User login
Prescriber’s guide to using 3 new antidepressants: Vilazodone, levomilnacipran, vortioxetine
With a prevalence >17%, depression is one of the most common mental disorders in the United States and the second leading cause of disability worldwide.1,2 For decades, primary care and mental health providers have used selective serotonin reuptake inhibitors (SSRIs) as first-line treatment for depression—yet the remission rate after the first trial of an antidepressant is <30%, and continues to decline after a first antidepressant failure.3
That is why clinicians continue to seek effective treatments for depression—ones that will provide quick and sustainable remission—and why scientists and pharmaceutical manufacturers have been competing to develop more effective antidepressant medications.
In the past 4 years, the FDA has approved 3 antidepressants—vilazodone, levomilnacipran, and vortioxetine—with the hope of increasing options for patients who suffer from major depression. These 3 antidepressants differ in their mechanisms of action from other available antidepressants, and all have been shown to have acceptable safety and tolerability profiles.
In this article, we review these novel antidepressants and present some clinical pearls for their use. We also present our observations that each agent appears to show particular advantage in a certain subpopulation of depressed patients who often do not respond, or who do not adequately respond, to other antidepressants.
Vilazodone
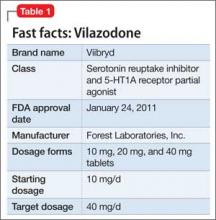
Vilazodone was approved by the FDA in 2011 (Table 1). The drug increases serotonin bioavailability in synapses through a strong dual action:
• blocking serotonin reuptake through the serotonin transporter
• partial agonism of the 5-HT1A presynaptic receptor.
Vilazodone also has a moderate effect on the 5-HT4 receptor and on dopamine and norepinephrine uptake inhibition.
The unique presynaptic 5-HT1A partial agonism of vilazodone is similar to that of buspirone, in which both drugs initially inhibit serotonin synthesis and neuronal firing.4 Researchers therefore expected that vilazodone would be more suitable for patients who have depression and a comorbid anxiety disorder; current FDA approval, however, is for depression only.
Adverse effects. The 5-HT4 receptor on which vilazodone acts is present in the gastrointestinal (GI) tract, and contributes to regulating symptoms in patients with irritable bowel syndrome (IBS)5; not surprisingly, the most frequent adverse effects of vilazodone are GI in nature (diarrhea, nausea, vomiting).
Headache is the most common non- GI side effect of vilazodone. Depressed patients who took vilazodone had no significant weight gain and did not report adverse sexual effects, compared with subjects given placebo.6
The following case—a patient with depression, significant anxiety, and IBS— exemplifies the type of patient for whom we find vilazodone most useful.
CASE Ms. A, age 19, is a college student with a history of major depressive disorder, social anxiety, and panic attacks for 2 years and IBS for 3 years. She was taking lubiprostone for IBS, with incomplete relief of GI symptoms. Because the family history included depression in Ms. A’s mother and sister, and both were doing well on escitalopram, we began a trial of that drug, 10 mg/d, that was quickly titrated to 20 mg/d.
Ms. A did not respond to 20 mg of escitalopram combined with psychotherapy.
We then started vilazodone, 10 mg/d after breakfast, for the first week, and reduced escitalopram to 10 mg/d. During Week 2, escitalopram was discontinued and vilazodone was increased to 20 mg/d. During Week 3, vilazodone was titrated to 40 mg/d.
Ms. A tolerated vilazodone well. Her depressive symptoms improved at the end of Week 2.
Unlike her experience with escitalopram, Ms. A’s anxiety symptoms—tenseness, racing thoughts, and panic attacks—all diminished when she switched to vilazodone. Notably, her IBS symptoms also were relieved, and she discontinued lubiprostone.
Ms. A’s depression remained in remission for 2 years, except for a brief period one summer, when she thought she “could do without any medication.” She tapered the vilazodone, week by week, to 10 mg/d, but her anxiety and bowel symptoms resurfaced to a degree that she resumed the 40-mg/d dosage.
Levomilnacipran
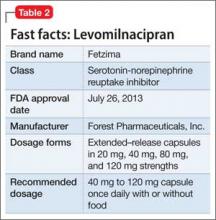
This drug is a 2013 addition to the small serotonin–norepinephrine reuptake inhibitor (SNRI) family of venlafaxine, desvenlafaxine, and duloxetine7 (Table 2). Levomilnacipran is the enantiomer of milnacipran, approved in Europe for depression but only for fibromyalgia pain and peripheral neuropathy in the United States.8 (Levomilnacipran is not FDA-approved for treating fibromyalgia pain.)
Levomilnacipran is unique because it is more of an NSRI, so to speak, than an SNRI: That is, the drug’s uptake inhibition of norepinephrine is more potent than its serotonin inhibition. Theoretically, levomilnacipran should help improve cognitive functions linked to the action of norepinephrine, such as concentration and motivation, and in turn, improve social function. The FDA also has approved levomilnacipran for treating functional impairment in depression.9
Adverse effects. The norepinephrine uptake inhibition of levomilnacipran might be responsible for observed increases in heart rate and blood pressure in some patients, and dose-dependent urinary hesitancy and erectile dysfunction in others. The drug has no significant effect on weight in depressed patients, compared with placebo.
Continue to: The benefits of levomilnacipran
The following case illustrates the benefits of levomilnacipran in a depressed patient who suffers from chronic pain and impaired social function.
CASE Mrs. C, age 44, was referred by her outpatient psychologist and her primary care provider for management of refractory depression. She did not respond to an SSRI, an SNRI, or augmentation with bupropion and aripiprazole.
Mrs. C was on disability leave from work because of depression and cervical spine pain that might have been related to repetitive movement as a telephone customer service representative. She complained of loss of motivation, fatigue, and high anxiety about returning to work because of the many unhappy customers she felt she had to soothe.
Levomilnacipran was started at 20 mg/d for 2 days, then titrated to 40 mg/d for 5 days, 80 mg/d for 1 week, and 120 mg/d thereafter. Her previous antidepressants, fluoxetine and bupropion, were discontinued while levomilnacipran was titrated.
Mrs. C continued to receive weekly psychotherapy and physical therapy and to take tizanidine, a muscle relaxant, and over-the-counter medications for pain. Her Patient Health Questionnaire (PHQ-9) score declined from 13 when levomilnacipran was started to 5 at the next visit, 6 weeks later.
Within 4 months of initiating levomilnacipran, Mrs. C returned to work with a series of cue cards to use when speaking with irate or unhappy customers. At that point, her cervical spine pain was barely noticeable and no longer interfered with function.
Vortioxetine
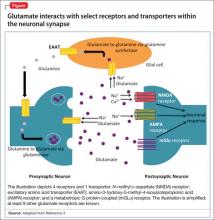
This agent has a novel multimodal mechanism of action (Table 3). It is an SSRI as well as a 5-HT1A full agonist and 5-HT3 receptor antagonist. Vortioxetine also has an inhibitory effect on 5-HT7 and 5-HT1D receptors and partial agonism of 5-HT1B receptors.
The downstream effect of this multimodal action is an increase in dopamine, norepinephrine, and acetylcholine activity in the prefrontal cortex.10 These downstream effects are thought to help restore some cognitive deficits associated with depression.11
Vortioxetine is the only antidepressant among the 3 discussed in this article that was studied over a long period to ensure that short-term benefits continue beyond the 6- to 8-week acute Phase-III studies. A high remission rate (61%) was observed in patients who were treated on an open-label basis with vortioxetine, 10 mg/d, then randomized to maintenance with vortioxetine or placebo.12
Older patients. Vortioxetine is unique among these 3 antidepressants in that it is the only one studied separately in geriatric patients: In an 8-week Phase-III trial, 452 geriatric patients age 64 to 88 were randomized to 5 mg/d of vortioxetine or placebo.13 Vortioxetine was significantly more effective than placebo at Week 6.
Vortioxetine also is the only antidepressant investigated for an effect on cognitive deficits: In a Phase-III double-blind, placebo-controlled study of 602 patients with major depressive disorder, using duloxetine as active reference, vortioxetine was found to have a significant effect on Digit Symbol Substitution Test scores, compared with placebo, independent of its antidepressant effect (ie, patients who did not show any antidepressant benefit still showed an improvement in attention, speed processing, memory, and executive function).14
We have found, therefore, that vortioxetine is helpful for depressed patients who have cognitive deficits, especially geriatric patients.
CASE Mrs. B, age 84, married, has a 4-year history of depression. She has taken several antidepressants with little consistent relief.
A brief psychiatric hospitalization 2 years ago temporarily reduced the severity of Mrs. B’s depression; gradually, she relapsed. She felt hopeless and resisted another psychiatric evaluation. Mrs. B’s family includes several clinicians, who wondered if she was developing cognitive deficits that were interfering with her recovery.
At initial evaluation, Mrs. B failed to recall 2 of 3 objects but performed the clock drawing test perfectly—qualifying her for a diagnosis of mild cognitive impairment in addition to major depression. Her PHQ-9 score at baseline was 22.
On the assumption that the severity of her depression was contributing to cognitive deficits, vortioxetine, 5 mg/d, was initiated for 2 weeks and then titrated to 10 mg/d.
At 4 weeks’ follow-up, Mrs. B passed the Mini-Cog test; her PHQ-9 score fell to 8. She has remained asymptomatic for 6 months at the 10-mg/d dosage; her lowest PHQ-9 score was 5.
Adverse effects. The most common adverse effects are mild or moderate GI in nature. Weight gain and adverse sexual effects were not significantly different among patients receiving vortioxetine than among patients given placebo.
A note about the safety of these agents
All 3 of these antidepressants carry the standard black-box warning about the elevated risk of suicide in patients taking an antidepressant. None of them are approved for patients age <18.
Continue to: Suicidal ideation was reported
Suicidal ideation was reported in 11.2% of patients taking vortioxetine, compared with 12.5% of those given placebo15; 24% of patients taking levomilnacipran reported suicidal ideation, compared with 22% of those who took placebo.16 In a long-term study of 599 patients taking vilazodone, 4 given placebo exhibited suicidal behavior, compared with 2 who took vilazodone.17
Drug-drug interactions are an important consideration when vilazodone, levomilnacipran, and vortioxetine are prescribed in combination with other medications. See the following discussion.
Vilazodone should be taken with food because it has 72% bioavailability after a meal.18 The drug is metabolized primarily by cytochrome P (CYP) 3A4 and CYP3A5; it does not affect CYP substrates or, it’s likely, produce significant changes to other medications metabolized by the CYP pathway.
Conversely, the dosage of vilazodone should be reduced to 20 mg/d if it is co- administered with a strong CYP3A4 inhibitor (eg, ketoconazole). The dosage should be increased as much as 2-fold when vilazodone is used concomitantly used with a strong CYP3A4 inducer (eg, carbamazepine) for >14 days. The maximum daily dosage should not exceed 80 mg/d.
Levomilnacipran. Unlike vilazodone and vortioxetine, levomilnacipran is affected by renal function.19 Concomitant medications, however, including those that influence CYP renal transporters (particularly CYP3A4, which metabolizes levomilnacipran), do not show an impact on the blood level of levomilnacipran.
No dosage adjustment is needed for patients who have mild renal impairment, but the maintenance dosage of levomilnacipran for patients who have moderate or severe renal impairment should not exceed 80 mg/d in 1 dose, and 60 mg/d in 1 dose, respectively.20
Vortioxetine. Seventy percent of a dose of vortioxetine is absorbed independent of food; the drug has a half-life of 66 hours. Vortioxetine is metabolized primarily by the CYP450 enzyme system, including 2D6, and, to a lesser extent, by CYP3A4, CYP3A5, CYP2C9, and CYP2C19.21
Vortioxetine has minimal effect on P450 substrates in in vitro studies, which was confirmed in 4 other in vivo studies.21-23 In studies of hormonal contraception, bupropion, and omeprazole, vortioxetine did not produce significant changes in the blood level of the other medications. The blood level of vortioxetine increased by 128% when taken with the CYP2D6 inhibitor bupropion,24 but the blood level did not markedly change with other inhibitors because the drug utilizes uses several CYP pathways. Use caution, therefore, when adding bupropion to vortioxetine because the combination elevates the risk of nausea, diarrhea, and headache.
With each agent, specific benefit
Vilazodone, levomilnacipran, and vortioxetine each add distinct benefit to the clinician’s toolbox of treatments for major depressive disorder. Although all antidepressants to some extent alleviate anxiety and pain and reverse cognitive decline associated with depression, our experience suggests using vilazodone for anxious depressed patients; levomilnacipran for depressed patients who experience pain; and vortioxetine for depressed patients who suffer cognitive decline and for geriatric patients.
Bottom Line
The FDA has approved 3 antidepressants in the past 4 years: vilazodone, levomilnacipran, and vortioxetine. The hope is that these agents will bolster treatment options for major depression—perhaps especially so, as we have seen, in the anxious depressed (vilazodone), the depressed in pain (levomilnacipran), and the depressed with cognitive decline, or geriatric patients (vortioxetine).
Related Resources
• Kalia R, Mittal M, Preskorn S. Vilazodone for major depressive disorder. Current Psychiatry. 2011;10(4):84-86,88.
• Lincoln J, Wehler C. Vortioxetine for major depressive disorder. Current Psychiatry. 2014;13(2):67-70.
• Macaluso M, Kazanchi H, Malhotra V. Levomilnacipran for the treatment of major depressive disorder. Current Psychiatry. 2013;12(12):50-52,54,55.
• McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
• Thase ME, Chen D, Edwards J, et al. Efficacy of vilazodone on anxiety symptoms in patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(6):351-356.
Drug Brand Names
Aripiprazole • Abilify Levomilnacipran • Fetzima
Bupropion • Wellbutrin, Zyban Lubiprostone • Amitiza
Buspirone • BuSpar Milnacipran • Savella
Carbamazepine • Tegretol, Equetro Omeprazole • Prilosec
Desvenlafaxine • Pristiq Tizanidine • Zanaflex
Duloxetine • Cymbalta Venlafaxine • Effexor
Escitalopram • Lexapro Vilazodone • Viibryd
Fluoxetine • Prozac Vortioxetine • Brintellix
Ketoconazole • Nizoral
1. Andrade L, Caraveo-Anduaga JJ, Berglund P, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12(1):3-21.
2. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547.
3. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-459.
4. Khan A. Vilazodone, a novel dual-acting serotonergic antidepressant for managing major depression. Expert Opin Investig Drugs. 2009;18(11):1753-1764.
5. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
6. Robinson DS, Kajdasz DK, Gallipoli S, et al. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643-646.
7. Saraceni MM, Venci JV, Gandhi MA. Levomilnacipran (Fetzima): a new serotonin-norepinephrine reuptake inhibitor for the treatment of major depressive disorder. J Pharm Pract. 2013;27(4):389-395.
8. Deardorff WJ, Grossberg GT. A review of the clinical efficacy, safety and tolerability of the antidepressants vilazodone, levomilnacipran and vortioxetine. Expert Opin Pharmacother. 2014;15(17):2525-2542.
9. Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2013;67(11):1089-1104.
10. Mørk A, Pehrson A, Brennum LT, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther. 2012;340(3):666-675.
11. Pehrson AL, Leiser SC, Gulinello M, et al. Treatment of cognitive dysfunction in major depressive disorder-a review of the preclinical evidence for efficacy of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and the multimodal-acting antidepressant vortioxetine [published online August 5, 2014]. Eur J Pharmacol. doi: 10.1016/j.ejphar.2014.07.044.
12. Baldwin DS, Hansen T, Florea I. Vortioxetine (Lu AA21004) in the long-term open-label treatment of major depressive disorder. Curr Med Res Opin. 2012;28(10):1717-1724.
13. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-523.
14. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6): 900-909.
15. Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138-149.
16. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
17. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
18. Boinpally R, Gad N, Gupta S, et al. Influence of CYP3A4 induction/inhibition on the pharmacokinetics of vilazodone in healthy subjects. Clin Ther. 2014; 36(11):1638-1649.
19. Chen L, Boinpally R, Greenberg WM, et al. Effect of hepatic impairment on the pharmacokinetics of levomilnacipran following a single oral dose of a levomilnacipran extended-release capsule in human participants. Clin Drug Investig. 2014;34(5):351-359.
20. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
21. Hvenegaard MG, Bang-Andersen B, Pedersen H, et al. Identification of the cytochrome P450 and other enzymes involved in the in vitro oxidative metabolism of a novel antidepressant, Lu AA21004. Drug Metab Dispos. 2012; 40(7):1357-1365.
22. Chen G, Lee R, Højer AM, et al. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013; 33(10):727-736.
23. Areberg J, Søgaard B, Højer AM. The clinical pharmacokinetics of Lu AA21004 and its major metabolite in healthy young volunteers. Basic Clin Pharmacol Toxicol. 2012;111(3):198-205.
24. Areberg J, Petersen KB, Chen G, et al. Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin Pharmacol Toxicol. 2014;115(6):552-559.
With a prevalence >17%, depression is one of the most common mental disorders in the United States and the second leading cause of disability worldwide.1,2 For decades, primary care and mental health providers have used selective serotonin reuptake inhibitors (SSRIs) as first-line treatment for depression—yet the remission rate after the first trial of an antidepressant is <30%, and continues to decline after a first antidepressant failure.3
That is why clinicians continue to seek effective treatments for depression—ones that will provide quick and sustainable remission—and why scientists and pharmaceutical manufacturers have been competing to develop more effective antidepressant medications.
In the past 4 years, the FDA has approved 3 antidepressants—vilazodone, levomilnacipran, and vortioxetine—with the hope of increasing options for patients who suffer from major depression. These 3 antidepressants differ in their mechanisms of action from other available antidepressants, and all have been shown to have acceptable safety and tolerability profiles.
In this article, we review these novel antidepressants and present some clinical pearls for their use. We also present our observations that each agent appears to show particular advantage in a certain subpopulation of depressed patients who often do not respond, or who do not adequately respond, to other antidepressants.
Vilazodone
Vilazodone was approved by the FDA in 2011 (Table 1). The drug increases serotonin bioavailability in synapses through a strong dual action:
• blocking serotonin reuptake through the serotonin transporter
• partial agonism of the 5-HT1A presynaptic receptor.
Vilazodone also has a moderate effect on the 5-HT4 receptor and on dopamine and norepinephrine uptake inhibition.
The unique presynaptic 5-HT1A partial agonism of vilazodone is similar to that of buspirone, in which both drugs initially inhibit serotonin synthesis and neuronal firing.4 Researchers therefore expected that vilazodone would be more suitable for patients who have depression and a comorbid anxiety disorder; current FDA approval, however, is for depression only.
Adverse effects. The 5-HT4 receptor on which vilazodone acts is present in the gastrointestinal (GI) tract, and contributes to regulating symptoms in patients with irritable bowel syndrome (IBS)5; not surprisingly, the most frequent adverse effects of vilazodone are GI in nature (diarrhea, nausea, vomiting).
Headache is the most common non- GI side effect of vilazodone. Depressed patients who took vilazodone had no significant weight gain and did not report adverse sexual effects, compared with subjects given placebo.6
The following case—a patient with depression, significant anxiety, and IBS— exemplifies the type of patient for whom we find vilazodone most useful.
CASE Ms. A, age 19, is a college student with a history of major depressive disorder, social anxiety, and panic attacks for 2 years and IBS for 3 years. She was taking lubiprostone for IBS, with incomplete relief of GI symptoms. Because the family history included depression in Ms. A’s mother and sister, and both were doing well on escitalopram, we began a trial of that drug, 10 mg/d, that was quickly titrated to 20 mg/d.
Ms. A did not respond to 20 mg of escitalopram combined with psychotherapy.
We then started vilazodone, 10 mg/d after breakfast, for the first week, and reduced escitalopram to 10 mg/d. During Week 2, escitalopram was discontinued and vilazodone was increased to 20 mg/d. During Week 3, vilazodone was titrated to 40 mg/d.
Ms. A tolerated vilazodone well. Her depressive symptoms improved at the end of Week 2.
Unlike her experience with escitalopram, Ms. A’s anxiety symptoms—tenseness, racing thoughts, and panic attacks—all diminished when she switched to vilazodone. Notably, her IBS symptoms also were relieved, and she discontinued lubiprostone.
Ms. A’s depression remained in remission for 2 years, except for a brief period one summer, when she thought she “could do without any medication.” She tapered the vilazodone, week by week, to 10 mg/d, but her anxiety and bowel symptoms resurfaced to a degree that she resumed the 40-mg/d dosage.
Levomilnacipran
This drug is a 2013 addition to the small serotonin–norepinephrine reuptake inhibitor (SNRI) family of venlafaxine, desvenlafaxine, and duloxetine7 (Table 2). Levomilnacipran is the enantiomer of milnacipran, approved in Europe for depression but only for fibromyalgia pain and peripheral neuropathy in the United States.8 (Levomilnacipran is not FDA-approved for treating fibromyalgia pain.)
Levomilnacipran is unique because it is more of an NSRI, so to speak, than an SNRI: That is, the drug’s uptake inhibition of norepinephrine is more potent than its serotonin inhibition. Theoretically, levomilnacipran should help improve cognitive functions linked to the action of norepinephrine, such as concentration and motivation, and in turn, improve social function. The FDA also has approved levomilnacipran for treating functional impairment in depression.9
Adverse effects. The norepinephrine uptake inhibition of levomilnacipran might be responsible for observed increases in heart rate and blood pressure in some patients, and dose-dependent urinary hesitancy and erectile dysfunction in others. The drug has no significant effect on weight in depressed patients, compared with placebo.
Continue to: The benefits of levomilnacipran
The following case illustrates the benefits of levomilnacipran in a depressed patient who suffers from chronic pain and impaired social function.
CASE Mrs. C, age 44, was referred by her outpatient psychologist and her primary care provider for management of refractory depression. She did not respond to an SSRI, an SNRI, or augmentation with bupropion and aripiprazole.
Mrs. C was on disability leave from work because of depression and cervical spine pain that might have been related to repetitive movement as a telephone customer service representative. She complained of loss of motivation, fatigue, and high anxiety about returning to work because of the many unhappy customers she felt she had to soothe.
Levomilnacipran was started at 20 mg/d for 2 days, then titrated to 40 mg/d for 5 days, 80 mg/d for 1 week, and 120 mg/d thereafter. Her previous antidepressants, fluoxetine and bupropion, were discontinued while levomilnacipran was titrated.
Mrs. C continued to receive weekly psychotherapy and physical therapy and to take tizanidine, a muscle relaxant, and over-the-counter medications for pain. Her Patient Health Questionnaire (PHQ-9) score declined from 13 when levomilnacipran was started to 5 at the next visit, 6 weeks later.
Within 4 months of initiating levomilnacipran, Mrs. C returned to work with a series of cue cards to use when speaking with irate or unhappy customers. At that point, her cervical spine pain was barely noticeable and no longer interfered with function.
Vortioxetine
This agent has a novel multimodal mechanism of action (Table 3). It is an SSRI as well as a 5-HT1A full agonist and 5-HT3 receptor antagonist. Vortioxetine also has an inhibitory effect on 5-HT7 and 5-HT1D receptors and partial agonism of 5-HT1B receptors.
The downstream effect of this multimodal action is an increase in dopamine, norepinephrine, and acetylcholine activity in the prefrontal cortex.10 These downstream effects are thought to help restore some cognitive deficits associated with depression.11
Vortioxetine is the only antidepressant among the 3 discussed in this article that was studied over a long period to ensure that short-term benefits continue beyond the 6- to 8-week acute Phase-III studies. A high remission rate (61%) was observed in patients who were treated on an open-label basis with vortioxetine, 10 mg/d, then randomized to maintenance with vortioxetine or placebo.12
Older patients. Vortioxetine is unique among these 3 antidepressants in that it is the only one studied separately in geriatric patients: In an 8-week Phase-III trial, 452 geriatric patients age 64 to 88 were randomized to 5 mg/d of vortioxetine or placebo.13 Vortioxetine was significantly more effective than placebo at Week 6.
Vortioxetine also is the only antidepressant investigated for an effect on cognitive deficits: In a Phase-III double-blind, placebo-controlled study of 602 patients with major depressive disorder, using duloxetine as active reference, vortioxetine was found to have a significant effect on Digit Symbol Substitution Test scores, compared with placebo, independent of its antidepressant effect (ie, patients who did not show any antidepressant benefit still showed an improvement in attention, speed processing, memory, and executive function).14
We have found, therefore, that vortioxetine is helpful for depressed patients who have cognitive deficits, especially geriatric patients.
CASE Mrs. B, age 84, married, has a 4-year history of depression. She has taken several antidepressants with little consistent relief.
A brief psychiatric hospitalization 2 years ago temporarily reduced the severity of Mrs. B’s depression; gradually, she relapsed. She felt hopeless and resisted another psychiatric evaluation. Mrs. B’s family includes several clinicians, who wondered if she was developing cognitive deficits that were interfering with her recovery.
At initial evaluation, Mrs. B failed to recall 2 of 3 objects but performed the clock drawing test perfectly—qualifying her for a diagnosis of mild cognitive impairment in addition to major depression. Her PHQ-9 score at baseline was 22.
On the assumption that the severity of her depression was contributing to cognitive deficits, vortioxetine, 5 mg/d, was initiated for 2 weeks and then titrated to 10 mg/d.
At 4 weeks’ follow-up, Mrs. B passed the Mini-Cog test; her PHQ-9 score fell to 8. She has remained asymptomatic for 6 months at the 10-mg/d dosage; her lowest PHQ-9 score was 5.
Adverse effects. The most common adverse effects are mild or moderate GI in nature. Weight gain and adverse sexual effects were not significantly different among patients receiving vortioxetine than among patients given placebo.
A note about the safety of these agents
All 3 of these antidepressants carry the standard black-box warning about the elevated risk of suicide in patients taking an antidepressant. None of them are approved for patients age <18.
Continue to: Suicidal ideation was reported
Suicidal ideation was reported in 11.2% of patients taking vortioxetine, compared with 12.5% of those given placebo15; 24% of patients taking levomilnacipran reported suicidal ideation, compared with 22% of those who took placebo.16 In a long-term study of 599 patients taking vilazodone, 4 given placebo exhibited suicidal behavior, compared with 2 who took vilazodone.17
Drug-drug interactions are an important consideration when vilazodone, levomilnacipran, and vortioxetine are prescribed in combination with other medications. See the following discussion.
Vilazodone should be taken with food because it has 72% bioavailability after a meal.18 The drug is metabolized primarily by cytochrome P (CYP) 3A4 and CYP3A5; it does not affect CYP substrates or, it’s likely, produce significant changes to other medications metabolized by the CYP pathway.
Conversely, the dosage of vilazodone should be reduced to 20 mg/d if it is co- administered with a strong CYP3A4 inhibitor (eg, ketoconazole). The dosage should be increased as much as 2-fold when vilazodone is used concomitantly used with a strong CYP3A4 inducer (eg, carbamazepine) for >14 days. The maximum daily dosage should not exceed 80 mg/d.
Levomilnacipran. Unlike vilazodone and vortioxetine, levomilnacipran is affected by renal function.19 Concomitant medications, however, including those that influence CYP renal transporters (particularly CYP3A4, which metabolizes levomilnacipran), do not show an impact on the blood level of levomilnacipran.
No dosage adjustment is needed for patients who have mild renal impairment, but the maintenance dosage of levomilnacipran for patients who have moderate or severe renal impairment should not exceed 80 mg/d in 1 dose, and 60 mg/d in 1 dose, respectively.20
Vortioxetine. Seventy percent of a dose of vortioxetine is absorbed independent of food; the drug has a half-life of 66 hours. Vortioxetine is metabolized primarily by the CYP450 enzyme system, including 2D6, and, to a lesser extent, by CYP3A4, CYP3A5, CYP2C9, and CYP2C19.21
Vortioxetine has minimal effect on P450 substrates in in vitro studies, which was confirmed in 4 other in vivo studies.21-23 In studies of hormonal contraception, bupropion, and omeprazole, vortioxetine did not produce significant changes in the blood level of the other medications. The blood level of vortioxetine increased by 128% when taken with the CYP2D6 inhibitor bupropion,24 but the blood level did not markedly change with other inhibitors because the drug utilizes uses several CYP pathways. Use caution, therefore, when adding bupropion to vortioxetine because the combination elevates the risk of nausea, diarrhea, and headache.
With each agent, specific benefit
Vilazodone, levomilnacipran, and vortioxetine each add distinct benefit to the clinician’s toolbox of treatments for major depressive disorder. Although all antidepressants to some extent alleviate anxiety and pain and reverse cognitive decline associated with depression, our experience suggests using vilazodone for anxious depressed patients; levomilnacipran for depressed patients who experience pain; and vortioxetine for depressed patients who suffer cognitive decline and for geriatric patients.
Bottom Line
The FDA has approved 3 antidepressants in the past 4 years: vilazodone, levomilnacipran, and vortioxetine. The hope is that these agents will bolster treatment options for major depression—perhaps especially so, as we have seen, in the anxious depressed (vilazodone), the depressed in pain (levomilnacipran), and the depressed with cognitive decline, or geriatric patients (vortioxetine).
Related Resources
• Kalia R, Mittal M, Preskorn S. Vilazodone for major depressive disorder. Current Psychiatry. 2011;10(4):84-86,88.
• Lincoln J, Wehler C. Vortioxetine for major depressive disorder. Current Psychiatry. 2014;13(2):67-70.
• Macaluso M, Kazanchi H, Malhotra V. Levomilnacipran for the treatment of major depressive disorder. Current Psychiatry. 2013;12(12):50-52,54,55.
• McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
• Thase ME, Chen D, Edwards J, et al. Efficacy of vilazodone on anxiety symptoms in patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(6):351-356.
Drug Brand Names
Aripiprazole • Abilify Levomilnacipran • Fetzima
Bupropion • Wellbutrin, Zyban Lubiprostone • Amitiza
Buspirone • BuSpar Milnacipran • Savella
Carbamazepine • Tegretol, Equetro Omeprazole • Prilosec
Desvenlafaxine • Pristiq Tizanidine • Zanaflex
Duloxetine • Cymbalta Venlafaxine • Effexor
Escitalopram • Lexapro Vilazodone • Viibryd
Fluoxetine • Prozac Vortioxetine • Brintellix
Ketoconazole • Nizoral
With a prevalence >17%, depression is one of the most common mental disorders in the United States and the second leading cause of disability worldwide.1,2 For decades, primary care and mental health providers have used selective serotonin reuptake inhibitors (SSRIs) as first-line treatment for depression—yet the remission rate after the first trial of an antidepressant is <30%, and continues to decline after a first antidepressant failure.3
That is why clinicians continue to seek effective treatments for depression—ones that will provide quick and sustainable remission—and why scientists and pharmaceutical manufacturers have been competing to develop more effective antidepressant medications.
In the past 4 years, the FDA has approved 3 antidepressants—vilazodone, levomilnacipran, and vortioxetine—with the hope of increasing options for patients who suffer from major depression. These 3 antidepressants differ in their mechanisms of action from other available antidepressants, and all have been shown to have acceptable safety and tolerability profiles.
In this article, we review these novel antidepressants and present some clinical pearls for their use. We also present our observations that each agent appears to show particular advantage in a certain subpopulation of depressed patients who often do not respond, or who do not adequately respond, to other antidepressants.
Vilazodone
Vilazodone was approved by the FDA in 2011 (Table 1). The drug increases serotonin bioavailability in synapses through a strong dual action:
• blocking serotonin reuptake through the serotonin transporter
• partial agonism of the 5-HT1A presynaptic receptor.
Vilazodone also has a moderate effect on the 5-HT4 receptor and on dopamine and norepinephrine uptake inhibition.
The unique presynaptic 5-HT1A partial agonism of vilazodone is similar to that of buspirone, in which both drugs initially inhibit serotonin synthesis and neuronal firing.4 Researchers therefore expected that vilazodone would be more suitable for patients who have depression and a comorbid anxiety disorder; current FDA approval, however, is for depression only.
Adverse effects. The 5-HT4 receptor on which vilazodone acts is present in the gastrointestinal (GI) tract, and contributes to regulating symptoms in patients with irritable bowel syndrome (IBS)5; not surprisingly, the most frequent adverse effects of vilazodone are GI in nature (diarrhea, nausea, vomiting).
Headache is the most common non- GI side effect of vilazodone. Depressed patients who took vilazodone had no significant weight gain and did not report adverse sexual effects, compared with subjects given placebo.6
The following case—a patient with depression, significant anxiety, and IBS— exemplifies the type of patient for whom we find vilazodone most useful.
CASE Ms. A, age 19, is a college student with a history of major depressive disorder, social anxiety, and panic attacks for 2 years and IBS for 3 years. She was taking lubiprostone for IBS, with incomplete relief of GI symptoms. Because the family history included depression in Ms. A’s mother and sister, and both were doing well on escitalopram, we began a trial of that drug, 10 mg/d, that was quickly titrated to 20 mg/d.
Ms. A did not respond to 20 mg of escitalopram combined with psychotherapy.
We then started vilazodone, 10 mg/d after breakfast, for the first week, and reduced escitalopram to 10 mg/d. During Week 2, escitalopram was discontinued and vilazodone was increased to 20 mg/d. During Week 3, vilazodone was titrated to 40 mg/d.
Ms. A tolerated vilazodone well. Her depressive symptoms improved at the end of Week 2.
Unlike her experience with escitalopram, Ms. A’s anxiety symptoms—tenseness, racing thoughts, and panic attacks—all diminished when she switched to vilazodone. Notably, her IBS symptoms also were relieved, and she discontinued lubiprostone.
Ms. A’s depression remained in remission for 2 years, except for a brief period one summer, when she thought she “could do without any medication.” She tapered the vilazodone, week by week, to 10 mg/d, but her anxiety and bowel symptoms resurfaced to a degree that she resumed the 40-mg/d dosage.
Levomilnacipran
This drug is a 2013 addition to the small serotonin–norepinephrine reuptake inhibitor (SNRI) family of venlafaxine, desvenlafaxine, and duloxetine7 (Table 2). Levomilnacipran is the enantiomer of milnacipran, approved in Europe for depression but only for fibromyalgia pain and peripheral neuropathy in the United States.8 (Levomilnacipran is not FDA-approved for treating fibromyalgia pain.)
Levomilnacipran is unique because it is more of an NSRI, so to speak, than an SNRI: That is, the drug’s uptake inhibition of norepinephrine is more potent than its serotonin inhibition. Theoretically, levomilnacipran should help improve cognitive functions linked to the action of norepinephrine, such as concentration and motivation, and in turn, improve social function. The FDA also has approved levomilnacipran for treating functional impairment in depression.9
Adverse effects. The norepinephrine uptake inhibition of levomilnacipran might be responsible for observed increases in heart rate and blood pressure in some patients, and dose-dependent urinary hesitancy and erectile dysfunction in others. The drug has no significant effect on weight in depressed patients, compared with placebo.
Continue to: The benefits of levomilnacipran
The following case illustrates the benefits of levomilnacipran in a depressed patient who suffers from chronic pain and impaired social function.
CASE Mrs. C, age 44, was referred by her outpatient psychologist and her primary care provider for management of refractory depression. She did not respond to an SSRI, an SNRI, or augmentation with bupropion and aripiprazole.
Mrs. C was on disability leave from work because of depression and cervical spine pain that might have been related to repetitive movement as a telephone customer service representative. She complained of loss of motivation, fatigue, and high anxiety about returning to work because of the many unhappy customers she felt she had to soothe.
Levomilnacipran was started at 20 mg/d for 2 days, then titrated to 40 mg/d for 5 days, 80 mg/d for 1 week, and 120 mg/d thereafter. Her previous antidepressants, fluoxetine and bupropion, were discontinued while levomilnacipran was titrated.
Mrs. C continued to receive weekly psychotherapy and physical therapy and to take tizanidine, a muscle relaxant, and over-the-counter medications for pain. Her Patient Health Questionnaire (PHQ-9) score declined from 13 when levomilnacipran was started to 5 at the next visit, 6 weeks later.
Within 4 months of initiating levomilnacipran, Mrs. C returned to work with a series of cue cards to use when speaking with irate or unhappy customers. At that point, her cervical spine pain was barely noticeable and no longer interfered with function.
Vortioxetine
This agent has a novel multimodal mechanism of action (Table 3). It is an SSRI as well as a 5-HT1A full agonist and 5-HT3 receptor antagonist. Vortioxetine also has an inhibitory effect on 5-HT7 and 5-HT1D receptors and partial agonism of 5-HT1B receptors.
The downstream effect of this multimodal action is an increase in dopamine, norepinephrine, and acetylcholine activity in the prefrontal cortex.10 These downstream effects are thought to help restore some cognitive deficits associated with depression.11
Vortioxetine is the only antidepressant among the 3 discussed in this article that was studied over a long period to ensure that short-term benefits continue beyond the 6- to 8-week acute Phase-III studies. A high remission rate (61%) was observed in patients who were treated on an open-label basis with vortioxetine, 10 mg/d, then randomized to maintenance with vortioxetine or placebo.12
Older patients. Vortioxetine is unique among these 3 antidepressants in that it is the only one studied separately in geriatric patients: In an 8-week Phase-III trial, 452 geriatric patients age 64 to 88 were randomized to 5 mg/d of vortioxetine or placebo.13 Vortioxetine was significantly more effective than placebo at Week 6.
Vortioxetine also is the only antidepressant investigated for an effect on cognitive deficits: In a Phase-III double-blind, placebo-controlled study of 602 patients with major depressive disorder, using duloxetine as active reference, vortioxetine was found to have a significant effect on Digit Symbol Substitution Test scores, compared with placebo, independent of its antidepressant effect (ie, patients who did not show any antidepressant benefit still showed an improvement in attention, speed processing, memory, and executive function).14
We have found, therefore, that vortioxetine is helpful for depressed patients who have cognitive deficits, especially geriatric patients.
CASE Mrs. B, age 84, married, has a 4-year history of depression. She has taken several antidepressants with little consistent relief.
A brief psychiatric hospitalization 2 years ago temporarily reduced the severity of Mrs. B’s depression; gradually, she relapsed. She felt hopeless and resisted another psychiatric evaluation. Mrs. B’s family includes several clinicians, who wondered if she was developing cognitive deficits that were interfering with her recovery.
At initial evaluation, Mrs. B failed to recall 2 of 3 objects but performed the clock drawing test perfectly—qualifying her for a diagnosis of mild cognitive impairment in addition to major depression. Her PHQ-9 score at baseline was 22.
On the assumption that the severity of her depression was contributing to cognitive deficits, vortioxetine, 5 mg/d, was initiated for 2 weeks and then titrated to 10 mg/d.
At 4 weeks’ follow-up, Mrs. B passed the Mini-Cog test; her PHQ-9 score fell to 8. She has remained asymptomatic for 6 months at the 10-mg/d dosage; her lowest PHQ-9 score was 5.
Adverse effects. The most common adverse effects are mild or moderate GI in nature. Weight gain and adverse sexual effects were not significantly different among patients receiving vortioxetine than among patients given placebo.
A note about the safety of these agents
All 3 of these antidepressants carry the standard black-box warning about the elevated risk of suicide in patients taking an antidepressant. None of them are approved for patients age <18.
Continue to: Suicidal ideation was reported
Suicidal ideation was reported in 11.2% of patients taking vortioxetine, compared with 12.5% of those given placebo15; 24% of patients taking levomilnacipran reported suicidal ideation, compared with 22% of those who took placebo.16 In a long-term study of 599 patients taking vilazodone, 4 given placebo exhibited suicidal behavior, compared with 2 who took vilazodone.17
Drug-drug interactions are an important consideration when vilazodone, levomilnacipran, and vortioxetine are prescribed in combination with other medications. See the following discussion.
Vilazodone should be taken with food because it has 72% bioavailability after a meal.18 The drug is metabolized primarily by cytochrome P (CYP) 3A4 and CYP3A5; it does not affect CYP substrates or, it’s likely, produce significant changes to other medications metabolized by the CYP pathway.
Conversely, the dosage of vilazodone should be reduced to 20 mg/d if it is co- administered with a strong CYP3A4 inhibitor (eg, ketoconazole). The dosage should be increased as much as 2-fold when vilazodone is used concomitantly used with a strong CYP3A4 inducer (eg, carbamazepine) for >14 days. The maximum daily dosage should not exceed 80 mg/d.
Levomilnacipran. Unlike vilazodone and vortioxetine, levomilnacipran is affected by renal function.19 Concomitant medications, however, including those that influence CYP renal transporters (particularly CYP3A4, which metabolizes levomilnacipran), do not show an impact on the blood level of levomilnacipran.
No dosage adjustment is needed for patients who have mild renal impairment, but the maintenance dosage of levomilnacipran for patients who have moderate or severe renal impairment should not exceed 80 mg/d in 1 dose, and 60 mg/d in 1 dose, respectively.20
Vortioxetine. Seventy percent of a dose of vortioxetine is absorbed independent of food; the drug has a half-life of 66 hours. Vortioxetine is metabolized primarily by the CYP450 enzyme system, including 2D6, and, to a lesser extent, by CYP3A4, CYP3A5, CYP2C9, and CYP2C19.21
Vortioxetine has minimal effect on P450 substrates in in vitro studies, which was confirmed in 4 other in vivo studies.21-23 In studies of hormonal contraception, bupropion, and omeprazole, vortioxetine did not produce significant changes in the blood level of the other medications. The blood level of vortioxetine increased by 128% when taken with the CYP2D6 inhibitor bupropion,24 but the blood level did not markedly change with other inhibitors because the drug utilizes uses several CYP pathways. Use caution, therefore, when adding bupropion to vortioxetine because the combination elevates the risk of nausea, diarrhea, and headache.
With each agent, specific benefit
Vilazodone, levomilnacipran, and vortioxetine each add distinct benefit to the clinician’s toolbox of treatments for major depressive disorder. Although all antidepressants to some extent alleviate anxiety and pain and reverse cognitive decline associated with depression, our experience suggests using vilazodone for anxious depressed patients; levomilnacipran for depressed patients who experience pain; and vortioxetine for depressed patients who suffer cognitive decline and for geriatric patients.
Bottom Line
The FDA has approved 3 antidepressants in the past 4 years: vilazodone, levomilnacipran, and vortioxetine. The hope is that these agents will bolster treatment options for major depression—perhaps especially so, as we have seen, in the anxious depressed (vilazodone), the depressed in pain (levomilnacipran), and the depressed with cognitive decline, or geriatric patients (vortioxetine).
Related Resources
• Kalia R, Mittal M, Preskorn S. Vilazodone for major depressive disorder. Current Psychiatry. 2011;10(4):84-86,88.
• Lincoln J, Wehler C. Vortioxetine for major depressive disorder. Current Psychiatry. 2014;13(2):67-70.
• Macaluso M, Kazanchi H, Malhotra V. Levomilnacipran for the treatment of major depressive disorder. Current Psychiatry. 2013;12(12):50-52,54,55.
• McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
• Thase ME, Chen D, Edwards J, et al. Efficacy of vilazodone on anxiety symptoms in patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(6):351-356.
Drug Brand Names
Aripiprazole • Abilify Levomilnacipran • Fetzima
Bupropion • Wellbutrin, Zyban Lubiprostone • Amitiza
Buspirone • BuSpar Milnacipran • Savella
Carbamazepine • Tegretol, Equetro Omeprazole • Prilosec
Desvenlafaxine • Pristiq Tizanidine • Zanaflex
Duloxetine • Cymbalta Venlafaxine • Effexor
Escitalopram • Lexapro Vilazodone • Viibryd
Fluoxetine • Prozac Vortioxetine • Brintellix
Ketoconazole • Nizoral
1. Andrade L, Caraveo-Anduaga JJ, Berglund P, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12(1):3-21.
2. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547.
3. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-459.
4. Khan A. Vilazodone, a novel dual-acting serotonergic antidepressant for managing major depression. Expert Opin Investig Drugs. 2009;18(11):1753-1764.
5. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
6. Robinson DS, Kajdasz DK, Gallipoli S, et al. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643-646.
7. Saraceni MM, Venci JV, Gandhi MA. Levomilnacipran (Fetzima): a new serotonin-norepinephrine reuptake inhibitor for the treatment of major depressive disorder. J Pharm Pract. 2013;27(4):389-395.
8. Deardorff WJ, Grossberg GT. A review of the clinical efficacy, safety and tolerability of the antidepressants vilazodone, levomilnacipran and vortioxetine. Expert Opin Pharmacother. 2014;15(17):2525-2542.
9. Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2013;67(11):1089-1104.
10. Mørk A, Pehrson A, Brennum LT, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther. 2012;340(3):666-675.
11. Pehrson AL, Leiser SC, Gulinello M, et al. Treatment of cognitive dysfunction in major depressive disorder-a review of the preclinical evidence for efficacy of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and the multimodal-acting antidepressant vortioxetine [published online August 5, 2014]. Eur J Pharmacol. doi: 10.1016/j.ejphar.2014.07.044.
12. Baldwin DS, Hansen T, Florea I. Vortioxetine (Lu AA21004) in the long-term open-label treatment of major depressive disorder. Curr Med Res Opin. 2012;28(10):1717-1724.
13. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-523.
14. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6): 900-909.
15. Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138-149.
16. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
17. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
18. Boinpally R, Gad N, Gupta S, et al. Influence of CYP3A4 induction/inhibition on the pharmacokinetics of vilazodone in healthy subjects. Clin Ther. 2014; 36(11):1638-1649.
19. Chen L, Boinpally R, Greenberg WM, et al. Effect of hepatic impairment on the pharmacokinetics of levomilnacipran following a single oral dose of a levomilnacipran extended-release capsule in human participants. Clin Drug Investig. 2014;34(5):351-359.
20. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
21. Hvenegaard MG, Bang-Andersen B, Pedersen H, et al. Identification of the cytochrome P450 and other enzymes involved in the in vitro oxidative metabolism of a novel antidepressant, Lu AA21004. Drug Metab Dispos. 2012; 40(7):1357-1365.
22. Chen G, Lee R, Højer AM, et al. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013; 33(10):727-736.
23. Areberg J, Søgaard B, Højer AM. The clinical pharmacokinetics of Lu AA21004 and its major metabolite in healthy young volunteers. Basic Clin Pharmacol Toxicol. 2012;111(3):198-205.
24. Areberg J, Petersen KB, Chen G, et al. Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin Pharmacol Toxicol. 2014;115(6):552-559.
1. Andrade L, Caraveo-Anduaga JJ, Berglund P, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12(1):3-21.
2. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547.
3. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-459.
4. Khan A. Vilazodone, a novel dual-acting serotonergic antidepressant for managing major depression. Expert Opin Investig Drugs. 2009;18(11):1753-1764.
5. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
6. Robinson DS, Kajdasz DK, Gallipoli S, et al. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643-646.
7. Saraceni MM, Venci JV, Gandhi MA. Levomilnacipran (Fetzima): a new serotonin-norepinephrine reuptake inhibitor for the treatment of major depressive disorder. J Pharm Pract. 2013;27(4):389-395.
8. Deardorff WJ, Grossberg GT. A review of the clinical efficacy, safety and tolerability of the antidepressants vilazodone, levomilnacipran and vortioxetine. Expert Opin Pharmacother. 2014;15(17):2525-2542.
9. Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2013;67(11):1089-1104.
10. Mørk A, Pehrson A, Brennum LT, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther. 2012;340(3):666-675.
11. Pehrson AL, Leiser SC, Gulinello M, et al. Treatment of cognitive dysfunction in major depressive disorder-a review of the preclinical evidence for efficacy of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and the multimodal-acting antidepressant vortioxetine [published online August 5, 2014]. Eur J Pharmacol. doi: 10.1016/j.ejphar.2014.07.044.
12. Baldwin DS, Hansen T, Florea I. Vortioxetine (Lu AA21004) in the long-term open-label treatment of major depressive disorder. Curr Med Res Opin. 2012;28(10):1717-1724.
13. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-523.
14. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6): 900-909.
15. Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138-149.
16. Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
17. Khan A, Sambunaris A, Edwards J, et al. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. Int Clin Psychopharmacol. 2014;29(2):86-92.
18. Boinpally R, Gad N, Gupta S, et al. Influence of CYP3A4 induction/inhibition on the pharmacokinetics of vilazodone in healthy subjects. Clin Ther. 2014; 36(11):1638-1649.
19. Chen L, Boinpally R, Greenberg WM, et al. Effect of hepatic impairment on the pharmacokinetics of levomilnacipran following a single oral dose of a levomilnacipran extended-release capsule in human participants. Clin Drug Investig. 2014;34(5):351-359.
20. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
21. Hvenegaard MG, Bang-Andersen B, Pedersen H, et al. Identification of the cytochrome P450 and other enzymes involved in the in vitro oxidative metabolism of a novel antidepressant, Lu AA21004. Drug Metab Dispos. 2012; 40(7):1357-1365.
22. Chen G, Lee R, Højer AM, et al. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013; 33(10):727-736.
23. Areberg J, Søgaard B, Højer AM. The clinical pharmacokinetics of Lu AA21004 and its major metabolite in healthy young volunteers. Basic Clin Pharmacol Toxicol. 2012;111(3):198-205.
24. Areberg J, Petersen KB, Chen G, et al. Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin Pharmacol Toxicol. 2014;115(6):552-559.
Court allows generic colchicine to enter market
After a 5-year monopoly on the sale of colchicine put the gout medication out of reach for many patients, a federal judge in January denied an injunction request by Takeda Pharmaceuticals U.S.A. to halt the distribution of colchicine products by Hikma Pharmaceuticals PLC.
The availability of a generic colchicine will introduce competition into the marketplace and drive down costs, said Dr. E. William St.Clair, president of the American College of Rheumatology and chief of the division of rheumatology and immunology at Duke University, Durham, N.C. The ACR issued a friend-of-the-court brief to the federal district court in support of a generic colchicine product entering the market.
“With the steep price increase in colchicine, many patients with gout were now unable to afford chronic colchicine therapy,” Dr. St.Clair said in an interview. “Improving access to colchicine by making it more affordable will increase patient compliance and reduce the suffering and disability associated with repeated gout flares.”
The debate over colchicine and the right to market the medication has a lengthy history. The drug has been prescribed to treat gout for decades, predating the law that requires drugs to be approved by the Food and Drug Administration. In 2009, the FDA approved a brand name colchicine product (Colcrys) by Mutual Pharmaceutical Company/URL Pharmacy Inc. – now Takeda Pharmaceuticals U.S.A. – after the company conducted clinical trials on dosing regimens and performed drug interaction studies. The FDA’s approval of Colcrys came with exclusive marketing rights for gout for 3 years and for familial Mediterranean fever for 7 years.
Mutual Pharmaceutical Company then sued other manufacturers of colchicine, claiming the drug makers were falsely implying that their products were FDA approved. Shortly later, the FDA ordered companies marketing single-ingredient oral colchicine to remove their unapproved products from the market. Physicians and patients meanwhile saw the price of colchicine increase from about 10 cents per tablet to $5 per tablet.
In September 2014, the FDA granted approval for Hikma to market and sell Mitigare, a colchicine capsule for the prophylactic treatment of gout. Hikma had also planned to launch an authorized generic of Mitigare. Before Mitigare could fully launch, Takeda obtained a temporary restraining order against the sale of colchicine products by Hikma, citing Takeda’s patents for acute gout treatment. Takeda simultaneously sued the FDA in a separate proceeding. Takeda said the FDA’s approval of Hikma’s colchicine product was legally impermissible.
In its brief to the federal court, the ACR argued the public interest would be severely disserved by the barring of Hikma’s colchicine product.
“The unfortunate reality is that nearly 30% of patients in the United States take risky and potentially dangerous steps to save money on prescription medicines, with many choosing to skip doses, or not fill their prescriptions altogether,” the ACR said in its brief. “Takeda’s monopoly and the associated price increase for colchicine has resulted in precisely the sorts of risky behavior described.”
A lower court denied Takeda’s preliminary injunction request and the decision was upheld by the U.S. Court of Appeals for the Federal Circuit on Jan. 9. Pending the outcome of further litigation, the federal court ruled that both Takeda and Hikma are free to immediately offer colchicine products for prophylactic use. Also on Jan. 9, the U.S. District Court for the District of Columbia denied Takeda’s request to overturn the FDA’s approval of Mitigare.
In an interview, a Takeda spokeswoman said the company will continue its patent infringement litigation against Hikma and its U.S. subsidiary, West-Ward Pharmaceuticals, along with its lawsuit against the FDA. The company offered no comment on the judge’s decision to deny the injunction.
West-Ward, meanwhile, launched its authorized generic to Mitigare following the judge’s Jan. 9 decision, and Mitigare’s entry to the market was resumed.
“We immediately sought to launch a generic of Mitigare to ensure that adult patients in need of treatment for the prophylaxis of gout flares had access to a lower-cost, alternative colchicine capsule product,” said Spiro Gavaris, vice president of sales and marketing for West-Ward Pharmaceuticals. “We understood that in recent years, some patients may have lost access to, or became frustrated with, colchicine when there was one brand product at a significantly higher price point. With our launch of the authorized generic of Mitigare capsules, doctors can now choose to prescribe Mitigare for the prophylaxis of gout flares in adults, thereby providing these patients with a lower cost generic medication.”
Days after the decision, Takeda announced that it also would be offering access to a generic colchicine. In a statement, the company said it would partner with Prasco Laboratories to distribute Colchicine Tablets, USP, an authorized generic version of Colcrys. The product is being marketed under the Prasco label and became available in U.S. pharmacies in mid-January.
“At Takeda, we remain committed to providing patients with therapies that are safe, efficacious and meet high quality standards,” Douglas Cole, Takeda Pharmaceuticals President, said in a statement. “This new partnership will help enhance patient access to an important gout medicine by supplying Prasco with Colchicine Tablets, USP, manufactured under the same rigorous standards and processes as Colcrys.”
Prices for the Mitigare authorized generic and the Colcrys authorized generic have not been publicly announced. In an interview, West-Ward said it could not comment on exact savings to patients as savings will vary between insurance plans and pharmacy distribution channels.
“Our goal is to provide the most aggressive discounts on generic colchicine in the market with the intent for those discounts to be passed on to adult patients in need of treatment for the prophylaxis of gout flares,” Mr. Gavaris said.
At this article’s deadline, Takeda and Prasco had not responded to a question about the price of the generic Colcrys.
While rheumatologists expressed relief that generic colchicine products have finally become available, they also voiced concerns about potential barriers to access.
“I am proud of what the ACR did here and would hope that it serves as a model for future efforts by professional medical organizations to get into the (often legal) trenches and truly help their patients get affordable care. … [They] put their mouth where the money is and stood up for patients,” said Dr. Christopher M. Burns, a rheumatologist at the Geisel School of Medicine at Dartmouth College, Lebanon, N.H.
“I hope that this will, in fact, reduce the pricing for colchicine, and that it will not allow payers to add an onerous out-of-pocket cost for the drug far in excess of the cost if paid without utilizing pharmacy benefits,” said Dr. Norman B. Gaylis, a rheumatologist in private practice in Aventura, Fla.
Dr. St.Clair added that the future availability and cost of generic colchicine is not certain given common supply-and-demand problems that can arise with generic drugs.
“We have observed critical shortages of several generic medications during the past several years that have drastically affected medical therapy for many common conditions,” he said in an interview. “We will need to keep a close eye on the supply of generic colchicine to ensure it keeps up with the demand. The FDA and the pharmaceutical industry has an obligation to ensure that patients have access to these critical generic drugs.”
On Twitter @legal_med
After a 5-year monopoly on the sale of colchicine put the gout medication out of reach for many patients, a federal judge in January denied an injunction request by Takeda Pharmaceuticals U.S.A. to halt the distribution of colchicine products by Hikma Pharmaceuticals PLC.
The availability of a generic colchicine will introduce competition into the marketplace and drive down costs, said Dr. E. William St.Clair, president of the American College of Rheumatology and chief of the division of rheumatology and immunology at Duke University, Durham, N.C. The ACR issued a friend-of-the-court brief to the federal district court in support of a generic colchicine product entering the market.
“With the steep price increase in colchicine, many patients with gout were now unable to afford chronic colchicine therapy,” Dr. St.Clair said in an interview. “Improving access to colchicine by making it more affordable will increase patient compliance and reduce the suffering and disability associated with repeated gout flares.”
The debate over colchicine and the right to market the medication has a lengthy history. The drug has been prescribed to treat gout for decades, predating the law that requires drugs to be approved by the Food and Drug Administration. In 2009, the FDA approved a brand name colchicine product (Colcrys) by Mutual Pharmaceutical Company/URL Pharmacy Inc. – now Takeda Pharmaceuticals U.S.A. – after the company conducted clinical trials on dosing regimens and performed drug interaction studies. The FDA’s approval of Colcrys came with exclusive marketing rights for gout for 3 years and for familial Mediterranean fever for 7 years.
Mutual Pharmaceutical Company then sued other manufacturers of colchicine, claiming the drug makers were falsely implying that their products were FDA approved. Shortly later, the FDA ordered companies marketing single-ingredient oral colchicine to remove their unapproved products from the market. Physicians and patients meanwhile saw the price of colchicine increase from about 10 cents per tablet to $5 per tablet.
In September 2014, the FDA granted approval for Hikma to market and sell Mitigare, a colchicine capsule for the prophylactic treatment of gout. Hikma had also planned to launch an authorized generic of Mitigare. Before Mitigare could fully launch, Takeda obtained a temporary restraining order against the sale of colchicine products by Hikma, citing Takeda’s patents for acute gout treatment. Takeda simultaneously sued the FDA in a separate proceeding. Takeda said the FDA’s approval of Hikma’s colchicine product was legally impermissible.
In its brief to the federal court, the ACR argued the public interest would be severely disserved by the barring of Hikma’s colchicine product.
“The unfortunate reality is that nearly 30% of patients in the United States take risky and potentially dangerous steps to save money on prescription medicines, with many choosing to skip doses, or not fill their prescriptions altogether,” the ACR said in its brief. “Takeda’s monopoly and the associated price increase for colchicine has resulted in precisely the sorts of risky behavior described.”
A lower court denied Takeda’s preliminary injunction request and the decision was upheld by the U.S. Court of Appeals for the Federal Circuit on Jan. 9. Pending the outcome of further litigation, the federal court ruled that both Takeda and Hikma are free to immediately offer colchicine products for prophylactic use. Also on Jan. 9, the U.S. District Court for the District of Columbia denied Takeda’s request to overturn the FDA’s approval of Mitigare.
In an interview, a Takeda spokeswoman said the company will continue its patent infringement litigation against Hikma and its U.S. subsidiary, West-Ward Pharmaceuticals, along with its lawsuit against the FDA. The company offered no comment on the judge’s decision to deny the injunction.
West-Ward, meanwhile, launched its authorized generic to Mitigare following the judge’s Jan. 9 decision, and Mitigare’s entry to the market was resumed.
“We immediately sought to launch a generic of Mitigare to ensure that adult patients in need of treatment for the prophylaxis of gout flares had access to a lower-cost, alternative colchicine capsule product,” said Spiro Gavaris, vice president of sales and marketing for West-Ward Pharmaceuticals. “We understood that in recent years, some patients may have lost access to, or became frustrated with, colchicine when there was one brand product at a significantly higher price point. With our launch of the authorized generic of Mitigare capsules, doctors can now choose to prescribe Mitigare for the prophylaxis of gout flares in adults, thereby providing these patients with a lower cost generic medication.”
Days after the decision, Takeda announced that it also would be offering access to a generic colchicine. In a statement, the company said it would partner with Prasco Laboratories to distribute Colchicine Tablets, USP, an authorized generic version of Colcrys. The product is being marketed under the Prasco label and became available in U.S. pharmacies in mid-January.
“At Takeda, we remain committed to providing patients with therapies that are safe, efficacious and meet high quality standards,” Douglas Cole, Takeda Pharmaceuticals President, said in a statement. “This new partnership will help enhance patient access to an important gout medicine by supplying Prasco with Colchicine Tablets, USP, manufactured under the same rigorous standards and processes as Colcrys.”
Prices for the Mitigare authorized generic and the Colcrys authorized generic have not been publicly announced. In an interview, West-Ward said it could not comment on exact savings to patients as savings will vary between insurance plans and pharmacy distribution channels.
“Our goal is to provide the most aggressive discounts on generic colchicine in the market with the intent for those discounts to be passed on to adult patients in need of treatment for the prophylaxis of gout flares,” Mr. Gavaris said.
At this article’s deadline, Takeda and Prasco had not responded to a question about the price of the generic Colcrys.
While rheumatologists expressed relief that generic colchicine products have finally become available, they also voiced concerns about potential barriers to access.
“I am proud of what the ACR did here and would hope that it serves as a model for future efforts by professional medical organizations to get into the (often legal) trenches and truly help their patients get affordable care. … [They] put their mouth where the money is and stood up for patients,” said Dr. Christopher M. Burns, a rheumatologist at the Geisel School of Medicine at Dartmouth College, Lebanon, N.H.
“I hope that this will, in fact, reduce the pricing for colchicine, and that it will not allow payers to add an onerous out-of-pocket cost for the drug far in excess of the cost if paid without utilizing pharmacy benefits,” said Dr. Norman B. Gaylis, a rheumatologist in private practice in Aventura, Fla.
Dr. St.Clair added that the future availability and cost of generic colchicine is not certain given common supply-and-demand problems that can arise with generic drugs.
“We have observed critical shortages of several generic medications during the past several years that have drastically affected medical therapy for many common conditions,” he said in an interview. “We will need to keep a close eye on the supply of generic colchicine to ensure it keeps up with the demand. The FDA and the pharmaceutical industry has an obligation to ensure that patients have access to these critical generic drugs.”
On Twitter @legal_med
After a 5-year monopoly on the sale of colchicine put the gout medication out of reach for many patients, a federal judge in January denied an injunction request by Takeda Pharmaceuticals U.S.A. to halt the distribution of colchicine products by Hikma Pharmaceuticals PLC.
The availability of a generic colchicine will introduce competition into the marketplace and drive down costs, said Dr. E. William St.Clair, president of the American College of Rheumatology and chief of the division of rheumatology and immunology at Duke University, Durham, N.C. The ACR issued a friend-of-the-court brief to the federal district court in support of a generic colchicine product entering the market.
“With the steep price increase in colchicine, many patients with gout were now unable to afford chronic colchicine therapy,” Dr. St.Clair said in an interview. “Improving access to colchicine by making it more affordable will increase patient compliance and reduce the suffering and disability associated with repeated gout flares.”
The debate over colchicine and the right to market the medication has a lengthy history. The drug has been prescribed to treat gout for decades, predating the law that requires drugs to be approved by the Food and Drug Administration. In 2009, the FDA approved a brand name colchicine product (Colcrys) by Mutual Pharmaceutical Company/URL Pharmacy Inc. – now Takeda Pharmaceuticals U.S.A. – after the company conducted clinical trials on dosing regimens and performed drug interaction studies. The FDA’s approval of Colcrys came with exclusive marketing rights for gout for 3 years and for familial Mediterranean fever for 7 years.
Mutual Pharmaceutical Company then sued other manufacturers of colchicine, claiming the drug makers were falsely implying that their products were FDA approved. Shortly later, the FDA ordered companies marketing single-ingredient oral colchicine to remove their unapproved products from the market. Physicians and patients meanwhile saw the price of colchicine increase from about 10 cents per tablet to $5 per tablet.
In September 2014, the FDA granted approval for Hikma to market and sell Mitigare, a colchicine capsule for the prophylactic treatment of gout. Hikma had also planned to launch an authorized generic of Mitigare. Before Mitigare could fully launch, Takeda obtained a temporary restraining order against the sale of colchicine products by Hikma, citing Takeda’s patents for acute gout treatment. Takeda simultaneously sued the FDA in a separate proceeding. Takeda said the FDA’s approval of Hikma’s colchicine product was legally impermissible.
In its brief to the federal court, the ACR argued the public interest would be severely disserved by the barring of Hikma’s colchicine product.
“The unfortunate reality is that nearly 30% of patients in the United States take risky and potentially dangerous steps to save money on prescription medicines, with many choosing to skip doses, or not fill their prescriptions altogether,” the ACR said in its brief. “Takeda’s monopoly and the associated price increase for colchicine has resulted in precisely the sorts of risky behavior described.”
A lower court denied Takeda’s preliminary injunction request and the decision was upheld by the U.S. Court of Appeals for the Federal Circuit on Jan. 9. Pending the outcome of further litigation, the federal court ruled that both Takeda and Hikma are free to immediately offer colchicine products for prophylactic use. Also on Jan. 9, the U.S. District Court for the District of Columbia denied Takeda’s request to overturn the FDA’s approval of Mitigare.
In an interview, a Takeda spokeswoman said the company will continue its patent infringement litigation against Hikma and its U.S. subsidiary, West-Ward Pharmaceuticals, along with its lawsuit against the FDA. The company offered no comment on the judge’s decision to deny the injunction.
West-Ward, meanwhile, launched its authorized generic to Mitigare following the judge’s Jan. 9 decision, and Mitigare’s entry to the market was resumed.
“We immediately sought to launch a generic of Mitigare to ensure that adult patients in need of treatment for the prophylaxis of gout flares had access to a lower-cost, alternative colchicine capsule product,” said Spiro Gavaris, vice president of sales and marketing for West-Ward Pharmaceuticals. “We understood that in recent years, some patients may have lost access to, or became frustrated with, colchicine when there was one brand product at a significantly higher price point. With our launch of the authorized generic of Mitigare capsules, doctors can now choose to prescribe Mitigare for the prophylaxis of gout flares in adults, thereby providing these patients with a lower cost generic medication.”
Days after the decision, Takeda announced that it also would be offering access to a generic colchicine. In a statement, the company said it would partner with Prasco Laboratories to distribute Colchicine Tablets, USP, an authorized generic version of Colcrys. The product is being marketed under the Prasco label and became available in U.S. pharmacies in mid-January.
“At Takeda, we remain committed to providing patients with therapies that are safe, efficacious and meet high quality standards,” Douglas Cole, Takeda Pharmaceuticals President, said in a statement. “This new partnership will help enhance patient access to an important gout medicine by supplying Prasco with Colchicine Tablets, USP, manufactured under the same rigorous standards and processes as Colcrys.”
Prices for the Mitigare authorized generic and the Colcrys authorized generic have not been publicly announced. In an interview, West-Ward said it could not comment on exact savings to patients as savings will vary between insurance plans and pharmacy distribution channels.
“Our goal is to provide the most aggressive discounts on generic colchicine in the market with the intent for those discounts to be passed on to adult patients in need of treatment for the prophylaxis of gout flares,” Mr. Gavaris said.
At this article’s deadline, Takeda and Prasco had not responded to a question about the price of the generic Colcrys.
While rheumatologists expressed relief that generic colchicine products have finally become available, they also voiced concerns about potential barriers to access.
“I am proud of what the ACR did here and would hope that it serves as a model for future efforts by professional medical organizations to get into the (often legal) trenches and truly help their patients get affordable care. … [They] put their mouth where the money is and stood up for patients,” said Dr. Christopher M. Burns, a rheumatologist at the Geisel School of Medicine at Dartmouth College, Lebanon, N.H.
“I hope that this will, in fact, reduce the pricing for colchicine, and that it will not allow payers to add an onerous out-of-pocket cost for the drug far in excess of the cost if paid without utilizing pharmacy benefits,” said Dr. Norman B. Gaylis, a rheumatologist in private practice in Aventura, Fla.
Dr. St.Clair added that the future availability and cost of generic colchicine is not certain given common supply-and-demand problems that can arise with generic drugs.
“We have observed critical shortages of several generic medications during the past several years that have drastically affected medical therapy for many common conditions,” he said in an interview. “We will need to keep a close eye on the supply of generic colchicine to ensure it keeps up with the demand. The FDA and the pharmaceutical industry has an obligation to ensure that patients have access to these critical generic drugs.”
On Twitter @legal_med
Patient-led teledermoscopy appears feasible and effective
Patient-administered teledermoscopy using an iPhone-based mobile dermatoscope attachment and app is an effective and feasible method for short-term monitoring of clinically atypical nevi, with the added benefit of improving patient and physician convenience, based on data from a pilot study of 29 patients.
Researchers found a high level of diagnostic concordance (0.87) between dermatoscope images taken and assessed by an office-based dermatologist and those taken by the patient – albeit in the clinic setting – using the mobile dermatoscope and assessed by a teledermatologist.
All but one of the 29 patients with clinically atypical nevi who completed the study were able to acquire evaluable baseline and follow-up images, the researchers noted. In addition, most of the patients reported that the device was easy to use and that it saved them a trip to the doctor’s office. The study findings were published online Jan. 28 in JAMA Dermatology (doi:10.1001/jamadermatol.2014.3837).
“Under our modality of care, patients needing short-term monitoring will have an established relationship with their dermatologists, who will be the ones identifying concerning lesions that need to be monitored and the ones who evaluate the lesions via teledermoscopy and communicate treatment options directly with the patients,” wrote Xinyuan Wu of Memorial Sloan Kettering Cancer Center, New York, and colleagues.
The authors of an accompanying editorial wrote that recommendations for screening and follow-up for melanoma placed considerable burdens on patients, physicians, and the health care system, and that the patient-led mobile teledermoscopy described in the study was one of a number of options being considered to reduce that burden.
“The study by Wu and colleagues in this issue adds significantly to the discussion on whether regular follow-up visits with clinicians could be replaced by patient self-monitoring with remote feedback by a teledermatologist,” wrote Monika Janda, Ph.D., of the Queensland University of Technology in Brisbane, Australia, and colleagues.
One editorial author reported shares and consultancies with e-derm-consult GmbH and MoleMap, but there were no other conflicts of interest declared.
Patient-administered teledermoscopy using an iPhone-based mobile dermatoscope attachment and app is an effective and feasible method for short-term monitoring of clinically atypical nevi, with the added benefit of improving patient and physician convenience, based on data from a pilot study of 29 patients.
Researchers found a high level of diagnostic concordance (0.87) between dermatoscope images taken and assessed by an office-based dermatologist and those taken by the patient – albeit in the clinic setting – using the mobile dermatoscope and assessed by a teledermatologist.
All but one of the 29 patients with clinically atypical nevi who completed the study were able to acquire evaluable baseline and follow-up images, the researchers noted. In addition, most of the patients reported that the device was easy to use and that it saved them a trip to the doctor’s office. The study findings were published online Jan. 28 in JAMA Dermatology (doi:10.1001/jamadermatol.2014.3837).
“Under our modality of care, patients needing short-term monitoring will have an established relationship with their dermatologists, who will be the ones identifying concerning lesions that need to be monitored and the ones who evaluate the lesions via teledermoscopy and communicate treatment options directly with the patients,” wrote Xinyuan Wu of Memorial Sloan Kettering Cancer Center, New York, and colleagues.
The authors of an accompanying editorial wrote that recommendations for screening and follow-up for melanoma placed considerable burdens on patients, physicians, and the health care system, and that the patient-led mobile teledermoscopy described in the study was one of a number of options being considered to reduce that burden.
“The study by Wu and colleagues in this issue adds significantly to the discussion on whether regular follow-up visits with clinicians could be replaced by patient self-monitoring with remote feedback by a teledermatologist,” wrote Monika Janda, Ph.D., of the Queensland University of Technology in Brisbane, Australia, and colleagues.
One editorial author reported shares and consultancies with e-derm-consult GmbH and MoleMap, but there were no other conflicts of interest declared.
Patient-administered teledermoscopy using an iPhone-based mobile dermatoscope attachment and app is an effective and feasible method for short-term monitoring of clinically atypical nevi, with the added benefit of improving patient and physician convenience, based on data from a pilot study of 29 patients.
Researchers found a high level of diagnostic concordance (0.87) between dermatoscope images taken and assessed by an office-based dermatologist and those taken by the patient – albeit in the clinic setting – using the mobile dermatoscope and assessed by a teledermatologist.
All but one of the 29 patients with clinically atypical nevi who completed the study were able to acquire evaluable baseline and follow-up images, the researchers noted. In addition, most of the patients reported that the device was easy to use and that it saved them a trip to the doctor’s office. The study findings were published online Jan. 28 in JAMA Dermatology (doi:10.1001/jamadermatol.2014.3837).
“Under our modality of care, patients needing short-term monitoring will have an established relationship with their dermatologists, who will be the ones identifying concerning lesions that need to be monitored and the ones who evaluate the lesions via teledermoscopy and communicate treatment options directly with the patients,” wrote Xinyuan Wu of Memorial Sloan Kettering Cancer Center, New York, and colleagues.
The authors of an accompanying editorial wrote that recommendations for screening and follow-up for melanoma placed considerable burdens on patients, physicians, and the health care system, and that the patient-led mobile teledermoscopy described in the study was one of a number of options being considered to reduce that burden.
“The study by Wu and colleagues in this issue adds significantly to the discussion on whether regular follow-up visits with clinicians could be replaced by patient self-monitoring with remote feedback by a teledermatologist,” wrote Monika Janda, Ph.D., of the Queensland University of Technology in Brisbane, Australia, and colleagues.
One editorial author reported shares and consultancies with e-derm-consult GmbH and MoleMap, but there were no other conflicts of interest declared.
FROM JAMA DERMATOLOGY
Key clinical point: Patient-administered teledermoscopy using an iPhone-based mobile dermatoscope attachment and app is an effective and feasible method for short-term monitoring of clinically atypical nevi.
Major finding: Researchers found a high level of diagnostic concordance (0.87) between dermatoscope images taken and assessed by the office-based dermatologist and those taken by the patient using an iPhone.
Data source:A prospective cohort study in 34 patients – 29 of whom completed follow-up – with clinically atypical nevi.
Disclosures: One editorial author reported shares and consultancies with e-derm-consult GmbH and MoleMap. No other conflicts of interest were declared.
Marijuana: The good, the bad, and the ugly
With the recent legalization of marijuana in many states, marijuana and its uses are a hot topic in most social circles. As physicians, we see the full spectrum, from its healing properties to its destructive ones. The goal of this article is not to persuade you into changing positions on its legalization, but rather to stress the importance of remaining neutral and educating families on the facts and potential pros and cons as they relate to the health of their children.
On Jan. 26, 2015,* the American Academy of Pediatrics released its policy statement on marijuana and its use (Pediatrics 2014 [doi:10.1542/peds.2014-4146]). The AAP does not support the legalization of marijuana because of the harm that it poses to children and adolescents, nor does it support legalization of medical marijuana outside the regulatory process of the Food and Drug Administration. It does recognize that marijuana may be an option for children with life-threatening or debilitating illnesses. The AAP does support the decriminalization of marijuana use or possession and advocates for less-harsh criminal penalties. Many of the recommendations were made because of the current research on marijuana and its use.
According to 2014’s Monitoring the Future survey of drug use and attitudes among American 8th, 10th, and 12th graders, marijuana is the most common illegal drug used by adolescents. Among 8th graders, 6.5% reported use; among 10th graders, 16.6% reported use; and 21.2 % of 12th graders reported use. A total of 81% of 12th grade students stated it was easy to get. Marijuana use at all three grade levels was higher than cigarette use (National Institute on Drug Abuse. Drug Facts, 2014). Another study found that early initiation of marijuana use was 6.5 times more likely to result in addiction than if it was initiated after the age of 21 years (Adolescent substance use: America’s #1 public health problem. CASA Columbia, 2011).
One thing we can agree upon is that an adolescent using any substance to mask or lessen the pain of a situation is in trouble. Whether adolescents are overeating or denying themselves food, or using drugs to get high, or behaving promiscuously to get attention, overindulgence is never good. So when we evaluate the effects of marijuana use among teens, we have to separate out the underlying emotional issues from the effects related to the drug. Adolescents are at particular risk for overuse because most lack the experience or maturity to stop when things get out of hand. And they are at risk when using anything that will give them a “high.” Substances like glue, gasoline, and cold medicine can bring them that high, and marijuana is no different – except that it is illegal.
Alcohol, cigarettes, and prescription medications are also vehicles to that desired high. Each has greater addictive properties than marijuana does. According to the Monitoring the Future study, most high school seniors do not think occasional use of marijuana is harmful, with only 36% saying regular use puts you at greater risk, compared with 39.5% in 2013 and 52% in 2009. The perception that marijuana is harmful has definitely declined.
Cannabis smoke contains three times the amount of tar found in tobacco smoke and 50% more carcinogens (N. Engl. J. Med. 1988;318:347). It also can irritate the airways, causing exacerbations of asthma, cystic fibrosis, sputum production, and pharyngitis (Arch. Intern. Med. 2007;167:221). Long-term studies showed that extended use was associated with increased obstructive lung diseases.
There is substantial evidence that indicates that cannabis use can cause psychosis. One review noted evidence that genetic factors may influence the risk of psychosis in adults who used cannabis as adolescents (Biol. Psychiatry 2005;57:1117). Cannabis is believed to release dopamine in the body, which may lead to the psychosis. Another study found that the onset of psychotic illness occurred more than 2 years earlier in patients who were heavy cannabis users (Arch. Gen. Psychiatry 2011;68:555).
Another important finding is that marijuana can suppress testosterone secretion in men, which may result in decreased libido, impotence, and gynecomastia (N. Engl. J. Med. 1974;290:872). Many teens believe cannabis is safe because it’s a plant, and consequently, may not relate these symptoms to its use.
The research on cannabis smoke and its relationship to cancer are limited by inadequate sample sizes and confounding factors not taken into account, but there does seem to be a relationship between cannabis smoke and lung cancer and bladder cancer (J. Psychoactive Drugs 1994;26:285; Urology 2006;67:100). However, head and neck cancers have not shown a relationship to marijuana use (Cancer Epidemiol. Biomarkers Prev. 2009;18:1544-51). Cardiovascular effects have been related to the increased sympathetic activity and decreased parasympathetic activity that can result in bradycardia and hypotension with high doses. This may be of particular concern in older people with coronary artery disease (J. Clin. Pharmacol. 2002;42:58S).
The medicinal properties of marijuana are an important consideration. Marijuana has been shown to be particularly effective in controlling some forms of seizure, pain, nausea from chemotherapy, muscle spasms caused by multiple sclerosis, and Crohn’s disease. The FDA has approved tetrahydrocannabinol, or THC, a key ingredient in marijuana, to treat nausea and improve appetite. In states that have legalized cannabis, qualifying patients can get prescriptions from their physicians to use at authorized dispensaries. For some patients, the effects can be life changing; for others, it can help with pain management and the discomfort associated with certain illnesses.
Beyond the scope of medicine is the economics of the legalization of marijuana. States that have already legalized it have seen revenues in the billions. Marijuana cash crops are estimated at $14 billion in revenue. Jon Gettman’s 2007 study, “Lost Taxes and Other Costs of Marijuana,” states that the prohibition of marijuana costs the government $113 billion, while it costs taxpayers $31.1 billion each year. The study projects that legalization of cannabis may save the criminal justice system $10.7 billion and an additional $6.2 billion for taxpayers. That sort of money does talk: Regardless of current opposition to the legalization of marijuana, it is probably just a matter of time before marijuana is legalized in every state.
The scope of marijuana issues is broad and, for many, controversial. The drug can serve as a healer, create health challenges, lead to drug addiction, or even become a significant revenue source to a state’s coffers. As providers, we need to be able to provide our patients with research-based information and resources, and dispel myths, so that they can make informed decisions for themselves that are in the best interests of their children.
Dr. Pearce is a pediatrician in Frankfort, Ill. She had no relevant financial disclosures. E-mail her at [email protected].
*Correction, 1/29/2015: An earlier version of this story had the incorrect date of publication of the AAP's policy statement.
With the recent legalization of marijuana in many states, marijuana and its uses are a hot topic in most social circles. As physicians, we see the full spectrum, from its healing properties to its destructive ones. The goal of this article is not to persuade you into changing positions on its legalization, but rather to stress the importance of remaining neutral and educating families on the facts and potential pros and cons as they relate to the health of their children.
On Jan. 26, 2015,* the American Academy of Pediatrics released its policy statement on marijuana and its use (Pediatrics 2014 [doi:10.1542/peds.2014-4146]). The AAP does not support the legalization of marijuana because of the harm that it poses to children and adolescents, nor does it support legalization of medical marijuana outside the regulatory process of the Food and Drug Administration. It does recognize that marijuana may be an option for children with life-threatening or debilitating illnesses. The AAP does support the decriminalization of marijuana use or possession and advocates for less-harsh criminal penalties. Many of the recommendations were made because of the current research on marijuana and its use.
According to 2014’s Monitoring the Future survey of drug use and attitudes among American 8th, 10th, and 12th graders, marijuana is the most common illegal drug used by adolescents. Among 8th graders, 6.5% reported use; among 10th graders, 16.6% reported use; and 21.2 % of 12th graders reported use. A total of 81% of 12th grade students stated it was easy to get. Marijuana use at all three grade levels was higher than cigarette use (National Institute on Drug Abuse. Drug Facts, 2014). Another study found that early initiation of marijuana use was 6.5 times more likely to result in addiction than if it was initiated after the age of 21 years (Adolescent substance use: America’s #1 public health problem. CASA Columbia, 2011).
One thing we can agree upon is that an adolescent using any substance to mask or lessen the pain of a situation is in trouble. Whether adolescents are overeating or denying themselves food, or using drugs to get high, or behaving promiscuously to get attention, overindulgence is never good. So when we evaluate the effects of marijuana use among teens, we have to separate out the underlying emotional issues from the effects related to the drug. Adolescents are at particular risk for overuse because most lack the experience or maturity to stop when things get out of hand. And they are at risk when using anything that will give them a “high.” Substances like glue, gasoline, and cold medicine can bring them that high, and marijuana is no different – except that it is illegal.
Alcohol, cigarettes, and prescription medications are also vehicles to that desired high. Each has greater addictive properties than marijuana does. According to the Monitoring the Future study, most high school seniors do not think occasional use of marijuana is harmful, with only 36% saying regular use puts you at greater risk, compared with 39.5% in 2013 and 52% in 2009. The perception that marijuana is harmful has definitely declined.
Cannabis smoke contains three times the amount of tar found in tobacco smoke and 50% more carcinogens (N. Engl. J. Med. 1988;318:347). It also can irritate the airways, causing exacerbations of asthma, cystic fibrosis, sputum production, and pharyngitis (Arch. Intern. Med. 2007;167:221). Long-term studies showed that extended use was associated with increased obstructive lung diseases.
There is substantial evidence that indicates that cannabis use can cause psychosis. One review noted evidence that genetic factors may influence the risk of psychosis in adults who used cannabis as adolescents (Biol. Psychiatry 2005;57:1117). Cannabis is believed to release dopamine in the body, which may lead to the psychosis. Another study found that the onset of psychotic illness occurred more than 2 years earlier in patients who were heavy cannabis users (Arch. Gen. Psychiatry 2011;68:555).
Another important finding is that marijuana can suppress testosterone secretion in men, which may result in decreased libido, impotence, and gynecomastia (N. Engl. J. Med. 1974;290:872). Many teens believe cannabis is safe because it’s a plant, and consequently, may not relate these symptoms to its use.
The research on cannabis smoke and its relationship to cancer are limited by inadequate sample sizes and confounding factors not taken into account, but there does seem to be a relationship between cannabis smoke and lung cancer and bladder cancer (J. Psychoactive Drugs 1994;26:285; Urology 2006;67:100). However, head and neck cancers have not shown a relationship to marijuana use (Cancer Epidemiol. Biomarkers Prev. 2009;18:1544-51). Cardiovascular effects have been related to the increased sympathetic activity and decreased parasympathetic activity that can result in bradycardia and hypotension with high doses. This may be of particular concern in older people with coronary artery disease (J. Clin. Pharmacol. 2002;42:58S).
The medicinal properties of marijuana are an important consideration. Marijuana has been shown to be particularly effective in controlling some forms of seizure, pain, nausea from chemotherapy, muscle spasms caused by multiple sclerosis, and Crohn’s disease. The FDA has approved tetrahydrocannabinol, or THC, a key ingredient in marijuana, to treat nausea and improve appetite. In states that have legalized cannabis, qualifying patients can get prescriptions from their physicians to use at authorized dispensaries. For some patients, the effects can be life changing; for others, it can help with pain management and the discomfort associated with certain illnesses.
Beyond the scope of medicine is the economics of the legalization of marijuana. States that have already legalized it have seen revenues in the billions. Marijuana cash crops are estimated at $14 billion in revenue. Jon Gettman’s 2007 study, “Lost Taxes and Other Costs of Marijuana,” states that the prohibition of marijuana costs the government $113 billion, while it costs taxpayers $31.1 billion each year. The study projects that legalization of cannabis may save the criminal justice system $10.7 billion and an additional $6.2 billion for taxpayers. That sort of money does talk: Regardless of current opposition to the legalization of marijuana, it is probably just a matter of time before marijuana is legalized in every state.
The scope of marijuana issues is broad and, for many, controversial. The drug can serve as a healer, create health challenges, lead to drug addiction, or even become a significant revenue source to a state’s coffers. As providers, we need to be able to provide our patients with research-based information and resources, and dispel myths, so that they can make informed decisions for themselves that are in the best interests of their children.
Dr. Pearce is a pediatrician in Frankfort, Ill. She had no relevant financial disclosures. E-mail her at [email protected].
*Correction, 1/29/2015: An earlier version of this story had the incorrect date of publication of the AAP's policy statement.
With the recent legalization of marijuana in many states, marijuana and its uses are a hot topic in most social circles. As physicians, we see the full spectrum, from its healing properties to its destructive ones. The goal of this article is not to persuade you into changing positions on its legalization, but rather to stress the importance of remaining neutral and educating families on the facts and potential pros and cons as they relate to the health of their children.
On Jan. 26, 2015,* the American Academy of Pediatrics released its policy statement on marijuana and its use (Pediatrics 2014 [doi:10.1542/peds.2014-4146]). The AAP does not support the legalization of marijuana because of the harm that it poses to children and adolescents, nor does it support legalization of medical marijuana outside the regulatory process of the Food and Drug Administration. It does recognize that marijuana may be an option for children with life-threatening or debilitating illnesses. The AAP does support the decriminalization of marijuana use or possession and advocates for less-harsh criminal penalties. Many of the recommendations were made because of the current research on marijuana and its use.
According to 2014’s Monitoring the Future survey of drug use and attitudes among American 8th, 10th, and 12th graders, marijuana is the most common illegal drug used by adolescents. Among 8th graders, 6.5% reported use; among 10th graders, 16.6% reported use; and 21.2 % of 12th graders reported use. A total of 81% of 12th grade students stated it was easy to get. Marijuana use at all three grade levels was higher than cigarette use (National Institute on Drug Abuse. Drug Facts, 2014). Another study found that early initiation of marijuana use was 6.5 times more likely to result in addiction than if it was initiated after the age of 21 years (Adolescent substance use: America’s #1 public health problem. CASA Columbia, 2011).
One thing we can agree upon is that an adolescent using any substance to mask or lessen the pain of a situation is in trouble. Whether adolescents are overeating or denying themselves food, or using drugs to get high, or behaving promiscuously to get attention, overindulgence is never good. So when we evaluate the effects of marijuana use among teens, we have to separate out the underlying emotional issues from the effects related to the drug. Adolescents are at particular risk for overuse because most lack the experience or maturity to stop when things get out of hand. And they are at risk when using anything that will give them a “high.” Substances like glue, gasoline, and cold medicine can bring them that high, and marijuana is no different – except that it is illegal.
Alcohol, cigarettes, and prescription medications are also vehicles to that desired high. Each has greater addictive properties than marijuana does. According to the Monitoring the Future study, most high school seniors do not think occasional use of marijuana is harmful, with only 36% saying regular use puts you at greater risk, compared with 39.5% in 2013 and 52% in 2009. The perception that marijuana is harmful has definitely declined.
Cannabis smoke contains three times the amount of tar found in tobacco smoke and 50% more carcinogens (N. Engl. J. Med. 1988;318:347). It also can irritate the airways, causing exacerbations of asthma, cystic fibrosis, sputum production, and pharyngitis (Arch. Intern. Med. 2007;167:221). Long-term studies showed that extended use was associated with increased obstructive lung diseases.
There is substantial evidence that indicates that cannabis use can cause psychosis. One review noted evidence that genetic factors may influence the risk of psychosis in adults who used cannabis as adolescents (Biol. Psychiatry 2005;57:1117). Cannabis is believed to release dopamine in the body, which may lead to the psychosis. Another study found that the onset of psychotic illness occurred more than 2 years earlier in patients who were heavy cannabis users (Arch. Gen. Psychiatry 2011;68:555).
Another important finding is that marijuana can suppress testosterone secretion in men, which may result in decreased libido, impotence, and gynecomastia (N. Engl. J. Med. 1974;290:872). Many teens believe cannabis is safe because it’s a plant, and consequently, may not relate these symptoms to its use.
The research on cannabis smoke and its relationship to cancer are limited by inadequate sample sizes and confounding factors not taken into account, but there does seem to be a relationship between cannabis smoke and lung cancer and bladder cancer (J. Psychoactive Drugs 1994;26:285; Urology 2006;67:100). However, head and neck cancers have not shown a relationship to marijuana use (Cancer Epidemiol. Biomarkers Prev. 2009;18:1544-51). Cardiovascular effects have been related to the increased sympathetic activity and decreased parasympathetic activity that can result in bradycardia and hypotension with high doses. This may be of particular concern in older people with coronary artery disease (J. Clin. Pharmacol. 2002;42:58S).
The medicinal properties of marijuana are an important consideration. Marijuana has been shown to be particularly effective in controlling some forms of seizure, pain, nausea from chemotherapy, muscle spasms caused by multiple sclerosis, and Crohn’s disease. The FDA has approved tetrahydrocannabinol, or THC, a key ingredient in marijuana, to treat nausea and improve appetite. In states that have legalized cannabis, qualifying patients can get prescriptions from their physicians to use at authorized dispensaries. For some patients, the effects can be life changing; for others, it can help with pain management and the discomfort associated with certain illnesses.
Beyond the scope of medicine is the economics of the legalization of marijuana. States that have already legalized it have seen revenues in the billions. Marijuana cash crops are estimated at $14 billion in revenue. Jon Gettman’s 2007 study, “Lost Taxes and Other Costs of Marijuana,” states that the prohibition of marijuana costs the government $113 billion, while it costs taxpayers $31.1 billion each year. The study projects that legalization of cannabis may save the criminal justice system $10.7 billion and an additional $6.2 billion for taxpayers. That sort of money does talk: Regardless of current opposition to the legalization of marijuana, it is probably just a matter of time before marijuana is legalized in every state.
The scope of marijuana issues is broad and, for many, controversial. The drug can serve as a healer, create health challenges, lead to drug addiction, or even become a significant revenue source to a state’s coffers. As providers, we need to be able to provide our patients with research-based information and resources, and dispel myths, so that they can make informed decisions for themselves that are in the best interests of their children.
Dr. Pearce is a pediatrician in Frankfort, Ill. She had no relevant financial disclosures. E-mail her at [email protected].
*Correction, 1/29/2015: An earlier version of this story had the incorrect date of publication of the AAP's policy statement.
Delusional and aggressive, while playing the lottery
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Delusional and aggressive
Mr. P, age 78, of Filipino heritage, is brought to the psychiatric hospital because he has been verbally aggressive toward his wife for several weeks. He has no history of a psychiatric diagnosis or inpatient psychiatric hospitalization, and no history of taking any psychotropic medications.
According to his wife, Mr. P has been ruminating about his father, who died in World War II, saying that “the Japanese never gave his body back” to him. Also, his wife describes 3 weeks of physically aggressive behavior, such as throwing punches; the last episode was 2 days before admission.
Mr. P is not bathing, eating, taking his medications, and attending to his activities of daily living. He sleeps for only 1 to 2 hours a night; is irritable and easily distractible; and experiences flight of ideas. Mr. P has been buying lottery tickets, telling his daughter that he will become a millionaire and then buy a house in the Philippines.
Mr. P reports depressed mood, but no other depressive symptoms are present. He reports no suicidal or homicidal ideations, auditory or visual hallucinations, or anxiety symptoms. He has no history of substance abuse.
What diagnosis would you give Mr. P?
a) late-onset bipolar disorder
b) Alzheimer’s disease
c) major depressive disorder
d) frontotemporal dementia
The authors’ observations
Bipolar disorder in later life is a complex and confounding neuropsychiatric syndrome with diagnostic and therapeutic challenges. The disorder can affect people of all ages and is not uncommon among geriatric patients, with a 1-year prevalence in United States of 0.4%.1 In one study, 10% of new bipolar disorder cases were found to occur after age 50.2 As the American population grows older, the number of bipolar disorder cases among seniors is expected to increase.3
It was once thought that symptoms of bipolar disorder disappear with age; newer research has disproved this theory, and proposes that untreated bipolar disorder worsens over time.4 Persons who are given the diagnosis later in life could have had bipolar disorder for decades, but symptoms became more noticeable and problematic with age.5
Common symptoms in geriatric patients can differ from what we might expect in younger patients: agitation, hyperactivity, irritability, confusion, and psychosis.6 When the disorder presents in patients age >60, it can be severe, with significant changes in cognitive function, including difficulties with memory, perception, judgment, and problem-solving.7,8
HISTORY Medical comorbidities
Mr. P emigrated from the Philippines 20 years ago, is married, and lives with his wife. He has 3 brothers; his parents were divorced, and his mother remarried. Mr. P completed high school.
Mr. P has an extensive medical history: diabetes mellitus, hypertension, dyslipidemia, and recent double coronary artery bypass grafting. He is taking several medications: sitagliptin, 25 mg/d; pantoprazole, 5 mg/d; metformin, 1,000 mg/d; rivaroxaban, 20 mg/d; amiodarone, 200 mg/d; metoprolol, 12.5 mg/d; olmesartan medoxomil, 40 mg/d; aspirin, 81 mg/d; simvastatin, 10 mg/d; eszopiclone, 3 mg at bedtime; and amlodipine, 5 mg at bedtime.
Mr. P was following up with his primary care physician for his medical conditions and was adherent with treatment until 1 week before he was admitted to our facility.
The authors’ observations
Always rule out medical causes in a case of new-onset mania, which is particularly important in geriatric patients. Older patients with new-onset mania are more than twice as likely to have a comorbid neurologic disorder.9 Neurologic causes of late-onset mania include:
• stroke
• tumor
• epilepsy
• Huntington’s disease and other movement disorders
• multiple sclerosis and other white-matter diseases
• head trauma
• infection (such as neurosyphilis)
• Creutzfeldt-Jakob disease
• frontotemporal dementia.10
Mr. P’s presentation of psychomotor agitation, impaired functioning, decreased need for sleep, increased energy, hyperverbal speech, and complex paranoid delusions meets DSM-5 criteria for bipolar disorder, manic phase. In addition, older manic patients frequently present with confusion, disorientation, and distractibility. Younger patients with mania often present with euphoric moods and grandiosity; in contrast, geriatric patients are more likely to show a mixture of depressed affect and manic symptoms (pressured speech and a decreased need for sleep).11-15
We considered an emerging neurodegenerative process, because dementia can present early with disinhibition, lability, and other behavioral disturbances, including classic manic syndromes.16 Although we could not fully rule out a neurodegenerative process in the initial phase of treatment, Mr. P’s longitudinal course demonstrated no change in baseline cognitive function and no evidence of subsequent decline, making dementia unlikely.17
Patients with frontotemporal dementia are more likely to present initially to a psychiatrist than to a neurologist.18
Frontotemporal dementia is a progressive neurodegenerative disease that affects the frontal and temporal cortices; it is a common cause of dementia in patients age <65.19 Frontotemporal dementia is characterized by insidious behavioral and personality changes; often, the initial presentation lacks any clear neurologic signs or symptoms. Key features include apathy, disinhibition, loss of sympathy and empathy, repetitive motor behaviors, and overeating.20
Mr. P’s symptoms stabilized with divalproex sprinkles and risperidone. There was no evidence of decline in memory, social interaction, or behavior.
EVALUATION Paranoia
On mental status exam, Mr. P has an appropriate appearance; he is clean and shaven, with good eye contact. Muscular tone and gait are within normal limits. Level of activity is increased; he exhibits psychomotor agitation. Speech is rapid, over-productive, and loud; thought process shows flight of ideas, and thought associations are circumstantial.
Mr. P has paranoid delusions about the staff trying to hurt him. His judgment is poor, evidenced by an inability to take care of himself. Insight is minimal, as seen by noncompliance with treatment. Mr. P is oriented only to person and place. His mood is anxious; affect is labile.
Complete blood count, comprehensive metabolic profile, blood alcohol level, urine analysis, urine toxicology, electrocardiogram, and CT scan of the head are within normal limits.
Mr. P is given a diagnosis of mood disorder due to general medical condition, psychotic disorder due to general medical condition. The team rules out acute delirium, bipolar I disorder, and neurodegenerative disorders such as frontotemporal dementia.
Mr. P is maintained on pre-admission medications for his medical conditions. A mood stabilizer, divalproex sprinkles, 250 mg/d, is added.
Once on the unit, Mr. P is re-evaluated. Divalproex is increased to 500 mg/d; risperidone, 0.5 mg/d, is added to address paranoia. Mr. P also receives group and individual psychotherapy. He does not participate in neuropsychological testing, and no single-photon emission CT analysis is done. Mr. P remains in the hospital for 2 weeks. After a family meeting, his daughter says she feels comfortable taking Mr. P home. He follows up in the outpatient clinic and is doing well.
The authors’ observations
Treating geriatric patients with bipolar disorder requires attention to several factors (Table). Older patients might tolerate or metabolize medications differently than younger adults, and therefore may need a different dosage. Older patients are more likely to have comorbid medical conditions and to be taking medications for those ailments. Treatment is much more complicated for this age group because physicians need to account for possible drug-drug interactions.21
A number of medications can be helpful in treating older patients who have bipolar disorder.11 Ongoing research compares lithium with anticonvulsants in older bipolar disorder patients to determine which drug has the greatest benefit with the lowest risk of side effects.
Psychotherapy can be a valuable addition to pharmacotherapy in older adults. Some psychotherapy programs are specifically geared to older bipolar disorder patients.22,23
Use of divalproex sodium in older patients
First, perform baseline laboratory tests: complete blood count, liver function, and electrocardiogram. Initiate divalproex sodium, 250 mg at bedtime, increasing the dosage every 3 to 5 days by 250 mg, with a target dose of 500 to 2,000 mg/d (divided into 2 or 3 doses). Monitor serum levels; levels of 29 to 100 μg/mL are effective and well tolerated. Common side effects include excess sedation, ataxia, tremor, nausea, and, rarely, hepatotoxicity, leukopenia, and thrombocytopenia.24
Use of lithium in geriatric patients
First, perform baseline laboratory tests: electrolytes, creatinine, blood urea nitrogen, urine, thyroid stimulating hormone, and electrocardiogram. Starting dosage is 300 mg at bedtime (150 mg for frail cachectic patients). Monitor serum levels 12 hours after last dose, adjusting dosage every 5 days until a target serum level of 0.5 to 0.8 mEq/L is reached. Common dosages for geriatric patients are 300 to 600 mg/d, which often can be given as a single bedtime dose. Cautions: When using lithium with a thiazide diuretic or nonsteroidal anti-inflammatory drug, watch for dehydration, vomiting, and diarrhea, which will elevate the serum lithium level. Side effects include ataxia, tremor, urinary frequency, thirst, nausea, diarrhea, hypothyroidism, and exacerbation of psoriasis. Once stabilized, monitor the serum lithium level, thyroid-stimulating hormone, and kidney function every 3 to 6 months.24
Bottom Line
In geriatric patients, bipolar disorder can present with agitation, irritability, confusion, and psychosis, rather than euphoric mood and grandiosity. When you suspect bipolar disorder in an older patient, first rule out medical causes of symptoms. When selecting treatment, consider comorbid medical conditions and possible drug-drug interactions.
Related Resources
• Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
• Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
Drug Brand Names
Amiodarone • Cordarone Olanzapine • Zyprexa
Amlodipine • Norvasc Olmesartan medoxomil • Benicar
Divalproex sodium • Depakote Pantoprazole • Protonix
Eszopiclone • Lunesta Risperidone • Risperdal
Lithium • Eskalith, Lithobid Rivaroxaban • Xarelto
Lorazepam • Ativan Simvastatin • Zocor
Metformin • Glucophage Sitagliptin • Januvia
Metoprolol • Lopressor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
1. Weissman MM, Leaf PJ, Tischler GL, et al. Affective disorders in five United States communities. Psychol Med. 1988;18(1):141-153.
2. Yassa R, Nair NP, Iskandar H. Late-onset bipolar disorder. Psychiatr Clin North Am. 1988;11(1):117-131.
3. Verdoux H, Bourgeois M. Secondary mania caused by cerebral organic pathology [in French]. Ann Med Psychol (Paris). 1995;153(3):161-168.
4. Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness in the patient’s family. Br J Psychiatry. 1987;150:285-292.
5. Yassa R, Nair V, Nastase C, et al. Prevalence of bipolar disorder in a psychogeriatric population. J Affect Disord. 1988;14(3):197-201.
6. Robinson RG, Boston JD, Starkstein SE, et al. Comparison of mania with depression following brain injury: casual factors. Am J Psychiatry. 1988;145(2):172-178.
7. Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis. 1988;176(2):87-100.
8. Herrmann N, Bremner KE, Naranjo CA. Pharmacotherapy of late life mood disorders. Clin Neurosci. 1997;4(1):41-47.
9. Tohen M, Shulman KI, Satlin A. First-episode mania in late life. Am J Psychiatry. 1994;151(1):130-132.
10. Mendez MF. Mania in neurologic disorders. Curr Psychiatry Rep. 2000;2(5):440-445.
11. Eagles JM, Whalley LJ. Aging and affective disorders: the age at first onset of affective disorders in Scotland, 1969- 1978. Br J Psychiatry. 1985;147:180-187.
12. Snowdon J. A retrospective case-note study of bipolar disorder in old age. Br J Psychiatry. 1991;158:485-490.
13. Winokur G. The Iowa 500: heterogeneity and course in manic-depressive illness (bipolar). Compr Psychiatry. 1975;16(2):125-131.
14. Shulman K, Post F. Bipolar affective disorder in old age. Br J Psychiatry. 1980;136:26-32.
15. Young RC, Falk JR. Age, manic psychopathology, and treatment response. Int J Geriatr Psychiatry. 1989;4(2):73-78.
16. Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder [in Portuguese]? Rev Bras Psiquiatr. 2004;26(suppl 3):27-30.
17. Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
18. Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811-1816.
19. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615-1621.
20. Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl. 1996;47:103-123.
21. Broadhead J, Jacoby R. Mania in old age: a first prospective study. Int J Geriatr Psychiatry. 1990;5(4):215-222.
22. Dhingra U, Rabins PV. Mania in the elderly: a 5-7 year follow-up. J Am Geriatr Soc. 1991;39(6):581-583.
23. Shulman KI. Neurologic comorbidity and mania in old age. Clin Neurosci. 1997;4(1):37-40.
24. Shulman KI, Herrmann N. Bipolar disorder in old age. Can Fam Physician. 1999;45:1229-1237.
Do glutamatergic drugs have a role in treating depression?
Mrs. S, age 46, has been struggling to manage depression for 7 years. She completed adequate trials of several selective serotonin reuptake inhibitors and bupropion. Currently, she is taking duloxetine, 60 mg/d, and aripiprazole, 5 mg/d.
At her most recent clinic visit, Mrs. S reports that she is doing “OK,” but that she still feels sad and disengaged most days of the week. She wants to know more about ketamine for treating depression after reading about it on the Internet and hearing it mentioned in a support group she attends. She asks if you think it would work for her, and gives you with a copy of an article about its use in patients with treatment-resistant depression. Mrs. S has no other health conditions and takes a daily vitamin D and calcium supplement.
The monoamine hypothesis of depression postulates that symptoms originate from underactivity of monoamines, such as serotonin, norepinephrine, and dopamine, in the brain. This hypothesis was formulated in the 1960s after researchers observed that monoamine oxidase inhibitors and tricyclic antidepressants relieved depressive symptoms; both were known to increase monoamine concentrations in the synaptic cleft.1
Regrettably, these medications do not adequately relieve depressive symptoms for many people. In fact, symptom remission occurs in only one-third of treated patients.2 This low remission rate reflects a lack of understanding of the pathophysiology of depression, and the need for drugs with unique mechanisms of action.
One of the newest drug targets shown to be relevant in psychiatric illness is the
glutamatergic system. Glutamate is the predominant excitatory neurotransmitter in the CNS, and it is responsible for many key functions, including synaptic plasticity, learning, memory, and locomotion.3 Normally, the glutamatergic system tightly regulates the amount of glutamate in the neuronal synapse via receptors on presynaptic and postsynaptic neurons, as well as on glial cells (Figure). When this equilibrium is disrupted in stressful situations, such as ischemia, trauma, or seizures, excess glutamate is released into the synapse. The resulting glutamatergic hyperactivity can lead to neurotoxicity and cell death when neuronal receptors are activated for an extended period.
A key component of the glutamatergic system that is responsible for removing excess glutamate from the synapse is membrane-bound transporters, which are similar to serotonin and norepinephrine transporters. These excitatory amino acid transporters (EAATs) are important because glutamate metabolism does not occur within the synapse and EAATS are responsible for removing most of the glutamate from the synapse into glial cells.3
The network of receptors within the synapse that are activated by glutamate is extensive and complex. There are at least 11 glutamate-responsive receptors: 3 are ionotropic action channels, and the remaining 8 are metabotropic G protein-coupled receptors. Previous studies have shown regional changes in glutamate receptors, as well as elevated levels of glutamate, in the brains of patients with major depressive disorder (MDD).4
Ketamine. The ionotropic receptor N-methyl-d-aspartate (NMDA) is one of the most studied glutamate receptors. Pharmacologically, ketamine is a noncompetitive NMDA receptor antagonist that also activates the amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, which is another subtype of ionotropic glutamate receptors. In open-label clinical trials, ketamine has demonstrated rapid antidepressant action in patients with treatment-resistant MDD.4,5
Recently, Murrough et al6 performed the first randomized, psychoactive controlled trial using a single IV infusion of ketamine dosed below anesthesia ranges (0.5 mg/kg), or midazolam (0.045 mg/kg), in patients with treatment-resistant depression who had been antidepressant-free for at least 4 weeks. They found that 24 hours after medication administration, the likelihood of response to ketamine was significantly higher than the response to midazolam (OR: 2.18; 95% CI: 1.21 to 4.14), with a response rate of 64% in the ketamine group and 28% in the midazolam group.6
Psychotropic side effects, such as hallucinations, are a major concern with ketamine tolerability and abuse potential. This is largely because of ketamine’s antagonism of the NMDA receptor, which is a property shared with other abused drugs such as phencyclidine (PCP) and dextromethorphan. In the Murrough et al6 study, there were no reported cases of paranoia or hallucinations, but dissociative symptoms were relatively common (17%).
Although the results in this trial appear encouraging, there are several limitations to using ketamine to treat MDD, especially in an ambulatory setting. Concerns include ketamine’s IV administration, potential for abuse, long-term efficacy, and side-effect profile—particularly psychotic symptoms and hemodynamic changes. An ideal compound would have the rapid efficacy of ketamine, but with a safer side-effect profile, easier administration, and less potential for abuse.
Riluzole also acts on the glutamatergic system, but has not shown antidepressant efficacy as consistently as ketamine. Riluzole is FDA-approved for treating amyotrophic lateral sclerosis.5 Pharmacologically, riluzole is a glutamatergic modulator that increases glutamate reuptake into glial cells, decreases glutamate release, and increases AMPA trafficking. In open-label studies riluzole has shown efficacy in reducing depressive symptoms.4,5 However, when compared with placebo as a means of sustaining treatment response after a 1-time dose of ketamine, riluzole showed was no significant improvement in time to depressive relapse.7
Acamprosate, often used for treating alcohol abuse, is another a drug with glutamatergic activity that has been studied for possible use as an antidepressant.5
A review by Lapidus et al5 has a more extensive listing of current medications and investigational compounds that modulate glutamate transmission, and are of interest for their possible antidepressant activity. Given the relatively new “glutamatergic hypothesis” of depression, it is exciting that so many current and novel glutamatergic drug therapies are being evaluated.
Future of ketamine treatment
Glutamate has been shown to play an important part in the pathophysiology of depression. The rapid antidepressant efficacy of ketamine provides evidence that future medications with glutamate-modulating activity could be useful for patients who struggle to achieve symptom relief using available antidepressants. Several limitations exist regarding ketamine use, and more work in this important therapeutic area needs to be done. This last point is important to remember when speaking with patients such as Mrs. S. Although it is understandable for her to be excited about novel treatment options such as ketamine, stress to her that treating depression with ketamine at this time is strictly investigational, and that the drug needs to be thoroughly evaluated for safety and efficacy before it can be prescribed for this indication.
CASE CONTINUED
Mrs. S realizes that ketamine may not be the best next step for her, and she agrees to explore other approaches to treat her residual depressive symptoms.
Related Resources
• Machado-Vieira R, Ibrahim L, Henter ID, et al. Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol Biochem Behav. 2012;100(4):678-687.
• Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72(10):1313-1333.
Drug Brand Names
Acamprosate • Campral Duloxetine • Cymbalta
Aripiprazole • Abilify Ketamine • Ketalar
Bupropion • Wellbutrin, Zyban Riluzole • Rilutek
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. Niciu MJ, Ionescu DF, Richards EM, et al. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm. 2014;121(8):907-924.
2. Gaynes BN, Dusetzina SB, Ellis AR, et al. Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32(1):114-119.
3. Curry SC, Mills KC, Ruha A, et al. Neurotransmitters and neuromodulators. In: Nelson LS, Lewin NA, Howland MA, et al, eds. Goldfrank’s toxicologic emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:189-220.
4. Zarate C Jr, Machado-Vieira R, Henter I, et al. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18(5):293-303.
5. Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr Dis Treat. 2013;9:1101-1112.
6. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
7. Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37(6):1526-1533.
Mrs. S, age 46, has been struggling to manage depression for 7 years. She completed adequate trials of several selective serotonin reuptake inhibitors and bupropion. Currently, she is taking duloxetine, 60 mg/d, and aripiprazole, 5 mg/d.
At her most recent clinic visit, Mrs. S reports that she is doing “OK,” but that she still feels sad and disengaged most days of the week. She wants to know more about ketamine for treating depression after reading about it on the Internet and hearing it mentioned in a support group she attends. She asks if you think it would work for her, and gives you with a copy of an article about its use in patients with treatment-resistant depression. Mrs. S has no other health conditions and takes a daily vitamin D and calcium supplement.
The monoamine hypothesis of depression postulates that symptoms originate from underactivity of monoamines, such as serotonin, norepinephrine, and dopamine, in the brain. This hypothesis was formulated in the 1960s after researchers observed that monoamine oxidase inhibitors and tricyclic antidepressants relieved depressive symptoms; both were known to increase monoamine concentrations in the synaptic cleft.1
Regrettably, these medications do not adequately relieve depressive symptoms for many people. In fact, symptom remission occurs in only one-third of treated patients.2 This low remission rate reflects a lack of understanding of the pathophysiology of depression, and the need for drugs with unique mechanisms of action.
One of the newest drug targets shown to be relevant in psychiatric illness is the
glutamatergic system. Glutamate is the predominant excitatory neurotransmitter in the CNS, and it is responsible for many key functions, including synaptic plasticity, learning, memory, and locomotion.3 Normally, the glutamatergic system tightly regulates the amount of glutamate in the neuronal synapse via receptors on presynaptic and postsynaptic neurons, as well as on glial cells (Figure). When this equilibrium is disrupted in stressful situations, such as ischemia, trauma, or seizures, excess glutamate is released into the synapse. The resulting glutamatergic hyperactivity can lead to neurotoxicity and cell death when neuronal receptors are activated for an extended period.
A key component of the glutamatergic system that is responsible for removing excess glutamate from the synapse is membrane-bound transporters, which are similar to serotonin and norepinephrine transporters. These excitatory amino acid transporters (EAATs) are important because glutamate metabolism does not occur within the synapse and EAATS are responsible for removing most of the glutamate from the synapse into glial cells.3
The network of receptors within the synapse that are activated by glutamate is extensive and complex. There are at least 11 glutamate-responsive receptors: 3 are ionotropic action channels, and the remaining 8 are metabotropic G protein-coupled receptors. Previous studies have shown regional changes in glutamate receptors, as well as elevated levels of glutamate, in the brains of patients with major depressive disorder (MDD).4
Ketamine. The ionotropic receptor N-methyl-d-aspartate (NMDA) is one of the most studied glutamate receptors. Pharmacologically, ketamine is a noncompetitive NMDA receptor antagonist that also activates the amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, which is another subtype of ionotropic glutamate receptors. In open-label clinical trials, ketamine has demonstrated rapid antidepressant action in patients with treatment-resistant MDD.4,5
Recently, Murrough et al6 performed the first randomized, psychoactive controlled trial using a single IV infusion of ketamine dosed below anesthesia ranges (0.5 mg/kg), or midazolam (0.045 mg/kg), in patients with treatment-resistant depression who had been antidepressant-free for at least 4 weeks. They found that 24 hours after medication administration, the likelihood of response to ketamine was significantly higher than the response to midazolam (OR: 2.18; 95% CI: 1.21 to 4.14), with a response rate of 64% in the ketamine group and 28% in the midazolam group.6
Psychotropic side effects, such as hallucinations, are a major concern with ketamine tolerability and abuse potential. This is largely because of ketamine’s antagonism of the NMDA receptor, which is a property shared with other abused drugs such as phencyclidine (PCP) and dextromethorphan. In the Murrough et al6 study, there were no reported cases of paranoia or hallucinations, but dissociative symptoms were relatively common (17%).
Although the results in this trial appear encouraging, there are several limitations to using ketamine to treat MDD, especially in an ambulatory setting. Concerns include ketamine’s IV administration, potential for abuse, long-term efficacy, and side-effect profile—particularly psychotic symptoms and hemodynamic changes. An ideal compound would have the rapid efficacy of ketamine, but with a safer side-effect profile, easier administration, and less potential for abuse.
Riluzole also acts on the glutamatergic system, but has not shown antidepressant efficacy as consistently as ketamine. Riluzole is FDA-approved for treating amyotrophic lateral sclerosis.5 Pharmacologically, riluzole is a glutamatergic modulator that increases glutamate reuptake into glial cells, decreases glutamate release, and increases AMPA trafficking. In open-label studies riluzole has shown efficacy in reducing depressive symptoms.4,5 However, when compared with placebo as a means of sustaining treatment response after a 1-time dose of ketamine, riluzole showed was no significant improvement in time to depressive relapse.7
Acamprosate, often used for treating alcohol abuse, is another a drug with glutamatergic activity that has been studied for possible use as an antidepressant.5
A review by Lapidus et al5 has a more extensive listing of current medications and investigational compounds that modulate glutamate transmission, and are of interest for their possible antidepressant activity. Given the relatively new “glutamatergic hypothesis” of depression, it is exciting that so many current and novel glutamatergic drug therapies are being evaluated.
Future of ketamine treatment
Glutamate has been shown to play an important part in the pathophysiology of depression. The rapid antidepressant efficacy of ketamine provides evidence that future medications with glutamate-modulating activity could be useful for patients who struggle to achieve symptom relief using available antidepressants. Several limitations exist regarding ketamine use, and more work in this important therapeutic area needs to be done. This last point is important to remember when speaking with patients such as Mrs. S. Although it is understandable for her to be excited about novel treatment options such as ketamine, stress to her that treating depression with ketamine at this time is strictly investigational, and that the drug needs to be thoroughly evaluated for safety and efficacy before it can be prescribed for this indication.
CASE CONTINUED
Mrs. S realizes that ketamine may not be the best next step for her, and she agrees to explore other approaches to treat her residual depressive symptoms.
Related Resources
• Machado-Vieira R, Ibrahim L, Henter ID, et al. Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol Biochem Behav. 2012;100(4):678-687.
• Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72(10):1313-1333.
Drug Brand Names
Acamprosate • Campral Duloxetine • Cymbalta
Aripiprazole • Abilify Ketamine • Ketalar
Bupropion • Wellbutrin, Zyban Riluzole • Rilutek
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Mrs. S, age 46, has been struggling to manage depression for 7 years. She completed adequate trials of several selective serotonin reuptake inhibitors and bupropion. Currently, she is taking duloxetine, 60 mg/d, and aripiprazole, 5 mg/d.
At her most recent clinic visit, Mrs. S reports that she is doing “OK,” but that she still feels sad and disengaged most days of the week. She wants to know more about ketamine for treating depression after reading about it on the Internet and hearing it mentioned in a support group she attends. She asks if you think it would work for her, and gives you with a copy of an article about its use in patients with treatment-resistant depression. Mrs. S has no other health conditions and takes a daily vitamin D and calcium supplement.
The monoamine hypothesis of depression postulates that symptoms originate from underactivity of monoamines, such as serotonin, norepinephrine, and dopamine, in the brain. This hypothesis was formulated in the 1960s after researchers observed that monoamine oxidase inhibitors and tricyclic antidepressants relieved depressive symptoms; both were known to increase monoamine concentrations in the synaptic cleft.1
Regrettably, these medications do not adequately relieve depressive symptoms for many people. In fact, symptom remission occurs in only one-third of treated patients.2 This low remission rate reflects a lack of understanding of the pathophysiology of depression, and the need for drugs with unique mechanisms of action.
One of the newest drug targets shown to be relevant in psychiatric illness is the
glutamatergic system. Glutamate is the predominant excitatory neurotransmitter in the CNS, and it is responsible for many key functions, including synaptic plasticity, learning, memory, and locomotion.3 Normally, the glutamatergic system tightly regulates the amount of glutamate in the neuronal synapse via receptors on presynaptic and postsynaptic neurons, as well as on glial cells (Figure). When this equilibrium is disrupted in stressful situations, such as ischemia, trauma, or seizures, excess glutamate is released into the synapse. The resulting glutamatergic hyperactivity can lead to neurotoxicity and cell death when neuronal receptors are activated for an extended period.
A key component of the glutamatergic system that is responsible for removing excess glutamate from the synapse is membrane-bound transporters, which are similar to serotonin and norepinephrine transporters. These excitatory amino acid transporters (EAATs) are important because glutamate metabolism does not occur within the synapse and EAATS are responsible for removing most of the glutamate from the synapse into glial cells.3
The network of receptors within the synapse that are activated by glutamate is extensive and complex. There are at least 11 glutamate-responsive receptors: 3 are ionotropic action channels, and the remaining 8 are metabotropic G protein-coupled receptors. Previous studies have shown regional changes in glutamate receptors, as well as elevated levels of glutamate, in the brains of patients with major depressive disorder (MDD).4
Ketamine. The ionotropic receptor N-methyl-d-aspartate (NMDA) is one of the most studied glutamate receptors. Pharmacologically, ketamine is a noncompetitive NMDA receptor antagonist that also activates the amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, which is another subtype of ionotropic glutamate receptors. In open-label clinical trials, ketamine has demonstrated rapid antidepressant action in patients with treatment-resistant MDD.4,5
Recently, Murrough et al6 performed the first randomized, psychoactive controlled trial using a single IV infusion of ketamine dosed below anesthesia ranges (0.5 mg/kg), or midazolam (0.045 mg/kg), in patients with treatment-resistant depression who had been antidepressant-free for at least 4 weeks. They found that 24 hours after medication administration, the likelihood of response to ketamine was significantly higher than the response to midazolam (OR: 2.18; 95% CI: 1.21 to 4.14), with a response rate of 64% in the ketamine group and 28% in the midazolam group.6
Psychotropic side effects, such as hallucinations, are a major concern with ketamine tolerability and abuse potential. This is largely because of ketamine’s antagonism of the NMDA receptor, which is a property shared with other abused drugs such as phencyclidine (PCP) and dextromethorphan. In the Murrough et al6 study, there were no reported cases of paranoia or hallucinations, but dissociative symptoms were relatively common (17%).
Although the results in this trial appear encouraging, there are several limitations to using ketamine to treat MDD, especially in an ambulatory setting. Concerns include ketamine’s IV administration, potential for abuse, long-term efficacy, and side-effect profile—particularly psychotic symptoms and hemodynamic changes. An ideal compound would have the rapid efficacy of ketamine, but with a safer side-effect profile, easier administration, and less potential for abuse.
Riluzole also acts on the glutamatergic system, but has not shown antidepressant efficacy as consistently as ketamine. Riluzole is FDA-approved for treating amyotrophic lateral sclerosis.5 Pharmacologically, riluzole is a glutamatergic modulator that increases glutamate reuptake into glial cells, decreases glutamate release, and increases AMPA trafficking. In open-label studies riluzole has shown efficacy in reducing depressive symptoms.4,5 However, when compared with placebo as a means of sustaining treatment response after a 1-time dose of ketamine, riluzole showed was no significant improvement in time to depressive relapse.7
Acamprosate, often used for treating alcohol abuse, is another a drug with glutamatergic activity that has been studied for possible use as an antidepressant.5
A review by Lapidus et al5 has a more extensive listing of current medications and investigational compounds that modulate glutamate transmission, and are of interest for their possible antidepressant activity. Given the relatively new “glutamatergic hypothesis” of depression, it is exciting that so many current and novel glutamatergic drug therapies are being evaluated.
Future of ketamine treatment
Glutamate has been shown to play an important part in the pathophysiology of depression. The rapid antidepressant efficacy of ketamine provides evidence that future medications with glutamate-modulating activity could be useful for patients who struggle to achieve symptom relief using available antidepressants. Several limitations exist regarding ketamine use, and more work in this important therapeutic area needs to be done. This last point is important to remember when speaking with patients such as Mrs. S. Although it is understandable for her to be excited about novel treatment options such as ketamine, stress to her that treating depression with ketamine at this time is strictly investigational, and that the drug needs to be thoroughly evaluated for safety and efficacy before it can be prescribed for this indication.
CASE CONTINUED
Mrs. S realizes that ketamine may not be the best next step for her, and she agrees to explore other approaches to treat her residual depressive symptoms.
Related Resources
• Machado-Vieira R, Ibrahim L, Henter ID, et al. Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol Biochem Behav. 2012;100(4):678-687.
• Mathews DC, Henter ID, Zarate CA. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs. 2012;72(10):1313-1333.
Drug Brand Names
Acamprosate • Campral Duloxetine • Cymbalta
Aripiprazole • Abilify Ketamine • Ketalar
Bupropion • Wellbutrin, Zyban Riluzole • Rilutek
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. Niciu MJ, Ionescu DF, Richards EM, et al. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm. 2014;121(8):907-924.
2. Gaynes BN, Dusetzina SB, Ellis AR, et al. Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32(1):114-119.
3. Curry SC, Mills KC, Ruha A, et al. Neurotransmitters and neuromodulators. In: Nelson LS, Lewin NA, Howland MA, et al, eds. Goldfrank’s toxicologic emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:189-220.
4. Zarate C Jr, Machado-Vieira R, Henter I, et al. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18(5):293-303.
5. Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr Dis Treat. 2013;9:1101-1112.
6. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
7. Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37(6):1526-1533.
1. Niciu MJ, Ionescu DF, Richards EM, et al. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm. 2014;121(8):907-924.
2. Gaynes BN, Dusetzina SB, Ellis AR, et al. Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32(1):114-119.
3. Curry SC, Mills KC, Ruha A, et al. Neurotransmitters and neuromodulators. In: Nelson LS, Lewin NA, Howland MA, et al, eds. Goldfrank’s toxicologic emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:189-220.
4. Zarate C Jr, Machado-Vieira R, Henter I, et al. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18(5):293-303.
5. Lapidus KA, Soleimani L, Murrough JW. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr Dis Treat. 2013;9:1101-1112.
6. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
7. Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37(6):1526-1533.
More on insomnia disorders in older patients
Regarding Drs. Irene S. Hong’s and Jeffrey R. Bishop’s article, “Sedative-hypnotics for sleepless geriatric patients: Choose wisely” (Current Psychiatry, 2014;13(10):36-39, 46-50, 52 [http://bit.ly/1ApmcoO]), which undertook a comprehensive review of current therapies for insomnia in geriatric patients, here are 3 clarifications.
• I want to reinforce the latest thinking about the nature and pathophysiology of insomnia. DSM-5 classifies insomnia as a disorder, not as a symptom of other problems; the concept of “secondary insomnia” is rejected in DSM-5. Insomnia typically is seen as comorbid with other medical and psychiatric disorders. Often, insomnia predates the comorbid disorder (eg, depression), but rarely is it resolved by treating the comorbid condition.
• Good clinical practice, therefore, requires treating the comorbid condition and the insomnia each directly.
• The insomnia disorder manifests itself, in part, by a report of difficulty falling asleep or staying asleep. The authors use the example of sleep-onset insomnia as typical in older adults. However, sleep maintenance and early morning awakenings are the most common symptoms among geriatric insomnia patients.
• The authors mention only in passing an important medication for sleep maintenance in adults and in the geriatric patient specifically: doxepin. Low-dose doxepin, at 3 mg (for the geriatric patient) and 6 mg, is FDA-approved as a nonscheduled hypnotic for sleep maintenance insomnia. This formulationa is the only hypnotic classified as safe for geriatric patients in the 2012 Beers Criteria Update of the American Geriatrics Society.1 Unlike higher dosages of doxepin, the action of low-dose doxepin is, essentially, selective H1 antagonism.
aSold as Silenor.
Reference
1. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
Regarding Drs. Irene S. Hong’s and Jeffrey R. Bishop’s article, “Sedative-hypnotics for sleepless geriatric patients: Choose wisely” (Current Psychiatry, 2014;13(10):36-39, 46-50, 52 [http://bit.ly/1ApmcoO]), which undertook a comprehensive review of current therapies for insomnia in geriatric patients, here are 3 clarifications.
• I want to reinforce the latest thinking about the nature and pathophysiology of insomnia. DSM-5 classifies insomnia as a disorder, not as a symptom of other problems; the concept of “secondary insomnia” is rejected in DSM-5. Insomnia typically is seen as comorbid with other medical and psychiatric disorders. Often, insomnia predates the comorbid disorder (eg, depression), but rarely is it resolved by treating the comorbid condition.
• Good clinical practice, therefore, requires treating the comorbid condition and the insomnia each directly.
• The insomnia disorder manifests itself, in part, by a report of difficulty falling asleep or staying asleep. The authors use the example of sleep-onset insomnia as typical in older adults. However, sleep maintenance and early morning awakenings are the most common symptoms among geriatric insomnia patients.
• The authors mention only in passing an important medication for sleep maintenance in adults and in the geriatric patient specifically: doxepin. Low-dose doxepin, at 3 mg (for the geriatric patient) and 6 mg, is FDA-approved as a nonscheduled hypnotic for sleep maintenance insomnia. This formulationa is the only hypnotic classified as safe for geriatric patients in the 2012 Beers Criteria Update of the American Geriatrics Society.1 Unlike higher dosages of doxepin, the action of low-dose doxepin is, essentially, selective H1 antagonism.
aSold as Silenor.
Regarding Drs. Irene S. Hong’s and Jeffrey R. Bishop’s article, “Sedative-hypnotics for sleepless geriatric patients: Choose wisely” (Current Psychiatry, 2014;13(10):36-39, 46-50, 52 [http://bit.ly/1ApmcoO]), which undertook a comprehensive review of current therapies for insomnia in geriatric patients, here are 3 clarifications.
• I want to reinforce the latest thinking about the nature and pathophysiology of insomnia. DSM-5 classifies insomnia as a disorder, not as a symptom of other problems; the concept of “secondary insomnia” is rejected in DSM-5. Insomnia typically is seen as comorbid with other medical and psychiatric disorders. Often, insomnia predates the comorbid disorder (eg, depression), but rarely is it resolved by treating the comorbid condition.
• Good clinical practice, therefore, requires treating the comorbid condition and the insomnia each directly.
• The insomnia disorder manifests itself, in part, by a report of difficulty falling asleep or staying asleep. The authors use the example of sleep-onset insomnia as typical in older adults. However, sleep maintenance and early morning awakenings are the most common symptoms among geriatric insomnia patients.
• The authors mention only in passing an important medication for sleep maintenance in adults and in the geriatric patient specifically: doxepin. Low-dose doxepin, at 3 mg (for the geriatric patient) and 6 mg, is FDA-approved as a nonscheduled hypnotic for sleep maintenance insomnia. This formulationa is the only hypnotic classified as safe for geriatric patients in the 2012 Beers Criteria Update of the American Geriatrics Society.1 Unlike higher dosages of doxepin, the action of low-dose doxepin is, essentially, selective H1 antagonism.
aSold as Silenor.
Reference
1. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
Reference
1. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
Gene variation explains drug toxicity in ALL

Credit: Peter Barta
Inherited variations in the NUDT15 gene can reduce tolerance of the drug mercaptopurine in children with acute lymphoblastic leukemia (ALL), according to research published in the Journal of Clinical Oncology.
The study showed that patients who inherited one or two copies of the newly identified variation in the NUDT15 gene were extremely sensitive to mercaptopurine.
The patients required dose reductions of as much as 92%.
And when mercaptopurine was given at standard doses, the patients developed side effects that caused treatment delays.
These findings should aid efforts to improve the identification and treatment of patients who need reduced doses of mercaptopurine, according to researchers.
“Mercaptopurine intolerance has been suspected to be a problem for young ALL patients of East Asian ancestry,” said study author Jun J. Yang, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee.
“Even at very low doses, the patients often develop toxicity that delays treatment. But, until now, the genetic basis of the problem was unknown.”
With that in mind, Dr Yang and his colleagues performed a genome-wide association study in children with ALL who received mercaptopurine treatment regimens. The discovery and replication cohorts included 657 and 371 children, respectively, from two prospective trials.
The research revealed that patients of East Asian and Hispanic background were more likely to inherit the NUDT15 variant than patients from other racial and ethnic groups.
Among patients of East Asian ancestry, 9.8% carried at least one copy of the NUDT15 variant, compared to 3.9% of Hispanic patients. (East Asia includes China, Japan, and Korea.)
The NUDT15 variant was rarer among patients of European or African ancestry.
This study also confirmed previous research that showed variations in another gene, TPMT, are associated with an increased risk of mercaptopurine toxicity.
TPMT carries instructions for assembling an enzyme of the same name that inactivates mercaptopurine and related drugs. The TPMT variants are less able to inactivate the drug, which can lead to a dangerous build-up of mercaptopurine and suppression of the immune system.
The researchers suspect the NUDT15 variant acts in a similar fashion.
Regardless, the team found that 100% of children who were homozygous for either TPMT or NUDT15 variants or heterozygous for both required at least a 50% reduction in mercaptopurine dose. Only 7.7% of the other patients required similar reductions.
“The results of this study confirm that TPMT genetic variation is one of the most critical determinants of mercaptopurine tolerance, particularly in non-East Asian populations,” said senior study author Mary Relling, PharmD, of St Jude.
“But we also found that TPMT variants do not completely explain mercaptopurine intolerance, particularly in patients of East Asian ancestry. Other factors, both genetic and non-genetic, are still to be discovered to improve the safety and effectiveness of mercaptopurine treatment for children with ALL.” ![]()

Credit: Peter Barta
Inherited variations in the NUDT15 gene can reduce tolerance of the drug mercaptopurine in children with acute lymphoblastic leukemia (ALL), according to research published in the Journal of Clinical Oncology.
The study showed that patients who inherited one or two copies of the newly identified variation in the NUDT15 gene were extremely sensitive to mercaptopurine.
The patients required dose reductions of as much as 92%.
And when mercaptopurine was given at standard doses, the patients developed side effects that caused treatment delays.
These findings should aid efforts to improve the identification and treatment of patients who need reduced doses of mercaptopurine, according to researchers.
“Mercaptopurine intolerance has been suspected to be a problem for young ALL patients of East Asian ancestry,” said study author Jun J. Yang, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee.
“Even at very low doses, the patients often develop toxicity that delays treatment. But, until now, the genetic basis of the problem was unknown.”
With that in mind, Dr Yang and his colleagues performed a genome-wide association study in children with ALL who received mercaptopurine treatment regimens. The discovery and replication cohorts included 657 and 371 children, respectively, from two prospective trials.
The research revealed that patients of East Asian and Hispanic background were more likely to inherit the NUDT15 variant than patients from other racial and ethnic groups.
Among patients of East Asian ancestry, 9.8% carried at least one copy of the NUDT15 variant, compared to 3.9% of Hispanic patients. (East Asia includes China, Japan, and Korea.)
The NUDT15 variant was rarer among patients of European or African ancestry.
This study also confirmed previous research that showed variations in another gene, TPMT, are associated with an increased risk of mercaptopurine toxicity.
TPMT carries instructions for assembling an enzyme of the same name that inactivates mercaptopurine and related drugs. The TPMT variants are less able to inactivate the drug, which can lead to a dangerous build-up of mercaptopurine and suppression of the immune system.
The researchers suspect the NUDT15 variant acts in a similar fashion.
Regardless, the team found that 100% of children who were homozygous for either TPMT or NUDT15 variants or heterozygous for both required at least a 50% reduction in mercaptopurine dose. Only 7.7% of the other patients required similar reductions.
“The results of this study confirm that TPMT genetic variation is one of the most critical determinants of mercaptopurine tolerance, particularly in non-East Asian populations,” said senior study author Mary Relling, PharmD, of St Jude.
“But we also found that TPMT variants do not completely explain mercaptopurine intolerance, particularly in patients of East Asian ancestry. Other factors, both genetic and non-genetic, are still to be discovered to improve the safety and effectiveness of mercaptopurine treatment for children with ALL.” ![]()

Credit: Peter Barta
Inherited variations in the NUDT15 gene can reduce tolerance of the drug mercaptopurine in children with acute lymphoblastic leukemia (ALL), according to research published in the Journal of Clinical Oncology.
The study showed that patients who inherited one or two copies of the newly identified variation in the NUDT15 gene were extremely sensitive to mercaptopurine.
The patients required dose reductions of as much as 92%.
And when mercaptopurine was given at standard doses, the patients developed side effects that caused treatment delays.
These findings should aid efforts to improve the identification and treatment of patients who need reduced doses of mercaptopurine, according to researchers.
“Mercaptopurine intolerance has been suspected to be a problem for young ALL patients of East Asian ancestry,” said study author Jun J. Yang, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee.
“Even at very low doses, the patients often develop toxicity that delays treatment. But, until now, the genetic basis of the problem was unknown.”
With that in mind, Dr Yang and his colleagues performed a genome-wide association study in children with ALL who received mercaptopurine treatment regimens. The discovery and replication cohorts included 657 and 371 children, respectively, from two prospective trials.
The research revealed that patients of East Asian and Hispanic background were more likely to inherit the NUDT15 variant than patients from other racial and ethnic groups.
Among patients of East Asian ancestry, 9.8% carried at least one copy of the NUDT15 variant, compared to 3.9% of Hispanic patients. (East Asia includes China, Japan, and Korea.)
The NUDT15 variant was rarer among patients of European or African ancestry.
This study also confirmed previous research that showed variations in another gene, TPMT, are associated with an increased risk of mercaptopurine toxicity.
TPMT carries instructions for assembling an enzyme of the same name that inactivates mercaptopurine and related drugs. The TPMT variants are less able to inactivate the drug, which can lead to a dangerous build-up of mercaptopurine and suppression of the immune system.
The researchers suspect the NUDT15 variant acts in a similar fashion.
Regardless, the team found that 100% of children who were homozygous for either TPMT or NUDT15 variants or heterozygous for both required at least a 50% reduction in mercaptopurine dose. Only 7.7% of the other patients required similar reductions.
“The results of this study confirm that TPMT genetic variation is one of the most critical determinants of mercaptopurine tolerance, particularly in non-East Asian populations,” said senior study author Mary Relling, PharmD, of St Jude.
“But we also found that TPMT variants do not completely explain mercaptopurine intolerance, particularly in patients of East Asian ancestry. Other factors, both genetic and non-genetic, are still to be discovered to improve the safety and effectiveness of mercaptopurine treatment for children with ALL.” ![]()
Why EBV-positive lymphomas resist IFN therapy

Credit: Ed Uthman
New research has revealed how Epstein Barr virus (EBV) and other herpes viruses outwit the body’s immune response.
It seems these viruses carry microRNAs (miRNAs) that block the interferon (IFN) response—when immune cells release IFN to prevent viral replication, which often kills or slows the growth of infected host cells.
This appears to explain why patients with EBV-positive lymphomas and other viral cancers may resist treatment with IFN.
Jennifer Cox, a graduate student at the University of Texas Austin, and her colleagues recounted these findings in PNAS.
The team noted that many viruses, including EBV, carry miRNAs they use to hijack natural processes in a host’s cells during an infection.
Viral miRNAs are known to prevent host cell death, promote host cell growth, and dampen the host cell’s viral defenses. However, scientists don’t yet know which viral miRNAs perform which functions.
To gain some insight, Cox and her colleagues screened a library of more than 70 human viral miRNAs. This revealed 3 unrelated miRNAs from distantly related herpes viruses that significantly inhibited IFN signaling.
The 5’ and 3’ derivatives from EBV-encoded miR-BART-18 precursor miRNA and the orthologous precursor miRNA from Rhesus lymphocryptovirus all reduced expression of the cyclic AMP-responsive element-binding protein (CBP), which, as part of the p300-CBP complex, mediates IFN signaling.
When the researchers restored miR-BART-18 to cells infected with an EBV miRNA mutant, they observed a cellular growth advantage upon IFN treatment. And they found that miRNAs from other herpes viruses were able to complement this activity.
The team also showed that blocking miR-BART-18 function in an EBV-positive tumor cell line rendered cells more susceptible to IFN-mediated effects.
“[These findings] could explain the variability seen in the success of previous interferon-based cancer treatments,” Cox said. “While this work does not immediately identify new drugs, the fact that such different tumor viruses have converged on the same strategy makes this an exciting pursuit for future therapies against viral cancers.” ![]()

Credit: Ed Uthman
New research has revealed how Epstein Barr virus (EBV) and other herpes viruses outwit the body’s immune response.
It seems these viruses carry microRNAs (miRNAs) that block the interferon (IFN) response—when immune cells release IFN to prevent viral replication, which often kills or slows the growth of infected host cells.
This appears to explain why patients with EBV-positive lymphomas and other viral cancers may resist treatment with IFN.
Jennifer Cox, a graduate student at the University of Texas Austin, and her colleagues recounted these findings in PNAS.
The team noted that many viruses, including EBV, carry miRNAs they use to hijack natural processes in a host’s cells during an infection.
Viral miRNAs are known to prevent host cell death, promote host cell growth, and dampen the host cell’s viral defenses. However, scientists don’t yet know which viral miRNAs perform which functions.
To gain some insight, Cox and her colleagues screened a library of more than 70 human viral miRNAs. This revealed 3 unrelated miRNAs from distantly related herpes viruses that significantly inhibited IFN signaling.
The 5’ and 3’ derivatives from EBV-encoded miR-BART-18 precursor miRNA and the orthologous precursor miRNA from Rhesus lymphocryptovirus all reduced expression of the cyclic AMP-responsive element-binding protein (CBP), which, as part of the p300-CBP complex, mediates IFN signaling.
When the researchers restored miR-BART-18 to cells infected with an EBV miRNA mutant, they observed a cellular growth advantage upon IFN treatment. And they found that miRNAs from other herpes viruses were able to complement this activity.
The team also showed that blocking miR-BART-18 function in an EBV-positive tumor cell line rendered cells more susceptible to IFN-mediated effects.
“[These findings] could explain the variability seen in the success of previous interferon-based cancer treatments,” Cox said. “While this work does not immediately identify new drugs, the fact that such different tumor viruses have converged on the same strategy makes this an exciting pursuit for future therapies against viral cancers.” ![]()

Credit: Ed Uthman
New research has revealed how Epstein Barr virus (EBV) and other herpes viruses outwit the body’s immune response.
It seems these viruses carry microRNAs (miRNAs) that block the interferon (IFN) response—when immune cells release IFN to prevent viral replication, which often kills or slows the growth of infected host cells.
This appears to explain why patients with EBV-positive lymphomas and other viral cancers may resist treatment with IFN.
Jennifer Cox, a graduate student at the University of Texas Austin, and her colleagues recounted these findings in PNAS.
The team noted that many viruses, including EBV, carry miRNAs they use to hijack natural processes in a host’s cells during an infection.
Viral miRNAs are known to prevent host cell death, promote host cell growth, and dampen the host cell’s viral defenses. However, scientists don’t yet know which viral miRNAs perform which functions.
To gain some insight, Cox and her colleagues screened a library of more than 70 human viral miRNAs. This revealed 3 unrelated miRNAs from distantly related herpes viruses that significantly inhibited IFN signaling.
The 5’ and 3’ derivatives from EBV-encoded miR-BART-18 precursor miRNA and the orthologous precursor miRNA from Rhesus lymphocryptovirus all reduced expression of the cyclic AMP-responsive element-binding protein (CBP), which, as part of the p300-CBP complex, mediates IFN signaling.
When the researchers restored miR-BART-18 to cells infected with an EBV miRNA mutant, they observed a cellular growth advantage upon IFN treatment. And they found that miRNAs from other herpes viruses were able to complement this activity.
The team also showed that blocking miR-BART-18 function in an EBV-positive tumor cell line rendered cells more susceptible to IFN-mediated effects.
“[These findings] could explain the variability seen in the success of previous interferon-based cancer treatments,” Cox said. “While this work does not immediately identify new drugs, the fact that such different tumor viruses have converged on the same strategy makes this an exciting pursuit for future therapies against viral cancers.” ![]()
Drug on the fast track to treat HAE

within pancreatic tissue
Credit: Louisa Howard
The US Food and Drug Administration (FDA) has granted fast track designation for BCX4161, an oral inhibitor of plasma kallikrein intended to treat hereditary angioedema (HAE).
Uncontrolled activation of plasma kallikrein, caused by deficiency of its physiological inhibitor (C1 inhibitor) in HAE, results in acute systemic edema.
By inhibiting plasma kallikrein, BCX4161 suppresses the production of bradykinin, the mediator of acute swelling attacks in HAE patients.
HAE is a severely debilitating and potentially fatal condition that occurs in approximately 1 in 50,000 people. Symptoms include recurrent episodes of edema in various locations, as well as bouts of excruciating abdominal pain, nausea, and vomiting that are caused by swelling in the intestinal walls.
HAE patients have a defect in the gene that controls C1 inhibitor, and this results in the production of inadequate or non-functioning C1 inhibitor protein.
Normal C1 inhibitor helps regulate the biochemical interactions of blood-based systems involved in disease-fighting, inflammatory response, and coagulation.
Because defective C1 inhibitor does not adequately perform its regulatory function, a biochemical imbalance can occur and produce unwanted peptides that induce the capillaries to release fluids into surrounding tissue, causing edema.
BCX4161 trials
In May 2014, BioCryst Pharmaceuticals, the company developing BCX4161, announced results from the phase 2a OPuS-1 trial.
OPuS-1 investigators evaluated 400 mg of BCX4161 administered 3 times a day for 28 days in HAE patients with a high angioedema attack frequency (≥ 1 per week), in a randomized, placebo-controlled, 2-period cross-over design.
BCX4161 demonstrated a significant reduction in mean attack rate compared to placebo. The mean attack rate per patient-week was 0.82 on BCX4161 treatment and 1.27 on placebo (P<0.001).
The mean number of attack-free days during each treatment period improved from 19 for placebo to 22 for BCX4161 (P=0.008). Three subjects were attack-free during the BCX4161 period, compared to none during the placebo period.
BCX4161 was generally well-tolerated, BioCryst reported, with an adverse event profile similar to that observed for placebo. There was one serious adverse event reported, an abdominal HAE attack during the placebo period.
In December, the first patient was dosed in the OPuS-2 trial, a double-blind, randomized, placebo- controlled trial conducted in the US and European Union.
Study investigators will evaluate the efficacy and safety of BCX4161 treatment for 12 weeks in patients with HAE. BioCryst expects to report results from OPuS-2 by the end of 2015.
About fast track designation
The FDA’s fast track process is designed to facilitate the development and expedite the review and approval of drugs intended to treat serious or life-threatening conditions that also address unmet medical needs.
A drug that receives fast track designation is usually eligible for more frequent written communication and meetings with the FDA to discuss the drug’s development plan and the collection of appropriate data supporting drug approval.
Priority review and rolling review may be granted if relevant criteria are met. Rolling review allows a drug company to submit completed sections of its new drug application on an ongoing basis, rather than wait until the entire application is complete. ![]()

within pancreatic tissue
Credit: Louisa Howard
The US Food and Drug Administration (FDA) has granted fast track designation for BCX4161, an oral inhibitor of plasma kallikrein intended to treat hereditary angioedema (HAE).
Uncontrolled activation of plasma kallikrein, caused by deficiency of its physiological inhibitor (C1 inhibitor) in HAE, results in acute systemic edema.
By inhibiting plasma kallikrein, BCX4161 suppresses the production of bradykinin, the mediator of acute swelling attacks in HAE patients.
HAE is a severely debilitating and potentially fatal condition that occurs in approximately 1 in 50,000 people. Symptoms include recurrent episodes of edema in various locations, as well as bouts of excruciating abdominal pain, nausea, and vomiting that are caused by swelling in the intestinal walls.
HAE patients have a defect in the gene that controls C1 inhibitor, and this results in the production of inadequate or non-functioning C1 inhibitor protein.
Normal C1 inhibitor helps regulate the biochemical interactions of blood-based systems involved in disease-fighting, inflammatory response, and coagulation.
Because defective C1 inhibitor does not adequately perform its regulatory function, a biochemical imbalance can occur and produce unwanted peptides that induce the capillaries to release fluids into surrounding tissue, causing edema.
BCX4161 trials
In May 2014, BioCryst Pharmaceuticals, the company developing BCX4161, announced results from the phase 2a OPuS-1 trial.
OPuS-1 investigators evaluated 400 mg of BCX4161 administered 3 times a day for 28 days in HAE patients with a high angioedema attack frequency (≥ 1 per week), in a randomized, placebo-controlled, 2-period cross-over design.
BCX4161 demonstrated a significant reduction in mean attack rate compared to placebo. The mean attack rate per patient-week was 0.82 on BCX4161 treatment and 1.27 on placebo (P<0.001).
The mean number of attack-free days during each treatment period improved from 19 for placebo to 22 for BCX4161 (P=0.008). Three subjects were attack-free during the BCX4161 period, compared to none during the placebo period.
BCX4161 was generally well-tolerated, BioCryst reported, with an adverse event profile similar to that observed for placebo. There was one serious adverse event reported, an abdominal HAE attack during the placebo period.
In December, the first patient was dosed in the OPuS-2 trial, a double-blind, randomized, placebo- controlled trial conducted in the US and European Union.
Study investigators will evaluate the efficacy and safety of BCX4161 treatment for 12 weeks in patients with HAE. BioCryst expects to report results from OPuS-2 by the end of 2015.
About fast track designation
The FDA’s fast track process is designed to facilitate the development and expedite the review and approval of drugs intended to treat serious or life-threatening conditions that also address unmet medical needs.
A drug that receives fast track designation is usually eligible for more frequent written communication and meetings with the FDA to discuss the drug’s development plan and the collection of appropriate data supporting drug approval.
Priority review and rolling review may be granted if relevant criteria are met. Rolling review allows a drug company to submit completed sections of its new drug application on an ongoing basis, rather than wait until the entire application is complete. ![]()

within pancreatic tissue
Credit: Louisa Howard
The US Food and Drug Administration (FDA) has granted fast track designation for BCX4161, an oral inhibitor of plasma kallikrein intended to treat hereditary angioedema (HAE).
Uncontrolled activation of plasma kallikrein, caused by deficiency of its physiological inhibitor (C1 inhibitor) in HAE, results in acute systemic edema.
By inhibiting plasma kallikrein, BCX4161 suppresses the production of bradykinin, the mediator of acute swelling attacks in HAE patients.
HAE is a severely debilitating and potentially fatal condition that occurs in approximately 1 in 50,000 people. Symptoms include recurrent episodes of edema in various locations, as well as bouts of excruciating abdominal pain, nausea, and vomiting that are caused by swelling in the intestinal walls.
HAE patients have a defect in the gene that controls C1 inhibitor, and this results in the production of inadequate or non-functioning C1 inhibitor protein.
Normal C1 inhibitor helps regulate the biochemical interactions of blood-based systems involved in disease-fighting, inflammatory response, and coagulation.
Because defective C1 inhibitor does not adequately perform its regulatory function, a biochemical imbalance can occur and produce unwanted peptides that induce the capillaries to release fluids into surrounding tissue, causing edema.
BCX4161 trials
In May 2014, BioCryst Pharmaceuticals, the company developing BCX4161, announced results from the phase 2a OPuS-1 trial.
OPuS-1 investigators evaluated 400 mg of BCX4161 administered 3 times a day for 28 days in HAE patients with a high angioedema attack frequency (≥ 1 per week), in a randomized, placebo-controlled, 2-period cross-over design.
BCX4161 demonstrated a significant reduction in mean attack rate compared to placebo. The mean attack rate per patient-week was 0.82 on BCX4161 treatment and 1.27 on placebo (P<0.001).
The mean number of attack-free days during each treatment period improved from 19 for placebo to 22 for BCX4161 (P=0.008). Three subjects were attack-free during the BCX4161 period, compared to none during the placebo period.
BCX4161 was generally well-tolerated, BioCryst reported, with an adverse event profile similar to that observed for placebo. There was one serious adverse event reported, an abdominal HAE attack during the placebo period.
In December, the first patient was dosed in the OPuS-2 trial, a double-blind, randomized, placebo- controlled trial conducted in the US and European Union.
Study investigators will evaluate the efficacy and safety of BCX4161 treatment for 12 weeks in patients with HAE. BioCryst expects to report results from OPuS-2 by the end of 2015.
About fast track designation
The FDA’s fast track process is designed to facilitate the development and expedite the review and approval of drugs intended to treat serious or life-threatening conditions that also address unmet medical needs.
A drug that receives fast track designation is usually eligible for more frequent written communication and meetings with the FDA to discuss the drug’s development plan and the collection of appropriate data supporting drug approval.
Priority review and rolling review may be granted if relevant criteria are met. Rolling review allows a drug company to submit completed sections of its new drug application on an ongoing basis, rather than wait until the entire application is complete. ![]()