User login
Daily episodes of confusion • altered behavior • chronic sleep deprivation • Dx?
THE CASE
A 60-year-old man with hypertension, gout, hyperlipidemia, and chronic sleep deprivation was referred to our neurology department for evaluation because he’d recently developed episodes of confusion and altered behavior that occurred daily. According to the patient’s wife, these episodes had started 4 weeks earlier while the patient was driving. He drove off the road while staring ahead with a “Joker-like” smile on his face. He was unable to utter more than a few words or respond to his wife, who was able to safely bring the car to a stop. The patient had spotty memory of this 40-minute episode.
Since then, he’d had similar but shorter episodes each morning, 20 to 75 minutes after taking his prescribed medications (lisinopril, simvastatin, and allopurinol). According to the patient’s wife, during these episodes, the patient would “act childish.” He would develop a voracious appetite and experience double or distorted vision, an unsteady gait, and poor muscle tone. These episodes were always followed by a long nap.
The man denied drinking, head trauma, acute illness, or taking illicit substances or any medications other than lisinopril, simvastatin, and allopurinol. Computed tomography, magnetic resonance imaging/magnetic resonance angiography, carotid Doppler ultrasound, and routine and 24-hour ambulatory electroencephalography (EEG) were normal.
Before the patient was referred to our neurology department, he had been prescribed a short course of the antiepileptic/mood stabilizer valproate and the wakefulness agent armodafinil, but neither medication had helped. The patient’s episodes continued daily, usually 20 to 75 minutes after taking his regular medications. When he decided to take them at night, the episodes began to occur at night.
His neurologic exam was normal. Family history was positive for a cousin with narcolepsy but negative for seizures and obstructive sleep apnea (OSA). Polysomnography revealed moderate OSA with minimal oxygen desaturation. Inpatient video EEG monitoring captured several of the events that the patient and his wife had described; the patient seemed “uninhibited” in his behavior. His EEG, cardiac telemetry, oxygen saturation, blood pressure, and serum glucose level remained normal.
The episodes’ sudden onset, peculiar symptoms, and duration—and the fact that they occurred after he took his usual medications—made complex partial seizures unlikely. The patient’s chronic sleep deprivation and family history of narcolepsy raised the possibility of “sleep attacks,” but the sudden onset and age of onset of his symptoms made those conditions less likely to explain the complete clinical picture. No particular hormonal disturbance could explain his presentation, and blood work was normal.
THE DIAGNOSIS
Because the patient’s episodes had been occurring shortly after the patient took his lisinopril, simvastatin, and allopurinol, and because his blood pressure and lipid levels were normal and his gout was asymptomatic, we decided to stop these medications. Later that day, the patient reported that he had discovered that his vial of lisinopril, which he had obtained from his regular pharmacy the day before his first episode, contained a different medication. He consulted a pharmacist, who determined that the vial contained extended release zolpidem 12.5 mg, and not his antihypertensive.
DISCUSSION
Although the true incidence of medication errors is difficult to determine, a 2006 Institute of Medicine report estimated that there are at least 1.5 million cases of preventable adverse drug events in the United States each year.1 In light of these statistics, medication errors need to be near the top of our differential diagnosis when patients suddenly develop symptoms for which there is no obvious cause.
Cause to pause? If you observe a temporal association between the onset of a patient’s symptoms and medication administration, consider possible adverse effects of the medication before ordering tests.
In this case … Our patient’s peculiar presentation correlated with regular ingestion of a high dose of zolpidem, a short-acting non-benzodiazepine gamma-aminobutyric acid (GABA) agonist. Zolpidem binds to the same GABAA receptor as benzodiazepines and therefore acts as a hypnotic by increasing GABA transmission.2 Neuropsychiatric adverse events associated with zolpidem include hallucinations, amnesia, parasomnia, psychomotor impairment, and complex behaviors (eg, sleepwalking or sleep-driving).2 Higher doses may cause coma or (rarely) death.2 One case report describes a patient who heard command hallucinations and stabbed himself after ingesting a large dose of zolpidem.3
Our patient
The patient’s episodes stopped after he discontinued the zolpidem. He subsequently received a correct prescription for lisinopril, and did not experience any additional episodes.
THE TAKEAWAY
Consider medication errors and adverse drug events in the differential diagnosis for patients who develop symptoms for which there is no obvious etiology. Educate patients, as well, to question their pharmacist if a recently filled prescription doesn’t look like the pill they usually take or makes them feel different than usual when they take it.
Of course, patients should be reminded that a generic medication may not always look the same as a brand-name drug or a previous generic prescription. But it can’t hurt for the patient to ask whether that medication that “looks different” is just a different generic—or a sign of a more worrisome mix-up.
1. Institute of Medicine. Preventing medication errors. Report Brief. July 2006. Institute of Medicine Web site. Available at: http://iom.edu/~/media/Files/Report%20Files/2006/Preventing-Medication-Errors-Quality-Chasm-Series/medicationerrorsnew.pdf. Accessed January 13, 2015.
2. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
3. Manfredi G, Kotzalidis GD, Lazanio S, et al. Command hallucinations with self-stabbing associated with zolpidem overdose. J Clin Psychiatry. 2010;71:92-93.
THE CASE
A 60-year-old man with hypertension, gout, hyperlipidemia, and chronic sleep deprivation was referred to our neurology department for evaluation because he’d recently developed episodes of confusion and altered behavior that occurred daily. According to the patient’s wife, these episodes had started 4 weeks earlier while the patient was driving. He drove off the road while staring ahead with a “Joker-like” smile on his face. He was unable to utter more than a few words or respond to his wife, who was able to safely bring the car to a stop. The patient had spotty memory of this 40-minute episode.
Since then, he’d had similar but shorter episodes each morning, 20 to 75 minutes after taking his prescribed medications (lisinopril, simvastatin, and allopurinol). According to the patient’s wife, during these episodes, the patient would “act childish.” He would develop a voracious appetite and experience double or distorted vision, an unsteady gait, and poor muscle tone. These episodes were always followed by a long nap.
The man denied drinking, head trauma, acute illness, or taking illicit substances or any medications other than lisinopril, simvastatin, and allopurinol. Computed tomography, magnetic resonance imaging/magnetic resonance angiography, carotid Doppler ultrasound, and routine and 24-hour ambulatory electroencephalography (EEG) were normal.
Before the patient was referred to our neurology department, he had been prescribed a short course of the antiepileptic/mood stabilizer valproate and the wakefulness agent armodafinil, but neither medication had helped. The patient’s episodes continued daily, usually 20 to 75 minutes after taking his regular medications. When he decided to take them at night, the episodes began to occur at night.
His neurologic exam was normal. Family history was positive for a cousin with narcolepsy but negative for seizures and obstructive sleep apnea (OSA). Polysomnography revealed moderate OSA with minimal oxygen desaturation. Inpatient video EEG monitoring captured several of the events that the patient and his wife had described; the patient seemed “uninhibited” in his behavior. His EEG, cardiac telemetry, oxygen saturation, blood pressure, and serum glucose level remained normal.
The episodes’ sudden onset, peculiar symptoms, and duration—and the fact that they occurred after he took his usual medications—made complex partial seizures unlikely. The patient’s chronic sleep deprivation and family history of narcolepsy raised the possibility of “sleep attacks,” but the sudden onset and age of onset of his symptoms made those conditions less likely to explain the complete clinical picture. No particular hormonal disturbance could explain his presentation, and blood work was normal.
THE DIAGNOSIS
Because the patient’s episodes had been occurring shortly after the patient took his lisinopril, simvastatin, and allopurinol, and because his blood pressure and lipid levels were normal and his gout was asymptomatic, we decided to stop these medications. Later that day, the patient reported that he had discovered that his vial of lisinopril, which he had obtained from his regular pharmacy the day before his first episode, contained a different medication. He consulted a pharmacist, who determined that the vial contained extended release zolpidem 12.5 mg, and not his antihypertensive.
DISCUSSION
Although the true incidence of medication errors is difficult to determine, a 2006 Institute of Medicine report estimated that there are at least 1.5 million cases of preventable adverse drug events in the United States each year.1 In light of these statistics, medication errors need to be near the top of our differential diagnosis when patients suddenly develop symptoms for which there is no obvious cause.
Cause to pause? If you observe a temporal association between the onset of a patient’s symptoms and medication administration, consider possible adverse effects of the medication before ordering tests.
In this case … Our patient’s peculiar presentation correlated with regular ingestion of a high dose of zolpidem, a short-acting non-benzodiazepine gamma-aminobutyric acid (GABA) agonist. Zolpidem binds to the same GABAA receptor as benzodiazepines and therefore acts as a hypnotic by increasing GABA transmission.2 Neuropsychiatric adverse events associated with zolpidem include hallucinations, amnesia, parasomnia, psychomotor impairment, and complex behaviors (eg, sleepwalking or sleep-driving).2 Higher doses may cause coma or (rarely) death.2 One case report describes a patient who heard command hallucinations and stabbed himself after ingesting a large dose of zolpidem.3
Our patient
The patient’s episodes stopped after he discontinued the zolpidem. He subsequently received a correct prescription for lisinopril, and did not experience any additional episodes.
THE TAKEAWAY
Consider medication errors and adverse drug events in the differential diagnosis for patients who develop symptoms for which there is no obvious etiology. Educate patients, as well, to question their pharmacist if a recently filled prescription doesn’t look like the pill they usually take or makes them feel different than usual when they take it.
Of course, patients should be reminded that a generic medication may not always look the same as a brand-name drug or a previous generic prescription. But it can’t hurt for the patient to ask whether that medication that “looks different” is just a different generic—or a sign of a more worrisome mix-up.
THE CASE
A 60-year-old man with hypertension, gout, hyperlipidemia, and chronic sleep deprivation was referred to our neurology department for evaluation because he’d recently developed episodes of confusion and altered behavior that occurred daily. According to the patient’s wife, these episodes had started 4 weeks earlier while the patient was driving. He drove off the road while staring ahead with a “Joker-like” smile on his face. He was unable to utter more than a few words or respond to his wife, who was able to safely bring the car to a stop. The patient had spotty memory of this 40-minute episode.
Since then, he’d had similar but shorter episodes each morning, 20 to 75 minutes after taking his prescribed medications (lisinopril, simvastatin, and allopurinol). According to the patient’s wife, during these episodes, the patient would “act childish.” He would develop a voracious appetite and experience double or distorted vision, an unsteady gait, and poor muscle tone. These episodes were always followed by a long nap.
The man denied drinking, head trauma, acute illness, or taking illicit substances or any medications other than lisinopril, simvastatin, and allopurinol. Computed tomography, magnetic resonance imaging/magnetic resonance angiography, carotid Doppler ultrasound, and routine and 24-hour ambulatory electroencephalography (EEG) were normal.
Before the patient was referred to our neurology department, he had been prescribed a short course of the antiepileptic/mood stabilizer valproate and the wakefulness agent armodafinil, but neither medication had helped. The patient’s episodes continued daily, usually 20 to 75 minutes after taking his regular medications. When he decided to take them at night, the episodes began to occur at night.
His neurologic exam was normal. Family history was positive for a cousin with narcolepsy but negative for seizures and obstructive sleep apnea (OSA). Polysomnography revealed moderate OSA with minimal oxygen desaturation. Inpatient video EEG monitoring captured several of the events that the patient and his wife had described; the patient seemed “uninhibited” in his behavior. His EEG, cardiac telemetry, oxygen saturation, blood pressure, and serum glucose level remained normal.
The episodes’ sudden onset, peculiar symptoms, and duration—and the fact that they occurred after he took his usual medications—made complex partial seizures unlikely. The patient’s chronic sleep deprivation and family history of narcolepsy raised the possibility of “sleep attacks,” but the sudden onset and age of onset of his symptoms made those conditions less likely to explain the complete clinical picture. No particular hormonal disturbance could explain his presentation, and blood work was normal.
THE DIAGNOSIS
Because the patient’s episodes had been occurring shortly after the patient took his lisinopril, simvastatin, and allopurinol, and because his blood pressure and lipid levels were normal and his gout was asymptomatic, we decided to stop these medications. Later that day, the patient reported that he had discovered that his vial of lisinopril, which he had obtained from his regular pharmacy the day before his first episode, contained a different medication. He consulted a pharmacist, who determined that the vial contained extended release zolpidem 12.5 mg, and not his antihypertensive.
DISCUSSION
Although the true incidence of medication errors is difficult to determine, a 2006 Institute of Medicine report estimated that there are at least 1.5 million cases of preventable adverse drug events in the United States each year.1 In light of these statistics, medication errors need to be near the top of our differential diagnosis when patients suddenly develop symptoms for which there is no obvious cause.
Cause to pause? If you observe a temporal association between the onset of a patient’s symptoms and medication administration, consider possible adverse effects of the medication before ordering tests.
In this case … Our patient’s peculiar presentation correlated with regular ingestion of a high dose of zolpidem, a short-acting non-benzodiazepine gamma-aminobutyric acid (GABA) agonist. Zolpidem binds to the same GABAA receptor as benzodiazepines and therefore acts as a hypnotic by increasing GABA transmission.2 Neuropsychiatric adverse events associated with zolpidem include hallucinations, amnesia, parasomnia, psychomotor impairment, and complex behaviors (eg, sleepwalking or sleep-driving).2 Higher doses may cause coma or (rarely) death.2 One case report describes a patient who heard command hallucinations and stabbed himself after ingesting a large dose of zolpidem.3
Our patient
The patient’s episodes stopped after he discontinued the zolpidem. He subsequently received a correct prescription for lisinopril, and did not experience any additional episodes.
THE TAKEAWAY
Consider medication errors and adverse drug events in the differential diagnosis for patients who develop symptoms for which there is no obvious etiology. Educate patients, as well, to question their pharmacist if a recently filled prescription doesn’t look like the pill they usually take or makes them feel different than usual when they take it.
Of course, patients should be reminded that a generic medication may not always look the same as a brand-name drug or a previous generic prescription. But it can’t hurt for the patient to ask whether that medication that “looks different” is just a different generic—or a sign of a more worrisome mix-up.
1. Institute of Medicine. Preventing medication errors. Report Brief. July 2006. Institute of Medicine Web site. Available at: http://iom.edu/~/media/Files/Report%20Files/2006/Preventing-Medication-Errors-Quality-Chasm-Series/medicationerrorsnew.pdf. Accessed January 13, 2015.
2. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
3. Manfredi G, Kotzalidis GD, Lazanio S, et al. Command hallucinations with self-stabbing associated with zolpidem overdose. J Clin Psychiatry. 2010;71:92-93.
1. Institute of Medicine. Preventing medication errors. Report Brief. July 2006. Institute of Medicine Web site. Available at: http://iom.edu/~/media/Files/Report%20Files/2006/Preventing-Medication-Errors-Quality-Chasm-Series/medicationerrorsnew.pdf. Accessed January 13, 2015.
2. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
3. Manfredi G, Kotzalidis GD, Lazanio S, et al. Command hallucinations with self-stabbing associated with zolpidem overdose. J Clin Psychiatry. 2010;71:92-93.
Is red-yeast rice a safe and effective alternative to statins?
Yes, but perhaps not the red-yeast rice extracts available in the United States.
In patients with known coronary artery disease and dyslipidemia (secondary prevention), therapy with red-yeast rice extract containing naturally-occurring lovastatin is associated with a 30% reduction in coronary heart disease (CHD) mortality and a 60% reduction in myocardial infarction (MI), similar to the effect of statin medications (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] in China).
In patients older than 65 years with hypertension and a previous MI, the rate of adverse effects from lovastatin-containing red-yeast rice is 2.1% (SOR: B, RCT in China).
In patients with previous statin intolerance, the rates of myalgias and treatment discontinuation with lovastatin-containing red-yeast rice therapy are similar to either placebo or another statin (SOR: C, low-powered RCTs).
The US Food and Drug Administration (FDA) doesn’t allow lovastatin-containing red-yeast rice products on the US market; physicians should be aware that products purchased by patients online contain variable amounts of lovastatin.
EVIDENCE SUMMARY
Red-yeast rice is a Chinese dietary and medicinal product of yeast (Monascus purpureus) grown on rice. It contains a wide range of biologically active compounds, including lovastatin (monacolin K). The FDA has banned the sale of red-yeast rice products with more than trace amounts of lovastatin.1
Red-yeast rice beats placebo, similar to statins
A systematic review of 22 RCTs (N=6520), primarily conducted in China using 600 to 2400 mg red-yeast rice extract daily (lovastatin content 5-20 mg), assessed outcomes in patients with known CHD and dyslipidemia.2 In one trial of 4870 patients, users of red-yeast rice had significant reductions in CHD mortality (relative risk [RR]=0.69; 95% confidence interval [CI], 0.54-0.89), incidence of MI (RR=0.39; 95% CI, 0.28-0.55), and revascularization (RR=0.67; 95% CI, 0.50-0.89) compared with placebo users.
However, when compared with statin therapy, red-yeast rice didn’t yield statistically significant differences in CHD mortality (2 trials, N=220; RR=0.26; 95% CI, 0.06-1.21), incidence of MI (1 trial, N=84; RR=0.95; 95% CI, 0.30-3.05) or revascularization (1 trial, N=84; RR=1.14; 95% CI, 0.38-3.46).
Red-yeast rice outperforms placebo in CHD and MI—but not stroke
A secondary analysis of an RCT evaluated the impact of red-yeast rice extract (600 mg twice a day) for 4.5 years on cardiovascular events and mortality in 1530 Chinese patients 60 years of age and older with hypertension and a previous MI.3 The lovastatin content of the red-yeast rice was 5 to 6.4 mg/d.
Compared with placebo, red-yeast rice was associated with a lower incidence of CHD events (RR=0.63; 95% CI, 0.36-0.83), nonfatal MI (RR=0.48; 95% CI, 0.37-0.71), and all-cause mortality (RR=0.65; 95% CI, 0.49-0.83) but not with a statistically significant difference in stroke (RR=0.63; 95% CI, 0.47-1.09) or cardiac revascularization (RR=0.68; 95% CI, 0.52-1.19).
Total adverse events in this study were similar for red-yeast rice and placebo (2.1% vs 1.2%, respectively; P>.05). They included gastrointestinal discomfort, allergic reactions, myalgias, edema, erectile dysfunction, and neuropsychological symptoms.
Red-yeast rice is similar to placebo or another statin in statin-induced myalgia
In a small community-based trial of 62 adults with dyslipidemia and a history of statin-induced myalgia, investigators randomized patients to receive either red-yeast rice extract at 1800 mg (with 3.1 mg lovastatin) or placebo twice daily for 24 weeks.4 Patients’ weekly self-reports of pain (on a 10-point scale) were skewed at baseline (1.4 in the red-yeast rice group vs 2.6 in the placebo group; P=.026) but similar at 12 weeks (1.4 with red-yeast rice vs 1.9 with placebo; P=.30) and 24 weeks (1.2 with red-yeast rice vs 2.0 with placebo; P=.120).
An RCT of 43 adults with dyslipidemia and history of statin intolerance compared red-yeast rice extract (2400 mg, with 10 mg lovastatin) with pravastatin (20 mg) dosed twice a day.5 At the end of 12 weeks, mean self-reported pain scores (on a 10-point scale) were similar (1.4 with red-yeast rice vs 1.1 with pravastatin; P=.82), as were discontinuation rates because of myalgia (5% with red-yeast rice vs 9% with pravastatin; P=.99).
RECOMMENDATIONS
A narrative review of alternative therapies for heart failure and hypercholesterolemia states that red yeast rice may be a cost-saving option for hypercholesterolemia in patients who can’t afford other medications (purchased mostly online, cost $8-$20/month for a dosage equivalent to lovastatin 20 mg/d).6
A ConsumerLab review of red yeast rice products available since the FDA ban in 2011 tested products marketed in the United States and found variable amounts of lovastatin.1,7 The group determined that labeling was a poor guide to lovastatin content, which ranged from 0 to 20 mg per daily dose, and that the products may not have been standardized. The group concluded that therapeutic effects weren’t predictable.
1. National Institutes of Health. Red yeast rice: An introduction. National Center for Complementary and Integrative Health Web site. Available at: http://nccam.nih.gov/health/redyeastrice. Accessed October 28, 2013.
2. Shang Q, Liu Z, Chen K, et al. A systematic review of xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid Based Complement Alternat Med. 2012;2012:636547.
3. Li JJ, Lu ZL, Kou WR, et al. Beneficial impact of xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J Clin Pharmacol. 2009;49:947-956.
4. Becker DJ, Gordon RY, Halbert SC, et al. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150:830-839,
W147-W149.
5. Halbert SC, French B, Gordon RY, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105:198-204.
6. Morelli V, Zoorob RJ. Alternative therapies: Part II. Congestive heart failure and hypercholesterolemia. Am Fam Physician. 2000;62:1325-1330.
7. Consumerlab.com. Product Review: Red yeast rice supplements review. ConsumerLab Web site. Available at: https://www.consumerlab.com/reviews/Red-Yeast-Rice-Supplements-Review/Red_Yeast_Rice. Accessed January 20, 2015.
Yes, but perhaps not the red-yeast rice extracts available in the United States.
In patients with known coronary artery disease and dyslipidemia (secondary prevention), therapy with red-yeast rice extract containing naturally-occurring lovastatin is associated with a 30% reduction in coronary heart disease (CHD) mortality and a 60% reduction in myocardial infarction (MI), similar to the effect of statin medications (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] in China).
In patients older than 65 years with hypertension and a previous MI, the rate of adverse effects from lovastatin-containing red-yeast rice is 2.1% (SOR: B, RCT in China).
In patients with previous statin intolerance, the rates of myalgias and treatment discontinuation with lovastatin-containing red-yeast rice therapy are similar to either placebo or another statin (SOR: C, low-powered RCTs).
The US Food and Drug Administration (FDA) doesn’t allow lovastatin-containing red-yeast rice products on the US market; physicians should be aware that products purchased by patients online contain variable amounts of lovastatin.
EVIDENCE SUMMARY
Red-yeast rice is a Chinese dietary and medicinal product of yeast (Monascus purpureus) grown on rice. It contains a wide range of biologically active compounds, including lovastatin (monacolin K). The FDA has banned the sale of red-yeast rice products with more than trace amounts of lovastatin.1
Red-yeast rice beats placebo, similar to statins
A systematic review of 22 RCTs (N=6520), primarily conducted in China using 600 to 2400 mg red-yeast rice extract daily (lovastatin content 5-20 mg), assessed outcomes in patients with known CHD and dyslipidemia.2 In one trial of 4870 patients, users of red-yeast rice had significant reductions in CHD mortality (relative risk [RR]=0.69; 95% confidence interval [CI], 0.54-0.89), incidence of MI (RR=0.39; 95% CI, 0.28-0.55), and revascularization (RR=0.67; 95% CI, 0.50-0.89) compared with placebo users.
However, when compared with statin therapy, red-yeast rice didn’t yield statistically significant differences in CHD mortality (2 trials, N=220; RR=0.26; 95% CI, 0.06-1.21), incidence of MI (1 trial, N=84; RR=0.95; 95% CI, 0.30-3.05) or revascularization (1 trial, N=84; RR=1.14; 95% CI, 0.38-3.46).
Red-yeast rice outperforms placebo in CHD and MI—but not stroke
A secondary analysis of an RCT evaluated the impact of red-yeast rice extract (600 mg twice a day) for 4.5 years on cardiovascular events and mortality in 1530 Chinese patients 60 years of age and older with hypertension and a previous MI.3 The lovastatin content of the red-yeast rice was 5 to 6.4 mg/d.
Compared with placebo, red-yeast rice was associated with a lower incidence of CHD events (RR=0.63; 95% CI, 0.36-0.83), nonfatal MI (RR=0.48; 95% CI, 0.37-0.71), and all-cause mortality (RR=0.65; 95% CI, 0.49-0.83) but not with a statistically significant difference in stroke (RR=0.63; 95% CI, 0.47-1.09) or cardiac revascularization (RR=0.68; 95% CI, 0.52-1.19).
Total adverse events in this study were similar for red-yeast rice and placebo (2.1% vs 1.2%, respectively; P>.05). They included gastrointestinal discomfort, allergic reactions, myalgias, edema, erectile dysfunction, and neuropsychological symptoms.
Red-yeast rice is similar to placebo or another statin in statin-induced myalgia
In a small community-based trial of 62 adults with dyslipidemia and a history of statin-induced myalgia, investigators randomized patients to receive either red-yeast rice extract at 1800 mg (with 3.1 mg lovastatin) or placebo twice daily for 24 weeks.4 Patients’ weekly self-reports of pain (on a 10-point scale) were skewed at baseline (1.4 in the red-yeast rice group vs 2.6 in the placebo group; P=.026) but similar at 12 weeks (1.4 with red-yeast rice vs 1.9 with placebo; P=.30) and 24 weeks (1.2 with red-yeast rice vs 2.0 with placebo; P=.120).
An RCT of 43 adults with dyslipidemia and history of statin intolerance compared red-yeast rice extract (2400 mg, with 10 mg lovastatin) with pravastatin (20 mg) dosed twice a day.5 At the end of 12 weeks, mean self-reported pain scores (on a 10-point scale) were similar (1.4 with red-yeast rice vs 1.1 with pravastatin; P=.82), as were discontinuation rates because of myalgia (5% with red-yeast rice vs 9% with pravastatin; P=.99).
RECOMMENDATIONS
A narrative review of alternative therapies for heart failure and hypercholesterolemia states that red yeast rice may be a cost-saving option for hypercholesterolemia in patients who can’t afford other medications (purchased mostly online, cost $8-$20/month for a dosage equivalent to lovastatin 20 mg/d).6
A ConsumerLab review of red yeast rice products available since the FDA ban in 2011 tested products marketed in the United States and found variable amounts of lovastatin.1,7 The group determined that labeling was a poor guide to lovastatin content, which ranged from 0 to 20 mg per daily dose, and that the products may not have been standardized. The group concluded that therapeutic effects weren’t predictable.
Yes, but perhaps not the red-yeast rice extracts available in the United States.
In patients with known coronary artery disease and dyslipidemia (secondary prevention), therapy with red-yeast rice extract containing naturally-occurring lovastatin is associated with a 30% reduction in coronary heart disease (CHD) mortality and a 60% reduction in myocardial infarction (MI), similar to the effect of statin medications (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] in China).
In patients older than 65 years with hypertension and a previous MI, the rate of adverse effects from lovastatin-containing red-yeast rice is 2.1% (SOR: B, RCT in China).
In patients with previous statin intolerance, the rates of myalgias and treatment discontinuation with lovastatin-containing red-yeast rice therapy are similar to either placebo or another statin (SOR: C, low-powered RCTs).
The US Food and Drug Administration (FDA) doesn’t allow lovastatin-containing red-yeast rice products on the US market; physicians should be aware that products purchased by patients online contain variable amounts of lovastatin.
EVIDENCE SUMMARY
Red-yeast rice is a Chinese dietary and medicinal product of yeast (Monascus purpureus) grown on rice. It contains a wide range of biologically active compounds, including lovastatin (monacolin K). The FDA has banned the sale of red-yeast rice products with more than trace amounts of lovastatin.1
Red-yeast rice beats placebo, similar to statins
A systematic review of 22 RCTs (N=6520), primarily conducted in China using 600 to 2400 mg red-yeast rice extract daily (lovastatin content 5-20 mg), assessed outcomes in patients with known CHD and dyslipidemia.2 In one trial of 4870 patients, users of red-yeast rice had significant reductions in CHD mortality (relative risk [RR]=0.69; 95% confidence interval [CI], 0.54-0.89), incidence of MI (RR=0.39; 95% CI, 0.28-0.55), and revascularization (RR=0.67; 95% CI, 0.50-0.89) compared with placebo users.
However, when compared with statin therapy, red-yeast rice didn’t yield statistically significant differences in CHD mortality (2 trials, N=220; RR=0.26; 95% CI, 0.06-1.21), incidence of MI (1 trial, N=84; RR=0.95; 95% CI, 0.30-3.05) or revascularization (1 trial, N=84; RR=1.14; 95% CI, 0.38-3.46).
Red-yeast rice outperforms placebo in CHD and MI—but not stroke
A secondary analysis of an RCT evaluated the impact of red-yeast rice extract (600 mg twice a day) for 4.5 years on cardiovascular events and mortality in 1530 Chinese patients 60 years of age and older with hypertension and a previous MI.3 The lovastatin content of the red-yeast rice was 5 to 6.4 mg/d.
Compared with placebo, red-yeast rice was associated with a lower incidence of CHD events (RR=0.63; 95% CI, 0.36-0.83), nonfatal MI (RR=0.48; 95% CI, 0.37-0.71), and all-cause mortality (RR=0.65; 95% CI, 0.49-0.83) but not with a statistically significant difference in stroke (RR=0.63; 95% CI, 0.47-1.09) or cardiac revascularization (RR=0.68; 95% CI, 0.52-1.19).
Total adverse events in this study were similar for red-yeast rice and placebo (2.1% vs 1.2%, respectively; P>.05). They included gastrointestinal discomfort, allergic reactions, myalgias, edema, erectile dysfunction, and neuropsychological symptoms.
Red-yeast rice is similar to placebo or another statin in statin-induced myalgia
In a small community-based trial of 62 adults with dyslipidemia and a history of statin-induced myalgia, investigators randomized patients to receive either red-yeast rice extract at 1800 mg (with 3.1 mg lovastatin) or placebo twice daily for 24 weeks.4 Patients’ weekly self-reports of pain (on a 10-point scale) were skewed at baseline (1.4 in the red-yeast rice group vs 2.6 in the placebo group; P=.026) but similar at 12 weeks (1.4 with red-yeast rice vs 1.9 with placebo; P=.30) and 24 weeks (1.2 with red-yeast rice vs 2.0 with placebo; P=.120).
An RCT of 43 adults with dyslipidemia and history of statin intolerance compared red-yeast rice extract (2400 mg, with 10 mg lovastatin) with pravastatin (20 mg) dosed twice a day.5 At the end of 12 weeks, mean self-reported pain scores (on a 10-point scale) were similar (1.4 with red-yeast rice vs 1.1 with pravastatin; P=.82), as were discontinuation rates because of myalgia (5% with red-yeast rice vs 9% with pravastatin; P=.99).
RECOMMENDATIONS
A narrative review of alternative therapies for heart failure and hypercholesterolemia states that red yeast rice may be a cost-saving option for hypercholesterolemia in patients who can’t afford other medications (purchased mostly online, cost $8-$20/month for a dosage equivalent to lovastatin 20 mg/d).6
A ConsumerLab review of red yeast rice products available since the FDA ban in 2011 tested products marketed in the United States and found variable amounts of lovastatin.1,7 The group determined that labeling was a poor guide to lovastatin content, which ranged from 0 to 20 mg per daily dose, and that the products may not have been standardized. The group concluded that therapeutic effects weren’t predictable.
1. National Institutes of Health. Red yeast rice: An introduction. National Center for Complementary and Integrative Health Web site. Available at: http://nccam.nih.gov/health/redyeastrice. Accessed October 28, 2013.
2. Shang Q, Liu Z, Chen K, et al. A systematic review of xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid Based Complement Alternat Med. 2012;2012:636547.
3. Li JJ, Lu ZL, Kou WR, et al. Beneficial impact of xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J Clin Pharmacol. 2009;49:947-956.
4. Becker DJ, Gordon RY, Halbert SC, et al. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150:830-839,
W147-W149.
5. Halbert SC, French B, Gordon RY, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105:198-204.
6. Morelli V, Zoorob RJ. Alternative therapies: Part II. Congestive heart failure and hypercholesterolemia. Am Fam Physician. 2000;62:1325-1330.
7. Consumerlab.com. Product Review: Red yeast rice supplements review. ConsumerLab Web site. Available at: https://www.consumerlab.com/reviews/Red-Yeast-Rice-Supplements-Review/Red_Yeast_Rice. Accessed January 20, 2015.
1. National Institutes of Health. Red yeast rice: An introduction. National Center for Complementary and Integrative Health Web site. Available at: http://nccam.nih.gov/health/redyeastrice. Accessed October 28, 2013.
2. Shang Q, Liu Z, Chen K, et al. A systematic review of xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid Based Complement Alternat Med. 2012;2012:636547.
3. Li JJ, Lu ZL, Kou WR, et al. Beneficial impact of xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J Clin Pharmacol. 2009;49:947-956.
4. Becker DJ, Gordon RY, Halbert SC, et al. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150:830-839,
W147-W149.
5. Halbert SC, French B, Gordon RY, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol. 2010;105:198-204.
6. Morelli V, Zoorob RJ. Alternative therapies: Part II. Congestive heart failure and hypercholesterolemia. Am Fam Physician. 2000;62:1325-1330.
7. Consumerlab.com. Product Review: Red yeast rice supplements review. ConsumerLab Web site. Available at: https://www.consumerlab.com/reviews/Red-Yeast-Rice-Supplements-Review/Red_Yeast_Rice. Accessed January 20, 2015.
Evidence-based answers from the Family Physicians Inquiries Network
Does frenotomy help infants with tongue-tie overcome breastfeeding difficulties?
Probably not. No evidence exists for improved latching after frenotomy, and evidence concerning improvements in maternal comfort is conflicting. At best, frenotomy improves maternal nipple pain by 10% and maternal subjective sense of improvement over the short term (0 to 2 weeks) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results for maternal nipple pain and overall feeding).
No studies have evaluated outcomes such as infant weight gain following frenotomy.
Experts don’t recommend frenotomy unless a clear association exists between ankyloglossia (tongue-tie) and breastfeeding problems. Frenotomy should be performed with anesthesia by an experienced clinician to minimize the risk of complications (SOR: C, a practice guideline.)
EVIDENCE SUMMARY
Two RCTs found short-term (0-14 days) improvement in breastfeeding after frenotomy. One, which evaluated the effect of frenotomy on infants with significant ankyloglossia and breastfeeding difficulties, found short-term improvement in maternal nipple pain. Investigators randomized 58 infants (mean age 6 days) with ankyloglossia (rated 8 out of 10 on a standardized severity scale) to receive either frenotomy or no intervention.1 They used the 50-point Short Form McGill Pain Questionnaire to measure maternal nipple pain at baseline, immediately after, and at 2, 4, 8, and 52 weeks.
Mothers in the intervention group reported a 10% greater reduction in nipple pain after frenotomy compared with the control group (11 points vs 6 points; P=.001). The improvement persisted at 2 weeks (graphic representation in study, P value not supplied) but not at 4 weeks or beyond.
An earlier, unblinded RCT randomized 40 infants (mean age 14 days) with ankyloglossia and breastfeeding problems to frenotomy or lactation support.2 It found maternal subjective ratings of “improvement” (not quantified) by telephone interview at 24 hours (85% vs 3%; P<.01). Investigators performed frenotomy on all 19 of the unimproved control infants at 48 hours.
Frenotomy doesn’t improve breastfeeding overall
Two newer RCTs evaluating frenotomy and LATCH (Latch, Audible swallowing, nipple Type, Comfort, and Hold) scores, which include a component measuring maternal comfort, found no breastfeeding improvements. (LATCH is a validated 10-point score with moderate predictive value for identifying mothers at risk for early weaning because of sore nipples.3)
A double-blind RCT that assessed frenotomy in 57 infants (mean age 32 days) with ankyloglossia and breastfeeding problems (severity of both unspecified) found no improvement in breastfeeding overall or nipple pain.4 Investigators randomized infants to frenotomy or sham frenotomy and used independent observers to measure outcomes with the LATCH score and the Infant Breastfeeding Assessment Tool (IBFAT), a standardized method of assessing overall feeding.
They observed no significant differences in LATCH or IBFAT scores between groups. More mothers in the frenotomy group reported improved breastfeeding, but most were able to determine whether their baby had undergone frenotomy.
A single-blinded, RCT of early frenotomy in 107 younger infants with breastfeeding difficulties and mild to moderate ankyloglossia also found no improvement in LATCH scores.5 Researchers randomized infants younger than 2 weeks (blinded to researchers and unblinded to mothers) to either immediate frenotomy or standard care. They measured LATCH scores at baseline and after 5 days (by intention to treat).
Investigators found no difference in LATCH scores at 5 days postfrenotomy (pretreatment score 6.4 ± 2.3, posttreatment 6.8 ± 2.0; not significant).
RECOMMENDATIONS
A 2011 position statement from the Community Paediatrics Committee of the Canadian Paediatric Society notes that ankyloglossia is a relatively uncommon congenital anomaly, and associations between ankyloglossia and breastfeeding problems in infants have been inconsistent.6 For these reasons, the Committee doesn’t recommend frenotomy.
However, if the clinician deems surgical intervention necessary based on a clear association between significant tongue-tie and major breastfeeding problems, then frenotomy should be performed by a clinician experienced in the procedure and with appropriate analgesia. The Committee states that although ankyloglossia release appears to be a minor procedure, it may cause complications such as bleeding, infection, or injury to the Wharton’s duct.
The Academy of Breastfeeding Medicine, a worldwide organization of physicians dedicated to the promotion, protection, and support of breastfeeding and human lactation, is currently revising its guidelines on neonatal ankyloglossia.7
1. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
2. Hogan M, Westcott C, Griffiths M. Randomized, controlled trial of division of tongue-tie in infants with feeding problems. Paediatr Child Health. 2005;41:246-250.
3. Emond A, Ingram J, Johnson D, et al. Randomised controlled trial of early frenotomy in breastfed infants with mild-moderate tongue-tie. Arch Dis Child Fetal Neonatal Ed. 2014;99:F189-F195.
4. Berry J, Griffiths M, Westcott C. A double-blind, randomized, controlled trial of tongue-tie division and its immediate effect on breastfeeding. Breastfeed Med. 2012;7:189-193.
5. Riordan J, Bibb D, Miller M, et al. Predicting breastfeeding duration using the LATCH breastfeeding assessment tool. J Hum Lact. 2001;17:20-23.
6. Rowan-Legg A. Ankyloglossia and breastfeeding. Paediatr Child Health. 2011;16:222.
7. Academy of Breastfeeding Medicine. Statements. Academy of Breastfeeding Medicine Web site. Available at: www.bfmed.org/Resources/Protocols.aspx. Accessed January 10, 2015.
Probably not. No evidence exists for improved latching after frenotomy, and evidence concerning improvements in maternal comfort is conflicting. At best, frenotomy improves maternal nipple pain by 10% and maternal subjective sense of improvement over the short term (0 to 2 weeks) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results for maternal nipple pain and overall feeding).
No studies have evaluated outcomes such as infant weight gain following frenotomy.
Experts don’t recommend frenotomy unless a clear association exists between ankyloglossia (tongue-tie) and breastfeeding problems. Frenotomy should be performed with anesthesia by an experienced clinician to minimize the risk of complications (SOR: C, a practice guideline.)
EVIDENCE SUMMARY
Two RCTs found short-term (0-14 days) improvement in breastfeeding after frenotomy. One, which evaluated the effect of frenotomy on infants with significant ankyloglossia and breastfeeding difficulties, found short-term improvement in maternal nipple pain. Investigators randomized 58 infants (mean age 6 days) with ankyloglossia (rated 8 out of 10 on a standardized severity scale) to receive either frenotomy or no intervention.1 They used the 50-point Short Form McGill Pain Questionnaire to measure maternal nipple pain at baseline, immediately after, and at 2, 4, 8, and 52 weeks.
Mothers in the intervention group reported a 10% greater reduction in nipple pain after frenotomy compared with the control group (11 points vs 6 points; P=.001). The improvement persisted at 2 weeks (graphic representation in study, P value not supplied) but not at 4 weeks or beyond.
An earlier, unblinded RCT randomized 40 infants (mean age 14 days) with ankyloglossia and breastfeeding problems to frenotomy or lactation support.2 It found maternal subjective ratings of “improvement” (not quantified) by telephone interview at 24 hours (85% vs 3%; P<.01). Investigators performed frenotomy on all 19 of the unimproved control infants at 48 hours.
Frenotomy doesn’t improve breastfeeding overall
Two newer RCTs evaluating frenotomy and LATCH (Latch, Audible swallowing, nipple Type, Comfort, and Hold) scores, which include a component measuring maternal comfort, found no breastfeeding improvements. (LATCH is a validated 10-point score with moderate predictive value for identifying mothers at risk for early weaning because of sore nipples.3)
A double-blind RCT that assessed frenotomy in 57 infants (mean age 32 days) with ankyloglossia and breastfeeding problems (severity of both unspecified) found no improvement in breastfeeding overall or nipple pain.4 Investigators randomized infants to frenotomy or sham frenotomy and used independent observers to measure outcomes with the LATCH score and the Infant Breastfeeding Assessment Tool (IBFAT), a standardized method of assessing overall feeding.
They observed no significant differences in LATCH or IBFAT scores between groups. More mothers in the frenotomy group reported improved breastfeeding, but most were able to determine whether their baby had undergone frenotomy.
A single-blinded, RCT of early frenotomy in 107 younger infants with breastfeeding difficulties and mild to moderate ankyloglossia also found no improvement in LATCH scores.5 Researchers randomized infants younger than 2 weeks (blinded to researchers and unblinded to mothers) to either immediate frenotomy or standard care. They measured LATCH scores at baseline and after 5 days (by intention to treat).
Investigators found no difference in LATCH scores at 5 days postfrenotomy (pretreatment score 6.4 ± 2.3, posttreatment 6.8 ± 2.0; not significant).
RECOMMENDATIONS
A 2011 position statement from the Community Paediatrics Committee of the Canadian Paediatric Society notes that ankyloglossia is a relatively uncommon congenital anomaly, and associations between ankyloglossia and breastfeeding problems in infants have been inconsistent.6 For these reasons, the Committee doesn’t recommend frenotomy.
However, if the clinician deems surgical intervention necessary based on a clear association between significant tongue-tie and major breastfeeding problems, then frenotomy should be performed by a clinician experienced in the procedure and with appropriate analgesia. The Committee states that although ankyloglossia release appears to be a minor procedure, it may cause complications such as bleeding, infection, or injury to the Wharton’s duct.
The Academy of Breastfeeding Medicine, a worldwide organization of physicians dedicated to the promotion, protection, and support of breastfeeding and human lactation, is currently revising its guidelines on neonatal ankyloglossia.7
Probably not. No evidence exists for improved latching after frenotomy, and evidence concerning improvements in maternal comfort is conflicting. At best, frenotomy improves maternal nipple pain by 10% and maternal subjective sense of improvement over the short term (0 to 2 weeks) (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with conflicting results for maternal nipple pain and overall feeding).
No studies have evaluated outcomes such as infant weight gain following frenotomy.
Experts don’t recommend frenotomy unless a clear association exists between ankyloglossia (tongue-tie) and breastfeeding problems. Frenotomy should be performed with anesthesia by an experienced clinician to minimize the risk of complications (SOR: C, a practice guideline.)
EVIDENCE SUMMARY
Two RCTs found short-term (0-14 days) improvement in breastfeeding after frenotomy. One, which evaluated the effect of frenotomy on infants with significant ankyloglossia and breastfeeding difficulties, found short-term improvement in maternal nipple pain. Investigators randomized 58 infants (mean age 6 days) with ankyloglossia (rated 8 out of 10 on a standardized severity scale) to receive either frenotomy or no intervention.1 They used the 50-point Short Form McGill Pain Questionnaire to measure maternal nipple pain at baseline, immediately after, and at 2, 4, 8, and 52 weeks.
Mothers in the intervention group reported a 10% greater reduction in nipple pain after frenotomy compared with the control group (11 points vs 6 points; P=.001). The improvement persisted at 2 weeks (graphic representation in study, P value not supplied) but not at 4 weeks or beyond.
An earlier, unblinded RCT randomized 40 infants (mean age 14 days) with ankyloglossia and breastfeeding problems to frenotomy or lactation support.2 It found maternal subjective ratings of “improvement” (not quantified) by telephone interview at 24 hours (85% vs 3%; P<.01). Investigators performed frenotomy on all 19 of the unimproved control infants at 48 hours.
Frenotomy doesn’t improve breastfeeding overall
Two newer RCTs evaluating frenotomy and LATCH (Latch, Audible swallowing, nipple Type, Comfort, and Hold) scores, which include a component measuring maternal comfort, found no breastfeeding improvements. (LATCH is a validated 10-point score with moderate predictive value for identifying mothers at risk for early weaning because of sore nipples.3)
A double-blind RCT that assessed frenotomy in 57 infants (mean age 32 days) with ankyloglossia and breastfeeding problems (severity of both unspecified) found no improvement in breastfeeding overall or nipple pain.4 Investigators randomized infants to frenotomy or sham frenotomy and used independent observers to measure outcomes with the LATCH score and the Infant Breastfeeding Assessment Tool (IBFAT), a standardized method of assessing overall feeding.
They observed no significant differences in LATCH or IBFAT scores between groups. More mothers in the frenotomy group reported improved breastfeeding, but most were able to determine whether their baby had undergone frenotomy.
A single-blinded, RCT of early frenotomy in 107 younger infants with breastfeeding difficulties and mild to moderate ankyloglossia also found no improvement in LATCH scores.5 Researchers randomized infants younger than 2 weeks (blinded to researchers and unblinded to mothers) to either immediate frenotomy or standard care. They measured LATCH scores at baseline and after 5 days (by intention to treat).
Investigators found no difference in LATCH scores at 5 days postfrenotomy (pretreatment score 6.4 ± 2.3, posttreatment 6.8 ± 2.0; not significant).
RECOMMENDATIONS
A 2011 position statement from the Community Paediatrics Committee of the Canadian Paediatric Society notes that ankyloglossia is a relatively uncommon congenital anomaly, and associations between ankyloglossia and breastfeeding problems in infants have been inconsistent.6 For these reasons, the Committee doesn’t recommend frenotomy.
However, if the clinician deems surgical intervention necessary based on a clear association between significant tongue-tie and major breastfeeding problems, then frenotomy should be performed by a clinician experienced in the procedure and with appropriate analgesia. The Committee states that although ankyloglossia release appears to be a minor procedure, it may cause complications such as bleeding, infection, or injury to the Wharton’s duct.
The Academy of Breastfeeding Medicine, a worldwide organization of physicians dedicated to the promotion, protection, and support of breastfeeding and human lactation, is currently revising its guidelines on neonatal ankyloglossia.7
1. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
2. Hogan M, Westcott C, Griffiths M. Randomized, controlled trial of division of tongue-tie in infants with feeding problems. Paediatr Child Health. 2005;41:246-250.
3. Emond A, Ingram J, Johnson D, et al. Randomised controlled trial of early frenotomy in breastfed infants with mild-moderate tongue-tie. Arch Dis Child Fetal Neonatal Ed. 2014;99:F189-F195.
4. Berry J, Griffiths M, Westcott C. A double-blind, randomized, controlled trial of tongue-tie division and its immediate effect on breastfeeding. Breastfeed Med. 2012;7:189-193.
5. Riordan J, Bibb D, Miller M, et al. Predicting breastfeeding duration using the LATCH breastfeeding assessment tool. J Hum Lact. 2001;17:20-23.
6. Rowan-Legg A. Ankyloglossia and breastfeeding. Paediatr Child Health. 2011;16:222.
7. Academy of Breastfeeding Medicine. Statements. Academy of Breastfeeding Medicine Web site. Available at: www.bfmed.org/Resources/Protocols.aspx. Accessed January 10, 2015.
1. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
2. Hogan M, Westcott C, Griffiths M. Randomized, controlled trial of division of tongue-tie in infants with feeding problems. Paediatr Child Health. 2005;41:246-250.
3. Emond A, Ingram J, Johnson D, et al. Randomised controlled trial of early frenotomy in breastfed infants with mild-moderate tongue-tie. Arch Dis Child Fetal Neonatal Ed. 2014;99:F189-F195.
4. Berry J, Griffiths M, Westcott C. A double-blind, randomized, controlled trial of tongue-tie division and its immediate effect on breastfeeding. Breastfeed Med. 2012;7:189-193.
5. Riordan J, Bibb D, Miller M, et al. Predicting breastfeeding duration using the LATCH breastfeeding assessment tool. J Hum Lact. 2001;17:20-23.
6. Rowan-Legg A. Ankyloglossia and breastfeeding. Paediatr Child Health. 2011;16:222.
7. Academy of Breastfeeding Medicine. Statements. Academy of Breastfeeding Medicine Web site. Available at: www.bfmed.org/Resources/Protocols.aspx. Accessed January 10, 2015.
Evidence-based answers from the Family Physicians Inquiries Network
Does topical diclofenac relieve osteoarthritis pain?
Yes, at least in the short term. Topical diclofenac, with and without dimethyl sulfoxide (DMSO), modestly improves pain and function scores (by 4%-8%) for as long as 12 weeks in patients with osteoarthritis (OA) of the knee (strength of recommendation [SOR]: A, meta-analyses of multiple randomized controlled trials [RCTs]).
Topical diclofenac modestly decreases pain scores in patients with OA of the hand in the short term (by 9% at 6 weeks) but no more than placebo at 8 weeks (SOR: B, RCT).
Both topical diclofenac with DMSO and oral diclofenac produce similar pain and function scores in patients with OA of the knee. In addition to minor skin dryness, topical diclofenac causes gastrointestinal (GI) adverse effects in about a third of patients (SOR: B, RCT).
EVIDENCE SUMMARY
Diclofenac gel ($260-$330 per 150-mL bottle) and diclofenac with DMSO solution are the only topical nonsteroidal anti-inflammatory drugs (NSAIDs) available in the United States.
Topical diclofenac with DMSO beats placebo
In a meta-analysis of 3 RCTs (697 patients, mean age 63.2, 37% male) with knee OA, topical diclofenac solution with DMSO (Pennsaid, 40 drops applied 4 times daily) demonstrated superiority to vehicle-controlled placebo at 4 to 12 weeks (mean 8.5 weeks) using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index.1 The WOMAC is a standardized patient questionnaire measuring 5 items for pain (score range 0-20), 2 for stiffness (score range 0-8), and 17 for functional limitation (score range 0-68).
Compared with placebo, topical diclofenac with DMSO resulted in 1.6 units greater reduction in pain (8% difference), 0.6 units greater reduction in stiffness (7.5% difference), and 5.5 units greater improvement in physical function (8% difference). Patients using diclofenac reported more minor skin dryness than patients using placebo (number needed to harm [NNH]=6).
Diclofenac gel is also effective, but may cause dermatitis
A pooled analysis of 3 12-week randomized, double-blind, parallel-group, multicenter trials of 1426 patients with OA of the knee compared topical diclofenac gel (4 g applied 4 times a day) with vehicle placebo for patients older than 25 years and patients older than 65 years.2 Investigators evaluated 972 patients who suffered a symptom flare at 1, 4, 8, and 12 weeks after a one-week washout period.
Diclofenac demonstrated statistical superiority across all age groups when compared with placebo, based on pain and physical function measured on the WOMAC Index. Its effects were modest, however.
At 12 weeks, patients younger than 65 years showed pain improvement of -5.8 vs -4.7 for placebo (a 5.5% improvement on the 20-point scale) and improvement in physical function of -17.9 vs -14.2 (a 5.4% improvement on the 68-point scale). Patients older than 65 years demonstrated pain improvement of -5.3 vs -4.1 for placebo (6% improvement on the 20-point scale) and physical function improvement of -15.5 vs -11.0 for placebo (6.6% improvement on the 68-point scale).
Dermatitis was more common in the diclofenac groups, with a NNH of 30 in patients younger than 65 years and 19 in patients older than 65 years.
Diclofenac gel effectively treated hand OA for as long as 6 weeks in a randomized, double-blind, placebo-controlled trial of 809 men and women older than 39 years. Pain scores on a 100-point visual analog scale improved when compared with placebo alone.3 At 6 weeks, topical diclofenac reduced pain scores by 45% compared with 36% for placebo (P=.023). Pain reductions also were greater in the diclofenac group at 8 weeks, although not statistically different.
Oral diclofenac works well, too, but has more GI adverse effects
An RCT of 622 patients (40-85 years of age) with symptomatic and radiographically diagnosed OA of the knee compared topical diclofenac solution (75 mg/d) with oral diclofenac (50 mg 3 times a day) and found similar efficacy at 12 weeks, with no significant difference between oral and topical preparations for pain, physical function, and stiffness measured with the WOMAC Index (P=.23, .06, .24, respectively).4 Oral diclofenac produced more adverse GI side effects than the topical solution (48% vs 35%; P=.0006).
RECOMMENDATIONS
The Agency for Healthcare Research and Quality states that topical and oral NSAIDs reduce knee OA pain equally.5
The Guidelines of the American Academy of Orthopaedic Surgeons, American College of Rheumatology, European League Against Rheumatism, Osteoarthritis Research Society International, and National Institute for Health and Care Excellence all state that clinicians may consider topical NSAIDs for patients with mild to moderate OA of the knee or hand, particularly in patients with few affected joints or a history of sensitivity to oral NSAIDs.6
1. Towheed TE. Pennsaid therapy for osteoarthritis of the knee: a systematic review and meta-analysis of randomized controlled trials. J Rheumatol. 2006;33:567-573.
2. Baraf HS, Gloth FM, Barthel HR, et al. Safety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trials. Drugs Aging. 2011;28:27-40.
3. Altman RD, Dreiser RL, Fisher CL, et al. Diclofenac sodium gel in patients with primary hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:1991-1999.
4. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (Pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
5. Chou R, McDonagh MS, Nakamoto E, et al. Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review. Rockville, MD: Agency for Healthcare Research and Quality; 2011. AHRQ Publication No. 11(12)-EHC076-EF.
6. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis. part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476-499
Yes, at least in the short term. Topical diclofenac, with and without dimethyl sulfoxide (DMSO), modestly improves pain and function scores (by 4%-8%) for as long as 12 weeks in patients with osteoarthritis (OA) of the knee (strength of recommendation [SOR]: A, meta-analyses of multiple randomized controlled trials [RCTs]).
Topical diclofenac modestly decreases pain scores in patients with OA of the hand in the short term (by 9% at 6 weeks) but no more than placebo at 8 weeks (SOR: B, RCT).
Both topical diclofenac with DMSO and oral diclofenac produce similar pain and function scores in patients with OA of the knee. In addition to minor skin dryness, topical diclofenac causes gastrointestinal (GI) adverse effects in about a third of patients (SOR: B, RCT).
EVIDENCE SUMMARY
Diclofenac gel ($260-$330 per 150-mL bottle) and diclofenac with DMSO solution are the only topical nonsteroidal anti-inflammatory drugs (NSAIDs) available in the United States.
Topical diclofenac with DMSO beats placebo
In a meta-analysis of 3 RCTs (697 patients, mean age 63.2, 37% male) with knee OA, topical diclofenac solution with DMSO (Pennsaid, 40 drops applied 4 times daily) demonstrated superiority to vehicle-controlled placebo at 4 to 12 weeks (mean 8.5 weeks) using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index.1 The WOMAC is a standardized patient questionnaire measuring 5 items for pain (score range 0-20), 2 for stiffness (score range 0-8), and 17 for functional limitation (score range 0-68).
Compared with placebo, topical diclofenac with DMSO resulted in 1.6 units greater reduction in pain (8% difference), 0.6 units greater reduction in stiffness (7.5% difference), and 5.5 units greater improvement in physical function (8% difference). Patients using diclofenac reported more minor skin dryness than patients using placebo (number needed to harm [NNH]=6).
Diclofenac gel is also effective, but may cause dermatitis
A pooled analysis of 3 12-week randomized, double-blind, parallel-group, multicenter trials of 1426 patients with OA of the knee compared topical diclofenac gel (4 g applied 4 times a day) with vehicle placebo for patients older than 25 years and patients older than 65 years.2 Investigators evaluated 972 patients who suffered a symptom flare at 1, 4, 8, and 12 weeks after a one-week washout period.
Diclofenac demonstrated statistical superiority across all age groups when compared with placebo, based on pain and physical function measured on the WOMAC Index. Its effects were modest, however.
At 12 weeks, patients younger than 65 years showed pain improvement of -5.8 vs -4.7 for placebo (a 5.5% improvement on the 20-point scale) and improvement in physical function of -17.9 vs -14.2 (a 5.4% improvement on the 68-point scale). Patients older than 65 years demonstrated pain improvement of -5.3 vs -4.1 for placebo (6% improvement on the 20-point scale) and physical function improvement of -15.5 vs -11.0 for placebo (6.6% improvement on the 68-point scale).
Dermatitis was more common in the diclofenac groups, with a NNH of 30 in patients younger than 65 years and 19 in patients older than 65 years.
Diclofenac gel effectively treated hand OA for as long as 6 weeks in a randomized, double-blind, placebo-controlled trial of 809 men and women older than 39 years. Pain scores on a 100-point visual analog scale improved when compared with placebo alone.3 At 6 weeks, topical diclofenac reduced pain scores by 45% compared with 36% for placebo (P=.023). Pain reductions also were greater in the diclofenac group at 8 weeks, although not statistically different.
Oral diclofenac works well, too, but has more GI adverse effects
An RCT of 622 patients (40-85 years of age) with symptomatic and radiographically diagnosed OA of the knee compared topical diclofenac solution (75 mg/d) with oral diclofenac (50 mg 3 times a day) and found similar efficacy at 12 weeks, with no significant difference between oral and topical preparations for pain, physical function, and stiffness measured with the WOMAC Index (P=.23, .06, .24, respectively).4 Oral diclofenac produced more adverse GI side effects than the topical solution (48% vs 35%; P=.0006).
RECOMMENDATIONS
The Agency for Healthcare Research and Quality states that topical and oral NSAIDs reduce knee OA pain equally.5
The Guidelines of the American Academy of Orthopaedic Surgeons, American College of Rheumatology, European League Against Rheumatism, Osteoarthritis Research Society International, and National Institute for Health and Care Excellence all state that clinicians may consider topical NSAIDs for patients with mild to moderate OA of the knee or hand, particularly in patients with few affected joints or a history of sensitivity to oral NSAIDs.6
Yes, at least in the short term. Topical diclofenac, with and without dimethyl sulfoxide (DMSO), modestly improves pain and function scores (by 4%-8%) for as long as 12 weeks in patients with osteoarthritis (OA) of the knee (strength of recommendation [SOR]: A, meta-analyses of multiple randomized controlled trials [RCTs]).
Topical diclofenac modestly decreases pain scores in patients with OA of the hand in the short term (by 9% at 6 weeks) but no more than placebo at 8 weeks (SOR: B, RCT).
Both topical diclofenac with DMSO and oral diclofenac produce similar pain and function scores in patients with OA of the knee. In addition to minor skin dryness, topical diclofenac causes gastrointestinal (GI) adverse effects in about a third of patients (SOR: B, RCT).
EVIDENCE SUMMARY
Diclofenac gel ($260-$330 per 150-mL bottle) and diclofenac with DMSO solution are the only topical nonsteroidal anti-inflammatory drugs (NSAIDs) available in the United States.
Topical diclofenac with DMSO beats placebo
In a meta-analysis of 3 RCTs (697 patients, mean age 63.2, 37% male) with knee OA, topical diclofenac solution with DMSO (Pennsaid, 40 drops applied 4 times daily) demonstrated superiority to vehicle-controlled placebo at 4 to 12 weeks (mean 8.5 weeks) using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index.1 The WOMAC is a standardized patient questionnaire measuring 5 items for pain (score range 0-20), 2 for stiffness (score range 0-8), and 17 for functional limitation (score range 0-68).
Compared with placebo, topical diclofenac with DMSO resulted in 1.6 units greater reduction in pain (8% difference), 0.6 units greater reduction in stiffness (7.5% difference), and 5.5 units greater improvement in physical function (8% difference). Patients using diclofenac reported more minor skin dryness than patients using placebo (number needed to harm [NNH]=6).
Diclofenac gel is also effective, but may cause dermatitis
A pooled analysis of 3 12-week randomized, double-blind, parallel-group, multicenter trials of 1426 patients with OA of the knee compared topical diclofenac gel (4 g applied 4 times a day) with vehicle placebo for patients older than 25 years and patients older than 65 years.2 Investigators evaluated 972 patients who suffered a symptom flare at 1, 4, 8, and 12 weeks after a one-week washout period.
Diclofenac demonstrated statistical superiority across all age groups when compared with placebo, based on pain and physical function measured on the WOMAC Index. Its effects were modest, however.
At 12 weeks, patients younger than 65 years showed pain improvement of -5.8 vs -4.7 for placebo (a 5.5% improvement on the 20-point scale) and improvement in physical function of -17.9 vs -14.2 (a 5.4% improvement on the 68-point scale). Patients older than 65 years demonstrated pain improvement of -5.3 vs -4.1 for placebo (6% improvement on the 20-point scale) and physical function improvement of -15.5 vs -11.0 for placebo (6.6% improvement on the 68-point scale).
Dermatitis was more common in the diclofenac groups, with a NNH of 30 in patients younger than 65 years and 19 in patients older than 65 years.
Diclofenac gel effectively treated hand OA for as long as 6 weeks in a randomized, double-blind, placebo-controlled trial of 809 men and women older than 39 years. Pain scores on a 100-point visual analog scale improved when compared with placebo alone.3 At 6 weeks, topical diclofenac reduced pain scores by 45% compared with 36% for placebo (P=.023). Pain reductions also were greater in the diclofenac group at 8 weeks, although not statistically different.
Oral diclofenac works well, too, but has more GI adverse effects
An RCT of 622 patients (40-85 years of age) with symptomatic and radiographically diagnosed OA of the knee compared topical diclofenac solution (75 mg/d) with oral diclofenac (50 mg 3 times a day) and found similar efficacy at 12 weeks, with no significant difference between oral and topical preparations for pain, physical function, and stiffness measured with the WOMAC Index (P=.23, .06, .24, respectively).4 Oral diclofenac produced more adverse GI side effects than the topical solution (48% vs 35%; P=.0006).
RECOMMENDATIONS
The Agency for Healthcare Research and Quality states that topical and oral NSAIDs reduce knee OA pain equally.5
The Guidelines of the American Academy of Orthopaedic Surgeons, American College of Rheumatology, European League Against Rheumatism, Osteoarthritis Research Society International, and National Institute for Health and Care Excellence all state that clinicians may consider topical NSAIDs for patients with mild to moderate OA of the knee or hand, particularly in patients with few affected joints or a history of sensitivity to oral NSAIDs.6
1. Towheed TE. Pennsaid therapy for osteoarthritis of the knee: a systematic review and meta-analysis of randomized controlled trials. J Rheumatol. 2006;33:567-573.
2. Baraf HS, Gloth FM, Barthel HR, et al. Safety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trials. Drugs Aging. 2011;28:27-40.
3. Altman RD, Dreiser RL, Fisher CL, et al. Diclofenac sodium gel in patients with primary hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:1991-1999.
4. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (Pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
5. Chou R, McDonagh MS, Nakamoto E, et al. Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review. Rockville, MD: Agency for Healthcare Research and Quality; 2011. AHRQ Publication No. 11(12)-EHC076-EF.
6. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis. part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476-499
1. Towheed TE. Pennsaid therapy for osteoarthritis of the knee: a systematic review and meta-analysis of randomized controlled trials. J Rheumatol. 2006;33:567-573.
2. Baraf HS, Gloth FM, Barthel HR, et al. Safety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trials. Drugs Aging. 2011;28:27-40.
3. Altman RD, Dreiser RL, Fisher CL, et al. Diclofenac sodium gel in patients with primary hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:1991-1999.
4. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (Pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
5. Chou R, McDonagh MS, Nakamoto E, et al. Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review. Rockville, MD: Agency for Healthcare Research and Quality; 2011. AHRQ Publication No. 11(12)-EHC076-EF.
6. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis. part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476-499
Evidence-based answers from the Family Physicians Inquiries Network
What is the best beta-blocker for systolic heart failure?
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
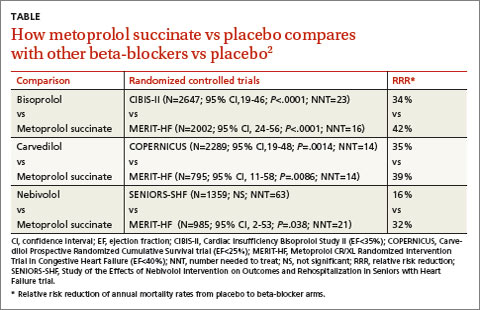
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3
RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3

RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3

RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
Evidence-based answers from the Family Physicians Inquiries Network
February 2015 Quiz 1
ANSWER: D
Critique
Cough is considered to be one of the atypical manifestations of reflux disease. The mechanisms include regurgitation with tracheal aspiration of gastric content, but also triggering of reflex bronchial reactivity with small amounts of acid in the distal esophagus in predisposed individuals. However, environmental allergens, postnasal drip, and airway disorders can also trigger chronic cough. Upper endoscopy with esophageal biopsies may demonstrate reflux esophagitis, but this does not establish whether cough is triggered by reflux events. A solid bolus barium swallow is useful in the evaluation of esophageal dysphagia. Impedance planimetry assesses biomechanical properties of the esophageal wall, and does not help assess the role of reflux in chronic cough. Esophageal high-resolution manometry will demonstrate esophageal motor patterns, but does not determine causality of chronic cough. Wireless pH testing off PPI therapy has the potential to determine whether cough events correlate with acidic reflux events. Some investigators have combined this with a cough monitor that can precisely time cough events, allowing for more accurate assessments of the association between cough and reflux events.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
ANSWER: D
Critique
Cough is considered to be one of the atypical manifestations of reflux disease. The mechanisms include regurgitation with tracheal aspiration of gastric content, but also triggering of reflex bronchial reactivity with small amounts of acid in the distal esophagus in predisposed individuals. However, environmental allergens, postnasal drip, and airway disorders can also trigger chronic cough. Upper endoscopy with esophageal biopsies may demonstrate reflux esophagitis, but this does not establish whether cough is triggered by reflux events. A solid bolus barium swallow is useful in the evaluation of esophageal dysphagia. Impedance planimetry assesses biomechanical properties of the esophageal wall, and does not help assess the role of reflux in chronic cough. Esophageal high-resolution manometry will demonstrate esophageal motor patterns, but does not determine causality of chronic cough. Wireless pH testing off PPI therapy has the potential to determine whether cough events correlate with acidic reflux events. Some investigators have combined this with a cough monitor that can precisely time cough events, allowing for more accurate assessments of the association between cough and reflux events.
ANSWER: D
Critique
Cough is considered to be one of the atypical manifestations of reflux disease. The mechanisms include regurgitation with tracheal aspiration of gastric content, but also triggering of reflex bronchial reactivity with small amounts of acid in the distal esophagus in predisposed individuals. However, environmental allergens, postnasal drip, and airway disorders can also trigger chronic cough. Upper endoscopy with esophageal biopsies may demonstrate reflux esophagitis, but this does not establish whether cough is triggered by reflux events. A solid bolus barium swallow is useful in the evaluation of esophageal dysphagia. Impedance planimetry assesses biomechanical properties of the esophageal wall, and does not help assess the role of reflux in chronic cough. Esophageal high-resolution manometry will demonstrate esophageal motor patterns, but does not determine causality of chronic cough. Wireless pH testing off PPI therapy has the potential to determine whether cough events correlate with acidic reflux events. Some investigators have combined this with a cough monitor that can precisely time cough events, allowing for more accurate assessments of the association between cough and reflux events.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
February 2015 Quiz 2
ANSWER: C
Critique
The diagnosis of H. pylori may be made using either invasive or noninvasive methods. Invasive diagnostic methods either detect organisms directly (i.e., histological identification with appropriate stains or bacterial culture) or indirectly (i.e., rapid urease testing of biopsy specimens). Noninvasive methods include serum antibody, stool antigen, and detecting the metabolites of the bacterial enzyme urease (i.e., urea breath testing). It should be noted that serology should not be used to test for eradication as the antibodies may remain elevated for years after successful eradication therapy.
Treatment of H. pylori infection has become problematic recently primarily because of increasing antibiotic resistance. The eradication rate of standard triple therapy consisting of a PPI combined with clarithromycin (500 mg) and amoxicillin (1 g) (or metronidazole (500 mg)), all given b.i.d. for 7-14 days has now declined to unacceptable levels, averaging 70%-80% but reported to be as low as 55%. It is currently recommended that a noninvasive method be used (other than serology) to confirm eradication in patients in whom eradication is deemed necessary.
- Malfertheiner P, Megraud F, O’Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection – the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646-64.
- De F, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Lerardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J. Gastrointest. Liver Dis. 2010;19:409-14.
- Kearney DJ, Brousal A. Treatment of Helicobacter pylori infection in clinical practice in the United States – Results from 224 patients. Dig. Dis. Sci. 2000;45:265-71.
ANSWER: C
Critique
The diagnosis of H. pylori may be made using either invasive or noninvasive methods. Invasive diagnostic methods either detect organisms directly (i.e., histological identification with appropriate stains or bacterial culture) or indirectly (i.e., rapid urease testing of biopsy specimens). Noninvasive methods include serum antibody, stool antigen, and detecting the metabolites of the bacterial enzyme urease (i.e., urea breath testing). It should be noted that serology should not be used to test for eradication as the antibodies may remain elevated for years after successful eradication therapy.
Treatment of H. pylori infection has become problematic recently primarily because of increasing antibiotic resistance. The eradication rate of standard triple therapy consisting of a PPI combined with clarithromycin (500 mg) and amoxicillin (1 g) (or metronidazole (500 mg)), all given b.i.d. for 7-14 days has now declined to unacceptable levels, averaging 70%-80% but reported to be as low as 55%. It is currently recommended that a noninvasive method be used (other than serology) to confirm eradication in patients in whom eradication is deemed necessary.
ANSWER: C
Critique
The diagnosis of H. pylori may be made using either invasive or noninvasive methods. Invasive diagnostic methods either detect organisms directly (i.e., histological identification with appropriate stains or bacterial culture) or indirectly (i.e., rapid urease testing of biopsy specimens). Noninvasive methods include serum antibody, stool antigen, and detecting the metabolites of the bacterial enzyme urease (i.e., urea breath testing). It should be noted that serology should not be used to test for eradication as the antibodies may remain elevated for years after successful eradication therapy.
Treatment of H. pylori infection has become problematic recently primarily because of increasing antibiotic resistance. The eradication rate of standard triple therapy consisting of a PPI combined with clarithromycin (500 mg) and amoxicillin (1 g) (or metronidazole (500 mg)), all given b.i.d. for 7-14 days has now declined to unacceptable levels, averaging 70%-80% but reported to be as low as 55%. It is currently recommended that a noninvasive method be used (other than serology) to confirm eradication in patients in whom eradication is deemed necessary.
- Malfertheiner P, Megraud F, O’Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection – the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646-64.
- De F, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Lerardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J. Gastrointest. Liver Dis. 2010;19:409-14.
- Kearney DJ, Brousal A. Treatment of Helicobacter pylori infection in clinical practice in the United States – Results from 224 patients. Dig. Dis. Sci. 2000;45:265-71.
- Malfertheiner P, Megraud F, O’Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection – the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646-64.
- De F, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Lerardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J. Gastrointest. Liver Dis. 2010;19:409-14.
- Kearney DJ, Brousal A. Treatment of Helicobacter pylori infection in clinical practice in the United States – Results from 224 patients. Dig. Dis. Sci. 2000;45:265-71.
Team touts a new, improved hydrogel
Louis Heiser & Robert Ackland
A new hydrogel improves on previous models by enabling the generation of more mature blood vessels, according to research published in ACS Nano.
The hydrogel also overcomes several other issues that have kept previous hydrogels from reaching their potential to treat injuries and forming new vasculature to treat heart attack, stroke, and ischemic tissue diseases.
Like earlier versions, the new hydrogel can be injected in liquid form and turns into a nanofiber-infused gel at the site of the injury. The difference with this hydrogel, according to researchers, is the quality of the blood vessels that are formed.
This hydrogel is made of self-assembling synthetic peptides that form nanofiber scaffolds. And the peptides incorporate a mimic of vascular endothelial growth factor, a signal protein that promotes angiogenesis.
Furthermore, the hydrogel can be easily delivered by syringe, is quickly infiltrated by hematopoietic and mesenchymal cells, and quickly forms a mature vascular network.
“In a lot of the published literature, you see rings that only have the endothelial cell lining, and that indicates a very immature blood vessel,” said study author Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“These types of vessels usually don’t persist and disappear shortly after they show up. In ours, you see that same endothelial cell layer, but surrounding it is a smooth muscle cell layer that indicates a much more mature vessel that’s likely to persist.”
Furthermore, the scaffolds the hydrogel forms show no signs of fibrous encapsulation. After 3 weeks, they are resorbed into the native tissue.
In previous studies, implanted synthetic materials tended to become encapsulated by fibrous barriers that kept cells and blood vessels from infiltrating the scaffold, Dr Hartgerink said.
“That is an extremely common problem in synthetic materials put into the body,” he explained. “Some avoid this problem, but if the body doesn’t like a material and isn’t able to destroy it, the solution is to wall it off.”
“As soon as that happens, the flow of nutrients across that barrier decreases to almost nothing. So the fact that we’ve developed syringe-directed delivery of a material that doesn’t develop fibrous encapsulation is really important.”
Other negative characteristics of earlier hydrogels—unwanted immune responses, surface degradation preceding their integration into biological systems, and the release of artificial degradation byproducts—have been eliminated as well, Dr Hartgerink said.
“There are a lot of features about this hydrogel that come together to make it a unique system,” he added. “If you look through the literature at what other people have done, each concept that is involved in our system probably exists somewhere already. The difference is that we have all these features in one place working together.” ![]()

Louis Heiser & Robert Ackland
A new hydrogel improves on previous models by enabling the generation of more mature blood vessels, according to research published in ACS Nano.
The hydrogel also overcomes several other issues that have kept previous hydrogels from reaching their potential to treat injuries and forming new vasculature to treat heart attack, stroke, and ischemic tissue diseases.
Like earlier versions, the new hydrogel can be injected in liquid form and turns into a nanofiber-infused gel at the site of the injury. The difference with this hydrogel, according to researchers, is the quality of the blood vessels that are formed.
This hydrogel is made of self-assembling synthetic peptides that form nanofiber scaffolds. And the peptides incorporate a mimic of vascular endothelial growth factor, a signal protein that promotes angiogenesis.
Furthermore, the hydrogel can be easily delivered by syringe, is quickly infiltrated by hematopoietic and mesenchymal cells, and quickly forms a mature vascular network.
“In a lot of the published literature, you see rings that only have the endothelial cell lining, and that indicates a very immature blood vessel,” said study author Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“These types of vessels usually don’t persist and disappear shortly after they show up. In ours, you see that same endothelial cell layer, but surrounding it is a smooth muscle cell layer that indicates a much more mature vessel that’s likely to persist.”
Furthermore, the scaffolds the hydrogel forms show no signs of fibrous encapsulation. After 3 weeks, they are resorbed into the native tissue.
In previous studies, implanted synthetic materials tended to become encapsulated by fibrous barriers that kept cells and blood vessels from infiltrating the scaffold, Dr Hartgerink said.
“That is an extremely common problem in synthetic materials put into the body,” he explained. “Some avoid this problem, but if the body doesn’t like a material and isn’t able to destroy it, the solution is to wall it off.”
“As soon as that happens, the flow of nutrients across that barrier decreases to almost nothing. So the fact that we’ve developed syringe-directed delivery of a material that doesn’t develop fibrous encapsulation is really important.”
Other negative characteristics of earlier hydrogels—unwanted immune responses, surface degradation preceding their integration into biological systems, and the release of artificial degradation byproducts—have been eliminated as well, Dr Hartgerink said.
“There are a lot of features about this hydrogel that come together to make it a unique system,” he added. “If you look through the literature at what other people have done, each concept that is involved in our system probably exists somewhere already. The difference is that we have all these features in one place working together.” ![]()

Louis Heiser & Robert Ackland
A new hydrogel improves on previous models by enabling the generation of more mature blood vessels, according to research published in ACS Nano.
The hydrogel also overcomes several other issues that have kept previous hydrogels from reaching their potential to treat injuries and forming new vasculature to treat heart attack, stroke, and ischemic tissue diseases.
Like earlier versions, the new hydrogel can be injected in liquid form and turns into a nanofiber-infused gel at the site of the injury. The difference with this hydrogel, according to researchers, is the quality of the blood vessels that are formed.
This hydrogel is made of self-assembling synthetic peptides that form nanofiber scaffolds. And the peptides incorporate a mimic of vascular endothelial growth factor, a signal protein that promotes angiogenesis.
Furthermore, the hydrogel can be easily delivered by syringe, is quickly infiltrated by hematopoietic and mesenchymal cells, and quickly forms a mature vascular network.
“In a lot of the published literature, you see rings that only have the endothelial cell lining, and that indicates a very immature blood vessel,” said study author Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“These types of vessels usually don’t persist and disappear shortly after they show up. In ours, you see that same endothelial cell layer, but surrounding it is a smooth muscle cell layer that indicates a much more mature vessel that’s likely to persist.”
Furthermore, the scaffolds the hydrogel forms show no signs of fibrous encapsulation. After 3 weeks, they are resorbed into the native tissue.
In previous studies, implanted synthetic materials tended to become encapsulated by fibrous barriers that kept cells and blood vessels from infiltrating the scaffold, Dr Hartgerink said.
“That is an extremely common problem in synthetic materials put into the body,” he explained. “Some avoid this problem, but if the body doesn’t like a material and isn’t able to destroy it, the solution is to wall it off.”
“As soon as that happens, the flow of nutrients across that barrier decreases to almost nothing. So the fact that we’ve developed syringe-directed delivery of a material that doesn’t develop fibrous encapsulation is really important.”
Other negative characteristics of earlier hydrogels—unwanted immune responses, surface degradation preceding their integration into biological systems, and the release of artificial degradation byproducts—have been eliminated as well, Dr Hartgerink said.
“There are a lot of features about this hydrogel that come together to make it a unique system,” he added. “If you look through the literature at what other people have done, each concept that is involved in our system probably exists somewhere already. The difference is that we have all these features in one place working together.” ![]()
Would a cholesterol medication have made a difference? … More
Would a cholesterol medication have made a difference?
A WOMAN WITH A HISTORY OF HYPERTENSION and hyperlipidemia sought treatment from her family physician (FP) for a protracted, nonproductive cough. The FP diagnosed sinusitis and reactive airway disease and prescribed steroids and antibiotics. The patient returned to the FP 5 more times over the next 9 weeks. The patient’s symptoms waxed and waned, but her cough continued. She reported chest tightness and shortness of breath on exertion. A chest x-ray revealed moderate heart enlargement. An echocardiogram was scheduled.
During the patient’s last visit, her FP noted that she had shortness of breath on exertion, but no chest pain. Three days later she suffered a massive myocardial infarction (MI). Cardiac catheterization found 80% occlusion of the left anterior descending artery. She underwent angioplasty and stent placement; after this procedure her ejection fraction was 25% to 30%. One month later, the patient received a pacemaker/defibrillator. The patient’s cardiac symptoms returned 7 months later, and she underwent another angioplasty. She improved and her last echocardiogram showed near-normal heart function.
PLAINTIFF’S CLAIM Although the patient had persistently elevated cholesterol levels, the FP failed to order repeat cholesterol studies and arrange for drug therapy. If the patient’s hyperlipidemia had been medically managed, her coronary artery disease would not have progressed to unstable angina and MI. The FP also failed to obtain routine electrocardiograms or an urgent cardiac consult after a chest x-ray showed an enlarged heart. The FP also failed to send the patient to an emergency department when she complained of shortness of breath on exertion.
THE DEFENSE An urgent cardiac work-up was not indicated and the patient’s cholesterol levels were only mildly elevated and did not require medical management. Her MI was unavoidable since most infarctions are due to plaque rupture in coronary vessels that aren’t occluded enough to require treatment.
VERDICT $1.6 million Michigan verdict.
COMMENT I think the key issue in this difficult diagnostic case is not the lack of prescribing cholesterol medication, but the repeated office visits with no definite diagnosis. If the physician had escalated the evaluation more quickly, the MI might have been avoided.
Narcotic misstep has tragic consequences
A 47-YEAR-OLD MAN SOUGHT TREATMENT FOR DRUG ADDICTION. His physician prescribed methadone, despite not being licensed to do so. After 4 days of taking methadone, the patient went to the hospital because he felt dizzy and was having difficulty breathing. Two days after being examined and discharged, he died from methadone toxicity.
PLAINTIFF’S CLAIM The toxicity was caused by simultaneous use of methadone and alprazolam, which the patient also had been prescribed. The physician failed to recognize the potential toxicity and should have performed testing that could have revealed the simultaneous use of other drugs. In addition, the physician was not licensed to prescribe methadone.
THE DEFENSE The physician had recommended a licensed, qualified facility that could have treated the plaintiff, but the plaintiff preferred treatment in a setting that allowed him to remain anonymous.
VERDICT $1.15 million New York settlement.
COMMENT Don’t break the law, even if your patient asks you to. Know your state laws regarding narcotic prescribing. These are getting more stringent due to the rapid rise in prescription narcotic overdose deaths in the United States.
Would a cholesterol medication have made a difference?
A WOMAN WITH A HISTORY OF HYPERTENSION and hyperlipidemia sought treatment from her family physician (FP) for a protracted, nonproductive cough. The FP diagnosed sinusitis and reactive airway disease and prescribed steroids and antibiotics. The patient returned to the FP 5 more times over the next 9 weeks. The patient’s symptoms waxed and waned, but her cough continued. She reported chest tightness and shortness of breath on exertion. A chest x-ray revealed moderate heart enlargement. An echocardiogram was scheduled.
During the patient’s last visit, her FP noted that she had shortness of breath on exertion, but no chest pain. Three days later she suffered a massive myocardial infarction (MI). Cardiac catheterization found 80% occlusion of the left anterior descending artery. She underwent angioplasty and stent placement; after this procedure her ejection fraction was 25% to 30%. One month later, the patient received a pacemaker/defibrillator. The patient’s cardiac symptoms returned 7 months later, and she underwent another angioplasty. She improved and her last echocardiogram showed near-normal heart function.
PLAINTIFF’S CLAIM Although the patient had persistently elevated cholesterol levels, the FP failed to order repeat cholesterol studies and arrange for drug therapy. If the patient’s hyperlipidemia had been medically managed, her coronary artery disease would not have progressed to unstable angina and MI. The FP also failed to obtain routine electrocardiograms or an urgent cardiac consult after a chest x-ray showed an enlarged heart. The FP also failed to send the patient to an emergency department when she complained of shortness of breath on exertion.
THE DEFENSE An urgent cardiac work-up was not indicated and the patient’s cholesterol levels were only mildly elevated and did not require medical management. Her MI was unavoidable since most infarctions are due to plaque rupture in coronary vessels that aren’t occluded enough to require treatment.
VERDICT $1.6 million Michigan verdict.
COMMENT I think the key issue in this difficult diagnostic case is not the lack of prescribing cholesterol medication, but the repeated office visits with no definite diagnosis. If the physician had escalated the evaluation more quickly, the MI might have been avoided.
Narcotic misstep has tragic consequences
A 47-YEAR-OLD MAN SOUGHT TREATMENT FOR DRUG ADDICTION. His physician prescribed methadone, despite not being licensed to do so. After 4 days of taking methadone, the patient went to the hospital because he felt dizzy and was having difficulty breathing. Two days after being examined and discharged, he died from methadone toxicity.
PLAINTIFF’S CLAIM The toxicity was caused by simultaneous use of methadone and alprazolam, which the patient also had been prescribed. The physician failed to recognize the potential toxicity and should have performed testing that could have revealed the simultaneous use of other drugs. In addition, the physician was not licensed to prescribe methadone.
THE DEFENSE The physician had recommended a licensed, qualified facility that could have treated the plaintiff, but the plaintiff preferred treatment in a setting that allowed him to remain anonymous.
VERDICT $1.15 million New York settlement.
COMMENT Don’t break the law, even if your patient asks you to. Know your state laws regarding narcotic prescribing. These are getting more stringent due to the rapid rise in prescription narcotic overdose deaths in the United States.
Would a cholesterol medication have made a difference?
A WOMAN WITH A HISTORY OF HYPERTENSION and hyperlipidemia sought treatment from her family physician (FP) for a protracted, nonproductive cough. The FP diagnosed sinusitis and reactive airway disease and prescribed steroids and antibiotics. The patient returned to the FP 5 more times over the next 9 weeks. The patient’s symptoms waxed and waned, but her cough continued. She reported chest tightness and shortness of breath on exertion. A chest x-ray revealed moderate heart enlargement. An echocardiogram was scheduled.
During the patient’s last visit, her FP noted that she had shortness of breath on exertion, but no chest pain. Three days later she suffered a massive myocardial infarction (MI). Cardiac catheterization found 80% occlusion of the left anterior descending artery. She underwent angioplasty and stent placement; after this procedure her ejection fraction was 25% to 30%. One month later, the patient received a pacemaker/defibrillator. The patient’s cardiac symptoms returned 7 months later, and she underwent another angioplasty. She improved and her last echocardiogram showed near-normal heart function.
PLAINTIFF’S CLAIM Although the patient had persistently elevated cholesterol levels, the FP failed to order repeat cholesterol studies and arrange for drug therapy. If the patient’s hyperlipidemia had been medically managed, her coronary artery disease would not have progressed to unstable angina and MI. The FP also failed to obtain routine electrocardiograms or an urgent cardiac consult after a chest x-ray showed an enlarged heart. The FP also failed to send the patient to an emergency department when she complained of shortness of breath on exertion.
THE DEFENSE An urgent cardiac work-up was not indicated and the patient’s cholesterol levels were only mildly elevated and did not require medical management. Her MI was unavoidable since most infarctions are due to plaque rupture in coronary vessels that aren’t occluded enough to require treatment.
VERDICT $1.6 million Michigan verdict.
COMMENT I think the key issue in this difficult diagnostic case is not the lack of prescribing cholesterol medication, but the repeated office visits with no definite diagnosis. If the physician had escalated the evaluation more quickly, the MI might have been avoided.
Narcotic misstep has tragic consequences
A 47-YEAR-OLD MAN SOUGHT TREATMENT FOR DRUG ADDICTION. His physician prescribed methadone, despite not being licensed to do so. After 4 days of taking methadone, the patient went to the hospital because he felt dizzy and was having difficulty breathing. Two days after being examined and discharged, he died from methadone toxicity.
PLAINTIFF’S CLAIM The toxicity was caused by simultaneous use of methadone and alprazolam, which the patient also had been prescribed. The physician failed to recognize the potential toxicity and should have performed testing that could have revealed the simultaneous use of other drugs. In addition, the physician was not licensed to prescribe methadone.
THE DEFENSE The physician had recommended a licensed, qualified facility that could have treated the plaintiff, but the plaintiff preferred treatment in a setting that allowed him to remain anonymous.
VERDICT $1.15 million New York settlement.
COMMENT Don’t break the law, even if your patient asks you to. Know your state laws regarding narcotic prescribing. These are getting more stringent due to the rapid rise in prescription narcotic overdose deaths in the United States.
Malpractice Counsel
Traumatic Back Pain
An 84-year-old man with low-back pain following a motor vehicle crash was brought to the ED by emergency medical services (EMS). He had been the restrained driver, stopped at a traffic light, when he was struck from behind by a second vehicle.
In the ED, the patient only complained of low-back pain. He denied any radiation of pain or lower-extremity numbness or weakness. He also denied any head injury, loss of consciousness, neck pain, or abdominal pain. His past medical history was significant for hypertension, arthritis, and coronary artery disease.
On physical examination, the patient’s vital signs were normal. The head, eyes, ears, nose, and throat (HEENT) examination was also normal; specifically, there was no tenderness to palpation of the cervical spine in the posterior midline. Regarding the cardiopulmonary examination, auscultation of the lungs revealed clear, bilateral breath sounds; the heart examination was normal. The patient had a soft abdomen, without tenderness, guarding, or rebound. His pelvis was stable, but he did exhibit some tenderness on palpation of the lower-thoracic and upper-lumbar spine. The neurological examination revealed normal motor strength and sensation in the lower extremities.
The emergency physician (EP) ordered X-rays of the thoracic and lumbar spine and a urinalysis. The films were interpreted by both the EP and radiologist as normal; the results of the urinalysis were also normal. The patient was diagnosed with a lower back strain secondary to the motor vehicle crash and was discharged home with an analgesic.
The next day, however, the patient began to complain of increased back pain and lower-extremity numbness and weakness. He was brought back to the same hospital ED where he was noted to have severe weakness of both lower extremities and decreased sensation to touch. Additional imaging was performed, which demonstrated a fracture of T11 with spinal cord impingement. He was taken to surgery, but unfortunately the injury was permanent, and the patient was left with lower-extremity paralysis and bowel and bladder incontinence.
The plaintiff sued the EP and the radiologist for not properly interpreting the initial X-rays. The defendants denied liability, asserting the patient’s injury was a result of the collision and that nothing could have prevented it. According to a published account, the jury returned a verdict finding the EP to be 40% at fault and the radiologist 60% at fault.
Discussion
Emergency physicians frequently manage patients experiencing pain or injury following a motor vehicle crash. If the patient is complaining of neck or back pain, the prehospital providers will immobilize the patient with a rigid cervical collar (ie, if neck pain is present) and a long backboard if pain anywhere along the spine is present (ie, cervical, thoracic, or lumbar).
When the initial airway, breathing, circulation, and disability assessment for the trauma patient is performed and found to be normal, a secondary examination should be performed. Trauma patients with back pain should be log-rolled onto their side, with spinal immobilization followed by visual inspection and palpation/percussion of the midline of the thoracic and lumbar spine. The presence of midline tenderness suggests an acute injury and the need to keep the patient immobilized. Patients should be removed off the backboard and onto the gurney mattress while immobilizing the spine. The standard hospital mattress provides acceptable spinal support.1
Historically, plain radiographs of the thoracic and lumbar spine have been the imaging test of choice in the initial evaluation of suspected traumatic spinal column injury. However, similar to cervical spine trauma, computed tomography (CT) is assuming a larger role in the evaluation of patients with suspected thoracic or lumbar spine injury. When thoracic and abdominal CT scans are performed to evaluate for possible chest or abdominal trauma, those images can be reformatted and used to reconstruct images of the thoracic and lumbar spine, significantly reducing radiation exposure.1 While CT is the gold standard imaging study for evaluation of bony or ligamentous injury of the spine, magnetic resonance imaging (MRI) is the study of choice for patients with neurological deficits or suspected spinal cord injury.
This patient had a completely normal neurological examination at initial presentation, so there was no indication for an MRI. The bony injury to T11 must have been very subtle for both the EP and the radiologist to have missed it. Unfortunately, the jury appears to have used the standard of “perfection,” rather than the “reasonable and prudent physician” in judging that the injury should have been detected. This case serves as a reminder that EPs cannot rely on consulting specialists to consistently and reliably provide accurate information. Moreover, this case emphasizes the need to consider CT imaging of the spine in the evaluation of patients with severe back pain of traumatic origin when plain radiographs appear normal.
Hip-Reduction Problem
A 79-year-old man with left hip pain presented to the ED via EMS. The patient stated that when he had bent over to retrieve his dropped glasses, he experienced the immediate onset of left hip pain and fell to the floor. He was unable to get up on his own and called EMS. The patient had undergone total left hip replacement 1 month prior. At presentation, he complained only of severe pain in his left hip; he denied head injury, neck pain or stiffness, chest pain, or abdominal pain. His past medical history was significant for hypertension and type 2 diabetes mellitus. The patient had no known drug allergies.
On physical examination, he was mildly tachycardic. His vital signs were: heart rate, 102 beats/minute; blood pressure, 156/88 mm Hg; respiratory rate, 20 breaths/minutes; and temperature, afebrile. His pulse oximetry was 98% on room air. The HEENT, lung, heart, and abdominal examinations were all normal. Standing at the foot of the bed, the patient had obvious shortening, internal rotation, and adduction of the left leg. The left knee was without tenderness or swelling. The neurovascular examination of the left lower extremity was completely normal.
Plain radiographs of the pelvis and left hip ordered by the EP demonstrated a posterior hip dislocation with intact hardware. The EP consulted the patient’s orthopedic physician, and both agreed the EP should attempt to reduce the dislocation in the ED. Using conscious sedation, the EP was able to reduce the dislocation, but postreduction films demonstrated a new fracture requiring orthopedic surgery. Unfortunately, the patient had a very difficult recovery, ultimately resulting in death.
The patient’s estate sued the EP, stating he should have had the orthopedic physician reduce the dislocation. The defense argued that fracture is a known complication of reduction of a dislocated hip. A defense verdict was returned.
Discussion
Approximately 85% to 90% of hip dislocations are posterior; the remaining 10% are anterior. Posterior hip dislocations are a common complication following total hip-replacement surgery.1 Hip dislocation is a true orthopedic and time-dependent emergency. The longer the hip remains dislocated, the more likely complications are to occur, including osteonecrosis of the femoral head, arthritic degeneration of the hip joint, and long-term neurological sequelae.2 The treatment of posterior hip dislocation (without fracture) is closed reduction as quickly as possible, and preferably within 6 hours.3 As this case demonstrates, minimal forces can result in a hip dislocation following a total hip replacement. In healthy patients, however, significant forces (eg, high-speed motor vehicle crashes) are required to cause posterior hip dislocation.
Patients with a posterior hip dislocation will present in severe pain and an inability to ambulate. In most cases of posterior hip dislocation, the affected lower extremity will be visibly shortened, internally rotated, and adducted. The knee should always be examined for injury, as well as performance of a thorough neurovascular examination of the affected extremity.
Plain X-ray films will usually identify a posterior hip dislocation. On an anteroposterior pelvis X-ray, the femoral head will be seen outside and just superior to the acetabulum. Special attention should be made to the acetabulum to ensure a concomitant acetabular fracture is not missed.
Indications for closed reduction of a posterior hip dislocation include dislocation with or without neurological deficit and no associated fracture, or dislocation with an associated fracture if no neurological deficits are present.2 An open traumatic hip dislocation should only be reduced in the operating room.
It is certainly within the purview of the EP to attempt a closed reduction for a posterior hip dislocation if no contraindications exist. The patient will need to be sedated (ie, procedural sedation, conscious sedation, or moderate sedation) for any chance of success at reduction. While it is beyond the scope of this article to review the various techniques used to reduce a posterior hip dislocation, one of the guiding principles is that after two or three unsuccessful attempts by the EP to reduce the dislocation, no further attempts should be made and orthopedic surgery services should be consulted. This is because the risk of complications increases as the number of failed attempts increase.
It is unclear how many attempts the EP made in this case. Fracture is a known complication when attempting reduction for a hip dislocation, be it an orthopedic surgeon or an EP. It was certainly appropriate for the EP in this case to attempt closed reduction, given the importance of timely reduction.
Reference (Traumatic Back Pain)
- Baron BJ, McSherry KJ, Larson JL, Scalea TM. Spinal and spinal cord trauma In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1709-1730.
(Hip-Reduction Problem)
- Dela Cruz JE, Sullivan DN, Varboncouer E, et al. Comparison of proceduralsedation for the reduction of dislocated total hip arthroplasty.West J Emerg Med. 2014:15(1):76-80.
- Davenport M. Joint reduction, hip dislocation, posterior. Medscape Web site. eMedicine.medscape.com/article/109225. Updated February 11, 2014. Accessed January 27, 2015.
- Steele MT, Stubbs AM. Hip and femur injuries. In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1848-1856.
Traumatic Back Pain
An 84-year-old man with low-back pain following a motor vehicle crash was brought to the ED by emergency medical services (EMS). He had been the restrained driver, stopped at a traffic light, when he was struck from behind by a second vehicle.
In the ED, the patient only complained of low-back pain. He denied any radiation of pain or lower-extremity numbness or weakness. He also denied any head injury, loss of consciousness, neck pain, or abdominal pain. His past medical history was significant for hypertension, arthritis, and coronary artery disease.
On physical examination, the patient’s vital signs were normal. The head, eyes, ears, nose, and throat (HEENT) examination was also normal; specifically, there was no tenderness to palpation of the cervical spine in the posterior midline. Regarding the cardiopulmonary examination, auscultation of the lungs revealed clear, bilateral breath sounds; the heart examination was normal. The patient had a soft abdomen, without tenderness, guarding, or rebound. His pelvis was stable, but he did exhibit some tenderness on palpation of the lower-thoracic and upper-lumbar spine. The neurological examination revealed normal motor strength and sensation in the lower extremities.
The emergency physician (EP) ordered X-rays of the thoracic and lumbar spine and a urinalysis. The films were interpreted by both the EP and radiologist as normal; the results of the urinalysis were also normal. The patient was diagnosed with a lower back strain secondary to the motor vehicle crash and was discharged home with an analgesic.
The next day, however, the patient began to complain of increased back pain and lower-extremity numbness and weakness. He was brought back to the same hospital ED where he was noted to have severe weakness of both lower extremities and decreased sensation to touch. Additional imaging was performed, which demonstrated a fracture of T11 with spinal cord impingement. He was taken to surgery, but unfortunately the injury was permanent, and the patient was left with lower-extremity paralysis and bowel and bladder incontinence.
The plaintiff sued the EP and the radiologist for not properly interpreting the initial X-rays. The defendants denied liability, asserting the patient’s injury was a result of the collision and that nothing could have prevented it. According to a published account, the jury returned a verdict finding the EP to be 40% at fault and the radiologist 60% at fault.
Discussion
Emergency physicians frequently manage patients experiencing pain or injury following a motor vehicle crash. If the patient is complaining of neck or back pain, the prehospital providers will immobilize the patient with a rigid cervical collar (ie, if neck pain is present) and a long backboard if pain anywhere along the spine is present (ie, cervical, thoracic, or lumbar).
When the initial airway, breathing, circulation, and disability assessment for the trauma patient is performed and found to be normal, a secondary examination should be performed. Trauma patients with back pain should be log-rolled onto their side, with spinal immobilization followed by visual inspection and palpation/percussion of the midline of the thoracic and lumbar spine. The presence of midline tenderness suggests an acute injury and the need to keep the patient immobilized. Patients should be removed off the backboard and onto the gurney mattress while immobilizing the spine. The standard hospital mattress provides acceptable spinal support.1
Historically, plain radiographs of the thoracic and lumbar spine have been the imaging test of choice in the initial evaluation of suspected traumatic spinal column injury. However, similar to cervical spine trauma, computed tomography (CT) is assuming a larger role in the evaluation of patients with suspected thoracic or lumbar spine injury. When thoracic and abdominal CT scans are performed to evaluate for possible chest or abdominal trauma, those images can be reformatted and used to reconstruct images of the thoracic and lumbar spine, significantly reducing radiation exposure.1 While CT is the gold standard imaging study for evaluation of bony or ligamentous injury of the spine, magnetic resonance imaging (MRI) is the study of choice for patients with neurological deficits or suspected spinal cord injury.
This patient had a completely normal neurological examination at initial presentation, so there was no indication for an MRI. The bony injury to T11 must have been very subtle for both the EP and the radiologist to have missed it. Unfortunately, the jury appears to have used the standard of “perfection,” rather than the “reasonable and prudent physician” in judging that the injury should have been detected. This case serves as a reminder that EPs cannot rely on consulting specialists to consistently and reliably provide accurate information. Moreover, this case emphasizes the need to consider CT imaging of the spine in the evaluation of patients with severe back pain of traumatic origin when plain radiographs appear normal.
Hip-Reduction Problem
A 79-year-old man with left hip pain presented to the ED via EMS. The patient stated that when he had bent over to retrieve his dropped glasses, he experienced the immediate onset of left hip pain and fell to the floor. He was unable to get up on his own and called EMS. The patient had undergone total left hip replacement 1 month prior. At presentation, he complained only of severe pain in his left hip; he denied head injury, neck pain or stiffness, chest pain, or abdominal pain. His past medical history was significant for hypertension and type 2 diabetes mellitus. The patient had no known drug allergies.
On physical examination, he was mildly tachycardic. His vital signs were: heart rate, 102 beats/minute; blood pressure, 156/88 mm Hg; respiratory rate, 20 breaths/minutes; and temperature, afebrile. His pulse oximetry was 98% on room air. The HEENT, lung, heart, and abdominal examinations were all normal. Standing at the foot of the bed, the patient had obvious shortening, internal rotation, and adduction of the left leg. The left knee was without tenderness or swelling. The neurovascular examination of the left lower extremity was completely normal.
Plain radiographs of the pelvis and left hip ordered by the EP demonstrated a posterior hip dislocation with intact hardware. The EP consulted the patient’s orthopedic physician, and both agreed the EP should attempt to reduce the dislocation in the ED. Using conscious sedation, the EP was able to reduce the dislocation, but postreduction films demonstrated a new fracture requiring orthopedic surgery. Unfortunately, the patient had a very difficult recovery, ultimately resulting in death.
The patient’s estate sued the EP, stating he should have had the orthopedic physician reduce the dislocation. The defense argued that fracture is a known complication of reduction of a dislocated hip. A defense verdict was returned.
Discussion
Approximately 85% to 90% of hip dislocations are posterior; the remaining 10% are anterior. Posterior hip dislocations are a common complication following total hip-replacement surgery.1 Hip dislocation is a true orthopedic and time-dependent emergency. The longer the hip remains dislocated, the more likely complications are to occur, including osteonecrosis of the femoral head, arthritic degeneration of the hip joint, and long-term neurological sequelae.2 The treatment of posterior hip dislocation (without fracture) is closed reduction as quickly as possible, and preferably within 6 hours.3 As this case demonstrates, minimal forces can result in a hip dislocation following a total hip replacement. In healthy patients, however, significant forces (eg, high-speed motor vehicle crashes) are required to cause posterior hip dislocation.
Patients with a posterior hip dislocation will present in severe pain and an inability to ambulate. In most cases of posterior hip dislocation, the affected lower extremity will be visibly shortened, internally rotated, and adducted. The knee should always be examined for injury, as well as performance of a thorough neurovascular examination of the affected extremity.
Plain X-ray films will usually identify a posterior hip dislocation. On an anteroposterior pelvis X-ray, the femoral head will be seen outside and just superior to the acetabulum. Special attention should be made to the acetabulum to ensure a concomitant acetabular fracture is not missed.
Indications for closed reduction of a posterior hip dislocation include dislocation with or without neurological deficit and no associated fracture, or dislocation with an associated fracture if no neurological deficits are present.2 An open traumatic hip dislocation should only be reduced in the operating room.
It is certainly within the purview of the EP to attempt a closed reduction for a posterior hip dislocation if no contraindications exist. The patient will need to be sedated (ie, procedural sedation, conscious sedation, or moderate sedation) for any chance of success at reduction. While it is beyond the scope of this article to review the various techniques used to reduce a posterior hip dislocation, one of the guiding principles is that after two or three unsuccessful attempts by the EP to reduce the dislocation, no further attempts should be made and orthopedic surgery services should be consulted. This is because the risk of complications increases as the number of failed attempts increase.
It is unclear how many attempts the EP made in this case. Fracture is a known complication when attempting reduction for a hip dislocation, be it an orthopedic surgeon or an EP. It was certainly appropriate for the EP in this case to attempt closed reduction, given the importance of timely reduction.
Traumatic Back Pain
An 84-year-old man with low-back pain following a motor vehicle crash was brought to the ED by emergency medical services (EMS). He had been the restrained driver, stopped at a traffic light, when he was struck from behind by a second vehicle.
In the ED, the patient only complained of low-back pain. He denied any radiation of pain or lower-extremity numbness or weakness. He also denied any head injury, loss of consciousness, neck pain, or abdominal pain. His past medical history was significant for hypertension, arthritis, and coronary artery disease.
On physical examination, the patient’s vital signs were normal. The head, eyes, ears, nose, and throat (HEENT) examination was also normal; specifically, there was no tenderness to palpation of the cervical spine in the posterior midline. Regarding the cardiopulmonary examination, auscultation of the lungs revealed clear, bilateral breath sounds; the heart examination was normal. The patient had a soft abdomen, without tenderness, guarding, or rebound. His pelvis was stable, but he did exhibit some tenderness on palpation of the lower-thoracic and upper-lumbar spine. The neurological examination revealed normal motor strength and sensation in the lower extremities.
The emergency physician (EP) ordered X-rays of the thoracic and lumbar spine and a urinalysis. The films were interpreted by both the EP and radiologist as normal; the results of the urinalysis were also normal. The patient was diagnosed with a lower back strain secondary to the motor vehicle crash and was discharged home with an analgesic.
The next day, however, the patient began to complain of increased back pain and lower-extremity numbness and weakness. He was brought back to the same hospital ED where he was noted to have severe weakness of both lower extremities and decreased sensation to touch. Additional imaging was performed, which demonstrated a fracture of T11 with spinal cord impingement. He was taken to surgery, but unfortunately the injury was permanent, and the patient was left with lower-extremity paralysis and bowel and bladder incontinence.
The plaintiff sued the EP and the radiologist for not properly interpreting the initial X-rays. The defendants denied liability, asserting the patient’s injury was a result of the collision and that nothing could have prevented it. According to a published account, the jury returned a verdict finding the EP to be 40% at fault and the radiologist 60% at fault.
Discussion
Emergency physicians frequently manage patients experiencing pain or injury following a motor vehicle crash. If the patient is complaining of neck or back pain, the prehospital providers will immobilize the patient with a rigid cervical collar (ie, if neck pain is present) and a long backboard if pain anywhere along the spine is present (ie, cervical, thoracic, or lumbar).
When the initial airway, breathing, circulation, and disability assessment for the trauma patient is performed and found to be normal, a secondary examination should be performed. Trauma patients with back pain should be log-rolled onto their side, with spinal immobilization followed by visual inspection and palpation/percussion of the midline of the thoracic and lumbar spine. The presence of midline tenderness suggests an acute injury and the need to keep the patient immobilized. Patients should be removed off the backboard and onto the gurney mattress while immobilizing the spine. The standard hospital mattress provides acceptable spinal support.1
Historically, plain radiographs of the thoracic and lumbar spine have been the imaging test of choice in the initial evaluation of suspected traumatic spinal column injury. However, similar to cervical spine trauma, computed tomography (CT) is assuming a larger role in the evaluation of patients with suspected thoracic or lumbar spine injury. When thoracic and abdominal CT scans are performed to evaluate for possible chest or abdominal trauma, those images can be reformatted and used to reconstruct images of the thoracic and lumbar spine, significantly reducing radiation exposure.1 While CT is the gold standard imaging study for evaluation of bony or ligamentous injury of the spine, magnetic resonance imaging (MRI) is the study of choice for patients with neurological deficits or suspected spinal cord injury.
This patient had a completely normal neurological examination at initial presentation, so there was no indication for an MRI. The bony injury to T11 must have been very subtle for both the EP and the radiologist to have missed it. Unfortunately, the jury appears to have used the standard of “perfection,” rather than the “reasonable and prudent physician” in judging that the injury should have been detected. This case serves as a reminder that EPs cannot rely on consulting specialists to consistently and reliably provide accurate information. Moreover, this case emphasizes the need to consider CT imaging of the spine in the evaluation of patients with severe back pain of traumatic origin when plain radiographs appear normal.
Hip-Reduction Problem
A 79-year-old man with left hip pain presented to the ED via EMS. The patient stated that when he had bent over to retrieve his dropped glasses, he experienced the immediate onset of left hip pain and fell to the floor. He was unable to get up on his own and called EMS. The patient had undergone total left hip replacement 1 month prior. At presentation, he complained only of severe pain in his left hip; he denied head injury, neck pain or stiffness, chest pain, or abdominal pain. His past medical history was significant for hypertension and type 2 diabetes mellitus. The patient had no known drug allergies.
On physical examination, he was mildly tachycardic. His vital signs were: heart rate, 102 beats/minute; blood pressure, 156/88 mm Hg; respiratory rate, 20 breaths/minutes; and temperature, afebrile. His pulse oximetry was 98% on room air. The HEENT, lung, heart, and abdominal examinations were all normal. Standing at the foot of the bed, the patient had obvious shortening, internal rotation, and adduction of the left leg. The left knee was without tenderness or swelling. The neurovascular examination of the left lower extremity was completely normal.
Plain radiographs of the pelvis and left hip ordered by the EP demonstrated a posterior hip dislocation with intact hardware. The EP consulted the patient’s orthopedic physician, and both agreed the EP should attempt to reduce the dislocation in the ED. Using conscious sedation, the EP was able to reduce the dislocation, but postreduction films demonstrated a new fracture requiring orthopedic surgery. Unfortunately, the patient had a very difficult recovery, ultimately resulting in death.
The patient’s estate sued the EP, stating he should have had the orthopedic physician reduce the dislocation. The defense argued that fracture is a known complication of reduction of a dislocated hip. A defense verdict was returned.
Discussion
Approximately 85% to 90% of hip dislocations are posterior; the remaining 10% are anterior. Posterior hip dislocations are a common complication following total hip-replacement surgery.1 Hip dislocation is a true orthopedic and time-dependent emergency. The longer the hip remains dislocated, the more likely complications are to occur, including osteonecrosis of the femoral head, arthritic degeneration of the hip joint, and long-term neurological sequelae.2 The treatment of posterior hip dislocation (without fracture) is closed reduction as quickly as possible, and preferably within 6 hours.3 As this case demonstrates, minimal forces can result in a hip dislocation following a total hip replacement. In healthy patients, however, significant forces (eg, high-speed motor vehicle crashes) are required to cause posterior hip dislocation.
Patients with a posterior hip dislocation will present in severe pain and an inability to ambulate. In most cases of posterior hip dislocation, the affected lower extremity will be visibly shortened, internally rotated, and adducted. The knee should always be examined for injury, as well as performance of a thorough neurovascular examination of the affected extremity.
Plain X-ray films will usually identify a posterior hip dislocation. On an anteroposterior pelvis X-ray, the femoral head will be seen outside and just superior to the acetabulum. Special attention should be made to the acetabulum to ensure a concomitant acetabular fracture is not missed.
Indications for closed reduction of a posterior hip dislocation include dislocation with or without neurological deficit and no associated fracture, or dislocation with an associated fracture if no neurological deficits are present.2 An open traumatic hip dislocation should only be reduced in the operating room.
It is certainly within the purview of the EP to attempt a closed reduction for a posterior hip dislocation if no contraindications exist. The patient will need to be sedated (ie, procedural sedation, conscious sedation, or moderate sedation) for any chance of success at reduction. While it is beyond the scope of this article to review the various techniques used to reduce a posterior hip dislocation, one of the guiding principles is that after two or three unsuccessful attempts by the EP to reduce the dislocation, no further attempts should be made and orthopedic surgery services should be consulted. This is because the risk of complications increases as the number of failed attempts increase.
It is unclear how many attempts the EP made in this case. Fracture is a known complication when attempting reduction for a hip dislocation, be it an orthopedic surgeon or an EP. It was certainly appropriate for the EP in this case to attempt closed reduction, given the importance of timely reduction.
Reference (Traumatic Back Pain)
- Baron BJ, McSherry KJ, Larson JL, Scalea TM. Spinal and spinal cord trauma In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1709-1730.
(Hip-Reduction Problem)
- Dela Cruz JE, Sullivan DN, Varboncouer E, et al. Comparison of proceduralsedation for the reduction of dislocated total hip arthroplasty.West J Emerg Med. 2014:15(1):76-80.
- Davenport M. Joint reduction, hip dislocation, posterior. Medscape Web site. eMedicine.medscape.com/article/109225. Updated February 11, 2014. Accessed January 27, 2015.
- Steele MT, Stubbs AM. Hip and femur injuries. In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1848-1856.
Reference (Traumatic Back Pain)
- Baron BJ, McSherry KJ, Larson JL, Scalea TM. Spinal and spinal cord trauma In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1709-1730.
(Hip-Reduction Problem)
- Dela Cruz JE, Sullivan DN, Varboncouer E, et al. Comparison of proceduralsedation for the reduction of dislocated total hip arthroplasty.West J Emerg Med. 2014:15(1):76-80.
- Davenport M. Joint reduction, hip dislocation, posterior. Medscape Web site. eMedicine.medscape.com/article/109225. Updated February 11, 2014. Accessed January 27, 2015.
- Steele MT, Stubbs AM. Hip and femur injuries. In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1848-1856.



