User login
Real-world CAS results in Medicare patients not up to trial standards
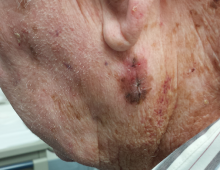
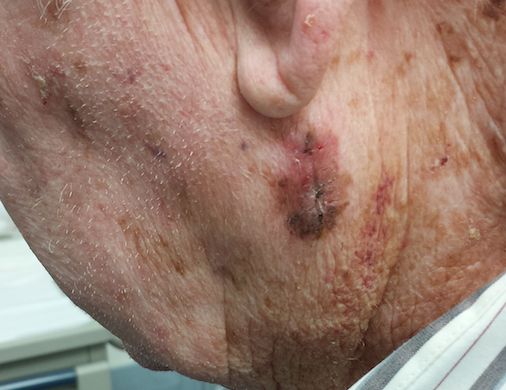
The presence of competing risks and overall lower levels of provider proficiency appeared to limit the benefits of carotid artery stenting in Medicare beneficiaries, according to the results of a large retrospective cohort study of the Centers for Medicare & Medicaid Services CAS database (2005-2009).
Periprocedural mortality was more than twice the rate in this patient population than in those earlier patients those involved in the pivotal CREST and SAPPHIRE clinical trials, according to a report published online Jan. 12 in JAMA Neurology [doi:10.1001/jamaneurol.2014.3638].
“The higher risk of periprocedural complications and the burden of competing risks owing to age and comorbidity burden must be carefully considered when deciding between carotid stenosis treatments for Medicare beneficiaries,” according to Jessica J. Jalbert, Ph.D., of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues.
Over 22,000 patients were assessed in the study. The mean patient age was just over 76 years, 60.5% were men, and 94% were white. Approximately half were symptomatic, 91.2% were at high surgical risk, and 97.4% had carotid stenosis of at least 70%.
Almost 80% of the patients undergoing carotid artery stenting (CAS) met the SAPPHIRE trial indications and about half met at least one of the SAPPHIRE criteria for high surgical risk.
In the mean follow-up of approximately 2 years, mortality risks exceeded one-third for patients who were 80 years of age or older (41.5% mortality risk), symptomatic (37.3% risk), at high surgical risk with symptomatic carotid stenosis of at least 50% (37.3% risk), or admitted nonelectively (36.2% risk). In addition, among asymptomatic patients, mortality after the periprocedural period exceeded one-third for patients at least 80 years old.
Of particular concern, few of these Medicare beneficiaries undergoing CAS as per the National Coverage Determinations were treated by providers with proficiency levels similar to those required in the clinical trials. This is a potential problem because lower annual volume and early operator experience are associated with increased periprocedural mortality, the authors wrote.
CAS was performed primarily by male physicians (98.4%), specializing in cardiology (52.9%), practicing within a group (79.4%), and residing in the South (42.5%). The mean number of past-year CAS procedures performed was only 13.9 for physicians and 29.8 for hospitals. This translated to more than 80% of the physicians not meeting the minimum CAS volume requirements and/or minimum complication rates of the SAPPHIRE trial, and more than 90% not meeting the requirements of the CREST trial.
“Our results may support concerns about the limited generalizability of [randomized clinical trial] findings,” the researchers stated.
“Real-world observational studies comparing CAS, carotid endarterectomy, and medical management are needed to determine the performance of carotid stenosis treatment options for Medicare beneficiaries,” Dr. Jalbert and her colleagues concluded.
The authors reported no relevant disclosures. The study was funded by the Agency for Healthcare Research and Quality, U.S. Department of Health & Human Services.
The presence of competing risks and overall lower levels of provider proficiency appeared to limit the benefits of carotid artery stenting in Medicare beneficiaries, according to the results of a large retrospective cohort study of the Centers for Medicare & Medicaid Services CAS database (2005-2009).
Periprocedural mortality was more than twice the rate in this patient population than in those earlier patients those involved in the pivotal CREST and SAPPHIRE clinical trials, according to a report published online Jan. 12 in JAMA Neurology [doi:10.1001/jamaneurol.2014.3638].
“The higher risk of periprocedural complications and the burden of competing risks owing to age and comorbidity burden must be carefully considered when deciding between carotid stenosis treatments for Medicare beneficiaries,” according to Jessica J. Jalbert, Ph.D., of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues.
Over 22,000 patients were assessed in the study. The mean patient age was just over 76 years, 60.5% were men, and 94% were white. Approximately half were symptomatic, 91.2% were at high surgical risk, and 97.4% had carotid stenosis of at least 70%.
Almost 80% of the patients undergoing carotid artery stenting (CAS) met the SAPPHIRE trial indications and about half met at least one of the SAPPHIRE criteria for high surgical risk.
In the mean follow-up of approximately 2 years, mortality risks exceeded one-third for patients who were 80 years of age or older (41.5% mortality risk), symptomatic (37.3% risk), at high surgical risk with symptomatic carotid stenosis of at least 50% (37.3% risk), or admitted nonelectively (36.2% risk). In addition, among asymptomatic patients, mortality after the periprocedural period exceeded one-third for patients at least 80 years old.
Of particular concern, few of these Medicare beneficiaries undergoing CAS as per the National Coverage Determinations were treated by providers with proficiency levels similar to those required in the clinical trials. This is a potential problem because lower annual volume and early operator experience are associated with increased periprocedural mortality, the authors wrote.
CAS was performed primarily by male physicians (98.4%), specializing in cardiology (52.9%), practicing within a group (79.4%), and residing in the South (42.5%). The mean number of past-year CAS procedures performed was only 13.9 for physicians and 29.8 for hospitals. This translated to more than 80% of the physicians not meeting the minimum CAS volume requirements and/or minimum complication rates of the SAPPHIRE trial, and more than 90% not meeting the requirements of the CREST trial.
“Our results may support concerns about the limited generalizability of [randomized clinical trial] findings,” the researchers stated.
“Real-world observational studies comparing CAS, carotid endarterectomy, and medical management are needed to determine the performance of carotid stenosis treatment options for Medicare beneficiaries,” Dr. Jalbert and her colleagues concluded.
The authors reported no relevant disclosures. The study was funded by the Agency for Healthcare Research and Quality, U.S. Department of Health & Human Services.
The presence of competing risks and overall lower levels of provider proficiency appeared to limit the benefits of carotid artery stenting in Medicare beneficiaries, according to the results of a large retrospective cohort study of the Centers for Medicare & Medicaid Services CAS database (2005-2009).
Periprocedural mortality was more than twice the rate in this patient population than in those earlier patients those involved in the pivotal CREST and SAPPHIRE clinical trials, according to a report published online Jan. 12 in JAMA Neurology [doi:10.1001/jamaneurol.2014.3638].
“The higher risk of periprocedural complications and the burden of competing risks owing to age and comorbidity burden must be carefully considered when deciding between carotid stenosis treatments for Medicare beneficiaries,” according to Jessica J. Jalbert, Ph.D., of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues.
Over 22,000 patients were assessed in the study. The mean patient age was just over 76 years, 60.5% were men, and 94% were white. Approximately half were symptomatic, 91.2% were at high surgical risk, and 97.4% had carotid stenosis of at least 70%.
Almost 80% of the patients undergoing carotid artery stenting (CAS) met the SAPPHIRE trial indications and about half met at least one of the SAPPHIRE criteria for high surgical risk.
In the mean follow-up of approximately 2 years, mortality risks exceeded one-third for patients who were 80 years of age or older (41.5% mortality risk), symptomatic (37.3% risk), at high surgical risk with symptomatic carotid stenosis of at least 50% (37.3% risk), or admitted nonelectively (36.2% risk). In addition, among asymptomatic patients, mortality after the periprocedural period exceeded one-third for patients at least 80 years old.
Of particular concern, few of these Medicare beneficiaries undergoing CAS as per the National Coverage Determinations were treated by providers with proficiency levels similar to those required in the clinical trials. This is a potential problem because lower annual volume and early operator experience are associated with increased periprocedural mortality, the authors wrote.
CAS was performed primarily by male physicians (98.4%), specializing in cardiology (52.9%), practicing within a group (79.4%), and residing in the South (42.5%). The mean number of past-year CAS procedures performed was only 13.9 for physicians and 29.8 for hospitals. This translated to more than 80% of the physicians not meeting the minimum CAS volume requirements and/or minimum complication rates of the SAPPHIRE trial, and more than 90% not meeting the requirements of the CREST trial.
“Our results may support concerns about the limited generalizability of [randomized clinical trial] findings,” the researchers stated.
“Real-world observational studies comparing CAS, carotid endarterectomy, and medical management are needed to determine the performance of carotid stenosis treatment options for Medicare beneficiaries,” Dr. Jalbert and her colleagues concluded.
The authors reported no relevant disclosures. The study was funded by the Agency for Healthcare Research and Quality, U.S. Department of Health & Human Services.
FROM JAMA NEUROLOGY
Key clinical point: Mortality risks exceeded one-third for patients who were 80 years of age or older, symptomatic, at high surgical risk with symptomatic carotid stenosis of at least 50%, or admitted nonelectively.
Major finding: More than 80% of the physicians performing CAS in the real world did not meet the minimum CAS volume requirements and/or minimum complication rates of the SAPPPHIRE trial.
Data source: Data were obtained from a large retrospective cohort study of the Centers for Medicare and Medicaid Services CAS database (2005-2009).
Disclosures: The authors reported no relevant disclosures.
MicroRNA may be therapeutic target for ALK- ALCL
SAN FRANCISCO—MicroRNA-155 (miR-155) can act as an oncogenic driver in ALK− anaplastic large-cell lymphoma (ALCL) and may therefore be a therapeutic target for the disease, according to a presentation at the 7th Annual T-cell Lymphoma Forum.
Analyzing patient samples and cell lines, researchers discovered that miR-155 is highly expressed in ALK− ALCL but is nearly absent in ALK+ ALCL.
They also found evidence suggesting that miR-155 drives tumor growth in mouse models of ALK− ALCL.
Philipp Staber, MD, PhD, of the Medical University of Vienna in Austria, presented these findings at the meeting.
Dr Staber and his colleagues previously found (Merkel et al, PNAS 2010) that miR-155 was highly expressed in ALK− ALCL. So they decided to take a closer look at the phenomenon.
They assessed miR-155 expression in samples from patients with ALK+ or ALK− ALCL, as well as 6 ALCL cell lines.
miR-155 expression was significantly higher in the ALK− patient samples than in the ALK+ samples (P<0.001). And it was significantly higher (P<0.001) in the ALK− cell lines (DL-40, Mac1, and Mac2a) than in the ALK+ cell lines (K299, SR789, and SUP-M2).
Dr Staber and his colleagues then overexpressed miR-155 in ALK+ ALCL cell lines (K299 and SR789). And they observed a decrease in known target genes of miR-155—C/EBPβ, SOCS1, and SHIP1.
The researchers also observed an inverse correlation between miR-155 host gene promoter methylation and miR-155 expression in an ALCL+ cell line, which suggested that ALK activity has no direct effect on miR-155 levels.
The team treated the K299 cell line with the ALK inhibitor crizotinib at 100 nM, 200 nM, and 400 nM doses and found that miR-155 did not increase at any dose. Dr Staber noted, however, that the researchers were only able to treat cells for a maximum of 24 hours.
The group then discovered that anti-miR-155 mimics could reduce tumor growth in mouse models of ALK- ALCL. Mice were injected with Mac1 or Mac2a cells transfected with anti-miR-155, control RNA, or pre-miR-155 oligos.
In both Mac1 and Mac2 models, tumors were substantially smaller in the anti-mir-155 mice than in the pre-miR-155 mice (P=0.038 and P=0.006, respectively). But tumor growth was not significantly reduced compared to controls.
“Immunohistochemistry in these tumors shows quite a clear picture,” Dr Staber said. “The C/EBPβ target gene is overexpressed when using anti-miR-155, and [expression is decreased] with overexpression of miR-155. And the same is true for SOCS1. STAT3 signaling is increased through overexpression of miR-155.”
The researchers observed the same effect in ALK− ALCL patient samples.
Using ALK+ cell lines (K299 and SR789), the team went on to show that miR-155 suppresses IL-8 expression and induces IL-22 expression, a finding they verified in mice.
“IL-8 is a direct target of C/EBPβ, and C/EBPβ, as shown before, is a target of miR-155, so this makes sense,” Dr Staber said. “On the other hand, IL-22 is a strong inducer of STAT3 signaling, which is strongly induced when we increase miR-155 expression.”
Dr Staber and his colleagues concluded that these findings, when taken together, suggest that miR-155 could be a therapeutic target for ALK− ALCL. ![]()
SAN FRANCISCO—MicroRNA-155 (miR-155) can act as an oncogenic driver in ALK− anaplastic large-cell lymphoma (ALCL) and may therefore be a therapeutic target for the disease, according to a presentation at the 7th Annual T-cell Lymphoma Forum.
Analyzing patient samples and cell lines, researchers discovered that miR-155 is highly expressed in ALK− ALCL but is nearly absent in ALK+ ALCL.
They also found evidence suggesting that miR-155 drives tumor growth in mouse models of ALK− ALCL.
Philipp Staber, MD, PhD, of the Medical University of Vienna in Austria, presented these findings at the meeting.
Dr Staber and his colleagues previously found (Merkel et al, PNAS 2010) that miR-155 was highly expressed in ALK− ALCL. So they decided to take a closer look at the phenomenon.
They assessed miR-155 expression in samples from patients with ALK+ or ALK− ALCL, as well as 6 ALCL cell lines.
miR-155 expression was significantly higher in the ALK− patient samples than in the ALK+ samples (P<0.001). And it was significantly higher (P<0.001) in the ALK− cell lines (DL-40, Mac1, and Mac2a) than in the ALK+ cell lines (K299, SR789, and SUP-M2).
Dr Staber and his colleagues then overexpressed miR-155 in ALK+ ALCL cell lines (K299 and SR789). And they observed a decrease in known target genes of miR-155—C/EBPβ, SOCS1, and SHIP1.
The researchers also observed an inverse correlation between miR-155 host gene promoter methylation and miR-155 expression in an ALCL+ cell line, which suggested that ALK activity has no direct effect on miR-155 levels.
The team treated the K299 cell line with the ALK inhibitor crizotinib at 100 nM, 200 nM, and 400 nM doses and found that miR-155 did not increase at any dose. Dr Staber noted, however, that the researchers were only able to treat cells for a maximum of 24 hours.
The group then discovered that anti-miR-155 mimics could reduce tumor growth in mouse models of ALK- ALCL. Mice were injected with Mac1 or Mac2a cells transfected with anti-miR-155, control RNA, or pre-miR-155 oligos.
In both Mac1 and Mac2 models, tumors were substantially smaller in the anti-mir-155 mice than in the pre-miR-155 mice (P=0.038 and P=0.006, respectively). But tumor growth was not significantly reduced compared to controls.
“Immunohistochemistry in these tumors shows quite a clear picture,” Dr Staber said. “The C/EBPβ target gene is overexpressed when using anti-miR-155, and [expression is decreased] with overexpression of miR-155. And the same is true for SOCS1. STAT3 signaling is increased through overexpression of miR-155.”
The researchers observed the same effect in ALK− ALCL patient samples.
Using ALK+ cell lines (K299 and SR789), the team went on to show that miR-155 suppresses IL-8 expression and induces IL-22 expression, a finding they verified in mice.
“IL-8 is a direct target of C/EBPβ, and C/EBPβ, as shown before, is a target of miR-155, so this makes sense,” Dr Staber said. “On the other hand, IL-22 is a strong inducer of STAT3 signaling, which is strongly induced when we increase miR-155 expression.”
Dr Staber and his colleagues concluded that these findings, when taken together, suggest that miR-155 could be a therapeutic target for ALK− ALCL. ![]()
SAN FRANCISCO—MicroRNA-155 (miR-155) can act as an oncogenic driver in ALK− anaplastic large-cell lymphoma (ALCL) and may therefore be a therapeutic target for the disease, according to a presentation at the 7th Annual T-cell Lymphoma Forum.
Analyzing patient samples and cell lines, researchers discovered that miR-155 is highly expressed in ALK− ALCL but is nearly absent in ALK+ ALCL.
They also found evidence suggesting that miR-155 drives tumor growth in mouse models of ALK− ALCL.
Philipp Staber, MD, PhD, of the Medical University of Vienna in Austria, presented these findings at the meeting.
Dr Staber and his colleagues previously found (Merkel et al, PNAS 2010) that miR-155 was highly expressed in ALK− ALCL. So they decided to take a closer look at the phenomenon.
They assessed miR-155 expression in samples from patients with ALK+ or ALK− ALCL, as well as 6 ALCL cell lines.
miR-155 expression was significantly higher in the ALK− patient samples than in the ALK+ samples (P<0.001). And it was significantly higher (P<0.001) in the ALK− cell lines (DL-40, Mac1, and Mac2a) than in the ALK+ cell lines (K299, SR789, and SUP-M2).
Dr Staber and his colleagues then overexpressed miR-155 in ALK+ ALCL cell lines (K299 and SR789). And they observed a decrease in known target genes of miR-155—C/EBPβ, SOCS1, and SHIP1.
The researchers also observed an inverse correlation between miR-155 host gene promoter methylation and miR-155 expression in an ALCL+ cell line, which suggested that ALK activity has no direct effect on miR-155 levels.
The team treated the K299 cell line with the ALK inhibitor crizotinib at 100 nM, 200 nM, and 400 nM doses and found that miR-155 did not increase at any dose. Dr Staber noted, however, that the researchers were only able to treat cells for a maximum of 24 hours.
The group then discovered that anti-miR-155 mimics could reduce tumor growth in mouse models of ALK- ALCL. Mice were injected with Mac1 or Mac2a cells transfected with anti-miR-155, control RNA, or pre-miR-155 oligos.
In both Mac1 and Mac2 models, tumors were substantially smaller in the anti-mir-155 mice than in the pre-miR-155 mice (P=0.038 and P=0.006, respectively). But tumor growth was not significantly reduced compared to controls.
“Immunohistochemistry in these tumors shows quite a clear picture,” Dr Staber said. “The C/EBPβ target gene is overexpressed when using anti-miR-155, and [expression is decreased] with overexpression of miR-155. And the same is true for SOCS1. STAT3 signaling is increased through overexpression of miR-155.”
The researchers observed the same effect in ALK− ALCL patient samples.
Using ALK+ cell lines (K299 and SR789), the team went on to show that miR-155 suppresses IL-8 expression and induces IL-22 expression, a finding they verified in mice.
“IL-8 is a direct target of C/EBPβ, and C/EBPβ, as shown before, is a target of miR-155, so this makes sense,” Dr Staber said. “On the other hand, IL-22 is a strong inducer of STAT3 signaling, which is strongly induced when we increase miR-155 expression.”
Dr Staber and his colleagues concluded that these findings, when taken together, suggest that miR-155 could be a therapeutic target for ALK− ALCL. ![]()
Memory T cells can fight CMV infection

CMV infection
Transplanting a small number of antiviral memory T cells along with donor hematopoietic stem cells can fight and may even prevent cytomegalovirus (CMV) disease in transplant recipients, according to preclinical research published in the Journal of Immunology.
To date, researchers have focused on developing anti-CMV immunotherapy with effector-phenotype CD8+ T cells (TEFF cells), which attack and kill virally infected host cells.
But Christopher Snyder, PhD, of Thomas Jefferson University in Philadelphia, Pennsylvania, and his colleagues found that CMV-specific TEFF cells divide poorly in response to CMV infection or reactivation in mouse models.
So they wondered if CMV-specific memory-phenotype CD8+ T cells (TM cells)—which act more like stem cells—could help control the infection long-term.
The group showed that a small number of TM cells were enough to produce and repeatedly replenish all of the TEFF cells needed to fight CMV.
The infused TM cells became major contributors to the recipient antiviral immune response, persisting for at least 3 months’ time and producing TEFF cells at a steady stream.
To determine whether these cells have counterparts in humans, Dr Snyder and his colleagues compared the genomic fingerprints of mouse and human TM cells that were specific for CMV. Results showed the two had similar profiles.
“This suggested that human and mouse CMV-specific memory T cells are very similar populations,” said study author Michael Quinn, an MD/PhD student at Thomas Jefferson University.
“Therefore, infusing similar cells into humans could improve on immunotherapeutic methods for controlling CMV infection. This may be a valuable approach to keep the disease from emerging in people.”
Dr Snyder added, “Our data argue for developing new clinical trials focused specifically on using these T memory cells, in order to determine if it would indeed be better than current therapeutic options.” ![]()

CMV infection
Transplanting a small number of antiviral memory T cells along with donor hematopoietic stem cells can fight and may even prevent cytomegalovirus (CMV) disease in transplant recipients, according to preclinical research published in the Journal of Immunology.
To date, researchers have focused on developing anti-CMV immunotherapy with effector-phenotype CD8+ T cells (TEFF cells), which attack and kill virally infected host cells.
But Christopher Snyder, PhD, of Thomas Jefferson University in Philadelphia, Pennsylvania, and his colleagues found that CMV-specific TEFF cells divide poorly in response to CMV infection or reactivation in mouse models.
So they wondered if CMV-specific memory-phenotype CD8+ T cells (TM cells)—which act more like stem cells—could help control the infection long-term.
The group showed that a small number of TM cells were enough to produce and repeatedly replenish all of the TEFF cells needed to fight CMV.
The infused TM cells became major contributors to the recipient antiviral immune response, persisting for at least 3 months’ time and producing TEFF cells at a steady stream.
To determine whether these cells have counterparts in humans, Dr Snyder and his colleagues compared the genomic fingerprints of mouse and human TM cells that were specific for CMV. Results showed the two had similar profiles.
“This suggested that human and mouse CMV-specific memory T cells are very similar populations,” said study author Michael Quinn, an MD/PhD student at Thomas Jefferson University.
“Therefore, infusing similar cells into humans could improve on immunotherapeutic methods for controlling CMV infection. This may be a valuable approach to keep the disease from emerging in people.”
Dr Snyder added, “Our data argue for developing new clinical trials focused specifically on using these T memory cells, in order to determine if it would indeed be better than current therapeutic options.” ![]()

CMV infection
Transplanting a small number of antiviral memory T cells along with donor hematopoietic stem cells can fight and may even prevent cytomegalovirus (CMV) disease in transplant recipients, according to preclinical research published in the Journal of Immunology.
To date, researchers have focused on developing anti-CMV immunotherapy with effector-phenotype CD8+ T cells (TEFF cells), which attack and kill virally infected host cells.
But Christopher Snyder, PhD, of Thomas Jefferson University in Philadelphia, Pennsylvania, and his colleagues found that CMV-specific TEFF cells divide poorly in response to CMV infection or reactivation in mouse models.
So they wondered if CMV-specific memory-phenotype CD8+ T cells (TM cells)—which act more like stem cells—could help control the infection long-term.
The group showed that a small number of TM cells were enough to produce and repeatedly replenish all of the TEFF cells needed to fight CMV.
The infused TM cells became major contributors to the recipient antiviral immune response, persisting for at least 3 months’ time and producing TEFF cells at a steady stream.
To determine whether these cells have counterparts in humans, Dr Snyder and his colleagues compared the genomic fingerprints of mouse and human TM cells that were specific for CMV. Results showed the two had similar profiles.
“This suggested that human and mouse CMV-specific memory T cells are very similar populations,” said study author Michael Quinn, an MD/PhD student at Thomas Jefferson University.
“Therefore, infusing similar cells into humans could improve on immunotherapeutic methods for controlling CMV infection. This may be a valuable approach to keep the disease from emerging in people.”
Dr Snyder added, “Our data argue for developing new clinical trials focused specifically on using these T memory cells, in order to determine if it would indeed be better than current therapeutic options.” ![]()
mAb shows ‘modest activity’ in rel/ref MM

Photo by Linda Bartlett
The monoclonal IgM antibody PAT-SM6 was “well-tolerated” and showed “modest clinical activity” in patients with relapsed or refractory multiple myeloma (MM), researchers reported in haematologica.
Adverse events occurred in all 12 patients enrolled in the phase 1/2a study, but most were considered unrelated to treatment.
A third of patients, all of whom had progressive disease upon study entry, achieved stable disease after receiving PAT-SM6. The remaining patients progressed.
Leo Rasche, MD, of University Hospital Wurzburg in Germany, and his colleagues conducted this study. It was funded, in part, by Patrys Limited, the company developing PAT-SM6.
The study included 12 heavily pretreated MM patients. They had a median age of 69.5 years and a long-standing history of MM (range, 3.25 to 15.75 years). They had received a median of 3.9 prior lines of therapy (range, 2-7).
Patients received 4 escalating doses of PAT-SM6, over a period of 2 weeks, via intravenous infusions at 0.3 mg/kg, 1 mg/kg, 3 mg/kg, and 6 mg/kg.
Safety data
There were 54 treatment-emergent adverse events in all 12 patients. However, there were no dose-limiting toxicities and no deaths. The maximum tolerated dose has not been reached.
More than 80% of the adverse events were of mild to moderate intensity. Two patients (16.6%) each experienced a single serious event. One patient had acute back pain, and one had a bile duct stone. Neither of these events was considered treatment-related.
Twenty-one adverse events were considered treatment-related. This included leukopenia (66.6%), neutropenia (50%), hypertension (16.6%), catheter-related thrombophlebitis (8.3%), injection site erythema (8.3%), slight headache (8.3%), C-reactive protein increase (8.3%), and hypertriglyceridemia (8.3%).
Efficacy data
Most patients progressed following treatment, but 4 (33.3%) had stable disease. The investigators noted that stable disease is not necessarily connected with a clinical benefit, so they analyzed the 4 patients in detail.
Patient 4, who received PAT-SM6 at 1 mg/kg, entered the study with high-risk disease. The patient had 13q deletion and 1q21 gain, had received 5 prior lines of therapy, and was refractory to novel agents, including pomalidomide and bortezomib.
The patient was treatment-free for 1 month prior to receiving PAT-SM6. During treatment, there were no symptoms of active myeloma, and the patient asked to continue salvage therapy 1 week after the end of the study.
Patient 7, who received PAT-SM6 at 3 mg/kg, had been diagnosed with MM for 15 years and had received 4 prior lines of therapy.
The patient’s treatment-free interval before receiving PAT-SM6 was 30 months. After PAT-SM6, the patient was therapy-free for 4.6 months.
Patient 10, who received PAT-SM6 at 6mg/kg, had 6 prior lines of therapy, including tandem autologous stem cell transplant and several polychemotherapeutic regimens.
The patient was therapy-free for 4 months prior to receiving PAT-SM6. The patient requested salvage therapy 1 month after receiving PAT-SM6.
Patient 11, who received PAT-SM6 at 6mg/kg, had 4 prior lines of therapy and was refractory to both lenalidomide and thalidomide.
The patient’s treatment-free interval prior to PAT-SM6 was 12 months. After PAT-SM6, the patient was therapy-free for 5.2 months.
The researchers said additional trials testing PAT-SM6 in combination with other MM therapies are planned. PAT-SM6 has received orphan drug designation for MM in the US and the European Union. ![]()

Photo by Linda Bartlett
The monoclonal IgM antibody PAT-SM6 was “well-tolerated” and showed “modest clinical activity” in patients with relapsed or refractory multiple myeloma (MM), researchers reported in haematologica.
Adverse events occurred in all 12 patients enrolled in the phase 1/2a study, but most were considered unrelated to treatment.
A third of patients, all of whom had progressive disease upon study entry, achieved stable disease after receiving PAT-SM6. The remaining patients progressed.
Leo Rasche, MD, of University Hospital Wurzburg in Germany, and his colleagues conducted this study. It was funded, in part, by Patrys Limited, the company developing PAT-SM6.
The study included 12 heavily pretreated MM patients. They had a median age of 69.5 years and a long-standing history of MM (range, 3.25 to 15.75 years). They had received a median of 3.9 prior lines of therapy (range, 2-7).
Patients received 4 escalating doses of PAT-SM6, over a period of 2 weeks, via intravenous infusions at 0.3 mg/kg, 1 mg/kg, 3 mg/kg, and 6 mg/kg.
Safety data
There were 54 treatment-emergent adverse events in all 12 patients. However, there were no dose-limiting toxicities and no deaths. The maximum tolerated dose has not been reached.
More than 80% of the adverse events were of mild to moderate intensity. Two patients (16.6%) each experienced a single serious event. One patient had acute back pain, and one had a bile duct stone. Neither of these events was considered treatment-related.
Twenty-one adverse events were considered treatment-related. This included leukopenia (66.6%), neutropenia (50%), hypertension (16.6%), catheter-related thrombophlebitis (8.3%), injection site erythema (8.3%), slight headache (8.3%), C-reactive protein increase (8.3%), and hypertriglyceridemia (8.3%).
Efficacy data
Most patients progressed following treatment, but 4 (33.3%) had stable disease. The investigators noted that stable disease is not necessarily connected with a clinical benefit, so they analyzed the 4 patients in detail.
Patient 4, who received PAT-SM6 at 1 mg/kg, entered the study with high-risk disease. The patient had 13q deletion and 1q21 gain, had received 5 prior lines of therapy, and was refractory to novel agents, including pomalidomide and bortezomib.
The patient was treatment-free for 1 month prior to receiving PAT-SM6. During treatment, there were no symptoms of active myeloma, and the patient asked to continue salvage therapy 1 week after the end of the study.
Patient 7, who received PAT-SM6 at 3 mg/kg, had been diagnosed with MM for 15 years and had received 4 prior lines of therapy.
The patient’s treatment-free interval before receiving PAT-SM6 was 30 months. After PAT-SM6, the patient was therapy-free for 4.6 months.
Patient 10, who received PAT-SM6 at 6mg/kg, had 6 prior lines of therapy, including tandem autologous stem cell transplant and several polychemotherapeutic regimens.
The patient was therapy-free for 4 months prior to receiving PAT-SM6. The patient requested salvage therapy 1 month after receiving PAT-SM6.
Patient 11, who received PAT-SM6 at 6mg/kg, had 4 prior lines of therapy and was refractory to both lenalidomide and thalidomide.
The patient’s treatment-free interval prior to PAT-SM6 was 12 months. After PAT-SM6, the patient was therapy-free for 5.2 months.
The researchers said additional trials testing PAT-SM6 in combination with other MM therapies are planned. PAT-SM6 has received orphan drug designation for MM in the US and the European Union. ![]()

Photo by Linda Bartlett
The monoclonal IgM antibody PAT-SM6 was “well-tolerated” and showed “modest clinical activity” in patients with relapsed or refractory multiple myeloma (MM), researchers reported in haematologica.
Adverse events occurred in all 12 patients enrolled in the phase 1/2a study, but most were considered unrelated to treatment.
A third of patients, all of whom had progressive disease upon study entry, achieved stable disease after receiving PAT-SM6. The remaining patients progressed.
Leo Rasche, MD, of University Hospital Wurzburg in Germany, and his colleagues conducted this study. It was funded, in part, by Patrys Limited, the company developing PAT-SM6.
The study included 12 heavily pretreated MM patients. They had a median age of 69.5 years and a long-standing history of MM (range, 3.25 to 15.75 years). They had received a median of 3.9 prior lines of therapy (range, 2-7).
Patients received 4 escalating doses of PAT-SM6, over a period of 2 weeks, via intravenous infusions at 0.3 mg/kg, 1 mg/kg, 3 mg/kg, and 6 mg/kg.
Safety data
There were 54 treatment-emergent adverse events in all 12 patients. However, there were no dose-limiting toxicities and no deaths. The maximum tolerated dose has not been reached.
More than 80% of the adverse events were of mild to moderate intensity. Two patients (16.6%) each experienced a single serious event. One patient had acute back pain, and one had a bile duct stone. Neither of these events was considered treatment-related.
Twenty-one adverse events were considered treatment-related. This included leukopenia (66.6%), neutropenia (50%), hypertension (16.6%), catheter-related thrombophlebitis (8.3%), injection site erythema (8.3%), slight headache (8.3%), C-reactive protein increase (8.3%), and hypertriglyceridemia (8.3%).
Efficacy data
Most patients progressed following treatment, but 4 (33.3%) had stable disease. The investigators noted that stable disease is not necessarily connected with a clinical benefit, so they analyzed the 4 patients in detail.
Patient 4, who received PAT-SM6 at 1 mg/kg, entered the study with high-risk disease. The patient had 13q deletion and 1q21 gain, had received 5 prior lines of therapy, and was refractory to novel agents, including pomalidomide and bortezomib.
The patient was treatment-free for 1 month prior to receiving PAT-SM6. During treatment, there were no symptoms of active myeloma, and the patient asked to continue salvage therapy 1 week after the end of the study.
Patient 7, who received PAT-SM6 at 3 mg/kg, had been diagnosed with MM for 15 years and had received 4 prior lines of therapy.
The patient’s treatment-free interval before receiving PAT-SM6 was 30 months. After PAT-SM6, the patient was therapy-free for 4.6 months.
Patient 10, who received PAT-SM6 at 6mg/kg, had 6 prior lines of therapy, including tandem autologous stem cell transplant and several polychemotherapeutic regimens.
The patient was therapy-free for 4 months prior to receiving PAT-SM6. The patient requested salvage therapy 1 month after receiving PAT-SM6.
Patient 11, who received PAT-SM6 at 6mg/kg, had 4 prior lines of therapy and was refractory to both lenalidomide and thalidomide.
The patient’s treatment-free interval prior to PAT-SM6 was 12 months. After PAT-SM6, the patient was therapy-free for 5.2 months.
The researchers said additional trials testing PAT-SM6 in combination with other MM therapies are planned. PAT-SM6 has received orphan drug designation for MM in the US and the European Union. ![]()
Vitamin A as malaria prophylaxis

Photo by Sarah Mattison
New research suggests vitamin A may reduce the risk of malaria in young children.
The study showed that children under 5 living in sub-Saharan Africa were 54% less likely to develop malaria if they had been given a single, large dose of vitamin A.
The finding, published in eLife, indicates that vitamin A may be able to protect children from the malaria parasite, especially if administered during the wet season, when malaria-infected mosquitos are most prevalent.
“Now, we need to test vitamin A in a randomized, controlled, clinical trial to better understand whether this could really be an effective way to prevent this disease,” said study author Maria-Graciela Hollm-Delgado, PhD, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland.
Dr Hollm-Delgado and her colleagues analyzed national survey data from 4 sub-Saharan countries—Burkina Faso, Mozambique, Rwanda, and Senegal—on children between the ages of 6 months and 59 months.
The goal was to determine the risk of Plasmodium parasitemia (n=8390) and Plasmodium falciparum-specific antigenemia (n=6121) following vitamin A supplementation and vaccinations.
The researchers found the measles and polio vaccines were not associated with malaria. And Bacille Calmette Guerin vaccination was associated with an increased risk of antigenemia (relative risk [RR]=4.06) but not parasitemia.
Only vitamin A was protective against malaria. Children who received vitamin A were less likely to present with parasitemia (RR=0.46) and antigenemia (RR=0.23).
Vitamin A appeared to be more protective under certain circumstances, including when administered during the rainy season, as well as when given to older children and when more time had passed since supplementation.
The researchers aren’t certain why vitamin A would reduce the rate of malaria infection, but they suspect it is because vitamin A, which is known to boost immunity and improve the ability to fight off infection, may help the body clear out the malaria parasite more quickly.
Only 62% of children in the study had received vitamin A supplementation, even though World Health Organization guidelines recommend that all children in sub-Saharan Africa receive a single, large dose of vitamin A.
Rates were higher for many vaccinations, Dr Hollm-Delgado said, noting that the guidelines for vitamin A aren’t as specific as they are for most vaccinations. ![]()

Photo by Sarah Mattison
New research suggests vitamin A may reduce the risk of malaria in young children.
The study showed that children under 5 living in sub-Saharan Africa were 54% less likely to develop malaria if they had been given a single, large dose of vitamin A.
The finding, published in eLife, indicates that vitamin A may be able to protect children from the malaria parasite, especially if administered during the wet season, when malaria-infected mosquitos are most prevalent.
“Now, we need to test vitamin A in a randomized, controlled, clinical trial to better understand whether this could really be an effective way to prevent this disease,” said study author Maria-Graciela Hollm-Delgado, PhD, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland.
Dr Hollm-Delgado and her colleagues analyzed national survey data from 4 sub-Saharan countries—Burkina Faso, Mozambique, Rwanda, and Senegal—on children between the ages of 6 months and 59 months.
The goal was to determine the risk of Plasmodium parasitemia (n=8390) and Plasmodium falciparum-specific antigenemia (n=6121) following vitamin A supplementation and vaccinations.
The researchers found the measles and polio vaccines were not associated with malaria. And Bacille Calmette Guerin vaccination was associated with an increased risk of antigenemia (relative risk [RR]=4.06) but not parasitemia.
Only vitamin A was protective against malaria. Children who received vitamin A were less likely to present with parasitemia (RR=0.46) and antigenemia (RR=0.23).
Vitamin A appeared to be more protective under certain circumstances, including when administered during the rainy season, as well as when given to older children and when more time had passed since supplementation.
The researchers aren’t certain why vitamin A would reduce the rate of malaria infection, but they suspect it is because vitamin A, which is known to boost immunity and improve the ability to fight off infection, may help the body clear out the malaria parasite more quickly.
Only 62% of children in the study had received vitamin A supplementation, even though World Health Organization guidelines recommend that all children in sub-Saharan Africa receive a single, large dose of vitamin A.
Rates were higher for many vaccinations, Dr Hollm-Delgado said, noting that the guidelines for vitamin A aren’t as specific as they are for most vaccinations. ![]()

Photo by Sarah Mattison
New research suggests vitamin A may reduce the risk of malaria in young children.
The study showed that children under 5 living in sub-Saharan Africa were 54% less likely to develop malaria if they had been given a single, large dose of vitamin A.
The finding, published in eLife, indicates that vitamin A may be able to protect children from the malaria parasite, especially if administered during the wet season, when malaria-infected mosquitos are most prevalent.
“Now, we need to test vitamin A in a randomized, controlled, clinical trial to better understand whether this could really be an effective way to prevent this disease,” said study author Maria-Graciela Hollm-Delgado, PhD, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland.
Dr Hollm-Delgado and her colleagues analyzed national survey data from 4 sub-Saharan countries—Burkina Faso, Mozambique, Rwanda, and Senegal—on children between the ages of 6 months and 59 months.
The goal was to determine the risk of Plasmodium parasitemia (n=8390) and Plasmodium falciparum-specific antigenemia (n=6121) following vitamin A supplementation and vaccinations.
The researchers found the measles and polio vaccines were not associated with malaria. And Bacille Calmette Guerin vaccination was associated with an increased risk of antigenemia (relative risk [RR]=4.06) but not parasitemia.
Only vitamin A was protective against malaria. Children who received vitamin A were less likely to present with parasitemia (RR=0.46) and antigenemia (RR=0.23).
Vitamin A appeared to be more protective under certain circumstances, including when administered during the rainy season, as well as when given to older children and when more time had passed since supplementation.
The researchers aren’t certain why vitamin A would reduce the rate of malaria infection, but they suspect it is because vitamin A, which is known to boost immunity and improve the ability to fight off infection, may help the body clear out the malaria parasite more quickly.
Only 62% of children in the study had received vitamin A supplementation, even though World Health Organization guidelines recommend that all children in sub-Saharan Africa receive a single, large dose of vitamin A.
Rates were higher for many vaccinations, Dr Hollm-Delgado said, noting that the guidelines for vitamin A aren’t as specific as they are for most vaccinations. ![]()
Hospital Renovation Patient Satisfaction
Hospitals are expensive and complex facilities to build and renovate. It is estimated $200 billion is being spent in the United States during this decade on hospital construction and renovation, and further expenditures in this area are expected.[1] Aging hospital infrastructure, competition, and health system expansion have motivated institutions to invest in renovation and new hospital building construction.[2, 3, 4, 5, 6, 7] There is a trend toward patient‐centered design in new hospital construction. Features of this trend include same‐handed design (ie, rooms on a unit have all beds oriented in the same direction and do not share headwalls); use of sound absorbent materials to reduced ambient noise[7, 8, 9]; rooms with improved view and increased natural lighting to reduce anxiety, decrease delirium, and increase sense of wellbeing[10, 11, 12]; incorporation of natural elements like gardens, water features, and art[12, 13, 14, 15, 16, 17, 18]; single‐patient rooms to reduce transmission of infection and enhance privacy and visitor comfort[7, 19, 20]; presence of comfortable waiting rooms and visitor accommodations to enhance comfort and family participation[21, 22, 23]; and hotel‐like amenities such as on‐demand entertainment and room service menus.[24, 25]
There is a belief among some hospital leaders that patients are generally unable to distinguish their positive experience with a pleasing healthcare environment from their positive experience with care, and thus improving facilities will lead to improved satisfaction across the board.[26, 27] In a controlled study of hospitalized patients, appealing rooms were associated with increased satisfaction with services including housekeeping and food service staff, meals, as well as physicians and overall satisfaction.[26] A 2012 survey of hospital leadership found that expanding and renovating facilities was considered a top priority in improving patient satisfaction, with 82% of the respondents stating that this was important.[27]
Despite these attitudes, the impact of patient‐centered design on patient satisfaction is not well understood. Studies have shown that renovations and hospital construction that incorporates noise reduction strategies, positive distraction, patient and caregiver control, attractive waiting rooms, improved patient room appearance, private rooms, and large windows result in improved satisfaction with nursing, noise level, unit environment and cleanliness, perceived wait time, discharge preparedness, and overall care. [7, 19, 20, 23, 28] However, these studies were limited by small sample size, inclusion of a narrow group of patients (eg, ambulatory, obstetric, geriatric rehabilitation, intensive care unit), and concurrent use of interventions other than design improvement (eg, nurse and patient education). Many of these studies did not use the ubiquitous Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) and Press Ganey patient satisfaction surveys.
We sought to determine the changes in patient satisfaction that occurred during a natural experiment, in which clinical units (comprising stable nursing, physician, and unit teams) were relocated from an historic clinical building to a new clinical building that featured patient‐centered design, using HCAHPS and Press Ganey surveys and a large study population. We hypothesized that new building features would positively impact both facility related (eg, noise level), nonfacility related (eg, physician and housekeeping service related), and overall satisfaction.
METHODS
This was a retrospective analysis of prospectively collected Press Ganey and HCAPHS patient satisfaction survey data for a single academic tertiary care hospital.[29] The research project was reviewed and approved by the institutional review board.
Participants
All patients discharged from 12 clinical units that relocated to the new clinical building and returned patient satisfaction surveys served as study patients. The moved units included the coronary care unit, cardiac step down unit, medical intensive care unit, neuro critical care unit, surgical intensive care unit, orthopedic unit, neurology unit, neurosurgery unit, obstetrics units, gynecology unit, urology unit, cardiothoracic surgery unit, and the transplant surgery and renal transplant unit. Patients on clinical units that did not move served as concurrent controls.
Exposure
Patients admitted to the new clinical building experienced several patient‐centered design features. These features included easy access to healing gardens with a water feature, soaring lobbies, a collection of more than 500 works of art, well‐decorated and light‐filled patient rooms with sleeping accommodations for family members, sound‐absorbing features in patient care corridors ranging from acoustical ceiling tiles to a quiet nurse‐call system, and an interactive television network with Internet, movies, and games. All patients during the baseline period and control patients during the study period were located in typical patient rooms with standard hospital amenities. No other major patient satisfaction interventions were initiated during the pre‐ or postperiod in either arm of the study; ongoing patient satisfaction efforts (such as unit‐based customer care representatives) were deployed broadly and not restricted to the new clinical building. Clinical teams comprised of physicians, nurses, and ancillary staff did not change significantly after the move.
Time Periods
The move to new clinical building occurred on May 1, 2012. After allowing for a 15‐day washout period, the postmove period included Press Ganey and HCAHPS surveys returned for discharges that occurred during a 7.5‐month period between May 15, 2102 and December 31, 2012. Baseline data included Press Ganey and HCAHPS surveys returned for discharges in the preceding 12 months (May 1, 2011 to April 30, 2012). Sensitivity analysis using only 7.5 months of baseline data did not reveal any significant difference when compared with 12‐month baseline data, and we report only data from the 12‐month baseline period.
Instruments
Press Ganey and HCAHPS patient satisfaction surveys were sent via mail in the same envelope. Fifty percent of the discharged patients were randomized to receive the surveys. The Press Ganey survey contained 33 items covering across several subdomains including room, meal, nursing, physician, ancillary staff, visitor, discharge, and overall satisfaction. The HCAHPS survey contained 29 Centers for Medicare and Medicaid Services (CMS)‐mandated items, of which 21 are related to patient satisfaction. The development and testing and methods for administration and reporting of the HCAHPS survey have been previously described.[30, 31] Press Ganey patient satisfaction survey results have been reported in the literature.[32, 33]
Outcome Variables
Press Ganey and HCAHPS patient satisfaction survey responses were the primary outcome variables of the study. The survey items were categorized as facility related (eg, noise level), nonfacility related (eg, physician and nursing staff satisfaction), and overall satisfaction related.
Covariates
Age, sex, length of stay (LOS), insurance type, and all‐payer refined diagnosis‐related groupassociated illness complexity were included as covariates.
Statistical Analysis
Percent top‐box scores were calculated for each survey item as the percent of patients who responded very good for a given item on Press Ganey survey items and always or definitely yes or 9 or 10 on HCAHPS survey items. CMS utilizes percent top‐box scores to calculate payments under the Value Based Purchasing (VBP) program and to report the results publicly. Numerous studies have also reported percent top‐box scores for HCAHPS survey results.[31, 32, 33, 34]
Odds ratios of premove versus postmove percentage of top‐box scores, adjusted for age, sex, LOS, complexity of illness, and insurance type were determined using logistic regression for the units that moved. Similar scores were calculated for unmoved units to detect secular trends. To determine whether the differences between the moved and unmoved units were significant, we introduced the interaction term (moved vs unmoved unit status) (pre‐ vs postmove time period) into the logistic regression models and examined the adjusted P value for this term. All statistical analysis was performed using SAS Institute Inc.'s (Cary, NC) JMP Pro 10.0.0.
RESULTS
The study included 1648 respondents in the moved units in the baseline period (ie, units designated to move to a new clinical building) and 1373 respondents in the postmove period. There were 1593 respondents in the control group during the baseline period and 1049 respondents in the postmove period. For the units that moved, survey response rates were 28.5% prior to the move and 28.3% after the move. For the units that did not move, survey response rates were 20.9% prior to the move and 22.7% after the move. A majority of survey respondents on the nursing units that moved were white, male, and had private insurance (Table 1). There were no significant differences between respondents across these characteristics between the pre‐ and postmove periods. Mean age and LOS were also similar. For these units, there were 70.5% private rooms prior to the move and 100% after the move. For the unmoved units, 58.9% of the rooms were private in the baseline period and 72.7% were private in the study period. Similar to the units that moved, characteristics of the respondents on the unmoved units also did not differ significantly in the postmove period.
| Patient demographics | Moved Units (N=3,021) | Unmoved Units (N=2,642) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | P Value | Pre | Post | P Value | |
| ||||||
| White | 75.3% | 78.2% | 0.07 | 66.7% | 68.5% | 0.31 |
| Mean age, y | 57.3 | 57.4 | 0.84 | 57.3 | 57.1 | 0.81 |
| Male | 54.3% | 53.0% | 0.48 | 40.5% | 42.3% | 0.23 |
| Self‐reported health | ||||||
| Excellent or very good | 54.7% | 51.2% | 0.04 | 38.7% | 39.5% | 0.11 |
| Good | 27.8% | 32.0% | 29.3% | 32.2% | ||
| Fair or poor | 17.5% | 16.9% | 32.0% | 28.3% | ||
| Self‐reported language | ||||||
| English | 96.0% | 97.2% | 0.06 | 96.8% | 97.1% | 0.63 |
| Other | 4.0% | 2.8% | 3.2% | 2.9% | ||
| Self‐reported education | ||||||
| Less than high school | 5.8% | 5.0% | 0.24 | 10.8% | 10.4% | 0.24 |
| High school grad | 46.4% | 44.2% | 48.6% | 45.5% | ||
| College grad or more | 47.7% | 50.7% | 40.7% | 44.7% | ||
| Insurance type | ||||||
| Medicaid | 6.7% | 5.5% | 0.11 | 10.8% | 9.0% | 0.32 |
| Medicare | 32.0% | 35.5% | 36.0% | 36.1% | ||
| Private insurance | 55.6% | 52.8% | 48.0% | 50.3% | ||
| Mean APRDRG complexity* | 2.1 | 2.1 | 0.09 | 2.3 | 2.3 | 0.14 |
| Mean LOS | 4.7 | 5.0 | 0.12 | 4.9 | 5.0 | 0.77 |
| Service | ||||||
| Medicine | 15.4% | 16.2% | 0.51 | 40.0% | 34.5% | 0.10 |
| Surgery | 50.7% | 45.7% | 40.1% | 44.1% | ||
| Neurosciences | 20.3% | 24.1% | 6.0% | 6.0% | ||
| Obstetrics/gynecology | 7.5% | 8.2% | 5.7% | 5.6% | ||
The move was associated with significant improvements in facility‐related satisfaction (Tables 2 and 3). The most prominent increases in satisfaction were with pleasantness of dcor (33.6% vs 66.2%), noise level (39.9% vs 59.3%), and visitor accommodation and comfort (50.0% vs 70.3 %). There was improvement in satisfaction related to cleanliness of the room (49.0% vs 68.6 %), but no significant increase in satisfaction with courtesy of the person cleaning the room (59.8% vs 67.7%) when compared with units that did move.
| Satisfaction Domain | Moved Units | Unmoved Units | P Value of the Difference in Odds Ratio Between Moved and Unmoved Units | |||||
|---|---|---|---|---|---|---|---|---|
| % Top Box | Adjusted Odds Ratio* (95% CI) | % Top Box | Adjusted Odds Ratio* (95% CI) | |||||
| Pre | Post | Pre | Post | |||||
| ||||||||
| FACILITY RELATED | ||||||||
| Hospital environment | ||||||||
| Cleanliness of the room and bathroom | 61.0 | 70.8 | 1.62 (1.40‐1.90) | 64.0 | 69.2 | 1.24 (1.03‐1.48) | 0.03 | |
| Quietness of the room | 51.3 | 65.4 | 1.89 (1.63‐2.19) | 58.6 | 60.3 | 1.08 (0.90‐1.28) | <0.0001 | |
| NONFACILITY RELATED | ||||||||
| Nursing communication | ||||||||
| Nurses treated with courtesy/respect | 84.0 | 86.7 | 1.28 (1.05‐1.57) | 83.6 | 87.1 | 1.29 (1.02‐1.64) | 0.92 | |
| Nurses listened | 73.1 | 76.4 | 1.21 (1.03‐1.43) | 74.2 | 75.5 | 1.05 (0.86‐1.27) | 0.26 | |
| Nurses explained | 75.0 | 76.6 | 1.10 (0.94‐1.30) | 76.0 | 76.2 | 1.00 (0.82‐1.21) | 0.43 | |
| Physician communication | ||||||||
| Doctors treated with courtesy/respect | 89.5 | 90.5 | 1.13 (0.89‐1.42) | 84.9 | 87.3 | 1.20 (0.94‐1.53) | 0.77 | |
| Doctors listened | 81.4 | 81.0 | 0.93 (0.83‐1.19) | 77.7 | 77.1 | 0.94 (0.77‐1.15) | 0.68 | |
| Doctors explained | 79.2 | 79.0 | 1.00(0.84‐1.19) | 75.7 | 74.4 | 0.92 (0.76‐1.12) | 0.49 | |
| Other | ||||||||
| Help toileting as soon as you wanted | 61.8 | 63.7 | 1.08 (0.89‐1.32) | 62.3 | 60.6 | 0.92 (0.71‐1.18) | 0.31 | |
| Pain well controlled | 63.2 | 63.8 | 1.06 (0.90‐1.25) | 62.0 | 62.6 | 0.99 (0.81‐1.20) | 060 | |
| Staff do everything to help with pain | 77.7 | 80.1 | 1.19 (0.99‐1.44) | 76.8 | 75.7 | 0.90 (0.75‐1.13) | 0.07 | |
| Staff describe medicine side effects | 47.0 | 47.6 | 1.05 (0.89‐1.24) | 49.2 | 47.1 | 0.91 (0.74‐1.11) | 0.32 | |
| Tell you what new medicine was for | 76.4 | 76.4 | 1.02 (0.84‐1.25) | 77.1 | 78.8 | 1.09(0.85‐1.39) | 0.65 | |
| Overall | ||||||||
| Rate hospital (010) | 75.0 | 83.3 | 1.71 (1.44‐2.05) | 75.7 | 77.6 | 1.06 (0.87‐1.29) | 0.006 | |
| Recommend hospital | 82.5 | 87.1 | 1.43 (1.18‐1.76) | 81.4 | 82.0 | 0.98 (0.79‐1.22) | 0.03 | |
| Satisfaction Domain | Moved Unit | Unmoved Unit | P Value of the Difference in Odds Ratio Between Moved and Unmoved Units | ||||
|---|---|---|---|---|---|---|---|
| % Top Box | Adjusted Odds Ratio* (95% CI) | % Top Box | Adjusted Odds Ratio* (95% CI) | ||||
| Pre | Post | Pre | Post | ||||
| |||||||
| FACILITY RELATED | |||||||
| Room | |||||||
| Pleasantness of room dcor | 33.6 | 64.8 | 3.77 (3.24‐4.38) | 41.6 | 47.0 | 1.21 (1.02‐1.44) | <0.0001 |
| Room cleanliness | 49.0 | 68.6 | 2.35 (2.02‐2.73) | 51.6 | 59.1 | 1.32 (1.12‐1.58) | <0.0001 |
| Room temperature | 43.1 | 54.9 | 1.64 (1.43‐1.90) | 45.0 | 48.8 | 1.14 (0.96‐1.36) | 0.002 |
| Noise level in and around the room | 40.2 | 59.2 | 2.23 (1.92‐2.58) | 45.5 | 47.6 | 1.07 (0.90‐1.22) | <0.0001 |
| Visitor related | |||||||
| Accommodations and comfort of visitors | 50.0 | 70.3 | 2.44 (2.10‐2.83) | 55.3 | 59.1 | 1.14 (0.96‐1.35) | <0.0001 |
| NONFACILITY RELATED | |||||||
| Food | |||||||
| Temperature of the food | 31.1 | 33.6 | 1.15 (0.99‐1.34) | 34.0 | 38.9 | 1.23 (1.02‐1.47) | 0.51 |
| Quality of the food | 25.8 | 27.1 | 1.10 (0.93‐1.30) | 30.2 | 36.2 | 1.32 (1.10‐1.59) | 0.12 |
| Courtesy of the person who served food | 63.9 | 62.3 | 0.93 (0.80‐1.10) | 66.0 | 61.4 | 0.82 (0.69‐0.98) | 0.26 |
| Nursing | |||||||
| Friendliness/courtesy of the nurses | 76.3 | 82.8 | 1.49 (1.26‐1.79) | 77.7 | 80.1 | 1.10 (0.90‐1.37) | 0.04 |
| Promptness of response to call | 60.1 | 62.6 | 1.14 (0.98‐1.33) | 59.2 | 62.0 | 1.10 (0.91‐1.31) | 0.80 |
| Nurses' attitude toward requests | 71.0 | 75.8 | 1.30 (1.11‐1.54) | 70.5 | 72.4 | 1.06 (0.88‐1.28) | 0.13 |
| Attention to special/personal needs | 66.7 | 72.2 | 1.32 (1.13‐1.54) | 67.8 | 70.3 | 1.09 (0.91‐1.31) | 0.16 |
| Nurses kept you informed | 64.3 | 72.2 | 1.46 (1.25‐1.70) | 65.8 | 69.8 | 1.17 (0.98‐1.41) | 0.88 |
| Skill of the nurses | 75.3 | 79.5 | 1.28 (1.08‐1.52) | 74.3 | 78.6 | 1.23 (1.01‐1.51) | 0.89 |
| Ancillary staff | |||||||
| Courtesy of the person cleaning the room | 59.8 | 67.7 | 1.41 (1.21‐1.65) | 61.2 | 66.5 | 1.24 (1.03‐1.49) | 0.28 |
| Courtesy of the person who took blood | 66.5 | 68.1 | 1.10 (0.94‐1.28) | 63.2 | 63.1 | 0.96 (0.76‐1.08) | 0.34 |
| Courtesy of the person who started the IV | 70.0 | 71.7 | 1.09 (0.93‐1.28) | 66.6 | 69.3 | 1.11 (0.92‐1.33) | 0.88 |
| Visitor related | |||||||
| Staff attitude toward visitors | 68.1 | 79.4 | 1.84 (1.56‐2.18) | 70.3 | 72.2 | 1.06 (0.87‐1.28) | <0.0001 |
| Physician | |||||||
| Time physician spent with you | 55.0 | 58.9 | 1.20 (1.04‐1.39) | 53.2 | 55.9 | 1.10 (0.92‐1.30) | 0.46 |
| Physician concern questions/worries | 67.2 | 70.7 | 1.20 (1.03‐1.40) | 64.3 | 66.1 | 1.05 (0.88‐1.26) | 0.31 |
| Physician kept you informed | 65.3 | 67.5 | 1.12 (0.96‐1.30) | 61.6 | 63.2 | 1.05 (0.88‐1.25) | 0.58 |
| Friendliness/courtesy of physician | 76.3 | 78.1 | 1.11 (0.93‐1.31) | 71.0 | 73.3 | 1.08 (0.90‐1.31) | 0.89 |
| Skill of physician | 85.4 | 88.5 | 1.35 (1.09‐1.68) | 78.0 | 81.0 | 1.15 (0.93‐1.43) | 0.34 |
| Discharge | |||||||
| Extent felt ready for discharge | 62.0 | 66.7 | 1.23 (1.07‐1.44) | 59.2 | 62.3 | 1.10 (0.92‐1.30) | 0.35 |
| Speed of discharge process | 50.7 | 54.2 | 1.16 (1.01‐1.33) | 47.8 | 50.0 | 1.07 (0.90‐1.27) | 0.49 |
| Instructions for care at home | 66.4 | 71.1 | 1.25 (1.06‐1.46) | 64.0 | 67.7 | 1.16 (0.97‐1.39) | 0.54 |
| Staff concern for your privacy | 65.3 | 71.8 | 1.37 (1.17‐0.85) | 63.6 | 66.2 | 1.10 (0.91‐1.31) | 0.07 |
| Miscellaneous | |||||||
| How well your pain was controlled | 64.2 | 66.5 | 1.14 (0.97‐1.32) | 60.2 | 62.6 | 1.07 (0.89‐1.28) | 0.66 |
| Staff addressed emotional needs | 60.0 | 63.4 | 1.19 (1.02‐1.38) | 55.1 | 60.2 | 1.20 (1.01‐1.42) | 0.90 |
| Response to concerns/complaints | 61.1 | 64.5 | 1.19 (1.02‐1.38) | 57.2 | 60.1 | 1.10 (0.92‐1.31) | 0.57 |
| Overall | |||||||
| Staff worked together to care for you | 72.6 | 77.2 | 1.29 (1.10‐1.52) | 70.3 | 73.2 | 1.13 (0.93‐1.37) | 0.30 |
| Likelihood of recommending hospital | 79.1 | 84.3 | 1.44 (1.20‐1.74) | 76.3 | 79.2 | 1.14 (0.93‐1.39) | 0.10 |
| Overall rating of care given | 76.8 | 83.0 | 1.50 (1.25‐1.80) | 74.7 | 77.2 | 1.10 (0.90‐1.34) | 0.03 |
With regard to nonfacility‐related satisfaction, there were statistically higher scores in several nursing, physician, and discharge‐related satisfaction domains after the move. However, these changes were not associated with the move to the new clinical building as they were not significantly different from improvements on the unmoved units. Among nonfacility‐related items, only staff attitude toward visitors showed significant improvement (68.1% vs 79.4%). There was a significant improvement in hospital rating (75.0% vs 83.3% in the moved units and 75.7% vs 77.6% in the unmoved units). However, the other 3 measures of overall satisfaction did not show significant improvement associated with the move to the new clinical building when compared to the concurrent controls.
DISCUSSION
Contrary to our hypothesis and a belief held by many, we found that patients appeared able to distinguish their experience with hospital environment from their experience with providers and other services. Improvement in hospital facilities with incorporation of patient‐centered features was associated with improvements that were largely limited to increases in satisfaction with quietness, cleanliness, temperature, and dcor of the room along with visitor‐related satisfaction. Notably, there was no significant improvement in satisfaction related to physicians, nurses, housekeeping, and other service staff. There was improvement in satisfaction with staff attitude toward visitors, but this can be attributed to availability of visitor‐friendly facilities. There was a significant improvement in 1 of the 4 measures of overall satisfaction. Our findings also support the construct validity of HCAHPS and Press Ganey patient satisfaction surveys.
Ours is one of the largest studies on patient satisfaction related to patient‐centered design features in the inpatient acute care setting. Swan et al. also studied patients in an acute inpatient setting and compared satisfaction related to appealing versus typical hospital rooms. Patients were matched for case mix, insurance, gender, types of medical services received and LOS, and were served by the same set of physicians and similar food service and housekeeping staff.[26] Unlike our study, they found improved satisfaction related to physicians, housekeeping staff, food service staff, meals, and overall satisfaction. However, the study had some limitations. In particular, the study sample was self‐selected because the patients in this group were required to pay an extra daily fee to utilize the appealing room. Additionally, there were only 177 patients across the 2 groups, and the actual differences in satisfaction scores were small. Our sample was larger and patients in the study group were admitted to units in the new clinical buildings by the same criteria as they were admitted to the historic building prior to the move, and there were no significant differences in baseline characteristics between the comparison groups.
Jansen et al. also found broad improvements in patient satisfaction in a study of over 309 maternity unit patients in a new construction, all private‐room maternity unit with more appealing design elements and comfort features for visitors.[7] Improved satisfaction was noted with the physical environment, nursing care, assistance with feeding, respect for privacy, and discharge planning. However, it is difficult to extrapolate the results of this study to other settings, as maternity unit patients constitute a unique patient demographic with unique care needs. Additionally, when compared with patients in the control group, the patients in the study group were cared for by nurses who had a lower workload and who were not assigned other patients with more complex needs. Because nursing availability may be expected to impact satisfaction with clinical domains, the impact of private and appealing room may very well have been limited to improved satisfaction with the physical environment.
Despite the widespread belief among healthcare leadership that facility renovation or expansion is a vital strategy for improving patient satisfaction, our study shows that this may not be a dominant factor.[27] In fact, the Planetree model showed that improvement in satisfaction related to physical environment and nursing care was associated with implementation of both patient‐centered design features as well as with utilization of nurses that were trained to provide personalized care, educate patients, and involve patients and family.[28] It is more likely that provider‐level interventions will have a greater impact on provider level and overall satisfaction. This idea is supported by a recent JD Powers study suggesting that facilities represent only 19% of overall satisfaction in the inpatient setting.[35]
Although our study focused on patient‐centered design features, several renovation and construction projects have also focused on design features that improve patient safety and provider satisfaction, workflow, efficiency, productivity, stress, and time spent in direct care.[9] Interventions in these areas may lead to improvement in patient outcomes and perhaps lead to improvement in patient satisfaction; however, this relationship has not been well established at present.
In an era of cost containment, healthcare administrators are faced with high‐priced interventions, competing needs, limited resources, low profit margins, and often unclear evidence on cost‐effectiveness and return on investment of healthcare design features. Benefits are related to competitive advantage, higher reputation, patient retention, decreased malpractice costs, and increased Medicare payments through VBP programs that incentivize improved performance on quality metrics and patient satisfaction surveys. Our study supports the idea that a significant improvement in patient satisfaction related to creature comforts can be achieved with investment in patient‐centered design features. However, our findings also suggest that institutions should perform an individualized cost‐benefit analysis related to improvements in this narrow area of patient satisfaction. In our study, incorporation of patient‐centered design features resulted in improvement on 2 VBP HCAHPS measures, and its contribution toward total performance score under the VBP program would be limited.
Strengths of our study include the use of concurrent controls and our ability to capitalize on a natural experiment in which care teams remained constant before and after a move to a new clinical building. However, our study has some limitations. It was conducted at a single tertiary care academic center that predominantly serves an inner city population and referral patients seeking specialized care. Drivers of patient satisfaction may be different in community hospitals, and a different relationship may be observed between patient‐centered design and domains of patient satisfaction in this setting. Further studies in different hospital settings are needed to confirm our findings. Additionally, we were limited by the low response rate of the surveys. However, this is a widespread problem with all patient satisfaction research utilizing voluntary surveys, and our response rates are consistent with those previously reported.[34, 36, 37, 38] Furthermore, low response rates have not impeded the implementation of pay‐for‐performance programs on a national scale using HCHAPS.
In conclusion, our study suggests that hospitals should not use outdated facilities as an excuse for achievement of suboptimal satisfaction scores. Patients respond positively to creature comforts, pleasing surroundings, and visitor‐friendly facilities but can distinguish these positive experiences from experiences in other patient satisfaction domains. In our study, the move to a higher‐amenity building had only a modest impact on overall patient satisfaction, perhaps because clinical care is the primary driver of this outcome. Contrary to belief held by some hospital leaders, major strides in overall satisfaction across the board and other subdomains of satisfaction likely require intervention in areas other than facility renovation and expansion.
Disclosures
Zishan Siddiqui, MD, was supported by the Osler Center of Clinical Excellence Faculty Scholarship Grant. Funds from Johns Hopkins Hospitalist Scholars Program supported the research project. The authors have no conflict of interests to disclose.
- , . Create a blueprint for successful hospital construction. Nurs Manage. 2006;37(6):39–44.
- Walter Reed National Military Medical Center website. Facts at a glance. Available at: http://www.wrnmmc.capmed.mil/About%20Us/SitePages/Facts.aspx. Accessed June 19, 2013.
- . Keys to collaboration. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/keys‐collaboration. Accessed June 19, 2013.
- . A tale of 4 hospitals. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/tale‐4‐hospitals. Accessed June 19, 2013.
- . Gateway to the east. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/gateway‐east. Accessed June 19, 2013.
- . Lessons learned. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/lessons‐learned. Accessed June 19, 2013.
- , , , , . Single room maternity care and client satisfaction. Birth. 2000;27(4):235–243.
- , , , . Same‐handed and mirrored unit configurations: is there a difference in patient and nurse outcomes? J Nurs Adm. 2011;41(6):273–279.
- , . The Pebble Projects: coordinated evidence‐based case studies. Build Res Inform. 2008;36(2):129–145.
- , , . Effects of exposure to nature and abstract pictures on patients recovering from open heart surgery. J Soc Psychophysiol Res. 1993;30:7.
- , , , . Postoperative delirium. Curr Drug Targets. 2005;6(7):807–814.
- . Stimulus deprivation in windowless rooms. Anaesthesia. 1977;32(7):598–602.
- , , , . Post‐occupancy evaluation of healing gardens in a pediatric cancer center. Landsc Urban Plan. 2005;73(2):167–183.
- . Healing gardens in hospitals. Interdiscip Des Res J. 2007;1(1):1–27.
- , . Restorative gardens. BMJ. 1993;306(6885):1080–1081.
- . Effects of interior design on wellness: theory and recent scientific research. J Health Care Inter Des. 1991;3:97–109.
- , . Sunny hospital rooms expedite recovery from severe and refractory depressions. J Affect Disord. 1996;40(1‐2):49–51.
- . Art in hospital spaces: the role of hospitals in an aestheticised society. Int J Cult Policy. 2007;13(1):85–101.
- , , . Renovation of a semiprivate patient room. Bowman Center Geriatric Rehabilitation Unit. Nurs Clin North Am 1995;30(1):97–115.
- , , , et al. (2013). Effect of intensive care environment on family and patient satisfaction: a before‐after study. Intensive Care Med. 2013;39(9):1626–1634.
- , , , , . Outcomes of environmental appraisal of different hospital waiting areas. Environ Behav. 2003;35(6):842–869.
- . Redesigning the neurocritical care unit to enhance family participation and improve outcomes. Cleve Clin J Med. 2009;76(suppl 2):S70–S74.
- , . The ecology of the patient visit: physical attractiveness, waiting times, and perceived quality of care. J Ambul Care Manage. 2008;31(2):128–141.
- . Patient satisfaction and the new consumer. Hosp Health Netw. 2006;80(57):59–62.
- . Patient satisfaction. Hospitals embrace hotel‐like amenities. Hosp Health Netw. 2007;81(11):24–26.
- , , . Do appealing hospital rooms increase patient evaluations of physicians, nurses, and hospital services? Health Care Manage Rev. 2003;28(3):254–264.
- . Patient experience and HCAHPS: little consensus on a top priority. Health Leaders Media website. Available at http://www.healthleadersmedia.com/intelligence/detail.cfm?content_id=28289334(2):125–133.
- Centers for Medicare 67:27–37.
- Hospital Consumer Assessment of Healthcare Providers and Systems. Summary analysis. http://www.hcahpsonline.org/SummaryAnalyses.aspx. Accessed October 1, 2014.
- Centers for Medicare 44(2 pt 1):501–518.
- J.D. Power and Associates. Patient satisfaction influenced more by hospital staff than by the hospital facilities. Available at: http://www.jdpower.com/press‐releases/2012‐national‐patient‐experience‐study#sthash.gSv6wAdc.dpuf. Accessed December 10, 2013.
- , , , , . Racial and ethnic differences in a patient survey: patients' values, ratings, and reports regarding physician primary care performance in a large health maintenance organization. Med Care. 2000;38(3): 300–310.
- , , , . Patient experience in safety‐net hospitals implications for improving care and Value‐Based Purchasing patient experience in safety‐net hospitals. Arch Intern Med. 2012;172(16):1204–1210.
- , , , . Comparison of Hospital Consumer Assessment of Healthcare Providers and Systems patient satisfaction scores for specialty hospitals and general medical hospitals: confounding effect of survey response rate. J Hosp Med. 2014;9(9):590–593.
Hospitals are expensive and complex facilities to build and renovate. It is estimated $200 billion is being spent in the United States during this decade on hospital construction and renovation, and further expenditures in this area are expected.[1] Aging hospital infrastructure, competition, and health system expansion have motivated institutions to invest in renovation and new hospital building construction.[2, 3, 4, 5, 6, 7] There is a trend toward patient‐centered design in new hospital construction. Features of this trend include same‐handed design (ie, rooms on a unit have all beds oriented in the same direction and do not share headwalls); use of sound absorbent materials to reduced ambient noise[7, 8, 9]; rooms with improved view and increased natural lighting to reduce anxiety, decrease delirium, and increase sense of wellbeing[10, 11, 12]; incorporation of natural elements like gardens, water features, and art[12, 13, 14, 15, 16, 17, 18]; single‐patient rooms to reduce transmission of infection and enhance privacy and visitor comfort[7, 19, 20]; presence of comfortable waiting rooms and visitor accommodations to enhance comfort and family participation[21, 22, 23]; and hotel‐like amenities such as on‐demand entertainment and room service menus.[24, 25]
There is a belief among some hospital leaders that patients are generally unable to distinguish their positive experience with a pleasing healthcare environment from their positive experience with care, and thus improving facilities will lead to improved satisfaction across the board.[26, 27] In a controlled study of hospitalized patients, appealing rooms were associated with increased satisfaction with services including housekeeping and food service staff, meals, as well as physicians and overall satisfaction.[26] A 2012 survey of hospital leadership found that expanding and renovating facilities was considered a top priority in improving patient satisfaction, with 82% of the respondents stating that this was important.[27]
Despite these attitudes, the impact of patient‐centered design on patient satisfaction is not well understood. Studies have shown that renovations and hospital construction that incorporates noise reduction strategies, positive distraction, patient and caregiver control, attractive waiting rooms, improved patient room appearance, private rooms, and large windows result in improved satisfaction with nursing, noise level, unit environment and cleanliness, perceived wait time, discharge preparedness, and overall care. [7, 19, 20, 23, 28] However, these studies were limited by small sample size, inclusion of a narrow group of patients (eg, ambulatory, obstetric, geriatric rehabilitation, intensive care unit), and concurrent use of interventions other than design improvement (eg, nurse and patient education). Many of these studies did not use the ubiquitous Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) and Press Ganey patient satisfaction surveys.
We sought to determine the changes in patient satisfaction that occurred during a natural experiment, in which clinical units (comprising stable nursing, physician, and unit teams) were relocated from an historic clinical building to a new clinical building that featured patient‐centered design, using HCAHPS and Press Ganey surveys and a large study population. We hypothesized that new building features would positively impact both facility related (eg, noise level), nonfacility related (eg, physician and housekeeping service related), and overall satisfaction.
METHODS
This was a retrospective analysis of prospectively collected Press Ganey and HCAPHS patient satisfaction survey data for a single academic tertiary care hospital.[29] The research project was reviewed and approved by the institutional review board.
Participants
All patients discharged from 12 clinical units that relocated to the new clinical building and returned patient satisfaction surveys served as study patients. The moved units included the coronary care unit, cardiac step down unit, medical intensive care unit, neuro critical care unit, surgical intensive care unit, orthopedic unit, neurology unit, neurosurgery unit, obstetrics units, gynecology unit, urology unit, cardiothoracic surgery unit, and the transplant surgery and renal transplant unit. Patients on clinical units that did not move served as concurrent controls.
Exposure
Patients admitted to the new clinical building experienced several patient‐centered design features. These features included easy access to healing gardens with a water feature, soaring lobbies, a collection of more than 500 works of art, well‐decorated and light‐filled patient rooms with sleeping accommodations for family members, sound‐absorbing features in patient care corridors ranging from acoustical ceiling tiles to a quiet nurse‐call system, and an interactive television network with Internet, movies, and games. All patients during the baseline period and control patients during the study period were located in typical patient rooms with standard hospital amenities. No other major patient satisfaction interventions were initiated during the pre‐ or postperiod in either arm of the study; ongoing patient satisfaction efforts (such as unit‐based customer care representatives) were deployed broadly and not restricted to the new clinical building. Clinical teams comprised of physicians, nurses, and ancillary staff did not change significantly after the move.
Time Periods
The move to new clinical building occurred on May 1, 2012. After allowing for a 15‐day washout period, the postmove period included Press Ganey and HCAHPS surveys returned for discharges that occurred during a 7.5‐month period between May 15, 2102 and December 31, 2012. Baseline data included Press Ganey and HCAHPS surveys returned for discharges in the preceding 12 months (May 1, 2011 to April 30, 2012). Sensitivity analysis using only 7.5 months of baseline data did not reveal any significant difference when compared with 12‐month baseline data, and we report only data from the 12‐month baseline period.
Instruments
Press Ganey and HCAHPS patient satisfaction surveys were sent via mail in the same envelope. Fifty percent of the discharged patients were randomized to receive the surveys. The Press Ganey survey contained 33 items covering across several subdomains including room, meal, nursing, physician, ancillary staff, visitor, discharge, and overall satisfaction. The HCAHPS survey contained 29 Centers for Medicare and Medicaid Services (CMS)‐mandated items, of which 21 are related to patient satisfaction. The development and testing and methods for administration and reporting of the HCAHPS survey have been previously described.[30, 31] Press Ganey patient satisfaction survey results have been reported in the literature.[32, 33]
Outcome Variables
Press Ganey and HCAHPS patient satisfaction survey responses were the primary outcome variables of the study. The survey items were categorized as facility related (eg, noise level), nonfacility related (eg, physician and nursing staff satisfaction), and overall satisfaction related.
Covariates
Age, sex, length of stay (LOS), insurance type, and all‐payer refined diagnosis‐related groupassociated illness complexity were included as covariates.
Statistical Analysis
Percent top‐box scores were calculated for each survey item as the percent of patients who responded very good for a given item on Press Ganey survey items and always or definitely yes or 9 or 10 on HCAHPS survey items. CMS utilizes percent top‐box scores to calculate payments under the Value Based Purchasing (VBP) program and to report the results publicly. Numerous studies have also reported percent top‐box scores for HCAHPS survey results.[31, 32, 33, 34]
Odds ratios of premove versus postmove percentage of top‐box scores, adjusted for age, sex, LOS, complexity of illness, and insurance type were determined using logistic regression for the units that moved. Similar scores were calculated for unmoved units to detect secular trends. To determine whether the differences between the moved and unmoved units were significant, we introduced the interaction term (moved vs unmoved unit status) (pre‐ vs postmove time period) into the logistic regression models and examined the adjusted P value for this term. All statistical analysis was performed using SAS Institute Inc.'s (Cary, NC) JMP Pro 10.0.0.
RESULTS
The study included 1648 respondents in the moved units in the baseline period (ie, units designated to move to a new clinical building) and 1373 respondents in the postmove period. There were 1593 respondents in the control group during the baseline period and 1049 respondents in the postmove period. For the units that moved, survey response rates were 28.5% prior to the move and 28.3% after the move. For the units that did not move, survey response rates were 20.9% prior to the move and 22.7% after the move. A majority of survey respondents on the nursing units that moved were white, male, and had private insurance (Table 1). There were no significant differences between respondents across these characteristics between the pre‐ and postmove periods. Mean age and LOS were also similar. For these units, there were 70.5% private rooms prior to the move and 100% after the move. For the unmoved units, 58.9% of the rooms were private in the baseline period and 72.7% were private in the study period. Similar to the units that moved, characteristics of the respondents on the unmoved units also did not differ significantly in the postmove period.
| Patient demographics | Moved Units (N=3,021) | Unmoved Units (N=2,642) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | P Value | Pre | Post | P Value | |
| ||||||
| White | 75.3% | 78.2% | 0.07 | 66.7% | 68.5% | 0.31 |
| Mean age, y | 57.3 | 57.4 | 0.84 | 57.3 | 57.1 | 0.81 |
| Male | 54.3% | 53.0% | 0.48 | 40.5% | 42.3% | 0.23 |
| Self‐reported health | ||||||
| Excellent or very good | 54.7% | 51.2% | 0.04 | 38.7% | 39.5% | 0.11 |
| Good | 27.8% | 32.0% | 29.3% | 32.2% | ||
| Fair or poor | 17.5% | 16.9% | 32.0% | 28.3% | ||
| Self‐reported language | ||||||
| English | 96.0% | 97.2% | 0.06 | 96.8% | 97.1% | 0.63 |
| Other | 4.0% | 2.8% | 3.2% | 2.9% | ||
| Self‐reported education | ||||||
| Less than high school | 5.8% | 5.0% | 0.24 | 10.8% | 10.4% | 0.24 |
| High school grad | 46.4% | 44.2% | 48.6% | 45.5% | ||
| College grad or more | 47.7% | 50.7% | 40.7% | 44.7% | ||
| Insurance type | ||||||
| Medicaid | 6.7% | 5.5% | 0.11 | 10.8% | 9.0% | 0.32 |
| Medicare | 32.0% | 35.5% | 36.0% | 36.1% | ||
| Private insurance | 55.6% | 52.8% | 48.0% | 50.3% | ||
| Mean APRDRG complexity* | 2.1 | 2.1 | 0.09 | 2.3 | 2.3 | 0.14 |
| Mean LOS | 4.7 | 5.0 | 0.12 | 4.9 | 5.0 | 0.77 |
| Service | ||||||
| Medicine | 15.4% | 16.2% | 0.51 | 40.0% | 34.5% | 0.10 |
| Surgery | 50.7% | 45.7% | 40.1% | 44.1% | ||
| Neurosciences | 20.3% | 24.1% | 6.0% | 6.0% | ||
| Obstetrics/gynecology | 7.5% | 8.2% | 5.7% | 5.6% | ||
The move was associated with significant improvements in facility‐related satisfaction (Tables 2 and 3). The most prominent increases in satisfaction were with pleasantness of dcor (33.6% vs 66.2%), noise level (39.9% vs 59.3%), and visitor accommodation and comfort (50.0% vs 70.3 %). There was improvement in satisfaction related to cleanliness of the room (49.0% vs 68.6 %), but no significant increase in satisfaction with courtesy of the person cleaning the room (59.8% vs 67.7%) when compared with units that did move.
| Satisfaction Domain | Moved Units | Unmoved Units | P Value of the Difference in Odds Ratio Between Moved and Unmoved Units | |||||
|---|---|---|---|---|---|---|---|---|
| % Top Box | Adjusted Odds Ratio* (95% CI) | % Top Box | Adjusted Odds Ratio* (95% CI) | |||||
| Pre | Post | Pre | Post | |||||
| ||||||||
| FACILITY RELATED | ||||||||
| Hospital environment | ||||||||
| Cleanliness of the room and bathroom | 61.0 | 70.8 | 1.62 (1.40‐1.90) | 64.0 | 69.2 | 1.24 (1.03‐1.48) | 0.03 | |
| Quietness of the room | 51.3 | 65.4 | 1.89 (1.63‐2.19) | 58.6 | 60.3 | 1.08 (0.90‐1.28) | <0.0001 | |
| NONFACILITY RELATED | ||||||||
| Nursing communication | ||||||||
| Nurses treated with courtesy/respect | 84.0 | 86.7 | 1.28 (1.05‐1.57) | 83.6 | 87.1 | 1.29 (1.02‐1.64) | 0.92 | |
| Nurses listened | 73.1 | 76.4 | 1.21 (1.03‐1.43) | 74.2 | 75.5 | 1.05 (0.86‐1.27) | 0.26 | |
| Nurses explained | 75.0 | 76.6 | 1.10 (0.94‐1.30) | 76.0 | 76.2 | 1.00 (0.82‐1.21) | 0.43 | |
| Physician communication | ||||||||
| Doctors treated with courtesy/respect | 89.5 | 90.5 | 1.13 (0.89‐1.42) | 84.9 | 87.3 | 1.20 (0.94‐1.53) | 0.77 | |
| Doctors listened | 81.4 | 81.0 | 0.93 (0.83‐1.19) | 77.7 | 77.1 | 0.94 (0.77‐1.15) | 0.68 | |
| Doctors explained | 79.2 | 79.0 | 1.00(0.84‐1.19) | 75.7 | 74.4 | 0.92 (0.76‐1.12) | 0.49 | |
| Other | ||||||||
| Help toileting as soon as you wanted | 61.8 | 63.7 | 1.08 (0.89‐1.32) | 62.3 | 60.6 | 0.92 (0.71‐1.18) | 0.31 | |
| Pain well controlled | 63.2 | 63.8 | 1.06 (0.90‐1.25) | 62.0 | 62.6 | 0.99 (0.81‐1.20) | 060 | |
| Staff do everything to help with pain | 77.7 | 80.1 | 1.19 (0.99‐1.44) | 76.8 | 75.7 | 0.90 (0.75‐1.13) | 0.07 | |
| Staff describe medicine side effects | 47.0 | 47.6 | 1.05 (0.89‐1.24) | 49.2 | 47.1 | 0.91 (0.74‐1.11) | 0.32 | |
| Tell you what new medicine was for | 76.4 | 76.4 | 1.02 (0.84‐1.25) | 77.1 | 78.8 | 1.09(0.85‐1.39) | 0.65 | |
| Overall | ||||||||
| Rate hospital (010) | 75.0 | 83.3 | 1.71 (1.44‐2.05) | 75.7 | 77.6 | 1.06 (0.87‐1.29) | 0.006 | |
| Recommend hospital | 82.5 | 87.1 | 1.43 (1.18‐1.76) | 81.4 | 82.0 | 0.98 (0.79‐1.22) | 0.03 | |
| Satisfaction Domain | Moved Unit | Unmoved Unit | P Value of the Difference in Odds Ratio Between Moved and Unmoved Units | ||||
|---|---|---|---|---|---|---|---|
| % Top Box | Adjusted Odds Ratio* (95% CI) | % Top Box | Adjusted Odds Ratio* (95% CI) | ||||
| Pre | Post | Pre | Post | ||||
| |||||||
| FACILITY RELATED | |||||||
| Room | |||||||
| Pleasantness of room dcor | 33.6 | 64.8 | 3.77 (3.24‐4.38) | 41.6 | 47.0 | 1.21 (1.02‐1.44) | <0.0001 |
| Room cleanliness | 49.0 | 68.6 | 2.35 (2.02‐2.73) | 51.6 | 59.1 | 1.32 (1.12‐1.58) | <0.0001 |
| Room temperature | 43.1 | 54.9 | 1.64 (1.43‐1.90) | 45.0 | 48.8 | 1.14 (0.96‐1.36) | 0.002 |
| Noise level in and around the room | 40.2 | 59.2 | 2.23 (1.92‐2.58) | 45.5 | 47.6 | 1.07 (0.90‐1.22) | <0.0001 |
| Visitor related | |||||||
| Accommodations and comfort of visitors | 50.0 | 70.3 | 2.44 (2.10‐2.83) | 55.3 | 59.1 | 1.14 (0.96‐1.35) | <0.0001 |
| NONFACILITY RELATED | |||||||
| Food | |||||||
| Temperature of the food | 31.1 | 33.6 | 1.15 (0.99‐1.34) | 34.0 | 38.9 | 1.23 (1.02‐1.47) | 0.51 |
| Quality of the food | 25.8 | 27.1 | 1.10 (0.93‐1.30) | 30.2 | 36.2 | 1.32 (1.10‐1.59) | 0.12 |
| Courtesy of the person who served food | 63.9 | 62.3 | 0.93 (0.80‐1.10) | 66.0 | 61.4 | 0.82 (0.69‐0.98) | 0.26 |
| Nursing | |||||||
| Friendliness/courtesy of the nurses | 76.3 | 82.8 | 1.49 (1.26‐1.79) | 77.7 | 80.1 | 1.10 (0.90‐1.37) | 0.04 |
| Promptness of response to call | 60.1 | 62.6 | 1.14 (0.98‐1.33) | 59.2 | 62.0 | 1.10 (0.91‐1.31) | 0.80 |
| Nurses' attitude toward requests | 71.0 | 75.8 | 1.30 (1.11‐1.54) | 70.5 | 72.4 | 1.06 (0.88‐1.28) | 0.13 |
| Attention to special/personal needs | 66.7 | 72.2 | 1.32 (1.13‐1.54) | 67.8 | 70.3 | 1.09 (0.91‐1.31) | 0.16 |
| Nurses kept you informed | 64.3 | 72.2 | 1.46 (1.25‐1.70) | 65.8 | 69.8 | 1.17 (0.98‐1.41) | 0.88 |
| Skill of the nurses | 75.3 | 79.5 | 1.28 (1.08‐1.52) | 74.3 | 78.6 | 1.23 (1.01‐1.51) | 0.89 |
| Ancillary staff | |||||||
| Courtesy of the person cleaning the room | 59.8 | 67.7 | 1.41 (1.21‐1.65) | 61.2 | 66.5 | 1.24 (1.03‐1.49) | 0.28 |
| Courtesy of the person who took blood | 66.5 | 68.1 | 1.10 (0.94‐1.28) | 63.2 | 63.1 | 0.96 (0.76‐1.08) | 0.34 |
| Courtesy of the person who started the IV | 70.0 | 71.7 | 1.09 (0.93‐1.28) | 66.6 | 69.3 | 1.11 (0.92‐1.33) | 0.88 |
| Visitor related | |||||||
| Staff attitude toward visitors | 68.1 | 79.4 | 1.84 (1.56‐2.18) | 70.3 | 72.2 | 1.06 (0.87‐1.28) | <0.0001 |
| Physician | |||||||
| Time physician spent with you | 55.0 | 58.9 | 1.20 (1.04‐1.39) | 53.2 | 55.9 | 1.10 (0.92‐1.30) | 0.46 |
| Physician concern questions/worries | 67.2 | 70.7 | 1.20 (1.03‐1.40) | 64.3 | 66.1 | 1.05 (0.88‐1.26) | 0.31 |
| Physician kept you informed | 65.3 | 67.5 | 1.12 (0.96‐1.30) | 61.6 | 63.2 | 1.05 (0.88‐1.25) | 0.58 |
| Friendliness/courtesy of physician | 76.3 | 78.1 | 1.11 (0.93‐1.31) | 71.0 | 73.3 | 1.08 (0.90‐1.31) | 0.89 |
| Skill of physician | 85.4 | 88.5 | 1.35 (1.09‐1.68) | 78.0 | 81.0 | 1.15 (0.93‐1.43) | 0.34 |
| Discharge | |||||||
| Extent felt ready for discharge | 62.0 | 66.7 | 1.23 (1.07‐1.44) | 59.2 | 62.3 | 1.10 (0.92‐1.30) | 0.35 |
| Speed of discharge process | 50.7 | 54.2 | 1.16 (1.01‐1.33) | 47.8 | 50.0 | 1.07 (0.90‐1.27) | 0.49 |
| Instructions for care at home | 66.4 | 71.1 | 1.25 (1.06‐1.46) | 64.0 | 67.7 | 1.16 (0.97‐1.39) | 0.54 |
| Staff concern for your privacy | 65.3 | 71.8 | 1.37 (1.17‐0.85) | 63.6 | 66.2 | 1.10 (0.91‐1.31) | 0.07 |
| Miscellaneous | |||||||
| How well your pain was controlled | 64.2 | 66.5 | 1.14 (0.97‐1.32) | 60.2 | 62.6 | 1.07 (0.89‐1.28) | 0.66 |
| Staff addressed emotional needs | 60.0 | 63.4 | 1.19 (1.02‐1.38) | 55.1 | 60.2 | 1.20 (1.01‐1.42) | 0.90 |
| Response to concerns/complaints | 61.1 | 64.5 | 1.19 (1.02‐1.38) | 57.2 | 60.1 | 1.10 (0.92‐1.31) | 0.57 |
| Overall | |||||||
| Staff worked together to care for you | 72.6 | 77.2 | 1.29 (1.10‐1.52) | 70.3 | 73.2 | 1.13 (0.93‐1.37) | 0.30 |
| Likelihood of recommending hospital | 79.1 | 84.3 | 1.44 (1.20‐1.74) | 76.3 | 79.2 | 1.14 (0.93‐1.39) | 0.10 |
| Overall rating of care given | 76.8 | 83.0 | 1.50 (1.25‐1.80) | 74.7 | 77.2 | 1.10 (0.90‐1.34) | 0.03 |
With regard to nonfacility‐related satisfaction, there were statistically higher scores in several nursing, physician, and discharge‐related satisfaction domains after the move. However, these changes were not associated with the move to the new clinical building as they were not significantly different from improvements on the unmoved units. Among nonfacility‐related items, only staff attitude toward visitors showed significant improvement (68.1% vs 79.4%). There was a significant improvement in hospital rating (75.0% vs 83.3% in the moved units and 75.7% vs 77.6% in the unmoved units). However, the other 3 measures of overall satisfaction did not show significant improvement associated with the move to the new clinical building when compared to the concurrent controls.
DISCUSSION
Contrary to our hypothesis and a belief held by many, we found that patients appeared able to distinguish their experience with hospital environment from their experience with providers and other services. Improvement in hospital facilities with incorporation of patient‐centered features was associated with improvements that were largely limited to increases in satisfaction with quietness, cleanliness, temperature, and dcor of the room along with visitor‐related satisfaction. Notably, there was no significant improvement in satisfaction related to physicians, nurses, housekeeping, and other service staff. There was improvement in satisfaction with staff attitude toward visitors, but this can be attributed to availability of visitor‐friendly facilities. There was a significant improvement in 1 of the 4 measures of overall satisfaction. Our findings also support the construct validity of HCAHPS and Press Ganey patient satisfaction surveys.
Ours is one of the largest studies on patient satisfaction related to patient‐centered design features in the inpatient acute care setting. Swan et al. also studied patients in an acute inpatient setting and compared satisfaction related to appealing versus typical hospital rooms. Patients were matched for case mix, insurance, gender, types of medical services received and LOS, and were served by the same set of physicians and similar food service and housekeeping staff.[26] Unlike our study, they found improved satisfaction related to physicians, housekeeping staff, food service staff, meals, and overall satisfaction. However, the study had some limitations. In particular, the study sample was self‐selected because the patients in this group were required to pay an extra daily fee to utilize the appealing room. Additionally, there were only 177 patients across the 2 groups, and the actual differences in satisfaction scores were small. Our sample was larger and patients in the study group were admitted to units in the new clinical buildings by the same criteria as they were admitted to the historic building prior to the move, and there were no significant differences in baseline characteristics between the comparison groups.
Jansen et al. also found broad improvements in patient satisfaction in a study of over 309 maternity unit patients in a new construction, all private‐room maternity unit with more appealing design elements and comfort features for visitors.[7] Improved satisfaction was noted with the physical environment, nursing care, assistance with feeding, respect for privacy, and discharge planning. However, it is difficult to extrapolate the results of this study to other settings, as maternity unit patients constitute a unique patient demographic with unique care needs. Additionally, when compared with patients in the control group, the patients in the study group were cared for by nurses who had a lower workload and who were not assigned other patients with more complex needs. Because nursing availability may be expected to impact satisfaction with clinical domains, the impact of private and appealing room may very well have been limited to improved satisfaction with the physical environment.
Despite the widespread belief among healthcare leadership that facility renovation or expansion is a vital strategy for improving patient satisfaction, our study shows that this may not be a dominant factor.[27] In fact, the Planetree model showed that improvement in satisfaction related to physical environment and nursing care was associated with implementation of both patient‐centered design features as well as with utilization of nurses that were trained to provide personalized care, educate patients, and involve patients and family.[28] It is more likely that provider‐level interventions will have a greater impact on provider level and overall satisfaction. This idea is supported by a recent JD Powers study suggesting that facilities represent only 19% of overall satisfaction in the inpatient setting.[35]
Although our study focused on patient‐centered design features, several renovation and construction projects have also focused on design features that improve patient safety and provider satisfaction, workflow, efficiency, productivity, stress, and time spent in direct care.[9] Interventions in these areas may lead to improvement in patient outcomes and perhaps lead to improvement in patient satisfaction; however, this relationship has not been well established at present.
In an era of cost containment, healthcare administrators are faced with high‐priced interventions, competing needs, limited resources, low profit margins, and often unclear evidence on cost‐effectiveness and return on investment of healthcare design features. Benefits are related to competitive advantage, higher reputation, patient retention, decreased malpractice costs, and increased Medicare payments through VBP programs that incentivize improved performance on quality metrics and patient satisfaction surveys. Our study supports the idea that a significant improvement in patient satisfaction related to creature comforts can be achieved with investment in patient‐centered design features. However, our findings also suggest that institutions should perform an individualized cost‐benefit analysis related to improvements in this narrow area of patient satisfaction. In our study, incorporation of patient‐centered design features resulted in improvement on 2 VBP HCAHPS measures, and its contribution toward total performance score under the VBP program would be limited.
Strengths of our study include the use of concurrent controls and our ability to capitalize on a natural experiment in which care teams remained constant before and after a move to a new clinical building. However, our study has some limitations. It was conducted at a single tertiary care academic center that predominantly serves an inner city population and referral patients seeking specialized care. Drivers of patient satisfaction may be different in community hospitals, and a different relationship may be observed between patient‐centered design and domains of patient satisfaction in this setting. Further studies in different hospital settings are needed to confirm our findings. Additionally, we were limited by the low response rate of the surveys. However, this is a widespread problem with all patient satisfaction research utilizing voluntary surveys, and our response rates are consistent with those previously reported.[34, 36, 37, 38] Furthermore, low response rates have not impeded the implementation of pay‐for‐performance programs on a national scale using HCHAPS.
In conclusion, our study suggests that hospitals should not use outdated facilities as an excuse for achievement of suboptimal satisfaction scores. Patients respond positively to creature comforts, pleasing surroundings, and visitor‐friendly facilities but can distinguish these positive experiences from experiences in other patient satisfaction domains. In our study, the move to a higher‐amenity building had only a modest impact on overall patient satisfaction, perhaps because clinical care is the primary driver of this outcome. Contrary to belief held by some hospital leaders, major strides in overall satisfaction across the board and other subdomains of satisfaction likely require intervention in areas other than facility renovation and expansion.
Disclosures
Zishan Siddiqui, MD, was supported by the Osler Center of Clinical Excellence Faculty Scholarship Grant. Funds from Johns Hopkins Hospitalist Scholars Program supported the research project. The authors have no conflict of interests to disclose.
Hospitals are expensive and complex facilities to build and renovate. It is estimated $200 billion is being spent in the United States during this decade on hospital construction and renovation, and further expenditures in this area are expected.[1] Aging hospital infrastructure, competition, and health system expansion have motivated institutions to invest in renovation and new hospital building construction.[2, 3, 4, 5, 6, 7] There is a trend toward patient‐centered design in new hospital construction. Features of this trend include same‐handed design (ie, rooms on a unit have all beds oriented in the same direction and do not share headwalls); use of sound absorbent materials to reduced ambient noise[7, 8, 9]; rooms with improved view and increased natural lighting to reduce anxiety, decrease delirium, and increase sense of wellbeing[10, 11, 12]; incorporation of natural elements like gardens, water features, and art[12, 13, 14, 15, 16, 17, 18]; single‐patient rooms to reduce transmission of infection and enhance privacy and visitor comfort[7, 19, 20]; presence of comfortable waiting rooms and visitor accommodations to enhance comfort and family participation[21, 22, 23]; and hotel‐like amenities such as on‐demand entertainment and room service menus.[24, 25]
There is a belief among some hospital leaders that patients are generally unable to distinguish their positive experience with a pleasing healthcare environment from their positive experience with care, and thus improving facilities will lead to improved satisfaction across the board.[26, 27] In a controlled study of hospitalized patients, appealing rooms were associated with increased satisfaction with services including housekeeping and food service staff, meals, as well as physicians and overall satisfaction.[26] A 2012 survey of hospital leadership found that expanding and renovating facilities was considered a top priority in improving patient satisfaction, with 82% of the respondents stating that this was important.[27]
Despite these attitudes, the impact of patient‐centered design on patient satisfaction is not well understood. Studies have shown that renovations and hospital construction that incorporates noise reduction strategies, positive distraction, patient and caregiver control, attractive waiting rooms, improved patient room appearance, private rooms, and large windows result in improved satisfaction with nursing, noise level, unit environment and cleanliness, perceived wait time, discharge preparedness, and overall care. [7, 19, 20, 23, 28] However, these studies were limited by small sample size, inclusion of a narrow group of patients (eg, ambulatory, obstetric, geriatric rehabilitation, intensive care unit), and concurrent use of interventions other than design improvement (eg, nurse and patient education). Many of these studies did not use the ubiquitous Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) and Press Ganey patient satisfaction surveys.
We sought to determine the changes in patient satisfaction that occurred during a natural experiment, in which clinical units (comprising stable nursing, physician, and unit teams) were relocated from an historic clinical building to a new clinical building that featured patient‐centered design, using HCAHPS and Press Ganey surveys and a large study population. We hypothesized that new building features would positively impact both facility related (eg, noise level), nonfacility related (eg, physician and housekeeping service related), and overall satisfaction.
METHODS
This was a retrospective analysis of prospectively collected Press Ganey and HCAPHS patient satisfaction survey data for a single academic tertiary care hospital.[29] The research project was reviewed and approved by the institutional review board.
Participants
All patients discharged from 12 clinical units that relocated to the new clinical building and returned patient satisfaction surveys served as study patients. The moved units included the coronary care unit, cardiac step down unit, medical intensive care unit, neuro critical care unit, surgical intensive care unit, orthopedic unit, neurology unit, neurosurgery unit, obstetrics units, gynecology unit, urology unit, cardiothoracic surgery unit, and the transplant surgery and renal transplant unit. Patients on clinical units that did not move served as concurrent controls.
Exposure
Patients admitted to the new clinical building experienced several patient‐centered design features. These features included easy access to healing gardens with a water feature, soaring lobbies, a collection of more than 500 works of art, well‐decorated and light‐filled patient rooms with sleeping accommodations for family members, sound‐absorbing features in patient care corridors ranging from acoustical ceiling tiles to a quiet nurse‐call system, and an interactive television network with Internet, movies, and games. All patients during the baseline period and control patients during the study period were located in typical patient rooms with standard hospital amenities. No other major patient satisfaction interventions were initiated during the pre‐ or postperiod in either arm of the study; ongoing patient satisfaction efforts (such as unit‐based customer care representatives) were deployed broadly and not restricted to the new clinical building. Clinical teams comprised of physicians, nurses, and ancillary staff did not change significantly after the move.
Time Periods
The move to new clinical building occurred on May 1, 2012. After allowing for a 15‐day washout period, the postmove period included Press Ganey and HCAHPS surveys returned for discharges that occurred during a 7.5‐month period between May 15, 2102 and December 31, 2012. Baseline data included Press Ganey and HCAHPS surveys returned for discharges in the preceding 12 months (May 1, 2011 to April 30, 2012). Sensitivity analysis using only 7.5 months of baseline data did not reveal any significant difference when compared with 12‐month baseline data, and we report only data from the 12‐month baseline period.
Instruments
Press Ganey and HCAHPS patient satisfaction surveys were sent via mail in the same envelope. Fifty percent of the discharged patients were randomized to receive the surveys. The Press Ganey survey contained 33 items covering across several subdomains including room, meal, nursing, physician, ancillary staff, visitor, discharge, and overall satisfaction. The HCAHPS survey contained 29 Centers for Medicare and Medicaid Services (CMS)‐mandated items, of which 21 are related to patient satisfaction. The development and testing and methods for administration and reporting of the HCAHPS survey have been previously described.[30, 31] Press Ganey patient satisfaction survey results have been reported in the literature.[32, 33]
Outcome Variables
Press Ganey and HCAHPS patient satisfaction survey responses were the primary outcome variables of the study. The survey items were categorized as facility related (eg, noise level), nonfacility related (eg, physician and nursing staff satisfaction), and overall satisfaction related.
Covariates
Age, sex, length of stay (LOS), insurance type, and all‐payer refined diagnosis‐related groupassociated illness complexity were included as covariates.
Statistical Analysis
Percent top‐box scores were calculated for each survey item as the percent of patients who responded very good for a given item on Press Ganey survey items and always or definitely yes or 9 or 10 on HCAHPS survey items. CMS utilizes percent top‐box scores to calculate payments under the Value Based Purchasing (VBP) program and to report the results publicly. Numerous studies have also reported percent top‐box scores for HCAHPS survey results.[31, 32, 33, 34]
Odds ratios of premove versus postmove percentage of top‐box scores, adjusted for age, sex, LOS, complexity of illness, and insurance type were determined using logistic regression for the units that moved. Similar scores were calculated for unmoved units to detect secular trends. To determine whether the differences between the moved and unmoved units were significant, we introduced the interaction term (moved vs unmoved unit status) (pre‐ vs postmove time period) into the logistic regression models and examined the adjusted P value for this term. All statistical analysis was performed using SAS Institute Inc.'s (Cary, NC) JMP Pro 10.0.0.
RESULTS
The study included 1648 respondents in the moved units in the baseline period (ie, units designated to move to a new clinical building) and 1373 respondents in the postmove period. There were 1593 respondents in the control group during the baseline period and 1049 respondents in the postmove period. For the units that moved, survey response rates were 28.5% prior to the move and 28.3% after the move. For the units that did not move, survey response rates were 20.9% prior to the move and 22.7% after the move. A majority of survey respondents on the nursing units that moved were white, male, and had private insurance (Table 1). There were no significant differences between respondents across these characteristics between the pre‐ and postmove periods. Mean age and LOS were also similar. For these units, there were 70.5% private rooms prior to the move and 100% after the move. For the unmoved units, 58.9% of the rooms were private in the baseline period and 72.7% were private in the study period. Similar to the units that moved, characteristics of the respondents on the unmoved units also did not differ significantly in the postmove period.
| Patient demographics | Moved Units (N=3,021) | Unmoved Units (N=2,642) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | P Value | Pre | Post | P Value | |
| ||||||
| White | 75.3% | 78.2% | 0.07 | 66.7% | 68.5% | 0.31 |
| Mean age, y | 57.3 | 57.4 | 0.84 | 57.3 | 57.1 | 0.81 |
| Male | 54.3% | 53.0% | 0.48 | 40.5% | 42.3% | 0.23 |
| Self‐reported health | ||||||
| Excellent or very good | 54.7% | 51.2% | 0.04 | 38.7% | 39.5% | 0.11 |
| Good | 27.8% | 32.0% | 29.3% | 32.2% | ||
| Fair or poor | 17.5% | 16.9% | 32.0% | 28.3% | ||
| Self‐reported language | ||||||
| English | 96.0% | 97.2% | 0.06 | 96.8% | 97.1% | 0.63 |
| Other | 4.0% | 2.8% | 3.2% | 2.9% | ||
| Self‐reported education | ||||||
| Less than high school | 5.8% | 5.0% | 0.24 | 10.8% | 10.4% | 0.24 |
| High school grad | 46.4% | 44.2% | 48.6% | 45.5% | ||
| College grad or more | 47.7% | 50.7% | 40.7% | 44.7% | ||
| Insurance type | ||||||
| Medicaid | 6.7% | 5.5% | 0.11 | 10.8% | 9.0% | 0.32 |
| Medicare | 32.0% | 35.5% | 36.0% | 36.1% | ||
| Private insurance | 55.6% | 52.8% | 48.0% | 50.3% | ||
| Mean APRDRG complexity* | 2.1 | 2.1 | 0.09 | 2.3 | 2.3 | 0.14 |
| Mean LOS | 4.7 | 5.0 | 0.12 | 4.9 | 5.0 | 0.77 |
| Service | ||||||
| Medicine | 15.4% | 16.2% | 0.51 | 40.0% | 34.5% | 0.10 |
| Surgery | 50.7% | 45.7% | 40.1% | 44.1% | ||
| Neurosciences | 20.3% | 24.1% | 6.0% | 6.0% | ||
| Obstetrics/gynecology | 7.5% | 8.2% | 5.7% | 5.6% | ||
The move was associated with significant improvements in facility‐related satisfaction (Tables 2 and 3). The most prominent increases in satisfaction were with pleasantness of dcor (33.6% vs 66.2%), noise level (39.9% vs 59.3%), and visitor accommodation and comfort (50.0% vs 70.3 %). There was improvement in satisfaction related to cleanliness of the room (49.0% vs 68.6 %), but no significant increase in satisfaction with courtesy of the person cleaning the room (59.8% vs 67.7%) when compared with units that did move.
| Satisfaction Domain | Moved Units | Unmoved Units | P Value of the Difference in Odds Ratio Between Moved and Unmoved Units | |||||
|---|---|---|---|---|---|---|---|---|
| % Top Box | Adjusted Odds Ratio* (95% CI) | % Top Box | Adjusted Odds Ratio* (95% CI) | |||||
| Pre | Post | Pre | Post | |||||
| ||||||||
| FACILITY RELATED | ||||||||
| Hospital environment | ||||||||
| Cleanliness of the room and bathroom | 61.0 | 70.8 | 1.62 (1.40‐1.90) | 64.0 | 69.2 | 1.24 (1.03‐1.48) | 0.03 | |
| Quietness of the room | 51.3 | 65.4 | 1.89 (1.63‐2.19) | 58.6 | 60.3 | 1.08 (0.90‐1.28) | <0.0001 | |
| NONFACILITY RELATED | ||||||||
| Nursing communication | ||||||||
| Nurses treated with courtesy/respect | 84.0 | 86.7 | 1.28 (1.05‐1.57) | 83.6 | 87.1 | 1.29 (1.02‐1.64) | 0.92 | |
| Nurses listened | 73.1 | 76.4 | 1.21 (1.03‐1.43) | 74.2 | 75.5 | 1.05 (0.86‐1.27) | 0.26 | |
| Nurses explained | 75.0 | 76.6 | 1.10 (0.94‐1.30) | 76.0 | 76.2 | 1.00 (0.82‐1.21) | 0.43 | |
| Physician communication | ||||||||
| Doctors treated with courtesy/respect | 89.5 | 90.5 | 1.13 (0.89‐1.42) | 84.9 | 87.3 | 1.20 (0.94‐1.53) | 0.77 | |
| Doctors listened | 81.4 | 81.0 | 0.93 (0.83‐1.19) | 77.7 | 77.1 | 0.94 (0.77‐1.15) | 0.68 | |
| Doctors explained | 79.2 | 79.0 | 1.00(0.84‐1.19) | 75.7 | 74.4 | 0.92 (0.76‐1.12) | 0.49 | |
| Other | ||||||||
| Help toileting as soon as you wanted | 61.8 | 63.7 | 1.08 (0.89‐1.32) | 62.3 | 60.6 | 0.92 (0.71‐1.18) | 0.31 | |
| Pain well controlled | 63.2 | 63.8 | 1.06 (0.90‐1.25) | 62.0 | 62.6 | 0.99 (0.81‐1.20) | 060 | |
| Staff do everything to help with pain | 77.7 | 80.1 | 1.19 (0.99‐1.44) | 76.8 | 75.7 | 0.90 (0.75‐1.13) | 0.07 | |
| Staff describe medicine side effects | 47.0 | 47.6 | 1.05 (0.89‐1.24) | 49.2 | 47.1 | 0.91 (0.74‐1.11) | 0.32 | |
| Tell you what new medicine was for | 76.4 | 76.4 | 1.02 (0.84‐1.25) | 77.1 | 78.8 | 1.09(0.85‐1.39) | 0.65 | |
| Overall | ||||||||
| Rate hospital (010) | 75.0 | 83.3 | 1.71 (1.44‐2.05) | 75.7 | 77.6 | 1.06 (0.87‐1.29) | 0.006 | |
| Recommend hospital | 82.5 | 87.1 | 1.43 (1.18‐1.76) | 81.4 | 82.0 | 0.98 (0.79‐1.22) | 0.03 | |
| Satisfaction Domain | Moved Unit | Unmoved Unit | P Value of the Difference in Odds Ratio Between Moved and Unmoved Units | ||||
|---|---|---|---|---|---|---|---|
| % Top Box | Adjusted Odds Ratio* (95% CI) | % Top Box | Adjusted Odds Ratio* (95% CI) | ||||
| Pre | Post | Pre | Post | ||||
| |||||||
| FACILITY RELATED | |||||||
| Room | |||||||
| Pleasantness of room dcor | 33.6 | 64.8 | 3.77 (3.24‐4.38) | 41.6 | 47.0 | 1.21 (1.02‐1.44) | <0.0001 |
| Room cleanliness | 49.0 | 68.6 | 2.35 (2.02‐2.73) | 51.6 | 59.1 | 1.32 (1.12‐1.58) | <0.0001 |
| Room temperature | 43.1 | 54.9 | 1.64 (1.43‐1.90) | 45.0 | 48.8 | 1.14 (0.96‐1.36) | 0.002 |
| Noise level in and around the room | 40.2 | 59.2 | 2.23 (1.92‐2.58) | 45.5 | 47.6 | 1.07 (0.90‐1.22) | <0.0001 |
| Visitor related | |||||||
| Accommodations and comfort of visitors | 50.0 | 70.3 | 2.44 (2.10‐2.83) | 55.3 | 59.1 | 1.14 (0.96‐1.35) | <0.0001 |
| NONFACILITY RELATED | |||||||
| Food | |||||||
| Temperature of the food | 31.1 | 33.6 | 1.15 (0.99‐1.34) | 34.0 | 38.9 | 1.23 (1.02‐1.47) | 0.51 |
| Quality of the food | 25.8 | 27.1 | 1.10 (0.93‐1.30) | 30.2 | 36.2 | 1.32 (1.10‐1.59) | 0.12 |
| Courtesy of the person who served food | 63.9 | 62.3 | 0.93 (0.80‐1.10) | 66.0 | 61.4 | 0.82 (0.69‐0.98) | 0.26 |
| Nursing | |||||||
| Friendliness/courtesy of the nurses | 76.3 | 82.8 | 1.49 (1.26‐1.79) | 77.7 | 80.1 | 1.10 (0.90‐1.37) | 0.04 |
| Promptness of response to call | 60.1 | 62.6 | 1.14 (0.98‐1.33) | 59.2 | 62.0 | 1.10 (0.91‐1.31) | 0.80 |
| Nurses' attitude toward requests | 71.0 | 75.8 | 1.30 (1.11‐1.54) | 70.5 | 72.4 | 1.06 (0.88‐1.28) | 0.13 |
| Attention to special/personal needs | 66.7 | 72.2 | 1.32 (1.13‐1.54) | 67.8 | 70.3 | 1.09 (0.91‐1.31) | 0.16 |
| Nurses kept you informed | 64.3 | 72.2 | 1.46 (1.25‐1.70) | 65.8 | 69.8 | 1.17 (0.98‐1.41) | 0.88 |
| Skill of the nurses | 75.3 | 79.5 | 1.28 (1.08‐1.52) | 74.3 | 78.6 | 1.23 (1.01‐1.51) | 0.89 |
| Ancillary staff | |||||||
| Courtesy of the person cleaning the room | 59.8 | 67.7 | 1.41 (1.21‐1.65) | 61.2 | 66.5 | 1.24 (1.03‐1.49) | 0.28 |
| Courtesy of the person who took blood | 66.5 | 68.1 | 1.10 (0.94‐1.28) | 63.2 | 63.1 | 0.96 (0.76‐1.08) | 0.34 |
| Courtesy of the person who started the IV | 70.0 | 71.7 | 1.09 (0.93‐1.28) | 66.6 | 69.3 | 1.11 (0.92‐1.33) | 0.88 |
| Visitor related | |||||||
| Staff attitude toward visitors | 68.1 | 79.4 | 1.84 (1.56‐2.18) | 70.3 | 72.2 | 1.06 (0.87‐1.28) | <0.0001 |
| Physician | |||||||
| Time physician spent with you | 55.0 | 58.9 | 1.20 (1.04‐1.39) | 53.2 | 55.9 | 1.10 (0.92‐1.30) | 0.46 |
| Physician concern questions/worries | 67.2 | 70.7 | 1.20 (1.03‐1.40) | 64.3 | 66.1 | 1.05 (0.88‐1.26) | 0.31 |
| Physician kept you informed | 65.3 | 67.5 | 1.12 (0.96‐1.30) | 61.6 | 63.2 | 1.05 (0.88‐1.25) | 0.58 |
| Friendliness/courtesy of physician | 76.3 | 78.1 | 1.11 (0.93‐1.31) | 71.0 | 73.3 | 1.08 (0.90‐1.31) | 0.89 |
| Skill of physician | 85.4 | 88.5 | 1.35 (1.09‐1.68) | 78.0 | 81.0 | 1.15 (0.93‐1.43) | 0.34 |
| Discharge | |||||||
| Extent felt ready for discharge | 62.0 | 66.7 | 1.23 (1.07‐1.44) | 59.2 | 62.3 | 1.10 (0.92‐1.30) | 0.35 |
| Speed of discharge process | 50.7 | 54.2 | 1.16 (1.01‐1.33) | 47.8 | 50.0 | 1.07 (0.90‐1.27) | 0.49 |
| Instructions for care at home | 66.4 | 71.1 | 1.25 (1.06‐1.46) | 64.0 | 67.7 | 1.16 (0.97‐1.39) | 0.54 |
| Staff concern for your privacy | 65.3 | 71.8 | 1.37 (1.17‐0.85) | 63.6 | 66.2 | 1.10 (0.91‐1.31) | 0.07 |
| Miscellaneous | |||||||
| How well your pain was controlled | 64.2 | 66.5 | 1.14 (0.97‐1.32) | 60.2 | 62.6 | 1.07 (0.89‐1.28) | 0.66 |
| Staff addressed emotional needs | 60.0 | 63.4 | 1.19 (1.02‐1.38) | 55.1 | 60.2 | 1.20 (1.01‐1.42) | 0.90 |
| Response to concerns/complaints | 61.1 | 64.5 | 1.19 (1.02‐1.38) | 57.2 | 60.1 | 1.10 (0.92‐1.31) | 0.57 |
| Overall | |||||||
| Staff worked together to care for you | 72.6 | 77.2 | 1.29 (1.10‐1.52) | 70.3 | 73.2 | 1.13 (0.93‐1.37) | 0.30 |
| Likelihood of recommending hospital | 79.1 | 84.3 | 1.44 (1.20‐1.74) | 76.3 | 79.2 | 1.14 (0.93‐1.39) | 0.10 |
| Overall rating of care given | 76.8 | 83.0 | 1.50 (1.25‐1.80) | 74.7 | 77.2 | 1.10 (0.90‐1.34) | 0.03 |
With regard to nonfacility‐related satisfaction, there were statistically higher scores in several nursing, physician, and discharge‐related satisfaction domains after the move. However, these changes were not associated with the move to the new clinical building as they were not significantly different from improvements on the unmoved units. Among nonfacility‐related items, only staff attitude toward visitors showed significant improvement (68.1% vs 79.4%). There was a significant improvement in hospital rating (75.0% vs 83.3% in the moved units and 75.7% vs 77.6% in the unmoved units). However, the other 3 measures of overall satisfaction did not show significant improvement associated with the move to the new clinical building when compared to the concurrent controls.
DISCUSSION
Contrary to our hypothesis and a belief held by many, we found that patients appeared able to distinguish their experience with hospital environment from their experience with providers and other services. Improvement in hospital facilities with incorporation of patient‐centered features was associated with improvements that were largely limited to increases in satisfaction with quietness, cleanliness, temperature, and dcor of the room along with visitor‐related satisfaction. Notably, there was no significant improvement in satisfaction related to physicians, nurses, housekeeping, and other service staff. There was improvement in satisfaction with staff attitude toward visitors, but this can be attributed to availability of visitor‐friendly facilities. There was a significant improvement in 1 of the 4 measures of overall satisfaction. Our findings also support the construct validity of HCAHPS and Press Ganey patient satisfaction surveys.
Ours is one of the largest studies on patient satisfaction related to patient‐centered design features in the inpatient acute care setting. Swan et al. also studied patients in an acute inpatient setting and compared satisfaction related to appealing versus typical hospital rooms. Patients were matched for case mix, insurance, gender, types of medical services received and LOS, and were served by the same set of physicians and similar food service and housekeeping staff.[26] Unlike our study, they found improved satisfaction related to physicians, housekeeping staff, food service staff, meals, and overall satisfaction. However, the study had some limitations. In particular, the study sample was self‐selected because the patients in this group were required to pay an extra daily fee to utilize the appealing room. Additionally, there were only 177 patients across the 2 groups, and the actual differences in satisfaction scores were small. Our sample was larger and patients in the study group were admitted to units in the new clinical buildings by the same criteria as they were admitted to the historic building prior to the move, and there were no significant differences in baseline characteristics between the comparison groups.
Jansen et al. also found broad improvements in patient satisfaction in a study of over 309 maternity unit patients in a new construction, all private‐room maternity unit with more appealing design elements and comfort features for visitors.[7] Improved satisfaction was noted with the physical environment, nursing care, assistance with feeding, respect for privacy, and discharge planning. However, it is difficult to extrapolate the results of this study to other settings, as maternity unit patients constitute a unique patient demographic with unique care needs. Additionally, when compared with patients in the control group, the patients in the study group were cared for by nurses who had a lower workload and who were not assigned other patients with more complex needs. Because nursing availability may be expected to impact satisfaction with clinical domains, the impact of private and appealing room may very well have been limited to improved satisfaction with the physical environment.
Despite the widespread belief among healthcare leadership that facility renovation or expansion is a vital strategy for improving patient satisfaction, our study shows that this may not be a dominant factor.[27] In fact, the Planetree model showed that improvement in satisfaction related to physical environment and nursing care was associated with implementation of both patient‐centered design features as well as with utilization of nurses that were trained to provide personalized care, educate patients, and involve patients and family.[28] It is more likely that provider‐level interventions will have a greater impact on provider level and overall satisfaction. This idea is supported by a recent JD Powers study suggesting that facilities represent only 19% of overall satisfaction in the inpatient setting.[35]
Although our study focused on patient‐centered design features, several renovation and construction projects have also focused on design features that improve patient safety and provider satisfaction, workflow, efficiency, productivity, stress, and time spent in direct care.[9] Interventions in these areas may lead to improvement in patient outcomes and perhaps lead to improvement in patient satisfaction; however, this relationship has not been well established at present.
In an era of cost containment, healthcare administrators are faced with high‐priced interventions, competing needs, limited resources, low profit margins, and often unclear evidence on cost‐effectiveness and return on investment of healthcare design features. Benefits are related to competitive advantage, higher reputation, patient retention, decreased malpractice costs, and increased Medicare payments through VBP programs that incentivize improved performance on quality metrics and patient satisfaction surveys. Our study supports the idea that a significant improvement in patient satisfaction related to creature comforts can be achieved with investment in patient‐centered design features. However, our findings also suggest that institutions should perform an individualized cost‐benefit analysis related to improvements in this narrow area of patient satisfaction. In our study, incorporation of patient‐centered design features resulted in improvement on 2 VBP HCAHPS measures, and its contribution toward total performance score under the VBP program would be limited.
Strengths of our study include the use of concurrent controls and our ability to capitalize on a natural experiment in which care teams remained constant before and after a move to a new clinical building. However, our study has some limitations. It was conducted at a single tertiary care academic center that predominantly serves an inner city population and referral patients seeking specialized care. Drivers of patient satisfaction may be different in community hospitals, and a different relationship may be observed between patient‐centered design and domains of patient satisfaction in this setting. Further studies in different hospital settings are needed to confirm our findings. Additionally, we were limited by the low response rate of the surveys. However, this is a widespread problem with all patient satisfaction research utilizing voluntary surveys, and our response rates are consistent with those previously reported.[34, 36, 37, 38] Furthermore, low response rates have not impeded the implementation of pay‐for‐performance programs on a national scale using HCHAPS.
In conclusion, our study suggests that hospitals should not use outdated facilities as an excuse for achievement of suboptimal satisfaction scores. Patients respond positively to creature comforts, pleasing surroundings, and visitor‐friendly facilities but can distinguish these positive experiences from experiences in other patient satisfaction domains. In our study, the move to a higher‐amenity building had only a modest impact on overall patient satisfaction, perhaps because clinical care is the primary driver of this outcome. Contrary to belief held by some hospital leaders, major strides in overall satisfaction across the board and other subdomains of satisfaction likely require intervention in areas other than facility renovation and expansion.
Disclosures
Zishan Siddiqui, MD, was supported by the Osler Center of Clinical Excellence Faculty Scholarship Grant. Funds from Johns Hopkins Hospitalist Scholars Program supported the research project. The authors have no conflict of interests to disclose.
- , . Create a blueprint for successful hospital construction. Nurs Manage. 2006;37(6):39–44.
- Walter Reed National Military Medical Center website. Facts at a glance. Available at: http://www.wrnmmc.capmed.mil/About%20Us/SitePages/Facts.aspx. Accessed June 19, 2013.
- . Keys to collaboration. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/keys‐collaboration. Accessed June 19, 2013.
- . A tale of 4 hospitals. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/tale‐4‐hospitals. Accessed June 19, 2013.
- . Gateway to the east. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/gateway‐east. Accessed June 19, 2013.
- . Lessons learned. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/lessons‐learned. Accessed June 19, 2013.
- , , , , . Single room maternity care and client satisfaction. Birth. 2000;27(4):235–243.
- , , , . Same‐handed and mirrored unit configurations: is there a difference in patient and nurse outcomes? J Nurs Adm. 2011;41(6):273–279.
- , . The Pebble Projects: coordinated evidence‐based case studies. Build Res Inform. 2008;36(2):129–145.
- , , . Effects of exposure to nature and abstract pictures on patients recovering from open heart surgery. J Soc Psychophysiol Res. 1993;30:7.
- , , , . Postoperative delirium. Curr Drug Targets. 2005;6(7):807–814.
- . Stimulus deprivation in windowless rooms. Anaesthesia. 1977;32(7):598–602.
- , , , . Post‐occupancy evaluation of healing gardens in a pediatric cancer center. Landsc Urban Plan. 2005;73(2):167–183.
- . Healing gardens in hospitals. Interdiscip Des Res J. 2007;1(1):1–27.
- , . Restorative gardens. BMJ. 1993;306(6885):1080–1081.
- . Effects of interior design on wellness: theory and recent scientific research. J Health Care Inter Des. 1991;3:97–109.
- , . Sunny hospital rooms expedite recovery from severe and refractory depressions. J Affect Disord. 1996;40(1‐2):49–51.
- . Art in hospital spaces: the role of hospitals in an aestheticised society. Int J Cult Policy. 2007;13(1):85–101.
- , , . Renovation of a semiprivate patient room. Bowman Center Geriatric Rehabilitation Unit. Nurs Clin North Am 1995;30(1):97–115.
- , , , et al. (2013). Effect of intensive care environment on family and patient satisfaction: a before‐after study. Intensive Care Med. 2013;39(9):1626–1634.
- , , , , . Outcomes of environmental appraisal of different hospital waiting areas. Environ Behav. 2003;35(6):842–869.
- . Redesigning the neurocritical care unit to enhance family participation and improve outcomes. Cleve Clin J Med. 2009;76(suppl 2):S70–S74.
- , . The ecology of the patient visit: physical attractiveness, waiting times, and perceived quality of care. J Ambul Care Manage. 2008;31(2):128–141.
- . Patient satisfaction and the new consumer. Hosp Health Netw. 2006;80(57):59–62.
- . Patient satisfaction. Hospitals embrace hotel‐like amenities. Hosp Health Netw. 2007;81(11):24–26.
- , , . Do appealing hospital rooms increase patient evaluations of physicians, nurses, and hospital services? Health Care Manage Rev. 2003;28(3):254–264.
- . Patient experience and HCAHPS: little consensus on a top priority. Health Leaders Media website. Available at http://www.healthleadersmedia.com/intelligence/detail.cfm?content_id=28289334(2):125–133.
- Centers for Medicare 67:27–37.
- Hospital Consumer Assessment of Healthcare Providers and Systems. Summary analysis. http://www.hcahpsonline.org/SummaryAnalyses.aspx. Accessed October 1, 2014.
- Centers for Medicare 44(2 pt 1):501–518.
- J.D. Power and Associates. Patient satisfaction influenced more by hospital staff than by the hospital facilities. Available at: http://www.jdpower.com/press‐releases/2012‐national‐patient‐experience‐study#sthash.gSv6wAdc.dpuf. Accessed December 10, 2013.
- , , , , . Racial and ethnic differences in a patient survey: patients' values, ratings, and reports regarding physician primary care performance in a large health maintenance organization. Med Care. 2000;38(3): 300–310.
- , , , . Patient experience in safety‐net hospitals implications for improving care and Value‐Based Purchasing patient experience in safety‐net hospitals. Arch Intern Med. 2012;172(16):1204–1210.
- , , , . Comparison of Hospital Consumer Assessment of Healthcare Providers and Systems patient satisfaction scores for specialty hospitals and general medical hospitals: confounding effect of survey response rate. J Hosp Med. 2014;9(9):590–593.
- , . Create a blueprint for successful hospital construction. Nurs Manage. 2006;37(6):39–44.
- Walter Reed National Military Medical Center website. Facts at a glance. Available at: http://www.wrnmmc.capmed.mil/About%20Us/SitePages/Facts.aspx. Accessed June 19, 2013.
- . Keys to collaboration. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/keys‐collaboration. Accessed June 19, 2013.
- . A tale of 4 hospitals. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/tale‐4‐hospitals. Accessed June 19, 2013.
- . Gateway to the east. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/gateway‐east. Accessed June 19, 2013.
- . Lessons learned. Healthcare Design website. Available at: http://www.healthcaredesignmagazine.com/building‐ideas/lessons‐learned. Accessed June 19, 2013.
- , , , , . Single room maternity care and client satisfaction. Birth. 2000;27(4):235–243.
- , , , . Same‐handed and mirrored unit configurations: is there a difference in patient and nurse outcomes? J Nurs Adm. 2011;41(6):273–279.
- , . The Pebble Projects: coordinated evidence‐based case studies. Build Res Inform. 2008;36(2):129–145.
- , , . Effects of exposure to nature and abstract pictures on patients recovering from open heart surgery. J Soc Psychophysiol Res. 1993;30:7.
- , , , . Postoperative delirium. Curr Drug Targets. 2005;6(7):807–814.
- . Stimulus deprivation in windowless rooms. Anaesthesia. 1977;32(7):598–602.
- , , , . Post‐occupancy evaluation of healing gardens in a pediatric cancer center. Landsc Urban Plan. 2005;73(2):167–183.
- . Healing gardens in hospitals. Interdiscip Des Res J. 2007;1(1):1–27.
- , . Restorative gardens. BMJ. 1993;306(6885):1080–1081.
- . Effects of interior design on wellness: theory and recent scientific research. J Health Care Inter Des. 1991;3:97–109.
- , . Sunny hospital rooms expedite recovery from severe and refractory depressions. J Affect Disord. 1996;40(1‐2):49–51.
- . Art in hospital spaces: the role of hospitals in an aestheticised society. Int J Cult Policy. 2007;13(1):85–101.
- , , . Renovation of a semiprivate patient room. Bowman Center Geriatric Rehabilitation Unit. Nurs Clin North Am 1995;30(1):97–115.
- , , , et al. (2013). Effect of intensive care environment on family and patient satisfaction: a before‐after study. Intensive Care Med. 2013;39(9):1626–1634.
- , , , , . Outcomes of environmental appraisal of different hospital waiting areas. Environ Behav. 2003;35(6):842–869.
- . Redesigning the neurocritical care unit to enhance family participation and improve outcomes. Cleve Clin J Med. 2009;76(suppl 2):S70–S74.
- , . The ecology of the patient visit: physical attractiveness, waiting times, and perceived quality of care. J Ambul Care Manage. 2008;31(2):128–141.
- . Patient satisfaction and the new consumer. Hosp Health Netw. 2006;80(57):59–62.
- . Patient satisfaction. Hospitals embrace hotel‐like amenities. Hosp Health Netw. 2007;81(11):24–26.
- , , . Do appealing hospital rooms increase patient evaluations of physicians, nurses, and hospital services? Health Care Manage Rev. 2003;28(3):254–264.
- . Patient experience and HCAHPS: little consensus on a top priority. Health Leaders Media website. Available at http://www.healthleadersmedia.com/intelligence/detail.cfm?content_id=28289334(2):125–133.
- Centers for Medicare 67:27–37.
- Hospital Consumer Assessment of Healthcare Providers and Systems. Summary analysis. http://www.hcahpsonline.org/SummaryAnalyses.aspx. Accessed October 1, 2014.
- Centers for Medicare 44(2 pt 1):501–518.
- J.D. Power and Associates. Patient satisfaction influenced more by hospital staff than by the hospital facilities. Available at: http://www.jdpower.com/press‐releases/2012‐national‐patient‐experience‐study#sthash.gSv6wAdc.dpuf. Accessed December 10, 2013.
- , , , , . Racial and ethnic differences in a patient survey: patients' values, ratings, and reports regarding physician primary care performance in a large health maintenance organization. Med Care. 2000;38(3): 300–310.
- , , , . Patient experience in safety‐net hospitals implications for improving care and Value‐Based Purchasing patient experience in safety‐net hospitals. Arch Intern Med. 2012;172(16):1204–1210.
- , , , . Comparison of Hospital Consumer Assessment of Healthcare Providers and Systems patient satisfaction scores for specialty hospitals and general medical hospitals: confounding effect of survey response rate. J Hosp Med. 2014;9(9):590–593.
© 2014 Society of Hospital Medicine
What Is This Large, Oddly Pigmented “Freckle”?
A 79-year-old man presents for a routine skin check, in the context of his 40-year history of nonmelanoma skin cancer. He grew up on a farm and then became a farmer himself, spending almost every day in the sun (usually without a hat). His skin burned easily but would take on a “tan” by the start of summer.
In the succeeding years, he developed so many skin cancers (and had them removed) that he lost count. All were basal cell or squamous cell carcinomas, predominately manifesting on his face, arms, and ears. Several required Mohs surgery for removal.
EXAMINATION
Abundant evidence of excessive sun exposure is seen on the patient’s skin: actinic keratoses on the forehead, ears, and neck, and multiple solar lentigines on the face, neck, and arms. On his left neck, below the ear, is a large, oddly pigmented, dark macular patch. Dermatoscopic examination reveals focal pigmentary clumping and streaming, which prompts the decision to perform an incisional biopsy. The darkest and most irregular part of the lesion is taken as a sample. The pathology report shows lentigo maligna.
What is lentigo maligna?
DISCUSSION
Lentigo maligna (LM), also known as Hutchinson freckle, is a type of melanoma in situ that is typically seen on sun-exposed skin. It has indistinct margins with predominantly brown and black coloration. LM is usually seen on older, mostly fair-skinned patients who have a history of extensive sun exposure. Its preferred sites include the face, ears, neck, and upper extremities.
LM is, by definition, entirely superficial and therefore safe. Its main significance is that it can become focally invasive to the dermis, a phenomenon termed lentigo maligna melanoma (LMM). When that occurs, the clusters of spindle-shaped atypical cells can then progress to a vertical growth phase, resulting in intravascular invasion that eventuates in metastasis.
LMs grow so slowly that they often escape detection; they are sometimes mistaken for solar lentigines (SL). They can “collide” with SLs or other benign lesions (eg, seborrheic keratoses), which can effectively camouflage them. Suspicion is usually triggered by change (color, size) in a lesion.
Biopsy is the only method of detection. In this case, the central portion of this large, oddly pigmented and bordered, multicolored patch was excised and the darkest, most irregular part of the lesion collected. This is the gold standard for biopsy of a potential melanoma. A large deep shave biopsy (“saucerization”) would have accomplished the same thing; studies show that neither process will cause metastasis. The main mistake to avoid is performing a single punch biopsy, which risks a false-negative result.
The definitive surgical approach is to remove the lesion with a 1-cm margin around it and into the underlying adipose layer. The wound can either be left to heal by secondary intention or closed primarily. Either way, this patient’s prognosis is excellent, unless the final pathology report indicates focal invasion. With a patient of this age, the LM could have been left alone—although, once discovered, LM is irresistibly compelling to treat.
TAKE-HOME LEARNING POINTS
• Lentigo maligna (LM) is a type of melanoma in situ with the potential to progress to lentigo maligna melanoma, an invasive form of early melanoma.
• LM is common on sun-exposed skin of older patients with a history of excessive sun exposure.
• Faces, ears, arms, and necks are common areas for LM to manifest.
• Biopsy of suspected melanoma should incorporate a significant portion of the darkest, most irregular part of the lesion; it can be done by incisional technique or deep saucerization.
• LM is also known as Hutchinson freckle, since it often resembles a large, irregularly bordered and pigmented freckle.
A 79-year-old man presents for a routine skin check, in the context of his 40-year history of nonmelanoma skin cancer. He grew up on a farm and then became a farmer himself, spending almost every day in the sun (usually without a hat). His skin burned easily but would take on a “tan” by the start of summer.
In the succeeding years, he developed so many skin cancers (and had them removed) that he lost count. All were basal cell or squamous cell carcinomas, predominately manifesting on his face, arms, and ears. Several required Mohs surgery for removal.
EXAMINATION
Abundant evidence of excessive sun exposure is seen on the patient’s skin: actinic keratoses on the forehead, ears, and neck, and multiple solar lentigines on the face, neck, and arms. On his left neck, below the ear, is a large, oddly pigmented, dark macular patch. Dermatoscopic examination reveals focal pigmentary clumping and streaming, which prompts the decision to perform an incisional biopsy. The darkest and most irregular part of the lesion is taken as a sample. The pathology report shows lentigo maligna.
What is lentigo maligna?
DISCUSSION
Lentigo maligna (LM), also known as Hutchinson freckle, is a type of melanoma in situ that is typically seen on sun-exposed skin. It has indistinct margins with predominantly brown and black coloration. LM is usually seen on older, mostly fair-skinned patients who have a history of extensive sun exposure. Its preferred sites include the face, ears, neck, and upper extremities.
LM is, by definition, entirely superficial and therefore safe. Its main significance is that it can become focally invasive to the dermis, a phenomenon termed lentigo maligna melanoma (LMM). When that occurs, the clusters of spindle-shaped atypical cells can then progress to a vertical growth phase, resulting in intravascular invasion that eventuates in metastasis.
LMs grow so slowly that they often escape detection; they are sometimes mistaken for solar lentigines (SL). They can “collide” with SLs or other benign lesions (eg, seborrheic keratoses), which can effectively camouflage them. Suspicion is usually triggered by change (color, size) in a lesion.
Biopsy is the only method of detection. In this case, the central portion of this large, oddly pigmented and bordered, multicolored patch was excised and the darkest, most irregular part of the lesion collected. This is the gold standard for biopsy of a potential melanoma. A large deep shave biopsy (“saucerization”) would have accomplished the same thing; studies show that neither process will cause metastasis. The main mistake to avoid is performing a single punch biopsy, which risks a false-negative result.
The definitive surgical approach is to remove the lesion with a 1-cm margin around it and into the underlying adipose layer. The wound can either be left to heal by secondary intention or closed primarily. Either way, this patient’s prognosis is excellent, unless the final pathology report indicates focal invasion. With a patient of this age, the LM could have been left alone—although, once discovered, LM is irresistibly compelling to treat.
TAKE-HOME LEARNING POINTS
• Lentigo maligna (LM) is a type of melanoma in situ with the potential to progress to lentigo maligna melanoma, an invasive form of early melanoma.
• LM is common on sun-exposed skin of older patients with a history of excessive sun exposure.
• Faces, ears, arms, and necks are common areas for LM to manifest.
• Biopsy of suspected melanoma should incorporate a significant portion of the darkest, most irregular part of the lesion; it can be done by incisional technique or deep saucerization.
• LM is also known as Hutchinson freckle, since it often resembles a large, irregularly bordered and pigmented freckle.
A 79-year-old man presents for a routine skin check, in the context of his 40-year history of nonmelanoma skin cancer. He grew up on a farm and then became a farmer himself, spending almost every day in the sun (usually without a hat). His skin burned easily but would take on a “tan” by the start of summer.
In the succeeding years, he developed so many skin cancers (and had them removed) that he lost count. All were basal cell or squamous cell carcinomas, predominately manifesting on his face, arms, and ears. Several required Mohs surgery for removal.
EXAMINATION
Abundant evidence of excessive sun exposure is seen on the patient’s skin: actinic keratoses on the forehead, ears, and neck, and multiple solar lentigines on the face, neck, and arms. On his left neck, below the ear, is a large, oddly pigmented, dark macular patch. Dermatoscopic examination reveals focal pigmentary clumping and streaming, which prompts the decision to perform an incisional biopsy. The darkest and most irregular part of the lesion is taken as a sample. The pathology report shows lentigo maligna.
What is lentigo maligna?
DISCUSSION
Lentigo maligna (LM), also known as Hutchinson freckle, is a type of melanoma in situ that is typically seen on sun-exposed skin. It has indistinct margins with predominantly brown and black coloration. LM is usually seen on older, mostly fair-skinned patients who have a history of extensive sun exposure. Its preferred sites include the face, ears, neck, and upper extremities.
LM is, by definition, entirely superficial and therefore safe. Its main significance is that it can become focally invasive to the dermis, a phenomenon termed lentigo maligna melanoma (LMM). When that occurs, the clusters of spindle-shaped atypical cells can then progress to a vertical growth phase, resulting in intravascular invasion that eventuates in metastasis.
LMs grow so slowly that they often escape detection; they are sometimes mistaken for solar lentigines (SL). They can “collide” with SLs or other benign lesions (eg, seborrheic keratoses), which can effectively camouflage them. Suspicion is usually triggered by change (color, size) in a lesion.
Biopsy is the only method of detection. In this case, the central portion of this large, oddly pigmented and bordered, multicolored patch was excised and the darkest, most irregular part of the lesion collected. This is the gold standard for biopsy of a potential melanoma. A large deep shave biopsy (“saucerization”) would have accomplished the same thing; studies show that neither process will cause metastasis. The main mistake to avoid is performing a single punch biopsy, which risks a false-negative result.
The definitive surgical approach is to remove the lesion with a 1-cm margin around it and into the underlying adipose layer. The wound can either be left to heal by secondary intention or closed primarily. Either way, this patient’s prognosis is excellent, unless the final pathology report indicates focal invasion. With a patient of this age, the LM could have been left alone—although, once discovered, LM is irresistibly compelling to treat.
TAKE-HOME LEARNING POINTS
• Lentigo maligna (LM) is a type of melanoma in situ with the potential to progress to lentigo maligna melanoma, an invasive form of early melanoma.
• LM is common on sun-exposed skin of older patients with a history of excessive sun exposure.
• Faces, ears, arms, and necks are common areas for LM to manifest.
• Biopsy of suspected melanoma should incorporate a significant portion of the darkest, most irregular part of the lesion; it can be done by incisional technique or deep saucerization.
• LM is also known as Hutchinson freckle, since it often resembles a large, irregularly bordered and pigmented freckle.
Conference News Update—Radiological Society of North America 2015
DTI Reveals Changes in Brain Connections in Early Alzheimer’s Disease
Changes in brain connections visible on MRI could represent an imaging biomarker of Alzheimer’s disease, according to a study presented at the meeting.
As many as five million Americans have Alzheimer’s disease, and this number is expected to increase to 14 million by 2050, according to the Centers for Disease Control and Prevention. Preventive treatments may be most effective before Alzheimer’s disease is diagnosed, such as when a person is experiencing mild cognitive impairment.
Previous efforts at early detection have focused on beta amyloid. For the current study, researchers looked at the brain’s structural connectome, a map of white matter tracts that carry signals between various areas of the brain.
“The structural connectome provides us with a way to characterize and measure these connections and how they change through disease or age,” said Jeffrey W. Prescott, MD, PhD, a radiology resident at Duke University Medical Center in Durham, North Carolina, and a coauthor of the study.
Dr. Prescott and colleagues analyzed data for 102 patients enrolled in a national study called the Alzheimer’s Disease Neuroimaging Initiative 2. The patients had undergone diffusion tensor imaging (DTI), which assesses the integrity of white matter tracts in the brain by measuring how easy it is for water to move along them. “Water prefers moving along the defined physical connections between regions in the brain, which makes DTI a great tool for evaluating the structural connectome,” said Dr. Prescott.
The researchers compared changes in the structural connectome with results from florbetapir PET imaging, a technique that measures the amount of beta amyloid plaque in the brain. The results showed a strong association between florbetapir uptake and decreases in the strength of the structural connectome in each of the five areas of the brain studied.
“This study ties together two of the major changes in the Alzheimer’s brain—structural tissue changes and pathologic amyloid plaque deposition—and suggests a promising role for DTI as a possible diagnostic adjunct,” said Dr. Prescott.
Based on these findings, DTI may have a role in assessing brain damage in early Alzheimer’s disease and in monitoring the effect of new therapies.
“Traditionally, Alzheimer’s disease is believed to exert its effects on thinking via damage to the brain’s gray matter, where most of the nerve cells are concentrated,” said Jeffrey R. Petrella, MD, Professor of Radiology at Duke University and senior author of the research. “This study suggests that amyloid deposition in the gray matter affects the associated white matter connections, which are essential for conducting messages across the billions of nerve cells in the brain, allowing for all aspects of mental function.”
“We suspect that as amyloid plaque load in the gray matter increases, the brain’s white matter starts to break down or malfunction and lose its ability to move water and neurochemicals efficiently,” added Dr. Prescott.
The researchers plan to continue studying this cohort of patients over time to gain a better understanding of how the disease evolves in individual patients. They also intend to incorporate functional imaging into their research to learn about how the relationship between function and structure changes with increasing amyloid burden.
Asymptomatic Atherosclerosis May Be Associated With Cognitive Impairment
A buildup of plaque in the body’s major arteries is associated with mild cognitive impairment, according to a study of approximately 2,000 adults conducted at the University of Texas (UT) Southwestern Medical Center.
“It is well established that plaque buildup in the arteries is a predictor of heart disease, but the relationship between atherosclerosis and brain health is less clear,” said Christopher D. Maroules, MD, a radiology resident at UT Southwestern Medical Center in Dallas. “Our findings suggest that atherosclerosis not only affects the heart, but also brain health.”
Researchers analyzed the test results of 1,903 participants (mean age, 44) in the Dallas Heart Study, a multiethnic population-based study of adults from Dallas County, Texas. The participants included men and women who had no symptoms of cardiovascular disease.
Study participants completed the Montreal Cognitive Assessment (MoCA), a 30-point standardized test for detecting mild cognitive impairment, and underwent MRI of the brain to measure white matter hyperintensity volume. Bright white spots known as high signal intensity areas on a brain MRI indicate abnormal changes within the white matter.
“Increased white matter hyperintensity volume is part of the normal aging process,” explained Dr. Maroules. “But excessive white matter hyperintensity volume is a marker for cognitive impairment.”
Study participants also underwent imaging exams to measure the buildup of plaque in the arteries in three distinct vascular areas of the body. They underwent MRI to measure wall thickness in the carotid arteries and in the abdominal aorta, and received CT to measure coronary artery calcium.
Using the results, researchers performed a statistical regression to understand the relationship between the incidence of atherosclerosis and mild cognitive impairment. After adjusting for traditional risk factors for atherosclerosis, including age, ethnicity, male sex, diabetes, hypertension, smoking, and BMI, the investigators found independent relationships between atherosclerosis in all three vascular areas of the body and cognitive health, as measured by MoCA scores, and white matter hyperintensity volume on MRI.
Individuals in the highest quartile of internal carotid wall thickness were 21% more likely to have cognitive impairment, as indicated by a low MoCA score. An increasing coronary artery calcium score was predictive of large white matter intensity volume on MRI.
“These results underscore the importance of identifying atherosclerosis in its early stages, not just to help preserve heart function, but also to preserve cognition and brain health,” said Dr. Maroules. The MRI and CT imaging techniques provide valuable prognostic information about an individual’s downstream health risks, he added.
“Plaque buildup in blood vessels throughout the body offers us a window into brain health. Imaging with CT and MRI has an important role in identifying patients who are at a higher risk for cognitive impairment.”
A Season of High School Football Without Concussion May Cause Brain Changes
Some high school football players exhibit measurable brain changes after a single season of play, even in the absence of concussion, according to a study presented at the meeting.
“This study adds to the growing body of evidence that a season of play in a contact sport can affect the brain in the absence of clinical findings,” said Christopher T. Whitlow, MD, PhD, MHA, Associate Professor of Radiology at Wake Forest School of Medicine and radiologist at Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
In recent years, various reports have suggested the potential effects that participation in youth sports may have on the developing brain. Most of these studies have looked at brain changes as a result of concussion, however. Dr. Whitlow and colleagues set out to determine whether head impacts withstood in the course of a season of high school football produce white matter changes in the brain in the absence of clinically diagnosed concussion.
The researchers studied 24 high school football players between the ages of 16 and 18. For all games and practices, players were monitored with Head Impact Telemetry System (HITs) helmet-mounted accelerometers, which are used in youth and collegiate football to assess the frequency and severity of helmet impacts.
Risk-weighted cumulative exposure was computed from the HITs data and represented the risk of concussion over the course of the season. These data, along with the total number of impacts, were used to categorize the players as heavy hitters or light hitters. The researchers identified nine of the 24 participants as heavy hitters and 15 as light hitters. None of the players had concussion during the season.
All players underwent pre- and post-season evaluation with diffusion tensor imaging (DTI) of the brain. Diffusion tensor imaging measures fractional anisotropy, which indicates the movement of water molecules along axons. In healthy white matter, the direction of water movement is fairly uniform, and fractional anisotropy is high. When water movement is more random, fractional anisotropy values decrease, thus suggesting microstructural abnormalities.
The results showed that both groups demonstrated global increases of fractional anisotropy over time, likely reflecting the effects of brain development. However, the heavy-hitter group showed statistically significant areas of decreased fractional anisotropy post-season in specific areas of the brain, including the splenium of the corpus callosum and deep white matter tracts.
“Our study found that players experiencing greater levels of head impacts have more fractional anisotropy loss, compared with players with lower impact exposure,” said Dr. Whitlow. “Similar brain MRI changes have been previously associated with mild traumatic brain injury. However, it is unclear whether or not these effects will be associated with any negative long-term consequences.” These findings are preliminary, and more study needs to be performed, concluded Dr. Whitlow.
Mild Coronary Artery Disease Increases Risk of Cardiovascular Events
Patients with diabetes and mild coronary artery disease have the same relative risk for a heart attack or other major adverse heart event as patients with diabetes and serious single-vessel obstructive disease, according to a long-term study.
Researchers at the University of British Columbia and St. Paul’s Hospital in Vancouver analyzed data from the Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter (CONFIRM) Registry, which was developed to examine the prognostic value of cardiac computed tomography angiography (CCTA) for predicting adverse cardiac events related to coronary artery disease. The registry, which has CCTA data for 40,000 patients from 17 centers around the world, now has five-year follow-up data for 14,000 patients.
“The CONFIRM Registry is the largest long-term data set available and allowed us to evaluate the long-term prognostic value of CCTA in diabetic patients,” said Jonathan Leipsic, MD, vice chairman of the Department of Radiology at the University of British Columbia and study coauthor.
The researchers analyzed data for 1,823 patients with diabetes who underwent CCTA to detect and determine the extent of coronary artery disease. Men and women (median age, 61.7) in the study were categorized as having no coronary artery disease, mild disease (ie, coronary artery narrowed by less than 50%), or obstructive disease (ie, obstruction of more than 50% of the artery). Over a 5.2-year follow-up period, 246 deaths occurred, representing 13.5% of the total study group.
Major adverse cardiovascular event (MACE) data were available for 973 patients. During the follow-up period, 295 (30.3%) of the patients had a MACE, such as heart attack or a coronary revascularization.
The researchers found that both obstructive and mild, or nonobstructive, coronary artery disease, as determined by CCTA, were associated with patient deaths and MACE. Most importantly, the researchers found that the relative risk for death or MACE for a patient with mild coronary artery disease was comparable to that of patients with single vessel obstructive disease.
“Until now, two-year follow-up studies suggested that a diabetic patient with mild or nonobstructive coronary artery disease had a lower risk of major adverse cardiovascular events and death than patients with obstructive disease,” said Philipp Blanke, MD, a radiologist at the University of British Columbia and St. Paul’s Hospital and a coauthor of the study. “Our five-year follow-up data suggest that nonobstructive and obstructive coronary artery disease, as detected by cardiac CTA in diabetic patients, are both associated with higher rates of mortality.”
Researchers need a better understanding of the evolution of plaque in the arteries and of patient response to therapies, said Dr. Leipsic. “Cardiac CT angiography is helpful for identifying diabetic patients who are at higher risk for heart events and who may benefit from more aggressive therapy to help modify that risk,” he added.
Patients Prefer Direct Access to Imaging Records
Patients value direct, independent access to their medical exams, researchers reported.
Giampaolo Greco, PhD, MPH, Assistant Professor in the Department of Population Health Science and Policy at the Mount Sinai School of Medicine in New York City, and colleagues set out to evaluate patient and provider satisfaction with RSNA Image Share, an Internet-based interoperable image exchange system that gives patients ownership of their imaging exams and control over access to their imaging records. The network enables radiology sites to make results of imaging exams available for patients to incorporate in personal health record (PHR) accounts they can use to securely store, manage, and share their imaging records. Sites also can use the network to send patient imaging records to other participating sites to support better informed care.
For the study, patients undergoing radiologic exams at four academic centers were eligible to establish online PHR accounts using the RSNA Image Share network. Patients could then use their PHR accounts to maintain and share their images with selected providers, creating a detailed medical history accessible through any secure Internet connection.
Between July 2012 and August 2013, the study enrolled 2,562 patients, mean age 50.4, including a significant representation of older individuals. Older individuals have the highest healthcare utilization and often experience or perceive a significant barrier in using information technology.
The median number of exams uploaded per patient was six. Study participants were provided a brief survey to assess patient and physician experience with the exchange of images, and 502 patients completed and returned their surveys. Of these respondents, 448 patients identified the method used at the visit to share images: Internet, CDs, both Internet and CDs, or other, and 165 included a section completed by their physician.
Nearly all (96%) of the patients responded positively to having direct access to their medical images, and 78% viewed their images independently. There was no difference between Internet and CD users in satisfaction with privacy and security and timeliness of access to medical images. A greater percentage of Internet users reported being able to access their images without difficulty, compared with CD users (88.3% vs 77.5%).
DTI Reveals Changes in Brain Connections in Early Alzheimer’s Disease
Changes in brain connections visible on MRI could represent an imaging biomarker of Alzheimer’s disease, according to a study presented at the meeting.
As many as five million Americans have Alzheimer’s disease, and this number is expected to increase to 14 million by 2050, according to the Centers for Disease Control and Prevention. Preventive treatments may be most effective before Alzheimer’s disease is diagnosed, such as when a person is experiencing mild cognitive impairment.
Previous efforts at early detection have focused on beta amyloid. For the current study, researchers looked at the brain’s structural connectome, a map of white matter tracts that carry signals between various areas of the brain.
“The structural connectome provides us with a way to characterize and measure these connections and how they change through disease or age,” said Jeffrey W. Prescott, MD, PhD, a radiology resident at Duke University Medical Center in Durham, North Carolina, and a coauthor of the study.
Dr. Prescott and colleagues analyzed data for 102 patients enrolled in a national study called the Alzheimer’s Disease Neuroimaging Initiative 2. The patients had undergone diffusion tensor imaging (DTI), which assesses the integrity of white matter tracts in the brain by measuring how easy it is for water to move along them. “Water prefers moving along the defined physical connections between regions in the brain, which makes DTI a great tool for evaluating the structural connectome,” said Dr. Prescott.
The researchers compared changes in the structural connectome with results from florbetapir PET imaging, a technique that measures the amount of beta amyloid plaque in the brain. The results showed a strong association between florbetapir uptake and decreases in the strength of the structural connectome in each of the five areas of the brain studied.
“This study ties together two of the major changes in the Alzheimer’s brain—structural tissue changes and pathologic amyloid plaque deposition—and suggests a promising role for DTI as a possible diagnostic adjunct,” said Dr. Prescott.
Based on these findings, DTI may have a role in assessing brain damage in early Alzheimer’s disease and in monitoring the effect of new therapies.
“Traditionally, Alzheimer’s disease is believed to exert its effects on thinking via damage to the brain’s gray matter, where most of the nerve cells are concentrated,” said Jeffrey R. Petrella, MD, Professor of Radiology at Duke University and senior author of the research. “This study suggests that amyloid deposition in the gray matter affects the associated white matter connections, which are essential for conducting messages across the billions of nerve cells in the brain, allowing for all aspects of mental function.”
“We suspect that as amyloid plaque load in the gray matter increases, the brain’s white matter starts to break down or malfunction and lose its ability to move water and neurochemicals efficiently,” added Dr. Prescott.
The researchers plan to continue studying this cohort of patients over time to gain a better understanding of how the disease evolves in individual patients. They also intend to incorporate functional imaging into their research to learn about how the relationship between function and structure changes with increasing amyloid burden.
Asymptomatic Atherosclerosis May Be Associated With Cognitive Impairment
A buildup of plaque in the body’s major arteries is associated with mild cognitive impairment, according to a study of approximately 2,000 adults conducted at the University of Texas (UT) Southwestern Medical Center.
“It is well established that plaque buildup in the arteries is a predictor of heart disease, but the relationship between atherosclerosis and brain health is less clear,” said Christopher D. Maroules, MD, a radiology resident at UT Southwestern Medical Center in Dallas. “Our findings suggest that atherosclerosis not only affects the heart, but also brain health.”
Researchers analyzed the test results of 1,903 participants (mean age, 44) in the Dallas Heart Study, a multiethnic population-based study of adults from Dallas County, Texas. The participants included men and women who had no symptoms of cardiovascular disease.
Study participants completed the Montreal Cognitive Assessment (MoCA), a 30-point standardized test for detecting mild cognitive impairment, and underwent MRI of the brain to measure white matter hyperintensity volume. Bright white spots known as high signal intensity areas on a brain MRI indicate abnormal changes within the white matter.
“Increased white matter hyperintensity volume is part of the normal aging process,” explained Dr. Maroules. “But excessive white matter hyperintensity volume is a marker for cognitive impairment.”
Study participants also underwent imaging exams to measure the buildup of plaque in the arteries in three distinct vascular areas of the body. They underwent MRI to measure wall thickness in the carotid arteries and in the abdominal aorta, and received CT to measure coronary artery calcium.
Using the results, researchers performed a statistical regression to understand the relationship between the incidence of atherosclerosis and mild cognitive impairment. After adjusting for traditional risk factors for atherosclerosis, including age, ethnicity, male sex, diabetes, hypertension, smoking, and BMI, the investigators found independent relationships between atherosclerosis in all three vascular areas of the body and cognitive health, as measured by MoCA scores, and white matter hyperintensity volume on MRI.
Individuals in the highest quartile of internal carotid wall thickness were 21% more likely to have cognitive impairment, as indicated by a low MoCA score. An increasing coronary artery calcium score was predictive of large white matter intensity volume on MRI.
“These results underscore the importance of identifying atherosclerosis in its early stages, not just to help preserve heart function, but also to preserve cognition and brain health,” said Dr. Maroules. The MRI and CT imaging techniques provide valuable prognostic information about an individual’s downstream health risks, he added.
“Plaque buildup in blood vessels throughout the body offers us a window into brain health. Imaging with CT and MRI has an important role in identifying patients who are at a higher risk for cognitive impairment.”
A Season of High School Football Without Concussion May Cause Brain Changes
Some high school football players exhibit measurable brain changes after a single season of play, even in the absence of concussion, according to a study presented at the meeting.
“This study adds to the growing body of evidence that a season of play in a contact sport can affect the brain in the absence of clinical findings,” said Christopher T. Whitlow, MD, PhD, MHA, Associate Professor of Radiology at Wake Forest School of Medicine and radiologist at Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
In recent years, various reports have suggested the potential effects that participation in youth sports may have on the developing brain. Most of these studies have looked at brain changes as a result of concussion, however. Dr. Whitlow and colleagues set out to determine whether head impacts withstood in the course of a season of high school football produce white matter changes in the brain in the absence of clinically diagnosed concussion.
The researchers studied 24 high school football players between the ages of 16 and 18. For all games and practices, players were monitored with Head Impact Telemetry System (HITs) helmet-mounted accelerometers, which are used in youth and collegiate football to assess the frequency and severity of helmet impacts.
Risk-weighted cumulative exposure was computed from the HITs data and represented the risk of concussion over the course of the season. These data, along with the total number of impacts, were used to categorize the players as heavy hitters or light hitters. The researchers identified nine of the 24 participants as heavy hitters and 15 as light hitters. None of the players had concussion during the season.
All players underwent pre- and post-season evaluation with diffusion tensor imaging (DTI) of the brain. Diffusion tensor imaging measures fractional anisotropy, which indicates the movement of water molecules along axons. In healthy white matter, the direction of water movement is fairly uniform, and fractional anisotropy is high. When water movement is more random, fractional anisotropy values decrease, thus suggesting microstructural abnormalities.
The results showed that both groups demonstrated global increases of fractional anisotropy over time, likely reflecting the effects of brain development. However, the heavy-hitter group showed statistically significant areas of decreased fractional anisotropy post-season in specific areas of the brain, including the splenium of the corpus callosum and deep white matter tracts.
“Our study found that players experiencing greater levels of head impacts have more fractional anisotropy loss, compared with players with lower impact exposure,” said Dr. Whitlow. “Similar brain MRI changes have been previously associated with mild traumatic brain injury. However, it is unclear whether or not these effects will be associated with any negative long-term consequences.” These findings are preliminary, and more study needs to be performed, concluded Dr. Whitlow.
Mild Coronary Artery Disease Increases Risk of Cardiovascular Events
Patients with diabetes and mild coronary artery disease have the same relative risk for a heart attack or other major adverse heart event as patients with diabetes and serious single-vessel obstructive disease, according to a long-term study.
Researchers at the University of British Columbia and St. Paul’s Hospital in Vancouver analyzed data from the Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter (CONFIRM) Registry, which was developed to examine the prognostic value of cardiac computed tomography angiography (CCTA) for predicting adverse cardiac events related to coronary artery disease. The registry, which has CCTA data for 40,000 patients from 17 centers around the world, now has five-year follow-up data for 14,000 patients.
“The CONFIRM Registry is the largest long-term data set available and allowed us to evaluate the long-term prognostic value of CCTA in diabetic patients,” said Jonathan Leipsic, MD, vice chairman of the Department of Radiology at the University of British Columbia and study coauthor.
The researchers analyzed data for 1,823 patients with diabetes who underwent CCTA to detect and determine the extent of coronary artery disease. Men and women (median age, 61.7) in the study were categorized as having no coronary artery disease, mild disease (ie, coronary artery narrowed by less than 50%), or obstructive disease (ie, obstruction of more than 50% of the artery). Over a 5.2-year follow-up period, 246 deaths occurred, representing 13.5% of the total study group.
Major adverse cardiovascular event (MACE) data were available for 973 patients. During the follow-up period, 295 (30.3%) of the patients had a MACE, such as heart attack or a coronary revascularization.
The researchers found that both obstructive and mild, or nonobstructive, coronary artery disease, as determined by CCTA, were associated with patient deaths and MACE. Most importantly, the researchers found that the relative risk for death or MACE for a patient with mild coronary artery disease was comparable to that of patients with single vessel obstructive disease.
“Until now, two-year follow-up studies suggested that a diabetic patient with mild or nonobstructive coronary artery disease had a lower risk of major adverse cardiovascular events and death than patients with obstructive disease,” said Philipp Blanke, MD, a radiologist at the University of British Columbia and St. Paul’s Hospital and a coauthor of the study. “Our five-year follow-up data suggest that nonobstructive and obstructive coronary artery disease, as detected by cardiac CTA in diabetic patients, are both associated with higher rates of mortality.”
Researchers need a better understanding of the evolution of plaque in the arteries and of patient response to therapies, said Dr. Leipsic. “Cardiac CT angiography is helpful for identifying diabetic patients who are at higher risk for heart events and who may benefit from more aggressive therapy to help modify that risk,” he added.
Patients Prefer Direct Access to Imaging Records
Patients value direct, independent access to their medical exams, researchers reported.
Giampaolo Greco, PhD, MPH, Assistant Professor in the Department of Population Health Science and Policy at the Mount Sinai School of Medicine in New York City, and colleagues set out to evaluate patient and provider satisfaction with RSNA Image Share, an Internet-based interoperable image exchange system that gives patients ownership of their imaging exams and control over access to their imaging records. The network enables radiology sites to make results of imaging exams available for patients to incorporate in personal health record (PHR) accounts they can use to securely store, manage, and share their imaging records. Sites also can use the network to send patient imaging records to other participating sites to support better informed care.
For the study, patients undergoing radiologic exams at four academic centers were eligible to establish online PHR accounts using the RSNA Image Share network. Patients could then use their PHR accounts to maintain and share their images with selected providers, creating a detailed medical history accessible through any secure Internet connection.
Between July 2012 and August 2013, the study enrolled 2,562 patients, mean age 50.4, including a significant representation of older individuals. Older individuals have the highest healthcare utilization and often experience or perceive a significant barrier in using information technology.
The median number of exams uploaded per patient was six. Study participants were provided a brief survey to assess patient and physician experience with the exchange of images, and 502 patients completed and returned their surveys. Of these respondents, 448 patients identified the method used at the visit to share images: Internet, CDs, both Internet and CDs, or other, and 165 included a section completed by their physician.
Nearly all (96%) of the patients responded positively to having direct access to their medical images, and 78% viewed their images independently. There was no difference between Internet and CD users in satisfaction with privacy and security and timeliness of access to medical images. A greater percentage of Internet users reported being able to access their images without difficulty, compared with CD users (88.3% vs 77.5%).
DTI Reveals Changes in Brain Connections in Early Alzheimer’s Disease
Changes in brain connections visible on MRI could represent an imaging biomarker of Alzheimer’s disease, according to a study presented at the meeting.
As many as five million Americans have Alzheimer’s disease, and this number is expected to increase to 14 million by 2050, according to the Centers for Disease Control and Prevention. Preventive treatments may be most effective before Alzheimer’s disease is diagnosed, such as when a person is experiencing mild cognitive impairment.
Previous efforts at early detection have focused on beta amyloid. For the current study, researchers looked at the brain’s structural connectome, a map of white matter tracts that carry signals between various areas of the brain.
“The structural connectome provides us with a way to characterize and measure these connections and how they change through disease or age,” said Jeffrey W. Prescott, MD, PhD, a radiology resident at Duke University Medical Center in Durham, North Carolina, and a coauthor of the study.
Dr. Prescott and colleagues analyzed data for 102 patients enrolled in a national study called the Alzheimer’s Disease Neuroimaging Initiative 2. The patients had undergone diffusion tensor imaging (DTI), which assesses the integrity of white matter tracts in the brain by measuring how easy it is for water to move along them. “Water prefers moving along the defined physical connections between regions in the brain, which makes DTI a great tool for evaluating the structural connectome,” said Dr. Prescott.
The researchers compared changes in the structural connectome with results from florbetapir PET imaging, a technique that measures the amount of beta amyloid plaque in the brain. The results showed a strong association between florbetapir uptake and decreases in the strength of the structural connectome in each of the five areas of the brain studied.
“This study ties together two of the major changes in the Alzheimer’s brain—structural tissue changes and pathologic amyloid plaque deposition—and suggests a promising role for DTI as a possible diagnostic adjunct,” said Dr. Prescott.
Based on these findings, DTI may have a role in assessing brain damage in early Alzheimer’s disease and in monitoring the effect of new therapies.
“Traditionally, Alzheimer’s disease is believed to exert its effects on thinking via damage to the brain’s gray matter, where most of the nerve cells are concentrated,” said Jeffrey R. Petrella, MD, Professor of Radiology at Duke University and senior author of the research. “This study suggests that amyloid deposition in the gray matter affects the associated white matter connections, which are essential for conducting messages across the billions of nerve cells in the brain, allowing for all aspects of mental function.”
“We suspect that as amyloid plaque load in the gray matter increases, the brain’s white matter starts to break down or malfunction and lose its ability to move water and neurochemicals efficiently,” added Dr. Prescott.
The researchers plan to continue studying this cohort of patients over time to gain a better understanding of how the disease evolves in individual patients. They also intend to incorporate functional imaging into their research to learn about how the relationship between function and structure changes with increasing amyloid burden.
Asymptomatic Atherosclerosis May Be Associated With Cognitive Impairment
A buildup of plaque in the body’s major arteries is associated with mild cognitive impairment, according to a study of approximately 2,000 adults conducted at the University of Texas (UT) Southwestern Medical Center.
“It is well established that plaque buildup in the arteries is a predictor of heart disease, but the relationship between atherosclerosis and brain health is less clear,” said Christopher D. Maroules, MD, a radiology resident at UT Southwestern Medical Center in Dallas. “Our findings suggest that atherosclerosis not only affects the heart, but also brain health.”
Researchers analyzed the test results of 1,903 participants (mean age, 44) in the Dallas Heart Study, a multiethnic population-based study of adults from Dallas County, Texas. The participants included men and women who had no symptoms of cardiovascular disease.
Study participants completed the Montreal Cognitive Assessment (MoCA), a 30-point standardized test for detecting mild cognitive impairment, and underwent MRI of the brain to measure white matter hyperintensity volume. Bright white spots known as high signal intensity areas on a brain MRI indicate abnormal changes within the white matter.
“Increased white matter hyperintensity volume is part of the normal aging process,” explained Dr. Maroules. “But excessive white matter hyperintensity volume is a marker for cognitive impairment.”
Study participants also underwent imaging exams to measure the buildup of plaque in the arteries in three distinct vascular areas of the body. They underwent MRI to measure wall thickness in the carotid arteries and in the abdominal aorta, and received CT to measure coronary artery calcium.
Using the results, researchers performed a statistical regression to understand the relationship between the incidence of atherosclerosis and mild cognitive impairment. After adjusting for traditional risk factors for atherosclerosis, including age, ethnicity, male sex, diabetes, hypertension, smoking, and BMI, the investigators found independent relationships between atherosclerosis in all three vascular areas of the body and cognitive health, as measured by MoCA scores, and white matter hyperintensity volume on MRI.
Individuals in the highest quartile of internal carotid wall thickness were 21% more likely to have cognitive impairment, as indicated by a low MoCA score. An increasing coronary artery calcium score was predictive of large white matter intensity volume on MRI.
“These results underscore the importance of identifying atherosclerosis in its early stages, not just to help preserve heart function, but also to preserve cognition and brain health,” said Dr. Maroules. The MRI and CT imaging techniques provide valuable prognostic information about an individual’s downstream health risks, he added.
“Plaque buildup in blood vessels throughout the body offers us a window into brain health. Imaging with CT and MRI has an important role in identifying patients who are at a higher risk for cognitive impairment.”
A Season of High School Football Without Concussion May Cause Brain Changes
Some high school football players exhibit measurable brain changes after a single season of play, even in the absence of concussion, according to a study presented at the meeting.
“This study adds to the growing body of evidence that a season of play in a contact sport can affect the brain in the absence of clinical findings,” said Christopher T. Whitlow, MD, PhD, MHA, Associate Professor of Radiology at Wake Forest School of Medicine and radiologist at Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
In recent years, various reports have suggested the potential effects that participation in youth sports may have on the developing brain. Most of these studies have looked at brain changes as a result of concussion, however. Dr. Whitlow and colleagues set out to determine whether head impacts withstood in the course of a season of high school football produce white matter changes in the brain in the absence of clinically diagnosed concussion.
The researchers studied 24 high school football players between the ages of 16 and 18. For all games and practices, players were monitored with Head Impact Telemetry System (HITs) helmet-mounted accelerometers, which are used in youth and collegiate football to assess the frequency and severity of helmet impacts.
Risk-weighted cumulative exposure was computed from the HITs data and represented the risk of concussion over the course of the season. These data, along with the total number of impacts, were used to categorize the players as heavy hitters or light hitters. The researchers identified nine of the 24 participants as heavy hitters and 15 as light hitters. None of the players had concussion during the season.
All players underwent pre- and post-season evaluation with diffusion tensor imaging (DTI) of the brain. Diffusion tensor imaging measures fractional anisotropy, which indicates the movement of water molecules along axons. In healthy white matter, the direction of water movement is fairly uniform, and fractional anisotropy is high. When water movement is more random, fractional anisotropy values decrease, thus suggesting microstructural abnormalities.
The results showed that both groups demonstrated global increases of fractional anisotropy over time, likely reflecting the effects of brain development. However, the heavy-hitter group showed statistically significant areas of decreased fractional anisotropy post-season in specific areas of the brain, including the splenium of the corpus callosum and deep white matter tracts.
“Our study found that players experiencing greater levels of head impacts have more fractional anisotropy loss, compared with players with lower impact exposure,” said Dr. Whitlow. “Similar brain MRI changes have been previously associated with mild traumatic brain injury. However, it is unclear whether or not these effects will be associated with any negative long-term consequences.” These findings are preliminary, and more study needs to be performed, concluded Dr. Whitlow.
Mild Coronary Artery Disease Increases Risk of Cardiovascular Events
Patients with diabetes and mild coronary artery disease have the same relative risk for a heart attack or other major adverse heart event as patients with diabetes and serious single-vessel obstructive disease, according to a long-term study.
Researchers at the University of British Columbia and St. Paul’s Hospital in Vancouver analyzed data from the Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter (CONFIRM) Registry, which was developed to examine the prognostic value of cardiac computed tomography angiography (CCTA) for predicting adverse cardiac events related to coronary artery disease. The registry, which has CCTA data for 40,000 patients from 17 centers around the world, now has five-year follow-up data for 14,000 patients.
“The CONFIRM Registry is the largest long-term data set available and allowed us to evaluate the long-term prognostic value of CCTA in diabetic patients,” said Jonathan Leipsic, MD, vice chairman of the Department of Radiology at the University of British Columbia and study coauthor.
The researchers analyzed data for 1,823 patients with diabetes who underwent CCTA to detect and determine the extent of coronary artery disease. Men and women (median age, 61.7) in the study were categorized as having no coronary artery disease, mild disease (ie, coronary artery narrowed by less than 50%), or obstructive disease (ie, obstruction of more than 50% of the artery). Over a 5.2-year follow-up period, 246 deaths occurred, representing 13.5% of the total study group.
Major adverse cardiovascular event (MACE) data were available for 973 patients. During the follow-up period, 295 (30.3%) of the patients had a MACE, such as heart attack or a coronary revascularization.
The researchers found that both obstructive and mild, or nonobstructive, coronary artery disease, as determined by CCTA, were associated with patient deaths and MACE. Most importantly, the researchers found that the relative risk for death or MACE for a patient with mild coronary artery disease was comparable to that of patients with single vessel obstructive disease.
“Until now, two-year follow-up studies suggested that a diabetic patient with mild or nonobstructive coronary artery disease had a lower risk of major adverse cardiovascular events and death than patients with obstructive disease,” said Philipp Blanke, MD, a radiologist at the University of British Columbia and St. Paul’s Hospital and a coauthor of the study. “Our five-year follow-up data suggest that nonobstructive and obstructive coronary artery disease, as detected by cardiac CTA in diabetic patients, are both associated with higher rates of mortality.”
Researchers need a better understanding of the evolution of plaque in the arteries and of patient response to therapies, said Dr. Leipsic. “Cardiac CT angiography is helpful for identifying diabetic patients who are at higher risk for heart events and who may benefit from more aggressive therapy to help modify that risk,” he added.
Patients Prefer Direct Access to Imaging Records
Patients value direct, independent access to their medical exams, researchers reported.
Giampaolo Greco, PhD, MPH, Assistant Professor in the Department of Population Health Science and Policy at the Mount Sinai School of Medicine in New York City, and colleagues set out to evaluate patient and provider satisfaction with RSNA Image Share, an Internet-based interoperable image exchange system that gives patients ownership of their imaging exams and control over access to their imaging records. The network enables radiology sites to make results of imaging exams available for patients to incorporate in personal health record (PHR) accounts they can use to securely store, manage, and share their imaging records. Sites also can use the network to send patient imaging records to other participating sites to support better informed care.
For the study, patients undergoing radiologic exams at four academic centers were eligible to establish online PHR accounts using the RSNA Image Share network. Patients could then use their PHR accounts to maintain and share their images with selected providers, creating a detailed medical history accessible through any secure Internet connection.
Between July 2012 and August 2013, the study enrolled 2,562 patients, mean age 50.4, including a significant representation of older individuals. Older individuals have the highest healthcare utilization and often experience or perceive a significant barrier in using information technology.
The median number of exams uploaded per patient was six. Study participants were provided a brief survey to assess patient and physician experience with the exchange of images, and 502 patients completed and returned their surveys. Of these respondents, 448 patients identified the method used at the visit to share images: Internet, CDs, both Internet and CDs, or other, and 165 included a section completed by their physician.
Nearly all (96%) of the patients responded positively to having direct access to their medical images, and 78% viewed their images independently. There was no difference between Internet and CD users in satisfaction with privacy and security and timeliness of access to medical images. A greater percentage of Internet users reported being able to access their images without difficulty, compared with CD users (88.3% vs 77.5%).
Xerosis is significant risk during targeted anticancer treatments
Patients receiving targeted anticancer treatments are at a significant risk of developing xerosis, or abnormal dryness, according to Dr. Johannah Valentine and her associates.
In a systematic review and meta-analysis of clinical trials involving 58 targeted agents, nearly 18% of all patients developed xerosis, with 1% of patients developing high-grade xerosis. The incidence may be affected by age, concomitant medications, comorbidities, and underlying malignancies or skin conditions, and reporting may vary among physicians and institutions, the researchers said.
Patients should be counseled and treated early for this symptom to prevent suboptimal dosing and quality-of-life impairment, the investigators recommended.
Read the full article at the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2014.12.010).
Patients receiving targeted anticancer treatments are at a significant risk of developing xerosis, or abnormal dryness, according to Dr. Johannah Valentine and her associates.
In a systematic review and meta-analysis of clinical trials involving 58 targeted agents, nearly 18% of all patients developed xerosis, with 1% of patients developing high-grade xerosis. The incidence may be affected by age, concomitant medications, comorbidities, and underlying malignancies or skin conditions, and reporting may vary among physicians and institutions, the researchers said.
Patients should be counseled and treated early for this symptom to prevent suboptimal dosing and quality-of-life impairment, the investigators recommended.
Read the full article at the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2014.12.010).
Patients receiving targeted anticancer treatments are at a significant risk of developing xerosis, or abnormal dryness, according to Dr. Johannah Valentine and her associates.
In a systematic review and meta-analysis of clinical trials involving 58 targeted agents, nearly 18% of all patients developed xerosis, with 1% of patients developing high-grade xerosis. The incidence may be affected by age, concomitant medications, comorbidities, and underlying malignancies or skin conditions, and reporting may vary among physicians and institutions, the researchers said.
Patients should be counseled and treated early for this symptom to prevent suboptimal dosing and quality-of-life impairment, the investigators recommended.
Read the full article at the Journal of the American Academy of Dermatology (doi:10.1016/j.jaad.2014.12.010).