User login
Expert tips on retropubic vs. transobturator sling approaches
Midurethral slings – both retropubic and transobturator – have been extensively studied and have evolved to become standard therapies for the treatment of stress urinary incontinence. The two approaches utilize different routes for sling delivery, but in many other respects, they are similar. Improvements in technique are continually being developed. In this column, Dr. Sokol and Dr. Rardin share key parts of their technique and give their pearls of advice for midurethral sling surgery.
Dr. Sokol’s retropubic approach
I use newer retropubic midurethral slings with smaller trocars that have evolved from first-generation tension-free vaginal tape (TVT) slings. The slings I prefer are placed in a bottom-up fashion, with curved needles passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
I find it helpful to place patients in a high lithotomy position with the legs supported in candy cane stirrups rather than Allen-type stirrups; sling placement is a short procedure with minimal to no risk of neuropathy.
To precisely identify the midurethral point, I use a 16-FR Foley catheter. When the catheter balloon is filled with 10 mm of fluid and gently pulled back, the urethrovesical junction can be identified. Then, by looking at the urethral meatus relative to the bladder neck, I can mark the midpoint between the two.
The suprapubic exit points are marked at two finger-widths lateral to the midline, just above the pubic symphysis. For precise identification of these points, the Foley catheter may be pulled up exactly midline (with the collection bag detached), and the two finger-widths measured on either side. I also aim for the ipsilateral shoulder, imagining a straight line from the urethral meatus to the ipsilateral shoulder on each side. Together, these measurements and visual cues serve as a good safety check.
With two Allis clamps, the vaginal wall on either side of the midline is grasped transversely at the level of the midurethral mark. The clamps will sit a couple of centimeters apart so that the midurethral point can be visualized. This helps to stabilize and elevate the midurethra.
To safely and efficiently develop a paraurethral passage, I perform a hydrodissection and hemostatic injection at the level of the midurethra using a control top 10-cc syringe with a 22-gauge needle. I find that dilute vasopressin saline solution affords better hemostasis than does a dilute lidocaine epinephrine solution, though I use dilute lidocaine if the sling is being done under local anesthesia.
The needle is inserted into a full thickness of skin, to a point shy of the urethra, and 10 cc is rapidly injected. The vaginal epithelium will appear blanched and will balloon out, like a white marble. The process basically lifts the vaginal skin away from the urethra itself, not only creating hemostasis but also providing a zone of safety to help avoid a urethral injury.
With a second syringe identical to the first, I inject 5 cc on each side of the midurethral point, aiming precisely at the underside of the pubic bone toward the ipsilateral shoulder. This creates a hydrodissected tunnel around each side of the midurethra. With a final syringe, I then inject 5 cc on each side suprapubically at my marked exit points.
With a #15 blade scalpel, I make two very small “poke” incisions transversely at the suprapubic sites. The suburethral incision is larger – just over a centimeter – and is made through a full thickness of skin under the area of hydrodissection, but not so deep as to injure the urethra. To finish development of the paraurethral passage, I pass standard Metzenbaum scissors through each hydrodissected tunnel until I feel the underside of the pubic bone, but no further.
For sling placement (after ensuring the bladder is completely empty), I lower the table such that my arm will be at a right angle to pass the sling while standing.
With my index finger underneath the pubic bone, the trocar tip, with the attached sling, is advanced with my thumb directly toward the ipsilateral shoulder just until it pops through the retropubic space. The depth of the trocar tip can be palpated with the index finger of the same hand, which is positioned just below the pelvic bone.
After the sling “pops” into the retropubic space, I remove my hand from the vagina and place it on the abdominal wall at the ipsilateral suprapubic poke site. In one smooth pass, I hug the pubic bone and advance the sling, again aiming directly and consistently at the shoulder. The trocar handle stays steady, never deviating in any direction. Cystoscopy is performed after the sling is placed on both sides to ensure bladder and urethral integrity.
For tensioning, I raise the table back up and, after reinserting the Foley catheter and a Sims retractor, I place my finger in the middle of the sling and pull the suprapubic ends of the sling up until my finger rests right under the urethra.
I then remove the vaginal clamps and use Metzenbaum scissors as a spacer between the sling and the urethra. With the scissors parallel to and right under the Foley catheter, at the same angle as the urethra, I tighten the sling and remove the plastic sheaths.
Dr. Sokol’s transobturator (TOT) approach
I most often use an outside-in sling. I utilize the same patient positioning and identify the midurethral point in the same way as with the retropubic approach.
On the thigh, I identify the adductor longus tendon as well as a little soft spot or depression just beneath the tendon and lateral to the descending ischial pubic ramus. With my thumb on the soft spot, I can actually grasp the adductor longus tendon between my thumb and index finger. This spot, which is also approximately at the level of the clitoris, marks the entry point for sling placement. It is the thinnest point between the groin and the vagina at the level of the midurethra.
I perform a similar hydrodissection under the urethra as I do in a retropubic procedure, though instead of injecting 5 cc’s to the underside of the pubic symphysis on each side, I instead inject toward the obturator internus muscles. I then inject my final syringe of dilute vasopressin saline solution at the groin poke incision sites, directed toward the projected trocar path, as opposed to suprapubically.
After the full-thickness vaginal incision is made with the scalpel, the dissection is performed sharply with Metzenbaum scissors and is more like the dissection done for cystocele repair than for a retropubic sling. Rather than a poke, the midurethral incision is long enough – about 1.5 cm – for me to reach a finger behind the obturator internus muscle after having sharply dissected the suburethral tissue and fascia. The angle of the dissection is more lateral than for a retropubic sling, toward the underside of the descending ischiopubic ramus and obturator internus muscle.
To place the sling, I have one hand with an index finger in the midurethral tunnel under the obturator internus muscle to protect the urethra. The thumb of that same hand is used to push the helical trocar straight through the thigh poke incision with the handle starting at a 35-degree angle from vertical. The trocar tip is pushed until it can no longer go straight and is ready to be tightly turned around the descending ischiopubic ramus with the opposite hand. A distinct pop can be felt as the trocar tip advances through the obturator membrane and muscles. As the tip is advanced, the angle of the trocar is rotated from 35 degrees to vertical, almost perpendicular to the floor. At this point, the tip of the trocar should be guided out of the midurethral tunnel against the opposite index finger.
I utilize the same technique for tensioning a TOT sling as I do the retropubic sling.
Dr. Rardin’s retropubic approach
I continue to use the original TVT sling with a 5-mm stainless steel, mechanically cut trocar and reusable handle. The newer slender needles may advance with less pressure, but I worry about them bending during passage. I feel more assured and comfortable using the older trocars.
I perform retropubic hydrodissection with a spinal needle using a top-down approach. With 40 cc of a very dilute solution of bupivacaine (Marcaine) with epinephrine on each side of the urethra, I create columns of hydrodissected space. Studies are inconsistent about the benefits of hydrodissection, but theoretically, it decreases the risk of bladder injury by pushing the bladder away from the pubic bone, creates effective hemostasis, and can provide analgesia that will be on board when the patient wakes up.
I bring the spinal needle down behind the pubic bone to the location of the urethral incision site, with my finger in the vagina, so I can feel the tip of the needle next to the urethra/Foley catheter. For each side, I will inject 20 cc of the solution in this location, and the other 20 cc as I withdraw the needle upward.
For trocar passage, some surgeons are taught to advance the trocar until a pop is felt, then drop the handle and push upward. I view the maneuver as a consistent, smooth arch; for every degree that I advance the trocar, I drop the handle slightly in order to maintain contact with the back of the pubic bone throughout the pass. I continuously and simultaneously drop the handle and advance the trocar. Contact with the back of the pubic bone is maintained with a slight pulling on the back end of the trocar handle, while the forward hand pushes the trocar upward.
The target that I visualize for a retropubic pass is the patient’s ipsilateral shoulder. A cadaver study showed that if you aim as far lateral as the patient’s outstretched elbow, you can enter the iliac vasculature (Obstet. Gynecol. 2003;101:933-6).
If the patient is draped such that I cannot see the ipsilateral shoulder, I ask the anesthesia team to show me. I have also identified and marked the suprapubic points about 2-3 cm from each other on either side of the midline just about the pubic symphysis, but I consider the broader anatomic picture and purposeful visualization toward the ipsilateral shoulder to be an essential part of safe technique. In general, it is safer to be more medial than more lateral for the needle passage.
I continue to use a rigid catheter guide to deflect the bladder neck while passing the needles.
Cystoscopy is performed after both needles are placed but not yet pulled through. I fill the bladder until the ureteral orifices appear flattened, which confirms that the bladder is under enough distension to preclude any mucosal wrinkles.
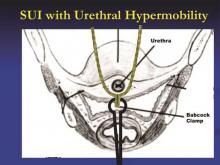
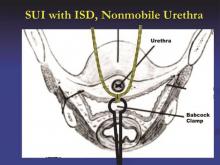
The technique I utilize for adjusting the tension of the TVT sling was taught to me by Dr. Peter L. Rosenblatt of Mt. Auburn Hospital in Cambridge, Mass. At the midline of the sling, I advance the sheaths just enough so that I can grasp a 2-3 mm “knuckle” of the midportion of the sling with a Babcock clamp. I then pull the sling ends until the Babcock comes into gentle contact with the suburethral tissue. The sheaths encasing the sling are then removed and the Babcock clamp is released to assure a tension-free deployment.
The amount of tape that is pinched with the Babcock – the size of the “knuckle” – determines the tension. For a patient with a more profound problem, such as intrinsic sphincter deficiency or a lack or urethral hypermobility, I will grasp a smaller knuckle.
These steps ensure that the midportion of the tape will not tighten or become deformed under tension. Rather than use a spacer, I like to protect the midportion of the tape and prevent it from being stretched. I find that the approach is reproducible and results in a reliable amount of space between the urethra and sling when the procedure is completed.
Dr. Rardin’s TOT approach
I employ an inside-out technique to the TOT procedure, and I utilize devices with segment of mesh that is shorter – only about 13 cm in length – than the original full-length mesh used in many TOT procedures. Once placed, the ends of the mesh penetrate the obturator membrane and obturator externus but not the adductor compartment of the thigh and groin.
In a study we presented last year at the annual meeting of the Society of Gynecologic Surgeons meeting, the shortened tape reduced postoperative groin pain, compared with full-length TOT tape without any reduction in subjective benefit. It appears that with shortened tape, we are anchoring the sling in tissues that provide critical support while avoiding the muscles that relate to the inner thigh/groin pain experienced by some patients. Effectiveness was not reduced, compared with full-length TOT slings.
These shortened slings are distinct from a single-incision sling, which is basically pushed into place. We still pass the needle all the way through a vaginal incision and out through the obturator foramen, and we pull the sling into place as we would any other TOT sling. The difference is that we’re not leaving any mesh in the groin.
I prefer an inside-out approach for two reasons: I always feel that I have more control over where a needle enters than where it exits, and precision is important with suburethral slings. Secondly, the dissection tunnel created for an inside-out pass is much smaller than the tunnel that must be dissected for an outside-in approach. In theory, less dissection means less devascularization, less denervation, and less opportunity for erosion.
Hydrodissection for TOT slings is more minimal and involves less fluid than does hydrodissection for retropubic slings, mainly because we do not want to anesthetize the obturator nerve. I pass the spinal needle from the outside in. At the start, prior to making any incisions, it is important to identify the arcus tendineus, a linear thickening of the superior fascia that is sometimes called the “white line.” This is where the sulcus is affixed to the sidewall. I will be sure to penetrate the sidewall at or above the level of the arcus.
With the TOT approach, the likelihood of bladder injury is so low that I usually drive the trocars all the way through prior to cystoscopy, as opposed to leaving the needles in place as I do with the retropubic approach.
Tensioning is achieved in the same manner, by using the Babcock clamp to avoid distortion of the critical part of the mesh while creating the space needed given the patient’s clinical scenario. It is worth remembering, at this point, that overall risks for retention appear to be lower for obturator slings, compared with retropubic slings.
I place most of my patients receiving retropubic slings in dorsal lithotomy position; but for obturator sling placement, I favor a few degrees into higher lithotomy because this pulls the obturator neurovascular bundle a little further out of the path of the needle.
Dr. Sokol reported that he owns stock in Pelvalon and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems and the recipient of research grants from Acell and several other companies. Dr. Rardin reported that he has no relevant financial disclosures. To view a video related to this article, go to SurgeryU at aagl.org/obgyn-news.
Midurethral slings – both retropubic and transobturator – have been extensively studied and have evolved to become standard therapies for the treatment of stress urinary incontinence. The two approaches utilize different routes for sling delivery, but in many other respects, they are similar. Improvements in technique are continually being developed. In this column, Dr. Sokol and Dr. Rardin share key parts of their technique and give their pearls of advice for midurethral sling surgery.
Dr. Sokol’s retropubic approach
I use newer retropubic midurethral slings with smaller trocars that have evolved from first-generation tension-free vaginal tape (TVT) slings. The slings I prefer are placed in a bottom-up fashion, with curved needles passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
I find it helpful to place patients in a high lithotomy position with the legs supported in candy cane stirrups rather than Allen-type stirrups; sling placement is a short procedure with minimal to no risk of neuropathy.
To precisely identify the midurethral point, I use a 16-FR Foley catheter. When the catheter balloon is filled with 10 mm of fluid and gently pulled back, the urethrovesical junction can be identified. Then, by looking at the urethral meatus relative to the bladder neck, I can mark the midpoint between the two.
The suprapubic exit points are marked at two finger-widths lateral to the midline, just above the pubic symphysis. For precise identification of these points, the Foley catheter may be pulled up exactly midline (with the collection bag detached), and the two finger-widths measured on either side. I also aim for the ipsilateral shoulder, imagining a straight line from the urethral meatus to the ipsilateral shoulder on each side. Together, these measurements and visual cues serve as a good safety check.
With two Allis clamps, the vaginal wall on either side of the midline is grasped transversely at the level of the midurethral mark. The clamps will sit a couple of centimeters apart so that the midurethral point can be visualized. This helps to stabilize and elevate the midurethra.
To safely and efficiently develop a paraurethral passage, I perform a hydrodissection and hemostatic injection at the level of the midurethra using a control top 10-cc syringe with a 22-gauge needle. I find that dilute vasopressin saline solution affords better hemostasis than does a dilute lidocaine epinephrine solution, though I use dilute lidocaine if the sling is being done under local anesthesia.
The needle is inserted into a full thickness of skin, to a point shy of the urethra, and 10 cc is rapidly injected. The vaginal epithelium will appear blanched and will balloon out, like a white marble. The process basically lifts the vaginal skin away from the urethra itself, not only creating hemostasis but also providing a zone of safety to help avoid a urethral injury.
With a second syringe identical to the first, I inject 5 cc on each side of the midurethral point, aiming precisely at the underside of the pubic bone toward the ipsilateral shoulder. This creates a hydrodissected tunnel around each side of the midurethra. With a final syringe, I then inject 5 cc on each side suprapubically at my marked exit points.
With a #15 blade scalpel, I make two very small “poke” incisions transversely at the suprapubic sites. The suburethral incision is larger – just over a centimeter – and is made through a full thickness of skin under the area of hydrodissection, but not so deep as to injure the urethra. To finish development of the paraurethral passage, I pass standard Metzenbaum scissors through each hydrodissected tunnel until I feel the underside of the pubic bone, but no further.
For sling placement (after ensuring the bladder is completely empty), I lower the table such that my arm will be at a right angle to pass the sling while standing.
With my index finger underneath the pubic bone, the trocar tip, with the attached sling, is advanced with my thumb directly toward the ipsilateral shoulder just until it pops through the retropubic space. The depth of the trocar tip can be palpated with the index finger of the same hand, which is positioned just below the pelvic bone.
After the sling “pops” into the retropubic space, I remove my hand from the vagina and place it on the abdominal wall at the ipsilateral suprapubic poke site. In one smooth pass, I hug the pubic bone and advance the sling, again aiming directly and consistently at the shoulder. The trocar handle stays steady, never deviating in any direction. Cystoscopy is performed after the sling is placed on both sides to ensure bladder and urethral integrity.
For tensioning, I raise the table back up and, after reinserting the Foley catheter and a Sims retractor, I place my finger in the middle of the sling and pull the suprapubic ends of the sling up until my finger rests right under the urethra.
I then remove the vaginal clamps and use Metzenbaum scissors as a spacer between the sling and the urethra. With the scissors parallel to and right under the Foley catheter, at the same angle as the urethra, I tighten the sling and remove the plastic sheaths.
Dr. Sokol’s transobturator (TOT) approach
I most often use an outside-in sling. I utilize the same patient positioning and identify the midurethral point in the same way as with the retropubic approach.
On the thigh, I identify the adductor longus tendon as well as a little soft spot or depression just beneath the tendon and lateral to the descending ischial pubic ramus. With my thumb on the soft spot, I can actually grasp the adductor longus tendon between my thumb and index finger. This spot, which is also approximately at the level of the clitoris, marks the entry point for sling placement. It is the thinnest point between the groin and the vagina at the level of the midurethra.
I perform a similar hydrodissection under the urethra as I do in a retropubic procedure, though instead of injecting 5 cc’s to the underside of the pubic symphysis on each side, I instead inject toward the obturator internus muscles. I then inject my final syringe of dilute vasopressin saline solution at the groin poke incision sites, directed toward the projected trocar path, as opposed to suprapubically.
After the full-thickness vaginal incision is made with the scalpel, the dissection is performed sharply with Metzenbaum scissors and is more like the dissection done for cystocele repair than for a retropubic sling. Rather than a poke, the midurethral incision is long enough – about 1.5 cm – for me to reach a finger behind the obturator internus muscle after having sharply dissected the suburethral tissue and fascia. The angle of the dissection is more lateral than for a retropubic sling, toward the underside of the descending ischiopubic ramus and obturator internus muscle.
To place the sling, I have one hand with an index finger in the midurethral tunnel under the obturator internus muscle to protect the urethra. The thumb of that same hand is used to push the helical trocar straight through the thigh poke incision with the handle starting at a 35-degree angle from vertical. The trocar tip is pushed until it can no longer go straight and is ready to be tightly turned around the descending ischiopubic ramus with the opposite hand. A distinct pop can be felt as the trocar tip advances through the obturator membrane and muscles. As the tip is advanced, the angle of the trocar is rotated from 35 degrees to vertical, almost perpendicular to the floor. At this point, the tip of the trocar should be guided out of the midurethral tunnel against the opposite index finger.
I utilize the same technique for tensioning a TOT sling as I do the retropubic sling.
Dr. Rardin’s retropubic approach
I continue to use the original TVT sling with a 5-mm stainless steel, mechanically cut trocar and reusable handle. The newer slender needles may advance with less pressure, but I worry about them bending during passage. I feel more assured and comfortable using the older trocars.
I perform retropubic hydrodissection with a spinal needle using a top-down approach. With 40 cc of a very dilute solution of bupivacaine (Marcaine) with epinephrine on each side of the urethra, I create columns of hydrodissected space. Studies are inconsistent about the benefits of hydrodissection, but theoretically, it decreases the risk of bladder injury by pushing the bladder away from the pubic bone, creates effective hemostasis, and can provide analgesia that will be on board when the patient wakes up.
I bring the spinal needle down behind the pubic bone to the location of the urethral incision site, with my finger in the vagina, so I can feel the tip of the needle next to the urethra/Foley catheter. For each side, I will inject 20 cc of the solution in this location, and the other 20 cc as I withdraw the needle upward.
For trocar passage, some surgeons are taught to advance the trocar until a pop is felt, then drop the handle and push upward. I view the maneuver as a consistent, smooth arch; for every degree that I advance the trocar, I drop the handle slightly in order to maintain contact with the back of the pubic bone throughout the pass. I continuously and simultaneously drop the handle and advance the trocar. Contact with the back of the pubic bone is maintained with a slight pulling on the back end of the trocar handle, while the forward hand pushes the trocar upward.
The target that I visualize for a retropubic pass is the patient’s ipsilateral shoulder. A cadaver study showed that if you aim as far lateral as the patient’s outstretched elbow, you can enter the iliac vasculature (Obstet. Gynecol. 2003;101:933-6).
If the patient is draped such that I cannot see the ipsilateral shoulder, I ask the anesthesia team to show me. I have also identified and marked the suprapubic points about 2-3 cm from each other on either side of the midline just about the pubic symphysis, but I consider the broader anatomic picture and purposeful visualization toward the ipsilateral shoulder to be an essential part of safe technique. In general, it is safer to be more medial than more lateral for the needle passage.
I continue to use a rigid catheter guide to deflect the bladder neck while passing the needles.
Cystoscopy is performed after both needles are placed but not yet pulled through. I fill the bladder until the ureteral orifices appear flattened, which confirms that the bladder is under enough distension to preclude any mucosal wrinkles.
The technique I utilize for adjusting the tension of the TVT sling was taught to me by Dr. Peter L. Rosenblatt of Mt. Auburn Hospital in Cambridge, Mass. At the midline of the sling, I advance the sheaths just enough so that I can grasp a 2-3 mm “knuckle” of the midportion of the sling with a Babcock clamp. I then pull the sling ends until the Babcock comes into gentle contact with the suburethral tissue. The sheaths encasing the sling are then removed and the Babcock clamp is released to assure a tension-free deployment.
The amount of tape that is pinched with the Babcock – the size of the “knuckle” – determines the tension. For a patient with a more profound problem, such as intrinsic sphincter deficiency or a lack or urethral hypermobility, I will grasp a smaller knuckle.
These steps ensure that the midportion of the tape will not tighten or become deformed under tension. Rather than use a spacer, I like to protect the midportion of the tape and prevent it from being stretched. I find that the approach is reproducible and results in a reliable amount of space between the urethra and sling when the procedure is completed.
Dr. Rardin’s TOT approach
I employ an inside-out technique to the TOT procedure, and I utilize devices with segment of mesh that is shorter – only about 13 cm in length – than the original full-length mesh used in many TOT procedures. Once placed, the ends of the mesh penetrate the obturator membrane and obturator externus but not the adductor compartment of the thigh and groin.
In a study we presented last year at the annual meeting of the Society of Gynecologic Surgeons meeting, the shortened tape reduced postoperative groin pain, compared with full-length TOT tape without any reduction in subjective benefit. It appears that with shortened tape, we are anchoring the sling in tissues that provide critical support while avoiding the muscles that relate to the inner thigh/groin pain experienced by some patients. Effectiveness was not reduced, compared with full-length TOT slings.
These shortened slings are distinct from a single-incision sling, which is basically pushed into place. We still pass the needle all the way through a vaginal incision and out through the obturator foramen, and we pull the sling into place as we would any other TOT sling. The difference is that we’re not leaving any mesh in the groin.
I prefer an inside-out approach for two reasons: I always feel that I have more control over where a needle enters than where it exits, and precision is important with suburethral slings. Secondly, the dissection tunnel created for an inside-out pass is much smaller than the tunnel that must be dissected for an outside-in approach. In theory, less dissection means less devascularization, less denervation, and less opportunity for erosion.
Hydrodissection for TOT slings is more minimal and involves less fluid than does hydrodissection for retropubic slings, mainly because we do not want to anesthetize the obturator nerve. I pass the spinal needle from the outside in. At the start, prior to making any incisions, it is important to identify the arcus tendineus, a linear thickening of the superior fascia that is sometimes called the “white line.” This is where the sulcus is affixed to the sidewall. I will be sure to penetrate the sidewall at or above the level of the arcus.
With the TOT approach, the likelihood of bladder injury is so low that I usually drive the trocars all the way through prior to cystoscopy, as opposed to leaving the needles in place as I do with the retropubic approach.
Tensioning is achieved in the same manner, by using the Babcock clamp to avoid distortion of the critical part of the mesh while creating the space needed given the patient’s clinical scenario. It is worth remembering, at this point, that overall risks for retention appear to be lower for obturator slings, compared with retropubic slings.
I place most of my patients receiving retropubic slings in dorsal lithotomy position; but for obturator sling placement, I favor a few degrees into higher lithotomy because this pulls the obturator neurovascular bundle a little further out of the path of the needle.
Dr. Sokol reported that he owns stock in Pelvalon and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems and the recipient of research grants from Acell and several other companies. Dr. Rardin reported that he has no relevant financial disclosures. To view a video related to this article, go to SurgeryU at aagl.org/obgyn-news.
Midurethral slings – both retropubic and transobturator – have been extensively studied and have evolved to become standard therapies for the treatment of stress urinary incontinence. The two approaches utilize different routes for sling delivery, but in many other respects, they are similar. Improvements in technique are continually being developed. In this column, Dr. Sokol and Dr. Rardin share key parts of their technique and give their pearls of advice for midurethral sling surgery.
Dr. Sokol’s retropubic approach
I use newer retropubic midurethral slings with smaller trocars that have evolved from first-generation tension-free vaginal tape (TVT) slings. The slings I prefer are placed in a bottom-up fashion, with curved needles passed from a small vaginal incision up through the retropubic space to exit through two suprapubic incisions.
I find it helpful to place patients in a high lithotomy position with the legs supported in candy cane stirrups rather than Allen-type stirrups; sling placement is a short procedure with minimal to no risk of neuropathy.
To precisely identify the midurethral point, I use a 16-FR Foley catheter. When the catheter balloon is filled with 10 mm of fluid and gently pulled back, the urethrovesical junction can be identified. Then, by looking at the urethral meatus relative to the bladder neck, I can mark the midpoint between the two.
The suprapubic exit points are marked at two finger-widths lateral to the midline, just above the pubic symphysis. For precise identification of these points, the Foley catheter may be pulled up exactly midline (with the collection bag detached), and the two finger-widths measured on either side. I also aim for the ipsilateral shoulder, imagining a straight line from the urethral meatus to the ipsilateral shoulder on each side. Together, these measurements and visual cues serve as a good safety check.
With two Allis clamps, the vaginal wall on either side of the midline is grasped transversely at the level of the midurethral mark. The clamps will sit a couple of centimeters apart so that the midurethral point can be visualized. This helps to stabilize and elevate the midurethra.
To safely and efficiently develop a paraurethral passage, I perform a hydrodissection and hemostatic injection at the level of the midurethra using a control top 10-cc syringe with a 22-gauge needle. I find that dilute vasopressin saline solution affords better hemostasis than does a dilute lidocaine epinephrine solution, though I use dilute lidocaine if the sling is being done under local anesthesia.
The needle is inserted into a full thickness of skin, to a point shy of the urethra, and 10 cc is rapidly injected. The vaginal epithelium will appear blanched and will balloon out, like a white marble. The process basically lifts the vaginal skin away from the urethra itself, not only creating hemostasis but also providing a zone of safety to help avoid a urethral injury.
With a second syringe identical to the first, I inject 5 cc on each side of the midurethral point, aiming precisely at the underside of the pubic bone toward the ipsilateral shoulder. This creates a hydrodissected tunnel around each side of the midurethra. With a final syringe, I then inject 5 cc on each side suprapubically at my marked exit points.
With a #15 blade scalpel, I make two very small “poke” incisions transversely at the suprapubic sites. The suburethral incision is larger – just over a centimeter – and is made through a full thickness of skin under the area of hydrodissection, but not so deep as to injure the urethra. To finish development of the paraurethral passage, I pass standard Metzenbaum scissors through each hydrodissected tunnel until I feel the underside of the pubic bone, but no further.
For sling placement (after ensuring the bladder is completely empty), I lower the table such that my arm will be at a right angle to pass the sling while standing.
With my index finger underneath the pubic bone, the trocar tip, with the attached sling, is advanced with my thumb directly toward the ipsilateral shoulder just until it pops through the retropubic space. The depth of the trocar tip can be palpated with the index finger of the same hand, which is positioned just below the pelvic bone.
After the sling “pops” into the retropubic space, I remove my hand from the vagina and place it on the abdominal wall at the ipsilateral suprapubic poke site. In one smooth pass, I hug the pubic bone and advance the sling, again aiming directly and consistently at the shoulder. The trocar handle stays steady, never deviating in any direction. Cystoscopy is performed after the sling is placed on both sides to ensure bladder and urethral integrity.
For tensioning, I raise the table back up and, after reinserting the Foley catheter and a Sims retractor, I place my finger in the middle of the sling and pull the suprapubic ends of the sling up until my finger rests right under the urethra.
I then remove the vaginal clamps and use Metzenbaum scissors as a spacer between the sling and the urethra. With the scissors parallel to and right under the Foley catheter, at the same angle as the urethra, I tighten the sling and remove the plastic sheaths.
Dr. Sokol’s transobturator (TOT) approach
I most often use an outside-in sling. I utilize the same patient positioning and identify the midurethral point in the same way as with the retropubic approach.
On the thigh, I identify the adductor longus tendon as well as a little soft spot or depression just beneath the tendon and lateral to the descending ischial pubic ramus. With my thumb on the soft spot, I can actually grasp the adductor longus tendon between my thumb and index finger. This spot, which is also approximately at the level of the clitoris, marks the entry point for sling placement. It is the thinnest point between the groin and the vagina at the level of the midurethra.
I perform a similar hydrodissection under the urethra as I do in a retropubic procedure, though instead of injecting 5 cc’s to the underside of the pubic symphysis on each side, I instead inject toward the obturator internus muscles. I then inject my final syringe of dilute vasopressin saline solution at the groin poke incision sites, directed toward the projected trocar path, as opposed to suprapubically.
After the full-thickness vaginal incision is made with the scalpel, the dissection is performed sharply with Metzenbaum scissors and is more like the dissection done for cystocele repair than for a retropubic sling. Rather than a poke, the midurethral incision is long enough – about 1.5 cm – for me to reach a finger behind the obturator internus muscle after having sharply dissected the suburethral tissue and fascia. The angle of the dissection is more lateral than for a retropubic sling, toward the underside of the descending ischiopubic ramus and obturator internus muscle.
To place the sling, I have one hand with an index finger in the midurethral tunnel under the obturator internus muscle to protect the urethra. The thumb of that same hand is used to push the helical trocar straight through the thigh poke incision with the handle starting at a 35-degree angle from vertical. The trocar tip is pushed until it can no longer go straight and is ready to be tightly turned around the descending ischiopubic ramus with the opposite hand. A distinct pop can be felt as the trocar tip advances through the obturator membrane and muscles. As the tip is advanced, the angle of the trocar is rotated from 35 degrees to vertical, almost perpendicular to the floor. At this point, the tip of the trocar should be guided out of the midurethral tunnel against the opposite index finger.
I utilize the same technique for tensioning a TOT sling as I do the retropubic sling.
Dr. Rardin’s retropubic approach
I continue to use the original TVT sling with a 5-mm stainless steel, mechanically cut trocar and reusable handle. The newer slender needles may advance with less pressure, but I worry about them bending during passage. I feel more assured and comfortable using the older trocars.
I perform retropubic hydrodissection with a spinal needle using a top-down approach. With 40 cc of a very dilute solution of bupivacaine (Marcaine) with epinephrine on each side of the urethra, I create columns of hydrodissected space. Studies are inconsistent about the benefits of hydrodissection, but theoretically, it decreases the risk of bladder injury by pushing the bladder away from the pubic bone, creates effective hemostasis, and can provide analgesia that will be on board when the patient wakes up.
I bring the spinal needle down behind the pubic bone to the location of the urethral incision site, with my finger in the vagina, so I can feel the tip of the needle next to the urethra/Foley catheter. For each side, I will inject 20 cc of the solution in this location, and the other 20 cc as I withdraw the needle upward.
For trocar passage, some surgeons are taught to advance the trocar until a pop is felt, then drop the handle and push upward. I view the maneuver as a consistent, smooth arch; for every degree that I advance the trocar, I drop the handle slightly in order to maintain contact with the back of the pubic bone throughout the pass. I continuously and simultaneously drop the handle and advance the trocar. Contact with the back of the pubic bone is maintained with a slight pulling on the back end of the trocar handle, while the forward hand pushes the trocar upward.
The target that I visualize for a retropubic pass is the patient’s ipsilateral shoulder. A cadaver study showed that if you aim as far lateral as the patient’s outstretched elbow, you can enter the iliac vasculature (Obstet. Gynecol. 2003;101:933-6).
If the patient is draped such that I cannot see the ipsilateral shoulder, I ask the anesthesia team to show me. I have also identified and marked the suprapubic points about 2-3 cm from each other on either side of the midline just about the pubic symphysis, but I consider the broader anatomic picture and purposeful visualization toward the ipsilateral shoulder to be an essential part of safe technique. In general, it is safer to be more medial than more lateral for the needle passage.
I continue to use a rigid catheter guide to deflect the bladder neck while passing the needles.
Cystoscopy is performed after both needles are placed but not yet pulled through. I fill the bladder until the ureteral orifices appear flattened, which confirms that the bladder is under enough distension to preclude any mucosal wrinkles.
The technique I utilize for adjusting the tension of the TVT sling was taught to me by Dr. Peter L. Rosenblatt of Mt. Auburn Hospital in Cambridge, Mass. At the midline of the sling, I advance the sheaths just enough so that I can grasp a 2-3 mm “knuckle” of the midportion of the sling with a Babcock clamp. I then pull the sling ends until the Babcock comes into gentle contact with the suburethral tissue. The sheaths encasing the sling are then removed and the Babcock clamp is released to assure a tension-free deployment.
The amount of tape that is pinched with the Babcock – the size of the “knuckle” – determines the tension. For a patient with a more profound problem, such as intrinsic sphincter deficiency or a lack or urethral hypermobility, I will grasp a smaller knuckle.
These steps ensure that the midportion of the tape will not tighten or become deformed under tension. Rather than use a spacer, I like to protect the midportion of the tape and prevent it from being stretched. I find that the approach is reproducible and results in a reliable amount of space between the urethra and sling when the procedure is completed.
Dr. Rardin’s TOT approach
I employ an inside-out technique to the TOT procedure, and I utilize devices with segment of mesh that is shorter – only about 13 cm in length – than the original full-length mesh used in many TOT procedures. Once placed, the ends of the mesh penetrate the obturator membrane and obturator externus but not the adductor compartment of the thigh and groin.
In a study we presented last year at the annual meeting of the Society of Gynecologic Surgeons meeting, the shortened tape reduced postoperative groin pain, compared with full-length TOT tape without any reduction in subjective benefit. It appears that with shortened tape, we are anchoring the sling in tissues that provide critical support while avoiding the muscles that relate to the inner thigh/groin pain experienced by some patients. Effectiveness was not reduced, compared with full-length TOT slings.
These shortened slings are distinct from a single-incision sling, which is basically pushed into place. We still pass the needle all the way through a vaginal incision and out through the obturator foramen, and we pull the sling into place as we would any other TOT sling. The difference is that we’re not leaving any mesh in the groin.
I prefer an inside-out approach for two reasons: I always feel that I have more control over where a needle enters than where it exits, and precision is important with suburethral slings. Secondly, the dissection tunnel created for an inside-out pass is much smaller than the tunnel that must be dissected for an outside-in approach. In theory, less dissection means less devascularization, less denervation, and less opportunity for erosion.
Hydrodissection for TOT slings is more minimal and involves less fluid than does hydrodissection for retropubic slings, mainly because we do not want to anesthetize the obturator nerve. I pass the spinal needle from the outside in. At the start, prior to making any incisions, it is important to identify the arcus tendineus, a linear thickening of the superior fascia that is sometimes called the “white line.” This is where the sulcus is affixed to the sidewall. I will be sure to penetrate the sidewall at or above the level of the arcus.
With the TOT approach, the likelihood of bladder injury is so low that I usually drive the trocars all the way through prior to cystoscopy, as opposed to leaving the needles in place as I do with the retropubic approach.
Tensioning is achieved in the same manner, by using the Babcock clamp to avoid distortion of the critical part of the mesh while creating the space needed given the patient’s clinical scenario. It is worth remembering, at this point, that overall risks for retention appear to be lower for obturator slings, compared with retropubic slings.
I place most of my patients receiving retropubic slings in dorsal lithotomy position; but for obturator sling placement, I favor a few degrees into higher lithotomy because this pulls the obturator neurovascular bundle a little further out of the path of the needle.
Dr. Sokol reported that he owns stock in Pelvalon and is a clinical adviser to that company. He also is a national principal investigator for American Medical Systems and the recipient of research grants from Acell and several other companies. Dr. Rardin reported that he has no relevant financial disclosures. To view a video related to this article, go to SurgeryU at aagl.org/obgyn-news.
Team discovers new mechanisms of platelet formation

in the bone marrow
Researchers have found that megakaryocytes can produce platelets via different polyploidization mechanisms.
Experiments suggested that endomitosis is the major megakaryocyte polyploidization mechanism, but megakaryocytes can generate platelets even in the absence of endomitosis—via endocycles and re-replication.
These findings may have implications for treating cancers as well as thrombocytopenia, the researchers said.
They described their discoveries in Developmental Cell.
Marcos Malumbres, PhD, of Centro Nacional de Investigaciones Oncologicas (CNIO) in Madrid, Spain, and his colleagues performed a genetic analysis of endomitosis, in which megakaryocytes become polyploid by entering mitosis but aborting anaphase.
The team studied mice lacking key proteins involved in mitotic entry (Cdk1 and Cdk2), mitotic progression (Aurora B), or mitotic exit (the anaphase-promoting complex [APC/C] cofactor Cdc20) during megakaryocyte maturation.
They found that Aurora B is not required for megakaryocyte maturation, but Cdc20 is. In mice lacking Cdc20, the researchers observed mitotic arrest and severe thrombocytopenia. The team said this suggests endomitosis is the major megakaryocyte polyploidization mechanism in vivo.
So they were surprised to discover that deleting Cdk1 prevents endomitosis but does not hinder platelet formation.
“Whilst the elimination of the main proteins that regulate megakaryocyte growth generates, as we thought, a reduction in the production of platelets, this didn’t happen when we removed Cdk1,” Dr Malumbres said. “[Even] when Cdk1 was absent, the megakaryocytes were able to grow in size in a similar way to normal cells.”
“[Further] analysis revealed that cells deficient in Cdk1 underwent cellular reprogramming towards a process known as endocycles, which can also be seen in other types of cells, such as certain cells in the placenta,” said Marianna Trakala, also of CNIO.
In endocycles, cells successively replicate their genomes without segregating chromosomes during mitosis.
The researchers uncovered an additional mechanism for megakaryocyte polyploidy when they studied mice lacking both Cdk1 and Cdk2. The deletion of both Cdks resulted in aberrant re-replication, in which the genome is replicated more than once per cell cycle, but platelet levels were not affected.
In fact, the team found that the loss of Cdk1 alone or Cdk1 and Cdk2 together can significantly rescue proplatelet formation and increase platelet levels in Cdc20-null mice.
“We immediately asked ourselves if, by reprogramming the cell cycle towards endocycles, we could correct the thrombocytopenia induced in other models,” Dr Malumbres said.
And when the researchers eliminated Cdk1 in Cdc20-null mice with severe thrombocytopenia, they observed an increase in platelet production. Results were similar when the team ablated Cdk1 and Cdk2 in Cdc20-null mice.
In addition to their implications for treating thrombocytopenia, these results could aid the design of cancer treatments, the researchers said. The findings reveal the different requirements that normal or tumor cells have toward cell-cycle regulators.
Specifically, the research suggests that deleting Cdk1 or other cell-cycle proteins is lethal for tumor cells but does not affect megakaryocytes. So drugs that inhibit these proteins could be used to treat malignancies such as pro-megakaryocytic leukemias. ![]()

in the bone marrow
Researchers have found that megakaryocytes can produce platelets via different polyploidization mechanisms.
Experiments suggested that endomitosis is the major megakaryocyte polyploidization mechanism, but megakaryocytes can generate platelets even in the absence of endomitosis—via endocycles and re-replication.
These findings may have implications for treating cancers as well as thrombocytopenia, the researchers said.
They described their discoveries in Developmental Cell.
Marcos Malumbres, PhD, of Centro Nacional de Investigaciones Oncologicas (CNIO) in Madrid, Spain, and his colleagues performed a genetic analysis of endomitosis, in which megakaryocytes become polyploid by entering mitosis but aborting anaphase.
The team studied mice lacking key proteins involved in mitotic entry (Cdk1 and Cdk2), mitotic progression (Aurora B), or mitotic exit (the anaphase-promoting complex [APC/C] cofactor Cdc20) during megakaryocyte maturation.
They found that Aurora B is not required for megakaryocyte maturation, but Cdc20 is. In mice lacking Cdc20, the researchers observed mitotic arrest and severe thrombocytopenia. The team said this suggests endomitosis is the major megakaryocyte polyploidization mechanism in vivo.
So they were surprised to discover that deleting Cdk1 prevents endomitosis but does not hinder platelet formation.
“Whilst the elimination of the main proteins that regulate megakaryocyte growth generates, as we thought, a reduction in the production of platelets, this didn’t happen when we removed Cdk1,” Dr Malumbres said. “[Even] when Cdk1 was absent, the megakaryocytes were able to grow in size in a similar way to normal cells.”
“[Further] analysis revealed that cells deficient in Cdk1 underwent cellular reprogramming towards a process known as endocycles, which can also be seen in other types of cells, such as certain cells in the placenta,” said Marianna Trakala, also of CNIO.
In endocycles, cells successively replicate their genomes without segregating chromosomes during mitosis.
The researchers uncovered an additional mechanism for megakaryocyte polyploidy when they studied mice lacking both Cdk1 and Cdk2. The deletion of both Cdks resulted in aberrant re-replication, in which the genome is replicated more than once per cell cycle, but platelet levels were not affected.
In fact, the team found that the loss of Cdk1 alone or Cdk1 and Cdk2 together can significantly rescue proplatelet formation and increase platelet levels in Cdc20-null mice.
“We immediately asked ourselves if, by reprogramming the cell cycle towards endocycles, we could correct the thrombocytopenia induced in other models,” Dr Malumbres said.
And when the researchers eliminated Cdk1 in Cdc20-null mice with severe thrombocytopenia, they observed an increase in platelet production. Results were similar when the team ablated Cdk1 and Cdk2 in Cdc20-null mice.
In addition to their implications for treating thrombocytopenia, these results could aid the design of cancer treatments, the researchers said. The findings reveal the different requirements that normal or tumor cells have toward cell-cycle regulators.
Specifically, the research suggests that deleting Cdk1 or other cell-cycle proteins is lethal for tumor cells but does not affect megakaryocytes. So drugs that inhibit these proteins could be used to treat malignancies such as pro-megakaryocytic leukemias. ![]()

in the bone marrow
Researchers have found that megakaryocytes can produce platelets via different polyploidization mechanisms.
Experiments suggested that endomitosis is the major megakaryocyte polyploidization mechanism, but megakaryocytes can generate platelets even in the absence of endomitosis—via endocycles and re-replication.
These findings may have implications for treating cancers as well as thrombocytopenia, the researchers said.
They described their discoveries in Developmental Cell.
Marcos Malumbres, PhD, of Centro Nacional de Investigaciones Oncologicas (CNIO) in Madrid, Spain, and his colleagues performed a genetic analysis of endomitosis, in which megakaryocytes become polyploid by entering mitosis but aborting anaphase.
The team studied mice lacking key proteins involved in mitotic entry (Cdk1 and Cdk2), mitotic progression (Aurora B), or mitotic exit (the anaphase-promoting complex [APC/C] cofactor Cdc20) during megakaryocyte maturation.
They found that Aurora B is not required for megakaryocyte maturation, but Cdc20 is. In mice lacking Cdc20, the researchers observed mitotic arrest and severe thrombocytopenia. The team said this suggests endomitosis is the major megakaryocyte polyploidization mechanism in vivo.
So they were surprised to discover that deleting Cdk1 prevents endomitosis but does not hinder platelet formation.
“Whilst the elimination of the main proteins that regulate megakaryocyte growth generates, as we thought, a reduction in the production of platelets, this didn’t happen when we removed Cdk1,” Dr Malumbres said. “[Even] when Cdk1 was absent, the megakaryocytes were able to grow in size in a similar way to normal cells.”
“[Further] analysis revealed that cells deficient in Cdk1 underwent cellular reprogramming towards a process known as endocycles, which can also be seen in other types of cells, such as certain cells in the placenta,” said Marianna Trakala, also of CNIO.
In endocycles, cells successively replicate their genomes without segregating chromosomes during mitosis.
The researchers uncovered an additional mechanism for megakaryocyte polyploidy when they studied mice lacking both Cdk1 and Cdk2. The deletion of both Cdks resulted in aberrant re-replication, in which the genome is replicated more than once per cell cycle, but platelet levels were not affected.
In fact, the team found that the loss of Cdk1 alone or Cdk1 and Cdk2 together can significantly rescue proplatelet formation and increase platelet levels in Cdc20-null mice.
“We immediately asked ourselves if, by reprogramming the cell cycle towards endocycles, we could correct the thrombocytopenia induced in other models,” Dr Malumbres said.
And when the researchers eliminated Cdk1 in Cdc20-null mice with severe thrombocytopenia, they observed an increase in platelet production. Results were similar when the team ablated Cdk1 and Cdk2 in Cdc20-null mice.
In addition to their implications for treating thrombocytopenia, these results could aid the design of cancer treatments, the researchers said. The findings reveal the different requirements that normal or tumor cells have toward cell-cycle regulators.
Specifically, the research suggests that deleting Cdk1 or other cell-cycle proteins is lethal for tumor cells but does not affect megakaryocytes. So drugs that inhibit these proteins could be used to treat malignancies such as pro-megakaryocytic leukemias. ![]()
Fusion protein fights resistant ALL

In a preclinical study, a fusion protein targeted treatment-resistant leukemia, demonstrating superiority over both chemotherapy and radiation.
The protein, CD19L-sTRAIL, induced apoptosis in radiation-resistant cells from patients with B-precursor acute lymphoblastic leukemia (ALL) and in
mouse models of the disease.
Additionally, mice that received CD19L-sTRAIL had significantly longer event-free survival than mice that received chemotherapy.
An account of this study appears in The Journal of Clinical Investigation.
Study investigators knew that TNF-related apoptosis-inducing ligand (TRAIL) can cause apoptosis in cancer cells by binding to TRAIL-receptor 1 and TRAIL-receptor 2.
“TRAIL is a naturally occurring part of the body’s immune system that kills cancer cells without toxicity to normal cells,” said Faith Uckun, MD, PhD, of Children’s Hospital Los Angeles in California.
“However, earlier clinical trials using TRAIL as a potential anticancer medicine candidate have not been successful, largely because of its propensity to bind, not only to cancer cells, but also to ‘decoy’ receptors.”
But Dr Uckun and her colleagues had discovered CD19-ligand (CD19L), a natural ligand of human CD19 that is expressed by almost all ALL cells. And they hypothesized that fusing CD19L to the portion of TRAIL that can kill cancer cells (known as sTRAIL) would produce a powerful weapon against leukemia cells.
The resulting fusion protein, CD19L-sTRAIL, would seek out, bind, and destroy only leukemia cells carrying CD19 as the target docking site.
In experiments, the investigators showed that their engineering converted sTRAIL into a much more potent “membrane-anchored” form that is capable of triggering apoptosis, even in the most aggressive and therapy-resistant ALL cells.
“Due to its ability to anchor to the surface of cancer cells via CD19, CD19L-sTRAIL was 100,000-fold more potent than sTRAIL and consistently killed more than 99% of aggressive leukemia cells taken directly from children with ALL—not only in the test tube but also in mice,” Dr Uckun said.
At a 2.1 pM concentration, CD19L-sTRAIL caused 84.0±4.7% apoptosis in leukemia cells from patients with B-precursor ALL, whereas radiation with 2 Gy γ-rays caused 45.0±9.0% apoptosis (P<0.0001). Higher concentrations of CD19L-sTRAIL prompted an apoptosis rate of more than 90%.
In cells from mouse models of B-precursor ALL, 2.1 pM of CD19L-sTRAIL caused 91.4±5.4% apoptosis, compared to 16.0±4.6% with 2 Gy γ-rays (P<0.0001).
In addition, administering 2 or 3 doses of CD19L-sTRAIL significantly improved event-free survival in mice challenged with an otherwise fatal dose of leukemia cells.
The median event-free survival was 17 days in control mice; 20 days in mice treated with either vincristine, dexamethasone, and peg-asparaginase or vincristine, doxorubicin, and peg-asparaginase; and 58 days in mice that received 2- to 3-day treatment with CD19L-sTRAIL (P<0.0001 vs controls; P=0.0002 vs chemo).
The investigators said these results support the clinical potential of CD19L-sTRAIL as a new agent against B-precursor ALL.
“We are hopeful that the knowledge gained from this study will open a new range of effective treatment opportunities for children with recurrent leukemia,” Dr Uckun said. ![]()

In a preclinical study, a fusion protein targeted treatment-resistant leukemia, demonstrating superiority over both chemotherapy and radiation.
The protein, CD19L-sTRAIL, induced apoptosis in radiation-resistant cells from patients with B-precursor acute lymphoblastic leukemia (ALL) and in
mouse models of the disease.
Additionally, mice that received CD19L-sTRAIL had significantly longer event-free survival than mice that received chemotherapy.
An account of this study appears in The Journal of Clinical Investigation.
Study investigators knew that TNF-related apoptosis-inducing ligand (TRAIL) can cause apoptosis in cancer cells by binding to TRAIL-receptor 1 and TRAIL-receptor 2.
“TRAIL is a naturally occurring part of the body’s immune system that kills cancer cells without toxicity to normal cells,” said Faith Uckun, MD, PhD, of Children’s Hospital Los Angeles in California.
“However, earlier clinical trials using TRAIL as a potential anticancer medicine candidate have not been successful, largely because of its propensity to bind, not only to cancer cells, but also to ‘decoy’ receptors.”
But Dr Uckun and her colleagues had discovered CD19-ligand (CD19L), a natural ligand of human CD19 that is expressed by almost all ALL cells. And they hypothesized that fusing CD19L to the portion of TRAIL that can kill cancer cells (known as sTRAIL) would produce a powerful weapon against leukemia cells.
The resulting fusion protein, CD19L-sTRAIL, would seek out, bind, and destroy only leukemia cells carrying CD19 as the target docking site.
In experiments, the investigators showed that their engineering converted sTRAIL into a much more potent “membrane-anchored” form that is capable of triggering apoptosis, even in the most aggressive and therapy-resistant ALL cells.
“Due to its ability to anchor to the surface of cancer cells via CD19, CD19L-sTRAIL was 100,000-fold more potent than sTRAIL and consistently killed more than 99% of aggressive leukemia cells taken directly from children with ALL—not only in the test tube but also in mice,” Dr Uckun said.
At a 2.1 pM concentration, CD19L-sTRAIL caused 84.0±4.7% apoptosis in leukemia cells from patients with B-precursor ALL, whereas radiation with 2 Gy γ-rays caused 45.0±9.0% apoptosis (P<0.0001). Higher concentrations of CD19L-sTRAIL prompted an apoptosis rate of more than 90%.
In cells from mouse models of B-precursor ALL, 2.1 pM of CD19L-sTRAIL caused 91.4±5.4% apoptosis, compared to 16.0±4.6% with 2 Gy γ-rays (P<0.0001).
In addition, administering 2 or 3 doses of CD19L-sTRAIL significantly improved event-free survival in mice challenged with an otherwise fatal dose of leukemia cells.
The median event-free survival was 17 days in control mice; 20 days in mice treated with either vincristine, dexamethasone, and peg-asparaginase or vincristine, doxorubicin, and peg-asparaginase; and 58 days in mice that received 2- to 3-day treatment with CD19L-sTRAIL (P<0.0001 vs controls; P=0.0002 vs chemo).
The investigators said these results support the clinical potential of CD19L-sTRAIL as a new agent against B-precursor ALL.
“We are hopeful that the knowledge gained from this study will open a new range of effective treatment opportunities for children with recurrent leukemia,” Dr Uckun said. ![]()

In a preclinical study, a fusion protein targeted treatment-resistant leukemia, demonstrating superiority over both chemotherapy and radiation.
The protein, CD19L-sTRAIL, induced apoptosis in radiation-resistant cells from patients with B-precursor acute lymphoblastic leukemia (ALL) and in
mouse models of the disease.
Additionally, mice that received CD19L-sTRAIL had significantly longer event-free survival than mice that received chemotherapy.
An account of this study appears in The Journal of Clinical Investigation.
Study investigators knew that TNF-related apoptosis-inducing ligand (TRAIL) can cause apoptosis in cancer cells by binding to TRAIL-receptor 1 and TRAIL-receptor 2.
“TRAIL is a naturally occurring part of the body’s immune system that kills cancer cells without toxicity to normal cells,” said Faith Uckun, MD, PhD, of Children’s Hospital Los Angeles in California.
“However, earlier clinical trials using TRAIL as a potential anticancer medicine candidate have not been successful, largely because of its propensity to bind, not only to cancer cells, but also to ‘decoy’ receptors.”
But Dr Uckun and her colleagues had discovered CD19-ligand (CD19L), a natural ligand of human CD19 that is expressed by almost all ALL cells. And they hypothesized that fusing CD19L to the portion of TRAIL that can kill cancer cells (known as sTRAIL) would produce a powerful weapon against leukemia cells.
The resulting fusion protein, CD19L-sTRAIL, would seek out, bind, and destroy only leukemia cells carrying CD19 as the target docking site.
In experiments, the investigators showed that their engineering converted sTRAIL into a much more potent “membrane-anchored” form that is capable of triggering apoptosis, even in the most aggressive and therapy-resistant ALL cells.
“Due to its ability to anchor to the surface of cancer cells via CD19, CD19L-sTRAIL was 100,000-fold more potent than sTRAIL and consistently killed more than 99% of aggressive leukemia cells taken directly from children with ALL—not only in the test tube but also in mice,” Dr Uckun said.
At a 2.1 pM concentration, CD19L-sTRAIL caused 84.0±4.7% apoptosis in leukemia cells from patients with B-precursor ALL, whereas radiation with 2 Gy γ-rays caused 45.0±9.0% apoptosis (P<0.0001). Higher concentrations of CD19L-sTRAIL prompted an apoptosis rate of more than 90%.
In cells from mouse models of B-precursor ALL, 2.1 pM of CD19L-sTRAIL caused 91.4±5.4% apoptosis, compared to 16.0±4.6% with 2 Gy γ-rays (P<0.0001).
In addition, administering 2 or 3 doses of CD19L-sTRAIL significantly improved event-free survival in mice challenged with an otherwise fatal dose of leukemia cells.
The median event-free survival was 17 days in control mice; 20 days in mice treated with either vincristine, dexamethasone, and peg-asparaginase or vincristine, doxorubicin, and peg-asparaginase; and 58 days in mice that received 2- to 3-day treatment with CD19L-sTRAIL (P<0.0001 vs controls; P=0.0002 vs chemo).
The investigators said these results support the clinical potential of CD19L-sTRAIL as a new agent against B-precursor ALL.
“We are hopeful that the knowledge gained from this study will open a new range of effective treatment opportunities for children with recurrent leukemia,” Dr Uckun said. ![]()
Product granted orphan designation for GVHD

Credit: PLOS ONE
The European Medicines Agency (EMA) has granted orphan drug status to ApoCell for the prevention of graft-vs-host disease (GVHD).
ApoCell consists of matched-donor, mononuclear-enriched, early apoptotic cells. It has been shown to immunomodulate macrophages and dendritic cells, both of which are involved in GVHD pathogenesis.
ApoCell showed early promise in a phase 1/2a study, and a phase 2b/3 trial of the product is set to begin this year.
ApoCell, which is under development by Enlivex Therapeutics, received orphan drug designation in the US in 2013.
In the European Union (EU), orphan designation is granted to products intended for the treatment, prevention, or diagnosis of a life-threatening or chronically debilitating disease with a prevalence of no more than 5 in 10,000, with no satisfactory method of diagnosis, prevention, or treatment authorized by the EMA.
Orphan designation in the EU comes with a number of benefits, including protocol assistance and 10-year market exclusivity following regulatory approval.
Phase 1/2a trial of ApoCell
For this study, researchers tested an infusion of ApoCell, in addition to cyclosporine and methotrexate, as prophylaxis for acute GVHD.
The trial enrolled 13 leukemia patients who had a median age of 37 (range, 20-59). All of the patients received an HLA-matched, myeloablative, allogeneic hematopoietic stem cell transplant (HSCT) from a related donor.
All patients engrafted. The median time to neutrophil recovery was 13 days after HSCT (range, 11 to 19), and the median time to platelet recovery was 15 days (range, 11 to 59).
At 100 days post-HSCT, the cumulative incidence of acute grade 2-4 GVHD was 23.1%, and the incidence of acute grade 3-4 GVHD was 15.4%. None of the patients developed acute GVHD beyond day 100.
Among patients who received the 2 higher doses of ApoCell (n=6), the rate of acute grade 2-4 GVHD was 0%.
Ten patients experienced serious adverse events, but 7 were considered unrelated to ApoCell, and 3 were likely unrelated to the treatment.
The nonrelapse mortality rate was 7.7% at both 100 and 180 days after HSCT. The overall survival rate was 92% at 100 days and 85% at 180 days.
The researchers said these results suggest a single infusion of ApoCell is safe and may effectively prevent acute GVHD. ![]()

Credit: PLOS ONE
The European Medicines Agency (EMA) has granted orphan drug status to ApoCell for the prevention of graft-vs-host disease (GVHD).
ApoCell consists of matched-donor, mononuclear-enriched, early apoptotic cells. It has been shown to immunomodulate macrophages and dendritic cells, both of which are involved in GVHD pathogenesis.
ApoCell showed early promise in a phase 1/2a study, and a phase 2b/3 trial of the product is set to begin this year.
ApoCell, which is under development by Enlivex Therapeutics, received orphan drug designation in the US in 2013.
In the European Union (EU), orphan designation is granted to products intended for the treatment, prevention, or diagnosis of a life-threatening or chronically debilitating disease with a prevalence of no more than 5 in 10,000, with no satisfactory method of diagnosis, prevention, or treatment authorized by the EMA.
Orphan designation in the EU comes with a number of benefits, including protocol assistance and 10-year market exclusivity following regulatory approval.
Phase 1/2a trial of ApoCell
For this study, researchers tested an infusion of ApoCell, in addition to cyclosporine and methotrexate, as prophylaxis for acute GVHD.
The trial enrolled 13 leukemia patients who had a median age of 37 (range, 20-59). All of the patients received an HLA-matched, myeloablative, allogeneic hematopoietic stem cell transplant (HSCT) from a related donor.
All patients engrafted. The median time to neutrophil recovery was 13 days after HSCT (range, 11 to 19), and the median time to platelet recovery was 15 days (range, 11 to 59).
At 100 days post-HSCT, the cumulative incidence of acute grade 2-4 GVHD was 23.1%, and the incidence of acute grade 3-4 GVHD was 15.4%. None of the patients developed acute GVHD beyond day 100.
Among patients who received the 2 higher doses of ApoCell (n=6), the rate of acute grade 2-4 GVHD was 0%.
Ten patients experienced serious adverse events, but 7 were considered unrelated to ApoCell, and 3 were likely unrelated to the treatment.
The nonrelapse mortality rate was 7.7% at both 100 and 180 days after HSCT. The overall survival rate was 92% at 100 days and 85% at 180 days.
The researchers said these results suggest a single infusion of ApoCell is safe and may effectively prevent acute GVHD. ![]()

Credit: PLOS ONE
The European Medicines Agency (EMA) has granted orphan drug status to ApoCell for the prevention of graft-vs-host disease (GVHD).
ApoCell consists of matched-donor, mononuclear-enriched, early apoptotic cells. It has been shown to immunomodulate macrophages and dendritic cells, both of which are involved in GVHD pathogenesis.
ApoCell showed early promise in a phase 1/2a study, and a phase 2b/3 trial of the product is set to begin this year.
ApoCell, which is under development by Enlivex Therapeutics, received orphan drug designation in the US in 2013.
In the European Union (EU), orphan designation is granted to products intended for the treatment, prevention, or diagnosis of a life-threatening or chronically debilitating disease with a prevalence of no more than 5 in 10,000, with no satisfactory method of diagnosis, prevention, or treatment authorized by the EMA.
Orphan designation in the EU comes with a number of benefits, including protocol assistance and 10-year market exclusivity following regulatory approval.
Phase 1/2a trial of ApoCell
For this study, researchers tested an infusion of ApoCell, in addition to cyclosporine and methotrexate, as prophylaxis for acute GVHD.
The trial enrolled 13 leukemia patients who had a median age of 37 (range, 20-59). All of the patients received an HLA-matched, myeloablative, allogeneic hematopoietic stem cell transplant (HSCT) from a related donor.
All patients engrafted. The median time to neutrophil recovery was 13 days after HSCT (range, 11 to 19), and the median time to platelet recovery was 15 days (range, 11 to 59).
At 100 days post-HSCT, the cumulative incidence of acute grade 2-4 GVHD was 23.1%, and the incidence of acute grade 3-4 GVHD was 15.4%. None of the patients developed acute GVHD beyond day 100.
Among patients who received the 2 higher doses of ApoCell (n=6), the rate of acute grade 2-4 GVHD was 0%.
Ten patients experienced serious adverse events, but 7 were considered unrelated to ApoCell, and 3 were likely unrelated to the treatment.
The nonrelapse mortality rate was 7.7% at both 100 and 180 days after HSCT. The overall survival rate was 92% at 100 days and 85% at 180 days.
The researchers said these results suggest a single infusion of ApoCell is safe and may effectively prevent acute GVHD. ![]()
NICE recommends rivaroxaban for ACS
Credit: NHS
In its final draft guidance, the UK’s National Institute for Health and Care Excellence (NICE) is recommending rivaroxaban (Xarelto) as an option for preventing adverse outcomes in patients with acute coronary syndromes (ACS).
The agency said it supports use of the factor Xa inhibitor in combination with aspirin and clopidogrel or aspirin alone to prevent atherothrombotic events in ACS patients with elevated cardiac biomarkers.
This includes patients who have had ST-segment-elevation myocardial infarctions (STEMIs) or non-ST-segment myocardial infarctions (NSTEMIs) but not unstable angina. In unstable angina, damage to the heart is not severe enough to result in the release of biomarkers into the blood, so this condition is not included in the guidance.
“Because rivaroxaban is associated with a higher risk of causing bleeding than clopidogrel in combination with aspirin or aspirin alone, the draft guidance recommends that, before starting treatment, doctors should carry out a careful assessment of a person’s bleeding risk,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“The decision to start treatment should be made after an informed discussion between the doctor and patient about the benefits and risks of rivaroxaban. Also, because there is limited experience of treatment with rivaroxaban up to 24 months, the draft guidance recommends careful consideration should be given to whether treatment is continued beyond 12 months.”
The final draft guidance is now with consultees, who have the opportunity to appeal against it. Once NICE issues its final guidance on a technology, it replaces local recommendations.
Cost and clinical effectiveness
An appraisal committee advising NICE concluded that rivaroxaban given at 2.5 mg twice daily in combination with aspirin plus clopidogrel or with aspirin alone was more effective than aspirin plus clopidogrel or aspirin alone for preventing further cardiovascular deaths and myocardial infarction in patients with ACS and raised cardiac biomarkers.
The committee also found rivaroxaban to be cost-effective. According to Bayer Healthcare (the company co-developing rivaroxaban with Janssen Research & Development, LLC), the base-case incremental cost-effectiveness ratio (ICER) was £6203 per quality-adjusted life-year (QALY) gained. The evidence review group’s preferred base-case estimate was £5622 per QALY gained.
The committee said there is uncertainty about the validity of these results, which were based on the ATLAS-ACS 2-TIMI 51 trial, because of the risk of bias resulting from missing trial data and informative censoring.
However, the committee considered that the ICERs presented were all within the range that could be considered cost-effective, and adjusting for the various types of bias that might have occurred was unlikely to increase the ICER to the extent that it would become unacceptable.
The list price of rivaroxaban is £58.88 per 2.5 mg, 56-capsule pack (excluding value-added tax). The license dose is 2.5 mg twice daily, which equates to a price of £2.10 per day.
Assuming a treatment duration of 12 months, total acquisition costs are £766.50. Costs may vary in different settings because of negotiated procurement discounts. ![]()

Credit: NHS
In its final draft guidance, the UK’s National Institute for Health and Care Excellence (NICE) is recommending rivaroxaban (Xarelto) as an option for preventing adverse outcomes in patients with acute coronary syndromes (ACS).
The agency said it supports use of the factor Xa inhibitor in combination with aspirin and clopidogrel or aspirin alone to prevent atherothrombotic events in ACS patients with elevated cardiac biomarkers.
This includes patients who have had ST-segment-elevation myocardial infarctions (STEMIs) or non-ST-segment myocardial infarctions (NSTEMIs) but not unstable angina. In unstable angina, damage to the heart is not severe enough to result in the release of biomarkers into the blood, so this condition is not included in the guidance.
“Because rivaroxaban is associated with a higher risk of causing bleeding than clopidogrel in combination with aspirin or aspirin alone, the draft guidance recommends that, before starting treatment, doctors should carry out a careful assessment of a person’s bleeding risk,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“The decision to start treatment should be made after an informed discussion between the doctor and patient about the benefits and risks of rivaroxaban. Also, because there is limited experience of treatment with rivaroxaban up to 24 months, the draft guidance recommends careful consideration should be given to whether treatment is continued beyond 12 months.”
The final draft guidance is now with consultees, who have the opportunity to appeal against it. Once NICE issues its final guidance on a technology, it replaces local recommendations.
Cost and clinical effectiveness
An appraisal committee advising NICE concluded that rivaroxaban given at 2.5 mg twice daily in combination with aspirin plus clopidogrel or with aspirin alone was more effective than aspirin plus clopidogrel or aspirin alone for preventing further cardiovascular deaths and myocardial infarction in patients with ACS and raised cardiac biomarkers.
The committee also found rivaroxaban to be cost-effective. According to Bayer Healthcare (the company co-developing rivaroxaban with Janssen Research & Development, LLC), the base-case incremental cost-effectiveness ratio (ICER) was £6203 per quality-adjusted life-year (QALY) gained. The evidence review group’s preferred base-case estimate was £5622 per QALY gained.
The committee said there is uncertainty about the validity of these results, which were based on the ATLAS-ACS 2-TIMI 51 trial, because of the risk of bias resulting from missing trial data and informative censoring.
However, the committee considered that the ICERs presented were all within the range that could be considered cost-effective, and adjusting for the various types of bias that might have occurred was unlikely to increase the ICER to the extent that it would become unacceptable.
The list price of rivaroxaban is £58.88 per 2.5 mg, 56-capsule pack (excluding value-added tax). The license dose is 2.5 mg twice daily, which equates to a price of £2.10 per day.
Assuming a treatment duration of 12 months, total acquisition costs are £766.50. Costs may vary in different settings because of negotiated procurement discounts. ![]()

Credit: NHS
In its final draft guidance, the UK’s National Institute for Health and Care Excellence (NICE) is recommending rivaroxaban (Xarelto) as an option for preventing adverse outcomes in patients with acute coronary syndromes (ACS).
The agency said it supports use of the factor Xa inhibitor in combination with aspirin and clopidogrel or aspirin alone to prevent atherothrombotic events in ACS patients with elevated cardiac biomarkers.
This includes patients who have had ST-segment-elevation myocardial infarctions (STEMIs) or non-ST-segment myocardial infarctions (NSTEMIs) but not unstable angina. In unstable angina, damage to the heart is not severe enough to result in the release of biomarkers into the blood, so this condition is not included in the guidance.
“Because rivaroxaban is associated with a higher risk of causing bleeding than clopidogrel in combination with aspirin or aspirin alone, the draft guidance recommends that, before starting treatment, doctors should carry out a careful assessment of a person’s bleeding risk,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“The decision to start treatment should be made after an informed discussion between the doctor and patient about the benefits and risks of rivaroxaban. Also, because there is limited experience of treatment with rivaroxaban up to 24 months, the draft guidance recommends careful consideration should be given to whether treatment is continued beyond 12 months.”
The final draft guidance is now with consultees, who have the opportunity to appeal against it. Once NICE issues its final guidance on a technology, it replaces local recommendations.
Cost and clinical effectiveness
An appraisal committee advising NICE concluded that rivaroxaban given at 2.5 mg twice daily in combination with aspirin plus clopidogrel or with aspirin alone was more effective than aspirin plus clopidogrel or aspirin alone for preventing further cardiovascular deaths and myocardial infarction in patients with ACS and raised cardiac biomarkers.
The committee also found rivaroxaban to be cost-effective. According to Bayer Healthcare (the company co-developing rivaroxaban with Janssen Research & Development, LLC), the base-case incremental cost-effectiveness ratio (ICER) was £6203 per quality-adjusted life-year (QALY) gained. The evidence review group’s preferred base-case estimate was £5622 per QALY gained.
The committee said there is uncertainty about the validity of these results, which were based on the ATLAS-ACS 2-TIMI 51 trial, because of the risk of bias resulting from missing trial data and informative censoring.
However, the committee considered that the ICERs presented were all within the range that could be considered cost-effective, and adjusting for the various types of bias that might have occurred was unlikely to increase the ICER to the extent that it would become unacceptable.
The list price of rivaroxaban is £58.88 per 2.5 mg, 56-capsule pack (excluding value-added tax). The license dose is 2.5 mg twice daily, which equates to a price of £2.10 per day.
Assuming a treatment duration of 12 months, total acquisition costs are £766.50. Costs may vary in different settings because of negotiated procurement discounts. ![]()
Pituitary Incidentaloma
Brian, 46, is referred to endocrinology for evaluation of a pituitary “mass.” The mass was an incidental finding of head and neck CT performed three months ago, when Brian went to an emergency department following a motor vehicle accident. He has fully recovered from the accident and feels well. He describes himself as a “completely healthy” person who has no chronic medical conditions and takes neither prescription nor OTC medications.
Brian denies significant headache, visual disturbance, change in appetite, unexplained weight change, skin rash (wide purple striae) or color changes (hyperpigmentation), polyuria or polydipsia, dizziness, syncopal episodes, low libido, erectile dysfunction, joint pain, and changes in ring or shoe size. He does not wear a hat or cap and is unaware of head size changes. He has not experienced changes in his facial features or trouble with chewing.
He is a happily married engineer with two healthy children and reports that he feels well except for this “brain tumor” finding that has been a shock to him and his family. There is no family history of pituitary adenoma or multiple endocrine neoplasia syndrome.
His vital signs, all within normal ranges, include a blood pressure of 103/65 mm Hg. His height is 6 ft and his weight, 180 lb. His BMI is 24.4.
HOW COMMON IS PITUITARY INCIDENTALOMA?
A pituitary incidentaloma is a lesion in the pituitary gland that was not previously suspected and was found through an imaging study ordered for other reasons. Pituitary incidentaloma is surprisingly common, with an average prevalence of 10.6% (as estimated from combined autopsy data), although it has been found in up to 20% of patients undergoing CT and 38% undergoing MRI.1,2 Most are microadenomas (< 1 cm in size).1
Continue for recommendations from the Endocrine Society >>
SHOULD AN ASYMPTOMATIC PATIENT BE EVALUATED FURTHER?
Endocrine Society guidelines2 recommend that all patients with pituitary incidentaloma, with or without symptoms, should undergo a complete history and physical examination and laboratory evaluation to exclude hypersecretion and hyposecretion of pituitary hormones.
The “classic” presentation of pituitary hormone hypersecretion—in the form of prolactinoma, adrenocorticotropic hormone (ACTH) excess (Cushing disease), growth hormone (GH) excess (gigantism/acromegaly), and TSH excess (secondary hyperthyroidism)—may be readily detectable on history and physical examination. Subtle cases, so-called subclinical disease, however, may exhibit little or no signs and symptoms initially but can be detrimental to the patient’s health if left untreated. For example, the estimated time from onset to diagnosis of acromegaly is approximately seven to 10 years—a delay that can significantly impact the patient’s morbidity and mortality.3
Prolactinoma can be more clinically apparent in premenopausal females due to irregular menstrual cycles (oligomenorrhea/amenorrhea). However, galactorrhea, or “milky” nipple discharge, occurs in only about 50% of women with prolactinoma and is extremely rare in men. Furthermore, the clinical presentation of prolactinoma in men is vague and related to hypogonadism, resulting from increased prolactin levels. Since men are essentially asymptomatic, these tumors can grow extensively (macroadenoma) and cause “mass effect,” such as headaches and visual impairment.
Therefore, without laboratory testing, abnormal pituitary function may go unrecognized.
WHAT LABS SHOULD I ORDER FOR THIS PATIENT?
Guidelines suggest an initial screening panel of prolactin, GH, insulin-like growth factor 1 (IGF-1), ACTH, early-morning cortisol, TSH, free T4, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone.2
Note the use of “suggest” rather than “recommend.” Even among guideline task force members, there were differences in opinion as to whether certain tests (eg, TSH, LH, and FSH) should be included in initial screening. Those tests can be ordered at the clinician’s discretion, according to the level of suspicion, or can be added later if necessary.
Brian’s sample for laboratory testing is drawn at 7:50 am. Results can be found in the table (previous page).
Next page: Caveats and concerns >>
ARE THERE ANY CAVEATS TO THE INTERPRETATION OF LAB VALUES?
It is important to note that in postmenopausal women who are not taking hormone replacement therapy, LH and FSH will be elevated and estradiol may provide an additional clue in the detection of abnormal function. Conversely, low LH and FSH in postmenopausal women should raise a flag for hypopituitarism.
Another caveat is that GH secretion is pulsatile and serum levels are undetectable between pulses. Therefore, low/undetectable GH does not necessarily suggest deficiency. The GH measurement would only be helpful if it is significantly elevated (suggestive of hypersecretion—gigantism/acromegaly). Otherwise, GH has little value as a screening test.
Instead, IGF-1, which is secreted from the liver in response to GH secretion, has a longer half-life and serves as a better screening tool. IGF-1 has age- and sex-adjusted reference ranges, which are often reported by the lab or given as a Z score.
WHAT IS THE PREFERRED IMAGING STUDY FOR THE PITUITARY GLAND?
The best choice is MRI of the pituitary gland (not the whole brain) with gadolinium. If the incidentaloma was initially diagnosed by a CT, additional testing with MRI should be performed, unless contraindicated.2
Brian is referred for MRI with gadolinium. The radiologist’s report describes a 5 x 4 x 4–mm pituitary microadenoma without sellar extension or involvement of the optic chiasm.
AT WHAT POINT SHOULD OPTIC CHIASM BE A CONCERN?
Since the pituitary gland is located directly beneath the optic chiasm, any compressive effect of growth against the optic nerve(s) can cause visual impairment. This includes bitemporal hemianopsia (loss of peripheral vision) or ophthalmoplegia (abnormal movement of the ocular muscle). Since clinical signs and symptoms can be subtle or absent, all patients with evidence of a pituitary lesion abutting or compressing the optic chiasm should have a formal visual field exam.2
Continue for surgical intervention >>
WHO REQUIRES SURGICAL INTERVENTION?
Patients with mass effect (headache, increased intracranial pressure, compromised optic chiasm) and those with hyperfunctioning nonprolactin adenomas, some (but not all) macroprolactinomas, or pituitary apoplexy should be referred for surgery.2 Almost all cases involve macroadenomas rather than microadenomas.
The preferred treatment for GH-secreting tumors and ACTH-secreting tumors is surgery. However, prolactinoma can be well controlled with pharmacologic agents (dopamine agonists) in most cases. For prolactinomas refractory to these medications, surgical resection is recommended. (Detailed treatment approaches are available elsewhere; those for hyperprolactinoma can be found on the Clinician Reviews website: http://bit.ly/1HOb9Jf.)
Pituitary apoplexy, a life-threatening emergency that requires prompt surgical decompression, is an infarction of the gland due to abrupt cessation of the blood supply, caused by either pituitary artery hemorrhage or sudden hypovolemia. Increased blood supply is needed due to the extra tissue and volume of the pituitary mass; this may stress the pituitary arteries, which are not equipped for this increased flow, causing them to rupture. Hemorrhage anywhere else in the body can lead to hypovolemia and decrease the blood supply to the pituitary gland. A classic example would be postpartum hemorrhage causing pituitary infarct, called Sheehan syndrome.
Due to increased estrogen levels, the pituitary gland doubles in size during pregnancy.4 A preexisting mass may further develop and compress the optic chiasm. Therefore, women of childbearing age should be engaged in discussion of the potential risks and benefits of decompression surgery before actively pursuing pregnancy—especially if the lesion is close to the optic chiasm.
Surgery can also be considered for patients with significant growth in adenoma size during monitoring, loss of endocrinologic function due to mass effect on other pituitary cells, or unremitting headache.2
Next: How should patients be monitored?
HOW SHOULD PATIENTS BE MONITORED?
Those who do not meet criteria for surgery can be closely monitored with periodic testing. Imaging can be repeated six months after the first scan for macroadenoma and in one year for microadenoma. If there is no change in the size of the mass, imaging can be done yearly for macroadenoma and for microadenoma, every one to two years for three years and then gradually less often thereafter.2
Unless the lesion is abutting the optic chiasm (seen via imaging) or the patient reports symptoms, visual field testing does not need to be repeated.
Lab testing should be repeated six months after initial testing for macroadenoma and yearly thereafter. No further testing is suggested for nonsecretory microadenoma, unless clinically indicated.2
If there are any changes in status—noted clinically or via imaging—more frequent testing is suggested.
Brian is reassured that pituitary adenoma is not an uncommon finding and that his adenoma is relatively small in size and nonsecretory. Repeat pituitary MRI in one year is recommended.
CONCLUSION
Most pituitary incidentalomas have no consequences to a patient’s health. However, patients often become highly anxious about the “brain tumor” they were told they have. Appropriate patient education and thorough evaluation can reassure patients and alleviate their concerns.
REFERENCES
1. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37(1):151-171.
2. Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(4):894-904.
3. Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists Medical Guidelines For Clinical Practice For The Diagnosis And Treatment Of Acromegaly–2011 Update. Endocr Pract. 2011;17(suppl 4).
4. Jameson JL. Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010:16-49.
Brian, 46, is referred to endocrinology for evaluation of a pituitary “mass.” The mass was an incidental finding of head and neck CT performed three months ago, when Brian went to an emergency department following a motor vehicle accident. He has fully recovered from the accident and feels well. He describes himself as a “completely healthy” person who has no chronic medical conditions and takes neither prescription nor OTC medications.
Brian denies significant headache, visual disturbance, change in appetite, unexplained weight change, skin rash (wide purple striae) or color changes (hyperpigmentation), polyuria or polydipsia, dizziness, syncopal episodes, low libido, erectile dysfunction, joint pain, and changes in ring or shoe size. He does not wear a hat or cap and is unaware of head size changes. He has not experienced changes in his facial features or trouble with chewing.
He is a happily married engineer with two healthy children and reports that he feels well except for this “brain tumor” finding that has been a shock to him and his family. There is no family history of pituitary adenoma or multiple endocrine neoplasia syndrome.
His vital signs, all within normal ranges, include a blood pressure of 103/65 mm Hg. His height is 6 ft and his weight, 180 lb. His BMI is 24.4.
HOW COMMON IS PITUITARY INCIDENTALOMA?
A pituitary incidentaloma is a lesion in the pituitary gland that was not previously suspected and was found through an imaging study ordered for other reasons. Pituitary incidentaloma is surprisingly common, with an average prevalence of 10.6% (as estimated from combined autopsy data), although it has been found in up to 20% of patients undergoing CT and 38% undergoing MRI.1,2 Most are microadenomas (< 1 cm in size).1
Continue for recommendations from the Endocrine Society >>
SHOULD AN ASYMPTOMATIC PATIENT BE EVALUATED FURTHER?
Endocrine Society guidelines2 recommend that all patients with pituitary incidentaloma, with or without symptoms, should undergo a complete history and physical examination and laboratory evaluation to exclude hypersecretion and hyposecretion of pituitary hormones.
The “classic” presentation of pituitary hormone hypersecretion—in the form of prolactinoma, adrenocorticotropic hormone (ACTH) excess (Cushing disease), growth hormone (GH) excess (gigantism/acromegaly), and TSH excess (secondary hyperthyroidism)—may be readily detectable on history and physical examination. Subtle cases, so-called subclinical disease, however, may exhibit little or no signs and symptoms initially but can be detrimental to the patient’s health if left untreated. For example, the estimated time from onset to diagnosis of acromegaly is approximately seven to 10 years—a delay that can significantly impact the patient’s morbidity and mortality.3
Prolactinoma can be more clinically apparent in premenopausal females due to irregular menstrual cycles (oligomenorrhea/amenorrhea). However, galactorrhea, or “milky” nipple discharge, occurs in only about 50% of women with prolactinoma and is extremely rare in men. Furthermore, the clinical presentation of prolactinoma in men is vague and related to hypogonadism, resulting from increased prolactin levels. Since men are essentially asymptomatic, these tumors can grow extensively (macroadenoma) and cause “mass effect,” such as headaches and visual impairment.
Therefore, without laboratory testing, abnormal pituitary function may go unrecognized.
WHAT LABS SHOULD I ORDER FOR THIS PATIENT?
Guidelines suggest an initial screening panel of prolactin, GH, insulin-like growth factor 1 (IGF-1), ACTH, early-morning cortisol, TSH, free T4, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone.2
Note the use of “suggest” rather than “recommend.” Even among guideline task force members, there were differences in opinion as to whether certain tests (eg, TSH, LH, and FSH) should be included in initial screening. Those tests can be ordered at the clinician’s discretion, according to the level of suspicion, or can be added later if necessary.
Brian’s sample for laboratory testing is drawn at 7:50 am. Results can be found in the table (previous page).
Next page: Caveats and concerns >>
ARE THERE ANY CAVEATS TO THE INTERPRETATION OF LAB VALUES?
It is important to note that in postmenopausal women who are not taking hormone replacement therapy, LH and FSH will be elevated and estradiol may provide an additional clue in the detection of abnormal function. Conversely, low LH and FSH in postmenopausal women should raise a flag for hypopituitarism.
Another caveat is that GH secretion is pulsatile and serum levels are undetectable between pulses. Therefore, low/undetectable GH does not necessarily suggest deficiency. The GH measurement would only be helpful if it is significantly elevated (suggestive of hypersecretion—gigantism/acromegaly). Otherwise, GH has little value as a screening test.
Instead, IGF-1, which is secreted from the liver in response to GH secretion, has a longer half-life and serves as a better screening tool. IGF-1 has age- and sex-adjusted reference ranges, which are often reported by the lab or given as a Z score.
WHAT IS THE PREFERRED IMAGING STUDY FOR THE PITUITARY GLAND?
The best choice is MRI of the pituitary gland (not the whole brain) with gadolinium. If the incidentaloma was initially diagnosed by a CT, additional testing with MRI should be performed, unless contraindicated.2
Brian is referred for MRI with gadolinium. The radiologist’s report describes a 5 x 4 x 4–mm pituitary microadenoma without sellar extension or involvement of the optic chiasm.
AT WHAT POINT SHOULD OPTIC CHIASM BE A CONCERN?
Since the pituitary gland is located directly beneath the optic chiasm, any compressive effect of growth against the optic nerve(s) can cause visual impairment. This includes bitemporal hemianopsia (loss of peripheral vision) or ophthalmoplegia (abnormal movement of the ocular muscle). Since clinical signs and symptoms can be subtle or absent, all patients with evidence of a pituitary lesion abutting or compressing the optic chiasm should have a formal visual field exam.2
Continue for surgical intervention >>
WHO REQUIRES SURGICAL INTERVENTION?
Patients with mass effect (headache, increased intracranial pressure, compromised optic chiasm) and those with hyperfunctioning nonprolactin adenomas, some (but not all) macroprolactinomas, or pituitary apoplexy should be referred for surgery.2 Almost all cases involve macroadenomas rather than microadenomas.
The preferred treatment for GH-secreting tumors and ACTH-secreting tumors is surgery. However, prolactinoma can be well controlled with pharmacologic agents (dopamine agonists) in most cases. For prolactinomas refractory to these medications, surgical resection is recommended. (Detailed treatment approaches are available elsewhere; those for hyperprolactinoma can be found on the Clinician Reviews website: http://bit.ly/1HOb9Jf.)
Pituitary apoplexy, a life-threatening emergency that requires prompt surgical decompression, is an infarction of the gland due to abrupt cessation of the blood supply, caused by either pituitary artery hemorrhage or sudden hypovolemia. Increased blood supply is needed due to the extra tissue and volume of the pituitary mass; this may stress the pituitary arteries, which are not equipped for this increased flow, causing them to rupture. Hemorrhage anywhere else in the body can lead to hypovolemia and decrease the blood supply to the pituitary gland. A classic example would be postpartum hemorrhage causing pituitary infarct, called Sheehan syndrome.
Due to increased estrogen levels, the pituitary gland doubles in size during pregnancy.4 A preexisting mass may further develop and compress the optic chiasm. Therefore, women of childbearing age should be engaged in discussion of the potential risks and benefits of decompression surgery before actively pursuing pregnancy—especially if the lesion is close to the optic chiasm.
Surgery can also be considered for patients with significant growth in adenoma size during monitoring, loss of endocrinologic function due to mass effect on other pituitary cells, or unremitting headache.2
Next: How should patients be monitored?
HOW SHOULD PATIENTS BE MONITORED?
Those who do not meet criteria for surgery can be closely monitored with periodic testing. Imaging can be repeated six months after the first scan for macroadenoma and in one year for microadenoma. If there is no change in the size of the mass, imaging can be done yearly for macroadenoma and for microadenoma, every one to two years for three years and then gradually less often thereafter.2
Unless the lesion is abutting the optic chiasm (seen via imaging) or the patient reports symptoms, visual field testing does not need to be repeated.
Lab testing should be repeated six months after initial testing for macroadenoma and yearly thereafter. No further testing is suggested for nonsecretory microadenoma, unless clinically indicated.2
If there are any changes in status—noted clinically or via imaging—more frequent testing is suggested.
Brian is reassured that pituitary adenoma is not an uncommon finding and that his adenoma is relatively small in size and nonsecretory. Repeat pituitary MRI in one year is recommended.
CONCLUSION
Most pituitary incidentalomas have no consequences to a patient’s health. However, patients often become highly anxious about the “brain tumor” they were told they have. Appropriate patient education and thorough evaluation can reassure patients and alleviate their concerns.
REFERENCES
1. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37(1):151-171.
2. Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(4):894-904.
3. Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists Medical Guidelines For Clinical Practice For The Diagnosis And Treatment Of Acromegaly–2011 Update. Endocr Pract. 2011;17(suppl 4).
4. Jameson JL. Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010:16-49.
Brian, 46, is referred to endocrinology for evaluation of a pituitary “mass.” The mass was an incidental finding of head and neck CT performed three months ago, when Brian went to an emergency department following a motor vehicle accident. He has fully recovered from the accident and feels well. He describes himself as a “completely healthy” person who has no chronic medical conditions and takes neither prescription nor OTC medications.
Brian denies significant headache, visual disturbance, change in appetite, unexplained weight change, skin rash (wide purple striae) or color changes (hyperpigmentation), polyuria or polydipsia, dizziness, syncopal episodes, low libido, erectile dysfunction, joint pain, and changes in ring or shoe size. He does not wear a hat or cap and is unaware of head size changes. He has not experienced changes in his facial features or trouble with chewing.
He is a happily married engineer with two healthy children and reports that he feels well except for this “brain tumor” finding that has been a shock to him and his family. There is no family history of pituitary adenoma or multiple endocrine neoplasia syndrome.
His vital signs, all within normal ranges, include a blood pressure of 103/65 mm Hg. His height is 6 ft and his weight, 180 lb. His BMI is 24.4.
HOW COMMON IS PITUITARY INCIDENTALOMA?
A pituitary incidentaloma is a lesion in the pituitary gland that was not previously suspected and was found through an imaging study ordered for other reasons. Pituitary incidentaloma is surprisingly common, with an average prevalence of 10.6% (as estimated from combined autopsy data), although it has been found in up to 20% of patients undergoing CT and 38% undergoing MRI.1,2 Most are microadenomas (< 1 cm in size).1
Continue for recommendations from the Endocrine Society >>
SHOULD AN ASYMPTOMATIC PATIENT BE EVALUATED FURTHER?
Endocrine Society guidelines2 recommend that all patients with pituitary incidentaloma, with or without symptoms, should undergo a complete history and physical examination and laboratory evaluation to exclude hypersecretion and hyposecretion of pituitary hormones.
The “classic” presentation of pituitary hormone hypersecretion—in the form of prolactinoma, adrenocorticotropic hormone (ACTH) excess (Cushing disease), growth hormone (GH) excess (gigantism/acromegaly), and TSH excess (secondary hyperthyroidism)—may be readily detectable on history and physical examination. Subtle cases, so-called subclinical disease, however, may exhibit little or no signs and symptoms initially but can be detrimental to the patient’s health if left untreated. For example, the estimated time from onset to diagnosis of acromegaly is approximately seven to 10 years—a delay that can significantly impact the patient’s morbidity and mortality.3
Prolactinoma can be more clinically apparent in premenopausal females due to irregular menstrual cycles (oligomenorrhea/amenorrhea). However, galactorrhea, or “milky” nipple discharge, occurs in only about 50% of women with prolactinoma and is extremely rare in men. Furthermore, the clinical presentation of prolactinoma in men is vague and related to hypogonadism, resulting from increased prolactin levels. Since men are essentially asymptomatic, these tumors can grow extensively (macroadenoma) and cause “mass effect,” such as headaches and visual impairment.
Therefore, without laboratory testing, abnormal pituitary function may go unrecognized.
WHAT LABS SHOULD I ORDER FOR THIS PATIENT?
Guidelines suggest an initial screening panel of prolactin, GH, insulin-like growth factor 1 (IGF-1), ACTH, early-morning cortisol, TSH, free T4, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone.2
Note the use of “suggest” rather than “recommend.” Even among guideline task force members, there were differences in opinion as to whether certain tests (eg, TSH, LH, and FSH) should be included in initial screening. Those tests can be ordered at the clinician’s discretion, according to the level of suspicion, or can be added later if necessary.
Brian’s sample for laboratory testing is drawn at 7:50 am. Results can be found in the table (previous page).
Next page: Caveats and concerns >>
ARE THERE ANY CAVEATS TO THE INTERPRETATION OF LAB VALUES?
It is important to note that in postmenopausal women who are not taking hormone replacement therapy, LH and FSH will be elevated and estradiol may provide an additional clue in the detection of abnormal function. Conversely, low LH and FSH in postmenopausal women should raise a flag for hypopituitarism.
Another caveat is that GH secretion is pulsatile and serum levels are undetectable between pulses. Therefore, low/undetectable GH does not necessarily suggest deficiency. The GH measurement would only be helpful if it is significantly elevated (suggestive of hypersecretion—gigantism/acromegaly). Otherwise, GH has little value as a screening test.
Instead, IGF-1, which is secreted from the liver in response to GH secretion, has a longer half-life and serves as a better screening tool. IGF-1 has age- and sex-adjusted reference ranges, which are often reported by the lab or given as a Z score.
WHAT IS THE PREFERRED IMAGING STUDY FOR THE PITUITARY GLAND?
The best choice is MRI of the pituitary gland (not the whole brain) with gadolinium. If the incidentaloma was initially diagnosed by a CT, additional testing with MRI should be performed, unless contraindicated.2
Brian is referred for MRI with gadolinium. The radiologist’s report describes a 5 x 4 x 4–mm pituitary microadenoma without sellar extension or involvement of the optic chiasm.
AT WHAT POINT SHOULD OPTIC CHIASM BE A CONCERN?
Since the pituitary gland is located directly beneath the optic chiasm, any compressive effect of growth against the optic nerve(s) can cause visual impairment. This includes bitemporal hemianopsia (loss of peripheral vision) or ophthalmoplegia (abnormal movement of the ocular muscle). Since clinical signs and symptoms can be subtle or absent, all patients with evidence of a pituitary lesion abutting or compressing the optic chiasm should have a formal visual field exam.2
Continue for surgical intervention >>
WHO REQUIRES SURGICAL INTERVENTION?
Patients with mass effect (headache, increased intracranial pressure, compromised optic chiasm) and those with hyperfunctioning nonprolactin adenomas, some (but not all) macroprolactinomas, or pituitary apoplexy should be referred for surgery.2 Almost all cases involve macroadenomas rather than microadenomas.
The preferred treatment for GH-secreting tumors and ACTH-secreting tumors is surgery. However, prolactinoma can be well controlled with pharmacologic agents (dopamine agonists) in most cases. For prolactinomas refractory to these medications, surgical resection is recommended. (Detailed treatment approaches are available elsewhere; those for hyperprolactinoma can be found on the Clinician Reviews website: http://bit.ly/1HOb9Jf.)
Pituitary apoplexy, a life-threatening emergency that requires prompt surgical decompression, is an infarction of the gland due to abrupt cessation of the blood supply, caused by either pituitary artery hemorrhage or sudden hypovolemia. Increased blood supply is needed due to the extra tissue and volume of the pituitary mass; this may stress the pituitary arteries, which are not equipped for this increased flow, causing them to rupture. Hemorrhage anywhere else in the body can lead to hypovolemia and decrease the blood supply to the pituitary gland. A classic example would be postpartum hemorrhage causing pituitary infarct, called Sheehan syndrome.
Due to increased estrogen levels, the pituitary gland doubles in size during pregnancy.4 A preexisting mass may further develop and compress the optic chiasm. Therefore, women of childbearing age should be engaged in discussion of the potential risks and benefits of decompression surgery before actively pursuing pregnancy—especially if the lesion is close to the optic chiasm.
Surgery can also be considered for patients with significant growth in adenoma size during monitoring, loss of endocrinologic function due to mass effect on other pituitary cells, or unremitting headache.2
Next: How should patients be monitored?
HOW SHOULD PATIENTS BE MONITORED?
Those who do not meet criteria for surgery can be closely monitored with periodic testing. Imaging can be repeated six months after the first scan for macroadenoma and in one year for microadenoma. If there is no change in the size of the mass, imaging can be done yearly for macroadenoma and for microadenoma, every one to two years for three years and then gradually less often thereafter.2
Unless the lesion is abutting the optic chiasm (seen via imaging) or the patient reports symptoms, visual field testing does not need to be repeated.
Lab testing should be repeated six months after initial testing for macroadenoma and yearly thereafter. No further testing is suggested for nonsecretory microadenoma, unless clinically indicated.2
If there are any changes in status—noted clinically or via imaging—more frequent testing is suggested.
Brian is reassured that pituitary adenoma is not an uncommon finding and that his adenoma is relatively small in size and nonsecretory. Repeat pituitary MRI in one year is recommended.
CONCLUSION
Most pituitary incidentalomas have no consequences to a patient’s health. However, patients often become highly anxious about the “brain tumor” they were told they have. Appropriate patient education and thorough evaluation can reassure patients and alleviate their concerns.
REFERENCES
1. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37(1):151-171.
2. Freda PU, Beckers AM, Katznelson L, et al. Pituitary incidentaloma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(4):894-904.
3. Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists Medical Guidelines For Clinical Practice For The Diagnosis And Treatment Of Acromegaly–2011 Update. Endocr Pract. 2011;17(suppl 4).
4. Jameson JL. Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010:16-49.
Teledermoscopy referrals surpass paper for managing skin cancer patients
Smartphone teledermoscopy referrals were faster and allowed for more efficient management of patients with skin cancer, compared with paper referrals, according to Dr. Alexander Börve of the University of Gothenburg, Sweden, and his associates.
The waiting time was significantly shorter using teledermoscopy for patients with various melanomas and carcinomas when surgical treatment was necessary. “Triage decisions were also more reliable with teledermoscopy, and over 40% of the teledermoscopy patients could potentially have avoided face-to-face visits,” the researchers noted (Acta. Derm. Venereol. 2015;95:186-90).
Less than 1% of teledermoscopy referrals were excluded because of poor image quality, they said.
Read the full article at Acta Dermato-Venereologica (doi:10.2340/00015555-1906).
Smartphone teledermoscopy referrals were faster and allowed for more efficient management of patients with skin cancer, compared with paper referrals, according to Dr. Alexander Börve of the University of Gothenburg, Sweden, and his associates.
The waiting time was significantly shorter using teledermoscopy for patients with various melanomas and carcinomas when surgical treatment was necessary. “Triage decisions were also more reliable with teledermoscopy, and over 40% of the teledermoscopy patients could potentially have avoided face-to-face visits,” the researchers noted (Acta. Derm. Venereol. 2015;95:186-90).
Less than 1% of teledermoscopy referrals were excluded because of poor image quality, they said.
Read the full article at Acta Dermato-Venereologica (doi:10.2340/00015555-1906).
Smartphone teledermoscopy referrals were faster and allowed for more efficient management of patients with skin cancer, compared with paper referrals, according to Dr. Alexander Börve of the University of Gothenburg, Sweden, and his associates.
The waiting time was significantly shorter using teledermoscopy for patients with various melanomas and carcinomas when surgical treatment was necessary. “Triage decisions were also more reliable with teledermoscopy, and over 40% of the teledermoscopy patients could potentially have avoided face-to-face visits,” the researchers noted (Acta. Derm. Venereol. 2015;95:186-90).
Less than 1% of teledermoscopy referrals were excluded because of poor image quality, they said.
Read the full article at Acta Dermato-Venereologica (doi:10.2340/00015555-1906).
Residents reluctant to recommend DNR to patients
BOSTON – Medical residents in the United States appear to understand that cardiopulmonary resuscitation or intubation is highly unlikely to benefit patients with advanced cancers at the end of life, but the majority of residents surveyed said that they do not discuss code-status options or potentially beneficial palliative care with their dying patients.
“This was primarily due to residents’ perceptions of patient autonomy: Residents wanted patients to make their own decisions, without any influence from the doctor, which misses the concept of informed decision making. These incomplete discussions can cause at minimum improper documentation of patients’ wishes, and at most psychological harm, damage to the physician-patient relationship, and the potential for unwanted attempts at resuscitation,” said Dr. David J. Einstein, a resident at Beth Israel Deaconess Medical Center and Tufts Medical Center, both in Boston.
Despite their reluctance to have the discussion, however, the majority of residents said they preferred to discuss code status with patients themselves rather than hand it off to the attending physician, primarily out of a sense that it is their responsibility as physicians.
Yet these physicians in training did not seem to feel that they were also responsible for providing guidance to patients, Dr. Einstein said at the Palliative Care in Oncology Symposium.
“We felt that this represented an unmet need in training and practice. Residents and attendings should be providing guidance on all medical interventions, including CPR, and if they aren’t sure what to recommend, then they themselves should be seeking guidance from other experts, before asking a patient to falsely choose between an intervention and death,” he said.
The first discussion of code status – do not resuscitate (DNR) or do not intubate (DNI) – may occur in the hospital, and is often left to a resident physician. Ideally, the physician and patient should discuss the patient’s prognosis, goals for care, evaluation of CPR as a means of meeting those goals, and a recommendation.
But many residents lack training in the end-of-life discussion, which can have a significant impact on the quality of the patients’ remaining weeks or months of life.
Nationwide survey
Dr. Einstein and his colleagues conducted a nationwide survey to measure the likelihood that residents would discuss prognostic information and offer recommendations to patients with limited life expectancy. They also sought to determine why residents might be reluctant to provide discussion, and to evaluate their satisfaction with code-status discussions that both they and their attending physicians have conducted.
The survey presented respondents with a hypothetical case of a patient with stage IV adenocarcinoma of the lung metastatic to the brain. The patient, who has disease progression despite receiving first- and second-line therapy, presents to the emergency department with dyspnea and is slightly hypoxemic, but is not in distress. The patient has not previously established a code-status preference.
The investigators contacted 387 residency program directors by mail, 19 of whom agreed to participate and responded. They sent surveys to a total of 1,627 residents, 358 of which were completed and included.
The investigators found that slightly less than half of the respondents said they would share information with the patient about his/her prognosis and the relative benefit of CPR, and more than two-thirds said they would be unlikely to offer a specific recommendation.
“So even in the situation with a clearly declining patient, residents were as likely as not to provide the information needed to make an informed decision, and were far less likely to provide guidance on this decision,” Dr. Einstein said.
Asked the reason for their decisions, 69% of the residents who would not offer a recommendation said that the patient should make his/her own decision without any influence, and 26.5% said that the attending would not want them to offer a recommendation. Nonetheless, only 1.3% of this group said they believed that CPR would offer the patient a reasonable chance of resuscitation.
The majority of respondents who would offer a recommendation (93.5%) said they would recommend DNR and DNI.
Code-status talk a ‘responsibility’
When they were asked whether they would prefer the attending to discuss code status, nearly 70% of respondents disagreed.
Of those residents who said they preferred to retain the code-status discussion, 93.4% said they thought it was part of their responsibility as a physician, and 65.8% said they thought they had sufficient training and knowledge to do it. A minority in this group (2.5%) said that they would be likely to disagree with the attending’s estimate of prognosis, and 4.9% said they thought the attending would not share his/her estimate honestly.
When the authors asked about the residents’ general satisfaction with discussion of code status, “we learned two things: One, the residents are significantly more satisfied with their own discussions than their attendings’ discussions; and two, there is a substantial minority that is dissatisfied with all discussions, and a small number who are actually very satisfied,” Dr. Einstein said.
In a linear regression analysis testing for hypothesized correlations, the investigators found that more-senior residents were more likely to share prognostic information and make recommendations (P = .002). Residents who expressed an interest in hematology/oncology or palliative care specialization were also more likely to offer prognostic information, but not to make a recommendation about code status.
More-senior year of training correlated negatively with satisfaction with both the resident’s own and the attending’s discussion of code status.
“We found substantial dissatisfaction with code-status discussions in general, and we hypothesize that this is due to an internal conflict. When a resident knows that an intervention may be more harmful than beneficial, but thinks that the patient should make their own decision alone, then one may experience substantial frustration, and this would increase as training goes on and one becomes more sure of the outcomes of interventions like CPR,” Dr. Einstein said.
Generation gap
Evoking a potential generation gap between old-school doctors and the up-and-coming young physicians who by statute work fewer hours than their mentors had to, “I’m struck that [residents] don’t trust the attendings. When I was a resident, you didn’t do anything without asking the attending,” said Dr. Michael H. Levy, an invited discussant who is vice chair of medical oncology and director of the pain and palliative care program at Fox Chase Cancer Center, Philadelphia.
“I’m glad that the residents want to do it, but they have the same arrogance/ignorance that they don’t know how, so if we want them to do it, we have to train them,” he said. The symposium was cosponsored by AAHPM, ASCO, ASTRO, and MASCC. The study was supported in part by the Conquer Cancer Foundation. Dr. Einstein and Dr. Levy reported having no relevant disclosures.
Can you imagine a health care system where your treating physician cannot offer their patients information on code-status and end of life decisions? Seems somewhat preposterous, but in a national survey in the United States, more than two-thirds of medical residents said they would not offer end-of-life code-status prognostic information and recommendations to their patients with limited life expectancy. Granted that medical residents thought that invasive interventions in an individual with limited life expectancy were not warranted; they wanted patients to make these decisions on their own without influence from health care professionals. Although patient autonomy is one of the pillars in medical ethics, decisions made without undue influence should be made with the most accurate information at hand.
 |
| Dr. Laura Drudi |
In vascular surgery, specialists are performing moderate- to high-risk interventions on an exponentially growing elderly population plagued with a significant cardiovascular disease burden. It is therefore essential that physicians, where appropriate, discuss end-of-life and code status with their patients. In my experience, surgical residents are inexperienced when it comes to having these dialogues with patients largely because there are no teaching opportunities to learn these communication skills. Further, there are few opportunities to learn by observing these sensitive discussions that are often performed out of earshot. Currently, there is an unmet need in training programs when it comes to providing appropriate guidance to residents who may need to have these difficult discussions.
It is our responsibility as medical professionals to provide the best medical as well as end of life care. Residents who are appropriately trained to participate in code- status discussions and end-of-life decisions will enable our patients to make well-advised and truly autonomous decisions about their ultimate care.
Dr. Laura Drudi is the resident medical editor for Vascular Specialist.
Can you imagine a health care system where your treating physician cannot offer their patients information on code-status and end of life decisions? Seems somewhat preposterous, but in a national survey in the United States, more than two-thirds of medical residents said they would not offer end-of-life code-status prognostic information and recommendations to their patients with limited life expectancy. Granted that medical residents thought that invasive interventions in an individual with limited life expectancy were not warranted; they wanted patients to make these decisions on their own without influence from health care professionals. Although patient autonomy is one of the pillars in medical ethics, decisions made without undue influence should be made with the most accurate information at hand.
 |
| Dr. Laura Drudi |
In vascular surgery, specialists are performing moderate- to high-risk interventions on an exponentially growing elderly population plagued with a significant cardiovascular disease burden. It is therefore essential that physicians, where appropriate, discuss end-of-life and code status with their patients. In my experience, surgical residents are inexperienced when it comes to having these dialogues with patients largely because there are no teaching opportunities to learn these communication skills. Further, there are few opportunities to learn by observing these sensitive discussions that are often performed out of earshot. Currently, there is an unmet need in training programs when it comes to providing appropriate guidance to residents who may need to have these difficult discussions.
It is our responsibility as medical professionals to provide the best medical as well as end of life care. Residents who are appropriately trained to participate in code- status discussions and end-of-life decisions will enable our patients to make well-advised and truly autonomous decisions about their ultimate care.
Dr. Laura Drudi is the resident medical editor for Vascular Specialist.
Can you imagine a health care system where your treating physician cannot offer their patients information on code-status and end of life decisions? Seems somewhat preposterous, but in a national survey in the United States, more than two-thirds of medical residents said they would not offer end-of-life code-status prognostic information and recommendations to their patients with limited life expectancy. Granted that medical residents thought that invasive interventions in an individual with limited life expectancy were not warranted; they wanted patients to make these decisions on their own without influence from health care professionals. Although patient autonomy is one of the pillars in medical ethics, decisions made without undue influence should be made with the most accurate information at hand.
 |
| Dr. Laura Drudi |
In vascular surgery, specialists are performing moderate- to high-risk interventions on an exponentially growing elderly population plagued with a significant cardiovascular disease burden. It is therefore essential that physicians, where appropriate, discuss end-of-life and code status with their patients. In my experience, surgical residents are inexperienced when it comes to having these dialogues with patients largely because there are no teaching opportunities to learn these communication skills. Further, there are few opportunities to learn by observing these sensitive discussions that are often performed out of earshot. Currently, there is an unmet need in training programs when it comes to providing appropriate guidance to residents who may need to have these difficult discussions.
It is our responsibility as medical professionals to provide the best medical as well as end of life care. Residents who are appropriately trained to participate in code- status discussions and end-of-life decisions will enable our patients to make well-advised and truly autonomous decisions about their ultimate care.
Dr. Laura Drudi is the resident medical editor for Vascular Specialist.
BOSTON – Medical residents in the United States appear to understand that cardiopulmonary resuscitation or intubation is highly unlikely to benefit patients with advanced cancers at the end of life, but the majority of residents surveyed said that they do not discuss code-status options or potentially beneficial palliative care with their dying patients.
“This was primarily due to residents’ perceptions of patient autonomy: Residents wanted patients to make their own decisions, without any influence from the doctor, which misses the concept of informed decision making. These incomplete discussions can cause at minimum improper documentation of patients’ wishes, and at most psychological harm, damage to the physician-patient relationship, and the potential for unwanted attempts at resuscitation,” said Dr. David J. Einstein, a resident at Beth Israel Deaconess Medical Center and Tufts Medical Center, both in Boston.
Despite their reluctance to have the discussion, however, the majority of residents said they preferred to discuss code status with patients themselves rather than hand it off to the attending physician, primarily out of a sense that it is their responsibility as physicians.
Yet these physicians in training did not seem to feel that they were also responsible for providing guidance to patients, Dr. Einstein said at the Palliative Care in Oncology Symposium.
“We felt that this represented an unmet need in training and practice. Residents and attendings should be providing guidance on all medical interventions, including CPR, and if they aren’t sure what to recommend, then they themselves should be seeking guidance from other experts, before asking a patient to falsely choose between an intervention and death,” he said.
The first discussion of code status – do not resuscitate (DNR) or do not intubate (DNI) – may occur in the hospital, and is often left to a resident physician. Ideally, the physician and patient should discuss the patient’s prognosis, goals for care, evaluation of CPR as a means of meeting those goals, and a recommendation.
But many residents lack training in the end-of-life discussion, which can have a significant impact on the quality of the patients’ remaining weeks or months of life.
Nationwide survey
Dr. Einstein and his colleagues conducted a nationwide survey to measure the likelihood that residents would discuss prognostic information and offer recommendations to patients with limited life expectancy. They also sought to determine why residents might be reluctant to provide discussion, and to evaluate their satisfaction with code-status discussions that both they and their attending physicians have conducted.
The survey presented respondents with a hypothetical case of a patient with stage IV adenocarcinoma of the lung metastatic to the brain. The patient, who has disease progression despite receiving first- and second-line therapy, presents to the emergency department with dyspnea and is slightly hypoxemic, but is not in distress. The patient has not previously established a code-status preference.
The investigators contacted 387 residency program directors by mail, 19 of whom agreed to participate and responded. They sent surveys to a total of 1,627 residents, 358 of which were completed and included.
The investigators found that slightly less than half of the respondents said they would share information with the patient about his/her prognosis and the relative benefit of CPR, and more than two-thirds said they would be unlikely to offer a specific recommendation.
“So even in the situation with a clearly declining patient, residents were as likely as not to provide the information needed to make an informed decision, and were far less likely to provide guidance on this decision,” Dr. Einstein said.
Asked the reason for their decisions, 69% of the residents who would not offer a recommendation said that the patient should make his/her own decision without any influence, and 26.5% said that the attending would not want them to offer a recommendation. Nonetheless, only 1.3% of this group said they believed that CPR would offer the patient a reasonable chance of resuscitation.
The majority of respondents who would offer a recommendation (93.5%) said they would recommend DNR and DNI.
Code-status talk a ‘responsibility’
When they were asked whether they would prefer the attending to discuss code status, nearly 70% of respondents disagreed.
Of those residents who said they preferred to retain the code-status discussion, 93.4% said they thought it was part of their responsibility as a physician, and 65.8% said they thought they had sufficient training and knowledge to do it. A minority in this group (2.5%) said that they would be likely to disagree with the attending’s estimate of prognosis, and 4.9% said they thought the attending would not share his/her estimate honestly.
When the authors asked about the residents’ general satisfaction with discussion of code status, “we learned two things: One, the residents are significantly more satisfied with their own discussions than their attendings’ discussions; and two, there is a substantial minority that is dissatisfied with all discussions, and a small number who are actually very satisfied,” Dr. Einstein said.
In a linear regression analysis testing for hypothesized correlations, the investigators found that more-senior residents were more likely to share prognostic information and make recommendations (P = .002). Residents who expressed an interest in hematology/oncology or palliative care specialization were also more likely to offer prognostic information, but not to make a recommendation about code status.
More-senior year of training correlated negatively with satisfaction with both the resident’s own and the attending’s discussion of code status.
“We found substantial dissatisfaction with code-status discussions in general, and we hypothesize that this is due to an internal conflict. When a resident knows that an intervention may be more harmful than beneficial, but thinks that the patient should make their own decision alone, then one may experience substantial frustration, and this would increase as training goes on and one becomes more sure of the outcomes of interventions like CPR,” Dr. Einstein said.
Generation gap
Evoking a potential generation gap between old-school doctors and the up-and-coming young physicians who by statute work fewer hours than their mentors had to, “I’m struck that [residents] don’t trust the attendings. When I was a resident, you didn’t do anything without asking the attending,” said Dr. Michael H. Levy, an invited discussant who is vice chair of medical oncology and director of the pain and palliative care program at Fox Chase Cancer Center, Philadelphia.
“I’m glad that the residents want to do it, but they have the same arrogance/ignorance that they don’t know how, so if we want them to do it, we have to train them,” he said. The symposium was cosponsored by AAHPM, ASCO, ASTRO, and MASCC. The study was supported in part by the Conquer Cancer Foundation. Dr. Einstein and Dr. Levy reported having no relevant disclosures.
BOSTON – Medical residents in the United States appear to understand that cardiopulmonary resuscitation or intubation is highly unlikely to benefit patients with advanced cancers at the end of life, but the majority of residents surveyed said that they do not discuss code-status options or potentially beneficial palliative care with their dying patients.
“This was primarily due to residents’ perceptions of patient autonomy: Residents wanted patients to make their own decisions, without any influence from the doctor, which misses the concept of informed decision making. These incomplete discussions can cause at minimum improper documentation of patients’ wishes, and at most psychological harm, damage to the physician-patient relationship, and the potential for unwanted attempts at resuscitation,” said Dr. David J. Einstein, a resident at Beth Israel Deaconess Medical Center and Tufts Medical Center, both in Boston.
Despite their reluctance to have the discussion, however, the majority of residents said they preferred to discuss code status with patients themselves rather than hand it off to the attending physician, primarily out of a sense that it is their responsibility as physicians.
Yet these physicians in training did not seem to feel that they were also responsible for providing guidance to patients, Dr. Einstein said at the Palliative Care in Oncology Symposium.
“We felt that this represented an unmet need in training and practice. Residents and attendings should be providing guidance on all medical interventions, including CPR, and if they aren’t sure what to recommend, then they themselves should be seeking guidance from other experts, before asking a patient to falsely choose between an intervention and death,” he said.
The first discussion of code status – do not resuscitate (DNR) or do not intubate (DNI) – may occur in the hospital, and is often left to a resident physician. Ideally, the physician and patient should discuss the patient’s prognosis, goals for care, evaluation of CPR as a means of meeting those goals, and a recommendation.
But many residents lack training in the end-of-life discussion, which can have a significant impact on the quality of the patients’ remaining weeks or months of life.
Nationwide survey
Dr. Einstein and his colleagues conducted a nationwide survey to measure the likelihood that residents would discuss prognostic information and offer recommendations to patients with limited life expectancy. They also sought to determine why residents might be reluctant to provide discussion, and to evaluate their satisfaction with code-status discussions that both they and their attending physicians have conducted.
The survey presented respondents with a hypothetical case of a patient with stage IV adenocarcinoma of the lung metastatic to the brain. The patient, who has disease progression despite receiving first- and second-line therapy, presents to the emergency department with dyspnea and is slightly hypoxemic, but is not in distress. The patient has not previously established a code-status preference.
The investigators contacted 387 residency program directors by mail, 19 of whom agreed to participate and responded. They sent surveys to a total of 1,627 residents, 358 of which were completed and included.
The investigators found that slightly less than half of the respondents said they would share information with the patient about his/her prognosis and the relative benefit of CPR, and more than two-thirds said they would be unlikely to offer a specific recommendation.
“So even in the situation with a clearly declining patient, residents were as likely as not to provide the information needed to make an informed decision, and were far less likely to provide guidance on this decision,” Dr. Einstein said.
Asked the reason for their decisions, 69% of the residents who would not offer a recommendation said that the patient should make his/her own decision without any influence, and 26.5% said that the attending would not want them to offer a recommendation. Nonetheless, only 1.3% of this group said they believed that CPR would offer the patient a reasonable chance of resuscitation.
The majority of respondents who would offer a recommendation (93.5%) said they would recommend DNR and DNI.
Code-status talk a ‘responsibility’
When they were asked whether they would prefer the attending to discuss code status, nearly 70% of respondents disagreed.
Of those residents who said they preferred to retain the code-status discussion, 93.4% said they thought it was part of their responsibility as a physician, and 65.8% said they thought they had sufficient training and knowledge to do it. A minority in this group (2.5%) said that they would be likely to disagree with the attending’s estimate of prognosis, and 4.9% said they thought the attending would not share his/her estimate honestly.
When the authors asked about the residents’ general satisfaction with discussion of code status, “we learned two things: One, the residents are significantly more satisfied with their own discussions than their attendings’ discussions; and two, there is a substantial minority that is dissatisfied with all discussions, and a small number who are actually very satisfied,” Dr. Einstein said.
In a linear regression analysis testing for hypothesized correlations, the investigators found that more-senior residents were more likely to share prognostic information and make recommendations (P = .002). Residents who expressed an interest in hematology/oncology or palliative care specialization were also more likely to offer prognostic information, but not to make a recommendation about code status.
More-senior year of training correlated negatively with satisfaction with both the resident’s own and the attending’s discussion of code status.
“We found substantial dissatisfaction with code-status discussions in general, and we hypothesize that this is due to an internal conflict. When a resident knows that an intervention may be more harmful than beneficial, but thinks that the patient should make their own decision alone, then one may experience substantial frustration, and this would increase as training goes on and one becomes more sure of the outcomes of interventions like CPR,” Dr. Einstein said.
Generation gap
Evoking a potential generation gap between old-school doctors and the up-and-coming young physicians who by statute work fewer hours than their mentors had to, “I’m struck that [residents] don’t trust the attendings. When I was a resident, you didn’t do anything without asking the attending,” said Dr. Michael H. Levy, an invited discussant who is vice chair of medical oncology and director of the pain and palliative care program at Fox Chase Cancer Center, Philadelphia.
“I’m glad that the residents want to do it, but they have the same arrogance/ignorance that they don’t know how, so if we want them to do it, we have to train them,” he said. The symposium was cosponsored by AAHPM, ASCO, ASTRO, and MASCC. The study was supported in part by the Conquer Cancer Foundation. Dr. Einstein and Dr. Levy reported having no relevant disclosures.
Ibuprofen superior to morphine following pediatric tonsillectomy
Ibuprofen appears to be an effective and safer pain reliever than morphine in children undergoing tonsillectomies, according to a recent study.
Although the two medications, administered with acetaminophen, treated pain about equally, morphine showed a greater risk for oxygen desaturation the night after surgery.
“Given the unpredictable posttonsillectomy respiratory response to opioids (codeine, morphine, and hydrocodone) and the analgesic effectiveness of ibuprofen, perhaps the time has come to question the postoperative use of all opioids in this population,” wrote Lauren Kelly, Ph.D., of Western University in London, Ontario, and her associates. The study was published online Jan. 26 (Pediatrics 2014 Jan. 26 [doi: 10.1542/peds.2014-1906].
The researchers randomized 91 children aged 1-10 years to acetaminophen (10-15 mg/kg per dose every 4 hours) with either an age appropriate dose of 0.2-0.5 mg/kg oral morphine every 4 hours or 10 mg/kg of oral ibuprofen every 6 hours after the children underwent a tonsillectomy with or without an adenoidectomy to treat sleep disordered breathing. The study ran from September 2012 to January 2014.
Parents put pulse oximeters on their children the nights before and after surgery to monitor oxygen saturation and apnea events.
The first evening after the surgery, 68% of children receiving ibuprofen showed improvement in oxygen desaturations, compared with 14% of children receiving morphine. The children receiving ibuprofen experienced an average 1.79 fewer desaturation events per hour, compared to an average 11.17 more desaturation events per hour in the morphine group.
No differences in average postsurgical oxygen saturation, pain relieving effectiveness, tonsillar bleeding or drug adverse events were identified.
“The results of this study support effective posttonsillectomy analgesia in children by using ibuprofen in combination with acetaminophen,” Dr. Kelly and her team wrote.
Ibuprofen appears to be an effective and safer pain reliever than morphine in children undergoing tonsillectomies, according to a recent study.
Although the two medications, administered with acetaminophen, treated pain about equally, morphine showed a greater risk for oxygen desaturation the night after surgery.
“Given the unpredictable posttonsillectomy respiratory response to opioids (codeine, morphine, and hydrocodone) and the analgesic effectiveness of ibuprofen, perhaps the time has come to question the postoperative use of all opioids in this population,” wrote Lauren Kelly, Ph.D., of Western University in London, Ontario, and her associates. The study was published online Jan. 26 (Pediatrics 2014 Jan. 26 [doi: 10.1542/peds.2014-1906].
The researchers randomized 91 children aged 1-10 years to acetaminophen (10-15 mg/kg per dose every 4 hours) with either an age appropriate dose of 0.2-0.5 mg/kg oral morphine every 4 hours or 10 mg/kg of oral ibuprofen every 6 hours after the children underwent a tonsillectomy with or without an adenoidectomy to treat sleep disordered breathing. The study ran from September 2012 to January 2014.
Parents put pulse oximeters on their children the nights before and after surgery to monitor oxygen saturation and apnea events.
The first evening after the surgery, 68% of children receiving ibuprofen showed improvement in oxygen desaturations, compared with 14% of children receiving morphine. The children receiving ibuprofen experienced an average 1.79 fewer desaturation events per hour, compared to an average 11.17 more desaturation events per hour in the morphine group.
No differences in average postsurgical oxygen saturation, pain relieving effectiveness, tonsillar bleeding or drug adverse events were identified.
“The results of this study support effective posttonsillectomy analgesia in children by using ibuprofen in combination with acetaminophen,” Dr. Kelly and her team wrote.
Ibuprofen appears to be an effective and safer pain reliever than morphine in children undergoing tonsillectomies, according to a recent study.
Although the two medications, administered with acetaminophen, treated pain about equally, morphine showed a greater risk for oxygen desaturation the night after surgery.
“Given the unpredictable posttonsillectomy respiratory response to opioids (codeine, morphine, and hydrocodone) and the analgesic effectiveness of ibuprofen, perhaps the time has come to question the postoperative use of all opioids in this population,” wrote Lauren Kelly, Ph.D., of Western University in London, Ontario, and her associates. The study was published online Jan. 26 (Pediatrics 2014 Jan. 26 [doi: 10.1542/peds.2014-1906].
The researchers randomized 91 children aged 1-10 years to acetaminophen (10-15 mg/kg per dose every 4 hours) with either an age appropriate dose of 0.2-0.5 mg/kg oral morphine every 4 hours or 10 mg/kg of oral ibuprofen every 6 hours after the children underwent a tonsillectomy with or without an adenoidectomy to treat sleep disordered breathing. The study ran from September 2012 to January 2014.
Parents put pulse oximeters on their children the nights before and after surgery to monitor oxygen saturation and apnea events.
The first evening after the surgery, 68% of children receiving ibuprofen showed improvement in oxygen desaturations, compared with 14% of children receiving morphine. The children receiving ibuprofen experienced an average 1.79 fewer desaturation events per hour, compared to an average 11.17 more desaturation events per hour in the morphine group.
No differences in average postsurgical oxygen saturation, pain relieving effectiveness, tonsillar bleeding or drug adverse events were identified.
“The results of this study support effective posttonsillectomy analgesia in children by using ibuprofen in combination with acetaminophen,” Dr. Kelly and her team wrote.
FROM PEDIATRICS
Key clinical point: Ibuprofen safely and effectively replaces morphine for children’s pain relief following tonsillectomy.
Major finding: Among children receiving ibuprofen, 68% improved the first night post surgery, compared with 14% of children receiving morphine.
Data source: A prospective randomized clinical trial of 91 children, aged 1-10 years, assigned to receive 10-15 mg/kg acetaminophen with either 0.2-0.5 mg/kg oral morphine or 10 mg/kg of oral ibuprofen following a tonsillectomy with or without adenoidectomy.
Disclosures: The Canadian Institutes for Health Research Drug Safety and Effectiveness Network funded the study. The authors reported no relevant financial disclosures.
Deaths in young leukemia patients on decline in UK

Credit: Bill Branson
Cancer deaths in children, adolescents, and young adults in the UK have fallen by 58% in the past 40 years, according to new figures from Cancer Research UK.*
Among cancer patients age 24 and under, the number of deaths each year dropped from about 1300 in the mid-1970s (1975-1977) to around 550 in more recent years (2010-2012).
And patients with leukemia have seen the steepest decline in mortality.
This is key because, according to Cancer Research UK, leukemia is the most commonly diagnosed cancer in children aged 14 and under and the sixth most commonly diagnosed cancer among 15- to 24-year-olds.
“These figures are testament to the real progress we’re making in treating children and young people with cancer,” said Pam Kearns, PhD, director of the Cancer Research UK Clinical Trials Unit in Birmingham.
“But hundreds of young people are dying from cancer each year in the UK, which means there’s still much more we need to do.”
Around 1600 children and 2200 adolescents and young adults are diagnosed with cancer every year in the UK.
And cancer remains the biggest killer of children and young people in the UK. It is the most common cause of death in children and young women aged 15 to 24 and the fourth most common cause of death in young men aged 15 to 24.
Still, the latest figures from Cancer Research UK show progress.
The mortality rate for all cancer patients ages 0 to 24 fell from 63.7 per million in 1975-1977 to 36.7 per million in 2001-2003 and then to 27.9 per million in 2010-2012. So the mortality rate has fallen 56% since 1975 and 24% in the last decade.
The average number of cancer deaths among children age 0 to 24 fell from 1325 in 1975-1977 to 681 in 2001-2003 and then to 550 in 2010-2012. That translates to a 58% decrease in deaths from 1975 and a 19% decrease in the last decade.
For leukemia patients ages 0 to 24, the mortality rate fell from 9.6 per million in 2001-2003 to 5.6 per million in 2010-2012, which translates to a 42% decrease. The annual average number of deaths dropped from 179 in 2001-2003 to 110 in 2010-2012, which translates to a 39% decrease.
“Cancer causes more deaths among children and young people than any other disease in the UK, so it’s hugely encouraging to see that death toll now falling steadily,” said Harpal Kumar, DSc, Cancer Research UK’s chief executive.
“But as the largest funder of research into children’s cancers in the UK, we will keep going until no young lives are lost to cancer.”
To that end, Cancer Research UK has launched “Cancer Research UK Kids & Teens,” an ongoing campaign to fund more research to find better treatments for young cancer patients.
“Money raised by ‘Cancer Research UK Kids & Teens’ will be restricted to research into cancers affecting children, teenagers, and young adults, enabling us to better understand these cancers and find better and kinder treatments and cures,” Dr Kumar said. “In the next 5 to 10 years, Cancer Research UK hopes to double the amount it spends on these cancers.” ![]()
*Some of the information in this article is not available on the Cancer Research UK website (and was sent to HematologyTimes upon request), but similar data and additional details are available on the organization’s Cancer Stats page.

Credit: Bill Branson
Cancer deaths in children, adolescents, and young adults in the UK have fallen by 58% in the past 40 years, according to new figures from Cancer Research UK.*
Among cancer patients age 24 and under, the number of deaths each year dropped from about 1300 in the mid-1970s (1975-1977) to around 550 in more recent years (2010-2012).
And patients with leukemia have seen the steepest decline in mortality.
This is key because, according to Cancer Research UK, leukemia is the most commonly diagnosed cancer in children aged 14 and under and the sixth most commonly diagnosed cancer among 15- to 24-year-olds.
“These figures are testament to the real progress we’re making in treating children and young people with cancer,” said Pam Kearns, PhD, director of the Cancer Research UK Clinical Trials Unit in Birmingham.
“But hundreds of young people are dying from cancer each year in the UK, which means there’s still much more we need to do.”
Around 1600 children and 2200 adolescents and young adults are diagnosed with cancer every year in the UK.
And cancer remains the biggest killer of children and young people in the UK. It is the most common cause of death in children and young women aged 15 to 24 and the fourth most common cause of death in young men aged 15 to 24.
Still, the latest figures from Cancer Research UK show progress.
The mortality rate for all cancer patients ages 0 to 24 fell from 63.7 per million in 1975-1977 to 36.7 per million in 2001-2003 and then to 27.9 per million in 2010-2012. So the mortality rate has fallen 56% since 1975 and 24% in the last decade.
The average number of cancer deaths among children age 0 to 24 fell from 1325 in 1975-1977 to 681 in 2001-2003 and then to 550 in 2010-2012. That translates to a 58% decrease in deaths from 1975 and a 19% decrease in the last decade.
For leukemia patients ages 0 to 24, the mortality rate fell from 9.6 per million in 2001-2003 to 5.6 per million in 2010-2012, which translates to a 42% decrease. The annual average number of deaths dropped from 179 in 2001-2003 to 110 in 2010-2012, which translates to a 39% decrease.
“Cancer causes more deaths among children and young people than any other disease in the UK, so it’s hugely encouraging to see that death toll now falling steadily,” said Harpal Kumar, DSc, Cancer Research UK’s chief executive.
“But as the largest funder of research into children’s cancers in the UK, we will keep going until no young lives are lost to cancer.”
To that end, Cancer Research UK has launched “Cancer Research UK Kids & Teens,” an ongoing campaign to fund more research to find better treatments for young cancer patients.
“Money raised by ‘Cancer Research UK Kids & Teens’ will be restricted to research into cancers affecting children, teenagers, and young adults, enabling us to better understand these cancers and find better and kinder treatments and cures,” Dr Kumar said. “In the next 5 to 10 years, Cancer Research UK hopes to double the amount it spends on these cancers.” ![]()
*Some of the information in this article is not available on the Cancer Research UK website (and was sent to HematologyTimes upon request), but similar data and additional details are available on the organization’s Cancer Stats page.

Credit: Bill Branson
Cancer deaths in children, adolescents, and young adults in the UK have fallen by 58% in the past 40 years, according to new figures from Cancer Research UK.*
Among cancer patients age 24 and under, the number of deaths each year dropped from about 1300 in the mid-1970s (1975-1977) to around 550 in more recent years (2010-2012).
And patients with leukemia have seen the steepest decline in mortality.
This is key because, according to Cancer Research UK, leukemia is the most commonly diagnosed cancer in children aged 14 and under and the sixth most commonly diagnosed cancer among 15- to 24-year-olds.
“These figures are testament to the real progress we’re making in treating children and young people with cancer,” said Pam Kearns, PhD, director of the Cancer Research UK Clinical Trials Unit in Birmingham.
“But hundreds of young people are dying from cancer each year in the UK, which means there’s still much more we need to do.”
Around 1600 children and 2200 adolescents and young adults are diagnosed with cancer every year in the UK.
And cancer remains the biggest killer of children and young people in the UK. It is the most common cause of death in children and young women aged 15 to 24 and the fourth most common cause of death in young men aged 15 to 24.
Still, the latest figures from Cancer Research UK show progress.
The mortality rate for all cancer patients ages 0 to 24 fell from 63.7 per million in 1975-1977 to 36.7 per million in 2001-2003 and then to 27.9 per million in 2010-2012. So the mortality rate has fallen 56% since 1975 and 24% in the last decade.
The average number of cancer deaths among children age 0 to 24 fell from 1325 in 1975-1977 to 681 in 2001-2003 and then to 550 in 2010-2012. That translates to a 58% decrease in deaths from 1975 and a 19% decrease in the last decade.
For leukemia patients ages 0 to 24, the mortality rate fell from 9.6 per million in 2001-2003 to 5.6 per million in 2010-2012, which translates to a 42% decrease. The annual average number of deaths dropped from 179 in 2001-2003 to 110 in 2010-2012, which translates to a 39% decrease.
“Cancer causes more deaths among children and young people than any other disease in the UK, so it’s hugely encouraging to see that death toll now falling steadily,” said Harpal Kumar, DSc, Cancer Research UK’s chief executive.
“But as the largest funder of research into children’s cancers in the UK, we will keep going until no young lives are lost to cancer.”
To that end, Cancer Research UK has launched “Cancer Research UK Kids & Teens,” an ongoing campaign to fund more research to find better treatments for young cancer patients.
“Money raised by ‘Cancer Research UK Kids & Teens’ will be restricted to research into cancers affecting children, teenagers, and young adults, enabling us to better understand these cancers and find better and kinder treatments and cures,” Dr Kumar said. “In the next 5 to 10 years, Cancer Research UK hopes to double the amount it spends on these cancers.” ![]()
*Some of the information in this article is not available on the Cancer Research UK website (and was sent to HematologyTimes upon request), but similar data and additional details are available on the organization’s Cancer Stats page.