genotype) than in those who are heterozygous (CT) or homozygous for thymine in this location (the TT genotype) (Table 1).
Adding one of the new protease inhibitors, telaprevir or boceprevir, to the standard hepatitis C treatment substantially improves the cure rates in all three IL28B genotypes, but especially in people with CT or TT, in whom the response rate almost triples with the addition of one of these drugs. Those with the CC genotype (who are more likely to be cured with pegylated interferon and ribavirin alone) also achieve an increase (although minimal) in cure rates when a protease inhibitor is included in the regimen (TABLE 1).13–15 Thus, it remains unclear if adding a protease inhibitor to pegylated interferon plus ribavirin in patients with the IL28B CC genotype translates into added effectiveness worth the additional cost of the protease inhibitor in previously untreated patients.
Additionally, the effect of the IL28B genotype on telaprevir-based triple therapy has been disputed in more recent studies. In a subgroup analysis of the results of a trial that evaluated telaprevir in the treatment of hepatitis C, researchers found that sustained virologic response rates were significantly higher in the telaprevir group, and this was similar across the different IL28B polymorphisms.16
The favorable IL28B CC genotype is associated with higher rates of rapid virologic response to antiviral therapy.13–15 Of note, almost all patients who achieve a rapid virologic response do well, with a high rate of sustained virologic response even after a shorter duration of therapy (24 vs 48 weeks). Therefore, in addition to predicting response to interferon before starting treatment, the IL28B CC genotype may also identify patients who need only a shorter duration of therapy.
Interestingly, the C allele is much more frequent in white than in African American populations, an important observation that explains the racial difference in response to hepatitis C therapy.8
Two other research groups, from Asia and Australia, performed independent genome-wide association studies that identified different single-nucleotide polymorphisms (eg, rs8099917) in the same IL28B gene as predictors of response to treatment in patients with hepatitis C virus infection.17,18 These findings may be explained by linkage disequilibrium, which means that these single-nucleotide polymorphisms are found more frequently together in the same patient due to their proximity to each other. In this review, we will focus on the rs12979860 polymorphism; hence “IL28B genotype” will refer to the single-nucleotide polymorphism at rs12979860, unless otherwise specified.
The favorable CC genotype is less common in African Americans than in patients of other ethnicities.19 Moreover, although IL28B CC is associated with a better response rate to interferon-based antiviral therapy across all ethnicities, those of African American descent with the CC genotype are less likely to achieve a sustained virologic response than white or Hispanic Americans.8
BIOLOGIC ASSOCIATION: IL28B POLYMORPHISM AND HEPATITIS C
The interferon lambda family consists of three cytokines:
- Interleukin 29 (interferon lambda 1)
- Interleukin 28A (interferon lambda 2)
- Interleukin 28B (interferon lambda 3).
Production of these three molecules can be triggered by viral infection, and they induce antiviral activity through both innate and adaptive immune pathways. They signal through the IL10R-IL28R receptor complex.20–22 This receptor activates the JAK-STAT (Janus kinase-signal transducer and activator of transcription) pathway, which regulates a large number of interferon-stimulated genes, primarily through the interferon-stimulated response element (Figure 2).
A 2013 study found that interferon-stimulated gene expression levels in patients with normal livers were highest in those with the CC genotype, intermediate with CT, and lowest with TT. Interestingly, this pattern was reversed in those with hepatitis C virus infection, indicating a relationship between the IL28B genotype and gene expression before infection.23
The mechanism underlying the association between the IL28B polymorphism and response to hepatitis C treatment is not well understood. The unfavorable TT genotype seems to lead to continuous activation of a subset of interferon-stimulated genes in the presence of intracellular hepatitis C viral RNA. But this level of expression is not sufficient to eliminate the virus from the cells. Instead, it might lead to up-regulation of interferon-inhibitory molecules that suppress JAK-STAT signaling, thereby reducing sensitivity to interferon signaling. Therefore, the hepatocyte not only cannot clear the virus by itself, but also cannot induce strong interferon-stimulated gene expression when interferon is given during therapy.20–22
The recently identified ss469425590 polymorphism, which is located in close proximity to rs12979860 in the IL28B gene, is particularly interesting, as it suggests a possible molecular mechanism. The delta G frameshift variant creates a novel gene called IFNL4, which is transiently activated in response to hepatitis C virus infection.24IFNL4 stimulates STAT1 and STAT2 phosphorylation and induces the expression of interferon-stimulated genes. Increased interferon-stimulated gene expression has been shown to be associated with decreased response to pegylated interferon-ribavirin treatment. These observations suggest that the ss469425590 delta G allele is responsible for the increased activation of interferon-stimulated genes and the lower sustained virologic response rate observed in patients who receive pegylated interferon-ribavirin treatment. It is possible that the activation of interferon-stimulated genes in patients with the ss469425590 delta G/delta G genotype reduces interferon-stimulated gene responsiveness to interferon alpha, which normally activates interferon-stimulated genes and inhibits hepatitis C progression.24
IL28B POLYMORPHISM AND ACUTE HEPATITIS C VIRUS INFECTION
From 70% to 80% of acute hepatitis C virus infections persist and become chronic, while 20% to 30% spontaneously resolve. Epidemiologic, viral, and host factors have been associated with the differences in viral clearance or persistence, and studies have found that a strong host immune response against the virus favors viral clearance. Thus, variation in the genes involved in the immune response may contribute to one’s ability to clear the virus. Consistent with these observations, recent studies have shown that the polymorphism in the IL28 gene region encoding interferon lambda 3 strongly predicts spontaneous resolution of acute hepatitis C virus infection. People who have the IL28B CC genotype are three times more likely to spontaneously clear the virus than those with the CT or TT genotype (Figure 3).24
IL28B POLYMORPHISM AND THE NATURAL HISTORY OF HEPATITIS C
In people in whom hepatitis C virus infection persists, up to 20% develop progressive liver fibrosis and eventually cirrhosis over 10 to 20 years.19,25,26 The speed at which fibrosis develops in these patients is variable and unpredictable.25 The relationship between IL28B polymorphisms and hepatic fibrosis in patients with chronic hepatitis C virus infection has not been clearly established, although a study indicated that in patients with a known date of infection, the IL28B genotype is not associated with progression of hepatic fibrosis.27 Obstacles in this field of study are that it is difficult to determine accurately when the patient contracted the virus, and that serial liver biopsies are needed to investigate the progression of hepatic fibrosis.
Patients with chronic hepatitis C virus infection are also at higher risk of hepatocellular carcinoma compared with the general population.28 An analysis of explanted livers of patients with hepatitis C found that the prevalence of hepatocellular carcinoma in those with the unfavorable TT genotype was significantly higher than with the other genotypes.29 Similarly, an earlier study demonstrated that patients with hepatitis C-associated hepatocellular carcinoma carried the T allele more frequently.30 As with other aspects of IL28B associations with hepatitis C, these findings indicate that the C allele confers a certain degree of protection.
An important implication of these relationships is that they may eventually help identify patients at greater risk, who therefore need earlier intervention.
IL28B POLYMORPHISM AND LIVER TRANSPLANTATION
Hepatitis C virus infection always recurs after liver transplantation, with serious consequences that include cirrhosis and liver failure. Recurrent hepatitis C virus infection has become an important reason for repeat transplantation in the United States.
Results of treatment with pegylated interferon and ribavirin for recurrent hepatitis C after liver transplantation have been disappointing, with response rates lower than 30% and significant side effects.31 Identifying the factors that predict the response to therapy allows for better selection of treatment candidates.
Similar to the way the IL28B genotype predicts response to antiviral therapy in the nontransplant setting, the IL28B genotypes of both the recipient and the donor are strongly and independently associated with response to interferon-based treatment in patients with hepatitis C after liver transplantation. The IL28B CC genotype in either the recipient or the donor is associated with a higher rate of response to pegylated interferon and ribavirin combination therapy after liver transplantation.30,32 For example, the response rate to therapy after liver transplantation reaches 86% in CC-donor and CC-recipient livers, compared with 0% in TT-donor and TT-recipient livers.
Additionally, the IL28B genotype of the recipient may determine the severity of histologic recurrence of hepatitis C, as indicated by progressive hepatic fibrosis. A recipient IL28B TT genotype is associated with more severe histologic recurrence of hepatitis C.33
These data suggest that CC donor livers might be preferentially allocated to patients with hepatitis C virus infection.
IL28B AND OTHER FACTORS IN HEPATITIS C VIRUS INFECTION
Although it is tempting to think that the IL28B polymorphism is the sole predictor of response to antiviral therapy, it is but one of several known factors in the virus and the host.
While IL28B polymorphisms are the most important predictor of sustained virologic response with an interferon-based regimen, a rapid virologic response (undetectable viral load at 4 weeks) had superior predictive value and specificity in one study.34 In fact, for patients with chronic hepatitis C infection who achieved a rapid virologic response with pegylated interferon and ribavirin, the IL28B polymorphism had no effect on the rate of sustained virologic response. However, it did predict a sustained virologic response in the group who did not achieve rapid virologic response.
In a study of patients with acute hepatitis C infection,35 jaundice and the IL28 rs12979860 CC genotype both predicted spontaneous clearance. The best predictor of viral persistence was the combination of the CT or TT genotype plus the absence of jaundice, which had a predictive value of 98%.
IL28B AND THE FUTURE OF HEPATITIS C VIRUS THERAPY
New oral agents were recently approved for treating hepatitis C. As of November 2014, these included simeprevir, sofosbuvir, and ledipasvir.
Simeprevir is a second-generation NS3/4A protease inhibitor approved for use in combination with pegylated interferon and ribavirin. A recent phase 3 trial evaluating simeprevir in patients who had relapsed after prior therapy found sustained virologic response rates to be higher with simeprevir than with placebo, irrespective of IL28B status.36 This finding was similar to that of a trial of telaprevir.16
Sofosbuvir is a nucleotide analogue NS5B polymerase inhibitor that becomes incorporated into the growing RNA, inducing a chain termination event.37 In phase 3 trials,38,39 researchers found an initial rapid decrease in viral load for patients treated with this agent regardless of IL28B status.
In the NEUTRINO trial (Sofosbuvir With Peginterferon Alfa 2a and Ribavirin for 12 Weeks in Treatment-Naive Subjects With Chronic Genotype 1, 4, 5, or 6 HCV Infection),38 which used sofusbuvir in combination with interferon and ribavirin, the rate of sustained virologic response was higher in those with the favorable CC genotype (98%) than with a non-CC genotype (87%).
In COSMOS (A Study of TMC435 in Combination With PSI-7977 [GS7977] in Chronic Hepatitis C Genotype 1-Infected Prior Null Responders to Peginterferon/Ribavirin Therapy or HCV Treatment-Naive Patients),39 which used a combination of simeprevir, sofosbuvir, and ribavirin, the rate of sustained virologic response was higher in those with the CC genotype (100%) than with the TT genotype (83%; Table 1).
These new medications have radically changed the landscape of hepatitis C therapy and have also unlocked the potential for developing completely interferon-free regimens.
Other new interferon-free regimens such as ledipasvir, daclatasvir, and asunaprevir promise high rates of sustained virologic response, which makes the utility of testing for IL28B polymorphisms to predict sustained virologic response very much diminished (Table 1).40,41 However, these new drugs are expected to be expensive, and IL28B polymorphisms may be used to identify candidates who are more likely to respond to pegylated interferon and ribavirin, particularly in resource-poor settings and in developing countries. Additionally, patients who have contraindications to these newer therapies will still likely need an interferon-based regimen, and thus the IL28B polymorphism will still be important in predicting treatment response and prognosis.
IL28B WILL STILL BE RELEVANT IN THE INTERFERON-FREE AGE
The IL28B polymorphism is a strong predictor of spontaneous clearance of hepatitis C virus and responsiveness to interferon-based therapy, and testing for it has demonstrated a great potential to improve patient care. IL28B testing has become available for clinical use and may optimize the outcome of hepatitis C treatment by helping us to select the best treatment for individual patients and minimizing the duration of therapy and the side effects associated with interferon-based antiviral medications.
As newer therapies have shifted toward interferon-free regimens that offer very high sustained virologic response rates, the usefulness of IL28B polymorphism as a clinical test to predict the response rate to antiviral therapy is minimized substantially. It may remain clinically relevant in resource-poor settings and in developing countries, especially in light of the potentially prohibitive costs of the newer regimens, and for patients in whom these treatments are contraindicated. This does not minimize the lesson we learned from the discovery of the IL28B gene and the impact on our understanding of the pathogenesis of hepatitis C virus infection.
- Attia J, Ioannidis JP, Thakkinstian A, et al. How to use an article about genetic association: A: background concepts. JAMA 2009; 301:74–81.
- Samani NJ, Erdmann J, Hall AS, et al; WTCCC and the Cardiogenics Consortium. Genomewide association analysis of coronary artery disease. N Engl J Med 2007; 357:443–453.
- Zeggini E, Weedon MN, Lindgren CM, et al; Wellcome Trust Case Control Consortium (WTCCC). Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316:1336–1341.
- Matarín M, Brown WM, Scholz S, et al. A genome-wide genotyping study in patients with ischaemic stroke: initial analysis and data release. Lancet Neurol 2007; 6:414–420.
- Easton DF, Pooley KA, Dunning AM, et al; AOCS Management Group. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007; 447:1087–1093.
- Plenge RM, Seielstad M, Padyukov L, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N Engl J Med 2007; 357:1199–1209.
- Coon KD, Myers AJ, Craig DW, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J Clin Psychiatry 2007; 68:613–618.
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance (letter). Nature 2009; 461:399–401.
- Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahepatic manifestations. Cleve Clin J Med 2005; 72:1005-1019.
- Hanouneh IA, Feldstein AE, Lopez R, et al. Clinical significance of metabolic syndrome in the setting of chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 2008; 6:584–589.
- Elgouhari HM, Zein CO, Hanouneh I, Feldstein AE, Zein NN. Diabetes mellitus is associated with impaired response to antiviral therapy in chronic hepatitis C infection. Dig Dis Sci 2009; 54:2699–2705.
- Alkhouri N, Zein NN. Protease inhibitors: silver bullets for chronic hepatitis C infection? Cleve Clin J Med 2012; 79:213–222.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364:2405–2416.
- Jacobson IM, Catlett I, Marcellin P, et al. Telaprevir substantially improved SVR rates across all IL28B genotypes in the ADVANCE trial. J Hepatol 2011; 54(suppl 1):S542–S543.
- Pol S, Aerssens J, Zeuzem S, et al. Limited impact of IL28B genotype on response rates in telaprevir-treated patients with prior treatment failure. J Hepatol 2013; 58:883–889.
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009; 41:1100–1104.
- Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009; 41:1105–1109.
- Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009; 461:798–801.
- Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest 2009; 119:1745–1754.
- Marcello T, Grakoui A, Barba-Spaeth G, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 2006; 131:1887–1898.
- Doyle SE, Schreckhise H, Khuu-Duong K, et al. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 2006; 44:896–906.
- Raglow Z, Thoma-Perry C, Gilroy R, Wan YJ. IL28B genotype and the expression of ISGs in normal liver. Liver Int 2013; 33:991–998.
- Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet 2013; 45:164–171.
- Hanouneh IA, Zein NN, Askar M, Lopez R, John B. Interleukin-28B polymorphisms are associated with fibrosing cholestatic hepatitis in recurrent hepatitis C after liver transplantation. Clin Transplant 2012; 26:E335–E336.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825–832.
- Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000; 284:450–456.
- Bochud PY, Cai T, Overbeck K, et al; Swiss Hepatitis C Cohort Study Group. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol 2009; 51:655–666.
- Marabita F, Aghemo A, De Nicola S, et al. Genetic variation in the interleukin-28B gene is not associated with fibrosis progression in patients with chronic hepatitis C and known date of infection. Hepatology 2011; 54:1127–1134.
- Fabris C, Falleti E, Cussigh A, et al. IL-28B rs12979860 C/T allele distribution in patients with liver cirrhosis: role in the course of chronic viral hepatitis and the development of HCC. J Hepatol 2011; 54:716–722.
- Eurich D, Boas-Knoop S, Bahra M, et al. Role of IL28B polymorphism in the development of hepatitis C virus-induced hepatocellular carcinoma, graft fibrosis, and posttransplant antiviral therapy. Transplantation 2012; 93:644–649.
- Hanouneh IA, Miller C, Aucejo F, Lopez R, Quinn MK, Zein NN. Recurrent hepatitis C after liver transplantation: on-treatment prediction of response to peginterferon/ribavirin therapy. Liver Transpl 2008; 14:53–58.
- Charlton MR, Thompson A, Veldt BJ, et al. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology 2011; 53:317–324.
- Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 2010; 139:120–129.e18.
- Beinhardt S, Payer BA, Datz C, et al. A diagnostic score for the prediction of spontaneous resolution of acute hepatitis C virus infection. J Hepatol 2013; 59:972–977.
- Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology 2014; 146:1669–1679.e3.
- Sofia MJ, Bao D, Chang W, et al. Discovery of a ß-d-2’-deoxy-2’-ß-fluoro-2’-ß-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J Med Chem 2010; 53:7202–7218.
- Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–1887.
- Sulkowski MS, Jacobson IM, Ghalib R, et al. Once-daily simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in HCV genotype 1 prior null responders with metavir F0-2: COSMOS study subgroup analysis. 49th EASL, April 2014, London. Oral abstract O7. www.natap.org/2014/EASL/EASL_46.htm. Accesed January 9, 2015.
- Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 2012; 366:216–224.
- Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–1898.
- Attia J, Ioannidis JP, Thakkinstian A, et al. How to use an article about genetic association: A: background concepts. JAMA 2009; 301:74–81.
- Samani NJ, Erdmann J, Hall AS, et al; WTCCC and the Cardiogenics Consortium. Genomewide association analysis of coronary artery disease. N Engl J Med 2007; 357:443–453.
- Zeggini E, Weedon MN, Lindgren CM, et al; Wellcome Trust Case Control Consortium (WTCCC). Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316:1336–1341.
- Matarín M, Brown WM, Scholz S, et al. A genome-wide genotyping study in patients with ischaemic stroke: initial analysis and data release. Lancet Neurol 2007; 6:414–420.
- Easton DF, Pooley KA, Dunning AM, et al; AOCS Management Group. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007; 447:1087–1093.
- Plenge RM, Seielstad M, Padyukov L, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N Engl J Med 2007; 357:1199–1209.
- Coon KD, Myers AJ, Craig DW, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J Clin Psychiatry 2007; 68:613–618.
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance (letter). Nature 2009; 461:399–401.
- Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahepatic manifestations. Cleve Clin J Med 2005; 72:1005-1019.
- Hanouneh IA, Feldstein AE, Lopez R, et al. Clinical significance of metabolic syndrome in the setting of chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 2008; 6:584–589.
- Elgouhari HM, Zein CO, Hanouneh I, Feldstein AE, Zein NN. Diabetes mellitus is associated with impaired response to antiviral therapy in chronic hepatitis C infection. Dig Dis Sci 2009; 54:2699–2705.
- Alkhouri N, Zein NN. Protease inhibitors: silver bullets for chronic hepatitis C infection? Cleve Clin J Med 2012; 79:213–222.
- McHutchison JG, Everson GT, Gordon SC, et al; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364:2405–2416.
- Jacobson IM, Catlett I, Marcellin P, et al. Telaprevir substantially improved SVR rates across all IL28B genotypes in the ADVANCE trial. J Hepatol 2011; 54(suppl 1):S542–S543.
- Pol S, Aerssens J, Zeuzem S, et al. Limited impact of IL28B genotype on response rates in telaprevir-treated patients with prior treatment failure. J Hepatol 2013; 58:883–889.
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009; 41:1100–1104.
- Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009; 41:1105–1109.
- Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009; 461:798–801.
- Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest 2009; 119:1745–1754.
- Marcello T, Grakoui A, Barba-Spaeth G, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 2006; 131:1887–1898.
- Doyle SE, Schreckhise H, Khuu-Duong K, et al. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 2006; 44:896–906.
- Raglow Z, Thoma-Perry C, Gilroy R, Wan YJ. IL28B genotype and the expression of ISGs in normal liver. Liver Int 2013; 33:991–998.
- Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet 2013; 45:164–171.
- Hanouneh IA, Zein NN, Askar M, Lopez R, John B. Interleukin-28B polymorphisms are associated with fibrosing cholestatic hepatitis in recurrent hepatitis C after liver transplantation. Clin Transplant 2012; 26:E335–E336.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825–832.
- Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000; 284:450–456.
- Bochud PY, Cai T, Overbeck K, et al; Swiss Hepatitis C Cohort Study Group. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol 2009; 51:655–666.
- Marabita F, Aghemo A, De Nicola S, et al. Genetic variation in the interleukin-28B gene is not associated with fibrosis progression in patients with chronic hepatitis C and known date of infection. Hepatology 2011; 54:1127–1134.
- Fabris C, Falleti E, Cussigh A, et al. IL-28B rs12979860 C/T allele distribution in patients with liver cirrhosis: role in the course of chronic viral hepatitis and the development of HCC. J Hepatol 2011; 54:716–722.
- Eurich D, Boas-Knoop S, Bahra M, et al. Role of IL28B polymorphism in the development of hepatitis C virus-induced hepatocellular carcinoma, graft fibrosis, and posttransplant antiviral therapy. Transplantation 2012; 93:644–649.
- Hanouneh IA, Miller C, Aucejo F, Lopez R, Quinn MK, Zein NN. Recurrent hepatitis C after liver transplantation: on-treatment prediction of response to peginterferon/ribavirin therapy. Liver Transpl 2008; 14:53–58.
- Charlton MR, Thompson A, Veldt BJ, et al. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology 2011; 53:317–324.
- Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 2010; 139:120–129.e18.
- Beinhardt S, Payer BA, Datz C, et al. A diagnostic score for the prediction of spontaneous resolution of acute hepatitis C virus infection. J Hepatol 2013; 59:972–977.
- Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology 2014; 146:1669–1679.e3.
- Sofia MJ, Bao D, Chang W, et al. Discovery of a ß-d-2’-deoxy-2’-ß-fluoro-2’-ß-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J Med Chem 2010; 53:7202–7218.
- Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–1887.
- Sulkowski MS, Jacobson IM, Ghalib R, et al. Once-daily simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in HCV genotype 1 prior null responders with metavir F0-2: COSMOS study subgroup analysis. 49th EASL, April 2014, London. Oral abstract O7. www.natap.org/2014/EASL/EASL_46.htm. Accesed January 9, 2015.
- Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 2012; 366:216–224.
- Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–1898.
KEY POINTS
- In IL28B, the rs12979860 location can be occupied by either cytosine (C) or thymine (T). The CC genotype is more favorable than the CT or TT genotype.
- Testing for the IL28B polymorphism is currently available and allows for better outcomes through proper selection of treatment, particularly with interferon-based treatment.
- Although newer therapies have shifted toward regimens that do not use interferon, the IL28B polymorphism remains clinically significant, especially in light of the potentially prohibitive costs of the newer regimens, and for patients in whom these treatments are contraindicated.
The ‘skinny’ on eosinophilic esophagitis
Eosinophilic esophagitis is a new disease defined by specific criteria that include a constellation of symptoms. Consensus guidelines define it as a chronic antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.1
Ten years ago, a biopsy that revealed eosinophils in the esophagus was diagnostic, because normally eosinophils are not seen in the esophagus. The current definition has evolved to become more comprehensive and includes clinical, demographic, and radiographic criteria.
This article presents an overview of eosinophilic esophagitis—its pathogenesis, epidemiology, clinical presentation, diagnosis, and management.
ALLERGIC ORIGIN
Eosinophilic esophagitis is best regarded as a systemic rather than a single-organ disease, although current treatments are mostly directed specifically at esophageal inflammation. Evidence is clear that eosinophilic esophagitis is allergy-mediated.
The current “two-hit” etiologic model involves exposure first to aeroallergens that prime the esophagus, followed by food allergens that cause an eosinophilic response with antigen recognition and stimulation of immune cells from the bone marrow. Other allergic avenues may also be present, including those involved with atopy, asthma, eczema, and food allergies, which stimulate the Th2 pathway and lead to esophageal eosinophilia and inflammation.2
The two-hit model is supported experimentally: the disease can be induced in mice by injecting ovalbumin under the skin as a sensitizing agent, then exposing the airway to an aerosol of Aspergillus fumigatus, producing an allergic reaction involving classic Th2 allergy pathways.3 Further evidence is that many patients report that asthma or rhinitis developed years before esophageal disease began.
Patients with eosinophilic esophagitis and their family members have a high prevalence of allergies, and the disease frequently flares up during allergy season. Endoscopic biopsy specimens from patients often reveal increased T cells, mast cells, interleukin (IL)-5, and tumor necrosis factor alpha, all of which stimulate eotaxin and are essential to an allergic reaction. They also have high levels of CD3, CDA, and CD1A antigen-presenting lymphocytes, which are all associated with allergy.
Eosinophilic esophagitis responds to allergy medications, including corticosteroids and IL-5 or IL-13 mast-cell inhibitors. The strongest evidence for an allergic etiology is that withdrawing culpable food allergens leads to resolution of the disease. Peterson et al4 gave 18 adults with eosinophilic esophagitis an elemental diet (ie, a pure amino acid, carbohydrate-based diet in which all suspected allergens have been removed), and in 2 to 4 weeks, the mean number of eosinophils seen histologically fell from 54 to 10 cells per high-power field. The response was nearly complete (≤ 10 eosinophils per high-power field) in 72% of patients. When patients resumed a normal diet, the eosinophil content increased substantially within a few days.
Role of leaky tight junctions
Normally, the junctions between epithelial cells are tight, but many conditions, including allergic and autoimmune diseases, are now believed to involve altered permeability of this tissue. Tight-junction proteins play an important role in regulating antigen delivery and are modulated by cytokines. Activation of cytokines causes the membrane to become more permeable, allowing antigens to get through, leading to an enhanced reaction. In eosinophilic esophagitis, it is postulated that food antigens that pass through the leaky membrane activate CD1-antigen-presenting cells, which then initiate an allergic reaction.5–9
PREVALENCE IS INCREASING
Eosinophilic esophagitis was first described in 1993 with a report of 12 patients who had dysphagia, normal endoscopy, no acid reflux, and intraepithelial eosinophilia.10 The authors recognized that these patients had a distinct disease.
Since then, the disease has increased in prevalence. Kapel et al11 reviewed more than 74,000 endoscopy slides from a national pathology database and found 363 cases, with increasing prevalence during the study period from 2002 to 2005. Looking back further in a similar study, Whitney-Miller et al12 found a 0.3% prevalence from the years 1992 to 2000 vs 3.8% from 2001 to 2004.
Sealock et al13 reviewed the literature to assess the prevalence of eosinophilic esophagitis and found considerable variation depending on the populations sampled. One study from Sweden14 found a prevalence of 0.4% by performing endoscopy in 1,000 randomly selected people from nearly 3,000 responders to a questionnaire on abdominal symptoms. A study based on a Swiss database15 found only a 0.02% prevalence. Other studies show higher rates: a study from Florida that examined biopsy specimens from patients who underwent endoscopy for any reason found a prevalence of 1%.16 Another US study found a 15% prevalence in patients with dysphagia.17 Since these studies were done nearly a decade ago, we can expect the prevalence to be higher today.
Celiac disease has also been increasing in recent decades, as has gluten sensitivity. Allergies in general are on the rise worldwide, including asthma and atopic dermatitis. Theories as to the cause of these increases have focused on ambient antigens, food additives, proton pump inhibitors (PPIs), and the microbiome.18,19
DIAGNOSING EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis is diagnosed with a combination of symptomatic, histologic, and radiographic findings (Table 1). The classic patient is a white male—a child, teenager, or young adult—with dysphagia.
A case series of 23 adult patients20 found a mean age of 35 (age range 18 to 57), with a male preponderance (14:9). There is commonly a history of other allergies, including asthma, allergic rhinitis, and atopic dermatitis. Patients more commonly present with dysphagia than heartburn or other esophageal symptoms.11
Endoscopic findings—eosinophils, later fibrosis
Finding eosinophils in the esophagus is nonspecific and is not sufficient to make the diagnosis. Other systemic diseases can involve esophageal eosinophilia, including Churg-Strauss syndrome, Crohn disease, and helminthic diseases. Whether some are related to eosinophilic esophagitis or are independent is not well understood.
Characteristic findings on endoscopy include a corrugated or ringed appearance and linear furrows, resulting from fibrosis and scarring. “Micro-tears” may also be visible projecting linearly up the esophagus. Multiple white specks are signs of conglomerations of eosinophils and are easily confused with yeast infection. Strictures from scar tissue cause the mucosa to be tight and fragile, making the esophagus very susceptible to tearing during endoscopy.
After years of untreated disease, the esophagus becomes increasingly inflamed and fibrotic. Adult patients with eosinophilic esophagitis who were followed for a decade were found to develop increasing collagen deposition in which the submucosa or even the entire esophageal wall was diffusely fibrotic.21
Radiographic findings—a narrow esophagus
On radiography, the esophagus may appear narrow—not uncommonly one-third to one-quarter the caliber of a normal esophagus. As the esophagus progressively narrows, both eating and treatment become extremely difficult.
Symptoms are different in children and adults
Symptoms reflect the endoscopic changes over time. In children, the condition manifests with feeding difficulties, vomiting, symptoms of gastroesophageal reflux, and abdominal pain as signs of inflammation. As the esophagus becomes fibrotic, teenagers and young adults tend to present with strictures, dysphagia, and food impaction. Of patients who present to an emergency department with food impaction, the major cause is now eosinophilic esophagitis.22
It is important to pay attention to symptoms in children to diagnose the condition and start treatment early to prevent or postpone disease advancement. Medical therapy does not clearly reverse the fibrosis.
As in many chronic benign diseases, patients learn to compensate, so a careful history is essential. Many deny having a swallowing problem, but questioning may reveal that they have always been slow, picky eaters, consuming mostly soft foods and drinking fluids with every bite.
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease can be a challenge, as signs and symptoms overlap.
Veerappan et al23 looked for predictors of eosinophilic esophagitis in 400 adults who underwent routine upper endoscopy, 6.5% of whom had eosinophilic esophagitis. They found significant overlap in medical history for patients with and without the disease; while a higher proportion of patients with eosinophilic esophagitis had a history of asthma, dysphagia, food impactions, dermatitis, and food allergies, these conditions also occurred in other patients.
Similarly, the classic endoscopic findings of eosinophilic esophagitis—rings, furrows, strictures, and plaques—also occur in other conditions.23 Reflux disease can cause scarring from excess acid and may even be associated with eosinophils in the esophagus, indicative of a combination of allergy and reflux. A small-caliber esophagus is also occasionally present in patients with reflux disease.
Ambulatory pH monitoring has been recommended to help determine if gastroesophageal reflux is the cause of esophageal eosinophilia and to guide therapy. However, in a prospective study of 51 patients,24 neither positive nor negative results of initial pH monitoring accurately predicted response to PPIs or steroid therapy. Another study found that half of patients with an eosinophilic esophagitis profile without evidence of acid reflux by pH monitoring responded to treatment with a PPI.25
This raises the question of whether some patients with eosinophilic esophagitis have more acid reflux than is detected by pH monitoring, or alternatively, whether PPIs have other, less-recognized effects besides reducing acidity. Investigators are now ascribing a host of anti-inflammatory actions to PPIs, including effects on antioxidants, inflammatory cells, endothelial cells, and the gut microflora.26 And PPIs may alleviate eosinophilic esophagitis through anti-inflammatory effects rather than by inhibiting secretion of gastric acid.
THREE TYPES OF THERAPY
In general, three types of therapy are available for patients with eosinophilic esophagitis: medications, allergen avoidance, and esophageal dilation (Table 2).
Medications: Try a PPI first, then a corticosteroid
A PPI should be tried even for patients with a classic presentation of eosinophilic esophagitis because some will respond, and long-term PPI therapy is preferable to long-term steroid treatment. Patients should be put on a 2-month course and should then undergo repeat biopsy.
For patients who do not respond to a PPI, a corticosteroid or montelukast can be tried. Topical therapy is showing promise as both a short- and long-term option to bring about remission.27 For administration, a corticosteroid (budesonide or fluticasone) is mixed with a viscous solution, such as water with honey or chocolate syrup, making it thick so it better coats the esophagus. The therapy can be very effective: in up to 8 weeks some patients have a 90% resolution of esophageal eosinophilia. However, about 5% of patients develop a yeast infection, and adrenal suppression is a concern but appears to be uncommon.
Avoidance of allergens
Because eosinophilic esophagitis is an allergic disease, eliminating allergens should be an effective treatment. Unfortunately, from a practical standpoint, elimination is very difficult. The elemental diet formula is expensive and unpalatable, making it impractical for a prolonged period.
Gonsalves et al28 put 50 adult patients with eosinophilic esophagitis on a diet eliminating the six most common foods believed to trigger the disease—wheat, milk, nuts, eggs, soy, and seafood—and found a marked reduction in eosinophils in the proximal and distal esophagus after 6 weeks. Additional triggers that have been identified include rice, corn, and legumes.29
Unfortunately, maintaining a diet without the most commonly identified allergens is not easy. Although some very motivated patients can do it, it is especially hard for teens and young adults. Variations of the diet, such as eliminating just two foods, make following a plan easier. Omitting milk alone would benefit an estimated 20% of patients with eosinophilic esophagitis.
Identifying food triggers is a challenge in itself as there is no good noninvasive method of identifying the allergens. The radioallergosorbent test measures immunoglobulin (Ig) E, and the skin-prick test measures acute hypersensitivity, but neither is very sensitive for the Th2-mediated reaction involved in eosinophilic esophagitis. In early trials, endoscopy and biopsy were painstakingly performed with the removal and reintroduction of every suspected food allergen, requiring multiple biopsies weekly, which is impractical for safety and economic reasons.
Attempts are being made to devise less invasive methods of sampling the esophageal mucosa. Transnasal endoscopy—done as an outpatient procedure with topical anesthesia—is a possibility. Another possibility is the esophageal string test,30 which involves swalling a weighted capsule on a string and then, after an hour, pulling it up again and testing the tissue on the string.
The “cytosponge,” a new device currently under investigation, also uses a string delivery system. The patient swallows a sponge contained in a gelatin capsule and attached to a string. When the capsule dissolves in the stomach—a process that takes only a few minutes—the sponge expands. The string is then pulled up, causing the sponge to sample the esophageal mucosa and thus obtaining a histologic specimen. This method shows promise as an inexpensive and noninvasive way to monitor the disease, although larger studies are needed to establish efficacy.31
Dilation—proceed with caution
Dilation can be an important therapy, especially in teenagers and adults with a fibrotic, narrowed esophagus.
Early on, the procedure often resulted in complications such as deep mucosal tears and perforations. Jung et al32 retrospectively analyzed 293 dilations in 161 patients with eosinophilic esophagitis and found a deep mucosal tear in 27 patients (9%), three perforations, and one incidence of major bleeding. All complications resolved without surgery. Factors associated with increased risk of complications were luminal narrowing in the upper and middle third of the esophagus, a luminal stricture that could not be traversed with a standard upper endoscope, and use of a Savary dilator.
It is critical that dilation be done slowly—a few millimeters at a time. Several sessions may be needed.
TREATMENT DURING REMISSION IS CONTROVERSIAL
Unless the patient with eosinophilic esophagitis can consistently control the disease by avoiding allergens, the question arises of whether to continue treating a patient who is in remission.
On the one hand, there is no known risk of Barrett esophagus or malignancy when the condition is not treated, and weight loss is uncommon because patients tend to accommodate to the condition. However, the long-term consequences are uncertain. Allergies are chronic, and disease progression with more fibrosis should be prevented. Also, food impaction commonly occurs and this requires aggressive dilation, which is risky.
On the other hand, chronic steroid therapy involves risk. The optimum steroid dosage during remission and whether alternate-day dosing is adequate have yet to be determined.
Long-term trials are needed to answer these questions. In the meantime, most physicians tend to aggressively treat this disease, if not with specific food avoidance, then with steroid maintenance therapy.
MONITORING THE DISEASE
Monitoring eosinophilic esophagitis by clinical indicators is difficult. Once fibrosis develops, symptoms often do not reflect underlying pathology. It may turn out that, as in Crohn disease, monitoring mucosal healing rather than symptoms may be best.
Until we know more about this condition, careful monitoring of patients is important. However, it is too early to give specific guidance, such as endoscopy every 2 months or annually. Whether the eosinophil count should be the critical consideration is also unknown.
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20.
- Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009; 137:1238–1249.
- Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001; 107:83–90.
- Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013; 108:759–766.
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 2010; 20:142–149.
- Chang F, Anderson S. Clinical and pathological features of eosinophilic oesophagitis: a review. Pathology 2008; 40:3–8.
- Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep 2009; 11:190–194.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet 2010; 42:289–291.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38:109–116.
- Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008; 134:1316–1321.
- Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol 2009; 131:788–792.
- Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther 2010; 32:712–719.
- Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007; 56:615–620.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007; 102:2627–2632.
- Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology 2011; 141:1586–1592.
- Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy 1999; 29:342–346.
- Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2008; 6:531–535.
- Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophiic inflammation with esophageal food impaction in adults. Gastrointest Endosc 2005; 61:795–801.
- Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009; 7:420–426.
- Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012; 35:300–307.
- Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9:110–117.
- Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 2009; 54:2312–2317.
- Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139:1526–1537.
- Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142:1451–1459.
- Lucendo AJ, Arias Á, González-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131:797–804.
- Fillon SA, Harris JK, Wagner BD, et al. Novel device to sample the esophageal microbiome—the esophageal string test. PLoS One 2012; 7:e42938.
- Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014. pii: S1542-3565(14)00933-1. doi: 10.1016/j.cgh.2014.06.026. [Epub ahead of print]
- Jung KW, Gundersen N, Kopacova J, et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc 2011; 73:15–21.
Eosinophilic esophagitis is a new disease defined by specific criteria that include a constellation of symptoms. Consensus guidelines define it as a chronic antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.1
Ten years ago, a biopsy that revealed eosinophils in the esophagus was diagnostic, because normally eosinophils are not seen in the esophagus. The current definition has evolved to become more comprehensive and includes clinical, demographic, and radiographic criteria.
This article presents an overview of eosinophilic esophagitis—its pathogenesis, epidemiology, clinical presentation, diagnosis, and management.
ALLERGIC ORIGIN
Eosinophilic esophagitis is best regarded as a systemic rather than a single-organ disease, although current treatments are mostly directed specifically at esophageal inflammation. Evidence is clear that eosinophilic esophagitis is allergy-mediated.
The current “two-hit” etiologic model involves exposure first to aeroallergens that prime the esophagus, followed by food allergens that cause an eosinophilic response with antigen recognition and stimulation of immune cells from the bone marrow. Other allergic avenues may also be present, including those involved with atopy, asthma, eczema, and food allergies, which stimulate the Th2 pathway and lead to esophageal eosinophilia and inflammation.2
The two-hit model is supported experimentally: the disease can be induced in mice by injecting ovalbumin under the skin as a sensitizing agent, then exposing the airway to an aerosol of Aspergillus fumigatus, producing an allergic reaction involving classic Th2 allergy pathways.3 Further evidence is that many patients report that asthma or rhinitis developed years before esophageal disease began.
Patients with eosinophilic esophagitis and their family members have a high prevalence of allergies, and the disease frequently flares up during allergy season. Endoscopic biopsy specimens from patients often reveal increased T cells, mast cells, interleukin (IL)-5, and tumor necrosis factor alpha, all of which stimulate eotaxin and are essential to an allergic reaction. They also have high levels of CD3, CDA, and CD1A antigen-presenting lymphocytes, which are all associated with allergy.
Eosinophilic esophagitis responds to allergy medications, including corticosteroids and IL-5 or IL-13 mast-cell inhibitors. The strongest evidence for an allergic etiology is that withdrawing culpable food allergens leads to resolution of the disease. Peterson et al4 gave 18 adults with eosinophilic esophagitis an elemental diet (ie, a pure amino acid, carbohydrate-based diet in which all suspected allergens have been removed), and in 2 to 4 weeks, the mean number of eosinophils seen histologically fell from 54 to 10 cells per high-power field. The response was nearly complete (≤ 10 eosinophils per high-power field) in 72% of patients. When patients resumed a normal diet, the eosinophil content increased substantially within a few days.
Role of leaky tight junctions
Normally, the junctions between epithelial cells are tight, but many conditions, including allergic and autoimmune diseases, are now believed to involve altered permeability of this tissue. Tight-junction proteins play an important role in regulating antigen delivery and are modulated by cytokines. Activation of cytokines causes the membrane to become more permeable, allowing antigens to get through, leading to an enhanced reaction. In eosinophilic esophagitis, it is postulated that food antigens that pass through the leaky membrane activate CD1-antigen-presenting cells, which then initiate an allergic reaction.5–9
PREVALENCE IS INCREASING
Eosinophilic esophagitis was first described in 1993 with a report of 12 patients who had dysphagia, normal endoscopy, no acid reflux, and intraepithelial eosinophilia.10 The authors recognized that these patients had a distinct disease.
Since then, the disease has increased in prevalence. Kapel et al11 reviewed more than 74,000 endoscopy slides from a national pathology database and found 363 cases, with increasing prevalence during the study period from 2002 to 2005. Looking back further in a similar study, Whitney-Miller et al12 found a 0.3% prevalence from the years 1992 to 2000 vs 3.8% from 2001 to 2004.
Sealock et al13 reviewed the literature to assess the prevalence of eosinophilic esophagitis and found considerable variation depending on the populations sampled. One study from Sweden14 found a prevalence of 0.4% by performing endoscopy in 1,000 randomly selected people from nearly 3,000 responders to a questionnaire on abdominal symptoms. A study based on a Swiss database15 found only a 0.02% prevalence. Other studies show higher rates: a study from Florida that examined biopsy specimens from patients who underwent endoscopy for any reason found a prevalence of 1%.16 Another US study found a 15% prevalence in patients with dysphagia.17 Since these studies were done nearly a decade ago, we can expect the prevalence to be higher today.
Celiac disease has also been increasing in recent decades, as has gluten sensitivity. Allergies in general are on the rise worldwide, including asthma and atopic dermatitis. Theories as to the cause of these increases have focused on ambient antigens, food additives, proton pump inhibitors (PPIs), and the microbiome.18,19
DIAGNOSING EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis is diagnosed with a combination of symptomatic, histologic, and radiographic findings (Table 1). The classic patient is a white male—a child, teenager, or young adult—with dysphagia.
A case series of 23 adult patients20 found a mean age of 35 (age range 18 to 57), with a male preponderance (14:9). There is commonly a history of other allergies, including asthma, allergic rhinitis, and atopic dermatitis. Patients more commonly present with dysphagia than heartburn or other esophageal symptoms.11
Endoscopic findings—eosinophils, later fibrosis
Finding eosinophils in the esophagus is nonspecific and is not sufficient to make the diagnosis. Other systemic diseases can involve esophageal eosinophilia, including Churg-Strauss syndrome, Crohn disease, and helminthic diseases. Whether some are related to eosinophilic esophagitis or are independent is not well understood.
Characteristic findings on endoscopy include a corrugated or ringed appearance and linear furrows, resulting from fibrosis and scarring. “Micro-tears” may also be visible projecting linearly up the esophagus. Multiple white specks are signs of conglomerations of eosinophils and are easily confused with yeast infection. Strictures from scar tissue cause the mucosa to be tight and fragile, making the esophagus very susceptible to tearing during endoscopy.
After years of untreated disease, the esophagus becomes increasingly inflamed and fibrotic. Adult patients with eosinophilic esophagitis who were followed for a decade were found to develop increasing collagen deposition in which the submucosa or even the entire esophageal wall was diffusely fibrotic.21
Radiographic findings—a narrow esophagus
On radiography, the esophagus may appear narrow—not uncommonly one-third to one-quarter the caliber of a normal esophagus. As the esophagus progressively narrows, both eating and treatment become extremely difficult.
Symptoms are different in children and adults
Symptoms reflect the endoscopic changes over time. In children, the condition manifests with feeding difficulties, vomiting, symptoms of gastroesophageal reflux, and abdominal pain as signs of inflammation. As the esophagus becomes fibrotic, teenagers and young adults tend to present with strictures, dysphagia, and food impaction. Of patients who present to an emergency department with food impaction, the major cause is now eosinophilic esophagitis.22
It is important to pay attention to symptoms in children to diagnose the condition and start treatment early to prevent or postpone disease advancement. Medical therapy does not clearly reverse the fibrosis.
As in many chronic benign diseases, patients learn to compensate, so a careful history is essential. Many deny having a swallowing problem, but questioning may reveal that they have always been slow, picky eaters, consuming mostly soft foods and drinking fluids with every bite.
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease can be a challenge, as signs and symptoms overlap.
Veerappan et al23 looked for predictors of eosinophilic esophagitis in 400 adults who underwent routine upper endoscopy, 6.5% of whom had eosinophilic esophagitis. They found significant overlap in medical history for patients with and without the disease; while a higher proportion of patients with eosinophilic esophagitis had a history of asthma, dysphagia, food impactions, dermatitis, and food allergies, these conditions also occurred in other patients.
Similarly, the classic endoscopic findings of eosinophilic esophagitis—rings, furrows, strictures, and plaques—also occur in other conditions.23 Reflux disease can cause scarring from excess acid and may even be associated with eosinophils in the esophagus, indicative of a combination of allergy and reflux. A small-caliber esophagus is also occasionally present in patients with reflux disease.
Ambulatory pH monitoring has been recommended to help determine if gastroesophageal reflux is the cause of esophageal eosinophilia and to guide therapy. However, in a prospective study of 51 patients,24 neither positive nor negative results of initial pH monitoring accurately predicted response to PPIs or steroid therapy. Another study found that half of patients with an eosinophilic esophagitis profile without evidence of acid reflux by pH monitoring responded to treatment with a PPI.25
This raises the question of whether some patients with eosinophilic esophagitis have more acid reflux than is detected by pH monitoring, or alternatively, whether PPIs have other, less-recognized effects besides reducing acidity. Investigators are now ascribing a host of anti-inflammatory actions to PPIs, including effects on antioxidants, inflammatory cells, endothelial cells, and the gut microflora.26 And PPIs may alleviate eosinophilic esophagitis through anti-inflammatory effects rather than by inhibiting secretion of gastric acid.
THREE TYPES OF THERAPY
In general, three types of therapy are available for patients with eosinophilic esophagitis: medications, allergen avoidance, and esophageal dilation (Table 2).
Medications: Try a PPI first, then a corticosteroid
A PPI should be tried even for patients with a classic presentation of eosinophilic esophagitis because some will respond, and long-term PPI therapy is preferable to long-term steroid treatment. Patients should be put on a 2-month course and should then undergo repeat biopsy.
For patients who do not respond to a PPI, a corticosteroid or montelukast can be tried. Topical therapy is showing promise as both a short- and long-term option to bring about remission.27 For administration, a corticosteroid (budesonide or fluticasone) is mixed with a viscous solution, such as water with honey or chocolate syrup, making it thick so it better coats the esophagus. The therapy can be very effective: in up to 8 weeks some patients have a 90% resolution of esophageal eosinophilia. However, about 5% of patients develop a yeast infection, and adrenal suppression is a concern but appears to be uncommon.
Avoidance of allergens
Because eosinophilic esophagitis is an allergic disease, eliminating allergens should be an effective treatment. Unfortunately, from a practical standpoint, elimination is very difficult. The elemental diet formula is expensive and unpalatable, making it impractical for a prolonged period.
Gonsalves et al28 put 50 adult patients with eosinophilic esophagitis on a diet eliminating the six most common foods believed to trigger the disease—wheat, milk, nuts, eggs, soy, and seafood—and found a marked reduction in eosinophils in the proximal and distal esophagus after 6 weeks. Additional triggers that have been identified include rice, corn, and legumes.29
Unfortunately, maintaining a diet without the most commonly identified allergens is not easy. Although some very motivated patients can do it, it is especially hard for teens and young adults. Variations of the diet, such as eliminating just two foods, make following a plan easier. Omitting milk alone would benefit an estimated 20% of patients with eosinophilic esophagitis.
Identifying food triggers is a challenge in itself as there is no good noninvasive method of identifying the allergens. The radioallergosorbent test measures immunoglobulin (Ig) E, and the skin-prick test measures acute hypersensitivity, but neither is very sensitive for the Th2-mediated reaction involved in eosinophilic esophagitis. In early trials, endoscopy and biopsy were painstakingly performed with the removal and reintroduction of every suspected food allergen, requiring multiple biopsies weekly, which is impractical for safety and economic reasons.
Attempts are being made to devise less invasive methods of sampling the esophageal mucosa. Transnasal endoscopy—done as an outpatient procedure with topical anesthesia—is a possibility. Another possibility is the esophageal string test,30 which involves swalling a weighted capsule on a string and then, after an hour, pulling it up again and testing the tissue on the string.
The “cytosponge,” a new device currently under investigation, also uses a string delivery system. The patient swallows a sponge contained in a gelatin capsule and attached to a string. When the capsule dissolves in the stomach—a process that takes only a few minutes—the sponge expands. The string is then pulled up, causing the sponge to sample the esophageal mucosa and thus obtaining a histologic specimen. This method shows promise as an inexpensive and noninvasive way to monitor the disease, although larger studies are needed to establish efficacy.31
Dilation—proceed with caution
Dilation can be an important therapy, especially in teenagers and adults with a fibrotic, narrowed esophagus.
Early on, the procedure often resulted in complications such as deep mucosal tears and perforations. Jung et al32 retrospectively analyzed 293 dilations in 161 patients with eosinophilic esophagitis and found a deep mucosal tear in 27 patients (9%), three perforations, and one incidence of major bleeding. All complications resolved without surgery. Factors associated with increased risk of complications were luminal narrowing in the upper and middle third of the esophagus, a luminal stricture that could not be traversed with a standard upper endoscope, and use of a Savary dilator.
It is critical that dilation be done slowly—a few millimeters at a time. Several sessions may be needed.
TREATMENT DURING REMISSION IS CONTROVERSIAL
Unless the patient with eosinophilic esophagitis can consistently control the disease by avoiding allergens, the question arises of whether to continue treating a patient who is in remission.
On the one hand, there is no known risk of Barrett esophagus or malignancy when the condition is not treated, and weight loss is uncommon because patients tend to accommodate to the condition. However, the long-term consequences are uncertain. Allergies are chronic, and disease progression with more fibrosis should be prevented. Also, food impaction commonly occurs and this requires aggressive dilation, which is risky.
On the other hand, chronic steroid therapy involves risk. The optimum steroid dosage during remission and whether alternate-day dosing is adequate have yet to be determined.
Long-term trials are needed to answer these questions. In the meantime, most physicians tend to aggressively treat this disease, if not with specific food avoidance, then with steroid maintenance therapy.
MONITORING THE DISEASE
Monitoring eosinophilic esophagitis by clinical indicators is difficult. Once fibrosis develops, symptoms often do not reflect underlying pathology. It may turn out that, as in Crohn disease, monitoring mucosal healing rather than symptoms may be best.
Until we know more about this condition, careful monitoring of patients is important. However, it is too early to give specific guidance, such as endoscopy every 2 months or annually. Whether the eosinophil count should be the critical consideration is also unknown.
Eosinophilic esophagitis is a new disease defined by specific criteria that include a constellation of symptoms. Consensus guidelines define it as a chronic antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.1
Ten years ago, a biopsy that revealed eosinophils in the esophagus was diagnostic, because normally eosinophils are not seen in the esophagus. The current definition has evolved to become more comprehensive and includes clinical, demographic, and radiographic criteria.
This article presents an overview of eosinophilic esophagitis—its pathogenesis, epidemiology, clinical presentation, diagnosis, and management.
ALLERGIC ORIGIN
Eosinophilic esophagitis is best regarded as a systemic rather than a single-organ disease, although current treatments are mostly directed specifically at esophageal inflammation. Evidence is clear that eosinophilic esophagitis is allergy-mediated.
The current “two-hit” etiologic model involves exposure first to aeroallergens that prime the esophagus, followed by food allergens that cause an eosinophilic response with antigen recognition and stimulation of immune cells from the bone marrow. Other allergic avenues may also be present, including those involved with atopy, asthma, eczema, and food allergies, which stimulate the Th2 pathway and lead to esophageal eosinophilia and inflammation.2
The two-hit model is supported experimentally: the disease can be induced in mice by injecting ovalbumin under the skin as a sensitizing agent, then exposing the airway to an aerosol of Aspergillus fumigatus, producing an allergic reaction involving classic Th2 allergy pathways.3 Further evidence is that many patients report that asthma or rhinitis developed years before esophageal disease began.
Patients with eosinophilic esophagitis and their family members have a high prevalence of allergies, and the disease frequently flares up during allergy season. Endoscopic biopsy specimens from patients often reveal increased T cells, mast cells, interleukin (IL)-5, and tumor necrosis factor alpha, all of which stimulate eotaxin and are essential to an allergic reaction. They also have high levels of CD3, CDA, and CD1A antigen-presenting lymphocytes, which are all associated with allergy.
Eosinophilic esophagitis responds to allergy medications, including corticosteroids and IL-5 or IL-13 mast-cell inhibitors. The strongest evidence for an allergic etiology is that withdrawing culpable food allergens leads to resolution of the disease. Peterson et al4 gave 18 adults with eosinophilic esophagitis an elemental diet (ie, a pure amino acid, carbohydrate-based diet in which all suspected allergens have been removed), and in 2 to 4 weeks, the mean number of eosinophils seen histologically fell from 54 to 10 cells per high-power field. The response was nearly complete (≤ 10 eosinophils per high-power field) in 72% of patients. When patients resumed a normal diet, the eosinophil content increased substantially within a few days.
Role of leaky tight junctions
Normally, the junctions between epithelial cells are tight, but many conditions, including allergic and autoimmune diseases, are now believed to involve altered permeability of this tissue. Tight-junction proteins play an important role in regulating antigen delivery and are modulated by cytokines. Activation of cytokines causes the membrane to become more permeable, allowing antigens to get through, leading to an enhanced reaction. In eosinophilic esophagitis, it is postulated that food antigens that pass through the leaky membrane activate CD1-antigen-presenting cells, which then initiate an allergic reaction.5–9
PREVALENCE IS INCREASING
Eosinophilic esophagitis was first described in 1993 with a report of 12 patients who had dysphagia, normal endoscopy, no acid reflux, and intraepithelial eosinophilia.10 The authors recognized that these patients had a distinct disease.
Since then, the disease has increased in prevalence. Kapel et al11 reviewed more than 74,000 endoscopy slides from a national pathology database and found 363 cases, with increasing prevalence during the study period from 2002 to 2005. Looking back further in a similar study, Whitney-Miller et al12 found a 0.3% prevalence from the years 1992 to 2000 vs 3.8% from 2001 to 2004.
Sealock et al13 reviewed the literature to assess the prevalence of eosinophilic esophagitis and found considerable variation depending on the populations sampled. One study from Sweden14 found a prevalence of 0.4% by performing endoscopy in 1,000 randomly selected people from nearly 3,000 responders to a questionnaire on abdominal symptoms. A study based on a Swiss database15 found only a 0.02% prevalence. Other studies show higher rates: a study from Florida that examined biopsy specimens from patients who underwent endoscopy for any reason found a prevalence of 1%.16 Another US study found a 15% prevalence in patients with dysphagia.17 Since these studies were done nearly a decade ago, we can expect the prevalence to be higher today.
Celiac disease has also been increasing in recent decades, as has gluten sensitivity. Allergies in general are on the rise worldwide, including asthma and atopic dermatitis. Theories as to the cause of these increases have focused on ambient antigens, food additives, proton pump inhibitors (PPIs), and the microbiome.18,19
DIAGNOSING EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis is diagnosed with a combination of symptomatic, histologic, and radiographic findings (Table 1). The classic patient is a white male—a child, teenager, or young adult—with dysphagia.
A case series of 23 adult patients20 found a mean age of 35 (age range 18 to 57), with a male preponderance (14:9). There is commonly a history of other allergies, including asthma, allergic rhinitis, and atopic dermatitis. Patients more commonly present with dysphagia than heartburn or other esophageal symptoms.11
Endoscopic findings—eosinophils, later fibrosis
Finding eosinophils in the esophagus is nonspecific and is not sufficient to make the diagnosis. Other systemic diseases can involve esophageal eosinophilia, including Churg-Strauss syndrome, Crohn disease, and helminthic diseases. Whether some are related to eosinophilic esophagitis or are independent is not well understood.
Characteristic findings on endoscopy include a corrugated or ringed appearance and linear furrows, resulting from fibrosis and scarring. “Micro-tears” may also be visible projecting linearly up the esophagus. Multiple white specks are signs of conglomerations of eosinophils and are easily confused with yeast infection. Strictures from scar tissue cause the mucosa to be tight and fragile, making the esophagus very susceptible to tearing during endoscopy.
After years of untreated disease, the esophagus becomes increasingly inflamed and fibrotic. Adult patients with eosinophilic esophagitis who were followed for a decade were found to develop increasing collagen deposition in which the submucosa or even the entire esophageal wall was diffusely fibrotic.21
Radiographic findings—a narrow esophagus
On radiography, the esophagus may appear narrow—not uncommonly one-third to one-quarter the caliber of a normal esophagus. As the esophagus progressively narrows, both eating and treatment become extremely difficult.
Symptoms are different in children and adults
Symptoms reflect the endoscopic changes over time. In children, the condition manifests with feeding difficulties, vomiting, symptoms of gastroesophageal reflux, and abdominal pain as signs of inflammation. As the esophagus becomes fibrotic, teenagers and young adults tend to present with strictures, dysphagia, and food impaction. Of patients who present to an emergency department with food impaction, the major cause is now eosinophilic esophagitis.22
It is important to pay attention to symptoms in children to diagnose the condition and start treatment early to prevent or postpone disease advancement. Medical therapy does not clearly reverse the fibrosis.
As in many chronic benign diseases, patients learn to compensate, so a careful history is essential. Many deny having a swallowing problem, but questioning may reveal that they have always been slow, picky eaters, consuming mostly soft foods and drinking fluids with every bite.
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease can be a challenge, as signs and symptoms overlap.
Veerappan et al23 looked for predictors of eosinophilic esophagitis in 400 adults who underwent routine upper endoscopy, 6.5% of whom had eosinophilic esophagitis. They found significant overlap in medical history for patients with and without the disease; while a higher proportion of patients with eosinophilic esophagitis had a history of asthma, dysphagia, food impactions, dermatitis, and food allergies, these conditions also occurred in other patients.
Similarly, the classic endoscopic findings of eosinophilic esophagitis—rings, furrows, strictures, and plaques—also occur in other conditions.23 Reflux disease can cause scarring from excess acid and may even be associated with eosinophils in the esophagus, indicative of a combination of allergy and reflux. A small-caliber esophagus is also occasionally present in patients with reflux disease.
Ambulatory pH monitoring has been recommended to help determine if gastroesophageal reflux is the cause of esophageal eosinophilia and to guide therapy. However, in a prospective study of 51 patients,24 neither positive nor negative results of initial pH monitoring accurately predicted response to PPIs or steroid therapy. Another study found that half of patients with an eosinophilic esophagitis profile without evidence of acid reflux by pH monitoring responded to treatment with a PPI.25
This raises the question of whether some patients with eosinophilic esophagitis have more acid reflux than is detected by pH monitoring, or alternatively, whether PPIs have other, less-recognized effects besides reducing acidity. Investigators are now ascribing a host of anti-inflammatory actions to PPIs, including effects on antioxidants, inflammatory cells, endothelial cells, and the gut microflora.26 And PPIs may alleviate eosinophilic esophagitis through anti-inflammatory effects rather than by inhibiting secretion of gastric acid.
THREE TYPES OF THERAPY
In general, three types of therapy are available for patients with eosinophilic esophagitis: medications, allergen avoidance, and esophageal dilation (Table 2).
Medications: Try a PPI first, then a corticosteroid
A PPI should be tried even for patients with a classic presentation of eosinophilic esophagitis because some will respond, and long-term PPI therapy is preferable to long-term steroid treatment. Patients should be put on a 2-month course and should then undergo repeat biopsy.
For patients who do not respond to a PPI, a corticosteroid or montelukast can be tried. Topical therapy is showing promise as both a short- and long-term option to bring about remission.27 For administration, a corticosteroid (budesonide or fluticasone) is mixed with a viscous solution, such as water with honey or chocolate syrup, making it thick so it better coats the esophagus. The therapy can be very effective: in up to 8 weeks some patients have a 90% resolution of esophageal eosinophilia. However, about 5% of patients develop a yeast infection, and adrenal suppression is a concern but appears to be uncommon.
Avoidance of allergens
Because eosinophilic esophagitis is an allergic disease, eliminating allergens should be an effective treatment. Unfortunately, from a practical standpoint, elimination is very difficult. The elemental diet formula is expensive and unpalatable, making it impractical for a prolonged period.
Gonsalves et al28 put 50 adult patients with eosinophilic esophagitis on a diet eliminating the six most common foods believed to trigger the disease—wheat, milk, nuts, eggs, soy, and seafood—and found a marked reduction in eosinophils in the proximal and distal esophagus after 6 weeks. Additional triggers that have been identified include rice, corn, and legumes.29
Unfortunately, maintaining a diet without the most commonly identified allergens is not easy. Although some very motivated patients can do it, it is especially hard for teens and young adults. Variations of the diet, such as eliminating just two foods, make following a plan easier. Omitting milk alone would benefit an estimated 20% of patients with eosinophilic esophagitis.
Identifying food triggers is a challenge in itself as there is no good noninvasive method of identifying the allergens. The radioallergosorbent test measures immunoglobulin (Ig) E, and the skin-prick test measures acute hypersensitivity, but neither is very sensitive for the Th2-mediated reaction involved in eosinophilic esophagitis. In early trials, endoscopy and biopsy were painstakingly performed with the removal and reintroduction of every suspected food allergen, requiring multiple biopsies weekly, which is impractical for safety and economic reasons.
Attempts are being made to devise less invasive methods of sampling the esophageal mucosa. Transnasal endoscopy—done as an outpatient procedure with topical anesthesia—is a possibility. Another possibility is the esophageal string test,30 which involves swalling a weighted capsule on a string and then, after an hour, pulling it up again and testing the tissue on the string.
The “cytosponge,” a new device currently under investigation, also uses a string delivery system. The patient swallows a sponge contained in a gelatin capsule and attached to a string. When the capsule dissolves in the stomach—a process that takes only a few minutes—the sponge expands. The string is then pulled up, causing the sponge to sample the esophageal mucosa and thus obtaining a histologic specimen. This method shows promise as an inexpensive and noninvasive way to monitor the disease, although larger studies are needed to establish efficacy.31
Dilation—proceed with caution
Dilation can be an important therapy, especially in teenagers and adults with a fibrotic, narrowed esophagus.
Early on, the procedure often resulted in complications such as deep mucosal tears and perforations. Jung et al32 retrospectively analyzed 293 dilations in 161 patients with eosinophilic esophagitis and found a deep mucosal tear in 27 patients (9%), three perforations, and one incidence of major bleeding. All complications resolved without surgery. Factors associated with increased risk of complications were luminal narrowing in the upper and middle third of the esophagus, a luminal stricture that could not be traversed with a standard upper endoscope, and use of a Savary dilator.
It is critical that dilation be done slowly—a few millimeters at a time. Several sessions may be needed.
TREATMENT DURING REMISSION IS CONTROVERSIAL
Unless the patient with eosinophilic esophagitis can consistently control the disease by avoiding allergens, the question arises of whether to continue treating a patient who is in remission.
On the one hand, there is no known risk of Barrett esophagus or malignancy when the condition is not treated, and weight loss is uncommon because patients tend to accommodate to the condition. However, the long-term consequences are uncertain. Allergies are chronic, and disease progression with more fibrosis should be prevented. Also, food impaction commonly occurs and this requires aggressive dilation, which is risky.
On the other hand, chronic steroid therapy involves risk. The optimum steroid dosage during remission and whether alternate-day dosing is adequate have yet to be determined.
Long-term trials are needed to answer these questions. In the meantime, most physicians tend to aggressively treat this disease, if not with specific food avoidance, then with steroid maintenance therapy.
MONITORING THE DISEASE
Monitoring eosinophilic esophagitis by clinical indicators is difficult. Once fibrosis develops, symptoms often do not reflect underlying pathology. It may turn out that, as in Crohn disease, monitoring mucosal healing rather than symptoms may be best.
Until we know more about this condition, careful monitoring of patients is important. However, it is too early to give specific guidance, such as endoscopy every 2 months or annually. Whether the eosinophil count should be the critical consideration is also unknown.
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20.
- Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009; 137:1238–1249.
- Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001; 107:83–90.
- Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013; 108:759–766.
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 2010; 20:142–149.
- Chang F, Anderson S. Clinical and pathological features of eosinophilic oesophagitis: a review. Pathology 2008; 40:3–8.
- Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep 2009; 11:190–194.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet 2010; 42:289–291.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38:109–116.
- Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008; 134:1316–1321.
- Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol 2009; 131:788–792.
- Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther 2010; 32:712–719.
- Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007; 56:615–620.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007; 102:2627–2632.
- Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology 2011; 141:1586–1592.
- Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy 1999; 29:342–346.
- Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2008; 6:531–535.
- Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophiic inflammation with esophageal food impaction in adults. Gastrointest Endosc 2005; 61:795–801.
- Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009; 7:420–426.
- Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012; 35:300–307.
- Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9:110–117.
- Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 2009; 54:2312–2317.
- Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139:1526–1537.
- Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142:1451–1459.
- Lucendo AJ, Arias Á, González-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131:797–804.
- Fillon SA, Harris JK, Wagner BD, et al. Novel device to sample the esophageal microbiome—the esophageal string test. PLoS One 2012; 7:e42938.
- Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014. pii: S1542-3565(14)00933-1. doi: 10.1016/j.cgh.2014.06.026. [Epub ahead of print]
- Jung KW, Gundersen N, Kopacova J, et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc 2011; 73:15–21.
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20.
- Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009; 137:1238–1249.
- Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001; 107:83–90.
- Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013; 108:759–766.
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 2010; 20:142–149.
- Chang F, Anderson S. Clinical and pathological features of eosinophilic oesophagitis: a review. Pathology 2008; 40:3–8.
- Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep 2009; 11:190–194.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet 2010; 42:289–291.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38:109–116.
- Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008; 134:1316–1321.
- Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol 2009; 131:788–792.
- Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther 2010; 32:712–719.
- Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007; 56:615–620.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007; 102:2627–2632.
- Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology 2011; 141:1586–1592.
- Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy 1999; 29:342–346.
- Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2008; 6:531–535.
- Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophiic inflammation with esophageal food impaction in adults. Gastrointest Endosc 2005; 61:795–801.
- Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009; 7:420–426.
- Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012; 35:300–307.
- Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9:110–117.
- Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 2009; 54:2312–2317.
- Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139:1526–1537.
- Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142:1451–1459.
- Lucendo AJ, Arias Á, González-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131:797–804.
- Fillon SA, Harris JK, Wagner BD, et al. Novel device to sample the esophageal microbiome—the esophageal string test. PLoS One 2012; 7:e42938.
- Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014. pii: S1542-3565(14)00933-1. doi: 10.1016/j.cgh.2014.06.026. [Epub ahead of print]
- Jung KW, Gundersen N, Kopacova J, et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc 2011; 73:15–21.
KEY POINTS
- Eosinophilic esophagitis is an allergy-mediated, systemic disease.
- It is diagnosed by characteristic symptoms, esophageal biopsy (peak value ≥ 15 eosinophils per high-power field), and response to allergen avoidance or treatment with steroids.
- Therapy with a proton pump inhibitor should be tried even for patients with a classic presentation.
- Strict dietary avoidance of allergens has been shown to resolve the disease but is often impractical.
- Dilation is indicated for a narrowed esophagus but must be done cautiously because of the risk of tearing.
- How best to monitor the disease (eg, by annual endoscopy) is still uncertain.
Diagnostic certainty and the eosinophil
This issue of the Journal contains an article by Dr. David A. Katzka, titled “The ‘skinny’ on eosinophilic esophagitis.” Reading it, I was struck by two messages, one clinical and one biological.
The clinical message relates to the psychology of diagnosis, or as Dr. Jerome Groopman discussed in his book How Doctors Think, misdiagnosis. In many patients, eosinophilic esophagitis, especially early in its course, can mimic gastroesophageal reflux disease (GERD), causing dysphagia and discomfort with eating that may be relieved at least in part with a proton pump inhibitor. When evaluating a patient who relates a history compatible with a common condition, we instinctively tend to embrace the diagnosis of that common syndrome, in this case GERD, rather than initially explore in depth the possibility of less-common mimics. Once the disease has progressed, with the patient experiencing frequent postprandial emesis or needing to dramatically limit the size of meals despite taking a full dose of a proton pump inhibitor, we will hopefully revisit and reassess our initial diagnosis, often with endoscopy and biopsy. But that may not always occur promptly, because we may have committed (per Groopman) an “anchoring error,” seizing on an initial symptom or finding, allowing it to cloud our clinical judgment, reaching “premature closure,” and not keeping our minds open to alternative diagnoses such as eosinophilic esophagitis. I wonder how many of the younger patients I have diagnosed with GERD who had histories of “food intolerances” actually had eosinophilic esophagitis.
The biological message is that the eosinophil is a fascinating and generally misunderstood cell, not just a marker and mediator of allergy. As an apparent defender against the macro-invaders—worms and other parasites—it carries an arsenal of defensive weapons. But eosinophil-dominant inflammatory reactions started by various molecular triggers and perpetuated by interleukin 5 and other promoters of eosinophil proliferation and chemotaxis have a common histopathologic footprint—fibrosis.
Long-standing significant asthma is characterized as much by airway remodeling and fibrosis as it is by bronchospasm. A myocardial hallmark of hypereosinophilic syndrome is fibrosis. Eosinophilic pneumonia can be followed by local scarring. Eosinophils have been implicated in the pathogenesis of primary biliary cirrhosis and the granulomatous cirrhosis of schistosomiasis. And as Dr. Katzka reminds us, the confluence of food hypersensitivity, gastric acid, and the products of eosinophil activation (likely including transforming growth factor beta) in the esophageal wall can result in a marked fibrotic reaction with dysmotility. It is unclear whether this is a dysregulated attempt at healing with resultant maladaptive “scar” formation, or perhaps a misdirected inflammatory response, with the goal of walling off a perceived invader (an allergen is not a worm).
There are probably many other mimic diseases that we are not recognizing often enough. And tissue eosinophils may portend detrimental fibrotic remodeling.
This issue of the Journal contains an article by Dr. David A. Katzka, titled “The ‘skinny’ on eosinophilic esophagitis.” Reading it, I was struck by two messages, one clinical and one biological.
The clinical message relates to the psychology of diagnosis, or as Dr. Jerome Groopman discussed in his book How Doctors Think, misdiagnosis. In many patients, eosinophilic esophagitis, especially early in its course, can mimic gastroesophageal reflux disease (GERD), causing dysphagia and discomfort with eating that may be relieved at least in part with a proton pump inhibitor. When evaluating a patient who relates a history compatible with a common condition, we instinctively tend to embrace the diagnosis of that common syndrome, in this case GERD, rather than initially explore in depth the possibility of less-common mimics. Once the disease has progressed, with the patient experiencing frequent postprandial emesis or needing to dramatically limit the size of meals despite taking a full dose of a proton pump inhibitor, we will hopefully revisit and reassess our initial diagnosis, often with endoscopy and biopsy. But that may not always occur promptly, because we may have committed (per Groopman) an “anchoring error,” seizing on an initial symptom or finding, allowing it to cloud our clinical judgment, reaching “premature closure,” and not keeping our minds open to alternative diagnoses such as eosinophilic esophagitis. I wonder how many of the younger patients I have diagnosed with GERD who had histories of “food intolerances” actually had eosinophilic esophagitis.
The biological message is that the eosinophil is a fascinating and generally misunderstood cell, not just a marker and mediator of allergy. As an apparent defender against the macro-invaders—worms and other parasites—it carries an arsenal of defensive weapons. But eosinophil-dominant inflammatory reactions started by various molecular triggers and perpetuated by interleukin 5 and other promoters of eosinophil proliferation and chemotaxis have a common histopathologic footprint—fibrosis.
Long-standing significant asthma is characterized as much by airway remodeling and fibrosis as it is by bronchospasm. A myocardial hallmark of hypereosinophilic syndrome is fibrosis. Eosinophilic pneumonia can be followed by local scarring. Eosinophils have been implicated in the pathogenesis of primary biliary cirrhosis and the granulomatous cirrhosis of schistosomiasis. And as Dr. Katzka reminds us, the confluence of food hypersensitivity, gastric acid, and the products of eosinophil activation (likely including transforming growth factor beta) in the esophageal wall can result in a marked fibrotic reaction with dysmotility. It is unclear whether this is a dysregulated attempt at healing with resultant maladaptive “scar” formation, or perhaps a misdirected inflammatory response, with the goal of walling off a perceived invader (an allergen is not a worm).
There are probably many other mimic diseases that we are not recognizing often enough. And tissue eosinophils may portend detrimental fibrotic remodeling.
This issue of the Journal contains an article by Dr. David A. Katzka, titled “The ‘skinny’ on eosinophilic esophagitis.” Reading it, I was struck by two messages, one clinical and one biological.
The clinical message relates to the psychology of diagnosis, or as Dr. Jerome Groopman discussed in his book How Doctors Think, misdiagnosis. In many patients, eosinophilic esophagitis, especially early in its course, can mimic gastroesophageal reflux disease (GERD), causing dysphagia and discomfort with eating that may be relieved at least in part with a proton pump inhibitor. When evaluating a patient who relates a history compatible with a common condition, we instinctively tend to embrace the diagnosis of that common syndrome, in this case GERD, rather than initially explore in depth the possibility of less-common mimics. Once the disease has progressed, with the patient experiencing frequent postprandial emesis or needing to dramatically limit the size of meals despite taking a full dose of a proton pump inhibitor, we will hopefully revisit and reassess our initial diagnosis, often with endoscopy and biopsy. But that may not always occur promptly, because we may have committed (per Groopman) an “anchoring error,” seizing on an initial symptom or finding, allowing it to cloud our clinical judgment, reaching “premature closure,” and not keeping our minds open to alternative diagnoses such as eosinophilic esophagitis. I wonder how many of the younger patients I have diagnosed with GERD who had histories of “food intolerances” actually had eosinophilic esophagitis.
The biological message is that the eosinophil is a fascinating and generally misunderstood cell, not just a marker and mediator of allergy. As an apparent defender against the macro-invaders—worms and other parasites—it carries an arsenal of defensive weapons. But eosinophil-dominant inflammatory reactions started by various molecular triggers and perpetuated by interleukin 5 and other promoters of eosinophil proliferation and chemotaxis have a common histopathologic footprint—fibrosis.
Long-standing significant asthma is characterized as much by airway remodeling and fibrosis as it is by bronchospasm. A myocardial hallmark of hypereosinophilic syndrome is fibrosis. Eosinophilic pneumonia can be followed by local scarring. Eosinophils have been implicated in the pathogenesis of primary biliary cirrhosis and the granulomatous cirrhosis of schistosomiasis. And as Dr. Katzka reminds us, the confluence of food hypersensitivity, gastric acid, and the products of eosinophil activation (likely including transforming growth factor beta) in the esophageal wall can result in a marked fibrotic reaction with dysmotility. It is unclear whether this is a dysregulated attempt at healing with resultant maladaptive “scar” formation, or perhaps a misdirected inflammatory response, with the goal of walling off a perceived invader (an allergen is not a worm).
There are probably many other mimic diseases that we are not recognizing often enough. And tissue eosinophils may portend detrimental fibrotic remodeling.
David Henry's JCSO podcast, January 2015
In his monthly podcast for The Journal of Community and Supportive Oncology, Dr David Henry looks at Original Reports on the comparison of atropine-diphenoxylate and hyoscyamine in lowering the rates of irinotecan-related cholinergic syndrome; the effects of age and comorbidities in the management of rectal cancer in elderly patients at an institution in Portugal; the impact of a telehealth intervention on quality of life and symptom distress in patients with head and neck cancer; and the beneficial effects of animal-assisted visits on quality of life during multimodal radiation-chemotherapy regimens. He also discusses a Research Report in which the authors attempt, possibly for the first time, to quantify radiation exposure from diagnostic procedures in patients with newly diagnosed breast cancer, as well as two feature articles – a round-up of some of the presentations at the 2014 San Antonio Breast Cancer Symposium and a Journal Club presentation of therapies for lymphoproliferative disorders.
In his monthly podcast for The Journal of Community and Supportive Oncology, Dr David Henry looks at Original Reports on the comparison of atropine-diphenoxylate and hyoscyamine in lowering the rates of irinotecan-related cholinergic syndrome; the effects of age and comorbidities in the management of rectal cancer in elderly patients at an institution in Portugal; the impact of a telehealth intervention on quality of life and symptom distress in patients with head and neck cancer; and the beneficial effects of animal-assisted visits on quality of life during multimodal radiation-chemotherapy regimens. He also discusses a Research Report in which the authors attempt, possibly for the first time, to quantify radiation exposure from diagnostic procedures in patients with newly diagnosed breast cancer, as well as two feature articles – a round-up of some of the presentations at the 2014 San Antonio Breast Cancer Symposium and a Journal Club presentation of therapies for lymphoproliferative disorders.
In his monthly podcast for The Journal of Community and Supportive Oncology, Dr David Henry looks at Original Reports on the comparison of atropine-diphenoxylate and hyoscyamine in lowering the rates of irinotecan-related cholinergic syndrome; the effects of age and comorbidities in the management of rectal cancer in elderly patients at an institution in Portugal; the impact of a telehealth intervention on quality of life and symptom distress in patients with head and neck cancer; and the beneficial effects of animal-assisted visits on quality of life during multimodal radiation-chemotherapy regimens. He also discusses a Research Report in which the authors attempt, possibly for the first time, to quantify radiation exposure from diagnostic procedures in patients with newly diagnosed breast cancer, as well as two feature articles – a round-up of some of the presentations at the 2014 San Antonio Breast Cancer Symposium and a Journal Club presentation of therapies for lymphoproliferative disorders.
Hysteroscopic myomectomy using a mechanical approach
Uterine fibroids are a common complaint in gynecology, with an incidence of approximately 30% in women aged 25 to 45 years and a cumulative incidence of 70% to 80% by age 50.1,2 They are more prevalent in women of African descent and are a leading indication for hysterectomy.
Although they can be asymptomatic, submucosal fibroids are frequently associated with:
- abnormal uterine bleeding (AUB)
- dysmenorrhea
- expulsion of an intrauterine device (IUD)
- leukorrhea
- pelvic pain
- urinary frequency
- infertility
- premature labor
- reproductive wastage
- bleeding during hormone replacement therapy.
In postmenopausal women, the risk of malignancy in a leiomyoma ranges from 0.2% to 0.5%.1 The risk is lower in premenopausal women.
In this article, I describe the technique for hysteroscopic myomectomy using a mechanical approach (Truclear Tissue Removal System, Smith & Nephew, Andover, MA), which offers hysteroscopic morcellation as well as quick resection and efficient fluid management. (Note: Unlike open intraperitoneal morcellation, hysteroscopic morcellation carries a low risk of tissue spread.)
Classification of fibroids
Preoperative classification of leiomyomas makes it possible to determine the best route for surgery. The most commonly used classification system was developed by the European Society of Gynaecological Endoscopy (ESGE) (FIGURE 1), which considers the extent of intramural extension. Each fibroid under that system is classified as:
- Type 0 – no intramural extension
- Type I – less than 50% extension
- Type II – more than 50% extension.
A second classification system recently was devised to take into account additional features of the fibroid. The STEP-W classification considers size, topography, extension, penetration, and the lateral wall (FIGURE 2). In general, the lower the score, the less complex the procedure will be, with a lower risk of fluid intravasation, shorter operative time, and a greater likelihood of complete removal of the fibroid.
A multicenter, prospective study of 449 women who underwent hysteroscopic resection of their fibroids correlated the ESGE and STEP-W systems. All 320 fibroids (100%) with a score of 4 or below on the STEP-W classification system were completely removed, compared with 112 of 145 fibroids (77.2%) with a score greater than 4. All 33 cases of incomplete hysteroscopic resection (100%) had a STEP-W score above 4.3
In the same study, 85 of 86 cases (98.9%) with Type 0 fibroids under the ESGE system had complete resection, along with 278 of 298 Type I fibroids (93.3%), and 69 of 81 Type II fibroids (85.2%).3 Complete removal is a goal because it relieves symptoms and averts the need for additional procedures.
Patient selection
Proper patient selection for hysteroscopic myomectomy is extremely important. The most common indications are AUB, pelvic pain or discomfort, recurrent pregnancy loss, and infertility. In addition, the patient should have a strong wish for uterine preservation and desire a minimally invasive transcervical approach.
AAGL guidelines on the diagnosis and management of submucous fibroids note that, in general, submucous leiomyomas as large as 4 or 5 cm in diameter can be removed hysteroscopically by experienced surgeons.4
A hysteroscopic approach is not advised for women in whom hysteroscopic surgery is contraindicated, such as women with intrauterine pregnancy, active pelvic infection, active herpes infection, or cervical or uterine cancer. Women who have medical comorbidities such as coronary heart disease, significant renal disease, or bleeding diathesis may need perioperative clearance from anesthesia or hematology prior to hysteroscopic surgery and close fluid monitoring during the procedure.
Consider the leiomyoma
Penetration into the myometrium. Women who have a fibroid that penetrates more than 50% into the myometrium may benefit from hysteroscopic myomectomy, provided the surgeon is highly experienced. A skilled hysteroscopist can ensure complete enucleation of a penetrating fibroid in these cases.
If you are still in the learning process for hysteroscopy, however, start with easier cases—ie, polyps and Type 0 and Type I fibroids. Type II fibroids require longer operative time, are associated with increased fluid absorption and intravasation, carry an increased risk of perioperative complications, and may not always be completely resected.
Size of the fibroid also is relevant. As size increases, so does the volume of tissue needing to be removed, adding to overall operative time.
Presence of other fibroids. When a woman has an intracavitary fibroid as well as myomas in other locations, the surgeon should consider whether hysteroscopic removal of the intracavitary lesion alone can provide significant relief of all fibroid-related symptoms. In such cases, laparoscopic, robotic, or abdominal myomectomy may be preferable, especially if the volume of the additional myomas is considerable.
To determine the optimal surgical route, the physician must consider the symptoms present—is AUB the only symptom, or are other fibroid-related conditions present as well, such as bulk, pelvic pain, and other quality-of-life issues? If multiple symptoms exist, then other approaches may be better.
How fibroids affect fertility
Fibroids are present in 5% to 10% of women with infertility. In this population, fibroids are the only abnormal finding in 1.0% to 2.4% of cases.4
In a meta-analysis of 23 studies evaluating women with fibroids and infertility, Pritts and colleagues found nine studies involving submucosal fibroids.5 These studies included one randomized controlled trial, two prospective studies, and six retrospective analyses. They found that women who had fibroids with a submucosal component had lower pregnancy and implantation rates, compared with their infertile, myoma-free counterparts. Pritts and colleagues concluded that myomectomy is likely to improve fertility in these cases (TABLE).5
Instrumentation
Among the options are monopolar and bipolar resectoscopy and the mechanical approach using the Truclear System, which includes a morcellator. With conventional resectoscopy all chips must be removed, necessitating multiple insertions of the hysteroscope. Monopolar instrumentation, in particular, carries a risk of energy discharge to healthy tissue. The monopolar resectoscope also has a longer learning curve, compared with the mechanical approach.6
In contrast, the Truclear System requires fewer insertions, has a short learning curve, and omits the need for capture of individual chips, as the mechanical morcellator suctions and captures them throughout the procedure.7 In addition, because resection is performed mechanically, there is no risk of energy discharge to healthy tissue.
The Truclear system also is associated with a significantly shorter operative time, compared with resectoscopy, which may be advantageous for residents, fellows, and other physicians learning the procedure (FIGURE 3).7 Shorter operative time also may result in lower fluid deficits. In addition, saline distension may reduce the risk of fluid absorption and hyponatremia. The tissue-capture feature allows evaluation of the entire pathologic specimen.
Besides hysteroscopic myomectomy, the Truclear System is appropriate for visual dilatation and curettage (D&C), adhesiolysis, polypectomy, and evacuation of retained products of conception.
Preoperative evaluation
A complete history is vital to document which fibroid-related symptoms are present and how they affect quality of life.
Preoperative imaging also is imperative—using either 2D or 3D saline infusion sonography or a combination of diagnostic hysteroscopy and transvaginal ultrasound—to select patients for hysteroscopy, anticipate blood loss, and ensure that the proper instrumentation is available at the time of surgery. Magnetic resonance imaging, computed tomography, and hysterosalpingography are either prohibitively expensive or of limited value in the initial preoperative assessment of uterine fibroids.
Any woman who has AUB and a risk for endometrial hyperplasia or cancer should undergo endometrial assessment as well.
Use of preoperative medications
In most cases, prophylactic administration of antibiotics is not warranted to prevent infection or endocarditis.
Although some clinicians give gonadotropin-releasing hormone (GnRH) agonists to reduce the size of large fibroids, the drug complicates dissection of the fibroid from the surrounding capsule. For this reason, and because we lack data demonstrating that GnRH agonists decrease blood loss and limit absorption of distension media, I do not administer them to patients.8–12 Moreover, this drug can cause vasomotor symptoms, cervical stenosis, and vaginal hemorrhage (related to estrogen flare).
GnRH agonists may be of value to stimulate transient amenorrhea for several months preoperatively in order to correct iron-deficiency anemia. Intravenous iron also can be administered during this interval.
The risk of bleeding in hysteroscopic myomectomy is 2% to 3%.1 When the mechanical approach is used, rather than resectoscopy, continuous flow coupled with suctioning of the chips during the procedure keeps the image clear. Post-procedure contraction of the uterus stops most bleeding. Intrauterine pressure of the pump can be increased to help tamponade any oozing.
Misoprostol. Cervical stenosis is not uncommon in menopausal women. It can also pose a challenge in nulliparous women. Attempting hysteroscopy in the setting of cervical stenosis increases the risk of cervical laceration, creation of a false passage, and uterine perforation. For this reason, I prescribe oral or vaginal misoprostol 200 to 400 µg nightly for 1 to 2 days before the procedure.
Vasopressin can reduce blood loss during hysteroscopic myomectomy when it is injected into the cervical stroma preoperatively. It also reduces absorption of distension fluid and facilitates cervical dilation.
However, vasopressin must be injected with extreme care, with aspiration to confirm the absence of blood prior to each injection, as intravascular injection can lead to bradycardia, profound hypertension, and even death.13 Always notify the anesthesiologist prior to injection when vasopressin will be administered.
I routinely use vasopressin before hysteroscopic myomectomy (0.5 mg in 20 cc of saline or 20 U in 100 cc), injecting 5 cc of the solution at 3, 6, 9, and 12 o’clock positions.
Anesthesia during hysteroscopic myomectomy typically is “monitored anesthesia care,” or MAC, which consists of local anesthesia with sedation and analgesia. The need for regional or general anesthesia is rare. Consider adding a pericervical block or intravenous ketorolac (Toradol) to provide postoperative analgesia.
Surgical technique
Strict attention to fluid management is required throughout the procedure, preferably in accordance with AAGL guidelines on the management of hysteroscopic distending media.14 With the mechanical approach, because the distension fluid is isotonic (normal saline), it does not increase the risk of hyponatremia but can cause pulmonary edema or congestive heart failure. Intravasation usually is the result of excessive operative time, treatment of deeper myometrial fibroids (Type I or II), or high intrauterine pressure. I operate using intrauterine pressure in the range of 75 to 125 mm Hg.
The steps involved in the mechanical hysteroscopy approach are:
- Insert the hysteroscope into the uterus under direct visualization. In general, the greater the number of insertions, the greater the risk of uterine perforation. Preoperative cervical ripening helps facilitate insertion (see “Misoprostol” above).
- Distend the uterus with saline and inspect the uterine cavity, noting again the size and location of the fibroids and whether they are sessile or pedunculated.
- Locate the fibroid or other pathology to be removed, and place the morcellator window against it to begin cutting. Use the tip of the morcellator to elevate the fibroid for easier cutting. Enucleation is accomplished largely by varying the intrauterine pressure, which permits uterine decompression and myometrial contraction and renders the fibroid capsule more visible. If necessary, the hysteroscope can be withdrawn to stimulate myometrial contraction, which also helps to delineate the fibroid capsule.
- Reinspect the uterus to rule out perforation and remove any additional intrauterine pathology with a targeted view.
- Once all designated fibroids have been removed, withdraw the morcellator and hysteroscope from the uterus.
- Inspect the endocervical landscape to rule out injury and other pathology.
- Careful preoperative evaluation is important, preferably using diagnostic hysteroscopy or saline infusion sonography, to choose the optimal route of myomectomy and plan the surgical approach.
- During the myomectomy, pay close attention to fluid management and adhere strictly to predetermined limits.
- Complete removal of the fibroid is essential to relieve symptoms and avert the need for additional procedures.
Postoperative care
A nonsteroidal anti-inflammatory drug or limited use of narcotics usually is sufficient to relieve any postoperative cramping or vaginal discomfort.
Advise the patient to notify you in the event of increasing pain, foul-smelling vaginal discharge, or fever.
Also counsel her that she can return to most normal activities within 24 to 48 hours. Sexual activity is permissible 1 week after surgery. Early and frequent ambulation is important.
Schedule a follow-up visit 4 to 6 weeks after the procedure.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Perez-Medina T, Font EC, eds. Diagnostic and Operative Hysteroscopy. Tunbridge Wells, Kent, UK: Anshan Publishing; 2007:13.
2. Management of uterine fibroids: an update of the evidence. Agency for Healthcare Research and Quality. http://archive.ahrq.gov/clinic/tp/uteruptp.htm. Published July 2007. Accessed January 14, 2015.
3. Lasmar RB, Zinmei Z, Indman PD, Celeste RK, Di Spiezo Sardo A. Feasibility of a new system of classification of submucous myomas: a multicenter study. Fertil Steril. 2011;95(6):2073–2077.
4. AAGL Practice Report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol. 2012;19(2):152–171.
5. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223.
6. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466–471.
7. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62–66.
8. Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil Steril. 1997;68(5):881–886.
9. Taskin O, Sadik S, Onoglu A, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7(3):351.
10. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96(4):517–520.
11. Perino A, Chianchiano N, Petronio M, Cittadini E. Role of leuprolide acetate depot in hysteroscopic surgery: a controlled study. Fertil Steril. 1993;59(3):507–510.
12. Mencaglia L, Tantini C. GnRH agonist analogs and hysteroscopic resection of myomas. Int J Gynaecol Obstet. 1993;43(3):285–288.
13. Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 Pt 2):484–486.
14. Munro MD, Storz K, Abbott JA, et al; AAGL. AAGL Practice Report: practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013;20(2):137–148.
Uterine fibroids are a common complaint in gynecology, with an incidence of approximately 30% in women aged 25 to 45 years and a cumulative incidence of 70% to 80% by age 50.1,2 They are more prevalent in women of African descent and are a leading indication for hysterectomy.
Although they can be asymptomatic, submucosal fibroids are frequently associated with:
- abnormal uterine bleeding (AUB)
- dysmenorrhea
- expulsion of an intrauterine device (IUD)
- leukorrhea
- pelvic pain
- urinary frequency
- infertility
- premature labor
- reproductive wastage
- bleeding during hormone replacement therapy.
In postmenopausal women, the risk of malignancy in a leiomyoma ranges from 0.2% to 0.5%.1 The risk is lower in premenopausal women.
In this article, I describe the technique for hysteroscopic myomectomy using a mechanical approach (Truclear Tissue Removal System, Smith & Nephew, Andover, MA), which offers hysteroscopic morcellation as well as quick resection and efficient fluid management. (Note: Unlike open intraperitoneal morcellation, hysteroscopic morcellation carries a low risk of tissue spread.)
Classification of fibroids
Preoperative classification of leiomyomas makes it possible to determine the best route for surgery. The most commonly used classification system was developed by the European Society of Gynaecological Endoscopy (ESGE) (FIGURE 1), which considers the extent of intramural extension. Each fibroid under that system is classified as:
- Type 0 – no intramural extension
- Type I – less than 50% extension
- Type II – more than 50% extension.
A second classification system recently was devised to take into account additional features of the fibroid. The STEP-W classification considers size, topography, extension, penetration, and the lateral wall (FIGURE 2). In general, the lower the score, the less complex the procedure will be, with a lower risk of fluid intravasation, shorter operative time, and a greater likelihood of complete removal of the fibroid.
A multicenter, prospective study of 449 women who underwent hysteroscopic resection of their fibroids correlated the ESGE and STEP-W systems. All 320 fibroids (100%) with a score of 4 or below on the STEP-W classification system were completely removed, compared with 112 of 145 fibroids (77.2%) with a score greater than 4. All 33 cases of incomplete hysteroscopic resection (100%) had a STEP-W score above 4.3
In the same study, 85 of 86 cases (98.9%) with Type 0 fibroids under the ESGE system had complete resection, along with 278 of 298 Type I fibroids (93.3%), and 69 of 81 Type II fibroids (85.2%).3 Complete removal is a goal because it relieves symptoms and averts the need for additional procedures.
Patient selection
Proper patient selection for hysteroscopic myomectomy is extremely important. The most common indications are AUB, pelvic pain or discomfort, recurrent pregnancy loss, and infertility. In addition, the patient should have a strong wish for uterine preservation and desire a minimally invasive transcervical approach.
AAGL guidelines on the diagnosis and management of submucous fibroids note that, in general, submucous leiomyomas as large as 4 or 5 cm in diameter can be removed hysteroscopically by experienced surgeons.4
A hysteroscopic approach is not advised for women in whom hysteroscopic surgery is contraindicated, such as women with intrauterine pregnancy, active pelvic infection, active herpes infection, or cervical or uterine cancer. Women who have medical comorbidities such as coronary heart disease, significant renal disease, or bleeding diathesis may need perioperative clearance from anesthesia or hematology prior to hysteroscopic surgery and close fluid monitoring during the procedure.
Consider the leiomyoma
Penetration into the myometrium. Women who have a fibroid that penetrates more than 50% into the myometrium may benefit from hysteroscopic myomectomy, provided the surgeon is highly experienced. A skilled hysteroscopist can ensure complete enucleation of a penetrating fibroid in these cases.
If you are still in the learning process for hysteroscopy, however, start with easier cases—ie, polyps and Type 0 and Type I fibroids. Type II fibroids require longer operative time, are associated with increased fluid absorption and intravasation, carry an increased risk of perioperative complications, and may not always be completely resected.
Size of the fibroid also is relevant. As size increases, so does the volume of tissue needing to be removed, adding to overall operative time.
Presence of other fibroids. When a woman has an intracavitary fibroid as well as myomas in other locations, the surgeon should consider whether hysteroscopic removal of the intracavitary lesion alone can provide significant relief of all fibroid-related symptoms. In such cases, laparoscopic, robotic, or abdominal myomectomy may be preferable, especially if the volume of the additional myomas is considerable.
To determine the optimal surgical route, the physician must consider the symptoms present—is AUB the only symptom, or are other fibroid-related conditions present as well, such as bulk, pelvic pain, and other quality-of-life issues? If multiple symptoms exist, then other approaches may be better.
How fibroids affect fertility
Fibroids are present in 5% to 10% of women with infertility. In this population, fibroids are the only abnormal finding in 1.0% to 2.4% of cases.4
In a meta-analysis of 23 studies evaluating women with fibroids and infertility, Pritts and colleagues found nine studies involving submucosal fibroids.5 These studies included one randomized controlled trial, two prospective studies, and six retrospective analyses. They found that women who had fibroids with a submucosal component had lower pregnancy and implantation rates, compared with their infertile, myoma-free counterparts. Pritts and colleagues concluded that myomectomy is likely to improve fertility in these cases (TABLE).5
Instrumentation
Among the options are monopolar and bipolar resectoscopy and the mechanical approach using the Truclear System, which includes a morcellator. With conventional resectoscopy all chips must be removed, necessitating multiple insertions of the hysteroscope. Monopolar instrumentation, in particular, carries a risk of energy discharge to healthy tissue. The monopolar resectoscope also has a longer learning curve, compared with the mechanical approach.6
In contrast, the Truclear System requires fewer insertions, has a short learning curve, and omits the need for capture of individual chips, as the mechanical morcellator suctions and captures them throughout the procedure.7 In addition, because resection is performed mechanically, there is no risk of energy discharge to healthy tissue.
The Truclear system also is associated with a significantly shorter operative time, compared with resectoscopy, which may be advantageous for residents, fellows, and other physicians learning the procedure (FIGURE 3).7 Shorter operative time also may result in lower fluid deficits. In addition, saline distension may reduce the risk of fluid absorption and hyponatremia. The tissue-capture feature allows evaluation of the entire pathologic specimen.
Besides hysteroscopic myomectomy, the Truclear System is appropriate for visual dilatation and curettage (D&C), adhesiolysis, polypectomy, and evacuation of retained products of conception.
Preoperative evaluation
A complete history is vital to document which fibroid-related symptoms are present and how they affect quality of life.
Preoperative imaging also is imperative—using either 2D or 3D saline infusion sonography or a combination of diagnostic hysteroscopy and transvaginal ultrasound—to select patients for hysteroscopy, anticipate blood loss, and ensure that the proper instrumentation is available at the time of surgery. Magnetic resonance imaging, computed tomography, and hysterosalpingography are either prohibitively expensive or of limited value in the initial preoperative assessment of uterine fibroids.
Any woman who has AUB and a risk for endometrial hyperplasia or cancer should undergo endometrial assessment as well.
Use of preoperative medications
In most cases, prophylactic administration of antibiotics is not warranted to prevent infection or endocarditis.
Although some clinicians give gonadotropin-releasing hormone (GnRH) agonists to reduce the size of large fibroids, the drug complicates dissection of the fibroid from the surrounding capsule. For this reason, and because we lack data demonstrating that GnRH agonists decrease blood loss and limit absorption of distension media, I do not administer them to patients.8–12 Moreover, this drug can cause vasomotor symptoms, cervical stenosis, and vaginal hemorrhage (related to estrogen flare).
GnRH agonists may be of value to stimulate transient amenorrhea for several months preoperatively in order to correct iron-deficiency anemia. Intravenous iron also can be administered during this interval.
The risk of bleeding in hysteroscopic myomectomy is 2% to 3%.1 When the mechanical approach is used, rather than resectoscopy, continuous flow coupled with suctioning of the chips during the procedure keeps the image clear. Post-procedure contraction of the uterus stops most bleeding. Intrauterine pressure of the pump can be increased to help tamponade any oozing.
Misoprostol. Cervical stenosis is not uncommon in menopausal women. It can also pose a challenge in nulliparous women. Attempting hysteroscopy in the setting of cervical stenosis increases the risk of cervical laceration, creation of a false passage, and uterine perforation. For this reason, I prescribe oral or vaginal misoprostol 200 to 400 µg nightly for 1 to 2 days before the procedure.
Vasopressin can reduce blood loss during hysteroscopic myomectomy when it is injected into the cervical stroma preoperatively. It also reduces absorption of distension fluid and facilitates cervical dilation.
However, vasopressin must be injected with extreme care, with aspiration to confirm the absence of blood prior to each injection, as intravascular injection can lead to bradycardia, profound hypertension, and even death.13 Always notify the anesthesiologist prior to injection when vasopressin will be administered.
I routinely use vasopressin before hysteroscopic myomectomy (0.5 mg in 20 cc of saline or 20 U in 100 cc), injecting 5 cc of the solution at 3, 6, 9, and 12 o’clock positions.
Anesthesia during hysteroscopic myomectomy typically is “monitored anesthesia care,” or MAC, which consists of local anesthesia with sedation and analgesia. The need for regional or general anesthesia is rare. Consider adding a pericervical block or intravenous ketorolac (Toradol) to provide postoperative analgesia.
Surgical technique
Strict attention to fluid management is required throughout the procedure, preferably in accordance with AAGL guidelines on the management of hysteroscopic distending media.14 With the mechanical approach, because the distension fluid is isotonic (normal saline), it does not increase the risk of hyponatremia but can cause pulmonary edema or congestive heart failure. Intravasation usually is the result of excessive operative time, treatment of deeper myometrial fibroids (Type I or II), or high intrauterine pressure. I operate using intrauterine pressure in the range of 75 to 125 mm Hg.
The steps involved in the mechanical hysteroscopy approach are:
- Insert the hysteroscope into the uterus under direct visualization. In general, the greater the number of insertions, the greater the risk of uterine perforation. Preoperative cervical ripening helps facilitate insertion (see “Misoprostol” above).
- Distend the uterus with saline and inspect the uterine cavity, noting again the size and location of the fibroids and whether they are sessile or pedunculated.
- Locate the fibroid or other pathology to be removed, and place the morcellator window against it to begin cutting. Use the tip of the morcellator to elevate the fibroid for easier cutting. Enucleation is accomplished largely by varying the intrauterine pressure, which permits uterine decompression and myometrial contraction and renders the fibroid capsule more visible. If necessary, the hysteroscope can be withdrawn to stimulate myometrial contraction, which also helps to delineate the fibroid capsule.
- Reinspect the uterus to rule out perforation and remove any additional intrauterine pathology with a targeted view.
- Once all designated fibroids have been removed, withdraw the morcellator and hysteroscope from the uterus.
- Inspect the endocervical landscape to rule out injury and other pathology.
- Careful preoperative evaluation is important, preferably using diagnostic hysteroscopy or saline infusion sonography, to choose the optimal route of myomectomy and plan the surgical approach.
- During the myomectomy, pay close attention to fluid management and adhere strictly to predetermined limits.
- Complete removal of the fibroid is essential to relieve symptoms and avert the need for additional procedures.
Postoperative care
A nonsteroidal anti-inflammatory drug or limited use of narcotics usually is sufficient to relieve any postoperative cramping or vaginal discomfort.
Advise the patient to notify you in the event of increasing pain, foul-smelling vaginal discharge, or fever.
Also counsel her that she can return to most normal activities within 24 to 48 hours. Sexual activity is permissible 1 week after surgery. Early and frequent ambulation is important.
Schedule a follow-up visit 4 to 6 weeks after the procedure.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Uterine fibroids are a common complaint in gynecology, with an incidence of approximately 30% in women aged 25 to 45 years and a cumulative incidence of 70% to 80% by age 50.1,2 They are more prevalent in women of African descent and are a leading indication for hysterectomy.
Although they can be asymptomatic, submucosal fibroids are frequently associated with:
- abnormal uterine bleeding (AUB)
- dysmenorrhea
- expulsion of an intrauterine device (IUD)
- leukorrhea
- pelvic pain
- urinary frequency
- infertility
- premature labor
- reproductive wastage
- bleeding during hormone replacement therapy.
In postmenopausal women, the risk of malignancy in a leiomyoma ranges from 0.2% to 0.5%.1 The risk is lower in premenopausal women.
In this article, I describe the technique for hysteroscopic myomectomy using a mechanical approach (Truclear Tissue Removal System, Smith & Nephew, Andover, MA), which offers hysteroscopic morcellation as well as quick resection and efficient fluid management. (Note: Unlike open intraperitoneal morcellation, hysteroscopic morcellation carries a low risk of tissue spread.)
Classification of fibroids
Preoperative classification of leiomyomas makes it possible to determine the best route for surgery. The most commonly used classification system was developed by the European Society of Gynaecological Endoscopy (ESGE) (FIGURE 1), which considers the extent of intramural extension. Each fibroid under that system is classified as:
- Type 0 – no intramural extension
- Type I – less than 50% extension
- Type II – more than 50% extension.
A second classification system recently was devised to take into account additional features of the fibroid. The STEP-W classification considers size, topography, extension, penetration, and the lateral wall (FIGURE 2). In general, the lower the score, the less complex the procedure will be, with a lower risk of fluid intravasation, shorter operative time, and a greater likelihood of complete removal of the fibroid.
A multicenter, prospective study of 449 women who underwent hysteroscopic resection of their fibroids correlated the ESGE and STEP-W systems. All 320 fibroids (100%) with a score of 4 or below on the STEP-W classification system were completely removed, compared with 112 of 145 fibroids (77.2%) with a score greater than 4. All 33 cases of incomplete hysteroscopic resection (100%) had a STEP-W score above 4.3
In the same study, 85 of 86 cases (98.9%) with Type 0 fibroids under the ESGE system had complete resection, along with 278 of 298 Type I fibroids (93.3%), and 69 of 81 Type II fibroids (85.2%).3 Complete removal is a goal because it relieves symptoms and averts the need for additional procedures.
Patient selection
Proper patient selection for hysteroscopic myomectomy is extremely important. The most common indications are AUB, pelvic pain or discomfort, recurrent pregnancy loss, and infertility. In addition, the patient should have a strong wish for uterine preservation and desire a minimally invasive transcervical approach.
AAGL guidelines on the diagnosis and management of submucous fibroids note that, in general, submucous leiomyomas as large as 4 or 5 cm in diameter can be removed hysteroscopically by experienced surgeons.4
A hysteroscopic approach is not advised for women in whom hysteroscopic surgery is contraindicated, such as women with intrauterine pregnancy, active pelvic infection, active herpes infection, or cervical or uterine cancer. Women who have medical comorbidities such as coronary heart disease, significant renal disease, or bleeding diathesis may need perioperative clearance from anesthesia or hematology prior to hysteroscopic surgery and close fluid monitoring during the procedure.
Consider the leiomyoma
Penetration into the myometrium. Women who have a fibroid that penetrates more than 50% into the myometrium may benefit from hysteroscopic myomectomy, provided the surgeon is highly experienced. A skilled hysteroscopist can ensure complete enucleation of a penetrating fibroid in these cases.
If you are still in the learning process for hysteroscopy, however, start with easier cases—ie, polyps and Type 0 and Type I fibroids. Type II fibroids require longer operative time, are associated with increased fluid absorption and intravasation, carry an increased risk of perioperative complications, and may not always be completely resected.
Size of the fibroid also is relevant. As size increases, so does the volume of tissue needing to be removed, adding to overall operative time.
Presence of other fibroids. When a woman has an intracavitary fibroid as well as myomas in other locations, the surgeon should consider whether hysteroscopic removal of the intracavitary lesion alone can provide significant relief of all fibroid-related symptoms. In such cases, laparoscopic, robotic, or abdominal myomectomy may be preferable, especially if the volume of the additional myomas is considerable.
To determine the optimal surgical route, the physician must consider the symptoms present—is AUB the only symptom, or are other fibroid-related conditions present as well, such as bulk, pelvic pain, and other quality-of-life issues? If multiple symptoms exist, then other approaches may be better.
How fibroids affect fertility
Fibroids are present in 5% to 10% of women with infertility. In this population, fibroids are the only abnormal finding in 1.0% to 2.4% of cases.4
In a meta-analysis of 23 studies evaluating women with fibroids and infertility, Pritts and colleagues found nine studies involving submucosal fibroids.5 These studies included one randomized controlled trial, two prospective studies, and six retrospective analyses. They found that women who had fibroids with a submucosal component had lower pregnancy and implantation rates, compared with their infertile, myoma-free counterparts. Pritts and colleagues concluded that myomectomy is likely to improve fertility in these cases (TABLE).5
Instrumentation
Among the options are monopolar and bipolar resectoscopy and the mechanical approach using the Truclear System, which includes a morcellator. With conventional resectoscopy all chips must be removed, necessitating multiple insertions of the hysteroscope. Monopolar instrumentation, in particular, carries a risk of energy discharge to healthy tissue. The monopolar resectoscope also has a longer learning curve, compared with the mechanical approach.6
In contrast, the Truclear System requires fewer insertions, has a short learning curve, and omits the need for capture of individual chips, as the mechanical morcellator suctions and captures them throughout the procedure.7 In addition, because resection is performed mechanically, there is no risk of energy discharge to healthy tissue.
The Truclear system also is associated with a significantly shorter operative time, compared with resectoscopy, which may be advantageous for residents, fellows, and other physicians learning the procedure (FIGURE 3).7 Shorter operative time also may result in lower fluid deficits. In addition, saline distension may reduce the risk of fluid absorption and hyponatremia. The tissue-capture feature allows evaluation of the entire pathologic specimen.
Besides hysteroscopic myomectomy, the Truclear System is appropriate for visual dilatation and curettage (D&C), adhesiolysis, polypectomy, and evacuation of retained products of conception.
Preoperative evaluation
A complete history is vital to document which fibroid-related symptoms are present and how they affect quality of life.
Preoperative imaging also is imperative—using either 2D or 3D saline infusion sonography or a combination of diagnostic hysteroscopy and transvaginal ultrasound—to select patients for hysteroscopy, anticipate blood loss, and ensure that the proper instrumentation is available at the time of surgery. Magnetic resonance imaging, computed tomography, and hysterosalpingography are either prohibitively expensive or of limited value in the initial preoperative assessment of uterine fibroids.
Any woman who has AUB and a risk for endometrial hyperplasia or cancer should undergo endometrial assessment as well.
Use of preoperative medications
In most cases, prophylactic administration of antibiotics is not warranted to prevent infection or endocarditis.
Although some clinicians give gonadotropin-releasing hormone (GnRH) agonists to reduce the size of large fibroids, the drug complicates dissection of the fibroid from the surrounding capsule. For this reason, and because we lack data demonstrating that GnRH agonists decrease blood loss and limit absorption of distension media, I do not administer them to patients.8–12 Moreover, this drug can cause vasomotor symptoms, cervical stenosis, and vaginal hemorrhage (related to estrogen flare).
GnRH agonists may be of value to stimulate transient amenorrhea for several months preoperatively in order to correct iron-deficiency anemia. Intravenous iron also can be administered during this interval.
The risk of bleeding in hysteroscopic myomectomy is 2% to 3%.1 When the mechanical approach is used, rather than resectoscopy, continuous flow coupled with suctioning of the chips during the procedure keeps the image clear. Post-procedure contraction of the uterus stops most bleeding. Intrauterine pressure of the pump can be increased to help tamponade any oozing.
Misoprostol. Cervical stenosis is not uncommon in menopausal women. It can also pose a challenge in nulliparous women. Attempting hysteroscopy in the setting of cervical stenosis increases the risk of cervical laceration, creation of a false passage, and uterine perforation. For this reason, I prescribe oral or vaginal misoprostol 200 to 400 µg nightly for 1 to 2 days before the procedure.
Vasopressin can reduce blood loss during hysteroscopic myomectomy when it is injected into the cervical stroma preoperatively. It also reduces absorption of distension fluid and facilitates cervical dilation.
However, vasopressin must be injected with extreme care, with aspiration to confirm the absence of blood prior to each injection, as intravascular injection can lead to bradycardia, profound hypertension, and even death.13 Always notify the anesthesiologist prior to injection when vasopressin will be administered.
I routinely use vasopressin before hysteroscopic myomectomy (0.5 mg in 20 cc of saline or 20 U in 100 cc), injecting 5 cc of the solution at 3, 6, 9, and 12 o’clock positions.
Anesthesia during hysteroscopic myomectomy typically is “monitored anesthesia care,” or MAC, which consists of local anesthesia with sedation and analgesia. The need for regional or general anesthesia is rare. Consider adding a pericervical block or intravenous ketorolac (Toradol) to provide postoperative analgesia.
Surgical technique
Strict attention to fluid management is required throughout the procedure, preferably in accordance with AAGL guidelines on the management of hysteroscopic distending media.14 With the mechanical approach, because the distension fluid is isotonic (normal saline), it does not increase the risk of hyponatremia but can cause pulmonary edema or congestive heart failure. Intravasation usually is the result of excessive operative time, treatment of deeper myometrial fibroids (Type I or II), or high intrauterine pressure. I operate using intrauterine pressure in the range of 75 to 125 mm Hg.
The steps involved in the mechanical hysteroscopy approach are:
- Insert the hysteroscope into the uterus under direct visualization. In general, the greater the number of insertions, the greater the risk of uterine perforation. Preoperative cervical ripening helps facilitate insertion (see “Misoprostol” above).
- Distend the uterus with saline and inspect the uterine cavity, noting again the size and location of the fibroids and whether they are sessile or pedunculated.
- Locate the fibroid or other pathology to be removed, and place the morcellator window against it to begin cutting. Use the tip of the morcellator to elevate the fibroid for easier cutting. Enucleation is accomplished largely by varying the intrauterine pressure, which permits uterine decompression and myometrial contraction and renders the fibroid capsule more visible. If necessary, the hysteroscope can be withdrawn to stimulate myometrial contraction, which also helps to delineate the fibroid capsule.
- Reinspect the uterus to rule out perforation and remove any additional intrauterine pathology with a targeted view.
- Once all designated fibroids have been removed, withdraw the morcellator and hysteroscope from the uterus.
- Inspect the endocervical landscape to rule out injury and other pathology.
- Careful preoperative evaluation is important, preferably using diagnostic hysteroscopy or saline infusion sonography, to choose the optimal route of myomectomy and plan the surgical approach.
- During the myomectomy, pay close attention to fluid management and adhere strictly to predetermined limits.
- Complete removal of the fibroid is essential to relieve symptoms and avert the need for additional procedures.
Postoperative care
A nonsteroidal anti-inflammatory drug or limited use of narcotics usually is sufficient to relieve any postoperative cramping or vaginal discomfort.
Advise the patient to notify you in the event of increasing pain, foul-smelling vaginal discharge, or fever.
Also counsel her that she can return to most normal activities within 24 to 48 hours. Sexual activity is permissible 1 week after surgery. Early and frequent ambulation is important.
Schedule a follow-up visit 4 to 6 weeks after the procedure.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Perez-Medina T, Font EC, eds. Diagnostic and Operative Hysteroscopy. Tunbridge Wells, Kent, UK: Anshan Publishing; 2007:13.
2. Management of uterine fibroids: an update of the evidence. Agency for Healthcare Research and Quality. http://archive.ahrq.gov/clinic/tp/uteruptp.htm. Published July 2007. Accessed January 14, 2015.
3. Lasmar RB, Zinmei Z, Indman PD, Celeste RK, Di Spiezo Sardo A. Feasibility of a new system of classification of submucous myomas: a multicenter study. Fertil Steril. 2011;95(6):2073–2077.
4. AAGL Practice Report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol. 2012;19(2):152–171.
5. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223.
6. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466–471.
7. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62–66.
8. Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil Steril. 1997;68(5):881–886.
9. Taskin O, Sadik S, Onoglu A, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7(3):351.
10. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96(4):517–520.
11. Perino A, Chianchiano N, Petronio M, Cittadini E. Role of leuprolide acetate depot in hysteroscopic surgery: a controlled study. Fertil Steril. 1993;59(3):507–510.
12. Mencaglia L, Tantini C. GnRH agonist analogs and hysteroscopic resection of myomas. Int J Gynaecol Obstet. 1993;43(3):285–288.
13. Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 Pt 2):484–486.
14. Munro MD, Storz K, Abbott JA, et al; AAGL. AAGL Practice Report: practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013;20(2):137–148.
1. Perez-Medina T, Font EC, eds. Diagnostic and Operative Hysteroscopy. Tunbridge Wells, Kent, UK: Anshan Publishing; 2007:13.
2. Management of uterine fibroids: an update of the evidence. Agency for Healthcare Research and Quality. http://archive.ahrq.gov/clinic/tp/uteruptp.htm. Published July 2007. Accessed January 14, 2015.
3. Lasmar RB, Zinmei Z, Indman PD, Celeste RK, Di Spiezo Sardo A. Feasibility of a new system of classification of submucous myomas: a multicenter study. Fertil Steril. 2011;95(6):2073–2077.
4. AAGL Practice Report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol. 2012;19(2):152–171.
5. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223.
6. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466–471.
7. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62–66.
8. Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil Steril. 1997;68(5):881–886.
9. Taskin O, Sadik S, Onoglu A, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7(3):351.
10. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96(4):517–520.
11. Perino A, Chianchiano N, Petronio M, Cittadini E. Role of leuprolide acetate depot in hysteroscopic surgery: a controlled study. Fertil Steril. 1993;59(3):507–510.
12. Mencaglia L, Tantini C. GnRH agonist analogs and hysteroscopic resection of myomas. Int J Gynaecol Obstet. 1993;43(3):285–288.
13. Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 Pt 2):484–486.
14. Munro MD, Storz K, Abbott JA, et al; AAGL. AAGL Practice Report: practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013;20(2):137–148.
Simple versus radical hysterectomy
Hysterectomy is one of the fundamental surgical procedures in gynecology. Understanding the nuances of both the anatomy and the surgical dissection techniques of this procedure is especially important when approaching complex cases in either benign or oncologic settings.
This month’s surgical video contribution is by my gynecologic oncology colleagues, who highlight the key differences between the simple and radical hysterectomy. They emphasize key surgical principles for the benefit of both benign and oncologic surgeons.
The objectives of this video are to:
- compare the surgical techniques of a simple versus radical hysterectomy
- review the relevant anatomy as it relates to the varying types of hysterectomy
- provide an educational review of the different types of hysterectomy.
This video does an excellent job of achieving its objectives. I hope you share it with your colleagues and residents.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Hysterectomy is one of the fundamental surgical procedures in gynecology. Understanding the nuances of both the anatomy and the surgical dissection techniques of this procedure is especially important when approaching complex cases in either benign or oncologic settings.
This month’s surgical video contribution is by my gynecologic oncology colleagues, who highlight the key differences between the simple and radical hysterectomy. They emphasize key surgical principles for the benefit of both benign and oncologic surgeons.
The objectives of this video are to:
- compare the surgical techniques of a simple versus radical hysterectomy
- review the relevant anatomy as it relates to the varying types of hysterectomy
- provide an educational review of the different types of hysterectomy.
This video does an excellent job of achieving its objectives. I hope you share it with your colleagues and residents.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Hysterectomy is one of the fundamental surgical procedures in gynecology. Understanding the nuances of both the anatomy and the surgical dissection techniques of this procedure is especially important when approaching complex cases in either benign or oncologic settings.
This month’s surgical video contribution is by my gynecologic oncology colleagues, who highlight the key differences between the simple and radical hysterectomy. They emphasize key surgical principles for the benefit of both benign and oncologic surgeons.
The objectives of this video are to:
- compare the surgical techniques of a simple versus radical hysterectomy
- review the relevant anatomy as it relates to the varying types of hysterectomy
- provide an educational review of the different types of hysterectomy.
This video does an excellent job of achieving its objectives. I hope you share it with your colleagues and residents.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Product Update
LITHOTOMY STIRRUPS FOR PATIENT COMFORT
Frontier Medical Innovations says its new GStirrup® is designed to provide patients stable and comfortable foot positioning for extended periods of time during office-based surgical procedures. The GStirrup is a pair of cushioned lithotomy boots that attach to examination-table foot rests. Straps secure the patient’s feet and legs; weights in the base make it difficult for the patient to lift the boot off the footrest. GStirrups fit most footrests and are helpful for patients who have hip or knee replacement, low back pain, arthritis, or neurologic conditions such as multiple sclerosis or Parkinson’s disease.
FOR MORE INFORMATION, VISIT www.gstirrup.com
PREGNANCY, BIRTH, AND BREASTFEEDING APP
Totally Pregnant, an app for pregnant women and health-care providers, is now partnering with Lamaze International to provide access to online parenting classes for pregnancy, childbirth, and early parenting. By using the Totally Pregnant app, women can personalize their pregnancy experience and clinicians can connect with their patients. Available for iPhone, iPad, Android, and desktop.
FOR MORE INFORMATION, VISIT www.iamtotally.com
PREDICTING IVF SUCCESS
Univfy®offers fertility predictive analytics to help prospective parents understand the probability for the success of in vitro fertilization (IVF) and estimated costs. The PreIVF™ calculator helps to decide whether or when to start IVF; the PredictIVF™ helps determine if another IVF cycle is the right option. The IVF Cost Calculator offers a cost comparison tailored to the patient’s IVF success rate. Your Fertility™ is an interactive multimedia blog offering educational material.
FOR MORE INFORMATION, VISIT www.univfy.com
SECURE WAY TO CARRY MEDS
FusionWrap is a waist/hip wrap with two 7-inch pockets to carry small personal belongings. Besides providing secure space for identification and money, it also allows those with asthma, diabetes, allergies, or other chronic diseases to carry medications at all times. Made of stretch fabric that is moisture wicking and antimicrobial, FusionWrap comes in various colors and sizes for women, men, and children.
FOR MORE INFORMATION, VISIT www.fusion-wrap.com
BREAST IMAGING TOOLS
Volpara Solutions offers multiple volumetric breast-imaging software tools designed to improve clinical decision making and the early detection of breast cancer. Volpara®Density™ is a breast-density assessment tool available for clinical use with 2D mammography and digital breast tomosynthesis (3D mammography) platforms from multiple manufacturers.
FOR MORE INFORMATION, VISIT www.volparadensity.com
SMOKE EVACUATION PENCIL
The PlumePen® Elite is an electrosurgical smoke pencil with a compact, ergonomic design that is smaller but offers more flow than competitive smoke pencils, claims Buffalo Filter. The adjustable capture port allows for optimum visibility regardless of blade length. The one-piece molded design prevents buttons from sticking and improves grip. The PlumePen Elite connects to most surgical plume evacuators and generators.
FOR MORE INFORMATION, VISIT www.buffalofilter.com
SURGICAL CO2 LASER
Lumenis designed the AcuPulse Smart CO2 Laser with SurgiTouch™ Automation System for tissue ablation during gynecologic surgery to increase speed, precision, and convenience over other electrosurgical devices. Robotic, computer-controlled laser-beam movement provides more precision than hand-held lasers and offers reproducible outcomes. Brief laser-tissue interaction reduces thermal damage.
FOR MORE INFORMATION, VISIT www.lumenis.com
REUSABLE FORNIX FOR LAP HYSTERECTOMY
The Banyan Colpo-Port Vaginal Fornix Delineator is a reusable uterine elevator/vaginal fornix delineator for laparoscopic hysterectomy. Inserted vaginally, the distal tip has a canted, beveled cup that fits securely in the vaginal fornix. The Calibrated Uterine Elevator (CUE) passes through the inner diameter of the delineator body, through the cervix, and into the uterine cavity. The CUE can be locked at preset depths to prevent uterine perforation. The device is easy to clean using standard sterilization procedures.
FOR MORE INFORMATION, VISIT www.banyanmedllc.com
OVARIAN MALIGNANCY ALGORITHM
The Risk of Ovarian Malignancy Algorithm (ROMA™) from Fujirebio is a quantitative serum test intended to assess the risk of finding malignancy at surgery in a premenopausal or postmenopausal woman with an ovarian mass. ROMA, a risk stratification tool, combines the results of human epididymis protein 4 (HE4), cancer antigen 125 (CA125), and menopausal status into a numerical score.
FOR MORE INFORMATION, VISIT www.he4test.com
PORT-SITE CLOSURE DEVICE
neoClose® AnchorGuide facilitates rapid trocar exchange and precise AutoAnchor placement to help prevent port-site hernia. The AnchorGuide design facilitates the delivery of absorbable AutoAnchors through soft tissue during surgery; allows for the VectorX method of port approximation for reduced tension at wound sites; and helps desufflate to remove CO2 at the end of surgery. AnchorGuide comes in 8–15 mm and 5–12 mm sizes compatible with 8 mm robotic ports.
FOR MORE INFORMATION, VISIT www.neosurgical.com
LITHOTOMY STIRRUPS FOR PATIENT COMFORT
Frontier Medical Innovations says its new GStirrup® is designed to provide patients stable and comfortable foot positioning for extended periods of time during office-based surgical procedures. The GStirrup is a pair of cushioned lithotomy boots that attach to examination-table foot rests. Straps secure the patient’s feet and legs; weights in the base make it difficult for the patient to lift the boot off the footrest. GStirrups fit most footrests and are helpful for patients who have hip or knee replacement, low back pain, arthritis, or neurologic conditions such as multiple sclerosis or Parkinson’s disease.
FOR MORE INFORMATION, VISIT www.gstirrup.com
PREGNANCY, BIRTH, AND BREASTFEEDING APP
Totally Pregnant, an app for pregnant women and health-care providers, is now partnering with Lamaze International to provide access to online parenting classes for pregnancy, childbirth, and early parenting. By using the Totally Pregnant app, women can personalize their pregnancy experience and clinicians can connect with their patients. Available for iPhone, iPad, Android, and desktop.
FOR MORE INFORMATION, VISIT www.iamtotally.com
PREDICTING IVF SUCCESS
Univfy®offers fertility predictive analytics to help prospective parents understand the probability for the success of in vitro fertilization (IVF) and estimated costs. The PreIVF™ calculator helps to decide whether or when to start IVF; the PredictIVF™ helps determine if another IVF cycle is the right option. The IVF Cost Calculator offers a cost comparison tailored to the patient’s IVF success rate. Your Fertility™ is an interactive multimedia blog offering educational material.
FOR MORE INFORMATION, VISIT www.univfy.com
SECURE WAY TO CARRY MEDS
FusionWrap is a waist/hip wrap with two 7-inch pockets to carry small personal belongings. Besides providing secure space for identification and money, it also allows those with asthma, diabetes, allergies, or other chronic diseases to carry medications at all times. Made of stretch fabric that is moisture wicking and antimicrobial, FusionWrap comes in various colors and sizes for women, men, and children.
FOR MORE INFORMATION, VISIT www.fusion-wrap.com
BREAST IMAGING TOOLS
Volpara Solutions offers multiple volumetric breast-imaging software tools designed to improve clinical decision making and the early detection of breast cancer. Volpara®Density™ is a breast-density assessment tool available for clinical use with 2D mammography and digital breast tomosynthesis (3D mammography) platforms from multiple manufacturers.
FOR MORE INFORMATION, VISIT www.volparadensity.com
SMOKE EVACUATION PENCIL
The PlumePen® Elite is an electrosurgical smoke pencil with a compact, ergonomic design that is smaller but offers more flow than competitive smoke pencils, claims Buffalo Filter. The adjustable capture port allows for optimum visibility regardless of blade length. The one-piece molded design prevents buttons from sticking and improves grip. The PlumePen Elite connects to most surgical plume evacuators and generators.
FOR MORE INFORMATION, VISIT www.buffalofilter.com
SURGICAL CO2 LASER
Lumenis designed the AcuPulse Smart CO2 Laser with SurgiTouch™ Automation System for tissue ablation during gynecologic surgery to increase speed, precision, and convenience over other electrosurgical devices. Robotic, computer-controlled laser-beam movement provides more precision than hand-held lasers and offers reproducible outcomes. Brief laser-tissue interaction reduces thermal damage.
FOR MORE INFORMATION, VISIT www.lumenis.com
REUSABLE FORNIX FOR LAP HYSTERECTOMY
The Banyan Colpo-Port Vaginal Fornix Delineator is a reusable uterine elevator/vaginal fornix delineator for laparoscopic hysterectomy. Inserted vaginally, the distal tip has a canted, beveled cup that fits securely in the vaginal fornix. The Calibrated Uterine Elevator (CUE) passes through the inner diameter of the delineator body, through the cervix, and into the uterine cavity. The CUE can be locked at preset depths to prevent uterine perforation. The device is easy to clean using standard sterilization procedures.
FOR MORE INFORMATION, VISIT www.banyanmedllc.com
OVARIAN MALIGNANCY ALGORITHM
The Risk of Ovarian Malignancy Algorithm (ROMA™) from Fujirebio is a quantitative serum test intended to assess the risk of finding malignancy at surgery in a premenopausal or postmenopausal woman with an ovarian mass. ROMA, a risk stratification tool, combines the results of human epididymis protein 4 (HE4), cancer antigen 125 (CA125), and menopausal status into a numerical score.
FOR MORE INFORMATION, VISIT www.he4test.com
PORT-SITE CLOSURE DEVICE
neoClose® AnchorGuide facilitates rapid trocar exchange and precise AutoAnchor placement to help prevent port-site hernia. The AnchorGuide design facilitates the delivery of absorbable AutoAnchors through soft tissue during surgery; allows for the VectorX method of port approximation for reduced tension at wound sites; and helps desufflate to remove CO2 at the end of surgery. AnchorGuide comes in 8–15 mm and 5–12 mm sizes compatible with 8 mm robotic ports.
FOR MORE INFORMATION, VISIT www.neosurgical.com
LITHOTOMY STIRRUPS FOR PATIENT COMFORT
Frontier Medical Innovations says its new GStirrup® is designed to provide patients stable and comfortable foot positioning for extended periods of time during office-based surgical procedures. The GStirrup is a pair of cushioned lithotomy boots that attach to examination-table foot rests. Straps secure the patient’s feet and legs; weights in the base make it difficult for the patient to lift the boot off the footrest. GStirrups fit most footrests and are helpful for patients who have hip or knee replacement, low back pain, arthritis, or neurologic conditions such as multiple sclerosis or Parkinson’s disease.
FOR MORE INFORMATION, VISIT www.gstirrup.com
PREGNANCY, BIRTH, AND BREASTFEEDING APP
Totally Pregnant, an app for pregnant women and health-care providers, is now partnering with Lamaze International to provide access to online parenting classes for pregnancy, childbirth, and early parenting. By using the Totally Pregnant app, women can personalize their pregnancy experience and clinicians can connect with their patients. Available for iPhone, iPad, Android, and desktop.
FOR MORE INFORMATION, VISIT www.iamtotally.com
PREDICTING IVF SUCCESS
Univfy®offers fertility predictive analytics to help prospective parents understand the probability for the success of in vitro fertilization (IVF) and estimated costs. The PreIVF™ calculator helps to decide whether or when to start IVF; the PredictIVF™ helps determine if another IVF cycle is the right option. The IVF Cost Calculator offers a cost comparison tailored to the patient’s IVF success rate. Your Fertility™ is an interactive multimedia blog offering educational material.
FOR MORE INFORMATION, VISIT www.univfy.com
SECURE WAY TO CARRY MEDS
FusionWrap is a waist/hip wrap with two 7-inch pockets to carry small personal belongings. Besides providing secure space for identification and money, it also allows those with asthma, diabetes, allergies, or other chronic diseases to carry medications at all times. Made of stretch fabric that is moisture wicking and antimicrobial, FusionWrap comes in various colors and sizes for women, men, and children.
FOR MORE INFORMATION, VISIT www.fusion-wrap.com
BREAST IMAGING TOOLS
Volpara Solutions offers multiple volumetric breast-imaging software tools designed to improve clinical decision making and the early detection of breast cancer. Volpara®Density™ is a breast-density assessment tool available for clinical use with 2D mammography and digital breast tomosynthesis (3D mammography) platforms from multiple manufacturers.
FOR MORE INFORMATION, VISIT www.volparadensity.com
SMOKE EVACUATION PENCIL
The PlumePen® Elite is an electrosurgical smoke pencil with a compact, ergonomic design that is smaller but offers more flow than competitive smoke pencils, claims Buffalo Filter. The adjustable capture port allows for optimum visibility regardless of blade length. The one-piece molded design prevents buttons from sticking and improves grip. The PlumePen Elite connects to most surgical plume evacuators and generators.
FOR MORE INFORMATION, VISIT www.buffalofilter.com
SURGICAL CO2 LASER
Lumenis designed the AcuPulse Smart CO2 Laser with SurgiTouch™ Automation System for tissue ablation during gynecologic surgery to increase speed, precision, and convenience over other electrosurgical devices. Robotic, computer-controlled laser-beam movement provides more precision than hand-held lasers and offers reproducible outcomes. Brief laser-tissue interaction reduces thermal damage.
FOR MORE INFORMATION, VISIT www.lumenis.com
REUSABLE FORNIX FOR LAP HYSTERECTOMY
The Banyan Colpo-Port Vaginal Fornix Delineator is a reusable uterine elevator/vaginal fornix delineator for laparoscopic hysterectomy. Inserted vaginally, the distal tip has a canted, beveled cup that fits securely in the vaginal fornix. The Calibrated Uterine Elevator (CUE) passes through the inner diameter of the delineator body, through the cervix, and into the uterine cavity. The CUE can be locked at preset depths to prevent uterine perforation. The device is easy to clean using standard sterilization procedures.
FOR MORE INFORMATION, VISIT www.banyanmedllc.com
OVARIAN MALIGNANCY ALGORITHM
The Risk of Ovarian Malignancy Algorithm (ROMA™) from Fujirebio is a quantitative serum test intended to assess the risk of finding malignancy at surgery in a premenopausal or postmenopausal woman with an ovarian mass. ROMA, a risk stratification tool, combines the results of human epididymis protein 4 (HE4), cancer antigen 125 (CA125), and menopausal status into a numerical score.
FOR MORE INFORMATION, VISIT www.he4test.com
PORT-SITE CLOSURE DEVICE
neoClose® AnchorGuide facilitates rapid trocar exchange and precise AutoAnchor placement to help prevent port-site hernia. The AnchorGuide design facilitates the delivery of absorbable AutoAnchors through soft tissue during surgery; allows for the VectorX method of port approximation for reduced tension at wound sites; and helps desufflate to remove CO2 at the end of surgery. AnchorGuide comes in 8–15 mm and 5–12 mm sizes compatible with 8 mm robotic ports.
FOR MORE INFORMATION, VISIT www.neosurgical.com
RBC transfusions during CABG increase risk of pneumonia

Credit: Elise Amendola
SAN DIEGO—Patients who receive red blood cell (RBC) transfusions during coronary artery bypass grafting (CABG) surgery are at an increased risk of
developing pneumonia, according to research presented at the 51st Annual Meeting of The Society of Thoracic Surgeons.
And the risk appears to increase with the volume of RBCs transfused. Patients who received 6 or more units had a 14 times higher risk of developing pneumonia than their untransfused peers.
“Pneumonia is a known risk following CABG surgery, and developing it has been shown to significantly increase a patient’s risk of morbidity and mortality,” said study investigator Donald S. Likosky, PhD, of the University of Michigan Health System in Ann Arbor.
“Previous research has shown that 1 in every 20 CABG patients develop a major infection, with pneumonia being the most common type of infection.”
For this study, Dr Liksoky and his colleagues examined data on 16,182 patients who underwent CABG between 2011 and 2013 at any of the 33 hospitals participating in the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
They used propensity scoring to match the 4585 patients (32.3%) who received RBCs to the 9612 who did not (total=14,197). The team matched patients based on age, sex, body mass index, history of smoking, congestive heart failure, chronic obstructive pulmonary disease, diabetes, prior cardiac surgery, vascular disease, ejection fraction, preoperative hematocrit, and preoperative pneumonia.
The researchers then calculated adjusted odds ratios (ORs) reflecting the association between the number of RBC units transfused (0 to 6+) and postoperative pneumonia.
In all, 450 patients (3.2%) developed pneumonia. And the analysis revealed a significant association between any RBC transfusion and pneumonia (OR=4.0, P<0.001), as well as associations between the number of units transfused and the odds of developing pneumonia.
The OR was 1.6 (P=0.02) for patients who received 1 RBC unit, 2.1 for those who received 2 units (P<0.001), 4.9 for those who received 3 units (P<0.001), 5.5 for those who received 4 units (P<0.001), 8.9 for those who received 5 units (P<0.001), and 14.4 for patients who received 6 or more units (P<0.001).
“The ability to store and transfuse blood is one of medicine’s greatest accomplishments, but we are continuing to see that receiving a blood transfusion may alter a patient’s ability to fight infection,” said James R. Edgerton, MD, from The Heart Hospital Baylor Plano in Texas, who was not affiliated with this study.
“In their study, Dr Likosky and colleagues have identified an increased risk of pneumonia after transfusion, which is an important breakthrough because it allows physicians to remain vigilant for the onset of pneumonia and initiate therapy early in hopes of shortening its course and severity. It also enables physicians to initiate preventive therapies in patients who have been transfused, which will contribute to better care of our patients.”
“Patients should receive red blood cell transfusions based on clinical need,” Dr Likosky added. “Surgical teams may have opportunities to reduce the need for transfusions among patients, thereby reducing the risk of secondary complications.” ![]()

Credit: Elise Amendola
SAN DIEGO—Patients who receive red blood cell (RBC) transfusions during coronary artery bypass grafting (CABG) surgery are at an increased risk of
developing pneumonia, according to research presented at the 51st Annual Meeting of The Society of Thoracic Surgeons.
And the risk appears to increase with the volume of RBCs transfused. Patients who received 6 or more units had a 14 times higher risk of developing pneumonia than their untransfused peers.
“Pneumonia is a known risk following CABG surgery, and developing it has been shown to significantly increase a patient’s risk of morbidity and mortality,” said study investigator Donald S. Likosky, PhD, of the University of Michigan Health System in Ann Arbor.
“Previous research has shown that 1 in every 20 CABG patients develop a major infection, with pneumonia being the most common type of infection.”
For this study, Dr Liksoky and his colleagues examined data on 16,182 patients who underwent CABG between 2011 and 2013 at any of the 33 hospitals participating in the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
They used propensity scoring to match the 4585 patients (32.3%) who received RBCs to the 9612 who did not (total=14,197). The team matched patients based on age, sex, body mass index, history of smoking, congestive heart failure, chronic obstructive pulmonary disease, diabetes, prior cardiac surgery, vascular disease, ejection fraction, preoperative hematocrit, and preoperative pneumonia.
The researchers then calculated adjusted odds ratios (ORs) reflecting the association between the number of RBC units transfused (0 to 6+) and postoperative pneumonia.
In all, 450 patients (3.2%) developed pneumonia. And the analysis revealed a significant association between any RBC transfusion and pneumonia (OR=4.0, P<0.001), as well as associations between the number of units transfused and the odds of developing pneumonia.
The OR was 1.6 (P=0.02) for patients who received 1 RBC unit, 2.1 for those who received 2 units (P<0.001), 4.9 for those who received 3 units (P<0.001), 5.5 for those who received 4 units (P<0.001), 8.9 for those who received 5 units (P<0.001), and 14.4 for patients who received 6 or more units (P<0.001).
“The ability to store and transfuse blood is one of medicine’s greatest accomplishments, but we are continuing to see that receiving a blood transfusion may alter a patient’s ability to fight infection,” said James R. Edgerton, MD, from The Heart Hospital Baylor Plano in Texas, who was not affiliated with this study.
“In their study, Dr Likosky and colleagues have identified an increased risk of pneumonia after transfusion, which is an important breakthrough because it allows physicians to remain vigilant for the onset of pneumonia and initiate therapy early in hopes of shortening its course and severity. It also enables physicians to initiate preventive therapies in patients who have been transfused, which will contribute to better care of our patients.”
“Patients should receive red blood cell transfusions based on clinical need,” Dr Likosky added. “Surgical teams may have opportunities to reduce the need for transfusions among patients, thereby reducing the risk of secondary complications.” ![]()

Credit: Elise Amendola
SAN DIEGO—Patients who receive red blood cell (RBC) transfusions during coronary artery bypass grafting (CABG) surgery are at an increased risk of
developing pneumonia, according to research presented at the 51st Annual Meeting of The Society of Thoracic Surgeons.
And the risk appears to increase with the volume of RBCs transfused. Patients who received 6 or more units had a 14 times higher risk of developing pneumonia than their untransfused peers.
“Pneumonia is a known risk following CABG surgery, and developing it has been shown to significantly increase a patient’s risk of morbidity and mortality,” said study investigator Donald S. Likosky, PhD, of the University of Michigan Health System in Ann Arbor.
“Previous research has shown that 1 in every 20 CABG patients develop a major infection, with pneumonia being the most common type of infection.”
For this study, Dr Liksoky and his colleagues examined data on 16,182 patients who underwent CABG between 2011 and 2013 at any of the 33 hospitals participating in the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
They used propensity scoring to match the 4585 patients (32.3%) who received RBCs to the 9612 who did not (total=14,197). The team matched patients based on age, sex, body mass index, history of smoking, congestive heart failure, chronic obstructive pulmonary disease, diabetes, prior cardiac surgery, vascular disease, ejection fraction, preoperative hematocrit, and preoperative pneumonia.
The researchers then calculated adjusted odds ratios (ORs) reflecting the association between the number of RBC units transfused (0 to 6+) and postoperative pneumonia.
In all, 450 patients (3.2%) developed pneumonia. And the analysis revealed a significant association between any RBC transfusion and pneumonia (OR=4.0, P<0.001), as well as associations between the number of units transfused and the odds of developing pneumonia.
The OR was 1.6 (P=0.02) for patients who received 1 RBC unit, 2.1 for those who received 2 units (P<0.001), 4.9 for those who received 3 units (P<0.001), 5.5 for those who received 4 units (P<0.001), 8.9 for those who received 5 units (P<0.001), and 14.4 for patients who received 6 or more units (P<0.001).
“The ability to store and transfuse blood is one of medicine’s greatest accomplishments, but we are continuing to see that receiving a blood transfusion may alter a patient’s ability to fight infection,” said James R. Edgerton, MD, from The Heart Hospital Baylor Plano in Texas, who was not affiliated with this study.
“In their study, Dr Likosky and colleagues have identified an increased risk of pneumonia after transfusion, which is an important breakthrough because it allows physicians to remain vigilant for the onset of pneumonia and initiate therapy early in hopes of shortening its course and severity. It also enables physicians to initiate preventive therapies in patients who have been transfused, which will contribute to better care of our patients.”
“Patients should receive red blood cell transfusions based on clinical need,” Dr Likosky added. “Surgical teams may have opportunities to reduce the need for transfusions among patients, thereby reducing the risk of secondary complications.” ![]()