User login
Pharmacist Involvement in Transitional Care Can Reduce Return ED Visits, Inpatient Readmissions
Clinical question: Does pharmacist involvement in transitions of care decrease medication errors (MEs), adverse drug events (ADEs), and 30-day ED visits and inpatient readmissions?
Background: Previous studies show pharmacist involvement in discharge can reduce ADEs and improve patient satisfaction, but there have been inconsistent data on the impact of pharmacist involvement on readmissions, ADEs, and MEs.
Study design: Prospective, randomized, single-period, longitudinal study.
Setting: Northwestern Memorial Hospital, Chicago.
Synopsis: Investigators included 278 patients (137 in study arm, 141 in control arm) in the final analysis. The study arm received intensive pharmacist involvement on admission and discharge, followed by phone calls at three, 14, and 30 days post-discharge. The study arm had lower composite 30-day ED visits and inpatient readmission rates compared to the control group (25% vs. 39%; P=0.001) but did not have lower isolated inpatient readmission rates (20% vs. 24%; P=0.43). ADEs and MEs were not significantly different between the two groups.
This study had extensive exclusion criteria, limiting the patient population to which these results can be applied. It was underpowered, which could have prevented the detection of a significant improvement in readmission rates.
Care transitions are high-risk periods in patient care, and there is benefit to continuity of care of an interdisciplinary team, including pharmacists.
Bottom line: Pharmacist involvement in transitions of care was shown to reduce the composite of ED visits and inpatient readmissions.
Citation: Phatak A, Prusi R, Ward B, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study). J Hosp Med. 2016;11(1):39-44. doi:10.1002/jhm.2493.
Clinical question: Does pharmacist involvement in transitions of care decrease medication errors (MEs), adverse drug events (ADEs), and 30-day ED visits and inpatient readmissions?
Background: Previous studies show pharmacist involvement in discharge can reduce ADEs and improve patient satisfaction, but there have been inconsistent data on the impact of pharmacist involvement on readmissions, ADEs, and MEs.
Study design: Prospective, randomized, single-period, longitudinal study.
Setting: Northwestern Memorial Hospital, Chicago.
Synopsis: Investigators included 278 patients (137 in study arm, 141 in control arm) in the final analysis. The study arm received intensive pharmacist involvement on admission and discharge, followed by phone calls at three, 14, and 30 days post-discharge. The study arm had lower composite 30-day ED visits and inpatient readmission rates compared to the control group (25% vs. 39%; P=0.001) but did not have lower isolated inpatient readmission rates (20% vs. 24%; P=0.43). ADEs and MEs were not significantly different between the two groups.
This study had extensive exclusion criteria, limiting the patient population to which these results can be applied. It was underpowered, which could have prevented the detection of a significant improvement in readmission rates.
Care transitions are high-risk periods in patient care, and there is benefit to continuity of care of an interdisciplinary team, including pharmacists.
Bottom line: Pharmacist involvement in transitions of care was shown to reduce the composite of ED visits and inpatient readmissions.
Citation: Phatak A, Prusi R, Ward B, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study). J Hosp Med. 2016;11(1):39-44. doi:10.1002/jhm.2493.
Clinical question: Does pharmacist involvement in transitions of care decrease medication errors (MEs), adverse drug events (ADEs), and 30-day ED visits and inpatient readmissions?
Background: Previous studies show pharmacist involvement in discharge can reduce ADEs and improve patient satisfaction, but there have been inconsistent data on the impact of pharmacist involvement on readmissions, ADEs, and MEs.
Study design: Prospective, randomized, single-period, longitudinal study.
Setting: Northwestern Memorial Hospital, Chicago.
Synopsis: Investigators included 278 patients (137 in study arm, 141 in control arm) in the final analysis. The study arm received intensive pharmacist involvement on admission and discharge, followed by phone calls at three, 14, and 30 days post-discharge. The study arm had lower composite 30-day ED visits and inpatient readmission rates compared to the control group (25% vs. 39%; P=0.001) but did not have lower isolated inpatient readmission rates (20% vs. 24%; P=0.43). ADEs and MEs were not significantly different between the two groups.
This study had extensive exclusion criteria, limiting the patient population to which these results can be applied. It was underpowered, which could have prevented the detection of a significant improvement in readmission rates.
Care transitions are high-risk periods in patient care, and there is benefit to continuity of care of an interdisciplinary team, including pharmacists.
Bottom line: Pharmacist involvement in transitions of care was shown to reduce the composite of ED visits and inpatient readmissions.
Citation: Phatak A, Prusi R, Ward B, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study). J Hosp Med. 2016;11(1):39-44. doi:10.1002/jhm.2493.
Protein’s role in thrombosis elucidated

Image by Andre E.X. Brown
Preclinical research helps explain how the protein lysyl oxidase (LOX) enhances platelet activation and thrombosis.
Previous studies showed that LOX is overexpressed in pathologies associated with thrombosis, including myeloproliferative neoplasms.
However, it wasn’t clear exactly how LOX leads to thrombosis.
So Shinobu Matsuuras, PhD, of Boston University School of Medicine in Massachusetts, and her colleagues attempted to find out.
The team reported their findings in Blood.
The researchers wanted to determine the role of LOX in thrombosis and platelet function without the compounding influences of other pathologies.
So they generated mice that expressed LOX in wild-type megakaryocytes and platelets—Pf4-Loxtg/tg mice.
These mice had the same number of platelets as control mice, but the platelets in Pf4-Loxtg/tg mice were more likely than normal platelets to form a thrombus.
The time to vessel occlusion after endothelial injury was significantly shorter in Pf4-Loxtg/tg mice than control mice. The average time to occlusion was about 35% shorter in Pf4-Loxtg/tg mice.
The reason for this, according to the researchers’ experiments, is that platelets from Pf4-Loxtg/tg mice adhere better to collagen and have a greater aggregation response to lower doses of collagen.
The researchers were also able to pinpoint the receptor on the platelets that was affected by the oxidation activity of LOX—integrin α2β1.
The team said this is the first study to show that LOX expression enhances platelet adhesion to collagen through integrin α2β1.
And the results suggest LOX could be a target for antithrombotic therapy in myeloproliferative neoplasms and other pathologies associated with LOX overexpression. ![]()

Image by Andre E.X. Brown
Preclinical research helps explain how the protein lysyl oxidase (LOX) enhances platelet activation and thrombosis.
Previous studies showed that LOX is overexpressed in pathologies associated with thrombosis, including myeloproliferative neoplasms.
However, it wasn’t clear exactly how LOX leads to thrombosis.
So Shinobu Matsuuras, PhD, of Boston University School of Medicine in Massachusetts, and her colleagues attempted to find out.
The team reported their findings in Blood.
The researchers wanted to determine the role of LOX in thrombosis and platelet function without the compounding influences of other pathologies.
So they generated mice that expressed LOX in wild-type megakaryocytes and platelets—Pf4-Loxtg/tg mice.
These mice had the same number of platelets as control mice, but the platelets in Pf4-Loxtg/tg mice were more likely than normal platelets to form a thrombus.
The time to vessel occlusion after endothelial injury was significantly shorter in Pf4-Loxtg/tg mice than control mice. The average time to occlusion was about 35% shorter in Pf4-Loxtg/tg mice.
The reason for this, according to the researchers’ experiments, is that platelets from Pf4-Loxtg/tg mice adhere better to collagen and have a greater aggregation response to lower doses of collagen.
The researchers were also able to pinpoint the receptor on the platelets that was affected by the oxidation activity of LOX—integrin α2β1.
The team said this is the first study to show that LOX expression enhances platelet adhesion to collagen through integrin α2β1.
And the results suggest LOX could be a target for antithrombotic therapy in myeloproliferative neoplasms and other pathologies associated with LOX overexpression. ![]()

Image by Andre E.X. Brown
Preclinical research helps explain how the protein lysyl oxidase (LOX) enhances platelet activation and thrombosis.
Previous studies showed that LOX is overexpressed in pathologies associated with thrombosis, including myeloproliferative neoplasms.
However, it wasn’t clear exactly how LOX leads to thrombosis.
So Shinobu Matsuuras, PhD, of Boston University School of Medicine in Massachusetts, and her colleagues attempted to find out.
The team reported their findings in Blood.
The researchers wanted to determine the role of LOX in thrombosis and platelet function without the compounding influences of other pathologies.
So they generated mice that expressed LOX in wild-type megakaryocytes and platelets—Pf4-Loxtg/tg mice.
These mice had the same number of platelets as control mice, but the platelets in Pf4-Loxtg/tg mice were more likely than normal platelets to form a thrombus.
The time to vessel occlusion after endothelial injury was significantly shorter in Pf4-Loxtg/tg mice than control mice. The average time to occlusion was about 35% shorter in Pf4-Loxtg/tg mice.
The reason for this, according to the researchers’ experiments, is that platelets from Pf4-Loxtg/tg mice adhere better to collagen and have a greater aggregation response to lower doses of collagen.
The researchers were also able to pinpoint the receptor on the platelets that was affected by the oxidation activity of LOX—integrin α2β1.
The team said this is the first study to show that LOX expression enhances platelet adhesion to collagen through integrin α2β1.
And the results suggest LOX could be a target for antithrombotic therapy in myeloproliferative neoplasms and other pathologies associated with LOX overexpression. ![]()
Converting hemoglobin to fetal-type state

A pair of transcription factors repress the expression of fetal hemoglobin and might therefore be targets for the treatment of hemoglobinopathies, according to research published in Science.
Previous studies have indicated that hemoglobinopathies may be treated by inducing fetal-type hemoglobin.
However, researchers have been unable to achieve this because they don’t fully understand the factors that influence adult hemoglobin, including those that repress fetal hemoglobin.
Takeshi Masuda, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues suspected that the transcription factor LRF might play a role in the differentiation between hemoglobin types.
To test this theory, the team knocked out the gene that encodes LRF, ZBTB7A, in mice.
This boosted the expression of genes known to play a role in the production of fetal-type hemoglobin but not adult-type hemoglobin. Knocking out ZBTB7A in human cells produced similar results.
The researchers said LRF/ZBTB7A occupies fetal γ-globin genes and maintains the nucleosome density necessary for γ-globin gene silencing in adults.
They also discovered that LRF represses the expression of fetal-type hemoglobin through a NuRD repressor complex independent of BCL11A, a transcription factor already known to repress fetal-type hemoglobin.
However, in mice with both ZBTB7A and BCL11A knocked out, fetal-type hemoglobin represented a greater percentage of total hemoglobin (91% to 94%) than when either gene was knocked out alone.
Therefore, the researchers believe that therapies targeting the 2 proteins these genes express—LRF and BCL11A—may offer a viable way to induce fetal-type hemoglobin in patients with hemoglobinopathies. ![]()

A pair of transcription factors repress the expression of fetal hemoglobin and might therefore be targets for the treatment of hemoglobinopathies, according to research published in Science.
Previous studies have indicated that hemoglobinopathies may be treated by inducing fetal-type hemoglobin.
However, researchers have been unable to achieve this because they don’t fully understand the factors that influence adult hemoglobin, including those that repress fetal hemoglobin.
Takeshi Masuda, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues suspected that the transcription factor LRF might play a role in the differentiation between hemoglobin types.
To test this theory, the team knocked out the gene that encodes LRF, ZBTB7A, in mice.
This boosted the expression of genes known to play a role in the production of fetal-type hemoglobin but not adult-type hemoglobin. Knocking out ZBTB7A in human cells produced similar results.
The researchers said LRF/ZBTB7A occupies fetal γ-globin genes and maintains the nucleosome density necessary for γ-globin gene silencing in adults.
They also discovered that LRF represses the expression of fetal-type hemoglobin through a NuRD repressor complex independent of BCL11A, a transcription factor already known to repress fetal-type hemoglobin.
However, in mice with both ZBTB7A and BCL11A knocked out, fetal-type hemoglobin represented a greater percentage of total hemoglobin (91% to 94%) than when either gene was knocked out alone.
Therefore, the researchers believe that therapies targeting the 2 proteins these genes express—LRF and BCL11A—may offer a viable way to induce fetal-type hemoglobin in patients with hemoglobinopathies. ![]()

A pair of transcription factors repress the expression of fetal hemoglobin and might therefore be targets for the treatment of hemoglobinopathies, according to research published in Science.
Previous studies have indicated that hemoglobinopathies may be treated by inducing fetal-type hemoglobin.
However, researchers have been unable to achieve this because they don’t fully understand the factors that influence adult hemoglobin, including those that repress fetal hemoglobin.
Takeshi Masuda, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues suspected that the transcription factor LRF might play a role in the differentiation between hemoglobin types.
To test this theory, the team knocked out the gene that encodes LRF, ZBTB7A, in mice.
This boosted the expression of genes known to play a role in the production of fetal-type hemoglobin but not adult-type hemoglobin. Knocking out ZBTB7A in human cells produced similar results.
The researchers said LRF/ZBTB7A occupies fetal γ-globin genes and maintains the nucleosome density necessary for γ-globin gene silencing in adults.
They also discovered that LRF represses the expression of fetal-type hemoglobin through a NuRD repressor complex independent of BCL11A, a transcription factor already known to repress fetal-type hemoglobin.
However, in mice with both ZBTB7A and BCL11A knocked out, fetal-type hemoglobin represented a greater percentage of total hemoglobin (91% to 94%) than when either gene was knocked out alone.
Therefore, the researchers believe that therapies targeting the 2 proteins these genes express—LRF and BCL11A—may offer a viable way to induce fetal-type hemoglobin in patients with hemoglobinopathies. ![]()
NICE releases guideline on sepsis

Photo courtesy of the CDC
The National Institute for Health and Care Excellence (NICE) has published a new draft guideline to help healthcare professionals recognize sepsis and provide early treatment.
Although treatable in many cases, sepsis can be difficult to recognize and diagnose.
A recent study* of more than 3000 patients treated at UK hospitals revealed delays in identifying sepsis in more than a third of cases.
“There are around 123,000 cases of sepsis in England every year, and, unfortunately, thousands of people die after developing the condition,” said Mark Baker, director of the centre for clinical practice at NICE.
“Many of these deaths might be prevented if sepsis was recognized quickly and treatment started early. We know that when hospitals are well-prepared, clinicians do better at responding to patients with sepsis. However, recent reports have revealed that a third of hospitals have no formal protocols for recognizing and responding to sepsis.”
NICE created its draft guidance with that in mind. The guidance provides recommendations to aid in the recognition, diagnosis, and early management of sepsis, including:
- What signs and symptoms to look out for
- How to identify patients at high risk of developing sepsis
- Which tests to use to diagnose and monitor patients
- How to care for people with suspected sepsis outside of the hospital
- When patients should be referred for emergency care
- Appropriate use of antibiotics and other supportive treatments, such as fluids and oxygen
- The type of information that should be given to patients, families, and caregivers.
“We want all healthcare professionals to see sepsis as an immediate, life-threatening condition and make sure there are systems in place across the NHS for it to be recognized and treated as an emergency,” Baker said.
“This new guideline will be the first to provide evidence-based best practice advice on how to quickly identify and treat people with sepsis. We now urge all healthcare professionals and organizations with an interest in this area to comment on the proposed recommendations.”
The draft version of this guideline has been published for consultation. Organizations can register as a stakeholder on the NICE website and have until February 22, 2016, to submit their comments. Individuals are advised to pass comments through a registered stakeholder organization that most closely represents them. ![]()
*Published in “Just Say Sepsis!”—a report by the National Confidential Enquiry into Patient Outcome and Death.

Photo courtesy of the CDC
The National Institute for Health and Care Excellence (NICE) has published a new draft guideline to help healthcare professionals recognize sepsis and provide early treatment.
Although treatable in many cases, sepsis can be difficult to recognize and diagnose.
A recent study* of more than 3000 patients treated at UK hospitals revealed delays in identifying sepsis in more than a third of cases.
“There are around 123,000 cases of sepsis in England every year, and, unfortunately, thousands of people die after developing the condition,” said Mark Baker, director of the centre for clinical practice at NICE.
“Many of these deaths might be prevented if sepsis was recognized quickly and treatment started early. We know that when hospitals are well-prepared, clinicians do better at responding to patients with sepsis. However, recent reports have revealed that a third of hospitals have no formal protocols for recognizing and responding to sepsis.”
NICE created its draft guidance with that in mind. The guidance provides recommendations to aid in the recognition, diagnosis, and early management of sepsis, including:
- What signs and symptoms to look out for
- How to identify patients at high risk of developing sepsis
- Which tests to use to diagnose and monitor patients
- How to care for people with suspected sepsis outside of the hospital
- When patients should be referred for emergency care
- Appropriate use of antibiotics and other supportive treatments, such as fluids and oxygen
- The type of information that should be given to patients, families, and caregivers.
“We want all healthcare professionals to see sepsis as an immediate, life-threatening condition and make sure there are systems in place across the NHS for it to be recognized and treated as an emergency,” Baker said.
“This new guideline will be the first to provide evidence-based best practice advice on how to quickly identify and treat people with sepsis. We now urge all healthcare professionals and organizations with an interest in this area to comment on the proposed recommendations.”
The draft version of this guideline has been published for consultation. Organizations can register as a stakeholder on the NICE website and have until February 22, 2016, to submit their comments. Individuals are advised to pass comments through a registered stakeholder organization that most closely represents them. ![]()
*Published in “Just Say Sepsis!”—a report by the National Confidential Enquiry into Patient Outcome and Death.

Photo courtesy of the CDC
The National Institute for Health and Care Excellence (NICE) has published a new draft guideline to help healthcare professionals recognize sepsis and provide early treatment.
Although treatable in many cases, sepsis can be difficult to recognize and diagnose.
A recent study* of more than 3000 patients treated at UK hospitals revealed delays in identifying sepsis in more than a third of cases.
“There are around 123,000 cases of sepsis in England every year, and, unfortunately, thousands of people die after developing the condition,” said Mark Baker, director of the centre for clinical practice at NICE.
“Many of these deaths might be prevented if sepsis was recognized quickly and treatment started early. We know that when hospitals are well-prepared, clinicians do better at responding to patients with sepsis. However, recent reports have revealed that a third of hospitals have no formal protocols for recognizing and responding to sepsis.”
NICE created its draft guidance with that in mind. The guidance provides recommendations to aid in the recognition, diagnosis, and early management of sepsis, including:
- What signs and symptoms to look out for
- How to identify patients at high risk of developing sepsis
- Which tests to use to diagnose and monitor patients
- How to care for people with suspected sepsis outside of the hospital
- When patients should be referred for emergency care
- Appropriate use of antibiotics and other supportive treatments, such as fluids and oxygen
- The type of information that should be given to patients, families, and caregivers.
“We want all healthcare professionals to see sepsis as an immediate, life-threatening condition and make sure there are systems in place across the NHS for it to be recognized and treated as an emergency,” Baker said.
“This new guideline will be the first to provide evidence-based best practice advice on how to quickly identify and treat people with sepsis. We now urge all healthcare professionals and organizations with an interest in this area to comment on the proposed recommendations.”
The draft version of this guideline has been published for consultation. Organizations can register as a stakeholder on the NICE website and have until February 22, 2016, to submit their comments. Individuals are advised to pass comments through a registered stakeholder organization that most closely represents them. ![]()
*Published in “Just Say Sepsis!”—a report by the National Confidential Enquiry into Patient Outcome and Death.
Cancer survival tied to reduction in treatment

Photo by Bill Branson
Results of a large study suggest that long-term survivors of childhood cancer are living longer, partly due to a reduction in the use of certain treatments.
The 15-year death rate among the more than 34,000 childhood cancer survivors studied decreased steadily from 1970 onward.
And this decline coincided with changes in pediatric cancer therapy, including reductions in the use and dose of radiation therapy and anthracyclines.
These therapies are known to put cancer survivors at increased risk for developing second malignancies, heart failure, and other serious health problems.
“This study is the first to show that younger survivors from more recent treatment eras are less likely to die from the late effects of cancer treatment and more likely to enjoy longer lives,” said study author Greg Armstrong, MD, of St. Jude Children's Research Hospital in Memphis, Tennessee.
He and his colleagues reported these results in NEJM.
The study included 34,033 subjects who had been diagnosed with cancer and received treatment between 1970 and 1999 when they were age 20 or younger. All patients lived at least 5 years after their cancers were discovered and were considered long-term survivors.
Changes in mortality
At a median follow-up of 21 years (range, 5 to 38), there were 3958 deaths. Forty-one percent of deaths (n=1618) were considered health-related. This included 746 deaths from subsequent neoplasms, 241 from cardiac causes, 137 from pulmonary causes, and 494 from other causes.
The 15-year death rate (death from any cause) fell from 12.4% in the early 1970s to 6% in the 1990s (P<0.001). During the same period, the rate of death from health-related causes fell from 3.5% to 2.1% (P<0.001).
The researchers said there were significant reductions across treatment eras in the rates of death from any health-related cause among patients with acute lymphoblastic leukemia (ALL), Hodgkin lymphoma (HL), Wilms’ tumor, and astrocytoma, but not among patients with other cancers.
The rate of health-related death among ALL patients fell from 3.2% in the early 1970s to 2.1% in the 1990s (P<0.001). The rate fell from 5.3% to 2.6% (P=0.006) for HL patients, from 2.6% to 0.4% (P=0.005) for Wilms’ tumor patients, and from 4.7% to 1.8% (P=0.02) for astrocytoma patients.
The researchers said these reductions in mortality were attributable to decreases in the rates of death from subsequent neoplasm (P<0.001), cardiac causes (P<0.001), and pulmonary causes (P=0.04).
Treatment changes
The overall use of anthracyclines fell from 73% in the 1970s to 42% in the 1990s. And the use of any radiation decreased from 77% to 41%.
The use of cranial radiotherapy for ALL fell from 85% to 19%. The use of abdominal radiotherapy for Wilms’ tumor decreased from 78% to 43%. And the use of chest radiotherapy for HL fell from 87% to 61%.
The researchers noted that temporal reductions in 15-year rates of death from health-related causes followed temporal reductions in therapeutic exposure for patients with ALL, HL, Wilms’ tumor, and astrocytoma.
However, when the team adjusted their analysis for therapy (eg, anthracycline dose), the effect of the treatment era on the relative rate of death from health-related causes was attenuated in ALL (unadjusted relative rate=0.88, adjusted relative rate=1.02) and Wilms’ tumor (0.68 and 0.80, respectively) but not in HL (0.79 in both models) and astrocytoma (0.81 and 0.82, respectively).
Still, the researchers said the results of this study suggest the strategy of lowering therapeutic exposure has contributed to the decline in late mortality among 5-year survivors of childhood cancer. ![]()

Photo by Bill Branson
Results of a large study suggest that long-term survivors of childhood cancer are living longer, partly due to a reduction in the use of certain treatments.
The 15-year death rate among the more than 34,000 childhood cancer survivors studied decreased steadily from 1970 onward.
And this decline coincided with changes in pediatric cancer therapy, including reductions in the use and dose of radiation therapy and anthracyclines.
These therapies are known to put cancer survivors at increased risk for developing second malignancies, heart failure, and other serious health problems.
“This study is the first to show that younger survivors from more recent treatment eras are less likely to die from the late effects of cancer treatment and more likely to enjoy longer lives,” said study author Greg Armstrong, MD, of St. Jude Children's Research Hospital in Memphis, Tennessee.
He and his colleagues reported these results in NEJM.
The study included 34,033 subjects who had been diagnosed with cancer and received treatment between 1970 and 1999 when they were age 20 or younger. All patients lived at least 5 years after their cancers were discovered and were considered long-term survivors.
Changes in mortality
At a median follow-up of 21 years (range, 5 to 38), there were 3958 deaths. Forty-one percent of deaths (n=1618) were considered health-related. This included 746 deaths from subsequent neoplasms, 241 from cardiac causes, 137 from pulmonary causes, and 494 from other causes.
The 15-year death rate (death from any cause) fell from 12.4% in the early 1970s to 6% in the 1990s (P<0.001). During the same period, the rate of death from health-related causes fell from 3.5% to 2.1% (P<0.001).
The researchers said there were significant reductions across treatment eras in the rates of death from any health-related cause among patients with acute lymphoblastic leukemia (ALL), Hodgkin lymphoma (HL), Wilms’ tumor, and astrocytoma, but not among patients with other cancers.
The rate of health-related death among ALL patients fell from 3.2% in the early 1970s to 2.1% in the 1990s (P<0.001). The rate fell from 5.3% to 2.6% (P=0.006) for HL patients, from 2.6% to 0.4% (P=0.005) for Wilms’ tumor patients, and from 4.7% to 1.8% (P=0.02) for astrocytoma patients.
The researchers said these reductions in mortality were attributable to decreases in the rates of death from subsequent neoplasm (P<0.001), cardiac causes (P<0.001), and pulmonary causes (P=0.04).
Treatment changes
The overall use of anthracyclines fell from 73% in the 1970s to 42% in the 1990s. And the use of any radiation decreased from 77% to 41%.
The use of cranial radiotherapy for ALL fell from 85% to 19%. The use of abdominal radiotherapy for Wilms’ tumor decreased from 78% to 43%. And the use of chest radiotherapy for HL fell from 87% to 61%.
The researchers noted that temporal reductions in 15-year rates of death from health-related causes followed temporal reductions in therapeutic exposure for patients with ALL, HL, Wilms’ tumor, and astrocytoma.
However, when the team adjusted their analysis for therapy (eg, anthracycline dose), the effect of the treatment era on the relative rate of death from health-related causes was attenuated in ALL (unadjusted relative rate=0.88, adjusted relative rate=1.02) and Wilms’ tumor (0.68 and 0.80, respectively) but not in HL (0.79 in both models) and astrocytoma (0.81 and 0.82, respectively).
Still, the researchers said the results of this study suggest the strategy of lowering therapeutic exposure has contributed to the decline in late mortality among 5-year survivors of childhood cancer. ![]()

Photo by Bill Branson
Results of a large study suggest that long-term survivors of childhood cancer are living longer, partly due to a reduction in the use of certain treatments.
The 15-year death rate among the more than 34,000 childhood cancer survivors studied decreased steadily from 1970 onward.
And this decline coincided with changes in pediatric cancer therapy, including reductions in the use and dose of radiation therapy and anthracyclines.
These therapies are known to put cancer survivors at increased risk for developing second malignancies, heart failure, and other serious health problems.
“This study is the first to show that younger survivors from more recent treatment eras are less likely to die from the late effects of cancer treatment and more likely to enjoy longer lives,” said study author Greg Armstrong, MD, of St. Jude Children's Research Hospital in Memphis, Tennessee.
He and his colleagues reported these results in NEJM.
The study included 34,033 subjects who had been diagnosed with cancer and received treatment between 1970 and 1999 when they were age 20 or younger. All patients lived at least 5 years after their cancers were discovered and were considered long-term survivors.
Changes in mortality
At a median follow-up of 21 years (range, 5 to 38), there were 3958 deaths. Forty-one percent of deaths (n=1618) were considered health-related. This included 746 deaths from subsequent neoplasms, 241 from cardiac causes, 137 from pulmonary causes, and 494 from other causes.
The 15-year death rate (death from any cause) fell from 12.4% in the early 1970s to 6% in the 1990s (P<0.001). During the same period, the rate of death from health-related causes fell from 3.5% to 2.1% (P<0.001).
The researchers said there were significant reductions across treatment eras in the rates of death from any health-related cause among patients with acute lymphoblastic leukemia (ALL), Hodgkin lymphoma (HL), Wilms’ tumor, and astrocytoma, but not among patients with other cancers.
The rate of health-related death among ALL patients fell from 3.2% in the early 1970s to 2.1% in the 1990s (P<0.001). The rate fell from 5.3% to 2.6% (P=0.006) for HL patients, from 2.6% to 0.4% (P=0.005) for Wilms’ tumor patients, and from 4.7% to 1.8% (P=0.02) for astrocytoma patients.
The researchers said these reductions in mortality were attributable to decreases in the rates of death from subsequent neoplasm (P<0.001), cardiac causes (P<0.001), and pulmonary causes (P=0.04).
Treatment changes
The overall use of anthracyclines fell from 73% in the 1970s to 42% in the 1990s. And the use of any radiation decreased from 77% to 41%.
The use of cranial radiotherapy for ALL fell from 85% to 19%. The use of abdominal radiotherapy for Wilms’ tumor decreased from 78% to 43%. And the use of chest radiotherapy for HL fell from 87% to 61%.
The researchers noted that temporal reductions in 15-year rates of death from health-related causes followed temporal reductions in therapeutic exposure for patients with ALL, HL, Wilms’ tumor, and astrocytoma.
However, when the team adjusted their analysis for therapy (eg, anthracycline dose), the effect of the treatment era on the relative rate of death from health-related causes was attenuated in ALL (unadjusted relative rate=0.88, adjusted relative rate=1.02) and Wilms’ tumor (0.68 and 0.80, respectively) but not in HL (0.79 in both models) and astrocytoma (0.81 and 0.82, respectively).
Still, the researchers said the results of this study suggest the strategy of lowering therapeutic exposure has contributed to the decline in late mortality among 5-year survivors of childhood cancer. ![]()
No benefit in open massage over closed compressions in trauma cardiac arrest
SAN ANTONIO – Open-chest cardiac massage offers no benefit over closed-chest compressions in patients with traumatic cardiac arrest, according to a prospective observational study from the University of Maryland Shock Trauma Center in Baltimore.
The investigators compared 16 open-chest cardiac massage (OCCM) patients with 17 closed-chest compression (CCC) patients delivered directly to the level 1 trauma center in cardiac arrest. The open-massage group received closed compressions for a mean of 66 seconds before being converted to open massage for reasons that weren’t captured by the data.
End-tidal carbon dioxide (ETCO2) – the gold standard for determining the effectiveness of chest compressions and return of spontaneous circulation – was used as a surrogate for cardiac output and adequacy of resuscitation. Continuous high-resolution ETCO2 measurements were collected every 6 seconds in both groups.
When periods of OCCM were compared to equivalent periods of CCC, there were no differences in the initial, final, peak, or mean ETCO2 values, and there was no difference in return of spontaneous circulation (OCCM, 23.5% versus CCC, 38.9%; P = .53).
“Unless the patient has a thoracic injury that you need to get into the chest to fix, we didn’t see any benefit in opening the chest just to massage the heart. The data suggest that maybe we shouldn’t be so aggressive in doing open cardiac massage. There’s renewed interest in performing endovascular balloon occlusion techniques for the aorta to obtain hemorrhage control; if you do that and you do closed-chest compressions, it’s just as effective as opening up the chest and doing cardiac massage,” said investigator Dr. Matthew Bradley, a trauma surgeon at the Shock Trauma Center, at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Most of the patients were men, and there was a higher percentage of penetrating trauma in the OCCM group (81% versus 47%; P = .04).
The results were the same, however, in subgroup analyses limited to blunt and penetrating trauma.
All of the open massage patients died, but there were a few survivors in the CCC group. Dr. Bradley didn’t think the closed versus open approach was the reason for the survival difference.
Resuscitative endovascular balloon occlusion of the aorta patients were excluded from the trial to prevent confounding.
The investigators have no relevant disclosures.
SAN ANTONIO – Open-chest cardiac massage offers no benefit over closed-chest compressions in patients with traumatic cardiac arrest, according to a prospective observational study from the University of Maryland Shock Trauma Center in Baltimore.
The investigators compared 16 open-chest cardiac massage (OCCM) patients with 17 closed-chest compression (CCC) patients delivered directly to the level 1 trauma center in cardiac arrest. The open-massage group received closed compressions for a mean of 66 seconds before being converted to open massage for reasons that weren’t captured by the data.
End-tidal carbon dioxide (ETCO2) – the gold standard for determining the effectiveness of chest compressions and return of spontaneous circulation – was used as a surrogate for cardiac output and adequacy of resuscitation. Continuous high-resolution ETCO2 measurements were collected every 6 seconds in both groups.
When periods of OCCM were compared to equivalent periods of CCC, there were no differences in the initial, final, peak, or mean ETCO2 values, and there was no difference in return of spontaneous circulation (OCCM, 23.5% versus CCC, 38.9%; P = .53).
“Unless the patient has a thoracic injury that you need to get into the chest to fix, we didn’t see any benefit in opening the chest just to massage the heart. The data suggest that maybe we shouldn’t be so aggressive in doing open cardiac massage. There’s renewed interest in performing endovascular balloon occlusion techniques for the aorta to obtain hemorrhage control; if you do that and you do closed-chest compressions, it’s just as effective as opening up the chest and doing cardiac massage,” said investigator Dr. Matthew Bradley, a trauma surgeon at the Shock Trauma Center, at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Most of the patients were men, and there was a higher percentage of penetrating trauma in the OCCM group (81% versus 47%; P = .04).
The results were the same, however, in subgroup analyses limited to blunt and penetrating trauma.
All of the open massage patients died, but there were a few survivors in the CCC group. Dr. Bradley didn’t think the closed versus open approach was the reason for the survival difference.
Resuscitative endovascular balloon occlusion of the aorta patients were excluded from the trial to prevent confounding.
The investigators have no relevant disclosures.
SAN ANTONIO – Open-chest cardiac massage offers no benefit over closed-chest compressions in patients with traumatic cardiac arrest, according to a prospective observational study from the University of Maryland Shock Trauma Center in Baltimore.
The investigators compared 16 open-chest cardiac massage (OCCM) patients with 17 closed-chest compression (CCC) patients delivered directly to the level 1 trauma center in cardiac arrest. The open-massage group received closed compressions for a mean of 66 seconds before being converted to open massage for reasons that weren’t captured by the data.
End-tidal carbon dioxide (ETCO2) – the gold standard for determining the effectiveness of chest compressions and return of spontaneous circulation – was used as a surrogate for cardiac output and adequacy of resuscitation. Continuous high-resolution ETCO2 measurements were collected every 6 seconds in both groups.
When periods of OCCM were compared to equivalent periods of CCC, there were no differences in the initial, final, peak, or mean ETCO2 values, and there was no difference in return of spontaneous circulation (OCCM, 23.5% versus CCC, 38.9%; P = .53).
“Unless the patient has a thoracic injury that you need to get into the chest to fix, we didn’t see any benefit in opening the chest just to massage the heart. The data suggest that maybe we shouldn’t be so aggressive in doing open cardiac massage. There’s renewed interest in performing endovascular balloon occlusion techniques for the aorta to obtain hemorrhage control; if you do that and you do closed-chest compressions, it’s just as effective as opening up the chest and doing cardiac massage,” said investigator Dr. Matthew Bradley, a trauma surgeon at the Shock Trauma Center, at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Most of the patients were men, and there was a higher percentage of penetrating trauma in the OCCM group (81% versus 47%; P = .04).
The results were the same, however, in subgroup analyses limited to blunt and penetrating trauma.
All of the open massage patients died, but there were a few survivors in the CCC group. Dr. Bradley didn’t think the closed versus open approach was the reason for the survival difference.
Resuscitative endovascular balloon occlusion of the aorta patients were excluded from the trial to prevent confounding.
The investigators have no relevant disclosures.
AT THE EAST SCIENTIFIC ASSEMBLY
Key clinical point: There’s no benefit in opening the chest just to massage the heart.
Major finding: When periods of OCCM were compared to equivalent periods of CCC, there were no differences in initial, final, peak, or mean ETCO2 values, and there was no difference in return of spontaneous circulation (OCCM, 23.5% versus CCC, 38.9%; P = .53).
Data source: Prospective observational study in 33 patients
Disclosures: The investigators have no relevant disclosures.
Trauma hospitalists reduce mortality, readmissions
SAN ANTONIO – Embedding a hospitalist in the trauma service reduces mortality and readmissions, according to a review from Christiana Care Health System’s Level 1 trauma center in Newark, Del.
Investigators wanted to see how their trauma hospitalist program – launched in 2013 – was working, so they matched 469 patients who were comanaged by a hospitalist in 2014 to 938 patients who were not, based on age, injury severity score, and comorbidities.
“We were pleasantly surprised to see a dramatic reduction in mortality [2.9% to 0.4%] and 30-day trauma-related readmissions [2.3% to 0.6%]” when hospitalists were involved with care, said Dr. Mark Cipolle, chief of trauma surgery at the center. The findings were statistically significant.
More hospitalist patients were upgraded to the ICU [4.3% versus 2.1%], and hospitalist patients stayed in the hospital about a day and half longer. The increased ICU upgrades is probably from the extra vigilance trauma hospitalists bring to the team. As for length of stay, hospitalists probably “kept patients an extra day to tune up their diabetes, hypertension,” and other problems, and ensure they had good follow-up. “We strongly feel that many of these patients go home with their comorbidities better managed than when they came in,” Dr. Cipolle said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Hospitalists also seemed to improve patient satisfaction on surveys.
Christiana currently employs about eight trauma hospitalists in Newark who rotate through week-long shifts. They’ve become so central to trauma care there that Dr. Cipolle suspects it would be difficult to find patients without hospitalist management for a new control group.
His hospitalists tend to work with older patients and manage comorbidities, especially insulin-dependent diabetes, heart failure, other significant heart disease, and chronic renal injury. Trauma surgeons, meanwhile, manage injury-related issues, such as additional diagnostics and pain control.
There can be some disagreements when two attendings care for the same patient, but, overall, “we get along pretty darn well,” Dr. Cipolle said.
Hospitalists in the study made no significant difference in the frequency of cardiology, nephrology, neurology, or endocrinology consultations. There was no difference in the development of venous thromboembolism, pneumonia, stroke, urinary tract infection, bacteremia, or alcohol withdrawal.
The patients were about 72 years old, on average, with a mean injury severity score of 10. Roughly 8% were recovering from a stroke, about 10% had heart failure, a quarter were diabetic, and three-quarters were hypertensive.
Dr. Cipolle said he has no relevant disclosures.
SAN ANTONIO – Embedding a hospitalist in the trauma service reduces mortality and readmissions, according to a review from Christiana Care Health System’s Level 1 trauma center in Newark, Del.
Investigators wanted to see how their trauma hospitalist program – launched in 2013 – was working, so they matched 469 patients who were comanaged by a hospitalist in 2014 to 938 patients who were not, based on age, injury severity score, and comorbidities.
“We were pleasantly surprised to see a dramatic reduction in mortality [2.9% to 0.4%] and 30-day trauma-related readmissions [2.3% to 0.6%]” when hospitalists were involved with care, said Dr. Mark Cipolle, chief of trauma surgery at the center. The findings were statistically significant.
More hospitalist patients were upgraded to the ICU [4.3% versus 2.1%], and hospitalist patients stayed in the hospital about a day and half longer. The increased ICU upgrades is probably from the extra vigilance trauma hospitalists bring to the team. As for length of stay, hospitalists probably “kept patients an extra day to tune up their diabetes, hypertension,” and other problems, and ensure they had good follow-up. “We strongly feel that many of these patients go home with their comorbidities better managed than when they came in,” Dr. Cipolle said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Hospitalists also seemed to improve patient satisfaction on surveys.
Christiana currently employs about eight trauma hospitalists in Newark who rotate through week-long shifts. They’ve become so central to trauma care there that Dr. Cipolle suspects it would be difficult to find patients without hospitalist management for a new control group.
His hospitalists tend to work with older patients and manage comorbidities, especially insulin-dependent diabetes, heart failure, other significant heart disease, and chronic renal injury. Trauma surgeons, meanwhile, manage injury-related issues, such as additional diagnostics and pain control.
There can be some disagreements when two attendings care for the same patient, but, overall, “we get along pretty darn well,” Dr. Cipolle said.
Hospitalists in the study made no significant difference in the frequency of cardiology, nephrology, neurology, or endocrinology consultations. There was no difference in the development of venous thromboembolism, pneumonia, stroke, urinary tract infection, bacteremia, or alcohol withdrawal.
The patients were about 72 years old, on average, with a mean injury severity score of 10. Roughly 8% were recovering from a stroke, about 10% had heart failure, a quarter were diabetic, and three-quarters were hypertensive.
Dr. Cipolle said he has no relevant disclosures.
SAN ANTONIO – Embedding a hospitalist in the trauma service reduces mortality and readmissions, according to a review from Christiana Care Health System’s Level 1 trauma center in Newark, Del.
Investigators wanted to see how their trauma hospitalist program – launched in 2013 – was working, so they matched 469 patients who were comanaged by a hospitalist in 2014 to 938 patients who were not, based on age, injury severity score, and comorbidities.
“We were pleasantly surprised to see a dramatic reduction in mortality [2.9% to 0.4%] and 30-day trauma-related readmissions [2.3% to 0.6%]” when hospitalists were involved with care, said Dr. Mark Cipolle, chief of trauma surgery at the center. The findings were statistically significant.
More hospitalist patients were upgraded to the ICU [4.3% versus 2.1%], and hospitalist patients stayed in the hospital about a day and half longer. The increased ICU upgrades is probably from the extra vigilance trauma hospitalists bring to the team. As for length of stay, hospitalists probably “kept patients an extra day to tune up their diabetes, hypertension,” and other problems, and ensure they had good follow-up. “We strongly feel that many of these patients go home with their comorbidities better managed than when they came in,” Dr. Cipolle said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Hospitalists also seemed to improve patient satisfaction on surveys.
Christiana currently employs about eight trauma hospitalists in Newark who rotate through week-long shifts. They’ve become so central to trauma care there that Dr. Cipolle suspects it would be difficult to find patients without hospitalist management for a new control group.
His hospitalists tend to work with older patients and manage comorbidities, especially insulin-dependent diabetes, heart failure, other significant heart disease, and chronic renal injury. Trauma surgeons, meanwhile, manage injury-related issues, such as additional diagnostics and pain control.
There can be some disagreements when two attendings care for the same patient, but, overall, “we get along pretty darn well,” Dr. Cipolle said.
Hospitalists in the study made no significant difference in the frequency of cardiology, nephrology, neurology, or endocrinology consultations. There was no difference in the development of venous thromboembolism, pneumonia, stroke, urinary tract infection, bacteremia, or alcohol withdrawal.
The patients were about 72 years old, on average, with a mean injury severity score of 10. Roughly 8% were recovering from a stroke, about 10% had heart failure, a quarter were diabetic, and three-quarters were hypertensive.
Dr. Cipolle said he has no relevant disclosures.
AT THE EAST SCIENTIFIC ASSEMBLY
Key clinical point: The addition of a hospitalist to the trauma service resulted in statistically significant improvements in mortality and readmissions.
Major finding: When hospitalists were added to the trauma team, mortality fell from 2.9 to 0.4%, and 30-day readmissions fell from 2.3 to 0.6%.
Data source: Retrospective, single-center study of 1,407 trauma patients.
Disclosures: Dr. Cipolle said he has no relevant disclosures.
The Diagnosis Either You Know or You Don’t
A 54-year-old woman self-refers to dermatology for evaluation of asymptomatic lesions on her legs. Over the past several years, they have slowly grown larger, redder, and shinier. She denies any other skin problems. Medical history is significant for type 2 diabetes, which, according to the patient, is under good control.
The patient has sought care from a variety of professionals, including her primary care provider, her endocrinologist, and her gynecologist. Various diagnoses, including “fungal infection,” have been posited; treatment with antifungal cream and oral medications produced no effect.
She also consulted the proprietor of her local health food store, who actually examined her before diagnosing “yeast infection” and advising her to change her diet.
EXAMINATION
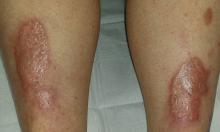
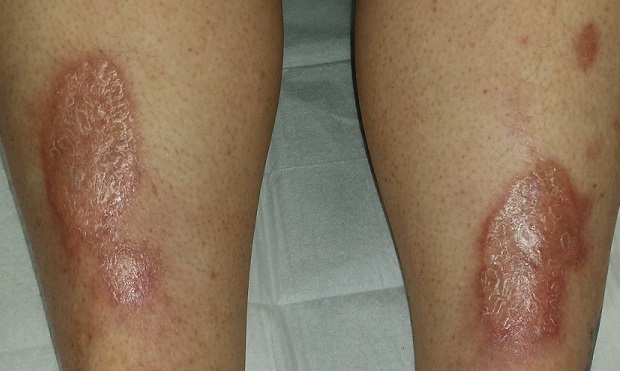
There are large, oval, reddish brown shiny plaques, measuring about 8 x 4 cm, on both anterior tibial areas. On closer inspection, the affected skin is yellowish pink and appears quite atrophic. This effect is more pronounced toward the centers of the lesions, which also display distinct telangiectasias. The borders of the plaques are slightly raised.
Examination of the rest of the patient’s skin reveals no other abnormalities.
What is the diagnosis?
DISCUSSION
Necrobiosis lipoidica (NL), though uncommon, is far from rare; it is seen somewhat regularly in dermatology practices. As this case demonstrates, it is one of hundreds of dermatologic diagnoses that you either know about or you don’t. Providers in the latter group invariably call it “fungal infection” because of its rounded borders—and because they simply have nothing else to call it.
True fungal infections are usually very superficial, involving only the outer layer of skin. This means they will manifest with scaling, a feature notably absent in this case. But more to the point, what was needed was a specific diagnosis, rather than another blind attempt at empiric treatment. Correct diagnosis dictates correct treatment.
NL was first described by Oppenhein in 1929, when he saw it in a diabetic patient. But it got its modern name in 1932 from Urbach, who thought it was invariably connected to diabetes. Now we know nondiabetic persons can develop it as well.
A number of theories exist as to the origins of this condition. One pinpoints microangiopathy of the same sort seen in the kidneys and eyes of diabetic patients. Another, proposed to account for the fact that NL is not exclusively seen in diabetes, holds that an autoimmune process might be involved—an opinion bolstered by the finding of increased TNF-alpha in the sera and skin of NL patients.
In any case, these atrophic plaques can grow quite large, eventually breaking down and ulcerating focally. Although the condition is usually asymptomatic, 25% of NL patients with advanced disease report pain.
Definitive diagnosis is made by punch biopsy, though visual diagnosis is quite adequate for those who can recognize the condition. NL lesions are most commonly seen on bilateral anterior tibial areas but can occasionally develop on the arm, face, or even genitals. The lesions can also koebnerize (ie, form along lines of trauma), occasionally being seen in surgical scars or even insulin injection sites.
Treatment, though largely unsatisfactory, includes topical and intralesional steroids. Particular care is taken to avoid or at least limit the formation of ulcers. In advanced cases, patients are referred to a wound care specialist. Oddly enough, neither the severity nor the prognosis of NL seem to have anything to do with how well or poorly the patient’s diabetes is controlled.
This patient was started on topical clobetasol cream, to be applied mostly to the actively advancing peripheral margin. Her prognosis, as with all NL patients, is guarded.
TAKE-HOME LEARNING POINTS
• Necrobiosis lipoidica (NL) is a disease of collagen degeneration that induces a granulomatous response that manifests microscopically with microangiopathy.
• NL usually appears on bilateral anterior tibial areas (but can affect the arms, face, or genitalia), as pinkish brown plaques with atrophic centers that tend to be yellow and telangiectatic.
• Though NL is usually associated with diabetes, it can develop in nondiabetic persons as well.
• The most feared complication of NL is eventual ulceration, which is why treatment is directed at taking care to avoid wounds to the plaques.
A 54-year-old woman self-refers to dermatology for evaluation of asymptomatic lesions on her legs. Over the past several years, they have slowly grown larger, redder, and shinier. She denies any other skin problems. Medical history is significant for type 2 diabetes, which, according to the patient, is under good control.
The patient has sought care from a variety of professionals, including her primary care provider, her endocrinologist, and her gynecologist. Various diagnoses, including “fungal infection,” have been posited; treatment with antifungal cream and oral medications produced no effect.
She also consulted the proprietor of her local health food store, who actually examined her before diagnosing “yeast infection” and advising her to change her diet.
EXAMINATION
There are large, oval, reddish brown shiny plaques, measuring about 8 x 4 cm, on both anterior tibial areas. On closer inspection, the affected skin is yellowish pink and appears quite atrophic. This effect is more pronounced toward the centers of the lesions, which also display distinct telangiectasias. The borders of the plaques are slightly raised.
Examination of the rest of the patient’s skin reveals no other abnormalities.
What is the diagnosis?
DISCUSSION
Necrobiosis lipoidica (NL), though uncommon, is far from rare; it is seen somewhat regularly in dermatology practices. As this case demonstrates, it is one of hundreds of dermatologic diagnoses that you either know about or you don’t. Providers in the latter group invariably call it “fungal infection” because of its rounded borders—and because they simply have nothing else to call it.
True fungal infections are usually very superficial, involving only the outer layer of skin. This means they will manifest with scaling, a feature notably absent in this case. But more to the point, what was needed was a specific diagnosis, rather than another blind attempt at empiric treatment. Correct diagnosis dictates correct treatment.
NL was first described by Oppenhein in 1929, when he saw it in a diabetic patient. But it got its modern name in 1932 from Urbach, who thought it was invariably connected to diabetes. Now we know nondiabetic persons can develop it as well.
A number of theories exist as to the origins of this condition. One pinpoints microangiopathy of the same sort seen in the kidneys and eyes of diabetic patients. Another, proposed to account for the fact that NL is not exclusively seen in diabetes, holds that an autoimmune process might be involved—an opinion bolstered by the finding of increased TNF-alpha in the sera and skin of NL patients.
In any case, these atrophic plaques can grow quite large, eventually breaking down and ulcerating focally. Although the condition is usually asymptomatic, 25% of NL patients with advanced disease report pain.
Definitive diagnosis is made by punch biopsy, though visual diagnosis is quite adequate for those who can recognize the condition. NL lesions are most commonly seen on bilateral anterior tibial areas but can occasionally develop on the arm, face, or even genitals. The lesions can also koebnerize (ie, form along lines of trauma), occasionally being seen in surgical scars or even insulin injection sites.
Treatment, though largely unsatisfactory, includes topical and intralesional steroids. Particular care is taken to avoid or at least limit the formation of ulcers. In advanced cases, patients are referred to a wound care specialist. Oddly enough, neither the severity nor the prognosis of NL seem to have anything to do with how well or poorly the patient’s diabetes is controlled.
This patient was started on topical clobetasol cream, to be applied mostly to the actively advancing peripheral margin. Her prognosis, as with all NL patients, is guarded.
TAKE-HOME LEARNING POINTS
• Necrobiosis lipoidica (NL) is a disease of collagen degeneration that induces a granulomatous response that manifests microscopically with microangiopathy.
• NL usually appears on bilateral anterior tibial areas (but can affect the arms, face, or genitalia), as pinkish brown plaques with atrophic centers that tend to be yellow and telangiectatic.
• Though NL is usually associated with diabetes, it can develop in nondiabetic persons as well.
• The most feared complication of NL is eventual ulceration, which is why treatment is directed at taking care to avoid wounds to the plaques.
A 54-year-old woman self-refers to dermatology for evaluation of asymptomatic lesions on her legs. Over the past several years, they have slowly grown larger, redder, and shinier. She denies any other skin problems. Medical history is significant for type 2 diabetes, which, according to the patient, is under good control.
The patient has sought care from a variety of professionals, including her primary care provider, her endocrinologist, and her gynecologist. Various diagnoses, including “fungal infection,” have been posited; treatment with antifungal cream and oral medications produced no effect.
She also consulted the proprietor of her local health food store, who actually examined her before diagnosing “yeast infection” and advising her to change her diet.
EXAMINATION
There are large, oval, reddish brown shiny plaques, measuring about 8 x 4 cm, on both anterior tibial areas. On closer inspection, the affected skin is yellowish pink and appears quite atrophic. This effect is more pronounced toward the centers of the lesions, which also display distinct telangiectasias. The borders of the plaques are slightly raised.
Examination of the rest of the patient’s skin reveals no other abnormalities.
What is the diagnosis?
DISCUSSION
Necrobiosis lipoidica (NL), though uncommon, is far from rare; it is seen somewhat regularly in dermatology practices. As this case demonstrates, it is one of hundreds of dermatologic diagnoses that you either know about or you don’t. Providers in the latter group invariably call it “fungal infection” because of its rounded borders—and because they simply have nothing else to call it.
True fungal infections are usually very superficial, involving only the outer layer of skin. This means they will manifest with scaling, a feature notably absent in this case. But more to the point, what was needed was a specific diagnosis, rather than another blind attempt at empiric treatment. Correct diagnosis dictates correct treatment.
NL was first described by Oppenhein in 1929, when he saw it in a diabetic patient. But it got its modern name in 1932 from Urbach, who thought it was invariably connected to diabetes. Now we know nondiabetic persons can develop it as well.
A number of theories exist as to the origins of this condition. One pinpoints microangiopathy of the same sort seen in the kidneys and eyes of diabetic patients. Another, proposed to account for the fact that NL is not exclusively seen in diabetes, holds that an autoimmune process might be involved—an opinion bolstered by the finding of increased TNF-alpha in the sera and skin of NL patients.
In any case, these atrophic plaques can grow quite large, eventually breaking down and ulcerating focally. Although the condition is usually asymptomatic, 25% of NL patients with advanced disease report pain.
Definitive diagnosis is made by punch biopsy, though visual diagnosis is quite adequate for those who can recognize the condition. NL lesions are most commonly seen on bilateral anterior tibial areas but can occasionally develop on the arm, face, or even genitals. The lesions can also koebnerize (ie, form along lines of trauma), occasionally being seen in surgical scars or even insulin injection sites.
Treatment, though largely unsatisfactory, includes topical and intralesional steroids. Particular care is taken to avoid or at least limit the formation of ulcers. In advanced cases, patients are referred to a wound care specialist. Oddly enough, neither the severity nor the prognosis of NL seem to have anything to do with how well or poorly the patient’s diabetes is controlled.
This patient was started on topical clobetasol cream, to be applied mostly to the actively advancing peripheral margin. Her prognosis, as with all NL patients, is guarded.
TAKE-HOME LEARNING POINTS
• Necrobiosis lipoidica (NL) is a disease of collagen degeneration that induces a granulomatous response that manifests microscopically with microangiopathy.
• NL usually appears on bilateral anterior tibial areas (but can affect the arms, face, or genitalia), as pinkish brown plaques with atrophic centers that tend to be yellow and telangiectatic.
• Though NL is usually associated with diabetes, it can develop in nondiabetic persons as well.
• The most feared complication of NL is eventual ulceration, which is why treatment is directed at taking care to avoid wounds to the plaques.
Blocking two targets boosted fetal hemoglobin expression
Two distinct proteins appear to control the switch from fetal to adult globin, based on studies performed in a humanized mouse model and human cells. The findings suggest therapies that target both proteins might induce a fetal-type globin state, which could prove therapeutically useful in individuals with human hemoglobinopathies such as sickle cell disease and thalassemia.
The leukemia/lymphoma-related factor (LRF) and B-cell lymphoma/leukemia 11A (BCL11A) are independently involved in the switch, and blocking their production may turn on fetal globin expression, Takeshi Masuda, Ph.D., of Brigham and Women’s Hospital and Harvard Medical School, Boston, and his colleagues report (SCIENCE. 2015 Jan 15;351[6270]:285-9)
Using a humanized mouse model, the researchers knocked out the ZBTB7A gene, which is responsible for producing LRF. This action boosted the expression of genes that control fetal but not adult-type hemoglobin. Knocking out the gene in human cells also resulted in an increase in fetal hemoglobin proteins.
The researchers then examined BCL11A, which is involved with fetal hemoglobin but does not suppress it. When genes were knocked out for both ZBTB7A and BCL11A in the mice, fetal hemoglobin represented a 91%-94% greater percentage of total hemoglobin than when either gene alone was knocked out.
The research was supported by awards and/or grants from the National Institute of Diabetes and Digestive and Kidney Disease, the Doris Duke Charitable Foundation, the National Institutes of Health, and the American Society of Hematology. Dr. Masuda, along with two other study authors, is a contributor to a patent application filed on behalf of Brigham and Women’s Hospital related to therapeutic targeting of the pathways.
Click here to read the study at Science.
Two distinct proteins appear to control the switch from fetal to adult globin, based on studies performed in a humanized mouse model and human cells. The findings suggest therapies that target both proteins might induce a fetal-type globin state, which could prove therapeutically useful in individuals with human hemoglobinopathies such as sickle cell disease and thalassemia.
The leukemia/lymphoma-related factor (LRF) and B-cell lymphoma/leukemia 11A (BCL11A) are independently involved in the switch, and blocking their production may turn on fetal globin expression, Takeshi Masuda, Ph.D., of Brigham and Women’s Hospital and Harvard Medical School, Boston, and his colleagues report (SCIENCE. 2015 Jan 15;351[6270]:285-9)
Using a humanized mouse model, the researchers knocked out the ZBTB7A gene, which is responsible for producing LRF. This action boosted the expression of genes that control fetal but not adult-type hemoglobin. Knocking out the gene in human cells also resulted in an increase in fetal hemoglobin proteins.
The researchers then examined BCL11A, which is involved with fetal hemoglobin but does not suppress it. When genes were knocked out for both ZBTB7A and BCL11A in the mice, fetal hemoglobin represented a 91%-94% greater percentage of total hemoglobin than when either gene alone was knocked out.
The research was supported by awards and/or grants from the National Institute of Diabetes and Digestive and Kidney Disease, the Doris Duke Charitable Foundation, the National Institutes of Health, and the American Society of Hematology. Dr. Masuda, along with two other study authors, is a contributor to a patent application filed on behalf of Brigham and Women’s Hospital related to therapeutic targeting of the pathways.
Click here to read the study at Science.
Two distinct proteins appear to control the switch from fetal to adult globin, based on studies performed in a humanized mouse model and human cells. The findings suggest therapies that target both proteins might induce a fetal-type globin state, which could prove therapeutically useful in individuals with human hemoglobinopathies such as sickle cell disease and thalassemia.
The leukemia/lymphoma-related factor (LRF) and B-cell lymphoma/leukemia 11A (BCL11A) are independently involved in the switch, and blocking their production may turn on fetal globin expression, Takeshi Masuda, Ph.D., of Brigham and Women’s Hospital and Harvard Medical School, Boston, and his colleagues report (SCIENCE. 2015 Jan 15;351[6270]:285-9)
Using a humanized mouse model, the researchers knocked out the ZBTB7A gene, which is responsible for producing LRF. This action boosted the expression of genes that control fetal but not adult-type hemoglobin. Knocking out the gene in human cells also resulted in an increase in fetal hemoglobin proteins.
The researchers then examined BCL11A, which is involved with fetal hemoglobin but does not suppress it. When genes were knocked out for both ZBTB7A and BCL11A in the mice, fetal hemoglobin represented a 91%-94% greater percentage of total hemoglobin than when either gene alone was knocked out.
The research was supported by awards and/or grants from the National Institute of Diabetes and Digestive and Kidney Disease, the Doris Duke Charitable Foundation, the National Institutes of Health, and the American Society of Hematology. Dr. Masuda, along with two other study authors, is a contributor to a patent application filed on behalf of Brigham and Women’s Hospital related to therapeutic targeting of the pathways.
Click here to read the study at Science.
FROM SCIENCE
FDA rejects antisense oligonucleotide drug for Duchenne muscular dystrophy
The Food and Drug Administration has decided against approving the exon 51-skipping antisense oligonucleotide drug drisapersen for forms of Duchenne muscular dystrophy amenable to exon skipping.
The agency’s complete response letter to BioMarin Pharmaceutical, the developer of drisapersen (Kyndrisa), said that the standard of substantial evidence of effectiveness had not been met, according to a written statement from the company on Jan. 14.
In a Nov. 24, 2015, meeting of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee, panel members generally felt that drisapersen’s efficacy data were not persuasive enough for an approval.
Drisapersen targets frame-disrupting mutations found in exon 51 of the dystrophin gene, which produces a nonfunctional protein in individuals with a certain form of Duchenne, by restoring expression of the mutated dystrophin gene. There are currently no FDA-approved drugs to treat Duchenne, which affects approximately 1 in every 3,500 to 1 in 5,000 male children, making it the most common fatal genetic disorder diagnosed in childhood.
BioMarin said that extension studies of drisapersen will continue as the company determines the next steps in its new drug application, as will the ongoing clinical trials for other exon-skipping oligonucleotides it is developing. The drug is still under review by the European Medicines Agency.
The Food and Drug Administration has decided against approving the exon 51-skipping antisense oligonucleotide drug drisapersen for forms of Duchenne muscular dystrophy amenable to exon skipping.
The agency’s complete response letter to BioMarin Pharmaceutical, the developer of drisapersen (Kyndrisa), said that the standard of substantial evidence of effectiveness had not been met, according to a written statement from the company on Jan. 14.
In a Nov. 24, 2015, meeting of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee, panel members generally felt that drisapersen’s efficacy data were not persuasive enough for an approval.
Drisapersen targets frame-disrupting mutations found in exon 51 of the dystrophin gene, which produces a nonfunctional protein in individuals with a certain form of Duchenne, by restoring expression of the mutated dystrophin gene. There are currently no FDA-approved drugs to treat Duchenne, which affects approximately 1 in every 3,500 to 1 in 5,000 male children, making it the most common fatal genetic disorder diagnosed in childhood.
BioMarin said that extension studies of drisapersen will continue as the company determines the next steps in its new drug application, as will the ongoing clinical trials for other exon-skipping oligonucleotides it is developing. The drug is still under review by the European Medicines Agency.
The Food and Drug Administration has decided against approving the exon 51-skipping antisense oligonucleotide drug drisapersen for forms of Duchenne muscular dystrophy amenable to exon skipping.
The agency’s complete response letter to BioMarin Pharmaceutical, the developer of drisapersen (Kyndrisa), said that the standard of substantial evidence of effectiveness had not been met, according to a written statement from the company on Jan. 14.
In a Nov. 24, 2015, meeting of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee, panel members generally felt that drisapersen’s efficacy data were not persuasive enough for an approval.
Drisapersen targets frame-disrupting mutations found in exon 51 of the dystrophin gene, which produces a nonfunctional protein in individuals with a certain form of Duchenne, by restoring expression of the mutated dystrophin gene. There are currently no FDA-approved drugs to treat Duchenne, which affects approximately 1 in every 3,500 to 1 in 5,000 male children, making it the most common fatal genetic disorder diagnosed in childhood.
BioMarin said that extension studies of drisapersen will continue as the company determines the next steps in its new drug application, as will the ongoing clinical trials for other exon-skipping oligonucleotides it is developing. The drug is still under review by the European Medicines Agency.