User login
Dupilumab gains off-label uses as clinicians turn to drug for more indications
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
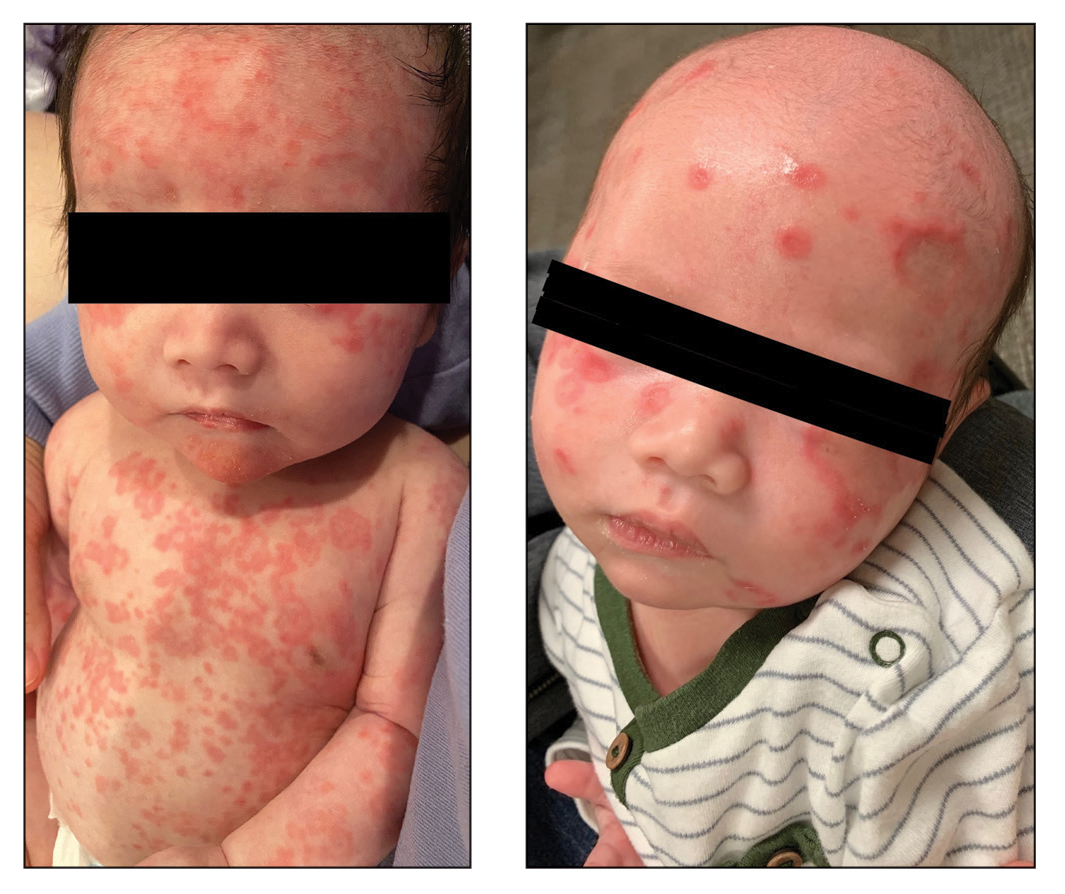
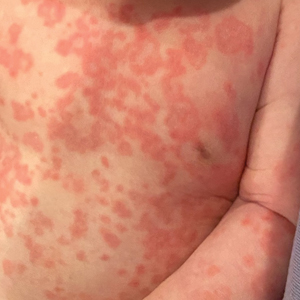
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Getting COVID shots in same arm may be more effective, study says
Scientists in Germany looked at health data for 303 people who got the mRNA vaccine and then a booster shot. Their antibody levels were measured two weeks after the second shot. None of the people had had COVID before the vaccinations.
Scientists found that the number of protective “killer T cells” was higher in the 147 study participants who got both shots in the same arm, said the study published in EBioMedicine.
The killer cells were found in 67% of cases in which both shots went into the same arm, compared with 43% of cases with different arms.
“That may suggest that that ipsilateral vaccination (in the same arm) is more likely to provide better protection should the vaccinated person become infected with the SARS-CoV-2 virus,” Laura Ziegler, a doctoral student at Saarland University, Germany, said in a news release.
William Schaffner, MD, a professor in the Division of Infectious Diseases at Vanderbilt University Medical Center, Nashville, Tenn., told CBS News that same-arm vaccinations may work better because the cells that provide the immune response are in local lymph nodes.
There’s greater immunological response if the immune cells in the lymph nodes are restimulated in the same place, said Dr. Schaffner, who was not involved in the German study.
The scientists from Saarland University said more research is needed before they can be certain that having vaccinations in the same arm is actually more effective for COVID shots and sequential vaccinations against diseases such as the flu.
A version of this article first appeared on Medscape.com.
Scientists in Germany looked at health data for 303 people who got the mRNA vaccine and then a booster shot. Their antibody levels were measured two weeks after the second shot. None of the people had had COVID before the vaccinations.
Scientists found that the number of protective “killer T cells” was higher in the 147 study participants who got both shots in the same arm, said the study published in EBioMedicine.
The killer cells were found in 67% of cases in which both shots went into the same arm, compared with 43% of cases with different arms.
“That may suggest that that ipsilateral vaccination (in the same arm) is more likely to provide better protection should the vaccinated person become infected with the SARS-CoV-2 virus,” Laura Ziegler, a doctoral student at Saarland University, Germany, said in a news release.
William Schaffner, MD, a professor in the Division of Infectious Diseases at Vanderbilt University Medical Center, Nashville, Tenn., told CBS News that same-arm vaccinations may work better because the cells that provide the immune response are in local lymph nodes.
There’s greater immunological response if the immune cells in the lymph nodes are restimulated in the same place, said Dr. Schaffner, who was not involved in the German study.
The scientists from Saarland University said more research is needed before they can be certain that having vaccinations in the same arm is actually more effective for COVID shots and sequential vaccinations against diseases such as the flu.
A version of this article first appeared on Medscape.com.
Scientists in Germany looked at health data for 303 people who got the mRNA vaccine and then a booster shot. Their antibody levels were measured two weeks after the second shot. None of the people had had COVID before the vaccinations.
Scientists found that the number of protective “killer T cells” was higher in the 147 study participants who got both shots in the same arm, said the study published in EBioMedicine.
The killer cells were found in 67% of cases in which both shots went into the same arm, compared with 43% of cases with different arms.
“That may suggest that that ipsilateral vaccination (in the same arm) is more likely to provide better protection should the vaccinated person become infected with the SARS-CoV-2 virus,” Laura Ziegler, a doctoral student at Saarland University, Germany, said in a news release.
William Schaffner, MD, a professor in the Division of Infectious Diseases at Vanderbilt University Medical Center, Nashville, Tenn., told CBS News that same-arm vaccinations may work better because the cells that provide the immune response are in local lymph nodes.
There’s greater immunological response if the immune cells in the lymph nodes are restimulated in the same place, said Dr. Schaffner, who was not involved in the German study.
The scientists from Saarland University said more research is needed before they can be certain that having vaccinations in the same arm is actually more effective for COVID shots and sequential vaccinations against diseases such as the flu.
A version of this article first appeared on Medscape.com.
FROM EBIOMEDICINE
Applications for the CUTIS 2024 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2024 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2024.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2024 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2024.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2024 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2024.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
AHA advocates normothermia for most comatose OHCA patients
a new American Heart Association (AHA) scientific advisory suggests.
On the basis of data from recent trials, the International Liaison Committee on Resuscitation and other organizations have altered their treatment recommendations for temperature management after cardiac arrest.
The AHA will present guidelines on this topic in a focused update to be published later in the year. Meanwhile, AHA’s Emergency Cardiovascular Care Committee convened a writing group to review the Hypothermia Versus Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial in the context of other recent evidence and rendered an expert opinion on how the trial may influence clinical practice. These findings will be incorporated into the upcoming guidelines.
“Many centers have already moved toward controlled normothermia for post-arrest patients, so we think this guidance will be welcomed by many,” said Sarah Perman, MD, of the Yale University, and Kate Berg, MD, of Beth Israel Deaconess Medical Center, who are both members of the AHA Emergency Cardiovascular Care Committee that authored the advisory.
“For those who continue to favor temperatures in the 32° to 36° range for some or even all patients, the guidance that we have drafted leaves room for clinicians to make patient-centered decisions,” they told this news organization.
“Certainly, a finite guideline that recommends one temperature for all would be easier to apply,” the authors acknowledged. “However, cardiac arrest is a heterogeneous event and brain injury is variable, and definitive evidence that one temperature in the range of 32-37.5 is superior to another is lacking. We hope that clinicians find that this guidance supports and informs their practice.”
The advisory was published online in Circulation.
TTM2 key
The new guidance is based largely on findings from the TTM2 trial, a multicenter, randomized clinical trial of temperature management for neuroprotection after cardiac arrest that included 1,900 unresponsive adult patients successfully resuscitated from OHCA.
Patients were randomly assigned to receive hypothermia, defined as a target temperature of 33° C for 28 hours, followed by gradual rewarming to 37° C, or normothermia, defined as a target temperature < 37.8° C, with early treatment of fever.
No significant between-group difference was seen in the primary outcome of death at 6 months, nor were there any significant differences by subgroups of sex, age, time to return of spontaneous circulation, initial rhythm, or circulatory shock on admission.
Although it’s still not clear whether certain patients might benefit from lower target temperatures, the authors noted, major international organizations now suggest a target post–cardiac arrest temperature of less than 37.5° C.
By contrast, current AHA guidelines endorse targeting a temperature between 32° C and 36° C for 24 hours.
Between now and the forthcoming formal guidance in the “2023 American Heart Association Focused Update on Advanced Cardiovascular Life Support,” the scientific advisory writing group agreed: “For unresponsive post–cardiac arrest adult patients with characteristics similar to those of individuals included in the TTM2 trial (OHCA of cardiac or unknown cause, excluding those with unwitnessed asystole), controlling patient temperature to < 37.5° C is a reasonable and evidence-based approach.
“For the broader group of patients with in-hospital cardiac arrest or OHCA of noncardiac (other medical) cause, evidence for the ideal approach to temperature management after return of spontaneous circulation is less certain; whether some of these patients might benefit from temperature control at temperatures between 33° C and 37.5° C remains unclear.”
Unless a catastrophic brain injury results from OHCA, the group wrote, “strictly preventing fever with continuous temperature monitoring, providing comprehensive critical care support, and deploying multimodal evidence-based strategies for neuroprognostication at a minimum of 72 hours after normothermia remain essential ...”
Dr. Perman and Dr. Berg concluded, “We hope that this guidance continues to encourage aggressive post-arrest care that includes focus on temperature control as well as the other major contributors to post-arrest bundles of care including hemodynamic optimization and guideline concordant neuroprognostication.”
No funding was reported. Dr. Berg has received grant support from AHA/ILCOR, and Dr. Perman, from NIH/NHLBI.
A version of this article first appeared on Medscape.com.
a new American Heart Association (AHA) scientific advisory suggests.
On the basis of data from recent trials, the International Liaison Committee on Resuscitation and other organizations have altered their treatment recommendations for temperature management after cardiac arrest.
The AHA will present guidelines on this topic in a focused update to be published later in the year. Meanwhile, AHA’s Emergency Cardiovascular Care Committee convened a writing group to review the Hypothermia Versus Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial in the context of other recent evidence and rendered an expert opinion on how the trial may influence clinical practice. These findings will be incorporated into the upcoming guidelines.
“Many centers have already moved toward controlled normothermia for post-arrest patients, so we think this guidance will be welcomed by many,” said Sarah Perman, MD, of the Yale University, and Kate Berg, MD, of Beth Israel Deaconess Medical Center, who are both members of the AHA Emergency Cardiovascular Care Committee that authored the advisory.
“For those who continue to favor temperatures in the 32° to 36° range for some or even all patients, the guidance that we have drafted leaves room for clinicians to make patient-centered decisions,” they told this news organization.
“Certainly, a finite guideline that recommends one temperature for all would be easier to apply,” the authors acknowledged. “However, cardiac arrest is a heterogeneous event and brain injury is variable, and definitive evidence that one temperature in the range of 32-37.5 is superior to another is lacking. We hope that clinicians find that this guidance supports and informs their practice.”
The advisory was published online in Circulation.
TTM2 key
The new guidance is based largely on findings from the TTM2 trial, a multicenter, randomized clinical trial of temperature management for neuroprotection after cardiac arrest that included 1,900 unresponsive adult patients successfully resuscitated from OHCA.
Patients were randomly assigned to receive hypothermia, defined as a target temperature of 33° C for 28 hours, followed by gradual rewarming to 37° C, or normothermia, defined as a target temperature < 37.8° C, with early treatment of fever.
No significant between-group difference was seen in the primary outcome of death at 6 months, nor were there any significant differences by subgroups of sex, age, time to return of spontaneous circulation, initial rhythm, or circulatory shock on admission.
Although it’s still not clear whether certain patients might benefit from lower target temperatures, the authors noted, major international organizations now suggest a target post–cardiac arrest temperature of less than 37.5° C.
By contrast, current AHA guidelines endorse targeting a temperature between 32° C and 36° C for 24 hours.
Between now and the forthcoming formal guidance in the “2023 American Heart Association Focused Update on Advanced Cardiovascular Life Support,” the scientific advisory writing group agreed: “For unresponsive post–cardiac arrest adult patients with characteristics similar to those of individuals included in the TTM2 trial (OHCA of cardiac or unknown cause, excluding those with unwitnessed asystole), controlling patient temperature to < 37.5° C is a reasonable and evidence-based approach.
“For the broader group of patients with in-hospital cardiac arrest or OHCA of noncardiac (other medical) cause, evidence for the ideal approach to temperature management after return of spontaneous circulation is less certain; whether some of these patients might benefit from temperature control at temperatures between 33° C and 37.5° C remains unclear.”
Unless a catastrophic brain injury results from OHCA, the group wrote, “strictly preventing fever with continuous temperature monitoring, providing comprehensive critical care support, and deploying multimodal evidence-based strategies for neuroprognostication at a minimum of 72 hours after normothermia remain essential ...”
Dr. Perman and Dr. Berg concluded, “We hope that this guidance continues to encourage aggressive post-arrest care that includes focus on temperature control as well as the other major contributors to post-arrest bundles of care including hemodynamic optimization and guideline concordant neuroprognostication.”
No funding was reported. Dr. Berg has received grant support from AHA/ILCOR, and Dr. Perman, from NIH/NHLBI.
A version of this article first appeared on Medscape.com.
a new American Heart Association (AHA) scientific advisory suggests.
On the basis of data from recent trials, the International Liaison Committee on Resuscitation and other organizations have altered their treatment recommendations for temperature management after cardiac arrest.
The AHA will present guidelines on this topic in a focused update to be published later in the year. Meanwhile, AHA’s Emergency Cardiovascular Care Committee convened a writing group to review the Hypothermia Versus Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial in the context of other recent evidence and rendered an expert opinion on how the trial may influence clinical practice. These findings will be incorporated into the upcoming guidelines.
“Many centers have already moved toward controlled normothermia for post-arrest patients, so we think this guidance will be welcomed by many,” said Sarah Perman, MD, of the Yale University, and Kate Berg, MD, of Beth Israel Deaconess Medical Center, who are both members of the AHA Emergency Cardiovascular Care Committee that authored the advisory.
“For those who continue to favor temperatures in the 32° to 36° range for some or even all patients, the guidance that we have drafted leaves room for clinicians to make patient-centered decisions,” they told this news organization.
“Certainly, a finite guideline that recommends one temperature for all would be easier to apply,” the authors acknowledged. “However, cardiac arrest is a heterogeneous event and brain injury is variable, and definitive evidence that one temperature in the range of 32-37.5 is superior to another is lacking. We hope that clinicians find that this guidance supports and informs their practice.”
The advisory was published online in Circulation.
TTM2 key
The new guidance is based largely on findings from the TTM2 trial, a multicenter, randomized clinical trial of temperature management for neuroprotection after cardiac arrest that included 1,900 unresponsive adult patients successfully resuscitated from OHCA.
Patients were randomly assigned to receive hypothermia, defined as a target temperature of 33° C for 28 hours, followed by gradual rewarming to 37° C, or normothermia, defined as a target temperature < 37.8° C, with early treatment of fever.
No significant between-group difference was seen in the primary outcome of death at 6 months, nor were there any significant differences by subgroups of sex, age, time to return of spontaneous circulation, initial rhythm, or circulatory shock on admission.
Although it’s still not clear whether certain patients might benefit from lower target temperatures, the authors noted, major international organizations now suggest a target post–cardiac arrest temperature of less than 37.5° C.
By contrast, current AHA guidelines endorse targeting a temperature between 32° C and 36° C for 24 hours.
Between now and the forthcoming formal guidance in the “2023 American Heart Association Focused Update on Advanced Cardiovascular Life Support,” the scientific advisory writing group agreed: “For unresponsive post–cardiac arrest adult patients with characteristics similar to those of individuals included in the TTM2 trial (OHCA of cardiac or unknown cause, excluding those with unwitnessed asystole), controlling patient temperature to < 37.5° C is a reasonable and evidence-based approach.
“For the broader group of patients with in-hospital cardiac arrest or OHCA of noncardiac (other medical) cause, evidence for the ideal approach to temperature management after return of spontaneous circulation is less certain; whether some of these patients might benefit from temperature control at temperatures between 33° C and 37.5° C remains unclear.”
Unless a catastrophic brain injury results from OHCA, the group wrote, “strictly preventing fever with continuous temperature monitoring, providing comprehensive critical care support, and deploying multimodal evidence-based strategies for neuroprognostication at a minimum of 72 hours after normothermia remain essential ...”
Dr. Perman and Dr. Berg concluded, “We hope that this guidance continues to encourage aggressive post-arrest care that includes focus on temperature control as well as the other major contributors to post-arrest bundles of care including hemodynamic optimization and guideline concordant neuroprognostication.”
No funding was reported. Dr. Berg has received grant support from AHA/ILCOR, and Dr. Perman, from NIH/NHLBI.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Parental bias about a doctor can’t trump a patient’s health
This transcript has been edited for clarity.
I’d like to present you today with a case that raised a large amount of discussion and debate. I got involved as an ethics consultant on the case. I think you’ll find it very interesting and I also think there are going to be some differences of opinion about how to manage the case. I’ll be looking forward to getting comments and feedback on this.
The case involved a 14-year-old boy who had been brought into the hospital by his parents, suffering from severe bouts of anxiety that were just almost overwhelming to him. When he was brought in, he was assigned a health care provider who had a West African last name. Prior to meeting the patient, I have to say that the father of this kid told the intake department nurse that he requested someone else. He saw the name – he hadn’t even met the provider – and he said he wanted someone who might be Catholic.
The parents are both from the Dominican Republic. They identified as White, but they appeared to be non-White Latinx to the nurse who was doing some of the initial intake. They got reassigned to a different provider in the department who identified as African American.
The first month of treatment for the young boy went very well, and he seemed to be getting along extremely well with his provider. He was reporting relief to both parents of some of his anxiety, and the provider felt very connected to the child. A good doctor-patient alliance had been formed.
Nevertheless, at the end of the first month, the father connected back to one of the administrators at the hospital and complained, saying he still wanted a different provider. When asked why, he said, “Well, I don’t really want to answer that,” but getting pressed, he basically said he wasn’t comfortable with having an African American doctor take care of his child. He eventually went back to the argument that what he wanted was someone with a Catholic background, although I don’t know that he knew whether this particular provider was religious – Catholic or anything else.
Some people felt that, as the father in charge of the child’s care, if we could accommodate what he wanted in terms of the parents being comfortable, then that’s something we should do. I absolutely did not agree.
My view is that in a situation where a strong provider-patient relationship has been established, where trust is going both ways, where there are no issues coming up between this 14-year-old and the provider, and when a serious mental health issue is being adequately addressed, the patient’s interest must come first.
Once that therapeutic alliance had been established and both the patient and the provider felt satisfied, I don’t think the father’s wishes made any sense. He may have been acting more out of bigotry or just discomfort about difference in terms of who the provider was. I don’t think that’s something that any health system should have to accommodate unless it is getting in the way of patient care.
I hope that we treat all physicians as properly trained to deal with all kinds of patients, regardless of their religion, ethnicity, or skin color. They should have the skills to manage and do well with any patient. There may be situations where it just doesn’t work or where people don’t get along. Yes, I think we then should try, perhaps, to shift the doctor, get a different nurse, or have a different person do an exam. That’s because of the inability to get the patient’s health interests addressed.
Listening to this dad about what he preferred in terms of religion or ethnicity seemed to me to be interfering with medical success. Could I stop him from moving this patient out entirely from the care setting? Probably not, but I think the way to manage this is to try to talk to him – and, by the way, to talk to the mother.
When we did bring the mom into the situation, she was very happy with the health care provider. She didn’t agree with the dad and wanted to have a meeting with the social worker, the dad, and her to get him to get over the worries, concerns, and maybe even biases he was bringing in about the kind of provider he wanted. That’s exactly what we did.
I know that there are many instances where patients may say, “I don’t want a particular doctor or a particular type.” My view is that we shouldn’t accommodate that. We should say that our doctors are trained to help and care for all manner of people. Unless we can think of some reason that there might be a gap or a problem in the actual delivery of the quality of care, we are not going to accommodate racism, bigotry, or bias.
We certainly shouldn’t be accommodating that once a successful therapeutic relationship is established. Even when it’s a child, I would argue that the patient’s best interest has to trump parental desires, parental worries, and parental concerns about the background, ethnicity, and religion of the provider.
Dr. Caplan is director of the division of medical ethics at NYU Langone Medical Center, New York. He disclosed a conflict of interest with Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to present you today with a case that raised a large amount of discussion and debate. I got involved as an ethics consultant on the case. I think you’ll find it very interesting and I also think there are going to be some differences of opinion about how to manage the case. I’ll be looking forward to getting comments and feedback on this.
The case involved a 14-year-old boy who had been brought into the hospital by his parents, suffering from severe bouts of anxiety that were just almost overwhelming to him. When he was brought in, he was assigned a health care provider who had a West African last name. Prior to meeting the patient, I have to say that the father of this kid told the intake department nurse that he requested someone else. He saw the name – he hadn’t even met the provider – and he said he wanted someone who might be Catholic.
The parents are both from the Dominican Republic. They identified as White, but they appeared to be non-White Latinx to the nurse who was doing some of the initial intake. They got reassigned to a different provider in the department who identified as African American.
The first month of treatment for the young boy went very well, and he seemed to be getting along extremely well with his provider. He was reporting relief to both parents of some of his anxiety, and the provider felt very connected to the child. A good doctor-patient alliance had been formed.
Nevertheless, at the end of the first month, the father connected back to one of the administrators at the hospital and complained, saying he still wanted a different provider. When asked why, he said, “Well, I don’t really want to answer that,” but getting pressed, he basically said he wasn’t comfortable with having an African American doctor take care of his child. He eventually went back to the argument that what he wanted was someone with a Catholic background, although I don’t know that he knew whether this particular provider was religious – Catholic or anything else.
Some people felt that, as the father in charge of the child’s care, if we could accommodate what he wanted in terms of the parents being comfortable, then that’s something we should do. I absolutely did not agree.
My view is that in a situation where a strong provider-patient relationship has been established, where trust is going both ways, where there are no issues coming up between this 14-year-old and the provider, and when a serious mental health issue is being adequately addressed, the patient’s interest must come first.
Once that therapeutic alliance had been established and both the patient and the provider felt satisfied, I don’t think the father’s wishes made any sense. He may have been acting more out of bigotry or just discomfort about difference in terms of who the provider was. I don’t think that’s something that any health system should have to accommodate unless it is getting in the way of patient care.
I hope that we treat all physicians as properly trained to deal with all kinds of patients, regardless of their religion, ethnicity, or skin color. They should have the skills to manage and do well with any patient. There may be situations where it just doesn’t work or where people don’t get along. Yes, I think we then should try, perhaps, to shift the doctor, get a different nurse, or have a different person do an exam. That’s because of the inability to get the patient’s health interests addressed.
Listening to this dad about what he preferred in terms of religion or ethnicity seemed to me to be interfering with medical success. Could I stop him from moving this patient out entirely from the care setting? Probably not, but I think the way to manage this is to try to talk to him – and, by the way, to talk to the mother.
When we did bring the mom into the situation, she was very happy with the health care provider. She didn’t agree with the dad and wanted to have a meeting with the social worker, the dad, and her to get him to get over the worries, concerns, and maybe even biases he was bringing in about the kind of provider he wanted. That’s exactly what we did.
I know that there are many instances where patients may say, “I don’t want a particular doctor or a particular type.” My view is that we shouldn’t accommodate that. We should say that our doctors are trained to help and care for all manner of people. Unless we can think of some reason that there might be a gap or a problem in the actual delivery of the quality of care, we are not going to accommodate racism, bigotry, or bias.
We certainly shouldn’t be accommodating that once a successful therapeutic relationship is established. Even when it’s a child, I would argue that the patient’s best interest has to trump parental desires, parental worries, and parental concerns about the background, ethnicity, and religion of the provider.
Dr. Caplan is director of the division of medical ethics at NYU Langone Medical Center, New York. He disclosed a conflict of interest with Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to present you today with a case that raised a large amount of discussion and debate. I got involved as an ethics consultant on the case. I think you’ll find it very interesting and I also think there are going to be some differences of opinion about how to manage the case. I’ll be looking forward to getting comments and feedback on this.
The case involved a 14-year-old boy who had been brought into the hospital by his parents, suffering from severe bouts of anxiety that were just almost overwhelming to him. When he was brought in, he was assigned a health care provider who had a West African last name. Prior to meeting the patient, I have to say that the father of this kid told the intake department nurse that he requested someone else. He saw the name – he hadn’t even met the provider – and he said he wanted someone who might be Catholic.
The parents are both from the Dominican Republic. They identified as White, but they appeared to be non-White Latinx to the nurse who was doing some of the initial intake. They got reassigned to a different provider in the department who identified as African American.
The first month of treatment for the young boy went very well, and he seemed to be getting along extremely well with his provider. He was reporting relief to both parents of some of his anxiety, and the provider felt very connected to the child. A good doctor-patient alliance had been formed.
Nevertheless, at the end of the first month, the father connected back to one of the administrators at the hospital and complained, saying he still wanted a different provider. When asked why, he said, “Well, I don’t really want to answer that,” but getting pressed, he basically said he wasn’t comfortable with having an African American doctor take care of his child. He eventually went back to the argument that what he wanted was someone with a Catholic background, although I don’t know that he knew whether this particular provider was religious – Catholic or anything else.
Some people felt that, as the father in charge of the child’s care, if we could accommodate what he wanted in terms of the parents being comfortable, then that’s something we should do. I absolutely did not agree.
My view is that in a situation where a strong provider-patient relationship has been established, where trust is going both ways, where there are no issues coming up between this 14-year-old and the provider, and when a serious mental health issue is being adequately addressed, the patient’s interest must come first.
Once that therapeutic alliance had been established and both the patient and the provider felt satisfied, I don’t think the father’s wishes made any sense. He may have been acting more out of bigotry or just discomfort about difference in terms of who the provider was. I don’t think that’s something that any health system should have to accommodate unless it is getting in the way of patient care.
I hope that we treat all physicians as properly trained to deal with all kinds of patients, regardless of their religion, ethnicity, or skin color. They should have the skills to manage and do well with any patient. There may be situations where it just doesn’t work or where people don’t get along. Yes, I think we then should try, perhaps, to shift the doctor, get a different nurse, or have a different person do an exam. That’s because of the inability to get the patient’s health interests addressed.
Listening to this dad about what he preferred in terms of religion or ethnicity seemed to me to be interfering with medical success. Could I stop him from moving this patient out entirely from the care setting? Probably not, but I think the way to manage this is to try to talk to him – and, by the way, to talk to the mother.
When we did bring the mom into the situation, she was very happy with the health care provider. She didn’t agree with the dad and wanted to have a meeting with the social worker, the dad, and her to get him to get over the worries, concerns, and maybe even biases he was bringing in about the kind of provider he wanted. That’s exactly what we did.
I know that there are many instances where patients may say, “I don’t want a particular doctor or a particular type.” My view is that we shouldn’t accommodate that. We should say that our doctors are trained to help and care for all manner of people. Unless we can think of some reason that there might be a gap or a problem in the actual delivery of the quality of care, we are not going to accommodate racism, bigotry, or bias.
We certainly shouldn’t be accommodating that once a successful therapeutic relationship is established. Even when it’s a child, I would argue that the patient’s best interest has to trump parental desires, parental worries, and parental concerns about the background, ethnicity, and religion of the provider.
Dr. Caplan is director of the division of medical ethics at NYU Langone Medical Center, New York. He disclosed a conflict of interest with Johnson & Johnson.
A version of this article first appeared on Medscape.com.
Artificial sweeteners no help for weight loss: Review
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
FROM CURRENT OPINION IN CARDIOLOGY
Delayed introduction of allergens increases allergy risk
These findings were published in Allergy.
Launched in April 2011, the French ELFE study aims to monitor children from birth to adulthood to better understand the factors from the intrauterine period to adolescence that affect their development, health, social skills, and school career. Thanks to this cohort, a team of scientists has reviewed the relationship between complementary feeding practices and allergies in French children.
The study focused on 6,662 children who had no signs of an allergic reaction before 2 months of age. Data on feeding practices were collected monthly from ages 3 months to 10 months. Their age at complementary feeding introduction was calculated, and a food diversity score was determined at 8 and 10 months. The number of major allergenic foods (out of eggs, fish, wheat, and dairy products) not introduced at 8 and 10 months was also determined. Allergic diseases (food allergy, eczema, asthma, and rhinoconjunctivitis) were reported by parents at 2 months and at 1, 2, 3.5, and 5.5 years.
Initially, scientists determined that just 62% of children began complementary feeding in the recommended age window, which is between ages 4 months and 6 months. They then closely studied the link between delayed introduction of major allergenic foods and the risk of food allergies. They saw that for 1 in 10 children, at least two major allergens, from eggs, fish, wheat, and dairy products, had still not been introduced into the diet of infants by the age of 10 months. Now, these children have a risk of developing a food allergy before the age of 5.5 years that is two times greater than that of children in whom the four major allergens were introduced before the age of 10 months.
These findings therefore confirm the importance of not delaying the introduction of major food allergens to prevent the occurrence of childhood allergic diseases. They provide convincing arguments in support of new recommendations made by the French pediatric and allergy societies as well as those issued by Public Health France.
ELFE: The first cohort to follow children from birth to adulthood
ELFE is the first longitudinal nationwide French study dedicated to monitoring children from birth to adulthood. More than 18,000 children born in metropolitan France in 2011 were included in this study, which represents 1 in 50 children born in 2011. From the time that researchers first met the families in the maternity ward, the parents who agreed to participate in this great scientific adventure have been questioned at regular intervals to better understand how environment, family members, and living conditions affect the development, health, and socialization of children.
This article was translated from the Medscape French Edition. A version of this article first appeared on Medscape.com.
These findings were published in Allergy.
Launched in April 2011, the French ELFE study aims to monitor children from birth to adulthood to better understand the factors from the intrauterine period to adolescence that affect their development, health, social skills, and school career. Thanks to this cohort, a team of scientists has reviewed the relationship between complementary feeding practices and allergies in French children.
The study focused on 6,662 children who had no signs of an allergic reaction before 2 months of age. Data on feeding practices were collected monthly from ages 3 months to 10 months. Their age at complementary feeding introduction was calculated, and a food diversity score was determined at 8 and 10 months. The number of major allergenic foods (out of eggs, fish, wheat, and dairy products) not introduced at 8 and 10 months was also determined. Allergic diseases (food allergy, eczema, asthma, and rhinoconjunctivitis) were reported by parents at 2 months and at 1, 2, 3.5, and 5.5 years.
Initially, scientists determined that just 62% of children began complementary feeding in the recommended age window, which is between ages 4 months and 6 months. They then closely studied the link between delayed introduction of major allergenic foods and the risk of food allergies. They saw that for 1 in 10 children, at least two major allergens, from eggs, fish, wheat, and dairy products, had still not been introduced into the diet of infants by the age of 10 months. Now, these children have a risk of developing a food allergy before the age of 5.5 years that is two times greater than that of children in whom the four major allergens were introduced before the age of 10 months.
These findings therefore confirm the importance of not delaying the introduction of major food allergens to prevent the occurrence of childhood allergic diseases. They provide convincing arguments in support of new recommendations made by the French pediatric and allergy societies as well as those issued by Public Health France.
ELFE: The first cohort to follow children from birth to adulthood
ELFE is the first longitudinal nationwide French study dedicated to monitoring children from birth to adulthood. More than 18,000 children born in metropolitan France in 2011 were included in this study, which represents 1 in 50 children born in 2011. From the time that researchers first met the families in the maternity ward, the parents who agreed to participate in this great scientific adventure have been questioned at regular intervals to better understand how environment, family members, and living conditions affect the development, health, and socialization of children.
This article was translated from the Medscape French Edition. A version of this article first appeared on Medscape.com.
These findings were published in Allergy.
Launched in April 2011, the French ELFE study aims to monitor children from birth to adulthood to better understand the factors from the intrauterine period to adolescence that affect their development, health, social skills, and school career. Thanks to this cohort, a team of scientists has reviewed the relationship between complementary feeding practices and allergies in French children.
The study focused on 6,662 children who had no signs of an allergic reaction before 2 months of age. Data on feeding practices were collected monthly from ages 3 months to 10 months. Their age at complementary feeding introduction was calculated, and a food diversity score was determined at 8 and 10 months. The number of major allergenic foods (out of eggs, fish, wheat, and dairy products) not introduced at 8 and 10 months was also determined. Allergic diseases (food allergy, eczema, asthma, and rhinoconjunctivitis) were reported by parents at 2 months and at 1, 2, 3.5, and 5.5 years.
Initially, scientists determined that just 62% of children began complementary feeding in the recommended age window, which is between ages 4 months and 6 months. They then closely studied the link between delayed introduction of major allergenic foods and the risk of food allergies. They saw that for 1 in 10 children, at least two major allergens, from eggs, fish, wheat, and dairy products, had still not been introduced into the diet of infants by the age of 10 months. Now, these children have a risk of developing a food allergy before the age of 5.5 years that is two times greater than that of children in whom the four major allergens were introduced before the age of 10 months.
These findings therefore confirm the importance of not delaying the introduction of major food allergens to prevent the occurrence of childhood allergic diseases. They provide convincing arguments in support of new recommendations made by the French pediatric and allergy societies as well as those issued by Public Health France.
ELFE: The first cohort to follow children from birth to adulthood
ELFE is the first longitudinal nationwide French study dedicated to monitoring children from birth to adulthood. More than 18,000 children born in metropolitan France in 2011 were included in this study, which represents 1 in 50 children born in 2011. From the time that researchers first met the families in the maternity ward, the parents who agreed to participate in this great scientific adventure have been questioned at regular intervals to better understand how environment, family members, and living conditions affect the development, health, and socialization of children.
This article was translated from the Medscape French Edition. A version of this article first appeared on Medscape.com.
FROM ALLERGY
FDA approves first RSV vaccine for pregnancy
The vaccine, known as Abrysvo, can be given between weeks 32 and 36 of pregnancy and is designed to protect infants from the virus from birth to 6 months of age.
Administered as a single-dose, intramuscular injection, the FDA approved Abrysvo at the end of May for the prevention of lower respiratory tract illness caused by RSV in people aged 60 years and older.
However, “RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, pointed out in a news release. “This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
Most children are infected with the contagious virus at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis, and in clinical trials, the new vaccine reduced that risk by up to 82%.
Before the vaccine became available, up to 3% of infants infected with RSV needed to be hospitalized, according to the Centers for Disease Control and Prevention. In the hospital, treatment typically includes oxygen, intravenous fluids, and mechanical ventilation.
RSV often causes common cold symptoms, but the virus poses the risk of severe complications that can lead to death among young children and older people. The CDC estimates 100-300 deaths of children younger than 5 years and 6,000-10,000 deaths of people aged 65 years and older are linked to RSV annually.
This is also the first year that an antibody shot is available to be given after birth to prevent severe RSV in infants younger than 1 year.
In its approval announcement, the FDA pointed out that preeclampsia occurred in 1.8% of pregnancies after Abrysvo, compared with 1.4% of those who received placebo. The FDA also reported that, in infants, low birth weight and jaundice occurred at a higher rate among the pregnant Abrysvo recipients, compared with the placebo group.
Studies have also shown that pregnant vaccine recipients experienced preterm birth at a rate of 5.7%, compared with a rate of 4.7% among those who received placebo. The FDA called the difference “a numerical imbalance” but said in the approval announcement that a “causal relationship” could not be established.
The FDA also noted that people already at high risk of preterm birth were excluded from clinical trials and that Pfizer must conduct ongoing studies to monitor the risk of preeclampsia as well as preterm birth.
A version of this article first appeared on Medscape.com.
The vaccine, known as Abrysvo, can be given between weeks 32 and 36 of pregnancy and is designed to protect infants from the virus from birth to 6 months of age.
Administered as a single-dose, intramuscular injection, the FDA approved Abrysvo at the end of May for the prevention of lower respiratory tract illness caused by RSV in people aged 60 years and older.
However, “RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, pointed out in a news release. “This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
Most children are infected with the contagious virus at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis, and in clinical trials, the new vaccine reduced that risk by up to 82%.
Before the vaccine became available, up to 3% of infants infected with RSV needed to be hospitalized, according to the Centers for Disease Control and Prevention. In the hospital, treatment typically includes oxygen, intravenous fluids, and mechanical ventilation.
RSV often causes common cold symptoms, but the virus poses the risk of severe complications that can lead to death among young children and older people. The CDC estimates 100-300 deaths of children younger than 5 years and 6,000-10,000 deaths of people aged 65 years and older are linked to RSV annually.
This is also the first year that an antibody shot is available to be given after birth to prevent severe RSV in infants younger than 1 year.
In its approval announcement, the FDA pointed out that preeclampsia occurred in 1.8% of pregnancies after Abrysvo, compared with 1.4% of those who received placebo. The FDA also reported that, in infants, low birth weight and jaundice occurred at a higher rate among the pregnant Abrysvo recipients, compared with the placebo group.
Studies have also shown that pregnant vaccine recipients experienced preterm birth at a rate of 5.7%, compared with a rate of 4.7% among those who received placebo. The FDA called the difference “a numerical imbalance” but said in the approval announcement that a “causal relationship” could not be established.
The FDA also noted that people already at high risk of preterm birth were excluded from clinical trials and that Pfizer must conduct ongoing studies to monitor the risk of preeclampsia as well as preterm birth.
A version of this article first appeared on Medscape.com.
The vaccine, known as Abrysvo, can be given between weeks 32 and 36 of pregnancy and is designed to protect infants from the virus from birth to 6 months of age.
Administered as a single-dose, intramuscular injection, the FDA approved Abrysvo at the end of May for the prevention of lower respiratory tract illness caused by RSV in people aged 60 years and older.
However, “RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, pointed out in a news release. “This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
Most children are infected with the contagious virus at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis, and in clinical trials, the new vaccine reduced that risk by up to 82%.
Before the vaccine became available, up to 3% of infants infected with RSV needed to be hospitalized, according to the Centers for Disease Control and Prevention. In the hospital, treatment typically includes oxygen, intravenous fluids, and mechanical ventilation.
RSV often causes common cold symptoms, but the virus poses the risk of severe complications that can lead to death among young children and older people. The CDC estimates 100-300 deaths of children younger than 5 years and 6,000-10,000 deaths of people aged 65 years and older are linked to RSV annually.
This is also the first year that an antibody shot is available to be given after birth to prevent severe RSV in infants younger than 1 year.
In its approval announcement, the FDA pointed out that preeclampsia occurred in 1.8% of pregnancies after Abrysvo, compared with 1.4% of those who received placebo. The FDA also reported that, in infants, low birth weight and jaundice occurred at a higher rate among the pregnant Abrysvo recipients, compared with the placebo group.
Studies have also shown that pregnant vaccine recipients experienced preterm birth at a rate of 5.7%, compared with a rate of 4.7% among those who received placebo. The FDA called the difference “a numerical imbalance” but said in the approval announcement that a “causal relationship” could not be established.
The FDA also noted that people already at high risk of preterm birth were excluded from clinical trials and that Pfizer must conduct ongoing studies to monitor the risk of preeclampsia as well as preterm birth.
A version of this article first appeared on Medscape.com.
Gene therapy offers new way to fight alcohol use disorder
Researchers from Oregon Health & Science University, Portland implanted the therapy directly into the brains of rhesus monkeys that had been conditioned to drink 8-10 alcoholic drinks a day. A harmless virus that carried a specific gene was placed in the region of the brain that regulates dopamine, which provides feelings of reward and pleasure.
“We wanted to see if we could normalize the dopamine in these motivational areas – if, indeed, motivation to overdrink or drink heavily would be mitigated,” said study author Kathleen Grant, PhD, a professor and chief of the division of neuroscience at the university’s Oregon National Primate Research Center.
The need for new alcohol use disorder treatments may be more dire than ever. Alcohol-related deaths in the United States increased dramatically between 2007 and 2020, especially in women, according to research published in the journal JAMA Network Open. The next year, they spiked again, to 108,791 alcohol-related deaths in 2021 alone, according to the National Institutes of Health. That’s slightly more than the number of drug overdoses recorded in 2021.
For the 29.5 million Americans with alcohol use disorder, also known as alcohol abuse or dependence, the road to recovery can be challenging. One reason is that the reward systems in their brains are working against them.
At the first taste of alcohol, the body releases dopamine. But if a person drinks too much for too long, the brain reduces dopamine production and even more alcohol is needed to feel good again.
The gene researchers placed in the monkeys’ brains is called glial-derived neurotrophic factor. It is a growth factor, stimulating cells to multiply. It may help improve function of brain cells that synthesize dopamine, effectively resetting the whole system and reducing the urge to drink.
The study was surprisingly successful. Compared with primates that received a placebo, those that received the growth factor gene decreased their drinking by about 90%. They basically quit drinking, while the primates that got the placebo resumed their habit.
A similar procedure is already used in patients with Parkinson’s disease. But more animal studies, and human clinical trials, would be needed before this therapy could be used in humans with alcohol use disorder. This invasive treatment involves brain surgery, which has risks, so it would likely be reserved for those with the most severe, dangerous drinking habits.
“I think it’d be appropriate for individuals where other treatment modalities just weren’t effective, and they’re worried for their lives,” Dr. Grant said.
Alcohol use disorder treatments
Today, treatment for alcohol use disorder ranges from a brief conversation with a health care provider, in mild cases, to psychiatric treatment or medication in moderate or severe cases.
There are four Food and Drug Administration–approved treatments for alcohol use disorder and a few more medications that health care providers can prescribe off label.
“They’re not widely used,” said Henry Kranzler, MD, a professor of psychiatry and director of the Center for Studies of Addiction at the University of Pennsylvania, Philadelphia. “They’re shockingly underutilized.”
One reason: Just 4.6% of people with alcohol use disorder seek treatment each year, according to NIH data.
“Some of the issues include the ubiquity of alcohol, and its acceptance in American culture – and the fact that that makes it difficult for people to acknowledge that they have a problem with alcohol,” said Dr. Kranzler.
But another problem is that many health care professionals don’t recognize and treat alcohol use disorder in patients who do seek care. Those seeking treatment for alcohol use disorder can find a qualified provider at the American Academy of Addiction Psychiatry or American Society of Addiction Medicine directories.
The future of treatment
Ongoing research could lead to more treatments, and make them more available and more appealing.
Unlike many other drugs that work on a single receptor in the body – like opioids that target opioid receptors, or nicotine, which targets choline receptors – alcohol affects many different receptors, said Robert Swift, MD, PhD, a professor of psychiatry and human behavior at Brown University, Providence, R.I. It also penetrates cells at high doses.
“There are so many different effects of alcohol, which makes it very hard to treat,” he said. “But on the other hand, it gives us an advantage, and there are probably different points that we can attack.”
Other exciting developments are underway, although more research, including clinical trials in humans, is needed before they arrive.
Some of the most promising:
- Hallucinogens. In the 1950s, before they became illegal, these drugs helped people drink less. Even Bill Wilson, cofounder of Alcoholics Anonymous, used hallucinogenic treatment in his recovery; it helped him envision overcoming a challenge. Today, there is renewed interest in hallucinogens for alcohol use disorder. In a study published in , people with alcohol use disorder who were given the hallucinogen psilocybin along with therapy spent fewer days drinking heavily over the following 32 weeks than people who received a different medication. Don’t try to do this yourself, though. “It’s not just taking a hallucinogen and having a trip,” Dr. Swift said. “It’s a therapy-guided session, so it’s a combination of using the hallucinogenic substance with a skilled therapist, and sometimes two skilled therapists, helping to guide the experience.”
- Epigenetic editing. Alcohol exposure can affect the activity of a gene in the amygdala, a brain region involved in emotional processing. found that, by editing that gene in rats through an intravenous line of genetic material, they reduced the rodents’ drinking and anxiety.
- Oxytocin. The so-called love hormone could help reset the dopamine system to make alcohol less appealing. “There are oxytocin receptors on dopamine neurons, and oxytocin makes your dopamine system more effective,” Dr. Swift said. In a from the Medical University of South Carolina, Charleston, mice injected with oxytocin didn’t drink during a stressful situation that could have otherwise led to relapse.
- Ghrelin. This stomach hormone could help curb drinking. In a study published in , mice that received drugs that increased ghrelin reduced their alcohol intake.
A version of this article first appeared on WebMD.com.
Researchers from Oregon Health & Science University, Portland implanted the therapy directly into the brains of rhesus monkeys that had been conditioned to drink 8-10 alcoholic drinks a day. A harmless virus that carried a specific gene was placed in the region of the brain that regulates dopamine, which provides feelings of reward and pleasure.
“We wanted to see if we could normalize the dopamine in these motivational areas – if, indeed, motivation to overdrink or drink heavily would be mitigated,” said study author Kathleen Grant, PhD, a professor and chief of the division of neuroscience at the university’s Oregon National Primate Research Center.
The need for new alcohol use disorder treatments may be more dire than ever. Alcohol-related deaths in the United States increased dramatically between 2007 and 2020, especially in women, according to research published in the journal JAMA Network Open. The next year, they spiked again, to 108,791 alcohol-related deaths in 2021 alone, according to the National Institutes of Health. That’s slightly more than the number of drug overdoses recorded in 2021.
For the 29.5 million Americans with alcohol use disorder, also known as alcohol abuse or dependence, the road to recovery can be challenging. One reason is that the reward systems in their brains are working against them.
At the first taste of alcohol, the body releases dopamine. But if a person drinks too much for too long, the brain reduces dopamine production and even more alcohol is needed to feel good again.
The gene researchers placed in the monkeys’ brains is called glial-derived neurotrophic factor. It is a growth factor, stimulating cells to multiply. It may help improve function of brain cells that synthesize dopamine, effectively resetting the whole system and reducing the urge to drink.
The study was surprisingly successful. Compared with primates that received a placebo, those that received the growth factor gene decreased their drinking by about 90%. They basically quit drinking, while the primates that got the placebo resumed their habit.
A similar procedure is already used in patients with Parkinson’s disease. But more animal studies, and human clinical trials, would be needed before this therapy could be used in humans with alcohol use disorder. This invasive treatment involves brain surgery, which has risks, so it would likely be reserved for those with the most severe, dangerous drinking habits.
“I think it’d be appropriate for individuals where other treatment modalities just weren’t effective, and they’re worried for their lives,” Dr. Grant said.
Alcohol use disorder treatments
Today, treatment for alcohol use disorder ranges from a brief conversation with a health care provider, in mild cases, to psychiatric treatment or medication in moderate or severe cases.
There are four Food and Drug Administration–approved treatments for alcohol use disorder and a few more medications that health care providers can prescribe off label.
“They’re not widely used,” said Henry Kranzler, MD, a professor of psychiatry and director of the Center for Studies of Addiction at the University of Pennsylvania, Philadelphia. “They’re shockingly underutilized.”
One reason: Just 4.6% of people with alcohol use disorder seek treatment each year, according to NIH data.
“Some of the issues include the ubiquity of alcohol, and its acceptance in American culture – and the fact that that makes it difficult for people to acknowledge that they have a problem with alcohol,” said Dr. Kranzler.
But another problem is that many health care professionals don’t recognize and treat alcohol use disorder in patients who do seek care. Those seeking treatment for alcohol use disorder can find a qualified provider at the American Academy of Addiction Psychiatry or American Society of Addiction Medicine directories.
The future of treatment
Ongoing research could lead to more treatments, and make them more available and more appealing.
Unlike many other drugs that work on a single receptor in the body – like opioids that target opioid receptors, or nicotine, which targets choline receptors – alcohol affects many different receptors, said Robert Swift, MD, PhD, a professor of psychiatry and human behavior at Brown University, Providence, R.I. It also penetrates cells at high doses.
“There are so many different effects of alcohol, which makes it very hard to treat,” he said. “But on the other hand, it gives us an advantage, and there are probably different points that we can attack.”
Other exciting developments are underway, although more research, including clinical trials in humans, is needed before they arrive.
Some of the most promising:
- Hallucinogens. In the 1950s, before they became illegal, these drugs helped people drink less. Even Bill Wilson, cofounder of Alcoholics Anonymous, used hallucinogenic treatment in his recovery; it helped him envision overcoming a challenge. Today, there is renewed interest in hallucinogens for alcohol use disorder. In a study published in , people with alcohol use disorder who were given the hallucinogen psilocybin along with therapy spent fewer days drinking heavily over the following 32 weeks than people who received a different medication. Don’t try to do this yourself, though. “It’s not just taking a hallucinogen and having a trip,” Dr. Swift said. “It’s a therapy-guided session, so it’s a combination of using the hallucinogenic substance with a skilled therapist, and sometimes two skilled therapists, helping to guide the experience.”
- Epigenetic editing. Alcohol exposure can affect the activity of a gene in the amygdala, a brain region involved in emotional processing. found that, by editing that gene in rats through an intravenous line of genetic material, they reduced the rodents’ drinking and anxiety.
- Oxytocin. The so-called love hormone could help reset the dopamine system to make alcohol less appealing. “There are oxytocin receptors on dopamine neurons, and oxytocin makes your dopamine system more effective,” Dr. Swift said. In a from the Medical University of South Carolina, Charleston, mice injected with oxytocin didn’t drink during a stressful situation that could have otherwise led to relapse.
- Ghrelin. This stomach hormone could help curb drinking. In a study published in , mice that received drugs that increased ghrelin reduced their alcohol intake.
A version of this article first appeared on WebMD.com.
Researchers from Oregon Health & Science University, Portland implanted the therapy directly into the brains of rhesus monkeys that had been conditioned to drink 8-10 alcoholic drinks a day. A harmless virus that carried a specific gene was placed in the region of the brain that regulates dopamine, which provides feelings of reward and pleasure.
“We wanted to see if we could normalize the dopamine in these motivational areas – if, indeed, motivation to overdrink or drink heavily would be mitigated,” said study author Kathleen Grant, PhD, a professor and chief of the division of neuroscience at the university’s Oregon National Primate Research Center.
The need for new alcohol use disorder treatments may be more dire than ever. Alcohol-related deaths in the United States increased dramatically between 2007 and 2020, especially in women, according to research published in the journal JAMA Network Open. The next year, they spiked again, to 108,791 alcohol-related deaths in 2021 alone, according to the National Institutes of Health. That’s slightly more than the number of drug overdoses recorded in 2021.
For the 29.5 million Americans with alcohol use disorder, also known as alcohol abuse or dependence, the road to recovery can be challenging. One reason is that the reward systems in their brains are working against them.
At the first taste of alcohol, the body releases dopamine. But if a person drinks too much for too long, the brain reduces dopamine production and even more alcohol is needed to feel good again.
The gene researchers placed in the monkeys’ brains is called glial-derived neurotrophic factor. It is a growth factor, stimulating cells to multiply. It may help improve function of brain cells that synthesize dopamine, effectively resetting the whole system and reducing the urge to drink.
The study was surprisingly successful. Compared with primates that received a placebo, those that received the growth factor gene decreased their drinking by about 90%. They basically quit drinking, while the primates that got the placebo resumed their habit.
A similar procedure is already used in patients with Parkinson’s disease. But more animal studies, and human clinical trials, would be needed before this therapy could be used in humans with alcohol use disorder. This invasive treatment involves brain surgery, which has risks, so it would likely be reserved for those with the most severe, dangerous drinking habits.
“I think it’d be appropriate for individuals where other treatment modalities just weren’t effective, and they’re worried for their lives,” Dr. Grant said.
Alcohol use disorder treatments
Today, treatment for alcohol use disorder ranges from a brief conversation with a health care provider, in mild cases, to psychiatric treatment or medication in moderate or severe cases.
There are four Food and Drug Administration–approved treatments for alcohol use disorder and a few more medications that health care providers can prescribe off label.
“They’re not widely used,” said Henry Kranzler, MD, a professor of psychiatry and director of the Center for Studies of Addiction at the University of Pennsylvania, Philadelphia. “They’re shockingly underutilized.”
One reason: Just 4.6% of people with alcohol use disorder seek treatment each year, according to NIH data.
“Some of the issues include the ubiquity of alcohol, and its acceptance in American culture – and the fact that that makes it difficult for people to acknowledge that they have a problem with alcohol,” said Dr. Kranzler.
But another problem is that many health care professionals don’t recognize and treat alcohol use disorder in patients who do seek care. Those seeking treatment for alcohol use disorder can find a qualified provider at the American Academy of Addiction Psychiatry or American Society of Addiction Medicine directories.
The future of treatment
Ongoing research could lead to more treatments, and make them more available and more appealing.
Unlike many other drugs that work on a single receptor in the body – like opioids that target opioid receptors, or nicotine, which targets choline receptors – alcohol affects many different receptors, said Robert Swift, MD, PhD, a professor of psychiatry and human behavior at Brown University, Providence, R.I. It also penetrates cells at high doses.
“There are so many different effects of alcohol, which makes it very hard to treat,” he said. “But on the other hand, it gives us an advantage, and there are probably different points that we can attack.”
Other exciting developments are underway, although more research, including clinical trials in humans, is needed before they arrive.
Some of the most promising:
- Hallucinogens. In the 1950s, before they became illegal, these drugs helped people drink less. Even Bill Wilson, cofounder of Alcoholics Anonymous, used hallucinogenic treatment in his recovery; it helped him envision overcoming a challenge. Today, there is renewed interest in hallucinogens for alcohol use disorder. In a study published in , people with alcohol use disorder who were given the hallucinogen psilocybin along with therapy spent fewer days drinking heavily over the following 32 weeks than people who received a different medication. Don’t try to do this yourself, though. “It’s not just taking a hallucinogen and having a trip,” Dr. Swift said. “It’s a therapy-guided session, so it’s a combination of using the hallucinogenic substance with a skilled therapist, and sometimes two skilled therapists, helping to guide the experience.”
- Epigenetic editing. Alcohol exposure can affect the activity of a gene in the amygdala, a brain region involved in emotional processing. found that, by editing that gene in rats through an intravenous line of genetic material, they reduced the rodents’ drinking and anxiety.
- Oxytocin. The so-called love hormone could help reset the dopamine system to make alcohol less appealing. “There are oxytocin receptors on dopamine neurons, and oxytocin makes your dopamine system more effective,” Dr. Swift said. In a from the Medical University of South Carolina, Charleston, mice injected with oxytocin didn’t drink during a stressful situation that could have otherwise led to relapse.
- Ghrelin. This stomach hormone could help curb drinking. In a study published in , mice that received drugs that increased ghrelin reduced their alcohol intake.
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
Diffuse Annular Plaques in an Infant
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
A 5-week-old infant boy presented with a rash at birth (left). The pregnancy was full term without complications, and he was otherwise healthy. A family history revealed that his older brother developed a similar rash 2 weeks after birth (right). Physical examination revealed polycyclic annular patches with an erythematous border and central clearing diffusely located on the trunk, extremities, scalp, and face with periorbital edema.