User login
New test can better predict cytokine storm, team says

Photo by Juan D. Alfonso
Scientists have developed a test that uses cells from a single donor’s blood to predict whether a new drug will cause a cytokine storm.
The group says this is an improvement over current tests, which use endothelial cells and peripheral blood mononuclear cells (PBMCs) from two separate donors and can therefore produce inaccurate results.
Furthermore, current tests cannot differentiate drugs that induce a mild cytokine storm from those that induce a severe one, but the new test can.
Jane Mitchell, PhD, of the National Heart and Lung Institute at Imperial College London in the UK, and her colleagues described the new test in The FASEB Journal.
Current tests for cytokine storm reactions use endothelial cells taken from the vessels of one donor and PBMCs from a different donor because endothelial cells are normally only grown from tissue removed in surgery or post-mortem, or from umbilical vessels after birth.
When cells from two different donors are used, one may have an immune reaction to the other. And this can result in the test falsely showing a severe immune reaction to a drug that is safe.
Dr Mitchell and her colleagues say they have solved this problem by isolating stem cells from the blood of a volunteer and using them to grow endothelial cells in a dish. The team then added PBMCs to the donor’s own endothelial cells to recreate the unique conditions found in their blood vessels.
When the scientists added the immunomodulatory drug TGN1412, the mixture of cells released a cytokine storm, as would happen inside the human body.
Responses to other drugs were consistent with those observed in humans as well. There was a modest response to alemtuzumab (Campath) and no response to the control antibodies trastuzumab (Herceptin), bevacizumab (Avastin), and ofatumumab (Arzerra).
“As biological therapies become more mainstream, it’s more likely that drugs being tested on humans for the first time will have unexpected and potentially catastrophic effects,” Dr Mitchell said.
“We’ve used adult stem cell technology to develop a laboratory test that could prevent another disaster like the TGN1412 trial [in which 6 healthy young men developed multi-organ failure]. Drug companies have the technical capacity to start using this test now, but we’re working on developing an off-the-shelf kit, which will make it easy to use on a large scale.”
The team has collaborated with the National Institute for Biological Standards and Control to validate the test and are now working with the clinical trials company Quintiles to develop the technology further. ![]()

Photo by Juan D. Alfonso
Scientists have developed a test that uses cells from a single donor’s blood to predict whether a new drug will cause a cytokine storm.
The group says this is an improvement over current tests, which use endothelial cells and peripheral blood mononuclear cells (PBMCs) from two separate donors and can therefore produce inaccurate results.
Furthermore, current tests cannot differentiate drugs that induce a mild cytokine storm from those that induce a severe one, but the new test can.
Jane Mitchell, PhD, of the National Heart and Lung Institute at Imperial College London in the UK, and her colleagues described the new test in The FASEB Journal.
Current tests for cytokine storm reactions use endothelial cells taken from the vessels of one donor and PBMCs from a different donor because endothelial cells are normally only grown from tissue removed in surgery or post-mortem, or from umbilical vessels after birth.
When cells from two different donors are used, one may have an immune reaction to the other. And this can result in the test falsely showing a severe immune reaction to a drug that is safe.
Dr Mitchell and her colleagues say they have solved this problem by isolating stem cells from the blood of a volunteer and using them to grow endothelial cells in a dish. The team then added PBMCs to the donor’s own endothelial cells to recreate the unique conditions found in their blood vessels.
When the scientists added the immunomodulatory drug TGN1412, the mixture of cells released a cytokine storm, as would happen inside the human body.
Responses to other drugs were consistent with those observed in humans as well. There was a modest response to alemtuzumab (Campath) and no response to the control antibodies trastuzumab (Herceptin), bevacizumab (Avastin), and ofatumumab (Arzerra).
“As biological therapies become more mainstream, it’s more likely that drugs being tested on humans for the first time will have unexpected and potentially catastrophic effects,” Dr Mitchell said.
“We’ve used adult stem cell technology to develop a laboratory test that could prevent another disaster like the TGN1412 trial [in which 6 healthy young men developed multi-organ failure]. Drug companies have the technical capacity to start using this test now, but we’re working on developing an off-the-shelf kit, which will make it easy to use on a large scale.”
The team has collaborated with the National Institute for Biological Standards and Control to validate the test and are now working with the clinical trials company Quintiles to develop the technology further. ![]()

Photo by Juan D. Alfonso
Scientists have developed a test that uses cells from a single donor’s blood to predict whether a new drug will cause a cytokine storm.
The group says this is an improvement over current tests, which use endothelial cells and peripheral blood mononuclear cells (PBMCs) from two separate donors and can therefore produce inaccurate results.
Furthermore, current tests cannot differentiate drugs that induce a mild cytokine storm from those that induce a severe one, but the new test can.
Jane Mitchell, PhD, of the National Heart and Lung Institute at Imperial College London in the UK, and her colleagues described the new test in The FASEB Journal.
Current tests for cytokine storm reactions use endothelial cells taken from the vessels of one donor and PBMCs from a different donor because endothelial cells are normally only grown from tissue removed in surgery or post-mortem, or from umbilical vessels after birth.
When cells from two different donors are used, one may have an immune reaction to the other. And this can result in the test falsely showing a severe immune reaction to a drug that is safe.
Dr Mitchell and her colleagues say they have solved this problem by isolating stem cells from the blood of a volunteer and using them to grow endothelial cells in a dish. The team then added PBMCs to the donor’s own endothelial cells to recreate the unique conditions found in their blood vessels.
When the scientists added the immunomodulatory drug TGN1412, the mixture of cells released a cytokine storm, as would happen inside the human body.
Responses to other drugs were consistent with those observed in humans as well. There was a modest response to alemtuzumab (Campath) and no response to the control antibodies trastuzumab (Herceptin), bevacizumab (Avastin), and ofatumumab (Arzerra).
“As biological therapies become more mainstream, it’s more likely that drugs being tested on humans for the first time will have unexpected and potentially catastrophic effects,” Dr Mitchell said.
“We’ve used adult stem cell technology to develop a laboratory test that could prevent another disaster like the TGN1412 trial [in which 6 healthy young men developed multi-organ failure]. Drug companies have the technical capacity to start using this test now, but we’re working on developing an off-the-shelf kit, which will make it easy to use on a large scale.”
The team has collaborated with the National Institute for Biological Standards and Control to validate the test and are now working with the clinical trials company Quintiles to develop the technology further. ![]()
Schizophrenia patients twice as likely to be at risk for DVT and PE
Schizophrenia patients exhibited a twofold higher adjusted risk of deep vein thrombosis and pulmonary embolism development than those without schizophrenia, according to research by Dr. Wen-Yu Hsu of China Medical University in Taiwan published in Schizophrenia Research.
The schizophrenia cohort exhibited a 2.02-fold higher adjusted hazard ratio (HR) for developing DVT and a 1.99-fold higher adjusted HR for developing PE. The population-based cohort study included 60,264 schizophrenia patients in Taiwan, compared with 60,264 control patients, obtained from the National Health Insurance Research Database in Taiwan from 1996 to 2011.
The researchers identified several lifestyle factors that could contribute to the development of DVT and PE, including a decrease in activities of daily living because of either antipsychotics or negative symptoms of schizophrenia, higher rates of smoking and metabolic syndrome among schizophrenia patients, and prolonged antipsychotic exposure. In addition, patients who are immobile could be at greater risk of developing DVT and PE. “Based on the findings of this study, receiving first-generation antipsychotics or receiving second-generation antipsychotics should be considered a contributing factor of DVT and PE development,” the researchers said.
Read more here: (Schizophr. Res. 2015;162:248-52 [doi:10.1016/j.schres.2015.01.012]).
Schizophrenia patients exhibited a twofold higher adjusted risk of deep vein thrombosis and pulmonary embolism development than those without schizophrenia, according to research by Dr. Wen-Yu Hsu of China Medical University in Taiwan published in Schizophrenia Research.
The schizophrenia cohort exhibited a 2.02-fold higher adjusted hazard ratio (HR) for developing DVT and a 1.99-fold higher adjusted HR for developing PE. The population-based cohort study included 60,264 schizophrenia patients in Taiwan, compared with 60,264 control patients, obtained from the National Health Insurance Research Database in Taiwan from 1996 to 2011.
The researchers identified several lifestyle factors that could contribute to the development of DVT and PE, including a decrease in activities of daily living because of either antipsychotics or negative symptoms of schizophrenia, higher rates of smoking and metabolic syndrome among schizophrenia patients, and prolonged antipsychotic exposure. In addition, patients who are immobile could be at greater risk of developing DVT and PE. “Based on the findings of this study, receiving first-generation antipsychotics or receiving second-generation antipsychotics should be considered a contributing factor of DVT and PE development,” the researchers said.
Read more here: (Schizophr. Res. 2015;162:248-52 [doi:10.1016/j.schres.2015.01.012]).
Schizophrenia patients exhibited a twofold higher adjusted risk of deep vein thrombosis and pulmonary embolism development than those without schizophrenia, according to research by Dr. Wen-Yu Hsu of China Medical University in Taiwan published in Schizophrenia Research.
The schizophrenia cohort exhibited a 2.02-fold higher adjusted hazard ratio (HR) for developing DVT and a 1.99-fold higher adjusted HR for developing PE. The population-based cohort study included 60,264 schizophrenia patients in Taiwan, compared with 60,264 control patients, obtained from the National Health Insurance Research Database in Taiwan from 1996 to 2011.
The researchers identified several lifestyle factors that could contribute to the development of DVT and PE, including a decrease in activities of daily living because of either antipsychotics or negative symptoms of schizophrenia, higher rates of smoking and metabolic syndrome among schizophrenia patients, and prolonged antipsychotic exposure. In addition, patients who are immobile could be at greater risk of developing DVT and PE. “Based on the findings of this study, receiving first-generation antipsychotics or receiving second-generation antipsychotics should be considered a contributing factor of DVT and PE development,” the researchers said.
Read more here: (Schizophr. Res. 2015;162:248-52 [doi:10.1016/j.schres.2015.01.012]).
What’s Eating You? Cutaneous Larva Migrans
Cutaneous larva migrans (CLM), also known as creeping eruption, is a pruritic serpiginous eruption caused by the migration of animal hookworm larvae through the epidermis.1,2 The most common parasites are Ancylostoma braziliense (common in dogs and cats) and Ancylostoma caninum (common in dogs).1
Disease Transmission
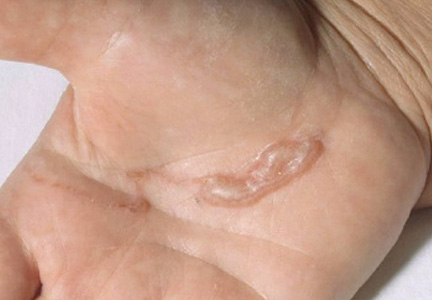
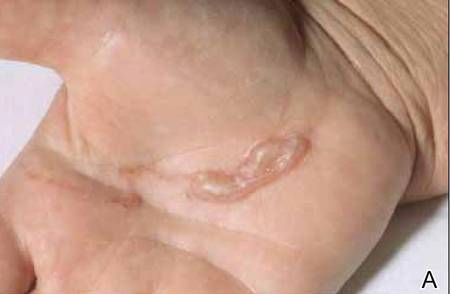
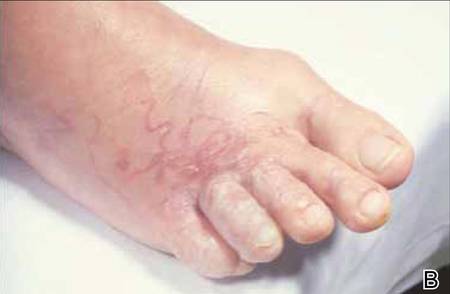
The infection is typically acquired in warm climates and tropical areas after coming in direct contact with sand or soil that is contaminated with animal feces. Therefore, the eruption most commonly occurs as a single or unilateral erythematous, pruritic, serpiginous tract on the feet, hands, or buttocks (Figure).2 The larval tract typically migrates at a rate of 1 to 2 cm per day,3 which is in contrast to the serpiginous urticarial rash of larva currens of strongyloidiasis that can travel up to 10 cm per hour.4
|
Clinical Presentation
Rarely, CLM can present with bilateral lesions5; in severe cases a single patient can have hundreds of lesions. It also may present as folliculitis and urticarial papules.6 Shih et al7 reported a patient with CLM that presented as a diffuse papular urticarialike eruption following a trip to Thailand. This case may represent an underdiagnosed presentation of CLM. Patients with a history of exposure to contaminated sand or soil diffusely on the body may exhibit lesions in less classic locations, such as the trunk and upper proximal extremities.3
Cutaneous larva migrans is a self-limited eruption, as the larvae cannot complete their lifecycles in the human body and typically die within 2 to 8 weeks.2 However, rare cases lasting up to a year have been reported.3 Sarasombath and Young2 reported a case of CLM that persisted for 4 months with intermittent symptoms characterized by several weeklong intervals with no symptoms or visible rash.
Cutaneous larva migrans typically presents with isolated dermatologic symptoms. Rare cases associated with Löffler syndrome characterized by migratory pulmonary infiltrates and peripheral eosinophilia have been reported.8 Two proposed mechanisms for pulmonary involvement include direct invasion of the lungs by the helminths and a systemic immunologic process triggered by the helminths, resulting in eosinophilic pulmonary infiltration.9
Diagnosis
Cutaneous larva migrans is a clinical diagnosis and skin biopsy usually is not obtained because the larvae often are located 1 to 2 cm beyond the visible erythematous border.3,5 Rarely, the parasites are found on biopsy, revealing larvae that are 0.5-mm thick and up to 10-mm long.10 The larvae typically are confined to the deep epidermis because the parasite lacks the collagenase required to penetrate the basement membrane.2
Langley et al11 showed that confocal scanning laser microscopy can be an effective method for identifying the highly refractile oval larva that disrupt the normal honeycomb pattern of the epidermis. Performing a 4-mm punch biopsy over the identified site can allow for precise excision and treatment of the intact hookworm larvae of CLM. There also are limited reports of dermoscopy being used to facilitate diagnosis of CLM.12 Dermoscopic features of CLM include translucent, brown, structureless areas in a segmental arrangement corresponding to the larval bodies and red-dotted vessels corresponding to an empty burrow.13 However, Zalaudek et al13 concluded that the efficacy of dermoscopy in aiding in the diagnosis of CLM has not been fully established.
Treatment
Cutaneous larva migrans is a self-limited condition that often resolves within 2 to 8 weeks; however, pruritus can be intense and patients therefore are seldom willing to forego treatment. Treatment options include a single oral dose of albendazole 400 mg in adults, with increased efficacy if administered daily for 3 to 5 days (or 10–15 mg/kg, with a maximum dose of 800 mg daily in children), a single oral dose of ivermectin 12 mg in adults (or 150 µg/kg in children), or topical application of thiabendazole 10% to 15% three times daily for at least 15 days.14 Cases of CLM complicated by Löffler syndrome may require a longer treatment course, such as a 7-day course of albendazole 400 mg daily. Tan and Liu9 reported a case of CLM complicated by Löffler syndrome that was successfully treated with albendazole. In this patient, initial treatment with 2 courses of mebendazole (3 days each for a total of 6 days) resulted in improvement of cutaneous lesions but not the pulmonary infiltrate. A subsequent prolonged course of albendazole and intravenous hydrocortisone for 5 days resulted in complete resolution of the pulmonary infiltrate and peripheral eosinophilia. The authors concluded that inadequacy of treatment with mebendazole may be related to differences in the rate of absorption and efficacy when compared to albendazole.9
Conclusion
Cutaneous larva migrans is a self-limited and pruritic skin eruption that is acquired after direct inoculation with sand or soil that is contaminated with feces containing A braziliense or A caninum. Although the classic presentation is readily identifiable, there are a variety of atypical presentations that may go undiagnosed. Symptomatic relief usually can be achieved with short courses of oral or topical antihelminth medications.
1. Berlin JM, Goldberg SJ, McDonough RD, et al. JAAD grand rounds quiz. serpiginous eruption on the leg. J Am Acad Dermatol. 2010;63:921-922.
2. Sarasombath PA, Young PK. An unusual presentation of cutaneous larva migrans. Arch Dermatol. 2007;143:955.
3. Patel S, Aboutalebi S, Vindhya PL, et al. What’s eating you? extensive cutaneous larva migrans (Ancylostoma braziliense). Cutis. 2008;82:239-240.
4. Elston DM, Czarnik K, Brockett R, et al. What’s eating you? Strongyloides stercoralis. Cutis. 2003;71:22-24.
5. Duarte De Sousa ICV, De La Pascua L. Bilateral cutaneous larva migrans [poster reference number 4677]. J Am Acad Dermatol. 2012;66(4, suppl 1):AB106.
6. Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of seven cases and review of the literature. Br J Dermatol. 2002;146:314-316.
7. Shih PY, Hsieh MY, Huang YH, et al. Multiple pruritic erythematous papules on the trunk after a trip to Thailand–quiz case. Arch Dermatol. 2010;146:557-562.
8. Wright DO, Gold ED. Löffler’s syndrome associated with creeping eruption (cutaneous helminthiasis): report of twenty-six cases. Arch Intern Med. 1946;78:303-312.
9. Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler’s syndrome. Arch Dermatol. 2010;146:210-212.
10. Rapini RP, ed. Practical Dermatopathology. Philadelphia, PA: Elsevier; 2005.
11. Langley R, Webb A, Haldane D, et al. Confocal microscopy of cutaneous larva migrans. J Am Acad Dermatol. 2011;64(2, suppl 1):AB100.
12. Aljasser MI, Lui H, Zeng H, et al. Dermoscopy and near-infrared fluorescence imaging of cutaneous larva migrans. Photodermatol Photoimmunol Photomed. 2013;29:337-338.
13. Zalaudek I, Giacomel J, Cabo H, et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology. 2008;216:14-23.
14. Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814.
Cutaneous larva migrans (CLM), also known as creeping eruption, is a pruritic serpiginous eruption caused by the migration of animal hookworm larvae through the epidermis.1,2 The most common parasites are Ancylostoma braziliense (common in dogs and cats) and Ancylostoma caninum (common in dogs).1
Disease Transmission
The infection is typically acquired in warm climates and tropical areas after coming in direct contact with sand or soil that is contaminated with animal feces. Therefore, the eruption most commonly occurs as a single or unilateral erythematous, pruritic, serpiginous tract on the feet, hands, or buttocks (Figure).2 The larval tract typically migrates at a rate of 1 to 2 cm per day,3 which is in contrast to the serpiginous urticarial rash of larva currens of strongyloidiasis that can travel up to 10 cm per hour.4


|
Clinical Presentation
Rarely, CLM can present with bilateral lesions5; in severe cases a single patient can have hundreds of lesions. It also may present as folliculitis and urticarial papules.6 Shih et al7 reported a patient with CLM that presented as a diffuse papular urticarialike eruption following a trip to Thailand. This case may represent an underdiagnosed presentation of CLM. Patients with a history of exposure to contaminated sand or soil diffusely on the body may exhibit lesions in less classic locations, such as the trunk and upper proximal extremities.3
Cutaneous larva migrans is a self-limited eruption, as the larvae cannot complete their lifecycles in the human body and typically die within 2 to 8 weeks.2 However, rare cases lasting up to a year have been reported.3 Sarasombath and Young2 reported a case of CLM that persisted for 4 months with intermittent symptoms characterized by several weeklong intervals with no symptoms or visible rash.
Cutaneous larva migrans typically presents with isolated dermatologic symptoms. Rare cases associated with Löffler syndrome characterized by migratory pulmonary infiltrates and peripheral eosinophilia have been reported.8 Two proposed mechanisms for pulmonary involvement include direct invasion of the lungs by the helminths and a systemic immunologic process triggered by the helminths, resulting in eosinophilic pulmonary infiltration.9
Diagnosis
Cutaneous larva migrans is a clinical diagnosis and skin biopsy usually is not obtained because the larvae often are located 1 to 2 cm beyond the visible erythematous border.3,5 Rarely, the parasites are found on biopsy, revealing larvae that are 0.5-mm thick and up to 10-mm long.10 The larvae typically are confined to the deep epidermis because the parasite lacks the collagenase required to penetrate the basement membrane.2
Langley et al11 showed that confocal scanning laser microscopy can be an effective method for identifying the highly refractile oval larva that disrupt the normal honeycomb pattern of the epidermis. Performing a 4-mm punch biopsy over the identified site can allow for precise excision and treatment of the intact hookworm larvae of CLM. There also are limited reports of dermoscopy being used to facilitate diagnosis of CLM.12 Dermoscopic features of CLM include translucent, brown, structureless areas in a segmental arrangement corresponding to the larval bodies and red-dotted vessels corresponding to an empty burrow.13 However, Zalaudek et al13 concluded that the efficacy of dermoscopy in aiding in the diagnosis of CLM has not been fully established.
Treatment
Cutaneous larva migrans is a self-limited condition that often resolves within 2 to 8 weeks; however, pruritus can be intense and patients therefore are seldom willing to forego treatment. Treatment options include a single oral dose of albendazole 400 mg in adults, with increased efficacy if administered daily for 3 to 5 days (or 10–15 mg/kg, with a maximum dose of 800 mg daily in children), a single oral dose of ivermectin 12 mg in adults (or 150 µg/kg in children), or topical application of thiabendazole 10% to 15% three times daily for at least 15 days.14 Cases of CLM complicated by Löffler syndrome may require a longer treatment course, such as a 7-day course of albendazole 400 mg daily. Tan and Liu9 reported a case of CLM complicated by Löffler syndrome that was successfully treated with albendazole. In this patient, initial treatment with 2 courses of mebendazole (3 days each for a total of 6 days) resulted in improvement of cutaneous lesions but not the pulmonary infiltrate. A subsequent prolonged course of albendazole and intravenous hydrocortisone for 5 days resulted in complete resolution of the pulmonary infiltrate and peripheral eosinophilia. The authors concluded that inadequacy of treatment with mebendazole may be related to differences in the rate of absorption and efficacy when compared to albendazole.9
Conclusion
Cutaneous larva migrans is a self-limited and pruritic skin eruption that is acquired after direct inoculation with sand or soil that is contaminated with feces containing A braziliense or A caninum. Although the classic presentation is readily identifiable, there are a variety of atypical presentations that may go undiagnosed. Symptomatic relief usually can be achieved with short courses of oral or topical antihelminth medications.
Cutaneous larva migrans (CLM), also known as creeping eruption, is a pruritic serpiginous eruption caused by the migration of animal hookworm larvae through the epidermis.1,2 The most common parasites are Ancylostoma braziliense (common in dogs and cats) and Ancylostoma caninum (common in dogs).1
Disease Transmission
The infection is typically acquired in warm climates and tropical areas after coming in direct contact with sand or soil that is contaminated with animal feces. Therefore, the eruption most commonly occurs as a single or unilateral erythematous, pruritic, serpiginous tract on the feet, hands, or buttocks (Figure).2 The larval tract typically migrates at a rate of 1 to 2 cm per day,3 which is in contrast to the serpiginous urticarial rash of larva currens of strongyloidiasis that can travel up to 10 cm per hour.4


|
Clinical Presentation
Rarely, CLM can present with bilateral lesions5; in severe cases a single patient can have hundreds of lesions. It also may present as folliculitis and urticarial papules.6 Shih et al7 reported a patient with CLM that presented as a diffuse papular urticarialike eruption following a trip to Thailand. This case may represent an underdiagnosed presentation of CLM. Patients with a history of exposure to contaminated sand or soil diffusely on the body may exhibit lesions in less classic locations, such as the trunk and upper proximal extremities.3
Cutaneous larva migrans is a self-limited eruption, as the larvae cannot complete their lifecycles in the human body and typically die within 2 to 8 weeks.2 However, rare cases lasting up to a year have been reported.3 Sarasombath and Young2 reported a case of CLM that persisted for 4 months with intermittent symptoms characterized by several weeklong intervals with no symptoms or visible rash.
Cutaneous larva migrans typically presents with isolated dermatologic symptoms. Rare cases associated with Löffler syndrome characterized by migratory pulmonary infiltrates and peripheral eosinophilia have been reported.8 Two proposed mechanisms for pulmonary involvement include direct invasion of the lungs by the helminths and a systemic immunologic process triggered by the helminths, resulting in eosinophilic pulmonary infiltration.9
Diagnosis
Cutaneous larva migrans is a clinical diagnosis and skin biopsy usually is not obtained because the larvae often are located 1 to 2 cm beyond the visible erythematous border.3,5 Rarely, the parasites are found on biopsy, revealing larvae that are 0.5-mm thick and up to 10-mm long.10 The larvae typically are confined to the deep epidermis because the parasite lacks the collagenase required to penetrate the basement membrane.2
Langley et al11 showed that confocal scanning laser microscopy can be an effective method for identifying the highly refractile oval larva that disrupt the normal honeycomb pattern of the epidermis. Performing a 4-mm punch biopsy over the identified site can allow for precise excision and treatment of the intact hookworm larvae of CLM. There also are limited reports of dermoscopy being used to facilitate diagnosis of CLM.12 Dermoscopic features of CLM include translucent, brown, structureless areas in a segmental arrangement corresponding to the larval bodies and red-dotted vessels corresponding to an empty burrow.13 However, Zalaudek et al13 concluded that the efficacy of dermoscopy in aiding in the diagnosis of CLM has not been fully established.
Treatment
Cutaneous larva migrans is a self-limited condition that often resolves within 2 to 8 weeks; however, pruritus can be intense and patients therefore are seldom willing to forego treatment. Treatment options include a single oral dose of albendazole 400 mg in adults, with increased efficacy if administered daily for 3 to 5 days (or 10–15 mg/kg, with a maximum dose of 800 mg daily in children), a single oral dose of ivermectin 12 mg in adults (or 150 µg/kg in children), or topical application of thiabendazole 10% to 15% three times daily for at least 15 days.14 Cases of CLM complicated by Löffler syndrome may require a longer treatment course, such as a 7-day course of albendazole 400 mg daily. Tan and Liu9 reported a case of CLM complicated by Löffler syndrome that was successfully treated with albendazole. In this patient, initial treatment with 2 courses of mebendazole (3 days each for a total of 6 days) resulted in improvement of cutaneous lesions but not the pulmonary infiltrate. A subsequent prolonged course of albendazole and intravenous hydrocortisone for 5 days resulted in complete resolution of the pulmonary infiltrate and peripheral eosinophilia. The authors concluded that inadequacy of treatment with mebendazole may be related to differences in the rate of absorption and efficacy when compared to albendazole.9
Conclusion
Cutaneous larva migrans is a self-limited and pruritic skin eruption that is acquired after direct inoculation with sand or soil that is contaminated with feces containing A braziliense or A caninum. Although the classic presentation is readily identifiable, there are a variety of atypical presentations that may go undiagnosed. Symptomatic relief usually can be achieved with short courses of oral or topical antihelminth medications.
1. Berlin JM, Goldberg SJ, McDonough RD, et al. JAAD grand rounds quiz. serpiginous eruption on the leg. J Am Acad Dermatol. 2010;63:921-922.
2. Sarasombath PA, Young PK. An unusual presentation of cutaneous larva migrans. Arch Dermatol. 2007;143:955.
3. Patel S, Aboutalebi S, Vindhya PL, et al. What’s eating you? extensive cutaneous larva migrans (Ancylostoma braziliense). Cutis. 2008;82:239-240.
4. Elston DM, Czarnik K, Brockett R, et al. What’s eating you? Strongyloides stercoralis. Cutis. 2003;71:22-24.
5. Duarte De Sousa ICV, De La Pascua L. Bilateral cutaneous larva migrans [poster reference number 4677]. J Am Acad Dermatol. 2012;66(4, suppl 1):AB106.
6. Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of seven cases and review of the literature. Br J Dermatol. 2002;146:314-316.
7. Shih PY, Hsieh MY, Huang YH, et al. Multiple pruritic erythematous papules on the trunk after a trip to Thailand–quiz case. Arch Dermatol. 2010;146:557-562.
8. Wright DO, Gold ED. Löffler’s syndrome associated with creeping eruption (cutaneous helminthiasis): report of twenty-six cases. Arch Intern Med. 1946;78:303-312.
9. Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler’s syndrome. Arch Dermatol. 2010;146:210-212.
10. Rapini RP, ed. Practical Dermatopathology. Philadelphia, PA: Elsevier; 2005.
11. Langley R, Webb A, Haldane D, et al. Confocal microscopy of cutaneous larva migrans. J Am Acad Dermatol. 2011;64(2, suppl 1):AB100.
12. Aljasser MI, Lui H, Zeng H, et al. Dermoscopy and near-infrared fluorescence imaging of cutaneous larva migrans. Photodermatol Photoimmunol Photomed. 2013;29:337-338.
13. Zalaudek I, Giacomel J, Cabo H, et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology. 2008;216:14-23.
14. Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814.
1. Berlin JM, Goldberg SJ, McDonough RD, et al. JAAD grand rounds quiz. serpiginous eruption on the leg. J Am Acad Dermatol. 2010;63:921-922.
2. Sarasombath PA, Young PK. An unusual presentation of cutaneous larva migrans. Arch Dermatol. 2007;143:955.
3. Patel S, Aboutalebi S, Vindhya PL, et al. What’s eating you? extensive cutaneous larva migrans (Ancylostoma braziliense). Cutis. 2008;82:239-240.
4. Elston DM, Czarnik K, Brockett R, et al. What’s eating you? Strongyloides stercoralis. Cutis. 2003;71:22-24.
5. Duarte De Sousa ICV, De La Pascua L. Bilateral cutaneous larva migrans [poster reference number 4677]. J Am Acad Dermatol. 2012;66(4, suppl 1):AB106.
6. Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of seven cases and review of the literature. Br J Dermatol. 2002;146:314-316.
7. Shih PY, Hsieh MY, Huang YH, et al. Multiple pruritic erythematous papules on the trunk after a trip to Thailand–quiz case. Arch Dermatol. 2010;146:557-562.
8. Wright DO, Gold ED. Löffler’s syndrome associated with creeping eruption (cutaneous helminthiasis): report of twenty-six cases. Arch Intern Med. 1946;78:303-312.
9. Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler’s syndrome. Arch Dermatol. 2010;146:210-212.
10. Rapini RP, ed. Practical Dermatopathology. Philadelphia, PA: Elsevier; 2005.
11. Langley R, Webb A, Haldane D, et al. Confocal microscopy of cutaneous larva migrans. J Am Acad Dermatol. 2011;64(2, suppl 1):AB100.
12. Aljasser MI, Lui H, Zeng H, et al. Dermoscopy and near-infrared fluorescence imaging of cutaneous larva migrans. Photodermatol Photoimmunol Photomed. 2013;29:337-338.
13. Zalaudek I, Giacomel J, Cabo H, et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology. 2008;216:14-23.
14. Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814.
Practice Points
- Classic cutaneous larva migrans (CLM) presents with a unilateral, serpiginous, pruritic eruption on the hands, feet, or buttocks following direct contact with sand or soil that is contaminated with Ancylostoma braziliense or Ancylostoma caninum.
- Atypical presentations of CLM include bilateral distribution; folliculitis and urticarial plaques; prolonged cases lasting up to 1 year; and Löffler syndrome characterized by migratory pulmonary infiltrates and peripheral eosinophilia.
- Cutaneous larva migrans is self-limited, but treatment often is necessary due to intense pruritus. Treatment options include a single oral dose of albendazole or ivermectin, topical thiabendazole, and prolonged courses of oral albendazole in cases complicated by Löffler syndrome.
Tide turns in favor of multivessel PCI in STEMI
SNOWMASS, COLO. – Recent data seem to refute the 2013 American Heart Association/American College of Cardiology class III recommendation to avoid multivessel percutaneous coronary intervention at the time of primary PCI for ST-elevation MI, Dr. David R. Holmes Jr. observed at the Annual Cardiovascular Conference at Snowmass.
“The current AHA/ACC guidelines for STEMI should be and are being reevaluated regarding clarifications for the indications and timing of non–infarct artery revascularization,” according to Dr. Holmes, a cardiologist at the Mayo Clinic in Rochester, Minn., and an ACC past president.
Indeed, the ACC has already withdrawn from its ‘Choosing Wisely’ campaign its former recommendation discouraging multivessel revascularization at the time of primary PCI for STEMI. The college cited “new science showing that complete revascularization of all significant blocked arteries leads to better outcomes in some heart attack patients.”
Dr. Holmes was coauthor of a meta-analysis of 4 prospective and 14 retrospective studies involving more than 40,000 patients that concluded multivessel PCI for STEMI should be discouraged, and that significant nonculprit lesions should only be treated during staged procedures (J. Am. Coll. Cardiol. 2011;58:692-703). This meta-analysis was influential in the creation of the class III ‘don’t do it’ recommendation in the AHA/ACC guidelines. But Dr. Holmes said that in hindsight, the data included in the meta-analysis were something of a mishmash and “wound up being very hard to interpret.”
Greater clarity has been brought by two more recent randomized trials: PRAMI and CvLPRIT. Both were relatively small by cardiology standards, but they ended up showing similarly striking advantages in favor of using the STEMI hospitalization to perform preventive PCI of both the infarct-related artery and non–infarct arteries with major stenoses.
PRAMI included 465 acute STEMI patients who underwent infarct artery PCI and were then randomized to preventive PCI or infarct artery–only PCI. At a mean follow-up of 23 months, the preventive multivessel PCI group had a 65% reduction in the relative risk of the primary outcome, a composite of cardiac death, nonfatal MI, or refractory angina (N. Engl. J. Med. 2013;369:1115-23).
The yet-to-be-published CvLPRIT study was presented at the 2014 European Society of Cardiology meeting in Barcelona. The multicenter study included 296 STEMI patients with angiographically established significant multivessel disease who were randomized to primary PCI of the culprit vessel only or to complete revascularization. The primary outcome, the 12-month composite of all-cause mortality, recurrent MI, heart failure, or ischemia-driven revascularization, occurred in 10% of the complete revascularization group, compared with 21.2% of patients assigned to culprit artery–only PCI.
Also at the ESC conference, CvLPRIT investigator Dr. Anthony Gershlick of the University of Leicester (England) presented a meta-analysis combining the weighted results of PRAMI, CvLPRIT, and two earlier randomized trials: HELP AMI (Int. J. Cardiovasc. Intervent. 2004;6:128-33) and an Italian trial (Heart 2010;96:662-7). The results strongly favored multivessel PCI, with a 45% reduction in mortality and a 61% decrease in recurrent MI, compared with culprit vessel–only PCI at the time of admission for STEMI.
“Maybe there aren’t any innocent bystanders,” commented Dr. Holmes. “Maybe if you have somebody who has multivessel disease and you see something you think might be an innocent bystander but is a significant lesion, maybe it’s not so innocent. Maybe by treating them all at the time of the initial intervention the patient is going to do better.”
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – Recent data seem to refute the 2013 American Heart Association/American College of Cardiology class III recommendation to avoid multivessel percutaneous coronary intervention at the time of primary PCI for ST-elevation MI, Dr. David R. Holmes Jr. observed at the Annual Cardiovascular Conference at Snowmass.
“The current AHA/ACC guidelines for STEMI should be and are being reevaluated regarding clarifications for the indications and timing of non–infarct artery revascularization,” according to Dr. Holmes, a cardiologist at the Mayo Clinic in Rochester, Minn., and an ACC past president.
Indeed, the ACC has already withdrawn from its ‘Choosing Wisely’ campaign its former recommendation discouraging multivessel revascularization at the time of primary PCI for STEMI. The college cited “new science showing that complete revascularization of all significant blocked arteries leads to better outcomes in some heart attack patients.”
Dr. Holmes was coauthor of a meta-analysis of 4 prospective and 14 retrospective studies involving more than 40,000 patients that concluded multivessel PCI for STEMI should be discouraged, and that significant nonculprit lesions should only be treated during staged procedures (J. Am. Coll. Cardiol. 2011;58:692-703). This meta-analysis was influential in the creation of the class III ‘don’t do it’ recommendation in the AHA/ACC guidelines. But Dr. Holmes said that in hindsight, the data included in the meta-analysis were something of a mishmash and “wound up being very hard to interpret.”
Greater clarity has been brought by two more recent randomized trials: PRAMI and CvLPRIT. Both were relatively small by cardiology standards, but they ended up showing similarly striking advantages in favor of using the STEMI hospitalization to perform preventive PCI of both the infarct-related artery and non–infarct arteries with major stenoses.
PRAMI included 465 acute STEMI patients who underwent infarct artery PCI and were then randomized to preventive PCI or infarct artery–only PCI. At a mean follow-up of 23 months, the preventive multivessel PCI group had a 65% reduction in the relative risk of the primary outcome, a composite of cardiac death, nonfatal MI, or refractory angina (N. Engl. J. Med. 2013;369:1115-23).
The yet-to-be-published CvLPRIT study was presented at the 2014 European Society of Cardiology meeting in Barcelona. The multicenter study included 296 STEMI patients with angiographically established significant multivessel disease who were randomized to primary PCI of the culprit vessel only or to complete revascularization. The primary outcome, the 12-month composite of all-cause mortality, recurrent MI, heart failure, or ischemia-driven revascularization, occurred in 10% of the complete revascularization group, compared with 21.2% of patients assigned to culprit artery–only PCI.
Also at the ESC conference, CvLPRIT investigator Dr. Anthony Gershlick of the University of Leicester (England) presented a meta-analysis combining the weighted results of PRAMI, CvLPRIT, and two earlier randomized trials: HELP AMI (Int. J. Cardiovasc. Intervent. 2004;6:128-33) and an Italian trial (Heart 2010;96:662-7). The results strongly favored multivessel PCI, with a 45% reduction in mortality and a 61% decrease in recurrent MI, compared with culprit vessel–only PCI at the time of admission for STEMI.
“Maybe there aren’t any innocent bystanders,” commented Dr. Holmes. “Maybe if you have somebody who has multivessel disease and you see something you think might be an innocent bystander but is a significant lesion, maybe it’s not so innocent. Maybe by treating them all at the time of the initial intervention the patient is going to do better.”
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – Recent data seem to refute the 2013 American Heart Association/American College of Cardiology class III recommendation to avoid multivessel percutaneous coronary intervention at the time of primary PCI for ST-elevation MI, Dr. David R. Holmes Jr. observed at the Annual Cardiovascular Conference at Snowmass.
“The current AHA/ACC guidelines for STEMI should be and are being reevaluated regarding clarifications for the indications and timing of non–infarct artery revascularization,” according to Dr. Holmes, a cardiologist at the Mayo Clinic in Rochester, Minn., and an ACC past president.
Indeed, the ACC has already withdrawn from its ‘Choosing Wisely’ campaign its former recommendation discouraging multivessel revascularization at the time of primary PCI for STEMI. The college cited “new science showing that complete revascularization of all significant blocked arteries leads to better outcomes in some heart attack patients.”
Dr. Holmes was coauthor of a meta-analysis of 4 prospective and 14 retrospective studies involving more than 40,000 patients that concluded multivessel PCI for STEMI should be discouraged, and that significant nonculprit lesions should only be treated during staged procedures (J. Am. Coll. Cardiol. 2011;58:692-703). This meta-analysis was influential in the creation of the class III ‘don’t do it’ recommendation in the AHA/ACC guidelines. But Dr. Holmes said that in hindsight, the data included in the meta-analysis were something of a mishmash and “wound up being very hard to interpret.”
Greater clarity has been brought by two more recent randomized trials: PRAMI and CvLPRIT. Both were relatively small by cardiology standards, but they ended up showing similarly striking advantages in favor of using the STEMI hospitalization to perform preventive PCI of both the infarct-related artery and non–infarct arteries with major stenoses.
PRAMI included 465 acute STEMI patients who underwent infarct artery PCI and were then randomized to preventive PCI or infarct artery–only PCI. At a mean follow-up of 23 months, the preventive multivessel PCI group had a 65% reduction in the relative risk of the primary outcome, a composite of cardiac death, nonfatal MI, or refractory angina (N. Engl. J. Med. 2013;369:1115-23).
The yet-to-be-published CvLPRIT study was presented at the 2014 European Society of Cardiology meeting in Barcelona. The multicenter study included 296 STEMI patients with angiographically established significant multivessel disease who were randomized to primary PCI of the culprit vessel only or to complete revascularization. The primary outcome, the 12-month composite of all-cause mortality, recurrent MI, heart failure, or ischemia-driven revascularization, occurred in 10% of the complete revascularization group, compared with 21.2% of patients assigned to culprit artery–only PCI.
Also at the ESC conference, CvLPRIT investigator Dr. Anthony Gershlick of the University of Leicester (England) presented a meta-analysis combining the weighted results of PRAMI, CvLPRIT, and two earlier randomized trials: HELP AMI (Int. J. Cardiovasc. Intervent. 2004;6:128-33) and an Italian trial (Heart 2010;96:662-7). The results strongly favored multivessel PCI, with a 45% reduction in mortality and a 61% decrease in recurrent MI, compared with culprit vessel–only PCI at the time of admission for STEMI.
“Maybe there aren’t any innocent bystanders,” commented Dr. Holmes. “Maybe if you have somebody who has multivessel disease and you see something you think might be an innocent bystander but is a significant lesion, maybe it’s not so innocent. Maybe by treating them all at the time of the initial intervention the patient is going to do better.”
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Photographic AK counting isn’t ready for prime time
Can photographs be as good as clinical examination for counting actinic keratoses?
Depending on the physician, AK counts varied widely on clinical assessment as well as in photos, reported Dr. Sudipta Sinnya of the Dermatology Research Centre at the University of Queensland, Brisbane, and associates. However, based on current two-dimensional technology, clinical counting yields superior results, said the researchers, who studied the counts of five trained observers carried out in two sessions with six patients.
“As technological advancements occur, three-dimensional photography will largely supersede two-dimensional photographs in clinical practice, and more robust image-capturing techniques should improve the accuracy of photographic counting,” the researchers noted.
Read the full article from Acta Dermato-Venereologica (2015 [doi:10.2340/00015555-2040]) here.
Can photographs be as good as clinical examination for counting actinic keratoses?
Depending on the physician, AK counts varied widely on clinical assessment as well as in photos, reported Dr. Sudipta Sinnya of the Dermatology Research Centre at the University of Queensland, Brisbane, and associates. However, based on current two-dimensional technology, clinical counting yields superior results, said the researchers, who studied the counts of five trained observers carried out in two sessions with six patients.
“As technological advancements occur, three-dimensional photography will largely supersede two-dimensional photographs in clinical practice, and more robust image-capturing techniques should improve the accuracy of photographic counting,” the researchers noted.
Read the full article from Acta Dermato-Venereologica (2015 [doi:10.2340/00015555-2040]) here.
Can photographs be as good as clinical examination for counting actinic keratoses?
Depending on the physician, AK counts varied widely on clinical assessment as well as in photos, reported Dr. Sudipta Sinnya of the Dermatology Research Centre at the University of Queensland, Brisbane, and associates. However, based on current two-dimensional technology, clinical counting yields superior results, said the researchers, who studied the counts of five trained observers carried out in two sessions with six patients.
“As technological advancements occur, three-dimensional photography will largely supersede two-dimensional photographs in clinical practice, and more robust image-capturing techniques should improve the accuracy of photographic counting,” the researchers noted.
Read the full article from Acta Dermato-Venereologica (2015 [doi:10.2340/00015555-2040]) here.
Vagal stimulation may help upper limb stroke recovery
NASHVILLE, TENN. – Patients with upper limbs affected by ischemic stroke who paired traditional rehabilitation exercises with pulsed vagus nerve stimulation boosted functional scores significantly higher than did those who performed exercises alone in a small, randomized pilot trial.
Taken together with the low rate of adverse events associated with device implantation, the study suggests that coupling the interventions is feasible and likely to be beneficial, Dr. Jesse Dawson of the University of Glasgow, Scotland, said at the International Stroke Conference, sponsored by the American Heart Association.
The vagus nerve stimulator (VNS) is typically used to suppress epileptiform discharges and circumvent seizures. The usual stimulation pattern is continuous cycles of 30 seconds on and 5 minutes off. In his randomized, controlled trial, Dr. Dawson set the device to deliver 0.5-second pulses that coincided with each repetition of a rehabilitative movement. When simulated, the nerve releases two proneuroplastic neurotransmitters, acetylcholine and norepinephrine, which then disperse over the cerebral cortex.
“Our theory was that if we timed these releases at specific periods during rehabilitation therapy, we might be able to drive neuroplasticity toward those specific tasks,” Dr. Dawson said at the conference. The technique has proved effective in both aged rats and rat stroke models, he added.
The trial comprised 20 patients who had experienced an ischemic stroke about 2 years prior. Each was left with residual dysfunction in an upper extremity; seven had a paretic limb. The mean Action Research Arm Test (ARAT) score was 33, and the mean upper extremity Fugl-Meyer score was 43, indicating moderate impairment.
Ten patients underwent VNS implantation. Nine completed the trial. One withdrew after 2 weeks because of a transient vocal cord palsy. This later resolved spontaneously.
Other adverse events related to the VNS were also transient. They included taste disturbance, chest pain, mild dysphagia, and nausea after a therapy session.
The 6-week intervention consisted of 18 sessions, each lasting 2 hours. In each, the rehabilitative movement was repeated 300-400 times.
In a per-protocol analysis, there was no significant difference in the upper extremity Fugl-Meyer score at the study’s end. However, when the patient who had withdrawn was excluded from the analysis, the results did become statistically significant. Patients in the dual-therapy group gained almost 10 points, compared with a 3-point gain in the exercise-only group. The ARAT scores were not different at study’s end.
In light of the positive initial results, a sham-controlled, randomized trial is in the works. The trial will randomize 20-25 patients to either the VNS-paired exercise or exercise-only interventions. All participants will receive the VNS device, but only the paired intervention group will receive actual stimuli.
Patricia Smith, Ph.D., the Doris E. Porter Professor in Physical Therapy at the University of Texas Southwestern, Dallas, is the lead investigator.
MicroTransponder, which makes the VNS unit, is sponsoring both the studies. Neither Dr. Dawson nor Dr. Smith have any financial ties to the company.
On Twitter @alz_gal
NASHVILLE, TENN. – Patients with upper limbs affected by ischemic stroke who paired traditional rehabilitation exercises with pulsed vagus nerve stimulation boosted functional scores significantly higher than did those who performed exercises alone in a small, randomized pilot trial.
Taken together with the low rate of adverse events associated with device implantation, the study suggests that coupling the interventions is feasible and likely to be beneficial, Dr. Jesse Dawson of the University of Glasgow, Scotland, said at the International Stroke Conference, sponsored by the American Heart Association.
The vagus nerve stimulator (VNS) is typically used to suppress epileptiform discharges and circumvent seizures. The usual stimulation pattern is continuous cycles of 30 seconds on and 5 minutes off. In his randomized, controlled trial, Dr. Dawson set the device to deliver 0.5-second pulses that coincided with each repetition of a rehabilitative movement. When simulated, the nerve releases two proneuroplastic neurotransmitters, acetylcholine and norepinephrine, which then disperse over the cerebral cortex.
“Our theory was that if we timed these releases at specific periods during rehabilitation therapy, we might be able to drive neuroplasticity toward those specific tasks,” Dr. Dawson said at the conference. The technique has proved effective in both aged rats and rat stroke models, he added.
The trial comprised 20 patients who had experienced an ischemic stroke about 2 years prior. Each was left with residual dysfunction in an upper extremity; seven had a paretic limb. The mean Action Research Arm Test (ARAT) score was 33, and the mean upper extremity Fugl-Meyer score was 43, indicating moderate impairment.
Ten patients underwent VNS implantation. Nine completed the trial. One withdrew after 2 weeks because of a transient vocal cord palsy. This later resolved spontaneously.
Other adverse events related to the VNS were also transient. They included taste disturbance, chest pain, mild dysphagia, and nausea after a therapy session.
The 6-week intervention consisted of 18 sessions, each lasting 2 hours. In each, the rehabilitative movement was repeated 300-400 times.
In a per-protocol analysis, there was no significant difference in the upper extremity Fugl-Meyer score at the study’s end. However, when the patient who had withdrawn was excluded from the analysis, the results did become statistically significant. Patients in the dual-therapy group gained almost 10 points, compared with a 3-point gain in the exercise-only group. The ARAT scores were not different at study’s end.
In light of the positive initial results, a sham-controlled, randomized trial is in the works. The trial will randomize 20-25 patients to either the VNS-paired exercise or exercise-only interventions. All participants will receive the VNS device, but only the paired intervention group will receive actual stimuli.
Patricia Smith, Ph.D., the Doris E. Porter Professor in Physical Therapy at the University of Texas Southwestern, Dallas, is the lead investigator.
MicroTransponder, which makes the VNS unit, is sponsoring both the studies. Neither Dr. Dawson nor Dr. Smith have any financial ties to the company.
On Twitter @alz_gal
NASHVILLE, TENN. – Patients with upper limbs affected by ischemic stroke who paired traditional rehabilitation exercises with pulsed vagus nerve stimulation boosted functional scores significantly higher than did those who performed exercises alone in a small, randomized pilot trial.
Taken together with the low rate of adverse events associated with device implantation, the study suggests that coupling the interventions is feasible and likely to be beneficial, Dr. Jesse Dawson of the University of Glasgow, Scotland, said at the International Stroke Conference, sponsored by the American Heart Association.
The vagus nerve stimulator (VNS) is typically used to suppress epileptiform discharges and circumvent seizures. The usual stimulation pattern is continuous cycles of 30 seconds on and 5 minutes off. In his randomized, controlled trial, Dr. Dawson set the device to deliver 0.5-second pulses that coincided with each repetition of a rehabilitative movement. When simulated, the nerve releases two proneuroplastic neurotransmitters, acetylcholine and norepinephrine, which then disperse over the cerebral cortex.
“Our theory was that if we timed these releases at specific periods during rehabilitation therapy, we might be able to drive neuroplasticity toward those specific tasks,” Dr. Dawson said at the conference. The technique has proved effective in both aged rats and rat stroke models, he added.
The trial comprised 20 patients who had experienced an ischemic stroke about 2 years prior. Each was left with residual dysfunction in an upper extremity; seven had a paretic limb. The mean Action Research Arm Test (ARAT) score was 33, and the mean upper extremity Fugl-Meyer score was 43, indicating moderate impairment.
Ten patients underwent VNS implantation. Nine completed the trial. One withdrew after 2 weeks because of a transient vocal cord palsy. This later resolved spontaneously.
Other adverse events related to the VNS were also transient. They included taste disturbance, chest pain, mild dysphagia, and nausea after a therapy session.
The 6-week intervention consisted of 18 sessions, each lasting 2 hours. In each, the rehabilitative movement was repeated 300-400 times.
In a per-protocol analysis, there was no significant difference in the upper extremity Fugl-Meyer score at the study’s end. However, when the patient who had withdrawn was excluded from the analysis, the results did become statistically significant. Patients in the dual-therapy group gained almost 10 points, compared with a 3-point gain in the exercise-only group. The ARAT scores were not different at study’s end.
In light of the positive initial results, a sham-controlled, randomized trial is in the works. The trial will randomize 20-25 patients to either the VNS-paired exercise or exercise-only interventions. All participants will receive the VNS device, but only the paired intervention group will receive actual stimuli.
Patricia Smith, Ph.D., the Doris E. Porter Professor in Physical Therapy at the University of Texas Southwestern, Dallas, is the lead investigator.
MicroTransponder, which makes the VNS unit, is sponsoring both the studies. Neither Dr. Dawson nor Dr. Smith have any financial ties to the company.
On Twitter @alz_gal
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: Vagus nerve stimulation paired with physical therapy may improve functional recovery in upper limbs after stroke.
Major finding: The paired intervention boosted the upper extremity Fugl-Meyer score by 10 points in the intervention group and 3 points in the control group.
Data source: A randomized trial of 20 patients about 2 years post stroke who had residual dysfunction in an upper extremity.
Disclosures: MicroTransponder, which makes the VNS unit, is sponsoring both the studies. Dr. Dawson has no financial ties to the company.
2011 Resident Work Hour Reforms Had No Effect on Mortality or Readmissions
Clinical question
Did the 2011 Accreditation Council for Graduate Medical Education resident work hour reforms affect patient outcomes?
Bottom line
Resident work hour reforms were proposed by the Accreditation Council for Graduate Medical Education (ACGME) to reduce resident fatigue (and thus potentially reduce the risk of medical errors), but implementation of the work hour changes also led to concerns over patient safety because of increased handoffs in care. This study shows that work hour reforms had no impact, either positive or negative, on the important patient outcomes of mortality and readmission rates. Other outcomes such as length of stay and number of intensive care unit transfers may need to be examined in future studies to detect more subtle differences. (LOE = 2b)
Reference
Study design: Cohort (retrospective)
Funding source: Government
Allocation: Uncertain
Setting: Inpatient (any location)
Synopsis
In 2011, the ACGME instituted work hour reforms for residents that reduced the work hour limit from 30 consecutive hours to 16 hours for first-year residents and 24 hours for all other residents. Investigators in this study evaluated the effect of the 2011 ACGME reforms on 30-day all-location mortality and 30-day all-cause readmissions. Patients included in the study were Medicare patients who were admitted to acute care US hospitals from 2009 to 2012 with acute myocardial infarction, stroke, gastrointestinal bleeding, or congestive heart failure, or those admitted for general, orthopedic, or vascular surgery. Hospitals were classified by their level of teaching intensity using a resident-to-bed ratio defined as the number of residents divided by the number of staffed beds.
In an analysis that adjusted for demographics, co-morbidities, and the presence of surgical complications, the implementation of work hour reforms did not affect 30-day mortality or readmissions in more-intensive teaching hospitals relative to less-intensive teaching hospitals during the postreform year as compared with 2 years before the reform. Multiple factors beyond the implementation of work hour reforms, may have contributed to this lack of effect. First, adherence to the new reforms by residency programs in the first year is unclear. Second, concurrent initiatives to improve patient outcomes during this time may have affected all hospitals, teaching and nonteaching. Finally, the authors suggest that the greater emphasis on resident supervision with the new reforms may have counterbalanced any negative effects of increased resident handoffs.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Did the 2011 Accreditation Council for Graduate Medical Education resident work hour reforms affect patient outcomes?
Bottom line
Resident work hour reforms were proposed by the Accreditation Council for Graduate Medical Education (ACGME) to reduce resident fatigue (and thus potentially reduce the risk of medical errors), but implementation of the work hour changes also led to concerns over patient safety because of increased handoffs in care. This study shows that work hour reforms had no impact, either positive or negative, on the important patient outcomes of mortality and readmission rates. Other outcomes such as length of stay and number of intensive care unit transfers may need to be examined in future studies to detect more subtle differences. (LOE = 2b)
Reference
Study design: Cohort (retrospective)
Funding source: Government
Allocation: Uncertain
Setting: Inpatient (any location)
Synopsis
In 2011, the ACGME instituted work hour reforms for residents that reduced the work hour limit from 30 consecutive hours to 16 hours for first-year residents and 24 hours for all other residents. Investigators in this study evaluated the effect of the 2011 ACGME reforms on 30-day all-location mortality and 30-day all-cause readmissions. Patients included in the study were Medicare patients who were admitted to acute care US hospitals from 2009 to 2012 with acute myocardial infarction, stroke, gastrointestinal bleeding, or congestive heart failure, or those admitted for general, orthopedic, or vascular surgery. Hospitals were classified by their level of teaching intensity using a resident-to-bed ratio defined as the number of residents divided by the number of staffed beds.
In an analysis that adjusted for demographics, co-morbidities, and the presence of surgical complications, the implementation of work hour reforms did not affect 30-day mortality or readmissions in more-intensive teaching hospitals relative to less-intensive teaching hospitals during the postreform year as compared with 2 years before the reform. Multiple factors beyond the implementation of work hour reforms, may have contributed to this lack of effect. First, adherence to the new reforms by residency programs in the first year is unclear. Second, concurrent initiatives to improve patient outcomes during this time may have affected all hospitals, teaching and nonteaching. Finally, the authors suggest that the greater emphasis on resident supervision with the new reforms may have counterbalanced any negative effects of increased resident handoffs.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Did the 2011 Accreditation Council for Graduate Medical Education resident work hour reforms affect patient outcomes?
Bottom line
Resident work hour reforms were proposed by the Accreditation Council for Graduate Medical Education (ACGME) to reduce resident fatigue (and thus potentially reduce the risk of medical errors), but implementation of the work hour changes also led to concerns over patient safety because of increased handoffs in care. This study shows that work hour reforms had no impact, either positive or negative, on the important patient outcomes of mortality and readmission rates. Other outcomes such as length of stay and number of intensive care unit transfers may need to be examined in future studies to detect more subtle differences. (LOE = 2b)
Reference
Study design: Cohort (retrospective)
Funding source: Government
Allocation: Uncertain
Setting: Inpatient (any location)
Synopsis
In 2011, the ACGME instituted work hour reforms for residents that reduced the work hour limit from 30 consecutive hours to 16 hours for first-year residents and 24 hours for all other residents. Investigators in this study evaluated the effect of the 2011 ACGME reforms on 30-day all-location mortality and 30-day all-cause readmissions. Patients included in the study were Medicare patients who were admitted to acute care US hospitals from 2009 to 2012 with acute myocardial infarction, stroke, gastrointestinal bleeding, or congestive heart failure, or those admitted for general, orthopedic, or vascular surgery. Hospitals were classified by their level of teaching intensity using a resident-to-bed ratio defined as the number of residents divided by the number of staffed beds.
In an analysis that adjusted for demographics, co-morbidities, and the presence of surgical complications, the implementation of work hour reforms did not affect 30-day mortality or readmissions in more-intensive teaching hospitals relative to less-intensive teaching hospitals during the postreform year as compared with 2 years before the reform. Multiple factors beyond the implementation of work hour reforms, may have contributed to this lack of effect. First, adherence to the new reforms by residency programs in the first year is unclear. Second, concurrent initiatives to improve patient outcomes during this time may have affected all hospitals, teaching and nonteaching. Finally, the authors suggest that the greater emphasis on resident supervision with the new reforms may have counterbalanced any negative effects of increased resident handoffs.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Chlorhexidine Bathing Does Not Reduce Nosocomial Infections
Clinical question: For critically ill patients, does daily bathing with chlorhexidine reduce health care–associated infections?
Bottom line
These results show that daily chlorhexidine bathing does not significantly affect the incidence of health care–associated infections. These data conflict with data from prior research, suggesting that more investigation is needed before incorporating chlorhexidine bathing into routine practice, especially given the increased cost with its use and the possibility of the development of chlorhexidine resistance. (LOE = 1b)
Study design: Cross-over trial (randomized)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (ICU only)
Synopsis
Previous studies have shown benefit of daily chlorhexidine bathing in patients at high risk of nosocomial blood stream infections (Daily POEM 7-31-2013; Daily POEM 4-26-2013). In this study, investigators randomized 5 intensive care units at a tertiary care hospital to provide daily bathing of all patients with either 2% chlorhexidine-impregnated cloths or with nonantimicrobial cloths. Each unit followed the assigned protocol for 10 weeks, followed by a 2-week washout period, and then crossed over to the alternate protocol for another 10 weeks. All units crossed over 3 times during the study. Almost 10,000 patients were included in the study. The primary outcome was a composite of health-care associated infections, including central-line associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and Clostridium difficile infections. There was no significant difference detected in the rate of the primary outcome between the chlorhexidine group and the control group with approximately 3 infections per 1000 patient-days in both groups. Adjusting for factors including demographics, co-morbidities, and the unit of admission also did not reveal a difference.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: For critically ill patients, does daily bathing with chlorhexidine reduce health care–associated infections?
Bottom line
These results show that daily chlorhexidine bathing does not significantly affect the incidence of health care–associated infections. These data conflict with data from prior research, suggesting that more investigation is needed before incorporating chlorhexidine bathing into routine practice, especially given the increased cost with its use and the possibility of the development of chlorhexidine resistance. (LOE = 1b)
Study design: Cross-over trial (randomized)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (ICU only)
Synopsis
Previous studies have shown benefit of daily chlorhexidine bathing in patients at high risk of nosocomial blood stream infections (Daily POEM 7-31-2013; Daily POEM 4-26-2013). In this study, investigators randomized 5 intensive care units at a tertiary care hospital to provide daily bathing of all patients with either 2% chlorhexidine-impregnated cloths or with nonantimicrobial cloths. Each unit followed the assigned protocol for 10 weeks, followed by a 2-week washout period, and then crossed over to the alternate protocol for another 10 weeks. All units crossed over 3 times during the study. Almost 10,000 patients were included in the study. The primary outcome was a composite of health-care associated infections, including central-line associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and Clostridium difficile infections. There was no significant difference detected in the rate of the primary outcome between the chlorhexidine group and the control group with approximately 3 infections per 1000 patient-days in both groups. Adjusting for factors including demographics, co-morbidities, and the unit of admission also did not reveal a difference.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: For critically ill patients, does daily bathing with chlorhexidine reduce health care–associated infections?
Bottom line
These results show that daily chlorhexidine bathing does not significantly affect the incidence of health care–associated infections. These data conflict with data from prior research, suggesting that more investigation is needed before incorporating chlorhexidine bathing into routine practice, especially given the increased cost with its use and the possibility of the development of chlorhexidine resistance. (LOE = 1b)
Study design: Cross-over trial (randomized)
Funding source: Government
Allocation: Concealed
Setting: Inpatient (ICU only)
Synopsis
Previous studies have shown benefit of daily chlorhexidine bathing in patients at high risk of nosocomial blood stream infections (Daily POEM 7-31-2013; Daily POEM 4-26-2013). In this study, investigators randomized 5 intensive care units at a tertiary care hospital to provide daily bathing of all patients with either 2% chlorhexidine-impregnated cloths or with nonantimicrobial cloths. Each unit followed the assigned protocol for 10 weeks, followed by a 2-week washout period, and then crossed over to the alternate protocol for another 10 weeks. All units crossed over 3 times during the study. Almost 10,000 patients were included in the study. The primary outcome was a composite of health-care associated infections, including central-line associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and Clostridium difficile infections. There was no significant difference detected in the rate of the primary outcome between the chlorhexidine group and the control group with approximately 3 infections per 1000 patient-days in both groups. Adjusting for factors including demographics, co-morbidities, and the unit of admission also did not reveal a difference.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Aggressive infant leukemia has few mutations

Photo by Vera Kratochvil
Infants who have acute lymphoblastic leukemia (ALL) with MLL rearrangements have few other mutations, according to new research.
The findings suggest that targeting MLL rearrangements in these patients is likely the key to improving their survival.
“We frequently associate a cancer’s aggressiveness with its mutation rate, but this work indicates that the two don’t always go hand-in-hand,” said Richard K. Wilson, PhD, of the Washington University School of Medicine in St Louis, Missouri.
“Still, our findings provide a new direction for developing more effective treatments for these very young patients.”
Dr Wilson and his colleagues reported their findings in Nature Genetics.
The researchers performed whole-genome, exome, RNA, and targeted DNA sequencing to identify genetic alterations in 65 infants with ALL, including 47 with the MLL rearrangement.
The team was surprised to find that, despite being an aggressive leukemia, the MLL-rearranged subtype had among the lowest mutation rates reported for any cancer. The predominant leukemic clone carried a mean of 1.3 non-silent mutations.
“These results show that, to improve survival for patients with this aggressive leukemia, we need to develop drugs that target the abnormal proteins produced by the MLL fusion gene or that interact with the abnormal MLL fusion protein to shut down the cellular machinery that drives their tumors,” said James R. Downing, MD, of St Jude Research Hospital in Memphis, Tennessee. “That will not be easy, but this study found no obvious cooperating mutations to target.”
Almost half of infants with MLL-rearranged ALL (47%) had activating mutations in the kinase-PI3K-RAS signaling pathway. But the mutations were often present in only some of the leukemic cells.
Furthermore, the researchers analyzed leukemia cells in infants whose cancer returned after treatment and found that, at the time of relapse, the cells lacked these mutations.
“The fact that the mutations were often lost at relapse suggests that patients are unlikely to benefit from therapeutically targeting these mutations at diagnosis,” Dr Downing said.
The researchers also found that older children with MLL-rearranged leukemia had significantly more mutations than infants—a mean of 6.5 mutations per case (P=7.15 × 10−5).
Furthermore, 45% of the older children had mutations in genes that encode epigenetic regulatory proteins. And, aside from MLL, epigenetic regulators were rarely mutated in infants with MLL-rearranged ALL.
“While MLL belongs to a family of genes that encode epigenetic regulatory proteins, there was a striking difference between infants and older children regarding the frequency of mutations in other epigenetic regulatory genes,” said Anna Andersson, PhD, of Lund University in Sweden.
“This observation raises the possibility of a fundamental difference in the cell targeted for transformation in infants versus older patients,” said Tanja Gruber, MD, PhD, of St Jude.
“Our working hypothesis is that, in infants, the MLL rearrangement occurs in a developing blood cell, a prenatal progenitor cell, which requires fewer additional mutations to fully transform into leukemia. In contrast, in older patients, the MLL rearrangement isn’t enough on its own.” ![]()

Photo by Vera Kratochvil
Infants who have acute lymphoblastic leukemia (ALL) with MLL rearrangements have few other mutations, according to new research.
The findings suggest that targeting MLL rearrangements in these patients is likely the key to improving their survival.
“We frequently associate a cancer’s aggressiveness with its mutation rate, but this work indicates that the two don’t always go hand-in-hand,” said Richard K. Wilson, PhD, of the Washington University School of Medicine in St Louis, Missouri.
“Still, our findings provide a new direction for developing more effective treatments for these very young patients.”
Dr Wilson and his colleagues reported their findings in Nature Genetics.
The researchers performed whole-genome, exome, RNA, and targeted DNA sequencing to identify genetic alterations in 65 infants with ALL, including 47 with the MLL rearrangement.
The team was surprised to find that, despite being an aggressive leukemia, the MLL-rearranged subtype had among the lowest mutation rates reported for any cancer. The predominant leukemic clone carried a mean of 1.3 non-silent mutations.
“These results show that, to improve survival for patients with this aggressive leukemia, we need to develop drugs that target the abnormal proteins produced by the MLL fusion gene or that interact with the abnormal MLL fusion protein to shut down the cellular machinery that drives their tumors,” said James R. Downing, MD, of St Jude Research Hospital in Memphis, Tennessee. “That will not be easy, but this study found no obvious cooperating mutations to target.”
Almost half of infants with MLL-rearranged ALL (47%) had activating mutations in the kinase-PI3K-RAS signaling pathway. But the mutations were often present in only some of the leukemic cells.
Furthermore, the researchers analyzed leukemia cells in infants whose cancer returned after treatment and found that, at the time of relapse, the cells lacked these mutations.
“The fact that the mutations were often lost at relapse suggests that patients are unlikely to benefit from therapeutically targeting these mutations at diagnosis,” Dr Downing said.
The researchers also found that older children with MLL-rearranged leukemia had significantly more mutations than infants—a mean of 6.5 mutations per case (P=7.15 × 10−5).
Furthermore, 45% of the older children had mutations in genes that encode epigenetic regulatory proteins. And, aside from MLL, epigenetic regulators were rarely mutated in infants with MLL-rearranged ALL.
“While MLL belongs to a family of genes that encode epigenetic regulatory proteins, there was a striking difference between infants and older children regarding the frequency of mutations in other epigenetic regulatory genes,” said Anna Andersson, PhD, of Lund University in Sweden.
“This observation raises the possibility of a fundamental difference in the cell targeted for transformation in infants versus older patients,” said Tanja Gruber, MD, PhD, of St Jude.
“Our working hypothesis is that, in infants, the MLL rearrangement occurs in a developing blood cell, a prenatal progenitor cell, which requires fewer additional mutations to fully transform into leukemia. In contrast, in older patients, the MLL rearrangement isn’t enough on its own.” ![]()

Photo by Vera Kratochvil
Infants who have acute lymphoblastic leukemia (ALL) with MLL rearrangements have few other mutations, according to new research.
The findings suggest that targeting MLL rearrangements in these patients is likely the key to improving their survival.
“We frequently associate a cancer’s aggressiveness with its mutation rate, but this work indicates that the two don’t always go hand-in-hand,” said Richard K. Wilson, PhD, of the Washington University School of Medicine in St Louis, Missouri.
“Still, our findings provide a new direction for developing more effective treatments for these very young patients.”
Dr Wilson and his colleagues reported their findings in Nature Genetics.
The researchers performed whole-genome, exome, RNA, and targeted DNA sequencing to identify genetic alterations in 65 infants with ALL, including 47 with the MLL rearrangement.
The team was surprised to find that, despite being an aggressive leukemia, the MLL-rearranged subtype had among the lowest mutation rates reported for any cancer. The predominant leukemic clone carried a mean of 1.3 non-silent mutations.
“These results show that, to improve survival for patients with this aggressive leukemia, we need to develop drugs that target the abnormal proteins produced by the MLL fusion gene or that interact with the abnormal MLL fusion protein to shut down the cellular machinery that drives their tumors,” said James R. Downing, MD, of St Jude Research Hospital in Memphis, Tennessee. “That will not be easy, but this study found no obvious cooperating mutations to target.”
Almost half of infants with MLL-rearranged ALL (47%) had activating mutations in the kinase-PI3K-RAS signaling pathway. But the mutations were often present in only some of the leukemic cells.
Furthermore, the researchers analyzed leukemia cells in infants whose cancer returned after treatment and found that, at the time of relapse, the cells lacked these mutations.
“The fact that the mutations were often lost at relapse suggests that patients are unlikely to benefit from therapeutically targeting these mutations at diagnosis,” Dr Downing said.
The researchers also found that older children with MLL-rearranged leukemia had significantly more mutations than infants—a mean of 6.5 mutations per case (P=7.15 × 10−5).
Furthermore, 45% of the older children had mutations in genes that encode epigenetic regulatory proteins. And, aside from MLL, epigenetic regulators were rarely mutated in infants with MLL-rearranged ALL.
“While MLL belongs to a family of genes that encode epigenetic regulatory proteins, there was a striking difference between infants and older children regarding the frequency of mutations in other epigenetic regulatory genes,” said Anna Andersson, PhD, of Lund University in Sweden.
“This observation raises the possibility of a fundamental difference in the cell targeted for transformation in infants versus older patients,” said Tanja Gruber, MD, PhD, of St Jude.
“Our working hypothesis is that, in infants, the MLL rearrangement occurs in a developing blood cell, a prenatal progenitor cell, which requires fewer additional mutations to fully transform into leukemia. In contrast, in older patients, the MLL rearrangement isn’t enough on its own.” ![]()
FDA approves first biosimilar product
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to be approved in the US.
A biosimilar product is approved based on data showing that it is highly similar to an already-approved biological product.
Sandoz Inc’s Zarxio is biosimilar to Amgen Inc’s Neupogen (filgrastim), which was originally licensed in 1991. Zarxio is now approved for the same indications as Neupogen.
Zarxio can be prescribed for:
- patients with cancer receiving myelosuppressive chemotherapy
- patients with acute myeloid leukemia receiving induction or consolidation chemotherapy
- patients with cancer undergoing bone marrow transplant
- patients undergoing autologous peripheral blood progenitor cell collection and therapy
- patients with severe chronic neutropenia.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
“Biosimilars will provide access to important therapies for patients who need them,” said FDA Commissioner Margaret A. Hamburg, MD.
“Patients and the healthcare community can be confident that biosimilar products approved by the FDA meet the agency’s rigorous safety, efficacy, and quality standards.”
Zarxio data
The FDA’s approval of Zarxio is based on a review of evidence that included structural and functional characterization, in vivo data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates Zarxio is biosimilar to Neupogen.
The PIONEER study was the final piece of data the FDA used to approve Zarxio as biosimilar to Neupogen. The data was sufficient to allow extrapolation of the use of Zarxio to all indications of Neupogen.
In the PIONEER study, Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in cancer patients undergoing myelosuppressive chemotherapy—1.17 and 1.20 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The most common side effects of Zarxio are aching in the bones or muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
About biosimilar approval
The Biologics Price Competition and Innovation Act of 2009 (BPCI Act) was passed as part of the Affordable Care Act that President Barack Obama signed into law in March 2010. The BPCI Act created an abbreviated licensure pathway for biological products shown to be “biosimilar” to or “interchangeable” with an FDA-licensed biological product, known as the reference product.
This abbreviated licensure pathway under section 351(k) of the Public Health Service Act permits reliance on certain existing scientific knowledge about the safety and effectiveness of the reference product, and it enables a biosimilar biological product to be licensed based on less than a full complement of product-specific preclinical and clinical data.
A biosimilar product can only be approved by the FDA if it has the same mechanism(s) of action, route(s) of administration, dosage form(s) and strength(s) as the reference product, and only for the indication(s) and condition(s) of use that have been approved for the reference product. The facilities where biosimilars are manufactured must also meet the FDA’s standards.
There must be no clinically meaningful differences between the biosimilar and the reference product in terms of safety and effectiveness. Only minor differences in clinically inactive components are allowable.
Zarxio has been approved as a biosimilar, not an interchangeable product. Under the BPCI Act, a biological product that has been approved as “interchangeable” may be substituted for the reference product without the intervention of the healthcare provider who prescribed the reference product.
For Zarxio’s approval, the FDA has designated a placeholder nonproprietary name for this product as “filgrastim-sndz.” The provision of a placeholder nonproprietary name should not be viewed as reflective of the agency’s decision on a comprehensive naming policy for biosimilars and other biological products.
While the FDA has not yet issued draft guidance on how current and future biological products marketed in the US should be named, the agency intends to do so in the near future.
For more details on Zarxio, see the full prescribing information. ![]()
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to be approved in the US.
A biosimilar product is approved based on data showing that it is highly similar to an already-approved biological product.
Sandoz Inc’s Zarxio is biosimilar to Amgen Inc’s Neupogen (filgrastim), which was originally licensed in 1991. Zarxio is now approved for the same indications as Neupogen.
Zarxio can be prescribed for:
- patients with cancer receiving myelosuppressive chemotherapy
- patients with acute myeloid leukemia receiving induction or consolidation chemotherapy
- patients with cancer undergoing bone marrow transplant
- patients undergoing autologous peripheral blood progenitor cell collection and therapy
- patients with severe chronic neutropenia.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
“Biosimilars will provide access to important therapies for patients who need them,” said FDA Commissioner Margaret A. Hamburg, MD.
“Patients and the healthcare community can be confident that biosimilar products approved by the FDA meet the agency’s rigorous safety, efficacy, and quality standards.”
Zarxio data
The FDA’s approval of Zarxio is based on a review of evidence that included structural and functional characterization, in vivo data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates Zarxio is biosimilar to Neupogen.
The PIONEER study was the final piece of data the FDA used to approve Zarxio as biosimilar to Neupogen. The data was sufficient to allow extrapolation of the use of Zarxio to all indications of Neupogen.
In the PIONEER study, Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in cancer patients undergoing myelosuppressive chemotherapy—1.17 and 1.20 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The most common side effects of Zarxio are aching in the bones or muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
About biosimilar approval
The Biologics Price Competition and Innovation Act of 2009 (BPCI Act) was passed as part of the Affordable Care Act that President Barack Obama signed into law in March 2010. The BPCI Act created an abbreviated licensure pathway for biological products shown to be “biosimilar” to or “interchangeable” with an FDA-licensed biological product, known as the reference product.
This abbreviated licensure pathway under section 351(k) of the Public Health Service Act permits reliance on certain existing scientific knowledge about the safety and effectiveness of the reference product, and it enables a biosimilar biological product to be licensed based on less than a full complement of product-specific preclinical and clinical data.
A biosimilar product can only be approved by the FDA if it has the same mechanism(s) of action, route(s) of administration, dosage form(s) and strength(s) as the reference product, and only for the indication(s) and condition(s) of use that have been approved for the reference product. The facilities where biosimilars are manufactured must also meet the FDA’s standards.
There must be no clinically meaningful differences between the biosimilar and the reference product in terms of safety and effectiveness. Only minor differences in clinically inactive components are allowable.
Zarxio has been approved as a biosimilar, not an interchangeable product. Under the BPCI Act, a biological product that has been approved as “interchangeable” may be substituted for the reference product without the intervention of the healthcare provider who prescribed the reference product.
For Zarxio’s approval, the FDA has designated a placeholder nonproprietary name for this product as “filgrastim-sndz.” The provision of a placeholder nonproprietary name should not be viewed as reflective of the agency’s decision on a comprehensive naming policy for biosimilars and other biological products.
While the FDA has not yet issued draft guidance on how current and future biological products marketed in the US should be named, the agency intends to do so in the near future.
For more details on Zarxio, see the full prescribing information. ![]()
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to be approved in the US.
A biosimilar product is approved based on data showing that it is highly similar to an already-approved biological product.
Sandoz Inc’s Zarxio is biosimilar to Amgen Inc’s Neupogen (filgrastim), which was originally licensed in 1991. Zarxio is now approved for the same indications as Neupogen.
Zarxio can be prescribed for:
- patients with cancer receiving myelosuppressive chemotherapy
- patients with acute myeloid leukemia receiving induction or consolidation chemotherapy
- patients with cancer undergoing bone marrow transplant
- patients undergoing autologous peripheral blood progenitor cell collection and therapy
- patients with severe chronic neutropenia.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
“Biosimilars will provide access to important therapies for patients who need them,” said FDA Commissioner Margaret A. Hamburg, MD.
“Patients and the healthcare community can be confident that biosimilar products approved by the FDA meet the agency’s rigorous safety, efficacy, and quality standards.”
Zarxio data
The FDA’s approval of Zarxio is based on a review of evidence that included structural and functional characterization, in vivo data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates Zarxio is biosimilar to Neupogen.
The PIONEER study was the final piece of data the FDA used to approve Zarxio as biosimilar to Neupogen. The data was sufficient to allow extrapolation of the use of Zarxio to all indications of Neupogen.
In the PIONEER study, Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in cancer patients undergoing myelosuppressive chemotherapy—1.17 and 1.20 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The most common side effects of Zarxio are aching in the bones or muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
About biosimilar approval
The Biologics Price Competition and Innovation Act of 2009 (BPCI Act) was passed as part of the Affordable Care Act that President Barack Obama signed into law in March 2010. The BPCI Act created an abbreviated licensure pathway for biological products shown to be “biosimilar” to or “interchangeable” with an FDA-licensed biological product, known as the reference product.
This abbreviated licensure pathway under section 351(k) of the Public Health Service Act permits reliance on certain existing scientific knowledge about the safety and effectiveness of the reference product, and it enables a biosimilar biological product to be licensed based on less than a full complement of product-specific preclinical and clinical data.
A biosimilar product can only be approved by the FDA if it has the same mechanism(s) of action, route(s) of administration, dosage form(s) and strength(s) as the reference product, and only for the indication(s) and condition(s) of use that have been approved for the reference product. The facilities where biosimilars are manufactured must also meet the FDA’s standards.
There must be no clinically meaningful differences between the biosimilar and the reference product in terms of safety and effectiveness. Only minor differences in clinically inactive components are allowable.
Zarxio has been approved as a biosimilar, not an interchangeable product. Under the BPCI Act, a biological product that has been approved as “interchangeable” may be substituted for the reference product without the intervention of the healthcare provider who prescribed the reference product.
For Zarxio’s approval, the FDA has designated a placeholder nonproprietary name for this product as “filgrastim-sndz.” The provision of a placeholder nonproprietary name should not be viewed as reflective of the agency’s decision on a comprehensive naming policy for biosimilars and other biological products.
While the FDA has not yet issued draft guidance on how current and future biological products marketed in the US should be named, the agency intends to do so in the near future.
For more details on Zarxio, see the full prescribing information. ![]()