User login
Model helps explain chaotic angiogenesis

Image by Louis Heiser
and Robert Ackland
Scientists say they have developed a model that provides new insight into chaotic angiogenesis, a hallmark of cancer.
The model shows how proteins involved in angiogenesis communicate with each other and how tumors take charge of the protein signaling chain that controls blood vessel growth.
The work, published in PNAS, suggests that Jagged ligands play a major role in the chaotic vessel growth observed around tumors.
In normal growth, an endothelial cell sprouts from an existing blood vessel as a tip. Others that follow the tip cell become the stalk cells that ultimately form vessel walls. The Notch signaling pathway directs the endothelial cell’s decision to become a tip or stalk.
Notch receptors bind with Delta ligand or Jagged ligand molecules produced by cells. How they interact determines the cell’s fate.
When Notch and Delta bind, they prompt a few cells to be tips and adjacent ones to be stalks. The Notch-Delta pathway has been studied extensively and is the target of many angiogenesis inhibitors now in use, according to the scientists.
“We wondered exactly what Notch-Jagged signaling does that is not done in Notch-Delta signaling,” said study author Marcelo Boareto, PhD, of the Swiss Federal Institute of Technology in Zurich.
“We find that when the cells communicate mostly via Jagged, we see a new kind of cell that is not exactly tip and not exactly stalk, but somewhere in between. This compromised cell is the major difference between normal and tumor angiogenesis and suggests that if Notch-Jagged signaling can be somehow suppressed without affecting Notch-Delta, we can probably disrupt tumor angiogenesis.”
The scientists found the tip/stalk hybrid cells do form new blood vessels, but these vessels rarely mature.
“High levels of Jagged in the environment can trigger the formation of blood vessels that are useful to the tumor: fast-developing, leaky, and spread chaotically all over the tumor mass,” Dr Boareto said.
“Tumors don’t have to wait for the vessels to develop,” added study author José Onuchic, PhD, of Rice University in Houston, Texas. “They take advantage of the leakiness of the structure.”
The scientists’ model also takes into account the effect of vascular endothelial growth factor, which triggers angiogenesis and is overexpressed by tumor cells.
“It is very interesting how the tumor hijacks this important mechanism, which is needed for the development of a functional vessel, and amplifies it to generate pathological angiogenesis that leads to uncontrolled growth,” Dr Onuchic said. ![]()

Image by Louis Heiser
and Robert Ackland
Scientists say they have developed a model that provides new insight into chaotic angiogenesis, a hallmark of cancer.
The model shows how proteins involved in angiogenesis communicate with each other and how tumors take charge of the protein signaling chain that controls blood vessel growth.
The work, published in PNAS, suggests that Jagged ligands play a major role in the chaotic vessel growth observed around tumors.
In normal growth, an endothelial cell sprouts from an existing blood vessel as a tip. Others that follow the tip cell become the stalk cells that ultimately form vessel walls. The Notch signaling pathway directs the endothelial cell’s decision to become a tip or stalk.
Notch receptors bind with Delta ligand or Jagged ligand molecules produced by cells. How they interact determines the cell’s fate.
When Notch and Delta bind, they prompt a few cells to be tips and adjacent ones to be stalks. The Notch-Delta pathway has been studied extensively and is the target of many angiogenesis inhibitors now in use, according to the scientists.
“We wondered exactly what Notch-Jagged signaling does that is not done in Notch-Delta signaling,” said study author Marcelo Boareto, PhD, of the Swiss Federal Institute of Technology in Zurich.
“We find that when the cells communicate mostly via Jagged, we see a new kind of cell that is not exactly tip and not exactly stalk, but somewhere in between. This compromised cell is the major difference between normal and tumor angiogenesis and suggests that if Notch-Jagged signaling can be somehow suppressed without affecting Notch-Delta, we can probably disrupt tumor angiogenesis.”
The scientists found the tip/stalk hybrid cells do form new blood vessels, but these vessels rarely mature.
“High levels of Jagged in the environment can trigger the formation of blood vessels that are useful to the tumor: fast-developing, leaky, and spread chaotically all over the tumor mass,” Dr Boareto said.
“Tumors don’t have to wait for the vessels to develop,” added study author José Onuchic, PhD, of Rice University in Houston, Texas. “They take advantage of the leakiness of the structure.”
The scientists’ model also takes into account the effect of vascular endothelial growth factor, which triggers angiogenesis and is overexpressed by tumor cells.
“It is very interesting how the tumor hijacks this important mechanism, which is needed for the development of a functional vessel, and amplifies it to generate pathological angiogenesis that leads to uncontrolled growth,” Dr Onuchic said. ![]()

Image by Louis Heiser
and Robert Ackland
Scientists say they have developed a model that provides new insight into chaotic angiogenesis, a hallmark of cancer.
The model shows how proteins involved in angiogenesis communicate with each other and how tumors take charge of the protein signaling chain that controls blood vessel growth.
The work, published in PNAS, suggests that Jagged ligands play a major role in the chaotic vessel growth observed around tumors.
In normal growth, an endothelial cell sprouts from an existing blood vessel as a tip. Others that follow the tip cell become the stalk cells that ultimately form vessel walls. The Notch signaling pathway directs the endothelial cell’s decision to become a tip or stalk.
Notch receptors bind with Delta ligand or Jagged ligand molecules produced by cells. How they interact determines the cell’s fate.
When Notch and Delta bind, they prompt a few cells to be tips and adjacent ones to be stalks. The Notch-Delta pathway has been studied extensively and is the target of many angiogenesis inhibitors now in use, according to the scientists.
“We wondered exactly what Notch-Jagged signaling does that is not done in Notch-Delta signaling,” said study author Marcelo Boareto, PhD, of the Swiss Federal Institute of Technology in Zurich.
“We find that when the cells communicate mostly via Jagged, we see a new kind of cell that is not exactly tip and not exactly stalk, but somewhere in between. This compromised cell is the major difference between normal and tumor angiogenesis and suggests that if Notch-Jagged signaling can be somehow suppressed without affecting Notch-Delta, we can probably disrupt tumor angiogenesis.”
The scientists found the tip/stalk hybrid cells do form new blood vessels, but these vessels rarely mature.
“High levels of Jagged in the environment can trigger the formation of blood vessels that are useful to the tumor: fast-developing, leaky, and spread chaotically all over the tumor mass,” Dr Boareto said.
“Tumors don’t have to wait for the vessels to develop,” added study author José Onuchic, PhD, of Rice University in Houston, Texas. “They take advantage of the leakiness of the structure.”
The scientists’ model also takes into account the effect of vascular endothelial growth factor, which triggers angiogenesis and is overexpressed by tumor cells.
“It is very interesting how the tumor hijacks this important mechanism, which is needed for the development of a functional vessel, and amplifies it to generate pathological angiogenesis that leads to uncontrolled growth,” Dr Onuchic said. ![]()
Sepsis readmissions common and costly, group says

Photo courtesy of the CDC
Hospital readmissions for sepsis are under-recognized, and additional measures are needed to prevent these readmissions, researchers say.
The group conducted a study of California hospitals that showed sepsis accounts for roughly the same percentage of readmissions as heart attacks and congestive heart failure.
However, sepsis readmissions cost the healthcare system more than heart attack and congestive heart failure readmissions combined.
The research was published in Critical Care Medicine.
“Our study shows how common sepsis readmissions are and [elucidates] some of the factors that are associated with higher risk of readmission after these severe infections,” said study author Dong Chang, MD, of Harbor-UCLA Medical Center in Los Angeles, California.
“In addition, we show that sepsis readmissions have a significant impact on healthcare expenditures relative to other high-risk conditions that are receiving active attention and interventions. Based on these results, we believe that sepsis readmissions are under-recognized and should be among the conditions that are targeted for intervention by policymakers.”
The researchers analyzed data on sepsis admissions for adults 18 years of age and older at all California hospitals from 2009 through 2011. The team also analyzed data for congestive heart failure and heart attack admissions during the same period.
There were 240,198 admissions for sepsis, 193,153 for congestive heart failure, and 105,684 for heart attacks. The all-cause, 30-day readmission rate was 20.4% for sepsis, 23.6% for congestive heart failure, and 17.7% for heart attacks.
Patients with sepsis were readmitted because of respiratory failure; pneumonia; urinary tract infections; renal infections; renal failure; intestinal infections; complications with devices, implants, or grafts; and other causes.
Sepsis readmission rates were higher among young adults than older adults, among men than women, among black and Native American patients than other racial groups, and among lower-income patients than those with higher incomes. In addition, patients with other concurrent health problems were more likely to be readmitted than those with sepsis alone.
The estimated annual cost of sepsis-related readmissions in California during the study period was $500 million, compared with $229 million for congestive heart failure and $142 million for heart attacks.
“These findings suggest that efforts to reduce hospital readmissions need to include sepsis prominently, at least on par with heart failure and myocardial infarction,” said study author Martin Shapiro, MD, PhD, of the David Geffen School of Medicine at UCLA.
Next, the researchers plan to examine why patients are readmitted after sepsis and the percentage of those readmissions that are due to processes that can be improved upon, such as discharge practices, follow-up care, and teaching patients how to take their medications. ![]()

Photo courtesy of the CDC
Hospital readmissions for sepsis are under-recognized, and additional measures are needed to prevent these readmissions, researchers say.
The group conducted a study of California hospitals that showed sepsis accounts for roughly the same percentage of readmissions as heart attacks and congestive heart failure.
However, sepsis readmissions cost the healthcare system more than heart attack and congestive heart failure readmissions combined.
The research was published in Critical Care Medicine.
“Our study shows how common sepsis readmissions are and [elucidates] some of the factors that are associated with higher risk of readmission after these severe infections,” said study author Dong Chang, MD, of Harbor-UCLA Medical Center in Los Angeles, California.
“In addition, we show that sepsis readmissions have a significant impact on healthcare expenditures relative to other high-risk conditions that are receiving active attention and interventions. Based on these results, we believe that sepsis readmissions are under-recognized and should be among the conditions that are targeted for intervention by policymakers.”
The researchers analyzed data on sepsis admissions for adults 18 years of age and older at all California hospitals from 2009 through 2011. The team also analyzed data for congestive heart failure and heart attack admissions during the same period.
There were 240,198 admissions for sepsis, 193,153 for congestive heart failure, and 105,684 for heart attacks. The all-cause, 30-day readmission rate was 20.4% for sepsis, 23.6% for congestive heart failure, and 17.7% for heart attacks.
Patients with sepsis were readmitted because of respiratory failure; pneumonia; urinary tract infections; renal infections; renal failure; intestinal infections; complications with devices, implants, or grafts; and other causes.
Sepsis readmission rates were higher among young adults than older adults, among men than women, among black and Native American patients than other racial groups, and among lower-income patients than those with higher incomes. In addition, patients with other concurrent health problems were more likely to be readmitted than those with sepsis alone.
The estimated annual cost of sepsis-related readmissions in California during the study period was $500 million, compared with $229 million for congestive heart failure and $142 million for heart attacks.
“These findings suggest that efforts to reduce hospital readmissions need to include sepsis prominently, at least on par with heart failure and myocardial infarction,” said study author Martin Shapiro, MD, PhD, of the David Geffen School of Medicine at UCLA.
Next, the researchers plan to examine why patients are readmitted after sepsis and the percentage of those readmissions that are due to processes that can be improved upon, such as discharge practices, follow-up care, and teaching patients how to take their medications. ![]()

Photo courtesy of the CDC
Hospital readmissions for sepsis are under-recognized, and additional measures are needed to prevent these readmissions, researchers say.
The group conducted a study of California hospitals that showed sepsis accounts for roughly the same percentage of readmissions as heart attacks and congestive heart failure.
However, sepsis readmissions cost the healthcare system more than heart attack and congestive heart failure readmissions combined.
The research was published in Critical Care Medicine.
“Our study shows how common sepsis readmissions are and [elucidates] some of the factors that are associated with higher risk of readmission after these severe infections,” said study author Dong Chang, MD, of Harbor-UCLA Medical Center in Los Angeles, California.
“In addition, we show that sepsis readmissions have a significant impact on healthcare expenditures relative to other high-risk conditions that are receiving active attention and interventions. Based on these results, we believe that sepsis readmissions are under-recognized and should be among the conditions that are targeted for intervention by policymakers.”
The researchers analyzed data on sepsis admissions for adults 18 years of age and older at all California hospitals from 2009 through 2011. The team also analyzed data for congestive heart failure and heart attack admissions during the same period.
There were 240,198 admissions for sepsis, 193,153 for congestive heart failure, and 105,684 for heart attacks. The all-cause, 30-day readmission rate was 20.4% for sepsis, 23.6% for congestive heart failure, and 17.7% for heart attacks.
Patients with sepsis were readmitted because of respiratory failure; pneumonia; urinary tract infections; renal infections; renal failure; intestinal infections; complications with devices, implants, or grafts; and other causes.
Sepsis readmission rates were higher among young adults than older adults, among men than women, among black and Native American patients than other racial groups, and among lower-income patients than those with higher incomes. In addition, patients with other concurrent health problems were more likely to be readmitted than those with sepsis alone.
The estimated annual cost of sepsis-related readmissions in California during the study period was $500 million, compared with $229 million for congestive heart failure and $142 million for heart attacks.
“These findings suggest that efforts to reduce hospital readmissions need to include sepsis prominently, at least on par with heart failure and myocardial infarction,” said study author Martin Shapiro, MD, PhD, of the David Geffen School of Medicine at UCLA.
Next, the researchers plan to examine why patients are readmitted after sepsis and the percentage of those readmissions that are due to processes that can be improved upon, such as discharge practices, follow-up care, and teaching patients how to take their medications. ![]()
Early progression predicts overall survival in FL

Photo by Rhoda Baer
The goal for many cancer patients is to reach the 5-year mark without progression, but a new study suggests 2 years might be a more appropriate goal for patients with follicular lymphoma (FL).
Previous research indicated that about 20% of FL patients relapse within 2 years of treatment.
Now, researchers have found these patients have a significantly worse 5-year survival rate than patients who make it past the 2-year mark without progressing.
Carla Casulo, MD, of the University of Rochester in New York, and her colleagues recounted these findings in the Journal of Clinical Oncology.
The team analyzed data from 588 patients with stage 2-4 FL who received first-line therapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
These patients could be separated into 2 groups: those with disease progression within 2 years of diagnosis (19%, n=110) and those who did not progress within the 2-year period (71%, n=420).
Eight percent of patients (n=46) were lost to follow-up, and 2% (n=12) died of causes other than progression within the 2-year period.
At 2 years, the overall survival (OS) rate was 68% in the early progression group and 97% in the group that did not progress. At 5 years, OS rates were 50% and 90%, respectively.
In unadjusted Cox regression analysis, early progression was associated with lower OS (hazard ratio[HR]=7.17). The same was true after the researchers adjusted for FLIPI score (HR=6.44).
The team observed similar results in a validation cohort of 147 FL patients who received first-line R-CHOP. At 2 years, the OS rate was 64% in the early progression group and 98% in the group that did not progress. At 5 years, OS rates were 34% and 94%, respectively.
Again, in an unadjusted analysis, early progression was associated with lower OS (HR=20.0). And this trend was maintained after the researchers adjusted for FLIPI score (HR=19.8).
The researchers also found that, for patients in the early progression group, clinical factors that were predictive of inferior OS were age, ECOG performance score, nodal sites, and disease stage. For the group that did not progress within 2 years, clinical factors that were predictive of OS were age and extranodal sites.
In a Cox regression analysis that encompassed these factors and early progression, only early progression, age, and ECOG performance scores remained significantly predictive of OS.
“[W]e have confirmed that all relapsed patients are not equal and therefore should not be approached the same at diagnosis, nor at the time of relapse, in terms of therapies,” Dr Casulo said.
“It will be critical to predict who is most likely to relapse early. We believe that targeted sequencing or gene-expression profiling will be important to understanding how to improve the outcomes of this group.” ![]()

Photo by Rhoda Baer
The goal for many cancer patients is to reach the 5-year mark without progression, but a new study suggests 2 years might be a more appropriate goal for patients with follicular lymphoma (FL).
Previous research indicated that about 20% of FL patients relapse within 2 years of treatment.
Now, researchers have found these patients have a significantly worse 5-year survival rate than patients who make it past the 2-year mark without progressing.
Carla Casulo, MD, of the University of Rochester in New York, and her colleagues recounted these findings in the Journal of Clinical Oncology.
The team analyzed data from 588 patients with stage 2-4 FL who received first-line therapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
These patients could be separated into 2 groups: those with disease progression within 2 years of diagnosis (19%, n=110) and those who did not progress within the 2-year period (71%, n=420).
Eight percent of patients (n=46) were lost to follow-up, and 2% (n=12) died of causes other than progression within the 2-year period.
At 2 years, the overall survival (OS) rate was 68% in the early progression group and 97% in the group that did not progress. At 5 years, OS rates were 50% and 90%, respectively.
In unadjusted Cox regression analysis, early progression was associated with lower OS (hazard ratio[HR]=7.17). The same was true after the researchers adjusted for FLIPI score (HR=6.44).
The team observed similar results in a validation cohort of 147 FL patients who received first-line R-CHOP. At 2 years, the OS rate was 64% in the early progression group and 98% in the group that did not progress. At 5 years, OS rates were 34% and 94%, respectively.
Again, in an unadjusted analysis, early progression was associated with lower OS (HR=20.0). And this trend was maintained after the researchers adjusted for FLIPI score (HR=19.8).
The researchers also found that, for patients in the early progression group, clinical factors that were predictive of inferior OS were age, ECOG performance score, nodal sites, and disease stage. For the group that did not progress within 2 years, clinical factors that were predictive of OS were age and extranodal sites.
In a Cox regression analysis that encompassed these factors and early progression, only early progression, age, and ECOG performance scores remained significantly predictive of OS.
“[W]e have confirmed that all relapsed patients are not equal and therefore should not be approached the same at diagnosis, nor at the time of relapse, in terms of therapies,” Dr Casulo said.
“It will be critical to predict who is most likely to relapse early. We believe that targeted sequencing or gene-expression profiling will be important to understanding how to improve the outcomes of this group.” ![]()

Photo by Rhoda Baer
The goal for many cancer patients is to reach the 5-year mark without progression, but a new study suggests 2 years might be a more appropriate goal for patients with follicular lymphoma (FL).
Previous research indicated that about 20% of FL patients relapse within 2 years of treatment.
Now, researchers have found these patients have a significantly worse 5-year survival rate than patients who make it past the 2-year mark without progressing.
Carla Casulo, MD, of the University of Rochester in New York, and her colleagues recounted these findings in the Journal of Clinical Oncology.
The team analyzed data from 588 patients with stage 2-4 FL who received first-line therapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
These patients could be separated into 2 groups: those with disease progression within 2 years of diagnosis (19%, n=110) and those who did not progress within the 2-year period (71%, n=420).
Eight percent of patients (n=46) were lost to follow-up, and 2% (n=12) died of causes other than progression within the 2-year period.
At 2 years, the overall survival (OS) rate was 68% in the early progression group and 97% in the group that did not progress. At 5 years, OS rates were 50% and 90%, respectively.
In unadjusted Cox regression analysis, early progression was associated with lower OS (hazard ratio[HR]=7.17). The same was true after the researchers adjusted for FLIPI score (HR=6.44).
The team observed similar results in a validation cohort of 147 FL patients who received first-line R-CHOP. At 2 years, the OS rate was 64% in the early progression group and 98% in the group that did not progress. At 5 years, OS rates were 34% and 94%, respectively.
Again, in an unadjusted analysis, early progression was associated with lower OS (HR=20.0). And this trend was maintained after the researchers adjusted for FLIPI score (HR=19.8).
The researchers also found that, for patients in the early progression group, clinical factors that were predictive of inferior OS were age, ECOG performance score, nodal sites, and disease stage. For the group that did not progress within 2 years, clinical factors that were predictive of OS were age and extranodal sites.
In a Cox regression analysis that encompassed these factors and early progression, only early progression, age, and ECOG performance scores remained significantly predictive of OS.
“[W]e have confirmed that all relapsed patients are not equal and therefore should not be approached the same at diagnosis, nor at the time of relapse, in terms of therapies,” Dr Casulo said.
“It will be critical to predict who is most likely to relapse early. We believe that targeted sequencing or gene-expression profiling will be important to understanding how to improve the outcomes of this group.” ![]()
Interim PET results guide ongoing therapy in Hodgkin lymphoma
Bleomycin can be eliminated after two cycles of the ABVD chemotherapeutic regimen based on a negative interim PET scan finding in patients with Hodgkin lymphoma, according to the 3-year findings of the RATHL study.
Being able to omit bleomycin after a negative interim PET scan was associated with a lower rate of pulmonary toxicity, but no loss in efficacy. For patients with positive interim PET scans, a more aggressive therapy was associated with good outcomes, suggesting that response-adapted therapy can yield good results, Dr. Peter Johnson said at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
In the large international RATHL study (Response-Adapted Therapy in Hodgkin Lymphoma study) 1,137 adults with newly diagnosed disease (41% stage II, 31% stage III, 28% stage IV) underwent PET-CT scans at baseline and after completing two cycles of ABVD (adriamycin, bleomycin, vinblastine, dacarbazine). The patients’ PET images were centrally reviewed using a 5-point scale as either negative (1-3) or positive (4-5),
The majority of patients (84%) had negative scans after two cycles of the ABVD regimen and were randomized to receive four additional cycles either with or without bleomycin (ABVD or AVD). Consolidation radiotherapy was not advised for patients whose interim PET scans were negative, regardless of baseline bulk or residual masses, Dr. Johnson, of the Cancer Research UK Centre at University of Southampton, England, reported.
Patients with positive interim PET scans received escalated therapy with a BEACOPP (bleomycin, etoposide, doxorubicin [Adriamycin], cyclophosphamide, vincristine [Oncovin], procarbazine, prednisolone) regimen. They received either eBEACOPP and BEASCOPP-14.
At the 3-year follow-up, progression-free survival in the PET-negative group was 85% for both the ABVD- and AVD-treated patients. Similarly, overall survival was 97% for both groups.
Factors that predicted treatment failure after a negative interim PET scan were initial tumor stage and international prognostic score, but not bulk, B symptoms, or score of the interim PET scan.
ABVD was associated with more pulmonary toxicity than was AVD.
Of 174 patients who had a positive interim PET scan and received escalated therapy, 74% had a subsequent negative PET scan after treatment. Their 3-year, progression-free survival rate was 68%, and their overall survival was 86% with no difference in outcome between two variations of BEACOPP (eBEACOPP and BEASCOPP-14).
Of the 53 deaths in the study, 19 were caused by Hodgkin lymphoma. The overall 3-year progression-free survival is 83%, and overall survival is 95%.
The results of the RATHL study have important implications for therapy of Hodgkin lymphoma, Dr. Johnson stated. First, interim PET scans are highly predictive for response to ABVD, providing valuable prognostic information to support decisions related to escalation of therapy. Secondly, after two cycles of ABVD, “it is safe to omit bleomycin from subsequent cycles, without consolidation radiotherapy,” he reported.
Omitting bleomycin has the potential to reduce pulmonary toxicity from chemotherapy, especially dyspnea, thromboembolism, and neutropenic fever, Dr. Johnson added. In the RATHL study, rates of pulmonary toxicity were significantly higher in the group receiving bleomycin.
This large randomized phase 3 RATHL trial has practice changing implications for advanced stage Hodgkin lymphoma. This trial had a simple straightforward design uniting two themes in Hodgkin research: (1) desire to minimizeor omit bleomycin due to its somewhat unpredictable pulmonary toxicity; and (2) utilizing early PET response- adapted strategies, though most such studies have focused on early stage patients. This trial demonstrates that patients with advanced stage Hodgkin lymphoma who achieve PET negativity after 2 cycles of ABVD, representing 84% of patients, do not need to be exposed to bleomycin during the last 4 cycles, reducing pulmonary toxicity. This has immediate clinical utility. Whether this can be extrapolated to early stage patients remains an interesting research question.
This large randomized phase 3 RATHL trial has practice changing implications for advanced stage Hodgkin lymphoma. This trial had a simple straightforward design uniting two themes in Hodgkin research: (1) desire to minimizeor omit bleomycin due to its somewhat unpredictable pulmonary toxicity; and (2) utilizing early PET response- adapted strategies, though most such studies have focused on early stage patients. This trial demonstrates that patients with advanced stage Hodgkin lymphoma who achieve PET negativity after 2 cycles of ABVD, representing 84% of patients, do not need to be exposed to bleomycin during the last 4 cycles, reducing pulmonary toxicity. This has immediate clinical utility. Whether this can be extrapolated to early stage patients remains an interesting research question.
This large randomized phase 3 RATHL trial has practice changing implications for advanced stage Hodgkin lymphoma. This trial had a simple straightforward design uniting two themes in Hodgkin research: (1) desire to minimizeor omit bleomycin due to its somewhat unpredictable pulmonary toxicity; and (2) utilizing early PET response- adapted strategies, though most such studies have focused on early stage patients. This trial demonstrates that patients with advanced stage Hodgkin lymphoma who achieve PET negativity after 2 cycles of ABVD, representing 84% of patients, do not need to be exposed to bleomycin during the last 4 cycles, reducing pulmonary toxicity. This has immediate clinical utility. Whether this can be extrapolated to early stage patients remains an interesting research question.
Bleomycin can be eliminated after two cycles of the ABVD chemotherapeutic regimen based on a negative interim PET scan finding in patients with Hodgkin lymphoma, according to the 3-year findings of the RATHL study.
Being able to omit bleomycin after a negative interim PET scan was associated with a lower rate of pulmonary toxicity, but no loss in efficacy. For patients with positive interim PET scans, a more aggressive therapy was associated with good outcomes, suggesting that response-adapted therapy can yield good results, Dr. Peter Johnson said at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
In the large international RATHL study (Response-Adapted Therapy in Hodgkin Lymphoma study) 1,137 adults with newly diagnosed disease (41% stage II, 31% stage III, 28% stage IV) underwent PET-CT scans at baseline and after completing two cycles of ABVD (adriamycin, bleomycin, vinblastine, dacarbazine). The patients’ PET images were centrally reviewed using a 5-point scale as either negative (1-3) or positive (4-5),
The majority of patients (84%) had negative scans after two cycles of the ABVD regimen and were randomized to receive four additional cycles either with or without bleomycin (ABVD or AVD). Consolidation radiotherapy was not advised for patients whose interim PET scans were negative, regardless of baseline bulk or residual masses, Dr. Johnson, of the Cancer Research UK Centre at University of Southampton, England, reported.
Patients with positive interim PET scans received escalated therapy with a BEACOPP (bleomycin, etoposide, doxorubicin [Adriamycin], cyclophosphamide, vincristine [Oncovin], procarbazine, prednisolone) regimen. They received either eBEACOPP and BEASCOPP-14.
At the 3-year follow-up, progression-free survival in the PET-negative group was 85% for both the ABVD- and AVD-treated patients. Similarly, overall survival was 97% for both groups.
Factors that predicted treatment failure after a negative interim PET scan were initial tumor stage and international prognostic score, but not bulk, B symptoms, or score of the interim PET scan.
ABVD was associated with more pulmonary toxicity than was AVD.
Of 174 patients who had a positive interim PET scan and received escalated therapy, 74% had a subsequent negative PET scan after treatment. Their 3-year, progression-free survival rate was 68%, and their overall survival was 86% with no difference in outcome between two variations of BEACOPP (eBEACOPP and BEASCOPP-14).
Of the 53 deaths in the study, 19 were caused by Hodgkin lymphoma. The overall 3-year progression-free survival is 83%, and overall survival is 95%.
The results of the RATHL study have important implications for therapy of Hodgkin lymphoma, Dr. Johnson stated. First, interim PET scans are highly predictive for response to ABVD, providing valuable prognostic information to support decisions related to escalation of therapy. Secondly, after two cycles of ABVD, “it is safe to omit bleomycin from subsequent cycles, without consolidation radiotherapy,” he reported.
Omitting bleomycin has the potential to reduce pulmonary toxicity from chemotherapy, especially dyspnea, thromboembolism, and neutropenic fever, Dr. Johnson added. In the RATHL study, rates of pulmonary toxicity were significantly higher in the group receiving bleomycin.
Bleomycin can be eliminated after two cycles of the ABVD chemotherapeutic regimen based on a negative interim PET scan finding in patients with Hodgkin lymphoma, according to the 3-year findings of the RATHL study.
Being able to omit bleomycin after a negative interim PET scan was associated with a lower rate of pulmonary toxicity, but no loss in efficacy. For patients with positive interim PET scans, a more aggressive therapy was associated with good outcomes, suggesting that response-adapted therapy can yield good results, Dr. Peter Johnson said at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
In the large international RATHL study (Response-Adapted Therapy in Hodgkin Lymphoma study) 1,137 adults with newly diagnosed disease (41% stage II, 31% stage III, 28% stage IV) underwent PET-CT scans at baseline and after completing two cycles of ABVD (adriamycin, bleomycin, vinblastine, dacarbazine). The patients’ PET images were centrally reviewed using a 5-point scale as either negative (1-3) or positive (4-5),
The majority of patients (84%) had negative scans after two cycles of the ABVD regimen and were randomized to receive four additional cycles either with or without bleomycin (ABVD or AVD). Consolidation radiotherapy was not advised for patients whose interim PET scans were negative, regardless of baseline bulk or residual masses, Dr. Johnson, of the Cancer Research UK Centre at University of Southampton, England, reported.
Patients with positive interim PET scans received escalated therapy with a BEACOPP (bleomycin, etoposide, doxorubicin [Adriamycin], cyclophosphamide, vincristine [Oncovin], procarbazine, prednisolone) regimen. They received either eBEACOPP and BEASCOPP-14.
At the 3-year follow-up, progression-free survival in the PET-negative group was 85% for both the ABVD- and AVD-treated patients. Similarly, overall survival was 97% for both groups.
Factors that predicted treatment failure after a negative interim PET scan were initial tumor stage and international prognostic score, but not bulk, B symptoms, or score of the interim PET scan.
ABVD was associated with more pulmonary toxicity than was AVD.
Of 174 patients who had a positive interim PET scan and received escalated therapy, 74% had a subsequent negative PET scan after treatment. Their 3-year, progression-free survival rate was 68%, and their overall survival was 86% with no difference in outcome between two variations of BEACOPP (eBEACOPP and BEASCOPP-14).
Of the 53 deaths in the study, 19 were caused by Hodgkin lymphoma. The overall 3-year progression-free survival is 83%, and overall survival is 95%.
The results of the RATHL study have important implications for therapy of Hodgkin lymphoma, Dr. Johnson stated. First, interim PET scans are highly predictive for response to ABVD, providing valuable prognostic information to support decisions related to escalation of therapy. Secondly, after two cycles of ABVD, “it is safe to omit bleomycin from subsequent cycles, without consolidation radiotherapy,” he reported.
Omitting bleomycin has the potential to reduce pulmonary toxicity from chemotherapy, especially dyspnea, thromboembolism, and neutropenic fever, Dr. Johnson added. In the RATHL study, rates of pulmonary toxicity were significantly higher in the group receiving bleomycin.
AT 13-ICML
Key clinical point: Bleomycin can be eliminated after two cycles of the ABVD chemotherapeutic regimen based on a negative interim PET scan finding in patients with Hodgkin lymphoma.
Major finding: At the 3-year follow-up, progression-free survival in the PET-negative group was 85% for both the ABVD- and AVD-treated patients. Similarly, overall survival was 97% for both groups.
Data source: The international RATHL study (Response-Adapted Therapy in Hodgkin Lymphoma study).
Disclosures: The study was supported by Cancer Research UK, Experimental Cancer Medicine Centre (ECMC), and the National Institute for Health Research Cancer Research Network (NCRN). The researchers had no relevant financial disclosures.
Listen Now: Teaching Value-Based Care: A Med-Ed Perspective
Adolescent cancer survivors report more emotional, neurocognitive impairment than do siblings
Adult survivors of cancer who were diagnosed between the ages of 11 and 21 years self-reported higher rates of impairment in emotional and neurocognitive outcomes compared with their sibling counterparts, according to researchers.
Compared with siblings, survivors reported greater anxiety (odds ratio [OR], 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89). Survivors were significantly less likely to be employed (P < .001).
Previous reports have shown that survivors of childhood cancers have increased risk for impaired neurocognitive functioning, but this is the first study to examine outcomes in survivors who were diagnosed during adolescence and early young adulthood.
“Cancer treatment during this time has the potential to interfere with adolescents’ separation from caregivers, autonomy with regard to planning social and academic schedules, participation in social activities, and maintaining privacy, particularly of their bodies,” wrote Dr. Pinki Prasad, assistant professor of pediatrics at Louisiana State University, New Orleans.
Survivors who were diagnosed with CNS tumors or leukemia during adolescence reported rates of emotional distress and neurocognitive dysfunction similar to rates of those diagnosed during early childhood, whereas diagnoses of lymphoma/sarcoma during adolescence resulted in lower risk of impairment compared with early childhood diagnoses. This may be due to the fact that the leukemia/CNS tumor group was more likely to receive cranial radiation therapy, a predictor of neurocognitive late effects, Dr. Prasad and colleagues wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.57.7528]).
Among those diagnosed with lymphomas or sarcomas during adolescence, treatment with corticosteroids was associated with greater risk of self-reported difficulties with somatization, anxiety, task efficiency, and memory.
The Childhood Cancer Survivor Study (CCSS) is a multicenter, retrospective cohort study that comprised 2,589 survivors who were diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts. Participants completed the Brief Symptom Inventory–18, which measures symptoms of emotional distress, and the CCSS Neurocognitive Questionnaire.
The authors noted that the results indicate “high rates of self-reported impairment in neurocognitive function and psychological distress that are associated with limitation in development of adult social milestones,” and that further follow-up with survivors of adolescent and early young adult cancers may be necessary.
Adult survivors of cancer who were diagnosed between the ages of 11 and 21 years self-reported higher rates of impairment in emotional and neurocognitive outcomes compared with their sibling counterparts, according to researchers.
Compared with siblings, survivors reported greater anxiety (odds ratio [OR], 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89). Survivors were significantly less likely to be employed (P < .001).
Previous reports have shown that survivors of childhood cancers have increased risk for impaired neurocognitive functioning, but this is the first study to examine outcomes in survivors who were diagnosed during adolescence and early young adulthood.
“Cancer treatment during this time has the potential to interfere with adolescents’ separation from caregivers, autonomy with regard to planning social and academic schedules, participation in social activities, and maintaining privacy, particularly of their bodies,” wrote Dr. Pinki Prasad, assistant professor of pediatrics at Louisiana State University, New Orleans.
Survivors who were diagnosed with CNS tumors or leukemia during adolescence reported rates of emotional distress and neurocognitive dysfunction similar to rates of those diagnosed during early childhood, whereas diagnoses of lymphoma/sarcoma during adolescence resulted in lower risk of impairment compared with early childhood diagnoses. This may be due to the fact that the leukemia/CNS tumor group was more likely to receive cranial radiation therapy, a predictor of neurocognitive late effects, Dr. Prasad and colleagues wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.57.7528]).
Among those diagnosed with lymphomas or sarcomas during adolescence, treatment with corticosteroids was associated with greater risk of self-reported difficulties with somatization, anxiety, task efficiency, and memory.
The Childhood Cancer Survivor Study (CCSS) is a multicenter, retrospective cohort study that comprised 2,589 survivors who were diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts. Participants completed the Brief Symptom Inventory–18, which measures symptoms of emotional distress, and the CCSS Neurocognitive Questionnaire.
The authors noted that the results indicate “high rates of self-reported impairment in neurocognitive function and psychological distress that are associated with limitation in development of adult social milestones,” and that further follow-up with survivors of adolescent and early young adult cancers may be necessary.
Adult survivors of cancer who were diagnosed between the ages of 11 and 21 years self-reported higher rates of impairment in emotional and neurocognitive outcomes compared with their sibling counterparts, according to researchers.
Compared with siblings, survivors reported greater anxiety (odds ratio [OR], 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89). Survivors were significantly less likely to be employed (P < .001).
Previous reports have shown that survivors of childhood cancers have increased risk for impaired neurocognitive functioning, but this is the first study to examine outcomes in survivors who were diagnosed during adolescence and early young adulthood.
“Cancer treatment during this time has the potential to interfere with adolescents’ separation from caregivers, autonomy with regard to planning social and academic schedules, participation in social activities, and maintaining privacy, particularly of their bodies,” wrote Dr. Pinki Prasad, assistant professor of pediatrics at Louisiana State University, New Orleans.
Survivors who were diagnosed with CNS tumors or leukemia during adolescence reported rates of emotional distress and neurocognitive dysfunction similar to rates of those diagnosed during early childhood, whereas diagnoses of lymphoma/sarcoma during adolescence resulted in lower risk of impairment compared with early childhood diagnoses. This may be due to the fact that the leukemia/CNS tumor group was more likely to receive cranial radiation therapy, a predictor of neurocognitive late effects, Dr. Prasad and colleagues wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.57.7528]).
Among those diagnosed with lymphomas or sarcomas during adolescence, treatment with corticosteroids was associated with greater risk of self-reported difficulties with somatization, anxiety, task efficiency, and memory.
The Childhood Cancer Survivor Study (CCSS) is a multicenter, retrospective cohort study that comprised 2,589 survivors who were diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts. Participants completed the Brief Symptom Inventory–18, which measures symptoms of emotional distress, and the CCSS Neurocognitive Questionnaire.
The authors noted that the results indicate “high rates of self-reported impairment in neurocognitive function and psychological distress that are associated with limitation in development of adult social milestones,” and that further follow-up with survivors of adolescent and early young adult cancers may be necessary.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Adult cancer survivors diagnosed between ages 11 and 21 years self-reported higher rates of emotional distress and neurocognitive dysfunction compared with sibling counterparts.
Major finding: Compared with siblings, survivors reported greater anxiety (odds ratio, 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89).
Data source: The Childhood Cancer Survivor Study, a multicenter, retrospective cohort study of 2,589 survivors diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts.
Disclosures: The National Cancer Institute supported the study. Dr. Prasad reported having no disclosures.
Repigmentation of Gray Hair in Lesions of Annular Elastolytic Giant Cell Granuloma
Hair pigmentation is a complex phenomenon that involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu.1 Repigmentation of gray hair has been associated with herpes zoster infection,2 use of systemic corticosteroids,3 thyroid hormone therapy,4 or treatment with interferon and ribavirin.5 We report a case of repigmentation of gray hairs in lesions of annular elastolytic giant cell granuloma (AEGCG) on the scalp of a 67-year-old man.
Case Report
A 67-year-old man presented to the dermatology department for evaluation of pruritic lesions on the face and scalp of 1 year’s duration. The patient reported that hairs in the involved areas of the scalp had turned from gray to a dark color since the appearance of the lesions. The patient had a history of hypertension and type 2 diabetes mellitus. His current medications included irbesartan, atorvastatin, metformin, acetylsalicylic acid, omeprazole, and repaglinide.
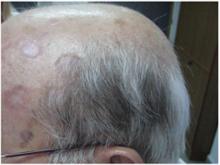
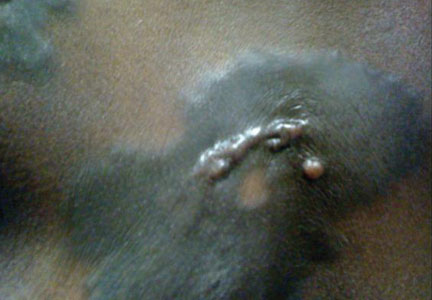
Physical examination revealed plaques on the scalp and cheeks that were 2 to 10 mm in diameter. Some of the plaques had an atrophic center and a desquamative peripheral border. The patient had androgenetic alopecia. The remaining hair was dark in the areas affected by the inflammatory plaques while it remained white-gray in the uninvolved areas (Figure 1).
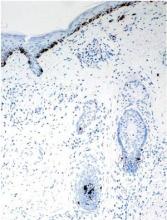
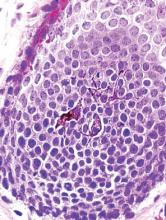
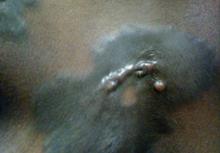
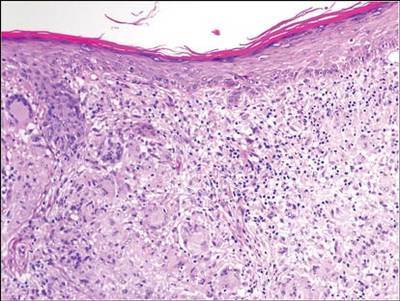
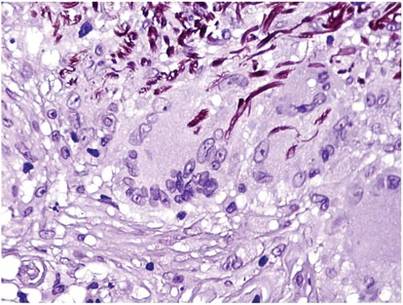
A biopsy of one of the lesions was performed. Histopathology revealed a granulomatous dermatitis involving mostly the upper and mid dermis (Figure 2). Granulomas were epithelioid with many giant cells, some of which contained many nuclei. A ringed array of nuclei was noted in some histiocytes. Elastic fibers were absent in the central zone of the granulomas, a finding that was better evidenced on orcein staining (Figure 3). On the contrary, the peripheral zone of the granulomas showed an increased amount of thick elastotic material. Elastophagocytosis was observed, but no asteroid bodies, Schaumann bodies, or mucin deposits were noted. Histochemistry for microorganisms with Ziehl-Neelsen and periodic acid–Schiff staining was negative. Other findings included a mild infiltrate of melanophages in the papillary dermis as well as a mild superficial dermal inflammatory infiltrate that was rich in plasma cells. Immunostaining for Treponema pallidum was negative. The lymphocytic infiltrate was CD4+predominant. A prominent dermal elastosis also was noted. Hair follicles within the plaques were small in size, penetrating just the dermis. Immunostaining for HMB-45, melan-A, and S-100 demonstrated preserved melanocytes in the hair bulbs (Figure 4). CD68 immunostaining made the infiltrate of macrophages stand out. Based on the results of the histopathologic evaluation, a diagnosis of AEGCG was made.
| 
|
Comment
Annular elastolytic giant cell granuloma is a controversial entity that was first described by O’Brien6 in 1975 as actinic granuloma. Hanke et al7 proposed the term annular elastolytic giant cell granuloma to encompass lesions previously called actinic granuloma, atypical necrobiosis lipoidica, and Miescher granuloma. Some researchers have claimed that AEGCG is an independent entity, therefore separate and distinguishable from granuloma annulare. Histopathologic clues to distinguish AEGCG from granuloma annulare have been noted in the literature.7-9 Other investigators believe AEGCG is a type of granuloma annulare that appears on exposed skin.10 There are several variants of the classic clinical presentation of AEGCG, such as cases including presentation in unexposed areas of the skin,11 a papular variant,12 a rapidly regressive variant,13 a reticular variant,14 a variant of early childhood,15 a generalized variant,16 presentation in a necklace distribution,17 presentation as alopecia,18 a sarcoid variant,19 or presentation as reticulate erythema.20 However, no variant has been associated with hair repigmentation.
Melanin units from the proximal hair bulb are responsible for pigmentation in adult hair follicles and are integrated by the hair matrix, melanocytes, keratinocytes, and fibroblasts.21 Hair bulb melanocytes are larger and more dendritic than epidermal melanocytes (Figure 5). The hair only pigments during the anagen phase; therefore, its pigmentation is cyclic, as opposed to epidermal pigmentation, which is ongoing. Hair pigmentation is the result of a complex interaction between the epithelium, the mesenchyme, and the neuroectoderm. This complex pigmentation results from the interaction between follicular melanocytes, keratinocytes, and the fibroblasts from the hair papilla.22 Hair pigmentation involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu1,23-25 (Table), and it is regulated by autocrines, paracrines, or intracrines.21 Therefore, it is likely that many environmental factors may affect hair pigmentation, which may explain why repigmentation of the hair has been seen in the setting of herpes zoster infection,2 use of systemic corticosteroids in the treatment of bullous pemphigoid,3 thyroid hormone therapy,4 treatment with interferon and ribavirin,5 porphyria cutanea tarda,26 or lentigo maligna.27 In our patient, AEGCG might have induced some changes in the dermal environment that were responsible for the repigmentation of the patient’s gray hair. It is speculated that solar radiation and other factors can transform the antigenicity of elastic fibers and induce an immune response in AEGCG.12,15 The lymphocytic infiltrate in these lesions is predominantly CD4+, as seen in our patient, which is consistent with an autoimmune hypothesis.15 Nevertheless, it most likely is too simplistic to attribute the repigmentation to the influence of just these cells.
1. Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155-1228.
2. Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
3. Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatol Venereol. 2008;22:1018-1020.
4. Redondo P, Guzmán M, Marquina M, et al. Repigmentation of gray hair after thyroid hormone treatment [in Spanish]. Actas Dermosifiliogr. 2007;98:603-610.
5. Kavak A, Akcan Y, Korkmaz U. Hair repigmentation in a hepatitis C patient treated with interferon and ribavirin. Dermatology. 2005;211:171-172.
6. O’Brien JP. Actinic granuloma. an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
7. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
8. Al-Hoqail IA, Al-Ghamdi AM, Martinka M, et al. Actinic granuloma is a unique and distinct entity: a comparative study with granuloma annulare. Am J Dermatopathol. 2002;24:209-212.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
11. Muramatsu T, Shirai T, Yamashina Y, et al. Annular elastolytic giant cell granuloma: an unusual case with lesions arising in non-sun-exposed areas. J Dermatol. 1987;14:54-58.
12. Kelly BJ, Mrstik ME, Ramos-Caro FA, et al. Papular elastolytic giant cell granuloma responding to hydroxychloroquine and quinacrine. Int J Dermatol. 2004;43:964-966.
13. Misago N, Ohtsuka Y, Ishii K, et al. Papular and reticular elastolytic giant cell granuloma: rapid spontaneous regression. Acta Derm Venereol. 2007;87:89-90.
14. Hinrichs R, Weiss T, Peschke E, et al. A reticular variant of elastolytic giant cell granuloma. Clin Exp Dermatol. 2006;31:42-44.
15. Lee HW, Lee MW, Choi JH, et al. Annular elastolytic giant cell granuloma in an infant: improvement after treatment with oral tranilast andtopical pimecrolimus. J Am Acad Dermatol. 2005;53(5, suppl 1):S244-S246.
16. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422.
17. Meadows KP, O’Reilly MA, Harris RM, et al. Erythematous annular plaques in a necklace distribution. annular elastolytic giant cell granuloma. Arch Dermatol. 2001;137:1647-1652.
18. Delgado-Jimenez Y, Perez-Gala S, Peñas PF, et al. O’Brien actinic granuloma presenting as alopecia. J Eur Acad Dermatol Venereol. 2006;20:226-227.
19. Gambichler T, Herde M, Hoffmann K, et al. Sarcoid variant of actinic granuloma: is it annular sarcoidosis? Dermatology. 2001;203:353-354.
20. Bannister MJ, Rubel DM, Kossard S. Mid-dermal elastophagocytosis presenting as a persistent reticulate erythema. Australas J Dermatol. 2001;42:50-54.
21. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 suppl):90S-97S.
22. Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
23. Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4:24-28.
24. Slominski A, Wortsman J. Neuroendocrinology of the skin [published correction appears in Endocr Rev. 2002;23:364]. Endocr Rev. 2000;21:457-487.
25. Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979-1020.
26. Shaffrali FC, McDonagh AJ, Messenger AG. Hair darkening in porphyria cutanea tarda. Br J Dermatol. 2002;146:325-329.
27. Dummer R. Clinical picture: hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
Hair pigmentation is a complex phenomenon that involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu.1 Repigmentation of gray hair has been associated with herpes zoster infection,2 use of systemic corticosteroids,3 thyroid hormone therapy,4 or treatment with interferon and ribavirin.5 We report a case of repigmentation of gray hairs in lesions of annular elastolytic giant cell granuloma (AEGCG) on the scalp of a 67-year-old man.
Case Report
A 67-year-old man presented to the dermatology department for evaluation of pruritic lesions on the face and scalp of 1 year’s duration. The patient reported that hairs in the involved areas of the scalp had turned from gray to a dark color since the appearance of the lesions. The patient had a history of hypertension and type 2 diabetes mellitus. His current medications included irbesartan, atorvastatin, metformin, acetylsalicylic acid, omeprazole, and repaglinide.
Physical examination revealed plaques on the scalp and cheeks that were 2 to 10 mm in diameter. Some of the plaques had an atrophic center and a desquamative peripheral border. The patient had androgenetic alopecia. The remaining hair was dark in the areas affected by the inflammatory plaques while it remained white-gray in the uninvolved areas (Figure 1).
A biopsy of one of the lesions was performed. Histopathology revealed a granulomatous dermatitis involving mostly the upper and mid dermis (Figure 2). Granulomas were epithelioid with many giant cells, some of which contained many nuclei. A ringed array of nuclei was noted in some histiocytes. Elastic fibers were absent in the central zone of the granulomas, a finding that was better evidenced on orcein staining (Figure 3). On the contrary, the peripheral zone of the granulomas showed an increased amount of thick elastotic material. Elastophagocytosis was observed, but no asteroid bodies, Schaumann bodies, or mucin deposits were noted. Histochemistry for microorganisms with Ziehl-Neelsen and periodic acid–Schiff staining was negative. Other findings included a mild infiltrate of melanophages in the papillary dermis as well as a mild superficial dermal inflammatory infiltrate that was rich in plasma cells. Immunostaining for Treponema pallidum was negative. The lymphocytic infiltrate was CD4+predominant. A prominent dermal elastosis also was noted. Hair follicles within the plaques were small in size, penetrating just the dermis. Immunostaining for HMB-45, melan-A, and S-100 demonstrated preserved melanocytes in the hair bulbs (Figure 4). CD68 immunostaining made the infiltrate of macrophages stand out. Based on the results of the histopathologic evaluation, a diagnosis of AEGCG was made.

| 
|
Comment
Annular elastolytic giant cell granuloma is a controversial entity that was first described by O’Brien6 in 1975 as actinic granuloma. Hanke et al7 proposed the term annular elastolytic giant cell granuloma to encompass lesions previously called actinic granuloma, atypical necrobiosis lipoidica, and Miescher granuloma. Some researchers have claimed that AEGCG is an independent entity, therefore separate and distinguishable from granuloma annulare. Histopathologic clues to distinguish AEGCG from granuloma annulare have been noted in the literature.7-9 Other investigators believe AEGCG is a type of granuloma annulare that appears on exposed skin.10 There are several variants of the classic clinical presentation of AEGCG, such as cases including presentation in unexposed areas of the skin,11 a papular variant,12 a rapidly regressive variant,13 a reticular variant,14 a variant of early childhood,15 a generalized variant,16 presentation in a necklace distribution,17 presentation as alopecia,18 a sarcoid variant,19 or presentation as reticulate erythema.20 However, no variant has been associated with hair repigmentation.
Melanin units from the proximal hair bulb are responsible for pigmentation in adult hair follicles and are integrated by the hair matrix, melanocytes, keratinocytes, and fibroblasts.21 Hair bulb melanocytes are larger and more dendritic than epidermal melanocytes (Figure 5). The hair only pigments during the anagen phase; therefore, its pigmentation is cyclic, as opposed to epidermal pigmentation, which is ongoing. Hair pigmentation is the result of a complex interaction between the epithelium, the mesenchyme, and the neuroectoderm. This complex pigmentation results from the interaction between follicular melanocytes, keratinocytes, and the fibroblasts from the hair papilla.22 Hair pigmentation involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu1,23-25 (Table), and it is regulated by autocrines, paracrines, or intracrines.21 Therefore, it is likely that many environmental factors may affect hair pigmentation, which may explain why repigmentation of the hair has been seen in the setting of herpes zoster infection,2 use of systemic corticosteroids in the treatment of bullous pemphigoid,3 thyroid hormone therapy,4 treatment with interferon and ribavirin,5 porphyria cutanea tarda,26 or lentigo maligna.27 In our patient, AEGCG might have induced some changes in the dermal environment that were responsible for the repigmentation of the patient’s gray hair. It is speculated that solar radiation and other factors can transform the antigenicity of elastic fibers and induce an immune response in AEGCG.12,15 The lymphocytic infiltrate in these lesions is predominantly CD4+, as seen in our patient, which is consistent with an autoimmune hypothesis.15 Nevertheless, it most likely is too simplistic to attribute the repigmentation to the influence of just these cells.
Hair pigmentation is a complex phenomenon that involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu.1 Repigmentation of gray hair has been associated with herpes zoster infection,2 use of systemic corticosteroids,3 thyroid hormone therapy,4 or treatment with interferon and ribavirin.5 We report a case of repigmentation of gray hairs in lesions of annular elastolytic giant cell granuloma (AEGCG) on the scalp of a 67-year-old man.
Case Report
A 67-year-old man presented to the dermatology department for evaluation of pruritic lesions on the face and scalp of 1 year’s duration. The patient reported that hairs in the involved areas of the scalp had turned from gray to a dark color since the appearance of the lesions. The patient had a history of hypertension and type 2 diabetes mellitus. His current medications included irbesartan, atorvastatin, metformin, acetylsalicylic acid, omeprazole, and repaglinide.
Physical examination revealed plaques on the scalp and cheeks that were 2 to 10 mm in diameter. Some of the plaques had an atrophic center and a desquamative peripheral border. The patient had androgenetic alopecia. The remaining hair was dark in the areas affected by the inflammatory plaques while it remained white-gray in the uninvolved areas (Figure 1).
A biopsy of one of the lesions was performed. Histopathology revealed a granulomatous dermatitis involving mostly the upper and mid dermis (Figure 2). Granulomas were epithelioid with many giant cells, some of which contained many nuclei. A ringed array of nuclei was noted in some histiocytes. Elastic fibers were absent in the central zone of the granulomas, a finding that was better evidenced on orcein staining (Figure 3). On the contrary, the peripheral zone of the granulomas showed an increased amount of thick elastotic material. Elastophagocytosis was observed, but no asteroid bodies, Schaumann bodies, or mucin deposits were noted. Histochemistry for microorganisms with Ziehl-Neelsen and periodic acid–Schiff staining was negative. Other findings included a mild infiltrate of melanophages in the papillary dermis as well as a mild superficial dermal inflammatory infiltrate that was rich in plasma cells. Immunostaining for Treponema pallidum was negative. The lymphocytic infiltrate was CD4+predominant. A prominent dermal elastosis also was noted. Hair follicles within the plaques were small in size, penetrating just the dermis. Immunostaining for HMB-45, melan-A, and S-100 demonstrated preserved melanocytes in the hair bulbs (Figure 4). CD68 immunostaining made the infiltrate of macrophages stand out. Based on the results of the histopathologic evaluation, a diagnosis of AEGCG was made.

| 
|
Comment
Annular elastolytic giant cell granuloma is a controversial entity that was first described by O’Brien6 in 1975 as actinic granuloma. Hanke et al7 proposed the term annular elastolytic giant cell granuloma to encompass lesions previously called actinic granuloma, atypical necrobiosis lipoidica, and Miescher granuloma. Some researchers have claimed that AEGCG is an independent entity, therefore separate and distinguishable from granuloma annulare. Histopathologic clues to distinguish AEGCG from granuloma annulare have been noted in the literature.7-9 Other investigators believe AEGCG is a type of granuloma annulare that appears on exposed skin.10 There are several variants of the classic clinical presentation of AEGCG, such as cases including presentation in unexposed areas of the skin,11 a papular variant,12 a rapidly regressive variant,13 a reticular variant,14 a variant of early childhood,15 a generalized variant,16 presentation in a necklace distribution,17 presentation as alopecia,18 a sarcoid variant,19 or presentation as reticulate erythema.20 However, no variant has been associated with hair repigmentation.
Melanin units from the proximal hair bulb are responsible for pigmentation in adult hair follicles and are integrated by the hair matrix, melanocytes, keratinocytes, and fibroblasts.21 Hair bulb melanocytes are larger and more dendritic than epidermal melanocytes (Figure 5). The hair only pigments during the anagen phase; therefore, its pigmentation is cyclic, as opposed to epidermal pigmentation, which is ongoing. Hair pigmentation is the result of a complex interaction between the epithelium, the mesenchyme, and the neuroectoderm. This complex pigmentation results from the interaction between follicular melanocytes, keratinocytes, and the fibroblasts from the hair papilla.22 Hair pigmentation involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu1,23-25 (Table), and it is regulated by autocrines, paracrines, or intracrines.21 Therefore, it is likely that many environmental factors may affect hair pigmentation, which may explain why repigmentation of the hair has been seen in the setting of herpes zoster infection,2 use of systemic corticosteroids in the treatment of bullous pemphigoid,3 thyroid hormone therapy,4 treatment with interferon and ribavirin,5 porphyria cutanea tarda,26 or lentigo maligna.27 In our patient, AEGCG might have induced some changes in the dermal environment that were responsible for the repigmentation of the patient’s gray hair. It is speculated that solar radiation and other factors can transform the antigenicity of elastic fibers and induce an immune response in AEGCG.12,15 The lymphocytic infiltrate in these lesions is predominantly CD4+, as seen in our patient, which is consistent with an autoimmune hypothesis.15 Nevertheless, it most likely is too simplistic to attribute the repigmentation to the influence of just these cells.
1. Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155-1228.
2. Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
3. Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatol Venereol. 2008;22:1018-1020.
4. Redondo P, Guzmán M, Marquina M, et al. Repigmentation of gray hair after thyroid hormone treatment [in Spanish]. Actas Dermosifiliogr. 2007;98:603-610.
5. Kavak A, Akcan Y, Korkmaz U. Hair repigmentation in a hepatitis C patient treated with interferon and ribavirin. Dermatology. 2005;211:171-172.
6. O’Brien JP. Actinic granuloma. an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
7. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
8. Al-Hoqail IA, Al-Ghamdi AM, Martinka M, et al. Actinic granuloma is a unique and distinct entity: a comparative study with granuloma annulare. Am J Dermatopathol. 2002;24:209-212.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
11. Muramatsu T, Shirai T, Yamashina Y, et al. Annular elastolytic giant cell granuloma: an unusual case with lesions arising in non-sun-exposed areas. J Dermatol. 1987;14:54-58.
12. Kelly BJ, Mrstik ME, Ramos-Caro FA, et al. Papular elastolytic giant cell granuloma responding to hydroxychloroquine and quinacrine. Int J Dermatol. 2004;43:964-966.
13. Misago N, Ohtsuka Y, Ishii K, et al. Papular and reticular elastolytic giant cell granuloma: rapid spontaneous regression. Acta Derm Venereol. 2007;87:89-90.
14. Hinrichs R, Weiss T, Peschke E, et al. A reticular variant of elastolytic giant cell granuloma. Clin Exp Dermatol. 2006;31:42-44.
15. Lee HW, Lee MW, Choi JH, et al. Annular elastolytic giant cell granuloma in an infant: improvement after treatment with oral tranilast andtopical pimecrolimus. J Am Acad Dermatol. 2005;53(5, suppl 1):S244-S246.
16. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422.
17. Meadows KP, O’Reilly MA, Harris RM, et al. Erythematous annular plaques in a necklace distribution. annular elastolytic giant cell granuloma. Arch Dermatol. 2001;137:1647-1652.
18. Delgado-Jimenez Y, Perez-Gala S, Peñas PF, et al. O’Brien actinic granuloma presenting as alopecia. J Eur Acad Dermatol Venereol. 2006;20:226-227.
19. Gambichler T, Herde M, Hoffmann K, et al. Sarcoid variant of actinic granuloma: is it annular sarcoidosis? Dermatology. 2001;203:353-354.
20. Bannister MJ, Rubel DM, Kossard S. Mid-dermal elastophagocytosis presenting as a persistent reticulate erythema. Australas J Dermatol. 2001;42:50-54.
21. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 suppl):90S-97S.
22. Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
23. Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4:24-28.
24. Slominski A, Wortsman J. Neuroendocrinology of the skin [published correction appears in Endocr Rev. 2002;23:364]. Endocr Rev. 2000;21:457-487.
25. Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979-1020.
26. Shaffrali FC, McDonagh AJ, Messenger AG. Hair darkening in porphyria cutanea tarda. Br J Dermatol. 2002;146:325-329.
27. Dummer R. Clinical picture: hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
1. Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155-1228.
2. Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
3. Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatol Venereol. 2008;22:1018-1020.
4. Redondo P, Guzmán M, Marquina M, et al. Repigmentation of gray hair after thyroid hormone treatment [in Spanish]. Actas Dermosifiliogr. 2007;98:603-610.
5. Kavak A, Akcan Y, Korkmaz U. Hair repigmentation in a hepatitis C patient treated with interferon and ribavirin. Dermatology. 2005;211:171-172.
6. O’Brien JP. Actinic granuloma. an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
7. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
8. Al-Hoqail IA, Al-Ghamdi AM, Martinka M, et al. Actinic granuloma is a unique and distinct entity: a comparative study with granuloma annulare. Am J Dermatopathol. 2002;24:209-212.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
11. Muramatsu T, Shirai T, Yamashina Y, et al. Annular elastolytic giant cell granuloma: an unusual case with lesions arising in non-sun-exposed areas. J Dermatol. 1987;14:54-58.
12. Kelly BJ, Mrstik ME, Ramos-Caro FA, et al. Papular elastolytic giant cell granuloma responding to hydroxychloroquine and quinacrine. Int J Dermatol. 2004;43:964-966.
13. Misago N, Ohtsuka Y, Ishii K, et al. Papular and reticular elastolytic giant cell granuloma: rapid spontaneous regression. Acta Derm Venereol. 2007;87:89-90.
14. Hinrichs R, Weiss T, Peschke E, et al. A reticular variant of elastolytic giant cell granuloma. Clin Exp Dermatol. 2006;31:42-44.
15. Lee HW, Lee MW, Choi JH, et al. Annular elastolytic giant cell granuloma in an infant: improvement after treatment with oral tranilast andtopical pimecrolimus. J Am Acad Dermatol. 2005;53(5, suppl 1):S244-S246.
16. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422.
17. Meadows KP, O’Reilly MA, Harris RM, et al. Erythematous annular plaques in a necklace distribution. annular elastolytic giant cell granuloma. Arch Dermatol. 2001;137:1647-1652.
18. Delgado-Jimenez Y, Perez-Gala S, Peñas PF, et al. O’Brien actinic granuloma presenting as alopecia. J Eur Acad Dermatol Venereol. 2006;20:226-227.
19. Gambichler T, Herde M, Hoffmann K, et al. Sarcoid variant of actinic granuloma: is it annular sarcoidosis? Dermatology. 2001;203:353-354.
20. Bannister MJ, Rubel DM, Kossard S. Mid-dermal elastophagocytosis presenting as a persistent reticulate erythema. Australas J Dermatol. 2001;42:50-54.
21. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 suppl):90S-97S.
22. Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
23. Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4:24-28.
24. Slominski A, Wortsman J. Neuroendocrinology of the skin [published correction appears in Endocr Rev. 2002;23:364]. Endocr Rev. 2000;21:457-487.
25. Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979-1020.
26. Shaffrali FC, McDonagh AJ, Messenger AG. Hair darkening in porphyria cutanea tarda. Br J Dermatol. 2002;146:325-329.
27. Dummer R. Clinical picture: hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
Practice Points
- Hair repigmentation can be a clinical clue to a subjacent inflammatory disease.
- Hair depigmentation associated with aging may be a reversible condition under proper stimulation.
Get more patients with backlinks
The medical profession has its jargon. So does the Internet world. Some of that jargon can be important to your success. “Backlinks” or “inbound links” are terms that should get your attention.
Why?
By developing them, you can attract more patients to your practice.
Backlinks are one piece of the Internet marketing puzzle that can help get your Web site on the first page of Google search results.
And just how important is it to be on page 1?
Well, consider that 91.5% of Web surfers do not go beyond the first page of results. That’s what an online advertising network called Chitika found when it examined tens of millions of online ad impressions in which the user was referred to the page via a Google search.1
Just what are backlinks? They are links to your Web page from another site. In basic link terminology, a backlink is any link received by a Web page, directory, Web site, or top-level domain from another, similar site. In this article we discuss the importance of these links and ways to use them in your social media to attract new patients to your site and your practice.
Start with good site design
If you can get listed on the first page of Google search results for the keywords your patients are using, more traffic will come to your Web site. That won’t help if you have a poorly designed site that has no patient conversion strategies, techniques, and systems to transform Web site visitors to patients.
You see, everything has to work together in a coordinated, integrated manner if you want to increase the number of patients who are looking for your services online. We’ve covered many of the basics in earlier articles on Web site design and improvement (see the box below). If you have a nonoptimal site, consider starting with these articles.
Articles on Web design and Internet usage by Ron Romano and Neil H. Baum, MD
5 ways to wake up your Web site
April 2015
Using the Internet in your practice
Part 1: Why social media are important and how to get started
February 2014
Part 2: Generating new patients using social media
April 2014
Part 3: Maximizing your online reach through SEO and pay-per-click
September 2014
Part 4: Reputation management: How to gather kudos and combat negative online reviews
December 2014
These articles are available in the archive at obgmanagement.com
Why backlinks are important
Google uses more than 200 algorithms to rank your Web site. Some are more important than others and have a greater influence on search engine positioning. Backlinks are one of those important influencers.
The number of backlinks you have is an indication of the popularity or importance of your Web site. Google considers a site more significant or relevant than others if it has a large number of quality backlinks from other directories, ezines, blogs, and social media Web sites. These backlinks must be relevant to your keywords. For example, because you are a medical professional, a link to your site from the American Cancer Society or the Mayo Clinic is considered more credible than a link from a local spa or health club.
A search engine such as Google considers the content of the sites it places at the top of the search results page. When links to your site come from other credible and popular sites, and those sites have content related to your site, these backlinks are considered more relevant to your site.
If backlinks come from sites with unrelated content, they are considered less relevant. You may even be penalized by Google for adding backlinks that have no content value.
For example, if a Web master has a site that focuses on urinary incontinence and receives a backlink from another site with information or articles about urinary incontinence, that backlink will be considered more relevant than a link from a site about mortgages that somehow also includes urinary incontinence on its page. Therefore, the higher the relevance of the site linking back to yours, the better the quality of that link.
Top 7 inbound links—and how to obtain them
1. Directories
Directories are indexes of online sites, typically organized by category. You want to ensure that each of your keywords is manually submitted to each directory so it is listed separately. This way you get maximum link value for each keyword.
Links back to your site from directories such as Yahoo Directory and DMOZ.org are valuable. DMOZ.org is edited by humans. Although it is free, it may take some time for your site to be added. A listing in Yahoo’s Directory costs $299 per year.
2. Press releases
If you are writing press releases, make sure they contain keywords that someone would use to find a business like yours. Also ensure that they include links back to your site.
Once the press release is written, submit it to all the news agencies. Then you must wait and see if any of them pick it up and publish it.
You may want to consider having a press release professionally written and distributed by a public relations firm to boost your chances of having the release picked up. PRWeb.com has an excellent reputation. Its distribution network includes the search engines Google, Yahoo, and Bing; media outlets such as USA Today, CNN, and the Wall Street Journal; Associated Press distribution through major newspapers; and health and medical digests such as the Mayo Clinic, WebMD, Women’s Health, and many more.
3. Article directories
By writing and distributing articles through high-traffic article directories, such as EzineArticles.com, Articles.org, and Hubpages.com, you can attract valuable inbound links from a high-traffic site. Craft an effective link at the close of your article to drive traffic back to your site. An example of what your link might say is, “To view a short video on Kegel exercises for pelvic organ prolapse, visit our Web site at www.neilbaum.com/videos.”
4. Social bookmarking
Like Web browser bookmarks, social bookmarking sites such as Digg.com, Reddit.com, and Del.icio.us.com store individual pages (bookmarks) online and allow users to tag (with keywords), organize, search, and manage these bookmarks as well as share them with others. If you bookmark your content on these sites, you get a link from the service. By producing content that your readers enjoy and bookmark to their friends, you gain a link that increases in search engine optimization (SEO) value.
5. Blog comments
To find blog posts on which to comment, you can use blog-specific search engines such as Google Blog Search. Make sure these are blogs read by your target market, not your colleagues. Brand yourself by always using the same name and remember to link back to your site. Always leave a comment that adds to the conversation.
6. Social media
Google also indexes your Twitter updates and social networking profiles. Add that to Web 2.0 hubsites like Scribd or HubPage and you’ve got a way to create many inbound links in a very short time. Scribd is a digital library featuring an ebook and audiobook subscription service that includes New York Times best sellers and classics. HubPages is a user-generated content, revenue-sharing Web site.
7. Video syndication
YouTube is one of the most visited sites online, and the number of sites that syndicate videos is growing every day. These sites often allow you to link to your site in your video’s description, on your profile page, or both.
The importance of being consistent—and honest
For best results, you need to build these links monthly with regularity, and over time, you will reap the benefits of improved rankings. While it is fairly easy to modify your Web pages to make them more SEO-friendly, it is harder to influence other Web sites and get them to link to yours. This is the reason search engines consider backlinks such an important factor.
Moreover, search engines’ criteria for quality backlinks have gotten tougher, thanks to unscrupulous Web masters trying to achieve these backlinks by deceptive techniques, such as hidden links or automatically generated pages whose sole purpose is to provide backlinks to Web sites. These pages are called link farms. Not only are they disregarded by search engines, but linking to one could get your site banned entirely. This strategy is often referred to as “black hat” linking and is to be avoided.
“White hat” methods to increase backlinks
Blog posting is one of the easiest, least expensive, and most effective ways to garner links from other sites. However, to reap this benefit, you must post blog entries consistently. We suggest posting at least once weekly. Your blog will gain more attention if you have something newsworthy to report. For example, if you attend a meeting where a revolutionary new development is reported, and you write about it before the media, you can be sure others will want to connect and link to your site.
Conduct a survey and share your results on your site. Others will want to link to your report.
Share any templates your office uses to be more efficient and productive. For example, Dr. Baum has a template, or checklist, for starting and ending every day in the office. It is shared on his Web site so that other sites can link to it and make use of it.
Show your funny bone. Humor often travels in a viral direction. If something funny happens in your practice, share it with others and they will frequently link to the source.
Join a forum. Forums are a great source of high-quality traffic and links. You can use a forum to reach out to a specific community.By placing valid, useful contributions, you gain legitimate authority for your site.
The bottom line
You want to attract as many visitors to your Web site as possible. Your own content and the frequency of your postings are mainstays of making your Web site relevant to existing and potential patients. Also useful are backlinks. The number and quality of your inbound links are major factors in SEO. Search engines place high value on trust and authority, and an inbound link from a very high-ranking and trusted Web site tells the search engine that someone trusted also trusts you. So start linking.
Reference
1. The value of Google result positioning. Chitika.com. http://chitika.com/google-positioning-value. Updated June 12, 2013. Accessed June 9, 2015.
The medical profession has its jargon. So does the Internet world. Some of that jargon can be important to your success. “Backlinks” or “inbound links” are terms that should get your attention.
Why?
By developing them, you can attract more patients to your practice.
Backlinks are one piece of the Internet marketing puzzle that can help get your Web site on the first page of Google search results.
And just how important is it to be on page 1?
Well, consider that 91.5% of Web surfers do not go beyond the first page of results. That’s what an online advertising network called Chitika found when it examined tens of millions of online ad impressions in which the user was referred to the page via a Google search.1
Just what are backlinks? They are links to your Web page from another site. In basic link terminology, a backlink is any link received by a Web page, directory, Web site, or top-level domain from another, similar site. In this article we discuss the importance of these links and ways to use them in your social media to attract new patients to your site and your practice.
Start with good site design
If you can get listed on the first page of Google search results for the keywords your patients are using, more traffic will come to your Web site. That won’t help if you have a poorly designed site that has no patient conversion strategies, techniques, and systems to transform Web site visitors to patients.
You see, everything has to work together in a coordinated, integrated manner if you want to increase the number of patients who are looking for your services online. We’ve covered many of the basics in earlier articles on Web site design and improvement (see the box below). If you have a nonoptimal site, consider starting with these articles.
Articles on Web design and Internet usage by Ron Romano and Neil H. Baum, MD
5 ways to wake up your Web site
April 2015
Using the Internet in your practice
Part 1: Why social media are important and how to get started
February 2014
Part 2: Generating new patients using social media
April 2014
Part 3: Maximizing your online reach through SEO and pay-per-click
September 2014
Part 4: Reputation management: How to gather kudos and combat negative online reviews
December 2014
These articles are available in the archive at obgmanagement.com
Why backlinks are important
Google uses more than 200 algorithms to rank your Web site. Some are more important than others and have a greater influence on search engine positioning. Backlinks are one of those important influencers.
The number of backlinks you have is an indication of the popularity or importance of your Web site. Google considers a site more significant or relevant than others if it has a large number of quality backlinks from other directories, ezines, blogs, and social media Web sites. These backlinks must be relevant to your keywords. For example, because you are a medical professional, a link to your site from the American Cancer Society or the Mayo Clinic is considered more credible than a link from a local spa or health club.
A search engine such as Google considers the content of the sites it places at the top of the search results page. When links to your site come from other credible and popular sites, and those sites have content related to your site, these backlinks are considered more relevant to your site.
If backlinks come from sites with unrelated content, they are considered less relevant. You may even be penalized by Google for adding backlinks that have no content value.
For example, if a Web master has a site that focuses on urinary incontinence and receives a backlink from another site with information or articles about urinary incontinence, that backlink will be considered more relevant than a link from a site about mortgages that somehow also includes urinary incontinence on its page. Therefore, the higher the relevance of the site linking back to yours, the better the quality of that link.
Top 7 inbound links—and how to obtain them
1. Directories
Directories are indexes of online sites, typically organized by category. You want to ensure that each of your keywords is manually submitted to each directory so it is listed separately. This way you get maximum link value for each keyword.
Links back to your site from directories such as Yahoo Directory and DMOZ.org are valuable. DMOZ.org is edited by humans. Although it is free, it may take some time for your site to be added. A listing in Yahoo’s Directory costs $299 per year.
2. Press releases
If you are writing press releases, make sure they contain keywords that someone would use to find a business like yours. Also ensure that they include links back to your site.
Once the press release is written, submit it to all the news agencies. Then you must wait and see if any of them pick it up and publish it.
You may want to consider having a press release professionally written and distributed by a public relations firm to boost your chances of having the release picked up. PRWeb.com has an excellent reputation. Its distribution network includes the search engines Google, Yahoo, and Bing; media outlets such as USA Today, CNN, and the Wall Street Journal; Associated Press distribution through major newspapers; and health and medical digests such as the Mayo Clinic, WebMD, Women’s Health, and many more.
3. Article directories
By writing and distributing articles through high-traffic article directories, such as EzineArticles.com, Articles.org, and Hubpages.com, you can attract valuable inbound links from a high-traffic site. Craft an effective link at the close of your article to drive traffic back to your site. An example of what your link might say is, “To view a short video on Kegel exercises for pelvic organ prolapse, visit our Web site at www.neilbaum.com/videos.”
4. Social bookmarking
Like Web browser bookmarks, social bookmarking sites such as Digg.com, Reddit.com, and Del.icio.us.com store individual pages (bookmarks) online and allow users to tag (with keywords), organize, search, and manage these bookmarks as well as share them with others. If you bookmark your content on these sites, you get a link from the service. By producing content that your readers enjoy and bookmark to their friends, you gain a link that increases in search engine optimization (SEO) value.
5. Blog comments
To find blog posts on which to comment, you can use blog-specific search engines such as Google Blog Search. Make sure these are blogs read by your target market, not your colleagues. Brand yourself by always using the same name and remember to link back to your site. Always leave a comment that adds to the conversation.
6. Social media
Google also indexes your Twitter updates and social networking profiles. Add that to Web 2.0 hubsites like Scribd or HubPage and you’ve got a way to create many inbound links in a very short time. Scribd is a digital library featuring an ebook and audiobook subscription service that includes New York Times best sellers and classics. HubPages is a user-generated content, revenue-sharing Web site.
7. Video syndication
YouTube is one of the most visited sites online, and the number of sites that syndicate videos is growing every day. These sites often allow you to link to your site in your video’s description, on your profile page, or both.
The importance of being consistent—and honest
For best results, you need to build these links monthly with regularity, and over time, you will reap the benefits of improved rankings. While it is fairly easy to modify your Web pages to make them more SEO-friendly, it is harder to influence other Web sites and get them to link to yours. This is the reason search engines consider backlinks such an important factor.
Moreover, search engines’ criteria for quality backlinks have gotten tougher, thanks to unscrupulous Web masters trying to achieve these backlinks by deceptive techniques, such as hidden links or automatically generated pages whose sole purpose is to provide backlinks to Web sites. These pages are called link farms. Not only are they disregarded by search engines, but linking to one could get your site banned entirely. This strategy is often referred to as “black hat” linking and is to be avoided.
“White hat” methods to increase backlinks
Blog posting is one of the easiest, least expensive, and most effective ways to garner links from other sites. However, to reap this benefit, you must post blog entries consistently. We suggest posting at least once weekly. Your blog will gain more attention if you have something newsworthy to report. For example, if you attend a meeting where a revolutionary new development is reported, and you write about it before the media, you can be sure others will want to connect and link to your site.
Conduct a survey and share your results on your site. Others will want to link to your report.
Share any templates your office uses to be more efficient and productive. For example, Dr. Baum has a template, or checklist, for starting and ending every day in the office. It is shared on his Web site so that other sites can link to it and make use of it.
Show your funny bone. Humor often travels in a viral direction. If something funny happens in your practice, share it with others and they will frequently link to the source.
Join a forum. Forums are a great source of high-quality traffic and links. You can use a forum to reach out to a specific community.By placing valid, useful contributions, you gain legitimate authority for your site.
The bottom line
You want to attract as many visitors to your Web site as possible. Your own content and the frequency of your postings are mainstays of making your Web site relevant to existing and potential patients. Also useful are backlinks. The number and quality of your inbound links are major factors in SEO. Search engines place high value on trust and authority, and an inbound link from a very high-ranking and trusted Web site tells the search engine that someone trusted also trusts you. So start linking.
The medical profession has its jargon. So does the Internet world. Some of that jargon can be important to your success. “Backlinks” or “inbound links” are terms that should get your attention.
Why?
By developing them, you can attract more patients to your practice.
Backlinks are one piece of the Internet marketing puzzle that can help get your Web site on the first page of Google search results.
And just how important is it to be on page 1?
Well, consider that 91.5% of Web surfers do not go beyond the first page of results. That’s what an online advertising network called Chitika found when it examined tens of millions of online ad impressions in which the user was referred to the page via a Google search.1
Just what are backlinks? They are links to your Web page from another site. In basic link terminology, a backlink is any link received by a Web page, directory, Web site, or top-level domain from another, similar site. In this article we discuss the importance of these links and ways to use them in your social media to attract new patients to your site and your practice.
Start with good site design
If you can get listed on the first page of Google search results for the keywords your patients are using, more traffic will come to your Web site. That won’t help if you have a poorly designed site that has no patient conversion strategies, techniques, and systems to transform Web site visitors to patients.
You see, everything has to work together in a coordinated, integrated manner if you want to increase the number of patients who are looking for your services online. We’ve covered many of the basics in earlier articles on Web site design and improvement (see the box below). If you have a nonoptimal site, consider starting with these articles.
Articles on Web design and Internet usage by Ron Romano and Neil H. Baum, MD
5 ways to wake up your Web site
April 2015
Using the Internet in your practice
Part 1: Why social media are important and how to get started
February 2014
Part 2: Generating new patients using social media
April 2014
Part 3: Maximizing your online reach through SEO and pay-per-click
September 2014
Part 4: Reputation management: How to gather kudos and combat negative online reviews
December 2014
These articles are available in the archive at obgmanagement.com
Why backlinks are important
Google uses more than 200 algorithms to rank your Web site. Some are more important than others and have a greater influence on search engine positioning. Backlinks are one of those important influencers.
The number of backlinks you have is an indication of the popularity or importance of your Web site. Google considers a site more significant or relevant than others if it has a large number of quality backlinks from other directories, ezines, blogs, and social media Web sites. These backlinks must be relevant to your keywords. For example, because you are a medical professional, a link to your site from the American Cancer Society or the Mayo Clinic is considered more credible than a link from a local spa or health club.
A search engine such as Google considers the content of the sites it places at the top of the search results page. When links to your site come from other credible and popular sites, and those sites have content related to your site, these backlinks are considered more relevant to your site.
If backlinks come from sites with unrelated content, they are considered less relevant. You may even be penalized by Google for adding backlinks that have no content value.
For example, if a Web master has a site that focuses on urinary incontinence and receives a backlink from another site with information or articles about urinary incontinence, that backlink will be considered more relevant than a link from a site about mortgages that somehow also includes urinary incontinence on its page. Therefore, the higher the relevance of the site linking back to yours, the better the quality of that link.
Top 7 inbound links—and how to obtain them
1. Directories
Directories are indexes of online sites, typically organized by category. You want to ensure that each of your keywords is manually submitted to each directory so it is listed separately. This way you get maximum link value for each keyword.
Links back to your site from directories such as Yahoo Directory and DMOZ.org are valuable. DMOZ.org is edited by humans. Although it is free, it may take some time for your site to be added. A listing in Yahoo’s Directory costs $299 per year.
2. Press releases
If you are writing press releases, make sure they contain keywords that someone would use to find a business like yours. Also ensure that they include links back to your site.
Once the press release is written, submit it to all the news agencies. Then you must wait and see if any of them pick it up and publish it.
You may want to consider having a press release professionally written and distributed by a public relations firm to boost your chances of having the release picked up. PRWeb.com has an excellent reputation. Its distribution network includes the search engines Google, Yahoo, and Bing; media outlets such as USA Today, CNN, and the Wall Street Journal; Associated Press distribution through major newspapers; and health and medical digests such as the Mayo Clinic, WebMD, Women’s Health, and many more.
3. Article directories
By writing and distributing articles through high-traffic article directories, such as EzineArticles.com, Articles.org, and Hubpages.com, you can attract valuable inbound links from a high-traffic site. Craft an effective link at the close of your article to drive traffic back to your site. An example of what your link might say is, “To view a short video on Kegel exercises for pelvic organ prolapse, visit our Web site at www.neilbaum.com/videos.”
4. Social bookmarking
Like Web browser bookmarks, social bookmarking sites such as Digg.com, Reddit.com, and Del.icio.us.com store individual pages (bookmarks) online and allow users to tag (with keywords), organize, search, and manage these bookmarks as well as share them with others. If you bookmark your content on these sites, you get a link from the service. By producing content that your readers enjoy and bookmark to their friends, you gain a link that increases in search engine optimization (SEO) value.
5. Blog comments
To find blog posts on which to comment, you can use blog-specific search engines such as Google Blog Search. Make sure these are blogs read by your target market, not your colleagues. Brand yourself by always using the same name and remember to link back to your site. Always leave a comment that adds to the conversation.
6. Social media
Google also indexes your Twitter updates and social networking profiles. Add that to Web 2.0 hubsites like Scribd or HubPage and you’ve got a way to create many inbound links in a very short time. Scribd is a digital library featuring an ebook and audiobook subscription service that includes New York Times best sellers and classics. HubPages is a user-generated content, revenue-sharing Web site.
7. Video syndication
YouTube is one of the most visited sites online, and the number of sites that syndicate videos is growing every day. These sites often allow you to link to your site in your video’s description, on your profile page, or both.
The importance of being consistent—and honest
For best results, you need to build these links monthly with regularity, and over time, you will reap the benefits of improved rankings. While it is fairly easy to modify your Web pages to make them more SEO-friendly, it is harder to influence other Web sites and get them to link to yours. This is the reason search engines consider backlinks such an important factor.
Moreover, search engines’ criteria for quality backlinks have gotten tougher, thanks to unscrupulous Web masters trying to achieve these backlinks by deceptive techniques, such as hidden links or automatically generated pages whose sole purpose is to provide backlinks to Web sites. These pages are called link farms. Not only are they disregarded by search engines, but linking to one could get your site banned entirely. This strategy is often referred to as “black hat” linking and is to be avoided.
“White hat” methods to increase backlinks
Blog posting is one of the easiest, least expensive, and most effective ways to garner links from other sites. However, to reap this benefit, you must post blog entries consistently. We suggest posting at least once weekly. Your blog will gain more attention if you have something newsworthy to report. For example, if you attend a meeting where a revolutionary new development is reported, and you write about it before the media, you can be sure others will want to connect and link to your site.
Conduct a survey and share your results on your site. Others will want to link to your report.
Share any templates your office uses to be more efficient and productive. For example, Dr. Baum has a template, or checklist, for starting and ending every day in the office. It is shared on his Web site so that other sites can link to it and make use of it.
Show your funny bone. Humor often travels in a viral direction. If something funny happens in your practice, share it with others and they will frequently link to the source.
Join a forum. Forums are a great source of high-quality traffic and links. You can use a forum to reach out to a specific community.By placing valid, useful contributions, you gain legitimate authority for your site.
The bottom line
You want to attract as many visitors to your Web site as possible. Your own content and the frequency of your postings are mainstays of making your Web site relevant to existing and potential patients. Also useful are backlinks. The number and quality of your inbound links are major factors in SEO. Search engines place high value on trust and authority, and an inbound link from a very high-ranking and trusted Web site tells the search engine that someone trusted also trusts you. So start linking.
Reference
1. The value of Google result positioning. Chitika.com. http://chitika.com/google-positioning-value. Updated June 12, 2013. Accessed June 9, 2015.
Reference
1. The value of Google result positioning. Chitika.com. http://chitika.com/google-positioning-value. Updated June 12, 2013. Accessed June 9, 2015.
Tired knees
Last week, one of my patients presented with a BMI of 49 and two canes. Knee x-ray shows marked medial compartment narrowing bilaterally. We will inject her knees with steroids, but this will be temporary.
As the obesity epidemic continues to rage, native joints are rapidly being replaced with metal ones. Our pitiful homegrown joints were not designed to carry all this human weight. Joint forces in the hip and knee have been estimated to be 3 times body weight when walking on level ground and 6-10 times body weight when stooping or bending. Combine this with all the ‘screen time’ (average 8 hours a day for U.S. adults) and all the trips to the bathroom from the poorly controlled diabetes, and we are set up for needing a lot more orthopedic surgeons.
So should we push for surgery?
I am reluctant to immediately and eagerly pursue surgery based upon data from Ward et al. elucidating the increased risk for complications after joint surgery among patients with a BMI > 40 (J.Arthroplasty. 2015 Jun 3. pii: S0883-5403(15)00474-X. doi: 10.1016/j.arth.2015.03.045. [Epub ahead of print]). Data from the bariatric literature suggest that the risk of complications following joint replacement is lower if bariatric surgery is performed first. Weight loss as we look toward joint replacement is a good idea for both our orthopedic colleagues and our patients.
So we will work on weight loss first
In patients with osteoarthritis, a moderate amount of weight loss can significantly improve knee function. The short term efficacy of weight loss is comparable to joint replacement. But clinicians need to be wary of the “pain-exercise block”: patients telling us they cannot lose weight because the pain prevents them from exercising. I tell my patients that weight loss and weight maintenance can be managed effectively through dietary modification and that they do not have to run a marathon, they just need to walk if they can. But patients do not always want to hear this. Caloric restriction is psychologically painful for many. I remind them that 30 minutes of exercise can be undone in 30 seconds with a bar of chocolate, so we need to skip the chocolate bar if we do light exercise or forgo exercise altogether. Exercise is important for a million other reasons, but many of our patients can’t engage, especially when presenting with gait assist devices.
My patient and I started the discussion of bariatric surgery. In the meantime, we are going to try a trial of lorcaserin and hope the knees hold out. We are likely going to need more steroids.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter: @jonebbert.
Last week, one of my patients presented with a BMI of 49 and two canes. Knee x-ray shows marked medial compartment narrowing bilaterally. We will inject her knees with steroids, but this will be temporary.
As the obesity epidemic continues to rage, native joints are rapidly being replaced with metal ones. Our pitiful homegrown joints were not designed to carry all this human weight. Joint forces in the hip and knee have been estimated to be 3 times body weight when walking on level ground and 6-10 times body weight when stooping or bending. Combine this with all the ‘screen time’ (average 8 hours a day for U.S. adults) and all the trips to the bathroom from the poorly controlled diabetes, and we are set up for needing a lot more orthopedic surgeons.
So should we push for surgery?
I am reluctant to immediately and eagerly pursue surgery based upon data from Ward et al. elucidating the increased risk for complications after joint surgery among patients with a BMI > 40 (J.Arthroplasty. 2015 Jun 3. pii: S0883-5403(15)00474-X. doi: 10.1016/j.arth.2015.03.045. [Epub ahead of print]). Data from the bariatric literature suggest that the risk of complications following joint replacement is lower if bariatric surgery is performed first. Weight loss as we look toward joint replacement is a good idea for both our orthopedic colleagues and our patients.
So we will work on weight loss first
In patients with osteoarthritis, a moderate amount of weight loss can significantly improve knee function. The short term efficacy of weight loss is comparable to joint replacement. But clinicians need to be wary of the “pain-exercise block”: patients telling us they cannot lose weight because the pain prevents them from exercising. I tell my patients that weight loss and weight maintenance can be managed effectively through dietary modification and that they do not have to run a marathon, they just need to walk if they can. But patients do not always want to hear this. Caloric restriction is psychologically painful for many. I remind them that 30 minutes of exercise can be undone in 30 seconds with a bar of chocolate, so we need to skip the chocolate bar if we do light exercise or forgo exercise altogether. Exercise is important for a million other reasons, but many of our patients can’t engage, especially when presenting with gait assist devices.
My patient and I started the discussion of bariatric surgery. In the meantime, we are going to try a trial of lorcaserin and hope the knees hold out. We are likely going to need more steroids.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter: @jonebbert.
Last week, one of my patients presented with a BMI of 49 and two canes. Knee x-ray shows marked medial compartment narrowing bilaterally. We will inject her knees with steroids, but this will be temporary.
As the obesity epidemic continues to rage, native joints are rapidly being replaced with metal ones. Our pitiful homegrown joints were not designed to carry all this human weight. Joint forces in the hip and knee have been estimated to be 3 times body weight when walking on level ground and 6-10 times body weight when stooping or bending. Combine this with all the ‘screen time’ (average 8 hours a day for U.S. adults) and all the trips to the bathroom from the poorly controlled diabetes, and we are set up for needing a lot more orthopedic surgeons.
So should we push for surgery?
I am reluctant to immediately and eagerly pursue surgery based upon data from Ward et al. elucidating the increased risk for complications after joint surgery among patients with a BMI > 40 (J.Arthroplasty. 2015 Jun 3. pii: S0883-5403(15)00474-X. doi: 10.1016/j.arth.2015.03.045. [Epub ahead of print]). Data from the bariatric literature suggest that the risk of complications following joint replacement is lower if bariatric surgery is performed first. Weight loss as we look toward joint replacement is a good idea for both our orthopedic colleagues and our patients.
So we will work on weight loss first
In patients with osteoarthritis, a moderate amount of weight loss can significantly improve knee function. The short term efficacy of weight loss is comparable to joint replacement. But clinicians need to be wary of the “pain-exercise block”: patients telling us they cannot lose weight because the pain prevents them from exercising. I tell my patients that weight loss and weight maintenance can be managed effectively through dietary modification and that they do not have to run a marathon, they just need to walk if they can. But patients do not always want to hear this. Caloric restriction is psychologically painful for many. I remind them that 30 minutes of exercise can be undone in 30 seconds with a bar of chocolate, so we need to skip the chocolate bar if we do light exercise or forgo exercise altogether. Exercise is important for a million other reasons, but many of our patients can’t engage, especially when presenting with gait assist devices.
My patient and I started the discussion of bariatric surgery. In the meantime, we are going to try a trial of lorcaserin and hope the knees hold out. We are likely going to need more steroids.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter: @jonebbert.
Nephrogenic Systemic Fibrosis Following Gadolinium Administration
To the Editor:
Nephrogenic systemic fibrosis (NSF) is an emerging medical entity in patients with renal disease, which results in progressive cutaneous and systemic fibrosis. It is a rare disorder that has been recognized in patients with renal impairment since 2000.1 Patients with NSF demonstrate symmetric dermal and subcutaneous fibrosis evidenced by increasing skin induration on clinical examination. Nephrogenic systemic fibrosis most commonly involves the lower extremities, and after extending to the upper extremities and trunk, it sporadically involves the head and neck.
The clinical manifestation of NSF begins with edema, followed by marked dermal induration, sclerotic plaques, and joint contractures that can lead to considerable disability. Pathogenesis remains to be elucidated; it has been hypothesized that it could be related to gadolinium (Gd). Currently, there is no treatment of this unremitting disease.1-3 We report the case of a patient affected by NSF after administration of Gd for magnetic resonance angiography.
A 56-year-old woman was referred to the department of dermatology at the University of Maryland (Baltimore, Maryland) with persistent swelling of the lower legs, forearms, and trunk of 5 months’ duration. She had end-stage renal disease of nonspecific origin. Five months prior to presentation, she had magnetic resonance angiography, during which 10 mmol of Gd was administered. After, she developed a persistent rash and swelling of the lower legs. On presentation, physical examination revealed symmetric, shiny, pigmented papules and plaques on the forearms, buttocks, thighs, and legs, with no facial involvement (Figure).
A skin biopsy of thigh lesions showed a diffuse dermal proliferation of bland spindle cells associated with dermal fibrosis. A CD4+ cellular infiltrate showed extension into the subcutaneous tissue. Deposition of Gd also was noted in the skin. She was treated with corticosteroid therapy, and after 2 months she reported softening of the affected skin. On 6-month follow-up, her skin lesions did not progress and there was no evidence of systemic involvement. Additionally, renal function had improved.
Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, was first described by Cowper et al1 in 2000. Since then, more than 215 cases have been reported in the literature. Clinically, it is characterized by acute onset of cutaneous hardening and thickening of the extremities and the trunk, often resulting in flexion contractures. There may be varying surface changes such as pigmentation, peau d’orange texture, and shiny sclerosis. Patients often experience unpleasant symptoms such as pain, pruritus, stiffness, and paresthesia. Systemic involvement has been documented in the heart, lung, tendons, muscle, testes, and lamina dura.1-4
Histologic findings of NSF are diffuse dermal fibrosis with increased cellular infiltrates comprised of bland spindled fibrocytes. These fibrocytes express CD34 and type I procollagen. Collagen bundles are thickened but retain clefting, and elastic fibers often are prominent. This fibrotic pattern typically extends to the subcutaneous fat septa, which are widened and collagenous. The epidermis generally is uninvolved. Other findings include dermal mucin deposition, calcification of collagen and vessels, increased CD68+ histiocytes, increased factor XIIIa and dendritic cells, and neoangiogenesis. Rarely, multinucleated giant cells and Miescher radial granulomas with lymphocytic aggregates mimicking erythema nodosum have been described.2-4
Dermatologic entities with similar clinical and histopathologic features, including scleroderma, scleromyxedema, lipodermatosclerosis, erythema nodosum, eosinophilic fasciitis, and spindle cell neoplasms, should be excluded.1-4 The exact pathogenetic mechanisms of NSF have yet to be determined, but there is strong evidence that Gd plays an important causative role.1 In fact, almost all patients with NSF have been exposed to Gd. Gadolinium has been documented in affected skin of patients with NSF and has been shown to induce NSF-like changes in rat models.
Other clinical factors that have been associated with NSF include erythropoietin, elevated serum calcium and phosphate levels, vascular injury or surgery, iron metabolic abnormalities, and metabolic acidosis. It is likely that many factors in the unique physiologic state of patients with renal failure contribute to the abnormal fibrotic reaction to Gd-containing contrast agents in NSF. Gadolinium is a member of a group of 15 elemental metals termed lanthanoids and has been used extensively worldwide in magnetic resonance imaging as a component of intravenously administered contrast agents. Currently, 6 such agents are approved for use in the United States: gadopentetate dimeglumine, gadoteridol, gadodiamide, gadoversetamide, gadobenate dimeglumine, and gadoxetate sodium. All are chelated Gd products, with the chelate serving to prevent toxicity from free Gd ions.
In patients with no renal function abnormalities, the biologic half-life of Gd-based magnetic resonance contrast agents (GBCAs) is 1.5 to 2.0 hours. However, in patients with abnormal kidney function, this half-life is inversely prolonged, proportional to the glomerular filtration rate.5-7 The link between GBCA administration and NSF is compelling, though other etiologic associations have been reported. Surgical or vascular procedures, history of a hypercoagulable state, erythropoietin administration, and immune suppression have been proposed as triggering factors in NSF. The proposed mechanisms responsible for fibrosis in NSF have centered on a collagen-producing cell in the peripheral blood termed the circulating fibrocyte. These cells express CD34 and CD45RO antigens and are capable of producing type I collagen.
Circulating fibrocytes traffic to areas of chronic antigenic stimulation promoting wound repair and fibrotic reactions. Some authors have proposed that materials deposited in the skin might serve as targets for circulating fibrocytes.8 Circulating fibrocytes also are known to produce inflammatory cytokines including IL-1 and chemokines such as platelet-derived growth factor, transforming growth factor b, and others capable of propagating fibrotic responses. Increased expression of transforming growth factor has been reported in dendritic cells in NSF lesions and Parsons et al9 postulated that transglutaminase-2 activation of this protein may be responsible for inciting fibrosis in NSF. Transglutaminases also are known to be directly activated by Gd.10,11
Transmetalation has been proposed as a possible operative phenomenon responsible for NSF. Several cations including zinc, copper, iron, and carbon are known to compete with Gd and may displace it from the ligand, with anions such as OHe, PO4 3e, and CO3 2e binding the resultant free Gd. Some GBCAs contain excess ligand to diminish potential free Gd concentrations. In fact, substantial elevations of serum calcium and phosphorus in patients with NSF have been noted in a large series of patients with NSF. Calciphylaxis, an often catastrophic condition arising in patients with renal failure, has been described in association with NSF, and sodium thiosulfate has been used with success in treating both conditions.10 In addition, Sanyal et al12 noted a substantially higher serum calcium in NSF cases compared with controls.
Gadolinium plays an important role in the pathology of NSF and is confirmed by the presence of Gd in skin biopsies.
1. Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000-1001.
2. Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathologiocal definition and workup recommendations [published online ahead of print July 2, 2011]. J Am Acad Dermatol. 2011;65:1095-1106.
3. Gupta A, Shamseddin MK, Khaira A. Pathomechanisms of nephrogenic systemic fibrosis: new insights [published online ahead of print July 25, 2011]. Clin Exp Dermatol. 2011;36:763-768.
4. Zou Z, Ma L. Nephrogenic systemic fibrosis: review of 408 biopsy-confirmed cases. Indian J Dermatol. 2011;56:65-73.
5. Pan D, Schmieder AH, Wickline SA, et al. Manganese-based MRI contrast agents: past, present and future. Tetrahedron. 2011;67:8431-8444.
6. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18:188-198.
7. Wang Y, Alkasab TK, Narin O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines [published online ahead of print May 17, 2011]. Radiology. 2011;260:105-111.
8. Ortonne N, Lipsker D, Chantrel F, et al. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150:1050-1052.
9. Parsons AC, Yosipovitch G, Sheehan DJ, et al. Transglutaminases: the missing link in nephrogenic systemic fibrosis. Am J Dermatopathol. 2007;29:433-436.
10. Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature [published online ahead of print August 16, 2007]. Am J Transplant. 2007;7:2425-2432.
11. Goveia M, Chan BP, Patel PR. Evaluating the role of recombinant erythropoietin in nephrogenic systemic fibrosis [published online ahead of print August 8, 2007]. J Am Acad Dermatol. 2007;57:725-727.
12. Sanyal S, Marckmann P, Scherer S, et al. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis–an autopsy-based review [published online ahead of print March 25, 2011]. Nephrol Dial Transplant. 2011;26:3616-3626.
To the Editor:
Nephrogenic systemic fibrosis (NSF) is an emerging medical entity in patients with renal disease, which results in progressive cutaneous and systemic fibrosis. It is a rare disorder that has been recognized in patients with renal impairment since 2000.1 Patients with NSF demonstrate symmetric dermal and subcutaneous fibrosis evidenced by increasing skin induration on clinical examination. Nephrogenic systemic fibrosis most commonly involves the lower extremities, and after extending to the upper extremities and trunk, it sporadically involves the head and neck.
The clinical manifestation of NSF begins with edema, followed by marked dermal induration, sclerotic plaques, and joint contractures that can lead to considerable disability. Pathogenesis remains to be elucidated; it has been hypothesized that it could be related to gadolinium (Gd). Currently, there is no treatment of this unremitting disease.1-3 We report the case of a patient affected by NSF after administration of Gd for magnetic resonance angiography.
A 56-year-old woman was referred to the department of dermatology at the University of Maryland (Baltimore, Maryland) with persistent swelling of the lower legs, forearms, and trunk of 5 months’ duration. She had end-stage renal disease of nonspecific origin. Five months prior to presentation, she had magnetic resonance angiography, during which 10 mmol of Gd was administered. After, she developed a persistent rash and swelling of the lower legs. On presentation, physical examination revealed symmetric, shiny, pigmented papules and plaques on the forearms, buttocks, thighs, and legs, with no facial involvement (Figure).
A skin biopsy of thigh lesions showed a diffuse dermal proliferation of bland spindle cells associated with dermal fibrosis. A CD4+ cellular infiltrate showed extension into the subcutaneous tissue. Deposition of Gd also was noted in the skin. She was treated with corticosteroid therapy, and after 2 months she reported softening of the affected skin. On 6-month follow-up, her skin lesions did not progress and there was no evidence of systemic involvement. Additionally, renal function had improved.
Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, was first described by Cowper et al1 in 2000. Since then, more than 215 cases have been reported in the literature. Clinically, it is characterized by acute onset of cutaneous hardening and thickening of the extremities and the trunk, often resulting in flexion contractures. There may be varying surface changes such as pigmentation, peau d’orange texture, and shiny sclerosis. Patients often experience unpleasant symptoms such as pain, pruritus, stiffness, and paresthesia. Systemic involvement has been documented in the heart, lung, tendons, muscle, testes, and lamina dura.1-4
Histologic findings of NSF are diffuse dermal fibrosis with increased cellular infiltrates comprised of bland spindled fibrocytes. These fibrocytes express CD34 and type I procollagen. Collagen bundles are thickened but retain clefting, and elastic fibers often are prominent. This fibrotic pattern typically extends to the subcutaneous fat septa, which are widened and collagenous. The epidermis generally is uninvolved. Other findings include dermal mucin deposition, calcification of collagen and vessels, increased CD68+ histiocytes, increased factor XIIIa and dendritic cells, and neoangiogenesis. Rarely, multinucleated giant cells and Miescher radial granulomas with lymphocytic aggregates mimicking erythema nodosum have been described.2-4
Dermatologic entities with similar clinical and histopathologic features, including scleroderma, scleromyxedema, lipodermatosclerosis, erythema nodosum, eosinophilic fasciitis, and spindle cell neoplasms, should be excluded.1-4 The exact pathogenetic mechanisms of NSF have yet to be determined, but there is strong evidence that Gd plays an important causative role.1 In fact, almost all patients with NSF have been exposed to Gd. Gadolinium has been documented in affected skin of patients with NSF and has been shown to induce NSF-like changes in rat models.
Other clinical factors that have been associated with NSF include erythropoietin, elevated serum calcium and phosphate levels, vascular injury or surgery, iron metabolic abnormalities, and metabolic acidosis. It is likely that many factors in the unique physiologic state of patients with renal failure contribute to the abnormal fibrotic reaction to Gd-containing contrast agents in NSF. Gadolinium is a member of a group of 15 elemental metals termed lanthanoids and has been used extensively worldwide in magnetic resonance imaging as a component of intravenously administered contrast agents. Currently, 6 such agents are approved for use in the United States: gadopentetate dimeglumine, gadoteridol, gadodiamide, gadoversetamide, gadobenate dimeglumine, and gadoxetate sodium. All are chelated Gd products, with the chelate serving to prevent toxicity from free Gd ions.
In patients with no renal function abnormalities, the biologic half-life of Gd-based magnetic resonance contrast agents (GBCAs) is 1.5 to 2.0 hours. However, in patients with abnormal kidney function, this half-life is inversely prolonged, proportional to the glomerular filtration rate.5-7 The link between GBCA administration and NSF is compelling, though other etiologic associations have been reported. Surgical or vascular procedures, history of a hypercoagulable state, erythropoietin administration, and immune suppression have been proposed as triggering factors in NSF. The proposed mechanisms responsible for fibrosis in NSF have centered on a collagen-producing cell in the peripheral blood termed the circulating fibrocyte. These cells express CD34 and CD45RO antigens and are capable of producing type I collagen.
Circulating fibrocytes traffic to areas of chronic antigenic stimulation promoting wound repair and fibrotic reactions. Some authors have proposed that materials deposited in the skin might serve as targets for circulating fibrocytes.8 Circulating fibrocytes also are known to produce inflammatory cytokines including IL-1 and chemokines such as platelet-derived growth factor, transforming growth factor b, and others capable of propagating fibrotic responses. Increased expression of transforming growth factor has been reported in dendritic cells in NSF lesions and Parsons et al9 postulated that transglutaminase-2 activation of this protein may be responsible for inciting fibrosis in NSF. Transglutaminases also are known to be directly activated by Gd.10,11
Transmetalation has been proposed as a possible operative phenomenon responsible for NSF. Several cations including zinc, copper, iron, and carbon are known to compete with Gd and may displace it from the ligand, with anions such as OHe, PO4 3e, and CO3 2e binding the resultant free Gd. Some GBCAs contain excess ligand to diminish potential free Gd concentrations. In fact, substantial elevations of serum calcium and phosphorus in patients with NSF have been noted in a large series of patients with NSF. Calciphylaxis, an often catastrophic condition arising in patients with renal failure, has been described in association with NSF, and sodium thiosulfate has been used with success in treating both conditions.10 In addition, Sanyal et al12 noted a substantially higher serum calcium in NSF cases compared with controls.
Gadolinium plays an important role in the pathology of NSF and is confirmed by the presence of Gd in skin biopsies.
To the Editor:
Nephrogenic systemic fibrosis (NSF) is an emerging medical entity in patients with renal disease, which results in progressive cutaneous and systemic fibrosis. It is a rare disorder that has been recognized in patients with renal impairment since 2000.1 Patients with NSF demonstrate symmetric dermal and subcutaneous fibrosis evidenced by increasing skin induration on clinical examination. Nephrogenic systemic fibrosis most commonly involves the lower extremities, and after extending to the upper extremities and trunk, it sporadically involves the head and neck.
The clinical manifestation of NSF begins with edema, followed by marked dermal induration, sclerotic plaques, and joint contractures that can lead to considerable disability. Pathogenesis remains to be elucidated; it has been hypothesized that it could be related to gadolinium (Gd). Currently, there is no treatment of this unremitting disease.1-3 We report the case of a patient affected by NSF after administration of Gd for magnetic resonance angiography.
A 56-year-old woman was referred to the department of dermatology at the University of Maryland (Baltimore, Maryland) with persistent swelling of the lower legs, forearms, and trunk of 5 months’ duration. She had end-stage renal disease of nonspecific origin. Five months prior to presentation, she had magnetic resonance angiography, during which 10 mmol of Gd was administered. After, she developed a persistent rash and swelling of the lower legs. On presentation, physical examination revealed symmetric, shiny, pigmented papules and plaques on the forearms, buttocks, thighs, and legs, with no facial involvement (Figure).
A skin biopsy of thigh lesions showed a diffuse dermal proliferation of bland spindle cells associated with dermal fibrosis. A CD4+ cellular infiltrate showed extension into the subcutaneous tissue. Deposition of Gd also was noted in the skin. She was treated with corticosteroid therapy, and after 2 months she reported softening of the affected skin. On 6-month follow-up, her skin lesions did not progress and there was no evidence of systemic involvement. Additionally, renal function had improved.
Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, was first described by Cowper et al1 in 2000. Since then, more than 215 cases have been reported in the literature. Clinically, it is characterized by acute onset of cutaneous hardening and thickening of the extremities and the trunk, often resulting in flexion contractures. There may be varying surface changes such as pigmentation, peau d’orange texture, and shiny sclerosis. Patients often experience unpleasant symptoms such as pain, pruritus, stiffness, and paresthesia. Systemic involvement has been documented in the heart, lung, tendons, muscle, testes, and lamina dura.1-4
Histologic findings of NSF are diffuse dermal fibrosis with increased cellular infiltrates comprised of bland spindled fibrocytes. These fibrocytes express CD34 and type I procollagen. Collagen bundles are thickened but retain clefting, and elastic fibers often are prominent. This fibrotic pattern typically extends to the subcutaneous fat septa, which are widened and collagenous. The epidermis generally is uninvolved. Other findings include dermal mucin deposition, calcification of collagen and vessels, increased CD68+ histiocytes, increased factor XIIIa and dendritic cells, and neoangiogenesis. Rarely, multinucleated giant cells and Miescher radial granulomas with lymphocytic aggregates mimicking erythema nodosum have been described.2-4
Dermatologic entities with similar clinical and histopathologic features, including scleroderma, scleromyxedema, lipodermatosclerosis, erythema nodosum, eosinophilic fasciitis, and spindle cell neoplasms, should be excluded.1-4 The exact pathogenetic mechanisms of NSF have yet to be determined, but there is strong evidence that Gd plays an important causative role.1 In fact, almost all patients with NSF have been exposed to Gd. Gadolinium has been documented in affected skin of patients with NSF and has been shown to induce NSF-like changes in rat models.
Other clinical factors that have been associated with NSF include erythropoietin, elevated serum calcium and phosphate levels, vascular injury or surgery, iron metabolic abnormalities, and metabolic acidosis. It is likely that many factors in the unique physiologic state of patients with renal failure contribute to the abnormal fibrotic reaction to Gd-containing contrast agents in NSF. Gadolinium is a member of a group of 15 elemental metals termed lanthanoids and has been used extensively worldwide in magnetic resonance imaging as a component of intravenously administered contrast agents. Currently, 6 such agents are approved for use in the United States: gadopentetate dimeglumine, gadoteridol, gadodiamide, gadoversetamide, gadobenate dimeglumine, and gadoxetate sodium. All are chelated Gd products, with the chelate serving to prevent toxicity from free Gd ions.
In patients with no renal function abnormalities, the biologic half-life of Gd-based magnetic resonance contrast agents (GBCAs) is 1.5 to 2.0 hours. However, in patients with abnormal kidney function, this half-life is inversely prolonged, proportional to the glomerular filtration rate.5-7 The link between GBCA administration and NSF is compelling, though other etiologic associations have been reported. Surgical or vascular procedures, history of a hypercoagulable state, erythropoietin administration, and immune suppression have been proposed as triggering factors in NSF. The proposed mechanisms responsible for fibrosis in NSF have centered on a collagen-producing cell in the peripheral blood termed the circulating fibrocyte. These cells express CD34 and CD45RO antigens and are capable of producing type I collagen.
Circulating fibrocytes traffic to areas of chronic antigenic stimulation promoting wound repair and fibrotic reactions. Some authors have proposed that materials deposited in the skin might serve as targets for circulating fibrocytes.8 Circulating fibrocytes also are known to produce inflammatory cytokines including IL-1 and chemokines such as platelet-derived growth factor, transforming growth factor b, and others capable of propagating fibrotic responses. Increased expression of transforming growth factor has been reported in dendritic cells in NSF lesions and Parsons et al9 postulated that transglutaminase-2 activation of this protein may be responsible for inciting fibrosis in NSF. Transglutaminases also are known to be directly activated by Gd.10,11
Transmetalation has been proposed as a possible operative phenomenon responsible for NSF. Several cations including zinc, copper, iron, and carbon are known to compete with Gd and may displace it from the ligand, with anions such as OHe, PO4 3e, and CO3 2e binding the resultant free Gd. Some GBCAs contain excess ligand to diminish potential free Gd concentrations. In fact, substantial elevations of serum calcium and phosphorus in patients with NSF have been noted in a large series of patients with NSF. Calciphylaxis, an often catastrophic condition arising in patients with renal failure, has been described in association with NSF, and sodium thiosulfate has been used with success in treating both conditions.10 In addition, Sanyal et al12 noted a substantially higher serum calcium in NSF cases compared with controls.
Gadolinium plays an important role in the pathology of NSF and is confirmed by the presence of Gd in skin biopsies.
1. Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000-1001.
2. Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathologiocal definition and workup recommendations [published online ahead of print July 2, 2011]. J Am Acad Dermatol. 2011;65:1095-1106.
3. Gupta A, Shamseddin MK, Khaira A. Pathomechanisms of nephrogenic systemic fibrosis: new insights [published online ahead of print July 25, 2011]. Clin Exp Dermatol. 2011;36:763-768.
4. Zou Z, Ma L. Nephrogenic systemic fibrosis: review of 408 biopsy-confirmed cases. Indian J Dermatol. 2011;56:65-73.
5. Pan D, Schmieder AH, Wickline SA, et al. Manganese-based MRI contrast agents: past, present and future. Tetrahedron. 2011;67:8431-8444.
6. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18:188-198.
7. Wang Y, Alkasab TK, Narin O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines [published online ahead of print May 17, 2011]. Radiology. 2011;260:105-111.
8. Ortonne N, Lipsker D, Chantrel F, et al. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150:1050-1052.
9. Parsons AC, Yosipovitch G, Sheehan DJ, et al. Transglutaminases: the missing link in nephrogenic systemic fibrosis. Am J Dermatopathol. 2007;29:433-436.
10. Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature [published online ahead of print August 16, 2007]. Am J Transplant. 2007;7:2425-2432.
11. Goveia M, Chan BP, Patel PR. Evaluating the role of recombinant erythropoietin in nephrogenic systemic fibrosis [published online ahead of print August 8, 2007]. J Am Acad Dermatol. 2007;57:725-727.
12. Sanyal S, Marckmann P, Scherer S, et al. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis–an autopsy-based review [published online ahead of print March 25, 2011]. Nephrol Dial Transplant. 2011;26:3616-3626.
1. Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000-1001.
2. Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathologiocal definition and workup recommendations [published online ahead of print July 2, 2011]. J Am Acad Dermatol. 2011;65:1095-1106.
3. Gupta A, Shamseddin MK, Khaira A. Pathomechanisms of nephrogenic systemic fibrosis: new insights [published online ahead of print July 25, 2011]. Clin Exp Dermatol. 2011;36:763-768.
4. Zou Z, Ma L. Nephrogenic systemic fibrosis: review of 408 biopsy-confirmed cases. Indian J Dermatol. 2011;56:65-73.
5. Pan D, Schmieder AH, Wickline SA, et al. Manganese-based MRI contrast agents: past, present and future. Tetrahedron. 2011;67:8431-8444.
6. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18:188-198.
7. Wang Y, Alkasab TK, Narin O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines [published online ahead of print May 17, 2011]. Radiology. 2011;260:105-111.
8. Ortonne N, Lipsker D, Chantrel F, et al. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150:1050-1052.
9. Parsons AC, Yosipovitch G, Sheehan DJ, et al. Transglutaminases: the missing link in nephrogenic systemic fibrosis. Am J Dermatopathol. 2007;29:433-436.
10. Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature [published online ahead of print August 16, 2007]. Am J Transplant. 2007;7:2425-2432.
11. Goveia M, Chan BP, Patel PR. Evaluating the role of recombinant erythropoietin in nephrogenic systemic fibrosis [published online ahead of print August 8, 2007]. J Am Acad Dermatol. 2007;57:725-727.
12. Sanyal S, Marckmann P, Scherer S, et al. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis–an autopsy-based review [published online ahead of print March 25, 2011]. Nephrol Dial Transplant. 2011;26:3616-3626.