User login
A serious catch-22 for doctors prescribing pain meds
Recently, the West Virginia Supreme Court ruled that patients can sue doctors if they became addicted to a medication the doctors prescribed even if the patients have committed crimes, such as doctor shopping, to get there.
Apparently, no one can be held responsible for their own actions anymore.
This is a serious catch-22 for doctors. On the one hand, we have ethical considerations, and oaths, to help others and relieve suffering. Now, on the flip side, doing just that can open us to legal action.
I prescribe narcotics. I try to use them judiciously, and only in people for whom other options have failed or are contraindicated. I suspect most doctors are the same. Every drug has its risks and benefits, and we try to make a calculated decision for each patient. I ask for the patient’s input, too, since he or she is the one who’ll be taking it.
I also have to depend on patients’ honesty. Patients who sell drugs to others, take more than I’ve prescribed, or use other methods of getting them (doctor shopping, buying them off the street) are all committing serious offenses. The development of monitoring databases where I can check on such behaviors has helped me catch those who’ve abused the medications.
One person quoted in an article about the court decision said, “I lied to everybody. I would steal. I pawned my grandma’s wedding rings. I was breaking into houses, doing anything and everything to stay high.”
So, obviously, that was all the doctor’s fault. He was trying to help her, and apparently led her to commit theft and burglary. I suppose the next step in such insanity is that he could be charged as an accomplice in her crimes. After all, it’s not her fault that she decided to steal from others.
This opens up a gold mine. Crooks obtaining narcotics through illicit means can now sue the doctors who were originally trying to help them.
How will it affect me?
I’ll likely further decrease my writing for controlled pain meds. I really can’t give up my DEA number entirely, because I need it to write for several epilepsy medications. But the use of narcotics in my practice will decline. Other docs will probably do the same.
Sadly, this only hurts those who legitimately need pain relief. It will be harder for them to find doctors willing to prescribe narcotics, and even if they do, it’s possible those physicians won’t take their insurance.
Some will say my reaction to the ruling means I don’t care, which isn’t true. I do care. I signed up for this job to help people. But I also have a family to support and protect, and have to think of them, too.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, the West Virginia Supreme Court ruled that patients can sue doctors if they became addicted to a medication the doctors prescribed even if the patients have committed crimes, such as doctor shopping, to get there.
Apparently, no one can be held responsible for their own actions anymore.
This is a serious catch-22 for doctors. On the one hand, we have ethical considerations, and oaths, to help others and relieve suffering. Now, on the flip side, doing just that can open us to legal action.
I prescribe narcotics. I try to use them judiciously, and only in people for whom other options have failed or are contraindicated. I suspect most doctors are the same. Every drug has its risks and benefits, and we try to make a calculated decision for each patient. I ask for the patient’s input, too, since he or she is the one who’ll be taking it.
I also have to depend on patients’ honesty. Patients who sell drugs to others, take more than I’ve prescribed, or use other methods of getting them (doctor shopping, buying them off the street) are all committing serious offenses. The development of monitoring databases where I can check on such behaviors has helped me catch those who’ve abused the medications.
One person quoted in an article about the court decision said, “I lied to everybody. I would steal. I pawned my grandma’s wedding rings. I was breaking into houses, doing anything and everything to stay high.”
So, obviously, that was all the doctor’s fault. He was trying to help her, and apparently led her to commit theft and burglary. I suppose the next step in such insanity is that he could be charged as an accomplice in her crimes. After all, it’s not her fault that she decided to steal from others.
This opens up a gold mine. Crooks obtaining narcotics through illicit means can now sue the doctors who were originally trying to help them.
How will it affect me?
I’ll likely further decrease my writing for controlled pain meds. I really can’t give up my DEA number entirely, because I need it to write for several epilepsy medications. But the use of narcotics in my practice will decline. Other docs will probably do the same.
Sadly, this only hurts those who legitimately need pain relief. It will be harder for them to find doctors willing to prescribe narcotics, and even if they do, it’s possible those physicians won’t take their insurance.
Some will say my reaction to the ruling means I don’t care, which isn’t true. I do care. I signed up for this job to help people. But I also have a family to support and protect, and have to think of them, too.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, the West Virginia Supreme Court ruled that patients can sue doctors if they became addicted to a medication the doctors prescribed even if the patients have committed crimes, such as doctor shopping, to get there.
Apparently, no one can be held responsible for their own actions anymore.
This is a serious catch-22 for doctors. On the one hand, we have ethical considerations, and oaths, to help others and relieve suffering. Now, on the flip side, doing just that can open us to legal action.
I prescribe narcotics. I try to use them judiciously, and only in people for whom other options have failed or are contraindicated. I suspect most doctors are the same. Every drug has its risks and benefits, and we try to make a calculated decision for each patient. I ask for the patient’s input, too, since he or she is the one who’ll be taking it.
I also have to depend on patients’ honesty. Patients who sell drugs to others, take more than I’ve prescribed, or use other methods of getting them (doctor shopping, buying them off the street) are all committing serious offenses. The development of monitoring databases where I can check on such behaviors has helped me catch those who’ve abused the medications.
One person quoted in an article about the court decision said, “I lied to everybody. I would steal. I pawned my grandma’s wedding rings. I was breaking into houses, doing anything and everything to stay high.”
So, obviously, that was all the doctor’s fault. He was trying to help her, and apparently led her to commit theft and burglary. I suppose the next step in such insanity is that he could be charged as an accomplice in her crimes. After all, it’s not her fault that she decided to steal from others.
This opens up a gold mine. Crooks obtaining narcotics through illicit means can now sue the doctors who were originally trying to help them.
How will it affect me?
I’ll likely further decrease my writing for controlled pain meds. I really can’t give up my DEA number entirely, because I need it to write for several epilepsy medications. But the use of narcotics in my practice will decline. Other docs will probably do the same.
Sadly, this only hurts those who legitimately need pain relief. It will be harder for them to find doctors willing to prescribe narcotics, and even if they do, it’s possible those physicians won’t take their insurance.
Some will say my reaction to the ruling means I don’t care, which isn’t true. I do care. I signed up for this job to help people. But I also have a family to support and protect, and have to think of them, too.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
RBAC500 safe, effective for elderly patients with mantle cell lymphoma
Reducing the dose of cytarabine from 800 mg/m2 to 500 mg/m2 allowed a regimen of rituximab, bendamustine, and cytarabine to be safely administered as first-line therapy to elderly patients who had mantle cell lymphoma and were not candidates for autologous stem cell transplant, according to Dr. Carlo Visco of the San Bortolo Hospital in Vicenza, Italy.
“Hematologic toxicity was substantially reduced, compared to the earlier study, Dr. Visco said, calling the R-BAC500 regimen “a highly effective treatment” for patients with mantle cell lymphoma.
Speaking at the at the International Congress on Malignant Lymphoma in Lugano, Switzerland, Dr. Visco noted the “encouraging results, but high hematologic toxicity” seen in a previous study that employed the higher cytarabine dose. In that previous study, transient grades 3-4 thrombocytopenia occurred in 76% of cycles.
In an attempt to reduce hematologic toxicity, the Fondazione Italiana Linfomi designed a phase II trial in which the cytarabine dose was lowered to 500 mg/m2 (R-BAC500). The administration schedule of cytarabine (on days 2-4) and the other components of the original regimen (rituximab, 375 mg/m2, on day 1 and bendamustine, 70 mg/m2, on days 2 and 3) remained unchanged.
The 57 study subjects, median age 71, had newly diagnosed mantle cell lymphoma, and were not eligible for autologous transplant as determined by the comprehensive geriatric assessment; 75% of the patients were males and 91% had Ann Arbor stage III/IV disease.
The Mantle Cell International Prognostic Index (MIPI) was low in 15%, intermediate in 40%, and high in 45%; 9% had the blastoid variant of the disease.
The primary endpoints were complete remission rate, as measured by 18-fluorodeoxyglucose–PET, according to Cheson criteria 2007, and safety. Secondary endpoints included molecular response rate, progression-free survival, and overall survival.
The overall response rate was 96%, and the complete remission rate was 93%. The molecular response rate at the end of treatment was 76% on peripheral blood and 55% on bone marrow samples. With a median follow-up of 18 months, the projected 2-year progression-free survival was 83%, and the overall survival was 91% without maintenance therapy.
Nearly all patients, 53 of 57, received at least four cycles of therapy, and 36 had six cycles. Treatment was discontinued because of toxicity (primarily hematologic) in 15 patients. Only one patient discontinued because of progressive disease.
Grade 3 or 4 neutropenia and thrombocytopenia were observed in about half of administered cycles. Febrile neutropenia occurred in 6%. Extrahematologic toxicity was mainly cardiac (5%).
BR is a commonly used regimen for older, less fit patients with MCL. Inclusion of high dose cytarabine appears to be beneficial n younger patients with MCL, particularly in induction pre-SCT. The FIL has been investigating intermediate doses of cytarabine combined, rather than alternating, with BR. This phase 2 study utilized cytarabine 500 mg/m2 daily x 3 with BR (slightly lower than standard dose bendamustine). The patient population was older with predominantly intermediate-high MIPI, yet results were impressive, particularly the PET negative rate of 93% and marrow MRD negative rate of 55%. Follow-up is short, but remissions do appear durable. Concerns are the high number of patients unable to complete planned therapy, the high rate of grade 3 and 4 cytopenias, and the frequency of visits required for close blood count monitoring and blood product support.
BR is a commonly used regimen for older, less fit patients with MCL. Inclusion of high dose cytarabine appears to be beneficial n younger patients with MCL, particularly in induction pre-SCT. The FIL has been investigating intermediate doses of cytarabine combined, rather than alternating, with BR. This phase 2 study utilized cytarabine 500 mg/m2 daily x 3 with BR (slightly lower than standard dose bendamustine). The patient population was older with predominantly intermediate-high MIPI, yet results were impressive, particularly the PET negative rate of 93% and marrow MRD negative rate of 55%. Follow-up is short, but remissions do appear durable. Concerns are the high number of patients unable to complete planned therapy, the high rate of grade 3 and 4 cytopenias, and the frequency of visits required for close blood count monitoring and blood product support.
BR is a commonly used regimen for older, less fit patients with MCL. Inclusion of high dose cytarabine appears to be beneficial n younger patients with MCL, particularly in induction pre-SCT. The FIL has been investigating intermediate doses of cytarabine combined, rather than alternating, with BR. This phase 2 study utilized cytarabine 500 mg/m2 daily x 3 with BR (slightly lower than standard dose bendamustine). The patient population was older with predominantly intermediate-high MIPI, yet results were impressive, particularly the PET negative rate of 93% and marrow MRD negative rate of 55%. Follow-up is short, but remissions do appear durable. Concerns are the high number of patients unable to complete planned therapy, the high rate of grade 3 and 4 cytopenias, and the frequency of visits required for close blood count monitoring and blood product support.
Reducing the dose of cytarabine from 800 mg/m2 to 500 mg/m2 allowed a regimen of rituximab, bendamustine, and cytarabine to be safely administered as first-line therapy to elderly patients who had mantle cell lymphoma and were not candidates for autologous stem cell transplant, according to Dr. Carlo Visco of the San Bortolo Hospital in Vicenza, Italy.
“Hematologic toxicity was substantially reduced, compared to the earlier study, Dr. Visco said, calling the R-BAC500 regimen “a highly effective treatment” for patients with mantle cell lymphoma.
Speaking at the at the International Congress on Malignant Lymphoma in Lugano, Switzerland, Dr. Visco noted the “encouraging results, but high hematologic toxicity” seen in a previous study that employed the higher cytarabine dose. In that previous study, transient grades 3-4 thrombocytopenia occurred in 76% of cycles.
In an attempt to reduce hematologic toxicity, the Fondazione Italiana Linfomi designed a phase II trial in which the cytarabine dose was lowered to 500 mg/m2 (R-BAC500). The administration schedule of cytarabine (on days 2-4) and the other components of the original regimen (rituximab, 375 mg/m2, on day 1 and bendamustine, 70 mg/m2, on days 2 and 3) remained unchanged.
The 57 study subjects, median age 71, had newly diagnosed mantle cell lymphoma, and were not eligible for autologous transplant as determined by the comprehensive geriatric assessment; 75% of the patients were males and 91% had Ann Arbor stage III/IV disease.
The Mantle Cell International Prognostic Index (MIPI) was low in 15%, intermediate in 40%, and high in 45%; 9% had the blastoid variant of the disease.
The primary endpoints were complete remission rate, as measured by 18-fluorodeoxyglucose–PET, according to Cheson criteria 2007, and safety. Secondary endpoints included molecular response rate, progression-free survival, and overall survival.
The overall response rate was 96%, and the complete remission rate was 93%. The molecular response rate at the end of treatment was 76% on peripheral blood and 55% on bone marrow samples. With a median follow-up of 18 months, the projected 2-year progression-free survival was 83%, and the overall survival was 91% without maintenance therapy.
Nearly all patients, 53 of 57, received at least four cycles of therapy, and 36 had six cycles. Treatment was discontinued because of toxicity (primarily hematologic) in 15 patients. Only one patient discontinued because of progressive disease.
Grade 3 or 4 neutropenia and thrombocytopenia were observed in about half of administered cycles. Febrile neutropenia occurred in 6%. Extrahematologic toxicity was mainly cardiac (5%).
Reducing the dose of cytarabine from 800 mg/m2 to 500 mg/m2 allowed a regimen of rituximab, bendamustine, and cytarabine to be safely administered as first-line therapy to elderly patients who had mantle cell lymphoma and were not candidates for autologous stem cell transplant, according to Dr. Carlo Visco of the San Bortolo Hospital in Vicenza, Italy.
“Hematologic toxicity was substantially reduced, compared to the earlier study, Dr. Visco said, calling the R-BAC500 regimen “a highly effective treatment” for patients with mantle cell lymphoma.
Speaking at the at the International Congress on Malignant Lymphoma in Lugano, Switzerland, Dr. Visco noted the “encouraging results, but high hematologic toxicity” seen in a previous study that employed the higher cytarabine dose. In that previous study, transient grades 3-4 thrombocytopenia occurred in 76% of cycles.
In an attempt to reduce hematologic toxicity, the Fondazione Italiana Linfomi designed a phase II trial in which the cytarabine dose was lowered to 500 mg/m2 (R-BAC500). The administration schedule of cytarabine (on days 2-4) and the other components of the original regimen (rituximab, 375 mg/m2, on day 1 and bendamustine, 70 mg/m2, on days 2 and 3) remained unchanged.
The 57 study subjects, median age 71, had newly diagnosed mantle cell lymphoma, and were not eligible for autologous transplant as determined by the comprehensive geriatric assessment; 75% of the patients were males and 91% had Ann Arbor stage III/IV disease.
The Mantle Cell International Prognostic Index (MIPI) was low in 15%, intermediate in 40%, and high in 45%; 9% had the blastoid variant of the disease.
The primary endpoints were complete remission rate, as measured by 18-fluorodeoxyglucose–PET, according to Cheson criteria 2007, and safety. Secondary endpoints included molecular response rate, progression-free survival, and overall survival.
The overall response rate was 96%, and the complete remission rate was 93%. The molecular response rate at the end of treatment was 76% on peripheral blood and 55% on bone marrow samples. With a median follow-up of 18 months, the projected 2-year progression-free survival was 83%, and the overall survival was 91% without maintenance therapy.
Nearly all patients, 53 of 57, received at least four cycles of therapy, and 36 had six cycles. Treatment was discontinued because of toxicity (primarily hematologic) in 15 patients. Only one patient discontinued because of progressive disease.
Grade 3 or 4 neutropenia and thrombocytopenia were observed in about half of administered cycles. Febrile neutropenia occurred in 6%. Extrahematologic toxicity was mainly cardiac (5%).
FROM 13-ICML
Key clinical point: Reducing the dose of cytarabine from 800 mg/m2 to 500 mg/m2 allowed a regimen of rituximab, bendamustine, and cytarabine to be safely administered as first-line therapy to elderly patients with mantle cell lymphoma.
Major finding: Nearly all patients, 53 of 57, received at least four cycles of therapy, and 36 had six cycles. Treatment was discontinued because of toxicity (primarily hematologic) in 15 patients.
Data source: 57 study subjects, median age 71, who had newly diagnosed mantle cell lymphoma and were not eligible for autologous transplant as determined by the comprehensive geriatric assessment.
Disclosures: The trial was conducted by the Fondazione Italiana Linfomi. There were no relevant financial disclosures.
No signal for the superiority of autologous versus allogenic stem-cell transplants in T-cell lymphoma
A randomized trial designed to compare autologous to allogeneic stem cell transplantation as first-line therapy in younger patients with peripheral T-cell lymphoma was discontinued early because nearly 40% of the patients had early disease progression and did not undergo transplantation.
Peripheral T-cell lymphoma generally yields a poor prognosis when treated with conventional chemotherapy, but autologous or allogeneic stem cell transplants were thought to be an option for patients with relapsing or refractory disease. Based on this hypothesis, the AATT (Autologous or Allogeneic Transplantation in T-Cell Lymphoma) study explored stem cell transplant as a first-line therapy, enrolling 104 patients aged 18-60 between 2011 and 2014.
All patients received four courses of chemotherapy with CHOEP-14 (cyclophosphamide, adriamycin, vincristine, etoposide, and prednisone).
Those in the autologous stem cell group and those without a suitable donor proceeded to one course of DHAP (high-dose ara-C, cis-platinum, and dexamethasone) and stem cell collection. Patients randomized to autologous transplantation received high dose therapy (BCNU, etoposide, cytarabine, melphalan: BEAM) followed 4-6 weeks later by transplantation of autologous stem cells.
Patients randomized to allogeneic transplantation received high dose therapy (fludarabine, busulfan, cyclophosphamide: FBC) followed by transplantation of allogeneic stem cells. GvHD prophylaxis included antithymocyte globuline (ATG), cyclosporine A, and mycophenolate mofetil.
Among the 58 patients eligible for the interim analysis, the mean age was 50 and 64% were male. Thirteen of the 28 patients randomized for allogeneic transplants underwent transplants; the others were not allografted because of progressive disease or lack of a donor. Of the 30 patients randomized to autologous SCT, 19 had the procedure; 11 did not receive transplants because of progressive disease or infection, Dr. Norbert Schmitz of Asklepios Klinik St. Georg, Hamburg, Germany, reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
The primary outcome, 1-year event-free survival (EFS), was 41% in the intent-to-treat population (95% CI, 27%–54%).
Causes of death included lymphoma (seven autologous, five allogeneic), salvage therapy (two), early or late infections (four), and graft vs. host disease (two).
Survival rates did not significantly differ in the two stem cell transplant groups, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedure. Based on the low probability of meeting the primary outcome, the data safety monitoring board decided to stop patient accrual and discontinue the trial.
*This article was updated 7/8/2015.
As outcomes for patients with PTCL are suboptimal with standard therapy, usually CHOP/CHOEP, young and fit patients are commonly offered high dose chemotherapy with stem cell support (SCT) to consolidate 1st remission, though no firm data support this approach. As a trial of SCT vs observation would be difficult to accomplish, the AATT trail was undertaken to compare autologous vs allogeneic transplantation. The trial was not able to answer this question as it was halted early due to the high proportion of patients unable to proceed to SCT. One lesson here is that data reported for PTCL patients who receive SCT in 1st remission suffers from selection bias, unless accompanied by an intent-to-treat analysis. There is a clear need for improved induction therapy for PTCL.
As outcomes for patients with PTCL are suboptimal with standard therapy, usually CHOP/CHOEP, young and fit patients are commonly offered high dose chemotherapy with stem cell support (SCT) to consolidate 1st remission, though no firm data support this approach. As a trial of SCT vs observation would be difficult to accomplish, the AATT trail was undertaken to compare autologous vs allogeneic transplantation. The trial was not able to answer this question as it was halted early due to the high proportion of patients unable to proceed to SCT. One lesson here is that data reported for PTCL patients who receive SCT in 1st remission suffers from selection bias, unless accompanied by an intent-to-treat analysis. There is a clear need for improved induction therapy for PTCL.
As outcomes for patients with PTCL are suboptimal with standard therapy, usually CHOP/CHOEP, young and fit patients are commonly offered high dose chemotherapy with stem cell support (SCT) to consolidate 1st remission, though no firm data support this approach. As a trial of SCT vs observation would be difficult to accomplish, the AATT trail was undertaken to compare autologous vs allogeneic transplantation. The trial was not able to answer this question as it was halted early due to the high proportion of patients unable to proceed to SCT. One lesson here is that data reported for PTCL patients who receive SCT in 1st remission suffers from selection bias, unless accompanied by an intent-to-treat analysis. There is a clear need for improved induction therapy for PTCL.
A randomized trial designed to compare autologous to allogeneic stem cell transplantation as first-line therapy in younger patients with peripheral T-cell lymphoma was discontinued early because nearly 40% of the patients had early disease progression and did not undergo transplantation.
Peripheral T-cell lymphoma generally yields a poor prognosis when treated with conventional chemotherapy, but autologous or allogeneic stem cell transplants were thought to be an option for patients with relapsing or refractory disease. Based on this hypothesis, the AATT (Autologous or Allogeneic Transplantation in T-Cell Lymphoma) study explored stem cell transplant as a first-line therapy, enrolling 104 patients aged 18-60 between 2011 and 2014.
All patients received four courses of chemotherapy with CHOEP-14 (cyclophosphamide, adriamycin, vincristine, etoposide, and prednisone).
Those in the autologous stem cell group and those without a suitable donor proceeded to one course of DHAP (high-dose ara-C, cis-platinum, and dexamethasone) and stem cell collection. Patients randomized to autologous transplantation received high dose therapy (BCNU, etoposide, cytarabine, melphalan: BEAM) followed 4-6 weeks later by transplantation of autologous stem cells.
Patients randomized to allogeneic transplantation received high dose therapy (fludarabine, busulfan, cyclophosphamide: FBC) followed by transplantation of allogeneic stem cells. GvHD prophylaxis included antithymocyte globuline (ATG), cyclosporine A, and mycophenolate mofetil.
Among the 58 patients eligible for the interim analysis, the mean age was 50 and 64% were male. Thirteen of the 28 patients randomized for allogeneic transplants underwent transplants; the others were not allografted because of progressive disease or lack of a donor. Of the 30 patients randomized to autologous SCT, 19 had the procedure; 11 did not receive transplants because of progressive disease or infection, Dr. Norbert Schmitz of Asklepios Klinik St. Georg, Hamburg, Germany, reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
The primary outcome, 1-year event-free survival (EFS), was 41% in the intent-to-treat population (95% CI, 27%–54%).
Causes of death included lymphoma (seven autologous, five allogeneic), salvage therapy (two), early or late infections (four), and graft vs. host disease (two).
Survival rates did not significantly differ in the two stem cell transplant groups, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedure. Based on the low probability of meeting the primary outcome, the data safety monitoring board decided to stop patient accrual and discontinue the trial.
*This article was updated 7/8/2015.
A randomized trial designed to compare autologous to allogeneic stem cell transplantation as first-line therapy in younger patients with peripheral T-cell lymphoma was discontinued early because nearly 40% of the patients had early disease progression and did not undergo transplantation.
Peripheral T-cell lymphoma generally yields a poor prognosis when treated with conventional chemotherapy, but autologous or allogeneic stem cell transplants were thought to be an option for patients with relapsing or refractory disease. Based on this hypothesis, the AATT (Autologous or Allogeneic Transplantation in T-Cell Lymphoma) study explored stem cell transplant as a first-line therapy, enrolling 104 patients aged 18-60 between 2011 and 2014.
All patients received four courses of chemotherapy with CHOEP-14 (cyclophosphamide, adriamycin, vincristine, etoposide, and prednisone).
Those in the autologous stem cell group and those without a suitable donor proceeded to one course of DHAP (high-dose ara-C, cis-platinum, and dexamethasone) and stem cell collection. Patients randomized to autologous transplantation received high dose therapy (BCNU, etoposide, cytarabine, melphalan: BEAM) followed 4-6 weeks later by transplantation of autologous stem cells.
Patients randomized to allogeneic transplantation received high dose therapy (fludarabine, busulfan, cyclophosphamide: FBC) followed by transplantation of allogeneic stem cells. GvHD prophylaxis included antithymocyte globuline (ATG), cyclosporine A, and mycophenolate mofetil.
Among the 58 patients eligible for the interim analysis, the mean age was 50 and 64% were male. Thirteen of the 28 patients randomized for allogeneic transplants underwent transplants; the others were not allografted because of progressive disease or lack of a donor. Of the 30 patients randomized to autologous SCT, 19 had the procedure; 11 did not receive transplants because of progressive disease or infection, Dr. Norbert Schmitz of Asklepios Klinik St. Georg, Hamburg, Germany, reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
The primary outcome, 1-year event-free survival (EFS), was 41% in the intent-to-treat population (95% CI, 27%–54%).
Causes of death included lymphoma (seven autologous, five allogeneic), salvage therapy (two), early or late infections (four), and graft vs. host disease (two).
Survival rates did not significantly differ in the two stem cell transplant groups, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedure. Based on the low probability of meeting the primary outcome, the data safety monitoring board decided to stop patient accrual and discontinue the trial.
*This article was updated 7/8/2015.
FROM 13-ICML
Key clinical point: Survival rates did not significantly differ for autologous versus allogenic stem cell transplant in patients with peripheral T-cell lymphoma, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedures.
Major finding: Early disease progression led to the discontinuation of a randomized trial comparing autologous to allogeneic stem cell transplantation in younger patients with peripheral T-cell lymphoma.
Data source: Results from 58 patients eligible for the interim analysis.
Disclosures: There were no relevant financial disclosures.
Bendamustine regimen may be induction-therapy option in mantle cell lymphoma
Rituximab plus bendamustine may prove to be an induction-therapy option for younger patients with mantle cell lymphoma, Dr. Richard Chen and his colleagues in a SWOG (Southwest Oncology Group) trial reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
Compared with a more aggressive combination regimen, a rituximab plus bendamustine (Treanda) option is a simple regimen that can be given in an outpatient setting and was associated with fewer adverse events and similar 2-year outcomes, the researchers found. The more aggressive regimen, however, was associated with lower-than-expected stem cell mobilization rates and the trial was prematurely closed, allowing no significant results.
For this study, two induction-therapy regimens were compared in 53 patients with untreated stage III or IV (or bulky stage II) mantle cell lymphoma. All patients were less than age 65 years and received rituximab (R) in combination with one of two regimens: 18 patients received four cycles of R-HyperCVAD + methotrexate + cytarabine (R-HyperCVAD/MTX/ARA-C) and 35 patients received six cycles of R-bendamustine.
The overall response rate was 94% with R-HyperCVAD/MTX/ARA-C and 86% with R-bendamustine; the complete response rates were 31% and 43%, respectively; the partial response rates were 62% and 43%, respectively, Dr. Chen and his associates reported.
The median follow-up for surviving patients is nearly 24 months. The estimated 2-year progression-free survival was 87% for patients in both treatment groups.
Significantly higher rates of bone marrow toxicity occurred in the group receiving the R-HyperCVAD/MTX/ARA-C regimen, compared with the bendamustine regimen. Grade 3 and 4 thrombocytopenia occurred in 69% given R-HyperCVAD/MTX/ARA-C and 17% given R-bendamustine. Anemia affected 56% of those given R-HyperCVAD/MTX/ARA-C and 8.6% given R-bendamustine. Neutropenia was seen in 63% given R-HyperCVAD/MTX/ARA-C and 34% of patients given R-bendamustine. Febrile neutropenia occurred in 31% given R-HyperCVAD/MTX/ARA-C and 14% given R-bendamustine.
The study was discontinued prematurely because of the low mobilization of stem cells at the transplant phase of the study in patients given R-HyperCVAD/MTX/ARA-C. Just 4 of 16 patients on R-HyperCVAD/MTX/ARA-C and 21 of 35 patients given R-bendamustine underwent autologous stem cell transplants.
The R-bendamustine regimen seems less myelosuppressive. Because of the premature closure of the trial, the study did not reach statistical significance for 2-year progression-free survival, the researchers reported. Since bendamustine in combination with rituximab was associated with lower rates of hematologic toxicity, however, it warrants further study as an induction regimen, they concluded.
Young, fit patients with mantle cell lymphoma (MCL) are often treated with intensive, though non-curative, therapy. While some centers still use R-HyperCVAD/MA alone, most use alternating R-CHOP-based and high dose cytarabine-based regimens, followed by SCT. The U.S. Intergroup trial, led by SWOG, was designed to gather information about a strategy using a limited number of cycles of R-HyperCVAD/MA followed by SCT, and an alternative strategy using an effective but less-intense induction, bendamustine-rituximab (BR), also followed by SCT. The R-HyperCVAD/MA arm was closed early due to difficulties with stem cell collection. While there are technical reasons for this that likely could be overcome, results with other pre-SCT regimens are good enough that this is not likely to be further studied. The BR followed by SCT arm was closed after accrual of 35 patients, enough to get a sense that this was feasible, although it will be important to see further updates regarding how many of these patients did go on to SCT, and their ultimate outcomes. A key question is whether a study comparing BR induction with a different, commonly used intense regimen pre-SCT is worth the commitment of resources, given the range of novel agents now available for MCL.
Dr. Mitchell Smith is a medical oncologist affiliated with the Cleveland Clinic.
Young, fit patients with mantle cell lymphoma (MCL) are often treated with intensive, though non-curative, therapy. While some centers still use R-HyperCVAD/MA alone, most use alternating R-CHOP-based and high dose cytarabine-based regimens, followed by SCT. The U.S. Intergroup trial, led by SWOG, was designed to gather information about a strategy using a limited number of cycles of R-HyperCVAD/MA followed by SCT, and an alternative strategy using an effective but less-intense induction, bendamustine-rituximab (BR), also followed by SCT. The R-HyperCVAD/MA arm was closed early due to difficulties with stem cell collection. While there are technical reasons for this that likely could be overcome, results with other pre-SCT regimens are good enough that this is not likely to be further studied. The BR followed by SCT arm was closed after accrual of 35 patients, enough to get a sense that this was feasible, although it will be important to see further updates regarding how many of these patients did go on to SCT, and their ultimate outcomes. A key question is whether a study comparing BR induction with a different, commonly used intense regimen pre-SCT is worth the commitment of resources, given the range of novel agents now available for MCL.
Dr. Mitchell Smith is a medical oncologist affiliated with the Cleveland Clinic.
Young, fit patients with mantle cell lymphoma (MCL) are often treated with intensive, though non-curative, therapy. While some centers still use R-HyperCVAD/MA alone, most use alternating R-CHOP-based and high dose cytarabine-based regimens, followed by SCT. The U.S. Intergroup trial, led by SWOG, was designed to gather information about a strategy using a limited number of cycles of R-HyperCVAD/MA followed by SCT, and an alternative strategy using an effective but less-intense induction, bendamustine-rituximab (BR), also followed by SCT. The R-HyperCVAD/MA arm was closed early due to difficulties with stem cell collection. While there are technical reasons for this that likely could be overcome, results with other pre-SCT regimens are good enough that this is not likely to be further studied. The BR followed by SCT arm was closed after accrual of 35 patients, enough to get a sense that this was feasible, although it will be important to see further updates regarding how many of these patients did go on to SCT, and their ultimate outcomes. A key question is whether a study comparing BR induction with a different, commonly used intense regimen pre-SCT is worth the commitment of resources, given the range of novel agents now available for MCL.
Dr. Mitchell Smith is a medical oncologist affiliated with the Cleveland Clinic.
Rituximab plus bendamustine may prove to be an induction-therapy option for younger patients with mantle cell lymphoma, Dr. Richard Chen and his colleagues in a SWOG (Southwest Oncology Group) trial reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
Compared with a more aggressive combination regimen, a rituximab plus bendamustine (Treanda) option is a simple regimen that can be given in an outpatient setting and was associated with fewer adverse events and similar 2-year outcomes, the researchers found. The more aggressive regimen, however, was associated with lower-than-expected stem cell mobilization rates and the trial was prematurely closed, allowing no significant results.
For this study, two induction-therapy regimens were compared in 53 patients with untreated stage III or IV (or bulky stage II) mantle cell lymphoma. All patients were less than age 65 years and received rituximab (R) in combination with one of two regimens: 18 patients received four cycles of R-HyperCVAD + methotrexate + cytarabine (R-HyperCVAD/MTX/ARA-C) and 35 patients received six cycles of R-bendamustine.
The overall response rate was 94% with R-HyperCVAD/MTX/ARA-C and 86% with R-bendamustine; the complete response rates were 31% and 43%, respectively; the partial response rates were 62% and 43%, respectively, Dr. Chen and his associates reported.
The median follow-up for surviving patients is nearly 24 months. The estimated 2-year progression-free survival was 87% for patients in both treatment groups.
Significantly higher rates of bone marrow toxicity occurred in the group receiving the R-HyperCVAD/MTX/ARA-C regimen, compared with the bendamustine regimen. Grade 3 and 4 thrombocytopenia occurred in 69% given R-HyperCVAD/MTX/ARA-C and 17% given R-bendamustine. Anemia affected 56% of those given R-HyperCVAD/MTX/ARA-C and 8.6% given R-bendamustine. Neutropenia was seen in 63% given R-HyperCVAD/MTX/ARA-C and 34% of patients given R-bendamustine. Febrile neutropenia occurred in 31% given R-HyperCVAD/MTX/ARA-C and 14% given R-bendamustine.
The study was discontinued prematurely because of the low mobilization of stem cells at the transplant phase of the study in patients given R-HyperCVAD/MTX/ARA-C. Just 4 of 16 patients on R-HyperCVAD/MTX/ARA-C and 21 of 35 patients given R-bendamustine underwent autologous stem cell transplants.
The R-bendamustine regimen seems less myelosuppressive. Because of the premature closure of the trial, the study did not reach statistical significance for 2-year progression-free survival, the researchers reported. Since bendamustine in combination with rituximab was associated with lower rates of hematologic toxicity, however, it warrants further study as an induction regimen, they concluded.
Rituximab plus bendamustine may prove to be an induction-therapy option for younger patients with mantle cell lymphoma, Dr. Richard Chen and his colleagues in a SWOG (Southwest Oncology Group) trial reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
Compared with a more aggressive combination regimen, a rituximab plus bendamustine (Treanda) option is a simple regimen that can be given in an outpatient setting and was associated with fewer adverse events and similar 2-year outcomes, the researchers found. The more aggressive regimen, however, was associated with lower-than-expected stem cell mobilization rates and the trial was prematurely closed, allowing no significant results.
For this study, two induction-therapy regimens were compared in 53 patients with untreated stage III or IV (or bulky stage II) mantle cell lymphoma. All patients were less than age 65 years and received rituximab (R) in combination with one of two regimens: 18 patients received four cycles of R-HyperCVAD + methotrexate + cytarabine (R-HyperCVAD/MTX/ARA-C) and 35 patients received six cycles of R-bendamustine.
The overall response rate was 94% with R-HyperCVAD/MTX/ARA-C and 86% with R-bendamustine; the complete response rates were 31% and 43%, respectively; the partial response rates were 62% and 43%, respectively, Dr. Chen and his associates reported.
The median follow-up for surviving patients is nearly 24 months. The estimated 2-year progression-free survival was 87% for patients in both treatment groups.
Significantly higher rates of bone marrow toxicity occurred in the group receiving the R-HyperCVAD/MTX/ARA-C regimen, compared with the bendamustine regimen. Grade 3 and 4 thrombocytopenia occurred in 69% given R-HyperCVAD/MTX/ARA-C and 17% given R-bendamustine. Anemia affected 56% of those given R-HyperCVAD/MTX/ARA-C and 8.6% given R-bendamustine. Neutropenia was seen in 63% given R-HyperCVAD/MTX/ARA-C and 34% of patients given R-bendamustine. Febrile neutropenia occurred in 31% given R-HyperCVAD/MTX/ARA-C and 14% given R-bendamustine.
The study was discontinued prematurely because of the low mobilization of stem cells at the transplant phase of the study in patients given R-HyperCVAD/MTX/ARA-C. Just 4 of 16 patients on R-HyperCVAD/MTX/ARA-C and 21 of 35 patients given R-bendamustine underwent autologous stem cell transplants.
The R-bendamustine regimen seems less myelosuppressive. Because of the premature closure of the trial, the study did not reach statistical significance for 2-year progression-free survival, the researchers reported. Since bendamustine in combination with rituximab was associated with lower rates of hematologic toxicity, however, it warrants further study as an induction regimen, they concluded.
FROM 13-ICML
Key clinical point: Rituximab plus bendamustine may prove to be an option for induction therapy prior to autologous stem cell transplant in patients with mantle cell lymphoma.
Major finding: The overall response rate was 94% with R-HyperCVAD/MTX/ARA-C and 86% with R-bendamustine; the complete response rates were 31% and 43%, respectively; the partial response rates were 62% and 43%, respectively.
Data source: 53 patients with untreated stage III or IV (or bulky stage II) mantle cell lymphoma.
Disclosures: The investigators did not report any conflicts.
Benefit of extended anticoagulation is temporary, study shows

Results of the PADIS-PE trial suggest that patients with a first episode of pulmonary embolism (PE) benefit from extended anticoagulation therapy, but that benefit is lost after the treatment is stopped.
When patients received warfarin for 18 months after an initial 6 months of anticoagulation therapy, they had a lower risk of venous thromboembolism (VTE)
recurrence than patients who did not receive additional anticoagulation.
However, this benefit was lost once the patients stopped taking warfarin.
Francis Couturaud, MD, PhD, of the Universite de Bretagne Occidentale in Brest, France, and his colleagues reported these findings in JAMA.
The researchers analyzed 371 adult patients who had experienced a first episode of symptomatic, unprovoked PE and were initially treated for 6 months with a vitamin K antagonist.
The patients were then randomized to warfarin or placebo for 18 months. After randomization, 4 patients were lost to follow-up, all after month 18, and 1 withdrew due to an adverse event.
The median follow-up was 24 months. The primary outcome was a composite of recurrent VTE and major bleeding at 18 months after randomization.
During the 18-month treatment period, the primary outcome occurred in 3% (6/184) of patients in the warfarin arm and 13.5% (25/187) in the placebo arm (hazard ratio [HR]=0.22, P=0.001).
This was driven by a reduction in the risk of recurrent VTE, as the risk of major bleeding was higher in the warfarin arm. Recurrent VTE occurred in 3 patients in the warfarin arm and 25 in the placebo arm (HR=0.15, P<0.001). And major bleeding occurred in 4 patients in the warfarin arm and 1 in the placebo arm (HR=3.96, P=0.22).
Once patients stopped receiving warfarin, the reduction in risk of VTE recurrence was lost. During the entire study period (a median of 41 months), the primary outcome occurred in 21% (n=33) of patients in the warfarin arm and 24% (n=42) in the placebo arm (HR=0.75, P=0.22).
There was no significant difference between the treatment arms with regard to recurrent VTE (HR=0.69, P=0.14) or major bleeding (HR=1.12, P=0.85).
The researchers said these results suggest patients with an unprovoked PE require long-term VTE prevention measures. But further investigation is needed to determine whether these should include systematic treatment with vitamin K antagonists, new anticoagulants, or aspirin, or whether the measures should be tailored according to patient risk factors. ![]()

Results of the PADIS-PE trial suggest that patients with a first episode of pulmonary embolism (PE) benefit from extended anticoagulation therapy, but that benefit is lost after the treatment is stopped.
When patients received warfarin for 18 months after an initial 6 months of anticoagulation therapy, they had a lower risk of venous thromboembolism (VTE)
recurrence than patients who did not receive additional anticoagulation.
However, this benefit was lost once the patients stopped taking warfarin.
Francis Couturaud, MD, PhD, of the Universite de Bretagne Occidentale in Brest, France, and his colleagues reported these findings in JAMA.
The researchers analyzed 371 adult patients who had experienced a first episode of symptomatic, unprovoked PE and were initially treated for 6 months with a vitamin K antagonist.
The patients were then randomized to warfarin or placebo for 18 months. After randomization, 4 patients were lost to follow-up, all after month 18, and 1 withdrew due to an adverse event.
The median follow-up was 24 months. The primary outcome was a composite of recurrent VTE and major bleeding at 18 months after randomization.
During the 18-month treatment period, the primary outcome occurred in 3% (6/184) of patients in the warfarin arm and 13.5% (25/187) in the placebo arm (hazard ratio [HR]=0.22, P=0.001).
This was driven by a reduction in the risk of recurrent VTE, as the risk of major bleeding was higher in the warfarin arm. Recurrent VTE occurred in 3 patients in the warfarin arm and 25 in the placebo arm (HR=0.15, P<0.001). And major bleeding occurred in 4 patients in the warfarin arm and 1 in the placebo arm (HR=3.96, P=0.22).
Once patients stopped receiving warfarin, the reduction in risk of VTE recurrence was lost. During the entire study period (a median of 41 months), the primary outcome occurred in 21% (n=33) of patients in the warfarin arm and 24% (n=42) in the placebo arm (HR=0.75, P=0.22).
There was no significant difference between the treatment arms with regard to recurrent VTE (HR=0.69, P=0.14) or major bleeding (HR=1.12, P=0.85).
The researchers said these results suggest patients with an unprovoked PE require long-term VTE prevention measures. But further investigation is needed to determine whether these should include systematic treatment with vitamin K antagonists, new anticoagulants, or aspirin, or whether the measures should be tailored according to patient risk factors. ![]()

Results of the PADIS-PE trial suggest that patients with a first episode of pulmonary embolism (PE) benefit from extended anticoagulation therapy, but that benefit is lost after the treatment is stopped.
When patients received warfarin for 18 months after an initial 6 months of anticoagulation therapy, they had a lower risk of venous thromboembolism (VTE)
recurrence than patients who did not receive additional anticoagulation.
However, this benefit was lost once the patients stopped taking warfarin.
Francis Couturaud, MD, PhD, of the Universite de Bretagne Occidentale in Brest, France, and his colleagues reported these findings in JAMA.
The researchers analyzed 371 adult patients who had experienced a first episode of symptomatic, unprovoked PE and were initially treated for 6 months with a vitamin K antagonist.
The patients were then randomized to warfarin or placebo for 18 months. After randomization, 4 patients were lost to follow-up, all after month 18, and 1 withdrew due to an adverse event.
The median follow-up was 24 months. The primary outcome was a composite of recurrent VTE and major bleeding at 18 months after randomization.
During the 18-month treatment period, the primary outcome occurred in 3% (6/184) of patients in the warfarin arm and 13.5% (25/187) in the placebo arm (hazard ratio [HR]=0.22, P=0.001).
This was driven by a reduction in the risk of recurrent VTE, as the risk of major bleeding was higher in the warfarin arm. Recurrent VTE occurred in 3 patients in the warfarin arm and 25 in the placebo arm (HR=0.15, P<0.001). And major bleeding occurred in 4 patients in the warfarin arm and 1 in the placebo arm (HR=3.96, P=0.22).
Once patients stopped receiving warfarin, the reduction in risk of VTE recurrence was lost. During the entire study period (a median of 41 months), the primary outcome occurred in 21% (n=33) of patients in the warfarin arm and 24% (n=42) in the placebo arm (HR=0.75, P=0.22).
There was no significant difference between the treatment arms with regard to recurrent VTE (HR=0.69, P=0.14) or major bleeding (HR=1.12, P=0.85).
The researchers said these results suggest patients with an unprovoked PE require long-term VTE prevention measures. But further investigation is needed to determine whether these should include systematic treatment with vitamin K antagonists, new anticoagulants, or aspirin, or whether the measures should be tailored according to patient risk factors. ![]()
Novel mAb targeting CD70 shows activity in TCL

Photo by Linda Bartlett
LUGANO—The defucosylated monoclonal antibody (mAb) ARGX-110, which is active against CD70-bearing tumor cells and CD70-dependent stimulation of regulatory T cells, has shown activity in relapsed/refractory T-cell lymphoma (TCL), according to investigators.
Of the 8 TCL patients enrolled in a phase 1 trial of ARGX-110, 3 had a biological response to the mAb.
In this dose-escalation trial, the maximum tolerated dose of ARGX-110 was not reached.
Marie Maerevoet, MD, of the Institut Jules Bordet in Brussels, Belgium, presented results from the lymphoma cohort of this trial at the 13th International Congress on Malignant Lymphoma (abstract 040*). The study was sponsored by arGEN-X, the company developing ARGX-110.
Dr Maerevoet pointed out that more than half the tumor cells in 71% of patients with cutaneous T-cell lymphoma (CTCL) and 22% with peripheral T-cell lymphoma (PTCL) are CD70-positive. CD70 signaling occurs via CD27, and CD27 shedding is a biomarker for an active pathway.
Since ARGX-110 has an affinity for CD70, inhibits CD27 signaling, and mediates the lysis of TCL in Sézary syndrome (SS), mycosis fungoides, and anaplastic large cell lymphoma (ALCL) cell lines, researchers decided to investigate the safety and clinical pharmacology of ARGX-110 monotherapy in metastatic, relapsed or refractory, solid tumors and hematologic malignancies.
Patients’ tumors had to express CD70 by immunohistochemistry, defined as more than 10% tumor cells of 2+ or 3+ intensity.
The primary endpoint was to determine the maximum tolerated dose. Secondary endpoints were pharmacology, immunogenicity, and efficacy signals.
Patient demographics
Between February 2013 and April 2015, investigators assigned 63 patients to receive ARGX-110 at doses ranging from 0.1 to 10 mg/kg intravenously once every 3 weeks until disease progression or withdrawal due to toxicity. Patients were pre-medicated with corticoid regimens.
Eighteen patients had lymphoid malignancies—8 with B-cell lymphomas, 8 with TCL, and 2 with Hodgkin lymphoma.
The TCL cohort consisted of 1 patient with SS, 1 with transformed SS, 1 with T-helper CTCL, 2 with angioimmunoblastic T-cell lymphoma (AITL), 2 with PTCL not otherwise specified (NOS), and 1 with ALCL.
Patients were a median age of 62 (range, 55–78), had a median of 4 prior treatment regimens (range, 2–6), and received a median of 2 cycles of ARGX-110 (range, 1–6).
Dr Maerevoet noted that most lymphoma patients received a dose of 5 mg/kg every 3 weeks.
Safety
In the entire lymphoma cohort of 18 patients, 4 patients (22%) experienced a grade 1 or 2 infusion-related reaction. Three patients (18%) developed grade 3 sepsis—1 with Waldenstrom’s macroglobulinemia, 1 with AITL, and 1 with PTCL-NOS.
Two patients (11%) had hematologic toxicity consisting of a grade 3 decrease in hemoglobin and absolute neutrophil count, which was considered not related to treatment with ARGX-110.
“The maximum tolerated dose was not reached,” Dr Maerevoet said. “We didn’t observe auto-immune adverse events or impact on serum IgG or IgM.”
Efficacy outcomes
The main reason for withdrawal was progressive disease, which occurred in 14 lymphoma patients.
Two patients—1 with Waldenstrom’s macroglobulinemia and 1 with AITL—withdrew due to adverse events of sepsis (catheter infection, pneumonia), 1 patient with SS withdrew for social reasons, and 1 patient with follicular T-cell lymphoma (currently classified as PTCL-NOS) remains on study.
Dr Maerevoet described the 3 TCL patients who had a biologic response to ARGX-110. One patient with SS had a hematologic complete remission after 6 cycles at the 0.1 mg/kg dose.
Another patient with transformed SS experienced a depletion of circulating clones after 2 cycles of the 10 mg/kg dose. However, the patient ultimately died of progressive disease.
A third patient had resolution of autoimmune hemolytic anemia. This 61-year-old male with AITL achieved a partial response with normalization of LDH levels and an increase in hemoglobin to 7.9 g/dL without transfusion support after 2 doses of ARGX-110 at 5 mg/kg.
The patient became Coombs-negative and had a 16% reduction in tumor size by CT scan. However, the patient subsequently died of pneumonia.
The investigators also observed clinical activity in the peripheral blood, lymph nodes, and skin of 2 additional patients.
The biological activity of ARGX-110 as demonstrated by these TCL patients, in addition to the safety and tolerability of this mAb, led the team to conclude that further clinical investigation of ARGX-110 in TCL is warranted. ![]()
*Information in the abstract differs from that presented at the meeting.

Photo by Linda Bartlett
LUGANO—The defucosylated monoclonal antibody (mAb) ARGX-110, which is active against CD70-bearing tumor cells and CD70-dependent stimulation of regulatory T cells, has shown activity in relapsed/refractory T-cell lymphoma (TCL), according to investigators.
Of the 8 TCL patients enrolled in a phase 1 trial of ARGX-110, 3 had a biological response to the mAb.
In this dose-escalation trial, the maximum tolerated dose of ARGX-110 was not reached.
Marie Maerevoet, MD, of the Institut Jules Bordet in Brussels, Belgium, presented results from the lymphoma cohort of this trial at the 13th International Congress on Malignant Lymphoma (abstract 040*). The study was sponsored by arGEN-X, the company developing ARGX-110.
Dr Maerevoet pointed out that more than half the tumor cells in 71% of patients with cutaneous T-cell lymphoma (CTCL) and 22% with peripheral T-cell lymphoma (PTCL) are CD70-positive. CD70 signaling occurs via CD27, and CD27 shedding is a biomarker for an active pathway.
Since ARGX-110 has an affinity for CD70, inhibits CD27 signaling, and mediates the lysis of TCL in Sézary syndrome (SS), mycosis fungoides, and anaplastic large cell lymphoma (ALCL) cell lines, researchers decided to investigate the safety and clinical pharmacology of ARGX-110 monotherapy in metastatic, relapsed or refractory, solid tumors and hematologic malignancies.
Patients’ tumors had to express CD70 by immunohistochemistry, defined as more than 10% tumor cells of 2+ or 3+ intensity.
The primary endpoint was to determine the maximum tolerated dose. Secondary endpoints were pharmacology, immunogenicity, and efficacy signals.
Patient demographics
Between February 2013 and April 2015, investigators assigned 63 patients to receive ARGX-110 at doses ranging from 0.1 to 10 mg/kg intravenously once every 3 weeks until disease progression or withdrawal due to toxicity. Patients were pre-medicated with corticoid regimens.
Eighteen patients had lymphoid malignancies—8 with B-cell lymphomas, 8 with TCL, and 2 with Hodgkin lymphoma.
The TCL cohort consisted of 1 patient with SS, 1 with transformed SS, 1 with T-helper CTCL, 2 with angioimmunoblastic T-cell lymphoma (AITL), 2 with PTCL not otherwise specified (NOS), and 1 with ALCL.
Patients were a median age of 62 (range, 55–78), had a median of 4 prior treatment regimens (range, 2–6), and received a median of 2 cycles of ARGX-110 (range, 1–6).
Dr Maerevoet noted that most lymphoma patients received a dose of 5 mg/kg every 3 weeks.
Safety
In the entire lymphoma cohort of 18 patients, 4 patients (22%) experienced a grade 1 or 2 infusion-related reaction. Three patients (18%) developed grade 3 sepsis—1 with Waldenstrom’s macroglobulinemia, 1 with AITL, and 1 with PTCL-NOS.
Two patients (11%) had hematologic toxicity consisting of a grade 3 decrease in hemoglobin and absolute neutrophil count, which was considered not related to treatment with ARGX-110.
“The maximum tolerated dose was not reached,” Dr Maerevoet said. “We didn’t observe auto-immune adverse events or impact on serum IgG or IgM.”
Efficacy outcomes
The main reason for withdrawal was progressive disease, which occurred in 14 lymphoma patients.
Two patients—1 with Waldenstrom’s macroglobulinemia and 1 with AITL—withdrew due to adverse events of sepsis (catheter infection, pneumonia), 1 patient with SS withdrew for social reasons, and 1 patient with follicular T-cell lymphoma (currently classified as PTCL-NOS) remains on study.
Dr Maerevoet described the 3 TCL patients who had a biologic response to ARGX-110. One patient with SS had a hematologic complete remission after 6 cycles at the 0.1 mg/kg dose.
Another patient with transformed SS experienced a depletion of circulating clones after 2 cycles of the 10 mg/kg dose. However, the patient ultimately died of progressive disease.
A third patient had resolution of autoimmune hemolytic anemia. This 61-year-old male with AITL achieved a partial response with normalization of LDH levels and an increase in hemoglobin to 7.9 g/dL without transfusion support after 2 doses of ARGX-110 at 5 mg/kg.
The patient became Coombs-negative and had a 16% reduction in tumor size by CT scan. However, the patient subsequently died of pneumonia.
The investigators also observed clinical activity in the peripheral blood, lymph nodes, and skin of 2 additional patients.
The biological activity of ARGX-110 as demonstrated by these TCL patients, in addition to the safety and tolerability of this mAb, led the team to conclude that further clinical investigation of ARGX-110 in TCL is warranted. ![]()
*Information in the abstract differs from that presented at the meeting.

Photo by Linda Bartlett
LUGANO—The defucosylated monoclonal antibody (mAb) ARGX-110, which is active against CD70-bearing tumor cells and CD70-dependent stimulation of regulatory T cells, has shown activity in relapsed/refractory T-cell lymphoma (TCL), according to investigators.
Of the 8 TCL patients enrolled in a phase 1 trial of ARGX-110, 3 had a biological response to the mAb.
In this dose-escalation trial, the maximum tolerated dose of ARGX-110 was not reached.
Marie Maerevoet, MD, of the Institut Jules Bordet in Brussels, Belgium, presented results from the lymphoma cohort of this trial at the 13th International Congress on Malignant Lymphoma (abstract 040*). The study was sponsored by arGEN-X, the company developing ARGX-110.
Dr Maerevoet pointed out that more than half the tumor cells in 71% of patients with cutaneous T-cell lymphoma (CTCL) and 22% with peripheral T-cell lymphoma (PTCL) are CD70-positive. CD70 signaling occurs via CD27, and CD27 shedding is a biomarker for an active pathway.
Since ARGX-110 has an affinity for CD70, inhibits CD27 signaling, and mediates the lysis of TCL in Sézary syndrome (SS), mycosis fungoides, and anaplastic large cell lymphoma (ALCL) cell lines, researchers decided to investigate the safety and clinical pharmacology of ARGX-110 monotherapy in metastatic, relapsed or refractory, solid tumors and hematologic malignancies.
Patients’ tumors had to express CD70 by immunohistochemistry, defined as more than 10% tumor cells of 2+ or 3+ intensity.
The primary endpoint was to determine the maximum tolerated dose. Secondary endpoints were pharmacology, immunogenicity, and efficacy signals.
Patient demographics
Between February 2013 and April 2015, investigators assigned 63 patients to receive ARGX-110 at doses ranging from 0.1 to 10 mg/kg intravenously once every 3 weeks until disease progression or withdrawal due to toxicity. Patients were pre-medicated with corticoid regimens.
Eighteen patients had lymphoid malignancies—8 with B-cell lymphomas, 8 with TCL, and 2 with Hodgkin lymphoma.
The TCL cohort consisted of 1 patient with SS, 1 with transformed SS, 1 with T-helper CTCL, 2 with angioimmunoblastic T-cell lymphoma (AITL), 2 with PTCL not otherwise specified (NOS), and 1 with ALCL.
Patients were a median age of 62 (range, 55–78), had a median of 4 prior treatment regimens (range, 2–6), and received a median of 2 cycles of ARGX-110 (range, 1–6).
Dr Maerevoet noted that most lymphoma patients received a dose of 5 mg/kg every 3 weeks.
Safety
In the entire lymphoma cohort of 18 patients, 4 patients (22%) experienced a grade 1 or 2 infusion-related reaction. Three patients (18%) developed grade 3 sepsis—1 with Waldenstrom’s macroglobulinemia, 1 with AITL, and 1 with PTCL-NOS.
Two patients (11%) had hematologic toxicity consisting of a grade 3 decrease in hemoglobin and absolute neutrophil count, which was considered not related to treatment with ARGX-110.
“The maximum tolerated dose was not reached,” Dr Maerevoet said. “We didn’t observe auto-immune adverse events or impact on serum IgG or IgM.”
Efficacy outcomes
The main reason for withdrawal was progressive disease, which occurred in 14 lymphoma patients.
Two patients—1 with Waldenstrom’s macroglobulinemia and 1 with AITL—withdrew due to adverse events of sepsis (catheter infection, pneumonia), 1 patient with SS withdrew for social reasons, and 1 patient with follicular T-cell lymphoma (currently classified as PTCL-NOS) remains on study.
Dr Maerevoet described the 3 TCL patients who had a biologic response to ARGX-110. One patient with SS had a hematologic complete remission after 6 cycles at the 0.1 mg/kg dose.
Another patient with transformed SS experienced a depletion of circulating clones after 2 cycles of the 10 mg/kg dose. However, the patient ultimately died of progressive disease.
A third patient had resolution of autoimmune hemolytic anemia. This 61-year-old male with AITL achieved a partial response with normalization of LDH levels and an increase in hemoglobin to 7.9 g/dL without transfusion support after 2 doses of ARGX-110 at 5 mg/kg.
The patient became Coombs-negative and had a 16% reduction in tumor size by CT scan. However, the patient subsequently died of pneumonia.
The investigators also observed clinical activity in the peripheral blood, lymph nodes, and skin of 2 additional patients.
The biological activity of ARGX-110 as demonstrated by these TCL patients, in addition to the safety and tolerability of this mAb, led the team to conclude that further clinical investigation of ARGX-110 in TCL is warranted. ![]()
*Information in the abstract differs from that presented at the meeting.
NICE wants more info on PI3Kδ inhibitor

The UK’s National Institute for Health and Care Excellence (NICE) has opened a consultation on a preliminary draft guidance for the PI3Kδ inhibitor idelalisib (Zydelig).
The agency has requested additional information from Gilead Sciences, the company developing idelalisib, to inform a decision on the use of this drug
in combination with rituximab for adults with chronic lymphocytic
leukemia (CLL).
NICE said it has questions about the cost-effectiveness of this treatment that have not been answered. Idelalisib costs £3114.75 for sixty 150-mg tablets, and the mean cost of a 1-year treatment course is £37,922.
Until NICE receives the requested information, the agency said it cannot recommend idelalisib plus rituximab for adults with untreated CLL who have 17p deletion or TP53 mutation, adults with relapsed CLL, or adults whose CLL is refractory and retreatment with previous regimens is not considered appropriate.
“The independent appraisal committee, which is developing the guidance on behalf of NICE, considered evidence from the company, clinical experts, and patient representatives,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“It concluded there were still questions to be answered about the cost-effectiveness of the treatment. We have requested further analysis from the company. We want to ensure we have as much information as possible to make an informed recommendation.”
Specifically, NICE has requested:
- A revised cost-effectiveness analysis for the comparison of idelalisib plus rituximab with rituximab alone, best supportive care, and ofatumumab
- A sensitivity analysis exploring the length of treatment benefit of idelalisib plus rituximab from treatment discontinuation up to 5 years
- A sensitivity analysis exploring the effects of reducing the proportion of non-responders having intravenous immunoglobulin from 45% to 20% or less and increasing the number of responders having intravenous immunoglobulin from 0% to 20%
- A sensitivity analysis exploring the effect of using clinical effectiveness data from the subgroup of people in Study 116 whose disease is refractory.
The independent appraisal committee will review this information and develop further draft guidance.
Until final guidance is issued, National Health Service bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

The UK’s National Institute for Health and Care Excellence (NICE) has opened a consultation on a preliminary draft guidance for the PI3Kδ inhibitor idelalisib (Zydelig).
The agency has requested additional information from Gilead Sciences, the company developing idelalisib, to inform a decision on the use of this drug
in combination with rituximab for adults with chronic lymphocytic
leukemia (CLL).
NICE said it has questions about the cost-effectiveness of this treatment that have not been answered. Idelalisib costs £3114.75 for sixty 150-mg tablets, and the mean cost of a 1-year treatment course is £37,922.
Until NICE receives the requested information, the agency said it cannot recommend idelalisib plus rituximab for adults with untreated CLL who have 17p deletion or TP53 mutation, adults with relapsed CLL, or adults whose CLL is refractory and retreatment with previous regimens is not considered appropriate.
“The independent appraisal committee, which is developing the guidance on behalf of NICE, considered evidence from the company, clinical experts, and patient representatives,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“It concluded there were still questions to be answered about the cost-effectiveness of the treatment. We have requested further analysis from the company. We want to ensure we have as much information as possible to make an informed recommendation.”
Specifically, NICE has requested:
- A revised cost-effectiveness analysis for the comparison of idelalisib plus rituximab with rituximab alone, best supportive care, and ofatumumab
- A sensitivity analysis exploring the length of treatment benefit of idelalisib plus rituximab from treatment discontinuation up to 5 years
- A sensitivity analysis exploring the effects of reducing the proportion of non-responders having intravenous immunoglobulin from 45% to 20% or less and increasing the number of responders having intravenous immunoglobulin from 0% to 20%
- A sensitivity analysis exploring the effect of using clinical effectiveness data from the subgroup of people in Study 116 whose disease is refractory.
The independent appraisal committee will review this information and develop further draft guidance.
Until final guidance is issued, National Health Service bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()

The UK’s National Institute for Health and Care Excellence (NICE) has opened a consultation on a preliminary draft guidance for the PI3Kδ inhibitor idelalisib (Zydelig).
The agency has requested additional information from Gilead Sciences, the company developing idelalisib, to inform a decision on the use of this drug
in combination with rituximab for adults with chronic lymphocytic
leukemia (CLL).
NICE said it has questions about the cost-effectiveness of this treatment that have not been answered. Idelalisib costs £3114.75 for sixty 150-mg tablets, and the mean cost of a 1-year treatment course is £37,922.
Until NICE receives the requested information, the agency said it cannot recommend idelalisib plus rituximab for adults with untreated CLL who have 17p deletion or TP53 mutation, adults with relapsed CLL, or adults whose CLL is refractory and retreatment with previous regimens is not considered appropriate.
“The independent appraisal committee, which is developing the guidance on behalf of NICE, considered evidence from the company, clinical experts, and patient representatives,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“It concluded there were still questions to be answered about the cost-effectiveness of the treatment. We have requested further analysis from the company. We want to ensure we have as much information as possible to make an informed recommendation.”
Specifically, NICE has requested:
- A revised cost-effectiveness analysis for the comparison of idelalisib plus rituximab with rituximab alone, best supportive care, and ofatumumab
- A sensitivity analysis exploring the length of treatment benefit of idelalisib plus rituximab from treatment discontinuation up to 5 years
- A sensitivity analysis exploring the effects of reducing the proportion of non-responders having intravenous immunoglobulin from 45% to 20% or less and increasing the number of responders having intravenous immunoglobulin from 0% to 20%
- A sensitivity analysis exploring the effect of using clinical effectiveness data from the subgroup of people in Study 116 whose disease is refractory.
The independent appraisal committee will review this information and develop further draft guidance.
Until final guidance is issued, National Health Service bodies should make decisions locally on the funding of specific treatments. Once NICE issues its final guidance on a technology, it replaces local recommendations. ![]()
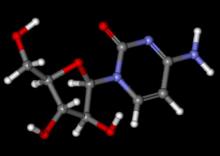
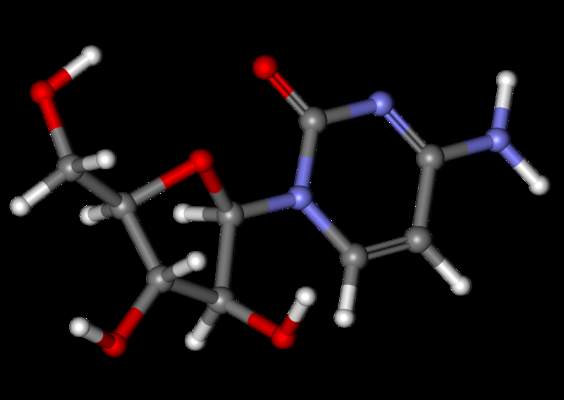
Drug may fight cancer by suppressing inflammation

Intermittent dosing with the immunosuppressant rapamycin can break an inflammatory loop that fuels cancer relapse, according to researchers.
The team found that rapamycin selectively breaks the cascade of inflammatory events that follow cellular senescence.
Once disrupted, it takes time for the inflammatory loop to re-establish itself. This suggests intermittent dosing could provide a way to reap the benefits of rapamycin while reducing side effects.
The researchers described these findings in Nature Cell Biology.
“We think this could provide a paradigm shift in the treatment of age-related disease, including cancer,” said study author Judith Campisi, PhD, of the Buck Institute for Research on Aging in Novato, California.
“Imagine the possibility of taking a pill for a few days or weeks every few years, as opposed to taking something with side effects every day for the rest of your life. It’s a new way of looking at how we could deal with age-related maladies.”
For this study, Dr Campisi and her colleagues administered rapamycin to mice with prostate cancer after they had received treatment with DNA-damaging chemotherapy.
The rapamycin reduced the secretion of inflammatory cytokines from senescent cells by suppressing the mTOR pathway. This suppressed the cells’ ability to stimulate tumor growth.
“DNA-damaging chemotherapy causes senescence, both to the tumor and its microenvironment,” Dr Campisi explained. “The tumor shrinks, but the immediate tissue environment is inflamed. We think signals from those inflamed cells trigger residual cancer cells to grow again. In the mice, rapamycin suppressed the ability of the tumor cells to relapse.”
The researchers said the paradigm-shifting potential of intermittent rapamycin dosing is based on the fact that it takes time for the inflammatory loop—fueled by the senescence-associated secretory phenotype (SASP)—to form and time for it to re-establish itself after a brief treatment period with rapamycin.
“Rapamycin blocks the production of a protein called IL-1α,” explained study author Remi-Martin Laberge, PhD, also of the Buck Institute.
“This in turn, suppresses IL6, a well-known inflammatory cytokine, at the level of transcription, which prevents the production of the IL6 protein. Because it acts at a deeper level within the cellular process, it takes longer for it to get started again.”
Dr Laberge also pointed out that treatment with rapamycin selectivity impacts the SASP, preserving the function of factors essential for wound healing.
“It’s an elegant solution,” Dr Laberge said. “Imagine using a small hammer to delicately knock out one thing that is causing problems. We knocked it out, and it stayed out long enough to benefit the health of the animal.”
“We have yet to fully understand why suppressing the mTOR pathway via rapamycin increases lifespan and health span in mice,” Dr Campisi added. “This work helps illuminate the puzzle. Perhaps the mice are living longer because they have less overall inflammation, and maybe intermittent dosing will make it possible for us to use [rapamycin] more widely in humans.” ![]()

Intermittent dosing with the immunosuppressant rapamycin can break an inflammatory loop that fuels cancer relapse, according to researchers.
The team found that rapamycin selectively breaks the cascade of inflammatory events that follow cellular senescence.
Once disrupted, it takes time for the inflammatory loop to re-establish itself. This suggests intermittent dosing could provide a way to reap the benefits of rapamycin while reducing side effects.
The researchers described these findings in Nature Cell Biology.
“We think this could provide a paradigm shift in the treatment of age-related disease, including cancer,” said study author Judith Campisi, PhD, of the Buck Institute for Research on Aging in Novato, California.
“Imagine the possibility of taking a pill for a few days or weeks every few years, as opposed to taking something with side effects every day for the rest of your life. It’s a new way of looking at how we could deal with age-related maladies.”
For this study, Dr Campisi and her colleagues administered rapamycin to mice with prostate cancer after they had received treatment with DNA-damaging chemotherapy.
The rapamycin reduced the secretion of inflammatory cytokines from senescent cells by suppressing the mTOR pathway. This suppressed the cells’ ability to stimulate tumor growth.
“DNA-damaging chemotherapy causes senescence, both to the tumor and its microenvironment,” Dr Campisi explained. “The tumor shrinks, but the immediate tissue environment is inflamed. We think signals from those inflamed cells trigger residual cancer cells to grow again. In the mice, rapamycin suppressed the ability of the tumor cells to relapse.”
The researchers said the paradigm-shifting potential of intermittent rapamycin dosing is based on the fact that it takes time for the inflammatory loop—fueled by the senescence-associated secretory phenotype (SASP)—to form and time for it to re-establish itself after a brief treatment period with rapamycin.
“Rapamycin blocks the production of a protein called IL-1α,” explained study author Remi-Martin Laberge, PhD, also of the Buck Institute.
“This in turn, suppresses IL6, a well-known inflammatory cytokine, at the level of transcription, which prevents the production of the IL6 protein. Because it acts at a deeper level within the cellular process, it takes longer for it to get started again.”
Dr Laberge also pointed out that treatment with rapamycin selectivity impacts the SASP, preserving the function of factors essential for wound healing.
“It’s an elegant solution,” Dr Laberge said. “Imagine using a small hammer to delicately knock out one thing that is causing problems. We knocked it out, and it stayed out long enough to benefit the health of the animal.”
“We have yet to fully understand why suppressing the mTOR pathway via rapamycin increases lifespan and health span in mice,” Dr Campisi added. “This work helps illuminate the puzzle. Perhaps the mice are living longer because they have less overall inflammation, and maybe intermittent dosing will make it possible for us to use [rapamycin] more widely in humans.” ![]()

Intermittent dosing with the immunosuppressant rapamycin can break an inflammatory loop that fuels cancer relapse, according to researchers.
The team found that rapamycin selectively breaks the cascade of inflammatory events that follow cellular senescence.
Once disrupted, it takes time for the inflammatory loop to re-establish itself. This suggests intermittent dosing could provide a way to reap the benefits of rapamycin while reducing side effects.
The researchers described these findings in Nature Cell Biology.
“We think this could provide a paradigm shift in the treatment of age-related disease, including cancer,” said study author Judith Campisi, PhD, of the Buck Institute for Research on Aging in Novato, California.
“Imagine the possibility of taking a pill for a few days or weeks every few years, as opposed to taking something with side effects every day for the rest of your life. It’s a new way of looking at how we could deal with age-related maladies.”
For this study, Dr Campisi and her colleagues administered rapamycin to mice with prostate cancer after they had received treatment with DNA-damaging chemotherapy.
The rapamycin reduced the secretion of inflammatory cytokines from senescent cells by suppressing the mTOR pathway. This suppressed the cells’ ability to stimulate tumor growth.
“DNA-damaging chemotherapy causes senescence, both to the tumor and its microenvironment,” Dr Campisi explained. “The tumor shrinks, but the immediate tissue environment is inflamed. We think signals from those inflamed cells trigger residual cancer cells to grow again. In the mice, rapamycin suppressed the ability of the tumor cells to relapse.”
The researchers said the paradigm-shifting potential of intermittent rapamycin dosing is based on the fact that it takes time for the inflammatory loop—fueled by the senescence-associated secretory phenotype (SASP)—to form and time for it to re-establish itself after a brief treatment period with rapamycin.
“Rapamycin blocks the production of a protein called IL-1α,” explained study author Remi-Martin Laberge, PhD, also of the Buck Institute.
“This in turn, suppresses IL6, a well-known inflammatory cytokine, at the level of transcription, which prevents the production of the IL6 protein. Because it acts at a deeper level within the cellular process, it takes longer for it to get started again.”
Dr Laberge also pointed out that treatment with rapamycin selectivity impacts the SASP, preserving the function of factors essential for wound healing.
“It’s an elegant solution,” Dr Laberge said. “Imagine using a small hammer to delicately knock out one thing that is causing problems. We knocked it out, and it stayed out long enough to benefit the health of the animal.”
“We have yet to fully understand why suppressing the mTOR pathway via rapamycin increases lifespan and health span in mice,” Dr Campisi added. “This work helps illuminate the puzzle. Perhaps the mice are living longer because they have less overall inflammation, and maybe intermittent dosing will make it possible for us to use [rapamycin] more widely in humans.” ![]()
School-based CBT program reduces depression, suicidality
Emotionally distressed Canadian students who completed a school-based cognitive-behavioral therapy program experienced significant improvements in depression and suicidality, according to a multicenter prospective study published in PLoS One.
At the end of the pilot program entitled Empowering a Multimodal Pathway Toward Healthy Youth (EMPATHY), the number of students who were actively suicidal fell by 73%, and depression scores dropped by about 15% across all schools and age groups, Dr. Peter Silverstone, a professor of psychiatry at the University of Alberta, Edmonton, said in an interview.
Depressive disorders affect at least 10% of U.S. young people, and about 8% who live in the United States attempt suicide each year, according to the Centers for Disease Control and Prevention. To explore solutions, Dr. Silverstone and his associates used tablets loaded with a specially designed software “app” to screen 3,244 Canadian students aged 11-18 years (6th-12th grades). The students attended the five middle schools and high schools in a single Alberta school district, and the screen incorporated questions from several short, free mental health scales (PLoS One 2015 [doi:10.1371/journal.pone.0125527]).
Trained staff then interviewed students who were assessed as actively suicidal and met with them and their parents in order to create a safety plan, make referrals to appropriate health services, and invite students to participate in the guided Internet cognitive behavioral therapy (CBT) program. Students who scored in the top 10% of a combined measure of depression, anxiety, and low self-esteem also were invited to participate in the CBT program, and an eight-session version of the program was deployed for all seventh and eighth graders.
Among 503 high-risk students who were offered the CBT program, 151 (30%) enrolled, and 90% completed the program.
Parents and students had only a week to sign and return the consent forms, which led to the low participation rate in the pilot phase of the study, Dr. Silverstone noted. “We changed the processes after the pilot and got much higher acceptance rates, nearer to 70% percent.”
At 12-week follow-up, program participants had significantly improved from baseline, compared with nonparticipants in terms of the combined mental health, depression, anxiety, self-esteem, and quality of life scales, but not in terms of self-reported use of drugs, alcohol, or tobacco, the investigators reported.
Of the 104 actively suicidal students at baseline who completed both assessments, 76 (73%) were in the no-risk group at 12 weeks, a significant difference.
The results are promising, but their durability remains unclear, as other studies have reported strong short-term results that did not hold several years later, the investigators noted.
“All staff hired in the schools to implement the program had to have some experience working with youth, and many had an undergraduate degree,” the investigators added. “However, it is important to note that they were specifically not highly trained individuals (such as psychologists or teachers), as it was felt that it would not be feasible for widespread expansion if such highly trained (and expensive) staff were required.”
“We have follow-up data for 15 months, until the end of June 2015, that we hope to be able to start analyzing before the end of the year,” Dr. Silverstone said. But in the meantime, the new provincial government has cut the program’s funding, which he called a “major disappointment. We have had to abandon all further plans for the program,” he said, adding that it will terminate at the end of 2015.
Alberta Health Services funded the study. The researchers declared having no relevant competing interests.
Emotionally distressed Canadian students who completed a school-based cognitive-behavioral therapy program experienced significant improvements in depression and suicidality, according to a multicenter prospective study published in PLoS One.
At the end of the pilot program entitled Empowering a Multimodal Pathway Toward Healthy Youth (EMPATHY), the number of students who were actively suicidal fell by 73%, and depression scores dropped by about 15% across all schools and age groups, Dr. Peter Silverstone, a professor of psychiatry at the University of Alberta, Edmonton, said in an interview.
Depressive disorders affect at least 10% of U.S. young people, and about 8% who live in the United States attempt suicide each year, according to the Centers for Disease Control and Prevention. To explore solutions, Dr. Silverstone and his associates used tablets loaded with a specially designed software “app” to screen 3,244 Canadian students aged 11-18 years (6th-12th grades). The students attended the five middle schools and high schools in a single Alberta school district, and the screen incorporated questions from several short, free mental health scales (PLoS One 2015 [doi:10.1371/journal.pone.0125527]).
Trained staff then interviewed students who were assessed as actively suicidal and met with them and their parents in order to create a safety plan, make referrals to appropriate health services, and invite students to participate in the guided Internet cognitive behavioral therapy (CBT) program. Students who scored in the top 10% of a combined measure of depression, anxiety, and low self-esteem also were invited to participate in the CBT program, and an eight-session version of the program was deployed for all seventh and eighth graders.
Among 503 high-risk students who were offered the CBT program, 151 (30%) enrolled, and 90% completed the program.
Parents and students had only a week to sign and return the consent forms, which led to the low participation rate in the pilot phase of the study, Dr. Silverstone noted. “We changed the processes after the pilot and got much higher acceptance rates, nearer to 70% percent.”
At 12-week follow-up, program participants had significantly improved from baseline, compared with nonparticipants in terms of the combined mental health, depression, anxiety, self-esteem, and quality of life scales, but not in terms of self-reported use of drugs, alcohol, or tobacco, the investigators reported.
Of the 104 actively suicidal students at baseline who completed both assessments, 76 (73%) were in the no-risk group at 12 weeks, a significant difference.
The results are promising, but their durability remains unclear, as other studies have reported strong short-term results that did not hold several years later, the investigators noted.
“All staff hired in the schools to implement the program had to have some experience working with youth, and many had an undergraduate degree,” the investigators added. “However, it is important to note that they were specifically not highly trained individuals (such as psychologists or teachers), as it was felt that it would not be feasible for widespread expansion if such highly trained (and expensive) staff were required.”
“We have follow-up data for 15 months, until the end of June 2015, that we hope to be able to start analyzing before the end of the year,” Dr. Silverstone said. But in the meantime, the new provincial government has cut the program’s funding, which he called a “major disappointment. We have had to abandon all further plans for the program,” he said, adding that it will terminate at the end of 2015.
Alberta Health Services funded the study. The researchers declared having no relevant competing interests.
Emotionally distressed Canadian students who completed a school-based cognitive-behavioral therapy program experienced significant improvements in depression and suicidality, according to a multicenter prospective study published in PLoS One.
At the end of the pilot program entitled Empowering a Multimodal Pathway Toward Healthy Youth (EMPATHY), the number of students who were actively suicidal fell by 73%, and depression scores dropped by about 15% across all schools and age groups, Dr. Peter Silverstone, a professor of psychiatry at the University of Alberta, Edmonton, said in an interview.
Depressive disorders affect at least 10% of U.S. young people, and about 8% who live in the United States attempt suicide each year, according to the Centers for Disease Control and Prevention. To explore solutions, Dr. Silverstone and his associates used tablets loaded with a specially designed software “app” to screen 3,244 Canadian students aged 11-18 years (6th-12th grades). The students attended the five middle schools and high schools in a single Alberta school district, and the screen incorporated questions from several short, free mental health scales (PLoS One 2015 [doi:10.1371/journal.pone.0125527]).
Trained staff then interviewed students who were assessed as actively suicidal and met with them and their parents in order to create a safety plan, make referrals to appropriate health services, and invite students to participate in the guided Internet cognitive behavioral therapy (CBT) program. Students who scored in the top 10% of a combined measure of depression, anxiety, and low self-esteem also were invited to participate in the CBT program, and an eight-session version of the program was deployed for all seventh and eighth graders.
Among 503 high-risk students who were offered the CBT program, 151 (30%) enrolled, and 90% completed the program.
Parents and students had only a week to sign and return the consent forms, which led to the low participation rate in the pilot phase of the study, Dr. Silverstone noted. “We changed the processes after the pilot and got much higher acceptance rates, nearer to 70% percent.”
At 12-week follow-up, program participants had significantly improved from baseline, compared with nonparticipants in terms of the combined mental health, depression, anxiety, self-esteem, and quality of life scales, but not in terms of self-reported use of drugs, alcohol, or tobacco, the investigators reported.
Of the 104 actively suicidal students at baseline who completed both assessments, 76 (73%) were in the no-risk group at 12 weeks, a significant difference.
The results are promising, but their durability remains unclear, as other studies have reported strong short-term results that did not hold several years later, the investigators noted.
“All staff hired in the schools to implement the program had to have some experience working with youth, and many had an undergraduate degree,” the investigators added. “However, it is important to note that they were specifically not highly trained individuals (such as psychologists or teachers), as it was felt that it would not be feasible for widespread expansion if such highly trained (and expensive) staff were required.”
“We have follow-up data for 15 months, until the end of June 2015, that we hope to be able to start analyzing before the end of the year,” Dr. Silverstone said. But in the meantime, the new provincial government has cut the program’s funding, which he called a “major disappointment. We have had to abandon all further plans for the program,” he said, adding that it will terminate at the end of 2015.
Alberta Health Services funded the study. The researchers declared having no relevant competing interests.
FROM PLOS ONE
Key clinical point: A school-based cognitive-behavioral therapy program was tied to substantial declines in adolescent depression and suicidality.
Major finding: At 12-week follow-up, the number of students who were actively suicidal was 73% lower than at baseline.
Data source: Multicenter Canadian prospective study of 3,244 students aged 11-18 years.
Disclosures: Alberta Health Services funded the study. The researchers declared having no relevant competing interests.
Cutaneous Infection With Mycobacterium kansasii in a Patient With Myelodysplastic Syndrome and Sweet Syndrome
To the Editor:
A 68-year-old man with a history of myelodysplastic syndrome and recurrent Sweet syndrome presented with left leg lesions of 3 months’ duration. The lesions originated as a solitary nodule on the left calf and subsequently developed into multiple nonpainful, nonpruritic, erythematous plaques of varying sizes with violaceous coloration and overlying necrotic eschar, occupying the entire anterior aspect of the left lower leg and left popliteal fossa (Figure). The patient denied any trauma or associated symptoms but had a history of Sweet syndrome that manifested as lesions on the arms and legs for which he took 6 mg of prednisone daily to prevent recurrence.
Histologic examination revealed nodular and diffuse chronic granulomatous and acute inflammatory infiltrate. Stains for bacteria, fungi, and acid-fast bacilli were negative. Cultures subsequently grew Mycobacterium kansasii, and the patient was started on isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 800 mg daily, and pyridoxine 50 mg daily. Chest radiograph and computed tomography showed no evidence of pulmonary disease and 2 blood cultures were negative for growth. The patient subsequently developed weakness that he attributed to the antibiotics and he decided to discontinue all treatment.
At 11 months the lesions showed no change; however, magnetic resonance imaging of the leg was suggestive of osteomyelitis. The patient was started on clarithromycin 500 mg twice daily with planned addition of isoniazid. The patient refused any additional antibiotics but agreed to continue the clarithromycin treatment for one year. He was subsequently lost to dermatology follow-up.
Nontuberculous mycobacteria (NTM) infection is a rare sequela of hematologic malignancy, seen in only 1.5% of patients.1 The NTM most commonly seen in hematologic malignancy are generally the fast-growing species Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, or Mycobacterium phlei, rather than slow growers Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium marinum, and Mycobacterium xenopi. Mycobacterium kansasii infection, such as seen in our patient, accounts for only 18% of cases.1 This case is further distinguished by the fact that cutaneous infections with NTM also are generally caused by fast-growing organisms such as Mycobacterium abscessus-chelonae complex and M fortuitum, rather than the slow-growing M kansasii.2,3
Mycobacterium kansasii is a slow-growing, acid-fast bacillus found in local water reservoirs, swimming pools, sewers, and tap water where it can live for up to 12 months.2,4,5Mycobacterium kansasii is traditionally considered the most virulent NTM.3,6 It most frequently causes a pulmonary infection in the immunosuppressed and patients with chronic bronchopulmonary disease.6,7 Disseminated disease is less common and is primarily seen in immunocompromised patients, particularly in human immunodeficiency virus–positive patients, transplant recipients, and patients with hematologic malignancies.1,6,8 Disseminated disease rarely has been seen in patients with normal immune function.2,3
Cutaneous M kansasii infection has only infrequently been described. Most patients tend to be middle-aged men, with a median affected age of 43 years.2,7,9,10 One review of cutaneous cases found that 72% had some form of altered immunity and more than 50% of those patients were on chronic steroids. The same review found that of the cases of cutaneous M kansasii in patients with altered immunity, only 30% had disseminated disease.10 Our patient was immunocompromised but showed no evidence of disseminated disease, as displayed by negative chest radiograph and computed tomography, lack of pulmonary symptoms, and negative blood cultures. As a 68-year-old man with myelodysplastic syndrome on chronic steroids with no disseminated disease, our patient fits well into these demographics, aside from his advanced age.
Cutaneous M kansasii infection has a variable presentation, manifesting as solitary lesions, nodules, pustules, seromas, erythematous plaques, verrucous lesions, ulcers, and as cellulitis.5,7,9-12 Immune competent individuals were more likely to present with raised lesions or ulcers, whereas immune compromised individuals had a more diffuse presentation of cellulitis or seromas with variable histology.6,8 Our patient, though immune compromised, presented with multiple erythematous plaques with eschars, which further endorses having a high clinical suspicion, as the lesions display marked heterogeneity.
Treatment of M kansasii infection consists of at least 1 year of isoniazid 300 mg daily, rifampin 600 mg daily, and ethambutol 15 mg/kg daily, with possible addition of streptomycin.8,13Mycobacterium kansasii infection necessitates multidrug treatment due to the broad range of resistance exhibited by different isolated strains.14
Response to treatment in cutaneous M kansasii greatly depends on the underlying disease state of the individual. Generally, immune competent individuals do very well, while the course in immune compromised patients depends on their degree of illness. Patients with disseminated disease generally do poorly.4,7,10 In at least one case of cutaneous disease, dissemination developed as a sequela, thus suggesting treatment is needed even in stable lesions.2 Dissemination was a concern with our patient given the magnetic resonance imaging findings suggestive of osteomyelitis. Although treatment generally consists of triple therapy with isoniazid, rifampin, and ethambutol, given the high frequency of adverse effects due to isoniazid or rifampin, as was seen in our patient, the drug regimen might have to be altered to suit the patient. Susceptibility testing is desirable to aid in tailoring the treatment.8,13 Furthermore, as the duration of treatment is at least 1 year, diligent follow-up must be maintained to avoid incomplete treatment.
The unpredictable presentation of cutaneous M kansasii infection coupled with the variable history necessitates a high level of clinical suspicion and a low threshold for culturing lesions. Furthermore, the long duration and complexity of the antibiotic regimen and the high incidence of adverse reactions demands strict follow-up, especially given the risk for progression to disseminated disease.
1. Chen CY, Sheng WH, Lai CC. Mycobacterial infections in adult patients with hematological malignancy. Eur J Clin Microbiol Infect Dis. 2012;31:1059-1066.
2. Han SH, Kim KM, Chin BS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: a case report and comprehensive review of the literature. J Korean Med Sci. 2010;25:304-308.
3. Razavi B, Cleveland MG. Cutaneous infection due to Mycobacterium kansasii. Diagn Microbiol Infect Dis. 2000;38:173-175.
4. Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995;13:207-222.
5. Nomura Y, Nishie W, Shibaki A, et al. Disseminated cutaneous Mycobacterium kansasii infection in a patient infected with the human immunodeficiency virus. Clin Exp Dermatol. 2009;34:625-626.
6. Bloch KC, Zwerling L, Pletcher MJ, et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129:698-704.
7. Breathnach A, Levell N, Munro C, et al. Cutaneous Mycobacterium kansasii infection: case report and review. Clin Infect Dis. 1995;20:812-817.
8. Pintado V, Gómez-Mampaso E, Martín-Dávila P. Mycobacterium kansasii infection in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1999;18:582-586.
9. Stengem J, Grande KK, Hsu S. Localized primary cutaneous Mycobacterium kansasii infection in an immunocompromised patient. J Am Acad Dermatol. 1999;41(5, pt 2):854-856.
10. Czelusta A, Moore AY. Cutaneous Mycobacterium kansasii infection in a patient with systemic lupus erythematosus: case report and review. J Am Acad Dermatol. 1999;40(2, pt 2):359-363.
11. Curcó N, Pagerols X, Gómez L, et al. Mycobacterium kansasii infection limited to the skin in a patient with AIDS. Br J Dermatol. 1996;135:324-326.
12. Hanke CW, Temofeew RK, Slama SL. Mycobacterium kansasii infection with multiple cutaneous lesions. J Am Acad Dermatol. 1987;16(5, pt 2):1122-1128.
13. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculousmycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
14. da Silva Telles MA, Chimara E, Ferrazoli L, Riley LW. Mycobacterium kansasii: antibiotic susceptibility and PCR-restriction analysis of clinical isolates. J Med Microbiol. 2005;54:975-979.
To the Editor:
A 68-year-old man with a history of myelodysplastic syndrome and recurrent Sweet syndrome presented with left leg lesions of 3 months’ duration. The lesions originated as a solitary nodule on the left calf and subsequently developed into multiple nonpainful, nonpruritic, erythematous plaques of varying sizes with violaceous coloration and overlying necrotic eschar, occupying the entire anterior aspect of the left lower leg and left popliteal fossa (Figure). The patient denied any trauma or associated symptoms but had a history of Sweet syndrome that manifested as lesions on the arms and legs for which he took 6 mg of prednisone daily to prevent recurrence.
Histologic examination revealed nodular and diffuse chronic granulomatous and acute inflammatory infiltrate. Stains for bacteria, fungi, and acid-fast bacilli were negative. Cultures subsequently grew Mycobacterium kansasii, and the patient was started on isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 800 mg daily, and pyridoxine 50 mg daily. Chest radiograph and computed tomography showed no evidence of pulmonary disease and 2 blood cultures were negative for growth. The patient subsequently developed weakness that he attributed to the antibiotics and he decided to discontinue all treatment.
At 11 months the lesions showed no change; however, magnetic resonance imaging of the leg was suggestive of osteomyelitis. The patient was started on clarithromycin 500 mg twice daily with planned addition of isoniazid. The patient refused any additional antibiotics but agreed to continue the clarithromycin treatment for one year. He was subsequently lost to dermatology follow-up.
Nontuberculous mycobacteria (NTM) infection is a rare sequela of hematologic malignancy, seen in only 1.5% of patients.1 The NTM most commonly seen in hematologic malignancy are generally the fast-growing species Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, or Mycobacterium phlei, rather than slow growers Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium marinum, and Mycobacterium xenopi. Mycobacterium kansasii infection, such as seen in our patient, accounts for only 18% of cases.1 This case is further distinguished by the fact that cutaneous infections with NTM also are generally caused by fast-growing organisms such as Mycobacterium abscessus-chelonae complex and M fortuitum, rather than the slow-growing M kansasii.2,3
Mycobacterium kansasii is a slow-growing, acid-fast bacillus found in local water reservoirs, swimming pools, sewers, and tap water where it can live for up to 12 months.2,4,5Mycobacterium kansasii is traditionally considered the most virulent NTM.3,6 It most frequently causes a pulmonary infection in the immunosuppressed and patients with chronic bronchopulmonary disease.6,7 Disseminated disease is less common and is primarily seen in immunocompromised patients, particularly in human immunodeficiency virus–positive patients, transplant recipients, and patients with hematologic malignancies.1,6,8 Disseminated disease rarely has been seen in patients with normal immune function.2,3
Cutaneous M kansasii infection has only infrequently been described. Most patients tend to be middle-aged men, with a median affected age of 43 years.2,7,9,10 One review of cutaneous cases found that 72% had some form of altered immunity and more than 50% of those patients were on chronic steroids. The same review found that of the cases of cutaneous M kansasii in patients with altered immunity, only 30% had disseminated disease.10 Our patient was immunocompromised but showed no evidence of disseminated disease, as displayed by negative chest radiograph and computed tomography, lack of pulmonary symptoms, and negative blood cultures. As a 68-year-old man with myelodysplastic syndrome on chronic steroids with no disseminated disease, our patient fits well into these demographics, aside from his advanced age.
Cutaneous M kansasii infection has a variable presentation, manifesting as solitary lesions, nodules, pustules, seromas, erythematous plaques, verrucous lesions, ulcers, and as cellulitis.5,7,9-12 Immune competent individuals were more likely to present with raised lesions or ulcers, whereas immune compromised individuals had a more diffuse presentation of cellulitis or seromas with variable histology.6,8 Our patient, though immune compromised, presented with multiple erythematous plaques with eschars, which further endorses having a high clinical suspicion, as the lesions display marked heterogeneity.
Treatment of M kansasii infection consists of at least 1 year of isoniazid 300 mg daily, rifampin 600 mg daily, and ethambutol 15 mg/kg daily, with possible addition of streptomycin.8,13Mycobacterium kansasii infection necessitates multidrug treatment due to the broad range of resistance exhibited by different isolated strains.14
Response to treatment in cutaneous M kansasii greatly depends on the underlying disease state of the individual. Generally, immune competent individuals do very well, while the course in immune compromised patients depends on their degree of illness. Patients with disseminated disease generally do poorly.4,7,10 In at least one case of cutaneous disease, dissemination developed as a sequela, thus suggesting treatment is needed even in stable lesions.2 Dissemination was a concern with our patient given the magnetic resonance imaging findings suggestive of osteomyelitis. Although treatment generally consists of triple therapy with isoniazid, rifampin, and ethambutol, given the high frequency of adverse effects due to isoniazid or rifampin, as was seen in our patient, the drug regimen might have to be altered to suit the patient. Susceptibility testing is desirable to aid in tailoring the treatment.8,13 Furthermore, as the duration of treatment is at least 1 year, diligent follow-up must be maintained to avoid incomplete treatment.
The unpredictable presentation of cutaneous M kansasii infection coupled with the variable history necessitates a high level of clinical suspicion and a low threshold for culturing lesions. Furthermore, the long duration and complexity of the antibiotic regimen and the high incidence of adverse reactions demands strict follow-up, especially given the risk for progression to disseminated disease.
To the Editor:
A 68-year-old man with a history of myelodysplastic syndrome and recurrent Sweet syndrome presented with left leg lesions of 3 months’ duration. The lesions originated as a solitary nodule on the left calf and subsequently developed into multiple nonpainful, nonpruritic, erythematous plaques of varying sizes with violaceous coloration and overlying necrotic eschar, occupying the entire anterior aspect of the left lower leg and left popliteal fossa (Figure). The patient denied any trauma or associated symptoms but had a history of Sweet syndrome that manifested as lesions on the arms and legs for which he took 6 mg of prednisone daily to prevent recurrence.
Histologic examination revealed nodular and diffuse chronic granulomatous and acute inflammatory infiltrate. Stains for bacteria, fungi, and acid-fast bacilli were negative. Cultures subsequently grew Mycobacterium kansasii, and the patient was started on isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 800 mg daily, and pyridoxine 50 mg daily. Chest radiograph and computed tomography showed no evidence of pulmonary disease and 2 blood cultures were negative for growth. The patient subsequently developed weakness that he attributed to the antibiotics and he decided to discontinue all treatment.
At 11 months the lesions showed no change; however, magnetic resonance imaging of the leg was suggestive of osteomyelitis. The patient was started on clarithromycin 500 mg twice daily with planned addition of isoniazid. The patient refused any additional antibiotics but agreed to continue the clarithromycin treatment for one year. He was subsequently lost to dermatology follow-up.
Nontuberculous mycobacteria (NTM) infection is a rare sequela of hematologic malignancy, seen in only 1.5% of patients.1 The NTM most commonly seen in hematologic malignancy are generally the fast-growing species Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, or Mycobacterium phlei, rather than slow growers Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium marinum, and Mycobacterium xenopi. Mycobacterium kansasii infection, such as seen in our patient, accounts for only 18% of cases.1 This case is further distinguished by the fact that cutaneous infections with NTM also are generally caused by fast-growing organisms such as Mycobacterium abscessus-chelonae complex and M fortuitum, rather than the slow-growing M kansasii.2,3
Mycobacterium kansasii is a slow-growing, acid-fast bacillus found in local water reservoirs, swimming pools, sewers, and tap water where it can live for up to 12 months.2,4,5Mycobacterium kansasii is traditionally considered the most virulent NTM.3,6 It most frequently causes a pulmonary infection in the immunosuppressed and patients with chronic bronchopulmonary disease.6,7 Disseminated disease is less common and is primarily seen in immunocompromised patients, particularly in human immunodeficiency virus–positive patients, transplant recipients, and patients with hematologic malignancies.1,6,8 Disseminated disease rarely has been seen in patients with normal immune function.2,3
Cutaneous M kansasii infection has only infrequently been described. Most patients tend to be middle-aged men, with a median affected age of 43 years.2,7,9,10 One review of cutaneous cases found that 72% had some form of altered immunity and more than 50% of those patients were on chronic steroids. The same review found that of the cases of cutaneous M kansasii in patients with altered immunity, only 30% had disseminated disease.10 Our patient was immunocompromised but showed no evidence of disseminated disease, as displayed by negative chest radiograph and computed tomography, lack of pulmonary symptoms, and negative blood cultures. As a 68-year-old man with myelodysplastic syndrome on chronic steroids with no disseminated disease, our patient fits well into these demographics, aside from his advanced age.
Cutaneous M kansasii infection has a variable presentation, manifesting as solitary lesions, nodules, pustules, seromas, erythematous plaques, verrucous lesions, ulcers, and as cellulitis.5,7,9-12 Immune competent individuals were more likely to present with raised lesions or ulcers, whereas immune compromised individuals had a more diffuse presentation of cellulitis or seromas with variable histology.6,8 Our patient, though immune compromised, presented with multiple erythematous plaques with eschars, which further endorses having a high clinical suspicion, as the lesions display marked heterogeneity.
Treatment of M kansasii infection consists of at least 1 year of isoniazid 300 mg daily, rifampin 600 mg daily, and ethambutol 15 mg/kg daily, with possible addition of streptomycin.8,13Mycobacterium kansasii infection necessitates multidrug treatment due to the broad range of resistance exhibited by different isolated strains.14
Response to treatment in cutaneous M kansasii greatly depends on the underlying disease state of the individual. Generally, immune competent individuals do very well, while the course in immune compromised patients depends on their degree of illness. Patients with disseminated disease generally do poorly.4,7,10 In at least one case of cutaneous disease, dissemination developed as a sequela, thus suggesting treatment is needed even in stable lesions.2 Dissemination was a concern with our patient given the magnetic resonance imaging findings suggestive of osteomyelitis. Although treatment generally consists of triple therapy with isoniazid, rifampin, and ethambutol, given the high frequency of adverse effects due to isoniazid or rifampin, as was seen in our patient, the drug regimen might have to be altered to suit the patient. Susceptibility testing is desirable to aid in tailoring the treatment.8,13 Furthermore, as the duration of treatment is at least 1 year, diligent follow-up must be maintained to avoid incomplete treatment.
The unpredictable presentation of cutaneous M kansasii infection coupled with the variable history necessitates a high level of clinical suspicion and a low threshold for culturing lesions. Furthermore, the long duration and complexity of the antibiotic regimen and the high incidence of adverse reactions demands strict follow-up, especially given the risk for progression to disseminated disease.
1. Chen CY, Sheng WH, Lai CC. Mycobacterial infections in adult patients with hematological malignancy. Eur J Clin Microbiol Infect Dis. 2012;31:1059-1066.
2. Han SH, Kim KM, Chin BS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: a case report and comprehensive review of the literature. J Korean Med Sci. 2010;25:304-308.
3. Razavi B, Cleveland MG. Cutaneous infection due to Mycobacterium kansasii. Diagn Microbiol Infect Dis. 2000;38:173-175.
4. Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995;13:207-222.
5. Nomura Y, Nishie W, Shibaki A, et al. Disseminated cutaneous Mycobacterium kansasii infection in a patient infected with the human immunodeficiency virus. Clin Exp Dermatol. 2009;34:625-626.
6. Bloch KC, Zwerling L, Pletcher MJ, et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129:698-704.
7. Breathnach A, Levell N, Munro C, et al. Cutaneous Mycobacterium kansasii infection: case report and review. Clin Infect Dis. 1995;20:812-817.
8. Pintado V, Gómez-Mampaso E, Martín-Dávila P. Mycobacterium kansasii infection in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1999;18:582-586.
9. Stengem J, Grande KK, Hsu S. Localized primary cutaneous Mycobacterium kansasii infection in an immunocompromised patient. J Am Acad Dermatol. 1999;41(5, pt 2):854-856.
10. Czelusta A, Moore AY. Cutaneous Mycobacterium kansasii infection in a patient with systemic lupus erythematosus: case report and review. J Am Acad Dermatol. 1999;40(2, pt 2):359-363.
11. Curcó N, Pagerols X, Gómez L, et al. Mycobacterium kansasii infection limited to the skin in a patient with AIDS. Br J Dermatol. 1996;135:324-326.
12. Hanke CW, Temofeew RK, Slama SL. Mycobacterium kansasii infection with multiple cutaneous lesions. J Am Acad Dermatol. 1987;16(5, pt 2):1122-1128.
13. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculousmycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
14. da Silva Telles MA, Chimara E, Ferrazoli L, Riley LW. Mycobacterium kansasii: antibiotic susceptibility and PCR-restriction analysis of clinical isolates. J Med Microbiol. 2005;54:975-979.
1. Chen CY, Sheng WH, Lai CC. Mycobacterial infections in adult patients with hematological malignancy. Eur J Clin Microbiol Infect Dis. 2012;31:1059-1066.
2. Han SH, Kim KM, Chin BS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: a case report and comprehensive review of the literature. J Korean Med Sci. 2010;25:304-308.
3. Razavi B, Cleveland MG. Cutaneous infection due to Mycobacterium kansasii. Diagn Microbiol Infect Dis. 2000;38:173-175.
4. Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995;13:207-222.
5. Nomura Y, Nishie W, Shibaki A, et al. Disseminated cutaneous Mycobacterium kansasii infection in a patient infected with the human immunodeficiency virus. Clin Exp Dermatol. 2009;34:625-626.
6. Bloch KC, Zwerling L, Pletcher MJ, et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129:698-704.
7. Breathnach A, Levell N, Munro C, et al. Cutaneous Mycobacterium kansasii infection: case report and review. Clin Infect Dis. 1995;20:812-817.
8. Pintado V, Gómez-Mampaso E, Martín-Dávila P. Mycobacterium kansasii infection in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1999;18:582-586.
9. Stengem J, Grande KK, Hsu S. Localized primary cutaneous Mycobacterium kansasii infection in an immunocompromised patient. J Am Acad Dermatol. 1999;41(5, pt 2):854-856.
10. Czelusta A, Moore AY. Cutaneous Mycobacterium kansasii infection in a patient with systemic lupus erythematosus: case report and review. J Am Acad Dermatol. 1999;40(2, pt 2):359-363.
11. Curcó N, Pagerols X, Gómez L, et al. Mycobacterium kansasii infection limited to the skin in a patient with AIDS. Br J Dermatol. 1996;135:324-326.
12. Hanke CW, Temofeew RK, Slama SL. Mycobacterium kansasii infection with multiple cutaneous lesions. J Am Acad Dermatol. 1987;16(5, pt 2):1122-1128.
13. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculousmycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
14. da Silva Telles MA, Chimara E, Ferrazoli L, Riley LW. Mycobacterium kansasii: antibiotic susceptibility and PCR-restriction analysis of clinical isolates. J Med Microbiol. 2005;54:975-979.