User login
Pearls from the ASDS meeting
The annual American Society for Dermatologic Surgery conference in Chicago Oct. 15-18 was one of the best attended meeting in years. From injectables to lasers to reconstruction, the newest information was distributed among the members.
Here are pearls gained from the ASDS conference that every dermatologist should know:
There are reports of temporary alopecia of the beard area in men after deoxycholic acid (Kybella) injections in the submentum. Patients should be counseled prior to injection. Deeper injections in males, pinching up the skin, and penetrating the needle to the hub are measures that have been suggested to help minimize the risk of this potential side effect.
More than 60 cases of blindness secondary to filler injections have been reported, but such cases are likely underreported. The majority of reports were from South Korea and most cases were due to autologous fat transfer. High risk areas include the glabella, nasal dorsum, and anteromedial cheek/tear trough due to retrograde flow of a filler embolus to the ophthalmic artery from anastomoses with the angular, dorsal nasal, and supratrochlear arteries. Cannulas are recommended as they are considered safer than needles, particularly when injecting either fat or fillers in the mid face area.
However, even cannulas are not foolproof. There are some areas where periosteal placement of filler is important and therefore the use of needles is required, such as the anterosuperior temple, zygomaticomalar cheek, and central chin. Expert knowledge of the vascular anatomy of the face, including location and depth of important vessels, is a must.
If a vascular occlusion occurs – particularly to the ophthalmic artery that can result in blindness – symptoms may include pain, visual disturbances, vomiting, and blanching/reticulation of blood vessels on the skin surface. Time is of the essence in preventing or reversing vision loss. If a hyaluronic acid filler was used, retrobulbar injection of at least 1,000 units of hyaluronidase and referral to an ophthalmologist should be done within minutes.
For body contouring and skin tightening, cryolipolysis and high-intensity focused ultrasound have shown results over the past several years. However, newer technologies including nonthermal focused ultrasound, multipolar radiofrequency, and fractional radiofrequency with microneedling, and a 1064 nm diode laser also show some promise.
The ablative fractional CO2 laser was shown to be helpful for hypopigmented scars.
Malpractice lawsuits against cosmetic procedures are highest among physician extenders (physician assistants, nurses, assistants, etc).
Dr. Wesley and Dr. Talakoub are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
This article was updated Nov. 16, 2015.
The annual American Society for Dermatologic Surgery conference in Chicago Oct. 15-18 was one of the best attended meeting in years. From injectables to lasers to reconstruction, the newest information was distributed among the members.
Here are pearls gained from the ASDS conference that every dermatologist should know:
There are reports of temporary alopecia of the beard area in men after deoxycholic acid (Kybella) injections in the submentum. Patients should be counseled prior to injection. Deeper injections in males, pinching up the skin, and penetrating the needle to the hub are measures that have been suggested to help minimize the risk of this potential side effect.
More than 60 cases of blindness secondary to filler injections have been reported, but such cases are likely underreported. The majority of reports were from South Korea and most cases were due to autologous fat transfer. High risk areas include the glabella, nasal dorsum, and anteromedial cheek/tear trough due to retrograde flow of a filler embolus to the ophthalmic artery from anastomoses with the angular, dorsal nasal, and supratrochlear arteries. Cannulas are recommended as they are considered safer than needles, particularly when injecting either fat or fillers in the mid face area.
However, even cannulas are not foolproof. There are some areas where periosteal placement of filler is important and therefore the use of needles is required, such as the anterosuperior temple, zygomaticomalar cheek, and central chin. Expert knowledge of the vascular anatomy of the face, including location and depth of important vessels, is a must.
If a vascular occlusion occurs – particularly to the ophthalmic artery that can result in blindness – symptoms may include pain, visual disturbances, vomiting, and blanching/reticulation of blood vessels on the skin surface. Time is of the essence in preventing or reversing vision loss. If a hyaluronic acid filler was used, retrobulbar injection of at least 1,000 units of hyaluronidase and referral to an ophthalmologist should be done within minutes.
For body contouring and skin tightening, cryolipolysis and high-intensity focused ultrasound have shown results over the past several years. However, newer technologies including nonthermal focused ultrasound, multipolar radiofrequency, and fractional radiofrequency with microneedling, and a 1064 nm diode laser also show some promise.
The ablative fractional CO2 laser was shown to be helpful for hypopigmented scars.
Malpractice lawsuits against cosmetic procedures are highest among physician extenders (physician assistants, nurses, assistants, etc).
Dr. Wesley and Dr. Talakoub are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
This article was updated Nov. 16, 2015.
The annual American Society for Dermatologic Surgery conference in Chicago Oct. 15-18 was one of the best attended meeting in years. From injectables to lasers to reconstruction, the newest information was distributed among the members.
Here are pearls gained from the ASDS conference that every dermatologist should know:
There are reports of temporary alopecia of the beard area in men after deoxycholic acid (Kybella) injections in the submentum. Patients should be counseled prior to injection. Deeper injections in males, pinching up the skin, and penetrating the needle to the hub are measures that have been suggested to help minimize the risk of this potential side effect.
More than 60 cases of blindness secondary to filler injections have been reported, but such cases are likely underreported. The majority of reports were from South Korea and most cases were due to autologous fat transfer. High risk areas include the glabella, nasal dorsum, and anteromedial cheek/tear trough due to retrograde flow of a filler embolus to the ophthalmic artery from anastomoses with the angular, dorsal nasal, and supratrochlear arteries. Cannulas are recommended as they are considered safer than needles, particularly when injecting either fat or fillers in the mid face area.
However, even cannulas are not foolproof. There are some areas where periosteal placement of filler is important and therefore the use of needles is required, such as the anterosuperior temple, zygomaticomalar cheek, and central chin. Expert knowledge of the vascular anatomy of the face, including location and depth of important vessels, is a must.
If a vascular occlusion occurs – particularly to the ophthalmic artery that can result in blindness – symptoms may include pain, visual disturbances, vomiting, and blanching/reticulation of blood vessels on the skin surface. Time is of the essence in preventing or reversing vision loss. If a hyaluronic acid filler was used, retrobulbar injection of at least 1,000 units of hyaluronidase and referral to an ophthalmologist should be done within minutes.
For body contouring and skin tightening, cryolipolysis and high-intensity focused ultrasound have shown results over the past several years. However, newer technologies including nonthermal focused ultrasound, multipolar radiofrequency, and fractional radiofrequency with microneedling, and a 1064 nm diode laser also show some promise.
The ablative fractional CO2 laser was shown to be helpful for hypopigmented scars.
Malpractice lawsuits against cosmetic procedures are highest among physician extenders (physician assistants, nurses, assistants, etc).
Dr. Wesley and Dr. Talakoub are co-contributors to a monthly Aesthetic Dermatology column in Dermatology News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
This article was updated Nov. 16, 2015.
EADV: Spotlight on alexithymia in psoriasis
COPENHAGEN – Alexithymia – difficulty in recognizing and describing one’s emotions – is exceptionally common among psoriasis patients and may represent a novel therapeutic target, according to Dr. Carle Paul.
“We found a significant association between alexithymia and more severe psoriasis, anxiety, depression, decreased quality of life, harmful alcohol consumption, and work impairment,” reported Dr. Paul, professor and chairman of the department of dermatology at the University of Toulouse (France).
Alexithymia, a personality construct sometimes referred to as “emotional blindness,” was first described by psychologists in the 1970s. The previous glaring lack of data on the prevalence and consequences of alexithymia in psoriasis patients served as the impetus for the ongoing EPIDEPSO study (Epidemiological Study in Patients With Recently Diagnosed Psoriasis), a prospective 1-year international, epidemiologic, noninterventional observational study involving 719 adults with moderate to severe plaque psoriasis of less than 10 years’ duration. Dr. Paul presented the baseline findings at the at the annual congress of the European Academy of Dermatology and Venereology.
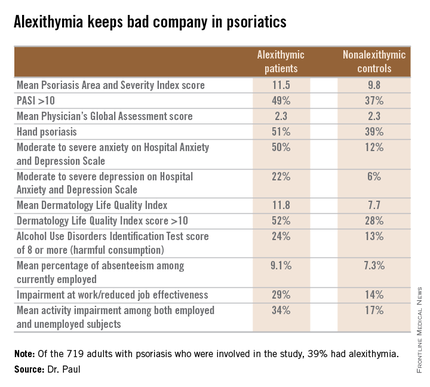
The first noteworthy finding was the strikingly high prevalence of alexithymia in this group of psoriasis patients: 39% of the 719 patients had alexithymia, as defined by a score of 61 or more on the validated, 20-item Toronto Alexithymia Scale.
Patients with alexithymia had slightly but significantly more severe psoriasis as evidenced by their mean Psoriasis Area and Severity Index score of 11.5, compared with 9.8 in unaffected patients. Hand psoriasis was more common in alexithymic patients by a margin of 51%-39%, although the prevalence of psoriasis of the face and neck was similar in the two groups.
Alexithymia was associated with significantly higher rates of several forms of psychiatric comorbidity and problems in living as assessed by validated tests. One comorbid condition stood out above the rest.
“The most striking feature is the very close relationship between alexithymia and anxiety,” according to Dr. Paul. “Among alexithymic patients, 50% had moderate to severe anxiety, as measured by the Hospital Anxiety and Depression Scale–A, whereas in psoriasis patients without alexithymia, this proportion was 12%.”
“Alexithymia identifies a patient population with a high burden of psoriasis, but at the same time, they have difficulty in expressing their emotions and feelings to their doctor. And I think this may explain the fact that some psoriasis patients have difficulties in interacting with doctors, because even though they have a high psoriasis burden, they cannot express how much they suffer from the disease,” the dermatologist continued.
Audience members at this standing-room-only EADV session on new research findings in psoriasis were clearly intrigued by the novel findings about a psychological condition unfamiliar to most. They wanted to know if the Toronto Alexithymia Scale is suitable for use in everyday clinical practice. The answer is yes, Dr. Paul replied, but the practical importance of identifying the large subgroup of patients with alexithymia has yet to be determined.
“We want to find out if we can modify alexithymia with interventions, but we don’t have the prospective data yet,” he added.
Even if alexithymia turns out to be a fixed characteristic not amenable to intervention, however, EPIDEPSO has shown that it could be useful as a red flag because it keeps company with several psychiatric conditions, which are anxiety, depression, and alcohol abuse.
What about causality? others asked. Does alexithymia cause anxiety and depression, or does the emotional toll of psoriasis promote anxiety and depression, which then causes alexithymia? Dr. Paul responded that it’s impossible to say at this point because these initial EPIDEPSO data are cross-sectional; however, the 1-year follow-up may yield insight.
EPIDEPSO is funded by Janssen. Dr. Paul reported receiving a research grant from the company to conduct the study.
COPENHAGEN – Alexithymia – difficulty in recognizing and describing one’s emotions – is exceptionally common among psoriasis patients and may represent a novel therapeutic target, according to Dr. Carle Paul.
“We found a significant association between alexithymia and more severe psoriasis, anxiety, depression, decreased quality of life, harmful alcohol consumption, and work impairment,” reported Dr. Paul, professor and chairman of the department of dermatology at the University of Toulouse (France).
Alexithymia, a personality construct sometimes referred to as “emotional blindness,” was first described by psychologists in the 1970s. The previous glaring lack of data on the prevalence and consequences of alexithymia in psoriasis patients served as the impetus for the ongoing EPIDEPSO study (Epidemiological Study in Patients With Recently Diagnosed Psoriasis), a prospective 1-year international, epidemiologic, noninterventional observational study involving 719 adults with moderate to severe plaque psoriasis of less than 10 years’ duration. Dr. Paul presented the baseline findings at the at the annual congress of the European Academy of Dermatology and Venereology.
The first noteworthy finding was the strikingly high prevalence of alexithymia in this group of psoriasis patients: 39% of the 719 patients had alexithymia, as defined by a score of 61 or more on the validated, 20-item Toronto Alexithymia Scale.
Patients with alexithymia had slightly but significantly more severe psoriasis as evidenced by their mean Psoriasis Area and Severity Index score of 11.5, compared with 9.8 in unaffected patients. Hand psoriasis was more common in alexithymic patients by a margin of 51%-39%, although the prevalence of psoriasis of the face and neck was similar in the two groups.
Alexithymia was associated with significantly higher rates of several forms of psychiatric comorbidity and problems in living as assessed by validated tests. One comorbid condition stood out above the rest.
“The most striking feature is the very close relationship between alexithymia and anxiety,” according to Dr. Paul. “Among alexithymic patients, 50% had moderate to severe anxiety, as measured by the Hospital Anxiety and Depression Scale–A, whereas in psoriasis patients without alexithymia, this proportion was 12%.”
“Alexithymia identifies a patient population with a high burden of psoriasis, but at the same time, they have difficulty in expressing their emotions and feelings to their doctor. And I think this may explain the fact that some psoriasis patients have difficulties in interacting with doctors, because even though they have a high psoriasis burden, they cannot express how much they suffer from the disease,” the dermatologist continued.

Audience members at this standing-room-only EADV session on new research findings in psoriasis were clearly intrigued by the novel findings about a psychological condition unfamiliar to most. They wanted to know if the Toronto Alexithymia Scale is suitable for use in everyday clinical practice. The answer is yes, Dr. Paul replied, but the practical importance of identifying the large subgroup of patients with alexithymia has yet to be determined.
“We want to find out if we can modify alexithymia with interventions, but we don’t have the prospective data yet,” he added.
Even if alexithymia turns out to be a fixed characteristic not amenable to intervention, however, EPIDEPSO has shown that it could be useful as a red flag because it keeps company with several psychiatric conditions, which are anxiety, depression, and alcohol abuse.
What about causality? others asked. Does alexithymia cause anxiety and depression, or does the emotional toll of psoriasis promote anxiety and depression, which then causes alexithymia? Dr. Paul responded that it’s impossible to say at this point because these initial EPIDEPSO data are cross-sectional; however, the 1-year follow-up may yield insight.
EPIDEPSO is funded by Janssen. Dr. Paul reported receiving a research grant from the company to conduct the study.
COPENHAGEN – Alexithymia – difficulty in recognizing and describing one’s emotions – is exceptionally common among psoriasis patients and may represent a novel therapeutic target, according to Dr. Carle Paul.
“We found a significant association between alexithymia and more severe psoriasis, anxiety, depression, decreased quality of life, harmful alcohol consumption, and work impairment,” reported Dr. Paul, professor and chairman of the department of dermatology at the University of Toulouse (France).
Alexithymia, a personality construct sometimes referred to as “emotional blindness,” was first described by psychologists in the 1970s. The previous glaring lack of data on the prevalence and consequences of alexithymia in psoriasis patients served as the impetus for the ongoing EPIDEPSO study (Epidemiological Study in Patients With Recently Diagnosed Psoriasis), a prospective 1-year international, epidemiologic, noninterventional observational study involving 719 adults with moderate to severe plaque psoriasis of less than 10 years’ duration. Dr. Paul presented the baseline findings at the at the annual congress of the European Academy of Dermatology and Venereology.
The first noteworthy finding was the strikingly high prevalence of alexithymia in this group of psoriasis patients: 39% of the 719 patients had alexithymia, as defined by a score of 61 or more on the validated, 20-item Toronto Alexithymia Scale.
Patients with alexithymia had slightly but significantly more severe psoriasis as evidenced by their mean Psoriasis Area and Severity Index score of 11.5, compared with 9.8 in unaffected patients. Hand psoriasis was more common in alexithymic patients by a margin of 51%-39%, although the prevalence of psoriasis of the face and neck was similar in the two groups.
Alexithymia was associated with significantly higher rates of several forms of psychiatric comorbidity and problems in living as assessed by validated tests. One comorbid condition stood out above the rest.
“The most striking feature is the very close relationship between alexithymia and anxiety,” according to Dr. Paul. “Among alexithymic patients, 50% had moderate to severe anxiety, as measured by the Hospital Anxiety and Depression Scale–A, whereas in psoriasis patients without alexithymia, this proportion was 12%.”
“Alexithymia identifies a patient population with a high burden of psoriasis, but at the same time, they have difficulty in expressing their emotions and feelings to their doctor. And I think this may explain the fact that some psoriasis patients have difficulties in interacting with doctors, because even though they have a high psoriasis burden, they cannot express how much they suffer from the disease,” the dermatologist continued.

Audience members at this standing-room-only EADV session on new research findings in psoriasis were clearly intrigued by the novel findings about a psychological condition unfamiliar to most. They wanted to know if the Toronto Alexithymia Scale is suitable for use in everyday clinical practice. The answer is yes, Dr. Paul replied, but the practical importance of identifying the large subgroup of patients with alexithymia has yet to be determined.
“We want to find out if we can modify alexithymia with interventions, but we don’t have the prospective data yet,” he added.
Even if alexithymia turns out to be a fixed characteristic not amenable to intervention, however, EPIDEPSO has shown that it could be useful as a red flag because it keeps company with several psychiatric conditions, which are anxiety, depression, and alcohol abuse.
What about causality? others asked. Does alexithymia cause anxiety and depression, or does the emotional toll of psoriasis promote anxiety and depression, which then causes alexithymia? Dr. Paul responded that it’s impossible to say at this point because these initial EPIDEPSO data are cross-sectional; however, the 1-year follow-up may yield insight.
EPIDEPSO is funded by Janssen. Dr. Paul reported receiving a research grant from the company to conduct the study.
AT THE EADV CONGRESS
Key clinical point: Alexithymia is strikingly common among psoriasis patients and is associated with multiple psychiatric comorbidities and problems in living.
Major finding: Thirty-nine percent of a large cohort of psoriasis patients met the criteria for alexithymia, an inability to identify and describe one’s emotions. Affected patients had markedly higher rates of anxiety, depression, problem drinking, and impairments of quality of life and work productivity.
Data source: The EPIDEPSO study, an ongoing 1-year prospective, observational international study involving 719 adults with moderate to severe psoriasis of less than 10 years’ duration.
Disclosures: The EPIDEPSO study is funded by Janssen, which provided the presenter with a research grant.
Increased Mortality in Megacolon C. diff Patients
NEW YORK - Health care professionals should be highly suspicious of megacolon in Clostridium difficile-infected patients and have a low threshold for transferring infected patients to intensive care units, Veterans Affairs researchers warn.
"The incidence of Clostridium difficile-associated megacolon has nearly tripled and mortality has nearly doubled over the past decade," Dr. SreyRam Kuy from the Overton Brooks VA Medical Center in Shreveport, Louisiana, said by email.
"It could be argued that this increased incidence may be due in part to improvements in detection of Clostridium difficile," she said. "However, this increase over the past decade . . . correlates with prior work by the Agency for Healthcare Research and Quality, which showed a 74% increase in the overall number of hospital discharges with Clostridium difficile infections from 1993-2001, in the decade prior to our study."
Dr. Kuy and colleagues analyzed records in the Nationwide Inpatient Sample (2000-2010) and identified patients with both C. difficile infection and megacolon.
They identified 28,219 cases of C. difficile infection, or 0.38% of all hospitalized patients, in 2000. That grew to more than 68,600 cases, or 0.88% of hospitalized patients, in 2010.
While the overall incidence of megacolon remained steady at 0.02% of hospitalized patients from 2000 to 2010, the rate of megacolon cases tied to C. diff infection increased from 3.61% in 2000 to 9.39% in 2010 (p<0.05).
"Compared with patients with megacolon but without C. difficile infection, patients with C. difficile-associated megacolon are significantly older, are more likely to have an urgent or emergent admission, are more likely to be admitted from the emergency department or transferred from another hospital, and are more likely to be treated at large, urban, teaching hospitals," the researchers write in an article online Oct. 7 in JAMA Surgery.
They report that the mean length of hospital stay for patients with C. difficile-associated megacolon was 16.13 days, the mean cost of hospitalization came to $41,968, and 50.7% required transitional care after hospital discharge. The mortality among these patients went up from 13.56% in 2000 to 24.45% in 2010, and peaked at 30.03% in 2007 (p<0.05).
"The rise in mortality is also alarming," Dr. Kuy said. "Potentially, this rise in mortality could be attributed to changes in virulence of Clostridium difficile strains, though we are unable to determine this due to limitations of the dataset utilized for this research. Other factors that can affect mortality are antibiotic regimen, severity of disease, physical exam findings, immunosuppression, presence of end organ failure, patient frailty status, APACHE (Acute Physiology and Chronic Health Evaluation) score, signs of sepsis, and leukocytosis, which have been identified as factors associated with mortality in prior published reports."
"This study draws attention to the tremendous burden of Clostridium difficile on patient mortality, health care costs and resources, transitional care utilization, and the extremely important need for aggressive prevention of this iatrogenic disease," she concluded.
The authors reported no funding or disclosures.
NEW YORK - Health care professionals should be highly suspicious of megacolon in Clostridium difficile-infected patients and have a low threshold for transferring infected patients to intensive care units, Veterans Affairs researchers warn.
"The incidence of Clostridium difficile-associated megacolon has nearly tripled and mortality has nearly doubled over the past decade," Dr. SreyRam Kuy from the Overton Brooks VA Medical Center in Shreveport, Louisiana, said by email.
"It could be argued that this increased incidence may be due in part to improvements in detection of Clostridium difficile," she said. "However, this increase over the past decade . . . correlates with prior work by the Agency for Healthcare Research and Quality, which showed a 74% increase in the overall number of hospital discharges with Clostridium difficile infections from 1993-2001, in the decade prior to our study."
Dr. Kuy and colleagues analyzed records in the Nationwide Inpatient Sample (2000-2010) and identified patients with both C. difficile infection and megacolon.
They identified 28,219 cases of C. difficile infection, or 0.38% of all hospitalized patients, in 2000. That grew to more than 68,600 cases, or 0.88% of hospitalized patients, in 2010.
While the overall incidence of megacolon remained steady at 0.02% of hospitalized patients from 2000 to 2010, the rate of megacolon cases tied to C. diff infection increased from 3.61% in 2000 to 9.39% in 2010 (p<0.05).
"Compared with patients with megacolon but without C. difficile infection, patients with C. difficile-associated megacolon are significantly older, are more likely to have an urgent or emergent admission, are more likely to be admitted from the emergency department or transferred from another hospital, and are more likely to be treated at large, urban, teaching hospitals," the researchers write in an article online Oct. 7 in JAMA Surgery.
They report that the mean length of hospital stay for patients with C. difficile-associated megacolon was 16.13 days, the mean cost of hospitalization came to $41,968, and 50.7% required transitional care after hospital discharge. The mortality among these patients went up from 13.56% in 2000 to 24.45% in 2010, and peaked at 30.03% in 2007 (p<0.05).
"The rise in mortality is also alarming," Dr. Kuy said. "Potentially, this rise in mortality could be attributed to changes in virulence of Clostridium difficile strains, though we are unable to determine this due to limitations of the dataset utilized for this research. Other factors that can affect mortality are antibiotic regimen, severity of disease, physical exam findings, immunosuppression, presence of end organ failure, patient frailty status, APACHE (Acute Physiology and Chronic Health Evaluation) score, signs of sepsis, and leukocytosis, which have been identified as factors associated with mortality in prior published reports."
"This study draws attention to the tremendous burden of Clostridium difficile on patient mortality, health care costs and resources, transitional care utilization, and the extremely important need for aggressive prevention of this iatrogenic disease," she concluded.
The authors reported no funding or disclosures.
NEW YORK - Health care professionals should be highly suspicious of megacolon in Clostridium difficile-infected patients and have a low threshold for transferring infected patients to intensive care units, Veterans Affairs researchers warn.
"The incidence of Clostridium difficile-associated megacolon has nearly tripled and mortality has nearly doubled over the past decade," Dr. SreyRam Kuy from the Overton Brooks VA Medical Center in Shreveport, Louisiana, said by email.
"It could be argued that this increased incidence may be due in part to improvements in detection of Clostridium difficile," she said. "However, this increase over the past decade . . . correlates with prior work by the Agency for Healthcare Research and Quality, which showed a 74% increase in the overall number of hospital discharges with Clostridium difficile infections from 1993-2001, in the decade prior to our study."
Dr. Kuy and colleagues analyzed records in the Nationwide Inpatient Sample (2000-2010) and identified patients with both C. difficile infection and megacolon.
They identified 28,219 cases of C. difficile infection, or 0.38% of all hospitalized patients, in 2000. That grew to more than 68,600 cases, or 0.88% of hospitalized patients, in 2010.
While the overall incidence of megacolon remained steady at 0.02% of hospitalized patients from 2000 to 2010, the rate of megacolon cases tied to C. diff infection increased from 3.61% in 2000 to 9.39% in 2010 (p<0.05).
"Compared with patients with megacolon but without C. difficile infection, patients with C. difficile-associated megacolon are significantly older, are more likely to have an urgent or emergent admission, are more likely to be admitted from the emergency department or transferred from another hospital, and are more likely to be treated at large, urban, teaching hospitals," the researchers write in an article online Oct. 7 in JAMA Surgery.
They report that the mean length of hospital stay for patients with C. difficile-associated megacolon was 16.13 days, the mean cost of hospitalization came to $41,968, and 50.7% required transitional care after hospital discharge. The mortality among these patients went up from 13.56% in 2000 to 24.45% in 2010, and peaked at 30.03% in 2007 (p<0.05).
"The rise in mortality is also alarming," Dr. Kuy said. "Potentially, this rise in mortality could be attributed to changes in virulence of Clostridium difficile strains, though we are unable to determine this due to limitations of the dataset utilized for this research. Other factors that can affect mortality are antibiotic regimen, severity of disease, physical exam findings, immunosuppression, presence of end organ failure, patient frailty status, APACHE (Acute Physiology and Chronic Health Evaluation) score, signs of sepsis, and leukocytosis, which have been identified as factors associated with mortality in prior published reports."
"This study draws attention to the tremendous burden of Clostridium difficile on patient mortality, health care costs and resources, transitional care utilization, and the extremely important need for aggressive prevention of this iatrogenic disease," she concluded.
The authors reported no funding or disclosures.
Million Veteran Program Sees Significant Research, Enrollment Progress
The Million Veterans Program (MVP) has reached 40% of its goal, registering more than 400,000 participants. Veterans who participate in the program donate blood for DNA extraction, which is linked to their health records. Created in 2012, MVP was expected to take 5 to 7 years to reach 1 million participants. Recently started research studies associated with MVP include cardiovascular risk factors, multisubstance use, pharmacogenomics of kidney disease, and metabolic conditions, among others.
“We are proud to see the progress being made in MVP, and we are confident the knowledge gained through this research will have a very tangible and positive impact on the health care that Veterans and all Americans receive,” said Secretary of Veterans Affairs Robert A. McDonald. “We applaud our Veterans participating in the program. The selfless sacrifice they are making will allow researchers to gain valuable, important information.”
Genomic programs such as MVP received a significant boost earlier this year with the announcement of President Obama’s Precision Medicine Initiative. Both the Precision Medicine Initiative and MVP are part of a larger effort to better tailor treatment to individual patients based in part on their genetics. “VA is thrilled to be working closely with the White House and other federal partners on the president’s Precision Medicine Initiative,” said VA Chief Research and Development Officer Timothy O’Leary, MD, PhD. “We are committed to making precision medicine a reality for veterans and the nation."
Federal Practitioner recently spoke with Robert Nussbaum, MD, of the University of California—San Francisco on the potential impact of MVP on genomics research. Watch the video below for more on the importance of MVP and its role in genomics.
The Million Veterans Program (MVP) has reached 40% of its goal, registering more than 400,000 participants. Veterans who participate in the program donate blood for DNA extraction, which is linked to their health records. Created in 2012, MVP was expected to take 5 to 7 years to reach 1 million participants. Recently started research studies associated with MVP include cardiovascular risk factors, multisubstance use, pharmacogenomics of kidney disease, and metabolic conditions, among others.
“We are proud to see the progress being made in MVP, and we are confident the knowledge gained through this research will have a very tangible and positive impact on the health care that Veterans and all Americans receive,” said Secretary of Veterans Affairs Robert A. McDonald. “We applaud our Veterans participating in the program. The selfless sacrifice they are making will allow researchers to gain valuable, important information.”
Genomic programs such as MVP received a significant boost earlier this year with the announcement of President Obama’s Precision Medicine Initiative. Both the Precision Medicine Initiative and MVP are part of a larger effort to better tailor treatment to individual patients based in part on their genetics. “VA is thrilled to be working closely with the White House and other federal partners on the president’s Precision Medicine Initiative,” said VA Chief Research and Development Officer Timothy O’Leary, MD, PhD. “We are committed to making precision medicine a reality for veterans and the nation."
Federal Practitioner recently spoke with Robert Nussbaum, MD, of the University of California—San Francisco on the potential impact of MVP on genomics research. Watch the video below for more on the importance of MVP and its role in genomics.
The Million Veterans Program (MVP) has reached 40% of its goal, registering more than 400,000 participants. Veterans who participate in the program donate blood for DNA extraction, which is linked to their health records. Created in 2012, MVP was expected to take 5 to 7 years to reach 1 million participants. Recently started research studies associated with MVP include cardiovascular risk factors, multisubstance use, pharmacogenomics of kidney disease, and metabolic conditions, among others.
“We are proud to see the progress being made in MVP, and we are confident the knowledge gained through this research will have a very tangible and positive impact on the health care that Veterans and all Americans receive,” said Secretary of Veterans Affairs Robert A. McDonald. “We applaud our Veterans participating in the program. The selfless sacrifice they are making will allow researchers to gain valuable, important information.”
Genomic programs such as MVP received a significant boost earlier this year with the announcement of President Obama’s Precision Medicine Initiative. Both the Precision Medicine Initiative and MVP are part of a larger effort to better tailor treatment to individual patients based in part on their genetics. “VA is thrilled to be working closely with the White House and other federal partners on the president’s Precision Medicine Initiative,” said VA Chief Research and Development Officer Timothy O’Leary, MD, PhD. “We are committed to making precision medicine a reality for veterans and the nation."
Federal Practitioner recently spoke with Robert Nussbaum, MD, of the University of California—San Francisco on the potential impact of MVP on genomics research. Watch the video below for more on the importance of MVP and its role in genomics.
Guidelines back multivessel PCI
New recommendations validate the treatment of partially blocked vessels along with the culprit vessel in patients undergoing a primary percutaneous coronary intervention for ST-elevation myocardial infarction (STEMI).
While 2013 guidelines by the American College of Cardiology and the American Heart Association cautioned against multivessel interventions as nonbeneficial in STEMI, evidence from four recent randomized controlled trials now supports the practice as “reasonable,” the updated guidelines say.
Partially blocked vessels may be treated in hemodynamically stable patients at the time of PCI or as a planned staged procedure.
The guidelines, issued Oct 21 by the ACC/AHA and the Society for Cardiovascular Angiography and Interventions, with collaboration from the American College of Emergency Physicians, have been published online in Journal of the American College of Cardiology (2015 Oct 21;10.1016/jacc.2015.10.005), Circulation, and Catheterization and Cardiovascular Interventions.
The guidelines also downgrade a prior recommendation on routine use of manual aspiration thrombectomy before primary PCI to implant a stent, citing evidence from three randomized trials (INFUSE-AMI, TASTE, and TOTAL) in support of the new class III “no benefit” recommendation. Previously, the organizations had considered this treatment strategy reasonable.
For the advice on primary PCI and multivessel treatment, the guideline authors, led by Dr. Glenn N. Levine of Baylor College of Medicine in Houston, identified four trials (PRAMI, CvLPRIT, DANAMI 3-PRIMULTI, PRAGUE-13) in which multivessel PCI, either staged or at the time of primary PCI, was shown to be nonharmful or beneficial in selected patients with STEMI. In three of these trials, multivessel treatment was shown associated with significant reductions in risk of death and other cardiac events compared to culprit-vessel-only treatment.
Previously, “differing inclusion criteria, study protocols, timing of multivessel PCI, statistical heterogeneity, and variable endpoints” made study results on culprit-only vs. multivessel PCI conflicting, Dr. Levine and colleagues wrote.
While the more recent RCTs have helped clarify a benefit or at least lack of harm, “there are insufficient observational data and no randomized data at this time to inform a recommendation with regard to the optimal timing of nonculprit vessel PCI,” the authors wrote, saying further studies were needed. Clinical data, lesion severity and complexity, and the risk of contrast nephropathy should be considered when determining whether to perform primary or staged multivessel PCI.
Earlier recommendations in 2011 and 2013 favoring aspiration thrombectomy before primary PCI had been based largely on the results of one single-center randomized study enrolling about 1,000 patients (Lancet 2008;371:1915-20).
Since then, much larger trials have shown no significant differences in major cardiac events or death in people who received aspiration thrombectomy prior to primary PCI compared with PCI alone, and a meta-analysis of more than 20,000 patients across 17 trials found no significant reduction in death, reinfarction, or stent thrombosis associated with routine aspiration thrombectomy vs. PCI alone (Circ Cardiovasc Interv. 2015;8:e002258).
The guideline authors clarified that the downgraded recommendation of “no benefit” applies only to routine use of aspiration thrombectomy before primary PCI. Current data remain inadequate to determine a benefit for selective or “bailout” aspiration thrombectomy, which is thrombectomy that, though unplanned, had to be used during the procedure because of an unsatisfactory initial result or a complication.
Several of the ACC/AHA/SCAI guideline authors or reviewers, including both vice chairs of the PCI writing committee, disclosed industry relationships.
New recommendations validate the treatment of partially blocked vessels along with the culprit vessel in patients undergoing a primary percutaneous coronary intervention for ST-elevation myocardial infarction (STEMI).
While 2013 guidelines by the American College of Cardiology and the American Heart Association cautioned against multivessel interventions as nonbeneficial in STEMI, evidence from four recent randomized controlled trials now supports the practice as “reasonable,” the updated guidelines say.
Partially blocked vessels may be treated in hemodynamically stable patients at the time of PCI or as a planned staged procedure.
The guidelines, issued Oct 21 by the ACC/AHA and the Society for Cardiovascular Angiography and Interventions, with collaboration from the American College of Emergency Physicians, have been published online in Journal of the American College of Cardiology (2015 Oct 21;10.1016/jacc.2015.10.005), Circulation, and Catheterization and Cardiovascular Interventions.
The guidelines also downgrade a prior recommendation on routine use of manual aspiration thrombectomy before primary PCI to implant a stent, citing evidence from three randomized trials (INFUSE-AMI, TASTE, and TOTAL) in support of the new class III “no benefit” recommendation. Previously, the organizations had considered this treatment strategy reasonable.
For the advice on primary PCI and multivessel treatment, the guideline authors, led by Dr. Glenn N. Levine of Baylor College of Medicine in Houston, identified four trials (PRAMI, CvLPRIT, DANAMI 3-PRIMULTI, PRAGUE-13) in which multivessel PCI, either staged or at the time of primary PCI, was shown to be nonharmful or beneficial in selected patients with STEMI. In three of these trials, multivessel treatment was shown associated with significant reductions in risk of death and other cardiac events compared to culprit-vessel-only treatment.
Previously, “differing inclusion criteria, study protocols, timing of multivessel PCI, statistical heterogeneity, and variable endpoints” made study results on culprit-only vs. multivessel PCI conflicting, Dr. Levine and colleagues wrote.
While the more recent RCTs have helped clarify a benefit or at least lack of harm, “there are insufficient observational data and no randomized data at this time to inform a recommendation with regard to the optimal timing of nonculprit vessel PCI,” the authors wrote, saying further studies were needed. Clinical data, lesion severity and complexity, and the risk of contrast nephropathy should be considered when determining whether to perform primary or staged multivessel PCI.
Earlier recommendations in 2011 and 2013 favoring aspiration thrombectomy before primary PCI had been based largely on the results of one single-center randomized study enrolling about 1,000 patients (Lancet 2008;371:1915-20).
Since then, much larger trials have shown no significant differences in major cardiac events or death in people who received aspiration thrombectomy prior to primary PCI compared with PCI alone, and a meta-analysis of more than 20,000 patients across 17 trials found no significant reduction in death, reinfarction, or stent thrombosis associated with routine aspiration thrombectomy vs. PCI alone (Circ Cardiovasc Interv. 2015;8:e002258).
The guideline authors clarified that the downgraded recommendation of “no benefit” applies only to routine use of aspiration thrombectomy before primary PCI. Current data remain inadequate to determine a benefit for selective or “bailout” aspiration thrombectomy, which is thrombectomy that, though unplanned, had to be used during the procedure because of an unsatisfactory initial result or a complication.
Several of the ACC/AHA/SCAI guideline authors or reviewers, including both vice chairs of the PCI writing committee, disclosed industry relationships.
New recommendations validate the treatment of partially blocked vessels along with the culprit vessel in patients undergoing a primary percutaneous coronary intervention for ST-elevation myocardial infarction (STEMI).
While 2013 guidelines by the American College of Cardiology and the American Heart Association cautioned against multivessel interventions as nonbeneficial in STEMI, evidence from four recent randomized controlled trials now supports the practice as “reasonable,” the updated guidelines say.
Partially blocked vessels may be treated in hemodynamically stable patients at the time of PCI or as a planned staged procedure.
The guidelines, issued Oct 21 by the ACC/AHA and the Society for Cardiovascular Angiography and Interventions, with collaboration from the American College of Emergency Physicians, have been published online in Journal of the American College of Cardiology (2015 Oct 21;10.1016/jacc.2015.10.005), Circulation, and Catheterization and Cardiovascular Interventions.
The guidelines also downgrade a prior recommendation on routine use of manual aspiration thrombectomy before primary PCI to implant a stent, citing evidence from three randomized trials (INFUSE-AMI, TASTE, and TOTAL) in support of the new class III “no benefit” recommendation. Previously, the organizations had considered this treatment strategy reasonable.
For the advice on primary PCI and multivessel treatment, the guideline authors, led by Dr. Glenn N. Levine of Baylor College of Medicine in Houston, identified four trials (PRAMI, CvLPRIT, DANAMI 3-PRIMULTI, PRAGUE-13) in which multivessel PCI, either staged or at the time of primary PCI, was shown to be nonharmful or beneficial in selected patients with STEMI. In three of these trials, multivessel treatment was shown associated with significant reductions in risk of death and other cardiac events compared to culprit-vessel-only treatment.
Previously, “differing inclusion criteria, study protocols, timing of multivessel PCI, statistical heterogeneity, and variable endpoints” made study results on culprit-only vs. multivessel PCI conflicting, Dr. Levine and colleagues wrote.
While the more recent RCTs have helped clarify a benefit or at least lack of harm, “there are insufficient observational data and no randomized data at this time to inform a recommendation with regard to the optimal timing of nonculprit vessel PCI,” the authors wrote, saying further studies were needed. Clinical data, lesion severity and complexity, and the risk of contrast nephropathy should be considered when determining whether to perform primary or staged multivessel PCI.
Earlier recommendations in 2011 and 2013 favoring aspiration thrombectomy before primary PCI had been based largely on the results of one single-center randomized study enrolling about 1,000 patients (Lancet 2008;371:1915-20).
Since then, much larger trials have shown no significant differences in major cardiac events or death in people who received aspiration thrombectomy prior to primary PCI compared with PCI alone, and a meta-analysis of more than 20,000 patients across 17 trials found no significant reduction in death, reinfarction, or stent thrombosis associated with routine aspiration thrombectomy vs. PCI alone (Circ Cardiovasc Interv. 2015;8:e002258).
The guideline authors clarified that the downgraded recommendation of “no benefit” applies only to routine use of aspiration thrombectomy before primary PCI. Current data remain inadequate to determine a benefit for selective or “bailout” aspiration thrombectomy, which is thrombectomy that, though unplanned, had to be used during the procedure because of an unsatisfactory initial result or a complication.
Several of the ACC/AHA/SCAI guideline authors or reviewers, including both vice chairs of the PCI writing committee, disclosed industry relationships.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
EADV: Best treatments for great saphenous vein reflux
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
AT THE EADV CONGRESS
Key clinical point: Long-term outcomes for treatment of great saphenous vein reflux were significantly better with conventional surgery or endovenous laser ablation than with ultrasound-guided foam sclerotherapy.
Major finding: Obliteration or absence of the treated great saphenous vein segment was achieved with conventional surgery in 85% of treated cases, with endovenous laser ablation (EVLA) in 77% of legs treated, and with ultrasound-guided foam sclerotherapy (UGFS) in 23%.
Data source: This multicenter clinical trial with 5-year follow-up included 224 legs randomized to one of three popular treatments for great saphenous varicose veins.
Disclosures: The study was sponsored by Erasmus University. The presenter reported having no financial conflicts of interest.
Ibrutinib may prove useful in MM, research shows

showing multiple myeloma
NEW YORK—Results of an open-label, phase 2, dose-escalation study of ibrutinib combined with low-dose dexamethasone suggest the Bruton’s tyrosine kinase (BTK) inhibitor may be useful in treating relapsed or relapsed and refractory patients with multiple myeloma (MM).
In the highest dose cohort, 23% of patients experienced a clinical benefit, which was defined as a minimal response or better by International Myeloma Working Group criteria.
“Ibrutinib is a remarkable agent and, through BTK inhibition, has been a game-changer in CLL [chronic lymphocytic leukemia],” said Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Richardson reported results with ibrutinib in MM at Lymphoma & Myeloma 2015.
The rationale for the use of ibrutinib in myeloma is the robust BTK expression in the plasma cells of MM patients as well as its regulation of myeloma “stemness” in the bone marrow.
Ibrutinib plus low-dose dexamethasone has previously demonstrated activity in myeloma at the highest dose tested in a phase 1 trial.
So investigators continued to evaluate ibrutinib in 4 dose cohorts, with and without dexamathesone, and enrolled 92 relapsed/refractory MM patients. Dr Richardson described the results of cohort 4, which met criteria for expansion as of March 2015.
The investigators enrolled 43 patients in cohort 4 to receive 840 mg daily of ibrutinib plus 40 mg of dexamethasone weekly. This dose of ibrutinib is double the dose approved by the US Food and Drug Administration (FDA) for treatment of CLL and Waldenström’s macroglobulinemia.
The patient population was “very typical” for a relapsed/refractory patient population, Dr Richardson noted. The median age was 65 years (range, 43–81), almost two-thirds were male, and they had a median of 4 (range, 2–10) prior therapies.
More than half of patients had an ECOG performance status of 0 or 1, and their median time since diagnosis was 6.5 years.
Nineteen percent had t(11;14), and other chromosomal abnormalities included del 13q14, t(4:14), and del 17p. Twenty-three patients were ISS stage I, 16 were ISS stage II, and 4 were ISS stage III.
Seventy-seven percent of patients had received an autologous stem cell transplant, 95% had prior alkylator treatment, 91% prior lenalidomide, 58% prior thalidomide, and 91% prior bortezomib treatment.
All patients were steroid-refractory. Thirty-five percent were refractory to alkylator treatment, 61% were refractory to lenalidomide, and 49% were refractory to bortezomib.
“And even a number of them had been exposed to pomalidomide and also to carfilzomib,” Dr Richardson said, “recognizing that these are the new and exciting drugs on the block, but, at the same time, a significant number of our patients already received those therapies.”
Five percent of patients achieved a partial response to ibrutinib and low-dose dexamethasone, and 18% achieved a minimal response, for a clinical benefit rate of 23%. Thirty percent had stable disease after 4 or more cycles.
“What was particularly striking,” Dr Richardson said, “was at the top dose, the 840-mg dose, the progression-free survival.”
The median time to disease progression was 5.4 months (range, 0.0–16.4), which the investigators thought was compelling in this large study.
Dr Richardson noted that there were no significant differences in safety observed across all dose cohorts, although 55% of patients experienced a grade 3 or greater treatment-emergent adverse event (AE), while 29% experienced at least 1 serious AE. And 10% of patients experienced peripheral neuropathy (PN), 8 of whom had a prior history of PN.
Treatment-emergent hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included anemia (23%) and thrombocytopenia (21%). Grade 3/4 anemia and thrombocytopenia occurred in 9% of patients in each category.
Treatment-emergent non-hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included diarrhea (63%), fatigue (49%), cough (26%), nausea (23%), and muscle spasms (21%). Two percent of patients had grade 3/4 diarrhea. There were no other grade 3/4 adverse events in the cohort.
Eighty-six percent of patients in cohort 4 discontinued treatment, 60% due to progressive disease, 12% due to an AE, and 2% at the discretion of the investigator. Twelve percent withdrew, were noncompliant, or required concomitant medication that was not permitted by the protocol.
Overall, Dr Richardson said, the safety profile was manageable, similar across the dosing cohorts, and consistent with those seen in CLL and Waldenström’s macroglobulinemia. The 840-mg dose did not increase toxicity and demonstrated activity in this heavily pretreated population.
“The real signal that struck us,” Dr Richardson emphasized, “was the progression-free survival at 5.4 months . . . for a 92-patient, multicenter experience, this is obviously, I think, an encouraging start.”
Investigators continue to explore ibrutinib in 2 ongoing combination studies, one with carfilzomib and dexamethasone (PCYC-1119) and another with pomalidomide and dexamethasone (PCYC-1138). Another trial with lenalidomide and dexamethasone is planned.
Ibrutinib now has 4 FDA-approved indications: patients with CLL who have received at least 1 prior therapy, CLL patients with del 17p, patients with mantle cell lymphoma, and patients with Waldenström’s macroglobulinemia.
Ibrutinib is distributed and marketed as Imbruvica by Pharmacyclics and also marketed by Janssen Biotech, Inc. ![]()

showing multiple myeloma
NEW YORK—Results of an open-label, phase 2, dose-escalation study of ibrutinib combined with low-dose dexamethasone suggest the Bruton’s tyrosine kinase (BTK) inhibitor may be useful in treating relapsed or relapsed and refractory patients with multiple myeloma (MM).
In the highest dose cohort, 23% of patients experienced a clinical benefit, which was defined as a minimal response or better by International Myeloma Working Group criteria.
“Ibrutinib is a remarkable agent and, through BTK inhibition, has been a game-changer in CLL [chronic lymphocytic leukemia],” said Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Richardson reported results with ibrutinib in MM at Lymphoma & Myeloma 2015.
The rationale for the use of ibrutinib in myeloma is the robust BTK expression in the plasma cells of MM patients as well as its regulation of myeloma “stemness” in the bone marrow.
Ibrutinib plus low-dose dexamethasone has previously demonstrated activity in myeloma at the highest dose tested in a phase 1 trial.
So investigators continued to evaluate ibrutinib in 4 dose cohorts, with and without dexamathesone, and enrolled 92 relapsed/refractory MM patients. Dr Richardson described the results of cohort 4, which met criteria for expansion as of March 2015.
The investigators enrolled 43 patients in cohort 4 to receive 840 mg daily of ibrutinib plus 40 mg of dexamethasone weekly. This dose of ibrutinib is double the dose approved by the US Food and Drug Administration (FDA) for treatment of CLL and Waldenström’s macroglobulinemia.
The patient population was “very typical” for a relapsed/refractory patient population, Dr Richardson noted. The median age was 65 years (range, 43–81), almost two-thirds were male, and they had a median of 4 (range, 2–10) prior therapies.
More than half of patients had an ECOG performance status of 0 or 1, and their median time since diagnosis was 6.5 years.
Nineteen percent had t(11;14), and other chromosomal abnormalities included del 13q14, t(4:14), and del 17p. Twenty-three patients were ISS stage I, 16 were ISS stage II, and 4 were ISS stage III.
Seventy-seven percent of patients had received an autologous stem cell transplant, 95% had prior alkylator treatment, 91% prior lenalidomide, 58% prior thalidomide, and 91% prior bortezomib treatment.
All patients were steroid-refractory. Thirty-five percent were refractory to alkylator treatment, 61% were refractory to lenalidomide, and 49% were refractory to bortezomib.
“And even a number of them had been exposed to pomalidomide and also to carfilzomib,” Dr Richardson said, “recognizing that these are the new and exciting drugs on the block, but, at the same time, a significant number of our patients already received those therapies.”
Five percent of patients achieved a partial response to ibrutinib and low-dose dexamethasone, and 18% achieved a minimal response, for a clinical benefit rate of 23%. Thirty percent had stable disease after 4 or more cycles.
“What was particularly striking,” Dr Richardson said, “was at the top dose, the 840-mg dose, the progression-free survival.”
The median time to disease progression was 5.4 months (range, 0.0–16.4), which the investigators thought was compelling in this large study.
Dr Richardson noted that there were no significant differences in safety observed across all dose cohorts, although 55% of patients experienced a grade 3 or greater treatment-emergent adverse event (AE), while 29% experienced at least 1 serious AE. And 10% of patients experienced peripheral neuropathy (PN), 8 of whom had a prior history of PN.
Treatment-emergent hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included anemia (23%) and thrombocytopenia (21%). Grade 3/4 anemia and thrombocytopenia occurred in 9% of patients in each category.
Treatment-emergent non-hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included diarrhea (63%), fatigue (49%), cough (26%), nausea (23%), and muscle spasms (21%). Two percent of patients had grade 3/4 diarrhea. There were no other grade 3/4 adverse events in the cohort.
Eighty-six percent of patients in cohort 4 discontinued treatment, 60% due to progressive disease, 12% due to an AE, and 2% at the discretion of the investigator. Twelve percent withdrew, were noncompliant, or required concomitant medication that was not permitted by the protocol.
Overall, Dr Richardson said, the safety profile was manageable, similar across the dosing cohorts, and consistent with those seen in CLL and Waldenström’s macroglobulinemia. The 840-mg dose did not increase toxicity and demonstrated activity in this heavily pretreated population.
“The real signal that struck us,” Dr Richardson emphasized, “was the progression-free survival at 5.4 months . . . for a 92-patient, multicenter experience, this is obviously, I think, an encouraging start.”
Investigators continue to explore ibrutinib in 2 ongoing combination studies, one with carfilzomib and dexamethasone (PCYC-1119) and another with pomalidomide and dexamethasone (PCYC-1138). Another trial with lenalidomide and dexamethasone is planned.
Ibrutinib now has 4 FDA-approved indications: patients with CLL who have received at least 1 prior therapy, CLL patients with del 17p, patients with mantle cell lymphoma, and patients with Waldenström’s macroglobulinemia.
Ibrutinib is distributed and marketed as Imbruvica by Pharmacyclics and also marketed by Janssen Biotech, Inc. ![]()

showing multiple myeloma
NEW YORK—Results of an open-label, phase 2, dose-escalation study of ibrutinib combined with low-dose dexamethasone suggest the Bruton’s tyrosine kinase (BTK) inhibitor may be useful in treating relapsed or relapsed and refractory patients with multiple myeloma (MM).
In the highest dose cohort, 23% of patients experienced a clinical benefit, which was defined as a minimal response or better by International Myeloma Working Group criteria.
“Ibrutinib is a remarkable agent and, through BTK inhibition, has been a game-changer in CLL [chronic lymphocytic leukemia],” said Paul Richardson, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts.
Dr Richardson reported results with ibrutinib in MM at Lymphoma & Myeloma 2015.
The rationale for the use of ibrutinib in myeloma is the robust BTK expression in the plasma cells of MM patients as well as its regulation of myeloma “stemness” in the bone marrow.
Ibrutinib plus low-dose dexamethasone has previously demonstrated activity in myeloma at the highest dose tested in a phase 1 trial.
So investigators continued to evaluate ibrutinib in 4 dose cohorts, with and without dexamathesone, and enrolled 92 relapsed/refractory MM patients. Dr Richardson described the results of cohort 4, which met criteria for expansion as of March 2015.
The investigators enrolled 43 patients in cohort 4 to receive 840 mg daily of ibrutinib plus 40 mg of dexamethasone weekly. This dose of ibrutinib is double the dose approved by the US Food and Drug Administration (FDA) for treatment of CLL and Waldenström’s macroglobulinemia.
The patient population was “very typical” for a relapsed/refractory patient population, Dr Richardson noted. The median age was 65 years (range, 43–81), almost two-thirds were male, and they had a median of 4 (range, 2–10) prior therapies.
More than half of patients had an ECOG performance status of 0 or 1, and their median time since diagnosis was 6.5 years.
Nineteen percent had t(11;14), and other chromosomal abnormalities included del 13q14, t(4:14), and del 17p. Twenty-three patients were ISS stage I, 16 were ISS stage II, and 4 were ISS stage III.
Seventy-seven percent of patients had received an autologous stem cell transplant, 95% had prior alkylator treatment, 91% prior lenalidomide, 58% prior thalidomide, and 91% prior bortezomib treatment.
All patients were steroid-refractory. Thirty-five percent were refractory to alkylator treatment, 61% were refractory to lenalidomide, and 49% were refractory to bortezomib.
“And even a number of them had been exposed to pomalidomide and also to carfilzomib,” Dr Richardson said, “recognizing that these are the new and exciting drugs on the block, but, at the same time, a significant number of our patients already received those therapies.”
Five percent of patients achieved a partial response to ibrutinib and low-dose dexamethasone, and 18% achieved a minimal response, for a clinical benefit rate of 23%. Thirty percent had stable disease after 4 or more cycles.
“What was particularly striking,” Dr Richardson said, “was at the top dose, the 840-mg dose, the progression-free survival.”
The median time to disease progression was 5.4 months (range, 0.0–16.4), which the investigators thought was compelling in this large study.
Dr Richardson noted that there were no significant differences in safety observed across all dose cohorts, although 55% of patients experienced a grade 3 or greater treatment-emergent adverse event (AE), while 29% experienced at least 1 serious AE. And 10% of patients experienced peripheral neuropathy (PN), 8 of whom had a prior history of PN.
Treatment-emergent hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included anemia (23%) and thrombocytopenia (21%). Grade 3/4 anemia and thrombocytopenia occurred in 9% of patients in each category.
Treatment-emergent non-hematologic AEs of any grade in cohort 4 occurring in more than 20% of patients included diarrhea (63%), fatigue (49%), cough (26%), nausea (23%), and muscle spasms (21%). Two percent of patients had grade 3/4 diarrhea. There were no other grade 3/4 adverse events in the cohort.
Eighty-six percent of patients in cohort 4 discontinued treatment, 60% due to progressive disease, 12% due to an AE, and 2% at the discretion of the investigator. Twelve percent withdrew, were noncompliant, or required concomitant medication that was not permitted by the protocol.
Overall, Dr Richardson said, the safety profile was manageable, similar across the dosing cohorts, and consistent with those seen in CLL and Waldenström’s macroglobulinemia. The 840-mg dose did not increase toxicity and demonstrated activity in this heavily pretreated population.
“The real signal that struck us,” Dr Richardson emphasized, “was the progression-free survival at 5.4 months . . . for a 92-patient, multicenter experience, this is obviously, I think, an encouraging start.”
Investigators continue to explore ibrutinib in 2 ongoing combination studies, one with carfilzomib and dexamethasone (PCYC-1119) and another with pomalidomide and dexamethasone (PCYC-1138). Another trial with lenalidomide and dexamethasone is planned.
Ibrutinib now has 4 FDA-approved indications: patients with CLL who have received at least 1 prior therapy, CLL patients with del 17p, patients with mantle cell lymphoma, and patients with Waldenström’s macroglobulinemia.
Ibrutinib is distributed and marketed as Imbruvica by Pharmacyclics and also marketed by Janssen Biotech, Inc. ![]()
Snake venom helps hydrogels stop bleeding

Hartgerink, PhD, (left)
and Vivek Kumar, PhD
Photo courtesy of
Jeff Fitlow/Rice University
A nanofiber hydrogel infused with snake venom can stop bleeding quickly, even in the presence of anticoagulants, according to researchers.
The hydrogel, SB50, incorporates batroxobin, a venom produced by 2 species of South American pit viper.
SB50 can be injected as a liquid and transforms into a gel that conforms to the site of a wound, keeping it closed and promoting clotting within seconds.
The researchers described this hydrogel in ACS Biomaterials Science and Engineering.
Batroxobin was recognized for its properties as a coagulant in 1936. It has been used to remove excess fibrin proteins from the blood to treat thrombosis and as a topical hemostat. It has also been used as a diagnostic tool to determine blood-clotting time in the presence of heparin.
“From a clinical perspective, that’s far and away the most important issue here,” said study author
Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“There’s a lot of different things that can trigger blood coagulation, but when you’re on heparin, most of them don’t work or they work slowly or poorly. The use of batroxobin allows us to get around this problem because it can immediately start the clotting process, regardless of whether heparin is there or not.”
The batroxobin combined with the researchers’ hydrogels isn’t taken directly from snakes, Dr Hartgerink noted. The substance used for medicine is produced by genetically modified bacteria and then purified, avoiding the risk of other contaminant toxins.
The researchers combined batroxobin with their synthetic, self-assembling nanofibers, which can be loaded into a syringe and injected at the site of a wound, where they reassemble themselves into a gel.
Tests showed the new material stopped a wound from bleeding in as little as 6 seconds, and further prodding of the wound minutes later did not reopen it.
The researchers also tested several other options: the hydrogel without batroxobin, the batroxobin without the hydrogel, a current clinical hemostat known as GelFoam, and an alternative self-assembling hemostat known as Puramatrix. None of these options were as effective, especially in the presence of anticoagulants.
The new work builds upon the researchers’ development of injectable hydrogel scaffolds that help wounds heal and grow natural tissue. The synthetic scaffolds are built from the peptide sequences to mimic natural processes.
“We think SB50 has great potential to stop surgical bleeding, particularly in difficult cases in which the patient is taking heparin or other anticoagulants,” Dr Hartgerink said. “SB50 takes the powerful clotting ability of this snake venom and makes it far more effective by delivering it in an easily localized hydrogel that prevents possible unwanted systemic effects from using batroxobin alone.” ![]()

Hartgerink, PhD, (left)
and Vivek Kumar, PhD
Photo courtesy of
Jeff Fitlow/Rice University
A nanofiber hydrogel infused with snake venom can stop bleeding quickly, even in the presence of anticoagulants, according to researchers.
The hydrogel, SB50, incorporates batroxobin, a venom produced by 2 species of South American pit viper.
SB50 can be injected as a liquid and transforms into a gel that conforms to the site of a wound, keeping it closed and promoting clotting within seconds.
The researchers described this hydrogel in ACS Biomaterials Science and Engineering.
Batroxobin was recognized for its properties as a coagulant in 1936. It has been used to remove excess fibrin proteins from the blood to treat thrombosis and as a topical hemostat. It has also been used as a diagnostic tool to determine blood-clotting time in the presence of heparin.
“From a clinical perspective, that’s far and away the most important issue here,” said study author
Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“There’s a lot of different things that can trigger blood coagulation, but when you’re on heparin, most of them don’t work or they work slowly or poorly. The use of batroxobin allows us to get around this problem because it can immediately start the clotting process, regardless of whether heparin is there or not.”
The batroxobin combined with the researchers’ hydrogels isn’t taken directly from snakes, Dr Hartgerink noted. The substance used for medicine is produced by genetically modified bacteria and then purified, avoiding the risk of other contaminant toxins.
The researchers combined batroxobin with their synthetic, self-assembling nanofibers, which can be loaded into a syringe and injected at the site of a wound, where they reassemble themselves into a gel.
Tests showed the new material stopped a wound from bleeding in as little as 6 seconds, and further prodding of the wound minutes later did not reopen it.
The researchers also tested several other options: the hydrogel without batroxobin, the batroxobin without the hydrogel, a current clinical hemostat known as GelFoam, and an alternative self-assembling hemostat known as Puramatrix. None of these options were as effective, especially in the presence of anticoagulants.
The new work builds upon the researchers’ development of injectable hydrogel scaffolds that help wounds heal and grow natural tissue. The synthetic scaffolds are built from the peptide sequences to mimic natural processes.
“We think SB50 has great potential to stop surgical bleeding, particularly in difficult cases in which the patient is taking heparin or other anticoagulants,” Dr Hartgerink said. “SB50 takes the powerful clotting ability of this snake venom and makes it far more effective by delivering it in an easily localized hydrogel that prevents possible unwanted systemic effects from using batroxobin alone.” ![]()

Hartgerink, PhD, (left)
and Vivek Kumar, PhD
Photo courtesy of
Jeff Fitlow/Rice University
A nanofiber hydrogel infused with snake venom can stop bleeding quickly, even in the presence of anticoagulants, according to researchers.
The hydrogel, SB50, incorporates batroxobin, a venom produced by 2 species of South American pit viper.
SB50 can be injected as a liquid and transforms into a gel that conforms to the site of a wound, keeping it closed and promoting clotting within seconds.
The researchers described this hydrogel in ACS Biomaterials Science and Engineering.
Batroxobin was recognized for its properties as a coagulant in 1936. It has been used to remove excess fibrin proteins from the blood to treat thrombosis and as a topical hemostat. It has also been used as a diagnostic tool to determine blood-clotting time in the presence of heparin.
“From a clinical perspective, that’s far and away the most important issue here,” said study author
Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“There’s a lot of different things that can trigger blood coagulation, but when you’re on heparin, most of them don’t work or they work slowly or poorly. The use of batroxobin allows us to get around this problem because it can immediately start the clotting process, regardless of whether heparin is there or not.”
The batroxobin combined with the researchers’ hydrogels isn’t taken directly from snakes, Dr Hartgerink noted. The substance used for medicine is produced by genetically modified bacteria and then purified, avoiding the risk of other contaminant toxins.
The researchers combined batroxobin with their synthetic, self-assembling nanofibers, which can be loaded into a syringe and injected at the site of a wound, where they reassemble themselves into a gel.
Tests showed the new material stopped a wound from bleeding in as little as 6 seconds, and further prodding of the wound minutes later did not reopen it.
The researchers also tested several other options: the hydrogel without batroxobin, the batroxobin without the hydrogel, a current clinical hemostat known as GelFoam, and an alternative self-assembling hemostat known as Puramatrix. None of these options were as effective, especially in the presence of anticoagulants.
The new work builds upon the researchers’ development of injectable hydrogel scaffolds that help wounds heal and grow natural tissue. The synthetic scaffolds are built from the peptide sequences to mimic natural processes.
“We think SB50 has great potential to stop surgical bleeding, particularly in difficult cases in which the patient is taking heparin or other anticoagulants,” Dr Hartgerink said. “SB50 takes the powerful clotting ability of this snake venom and makes it far more effective by delivering it in an easily localized hydrogel that prevents possible unwanted systemic effects from using batroxobin alone.” ![]()
New test could help fight leukemia

Image courtesy of NIAID
Researchers say they have developed a test that can reveal how the immune system would respond to vaccines for leukemia.
To conduct this test, cancer-specific-proteins are spotted onto a microscope slide.
They are then incubated with a patient blood sample to show whether the immune system can recognize the proteins.
The researchers believe this test could inform immunotherapy trial development and eventually direct the treatment of leukemia.
They described the test in PLOS ONE.
The team explained that cellular arrays using peptide-MHC (pMHC) tetramers allow the simultaneous detection of different antigen-specific T-cell populations that are naturally circulating in leukemia patients and healthy individuals.
The researchers developed a pMHC array to detect CD8+ T-cell populations in leukemia patients that recognize epitopes within viral antigens and leukemia antigens.
Experiments showed this test was at least as sensitive as flow cytometry.
The pMHC array successfully identified more than 40 T-cell populations. It identified T cells that recognized various tumor antigen epitopes in patients with acute myeloid leukemia and acute lymphoblastic leukemia.
“This [test] would allow us to know how good a patients’ immune system is and potentially which proteins their immune system will react to, allowing us to prioritize which proteins we use to develop anticancer vaccines,” said study author Barbara Guinn, PhD, of the University of Southampton in the UK.
“In the future, we may be able to monitor patient immune responses as they are treated in clinical trials, helping us to direct the immune system more efficiently against cancer cells.”
Dr Guinn has spent a large part of her career investigating the differences between cancer cells and normal cells in terms of the proteins they make. She has been able to identify a number of proteins that are overexpressed in tumor cells but not healthy cells.
“Some of these proteins act as biomarkers for patient survival,” she said, “and some of them have helped us understand more about how cancer develops in subgroups of patients with leukemia.” ![]()

Image courtesy of NIAID
Researchers say they have developed a test that can reveal how the immune system would respond to vaccines for leukemia.
To conduct this test, cancer-specific-proteins are spotted onto a microscope slide.
They are then incubated with a patient blood sample to show whether the immune system can recognize the proteins.
The researchers believe this test could inform immunotherapy trial development and eventually direct the treatment of leukemia.
They described the test in PLOS ONE.
The team explained that cellular arrays using peptide-MHC (pMHC) tetramers allow the simultaneous detection of different antigen-specific T-cell populations that are naturally circulating in leukemia patients and healthy individuals.
The researchers developed a pMHC array to detect CD8+ T-cell populations in leukemia patients that recognize epitopes within viral antigens and leukemia antigens.
Experiments showed this test was at least as sensitive as flow cytometry.
The pMHC array successfully identified more than 40 T-cell populations. It identified T cells that recognized various tumor antigen epitopes in patients with acute myeloid leukemia and acute lymphoblastic leukemia.
“This [test] would allow us to know how good a patients’ immune system is and potentially which proteins their immune system will react to, allowing us to prioritize which proteins we use to develop anticancer vaccines,” said study author Barbara Guinn, PhD, of the University of Southampton in the UK.
“In the future, we may be able to monitor patient immune responses as they are treated in clinical trials, helping us to direct the immune system more efficiently against cancer cells.”
Dr Guinn has spent a large part of her career investigating the differences between cancer cells and normal cells in terms of the proteins they make. She has been able to identify a number of proteins that are overexpressed in tumor cells but not healthy cells.
“Some of these proteins act as biomarkers for patient survival,” she said, “and some of them have helped us understand more about how cancer develops in subgroups of patients with leukemia.” ![]()

Image courtesy of NIAID
Researchers say they have developed a test that can reveal how the immune system would respond to vaccines for leukemia.
To conduct this test, cancer-specific-proteins are spotted onto a microscope slide.
They are then incubated with a patient blood sample to show whether the immune system can recognize the proteins.
The researchers believe this test could inform immunotherapy trial development and eventually direct the treatment of leukemia.
They described the test in PLOS ONE.
The team explained that cellular arrays using peptide-MHC (pMHC) tetramers allow the simultaneous detection of different antigen-specific T-cell populations that are naturally circulating in leukemia patients and healthy individuals.
The researchers developed a pMHC array to detect CD8+ T-cell populations in leukemia patients that recognize epitopes within viral antigens and leukemia antigens.
Experiments showed this test was at least as sensitive as flow cytometry.
The pMHC array successfully identified more than 40 T-cell populations. It identified T cells that recognized various tumor antigen epitopes in patients with acute myeloid leukemia and acute lymphoblastic leukemia.
“This [test] would allow us to know how good a patients’ immune system is and potentially which proteins their immune system will react to, allowing us to prioritize which proteins we use to develop anticancer vaccines,” said study author Barbara Guinn, PhD, of the University of Southampton in the UK.
“In the future, we may be able to monitor patient immune responses as they are treated in clinical trials, helping us to direct the immune system more efficiently against cancer cells.”
Dr Guinn has spent a large part of her career investigating the differences between cancer cells and normal cells in terms of the proteins they make. She has been able to identify a number of proteins that are overexpressed in tumor cells but not healthy cells.
“Some of these proteins act as biomarkers for patient survival,” she said, “and some of them have helped us understand more about how cancer develops in subgroups of patients with leukemia.” ![]()
Team aims to inhibit Notch safely

Photo courtesy of the
University of Michigan
A new study suggests a potential way to block one of the most common cancer-causing genes without causing severe side effects.
The Notch gene plays a role in many cancers, and it’s the most common cancer-causing gene in T-cell acute lymphoblastic leukemia (T-ALL).
About 60% of children and adults with T-ALL harbor a Notch mutation.
Unfortunately, drugs that inhibit Notch can cause serious side effects, such as skin cancers.
Now, investigators have discovered a potential new target to inhibit Notch without the toxic effects.
They found that a protein called Zmiz1 sticks to Notch, prompting the gene to turn on its cancer function. But Zmiz1 does not impact normal, healthy Notch functions.
“Notch controls the genes that cause cancer, but it’s also important for normal health,” said Mark Chiang, MD, PhD, of the University of Michigan in Ann Arbor.
“The challenge is to knock out the cancer function of Notch but preserve its normal function. If you unstick Zmiz1 from Notch, the cancer cells die. And Zmiz1 seems to be selective in turning on the cancer functions of Notch.”
Dr Chiang and his colleagues found that mice lived longer when Zmiz1 was deleted. The mice had normal body weight and no severe side effects from Zmiz1 deletion.
The investigators reported these results in Immunity.
“Our goal is to develop a drug to sit right between Notch and Zmiz1 that could break apart the bond,” Dr Chiang said. “We think this would block the Notch cancer pathway without causing toxic side effects, like we see with current Notch inhibitors.”
He noted that a majority of children with T-ALL are cured, but about 20% will relapse. Those children face a grim prognosis.
“We need to develop therapies against Notch to help kids with relapsed cancer and to cure kids with fewer toxicities or long-term effects,” Dr Chiang said. “Our current treatments may often be curative, but there can be a huge price to pay in late effects.”
To further this research, Dr Chiang and his colleagues plan to use X-ray crystallography to create a 3-dimensional image of Notch and Zmiz1 in an effort to understand how they are sticking together. This could help the team to design a drug to separate the proteins. ![]()

Photo courtesy of the
University of Michigan
A new study suggests a potential way to block one of the most common cancer-causing genes without causing severe side effects.
The Notch gene plays a role in many cancers, and it’s the most common cancer-causing gene in T-cell acute lymphoblastic leukemia (T-ALL).
About 60% of children and adults with T-ALL harbor a Notch mutation.
Unfortunately, drugs that inhibit Notch can cause serious side effects, such as skin cancers.
Now, investigators have discovered a potential new target to inhibit Notch without the toxic effects.
They found that a protein called Zmiz1 sticks to Notch, prompting the gene to turn on its cancer function. But Zmiz1 does not impact normal, healthy Notch functions.
“Notch controls the genes that cause cancer, but it’s also important for normal health,” said Mark Chiang, MD, PhD, of the University of Michigan in Ann Arbor.
“The challenge is to knock out the cancer function of Notch but preserve its normal function. If you unstick Zmiz1 from Notch, the cancer cells die. And Zmiz1 seems to be selective in turning on the cancer functions of Notch.”
Dr Chiang and his colleagues found that mice lived longer when Zmiz1 was deleted. The mice had normal body weight and no severe side effects from Zmiz1 deletion.
The investigators reported these results in Immunity.
“Our goal is to develop a drug to sit right between Notch and Zmiz1 that could break apart the bond,” Dr Chiang said. “We think this would block the Notch cancer pathway without causing toxic side effects, like we see with current Notch inhibitors.”
He noted that a majority of children with T-ALL are cured, but about 20% will relapse. Those children face a grim prognosis.
“We need to develop therapies against Notch to help kids with relapsed cancer and to cure kids with fewer toxicities or long-term effects,” Dr Chiang said. “Our current treatments may often be curative, but there can be a huge price to pay in late effects.”
To further this research, Dr Chiang and his colleagues plan to use X-ray crystallography to create a 3-dimensional image of Notch and Zmiz1 in an effort to understand how they are sticking together. This could help the team to design a drug to separate the proteins. ![]()

Photo courtesy of the
University of Michigan
A new study suggests a potential way to block one of the most common cancer-causing genes without causing severe side effects.
The Notch gene plays a role in many cancers, and it’s the most common cancer-causing gene in T-cell acute lymphoblastic leukemia (T-ALL).
About 60% of children and adults with T-ALL harbor a Notch mutation.
Unfortunately, drugs that inhibit Notch can cause serious side effects, such as skin cancers.
Now, investigators have discovered a potential new target to inhibit Notch without the toxic effects.
They found that a protein called Zmiz1 sticks to Notch, prompting the gene to turn on its cancer function. But Zmiz1 does not impact normal, healthy Notch functions.
“Notch controls the genes that cause cancer, but it’s also important for normal health,” said Mark Chiang, MD, PhD, of the University of Michigan in Ann Arbor.
“The challenge is to knock out the cancer function of Notch but preserve its normal function. If you unstick Zmiz1 from Notch, the cancer cells die. And Zmiz1 seems to be selective in turning on the cancer functions of Notch.”
Dr Chiang and his colleagues found that mice lived longer when Zmiz1 was deleted. The mice had normal body weight and no severe side effects from Zmiz1 deletion.
The investigators reported these results in Immunity.
“Our goal is to develop a drug to sit right between Notch and Zmiz1 that could break apart the bond,” Dr Chiang said. “We think this would block the Notch cancer pathway without causing toxic side effects, like we see with current Notch inhibitors.”
He noted that a majority of children with T-ALL are cured, but about 20% will relapse. Those children face a grim prognosis.
“We need to develop therapies against Notch to help kids with relapsed cancer and to cure kids with fewer toxicities or long-term effects,” Dr Chiang said. “Our current treatments may often be curative, but there can be a huge price to pay in late effects.”
To further this research, Dr Chiang and his colleagues plan to use X-ray crystallography to create a 3-dimensional image of Notch and Zmiz1 in an effort to understand how they are sticking together. This could help the team to design a drug to separate the proteins. ![]()






