User login
Alternative payment models: MedPAC says keep it simple
WASHINGTON – A federal advisory panel has straightforward advice for the government as it shifts towards value-based health care: Keep it simple.
At the Jan. 15 meeting of the Medicare Payment Advisory Commission (MedPAC), Commissioner Warner Thomas said, “I think one of our first principles should be to try to simplify this because it’s very complicated for a provider to try to understand what path to take and how to play in these situations. We want providers to engage in this and I think there is a lot of aversion because folks don’t understand it, and they’re not sure where to play or where not to play.”
Officials at the Centers for Medicare & Medicaid Services are drafting the regulations needed to implement the Medicare Access & CHIP Reauthorization Act (MACRA); the proposed regulations are expected in the Spring.
MedPAC staff offered a number of principles they hope to see incorporated into the final design for alternative payment models (APMs) under the law, including:
• Incentive payments to providers should be distributed only if the entities they are a part of are able to successfully control cost, improve quality, or both.
• Entities should have enough beneficiaries to detect changes in spending and quality.
• Entities should be at risk for total Part A and Part B spending; in the future, Part D also might be measured.
• Entities should be allowed to share savings with beneficiaries.
• Regulatory relief should be possible.
Several MedPAC commissioners focused on how physicians are viewing the risk they will be asked to assume in an APM.
APMs are “an attempt to sort of jump start a movement in the direction of risk taking from a group that I don’t think is very thrilled about taking financial risk and has shown it by their actual practice in the face of efforts at least to nudge them in this direction,” said Commissioner Bill Gradison Jr., a former congressman from Ohio who served on the House Committee on Ways and Means. “Having said that, I wish I had a better understanding of the motivation of physicians in terms of what might move them in this direction. The assumption here is that the money will move them, and maybe it’s that simple, but I am not too sure about that.”
Commissioner Jack Hoadley, Ph.D., of the Health Policy Institute at Georgetown University, Washington, noted that the if there is too little risk involved, there tends to be little movement in changing the behavior.
Dr. Hoadley suggested that CMS could consider “looking at some of the models by which risk can be structured so it puts plenty of money on the line but it doesn’t do it in a way that’s going to get complicated in terms of physicians owing money back.”
Commissioners also recommended a tight threshold, at least early on, to show that cost containment and/or quality improvement are well established before an entity can move into an APM and receive a financial incentive.
“Let’s keep the initial round and definition tight and actually demand a track record before we qualify something as an alternative payment mechanism,” said Commissioner Kathy Buto, a U.S. and international health policy consultant and former deputy executive secretary in the U.S. Health & Human Services Department. “Once you open this up, you can’t close it down. You cannot walk it back. We are talking about paying a lot more money, 5% more and if the definition is too loose and too many entities get into it, you can never walk it back and you will be just adding to the expenditures.”
WASHINGTON – A federal advisory panel has straightforward advice for the government as it shifts towards value-based health care: Keep it simple.
At the Jan. 15 meeting of the Medicare Payment Advisory Commission (MedPAC), Commissioner Warner Thomas said, “I think one of our first principles should be to try to simplify this because it’s very complicated for a provider to try to understand what path to take and how to play in these situations. We want providers to engage in this and I think there is a lot of aversion because folks don’t understand it, and they’re not sure where to play or where not to play.”
Officials at the Centers for Medicare & Medicaid Services are drafting the regulations needed to implement the Medicare Access & CHIP Reauthorization Act (MACRA); the proposed regulations are expected in the Spring.
MedPAC staff offered a number of principles they hope to see incorporated into the final design for alternative payment models (APMs) under the law, including:
• Incentive payments to providers should be distributed only if the entities they are a part of are able to successfully control cost, improve quality, or both.
• Entities should have enough beneficiaries to detect changes in spending and quality.
• Entities should be at risk for total Part A and Part B spending; in the future, Part D also might be measured.
• Entities should be allowed to share savings with beneficiaries.
• Regulatory relief should be possible.
Several MedPAC commissioners focused on how physicians are viewing the risk they will be asked to assume in an APM.
APMs are “an attempt to sort of jump start a movement in the direction of risk taking from a group that I don’t think is very thrilled about taking financial risk and has shown it by their actual practice in the face of efforts at least to nudge them in this direction,” said Commissioner Bill Gradison Jr., a former congressman from Ohio who served on the House Committee on Ways and Means. “Having said that, I wish I had a better understanding of the motivation of physicians in terms of what might move them in this direction. The assumption here is that the money will move them, and maybe it’s that simple, but I am not too sure about that.”
Commissioner Jack Hoadley, Ph.D., of the Health Policy Institute at Georgetown University, Washington, noted that the if there is too little risk involved, there tends to be little movement in changing the behavior.
Dr. Hoadley suggested that CMS could consider “looking at some of the models by which risk can be structured so it puts plenty of money on the line but it doesn’t do it in a way that’s going to get complicated in terms of physicians owing money back.”
Commissioners also recommended a tight threshold, at least early on, to show that cost containment and/or quality improvement are well established before an entity can move into an APM and receive a financial incentive.
“Let’s keep the initial round and definition tight and actually demand a track record before we qualify something as an alternative payment mechanism,” said Commissioner Kathy Buto, a U.S. and international health policy consultant and former deputy executive secretary in the U.S. Health & Human Services Department. “Once you open this up, you can’t close it down. You cannot walk it back. We are talking about paying a lot more money, 5% more and if the definition is too loose and too many entities get into it, you can never walk it back and you will be just adding to the expenditures.”
WASHINGTON – A federal advisory panel has straightforward advice for the government as it shifts towards value-based health care: Keep it simple.
At the Jan. 15 meeting of the Medicare Payment Advisory Commission (MedPAC), Commissioner Warner Thomas said, “I think one of our first principles should be to try to simplify this because it’s very complicated for a provider to try to understand what path to take and how to play in these situations. We want providers to engage in this and I think there is a lot of aversion because folks don’t understand it, and they’re not sure where to play or where not to play.”
Officials at the Centers for Medicare & Medicaid Services are drafting the regulations needed to implement the Medicare Access & CHIP Reauthorization Act (MACRA); the proposed regulations are expected in the Spring.
MedPAC staff offered a number of principles they hope to see incorporated into the final design for alternative payment models (APMs) under the law, including:
• Incentive payments to providers should be distributed only if the entities they are a part of are able to successfully control cost, improve quality, or both.
• Entities should have enough beneficiaries to detect changes in spending and quality.
• Entities should be at risk for total Part A and Part B spending; in the future, Part D also might be measured.
• Entities should be allowed to share savings with beneficiaries.
• Regulatory relief should be possible.
Several MedPAC commissioners focused on how physicians are viewing the risk they will be asked to assume in an APM.
APMs are “an attempt to sort of jump start a movement in the direction of risk taking from a group that I don’t think is very thrilled about taking financial risk and has shown it by their actual practice in the face of efforts at least to nudge them in this direction,” said Commissioner Bill Gradison Jr., a former congressman from Ohio who served on the House Committee on Ways and Means. “Having said that, I wish I had a better understanding of the motivation of physicians in terms of what might move them in this direction. The assumption here is that the money will move them, and maybe it’s that simple, but I am not too sure about that.”
Commissioner Jack Hoadley, Ph.D., of the Health Policy Institute at Georgetown University, Washington, noted that the if there is too little risk involved, there tends to be little movement in changing the behavior.
Dr. Hoadley suggested that CMS could consider “looking at some of the models by which risk can be structured so it puts plenty of money on the line but it doesn’t do it in a way that’s going to get complicated in terms of physicians owing money back.”
Commissioners also recommended a tight threshold, at least early on, to show that cost containment and/or quality improvement are well established before an entity can move into an APM and receive a financial incentive.
“Let’s keep the initial round and definition tight and actually demand a track record before we qualify something as an alternative payment mechanism,” said Commissioner Kathy Buto, a U.S. and international health policy consultant and former deputy executive secretary in the U.S. Health & Human Services Department. “Once you open this up, you can’t close it down. You cannot walk it back. We are talking about paying a lot more money, 5% more and if the definition is too loose and too many entities get into it, you can never walk it back and you will be just adding to the expenditures.”
AT A MEDPAC MEETING
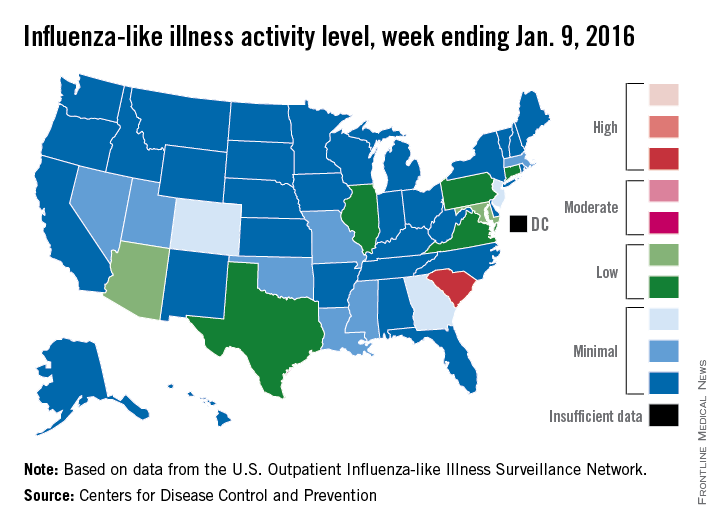
New year brings reduced flu activity
Influenza-like illness (ILI) activity was down during the first full week in January – a not-so-unlucky week 13 of the 2015-2016 flu season, the Centers for Disease Control and Prevention reported.
South Carolina was at level 8, making it the only state in the “high” range of activity for the week ending Jan. 9. Puerto Rico was also at level 8, but there was no state in the “moderate” range and only seven states in the “low” range: Arizona and Maryland at level 5 and Connecticut, Illinois, Pennsylvania, Texas, and Virginia at level 4. There were 18 states at level 2 or higher, compared with 24 the previous week, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
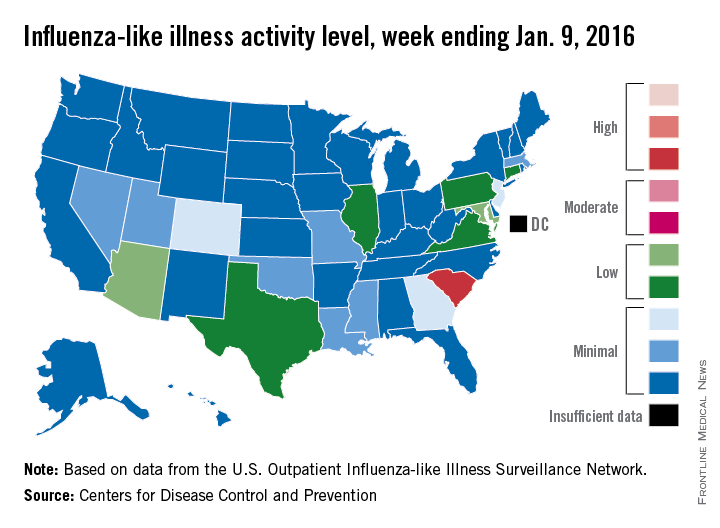
The proportion of outpatient visits for ILI was 2% for week 13, which was down from 2.8% in week 12 and below the national baseline of 2.1%, the CDC said. ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.
One ILI-related pediatric death was reported during week 13, although it actually occurred in December. So far, there have been seven pediatric deaths reported during the 2015-2016 flu season, the CDC report noted.
Since Oct. 1, 2015, there have been 423 laboratory-confirmed influenza-associated hospitalizations reported in the 13 states – including California, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 1.5 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
Influenza-like illness (ILI) activity was down during the first full week in January – a not-so-unlucky week 13 of the 2015-2016 flu season, the Centers for Disease Control and Prevention reported.
South Carolina was at level 8, making it the only state in the “high” range of activity for the week ending Jan. 9. Puerto Rico was also at level 8, but there was no state in the “moderate” range and only seven states in the “low” range: Arizona and Maryland at level 5 and Connecticut, Illinois, Pennsylvania, Texas, and Virginia at level 4. There were 18 states at level 2 or higher, compared with 24 the previous week, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.

The proportion of outpatient visits for ILI was 2% for week 13, which was down from 2.8% in week 12 and below the national baseline of 2.1%, the CDC said. ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.
One ILI-related pediatric death was reported during week 13, although it actually occurred in December. So far, there have been seven pediatric deaths reported during the 2015-2016 flu season, the CDC report noted.
Since Oct. 1, 2015, there have been 423 laboratory-confirmed influenza-associated hospitalizations reported in the 13 states – including California, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 1.5 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
Influenza-like illness (ILI) activity was down during the first full week in January – a not-so-unlucky week 13 of the 2015-2016 flu season, the Centers for Disease Control and Prevention reported.
South Carolina was at level 8, making it the only state in the “high” range of activity for the week ending Jan. 9. Puerto Rico was also at level 8, but there was no state in the “moderate” range and only seven states in the “low” range: Arizona and Maryland at level 5 and Connecticut, Illinois, Pennsylvania, Texas, and Virginia at level 4. There were 18 states at level 2 or higher, compared with 24 the previous week, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.

The proportion of outpatient visits for ILI was 2% for week 13, which was down from 2.8% in week 12 and below the national baseline of 2.1%, the CDC said. ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.
One ILI-related pediatric death was reported during week 13, although it actually occurred in December. So far, there have been seven pediatric deaths reported during the 2015-2016 flu season, the CDC report noted.
Since Oct. 1, 2015, there have been 423 laboratory-confirmed influenza-associated hospitalizations reported in the 13 states – including California, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 1.5 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
2016: Celebrating 20 Years of Hospital Medicine and Looking Toward a Bright Future
For hospitalists who were practicing medicine in 1996, it may seem like just a few years ago; however, that was when the term “hospitalist” was first used by Bob Wachter, MD, MHM, and Lee Goldman, MD, in a New England Journal of Medicine article. And for those who started their hospitalist careers since then, it may be inconceivable that there was a time before hospitalists were widely known.
In either case, 20 years later, the term has stuck and now identifies more than 44,000 hospitalists nationwide.
SHM will be commemorating the specialty’s milestone all year, referring to 2016 as the “Year of the Hospitalist.”
In addition to yearlong recognition of hospitalists, SHM will celebrate the Year of the Hospitalist in front of thousands at a plenary session at HM16 in San Diego. There, SHM co-founders John Nelson, MD, MHM, and Win Whitcomb, MD, MHM, will offer their own perspective on the specialty after just 20 years—and what’s to come.
“When Win Whitcomb and I began talking about forming a medical society, we wanted to make sure it would serve as a forum for exchange of ideas about the most effective ways to approach our work,” Dr. Nelson recalls. “It is really gratifying to see all the ways SHM has done that and to think about how it will be increasingly important for all in hospital medicine to have a way to stay connected given the ever-increasing pace of change in hospital care and healthcare generally.”
Dr. Whitcomb shares Dr. Nelson’s awe at the growth and influence of the specialty in a relatively short amount of time.
“A lot has changed in 20 years. Back then, we were surprised to observe that hospitalists, yet unnamed, could reliably decrease cost and length of stay,” Dr. Whitcomb says. “Then, in 1999, hospital medicine was transformed as patient safety emerged as a bona fide discipline.”
However, the transitions are not over for hospitalists, he says.
“As we see healthcare in the midst of a generational transition to alternative payment models like ACOs and bundled payment, hospitalists will once again reinvent themselves, this time to influence care over not just the inpatient stay but also patients’ transition out of the hospital and back into the primary care system,” Dr. Whitcomb says. TH
Brendon Shank is SHM’s associate vice president of communications.
For hospitalists who were practicing medicine in 1996, it may seem like just a few years ago; however, that was when the term “hospitalist” was first used by Bob Wachter, MD, MHM, and Lee Goldman, MD, in a New England Journal of Medicine article. And for those who started their hospitalist careers since then, it may be inconceivable that there was a time before hospitalists were widely known.
In either case, 20 years later, the term has stuck and now identifies more than 44,000 hospitalists nationwide.
SHM will be commemorating the specialty’s milestone all year, referring to 2016 as the “Year of the Hospitalist.”
In addition to yearlong recognition of hospitalists, SHM will celebrate the Year of the Hospitalist in front of thousands at a plenary session at HM16 in San Diego. There, SHM co-founders John Nelson, MD, MHM, and Win Whitcomb, MD, MHM, will offer their own perspective on the specialty after just 20 years—and what’s to come.
“When Win Whitcomb and I began talking about forming a medical society, we wanted to make sure it would serve as a forum for exchange of ideas about the most effective ways to approach our work,” Dr. Nelson recalls. “It is really gratifying to see all the ways SHM has done that and to think about how it will be increasingly important for all in hospital medicine to have a way to stay connected given the ever-increasing pace of change in hospital care and healthcare generally.”
Dr. Whitcomb shares Dr. Nelson’s awe at the growth and influence of the specialty in a relatively short amount of time.
“A lot has changed in 20 years. Back then, we were surprised to observe that hospitalists, yet unnamed, could reliably decrease cost and length of stay,” Dr. Whitcomb says. “Then, in 1999, hospital medicine was transformed as patient safety emerged as a bona fide discipline.”
However, the transitions are not over for hospitalists, he says.
“As we see healthcare in the midst of a generational transition to alternative payment models like ACOs and bundled payment, hospitalists will once again reinvent themselves, this time to influence care over not just the inpatient stay but also patients’ transition out of the hospital and back into the primary care system,” Dr. Whitcomb says. TH
Brendon Shank is SHM’s associate vice president of communications.
For hospitalists who were practicing medicine in 1996, it may seem like just a few years ago; however, that was when the term “hospitalist” was first used by Bob Wachter, MD, MHM, and Lee Goldman, MD, in a New England Journal of Medicine article. And for those who started their hospitalist careers since then, it may be inconceivable that there was a time before hospitalists were widely known.
In either case, 20 years later, the term has stuck and now identifies more than 44,000 hospitalists nationwide.
SHM will be commemorating the specialty’s milestone all year, referring to 2016 as the “Year of the Hospitalist.”
In addition to yearlong recognition of hospitalists, SHM will celebrate the Year of the Hospitalist in front of thousands at a plenary session at HM16 in San Diego. There, SHM co-founders John Nelson, MD, MHM, and Win Whitcomb, MD, MHM, will offer their own perspective on the specialty after just 20 years—and what’s to come.
“When Win Whitcomb and I began talking about forming a medical society, we wanted to make sure it would serve as a forum for exchange of ideas about the most effective ways to approach our work,” Dr. Nelson recalls. “It is really gratifying to see all the ways SHM has done that and to think about how it will be increasingly important for all in hospital medicine to have a way to stay connected given the ever-increasing pace of change in hospital care and healthcare generally.”
Dr. Whitcomb shares Dr. Nelson’s awe at the growth and influence of the specialty in a relatively short amount of time.
“A lot has changed in 20 years. Back then, we were surprised to observe that hospitalists, yet unnamed, could reliably decrease cost and length of stay,” Dr. Whitcomb says. “Then, in 1999, hospital medicine was transformed as patient safety emerged as a bona fide discipline.”
However, the transitions are not over for hospitalists, he says.
“As we see healthcare in the midst of a generational transition to alternative payment models like ACOs and bundled payment, hospitalists will once again reinvent themselves, this time to influence care over not just the inpatient stay but also patients’ transition out of the hospital and back into the primary care system,” Dr. Whitcomb says. TH
Brendon Shank is SHM’s associate vice president of communications.
Melissa Mattison, MD, SFHM, Offers Inside Scoop on HM16 Educational Offerings
HM16 course director Melissa Mattison, MD, SFHM, assistant professor of medicine at Harvard Medical School in Boston, took some time out of her busy schedule to chat with The Hospitalist about how the annual meeting program comes together, the continued relevance of SHM meetings, resisting the lure of San Diego beaches, and more.
Read more about what's new at HM16
Question: There’s a lot packed into just a few days at every annual meeting. What is the process for determining what makes it into the program?
Answer: The annual meeting committee starts meeting in the spring, basically around 12 to 13 months before the annual meeting. So even as the current annual meeting is going on, the next one is well under way in terms of planning.
The annual meeting committee then meets every single week, if not more frequently, by conference call to map out and assign jobs and whatnot to the group. We usually start by reviewing workshops. The workshop proposals are received by SHM through an open-access submission process, whereby pretty much anyone can submit a proposal for a workshop. The annual meeting committee reviews all of the workshops. This year, we had 160 or 170 workshop submissions. We were only able to accept 16, I believe. …
Listen to more of our interview with Dr. Mattison.
At the same time the workshops are being selected, we work with the leaders of practice management, the leaders of the academic and research committees, and the leaders of quality and pediatrics committees to have them help us identify content for their various tracks, suggest speakers and talks. After all of that’s done, we move on to the remaining didactic sessions, and we look at what things were popular in previous years, what things had good reviews, which speakers were highly regarded, etc., and spend some time thinking deliberately about tracks that we feel we ought to include again, like the “Young Hospitalists” track and tracks that we think deserve to be included that haven’t previously been included—those that warrant some sort of attention because of widespread appeal and usefulness to the attendee of SHM.
Q: It’s very easy for hospitalists to stream videos of talks, access literature online, and talk about important topics in online chat rooms. In this day and age, what is the advantage to physically taking part in an annual meeting in an actual brick-and-mortar building?
A: The content itself is enough to draw someone. It’s packed content in terms of topics that would be of interest and benefit to the average hospitalist; pretty much any hospitalist who’s practicing medicine will find multiple, multiple sessions of interest and value. And you don’t have to go far for them. You don’t have to go hunting for them. They’re all there. …
Aside from actual didactics and content of the annual meeting, there’s the value of networking and of collaboration and meeting and talking to hospitalists from around the country and around the world. I mean, I think that one of the biggest values I’ve had is just meeting other people who are facing challenges in their work environment that I have [and learning] how they solve their challenges.
I think the [special interest] groups that meet Monday evening at 4:30 p.m. … those are great opportunities to go and meet people who have interests that are similar to yours or concerns that are similar to yours.
Q: Technology has a presence in the program this year. Why is it so important to highlight this?
A: The challenge of healthcare and incorporating technology into providing care to patients in a way that is efficient and helpful is there. That is a challenge that has been written about by many, many folks. Dr. Bob Wachter gave a whole keynote on it last year. And we’re all seeking ways to work with the technology that we have and identify opportunities to improve the care we’re providing using and harnessing the technology that’s available to us.
So whether that’s with new apps or with figuring out ways to embed decision support into our local systems of care, we need to do that. I think hospitalists are, time and time again, looked at as leaders at their institutions in this domain. It’s going to be 2016, this is the world we live in, and to ignore technology would be foolhardy.
Q: One of the new tracks is focused on post-acute care. Is the importance of the post-acute setting a sign that hospital medicine is, in some ways, reinventing itself?
A: I wouldn’t say reinventing. I think that hospitalists and internists that have become hospitalists have filled the gap in care over the past 20 years. It’s been 20 years since the name “hospitalist” was used in the New England Journal of Medicine. And in that time, the breadth and depth of care that hospitalists provide across the continuum in the acute-care setting has grown. …
Our older patients are often discharged from the acute-care setting but unable to return directly to their home environment safely. [They] require a period of a week or two, or sometimes longer, in a post-acute-care setting to continue to receive both the medical and the physical rehabilitation care that they need. And we know that there are not enough geriatricians in the world, and hospitalists are really sort of stepping up to provide this post-acute care. And it makes sense because the patients are coming from the hospital directly, and a lot of folks would say they’re sicker than ever in the post-acute-care setting. You don’t stay in the hospital for long anymore, and when you get to the post-acute-care setting, often the illness is ongoing but stabilized, and the patient is on the mend from whatever has befallen them. But they still require a fair amount of medical management. So it makes sense that hospitalists are going into that sphere.
Q: How will you go about resisting the temptation to stealthily leave the convention center during the day and hit the beach? Or will you be able to resist?
A: [Laughter.] I do like that question. I think that … I think that the conference, while it’s busy, there’s some time in the evening to go out and have a nice meal or [to] the beach and see friends. And then the last day, if you have time, if you don’t need to race off, you have a good half a day where you could go to the beach, or you could come early and come a day before or stay an extra couple of days and enjoy San Diego.
But I think if you skip the conference for the beach, you’re not doing yourself a service. You’re going to miss out on the opportunity to learn new clinical information, new strategies for communication. You’re also going to miss out on opportunities to network with your colleagues from across the country. TH
Thomas R. Collins is a freelance writer in South Florida.
HM16 course director Melissa Mattison, MD, SFHM, assistant professor of medicine at Harvard Medical School in Boston, took some time out of her busy schedule to chat with The Hospitalist about how the annual meeting program comes together, the continued relevance of SHM meetings, resisting the lure of San Diego beaches, and more.
Read more about what's new at HM16
Question: There’s a lot packed into just a few days at every annual meeting. What is the process for determining what makes it into the program?
Answer: The annual meeting committee starts meeting in the spring, basically around 12 to 13 months before the annual meeting. So even as the current annual meeting is going on, the next one is well under way in terms of planning.
The annual meeting committee then meets every single week, if not more frequently, by conference call to map out and assign jobs and whatnot to the group. We usually start by reviewing workshops. The workshop proposals are received by SHM through an open-access submission process, whereby pretty much anyone can submit a proposal for a workshop. The annual meeting committee reviews all of the workshops. This year, we had 160 or 170 workshop submissions. We were only able to accept 16, I believe. …
Listen to more of our interview with Dr. Mattison.
At the same time the workshops are being selected, we work with the leaders of practice management, the leaders of the academic and research committees, and the leaders of quality and pediatrics committees to have them help us identify content for their various tracks, suggest speakers and talks. After all of that’s done, we move on to the remaining didactic sessions, and we look at what things were popular in previous years, what things had good reviews, which speakers were highly regarded, etc., and spend some time thinking deliberately about tracks that we feel we ought to include again, like the “Young Hospitalists” track and tracks that we think deserve to be included that haven’t previously been included—those that warrant some sort of attention because of widespread appeal and usefulness to the attendee of SHM.
Q: It’s very easy for hospitalists to stream videos of talks, access literature online, and talk about important topics in online chat rooms. In this day and age, what is the advantage to physically taking part in an annual meeting in an actual brick-and-mortar building?
A: The content itself is enough to draw someone. It’s packed content in terms of topics that would be of interest and benefit to the average hospitalist; pretty much any hospitalist who’s practicing medicine will find multiple, multiple sessions of interest and value. And you don’t have to go far for them. You don’t have to go hunting for them. They’re all there. …
Aside from actual didactics and content of the annual meeting, there’s the value of networking and of collaboration and meeting and talking to hospitalists from around the country and around the world. I mean, I think that one of the biggest values I’ve had is just meeting other people who are facing challenges in their work environment that I have [and learning] how they solve their challenges.
I think the [special interest] groups that meet Monday evening at 4:30 p.m. … those are great opportunities to go and meet people who have interests that are similar to yours or concerns that are similar to yours.
Q: Technology has a presence in the program this year. Why is it so important to highlight this?
A: The challenge of healthcare and incorporating technology into providing care to patients in a way that is efficient and helpful is there. That is a challenge that has been written about by many, many folks. Dr. Bob Wachter gave a whole keynote on it last year. And we’re all seeking ways to work with the technology that we have and identify opportunities to improve the care we’re providing using and harnessing the technology that’s available to us.
So whether that’s with new apps or with figuring out ways to embed decision support into our local systems of care, we need to do that. I think hospitalists are, time and time again, looked at as leaders at their institutions in this domain. It’s going to be 2016, this is the world we live in, and to ignore technology would be foolhardy.
Q: One of the new tracks is focused on post-acute care. Is the importance of the post-acute setting a sign that hospital medicine is, in some ways, reinventing itself?
A: I wouldn’t say reinventing. I think that hospitalists and internists that have become hospitalists have filled the gap in care over the past 20 years. It’s been 20 years since the name “hospitalist” was used in the New England Journal of Medicine. And in that time, the breadth and depth of care that hospitalists provide across the continuum in the acute-care setting has grown. …
Our older patients are often discharged from the acute-care setting but unable to return directly to their home environment safely. [They] require a period of a week or two, or sometimes longer, in a post-acute-care setting to continue to receive both the medical and the physical rehabilitation care that they need. And we know that there are not enough geriatricians in the world, and hospitalists are really sort of stepping up to provide this post-acute care. And it makes sense because the patients are coming from the hospital directly, and a lot of folks would say they’re sicker than ever in the post-acute-care setting. You don’t stay in the hospital for long anymore, and when you get to the post-acute-care setting, often the illness is ongoing but stabilized, and the patient is on the mend from whatever has befallen them. But they still require a fair amount of medical management. So it makes sense that hospitalists are going into that sphere.
Q: How will you go about resisting the temptation to stealthily leave the convention center during the day and hit the beach? Or will you be able to resist?
A: [Laughter.] I do like that question. I think that … I think that the conference, while it’s busy, there’s some time in the evening to go out and have a nice meal or [to] the beach and see friends. And then the last day, if you have time, if you don’t need to race off, you have a good half a day where you could go to the beach, or you could come early and come a day before or stay an extra couple of days and enjoy San Diego.
But I think if you skip the conference for the beach, you’re not doing yourself a service. You’re going to miss out on the opportunity to learn new clinical information, new strategies for communication. You’re also going to miss out on opportunities to network with your colleagues from across the country. TH
Thomas R. Collins is a freelance writer in South Florida.
HM16 course director Melissa Mattison, MD, SFHM, assistant professor of medicine at Harvard Medical School in Boston, took some time out of her busy schedule to chat with The Hospitalist about how the annual meeting program comes together, the continued relevance of SHM meetings, resisting the lure of San Diego beaches, and more.
Read more about what's new at HM16
Question: There’s a lot packed into just a few days at every annual meeting. What is the process for determining what makes it into the program?
Answer: The annual meeting committee starts meeting in the spring, basically around 12 to 13 months before the annual meeting. So even as the current annual meeting is going on, the next one is well under way in terms of planning.
The annual meeting committee then meets every single week, if not more frequently, by conference call to map out and assign jobs and whatnot to the group. We usually start by reviewing workshops. The workshop proposals are received by SHM through an open-access submission process, whereby pretty much anyone can submit a proposal for a workshop. The annual meeting committee reviews all of the workshops. This year, we had 160 or 170 workshop submissions. We were only able to accept 16, I believe. …
Listen to more of our interview with Dr. Mattison.
At the same time the workshops are being selected, we work with the leaders of practice management, the leaders of the academic and research committees, and the leaders of quality and pediatrics committees to have them help us identify content for their various tracks, suggest speakers and talks. After all of that’s done, we move on to the remaining didactic sessions, and we look at what things were popular in previous years, what things had good reviews, which speakers were highly regarded, etc., and spend some time thinking deliberately about tracks that we feel we ought to include again, like the “Young Hospitalists” track and tracks that we think deserve to be included that haven’t previously been included—those that warrant some sort of attention because of widespread appeal and usefulness to the attendee of SHM.
Q: It’s very easy for hospitalists to stream videos of talks, access literature online, and talk about important topics in online chat rooms. In this day and age, what is the advantage to physically taking part in an annual meeting in an actual brick-and-mortar building?
A: The content itself is enough to draw someone. It’s packed content in terms of topics that would be of interest and benefit to the average hospitalist; pretty much any hospitalist who’s practicing medicine will find multiple, multiple sessions of interest and value. And you don’t have to go far for them. You don’t have to go hunting for them. They’re all there. …
Aside from actual didactics and content of the annual meeting, there’s the value of networking and of collaboration and meeting and talking to hospitalists from around the country and around the world. I mean, I think that one of the biggest values I’ve had is just meeting other people who are facing challenges in their work environment that I have [and learning] how they solve their challenges.
I think the [special interest] groups that meet Monday evening at 4:30 p.m. … those are great opportunities to go and meet people who have interests that are similar to yours or concerns that are similar to yours.
Q: Technology has a presence in the program this year. Why is it so important to highlight this?
A: The challenge of healthcare and incorporating technology into providing care to patients in a way that is efficient and helpful is there. That is a challenge that has been written about by many, many folks. Dr. Bob Wachter gave a whole keynote on it last year. And we’re all seeking ways to work with the technology that we have and identify opportunities to improve the care we’re providing using and harnessing the technology that’s available to us.
So whether that’s with new apps or with figuring out ways to embed decision support into our local systems of care, we need to do that. I think hospitalists are, time and time again, looked at as leaders at their institutions in this domain. It’s going to be 2016, this is the world we live in, and to ignore technology would be foolhardy.
Q: One of the new tracks is focused on post-acute care. Is the importance of the post-acute setting a sign that hospital medicine is, in some ways, reinventing itself?
A: I wouldn’t say reinventing. I think that hospitalists and internists that have become hospitalists have filled the gap in care over the past 20 years. It’s been 20 years since the name “hospitalist” was used in the New England Journal of Medicine. And in that time, the breadth and depth of care that hospitalists provide across the continuum in the acute-care setting has grown. …
Our older patients are often discharged from the acute-care setting but unable to return directly to their home environment safely. [They] require a period of a week or two, or sometimes longer, in a post-acute-care setting to continue to receive both the medical and the physical rehabilitation care that they need. And we know that there are not enough geriatricians in the world, and hospitalists are really sort of stepping up to provide this post-acute care. And it makes sense because the patients are coming from the hospital directly, and a lot of folks would say they’re sicker than ever in the post-acute-care setting. You don’t stay in the hospital for long anymore, and when you get to the post-acute-care setting, often the illness is ongoing but stabilized, and the patient is on the mend from whatever has befallen them. But they still require a fair amount of medical management. So it makes sense that hospitalists are going into that sphere.
Q: How will you go about resisting the temptation to stealthily leave the convention center during the day and hit the beach? Or will you be able to resist?
A: [Laughter.] I do like that question. I think that … I think that the conference, while it’s busy, there’s some time in the evening to go out and have a nice meal or [to] the beach and see friends. And then the last day, if you have time, if you don’t need to race off, you have a good half a day where you could go to the beach, or you could come early and come a day before or stay an extra couple of days and enjoy San Diego.
But I think if you skip the conference for the beach, you’re not doing yourself a service. You’re going to miss out on the opportunity to learn new clinical information, new strategies for communication. You’re also going to miss out on opportunities to network with your colleagues from across the country. TH
Thomas R. Collins is a freelance writer in South Florida.
CIPN persists in female cancer survivors

Photo courtesy of NIH
SAN FRANCISCO—A study of female cancer survivors indicates that many still have chemotherapy-induced peripheral neuropathy (CIPN) symptoms years after completing cancer treatment.
In addition, CIPN was associated with worse physical functioning, poorer mobility, and a higher risk of falls.
Although more research is needed, investigators believe these findings may inform rehabilitation and fall prevention interventions for people with CIPN.
The findings were presented at the 2016 Cancer Survivorship Symposium (abstract 130*).
“We can’t dismiss neuropathy as a treatment side effect that goes away because symptoms persist for years in nearly half of women,” said Kerri M. Winters-Stone, PhD, of Oregon Health and Science University in Portland.
“While there are no effective treatments for this side effect, rehabilitative exercise programs may preserve physical functioning and mobility in the presence of neuropathy to help prevent falls and resulting injuries.”
For this study, Dr Winters-Stone and her colleagues assessed data from 512 women enrolled in exercise intervention trials designed to address fractures and falls in female cancer survivors. Most of the women had breast cancer, but there were also cases of lung, colorectal, ovarian, and hematologic cancers.
At an average of 6 years post-cancer diagnosis, 46% of the women (n=238) still reported some symptoms of CIPN, such as loss of feeling in their hands and feet.
The investigators noted significant relationships (P<0.01) between CIPN severity and gait speed, Physical Performance Battery score, self-reported physical functioning, and self-reported disability.
The team also compared measures of physical functioning in the women with CIPN to measures in women without CIPN (n=274). This analysis was adjusted for cancer type and time since diagnosis.
There was a significant difference (P<0.01) between the groups in one measure of lower-extremity fitness but not another. Namely, it took CIPN-positive women significantly longer to rise out of a chair (tested 5 times each). But women in both groups fared similarly on a test measuring maximal leg press strength.
The investigators also tested the women on mobility and physical functioning. The CIPN-positive women fared significantly worse than CIPN-negative women (P<0.01) when it came to walking speed, step number, stride length, percentage of gait cycle in double support, and Physical Performance Battery score. However, there was no significant difference between the groups with regard to base of support.
Finally, CIPN-positive women were significantly more likely than CIPN-negative women to report poor physical function and disability (P<0.01 for both). And CIPN-positive women had a higher rate of falls in the last year (P<0.01).
The investigators said women with CIPN have specific underlying impairments that put them at risk for falls, which may be different from the impairments that occur with other conditions or old age.
For example, CIPN does not cause muscle weakness, but it has a distinct effect on movement and gait patterns.
The team noted that the women with CIPN had difficulty rising from a chair, possibly because their brains do not get enough information from their feet about how quickly or forcefully to stand up.
Based on these findings, the investigators argued that commonly recommended exercise, such as walking, may be safer for women with CIPN when done on a treadmill with handrails because their altered gait puts them at an increased risk of falling.
The team also said that machine-based resistance training may not be beneficial because neuropathy does not appear to decrease leg strength. Instead, rehabilitation efforts should focus on improving balance during upright movement and specific gait training.
Furthermore, the investigators believe that, if the symptoms of CIPN are detected early, cancer treatments could potentially be changed to prevent these debilitating problems or early rehabilitation interventions could be started.
In addition, Dr Winters-Stone and her research team are developing a smartphone-driven device that patients can use to detect and quantify symptoms of neuropathy, such as gait and balance impairments. ![]()
*Data in the abstract differ from the presentation.

Photo courtesy of NIH
SAN FRANCISCO—A study of female cancer survivors indicates that many still have chemotherapy-induced peripheral neuropathy (CIPN) symptoms years after completing cancer treatment.
In addition, CIPN was associated with worse physical functioning, poorer mobility, and a higher risk of falls.
Although more research is needed, investigators believe these findings may inform rehabilitation and fall prevention interventions for people with CIPN.
The findings were presented at the 2016 Cancer Survivorship Symposium (abstract 130*).
“We can’t dismiss neuropathy as a treatment side effect that goes away because symptoms persist for years in nearly half of women,” said Kerri M. Winters-Stone, PhD, of Oregon Health and Science University in Portland.
“While there are no effective treatments for this side effect, rehabilitative exercise programs may preserve physical functioning and mobility in the presence of neuropathy to help prevent falls and resulting injuries.”
For this study, Dr Winters-Stone and her colleagues assessed data from 512 women enrolled in exercise intervention trials designed to address fractures and falls in female cancer survivors. Most of the women had breast cancer, but there were also cases of lung, colorectal, ovarian, and hematologic cancers.
At an average of 6 years post-cancer diagnosis, 46% of the women (n=238) still reported some symptoms of CIPN, such as loss of feeling in their hands and feet.
The investigators noted significant relationships (P<0.01) between CIPN severity and gait speed, Physical Performance Battery score, self-reported physical functioning, and self-reported disability.
The team also compared measures of physical functioning in the women with CIPN to measures in women without CIPN (n=274). This analysis was adjusted for cancer type and time since diagnosis.
There was a significant difference (P<0.01) between the groups in one measure of lower-extremity fitness but not another. Namely, it took CIPN-positive women significantly longer to rise out of a chair (tested 5 times each). But women in both groups fared similarly on a test measuring maximal leg press strength.
The investigators also tested the women on mobility and physical functioning. The CIPN-positive women fared significantly worse than CIPN-negative women (P<0.01) when it came to walking speed, step number, stride length, percentage of gait cycle in double support, and Physical Performance Battery score. However, there was no significant difference between the groups with regard to base of support.
Finally, CIPN-positive women were significantly more likely than CIPN-negative women to report poor physical function and disability (P<0.01 for both). And CIPN-positive women had a higher rate of falls in the last year (P<0.01).
The investigators said women with CIPN have specific underlying impairments that put them at risk for falls, which may be different from the impairments that occur with other conditions or old age.
For example, CIPN does not cause muscle weakness, but it has a distinct effect on movement and gait patterns.
The team noted that the women with CIPN had difficulty rising from a chair, possibly because their brains do not get enough information from their feet about how quickly or forcefully to stand up.
Based on these findings, the investigators argued that commonly recommended exercise, such as walking, may be safer for women with CIPN when done on a treadmill with handrails because their altered gait puts them at an increased risk of falling.
The team also said that machine-based resistance training may not be beneficial because neuropathy does not appear to decrease leg strength. Instead, rehabilitation efforts should focus on improving balance during upright movement and specific gait training.
Furthermore, the investigators believe that, if the symptoms of CIPN are detected early, cancer treatments could potentially be changed to prevent these debilitating problems or early rehabilitation interventions could be started.
In addition, Dr Winters-Stone and her research team are developing a smartphone-driven device that patients can use to detect and quantify symptoms of neuropathy, such as gait and balance impairments. ![]()
*Data in the abstract differ from the presentation.

Photo courtesy of NIH
SAN FRANCISCO—A study of female cancer survivors indicates that many still have chemotherapy-induced peripheral neuropathy (CIPN) symptoms years after completing cancer treatment.
In addition, CIPN was associated with worse physical functioning, poorer mobility, and a higher risk of falls.
Although more research is needed, investigators believe these findings may inform rehabilitation and fall prevention interventions for people with CIPN.
The findings were presented at the 2016 Cancer Survivorship Symposium (abstract 130*).
“We can’t dismiss neuropathy as a treatment side effect that goes away because symptoms persist for years in nearly half of women,” said Kerri M. Winters-Stone, PhD, of Oregon Health and Science University in Portland.
“While there are no effective treatments for this side effect, rehabilitative exercise programs may preserve physical functioning and mobility in the presence of neuropathy to help prevent falls and resulting injuries.”
For this study, Dr Winters-Stone and her colleagues assessed data from 512 women enrolled in exercise intervention trials designed to address fractures and falls in female cancer survivors. Most of the women had breast cancer, but there were also cases of lung, colorectal, ovarian, and hematologic cancers.
At an average of 6 years post-cancer diagnosis, 46% of the women (n=238) still reported some symptoms of CIPN, such as loss of feeling in their hands and feet.
The investigators noted significant relationships (P<0.01) between CIPN severity and gait speed, Physical Performance Battery score, self-reported physical functioning, and self-reported disability.
The team also compared measures of physical functioning in the women with CIPN to measures in women without CIPN (n=274). This analysis was adjusted for cancer type and time since diagnosis.
There was a significant difference (P<0.01) between the groups in one measure of lower-extremity fitness but not another. Namely, it took CIPN-positive women significantly longer to rise out of a chair (tested 5 times each). But women in both groups fared similarly on a test measuring maximal leg press strength.
The investigators also tested the women on mobility and physical functioning. The CIPN-positive women fared significantly worse than CIPN-negative women (P<0.01) when it came to walking speed, step number, stride length, percentage of gait cycle in double support, and Physical Performance Battery score. However, there was no significant difference between the groups with regard to base of support.
Finally, CIPN-positive women were significantly more likely than CIPN-negative women to report poor physical function and disability (P<0.01 for both). And CIPN-positive women had a higher rate of falls in the last year (P<0.01).
The investigators said women with CIPN have specific underlying impairments that put them at risk for falls, which may be different from the impairments that occur with other conditions or old age.
For example, CIPN does not cause muscle weakness, but it has a distinct effect on movement and gait patterns.
The team noted that the women with CIPN had difficulty rising from a chair, possibly because their brains do not get enough information from their feet about how quickly or forcefully to stand up.
Based on these findings, the investigators argued that commonly recommended exercise, such as walking, may be safer for women with CIPN when done on a treadmill with handrails because their altered gait puts them at an increased risk of falling.
The team also said that machine-based resistance training may not be beneficial because neuropathy does not appear to decrease leg strength. Instead, rehabilitation efforts should focus on improving balance during upright movement and specific gait training.
Furthermore, the investigators believe that, if the symptoms of CIPN are detected early, cancer treatments could potentially be changed to prevent these debilitating problems or early rehabilitation interventions could be started.
In addition, Dr Winters-Stone and her research team are developing a smartphone-driven device that patients can use to detect and quantify symptoms of neuropathy, such as gait and balance impairments. ![]()
*Data in the abstract differ from the presentation.
Acupuncture no better than sham for relief of hot flashes
Chinese medicine needle acupuncture was about as effective as a sham blunt needle treatment in the relief of hot flashes, although women reported a 40% drop in symptoms with both treatments.
The findings, published online Jan. 18 in Annals of Internal Medicine, add to a growing, but conflicting body of evidence about the benefits of acupuncture in the treatment of menopause symptoms.
Prior to this study, two trials had demonstrated the effectiveness of acupuncture, compared with self care. And a pilot study had shown the effectiveness of acupuncture, compared with a noninsertive sham control. However, a Cochrane review found that acupuncture was more effective, compared with no treatment, and it had a moderate effect size, but was not effective when compared with a sham control (Cochrane Database Syst Rev. 2013 Jul 30;7:CD007410. doi:10.1002/14651858.CD007410.pub2).
The current trial, conducted at multiple sites in Australia, sought to add to the evidence with an adequately powered trial involving a sham control. But Carolyn Ee and her associates at the University of Melbourne noted that their study did not control for the nonspecific effects of acupuncture, such as regular interaction with a therapist.
The researchers randomly assigned 327 women aged older than 40 years who were in late menopause transition or postmenopause and experiencing at least seven moderate daily hot flashes to receive either a standardized Chinese medicine acupuncture treatment or a noninsertive, blunt needle sham acupuncture treatment. Patients received 10 treatments over 8 weeks, and they were assessed at 4 weeks, at the end of treatment, and at 3 and 6 months after treatment (Ann Intern Med. 2016; Jan 18. doi:10.7326/M15-1380.).
Both groups had about a 40% improvement in their hot flashes at the end of treatment, compared with their mean baseline hot flash score. The improvement was sustained at 3 and 6 months after the trial. In the acupuncture group, the mean hot flash scores at the end of treatment were 15.36, compared with 15.04 in the sham group, which was not statistically different. The researchers also found no advantage for acupuncture in quality of life, anxiety, or depression.
“Unless further high-quality evidence emerges, we cannot recommend skin-penetrating acupuncture as an efficacious treatment of this indication; the effects, if any, of acupuncture on these symptoms seem to be unrelated to needling,” the researchers wrote.
Some of the researchers reported receiving grant, scholarship, or fellowship support from the National Health and Medical Research Council of Australia, which funded the study.
On Twitter @maryellenny
Chinese medicine needle acupuncture was about as effective as a sham blunt needle treatment in the relief of hot flashes, although women reported a 40% drop in symptoms with both treatments.
The findings, published online Jan. 18 in Annals of Internal Medicine, add to a growing, but conflicting body of evidence about the benefits of acupuncture in the treatment of menopause symptoms.
Prior to this study, two trials had demonstrated the effectiveness of acupuncture, compared with self care. And a pilot study had shown the effectiveness of acupuncture, compared with a noninsertive sham control. However, a Cochrane review found that acupuncture was more effective, compared with no treatment, and it had a moderate effect size, but was not effective when compared with a sham control (Cochrane Database Syst Rev. 2013 Jul 30;7:CD007410. doi:10.1002/14651858.CD007410.pub2).
The current trial, conducted at multiple sites in Australia, sought to add to the evidence with an adequately powered trial involving a sham control. But Carolyn Ee and her associates at the University of Melbourne noted that their study did not control for the nonspecific effects of acupuncture, such as regular interaction with a therapist.
The researchers randomly assigned 327 women aged older than 40 years who were in late menopause transition or postmenopause and experiencing at least seven moderate daily hot flashes to receive either a standardized Chinese medicine acupuncture treatment or a noninsertive, blunt needle sham acupuncture treatment. Patients received 10 treatments over 8 weeks, and they were assessed at 4 weeks, at the end of treatment, and at 3 and 6 months after treatment (Ann Intern Med. 2016; Jan 18. doi:10.7326/M15-1380.).
Both groups had about a 40% improvement in their hot flashes at the end of treatment, compared with their mean baseline hot flash score. The improvement was sustained at 3 and 6 months after the trial. In the acupuncture group, the mean hot flash scores at the end of treatment were 15.36, compared with 15.04 in the sham group, which was not statistically different. The researchers also found no advantage for acupuncture in quality of life, anxiety, or depression.
“Unless further high-quality evidence emerges, we cannot recommend skin-penetrating acupuncture as an efficacious treatment of this indication; the effects, if any, of acupuncture on these symptoms seem to be unrelated to needling,” the researchers wrote.
Some of the researchers reported receiving grant, scholarship, or fellowship support from the National Health and Medical Research Council of Australia, which funded the study.
On Twitter @maryellenny
Chinese medicine needle acupuncture was about as effective as a sham blunt needle treatment in the relief of hot flashes, although women reported a 40% drop in symptoms with both treatments.
The findings, published online Jan. 18 in Annals of Internal Medicine, add to a growing, but conflicting body of evidence about the benefits of acupuncture in the treatment of menopause symptoms.
Prior to this study, two trials had demonstrated the effectiveness of acupuncture, compared with self care. And a pilot study had shown the effectiveness of acupuncture, compared with a noninsertive sham control. However, a Cochrane review found that acupuncture was more effective, compared with no treatment, and it had a moderate effect size, but was not effective when compared with a sham control (Cochrane Database Syst Rev. 2013 Jul 30;7:CD007410. doi:10.1002/14651858.CD007410.pub2).
The current trial, conducted at multiple sites in Australia, sought to add to the evidence with an adequately powered trial involving a sham control. But Carolyn Ee and her associates at the University of Melbourne noted that their study did not control for the nonspecific effects of acupuncture, such as regular interaction with a therapist.
The researchers randomly assigned 327 women aged older than 40 years who were in late menopause transition or postmenopause and experiencing at least seven moderate daily hot flashes to receive either a standardized Chinese medicine acupuncture treatment or a noninsertive, blunt needle sham acupuncture treatment. Patients received 10 treatments over 8 weeks, and they were assessed at 4 weeks, at the end of treatment, and at 3 and 6 months after treatment (Ann Intern Med. 2016; Jan 18. doi:10.7326/M15-1380.).
Both groups had about a 40% improvement in their hot flashes at the end of treatment, compared with their mean baseline hot flash score. The improvement was sustained at 3 and 6 months after the trial. In the acupuncture group, the mean hot flash scores at the end of treatment were 15.36, compared with 15.04 in the sham group, which was not statistically different. The researchers also found no advantage for acupuncture in quality of life, anxiety, or depression.
“Unless further high-quality evidence emerges, we cannot recommend skin-penetrating acupuncture as an efficacious treatment of this indication; the effects, if any, of acupuncture on these symptoms seem to be unrelated to needling,” the researchers wrote.
Some of the researchers reported receiving grant, scholarship, or fellowship support from the National Health and Medical Research Council of Australia, which funded the study.
On Twitter @maryellenny
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Chinese medicine acupuncture was no better than a sham treatment for the relief of hot flashes.
Major finding: After 8 weeks of treatment, mean hot flash scores were 15.36 in the acupuncture group and 15.04 in the sham treatment group, which was not a statistically significant difference.
Data source: A stratified, blind, parallel, randomized, sham-controlled trial of 327 women in late menopause transition or postmenopause.
Disclosures: Some of the researchers reported receiving grant, scholarship, or fellowship support from the National Health and Medical Research Council of Australia, which funded the study.
Secukinumab receives FDA approval for psoriatic arthritis, ankylosing spondylitis
The Food and Drug Administration approved two new indications for the interleukin-17A inhibitor secukinumab (Cosentyx) – psoriatic arthritis in adults and ankylosing spondylitis in adults – on Jan. 15. These join the approval for moderate to severe plaque psoriasis in adults it received in January 2015, according to an announcement from the drug’s manufacturer, Novartis.
The approvals are based on the efficacy and safety outcomes from four placebo-controlled, phase III studies, which included more than 1,500 adult patients with ankylosing spondylitis (AS) or psoriatic arthritis (PsA) who were biologic treatment naive or had an inadequate response or were intolerant to anti-TNF agents.
Pivotal phase III studies in the secukinumab clinical trial program, which provided key data for the submission, were MEASURE 1 and MEASURE 2 involving 590 patients with AS, and FUTURE 1 and FUTURE 2 involving 1,003 patients with PsA. Novartis continues to investigate the fully human monoclonal antibody against IL-17A for its potential in preventing radiographic progression of spinal and joint structural damage in AS and PsA patients, respectively.
The European Medicines Agency approved secukinumab for PsA and AS in November 2015.
The Food and Drug Administration approved two new indications for the interleukin-17A inhibitor secukinumab (Cosentyx) – psoriatic arthritis in adults and ankylosing spondylitis in adults – on Jan. 15. These join the approval for moderate to severe plaque psoriasis in adults it received in January 2015, according to an announcement from the drug’s manufacturer, Novartis.
The approvals are based on the efficacy and safety outcomes from four placebo-controlled, phase III studies, which included more than 1,500 adult patients with ankylosing spondylitis (AS) or psoriatic arthritis (PsA) who were biologic treatment naive or had an inadequate response or were intolerant to anti-TNF agents.
Pivotal phase III studies in the secukinumab clinical trial program, which provided key data for the submission, were MEASURE 1 and MEASURE 2 involving 590 patients with AS, and FUTURE 1 and FUTURE 2 involving 1,003 patients with PsA. Novartis continues to investigate the fully human monoclonal antibody against IL-17A for its potential in preventing radiographic progression of spinal and joint structural damage in AS and PsA patients, respectively.
The European Medicines Agency approved secukinumab for PsA and AS in November 2015.
The Food and Drug Administration approved two new indications for the interleukin-17A inhibitor secukinumab (Cosentyx) – psoriatic arthritis in adults and ankylosing spondylitis in adults – on Jan. 15. These join the approval for moderate to severe plaque psoriasis in adults it received in January 2015, according to an announcement from the drug’s manufacturer, Novartis.
The approvals are based on the efficacy and safety outcomes from four placebo-controlled, phase III studies, which included more than 1,500 adult patients with ankylosing spondylitis (AS) or psoriatic arthritis (PsA) who were biologic treatment naive or had an inadequate response or were intolerant to anti-TNF agents.
Pivotal phase III studies in the secukinumab clinical trial program, which provided key data for the submission, were MEASURE 1 and MEASURE 2 involving 590 patients with AS, and FUTURE 1 and FUTURE 2 involving 1,003 patients with PsA. Novartis continues to investigate the fully human monoclonal antibody against IL-17A for its potential in preventing radiographic progression of spinal and joint structural damage in AS and PsA patients, respectively.
The European Medicines Agency approved secukinumab for PsA and AS in November 2015.
Ignore your insurance company reminder about healthcare costs
In a letter dated Nov. 20, 2015, a particular insurance company reminded me that I need to think about healthcare costs. I got a breakdown of how often in the quarter I used proprietary vs. generic drugs, how I compared to other rheumatologists, and how I compared to other physicians. I also got a list of the drugs that I used and alternatives that I should be thinking about instead. In principle, this is not a terrible idea. But let me describe some glaring mistakes that show that these letters are in fact a contradiction in themselves. They are a huge waste of resources.
The letter suggested that my number one “prescribed brand drug with potential member savings opportunities” was Uloric, costing an average of $302 per prescription. The suggested “generic” alternative was Colcrys, leading to a “potential annual cost savings” of $600.
Third drug on my list? Colcrys, coming in at $165 per prescription with a potential annual savings of $300. Listed alternative: allopurinol.
We rheumatologists know that Colcrys is not an alternative to Uloric, and allopurinol is not an alternative to Colcrys. Also, suggesting an alternative only to suggest an alternative to that alternative is idiotic. Obviously, the letter is generated by a data-crunching algorithm. But an algorithm can only be as good as the programmer creating it.
As for my other proprietary prescriptions: Lyrica was the second on my list, and Celebrex the fourth. Let me explain why this is both annoying and inefficient. Before I could prescribe those drugs, this insurer made me jump through hoops to get them. In other words, there already exists in their database proof that I had already tried their recommended alternatives.
I can only conclude that within the bowels of health insurance corporate offices, and probably in more places than I care to imagine, there are people who are either incompetent or lazy, or both, making healthcare decisions.
So, to the health insurer: Forgive me if I ignore your reminder. I already know that healthcare costs are bloated. I am already quite conscientious about my prescribing practices. I think I can speak for all rheumatologists who receive these notices: You’re barking up the wrong tree.
Dr. Chan practices rheumatology in Pawtucket, R.I.
In a letter dated Nov. 20, 2015, a particular insurance company reminded me that I need to think about healthcare costs. I got a breakdown of how often in the quarter I used proprietary vs. generic drugs, how I compared to other rheumatologists, and how I compared to other physicians. I also got a list of the drugs that I used and alternatives that I should be thinking about instead. In principle, this is not a terrible idea. But let me describe some glaring mistakes that show that these letters are in fact a contradiction in themselves. They are a huge waste of resources.
The letter suggested that my number one “prescribed brand drug with potential member savings opportunities” was Uloric, costing an average of $302 per prescription. The suggested “generic” alternative was Colcrys, leading to a “potential annual cost savings” of $600.
Third drug on my list? Colcrys, coming in at $165 per prescription with a potential annual savings of $300. Listed alternative: allopurinol.
We rheumatologists know that Colcrys is not an alternative to Uloric, and allopurinol is not an alternative to Colcrys. Also, suggesting an alternative only to suggest an alternative to that alternative is idiotic. Obviously, the letter is generated by a data-crunching algorithm. But an algorithm can only be as good as the programmer creating it.
As for my other proprietary prescriptions: Lyrica was the second on my list, and Celebrex the fourth. Let me explain why this is both annoying and inefficient. Before I could prescribe those drugs, this insurer made me jump through hoops to get them. In other words, there already exists in their database proof that I had already tried their recommended alternatives.
I can only conclude that within the bowels of health insurance corporate offices, and probably in more places than I care to imagine, there are people who are either incompetent or lazy, or both, making healthcare decisions.
So, to the health insurer: Forgive me if I ignore your reminder. I already know that healthcare costs are bloated. I am already quite conscientious about my prescribing practices. I think I can speak for all rheumatologists who receive these notices: You’re barking up the wrong tree.
Dr. Chan practices rheumatology in Pawtucket, R.I.
In a letter dated Nov. 20, 2015, a particular insurance company reminded me that I need to think about healthcare costs. I got a breakdown of how often in the quarter I used proprietary vs. generic drugs, how I compared to other rheumatologists, and how I compared to other physicians. I also got a list of the drugs that I used and alternatives that I should be thinking about instead. In principle, this is not a terrible idea. But let me describe some glaring mistakes that show that these letters are in fact a contradiction in themselves. They are a huge waste of resources.
The letter suggested that my number one “prescribed brand drug with potential member savings opportunities” was Uloric, costing an average of $302 per prescription. The suggested “generic” alternative was Colcrys, leading to a “potential annual cost savings” of $600.
Third drug on my list? Colcrys, coming in at $165 per prescription with a potential annual savings of $300. Listed alternative: allopurinol.
We rheumatologists know that Colcrys is not an alternative to Uloric, and allopurinol is not an alternative to Colcrys. Also, suggesting an alternative only to suggest an alternative to that alternative is idiotic. Obviously, the letter is generated by a data-crunching algorithm. But an algorithm can only be as good as the programmer creating it.
As for my other proprietary prescriptions: Lyrica was the second on my list, and Celebrex the fourth. Let me explain why this is both annoying and inefficient. Before I could prescribe those drugs, this insurer made me jump through hoops to get them. In other words, there already exists in their database proof that I had already tried their recommended alternatives.
I can only conclude that within the bowels of health insurance corporate offices, and probably in more places than I care to imagine, there are people who are either incompetent or lazy, or both, making healthcare decisions.
So, to the health insurer: Forgive me if I ignore your reminder. I already know that healthcare costs are bloated. I am already quite conscientious about my prescribing practices. I think I can speak for all rheumatologists who receive these notices: You’re barking up the wrong tree.
Dr. Chan practices rheumatology in Pawtucket, R.I.
Pruritic Dermatitis Caused by Bird Mite Infestation
To the Editor:
There are a wide variety of zoonotic diseases that can be transmitted from birds to humans. Pigeons, chickens, starlings, canaries, and parakeets are known reservoirs of one particular zoonotic infection caused by the parasitic arthropod Dermanyssus gallinae.1 Dermanyssus gallinae (chicken mite) and Ornithonyssus sylviarum (northern fowl mite) are collectively referred to as bird mites. When these mites are unable to take blood meals from birds, they search out alternative hosts2; in humans, this often leads to the development of pruritic dermatitis.3
A 30-year-old woman presented to our clinic for evaluation of severe generalized pruritus accompanied by a sensation of “bugs on the skin” of 2 weeks’ duration. She noted the pruritus worsened when she was sitting outside on her porch. A few days prior to presentation, she noticed a small, “pinpoint-sized bug” on her arm (<1 mm in size), which she brought in for identification (Figure).

The bug was identified as a bird mite (Dermanyssus gallinae) on light microscopy, which was later confirmed by a medical entomologist. After the diagnosis of bird mite dermatitis was made, the patient noted there was a nest of starlings above the light on her porch. When she later investigated the nest following the current presentation, she noted many small mites crawling around the nest. The nest was removed and her symptoms resolved completely within 2 weeks without treatment.
Bird mites belong to the Arachnida class, under the order Acari. In 1958, Williams4 noted D gallinae’s ability to feed on human blood. Bird mites have 5 stages of development: egg, larva, protonymph, deutonymph, and adult. Protonymphs, deutonymphs, and adults can bite humans for a blood meal.5 Bird mites range from 0.3 to 1 mm in length and have nonsegmented, egg-shaped bodies with 4 pairs of legs. Before taking a blood meal, bird mites generally are a translucent brown color, and appear red when engorged with blood.2 Their small size makes them barely visible to the unaided eye. Of note, D gallinae and O sylviarum can be distinguished from each other based on subtle differences in morphology; for instance, the posterior genitoventral shield of O sylviarum is narrowly rounded, whereas it is broadly rounded in D gallinae. The dorsal shield of O sylviarum abruptly narrows posteriorly but is more smoothly narrowed in D gallinae.6 Additionally, O sylviarum tends to cause more irritating dermatitis in humans than D gallinae.3
Although they can be found worldwide, D gallinae and O sylviarum undergo optimal development at 20°C to 25°C and 70% humidity.3,5,7 Bird mites generally develop over the course of 5 to 12 days; thus, the population of bird mites in a single nest may grow to the tens of thousands before young birds permanently leave. Dermanyssus gallinae can survive for months in abandoned nests without a blood meal, while O sylviarum can survive for several weeks.8 It is important to note that humans are not ideal hosts for bird mites, as they are unable to survive for extended periods of time or reproduce on human hosts.9
When bird mites are no longer able to obtain blood meals from nesting birds, they begin their nocturnal migration to find suitable hosts. Bird nests generally are abandoned in late spring; thus, most patients with bird mite dermatitis present to clinics with bird mite dermatitis in late spring and early summer.10 Mites often travel through cracks in doors, floors, walls, and ceilings but also can gain access to living areas through ventilation ducts and air conditioning units.1 The mite’s bite and crawling on the skin is sometimes noticed by the patient. In general, however, intense itching is not observed until about 1 to 3 days after the mite makes contact with the skin. Patients often report that pruritus is worst at night.9 Papules and vesicles (bite reactions) may accompany the pruritus, and physicians commonly find bloody crust and excoriations in particularly pruritic areas.5 Urticarial plaques and diffuse erythema occasionally also may be present.9 Bird mites sometimes can be scraped from the skin and observed under light microscopy.11 Blood eosinophilia is not found in bird mite dermatitis. On histologic examination, perivascular eosinophilic infiltration can be seen in the upper part of the dermis.12
The differential diagnosis in patients with pruritic dermatitis of unknown origin generally includes scabies, pediculosis, and dermatitis caused by other types of infestation. However, unlike scabies, bird mites do not cause burrows to form on the skin.9 The presence of a bird’s nest near the area where the patient lives places bird mite dermatitis higher in the differential.
Dermanyssus gallinae is a known vector of bacteria (eg, Salmonella, Shigella, Staphylococcus, Spirochaete, Rickettsia, Pasteurella, Chlamydia psittaci, Erysipelothrix rhusiopathiae) as well as the viruses that cause Eastern and Western equine encephalitis and St. Louis encephalitis. Transmission of these bacteria and viruses is known in birds, but transmission to humans has not been reported.2,5,9,13
The management of bird mite dermatitis is straightforward. Usually mites can be successfully removed from the skin simply by bathing. Symptomatic treatment for bites with antihistamines and topical corticosteroids is sometimes but not always necessary.2 Unlike scabies or lice, there is no need for treatment with lindane.1 In terms of the prevention of additional bites, any bird nests located near living areas should be removed. Because bird mites often retreat back to nests between blood meals, insecticide sprays generally are unnecessary in interior spaces. Synthetic pyrethroids (eg, bifenthrin, cyfluthrin, cypermethrin, deltamethrin, cyhalothrin) can be used outside and in attics where nests may be located.2,14,15 However, the ability of bird mites to develop resistance to repeated chemical control could become a future concern.16
Research regarding the true incidence of bird mite dermatitis is lacking. Some researchers believe that the condition is underreported, possibly due to its uncommon environmental origin.3 Reports of bird mite dermatitis in the literature also are scarce. Our case demonstrates the importance of taking a thorough patient history to rule out exposure to bird mites. All patients with pruritic dermatitis of unknown origin should be questioned about possible contact or proximity to bird nests. These simple questions can lead to the correct diagnosis and a treatment plan that will quickly and effectively resolve the pruritic skin eruption.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Bellanger AP, Boris C, Foulet F, et al. Nosocomial dermatitis caused by Dermanyssus gallinae. Infect Cont Hosp Ep. 2008;29:282-283.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778) (Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- DiPalma A, Giangaspero A, Cafiero MA, et al. A gallery of the key characteristics to ease identification of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) and allow differentiation from Ornithonyssus sylviarum (Acari: Gamasida: Macronyssidae). Parasites and Vectors. 2012;5:104.
- Maurer V, Baumgartner J. Temperature influence on life table statistics of the chicken mite Dermanyssus gallinae (Acari: Dermanyssidae). Exp Appl Acarol. 1992;15:27-40.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exper Dermatol. 2000;25:129-131.
- Auger P, Nantel J, Meunier N, et al. Skin acariasis caused by Dermanyssus gallinae (de Geer): an in-hospital outbreak. Can Med Assoc J. 1979;120:700-703.
- Kong TK, To WK. Bird mite infestation. N Engl J Med. 2006;354:1728.
- Koh WL, Liu TT, Tay YK. Formication due to true parasitic infection: bird mites. Arch Dermatol. 2011;147:508-509.
- Hidano A, Asanuma K. Letter: Acariasis caused by bird mites. Arch Dermatol. 1976;112:881-882.
- Valiente Moro C, Chauve C, Zenner L. Experimental infection of Salmonella Enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Fletcher MG, Axtell RC. Susceptibilities of northern fowl mite, Ornithonyssus sylviarum (Acarina: Macronyssidae),and chicken mite, Dermanyssus gallinae (Acarina: Dermanyssidae), to selected acaricides. Exp Appl Acarol. 1991;13:137-142.
- Thind BB, Ford HL. Assessment of susceptibility of the poultry red mite Dermanyssus gallinae (Acari: Dermanyssidae) to some acaricides using an adapted filter paper based bioassay. Vet Parasitol. 2007;144:344-348.
- Chauve C. The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Vet Parasitol. 1998;79:239-245.
To the Editor:
There are a wide variety of zoonotic diseases that can be transmitted from birds to humans. Pigeons, chickens, starlings, canaries, and parakeets are known reservoirs of one particular zoonotic infection caused by the parasitic arthropod Dermanyssus gallinae.1 Dermanyssus gallinae (chicken mite) and Ornithonyssus sylviarum (northern fowl mite) are collectively referred to as bird mites. When these mites are unable to take blood meals from birds, they search out alternative hosts2; in humans, this often leads to the development of pruritic dermatitis.3
A 30-year-old woman presented to our clinic for evaluation of severe generalized pruritus accompanied by a sensation of “bugs on the skin” of 2 weeks’ duration. She noted the pruritus worsened when she was sitting outside on her porch. A few days prior to presentation, she noticed a small, “pinpoint-sized bug” on her arm (<1 mm in size), which she brought in for identification (Figure).

The bug was identified as a bird mite (Dermanyssus gallinae) on light microscopy, which was later confirmed by a medical entomologist. After the diagnosis of bird mite dermatitis was made, the patient noted there was a nest of starlings above the light on her porch. When she later investigated the nest following the current presentation, she noted many small mites crawling around the nest. The nest was removed and her symptoms resolved completely within 2 weeks without treatment.
Bird mites belong to the Arachnida class, under the order Acari. In 1958, Williams4 noted D gallinae’s ability to feed on human blood. Bird mites have 5 stages of development: egg, larva, protonymph, deutonymph, and adult. Protonymphs, deutonymphs, and adults can bite humans for a blood meal.5 Bird mites range from 0.3 to 1 mm in length and have nonsegmented, egg-shaped bodies with 4 pairs of legs. Before taking a blood meal, bird mites generally are a translucent brown color, and appear red when engorged with blood.2 Their small size makes them barely visible to the unaided eye. Of note, D gallinae and O sylviarum can be distinguished from each other based on subtle differences in morphology; for instance, the posterior genitoventral shield of O sylviarum is narrowly rounded, whereas it is broadly rounded in D gallinae. The dorsal shield of O sylviarum abruptly narrows posteriorly but is more smoothly narrowed in D gallinae.6 Additionally, O sylviarum tends to cause more irritating dermatitis in humans than D gallinae.3
Although they can be found worldwide, D gallinae and O sylviarum undergo optimal development at 20°C to 25°C and 70% humidity.3,5,7 Bird mites generally develop over the course of 5 to 12 days; thus, the population of bird mites in a single nest may grow to the tens of thousands before young birds permanently leave. Dermanyssus gallinae can survive for months in abandoned nests without a blood meal, while O sylviarum can survive for several weeks.8 It is important to note that humans are not ideal hosts for bird mites, as they are unable to survive for extended periods of time or reproduce on human hosts.9
When bird mites are no longer able to obtain blood meals from nesting birds, they begin their nocturnal migration to find suitable hosts. Bird nests generally are abandoned in late spring; thus, most patients with bird mite dermatitis present to clinics with bird mite dermatitis in late spring and early summer.10 Mites often travel through cracks in doors, floors, walls, and ceilings but also can gain access to living areas through ventilation ducts and air conditioning units.1 The mite’s bite and crawling on the skin is sometimes noticed by the patient. In general, however, intense itching is not observed until about 1 to 3 days after the mite makes contact with the skin. Patients often report that pruritus is worst at night.9 Papules and vesicles (bite reactions) may accompany the pruritus, and physicians commonly find bloody crust and excoriations in particularly pruritic areas.5 Urticarial plaques and diffuse erythema occasionally also may be present.9 Bird mites sometimes can be scraped from the skin and observed under light microscopy.11 Blood eosinophilia is not found in bird mite dermatitis. On histologic examination, perivascular eosinophilic infiltration can be seen in the upper part of the dermis.12
The differential diagnosis in patients with pruritic dermatitis of unknown origin generally includes scabies, pediculosis, and dermatitis caused by other types of infestation. However, unlike scabies, bird mites do not cause burrows to form on the skin.9 The presence of a bird’s nest near the area where the patient lives places bird mite dermatitis higher in the differential.
Dermanyssus gallinae is a known vector of bacteria (eg, Salmonella, Shigella, Staphylococcus, Spirochaete, Rickettsia, Pasteurella, Chlamydia psittaci, Erysipelothrix rhusiopathiae) as well as the viruses that cause Eastern and Western equine encephalitis and St. Louis encephalitis. Transmission of these bacteria and viruses is known in birds, but transmission to humans has not been reported.2,5,9,13
The management of bird mite dermatitis is straightforward. Usually mites can be successfully removed from the skin simply by bathing. Symptomatic treatment for bites with antihistamines and topical corticosteroids is sometimes but not always necessary.2 Unlike scabies or lice, there is no need for treatment with lindane.1 In terms of the prevention of additional bites, any bird nests located near living areas should be removed. Because bird mites often retreat back to nests between blood meals, insecticide sprays generally are unnecessary in interior spaces. Synthetic pyrethroids (eg, bifenthrin, cyfluthrin, cypermethrin, deltamethrin, cyhalothrin) can be used outside and in attics where nests may be located.2,14,15 However, the ability of bird mites to develop resistance to repeated chemical control could become a future concern.16
Research regarding the true incidence of bird mite dermatitis is lacking. Some researchers believe that the condition is underreported, possibly due to its uncommon environmental origin.3 Reports of bird mite dermatitis in the literature also are scarce. Our case demonstrates the importance of taking a thorough patient history to rule out exposure to bird mites. All patients with pruritic dermatitis of unknown origin should be questioned about possible contact or proximity to bird nests. These simple questions can lead to the correct diagnosis and a treatment plan that will quickly and effectively resolve the pruritic skin eruption.
To the Editor:
There are a wide variety of zoonotic diseases that can be transmitted from birds to humans. Pigeons, chickens, starlings, canaries, and parakeets are known reservoirs of one particular zoonotic infection caused by the parasitic arthropod Dermanyssus gallinae.1 Dermanyssus gallinae (chicken mite) and Ornithonyssus sylviarum (northern fowl mite) are collectively referred to as bird mites. When these mites are unable to take blood meals from birds, they search out alternative hosts2; in humans, this often leads to the development of pruritic dermatitis.3
A 30-year-old woman presented to our clinic for evaluation of severe generalized pruritus accompanied by a sensation of “bugs on the skin” of 2 weeks’ duration. She noted the pruritus worsened when she was sitting outside on her porch. A few days prior to presentation, she noticed a small, “pinpoint-sized bug” on her arm (<1 mm in size), which she brought in for identification (Figure).

The bug was identified as a bird mite (Dermanyssus gallinae) on light microscopy, which was later confirmed by a medical entomologist. After the diagnosis of bird mite dermatitis was made, the patient noted there was a nest of starlings above the light on her porch. When she later investigated the nest following the current presentation, she noted many small mites crawling around the nest. The nest was removed and her symptoms resolved completely within 2 weeks without treatment.
Bird mites belong to the Arachnida class, under the order Acari. In 1958, Williams4 noted D gallinae’s ability to feed on human blood. Bird mites have 5 stages of development: egg, larva, protonymph, deutonymph, and adult. Protonymphs, deutonymphs, and adults can bite humans for a blood meal.5 Bird mites range from 0.3 to 1 mm in length and have nonsegmented, egg-shaped bodies with 4 pairs of legs. Before taking a blood meal, bird mites generally are a translucent brown color, and appear red when engorged with blood.2 Their small size makes them barely visible to the unaided eye. Of note, D gallinae and O sylviarum can be distinguished from each other based on subtle differences in morphology; for instance, the posterior genitoventral shield of O sylviarum is narrowly rounded, whereas it is broadly rounded in D gallinae. The dorsal shield of O sylviarum abruptly narrows posteriorly but is more smoothly narrowed in D gallinae.6 Additionally, O sylviarum tends to cause more irritating dermatitis in humans than D gallinae.3
Although they can be found worldwide, D gallinae and O sylviarum undergo optimal development at 20°C to 25°C and 70% humidity.3,5,7 Bird mites generally develop over the course of 5 to 12 days; thus, the population of bird mites in a single nest may grow to the tens of thousands before young birds permanently leave. Dermanyssus gallinae can survive for months in abandoned nests without a blood meal, while O sylviarum can survive for several weeks.8 It is important to note that humans are not ideal hosts for bird mites, as they are unable to survive for extended periods of time or reproduce on human hosts.9
When bird mites are no longer able to obtain blood meals from nesting birds, they begin their nocturnal migration to find suitable hosts. Bird nests generally are abandoned in late spring; thus, most patients with bird mite dermatitis present to clinics with bird mite dermatitis in late spring and early summer.10 Mites often travel through cracks in doors, floors, walls, and ceilings but also can gain access to living areas through ventilation ducts and air conditioning units.1 The mite’s bite and crawling on the skin is sometimes noticed by the patient. In general, however, intense itching is not observed until about 1 to 3 days after the mite makes contact with the skin. Patients often report that pruritus is worst at night.9 Papules and vesicles (bite reactions) may accompany the pruritus, and physicians commonly find bloody crust and excoriations in particularly pruritic areas.5 Urticarial plaques and diffuse erythema occasionally also may be present.9 Bird mites sometimes can be scraped from the skin and observed under light microscopy.11 Blood eosinophilia is not found in bird mite dermatitis. On histologic examination, perivascular eosinophilic infiltration can be seen in the upper part of the dermis.12
The differential diagnosis in patients with pruritic dermatitis of unknown origin generally includes scabies, pediculosis, and dermatitis caused by other types of infestation. However, unlike scabies, bird mites do not cause burrows to form on the skin.9 The presence of a bird’s nest near the area where the patient lives places bird mite dermatitis higher in the differential.
Dermanyssus gallinae is a known vector of bacteria (eg, Salmonella, Shigella, Staphylococcus, Spirochaete, Rickettsia, Pasteurella, Chlamydia psittaci, Erysipelothrix rhusiopathiae) as well as the viruses that cause Eastern and Western equine encephalitis and St. Louis encephalitis. Transmission of these bacteria and viruses is known in birds, but transmission to humans has not been reported.2,5,9,13
The management of bird mite dermatitis is straightforward. Usually mites can be successfully removed from the skin simply by bathing. Symptomatic treatment for bites with antihistamines and topical corticosteroids is sometimes but not always necessary.2 Unlike scabies or lice, there is no need for treatment with lindane.1 In terms of the prevention of additional bites, any bird nests located near living areas should be removed. Because bird mites often retreat back to nests between blood meals, insecticide sprays generally are unnecessary in interior spaces. Synthetic pyrethroids (eg, bifenthrin, cyfluthrin, cypermethrin, deltamethrin, cyhalothrin) can be used outside and in attics where nests may be located.2,14,15 However, the ability of bird mites to develop resistance to repeated chemical control could become a future concern.16
Research regarding the true incidence of bird mite dermatitis is lacking. Some researchers believe that the condition is underreported, possibly due to its uncommon environmental origin.3 Reports of bird mite dermatitis in the literature also are scarce. Our case demonstrates the importance of taking a thorough patient history to rule out exposure to bird mites. All patients with pruritic dermatitis of unknown origin should be questioned about possible contact or proximity to bird nests. These simple questions can lead to the correct diagnosis and a treatment plan that will quickly and effectively resolve the pruritic skin eruption.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Bellanger AP, Boris C, Foulet F, et al. Nosocomial dermatitis caused by Dermanyssus gallinae. Infect Cont Hosp Ep. 2008;29:282-283.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778) (Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- DiPalma A, Giangaspero A, Cafiero MA, et al. A gallery of the key characteristics to ease identification of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) and allow differentiation from Ornithonyssus sylviarum (Acari: Gamasida: Macronyssidae). Parasites and Vectors. 2012;5:104.
- Maurer V, Baumgartner J. Temperature influence on life table statistics of the chicken mite Dermanyssus gallinae (Acari: Dermanyssidae). Exp Appl Acarol. 1992;15:27-40.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exper Dermatol. 2000;25:129-131.
- Auger P, Nantel J, Meunier N, et al. Skin acariasis caused by Dermanyssus gallinae (de Geer): an in-hospital outbreak. Can Med Assoc J. 1979;120:700-703.
- Kong TK, To WK. Bird mite infestation. N Engl J Med. 2006;354:1728.
- Koh WL, Liu TT, Tay YK. Formication due to true parasitic infection: bird mites. Arch Dermatol. 2011;147:508-509.
- Hidano A, Asanuma K. Letter: Acariasis caused by bird mites. Arch Dermatol. 1976;112:881-882.
- Valiente Moro C, Chauve C, Zenner L. Experimental infection of Salmonella Enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Fletcher MG, Axtell RC. Susceptibilities of northern fowl mite, Ornithonyssus sylviarum (Acarina: Macronyssidae),and chicken mite, Dermanyssus gallinae (Acarina: Dermanyssidae), to selected acaricides. Exp Appl Acarol. 1991;13:137-142.
- Thind BB, Ford HL. Assessment of susceptibility of the poultry red mite Dermanyssus gallinae (Acari: Dermanyssidae) to some acaricides using an adapted filter paper based bioassay. Vet Parasitol. 2007;144:344-348.
- Chauve C. The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Vet Parasitol. 1998;79:239-245.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Bellanger AP, Boris C, Foulet F, et al. Nosocomial dermatitis caused by Dermanyssus gallinae. Infect Cont Hosp Ep. 2008;29:282-283.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778) (Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- DiPalma A, Giangaspero A, Cafiero MA, et al. A gallery of the key characteristics to ease identification of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) and allow differentiation from Ornithonyssus sylviarum (Acari: Gamasida: Macronyssidae). Parasites and Vectors. 2012;5:104.
- Maurer V, Baumgartner J. Temperature influence on life table statistics of the chicken mite Dermanyssus gallinae (Acari: Dermanyssidae). Exp Appl Acarol. 1992;15:27-40.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exper Dermatol. 2000;25:129-131.
- Auger P, Nantel J, Meunier N, et al. Skin acariasis caused by Dermanyssus gallinae (de Geer): an in-hospital outbreak. Can Med Assoc J. 1979;120:700-703.
- Kong TK, To WK. Bird mite infestation. N Engl J Med. 2006;354:1728.
- Koh WL, Liu TT, Tay YK. Formication due to true parasitic infection: bird mites. Arch Dermatol. 2011;147:508-509.
- Hidano A, Asanuma K. Letter: Acariasis caused by bird mites. Arch Dermatol. 1976;112:881-882.
- Valiente Moro C, Chauve C, Zenner L. Experimental infection of Salmonella Enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Fletcher MG, Axtell RC. Susceptibilities of northern fowl mite, Ornithonyssus sylviarum (Acarina: Macronyssidae),and chicken mite, Dermanyssus gallinae (Acarina: Dermanyssidae), to selected acaricides. Exp Appl Acarol. 1991;13:137-142.
- Thind BB, Ford HL. Assessment of susceptibility of the poultry red mite Dermanyssus gallinae (Acari: Dermanyssidae) to some acaricides using an adapted filter paper based bioassay. Vet Parasitol. 2007;144:344-348.
- Chauve C. The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Vet Parasitol. 1998;79:239-245.
Bias and knowing too much about your patient
Years ago, I had a colleague who’d once worked for the prison system, treating people who were some of the more dangerous elements of society.
Once I asked if he’d ever gotten curious about what they were in for. He answered that, while he was always curious, he never asked. He felt as if knowing might prejudice his care. Since a key part of being a doctor is being impartial and objective, he was afraid that knowing about their previous heinous behavior would make him less concerned about treating them properly. And I agree.
When I was a younger doctor, I’d sometimes Google patients. I’d be curious about their backgrounds, or I wanted to see if there was anything on their social media I should be aware of they hadn’t told me. Maybe something like “I scored 20 percs off a neurologist today!”
I stopped after a while, and haven’t done it since. I never saw anything that would affect my treatment plan. I did, however, often learn about their political and religious views, some of which were distasteful to me. I respect anyone’s right to have an opinion, but that doesn’t mean I have to agree with them.
Like I’ve written before, I specifically avoid any discussion of religion or politics with my patients because doing so can lead to antagonism and dislike, with the potential to impact my objectivity.
The same can be said about what else you might learn online: their habits and hobbies, unflattering pictures, stories about their backgrounds, etc. All of those things can, in the right circumstances, lead to a bias against them. Perhaps it may just exist subconsciously, but it’s still there. A recent Medscape report noted the number of physicians who admitted having biases against patients, as well as the things that can trigger our visceral reactions: emotional state, weight, and intelligence, to name a few. We try hard to overcome negative feelings to provide proper care, but are still human and 100% objectivity is often difficult.
To me, Googling a patient became the same thing as asking inmates what they’d been locked up for: You learn things about them that might change how you view and care for them.
The only way to effectively treat patients is to see them as just people, like yourself. Knowing too much about their background that isn’t medically relevant is just asking for trouble.
I’d rather know less and be more objective.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Years ago, I had a colleague who’d once worked for the prison system, treating people who were some of the more dangerous elements of society.
Once I asked if he’d ever gotten curious about what they were in for. He answered that, while he was always curious, he never asked. He felt as if knowing might prejudice his care. Since a key part of being a doctor is being impartial and objective, he was afraid that knowing about their previous heinous behavior would make him less concerned about treating them properly. And I agree.
When I was a younger doctor, I’d sometimes Google patients. I’d be curious about their backgrounds, or I wanted to see if there was anything on their social media I should be aware of they hadn’t told me. Maybe something like “I scored 20 percs off a neurologist today!”
I stopped after a while, and haven’t done it since. I never saw anything that would affect my treatment plan. I did, however, often learn about their political and religious views, some of which were distasteful to me. I respect anyone’s right to have an opinion, but that doesn’t mean I have to agree with them.
Like I’ve written before, I specifically avoid any discussion of religion or politics with my patients because doing so can lead to antagonism and dislike, with the potential to impact my objectivity.
The same can be said about what else you might learn online: their habits and hobbies, unflattering pictures, stories about their backgrounds, etc. All of those things can, in the right circumstances, lead to a bias against them. Perhaps it may just exist subconsciously, but it’s still there. A recent Medscape report noted the number of physicians who admitted having biases against patients, as well as the things that can trigger our visceral reactions: emotional state, weight, and intelligence, to name a few. We try hard to overcome negative feelings to provide proper care, but are still human and 100% objectivity is often difficult.
To me, Googling a patient became the same thing as asking inmates what they’d been locked up for: You learn things about them that might change how you view and care for them.
The only way to effectively treat patients is to see them as just people, like yourself. Knowing too much about their background that isn’t medically relevant is just asking for trouble.
I’d rather know less and be more objective.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Years ago, I had a colleague who’d once worked for the prison system, treating people who were some of the more dangerous elements of society.
Once I asked if he’d ever gotten curious about what they were in for. He answered that, while he was always curious, he never asked. He felt as if knowing might prejudice his care. Since a key part of being a doctor is being impartial and objective, he was afraid that knowing about their previous heinous behavior would make him less concerned about treating them properly. And I agree.
When I was a younger doctor, I’d sometimes Google patients. I’d be curious about their backgrounds, or I wanted to see if there was anything on their social media I should be aware of they hadn’t told me. Maybe something like “I scored 20 percs off a neurologist today!”
I stopped after a while, and haven’t done it since. I never saw anything that would affect my treatment plan. I did, however, often learn about their political and religious views, some of which were distasteful to me. I respect anyone’s right to have an opinion, but that doesn’t mean I have to agree with them.
Like I’ve written before, I specifically avoid any discussion of religion or politics with my patients because doing so can lead to antagonism and dislike, with the potential to impact my objectivity.
The same can be said about what else you might learn online: their habits and hobbies, unflattering pictures, stories about their backgrounds, etc. All of those things can, in the right circumstances, lead to a bias against them. Perhaps it may just exist subconsciously, but it’s still there. A recent Medscape report noted the number of physicians who admitted having biases against patients, as well as the things that can trigger our visceral reactions: emotional state, weight, and intelligence, to name a few. We try hard to overcome negative feelings to provide proper care, but are still human and 100% objectivity is often difficult.
To me, Googling a patient became the same thing as asking inmates what they’d been locked up for: You learn things about them that might change how you view and care for them.
The only way to effectively treat patients is to see them as just people, like yourself. Knowing too much about their background that isn’t medically relevant is just asking for trouble.
I’d rather know less and be more objective.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.