User login
Salpingectomy after vaginal hysterectomy: Technique, tips, and pearls
In this article, I describe my technique for a vaginal approach to right salpingectomy with ovarian preservation, as well as right salpingo-oophorectomy, in a patient lacking a left tube and ovary. This technique is fully illustrated on a cadaver in the Web-based master course in vaginal hysterectomy produced by the AAGL and co-sponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That course is available online at https://www.aagl.org/vaghystwebinar.
For a detailed description of vaginal hysterectomy technique, see the article entitled “Vaginal hysterectomy using basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. Next month, in the December 2015 issue of the journal, I will detail my strategies for managing complications associated with vaginal hysterectomy, salpingectomy, and salpingo-oophorectomy.
Right salpingectomy
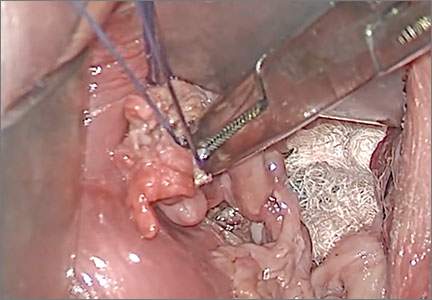
FIGURE 1 Locate the tube |
The fallopian tube will almost always be found on top of the ovary. |
FIGURE 2 Isolate the tube 
|
| Grasp the tube and bring it to the midline. |
Start with light traction
Begin by placing an instrument on the round ligament, tube, and uterine-ovarian pedicle, exerting light traction. Note that the tube will always be found on top of the ovary
(FIGURE 1). Take care during placement of packing material to avoid sweeping the fimbriae of the tube up and out of the surgical field. You may need to play with the packing a bit until you are able to deliver the tube.
Once you identify the tube, isolate it by bringing it down to the midline (FIGURE 2). One thing to note if you’re accustomed to performing bilateral salpingo-oophorectomy: The gonadal pedicle is fairly substantive and can sustain a bit of tugging. However, if you’re performing salpingectomy with ovarian preservation, you need to be much more careful in your handling of the tube because the mesosalpinx is extremely delicate.
After you bring the tube to the midline, grasp it using a Heaney or Shallcross clamp. You could use energy to take this pedicle or clamp and tie it.
Make sure that the packing material is out of the way and that you have most of the tube nicely isolated. Don’t take the tube too far up in the surgical field because, if you lose it, it can be hard to control the bleeding. Ensure that you have grasped the fimbriated end of the tube.
In some cases you can leave a portion of the tube right next to the round ligament (FIGURE 3). You can go back and take that portion later, if you desire. But when it comes to the potential for the fallopian tube to generate carcinoma, most of the concern involves the mid to distal end of the tube rather than the cornual portion.
Once the Shallcross clamp has a good purchase on the pedicle, bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle (FIGURE 4). It is extremely important during salpingectomy to tie this suture down gently but tightly. In the process, have your assistant flash the Shallcross clamp open when you tie the suture. Otherwise, the suture will tend to tear through the mesosalpinx. Be very careful in your handling of the specimen at this point. Next, cut right along the edge of the clamp to remove the tube.
FIGURE 3 Focus on the distal tube
| FIGURE 4 Clamp and tie the pedicle 
| |
| The cornual portion of the tube (proximal to the round ligament) can be left behind, if desired. The propensity for cancer centers on the distal end of the tube. | Bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle. |
If you prefer, you can stick-tie the remaining portion again, but usually one tie will suffice because there is such a small pedicle there. The distal portion of the pedicle eventually will necrose close to the tie. The next step is ensuring hemostasis.
On occasion, if you lose the pedicle high in the surgical field, you can try to oversew it. A 2-0 Vicryl suture may be used to place a figure-eight stitch to control bleeding around the mesosalpinx. Alternatively, an energy device may be used for hemostasis. Rarely, if you encounter bleeding that does not respond to the previous suggestions, you may need to remove the ovary to control bleeding if the tissue tears.
Transvaginal technique for salpingo-oophorectomy
Once the hysterectomy is completed, grasp the round ligament, tube, and uterine-ovarian pedicle, placing slight tension on the pedicle, and free the right round ligament to ease isolation of the gonadal vessels. Using electrocautery, carefully transect the round ligament. It is critical when isolating the round ligament to transect only the ligament and not to get deep into the underlying tissue or bleeding will ensue. If you “hug” just the round ligament, you will open into the broad ligament and easily be able to isolate the gonadal pedicle.
Once the pedicle is nicely isolated, readjust your retractors or lighting to improve visualization. Now the gonadal vessels can be isolated up high much more easily (FIGURE 1).
Next, use a Heaney clamp to grab the pedicle, making sure that the ovary is medial to the clamp (FIGURE 2).

| 
| |
| FIGURE 1: ISOLATE THE GONADAL VESSELS Once optimal visualization is achieved, the gonadal vessels can be isolated easily. | FIGURE 2: KEEP THE OVARY MEDIAL TO THE CLAMP Use a Heaney clamp to grab the pedicle, keeping the ovary medial to the clamp. |
In this setting, there are a number of techniques you can use to complete the salpingo-oophorectomy. I tend to doubly ligate the pedicle. To begin, cut the tagging suture to get it out of the way. Then place a free tie lateral to the clamp, bringing it down and underneath to fully encircle the pedicle. Ligate the pedicle then cut the free tie. Follow by cutting the pedicle beside the Heaney clamp and removing the specimen. Stick-tie the remaining pedicle.
Locate the free tie, which is easily identified. Place your needle between that free tie and the clamp so that you do not pierce the vessels proximal to the tie with that needle. Then doubly ligate the pedicle.
Check for hemostasis and, once confirmed, cut the pedicle tie. Because this patient does not have a left tube and ovary, the procedure is now completed.
Conclusion
The tubes are usually readily accessible for removal at the time of vaginal hysterectomy. There is evolving evidence that the tube may play a role in malignancy of the female genital tract. Thus, removal may be preventive. In addition, if there are paratubal cysts or hydrosalpinx from prior tubal ligation, it makes sense to remove the tube. There is little evidence to suggest that removal of the tubes accelerates the menopausal transition due to compromise of the blood supply to the ovaries.
You must be very gentle when handling and removing just the tubes. The mesosalpinx is delicate and easily torn or traumatized. A careful and deliberate approach is warranted.
Bilateral salpingectomy: Key take-aways
Locate the tube. The fallopian tube always lies on top of the ovary and should be found there. On occasion, the abdominal packing used to move the bowel out of the pelvis will “hide” the tube; readjusting this packing often solves the problem.
Be gentle with the mesosalpinx as it is very delicate and can easily avulse. It is very important to “flash the clamp” (open the clamp and then close it) as you free-tie the mesosalpinx to avoid cutting through the delicate pedicle.
Remove as much tube as possible. The fimbriae end of the tube usually is free and easy to identify. Try to remove as much of the tube as possible. Often, a bit of the proximal tube is left in the utero-ovarian pedicle tie.
Clean up. You will often find peritubal cysts or “tubal clips” from a sterilization procedure. I recommend that you remove any of these you encounter to avoid problems down the road. Often, these cysts and clip-like devices are removed as part of the specimen.
Dry up. Always confirm hemostasis before concluding the procedure. If there is bleeding, be sure to assess the mesosalpinx. Occasionally, the pedicle can be torn higher up, near the gonadal vessels. Investigate this region if bleeding seems to be an issue.
Transvaginal salpingo-oophorectomy: Key take-aways
Perfect a technique. There are many approaches to transvaginal removal of the adnexae; pick one and perfect it. The better you are, the fewer complications you will have. Recognize that a different approach (use of a stapler or energy sealing device, for example) may prove useful in some settings. Be surgically versatile and recognize situations that might call for something other than your usual approach.
Optimize visualization. The tubes and ovaries are usually very accessible vaginally. Use an abdominal pack to move the bowel out of the pelvis. Adequate retraction and use of a lighted retractor or suction irrigator will facilitate exposure.
Ligate the gonadal vessels. Retraction of the tube and ovary complex medially away from the pelvic sidewall will allow you to place a clamp (or stapler or energy device) lateral to secure the gonadal vessels and ensure complete removal of the adnexae.
Release the round ligament. Although this step is usually not required, it will allow you to isolate the adnexae more precisely, especially when dealing with an adnexal mass transvaginally. It is critical that you “hug” the round ligament and refrain from penetrating deeply into the underlying tissues, or bleeding will occur. Once the round ligament is released, the tube and ovary are isolated on the gonadal pedicle and can be completely excised with this technique.
Manage bleeding. If suturing, I prefer to doubly ligate the gonadal vessels. Once I clamp the pedicle, I “free-tie” the gonadal vessels with an initial suture. This suture secures the vascular pedicle and prevents retraction. The adnexae can then be removed, followed by placement of a “stick-tie” to re-ligate the pedicle. Although this vascular pedicle is more robust than the mesosalpinx, it, too, can be avulsed, so it is important to proceed with caution. I recommend having a long clamp (uterine packing forceps or MD Anderson clamp) available in your instrument pan to facilitate specific isolation of the gonadal vessels along the pelvic sidewall in the event avulsion does occur.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this article, I describe my technique for a vaginal approach to right salpingectomy with ovarian preservation, as well as right salpingo-oophorectomy, in a patient lacking a left tube and ovary. This technique is fully illustrated on a cadaver in the Web-based master course in vaginal hysterectomy produced by the AAGL and co-sponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That course is available online at https://www.aagl.org/vaghystwebinar.
For a detailed description of vaginal hysterectomy technique, see the article entitled “Vaginal hysterectomy using basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. Next month, in the December 2015 issue of the journal, I will detail my strategies for managing complications associated with vaginal hysterectomy, salpingectomy, and salpingo-oophorectomy.
Right salpingectomy
FIGURE 1 Locate the tube |
The fallopian tube will almost always be found on top of the ovary. |
FIGURE 2 Isolate the tube 
|
| Grasp the tube and bring it to the midline. |
Start with light traction
Begin by placing an instrument on the round ligament, tube, and uterine-ovarian pedicle, exerting light traction. Note that the tube will always be found on top of the ovary
(FIGURE 1). Take care during placement of packing material to avoid sweeping the fimbriae of the tube up and out of the surgical field. You may need to play with the packing a bit until you are able to deliver the tube.
Once you identify the tube, isolate it by bringing it down to the midline (FIGURE 2). One thing to note if you’re accustomed to performing bilateral salpingo-oophorectomy: The gonadal pedicle is fairly substantive and can sustain a bit of tugging. However, if you’re performing salpingectomy with ovarian preservation, you need to be much more careful in your handling of the tube because the mesosalpinx is extremely delicate.
After you bring the tube to the midline, grasp it using a Heaney or Shallcross clamp. You could use energy to take this pedicle or clamp and tie it.
Make sure that the packing material is out of the way and that you have most of the tube nicely isolated. Don’t take the tube too far up in the surgical field because, if you lose it, it can be hard to control the bleeding. Ensure that you have grasped the fimbriated end of the tube.
In some cases you can leave a portion of the tube right next to the round ligament (FIGURE 3). You can go back and take that portion later, if you desire. But when it comes to the potential for the fallopian tube to generate carcinoma, most of the concern involves the mid to distal end of the tube rather than the cornual portion.
Once the Shallcross clamp has a good purchase on the pedicle, bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle (FIGURE 4). It is extremely important during salpingectomy to tie this suture down gently but tightly. In the process, have your assistant flash the Shallcross clamp open when you tie the suture. Otherwise, the suture will tend to tear through the mesosalpinx. Be very careful in your handling of the specimen at this point. Next, cut right along the edge of the clamp to remove the tube.
FIGURE 3 Focus on the distal tube
| FIGURE 4 Clamp and tie the pedicle 
| |
| The cornual portion of the tube (proximal to the round ligament) can be left behind, if desired. The propensity for cancer centers on the distal end of the tube. | Bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle. |
If you prefer, you can stick-tie the remaining portion again, but usually one tie will suffice because there is such a small pedicle there. The distal portion of the pedicle eventually will necrose close to the tie. The next step is ensuring hemostasis.
On occasion, if you lose the pedicle high in the surgical field, you can try to oversew it. A 2-0 Vicryl suture may be used to place a figure-eight stitch to control bleeding around the mesosalpinx. Alternatively, an energy device may be used for hemostasis. Rarely, if you encounter bleeding that does not respond to the previous suggestions, you may need to remove the ovary to control bleeding if the tissue tears.
Transvaginal technique for salpingo-oophorectomy
Once the hysterectomy is completed, grasp the round ligament, tube, and uterine-ovarian pedicle, placing slight tension on the pedicle, and free the right round ligament to ease isolation of the gonadal vessels. Using electrocautery, carefully transect the round ligament. It is critical when isolating the round ligament to transect only the ligament and not to get deep into the underlying tissue or bleeding will ensue. If you “hug” just the round ligament, you will open into the broad ligament and easily be able to isolate the gonadal pedicle.
Once the pedicle is nicely isolated, readjust your retractors or lighting to improve visualization. Now the gonadal vessels can be isolated up high much more easily (FIGURE 1).
Next, use a Heaney clamp to grab the pedicle, making sure that the ovary is medial to the clamp (FIGURE 2).

| 
| |
| FIGURE 1: ISOLATE THE GONADAL VESSELS Once optimal visualization is achieved, the gonadal vessels can be isolated easily. | FIGURE 2: KEEP THE OVARY MEDIAL TO THE CLAMP Use a Heaney clamp to grab the pedicle, keeping the ovary medial to the clamp. |
In this setting, there are a number of techniques you can use to complete the salpingo-oophorectomy. I tend to doubly ligate the pedicle. To begin, cut the tagging suture to get it out of the way. Then place a free tie lateral to the clamp, bringing it down and underneath to fully encircle the pedicle. Ligate the pedicle then cut the free tie. Follow by cutting the pedicle beside the Heaney clamp and removing the specimen. Stick-tie the remaining pedicle.
Locate the free tie, which is easily identified. Place your needle between that free tie and the clamp so that you do not pierce the vessels proximal to the tie with that needle. Then doubly ligate the pedicle.
Check for hemostasis and, once confirmed, cut the pedicle tie. Because this patient does not have a left tube and ovary, the procedure is now completed.
Conclusion
The tubes are usually readily accessible for removal at the time of vaginal hysterectomy. There is evolving evidence that the tube may play a role in malignancy of the female genital tract. Thus, removal may be preventive. In addition, if there are paratubal cysts or hydrosalpinx from prior tubal ligation, it makes sense to remove the tube. There is little evidence to suggest that removal of the tubes accelerates the menopausal transition due to compromise of the blood supply to the ovaries.
You must be very gentle when handling and removing just the tubes. The mesosalpinx is delicate and easily torn or traumatized. A careful and deliberate approach is warranted.
Bilateral salpingectomy: Key take-aways
Locate the tube. The fallopian tube always lies on top of the ovary and should be found there. On occasion, the abdominal packing used to move the bowel out of the pelvis will “hide” the tube; readjusting this packing often solves the problem.
Be gentle with the mesosalpinx as it is very delicate and can easily avulse. It is very important to “flash the clamp” (open the clamp and then close it) as you free-tie the mesosalpinx to avoid cutting through the delicate pedicle.
Remove as much tube as possible. The fimbriae end of the tube usually is free and easy to identify. Try to remove as much of the tube as possible. Often, a bit of the proximal tube is left in the utero-ovarian pedicle tie.
Clean up. You will often find peritubal cysts or “tubal clips” from a sterilization procedure. I recommend that you remove any of these you encounter to avoid problems down the road. Often, these cysts and clip-like devices are removed as part of the specimen.
Dry up. Always confirm hemostasis before concluding the procedure. If there is bleeding, be sure to assess the mesosalpinx. Occasionally, the pedicle can be torn higher up, near the gonadal vessels. Investigate this region if bleeding seems to be an issue.
Transvaginal salpingo-oophorectomy: Key take-aways
Perfect a technique. There are many approaches to transvaginal removal of the adnexae; pick one and perfect it. The better you are, the fewer complications you will have. Recognize that a different approach (use of a stapler or energy sealing device, for example) may prove useful in some settings. Be surgically versatile and recognize situations that might call for something other than your usual approach.
Optimize visualization. The tubes and ovaries are usually very accessible vaginally. Use an abdominal pack to move the bowel out of the pelvis. Adequate retraction and use of a lighted retractor or suction irrigator will facilitate exposure.
Ligate the gonadal vessels. Retraction of the tube and ovary complex medially away from the pelvic sidewall will allow you to place a clamp (or stapler or energy device) lateral to secure the gonadal vessels and ensure complete removal of the adnexae.
Release the round ligament. Although this step is usually not required, it will allow you to isolate the adnexae more precisely, especially when dealing with an adnexal mass transvaginally. It is critical that you “hug” the round ligament and refrain from penetrating deeply into the underlying tissues, or bleeding will occur. Once the round ligament is released, the tube and ovary are isolated on the gonadal pedicle and can be completely excised with this technique.
Manage bleeding. If suturing, I prefer to doubly ligate the gonadal vessels. Once I clamp the pedicle, I “free-tie” the gonadal vessels with an initial suture. This suture secures the vascular pedicle and prevents retraction. The adnexae can then be removed, followed by placement of a “stick-tie” to re-ligate the pedicle. Although this vascular pedicle is more robust than the mesosalpinx, it, too, can be avulsed, so it is important to proceed with caution. I recommend having a long clamp (uterine packing forceps or MD Anderson clamp) available in your instrument pan to facilitate specific isolation of the gonadal vessels along the pelvic sidewall in the event avulsion does occur.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this article, I describe my technique for a vaginal approach to right salpingectomy with ovarian preservation, as well as right salpingo-oophorectomy, in a patient lacking a left tube and ovary. This technique is fully illustrated on a cadaver in the Web-based master course in vaginal hysterectomy produced by the AAGL and co-sponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That course is available online at https://www.aagl.org/vaghystwebinar.
For a detailed description of vaginal hysterectomy technique, see the article entitled “Vaginal hysterectomy using basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. Next month, in the December 2015 issue of the journal, I will detail my strategies for managing complications associated with vaginal hysterectomy, salpingectomy, and salpingo-oophorectomy.
Right salpingectomy
FIGURE 1 Locate the tube |
The fallopian tube will almost always be found on top of the ovary. |
FIGURE 2 Isolate the tube 
|
| Grasp the tube and bring it to the midline. |
Start with light traction
Begin by placing an instrument on the round ligament, tube, and uterine-ovarian pedicle, exerting light traction. Note that the tube will always be found on top of the ovary
(FIGURE 1). Take care during placement of packing material to avoid sweeping the fimbriae of the tube up and out of the surgical field. You may need to play with the packing a bit until you are able to deliver the tube.
Once you identify the tube, isolate it by bringing it down to the midline (FIGURE 2). One thing to note if you’re accustomed to performing bilateral salpingo-oophorectomy: The gonadal pedicle is fairly substantive and can sustain a bit of tugging. However, if you’re performing salpingectomy with ovarian preservation, you need to be much more careful in your handling of the tube because the mesosalpinx is extremely delicate.
After you bring the tube to the midline, grasp it using a Heaney or Shallcross clamp. You could use energy to take this pedicle or clamp and tie it.
Make sure that the packing material is out of the way and that you have most of the tube nicely isolated. Don’t take the tube too far up in the surgical field because, if you lose it, it can be hard to control the bleeding. Ensure that you have grasped the fimbriated end of the tube.
In some cases you can leave a portion of the tube right next to the round ligament (FIGURE 3). You can go back and take that portion later, if you desire. But when it comes to the potential for the fallopian tube to generate carcinoma, most of the concern involves the mid to distal end of the tube rather than the cornual portion.
Once the Shallcross clamp has a good purchase on the pedicle, bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle (FIGURE 4). It is extremely important during salpingectomy to tie this suture down gently but tightly. In the process, have your assistant flash the Shallcross clamp open when you tie the suture. Otherwise, the suture will tend to tear through the mesosalpinx. Be very careful in your handling of the specimen at this point. Next, cut right along the edge of the clamp to remove the tube.
FIGURE 3 Focus on the distal tube
| FIGURE 4 Clamp and tie the pedicle 
| |
| The cornual portion of the tube (proximal to the round ligament) can be left behind, if desired. The propensity for cancer centers on the distal end of the tube. | Bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle. |
If you prefer, you can stick-tie the remaining portion again, but usually one tie will suffice because there is such a small pedicle there. The distal portion of the pedicle eventually will necrose close to the tie. The next step is ensuring hemostasis.
On occasion, if you lose the pedicle high in the surgical field, you can try to oversew it. A 2-0 Vicryl suture may be used to place a figure-eight stitch to control bleeding around the mesosalpinx. Alternatively, an energy device may be used for hemostasis. Rarely, if you encounter bleeding that does not respond to the previous suggestions, you may need to remove the ovary to control bleeding if the tissue tears.
Transvaginal technique for salpingo-oophorectomy
Once the hysterectomy is completed, grasp the round ligament, tube, and uterine-ovarian pedicle, placing slight tension on the pedicle, and free the right round ligament to ease isolation of the gonadal vessels. Using electrocautery, carefully transect the round ligament. It is critical when isolating the round ligament to transect only the ligament and not to get deep into the underlying tissue or bleeding will ensue. If you “hug” just the round ligament, you will open into the broad ligament and easily be able to isolate the gonadal pedicle.
Once the pedicle is nicely isolated, readjust your retractors or lighting to improve visualization. Now the gonadal vessels can be isolated up high much more easily (FIGURE 1).
Next, use a Heaney clamp to grab the pedicle, making sure that the ovary is medial to the clamp (FIGURE 2).

| 
| |
| FIGURE 1: ISOLATE THE GONADAL VESSELS Once optimal visualization is achieved, the gonadal vessels can be isolated easily. | FIGURE 2: KEEP THE OVARY MEDIAL TO THE CLAMP Use a Heaney clamp to grab the pedicle, keeping the ovary medial to the clamp. |
In this setting, there are a number of techniques you can use to complete the salpingo-oophorectomy. I tend to doubly ligate the pedicle. To begin, cut the tagging suture to get it out of the way. Then place a free tie lateral to the clamp, bringing it down and underneath to fully encircle the pedicle. Ligate the pedicle then cut the free tie. Follow by cutting the pedicle beside the Heaney clamp and removing the specimen. Stick-tie the remaining pedicle.
Locate the free tie, which is easily identified. Place your needle between that free tie and the clamp so that you do not pierce the vessels proximal to the tie with that needle. Then doubly ligate the pedicle.
Check for hemostasis and, once confirmed, cut the pedicle tie. Because this patient does not have a left tube and ovary, the procedure is now completed.
Conclusion
The tubes are usually readily accessible for removal at the time of vaginal hysterectomy. There is evolving evidence that the tube may play a role in malignancy of the female genital tract. Thus, removal may be preventive. In addition, if there are paratubal cysts or hydrosalpinx from prior tubal ligation, it makes sense to remove the tube. There is little evidence to suggest that removal of the tubes accelerates the menopausal transition due to compromise of the blood supply to the ovaries.
You must be very gentle when handling and removing just the tubes. The mesosalpinx is delicate and easily torn or traumatized. A careful and deliberate approach is warranted.
Bilateral salpingectomy: Key take-aways
Locate the tube. The fallopian tube always lies on top of the ovary and should be found there. On occasion, the abdominal packing used to move the bowel out of the pelvis will “hide” the tube; readjusting this packing often solves the problem.
Be gentle with the mesosalpinx as it is very delicate and can easily avulse. It is very important to “flash the clamp” (open the clamp and then close it) as you free-tie the mesosalpinx to avoid cutting through the delicate pedicle.
Remove as much tube as possible. The fimbriae end of the tube usually is free and easy to identify. Try to remove as much of the tube as possible. Often, a bit of the proximal tube is left in the utero-ovarian pedicle tie.
Clean up. You will often find peritubal cysts or “tubal clips” from a sterilization procedure. I recommend that you remove any of these you encounter to avoid problems down the road. Often, these cysts and clip-like devices are removed as part of the specimen.
Dry up. Always confirm hemostasis before concluding the procedure. If there is bleeding, be sure to assess the mesosalpinx. Occasionally, the pedicle can be torn higher up, near the gonadal vessels. Investigate this region if bleeding seems to be an issue.
Transvaginal salpingo-oophorectomy: Key take-aways
Perfect a technique. There are many approaches to transvaginal removal of the adnexae; pick one and perfect it. The better you are, the fewer complications you will have. Recognize that a different approach (use of a stapler or energy sealing device, for example) may prove useful in some settings. Be surgically versatile and recognize situations that might call for something other than your usual approach.
Optimize visualization. The tubes and ovaries are usually very accessible vaginally. Use an abdominal pack to move the bowel out of the pelvis. Adequate retraction and use of a lighted retractor or suction irrigator will facilitate exposure.
Ligate the gonadal vessels. Retraction of the tube and ovary complex medially away from the pelvic sidewall will allow you to place a clamp (or stapler or energy device) lateral to secure the gonadal vessels and ensure complete removal of the adnexae.
Release the round ligament. Although this step is usually not required, it will allow you to isolate the adnexae more precisely, especially when dealing with an adnexal mass transvaginally. It is critical that you “hug” the round ligament and refrain from penetrating deeply into the underlying tissues, or bleeding will occur. Once the round ligament is released, the tube and ovary are isolated on the gonadal pedicle and can be completely excised with this technique.
Manage bleeding. If suturing, I prefer to doubly ligate the gonadal vessels. Once I clamp the pedicle, I “free-tie” the gonadal vessels with an initial suture. This suture secures the vascular pedicle and prevents retraction. The adnexae can then be removed, followed by placement of a “stick-tie” to re-ligate the pedicle. Although this vascular pedicle is more robust than the mesosalpinx, it, too, can be avulsed, so it is important to proceed with caution. I recommend having a long clamp (uterine packing forceps or MD Anderson clamp) available in your instrument pan to facilitate specific isolation of the gonadal vessels along the pelvic sidewall in the event avulsion does occur.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Article
- Technique for salpingo-oophorectomy
- Salpingectomy: Key take-aways
- Pointers for salpingo- oophorectomy
What you need to know (and do) to prescribe the new drug flibanserin
It was a long road to approval by the US Food and Drug Administration (FDA), but flibanserin (Addyi) got the nod on August 18, 2015. Its New Drug Application (NDA) originally was filed October 27, 2009. The drug launched October 17, 2015.
Although there has been a lot of fanfare about approval of this drug, most of the coverage has focused on its status as the “first female Viagra”—a less than accurate depiction. For a more realistic and practical assessment of the drug, OBG Management turned to Michael Krychman, MD, executive director of the Southern California Center for Sexual Health and Survivorship Medicine in Newport Beach, to determine the types of information clinicians need to know to begin prescribing flibanserin. This article highlights 11 questions (and answers) to help you get started.
1. How did the FDA arrive
at its approval?
In 2012, the agency determined that female sexual dysfunction was one of 20 disease areas that warranted focused attention. In October 2014, as part of its intensified look at female sexual dysfunction, the FDA convened a 2-day meeting “to advance our understanding,” reports Andrea Fischer, FDA press officer.
“During the first day of the meeting, the FDA solicited patients’ perspectives on their condition and its impact on daily life. While this meeting did not focus on flibanserin, it provided an opportunity for the FDA to hear directly from patients about the impact of their condition,” Ms. Fischer says. During the second day of the meeting, the FDA “discussed scientific issues and challenges with experts in sexual medicine.”
As a result, by the time of the FDA’s June 4, 2015 Advisory Committee meeting on the flibanserin NDA, FDA physician-scientists were well versed in many nuances of female sexual function. That meeting included an open public hearing “that provided an opportunity for members of the public, including patients, to provide input specifically on the flibanserin application,” Ms. Fischer notes.
Nuances of the deliberations
“The FDA’s regulatory decision making on any drug product is a science-based process that carefully weighs each drug in terms of its risks and benefits to the patient population for which the drug would be indicated,” says Ms. Fischer.
The challenge in the case of flibanserin was determining whether the drug provides “clinically meaningful” improvements in sexual activity and desire.
“For many conditions and diseases, what constitutes ‘clinically meaningful’ is well known and accepted,” Ms. Fischer notes, “such as when something is cured or a severe symptom that is life-altering resolves completely. For others, this is not the case. For example, a condition that has a wide range of degree of severity can offer challenges in assessing what constitutes a clinically meaningful treatment effect. Ascertaining this requires a comprehensive knowledge of the disease, affected patient population, management strategies and the drug in question, as well as an ability to look at the clinical trial data taking this all into account.”
“In clinical trials, an important method for assessing the impact of a treatment on a patient’s symptoms, mental state, or functional status is through direct self-report using well developed and thoughtfully integrated patient-reported outcome (PRO) assessments,” Ms. Fischer says. “PROs can provide valuable information on the patient perspective when determining whether benefits outweigh risks, and they also are used to support medical product labeling claims, which are a key source of information for both health care providers and patients. PROs have been and continue to be a high priority as part of FDA’s commitment to advance patient-focused drug development, and we fully expect this to continue. The clinical trials in the flibanserin NDA all utilized PRO assessments.”
Those assessments found that patients taking flibanserin had a significant increase in “sexually satisfying events.” Three 24-week randomized controlled trials explored this endpoint for flibanserin (studies 1–3).
As for improvements in desire, the first 2 trials utilized an e-diary to assess this aspect of sexual function, while the 3rd trial utilized the Female Sexual Function Index (FSFI).
Although the e-diary reflected no statistically significant improvement in desire in the first 2 trials, the FSFI did find significant improvement in the 3rd trial. In addition, when the FSFI was considered across all 3 trials, results in the desire domain were consistent. (The FSFI was used as a secondary tool in the first 2 trials.)
In addition, sexual distress, as measured by the Female Sexual Distress Scale (FSDS), was decreased in the trials with use of flibanserin, notes Dr. Krychman. The Advisory Committee determined that these findings were sufficient to demonstrate clinically meaningful improvements with use of the drug.
Although the drug was approved by the FDA, the agency was sufficiently concerned about some of its potential risks (see questions 4 and 5) that it implemented rigorous mitigation strategies (see question 7). Additional investigations were requested by the agency, including drug-drug interaction, alcohol challenge, and driving studies.
2. What are the indications?
Flibanserin is intended for use in premenopausal women who have acquired, generalized hypoactive sexual desire disorder (HSDD). That diagnosis no longer is included in the 5th edition of the Diagnostic and
Statistical Manual of Mental Disorders but is described in drug package labeling as “low sexual desire that causes marked distress or interpersonal difficulty and is not due to:
- a coexisting medical or psychiatric condition,
- problems within the relationship, or
- the effects of a medication or other drug substance.”1
- the effects of a medication or other drug substance.”
Although the drug has been tested in both premenopausal and postmenopausal women, it was approved for use only in premenopausal women. Also note inclusion of the term “acquired” before the diagnosis of HSDD, indicating that the drug is inappropriate for women who have never experienced a period of normal sexual desire.
3. How is HSDD diagnosed?
One of the best screening tools is the
Decreased Sexual Desire Screener, says
Dr. Krychman. It is available at http://obgynalliance.com/files/fsd/DSDS_Pocketcard.pdf. This tool is a validated instrument to help clinicians identify what HSDD is and is not.
4. Does the drug carry
any warnings?
Yes, it carries a black box warning about the risks of hypotension and syncope:
- when alcohol is consumed by users of the drug. (Alcohol use is contraindicated.)
- when the drug is taken in conjunction with moderate or strong CYP3A4 inhibitors or by patients with hepatic impairment. (The drug is contraindicated in both circumstances.) See question 9 for a list of drugs that are CYP3A4 inhibitors.
5. Are there any other risks worth noting?
The medication can increase the risks of hypotension and syncope even without concomitant use of alcohol. For example, in clinical trials, hypotension was reported in 0.2% of flibanserin-treated women versus less than 0.1% of placebo users. And syncope was reported in 0.4% of flibanserin users versus 0.2% of placebo-treated patients. Flibanserin is prescribed as a once-daily medication that is to be taken at bedtime; the risks of hypotension and syncope are increased if flibanserin is taken during waking hours.
The risk of adverse effects when flibanserin is taken with alcohol is highlighted by one case reported in package labeling: A 54-year-old postmenopausal woman died after taking flibanserin (100 mg daily at bedtime) for 14 days. This patient had a history of hypertension and hypercholesterolemia and consumed a baseline amount of 1 to 3 alcoholic beverages daily. She died of acute alcohol intoxication, with a blood alcohol concentration of 0.289 g/dL.1 Whether this patient’s death was related to flibanserin use is unknown.1
It is interesting to note that, in the studies of flibanserin leading up to the drug’s approval, alcohol use was not an exclusion, says Dr. Krychman. “Approximately 58% of women were self-described as mild to moderate drinkers. The clinical program was extremely large—more than 11,000 women were studied.”
Flibanserin is currently not approved for use in postmenopausal women, and concomitant alcohol consumption is contraindicated.
6. What is the dose?
The recommended dose is one tablet of
100 mg daily. The drug is to be taken at
bedtime to reduce the risks of hypotension, syncope, accidental injury, and central nervous system (CNS) depression, which can occur even in the absence of alcohol.
7. Are there any requirements for clinicians who want to prescribe the drug?
Yes. Because of the risks of hypotension, syncope, and CNS depression, the drug is subject to Risk Evaluation and Mitigation Strategies (REMS), as determined by the FDA. To prescribe the drug, providers must:
- review its prescribing information
- review the Provider and Pharmacy Training Program
- complete and submit the Knowledge Assessment Form
- enroll in REMS by completing and submitting the Prescriber Enrollment Form.
Before giving a patient her initial prescription, the provider must counsel her about the risks of hypotension and syncope and the interaction with alcohol using the Patient-Provider Agreement Form. The provider must then complete that form, provide a designated portion of it to the patient, and retain the remainder for the patient’s file.
For more information and to download the relevant forms, visit https://www.addyirems.com.
8. What are the most common
adverse reactions to the drug?
According to package labeling, the most common adverse reactions, with an incidence greater than 2%, are dizziness, somnolence, nausea, fatigue, insomnia, and dry mouth.
Less common reactions include anxiety, constipation, abdominal pain, rash, sedation, and vertigo.
In studies of the drug, appendicitis was reported among 0.2% of flibanserin-treated patients, compared with no reports of appendicitis among placebo-treated patients. The FDA has requested additional investigation of the association, if any, between flibanserin and appendicitis.
9. What drug interactions are notable?
As stated earlier, the concomitant use of flibanserin with alcohol or a moderate or strong CYP3A4 inhibitor can result in severe hypotension and syncope. Flibanserin also should not be prescribed for patients who use other CNS depressants such as diphenhydramine, opioids, benzodiazepines, and hypnotic agents.
Some examples of strong CYP3A4 inhibitors are ketoconazole, itraconazole, posaconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, boceprevir, telaprevir, telithromycin, and conivaptan.
Moderate CYP3A4 inhibitors include amprenavir, atazanavir, ciprofloxacin, diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil, and grapefruit juice.
In addition, the concomitant use of flibanserin with multiple weak CYP3A4 inhibitors—which include herbal supplements such as ginkgo and resveratrol and nonprescription drugs such as cimetidine—also may increase the risks of hypotension and syncope.
The concomitant use of flibanserin with digoxin increases the digoxin concentration and may lead to toxicity.
10. Is the drug safe in pregnancy
and lactation?
There are currently no data on the use of flibanserin in human pregnancy. In animals, fetal toxicity occurred only in the presence of significant maternal toxicity. Adverse effects included decreased fetal weight, structural anomalies, and increases in fetal loss when exposure exceeded 15 times the recommended human dosage.
As for the advisability of using flibanserin during lactation, it is unknown whether the drug is excreted in human milk, whether it might have adverse effects in the breastfed infant, or whether it affects milk production. Package labeling states: “Because of the potential for serious adverse reactions, including sedation in a breastfed infant, breastfeeding is not recommended during treatment with [flibanserin].”1
11. When should the drug
be discontinued?
If there is no improvement in sexual desire after an 8-week trial of flibanserin, the drug should be
discontinued.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Addyi [package insert]. Raleigh, NC: Sprout Pharmaceuticals; 2015.
It was a long road to approval by the US Food and Drug Administration (FDA), but flibanserin (Addyi) got the nod on August 18, 2015. Its New Drug Application (NDA) originally was filed October 27, 2009. The drug launched October 17, 2015.
Although there has been a lot of fanfare about approval of this drug, most of the coverage has focused on its status as the “first female Viagra”—a less than accurate depiction. For a more realistic and practical assessment of the drug, OBG Management turned to Michael Krychman, MD, executive director of the Southern California Center for Sexual Health and Survivorship Medicine in Newport Beach, to determine the types of information clinicians need to know to begin prescribing flibanserin. This article highlights 11 questions (and answers) to help you get started.
1. How did the FDA arrive
at its approval?
In 2012, the agency determined that female sexual dysfunction was one of 20 disease areas that warranted focused attention. In October 2014, as part of its intensified look at female sexual dysfunction, the FDA convened a 2-day meeting “to advance our understanding,” reports Andrea Fischer, FDA press officer.
“During the first day of the meeting, the FDA solicited patients’ perspectives on their condition and its impact on daily life. While this meeting did not focus on flibanserin, it provided an opportunity for the FDA to hear directly from patients about the impact of their condition,” Ms. Fischer says. During the second day of the meeting, the FDA “discussed scientific issues and challenges with experts in sexual medicine.”
As a result, by the time of the FDA’s June 4, 2015 Advisory Committee meeting on the flibanserin NDA, FDA physician-scientists were well versed in many nuances of female sexual function. That meeting included an open public hearing “that provided an opportunity for members of the public, including patients, to provide input specifically on the flibanserin application,” Ms. Fischer notes.
Nuances of the deliberations
“The FDA’s regulatory decision making on any drug product is a science-based process that carefully weighs each drug in terms of its risks and benefits to the patient population for which the drug would be indicated,” says Ms. Fischer.
The challenge in the case of flibanserin was determining whether the drug provides “clinically meaningful” improvements in sexual activity and desire.
“For many conditions and diseases, what constitutes ‘clinically meaningful’ is well known and accepted,” Ms. Fischer notes, “such as when something is cured or a severe symptom that is life-altering resolves completely. For others, this is not the case. For example, a condition that has a wide range of degree of severity can offer challenges in assessing what constitutes a clinically meaningful treatment effect. Ascertaining this requires a comprehensive knowledge of the disease, affected patient population, management strategies and the drug in question, as well as an ability to look at the clinical trial data taking this all into account.”
“In clinical trials, an important method for assessing the impact of a treatment on a patient’s symptoms, mental state, or functional status is through direct self-report using well developed and thoughtfully integrated patient-reported outcome (PRO) assessments,” Ms. Fischer says. “PROs can provide valuable information on the patient perspective when determining whether benefits outweigh risks, and they also are used to support medical product labeling claims, which are a key source of information for both health care providers and patients. PROs have been and continue to be a high priority as part of FDA’s commitment to advance patient-focused drug development, and we fully expect this to continue. The clinical trials in the flibanserin NDA all utilized PRO assessments.”
Those assessments found that patients taking flibanserin had a significant increase in “sexually satisfying events.” Three 24-week randomized controlled trials explored this endpoint for flibanserin (studies 1–3).
As for improvements in desire, the first 2 trials utilized an e-diary to assess this aspect of sexual function, while the 3rd trial utilized the Female Sexual Function Index (FSFI).
Although the e-diary reflected no statistically significant improvement in desire in the first 2 trials, the FSFI did find significant improvement in the 3rd trial. In addition, when the FSFI was considered across all 3 trials, results in the desire domain were consistent. (The FSFI was used as a secondary tool in the first 2 trials.)
In addition, sexual distress, as measured by the Female Sexual Distress Scale (FSDS), was decreased in the trials with use of flibanserin, notes Dr. Krychman. The Advisory Committee determined that these findings were sufficient to demonstrate clinically meaningful improvements with use of the drug.
Although the drug was approved by the FDA, the agency was sufficiently concerned about some of its potential risks (see questions 4 and 5) that it implemented rigorous mitigation strategies (see question 7). Additional investigations were requested by the agency, including drug-drug interaction, alcohol challenge, and driving studies.
2. What are the indications?
Flibanserin is intended for use in premenopausal women who have acquired, generalized hypoactive sexual desire disorder (HSDD). That diagnosis no longer is included in the 5th edition of the Diagnostic and
Statistical Manual of Mental Disorders but is described in drug package labeling as “low sexual desire that causes marked distress or interpersonal difficulty and is not due to:
- a coexisting medical or psychiatric condition,
- problems within the relationship, or
- the effects of a medication or other drug substance.”1
- the effects of a medication or other drug substance.”
Although the drug has been tested in both premenopausal and postmenopausal women, it was approved for use only in premenopausal women. Also note inclusion of the term “acquired” before the diagnosis of HSDD, indicating that the drug is inappropriate for women who have never experienced a period of normal sexual desire.
3. How is HSDD diagnosed?
One of the best screening tools is the
Decreased Sexual Desire Screener, says
Dr. Krychman. It is available at http://obgynalliance.com/files/fsd/DSDS_Pocketcard.pdf. This tool is a validated instrument to help clinicians identify what HSDD is and is not.
4. Does the drug carry
any warnings?
Yes, it carries a black box warning about the risks of hypotension and syncope:
- when alcohol is consumed by users of the drug. (Alcohol use is contraindicated.)
- when the drug is taken in conjunction with moderate or strong CYP3A4 inhibitors or by patients with hepatic impairment. (The drug is contraindicated in both circumstances.) See question 9 for a list of drugs that are CYP3A4 inhibitors.
5. Are there any other risks worth noting?
The medication can increase the risks of hypotension and syncope even without concomitant use of alcohol. For example, in clinical trials, hypotension was reported in 0.2% of flibanserin-treated women versus less than 0.1% of placebo users. And syncope was reported in 0.4% of flibanserin users versus 0.2% of placebo-treated patients. Flibanserin is prescribed as a once-daily medication that is to be taken at bedtime; the risks of hypotension and syncope are increased if flibanserin is taken during waking hours.
The risk of adverse effects when flibanserin is taken with alcohol is highlighted by one case reported in package labeling: A 54-year-old postmenopausal woman died after taking flibanserin (100 mg daily at bedtime) for 14 days. This patient had a history of hypertension and hypercholesterolemia and consumed a baseline amount of 1 to 3 alcoholic beverages daily. She died of acute alcohol intoxication, with a blood alcohol concentration of 0.289 g/dL.1 Whether this patient’s death was related to flibanserin use is unknown.1
It is interesting to note that, in the studies of flibanserin leading up to the drug’s approval, alcohol use was not an exclusion, says Dr. Krychman. “Approximately 58% of women were self-described as mild to moderate drinkers. The clinical program was extremely large—more than 11,000 women were studied.”
Flibanserin is currently not approved for use in postmenopausal women, and concomitant alcohol consumption is contraindicated.
6. What is the dose?
The recommended dose is one tablet of
100 mg daily. The drug is to be taken at
bedtime to reduce the risks of hypotension, syncope, accidental injury, and central nervous system (CNS) depression, which can occur even in the absence of alcohol.
7. Are there any requirements for clinicians who want to prescribe the drug?
Yes. Because of the risks of hypotension, syncope, and CNS depression, the drug is subject to Risk Evaluation and Mitigation Strategies (REMS), as determined by the FDA. To prescribe the drug, providers must:
- review its prescribing information
- review the Provider and Pharmacy Training Program
- complete and submit the Knowledge Assessment Form
- enroll in REMS by completing and submitting the Prescriber Enrollment Form.
Before giving a patient her initial prescription, the provider must counsel her about the risks of hypotension and syncope and the interaction with alcohol using the Patient-Provider Agreement Form. The provider must then complete that form, provide a designated portion of it to the patient, and retain the remainder for the patient’s file.
For more information and to download the relevant forms, visit https://www.addyirems.com.
8. What are the most common
adverse reactions to the drug?
According to package labeling, the most common adverse reactions, with an incidence greater than 2%, are dizziness, somnolence, nausea, fatigue, insomnia, and dry mouth.
Less common reactions include anxiety, constipation, abdominal pain, rash, sedation, and vertigo.
In studies of the drug, appendicitis was reported among 0.2% of flibanserin-treated patients, compared with no reports of appendicitis among placebo-treated patients. The FDA has requested additional investigation of the association, if any, between flibanserin and appendicitis.
9. What drug interactions are notable?
As stated earlier, the concomitant use of flibanserin with alcohol or a moderate or strong CYP3A4 inhibitor can result in severe hypotension and syncope. Flibanserin also should not be prescribed for patients who use other CNS depressants such as diphenhydramine, opioids, benzodiazepines, and hypnotic agents.
Some examples of strong CYP3A4 inhibitors are ketoconazole, itraconazole, posaconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, boceprevir, telaprevir, telithromycin, and conivaptan.
Moderate CYP3A4 inhibitors include amprenavir, atazanavir, ciprofloxacin, diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil, and grapefruit juice.
In addition, the concomitant use of flibanserin with multiple weak CYP3A4 inhibitors—which include herbal supplements such as ginkgo and resveratrol and nonprescription drugs such as cimetidine—also may increase the risks of hypotension and syncope.
The concomitant use of flibanserin with digoxin increases the digoxin concentration and may lead to toxicity.
10. Is the drug safe in pregnancy
and lactation?
There are currently no data on the use of flibanserin in human pregnancy. In animals, fetal toxicity occurred only in the presence of significant maternal toxicity. Adverse effects included decreased fetal weight, structural anomalies, and increases in fetal loss when exposure exceeded 15 times the recommended human dosage.
As for the advisability of using flibanserin during lactation, it is unknown whether the drug is excreted in human milk, whether it might have adverse effects in the breastfed infant, or whether it affects milk production. Package labeling states: “Because of the potential for serious adverse reactions, including sedation in a breastfed infant, breastfeeding is not recommended during treatment with [flibanserin].”1
11. When should the drug
be discontinued?
If there is no improvement in sexual desire after an 8-week trial of flibanserin, the drug should be
discontinued.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
It was a long road to approval by the US Food and Drug Administration (FDA), but flibanserin (Addyi) got the nod on August 18, 2015. Its New Drug Application (NDA) originally was filed October 27, 2009. The drug launched October 17, 2015.
Although there has been a lot of fanfare about approval of this drug, most of the coverage has focused on its status as the “first female Viagra”—a less than accurate depiction. For a more realistic and practical assessment of the drug, OBG Management turned to Michael Krychman, MD, executive director of the Southern California Center for Sexual Health and Survivorship Medicine in Newport Beach, to determine the types of information clinicians need to know to begin prescribing flibanserin. This article highlights 11 questions (and answers) to help you get started.
1. How did the FDA arrive
at its approval?
In 2012, the agency determined that female sexual dysfunction was one of 20 disease areas that warranted focused attention. In October 2014, as part of its intensified look at female sexual dysfunction, the FDA convened a 2-day meeting “to advance our understanding,” reports Andrea Fischer, FDA press officer.
“During the first day of the meeting, the FDA solicited patients’ perspectives on their condition and its impact on daily life. While this meeting did not focus on flibanserin, it provided an opportunity for the FDA to hear directly from patients about the impact of their condition,” Ms. Fischer says. During the second day of the meeting, the FDA “discussed scientific issues and challenges with experts in sexual medicine.”
As a result, by the time of the FDA’s June 4, 2015 Advisory Committee meeting on the flibanserin NDA, FDA physician-scientists were well versed in many nuances of female sexual function. That meeting included an open public hearing “that provided an opportunity for members of the public, including patients, to provide input specifically on the flibanserin application,” Ms. Fischer notes.
Nuances of the deliberations
“The FDA’s regulatory decision making on any drug product is a science-based process that carefully weighs each drug in terms of its risks and benefits to the patient population for which the drug would be indicated,” says Ms. Fischer.
The challenge in the case of flibanserin was determining whether the drug provides “clinically meaningful” improvements in sexual activity and desire.
“For many conditions and diseases, what constitutes ‘clinically meaningful’ is well known and accepted,” Ms. Fischer notes, “such as when something is cured or a severe symptom that is life-altering resolves completely. For others, this is not the case. For example, a condition that has a wide range of degree of severity can offer challenges in assessing what constitutes a clinically meaningful treatment effect. Ascertaining this requires a comprehensive knowledge of the disease, affected patient population, management strategies and the drug in question, as well as an ability to look at the clinical trial data taking this all into account.”
“In clinical trials, an important method for assessing the impact of a treatment on a patient’s symptoms, mental state, or functional status is through direct self-report using well developed and thoughtfully integrated patient-reported outcome (PRO) assessments,” Ms. Fischer says. “PROs can provide valuable information on the patient perspective when determining whether benefits outweigh risks, and they also are used to support medical product labeling claims, which are a key source of information for both health care providers and patients. PROs have been and continue to be a high priority as part of FDA’s commitment to advance patient-focused drug development, and we fully expect this to continue. The clinical trials in the flibanserin NDA all utilized PRO assessments.”
Those assessments found that patients taking flibanserin had a significant increase in “sexually satisfying events.” Three 24-week randomized controlled trials explored this endpoint for flibanserin (studies 1–3).
As for improvements in desire, the first 2 trials utilized an e-diary to assess this aspect of sexual function, while the 3rd trial utilized the Female Sexual Function Index (FSFI).
Although the e-diary reflected no statistically significant improvement in desire in the first 2 trials, the FSFI did find significant improvement in the 3rd trial. In addition, when the FSFI was considered across all 3 trials, results in the desire domain were consistent. (The FSFI was used as a secondary tool in the first 2 trials.)
In addition, sexual distress, as measured by the Female Sexual Distress Scale (FSDS), was decreased in the trials with use of flibanserin, notes Dr. Krychman. The Advisory Committee determined that these findings were sufficient to demonstrate clinically meaningful improvements with use of the drug.
Although the drug was approved by the FDA, the agency was sufficiently concerned about some of its potential risks (see questions 4 and 5) that it implemented rigorous mitigation strategies (see question 7). Additional investigations were requested by the agency, including drug-drug interaction, alcohol challenge, and driving studies.
2. What are the indications?
Flibanserin is intended for use in premenopausal women who have acquired, generalized hypoactive sexual desire disorder (HSDD). That diagnosis no longer is included in the 5th edition of the Diagnostic and
Statistical Manual of Mental Disorders but is described in drug package labeling as “low sexual desire that causes marked distress or interpersonal difficulty and is not due to:
- a coexisting medical or psychiatric condition,
- problems within the relationship, or
- the effects of a medication or other drug substance.”1
- the effects of a medication or other drug substance.”
Although the drug has been tested in both premenopausal and postmenopausal women, it was approved for use only in premenopausal women. Also note inclusion of the term “acquired” before the diagnosis of HSDD, indicating that the drug is inappropriate for women who have never experienced a period of normal sexual desire.
3. How is HSDD diagnosed?
One of the best screening tools is the
Decreased Sexual Desire Screener, says
Dr. Krychman. It is available at http://obgynalliance.com/files/fsd/DSDS_Pocketcard.pdf. This tool is a validated instrument to help clinicians identify what HSDD is and is not.
4. Does the drug carry
any warnings?
Yes, it carries a black box warning about the risks of hypotension and syncope:
- when alcohol is consumed by users of the drug. (Alcohol use is contraindicated.)
- when the drug is taken in conjunction with moderate or strong CYP3A4 inhibitors or by patients with hepatic impairment. (The drug is contraindicated in both circumstances.) See question 9 for a list of drugs that are CYP3A4 inhibitors.
5. Are there any other risks worth noting?
The medication can increase the risks of hypotension and syncope even without concomitant use of alcohol. For example, in clinical trials, hypotension was reported in 0.2% of flibanserin-treated women versus less than 0.1% of placebo users. And syncope was reported in 0.4% of flibanserin users versus 0.2% of placebo-treated patients. Flibanserin is prescribed as a once-daily medication that is to be taken at bedtime; the risks of hypotension and syncope are increased if flibanserin is taken during waking hours.
The risk of adverse effects when flibanserin is taken with alcohol is highlighted by one case reported in package labeling: A 54-year-old postmenopausal woman died after taking flibanserin (100 mg daily at bedtime) for 14 days. This patient had a history of hypertension and hypercholesterolemia and consumed a baseline amount of 1 to 3 alcoholic beverages daily. She died of acute alcohol intoxication, with a blood alcohol concentration of 0.289 g/dL.1 Whether this patient’s death was related to flibanserin use is unknown.1
It is interesting to note that, in the studies of flibanserin leading up to the drug’s approval, alcohol use was not an exclusion, says Dr. Krychman. “Approximately 58% of women were self-described as mild to moderate drinkers. The clinical program was extremely large—more than 11,000 women were studied.”
Flibanserin is currently not approved for use in postmenopausal women, and concomitant alcohol consumption is contraindicated.
6. What is the dose?
The recommended dose is one tablet of
100 mg daily. The drug is to be taken at
bedtime to reduce the risks of hypotension, syncope, accidental injury, and central nervous system (CNS) depression, which can occur even in the absence of alcohol.
7. Are there any requirements for clinicians who want to prescribe the drug?
Yes. Because of the risks of hypotension, syncope, and CNS depression, the drug is subject to Risk Evaluation and Mitigation Strategies (REMS), as determined by the FDA. To prescribe the drug, providers must:
- review its prescribing information
- review the Provider and Pharmacy Training Program
- complete and submit the Knowledge Assessment Form
- enroll in REMS by completing and submitting the Prescriber Enrollment Form.
Before giving a patient her initial prescription, the provider must counsel her about the risks of hypotension and syncope and the interaction with alcohol using the Patient-Provider Agreement Form. The provider must then complete that form, provide a designated portion of it to the patient, and retain the remainder for the patient’s file.
For more information and to download the relevant forms, visit https://www.addyirems.com.
8. What are the most common
adverse reactions to the drug?
According to package labeling, the most common adverse reactions, with an incidence greater than 2%, are dizziness, somnolence, nausea, fatigue, insomnia, and dry mouth.
Less common reactions include anxiety, constipation, abdominal pain, rash, sedation, and vertigo.
In studies of the drug, appendicitis was reported among 0.2% of flibanserin-treated patients, compared with no reports of appendicitis among placebo-treated patients. The FDA has requested additional investigation of the association, if any, between flibanserin and appendicitis.
9. What drug interactions are notable?
As stated earlier, the concomitant use of flibanserin with alcohol or a moderate or strong CYP3A4 inhibitor can result in severe hypotension and syncope. Flibanserin also should not be prescribed for patients who use other CNS depressants such as diphenhydramine, opioids, benzodiazepines, and hypnotic agents.
Some examples of strong CYP3A4 inhibitors are ketoconazole, itraconazole, posaconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, boceprevir, telaprevir, telithromycin, and conivaptan.
Moderate CYP3A4 inhibitors include amprenavir, atazanavir, ciprofloxacin, diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil, and grapefruit juice.
In addition, the concomitant use of flibanserin with multiple weak CYP3A4 inhibitors—which include herbal supplements such as ginkgo and resveratrol and nonprescription drugs such as cimetidine—also may increase the risks of hypotension and syncope.
The concomitant use of flibanserin with digoxin increases the digoxin concentration and may lead to toxicity.
10. Is the drug safe in pregnancy
and lactation?
There are currently no data on the use of flibanserin in human pregnancy. In animals, fetal toxicity occurred only in the presence of significant maternal toxicity. Adverse effects included decreased fetal weight, structural anomalies, and increases in fetal loss when exposure exceeded 15 times the recommended human dosage.
As for the advisability of using flibanserin during lactation, it is unknown whether the drug is excreted in human milk, whether it might have adverse effects in the breastfed infant, or whether it affects milk production. Package labeling states: “Because of the potential for serious adverse reactions, including sedation in a breastfed infant, breastfeeding is not recommended during treatment with [flibanserin].”1
11. When should the drug
be discontinued?
If there is no improvement in sexual desire after an 8-week trial of flibanserin, the drug should be
discontinued.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Addyi [package insert]. Raleigh, NC: Sprout Pharmaceuticals; 2015.
Reference
- Addyi [package insert]. Raleigh, NC: Sprout Pharmaceuticals; 2015.
In this Article
- How is HSDD diagnosed?
- What are clinicians required to do?
- Is the drug safe in pregnancy?
Signs of chorioamnionitis ignored? $3.5M settlement
Signs of chorioamnionitis ignored? $3.5M settlement
At 31 weeks’ gestation, a mother at risk for preterm labor was admitted to the hospital for 2 days. Examination and test results showed evidence of infection. She was given antenatal corticosteroids for fetal lung development in case of premature delivery. At discharge, bed rest was ordered and she complied. At 32 weeks’ gestation, she returned to the hospital with worsening symptoms, was prescribed antibiotics to treat a urinary tract infection, and was discharged. She went to the hospital a third time at almost 33 weeks’ gestation, experiencing contractions and leaking fluid. She was admitted with a plan to deliver the baby if any signs or symptoms of intra-amniotic infection (clinical chorioamnionitis) were present. Four days later, a cesarean delivery was ordered due to fetal tachycardia and decreased fetal heart rate. Imaging results performed in the neonatal intensive care unit showed that the baby received a brain injury. The child has physical and mental impairments including cerebral palsy, cortical blindness, and epilepsy.
Parents’ claim Hospital health care providers failed to communicate with each other or to obtain records from prior admissions, although the mother told them that she had been to the hospital twice within the past 2 weeks. Medical records from all 3 admissions showed clear signs and symptoms of a vaginal/cervical infection that had progressed to clinical chorioamnionitis 2 days before delivery. Examination of the placenta by a pathologist confirmed that the infection had spread to the umbilical cord, injuring the child.
Defendant’s defense The standard of care was met. There was no indication that an earlier delivery was needed.
Verdict A $3.5 million Michigan settlement was reached by the hospital during the trial.
Surgical approach questioned
A woman went to her ObGyn for tubal ligation and ventral hernia repair. The patient was concerned about infection and scarring. She agreed to a laparoscopic procedure, knowing that the procedure might have to be altered to laparotomy.
Patient’s claim The patient consented to laparoscopic surgery. However, surgery did not begin as laparoscopy but as an open procedure. The patient has a 6-inch scar on her abdomen. She accused both the ObGyn and the hospital of lack of informed consent for laparotomy.
Defendant’s defense The hospital claimed that their nurses’ role was to read the consent form signed by the patient in the ObGyn’s office. The ObGyn claimed that the patient signed a general consent form that permitted him to do what was reasonable. He had determined after surgery began that a laparoscopic procedure would have been more dangerous.
Verdict A $150,000 Louisiana verdict was returned against the ObGyn; the hospital was acquitted.
Macrosomic baby and mother both injured during delivery
Delivery of a mother’s fourth child was managed by a hospital-employed family physician (FP). Shoulder dystocia was encountered, and the FP made a 4th-degree extension of the episiotomy. The baby weighed 10 lb 14 oz at birth. The mother has fecal and urinary incontinence and pain as a result of the large episiotomy. The child has a right-sided brachial plexus injury.
Parents’ claim Failure to perform cesarean delivery caused injury to the mother and child. The FP should have recognized from the mother’s history of delivering 3 macrosomic babies and the progress of this pregnancy, that the baby was large.
Defendant’s defense The case was settled during trial.
Verdict A $1.5 million Minnesota settlement was reached that included $1.2 million for the child and $300,000 for the mother.
Surgical table folds during hysterectomy: $5.3M verdict
While a woman was undergoing a hysterectomy, the surgical table she was lying on folded up into a “U” position, causing the inserted speculum to tear the patient from vagina to rectum. The fall also caused a back injury usually attributed to falls from great distances. The patient has permanent pain, recurring diarrhea, and depression as a result of the injuries.
Patient’s claim The injuries occurred because of the defendants’ failure to read, understand, and follow the warning labels on the surgical table.
Defendant’s defense The case was settled before trial.
Verdict A $5.3 million settlement was reached with the hospital.
Signs of chorioamnionitis ignored? $3.5M settlement
At 31 weeks’ gestation, a mother at risk for preterm labor was admitted to the hospital for 2 days. Examination and test results showed evidence of infection. She was given antenatal corticosteroids for fetal lung development in case of premature delivery. At discharge, bed rest was ordered and she complied. At 32 weeks’ gestation, she returned to the hospital with worsening symptoms, was prescribed antibiotics to treat a urinary tract infection, and was discharged. She went to the hospital a third time at almost 33 weeks’ gestation, experiencing contractions and leaking fluid. She was admitted with a plan to deliver the baby if any signs or symptoms of intra-amniotic infection (clinical chorioamnionitis) were present. Four days later, a cesarean delivery was ordered due to fetal tachycardia and decreased fetal heart rate. Imaging results performed in the neonatal intensive care unit showed that the baby received a brain injury. The child has physical and mental impairments including cerebral palsy, cortical blindness, and epilepsy.
Parents’ claim Hospital health care providers failed to communicate with each other or to obtain records from prior admissions, although the mother told them that she had been to the hospital twice within the past 2 weeks. Medical records from all 3 admissions showed clear signs and symptoms of a vaginal/cervical infection that had progressed to clinical chorioamnionitis 2 days before delivery. Examination of the placenta by a pathologist confirmed that the infection had spread to the umbilical cord, injuring the child.
Defendant’s defense The standard of care was met. There was no indication that an earlier delivery was needed.
Verdict A $3.5 million Michigan settlement was reached by the hospital during the trial.
Surgical approach questioned
A woman went to her ObGyn for tubal ligation and ventral hernia repair. The patient was concerned about infection and scarring. She agreed to a laparoscopic procedure, knowing that the procedure might have to be altered to laparotomy.
Patient’s claim The patient consented to laparoscopic surgery. However, surgery did not begin as laparoscopy but as an open procedure. The patient has a 6-inch scar on her abdomen. She accused both the ObGyn and the hospital of lack of informed consent for laparotomy.
Defendant’s defense The hospital claimed that their nurses’ role was to read the consent form signed by the patient in the ObGyn’s office. The ObGyn claimed that the patient signed a general consent form that permitted him to do what was reasonable. He had determined after surgery began that a laparoscopic procedure would have been more dangerous.
Verdict A $150,000 Louisiana verdict was returned against the ObGyn; the hospital was acquitted.
Macrosomic baby and mother both injured during delivery
Delivery of a mother’s fourth child was managed by a hospital-employed family physician (FP). Shoulder dystocia was encountered, and the FP made a 4th-degree extension of the episiotomy. The baby weighed 10 lb 14 oz at birth. The mother has fecal and urinary incontinence and pain as a result of the large episiotomy. The child has a right-sided brachial plexus injury.
Parents’ claim Failure to perform cesarean delivery caused injury to the mother and child. The FP should have recognized from the mother’s history of delivering 3 macrosomic babies and the progress of this pregnancy, that the baby was large.
Defendant’s defense The case was settled during trial.
Verdict A $1.5 million Minnesota settlement was reached that included $1.2 million for the child and $300,000 for the mother.
Surgical table folds during hysterectomy: $5.3M verdict
While a woman was undergoing a hysterectomy, the surgical table she was lying on folded up into a “U” position, causing the inserted speculum to tear the patient from vagina to rectum. The fall also caused a back injury usually attributed to falls from great distances. The patient has permanent pain, recurring diarrhea, and depression as a result of the injuries.
Patient’s claim The injuries occurred because of the defendants’ failure to read, understand, and follow the warning labels on the surgical table.
Defendant’s defense The case was settled before trial.
Verdict A $5.3 million settlement was reached with the hospital.
Signs of chorioamnionitis ignored? $3.5M settlement
At 31 weeks’ gestation, a mother at risk for preterm labor was admitted to the hospital for 2 days. Examination and test results showed evidence of infection. She was given antenatal corticosteroids for fetal lung development in case of premature delivery. At discharge, bed rest was ordered and she complied. At 32 weeks’ gestation, she returned to the hospital with worsening symptoms, was prescribed antibiotics to treat a urinary tract infection, and was discharged. She went to the hospital a third time at almost 33 weeks’ gestation, experiencing contractions and leaking fluid. She was admitted with a plan to deliver the baby if any signs or symptoms of intra-amniotic infection (clinical chorioamnionitis) were present. Four days later, a cesarean delivery was ordered due to fetal tachycardia and decreased fetal heart rate. Imaging results performed in the neonatal intensive care unit showed that the baby received a brain injury. The child has physical and mental impairments including cerebral palsy, cortical blindness, and epilepsy.
Parents’ claim Hospital health care providers failed to communicate with each other or to obtain records from prior admissions, although the mother told them that she had been to the hospital twice within the past 2 weeks. Medical records from all 3 admissions showed clear signs and symptoms of a vaginal/cervical infection that had progressed to clinical chorioamnionitis 2 days before delivery. Examination of the placenta by a pathologist confirmed that the infection had spread to the umbilical cord, injuring the child.
Defendant’s defense The standard of care was met. There was no indication that an earlier delivery was needed.
Verdict A $3.5 million Michigan settlement was reached by the hospital during the trial.
Surgical approach questioned
A woman went to her ObGyn for tubal ligation and ventral hernia repair. The patient was concerned about infection and scarring. She agreed to a laparoscopic procedure, knowing that the procedure might have to be altered to laparotomy.
Patient’s claim The patient consented to laparoscopic surgery. However, surgery did not begin as laparoscopy but as an open procedure. The patient has a 6-inch scar on her abdomen. She accused both the ObGyn and the hospital of lack of informed consent for laparotomy.
Defendant’s defense The hospital claimed that their nurses’ role was to read the consent form signed by the patient in the ObGyn’s office. The ObGyn claimed that the patient signed a general consent form that permitted him to do what was reasonable. He had determined after surgery began that a laparoscopic procedure would have been more dangerous.
Verdict A $150,000 Louisiana verdict was returned against the ObGyn; the hospital was acquitted.
Macrosomic baby and mother both injured during delivery
Delivery of a mother’s fourth child was managed by a hospital-employed family physician (FP). Shoulder dystocia was encountered, and the FP made a 4th-degree extension of the episiotomy. The baby weighed 10 lb 14 oz at birth. The mother has fecal and urinary incontinence and pain as a result of the large episiotomy. The child has a right-sided brachial plexus injury.
Parents’ claim Failure to perform cesarean delivery caused injury to the mother and child. The FP should have recognized from the mother’s history of delivering 3 macrosomic babies and the progress of this pregnancy, that the baby was large.
Defendant’s defense The case was settled during trial.
Verdict A $1.5 million Minnesota settlement was reached that included $1.2 million for the child and $300,000 for the mother.
Surgical table folds during hysterectomy: $5.3M verdict
While a woman was undergoing a hysterectomy, the surgical table she was lying on folded up into a “U” position, causing the inserted speculum to tear the patient from vagina to rectum. The fall also caused a back injury usually attributed to falls from great distances. The patient has permanent pain, recurring diarrhea, and depression as a result of the injuries.
Patient’s claim The injuries occurred because of the defendants’ failure to read, understand, and follow the warning labels on the surgical table.
Defendant’s defense The case was settled before trial.
Verdict A $5.3 million settlement was reached with the hospital.
In this Article
- Surgical approach questioned
- Macrosomic baby and mother both injured during delivery
- Surgical table folds during hysterectomy: $5.3M verdict
BCG slows bladder cancer progression
Bacillus Calmette-Guérin (BCG) is the only intravesical therapy shown to be associated with decreased risk of bladder cancer progression, according to a report by the Agency for Healthcare Research and Quality (AHRQ), but the researchers cautioned that the therapy was associated with a high rate of adverse events.
The Comparative Effectiveness Review, headed by Dr. Roger Chou and based on research by the Pacific Northwest Evidence-Based Practice Center (EPC) under contract to the AHRQ, was conducted as a systematic review of trials identified via electronic databases and conducted from 1990 to 2014.
BCG was associated with a higher rate of local and systemic adverse events (granulomatous cystitis or irritative symptoms in 27%-84% of patients, macroscopic hematuria in 21%-72%, and fever in 27%-44%) when compared with no intravesical therapy, the researchers wrote. BCG was associated with decreased risk of bladder cancer progression, but no intravesical agent was associated with decreased risk of all-cause or bladder cancer mortality.
In addition, fluorescent cystoscopy was associated with decreased risk of subsequent bladder recurrence, compared with white light cystoscopy, but results were inconsistent through many of the trials identified.
Read the full report here.
Bacillus Calmette-Guérin (BCG) is the only intravesical therapy shown to be associated with decreased risk of bladder cancer progression, according to a report by the Agency for Healthcare Research and Quality (AHRQ), but the researchers cautioned that the therapy was associated with a high rate of adverse events.
The Comparative Effectiveness Review, headed by Dr. Roger Chou and based on research by the Pacific Northwest Evidence-Based Practice Center (EPC) under contract to the AHRQ, was conducted as a systematic review of trials identified via electronic databases and conducted from 1990 to 2014.
BCG was associated with a higher rate of local and systemic adverse events (granulomatous cystitis or irritative symptoms in 27%-84% of patients, macroscopic hematuria in 21%-72%, and fever in 27%-44%) when compared with no intravesical therapy, the researchers wrote. BCG was associated with decreased risk of bladder cancer progression, but no intravesical agent was associated with decreased risk of all-cause or bladder cancer mortality.
In addition, fluorescent cystoscopy was associated with decreased risk of subsequent bladder recurrence, compared with white light cystoscopy, but results were inconsistent through many of the trials identified.
Read the full report here.
Bacillus Calmette-Guérin (BCG) is the only intravesical therapy shown to be associated with decreased risk of bladder cancer progression, according to a report by the Agency for Healthcare Research and Quality (AHRQ), but the researchers cautioned that the therapy was associated with a high rate of adverse events.
The Comparative Effectiveness Review, headed by Dr. Roger Chou and based on research by the Pacific Northwest Evidence-Based Practice Center (EPC) under contract to the AHRQ, was conducted as a systematic review of trials identified via electronic databases and conducted from 1990 to 2014.
BCG was associated with a higher rate of local and systemic adverse events (granulomatous cystitis or irritative symptoms in 27%-84% of patients, macroscopic hematuria in 21%-72%, and fever in 27%-44%) when compared with no intravesical therapy, the researchers wrote. BCG was associated with decreased risk of bladder cancer progression, but no intravesical agent was associated with decreased risk of all-cause or bladder cancer mortality.
In addition, fluorescent cystoscopy was associated with decreased risk of subsequent bladder recurrence, compared with white light cystoscopy, but results were inconsistent through many of the trials identified.
Read the full report here.
ILDS establishes guidelines for treating AK patients
The International Leagues of Dermatological Societies (ILDS) in cooperation with the European Dermatology Forum has developed consensus-based guidelines for the treatment of actinic keratosis (AK), which are published in the Journal of the European Academy of Dermatology and Venereology.
“The guidelines were elaborated along adapted recommendations by the WHO guidelines review committee and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group,” say R. N. Werner and colleagues of the Medical University of Berlin. The guidelines include recommendations for treatment of different subgroups of AK patients, how to make an AK diagnosis, how to assess AK patients, and how to define AK.
The ILDS recommends or suggests the following interventions for treating patients who have single AK lesions:
• Cryotherapy
• Curettage (discrete, hyperkeratotic lesions)
• 0.5% 5-fluorouracil (5-FU)
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 3.75% imiquimod
• 5% imiquimod
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)
• 5-aminolevulinic acid-photodynamic therapy (ALA-PDT)
• methylaminolevulinate-photodynamic therapy (MAL-PDT)
For patients with multiple AK lesions/field cancerization, the ILDS recommends* or suggests that patients use the following therapies:
• 0.5% 5-FU*
• 3.75% imiquimod*
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)*
• ALA-PDT*
• MAL-PDT*
• Cryotherapy (patients with multiple lesions, especially for multiple discrete lesions; not suitable for the treatment of field cancerization)
• 3% diclofenac in 2.5% hyaluronic acid gel
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 5% imiquimod
• 2.5% imiquimod
• CO2 laser and Er:YAG laser
For immunosuppressed AK patients, the ILDS suggests the following treatments:
• Cryotherapy (especially for single lesions or multiple discrete lesions; not suitable for the treatment of field cancerization);
• Curettage (discrete, hyperkeratotic lesions)
• 5% 5-FU
• 5% imiquimod
• ALA-PDT
• MAL-PDT
The ILDS additionally recommends that immunosuppressed AK patients not use CO2 laser and Er:YAG laser.
“Deviation from the recommendations may be justified or inevitable in specific situations. The ultimate judgment regarding patient care must be individualized and must be made by the physician and patient in light of all presenting circumstances,” the authors said. “International guidelines are intended to be adapted to national or regional circumstances” (J Eur Acad Dermatol Venereol. 2015;29:2069-79).
The “long version of the guidelines” is available as an online supplement. Additionally, a methods report, results report, and declarations of interest of the guidelines development have been published at doi: 10.1111/jdv.13179 in the Journal of the European Academy of Dermatology and Venereology (2015).
The International Leagues of Dermatological Societies (ILDS) in cooperation with the European Dermatology Forum has developed consensus-based guidelines for the treatment of actinic keratosis (AK), which are published in the Journal of the European Academy of Dermatology and Venereology.
“The guidelines were elaborated along adapted recommendations by the WHO guidelines review committee and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group,” say R. N. Werner and colleagues of the Medical University of Berlin. The guidelines include recommendations for treatment of different subgroups of AK patients, how to make an AK diagnosis, how to assess AK patients, and how to define AK.
The ILDS recommends or suggests the following interventions for treating patients who have single AK lesions:
• Cryotherapy
• Curettage (discrete, hyperkeratotic lesions)
• 0.5% 5-fluorouracil (5-FU)
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 3.75% imiquimod
• 5% imiquimod
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)
• 5-aminolevulinic acid-photodynamic therapy (ALA-PDT)
• methylaminolevulinate-photodynamic therapy (MAL-PDT)
For patients with multiple AK lesions/field cancerization, the ILDS recommends* or suggests that patients use the following therapies:
• 0.5% 5-FU*
• 3.75% imiquimod*
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)*
• ALA-PDT*
• MAL-PDT*
• Cryotherapy (patients with multiple lesions, especially for multiple discrete lesions; not suitable for the treatment of field cancerization)
• 3% diclofenac in 2.5% hyaluronic acid gel
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 5% imiquimod
• 2.5% imiquimod
• CO2 laser and Er:YAG laser
For immunosuppressed AK patients, the ILDS suggests the following treatments:
• Cryotherapy (especially for single lesions or multiple discrete lesions; not suitable for the treatment of field cancerization);
• Curettage (discrete, hyperkeratotic lesions)
• 5% 5-FU
• 5% imiquimod
• ALA-PDT
• MAL-PDT
The ILDS additionally recommends that immunosuppressed AK patients not use CO2 laser and Er:YAG laser.
“Deviation from the recommendations may be justified or inevitable in specific situations. The ultimate judgment regarding patient care must be individualized and must be made by the physician and patient in light of all presenting circumstances,” the authors said. “International guidelines are intended to be adapted to national or regional circumstances” (J Eur Acad Dermatol Venereol. 2015;29:2069-79).
The “long version of the guidelines” is available as an online supplement. Additionally, a methods report, results report, and declarations of interest of the guidelines development have been published at doi: 10.1111/jdv.13179 in the Journal of the European Academy of Dermatology and Venereology (2015).
The International Leagues of Dermatological Societies (ILDS) in cooperation with the European Dermatology Forum has developed consensus-based guidelines for the treatment of actinic keratosis (AK), which are published in the Journal of the European Academy of Dermatology and Venereology.
“The guidelines were elaborated along adapted recommendations by the WHO guidelines review committee and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group,” say R. N. Werner and colleagues of the Medical University of Berlin. The guidelines include recommendations for treatment of different subgroups of AK patients, how to make an AK diagnosis, how to assess AK patients, and how to define AK.
The ILDS recommends or suggests the following interventions for treating patients who have single AK lesions:
• Cryotherapy
• Curettage (discrete, hyperkeratotic lesions)
• 0.5% 5-fluorouracil (5-FU)
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 3.75% imiquimod
• 5% imiquimod
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)
• 5-aminolevulinic acid-photodynamic therapy (ALA-PDT)
• methylaminolevulinate-photodynamic therapy (MAL-PDT)
For patients with multiple AK lesions/field cancerization, the ILDS recommends* or suggests that patients use the following therapies:
• 0.5% 5-FU*
• 3.75% imiquimod*
• Ingenol mebutate 0.015% (lesions on the face or scalp) and ingenol mebutate 0.05% (lesions on the trunk or extremities)*
• ALA-PDT*
• MAL-PDT*
• Cryotherapy (patients with multiple lesions, especially for multiple discrete lesions; not suitable for the treatment of field cancerization)
• 3% diclofenac in 2.5% hyaluronic acid gel
• 5% 5-FU
• 0.5% 5-FU + 10% salicylic acid (discrete, hyperkeratotic lesions)
• 5% imiquimod
• 2.5% imiquimod
• CO2 laser and Er:YAG laser
For immunosuppressed AK patients, the ILDS suggests the following treatments:
• Cryotherapy (especially for single lesions or multiple discrete lesions; not suitable for the treatment of field cancerization);
• Curettage (discrete, hyperkeratotic lesions)
• 5% 5-FU
• 5% imiquimod
• ALA-PDT
• MAL-PDT
The ILDS additionally recommends that immunosuppressed AK patients not use CO2 laser and Er:YAG laser.
“Deviation from the recommendations may be justified or inevitable in specific situations. The ultimate judgment regarding patient care must be individualized and must be made by the physician and patient in light of all presenting circumstances,” the authors said. “International guidelines are intended to be adapted to national or regional circumstances” (J Eur Acad Dermatol Venereol. 2015;29:2069-79).
The “long version of the guidelines” is available as an online supplement. Additionally, a methods report, results report, and declarations of interest of the guidelines development have been published at doi: 10.1111/jdv.13179 in the Journal of the European Academy of Dermatology and Venereology (2015).
FROM JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Increased surveillance may explain post-Fukushima pediatric thyroid cancers
LAKE BUENA VISTA, FLA. – More cases of thyroid cancer are being seen in Japanese youth after the Fukushima Daiichi nuclear power plant accident, but the increased incidence may be an artifact of heightened surveillance.
“The thyroid cancers appear to have already occurred prior to radiation exposure,” said Dr. Shinichi Suzuki of the department of thyroid and endocrinology at Fukushima (Japan) Medical University. Radiation-induced thyroid cancers take about 5 years to become detectable, so physicians should just now be seeing the earliest cases of thyroid cancer related to Fukushima radiation exposure, according to Dr. Suzuki. He presented interim results of Japan’s universal screening protocol for children potentially affected by the Fukushima incident at the International Thyroid Conference.
The protocol, designed to screen everyone residing in the Fukushima prefecture and aged 19 years or younger at the time of the 2011 incident, has been highly successful, with over 80% of those eligible receiving a baseline screening that included a thyroid ultrasound exam.
Screening consisted of an initial thyroid ultrasound exam performed with a portable ultrasound device. If no cyst or nodule was found, then the patient would be seen at the next scheduled thyroid ultrasound exam, 2 years later. Patients with cysts 20 mm or less in greatest diameter or nodules 5 mm or smaller also were deferred to the next scheduled examination. Patients with cysts larger than 20 mm or nodules larger than 5 mm received confirmatory examination by detailed ultrasound examination, blood work, and fine-needle aspiration.
Of the 300,476 patients who received the preliminary baseline survey, 2,294 (0.8%) had an abnormality that warranted confirmatory examination and 91.9% of patients went on to have the confirmatory exams. Of these, 113 were assessed as malignant or suspicious for malignancy. Ninety-nine patients had surgery, with findings of 98 cases of thyroid cancer and one benign tumor.
Patients examined after April 2014 were part of an expanded protocol. Under this protocol, 169,455 patients (44.7% participation) were examined and 1,223 patients (0.8%) had suspicious findings on thyroid ultrasound exam. Participation rate for confirmatory testing for this group was 62.7%, with 25 patients’ thyroids having malignant or potentially malignant findings. Six of these patients had surgery, and thyroid cancer was found in all six cases.
Pooling data from the 138 malignant or suspicious cases from the two groups, 105 patients in total have had surgery, 13 patients with small, noninvasive masses are being watched, and a further 20 are awaiting surgery, Dr. Suzuki said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Of the 97 patients with thyroid cancer who were treated at Fukushima University, 61 were female. The mean patient age at the time of the disaster was 14.8 ± 2.7 years (range, 6-18 years), while the mean age at diagnosis was 17.4 ± 2.8 years (range, 9-22 years). All patients were asymptomatic.
Tumors were unilateral in all but two patients. Mean tumor size was 15.1 ± 0.8 mm (range, 5-53 mm). Nearly all of the tumors (94/97) were papillary thyroid carcinoma, with 86 of those being classical-type papillary thyroid carcinoma. Three patients had poorly differentiated thyroid carcinoma. Fifty-eight patients (60%) had some intraglandular spread, while 71 (73%) had calcifications.
Dr. Suzuki and his collaborators compared these 97 cases with 37 cases of pediatric thyroid cancer in an historical Japanese cohort and to the 26 cases seen in a cohort from Belarus following the Chernobyl disaster. The Fukushima patients were significantly older than either comparison group, with mean age of 11.9 years for the historical Japanese cohort and 10.6 years for the children from Belarus. Tumor size was smaller than the historical Japanese cohort’s mean of 4.1 cm but about the same as that seen in Belarus (1.4 cm). Pulmonary metastases were more common in the historical Japanese cohort (19% vs. 4% in Belarus and 2% in Fukushima).
To have reference data that use similar techniques on a similar population, Japanese researchers are conducting thyroid ultrasound examsaccording to the Fukushima protocol concurrently in three other Japanese prefectures. This is especially important, Dr. Suzuki said, because rapid technological advances in ultrasound imaging mean that screening is much more likely to detect small abnormalities in the thyroid than would have been the case even a few years ago. For this reason, and also because much more radiation was released at the site of the Chernobyl nuclear disaster, only limited comparisons can be made between pediatric thyroid cancer rates from the two nuclear accidents.
Thyroid ultrasound exam “has the ability to detect a lot of thyroid cancers,” he said, so care must be taken to avoid overdiagnosis and overtreatment in this group of young people. Information to date from the Fukushima surveillance project does not yet “give us the clear view about the influence of radiation exposure after the accident on thyroid cancer occurrence,” he said.
Dr. Suzuki reported no relevant disclosures.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – More cases of thyroid cancer are being seen in Japanese youth after the Fukushima Daiichi nuclear power plant accident, but the increased incidence may be an artifact of heightened surveillance.
“The thyroid cancers appear to have already occurred prior to radiation exposure,” said Dr. Shinichi Suzuki of the department of thyroid and endocrinology at Fukushima (Japan) Medical University. Radiation-induced thyroid cancers take about 5 years to become detectable, so physicians should just now be seeing the earliest cases of thyroid cancer related to Fukushima radiation exposure, according to Dr. Suzuki. He presented interim results of Japan’s universal screening protocol for children potentially affected by the Fukushima incident at the International Thyroid Conference.
The protocol, designed to screen everyone residing in the Fukushima prefecture and aged 19 years or younger at the time of the 2011 incident, has been highly successful, with over 80% of those eligible receiving a baseline screening that included a thyroid ultrasound exam.
Screening consisted of an initial thyroid ultrasound exam performed with a portable ultrasound device. If no cyst or nodule was found, then the patient would be seen at the next scheduled thyroid ultrasound exam, 2 years later. Patients with cysts 20 mm or less in greatest diameter or nodules 5 mm or smaller also were deferred to the next scheduled examination. Patients with cysts larger than 20 mm or nodules larger than 5 mm received confirmatory examination by detailed ultrasound examination, blood work, and fine-needle aspiration.
Of the 300,476 patients who received the preliminary baseline survey, 2,294 (0.8%) had an abnormality that warranted confirmatory examination and 91.9% of patients went on to have the confirmatory exams. Of these, 113 were assessed as malignant or suspicious for malignancy. Ninety-nine patients had surgery, with findings of 98 cases of thyroid cancer and one benign tumor.
Patients examined after April 2014 were part of an expanded protocol. Under this protocol, 169,455 patients (44.7% participation) were examined and 1,223 patients (0.8%) had suspicious findings on thyroid ultrasound exam. Participation rate for confirmatory testing for this group was 62.7%, with 25 patients’ thyroids having malignant or potentially malignant findings. Six of these patients had surgery, and thyroid cancer was found in all six cases.
Pooling data from the 138 malignant or suspicious cases from the two groups, 105 patients in total have had surgery, 13 patients with small, noninvasive masses are being watched, and a further 20 are awaiting surgery, Dr. Suzuki said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Of the 97 patients with thyroid cancer who were treated at Fukushima University, 61 were female. The mean patient age at the time of the disaster was 14.8 ± 2.7 years (range, 6-18 years), while the mean age at diagnosis was 17.4 ± 2.8 years (range, 9-22 years). All patients were asymptomatic.
Tumors were unilateral in all but two patients. Mean tumor size was 15.1 ± 0.8 mm (range, 5-53 mm). Nearly all of the tumors (94/97) were papillary thyroid carcinoma, with 86 of those being classical-type papillary thyroid carcinoma. Three patients had poorly differentiated thyroid carcinoma. Fifty-eight patients (60%) had some intraglandular spread, while 71 (73%) had calcifications.
Dr. Suzuki and his collaborators compared these 97 cases with 37 cases of pediatric thyroid cancer in an historical Japanese cohort and to the 26 cases seen in a cohort from Belarus following the Chernobyl disaster. The Fukushima patients were significantly older than either comparison group, with mean age of 11.9 years for the historical Japanese cohort and 10.6 years for the children from Belarus. Tumor size was smaller than the historical Japanese cohort’s mean of 4.1 cm but about the same as that seen in Belarus (1.4 cm). Pulmonary metastases were more common in the historical Japanese cohort (19% vs. 4% in Belarus and 2% in Fukushima).
To have reference data that use similar techniques on a similar population, Japanese researchers are conducting thyroid ultrasound examsaccording to the Fukushima protocol concurrently in three other Japanese prefectures. This is especially important, Dr. Suzuki said, because rapid technological advances in ultrasound imaging mean that screening is much more likely to detect small abnormalities in the thyroid than would have been the case even a few years ago. For this reason, and also because much more radiation was released at the site of the Chernobyl nuclear disaster, only limited comparisons can be made between pediatric thyroid cancer rates from the two nuclear accidents.
Thyroid ultrasound exam “has the ability to detect a lot of thyroid cancers,” he said, so care must be taken to avoid overdiagnosis and overtreatment in this group of young people. Information to date from the Fukushima surveillance project does not yet “give us the clear view about the influence of radiation exposure after the accident on thyroid cancer occurrence,” he said.
Dr. Suzuki reported no relevant disclosures.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – More cases of thyroid cancer are being seen in Japanese youth after the Fukushima Daiichi nuclear power plant accident, but the increased incidence may be an artifact of heightened surveillance.
“The thyroid cancers appear to have already occurred prior to radiation exposure,” said Dr. Shinichi Suzuki of the department of thyroid and endocrinology at Fukushima (Japan) Medical University. Radiation-induced thyroid cancers take about 5 years to become detectable, so physicians should just now be seeing the earliest cases of thyroid cancer related to Fukushima radiation exposure, according to Dr. Suzuki. He presented interim results of Japan’s universal screening protocol for children potentially affected by the Fukushima incident at the International Thyroid Conference.
The protocol, designed to screen everyone residing in the Fukushima prefecture and aged 19 years or younger at the time of the 2011 incident, has been highly successful, with over 80% of those eligible receiving a baseline screening that included a thyroid ultrasound exam.
Screening consisted of an initial thyroid ultrasound exam performed with a portable ultrasound device. If no cyst or nodule was found, then the patient would be seen at the next scheduled thyroid ultrasound exam, 2 years later. Patients with cysts 20 mm or less in greatest diameter or nodules 5 mm or smaller also were deferred to the next scheduled examination. Patients with cysts larger than 20 mm or nodules larger than 5 mm received confirmatory examination by detailed ultrasound examination, blood work, and fine-needle aspiration.
Of the 300,476 patients who received the preliminary baseline survey, 2,294 (0.8%) had an abnormality that warranted confirmatory examination and 91.9% of patients went on to have the confirmatory exams. Of these, 113 were assessed as malignant or suspicious for malignancy. Ninety-nine patients had surgery, with findings of 98 cases of thyroid cancer and one benign tumor.
Patients examined after April 2014 were part of an expanded protocol. Under this protocol, 169,455 patients (44.7% participation) were examined and 1,223 patients (0.8%) had suspicious findings on thyroid ultrasound exam. Participation rate for confirmatory testing for this group was 62.7%, with 25 patients’ thyroids having malignant or potentially malignant findings. Six of these patients had surgery, and thyroid cancer was found in all six cases.
Pooling data from the 138 malignant or suspicious cases from the two groups, 105 patients in total have had surgery, 13 patients with small, noninvasive masses are being watched, and a further 20 are awaiting surgery, Dr. Suzuki said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Of the 97 patients with thyroid cancer who were treated at Fukushima University, 61 were female. The mean patient age at the time of the disaster was 14.8 ± 2.7 years (range, 6-18 years), while the mean age at diagnosis was 17.4 ± 2.8 years (range, 9-22 years). All patients were asymptomatic.
Tumors were unilateral in all but two patients. Mean tumor size was 15.1 ± 0.8 mm (range, 5-53 mm). Nearly all of the tumors (94/97) were papillary thyroid carcinoma, with 86 of those being classical-type papillary thyroid carcinoma. Three patients had poorly differentiated thyroid carcinoma. Fifty-eight patients (60%) had some intraglandular spread, while 71 (73%) had calcifications.
Dr. Suzuki and his collaborators compared these 97 cases with 37 cases of pediatric thyroid cancer in an historical Japanese cohort and to the 26 cases seen in a cohort from Belarus following the Chernobyl disaster. The Fukushima patients were significantly older than either comparison group, with mean age of 11.9 years for the historical Japanese cohort and 10.6 years for the children from Belarus. Tumor size was smaller than the historical Japanese cohort’s mean of 4.1 cm but about the same as that seen in Belarus (1.4 cm). Pulmonary metastases were more common in the historical Japanese cohort (19% vs. 4% in Belarus and 2% in Fukushima).
To have reference data that use similar techniques on a similar population, Japanese researchers are conducting thyroid ultrasound examsaccording to the Fukushima protocol concurrently in three other Japanese prefectures. This is especially important, Dr. Suzuki said, because rapid technological advances in ultrasound imaging mean that screening is much more likely to detect small abnormalities in the thyroid than would have been the case even a few years ago. For this reason, and also because much more radiation was released at the site of the Chernobyl nuclear disaster, only limited comparisons can be made between pediatric thyroid cancer rates from the two nuclear accidents.
Thyroid ultrasound exam “has the ability to detect a lot of thyroid cancers,” he said, so care must be taken to avoid overdiagnosis and overtreatment in this group of young people. Information to date from the Fukushima surveillance project does not yet “give us the clear view about the influence of radiation exposure after the accident on thyroid cancer occurrence,” he said.
Dr. Suzuki reported no relevant disclosures.
On Twitter @karioakes
AT ITC 2015
Key clinical point: The increased incidence of thyroid cancers in Japanese youth after the Fukushima nuclear accident may be an artifact of increased surveillance.
Major finding: A total of 138 thyroid cancers have been found when screening 469,931 children in Fukushima after the 2011 nuclear power plant accident.
Data source: Universal screening for thyroid cancer among individuals who were aged 18 years or younger and resident in Fukushima at the time of the accident.
Disclosures: Dr. Suzuki reported no relevant disclosures.
Heart Disease Linked to Loud Noise
NEW YORK - People with long-term exposure to loud noise at work or in leisure activities may be at increased risk of heart disease, a U.S. study finds.
Researchers found the strongest link in working-age people with high-frequency hearing loss, which is typically the result of chronic noise exposure.
"Compared with people with normal high-frequency hearing, people with bilateral high-frequency hearing loss were approximately two times more likely to have coronary heart disease," Dr. Wen Qi Gan of the University of Kentucky College of Public Health in Lexington, said by email.
Past research has already linked noise exposure, especially in workplaces, to coronary heart disease, hypertension, and other illnesses, Dr. Gan and his colleagues noted online September 15 in Occupational and Environmental Medicine. But many of these studies lacked individual information about actual noise exposure, relying instead on average decibel levels in the person's environment.
High-frequency hearing loss, the researchers wrote, is a better indicator of exposure to loud noise over time. To investigate the connection with heart disease, the researchers looked at data on 5223 individuals, ages 20 to 69, who participated in national health surveys between 1999 and 2004.
Overall, people with bilateral high-frequency hearing loss were about twice as likely to have coronary heart disease compared to those with normal high-frequency hearing. Among those age 50 and under, who were also most likely to be exposed to loud noise at work, the heart disease risk was increased four-fold.
There was no link to heart disease among people with one-sided hearing loss or loss of lower-frequency hearing, the study team noted, further supporting the idea that noise exposure is the culprit.
The study only looked at people at one time point, however, and cannot prove that noise or hearing loss are direct causes of heart disease. The researchers also acknowledged that they relied on study participants' own recollections about their work and leisure-time noise exposure.
Nonetheless, Dr. Gan said, accumulating evidence suggests that exposure to loud noise can increase the risk of coronary heart disease.
NEW YORK - People with long-term exposure to loud noise at work or in leisure activities may be at increased risk of heart disease, a U.S. study finds.
Researchers found the strongest link in working-age people with high-frequency hearing loss, which is typically the result of chronic noise exposure.
"Compared with people with normal high-frequency hearing, people with bilateral high-frequency hearing loss were approximately two times more likely to have coronary heart disease," Dr. Wen Qi Gan of the University of Kentucky College of Public Health in Lexington, said by email.
Past research has already linked noise exposure, especially in workplaces, to coronary heart disease, hypertension, and other illnesses, Dr. Gan and his colleagues noted online September 15 in Occupational and Environmental Medicine. But many of these studies lacked individual information about actual noise exposure, relying instead on average decibel levels in the person's environment.
High-frequency hearing loss, the researchers wrote, is a better indicator of exposure to loud noise over time. To investigate the connection with heart disease, the researchers looked at data on 5223 individuals, ages 20 to 69, who participated in national health surveys between 1999 and 2004.
Overall, people with bilateral high-frequency hearing loss were about twice as likely to have coronary heart disease compared to those with normal high-frequency hearing. Among those age 50 and under, who were also most likely to be exposed to loud noise at work, the heart disease risk was increased four-fold.
There was no link to heart disease among people with one-sided hearing loss or loss of lower-frequency hearing, the study team noted, further supporting the idea that noise exposure is the culprit.
The study only looked at people at one time point, however, and cannot prove that noise or hearing loss are direct causes of heart disease. The researchers also acknowledged that they relied on study participants' own recollections about their work and leisure-time noise exposure.
Nonetheless, Dr. Gan said, accumulating evidence suggests that exposure to loud noise can increase the risk of coronary heart disease.
NEW YORK - People with long-term exposure to loud noise at work or in leisure activities may be at increased risk of heart disease, a U.S. study finds.
Researchers found the strongest link in working-age people with high-frequency hearing loss, which is typically the result of chronic noise exposure.
"Compared with people with normal high-frequency hearing, people with bilateral high-frequency hearing loss were approximately two times more likely to have coronary heart disease," Dr. Wen Qi Gan of the University of Kentucky College of Public Health in Lexington, said by email.
Past research has already linked noise exposure, especially in workplaces, to coronary heart disease, hypertension, and other illnesses, Dr. Gan and his colleagues noted online September 15 in Occupational and Environmental Medicine. But many of these studies lacked individual information about actual noise exposure, relying instead on average decibel levels in the person's environment.
High-frequency hearing loss, the researchers wrote, is a better indicator of exposure to loud noise over time. To investigate the connection with heart disease, the researchers looked at data on 5223 individuals, ages 20 to 69, who participated in national health surveys between 1999 and 2004.
Overall, people with bilateral high-frequency hearing loss were about twice as likely to have coronary heart disease compared to those with normal high-frequency hearing. Among those age 50 and under, who were also most likely to be exposed to loud noise at work, the heart disease risk was increased four-fold.
There was no link to heart disease among people with one-sided hearing loss or loss of lower-frequency hearing, the study team noted, further supporting the idea that noise exposure is the culprit.
The study only looked at people at one time point, however, and cannot prove that noise or hearing loss are direct causes of heart disease. The researchers also acknowledged that they relied on study participants' own recollections about their work and leisure-time noise exposure.
Nonetheless, Dr. Gan said, accumulating evidence suggests that exposure to loud noise can increase the risk of coronary heart disease.
Could a complementary approach help your patient with insomnia?
Relaxation techniques, acupuncture, and dietary supplements are among the complementary health approaches that patients may consider when struggling with insomnia. But how effective—and safe—are they? A fact sheet from the National Center for Complementary and Alternative Medicine provides useful guidance and is available at: https://nccih.nih.gov/sites/nccam.nih.gov/files/Get_The_Facts_Sleep_Disorders_04-24-2014.pdf
Relaxation techniques, acupuncture, and dietary supplements are among the complementary health approaches that patients may consider when struggling with insomnia. But how effective—and safe—are they? A fact sheet from the National Center for Complementary and Alternative Medicine provides useful guidance and is available at: https://nccih.nih.gov/sites/nccam.nih.gov/files/Get_The_Facts_Sleep_Disorders_04-24-2014.pdf
Relaxation techniques, acupuncture, and dietary supplements are among the complementary health approaches that patients may consider when struggling with insomnia. But how effective—and safe—are they? A fact sheet from the National Center for Complementary and Alternative Medicine provides useful guidance and is available at: https://nccih.nih.gov/sites/nccam.nih.gov/files/Get_The_Facts_Sleep_Disorders_04-24-2014.pdf
Encouraging baby boomers to get tested for hepatitis C
More than 75% of adults infected with hepatitis C are baby boomers. To educate these patients on why they should get tested and how to make sense of test results, the Centers for Disease Control and Prevention has put together a fact sheet. It’s available at http://www.cdc.gov/knowmorehepatitis/media/pdfs/factsheet-boomers.pdf.
More than 75% of adults infected with hepatitis C are baby boomers. To educate these patients on why they should get tested and how to make sense of test results, the Centers for Disease Control and Prevention has put together a fact sheet. It’s available at http://www.cdc.gov/knowmorehepatitis/media/pdfs/factsheet-boomers.pdf.
More than 75% of adults infected with hepatitis C are baby boomers. To educate these patients on why they should get tested and how to make sense of test results, the Centers for Disease Control and Prevention has put together a fact sheet. It’s available at http://www.cdc.gov/knowmorehepatitis/media/pdfs/factsheet-boomers.pdf.
Surgical Management of Gorham-Stout Disease of the Pelvis Refractory to Medical and Radiation Therapy
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.