User login
Make the Diagnosis - November 2015
Diagnosis: Basal cell carcinoma
Basal cell carcinoma (BCC) is the most common skin cancer diagnosed in the United States, with approximately 2.8 million cases diagnosed annually, according to the Skin Cancer Foundation. While a minority of cases are linked to genetic syndromes (e.g., basal cell nevus syndrome), most cases result from ultraviolet sun exposure. A power law model was recently described linking ultraviolet exposure and incidence of basal cell carcinoma.
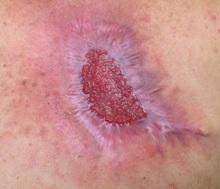
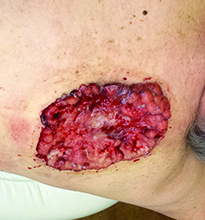
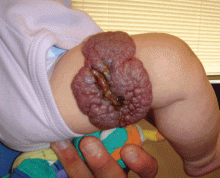
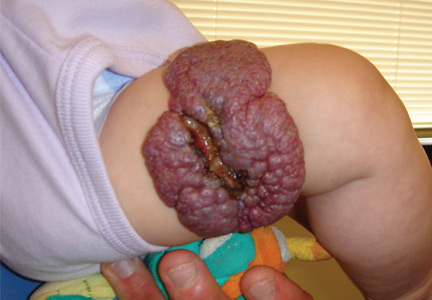
Many diagnosed BCC cases are small (< 1cm in size) and easily treated in the clinic. In cases where treatment is delayed for years due to financial, psychological, or psychiatric reasons, tumors can cause significant local tissue destruction and grow to alarming sizes.
The differential diagnosis of BCC may vary depending on the clinical sub-type (e.g., superficial, nodular, infiltrative, etc). Nodular BCC may mimic adnexal neoplasms, intradermal melanocytic nevi, Merkel cell carcinoma, or even amelanotic melanoma. Superficial BCC may mimic a lichenoid keratosis, Bowen’s disease, or other inflammatory conditions such as psoriasis and dermatitis. A larger lesion may mimic chronic infections such as mycetoma, or distant metastases from another primary carcinoma (breast or renal). Location and history can assist with teasing out the probable cause.
While diagnosis of this lesion can be made based on history and clinical appearance, this is best assisted with a biopsy, preferably a punch or incisional biopsy as chronic scarring and pseudoepitheliomatous hyperplasia may affect the pathologic diagnosis. Bacteria often colonize these large tumors and can lead to secondary infections. In this patient, maggots were noted in the skin.
While there are multiple modalities available for treating small tumors (e.g., topical imiquimod, 5-fluorouracil, electrodessication and curettage, excision, Mohs), larger tumors are often handled differently. While some might consider radiation therapy in this case, typically Mohs surgery is the treatment of choice. Oral vismodegib, a smoothened inhibitor, has been marketed for locally advanced basal cell carcinoma and has been used as a primary or adjunctive therapy with Mohs in tumors of this size. One important factor determining the choice of treatment is whether the primary tumor has spread. In tumors who have been left untreated for a long time, it is reasonable to image the patient to evaluate for metastases.
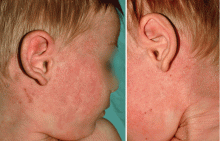
We present this case as an example where Mohs provided immediate definitive treatment. This tumor was cleared in 1 stage, with 67 slides read to evaluate the entire peripheral and deep margin. The Mohs surgery was performed under local anesthesia with no additional sedatives and 3-0 nylon sutures were used to approximate the wound. A two month follow-up photo is shown showing an almost healed surgical wound with an acceptable cosmetic result.
Diagnosis: Basal cell carcinoma
Basal cell carcinoma (BCC) is the most common skin cancer diagnosed in the United States, with approximately 2.8 million cases diagnosed annually, according to the Skin Cancer Foundation. While a minority of cases are linked to genetic syndromes (e.g., basal cell nevus syndrome), most cases result from ultraviolet sun exposure. A power law model was recently described linking ultraviolet exposure and incidence of basal cell carcinoma.
Many diagnosed BCC cases are small (< 1cm in size) and easily treated in the clinic. In cases where treatment is delayed for years due to financial, psychological, or psychiatric reasons, tumors can cause significant local tissue destruction and grow to alarming sizes.
The differential diagnosis of BCC may vary depending on the clinical sub-type (e.g., superficial, nodular, infiltrative, etc). Nodular BCC may mimic adnexal neoplasms, intradermal melanocytic nevi, Merkel cell carcinoma, or even amelanotic melanoma. Superficial BCC may mimic a lichenoid keratosis, Bowen’s disease, or other inflammatory conditions such as psoriasis and dermatitis. A larger lesion may mimic chronic infections such as mycetoma, or distant metastases from another primary carcinoma (breast or renal). Location and history can assist with teasing out the probable cause.
While diagnosis of this lesion can be made based on history and clinical appearance, this is best assisted with a biopsy, preferably a punch or incisional biopsy as chronic scarring and pseudoepitheliomatous hyperplasia may affect the pathologic diagnosis. Bacteria often colonize these large tumors and can lead to secondary infections. In this patient, maggots were noted in the skin.
While there are multiple modalities available for treating small tumors (e.g., topical imiquimod, 5-fluorouracil, electrodessication and curettage, excision, Mohs), larger tumors are often handled differently. While some might consider radiation therapy in this case, typically Mohs surgery is the treatment of choice. Oral vismodegib, a smoothened inhibitor, has been marketed for locally advanced basal cell carcinoma and has been used as a primary or adjunctive therapy with Mohs in tumors of this size. One important factor determining the choice of treatment is whether the primary tumor has spread. In tumors who have been left untreated for a long time, it is reasonable to image the patient to evaluate for metastases.
We present this case as an example where Mohs provided immediate definitive treatment. This tumor was cleared in 1 stage, with 67 slides read to evaluate the entire peripheral and deep margin. The Mohs surgery was performed under local anesthesia with no additional sedatives and 3-0 nylon sutures were used to approximate the wound. A two month follow-up photo is shown showing an almost healed surgical wound with an acceptable cosmetic result.
Diagnosis: Basal cell carcinoma
Basal cell carcinoma (BCC) is the most common skin cancer diagnosed in the United States, with approximately 2.8 million cases diagnosed annually, according to the Skin Cancer Foundation. While a minority of cases are linked to genetic syndromes (e.g., basal cell nevus syndrome), most cases result from ultraviolet sun exposure. A power law model was recently described linking ultraviolet exposure and incidence of basal cell carcinoma.
Many diagnosed BCC cases are small (< 1cm in size) and easily treated in the clinic. In cases where treatment is delayed for years due to financial, psychological, or psychiatric reasons, tumors can cause significant local tissue destruction and grow to alarming sizes.
The differential diagnosis of BCC may vary depending on the clinical sub-type (e.g., superficial, nodular, infiltrative, etc). Nodular BCC may mimic adnexal neoplasms, intradermal melanocytic nevi, Merkel cell carcinoma, or even amelanotic melanoma. Superficial BCC may mimic a lichenoid keratosis, Bowen’s disease, or other inflammatory conditions such as psoriasis and dermatitis. A larger lesion may mimic chronic infections such as mycetoma, or distant metastases from another primary carcinoma (breast or renal). Location and history can assist with teasing out the probable cause.
While diagnosis of this lesion can be made based on history and clinical appearance, this is best assisted with a biopsy, preferably a punch or incisional biopsy as chronic scarring and pseudoepitheliomatous hyperplasia may affect the pathologic diagnosis. Bacteria often colonize these large tumors and can lead to secondary infections. In this patient, maggots were noted in the skin.
While there are multiple modalities available for treating small tumors (e.g., topical imiquimod, 5-fluorouracil, electrodessication and curettage, excision, Mohs), larger tumors are often handled differently. While some might consider radiation therapy in this case, typically Mohs surgery is the treatment of choice. Oral vismodegib, a smoothened inhibitor, has been marketed for locally advanced basal cell carcinoma and has been used as a primary or adjunctive therapy with Mohs in tumors of this size. One important factor determining the choice of treatment is whether the primary tumor has spread. In tumors who have been left untreated for a long time, it is reasonable to image the patient to evaluate for metastases.
We present this case as an example where Mohs provided immediate definitive treatment. This tumor was cleared in 1 stage, with 67 slides read to evaluate the entire peripheral and deep margin. The Mohs surgery was performed under local anesthesia with no additional sedatives and 3-0 nylon sutures were used to approximate the wound. A two month follow-up photo is shown showing an almost healed surgical wound with an acceptable cosmetic result.
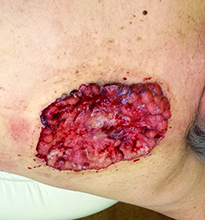
Case and photo courtesy of: Andrew R. Styperek MD; Houston Methodist Hospital and DermSurgery Associates, Houston TX Arash Kimyai-Asadi MD; DermSurgery Associates, Houston TX Dr. Bilu Martin is in private practice at Premier Dermatology, MD in Aventura, Fla. To submit your case for possible publication, send an e-mail to [email protected]. A 65 year old Caucasian male arrived with a decades-long history of a lesion on his back measuring 25 x 21 centimeters. In the last few years he has noticed discharge and an unpleasant smell. He denied any fatigue, shortness of breath, lymph node enlargement, or any other systemic symptoms. He had a history of hyperlipidemia and hypertension, which were controlled with daily oral medications. The patient was not taking aspirin or any other anticoagulant therapy.
Adjuvant Systemic Therapy for Early-Stage Breast Cancer
Over the past 20 years, substantial progress has been achieved in our understanding of breast cancer and in breast cancer treatment, with mortality from breast cancer declining by more than 25% over this time. This progress has been characterized by a greater understanding of the molecular biology of breast cancer, rational drug design, development of agents with specific cellular targets and pathways, development of better prognostic and predictive multigene assays, and marked improvements in supportive care.
To read the full article in PDF:
Over the past 20 years, substantial progress has been achieved in our understanding of breast cancer and in breast cancer treatment, with mortality from breast cancer declining by more than 25% over this time. This progress has been characterized by a greater understanding of the molecular biology of breast cancer, rational drug design, development of agents with specific cellular targets and pathways, development of better prognostic and predictive multigene assays, and marked improvements in supportive care.
To read the full article in PDF:
Over the past 20 years, substantial progress has been achieved in our understanding of breast cancer and in breast cancer treatment, with mortality from breast cancer declining by more than 25% over this time. This progress has been characterized by a greater understanding of the molecular biology of breast cancer, rational drug design, development of agents with specific cellular targets and pathways, development of better prognostic and predictive multigene assays, and marked improvements in supportive care.
To read the full article in PDF:
Perspectives in Pediatrics: From Theory to Practice
Supplement Editor:
Camille Sabella, MD
Contents
ADHD and behavioral disorders: Assessment, management, and an update from DSM-5
Joseph Austerman, DO
Use of long-acting reversible contraceptives to reduce the rate of teen pregnancy
Ellen Rome, MD, MPH
RSV infections: State of the art
Giovanni Piedimonte, MD
Dermatology for the pediatrician: Advances in diagnosis and treatment of common and not-so-common skin conditions
Joan Tamburro, DO
Learning disorders: How pediatricians can help
Elain E. Schulte, MD, MPH
Developmental delays and autism: Screening and surveillance
Carol Delahunty, MD
Supplement Editor:
Camille Sabella, MD
Contents
ADHD and behavioral disorders: Assessment, management, and an update from DSM-5
Joseph Austerman, DO
Use of long-acting reversible contraceptives to reduce the rate of teen pregnancy
Ellen Rome, MD, MPH
RSV infections: State of the art
Giovanni Piedimonte, MD
Dermatology for the pediatrician: Advances in diagnosis and treatment of common and not-so-common skin conditions
Joan Tamburro, DO
Learning disorders: How pediatricians can help
Elain E. Schulte, MD, MPH
Developmental delays and autism: Screening and surveillance
Carol Delahunty, MD
Supplement Editor:
Camille Sabella, MD
Contents
ADHD and behavioral disorders: Assessment, management, and an update from DSM-5
Joseph Austerman, DO
Use of long-acting reversible contraceptives to reduce the rate of teen pregnancy
Ellen Rome, MD, MPH
RSV infections: State of the art
Giovanni Piedimonte, MD
Dermatology for the pediatrician: Advances in diagnosis and treatment of common and not-so-common skin conditions
Joan Tamburro, DO
Learning disorders: How pediatricians can help
Elain E. Schulte, MD, MPH
Developmental delays and autism: Screening and surveillance
Carol Delahunty, MD
ADHD and behavioral disorders: Assessment, management, and an update from DSM-5
Behavioral disorders in pediatric patients—primarily attention deficit hyperactivity disorder (ADHD)—pose a clinical challenge for health care providers to accurately assess, diagnose, and treat. In 2013, the criteria for several disruptive behavioral disorders were updated in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5),1 their first major revisions since 1994. Among the most clinically relevant changes were revisions to the diagnosis of ADHD and the creation of a new diagnostic entity: disruptive mood dysregulation disorder (DMDD).
This article focuses on the updated diagnostic criteria published in the DSM-5 for behavioral disorders, describes the assessment of ADHD, and summarizes management strategies.
UPDATED DIAGNOSTIC CRITERIA
ADHD
This disorder is a chronic, neurologically based illness characterized by a persistent pattern of inattention and/or hyperactivity and impulsivity that are more inappropriate or disruptive than those in other children of a comparable age resulting in functional impairment in multiple settings, and these behaviors have been present for at least 6 months. Revised diagnostic criteria in DSM-5 used the same two categories for ADHD symptoms—inattention and hyperactivity-impulsive behaviors—but modified several diagnostic requirements.
Revised criteria
Impairment before age 12 instead of age 6. As a neurodevelopmental disorder, ADHD usually starts at a young age; teenagers presenting with newly developed ADHD-type symptoms probably do not have ADHD and efforts should be made to rule out other illnesses or social dynamics. The DSM-5 raised the age limit for onset of qualifying symptoms to before 12 years (previously by age 6) primarily to capture a cohort of pediatric patients, typically female, who present solely with inattention symptoms and may not display overt functional impairment early on.
Symptoms required in at least two settings. Symptoms must be present in at least two settings to qualify for a diagnosis of ADHD. This ensures that the behaviors occur globally; they do not occur just at school or at home but occur in both places.
Fewer symptoms required for diagnosis in adolescents. Although the diagnostic criteria retain the same symptoms as those in DSM-IV for different age groups, individuals aged 17 and older are now required to display only five or more inattentive or hyperactive-impulsive symptoms. Previously, at least six were required.
Partial remission criteria
The concept of partial remission was introduced in DSM-5. This acknowledges that two-thirds of children diagnosed with ADHD do not have symptoms that functionally impact activities of daily living beyond age 18.
Oppositional defiant disorder
In DSM-5, oppositional defiant disorder (ODD) is defined by emotional and behavioral symptoms grouped into three categories:
- Constant anger or irritability
- Argumentative or defiant behavior (arguing with authority figures)
- Vindictiveness.
Because defiant behavior may represent difficulty with self-control, ODD is associated with executive functioning deficits that are present in ADHD. Children with ODD tend to perform best in situations in which they can dominate or exert authority. To qualify as ODD, the pattern of behavior must be consistent for longer than 6 months. A severity rating was added based on pervasiveness of ODD symptoms. Otherwise the diagnosis did not change.
Conduct disorder: Purposeful aggression
The hallmarks of conduct disorder are purposeful aggression (eg, bullying), destruction of property, deceitfulness or theft, and serious violation of rules (eg, running away from home, repeat truancy). Some consider conduct disorder to be a separate illness from ODD, whereas others consider it a continuum of the same disorder. Conduct disorder can manifest as violence, as in initiating physical fights, or it can manifest in behaviors such as truancy, stealing, lying, and running away from home without the physical-aggression aspect.
Intermittent explosive disorder
Failure to control aggressive impulses defines intermittent explosive disorder (IED). The aggressive outbursts can be verbal or behavioral and tend to be impulsive. A small subset of children display isolated aggression out of proportion to provocation. The disorder tends to manifest at ages 3 or 4, and a diagnosis requires a stable environment with no significant early childhood trauma. Most often these symptoms are seen in children with intellectual disabilities or an autism spectrum disorder.
Disruptive mood dysregulation disorder
A new diagnostic category in DSM-5 is termed disruptive mood dysregulation disorder (DMDD). This captures many children who previously would have been diagnosed with pediatric bipolar disorder, even though most of them do not fulfill criteria for bipolar disorder as adults. The presence of baseline irritability separates this disorder from IED, which requires intermittent rapid and severe outbursts. The severe temper outbursts of DMDD must be recurrent, with an average of three occurrences per week, and have background irritability. The symptoms must have a duration of at least 12 months and be present in two settings. A diagnosis of DMDD cannot be made earlier than age 6, with onset before age 10.
ASSESSMENT OF ADHD
The clinical interview in conjunction with objective scales is the primary tool for diagnosing ADHD. The most frequent source of information is from the parents followed by the child’s schoolteachers. Patient interview, although unreliable in young children, should also be part of the assessment. Comparing the patient’s functional impairment against children of a similar age is necessary for an ADHD diagnosis.
The medical history can help rule out children with asthma or allergy being treated with corticosteroids and those with hypothyroidism and hyperthyroidism whose symptoms often fulfill the diagnostic criteria for ADHD.2,3 Symptoms of ADHD also may appear suddenly after a traumatic brain injury or other neurologic event.4 Other psychiatric illnesses, especially learning disorders, mood disorders, anxiety, other disruptive behavior disorders, or substance abuse, can mimic ADHD.
Ruling out other factors from a social history (eg, family conflict, bullying, sleep deprivation, being overscheduled with activities) adds to the reliability of an ADHD diagnosis. For example, repetitive uprooting and frequent changes in schools can cause academic problems that may be mistaken for ADHD, and use of stimulants may have failed to improve symptoms in these children.
Assessment scales
Pediatric assessment scales that can be performed in an office are more practical than standardized clinical assessments (Table 1). The Vanderbilt ADHD Diagnostic Teacher Rating Scale correlates highly with a diagnosis of ADHD. We use the Vanderbilt ADHD Diagnostic Parent Rating Scale for children up to age 1 year. Other scales track symptoms and functional impairment over time and can be administered before the patient’s appointment. The Conners Third Edition scale can be used to establish a baseline before initiating therapy and to help monitor changes over time.
Standardized tests to bolster the utility of the clinical interview include the Diagnostic Interview Schedule for Children and Adolescents and the Schedule for Affective Disorders and Schizophrenia in School-Age Children–Present and Lifetime Version. Free training is available regarding use of some of these standardized tests.
Developmental course, risk factors
The clinical course of ADHD is chronic. The onset of hyperactivity usually occurs at age 3 or 4, with combined hyperactivity and inattention usually appearing from ages 5 to 8.5,6 The evolution of symptoms is progressive and constant. Between 50% and 80% have symptoms that continue into adolescence, and in about 40%, symptoms continue into adulthood.7,8 Some children with ADHD have a temperament-neuropsychological profile characterized by aggressiveness, irritability, and mood lability. Deficits in planning, delayed aversion, and temporal processing are present.
Risk factors include prematurity, prenatal complications, an anoxic event, nutritional deficits (specifically iron and zinc), and lack of appropriate socialization.9–11 The disorder is heritable, which is usually clear from the clinical interview. Rates of delinquency and peer rejection are high. This may result in secondary comorbidity such as emotional, disruptive, or substance abuse problems.
MANAGEMENT STRATEGIES
Stimulants
The first-line pharmacologic treatment of ADHD is stimulants: methylphenidate, dexmethylphenidate, mixed amphetamine salts, dextroamphetamine, and lisdexamfetamine. Head-to-head trials of medications versus behavioral management favor medication use, even over the long term.12–14
Methylphenidate and amphetamines are equally effective and have similar adverse effect profiles. Insomnia and anorexia are the most common side effects of stimulants. Cardiac effects include tachycardia, chest pain, and hypertension. Very rarely, stimulants have been associated with sudden cardiac death syndrome in patients with underlying cardiac problems. The consensus is that stimulants are safe in the general population. The need to obtain an electrocardiogram before initiating a stimulant was removed by the US Food and Drug Administration (FDA) unless it is otherwise indicated.15
The response rate to stimulants is in the range of 70%. About one-third of patients have side effects, and approximately 15% have side effects severe enough to requiring changing or withdrawing the medication.16,17
Stimulants are available in several delivery systems. For the best effect, medications should be combined with behavioral management.
Alternatives to stimulants
If stimulants are ineffective, atomoxetine can be used to treat patients with inattention; however, its effect on hyperactivity and impulsivity is less pronounced than that of stimulants. Bupropion is another option for inattention. Both agents are well tolerated. Irritability and insomnia are side effects of atomoxetine, and liver damage is possible, so liver function tests must be ordered if the patient complains of upper-right-quadrant pain.
The evidence to support the use of modafinil is equivocal.18,19 Unlike stimulants, modafinil is associated with a slight increase in motivation.
Alpha-2 agonists are effective for treating aggression in the setting of ADHD, especially in younger children, and are well tolerated.20 Extended-release forms are available.
Combination therapy
Polypharmacy is sometimes indicated in the treatment of ADHD. A stimulant used in combination with atomoxetine was shown to be superior to either treatment alone in improving symptoms of hyperactivity and inattention.21 The combination, however, markedly increased the incidence of appetite loss, insomnia, and irritability.
A more promising combination is a stimulant with an alpha agonist. Symptoms of hyperactivity and inattention were improved more with this combination than with a stimulant plus placebo, with no difference in side effects.22
ADHD with comorbidities
Patients with ADHD, both adults and children, often have comorbid externalizing disorders and other emotional disorders, such as depression and anxiety, occurring in up to half of cases (Table 2).23,24 These comorbidities are important to consider when developing a treatment strategy. The following describes treatment options for the most common ADHD comorbidities (Table 3).
ODD or conduct disorder. The first-line therapy for these patients is a stimulant plus behavioral therapy. Adding an alpha agonist to this combination may be indicated if the comorbidity is severe. Second-generation antipsychotics also have been used as add-ons to stimulants with behavioral therapy, but weight gain and hormonal side effects are common.
Behavioral interventions are effective in targeting disruptive behavioral disorders, specifically multisystemic therapy. Multisystemic therapy is intensive therapy that involves working with the patient’s peer group or school, but most children must enter the legal system to receive this intervention. Multisystemic therapy is the only intervention shown to improve symptoms associated with comorbid ADHD and conduct disorder.25
Mood disorders. For these patients, the mood disorder is treated first. In doing so, symptoms of ADHD may disappear. For those with bipolar disease, a second-generation antipsychotic agent is superior to lithium in efficacy, maintenance of remission, and side effects in patients with a clear bipolar affective disorder, after which a stimulant can be added with less risk of developing manic symptoms. Using a stimulant first for this indication risks mood destabilization.
For patients with a major depressive disorder, bupropion can be used, although this indication is not FDA-approved, followed by the addition of a stimulant. One alternative is a selective serotonin reuptake inhibitor plus a stimulant; another is cognitive behavioral therapy plus atomoxetine and an alpha agonist.
Substance abuse. Patients with ADHD have high rates of substance abuse.26,27 Whether treatment of ADHD with stimulants reduces the risk of substance abuse is controversial. Because abuse of stimulants is common, start treatment with atomoxetine, bupropion, an alpha agonist, or a stimulant that is difficult to abuse (eg, lisdexamfetamine). Refer patients who are abusing substances to a specialist in substance abuse for behavioral management.
Anxiety. Atomoxetine is recommended for the treatment of anxiety that coexists with ADHD. A selective serotonin reuptake inhibitor in combination with a stimulant or alpha agonist, plus cognitive behavioral therapy, is another option for treating anxiety and ADHD. Tricyclic antidepressants have shown benefit in pediatric anxiety. Bupropion should not be used to target anxiety as it has been shown to have a limited effect on anxiety.
Tics. Stimulants may transiently exacerbate underlying tic disorders, but no longstanding difference in the course of tics has been observed with stimulant use.28 Alpha-2 antagonists target both tics and ADHD, so their use is preferred.29 Atomoxetine does not exacerbate tics but may reduce their frequency and severity.30
Dietary factors
Although challenging to accomplish, management of diet, specifically removal of artificial food coloring and sodium benzoate preservatives, has been more efficacious than behavioral management in the long-term reduction of core symptoms of ADHD.31,32 No herbal remedy has demonstrated efficacy in improving ADHD symptoms. The use of omega-3 fatty acids as a complement to stimulants has demonstrated efficacy in reducing core symptoms in ADHD.33
Behavioral therapy
Several forms of behavioral therapy have shown utility in improving symptoms in ADHD. Evidence supports that ADHD responds to cognitive behavioral therapy.34 In-school neurofeedback training for ADHD was shown to be better than cognitive training in improving inattention and hyperactivity-impulsivity at 6 months of follow-up.35
Parental training has the most evidence to support its use in children with ADHD. The two most common forms are Pathways Triple P (Positive Parenting Program) and The Incredible Years. Triple P is an early intervention designed to promote positive parent-child relationships to reduce behavior problems.36 The Incredible Years is a multicomponent program that emphasizes creating opportunities for active involvement, reinforcement of positive behavior, teaching skills, and setting clear limits, all of which are central to the social development strategy.37
Many children with ADHD respond to in-school interventions, at least an evaluation to rule out learning disorders, which typically have high morbidity. Children may qualify for Individualized Education Program (IEP) services, such as peer tutoring,38 computer-assisted instruction,39,40 and task-modification instruction.41 All of these have evidence to support their use.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Hak E, de Vries TW, Hoekstra PJ, Jick SS. Association of childhood attention-deficit/hyperactivity disorder with atopic diseases and skin infections? A matched case-control study using the General Practice Research Database. Ann Allergy Asthma Immunol 2013; 111:102–106.
- Pretorius E. Corticosteroids, depression and the role of serotonin. Rev Neurosci 2004; 15:109–116.
- Keenan HT, Hall GC, Marshall SW. Early head injury and attention deficit hyperactivity disorder: retrospective cohort study. BMJ 2008; 337:a1984.
- Applegate B, Lahey BB, Hart EL, et al. Validity of the age-of-onset criterion for ADHD: a report of the DSM-IV field trials. J Am Acad Child Adolesc Psychiatry 1997; 36:1211–1221.
- Loeber R, Green SM, Lahey BB, Christ MAG, Frick PJ. Developmental sequences in the age of onset of disruptive child behaviors. J Child Fam Studies 1992; 1:21–41.
- Barkley RA, Murphy KR, Fischer M. Adult ADHD: What the Science Says. New York, NY: Guilford Publications; 2008.
- Rasmussen P, Gillberg C. Natural outcome of ADHD with developmental coordination disorder at age 22 years: a controlled, longitudinal, community-based study. J Am Acad Child Adolesc Psychiatry 2000; 39:1424–1431.
- Silva D, Colvin L, Hagemann E, Bower C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics 2014; 133:e14–e22.
- Lindström K, Lindblad F, Hjern A. Preterm birth and attention-deficit/hyperactivity disorder in schoolchildren. Pediatrics 2011; 127:858–865.
- Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr 2007; 96:1269–1274.
- Brown RT, Amler RW, Freeman WS, et al; for the American Academy of Pediatrics Committee on Quality Improvement; American Academy of Pediatrics Subcommittee on Attention-Deficit/Hyperactivity Disorder. Treatment of attention-deficit/hyperactivity disorder: overview of the evidence. Pediatrics 2005; 115:e749–e757.
- The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1999; 56:1073–1086.
- MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics 2004; 113:754–761.
- Martinez-Raga J, Knecht C, Szerman N, Martinez MI. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs 2013; 27:15–30.
- Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr 2006; 27:1–10.
- Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ 2001; 165:1475–1488.
- Biederman J, Pliszka SR. Modafinil improves symptoms of attention-deficit/hyperactivity disorder across subtypes in children and adolescents. J Pediatr 2008; 152:394–399.
- Taylor FB, Russo J. Efficacy of modafinil compared to dextroamphetamine for the treatment of attention deficit hyperactivity disorder in adults. J Child Adolesc Psychopharmacol 2000; 10:311–320.
- Hirota T, Schwartz S, Correll CU. Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry 2014; 53:153–173.
- Wilens TE. Combined pharmacotherapy in pediatric psychopharmacology: friend or foe? J Child Adolesc Psychopharmacol 2009; 19:483–484.
- Wilens TE, Bukstein O, Brams M, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2012; 51:74–85.e2.
- Michielsen M, Comijs HC, Semeijn EJ, Beekman ATF, Deeg DJH, Kooij JJS. The comorbidity of anxiety and depressive symptoms in older adults with attention-deficit/hyperactivity disorder: a longitudinal study. J Affect Disord 2013; 148:220–227.
- Jensen PS, Hinshaw SP, Kraemer HC, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. J Am Acad Child Adolesc Psychiatry 2001; 40:147–158.
- Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry 2010; 18:556–559.
- Wilens TE, Martelon M, Joshi G, et al. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry 2011; 50:543–553.
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry 2011; 50:9–21.
- Spencer T, Biederman J, Wilens T, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001; 58:775–782.
- Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH. Systematic review: pharmacological treatment of tic disorders—efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev 2013; 37:1162–1171.
- Allen AJ, Kurlan RM, Gilbert DL, et al. Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology 2005; 65:1941–1949.
- McCann D, Barrett A, Cooper A, et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet 2007; 370:1560–1567.
- Schab DW, Trinh NH. Do artificial food colors promote hyperactivity in children with hyperactive syndromes? A meta-analysis of double-blind placebo-controlled trials. J Dev Behav Pediatr 2004; 25:423–434.
- Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 2006; 67:1954–1967.
- Muñoz-Solomando A, Kendall T, Whittington CJ. Cognitive behavioural therapy for children and adolescents. Curr Opin Psychiatry 2008; 21:332–337.
- Steiner NJ, Frenette EC, Rene KM, Brennan RT, Perrin EC. In-school neurofeedback training for ADHD: sustained improvements from a randomized control trial. Pediatrics 2014; 133:483–492.
- Wiggins TL, Sofronoff K, Sanders MR. Pathways Triple P-positive parenting program: effects on parent-child relationships and child behavior problems. Fam Process 2009; 48:517–530.
- Haggerty KP, McGlynn-Wright A, Klima T. Promising parenting programs for reducing adolescent problem behaviors. J Child Serv 2013; 8(4).
- Greenwood CR. Longitudinal analysis of time, engagement, and achievement in at-risk versus non-risk students. Except Child 1991; 57:521–535.
- Mautone JA, DuPaul GJ, Jitendra AK. The effects of computer-assisted instruction on the mathematics performance and classroom behavior of children with ADHD. J Atten Disord 2005; 9:301–312.
- Rabiner DL, Murray DW, Skinner AT, Malone PS. A randomized trial of two promising computer-based interventions for students with attention difficulties. J Abnorm Child Psychol 2010; 38:131–142.
- Dunlap G, dePerczel M, Clarke S, et al. Choice making to promote adaptive behavior for students with emotional and behavioral challenges. J Appl Behav Anal 1994; 27:505–518.
Behavioral disorders in pediatric patients—primarily attention deficit hyperactivity disorder (ADHD)—pose a clinical challenge for health care providers to accurately assess, diagnose, and treat. In 2013, the criteria for several disruptive behavioral disorders were updated in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5),1 their first major revisions since 1994. Among the most clinically relevant changes were revisions to the diagnosis of ADHD and the creation of a new diagnostic entity: disruptive mood dysregulation disorder (DMDD).
This article focuses on the updated diagnostic criteria published in the DSM-5 for behavioral disorders, describes the assessment of ADHD, and summarizes management strategies.
UPDATED DIAGNOSTIC CRITERIA
ADHD
This disorder is a chronic, neurologically based illness characterized by a persistent pattern of inattention and/or hyperactivity and impulsivity that are more inappropriate or disruptive than those in other children of a comparable age resulting in functional impairment in multiple settings, and these behaviors have been present for at least 6 months. Revised diagnostic criteria in DSM-5 used the same two categories for ADHD symptoms—inattention and hyperactivity-impulsive behaviors—but modified several diagnostic requirements.
Revised criteria
Impairment before age 12 instead of age 6. As a neurodevelopmental disorder, ADHD usually starts at a young age; teenagers presenting with newly developed ADHD-type symptoms probably do not have ADHD and efforts should be made to rule out other illnesses or social dynamics. The DSM-5 raised the age limit for onset of qualifying symptoms to before 12 years (previously by age 6) primarily to capture a cohort of pediatric patients, typically female, who present solely with inattention symptoms and may not display overt functional impairment early on.
Symptoms required in at least two settings. Symptoms must be present in at least two settings to qualify for a diagnosis of ADHD. This ensures that the behaviors occur globally; they do not occur just at school or at home but occur in both places.
Fewer symptoms required for diagnosis in adolescents. Although the diagnostic criteria retain the same symptoms as those in DSM-IV for different age groups, individuals aged 17 and older are now required to display only five or more inattentive or hyperactive-impulsive symptoms. Previously, at least six were required.
Partial remission criteria
The concept of partial remission was introduced in DSM-5. This acknowledges that two-thirds of children diagnosed with ADHD do not have symptoms that functionally impact activities of daily living beyond age 18.
Oppositional defiant disorder
In DSM-5, oppositional defiant disorder (ODD) is defined by emotional and behavioral symptoms grouped into three categories:
- Constant anger or irritability
- Argumentative or defiant behavior (arguing with authority figures)
- Vindictiveness.
Because defiant behavior may represent difficulty with self-control, ODD is associated with executive functioning deficits that are present in ADHD. Children with ODD tend to perform best in situations in which they can dominate or exert authority. To qualify as ODD, the pattern of behavior must be consistent for longer than 6 months. A severity rating was added based on pervasiveness of ODD symptoms. Otherwise the diagnosis did not change.
Conduct disorder: Purposeful aggression
The hallmarks of conduct disorder are purposeful aggression (eg, bullying), destruction of property, deceitfulness or theft, and serious violation of rules (eg, running away from home, repeat truancy). Some consider conduct disorder to be a separate illness from ODD, whereas others consider it a continuum of the same disorder. Conduct disorder can manifest as violence, as in initiating physical fights, or it can manifest in behaviors such as truancy, stealing, lying, and running away from home without the physical-aggression aspect.
Intermittent explosive disorder
Failure to control aggressive impulses defines intermittent explosive disorder (IED). The aggressive outbursts can be verbal or behavioral and tend to be impulsive. A small subset of children display isolated aggression out of proportion to provocation. The disorder tends to manifest at ages 3 or 4, and a diagnosis requires a stable environment with no significant early childhood trauma. Most often these symptoms are seen in children with intellectual disabilities or an autism spectrum disorder.
Disruptive mood dysregulation disorder
A new diagnostic category in DSM-5 is termed disruptive mood dysregulation disorder (DMDD). This captures many children who previously would have been diagnosed with pediatric bipolar disorder, even though most of them do not fulfill criteria for bipolar disorder as adults. The presence of baseline irritability separates this disorder from IED, which requires intermittent rapid and severe outbursts. The severe temper outbursts of DMDD must be recurrent, with an average of three occurrences per week, and have background irritability. The symptoms must have a duration of at least 12 months and be present in two settings. A diagnosis of DMDD cannot be made earlier than age 6, with onset before age 10.
ASSESSMENT OF ADHD
The clinical interview in conjunction with objective scales is the primary tool for diagnosing ADHD. The most frequent source of information is from the parents followed by the child’s schoolteachers. Patient interview, although unreliable in young children, should also be part of the assessment. Comparing the patient’s functional impairment against children of a similar age is necessary for an ADHD diagnosis.
The medical history can help rule out children with asthma or allergy being treated with corticosteroids and those with hypothyroidism and hyperthyroidism whose symptoms often fulfill the diagnostic criteria for ADHD.2,3 Symptoms of ADHD also may appear suddenly after a traumatic brain injury or other neurologic event.4 Other psychiatric illnesses, especially learning disorders, mood disorders, anxiety, other disruptive behavior disorders, or substance abuse, can mimic ADHD.
Ruling out other factors from a social history (eg, family conflict, bullying, sleep deprivation, being overscheduled with activities) adds to the reliability of an ADHD diagnosis. For example, repetitive uprooting and frequent changes in schools can cause academic problems that may be mistaken for ADHD, and use of stimulants may have failed to improve symptoms in these children.
Assessment scales
Pediatric assessment scales that can be performed in an office are more practical than standardized clinical assessments (Table 1). The Vanderbilt ADHD Diagnostic Teacher Rating Scale correlates highly with a diagnosis of ADHD. We use the Vanderbilt ADHD Diagnostic Parent Rating Scale for children up to age 1 year. Other scales track symptoms and functional impairment over time and can be administered before the patient’s appointment. The Conners Third Edition scale can be used to establish a baseline before initiating therapy and to help monitor changes over time.
Standardized tests to bolster the utility of the clinical interview include the Diagnostic Interview Schedule for Children and Adolescents and the Schedule for Affective Disorders and Schizophrenia in School-Age Children–Present and Lifetime Version. Free training is available regarding use of some of these standardized tests.
Developmental course, risk factors
The clinical course of ADHD is chronic. The onset of hyperactivity usually occurs at age 3 or 4, with combined hyperactivity and inattention usually appearing from ages 5 to 8.5,6 The evolution of symptoms is progressive and constant. Between 50% and 80% have symptoms that continue into adolescence, and in about 40%, symptoms continue into adulthood.7,8 Some children with ADHD have a temperament-neuropsychological profile characterized by aggressiveness, irritability, and mood lability. Deficits in planning, delayed aversion, and temporal processing are present.
Risk factors include prematurity, prenatal complications, an anoxic event, nutritional deficits (specifically iron and zinc), and lack of appropriate socialization.9–11 The disorder is heritable, which is usually clear from the clinical interview. Rates of delinquency and peer rejection are high. This may result in secondary comorbidity such as emotional, disruptive, or substance abuse problems.
MANAGEMENT STRATEGIES
Stimulants
The first-line pharmacologic treatment of ADHD is stimulants: methylphenidate, dexmethylphenidate, mixed amphetamine salts, dextroamphetamine, and lisdexamfetamine. Head-to-head trials of medications versus behavioral management favor medication use, even over the long term.12–14
Methylphenidate and amphetamines are equally effective and have similar adverse effect profiles. Insomnia and anorexia are the most common side effects of stimulants. Cardiac effects include tachycardia, chest pain, and hypertension. Very rarely, stimulants have been associated with sudden cardiac death syndrome in patients with underlying cardiac problems. The consensus is that stimulants are safe in the general population. The need to obtain an electrocardiogram before initiating a stimulant was removed by the US Food and Drug Administration (FDA) unless it is otherwise indicated.15
The response rate to stimulants is in the range of 70%. About one-third of patients have side effects, and approximately 15% have side effects severe enough to requiring changing or withdrawing the medication.16,17
Stimulants are available in several delivery systems. For the best effect, medications should be combined with behavioral management.
Alternatives to stimulants
If stimulants are ineffective, atomoxetine can be used to treat patients with inattention; however, its effect on hyperactivity and impulsivity is less pronounced than that of stimulants. Bupropion is another option for inattention. Both agents are well tolerated. Irritability and insomnia are side effects of atomoxetine, and liver damage is possible, so liver function tests must be ordered if the patient complains of upper-right-quadrant pain.
The evidence to support the use of modafinil is equivocal.18,19 Unlike stimulants, modafinil is associated with a slight increase in motivation.
Alpha-2 agonists are effective for treating aggression in the setting of ADHD, especially in younger children, and are well tolerated.20 Extended-release forms are available.
Combination therapy
Polypharmacy is sometimes indicated in the treatment of ADHD. A stimulant used in combination with atomoxetine was shown to be superior to either treatment alone in improving symptoms of hyperactivity and inattention.21 The combination, however, markedly increased the incidence of appetite loss, insomnia, and irritability.
A more promising combination is a stimulant with an alpha agonist. Symptoms of hyperactivity and inattention were improved more with this combination than with a stimulant plus placebo, with no difference in side effects.22
ADHD with comorbidities
Patients with ADHD, both adults and children, often have comorbid externalizing disorders and other emotional disorders, such as depression and anxiety, occurring in up to half of cases (Table 2).23,24 These comorbidities are important to consider when developing a treatment strategy. The following describes treatment options for the most common ADHD comorbidities (Table 3).
ODD or conduct disorder. The first-line therapy for these patients is a stimulant plus behavioral therapy. Adding an alpha agonist to this combination may be indicated if the comorbidity is severe. Second-generation antipsychotics also have been used as add-ons to stimulants with behavioral therapy, but weight gain and hormonal side effects are common.
Behavioral interventions are effective in targeting disruptive behavioral disorders, specifically multisystemic therapy. Multisystemic therapy is intensive therapy that involves working with the patient’s peer group or school, but most children must enter the legal system to receive this intervention. Multisystemic therapy is the only intervention shown to improve symptoms associated with comorbid ADHD and conduct disorder.25
Mood disorders. For these patients, the mood disorder is treated first. In doing so, symptoms of ADHD may disappear. For those with bipolar disease, a second-generation antipsychotic agent is superior to lithium in efficacy, maintenance of remission, and side effects in patients with a clear bipolar affective disorder, after which a stimulant can be added with less risk of developing manic symptoms. Using a stimulant first for this indication risks mood destabilization.
For patients with a major depressive disorder, bupropion can be used, although this indication is not FDA-approved, followed by the addition of a stimulant. One alternative is a selective serotonin reuptake inhibitor plus a stimulant; another is cognitive behavioral therapy plus atomoxetine and an alpha agonist.
Substance abuse. Patients with ADHD have high rates of substance abuse.26,27 Whether treatment of ADHD with stimulants reduces the risk of substance abuse is controversial. Because abuse of stimulants is common, start treatment with atomoxetine, bupropion, an alpha agonist, or a stimulant that is difficult to abuse (eg, lisdexamfetamine). Refer patients who are abusing substances to a specialist in substance abuse for behavioral management.
Anxiety. Atomoxetine is recommended for the treatment of anxiety that coexists with ADHD. A selective serotonin reuptake inhibitor in combination with a stimulant or alpha agonist, plus cognitive behavioral therapy, is another option for treating anxiety and ADHD. Tricyclic antidepressants have shown benefit in pediatric anxiety. Bupropion should not be used to target anxiety as it has been shown to have a limited effect on anxiety.
Tics. Stimulants may transiently exacerbate underlying tic disorders, but no longstanding difference in the course of tics has been observed with stimulant use.28 Alpha-2 antagonists target both tics and ADHD, so their use is preferred.29 Atomoxetine does not exacerbate tics but may reduce their frequency and severity.30
Dietary factors
Although challenging to accomplish, management of diet, specifically removal of artificial food coloring and sodium benzoate preservatives, has been more efficacious than behavioral management in the long-term reduction of core symptoms of ADHD.31,32 No herbal remedy has demonstrated efficacy in improving ADHD symptoms. The use of omega-3 fatty acids as a complement to stimulants has demonstrated efficacy in reducing core symptoms in ADHD.33
Behavioral therapy
Several forms of behavioral therapy have shown utility in improving symptoms in ADHD. Evidence supports that ADHD responds to cognitive behavioral therapy.34 In-school neurofeedback training for ADHD was shown to be better than cognitive training in improving inattention and hyperactivity-impulsivity at 6 months of follow-up.35
Parental training has the most evidence to support its use in children with ADHD. The two most common forms are Pathways Triple P (Positive Parenting Program) and The Incredible Years. Triple P is an early intervention designed to promote positive parent-child relationships to reduce behavior problems.36 The Incredible Years is a multicomponent program that emphasizes creating opportunities for active involvement, reinforcement of positive behavior, teaching skills, and setting clear limits, all of which are central to the social development strategy.37
Many children with ADHD respond to in-school interventions, at least an evaluation to rule out learning disorders, which typically have high morbidity. Children may qualify for Individualized Education Program (IEP) services, such as peer tutoring,38 computer-assisted instruction,39,40 and task-modification instruction.41 All of these have evidence to support their use.
Behavioral disorders in pediatric patients—primarily attention deficit hyperactivity disorder (ADHD)—pose a clinical challenge for health care providers to accurately assess, diagnose, and treat. In 2013, the criteria for several disruptive behavioral disorders were updated in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5),1 their first major revisions since 1994. Among the most clinically relevant changes were revisions to the diagnosis of ADHD and the creation of a new diagnostic entity: disruptive mood dysregulation disorder (DMDD).
This article focuses on the updated diagnostic criteria published in the DSM-5 for behavioral disorders, describes the assessment of ADHD, and summarizes management strategies.
UPDATED DIAGNOSTIC CRITERIA
ADHD
This disorder is a chronic, neurologically based illness characterized by a persistent pattern of inattention and/or hyperactivity and impulsivity that are more inappropriate or disruptive than those in other children of a comparable age resulting in functional impairment in multiple settings, and these behaviors have been present for at least 6 months. Revised diagnostic criteria in DSM-5 used the same two categories for ADHD symptoms—inattention and hyperactivity-impulsive behaviors—but modified several diagnostic requirements.
Revised criteria
Impairment before age 12 instead of age 6. As a neurodevelopmental disorder, ADHD usually starts at a young age; teenagers presenting with newly developed ADHD-type symptoms probably do not have ADHD and efforts should be made to rule out other illnesses or social dynamics. The DSM-5 raised the age limit for onset of qualifying symptoms to before 12 years (previously by age 6) primarily to capture a cohort of pediatric patients, typically female, who present solely with inattention symptoms and may not display overt functional impairment early on.
Symptoms required in at least two settings. Symptoms must be present in at least two settings to qualify for a diagnosis of ADHD. This ensures that the behaviors occur globally; they do not occur just at school or at home but occur in both places.
Fewer symptoms required for diagnosis in adolescents. Although the diagnostic criteria retain the same symptoms as those in DSM-IV for different age groups, individuals aged 17 and older are now required to display only five or more inattentive or hyperactive-impulsive symptoms. Previously, at least six were required.
Partial remission criteria
The concept of partial remission was introduced in DSM-5. This acknowledges that two-thirds of children diagnosed with ADHD do not have symptoms that functionally impact activities of daily living beyond age 18.
Oppositional defiant disorder
In DSM-5, oppositional defiant disorder (ODD) is defined by emotional and behavioral symptoms grouped into three categories:
- Constant anger or irritability
- Argumentative or defiant behavior (arguing with authority figures)
- Vindictiveness.
Because defiant behavior may represent difficulty with self-control, ODD is associated with executive functioning deficits that are present in ADHD. Children with ODD tend to perform best in situations in which they can dominate or exert authority. To qualify as ODD, the pattern of behavior must be consistent for longer than 6 months. A severity rating was added based on pervasiveness of ODD symptoms. Otherwise the diagnosis did not change.
Conduct disorder: Purposeful aggression
The hallmarks of conduct disorder are purposeful aggression (eg, bullying), destruction of property, deceitfulness or theft, and serious violation of rules (eg, running away from home, repeat truancy). Some consider conduct disorder to be a separate illness from ODD, whereas others consider it a continuum of the same disorder. Conduct disorder can manifest as violence, as in initiating physical fights, or it can manifest in behaviors such as truancy, stealing, lying, and running away from home without the physical-aggression aspect.
Intermittent explosive disorder
Failure to control aggressive impulses defines intermittent explosive disorder (IED). The aggressive outbursts can be verbal or behavioral and tend to be impulsive. A small subset of children display isolated aggression out of proportion to provocation. The disorder tends to manifest at ages 3 or 4, and a diagnosis requires a stable environment with no significant early childhood trauma. Most often these symptoms are seen in children with intellectual disabilities or an autism spectrum disorder.
Disruptive mood dysregulation disorder
A new diagnostic category in DSM-5 is termed disruptive mood dysregulation disorder (DMDD). This captures many children who previously would have been diagnosed with pediatric bipolar disorder, even though most of them do not fulfill criteria for bipolar disorder as adults. The presence of baseline irritability separates this disorder from IED, which requires intermittent rapid and severe outbursts. The severe temper outbursts of DMDD must be recurrent, with an average of three occurrences per week, and have background irritability. The symptoms must have a duration of at least 12 months and be present in two settings. A diagnosis of DMDD cannot be made earlier than age 6, with onset before age 10.
ASSESSMENT OF ADHD
The clinical interview in conjunction with objective scales is the primary tool for diagnosing ADHD. The most frequent source of information is from the parents followed by the child’s schoolteachers. Patient interview, although unreliable in young children, should also be part of the assessment. Comparing the patient’s functional impairment against children of a similar age is necessary for an ADHD diagnosis.
The medical history can help rule out children with asthma or allergy being treated with corticosteroids and those with hypothyroidism and hyperthyroidism whose symptoms often fulfill the diagnostic criteria for ADHD.2,3 Symptoms of ADHD also may appear suddenly after a traumatic brain injury or other neurologic event.4 Other psychiatric illnesses, especially learning disorders, mood disorders, anxiety, other disruptive behavior disorders, or substance abuse, can mimic ADHD.
Ruling out other factors from a social history (eg, family conflict, bullying, sleep deprivation, being overscheduled with activities) adds to the reliability of an ADHD diagnosis. For example, repetitive uprooting and frequent changes in schools can cause academic problems that may be mistaken for ADHD, and use of stimulants may have failed to improve symptoms in these children.
Assessment scales
Pediatric assessment scales that can be performed in an office are more practical than standardized clinical assessments (Table 1). The Vanderbilt ADHD Diagnostic Teacher Rating Scale correlates highly with a diagnosis of ADHD. We use the Vanderbilt ADHD Diagnostic Parent Rating Scale for children up to age 1 year. Other scales track symptoms and functional impairment over time and can be administered before the patient’s appointment. The Conners Third Edition scale can be used to establish a baseline before initiating therapy and to help monitor changes over time.
Standardized tests to bolster the utility of the clinical interview include the Diagnostic Interview Schedule for Children and Adolescents and the Schedule for Affective Disorders and Schizophrenia in School-Age Children–Present and Lifetime Version. Free training is available regarding use of some of these standardized tests.
Developmental course, risk factors
The clinical course of ADHD is chronic. The onset of hyperactivity usually occurs at age 3 or 4, with combined hyperactivity and inattention usually appearing from ages 5 to 8.5,6 The evolution of symptoms is progressive and constant. Between 50% and 80% have symptoms that continue into adolescence, and in about 40%, symptoms continue into adulthood.7,8 Some children with ADHD have a temperament-neuropsychological profile characterized by aggressiveness, irritability, and mood lability. Deficits in planning, delayed aversion, and temporal processing are present.
Risk factors include prematurity, prenatal complications, an anoxic event, nutritional deficits (specifically iron and zinc), and lack of appropriate socialization.9–11 The disorder is heritable, which is usually clear from the clinical interview. Rates of delinquency and peer rejection are high. This may result in secondary comorbidity such as emotional, disruptive, or substance abuse problems.
MANAGEMENT STRATEGIES
Stimulants
The first-line pharmacologic treatment of ADHD is stimulants: methylphenidate, dexmethylphenidate, mixed amphetamine salts, dextroamphetamine, and lisdexamfetamine. Head-to-head trials of medications versus behavioral management favor medication use, even over the long term.12–14
Methylphenidate and amphetamines are equally effective and have similar adverse effect profiles. Insomnia and anorexia are the most common side effects of stimulants. Cardiac effects include tachycardia, chest pain, and hypertension. Very rarely, stimulants have been associated with sudden cardiac death syndrome in patients with underlying cardiac problems. The consensus is that stimulants are safe in the general population. The need to obtain an electrocardiogram before initiating a stimulant was removed by the US Food and Drug Administration (FDA) unless it is otherwise indicated.15
The response rate to stimulants is in the range of 70%. About one-third of patients have side effects, and approximately 15% have side effects severe enough to requiring changing or withdrawing the medication.16,17
Stimulants are available in several delivery systems. For the best effect, medications should be combined with behavioral management.
Alternatives to stimulants
If stimulants are ineffective, atomoxetine can be used to treat patients with inattention; however, its effect on hyperactivity and impulsivity is less pronounced than that of stimulants. Bupropion is another option for inattention. Both agents are well tolerated. Irritability and insomnia are side effects of atomoxetine, and liver damage is possible, so liver function tests must be ordered if the patient complains of upper-right-quadrant pain.
The evidence to support the use of modafinil is equivocal.18,19 Unlike stimulants, modafinil is associated with a slight increase in motivation.
Alpha-2 agonists are effective for treating aggression in the setting of ADHD, especially in younger children, and are well tolerated.20 Extended-release forms are available.
Combination therapy
Polypharmacy is sometimes indicated in the treatment of ADHD. A stimulant used in combination with atomoxetine was shown to be superior to either treatment alone in improving symptoms of hyperactivity and inattention.21 The combination, however, markedly increased the incidence of appetite loss, insomnia, and irritability.
A more promising combination is a stimulant with an alpha agonist. Symptoms of hyperactivity and inattention were improved more with this combination than with a stimulant plus placebo, with no difference in side effects.22
ADHD with comorbidities
Patients with ADHD, both adults and children, often have comorbid externalizing disorders and other emotional disorders, such as depression and anxiety, occurring in up to half of cases (Table 2).23,24 These comorbidities are important to consider when developing a treatment strategy. The following describes treatment options for the most common ADHD comorbidities (Table 3).
ODD or conduct disorder. The first-line therapy for these patients is a stimulant plus behavioral therapy. Adding an alpha agonist to this combination may be indicated if the comorbidity is severe. Second-generation antipsychotics also have been used as add-ons to stimulants with behavioral therapy, but weight gain and hormonal side effects are common.
Behavioral interventions are effective in targeting disruptive behavioral disorders, specifically multisystemic therapy. Multisystemic therapy is intensive therapy that involves working with the patient’s peer group or school, but most children must enter the legal system to receive this intervention. Multisystemic therapy is the only intervention shown to improve symptoms associated with comorbid ADHD and conduct disorder.25
Mood disorders. For these patients, the mood disorder is treated first. In doing so, symptoms of ADHD may disappear. For those with bipolar disease, a second-generation antipsychotic agent is superior to lithium in efficacy, maintenance of remission, and side effects in patients with a clear bipolar affective disorder, after which a stimulant can be added with less risk of developing manic symptoms. Using a stimulant first for this indication risks mood destabilization.
For patients with a major depressive disorder, bupropion can be used, although this indication is not FDA-approved, followed by the addition of a stimulant. One alternative is a selective serotonin reuptake inhibitor plus a stimulant; another is cognitive behavioral therapy plus atomoxetine and an alpha agonist.
Substance abuse. Patients with ADHD have high rates of substance abuse.26,27 Whether treatment of ADHD with stimulants reduces the risk of substance abuse is controversial. Because abuse of stimulants is common, start treatment with atomoxetine, bupropion, an alpha agonist, or a stimulant that is difficult to abuse (eg, lisdexamfetamine). Refer patients who are abusing substances to a specialist in substance abuse for behavioral management.
Anxiety. Atomoxetine is recommended for the treatment of anxiety that coexists with ADHD. A selective serotonin reuptake inhibitor in combination with a stimulant or alpha agonist, plus cognitive behavioral therapy, is another option for treating anxiety and ADHD. Tricyclic antidepressants have shown benefit in pediatric anxiety. Bupropion should not be used to target anxiety as it has been shown to have a limited effect on anxiety.
Tics. Stimulants may transiently exacerbate underlying tic disorders, but no longstanding difference in the course of tics has been observed with stimulant use.28 Alpha-2 antagonists target both tics and ADHD, so their use is preferred.29 Atomoxetine does not exacerbate tics but may reduce their frequency and severity.30
Dietary factors
Although challenging to accomplish, management of diet, specifically removal of artificial food coloring and sodium benzoate preservatives, has been more efficacious than behavioral management in the long-term reduction of core symptoms of ADHD.31,32 No herbal remedy has demonstrated efficacy in improving ADHD symptoms. The use of omega-3 fatty acids as a complement to stimulants has demonstrated efficacy in reducing core symptoms in ADHD.33
Behavioral therapy
Several forms of behavioral therapy have shown utility in improving symptoms in ADHD. Evidence supports that ADHD responds to cognitive behavioral therapy.34 In-school neurofeedback training for ADHD was shown to be better than cognitive training in improving inattention and hyperactivity-impulsivity at 6 months of follow-up.35
Parental training has the most evidence to support its use in children with ADHD. The two most common forms are Pathways Triple P (Positive Parenting Program) and The Incredible Years. Triple P is an early intervention designed to promote positive parent-child relationships to reduce behavior problems.36 The Incredible Years is a multicomponent program that emphasizes creating opportunities for active involvement, reinforcement of positive behavior, teaching skills, and setting clear limits, all of which are central to the social development strategy.37
Many children with ADHD respond to in-school interventions, at least an evaluation to rule out learning disorders, which typically have high morbidity. Children may qualify for Individualized Education Program (IEP) services, such as peer tutoring,38 computer-assisted instruction,39,40 and task-modification instruction.41 All of these have evidence to support their use.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Hak E, de Vries TW, Hoekstra PJ, Jick SS. Association of childhood attention-deficit/hyperactivity disorder with atopic diseases and skin infections? A matched case-control study using the General Practice Research Database. Ann Allergy Asthma Immunol 2013; 111:102–106.
- Pretorius E. Corticosteroids, depression and the role of serotonin. Rev Neurosci 2004; 15:109–116.
- Keenan HT, Hall GC, Marshall SW. Early head injury and attention deficit hyperactivity disorder: retrospective cohort study. BMJ 2008; 337:a1984.
- Applegate B, Lahey BB, Hart EL, et al. Validity of the age-of-onset criterion for ADHD: a report of the DSM-IV field trials. J Am Acad Child Adolesc Psychiatry 1997; 36:1211–1221.
- Loeber R, Green SM, Lahey BB, Christ MAG, Frick PJ. Developmental sequences in the age of onset of disruptive child behaviors. J Child Fam Studies 1992; 1:21–41.
- Barkley RA, Murphy KR, Fischer M. Adult ADHD: What the Science Says. New York, NY: Guilford Publications; 2008.
- Rasmussen P, Gillberg C. Natural outcome of ADHD with developmental coordination disorder at age 22 years: a controlled, longitudinal, community-based study. J Am Acad Child Adolesc Psychiatry 2000; 39:1424–1431.
- Silva D, Colvin L, Hagemann E, Bower C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics 2014; 133:e14–e22.
- Lindström K, Lindblad F, Hjern A. Preterm birth and attention-deficit/hyperactivity disorder in schoolchildren. Pediatrics 2011; 127:858–865.
- Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr 2007; 96:1269–1274.
- Brown RT, Amler RW, Freeman WS, et al; for the American Academy of Pediatrics Committee on Quality Improvement; American Academy of Pediatrics Subcommittee on Attention-Deficit/Hyperactivity Disorder. Treatment of attention-deficit/hyperactivity disorder: overview of the evidence. Pediatrics 2005; 115:e749–e757.
- The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1999; 56:1073–1086.
- MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics 2004; 113:754–761.
- Martinez-Raga J, Knecht C, Szerman N, Martinez MI. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs 2013; 27:15–30.
- Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr 2006; 27:1–10.
- Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ 2001; 165:1475–1488.
- Biederman J, Pliszka SR. Modafinil improves symptoms of attention-deficit/hyperactivity disorder across subtypes in children and adolescents. J Pediatr 2008; 152:394–399.
- Taylor FB, Russo J. Efficacy of modafinil compared to dextroamphetamine for the treatment of attention deficit hyperactivity disorder in adults. J Child Adolesc Psychopharmacol 2000; 10:311–320.
- Hirota T, Schwartz S, Correll CU. Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry 2014; 53:153–173.
- Wilens TE. Combined pharmacotherapy in pediatric psychopharmacology: friend or foe? J Child Adolesc Psychopharmacol 2009; 19:483–484.
- Wilens TE, Bukstein O, Brams M, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2012; 51:74–85.e2.
- Michielsen M, Comijs HC, Semeijn EJ, Beekman ATF, Deeg DJH, Kooij JJS. The comorbidity of anxiety and depressive symptoms in older adults with attention-deficit/hyperactivity disorder: a longitudinal study. J Affect Disord 2013; 148:220–227.
- Jensen PS, Hinshaw SP, Kraemer HC, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. J Am Acad Child Adolesc Psychiatry 2001; 40:147–158.
- Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry 2010; 18:556–559.
- Wilens TE, Martelon M, Joshi G, et al. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry 2011; 50:543–553.
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry 2011; 50:9–21.
- Spencer T, Biederman J, Wilens T, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001; 58:775–782.
- Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH. Systematic review: pharmacological treatment of tic disorders—efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev 2013; 37:1162–1171.
- Allen AJ, Kurlan RM, Gilbert DL, et al. Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology 2005; 65:1941–1949.
- McCann D, Barrett A, Cooper A, et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet 2007; 370:1560–1567.
- Schab DW, Trinh NH. Do artificial food colors promote hyperactivity in children with hyperactive syndromes? A meta-analysis of double-blind placebo-controlled trials. J Dev Behav Pediatr 2004; 25:423–434.
- Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 2006; 67:1954–1967.
- Muñoz-Solomando A, Kendall T, Whittington CJ. Cognitive behavioural therapy for children and adolescents. Curr Opin Psychiatry 2008; 21:332–337.
- Steiner NJ, Frenette EC, Rene KM, Brennan RT, Perrin EC. In-school neurofeedback training for ADHD: sustained improvements from a randomized control trial. Pediatrics 2014; 133:483–492.
- Wiggins TL, Sofronoff K, Sanders MR. Pathways Triple P-positive parenting program: effects on parent-child relationships and child behavior problems. Fam Process 2009; 48:517–530.
- Haggerty KP, McGlynn-Wright A, Klima T. Promising parenting programs for reducing adolescent problem behaviors. J Child Serv 2013; 8(4).
- Greenwood CR. Longitudinal analysis of time, engagement, and achievement in at-risk versus non-risk students. Except Child 1991; 57:521–535.
- Mautone JA, DuPaul GJ, Jitendra AK. The effects of computer-assisted instruction on the mathematics performance and classroom behavior of children with ADHD. J Atten Disord 2005; 9:301–312.
- Rabiner DL, Murray DW, Skinner AT, Malone PS. A randomized trial of two promising computer-based interventions for students with attention difficulties. J Abnorm Child Psychol 2010; 38:131–142.
- Dunlap G, dePerczel M, Clarke S, et al. Choice making to promote adaptive behavior for students with emotional and behavioral challenges. J Appl Behav Anal 1994; 27:505–518.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Hak E, de Vries TW, Hoekstra PJ, Jick SS. Association of childhood attention-deficit/hyperactivity disorder with atopic diseases and skin infections? A matched case-control study using the General Practice Research Database. Ann Allergy Asthma Immunol 2013; 111:102–106.
- Pretorius E. Corticosteroids, depression and the role of serotonin. Rev Neurosci 2004; 15:109–116.
- Keenan HT, Hall GC, Marshall SW. Early head injury and attention deficit hyperactivity disorder: retrospective cohort study. BMJ 2008; 337:a1984.
- Applegate B, Lahey BB, Hart EL, et al. Validity of the age-of-onset criterion for ADHD: a report of the DSM-IV field trials. J Am Acad Child Adolesc Psychiatry 1997; 36:1211–1221.
- Loeber R, Green SM, Lahey BB, Christ MAG, Frick PJ. Developmental sequences in the age of onset of disruptive child behaviors. J Child Fam Studies 1992; 1:21–41.
- Barkley RA, Murphy KR, Fischer M. Adult ADHD: What the Science Says. New York, NY: Guilford Publications; 2008.
- Rasmussen P, Gillberg C. Natural outcome of ADHD with developmental coordination disorder at age 22 years: a controlled, longitudinal, community-based study. J Am Acad Child Adolesc Psychiatry 2000; 39:1424–1431.
- Silva D, Colvin L, Hagemann E, Bower C. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics 2014; 133:e14–e22.
- Lindström K, Lindblad F, Hjern A. Preterm birth and attention-deficit/hyperactivity disorder in schoolchildren. Pediatrics 2011; 127:858–865.
- Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr 2007; 96:1269–1274.
- Brown RT, Amler RW, Freeman WS, et al; for the American Academy of Pediatrics Committee on Quality Improvement; American Academy of Pediatrics Subcommittee on Attention-Deficit/Hyperactivity Disorder. Treatment of attention-deficit/hyperactivity disorder: overview of the evidence. Pediatrics 2005; 115:e749–e757.
- The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1999; 56:1073–1086.
- MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics 2004; 113:754–761.
- Martinez-Raga J, Knecht C, Szerman N, Martinez MI. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs 2013; 27:15–30.
- Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr 2006; 27:1–10.
- Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ 2001; 165:1475–1488.
- Biederman J, Pliszka SR. Modafinil improves symptoms of attention-deficit/hyperactivity disorder across subtypes in children and adolescents. J Pediatr 2008; 152:394–399.
- Taylor FB, Russo J. Efficacy of modafinil compared to dextroamphetamine for the treatment of attention deficit hyperactivity disorder in adults. J Child Adolesc Psychopharmacol 2000; 10:311–320.
- Hirota T, Schwartz S, Correll CU. Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry 2014; 53:153–173.
- Wilens TE. Combined pharmacotherapy in pediatric psychopharmacology: friend or foe? J Child Adolesc Psychopharmacol 2009; 19:483–484.
- Wilens TE, Bukstein O, Brams M, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2012; 51:74–85.e2.
- Michielsen M, Comijs HC, Semeijn EJ, Beekman ATF, Deeg DJH, Kooij JJS. The comorbidity of anxiety and depressive symptoms in older adults with attention-deficit/hyperactivity disorder: a longitudinal study. J Affect Disord 2013; 148:220–227.
- Jensen PS, Hinshaw SP, Kraemer HC, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. J Am Acad Child Adolesc Psychiatry 2001; 40:147–158.
- Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry 2010; 18:556–559.
- Wilens TE, Martelon M, Joshi G, et al. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry 2011; 50:543–553.
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry 2011; 50:9–21.
- Spencer T, Biederman J, Wilens T, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001; 58:775–782.
- Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH. Systematic review: pharmacological treatment of tic disorders—efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev 2013; 37:1162–1171.
- Allen AJ, Kurlan RM, Gilbert DL, et al. Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology 2005; 65:1941–1949.
- McCann D, Barrett A, Cooper A, et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet 2007; 370:1560–1567.
- Schab DW, Trinh NH. Do artificial food colors promote hyperactivity in children with hyperactive syndromes? A meta-analysis of double-blind placebo-controlled trials. J Dev Behav Pediatr 2004; 25:423–434.
- Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry 2006; 67:1954–1967.
- Muñoz-Solomando A, Kendall T, Whittington CJ. Cognitive behavioural therapy for children and adolescents. Curr Opin Psychiatry 2008; 21:332–337.
- Steiner NJ, Frenette EC, Rene KM, Brennan RT, Perrin EC. In-school neurofeedback training for ADHD: sustained improvements from a randomized control trial. Pediatrics 2014; 133:483–492.
- Wiggins TL, Sofronoff K, Sanders MR. Pathways Triple P-positive parenting program: effects on parent-child relationships and child behavior problems. Fam Process 2009; 48:517–530.
- Haggerty KP, McGlynn-Wright A, Klima T. Promising parenting programs for reducing adolescent problem behaviors. J Child Serv 2013; 8(4).
- Greenwood CR. Longitudinal analysis of time, engagement, and achievement in at-risk versus non-risk students. Except Child 1991; 57:521–535.
- Mautone JA, DuPaul GJ, Jitendra AK. The effects of computer-assisted instruction on the mathematics performance and classroom behavior of children with ADHD. J Atten Disord 2005; 9:301–312.
- Rabiner DL, Murray DW, Skinner AT, Malone PS. A randomized trial of two promising computer-based interventions for students with attention difficulties. J Abnorm Child Psychol 2010; 38:131–142.
- Dunlap G, dePerczel M, Clarke S, et al. Choice making to promote adaptive behavior for students with emotional and behavioral challenges. J Appl Behav Anal 1994; 27:505–518.
Use of long-acting reversible contraceptives to reduce the rate of teen pregnancy
Adolescents who are at risk of unintended pregnancy need access to highly effective contraceptives. Using a case study format, this article addresses the myths, misconceptions, and barriers to effective use of contraceptives, focusing on long-acting reversible contraceptives (LARCs) and suggesting ways to overcome these barriers.
CASE 1: TEEN WITH DYSMENORRHEA
Jessica is a 15-year-old girl presenting with complaints of severe cramps, causing her to miss school and other activities 3 to 4 days each month. She has had six sexual partners and believes that contraception would be a good idea. She also states that she hates shots and doesn’t swallow pills well. She asks you to help. What are her/your options?
In this case, options include chewable oral contraceptives, a contraceptive patch, the etonogestrel/ethinyl estradiol vaginal ring (NuvaRing), depot medroxyprogesterone acetate (DMPA; progestin-only, injectable, lasts 3 months), and LARCs, which are intrauterine systems (IUS), intrauterine devices (IUDs), and implants. If she can remember a chewable pill every day, that would be one option. The patch requires her to remember to change it weekly. The vaginal ring requires ability and motivation to insert and remove it vaginally each month. She has stated that she does not want shots, so DMPA is not a viable option.
In contrast, LARCs constitute “forgettable” contraception in that they are not dependent on daily or monthly investment of time and energy to use. With her dysmenorrhea, use of an LARC that contains a progestin to thin out her lining and/or induce amenorrhea has some additional advantages.
The Institute of Medicine has declared that expanding access to LARCs for young women is a national priority.1 In 2009, the American College of Obstetricians and Gynecologists encouraged implants and IUDs for nulliparous women and adolescents.2 The following review describes currently available LARCs.
Intrauterine systems (IUS)
The levonorgestrel-releasing IUS (Mirena) was approved by the US Food and Drug Administration (FDA) in 2000. It maintains efficacy for 5 years and has a failure rate of 0.2%. Contraception is reversible with its removal. The system consists of a small T-shaped frame with a steroid reservoir that releases 20 µg/day of levonorgestrel, resulting in high endometrial levels and low plasma levels of levonorgestrel. An alternate brand available in the US is Skyla, notable for its slightly smaller size, slightly higher expulsion rate, and similar side effect profile to Mirena.
The copper in the levonorgestrel-releasing IUS acts as a spermicide. The progestin thickens the cervical mucus and thins the endometrial lining to cause a marked reduction in uterine bleeding. Between 20% and 80% of recipients experience amenorrhea by 1 year.3–5 It is considered safe and effective, it provides prolonged relief of menstrual problems including menometrorrhagia. Because it contains only progestin, it can be used while breastfeeding. One drawback is the skill needed to insert the device, necessitating insertion by a clinician. Side effects include early spotting and rare instances of perforation of the uterus.3
Intrauterine devices (IUD)
The copper IUD (Paragard) was FDA approved in 1989 for 10 years of use, but it has been used off label for up to 12 years continuously. It is preferred by women who want to avoid hormones while achieving similar results as the levonorgestrel-releasing IUS, including reductions in menstrual bleeding. The copper IUD can be used in women with a history of ectopic pregnancy. Fertility returns after removal of the device. Its use has been associated with a reduction in the risk of endometrial cancer,6 which may be related to prevention of human papillomavirus infection. Insertion of the copper IUD is a relatively simple office procedure.
Implants
The etonorgestrel single-implant system (Implanon, Nexplanon) is a single rod containing 68 mg of the progestin etonorgestrel, which is the biologically active metabolite of desogestrel. The single rod eases implantation and removal compared with previous systems that contained six rods. The implant was FDA approved in 2006 but has been marketed worldwide since 1998. Nexplanon contains a single, radiopaque rod that is easier to localize and remove.
The duration of contraceptive efficacy for Nexplanon is 3 years. Etonorgestrel levels are undetectable within a few days of reversal. Breakthrough bleeding can occur, and depression and mood swings are potential side effects that are manageable with close follow-up. The implants can be removed at any time.
If breakthrough bleeding occurs while on progestin-only methods, an intermittent solution is to add estrogen by pill or patch for 3 weeks and then withdraw the estrogen until bleeding again occurs. This practice is usually not necessary by 12 months after implantation.
Implant use can reduce the repeat pregnancy rate among adolescents. In one study, researchers found that teenage mothers who chose a contraceptive implant during their first year postpartum, including the 37% who discontinued use, had a 2-year repeat pregnancy rate of 12% versus 46% among mothers using no method or other methods of contraception.7
Barriers to LARC use
Among adolescents attending an integrated prenatal and postpartum maternity clinic, 75% indicated intent to use LARCs postpartum. Approximately one-third chose an implant, one-third chose an IUS, and one-third chose either DMPA, oral contraceptives, a contraceptive patch, or a contraceptive ring. After 6 months, only 50% had received an LARC, leaving one-third at risk for rapid repeat pregnancy.8
Unfortunately, the safety, side effects, and efficacy of LARCs may be misunderstood by both clinicians and teens. A negative personal experience may dominate one’s thinking and act as a barrier to use. The adolescent may not be mature enough to understand the chance of pregnancy or its consequences. Use of an IUD or IUS requires planning, a visit to a clinic that can insert the device, and a substantial up-front expenditure, even though the average cost per year compares favorably to use of DMPA or oral contraceptives.
Lack of awareness of LARCs is another barrier to their use. Between 50% and 60% of young women have never heard of an IUD and 90% have no awareness of contraceptive implants.9–12 Of those who knew about them, only 25% knew that they were eligible to use LARCs.13
In addition, many practitioners still mistakenly believe that current IUDs can cause pelvic inflammatory disease (PID), despite there being no association between modern IUDs and PID after the first 20 days following insertion.14–16
Physicians may also be unaware of the medical eligibility criteria (MEC) for contraceptive use established by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC).15,16 Conditions affecting eligibility for the use of each contraceptive method are classified under four categories (Table 1).
Overall efficacy
The effectiveness of LARC use in young women has been established. In one large study,17 4,167 females aged 15 to 45 were offered contraception at no cost for 3 years. Of those who chose an LARC, the 12-month continuation rate was 86% compared with 55% among those choosing an oral contraceptive. Satisfaction rates reflect the continuation rates with more than 80% of LARC users being satisfied compared with 54% of oral contraceptive users being satisfied. The pregnancy rate was 22 times greater in women using short-acting contraceptives compared with LARC users. In women younger than 20, pregnancy rates were twice as high among oral contraceptive users.4,18
Case conclusion
Jessica chooses an IUS, and her adolescent-medicine physician inserts Mirena at her next visit. She has some irregular bleeding during the first 3 months, but by 1 year, she is having periods only every 5 to 6 months. She manages cramps with ibuprofen 400 mg orally every 6 hours and is careful not to miss ibuprofen doses when she starts cramping or bleeding. She has not had sexual activity since the insertion, but she plans to always use condoms when she chooses to have sex. At her 3-month visit after insertion, when considering whether to remove or continue with her IUS despite her initial unscheduled bleeding, she discusses the flexibility of IUS to allow her to change her mind: “It’s like changing my hairstyle; I can just come back and change it in 3 months or even sooner if it is really bothering me. I don’t have to think of it as permanent, just less of a daily bother.” She is pleased with her choice of LARC and plans to return in 6 months for follow-up.
CASE 2: TEEN REQUESTS RELIABLE CONTRACEPTION
Danielle is a 16-year-old nulliparous female currently using condoms for contraception but wants a more reliable method. Her options include an IUD/IUS (MEC 2 for women younger than 18 years), a contraceptive implant (MEC 1 for all ages), DMPA (MEC 2 for women younger than 18 years), and combined oral contraceptives (MEC 1 for all ages).
The use of DMPA by teenagers is worrisome because users experience a loss of 1% to 3% of bone mineral density (BMD) over 1 year, although BMD is regained after discontinuation.19 Whether BMD relates to fracture risk in adolescents is unclear, but there is no evidence that DMPA increases the risk. Nevertheless, a baseline BMD measurement repeated every other year is recommended for thin females taking DMPA. To slow potential bone loss, daily exercise and age-appropriate calcium and vitamin D intake should be encouraged in teens, who often do not get enough calcium.
Obese adolescents who use DMPA are more likely to gain weight than nonobese DMPA users and obese users of other contraceptive methods.20 Obese adolescents who use DMPA can gain as much as 10 kg.21
Any of the methods mentioned are options for contraception for Danielle, with continued use of condoms and counseling about dual protection. Compliance with the method chosen should be assessed at every visit.
Case conclusion
Danielle chooses DMPA, and in the first 6 months, she gains 20 pounds. She is frustrated by the weight gain and chooses to change to the contraceptive implant. She continues to use condoms always and remains satisfied with her choice 1 year later.
CASE 3: TEEN WITH HISTORY OF MULTIPLE SEXUAL PARTNERS
Yolanda is a 17-year-old female with a history of multiple sexual partners who lives in an area of high human immunodeficiency virus (HIV) presence. In addition to strong and supportive counseling about risk reduction and condom use, she also needs a highly effective contraceptive method. Available options include progestin-only implants, progestin-only injectables, and combined hormonal methods.
In 2010, the CDC and WHO stated that women at high risk of HIV and those already positive for HIV or acquired immunodeficiency syndrome (AIDS) are eligible for LARC use (MEC category 1).16 In January/February 2012, the recommendations were updated to address several key questions about hormonal contraception and HIV, including the risk of HIV acquisition in noninfected women, the risk of HIV disease and progression among HIV-positive women, the risk of transmission from infected to noninfected male partners, and the potential for interactions between hormonal contraception and antiretrovirals.
The revisions declared that contraceptive implants, injectables, pills, and IUDs/IUSs were still usable with HIV risk, HIV positivity, and AIDS, but that women using progestin-only injectable contraception should be strongly advised to also always use condoms (male or female) and other HIV preventive measures.22
Case conclusion
Yolanda chooses an IUS, which she uses successfully for the next few years. She uses condoms sporadically, but has fewer partners per year than in prior years. At last screening, she was HIV negative. Motivational interviewing and counseling are used to increase her condom usage and to decrease the number of partners with whom she has sexual activity. Her knowledge of sexually transmitted infections and contraceptive efficacy has increased, and she is less ambivalent about navigating condom use with her current partner. She is scheduled for monthly visits to continue to work on motivation to use condoms consistently in order to remain HIV negative.
DISCUSSION
Where LARC access is widespread and sex education is comprehensive, teen pregnancy rates and abortion rates tend to decline. An initiative to increase LARC use in 13 countries with significant need for contraceptives but with low IUD use resulted in significant increase in their use.23 Initiatives were tailored to each of the countries using a variety of models and means of distribution to provide LARCs. The data suggest that creating demand and linking it with delivery can significantly increase LARC use.
Prevention of disease, teen pregnancy, and sequelae of disease are goals of enhancing adolescent access to LARCs. To achieve this, LARCs should be prescribed before patients need them. Teachable moments, such as patients presenting with potential pelvic inflammatory disease or asking for a pregnancy test, should be recognized. Discussions with these patients should present the pros and cons of LARCs along with addressing any barriers they have to their use.
Educate not just colleagues but pharmacists, parents, patients, schools, and communities. Employ and engage social media tools to remind adolescents to be safer. Do not allow barriers to prevent LARC usage, and train residents and students to do the same.
SUMMARY
Adolescents who are at risk of unintended pregnancy need access to highly effective contraceptive methods. For adolescents eligible to use all methods of contraception, LARCs are safe and may be particularly suitable for this population. Dual protection should be encouraged for adolescents.
Myths and misconceptions about all contraceptives, including LARCS, remain barriers to effective use. Health care providers are in a unique position to provide confidential care to adolescents and to educate youth about the various contraceptive options while separating myth from fact. Use of LARCs requires the patient’s consent, access to care, and affordable options. This requires clinicians to be knowledgeable about the most recent data on contraceptive efficacy and side effect profiles.
- Institute of Medicine (US). Initial national priorities for comparative effectiveness research. Washington, DC: National Academies Press; 2009.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion no. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol 2009; 114:1434–1438.
- Mirena full prescribing information. http://labeling.bayerhealthcare.com/html/products/pi/Mirena_PI.pdf. Accessed: July 31, 2015.
- Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group, The American College of Obstetricians and Gynecologists. Committee opinion no. 539: adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012; 120:983–988.
- Heikinheimo O, Inki P, Schmelter T, Gemzell-Danielsson K. Bleeding pattern and user satisfaction in second consecutive levonorgestrel-releasing intrauterine system users: results of a prospective 5-year study. Hum Reprod 2014; 29:1182–1188.
- Curtis KM, Marchbanks PA, Peterson HB. Neoplasia with use of intrauterine devices. Contraception 2007; 75(suppl 6):S60–S69.
- Stevens-Simon C, Kelly L, Singer D. Preventing repeat adolescent pregnancies with early adoption of the contraceptive implant. Fam Plann Perspect 1999; 31:88–93.
- Tocce K, Sheeder J, Python J, Teal SB. Long acting reversible contraception in postpartum adolescents: early initiation of etonogestrel implant is superior to IUDs in the outpatient setting. J Pediatr Adolesc Gynecol 2012; 25:59–63.
- Fleming KL, Sokoloff A, Raine TR. Attitudes and beliefs about the intrauterine device among teenagers and young women. Contraception 2010; 82:178–182.
- Stanwood NL, Bradley KA. Young pregnant women’s knowledge of modern intrauterine devices. Obstet Gynecol 2006; 108:1417–1422.
- Spies EL, Askelson NM, Gelman E, Losch M. Young women’s knowledge, attitudes, and behaviors related to long-acting reversible contraceptives. Womens Health Issues 2010; 20:394–399.
- Whitaker AK, Johnson LM, Harwood B, Chiappetta L, Creinin MD, Gold MA. Adolescent and young adult women’s knowledge of and attitudes toward the intrauterine device. Contraception 2008; 78:211–217.
- Kavanaugh ML, Frohwirth L, Jerman J, Popkin R, Ethier K. Long-acting reversible contraception for adolescents and young adults: patient and provider perspectives. J Pediatr Adolesc Gynecol 2013; 26:86–95.
- Mohllajee AP, Curtis KM, Peterson HB. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception 2006; 73:145–153.
- World Health Organization. Medical eligibility criteria for contraceptive use. 4th ed. Geneva, Switzerland: WHO Press; 2010. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf?ua=1. Accessed July 2, 2015.
- Centers for Disease Control and Prevention (CDC). U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep 2010; 59(RR-4):1–86.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol 2011; 117:1105–1113.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012; 366:1998–2007.
- Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med 2005; 159:139–144.
- Bonny AE. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006; 160:40–45.
- Beksinska ME, Smit JA, Kleinschmidt I, Milford C, Farley TM. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception 2010; 81:30–34.
- Centers for Disease Control and Prevention (CDC). Update to CDC’s U.S. Medical Eligibility Criteria for Contraceptive Use, 2010: revised recommendations for the use of hormonal contraception among women at high risk for HIV infection or infected with HIV. MMWR Morb Mortal Wkly Rep 2012; 61:449–452.
- Blumenthal PD, Shah NM, Jain K, et al. Revitalizing long-acting reversible contraceptives in settings with high unmet need: a multicountry experience matching demand creation and service delivery. Contraception 2013; 87:170–175.
Adolescents who are at risk of unintended pregnancy need access to highly effective contraceptives. Using a case study format, this article addresses the myths, misconceptions, and barriers to effective use of contraceptives, focusing on long-acting reversible contraceptives (LARCs) and suggesting ways to overcome these barriers.
CASE 1: TEEN WITH DYSMENORRHEA
Jessica is a 15-year-old girl presenting with complaints of severe cramps, causing her to miss school and other activities 3 to 4 days each month. She has had six sexual partners and believes that contraception would be a good idea. She also states that she hates shots and doesn’t swallow pills well. She asks you to help. What are her/your options?
In this case, options include chewable oral contraceptives, a contraceptive patch, the etonogestrel/ethinyl estradiol vaginal ring (NuvaRing), depot medroxyprogesterone acetate (DMPA; progestin-only, injectable, lasts 3 months), and LARCs, which are intrauterine systems (IUS), intrauterine devices (IUDs), and implants. If she can remember a chewable pill every day, that would be one option. The patch requires her to remember to change it weekly. The vaginal ring requires ability and motivation to insert and remove it vaginally each month. She has stated that she does not want shots, so DMPA is not a viable option.
In contrast, LARCs constitute “forgettable” contraception in that they are not dependent on daily or monthly investment of time and energy to use. With her dysmenorrhea, use of an LARC that contains a progestin to thin out her lining and/or induce amenorrhea has some additional advantages.
The Institute of Medicine has declared that expanding access to LARCs for young women is a national priority.1 In 2009, the American College of Obstetricians and Gynecologists encouraged implants and IUDs for nulliparous women and adolescents.2 The following review describes currently available LARCs.
Intrauterine systems (IUS)
The levonorgestrel-releasing IUS (Mirena) was approved by the US Food and Drug Administration (FDA) in 2000. It maintains efficacy for 5 years and has a failure rate of 0.2%. Contraception is reversible with its removal. The system consists of a small T-shaped frame with a steroid reservoir that releases 20 µg/day of levonorgestrel, resulting in high endometrial levels and low plasma levels of levonorgestrel. An alternate brand available in the US is Skyla, notable for its slightly smaller size, slightly higher expulsion rate, and similar side effect profile to Mirena.
The copper in the levonorgestrel-releasing IUS acts as a spermicide. The progestin thickens the cervical mucus and thins the endometrial lining to cause a marked reduction in uterine bleeding. Between 20% and 80% of recipients experience amenorrhea by 1 year.3–5 It is considered safe and effective, it provides prolonged relief of menstrual problems including menometrorrhagia. Because it contains only progestin, it can be used while breastfeeding. One drawback is the skill needed to insert the device, necessitating insertion by a clinician. Side effects include early spotting and rare instances of perforation of the uterus.3
Intrauterine devices (IUD)
The copper IUD (Paragard) was FDA approved in 1989 for 10 years of use, but it has been used off label for up to 12 years continuously. It is preferred by women who want to avoid hormones while achieving similar results as the levonorgestrel-releasing IUS, including reductions in menstrual bleeding. The copper IUD can be used in women with a history of ectopic pregnancy. Fertility returns after removal of the device. Its use has been associated with a reduction in the risk of endometrial cancer,6 which may be related to prevention of human papillomavirus infection. Insertion of the copper IUD is a relatively simple office procedure.
Implants
The etonorgestrel single-implant system (Implanon, Nexplanon) is a single rod containing 68 mg of the progestin etonorgestrel, which is the biologically active metabolite of desogestrel. The single rod eases implantation and removal compared with previous systems that contained six rods. The implant was FDA approved in 2006 but has been marketed worldwide since 1998. Nexplanon contains a single, radiopaque rod that is easier to localize and remove.
The duration of contraceptive efficacy for Nexplanon is 3 years. Etonorgestrel levels are undetectable within a few days of reversal. Breakthrough bleeding can occur, and depression and mood swings are potential side effects that are manageable with close follow-up. The implants can be removed at any time.
If breakthrough bleeding occurs while on progestin-only methods, an intermittent solution is to add estrogen by pill or patch for 3 weeks and then withdraw the estrogen until bleeding again occurs. This practice is usually not necessary by 12 months after implantation.
Implant use can reduce the repeat pregnancy rate among adolescents. In one study, researchers found that teenage mothers who chose a contraceptive implant during their first year postpartum, including the 37% who discontinued use, had a 2-year repeat pregnancy rate of 12% versus 46% among mothers using no method or other methods of contraception.7
Barriers to LARC use
Among adolescents attending an integrated prenatal and postpartum maternity clinic, 75% indicated intent to use LARCs postpartum. Approximately one-third chose an implant, one-third chose an IUS, and one-third chose either DMPA, oral contraceptives, a contraceptive patch, or a contraceptive ring. After 6 months, only 50% had received an LARC, leaving one-third at risk for rapid repeat pregnancy.8
Unfortunately, the safety, side effects, and efficacy of LARCs may be misunderstood by both clinicians and teens. A negative personal experience may dominate one’s thinking and act as a barrier to use. The adolescent may not be mature enough to understand the chance of pregnancy or its consequences. Use of an IUD or IUS requires planning, a visit to a clinic that can insert the device, and a substantial up-front expenditure, even though the average cost per year compares favorably to use of DMPA or oral contraceptives.
Lack of awareness of LARCs is another barrier to their use. Between 50% and 60% of young women have never heard of an IUD and 90% have no awareness of contraceptive implants.9–12 Of those who knew about them, only 25% knew that they were eligible to use LARCs.13
In addition, many practitioners still mistakenly believe that current IUDs can cause pelvic inflammatory disease (PID), despite there being no association between modern IUDs and PID after the first 20 days following insertion.14–16
Physicians may also be unaware of the medical eligibility criteria (MEC) for contraceptive use established by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC).15,16 Conditions affecting eligibility for the use of each contraceptive method are classified under four categories (Table 1).
Overall efficacy
The effectiveness of LARC use in young women has been established. In one large study,17 4,167 females aged 15 to 45 were offered contraception at no cost for 3 years. Of those who chose an LARC, the 12-month continuation rate was 86% compared with 55% among those choosing an oral contraceptive. Satisfaction rates reflect the continuation rates with more than 80% of LARC users being satisfied compared with 54% of oral contraceptive users being satisfied. The pregnancy rate was 22 times greater in women using short-acting contraceptives compared with LARC users. In women younger than 20, pregnancy rates were twice as high among oral contraceptive users.4,18
Case conclusion
Jessica chooses an IUS, and her adolescent-medicine physician inserts Mirena at her next visit. She has some irregular bleeding during the first 3 months, but by 1 year, she is having periods only every 5 to 6 months. She manages cramps with ibuprofen 400 mg orally every 6 hours and is careful not to miss ibuprofen doses when she starts cramping or bleeding. She has not had sexual activity since the insertion, but she plans to always use condoms when she chooses to have sex. At her 3-month visit after insertion, when considering whether to remove or continue with her IUS despite her initial unscheduled bleeding, she discusses the flexibility of IUS to allow her to change her mind: “It’s like changing my hairstyle; I can just come back and change it in 3 months or even sooner if it is really bothering me. I don’t have to think of it as permanent, just less of a daily bother.” She is pleased with her choice of LARC and plans to return in 6 months for follow-up.
CASE 2: TEEN REQUESTS RELIABLE CONTRACEPTION
Danielle is a 16-year-old nulliparous female currently using condoms for contraception but wants a more reliable method. Her options include an IUD/IUS (MEC 2 for women younger than 18 years), a contraceptive implant (MEC 1 for all ages), DMPA (MEC 2 for women younger than 18 years), and combined oral contraceptives (MEC 1 for all ages).
The use of DMPA by teenagers is worrisome because users experience a loss of 1% to 3% of bone mineral density (BMD) over 1 year, although BMD is regained after discontinuation.19 Whether BMD relates to fracture risk in adolescents is unclear, but there is no evidence that DMPA increases the risk. Nevertheless, a baseline BMD measurement repeated every other year is recommended for thin females taking DMPA. To slow potential bone loss, daily exercise and age-appropriate calcium and vitamin D intake should be encouraged in teens, who often do not get enough calcium.
Obese adolescents who use DMPA are more likely to gain weight than nonobese DMPA users and obese users of other contraceptive methods.20 Obese adolescents who use DMPA can gain as much as 10 kg.21
Any of the methods mentioned are options for contraception for Danielle, with continued use of condoms and counseling about dual protection. Compliance with the method chosen should be assessed at every visit.
Case conclusion
Danielle chooses DMPA, and in the first 6 months, she gains 20 pounds. She is frustrated by the weight gain and chooses to change to the contraceptive implant. She continues to use condoms always and remains satisfied with her choice 1 year later.
CASE 3: TEEN WITH HISTORY OF MULTIPLE SEXUAL PARTNERS
Yolanda is a 17-year-old female with a history of multiple sexual partners who lives in an area of high human immunodeficiency virus (HIV) presence. In addition to strong and supportive counseling about risk reduction and condom use, she also needs a highly effective contraceptive method. Available options include progestin-only implants, progestin-only injectables, and combined hormonal methods.
In 2010, the CDC and WHO stated that women at high risk of HIV and those already positive for HIV or acquired immunodeficiency syndrome (AIDS) are eligible for LARC use (MEC category 1).16 In January/February 2012, the recommendations were updated to address several key questions about hormonal contraception and HIV, including the risk of HIV acquisition in noninfected women, the risk of HIV disease and progression among HIV-positive women, the risk of transmission from infected to noninfected male partners, and the potential for interactions between hormonal contraception and antiretrovirals.
The revisions declared that contraceptive implants, injectables, pills, and IUDs/IUSs were still usable with HIV risk, HIV positivity, and AIDS, but that women using progestin-only injectable contraception should be strongly advised to also always use condoms (male or female) and other HIV preventive measures.22
Case conclusion
Yolanda chooses an IUS, which she uses successfully for the next few years. She uses condoms sporadically, but has fewer partners per year than in prior years. At last screening, she was HIV negative. Motivational interviewing and counseling are used to increase her condom usage and to decrease the number of partners with whom she has sexual activity. Her knowledge of sexually transmitted infections and contraceptive efficacy has increased, and she is less ambivalent about navigating condom use with her current partner. She is scheduled for monthly visits to continue to work on motivation to use condoms consistently in order to remain HIV negative.
DISCUSSION
Where LARC access is widespread and sex education is comprehensive, teen pregnancy rates and abortion rates tend to decline. An initiative to increase LARC use in 13 countries with significant need for contraceptives but with low IUD use resulted in significant increase in their use.23 Initiatives were tailored to each of the countries using a variety of models and means of distribution to provide LARCs. The data suggest that creating demand and linking it with delivery can significantly increase LARC use.
Prevention of disease, teen pregnancy, and sequelae of disease are goals of enhancing adolescent access to LARCs. To achieve this, LARCs should be prescribed before patients need them. Teachable moments, such as patients presenting with potential pelvic inflammatory disease or asking for a pregnancy test, should be recognized. Discussions with these patients should present the pros and cons of LARCs along with addressing any barriers they have to their use.
Educate not just colleagues but pharmacists, parents, patients, schools, and communities. Employ and engage social media tools to remind adolescents to be safer. Do not allow barriers to prevent LARC usage, and train residents and students to do the same.
SUMMARY
Adolescents who are at risk of unintended pregnancy need access to highly effective contraceptive methods. For adolescents eligible to use all methods of contraception, LARCs are safe and may be particularly suitable for this population. Dual protection should be encouraged for adolescents.
Myths and misconceptions about all contraceptives, including LARCS, remain barriers to effective use. Health care providers are in a unique position to provide confidential care to adolescents and to educate youth about the various contraceptive options while separating myth from fact. Use of LARCs requires the patient’s consent, access to care, and affordable options. This requires clinicians to be knowledgeable about the most recent data on contraceptive efficacy and side effect profiles.
Adolescents who are at risk of unintended pregnancy need access to highly effective contraceptives. Using a case study format, this article addresses the myths, misconceptions, and barriers to effective use of contraceptives, focusing on long-acting reversible contraceptives (LARCs) and suggesting ways to overcome these barriers.
CASE 1: TEEN WITH DYSMENORRHEA
Jessica is a 15-year-old girl presenting with complaints of severe cramps, causing her to miss school and other activities 3 to 4 days each month. She has had six sexual partners and believes that contraception would be a good idea. She also states that she hates shots and doesn’t swallow pills well. She asks you to help. What are her/your options?
In this case, options include chewable oral contraceptives, a contraceptive patch, the etonogestrel/ethinyl estradiol vaginal ring (NuvaRing), depot medroxyprogesterone acetate (DMPA; progestin-only, injectable, lasts 3 months), and LARCs, which are intrauterine systems (IUS), intrauterine devices (IUDs), and implants. If she can remember a chewable pill every day, that would be one option. The patch requires her to remember to change it weekly. The vaginal ring requires ability and motivation to insert and remove it vaginally each month. She has stated that she does not want shots, so DMPA is not a viable option.
In contrast, LARCs constitute “forgettable” contraception in that they are not dependent on daily or monthly investment of time and energy to use. With her dysmenorrhea, use of an LARC that contains a progestin to thin out her lining and/or induce amenorrhea has some additional advantages.
The Institute of Medicine has declared that expanding access to LARCs for young women is a national priority.1 In 2009, the American College of Obstetricians and Gynecologists encouraged implants and IUDs for nulliparous women and adolescents.2 The following review describes currently available LARCs.
Intrauterine systems (IUS)
The levonorgestrel-releasing IUS (Mirena) was approved by the US Food and Drug Administration (FDA) in 2000. It maintains efficacy for 5 years and has a failure rate of 0.2%. Contraception is reversible with its removal. The system consists of a small T-shaped frame with a steroid reservoir that releases 20 µg/day of levonorgestrel, resulting in high endometrial levels and low plasma levels of levonorgestrel. An alternate brand available in the US is Skyla, notable for its slightly smaller size, slightly higher expulsion rate, and similar side effect profile to Mirena.
The copper in the levonorgestrel-releasing IUS acts as a spermicide. The progestin thickens the cervical mucus and thins the endometrial lining to cause a marked reduction in uterine bleeding. Between 20% and 80% of recipients experience amenorrhea by 1 year.3–5 It is considered safe and effective, it provides prolonged relief of menstrual problems including menometrorrhagia. Because it contains only progestin, it can be used while breastfeeding. One drawback is the skill needed to insert the device, necessitating insertion by a clinician. Side effects include early spotting and rare instances of perforation of the uterus.3
Intrauterine devices (IUD)
The copper IUD (Paragard) was FDA approved in 1989 for 10 years of use, but it has been used off label for up to 12 years continuously. It is preferred by women who want to avoid hormones while achieving similar results as the levonorgestrel-releasing IUS, including reductions in menstrual bleeding. The copper IUD can be used in women with a history of ectopic pregnancy. Fertility returns after removal of the device. Its use has been associated with a reduction in the risk of endometrial cancer,6 which may be related to prevention of human papillomavirus infection. Insertion of the copper IUD is a relatively simple office procedure.
Implants
The etonorgestrel single-implant system (Implanon, Nexplanon) is a single rod containing 68 mg of the progestin etonorgestrel, which is the biologically active metabolite of desogestrel. The single rod eases implantation and removal compared with previous systems that contained six rods. The implant was FDA approved in 2006 but has been marketed worldwide since 1998. Nexplanon contains a single, radiopaque rod that is easier to localize and remove.
The duration of contraceptive efficacy for Nexplanon is 3 years. Etonorgestrel levels are undetectable within a few days of reversal. Breakthrough bleeding can occur, and depression and mood swings are potential side effects that are manageable with close follow-up. The implants can be removed at any time.
If breakthrough bleeding occurs while on progestin-only methods, an intermittent solution is to add estrogen by pill or patch for 3 weeks and then withdraw the estrogen until bleeding again occurs. This practice is usually not necessary by 12 months after implantation.
Implant use can reduce the repeat pregnancy rate among adolescents. In one study, researchers found that teenage mothers who chose a contraceptive implant during their first year postpartum, including the 37% who discontinued use, had a 2-year repeat pregnancy rate of 12% versus 46% among mothers using no method or other methods of contraception.7
Barriers to LARC use
Among adolescents attending an integrated prenatal and postpartum maternity clinic, 75% indicated intent to use LARCs postpartum. Approximately one-third chose an implant, one-third chose an IUS, and one-third chose either DMPA, oral contraceptives, a contraceptive patch, or a contraceptive ring. After 6 months, only 50% had received an LARC, leaving one-third at risk for rapid repeat pregnancy.8
Unfortunately, the safety, side effects, and efficacy of LARCs may be misunderstood by both clinicians and teens. A negative personal experience may dominate one’s thinking and act as a barrier to use. The adolescent may not be mature enough to understand the chance of pregnancy or its consequences. Use of an IUD or IUS requires planning, a visit to a clinic that can insert the device, and a substantial up-front expenditure, even though the average cost per year compares favorably to use of DMPA or oral contraceptives.
Lack of awareness of LARCs is another barrier to their use. Between 50% and 60% of young women have never heard of an IUD and 90% have no awareness of contraceptive implants.9–12 Of those who knew about them, only 25% knew that they were eligible to use LARCs.13
In addition, many practitioners still mistakenly believe that current IUDs can cause pelvic inflammatory disease (PID), despite there being no association between modern IUDs and PID after the first 20 days following insertion.14–16
Physicians may also be unaware of the medical eligibility criteria (MEC) for contraceptive use established by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC).15,16 Conditions affecting eligibility for the use of each contraceptive method are classified under four categories (Table 1).
Overall efficacy
The effectiveness of LARC use in young women has been established. In one large study,17 4,167 females aged 15 to 45 were offered contraception at no cost for 3 years. Of those who chose an LARC, the 12-month continuation rate was 86% compared with 55% among those choosing an oral contraceptive. Satisfaction rates reflect the continuation rates with more than 80% of LARC users being satisfied compared with 54% of oral contraceptive users being satisfied. The pregnancy rate was 22 times greater in women using short-acting contraceptives compared with LARC users. In women younger than 20, pregnancy rates were twice as high among oral contraceptive users.4,18
Case conclusion
Jessica chooses an IUS, and her adolescent-medicine physician inserts Mirena at her next visit. She has some irregular bleeding during the first 3 months, but by 1 year, she is having periods only every 5 to 6 months. She manages cramps with ibuprofen 400 mg orally every 6 hours and is careful not to miss ibuprofen doses when she starts cramping or bleeding. She has not had sexual activity since the insertion, but she plans to always use condoms when she chooses to have sex. At her 3-month visit after insertion, when considering whether to remove or continue with her IUS despite her initial unscheduled bleeding, she discusses the flexibility of IUS to allow her to change her mind: “It’s like changing my hairstyle; I can just come back and change it in 3 months or even sooner if it is really bothering me. I don’t have to think of it as permanent, just less of a daily bother.” She is pleased with her choice of LARC and plans to return in 6 months for follow-up.
CASE 2: TEEN REQUESTS RELIABLE CONTRACEPTION
Danielle is a 16-year-old nulliparous female currently using condoms for contraception but wants a more reliable method. Her options include an IUD/IUS (MEC 2 for women younger than 18 years), a contraceptive implant (MEC 1 for all ages), DMPA (MEC 2 for women younger than 18 years), and combined oral contraceptives (MEC 1 for all ages).
The use of DMPA by teenagers is worrisome because users experience a loss of 1% to 3% of bone mineral density (BMD) over 1 year, although BMD is regained after discontinuation.19 Whether BMD relates to fracture risk in adolescents is unclear, but there is no evidence that DMPA increases the risk. Nevertheless, a baseline BMD measurement repeated every other year is recommended for thin females taking DMPA. To slow potential bone loss, daily exercise and age-appropriate calcium and vitamin D intake should be encouraged in teens, who often do not get enough calcium.
Obese adolescents who use DMPA are more likely to gain weight than nonobese DMPA users and obese users of other contraceptive methods.20 Obese adolescents who use DMPA can gain as much as 10 kg.21
Any of the methods mentioned are options for contraception for Danielle, with continued use of condoms and counseling about dual protection. Compliance with the method chosen should be assessed at every visit.
Case conclusion
Danielle chooses DMPA, and in the first 6 months, she gains 20 pounds. She is frustrated by the weight gain and chooses to change to the contraceptive implant. She continues to use condoms always and remains satisfied with her choice 1 year later.
CASE 3: TEEN WITH HISTORY OF MULTIPLE SEXUAL PARTNERS
Yolanda is a 17-year-old female with a history of multiple sexual partners who lives in an area of high human immunodeficiency virus (HIV) presence. In addition to strong and supportive counseling about risk reduction and condom use, she also needs a highly effective contraceptive method. Available options include progestin-only implants, progestin-only injectables, and combined hormonal methods.
In 2010, the CDC and WHO stated that women at high risk of HIV and those already positive for HIV or acquired immunodeficiency syndrome (AIDS) are eligible for LARC use (MEC category 1).16 In January/February 2012, the recommendations were updated to address several key questions about hormonal contraception and HIV, including the risk of HIV acquisition in noninfected women, the risk of HIV disease and progression among HIV-positive women, the risk of transmission from infected to noninfected male partners, and the potential for interactions between hormonal contraception and antiretrovirals.
The revisions declared that contraceptive implants, injectables, pills, and IUDs/IUSs were still usable with HIV risk, HIV positivity, and AIDS, but that women using progestin-only injectable contraception should be strongly advised to also always use condoms (male or female) and other HIV preventive measures.22
Case conclusion
Yolanda chooses an IUS, which she uses successfully for the next few years. She uses condoms sporadically, but has fewer partners per year than in prior years. At last screening, she was HIV negative. Motivational interviewing and counseling are used to increase her condom usage and to decrease the number of partners with whom she has sexual activity. Her knowledge of sexually transmitted infections and contraceptive efficacy has increased, and she is less ambivalent about navigating condom use with her current partner. She is scheduled for monthly visits to continue to work on motivation to use condoms consistently in order to remain HIV negative.
DISCUSSION
Where LARC access is widespread and sex education is comprehensive, teen pregnancy rates and abortion rates tend to decline. An initiative to increase LARC use in 13 countries with significant need for contraceptives but with low IUD use resulted in significant increase in their use.23 Initiatives were tailored to each of the countries using a variety of models and means of distribution to provide LARCs. The data suggest that creating demand and linking it with delivery can significantly increase LARC use.
Prevention of disease, teen pregnancy, and sequelae of disease are goals of enhancing adolescent access to LARCs. To achieve this, LARCs should be prescribed before patients need them. Teachable moments, such as patients presenting with potential pelvic inflammatory disease or asking for a pregnancy test, should be recognized. Discussions with these patients should present the pros and cons of LARCs along with addressing any barriers they have to their use.
Educate not just colleagues but pharmacists, parents, patients, schools, and communities. Employ and engage social media tools to remind adolescents to be safer. Do not allow barriers to prevent LARC usage, and train residents and students to do the same.
SUMMARY
Adolescents who are at risk of unintended pregnancy need access to highly effective contraceptive methods. For adolescents eligible to use all methods of contraception, LARCs are safe and may be particularly suitable for this population. Dual protection should be encouraged for adolescents.
Myths and misconceptions about all contraceptives, including LARCS, remain barriers to effective use. Health care providers are in a unique position to provide confidential care to adolescents and to educate youth about the various contraceptive options while separating myth from fact. Use of LARCs requires the patient’s consent, access to care, and affordable options. This requires clinicians to be knowledgeable about the most recent data on contraceptive efficacy and side effect profiles.
- Institute of Medicine (US). Initial national priorities for comparative effectiveness research. Washington, DC: National Academies Press; 2009.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion no. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol 2009; 114:1434–1438.
- Mirena full prescribing information. http://labeling.bayerhealthcare.com/html/products/pi/Mirena_PI.pdf. Accessed: July 31, 2015.
- Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group, The American College of Obstetricians and Gynecologists. Committee opinion no. 539: adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012; 120:983–988.
- Heikinheimo O, Inki P, Schmelter T, Gemzell-Danielsson K. Bleeding pattern and user satisfaction in second consecutive levonorgestrel-releasing intrauterine system users: results of a prospective 5-year study. Hum Reprod 2014; 29:1182–1188.
- Curtis KM, Marchbanks PA, Peterson HB. Neoplasia with use of intrauterine devices. Contraception 2007; 75(suppl 6):S60–S69.
- Stevens-Simon C, Kelly L, Singer D. Preventing repeat adolescent pregnancies with early adoption of the contraceptive implant. Fam Plann Perspect 1999; 31:88–93.
- Tocce K, Sheeder J, Python J, Teal SB. Long acting reversible contraception in postpartum adolescents: early initiation of etonogestrel implant is superior to IUDs in the outpatient setting. J Pediatr Adolesc Gynecol 2012; 25:59–63.
- Fleming KL, Sokoloff A, Raine TR. Attitudes and beliefs about the intrauterine device among teenagers and young women. Contraception 2010; 82:178–182.
- Stanwood NL, Bradley KA. Young pregnant women’s knowledge of modern intrauterine devices. Obstet Gynecol 2006; 108:1417–1422.
- Spies EL, Askelson NM, Gelman E, Losch M. Young women’s knowledge, attitudes, and behaviors related to long-acting reversible contraceptives. Womens Health Issues 2010; 20:394–399.
- Whitaker AK, Johnson LM, Harwood B, Chiappetta L, Creinin MD, Gold MA. Adolescent and young adult women’s knowledge of and attitudes toward the intrauterine device. Contraception 2008; 78:211–217.
- Kavanaugh ML, Frohwirth L, Jerman J, Popkin R, Ethier K. Long-acting reversible contraception for adolescents and young adults: patient and provider perspectives. J Pediatr Adolesc Gynecol 2013; 26:86–95.
- Mohllajee AP, Curtis KM, Peterson HB. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception 2006; 73:145–153.
- World Health Organization. Medical eligibility criteria for contraceptive use. 4th ed. Geneva, Switzerland: WHO Press; 2010. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf?ua=1. Accessed July 2, 2015.
- Centers for Disease Control and Prevention (CDC). U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep 2010; 59(RR-4):1–86.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol 2011; 117:1105–1113.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012; 366:1998–2007.
- Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med 2005; 159:139–144.
- Bonny AE. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006; 160:40–45.
- Beksinska ME, Smit JA, Kleinschmidt I, Milford C, Farley TM. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception 2010; 81:30–34.
- Centers for Disease Control and Prevention (CDC). Update to CDC’s U.S. Medical Eligibility Criteria for Contraceptive Use, 2010: revised recommendations for the use of hormonal contraception among women at high risk for HIV infection or infected with HIV. MMWR Morb Mortal Wkly Rep 2012; 61:449–452.
- Blumenthal PD, Shah NM, Jain K, et al. Revitalizing long-acting reversible contraceptives in settings with high unmet need: a multicountry experience matching demand creation and service delivery. Contraception 2013; 87:170–175.
- Institute of Medicine (US). Initial national priorities for comparative effectiveness research. Washington, DC: National Academies Press; 2009.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion no. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol 2009; 114:1434–1438.
- Mirena full prescribing information. http://labeling.bayerhealthcare.com/html/products/pi/Mirena_PI.pdf. Accessed: July 31, 2015.
- Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group, The American College of Obstetricians and Gynecologists. Committee opinion no. 539: adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012; 120:983–988.
- Heikinheimo O, Inki P, Schmelter T, Gemzell-Danielsson K. Bleeding pattern and user satisfaction in second consecutive levonorgestrel-releasing intrauterine system users: results of a prospective 5-year study. Hum Reprod 2014; 29:1182–1188.
- Curtis KM, Marchbanks PA, Peterson HB. Neoplasia with use of intrauterine devices. Contraception 2007; 75(suppl 6):S60–S69.
- Stevens-Simon C, Kelly L, Singer D. Preventing repeat adolescent pregnancies with early adoption of the contraceptive implant. Fam Plann Perspect 1999; 31:88–93.
- Tocce K, Sheeder J, Python J, Teal SB. Long acting reversible contraception in postpartum adolescents: early initiation of etonogestrel implant is superior to IUDs in the outpatient setting. J Pediatr Adolesc Gynecol 2012; 25:59–63.
- Fleming KL, Sokoloff A, Raine TR. Attitudes and beliefs about the intrauterine device among teenagers and young women. Contraception 2010; 82:178–182.
- Stanwood NL, Bradley KA. Young pregnant women’s knowledge of modern intrauterine devices. Obstet Gynecol 2006; 108:1417–1422.
- Spies EL, Askelson NM, Gelman E, Losch M. Young women’s knowledge, attitudes, and behaviors related to long-acting reversible contraceptives. Womens Health Issues 2010; 20:394–399.
- Whitaker AK, Johnson LM, Harwood B, Chiappetta L, Creinin MD, Gold MA. Adolescent and young adult women’s knowledge of and attitudes toward the intrauterine device. Contraception 2008; 78:211–217.
- Kavanaugh ML, Frohwirth L, Jerman J, Popkin R, Ethier K. Long-acting reversible contraception for adolescents and young adults: patient and provider perspectives. J Pediatr Adolesc Gynecol 2013; 26:86–95.
- Mohllajee AP, Curtis KM, Peterson HB. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception 2006; 73:145–153.
- World Health Organization. Medical eligibility criteria for contraceptive use. 4th ed. Geneva, Switzerland: WHO Press; 2010. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf?ua=1. Accessed July 2, 2015.
- Centers for Disease Control and Prevention (CDC). U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep 2010; 59(RR-4):1–86.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol 2011; 117:1105–1113.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012; 366:1998–2007.
- Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med 2005; 159:139–144.
- Bonny AE. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006; 160:40–45.
- Beksinska ME, Smit JA, Kleinschmidt I, Milford C, Farley TM. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception 2010; 81:30–34.
- Centers for Disease Control and Prevention (CDC). Update to CDC’s U.S. Medical Eligibility Criteria for Contraceptive Use, 2010: revised recommendations for the use of hormonal contraception among women at high risk for HIV infection or infected with HIV. MMWR Morb Mortal Wkly Rep 2012; 61:449–452.
- Blumenthal PD, Shah NM, Jain K, et al. Revitalizing long-acting reversible contraceptives in settings with high unmet need: a multicountry experience matching demand creation and service delivery. Contraception 2013; 87:170–175.
RSV infections: State of the art
Understanding of respiratory syncytial virus (RSV) infection has increased substantially since its discovery, but a curative treatment remains elusive. Insights into the impact of gestational age, epidemiologic patterns, virus incubation and proliferation, pathogenesis and pathophysiology, and host immune response have set the stage for preventive measures and effective therapy. In today’s clinical practice, a specific humanized antibody is administered to high-risk infants, which is safe and effective in preventing RSV-related acute and postacute symptoms.
VIRUS STRUCTURE AND CLASSIFICATION
Knowledge of the molecular structure of RSV is important because it gives clues as to how it infects the human airways (Figure 1). The virus is made of a single strand of RNA contained in a nucleocapsid made of only 11 proteins and covered by a lipid envelope. Essential for the virulence of RSV are the surface glycoproteins G and F (fusion) that attach to the host airway epithelial cells and merge the viral envelope to the membranes of multiple adjacent cells, creating the “syncytia” that give RSV its name. G and F proteins are also the principal antigens exposed to the host immune system, and therefore the patient’s neutralizing antibodies are primarily directed against these targets.1
Human RSV is a Pneumovirus belonging to the Paramyxoviridae family. There are two strains of RSV (A and B) found in infants. Approximately 60% of all lower respiratory infections in preschool-aged children worldwide are caused by RSV (Figure 2).2 All the other viruses—including influenza, parainfluenza, metapneumovirus, and adenoviruses—as well as bacterial infections are much less common during the first years of life.
VIROLOGY
The burden of RSV continues to grow worldwide, particularly among the youngest segment of the population.2 During the first year of life, infants are not adequately protected from RSV by maternal immunoglobulins. Thus, approximately 80% of lower respiratory infections in children younger than 1 year are due to RSV with peak incidence occurring at 2 to 3 months of age. In the United States, bronchiolitis-associated hospitalizations in infants younger than 12 months more than doubled between 1980 and 1996, and in 1996, accounted for approximately 3% of all pediatric hospitalizations. Up to 120,000 RSV-related pediatric hospitalizations per year occur during a typical season.3
Mortality rates have improved significantly—at least in industrialized countries—and now are probably less than 1%.4 In the United States, RSV is estimated to cause fewer than 100 deaths annually, primarily because of better supportive care and the use of mechanical ventilation.
The seasonality of RSV varies by region. In the northern United States from October through March, RSV causes up to 90% of lower respiratory infection cases in infants. In the southern United States and farther into the southern hemisphere, the virus becomes endemic and can be present throughout the year with peak incidence occurring from August to September.2
RSV RISK FACTORS
The severity and frequency of RSV infection is influenced by well-known risk factors including environmental overcrowding, absence of breastfeeding, and immunosuppression. Children with chronic lung disease are predisposed to clinically significant RSV infection by their limited respiratory reserve, and 60% of those affected will be hospitalized. Hemodynamically significant congenital heart defects associated with higher pulmonary blood flow also increase risk for severe RSV disease, with more than 50% of children infected requiring hospitalization.4
Prematurity and increased RSV risk
Premature-born infants have a 10-fold increased risk of RSV infection and account for 25% to 30% of RSV hospitalizations annually.4 Prematurity as a risk factor for RSV is primarily tied to the physiology of placental immunoglobulin G (IgG) transfer. The human placenta is not permeable to IgG during the first half of pregnancy because of low expression of the Fc receptor needed to bind immunoglobulins and transfer them into the fetal circulation. Further, maternal IgG is recognized as a foreign protein by the newborn and is progressively removed from the circulation by the liver.5
Thus, the IgG decline continues postnatally, reaching the lowest concentration at 2 to 3 months of age because newborns are unable to synthesize their own antibodies. During this time, full-term born babies are at the highest risk for developing RSV infection. The risk is logically higher for premature infants who lack the full benefit of IgG transfer occurring during the last trimester, rendering antibody levels even lower at nadir. The greater the prematurity, the less the antibody protection and the more the predisposition to RSV infection.5
CLINICAL MANIFESTATIONS
The hallmark signs of RSV bronchiolitis are wheezing, cough, and increased work of breathing caused by infection of the bronchiolar airways. However, the nasal phase of the infection may cause irritation and trigeminal nerve activation associated with sneezing, congestion, and apnea. Indeed, approximately 20% of infants manifest an apnea episode as the first symptom of infection.6
The specific etiology of RSV can be confirmed by antigen detection tests, currently being replaced by more sensitive polymerase chain reaction (PCR)-based assays. Chest radiography reveals obvious signs of RSV lower respiratory infection, typically patch atelectasis, increased peribronchial markings, and bilateral hyperinflation with flattening of the diaphragm. Current guidelines indicate that the diagnosis of acute bronchiolitis should be based exclusively on the history and physical exam; it does not require radiographic or laboratory studies.
Correct etiologic diagnosis, however, is crucial to avoid unnecessary therapies and rule out rare conditions that could be worsened by therapies commonly used for bronchiolitis. For example, infants with dilated cardiomyopathy and congestive heart failure may present with symptoms of wheezing that mimic an acute respiratory infection, but these patients are at risk of developing supraventricular tachycardia and even cardiopulmonary collapse associated with administration of beta-agonist agents. In such cases, a chest radiograph will show cardiomegaly suggesting a different diagnosis and therapy, and thus avoid significant complications.
Recurrent wheezing
Children infected with RSV during their first year of life develop a higher risk of subsequent episodes of bronchial obstruction. Several retrospective studies conducted in the 1980s explored the incidence of lower respiratory symptoms continuing for years after initial RSV infection. Particularly unexpected was the finding that at least one-third of children hospitalized with RSV bronchiolitis will continue to wheeze beyond 6 to 8 years of age (Figure 3).7–9
More recent prospective studies of recurrent wheezing following RSV infection have been conducted. A Swedish study of 47 infants hospitalized with culture-proven RSV bronchiolitis showed significantly increased physician-diagnosed asthma (38%) compared with the 93 controls (2%) at age 7.5 years (P < .0001).10 Follow-up studies of the same cohort found increased risk for recurrent wheezing or asthma in the RSV group still present at age 13 years11 and 18 years12 compared with controls.
Asthma
History of RSV infection is important both as a trigger of asthma attacks and in the inception of asthma. Family history of asthma is the most important marker of a genetic predisposition to develop asthma. A multivariate analysis of the Swedish cohort discussed above showed that children with neither previous RSV infection nor family history of asthma had approximately a 5% risk of developing asthma by age 7.5 years. None of the children with a family history of asthma but no history of RSV infection developed asthma during follow-up. Children who had an RSV infection without a familial predisposition to asthma had approximately a 20% risk of asthma development. Children with both previous RSV infection and a family history of asthma had the highest (~40%) risk of developing asthma.10
These results provide important information about asthma pathogenesis in early life. The main implication is that even if a child has a genetic predisposition to asthma development, clinical manifestations will not develop without exposure to environmental agents that allow the actual expression of the predisposing genes, such as pollution, unbalanced diet, or early-life RSV infection. On the other hand, children without genetic predisposition can present with clinical manifestations undistinguishable from atopic asthma if their respiratory tract is exposed to specific viral pathogens during crucial developmental windows in early life. Finally, the combination of genetic predisposition and adverse environmental exposures in infancy carries the highest risk of asthma.
RSV MANAGEMENT
Ribavirin
The life cycle of RSV after the initial infection affects treatment strategies. RSV produces no symptoms for at least 3 to 5 days following infection (“eclipse phase”), during which time it reproduces exponentially and reaches the lungs, causing the first symptoms to be observable after 5 to 7 days. By the time the first symptoms emerge, the virus is already rapidly disappearing from the system.13 If ribavirin is administered at this point, the pediatrician has provided a virostatic agent against a virus that is no longer present, and therapy will not only be ineffective, but it may trigger bronchospasms.
The American Academy of Pediatrics (AAP) Committee on Infectious Diseases supported the use of ribavirin in 1993 guidelines,14 but changed their recommendation to “may be considered” in 1996.15 The only exception for ribavirin use is in the immunocompromised patient with RSV. In this scenario, the virus continues to replicate for months after the initial infection unopposed by the host defense mechanisms; therefore, aerosolized ribavirin therapy should be considered, either alone or in combination with humanized anti-RSV antibody.
Other treatments
Corticosteroids have not been shown to have a significant effect on RSV bronchiolitis.16 Alpha- and beta-adrenergic bronchodilators, such as epinephrine and albuterol, have shown very little or no effect on RSV symptoms in multiple controlled trials. The only effective treatment with proven efficacy is supportive therapy with adequate fluid intake and oxygen. Oral feeding should be withheld in patients with high respiratory rates to prevent aspiration.
PROPHYLAXIS
Vaccines
Vaccines have been ineffective against RSV and can be dangerous in children. RSV is not a strongly cytopathic virus; illness results primarily from the host immune response against the infection rather than from the virus itself.16 Therefore, any vaccine carries the risk of creating a stronger and potentially dangerous immune response to the next infection.
In the 1960s, a formalin-inactivated RSV vaccine was introduced with deleterious outcomes—it was minimally protective and was responsible for infant deaths. Because mortality was not immediate, the vaccine continued to be administered throughout the season. Unfortunately, with their immunity modified, vaccinated children became severely ill when they came in contact with the wild-type virus during the year following vaccination. The withdrawal of the formalin-inactivated vaccine still represents an important precedent that makes investigators and regulatory bodies very cautious about active immunization against this virus.
Palivizumab
Palivizumab is a 96% human monoclonal antibody targeting the RSV F protein, and it offers passive immunity for infants at risk for severe infection. The Impact-RSV clinical trial of palivizumab showed that five monthly intramuscular injections effectively reduced RSV hospitalizations by 78% in premature infants from 32 to 35 weeks gestation without bronchopulmonary dysplasia (BPD). Treatment offered only a 39% reduction in premature infants with BPD.17
The most recent AAP guidelines18 recommend palivizumab prophylaxis with a maximum of five monthly doses only in the first year of life for otherwise healthy infants born before 29 weeks gestation and for infants born before 32 weeks gestation with chronic lung disease of prematurity (CLD) defined as a requirement for supplemental oxygen for at least 28 days after birth. Prophylaxis is no longer recommended in the second year of life, except for infants with CLD still requiring oxygen, corticosteroids, or diuretics. Palivizumab prophylaxis should be discontinued if a breakthrough RSV hospitalization occurs because the likelihood of a second RSV hospitalization in the same season is low.
Palivizumab also should be considered for children with hemodynamically significant congenital heart defects, profound immunodeficiency, and pulmonary or neuromuscular pathologies impairing airway clearance; however, no formal recommendation was made for patients with Down syndrome or cystic fibrosis due to insufficient data.18 The protective effect of palivizumab appears to be cumulative, with almost half of the breakthrough infections observed in the clinical trials occurring after the first injection or immediately following the second.17
A recent randomized, double-blind, placebo-controlled trial has shown that palivizumab given to premature infants during the first year of life provides a 40% to 60% reduction of wheezing episodes.19 This trial confirms the results of previous retrospective studies; however, further large multicenter trials of palivizumab prophylaxis in both premature and full-term infants are needed to assess protection against recurrent wheezing and asthma, and to formulate evidence-based recommendations.
FUTURE DIRECTIONS
Nebulization of 3% saline improves mucociliary clearance and is increasingly being used in airway diseases involving mucus plugging (eg, cystic fibrosis). It also has been reported to reduce length of hospital stay and provide symptomatic relief in patients with bronchiolitis, but its use remains controversial. In particular, it has not been shown to be effective at reducing hospitalization when used in emergency settings. Therefore, based on current evidence, the administration of hypertonic saline for bronchiolitis should be limited to hospitalized infants.
Anti-leukotrienes used during the acute phase of RSV bronchiolitis have been shown to improve post-bronchiolitis respiratory symptoms, especially in younger patients with high urinary leukotriene E4 (LTE4). However, a large, multicenter, randomized, double-blind, placebo-controlled trial with montelukast failed to show statistically significant clinical improvement. A post-hoc data analysis revealed that children with persistent respiratory symptoms after the acute phase of the infection may benefit from montelukast, but the manufacturer (Merck) is no longer pursuing this indication.
There is not enough scientific evidence at present to support the use of DNAse or exogenous surfactant in the setting of acute bronchiolitis. New generations of specific antivirals and vaccines based on immunogens offer hope as new options for prophylaxis and/or management of RSV infection in the not too distant future.
Recent basic science investigations have provided new findings that could influence future therapies. Some of these studies have suggested that the tropism of RSV is not solely for the airway epithelium; rather, this virus can spread hematogenously to infect extrapulmonary targets, particularly the bone marrow stromal cells where RSV finds a sanctuary niche shielding it from immune protection and allowing subclinical latency. In one study, RSV was found in the bone marrow of every child and in about 80% of adults tested.20 Similar findings were replicated with other pathogens like Mycobacterium tuberculosis.21 This discovery could direct future treatment strategies for a number of viral, bacterial, and parasitic infections.
In another study, transplacental transmission of RSV from the mother’s airways to fetal lung tissues was shown for the first time in an animal model of infection. When the virus enters fetal respiratory cells, it persists and induces the expression of neurotrophic factors and receptors resulting in postnatal airways with dramatically increased parasympathetic innervation and methacholine reactivity. These structural and functional changes provide a suitable model to explain the development of long-term bronchial hyperreactivity following re-exposure to RSV in early life.22
In conclusion, the search for a cure for the most common respiratory infection in children has humbled several generations of investigators through the more than 50 years since RSV was discovered. Nevertheless, the rapid evolution in the fields of virology and immunology and recent breakthrough discoveries promise to deliver new strategies that may make a difference in the management of this common infection and its long-term sequelae.
- Peters TR, Crowe JE Jr. Respiratory syncytial virus. In: Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008.
- Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35:519–530.
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999; 282:1440–1446.
- Simoes EA, Rieger CHL. RSV infection in developed and developing countries. Infect Med 1999; 16:S11–S17.
- Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1,500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res 1986; 20:899 –904.
- Sabogal C, Auais A, Napchan G, et al. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 2005; 57:819–825.
- Hall CB, Hall WJ, Gala CL, MaGill FB, Leddy JP. Long-term prospective study in children after respiratory syncytial virus infection. J Pediatr 1984; 105:358–364.
- Henry RL, Hodges IGC, Milner AD, Stokes GM. Respiratory problems 2 years after acute bronchiolitis in infancy. Arch Dis Child 1983; 58:713–716.
- Webb MSC, Henry RL, Milner AD, Stokes GM, Swarbrick AS. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child 1985; 60:1064–1067.
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161:1501–1507.
- Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005; 171:137–141.
- Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65:1045–1052.
- McIntosh K, Chanock RM. Respiratory syncytial virus. In: Fields BN, Knipe DM, eds. Virology. New York: Raven Press; 1990:1045.
- Committee on Infectious Diseases. American Academy of Pediatrics: use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics 1993; 92:501–504.
- Committee on Infectious Diseases. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996; 97:137–140.
- Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol 2011; 46:324–347.
- The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory synctial virus infection in high-risk infants. Pediatrics 1998; 102(3 Pt 1):531–537.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–e1502.
- Blanken MO, Rovers MM, Molenaar JM, et al; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–1799.
- Rezaee F, Gibson LF, Piktel D, Othumpangat S, Piedimonte G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol 2011; 45:277–286.
- Das B, Kashino SS, Pulu I, et al. CD271+ bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med 2013; 5:170ra13.
- Piedimonte G, Perez MK. Alternative mechanisms for respiratory syncytial virus (RSV) infection and persistence: could RSV be transmitted through the placenta and persist into developing fetal lungs? Curr Opin Pharmacol 2014; 16:82–88.
Understanding of respiratory syncytial virus (RSV) infection has increased substantially since its discovery, but a curative treatment remains elusive. Insights into the impact of gestational age, epidemiologic patterns, virus incubation and proliferation, pathogenesis and pathophysiology, and host immune response have set the stage for preventive measures and effective therapy. In today’s clinical practice, a specific humanized antibody is administered to high-risk infants, which is safe and effective in preventing RSV-related acute and postacute symptoms.
VIRUS STRUCTURE AND CLASSIFICATION
Knowledge of the molecular structure of RSV is important because it gives clues as to how it infects the human airways (Figure 1). The virus is made of a single strand of RNA contained in a nucleocapsid made of only 11 proteins and covered by a lipid envelope. Essential for the virulence of RSV are the surface glycoproteins G and F (fusion) that attach to the host airway epithelial cells and merge the viral envelope to the membranes of multiple adjacent cells, creating the “syncytia” that give RSV its name. G and F proteins are also the principal antigens exposed to the host immune system, and therefore the patient’s neutralizing antibodies are primarily directed against these targets.1
Human RSV is a Pneumovirus belonging to the Paramyxoviridae family. There are two strains of RSV (A and B) found in infants. Approximately 60% of all lower respiratory infections in preschool-aged children worldwide are caused by RSV (Figure 2).2 All the other viruses—including influenza, parainfluenza, metapneumovirus, and adenoviruses—as well as bacterial infections are much less common during the first years of life.
VIROLOGY
The burden of RSV continues to grow worldwide, particularly among the youngest segment of the population.2 During the first year of life, infants are not adequately protected from RSV by maternal immunoglobulins. Thus, approximately 80% of lower respiratory infections in children younger than 1 year are due to RSV with peak incidence occurring at 2 to 3 months of age. In the United States, bronchiolitis-associated hospitalizations in infants younger than 12 months more than doubled between 1980 and 1996, and in 1996, accounted for approximately 3% of all pediatric hospitalizations. Up to 120,000 RSV-related pediatric hospitalizations per year occur during a typical season.3
Mortality rates have improved significantly—at least in industrialized countries—and now are probably less than 1%.4 In the United States, RSV is estimated to cause fewer than 100 deaths annually, primarily because of better supportive care and the use of mechanical ventilation.
The seasonality of RSV varies by region. In the northern United States from October through March, RSV causes up to 90% of lower respiratory infection cases in infants. In the southern United States and farther into the southern hemisphere, the virus becomes endemic and can be present throughout the year with peak incidence occurring from August to September.2
RSV RISK FACTORS
The severity and frequency of RSV infection is influenced by well-known risk factors including environmental overcrowding, absence of breastfeeding, and immunosuppression. Children with chronic lung disease are predisposed to clinically significant RSV infection by their limited respiratory reserve, and 60% of those affected will be hospitalized. Hemodynamically significant congenital heart defects associated with higher pulmonary blood flow also increase risk for severe RSV disease, with more than 50% of children infected requiring hospitalization.4
Prematurity and increased RSV risk
Premature-born infants have a 10-fold increased risk of RSV infection and account for 25% to 30% of RSV hospitalizations annually.4 Prematurity as a risk factor for RSV is primarily tied to the physiology of placental immunoglobulin G (IgG) transfer. The human placenta is not permeable to IgG during the first half of pregnancy because of low expression of the Fc receptor needed to bind immunoglobulins and transfer them into the fetal circulation. Further, maternal IgG is recognized as a foreign protein by the newborn and is progressively removed from the circulation by the liver.5
Thus, the IgG decline continues postnatally, reaching the lowest concentration at 2 to 3 months of age because newborns are unable to synthesize their own antibodies. During this time, full-term born babies are at the highest risk for developing RSV infection. The risk is logically higher for premature infants who lack the full benefit of IgG transfer occurring during the last trimester, rendering antibody levels even lower at nadir. The greater the prematurity, the less the antibody protection and the more the predisposition to RSV infection.5
CLINICAL MANIFESTATIONS
The hallmark signs of RSV bronchiolitis are wheezing, cough, and increased work of breathing caused by infection of the bronchiolar airways. However, the nasal phase of the infection may cause irritation and trigeminal nerve activation associated with sneezing, congestion, and apnea. Indeed, approximately 20% of infants manifest an apnea episode as the first symptom of infection.6
The specific etiology of RSV can be confirmed by antigen detection tests, currently being replaced by more sensitive polymerase chain reaction (PCR)-based assays. Chest radiography reveals obvious signs of RSV lower respiratory infection, typically patch atelectasis, increased peribronchial markings, and bilateral hyperinflation with flattening of the diaphragm. Current guidelines indicate that the diagnosis of acute bronchiolitis should be based exclusively on the history and physical exam; it does not require radiographic or laboratory studies.
Correct etiologic diagnosis, however, is crucial to avoid unnecessary therapies and rule out rare conditions that could be worsened by therapies commonly used for bronchiolitis. For example, infants with dilated cardiomyopathy and congestive heart failure may present with symptoms of wheezing that mimic an acute respiratory infection, but these patients are at risk of developing supraventricular tachycardia and even cardiopulmonary collapse associated with administration of beta-agonist agents. In such cases, a chest radiograph will show cardiomegaly suggesting a different diagnosis and therapy, and thus avoid significant complications.
Recurrent wheezing
Children infected with RSV during their first year of life develop a higher risk of subsequent episodes of bronchial obstruction. Several retrospective studies conducted in the 1980s explored the incidence of lower respiratory symptoms continuing for years after initial RSV infection. Particularly unexpected was the finding that at least one-third of children hospitalized with RSV bronchiolitis will continue to wheeze beyond 6 to 8 years of age (Figure 3).7–9
More recent prospective studies of recurrent wheezing following RSV infection have been conducted. A Swedish study of 47 infants hospitalized with culture-proven RSV bronchiolitis showed significantly increased physician-diagnosed asthma (38%) compared with the 93 controls (2%) at age 7.5 years (P < .0001).10 Follow-up studies of the same cohort found increased risk for recurrent wheezing or asthma in the RSV group still present at age 13 years11 and 18 years12 compared with controls.
Asthma
History of RSV infection is important both as a trigger of asthma attacks and in the inception of asthma. Family history of asthma is the most important marker of a genetic predisposition to develop asthma. A multivariate analysis of the Swedish cohort discussed above showed that children with neither previous RSV infection nor family history of asthma had approximately a 5% risk of developing asthma by age 7.5 years. None of the children with a family history of asthma but no history of RSV infection developed asthma during follow-up. Children who had an RSV infection without a familial predisposition to asthma had approximately a 20% risk of asthma development. Children with both previous RSV infection and a family history of asthma had the highest (~40%) risk of developing asthma.10
These results provide important information about asthma pathogenesis in early life. The main implication is that even if a child has a genetic predisposition to asthma development, clinical manifestations will not develop without exposure to environmental agents that allow the actual expression of the predisposing genes, such as pollution, unbalanced diet, or early-life RSV infection. On the other hand, children without genetic predisposition can present with clinical manifestations undistinguishable from atopic asthma if their respiratory tract is exposed to specific viral pathogens during crucial developmental windows in early life. Finally, the combination of genetic predisposition and adverse environmental exposures in infancy carries the highest risk of asthma.
RSV MANAGEMENT
Ribavirin
The life cycle of RSV after the initial infection affects treatment strategies. RSV produces no symptoms for at least 3 to 5 days following infection (“eclipse phase”), during which time it reproduces exponentially and reaches the lungs, causing the first symptoms to be observable after 5 to 7 days. By the time the first symptoms emerge, the virus is already rapidly disappearing from the system.13 If ribavirin is administered at this point, the pediatrician has provided a virostatic agent against a virus that is no longer present, and therapy will not only be ineffective, but it may trigger bronchospasms.
The American Academy of Pediatrics (AAP) Committee on Infectious Diseases supported the use of ribavirin in 1993 guidelines,14 but changed their recommendation to “may be considered” in 1996.15 The only exception for ribavirin use is in the immunocompromised patient with RSV. In this scenario, the virus continues to replicate for months after the initial infection unopposed by the host defense mechanisms; therefore, aerosolized ribavirin therapy should be considered, either alone or in combination with humanized anti-RSV antibody.
Other treatments
Corticosteroids have not been shown to have a significant effect on RSV bronchiolitis.16 Alpha- and beta-adrenergic bronchodilators, such as epinephrine and albuterol, have shown very little or no effect on RSV symptoms in multiple controlled trials. The only effective treatment with proven efficacy is supportive therapy with adequate fluid intake and oxygen. Oral feeding should be withheld in patients with high respiratory rates to prevent aspiration.
PROPHYLAXIS
Vaccines
Vaccines have been ineffective against RSV and can be dangerous in children. RSV is not a strongly cytopathic virus; illness results primarily from the host immune response against the infection rather than from the virus itself.16 Therefore, any vaccine carries the risk of creating a stronger and potentially dangerous immune response to the next infection.
In the 1960s, a formalin-inactivated RSV vaccine was introduced with deleterious outcomes—it was minimally protective and was responsible for infant deaths. Because mortality was not immediate, the vaccine continued to be administered throughout the season. Unfortunately, with their immunity modified, vaccinated children became severely ill when they came in contact with the wild-type virus during the year following vaccination. The withdrawal of the formalin-inactivated vaccine still represents an important precedent that makes investigators and regulatory bodies very cautious about active immunization against this virus.
Palivizumab
Palivizumab is a 96% human monoclonal antibody targeting the RSV F protein, and it offers passive immunity for infants at risk for severe infection. The Impact-RSV clinical trial of palivizumab showed that five monthly intramuscular injections effectively reduced RSV hospitalizations by 78% in premature infants from 32 to 35 weeks gestation without bronchopulmonary dysplasia (BPD). Treatment offered only a 39% reduction in premature infants with BPD.17
The most recent AAP guidelines18 recommend palivizumab prophylaxis with a maximum of five monthly doses only in the first year of life for otherwise healthy infants born before 29 weeks gestation and for infants born before 32 weeks gestation with chronic lung disease of prematurity (CLD) defined as a requirement for supplemental oxygen for at least 28 days after birth. Prophylaxis is no longer recommended in the second year of life, except for infants with CLD still requiring oxygen, corticosteroids, or diuretics. Palivizumab prophylaxis should be discontinued if a breakthrough RSV hospitalization occurs because the likelihood of a second RSV hospitalization in the same season is low.
Palivizumab also should be considered for children with hemodynamically significant congenital heart defects, profound immunodeficiency, and pulmonary or neuromuscular pathologies impairing airway clearance; however, no formal recommendation was made for patients with Down syndrome or cystic fibrosis due to insufficient data.18 The protective effect of palivizumab appears to be cumulative, with almost half of the breakthrough infections observed in the clinical trials occurring after the first injection or immediately following the second.17
A recent randomized, double-blind, placebo-controlled trial has shown that palivizumab given to premature infants during the first year of life provides a 40% to 60% reduction of wheezing episodes.19 This trial confirms the results of previous retrospective studies; however, further large multicenter trials of palivizumab prophylaxis in both premature and full-term infants are needed to assess protection against recurrent wheezing and asthma, and to formulate evidence-based recommendations.
FUTURE DIRECTIONS
Nebulization of 3% saline improves mucociliary clearance and is increasingly being used in airway diseases involving mucus plugging (eg, cystic fibrosis). It also has been reported to reduce length of hospital stay and provide symptomatic relief in patients with bronchiolitis, but its use remains controversial. In particular, it has not been shown to be effective at reducing hospitalization when used in emergency settings. Therefore, based on current evidence, the administration of hypertonic saline for bronchiolitis should be limited to hospitalized infants.
Anti-leukotrienes used during the acute phase of RSV bronchiolitis have been shown to improve post-bronchiolitis respiratory symptoms, especially in younger patients with high urinary leukotriene E4 (LTE4). However, a large, multicenter, randomized, double-blind, placebo-controlled trial with montelukast failed to show statistically significant clinical improvement. A post-hoc data analysis revealed that children with persistent respiratory symptoms after the acute phase of the infection may benefit from montelukast, but the manufacturer (Merck) is no longer pursuing this indication.
There is not enough scientific evidence at present to support the use of DNAse or exogenous surfactant in the setting of acute bronchiolitis. New generations of specific antivirals and vaccines based on immunogens offer hope as new options for prophylaxis and/or management of RSV infection in the not too distant future.
Recent basic science investigations have provided new findings that could influence future therapies. Some of these studies have suggested that the tropism of RSV is not solely for the airway epithelium; rather, this virus can spread hematogenously to infect extrapulmonary targets, particularly the bone marrow stromal cells where RSV finds a sanctuary niche shielding it from immune protection and allowing subclinical latency. In one study, RSV was found in the bone marrow of every child and in about 80% of adults tested.20 Similar findings were replicated with other pathogens like Mycobacterium tuberculosis.21 This discovery could direct future treatment strategies for a number of viral, bacterial, and parasitic infections.
In another study, transplacental transmission of RSV from the mother’s airways to fetal lung tissues was shown for the first time in an animal model of infection. When the virus enters fetal respiratory cells, it persists and induces the expression of neurotrophic factors and receptors resulting in postnatal airways with dramatically increased parasympathetic innervation and methacholine reactivity. These structural and functional changes provide a suitable model to explain the development of long-term bronchial hyperreactivity following re-exposure to RSV in early life.22
In conclusion, the search for a cure for the most common respiratory infection in children has humbled several generations of investigators through the more than 50 years since RSV was discovered. Nevertheless, the rapid evolution in the fields of virology and immunology and recent breakthrough discoveries promise to deliver new strategies that may make a difference in the management of this common infection and its long-term sequelae.
Understanding of respiratory syncytial virus (RSV) infection has increased substantially since its discovery, but a curative treatment remains elusive. Insights into the impact of gestational age, epidemiologic patterns, virus incubation and proliferation, pathogenesis and pathophysiology, and host immune response have set the stage for preventive measures and effective therapy. In today’s clinical practice, a specific humanized antibody is administered to high-risk infants, which is safe and effective in preventing RSV-related acute and postacute symptoms.
VIRUS STRUCTURE AND CLASSIFICATION
Knowledge of the molecular structure of RSV is important because it gives clues as to how it infects the human airways (Figure 1). The virus is made of a single strand of RNA contained in a nucleocapsid made of only 11 proteins and covered by a lipid envelope. Essential for the virulence of RSV are the surface glycoproteins G and F (fusion) that attach to the host airway epithelial cells and merge the viral envelope to the membranes of multiple adjacent cells, creating the “syncytia” that give RSV its name. G and F proteins are also the principal antigens exposed to the host immune system, and therefore the patient’s neutralizing antibodies are primarily directed against these targets.1
Human RSV is a Pneumovirus belonging to the Paramyxoviridae family. There are two strains of RSV (A and B) found in infants. Approximately 60% of all lower respiratory infections in preschool-aged children worldwide are caused by RSV (Figure 2).2 All the other viruses—including influenza, parainfluenza, metapneumovirus, and adenoviruses—as well as bacterial infections are much less common during the first years of life.
VIROLOGY
The burden of RSV continues to grow worldwide, particularly among the youngest segment of the population.2 During the first year of life, infants are not adequately protected from RSV by maternal immunoglobulins. Thus, approximately 80% of lower respiratory infections in children younger than 1 year are due to RSV with peak incidence occurring at 2 to 3 months of age. In the United States, bronchiolitis-associated hospitalizations in infants younger than 12 months more than doubled between 1980 and 1996, and in 1996, accounted for approximately 3% of all pediatric hospitalizations. Up to 120,000 RSV-related pediatric hospitalizations per year occur during a typical season.3
Mortality rates have improved significantly—at least in industrialized countries—and now are probably less than 1%.4 In the United States, RSV is estimated to cause fewer than 100 deaths annually, primarily because of better supportive care and the use of mechanical ventilation.
The seasonality of RSV varies by region. In the northern United States from October through March, RSV causes up to 90% of lower respiratory infection cases in infants. In the southern United States and farther into the southern hemisphere, the virus becomes endemic and can be present throughout the year with peak incidence occurring from August to September.2
RSV RISK FACTORS
The severity and frequency of RSV infection is influenced by well-known risk factors including environmental overcrowding, absence of breastfeeding, and immunosuppression. Children with chronic lung disease are predisposed to clinically significant RSV infection by their limited respiratory reserve, and 60% of those affected will be hospitalized. Hemodynamically significant congenital heart defects associated with higher pulmonary blood flow also increase risk for severe RSV disease, with more than 50% of children infected requiring hospitalization.4
Prematurity and increased RSV risk
Premature-born infants have a 10-fold increased risk of RSV infection and account for 25% to 30% of RSV hospitalizations annually.4 Prematurity as a risk factor for RSV is primarily tied to the physiology of placental immunoglobulin G (IgG) transfer. The human placenta is not permeable to IgG during the first half of pregnancy because of low expression of the Fc receptor needed to bind immunoglobulins and transfer them into the fetal circulation. Further, maternal IgG is recognized as a foreign protein by the newborn and is progressively removed from the circulation by the liver.5
Thus, the IgG decline continues postnatally, reaching the lowest concentration at 2 to 3 months of age because newborns are unable to synthesize their own antibodies. During this time, full-term born babies are at the highest risk for developing RSV infection. The risk is logically higher for premature infants who lack the full benefit of IgG transfer occurring during the last trimester, rendering antibody levels even lower at nadir. The greater the prematurity, the less the antibody protection and the more the predisposition to RSV infection.5
CLINICAL MANIFESTATIONS
The hallmark signs of RSV bronchiolitis are wheezing, cough, and increased work of breathing caused by infection of the bronchiolar airways. However, the nasal phase of the infection may cause irritation and trigeminal nerve activation associated with sneezing, congestion, and apnea. Indeed, approximately 20% of infants manifest an apnea episode as the first symptom of infection.6
The specific etiology of RSV can be confirmed by antigen detection tests, currently being replaced by more sensitive polymerase chain reaction (PCR)-based assays. Chest radiography reveals obvious signs of RSV lower respiratory infection, typically patch atelectasis, increased peribronchial markings, and bilateral hyperinflation with flattening of the diaphragm. Current guidelines indicate that the diagnosis of acute bronchiolitis should be based exclusively on the history and physical exam; it does not require radiographic or laboratory studies.
Correct etiologic diagnosis, however, is crucial to avoid unnecessary therapies and rule out rare conditions that could be worsened by therapies commonly used for bronchiolitis. For example, infants with dilated cardiomyopathy and congestive heart failure may present with symptoms of wheezing that mimic an acute respiratory infection, but these patients are at risk of developing supraventricular tachycardia and even cardiopulmonary collapse associated with administration of beta-agonist agents. In such cases, a chest radiograph will show cardiomegaly suggesting a different diagnosis and therapy, and thus avoid significant complications.
Recurrent wheezing
Children infected with RSV during their first year of life develop a higher risk of subsequent episodes of bronchial obstruction. Several retrospective studies conducted in the 1980s explored the incidence of lower respiratory symptoms continuing for years after initial RSV infection. Particularly unexpected was the finding that at least one-third of children hospitalized with RSV bronchiolitis will continue to wheeze beyond 6 to 8 years of age (Figure 3).7–9
More recent prospective studies of recurrent wheezing following RSV infection have been conducted. A Swedish study of 47 infants hospitalized with culture-proven RSV bronchiolitis showed significantly increased physician-diagnosed asthma (38%) compared with the 93 controls (2%) at age 7.5 years (P < .0001).10 Follow-up studies of the same cohort found increased risk for recurrent wheezing or asthma in the RSV group still present at age 13 years11 and 18 years12 compared with controls.
Asthma
History of RSV infection is important both as a trigger of asthma attacks and in the inception of asthma. Family history of asthma is the most important marker of a genetic predisposition to develop asthma. A multivariate analysis of the Swedish cohort discussed above showed that children with neither previous RSV infection nor family history of asthma had approximately a 5% risk of developing asthma by age 7.5 years. None of the children with a family history of asthma but no history of RSV infection developed asthma during follow-up. Children who had an RSV infection without a familial predisposition to asthma had approximately a 20% risk of asthma development. Children with both previous RSV infection and a family history of asthma had the highest (~40%) risk of developing asthma.10
These results provide important information about asthma pathogenesis in early life. The main implication is that even if a child has a genetic predisposition to asthma development, clinical manifestations will not develop without exposure to environmental agents that allow the actual expression of the predisposing genes, such as pollution, unbalanced diet, or early-life RSV infection. On the other hand, children without genetic predisposition can present with clinical manifestations undistinguishable from atopic asthma if their respiratory tract is exposed to specific viral pathogens during crucial developmental windows in early life. Finally, the combination of genetic predisposition and adverse environmental exposures in infancy carries the highest risk of asthma.
RSV MANAGEMENT
Ribavirin
The life cycle of RSV after the initial infection affects treatment strategies. RSV produces no symptoms for at least 3 to 5 days following infection (“eclipse phase”), during which time it reproduces exponentially and reaches the lungs, causing the first symptoms to be observable after 5 to 7 days. By the time the first symptoms emerge, the virus is already rapidly disappearing from the system.13 If ribavirin is administered at this point, the pediatrician has provided a virostatic agent against a virus that is no longer present, and therapy will not only be ineffective, but it may trigger bronchospasms.
The American Academy of Pediatrics (AAP) Committee on Infectious Diseases supported the use of ribavirin in 1993 guidelines,14 but changed their recommendation to “may be considered” in 1996.15 The only exception for ribavirin use is in the immunocompromised patient with RSV. In this scenario, the virus continues to replicate for months after the initial infection unopposed by the host defense mechanisms; therefore, aerosolized ribavirin therapy should be considered, either alone or in combination with humanized anti-RSV antibody.
Other treatments
Corticosteroids have not been shown to have a significant effect on RSV bronchiolitis.16 Alpha- and beta-adrenergic bronchodilators, such as epinephrine and albuterol, have shown very little or no effect on RSV symptoms in multiple controlled trials. The only effective treatment with proven efficacy is supportive therapy with adequate fluid intake and oxygen. Oral feeding should be withheld in patients with high respiratory rates to prevent aspiration.
PROPHYLAXIS
Vaccines
Vaccines have been ineffective against RSV and can be dangerous in children. RSV is not a strongly cytopathic virus; illness results primarily from the host immune response against the infection rather than from the virus itself.16 Therefore, any vaccine carries the risk of creating a stronger and potentially dangerous immune response to the next infection.
In the 1960s, a formalin-inactivated RSV vaccine was introduced with deleterious outcomes—it was minimally protective and was responsible for infant deaths. Because mortality was not immediate, the vaccine continued to be administered throughout the season. Unfortunately, with their immunity modified, vaccinated children became severely ill when they came in contact with the wild-type virus during the year following vaccination. The withdrawal of the formalin-inactivated vaccine still represents an important precedent that makes investigators and regulatory bodies very cautious about active immunization against this virus.
Palivizumab
Palivizumab is a 96% human monoclonal antibody targeting the RSV F protein, and it offers passive immunity for infants at risk for severe infection. The Impact-RSV clinical trial of palivizumab showed that five monthly intramuscular injections effectively reduced RSV hospitalizations by 78% in premature infants from 32 to 35 weeks gestation without bronchopulmonary dysplasia (BPD). Treatment offered only a 39% reduction in premature infants with BPD.17
The most recent AAP guidelines18 recommend palivizumab prophylaxis with a maximum of five monthly doses only in the first year of life for otherwise healthy infants born before 29 weeks gestation and for infants born before 32 weeks gestation with chronic lung disease of prematurity (CLD) defined as a requirement for supplemental oxygen for at least 28 days after birth. Prophylaxis is no longer recommended in the second year of life, except for infants with CLD still requiring oxygen, corticosteroids, or diuretics. Palivizumab prophylaxis should be discontinued if a breakthrough RSV hospitalization occurs because the likelihood of a second RSV hospitalization in the same season is low.
Palivizumab also should be considered for children with hemodynamically significant congenital heart defects, profound immunodeficiency, and pulmonary or neuromuscular pathologies impairing airway clearance; however, no formal recommendation was made for patients with Down syndrome or cystic fibrosis due to insufficient data.18 The protective effect of palivizumab appears to be cumulative, with almost half of the breakthrough infections observed in the clinical trials occurring after the first injection or immediately following the second.17
A recent randomized, double-blind, placebo-controlled trial has shown that palivizumab given to premature infants during the first year of life provides a 40% to 60% reduction of wheezing episodes.19 This trial confirms the results of previous retrospective studies; however, further large multicenter trials of palivizumab prophylaxis in both premature and full-term infants are needed to assess protection against recurrent wheezing and asthma, and to formulate evidence-based recommendations.
FUTURE DIRECTIONS
Nebulization of 3% saline improves mucociliary clearance and is increasingly being used in airway diseases involving mucus plugging (eg, cystic fibrosis). It also has been reported to reduce length of hospital stay and provide symptomatic relief in patients with bronchiolitis, but its use remains controversial. In particular, it has not been shown to be effective at reducing hospitalization when used in emergency settings. Therefore, based on current evidence, the administration of hypertonic saline for bronchiolitis should be limited to hospitalized infants.
Anti-leukotrienes used during the acute phase of RSV bronchiolitis have been shown to improve post-bronchiolitis respiratory symptoms, especially in younger patients with high urinary leukotriene E4 (LTE4). However, a large, multicenter, randomized, double-blind, placebo-controlled trial with montelukast failed to show statistically significant clinical improvement. A post-hoc data analysis revealed that children with persistent respiratory symptoms after the acute phase of the infection may benefit from montelukast, but the manufacturer (Merck) is no longer pursuing this indication.
There is not enough scientific evidence at present to support the use of DNAse or exogenous surfactant in the setting of acute bronchiolitis. New generations of specific antivirals and vaccines based on immunogens offer hope as new options for prophylaxis and/or management of RSV infection in the not too distant future.
Recent basic science investigations have provided new findings that could influence future therapies. Some of these studies have suggested that the tropism of RSV is not solely for the airway epithelium; rather, this virus can spread hematogenously to infect extrapulmonary targets, particularly the bone marrow stromal cells where RSV finds a sanctuary niche shielding it from immune protection and allowing subclinical latency. In one study, RSV was found in the bone marrow of every child and in about 80% of adults tested.20 Similar findings were replicated with other pathogens like Mycobacterium tuberculosis.21 This discovery could direct future treatment strategies for a number of viral, bacterial, and parasitic infections.
In another study, transplacental transmission of RSV from the mother’s airways to fetal lung tissues was shown for the first time in an animal model of infection. When the virus enters fetal respiratory cells, it persists and induces the expression of neurotrophic factors and receptors resulting in postnatal airways with dramatically increased parasympathetic innervation and methacholine reactivity. These structural and functional changes provide a suitable model to explain the development of long-term bronchial hyperreactivity following re-exposure to RSV in early life.22
In conclusion, the search for a cure for the most common respiratory infection in children has humbled several generations of investigators through the more than 50 years since RSV was discovered. Nevertheless, the rapid evolution in the fields of virology and immunology and recent breakthrough discoveries promise to deliver new strategies that may make a difference in the management of this common infection and its long-term sequelae.
- Peters TR, Crowe JE Jr. Respiratory syncytial virus. In: Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008.
- Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35:519–530.
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999; 282:1440–1446.
- Simoes EA, Rieger CHL. RSV infection in developed and developing countries. Infect Med 1999; 16:S11–S17.
- Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1,500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res 1986; 20:899 –904.
- Sabogal C, Auais A, Napchan G, et al. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 2005; 57:819–825.
- Hall CB, Hall WJ, Gala CL, MaGill FB, Leddy JP. Long-term prospective study in children after respiratory syncytial virus infection. J Pediatr 1984; 105:358–364.
- Henry RL, Hodges IGC, Milner AD, Stokes GM. Respiratory problems 2 years after acute bronchiolitis in infancy. Arch Dis Child 1983; 58:713–716.
- Webb MSC, Henry RL, Milner AD, Stokes GM, Swarbrick AS. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child 1985; 60:1064–1067.
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161:1501–1507.
- Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005; 171:137–141.
- Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65:1045–1052.
- McIntosh K, Chanock RM. Respiratory syncytial virus. In: Fields BN, Knipe DM, eds. Virology. New York: Raven Press; 1990:1045.
- Committee on Infectious Diseases. American Academy of Pediatrics: use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics 1993; 92:501–504.
- Committee on Infectious Diseases. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996; 97:137–140.
- Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol 2011; 46:324–347.
- The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory synctial virus infection in high-risk infants. Pediatrics 1998; 102(3 Pt 1):531–537.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–e1502.
- Blanken MO, Rovers MM, Molenaar JM, et al; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–1799.
- Rezaee F, Gibson LF, Piktel D, Othumpangat S, Piedimonte G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol 2011; 45:277–286.
- Das B, Kashino SS, Pulu I, et al. CD271+ bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med 2013; 5:170ra13.
- Piedimonte G, Perez MK. Alternative mechanisms for respiratory syncytial virus (RSV) infection and persistence: could RSV be transmitted through the placenta and persist into developing fetal lungs? Curr Opin Pharmacol 2014; 16:82–88.
- Peters TR, Crowe JE Jr. Respiratory syncytial virus. In: Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008.
- Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35:519–530.
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999; 282:1440–1446.
- Simoes EA, Rieger CHL. RSV infection in developed and developing countries. Infect Med 1999; 16:S11–S17.
- Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1,500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res 1986; 20:899 –904.
- Sabogal C, Auais A, Napchan G, et al. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 2005; 57:819–825.
- Hall CB, Hall WJ, Gala CL, MaGill FB, Leddy JP. Long-term prospective study in children after respiratory syncytial virus infection. J Pediatr 1984; 105:358–364.
- Henry RL, Hodges IGC, Milner AD, Stokes GM. Respiratory problems 2 years after acute bronchiolitis in infancy. Arch Dis Child 1983; 58:713–716.
- Webb MSC, Henry RL, Milner AD, Stokes GM, Swarbrick AS. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child 1985; 60:1064–1067.
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161:1501–1507.
- Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005; 171:137–141.
- Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65:1045–1052.
- McIntosh K, Chanock RM. Respiratory syncytial virus. In: Fields BN, Knipe DM, eds. Virology. New York: Raven Press; 1990:1045.
- Committee on Infectious Diseases. American Academy of Pediatrics: use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics 1993; 92:501–504.
- Committee on Infectious Diseases. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996; 97:137–140.
- Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol 2011; 46:324–347.
- The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory synctial virus infection in high-risk infants. Pediatrics 1998; 102(3 Pt 1):531–537.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–e1502.
- Blanken MO, Rovers MM, Molenaar JM, et al; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–1799.
- Rezaee F, Gibson LF, Piktel D, Othumpangat S, Piedimonte G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol 2011; 45:277–286.
- Das B, Kashino SS, Pulu I, et al. CD271+ bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med 2013; 5:170ra13.
- Piedimonte G, Perez MK. Alternative mechanisms for respiratory syncytial virus (RSV) infection and persistence: could RSV be transmitted through the placenta and persist into developing fetal lungs? Curr Opin Pharmacol 2014; 16:82–88.
Dermatology for the pediatrician: Advances in diagnosis and treatment of common and not-so-common skin conditions
Primary care pediatricians are often asked about manifestations of both common and rare dermatologic disorders. They frequently encounter pediatric skin conditions, making it important to stay abreast of new developments in diagnosis and treatment. Common pediatric dermatologic diagnoses include atopic dermatitis, allergic contact dermatitis, tinea capitis, infantile hemangioma, and alopecia areata. Less common conditions include epidermal nevi and tuberous sclerosis.
ATOPIC DERMATITIS (ECZEMA)
Atopic dermatitis, also referred to as eczema, is a common pediatric skin condition characterized by erythema, pruritus, scaling, lichenification, and papulovesicles (Figure 1). It affects up to 17% of children in the United States.1,2 A wide range of environmental factors, such as contact allergens, stress, food, skin flora, and humidity, affect the development and severity of atopic dermatitis. Studies also support a genetic basis; when both parents are atopic, their children have a 70% risk for developing atopic dermatitis.3
Approximately 50% of European whites have a filaggrin mutation thought to cause pediatric atopic dermatitis or eczema. The filaggrin gene (chromosome 1q21) is located in the epidermal differentiation complex and encodes profilaggrin. The defect leads to a poor protein-lipid cell envelope and a loss of the filaggrin hygroscopic amino acids that act as a natural barrier.4
Additionally, these patients have increased surface pH. This is of particular importance because at a certain pH, there is decreased inhibition of Staphylococcus aureus. Decreased activity of ceramide metabolism enzymes occurs, resulting in increased water loss along with increased entry of foreign substances.
The best treatment for atopic dermatitis is daily bathing with a mild, nondrying soap. Unfortunately, many parents purposely do not bathe their atopic children daily because they believe it is harmful. Many mild cleansers are available today that do not aggravate the condition.
Another effective treatment for eczema is to apply a moisturizer. Previously, thick ointments likely to occlude water loss were applied, but research on the pathophysiological process of atopic dermatitis has led to the development of moisturizers and topical skin products targeted to correct reduced amounts of ceramides and natural moisturizing factors in the skin with natural moisturizing factors, ceramides, and pseudoceramide products.5 A ceramide-containing moisturizer should be applied immediately after giving the child a bath. These moisturizers are available over-the-counter but can be relatively expensive. Some popular ceramide-containing skin lubricants include CeraVe cream, Cetaphil RestoraDerm cream, Aveeno eczema cream, and Eucerin eczema cream. If cost is an issue, a thick emollient such as petroleum jelly can be used.
Prevention
Several theories have been proposed to explain the development of eczema beyond genetic predisposition, leading to attempts to prevent its development. Theories include breastfeeding—both nursing and not nursing. While it seems that breastfeeding should be protective against atopic dermatitis, unfortunately there is no proof of this. Also, withholding certain foods during the introduction of solid foods (and not eating certain foods during nursing) will not decrease the risk of developing atopic dermatitis. Early exposure to farm animals has been debated as causative, and there is evidence that early exposure to antibiotics increases atopic dermatitis risk. Lastly, maternal fish oil or probiotic ingestion during pregnancy has not changed the rates of atopic dermatitis in infants.6,7 Therefore, the best course of action is to provide simple skin care guidelines and treat children as they present with atopic dermatitis.
ALLERGIC CONTACT DERMATITIS
Contrary to previously held beliefs, allergic contact dermatitis is not rare in children. In fact, rates are equal between children and adults. Allergic contact dermatitis or irritant contact dermatitis is also associated with skin barrier breakdown common among atopic dermatitis patients. Additionally, atopic dermatitis patients may exhibit allergic contact dermatitis due to exposure to various topical preparations.8
Toilet seats
Recently, allergic contact dermatitis has been reported in association with toilet seats,9 possibly due to polyurethane or polypropylene in the seat.10 Another possible reaction may be to chemicals in antiseptic wipes used on the toilet seat. The presentation of allergic contact dermatitis due to toilet seat contact is higher on the posterior thigh for older children compared to that seen in toddlers and younger children. It can be differentially diagnosed from atopic dermatitis because it is limited to the posterior leg.11
Baby wipes
Some baby-wipe brands contain the allergen methylisothiazolinone, alone or in combination with methylchloroisothiazolinone.12 Even expensive brands marketed as hypoallergenic may contain these chemicals or other types of preservatives. Interestingly, some parents continue to use baby wipes on their children’s faces and bodies as they grow up to provide a quick clean up. So, in children who are toddler age or older, allergic contact dermatitis to baby wipes may not be localized to the anogenital area. Consider this condition in the differential diagnoses with “lip licker” dermatitis.
Car seats
Car seats also are associated with contact dermatitis but the specific allergen is unknown. The presentation is similar to toilet seat dermatitis as the posterior leg is affected while the anterior leg remains clear. This type of contact dermatitis is more frequently reported with the use of car seats made of tightly woven, shiny material. A cotton cloth barrier can be placed over the car seat to prevent skin contact.13
Sports equipment
Allergic contact dermatitis may be associated with the use of shin guards and neoprene wetsuits, with p-tert butylphenol formaldehyde resin as the likely allergen.14 There is obvious delineation of the affected and nonaffected skin, which can help rule out atopic dermatitis (Figure 2). However, there is the potential for atopic dermatitis to develop with use of these items because the skin is occluded for long periods and will sweat, causing a reaction. Again, providing a barrier between the surface of the shin guard or wetsuit and the skin will help.
Metals
Finally, nickel and other metals are known sources of allergic contact dermatitis.15
TINEA CAPITIS
The challenge for this dermatologic condition is finding an effective treatment without a prolonged course of medicine. If tinea capitis continues for too long, the inflammatory process can result in permanent alopecia. The condition is caused by fungus, most commonly Trycophyton tonsurans.16 Terbinafine (3–8 mg/kg/day for 2–4 weeks) is superior to griseofulvin for T tonsurans; however, griseofulvin is superior to terbinafine for treating Microsporum species.17 Health insurance coverage of terbinafine is a concern for some families. Finally, children with tinea capitis should use a sporicidal shampoo, selenium sulfide, or ketoconazole to decrease the spread of spores.
INFANTILE HEMANGIOMAS
Propranolol
The major breakthrough in pediatric dermatology of the past decade has been the use of propranolol to treat infantile hemangiomas. Propranolol hydrochloride (Hemangeol) received US Food and Drug Administration (FDA) approval in March 2014 for the treatment of proliferating infantile hemangioma. The proposed mechanism of action is suppression of vascular endothelial growth factor and basic fibroblast growth factor in in vitro hemangioma-derived stem cells.18
Propranolol is indicated for complex cutaneous, visceral, hepatic, and airway infantile hemangiomas. For most children, treatment with oral propranolol is preferable to prolonged treatment with systemic steroids. If propranolol is started early in the disease course, it can greatly reduce the need for extensive surgery, which is of particular importance when the nasal tip, oral mucosa, and face are affected. Children with large hemangiomas that are prone to ulceration also greatly benefit from propranolol treatment. Ulcerated hemangiomas are extremely painful and problematic, and can take months to heal (Figure 3).
Concomitant abnormalities and PHACE
A visceral evaluation is generally indicated for children with five or six lesions, but hemangioma size should also be considered when determining the need for additional tests. When a facial, scalp, or neck infantile hemangioma is larger than 5 cm, imaging studies are indicated to check for vascular anomalies in the brain and congenital heart defects (most commonly aortic problems).
A subgroup of these children have PHACE (posterior fossa anomalies, hemangioma, arterial lesions, cardiac abnormalities/aortic coarctation, and eye abnormalities) syndrome. A consensus guideline found that evidence supports treating these patients with propranolol.19 However, there have been isolated reports of acute ischemic stroke in PHACE syndrome patients on concomitant steroid therapy with severe arteriopathy. Therefore, before initiating propranolol therapy, infants with large facial hemangiomas who are at risk for PHACE should be evaluated with magnetic resonance angiography of the head and neck, and with cardiac imaging that includes the aortic arch.
Propranolol dosing
The FDA-approved dosing of propranolol hydrochloride is 1 to 3 mg/kg/day. Side effects include hypoglycemia, hypotension, exacerbation of asthma or respiratory infections, dental caries, cold extremities, and night terrors. These side effects should be closely monitored for, particularly in younger infants; however, propranolol has a relatively benign safety profile that should be taken into consideration in the risk/benefit analysis.
New pathogenesis insights
In addition to breakthrough treatment, a greater understanding of the pathogenesis of infantile hemangiomas has been attained. The most rapid lesion growth phase occurs at a younger age than was previously believed, and it is now thought to be greatest when patients are between 5.5 and 7.5 weeks old.20 Treatment initiation before the rapid growth period, rather than after, is crucial to successful outcomes.
Additionally, research has identified some of the clinical signs prior to ulceration of infantile hemangioma.21 A white central area occurring in a proliferating lesion is predictive of ulceration. This white coloration of the proliferation stage should not be mistaken for the graying of involution that occurs when infants are older than 3 months. Again, initiating early propranolol therapy should prevent some of the pain associated with hemangiomas that are likely to ulcerate.
ALOPECIA AREATA
Intralesional steroids have been the gold standard of treatment for localized alopecia areata. However, for children who cannot tolerate the injections, can topical steroids achieve enough penetrance to reduce lesion duration? A recent study compared twice-daily application of clobetasol propionate 0.05% cream or hydrocortisone 1% cream for 6 weeks on followed by 6 weeks off for 24 weeks in children with alopecia areata affecting at least 10% of scalp surface area. The clobetasol cream was superior in terms of decreasing alopetic surface area compared with hydrocortisone.22 In reference to the efficacy of clobetasol cream, only localized alopecia areata showed this response, and not alopecia totalis or universalis.
RELATIVELY RARE DIAGNOSES
Epidermal nevi
Epidermal nevi size and phenotype have guided treatment decisions when evaluating these lesions. It is believed that epidermal nevi are due to RAS/MAPK mutations, and further examination of genetic causes has become increasingly important. If the genetic mutation is known, clinical signs and genetic testing can be used to monitor children for conditions potentially related to this mosaic disorder, and to properly diagnose and treat the lesions.
Recently, researchers have been able to explain why some children with large epidermal nevi develop hypophosphatemic rickets and others do not. Mutations in HRAS or NRAS can lead to an increase in fibroblast growth factor-23, resulting in this bone abnormality.23
Tuberous sclerosis
Tuberous sclerosis (TS) is remarkable for noncancerous lesions occurring in skin, brain, kidneys, nervous system, heart, lungs, or retina. Skin presentations include patches of light-colored skin, thickened skin, growths under the nails, and facial lesions that resemble acne. Ash leaf or café au lait macules may occur, as well as large plaque angiofibromas.
While oral rapamycin has been used to treat internal tumors related to TS, a low-dose topical formula has been shown effective for treating facial angiofibromas of TS.24 This new therapy is important given the impact of facial anomalies on psychosocial development. In addition, topical rapamycin avoids the need for laser treatment, which is more painful and less effective.
SUMMARY
The understanding of common and rare skin conditions has increased during the past decade. Studies in dermatologic diagnoses and treatment have produced new insights into genetic mutations, including those involved in the development of epidermal nevi. Serendipitous findings, such as the use of propranolol for infantile hemangiomas, have also occurred. These advances have allowed for greater diagnostic accuracy and better treatments. Less invasive treatment strategies also have been developed, including clobetasol cream for localized alopecia areata and topical rapamycin for tuberous sclerosis, and these have led to greater compliance, fewer treatment-related adverse effects, and more efficacious outcomes.
- Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon school children. J Am Acad Dermatol 2000;43: 649–655.
- Hanifin JM, Reed ML; Eczema Prevalence and Impact Working Group. A population-based survey of eczema prevalence in the United States. Dermatitis 2007; 18:82–91.
- Ruzicka T. Atopic eczema between rationality and irrationality. Arch Dermatol 1998; 134:1462–1469.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization through barrier-disrupted skin elicits a TH2-dominant cytokine response. Eur J Immunol 1998; 28:769–779.
- Hon KL, Leung AK, Barankin B. Barrier repair therapy in atopic dermatitis: an overview. Am J Clin Dermatol 2013; 14:389–399.
- Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol 2004; 15(suppl 16):4–5, 9–32.
- Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years’ follow-up. Acta Paediatr Suppl 1996; 414:1–21.
- Admani S, Jacob SE. Allergic contact dermatitis in children: review of the past decade. Curr Allergy Asthma Rep 2014; 14:421.
- Holme SA, Stone NM, Mills CM. Toilet seat contact dermatitis. Pediatr Dermatol 2005; 22:344–345.
- Heilig S, Adams DR, Zaenglein AL. Persistent allergic contact dermatitis to plastic toilet seats. Pediatr Dermatol 2011; 28:587–590.
- Litvinov IV, Sugathan P, Cohen BA. Recognizing and treating toilet-seat contact dermatitis in children. Pediatrics 2010; 125:e419–e422.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis 2013; 24:2–6.
- Ghali FE. “Car seat dermatitis”: a newly described form of contact dermatitis. Pediatr Dermatol 2011; 28:321–326.
- Herro E, Jacob SE. p-tert-Butylphenol formaldehyde resin and its impact on children. Dermatitis 2012; 23:86–88.
- Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis. J Am Acad Dermatol 2013; 69:232–237.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol 2004; 50:748–752.
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol 2013; 30:1–6.
- Zhang L, Mai HM, Zheng J, et al. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol 2013; 7:48–55.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 2013; 131:128–140.
- Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents’ photographs tell us. Pediatrics 2012; 130:e314–e320.
- Maguiness SM, Hoffman WY, McCalmont TH, Frieden IJ. Early white discoloration of infantile hemangioma: a sign of impending ulceration. Arch Dermatol 2010; 146:1235–1239.
- Lenane P, Macarthur C, Parkin PC, et al. Clobetasol propionate, 0.05%, vs hydrocortisone, 1%, for alopecia areata in children: a randomized clinical trial. JAMA Dermatol 2014; 150:47–50.
- Lim YH, Ovejero D, Sugarman JS, et al. Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Hum Mol Genet 2014; 23:397–407.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double-blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs R D 2012; 12:121–126.
Primary care pediatricians are often asked about manifestations of both common and rare dermatologic disorders. They frequently encounter pediatric skin conditions, making it important to stay abreast of new developments in diagnosis and treatment. Common pediatric dermatologic diagnoses include atopic dermatitis, allergic contact dermatitis, tinea capitis, infantile hemangioma, and alopecia areata. Less common conditions include epidermal nevi and tuberous sclerosis.
ATOPIC DERMATITIS (ECZEMA)
Atopic dermatitis, also referred to as eczema, is a common pediatric skin condition characterized by erythema, pruritus, scaling, lichenification, and papulovesicles (Figure 1). It affects up to 17% of children in the United States.1,2 A wide range of environmental factors, such as contact allergens, stress, food, skin flora, and humidity, affect the development and severity of atopic dermatitis. Studies also support a genetic basis; when both parents are atopic, their children have a 70% risk for developing atopic dermatitis.3
Approximately 50% of European whites have a filaggrin mutation thought to cause pediatric atopic dermatitis or eczema. The filaggrin gene (chromosome 1q21) is located in the epidermal differentiation complex and encodes profilaggrin. The defect leads to a poor protein-lipid cell envelope and a loss of the filaggrin hygroscopic amino acids that act as a natural barrier.4
Additionally, these patients have increased surface pH. This is of particular importance because at a certain pH, there is decreased inhibition of Staphylococcus aureus. Decreased activity of ceramide metabolism enzymes occurs, resulting in increased water loss along with increased entry of foreign substances.
The best treatment for atopic dermatitis is daily bathing with a mild, nondrying soap. Unfortunately, many parents purposely do not bathe their atopic children daily because they believe it is harmful. Many mild cleansers are available today that do not aggravate the condition.
Another effective treatment for eczema is to apply a moisturizer. Previously, thick ointments likely to occlude water loss were applied, but research on the pathophysiological process of atopic dermatitis has led to the development of moisturizers and topical skin products targeted to correct reduced amounts of ceramides and natural moisturizing factors in the skin with natural moisturizing factors, ceramides, and pseudoceramide products.5 A ceramide-containing moisturizer should be applied immediately after giving the child a bath. These moisturizers are available over-the-counter but can be relatively expensive. Some popular ceramide-containing skin lubricants include CeraVe cream, Cetaphil RestoraDerm cream, Aveeno eczema cream, and Eucerin eczema cream. If cost is an issue, a thick emollient such as petroleum jelly can be used.
Prevention
Several theories have been proposed to explain the development of eczema beyond genetic predisposition, leading to attempts to prevent its development. Theories include breastfeeding—both nursing and not nursing. While it seems that breastfeeding should be protective against atopic dermatitis, unfortunately there is no proof of this. Also, withholding certain foods during the introduction of solid foods (and not eating certain foods during nursing) will not decrease the risk of developing atopic dermatitis. Early exposure to farm animals has been debated as causative, and there is evidence that early exposure to antibiotics increases atopic dermatitis risk. Lastly, maternal fish oil or probiotic ingestion during pregnancy has not changed the rates of atopic dermatitis in infants.6,7 Therefore, the best course of action is to provide simple skin care guidelines and treat children as they present with atopic dermatitis.
ALLERGIC CONTACT DERMATITIS
Contrary to previously held beliefs, allergic contact dermatitis is not rare in children. In fact, rates are equal between children and adults. Allergic contact dermatitis or irritant contact dermatitis is also associated with skin barrier breakdown common among atopic dermatitis patients. Additionally, atopic dermatitis patients may exhibit allergic contact dermatitis due to exposure to various topical preparations.8
Toilet seats
Recently, allergic contact dermatitis has been reported in association with toilet seats,9 possibly due to polyurethane or polypropylene in the seat.10 Another possible reaction may be to chemicals in antiseptic wipes used on the toilet seat. The presentation of allergic contact dermatitis due to toilet seat contact is higher on the posterior thigh for older children compared to that seen in toddlers and younger children. It can be differentially diagnosed from atopic dermatitis because it is limited to the posterior leg.11
Baby wipes
Some baby-wipe brands contain the allergen methylisothiazolinone, alone or in combination with methylchloroisothiazolinone.12 Even expensive brands marketed as hypoallergenic may contain these chemicals or other types of preservatives. Interestingly, some parents continue to use baby wipes on their children’s faces and bodies as they grow up to provide a quick clean up. So, in children who are toddler age or older, allergic contact dermatitis to baby wipes may not be localized to the anogenital area. Consider this condition in the differential diagnoses with “lip licker” dermatitis.
Car seats
Car seats also are associated with contact dermatitis but the specific allergen is unknown. The presentation is similar to toilet seat dermatitis as the posterior leg is affected while the anterior leg remains clear. This type of contact dermatitis is more frequently reported with the use of car seats made of tightly woven, shiny material. A cotton cloth barrier can be placed over the car seat to prevent skin contact.13
Sports equipment
Allergic contact dermatitis may be associated with the use of shin guards and neoprene wetsuits, with p-tert butylphenol formaldehyde resin as the likely allergen.14 There is obvious delineation of the affected and nonaffected skin, which can help rule out atopic dermatitis (Figure 2). However, there is the potential for atopic dermatitis to develop with use of these items because the skin is occluded for long periods and will sweat, causing a reaction. Again, providing a barrier between the surface of the shin guard or wetsuit and the skin will help.
Metals
Finally, nickel and other metals are known sources of allergic contact dermatitis.15
TINEA CAPITIS
The challenge for this dermatologic condition is finding an effective treatment without a prolonged course of medicine. If tinea capitis continues for too long, the inflammatory process can result in permanent alopecia. The condition is caused by fungus, most commonly Trycophyton tonsurans.16 Terbinafine (3–8 mg/kg/day for 2–4 weeks) is superior to griseofulvin for T tonsurans; however, griseofulvin is superior to terbinafine for treating Microsporum species.17 Health insurance coverage of terbinafine is a concern for some families. Finally, children with tinea capitis should use a sporicidal shampoo, selenium sulfide, or ketoconazole to decrease the spread of spores.
INFANTILE HEMANGIOMAS
Propranolol
The major breakthrough in pediatric dermatology of the past decade has been the use of propranolol to treat infantile hemangiomas. Propranolol hydrochloride (Hemangeol) received US Food and Drug Administration (FDA) approval in March 2014 for the treatment of proliferating infantile hemangioma. The proposed mechanism of action is suppression of vascular endothelial growth factor and basic fibroblast growth factor in in vitro hemangioma-derived stem cells.18
Propranolol is indicated for complex cutaneous, visceral, hepatic, and airway infantile hemangiomas. For most children, treatment with oral propranolol is preferable to prolonged treatment with systemic steroids. If propranolol is started early in the disease course, it can greatly reduce the need for extensive surgery, which is of particular importance when the nasal tip, oral mucosa, and face are affected. Children with large hemangiomas that are prone to ulceration also greatly benefit from propranolol treatment. Ulcerated hemangiomas are extremely painful and problematic, and can take months to heal (Figure 3).
Concomitant abnormalities and PHACE
A visceral evaluation is generally indicated for children with five or six lesions, but hemangioma size should also be considered when determining the need for additional tests. When a facial, scalp, or neck infantile hemangioma is larger than 5 cm, imaging studies are indicated to check for vascular anomalies in the brain and congenital heart defects (most commonly aortic problems).
A subgroup of these children have PHACE (posterior fossa anomalies, hemangioma, arterial lesions, cardiac abnormalities/aortic coarctation, and eye abnormalities) syndrome. A consensus guideline found that evidence supports treating these patients with propranolol.19 However, there have been isolated reports of acute ischemic stroke in PHACE syndrome patients on concomitant steroid therapy with severe arteriopathy. Therefore, before initiating propranolol therapy, infants with large facial hemangiomas who are at risk for PHACE should be evaluated with magnetic resonance angiography of the head and neck, and with cardiac imaging that includes the aortic arch.
Propranolol dosing
The FDA-approved dosing of propranolol hydrochloride is 1 to 3 mg/kg/day. Side effects include hypoglycemia, hypotension, exacerbation of asthma or respiratory infections, dental caries, cold extremities, and night terrors. These side effects should be closely monitored for, particularly in younger infants; however, propranolol has a relatively benign safety profile that should be taken into consideration in the risk/benefit analysis.
New pathogenesis insights
In addition to breakthrough treatment, a greater understanding of the pathogenesis of infantile hemangiomas has been attained. The most rapid lesion growth phase occurs at a younger age than was previously believed, and it is now thought to be greatest when patients are between 5.5 and 7.5 weeks old.20 Treatment initiation before the rapid growth period, rather than after, is crucial to successful outcomes.
Additionally, research has identified some of the clinical signs prior to ulceration of infantile hemangioma.21 A white central area occurring in a proliferating lesion is predictive of ulceration. This white coloration of the proliferation stage should not be mistaken for the graying of involution that occurs when infants are older than 3 months. Again, initiating early propranolol therapy should prevent some of the pain associated with hemangiomas that are likely to ulcerate.
ALOPECIA AREATA
Intralesional steroids have been the gold standard of treatment for localized alopecia areata. However, for children who cannot tolerate the injections, can topical steroids achieve enough penetrance to reduce lesion duration? A recent study compared twice-daily application of clobetasol propionate 0.05% cream or hydrocortisone 1% cream for 6 weeks on followed by 6 weeks off for 24 weeks in children with alopecia areata affecting at least 10% of scalp surface area. The clobetasol cream was superior in terms of decreasing alopetic surface area compared with hydrocortisone.22 In reference to the efficacy of clobetasol cream, only localized alopecia areata showed this response, and not alopecia totalis or universalis.
RELATIVELY RARE DIAGNOSES
Epidermal nevi
Epidermal nevi size and phenotype have guided treatment decisions when evaluating these lesions. It is believed that epidermal nevi are due to RAS/MAPK mutations, and further examination of genetic causes has become increasingly important. If the genetic mutation is known, clinical signs and genetic testing can be used to monitor children for conditions potentially related to this mosaic disorder, and to properly diagnose and treat the lesions.
Recently, researchers have been able to explain why some children with large epidermal nevi develop hypophosphatemic rickets and others do not. Mutations in HRAS or NRAS can lead to an increase in fibroblast growth factor-23, resulting in this bone abnormality.23
Tuberous sclerosis
Tuberous sclerosis (TS) is remarkable for noncancerous lesions occurring in skin, brain, kidneys, nervous system, heart, lungs, or retina. Skin presentations include patches of light-colored skin, thickened skin, growths under the nails, and facial lesions that resemble acne. Ash leaf or café au lait macules may occur, as well as large plaque angiofibromas.
While oral rapamycin has been used to treat internal tumors related to TS, a low-dose topical formula has been shown effective for treating facial angiofibromas of TS.24 This new therapy is important given the impact of facial anomalies on psychosocial development. In addition, topical rapamycin avoids the need for laser treatment, which is more painful and less effective.
SUMMARY
The understanding of common and rare skin conditions has increased during the past decade. Studies in dermatologic diagnoses and treatment have produced new insights into genetic mutations, including those involved in the development of epidermal nevi. Serendipitous findings, such as the use of propranolol for infantile hemangiomas, have also occurred. These advances have allowed for greater diagnostic accuracy and better treatments. Less invasive treatment strategies also have been developed, including clobetasol cream for localized alopecia areata and topical rapamycin for tuberous sclerosis, and these have led to greater compliance, fewer treatment-related adverse effects, and more efficacious outcomes.
Primary care pediatricians are often asked about manifestations of both common and rare dermatologic disorders. They frequently encounter pediatric skin conditions, making it important to stay abreast of new developments in diagnosis and treatment. Common pediatric dermatologic diagnoses include atopic dermatitis, allergic contact dermatitis, tinea capitis, infantile hemangioma, and alopecia areata. Less common conditions include epidermal nevi and tuberous sclerosis.
ATOPIC DERMATITIS (ECZEMA)
Atopic dermatitis, also referred to as eczema, is a common pediatric skin condition characterized by erythema, pruritus, scaling, lichenification, and papulovesicles (Figure 1). It affects up to 17% of children in the United States.1,2 A wide range of environmental factors, such as contact allergens, stress, food, skin flora, and humidity, affect the development and severity of atopic dermatitis. Studies also support a genetic basis; when both parents are atopic, their children have a 70% risk for developing atopic dermatitis.3
Approximately 50% of European whites have a filaggrin mutation thought to cause pediatric atopic dermatitis or eczema. The filaggrin gene (chromosome 1q21) is located in the epidermal differentiation complex and encodes profilaggrin. The defect leads to a poor protein-lipid cell envelope and a loss of the filaggrin hygroscopic amino acids that act as a natural barrier.4
Additionally, these patients have increased surface pH. This is of particular importance because at a certain pH, there is decreased inhibition of Staphylococcus aureus. Decreased activity of ceramide metabolism enzymes occurs, resulting in increased water loss along with increased entry of foreign substances.
The best treatment for atopic dermatitis is daily bathing with a mild, nondrying soap. Unfortunately, many parents purposely do not bathe their atopic children daily because they believe it is harmful. Many mild cleansers are available today that do not aggravate the condition.
Another effective treatment for eczema is to apply a moisturizer. Previously, thick ointments likely to occlude water loss were applied, but research on the pathophysiological process of atopic dermatitis has led to the development of moisturizers and topical skin products targeted to correct reduced amounts of ceramides and natural moisturizing factors in the skin with natural moisturizing factors, ceramides, and pseudoceramide products.5 A ceramide-containing moisturizer should be applied immediately after giving the child a bath. These moisturizers are available over-the-counter but can be relatively expensive. Some popular ceramide-containing skin lubricants include CeraVe cream, Cetaphil RestoraDerm cream, Aveeno eczema cream, and Eucerin eczema cream. If cost is an issue, a thick emollient such as petroleum jelly can be used.
Prevention
Several theories have been proposed to explain the development of eczema beyond genetic predisposition, leading to attempts to prevent its development. Theories include breastfeeding—both nursing and not nursing. While it seems that breastfeeding should be protective against atopic dermatitis, unfortunately there is no proof of this. Also, withholding certain foods during the introduction of solid foods (and not eating certain foods during nursing) will not decrease the risk of developing atopic dermatitis. Early exposure to farm animals has been debated as causative, and there is evidence that early exposure to antibiotics increases atopic dermatitis risk. Lastly, maternal fish oil or probiotic ingestion during pregnancy has not changed the rates of atopic dermatitis in infants.6,7 Therefore, the best course of action is to provide simple skin care guidelines and treat children as they present with atopic dermatitis.
ALLERGIC CONTACT DERMATITIS
Contrary to previously held beliefs, allergic contact dermatitis is not rare in children. In fact, rates are equal between children and adults. Allergic contact dermatitis or irritant contact dermatitis is also associated with skin barrier breakdown common among atopic dermatitis patients. Additionally, atopic dermatitis patients may exhibit allergic contact dermatitis due to exposure to various topical preparations.8
Toilet seats
Recently, allergic contact dermatitis has been reported in association with toilet seats,9 possibly due to polyurethane or polypropylene in the seat.10 Another possible reaction may be to chemicals in antiseptic wipes used on the toilet seat. The presentation of allergic contact dermatitis due to toilet seat contact is higher on the posterior thigh for older children compared to that seen in toddlers and younger children. It can be differentially diagnosed from atopic dermatitis because it is limited to the posterior leg.11
Baby wipes
Some baby-wipe brands contain the allergen methylisothiazolinone, alone or in combination with methylchloroisothiazolinone.12 Even expensive brands marketed as hypoallergenic may contain these chemicals or other types of preservatives. Interestingly, some parents continue to use baby wipes on their children’s faces and bodies as they grow up to provide a quick clean up. So, in children who are toddler age or older, allergic contact dermatitis to baby wipes may not be localized to the anogenital area. Consider this condition in the differential diagnoses with “lip licker” dermatitis.
Car seats
Car seats also are associated with contact dermatitis but the specific allergen is unknown. The presentation is similar to toilet seat dermatitis as the posterior leg is affected while the anterior leg remains clear. This type of contact dermatitis is more frequently reported with the use of car seats made of tightly woven, shiny material. A cotton cloth barrier can be placed over the car seat to prevent skin contact.13
Sports equipment
Allergic contact dermatitis may be associated with the use of shin guards and neoprene wetsuits, with p-tert butylphenol formaldehyde resin as the likely allergen.14 There is obvious delineation of the affected and nonaffected skin, which can help rule out atopic dermatitis (Figure 2). However, there is the potential for atopic dermatitis to develop with use of these items because the skin is occluded for long periods and will sweat, causing a reaction. Again, providing a barrier between the surface of the shin guard or wetsuit and the skin will help.
Metals
Finally, nickel and other metals are known sources of allergic contact dermatitis.15
TINEA CAPITIS
The challenge for this dermatologic condition is finding an effective treatment without a prolonged course of medicine. If tinea capitis continues for too long, the inflammatory process can result in permanent alopecia. The condition is caused by fungus, most commonly Trycophyton tonsurans.16 Terbinafine (3–8 mg/kg/day for 2–4 weeks) is superior to griseofulvin for T tonsurans; however, griseofulvin is superior to terbinafine for treating Microsporum species.17 Health insurance coverage of terbinafine is a concern for some families. Finally, children with tinea capitis should use a sporicidal shampoo, selenium sulfide, or ketoconazole to decrease the spread of spores.
INFANTILE HEMANGIOMAS
Propranolol
The major breakthrough in pediatric dermatology of the past decade has been the use of propranolol to treat infantile hemangiomas. Propranolol hydrochloride (Hemangeol) received US Food and Drug Administration (FDA) approval in March 2014 for the treatment of proliferating infantile hemangioma. The proposed mechanism of action is suppression of vascular endothelial growth factor and basic fibroblast growth factor in in vitro hemangioma-derived stem cells.18
Propranolol is indicated for complex cutaneous, visceral, hepatic, and airway infantile hemangiomas. For most children, treatment with oral propranolol is preferable to prolonged treatment with systemic steroids. If propranolol is started early in the disease course, it can greatly reduce the need for extensive surgery, which is of particular importance when the nasal tip, oral mucosa, and face are affected. Children with large hemangiomas that are prone to ulceration also greatly benefit from propranolol treatment. Ulcerated hemangiomas are extremely painful and problematic, and can take months to heal (Figure 3).
Concomitant abnormalities and PHACE
A visceral evaluation is generally indicated for children with five or six lesions, but hemangioma size should also be considered when determining the need for additional tests. When a facial, scalp, or neck infantile hemangioma is larger than 5 cm, imaging studies are indicated to check for vascular anomalies in the brain and congenital heart defects (most commonly aortic problems).
A subgroup of these children have PHACE (posterior fossa anomalies, hemangioma, arterial lesions, cardiac abnormalities/aortic coarctation, and eye abnormalities) syndrome. A consensus guideline found that evidence supports treating these patients with propranolol.19 However, there have been isolated reports of acute ischemic stroke in PHACE syndrome patients on concomitant steroid therapy with severe arteriopathy. Therefore, before initiating propranolol therapy, infants with large facial hemangiomas who are at risk for PHACE should be evaluated with magnetic resonance angiography of the head and neck, and with cardiac imaging that includes the aortic arch.
Propranolol dosing
The FDA-approved dosing of propranolol hydrochloride is 1 to 3 mg/kg/day. Side effects include hypoglycemia, hypotension, exacerbation of asthma or respiratory infections, dental caries, cold extremities, and night terrors. These side effects should be closely monitored for, particularly in younger infants; however, propranolol has a relatively benign safety profile that should be taken into consideration in the risk/benefit analysis.
New pathogenesis insights
In addition to breakthrough treatment, a greater understanding of the pathogenesis of infantile hemangiomas has been attained. The most rapid lesion growth phase occurs at a younger age than was previously believed, and it is now thought to be greatest when patients are between 5.5 and 7.5 weeks old.20 Treatment initiation before the rapid growth period, rather than after, is crucial to successful outcomes.
Additionally, research has identified some of the clinical signs prior to ulceration of infantile hemangioma.21 A white central area occurring in a proliferating lesion is predictive of ulceration. This white coloration of the proliferation stage should not be mistaken for the graying of involution that occurs when infants are older than 3 months. Again, initiating early propranolol therapy should prevent some of the pain associated with hemangiomas that are likely to ulcerate.
ALOPECIA AREATA
Intralesional steroids have been the gold standard of treatment for localized alopecia areata. However, for children who cannot tolerate the injections, can topical steroids achieve enough penetrance to reduce lesion duration? A recent study compared twice-daily application of clobetasol propionate 0.05% cream or hydrocortisone 1% cream for 6 weeks on followed by 6 weeks off for 24 weeks in children with alopecia areata affecting at least 10% of scalp surface area. The clobetasol cream was superior in terms of decreasing alopetic surface area compared with hydrocortisone.22 In reference to the efficacy of clobetasol cream, only localized alopecia areata showed this response, and not alopecia totalis or universalis.
RELATIVELY RARE DIAGNOSES
Epidermal nevi
Epidermal nevi size and phenotype have guided treatment decisions when evaluating these lesions. It is believed that epidermal nevi are due to RAS/MAPK mutations, and further examination of genetic causes has become increasingly important. If the genetic mutation is known, clinical signs and genetic testing can be used to monitor children for conditions potentially related to this mosaic disorder, and to properly diagnose and treat the lesions.
Recently, researchers have been able to explain why some children with large epidermal nevi develop hypophosphatemic rickets and others do not. Mutations in HRAS or NRAS can lead to an increase in fibroblast growth factor-23, resulting in this bone abnormality.23
Tuberous sclerosis
Tuberous sclerosis (TS) is remarkable for noncancerous lesions occurring in skin, brain, kidneys, nervous system, heart, lungs, or retina. Skin presentations include patches of light-colored skin, thickened skin, growths under the nails, and facial lesions that resemble acne. Ash leaf or café au lait macules may occur, as well as large plaque angiofibromas.
While oral rapamycin has been used to treat internal tumors related to TS, a low-dose topical formula has been shown effective for treating facial angiofibromas of TS.24 This new therapy is important given the impact of facial anomalies on psychosocial development. In addition, topical rapamycin avoids the need for laser treatment, which is more painful and less effective.
SUMMARY
The understanding of common and rare skin conditions has increased during the past decade. Studies in dermatologic diagnoses and treatment have produced new insights into genetic mutations, including those involved in the development of epidermal nevi. Serendipitous findings, such as the use of propranolol for infantile hemangiomas, have also occurred. These advances have allowed for greater diagnostic accuracy and better treatments. Less invasive treatment strategies also have been developed, including clobetasol cream for localized alopecia areata and topical rapamycin for tuberous sclerosis, and these have led to greater compliance, fewer treatment-related adverse effects, and more efficacious outcomes.
- Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon school children. J Am Acad Dermatol 2000;43: 649–655.
- Hanifin JM, Reed ML; Eczema Prevalence and Impact Working Group. A population-based survey of eczema prevalence in the United States. Dermatitis 2007; 18:82–91.
- Ruzicka T. Atopic eczema between rationality and irrationality. Arch Dermatol 1998; 134:1462–1469.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization through barrier-disrupted skin elicits a TH2-dominant cytokine response. Eur J Immunol 1998; 28:769–779.
- Hon KL, Leung AK, Barankin B. Barrier repair therapy in atopic dermatitis: an overview. Am J Clin Dermatol 2013; 14:389–399.
- Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol 2004; 15(suppl 16):4–5, 9–32.
- Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years’ follow-up. Acta Paediatr Suppl 1996; 414:1–21.
- Admani S, Jacob SE. Allergic contact dermatitis in children: review of the past decade. Curr Allergy Asthma Rep 2014; 14:421.
- Holme SA, Stone NM, Mills CM. Toilet seat contact dermatitis. Pediatr Dermatol 2005; 22:344–345.
- Heilig S, Adams DR, Zaenglein AL. Persistent allergic contact dermatitis to plastic toilet seats. Pediatr Dermatol 2011; 28:587–590.
- Litvinov IV, Sugathan P, Cohen BA. Recognizing and treating toilet-seat contact dermatitis in children. Pediatrics 2010; 125:e419–e422.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis 2013; 24:2–6.
- Ghali FE. “Car seat dermatitis”: a newly described form of contact dermatitis. Pediatr Dermatol 2011; 28:321–326.
- Herro E, Jacob SE. p-tert-Butylphenol formaldehyde resin and its impact on children. Dermatitis 2012; 23:86–88.
- Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis. J Am Acad Dermatol 2013; 69:232–237.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol 2004; 50:748–752.
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol 2013; 30:1–6.
- Zhang L, Mai HM, Zheng J, et al. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol 2013; 7:48–55.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 2013; 131:128–140.
- Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents’ photographs tell us. Pediatrics 2012; 130:e314–e320.
- Maguiness SM, Hoffman WY, McCalmont TH, Frieden IJ. Early white discoloration of infantile hemangioma: a sign of impending ulceration. Arch Dermatol 2010; 146:1235–1239.
- Lenane P, Macarthur C, Parkin PC, et al. Clobetasol propionate, 0.05%, vs hydrocortisone, 1%, for alopecia areata in children: a randomized clinical trial. JAMA Dermatol 2014; 150:47–50.
- Lim YH, Ovejero D, Sugarman JS, et al. Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Hum Mol Genet 2014; 23:397–407.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double-blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs R D 2012; 12:121–126.
- Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon school children. J Am Acad Dermatol 2000;43: 649–655.
- Hanifin JM, Reed ML; Eczema Prevalence and Impact Working Group. A population-based survey of eczema prevalence in the United States. Dermatitis 2007; 18:82–91.
- Ruzicka T. Atopic eczema between rationality and irrationality. Arch Dermatol 1998; 134:1462–1469.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization through barrier-disrupted skin elicits a TH2-dominant cytokine response. Eur J Immunol 1998; 28:769–779.
- Hon KL, Leung AK, Barankin B. Barrier repair therapy in atopic dermatitis: an overview. Am J Clin Dermatol 2013; 14:389–399.
- Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol 2004; 15(suppl 16):4–5, 9–32.
- Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years’ follow-up. Acta Paediatr Suppl 1996; 414:1–21.
- Admani S, Jacob SE. Allergic contact dermatitis in children: review of the past decade. Curr Allergy Asthma Rep 2014; 14:421.
- Holme SA, Stone NM, Mills CM. Toilet seat contact dermatitis. Pediatr Dermatol 2005; 22:344–345.
- Heilig S, Adams DR, Zaenglein AL. Persistent allergic contact dermatitis to plastic toilet seats. Pediatr Dermatol 2011; 28:587–590.
- Litvinov IV, Sugathan P, Cohen BA. Recognizing and treating toilet-seat contact dermatitis in children. Pediatrics 2010; 125:e419–e422.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis 2013; 24:2–6.
- Ghali FE. “Car seat dermatitis”: a newly described form of contact dermatitis. Pediatr Dermatol 2011; 28:321–326.
- Herro E, Jacob SE. p-tert-Butylphenol formaldehyde resin and its impact on children. Dermatitis 2012; 23:86–88.
- Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis. J Am Acad Dermatol 2013; 69:232–237.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol 2004; 50:748–752.
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol 2013; 30:1–6.
- Zhang L, Mai HM, Zheng J, et al. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol 2013; 7:48–55.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 2013; 131:128–140.
- Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents’ photographs tell us. Pediatrics 2012; 130:e314–e320.
- Maguiness SM, Hoffman WY, McCalmont TH, Frieden IJ. Early white discoloration of infantile hemangioma: a sign of impending ulceration. Arch Dermatol 2010; 146:1235–1239.
- Lenane P, Macarthur C, Parkin PC, et al. Clobetasol propionate, 0.05%, vs hydrocortisone, 1%, for alopecia areata in children: a randomized clinical trial. JAMA Dermatol 2014; 150:47–50.
- Lim YH, Ovejero D, Sugarman JS, et al. Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Hum Mol Genet 2014; 23:397–407.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double-blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs R D 2012; 12:121–126.
Learning disorders: How pediatricians can help
Learning disabilities can negatively affect the child, family, school, and, ultimately, society. Approximately 10% of US children have a learning disability.1 Unfortunately, learning disabilities are often unaddressed, under-addressed, or incorrectly addressed by family and schools.
Pediatricians are well positioned to address these concerns, refer for screenings and diagnoses, and provide additional support. This requires knowledge and skills to identify the risk factors for learning disabilities, recognize the early warning signs, and apply the appropriate diagnostic tools. Additionally, primary care pediatricians can support families with referrals to appropriate healthcare specialists and by communicating with patients’ schools.
LEARNING DISABILITIES DEFINED
There is no universal consensus regarding what constitutes a learning disability. The American Pediatrics Association defines specific learning disorder as reading, written expression, or mathematics skills that are substantially lower than expected for the individual’s age, measured intelligence, and age-appropriate education level or when achievement falls below a set standard.2
The Individuals with Disabilities Education Act (IDEA),3 which governs considerations schools must make for learning-disabled students, more broadly defines specific learning disability as impairment in one or more of the following: math, understanding or using written or spoken language, information processing, memory, and reading. Specific reading impairments include dyslexia, orthographic impairment (inability to memorize words), and hyperlexia disorder (comprehension difficulties). The IDEA also includes conditions such as perceptual disabilities, brain injury, minimal brain dysfunction, and developmental aphasia as learning disabilities.
EPIDEMIOLOGY
Approximately 10% of children in the United States have a learning disability. Of those affected, 40.7% have a learning disability in reading, language, math, information processing, or memory. Speech or language impairments affect 18.5%, of which dyslexia is the most common disability, affecting up to 80%. Mental retardation affects 7.4%, and 6.4% have serious emotional disturbances that prevent learning. In addition, up to 35% of those with a learning disorder have comorbid attention deficit hyperactivity disorder (ADHD) or other mental health difficulties including depression, anxiety, bipolar disorder, and obsessive compulsive disorder. Learning disabilities are twice as common in children with chronic health conditions.1,4,5
LEARNING DISABILITY ASSESSMENT
Medical conditions
The medical evaluation for suspected learning disability must be tailored to rule out obvious underlying or associated medical issues. Fetal alcohol syndrome, dysmorphisms, other syndromes, and apparent genetic causes should be ruled out.
Vision and hearing screens should be ordered and patients should be assessed for other potential sensory impairments that may resemble features of a learning disability. Medications such as anticonvulsants may have side effects that can cause learning difficulties and in older children, substance abuse must be ruled out.
Risk factors
A medical history should identify risk factors for learning disabilities. These include prematurity, low birth weight, early-life malnutrition, poverty and under-stimulating environments, head injury, epilepsy, and chronic health conditions. A family history of learning disabilities, including dyslexia or other learning disabilities, attention deficit, memory difficulty, and dropping out of school, are also risk factors.6
Early warning signs
Early warning signs of learning disability are listed in Table 1. Speech delay is particularly important to assess and parents should be asked about the child’s history of acquiring language. Children with problems discriminating sounds may present with difficulty in articulation.
Asking parents whether the child has trouble rhyming words, learning song lyrics, or carrying a tune is often helpful. For example, a child may be able to recite the words to “Happy Birthday to You” but cannot sing it. These questions provide information about ability to memorize words, follow directions, recite words back, and can indicate possible speech or hearing difficulties.
Written language ability can be assessed with pseudoword decoding or deciphering nonsense words based on phonemic awareness.
Although difficulty following directions can be an early warning sign, many children have problems in this area without a specific learning disability, especially if the direction is not in accordance with the level of neurological development (ie, complex instructions given to a younger child).
Fine motor skill impairment can be observed when children cannot easily hold utensils, button or buckle clothing, or manipulate small objects such as pencils and crayons. However, the ability to manipulate small objects should not rule out learning disability in institutionalized children or those from larger families, where small motor skills and independent dressing develop earlier. Other warning signs may be better indicators in these children.
Impaired visual-spatial processing may be manifested in a younger child as difficulty matching shapes and in an older child or adolescent as inability to copy information from a smart-board or whiteboard onto paper. Learning disabilities also may present as difficulty with processing of visual and auditory information.
Executive function
Learning disabilities can affect working memory and processing speed, which are basic cognitive processes subservient to the higher-order executive functions. The prefrontal cortex does not start to fully develop until a child is 7 to 8 years old. Impairment in this area may present as difficulties with time management, organization, or losing things. Those with right hemisphere involvement may have nonverbal learning disabilities exhibited as difficulty understanding math and word problems, and difficulty with perceptual reasoning. For example, the child may be able to repeat information in a rote manner but not grasp the meaning of what is said. This is sometimes termed a “cocktail personality,” in which a child’s conversation becomes nonsensical.
Nonverbal learning disabilities also are often seen in those with fetal alcohol syndrome or other fetal alcohol spectrum disorders such as alcohol-related neurodevelopmental disorder.
Attention deficit hyperactivity disorder
Learning disorders appear to have an established association with ADHD. Children with ADHD are twice as likely to have dyslexia, and, conversely, children with dyslexia are twice as likely to have ADHD. It is therefore difficult to establish which condition is primary. Did the ADHD behavior precede the dyslexia and accentuate a reading difficulty? Perhaps the dyslexia was primary, which led to inattentiveness on account of the inability to decipher words.
For these reasons, psychological assessments should be performed in a timely fashion to delineate the cause. It is easier for the general pediatrician to screen for attention problems than for dyslexia. Validated tests that screen for reading disorders in 3- to- 5-year-old children are not a routine part of well-child care.
SCHOOL SUPPORT
The IDEA requires that public schools assess children to determine their needs and provide the necessary support to address those needs.5 The goal is to ensure that all children receive free and appropriate education.
Assessment types
School districts’ assessments vary, but they are required to evaluate all areas of suspected disability, including during all parts of a school day, and to be carried out in a nonrestrictive environment (ie, educated in the regular classroom the student would attend if not disabled). The objective is to determine whether a child is eligible for an individualized education plan (IEP).
Schools have two options for learning disability assessment. The most common is a psychoeducational evaluation that includes cognitive and achievement assessment. Time limits for completing these assessments vary by state.
The alternative is to provide a response to intervention (RTI) approach. According to the National Center on RTI, “schools identify students at risk for poor learning outcomes, monitor student progress, provide evidence-based interventions, and adjust the intensity and nature of those interventions depending on a student’s responsiveness, and identify students with learning disabilities or other disabilities.”7 With RTI, there are varying levels of intensity, which may result in undue delays in education as a result of the observation period prior to intervention.
Any assessment requires evaluation of speech, language, and mathematics by a qualified professional, but the school is not required to do psychological or educational assessment. The role of school systems is not to provide comprehensive mental health support for students.
Individualized education plans
If an assessment finds a learning disability currently covered by IDEA (Table 2) and the disability affects effective educational progress, the school must develop an IEP for the student.
An IEP is a written contract outlining specific goals and measurable outcomes. It may include school placement determination and specific services like occupational therapy, physical therapy, speech, and/or special education services. The outcomes of an IEP can vary widely from state to state.
504 plans
When an assessment finds a covered disability but the disability is determined not to be a cause of educational impairment in terms of the child’s functioning in the school, the child will not qualify for an IEP but may be eligible for a 504 plan. Typically, a 504 plan involves making appropriate accommodation such as classroom seating location, homework modifications, and testing modifications.
Disagreement
There are options when the family and school disagree on the assessment outcome or the extent of an IEP plan. It is usually beneficial for the family to accept the IEP so that something is in place for the child while attempts are made to resolve the issue. In some cases, the family may choose to pursue their state-specific appeals process or mediation and/or to proceed to litigation. They also have the right to pursue independent assessment of their child’s suspected learning disability; however, this is an out-of-pocket expense that may not be covered by health insurance. Furthermore, the school is not required to accept independent assessor results or recommendations.
There may be benefits from independent neuropsychological evaluations because most school districts do not have access to neuropsychologists. Neuropsychological evaluations can provide a detailed assessment of higher-level cognitive abilities, such as executive function, memory, visual-spatial processing, visual-motor processing, language function, effort, and attention.
THE PEDIATRICIAN’S ROLE
School communication
The pediatrician can play a key role in requesting the initial multifactorial learning disability assessment on behalf of the family. Cleveland Clinic has template letters available for both the parents and the pediatrician to send to the school that can be adapted (Figure 1). Both should state the suspected learning disability ing disabilities and problems and should be addressed to the school principal.
Pediatricians can also communicate with the teacher about classroom strategies before an IEP or 504 Plan is determined, in order to help set the context of what may be coming. Surprisingly, many teachers are unfamiliar with requirements made for learning-disabled students, such as the benefits of seating the child toward the front of a class.
Talk to the child
The pediatrician should emphasize to the child that everyone learns differently and in a way best suited to him or her. The child should know that everyone’s brain works differently, and every child should try to work hard at school because it is their job in life at that age. Children can relate to an analogy that the brain is like a computer, with some models being faster than others and analyzing data differently.
Parents can help children build on their natural strengths and talents despite the learning disability. Point out that some children are good at math, others at reading, and some mainly excel at sports or the arts. It may be helpful to provide real-life examples of people who are successful despite learning disability, such as a celebrity with dyslexia.
Talk to the parents/guardians
Parents/guardians need to know that learning disability is not the result of poor parenting. Moreover, emphasize that the child is not lazy or stupid and that the condition can be very exhausting and frustrating for the child. Their child will have good and bad days, much like they do at their own work. Patience, rather than force, should be stressed.
Encourage parents to find alternative ways to teach their child that make it easier for them to learn. Online education programs may serve as beneficial supplements for these children, and field trips can provide more hands-on learning. In addition, talk to parents about the need for additional, independent testing and be able to refer them to appropriate sources for such testing as well as to neuropsychological resources, when warranted.
CONCLUSION
As families increasingly turn to pediatricians to address learning disabilities and problems children are having at school, physicians need to understand the various diagnoses and be able to assist with proper assessments. While schools must provide modifications when a diagnosis is made, there is sometimes disagreement about what the specific learning disability is and its extent of involvement in the child’s education. Diagnosing and addressing learning disability early can lessen various behavioral problems, help prevent dropping out of school, and enhance life outcomes for the child.
- Altarac M, Saroha E. Lifetime prevalence of learning disability among US children. Pediatrics 2007; 119(suppl 1):S77–S83.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Building the Legacy: IDEA 2004. U.S. Department of Education website. http://idea.ed.gov/. Accessed July 6, 2015.
- Pastor PN, Reuben CA. Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004–2006. Vital Health Stat 10 2008; 237:1–14. http://www.cdc.gov/nchs/data/series/sr_10/Sr10_237.pdf. Accessed July 6, 2015.
- U.S. Department of Education, Office of Special Education and Rehabilitative Services. 29th Annual Report to Congress on the Implementation of the Individuals with Disabilities Education Act, 2007, vol 1. Washington, DC; 2010. http://www2.ed.gov/about/reports/annual/osep/2007/parts-b-c/29th-vol-1.pdf. Accessed July 6, 2015.
- National Joint Committee on Learning Disabilities. Learning Disabilities and Young Children: Identification and Intervention (technical report). October 2007. http://www.asha.org/policy/TR2007-00307/. Accessed July 6, 2015.
- Response to intervention (RTI). Center for Parent Information and Resources website. http://www.parentcenterhub.org/repository/rti/. Updated August 2012. Accessed July 6, 2015.
Learning disabilities can negatively affect the child, family, school, and, ultimately, society. Approximately 10% of US children have a learning disability.1 Unfortunately, learning disabilities are often unaddressed, under-addressed, or incorrectly addressed by family and schools.
Pediatricians are well positioned to address these concerns, refer for screenings and diagnoses, and provide additional support. This requires knowledge and skills to identify the risk factors for learning disabilities, recognize the early warning signs, and apply the appropriate diagnostic tools. Additionally, primary care pediatricians can support families with referrals to appropriate healthcare specialists and by communicating with patients’ schools.
LEARNING DISABILITIES DEFINED
There is no universal consensus regarding what constitutes a learning disability. The American Pediatrics Association defines specific learning disorder as reading, written expression, or mathematics skills that are substantially lower than expected for the individual’s age, measured intelligence, and age-appropriate education level or when achievement falls below a set standard.2
The Individuals with Disabilities Education Act (IDEA),3 which governs considerations schools must make for learning-disabled students, more broadly defines specific learning disability as impairment in one or more of the following: math, understanding or using written or spoken language, information processing, memory, and reading. Specific reading impairments include dyslexia, orthographic impairment (inability to memorize words), and hyperlexia disorder (comprehension difficulties). The IDEA also includes conditions such as perceptual disabilities, brain injury, minimal brain dysfunction, and developmental aphasia as learning disabilities.
EPIDEMIOLOGY
Approximately 10% of children in the United States have a learning disability. Of those affected, 40.7% have a learning disability in reading, language, math, information processing, or memory. Speech or language impairments affect 18.5%, of which dyslexia is the most common disability, affecting up to 80%. Mental retardation affects 7.4%, and 6.4% have serious emotional disturbances that prevent learning. In addition, up to 35% of those with a learning disorder have comorbid attention deficit hyperactivity disorder (ADHD) or other mental health difficulties including depression, anxiety, bipolar disorder, and obsessive compulsive disorder. Learning disabilities are twice as common in children with chronic health conditions.1,4,5
LEARNING DISABILITY ASSESSMENT
Medical conditions
The medical evaluation for suspected learning disability must be tailored to rule out obvious underlying or associated medical issues. Fetal alcohol syndrome, dysmorphisms, other syndromes, and apparent genetic causes should be ruled out.
Vision and hearing screens should be ordered and patients should be assessed for other potential sensory impairments that may resemble features of a learning disability. Medications such as anticonvulsants may have side effects that can cause learning difficulties and in older children, substance abuse must be ruled out.
Risk factors
A medical history should identify risk factors for learning disabilities. These include prematurity, low birth weight, early-life malnutrition, poverty and under-stimulating environments, head injury, epilepsy, and chronic health conditions. A family history of learning disabilities, including dyslexia or other learning disabilities, attention deficit, memory difficulty, and dropping out of school, are also risk factors.6
Early warning signs
Early warning signs of learning disability are listed in Table 1. Speech delay is particularly important to assess and parents should be asked about the child’s history of acquiring language. Children with problems discriminating sounds may present with difficulty in articulation.
Asking parents whether the child has trouble rhyming words, learning song lyrics, or carrying a tune is often helpful. For example, a child may be able to recite the words to “Happy Birthday to You” but cannot sing it. These questions provide information about ability to memorize words, follow directions, recite words back, and can indicate possible speech or hearing difficulties.
Written language ability can be assessed with pseudoword decoding or deciphering nonsense words based on phonemic awareness.
Although difficulty following directions can be an early warning sign, many children have problems in this area without a specific learning disability, especially if the direction is not in accordance with the level of neurological development (ie, complex instructions given to a younger child).
Fine motor skill impairment can be observed when children cannot easily hold utensils, button or buckle clothing, or manipulate small objects such as pencils and crayons. However, the ability to manipulate small objects should not rule out learning disability in institutionalized children or those from larger families, where small motor skills and independent dressing develop earlier. Other warning signs may be better indicators in these children.
Impaired visual-spatial processing may be manifested in a younger child as difficulty matching shapes and in an older child or adolescent as inability to copy information from a smart-board or whiteboard onto paper. Learning disabilities also may present as difficulty with processing of visual and auditory information.
Executive function
Learning disabilities can affect working memory and processing speed, which are basic cognitive processes subservient to the higher-order executive functions. The prefrontal cortex does not start to fully develop until a child is 7 to 8 years old. Impairment in this area may present as difficulties with time management, organization, or losing things. Those with right hemisphere involvement may have nonverbal learning disabilities exhibited as difficulty understanding math and word problems, and difficulty with perceptual reasoning. For example, the child may be able to repeat information in a rote manner but not grasp the meaning of what is said. This is sometimes termed a “cocktail personality,” in which a child’s conversation becomes nonsensical.
Nonverbal learning disabilities also are often seen in those with fetal alcohol syndrome or other fetal alcohol spectrum disorders such as alcohol-related neurodevelopmental disorder.
Attention deficit hyperactivity disorder
Learning disorders appear to have an established association with ADHD. Children with ADHD are twice as likely to have dyslexia, and, conversely, children with dyslexia are twice as likely to have ADHD. It is therefore difficult to establish which condition is primary. Did the ADHD behavior precede the dyslexia and accentuate a reading difficulty? Perhaps the dyslexia was primary, which led to inattentiveness on account of the inability to decipher words.
For these reasons, psychological assessments should be performed in a timely fashion to delineate the cause. It is easier for the general pediatrician to screen for attention problems than for dyslexia. Validated tests that screen for reading disorders in 3- to- 5-year-old children are not a routine part of well-child care.
SCHOOL SUPPORT
The IDEA requires that public schools assess children to determine their needs and provide the necessary support to address those needs.5 The goal is to ensure that all children receive free and appropriate education.
Assessment types
School districts’ assessments vary, but they are required to evaluate all areas of suspected disability, including during all parts of a school day, and to be carried out in a nonrestrictive environment (ie, educated in the regular classroom the student would attend if not disabled). The objective is to determine whether a child is eligible for an individualized education plan (IEP).
Schools have two options for learning disability assessment. The most common is a psychoeducational evaluation that includes cognitive and achievement assessment. Time limits for completing these assessments vary by state.
The alternative is to provide a response to intervention (RTI) approach. According to the National Center on RTI, “schools identify students at risk for poor learning outcomes, monitor student progress, provide evidence-based interventions, and adjust the intensity and nature of those interventions depending on a student’s responsiveness, and identify students with learning disabilities or other disabilities.”7 With RTI, there are varying levels of intensity, which may result in undue delays in education as a result of the observation period prior to intervention.
Any assessment requires evaluation of speech, language, and mathematics by a qualified professional, but the school is not required to do psychological or educational assessment. The role of school systems is not to provide comprehensive mental health support for students.
Individualized education plans
If an assessment finds a learning disability currently covered by IDEA (Table 2) and the disability affects effective educational progress, the school must develop an IEP for the student.
An IEP is a written contract outlining specific goals and measurable outcomes. It may include school placement determination and specific services like occupational therapy, physical therapy, speech, and/or special education services. The outcomes of an IEP can vary widely from state to state.
504 plans
When an assessment finds a covered disability but the disability is determined not to be a cause of educational impairment in terms of the child’s functioning in the school, the child will not qualify for an IEP but may be eligible for a 504 plan. Typically, a 504 plan involves making appropriate accommodation such as classroom seating location, homework modifications, and testing modifications.
Disagreement
There are options when the family and school disagree on the assessment outcome or the extent of an IEP plan. It is usually beneficial for the family to accept the IEP so that something is in place for the child while attempts are made to resolve the issue. In some cases, the family may choose to pursue their state-specific appeals process or mediation and/or to proceed to litigation. They also have the right to pursue independent assessment of their child’s suspected learning disability; however, this is an out-of-pocket expense that may not be covered by health insurance. Furthermore, the school is not required to accept independent assessor results or recommendations.
There may be benefits from independent neuropsychological evaluations because most school districts do not have access to neuropsychologists. Neuropsychological evaluations can provide a detailed assessment of higher-level cognitive abilities, such as executive function, memory, visual-spatial processing, visual-motor processing, language function, effort, and attention.
THE PEDIATRICIAN’S ROLE
School communication
The pediatrician can play a key role in requesting the initial multifactorial learning disability assessment on behalf of the family. Cleveland Clinic has template letters available for both the parents and the pediatrician to send to the school that can be adapted (Figure 1). Both should state the suspected learning disability ing disabilities and problems and should be addressed to the school principal.
Pediatricians can also communicate with the teacher about classroom strategies before an IEP or 504 Plan is determined, in order to help set the context of what may be coming. Surprisingly, many teachers are unfamiliar with requirements made for learning-disabled students, such as the benefits of seating the child toward the front of a class.
Talk to the child
The pediatrician should emphasize to the child that everyone learns differently and in a way best suited to him or her. The child should know that everyone’s brain works differently, and every child should try to work hard at school because it is their job in life at that age. Children can relate to an analogy that the brain is like a computer, with some models being faster than others and analyzing data differently.
Parents can help children build on their natural strengths and talents despite the learning disability. Point out that some children are good at math, others at reading, and some mainly excel at sports or the arts. It may be helpful to provide real-life examples of people who are successful despite learning disability, such as a celebrity with dyslexia.
Talk to the parents/guardians
Parents/guardians need to know that learning disability is not the result of poor parenting. Moreover, emphasize that the child is not lazy or stupid and that the condition can be very exhausting and frustrating for the child. Their child will have good and bad days, much like they do at their own work. Patience, rather than force, should be stressed.
Encourage parents to find alternative ways to teach their child that make it easier for them to learn. Online education programs may serve as beneficial supplements for these children, and field trips can provide more hands-on learning. In addition, talk to parents about the need for additional, independent testing and be able to refer them to appropriate sources for such testing as well as to neuropsychological resources, when warranted.
CONCLUSION
As families increasingly turn to pediatricians to address learning disabilities and problems children are having at school, physicians need to understand the various diagnoses and be able to assist with proper assessments. While schools must provide modifications when a diagnosis is made, there is sometimes disagreement about what the specific learning disability is and its extent of involvement in the child’s education. Diagnosing and addressing learning disability early can lessen various behavioral problems, help prevent dropping out of school, and enhance life outcomes for the child.
Learning disabilities can negatively affect the child, family, school, and, ultimately, society. Approximately 10% of US children have a learning disability.1 Unfortunately, learning disabilities are often unaddressed, under-addressed, or incorrectly addressed by family and schools.
Pediatricians are well positioned to address these concerns, refer for screenings and diagnoses, and provide additional support. This requires knowledge and skills to identify the risk factors for learning disabilities, recognize the early warning signs, and apply the appropriate diagnostic tools. Additionally, primary care pediatricians can support families with referrals to appropriate healthcare specialists and by communicating with patients’ schools.
LEARNING DISABILITIES DEFINED
There is no universal consensus regarding what constitutes a learning disability. The American Pediatrics Association defines specific learning disorder as reading, written expression, or mathematics skills that are substantially lower than expected for the individual’s age, measured intelligence, and age-appropriate education level or when achievement falls below a set standard.2
The Individuals with Disabilities Education Act (IDEA),3 which governs considerations schools must make for learning-disabled students, more broadly defines specific learning disability as impairment in one or more of the following: math, understanding or using written or spoken language, information processing, memory, and reading. Specific reading impairments include dyslexia, orthographic impairment (inability to memorize words), and hyperlexia disorder (comprehension difficulties). The IDEA also includes conditions such as perceptual disabilities, brain injury, minimal brain dysfunction, and developmental aphasia as learning disabilities.
EPIDEMIOLOGY
Approximately 10% of children in the United States have a learning disability. Of those affected, 40.7% have a learning disability in reading, language, math, information processing, or memory. Speech or language impairments affect 18.5%, of which dyslexia is the most common disability, affecting up to 80%. Mental retardation affects 7.4%, and 6.4% have serious emotional disturbances that prevent learning. In addition, up to 35% of those with a learning disorder have comorbid attention deficit hyperactivity disorder (ADHD) or other mental health difficulties including depression, anxiety, bipolar disorder, and obsessive compulsive disorder. Learning disabilities are twice as common in children with chronic health conditions.1,4,5
LEARNING DISABILITY ASSESSMENT
Medical conditions
The medical evaluation for suspected learning disability must be tailored to rule out obvious underlying or associated medical issues. Fetal alcohol syndrome, dysmorphisms, other syndromes, and apparent genetic causes should be ruled out.
Vision and hearing screens should be ordered and patients should be assessed for other potential sensory impairments that may resemble features of a learning disability. Medications such as anticonvulsants may have side effects that can cause learning difficulties and in older children, substance abuse must be ruled out.
Risk factors
A medical history should identify risk factors for learning disabilities. These include prematurity, low birth weight, early-life malnutrition, poverty and under-stimulating environments, head injury, epilepsy, and chronic health conditions. A family history of learning disabilities, including dyslexia or other learning disabilities, attention deficit, memory difficulty, and dropping out of school, are also risk factors.6
Early warning signs
Early warning signs of learning disability are listed in Table 1. Speech delay is particularly important to assess and parents should be asked about the child’s history of acquiring language. Children with problems discriminating sounds may present with difficulty in articulation.
Asking parents whether the child has trouble rhyming words, learning song lyrics, or carrying a tune is often helpful. For example, a child may be able to recite the words to “Happy Birthday to You” but cannot sing it. These questions provide information about ability to memorize words, follow directions, recite words back, and can indicate possible speech or hearing difficulties.
Written language ability can be assessed with pseudoword decoding or deciphering nonsense words based on phonemic awareness.
Although difficulty following directions can be an early warning sign, many children have problems in this area without a specific learning disability, especially if the direction is not in accordance with the level of neurological development (ie, complex instructions given to a younger child).
Fine motor skill impairment can be observed when children cannot easily hold utensils, button or buckle clothing, or manipulate small objects such as pencils and crayons. However, the ability to manipulate small objects should not rule out learning disability in institutionalized children or those from larger families, where small motor skills and independent dressing develop earlier. Other warning signs may be better indicators in these children.
Impaired visual-spatial processing may be manifested in a younger child as difficulty matching shapes and in an older child or adolescent as inability to copy information from a smart-board or whiteboard onto paper. Learning disabilities also may present as difficulty with processing of visual and auditory information.
Executive function
Learning disabilities can affect working memory and processing speed, which are basic cognitive processes subservient to the higher-order executive functions. The prefrontal cortex does not start to fully develop until a child is 7 to 8 years old. Impairment in this area may present as difficulties with time management, organization, or losing things. Those with right hemisphere involvement may have nonverbal learning disabilities exhibited as difficulty understanding math and word problems, and difficulty with perceptual reasoning. For example, the child may be able to repeat information in a rote manner but not grasp the meaning of what is said. This is sometimes termed a “cocktail personality,” in which a child’s conversation becomes nonsensical.
Nonverbal learning disabilities also are often seen in those with fetal alcohol syndrome or other fetal alcohol spectrum disorders such as alcohol-related neurodevelopmental disorder.
Attention deficit hyperactivity disorder
Learning disorders appear to have an established association with ADHD. Children with ADHD are twice as likely to have dyslexia, and, conversely, children with dyslexia are twice as likely to have ADHD. It is therefore difficult to establish which condition is primary. Did the ADHD behavior precede the dyslexia and accentuate a reading difficulty? Perhaps the dyslexia was primary, which led to inattentiveness on account of the inability to decipher words.
For these reasons, psychological assessments should be performed in a timely fashion to delineate the cause. It is easier for the general pediatrician to screen for attention problems than for dyslexia. Validated tests that screen for reading disorders in 3- to- 5-year-old children are not a routine part of well-child care.
SCHOOL SUPPORT
The IDEA requires that public schools assess children to determine their needs and provide the necessary support to address those needs.5 The goal is to ensure that all children receive free and appropriate education.
Assessment types
School districts’ assessments vary, but they are required to evaluate all areas of suspected disability, including during all parts of a school day, and to be carried out in a nonrestrictive environment (ie, educated in the regular classroom the student would attend if not disabled). The objective is to determine whether a child is eligible for an individualized education plan (IEP).
Schools have two options for learning disability assessment. The most common is a psychoeducational evaluation that includes cognitive and achievement assessment. Time limits for completing these assessments vary by state.
The alternative is to provide a response to intervention (RTI) approach. According to the National Center on RTI, “schools identify students at risk for poor learning outcomes, monitor student progress, provide evidence-based interventions, and adjust the intensity and nature of those interventions depending on a student’s responsiveness, and identify students with learning disabilities or other disabilities.”7 With RTI, there are varying levels of intensity, which may result in undue delays in education as a result of the observation period prior to intervention.
Any assessment requires evaluation of speech, language, and mathematics by a qualified professional, but the school is not required to do psychological or educational assessment. The role of school systems is not to provide comprehensive mental health support for students.
Individualized education plans
If an assessment finds a learning disability currently covered by IDEA (Table 2) and the disability affects effective educational progress, the school must develop an IEP for the student.
An IEP is a written contract outlining specific goals and measurable outcomes. It may include school placement determination and specific services like occupational therapy, physical therapy, speech, and/or special education services. The outcomes of an IEP can vary widely from state to state.
504 plans
When an assessment finds a covered disability but the disability is determined not to be a cause of educational impairment in terms of the child’s functioning in the school, the child will not qualify for an IEP but may be eligible for a 504 plan. Typically, a 504 plan involves making appropriate accommodation such as classroom seating location, homework modifications, and testing modifications.
Disagreement
There are options when the family and school disagree on the assessment outcome or the extent of an IEP plan. It is usually beneficial for the family to accept the IEP so that something is in place for the child while attempts are made to resolve the issue. In some cases, the family may choose to pursue their state-specific appeals process or mediation and/or to proceed to litigation. They also have the right to pursue independent assessment of their child’s suspected learning disability; however, this is an out-of-pocket expense that may not be covered by health insurance. Furthermore, the school is not required to accept independent assessor results or recommendations.
There may be benefits from independent neuropsychological evaluations because most school districts do not have access to neuropsychologists. Neuropsychological evaluations can provide a detailed assessment of higher-level cognitive abilities, such as executive function, memory, visual-spatial processing, visual-motor processing, language function, effort, and attention.
THE PEDIATRICIAN’S ROLE
School communication
The pediatrician can play a key role in requesting the initial multifactorial learning disability assessment on behalf of the family. Cleveland Clinic has template letters available for both the parents and the pediatrician to send to the school that can be adapted (Figure 1). Both should state the suspected learning disability ing disabilities and problems and should be addressed to the school principal.
Pediatricians can also communicate with the teacher about classroom strategies before an IEP or 504 Plan is determined, in order to help set the context of what may be coming. Surprisingly, many teachers are unfamiliar with requirements made for learning-disabled students, such as the benefits of seating the child toward the front of a class.
Talk to the child
The pediatrician should emphasize to the child that everyone learns differently and in a way best suited to him or her. The child should know that everyone’s brain works differently, and every child should try to work hard at school because it is their job in life at that age. Children can relate to an analogy that the brain is like a computer, with some models being faster than others and analyzing data differently.
Parents can help children build on their natural strengths and talents despite the learning disability. Point out that some children are good at math, others at reading, and some mainly excel at sports or the arts. It may be helpful to provide real-life examples of people who are successful despite learning disability, such as a celebrity with dyslexia.
Talk to the parents/guardians
Parents/guardians need to know that learning disability is not the result of poor parenting. Moreover, emphasize that the child is not lazy or stupid and that the condition can be very exhausting and frustrating for the child. Their child will have good and bad days, much like they do at their own work. Patience, rather than force, should be stressed.
Encourage parents to find alternative ways to teach their child that make it easier for them to learn. Online education programs may serve as beneficial supplements for these children, and field trips can provide more hands-on learning. In addition, talk to parents about the need for additional, independent testing and be able to refer them to appropriate sources for such testing as well as to neuropsychological resources, when warranted.
CONCLUSION
As families increasingly turn to pediatricians to address learning disabilities and problems children are having at school, physicians need to understand the various diagnoses and be able to assist with proper assessments. While schools must provide modifications when a diagnosis is made, there is sometimes disagreement about what the specific learning disability is and its extent of involvement in the child’s education. Diagnosing and addressing learning disability early can lessen various behavioral problems, help prevent dropping out of school, and enhance life outcomes for the child.
- Altarac M, Saroha E. Lifetime prevalence of learning disability among US children. Pediatrics 2007; 119(suppl 1):S77–S83.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Building the Legacy: IDEA 2004. U.S. Department of Education website. http://idea.ed.gov/. Accessed July 6, 2015.
- Pastor PN, Reuben CA. Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004–2006. Vital Health Stat 10 2008; 237:1–14. http://www.cdc.gov/nchs/data/series/sr_10/Sr10_237.pdf. Accessed July 6, 2015.
- U.S. Department of Education, Office of Special Education and Rehabilitative Services. 29th Annual Report to Congress on the Implementation of the Individuals with Disabilities Education Act, 2007, vol 1. Washington, DC; 2010. http://www2.ed.gov/about/reports/annual/osep/2007/parts-b-c/29th-vol-1.pdf. Accessed July 6, 2015.
- National Joint Committee on Learning Disabilities. Learning Disabilities and Young Children: Identification and Intervention (technical report). October 2007. http://www.asha.org/policy/TR2007-00307/. Accessed July 6, 2015.
- Response to intervention (RTI). Center for Parent Information and Resources website. http://www.parentcenterhub.org/repository/rti/. Updated August 2012. Accessed July 6, 2015.
- Altarac M, Saroha E. Lifetime prevalence of learning disability among US children. Pediatrics 2007; 119(suppl 1):S77–S83.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Building the Legacy: IDEA 2004. U.S. Department of Education website. http://idea.ed.gov/. Accessed July 6, 2015.
- Pastor PN, Reuben CA. Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004–2006. Vital Health Stat 10 2008; 237:1–14. http://www.cdc.gov/nchs/data/series/sr_10/Sr10_237.pdf. Accessed July 6, 2015.
- U.S. Department of Education, Office of Special Education and Rehabilitative Services. 29th Annual Report to Congress on the Implementation of the Individuals with Disabilities Education Act, 2007, vol 1. Washington, DC; 2010. http://www2.ed.gov/about/reports/annual/osep/2007/parts-b-c/29th-vol-1.pdf. Accessed July 6, 2015.
- National Joint Committee on Learning Disabilities. Learning Disabilities and Young Children: Identification and Intervention (technical report). October 2007. http://www.asha.org/policy/TR2007-00307/. Accessed July 6, 2015.
- Response to intervention (RTI). Center for Parent Information and Resources website. http://www.parentcenterhub.org/repository/rti/. Updated August 2012. Accessed July 6, 2015.
Developmental delays and autism: Screening and surveillance
Approximately 1 in 6 children in the United States had a developmental disability or a chronic physical, behavioral, or emotional condition that placed them at risk for developmental disability in 2008.1 These data also show that the prevalence of these disabilities increased by 17.1% from 1997 to 2008. The trend is even greater for autism. In a study of 8-year-old children in Atlanta, GA,2 the estimated prevalence of autism spectrum disorder increased 269% from 1996 to 2010, an average increase of 9.3% each year.
Overall, the prevalence of any developmental disability was 13.9%. This includes the following prevalence percentages1:
- Learning disabilities, 7.7%
- Attention deficit hyperactivity disorder (ADHD), 6.7%
- Other developmental delay, 3.7%
- Autism spectrum disorder, 1.47%.
Despite the high prevalence of these disabilities, many children are not appropriately diagnosed and treated. More than 30% of parents referred to professionals reported that help was not offered for their children’s developmental disorders.3
IDENTIFICATION
Early identification through screening and surveillance is crucial because it enables early intervention, which improves outcomes for children with developmental disorders. Identifying a developmental disorder is the initial step in evaluating the disorder. It permits access to disease-specific intervention, and it helps parents understand their child’s needs.
Identification also allows for reproductive counseling. For example, if a child has an autism spectrum disorder, the parents can be informed about their risk of having another child with the disorder. Siblings of these children have increased risks of a learning disorder. Finally, early identification allows access to free behavioral intervention though the Individuals with Disabilities Education Act, parts B (school-aged children) and C (36 months and younger).4
SURVEILLANCE
Ongoing surveillance is advised in addition to screening for developmental disabilities.5 Surveillance is a flexible, continuous, longitudinal process performed at every well-child visit and aimed at identifying concerns. Screening involves administering a brief, standardized tool normalized for specific ages and stages of development to identify any concerns.
Surveillance components
The components of developmental surveillance include the following5:
- Eliciting and addressing parents’ concerns
- Obtaining a developmental history by asking about developmental changes since the previous visit and requesting age-specific information (eg, whether the child is pointing or walking)
- Making accurate developmental and behavioral observations on fine and gross motor skills, speech and language, and social engagement
- Conducting a neurologic examination
- Identifying environmental, genetic, biologic, social, and demographic factors that present potential developmental risks or protection
- Maintaining an accurate record of documenting the process and findings.
The developmental history should note milestones and any delay, deviation, or regression from standard development expectations. Specific patient risk factors such as preterm birth, prenatal substance exposure, seizures, and growth abnormalities should be documented. Family risk factors including parental mental health, developmental disorders, or history of substance abuse should be recorded.
Developmental patterns should be assessed at each visit and classified as normal, delayed (normal sequence but slower rate of acquisition skills), dissociation (delay in one area of development but not others), deviance (achievement of milestones but not in the typical sequence, such as occurs with cerebral palsy), or regression (loss of previously acquired skills or a slowing or cessation of acquiring new skills).
Surveillance should note any abnormalities of body posture, patterns of movement, and muscle tone. Surveillance also should include looking for stereotypic movements like hand flapping, rocking, pacing, spinning, toe walking, and repetitive behaviors (such as overly repetitive play). Eye contact should also be assessed. For example, does the child initiate eye contact, make only selective eye contact, or avoid eye contact?
Verbal and nonverbal communication should be assessed when observing how the child interacts with the physician and family members. Does the child use gestures such as pointing appropriately? Children with deficits in symbolic language, such as those with autism spectrum disorder (speech, gestures), may use hand-over-hand communication such as leading a parent by the hand or placing a parent’s hand on what they want. Tantrums also can occur in children with developmental disabilities who lack more appropriated ways to communicate.
Behavioral observation includes scrutinizing engagement, impulsivity, and attention span. Tantrums, irritability, oppositionality, unusual fearfulness, and anxious, sad, or flat affect are warning signs of potential developmental or behavioral disorders. Facial expressions should be appropriate to the circumstance.
SCREENING
All infants, toddlers, preschoolers, and early elementary-aged children should be screened at regular intervals. Older school-age children with developmental concerns or who are struggling in school may require testing by a psychologist.
The American Academy of Pediatrics (AAP) recommends routine developmental screenings for specific disorders at ages 9, 18, and 30 months (or at 24 months if a 30-month visit is not planned).5 The screening at 9 months is intended to uncover potential vision and hearing problems, cerebral palsy, and other neuromotor disorders. At 18 months, screening can help identify cerebral palsy, autism spectrum disorders, global developmental delays, and specific language disorders. Screening for autism, global developmental delays, and specific language disorders should be repeated at the 24- or 30-month visits. In addition, AAP recommends an academic readiness assessment at age 4 to 5 years.
Development also should be screened whenever a parent expresses concern. Parental concerns about a child’s speech, language, behavior, or other development have a sensitivity and specificity in detecting developmental deficits that approximates those of commonly used screening tools.3 The message is to act immediately if a parent expresses a concern. If a parent is not concerned, continue with routine surveillance and scheduled screening.
SCREENING TOOLS
The two types of screening are problem-specific screening and broadband developmental-behavioral screening.
- Problem-specific screens are available for autism, speech delay, cognitive delay, or motor delay; they do not assess multiple different areas of development.
- Broadband developmental-behavioral screening tests are general and assess multiple areas of development, including autism.
Standardized tools with known reliability, sensitivity, specificity, and validity should be used. If the parents express no specific concerns, broadband developmental-behavioral and autism screening should be conducted at the AAP-recommended intervals. If a concern is noted on screening using a problem-specific tool, broadband screening is recommended because an abnormality in one domain may be a red flag for other developmental abnormalities.
Factors to consider in the choice of a test are the age range for which the test is intended, cost, length of time it takes to complete and to score, whether the test is paper-based or electronic, and the language availability (many tests are available in several languages).
Screening tests may be administered by the primary care physician or trained office staff before the scheduled office visit and then interpreted by the physician. Alternatively, screening tests meant to be completed by parents, teachers, or daycare providers can be mailed to the home before a scheduled visit to be completed and then interpreted by the physician at the next office visit.
There are many resources for screening tools and algorithms, both broadband and autism-specific. Some commonly used tests are compared in Table 1.6,7
BILLING CODES
The CPT code 96110 (developmental testing; limited, with interpretation and report) is used for screenings completed by a parent (or other caregiver) and interpreted by the health care provider. The code can be used for each screening tool, if multiple tools are used per visit. Testing administered, scored, and interpreted by the provider can be billed under CPT code 96111 (developmental testing; extended, with interpretation and report). The CPT code 96111 can be used only once per encounter, regardless of the number of tests administered.
DISCOVERING AN ABNORMALITY: REFERRALS, RESOURCES
Appropriate referral is indicated if a developmental concern has been uncovered during screening. If a concern is raised, either by parent report or identified by screening/surveillance, the child should be referred to the appropriate specialist for diagnostic testing (eg, developmental and behavioral pediatrics, neurology).
If the child is younger than 3 years, the Help Me Grow program (www.Helpmegrow.org) can provide additional screening and connect families to developmental services. It uses a network of community providers for support and maintenance during the referral process. Children 3 years and older are eligible for evaluation through the public school system.
Referral to physical therapy, occupational therapy, and speech and language evaluation may be appropriate. An audiology referral for a hearing evaluation is recommended whenever a concern is raised.
Neurology or physiology can be useful for evaluating abnormal gait and other motor disabilities typically seen with muscular dystrophy, cerebral palsy, and spina bifida. A genetics consult may identify causes of developmental delays and autism spectrum disorders. Neurology or genetics also may offer testing to assess for neurologic and metabolic causes of developmental delays. Developmental pediatricians can also evaluate for developmental disorders including autism.
Recognize that verbal children also can have autism spectrum disorders. In these children, the quality of communication is abnormal. When a concern about developmental delay is expressed or uncovered during screening, refer immediately for a diagnostic evaluation to avoid a delay in diagnosis and access to services. Early identification permits early intervention, which offers the best chance to improve outcomes.
SUMMARY
Screening for developmental delays and autism spectrum disorders in addition to ongoing developmental surveillance is recommended for all children at regular intervals, and is indicated whenever a parent expresses concern. Multiple screening tests are available. If a developmental concern is identified during screening, appropriate referral is indicated for further assessment.
- Boyle CA, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 2011; 127:1034–1042.
- Van Naarden Braun K, Christensen D, Doernberg N, et al. Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan Atlanta, 1991–2010. PLoS One 2015; 10:e0124120.
- Filipek PA, Accardo PJ, Ashwal S, et al. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology 2000; 55:468–479.
- National Assessment of IDEA Overview (NCEE 2011–4026). Washington, DC: National Center for Education Evaluation and Regional Assistance, Institute of Education Sciences, U.S. Department of Education; July 2011. http://ies.ed.gov/ncee/pubs/20114026/pdf/20114026.pdf. Accessed July 7, 2015.
- Council on Children with Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children with Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics 2006; 118:405–420.
- Marks KP, LaRosa AC. Understanding developmental-behavioral screening measures. Pediatr Rev 2012; 33:448–457.
- Drotar D, Stancin T, Dworkin P. Pediatric developmental screening: understanding and selecting screening instruments. The Commonwealth Fund website. http://www.commonwealthfund.org/~/media/files/publications/fund-manual/2008/feb/pediatric-developmental-screening--understanding-and-selecting-screening-instruments/pediatric_developmental_screening-pdf.pdf. Updated February 1, 2008. Accessed July 7, 2015.
Approximately 1 in 6 children in the United States had a developmental disability or a chronic physical, behavioral, or emotional condition that placed them at risk for developmental disability in 2008.1 These data also show that the prevalence of these disabilities increased by 17.1% from 1997 to 2008. The trend is even greater for autism. In a study of 8-year-old children in Atlanta, GA,2 the estimated prevalence of autism spectrum disorder increased 269% from 1996 to 2010, an average increase of 9.3% each year.
Overall, the prevalence of any developmental disability was 13.9%. This includes the following prevalence percentages1:
- Learning disabilities, 7.7%
- Attention deficit hyperactivity disorder (ADHD), 6.7%
- Other developmental delay, 3.7%
- Autism spectrum disorder, 1.47%.
Despite the high prevalence of these disabilities, many children are not appropriately diagnosed and treated. More than 30% of parents referred to professionals reported that help was not offered for their children’s developmental disorders.3
IDENTIFICATION
Early identification through screening and surveillance is crucial because it enables early intervention, which improves outcomes for children with developmental disorders. Identifying a developmental disorder is the initial step in evaluating the disorder. It permits access to disease-specific intervention, and it helps parents understand their child’s needs.
Identification also allows for reproductive counseling. For example, if a child has an autism spectrum disorder, the parents can be informed about their risk of having another child with the disorder. Siblings of these children have increased risks of a learning disorder. Finally, early identification allows access to free behavioral intervention though the Individuals with Disabilities Education Act, parts B (school-aged children) and C (36 months and younger).4
SURVEILLANCE
Ongoing surveillance is advised in addition to screening for developmental disabilities.5 Surveillance is a flexible, continuous, longitudinal process performed at every well-child visit and aimed at identifying concerns. Screening involves administering a brief, standardized tool normalized for specific ages and stages of development to identify any concerns.
Surveillance components
The components of developmental surveillance include the following5:
- Eliciting and addressing parents’ concerns
- Obtaining a developmental history by asking about developmental changes since the previous visit and requesting age-specific information (eg, whether the child is pointing or walking)
- Making accurate developmental and behavioral observations on fine and gross motor skills, speech and language, and social engagement
- Conducting a neurologic examination
- Identifying environmental, genetic, biologic, social, and demographic factors that present potential developmental risks or protection
- Maintaining an accurate record of documenting the process and findings.
The developmental history should note milestones and any delay, deviation, or regression from standard development expectations. Specific patient risk factors such as preterm birth, prenatal substance exposure, seizures, and growth abnormalities should be documented. Family risk factors including parental mental health, developmental disorders, or history of substance abuse should be recorded.
Developmental patterns should be assessed at each visit and classified as normal, delayed (normal sequence but slower rate of acquisition skills), dissociation (delay in one area of development but not others), deviance (achievement of milestones but not in the typical sequence, such as occurs with cerebral palsy), or regression (loss of previously acquired skills or a slowing or cessation of acquiring new skills).
Surveillance should note any abnormalities of body posture, patterns of movement, and muscle tone. Surveillance also should include looking for stereotypic movements like hand flapping, rocking, pacing, spinning, toe walking, and repetitive behaviors (such as overly repetitive play). Eye contact should also be assessed. For example, does the child initiate eye contact, make only selective eye contact, or avoid eye contact?
Verbal and nonverbal communication should be assessed when observing how the child interacts with the physician and family members. Does the child use gestures such as pointing appropriately? Children with deficits in symbolic language, such as those with autism spectrum disorder (speech, gestures), may use hand-over-hand communication such as leading a parent by the hand or placing a parent’s hand on what they want. Tantrums also can occur in children with developmental disabilities who lack more appropriated ways to communicate.
Behavioral observation includes scrutinizing engagement, impulsivity, and attention span. Tantrums, irritability, oppositionality, unusual fearfulness, and anxious, sad, or flat affect are warning signs of potential developmental or behavioral disorders. Facial expressions should be appropriate to the circumstance.
SCREENING
All infants, toddlers, preschoolers, and early elementary-aged children should be screened at regular intervals. Older school-age children with developmental concerns or who are struggling in school may require testing by a psychologist.
The American Academy of Pediatrics (AAP) recommends routine developmental screenings for specific disorders at ages 9, 18, and 30 months (or at 24 months if a 30-month visit is not planned).5 The screening at 9 months is intended to uncover potential vision and hearing problems, cerebral palsy, and other neuromotor disorders. At 18 months, screening can help identify cerebral palsy, autism spectrum disorders, global developmental delays, and specific language disorders. Screening for autism, global developmental delays, and specific language disorders should be repeated at the 24- or 30-month visits. In addition, AAP recommends an academic readiness assessment at age 4 to 5 years.
Development also should be screened whenever a parent expresses concern. Parental concerns about a child’s speech, language, behavior, or other development have a sensitivity and specificity in detecting developmental deficits that approximates those of commonly used screening tools.3 The message is to act immediately if a parent expresses a concern. If a parent is not concerned, continue with routine surveillance and scheduled screening.
SCREENING TOOLS
The two types of screening are problem-specific screening and broadband developmental-behavioral screening.
- Problem-specific screens are available for autism, speech delay, cognitive delay, or motor delay; they do not assess multiple different areas of development.
- Broadband developmental-behavioral screening tests are general and assess multiple areas of development, including autism.
Standardized tools with known reliability, sensitivity, specificity, and validity should be used. If the parents express no specific concerns, broadband developmental-behavioral and autism screening should be conducted at the AAP-recommended intervals. If a concern is noted on screening using a problem-specific tool, broadband screening is recommended because an abnormality in one domain may be a red flag for other developmental abnormalities.
Factors to consider in the choice of a test are the age range for which the test is intended, cost, length of time it takes to complete and to score, whether the test is paper-based or electronic, and the language availability (many tests are available in several languages).
Screening tests may be administered by the primary care physician or trained office staff before the scheduled office visit and then interpreted by the physician. Alternatively, screening tests meant to be completed by parents, teachers, or daycare providers can be mailed to the home before a scheduled visit to be completed and then interpreted by the physician at the next office visit.
There are many resources for screening tools and algorithms, both broadband and autism-specific. Some commonly used tests are compared in Table 1.6,7
BILLING CODES
The CPT code 96110 (developmental testing; limited, with interpretation and report) is used for screenings completed by a parent (or other caregiver) and interpreted by the health care provider. The code can be used for each screening tool, if multiple tools are used per visit. Testing administered, scored, and interpreted by the provider can be billed under CPT code 96111 (developmental testing; extended, with interpretation and report). The CPT code 96111 can be used only once per encounter, regardless of the number of tests administered.
DISCOVERING AN ABNORMALITY: REFERRALS, RESOURCES
Appropriate referral is indicated if a developmental concern has been uncovered during screening. If a concern is raised, either by parent report or identified by screening/surveillance, the child should be referred to the appropriate specialist for diagnostic testing (eg, developmental and behavioral pediatrics, neurology).
If the child is younger than 3 years, the Help Me Grow program (www.Helpmegrow.org) can provide additional screening and connect families to developmental services. It uses a network of community providers for support and maintenance during the referral process. Children 3 years and older are eligible for evaluation through the public school system.
Referral to physical therapy, occupational therapy, and speech and language evaluation may be appropriate. An audiology referral for a hearing evaluation is recommended whenever a concern is raised.
Neurology or physiology can be useful for evaluating abnormal gait and other motor disabilities typically seen with muscular dystrophy, cerebral palsy, and spina bifida. A genetics consult may identify causes of developmental delays and autism spectrum disorders. Neurology or genetics also may offer testing to assess for neurologic and metabolic causes of developmental delays. Developmental pediatricians can also evaluate for developmental disorders including autism.
Recognize that verbal children also can have autism spectrum disorders. In these children, the quality of communication is abnormal. When a concern about developmental delay is expressed or uncovered during screening, refer immediately for a diagnostic evaluation to avoid a delay in diagnosis and access to services. Early identification permits early intervention, which offers the best chance to improve outcomes.
SUMMARY
Screening for developmental delays and autism spectrum disorders in addition to ongoing developmental surveillance is recommended for all children at regular intervals, and is indicated whenever a parent expresses concern. Multiple screening tests are available. If a developmental concern is identified during screening, appropriate referral is indicated for further assessment.
Approximately 1 in 6 children in the United States had a developmental disability or a chronic physical, behavioral, or emotional condition that placed them at risk for developmental disability in 2008.1 These data also show that the prevalence of these disabilities increased by 17.1% from 1997 to 2008. The trend is even greater for autism. In a study of 8-year-old children in Atlanta, GA,2 the estimated prevalence of autism spectrum disorder increased 269% from 1996 to 2010, an average increase of 9.3% each year.
Overall, the prevalence of any developmental disability was 13.9%. This includes the following prevalence percentages1:
- Learning disabilities, 7.7%
- Attention deficit hyperactivity disorder (ADHD), 6.7%
- Other developmental delay, 3.7%
- Autism spectrum disorder, 1.47%.
Despite the high prevalence of these disabilities, many children are not appropriately diagnosed and treated. More than 30% of parents referred to professionals reported that help was not offered for their children’s developmental disorders.3
IDENTIFICATION
Early identification through screening and surveillance is crucial because it enables early intervention, which improves outcomes for children with developmental disorders. Identifying a developmental disorder is the initial step in evaluating the disorder. It permits access to disease-specific intervention, and it helps parents understand their child’s needs.
Identification also allows for reproductive counseling. For example, if a child has an autism spectrum disorder, the parents can be informed about their risk of having another child with the disorder. Siblings of these children have increased risks of a learning disorder. Finally, early identification allows access to free behavioral intervention though the Individuals with Disabilities Education Act, parts B (school-aged children) and C (36 months and younger).4
SURVEILLANCE
Ongoing surveillance is advised in addition to screening for developmental disabilities.5 Surveillance is a flexible, continuous, longitudinal process performed at every well-child visit and aimed at identifying concerns. Screening involves administering a brief, standardized tool normalized for specific ages and stages of development to identify any concerns.
Surveillance components
The components of developmental surveillance include the following5:
- Eliciting and addressing parents’ concerns
- Obtaining a developmental history by asking about developmental changes since the previous visit and requesting age-specific information (eg, whether the child is pointing or walking)
- Making accurate developmental and behavioral observations on fine and gross motor skills, speech and language, and social engagement
- Conducting a neurologic examination
- Identifying environmental, genetic, biologic, social, and demographic factors that present potential developmental risks or protection
- Maintaining an accurate record of documenting the process and findings.
The developmental history should note milestones and any delay, deviation, or regression from standard development expectations. Specific patient risk factors such as preterm birth, prenatal substance exposure, seizures, and growth abnormalities should be documented. Family risk factors including parental mental health, developmental disorders, or history of substance abuse should be recorded.
Developmental patterns should be assessed at each visit and classified as normal, delayed (normal sequence but slower rate of acquisition skills), dissociation (delay in one area of development but not others), deviance (achievement of milestones but not in the typical sequence, such as occurs with cerebral palsy), or regression (loss of previously acquired skills or a slowing or cessation of acquiring new skills).
Surveillance should note any abnormalities of body posture, patterns of movement, and muscle tone. Surveillance also should include looking for stereotypic movements like hand flapping, rocking, pacing, spinning, toe walking, and repetitive behaviors (such as overly repetitive play). Eye contact should also be assessed. For example, does the child initiate eye contact, make only selective eye contact, or avoid eye contact?
Verbal and nonverbal communication should be assessed when observing how the child interacts with the physician and family members. Does the child use gestures such as pointing appropriately? Children with deficits in symbolic language, such as those with autism spectrum disorder (speech, gestures), may use hand-over-hand communication such as leading a parent by the hand or placing a parent’s hand on what they want. Tantrums also can occur in children with developmental disabilities who lack more appropriated ways to communicate.
Behavioral observation includes scrutinizing engagement, impulsivity, and attention span. Tantrums, irritability, oppositionality, unusual fearfulness, and anxious, sad, or flat affect are warning signs of potential developmental or behavioral disorders. Facial expressions should be appropriate to the circumstance.
SCREENING
All infants, toddlers, preschoolers, and early elementary-aged children should be screened at regular intervals. Older school-age children with developmental concerns or who are struggling in school may require testing by a psychologist.
The American Academy of Pediatrics (AAP) recommends routine developmental screenings for specific disorders at ages 9, 18, and 30 months (or at 24 months if a 30-month visit is not planned).5 The screening at 9 months is intended to uncover potential vision and hearing problems, cerebral palsy, and other neuromotor disorders. At 18 months, screening can help identify cerebral palsy, autism spectrum disorders, global developmental delays, and specific language disorders. Screening for autism, global developmental delays, and specific language disorders should be repeated at the 24- or 30-month visits. In addition, AAP recommends an academic readiness assessment at age 4 to 5 years.
Development also should be screened whenever a parent expresses concern. Parental concerns about a child’s speech, language, behavior, or other development have a sensitivity and specificity in detecting developmental deficits that approximates those of commonly used screening tools.3 The message is to act immediately if a parent expresses a concern. If a parent is not concerned, continue with routine surveillance and scheduled screening.
SCREENING TOOLS
The two types of screening are problem-specific screening and broadband developmental-behavioral screening.
- Problem-specific screens are available for autism, speech delay, cognitive delay, or motor delay; they do not assess multiple different areas of development.
- Broadband developmental-behavioral screening tests are general and assess multiple areas of development, including autism.
Standardized tools with known reliability, sensitivity, specificity, and validity should be used. If the parents express no specific concerns, broadband developmental-behavioral and autism screening should be conducted at the AAP-recommended intervals. If a concern is noted on screening using a problem-specific tool, broadband screening is recommended because an abnormality in one domain may be a red flag for other developmental abnormalities.
Factors to consider in the choice of a test are the age range for which the test is intended, cost, length of time it takes to complete and to score, whether the test is paper-based or electronic, and the language availability (many tests are available in several languages).
Screening tests may be administered by the primary care physician or trained office staff before the scheduled office visit and then interpreted by the physician. Alternatively, screening tests meant to be completed by parents, teachers, or daycare providers can be mailed to the home before a scheduled visit to be completed and then interpreted by the physician at the next office visit.
There are many resources for screening tools and algorithms, both broadband and autism-specific. Some commonly used tests are compared in Table 1.6,7
BILLING CODES
The CPT code 96110 (developmental testing; limited, with interpretation and report) is used for screenings completed by a parent (or other caregiver) and interpreted by the health care provider. The code can be used for each screening tool, if multiple tools are used per visit. Testing administered, scored, and interpreted by the provider can be billed under CPT code 96111 (developmental testing; extended, with interpretation and report). The CPT code 96111 can be used only once per encounter, regardless of the number of tests administered.
DISCOVERING AN ABNORMALITY: REFERRALS, RESOURCES
Appropriate referral is indicated if a developmental concern has been uncovered during screening. If a concern is raised, either by parent report or identified by screening/surveillance, the child should be referred to the appropriate specialist for diagnostic testing (eg, developmental and behavioral pediatrics, neurology).
If the child is younger than 3 years, the Help Me Grow program (www.Helpmegrow.org) can provide additional screening and connect families to developmental services. It uses a network of community providers for support and maintenance during the referral process. Children 3 years and older are eligible for evaluation through the public school system.
Referral to physical therapy, occupational therapy, and speech and language evaluation may be appropriate. An audiology referral for a hearing evaluation is recommended whenever a concern is raised.
Neurology or physiology can be useful for evaluating abnormal gait and other motor disabilities typically seen with muscular dystrophy, cerebral palsy, and spina bifida. A genetics consult may identify causes of developmental delays and autism spectrum disorders. Neurology or genetics also may offer testing to assess for neurologic and metabolic causes of developmental delays. Developmental pediatricians can also evaluate for developmental disorders including autism.
Recognize that verbal children also can have autism spectrum disorders. In these children, the quality of communication is abnormal. When a concern about developmental delay is expressed or uncovered during screening, refer immediately for a diagnostic evaluation to avoid a delay in diagnosis and access to services. Early identification permits early intervention, which offers the best chance to improve outcomes.
SUMMARY
Screening for developmental delays and autism spectrum disorders in addition to ongoing developmental surveillance is recommended for all children at regular intervals, and is indicated whenever a parent expresses concern. Multiple screening tests are available. If a developmental concern is identified during screening, appropriate referral is indicated for further assessment.
- Boyle CA, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 2011; 127:1034–1042.
- Van Naarden Braun K, Christensen D, Doernberg N, et al. Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan Atlanta, 1991–2010. PLoS One 2015; 10:e0124120.
- Filipek PA, Accardo PJ, Ashwal S, et al. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology 2000; 55:468–479.
- National Assessment of IDEA Overview (NCEE 2011–4026). Washington, DC: National Center for Education Evaluation and Regional Assistance, Institute of Education Sciences, U.S. Department of Education; July 2011. http://ies.ed.gov/ncee/pubs/20114026/pdf/20114026.pdf. Accessed July 7, 2015.
- Council on Children with Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children with Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics 2006; 118:405–420.
- Marks KP, LaRosa AC. Understanding developmental-behavioral screening measures. Pediatr Rev 2012; 33:448–457.
- Drotar D, Stancin T, Dworkin P. Pediatric developmental screening: understanding and selecting screening instruments. The Commonwealth Fund website. http://www.commonwealthfund.org/~/media/files/publications/fund-manual/2008/feb/pediatric-developmental-screening--understanding-and-selecting-screening-instruments/pediatric_developmental_screening-pdf.pdf. Updated February 1, 2008. Accessed July 7, 2015.
- Boyle CA, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 2011; 127:1034–1042.
- Van Naarden Braun K, Christensen D, Doernberg N, et al. Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan Atlanta, 1991–2010. PLoS One 2015; 10:e0124120.
- Filipek PA, Accardo PJ, Ashwal S, et al. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology 2000; 55:468–479.
- National Assessment of IDEA Overview (NCEE 2011–4026). Washington, DC: National Center for Education Evaluation and Regional Assistance, Institute of Education Sciences, U.S. Department of Education; July 2011. http://ies.ed.gov/ncee/pubs/20114026/pdf/20114026.pdf. Accessed July 7, 2015.
- Council on Children with Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children with Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics 2006; 118:405–420.
- Marks KP, LaRosa AC. Understanding developmental-behavioral screening measures. Pediatr Rev 2012; 33:448–457.
- Drotar D, Stancin T, Dworkin P. Pediatric developmental screening: understanding and selecting screening instruments. The Commonwealth Fund website. http://www.commonwealthfund.org/~/media/files/publications/fund-manual/2008/feb/pediatric-developmental-screening--understanding-and-selecting-screening-instruments/pediatric_developmental_screening-pdf.pdf. Updated February 1, 2008. Accessed July 7, 2015.
Reprogramming the immune system

showing Hodgkin lymphoma
NEW YORK—Using a 3-pronged approach to reprogram the immune system—inhibition of critical pathways, activation of others, and depletion of malignant cells—may be the best strategy to optimize immune function in B-cell lymphomas, according to Stephen M. Ansell, MD, PhD, of the Mayo Clinic in Rochester, Minnesota.
“[A]ll told, there are multiple immunological barriers to an effective immune response,” Dr Ansell said at Lymphoma & Myeloma 2015.
“So the questions are how can you use an immune checkpoint approach to try and modulate this and improve the outcome.”
Dr Ansell discussed checkpoint inhibitors, immune signal activators, and the potential of combining the approaches in Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) in a way that enhances rather than antagonizes their effects.
Blocking CTLA-4
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) functions as an immune checkpoint that downregulates the immune system. A receptor found on the surface of inhibitor T cells, it acts as an off switch when it binds to CD80 or CD86 on the surface of antigen-presenting cells.
Ipilimumab, an antibody that targets CTLA-4, has been approved by the US Food and Drug Administration for the treatment of melanoma and is in clinical trials for lung, bladder, and prostate cancer.
Investigators wanted to see whether it also works in lymphoma, so they conducted a phase 1 study in relapsed/refractory B-cell NHL.
Eighteen patients received 3 mg/kg of ipilimumab. Two patients responded, 1 with a complete response (CR) that lasted more than 31 months, and 1 with a partial response (PR) that lasted 19 months. In 5 of 16 patients (31%), T-cell proliferation to recall antigens increased more than 2-fold.
As Dr Ansell explained, “Immune response doesn’t always correlate directly with the clinical responses. So I think we really have a lot to learn about what is really a biomarker of efficacy.”
Ipilimumab was also evaluated to treat relapse after allogeneic hematopoietic stem cell transplantation (HSCT) in 29 patients with relapsed hematologic disease. Two patients with HL achieved a CR and 1 patient with mantle cell lymphoma achieved a PR.
The investigators observed that ipilimumab did not induce or exacerbate clinical graft-versus-host disease.
Blocking PD-1
Programmed cell death protein 1 (PD-1) is a surface receptor expressed on T cells and pro-B cells. PD-1 binds 2 ligands, PD-L1 and PD-L2.
PD-1 ligands are overexpressed in inflammatory environments and attenuate the immune response through PD-1 on immune effector cells. In addition, PD-L1 expressed on malignant cells or in the tumor microenvironment suppresses tumor-infiltrating lymphocyte activity.
Pidilizumab, a humanized monoclonal antibody that binds to PD-1, weakens the apoptotic processes in lymphocytes and augments the antitumor activities of NK cells.
Investigators conducted a phase 2 trial of pidilizumab in patients with diffuse large B-cell lymphoma (DLBCL) after autologous HSCT to modulate the immune system after a transplant.
The team treated 66 patients with the antibody. At 16 months, progression-free survival (PFS) was 72%. For the 24 high-risk patients who were PET-positive after salvage chemotherapy, the 16-month PFS was 70%.
“And I think that what was most interesting,” Dr Ansell said, when focusing on the 35 patients with measurable disease after transplant, pidilizumab produced a 51% response rate “even in patients that actually had active disease.”
When pidilizumab was combined with rituximab in another trial in patients with relapsed follicular lymphoma (FL), 19 of 29 evaluable patients (66%) achieved an objective response: 15 (52%) CRs and 4 (14%) PRs.
“You might say, ‘Who cares? That’s not that great,’” Dr Ansell said. “But I think what was pretty impressive is that 52% CR rate. And most of you who treat patients with rituximab would know that that’s quite surprising, suggesting that there may be additional benefit for the use of PD-1 blockade in this subset of patients.”
Nivolumab, another monoclonal antibody that blocks the PD-1 pathway, is being investigated in a number of lymphoid malignancies, including HL, DLBCL, and T-cell lymphomas.
In a phase 1 study of nivolumab in 81 patients with relapsed or refractory lymphoid malignancies, the best preliminary overall response has been in FL and DLBCL patients, with an objective response rate of 40% and 36%, respectively, including 1 PR and 3 PRs in each subtype.
“I think what is important,” Dr Ansell said, “is that the side effects, as expected, were mainly immune-mediated, not as dramatic as have been seen with other agents, and very similar to what has been seen in solid tumor studies.”
Dr Ansell pointed out that the response rates with nivolumab varied widely by histology, suggesting that “we have a lot to learn about why patients benefit and who exactly benefits.”
There were no responses in patients with multiple myeloma or primary mediastinal B-cell lymphoma, although many patients achieved stable disease.
“Hodgkin lymphoma was completely different,” Dr Ansell said, “and there were responses in virtually every patient.”
Of 23 patients treated with nivolumab, 20 responded—4 achieved a CR and 16 a PR—including patients who had failed autologous HSCT and brentuximab vedotin treatment. Eleven patients, including 2 with CRs, have an ongoing response, some approaching 2 years. So the responses have been durable, Dr Ansell noted.
Yet another PD-1 antibody, pembrolizumab, has prompted reduction in tumor burden in HL in all but 2 of 29 evaluable patients, including 6 CRs and 13 PRs. The median duration of response has not yet been reached, and the side effect profile was similar to what has been seen with nivolumab and in solid tumors.
Activating immune stimulatory signals
Another approach to boosting the immune system is to activate immune stimulatory signals, eg, CD27 and CD40, and get a benefit that way. Varlilumab (CDX-1127) is an unconjugated monoclonal antibody that binds CD27 and activates CD27-expressing T cells.
In a phase 1 trial of varlilumab in 24 lymphoma patients, investigators found no significant depletion in absolute lymphocyte counts, T cells, or B cells. “Not quite the same success story,” Dr Ansell said, with a response—a CR—in only 1 patient.
Investigators did observe, however, evidence of increased soluble CD27, a reduction of circulating Tregs, and the induction of pro-inflammatory cytokines.
And in a phase 1 study of the anti-CD40 monoclonal antibody dacetuzumab in recurrent NHL, “the response rate was disappointingly low,” Dr Ansell said.
Investigators observed 6 objective responses, including 1 CR and 5 PRs, and a decrease in tumor size in approximately one-third of the 50 patients treated.
The investigators of the subsequent phase 2 trial did not want to take the agent forward for further study, Dr Ansell noted, “but there are antibodies now being developed in this space that will hopefully be more effective and create a greater benefit.”
Optimizing immune function
Dr Ansell suggested there are 3 main approaches to treating patients. One is going directly after the malignant cells and depleting them. A second is to inhibit critical pathways that the malignant cell is dependent upon, “starving them, if you like.” The third way is to activate the immune system and thereby create a greater benefit for patients.
“[P]robably our best strategy is to use all 3 in a reprogram approach,” he said. “Because unless you target each one of these areas, the likelihood is that the other sides of the 3-legged stool will take over.”
“This is an encouraging and exciting time for immune checkpoints therapy and an encouraging and exciting time for immune therapies in general. I think this is really the new frontier in lymphomas.” ![]()

showing Hodgkin lymphoma
NEW YORK—Using a 3-pronged approach to reprogram the immune system—inhibition of critical pathways, activation of others, and depletion of malignant cells—may be the best strategy to optimize immune function in B-cell lymphomas, according to Stephen M. Ansell, MD, PhD, of the Mayo Clinic in Rochester, Minnesota.
“[A]ll told, there are multiple immunological barriers to an effective immune response,” Dr Ansell said at Lymphoma & Myeloma 2015.
“So the questions are how can you use an immune checkpoint approach to try and modulate this and improve the outcome.”
Dr Ansell discussed checkpoint inhibitors, immune signal activators, and the potential of combining the approaches in Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) in a way that enhances rather than antagonizes their effects.
Blocking CTLA-4
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) functions as an immune checkpoint that downregulates the immune system. A receptor found on the surface of inhibitor T cells, it acts as an off switch when it binds to CD80 or CD86 on the surface of antigen-presenting cells.
Ipilimumab, an antibody that targets CTLA-4, has been approved by the US Food and Drug Administration for the treatment of melanoma and is in clinical trials for lung, bladder, and prostate cancer.
Investigators wanted to see whether it also works in lymphoma, so they conducted a phase 1 study in relapsed/refractory B-cell NHL.
Eighteen patients received 3 mg/kg of ipilimumab. Two patients responded, 1 with a complete response (CR) that lasted more than 31 months, and 1 with a partial response (PR) that lasted 19 months. In 5 of 16 patients (31%), T-cell proliferation to recall antigens increased more than 2-fold.
As Dr Ansell explained, “Immune response doesn’t always correlate directly with the clinical responses. So I think we really have a lot to learn about what is really a biomarker of efficacy.”
Ipilimumab was also evaluated to treat relapse after allogeneic hematopoietic stem cell transplantation (HSCT) in 29 patients with relapsed hematologic disease. Two patients with HL achieved a CR and 1 patient with mantle cell lymphoma achieved a PR.
The investigators observed that ipilimumab did not induce or exacerbate clinical graft-versus-host disease.
Blocking PD-1
Programmed cell death protein 1 (PD-1) is a surface receptor expressed on T cells and pro-B cells. PD-1 binds 2 ligands, PD-L1 and PD-L2.
PD-1 ligands are overexpressed in inflammatory environments and attenuate the immune response through PD-1 on immune effector cells. In addition, PD-L1 expressed on malignant cells or in the tumor microenvironment suppresses tumor-infiltrating lymphocyte activity.
Pidilizumab, a humanized monoclonal antibody that binds to PD-1, weakens the apoptotic processes in lymphocytes and augments the antitumor activities of NK cells.
Investigators conducted a phase 2 trial of pidilizumab in patients with diffuse large B-cell lymphoma (DLBCL) after autologous HSCT to modulate the immune system after a transplant.
The team treated 66 patients with the antibody. At 16 months, progression-free survival (PFS) was 72%. For the 24 high-risk patients who were PET-positive after salvage chemotherapy, the 16-month PFS was 70%.
“And I think that what was most interesting,” Dr Ansell said, when focusing on the 35 patients with measurable disease after transplant, pidilizumab produced a 51% response rate “even in patients that actually had active disease.”
When pidilizumab was combined with rituximab in another trial in patients with relapsed follicular lymphoma (FL), 19 of 29 evaluable patients (66%) achieved an objective response: 15 (52%) CRs and 4 (14%) PRs.
“You might say, ‘Who cares? That’s not that great,’” Dr Ansell said. “But I think what was pretty impressive is that 52% CR rate. And most of you who treat patients with rituximab would know that that’s quite surprising, suggesting that there may be additional benefit for the use of PD-1 blockade in this subset of patients.”
Nivolumab, another monoclonal antibody that blocks the PD-1 pathway, is being investigated in a number of lymphoid malignancies, including HL, DLBCL, and T-cell lymphomas.
In a phase 1 study of nivolumab in 81 patients with relapsed or refractory lymphoid malignancies, the best preliminary overall response has been in FL and DLBCL patients, with an objective response rate of 40% and 36%, respectively, including 1 PR and 3 PRs in each subtype.
“I think what is important,” Dr Ansell said, “is that the side effects, as expected, were mainly immune-mediated, not as dramatic as have been seen with other agents, and very similar to what has been seen in solid tumor studies.”
Dr Ansell pointed out that the response rates with nivolumab varied widely by histology, suggesting that “we have a lot to learn about why patients benefit and who exactly benefits.”
There were no responses in patients with multiple myeloma or primary mediastinal B-cell lymphoma, although many patients achieved stable disease.
“Hodgkin lymphoma was completely different,” Dr Ansell said, “and there were responses in virtually every patient.”
Of 23 patients treated with nivolumab, 20 responded—4 achieved a CR and 16 a PR—including patients who had failed autologous HSCT and brentuximab vedotin treatment. Eleven patients, including 2 with CRs, have an ongoing response, some approaching 2 years. So the responses have been durable, Dr Ansell noted.
Yet another PD-1 antibody, pembrolizumab, has prompted reduction in tumor burden in HL in all but 2 of 29 evaluable patients, including 6 CRs and 13 PRs. The median duration of response has not yet been reached, and the side effect profile was similar to what has been seen with nivolumab and in solid tumors.
Activating immune stimulatory signals
Another approach to boosting the immune system is to activate immune stimulatory signals, eg, CD27 and CD40, and get a benefit that way. Varlilumab (CDX-1127) is an unconjugated monoclonal antibody that binds CD27 and activates CD27-expressing T cells.
In a phase 1 trial of varlilumab in 24 lymphoma patients, investigators found no significant depletion in absolute lymphocyte counts, T cells, or B cells. “Not quite the same success story,” Dr Ansell said, with a response—a CR—in only 1 patient.
Investigators did observe, however, evidence of increased soluble CD27, a reduction of circulating Tregs, and the induction of pro-inflammatory cytokines.
And in a phase 1 study of the anti-CD40 monoclonal antibody dacetuzumab in recurrent NHL, “the response rate was disappointingly low,” Dr Ansell said.
Investigators observed 6 objective responses, including 1 CR and 5 PRs, and a decrease in tumor size in approximately one-third of the 50 patients treated.
The investigators of the subsequent phase 2 trial did not want to take the agent forward for further study, Dr Ansell noted, “but there are antibodies now being developed in this space that will hopefully be more effective and create a greater benefit.”
Optimizing immune function
Dr Ansell suggested there are 3 main approaches to treating patients. One is going directly after the malignant cells and depleting them. A second is to inhibit critical pathways that the malignant cell is dependent upon, “starving them, if you like.” The third way is to activate the immune system and thereby create a greater benefit for patients.
“[P]robably our best strategy is to use all 3 in a reprogram approach,” he said. “Because unless you target each one of these areas, the likelihood is that the other sides of the 3-legged stool will take over.”
“This is an encouraging and exciting time for immune checkpoints therapy and an encouraging and exciting time for immune therapies in general. I think this is really the new frontier in lymphomas.” ![]()

showing Hodgkin lymphoma
NEW YORK—Using a 3-pronged approach to reprogram the immune system—inhibition of critical pathways, activation of others, and depletion of malignant cells—may be the best strategy to optimize immune function in B-cell lymphomas, according to Stephen M. Ansell, MD, PhD, of the Mayo Clinic in Rochester, Minnesota.
“[A]ll told, there are multiple immunological barriers to an effective immune response,” Dr Ansell said at Lymphoma & Myeloma 2015.
“So the questions are how can you use an immune checkpoint approach to try and modulate this and improve the outcome.”
Dr Ansell discussed checkpoint inhibitors, immune signal activators, and the potential of combining the approaches in Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) in a way that enhances rather than antagonizes their effects.
Blocking CTLA-4
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) functions as an immune checkpoint that downregulates the immune system. A receptor found on the surface of inhibitor T cells, it acts as an off switch when it binds to CD80 or CD86 on the surface of antigen-presenting cells.
Ipilimumab, an antibody that targets CTLA-4, has been approved by the US Food and Drug Administration for the treatment of melanoma and is in clinical trials for lung, bladder, and prostate cancer.
Investigators wanted to see whether it also works in lymphoma, so they conducted a phase 1 study in relapsed/refractory B-cell NHL.
Eighteen patients received 3 mg/kg of ipilimumab. Two patients responded, 1 with a complete response (CR) that lasted more than 31 months, and 1 with a partial response (PR) that lasted 19 months. In 5 of 16 patients (31%), T-cell proliferation to recall antigens increased more than 2-fold.
As Dr Ansell explained, “Immune response doesn’t always correlate directly with the clinical responses. So I think we really have a lot to learn about what is really a biomarker of efficacy.”
Ipilimumab was also evaluated to treat relapse after allogeneic hematopoietic stem cell transplantation (HSCT) in 29 patients with relapsed hematologic disease. Two patients with HL achieved a CR and 1 patient with mantle cell lymphoma achieved a PR.
The investigators observed that ipilimumab did not induce or exacerbate clinical graft-versus-host disease.
Blocking PD-1
Programmed cell death protein 1 (PD-1) is a surface receptor expressed on T cells and pro-B cells. PD-1 binds 2 ligands, PD-L1 and PD-L2.
PD-1 ligands are overexpressed in inflammatory environments and attenuate the immune response through PD-1 on immune effector cells. In addition, PD-L1 expressed on malignant cells or in the tumor microenvironment suppresses tumor-infiltrating lymphocyte activity.
Pidilizumab, a humanized monoclonal antibody that binds to PD-1, weakens the apoptotic processes in lymphocytes and augments the antitumor activities of NK cells.
Investigators conducted a phase 2 trial of pidilizumab in patients with diffuse large B-cell lymphoma (DLBCL) after autologous HSCT to modulate the immune system after a transplant.
The team treated 66 patients with the antibody. At 16 months, progression-free survival (PFS) was 72%. For the 24 high-risk patients who were PET-positive after salvage chemotherapy, the 16-month PFS was 70%.
“And I think that what was most interesting,” Dr Ansell said, when focusing on the 35 patients with measurable disease after transplant, pidilizumab produced a 51% response rate “even in patients that actually had active disease.”
When pidilizumab was combined with rituximab in another trial in patients with relapsed follicular lymphoma (FL), 19 of 29 evaluable patients (66%) achieved an objective response: 15 (52%) CRs and 4 (14%) PRs.
“You might say, ‘Who cares? That’s not that great,’” Dr Ansell said. “But I think what was pretty impressive is that 52% CR rate. And most of you who treat patients with rituximab would know that that’s quite surprising, suggesting that there may be additional benefit for the use of PD-1 blockade in this subset of patients.”
Nivolumab, another monoclonal antibody that blocks the PD-1 pathway, is being investigated in a number of lymphoid malignancies, including HL, DLBCL, and T-cell lymphomas.
In a phase 1 study of nivolumab in 81 patients with relapsed or refractory lymphoid malignancies, the best preliminary overall response has been in FL and DLBCL patients, with an objective response rate of 40% and 36%, respectively, including 1 PR and 3 PRs in each subtype.
“I think what is important,” Dr Ansell said, “is that the side effects, as expected, were mainly immune-mediated, not as dramatic as have been seen with other agents, and very similar to what has been seen in solid tumor studies.”
Dr Ansell pointed out that the response rates with nivolumab varied widely by histology, suggesting that “we have a lot to learn about why patients benefit and who exactly benefits.”
There were no responses in patients with multiple myeloma or primary mediastinal B-cell lymphoma, although many patients achieved stable disease.
“Hodgkin lymphoma was completely different,” Dr Ansell said, “and there were responses in virtually every patient.”
Of 23 patients treated with nivolumab, 20 responded—4 achieved a CR and 16 a PR—including patients who had failed autologous HSCT and brentuximab vedotin treatment. Eleven patients, including 2 with CRs, have an ongoing response, some approaching 2 years. So the responses have been durable, Dr Ansell noted.
Yet another PD-1 antibody, pembrolizumab, has prompted reduction in tumor burden in HL in all but 2 of 29 evaluable patients, including 6 CRs and 13 PRs. The median duration of response has not yet been reached, and the side effect profile was similar to what has been seen with nivolumab and in solid tumors.
Activating immune stimulatory signals
Another approach to boosting the immune system is to activate immune stimulatory signals, eg, CD27 and CD40, and get a benefit that way. Varlilumab (CDX-1127) is an unconjugated monoclonal antibody that binds CD27 and activates CD27-expressing T cells.
In a phase 1 trial of varlilumab in 24 lymphoma patients, investigators found no significant depletion in absolute lymphocyte counts, T cells, or B cells. “Not quite the same success story,” Dr Ansell said, with a response—a CR—in only 1 patient.
Investigators did observe, however, evidence of increased soluble CD27, a reduction of circulating Tregs, and the induction of pro-inflammatory cytokines.
And in a phase 1 study of the anti-CD40 monoclonal antibody dacetuzumab in recurrent NHL, “the response rate was disappointingly low,” Dr Ansell said.
Investigators observed 6 objective responses, including 1 CR and 5 PRs, and a decrease in tumor size in approximately one-third of the 50 patients treated.
The investigators of the subsequent phase 2 trial did not want to take the agent forward for further study, Dr Ansell noted, “but there are antibodies now being developed in this space that will hopefully be more effective and create a greater benefit.”
Optimizing immune function
Dr Ansell suggested there are 3 main approaches to treating patients. One is going directly after the malignant cells and depleting them. A second is to inhibit critical pathways that the malignant cell is dependent upon, “starving them, if you like.” The third way is to activate the immune system and thereby create a greater benefit for patients.
“[P]robably our best strategy is to use all 3 in a reprogram approach,” he said. “Because unless you target each one of these areas, the likelihood is that the other sides of the 3-legged stool will take over.”
“This is an encouraging and exciting time for immune checkpoints therapy and an encouraging and exciting time for immune therapies in general. I think this is really the new frontier in lymphomas.” ![]()