User login
Sleep disturbance may predict increased risk of suicidal thoughts
Suicide remains the second leading cause of death in young adults, but factors that may predict increased suicide risk have not been characterized, wrote Rebecca C. Cox, PhD, of the University of Colorado Boulder, and colleagues.
“Sleep disturbance is a promising modifiable risk factor for acute changes in suicide risk,” they noted. “Previous research has found multiple aspects of sleep disturbance are linked to elevated SI, including insomnia symptoms, both short and long sleep duration, nocturnal wakefulness, and nightmares.”
However, data on the impact of nightly sleep disturbance on suicide risk are limited, the researchers said. They hypothesized that use of ecological momentary assessment (EMA) to assess daily variability in sleep might offer more insight into the relationship between various components of sleep disturbance and changes in suicide risk.
In a study published in Psychiatry Research , the investigators recruited 102 young adults aged 18-35 years who had a history of suicidal behavior; 74.5% were female, 64.7% were White. Participants completed seven semi-random surveys per day for between wake and sleep schedules over 21 days. Each survey asked participants to report on whether they had experienced suicidal ideation (SI) since the last survey. The researchers examined within-person and between-person sleep variables including bedtime, sleep onset latency, sleep onset, number of awakenings, wake after sleep onset, sleep duration, sleep timing, sleep quality, and nightmares.
Overall, nightmares had a significant, positive effect on passive SI at both within- and between-person levels, but no significant effect on active SI. Sleep latency showed a significant, positive effect on passive and active SI at the between-person level, meaning that “participants who took longer to fall asleep on average were more likely to experience passive and active SI during the sampling period,” the researchers noted.
In addition, days following nights of more time awake between sleep onset and offset were days with increased likelihood of passive and active SI. Similarly, days following nights of worse sleep quality than normally reported for an individual were days with increased likelihood of passive and active SI. Sleep timing and duration had no significant effects on SI at the within- or between-person level.
“Notably, tests of reverse models found no relation between daily passive or active SI and any component of the subsequent night’s sleep, suggesting a unidirectional relation between sleep disturbance and subsequent SI,” the researchers wrote in their discussion. If future research replicates the study findings, the results could support the inclusion of sleep difficulties on standard risk assessments as a way to identify risk for SI and initiate prevention approaches, they said.
The findings were limited by several factors including the potential for unmeasured variables impacting the associations between sleep and SI, the researchers noted. Other limitations included the lack of data on more severe levels of SI such as planning and intent, and on suicidal behaviors such as preparatory behaviors, aborted attempts, and actual attempts. The findings also may not generalize to other age groups such as children, adolescents, or older adults, they said.
More research is needed to determine which sleep disturbance components are acute risk factors for which suicide-related outcomes, the researchers said. However, the study is the first to provide evidence for daily sleep disturbances as a near-term predictor of SI in young adults, they concluded.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
Suicide remains the second leading cause of death in young adults, but factors that may predict increased suicide risk have not been characterized, wrote Rebecca C. Cox, PhD, of the University of Colorado Boulder, and colleagues.
“Sleep disturbance is a promising modifiable risk factor for acute changes in suicide risk,” they noted. “Previous research has found multiple aspects of sleep disturbance are linked to elevated SI, including insomnia symptoms, both short and long sleep duration, nocturnal wakefulness, and nightmares.”
However, data on the impact of nightly sleep disturbance on suicide risk are limited, the researchers said. They hypothesized that use of ecological momentary assessment (EMA) to assess daily variability in sleep might offer more insight into the relationship between various components of sleep disturbance and changes in suicide risk.
In a study published in Psychiatry Research , the investigators recruited 102 young adults aged 18-35 years who had a history of suicidal behavior; 74.5% were female, 64.7% were White. Participants completed seven semi-random surveys per day for between wake and sleep schedules over 21 days. Each survey asked participants to report on whether they had experienced suicidal ideation (SI) since the last survey. The researchers examined within-person and between-person sleep variables including bedtime, sleep onset latency, sleep onset, number of awakenings, wake after sleep onset, sleep duration, sleep timing, sleep quality, and nightmares.
Overall, nightmares had a significant, positive effect on passive SI at both within- and between-person levels, but no significant effect on active SI. Sleep latency showed a significant, positive effect on passive and active SI at the between-person level, meaning that “participants who took longer to fall asleep on average were more likely to experience passive and active SI during the sampling period,” the researchers noted.
In addition, days following nights of more time awake between sleep onset and offset were days with increased likelihood of passive and active SI. Similarly, days following nights of worse sleep quality than normally reported for an individual were days with increased likelihood of passive and active SI. Sleep timing and duration had no significant effects on SI at the within- or between-person level.
“Notably, tests of reverse models found no relation between daily passive or active SI and any component of the subsequent night’s sleep, suggesting a unidirectional relation between sleep disturbance and subsequent SI,” the researchers wrote in their discussion. If future research replicates the study findings, the results could support the inclusion of sleep difficulties on standard risk assessments as a way to identify risk for SI and initiate prevention approaches, they said.
The findings were limited by several factors including the potential for unmeasured variables impacting the associations between sleep and SI, the researchers noted. Other limitations included the lack of data on more severe levels of SI such as planning and intent, and on suicidal behaviors such as preparatory behaviors, aborted attempts, and actual attempts. The findings also may not generalize to other age groups such as children, adolescents, or older adults, they said.
More research is needed to determine which sleep disturbance components are acute risk factors for which suicide-related outcomes, the researchers said. However, the study is the first to provide evidence for daily sleep disturbances as a near-term predictor of SI in young adults, they concluded.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
Suicide remains the second leading cause of death in young adults, but factors that may predict increased suicide risk have not been characterized, wrote Rebecca C. Cox, PhD, of the University of Colorado Boulder, and colleagues.
“Sleep disturbance is a promising modifiable risk factor for acute changes in suicide risk,” they noted. “Previous research has found multiple aspects of sleep disturbance are linked to elevated SI, including insomnia symptoms, both short and long sleep duration, nocturnal wakefulness, and nightmares.”
However, data on the impact of nightly sleep disturbance on suicide risk are limited, the researchers said. They hypothesized that use of ecological momentary assessment (EMA) to assess daily variability in sleep might offer more insight into the relationship between various components of sleep disturbance and changes in suicide risk.
In a study published in Psychiatry Research , the investigators recruited 102 young adults aged 18-35 years who had a history of suicidal behavior; 74.5% were female, 64.7% were White. Participants completed seven semi-random surveys per day for between wake and sleep schedules over 21 days. Each survey asked participants to report on whether they had experienced suicidal ideation (SI) since the last survey. The researchers examined within-person and between-person sleep variables including bedtime, sleep onset latency, sleep onset, number of awakenings, wake after sleep onset, sleep duration, sleep timing, sleep quality, and nightmares.
Overall, nightmares had a significant, positive effect on passive SI at both within- and between-person levels, but no significant effect on active SI. Sleep latency showed a significant, positive effect on passive and active SI at the between-person level, meaning that “participants who took longer to fall asleep on average were more likely to experience passive and active SI during the sampling period,” the researchers noted.
In addition, days following nights of more time awake between sleep onset and offset were days with increased likelihood of passive and active SI. Similarly, days following nights of worse sleep quality than normally reported for an individual were days with increased likelihood of passive and active SI. Sleep timing and duration had no significant effects on SI at the within- or between-person level.
“Notably, tests of reverse models found no relation between daily passive or active SI and any component of the subsequent night’s sleep, suggesting a unidirectional relation between sleep disturbance and subsequent SI,” the researchers wrote in their discussion. If future research replicates the study findings, the results could support the inclusion of sleep difficulties on standard risk assessments as a way to identify risk for SI and initiate prevention approaches, they said.
The findings were limited by several factors including the potential for unmeasured variables impacting the associations between sleep and SI, the researchers noted. Other limitations included the lack of data on more severe levels of SI such as planning and intent, and on suicidal behaviors such as preparatory behaviors, aborted attempts, and actual attempts. The findings also may not generalize to other age groups such as children, adolescents, or older adults, they said.
More research is needed to determine which sleep disturbance components are acute risk factors for which suicide-related outcomes, the researchers said. However, the study is the first to provide evidence for daily sleep disturbances as a near-term predictor of SI in young adults, they concluded.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRY RESEARCH
Lymphoma specialist to lead MD Anderson’s cancer medicine division
“My research uncovered a series of physicians who served as ‘clinical champions’ and dramatically sped the process of drug development,” Dr. Flowers recalled in an interview. “This early career research inspired me to become the type of clinical champion that I uncovered.”
Over his career, hematologist-oncologist Dr. Flowers has developed lifesaving therapies for lymphoma, which has transformed into a highly treatable and even curable disease. He’s listed as a coauthor of hundreds of peer-reviewed cancer studies, reports, and medical society guidelines. And he’s revealed stark disparities in blood cancer care: His research shows that non-White patients suffer from worse outcomes, regardless of factors like income and insurance coverage.
The University of Texas MD Anderson Cancer Center, Houston, recently named physician-scientist Dr. Flowers as division head of cancer medicine, a position he’s held on an interim basis. As of Sept. 1, he will permanently oversee 300 faculty and more than 2,000 staff members.
A running start in Seattle
For Dr. Flowers, track and field is a sport that runs in the family. His grandfather was a top runner in both high school and college, and both Dr. Flowers and his brother ran competitively in Seattle, where they grew up. But Dr. Flowers chose a career in oncology, earning a medical degree at Stanford and master’s degrees at both Stanford and the University of Washington, Seattle.
The late Kenneth Melmon, MD, a groundbreaking pharmacologist, was a major influence. “He was one of the first people that I met when I began as an undergraduate at Stanford. We grew to be long-standing friends, and he demonstrated what outstanding mentorship looks like. In our research collaboration, we investigated the work of Dr. Gertrude Elion and Dr. George Hitchings involving the translation of pharmacological data from cellular and animal models to clinically useful drugs including 6-mercaptopurine, allopurinol, azathioprine, acyclovir, and zidovudine.”
The late Oliver Press, MD, a blood cancer specialist, inspired Dr. Flower’s interest in lymphoma. “I began work with him during an internship at the University of Washington. Ollie was a great inspiration and a key leader in the development of innovative therapies for lymphoma. He embodied the role of a clinical champion translating work in radioimmunotherapy to new therapeutics for patients with lymphomas. Working with him ultimately led me to pursue a career in hematology and oncology with a focus on the care for patients with lymphomas.”
Career blooms as lymphoma care advances
Dr. Flowers went on to Emory University, Atlanta, where he served as scientific director of the Research Informatics Shared Resource and a faculty member in the department of biomedical informatics. “I applied my training in informatics and my clinical expertise to support active grants from the Burroughs Wellcome Fund for Innovation in Regulatory Science and from the National Cancer Institute to develop informatics tools for pathology image analysis and prognostic modeling.”
For 13 years, he also served the Winship Cancer Institute as director of the Emory Healthcare lymphoma program (where his patients included Kansas City Chiefs football star Eric Berry), and for 4 years as scientific director of research informatics. Meanwhile, Dr. Flowers helped develop national practice guidelines for the American Society of Clinical Oncology, the American Cancer Society, and the American College of Radiology. He also chaired the ASCO guideline on management of febrile neutropenia.
In 2019, MD Anderson hired Dr. Flowers as chair of the department of lymphoma/myeloma. A year later, he was appointed division head ad interim for cancer medicine.
“Chris is a unique leader who expertly combines mentorship, sponsorship, and bidirectional open, honest communication,” said Sairah Ahmed, MD, associate professor of lymphoma at MD Anderson. “He doesn’t just empower his team to reach their goals. He also inspires those around him to turn vision into reality.”
As Dr. Flowers noted, many patients with lymphoma are now able to recover and live normal lives. He himself played a direct role himself in boosting lifespans.
“I have been fortunate to play a role in the development of several treatments that have led to advances in first-line therapy for patients with aggressive lymphomas. I partnered with others at MD Anderson, including Dr. Sattva Neelapu and Dr. Jason Westin, who have developed novel therapies like chimeric antigen receptor T-cell therapy for patients with relapse lymphomas,” he said. “Leaders in the field at MD Anderson like Dr. Michael Wang have developed new oral treatments for patients with rare lymphoma subtypes like mantle cell lymphoma. Other colleagues such as Dr. Nathan Fowler and Dr. Loretta Nastoupil have focused on the care for patients with indolent lymphomas and developed less-toxic therapies that are now in common use.”
Exposing the disparities in blood cancer care
Dr. Flowers, who’s African American, has also been a leader in health disparity research. In 2016, for example, he was coauthor of a study into non-Hodgkin’s lymphoma that revealed that Blacks in the United States have dramatically lower survival rates than Whites. The 10-year survival rate for Black women with chronic lymphocytic leukemia was just 47%, for example, compared with 66% for White females. “Although incidence rates of lymphoid neoplasms are generally higher among Whites, Black men tend to have poorer survival,” Dr. Flowers and colleagues wrote.
In a 2021 report for the ASCO Educational Book, Dr. Flowers and hematologist-oncologist Demetria Smith-Graziani, MD, now with Emory University, explored disparities across blood cancers and barriers to minority enrollment in clinical trials. “Some approaches that clinicians can apply to address these disparities include increasing systems-level awareness, improving access to care, and reducing biases in clinical setting,” the authors wrote.
Luis Malpica Castillo, MD, assistant professor of lymphoma at MD Anderson Cancer Center, lauded the work of Dr. Flowers in expanding opportunities for minority patients with the disease.
“During the past years, Dr. Flowers’ work has not only had a positive impact on the Texan community, but minority populations living with cancer in the United States and abroad,” he said. “Currently, we are implementing cancer care networks aimed to increase diversity in clinical trials by enrolling a larger number of Hispanic and African American patients, who otherwise may not have benefited from novel therapies. The ultimate goal is to provide high-quality care to all patients living with cancer.”
In addition to his research work, Dr. Flowers is an advocate for diversity within the hematology community. He’s a founding member and former chair of the American Society of Hematology’s Committee on Diversity, Equity and Inclusion (formerly the Committee on Promoting Diversity), and he helped develop the society’s Minority Recruitment Initiative.
What’s next for Dr. Flowers? For one, he plans to continue working as a mentor; he received the ASH Mentor Award in honor of his service in 2022. “I am strongly committed to increasing the number of tenure-track investigators trained in clinical and translational cancer research and to promote their career development.”
And he looks forward to helping develop MD Anderson’s recently announced $2.5 billion hospital in Austin. “This will extend the exceptional care that we provide as the No. 1 cancer center in the United States,” he said. “It will also create new opportunities for research and collaboration with experts at UT Austin.”
When he’s not in clinic, Dr. Flowers embraces his lifelong love of speeding through life on his own two feet. He’s even inspired his children to share his passion. “I run most days of the week,” he said. “Running provides a great opportunity to think and process new research ideas, work through leadership challenges, and sometimes just to relax and let go of the day.”
“My research uncovered a series of physicians who served as ‘clinical champions’ and dramatically sped the process of drug development,” Dr. Flowers recalled in an interview. “This early career research inspired me to become the type of clinical champion that I uncovered.”
Over his career, hematologist-oncologist Dr. Flowers has developed lifesaving therapies for lymphoma, which has transformed into a highly treatable and even curable disease. He’s listed as a coauthor of hundreds of peer-reviewed cancer studies, reports, and medical society guidelines. And he’s revealed stark disparities in blood cancer care: His research shows that non-White patients suffer from worse outcomes, regardless of factors like income and insurance coverage.
The University of Texas MD Anderson Cancer Center, Houston, recently named physician-scientist Dr. Flowers as division head of cancer medicine, a position he’s held on an interim basis. As of Sept. 1, he will permanently oversee 300 faculty and more than 2,000 staff members.
A running start in Seattle
For Dr. Flowers, track and field is a sport that runs in the family. His grandfather was a top runner in both high school and college, and both Dr. Flowers and his brother ran competitively in Seattle, where they grew up. But Dr. Flowers chose a career in oncology, earning a medical degree at Stanford and master’s degrees at both Stanford and the University of Washington, Seattle.
The late Kenneth Melmon, MD, a groundbreaking pharmacologist, was a major influence. “He was one of the first people that I met when I began as an undergraduate at Stanford. We grew to be long-standing friends, and he demonstrated what outstanding mentorship looks like. In our research collaboration, we investigated the work of Dr. Gertrude Elion and Dr. George Hitchings involving the translation of pharmacological data from cellular and animal models to clinically useful drugs including 6-mercaptopurine, allopurinol, azathioprine, acyclovir, and zidovudine.”
The late Oliver Press, MD, a blood cancer specialist, inspired Dr. Flower’s interest in lymphoma. “I began work with him during an internship at the University of Washington. Ollie was a great inspiration and a key leader in the development of innovative therapies for lymphoma. He embodied the role of a clinical champion translating work in radioimmunotherapy to new therapeutics for patients with lymphomas. Working with him ultimately led me to pursue a career in hematology and oncology with a focus on the care for patients with lymphomas.”
Career blooms as lymphoma care advances
Dr. Flowers went on to Emory University, Atlanta, where he served as scientific director of the Research Informatics Shared Resource and a faculty member in the department of biomedical informatics. “I applied my training in informatics and my clinical expertise to support active grants from the Burroughs Wellcome Fund for Innovation in Regulatory Science and from the National Cancer Institute to develop informatics tools for pathology image analysis and prognostic modeling.”
For 13 years, he also served the Winship Cancer Institute as director of the Emory Healthcare lymphoma program (where his patients included Kansas City Chiefs football star Eric Berry), and for 4 years as scientific director of research informatics. Meanwhile, Dr. Flowers helped develop national practice guidelines for the American Society of Clinical Oncology, the American Cancer Society, and the American College of Radiology. He also chaired the ASCO guideline on management of febrile neutropenia.
In 2019, MD Anderson hired Dr. Flowers as chair of the department of lymphoma/myeloma. A year later, he was appointed division head ad interim for cancer medicine.
“Chris is a unique leader who expertly combines mentorship, sponsorship, and bidirectional open, honest communication,” said Sairah Ahmed, MD, associate professor of lymphoma at MD Anderson. “He doesn’t just empower his team to reach their goals. He also inspires those around him to turn vision into reality.”
As Dr. Flowers noted, many patients with lymphoma are now able to recover and live normal lives. He himself played a direct role himself in boosting lifespans.
“I have been fortunate to play a role in the development of several treatments that have led to advances in first-line therapy for patients with aggressive lymphomas. I partnered with others at MD Anderson, including Dr. Sattva Neelapu and Dr. Jason Westin, who have developed novel therapies like chimeric antigen receptor T-cell therapy for patients with relapse lymphomas,” he said. “Leaders in the field at MD Anderson like Dr. Michael Wang have developed new oral treatments for patients with rare lymphoma subtypes like mantle cell lymphoma. Other colleagues such as Dr. Nathan Fowler and Dr. Loretta Nastoupil have focused on the care for patients with indolent lymphomas and developed less-toxic therapies that are now in common use.”
Exposing the disparities in blood cancer care
Dr. Flowers, who’s African American, has also been a leader in health disparity research. In 2016, for example, he was coauthor of a study into non-Hodgkin’s lymphoma that revealed that Blacks in the United States have dramatically lower survival rates than Whites. The 10-year survival rate for Black women with chronic lymphocytic leukemia was just 47%, for example, compared with 66% for White females. “Although incidence rates of lymphoid neoplasms are generally higher among Whites, Black men tend to have poorer survival,” Dr. Flowers and colleagues wrote.
In a 2021 report for the ASCO Educational Book, Dr. Flowers and hematologist-oncologist Demetria Smith-Graziani, MD, now with Emory University, explored disparities across blood cancers and barriers to minority enrollment in clinical trials. “Some approaches that clinicians can apply to address these disparities include increasing systems-level awareness, improving access to care, and reducing biases in clinical setting,” the authors wrote.
Luis Malpica Castillo, MD, assistant professor of lymphoma at MD Anderson Cancer Center, lauded the work of Dr. Flowers in expanding opportunities for minority patients with the disease.
“During the past years, Dr. Flowers’ work has not only had a positive impact on the Texan community, but minority populations living with cancer in the United States and abroad,” he said. “Currently, we are implementing cancer care networks aimed to increase diversity in clinical trials by enrolling a larger number of Hispanic and African American patients, who otherwise may not have benefited from novel therapies. The ultimate goal is to provide high-quality care to all patients living with cancer.”
In addition to his research work, Dr. Flowers is an advocate for diversity within the hematology community. He’s a founding member and former chair of the American Society of Hematology’s Committee on Diversity, Equity and Inclusion (formerly the Committee on Promoting Diversity), and he helped develop the society’s Minority Recruitment Initiative.
What’s next for Dr. Flowers? For one, he plans to continue working as a mentor; he received the ASH Mentor Award in honor of his service in 2022. “I am strongly committed to increasing the number of tenure-track investigators trained in clinical and translational cancer research and to promote their career development.”
And he looks forward to helping develop MD Anderson’s recently announced $2.5 billion hospital in Austin. “This will extend the exceptional care that we provide as the No. 1 cancer center in the United States,” he said. “It will also create new opportunities for research and collaboration with experts at UT Austin.”
When he’s not in clinic, Dr. Flowers embraces his lifelong love of speeding through life on his own two feet. He’s even inspired his children to share his passion. “I run most days of the week,” he said. “Running provides a great opportunity to think and process new research ideas, work through leadership challenges, and sometimes just to relax and let go of the day.”
“My research uncovered a series of physicians who served as ‘clinical champions’ and dramatically sped the process of drug development,” Dr. Flowers recalled in an interview. “This early career research inspired me to become the type of clinical champion that I uncovered.”
Over his career, hematologist-oncologist Dr. Flowers has developed lifesaving therapies for lymphoma, which has transformed into a highly treatable and even curable disease. He’s listed as a coauthor of hundreds of peer-reviewed cancer studies, reports, and medical society guidelines. And he’s revealed stark disparities in blood cancer care: His research shows that non-White patients suffer from worse outcomes, regardless of factors like income and insurance coverage.
The University of Texas MD Anderson Cancer Center, Houston, recently named physician-scientist Dr. Flowers as division head of cancer medicine, a position he’s held on an interim basis. As of Sept. 1, he will permanently oversee 300 faculty and more than 2,000 staff members.
A running start in Seattle
For Dr. Flowers, track and field is a sport that runs in the family. His grandfather was a top runner in both high school and college, and both Dr. Flowers and his brother ran competitively in Seattle, where they grew up. But Dr. Flowers chose a career in oncology, earning a medical degree at Stanford and master’s degrees at both Stanford and the University of Washington, Seattle.
The late Kenneth Melmon, MD, a groundbreaking pharmacologist, was a major influence. “He was one of the first people that I met when I began as an undergraduate at Stanford. We grew to be long-standing friends, and he demonstrated what outstanding mentorship looks like. In our research collaboration, we investigated the work of Dr. Gertrude Elion and Dr. George Hitchings involving the translation of pharmacological data from cellular and animal models to clinically useful drugs including 6-mercaptopurine, allopurinol, azathioprine, acyclovir, and zidovudine.”
The late Oliver Press, MD, a blood cancer specialist, inspired Dr. Flower’s interest in lymphoma. “I began work with him during an internship at the University of Washington. Ollie was a great inspiration and a key leader in the development of innovative therapies for lymphoma. He embodied the role of a clinical champion translating work in radioimmunotherapy to new therapeutics for patients with lymphomas. Working with him ultimately led me to pursue a career in hematology and oncology with a focus on the care for patients with lymphomas.”
Career blooms as lymphoma care advances
Dr. Flowers went on to Emory University, Atlanta, where he served as scientific director of the Research Informatics Shared Resource and a faculty member in the department of biomedical informatics. “I applied my training in informatics and my clinical expertise to support active grants from the Burroughs Wellcome Fund for Innovation in Regulatory Science and from the National Cancer Institute to develop informatics tools for pathology image analysis and prognostic modeling.”
For 13 years, he also served the Winship Cancer Institute as director of the Emory Healthcare lymphoma program (where his patients included Kansas City Chiefs football star Eric Berry), and for 4 years as scientific director of research informatics. Meanwhile, Dr. Flowers helped develop national practice guidelines for the American Society of Clinical Oncology, the American Cancer Society, and the American College of Radiology. He also chaired the ASCO guideline on management of febrile neutropenia.
In 2019, MD Anderson hired Dr. Flowers as chair of the department of lymphoma/myeloma. A year later, he was appointed division head ad interim for cancer medicine.
“Chris is a unique leader who expertly combines mentorship, sponsorship, and bidirectional open, honest communication,” said Sairah Ahmed, MD, associate professor of lymphoma at MD Anderson. “He doesn’t just empower his team to reach their goals. He also inspires those around him to turn vision into reality.”
As Dr. Flowers noted, many patients with lymphoma are now able to recover and live normal lives. He himself played a direct role himself in boosting lifespans.
“I have been fortunate to play a role in the development of several treatments that have led to advances in first-line therapy for patients with aggressive lymphomas. I partnered with others at MD Anderson, including Dr. Sattva Neelapu and Dr. Jason Westin, who have developed novel therapies like chimeric antigen receptor T-cell therapy for patients with relapse lymphomas,” he said. “Leaders in the field at MD Anderson like Dr. Michael Wang have developed new oral treatments for patients with rare lymphoma subtypes like mantle cell lymphoma. Other colleagues such as Dr. Nathan Fowler and Dr. Loretta Nastoupil have focused on the care for patients with indolent lymphomas and developed less-toxic therapies that are now in common use.”
Exposing the disparities in blood cancer care
Dr. Flowers, who’s African American, has also been a leader in health disparity research. In 2016, for example, he was coauthor of a study into non-Hodgkin’s lymphoma that revealed that Blacks in the United States have dramatically lower survival rates than Whites. The 10-year survival rate for Black women with chronic lymphocytic leukemia was just 47%, for example, compared with 66% for White females. “Although incidence rates of lymphoid neoplasms are generally higher among Whites, Black men tend to have poorer survival,” Dr. Flowers and colleagues wrote.
In a 2021 report for the ASCO Educational Book, Dr. Flowers and hematologist-oncologist Demetria Smith-Graziani, MD, now with Emory University, explored disparities across blood cancers and barriers to minority enrollment in clinical trials. “Some approaches that clinicians can apply to address these disparities include increasing systems-level awareness, improving access to care, and reducing biases in clinical setting,” the authors wrote.
Luis Malpica Castillo, MD, assistant professor of lymphoma at MD Anderson Cancer Center, lauded the work of Dr. Flowers in expanding opportunities for minority patients with the disease.
“During the past years, Dr. Flowers’ work has not only had a positive impact on the Texan community, but minority populations living with cancer in the United States and abroad,” he said. “Currently, we are implementing cancer care networks aimed to increase diversity in clinical trials by enrolling a larger number of Hispanic and African American patients, who otherwise may not have benefited from novel therapies. The ultimate goal is to provide high-quality care to all patients living with cancer.”
In addition to his research work, Dr. Flowers is an advocate for diversity within the hematology community. He’s a founding member and former chair of the American Society of Hematology’s Committee on Diversity, Equity and Inclusion (formerly the Committee on Promoting Diversity), and he helped develop the society’s Minority Recruitment Initiative.
What’s next for Dr. Flowers? For one, he plans to continue working as a mentor; he received the ASH Mentor Award in honor of his service in 2022. “I am strongly committed to increasing the number of tenure-track investigators trained in clinical and translational cancer research and to promote their career development.”
And he looks forward to helping develop MD Anderson’s recently announced $2.5 billion hospital in Austin. “This will extend the exceptional care that we provide as the No. 1 cancer center in the United States,” he said. “It will also create new opportunities for research and collaboration with experts at UT Austin.”
When he’s not in clinic, Dr. Flowers embraces his lifelong love of speeding through life on his own two feet. He’s even inspired his children to share his passion. “I run most days of the week,” he said. “Running provides a great opportunity to think and process new research ideas, work through leadership challenges, and sometimes just to relax and let go of the day.”
Buyer beware
The invitation came to my house, addressed to “residential customer.” It was for my wife and me to attend a free dinner at a swanky local restaurant to learn about “revolutionary” treatments for memory loss. It was presented by a “wellness expert.”
Of course, I just had to check out the website.
The dinner was hosted by an internist pushing an unproven (except for the usual small noncontrolled studies) treatment. Although not stated, I’m sure when you call you’ll find out this is not covered by insurance; not Food and Drug Administration approved to treat, cure, diagnose, prevent any disease; your mileage may vary, etc.
The website was full of testimonials as to how well the treatments worked, primarily from people in their 20s-40s who are, realistically, unlikely to have a pathologically serious cause for memory problems. The site also mentions that you can use it to treat traumatic brain injury, ADHD, learning disorders, obsessive-compulsive disorder, PTSD, Parkinson’s disease, autism, dementia, and stroke, though it does clearly state that such use is not FDA approved.
Prices (I assume all cash pay) for the treatment weren’t listed. I guess you have to come to the “free” dinner for those, or submit an online form to the office.
I’m not going to say the advertised treatment doesn’t work. It might, for at least some of those things. A PubMed search tells me it’s under investigation for several of them.
But that doesn’t mean it works. It might, but a lot of things that look promising in early trials end up failing in the long run. So, at least to me, this is no different than people selling various over-the-counter supplements online with all kinds of extravagant claims and testimonials.
I also have to question a treatment targeting young people for memory loss. In neurology we see a lot of that, and know that true pathology is rare. Most of these patients have root issues with depression, or anxiety, or stress that are affecting their memory. That doesn’t make their memory issues any less real, but they shouldn’t be lumped in with neurodegenerative diseases. They need to be correctly diagnosed and treated for what they are.
Maybe it’s just me, but I often see this sort of thing as kind of sketchy – generating business for unproven treatments by selling fear – you need to do something NOW to keep from getting worse. And, of course there’s always the mysterious “they.” The treatments “they” offer don’t work. Why aren’t “they” telling you what really does?
Looking at the restaurant’s online menu, dinners are around $75 per person and the invitation says “seats are limited.” Doing some mental math gives you an idea how many diners need to come, what percentage of them need to sign up for the treatment, and how much it costs to recoup the investment.
Let the buyer beware.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The invitation came to my house, addressed to “residential customer.” It was for my wife and me to attend a free dinner at a swanky local restaurant to learn about “revolutionary” treatments for memory loss. It was presented by a “wellness expert.”
Of course, I just had to check out the website.
The dinner was hosted by an internist pushing an unproven (except for the usual small noncontrolled studies) treatment. Although not stated, I’m sure when you call you’ll find out this is not covered by insurance; not Food and Drug Administration approved to treat, cure, diagnose, prevent any disease; your mileage may vary, etc.
The website was full of testimonials as to how well the treatments worked, primarily from people in their 20s-40s who are, realistically, unlikely to have a pathologically serious cause for memory problems. The site also mentions that you can use it to treat traumatic brain injury, ADHD, learning disorders, obsessive-compulsive disorder, PTSD, Parkinson’s disease, autism, dementia, and stroke, though it does clearly state that such use is not FDA approved.
Prices (I assume all cash pay) for the treatment weren’t listed. I guess you have to come to the “free” dinner for those, or submit an online form to the office.
I’m not going to say the advertised treatment doesn’t work. It might, for at least some of those things. A PubMed search tells me it’s under investigation for several of them.
But that doesn’t mean it works. It might, but a lot of things that look promising in early trials end up failing in the long run. So, at least to me, this is no different than people selling various over-the-counter supplements online with all kinds of extravagant claims and testimonials.
I also have to question a treatment targeting young people for memory loss. In neurology we see a lot of that, and know that true pathology is rare. Most of these patients have root issues with depression, or anxiety, or stress that are affecting their memory. That doesn’t make their memory issues any less real, but they shouldn’t be lumped in with neurodegenerative diseases. They need to be correctly diagnosed and treated for what they are.
Maybe it’s just me, but I often see this sort of thing as kind of sketchy – generating business for unproven treatments by selling fear – you need to do something NOW to keep from getting worse. And, of course there’s always the mysterious “they.” The treatments “they” offer don’t work. Why aren’t “they” telling you what really does?
Looking at the restaurant’s online menu, dinners are around $75 per person and the invitation says “seats are limited.” Doing some mental math gives you an idea how many diners need to come, what percentage of them need to sign up for the treatment, and how much it costs to recoup the investment.
Let the buyer beware.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The invitation came to my house, addressed to “residential customer.” It was for my wife and me to attend a free dinner at a swanky local restaurant to learn about “revolutionary” treatments for memory loss. It was presented by a “wellness expert.”
Of course, I just had to check out the website.
The dinner was hosted by an internist pushing an unproven (except for the usual small noncontrolled studies) treatment. Although not stated, I’m sure when you call you’ll find out this is not covered by insurance; not Food and Drug Administration approved to treat, cure, diagnose, prevent any disease; your mileage may vary, etc.
The website was full of testimonials as to how well the treatments worked, primarily from people in their 20s-40s who are, realistically, unlikely to have a pathologically serious cause for memory problems. The site also mentions that you can use it to treat traumatic brain injury, ADHD, learning disorders, obsessive-compulsive disorder, PTSD, Parkinson’s disease, autism, dementia, and stroke, though it does clearly state that such use is not FDA approved.
Prices (I assume all cash pay) for the treatment weren’t listed. I guess you have to come to the “free” dinner for those, or submit an online form to the office.
I’m not going to say the advertised treatment doesn’t work. It might, for at least some of those things. A PubMed search tells me it’s under investigation for several of them.
But that doesn’t mean it works. It might, but a lot of things that look promising in early trials end up failing in the long run. So, at least to me, this is no different than people selling various over-the-counter supplements online with all kinds of extravagant claims and testimonials.
I also have to question a treatment targeting young people for memory loss. In neurology we see a lot of that, and know that true pathology is rare. Most of these patients have root issues with depression, or anxiety, or stress that are affecting their memory. That doesn’t make their memory issues any less real, but they shouldn’t be lumped in with neurodegenerative diseases. They need to be correctly diagnosed and treated for what they are.
Maybe it’s just me, but I often see this sort of thing as kind of sketchy – generating business for unproven treatments by selling fear – you need to do something NOW to keep from getting worse. And, of course there’s always the mysterious “they.” The treatments “they” offer don’t work. Why aren’t “they” telling you what really does?
Looking at the restaurant’s online menu, dinners are around $75 per person and the invitation says “seats are limited.” Doing some mental math gives you an idea how many diners need to come, what percentage of them need to sign up for the treatment, and how much it costs to recoup the investment.
Let the buyer beware.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Evaluating Pharmacists’ Time Collecting Self-Monitoring Blood Glucose Data
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
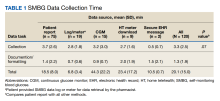
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
EMA validates marketing authorization application for delgocitinib cream
The which marks the beginning of the review process for the treatment by the EMA’s Committee for Medicinal Products for Human Use.
Delgocitinib is an investigational topical pan–Janus kinase inhibitor that inhibits activation of the JAK-STAT pathway.
The development follows results reported from two phase 3 clinical trials known as DELTA 1 and DELTA 2, which evaluated the safety and efficacy of delgocitinib cream applications twice per day compared with a vehicle cream in adults with mild to severe chronic hand eczema. Results of DELTA 1 were presented at the 2023 annual meeting of the American Academy of Dermatology. A multisite, open-label extension trial known as DELTA 3 is still in progress.
According to a press release from LEO Pharma, which is developing the product, the efficacy and safety of delgocitinib cream have not been evaluated by any regulatory authority. In 2020, the drug was granted fast-track designation by the Food and Drug Administration for the potential treatment of adults with moderate to severe chronic hand eczema. There are currently no treatment options available in the United States specifically approved for treating the condition.
The which marks the beginning of the review process for the treatment by the EMA’s Committee for Medicinal Products for Human Use.
Delgocitinib is an investigational topical pan–Janus kinase inhibitor that inhibits activation of the JAK-STAT pathway.
The development follows results reported from two phase 3 clinical trials known as DELTA 1 and DELTA 2, which evaluated the safety and efficacy of delgocitinib cream applications twice per day compared with a vehicle cream in adults with mild to severe chronic hand eczema. Results of DELTA 1 were presented at the 2023 annual meeting of the American Academy of Dermatology. A multisite, open-label extension trial known as DELTA 3 is still in progress.
According to a press release from LEO Pharma, which is developing the product, the efficacy and safety of delgocitinib cream have not been evaluated by any regulatory authority. In 2020, the drug was granted fast-track designation by the Food and Drug Administration for the potential treatment of adults with moderate to severe chronic hand eczema. There are currently no treatment options available in the United States specifically approved for treating the condition.
The which marks the beginning of the review process for the treatment by the EMA’s Committee for Medicinal Products for Human Use.
Delgocitinib is an investigational topical pan–Janus kinase inhibitor that inhibits activation of the JAK-STAT pathway.
The development follows results reported from two phase 3 clinical trials known as DELTA 1 and DELTA 2, which evaluated the safety and efficacy of delgocitinib cream applications twice per day compared with a vehicle cream in adults with mild to severe chronic hand eczema. Results of DELTA 1 were presented at the 2023 annual meeting of the American Academy of Dermatology. A multisite, open-label extension trial known as DELTA 3 is still in progress.
According to a press release from LEO Pharma, which is developing the product, the efficacy and safety of delgocitinib cream have not been evaluated by any regulatory authority. In 2020, the drug was granted fast-track designation by the Food and Drug Administration for the potential treatment of adults with moderate to severe chronic hand eczema. There are currently no treatment options available in the United States specifically approved for treating the condition.
Dementia diagnosis a good time to reduce polypharmacy
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
FROM JAMA INTERNAL MEDICINE
Big geographic access gaps for oncofertility services in U.S.
TOPLINE:
, a study shows.
METHODOLOGY:
- In this cross-sectional analysis, researchers identified 370 fertility centers in the United States (361 in the continental U.S.) that provide oncofertility services and that met criteria for an oncofertility center.
- Clinics were considered oncofertility centers if they offered oocyte cryopreservation, had performed at least one fertility preservation cycle in 2018, reported serving people without partners, and had an embryology laboratory that was accredited per Centers for Disease Control and Prevention guidelines.
- Researchers then quantified the number of young women potentially eligible for oncofertility services who lived farther than 2 hours from an oncofertility center.
- In a secondary analysis, the team assessed the association between geographic access and state fertility preservation insurance mandates.
TAKEAWAY:
- Overall, 3.63 million (5.7%) young women of reproductive age in the United States (aged 15-4) live more than 2 hours from an oncofertility center.
- The greatest gaps in access are in the Mountain West, West North Central, and Southwest regions; for instance, in Montana, North Dakota, and Wyoming fewer than half of the at-risk population has geographic access to an oncofertility center.
- Among the 11 states with fertility preservation insurance mandates, 98.5% of at-risk women have geographic access to an oncofertility center; in the 17 states without fertility preservation legislation, 79.6% of at-risk women have access to an oncofertility center.
IN PRACTICE:
Just over 3.6 million “reproductive-age female individuals lack geographic access to oncofertility services, especially in the Mountain West and West North Central regions,” the authors concluded. “Significant geographic disparities in access to fertility preservation in the U.S. require strategic expansion of care, especially given the growing demand for oncofertility services.”
SOURCE:
The study, led by Benjamin Peipert, MD, with Duke University, Durham, N.C., was published online in JAMA Oncology.
LIMITATIONS:
The authors relied on clinic data reported to the CDC, made assumptions about reasonable travel time, and may have underestimated access in some areas.
DISCLOSURES:
The study had no commercial funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a study shows.
METHODOLOGY:
- In this cross-sectional analysis, researchers identified 370 fertility centers in the United States (361 in the continental U.S.) that provide oncofertility services and that met criteria for an oncofertility center.
- Clinics were considered oncofertility centers if they offered oocyte cryopreservation, had performed at least one fertility preservation cycle in 2018, reported serving people without partners, and had an embryology laboratory that was accredited per Centers for Disease Control and Prevention guidelines.
- Researchers then quantified the number of young women potentially eligible for oncofertility services who lived farther than 2 hours from an oncofertility center.
- In a secondary analysis, the team assessed the association between geographic access and state fertility preservation insurance mandates.
TAKEAWAY:
- Overall, 3.63 million (5.7%) young women of reproductive age in the United States (aged 15-4) live more than 2 hours from an oncofertility center.
- The greatest gaps in access are in the Mountain West, West North Central, and Southwest regions; for instance, in Montana, North Dakota, and Wyoming fewer than half of the at-risk population has geographic access to an oncofertility center.
- Among the 11 states with fertility preservation insurance mandates, 98.5% of at-risk women have geographic access to an oncofertility center; in the 17 states without fertility preservation legislation, 79.6% of at-risk women have access to an oncofertility center.
IN PRACTICE:
Just over 3.6 million “reproductive-age female individuals lack geographic access to oncofertility services, especially in the Mountain West and West North Central regions,” the authors concluded. “Significant geographic disparities in access to fertility preservation in the U.S. require strategic expansion of care, especially given the growing demand for oncofertility services.”
SOURCE:
The study, led by Benjamin Peipert, MD, with Duke University, Durham, N.C., was published online in JAMA Oncology.
LIMITATIONS:
The authors relied on clinic data reported to the CDC, made assumptions about reasonable travel time, and may have underestimated access in some areas.
DISCLOSURES:
The study had no commercial funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a study shows.
METHODOLOGY:
- In this cross-sectional analysis, researchers identified 370 fertility centers in the United States (361 in the continental U.S.) that provide oncofertility services and that met criteria for an oncofertility center.
- Clinics were considered oncofertility centers if they offered oocyte cryopreservation, had performed at least one fertility preservation cycle in 2018, reported serving people without partners, and had an embryology laboratory that was accredited per Centers for Disease Control and Prevention guidelines.
- Researchers then quantified the number of young women potentially eligible for oncofertility services who lived farther than 2 hours from an oncofertility center.
- In a secondary analysis, the team assessed the association between geographic access and state fertility preservation insurance mandates.
TAKEAWAY:
- Overall, 3.63 million (5.7%) young women of reproductive age in the United States (aged 15-4) live more than 2 hours from an oncofertility center.
- The greatest gaps in access are in the Mountain West, West North Central, and Southwest regions; for instance, in Montana, North Dakota, and Wyoming fewer than half of the at-risk population has geographic access to an oncofertility center.
- Among the 11 states with fertility preservation insurance mandates, 98.5% of at-risk women have geographic access to an oncofertility center; in the 17 states without fertility preservation legislation, 79.6% of at-risk women have access to an oncofertility center.
IN PRACTICE:
Just over 3.6 million “reproductive-age female individuals lack geographic access to oncofertility services, especially in the Mountain West and West North Central regions,” the authors concluded. “Significant geographic disparities in access to fertility preservation in the U.S. require strategic expansion of care, especially given the growing demand for oncofertility services.”
SOURCE:
The study, led by Benjamin Peipert, MD, with Duke University, Durham, N.C., was published online in JAMA Oncology.
LIMITATIONS:
The authors relied on clinic data reported to the CDC, made assumptions about reasonable travel time, and may have underestimated access in some areas.
DISCLOSURES:
The study had no commercial funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Long COVID lawsuits coming, but not likely to succeed, experts predict
By now, concerns about COVID-related lawsuits have faded into the rear view mirror for most physicians.
While some say it’s doubtful the claims will succeed, the lawsuits could still create legal headaches for doctors in the form of time and money.
Long COVID claims are defined as complaints that allege that a diagnosis of long COVID was missed or delayed and that caused harm or injury. Lawsuits may also include claims in which patients allege that they were misdiagnosed as having long COVID when they were really suffering from another condition.
So far, a handful of long COVID claims have come down the pipeline, said Peter A. Kolbert, JD, senior vice president of claims and litigation services for Healthcare Risk Advisors, part of TDC Group.
“This is an area that is emerging as we speak,” Mr. Kolbert said. “We are starting to see these claims trickle in.”
In a recent case, for example, a patient sued her primary care physician for negligence, alleging her original SARS-CoV-2 infection was mismanaged and that this led to permanent neuropathy from long COVID. Had the patient been treated appropriately, the patient contends, she would not have developed long COVID or the resulting neuropathy, said Mr. Kolbert. An outcome in the case has not yet been reached, added Mr. Kolbert, who heard about the claim from a colleague.
The increase in the number of lawsuits raises concerns about how courts and juries might decide long COVID claims when so much about the condition is still unknown and best treatment practices are still developing. Research shows that long COVID occurs in at least 10% of cases of SARS-CoV-2 infection, and more than 200 symptoms have been identified. A Kaiser Family Foundation study found that 15% of the U.S. population believe they have experienced the symptoms of long COVID at some point, and 6% of people believe they currently have long COVID.
The risk of long COVID lawsuits underscores the importance of physicians taking proactive steps to protect themselves from liability when treating patients who might have the condition, say legal experts.
“There are legal standards that say new, unestablished scientific principles shouldn’t be first tested by a jury, they should be recognized and established within their [professional] area,” Mr. Kolbert said. “While we are seeing lawsuits related to long COVID, I think it is truly putting the cart before the horse, because there needs to be societal recognition that we’re still learning how to define and treat long COVID.”
What are patients alleging?
In the few long COVID claims that have arisen, some complaints have alleged delay in the recognition and treatment of long COVID, according to Mr. Kolbert. There have also been claims that physicians failed to refer a patient with long COVID to a specialist in a timely way and that this results in the patient’s experiencing chronic fatigue or a neuropathy.
Fatigue is one of the most common symptoms associated with long COVID, according to recent studies. Other symptoms include postexertional malaise, brain fog, and gastrointestinal problems.
Another rising legal theme is failure to adequately communicate with patients about what long COVID is and what it entails.
Whether plaintiffs who bring long COVID claims will be successful in court remains a question.
Andrew D. DeSimone, JD, a Lexington, Ky.–based medical malpractice defense attorney, said he has not seen any claims involving long COVID. He added that a long COVID claim would be challenging to prove, considering the standard of care for treating the condition is still evolving. Plaintiffs in a medical malpractice action must prove that physicians owed a duty of care to the patient, that the doctor breached that duty by failing to conform to the standard of care, and that the breach caused an injury that harmed the patient.
Mr. DeSimone also doubts whether juries would be very sympathetic to such plaintiffs.
“There’s a lot of fatigue around COVID still,” he said. “I don’t know if a jury would buy into someone claiming long COVID. I think the claim would have a hard time gaining traction. Not that it’s impossible.”
Another unanswered question is whether legal protections enacted by states during the pandemic might apply to long COVID claims.
Shortly after the pandemic started, most states enacted laws or executive orders that shielded physicians from liability claims relating to the prevention and treatment of COVID-19, unless gross negligence or willful misconduct is proved. The U.S. Department of Health and Human Services published a declaration under the Public Readiness and Emergency Preparedness Act (PREP Act) that provided liability immunity to health care professionals for any activity related to medical countermeasures against COVID-19.
Some of these state immunities have since expired. Other states have extended their legal protections for short periods. In Indiana, for example, physicians and businesses are protected until Dec. 31, 2024, from civil tort actions that allege damages arising from COVID-19.
It’s possible that in long COVID lawsuits, physicians would be protected by the immunities unless the cases come after the protections expire, said J. Richard Moore, a medical liability defense attorney based in Indianapolis.
“I could foresee long COVID claims that don’t accrue until after December 2024, meaning it only becomes clear that a patient is struggling with long COVID–related symptoms after that date,” he said. “That could result in COVID claims that do not fall under the immunities.”
Mr. Moore said that if long COVID claims become truly problematic, the legislature could extend the immunities.
Other states, such as Washington, have statutes in place that increase the burden of proof for plaintiffs in cases in which care is affected by COVID and/or the treating of COVID. Elizabeth A. Leedom, a Seattle-based medical liability defense attorney, said the law would likely encompass long COVID claims if the care and treatment at issue occurred during the COVID state of emergency.
Compliance with current treatment guidelines is likely to be a good defense against any claim of delay/failure to diagnose COVID, including long COVID, she said.
Mr. Kolbert, however, doubts that the state immunities would protect against the claims.
“Courts are enforcing qualified immunities as to [traditional] COVID claims. However, I suspect that long COVID claims will fall into a category of traditional medical malpractice claim, such as delay in or failure to diagnose,” he said. In such cases, physicians “may not be able to take advantage of state-qualified immunities. Of course, this will depend upon the language of each state’s qualified immunity provisions.”
As for the statute of limitations, the clock generally starts running either when the alleged negligent conduct occurred or when the patient knew or, in the exercise of ordinary diligence, should have known, that they had been harmed by the alleged negligence, Mr. Moore said. Statutes of limitations are state specific, but the majority of states mandate a 2- to 3-year limit between the injury and the filing of a claim.
So, while the statute of limitations may be soon expiring for alleged harm that occurred during the pandemic, for patients newly diagnosed with long COVID or who have just discovered associated injuries, the clock may have just started ticking.
How to protect yourself against suits
Avoiding liability associated with long COVID involves the traditional legal guidance physicians are used to hearing, but with an added factor, Mr. Kolbert said.
There always needs to be communication with patients regarding the disease process, but in this area, there needs to be strong communication as to whether patients have had COVID in the past and what symptoms they are experiencing, he said. Physicians should ensure that patients know that long COVID may present in a variety of ways and that there is no definitive test for long COVID.
Physicians should document when the patient has been instructed to follow up and should take necessary steps to ensure the patient returns for follow-up care, he added.
On the opposite side of the spectrum is making sure not to assume a condition or symptom is the result of long COVID, he said. Care should be taken not to diagnose long COVID without excluding traditional causes.
“Ensure that patients know that COVID is not over, per se, and that science supports vaccination,” Mr. Kolbert said. “The best defense here is a strong communicative offense, engaging with the patient and thoughtfully charting about this.”
A version of this article first appeared on Medscape.com.
By now, concerns about COVID-related lawsuits have faded into the rear view mirror for most physicians.
While some say it’s doubtful the claims will succeed, the lawsuits could still create legal headaches for doctors in the form of time and money.
Long COVID claims are defined as complaints that allege that a diagnosis of long COVID was missed or delayed and that caused harm or injury. Lawsuits may also include claims in which patients allege that they were misdiagnosed as having long COVID when they were really suffering from another condition.
So far, a handful of long COVID claims have come down the pipeline, said Peter A. Kolbert, JD, senior vice president of claims and litigation services for Healthcare Risk Advisors, part of TDC Group.
“This is an area that is emerging as we speak,” Mr. Kolbert said. “We are starting to see these claims trickle in.”
In a recent case, for example, a patient sued her primary care physician for negligence, alleging her original SARS-CoV-2 infection was mismanaged and that this led to permanent neuropathy from long COVID. Had the patient been treated appropriately, the patient contends, she would not have developed long COVID or the resulting neuropathy, said Mr. Kolbert. An outcome in the case has not yet been reached, added Mr. Kolbert, who heard about the claim from a colleague.
The increase in the number of lawsuits raises concerns about how courts and juries might decide long COVID claims when so much about the condition is still unknown and best treatment practices are still developing. Research shows that long COVID occurs in at least 10% of cases of SARS-CoV-2 infection, and more than 200 symptoms have been identified. A Kaiser Family Foundation study found that 15% of the U.S. population believe they have experienced the symptoms of long COVID at some point, and 6% of people believe they currently have long COVID.
The risk of long COVID lawsuits underscores the importance of physicians taking proactive steps to protect themselves from liability when treating patients who might have the condition, say legal experts.
“There are legal standards that say new, unestablished scientific principles shouldn’t be first tested by a jury, they should be recognized and established within their [professional] area,” Mr. Kolbert said. “While we are seeing lawsuits related to long COVID, I think it is truly putting the cart before the horse, because there needs to be societal recognition that we’re still learning how to define and treat long COVID.”
What are patients alleging?
In the few long COVID claims that have arisen, some complaints have alleged delay in the recognition and treatment of long COVID, according to Mr. Kolbert. There have also been claims that physicians failed to refer a patient with long COVID to a specialist in a timely way and that this results in the patient’s experiencing chronic fatigue or a neuropathy.
Fatigue is one of the most common symptoms associated with long COVID, according to recent studies. Other symptoms include postexertional malaise, brain fog, and gastrointestinal problems.
Another rising legal theme is failure to adequately communicate with patients about what long COVID is and what it entails.
Whether plaintiffs who bring long COVID claims will be successful in court remains a question.
Andrew D. DeSimone, JD, a Lexington, Ky.–based medical malpractice defense attorney, said he has not seen any claims involving long COVID. He added that a long COVID claim would be challenging to prove, considering the standard of care for treating the condition is still evolving. Plaintiffs in a medical malpractice action must prove that physicians owed a duty of care to the patient, that the doctor breached that duty by failing to conform to the standard of care, and that the breach caused an injury that harmed the patient.
Mr. DeSimone also doubts whether juries would be very sympathetic to such plaintiffs.
“There’s a lot of fatigue around COVID still,” he said. “I don’t know if a jury would buy into someone claiming long COVID. I think the claim would have a hard time gaining traction. Not that it’s impossible.”
Another unanswered question is whether legal protections enacted by states during the pandemic might apply to long COVID claims.
Shortly after the pandemic started, most states enacted laws or executive orders that shielded physicians from liability claims relating to the prevention and treatment of COVID-19, unless gross negligence or willful misconduct is proved. The U.S. Department of Health and Human Services published a declaration under the Public Readiness and Emergency Preparedness Act (PREP Act) that provided liability immunity to health care professionals for any activity related to medical countermeasures against COVID-19.
Some of these state immunities have since expired. Other states have extended their legal protections for short periods. In Indiana, for example, physicians and businesses are protected until Dec. 31, 2024, from civil tort actions that allege damages arising from COVID-19.
It’s possible that in long COVID lawsuits, physicians would be protected by the immunities unless the cases come after the protections expire, said J. Richard Moore, a medical liability defense attorney based in Indianapolis.
“I could foresee long COVID claims that don’t accrue until after December 2024, meaning it only becomes clear that a patient is struggling with long COVID–related symptoms after that date,” he said. “That could result in COVID claims that do not fall under the immunities.”
Mr. Moore said that if long COVID claims become truly problematic, the legislature could extend the immunities.
Other states, such as Washington, have statutes in place that increase the burden of proof for plaintiffs in cases in which care is affected by COVID and/or the treating of COVID. Elizabeth A. Leedom, a Seattle-based medical liability defense attorney, said the law would likely encompass long COVID claims if the care and treatment at issue occurred during the COVID state of emergency.
Compliance with current treatment guidelines is likely to be a good defense against any claim of delay/failure to diagnose COVID, including long COVID, she said.
Mr. Kolbert, however, doubts that the state immunities would protect against the claims.
“Courts are enforcing qualified immunities as to [traditional] COVID claims. However, I suspect that long COVID claims will fall into a category of traditional medical malpractice claim, such as delay in or failure to diagnose,” he said. In such cases, physicians “may not be able to take advantage of state-qualified immunities. Of course, this will depend upon the language of each state’s qualified immunity provisions.”
As for the statute of limitations, the clock generally starts running either when the alleged negligent conduct occurred or when the patient knew or, in the exercise of ordinary diligence, should have known, that they had been harmed by the alleged negligence, Mr. Moore said. Statutes of limitations are state specific, but the majority of states mandate a 2- to 3-year limit between the injury and the filing of a claim.
So, while the statute of limitations may be soon expiring for alleged harm that occurred during the pandemic, for patients newly diagnosed with long COVID or who have just discovered associated injuries, the clock may have just started ticking.
How to protect yourself against suits
Avoiding liability associated with long COVID involves the traditional legal guidance physicians are used to hearing, but with an added factor, Mr. Kolbert said.
There always needs to be communication with patients regarding the disease process, but in this area, there needs to be strong communication as to whether patients have had COVID in the past and what symptoms they are experiencing, he said. Physicians should ensure that patients know that long COVID may present in a variety of ways and that there is no definitive test for long COVID.
Physicians should document when the patient has been instructed to follow up and should take necessary steps to ensure the patient returns for follow-up care, he added.
On the opposite side of the spectrum is making sure not to assume a condition or symptom is the result of long COVID, he said. Care should be taken not to diagnose long COVID without excluding traditional causes.
“Ensure that patients know that COVID is not over, per se, and that science supports vaccination,” Mr. Kolbert said. “The best defense here is a strong communicative offense, engaging with the patient and thoughtfully charting about this.”
A version of this article first appeared on Medscape.com.
By now, concerns about COVID-related lawsuits have faded into the rear view mirror for most physicians.
While some say it’s doubtful the claims will succeed, the lawsuits could still create legal headaches for doctors in the form of time and money.
Long COVID claims are defined as complaints that allege that a diagnosis of long COVID was missed or delayed and that caused harm or injury. Lawsuits may also include claims in which patients allege that they were misdiagnosed as having long COVID when they were really suffering from another condition.
So far, a handful of long COVID claims have come down the pipeline, said Peter A. Kolbert, JD, senior vice president of claims and litigation services for Healthcare Risk Advisors, part of TDC Group.
“This is an area that is emerging as we speak,” Mr. Kolbert said. “We are starting to see these claims trickle in.”
In a recent case, for example, a patient sued her primary care physician for negligence, alleging her original SARS-CoV-2 infection was mismanaged and that this led to permanent neuropathy from long COVID. Had the patient been treated appropriately, the patient contends, she would not have developed long COVID or the resulting neuropathy, said Mr. Kolbert. An outcome in the case has not yet been reached, added Mr. Kolbert, who heard about the claim from a colleague.
The increase in the number of lawsuits raises concerns about how courts and juries might decide long COVID claims when so much about the condition is still unknown and best treatment practices are still developing. Research shows that long COVID occurs in at least 10% of cases of SARS-CoV-2 infection, and more than 200 symptoms have been identified. A Kaiser Family Foundation study found that 15% of the U.S. population believe they have experienced the symptoms of long COVID at some point, and 6% of people believe they currently have long COVID.
The risk of long COVID lawsuits underscores the importance of physicians taking proactive steps to protect themselves from liability when treating patients who might have the condition, say legal experts.
“There are legal standards that say new, unestablished scientific principles shouldn’t be first tested by a jury, they should be recognized and established within their [professional] area,” Mr. Kolbert said. “While we are seeing lawsuits related to long COVID, I think it is truly putting the cart before the horse, because there needs to be societal recognition that we’re still learning how to define and treat long COVID.”
What are patients alleging?
In the few long COVID claims that have arisen, some complaints have alleged delay in the recognition and treatment of long COVID, according to Mr. Kolbert. There have also been claims that physicians failed to refer a patient with long COVID to a specialist in a timely way and that this results in the patient’s experiencing chronic fatigue or a neuropathy.
Fatigue is one of the most common symptoms associated with long COVID, according to recent studies. Other symptoms include postexertional malaise, brain fog, and gastrointestinal problems.
Another rising legal theme is failure to adequately communicate with patients about what long COVID is and what it entails.
Whether plaintiffs who bring long COVID claims will be successful in court remains a question.
Andrew D. DeSimone, JD, a Lexington, Ky.–based medical malpractice defense attorney, said he has not seen any claims involving long COVID. He added that a long COVID claim would be challenging to prove, considering the standard of care for treating the condition is still evolving. Plaintiffs in a medical malpractice action must prove that physicians owed a duty of care to the patient, that the doctor breached that duty by failing to conform to the standard of care, and that the breach caused an injury that harmed the patient.
Mr. DeSimone also doubts whether juries would be very sympathetic to such plaintiffs.
“There’s a lot of fatigue around COVID still,” he said. “I don’t know if a jury would buy into someone claiming long COVID. I think the claim would have a hard time gaining traction. Not that it’s impossible.”
Another unanswered question is whether legal protections enacted by states during the pandemic might apply to long COVID claims.
Shortly after the pandemic started, most states enacted laws or executive orders that shielded physicians from liability claims relating to the prevention and treatment of COVID-19, unless gross negligence or willful misconduct is proved. The U.S. Department of Health and Human Services published a declaration under the Public Readiness and Emergency Preparedness Act (PREP Act) that provided liability immunity to health care professionals for any activity related to medical countermeasures against COVID-19.
Some of these state immunities have since expired. Other states have extended their legal protections for short periods. In Indiana, for example, physicians and businesses are protected until Dec. 31, 2024, from civil tort actions that allege damages arising from COVID-19.
It’s possible that in long COVID lawsuits, physicians would be protected by the immunities unless the cases come after the protections expire, said J. Richard Moore, a medical liability defense attorney based in Indianapolis.
“I could foresee long COVID claims that don’t accrue until after December 2024, meaning it only becomes clear that a patient is struggling with long COVID–related symptoms after that date,” he said. “That could result in COVID claims that do not fall under the immunities.”
Mr. Moore said that if long COVID claims become truly problematic, the legislature could extend the immunities.
Other states, such as Washington, have statutes in place that increase the burden of proof for plaintiffs in cases in which care is affected by COVID and/or the treating of COVID. Elizabeth A. Leedom, a Seattle-based medical liability defense attorney, said the law would likely encompass long COVID claims if the care and treatment at issue occurred during the COVID state of emergency.
Compliance with current treatment guidelines is likely to be a good defense against any claim of delay/failure to diagnose COVID, including long COVID, she said.
Mr. Kolbert, however, doubts that the state immunities would protect against the claims.
“Courts are enforcing qualified immunities as to [traditional] COVID claims. However, I suspect that long COVID claims will fall into a category of traditional medical malpractice claim, such as delay in or failure to diagnose,” he said. In such cases, physicians “may not be able to take advantage of state-qualified immunities. Of course, this will depend upon the language of each state’s qualified immunity provisions.”
As for the statute of limitations, the clock generally starts running either when the alleged negligent conduct occurred or when the patient knew or, in the exercise of ordinary diligence, should have known, that they had been harmed by the alleged negligence, Mr. Moore said. Statutes of limitations are state specific, but the majority of states mandate a 2- to 3-year limit between the injury and the filing of a claim.
So, while the statute of limitations may be soon expiring for alleged harm that occurred during the pandemic, for patients newly diagnosed with long COVID or who have just discovered associated injuries, the clock may have just started ticking.
How to protect yourself against suits
Avoiding liability associated with long COVID involves the traditional legal guidance physicians are used to hearing, but with an added factor, Mr. Kolbert said.
There always needs to be communication with patients regarding the disease process, but in this area, there needs to be strong communication as to whether patients have had COVID in the past and what symptoms they are experiencing, he said. Physicians should ensure that patients know that long COVID may present in a variety of ways and that there is no definitive test for long COVID.
Physicians should document when the patient has been instructed to follow up and should take necessary steps to ensure the patient returns for follow-up care, he added.
On the opposite side of the spectrum is making sure not to assume a condition or symptom is the result of long COVID, he said. Care should be taken not to diagnose long COVID without excluding traditional causes.
“Ensure that patients know that COVID is not over, per se, and that science supports vaccination,” Mr. Kolbert said. “The best defense here is a strong communicative offense, engaging with the patient and thoughtfully charting about this.”
A version of this article first appeared on Medscape.com.
Lobbying allowed insurers to charge physicians fees to receive payments online: Report
, according to a new investigation by the nonprofit news organization ProPublica.
The Affordable Care Act requires that health plans give providers the option of being paid electronically to improve efficiency and save money. In 2017, the Centers for Medicare & Medicaid Services issued guidance that prohibited insurers and their payment processing vendors from “engaging in unfair business practices that do not support an efficient healthcare system,” according to a recent Medical Group Management Association position paper.
But that guidance, which appeared to forbid requiring fees to receive payments online, disappeared from the CMS site 6 months later.
According to ProPublica’s reporting, the change was the result of a quiet insurance industry lobbying campaign led by Matthew Albright, a former CMS employee who left government service to work for Zelis, a payment processing company co-owned by private equity giant Bain Capital.
The details of the lobbying effort were discovered by Alex Shteynshlyuger, a New York urologist, who through public records requests received the email correspondence between Mr. Albright and CMS and shared that material with ProPublica.
Mr. Albright had been able to influence CMS policy to protect what ProPublica called a “crucial revenue stream” for payment processors. The fee notice was removed just 3 days after Mr. Albright requested the change, ProPublica found.
When CMS resisted further changes, including eliminating guidance forbidding insurers and payment processors from charging excess fees for online payments, Mr. Albright brought in a law firm. The threat of a lawsuit by deep-pocketed Zelis was enough to bring CMS in line, ProPublica reported. Today, these fees can cost larger medical practices more than $1 million a year, according to the MGMA report.
“It took less than a decade for a new industry of middlemen, owned by private equity funds and giant conglomerates like UnitedHealth Group, to cash in,” writes Cezary Podkul, the author of the ProPublica report.
Predatory practices
It might seem that avoiding the fees would be as simple as requesting to be paid by check. However, a 2021 poll by the MGMA found that 57% of doctors were being charged these fees when they hadn’t agreed to them. According to the ProPublica report, physicians who have requested to be paid by check often find themselves being bounced back to electronic fund transfer (EFT) payments, where they are again charged fees.
In October 2021, more than 90 physician organizations, including the American Medical Association and the MGMA, signed a letter calling on the Biden administration to reinstate guidance to protect physicians’ right to receive EFT payments without paying fees. The letter describes the practice as “outrageous” and analogous to “an employee being required to enroll in a program that would deduct a percentage of each paycheck to receive direct deposit payments from an employer.”
So far, however, the situation remains unchanged. The language on the CMS site has changed, though. In 2022, the guidelines were adjusted to clarify that EFT fees are allowed.
A version of this article first appeared on Medscape.com.
, according to a new investigation by the nonprofit news organization ProPublica.
The Affordable Care Act requires that health plans give providers the option of being paid electronically to improve efficiency and save money. In 2017, the Centers for Medicare & Medicaid Services issued guidance that prohibited insurers and their payment processing vendors from “engaging in unfair business practices that do not support an efficient healthcare system,” according to a recent Medical Group Management Association position paper.
But that guidance, which appeared to forbid requiring fees to receive payments online, disappeared from the CMS site 6 months later.
According to ProPublica’s reporting, the change was the result of a quiet insurance industry lobbying campaign led by Matthew Albright, a former CMS employee who left government service to work for Zelis, a payment processing company co-owned by private equity giant Bain Capital.
The details of the lobbying effort were discovered by Alex Shteynshlyuger, a New York urologist, who through public records requests received the email correspondence between Mr. Albright and CMS and shared that material with ProPublica.
Mr. Albright had been able to influence CMS policy to protect what ProPublica called a “crucial revenue stream” for payment processors. The fee notice was removed just 3 days after Mr. Albright requested the change, ProPublica found.
When CMS resisted further changes, including eliminating guidance forbidding insurers and payment processors from charging excess fees for online payments, Mr. Albright brought in a law firm. The threat of a lawsuit by deep-pocketed Zelis was enough to bring CMS in line, ProPublica reported. Today, these fees can cost larger medical practices more than $1 million a year, according to the MGMA report.
“It took less than a decade for a new industry of middlemen, owned by private equity funds and giant conglomerates like UnitedHealth Group, to cash in,” writes Cezary Podkul, the author of the ProPublica report.
Predatory practices
It might seem that avoiding the fees would be as simple as requesting to be paid by check. However, a 2021 poll by the MGMA found that 57% of doctors were being charged these fees when they hadn’t agreed to them. According to the ProPublica report, physicians who have requested to be paid by check often find themselves being bounced back to electronic fund transfer (EFT) payments, where they are again charged fees.
In October 2021, more than 90 physician organizations, including the American Medical Association and the MGMA, signed a letter calling on the Biden administration to reinstate guidance to protect physicians’ right to receive EFT payments without paying fees. The letter describes the practice as “outrageous” and analogous to “an employee being required to enroll in a program that would deduct a percentage of each paycheck to receive direct deposit payments from an employer.”
So far, however, the situation remains unchanged. The language on the CMS site has changed, though. In 2022, the guidelines were adjusted to clarify that EFT fees are allowed.
A version of this article first appeared on Medscape.com.
, according to a new investigation by the nonprofit news organization ProPublica.
The Affordable Care Act requires that health plans give providers the option of being paid electronically to improve efficiency and save money. In 2017, the Centers for Medicare & Medicaid Services issued guidance that prohibited insurers and their payment processing vendors from “engaging in unfair business practices that do not support an efficient healthcare system,” according to a recent Medical Group Management Association position paper.
But that guidance, which appeared to forbid requiring fees to receive payments online, disappeared from the CMS site 6 months later.
According to ProPublica’s reporting, the change was the result of a quiet insurance industry lobbying campaign led by Matthew Albright, a former CMS employee who left government service to work for Zelis, a payment processing company co-owned by private equity giant Bain Capital.
The details of the lobbying effort were discovered by Alex Shteynshlyuger, a New York urologist, who through public records requests received the email correspondence between Mr. Albright and CMS and shared that material with ProPublica.
Mr. Albright had been able to influence CMS policy to protect what ProPublica called a “crucial revenue stream” for payment processors. The fee notice was removed just 3 days after Mr. Albright requested the change, ProPublica found.
When CMS resisted further changes, including eliminating guidance forbidding insurers and payment processors from charging excess fees for online payments, Mr. Albright brought in a law firm. The threat of a lawsuit by deep-pocketed Zelis was enough to bring CMS in line, ProPublica reported. Today, these fees can cost larger medical practices more than $1 million a year, according to the MGMA report.
“It took less than a decade for a new industry of middlemen, owned by private equity funds and giant conglomerates like UnitedHealth Group, to cash in,” writes Cezary Podkul, the author of the ProPublica report.
Predatory practices
It might seem that avoiding the fees would be as simple as requesting to be paid by check. However, a 2021 poll by the MGMA found that 57% of doctors were being charged these fees when they hadn’t agreed to them. According to the ProPublica report, physicians who have requested to be paid by check often find themselves being bounced back to electronic fund transfer (EFT) payments, where they are again charged fees.
In October 2021, more than 90 physician organizations, including the American Medical Association and the MGMA, signed a letter calling on the Biden administration to reinstate guidance to protect physicians’ right to receive EFT payments without paying fees. The letter describes the practice as “outrageous” and analogous to “an employee being required to enroll in a program that would deduct a percentage of each paycheck to receive direct deposit payments from an employer.”
So far, however, the situation remains unchanged. The language on the CMS site has changed, though. In 2022, the guidelines were adjusted to clarify that EFT fees are allowed.
A version of this article first appeared on Medscape.com.
Structural changes may separate axial psoriatic arthritis from axial spondyloarthritis
Approximately 20% of adults with axial psoriatic arthritis (PsA) show active or structural spinal changes without changes in the sacroiliac joint, based on imaging data from 106 individuals.
Axial PsA has been historically grouped with axial spondyloarthritis (axSpA), but it has received more attention in recent years as a condition potentially distinct from axSpA, Henriette Käding, an MD and PhD student in the department of gastroenterology, infectiology, and rheumatology at Charité-Universitätsmedizin Berlin, said in her research presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). She added that the debate persists as to whether these conditions are on the same spectrum or should be separated.
Data from previous studies suggest differences in genetic, clinical, radiographic, and prognostic characteristics between axial PsA and axSpA that may affect patients’ response to available treatments. However, there are relatively little data available on distinguishing imaging and clinical features, and there’s a lack of classification criteria for axial PsA, Ms. Käding said.
Ms. Käding and colleagues prospectively collected data from 106 patients with axial PsA between August 2019 and June 2023 and presented the baseline data of this longitudinal project at the GRAPPA annual meeting in Dublin. At baseline, the researchers conducted clinical assessments of the participants, along with blood sampling, stool samples, and imaging protocols that included MRI of the whole spine and sacroiliac joint (SIJ).
The mean age of the included patients was 44.5 years; 55.7% were female. Inflammatory back pain was present in most of the patients at baseline (78.4%), and 48.1% were positive for HLA-B27, a genetic risk factor for both axSpA and axial PsA. Approximately one-third of the patients had elevated C-reactive protein (> 5 mg/L). In the baseline MRI scans, active inflammatory changes in the sacroiliac joints (SIJ) were seen in 51.9% of the patients and structural changes in 72.1%. MRI spine scans showed active changes in 58.7% of the patients. Notably, active and/or structural changes of the spine without changes in the SIJ appeared in 20% of the patients, Ms. Käding said.
With regard to existing classification criteria, the researchers observed that 92% of the patients met the CASPAR (Classification Criteria for Psoriatic Arthritis) criteria for PsA, 73% met the ASAS (Assessment of Spondyloarthritis International Society) criteria, while 66% of patients met both ASAS and CASPAR criteria.
The study will be the first to include longitudinal MRI scans of the whole spine and SIJ in addition to conventional radiographs, Ms. Käding said.
Better characterization should improve treatment
“Axial involvement in PsA might, on one hand, go unnoticed, but on the other hand, it could also be misdiagnosed in patients with degenerative spinal disease,” Denis Poddubnyy, MD, one of the study coauthors, also of Charité-Universitätsmedizin Berlin, said in an interview.
“By comprehending the unique characteristics, progression, and treatment responses within the axial domain, rheumatologists can customize interventions and therapies to effectively manage the psoriatic disease,” Dr. Poddubnyy said.
“One of the most significant findings [of the current study] is the relatively high frequency of spinal involvement without sacroiliac joint” involvement, Fabian Proft, MD, of Charité-Universitätsmedizin Berlin and senior author of the study, said in an interview. “This finding holds importance as, in primary axial SpA, the disease typically originates in the sacroiliac joints. In contrast, in PsA, the scenario differs, which has implications for the diagnostic approach in clinical practice.”
“In individuals with PsA, spinal involvement can occur independently of sacroiliac joint [involvement]. As a result, imaging studies conducted on patients suspected of having axial PsA should encompass not only the sacroiliac joints but also the spine,” Dr. Poddubnyy explained. “It is important to note, however, that imaging findings such as bony spurs and bone marrow edema might be caused by degeneration or mechanical issues and, therefore, need to be interpreted with caution within the clinical context.”
The study was supported in part by an unrestricted research grant from Novartis. Dr. Poddubnyy and Dr. Proft disclosed receiving research grants and consultancy payments from Novartis and serving on speaker bureaus for the company.
Approximately 20% of adults with axial psoriatic arthritis (PsA) show active or structural spinal changes without changes in the sacroiliac joint, based on imaging data from 106 individuals.
Axial PsA has been historically grouped with axial spondyloarthritis (axSpA), but it has received more attention in recent years as a condition potentially distinct from axSpA, Henriette Käding, an MD and PhD student in the department of gastroenterology, infectiology, and rheumatology at Charité-Universitätsmedizin Berlin, said in her research presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). She added that the debate persists as to whether these conditions are on the same spectrum or should be separated.
Data from previous studies suggest differences in genetic, clinical, radiographic, and prognostic characteristics between axial PsA and axSpA that may affect patients’ response to available treatments. However, there are relatively little data available on distinguishing imaging and clinical features, and there’s a lack of classification criteria for axial PsA, Ms. Käding said.
Ms. Käding and colleagues prospectively collected data from 106 patients with axial PsA between August 2019 and June 2023 and presented the baseline data of this longitudinal project at the GRAPPA annual meeting in Dublin. At baseline, the researchers conducted clinical assessments of the participants, along with blood sampling, stool samples, and imaging protocols that included MRI of the whole spine and sacroiliac joint (SIJ).
The mean age of the included patients was 44.5 years; 55.7% were female. Inflammatory back pain was present in most of the patients at baseline (78.4%), and 48.1% were positive for HLA-B27, a genetic risk factor for both axSpA and axial PsA. Approximately one-third of the patients had elevated C-reactive protein (> 5 mg/L). In the baseline MRI scans, active inflammatory changes in the sacroiliac joints (SIJ) were seen in 51.9% of the patients and structural changes in 72.1%. MRI spine scans showed active changes in 58.7% of the patients. Notably, active and/or structural changes of the spine without changes in the SIJ appeared in 20% of the patients, Ms. Käding said.
With regard to existing classification criteria, the researchers observed that 92% of the patients met the CASPAR (Classification Criteria for Psoriatic Arthritis) criteria for PsA, 73% met the ASAS (Assessment of Spondyloarthritis International Society) criteria, while 66% of patients met both ASAS and CASPAR criteria.
The study will be the first to include longitudinal MRI scans of the whole spine and SIJ in addition to conventional radiographs, Ms. Käding said.
Better characterization should improve treatment
“Axial involvement in PsA might, on one hand, go unnoticed, but on the other hand, it could also be misdiagnosed in patients with degenerative spinal disease,” Denis Poddubnyy, MD, one of the study coauthors, also of Charité-Universitätsmedizin Berlin, said in an interview.
“By comprehending the unique characteristics, progression, and treatment responses within the axial domain, rheumatologists can customize interventions and therapies to effectively manage the psoriatic disease,” Dr. Poddubnyy said.
“One of the most significant findings [of the current study] is the relatively high frequency of spinal involvement without sacroiliac joint” involvement, Fabian Proft, MD, of Charité-Universitätsmedizin Berlin and senior author of the study, said in an interview. “This finding holds importance as, in primary axial SpA, the disease typically originates in the sacroiliac joints. In contrast, in PsA, the scenario differs, which has implications for the diagnostic approach in clinical practice.”
“In individuals with PsA, spinal involvement can occur independently of sacroiliac joint [involvement]. As a result, imaging studies conducted on patients suspected of having axial PsA should encompass not only the sacroiliac joints but also the spine,” Dr. Poddubnyy explained. “It is important to note, however, that imaging findings such as bony spurs and bone marrow edema might be caused by degeneration or mechanical issues and, therefore, need to be interpreted with caution within the clinical context.”
The study was supported in part by an unrestricted research grant from Novartis. Dr. Poddubnyy and Dr. Proft disclosed receiving research grants and consultancy payments from Novartis and serving on speaker bureaus for the company.
Approximately 20% of adults with axial psoriatic arthritis (PsA) show active or structural spinal changes without changes in the sacroiliac joint, based on imaging data from 106 individuals.
Axial PsA has been historically grouped with axial spondyloarthritis (axSpA), but it has received more attention in recent years as a condition potentially distinct from axSpA, Henriette Käding, an MD and PhD student in the department of gastroenterology, infectiology, and rheumatology at Charité-Universitätsmedizin Berlin, said in her research presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). She added that the debate persists as to whether these conditions are on the same spectrum or should be separated.
Data from previous studies suggest differences in genetic, clinical, radiographic, and prognostic characteristics between axial PsA and axSpA that may affect patients’ response to available treatments. However, there are relatively little data available on distinguishing imaging and clinical features, and there’s a lack of classification criteria for axial PsA, Ms. Käding said.
Ms. Käding and colleagues prospectively collected data from 106 patients with axial PsA between August 2019 and June 2023 and presented the baseline data of this longitudinal project at the GRAPPA annual meeting in Dublin. At baseline, the researchers conducted clinical assessments of the participants, along with blood sampling, stool samples, and imaging protocols that included MRI of the whole spine and sacroiliac joint (SIJ).
The mean age of the included patients was 44.5 years; 55.7% were female. Inflammatory back pain was present in most of the patients at baseline (78.4%), and 48.1% were positive for HLA-B27, a genetic risk factor for both axSpA and axial PsA. Approximately one-third of the patients had elevated C-reactive protein (> 5 mg/L). In the baseline MRI scans, active inflammatory changes in the sacroiliac joints (SIJ) were seen in 51.9% of the patients and structural changes in 72.1%. MRI spine scans showed active changes in 58.7% of the patients. Notably, active and/or structural changes of the spine without changes in the SIJ appeared in 20% of the patients, Ms. Käding said.
With regard to existing classification criteria, the researchers observed that 92% of the patients met the CASPAR (Classification Criteria for Psoriatic Arthritis) criteria for PsA, 73% met the ASAS (Assessment of Spondyloarthritis International Society) criteria, while 66% of patients met both ASAS and CASPAR criteria.
The study will be the first to include longitudinal MRI scans of the whole spine and SIJ in addition to conventional radiographs, Ms. Käding said.
Better characterization should improve treatment
“Axial involvement in PsA might, on one hand, go unnoticed, but on the other hand, it could also be misdiagnosed in patients with degenerative spinal disease,” Denis Poddubnyy, MD, one of the study coauthors, also of Charité-Universitätsmedizin Berlin, said in an interview.
“By comprehending the unique characteristics, progression, and treatment responses within the axial domain, rheumatologists can customize interventions and therapies to effectively manage the psoriatic disease,” Dr. Poddubnyy said.
“One of the most significant findings [of the current study] is the relatively high frequency of spinal involvement without sacroiliac joint” involvement, Fabian Proft, MD, of Charité-Universitätsmedizin Berlin and senior author of the study, said in an interview. “This finding holds importance as, in primary axial SpA, the disease typically originates in the sacroiliac joints. In contrast, in PsA, the scenario differs, which has implications for the diagnostic approach in clinical practice.”
“In individuals with PsA, spinal involvement can occur independently of sacroiliac joint [involvement]. As a result, imaging studies conducted on patients suspected of having axial PsA should encompass not only the sacroiliac joints but also the spine,” Dr. Poddubnyy explained. “It is important to note, however, that imaging findings such as bony spurs and bone marrow edema might be caused by degeneration or mechanical issues and, therefore, need to be interpreted with caution within the clinical context.”
The study was supported in part by an unrestricted research grant from Novartis. Dr. Poddubnyy and Dr. Proft disclosed receiving research grants and consultancy payments from Novartis and serving on speaker bureaus for the company.
FROM GRAPPA 2023