User login
BEST-CLI: New Initiatives, Upcoming Meetings
A number of new initiatives have recently been incorporated into the Best Endovascular versus best Surgical Therapy in patients with Critical Limb Ischemia (BEST-CLI) trial. It is hoped the additions and changes, including a modification of the protocol exclusion criteria, will facilitate subject accrual, an expansion to 140 trial sites and distribution of additional funds to all participating and enrolling centers.
The landmark BEST-CLI trial is a prospective, randomized, pragmatic, open-label superiority trial that is currently enrolling patients at 120 active sites in the United States and Canada. The trial will compare treatment efficacy, functional outcomes and cost in patients with critical limb ischemia who are candidates for both infrainguinal bypass and endovascular therapy and who are randomly assigned to one of the two treatments. Of the target number of 2,100 patients, 295 have been assigned treatments.
Changes include:
- The presence of >50% stenosis of the ipsilateral common femoral artery (CFA) will no longer be an exclusion. Treatment of a severe stenosis or an occlusion of the CFA either by open (surgical endarterectomy and patch angioplasty) or endovascular means will now be allowed and will be irrespective of the randomized treatment of the more distal occlusive disease.
- Immnosuppressive medication, a femoropopliteal TASC II A pattern of disease and hypercoaguagility will no longer be exclusions.
- The required delay following a previous open vascular or endovascular procedure (surgical bypass, balloon angioplasty, atherectomy or stenting) performed on the index limb has been reduced from six to three months. The required delay following a previous surgical inflow procedures performed on the index limb has been reduced from six months to six weeks.
- Treatment of an aortic or ipsilateral iliac artery occlusion will now be allowed.
- The protocol changes serve to bring the trial criteria more in line with common practice, while not altering to any significant degree the study aims.
The National Heart Lung and Blood Institute is sponsoring the trial, designed to address a number of questions of ongoing clinical interest. It aspires to significantly impact clinical practice by defining an evidence-based standard of care for patients with CLI. Recent changes in the trial design should serve to facilitate patient enrollment. Vascular surgeons participating are asked to continue their efforts to achieve current enrollment targets. Those not participating who wish to do so are asked to email [email protected].
Drs. Alik Farber (Boston Medical Center), Matthew Menard (Brigham and Women’s Hospital) and Kenneth Rosenfield (Massachusetts General Hospital) are leading the BEST-CLI Trial.
All are invited to upcoming BEST-CLI Trial Investigators Meetings at VIVA (3 to 4:30 p.m. PST on Tuesday, Nov. 3) and VEITH (3 to 4:30 p.m. EST on Friday, Nov 20).
For more information visit www.bestcli.com
A number of new initiatives have recently been incorporated into the Best Endovascular versus best Surgical Therapy in patients with Critical Limb Ischemia (BEST-CLI) trial. It is hoped the additions and changes, including a modification of the protocol exclusion criteria, will facilitate subject accrual, an expansion to 140 trial sites and distribution of additional funds to all participating and enrolling centers.
The landmark BEST-CLI trial is a prospective, randomized, pragmatic, open-label superiority trial that is currently enrolling patients at 120 active sites in the United States and Canada. The trial will compare treatment efficacy, functional outcomes and cost in patients with critical limb ischemia who are candidates for both infrainguinal bypass and endovascular therapy and who are randomly assigned to one of the two treatments. Of the target number of 2,100 patients, 295 have been assigned treatments.
Changes include:
- The presence of >50% stenosis of the ipsilateral common femoral artery (CFA) will no longer be an exclusion. Treatment of a severe stenosis or an occlusion of the CFA either by open (surgical endarterectomy and patch angioplasty) or endovascular means will now be allowed and will be irrespective of the randomized treatment of the more distal occlusive disease.
- Immnosuppressive medication, a femoropopliteal TASC II A pattern of disease and hypercoaguagility will no longer be exclusions.
- The required delay following a previous open vascular or endovascular procedure (surgical bypass, balloon angioplasty, atherectomy or stenting) performed on the index limb has been reduced from six to three months. The required delay following a previous surgical inflow procedures performed on the index limb has been reduced from six months to six weeks.
- Treatment of an aortic or ipsilateral iliac artery occlusion will now be allowed.
- The protocol changes serve to bring the trial criteria more in line with common practice, while not altering to any significant degree the study aims.
The National Heart Lung and Blood Institute is sponsoring the trial, designed to address a number of questions of ongoing clinical interest. It aspires to significantly impact clinical practice by defining an evidence-based standard of care for patients with CLI. Recent changes in the trial design should serve to facilitate patient enrollment. Vascular surgeons participating are asked to continue their efforts to achieve current enrollment targets. Those not participating who wish to do so are asked to email [email protected].
Drs. Alik Farber (Boston Medical Center), Matthew Menard (Brigham and Women’s Hospital) and Kenneth Rosenfield (Massachusetts General Hospital) are leading the BEST-CLI Trial.
All are invited to upcoming BEST-CLI Trial Investigators Meetings at VIVA (3 to 4:30 p.m. PST on Tuesday, Nov. 3) and VEITH (3 to 4:30 p.m. EST on Friday, Nov 20).
For more information visit www.bestcli.com
A number of new initiatives have recently been incorporated into the Best Endovascular versus best Surgical Therapy in patients with Critical Limb Ischemia (BEST-CLI) trial. It is hoped the additions and changes, including a modification of the protocol exclusion criteria, will facilitate subject accrual, an expansion to 140 trial sites and distribution of additional funds to all participating and enrolling centers.
The landmark BEST-CLI trial is a prospective, randomized, pragmatic, open-label superiority trial that is currently enrolling patients at 120 active sites in the United States and Canada. The trial will compare treatment efficacy, functional outcomes and cost in patients with critical limb ischemia who are candidates for both infrainguinal bypass and endovascular therapy and who are randomly assigned to one of the two treatments. Of the target number of 2,100 patients, 295 have been assigned treatments.
Changes include:
- The presence of >50% stenosis of the ipsilateral common femoral artery (CFA) will no longer be an exclusion. Treatment of a severe stenosis or an occlusion of the CFA either by open (surgical endarterectomy and patch angioplasty) or endovascular means will now be allowed and will be irrespective of the randomized treatment of the more distal occlusive disease.
- Immnosuppressive medication, a femoropopliteal TASC II A pattern of disease and hypercoaguagility will no longer be exclusions.
- The required delay following a previous open vascular or endovascular procedure (surgical bypass, balloon angioplasty, atherectomy or stenting) performed on the index limb has been reduced from six to three months. The required delay following a previous surgical inflow procedures performed on the index limb has been reduced from six months to six weeks.
- Treatment of an aortic or ipsilateral iliac artery occlusion will now be allowed.
- The protocol changes serve to bring the trial criteria more in line with common practice, while not altering to any significant degree the study aims.
The National Heart Lung and Blood Institute is sponsoring the trial, designed to address a number of questions of ongoing clinical interest. It aspires to significantly impact clinical practice by defining an evidence-based standard of care for patients with CLI. Recent changes in the trial design should serve to facilitate patient enrollment. Vascular surgeons participating are asked to continue their efforts to achieve current enrollment targets. Those not participating who wish to do so are asked to email [email protected].
Drs. Alik Farber (Boston Medical Center), Matthew Menard (Brigham and Women’s Hospital) and Kenneth Rosenfield (Massachusetts General Hospital) are leading the BEST-CLI Trial.
All are invited to upcoming BEST-CLI Trial Investigators Meetings at VIVA (3 to 4:30 p.m. PST on Tuesday, Nov. 3) and VEITH (3 to 4:30 p.m. EST on Friday, Nov 20).
For more information visit www.bestcli.com
PTSD in Women and Men
Beginning in September, Federal Practitioner will focus on female health and women service members, including interviews with authors of chapters from Women at War. For more, visit www.fedprac.com/articles/women-at-war.html.
In addition to coediting the Women at War volume, Dr. Ritchie authored the chapter, “Female Soldiers and Post-Traumatic Stress Disorder” (PTSD). In this interview, she discusses the evolving definition of PTSD, military and civilian research into the condition, its stressors, and how PTSD may differ in women and men.
For a limited time, a discount is being offered to Federal Practitioner readers. Click here and use the promo code AMPROMD9 at checkout.
Beginning in September, Federal Practitioner will focus on female health and women service members, including interviews with authors of chapters from Women at War. For more, visit www.fedprac.com/articles/women-at-war.html.
In addition to coediting the Women at War volume, Dr. Ritchie authored the chapter, “Female Soldiers and Post-Traumatic Stress Disorder” (PTSD). In this interview, she discusses the evolving definition of PTSD, military and civilian research into the condition, its stressors, and how PTSD may differ in women and men.
For a limited time, a discount is being offered to Federal Practitioner readers. Click here and use the promo code AMPROMD9 at checkout.
Beginning in September, Federal Practitioner will focus on female health and women service members, including interviews with authors of chapters from Women at War. For more, visit www.fedprac.com/articles/women-at-war.html.
In addition to coediting the Women at War volume, Dr. Ritchie authored the chapter, “Female Soldiers and Post-Traumatic Stress Disorder” (PTSD). In this interview, she discusses the evolving definition of PTSD, military and civilian research into the condition, its stressors, and how PTSD may differ in women and men.
For a limited time, a discount is being offered to Federal Practitioner readers. Click here and use the promo code AMPROMD9 at checkout.
How a cancer diagnosis affects income

receiving treatment
Photo by Rhoda Baer
A new study indicates that when American adults are diagnosed with
cancer, they experience significant decreases in the probability of
working, in the number of hours they work, and correspondingly, in their
incomes.
Additionally, these effects appear to be more pronounced among men than women.
Anna Zajacova, PhD, of the University of Wyoming in Laramie, and her colleagues reported these findings in Cancer.
The researchers analyzed data from the Panel Study of Income Dynamics, a nationally representative, prospective, population-based, observational study with individual and family level economic information. The data spanned the period from 1999 to 2009.
The team used models to estimate the impact of cancer on employment, hours worked, individual income, and total family income.
The results showed that, after a cancer diagnosis, the probability of a patient being employed dropped by almost 10 percentage points, and hours worked declined by up to 200 hours, or about 5 weeks of full-time work, in the first year.
Annual labor market earnings dropped almost 40% within 2 years of diagnosis, and they remained lower than before diagnosis. Total family income declined by 20%, although it recovered within 4 years of cancer diagnosis.
These effects were primarily driven by losses among male cancer survivors. For women diagnosed with cancer, the losses were largely not statistically significant.
“Fifteen million American adults are cancer survivors, and American families need economic support while they are dealing with the rigors of cancer treatment,” Dr Zajacova said.
“Our paper suggests that families where an adult—especially a working-age male—is diagnosed with cancer suffer short-term and long-term declines in their economic well-being. We need to improve workplace and insurance safety nets so families can focus on dealing with the cancer treatment rather than deal with the financial and employment fallout.” ![]()

receiving treatment
Photo by Rhoda Baer
A new study indicates that when American adults are diagnosed with
cancer, they experience significant decreases in the probability of
working, in the number of hours they work, and correspondingly, in their
incomes.
Additionally, these effects appear to be more pronounced among men than women.
Anna Zajacova, PhD, of the University of Wyoming in Laramie, and her colleagues reported these findings in Cancer.
The researchers analyzed data from the Panel Study of Income Dynamics, a nationally representative, prospective, population-based, observational study with individual and family level economic information. The data spanned the period from 1999 to 2009.
The team used models to estimate the impact of cancer on employment, hours worked, individual income, and total family income.
The results showed that, after a cancer diagnosis, the probability of a patient being employed dropped by almost 10 percentage points, and hours worked declined by up to 200 hours, or about 5 weeks of full-time work, in the first year.
Annual labor market earnings dropped almost 40% within 2 years of diagnosis, and they remained lower than before diagnosis. Total family income declined by 20%, although it recovered within 4 years of cancer diagnosis.
These effects were primarily driven by losses among male cancer survivors. For women diagnosed with cancer, the losses were largely not statistically significant.
“Fifteen million American adults are cancer survivors, and American families need economic support while they are dealing with the rigors of cancer treatment,” Dr Zajacova said.
“Our paper suggests that families where an adult—especially a working-age male—is diagnosed with cancer suffer short-term and long-term declines in their economic well-being. We need to improve workplace and insurance safety nets so families can focus on dealing with the cancer treatment rather than deal with the financial and employment fallout.” ![]()

receiving treatment
Photo by Rhoda Baer
A new study indicates that when American adults are diagnosed with
cancer, they experience significant decreases in the probability of
working, in the number of hours they work, and correspondingly, in their
incomes.
Additionally, these effects appear to be more pronounced among men than women.
Anna Zajacova, PhD, of the University of Wyoming in Laramie, and her colleagues reported these findings in Cancer.
The researchers analyzed data from the Panel Study of Income Dynamics, a nationally representative, prospective, population-based, observational study with individual and family level economic information. The data spanned the period from 1999 to 2009.
The team used models to estimate the impact of cancer on employment, hours worked, individual income, and total family income.
The results showed that, after a cancer diagnosis, the probability of a patient being employed dropped by almost 10 percentage points, and hours worked declined by up to 200 hours, or about 5 weeks of full-time work, in the first year.
Annual labor market earnings dropped almost 40% within 2 years of diagnosis, and they remained lower than before diagnosis. Total family income declined by 20%, although it recovered within 4 years of cancer diagnosis.
These effects were primarily driven by losses among male cancer survivors. For women diagnosed with cancer, the losses were largely not statistically significant.
“Fifteen million American adults are cancer survivors, and American families need economic support while they are dealing with the rigors of cancer treatment,” Dr Zajacova said.
“Our paper suggests that families where an adult—especially a working-age male—is diagnosed with cancer suffer short-term and long-term declines in their economic well-being. We need to improve workplace and insurance safety nets so families can focus on dealing with the cancer treatment rather than deal with the financial and employment fallout.” ![]()
How a cancer diagnosis affects income

receiving treatment
Photo by Rhoda Baer
A new study indicates that when American adults are diagnosed with cancer, they experience significant decreases in the probability of working, in the number of hours they work, and correspondingly, in their incomes.
Additionally, these effects appear to be more pronounced among men than women.
Anna Zajacova, PhD, of the University of Wyoming in Laramie, and her colleagues reported these findings in Cancer.
The researchers analyzed data from the Panel Study of Income Dynamics, a nationally representative, prospective, population-based, observational study with individual and family level economic information. The data spanned the period from 1999 to 2009.
The team used models to estimate the impact of cancer on employment, hours worked, individual income, and total family income.
The results showed that, after a cancer diagnosis, the probability of a patient being employed dropped by almost 10 percentage points, and hours worked declined by up to 200 hours, or about 5 weeks of full-time work, in the first year.
Annual labor market earnings dropped almost 40% within 2 years of diagnosis, and they remained lower than before diagnosis. Total family income declined by 20%, although it recovered within 4 years of cancer diagnosis.
These effects were primarily driven by losses among male cancer survivors. For women diagnosed with cancer, the losses were largely not statistically significant.
“Fifteen million American adults are cancer survivors, and American families need economic support while they are dealing with the rigors of cancer treatment,” Dr Zajacova said.
“Our paper suggests that families where an adult—especially a working-age male—is diagnosed with cancer suffer short-term and long-term declines in their economic well-being. We need to improve workplace and insurance safety nets so families can focus on dealing with the cancer treatment rather than deal with the financial and employment fallout.” ![]()

receiving treatment
Photo by Rhoda Baer
A new study indicates that when American adults are diagnosed with cancer, they experience significant decreases in the probability of working, in the number of hours they work, and correspondingly, in their incomes.
Additionally, these effects appear to be more pronounced among men than women.
Anna Zajacova, PhD, of the University of Wyoming in Laramie, and her colleagues reported these findings in Cancer.
The researchers analyzed data from the Panel Study of Income Dynamics, a nationally representative, prospective, population-based, observational study with individual and family level economic information. The data spanned the period from 1999 to 2009.
The team used models to estimate the impact of cancer on employment, hours worked, individual income, and total family income.
The results showed that, after a cancer diagnosis, the probability of a patient being employed dropped by almost 10 percentage points, and hours worked declined by up to 200 hours, or about 5 weeks of full-time work, in the first year.
Annual labor market earnings dropped almost 40% within 2 years of diagnosis, and they remained lower than before diagnosis. Total family income declined by 20%, although it recovered within 4 years of cancer diagnosis.
These effects were primarily driven by losses among male cancer survivors. For women diagnosed with cancer, the losses were largely not statistically significant.
“Fifteen million American adults are cancer survivors, and American families need economic support while they are dealing with the rigors of cancer treatment,” Dr Zajacova said.
“Our paper suggests that families where an adult—especially a working-age male—is diagnosed with cancer suffer short-term and long-term declines in their economic well-being. We need to improve workplace and insurance safety nets so families can focus on dealing with the cancer treatment rather than deal with the financial and employment fallout.” ![]()

receiving treatment
Photo by Rhoda Baer
A new study indicates that when American adults are diagnosed with cancer, they experience significant decreases in the probability of working, in the number of hours they work, and correspondingly, in their incomes.
Additionally, these effects appear to be more pronounced among men than women.
Anna Zajacova, PhD, of the University of Wyoming in Laramie, and her colleagues reported these findings in Cancer.
The researchers analyzed data from the Panel Study of Income Dynamics, a nationally representative, prospective, population-based, observational study with individual and family level economic information. The data spanned the period from 1999 to 2009.
The team used models to estimate the impact of cancer on employment, hours worked, individual income, and total family income.
The results showed that, after a cancer diagnosis, the probability of a patient being employed dropped by almost 10 percentage points, and hours worked declined by up to 200 hours, or about 5 weeks of full-time work, in the first year.
Annual labor market earnings dropped almost 40% within 2 years of diagnosis, and they remained lower than before diagnosis. Total family income declined by 20%, although it recovered within 4 years of cancer diagnosis.
These effects were primarily driven by losses among male cancer survivors. For women diagnosed with cancer, the losses were largely not statistically significant.
“Fifteen million American adults are cancer survivors, and American families need economic support while they are dealing with the rigors of cancer treatment,” Dr Zajacova said.
“Our paper suggests that families where an adult—especially a working-age male—is diagnosed with cancer suffer short-term and long-term declines in their economic well-being. We need to improve workplace and insurance safety nets so families can focus on dealing with the cancer treatment rather than deal with the financial and employment fallout.” ![]()
Group identifies potential target for AML
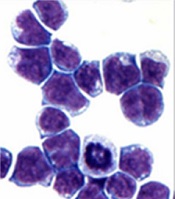
marrow cells generated by AE
Image courtesy of
The Rockefeller University
Preclinical research has revealed a potential therapeutic target for acute myeloid leukemia (AML)—the histone demethylase JMJD1C.
Investigators found that JMJD1C interacts with RUNX1–RUNX1T1 (formerly AML1-ETO and abbreviated here as AE), a transcription factor generated by the t(8;21) translocation in AML.
However, the team also found that JMJD1C is required for proliferation in many AML cell lines, not just those with AE.
Robert G. Roeder, PhD, of The Rockefeller University in New York, New York, and his colleagues detailed these findings in Genes and Development.
The investigators began this study by searching for proteins that interact with AE, and they identified JMJD1C. To investigate the relationship between JMJD1C and AE, the team explored the broader effects of removing JMJD1C.
“We found that numerous genes were downregulated upon loss of JMJD1C, and the set overlaps significantly with the genes that are normally activated by AE,” said study author Mo Chen, PhD, a researcher in Dr Roeder’s lab.
The investigators also found that AML cells are addicted to the presence of JMJD1C, and, without it, they cannot survive. The team observed an increase in apoptosis when JMJD1C was depleted from Kasumi-1 and SKNO-1 cell lines.
With subsequent experiments, the investigators confirmed that JMJD1C interacts with AE and demonstrated that JMJD1C is required for AE to exert its cancer-promoting effects. But they also found that JMJD1C plays an even broader role in AML.
“We were very surprised to find that JMJD1C is required for the proliferation of other acute myeloid leukemia cell lines, which do not have AE,” Dr Chen said.
The team found that depleting JMJD1C compromised the growth of the following cell lines: Kasumi-1 (AML1-ETO; AML M2), MOLM-13 (MLL-AF9, FLT3ITD; AML M5a), THP-1 (MLL-AF9, NRASmut; AML M5), HNT-34 (BCR-ABL1; AML M4), MV4-11 (MLL-AF4; AML M5), CMK (JAK3A527V; AML M7+ Down’s Syndrome), HEL (AML M6), NOMO-1 (MLL-AF9; AML M5a), NB4 (PML-RARα; AML M3), and HL-60 (MYC amplification; AML M2).
The only cell line that was not affected by depletion of JMJD1C was KG-1 (NRASmut; AML).
To build upon this discovery, the investigators looked for transcription factors aside from AE that might be responsible for JMJD1C addiction. The team found at least 2—LYL1 and HEB—that can recruit JMJD1C to target genes in diseased cells that lack AE, fueling leukemia growth.
The investigators said these results suggest JMJD1C may play a general role in promoting the growth of myeloid leukemias.
“We are excited because this type of general phenomena is an ideal target for drug development,” Dr Roeder said. “Our work will facilitate the development of selective inhibitors against JMJD1C, which is a highly promising therapeutic target for multiple types of leukemia.” ![]()

marrow cells generated by AE
Image courtesy of
The Rockefeller University
Preclinical research has revealed a potential therapeutic target for acute myeloid leukemia (AML)—the histone demethylase JMJD1C.
Investigators found that JMJD1C interacts with RUNX1–RUNX1T1 (formerly AML1-ETO and abbreviated here as AE), a transcription factor generated by the t(8;21) translocation in AML.
However, the team also found that JMJD1C is required for proliferation in many AML cell lines, not just those with AE.
Robert G. Roeder, PhD, of The Rockefeller University in New York, New York, and his colleagues detailed these findings in Genes and Development.
The investigators began this study by searching for proteins that interact with AE, and they identified JMJD1C. To investigate the relationship between JMJD1C and AE, the team explored the broader effects of removing JMJD1C.
“We found that numerous genes were downregulated upon loss of JMJD1C, and the set overlaps significantly with the genes that are normally activated by AE,” said study author Mo Chen, PhD, a researcher in Dr Roeder’s lab.
The investigators also found that AML cells are addicted to the presence of JMJD1C, and, without it, they cannot survive. The team observed an increase in apoptosis when JMJD1C was depleted from Kasumi-1 and SKNO-1 cell lines.
With subsequent experiments, the investigators confirmed that JMJD1C interacts with AE and demonstrated that JMJD1C is required for AE to exert its cancer-promoting effects. But they also found that JMJD1C plays an even broader role in AML.
“We were very surprised to find that JMJD1C is required for the proliferation of other acute myeloid leukemia cell lines, which do not have AE,” Dr Chen said.
The team found that depleting JMJD1C compromised the growth of the following cell lines: Kasumi-1 (AML1-ETO; AML M2), MOLM-13 (MLL-AF9, FLT3ITD; AML M5a), THP-1 (MLL-AF9, NRASmut; AML M5), HNT-34 (BCR-ABL1; AML M4), MV4-11 (MLL-AF4; AML M5), CMK (JAK3A527V; AML M7+ Down’s Syndrome), HEL (AML M6), NOMO-1 (MLL-AF9; AML M5a), NB4 (PML-RARα; AML M3), and HL-60 (MYC amplification; AML M2).
The only cell line that was not affected by depletion of JMJD1C was KG-1 (NRASmut; AML).
To build upon this discovery, the investigators looked for transcription factors aside from AE that might be responsible for JMJD1C addiction. The team found at least 2—LYL1 and HEB—that can recruit JMJD1C to target genes in diseased cells that lack AE, fueling leukemia growth.
The investigators said these results suggest JMJD1C may play a general role in promoting the growth of myeloid leukemias.
“We are excited because this type of general phenomena is an ideal target for drug development,” Dr Roeder said. “Our work will facilitate the development of selective inhibitors against JMJD1C, which is a highly promising therapeutic target for multiple types of leukemia.” ![]()

marrow cells generated by AE
Image courtesy of
The Rockefeller University
Preclinical research has revealed a potential therapeutic target for acute myeloid leukemia (AML)—the histone demethylase JMJD1C.
Investigators found that JMJD1C interacts with RUNX1–RUNX1T1 (formerly AML1-ETO and abbreviated here as AE), a transcription factor generated by the t(8;21) translocation in AML.
However, the team also found that JMJD1C is required for proliferation in many AML cell lines, not just those with AE.
Robert G. Roeder, PhD, of The Rockefeller University in New York, New York, and his colleagues detailed these findings in Genes and Development.
The investigators began this study by searching for proteins that interact with AE, and they identified JMJD1C. To investigate the relationship between JMJD1C and AE, the team explored the broader effects of removing JMJD1C.
“We found that numerous genes were downregulated upon loss of JMJD1C, and the set overlaps significantly with the genes that are normally activated by AE,” said study author Mo Chen, PhD, a researcher in Dr Roeder’s lab.
The investigators also found that AML cells are addicted to the presence of JMJD1C, and, without it, they cannot survive. The team observed an increase in apoptosis when JMJD1C was depleted from Kasumi-1 and SKNO-1 cell lines.
With subsequent experiments, the investigators confirmed that JMJD1C interacts with AE and demonstrated that JMJD1C is required for AE to exert its cancer-promoting effects. But they also found that JMJD1C plays an even broader role in AML.
“We were very surprised to find that JMJD1C is required for the proliferation of other acute myeloid leukemia cell lines, which do not have AE,” Dr Chen said.
The team found that depleting JMJD1C compromised the growth of the following cell lines: Kasumi-1 (AML1-ETO; AML M2), MOLM-13 (MLL-AF9, FLT3ITD; AML M5a), THP-1 (MLL-AF9, NRASmut; AML M5), HNT-34 (BCR-ABL1; AML M4), MV4-11 (MLL-AF4; AML M5), CMK (JAK3A527V; AML M7+ Down’s Syndrome), HEL (AML M6), NOMO-1 (MLL-AF9; AML M5a), NB4 (PML-RARα; AML M3), and HL-60 (MYC amplification; AML M2).
The only cell line that was not affected by depletion of JMJD1C was KG-1 (NRASmut; AML).
To build upon this discovery, the investigators looked for transcription factors aside from AE that might be responsible for JMJD1C addiction. The team found at least 2—LYL1 and HEB—that can recruit JMJD1C to target genes in diseased cells that lack AE, fueling leukemia growth.
The investigators said these results suggest JMJD1C may play a general role in promoting the growth of myeloid leukemias.
“We are excited because this type of general phenomena is an ideal target for drug development,” Dr Roeder said. “Our work will facilitate the development of selective inhibitors against JMJD1C, which is a highly promising therapeutic target for multiple types of leukemia.” ![]()
TCT: Immobilized leaflets on bioprosthetic aortic valves trigger concern
SAN FRANCISCO – The newly discovered issue of reduced leaflet motion and possible thrombus on bioprosthetic aortic heart valves, called by one expert “an imaging observation of uncertain clinical significance,” nonetheless drew lots of attention at the Transcatheter Cardiovascular Therapeutics annual meeting. Reduced leaflet motion was the focus of the meeting’s opening session as well as a specially scheduled press conference.
Much of the attention dealt with clarifying the situation and calling for calm after patient concerns were aroused by a report on Oct. 5 that examination of detailed CT scans from small series of patients who had recently undergone aortic valve replacement showed reduced-motion or immobilized valve leaflets on some of the bioprosthetic valves. The pattern of the finding, made using four-dimensional CT imaging, indicated that reduced-motion leaflets did not occur, and possibly even resolved, when patients were on anticoagulant therapy, suggesting that leaflet immobilization involved thrombus. Also, reduced-motion leaflets appeared following both transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), said Dr. Raj R. Makkar.
Dr. Makkar summarized his CT findings in several talks during the meeting and also in a report published a few days before the meeting (N Engl J Med. 2015 Oct 5. doi: 10.1056/NEJMoa1509233).
“We started with what we thought was an imaging artifact and established that is it real. We also established with reasonable certainty that it is related to thrombus,” said Dr. Makkar, professor at the University of California, Los Angeles, and director of the Cardiovascular Interventional Center at Cedars-Sinai Medical Center in Los Angeles. The evidence also indicates that this is a class effect that occurs with all types of TAVR systems as well as surgically placed valves.
What the evidence so far does not indicate is that patients with reduced-motion leaflets face any clinical consequence nor need for routine CT imaging of a newly-placed TAVR or SAVR valve. Also no need for routine anticoagulant therapy instead of standard treatment with dual antiplatelet therapy for several months following placement of a bioprosthetic aortic valve. “We should not make the leap that following TAVR, everyone should be on an anticoagulant” because anticoagulant treatment carries it own risks, said Dr. Makkar, who noted that roughly a quarter of TAVR patients receive anticoagulant treatment because of another indication, such as atrial fibrillation.
“The study did not show a temporal or causal relationship between the imaging findings and stroke. That needs emphasis,” commented Dr. Susheel Kodali, codirector of the Heart Valve Center at the Center for Interventional Vascular Therapy at Columbia University in New York. The possible link between leaflet immobility and strokes or other neurologic events “warrants further study,” as the data that Dr. Makkar reported involved a total of only six strokes or transient ischemic attacks. Data from all the TAVR trials and registries reported so far showed “no late signal of stroke,” said Dr. Kodali, who added that SAVR had a 30-year record of net benefit for appropriate patients.
“Is valve-leaflet thickening an important controversy or much ado about nothing?” wondered Dr. Martin B. Leon, director of the Center for Interventional Vascular Therapy of Columbia University.
“Patients should not feel at risk, and there is no need to do anything differently” for the time being in routine practice, commented Dr. Jeffrey J. Popma, professor at Harvard Medical School and director of interventional cardiology at Beth Israel Deaconess Medical Center, both in Boston.
Dr. Makkar said that in the days following the publication of his report, he had “a lot of phone calls and time spent allaying anxiety in patients and reassuring them.”
One reason why these leaflet-motion abnormalities may have shown up on CT examinations recently is that “the cameras have gotten better,” said Dr. Jonathon A. Leipsic, codirector of advanced cardiac imaging at the Providence Health Care Heart Center at St. Paul’s Hospital in Vancouver. Dr. Leipsic also highlighted that with state-of-the-art CT images, immobilized leaflets are easy to identify.
Despite that, Dr. Popma stressed that standardized imaging protocols are needed going forward to produce reliable incidence data.
The data that Dr. Makkar reported came from a review of four-dimensional CT imaging done on 187 replacement aortic valves, usually within 3 months of placement. Images for 55 aortic valves came from the device-approval trial for a new TAVR system, taken 30 days after patients underwent TAVR with any of three types of systems. The images showed reduced leaflet motion in 22 valves (40%).
CT images for another 132 valves came from a Cedar’s-Sinai registry and a second, independent registry maintained in Denmark. CT images showed that 17 (13%) of the replaced aortic valves showed a leaflet-motion abnormality, including two valves placed using SAVR. Half the registry patients had undergone CT imaging within 88 days of valve replacement. The only signal of a clinical outcome linked with reduced-motion leaflets was a small increase in the incidence of transient ischemic attacks, but Dr. Makkar cautioned that transient ischemic attacks “are hard to adjudicate.”
Dr. Makkar’s report was “a small but important study, one of the first reports of this phenomenon. You don’t want to lose sight of all the evidence of patient benefit” from aortic valve replacement, stressed Dr. Kodali at the meeting, sponsored by the Cardiovascular Research Foundation. “This needs to be investigated further, probably by a Food and Drug Administration–mandated trial with CT imaging.”
“Aortic valves are lifesaving devices. The last thing that should happen is patients not getting their aortic valves replaced” when their condition demands it, Dr. Makkar said.
The PORTICO IDE study and RESOLVE registry were funded by St. Jude. Dr. Makkar has received honoraria and research support from St. Jude, lecture fees from Edwards Lifesciences, research grants from Edwards and Medtronic, and has an equity interest in Entourage. Dr. Kodali has been a consultant to Edwards Lifesciences and Claret Medical and has an equity interest in Thubrikar Aortic Valve. Dr. Leon has been a consultant to Edwards. Dr. Popma has been a consultant to Abbott Laboratories, Boston Scientific, and St. Jude, and he has been a speaker for and received grants from Medtronic, Dr. Leipsic has been a consultant to Edwards and Heartflow and received grants from Edwards, Neovasc, and Tendyne.
On Twitter @mitchelzoler
SAN FRANCISCO – The newly discovered issue of reduced leaflet motion and possible thrombus on bioprosthetic aortic heart valves, called by one expert “an imaging observation of uncertain clinical significance,” nonetheless drew lots of attention at the Transcatheter Cardiovascular Therapeutics annual meeting. Reduced leaflet motion was the focus of the meeting’s opening session as well as a specially scheduled press conference.
Much of the attention dealt with clarifying the situation and calling for calm after patient concerns were aroused by a report on Oct. 5 that examination of detailed CT scans from small series of patients who had recently undergone aortic valve replacement showed reduced-motion or immobilized valve leaflets on some of the bioprosthetic valves. The pattern of the finding, made using four-dimensional CT imaging, indicated that reduced-motion leaflets did not occur, and possibly even resolved, when patients were on anticoagulant therapy, suggesting that leaflet immobilization involved thrombus. Also, reduced-motion leaflets appeared following both transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), said Dr. Raj R. Makkar.
Dr. Makkar summarized his CT findings in several talks during the meeting and also in a report published a few days before the meeting (N Engl J Med. 2015 Oct 5. doi: 10.1056/NEJMoa1509233).
“We started with what we thought was an imaging artifact and established that is it real. We also established with reasonable certainty that it is related to thrombus,” said Dr. Makkar, professor at the University of California, Los Angeles, and director of the Cardiovascular Interventional Center at Cedars-Sinai Medical Center in Los Angeles. The evidence also indicates that this is a class effect that occurs with all types of TAVR systems as well as surgically placed valves.
What the evidence so far does not indicate is that patients with reduced-motion leaflets face any clinical consequence nor need for routine CT imaging of a newly-placed TAVR or SAVR valve. Also no need for routine anticoagulant therapy instead of standard treatment with dual antiplatelet therapy for several months following placement of a bioprosthetic aortic valve. “We should not make the leap that following TAVR, everyone should be on an anticoagulant” because anticoagulant treatment carries it own risks, said Dr. Makkar, who noted that roughly a quarter of TAVR patients receive anticoagulant treatment because of another indication, such as atrial fibrillation.
“The study did not show a temporal or causal relationship between the imaging findings and stroke. That needs emphasis,” commented Dr. Susheel Kodali, codirector of the Heart Valve Center at the Center for Interventional Vascular Therapy at Columbia University in New York. The possible link between leaflet immobility and strokes or other neurologic events “warrants further study,” as the data that Dr. Makkar reported involved a total of only six strokes or transient ischemic attacks. Data from all the TAVR trials and registries reported so far showed “no late signal of stroke,” said Dr. Kodali, who added that SAVR had a 30-year record of net benefit for appropriate patients.
“Is valve-leaflet thickening an important controversy or much ado about nothing?” wondered Dr. Martin B. Leon, director of the Center for Interventional Vascular Therapy of Columbia University.
“Patients should not feel at risk, and there is no need to do anything differently” for the time being in routine practice, commented Dr. Jeffrey J. Popma, professor at Harvard Medical School and director of interventional cardiology at Beth Israel Deaconess Medical Center, both in Boston.
Dr. Makkar said that in the days following the publication of his report, he had “a lot of phone calls and time spent allaying anxiety in patients and reassuring them.”
One reason why these leaflet-motion abnormalities may have shown up on CT examinations recently is that “the cameras have gotten better,” said Dr. Jonathon A. Leipsic, codirector of advanced cardiac imaging at the Providence Health Care Heart Center at St. Paul’s Hospital in Vancouver. Dr. Leipsic also highlighted that with state-of-the-art CT images, immobilized leaflets are easy to identify.
Despite that, Dr. Popma stressed that standardized imaging protocols are needed going forward to produce reliable incidence data.
The data that Dr. Makkar reported came from a review of four-dimensional CT imaging done on 187 replacement aortic valves, usually within 3 months of placement. Images for 55 aortic valves came from the device-approval trial for a new TAVR system, taken 30 days after patients underwent TAVR with any of three types of systems. The images showed reduced leaflet motion in 22 valves (40%).
CT images for another 132 valves came from a Cedar’s-Sinai registry and a second, independent registry maintained in Denmark. CT images showed that 17 (13%) of the replaced aortic valves showed a leaflet-motion abnormality, including two valves placed using SAVR. Half the registry patients had undergone CT imaging within 88 days of valve replacement. The only signal of a clinical outcome linked with reduced-motion leaflets was a small increase in the incidence of transient ischemic attacks, but Dr. Makkar cautioned that transient ischemic attacks “are hard to adjudicate.”
Dr. Makkar’s report was “a small but important study, one of the first reports of this phenomenon. You don’t want to lose sight of all the evidence of patient benefit” from aortic valve replacement, stressed Dr. Kodali at the meeting, sponsored by the Cardiovascular Research Foundation. “This needs to be investigated further, probably by a Food and Drug Administration–mandated trial with CT imaging.”
“Aortic valves are lifesaving devices. The last thing that should happen is patients not getting their aortic valves replaced” when their condition demands it, Dr. Makkar said.
The PORTICO IDE study and RESOLVE registry were funded by St. Jude. Dr. Makkar has received honoraria and research support from St. Jude, lecture fees from Edwards Lifesciences, research grants from Edwards and Medtronic, and has an equity interest in Entourage. Dr. Kodali has been a consultant to Edwards Lifesciences and Claret Medical and has an equity interest in Thubrikar Aortic Valve. Dr. Leon has been a consultant to Edwards. Dr. Popma has been a consultant to Abbott Laboratories, Boston Scientific, and St. Jude, and he has been a speaker for and received grants from Medtronic, Dr. Leipsic has been a consultant to Edwards and Heartflow and received grants from Edwards, Neovasc, and Tendyne.
On Twitter @mitchelzoler
SAN FRANCISCO – The newly discovered issue of reduced leaflet motion and possible thrombus on bioprosthetic aortic heart valves, called by one expert “an imaging observation of uncertain clinical significance,” nonetheless drew lots of attention at the Transcatheter Cardiovascular Therapeutics annual meeting. Reduced leaflet motion was the focus of the meeting’s opening session as well as a specially scheduled press conference.
Much of the attention dealt with clarifying the situation and calling for calm after patient concerns were aroused by a report on Oct. 5 that examination of detailed CT scans from small series of patients who had recently undergone aortic valve replacement showed reduced-motion or immobilized valve leaflets on some of the bioprosthetic valves. The pattern of the finding, made using four-dimensional CT imaging, indicated that reduced-motion leaflets did not occur, and possibly even resolved, when patients were on anticoagulant therapy, suggesting that leaflet immobilization involved thrombus. Also, reduced-motion leaflets appeared following both transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), said Dr. Raj R. Makkar.
Dr. Makkar summarized his CT findings in several talks during the meeting and also in a report published a few days before the meeting (N Engl J Med. 2015 Oct 5. doi: 10.1056/NEJMoa1509233).
“We started with what we thought was an imaging artifact and established that is it real. We also established with reasonable certainty that it is related to thrombus,” said Dr. Makkar, professor at the University of California, Los Angeles, and director of the Cardiovascular Interventional Center at Cedars-Sinai Medical Center in Los Angeles. The evidence also indicates that this is a class effect that occurs with all types of TAVR systems as well as surgically placed valves.
What the evidence so far does not indicate is that patients with reduced-motion leaflets face any clinical consequence nor need for routine CT imaging of a newly-placed TAVR or SAVR valve. Also no need for routine anticoagulant therapy instead of standard treatment with dual antiplatelet therapy for several months following placement of a bioprosthetic aortic valve. “We should not make the leap that following TAVR, everyone should be on an anticoagulant” because anticoagulant treatment carries it own risks, said Dr. Makkar, who noted that roughly a quarter of TAVR patients receive anticoagulant treatment because of another indication, such as atrial fibrillation.
“The study did not show a temporal or causal relationship between the imaging findings and stroke. That needs emphasis,” commented Dr. Susheel Kodali, codirector of the Heart Valve Center at the Center for Interventional Vascular Therapy at Columbia University in New York. The possible link between leaflet immobility and strokes or other neurologic events “warrants further study,” as the data that Dr. Makkar reported involved a total of only six strokes or transient ischemic attacks. Data from all the TAVR trials and registries reported so far showed “no late signal of stroke,” said Dr. Kodali, who added that SAVR had a 30-year record of net benefit for appropriate patients.
“Is valve-leaflet thickening an important controversy or much ado about nothing?” wondered Dr. Martin B. Leon, director of the Center for Interventional Vascular Therapy of Columbia University.
“Patients should not feel at risk, and there is no need to do anything differently” for the time being in routine practice, commented Dr. Jeffrey J. Popma, professor at Harvard Medical School and director of interventional cardiology at Beth Israel Deaconess Medical Center, both in Boston.
Dr. Makkar said that in the days following the publication of his report, he had “a lot of phone calls and time spent allaying anxiety in patients and reassuring them.”
One reason why these leaflet-motion abnormalities may have shown up on CT examinations recently is that “the cameras have gotten better,” said Dr. Jonathon A. Leipsic, codirector of advanced cardiac imaging at the Providence Health Care Heart Center at St. Paul’s Hospital in Vancouver. Dr. Leipsic also highlighted that with state-of-the-art CT images, immobilized leaflets are easy to identify.
Despite that, Dr. Popma stressed that standardized imaging protocols are needed going forward to produce reliable incidence data.
The data that Dr. Makkar reported came from a review of four-dimensional CT imaging done on 187 replacement aortic valves, usually within 3 months of placement. Images for 55 aortic valves came from the device-approval trial for a new TAVR system, taken 30 days after patients underwent TAVR with any of three types of systems. The images showed reduced leaflet motion in 22 valves (40%).
CT images for another 132 valves came from a Cedar’s-Sinai registry and a second, independent registry maintained in Denmark. CT images showed that 17 (13%) of the replaced aortic valves showed a leaflet-motion abnormality, including two valves placed using SAVR. Half the registry patients had undergone CT imaging within 88 days of valve replacement. The only signal of a clinical outcome linked with reduced-motion leaflets was a small increase in the incidence of transient ischemic attacks, but Dr. Makkar cautioned that transient ischemic attacks “are hard to adjudicate.”
Dr. Makkar’s report was “a small but important study, one of the first reports of this phenomenon. You don’t want to lose sight of all the evidence of patient benefit” from aortic valve replacement, stressed Dr. Kodali at the meeting, sponsored by the Cardiovascular Research Foundation. “This needs to be investigated further, probably by a Food and Drug Administration–mandated trial with CT imaging.”
“Aortic valves are lifesaving devices. The last thing that should happen is patients not getting their aortic valves replaced” when their condition demands it, Dr. Makkar said.
The PORTICO IDE study and RESOLVE registry were funded by St. Jude. Dr. Makkar has received honoraria and research support from St. Jude, lecture fees from Edwards Lifesciences, research grants from Edwards and Medtronic, and has an equity interest in Entourage. Dr. Kodali has been a consultant to Edwards Lifesciences and Claret Medical and has an equity interest in Thubrikar Aortic Valve. Dr. Leon has been a consultant to Edwards. Dr. Popma has been a consultant to Abbott Laboratories, Boston Scientific, and St. Jude, and he has been a speaker for and received grants from Medtronic, Dr. Leipsic has been a consultant to Edwards and Heartflow and received grants from Edwards, Neovasc, and Tendyne.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM TCT 2015
Key clinical point: CT imaging of recently placed bioprosthetic aortic valves showed several cases of leaflets with reduced motion, suggesting possible clinical consequences.
Major finding: CT imaging showed reduced leaflet motion in 22 of 55 (40%) trial patients and 17 of 132 (13%) registry patients.
Data source: An observational study of CT images collected on 187 patients who had undergone aortic valve replacement from the PORTICO IDE study (55 patients), and the RESOLVE and SAVORY registries (132 total patients).
Disclosures: The PORTICO IDE study and RESOLVE registry were funded by St. Jude. Dr. Makkar has received honoraria and research support from St. Jude, lecture fees from Edwards Lifesciences, research grants from Edwards and Medtronic, and has an equity interest in Entourage.
WHO recommends pilot projects for malaria vaccine

a malaria-endemic region
Photo by Sarah Mattison
More testing is needed before the malaria vaccine candidate RTS,S/AS01 (Mosquirix) can be put into widespread use, according to a pair of World Health Organization (WHO) advisory committees.
The WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Committee (MPAC) are recommending that RTS,S be introduced in 3 to 5 pilot projects to determine the best way to deliver the vaccine to young children.
The issue, according to the committees, is that the vaccine must be administered in 4 doses and therefore requires repeat contact with the healthcare system.
The first 3 doses are given 1 month apart, and the last dose is given 18 months later. Without the fourth dose, children in a phase 3 study of RTS,S had no overall reduction in severe malaria.
So SAGE and MPAC want to be sure this vaccination schedule is feasible.
“The question about how the malaria vaccine may best be delivered still needs to be answered,” said Jon S. Abramson, chair of SAGE. “After detailed assessment of all the evidence, we recommended that this question is best addressed by having 3 to 5 large pilot implementation projects.”
This could delay widespread implementation of RTS,S for 3 to 5 years. Alternatively, if it is not possible to deliver all 4 doses of RTS,S consistently, Abramson said the vaccine may not be used at all.
RTS,S is being assessed as a complementary malaria control tool that could potentially be added to—but not replace—the core package of proven malaria preventive, diagnostic, and treatment measures.
The vaccine acts against Plasmodium falciparum, the most deadly malaria parasite globally and the most prevalent in Africa.
In a phase 3 trial, young children who received 4 doses of RTS,S had a 36% reduction in the number of clinical episodes of malaria at 4 years. Infants who received 4 doses of RTS,S had a 26% reduction in the number of clinical malaria episodes over 3 years.
Children had a significantly lower incidence of severe malaria only if they received all 4 doses of RTS,S. The vaccine did not confer the same benefit in infants, regardless of the doses given.
Results of a subsequent study suggested that genetic variation influences the vaccine’s ability to ward off malaria in young children but not in infants.
RTS,S was recently granted a positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) via Article 58 of Regulation No 726/2004.
This allows the CHMP, in cooperation with the WHO, to give a scientific opinion on a medicinal product intended for markets outside the European Union. This assessment requires products to meet the same standards as products intended for use in the European Union.
Once the CHMP issued a positive opinion of RTS,S, the WHO began formulating a policy recommendation on use of the vaccine in national immunization programs. RTS,S must pass the WHO pre-qualification process and be approved by national regulatory authorities before it can be used in such programs. ![]()

a malaria-endemic region
Photo by Sarah Mattison
More testing is needed before the malaria vaccine candidate RTS,S/AS01 (Mosquirix) can be put into widespread use, according to a pair of World Health Organization (WHO) advisory committees.
The WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Committee (MPAC) are recommending that RTS,S be introduced in 3 to 5 pilot projects to determine the best way to deliver the vaccine to young children.
The issue, according to the committees, is that the vaccine must be administered in 4 doses and therefore requires repeat contact with the healthcare system.
The first 3 doses are given 1 month apart, and the last dose is given 18 months later. Without the fourth dose, children in a phase 3 study of RTS,S had no overall reduction in severe malaria.
So SAGE and MPAC want to be sure this vaccination schedule is feasible.
“The question about how the malaria vaccine may best be delivered still needs to be answered,” said Jon S. Abramson, chair of SAGE. “After detailed assessment of all the evidence, we recommended that this question is best addressed by having 3 to 5 large pilot implementation projects.”
This could delay widespread implementation of RTS,S for 3 to 5 years. Alternatively, if it is not possible to deliver all 4 doses of RTS,S consistently, Abramson said the vaccine may not be used at all.
RTS,S is being assessed as a complementary malaria control tool that could potentially be added to—but not replace—the core package of proven malaria preventive, diagnostic, and treatment measures.
The vaccine acts against Plasmodium falciparum, the most deadly malaria parasite globally and the most prevalent in Africa.
In a phase 3 trial, young children who received 4 doses of RTS,S had a 36% reduction in the number of clinical episodes of malaria at 4 years. Infants who received 4 doses of RTS,S had a 26% reduction in the number of clinical malaria episodes over 3 years.
Children had a significantly lower incidence of severe malaria only if they received all 4 doses of RTS,S. The vaccine did not confer the same benefit in infants, regardless of the doses given.
Results of a subsequent study suggested that genetic variation influences the vaccine’s ability to ward off malaria in young children but not in infants.
RTS,S was recently granted a positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) via Article 58 of Regulation No 726/2004.
This allows the CHMP, in cooperation with the WHO, to give a scientific opinion on a medicinal product intended for markets outside the European Union. This assessment requires products to meet the same standards as products intended for use in the European Union.
Once the CHMP issued a positive opinion of RTS,S, the WHO began formulating a policy recommendation on use of the vaccine in national immunization programs. RTS,S must pass the WHO pre-qualification process and be approved by national regulatory authorities before it can be used in such programs. ![]()

a malaria-endemic region
Photo by Sarah Mattison
More testing is needed before the malaria vaccine candidate RTS,S/AS01 (Mosquirix) can be put into widespread use, according to a pair of World Health Organization (WHO) advisory committees.
The WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Committee (MPAC) are recommending that RTS,S be introduced in 3 to 5 pilot projects to determine the best way to deliver the vaccine to young children.
The issue, according to the committees, is that the vaccine must be administered in 4 doses and therefore requires repeat contact with the healthcare system.
The first 3 doses are given 1 month apart, and the last dose is given 18 months later. Without the fourth dose, children in a phase 3 study of RTS,S had no overall reduction in severe malaria.
So SAGE and MPAC want to be sure this vaccination schedule is feasible.
“The question about how the malaria vaccine may best be delivered still needs to be answered,” said Jon S. Abramson, chair of SAGE. “After detailed assessment of all the evidence, we recommended that this question is best addressed by having 3 to 5 large pilot implementation projects.”
This could delay widespread implementation of RTS,S for 3 to 5 years. Alternatively, if it is not possible to deliver all 4 doses of RTS,S consistently, Abramson said the vaccine may not be used at all.
RTS,S is being assessed as a complementary malaria control tool that could potentially be added to—but not replace—the core package of proven malaria preventive, diagnostic, and treatment measures.
The vaccine acts against Plasmodium falciparum, the most deadly malaria parasite globally and the most prevalent in Africa.
In a phase 3 trial, young children who received 4 doses of RTS,S had a 36% reduction in the number of clinical episodes of malaria at 4 years. Infants who received 4 doses of RTS,S had a 26% reduction in the number of clinical malaria episodes over 3 years.
Children had a significantly lower incidence of severe malaria only if they received all 4 doses of RTS,S. The vaccine did not confer the same benefit in infants, regardless of the doses given.
Results of a subsequent study suggested that genetic variation influences the vaccine’s ability to ward off malaria in young children but not in infants.
RTS,S was recently granted a positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) via Article 58 of Regulation No 726/2004.
This allows the CHMP, in cooperation with the WHO, to give a scientific opinion on a medicinal product intended for markets outside the European Union. This assessment requires products to meet the same standards as products intended for use in the European Union.
Once the CHMP issued a positive opinion of RTS,S, the WHO began formulating a policy recommendation on use of the vaccine in national immunization programs. RTS,S must pass the WHO pre-qualification process and be approved by national regulatory authorities before it can be used in such programs. ![]()
ACS: Loop ileostomy may give IBD colitis patients an alternative to urgent colectomy
CHICAGO – Diverting loop ileostomy may be a better option than urgent colectomy as the first surgical step for medically refractory severe ulcerative colitis and Crohn’s disease.
Investigators from the University of California, Los Angeles, have found that ileostomy gives patients a chance to recover from their acute illness – and their colons a chance to heal – so they’re in better shape for definitive surgery further down the road, if it’s even needed (J Am Coll Surg. 2015 Oct;221[4]:S37-S38).
“Urgent colectomy is standard practice for medically refractory severe ulcerative and Crohn’s colitis. However, immunosuppression and malnutrition can result in significant morbidity. This change in management strategy does not eliminate the potential need for definitive surgery, but it does allow for the more extensive procedure to be performed in an elective setting under optimized conditions, thereby improving clinical outcomes,” said the investigators, led by Dr. Amy Lightner, formerly of UCLA but now a colorectal surgery fellow at the Mayo Clinic in Rochester, Minn.
There were just eight patients in the series, so the results are tentative. Six had ulcerative colitis (UC) and two had Crohn’s disease (CD). On presentation, the patients were tachycardic, febrile, malnourished, and anemic, with severe mucosal disease confirmed by endoscopy. Steroids, immunomodulators, and biologics no longer helped. Overall, the patients were too sick to go home, but not quite sick enough for the ICU. Their average age was 29 years.
They underwent a single-incision, laparoscopic diverting loop ileostomy, which took about 45 minutes. The technique, and perhaps the thinking behind it, are similar to one gaining popularity for Clostridium difficile colitis, but without the colonic lavage.
Within 24-48 hours postop, tachycardia and fevers resolved, and patients tolerated oral intake. Narcotic use dropped, and bloody stools became less frequent, and then ceased in all but one patient. Within a month, the average hemoglobin level had climbed from a baseline of 9 g/dL to 11.5 g/dL, and average albumin from 2.5 g/dL to 4 g/dL. Within 2 months, patients’ bowels looked pink and healthy on repeat endoscopy.
“It was a remarkable turnaround. Within 48 hours, they looked markedly different. We are having very good results with this, and it’s much better for patients” than is colectomy during acute illness. “It’s a good change in management,” Dr. Lightner said.
After months of follow-up, two patients, one with UC and one with CD, haven’t needed a colectomy and are maintained on biologics. The other UC patients have had ileal pouch-anal anastomosis. The other CD patient had a subsequent ileorectal anastomosis. Patients were able to undergo those procedures laparoscopically and “have done really well,” Dr. Lightner said.
It’s unclear why loop ileostomy seems so helpful. Perhaps it has something to do with shifts in bacterial populations or decompression of the colon. Maybe it’s just about giving the colon a rest, she said.
The investigators will continue to study the approach. Since the initial report, 8 more patients have joined the series, for a current total of 16. “We are still seeing good results,” Dr. Lightner said.
Dr. Lightner has no disclosures, and there was no outside funding for the work.
These patients are challenging. Often, they are on multiple immunomodulators and are malnourished and anemic, with systemic manifestations of inflammatory disease. The abdomen may be hostile. None of these are favorable factors for doing a total abdominal colectomy, but that remains the standard even today.
This is truly a feasibility or pilot study, and as such, it’s difficult to draw definitive conclusions. Cost-effectiveness is unclear, and some patients are maintained on biologics when, in fact, they may have had a curative procedure with surgery. The follow-up isn’t long enough to look at recurrence of colitis. Nevertheless, it certainly is an intriguing and perhaps revolutionary approach to treating these patients.
Dr. Sean C. Glasgow is a colorectal surgeon and assistant professor of surgery at Washington University in St. Louis. He was not involved with the study.
These patients are challenging. Often, they are on multiple immunomodulators and are malnourished and anemic, with systemic manifestations of inflammatory disease. The abdomen may be hostile. None of these are favorable factors for doing a total abdominal colectomy, but that remains the standard even today.
This is truly a feasibility or pilot study, and as such, it’s difficult to draw definitive conclusions. Cost-effectiveness is unclear, and some patients are maintained on biologics when, in fact, they may have had a curative procedure with surgery. The follow-up isn’t long enough to look at recurrence of colitis. Nevertheless, it certainly is an intriguing and perhaps revolutionary approach to treating these patients.
Dr. Sean C. Glasgow is a colorectal surgeon and assistant professor of surgery at Washington University in St. Louis. He was not involved with the study.
These patients are challenging. Often, they are on multiple immunomodulators and are malnourished and anemic, with systemic manifestations of inflammatory disease. The abdomen may be hostile. None of these are favorable factors for doing a total abdominal colectomy, but that remains the standard even today.
This is truly a feasibility or pilot study, and as such, it’s difficult to draw definitive conclusions. Cost-effectiveness is unclear, and some patients are maintained on biologics when, in fact, they may have had a curative procedure with surgery. The follow-up isn’t long enough to look at recurrence of colitis. Nevertheless, it certainly is an intriguing and perhaps revolutionary approach to treating these patients.
Dr. Sean C. Glasgow is a colorectal surgeon and assistant professor of surgery at Washington University in St. Louis. He was not involved with the study.
CHICAGO – Diverting loop ileostomy may be a better option than urgent colectomy as the first surgical step for medically refractory severe ulcerative colitis and Crohn’s disease.
Investigators from the University of California, Los Angeles, have found that ileostomy gives patients a chance to recover from their acute illness – and their colons a chance to heal – so they’re in better shape for definitive surgery further down the road, if it’s even needed (J Am Coll Surg. 2015 Oct;221[4]:S37-S38).
“Urgent colectomy is standard practice for medically refractory severe ulcerative and Crohn’s colitis. However, immunosuppression and malnutrition can result in significant morbidity. This change in management strategy does not eliminate the potential need for definitive surgery, but it does allow for the more extensive procedure to be performed in an elective setting under optimized conditions, thereby improving clinical outcomes,” said the investigators, led by Dr. Amy Lightner, formerly of UCLA but now a colorectal surgery fellow at the Mayo Clinic in Rochester, Minn.
There were just eight patients in the series, so the results are tentative. Six had ulcerative colitis (UC) and two had Crohn’s disease (CD). On presentation, the patients were tachycardic, febrile, malnourished, and anemic, with severe mucosal disease confirmed by endoscopy. Steroids, immunomodulators, and biologics no longer helped. Overall, the patients were too sick to go home, but not quite sick enough for the ICU. Their average age was 29 years.
They underwent a single-incision, laparoscopic diverting loop ileostomy, which took about 45 minutes. The technique, and perhaps the thinking behind it, are similar to one gaining popularity for Clostridium difficile colitis, but without the colonic lavage.
Within 24-48 hours postop, tachycardia and fevers resolved, and patients tolerated oral intake. Narcotic use dropped, and bloody stools became less frequent, and then ceased in all but one patient. Within a month, the average hemoglobin level had climbed from a baseline of 9 g/dL to 11.5 g/dL, and average albumin from 2.5 g/dL to 4 g/dL. Within 2 months, patients’ bowels looked pink and healthy on repeat endoscopy.
“It was a remarkable turnaround. Within 48 hours, they looked markedly different. We are having very good results with this, and it’s much better for patients” than is colectomy during acute illness. “It’s a good change in management,” Dr. Lightner said.
After months of follow-up, two patients, one with UC and one with CD, haven’t needed a colectomy and are maintained on biologics. The other UC patients have had ileal pouch-anal anastomosis. The other CD patient had a subsequent ileorectal anastomosis. Patients were able to undergo those procedures laparoscopically and “have done really well,” Dr. Lightner said.
It’s unclear why loop ileostomy seems so helpful. Perhaps it has something to do with shifts in bacterial populations or decompression of the colon. Maybe it’s just about giving the colon a rest, she said.
The investigators will continue to study the approach. Since the initial report, 8 more patients have joined the series, for a current total of 16. “We are still seeing good results,” Dr. Lightner said.
Dr. Lightner has no disclosures, and there was no outside funding for the work.
CHICAGO – Diverting loop ileostomy may be a better option than urgent colectomy as the first surgical step for medically refractory severe ulcerative colitis and Crohn’s disease.
Investigators from the University of California, Los Angeles, have found that ileostomy gives patients a chance to recover from their acute illness – and their colons a chance to heal – so they’re in better shape for definitive surgery further down the road, if it’s even needed (J Am Coll Surg. 2015 Oct;221[4]:S37-S38).
“Urgent colectomy is standard practice for medically refractory severe ulcerative and Crohn’s colitis. However, immunosuppression and malnutrition can result in significant morbidity. This change in management strategy does not eliminate the potential need for definitive surgery, but it does allow for the more extensive procedure to be performed in an elective setting under optimized conditions, thereby improving clinical outcomes,” said the investigators, led by Dr. Amy Lightner, formerly of UCLA but now a colorectal surgery fellow at the Mayo Clinic in Rochester, Minn.
There were just eight patients in the series, so the results are tentative. Six had ulcerative colitis (UC) and two had Crohn’s disease (CD). On presentation, the patients were tachycardic, febrile, malnourished, and anemic, with severe mucosal disease confirmed by endoscopy. Steroids, immunomodulators, and biologics no longer helped. Overall, the patients were too sick to go home, but not quite sick enough for the ICU. Their average age was 29 years.
They underwent a single-incision, laparoscopic diverting loop ileostomy, which took about 45 minutes. The technique, and perhaps the thinking behind it, are similar to one gaining popularity for Clostridium difficile colitis, but without the colonic lavage.
Within 24-48 hours postop, tachycardia and fevers resolved, and patients tolerated oral intake. Narcotic use dropped, and bloody stools became less frequent, and then ceased in all but one patient. Within a month, the average hemoglobin level had climbed from a baseline of 9 g/dL to 11.5 g/dL, and average albumin from 2.5 g/dL to 4 g/dL. Within 2 months, patients’ bowels looked pink and healthy on repeat endoscopy.
“It was a remarkable turnaround. Within 48 hours, they looked markedly different. We are having very good results with this, and it’s much better for patients” than is colectomy during acute illness. “It’s a good change in management,” Dr. Lightner said.
After months of follow-up, two patients, one with UC and one with CD, haven’t needed a colectomy and are maintained on biologics. The other UC patients have had ileal pouch-anal anastomosis. The other CD patient had a subsequent ileorectal anastomosis. Patients were able to undergo those procedures laparoscopically and “have done really well,” Dr. Lightner said.
It’s unclear why loop ileostomy seems so helpful. Perhaps it has something to do with shifts in bacterial populations or decompression of the colon. Maybe it’s just about giving the colon a rest, she said.
The investigators will continue to study the approach. Since the initial report, 8 more patients have joined the series, for a current total of 16. “We are still seeing good results,” Dr. Lightner said.
Dr. Lightner has no disclosures, and there was no outside funding for the work.
AT THE ACS CLINICAL CONGRESS
Key clinical point: Patients with refractory inflammatory bowel disease may benefit from loop ileostomy in lieu of urgent colectomy as a first surgical step.
Major finding: Within 24-48 hours after diverting loop ileostomy, tachycardia and fevers resolved, and patients tolerated oral intake. Narcotic use dropped, and bloody stools became less frequent, then ceased.
Data source: Pilot study in eight patients with refractory inflammatory bowel disease
Disclosures: The lead investigator has no disclosures, and there was no outside funding for the work.
ACS: Don’t shy away from venovenous ECMO for trauma lung failure
CHICAGO – Venovenous extracorporeal membrane oxygenation will save perhaps a third of patients who – despite maximum ventilator support – go into end-stage respiratory failure after trauma, according to investigators from the University of Maryland, Baltimore.
“Institutions without the available expertise and ICU capabilities should promptly refer patients with end-stage respiratory failure secondary to trauma to a tertiary care center. Venovenous ECMO [extracorporeal membrane oxygenation] life support may be their only chance for survival and should not be overlooked due to fear of complications,” they concluded.
ECMO usually requires heparin anticoagulation to prevent clots; the fear of subsequent bleeding is one of the things that prevents ECMO’s widespread use in trauma. As a result, “a lot of patients who need ECMO lung support don’t get it,” said Dr. Sarwat Ahmad, of the university.
Dr. Ahmad and her colleagues, however, found that ECMO did not lead to worse outcomes in their lung failure patients.
Their conclusions come from a review of 39 adult blunt and penetrating trauma patients who received ECMO at the university’s Level I trauma center over the past 9 years.
Thirty-two patients had venovenous ECMO mostly for acute respiratory distress; maximal ventilator support, adjunctive medications, and chest therapy did not help. ECMO outflow was from the femoral vein, and blood was returned to the internal jugular vein. Twelve patients (38%) survived, which “is good in this scenario because they otherwise would have died,” Dr. Ahmad said.
The mean pre-ECMO P/F ratio – arterial oxygen partial pressure to fractional inspired oxygen – among the survivors was 98 mm Hg. Values below 100 mm Hg indicate severe lung injury, but some patients had values approaching 200 mm Hg, meaning that ECMO was a good idea even in patients with less severe lung injury.
Seven patients received venoarterial ECMO mostly for cardiac arrest, with outflow from the femoral vein and blood returned via the femoral artery. The patients were pulseless on arrival, so bypassing the heart seemed the only option, but none of them survived. Because of that, the investigators concluded that venoarterial ECMO is “not going to help” in trauma patients, Dr. Ahmad said.
One of the 12 survivors and over half of those who died had injury severity scores above 40 points. Also, Glasgow coma scores below 8 points were far more common among patients who died.
All 12 of the survivors and 14 of the 27 who died were anticoagulated with heparin. “We didn’t see an increased incidence of complications between those who got heparin and those who did not” and, overall, there wasn’t a higher incidence of complications in ECMO patients, compared with other trauma patients. “Even traumatic brain injury patients didn’t do any worse on ECMO. We don’t think that the fear of complications should turn you away from using ECMO,” Dr. Ahmad said.
Dr. Ahmad has no disclosures; there was no external funding for the work.
CHICAGO – Venovenous extracorporeal membrane oxygenation will save perhaps a third of patients who – despite maximum ventilator support – go into end-stage respiratory failure after trauma, according to investigators from the University of Maryland, Baltimore.
“Institutions without the available expertise and ICU capabilities should promptly refer patients with end-stage respiratory failure secondary to trauma to a tertiary care center. Venovenous ECMO [extracorporeal membrane oxygenation] life support may be their only chance for survival and should not be overlooked due to fear of complications,” they concluded.
ECMO usually requires heparin anticoagulation to prevent clots; the fear of subsequent bleeding is one of the things that prevents ECMO’s widespread use in trauma. As a result, “a lot of patients who need ECMO lung support don’t get it,” said Dr. Sarwat Ahmad, of the university.
Dr. Ahmad and her colleagues, however, found that ECMO did not lead to worse outcomes in their lung failure patients.
Their conclusions come from a review of 39 adult blunt and penetrating trauma patients who received ECMO at the university’s Level I trauma center over the past 9 years.
Thirty-two patients had venovenous ECMO mostly for acute respiratory distress; maximal ventilator support, adjunctive medications, and chest therapy did not help. ECMO outflow was from the femoral vein, and blood was returned to the internal jugular vein. Twelve patients (38%) survived, which “is good in this scenario because they otherwise would have died,” Dr. Ahmad said.
The mean pre-ECMO P/F ratio – arterial oxygen partial pressure to fractional inspired oxygen – among the survivors was 98 mm Hg. Values below 100 mm Hg indicate severe lung injury, but some patients had values approaching 200 mm Hg, meaning that ECMO was a good idea even in patients with less severe lung injury.
Seven patients received venoarterial ECMO mostly for cardiac arrest, with outflow from the femoral vein and blood returned via the femoral artery. The patients were pulseless on arrival, so bypassing the heart seemed the only option, but none of them survived. Because of that, the investigators concluded that venoarterial ECMO is “not going to help” in trauma patients, Dr. Ahmad said.
One of the 12 survivors and over half of those who died had injury severity scores above 40 points. Also, Glasgow coma scores below 8 points were far more common among patients who died.
All 12 of the survivors and 14 of the 27 who died were anticoagulated with heparin. “We didn’t see an increased incidence of complications between those who got heparin and those who did not” and, overall, there wasn’t a higher incidence of complications in ECMO patients, compared with other trauma patients. “Even traumatic brain injury patients didn’t do any worse on ECMO. We don’t think that the fear of complications should turn you away from using ECMO,” Dr. Ahmad said.
Dr. Ahmad has no disclosures; there was no external funding for the work.
CHICAGO – Venovenous extracorporeal membrane oxygenation will save perhaps a third of patients who – despite maximum ventilator support – go into end-stage respiratory failure after trauma, according to investigators from the University of Maryland, Baltimore.
“Institutions without the available expertise and ICU capabilities should promptly refer patients with end-stage respiratory failure secondary to trauma to a tertiary care center. Venovenous ECMO [extracorporeal membrane oxygenation] life support may be their only chance for survival and should not be overlooked due to fear of complications,” they concluded.
ECMO usually requires heparin anticoagulation to prevent clots; the fear of subsequent bleeding is one of the things that prevents ECMO’s widespread use in trauma. As a result, “a lot of patients who need ECMO lung support don’t get it,” said Dr. Sarwat Ahmad, of the university.
Dr. Ahmad and her colleagues, however, found that ECMO did not lead to worse outcomes in their lung failure patients.
Their conclusions come from a review of 39 adult blunt and penetrating trauma patients who received ECMO at the university’s Level I trauma center over the past 9 years.
Thirty-two patients had venovenous ECMO mostly for acute respiratory distress; maximal ventilator support, adjunctive medications, and chest therapy did not help. ECMO outflow was from the femoral vein, and blood was returned to the internal jugular vein. Twelve patients (38%) survived, which “is good in this scenario because they otherwise would have died,” Dr. Ahmad said.
The mean pre-ECMO P/F ratio – arterial oxygen partial pressure to fractional inspired oxygen – among the survivors was 98 mm Hg. Values below 100 mm Hg indicate severe lung injury, but some patients had values approaching 200 mm Hg, meaning that ECMO was a good idea even in patients with less severe lung injury.
Seven patients received venoarterial ECMO mostly for cardiac arrest, with outflow from the femoral vein and blood returned via the femoral artery. The patients were pulseless on arrival, so bypassing the heart seemed the only option, but none of them survived. Because of that, the investigators concluded that venoarterial ECMO is “not going to help” in trauma patients, Dr. Ahmad said.
One of the 12 survivors and over half of those who died had injury severity scores above 40 points. Also, Glasgow coma scores below 8 points were far more common among patients who died.
All 12 of the survivors and 14 of the 27 who died were anticoagulated with heparin. “We didn’t see an increased incidence of complications between those who got heparin and those who did not” and, overall, there wasn’t a higher incidence of complications in ECMO patients, compared with other trauma patients. “Even traumatic brain injury patients didn’t do any worse on ECMO. We don’t think that the fear of complications should turn you away from using ECMO,” Dr. Ahmad said.
Dr. Ahmad has no disclosures; there was no external funding for the work.
AT THE ACS CLINICAL CONGRESS
Key clinical point: Venovenous ECMO can be life saving when trauma patients go into respiratory failure.
Major finding: Thirty-two patients had venovenous ECMO, mostly for acute respiratory distress; twelve (38%) survived.
Data source: Review of ECMO in 39 trauma patients
Disclosures: The lead investigator has no disclosures, and there was no external funding for the work.
FDA approves low-dose meloxicam medication for osteoarthritis
The Food and Drug Administration has approved a low-dose formulation of meloxicam for the treatment of osteoarthritis pain, according to manufacturer, Iroko Pharmaceuticals.
The new medication, Vivlodex, is made using a proprietary technology for producing submicron-size particles of meloxicam that are 10 times smaller than their traditional size, decreasing dissolution time. In a 12-week, phase III trial of 402 osteoarthritis patients aged 40 years or older, Vivlodex in 5- and 10-mg doses achieved efficacy at 33% lower doses than with commercially available meloxicam medications. Vivlodex will be available as a once-daily medication in 5- or 10-mg doses.
The FDA and professional medical associations have recommended administering nonsteroidal anti-inflammatory drugs in as small a dose and for as short a duration as possible. Serious adverse events stemming from NSAID treatments are related to dose and risk can increase as treatment continues.
“Meloxicam is the second most commonly prescribed NSAID in the [United States]. The approval of Vivlodex is a welcome option that offers patients an effective, low-dose NSAID,” Dr. Byron Cryer, associate dean at the University of Texas Southwestern Medical Center at Dallas, said in a statement from Iroko.
The Food and Drug Administration has approved a low-dose formulation of meloxicam for the treatment of osteoarthritis pain, according to manufacturer, Iroko Pharmaceuticals.
The new medication, Vivlodex, is made using a proprietary technology for producing submicron-size particles of meloxicam that are 10 times smaller than their traditional size, decreasing dissolution time. In a 12-week, phase III trial of 402 osteoarthritis patients aged 40 years or older, Vivlodex in 5- and 10-mg doses achieved efficacy at 33% lower doses than with commercially available meloxicam medications. Vivlodex will be available as a once-daily medication in 5- or 10-mg doses.
The FDA and professional medical associations have recommended administering nonsteroidal anti-inflammatory drugs in as small a dose and for as short a duration as possible. Serious adverse events stemming from NSAID treatments are related to dose and risk can increase as treatment continues.
“Meloxicam is the second most commonly prescribed NSAID in the [United States]. The approval of Vivlodex is a welcome option that offers patients an effective, low-dose NSAID,” Dr. Byron Cryer, associate dean at the University of Texas Southwestern Medical Center at Dallas, said in a statement from Iroko.
The Food and Drug Administration has approved a low-dose formulation of meloxicam for the treatment of osteoarthritis pain, according to manufacturer, Iroko Pharmaceuticals.
The new medication, Vivlodex, is made using a proprietary technology for producing submicron-size particles of meloxicam that are 10 times smaller than their traditional size, decreasing dissolution time. In a 12-week, phase III trial of 402 osteoarthritis patients aged 40 years or older, Vivlodex in 5- and 10-mg doses achieved efficacy at 33% lower doses than with commercially available meloxicam medications. Vivlodex will be available as a once-daily medication in 5- or 10-mg doses.
The FDA and professional medical associations have recommended administering nonsteroidal anti-inflammatory drugs in as small a dose and for as short a duration as possible. Serious adverse events stemming from NSAID treatments are related to dose and risk can increase as treatment continues.
“Meloxicam is the second most commonly prescribed NSAID in the [United States]. The approval of Vivlodex is a welcome option that offers patients an effective, low-dose NSAID,” Dr. Byron Cryer, associate dean at the University of Texas Southwestern Medical Center at Dallas, said in a statement from Iroko.












