User login
Subpectoral Biceps Tenodesis
Tendinopathy of the long head of the biceps brachii (LHB) is a common source of anterior shoulder pain. The LHB tendon is an intra-articular yet extrasynovial structure, ensheathed by the synovial lining of the articular capsule.1 Branches of the anterior circumflex humeral artery course along the bicipital groove, but the gliding undersurface of the LHB remains avascular.2 Tendon irritation is most common within the groove and usually produces “tendinosis,” characterized by collagen fiber atrophy, fibrinoid necrosis, and fibrocyte proliferation.1 Neviaser and colleagues3 correlated such changes in the LHB tendon with rotator cuff pathology, as the 2 often coexist. Primary LHB tendinitis is less common and associated with younger patients who engage in overhead activities, such as baseball and volleyball.4
Nonoperative management, which is trialed initially, consists of rest, use of nonsteroidal anti-inflammatory drugs, and physical therapy. Corticosteroid injections are administered through the subacromial space or glenohumeral joint, which is continuous with the LHB sheath. Some physicians give ultrasound-guided injections into the LHB sheath. For fear of tendon atrophy from corticosteroid injections, some physicians prefer iontophoresis with a topical steroid over the bicipital groove. If conservative measures fail, the physician can choose from 2 primary surgical options: biceps tenotomy and tenodesis. Tenodesis can be performed within the groove (suprapectoral) or subpectoral. In this review, we highlight 5 key features of subpectoral biceps tenodesis to guide treatment and improve outcomes.
Examination and Indications
Management of LHB tendinopathy begins with a complete physical examination. Tenderness over the bicipital groove is the most consistent finding, but this region may be difficult to localize in large individuals. The arm should be internally rotated 10° to orient the groove anterior and palpated 7 cm below the acromion.5 Anterior shoulder pain after resisted elevation with the elbow extended and supinated represents a positive Speed test. A positive Yergason test produces pain with resisted forearm supination while the elbow is flexed to 90°.
Evaluation of biceps instability is important in deciding which type of management (operative or nonoperative) is appropriate for a patient. Medial biceps subluxation may be detected by bringing the flexed arm from abduction, external rotation into cross-body adduction, internal rotation with decreased arm flexion.6 Another maneuver that elicits biceps irritation is combined abduction–extension, which places tension on the biceps tendon. Similarly, coracoid impingement may disrupt the subscapularis roof of the biceps sheath and cause LHB instability. Dines and colleagues7 reproduced the painful clicking of coracoid impingement by placing the shoulder in forward elevation, internal rotation, and varying degrees of adduction. Belly-press, lift-off, and internal rotation strength are other tests that assess subscapularis integrity. Rotator cuff impingement signs should be evaluated, and the contralateral shoulder should be examined for comparison.
Plain radiographs may show a pathology, such as anterior acromial spurring or posterior overgrowth of the coracoid, for which surgery is more suited. T2-weighted magnetic resonance imaging (MRI) may show an increased LHB signal, but this has shown poor concordance with arthroscopic findings of biceps pathology.8 Magnetic resonance arthrography can better detect medial dislocation of the LHB tendon from subscapularis tears. Ultrasound is cost-effective but highly operator-dependent.
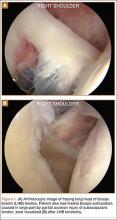
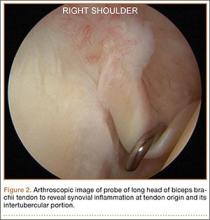
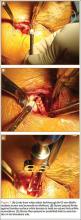
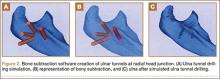
Indications for biceps tenotomy or tenodesis include failed conservative management, partial-thickness LHB tears more than 25% to 50% in diameter, and medial subluxation of the LHB tendon with or without a subscapularis tear. Superior labrum anterior to posterior (SLAP) tears in older patients are a relative indication. Intraoperative findings may also indicate the need for LHB surgery. During the diagnostic arthroscopy, the LHB tendon should be evaluated for synovial inflammation or fraying (Figures 1A, 1B). This may need to be done under dry conditions, as pump pressure can compress and blunt the inflamed appearance. The O’Brien maneuver can be performed to demonstrate incarceration of the LHB tendon within the anterior glenohumeral joint. A probe should be placed through an anterior portal to pull the intertubercular LHB tendon into view, as this region is most commonly inflamed (Figure 2). Probing of the tendon also allows assessment of the stability of the biceps sling.
Surgical Technique
When biceps surgery is indicated, the surgeon must choose between tenotomy and tenodesis. Tenotomy is a low-demand procedure indicated for low-demand patients. A “Popeye” deformity may occur in up to 62% of patients, but Boileau and colleagues9 reported that none of their patients were bothered by it. Another concern after tenotomy is fatigue-cramping of the biceps muscle belly. Kelly and colleagues10 reported that up to 40% of patients had soreness and decreased strength with elbow flexion. Such cramping is more common in patients under age 60 years. For these reasons, biceps tenotomy should be reserved for older, low-demand patients who are not concerned about cosmesis and less likely to comply with postoperative motion restrictions.2 We tend to perform tenotomy in obese patients, who may have a Popeye deformity that is not detectable, and in patients with diabetes; the goal is to avoid a wound infection resulting from the close proximity of tenodesis incision and axilla.
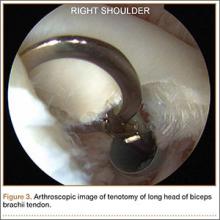
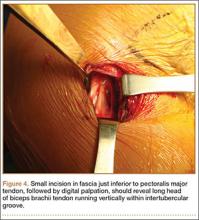
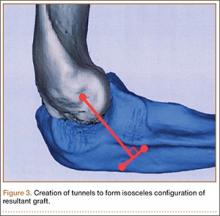
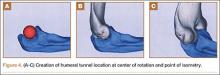
Biceps tenodesis should preserve the length–tension relationship of the biceps muscle and maintain its normal contour. Tenodesis location may be proximal or distal. Proximal fixation can be performed arthroscopically, and its advocates argue that keeping the LHB tendon within the bicipital groove preserves muscle strength. Boileau and Neyton11 found biceps strength to be 90% that of the contralateral arm after arthroscopic tenodesis. The bicipital groove, however, is lined with synovium and is a primary site of LHB pathology. Up to 78% of intra-articular biceps tears extend through the groove outside the joint.12 Proximal tenodesis thus retains a major pain generator. In a retrospective study of 188 patients, Sanders and colleagues13,14 found a 36% revision rate after proximal arthroscopic tenodesis and a 13% rate after proximal open tenodesis with an intact biceps sheath—significantly lower than the 3% after distal tenodesis outside the bicipital groove.1 For this reason, we advocate distal biceps tenodesis beneath the pectoralis major tendon. After tenotomy with an arthroscopic basket (Figure 3), the LHB tendon is retracted out of the glenohumeral joint by extending the elbow. For the mini-open incision, the head of the bed is lowered from the beach-chair position to 30°. The arm is abducted on a Mayo stand, and the inferior border of the pectoralis major tendon is palpated. A 3-cm vertical incision is made along the medial arm starting 1 cm superior to the inferior pectoralis edge. The subcutaneous tissues are mobilized, and dissection is carried down to the pectoralis major and coracobrachialis tendons. Visualization of the cephalic vein indicates that the exposure is too far lateral. The horizontal fibers of the pectoralis major are identified, and a small incision through the inferior overlying fascia is directed laterally and then distally in line with the long axis of the humerus. Digital palpation helps identify the anterior humerus and fusiform LHB tendon running vertically within the intertubercular groove (Figure 4). Cephalad retraction of the pectoralis major allows direct visualization of the LHB tendon. A right-angle clamp is positioned deep to the LHB tendon and directed medial to lateral to retrieve the LHB tendon out of the incision.
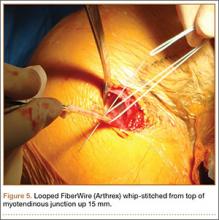
No. 2 looped Fiberwire (Arthrex) is then whip-stitched from the top of the myotendinous junction up 20 mm (Figure 5). The remaining 2 to 3 cm of LHB tendon proximal to the whip-stitching may be excised to remove inflammatory tissue. The pectoralis major is retracted superiorly with an Army-Navy retractor while a pointed Hohmann retractor is placed laterally. Medial retraction of the conjoined tendon should be done carefully with a Chandler elevator and minimal levering. In a cadaveric study, Dickens and colleagues15 found that the musculocutaneous nerve, radial nerve, and deep brachial artery were all within 1 cm of the standard medial retractor. Compared with internal rotation of the arm, external rotation moves the musculocutaneous nerve 11 mm farther from the tenodesis site.15
Once exposure is adequate, the appropriate length–tension of the LHB tendon must be established. The inferior edge of the pectoralis major is used as a landmark. Anatomical studies have shown that the top of the LHB myotendinous junction lies 20 to 31 mm proximal to the inferior pectoralis edge.16,17 Therefore, the tenodesis site should be 2 to 3 cm superior to the inferior pectoralis edge and centered on the humerus. Overall, the subpectoral location offers unique landmarks for LHB length-tensioning and provides soft-tissue coverage of the tenodesis site.
After identification of the appropriate tenodesis site, the surgeon chooses from a variety of fixation techniques. The “bone-tunnel technique” involves drilling an 8-mm unicortical hole through the anterior humerus followed by 2 smaller suture tunnels inferior to it; the LHB tendon with Krackow stitches is passed retrograde through the large hole by pulling the sutures through the smaller tunnels and tying them down.18 Despite the ease of performing this type of fixation, Mazzocca and colleagues19 found more cyclic displacement with bone tunnels than with interference screws and suture anchors. Other, less common techniques include the keyhole method (passing a rolled knot of LHB tendon through a keyhole in the bone)20 and soft-tissue tenodesis to the rotator interval or conjoined tendon.21,22 Recently, however, attention has turned mostly to interference screw and suture anchor fixation.
Multiple laboratory studies have demonstrated the superiority of interference screw fixation. Kilicoglu and colleagues23 and Ozalay and colleagues24 evaluated various fixation types in a sheep model, and both groups found the highest loads to failure with interference screws. Patzer and colleagues25 compared interference screws and knotless suture anchors in a human cadaveric study and noted significantly higher failure loads with interference screws. Some authors26,27 have presented conflicting laboratory data, and Millett and colleagues28 reported no difference in clinical outcomes between interference screws and suture anchors. However, these studies have not demonstrated inferiority of interference screws, and, in light of other evidence suggesting its biomechanical superiority, we prefer interference screw fixation.19,23-25,29
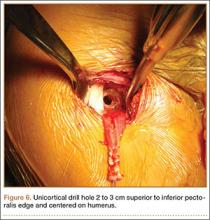
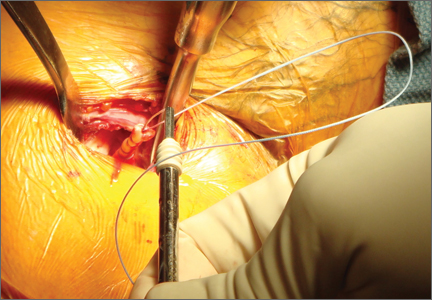
Exposing the bony surface for fixation involves electrocautery and subsequent use of a periosteal elevator to reflect a 1-cm periosteal window. A guide wire is drilled unicortically through the anterior cortex at the tenodesis site and is overreamed with an 8-mm cannulated reamer (Figure 6). This tunnel is then tapped, and bone debris is irrigated and suctioned from the wound. Cadaveric studies have shown no difference in failure loads with varying screw lengths or diameters.29,30 We use an 8×12-mm BioTenodesis screw (Arthrex) to match the typical width of the LHB tendon (Figures 7A-7C). One suture limb from the tendon whip-stitch is passed through the BioTenodesis screw and screwdriver. An assistant then uses a right-angle clamp as a pulley on the tendon so that the tendon may be visualized and “dunked” into the tunnel under direct visualization. As the screw is inserted, axial pressure is applied and the insertion paddle firmly held. Care should be taken to avoid overtightening the screw lest it become intramedullary. After the screw is flush to bone, the 2 whip-stitch suture limbs are tied for additional fixation.
Postoperative Rehabilitation
The optimal postoperative protocol for subpectoral biceps tenodesis has not been rigorously studied and is guided by the procedures performed with the biceps tenodesis. For the immediate postoperative period, Provencher and colleagues5 and Mazzocca and colleagues31 recommended immobilization in a sling during sleep and during the day if the patient is out in public or having difficulty maintaining the elbow flexed passively.
For isolated biceps tenodesis cases, passive- and active-assisted range of motion (ROM) of the glenohumeral, elbow, and wrist joints are permitted during the initial 4 weeks. At 3 weeks, the sling is discontinued and active ROM permitted. At 6 weeks, strengthening of the biceps, rotator cuff, deltoid, and periscapular muscles may begin with isometric contractions and progress to elastic bands and handheld weights. The same protocol is used if acromioplasty is performed at time of tenodesis. These patients may progress to active-assisted and active ROM earlier than 4 weeks if advised of the risks. However, sustained isometric biceps contraction, biceps strengthening, and resisted supination should not be performed until 6 weeks after surgery. If rotator cuff repair is performed, the patient is immobilized in a sling and passive ROM of the glenohumeral, elbow, and wrist joints is permitted during the first 6 weeks. The patient may progress to active-assisted and active ROM over the next 6 weeks, after motion is restored but before formal strengthening is initiated.32 For overhead athletes, Werner and colleagues33 advocated a throwing program starting 3 to 4 months after surgery.
Outcomes and Complications
Mini-open subpectoral biceps tenodesis is a safe, reliable, and effective treatment for LHB tendon pathology. This procedure provides excellent pain relief and functional outcomes32,34,35 and has a low complication rate.5,35-40 At a mean of 29 months after biceps tenodesis with an interference screw, Mazzocca and colleagues32 found statistically significant improvements on all clinical outcome measures: Rowe, American Shoulder and Elbow Surgeons (ASES), Simple Shoulder Test (SST), Constant-Murley, and Single Assessment Numeric Evaluation (SANE). Biceps symmetry was restored in 35 of 41 patients. Millett and colleagues28 reported that subpectoral biceps tenodesis relieved pain and improved function as measured by visual analog scale pain, ASES scores, and abbreviated Constant scores. Werner and colleagues34 compared open subpectoral and arthroscopic suprapectoral techniques and found excellent clinical and functional outcomes with both techniques at a mean of 3.1 years. There were no significant differences in ROM, strength, or clinical outcome scores between the 2 techniques.
Potential complications include hematoma, seroma, hardware failure, reaction to biodegradable screw, persistent anterior shoulder pain, stiffness, humeral fracture, reflex sympathetic dystrophy, infection, nerve injury, and brachial artery injury. The musculocutaneous nerve can be lacerated during screw placement or even avulsed if the surgeon attempts to retrieve the LHB tendon blindly.41 In the most comprehensive study of tenodesis complications, Nho and colleagues35 recorded a 2% complication rate in 353 patients over 3 years. Persistent bicipital pain and fixation failure causing a Popeye deformity were the 2 most common complications (0.57% each). In a study of 103 patients, Abtahi and colleagues39 found a 7% complication rate, with 4 superficial wound infections and 2 temporary nerve palsies. Millett and colleagues28 reported low complication rates with both interference screw and suture anchor fixation. Neither technique had a fixation failure, and persistent bicipital groove tenderness occurred in just 3% of patients after interference screw fixation and in 7% after suture anchor fixation. Mazzocca and colleagues32 documented 1 fixation failure (2%) 1 year after interference screw fixation.
Werner and colleagues34 encountered stiffness more than any other complication and found it to be more common in their arthroscopic group (9.4%) than in their open group (6.0%). They used intra-articular corticosteroid injections and physical therapy to successfully treat all cases of postoperative stiffness. Humeral fracture is uncommon after tenodesis.37,42 In a recent biomechanical study, however, Euler and colleagues40 found a significant reduction (25%) in humeral strength after a laterally eccentric, malpositioned biceps tenodesis. This decreased osseous strength may increase susceptibility to humeral shaft fracture, especially when interference screw fixation is used. Sears and colleagues37 and Dein and colleagues42 presented case reports of humeral fracture after biceps tenodesis with an interference screw.
For patients with fixation failure or continued anterior shoulder pain, revision biceps tenodesis is safe and effective. Heckman and colleagues43 and Gregory and colleagues44 showed revision tenodesis can lead to excellent pain relief and functional outcomes, for it allows complete removal of the biceps from the groove and preserves biceps function. Gregory and colleagues44 revised subpectoral biceps tenodesis for either continued pain or fixation failure and found significant improvements in pain and function a mean of 33.4 months after surgery. Anthony and colleagues45 performed biceps tenodesis for failed surgical tenotomies and autorupture of the LHB tendon. In their study of 11 patients, this surgery resulted in symptom improvement, patient satisfaction, resolution of Popeye deformity, and predictable return to activity.
Conclusion
LHB tendon pathology is a significant source of anterior shoulder pain and functional limitation. Diagnosis and treatment of this pathology can be challenging, and it is important to identify any concomitant pathologies or other pain sources. After failed nonoperative management, surgeons have the option of mini-open subpectoral biceps tenodesis—a safe, reliable, and effective treatment with excellent outcomes. Although multiple fixation options are available, we think that, based on the current literature, fixation with a bioabsorbable interference screw remains the best option. This procedure has demonstrated efficacy for revision biceps tenodesis, failed biceps tenotomy, and autorupture of the biceps.
1. Friedman DJ, Dunn JC, Higgins LD, Warner JJP. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Neviaser TJ, Neviaser RJ, Neviaser JS, Neviaser JS. The four-in-one arthroplasty for the painful arc syndrome. Clin Orthop Relat Res. 1982;163:107-112.
4. Patton WC, McCluskey GM 3rd. Biceps tendinitis and subluxation. Clin Sports Med. 2001;20(3):505-529.
5. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
6. Bennett WF. Arthroscopic repair of isolated subscapularis tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(2):131-143.
7. Dines DM, Warren RF, Inglis AE, Pavlov H. The coracoid impingement syndrome. Bone Joint J Br. 1990;72(2):314-316.
8. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
9. Boileau P, Baqué F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
10. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
11. Boileau P, Neyton L. Arthroscopic tenodesis for lesions of the long head of the biceps. Oper Orthop Traumatol. 2005;17(6):601-623.
12. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
13. Sanders B, Lavery K, Pennington S, Warner JJP. Biceps tendon tenodesis: success with proximal versus distal fixation (SS-16). Arthroscopy. 2008;24(6 suppl):e9.
14. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
15. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
16. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
17. Jarrett CD, McClelland WB, Xerogeanes JW. Minimally invasive proximal biceps tenodesis: an anatomical study for optimal placement and safe surgical technique. J Shoulder Elbow Surg. 2011;20(3):477-480.
18. Mazzocca AD, Noerdlinger MA, Romeo AA. Mini open and subpectoral biceps tenodesis. Oper Tech Sports Med. 2003;11(1):24-31.
19. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
20. Froimson AI, O I. Keyhole tenodesis of biceps origin at the shoulder. Clin Orthop Relat Res. 1975;(112):245-249.
21. Sekiya JK, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
22. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
23. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
24. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
25. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
26. Buchholz A, Martetschläger F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
27. Tashjian RZ, Henninger HB. Biomechanical evaluation of subpectoral biceps tenodesis: dual suture anchor versus interference screw fixation. J Shoulder Elbow Surg. 2013;22(10):1408-1412.
28. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJP. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9(1):121.
29. Sethi PM, Rajaram A, Beitzel K, Hackett TR, Chowaniec DM, Mazzocca AD. Biomechanical performance of subpectoral biceps tenodesis: a comparison of interference screw fixation, cortical button fixation, and interference screw diameter. J Shoulder Elbow Surg. 2013;22(4):451-457.
30. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
31. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
32. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
33. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
34. Werner BC, Evans CL, Holzgrefe RE, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of minimum 2-year clinical outcomes. Am J Sports Med. 2014;42(11):2583-2590.
35. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
36. Rhee PC, Spinner RJ, Bishop AT, Shin AY. Iatrogenic brachial plexus injuries associated with open subpectoral biceps tenodesis: a report of 4 cases. Am J Sports Med. 2013;41(9):2048-2053.
37. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
38. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
39. Abtahi AM, Granger EK, Tashjian RZ. Complications after subpectoral biceps tenodesis using a dual suture anchor technique. Int J Shoulder Surg. 2014;8(2):47-50.
40. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
41. Carofino BC, Brogan DM, Kircher MF, et al. Iatrogenic nerve injuries during shoulder surgery. J Bone Joint Surg Am. 2013;95(18):1667-1674.
42. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
43. Heckman DS, Creighton RA, Romeo AA. Management of failed biceps tenodesis or tenotomy: causation and treatment. Sports Med Arthrosc. 2010;18(3):173-180.
44. Gregory JM, Harwood DP, Gochanour E, Sherman SL, Romeo AA. Clinical outcomes of revision biceps tenodesis. Int J Shoulder Surg. 2012;6(2):45-50.
45. Anthony SG, McCormick F, Gross DJ, Golijanin P, Provencher MT. Biceps tenodesis for long head of the biceps after auto-rupture or failed surgical tenotomy: results in an active population. J Shoulder Elbow Surg. 2015;24(2):e36-e40.
Tendinopathy of the long head of the biceps brachii (LHB) is a common source of anterior shoulder pain. The LHB tendon is an intra-articular yet extrasynovial structure, ensheathed by the synovial lining of the articular capsule.1 Branches of the anterior circumflex humeral artery course along the bicipital groove, but the gliding undersurface of the LHB remains avascular.2 Tendon irritation is most common within the groove and usually produces “tendinosis,” characterized by collagen fiber atrophy, fibrinoid necrosis, and fibrocyte proliferation.1 Neviaser and colleagues3 correlated such changes in the LHB tendon with rotator cuff pathology, as the 2 often coexist. Primary LHB tendinitis is less common and associated with younger patients who engage in overhead activities, such as baseball and volleyball.4
Nonoperative management, which is trialed initially, consists of rest, use of nonsteroidal anti-inflammatory drugs, and physical therapy. Corticosteroid injections are administered through the subacromial space or glenohumeral joint, which is continuous with the LHB sheath. Some physicians give ultrasound-guided injections into the LHB sheath. For fear of tendon atrophy from corticosteroid injections, some physicians prefer iontophoresis with a topical steroid over the bicipital groove. If conservative measures fail, the physician can choose from 2 primary surgical options: biceps tenotomy and tenodesis. Tenodesis can be performed within the groove (suprapectoral) or subpectoral. In this review, we highlight 5 key features of subpectoral biceps tenodesis to guide treatment and improve outcomes.
Examination and Indications
Management of LHB tendinopathy begins with a complete physical examination. Tenderness over the bicipital groove is the most consistent finding, but this region may be difficult to localize in large individuals. The arm should be internally rotated 10° to orient the groove anterior and palpated 7 cm below the acromion.5 Anterior shoulder pain after resisted elevation with the elbow extended and supinated represents a positive Speed test. A positive Yergason test produces pain with resisted forearm supination while the elbow is flexed to 90°.
Evaluation of biceps instability is important in deciding which type of management (operative or nonoperative) is appropriate for a patient. Medial biceps subluxation may be detected by bringing the flexed arm from abduction, external rotation into cross-body adduction, internal rotation with decreased arm flexion.6 Another maneuver that elicits biceps irritation is combined abduction–extension, which places tension on the biceps tendon. Similarly, coracoid impingement may disrupt the subscapularis roof of the biceps sheath and cause LHB instability. Dines and colleagues7 reproduced the painful clicking of coracoid impingement by placing the shoulder in forward elevation, internal rotation, and varying degrees of adduction. Belly-press, lift-off, and internal rotation strength are other tests that assess subscapularis integrity. Rotator cuff impingement signs should be evaluated, and the contralateral shoulder should be examined for comparison.
Plain radiographs may show a pathology, such as anterior acromial spurring or posterior overgrowth of the coracoid, for which surgery is more suited. T2-weighted magnetic resonance imaging (MRI) may show an increased LHB signal, but this has shown poor concordance with arthroscopic findings of biceps pathology.8 Magnetic resonance arthrography can better detect medial dislocation of the LHB tendon from subscapularis tears. Ultrasound is cost-effective but highly operator-dependent.
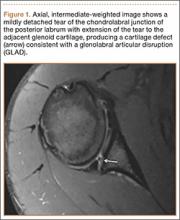
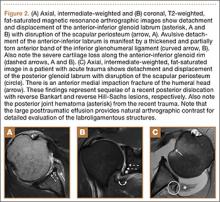
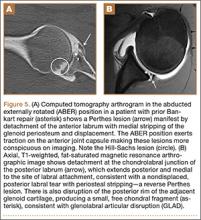
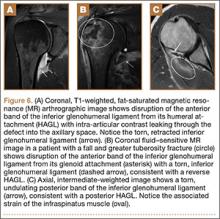
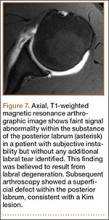
Indications for biceps tenotomy or tenodesis include failed conservative management, partial-thickness LHB tears more than 25% to 50% in diameter, and medial subluxation of the LHB tendon with or without a subscapularis tear. Superior labrum anterior to posterior (SLAP) tears in older patients are a relative indication. Intraoperative findings may also indicate the need for LHB surgery. During the diagnostic arthroscopy, the LHB tendon should be evaluated for synovial inflammation or fraying (Figures 1A, 1B). This may need to be done under dry conditions, as pump pressure can compress and blunt the inflamed appearance. The O’Brien maneuver can be performed to demonstrate incarceration of the LHB tendon within the anterior glenohumeral joint. A probe should be placed through an anterior portal to pull the intertubercular LHB tendon into view, as this region is most commonly inflamed (Figure 2). Probing of the tendon also allows assessment of the stability of the biceps sling.
Surgical Technique
When biceps surgery is indicated, the surgeon must choose between tenotomy and tenodesis. Tenotomy is a low-demand procedure indicated for low-demand patients. A “Popeye” deformity may occur in up to 62% of patients, but Boileau and colleagues9 reported that none of their patients were bothered by it. Another concern after tenotomy is fatigue-cramping of the biceps muscle belly. Kelly and colleagues10 reported that up to 40% of patients had soreness and decreased strength with elbow flexion. Such cramping is more common in patients under age 60 years. For these reasons, biceps tenotomy should be reserved for older, low-demand patients who are not concerned about cosmesis and less likely to comply with postoperative motion restrictions.2 We tend to perform tenotomy in obese patients, who may have a Popeye deformity that is not detectable, and in patients with diabetes; the goal is to avoid a wound infection resulting from the close proximity of tenodesis incision and axilla.
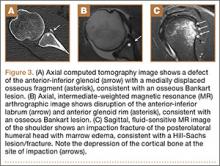
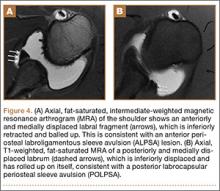
Biceps tenodesis should preserve the length–tension relationship of the biceps muscle and maintain its normal contour. Tenodesis location may be proximal or distal. Proximal fixation can be performed arthroscopically, and its advocates argue that keeping the LHB tendon within the bicipital groove preserves muscle strength. Boileau and Neyton11 found biceps strength to be 90% that of the contralateral arm after arthroscopic tenodesis. The bicipital groove, however, is lined with synovium and is a primary site of LHB pathology. Up to 78% of intra-articular biceps tears extend through the groove outside the joint.12 Proximal tenodesis thus retains a major pain generator. In a retrospective study of 188 patients, Sanders and colleagues13,14 found a 36% revision rate after proximal arthroscopic tenodesis and a 13% rate after proximal open tenodesis with an intact biceps sheath—significantly lower than the 3% after distal tenodesis outside the bicipital groove.1 For this reason, we advocate distal biceps tenodesis beneath the pectoralis major tendon. After tenotomy with an arthroscopic basket (Figure 3), the LHB tendon is retracted out of the glenohumeral joint by extending the elbow. For the mini-open incision, the head of the bed is lowered from the beach-chair position to 30°. The arm is abducted on a Mayo stand, and the inferior border of the pectoralis major tendon is palpated. A 3-cm vertical incision is made along the medial arm starting 1 cm superior to the inferior pectoralis edge. The subcutaneous tissues are mobilized, and dissection is carried down to the pectoralis major and coracobrachialis tendons. Visualization of the cephalic vein indicates that the exposure is too far lateral. The horizontal fibers of the pectoralis major are identified, and a small incision through the inferior overlying fascia is directed laterally and then distally in line with the long axis of the humerus. Digital palpation helps identify the anterior humerus and fusiform LHB tendon running vertically within the intertubercular groove (Figure 4). Cephalad retraction of the pectoralis major allows direct visualization of the LHB tendon. A right-angle clamp is positioned deep to the LHB tendon and directed medial to lateral to retrieve the LHB tendon out of the incision.
No. 2 looped Fiberwire (Arthrex) is then whip-stitched from the top of the myotendinous junction up 20 mm (Figure 5). The remaining 2 to 3 cm of LHB tendon proximal to the whip-stitching may be excised to remove inflammatory tissue. The pectoralis major is retracted superiorly with an Army-Navy retractor while a pointed Hohmann retractor is placed laterally. Medial retraction of the conjoined tendon should be done carefully with a Chandler elevator and minimal levering. In a cadaveric study, Dickens and colleagues15 found that the musculocutaneous nerve, radial nerve, and deep brachial artery were all within 1 cm of the standard medial retractor. Compared with internal rotation of the arm, external rotation moves the musculocutaneous nerve 11 mm farther from the tenodesis site.15
Once exposure is adequate, the appropriate length–tension of the LHB tendon must be established. The inferior edge of the pectoralis major is used as a landmark. Anatomical studies have shown that the top of the LHB myotendinous junction lies 20 to 31 mm proximal to the inferior pectoralis edge.16,17 Therefore, the tenodesis site should be 2 to 3 cm superior to the inferior pectoralis edge and centered on the humerus. Overall, the subpectoral location offers unique landmarks for LHB length-tensioning and provides soft-tissue coverage of the tenodesis site.
After identification of the appropriate tenodesis site, the surgeon chooses from a variety of fixation techniques. The “bone-tunnel technique” involves drilling an 8-mm unicortical hole through the anterior humerus followed by 2 smaller suture tunnels inferior to it; the LHB tendon with Krackow stitches is passed retrograde through the large hole by pulling the sutures through the smaller tunnels and tying them down.18 Despite the ease of performing this type of fixation, Mazzocca and colleagues19 found more cyclic displacement with bone tunnels than with interference screws and suture anchors. Other, less common techniques include the keyhole method (passing a rolled knot of LHB tendon through a keyhole in the bone)20 and soft-tissue tenodesis to the rotator interval or conjoined tendon.21,22 Recently, however, attention has turned mostly to interference screw and suture anchor fixation.
Multiple laboratory studies have demonstrated the superiority of interference screw fixation. Kilicoglu and colleagues23 and Ozalay and colleagues24 evaluated various fixation types in a sheep model, and both groups found the highest loads to failure with interference screws. Patzer and colleagues25 compared interference screws and knotless suture anchors in a human cadaveric study and noted significantly higher failure loads with interference screws. Some authors26,27 have presented conflicting laboratory data, and Millett and colleagues28 reported no difference in clinical outcomes between interference screws and suture anchors. However, these studies have not demonstrated inferiority of interference screws, and, in light of other evidence suggesting its biomechanical superiority, we prefer interference screw fixation.19,23-25,29
Exposing the bony surface for fixation involves electrocautery and subsequent use of a periosteal elevator to reflect a 1-cm periosteal window. A guide wire is drilled unicortically through the anterior cortex at the tenodesis site and is overreamed with an 8-mm cannulated reamer (Figure 6). This tunnel is then tapped, and bone debris is irrigated and suctioned from the wound. Cadaveric studies have shown no difference in failure loads with varying screw lengths or diameters.29,30 We use an 8×12-mm BioTenodesis screw (Arthrex) to match the typical width of the LHB tendon (Figures 7A-7C). One suture limb from the tendon whip-stitch is passed through the BioTenodesis screw and screwdriver. An assistant then uses a right-angle clamp as a pulley on the tendon so that the tendon may be visualized and “dunked” into the tunnel under direct visualization. As the screw is inserted, axial pressure is applied and the insertion paddle firmly held. Care should be taken to avoid overtightening the screw lest it become intramedullary. After the screw is flush to bone, the 2 whip-stitch suture limbs are tied for additional fixation.
Postoperative Rehabilitation
The optimal postoperative protocol for subpectoral biceps tenodesis has not been rigorously studied and is guided by the procedures performed with the biceps tenodesis. For the immediate postoperative period, Provencher and colleagues5 and Mazzocca and colleagues31 recommended immobilization in a sling during sleep and during the day if the patient is out in public or having difficulty maintaining the elbow flexed passively.
For isolated biceps tenodesis cases, passive- and active-assisted range of motion (ROM) of the glenohumeral, elbow, and wrist joints are permitted during the initial 4 weeks. At 3 weeks, the sling is discontinued and active ROM permitted. At 6 weeks, strengthening of the biceps, rotator cuff, deltoid, and periscapular muscles may begin with isometric contractions and progress to elastic bands and handheld weights. The same protocol is used if acromioplasty is performed at time of tenodesis. These patients may progress to active-assisted and active ROM earlier than 4 weeks if advised of the risks. However, sustained isometric biceps contraction, biceps strengthening, and resisted supination should not be performed until 6 weeks after surgery. If rotator cuff repair is performed, the patient is immobilized in a sling and passive ROM of the glenohumeral, elbow, and wrist joints is permitted during the first 6 weeks. The patient may progress to active-assisted and active ROM over the next 6 weeks, after motion is restored but before formal strengthening is initiated.32 For overhead athletes, Werner and colleagues33 advocated a throwing program starting 3 to 4 months after surgery.
Outcomes and Complications
Mini-open subpectoral biceps tenodesis is a safe, reliable, and effective treatment for LHB tendon pathology. This procedure provides excellent pain relief and functional outcomes32,34,35 and has a low complication rate.5,35-40 At a mean of 29 months after biceps tenodesis with an interference screw, Mazzocca and colleagues32 found statistically significant improvements on all clinical outcome measures: Rowe, American Shoulder and Elbow Surgeons (ASES), Simple Shoulder Test (SST), Constant-Murley, and Single Assessment Numeric Evaluation (SANE). Biceps symmetry was restored in 35 of 41 patients. Millett and colleagues28 reported that subpectoral biceps tenodesis relieved pain and improved function as measured by visual analog scale pain, ASES scores, and abbreviated Constant scores. Werner and colleagues34 compared open subpectoral and arthroscopic suprapectoral techniques and found excellent clinical and functional outcomes with both techniques at a mean of 3.1 years. There were no significant differences in ROM, strength, or clinical outcome scores between the 2 techniques.
Potential complications include hematoma, seroma, hardware failure, reaction to biodegradable screw, persistent anterior shoulder pain, stiffness, humeral fracture, reflex sympathetic dystrophy, infection, nerve injury, and brachial artery injury. The musculocutaneous nerve can be lacerated during screw placement or even avulsed if the surgeon attempts to retrieve the LHB tendon blindly.41 In the most comprehensive study of tenodesis complications, Nho and colleagues35 recorded a 2% complication rate in 353 patients over 3 years. Persistent bicipital pain and fixation failure causing a Popeye deformity were the 2 most common complications (0.57% each). In a study of 103 patients, Abtahi and colleagues39 found a 7% complication rate, with 4 superficial wound infections and 2 temporary nerve palsies. Millett and colleagues28 reported low complication rates with both interference screw and suture anchor fixation. Neither technique had a fixation failure, and persistent bicipital groove tenderness occurred in just 3% of patients after interference screw fixation and in 7% after suture anchor fixation. Mazzocca and colleagues32 documented 1 fixation failure (2%) 1 year after interference screw fixation.
Werner and colleagues34 encountered stiffness more than any other complication and found it to be more common in their arthroscopic group (9.4%) than in their open group (6.0%). They used intra-articular corticosteroid injections and physical therapy to successfully treat all cases of postoperative stiffness. Humeral fracture is uncommon after tenodesis.37,42 In a recent biomechanical study, however, Euler and colleagues40 found a significant reduction (25%) in humeral strength after a laterally eccentric, malpositioned biceps tenodesis. This decreased osseous strength may increase susceptibility to humeral shaft fracture, especially when interference screw fixation is used. Sears and colleagues37 and Dein and colleagues42 presented case reports of humeral fracture after biceps tenodesis with an interference screw.
For patients with fixation failure or continued anterior shoulder pain, revision biceps tenodesis is safe and effective. Heckman and colleagues43 and Gregory and colleagues44 showed revision tenodesis can lead to excellent pain relief and functional outcomes, for it allows complete removal of the biceps from the groove and preserves biceps function. Gregory and colleagues44 revised subpectoral biceps tenodesis for either continued pain or fixation failure and found significant improvements in pain and function a mean of 33.4 months after surgery. Anthony and colleagues45 performed biceps tenodesis for failed surgical tenotomies and autorupture of the LHB tendon. In their study of 11 patients, this surgery resulted in symptom improvement, patient satisfaction, resolution of Popeye deformity, and predictable return to activity.
Conclusion
LHB tendon pathology is a significant source of anterior shoulder pain and functional limitation. Diagnosis and treatment of this pathology can be challenging, and it is important to identify any concomitant pathologies or other pain sources. After failed nonoperative management, surgeons have the option of mini-open subpectoral biceps tenodesis—a safe, reliable, and effective treatment with excellent outcomes. Although multiple fixation options are available, we think that, based on the current literature, fixation with a bioabsorbable interference screw remains the best option. This procedure has demonstrated efficacy for revision biceps tenodesis, failed biceps tenotomy, and autorupture of the biceps.
Tendinopathy of the long head of the biceps brachii (LHB) is a common source of anterior shoulder pain. The LHB tendon is an intra-articular yet extrasynovial structure, ensheathed by the synovial lining of the articular capsule.1 Branches of the anterior circumflex humeral artery course along the bicipital groove, but the gliding undersurface of the LHB remains avascular.2 Tendon irritation is most common within the groove and usually produces “tendinosis,” characterized by collagen fiber atrophy, fibrinoid necrosis, and fibrocyte proliferation.1 Neviaser and colleagues3 correlated such changes in the LHB tendon with rotator cuff pathology, as the 2 often coexist. Primary LHB tendinitis is less common and associated with younger patients who engage in overhead activities, such as baseball and volleyball.4
Nonoperative management, which is trialed initially, consists of rest, use of nonsteroidal anti-inflammatory drugs, and physical therapy. Corticosteroid injections are administered through the subacromial space or glenohumeral joint, which is continuous with the LHB sheath. Some physicians give ultrasound-guided injections into the LHB sheath. For fear of tendon atrophy from corticosteroid injections, some physicians prefer iontophoresis with a topical steroid over the bicipital groove. If conservative measures fail, the physician can choose from 2 primary surgical options: biceps tenotomy and tenodesis. Tenodesis can be performed within the groove (suprapectoral) or subpectoral. In this review, we highlight 5 key features of subpectoral biceps tenodesis to guide treatment and improve outcomes.
Examination and Indications
Management of LHB tendinopathy begins with a complete physical examination. Tenderness over the bicipital groove is the most consistent finding, but this region may be difficult to localize in large individuals. The arm should be internally rotated 10° to orient the groove anterior and palpated 7 cm below the acromion.5 Anterior shoulder pain after resisted elevation with the elbow extended and supinated represents a positive Speed test. A positive Yergason test produces pain with resisted forearm supination while the elbow is flexed to 90°.
Evaluation of biceps instability is important in deciding which type of management (operative or nonoperative) is appropriate for a patient. Medial biceps subluxation may be detected by bringing the flexed arm from abduction, external rotation into cross-body adduction, internal rotation with decreased arm flexion.6 Another maneuver that elicits biceps irritation is combined abduction–extension, which places tension on the biceps tendon. Similarly, coracoid impingement may disrupt the subscapularis roof of the biceps sheath and cause LHB instability. Dines and colleagues7 reproduced the painful clicking of coracoid impingement by placing the shoulder in forward elevation, internal rotation, and varying degrees of adduction. Belly-press, lift-off, and internal rotation strength are other tests that assess subscapularis integrity. Rotator cuff impingement signs should be evaluated, and the contralateral shoulder should be examined for comparison.
Plain radiographs may show a pathology, such as anterior acromial spurring or posterior overgrowth of the coracoid, for which surgery is more suited. T2-weighted magnetic resonance imaging (MRI) may show an increased LHB signal, but this has shown poor concordance with arthroscopic findings of biceps pathology.8 Magnetic resonance arthrography can better detect medial dislocation of the LHB tendon from subscapularis tears. Ultrasound is cost-effective but highly operator-dependent.
Indications for biceps tenotomy or tenodesis include failed conservative management, partial-thickness LHB tears more than 25% to 50% in diameter, and medial subluxation of the LHB tendon with or without a subscapularis tear. Superior labrum anterior to posterior (SLAP) tears in older patients are a relative indication. Intraoperative findings may also indicate the need for LHB surgery. During the diagnostic arthroscopy, the LHB tendon should be evaluated for synovial inflammation or fraying (Figures 1A, 1B). This may need to be done under dry conditions, as pump pressure can compress and blunt the inflamed appearance. The O’Brien maneuver can be performed to demonstrate incarceration of the LHB tendon within the anterior glenohumeral joint. A probe should be placed through an anterior portal to pull the intertubercular LHB tendon into view, as this region is most commonly inflamed (Figure 2). Probing of the tendon also allows assessment of the stability of the biceps sling.
Surgical Technique
When biceps surgery is indicated, the surgeon must choose between tenotomy and tenodesis. Tenotomy is a low-demand procedure indicated for low-demand patients. A “Popeye” deformity may occur in up to 62% of patients, but Boileau and colleagues9 reported that none of their patients were bothered by it. Another concern after tenotomy is fatigue-cramping of the biceps muscle belly. Kelly and colleagues10 reported that up to 40% of patients had soreness and decreased strength with elbow flexion. Such cramping is more common in patients under age 60 years. For these reasons, biceps tenotomy should be reserved for older, low-demand patients who are not concerned about cosmesis and less likely to comply with postoperative motion restrictions.2 We tend to perform tenotomy in obese patients, who may have a Popeye deformity that is not detectable, and in patients with diabetes; the goal is to avoid a wound infection resulting from the close proximity of tenodesis incision and axilla.
Biceps tenodesis should preserve the length–tension relationship of the biceps muscle and maintain its normal contour. Tenodesis location may be proximal or distal. Proximal fixation can be performed arthroscopically, and its advocates argue that keeping the LHB tendon within the bicipital groove preserves muscle strength. Boileau and Neyton11 found biceps strength to be 90% that of the contralateral arm after arthroscopic tenodesis. The bicipital groove, however, is lined with synovium and is a primary site of LHB pathology. Up to 78% of intra-articular biceps tears extend through the groove outside the joint.12 Proximal tenodesis thus retains a major pain generator. In a retrospective study of 188 patients, Sanders and colleagues13,14 found a 36% revision rate after proximal arthroscopic tenodesis and a 13% rate after proximal open tenodesis with an intact biceps sheath—significantly lower than the 3% after distal tenodesis outside the bicipital groove.1 For this reason, we advocate distal biceps tenodesis beneath the pectoralis major tendon. After tenotomy with an arthroscopic basket (Figure 3), the LHB tendon is retracted out of the glenohumeral joint by extending the elbow. For the mini-open incision, the head of the bed is lowered from the beach-chair position to 30°. The arm is abducted on a Mayo stand, and the inferior border of the pectoralis major tendon is palpated. A 3-cm vertical incision is made along the medial arm starting 1 cm superior to the inferior pectoralis edge. The subcutaneous tissues are mobilized, and dissection is carried down to the pectoralis major and coracobrachialis tendons. Visualization of the cephalic vein indicates that the exposure is too far lateral. The horizontal fibers of the pectoralis major are identified, and a small incision through the inferior overlying fascia is directed laterally and then distally in line with the long axis of the humerus. Digital palpation helps identify the anterior humerus and fusiform LHB tendon running vertically within the intertubercular groove (Figure 4). Cephalad retraction of the pectoralis major allows direct visualization of the LHB tendon. A right-angle clamp is positioned deep to the LHB tendon and directed medial to lateral to retrieve the LHB tendon out of the incision.
No. 2 looped Fiberwire (Arthrex) is then whip-stitched from the top of the myotendinous junction up 20 mm (Figure 5). The remaining 2 to 3 cm of LHB tendon proximal to the whip-stitching may be excised to remove inflammatory tissue. The pectoralis major is retracted superiorly with an Army-Navy retractor while a pointed Hohmann retractor is placed laterally. Medial retraction of the conjoined tendon should be done carefully with a Chandler elevator and minimal levering. In a cadaveric study, Dickens and colleagues15 found that the musculocutaneous nerve, radial nerve, and deep brachial artery were all within 1 cm of the standard medial retractor. Compared with internal rotation of the arm, external rotation moves the musculocutaneous nerve 11 mm farther from the tenodesis site.15
Once exposure is adequate, the appropriate length–tension of the LHB tendon must be established. The inferior edge of the pectoralis major is used as a landmark. Anatomical studies have shown that the top of the LHB myotendinous junction lies 20 to 31 mm proximal to the inferior pectoralis edge.16,17 Therefore, the tenodesis site should be 2 to 3 cm superior to the inferior pectoralis edge and centered on the humerus. Overall, the subpectoral location offers unique landmarks for LHB length-tensioning and provides soft-tissue coverage of the tenodesis site.
After identification of the appropriate tenodesis site, the surgeon chooses from a variety of fixation techniques. The “bone-tunnel technique” involves drilling an 8-mm unicortical hole through the anterior humerus followed by 2 smaller suture tunnels inferior to it; the LHB tendon with Krackow stitches is passed retrograde through the large hole by pulling the sutures through the smaller tunnels and tying them down.18 Despite the ease of performing this type of fixation, Mazzocca and colleagues19 found more cyclic displacement with bone tunnels than with interference screws and suture anchors. Other, less common techniques include the keyhole method (passing a rolled knot of LHB tendon through a keyhole in the bone)20 and soft-tissue tenodesis to the rotator interval or conjoined tendon.21,22 Recently, however, attention has turned mostly to interference screw and suture anchor fixation.
Multiple laboratory studies have demonstrated the superiority of interference screw fixation. Kilicoglu and colleagues23 and Ozalay and colleagues24 evaluated various fixation types in a sheep model, and both groups found the highest loads to failure with interference screws. Patzer and colleagues25 compared interference screws and knotless suture anchors in a human cadaveric study and noted significantly higher failure loads with interference screws. Some authors26,27 have presented conflicting laboratory data, and Millett and colleagues28 reported no difference in clinical outcomes between interference screws and suture anchors. However, these studies have not demonstrated inferiority of interference screws, and, in light of other evidence suggesting its biomechanical superiority, we prefer interference screw fixation.19,23-25,29
Exposing the bony surface for fixation involves electrocautery and subsequent use of a periosteal elevator to reflect a 1-cm periosteal window. A guide wire is drilled unicortically through the anterior cortex at the tenodesis site and is overreamed with an 8-mm cannulated reamer (Figure 6). This tunnel is then tapped, and bone debris is irrigated and suctioned from the wound. Cadaveric studies have shown no difference in failure loads with varying screw lengths or diameters.29,30 We use an 8×12-mm BioTenodesis screw (Arthrex) to match the typical width of the LHB tendon (Figures 7A-7C). One suture limb from the tendon whip-stitch is passed through the BioTenodesis screw and screwdriver. An assistant then uses a right-angle clamp as a pulley on the tendon so that the tendon may be visualized and “dunked” into the tunnel under direct visualization. As the screw is inserted, axial pressure is applied and the insertion paddle firmly held. Care should be taken to avoid overtightening the screw lest it become intramedullary. After the screw is flush to bone, the 2 whip-stitch suture limbs are tied for additional fixation.
Postoperative Rehabilitation
The optimal postoperative protocol for subpectoral biceps tenodesis has not been rigorously studied and is guided by the procedures performed with the biceps tenodesis. For the immediate postoperative period, Provencher and colleagues5 and Mazzocca and colleagues31 recommended immobilization in a sling during sleep and during the day if the patient is out in public or having difficulty maintaining the elbow flexed passively.
For isolated biceps tenodesis cases, passive- and active-assisted range of motion (ROM) of the glenohumeral, elbow, and wrist joints are permitted during the initial 4 weeks. At 3 weeks, the sling is discontinued and active ROM permitted. At 6 weeks, strengthening of the biceps, rotator cuff, deltoid, and periscapular muscles may begin with isometric contractions and progress to elastic bands and handheld weights. The same protocol is used if acromioplasty is performed at time of tenodesis. These patients may progress to active-assisted and active ROM earlier than 4 weeks if advised of the risks. However, sustained isometric biceps contraction, biceps strengthening, and resisted supination should not be performed until 6 weeks after surgery. If rotator cuff repair is performed, the patient is immobilized in a sling and passive ROM of the glenohumeral, elbow, and wrist joints is permitted during the first 6 weeks. The patient may progress to active-assisted and active ROM over the next 6 weeks, after motion is restored but before formal strengthening is initiated.32 For overhead athletes, Werner and colleagues33 advocated a throwing program starting 3 to 4 months after surgery.
Outcomes and Complications
Mini-open subpectoral biceps tenodesis is a safe, reliable, and effective treatment for LHB tendon pathology. This procedure provides excellent pain relief and functional outcomes32,34,35 and has a low complication rate.5,35-40 At a mean of 29 months after biceps tenodesis with an interference screw, Mazzocca and colleagues32 found statistically significant improvements on all clinical outcome measures: Rowe, American Shoulder and Elbow Surgeons (ASES), Simple Shoulder Test (SST), Constant-Murley, and Single Assessment Numeric Evaluation (SANE). Biceps symmetry was restored in 35 of 41 patients. Millett and colleagues28 reported that subpectoral biceps tenodesis relieved pain and improved function as measured by visual analog scale pain, ASES scores, and abbreviated Constant scores. Werner and colleagues34 compared open subpectoral and arthroscopic suprapectoral techniques and found excellent clinical and functional outcomes with both techniques at a mean of 3.1 years. There were no significant differences in ROM, strength, or clinical outcome scores between the 2 techniques.
Potential complications include hematoma, seroma, hardware failure, reaction to biodegradable screw, persistent anterior shoulder pain, stiffness, humeral fracture, reflex sympathetic dystrophy, infection, nerve injury, and brachial artery injury. The musculocutaneous nerve can be lacerated during screw placement or even avulsed if the surgeon attempts to retrieve the LHB tendon blindly.41 In the most comprehensive study of tenodesis complications, Nho and colleagues35 recorded a 2% complication rate in 353 patients over 3 years. Persistent bicipital pain and fixation failure causing a Popeye deformity were the 2 most common complications (0.57% each). In a study of 103 patients, Abtahi and colleagues39 found a 7% complication rate, with 4 superficial wound infections and 2 temporary nerve palsies. Millett and colleagues28 reported low complication rates with both interference screw and suture anchor fixation. Neither technique had a fixation failure, and persistent bicipital groove tenderness occurred in just 3% of patients after interference screw fixation and in 7% after suture anchor fixation. Mazzocca and colleagues32 documented 1 fixation failure (2%) 1 year after interference screw fixation.
Werner and colleagues34 encountered stiffness more than any other complication and found it to be more common in their arthroscopic group (9.4%) than in their open group (6.0%). They used intra-articular corticosteroid injections and physical therapy to successfully treat all cases of postoperative stiffness. Humeral fracture is uncommon after tenodesis.37,42 In a recent biomechanical study, however, Euler and colleagues40 found a significant reduction (25%) in humeral strength after a laterally eccentric, malpositioned biceps tenodesis. This decreased osseous strength may increase susceptibility to humeral shaft fracture, especially when interference screw fixation is used. Sears and colleagues37 and Dein and colleagues42 presented case reports of humeral fracture after biceps tenodesis with an interference screw.
For patients with fixation failure or continued anterior shoulder pain, revision biceps tenodesis is safe and effective. Heckman and colleagues43 and Gregory and colleagues44 showed revision tenodesis can lead to excellent pain relief and functional outcomes, for it allows complete removal of the biceps from the groove and preserves biceps function. Gregory and colleagues44 revised subpectoral biceps tenodesis for either continued pain or fixation failure and found significant improvements in pain and function a mean of 33.4 months after surgery. Anthony and colleagues45 performed biceps tenodesis for failed surgical tenotomies and autorupture of the LHB tendon. In their study of 11 patients, this surgery resulted in symptom improvement, patient satisfaction, resolution of Popeye deformity, and predictable return to activity.
Conclusion
LHB tendon pathology is a significant source of anterior shoulder pain and functional limitation. Diagnosis and treatment of this pathology can be challenging, and it is important to identify any concomitant pathologies or other pain sources. After failed nonoperative management, surgeons have the option of mini-open subpectoral biceps tenodesis—a safe, reliable, and effective treatment with excellent outcomes. Although multiple fixation options are available, we think that, based on the current literature, fixation with a bioabsorbable interference screw remains the best option. This procedure has demonstrated efficacy for revision biceps tenodesis, failed biceps tenotomy, and autorupture of the biceps.
1. Friedman DJ, Dunn JC, Higgins LD, Warner JJP. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Neviaser TJ, Neviaser RJ, Neviaser JS, Neviaser JS. The four-in-one arthroplasty for the painful arc syndrome. Clin Orthop Relat Res. 1982;163:107-112.
4. Patton WC, McCluskey GM 3rd. Biceps tendinitis and subluxation. Clin Sports Med. 2001;20(3):505-529.
5. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
6. Bennett WF. Arthroscopic repair of isolated subscapularis tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(2):131-143.
7. Dines DM, Warren RF, Inglis AE, Pavlov H. The coracoid impingement syndrome. Bone Joint J Br. 1990;72(2):314-316.
8. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
9. Boileau P, Baqué F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
10. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
11. Boileau P, Neyton L. Arthroscopic tenodesis for lesions of the long head of the biceps. Oper Orthop Traumatol. 2005;17(6):601-623.
12. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
13. Sanders B, Lavery K, Pennington S, Warner JJP. Biceps tendon tenodesis: success with proximal versus distal fixation (SS-16). Arthroscopy. 2008;24(6 suppl):e9.
14. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
15. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
16. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
17. Jarrett CD, McClelland WB, Xerogeanes JW. Minimally invasive proximal biceps tenodesis: an anatomical study for optimal placement and safe surgical technique. J Shoulder Elbow Surg. 2011;20(3):477-480.
18. Mazzocca AD, Noerdlinger MA, Romeo AA. Mini open and subpectoral biceps tenodesis. Oper Tech Sports Med. 2003;11(1):24-31.
19. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
20. Froimson AI, O I. Keyhole tenodesis of biceps origin at the shoulder. Clin Orthop Relat Res. 1975;(112):245-249.
21. Sekiya JK, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
22. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
23. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
24. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
25. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
26. Buchholz A, Martetschläger F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
27. Tashjian RZ, Henninger HB. Biomechanical evaluation of subpectoral biceps tenodesis: dual suture anchor versus interference screw fixation. J Shoulder Elbow Surg. 2013;22(10):1408-1412.
28. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJP. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9(1):121.
29. Sethi PM, Rajaram A, Beitzel K, Hackett TR, Chowaniec DM, Mazzocca AD. Biomechanical performance of subpectoral biceps tenodesis: a comparison of interference screw fixation, cortical button fixation, and interference screw diameter. J Shoulder Elbow Surg. 2013;22(4):451-457.
30. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
31. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
32. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
33. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
34. Werner BC, Evans CL, Holzgrefe RE, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of minimum 2-year clinical outcomes. Am J Sports Med. 2014;42(11):2583-2590.
35. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
36. Rhee PC, Spinner RJ, Bishop AT, Shin AY. Iatrogenic brachial plexus injuries associated with open subpectoral biceps tenodesis: a report of 4 cases. Am J Sports Med. 2013;41(9):2048-2053.
37. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
38. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
39. Abtahi AM, Granger EK, Tashjian RZ. Complications after subpectoral biceps tenodesis using a dual suture anchor technique. Int J Shoulder Surg. 2014;8(2):47-50.
40. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
41. Carofino BC, Brogan DM, Kircher MF, et al. Iatrogenic nerve injuries during shoulder surgery. J Bone Joint Surg Am. 2013;95(18):1667-1674.
42. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
43. Heckman DS, Creighton RA, Romeo AA. Management of failed biceps tenodesis or tenotomy: causation and treatment. Sports Med Arthrosc. 2010;18(3):173-180.
44. Gregory JM, Harwood DP, Gochanour E, Sherman SL, Romeo AA. Clinical outcomes of revision biceps tenodesis. Int J Shoulder Surg. 2012;6(2):45-50.
45. Anthony SG, McCormick F, Gross DJ, Golijanin P, Provencher MT. Biceps tenodesis for long head of the biceps after auto-rupture or failed surgical tenotomy: results in an active population. J Shoulder Elbow Surg. 2015;24(2):e36-e40.
1. Friedman DJ, Dunn JC, Higgins LD, Warner JJP. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Neviaser TJ, Neviaser RJ, Neviaser JS, Neviaser JS. The four-in-one arthroplasty for the painful arc syndrome. Clin Orthop Relat Res. 1982;163:107-112.
4. Patton WC, McCluskey GM 3rd. Biceps tendinitis and subluxation. Clin Sports Med. 2001;20(3):505-529.
5. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
6. Bennett WF. Arthroscopic repair of isolated subscapularis tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(2):131-143.
7. Dines DM, Warren RF, Inglis AE, Pavlov H. The coracoid impingement syndrome. Bone Joint J Br. 1990;72(2):314-316.
8. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
9. Boileau P, Baqué F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
10. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
11. Boileau P, Neyton L. Arthroscopic tenodesis for lesions of the long head of the biceps. Oper Orthop Traumatol. 2005;17(6):601-623.
12. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
13. Sanders B, Lavery K, Pennington S, Warner JJP. Biceps tendon tenodesis: success with proximal versus distal fixation (SS-16). Arthroscopy. 2008;24(6 suppl):e9.
14. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
15. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
16. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
17. Jarrett CD, McClelland WB, Xerogeanes JW. Minimally invasive proximal biceps tenodesis: an anatomical study for optimal placement and safe surgical technique. J Shoulder Elbow Surg. 2011;20(3):477-480.
18. Mazzocca AD, Noerdlinger MA, Romeo AA. Mini open and subpectoral biceps tenodesis. Oper Tech Sports Med. 2003;11(1):24-31.
19. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
20. Froimson AI, O I. Keyhole tenodesis of biceps origin at the shoulder. Clin Orthop Relat Res. 1975;(112):245-249.
21. Sekiya JK, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
22. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
23. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
24. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
25. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
26. Buchholz A, Martetschläger F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
27. Tashjian RZ, Henninger HB. Biomechanical evaluation of subpectoral biceps tenodesis: dual suture anchor versus interference screw fixation. J Shoulder Elbow Surg. 2013;22(10):1408-1412.
28. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJP. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9(1):121.
29. Sethi PM, Rajaram A, Beitzel K, Hackett TR, Chowaniec DM, Mazzocca AD. Biomechanical performance of subpectoral biceps tenodesis: a comparison of interference screw fixation, cortical button fixation, and interference screw diameter. J Shoulder Elbow Surg. 2013;22(4):451-457.
30. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
31. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
32. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
33. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
34. Werner BC, Evans CL, Holzgrefe RE, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of minimum 2-year clinical outcomes. Am J Sports Med. 2014;42(11):2583-2590.
35. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
36. Rhee PC, Spinner RJ, Bishop AT, Shin AY. Iatrogenic brachial plexus injuries associated with open subpectoral biceps tenodesis: a report of 4 cases. Am J Sports Med. 2013;41(9):2048-2053.
37. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
38. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
39. Abtahi AM, Granger EK, Tashjian RZ. Complications after subpectoral biceps tenodesis using a dual suture anchor technique. Int J Shoulder Surg. 2014;8(2):47-50.
40. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
41. Carofino BC, Brogan DM, Kircher MF, et al. Iatrogenic nerve injuries during shoulder surgery. J Bone Joint Surg Am. 2013;95(18):1667-1674.
42. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
43. Heckman DS, Creighton RA, Romeo AA. Management of failed biceps tenodesis or tenotomy: causation and treatment. Sports Med Arthrosc. 2010;18(3):173-180.
44. Gregory JM, Harwood DP, Gochanour E, Sherman SL, Romeo AA. Clinical outcomes of revision biceps tenodesis. Int J Shoulder Surg. 2012;6(2):45-50.
45. Anthony SG, McCormick F, Gross DJ, Golijanin P, Provencher MT. Biceps tenodesis for long head of the biceps after auto-rupture or failed surgical tenotomy: results in an active population. J Shoulder Elbow Surg. 2015;24(2):e36-e40.
Navigating the Alphabet Soup of Labroligamentous Pathology of the Shoulder
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
Precision medicine in cardiology
The possibility that we can define the specific and unique treatment for an individual’s specific disease is the holy grail of therapeutics. Our current therapy is largely defined by randomized clinical trials (RCTs), which provide an average response of therapy in a given disease tested in hundreds or thousands of patients.
RCTs identify the statistical benefit of a particular treatment when compared with a placebo in heterogeneous patients who, at best, are demographically similar but do not truly represent the general population.
Dr. Sidney Goldstein
Although an RCT shows an average statistical benefit, some individuals may have a profound benefit; others in the trial may not benefit at all, and some may do worse than placebo. The reason for the differential response is poorly understood. Therefore, treatment programs based on RCT data by definition are crude and imprecise by design for a variety of conditions, including cancer and cardiovascular diseases.
There has been a call for more personalization and precision in defining and developing predictably successful therapy. It is proposed that precision medicine will lead to optimal targeted individualized treatment based on patients’ genetic profile. This has led oncologists to use genetic profiling of therapy based on cells derived from patient’s tumor. Cancer therapy is in the forefront of genomic analysis of tumor tissue in order to guide specific tumor therapy. Unique DNA gene mutations are now being identified that may explain the genesis, spread, and growth of a tumor. It also provides a potential link between the genetic characteristics of the tumor to specific therapy. As a result, a number of laboratories have developed genetic probes that can mitigate gene expression or overexpression and thereby modify progression of disease.
To some degree, the field of cardiology has found some precision in defining individual therapy for the treatment of a variety of expressions of cardiovascular disease. We have drugs that are aimed at the treatment of hypertension, hypercholesterolemia, heart failure, and vascular thrombogenesis, to name some of the major targets. Just as the oncologists are searching for specific treatments of individual tumors, cardiology is searching for specific targets based on our understanding of the pathophysiologic mechanism leading to the expression and progression of disease. We have been fortunate in a large part to be able to measure pathophysiology and therapy at the bedside.
For some time, investigators have been examining the genetic variants in human tissue in order to understand drug responsiveness in several cardiovascular environments. Single nucleotide polymorphisms (SNP) have been discovered in beta-receptors that may define risk factors for disease and function as modifiers of disease once it has occurred. These factors also have the potential to modify beta-receptor response to adrenergic agonists and antagonists. The understanding of the SNP expression is anticipated to lead to the individualization of drug therapy and define or predict an individual’s response to particular drug therapy. As a result, they may explain the spectrum of clinical response observed in RCTs. Similar observations have been observed with the genetic polymorphism, of renin-angiotensin-aldosterone system and sympathetic systems. The understanding of genetic polymorphism may also provide insight into the expression of disease in particular demographic groups.
So far, the cost of these drugs is huge when applied to just a few individuals who are potential beneficiaries of the therapy. Consequently, patient and society and your insurance company are asked to pay the cost of the drugs to treat just a few patients. Over time, it is possible that these unique genomic characteristics can be applied to larger populations and may spread the costs over a larger number of patients. So far, the potential for this to happen is limited.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
The possibility that we can define the specific and unique treatment for an individual’s specific disease is the holy grail of therapeutics. Our current therapy is largely defined by randomized clinical trials (RCTs), which provide an average response of therapy in a given disease tested in hundreds or thousands of patients.
RCTs identify the statistical benefit of a particular treatment when compared with a placebo in heterogeneous patients who, at best, are demographically similar but do not truly represent the general population.
Dr. Sidney Goldstein
Although an RCT shows an average statistical benefit, some individuals may have a profound benefit; others in the trial may not benefit at all, and some may do worse than placebo. The reason for the differential response is poorly understood. Therefore, treatment programs based on RCT data by definition are crude and imprecise by design for a variety of conditions, including cancer and cardiovascular diseases.
There has been a call for more personalization and precision in defining and developing predictably successful therapy. It is proposed that precision medicine will lead to optimal targeted individualized treatment based on patients’ genetic profile. This has led oncologists to use genetic profiling of therapy based on cells derived from patient’s tumor. Cancer therapy is in the forefront of genomic analysis of tumor tissue in order to guide specific tumor therapy. Unique DNA gene mutations are now being identified that may explain the genesis, spread, and growth of a tumor. It also provides a potential link between the genetic characteristics of the tumor to specific therapy. As a result, a number of laboratories have developed genetic probes that can mitigate gene expression or overexpression and thereby modify progression of disease.
To some degree, the field of cardiology has found some precision in defining individual therapy for the treatment of a variety of expressions of cardiovascular disease. We have drugs that are aimed at the treatment of hypertension, hypercholesterolemia, heart failure, and vascular thrombogenesis, to name some of the major targets. Just as the oncologists are searching for specific treatments of individual tumors, cardiology is searching for specific targets based on our understanding of the pathophysiologic mechanism leading to the expression and progression of disease. We have been fortunate in a large part to be able to measure pathophysiology and therapy at the bedside.
For some time, investigators have been examining the genetic variants in human tissue in order to understand drug responsiveness in several cardiovascular environments. Single nucleotide polymorphisms (SNP) have been discovered in beta-receptors that may define risk factors for disease and function as modifiers of disease once it has occurred. These factors also have the potential to modify beta-receptor response to adrenergic agonists and antagonists. The understanding of the SNP expression is anticipated to lead to the individualization of drug therapy and define or predict an individual’s response to particular drug therapy. As a result, they may explain the spectrum of clinical response observed in RCTs. Similar observations have been observed with the genetic polymorphism, of renin-angiotensin-aldosterone system and sympathetic systems. The understanding of genetic polymorphism may also provide insight into the expression of disease in particular demographic groups.
So far, the cost of these drugs is huge when applied to just a few individuals who are potential beneficiaries of the therapy. Consequently, patient and society and your insurance company are asked to pay the cost of the drugs to treat just a few patients. Over time, it is possible that these unique genomic characteristics can be applied to larger populations and may spread the costs over a larger number of patients. So far, the potential for this to happen is limited.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
The possibility that we can define the specific and unique treatment for an individual’s specific disease is the holy grail of therapeutics. Our current therapy is largely defined by randomized clinical trials (RCTs), which provide an average response of therapy in a given disease tested in hundreds or thousands of patients.
RCTs identify the statistical benefit of a particular treatment when compared with a placebo in heterogeneous patients who, at best, are demographically similar but do not truly represent the general population.
Dr. Sidney Goldstein
Although an RCT shows an average statistical benefit, some individuals may have a profound benefit; others in the trial may not benefit at all, and some may do worse than placebo. The reason for the differential response is poorly understood. Therefore, treatment programs based on RCT data by definition are crude and imprecise by design for a variety of conditions, including cancer and cardiovascular diseases.
There has been a call for more personalization and precision in defining and developing predictably successful therapy. It is proposed that precision medicine will lead to optimal targeted individualized treatment based on patients’ genetic profile. This has led oncologists to use genetic profiling of therapy based on cells derived from patient’s tumor. Cancer therapy is in the forefront of genomic analysis of tumor tissue in order to guide specific tumor therapy. Unique DNA gene mutations are now being identified that may explain the genesis, spread, and growth of a tumor. It also provides a potential link between the genetic characteristics of the tumor to specific therapy. As a result, a number of laboratories have developed genetic probes that can mitigate gene expression or overexpression and thereby modify progression of disease.
To some degree, the field of cardiology has found some precision in defining individual therapy for the treatment of a variety of expressions of cardiovascular disease. We have drugs that are aimed at the treatment of hypertension, hypercholesterolemia, heart failure, and vascular thrombogenesis, to name some of the major targets. Just as the oncologists are searching for specific treatments of individual tumors, cardiology is searching for specific targets based on our understanding of the pathophysiologic mechanism leading to the expression and progression of disease. We have been fortunate in a large part to be able to measure pathophysiology and therapy at the bedside.
For some time, investigators have been examining the genetic variants in human tissue in order to understand drug responsiveness in several cardiovascular environments. Single nucleotide polymorphisms (SNP) have been discovered in beta-receptors that may define risk factors for disease and function as modifiers of disease once it has occurred. These factors also have the potential to modify beta-receptor response to adrenergic agonists and antagonists. The understanding of the SNP expression is anticipated to lead to the individualization of drug therapy and define or predict an individual’s response to particular drug therapy. As a result, they may explain the spectrum of clinical response observed in RCTs. Similar observations have been observed with the genetic polymorphism, of renin-angiotensin-aldosterone system and sympathetic systems. The understanding of genetic polymorphism may also provide insight into the expression of disease in particular demographic groups.
So far, the cost of these drugs is huge when applied to just a few individuals who are potential beneficiaries of the therapy. Consequently, patient and society and your insurance company are asked to pay the cost of the drugs to treat just a few patients. Over time, it is possible that these unique genomic characteristics can be applied to larger populations and may spread the costs over a larger number of patients. So far, the potential for this to happen is limited.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Lateral Ulnar Collateral Ligament Reconstruction: An Analysis of Ulnar Tunnel Locations
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
A call for definitive trial of statins in breast cancer
SAN ANTONIO – A definitive phase III randomized controlled trial of statin therapy during adjuvant endocrine therapy for breast cancer is warranted in light of recent encouraging evidence pointing to a protective effect against disease recurrence, Melissa L. Bondy, Ph.D., said at the San Antonio Breast Cancer Symposium.
“The next phase of research in this area is to learn whether we can improve survival in breast cancer patients by having them take cholesterol-lowering medication. We really need to have a phase III trial in the adjuvant setting that includes these cholesterol-lowering drugs,” said Dr. Bondy, professor of cancer prevention and population sciences at Baylor Medical College, Houston.
Her call for a formal, randomized trial came while serving as discussant for two positive reports of an apparent beneficial effect of statins on breast cancer recurrence-free survival: one from the BIG 1-98 trial of adjuvant endocrine therapy for early-stage breast cancer, the other a meta-analysis of 12 published studies totaling 72,774 breast cancer patients.
Dr. Bondy found particularly compelling the Breast International Group (BIG) 1-98 study results presented by Dr. Signe Borgquist, an oncologist at Lund (Sweden) University. This secondary analysis of the impact of cholesterol-lowering medication included 7,963 postmenopausal women with early-stage, estrogen receptor–positive invasive breast cancer who were randomized to 5 years of tamoxifen, the aromatase inhibitor letrozole (Femara), or the two drugs in sequence. Serum total cholesterol levels and the use of cholesterol-lowering medications – a decision left up to individual physicians and patients – were assessed at baseline and again every 6 months for 5.5 years.
With a median 8 years of prospective follow-up, during which 1,432 patients experienced breast cancer recurrence, the disease-free survival rate was 18% higher in the 637 patients already on cholesterol-lowering medication – mainly statins – at enrollment, compared with those who were not. The distant recurrence-free interval was 19% better as well, with both analyses adjusted for their form of endocrine therapy, tumor size and grade, nodal status, local therapy, peritumoral vascular invasion, and other potential confounders.
Another 697 BIG 1-98 participants initiated cholesterol-lowering medication during their adjuvant endocrine therapy. In multivariate adjusted analyses, their disease-free survival rate was 21% greater and distant recurrence-free interval was 26% better than in participants not on a statin or other cholesterol-lowering medication during the trial.
As a possible mechanism by which statins might exert anticancer effects, Dr. Borgquist proposed that lowering cholesterol levels may deprive tumor cells of the ability to satisfy their increased demand for cholesterol uptake. This is a result of attenuated signaling through the estrogen receptor by the cholesterol metabolite 27-hydroxycholesterol, levels of which correlate with systemic total cholesterol.
Dr. Bondy offered another possible explanation for the reduced risk of breast cancer recurrence seen in women on cholesterol-lowering medication in BIG 1-98: “Many groups have shown that LDL affects the immune system by binding and inactivating all kinds of microorganisms and their toxic products. In other words, statins appear to affect the microbiome.”
She commented that, although the use of statins was nonrandomized in BIG 1-98, she was favorably impressed by the 8-year follow-up and careful monitoring of total cholesterol levels at 6-month intervals throughout the 5 years of adjuvant endocrine therapy.
Dr. Bondy noted that other studies – again, nonrandomized – suggest statins also interrupt the growth of colorectal cancer and other malignancies.
Dr. Sashidhar Manthravadi presented a meta-analysis of the 12 published studies that have reported data on the association of statins with recurrence-free and/or overall survival in breast cancer patients. Statin use was associated with a significant 34% improvement in recurrence-free survival.
Moreover, this benefit was confined to breast cancer patients on the lipophilic statins atorvastatin and simvastatin; the use of hydrophilic statins was not associated with a significant improvement in recurrence-free survival, according to Dr. Manthravadi, an internal medicine resident at the University of Missouri, Kansas City.
The use of statins also was associated with a 27% improvement in breast cancer–specific survival and a 33% improvement in overall survival, compared with statin nonusers; however, neither of these results achieved statistical significance, he added.
Dr. Bondy cautioned against making too much of the apparent distinction between lipophilic and hydrophilic statin in terms of protection against breast cancer recurrence, given the inherent limitations of a meta-analysis.
“There’s always a lot of missing information, as well as a failure to adjust for comorbidities. And follow-up times in these 12 studies ranged from 2.5 to 11.5 years,” she noted.
The ongoing BIG 1-98 trial is sponsored by the International Breast Cancer Study Group. Dr. Borgquist, Dr. Bondy, and Dr. Manthravadi reported having no financial conflicts of interest.
SAN ANTONIO – A definitive phase III randomized controlled trial of statin therapy during adjuvant endocrine therapy for breast cancer is warranted in light of recent encouraging evidence pointing to a protective effect against disease recurrence, Melissa L. Bondy, Ph.D., said at the San Antonio Breast Cancer Symposium.
“The next phase of research in this area is to learn whether we can improve survival in breast cancer patients by having them take cholesterol-lowering medication. We really need to have a phase III trial in the adjuvant setting that includes these cholesterol-lowering drugs,” said Dr. Bondy, professor of cancer prevention and population sciences at Baylor Medical College, Houston.
Her call for a formal, randomized trial came while serving as discussant for two positive reports of an apparent beneficial effect of statins on breast cancer recurrence-free survival: one from the BIG 1-98 trial of adjuvant endocrine therapy for early-stage breast cancer, the other a meta-analysis of 12 published studies totaling 72,774 breast cancer patients.
Dr. Bondy found particularly compelling the Breast International Group (BIG) 1-98 study results presented by Dr. Signe Borgquist, an oncologist at Lund (Sweden) University. This secondary analysis of the impact of cholesterol-lowering medication included 7,963 postmenopausal women with early-stage, estrogen receptor–positive invasive breast cancer who were randomized to 5 years of tamoxifen, the aromatase inhibitor letrozole (Femara), or the two drugs in sequence. Serum total cholesterol levels and the use of cholesterol-lowering medications – a decision left up to individual physicians and patients – were assessed at baseline and again every 6 months for 5.5 years.
With a median 8 years of prospective follow-up, during which 1,432 patients experienced breast cancer recurrence, the disease-free survival rate was 18% higher in the 637 patients already on cholesterol-lowering medication – mainly statins – at enrollment, compared with those who were not. The distant recurrence-free interval was 19% better as well, with both analyses adjusted for their form of endocrine therapy, tumor size and grade, nodal status, local therapy, peritumoral vascular invasion, and other potential confounders.
Another 697 BIG 1-98 participants initiated cholesterol-lowering medication during their adjuvant endocrine therapy. In multivariate adjusted analyses, their disease-free survival rate was 21% greater and distant recurrence-free interval was 26% better than in participants not on a statin or other cholesterol-lowering medication during the trial.
As a possible mechanism by which statins might exert anticancer effects, Dr. Borgquist proposed that lowering cholesterol levels may deprive tumor cells of the ability to satisfy their increased demand for cholesterol uptake. This is a result of attenuated signaling through the estrogen receptor by the cholesterol metabolite 27-hydroxycholesterol, levels of which correlate with systemic total cholesterol.
Dr. Bondy offered another possible explanation for the reduced risk of breast cancer recurrence seen in women on cholesterol-lowering medication in BIG 1-98: “Many groups have shown that LDL affects the immune system by binding and inactivating all kinds of microorganisms and their toxic products. In other words, statins appear to affect the microbiome.”
She commented that, although the use of statins was nonrandomized in BIG 1-98, she was favorably impressed by the 8-year follow-up and careful monitoring of total cholesterol levels at 6-month intervals throughout the 5 years of adjuvant endocrine therapy.
Dr. Bondy noted that other studies – again, nonrandomized – suggest statins also interrupt the growth of colorectal cancer and other malignancies.
Dr. Sashidhar Manthravadi presented a meta-analysis of the 12 published studies that have reported data on the association of statins with recurrence-free and/or overall survival in breast cancer patients. Statin use was associated with a significant 34% improvement in recurrence-free survival.
Moreover, this benefit was confined to breast cancer patients on the lipophilic statins atorvastatin and simvastatin; the use of hydrophilic statins was not associated with a significant improvement in recurrence-free survival, according to Dr. Manthravadi, an internal medicine resident at the University of Missouri, Kansas City.
The use of statins also was associated with a 27% improvement in breast cancer–specific survival and a 33% improvement in overall survival, compared with statin nonusers; however, neither of these results achieved statistical significance, he added.
Dr. Bondy cautioned against making too much of the apparent distinction between lipophilic and hydrophilic statin in terms of protection against breast cancer recurrence, given the inherent limitations of a meta-analysis.
“There’s always a lot of missing information, as well as a failure to adjust for comorbidities. And follow-up times in these 12 studies ranged from 2.5 to 11.5 years,” she noted.
The ongoing BIG 1-98 trial is sponsored by the International Breast Cancer Study Group. Dr. Borgquist, Dr. Bondy, and Dr. Manthravadi reported having no financial conflicts of interest.
SAN ANTONIO – A definitive phase III randomized controlled trial of statin therapy during adjuvant endocrine therapy for breast cancer is warranted in light of recent encouraging evidence pointing to a protective effect against disease recurrence, Melissa L. Bondy, Ph.D., said at the San Antonio Breast Cancer Symposium.
“The next phase of research in this area is to learn whether we can improve survival in breast cancer patients by having them take cholesterol-lowering medication. We really need to have a phase III trial in the adjuvant setting that includes these cholesterol-lowering drugs,” said Dr. Bondy, professor of cancer prevention and population sciences at Baylor Medical College, Houston.
Her call for a formal, randomized trial came while serving as discussant for two positive reports of an apparent beneficial effect of statins on breast cancer recurrence-free survival: one from the BIG 1-98 trial of adjuvant endocrine therapy for early-stage breast cancer, the other a meta-analysis of 12 published studies totaling 72,774 breast cancer patients.
Dr. Bondy found particularly compelling the Breast International Group (BIG) 1-98 study results presented by Dr. Signe Borgquist, an oncologist at Lund (Sweden) University. This secondary analysis of the impact of cholesterol-lowering medication included 7,963 postmenopausal women with early-stage, estrogen receptor–positive invasive breast cancer who were randomized to 5 years of tamoxifen, the aromatase inhibitor letrozole (Femara), or the two drugs in sequence. Serum total cholesterol levels and the use of cholesterol-lowering medications – a decision left up to individual physicians and patients – were assessed at baseline and again every 6 months for 5.5 years.
With a median 8 years of prospective follow-up, during which 1,432 patients experienced breast cancer recurrence, the disease-free survival rate was 18% higher in the 637 patients already on cholesterol-lowering medication – mainly statins – at enrollment, compared with those who were not. The distant recurrence-free interval was 19% better as well, with both analyses adjusted for their form of endocrine therapy, tumor size and grade, nodal status, local therapy, peritumoral vascular invasion, and other potential confounders.
Another 697 BIG 1-98 participants initiated cholesterol-lowering medication during their adjuvant endocrine therapy. In multivariate adjusted analyses, their disease-free survival rate was 21% greater and distant recurrence-free interval was 26% better than in participants not on a statin or other cholesterol-lowering medication during the trial.
As a possible mechanism by which statins might exert anticancer effects, Dr. Borgquist proposed that lowering cholesterol levels may deprive tumor cells of the ability to satisfy their increased demand for cholesterol uptake. This is a result of attenuated signaling through the estrogen receptor by the cholesterol metabolite 27-hydroxycholesterol, levels of which correlate with systemic total cholesterol.
Dr. Bondy offered another possible explanation for the reduced risk of breast cancer recurrence seen in women on cholesterol-lowering medication in BIG 1-98: “Many groups have shown that LDL affects the immune system by binding and inactivating all kinds of microorganisms and their toxic products. In other words, statins appear to affect the microbiome.”
She commented that, although the use of statins was nonrandomized in BIG 1-98, she was favorably impressed by the 8-year follow-up and careful monitoring of total cholesterol levels at 6-month intervals throughout the 5 years of adjuvant endocrine therapy.
Dr. Bondy noted that other studies – again, nonrandomized – suggest statins also interrupt the growth of colorectal cancer and other malignancies.
Dr. Sashidhar Manthravadi presented a meta-analysis of the 12 published studies that have reported data on the association of statins with recurrence-free and/or overall survival in breast cancer patients. Statin use was associated with a significant 34% improvement in recurrence-free survival.
Moreover, this benefit was confined to breast cancer patients on the lipophilic statins atorvastatin and simvastatin; the use of hydrophilic statins was not associated with a significant improvement in recurrence-free survival, according to Dr. Manthravadi, an internal medicine resident at the University of Missouri, Kansas City.
The use of statins also was associated with a 27% improvement in breast cancer–specific survival and a 33% improvement in overall survival, compared with statin nonusers; however, neither of these results achieved statistical significance, he added.
Dr. Bondy cautioned against making too much of the apparent distinction between lipophilic and hydrophilic statin in terms of protection against breast cancer recurrence, given the inherent limitations of a meta-analysis.
“There’s always a lot of missing information, as well as a failure to adjust for comorbidities. And follow-up times in these 12 studies ranged from 2.5 to 11.5 years,” she noted.
The ongoing BIG 1-98 trial is sponsored by the International Breast Cancer Study Group. Dr. Borgquist, Dr. Bondy, and Dr. Manthravadi reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM SABCS 2015
Lymphedema microsurgery gaining momentum
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
CHICAGO – Microsurgery does not cure lymphedema, in most cases. But, in most cases, it does improve the severity of lymphedema and reduce the complications of this chronic and debilitating disease. And “it certainly improves patients’ quality of life,” lymphedema treatment pioneer Dr. David W. Chang said at the 40th annual Northwestern Vascular Symposium.
Surgical treatment for limb lymphedema has come into its own since a lymphovenous shunt was first used in a dog model in 1962, with Dr. Chang and others now anastomosing subdermal lymphatics to subdermal venules less than 0.8 mm in diameter. The rationale behind “super-microsurgery” is that venous pressure is low in the subdermal venules and has minimal back flow, he said.
One of the big problems early on was knowing exactly where the lymphatic vessels were, but newer technology like indocyanine green (ICG) lymphangiography helps visualize functioning lymphatic channels for potential bypass and determine the severity of the disease. Understanding the disease stage is key to selecting the appropriate surgical procedure.
Lymphovenous bypass (LVB) is best in patients with stage 1 or 2 upper extremity lymphedema, while lymph node transfer (LNT) works for patients who are poor candidates for LVB or require combined breast reconstruction, said Dr. Chang, a plastic surgeon with the University of Chicago.
More recently, Dr. Chang has begun combining LVB and LNT, particularly for the more severe cases with stage 3 or 4 upper or lower extremity disease.
In Dr. Chang’s first 100 consecutive LVB cases while at the M.D. Anderson Cancer Center in Houston, quantitative improvement occurred in 74% of patients, symptom improvement in 96%, and the average volume differential reduction was 42% at 12 months (Plast Reconstr Surg. 2013 Nov;132:1305-14). The reduction was significantly larger in patients with earlier stage 1 or 2 vs. later stage 3 or 4 disease (61% vs. 17%).
During lymphovenous bypass, ICG is injected into the dermis of the web space and the superficial lymphatics evaluated with near-infrared fluorescence. It is easy to identify discrete functioning lymphatic channels in early-stage disease, but in late-stage disease significant dermal back flow is present, Dr. Chang said.
Dissection is performed under the microscope in the superficial subcutaneous plane to locate a good venule and lymph channel. Lymphatics are confirmed with isosulfan blue and ICG, and once the bypass site is determined, the lymphatic is anastomosed to the venule using 11-0 or 12-0 nylon, preferably in an end-to-side fashion. It’s thought this creates a more favorable flow pattern for the lymph to empty into the venule than an end-to-end anastomosis, he observed.
After the anastomosis is complete, patency is confirmed with isosulfan blue and ICG and the incision is closed under the microscope to ensure that the delicate anastomosis isn’t damaged. To avoid shear injury to the anastomosis, the limb is wrapped postoperatively for about a month without use of compression garments, he said.
Lymph node transfer (LNT) is increasingly being offered at centers to provide relief from lymphedema, although the mechanism by which it works is yet unclear; either the healthy lymph nodes act as a sponge to absorb lymphatic fluid or they induce lymphangiogenesis. Experience has shown, however, that rather than just grafting the lymph nodes, they need to be harvested with a vascular pedicle before transfer and anastomosed to the recipient artery and vein, although reconnecting the actual lymphatics may not be necessary, Dr. Chang observed.
Despite its popularity as a donor site, Dr. Chang said he is reluctant to use the groin because of the potential for iatrogenic lymphedema and prefers to harvest the supraclavicular nodes based off the transverse cervical artery. The external jugular vein can be harvested with the nodes if adequate venae comitantes are not present with the artery. Dissection of this flap can be difficult and care should be taken not to injure the lymphatic ducts, he noted.
It is also important to excise all scar tissue in the recipient site as this can impair lymphatic flow and inhibits lymphangiogenesis. If it is difficult to access or remove the scar, the vascularized lymph nodes are best placed just distal on the limb to the site of lymphatic obstruction, he added.
A recent meta-analysis (Plast Reconstr Surg. 2014 Apr;133:905-13) in five LNT studies reported that 91% of patients had a quantitative improvement, 78% discontinued compression garments, and complications were infection (8%), lymphorrhea (15%), and need for additional procedures (36%). There was great heterogeneity between studies, so the results should be interpreted with caution, Dr. Chang advised.
LNT is frequently combined with autologous breast reconstruction in patients with breast cancer, who comprise a significant percentage of Dr. Chang’s practice. The overall incidence of arm lymphedema after breast cancer can range from 8% to 56% at 2 years’ post-surgery, with the risk higher among women undergoing axillary lymph node dissection and/or axillary radiation.
Outcomes with combined LNT and breast reconstruction have been favorable, with one series reporting evidence of improved lymphatic flow on lymphoscintigraphy in five of six cases and one-third of patients no longer needing compression therapy (Ann Surg. 2012 Mar;255:468-73).
In cases where the patient requires a large skin paddle or seeks breast reconstruction after a previous mastectomy, lateral superficial groin lymph nodes can be harvested for transfer, leaving the deeper lymph nodes that drain the leg behind, Dr. Chang said. The nodes are usually clustered at the junction of the superior inferior epigastric and superficial circumflex iliac veins.
When combining LNT with breast reconstruction, this tissue is harvested together with the free abdominal flap used to reconstruct the breast. The superficial circumflex iliac vein is anastomosed in the axilla in addition to the arterial and venous anastomosis of the deep inferior epigastric vessels to the internal mammary vessels for the breast reconstruction. Reverse lymphatic mapping with technetium and ICG is used to decrease the risk of donor site lymphedema.
An algorithmic approach to simultaneous LNT with microvascular breast reconstruction proposed by Dr. Chang resulted in a 47% reduction in mean volume differential 12 months after reconstruction in 29 consecutive patients with refractory lymphedema following breast cancer treatment. These early results also showed no flap losses or donor-site lymphedema and donor-site wound complications in six patients (21%) that resolved with conservative measures (Ann Surg Oncol. 2015 Sep;22:2919-24).
The holy grail may be to strike lymphedema before it develops. To that end, Italian surgeons have proposed the Lymphatic Microsurgical Preventing Healing Approach (LYMPHA), which involves anastomosing arm lymphatics to a collateral branch of the axillary vein at the time of nodal dissection.
Over more than 4 years’ follow-up, only 3 of 74 breast cancer patients who underwent axillary nodal dissection with LYMPHA developed lymphedema, translating into a an exceptionally low 4% risk of lymphedema (Microsurgery. 2014 Sep;34:421-4). However, this approach is controversial because of unknown oncological risk and the uncertainty of its effectiveness in patients who may receive radiation after the surgery, Dr. Chang said in an interview.
Although these techniques show promise, currently no optimal solution exists and more research is needed to better understand lymphatic anatomy and physiology and the pathophysiology of lymphedema, concluded Dr. Chang, who reported no relevant conflicts of interest.
EXPERT ANALYSIS AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Neurosurgeon memoir illuminates the journey through cancer treatment and acceptance of mortality
Dr. Paul Kalanithi, a neurosurgeon who had just completed his residency at the Stanford (Calif.) University, died of metastatic lung cancer last year, but he left a memoir of his experiences as a physician, a patient, and a dying man that was published on Jan. 12. His book, “When Breath Becomes Air” (New York: Random House, 2016), recounts the many years of working to exhaustion and deferring of life experiences and pleasures that are necessary to complete medical training.
In a review of the book, Janet Maslin wrote, “One of the most poignant things about Dr. Kalanithi’s story is that he had postponed learning how to live while pursuing his career in neurosurgery. By the time he was ready to enjoy a life outside the operating room, what he needed to learn was how to die.”
Dr. Kalanithi reflected on the profound grief and sense of loss that comes with a diagnosis that he knew meant imminent death. The memoir also reveals his search for meaning and joy, and finally, his acceptance of mortality. He opted for palliative care and his memoir, along with the epilogue written by his wife, Dr. Lucy Kalanithi, gives insight into the value of the palliative path to patients and their families in dire medical crises.
Dr. Paul Kalanithi, a neurosurgeon who had just completed his residency at the Stanford (Calif.) University, died of metastatic lung cancer last year, but he left a memoir of his experiences as a physician, a patient, and a dying man that was published on Jan. 12. His book, “When Breath Becomes Air” (New York: Random House, 2016), recounts the many years of working to exhaustion and deferring of life experiences and pleasures that are necessary to complete medical training.
In a review of the book, Janet Maslin wrote, “One of the most poignant things about Dr. Kalanithi’s story is that he had postponed learning how to live while pursuing his career in neurosurgery. By the time he was ready to enjoy a life outside the operating room, what he needed to learn was how to die.”
Dr. Kalanithi reflected on the profound grief and sense of loss that comes with a diagnosis that he knew meant imminent death. The memoir also reveals his search for meaning and joy, and finally, his acceptance of mortality. He opted for palliative care and his memoir, along with the epilogue written by his wife, Dr. Lucy Kalanithi, gives insight into the value of the palliative path to patients and their families in dire medical crises.
Dr. Paul Kalanithi, a neurosurgeon who had just completed his residency at the Stanford (Calif.) University, died of metastatic lung cancer last year, but he left a memoir of his experiences as a physician, a patient, and a dying man that was published on Jan. 12. His book, “When Breath Becomes Air” (New York: Random House, 2016), recounts the many years of working to exhaustion and deferring of life experiences and pleasures that are necessary to complete medical training.
In a review of the book, Janet Maslin wrote, “One of the most poignant things about Dr. Kalanithi’s story is that he had postponed learning how to live while pursuing his career in neurosurgery. By the time he was ready to enjoy a life outside the operating room, what he needed to learn was how to die.”
Dr. Kalanithi reflected on the profound grief and sense of loss that comes with a diagnosis that he knew meant imminent death. The memoir also reveals his search for meaning and joy, and finally, his acceptance of mortality. He opted for palliative care and his memoir, along with the epilogue written by his wife, Dr. Lucy Kalanithi, gives insight into the value of the palliative path to patients and their families in dire medical crises.
David Bowie’s death inspires blog on palliative care
The death of David Bowie, iconic musician and artist, on Jan. 10 inspired palliative care specialist Dr. Mark Taubert to write a blog about end-of-life scenarios and the importance of advance care planning. The blog, which begins by thanking Mr. Bowie for his many artistic contributions, continues by suggesting that his planned death at home will inspire many people in similar health crises to consider palliative care. The palliative care conversation between a doctor and a patient facing death can be challenging but can lead to what Dr. Taubert called “a good death” at home with symptoms managed and loved ones nearby. Mr. Bowie’s son, Duncan Jones, tweeted a link to the blog in the days after his father’s death.
Dr. Taubert found himself speaking with a patient who was facing probable death in the near future, and both doctor and patient found inspiration in Mr. Bowie’s final music project and his death at home with his family. Dr. Taubert and his patient were able to have the conversation about palliative care at end-of-life in part because they were both impressed with what Mr. Bowie was able to achieve in his last months. “Your story became a way for us to communicate very openly about death, something many doctors and nurses struggle to introduce as a topic of conversation,” he wrote.
Dr. Taubert of the Velindre NHS Trust in Cardiff, Wales, noted that, although palliative care is a highly developed skill with many resources to help patients at the end of life, “this essential part of training is not always available for junior healthcare professionals, including doctors and nurses, and is sometimes overlooked or under-prioritized by those who plan their education. I think if you [David Bowie] were ever to return (as Lazarus did), you would be a firm advocate for good palliative care training being available everywhere.”
The death of David Bowie, iconic musician and artist, on Jan. 10 inspired palliative care specialist Dr. Mark Taubert to write a blog about end-of-life scenarios and the importance of advance care planning. The blog, which begins by thanking Mr. Bowie for his many artistic contributions, continues by suggesting that his planned death at home will inspire many people in similar health crises to consider palliative care. The palliative care conversation between a doctor and a patient facing death can be challenging but can lead to what Dr. Taubert called “a good death” at home with symptoms managed and loved ones nearby. Mr. Bowie’s son, Duncan Jones, tweeted a link to the blog in the days after his father’s death.
Dr. Taubert found himself speaking with a patient who was facing probable death in the near future, and both doctor and patient found inspiration in Mr. Bowie’s final music project and his death at home with his family. Dr. Taubert and his patient were able to have the conversation about palliative care at end-of-life in part because they were both impressed with what Mr. Bowie was able to achieve in his last months. “Your story became a way for us to communicate very openly about death, something many doctors and nurses struggle to introduce as a topic of conversation,” he wrote.
Dr. Taubert of the Velindre NHS Trust in Cardiff, Wales, noted that, although palliative care is a highly developed skill with many resources to help patients at the end of life, “this essential part of training is not always available for junior healthcare professionals, including doctors and nurses, and is sometimes overlooked or under-prioritized by those who plan their education. I think if you [David Bowie] were ever to return (as Lazarus did), you would be a firm advocate for good palliative care training being available everywhere.”
The death of David Bowie, iconic musician and artist, on Jan. 10 inspired palliative care specialist Dr. Mark Taubert to write a blog about end-of-life scenarios and the importance of advance care planning. The blog, which begins by thanking Mr. Bowie for his many artistic contributions, continues by suggesting that his planned death at home will inspire many people in similar health crises to consider palliative care. The palliative care conversation between a doctor and a patient facing death can be challenging but can lead to what Dr. Taubert called “a good death” at home with symptoms managed and loved ones nearby. Mr. Bowie’s son, Duncan Jones, tweeted a link to the blog in the days after his father’s death.
Dr. Taubert found himself speaking with a patient who was facing probable death in the near future, and both doctor and patient found inspiration in Mr. Bowie’s final music project and his death at home with his family. Dr. Taubert and his patient were able to have the conversation about palliative care at end-of-life in part because they were both impressed with what Mr. Bowie was able to achieve in his last months. “Your story became a way for us to communicate very openly about death, something many doctors and nurses struggle to introduce as a topic of conversation,” he wrote.
Dr. Taubert of the Velindre NHS Trust in Cardiff, Wales, noted that, although palliative care is a highly developed skill with many resources to help patients at the end of life, “this essential part of training is not always available for junior healthcare professionals, including doctors and nurses, and is sometimes overlooked or under-prioritized by those who plan their education. I think if you [David Bowie] were ever to return (as Lazarus did), you would be a firm advocate for good palliative care training being available everywhere.”
Families perceive few benefits from aggressive end-of-life care
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
FROM JAMA
Key clinical point: Bereaved family members were more satisfied with end-of-life cancer care when patients spent more than 3 days in hospice, died outside the hospital, and were not admitted to the ICU within 30 days of dying.
Major finding: Care was described as “excellent” about 9%-17% more often when these end-of-life quality indicators were met.
Data source: A multicenter, prospective, observational study of 1,146 family members of patients who died of lung or colorectal cancer.
Disclosures: The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the analysis. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
Law & Medicine: Health care costs and defensive medicine
Question: Which of the following choices is best?
A. Controversy exists over whether defensive medicine is widely practiced and whether it raises health care costs.
B. Everyone agrees that defensive medicine is widely practiced.
C. Doctors who spend more for their hospitalized patients have been reported to face a lower malpractice risk.
D. A and C.
E. B and C.
Answer: D. Virtually all doctors admit they practice defensive medicine, which describes medical care specifically directed to attenuate the threat of malpractice liability rather than for a proper medical indication. Survey studies generally put the prevalence at greater than 90%, but what separates true defensive medicine from careful practice or patient expectation/demand remains controversial.
A mail survey of 824 physicians in high-risk specialties in Pennsylvania, a state where doctors pay high malpractice premiums, revealed that nearly all reported practicing defensive medicine. The respondents admitted to both assurance behavior – such as ordering more tests, especially imaging studies – as well as avoidance behavior – that is, restricting or eliminating complex procedures or perceived litigious patients.1
In another study, emergency physicians in the upper third of “malpractice fear” used more diagnostic tests and were more likely to hospitalize patients at low risk for coronary artery disease.2
On the other hand, a report using simulated clinical scenarios concluded that the extent of defensive medicine was at most 8%, and another found no correlation between individual malpractice claims experience and resource use, physician concern about malpractice, tolerance for uncertainty, or perception of risk.3
Another area of contention is whether some of our soaring health care costs may reflect defensive medicine at play.
A recent survey of 2,000 U.S. orthopedic surgeons found that 96% admitted practicing defensive medicine, with 24% of all ordered tests being for defensive reasons. The authors estimated that this amounted to about $100,000 per surgeon per year, or a total annual sum of $2 billion for the 20,400 orthopedic surgeons in the U.S.4
By correlating professional liability insurance with cost of services, the AMA estimated that in the 1980s, defensive medicine cost $12.1 billion to $13.7 billion each year.5 In an oft-cited study by Kessler and McClellan, the authors measured the effects of malpractice liability reforms using data from elderly Medicare beneficiaries treated for serious heart disease.6 They found that reforms that directly reduced provider liability pressure led to reductions of 5%-9% in medical expenditures. If such Medicare savings, which amounted to $600 million per year for cardiac disease, were extrapolated across the health care system, the annual savings would total $50 billion.
A more conservative study estimated that systemwide savings from aggressive malpractice reform would approach $41 billion over 5 years.7
Dr. Anupam B. Jena and his coauthors are the latest investigators seeking to clarify the correlation between defensive medicine and health care costs.8 Using Florida hospital admission data from 2000-2009, which covered some 24,000 physicians in seven separate specialties, the authors found that higher spending by physicians was associated with reduced malpractice claims the following year. This pattern held true for six of the seven specialties, family practitioners being the sole exception.
For example, among internists, the malpractice risk probability was reduced from 1.5% in the bottom spending fifth ($19,725 per admission) to 0.3% in the top fifth ($39,379 per admission). Among obstetricians, a separate subgroup analysis of cesarean-section rates revealed that malpractice claims were approximately halved among obstetricians with rates in the highest fifth, compared with the lowest fifth. These results comport with previous reports of higher C-section rates in obstetricians who perceived themselves at higher malpractice risk, although other studies have found no correlation or an actual lower rate.
In concluding that higher resource use by physicians was associated with fewer malpractice claims, the authors acknowledged that a principal limitation of the study was the lack of information on illness severity. Importantly, they were unable to state whether higher spending was defensively motivated. A companion editorial questioned whether increased resource expenditure was a bona fide reflection of defensive medicine or may simply have resulted in fewer errors and adverse events.9
Unfortunately, the past malpractice experience of the doctors, which could provide insight into more defensive postures, better communication, etc., was not measured in the study. In addition, claims made just 1 year after an incident may not accurately reflect the real-world situation, where the filing of a lawsuit may lag any alleged negligence by a much longer period, especially in obstetric cases.
Worryingly, the results of this timely study may be used to imply that the more doctors spend, the less likely they are to be sued – a troubling notion in our current race to stem rising health care costs.
References
1. JAMA. 2005 Jun 1;293(21):2609-17.
2. Ann Emerg Med. 2005 Dec;46(6):525-33.
3. J Health Polit Policy Law. 1996 Summer;21(2):267-88.
4. Am J Orthop (Belle Mead NJ). 2012 Feb;41(2):69-73.
5. JAMA. 1987 May 22-29;257(20):2776-81.
6. Q J Econ. 1996 May; 111(2):353-90.
7. J Am Health Policy. 1994 Jul-Aug;4(4):7-15.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: Which of the following choices is best?
A. Controversy exists over whether defensive medicine is widely practiced and whether it raises health care costs.
B. Everyone agrees that defensive medicine is widely practiced.
C. Doctors who spend more for their hospitalized patients have been reported to face a lower malpractice risk.
D. A and C.
E. B and C.
Answer: D. Virtually all doctors admit they practice defensive medicine, which describes medical care specifically directed to attenuate the threat of malpractice liability rather than for a proper medical indication. Survey studies generally put the prevalence at greater than 90%, but what separates true defensive medicine from careful practice or patient expectation/demand remains controversial.
A mail survey of 824 physicians in high-risk specialties in Pennsylvania, a state where doctors pay high malpractice premiums, revealed that nearly all reported practicing defensive medicine. The respondents admitted to both assurance behavior – such as ordering more tests, especially imaging studies – as well as avoidance behavior – that is, restricting or eliminating complex procedures or perceived litigious patients.1
In another study, emergency physicians in the upper third of “malpractice fear” used more diagnostic tests and were more likely to hospitalize patients at low risk for coronary artery disease.2
On the other hand, a report using simulated clinical scenarios concluded that the extent of defensive medicine was at most 8%, and another found no correlation between individual malpractice claims experience and resource use, physician concern about malpractice, tolerance for uncertainty, or perception of risk.3
Another area of contention is whether some of our soaring health care costs may reflect defensive medicine at play.
A recent survey of 2,000 U.S. orthopedic surgeons found that 96% admitted practicing defensive medicine, with 24% of all ordered tests being for defensive reasons. The authors estimated that this amounted to about $100,000 per surgeon per year, or a total annual sum of $2 billion for the 20,400 orthopedic surgeons in the U.S.4
By correlating professional liability insurance with cost of services, the AMA estimated that in the 1980s, defensive medicine cost $12.1 billion to $13.7 billion each year.5 In an oft-cited study by Kessler and McClellan, the authors measured the effects of malpractice liability reforms using data from elderly Medicare beneficiaries treated for serious heart disease.6 They found that reforms that directly reduced provider liability pressure led to reductions of 5%-9% in medical expenditures. If such Medicare savings, which amounted to $600 million per year for cardiac disease, were extrapolated across the health care system, the annual savings would total $50 billion.
A more conservative study estimated that systemwide savings from aggressive malpractice reform would approach $41 billion over 5 years.7
Dr. Anupam B. Jena and his coauthors are the latest investigators seeking to clarify the correlation between defensive medicine and health care costs.8 Using Florida hospital admission data from 2000-2009, which covered some 24,000 physicians in seven separate specialties, the authors found that higher spending by physicians was associated with reduced malpractice claims the following year. This pattern held true for six of the seven specialties, family practitioners being the sole exception.
For example, among internists, the malpractice risk probability was reduced from 1.5% in the bottom spending fifth ($19,725 per admission) to 0.3% in the top fifth ($39,379 per admission). Among obstetricians, a separate subgroup analysis of cesarean-section rates revealed that malpractice claims were approximately halved among obstetricians with rates in the highest fifth, compared with the lowest fifth. These results comport with previous reports of higher C-section rates in obstetricians who perceived themselves at higher malpractice risk, although other studies have found no correlation or an actual lower rate.
In concluding that higher resource use by physicians was associated with fewer malpractice claims, the authors acknowledged that a principal limitation of the study was the lack of information on illness severity. Importantly, they were unable to state whether higher spending was defensively motivated. A companion editorial questioned whether increased resource expenditure was a bona fide reflection of defensive medicine or may simply have resulted in fewer errors and adverse events.9
Unfortunately, the past malpractice experience of the doctors, which could provide insight into more defensive postures, better communication, etc., was not measured in the study. In addition, claims made just 1 year after an incident may not accurately reflect the real-world situation, where the filing of a lawsuit may lag any alleged negligence by a much longer period, especially in obstetric cases.
Worryingly, the results of this timely study may be used to imply that the more doctors spend, the less likely they are to be sued – a troubling notion in our current race to stem rising health care costs.
References
1. JAMA. 2005 Jun 1;293(21):2609-17.
2. Ann Emerg Med. 2005 Dec;46(6):525-33.
3. J Health Polit Policy Law. 1996 Summer;21(2):267-88.
4. Am J Orthop (Belle Mead NJ). 2012 Feb;41(2):69-73.
5. JAMA. 1987 May 22-29;257(20):2776-81.
6. Q J Econ. 1996 May; 111(2):353-90.
7. J Am Health Policy. 1994 Jul-Aug;4(4):7-15.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: Which of the following choices is best?
A. Controversy exists over whether defensive medicine is widely practiced and whether it raises health care costs.
B. Everyone agrees that defensive medicine is widely practiced.
C. Doctors who spend more for their hospitalized patients have been reported to face a lower malpractice risk.
D. A and C.
E. B and C.
Answer: D. Virtually all doctors admit they practice defensive medicine, which describes medical care specifically directed to attenuate the threat of malpractice liability rather than for a proper medical indication. Survey studies generally put the prevalence at greater than 90%, but what separates true defensive medicine from careful practice or patient expectation/demand remains controversial.
A mail survey of 824 physicians in high-risk specialties in Pennsylvania, a state where doctors pay high malpractice premiums, revealed that nearly all reported practicing defensive medicine. The respondents admitted to both assurance behavior – such as ordering more tests, especially imaging studies – as well as avoidance behavior – that is, restricting or eliminating complex procedures or perceived litigious patients.1
In another study, emergency physicians in the upper third of “malpractice fear” used more diagnostic tests and were more likely to hospitalize patients at low risk for coronary artery disease.2
On the other hand, a report using simulated clinical scenarios concluded that the extent of defensive medicine was at most 8%, and another found no correlation between individual malpractice claims experience and resource use, physician concern about malpractice, tolerance for uncertainty, or perception of risk.3
Another area of contention is whether some of our soaring health care costs may reflect defensive medicine at play.
A recent survey of 2,000 U.S. orthopedic surgeons found that 96% admitted practicing defensive medicine, with 24% of all ordered tests being for defensive reasons. The authors estimated that this amounted to about $100,000 per surgeon per year, or a total annual sum of $2 billion for the 20,400 orthopedic surgeons in the U.S.4
By correlating professional liability insurance with cost of services, the AMA estimated that in the 1980s, defensive medicine cost $12.1 billion to $13.7 billion each year.5 In an oft-cited study by Kessler and McClellan, the authors measured the effects of malpractice liability reforms using data from elderly Medicare beneficiaries treated for serious heart disease.6 They found that reforms that directly reduced provider liability pressure led to reductions of 5%-9% in medical expenditures. If such Medicare savings, which amounted to $600 million per year for cardiac disease, were extrapolated across the health care system, the annual savings would total $50 billion.
A more conservative study estimated that systemwide savings from aggressive malpractice reform would approach $41 billion over 5 years.7
Dr. Anupam B. Jena and his coauthors are the latest investigators seeking to clarify the correlation between defensive medicine and health care costs.8 Using Florida hospital admission data from 2000-2009, which covered some 24,000 physicians in seven separate specialties, the authors found that higher spending by physicians was associated with reduced malpractice claims the following year. This pattern held true for six of the seven specialties, family practitioners being the sole exception.
For example, among internists, the malpractice risk probability was reduced from 1.5% in the bottom spending fifth ($19,725 per admission) to 0.3% in the top fifth ($39,379 per admission). Among obstetricians, a separate subgroup analysis of cesarean-section rates revealed that malpractice claims were approximately halved among obstetricians with rates in the highest fifth, compared with the lowest fifth. These results comport with previous reports of higher C-section rates in obstetricians who perceived themselves at higher malpractice risk, although other studies have found no correlation or an actual lower rate.
In concluding that higher resource use by physicians was associated with fewer malpractice claims, the authors acknowledged that a principal limitation of the study was the lack of information on illness severity. Importantly, they were unable to state whether higher spending was defensively motivated. A companion editorial questioned whether increased resource expenditure was a bona fide reflection of defensive medicine or may simply have resulted in fewer errors and adverse events.9
Unfortunately, the past malpractice experience of the doctors, which could provide insight into more defensive postures, better communication, etc., was not measured in the study. In addition, claims made just 1 year after an incident may not accurately reflect the real-world situation, where the filing of a lawsuit may lag any alleged negligence by a much longer period, especially in obstetric cases.
Worryingly, the results of this timely study may be used to imply that the more doctors spend, the less likely they are to be sued – a troubling notion in our current race to stem rising health care costs.
References
1. JAMA. 2005 Jun 1;293(21):2609-17.
2. Ann Emerg Med. 2005 Dec;46(6):525-33.
3. J Health Polit Policy Law. 1996 Summer;21(2):267-88.
4. Am J Orthop (Belle Mead NJ). 2012 Feb;41(2):69-73.
5. JAMA. 1987 May 22-29;257(20):2776-81.
6. Q J Econ. 1996 May; 111(2):353-90.
7. J Am Health Policy. 1994 Jul-Aug;4(4):7-15.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].