User login
Letter to the Editor
We complement Dr. Siddiqui et al. on their article published in the Journal of Hospital Medicine.[1] Analysis of the role of new physical environments on care and patient satisfaction is sparse and desperately needed for this high‐cost resource in healthcare delivery. A review of the original article led us to several observations/suggestions.
The focus of the study is on perceived patient satisfaction based on 2 survey tools. As noted by the authors, there are multiple factors that must be considered related to facilitiestheir potential contribution to patient infections and falls, the ability to accommodate new technology and procedures, and the shifting practice models such as the shift from inpatient to ambulatory care. Patient‐focused care concepts are only 1 element in the design challenge and costs.
The reputation of Johns Hopkins as a major tertiary referral center is well known internationally, and it would seem reasonable to assume that many of the patients were selected or referred to the institution based on its physicians. It does not seem unreasonable to assume that facilities would play a secondary role, and that perceived satisfaction would be high regardless of the physical environment. As noted by the authors, the transferability of this finding to community hospitals and other settings is unknown.
Patient satisfaction is an important element in design, but staff satisfaction and efficiency are also significant elements in maintaining a high‐quality healthcare system. We need tools to assess the relationship between staff retention, stress levels, and medical errors and the physical environment.
The focus of the article is on the transferability of perceived satisfaction with environment to satisfaction with physician care. Previously published studies have shown a correlation with environments and views from patients rooms with reduced patient stress levels and shorter lengths of stay. Physical space should not be disregarded as a component of effective patient care.[2]
We are committed to seeking designs that are effective, safe, and adaptable to long‐term needs. We support additional research in this and other related design issues. We hope that the improvements in patient and family environments labeled as patient focused will continue to evolve to respond to real healthcare needs. It would be unfortunate if progress is diverted by misinterpretation of the articles findings.
- , , , , . Changes in patient satisfaction related to hospital renovation: experience with a new clinical building. J Hosp Med. 2015;10(3):165–171.
- , , , et al. A review of the research literature on evidence‐based healthcare design. HERD. 2008;1(3):61–125.
We complement Dr. Siddiqui et al. on their article published in the Journal of Hospital Medicine.[1] Analysis of the role of new physical environments on care and patient satisfaction is sparse and desperately needed for this high‐cost resource in healthcare delivery. A review of the original article led us to several observations/suggestions.
The focus of the study is on perceived patient satisfaction based on 2 survey tools. As noted by the authors, there are multiple factors that must be considered related to facilitiestheir potential contribution to patient infections and falls, the ability to accommodate new technology and procedures, and the shifting practice models such as the shift from inpatient to ambulatory care. Patient‐focused care concepts are only 1 element in the design challenge and costs.
The reputation of Johns Hopkins as a major tertiary referral center is well known internationally, and it would seem reasonable to assume that many of the patients were selected or referred to the institution based on its physicians. It does not seem unreasonable to assume that facilities would play a secondary role, and that perceived satisfaction would be high regardless of the physical environment. As noted by the authors, the transferability of this finding to community hospitals and other settings is unknown.
Patient satisfaction is an important element in design, but staff satisfaction and efficiency are also significant elements in maintaining a high‐quality healthcare system. We need tools to assess the relationship between staff retention, stress levels, and medical errors and the physical environment.
The focus of the article is on the transferability of perceived satisfaction with environment to satisfaction with physician care. Previously published studies have shown a correlation with environments and views from patients rooms with reduced patient stress levels and shorter lengths of stay. Physical space should not be disregarded as a component of effective patient care.[2]
We are committed to seeking designs that are effective, safe, and adaptable to long‐term needs. We support additional research in this and other related design issues. We hope that the improvements in patient and family environments labeled as patient focused will continue to evolve to respond to real healthcare needs. It would be unfortunate if progress is diverted by misinterpretation of the articles findings.
We complement Dr. Siddiqui et al. on their article published in the Journal of Hospital Medicine.[1] Analysis of the role of new physical environments on care and patient satisfaction is sparse and desperately needed for this high‐cost resource in healthcare delivery. A review of the original article led us to several observations/suggestions.
The focus of the study is on perceived patient satisfaction based on 2 survey tools. As noted by the authors, there are multiple factors that must be considered related to facilitiestheir potential contribution to patient infections and falls, the ability to accommodate new technology and procedures, and the shifting practice models such as the shift from inpatient to ambulatory care. Patient‐focused care concepts are only 1 element in the design challenge and costs.
The reputation of Johns Hopkins as a major tertiary referral center is well known internationally, and it would seem reasonable to assume that many of the patients were selected or referred to the institution based on its physicians. It does not seem unreasonable to assume that facilities would play a secondary role, and that perceived satisfaction would be high regardless of the physical environment. As noted by the authors, the transferability of this finding to community hospitals and other settings is unknown.
Patient satisfaction is an important element in design, but staff satisfaction and efficiency are also significant elements in maintaining a high‐quality healthcare system. We need tools to assess the relationship between staff retention, stress levels, and medical errors and the physical environment.
The focus of the article is on the transferability of perceived satisfaction with environment to satisfaction with physician care. Previously published studies have shown a correlation with environments and views from patients rooms with reduced patient stress levels and shorter lengths of stay. Physical space should not be disregarded as a component of effective patient care.[2]
We are committed to seeking designs that are effective, safe, and adaptable to long‐term needs. We support additional research in this and other related design issues. We hope that the improvements in patient and family environments labeled as patient focused will continue to evolve to respond to real healthcare needs. It would be unfortunate if progress is diverted by misinterpretation of the articles findings.
- , , , , . Changes in patient satisfaction related to hospital renovation: experience with a new clinical building. J Hosp Med. 2015;10(3):165–171.
- , , , et al. A review of the research literature on evidence‐based healthcare design. HERD. 2008;1(3):61–125.
- , , , , . Changes in patient satisfaction related to hospital renovation: experience with a new clinical building. J Hosp Med. 2015;10(3):165–171.
- , , , et al. A review of the research literature on evidence‐based healthcare design. HERD. 2008;1(3):61–125.
ED Observation
Over the past 3 decades, emergency department observation units (EDOUs) have been increasingly implemented in the United States to supplement emergency department (ED) care in a time of increasing patient volume and hospital crowding. Given the limited availability of hospital resources, EDOUs provide emergency clinicians an extended period of time to evaluate and risk‐stratify patients without necessitating difficult‐to‐obtain outpatient follow‐up or a short‐stay hospitalization. Changes in Medicare and insurer reimbursement policies have incentivized the adoption of EDOUs, and now, over one‐third of EDs nationally offer an observation unit.[1]
Much of the observation‐science literature has been condition and institution specific, showing benefits with respect to cost, quality of care, safety, and patient satisfaction.[2, 3, 4, 5] Until now, there had not been a national study on the impact of EDOUs to investigate important outcome: hospital admission rates. Capp and colleagues, using the National Hospital Ambulatory Care Survey (NHAMCS), attempt to answer a very important question: Do EDs with observation units have lower hospital admission rates?[6] To do so, they first standardize admission rates to sociodemographic and clinical features of the patients, while adjusting for hospital‐level factors. Then they compare the risk‐standardized hospital admission rate between EDs with and without an observation unit as reported in the NHAMCS. The authors make creative and elegant use of this publicly available, national dataset to suggest that EDOUs do not decrease hospital admissions.
The authors appropriately identify some limitations of using such data to answer questions where nuanced, countervailing forces drive the outcome of interest. It is important to note the basic statistical premise that the inability to disprove the null hypothesis is not the same thing as proving that the null hypothesis is true. In other words, although this study was not able to detect a difference between admission rates for hospitals with EDOUs and those without, it cannot be absolutely taken to mean that there is no relationship. The authors clearly state that this study was underpowered given that the difference of ED risk‐standardized hospital admission rates was small and therefore is at risk of type II error. In addition, unmeasured confounding may hide a true association between EDOUs and admission rates. Both static and dynamic measures of ED volume, crowding, and boarding, as well as changes in case mix or acuity may drive adoption of EDOUs,[7] while simultaneously associated with risk of hospitalization. Without balance between the EDs with and without observation units, or longitudinal measures of EDs over time as they are implemented, we are left with potentially biased estimates.
It is also important to highlight that not all EDOUs are created equal.[8] EDs may admit patients to the observation unit based on prespecified conditions or include all comers at physician discretion. Once placed in observation status, patients may or may not be managed by specific protocols to provide guidance on timing, order, and scope of testing and decision making.
Finally, care in EDOUs may be provided by emergency physicians, hospitalists, or other clinicians such as advanced practice providers (eg, physician assistants, nurse practitioners), a distinction that likely impacts the ultimate patient disposition. In fact, the NHAMCS asks the question, What type of physicians make decisions for patients in this observation or clinical decision unit? Capp et al., however, did not include this variable to further stratify the data. Although we do not know whether or not inclusion of this factor may have ultimately changed the results, it could have implications for how distinctions in who manages EDOUs could affect admission rates.
Still, the negative findings of this study seem to raise a number of questions, which should spark a broader discussion on EDOUs. The current analysis provides an important first step toward a national understanding of EDOUs and their role in acute care. Future inquiries should account for variation in observation units and the hospitals in which they are housed as well as inclusion of meaningful outcomes beyond admission rates. A number of methodological approaches can be considered to achieve this; propensity score matching within observational data may provide better balance between facilities with and without EDOUs, whereas multicenter impact analyses using controlled before‐and‐after or cluster‐randomized trials should be considered the gold standard for studying observation unit implementation. Outcomes in these studies should include long‐term changes in health, aggregate healthcare utilization, overuse of resources that do not provide high‐value care, and impacts on how care and costs may be redistributed when patients receive more care in observation units.
Although cost containment is often touted as a cornerstone of EDOUs, it is critical to know how the costs are measured and who is paying. For example, when an option to place a patient in observation exists, might clinicians utilize it for some patients who do not require further evaluation and testing and could have been safely discharged?[9] This observation creep may arise because clinicians can use EDOUs, not because they should. Motivations may include delaying difficult disposition decisions, avoiding uncertainty or liability when discharging patients, limited access to outpatient follow‐up, or a desire to utilize observation status to justify the existence of EDOUs within the institution. In this way, EDOUs may, in fact, provide low‐value care at a time of soaring healthcare costs.
Perhaps even more perplexing is the question of how costs are shifted through use of EDOUs.[10, 11] Much of the literature advertising its cost savings are only from the perspective of the insurers' or hospitals' perspective,[12] with 1 study estimating a potential annual cost savings of $4.6 million for each hospital, or $3 billion nationally, associated with the implementation of observation care.[5] But are medical centers just passing costs on to patients to avoid penalties and disincentives associated with short‐stay hospitalizations? Both private insurers and the Centers for Medicare and Medicaid Services may deny payments for admissions deemed unnecessary. Further, under the Affordable Care Act, avoiding hospitalizations may mean fewer penalties when Medicare patients later require admission for certain conditions. As such, hospitals may find huge incentives and cost savings associated with observation units. However, using EDOUs to avoid the Medicare readmission penalty may backfire when less‐sick patients requiring care beyond the ED are treated and discharged from observation, leaving more medically complex and ill patients for hospitalization, a group potentially more likely to be rehospitalized within 30 days, making readmission rates appear higher.
Nonetheless, because services provided during observation status are billed as an outpatient visit, patients may be liable for a proportion of the overall visit. In contrast to inpatient stays where, in general, patients owe a single copay for most or all of services rendered, outpatient visits typically involve a la carte billing. When accounting for costs related to professional and facilities fees, medications, laboratory tests, and advanced diagnostics and procedures, patient bills may be markedly higher when they are placed in observation status. This is especially true for patients covered by Medicare, where observation stays are not covered under Part A.
Research will need to simultaneously identify best practices for how EDOUs are implemented and administered while appraising their impact on patient‐centered outcomes and true costs, from multiple perspectives, including the patient, hospital, and healthcare system. There is reason to be optimistic about EDOUs as potentially high‐value components of the acute care delivery system. However, the widespread implementation of observation units with the assumption that it is cost saving to hospitals and insurers, without high‐quality population studies to inform their impact more broadly, may undermine acceptance by patients and health‐policy experts.
Disclosure
Nothing to report.
- , , . National study of emergency department observation services. Acad Emerg Med. 2011;18(9):959–965.
- , , . Emergency department observation units: a clinical and financial benefit for hospitals. Health Care Manag Rev. 2011;36(1):28–37.
- , , , et al. Randomised controlled trial and economic evaluation of a chest pain observation unit compared with routine care. BMJ. 2004;328(7434):254.
- , , , , . Patient satisfaction with an emergency department asthma observation unit. Acad Emerg Med. 1999;6(3):178–183.
- , , , , , . Making greater use of dedicated hospital observation units for many short‐stay patients could save $3.1 billion a year. Health Aff (Millwood). 2012;31(10):2314–2323.
- , , , . The Impact of emergency department observation units on U.S. emergency department admission rates. J Hosp Med. 2015;10(11):738–742.
- , . Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med. 2008;52(2):126–136.
- , , , . A national survey of observation units in the United States. Am J Emerg Med. 2003;21(7):529–533.
- , , , . An evaluation of emergency physician selection of observation unit patients. Am J Emerg Med. 2006;24(3):271–279.
- , . Reducing patient financial liability for hospitalizations: the physician role. J Hosp Med. 2010;5(3):160–162.
- , , . Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251–1259.
- , , , , , . Revisiting the economic efficiencies of observation units. Manag Care. 2015;24(3):46–52.
Over the past 3 decades, emergency department observation units (EDOUs) have been increasingly implemented in the United States to supplement emergency department (ED) care in a time of increasing patient volume and hospital crowding. Given the limited availability of hospital resources, EDOUs provide emergency clinicians an extended period of time to evaluate and risk‐stratify patients without necessitating difficult‐to‐obtain outpatient follow‐up or a short‐stay hospitalization. Changes in Medicare and insurer reimbursement policies have incentivized the adoption of EDOUs, and now, over one‐third of EDs nationally offer an observation unit.[1]
Much of the observation‐science literature has been condition and institution specific, showing benefits with respect to cost, quality of care, safety, and patient satisfaction.[2, 3, 4, 5] Until now, there had not been a national study on the impact of EDOUs to investigate important outcome: hospital admission rates. Capp and colleagues, using the National Hospital Ambulatory Care Survey (NHAMCS), attempt to answer a very important question: Do EDs with observation units have lower hospital admission rates?[6] To do so, they first standardize admission rates to sociodemographic and clinical features of the patients, while adjusting for hospital‐level factors. Then they compare the risk‐standardized hospital admission rate between EDs with and without an observation unit as reported in the NHAMCS. The authors make creative and elegant use of this publicly available, national dataset to suggest that EDOUs do not decrease hospital admissions.
The authors appropriately identify some limitations of using such data to answer questions where nuanced, countervailing forces drive the outcome of interest. It is important to note the basic statistical premise that the inability to disprove the null hypothesis is not the same thing as proving that the null hypothesis is true. In other words, although this study was not able to detect a difference between admission rates for hospitals with EDOUs and those without, it cannot be absolutely taken to mean that there is no relationship. The authors clearly state that this study was underpowered given that the difference of ED risk‐standardized hospital admission rates was small and therefore is at risk of type II error. In addition, unmeasured confounding may hide a true association between EDOUs and admission rates. Both static and dynamic measures of ED volume, crowding, and boarding, as well as changes in case mix or acuity may drive adoption of EDOUs,[7] while simultaneously associated with risk of hospitalization. Without balance between the EDs with and without observation units, or longitudinal measures of EDs over time as they are implemented, we are left with potentially biased estimates.
It is also important to highlight that not all EDOUs are created equal.[8] EDs may admit patients to the observation unit based on prespecified conditions or include all comers at physician discretion. Once placed in observation status, patients may or may not be managed by specific protocols to provide guidance on timing, order, and scope of testing and decision making.
Finally, care in EDOUs may be provided by emergency physicians, hospitalists, or other clinicians such as advanced practice providers (eg, physician assistants, nurse practitioners), a distinction that likely impacts the ultimate patient disposition. In fact, the NHAMCS asks the question, What type of physicians make decisions for patients in this observation or clinical decision unit? Capp et al., however, did not include this variable to further stratify the data. Although we do not know whether or not inclusion of this factor may have ultimately changed the results, it could have implications for how distinctions in who manages EDOUs could affect admission rates.
Still, the negative findings of this study seem to raise a number of questions, which should spark a broader discussion on EDOUs. The current analysis provides an important first step toward a national understanding of EDOUs and their role in acute care. Future inquiries should account for variation in observation units and the hospitals in which they are housed as well as inclusion of meaningful outcomes beyond admission rates. A number of methodological approaches can be considered to achieve this; propensity score matching within observational data may provide better balance between facilities with and without EDOUs, whereas multicenter impact analyses using controlled before‐and‐after or cluster‐randomized trials should be considered the gold standard for studying observation unit implementation. Outcomes in these studies should include long‐term changes in health, aggregate healthcare utilization, overuse of resources that do not provide high‐value care, and impacts on how care and costs may be redistributed when patients receive more care in observation units.
Although cost containment is often touted as a cornerstone of EDOUs, it is critical to know how the costs are measured and who is paying. For example, when an option to place a patient in observation exists, might clinicians utilize it for some patients who do not require further evaluation and testing and could have been safely discharged?[9] This observation creep may arise because clinicians can use EDOUs, not because they should. Motivations may include delaying difficult disposition decisions, avoiding uncertainty or liability when discharging patients, limited access to outpatient follow‐up, or a desire to utilize observation status to justify the existence of EDOUs within the institution. In this way, EDOUs may, in fact, provide low‐value care at a time of soaring healthcare costs.
Perhaps even more perplexing is the question of how costs are shifted through use of EDOUs.[10, 11] Much of the literature advertising its cost savings are only from the perspective of the insurers' or hospitals' perspective,[12] with 1 study estimating a potential annual cost savings of $4.6 million for each hospital, or $3 billion nationally, associated with the implementation of observation care.[5] But are medical centers just passing costs on to patients to avoid penalties and disincentives associated with short‐stay hospitalizations? Both private insurers and the Centers for Medicare and Medicaid Services may deny payments for admissions deemed unnecessary. Further, under the Affordable Care Act, avoiding hospitalizations may mean fewer penalties when Medicare patients later require admission for certain conditions. As such, hospitals may find huge incentives and cost savings associated with observation units. However, using EDOUs to avoid the Medicare readmission penalty may backfire when less‐sick patients requiring care beyond the ED are treated and discharged from observation, leaving more medically complex and ill patients for hospitalization, a group potentially more likely to be rehospitalized within 30 days, making readmission rates appear higher.
Nonetheless, because services provided during observation status are billed as an outpatient visit, patients may be liable for a proportion of the overall visit. In contrast to inpatient stays where, in general, patients owe a single copay for most or all of services rendered, outpatient visits typically involve a la carte billing. When accounting for costs related to professional and facilities fees, medications, laboratory tests, and advanced diagnostics and procedures, patient bills may be markedly higher when they are placed in observation status. This is especially true for patients covered by Medicare, where observation stays are not covered under Part A.
Research will need to simultaneously identify best practices for how EDOUs are implemented and administered while appraising their impact on patient‐centered outcomes and true costs, from multiple perspectives, including the patient, hospital, and healthcare system. There is reason to be optimistic about EDOUs as potentially high‐value components of the acute care delivery system. However, the widespread implementation of observation units with the assumption that it is cost saving to hospitals and insurers, without high‐quality population studies to inform their impact more broadly, may undermine acceptance by patients and health‐policy experts.
Disclosure
Nothing to report.
Over the past 3 decades, emergency department observation units (EDOUs) have been increasingly implemented in the United States to supplement emergency department (ED) care in a time of increasing patient volume and hospital crowding. Given the limited availability of hospital resources, EDOUs provide emergency clinicians an extended period of time to evaluate and risk‐stratify patients without necessitating difficult‐to‐obtain outpatient follow‐up or a short‐stay hospitalization. Changes in Medicare and insurer reimbursement policies have incentivized the adoption of EDOUs, and now, over one‐third of EDs nationally offer an observation unit.[1]
Much of the observation‐science literature has been condition and institution specific, showing benefits with respect to cost, quality of care, safety, and patient satisfaction.[2, 3, 4, 5] Until now, there had not been a national study on the impact of EDOUs to investigate important outcome: hospital admission rates. Capp and colleagues, using the National Hospital Ambulatory Care Survey (NHAMCS), attempt to answer a very important question: Do EDs with observation units have lower hospital admission rates?[6] To do so, they first standardize admission rates to sociodemographic and clinical features of the patients, while adjusting for hospital‐level factors. Then they compare the risk‐standardized hospital admission rate between EDs with and without an observation unit as reported in the NHAMCS. The authors make creative and elegant use of this publicly available, national dataset to suggest that EDOUs do not decrease hospital admissions.
The authors appropriately identify some limitations of using such data to answer questions where nuanced, countervailing forces drive the outcome of interest. It is important to note the basic statistical premise that the inability to disprove the null hypothesis is not the same thing as proving that the null hypothesis is true. In other words, although this study was not able to detect a difference between admission rates for hospitals with EDOUs and those without, it cannot be absolutely taken to mean that there is no relationship. The authors clearly state that this study was underpowered given that the difference of ED risk‐standardized hospital admission rates was small and therefore is at risk of type II error. In addition, unmeasured confounding may hide a true association between EDOUs and admission rates. Both static and dynamic measures of ED volume, crowding, and boarding, as well as changes in case mix or acuity may drive adoption of EDOUs,[7] while simultaneously associated with risk of hospitalization. Without balance between the EDs with and without observation units, or longitudinal measures of EDs over time as they are implemented, we are left with potentially biased estimates.
It is also important to highlight that not all EDOUs are created equal.[8] EDs may admit patients to the observation unit based on prespecified conditions or include all comers at physician discretion. Once placed in observation status, patients may or may not be managed by specific protocols to provide guidance on timing, order, and scope of testing and decision making.
Finally, care in EDOUs may be provided by emergency physicians, hospitalists, or other clinicians such as advanced practice providers (eg, physician assistants, nurse practitioners), a distinction that likely impacts the ultimate patient disposition. In fact, the NHAMCS asks the question, What type of physicians make decisions for patients in this observation or clinical decision unit? Capp et al., however, did not include this variable to further stratify the data. Although we do not know whether or not inclusion of this factor may have ultimately changed the results, it could have implications for how distinctions in who manages EDOUs could affect admission rates.
Still, the negative findings of this study seem to raise a number of questions, which should spark a broader discussion on EDOUs. The current analysis provides an important first step toward a national understanding of EDOUs and their role in acute care. Future inquiries should account for variation in observation units and the hospitals in which they are housed as well as inclusion of meaningful outcomes beyond admission rates. A number of methodological approaches can be considered to achieve this; propensity score matching within observational data may provide better balance between facilities with and without EDOUs, whereas multicenter impact analyses using controlled before‐and‐after or cluster‐randomized trials should be considered the gold standard for studying observation unit implementation. Outcomes in these studies should include long‐term changes in health, aggregate healthcare utilization, overuse of resources that do not provide high‐value care, and impacts on how care and costs may be redistributed when patients receive more care in observation units.
Although cost containment is often touted as a cornerstone of EDOUs, it is critical to know how the costs are measured and who is paying. For example, when an option to place a patient in observation exists, might clinicians utilize it for some patients who do not require further evaluation and testing and could have been safely discharged?[9] This observation creep may arise because clinicians can use EDOUs, not because they should. Motivations may include delaying difficult disposition decisions, avoiding uncertainty or liability when discharging patients, limited access to outpatient follow‐up, or a desire to utilize observation status to justify the existence of EDOUs within the institution. In this way, EDOUs may, in fact, provide low‐value care at a time of soaring healthcare costs.
Perhaps even more perplexing is the question of how costs are shifted through use of EDOUs.[10, 11] Much of the literature advertising its cost savings are only from the perspective of the insurers' or hospitals' perspective,[12] with 1 study estimating a potential annual cost savings of $4.6 million for each hospital, or $3 billion nationally, associated with the implementation of observation care.[5] But are medical centers just passing costs on to patients to avoid penalties and disincentives associated with short‐stay hospitalizations? Both private insurers and the Centers for Medicare and Medicaid Services may deny payments for admissions deemed unnecessary. Further, under the Affordable Care Act, avoiding hospitalizations may mean fewer penalties when Medicare patients later require admission for certain conditions. As such, hospitals may find huge incentives and cost savings associated with observation units. However, using EDOUs to avoid the Medicare readmission penalty may backfire when less‐sick patients requiring care beyond the ED are treated and discharged from observation, leaving more medically complex and ill patients for hospitalization, a group potentially more likely to be rehospitalized within 30 days, making readmission rates appear higher.
Nonetheless, because services provided during observation status are billed as an outpatient visit, patients may be liable for a proportion of the overall visit. In contrast to inpatient stays where, in general, patients owe a single copay for most or all of services rendered, outpatient visits typically involve a la carte billing. When accounting for costs related to professional and facilities fees, medications, laboratory tests, and advanced diagnostics and procedures, patient bills may be markedly higher when they are placed in observation status. This is especially true for patients covered by Medicare, where observation stays are not covered under Part A.
Research will need to simultaneously identify best practices for how EDOUs are implemented and administered while appraising their impact on patient‐centered outcomes and true costs, from multiple perspectives, including the patient, hospital, and healthcare system. There is reason to be optimistic about EDOUs as potentially high‐value components of the acute care delivery system. However, the widespread implementation of observation units with the assumption that it is cost saving to hospitals and insurers, without high‐quality population studies to inform their impact more broadly, may undermine acceptance by patients and health‐policy experts.
Disclosure
Nothing to report.
- , , . National study of emergency department observation services. Acad Emerg Med. 2011;18(9):959–965.
- , , . Emergency department observation units: a clinical and financial benefit for hospitals. Health Care Manag Rev. 2011;36(1):28–37.
- , , , et al. Randomised controlled trial and economic evaluation of a chest pain observation unit compared with routine care. BMJ. 2004;328(7434):254.
- , , , , . Patient satisfaction with an emergency department asthma observation unit. Acad Emerg Med. 1999;6(3):178–183.
- , , , , , . Making greater use of dedicated hospital observation units for many short‐stay patients could save $3.1 billion a year. Health Aff (Millwood). 2012;31(10):2314–2323.
- , , , . The Impact of emergency department observation units on U.S. emergency department admission rates. J Hosp Med. 2015;10(11):738–742.
- , . Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med. 2008;52(2):126–136.
- , , , . A national survey of observation units in the United States. Am J Emerg Med. 2003;21(7):529–533.
- , , , . An evaluation of emergency physician selection of observation unit patients. Am J Emerg Med. 2006;24(3):271–279.
- , . Reducing patient financial liability for hospitalizations: the physician role. J Hosp Med. 2010;5(3):160–162.
- , , . Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251–1259.
- , , , , , . Revisiting the economic efficiencies of observation units. Manag Care. 2015;24(3):46–52.
- , , . National study of emergency department observation services. Acad Emerg Med. 2011;18(9):959–965.
- , , . Emergency department observation units: a clinical and financial benefit for hospitals. Health Care Manag Rev. 2011;36(1):28–37.
- , , , et al. Randomised controlled trial and economic evaluation of a chest pain observation unit compared with routine care. BMJ. 2004;328(7434):254.
- , , , , . Patient satisfaction with an emergency department asthma observation unit. Acad Emerg Med. 1999;6(3):178–183.
- , , , , , . Making greater use of dedicated hospital observation units for many short‐stay patients could save $3.1 billion a year. Health Aff (Millwood). 2012;31(10):2314–2323.
- , , , . The Impact of emergency department observation units on U.S. emergency department admission rates. J Hosp Med. 2015;10(11):738–742.
- , . Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med. 2008;52(2):126–136.
- , , , . A national survey of observation units in the United States. Am J Emerg Med. 2003;21(7):529–533.
- , , , . An evaluation of emergency physician selection of observation unit patients. Am J Emerg Med. 2006;24(3):271–279.
- , . Reducing patient financial liability for hospitalizations: the physician role. J Hosp Med. 2010;5(3):160–162.
- , , . Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251–1259.
- , , , , , . Revisiting the economic efficiencies of observation units. Manag Care. 2015;24(3):46–52.
ED Observation Units and Admission Rates
Today more than one‐third of emergency departments (EDs) in the United States have affiliated observation units, where patients can stay 24 to 48 hours without being admitted to the hospital.[1] Observation units experienced significant growth in the United States from 2005 to 2007, secondary to policy changes involving the Centers for Medicare and Medicaid Services (CMS), which expanded reimbursement for observation services to include any clinical condition. Furthermore, CMS implemented the Recovery Audit Contractor process, which was able to fine providers and facilities for inappropriate claims, with the principle method for charge recovery being inappropriate charges for short inpatient stays.
ED observation units (EDOUs) vary in the number of beds, but are often located adjacent to the emergency department.[2] It is estimated that EDOUs have the capacity for caring for 5% to 10% of any given ED volume.[2] Almost half of EDOUs are protocol driven, allowing these units to discharge up to 80% of all patients within 24 hours.[1, 2] Some studies have suggested that EDOUs are associated with a decrease in overall hospitalization rates, leading to cost savings.[1] However, these studies were limited by their single‐center design or simulated in nature. In addition, other studies show that EDOUs decrease inpatient admissions, length of stay, and costs related to specific clinical conditions such as chest pain, transient ischemic attack, and syncope.[3]
To further evaluate the association of observation units on ED hospital admission rates nationally, we analyzed the largest ED‐based survey, the 2010 National Hospital Ambulatory Medical Care Survey (NHAMCS), to assess the impact of observation units on hospital admissions from the ED. We hypothesized that observation units decrease overall hospital admissions from the ED.
METHODS
Study Design and Population
We performed a retrospective cross‐sectional analysis of ED visits from 2010. This study was exempt from institutional review board review by the University of Colorado and Yale University institutional review committee. The NHAMCS is an annual, national probability sample of ambulatory visits made to nonfederal, general, and short‐stay hospitals conducted by the Centers for Disease Control and Prevention (CDC), National Center for Health Statistics. The multistaged sample design was previously described elsewhere.[4] The 2010 NHAMCS dataset included 350 participating hospitals (unweighted sampling rate of 90%) and a total of 34,936 patient visits.[4]
Exclusions
We excluded patients who were less than 18 years old (n = 8015; 23%); left without being seen, left before examination completion, or left against medical advice (n = 813; 2%); transferred to another institution (n = 626; 1.7%); died on arrival or died in the ED (n = 60; 0.2%); and with missing data on discharge disposition (n = 100; 0.3%). Finally, we excluded hospitals with fewer than 30 visits per year (n = 307; 0.9%) to comply with reliable relative standard errors, as recommended by the CDC; after all of these exclusions there were 325 hospitals. Finally, we excluded hospitals with missing information on EDOUs (n = 783, 2.2%); our dataset at this point included 315 hospitals.
Outcomes
The primary outcome was hospital admission, either from the ED or admitted to an observation unit with subsequent hospital admission, defined as the ED risk‐standardized hospital admission rate (ED RSHAR).[5] This methodology allows for risk adjustment of case mix (ie, disease severity) for each hospital's ED admission rates and has been previously described in the evaluation of varying ED hospital admission rates using the same dataset.[5] To evaluate which hospitals had observation units, we used the following hospital survey question: Does your ED have an observation or clinical decision unit?
Identification of Variables
ED hospitalization rates were risk standardized for each hospital to account for each hospital's case mix and hospital factors such as socioeconomic status, clinical severity, and hospital characteristics. This methodology and dataset use have been previously described in detail.[5]
To account for common chief complaints leading to hospitalization and case‐mix distribution of these complaints among different hospitals, we analyzed all chief complaints and their relationship to hospital admission. We first identified those associated with an admission rate that exceeded 30% and was present in 1% or more of patient visits. The study team of researchers and clinicians determined the aforementioned cutoffs as clinically meaningful. Eight chief complaints met both criteria: chest pain and related symptoms, shortness of breath, other symptoms/probably related to psychological, general weakness, labored or difficulty breathing, fainting (syncope), unconscious arrival, and other symptoms referable to the nervous system. Chronic diseases, such as congestive heart failure, diabetes mellitus, renal disease on dialysis, and human immunodeficiency virus, were also included in the model.
Hospital factors included metropolitan status, geographic region of the country (limited to Northeast, Midwest, South, and West), teaching status, and urban or rural status.[6] We derived a new variable based on a previous study, teaching status, by combining nonprivate hospital status plus having at least 1 ED visit be evaluated by a resident.
Statistical Analyses
We used SAS version 9.2 (SAS Institute, Cary, NC) for all statistical analyses. Frequencies of all variables in the model were calculated to assess the distribution of data and quantify missing data. We did not want to have variables in the model with high collinearity. To investigate collinearity between independent variables, we calculated Spearman correlation coefficients; high collinearity was defined as r > 0.6. No variables included in the model had high collinearity.
To investigate the association of the candidate variables with hospitalization, we used survey logistic regression. Although some variables did not show an association with hospitalization, we felt they were clinically relevant and did not remove them from the model. Hierarchical logistic regression modeling (explained below) was used to calculate ED RSHAR based on the aforementioned selected variables associated with hospital admission.
Hierarchical logistic regression models (HLRM) were used to estimate RSHAR for each hospital. This approach reflects the assumption that a hospital‐specific component exists, and that it will affect the outcomes of patients at a particular institution. This method takes into consideration the hierarchical structure of the data to account for patient clustering within hospitals, and has been used by the CMS to publicly report hospital risk‐standardized rates of mortality and readmission for acute myocardial infarction, heart failure, and pneumonia.
We used a similar methodology as previously published.[5] In summary, the hospital RSHAR was calculated as a ratio of the number of predicted hospital admissions in the hospital to the number of expected hospital admissions in the hospital. This ratio is then multiplied by the national unadjusted rate of hospital admissions. We calculated the C statistic of the HLRM model to assess for overall adequacy of risk prediction. To analyze the association between ED RSHAR and EDOUs, we used analysis of variance, where the dependent variable was ED RSHAR and independent variable of interest was presence of EDOUs.
RESULTS
There were 24,232 ED visits from 315 hospitals in the United States in our study. Of these, 82 (20.6%) hospitals had an observation unit physically separate from the ED. Hospitals with and without observation units did not have different hospital patient level characteristics. There was no association between hospital ownership, teaching status, region location, urban or rural location, and hospitals with observation units when compared with hospitals without observation units (Table 1).
| Hospitals With Observation Units, W% (N = 82) | Hospitals Without Observation Units, W% (N = 233) | P Value | |
|---|---|---|---|
| |||
| Region of country | 0.54 | ||
| Northeast | 10.01 | 15.46 | |
| Midwest | 32.06 | 28.35 | |
| South | 41.84 | 36.33 | |
| West | 16.08 | 19.85 | |
| Ownership of hospitals | 0.4 | ||
| Voluntary, nonprofit | 77.28 | 72.35 | |
| Government, nonfederal | 18.78 | 16.11 | |
| Private | 3.94 | 11.55 | |
| Urban or rural location | 0.43 | ||
| Urban | 68.28 | 60.19 | |
| Rural | 31.72 | 39.81 | |
| Teaching hospital status | 0.56 | ||
| Teaching hospital | 63.22 | 68.28 | |
| Nonteaching hospital | 36.78 | 31.71 | |
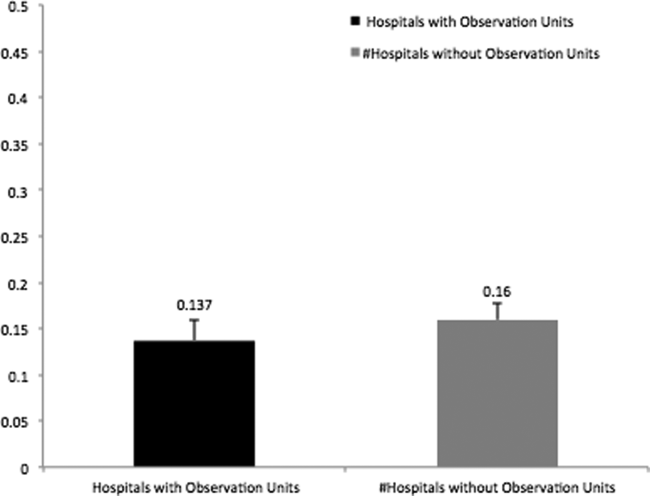
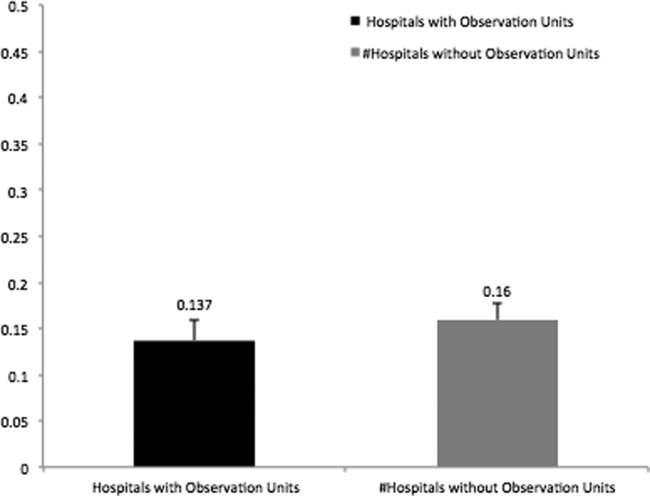
In addition, there was no association between patient characteristics at the ED visit level in hospitals with observation units when compared with patient characteristics at the ED visit level in hospitals without observation units (Table 2). The average ED risk‐standardized hospital admission rate for hospitals with observation units was 13.7% (95% confidence interval [CI]: 11.3 to 16.0) compared to 16.0% (95% CI: 14.1 to 17.7) for hospitals without observation units (Figure 1). This difference of 2.3% (95% CI: 0.1 to 4.7) was not statistically significant.
| Hospitals With Observation Units, W% (N = 6,067) | Hospitals Without Observation Units, W% (N = 18,165) | P Value | |
|---|---|---|---|
| |||
| Sex, female | 58.75 | 58.35 | 0.96 |
| Age, y | 45.17 | 46.08 | 0.32 |
| Race | 0.75 | ||
| Non‐Hispanic white | 63.54 | 66.41 | |
| Non‐Hispanic black | 23.67 | 18.77 | |
| Hispanic | 9.77 | 12.47 | |
| Other | 3.02 | 2.35 | |
| Source of payment | 0.87 | ||
| Private | 21.90 | 21.46 | |
| Medicare | 32.73 | 30.55 | |
| Medicaid | 22.15 | 23.23 | |
| Uninsured | 18.61 | 20.25 | |
| Unknown/missing | 4.61 | 4.51 | |
| Poverty level | 0.50 | ||
| 5% | 13.87 | 15.31 | |
| 5%9.9% | 32.57 | 23.38 | |
| 10%19.9% | 29.81 | 36.29 | |
| >20% | 20.32 | 20.18 | |
| Missing | 3.44 | 4.83 | |
| Arrival by ambulance | 0.06 | ||
| Yes | 20.01 | 18.61 | |
| No | 76.12 | 76.34 | |
| Unknown | 3.87 | 5.05 | |
| Severity of illness | 0.58 | ||
| Emergent | 16.58 | 16.62 | |
| Nonemergent | 44.09 | 43.85 | |
| Indeterminate | 1.18 | 1.17 | |
| Mental health, alcohol, unclassified | 38.15 | 38.37 | |
| Vital signs | |||
| Temperature | 0.91 | ||
| 9095F | 0.31 | 0.36 | |
| 95.1100.4F | 93.94 | 93.19 | |
| 100.4107F | 1.81 | 2.11 | |
| Missing | 3.94 | 4.35 | |
| Pulse | 0.60 | ||
| 1059 bpm | 3.39 | 3.93 | |
| 60100 bpm | 72.86 | 75.94 | |
| >101 bpm | 19.60 | 21.37 | |
| Missing | 4.16 | 7.67 | |
| Systolic blood pressure | 0.92 | ||
| 5090 mm Hg | 0.90 | 1.02 | |
| 91160 mm Hg | 85.49 | 84.03 | |
| 161260 mm Hg | 11.90 | 12.94 | |
| Missing | 1.71 | 2.01 | |
| Respiratory rate | 0.68 | ||
| 411 breaths/min | 0.24 | 0.19 | |
| 1220 breaths/min | 87.88 | 86.40 | |
| 2160 breaths/min | 8.90 | 10.09 | |
| Missing | 2.98 | 3.32 | |
| Chief complaint associated with hospitalization | |||
| Chest pain and related symptoms | 7.37 | 6.40 | 0.48 |
| Shortness of breath | 3.24 | 3.19 | 0.80 |
| Other symptoms/probably related to psychological | 1.28 | 0.97 | 0.19 |
| General weakness | 1.19 | 1.14 | 0.26 |
| Labored or difficult breathing | 0.56 | 0.88 | 0.93 |
| Fainting (syncope) | 0.44 | 0.42 | 0.09 |
| Unconscious on arrival | 0.35 | 0.38 | 0.17 |
| Other symptoms referable to the nervous system | 0.38 | 0.35 | 0.81 |
| Chronic diseases | |||
| Congestive heart failure | 4.13 | 4.05 | 0.05 |
| Cerebrovascular disease | 4.03 | 3.33 | 0.04 |
| Diabetes | 11.15 | 11.44 | 0.69 |
| HIV | 0.51 | 0.44 | 0.99 |
| On dialysis | 1.14 | 0.96 | 0.25 |
DISCUSSION
In this national study of hospital admissions from the ED, we did not find that hospitals with observation units had a statistically significant lower ED risk‐standardized admission rate when compared with hospitals that did not have observation units. However, the difference of ED risk‐standardized hospital admission rates between hospitals with observation units and those without observation units was very small, and we were likely underpowered to detect a statistically significant difference.
Recently, EDOUs have received much attention, in part because of increases in their numbers and frequency of use.[7] Prior studies, which did not report admission rates that were risk standardized, have also demonstrated no difference in the admission rates among hospitals with and without observation units.[6, 8] Although this result seems counterintuitive, several possible explanations exist.
One reason that there may not be a relation between the rate of inpatient admission and the presence of an observation unit is that the introduction of an EDOU appears to change physician behavior. When the option to admit to an observation unit is present, ED physicians are 2 times more likely to disposition patients to observation status without a statistically significant change in the rate of inpatient admission.[6] Studies have demonstrated that after the introduction of an observation unit, ED physicians tend to overutilize observation among patients who previously would have been discharged, while continuing to admit patients as inpatients who meet observation criteria, which could result in an increase in cost for payers and patients.[7, 9]
Observation units that are protocol driven have been associated with the best patient outcomes including shorter length of stay, lower likelihood of subsequent inpatient admission, and decreased cost.[10] Furthermore, studies evaluating EDOUs suggest increased patient satisfaction and improved patient safety, especially for protocol‐driven EDOUs.[2] However, currently, only half of dedicated observation units are protocol driven. It is also possible that the ED inpatient admission rate does not capture the full impact of an observation unit on care delivery and quality. Observation units are more likely to be present in EDs with a higher overall patient census, longer patient lengths of stay, and higher rates of ambulance diversion.[6, 8] Unfortunately, NHAMCS does not distinguish protocol‐driven versus nonprotocol‐driven observation units. From a policy standpoint, as EDOUs continue to emerge, there is an opportunity to standardize how EDOUs function by using best practices.
This study should be evaluated in the context of limitations such as heterogeneity in the management of EDOUs, limited hospital factor variables that may influence hospital admissions, and small sample size associated with each hospital. Because we were not able to determine which EDs used protocol‐driven observation units, we were not able to determine the impact of having a protocol‐driven observation unit on inpatient hospital admission rates. Additionally, the study may suffer from a selection bias, as EDs with observation units have been shown to have higher patient volume, longer patient lengths of stay, and greater rates of ED diversion. Despite the small sample size, our risk‐standardized model accounted for case mix and hospital factors associated with hospital admission rates and had a high C statistic value, which indicates that the predicted probability of being admitted from the ED highly correlates with the actual outcome of being admitted from the ED. We were unable to track hospitals longitudinally to determine if a hospital's high volume is associated with the creation of EDOUs as a means to offset its demand. However, in our analysis, we did control for overall patient volume when calculating the RHSAR. Finally, we were not able to limit the dataset to observation unit admission conditions because of the limited number of visits provided per hospital by NHAMCS. We conducted an analysis using 80% power and a P value of 0.05 to determine the sample size needed to have statistically significant results. We would require 920 hospitals to have statistically significant results, which suggests we were underpowered to detect a statistically significant difference.
In this preliminary study, we did not find an association between the presence of EDOUs and ED hospital admissions. Our study was limited by an inability to analyze administrative differences and to adjust for certain hospital factors that are likely to influence inpatient admissions via the ED. Nonetheless, our findings suggest that EDOUs merit further evaluation of their potential cost savings and the quality of the care they provide. An evaluation of ED observation departmental management is also needed to assess differences in care at observation units managed by emergency physicians versus nonemergency physicians.
Acknowledgments
Disclosures: R.C., B.S., and C.G. conceived the study. R.C. conducted the statistical analysis and was supervised by B.S. and C.G. All authors analyzed the results and interpreted findings. R.C. and D.B. drafted the manuscript, and all authors contributed substantially to its revision. All authors listed have contributed sufficiently to the project to be included as authors, and all those who are qualified to be authors are listed in the author byline. This work was previously presented at the 2013 Society for Academic Emergency Medicine Annual Meeting, Dallas, Texas. Dr. Capp is funded by a translational K award: KL2 TR001080. Dr. Gross reports grants from Johnson & Johnson, Medtronic Inc., and 21st Century Oncology during the conduct of this study. In addition, he received payment from Fair Health Inc. and ASTRO outside the submitted work. Dr. Sun receives National Institutes of Health funding. No conflicts of interest, financial or other, exist. This applies to all authors.
- , , . National study of emergency department observation services. Acad Emerg Med. 2011;18(9):959–965.
- , , . Emergency department observation units: a clinical and financial benefit for hospitals. Health Care Manage Rev 2011;36(1):28–37.
- , , , et al. Costs of an emergency department‐based accelerated diagnostic protocol vs hospitalization in patients with chest pain: a randomized controlled trial. JAMA. 1997;278(20):1670–1676.
- Centers for Disease Control and Prevention. National Hospital Ambulatory Medical Care Survey. Ambulatory health care data. Questionnaires, datasets, and related documentation. 2009. Available at: http://www.cdc.gov/nchs/ahcd/ahcd_questionnaires.htm. Accessed November 1, 2011.
- , , , et al. Hospital variation in risk‐standardized hospital admission rates from US EDs among adults. Am J Emerg Med. 2014;32(8):837–843.
- , , , , , . Use of observation care in US emergency departments, 2001 to 2008. PloS One. 2011;6(9):e24326.
- , , , , , . Making greater use of dedicated hospital observation units for many short‐stay patients could save $3.1 billion a year. Health Aff (Millwood). 2012;31(10):2314–2323.
- , , , . A national survey of observation units in the United States. Am J Emerg Med. 2003;21(7):529–533.
- , , , . An evaluation of emergency physician selection of observation unit patients. Am J Emerg Med. 2006;24(3):271–279.
- , , , , , . Protocol‐driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149–2156.
Today more than one‐third of emergency departments (EDs) in the United States have affiliated observation units, where patients can stay 24 to 48 hours without being admitted to the hospital.[1] Observation units experienced significant growth in the United States from 2005 to 2007, secondary to policy changes involving the Centers for Medicare and Medicaid Services (CMS), which expanded reimbursement for observation services to include any clinical condition. Furthermore, CMS implemented the Recovery Audit Contractor process, which was able to fine providers and facilities for inappropriate claims, with the principle method for charge recovery being inappropriate charges for short inpatient stays.
ED observation units (EDOUs) vary in the number of beds, but are often located adjacent to the emergency department.[2] It is estimated that EDOUs have the capacity for caring for 5% to 10% of any given ED volume.[2] Almost half of EDOUs are protocol driven, allowing these units to discharge up to 80% of all patients within 24 hours.[1, 2] Some studies have suggested that EDOUs are associated with a decrease in overall hospitalization rates, leading to cost savings.[1] However, these studies were limited by their single‐center design or simulated in nature. In addition, other studies show that EDOUs decrease inpatient admissions, length of stay, and costs related to specific clinical conditions such as chest pain, transient ischemic attack, and syncope.[3]
To further evaluate the association of observation units on ED hospital admission rates nationally, we analyzed the largest ED‐based survey, the 2010 National Hospital Ambulatory Medical Care Survey (NHAMCS), to assess the impact of observation units on hospital admissions from the ED. We hypothesized that observation units decrease overall hospital admissions from the ED.
METHODS
Study Design and Population
We performed a retrospective cross‐sectional analysis of ED visits from 2010. This study was exempt from institutional review board review by the University of Colorado and Yale University institutional review committee. The NHAMCS is an annual, national probability sample of ambulatory visits made to nonfederal, general, and short‐stay hospitals conducted by the Centers for Disease Control and Prevention (CDC), National Center for Health Statistics. The multistaged sample design was previously described elsewhere.[4] The 2010 NHAMCS dataset included 350 participating hospitals (unweighted sampling rate of 90%) and a total of 34,936 patient visits.[4]
Exclusions
We excluded patients who were less than 18 years old (n = 8015; 23%); left without being seen, left before examination completion, or left against medical advice (n = 813; 2%); transferred to another institution (n = 626; 1.7%); died on arrival or died in the ED (n = 60; 0.2%); and with missing data on discharge disposition (n = 100; 0.3%). Finally, we excluded hospitals with fewer than 30 visits per year (n = 307; 0.9%) to comply with reliable relative standard errors, as recommended by the CDC; after all of these exclusions there were 325 hospitals. Finally, we excluded hospitals with missing information on EDOUs (n = 783, 2.2%); our dataset at this point included 315 hospitals.
Outcomes
The primary outcome was hospital admission, either from the ED or admitted to an observation unit with subsequent hospital admission, defined as the ED risk‐standardized hospital admission rate (ED RSHAR).[5] This methodology allows for risk adjustment of case mix (ie, disease severity) for each hospital's ED admission rates and has been previously described in the evaluation of varying ED hospital admission rates using the same dataset.[5] To evaluate which hospitals had observation units, we used the following hospital survey question: Does your ED have an observation or clinical decision unit?
Identification of Variables
ED hospitalization rates were risk standardized for each hospital to account for each hospital's case mix and hospital factors such as socioeconomic status, clinical severity, and hospital characteristics. This methodology and dataset use have been previously described in detail.[5]
To account for common chief complaints leading to hospitalization and case‐mix distribution of these complaints among different hospitals, we analyzed all chief complaints and their relationship to hospital admission. We first identified those associated with an admission rate that exceeded 30% and was present in 1% or more of patient visits. The study team of researchers and clinicians determined the aforementioned cutoffs as clinically meaningful. Eight chief complaints met both criteria: chest pain and related symptoms, shortness of breath, other symptoms/probably related to psychological, general weakness, labored or difficulty breathing, fainting (syncope), unconscious arrival, and other symptoms referable to the nervous system. Chronic diseases, such as congestive heart failure, diabetes mellitus, renal disease on dialysis, and human immunodeficiency virus, were also included in the model.
Hospital factors included metropolitan status, geographic region of the country (limited to Northeast, Midwest, South, and West), teaching status, and urban or rural status.[6] We derived a new variable based on a previous study, teaching status, by combining nonprivate hospital status plus having at least 1 ED visit be evaluated by a resident.
Statistical Analyses
We used SAS version 9.2 (SAS Institute, Cary, NC) for all statistical analyses. Frequencies of all variables in the model were calculated to assess the distribution of data and quantify missing data. We did not want to have variables in the model with high collinearity. To investigate collinearity between independent variables, we calculated Spearman correlation coefficients; high collinearity was defined as r > 0.6. No variables included in the model had high collinearity.
To investigate the association of the candidate variables with hospitalization, we used survey logistic regression. Although some variables did not show an association with hospitalization, we felt they were clinically relevant and did not remove them from the model. Hierarchical logistic regression modeling (explained below) was used to calculate ED RSHAR based on the aforementioned selected variables associated with hospital admission.
Hierarchical logistic regression models (HLRM) were used to estimate RSHAR for each hospital. This approach reflects the assumption that a hospital‐specific component exists, and that it will affect the outcomes of patients at a particular institution. This method takes into consideration the hierarchical structure of the data to account for patient clustering within hospitals, and has been used by the CMS to publicly report hospital risk‐standardized rates of mortality and readmission for acute myocardial infarction, heart failure, and pneumonia.
We used a similar methodology as previously published.[5] In summary, the hospital RSHAR was calculated as a ratio of the number of predicted hospital admissions in the hospital to the number of expected hospital admissions in the hospital. This ratio is then multiplied by the national unadjusted rate of hospital admissions. We calculated the C statistic of the HLRM model to assess for overall adequacy of risk prediction. To analyze the association between ED RSHAR and EDOUs, we used analysis of variance, where the dependent variable was ED RSHAR and independent variable of interest was presence of EDOUs.
RESULTS
There were 24,232 ED visits from 315 hospitals in the United States in our study. Of these, 82 (20.6%) hospitals had an observation unit physically separate from the ED. Hospitals with and without observation units did not have different hospital patient level characteristics. There was no association between hospital ownership, teaching status, region location, urban or rural location, and hospitals with observation units when compared with hospitals without observation units (Table 1).
| Hospitals With Observation Units, W% (N = 82) | Hospitals Without Observation Units, W% (N = 233) | P Value | |
|---|---|---|---|
| |||
| Region of country | 0.54 | ||
| Northeast | 10.01 | 15.46 | |
| Midwest | 32.06 | 28.35 | |
| South | 41.84 | 36.33 | |
| West | 16.08 | 19.85 | |
| Ownership of hospitals | 0.4 | ||
| Voluntary, nonprofit | 77.28 | 72.35 | |
| Government, nonfederal | 18.78 | 16.11 | |
| Private | 3.94 | 11.55 | |
| Urban or rural location | 0.43 | ||
| Urban | 68.28 | 60.19 | |
| Rural | 31.72 | 39.81 | |
| Teaching hospital status | 0.56 | ||
| Teaching hospital | 63.22 | 68.28 | |
| Nonteaching hospital | 36.78 | 31.71 | |
In addition, there was no association between patient characteristics at the ED visit level in hospitals with observation units when compared with patient characteristics at the ED visit level in hospitals without observation units (Table 2). The average ED risk‐standardized hospital admission rate for hospitals with observation units was 13.7% (95% confidence interval [CI]: 11.3 to 16.0) compared to 16.0% (95% CI: 14.1 to 17.7) for hospitals without observation units (Figure 1). This difference of 2.3% (95% CI: 0.1 to 4.7) was not statistically significant.

| Hospitals With Observation Units, W% (N = 6,067) | Hospitals Without Observation Units, W% (N = 18,165) | P Value | |
|---|---|---|---|
| |||
| Sex, female | 58.75 | 58.35 | 0.96 |
| Age, y | 45.17 | 46.08 | 0.32 |
| Race | 0.75 | ||
| Non‐Hispanic white | 63.54 | 66.41 | |
| Non‐Hispanic black | 23.67 | 18.77 | |
| Hispanic | 9.77 | 12.47 | |
| Other | 3.02 | 2.35 | |
| Source of payment | 0.87 | ||
| Private | 21.90 | 21.46 | |
| Medicare | 32.73 | 30.55 | |
| Medicaid | 22.15 | 23.23 | |
| Uninsured | 18.61 | 20.25 | |
| Unknown/missing | 4.61 | 4.51 | |
| Poverty level | 0.50 | ||
| 5% | 13.87 | 15.31 | |
| 5%9.9% | 32.57 | 23.38 | |
| 10%19.9% | 29.81 | 36.29 | |
| >20% | 20.32 | 20.18 | |
| Missing | 3.44 | 4.83 | |
| Arrival by ambulance | 0.06 | ||
| Yes | 20.01 | 18.61 | |
| No | 76.12 | 76.34 | |
| Unknown | 3.87 | 5.05 | |
| Severity of illness | 0.58 | ||
| Emergent | 16.58 | 16.62 | |
| Nonemergent | 44.09 | 43.85 | |
| Indeterminate | 1.18 | 1.17 | |
| Mental health, alcohol, unclassified | 38.15 | 38.37 | |
| Vital signs | |||
| Temperature | 0.91 | ||
| 9095F | 0.31 | 0.36 | |
| 95.1100.4F | 93.94 | 93.19 | |
| 100.4107F | 1.81 | 2.11 | |
| Missing | 3.94 | 4.35 | |
| Pulse | 0.60 | ||
| 1059 bpm | 3.39 | 3.93 | |
| 60100 bpm | 72.86 | 75.94 | |
| >101 bpm | 19.60 | 21.37 | |
| Missing | 4.16 | 7.67 | |
| Systolic blood pressure | 0.92 | ||
| 5090 mm Hg | 0.90 | 1.02 | |
| 91160 mm Hg | 85.49 | 84.03 | |
| 161260 mm Hg | 11.90 | 12.94 | |
| Missing | 1.71 | 2.01 | |
| Respiratory rate | 0.68 | ||
| 411 breaths/min | 0.24 | 0.19 | |
| 1220 breaths/min | 87.88 | 86.40 | |
| 2160 breaths/min | 8.90 | 10.09 | |
| Missing | 2.98 | 3.32 | |
| Chief complaint associated with hospitalization | |||
| Chest pain and related symptoms | 7.37 | 6.40 | 0.48 |
| Shortness of breath | 3.24 | 3.19 | 0.80 |
| Other symptoms/probably related to psychological | 1.28 | 0.97 | 0.19 |
| General weakness | 1.19 | 1.14 | 0.26 |
| Labored or difficult breathing | 0.56 | 0.88 | 0.93 |
| Fainting (syncope) | 0.44 | 0.42 | 0.09 |
| Unconscious on arrival | 0.35 | 0.38 | 0.17 |
| Other symptoms referable to the nervous system | 0.38 | 0.35 | 0.81 |
| Chronic diseases | |||
| Congestive heart failure | 4.13 | 4.05 | 0.05 |
| Cerebrovascular disease | 4.03 | 3.33 | 0.04 |
| Diabetes | 11.15 | 11.44 | 0.69 |
| HIV | 0.51 | 0.44 | 0.99 |
| On dialysis | 1.14 | 0.96 | 0.25 |
DISCUSSION
In this national study of hospital admissions from the ED, we did not find that hospitals with observation units had a statistically significant lower ED risk‐standardized admission rate when compared with hospitals that did not have observation units. However, the difference of ED risk‐standardized hospital admission rates between hospitals with observation units and those without observation units was very small, and we were likely underpowered to detect a statistically significant difference.
Recently, EDOUs have received much attention, in part because of increases in their numbers and frequency of use.[7] Prior studies, which did not report admission rates that were risk standardized, have also demonstrated no difference in the admission rates among hospitals with and without observation units.[6, 8] Although this result seems counterintuitive, several possible explanations exist.
One reason that there may not be a relation between the rate of inpatient admission and the presence of an observation unit is that the introduction of an EDOU appears to change physician behavior. When the option to admit to an observation unit is present, ED physicians are 2 times more likely to disposition patients to observation status without a statistically significant change in the rate of inpatient admission.[6] Studies have demonstrated that after the introduction of an observation unit, ED physicians tend to overutilize observation among patients who previously would have been discharged, while continuing to admit patients as inpatients who meet observation criteria, which could result in an increase in cost for payers and patients.[7, 9]
Observation units that are protocol driven have been associated with the best patient outcomes including shorter length of stay, lower likelihood of subsequent inpatient admission, and decreased cost.[10] Furthermore, studies evaluating EDOUs suggest increased patient satisfaction and improved patient safety, especially for protocol‐driven EDOUs.[2] However, currently, only half of dedicated observation units are protocol driven. It is also possible that the ED inpatient admission rate does not capture the full impact of an observation unit on care delivery and quality. Observation units are more likely to be present in EDs with a higher overall patient census, longer patient lengths of stay, and higher rates of ambulance diversion.[6, 8] Unfortunately, NHAMCS does not distinguish protocol‐driven versus nonprotocol‐driven observation units. From a policy standpoint, as EDOUs continue to emerge, there is an opportunity to standardize how EDOUs function by using best practices.
This study should be evaluated in the context of limitations such as heterogeneity in the management of EDOUs, limited hospital factor variables that may influence hospital admissions, and small sample size associated with each hospital. Because we were not able to determine which EDs used protocol‐driven observation units, we were not able to determine the impact of having a protocol‐driven observation unit on inpatient hospital admission rates. Additionally, the study may suffer from a selection bias, as EDs with observation units have been shown to have higher patient volume, longer patient lengths of stay, and greater rates of ED diversion. Despite the small sample size, our risk‐standardized model accounted for case mix and hospital factors associated with hospital admission rates and had a high C statistic value, which indicates that the predicted probability of being admitted from the ED highly correlates with the actual outcome of being admitted from the ED. We were unable to track hospitals longitudinally to determine if a hospital's high volume is associated with the creation of EDOUs as a means to offset its demand. However, in our analysis, we did control for overall patient volume when calculating the RHSAR. Finally, we were not able to limit the dataset to observation unit admission conditions because of the limited number of visits provided per hospital by NHAMCS. We conducted an analysis using 80% power and a P value of 0.05 to determine the sample size needed to have statistically significant results. We would require 920 hospitals to have statistically significant results, which suggests we were underpowered to detect a statistically significant difference.
In this preliminary study, we did not find an association between the presence of EDOUs and ED hospital admissions. Our study was limited by an inability to analyze administrative differences and to adjust for certain hospital factors that are likely to influence inpatient admissions via the ED. Nonetheless, our findings suggest that EDOUs merit further evaluation of their potential cost savings and the quality of the care they provide. An evaluation of ED observation departmental management is also needed to assess differences in care at observation units managed by emergency physicians versus nonemergency physicians.
Acknowledgments
Disclosures: R.C., B.S., and C.G. conceived the study. R.C. conducted the statistical analysis and was supervised by B.S. and C.G. All authors analyzed the results and interpreted findings. R.C. and D.B. drafted the manuscript, and all authors contributed substantially to its revision. All authors listed have contributed sufficiently to the project to be included as authors, and all those who are qualified to be authors are listed in the author byline. This work was previously presented at the 2013 Society for Academic Emergency Medicine Annual Meeting, Dallas, Texas. Dr. Capp is funded by a translational K award: KL2 TR001080. Dr. Gross reports grants from Johnson & Johnson, Medtronic Inc., and 21st Century Oncology during the conduct of this study. In addition, he received payment from Fair Health Inc. and ASTRO outside the submitted work. Dr. Sun receives National Institutes of Health funding. No conflicts of interest, financial or other, exist. This applies to all authors.
Today more than one‐third of emergency departments (EDs) in the United States have affiliated observation units, where patients can stay 24 to 48 hours without being admitted to the hospital.[1] Observation units experienced significant growth in the United States from 2005 to 2007, secondary to policy changes involving the Centers for Medicare and Medicaid Services (CMS), which expanded reimbursement for observation services to include any clinical condition. Furthermore, CMS implemented the Recovery Audit Contractor process, which was able to fine providers and facilities for inappropriate claims, with the principle method for charge recovery being inappropriate charges for short inpatient stays.
ED observation units (EDOUs) vary in the number of beds, but are often located adjacent to the emergency department.[2] It is estimated that EDOUs have the capacity for caring for 5% to 10% of any given ED volume.[2] Almost half of EDOUs are protocol driven, allowing these units to discharge up to 80% of all patients within 24 hours.[1, 2] Some studies have suggested that EDOUs are associated with a decrease in overall hospitalization rates, leading to cost savings.[1] However, these studies were limited by their single‐center design or simulated in nature. In addition, other studies show that EDOUs decrease inpatient admissions, length of stay, and costs related to specific clinical conditions such as chest pain, transient ischemic attack, and syncope.[3]
To further evaluate the association of observation units on ED hospital admission rates nationally, we analyzed the largest ED‐based survey, the 2010 National Hospital Ambulatory Medical Care Survey (NHAMCS), to assess the impact of observation units on hospital admissions from the ED. We hypothesized that observation units decrease overall hospital admissions from the ED.
METHODS
Study Design and Population
We performed a retrospective cross‐sectional analysis of ED visits from 2010. This study was exempt from institutional review board review by the University of Colorado and Yale University institutional review committee. The NHAMCS is an annual, national probability sample of ambulatory visits made to nonfederal, general, and short‐stay hospitals conducted by the Centers for Disease Control and Prevention (CDC), National Center for Health Statistics. The multistaged sample design was previously described elsewhere.[4] The 2010 NHAMCS dataset included 350 participating hospitals (unweighted sampling rate of 90%) and a total of 34,936 patient visits.[4]
Exclusions
We excluded patients who were less than 18 years old (n = 8015; 23%); left without being seen, left before examination completion, or left against medical advice (n = 813; 2%); transferred to another institution (n = 626; 1.7%); died on arrival or died in the ED (n = 60; 0.2%); and with missing data on discharge disposition (n = 100; 0.3%). Finally, we excluded hospitals with fewer than 30 visits per year (n = 307; 0.9%) to comply with reliable relative standard errors, as recommended by the CDC; after all of these exclusions there were 325 hospitals. Finally, we excluded hospitals with missing information on EDOUs (n = 783, 2.2%); our dataset at this point included 315 hospitals.
Outcomes
The primary outcome was hospital admission, either from the ED or admitted to an observation unit with subsequent hospital admission, defined as the ED risk‐standardized hospital admission rate (ED RSHAR).[5] This methodology allows for risk adjustment of case mix (ie, disease severity) for each hospital's ED admission rates and has been previously described in the evaluation of varying ED hospital admission rates using the same dataset.[5] To evaluate which hospitals had observation units, we used the following hospital survey question: Does your ED have an observation or clinical decision unit?
Identification of Variables
ED hospitalization rates were risk standardized for each hospital to account for each hospital's case mix and hospital factors such as socioeconomic status, clinical severity, and hospital characteristics. This methodology and dataset use have been previously described in detail.[5]
To account for common chief complaints leading to hospitalization and case‐mix distribution of these complaints among different hospitals, we analyzed all chief complaints and their relationship to hospital admission. We first identified those associated with an admission rate that exceeded 30% and was present in 1% or more of patient visits. The study team of researchers and clinicians determined the aforementioned cutoffs as clinically meaningful. Eight chief complaints met both criteria: chest pain and related symptoms, shortness of breath, other symptoms/probably related to psychological, general weakness, labored or difficulty breathing, fainting (syncope), unconscious arrival, and other symptoms referable to the nervous system. Chronic diseases, such as congestive heart failure, diabetes mellitus, renal disease on dialysis, and human immunodeficiency virus, were also included in the model.
Hospital factors included metropolitan status, geographic region of the country (limited to Northeast, Midwest, South, and West), teaching status, and urban or rural status.[6] We derived a new variable based on a previous study, teaching status, by combining nonprivate hospital status plus having at least 1 ED visit be evaluated by a resident.
Statistical Analyses
We used SAS version 9.2 (SAS Institute, Cary, NC) for all statistical analyses. Frequencies of all variables in the model were calculated to assess the distribution of data and quantify missing data. We did not want to have variables in the model with high collinearity. To investigate collinearity between independent variables, we calculated Spearman correlation coefficients; high collinearity was defined as r > 0.6. No variables included in the model had high collinearity.
To investigate the association of the candidate variables with hospitalization, we used survey logistic regression. Although some variables did not show an association with hospitalization, we felt they were clinically relevant and did not remove them from the model. Hierarchical logistic regression modeling (explained below) was used to calculate ED RSHAR based on the aforementioned selected variables associated with hospital admission.
Hierarchical logistic regression models (HLRM) were used to estimate RSHAR for each hospital. This approach reflects the assumption that a hospital‐specific component exists, and that it will affect the outcomes of patients at a particular institution. This method takes into consideration the hierarchical structure of the data to account for patient clustering within hospitals, and has been used by the CMS to publicly report hospital risk‐standardized rates of mortality and readmission for acute myocardial infarction, heart failure, and pneumonia.
We used a similar methodology as previously published.[5] In summary, the hospital RSHAR was calculated as a ratio of the number of predicted hospital admissions in the hospital to the number of expected hospital admissions in the hospital. This ratio is then multiplied by the national unadjusted rate of hospital admissions. We calculated the C statistic of the HLRM model to assess for overall adequacy of risk prediction. To analyze the association between ED RSHAR and EDOUs, we used analysis of variance, where the dependent variable was ED RSHAR and independent variable of interest was presence of EDOUs.
RESULTS
There were 24,232 ED visits from 315 hospitals in the United States in our study. Of these, 82 (20.6%) hospitals had an observation unit physically separate from the ED. Hospitals with and without observation units did not have different hospital patient level characteristics. There was no association between hospital ownership, teaching status, region location, urban or rural location, and hospitals with observation units when compared with hospitals without observation units (Table 1).
| Hospitals With Observation Units, W% (N = 82) | Hospitals Without Observation Units, W% (N = 233) | P Value | |
|---|---|---|---|
| |||
| Region of country | 0.54 | ||
| Northeast | 10.01 | 15.46 | |
| Midwest | 32.06 | 28.35 | |
| South | 41.84 | 36.33 | |
| West | 16.08 | 19.85 | |
| Ownership of hospitals | 0.4 | ||
| Voluntary, nonprofit | 77.28 | 72.35 | |
| Government, nonfederal | 18.78 | 16.11 | |
| Private | 3.94 | 11.55 | |
| Urban or rural location | 0.43 | ||
| Urban | 68.28 | 60.19 | |
| Rural | 31.72 | 39.81 | |
| Teaching hospital status | 0.56 | ||
| Teaching hospital | 63.22 | 68.28 | |
| Nonteaching hospital | 36.78 | 31.71 | |
In addition, there was no association between patient characteristics at the ED visit level in hospitals with observation units when compared with patient characteristics at the ED visit level in hospitals without observation units (Table 2). The average ED risk‐standardized hospital admission rate for hospitals with observation units was 13.7% (95% confidence interval [CI]: 11.3 to 16.0) compared to 16.0% (95% CI: 14.1 to 17.7) for hospitals without observation units (Figure 1). This difference of 2.3% (95% CI: 0.1 to 4.7) was not statistically significant.

| Hospitals With Observation Units, W% (N = 6,067) | Hospitals Without Observation Units, W% (N = 18,165) | P Value | |
|---|---|---|---|
| |||
| Sex, female | 58.75 | 58.35 | 0.96 |
| Age, y | 45.17 | 46.08 | 0.32 |
| Race | 0.75 | ||
| Non‐Hispanic white | 63.54 | 66.41 | |
| Non‐Hispanic black | 23.67 | 18.77 | |
| Hispanic | 9.77 | 12.47 | |
| Other | 3.02 | 2.35 | |
| Source of payment | 0.87 | ||
| Private | 21.90 | 21.46 | |
| Medicare | 32.73 | 30.55 | |
| Medicaid | 22.15 | 23.23 | |
| Uninsured | 18.61 | 20.25 | |
| Unknown/missing | 4.61 | 4.51 | |
| Poverty level | 0.50 | ||
| 5% | 13.87 | 15.31 | |
| 5%9.9% | 32.57 | 23.38 | |
| 10%19.9% | 29.81 | 36.29 | |
| >20% | 20.32 | 20.18 | |
| Missing | 3.44 | 4.83 | |
| Arrival by ambulance | 0.06 | ||
| Yes | 20.01 | 18.61 | |
| No | 76.12 | 76.34 | |
| Unknown | 3.87 | 5.05 | |
| Severity of illness | 0.58 | ||
| Emergent | 16.58 | 16.62 | |
| Nonemergent | 44.09 | 43.85 | |
| Indeterminate | 1.18 | 1.17 | |
| Mental health, alcohol, unclassified | 38.15 | 38.37 | |
| Vital signs | |||
| Temperature | 0.91 | ||
| 9095F | 0.31 | 0.36 | |
| 95.1100.4F | 93.94 | 93.19 | |
| 100.4107F | 1.81 | 2.11 | |
| Missing | 3.94 | 4.35 | |
| Pulse | 0.60 | ||
| 1059 bpm | 3.39 | 3.93 | |
| 60100 bpm | 72.86 | 75.94 | |
| >101 bpm | 19.60 | 21.37 | |
| Missing | 4.16 | 7.67 | |
| Systolic blood pressure | 0.92 | ||
| 5090 mm Hg | 0.90 | 1.02 | |
| 91160 mm Hg | 85.49 | 84.03 | |
| 161260 mm Hg | 11.90 | 12.94 | |
| Missing | 1.71 | 2.01 | |
| Respiratory rate | 0.68 | ||
| 411 breaths/min | 0.24 | 0.19 | |
| 1220 breaths/min | 87.88 | 86.40 | |
| 2160 breaths/min | 8.90 | 10.09 | |
| Missing | 2.98 | 3.32 | |
| Chief complaint associated with hospitalization | |||
| Chest pain and related symptoms | 7.37 | 6.40 | 0.48 |
| Shortness of breath | 3.24 | 3.19 | 0.80 |
| Other symptoms/probably related to psychological | 1.28 | 0.97 | 0.19 |
| General weakness | 1.19 | 1.14 | 0.26 |
| Labored or difficult breathing | 0.56 | 0.88 | 0.93 |
| Fainting (syncope) | 0.44 | 0.42 | 0.09 |
| Unconscious on arrival | 0.35 | 0.38 | 0.17 |
| Other symptoms referable to the nervous system | 0.38 | 0.35 | 0.81 |
| Chronic diseases | |||
| Congestive heart failure | 4.13 | 4.05 | 0.05 |
| Cerebrovascular disease | 4.03 | 3.33 | 0.04 |
| Diabetes | 11.15 | 11.44 | 0.69 |
| HIV | 0.51 | 0.44 | 0.99 |
| On dialysis | 1.14 | 0.96 | 0.25 |
DISCUSSION
In this national study of hospital admissions from the ED, we did not find that hospitals with observation units had a statistically significant lower ED risk‐standardized admission rate when compared with hospitals that did not have observation units. However, the difference of ED risk‐standardized hospital admission rates between hospitals with observation units and those without observation units was very small, and we were likely underpowered to detect a statistically significant difference.
Recently, EDOUs have received much attention, in part because of increases in their numbers and frequency of use.[7] Prior studies, which did not report admission rates that were risk standardized, have also demonstrated no difference in the admission rates among hospitals with and without observation units.[6, 8] Although this result seems counterintuitive, several possible explanations exist.
One reason that there may not be a relation between the rate of inpatient admission and the presence of an observation unit is that the introduction of an EDOU appears to change physician behavior. When the option to admit to an observation unit is present, ED physicians are 2 times more likely to disposition patients to observation status without a statistically significant change in the rate of inpatient admission.[6] Studies have demonstrated that after the introduction of an observation unit, ED physicians tend to overutilize observation among patients who previously would have been discharged, while continuing to admit patients as inpatients who meet observation criteria, which could result in an increase in cost for payers and patients.[7, 9]
Observation units that are protocol driven have been associated with the best patient outcomes including shorter length of stay, lower likelihood of subsequent inpatient admission, and decreased cost.[10] Furthermore, studies evaluating EDOUs suggest increased patient satisfaction and improved patient safety, especially for protocol‐driven EDOUs.[2] However, currently, only half of dedicated observation units are protocol driven. It is also possible that the ED inpatient admission rate does not capture the full impact of an observation unit on care delivery and quality. Observation units are more likely to be present in EDs with a higher overall patient census, longer patient lengths of stay, and higher rates of ambulance diversion.[6, 8] Unfortunately, NHAMCS does not distinguish protocol‐driven versus nonprotocol‐driven observation units. From a policy standpoint, as EDOUs continue to emerge, there is an opportunity to standardize how EDOUs function by using best practices.
This study should be evaluated in the context of limitations such as heterogeneity in the management of EDOUs, limited hospital factor variables that may influence hospital admissions, and small sample size associated with each hospital. Because we were not able to determine which EDs used protocol‐driven observation units, we were not able to determine the impact of having a protocol‐driven observation unit on inpatient hospital admission rates. Additionally, the study may suffer from a selection bias, as EDs with observation units have been shown to have higher patient volume, longer patient lengths of stay, and greater rates of ED diversion. Despite the small sample size, our risk‐standardized model accounted for case mix and hospital factors associated with hospital admission rates and had a high C statistic value, which indicates that the predicted probability of being admitted from the ED highly correlates with the actual outcome of being admitted from the ED. We were unable to track hospitals longitudinally to determine if a hospital's high volume is associated with the creation of EDOUs as a means to offset its demand. However, in our analysis, we did control for overall patient volume when calculating the RHSAR. Finally, we were not able to limit the dataset to observation unit admission conditions because of the limited number of visits provided per hospital by NHAMCS. We conducted an analysis using 80% power and a P value of 0.05 to determine the sample size needed to have statistically significant results. We would require 920 hospitals to have statistically significant results, which suggests we were underpowered to detect a statistically significant difference.
In this preliminary study, we did not find an association between the presence of EDOUs and ED hospital admissions. Our study was limited by an inability to analyze administrative differences and to adjust for certain hospital factors that are likely to influence inpatient admissions via the ED. Nonetheless, our findings suggest that EDOUs merit further evaluation of their potential cost savings and the quality of the care they provide. An evaluation of ED observation departmental management is also needed to assess differences in care at observation units managed by emergency physicians versus nonemergency physicians.
Acknowledgments
Disclosures: R.C., B.S., and C.G. conceived the study. R.C. conducted the statistical analysis and was supervised by B.S. and C.G. All authors analyzed the results and interpreted findings. R.C. and D.B. drafted the manuscript, and all authors contributed substantially to its revision. All authors listed have contributed sufficiently to the project to be included as authors, and all those who are qualified to be authors are listed in the author byline. This work was previously presented at the 2013 Society for Academic Emergency Medicine Annual Meeting, Dallas, Texas. Dr. Capp is funded by a translational K award: KL2 TR001080. Dr. Gross reports grants from Johnson & Johnson, Medtronic Inc., and 21st Century Oncology during the conduct of this study. In addition, he received payment from Fair Health Inc. and ASTRO outside the submitted work. Dr. Sun receives National Institutes of Health funding. No conflicts of interest, financial or other, exist. This applies to all authors.
- , , . National study of emergency department observation services. Acad Emerg Med. 2011;18(9):959–965.
- , , . Emergency department observation units: a clinical and financial benefit for hospitals. Health Care Manage Rev 2011;36(1):28–37.
- , , , et al. Costs of an emergency department‐based accelerated diagnostic protocol vs hospitalization in patients with chest pain: a randomized controlled trial. JAMA. 1997;278(20):1670–1676.
- Centers for Disease Control and Prevention. National Hospital Ambulatory Medical Care Survey. Ambulatory health care data. Questionnaires, datasets, and related documentation. 2009. Available at: http://www.cdc.gov/nchs/ahcd/ahcd_questionnaires.htm. Accessed November 1, 2011.
- , , , et al. Hospital variation in risk‐standardized hospital admission rates from US EDs among adults. Am J Emerg Med. 2014;32(8):837–843.
- , , , , , . Use of observation care in US emergency departments, 2001 to 2008. PloS One. 2011;6(9):e24326.
- , , , , , . Making greater use of dedicated hospital observation units for many short‐stay patients could save $3.1 billion a year. Health Aff (Millwood). 2012;31(10):2314–2323.
- , , , . A national survey of observation units in the United States. Am J Emerg Med. 2003;21(7):529–533.
- , , , . An evaluation of emergency physician selection of observation unit patients. Am J Emerg Med. 2006;24(3):271–279.
- , , , , , . Protocol‐driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149–2156.
- , , . National study of emergency department observation services. Acad Emerg Med. 2011;18(9):959–965.
- , , . Emergency department observation units: a clinical and financial benefit for hospitals. Health Care Manage Rev 2011;36(1):28–37.
- , , , et al. Costs of an emergency department‐based accelerated diagnostic protocol vs hospitalization in patients with chest pain: a randomized controlled trial. JAMA. 1997;278(20):1670–1676.
- Centers for Disease Control and Prevention. National Hospital Ambulatory Medical Care Survey. Ambulatory health care data. Questionnaires, datasets, and related documentation. 2009. Available at: http://www.cdc.gov/nchs/ahcd/ahcd_questionnaires.htm. Accessed November 1, 2011.
- , , , et al. Hospital variation in risk‐standardized hospital admission rates from US EDs among adults. Am J Emerg Med. 2014;32(8):837–843.
- , , , , , . Use of observation care in US emergency departments, 2001 to 2008. PloS One. 2011;6(9):e24326.
- , , , , , . Making greater use of dedicated hospital observation units for many short‐stay patients could save $3.1 billion a year. Health Aff (Millwood). 2012;31(10):2314–2323.
- , , , . A national survey of observation units in the United States. Am J Emerg Med. 2003;21(7):529–533.
- , , , . An evaluation of emergency physician selection of observation unit patients. Am J Emerg Med. 2006;24(3):271–279.
- , , , , , . Protocol‐driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149–2156.
Frail elders at high mortality risk in the year following surgery
SAN DIEGO – Frail elderly patients face a significantly increased risk of mortality in the year after undergoing major elective noncardiac surgery, a large study from Canada showed.
“The current literature on perioperative frailty clearly shows that being frail before surgery substantially increases your risk of adverse postoperative outcomes,” Dr. Daniel I. McIsaac said in an interview prior to the annual meeting of the American Society of Anesthesiologists, where the study was presented. “In fact, frailty may underlie a lot of the associations between advanced age and adverse postoperative outcomes. Frailty increases in prevalence with increasing age, and as we all know, the population is aging. Therefore, we expect to see an increasing number of frail patients coming for surgery.”
In an effort to determine the risk of 1-year mortality in frail elderly patients having major elective surgery, the researchers used population-based health administrative data in Ontario, to identify 202,811 patients over the age of 65 who had intermediate- to high-risk elective noncardiac surgery between 2002 and 2012. They used the Johns Hopkins Adjusted Clinical Groups (ACG) frailty indicator and captured all deaths that occurred within 1 year of surgery. Proportional hazards regression models adjusted for age, gender, and socioeconomic status were used to evaluate the impact of frailty on 1-year postoperative mortality.
Of the 202,811 patients, 6,289 (3.1%) were frail, reported Dr. McIsaac of the department of anesthesiology at the University of Ottawa. The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23. Mortality was higher among frail patients for all types of surgery, compared with their nonfrail counterparts, with the exception of pancreaticoduodenectomy. Frailty had the strongest impact on the risk of mortality after total joint arthroplasty (adjusted hazard ratio of 3.79 for hip replacement and adjusted HR of 2.68 for knee replacement).
The risk of postoperative mortality for frail patients was much higher than for nonfrail patients in the early time period after surgery, especially during the first postoperative week. “Depending on how you control for other variables, a frail patient was 13-35 times more likely to die in the week after surgery than a nonfrail patient of the same age having the same surgery,” said Dr. McIsaac, who is also a staff anesthesiologist at the Ottawa Hospital. “This makes a lot of sense; frail patients are vulnerable to stressors, and surgery puts an enormous physiological stress on even healthy patients. Future work clearly needs to focus [on] addressing this high-risk time in the immediate postoperative period.”
He acknowledged certain limitations of the study, including its reliance on health administrative data and the fact that frailty “is a challenging exposure to study because there are a plethora of instruments that can be used to call someone frail. We used a validated set of frailty-defining diagnoses that have been shown to identify people with multidimensional frailty. That said, you can’t necessarily generalize our findings to patients identified as frail using other instruments.”
The findings, Dr. McIsaac concluded, suggest that clinicians should focus on identifying frail patients prior to surgery, “support them to ensure that they are more likely to derive benefit from surgery than harm, and focus on optimizing their care after surgery to address this early mortality risk.”
The study was funded by departments of anesthesiology at the University of Ottawa and at the Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
SAN DIEGO – Frail elderly patients face a significantly increased risk of mortality in the year after undergoing major elective noncardiac surgery, a large study from Canada showed.
“The current literature on perioperative frailty clearly shows that being frail before surgery substantially increases your risk of adverse postoperative outcomes,” Dr. Daniel I. McIsaac said in an interview prior to the annual meeting of the American Society of Anesthesiologists, where the study was presented. “In fact, frailty may underlie a lot of the associations between advanced age and adverse postoperative outcomes. Frailty increases in prevalence with increasing age, and as we all know, the population is aging. Therefore, we expect to see an increasing number of frail patients coming for surgery.”
In an effort to determine the risk of 1-year mortality in frail elderly patients having major elective surgery, the researchers used population-based health administrative data in Ontario, to identify 202,811 patients over the age of 65 who had intermediate- to high-risk elective noncardiac surgery between 2002 and 2012. They used the Johns Hopkins Adjusted Clinical Groups (ACG) frailty indicator and captured all deaths that occurred within 1 year of surgery. Proportional hazards regression models adjusted for age, gender, and socioeconomic status were used to evaluate the impact of frailty on 1-year postoperative mortality.
Of the 202,811 patients, 6,289 (3.1%) were frail, reported Dr. McIsaac of the department of anesthesiology at the University of Ottawa. The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23. Mortality was higher among frail patients for all types of surgery, compared with their nonfrail counterparts, with the exception of pancreaticoduodenectomy. Frailty had the strongest impact on the risk of mortality after total joint arthroplasty (adjusted hazard ratio of 3.79 for hip replacement and adjusted HR of 2.68 for knee replacement).
The risk of postoperative mortality for frail patients was much higher than for nonfrail patients in the early time period after surgery, especially during the first postoperative week. “Depending on how you control for other variables, a frail patient was 13-35 times more likely to die in the week after surgery than a nonfrail patient of the same age having the same surgery,” said Dr. McIsaac, who is also a staff anesthesiologist at the Ottawa Hospital. “This makes a lot of sense; frail patients are vulnerable to stressors, and surgery puts an enormous physiological stress on even healthy patients. Future work clearly needs to focus [on] addressing this high-risk time in the immediate postoperative period.”
He acknowledged certain limitations of the study, including its reliance on health administrative data and the fact that frailty “is a challenging exposure to study because there are a plethora of instruments that can be used to call someone frail. We used a validated set of frailty-defining diagnoses that have been shown to identify people with multidimensional frailty. That said, you can’t necessarily generalize our findings to patients identified as frail using other instruments.”
The findings, Dr. McIsaac concluded, suggest that clinicians should focus on identifying frail patients prior to surgery, “support them to ensure that they are more likely to derive benefit from surgery than harm, and focus on optimizing their care after surgery to address this early mortality risk.”
The study was funded by departments of anesthesiology at the University of Ottawa and at the Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
SAN DIEGO – Frail elderly patients face a significantly increased risk of mortality in the year after undergoing major elective noncardiac surgery, a large study from Canada showed.
“The current literature on perioperative frailty clearly shows that being frail before surgery substantially increases your risk of adverse postoperative outcomes,” Dr. Daniel I. McIsaac said in an interview prior to the annual meeting of the American Society of Anesthesiologists, where the study was presented. “In fact, frailty may underlie a lot of the associations between advanced age and adverse postoperative outcomes. Frailty increases in prevalence with increasing age, and as we all know, the population is aging. Therefore, we expect to see an increasing number of frail patients coming for surgery.”
In an effort to determine the risk of 1-year mortality in frail elderly patients having major elective surgery, the researchers used population-based health administrative data in Ontario, to identify 202,811 patients over the age of 65 who had intermediate- to high-risk elective noncardiac surgery between 2002 and 2012. They used the Johns Hopkins Adjusted Clinical Groups (ACG) frailty indicator and captured all deaths that occurred within 1 year of surgery. Proportional hazards regression models adjusted for age, gender, and socioeconomic status were used to evaluate the impact of frailty on 1-year postoperative mortality.
Of the 202,811 patients, 6,289 (3.1%) were frail, reported Dr. McIsaac of the department of anesthesiology at the University of Ottawa. The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23. Mortality was higher among frail patients for all types of surgery, compared with their nonfrail counterparts, with the exception of pancreaticoduodenectomy. Frailty had the strongest impact on the risk of mortality after total joint arthroplasty (adjusted hazard ratio of 3.79 for hip replacement and adjusted HR of 2.68 for knee replacement).
The risk of postoperative mortality for frail patients was much higher than for nonfrail patients in the early time period after surgery, especially during the first postoperative week. “Depending on how you control for other variables, a frail patient was 13-35 times more likely to die in the week after surgery than a nonfrail patient of the same age having the same surgery,” said Dr. McIsaac, who is also a staff anesthesiologist at the Ottawa Hospital. “This makes a lot of sense; frail patients are vulnerable to stressors, and surgery puts an enormous physiological stress on even healthy patients. Future work clearly needs to focus [on] addressing this high-risk time in the immediate postoperative period.”
He acknowledged certain limitations of the study, including its reliance on health administrative data and the fact that frailty “is a challenging exposure to study because there are a plethora of instruments that can be used to call someone frail. We used a validated set of frailty-defining diagnoses that have been shown to identify people with multidimensional frailty. That said, you can’t necessarily generalize our findings to patients identified as frail using other instruments.”
The findings, Dr. McIsaac concluded, suggest that clinicians should focus on identifying frail patients prior to surgery, “support them to ensure that they are more likely to derive benefit from surgery than harm, and focus on optimizing their care after surgery to address this early mortality risk.”
The study was funded by departments of anesthesiology at the University of Ottawa and at the Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
AT THE ASA ANNUAL MEETING
Key clinical point: Frail elderly patients face an increased risk of mortality within 1 year of undergoing noncardiac surgery.
Major finding: The 1-year postoperative mortality was 13.6% among frail patients, compared with 4.8% of nonfrail patients, for an adjusted hazard ratio of 2.23.
Data source: A study of 202,811 patients over the age of 65 years who underwent noncardiac surgery between 2002 and 2012.
Disclosures: The study was funded by departments of anesthesiology at the University of Ottawa and at The Ottawa Hospital. Dr. McIsaac reported having no financial disclosures.
TCT: Paclitaxel-coated balloon delivers durable SFA patency
SAN FRANCISCO – Treatment of femoropopliteal arterial disease with a paclitaxel-coated balloon produced durable, 2-year benefits compared with conventional balloon angioplasty during extended follow-up of the pivotal trial that led to U.S. approval of this drug-coated balloon.
The durability of the benefit first seen after 1 year when follow-up continued out to 2 years was an important finding that distinguishes the IN.PACT Admiral paclitaxel-covered balloon used in the current study from the first and only other drug-covered balloon (DCB) approved for U.S. practice, the Lutonix 035 DCB.
“Not all drug-coated balloons are the same,” Dr. John R. Laird said while reporting the IN.PACT Admiral DCB results at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although both the IN.PACT Admiral and Lutonix 035 DCB have paclitaxel coatings, the two devices differ by paclitaxel dose density on the balloon’s surface (3.5 mcg/mm2 and 2.0 mcg/mm2, respectively), type of excipient (carrier) used, and the balloon coating, noted Dr. Laird, professor and medical director of the Vascular Center at the University of California, Davis in Sacramento.
After the first year, primary patency ran 82% among the 220 patients randomized to the DCB and 52% in patients treated with percutaneous transluminal angioplasty, a statistically significant 30 percentage point difference in favor of the DCB. After 2 years, the rates were 79% in the DCB arm and 50% with a conventional balloon. “We saw no late catch-up that reduced the patency rate,” said Dr. Laird.
The INPACT SFA I(Randomized Trial of IN.PACT Admiral Drug Coated Balloon vs. Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease) trial enrolled 331 patients at 57 centers in the United States and Europe. Researchers reported the study’s primary efficacy and safety endpoints with 1-year follow-up earlier this year (Circulation. 2015 Feb 3;131:495-502). Concurrent with Dr. Laird’s report at the meeting, the 2-year results appeared online (J Amer Coll Card. 2015.doi:10.1016/j.jacc.2015.09.063).
Dr. Laird acknowledged that some types of stents also have shown good efficacy for treating femoropopliteal disease, but he had reservations about placing a stent when the DCB option exists.
“A lot of people have the sense that if we can avoid placing a stent in a femoral artery it helps preserve future treatment options for the patient. The problem with a stent is that once in-stent restenosis occurs in a leg artery, then the chances of getting a good result with an intravascular approach are poor,” Dr. Laird said at the meeting, sponsored by the Cardiovascular Research Foundation.
One potentially concerning finding from the 2-year follow-up was a statistically significant excess of all-cause mortality in the patients who received the DCB, with 16 deaths in the DCB arm and 1 death in the control, angioplasty arm. Dr. Laird dismissed the clinical importance of the finding, noting that all the deaths in the DCB arm had been independently adjudicated with none judged related to the device or procedure. In addition, the deaths occurred an average of 560 days following the procedure.
On Twitter @mitchelzoler
The IN.PACT Admiral paclitaxel-covered balloon provides a powerful new tool for treating superficial femoral artery and popliteal artery disease that works better than does a conventional balloon and avoids using a stent.
Not all drug-coated balloons (DCBs) are alike, even if they use the same antiproliferative drug, paclitaxel. The evidence suggests that the IN.PACT Admiral drug-coated balloon is superior to the performance of the Lutonix 035 DCB, although this has only been assessed in separate studies and not as a head-to-head comparison.

|
Dr. Gary Gershony |
Another option for treating superficial femoropopliteal disease is with any of a variety of stents. I think the general feeling among peripheral-artery specialists is that it’s better for patients to avoid having a stent permanently in their leg when other, equally-good options are available to try first. Sometimes placing a stent is unavoidable to produce a substantially better revascularization outcome, for example when a dissection occurs or for treating a significant residual stenosis.
The IN.PACT Admiral DCB has not yet been tested on complex or calcified lesions so its performance in those settings is not yet know. The basic message from this 2-year follow-up is that this paclitaxel-coated balloon had better results out to 2-years than a conventional balloon for lesions that were not especially complex and with an average length of 9 cm. For many patients with lesions like these a DCB is a good option because it may produce a durable result while maintaining the option to use a stent later if necessary.
Vascular specialists have been concerned about longer-term follow-up of the results from the IN.PACT SFA trial to see if a signal appeared of catchup restenosis between years 1 and 2. The results showed no evidence of this. It is reassuring to see this DCB technology can produce an effect that’s durable for 2 years without leaving behind a permanent implant. It strengthens the case for this particular DCB but should not be extrapolated to all drug-coated balloons or to all types of femoropopliteal lesions.
Dr. Gary Gershony is an interventional cardiologist and medical director of cardiovascular research, education and technology at John Muir Cardiovascular Institute of John Muir Health in Concord, Calif. He had no relevant disclosures. He made these comments as a discussant for the report and in an interview.
The IN.PACT Admiral paclitaxel-covered balloon provides a powerful new tool for treating superficial femoral artery and popliteal artery disease that works better than does a conventional balloon and avoids using a stent.
Not all drug-coated balloons (DCBs) are alike, even if they use the same antiproliferative drug, paclitaxel. The evidence suggests that the IN.PACT Admiral drug-coated balloon is superior to the performance of the Lutonix 035 DCB, although this has only been assessed in separate studies and not as a head-to-head comparison.

|
Dr. Gary Gershony |
Another option for treating superficial femoropopliteal disease is with any of a variety of stents. I think the general feeling among peripheral-artery specialists is that it’s better for patients to avoid having a stent permanently in their leg when other, equally-good options are available to try first. Sometimes placing a stent is unavoidable to produce a substantially better revascularization outcome, for example when a dissection occurs or for treating a significant residual stenosis.
The IN.PACT Admiral DCB has not yet been tested on complex or calcified lesions so its performance in those settings is not yet know. The basic message from this 2-year follow-up is that this paclitaxel-coated balloon had better results out to 2-years than a conventional balloon for lesions that were not especially complex and with an average length of 9 cm. For many patients with lesions like these a DCB is a good option because it may produce a durable result while maintaining the option to use a stent later if necessary.
Vascular specialists have been concerned about longer-term follow-up of the results from the IN.PACT SFA trial to see if a signal appeared of catchup restenosis between years 1 and 2. The results showed no evidence of this. It is reassuring to see this DCB technology can produce an effect that’s durable for 2 years without leaving behind a permanent implant. It strengthens the case for this particular DCB but should not be extrapolated to all drug-coated balloons or to all types of femoropopliteal lesions.
Dr. Gary Gershony is an interventional cardiologist and medical director of cardiovascular research, education and technology at John Muir Cardiovascular Institute of John Muir Health in Concord, Calif. He had no relevant disclosures. He made these comments as a discussant for the report and in an interview.
The IN.PACT Admiral paclitaxel-covered balloon provides a powerful new tool for treating superficial femoral artery and popliteal artery disease that works better than does a conventional balloon and avoids using a stent.
Not all drug-coated balloons (DCBs) are alike, even if they use the same antiproliferative drug, paclitaxel. The evidence suggests that the IN.PACT Admiral drug-coated balloon is superior to the performance of the Lutonix 035 DCB, although this has only been assessed in separate studies and not as a head-to-head comparison.

|
Dr. Gary Gershony |
Another option for treating superficial femoropopliteal disease is with any of a variety of stents. I think the general feeling among peripheral-artery specialists is that it’s better for patients to avoid having a stent permanently in their leg when other, equally-good options are available to try first. Sometimes placing a stent is unavoidable to produce a substantially better revascularization outcome, for example when a dissection occurs or for treating a significant residual stenosis.
The IN.PACT Admiral DCB has not yet been tested on complex or calcified lesions so its performance in those settings is not yet know. The basic message from this 2-year follow-up is that this paclitaxel-coated balloon had better results out to 2-years than a conventional balloon for lesions that were not especially complex and with an average length of 9 cm. For many patients with lesions like these a DCB is a good option because it may produce a durable result while maintaining the option to use a stent later if necessary.
Vascular specialists have been concerned about longer-term follow-up of the results from the IN.PACT SFA trial to see if a signal appeared of catchup restenosis between years 1 and 2. The results showed no evidence of this. It is reassuring to see this DCB technology can produce an effect that’s durable for 2 years without leaving behind a permanent implant. It strengthens the case for this particular DCB but should not be extrapolated to all drug-coated balloons or to all types of femoropopliteal lesions.
Dr. Gary Gershony is an interventional cardiologist and medical director of cardiovascular research, education and technology at John Muir Cardiovascular Institute of John Muir Health in Concord, Calif. He had no relevant disclosures. He made these comments as a discussant for the report and in an interview.
SAN FRANCISCO – Treatment of femoropopliteal arterial disease with a paclitaxel-coated balloon produced durable, 2-year benefits compared with conventional balloon angioplasty during extended follow-up of the pivotal trial that led to U.S. approval of this drug-coated balloon.
The durability of the benefit first seen after 1 year when follow-up continued out to 2 years was an important finding that distinguishes the IN.PACT Admiral paclitaxel-covered balloon used in the current study from the first and only other drug-covered balloon (DCB) approved for U.S. practice, the Lutonix 035 DCB.
“Not all drug-coated balloons are the same,” Dr. John R. Laird said while reporting the IN.PACT Admiral DCB results at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although both the IN.PACT Admiral and Lutonix 035 DCB have paclitaxel coatings, the two devices differ by paclitaxel dose density on the balloon’s surface (3.5 mcg/mm2 and 2.0 mcg/mm2, respectively), type of excipient (carrier) used, and the balloon coating, noted Dr. Laird, professor and medical director of the Vascular Center at the University of California, Davis in Sacramento.
After the first year, primary patency ran 82% among the 220 patients randomized to the DCB and 52% in patients treated with percutaneous transluminal angioplasty, a statistically significant 30 percentage point difference in favor of the DCB. After 2 years, the rates were 79% in the DCB arm and 50% with a conventional balloon. “We saw no late catch-up that reduced the patency rate,” said Dr. Laird.
The INPACT SFA I(Randomized Trial of IN.PACT Admiral Drug Coated Balloon vs. Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease) trial enrolled 331 patients at 57 centers in the United States and Europe. Researchers reported the study’s primary efficacy and safety endpoints with 1-year follow-up earlier this year (Circulation. 2015 Feb 3;131:495-502). Concurrent with Dr. Laird’s report at the meeting, the 2-year results appeared online (J Amer Coll Card. 2015.doi:10.1016/j.jacc.2015.09.063).
Dr. Laird acknowledged that some types of stents also have shown good efficacy for treating femoropopliteal disease, but he had reservations about placing a stent when the DCB option exists.
“A lot of people have the sense that if we can avoid placing a stent in a femoral artery it helps preserve future treatment options for the patient. The problem with a stent is that once in-stent restenosis occurs in a leg artery, then the chances of getting a good result with an intravascular approach are poor,” Dr. Laird said at the meeting, sponsored by the Cardiovascular Research Foundation.
One potentially concerning finding from the 2-year follow-up was a statistically significant excess of all-cause mortality in the patients who received the DCB, with 16 deaths in the DCB arm and 1 death in the control, angioplasty arm. Dr. Laird dismissed the clinical importance of the finding, noting that all the deaths in the DCB arm had been independently adjudicated with none judged related to the device or procedure. In addition, the deaths occurred an average of 560 days following the procedure.
On Twitter @mitchelzoler
SAN FRANCISCO – Treatment of femoropopliteal arterial disease with a paclitaxel-coated balloon produced durable, 2-year benefits compared with conventional balloon angioplasty during extended follow-up of the pivotal trial that led to U.S. approval of this drug-coated balloon.
The durability of the benefit first seen after 1 year when follow-up continued out to 2 years was an important finding that distinguishes the IN.PACT Admiral paclitaxel-covered balloon used in the current study from the first and only other drug-covered balloon (DCB) approved for U.S. practice, the Lutonix 035 DCB.
“Not all drug-coated balloons are the same,” Dr. John R. Laird said while reporting the IN.PACT Admiral DCB results at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although both the IN.PACT Admiral and Lutonix 035 DCB have paclitaxel coatings, the two devices differ by paclitaxel dose density on the balloon’s surface (3.5 mcg/mm2 and 2.0 mcg/mm2, respectively), type of excipient (carrier) used, and the balloon coating, noted Dr. Laird, professor and medical director of the Vascular Center at the University of California, Davis in Sacramento.
After the first year, primary patency ran 82% among the 220 patients randomized to the DCB and 52% in patients treated with percutaneous transluminal angioplasty, a statistically significant 30 percentage point difference in favor of the DCB. After 2 years, the rates were 79% in the DCB arm and 50% with a conventional balloon. “We saw no late catch-up that reduced the patency rate,” said Dr. Laird.
The INPACT SFA I(Randomized Trial of IN.PACT Admiral Drug Coated Balloon vs. Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease) trial enrolled 331 patients at 57 centers in the United States and Europe. Researchers reported the study’s primary efficacy and safety endpoints with 1-year follow-up earlier this year (Circulation. 2015 Feb 3;131:495-502). Concurrent with Dr. Laird’s report at the meeting, the 2-year results appeared online (J Amer Coll Card. 2015.doi:10.1016/j.jacc.2015.09.063).
Dr. Laird acknowledged that some types of stents also have shown good efficacy for treating femoropopliteal disease, but he had reservations about placing a stent when the DCB option exists.
“A lot of people have the sense that if we can avoid placing a stent in a femoral artery it helps preserve future treatment options for the patient. The problem with a stent is that once in-stent restenosis occurs in a leg artery, then the chances of getting a good result with an intravascular approach are poor,” Dr. Laird said at the meeting, sponsored by the Cardiovascular Research Foundation.
One potentially concerning finding from the 2-year follow-up was a statistically significant excess of all-cause mortality in the patients who received the DCB, with 16 deaths in the DCB arm and 1 death in the control, angioplasty arm. Dr. Laird dismissed the clinical importance of the finding, noting that all the deaths in the DCB arm had been independently adjudicated with none judged related to the device or procedure. In addition, the deaths occurred an average of 560 days following the procedure.
On Twitter @mitchelzoler
AT TCT 2015
Key clinical point: Two-year follow-up of paclitaxel-coated balloon treatment of femoropopliteal lesions showed durable and substantially better patency, compared with conventional balloon treatment.
Major finding: Two-year primary patency rate was 79% after treatment with the IN.PACT Admiral balloon and 50% with a conventional balloon.
Data source: INPACT SFA 1, a multicenter, randomized trial with 331 enrolled patients.
Disclosures: INPACT SFA I was sponsored by Medtronic, the company that markets the IN.PACT Admiral drug-coated balloon. Dr. Laird has been a consultant to Medtronic as well as to Bard, Abbott Vascular, Boston Scientific and Cordis. He also owns stock in several device companies.
Dose-intensive, multiagent regimen improves outcomes from low-risk rhabdomyosarcoma
A dose-intensive multiagent regimen including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation resulted in improved outcomes for patients with low-risk stage IV rhabdomyosarcoma (RMS), but not for patients with high-risk disease, according to results from the Children’s Oncology Group study.
For all patients with stage IV rhabdomyosarcoma, the 3-year event-free and overall survival rates were 38% and 56%, respectively. Patients with stage IV RMS with one or fewer Oberlin risk factors had 3-year event-free survival and overall survival rates of 69% and 79%, respectively; patients with two or more Oberlin risk factors had rates of 20% and 14%, respectively (Jour Clin Oncol. 2015 Oct 26. doi: 10.1200/JCO.2015.63.4048).
The study identified an expanded group of patients with low-risk metastatic RMS that included patients with embryonal RMS aged 10 years and older but with an Oberlin score of less than 2. The results in this group represent an improvement over previous study results. However, for the remainder of high-risk patients with alveolar RMS, different approaches are needed, according to Dr. Brenda Weigel of the University of Minnesota, Minneapolis, and her colleagues.
“Unfortunately, alveolar RMS has fewer genetic aberrations than embryonal RMS and no known recurrently mutated cancer consensus genes, which limits genetic targets available for therapeutic approaches,” they wrote.
The Children’s Oncology Group study ARST0431 enrolled 109 patients with metastatic RMS who had no prior chemotherapy or radiation treatment from 2006 to 2008.
The study combined three treatment strategies: dose intensification by interval compression, use of active agents identified in previous phase II window studies, and use of irinotecan as a radiation sensitizer. The 54-week treatment schedule began with two cycles of vincristine/irinotecan followed by interval-compressed vincristine/doxorubicin/cyclophosphamide and ifosfamide/etoposide (cycles began every 14 days), and finished with four cycles of standard vincristine/actinomycin/cyclophosphamide (VAC) and two more cycles of vincristine/irinotecan. The treatment plan also included radiation of primary and metastatic sites at week 19.
The most common nonhematologic adverse event of grade 3 or higher was diarrhea, reported in 20% of patients during the time period when they received irinotecan. Febrile neutropenia occurred in 63% of patients.
Dr. Weigel reported financial relationships with Genentech and Eli Lilly/ImClone System. Several of her coauthors reported having ties to industry sources.
A dose-intensive multiagent regimen including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation resulted in improved outcomes for patients with low-risk stage IV rhabdomyosarcoma (RMS), but not for patients with high-risk disease, according to results from the Children’s Oncology Group study.
For all patients with stage IV rhabdomyosarcoma, the 3-year event-free and overall survival rates were 38% and 56%, respectively. Patients with stage IV RMS with one or fewer Oberlin risk factors had 3-year event-free survival and overall survival rates of 69% and 79%, respectively; patients with two or more Oberlin risk factors had rates of 20% and 14%, respectively (Jour Clin Oncol. 2015 Oct 26. doi: 10.1200/JCO.2015.63.4048).
The study identified an expanded group of patients with low-risk metastatic RMS that included patients with embryonal RMS aged 10 years and older but with an Oberlin score of less than 2. The results in this group represent an improvement over previous study results. However, for the remainder of high-risk patients with alveolar RMS, different approaches are needed, according to Dr. Brenda Weigel of the University of Minnesota, Minneapolis, and her colleagues.
“Unfortunately, alveolar RMS has fewer genetic aberrations than embryonal RMS and no known recurrently mutated cancer consensus genes, which limits genetic targets available for therapeutic approaches,” they wrote.
The Children’s Oncology Group study ARST0431 enrolled 109 patients with metastatic RMS who had no prior chemotherapy or radiation treatment from 2006 to 2008.
The study combined three treatment strategies: dose intensification by interval compression, use of active agents identified in previous phase II window studies, and use of irinotecan as a radiation sensitizer. The 54-week treatment schedule began with two cycles of vincristine/irinotecan followed by interval-compressed vincristine/doxorubicin/cyclophosphamide and ifosfamide/etoposide (cycles began every 14 days), and finished with four cycles of standard vincristine/actinomycin/cyclophosphamide (VAC) and two more cycles of vincristine/irinotecan. The treatment plan also included radiation of primary and metastatic sites at week 19.
The most common nonhematologic adverse event of grade 3 or higher was diarrhea, reported in 20% of patients during the time period when they received irinotecan. Febrile neutropenia occurred in 63% of patients.
Dr. Weigel reported financial relationships with Genentech and Eli Lilly/ImClone System. Several of her coauthors reported having ties to industry sources.
A dose-intensive multiagent regimen including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation resulted in improved outcomes for patients with low-risk stage IV rhabdomyosarcoma (RMS), but not for patients with high-risk disease, according to results from the Children’s Oncology Group study.
For all patients with stage IV rhabdomyosarcoma, the 3-year event-free and overall survival rates were 38% and 56%, respectively. Patients with stage IV RMS with one or fewer Oberlin risk factors had 3-year event-free survival and overall survival rates of 69% and 79%, respectively; patients with two or more Oberlin risk factors had rates of 20% and 14%, respectively (Jour Clin Oncol. 2015 Oct 26. doi: 10.1200/JCO.2015.63.4048).
The study identified an expanded group of patients with low-risk metastatic RMS that included patients with embryonal RMS aged 10 years and older but with an Oberlin score of less than 2. The results in this group represent an improvement over previous study results. However, for the remainder of high-risk patients with alveolar RMS, different approaches are needed, according to Dr. Brenda Weigel of the University of Minnesota, Minneapolis, and her colleagues.
“Unfortunately, alveolar RMS has fewer genetic aberrations than embryonal RMS and no known recurrently mutated cancer consensus genes, which limits genetic targets available for therapeutic approaches,” they wrote.
The Children’s Oncology Group study ARST0431 enrolled 109 patients with metastatic RMS who had no prior chemotherapy or radiation treatment from 2006 to 2008.
The study combined three treatment strategies: dose intensification by interval compression, use of active agents identified in previous phase II window studies, and use of irinotecan as a radiation sensitizer. The 54-week treatment schedule began with two cycles of vincristine/irinotecan followed by interval-compressed vincristine/doxorubicin/cyclophosphamide and ifosfamide/etoposide (cycles began every 14 days), and finished with four cycles of standard vincristine/actinomycin/cyclophosphamide (VAC) and two more cycles of vincristine/irinotecan. The treatment plan also included radiation of primary and metastatic sites at week 19.
The most common nonhematologic adverse event of grade 3 or higher was diarrhea, reported in 20% of patients during the time period when they received irinotecan. Febrile neutropenia occurred in 63% of patients.
Dr. Weigel reported financial relationships with Genentech and Eli Lilly/ImClone System. Several of her coauthors reported having ties to industry sources.
Key clinical point: A dose-intensive, multiagent regimen including dose-compressed cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, irinotecan, and radiation resulted in improved outcomes for patients with low-risk stage IV rhabdomyosarcoma (RMS), but not for patients with high-risk disease.
Major finding: Patients with stage IV rhabdomyosarcoma with one or fewer Oberlin risk factors had 3-year event-free survival and overall survival rates of 69% and 79%, respectively; patients with two or more Oberlin risk factors had rates of 20% and 14%, respectively.
Data source: The Children’s Oncology Group study ARST0431 involving 109 patients with metastatic RMS who had no prior chemotherapy or radiation treatment from 2006 to 2008.
Disclosures: Dr. Weigel reported financial relationships with Genentech and Eli Lilly/ImClone System. Several of her coauthors reported having ties to industry sources.
High-radiation doses improve survival with inoperable intrahepatic cholangiocarcinoma
Using recent advances in radiotherapy (RT) planning and delivery, high-dose radiation delivered to hepatic tumors produced major survival benefits in patients with inoperable intrahepatic cholangiocarcinoma (IHCC), investigators reported online in Journal of Clinical Oncology.
“Treatment with ablative doses of RT using high-quality daily CT image guidance with inspiration breath-hold gating can achieve survival times comparable to those achieved with resection,” wrote Dr. Randa Tao, radiation oncologist at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.3778]).
From 2002 to 2014, 79 patients with inoperable IHCC were treated with definitive RT. The median survival time was 30 months; 1-, 2-, and 3-year overall survival rates were 87%, 61%, and 44%, respectively. Median progression-free survival was 30 months, and 1-, 2-, and 3-year progression-free survival rates were 88%, 61%, and 39%, respectively.
After completion of RT, 38 patients (48%) had primary tumor progression. Actuarial 1-, 2-, and 3-year local control rates were 81%, 45%, and 27%, respectively, with median duration of 23 months. The majority of patients (34) had recurrence within the high-dose radiation region, three had both in-field and marginal progression, and one had recurrence at the margin.
RT dose was the most important prognostic factor for overall survival and local control. Patients treated with doses higher than the conventional 50.4 Gy had a median survival of 43 months, compared with 23 months for patients treated with doses 50.4 Gy or less (P = .01).
Total biologically effective dose (BED) affected outcomes also. The 2- and 3-year overall survival rates for patients treated with BED greater than 80.5 Gy were both 73%, compared with 58% and 38% for those treated with BED of 80.5 Gy or less.
The treatment was well tolerated, with no cases of radiation induced liver disease observed.
The investigators recommend that higher total RT doses and higher doses delivered per fraction to achieve BED greater than 80.5 Gy should be considered for all patients as long as image guidance is used to ensure that the dose is delivered safely, and dose constraints to the liver, bile duct, stomach, and bowel can be met. The findings support the use of 67.5 Gy in 15 fractions (BED, 97.88 Gy).
Dr. Tao reported having no disclosures. Several of her coauthors reported having ties to industry sources.
Using recent advances in radiotherapy (RT) planning and delivery, high-dose radiation delivered to hepatic tumors produced major survival benefits in patients with inoperable intrahepatic cholangiocarcinoma (IHCC), investigators reported online in Journal of Clinical Oncology.
“Treatment with ablative doses of RT using high-quality daily CT image guidance with inspiration breath-hold gating can achieve survival times comparable to those achieved with resection,” wrote Dr. Randa Tao, radiation oncologist at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.3778]).
From 2002 to 2014, 79 patients with inoperable IHCC were treated with definitive RT. The median survival time was 30 months; 1-, 2-, and 3-year overall survival rates were 87%, 61%, and 44%, respectively. Median progression-free survival was 30 months, and 1-, 2-, and 3-year progression-free survival rates were 88%, 61%, and 39%, respectively.
After completion of RT, 38 patients (48%) had primary tumor progression. Actuarial 1-, 2-, and 3-year local control rates were 81%, 45%, and 27%, respectively, with median duration of 23 months. The majority of patients (34) had recurrence within the high-dose radiation region, three had both in-field and marginal progression, and one had recurrence at the margin.
RT dose was the most important prognostic factor for overall survival and local control. Patients treated with doses higher than the conventional 50.4 Gy had a median survival of 43 months, compared with 23 months for patients treated with doses 50.4 Gy or less (P = .01).
Total biologically effective dose (BED) affected outcomes also. The 2- and 3-year overall survival rates for patients treated with BED greater than 80.5 Gy were both 73%, compared with 58% and 38% for those treated with BED of 80.5 Gy or less.
The treatment was well tolerated, with no cases of radiation induced liver disease observed.
The investigators recommend that higher total RT doses and higher doses delivered per fraction to achieve BED greater than 80.5 Gy should be considered for all patients as long as image guidance is used to ensure that the dose is delivered safely, and dose constraints to the liver, bile duct, stomach, and bowel can be met. The findings support the use of 67.5 Gy in 15 fractions (BED, 97.88 Gy).
Dr. Tao reported having no disclosures. Several of her coauthors reported having ties to industry sources.
Using recent advances in radiotherapy (RT) planning and delivery, high-dose radiation delivered to hepatic tumors produced major survival benefits in patients with inoperable intrahepatic cholangiocarcinoma (IHCC), investigators reported online in Journal of Clinical Oncology.
“Treatment with ablative doses of RT using high-quality daily CT image guidance with inspiration breath-hold gating can achieve survival times comparable to those achieved with resection,” wrote Dr. Randa Tao, radiation oncologist at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.3778]).
From 2002 to 2014, 79 patients with inoperable IHCC were treated with definitive RT. The median survival time was 30 months; 1-, 2-, and 3-year overall survival rates were 87%, 61%, and 44%, respectively. Median progression-free survival was 30 months, and 1-, 2-, and 3-year progression-free survival rates were 88%, 61%, and 39%, respectively.
After completion of RT, 38 patients (48%) had primary tumor progression. Actuarial 1-, 2-, and 3-year local control rates were 81%, 45%, and 27%, respectively, with median duration of 23 months. The majority of patients (34) had recurrence within the high-dose radiation region, three had both in-field and marginal progression, and one had recurrence at the margin.
RT dose was the most important prognostic factor for overall survival and local control. Patients treated with doses higher than the conventional 50.4 Gy had a median survival of 43 months, compared with 23 months for patients treated with doses 50.4 Gy or less (P = .01).
Total biologically effective dose (BED) affected outcomes also. The 2- and 3-year overall survival rates for patients treated with BED greater than 80.5 Gy were both 73%, compared with 58% and 38% for those treated with BED of 80.5 Gy or less.
The treatment was well tolerated, with no cases of radiation induced liver disease observed.
The investigators recommend that higher total RT doses and higher doses delivered per fraction to achieve BED greater than 80.5 Gy should be considered for all patients as long as image guidance is used to ensure that the dose is delivered safely, and dose constraints to the liver, bile duct, stomach, and bowel can be met. The findings support the use of 67.5 Gy in 15 fractions (BED, 97.88 Gy).
Dr. Tao reported having no disclosures. Several of her coauthors reported having ties to industry sources.
Key clinical point: High-radiation doses delivered to hepatic tumors were well tolerated and survival outcomes were comparable to surgical resection.
Major finding: The median survival time was 30 months; 1-, 2-, and 3-year overall survival rates were 87%, 61%, and 44%, respectively.
Data source: From 2002 to 2014, 79 patients with IHCC were treated with definitive radiotherapy at the University of Texas MD Anderson Cancer Center.
Disclosures: Dr. Tao reported having no disclosures. Several of her coauthors reported having ties to industry sources.
Low BMI predicted worse survival in mCRC
In patients with metastatic colorectal cancer (mCRC), body mass index (BMI) was prognostic for overall survival (OS) and progression-free survival (PFS), investigators reported online in Journal of Clinical Oncology.
Risks were highest at the lowest BMI values, decreased as BMI increased to 28 kg/m2, and plateaued at higher BMI values.
By pooling data from more than 21,000 patients enrolled worldwide in 25 randomized trials for frontline treatment, “we have shown that BMI is prognostic for OS and PFS in this population, but with a shape of the risk curve across the BMI spectrum, different than that observed in the adjuvant setting,” wrote Lindsay Renfro, Ph.D., of the Mayo Clinic, Rochester, Minn., and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.6441]).
Patients with a BMI of 18.5 kg/m2 had a 50% increased risk of death (95% confidence interval, 43%-56%). After researchers adjusted for age, sex, performance status, and clinical characteristics, the prognostic significance of BMI remained (P less than .001).
Previous studies showed that obese patients with stage II or III colon cancer were at increased risk for disease recurrence or death, but results of the current study showed obese patients with mCRC were not at increased risk.
Men with low BMIs had a greater risk of death than did women. Both men and women with moderate and higher BMIs had similar risks. Previous studies have shown that the prognosis for women with colorectal cancer is improved over men, possibly because of the protective effect of estrogen.
The results suggest that patients with mCRC and low BMI are likely cachectic, a condition that affects approximately 50% of patients with colon cancer and is associated with a 20% mortality rate, the authors noted.
In patients with metastatic colorectal cancer (mCRC), body mass index (BMI) was prognostic for overall survival (OS) and progression-free survival (PFS), investigators reported online in Journal of Clinical Oncology.
Risks were highest at the lowest BMI values, decreased as BMI increased to 28 kg/m2, and plateaued at higher BMI values.
By pooling data from more than 21,000 patients enrolled worldwide in 25 randomized trials for frontline treatment, “we have shown that BMI is prognostic for OS and PFS in this population, but with a shape of the risk curve across the BMI spectrum, different than that observed in the adjuvant setting,” wrote Lindsay Renfro, Ph.D., of the Mayo Clinic, Rochester, Minn., and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.6441]).
Patients with a BMI of 18.5 kg/m2 had a 50% increased risk of death (95% confidence interval, 43%-56%). After researchers adjusted for age, sex, performance status, and clinical characteristics, the prognostic significance of BMI remained (P less than .001).
Previous studies showed that obese patients with stage II or III colon cancer were at increased risk for disease recurrence or death, but results of the current study showed obese patients with mCRC were not at increased risk.
Men with low BMIs had a greater risk of death than did women. Both men and women with moderate and higher BMIs had similar risks. Previous studies have shown that the prognosis for women with colorectal cancer is improved over men, possibly because of the protective effect of estrogen.
The results suggest that patients with mCRC and low BMI are likely cachectic, a condition that affects approximately 50% of patients with colon cancer and is associated with a 20% mortality rate, the authors noted.
In patients with metastatic colorectal cancer (mCRC), body mass index (BMI) was prognostic for overall survival (OS) and progression-free survival (PFS), investigators reported online in Journal of Clinical Oncology.
Risks were highest at the lowest BMI values, decreased as BMI increased to 28 kg/m2, and plateaued at higher BMI values.
By pooling data from more than 21,000 patients enrolled worldwide in 25 randomized trials for frontline treatment, “we have shown that BMI is prognostic for OS and PFS in this population, but with a shape of the risk curve across the BMI spectrum, different than that observed in the adjuvant setting,” wrote Lindsay Renfro, Ph.D., of the Mayo Clinic, Rochester, Minn., and her colleagues (Jour Clin Onc. 2015 Oct 26 [doi: 10.1200/JCO.2015.61.6441]).
Patients with a BMI of 18.5 kg/m2 had a 50% increased risk of death (95% confidence interval, 43%-56%). After researchers adjusted for age, sex, performance status, and clinical characteristics, the prognostic significance of BMI remained (P less than .001).
Previous studies showed that obese patients with stage II or III colon cancer were at increased risk for disease recurrence or death, but results of the current study showed obese patients with mCRC were not at increased risk.
Men with low BMIs had a greater risk of death than did women. Both men and women with moderate and higher BMIs had similar risks. Previous studies have shown that the prognosis for women with colorectal cancer is improved over men, possibly because of the protective effect of estrogen.
The results suggest that patients with mCRC and low BMI are likely cachectic, a condition that affects approximately 50% of patients with colon cancer and is associated with a 20% mortality rate, the authors noted.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Low BMI predicted worse overall and progression-free survival in patients with mCRC.
Major finding: Risks of death and disease progression were highest for patients with the lowest BMI, decreased as BMI increased to 28 kg/m2, and plateaued at higher BMI values.
Data source: Retrospective analysis of data from 25 first-line clinical trials that included 21,149 patients with metastatic colorectal cancer.
Disclosures: Dr. Renfro reported having no disclosures. Several of her coauthors reported having ties to industry sources.
MINIDEP: A simple, self-administered depression screening tool
Depression is a debilitating illness, and many cases go unrecognized and untreated. There are several depression inventories and questionnaires available for practitioners’ use, but many are long or require a specially trained rater or administrator.1-10
One well-known depression screening questionnaire is the Patient Health Questionnaire (PHQ-9). This instrument is a combination of a 2-item questionnaire and, if the 2-item questionnaire is positive, a 7-item questionnaire.2,3 Even if the PHQ-9 is used, it requires a trained healthcare professional to administer it, limiting its use.
On the other hand, the MINIDEP depression screening tool that I developed can be self-administered by the patient either online or while he (she) is in the waiting room. It can be used by any health care specialist (psychiatrist, psychologist, family practitioner, etc.) as part of the patient’s evaluation.
Unlike most conventional screening questionnaires, MINIDEP has only 7 questions but covers most of the DSM-5 criteria for major depressive disorder. It also includes a question on unexplained pains or aches, which often is the only symptom that patients report, but is absent in the PHQ-9 and in other screening questionnaires.
Having a simple, easy-to-remember mnemonic means that this questionnaire can be used by medical students, residents, allied health and mental health professionals, and primary care physicians to screen for depression in the community.11
MINIDEP Categories/areas of concern addressed
Mood (lowered) and emotional lability.
Interest and desires (anhedonia).
Nutrition, poor appetite, and weight loss or gain.
Insomnia or hypersomnia.
Death or dying (thinking of), feeling worthless or guilty, or making suicidal plans.
Energy (decreased), impaired daily activities, and worsened cognitive ability.
Pains and aches (in absence of unexplained medical illnesses).
I propose rating scores for this questionnaire (Figure) as follows:
0 to 3 Points: Patient is not clinically depressed. Evaluation by a mental health professional might be unnecessary.
4 to 9 Pointsa: Depression is suspected. Further evaluation by a mental health professional (not necessarily a psychiatrist) is warranted.
aThorough psychiatric evaluation also is warranted if the patient has scored 4 to 9 points, with at least 1 point from Question 5.
≥10 points: Depression is confirmed. The patient should be evaluated by a psychiatrist for suicidal thoughts.
Note that this proposed rating scale is based on my experience, although I believe it could be useful. To increase this screening tool’s sensitivity, in my experience, evaluation by a mental health professional might be necessary when a patient scores only 3 points on MINIDEP. The optimal number of points for triggering a clinical decision and this questionnaire’s sensitivity and specificity, however, need to be studied.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Depression in adults: screening. U.S. Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/depressionin-adults-screening. Updated July 2015. Accessed October 2, 2015.
2. Patient Health Questionnaire (PHQ-9). U.S. Preventive Services Task Force. http://www.integration.samhsa.gov/images/res/PHQ%20-%20Questions.pdf. Published October 4, 2005. Accessed September 30, 2015.
3. Patient Health Questionnaire (PHQ-9 & PHQ-2). American Psychological Association. http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/patient-health.aspx. Accessed October 2, 2015.
4. Online assessment measures. American Psychiatric Association. http://www.psychiatry.org/practice/dsm/dsm5/online-assessment-measures#Disorder. Accessed October 2, 2015.
5. Depression screening. Mental Health America. http://www.mentalhealthamerica.net/mental-health-screen/patient-health. Accessed October 2, 2015.
6. Major Depressive Disorder Diagnostic Criteria—SIGE CAPS. Family Medicine Reference. http://www.fammedref.org/mnemonic/major-depressive-disorder-
diagnostic-criteria-sigme-caps. Accessed October2, 2015.
7. Welcome to the Wakefield Self-Report Questionnaire, a screening test for depression. Counselling Resource. http://counsellingresource.com/lib/quizzes/depression-testing/wakefield. Accessed October 2, 2015.
8. Goldberg’s Depression and Mania Self-Rating Scales. Psy-World. http://www.psy-world.com/goldberg.htm. Published 1993. Accessed October 2, 2015.
9. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401.
10. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70.
11. Graypel EA. MINIDEP. http://www.minidep.com. Accessed October 2, 2015.
Depression is a debilitating illness, and many cases go unrecognized and untreated. There are several depression inventories and questionnaires available for practitioners’ use, but many are long or require a specially trained rater or administrator.1-10
One well-known depression screening questionnaire is the Patient Health Questionnaire (PHQ-9). This instrument is a combination of a 2-item questionnaire and, if the 2-item questionnaire is positive, a 7-item questionnaire.2,3 Even if the PHQ-9 is used, it requires a trained healthcare professional to administer it, limiting its use.
On the other hand, the MINIDEP depression screening tool that I developed can be self-administered by the patient either online or while he (she) is in the waiting room. It can be used by any health care specialist (psychiatrist, psychologist, family practitioner, etc.) as part of the patient’s evaluation.
Unlike most conventional screening questionnaires, MINIDEP has only 7 questions but covers most of the DSM-5 criteria for major depressive disorder. It also includes a question on unexplained pains or aches, which often is the only symptom that patients report, but is absent in the PHQ-9 and in other screening questionnaires.
Having a simple, easy-to-remember mnemonic means that this questionnaire can be used by medical students, residents, allied health and mental health professionals, and primary care physicians to screen for depression in the community.11
MINIDEP Categories/areas of concern addressed
Mood (lowered) and emotional lability.
Interest and desires (anhedonia).
Nutrition, poor appetite, and weight loss or gain.
Insomnia or hypersomnia.
Death or dying (thinking of), feeling worthless or guilty, or making suicidal plans.
Energy (decreased), impaired daily activities, and worsened cognitive ability.
Pains and aches (in absence of unexplained medical illnesses).
I propose rating scores for this questionnaire (Figure) as follows:
0 to 3 Points: Patient is not clinically depressed. Evaluation by a mental health professional might be unnecessary.
4 to 9 Pointsa: Depression is suspected. Further evaluation by a mental health professional (not necessarily a psychiatrist) is warranted.
aThorough psychiatric evaluation also is warranted if the patient has scored 4 to 9 points, with at least 1 point from Question 5.
≥10 points: Depression is confirmed. The patient should be evaluated by a psychiatrist for suicidal thoughts.
Note that this proposed rating scale is based on my experience, although I believe it could be useful. To increase this screening tool’s sensitivity, in my experience, evaluation by a mental health professional might be necessary when a patient scores only 3 points on MINIDEP. The optimal number of points for triggering a clinical decision and this questionnaire’s sensitivity and specificity, however, need to be studied.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Depression is a debilitating illness, and many cases go unrecognized and untreated. There are several depression inventories and questionnaires available for practitioners’ use, but many are long or require a specially trained rater or administrator.1-10
One well-known depression screening questionnaire is the Patient Health Questionnaire (PHQ-9). This instrument is a combination of a 2-item questionnaire and, if the 2-item questionnaire is positive, a 7-item questionnaire.2,3 Even if the PHQ-9 is used, it requires a trained healthcare professional to administer it, limiting its use.
On the other hand, the MINIDEP depression screening tool that I developed can be self-administered by the patient either online or while he (she) is in the waiting room. It can be used by any health care specialist (psychiatrist, psychologist, family practitioner, etc.) as part of the patient’s evaluation.
Unlike most conventional screening questionnaires, MINIDEP has only 7 questions but covers most of the DSM-5 criteria for major depressive disorder. It also includes a question on unexplained pains or aches, which often is the only symptom that patients report, but is absent in the PHQ-9 and in other screening questionnaires.
Having a simple, easy-to-remember mnemonic means that this questionnaire can be used by medical students, residents, allied health and mental health professionals, and primary care physicians to screen for depression in the community.11
MINIDEP Categories/areas of concern addressed
Mood (lowered) and emotional lability.
Interest and desires (anhedonia).
Nutrition, poor appetite, and weight loss or gain.
Insomnia or hypersomnia.
Death or dying (thinking of), feeling worthless or guilty, or making suicidal plans.
Energy (decreased), impaired daily activities, and worsened cognitive ability.
Pains and aches (in absence of unexplained medical illnesses).
I propose rating scores for this questionnaire (Figure) as follows:
0 to 3 Points: Patient is not clinically depressed. Evaluation by a mental health professional might be unnecessary.
4 to 9 Pointsa: Depression is suspected. Further evaluation by a mental health professional (not necessarily a psychiatrist) is warranted.
aThorough psychiatric evaluation also is warranted if the patient has scored 4 to 9 points, with at least 1 point from Question 5.
≥10 points: Depression is confirmed. The patient should be evaluated by a psychiatrist for suicidal thoughts.
Note that this proposed rating scale is based on my experience, although I believe it could be useful. To increase this screening tool’s sensitivity, in my experience, evaluation by a mental health professional might be necessary when a patient scores only 3 points on MINIDEP. The optimal number of points for triggering a clinical decision and this questionnaire’s sensitivity and specificity, however, need to be studied.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Depression in adults: screening. U.S. Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/depressionin-adults-screening. Updated July 2015. Accessed October 2, 2015.
2. Patient Health Questionnaire (PHQ-9). U.S. Preventive Services Task Force. http://www.integration.samhsa.gov/images/res/PHQ%20-%20Questions.pdf. Published October 4, 2005. Accessed September 30, 2015.
3. Patient Health Questionnaire (PHQ-9 & PHQ-2). American Psychological Association. http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/patient-health.aspx. Accessed October 2, 2015.
4. Online assessment measures. American Psychiatric Association. http://www.psychiatry.org/practice/dsm/dsm5/online-assessment-measures#Disorder. Accessed October 2, 2015.
5. Depression screening. Mental Health America. http://www.mentalhealthamerica.net/mental-health-screen/patient-health. Accessed October 2, 2015.
6. Major Depressive Disorder Diagnostic Criteria—SIGE CAPS. Family Medicine Reference. http://www.fammedref.org/mnemonic/major-depressive-disorder-
diagnostic-criteria-sigme-caps. Accessed October2, 2015.
7. Welcome to the Wakefield Self-Report Questionnaire, a screening test for depression. Counselling Resource. http://counsellingresource.com/lib/quizzes/depression-testing/wakefield. Accessed October 2, 2015.
8. Goldberg’s Depression and Mania Self-Rating Scales. Psy-World. http://www.psy-world.com/goldberg.htm. Published 1993. Accessed October 2, 2015.
9. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401.
10. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70.
11. Graypel EA. MINIDEP. http://www.minidep.com. Accessed October 2, 2015.
1. Depression in adults: screening. U.S. Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/depressionin-adults-screening. Updated July 2015. Accessed October 2, 2015.
2. Patient Health Questionnaire (PHQ-9). U.S. Preventive Services Task Force. http://www.integration.samhsa.gov/images/res/PHQ%20-%20Questions.pdf. Published October 4, 2005. Accessed September 30, 2015.
3. Patient Health Questionnaire (PHQ-9 & PHQ-2). American Psychological Association. http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/patient-health.aspx. Accessed October 2, 2015.
4. Online assessment measures. American Psychiatric Association. http://www.psychiatry.org/practice/dsm/dsm5/online-assessment-measures#Disorder. Accessed October 2, 2015.
5. Depression screening. Mental Health America. http://www.mentalhealthamerica.net/mental-health-screen/patient-health. Accessed October 2, 2015.
6. Major Depressive Disorder Diagnostic Criteria—SIGE CAPS. Family Medicine Reference. http://www.fammedref.org/mnemonic/major-depressive-disorder-
diagnostic-criteria-sigme-caps. Accessed October2, 2015.
7. Welcome to the Wakefield Self-Report Questionnaire, a screening test for depression. Counselling Resource. http://counsellingresource.com/lib/quizzes/depression-testing/wakefield. Accessed October 2, 2015.
8. Goldberg’s Depression and Mania Self-Rating Scales. Psy-World. http://www.psy-world.com/goldberg.htm. Published 1993. Accessed October 2, 2015.
9. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401.
10. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70.
11. Graypel EA. MINIDEP. http://www.minidep.com. Accessed October 2, 2015.
Venlafaxine discontinuation syndrome: Prevention and management
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Most antidepressants lead to adverse discontinuation symptoms when they are abruptly stopped or rapidly tapered. Antidepressants with a short half-life, such as paroxetine and venlafaxine, can cause significantly more severe discontinuation symptoms compared with antidepressants with a longer half-life.
One culprit in particular
Among serotonin-norepinephrine reuptake inhibitors (SNRIs), venlafaxine is notorious for severe discontinuation symptoms. Venlafaxine has a half-life of 3 to 7 hours, and its active metabolite, desvenlafaxine, possesses a half-life of 9 to 13 hours. Higher frequency of discontinuation symptoms is associated with the use of higher dosages of venlafaxine and longer duration of treatment.
Venlafaxine is available in immediate release (IR) and extended release (XR) formulations. Venlafaxine XR has a slower release, extending the time to peak plasma concentration and, therefore, has once daily dosing and fewer side effects; however, it offers no substantial advantage over IR formulation in terms of diminished withdrawal effects. Desvenlafaxine also is marketed as an antidepressant and, although one can speculate that the drug would have a lower rate of discontinuation symptoms than venlafaxine, no evidence supports this hypothesis.
A range of venlafaxine discontinuation symptoms have been reported (Table).1
Preventing discontinuation symptoms
Patients for whom venlafaxine is prescribed should be informed about discontinuation symptoms, especially those who have a history of noncompliance. Monitor patients closely for discontinuation symptoms when venlafaxine is stopped—even if the patient is switched to another antidepressant. A gradual dosage reduction is recommended rather than abrupt termination or rapid dosage reduction. Immediately switching from venlafaxine to a selective serotonin reuptake inhibitor (SSRI) generally is not recommended, although it could alleviate some discontinuation symptoms2; cross-taper medication over 2 to 3 weeks.
Switching from venlafaxine to another SNRI, such as duloxetine, is less well studied. At venlafaxine dosages of <150 mg/d, an immediate switch to another SNRI of equivalent dosage generally is well-tolerated. For higher dosages, a gradual cross-taper is advised.2
Most patients tolerate a venlafaxine dosage reduction by 75 mg/d, at 1-week intervals. For patients who experience severe discontinuation symptoms with a minor dosage reduction, venlafaxine can be tapered over 10 months with approximately 1% dosage reduction every 3 days. Stahl3 recommends dissolving the tablet in 100 mL of juice, discarding 1 mL, and drinking the rest. After 3 days, 2 mL can be discarded, etc.
Another strategy to prevent discontinuation syndrome is to initiate fluoxetine—an SSRI with a long half-life—before taper; maintain fluoxetine dosage while venlafaxine is tapered; and then taper fluoxetine.
Managing discontinuation symptoms
If your patient experiences significant discontinuation symptoms, resume the last prescribed venlafaxine dosage, with a plan for a more gradual taper. Acute discontinuation syndrome also can be treated by initiating fluoxetine, 10 to 20 mg/d; after symptoms resolve, fluoxetine can be tapered over 2 to 3 weeks.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.
1. Effexor (venlafaxine hydrochloride) [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2012.
2. Hirsch M, Birnbaum RJ. Antidepressant medication in adults: switching and discontinuing medication. http://www.uptodate.com/contents/antidepressant-medicationin-adults-switching-and-discontinuing-medication. Updated January 16, 2015. Accessed October 8, 2015.
3. Stahl SM. Venlafaxine. In: Stahl SM. The prescriber’s guide (Stahl’s essential psychopharmacology). 4th ed. New York, NY: Cambridge University Press; 2011:637-638.