User login
Find Answers, Share Stories at SHM's New Patient Experience Community
Have you had successes with improving the patient experience at your hospital? Or have you had a challenge or question? SHM’s new Patient Experience community on HMX is a great way to find answers and share your stories. In just the first few days of the community, participants are talking about the impact of computers and smartphones on the patient experience. To join the conversation, SHM members can find the Patient Experience community in HMX.
Have you had successes with improving the patient experience at your hospital? Or have you had a challenge or question? SHM’s new Patient Experience community on HMX is a great way to find answers and share your stories. In just the first few days of the community, participants are talking about the impact of computers and smartphones on the patient experience. To join the conversation, SHM members can find the Patient Experience community in HMX.
Have you had successes with improving the patient experience at your hospital? Or have you had a challenge or question? SHM’s new Patient Experience community on HMX is a great way to find answers and share your stories. In just the first few days of the community, participants are talking about the impact of computers and smartphones on the patient experience. To join the conversation, SHM members can find the Patient Experience community in HMX.
Post-Operative Transfusions after Noncardiac Surgery Associated with Increased Adverse Outcomes
Clinical question: Do transfusions affect post-operative outcomes after noncardiac surgery?
Background: Studies have demonstrated that a restrictive transfusion strategy is probably superior to a liberal transfusion strategy in many clinical settings. Despite this data, there continues to be wide variation in the use of blood transfusions in the peri-operative setting.
Study design: Retrospective cohort study.
Setting: Fifty-two community and academic hospitals in Michigan.
Synopsis: Demographic, operative, and outcomes data were extracted from the Michigan Surgical Quality Collaborative and reviewed for 48,720 patients who underwent noncardiac surgery between 2012-2014. A total of 4.6% of patients received a blood transfusion within 72 hours after surgery. The patients who received blood products were at increased risk for death at 30 days (3.6% excess absolute risk), for infectious complications (1% excess absolute risk), and for having at least one post-operative noninfectious complication (4.4% increased absolute risk).
Bottom line: Although observational in nature, this study adds to the increasing body of evidence supporting an increase in surgical morbidity and mortality associated with blood transfusions.
Citation: Abdelsattar ZM, Hendren S, Wong SL, Campbell DA Jr, Henke P. Variation in transfusion practices and the effect on outcomes after noncardiac surgery. Ann Surg. 2015;262(1):1-6.
Clinical question: Do transfusions affect post-operative outcomes after noncardiac surgery?
Background: Studies have demonstrated that a restrictive transfusion strategy is probably superior to a liberal transfusion strategy in many clinical settings. Despite this data, there continues to be wide variation in the use of blood transfusions in the peri-operative setting.
Study design: Retrospective cohort study.
Setting: Fifty-two community and academic hospitals in Michigan.
Synopsis: Demographic, operative, and outcomes data were extracted from the Michigan Surgical Quality Collaborative and reviewed for 48,720 patients who underwent noncardiac surgery between 2012-2014. A total of 4.6% of patients received a blood transfusion within 72 hours after surgery. The patients who received blood products were at increased risk for death at 30 days (3.6% excess absolute risk), for infectious complications (1% excess absolute risk), and for having at least one post-operative noninfectious complication (4.4% increased absolute risk).
Bottom line: Although observational in nature, this study adds to the increasing body of evidence supporting an increase in surgical morbidity and mortality associated with blood transfusions.
Citation: Abdelsattar ZM, Hendren S, Wong SL, Campbell DA Jr, Henke P. Variation in transfusion practices and the effect on outcomes after noncardiac surgery. Ann Surg. 2015;262(1):1-6.
Clinical question: Do transfusions affect post-operative outcomes after noncardiac surgery?
Background: Studies have demonstrated that a restrictive transfusion strategy is probably superior to a liberal transfusion strategy in many clinical settings. Despite this data, there continues to be wide variation in the use of blood transfusions in the peri-operative setting.
Study design: Retrospective cohort study.
Setting: Fifty-two community and academic hospitals in Michigan.
Synopsis: Demographic, operative, and outcomes data were extracted from the Michigan Surgical Quality Collaborative and reviewed for 48,720 patients who underwent noncardiac surgery between 2012-2014. A total of 4.6% of patients received a blood transfusion within 72 hours after surgery. The patients who received blood products were at increased risk for death at 30 days (3.6% excess absolute risk), for infectious complications (1% excess absolute risk), and for having at least one post-operative noninfectious complication (4.4% increased absolute risk).
Bottom line: Although observational in nature, this study adds to the increasing body of evidence supporting an increase in surgical morbidity and mortality associated with blood transfusions.
Citation: Abdelsattar ZM, Hendren S, Wong SL, Campbell DA Jr, Henke P. Variation in transfusion practices and the effect on outcomes after noncardiac surgery. Ann Surg. 2015;262(1):1-6.
Left Atrial Appendage Closure Favorable Over Warfarin for Atrial Fibrillation
Clinical question: Is there a favorable risk-benefit ratio for left atrial appendage closure (LAAC) compared to warfarin for prevention of stroke, systemic embolism, and cardiovascular death in nonvalvular atrial fibrillation?
Background: LAAC with the WATCHMAN device was shown to be noninferior to warfarin for the prevention of stroke, systemic embolism, and cardiovascular death in nonvalvular atrial fibrillation in two trials: PROTECT AF and PREVAIL. Further efficacy concerns were raised following routine regulatory filings, leading to the need for continued evaluation.
Study design: Meta-analysis.
Setting: Patient-level data were combined and analyzed from the PROTECT AF and PREVAIL trails and two nonrandomized registries of LAAC with the WATCHMAN device: the Continued Access PROTECT AF registry (CAP) and the Continued Access to PREVAIL registry (CAP2).
Synopsis: A total of 2,406 patients were enrolled from all four data sets from 2005-2014. Of those, 1,877 were treated with the WATCHMAN device and 382 were treated with warfarin. Annualized risk of stroke if untreated with anticoagulation for all patients was 5.7% to 7.6%, indicating that all were eligible to be treated with warfarin. Ninety percent of patients had moderate to high risk of bleeding. Analysis showed that LAAC was noninferior to warfarin for stroke, systemic embolism, and cardiovascular death.
A slight increase in ischemic stroke in the LAAC group was counterbalanced by the significant reduction in hemorrhagic stroke in the LAAC group versus the warfarin group. Cardiovascular deaths were significantly fewer in the LAAC cohort; all-cause mortality favored LAAC but did not reach statistical significance. There was also a significant reduction in nonprocedure-related major bleeding in the LAAC group. Limitations of this study include the limited number of patients treated with warfarin and lack of comparison to new oral anticoagulants (NOACs).
Bottom line: Patients with increased stroke risk from nonvalvular atrial fibrillation treated with the WATCHMAN device for LAAC have significant reductions in hemorrhagic stroke, cardiovascular death, and nonprocedure-related major bleeding, but slightly increased risk of ischemic stroke compared to those treated with warfarin.
Citaiton: Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65(24):2614-2623.
Clinical question: Is there a favorable risk-benefit ratio for left atrial appendage closure (LAAC) compared to warfarin for prevention of stroke, systemic embolism, and cardiovascular death in nonvalvular atrial fibrillation?
Background: LAAC with the WATCHMAN device was shown to be noninferior to warfarin for the prevention of stroke, systemic embolism, and cardiovascular death in nonvalvular atrial fibrillation in two trials: PROTECT AF and PREVAIL. Further efficacy concerns were raised following routine regulatory filings, leading to the need for continued evaluation.
Study design: Meta-analysis.
Setting: Patient-level data were combined and analyzed from the PROTECT AF and PREVAIL trails and two nonrandomized registries of LAAC with the WATCHMAN device: the Continued Access PROTECT AF registry (CAP) and the Continued Access to PREVAIL registry (CAP2).
Synopsis: A total of 2,406 patients were enrolled from all four data sets from 2005-2014. Of those, 1,877 were treated with the WATCHMAN device and 382 were treated with warfarin. Annualized risk of stroke if untreated with anticoagulation for all patients was 5.7% to 7.6%, indicating that all were eligible to be treated with warfarin. Ninety percent of patients had moderate to high risk of bleeding. Analysis showed that LAAC was noninferior to warfarin for stroke, systemic embolism, and cardiovascular death.
A slight increase in ischemic stroke in the LAAC group was counterbalanced by the significant reduction in hemorrhagic stroke in the LAAC group versus the warfarin group. Cardiovascular deaths were significantly fewer in the LAAC cohort; all-cause mortality favored LAAC but did not reach statistical significance. There was also a significant reduction in nonprocedure-related major bleeding in the LAAC group. Limitations of this study include the limited number of patients treated with warfarin and lack of comparison to new oral anticoagulants (NOACs).
Bottom line: Patients with increased stroke risk from nonvalvular atrial fibrillation treated with the WATCHMAN device for LAAC have significant reductions in hemorrhagic stroke, cardiovascular death, and nonprocedure-related major bleeding, but slightly increased risk of ischemic stroke compared to those treated with warfarin.
Citaiton: Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65(24):2614-2623.
Clinical question: Is there a favorable risk-benefit ratio for left atrial appendage closure (LAAC) compared to warfarin for prevention of stroke, systemic embolism, and cardiovascular death in nonvalvular atrial fibrillation?
Background: LAAC with the WATCHMAN device was shown to be noninferior to warfarin for the prevention of stroke, systemic embolism, and cardiovascular death in nonvalvular atrial fibrillation in two trials: PROTECT AF and PREVAIL. Further efficacy concerns were raised following routine regulatory filings, leading to the need for continued evaluation.
Study design: Meta-analysis.
Setting: Patient-level data were combined and analyzed from the PROTECT AF and PREVAIL trails and two nonrandomized registries of LAAC with the WATCHMAN device: the Continued Access PROTECT AF registry (CAP) and the Continued Access to PREVAIL registry (CAP2).
Synopsis: A total of 2,406 patients were enrolled from all four data sets from 2005-2014. Of those, 1,877 were treated with the WATCHMAN device and 382 were treated with warfarin. Annualized risk of stroke if untreated with anticoagulation for all patients was 5.7% to 7.6%, indicating that all were eligible to be treated with warfarin. Ninety percent of patients had moderate to high risk of bleeding. Analysis showed that LAAC was noninferior to warfarin for stroke, systemic embolism, and cardiovascular death.
A slight increase in ischemic stroke in the LAAC group was counterbalanced by the significant reduction in hemorrhagic stroke in the LAAC group versus the warfarin group. Cardiovascular deaths were significantly fewer in the LAAC cohort; all-cause mortality favored LAAC but did not reach statistical significance. There was also a significant reduction in nonprocedure-related major bleeding in the LAAC group. Limitations of this study include the limited number of patients treated with warfarin and lack of comparison to new oral anticoagulants (NOACs).
Bottom line: Patients with increased stroke risk from nonvalvular atrial fibrillation treated with the WATCHMAN device for LAAC have significant reductions in hemorrhagic stroke, cardiovascular death, and nonprocedure-related major bleeding, but slightly increased risk of ischemic stroke compared to those treated with warfarin.
Citaiton: Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65(24):2614-2623.
Pediatric Trigger Tool Helps Identify Inpatient Pediatric Harm
Clinical question: Can a trigger tool identify harms for hospitalized children?
Background: An estimated 400,000 people die annually in the United States as a result of hospital-associated harm. The Centers for Medicare and Medicaid Services define harm as “unintended physical injury … by medical care that required additional monitoring, treatment, or hospitalization or that resulted in death.” Although harm is common, voluntary reporting of events has been shown to capture only 2%-8% of harm. Global Trigger Tools (GTT) are an alternative to voluntary reports. These tools use “triggers,” or clues, to help reviewers identify potential harms when reviewing the electronic heath record. The Institute for Healthcare Improvement (IHI) has created an adult-focused GTT; however, no pediatric-focused GTT exists.
Study design: Cross-sectional, retrospective chart review.
Setting: Children <22 years old discharged from six freestanding U.S. children’s hospitals in February 2012.
Synopsis: In a prior paper, the authors described how they used a modified Delphi technique to develop a pediatric GTT based upon the IHI GTT. Here they piloted this new pediatric-focused GTT through a retrospective chart review. One clinical nonphysician reviewer and one physician reviewer were selected from each site and received training on use of the pediatric GTT and the identification of harms. One hundred charts from each site were randomly selected for application of the GTT. The reviewers examined the charts for harms and then applied the GTT. When reviewers found a harm, they determined the likelihood that the harm was preventable.
Of the 600 records reviewed, 240 harms were found. The GTT identified 1,093 potential harms, leading to identification of 204 harms. The remaining 36 harms did not cause a trigger and were found by chart review. The positive predictive value of the aggregate GTT was 22%. There were 40 harms per 100 patients, and 24.3% of patients had one or more harm. Sixty-eight percent of harms were of the least severe type, and only one led to a patient death. The most common harms were intravenous catheter infiltration, respiratory distress, constipation, pain, and surgical complications.
Bottom line: The pediatric GTT appears to be a moderately sensitive indicator for inpatient pediatric harm. Inpatient pediatric harm occurs frequently, with about one in four pediatric inpatients suffering from harm. Serious harm appears uncommon.
Citation: Stockwell DC, Bisarya H, Classen DC, et al. A trigger tool to detect harm in pediatric inpatient settings. Pediatrics. 2015;135(6):1036-1042.

Clinical question: Can a trigger tool identify harms for hospitalized children?
Background: An estimated 400,000 people die annually in the United States as a result of hospital-associated harm. The Centers for Medicare and Medicaid Services define harm as “unintended physical injury … by medical care that required additional monitoring, treatment, or hospitalization or that resulted in death.” Although harm is common, voluntary reporting of events has been shown to capture only 2%-8% of harm. Global Trigger Tools (GTT) are an alternative to voluntary reports. These tools use “triggers,” or clues, to help reviewers identify potential harms when reviewing the electronic heath record. The Institute for Healthcare Improvement (IHI) has created an adult-focused GTT; however, no pediatric-focused GTT exists.
Study design: Cross-sectional, retrospective chart review.
Setting: Children <22 years old discharged from six freestanding U.S. children’s hospitals in February 2012.
Synopsis: In a prior paper, the authors described how they used a modified Delphi technique to develop a pediatric GTT based upon the IHI GTT. Here they piloted this new pediatric-focused GTT through a retrospective chart review. One clinical nonphysician reviewer and one physician reviewer were selected from each site and received training on use of the pediatric GTT and the identification of harms. One hundred charts from each site were randomly selected for application of the GTT. The reviewers examined the charts for harms and then applied the GTT. When reviewers found a harm, they determined the likelihood that the harm was preventable.
Of the 600 records reviewed, 240 harms were found. The GTT identified 1,093 potential harms, leading to identification of 204 harms. The remaining 36 harms did not cause a trigger and were found by chart review. The positive predictive value of the aggregate GTT was 22%. There were 40 harms per 100 patients, and 24.3% of patients had one or more harm. Sixty-eight percent of harms were of the least severe type, and only one led to a patient death. The most common harms were intravenous catheter infiltration, respiratory distress, constipation, pain, and surgical complications.
Bottom line: The pediatric GTT appears to be a moderately sensitive indicator for inpatient pediatric harm. Inpatient pediatric harm occurs frequently, with about one in four pediatric inpatients suffering from harm. Serious harm appears uncommon.
Citation: Stockwell DC, Bisarya H, Classen DC, et al. A trigger tool to detect harm in pediatric inpatient settings. Pediatrics. 2015;135(6):1036-1042.

Clinical question: Can a trigger tool identify harms for hospitalized children?
Background: An estimated 400,000 people die annually in the United States as a result of hospital-associated harm. The Centers for Medicare and Medicaid Services define harm as “unintended physical injury … by medical care that required additional monitoring, treatment, or hospitalization or that resulted in death.” Although harm is common, voluntary reporting of events has been shown to capture only 2%-8% of harm. Global Trigger Tools (GTT) are an alternative to voluntary reports. These tools use “triggers,” or clues, to help reviewers identify potential harms when reviewing the electronic heath record. The Institute for Healthcare Improvement (IHI) has created an adult-focused GTT; however, no pediatric-focused GTT exists.
Study design: Cross-sectional, retrospective chart review.
Setting: Children <22 years old discharged from six freestanding U.S. children’s hospitals in February 2012.
Synopsis: In a prior paper, the authors described how they used a modified Delphi technique to develop a pediatric GTT based upon the IHI GTT. Here they piloted this new pediatric-focused GTT through a retrospective chart review. One clinical nonphysician reviewer and one physician reviewer were selected from each site and received training on use of the pediatric GTT and the identification of harms. One hundred charts from each site were randomly selected for application of the GTT. The reviewers examined the charts for harms and then applied the GTT. When reviewers found a harm, they determined the likelihood that the harm was preventable.
Of the 600 records reviewed, 240 harms were found. The GTT identified 1,093 potential harms, leading to identification of 204 harms. The remaining 36 harms did not cause a trigger and were found by chart review. The positive predictive value of the aggregate GTT was 22%. There were 40 harms per 100 patients, and 24.3% of patients had one or more harm. Sixty-eight percent of harms were of the least severe type, and only one led to a patient death. The most common harms were intravenous catheter infiltration, respiratory distress, constipation, pain, and surgical complications.
Bottom line: The pediatric GTT appears to be a moderately sensitive indicator for inpatient pediatric harm. Inpatient pediatric harm occurs frequently, with about one in four pediatric inpatients suffering from harm. Serious harm appears uncommon.
Citation: Stockwell DC, Bisarya H, Classen DC, et al. A trigger tool to detect harm in pediatric inpatient settings. Pediatrics. 2015;135(6):1036-1042.

Easing the Grieving Process for Families of Patients Dying in the ICU
Clinical question: Can we dignify death in the ICU and ease the grieving process by soliciting wishes from patients, families, and care team members?
Background: The death of the critically ill patient in the ICU can be dehumanizing and overwhelming for the patient’s family and friends, leading to prolonged physical and psychological stress. These deaths might have similar effects on the clinicians caring for the patients.
Study design: Mixed methods.
Setting: Medical-surgical ICU at a 21-bed, academic tertiary medical center in Ontario, Canada.
Synopsis: Semi-structured interviews were conducted with at least one family member and three clinicians per patient. A total of 40 patients were screened and deemed eligible for inclusion. Only seven patients were able to provide input on the wishes or interviews; the others had impaired consciousness. The team obtained 163 wishes from those individuals, and was able to implement 159 of them (97.5%). At least three wishes from each patient-family dyad were implemented.
The wishes were classified into five categories:
- Humanizing the environment;
- Personal tributes;
- Family reconnections;
- Rituals and observances; and
- Paying it forward.
These wishes were implemented before (51.6%) and after (48.4%) death and were generally inexpensive (less than $200 per patient).
From the 160 interviews of 170 individuals, the central theme that emerged was personalization of the dying process in the ICU through three related domains: dignifying the patient, giving the family a voice, and fostering clinician compassion.
The 3 Wishes Project provides a framework to foster discussion among care team members and families to ensure personalization and dignity in the dying process.
Bottom line: Solicitation of wishes from dying patients, their families, and their care team members can have a positive impact by allowing individualized end-of-life care.
Citation: Cook D, Swinton M, Toledo F, et al. Personalizing death in the intensive care unit: the 3 Wishes Project: a mixed-methods study. Ann Intern Med. 2015;163(4):271-279.
Clinical question: Can we dignify death in the ICU and ease the grieving process by soliciting wishes from patients, families, and care team members?
Background: The death of the critically ill patient in the ICU can be dehumanizing and overwhelming for the patient’s family and friends, leading to prolonged physical and psychological stress. These deaths might have similar effects on the clinicians caring for the patients.
Study design: Mixed methods.
Setting: Medical-surgical ICU at a 21-bed, academic tertiary medical center in Ontario, Canada.
Synopsis: Semi-structured interviews were conducted with at least one family member and three clinicians per patient. A total of 40 patients were screened and deemed eligible for inclusion. Only seven patients were able to provide input on the wishes or interviews; the others had impaired consciousness. The team obtained 163 wishes from those individuals, and was able to implement 159 of them (97.5%). At least three wishes from each patient-family dyad were implemented.
The wishes were classified into five categories:
- Humanizing the environment;
- Personal tributes;
- Family reconnections;
- Rituals and observances; and
- Paying it forward.
These wishes were implemented before (51.6%) and after (48.4%) death and were generally inexpensive (less than $200 per patient).
From the 160 interviews of 170 individuals, the central theme that emerged was personalization of the dying process in the ICU through three related domains: dignifying the patient, giving the family a voice, and fostering clinician compassion.
The 3 Wishes Project provides a framework to foster discussion among care team members and families to ensure personalization and dignity in the dying process.
Bottom line: Solicitation of wishes from dying patients, their families, and their care team members can have a positive impact by allowing individualized end-of-life care.
Citation: Cook D, Swinton M, Toledo F, et al. Personalizing death in the intensive care unit: the 3 Wishes Project: a mixed-methods study. Ann Intern Med. 2015;163(4):271-279.
Clinical question: Can we dignify death in the ICU and ease the grieving process by soliciting wishes from patients, families, and care team members?
Background: The death of the critically ill patient in the ICU can be dehumanizing and overwhelming for the patient’s family and friends, leading to prolonged physical and psychological stress. These deaths might have similar effects on the clinicians caring for the patients.
Study design: Mixed methods.
Setting: Medical-surgical ICU at a 21-bed, academic tertiary medical center in Ontario, Canada.
Synopsis: Semi-structured interviews were conducted with at least one family member and three clinicians per patient. A total of 40 patients were screened and deemed eligible for inclusion. Only seven patients were able to provide input on the wishes or interviews; the others had impaired consciousness. The team obtained 163 wishes from those individuals, and was able to implement 159 of them (97.5%). At least three wishes from each patient-family dyad were implemented.
The wishes were classified into five categories:
- Humanizing the environment;
- Personal tributes;
- Family reconnections;
- Rituals and observances; and
- Paying it forward.
These wishes were implemented before (51.6%) and after (48.4%) death and were generally inexpensive (less than $200 per patient).
From the 160 interviews of 170 individuals, the central theme that emerged was personalization of the dying process in the ICU through three related domains: dignifying the patient, giving the family a voice, and fostering clinician compassion.
The 3 Wishes Project provides a framework to foster discussion among care team members and families to ensure personalization and dignity in the dying process.
Bottom line: Solicitation of wishes from dying patients, their families, and their care team members can have a positive impact by allowing individualized end-of-life care.
Citation: Cook D, Swinton M, Toledo F, et al. Personalizing death in the intensive care unit: the 3 Wishes Project: a mixed-methods study. Ann Intern Med. 2015;163(4):271-279.
Germline genetic variation linked to pediatric ALL

Photo courtesy of
St. Jude/Seth Dixon
Germline variations in the ETV6 gene are associated with an increased risk of developing pediatric acute lymphoblastic leukemia (ALL), according to research published in The Lancet Oncology.
Researchers said the magnitude of the risk must still be determined, as well as how the variants identified may promote ALL.
The evidence suggests that ETV6 variation alone is not sufficient to cause ALL but may play a significant role in inherited predisposition to childhood ALL.
The researchers discovered the association between the ETV6 variants and childhood ALL by sequencing the whole exome of a family in which the mother and 2 of the 3 children have a history of pediatric ALL.
All were treated at St. Jude Children’s Research Hospital in Memphis, Tennessee, and are now cancer-free.
The researchers identified a novel non-sense ETV6 variant (p.Arg359X) in this mother and her 3 children, including a daughter who has not been diagnosed with cancer. The father does not have the variant.
This variant is predicted to result in the production of a shortened ETV6 protein that cannot fulfill its normal function of binding to DNA and regulating the expression of other genes.
The researchers screened an additional 4405 children with ALL and found 31 ETV6 exonic variants—21 missense, 5 frameshift, 4 non-sense, and 1 splice site—that are potentially related to leukemia risk in 35 patients, or almost 1% of the patients screened.
The variants identified were unique to ALL patients or extremely rare in the general population, the researchers said.
Patients with the variants tended to be older when diagnosed with ALL (10.2 years vs 4.7 years; P=0.017) and were more likely to have hyperdiploid leukemia. Sixty-four percent of ALL cases with germline ETV6 variants were hyperdiploid, compared to 27% of ALL cases without the variants (P=0.0050).
The variants were not associated with a particular ethnicity or with the outcome of ALL therapy.
The researchers also noted that almost half of the ETV6 variants identified (n=15) clustered in the erythroblast transformation specific domain.
“That suggests the loss or alteration of this DNA-binding function of ETV6 may be critical to cancer promotion,” said study author Jun J. Yang, PhD, of St. Jude.
“This is the latest example of the important role that genetic variation and inheritance plays in ALL risk. That has clear clinical implications and will help us understand the biology driving this cancer.”
These findings build on previous work that revealed an association between inherited ETV6 variations and thrombocytopenia in families with a susceptibility to hematologic malignancies. The researchers said this new study further solidifies the association between ETV6 and pediatric ALL. ![]()

Photo courtesy of
St. Jude/Seth Dixon
Germline variations in the ETV6 gene are associated with an increased risk of developing pediatric acute lymphoblastic leukemia (ALL), according to research published in The Lancet Oncology.
Researchers said the magnitude of the risk must still be determined, as well as how the variants identified may promote ALL.
The evidence suggests that ETV6 variation alone is not sufficient to cause ALL but may play a significant role in inherited predisposition to childhood ALL.
The researchers discovered the association between the ETV6 variants and childhood ALL by sequencing the whole exome of a family in which the mother and 2 of the 3 children have a history of pediatric ALL.
All were treated at St. Jude Children’s Research Hospital in Memphis, Tennessee, and are now cancer-free.
The researchers identified a novel non-sense ETV6 variant (p.Arg359X) in this mother and her 3 children, including a daughter who has not been diagnosed with cancer. The father does not have the variant.
This variant is predicted to result in the production of a shortened ETV6 protein that cannot fulfill its normal function of binding to DNA and regulating the expression of other genes.
The researchers screened an additional 4405 children with ALL and found 31 ETV6 exonic variants—21 missense, 5 frameshift, 4 non-sense, and 1 splice site—that are potentially related to leukemia risk in 35 patients, or almost 1% of the patients screened.
The variants identified were unique to ALL patients or extremely rare in the general population, the researchers said.
Patients with the variants tended to be older when diagnosed with ALL (10.2 years vs 4.7 years; P=0.017) and were more likely to have hyperdiploid leukemia. Sixty-four percent of ALL cases with germline ETV6 variants were hyperdiploid, compared to 27% of ALL cases without the variants (P=0.0050).
The variants were not associated with a particular ethnicity or with the outcome of ALL therapy.
The researchers also noted that almost half of the ETV6 variants identified (n=15) clustered in the erythroblast transformation specific domain.
“That suggests the loss or alteration of this DNA-binding function of ETV6 may be critical to cancer promotion,” said study author Jun J. Yang, PhD, of St. Jude.
“This is the latest example of the important role that genetic variation and inheritance plays in ALL risk. That has clear clinical implications and will help us understand the biology driving this cancer.”
These findings build on previous work that revealed an association between inherited ETV6 variations and thrombocytopenia in families with a susceptibility to hematologic malignancies. The researchers said this new study further solidifies the association between ETV6 and pediatric ALL. ![]()

Photo courtesy of
St. Jude/Seth Dixon
Germline variations in the ETV6 gene are associated with an increased risk of developing pediatric acute lymphoblastic leukemia (ALL), according to research published in The Lancet Oncology.
Researchers said the magnitude of the risk must still be determined, as well as how the variants identified may promote ALL.
The evidence suggests that ETV6 variation alone is not sufficient to cause ALL but may play a significant role in inherited predisposition to childhood ALL.
The researchers discovered the association between the ETV6 variants and childhood ALL by sequencing the whole exome of a family in which the mother and 2 of the 3 children have a history of pediatric ALL.
All were treated at St. Jude Children’s Research Hospital in Memphis, Tennessee, and are now cancer-free.
The researchers identified a novel non-sense ETV6 variant (p.Arg359X) in this mother and her 3 children, including a daughter who has not been diagnosed with cancer. The father does not have the variant.
This variant is predicted to result in the production of a shortened ETV6 protein that cannot fulfill its normal function of binding to DNA and regulating the expression of other genes.
The researchers screened an additional 4405 children with ALL and found 31 ETV6 exonic variants—21 missense, 5 frameshift, 4 non-sense, and 1 splice site—that are potentially related to leukemia risk in 35 patients, or almost 1% of the patients screened.
The variants identified were unique to ALL patients or extremely rare in the general population, the researchers said.
Patients with the variants tended to be older when diagnosed with ALL (10.2 years vs 4.7 years; P=0.017) and were more likely to have hyperdiploid leukemia. Sixty-four percent of ALL cases with germline ETV6 variants were hyperdiploid, compared to 27% of ALL cases without the variants (P=0.0050).
The variants were not associated with a particular ethnicity or with the outcome of ALL therapy.
The researchers also noted that almost half of the ETV6 variants identified (n=15) clustered in the erythroblast transformation specific domain.
“That suggests the loss or alteration of this DNA-binding function of ETV6 may be critical to cancer promotion,” said study author Jun J. Yang, PhD, of St. Jude.
“This is the latest example of the important role that genetic variation and inheritance plays in ALL risk. That has clear clinical implications and will help us understand the biology driving this cancer.”
These findings build on previous work that revealed an association between inherited ETV6 variations and thrombocytopenia in families with a susceptibility to hematologic malignancies. The researchers said this new study further solidifies the association between ETV6 and pediatric ALL. ![]()
Cancer care in Latin America

patient and her father
Photo by Rhoda Baer
Despite progress made in cancer care in Latin America in the last 2 years, substantial barriers remain to ensure optimal clinical management, according to a report commissioned by The Lancet Oncology.
The report, an update from a report published in 2013, details a number of improvements in cancer care in Latin America, either specifically related to cancer or to general healthcare initiatives that will also benefit cancer patients.
However, the updated report also suggests that major changes are needed in many areas to increase the standard of cancer care in Latin America.
Progress made
According to the report, progress has been made in the following areas.
The proportion of people in Latin America affiliated with any kind of health insurance program grew from 46% to 60% between 2008 and 2013.
For 2014, the World Health Organization (WHO) reported an 8% increase in the number of countries (60% of the whole region) with a National Cancer Plan. The following countries have newly adopted plans: Suriname, Ecuador, Dominican Republic, Trinidad and Tobago, Puerto Rico, Peru, El Salvador, and Colombia.
In addition, Latin America—most notably, Brazil and Argentina—has begun to address the shortage of cancer specialists.
Brazil has shown an increase of 77% in oncologists since 2011 (from 1457 to 2577). Concurrently, the number of hematologists has also increased by 40% (from 1420 in 2011 to 1985 in 2015), and that of radiotherapists by 12% (from 444 in 2011 to 497 in 2015). These rises are in the context of an 11% increase in cancer cases in Brazil (from 518,000 new cases in 2012 to 576,000 in 2014).
Many countries across Latin America have signed on to the Global Action Plan for the Prevention and Control of Non-Communicable Diseases 2013-2020, endorsed by the WHO, which aims to achieve a 25% reduction in premature mortality from non-communicable diseases (including cancer) by 2025.
The Colombian Ministry of Health and Social Protection has expanded its social insurance program to cover all types of cancer.
Since January 2014, the administration of chemotherapy and radiation treatments is free of charge in Uruguay.
The Atlas of Palliative Care was published in Latin America, which revealed a growth of more than 400% in the number of palliative services since 2006.
Room for improvement
The report indicates that the following issues are still problems in Latin America.
Compared with high-income countries, Latin America in 2015 remains behind in terms of public expenditure on health and cancer care.
Argentina and Mexico spend around 6% of their gross national product on healthcare, compared to 9% for the UK, 11% for Germany, and 17% for the US, which reflects a gap between Latin American and other countries not only proportionately but also in terms of absolute dollars. Only Brazil, at 9%, is close to the proportion spent in high-income countries.
In Latin America, only Brazil, Cuba, Costa Rica, and Uruguay are considered to have integration of social security and public insurance, and only Brazil, Cuba, and Costa Rica can be judged to have universal healthcare.
Many countries still have no specific training in palliative care (including Bolivia, El Salvador, Honduras, and Nicaragua).
Additionally, data from 2002 showed that Latin America accounted for less than 1% of the world’s opioid drug consumption for pain relief. Consumption of strong opioids still lags behind developed countries today, with no Latin American country exceeding 15 mg/capita per year.
Under-implementation of new technologies has not improved substantially since the previous Lancet Oncology Commission in 2013. There are a few exceptions, however, such as PET scanning technology improvements in Uruguay.
Pharmaceutical trials for expensive new anticancer therapies are largely unhelpful to most patients in Latin America. Patients participating in trials of expensive new anticancer therapies sometimes cannot complete treatment once their trial ends, and the trials often do not lead to approval in these regions.
There are often geographical disparities where most cancer specialists are located in major hospitals in big cities, requiring patients from rural and remote areas to travel far distances to these hospitals for cancer care.
In addition, waiting times in these centers can be unacceptably long, with reports from Mexico and Brazil describing median waiting times of 7 months or more for patients with breast cancer from symptomatic presentation to initial treatment.
Better cancer registries are desperately needed in all Latin American countries to more accurately quantify the cancer burden in the region and the resources required to combat it, according to the report. ![]()

patient and her father
Photo by Rhoda Baer
Despite progress made in cancer care in Latin America in the last 2 years, substantial barriers remain to ensure optimal clinical management, according to a report commissioned by The Lancet Oncology.
The report, an update from a report published in 2013, details a number of improvements in cancer care in Latin America, either specifically related to cancer or to general healthcare initiatives that will also benefit cancer patients.
However, the updated report also suggests that major changes are needed in many areas to increase the standard of cancer care in Latin America.
Progress made
According to the report, progress has been made in the following areas.
The proportion of people in Latin America affiliated with any kind of health insurance program grew from 46% to 60% between 2008 and 2013.
For 2014, the World Health Organization (WHO) reported an 8% increase in the number of countries (60% of the whole region) with a National Cancer Plan. The following countries have newly adopted plans: Suriname, Ecuador, Dominican Republic, Trinidad and Tobago, Puerto Rico, Peru, El Salvador, and Colombia.
In addition, Latin America—most notably, Brazil and Argentina—has begun to address the shortage of cancer specialists.
Brazil has shown an increase of 77% in oncologists since 2011 (from 1457 to 2577). Concurrently, the number of hematologists has also increased by 40% (from 1420 in 2011 to 1985 in 2015), and that of radiotherapists by 12% (from 444 in 2011 to 497 in 2015). These rises are in the context of an 11% increase in cancer cases in Brazil (from 518,000 new cases in 2012 to 576,000 in 2014).
Many countries across Latin America have signed on to the Global Action Plan for the Prevention and Control of Non-Communicable Diseases 2013-2020, endorsed by the WHO, which aims to achieve a 25% reduction in premature mortality from non-communicable diseases (including cancer) by 2025.
The Colombian Ministry of Health and Social Protection has expanded its social insurance program to cover all types of cancer.
Since January 2014, the administration of chemotherapy and radiation treatments is free of charge in Uruguay.
The Atlas of Palliative Care was published in Latin America, which revealed a growth of more than 400% in the number of palliative services since 2006.
Room for improvement
The report indicates that the following issues are still problems in Latin America.
Compared with high-income countries, Latin America in 2015 remains behind in terms of public expenditure on health and cancer care.
Argentina and Mexico spend around 6% of their gross national product on healthcare, compared to 9% for the UK, 11% for Germany, and 17% for the US, which reflects a gap between Latin American and other countries not only proportionately but also in terms of absolute dollars. Only Brazil, at 9%, is close to the proportion spent in high-income countries.
In Latin America, only Brazil, Cuba, Costa Rica, and Uruguay are considered to have integration of social security and public insurance, and only Brazil, Cuba, and Costa Rica can be judged to have universal healthcare.
Many countries still have no specific training in palliative care (including Bolivia, El Salvador, Honduras, and Nicaragua).
Additionally, data from 2002 showed that Latin America accounted for less than 1% of the world’s opioid drug consumption for pain relief. Consumption of strong opioids still lags behind developed countries today, with no Latin American country exceeding 15 mg/capita per year.
Under-implementation of new technologies has not improved substantially since the previous Lancet Oncology Commission in 2013. There are a few exceptions, however, such as PET scanning technology improvements in Uruguay.
Pharmaceutical trials for expensive new anticancer therapies are largely unhelpful to most patients in Latin America. Patients participating in trials of expensive new anticancer therapies sometimes cannot complete treatment once their trial ends, and the trials often do not lead to approval in these regions.
There are often geographical disparities where most cancer specialists are located in major hospitals in big cities, requiring patients from rural and remote areas to travel far distances to these hospitals for cancer care.
In addition, waiting times in these centers can be unacceptably long, with reports from Mexico and Brazil describing median waiting times of 7 months or more for patients with breast cancer from symptomatic presentation to initial treatment.
Better cancer registries are desperately needed in all Latin American countries to more accurately quantify the cancer burden in the region and the resources required to combat it, according to the report. ![]()

patient and her father
Photo by Rhoda Baer
Despite progress made in cancer care in Latin America in the last 2 years, substantial barriers remain to ensure optimal clinical management, according to a report commissioned by The Lancet Oncology.
The report, an update from a report published in 2013, details a number of improvements in cancer care in Latin America, either specifically related to cancer or to general healthcare initiatives that will also benefit cancer patients.
However, the updated report also suggests that major changes are needed in many areas to increase the standard of cancer care in Latin America.
Progress made
According to the report, progress has been made in the following areas.
The proportion of people in Latin America affiliated with any kind of health insurance program grew from 46% to 60% between 2008 and 2013.
For 2014, the World Health Organization (WHO) reported an 8% increase in the number of countries (60% of the whole region) with a National Cancer Plan. The following countries have newly adopted plans: Suriname, Ecuador, Dominican Republic, Trinidad and Tobago, Puerto Rico, Peru, El Salvador, and Colombia.
In addition, Latin America—most notably, Brazil and Argentina—has begun to address the shortage of cancer specialists.
Brazil has shown an increase of 77% in oncologists since 2011 (from 1457 to 2577). Concurrently, the number of hematologists has also increased by 40% (from 1420 in 2011 to 1985 in 2015), and that of radiotherapists by 12% (from 444 in 2011 to 497 in 2015). These rises are in the context of an 11% increase in cancer cases in Brazil (from 518,000 new cases in 2012 to 576,000 in 2014).
Many countries across Latin America have signed on to the Global Action Plan for the Prevention and Control of Non-Communicable Diseases 2013-2020, endorsed by the WHO, which aims to achieve a 25% reduction in premature mortality from non-communicable diseases (including cancer) by 2025.
The Colombian Ministry of Health and Social Protection has expanded its social insurance program to cover all types of cancer.
Since January 2014, the administration of chemotherapy and radiation treatments is free of charge in Uruguay.
The Atlas of Palliative Care was published in Latin America, which revealed a growth of more than 400% in the number of palliative services since 2006.
Room for improvement
The report indicates that the following issues are still problems in Latin America.
Compared with high-income countries, Latin America in 2015 remains behind in terms of public expenditure on health and cancer care.
Argentina and Mexico spend around 6% of their gross national product on healthcare, compared to 9% for the UK, 11% for Germany, and 17% for the US, which reflects a gap between Latin American and other countries not only proportionately but also in terms of absolute dollars. Only Brazil, at 9%, is close to the proportion spent in high-income countries.
In Latin America, only Brazil, Cuba, Costa Rica, and Uruguay are considered to have integration of social security and public insurance, and only Brazil, Cuba, and Costa Rica can be judged to have universal healthcare.
Many countries still have no specific training in palliative care (including Bolivia, El Salvador, Honduras, and Nicaragua).
Additionally, data from 2002 showed that Latin America accounted for less than 1% of the world’s opioid drug consumption for pain relief. Consumption of strong opioids still lags behind developed countries today, with no Latin American country exceeding 15 mg/capita per year.
Under-implementation of new technologies has not improved substantially since the previous Lancet Oncology Commission in 2013. There are a few exceptions, however, such as PET scanning technology improvements in Uruguay.
Pharmaceutical trials for expensive new anticancer therapies are largely unhelpful to most patients in Latin America. Patients participating in trials of expensive new anticancer therapies sometimes cannot complete treatment once their trial ends, and the trials often do not lead to approval in these regions.
There are often geographical disparities where most cancer specialists are located in major hospitals in big cities, requiring patients from rural and remote areas to travel far distances to these hospitals for cancer care.
In addition, waiting times in these centers can be unacceptably long, with reports from Mexico and Brazil describing median waiting times of 7 months or more for patients with breast cancer from symptomatic presentation to initial treatment.
Better cancer registries are desperately needed in all Latin American countries to more accurately quantify the cancer burden in the region and the resources required to combat it, according to the report. ![]()
Gene may be key to fighting sepsis

with a multi-pipettor
Photo courtesy of Stuart Hay/
Australian National University
Scientists have identified a gene that could potentially aid the development of new treatments for sepsis.
The team knew that sepsis occurs when lipopolysaccharides (LPS) on the surface of some bacteria infiltrate cells, triggering an immune response that causes the cells to self-destruct.
However, it was unclear exactly how this happens. That is, until the team found the protein gasdermin D plays a critical role in the pathway to sepsis.
“This finding is a key that could potentially unlock our ability to shut down this killer disease before it gets to a life-threatening stage,” said Chris Goodnow, PhD, of The Australian National University in Canberra.
Dr Goodnow and his colleagues described their discovery in Nature.
The scientists found that gasdermin D usually exists in cells in an inactive form.
But when the LPS molecules enter cells, they trigger caspase-11 to lop off the protective chemical cap of gasdermin D. This, in turn, prompts the cells to self-destruct.
The team said this suggests gasdermin D is a critical target of
caspase-11 and a key mediator of the host response against Gram-negative
bacteria.
To identify the source of this discovery, the scientists screened thousands of genes. In a little over a year, they isolated the gene that produces gasdermin D.
Lead study author Nobuhiko Kayagaki, PhD, of Genentech in San Francisco, California, said this work could have implications beyond sepsis.
“The identification of gasdermin D can give us a better understanding not only of lethal sepsis, but also of multiple other inflammatory diseases,” he said. ![]()

with a multi-pipettor
Photo courtesy of Stuart Hay/
Australian National University
Scientists have identified a gene that could potentially aid the development of new treatments for sepsis.
The team knew that sepsis occurs when lipopolysaccharides (LPS) on the surface of some bacteria infiltrate cells, triggering an immune response that causes the cells to self-destruct.
However, it was unclear exactly how this happens. That is, until the team found the protein gasdermin D plays a critical role in the pathway to sepsis.
“This finding is a key that could potentially unlock our ability to shut down this killer disease before it gets to a life-threatening stage,” said Chris Goodnow, PhD, of The Australian National University in Canberra.
Dr Goodnow and his colleagues described their discovery in Nature.
The scientists found that gasdermin D usually exists in cells in an inactive form.
But when the LPS molecules enter cells, they trigger caspase-11 to lop off the protective chemical cap of gasdermin D. This, in turn, prompts the cells to self-destruct.
The team said this suggests gasdermin D is a critical target of
caspase-11 and a key mediator of the host response against Gram-negative
bacteria.
To identify the source of this discovery, the scientists screened thousands of genes. In a little over a year, they isolated the gene that produces gasdermin D.
Lead study author Nobuhiko Kayagaki, PhD, of Genentech in San Francisco, California, said this work could have implications beyond sepsis.
“The identification of gasdermin D can give us a better understanding not only of lethal sepsis, but also of multiple other inflammatory diseases,” he said. ![]()

with a multi-pipettor
Photo courtesy of Stuart Hay/
Australian National University
Scientists have identified a gene that could potentially aid the development of new treatments for sepsis.
The team knew that sepsis occurs when lipopolysaccharides (LPS) on the surface of some bacteria infiltrate cells, triggering an immune response that causes the cells to self-destruct.
However, it was unclear exactly how this happens. That is, until the team found the protein gasdermin D plays a critical role in the pathway to sepsis.
“This finding is a key that could potentially unlock our ability to shut down this killer disease before it gets to a life-threatening stage,” said Chris Goodnow, PhD, of The Australian National University in Canberra.
Dr Goodnow and his colleagues described their discovery in Nature.
The scientists found that gasdermin D usually exists in cells in an inactive form.
But when the LPS molecules enter cells, they trigger caspase-11 to lop off the protective chemical cap of gasdermin D. This, in turn, prompts the cells to self-destruct.
The team said this suggests gasdermin D is a critical target of
caspase-11 and a key mediator of the host response against Gram-negative
bacteria.
To identify the source of this discovery, the scientists screened thousands of genes. In a little over a year, they isolated the gene that produces gasdermin D.
Lead study author Nobuhiko Kayagaki, PhD, of Genentech in San Francisco, California, said this work could have implications beyond sepsis.
“The identification of gasdermin D can give us a better understanding not only of lethal sepsis, but also of multiple other inflammatory diseases,” he said. ![]()
The search continues for additional targets in CLL
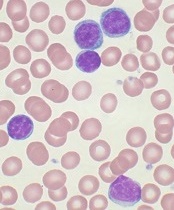
showing CLL
Image by Mary Ann Thompson
NEW YORK—Despite enormous advances in therapies for chronic lymphocytic leukemia (CLL) that target the B-cell receptor (BCR) signaling pathway, there is still room for improvement, according to investigators at the Mayo Clinic.
Bruton’s tyrosine kinase (BTK) and phosphoinositide-3 kinase delta (PI3Kδ) inhibitors are major players in mediating BCR signaling, yet both have off-target effects.
“These agents are not curative, and resistance develops,” explained Neil Kay, MD, of the Mayo Clinic in Rochester, Minnesota.
He described the Axl receptor tyrosine kinase and its inhibitor, TP-0903, at Lymphoma & Myeloma 2015, and discussed how it may be another promising target for CLL therapy.
Pros and cons of current therapies
Ibrutinib, a potent, irreversible, covalent inhibitor of BTK, inhibits interleukin 2 kinase, an essential enzyme in Th2 T cells.
“The potential benefit of this,” Dr Kay said, “is it shifts the balance between Th1 and Th2 T cells and potentially enhances antitumor immune responses.”
However, ibrutinib may cause off-target effects, including defects of platelet function, atrial fibrillation probably related to the inhibition of cardiac PI3K-AKT signaling, and decreased efficacy of anti-CD20 antibodies as mediated by natural killer cells.
In addition, long-term exposure to ibrutinib can induce signal mutations associated with resistance.
Idelalisib, a reversible inhibitor of the delta isoform of PI3K, modulates malignant B-cell signaling and inhibits the chemokines CCL3 and 4. It does not inhibit antibody-dependent cell-mediated cytotoxicity mediated by anti-CD20 antibodies, and it may stimulate antitumor responses.
However, idelalisib may also increase the incidence of diarrhea and colitis and can cause transaminitis and pneumonitis.
Axl receptor tyrosine kinase
So Dr Kay and fellow researchers explored whether CLL B cells express other active receptor tyrosine kinases (RTKs), and if so, what their functional implication is in CLL B-cell survival.
And what they detected is active Axl RTK and basic fibroblast growth factor receptor 3 (FGFR3) RTK in CLL B cells. The human Axl gene, a member of the TAM family of RTKs, is located on chromosome 19q13.2 and encodes a protein weighing 100–140 kD.
When Axl is activated, it initiates various signaling pathways, including cell survival, proliferation, apoptosis inhibition, migration, cell adhesion, and cytokine production. It does this through interactions with a number of signaling molecules, including PI3K and phospholipase C γ2 (PLCγ2), among others.
Most important, Axl is overexpressed in a number of human malignancies, including acute myeloid leukemia, and is associated with poor survivorship.
The research team immunoprecipitated Axl RTK from CLL B-cell lysates and examined them for co-localization by Western blot for the proteins PI3K, c-Src, Syk, PLCγ2, ZAP-70, and Lyn.
“What we think currently is that Axl provides a docking site for these key signaling molecules,” Dr Kay said.
The team found that, by inhibiting Axl with TP-0903, they were able to induce robust apoptosis in CLL B cells, including those from high-risk 17p– and 11q– CLL. They also observed that Axl inhibition resulted in significant reduction of the anti-apoptotic proteins Bcl-2, XIAP, and Mcl-1 and upregulation of the pro-apoptotic protein BIM in CLL B cells.
They then tested the BTK inhibitors ibrutinib and TP-4216 with and without the Axl inhibitor TP-0903 and found that concurrent administration of TP-0903 with the reversible BTK inhibitor TP-4216 had additive effects on apoptosis. This was not the case when it was added to ibrutinib.
“There was much more dramatic upregulation with the reversible BTK inhibitor,” Dr Kay emphasized.
He said Axl inhibition is a therapeutic opportunity in that these inhibitors are orally bioavailable and would be candidates for clinical testing. These inhibitors may minimize drug resistance and prevent the emergence of Richter’s transformation, he added.
The researchers received TP-0903 from Tolero Pharmaceuticals. ![]()

showing CLL
Image by Mary Ann Thompson
NEW YORK—Despite enormous advances in therapies for chronic lymphocytic leukemia (CLL) that target the B-cell receptor (BCR) signaling pathway, there is still room for improvement, according to investigators at the Mayo Clinic.
Bruton’s tyrosine kinase (BTK) and phosphoinositide-3 kinase delta (PI3Kδ) inhibitors are major players in mediating BCR signaling, yet both have off-target effects.
“These agents are not curative, and resistance develops,” explained Neil Kay, MD, of the Mayo Clinic in Rochester, Minnesota.
He described the Axl receptor tyrosine kinase and its inhibitor, TP-0903, at Lymphoma & Myeloma 2015, and discussed how it may be another promising target for CLL therapy.
Pros and cons of current therapies
Ibrutinib, a potent, irreversible, covalent inhibitor of BTK, inhibits interleukin 2 kinase, an essential enzyme in Th2 T cells.
“The potential benefit of this,” Dr Kay said, “is it shifts the balance between Th1 and Th2 T cells and potentially enhances antitumor immune responses.”
However, ibrutinib may cause off-target effects, including defects of platelet function, atrial fibrillation probably related to the inhibition of cardiac PI3K-AKT signaling, and decreased efficacy of anti-CD20 antibodies as mediated by natural killer cells.
In addition, long-term exposure to ibrutinib can induce signal mutations associated with resistance.
Idelalisib, a reversible inhibitor of the delta isoform of PI3K, modulates malignant B-cell signaling and inhibits the chemokines CCL3 and 4. It does not inhibit antibody-dependent cell-mediated cytotoxicity mediated by anti-CD20 antibodies, and it may stimulate antitumor responses.
However, idelalisib may also increase the incidence of diarrhea and colitis and can cause transaminitis and pneumonitis.
Axl receptor tyrosine kinase
So Dr Kay and fellow researchers explored whether CLL B cells express other active receptor tyrosine kinases (RTKs), and if so, what their functional implication is in CLL B-cell survival.
And what they detected is active Axl RTK and basic fibroblast growth factor receptor 3 (FGFR3) RTK in CLL B cells. The human Axl gene, a member of the TAM family of RTKs, is located on chromosome 19q13.2 and encodes a protein weighing 100–140 kD.
When Axl is activated, it initiates various signaling pathways, including cell survival, proliferation, apoptosis inhibition, migration, cell adhesion, and cytokine production. It does this through interactions with a number of signaling molecules, including PI3K and phospholipase C γ2 (PLCγ2), among others.
Most important, Axl is overexpressed in a number of human malignancies, including acute myeloid leukemia, and is associated with poor survivorship.
The research team immunoprecipitated Axl RTK from CLL B-cell lysates and examined them for co-localization by Western blot for the proteins PI3K, c-Src, Syk, PLCγ2, ZAP-70, and Lyn.
“What we think currently is that Axl provides a docking site for these key signaling molecules,” Dr Kay said.
The team found that, by inhibiting Axl with TP-0903, they were able to induce robust apoptosis in CLL B cells, including those from high-risk 17p– and 11q– CLL. They also observed that Axl inhibition resulted in significant reduction of the anti-apoptotic proteins Bcl-2, XIAP, and Mcl-1 and upregulation of the pro-apoptotic protein BIM in CLL B cells.
They then tested the BTK inhibitors ibrutinib and TP-4216 with and without the Axl inhibitor TP-0903 and found that concurrent administration of TP-0903 with the reversible BTK inhibitor TP-4216 had additive effects on apoptosis. This was not the case when it was added to ibrutinib.
“There was much more dramatic upregulation with the reversible BTK inhibitor,” Dr Kay emphasized.
He said Axl inhibition is a therapeutic opportunity in that these inhibitors are orally bioavailable and would be candidates for clinical testing. These inhibitors may minimize drug resistance and prevent the emergence of Richter’s transformation, he added.
The researchers received TP-0903 from Tolero Pharmaceuticals. ![]()

showing CLL
Image by Mary Ann Thompson
NEW YORK—Despite enormous advances in therapies for chronic lymphocytic leukemia (CLL) that target the B-cell receptor (BCR) signaling pathway, there is still room for improvement, according to investigators at the Mayo Clinic.
Bruton’s tyrosine kinase (BTK) and phosphoinositide-3 kinase delta (PI3Kδ) inhibitors are major players in mediating BCR signaling, yet both have off-target effects.
“These agents are not curative, and resistance develops,” explained Neil Kay, MD, of the Mayo Clinic in Rochester, Minnesota.
He described the Axl receptor tyrosine kinase and its inhibitor, TP-0903, at Lymphoma & Myeloma 2015, and discussed how it may be another promising target for CLL therapy.
Pros and cons of current therapies
Ibrutinib, a potent, irreversible, covalent inhibitor of BTK, inhibits interleukin 2 kinase, an essential enzyme in Th2 T cells.
“The potential benefit of this,” Dr Kay said, “is it shifts the balance between Th1 and Th2 T cells and potentially enhances antitumor immune responses.”
However, ibrutinib may cause off-target effects, including defects of platelet function, atrial fibrillation probably related to the inhibition of cardiac PI3K-AKT signaling, and decreased efficacy of anti-CD20 antibodies as mediated by natural killer cells.
In addition, long-term exposure to ibrutinib can induce signal mutations associated with resistance.
Idelalisib, a reversible inhibitor of the delta isoform of PI3K, modulates malignant B-cell signaling and inhibits the chemokines CCL3 and 4. It does not inhibit antibody-dependent cell-mediated cytotoxicity mediated by anti-CD20 antibodies, and it may stimulate antitumor responses.
However, idelalisib may also increase the incidence of diarrhea and colitis and can cause transaminitis and pneumonitis.
Axl receptor tyrosine kinase
So Dr Kay and fellow researchers explored whether CLL B cells express other active receptor tyrosine kinases (RTKs), and if so, what their functional implication is in CLL B-cell survival.
And what they detected is active Axl RTK and basic fibroblast growth factor receptor 3 (FGFR3) RTK in CLL B cells. The human Axl gene, a member of the TAM family of RTKs, is located on chromosome 19q13.2 and encodes a protein weighing 100–140 kD.
When Axl is activated, it initiates various signaling pathways, including cell survival, proliferation, apoptosis inhibition, migration, cell adhesion, and cytokine production. It does this through interactions with a number of signaling molecules, including PI3K and phospholipase C γ2 (PLCγ2), among others.
Most important, Axl is overexpressed in a number of human malignancies, including acute myeloid leukemia, and is associated with poor survivorship.
The research team immunoprecipitated Axl RTK from CLL B-cell lysates and examined them for co-localization by Western blot for the proteins PI3K, c-Src, Syk, PLCγ2, ZAP-70, and Lyn.
“What we think currently is that Axl provides a docking site for these key signaling molecules,” Dr Kay said.
The team found that, by inhibiting Axl with TP-0903, they were able to induce robust apoptosis in CLL B cells, including those from high-risk 17p– and 11q– CLL. They also observed that Axl inhibition resulted in significant reduction of the anti-apoptotic proteins Bcl-2, XIAP, and Mcl-1 and upregulation of the pro-apoptotic protein BIM in CLL B cells.
They then tested the BTK inhibitors ibrutinib and TP-4216 with and without the Axl inhibitor TP-0903 and found that concurrent administration of TP-0903 with the reversible BTK inhibitor TP-4216 had additive effects on apoptosis. This was not the case when it was added to ibrutinib.
“There was much more dramatic upregulation with the reversible BTK inhibitor,” Dr Kay emphasized.
He said Axl inhibition is a therapeutic opportunity in that these inhibitors are orally bioavailable and would be candidates for clinical testing. These inhibitors may minimize drug resistance and prevent the emergence of Richter’s transformation, he added.
The researchers received TP-0903 from Tolero Pharmaceuticals. ![]()
Acne as a Potential New Target for Soy Isoflavones
While the pathophysiology of acne vulgaris is, at a minimum, complex (and that’s putting it lightly), it is generally accepted that androgens such as dihydrotestosterone (DHT) can play a prominent role, especially in adult women with acne. Although it is not approved by the US Food and Drug Administration, the utilization of antiandrogens such as spironolactone (see my discussion of spironolactone use in adult females in the October issue of Cutis) has become standard practice for many US dermatologists who treat this patient population. Joined only by combined oral contraceptives, antihormonal therapies for acne are somewhat limited. Therefore, effective as well as safe additions are needed.
In a study published online on July 20 in Dermato-Endocrinology, Riyanto et al evaluated the potential of orally administered soy isoflavones for treatment of acne in adult women based on both lesion count over time and corresponding changes in DHT levels. Soy isoflavones such as genistein, daidzein, and glycitein have established effects on androgen metabolism through inhibition of 3β-hydroxysteroid dehydrogenase, 17β-hydroxysteroid dehydrogenase, and the 5α-reductases. The study was double-blinded and conducted over 12 weeks, and various confounders were accounted for, including body mass index and menstrual irregularities; however, the sample size was relatively small (N=40), with participants equally randomized to treatment with either a placebo or the soybean isoflavone (160 mg daily). The results were determined to be significant (P<.05) based on the statistical analysis, which found that the isoflavone group had a lower lesion count after 12 weeks as well as a drop in serum DHT levels. Baseline lesion counts and serum DHT levels were not statistically significant when compared to the placebo group.
What’s the Issue?
Am I saying you should recommend to all of your adult female acne patients that they should run out and buy soy isoflavone supplements? Probably not. Forgetting even the study limitations, we face a daily struggle with reproducibility when it comes to over-the-counter supplements given these products are not regulated with the same scrutiny as prescription products or devices. Unfortunately, the degree of variability between 1 manufacturer to another can be broad, with shelf life stability often being the greatest issue. Are all soy isoflavone supplements created equal? I don’t know, and I can assure you that most regulatory bodies don’t know either. Walking down the vitamin aisle with countless versions of the same product can be acne inducing in itself.
The data is certainly interesting and novel for this disease state. A larger study certainly is warranted, although as we increase the number of studies, I wonder if we will receive mixed data as witnessed with the breast cancer prevention studies with soy; some showed intake was advantageous, other did not (see suggested readings below if interested in learning more). To end on a positive note, the way I see it is that soy isoflavones could possibly become a cheaper addition to—not a replacement for—our vast yet active ingredient–lacking armament of acne treatments. Time will hopefully tell. How do you think these study results will impact the treatment of acne?
We want to know your views! Tell us what you think.
Suggested Readings
- Travis RC, Allen NE, Appleby PN, et al. A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. Int J Cancer. 2008;122:705-710.
- Key TJ, Sharp GB, Appleby PN, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. 1999;81:1248-1256.
- Zaineddin AK, Buck K, Vrieling A, et al. The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: a German case-control study. Nutr Cancer. 2012;64:652-665.
While the pathophysiology of acne vulgaris is, at a minimum, complex (and that’s putting it lightly), it is generally accepted that androgens such as dihydrotestosterone (DHT) can play a prominent role, especially in adult women with acne. Although it is not approved by the US Food and Drug Administration, the utilization of antiandrogens such as spironolactone (see my discussion of spironolactone use in adult females in the October issue of Cutis) has become standard practice for many US dermatologists who treat this patient population. Joined only by combined oral contraceptives, antihormonal therapies for acne are somewhat limited. Therefore, effective as well as safe additions are needed.
In a study published online on July 20 in Dermato-Endocrinology, Riyanto et al evaluated the potential of orally administered soy isoflavones for treatment of acne in adult women based on both lesion count over time and corresponding changes in DHT levels. Soy isoflavones such as genistein, daidzein, and glycitein have established effects on androgen metabolism through inhibition of 3β-hydroxysteroid dehydrogenase, 17β-hydroxysteroid dehydrogenase, and the 5α-reductases. The study was double-blinded and conducted over 12 weeks, and various confounders were accounted for, including body mass index and menstrual irregularities; however, the sample size was relatively small (N=40), with participants equally randomized to treatment with either a placebo or the soybean isoflavone (160 mg daily). The results were determined to be significant (P<.05) based on the statistical analysis, which found that the isoflavone group had a lower lesion count after 12 weeks as well as a drop in serum DHT levels. Baseline lesion counts and serum DHT levels were not statistically significant when compared to the placebo group.
What’s the Issue?
Am I saying you should recommend to all of your adult female acne patients that they should run out and buy soy isoflavone supplements? Probably not. Forgetting even the study limitations, we face a daily struggle with reproducibility when it comes to over-the-counter supplements given these products are not regulated with the same scrutiny as prescription products or devices. Unfortunately, the degree of variability between 1 manufacturer to another can be broad, with shelf life stability often being the greatest issue. Are all soy isoflavone supplements created equal? I don’t know, and I can assure you that most regulatory bodies don’t know either. Walking down the vitamin aisle with countless versions of the same product can be acne inducing in itself.
The data is certainly interesting and novel for this disease state. A larger study certainly is warranted, although as we increase the number of studies, I wonder if we will receive mixed data as witnessed with the breast cancer prevention studies with soy; some showed intake was advantageous, other did not (see suggested readings below if interested in learning more). To end on a positive note, the way I see it is that soy isoflavones could possibly become a cheaper addition to—not a replacement for—our vast yet active ingredient–lacking armament of acne treatments. Time will hopefully tell. How do you think these study results will impact the treatment of acne?
We want to know your views! Tell us what you think.
Suggested Readings
- Travis RC, Allen NE, Appleby PN, et al. A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. Int J Cancer. 2008;122:705-710.
- Key TJ, Sharp GB, Appleby PN, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. 1999;81:1248-1256.
- Zaineddin AK, Buck K, Vrieling A, et al. The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: a German case-control study. Nutr Cancer. 2012;64:652-665.
While the pathophysiology of acne vulgaris is, at a minimum, complex (and that’s putting it lightly), it is generally accepted that androgens such as dihydrotestosterone (DHT) can play a prominent role, especially in adult women with acne. Although it is not approved by the US Food and Drug Administration, the utilization of antiandrogens such as spironolactone (see my discussion of spironolactone use in adult females in the October issue of Cutis) has become standard practice for many US dermatologists who treat this patient population. Joined only by combined oral contraceptives, antihormonal therapies for acne are somewhat limited. Therefore, effective as well as safe additions are needed.
In a study published online on July 20 in Dermato-Endocrinology, Riyanto et al evaluated the potential of orally administered soy isoflavones for treatment of acne in adult women based on both lesion count over time and corresponding changes in DHT levels. Soy isoflavones such as genistein, daidzein, and glycitein have established effects on androgen metabolism through inhibition of 3β-hydroxysteroid dehydrogenase, 17β-hydroxysteroid dehydrogenase, and the 5α-reductases. The study was double-blinded and conducted over 12 weeks, and various confounders were accounted for, including body mass index and menstrual irregularities; however, the sample size was relatively small (N=40), with participants equally randomized to treatment with either a placebo or the soybean isoflavone (160 mg daily). The results were determined to be significant (P<.05) based on the statistical analysis, which found that the isoflavone group had a lower lesion count after 12 weeks as well as a drop in serum DHT levels. Baseline lesion counts and serum DHT levels were not statistically significant when compared to the placebo group.
What’s the Issue?
Am I saying you should recommend to all of your adult female acne patients that they should run out and buy soy isoflavone supplements? Probably not. Forgetting even the study limitations, we face a daily struggle with reproducibility when it comes to over-the-counter supplements given these products are not regulated with the same scrutiny as prescription products or devices. Unfortunately, the degree of variability between 1 manufacturer to another can be broad, with shelf life stability often being the greatest issue. Are all soy isoflavone supplements created equal? I don’t know, and I can assure you that most regulatory bodies don’t know either. Walking down the vitamin aisle with countless versions of the same product can be acne inducing in itself.
The data is certainly interesting and novel for this disease state. A larger study certainly is warranted, although as we increase the number of studies, I wonder if we will receive mixed data as witnessed with the breast cancer prevention studies with soy; some showed intake was advantageous, other did not (see suggested readings below if interested in learning more). To end on a positive note, the way I see it is that soy isoflavones could possibly become a cheaper addition to—not a replacement for—our vast yet active ingredient–lacking armament of acne treatments. Time will hopefully tell. How do you think these study results will impact the treatment of acne?
We want to know your views! Tell us what you think.
Suggested Readings
- Travis RC, Allen NE, Appleby PN, et al. A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. Int J Cancer. 2008;122:705-710.
- Key TJ, Sharp GB, Appleby PN, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. 1999;81:1248-1256.
- Zaineddin AK, Buck K, Vrieling A, et al. The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: a German case-control study. Nutr Cancer. 2012;64:652-665.
