User login
Distal Ulna Fracture With Delayed Ulnar Nerve Palsy in a Baseball Player
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
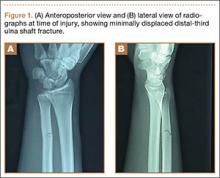
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
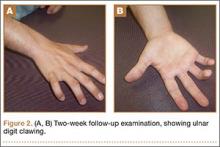
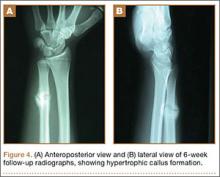
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
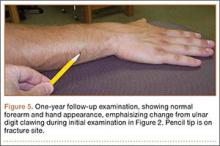
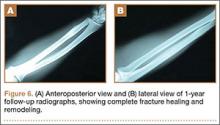
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
HM16 Takes a Look at Health IT, Post-Acute Care
Take a look at the HM16 program, and you get a snapshot of the most pressing topics in hospital medicine. Specifically, four new educational tracks are being rolled out at this year’s annual meeting, including a new track on the patient-doctor relationship, which is so crucial with today’s growing emphasis on patient satisfaction, and a track focused on perioperative medicine, an important area with a fast-moving frontier. Another new track covers post-acute care, a setting in which more and more hospitalists find themselves practicing. Then there’s the big daddy: health information technology (IT) for hospitalists.
Course Director Melissa Mattison, MD, SFHM, also points to a new twist in the way the conference will attempt to tackle the tough topic of work-life balance.
Read the full interview with Melissa Mattison, MD, SFHM.
Here’s a look at what’s new for HM16 attendees.
Health IT for Hospitalists
“There’s not a hospitalist in the country who’s not affected by IT and updates to their [electronic medical records (EMR)], new adoption of EMR technology, different vendors,” Dr. Mattison says. “We’re always searching for something to make our lives better and make the care that we provide more high quality.”
There will be sessions of a general nature, such as “There’s an App for That,” a review of mobile apps helpful to hospitalists. And there will be those for the more passionate technophiles, such as a session on clinical informatics and “Using IT to Help Drive the Shift from Volume to Value.”
“We’ve spent a lot of time trying to make sure there’s something for everyone,” says Kendall Rogers, MD, SFHM, chair of SHM’s IT Committee. “And even within each individual talk, we’ve tried to make sure that there is material that can be applicable from the frontline hospitalist to the CMIO of a hospital.”
Dr. Rogers says the committee has “really been pushing” to have its own track at the annual meeting.
Listen to more of our interview with Dr. Rogers.
“Health IT continues to be an area of great frustration and great promise,” he says. “I think most of the frustration that hospitalists have is because they realize the potential of health IT, and they see how far it is from the reality of what they’re working with every day.
“Hospitalists are well-suited for actively being involved in clinical informatics, but many of us would be far more effective in our roles with more formal education and training.”
Post-Acute Care
It’s estimated that as many as 35% of hospitalists work in the post-acute setting. The number very much surprised Dr. Mattison. When she heard of the figure, “[the committee] lobbied very hard to get a track for post-acute care.”
One session, “Building and Managing a PAC Practice,” will review setting up a staff, relevant regulations, billing, and collecting, and it should be of interest to both managers and physicians, says Sean Muldoon, MD, senior vice president and chief medical officer of the hospitalist division at Louisville, Ken.–based Kindred Healthcare and chair of SHM’s Post-Acute Care Task Force.
Another session, “Lost in Transitions,” will review information gaps and propose solutions “to the well-known voltage drop of information that can happen in transfer from the hospital to post-acute care.”
At Kindred, Dr. Muldoon says he has seen the benefits of hospitalist involvement in post-acute care.
“In many markets, we seek out and often are able to become a practice site for a large hospitalist medical group,” he says. “That’s really good for us, the patients, and, we think, the hospitalists because it allows the hospitalists to be exposed to the practice and benefits of post-acute care without having to make a full commitment to be a skilled-nursing physician or a long-term acute-care physician.”
It also makes transitions of care smoother and less disruptive, he says, “because a patient is simply transferred from one hospitalist in a group to another or often maintaining that same hospitalist in the post-acute-care setting.”
Dr. Muldoon says the new track is of value to any hospitalist, whether they actually work in post-acute care or not.
“A hospitalist would be hard-pressed to provide knowledgeable input into where a patient should receive post-acute care without a working knowledge of which patients should be directed to which post-acute-care setting,” he says.
Doctor-Patient Relationship
This topic was a pre-course last year, and organizers decided to make this a full track on the final day of the meeting schedule.
“It’s really about communication style,” Dr. Mattison says. “There’s one session called ‘The Language of Empathy and Engagement: Communication Essentials for Patient-Centered Care.’ There’s one on unconscious biases and our underlying assumptions and how it affects how we care for patients. [Another is focused] on improving the patient experience in the hospital.”
Co-Management/ Perioperative Medicine
“There are a lot of challenges around anticoagulation management, optimizing patients’ physical heath prior to the surgery, what things should we be doing, what medications should we be giving, what ones shouldn’t we be giving,” Dr. Mattison says. “It’s an evolving field that has, every year, new information.”
Hidden Gems
Dr. Mattison draws special attention to “Work-Life Balance: Is It Possible?” (Tuesday, March 8, 4:20–5:40 p.m.). This year, this problem—all too familiar to hospitalists—will be addressed in a panel discussion, which is a change from previous years.
“There’s been, year after year after year, a lot of discussion around, how can I make my job manageable if my boss isn’t listening to me or is not attuned to work-life balance? How can I navigate this process?” she says. “I’m hopeful that the panel discussion will provide people with some real examples and strategies for success.”
She also draws attention to the session “Perioperative Pitfalls: Overcoming Common Challenges in Managing Medical Problems in Surgical Patients” (Monday, March 7, 3:05–4:20 p.m.).
“There are some true leaders in perioperative management, and they’re going to come together and have a panel discussion,” she says. “It’ll be an opportunity to see some of the great minds think, if you will.” TH
Thomas R. Collins is a freelance writer in South Florida.
Take a look at the HM16 program, and you get a snapshot of the most pressing topics in hospital medicine. Specifically, four new educational tracks are being rolled out at this year’s annual meeting, including a new track on the patient-doctor relationship, which is so crucial with today’s growing emphasis on patient satisfaction, and a track focused on perioperative medicine, an important area with a fast-moving frontier. Another new track covers post-acute care, a setting in which more and more hospitalists find themselves practicing. Then there’s the big daddy: health information technology (IT) for hospitalists.
Course Director Melissa Mattison, MD, SFHM, also points to a new twist in the way the conference will attempt to tackle the tough topic of work-life balance.
Read the full interview with Melissa Mattison, MD, SFHM.
Here’s a look at what’s new for HM16 attendees.
Health IT for Hospitalists
“There’s not a hospitalist in the country who’s not affected by IT and updates to their [electronic medical records (EMR)], new adoption of EMR technology, different vendors,” Dr. Mattison says. “We’re always searching for something to make our lives better and make the care that we provide more high quality.”
There will be sessions of a general nature, such as “There’s an App for That,” a review of mobile apps helpful to hospitalists. And there will be those for the more passionate technophiles, such as a session on clinical informatics and “Using IT to Help Drive the Shift from Volume to Value.”
“We’ve spent a lot of time trying to make sure there’s something for everyone,” says Kendall Rogers, MD, SFHM, chair of SHM’s IT Committee. “And even within each individual talk, we’ve tried to make sure that there is material that can be applicable from the frontline hospitalist to the CMIO of a hospital.”
Dr. Rogers says the committee has “really been pushing” to have its own track at the annual meeting.
Listen to more of our interview with Dr. Rogers.
“Health IT continues to be an area of great frustration and great promise,” he says. “I think most of the frustration that hospitalists have is because they realize the potential of health IT, and they see how far it is from the reality of what they’re working with every day.
“Hospitalists are well-suited for actively being involved in clinical informatics, but many of us would be far more effective in our roles with more formal education and training.”
Post-Acute Care
It’s estimated that as many as 35% of hospitalists work in the post-acute setting. The number very much surprised Dr. Mattison. When she heard of the figure, “[the committee] lobbied very hard to get a track for post-acute care.”
One session, “Building and Managing a PAC Practice,” will review setting up a staff, relevant regulations, billing, and collecting, and it should be of interest to both managers and physicians, says Sean Muldoon, MD, senior vice president and chief medical officer of the hospitalist division at Louisville, Ken.–based Kindred Healthcare and chair of SHM’s Post-Acute Care Task Force.
Another session, “Lost in Transitions,” will review information gaps and propose solutions “to the well-known voltage drop of information that can happen in transfer from the hospital to post-acute care.”
At Kindred, Dr. Muldoon says he has seen the benefits of hospitalist involvement in post-acute care.
“In many markets, we seek out and often are able to become a practice site for a large hospitalist medical group,” he says. “That’s really good for us, the patients, and, we think, the hospitalists because it allows the hospitalists to be exposed to the practice and benefits of post-acute care without having to make a full commitment to be a skilled-nursing physician or a long-term acute-care physician.”
It also makes transitions of care smoother and less disruptive, he says, “because a patient is simply transferred from one hospitalist in a group to another or often maintaining that same hospitalist in the post-acute-care setting.”
Dr. Muldoon says the new track is of value to any hospitalist, whether they actually work in post-acute care or not.
“A hospitalist would be hard-pressed to provide knowledgeable input into where a patient should receive post-acute care without a working knowledge of which patients should be directed to which post-acute-care setting,” he says.
Doctor-Patient Relationship
This topic was a pre-course last year, and organizers decided to make this a full track on the final day of the meeting schedule.
“It’s really about communication style,” Dr. Mattison says. “There’s one session called ‘The Language of Empathy and Engagement: Communication Essentials for Patient-Centered Care.’ There’s one on unconscious biases and our underlying assumptions and how it affects how we care for patients. [Another is focused] on improving the patient experience in the hospital.”
Co-Management/ Perioperative Medicine
“There are a lot of challenges around anticoagulation management, optimizing patients’ physical heath prior to the surgery, what things should we be doing, what medications should we be giving, what ones shouldn’t we be giving,” Dr. Mattison says. “It’s an evolving field that has, every year, new information.”
Hidden Gems
Dr. Mattison draws special attention to “Work-Life Balance: Is It Possible?” (Tuesday, March 8, 4:20–5:40 p.m.). This year, this problem—all too familiar to hospitalists—will be addressed in a panel discussion, which is a change from previous years.
“There’s been, year after year after year, a lot of discussion around, how can I make my job manageable if my boss isn’t listening to me or is not attuned to work-life balance? How can I navigate this process?” she says. “I’m hopeful that the panel discussion will provide people with some real examples and strategies for success.”
She also draws attention to the session “Perioperative Pitfalls: Overcoming Common Challenges in Managing Medical Problems in Surgical Patients” (Monday, March 7, 3:05–4:20 p.m.).
“There are some true leaders in perioperative management, and they’re going to come together and have a panel discussion,” she says. “It’ll be an opportunity to see some of the great minds think, if you will.” TH
Thomas R. Collins is a freelance writer in South Florida.
Take a look at the HM16 program, and you get a snapshot of the most pressing topics in hospital medicine. Specifically, four new educational tracks are being rolled out at this year’s annual meeting, including a new track on the patient-doctor relationship, which is so crucial with today’s growing emphasis on patient satisfaction, and a track focused on perioperative medicine, an important area with a fast-moving frontier. Another new track covers post-acute care, a setting in which more and more hospitalists find themselves practicing. Then there’s the big daddy: health information technology (IT) for hospitalists.
Course Director Melissa Mattison, MD, SFHM, also points to a new twist in the way the conference will attempt to tackle the tough topic of work-life balance.
Read the full interview with Melissa Mattison, MD, SFHM.
Here’s a look at what’s new for HM16 attendees.
Health IT for Hospitalists
“There’s not a hospitalist in the country who’s not affected by IT and updates to their [electronic medical records (EMR)], new adoption of EMR technology, different vendors,” Dr. Mattison says. “We’re always searching for something to make our lives better and make the care that we provide more high quality.”
There will be sessions of a general nature, such as “There’s an App for That,” a review of mobile apps helpful to hospitalists. And there will be those for the more passionate technophiles, such as a session on clinical informatics and “Using IT to Help Drive the Shift from Volume to Value.”
“We’ve spent a lot of time trying to make sure there’s something for everyone,” says Kendall Rogers, MD, SFHM, chair of SHM’s IT Committee. “And even within each individual talk, we’ve tried to make sure that there is material that can be applicable from the frontline hospitalist to the CMIO of a hospital.”
Dr. Rogers says the committee has “really been pushing” to have its own track at the annual meeting.
Listen to more of our interview with Dr. Rogers.
“Health IT continues to be an area of great frustration and great promise,” he says. “I think most of the frustration that hospitalists have is because they realize the potential of health IT, and they see how far it is from the reality of what they’re working with every day.
“Hospitalists are well-suited for actively being involved in clinical informatics, but many of us would be far more effective in our roles with more formal education and training.”
Post-Acute Care
It’s estimated that as many as 35% of hospitalists work in the post-acute setting. The number very much surprised Dr. Mattison. When she heard of the figure, “[the committee] lobbied very hard to get a track for post-acute care.”
One session, “Building and Managing a PAC Practice,” will review setting up a staff, relevant regulations, billing, and collecting, and it should be of interest to both managers and physicians, says Sean Muldoon, MD, senior vice president and chief medical officer of the hospitalist division at Louisville, Ken.–based Kindred Healthcare and chair of SHM’s Post-Acute Care Task Force.
Another session, “Lost in Transitions,” will review information gaps and propose solutions “to the well-known voltage drop of information that can happen in transfer from the hospital to post-acute care.”
At Kindred, Dr. Muldoon says he has seen the benefits of hospitalist involvement in post-acute care.
“In many markets, we seek out and often are able to become a practice site for a large hospitalist medical group,” he says. “That’s really good for us, the patients, and, we think, the hospitalists because it allows the hospitalists to be exposed to the practice and benefits of post-acute care without having to make a full commitment to be a skilled-nursing physician or a long-term acute-care physician.”
It also makes transitions of care smoother and less disruptive, he says, “because a patient is simply transferred from one hospitalist in a group to another or often maintaining that same hospitalist in the post-acute-care setting.”
Dr. Muldoon says the new track is of value to any hospitalist, whether they actually work in post-acute care or not.
“A hospitalist would be hard-pressed to provide knowledgeable input into where a patient should receive post-acute care without a working knowledge of which patients should be directed to which post-acute-care setting,” he says.
Doctor-Patient Relationship
This topic was a pre-course last year, and organizers decided to make this a full track on the final day of the meeting schedule.
“It’s really about communication style,” Dr. Mattison says. “There’s one session called ‘The Language of Empathy and Engagement: Communication Essentials for Patient-Centered Care.’ There’s one on unconscious biases and our underlying assumptions and how it affects how we care for patients. [Another is focused] on improving the patient experience in the hospital.”
Co-Management/ Perioperative Medicine
“There are a lot of challenges around anticoagulation management, optimizing patients’ physical heath prior to the surgery, what things should we be doing, what medications should we be giving, what ones shouldn’t we be giving,” Dr. Mattison says. “It’s an evolving field that has, every year, new information.”
Hidden Gems
Dr. Mattison draws special attention to “Work-Life Balance: Is It Possible?” (Tuesday, March 8, 4:20–5:40 p.m.). This year, this problem—all too familiar to hospitalists—will be addressed in a panel discussion, which is a change from previous years.
“There’s been, year after year after year, a lot of discussion around, how can I make my job manageable if my boss isn’t listening to me or is not attuned to work-life balance? How can I navigate this process?” she says. “I’m hopeful that the panel discussion will provide people with some real examples and strategies for success.”
She also draws attention to the session “Perioperative Pitfalls: Overcoming Common Challenges in Managing Medical Problems in Surgical Patients” (Monday, March 7, 3:05–4:20 p.m.).
“There are some true leaders in perioperative management, and they’re going to come together and have a panel discussion,” she says. “It’ll be an opportunity to see some of the great minds think, if you will.” TH
Thomas R. Collins is a freelance writer in South Florida.
FDA approves maintenance therapy for CLL

Photo courtesy of GSK
The US Food and Drug Administration (FDA) has approved the use of ofatumumab (Arzerra) as maintenance therapy for patients with chronic lymphocytic leukemia (CLL).
The drug can now be given for an extended period to patients who are in complete or partial response after receiving at least 2 lines of therapy for recurrent or progressive CLL.
Ofatumumab is also FDA-approved as a single agent to treat CLL that is refractory to fludarabine and alemtuzumab.
And the drug is approved for use in combination with chlorambucil to treat previously untreated patients with CLL for whom fludarabine-based therapy is considered inappropriate.
The FDA granted the new approval for ofatumumab based on an interim analysis of the PROLONG study. The results suggested that ofatumumab maintenance can improve progression-free survival (PFS) in CLL patients when compared to observation.
Ofatumumab is marketed as Arzerra under a collaboration agreement between Genmab and Novartis. For more details on ofatumumab, see the full prescribing information.
PROLONG trial
The PROLONG trial was designed to compare ofatumumab maintenance to no further treatment in patients with a complete or partial response after second- or third-line treatment for CLL. Interim results of the study were presented at ASH 2014.
These results—in 474 patients—suggested that ofatumumab can significantly improve PFS. The median PFS was about 29 months in patients who received ofatumumab and about 15 months for patients who did not receive maintenance therapy (P<0.0001).
There was no significant difference in the median overall survival, which was not reached in either treatment arm.
The researchers said there were no unexpected safety findings. The most common adverse events (≥10%) were infusion reactions, neutropenia, and upper respiratory tract infection. ![]()

Photo courtesy of GSK
The US Food and Drug Administration (FDA) has approved the use of ofatumumab (Arzerra) as maintenance therapy for patients with chronic lymphocytic leukemia (CLL).
The drug can now be given for an extended period to patients who are in complete or partial response after receiving at least 2 lines of therapy for recurrent or progressive CLL.
Ofatumumab is also FDA-approved as a single agent to treat CLL that is refractory to fludarabine and alemtuzumab.
And the drug is approved for use in combination with chlorambucil to treat previously untreated patients with CLL for whom fludarabine-based therapy is considered inappropriate.
The FDA granted the new approval for ofatumumab based on an interim analysis of the PROLONG study. The results suggested that ofatumumab maintenance can improve progression-free survival (PFS) in CLL patients when compared to observation.
Ofatumumab is marketed as Arzerra under a collaboration agreement between Genmab and Novartis. For more details on ofatumumab, see the full prescribing information.
PROLONG trial
The PROLONG trial was designed to compare ofatumumab maintenance to no further treatment in patients with a complete or partial response after second- or third-line treatment for CLL. Interim results of the study were presented at ASH 2014.
These results—in 474 patients—suggested that ofatumumab can significantly improve PFS. The median PFS was about 29 months in patients who received ofatumumab and about 15 months for patients who did not receive maintenance therapy (P<0.0001).
There was no significant difference in the median overall survival, which was not reached in either treatment arm.
The researchers said there were no unexpected safety findings. The most common adverse events (≥10%) were infusion reactions, neutropenia, and upper respiratory tract infection. ![]()

Photo courtesy of GSK
The US Food and Drug Administration (FDA) has approved the use of ofatumumab (Arzerra) as maintenance therapy for patients with chronic lymphocytic leukemia (CLL).
The drug can now be given for an extended period to patients who are in complete or partial response after receiving at least 2 lines of therapy for recurrent or progressive CLL.
Ofatumumab is also FDA-approved as a single agent to treat CLL that is refractory to fludarabine and alemtuzumab.
And the drug is approved for use in combination with chlorambucil to treat previously untreated patients with CLL for whom fludarabine-based therapy is considered inappropriate.
The FDA granted the new approval for ofatumumab based on an interim analysis of the PROLONG study. The results suggested that ofatumumab maintenance can improve progression-free survival (PFS) in CLL patients when compared to observation.
Ofatumumab is marketed as Arzerra under a collaboration agreement between Genmab and Novartis. For more details on ofatumumab, see the full prescribing information.
PROLONG trial
The PROLONG trial was designed to compare ofatumumab maintenance to no further treatment in patients with a complete or partial response after second- or third-line treatment for CLL. Interim results of the study were presented at ASH 2014.
These results—in 474 patients—suggested that ofatumumab can significantly improve PFS. The median PFS was about 29 months in patients who received ofatumumab and about 15 months for patients who did not receive maintenance therapy (P<0.0001).
There was no significant difference in the median overall survival, which was not reached in either treatment arm.
The researchers said there were no unexpected safety findings. The most common adverse events (≥10%) were infusion reactions, neutropenia, and upper respiratory tract infection. ![]()
FDA approves generic drug for hemophilia

The US Food and Drug Administration (FDA) has approved a generic version of tranexamic acid for short-term control of bleeding in patients with hemophilia.
The drug, tranexamic acid injection (100 mg/mL) 1000 mg/10 mL single-dose vial, is a product of Aurobindo Pharma Limited.
The drug has been deemed bioequivalent and therapeutically equivalent to Cyklokapron® injection, 100 mg/mL, a product of Pharmacia and Upjohn Company.
Aurobindo Pharma Limited said the generic drug should be launched in the US by the end of March. ![]()

The US Food and Drug Administration (FDA) has approved a generic version of tranexamic acid for short-term control of bleeding in patients with hemophilia.
The drug, tranexamic acid injection (100 mg/mL) 1000 mg/10 mL single-dose vial, is a product of Aurobindo Pharma Limited.
The drug has been deemed bioequivalent and therapeutically equivalent to Cyklokapron® injection, 100 mg/mL, a product of Pharmacia and Upjohn Company.
Aurobindo Pharma Limited said the generic drug should be launched in the US by the end of March. ![]()

The US Food and Drug Administration (FDA) has approved a generic version of tranexamic acid for short-term control of bleeding in patients with hemophilia.
The drug, tranexamic acid injection (100 mg/mL) 1000 mg/10 mL single-dose vial, is a product of Aurobindo Pharma Limited.
The drug has been deemed bioequivalent and therapeutically equivalent to Cyklokapron® injection, 100 mg/mL, a product of Pharmacia and Upjohn Company.
Aurobindo Pharma Limited said the generic drug should be launched in the US by the end of March. ![]()
Research helps explain how RBCs move

Scientists say they have determined how red blood cells (RBCs) move, showing that RBCs can be moved by external forces and actively “wriggle” on their own.
Linking physical principles and biological reality, the team found that fast molecules in the vicinity of RBCs make the cell membranes wriggle, but the cells themselves also become active when they have enough reaction time.
The group recounted these findings in Nature Physics.
Previously, scientists had only shown that RBCs’ constant wriggling was caused by external forces. But biological considerations suggested that internal forces might also be responsible for the RBCs’ membranes changing shape.
“So we started with the following question, ‘As blood cells are living cells, why shouldn’t internal forces inside the cell also have an impact on the membrane?’” said study author Timo Betz, PhD, of Münster University in Münster, Germany.
“For biologists, this is all clear, but these forces were just never a part of any physical equation.”
Dr Betz and his colleagues wanted to find out more about the mechanics of blood cells and gain a detailed understanding of the forces that move and shape cells.
The team said it is important to learn about RBCs’ properties and their internal forces because they are unusually soft and elastic and must change their shape to pass through blood vessels. It is precisely because RBCs are normally so soft that, in previous studies, physicists measured large thermal fluctuations at the outer membrane of the cells.
These natural movements of molecules are defined by the ambient temperature. In other words, the cell membrane moves because molecules in the vicinity jog it. Under the microscope, this makes the RBCs appear to be wriggling.
Although this explains why RBCs move, it does not address the question of possible internal forces being a contributing factor.
So Dr Betz and his colleagues used optical tweezers to take a close look at the fluctuations of RBCs. The team stretched RBCs in a petri dish and analyzed the behavior of the cells.
The result was that, if the RBCs had enough reaction time, they became active themselves and were able to counteract the force of the optical tweezers. If they did not have this time, they were at the mercy of their environment, and only temperature-related forces were measured.
“By comparing both sets of measurements, we can exactly define how fast the cells become active themselves and what force they generate in order to change shape,” Dr Betz explained.
He and his colleagues have a theory as to which forces inside RBCs cause the cell membrane to change shape.
“Transport proteins could generate such forces in the membrane by moving ions from one side of the membrane to the other,” said study author Gerhard Gompper, PhD, of the Jülich Institute of Complex Systems in Jülich, Germany.
“Now, it’s up to the biologists, because we physicists only have a rough idea about which proteins might be the drivers for this movement,” Dr Betz added. “On the other hand, we can predict exactly how fast and how strong they are.” ![]()

Scientists say they have determined how red blood cells (RBCs) move, showing that RBCs can be moved by external forces and actively “wriggle” on their own.
Linking physical principles and biological reality, the team found that fast molecules in the vicinity of RBCs make the cell membranes wriggle, but the cells themselves also become active when they have enough reaction time.
The group recounted these findings in Nature Physics.
Previously, scientists had only shown that RBCs’ constant wriggling was caused by external forces. But biological considerations suggested that internal forces might also be responsible for the RBCs’ membranes changing shape.
“So we started with the following question, ‘As blood cells are living cells, why shouldn’t internal forces inside the cell also have an impact on the membrane?’” said study author Timo Betz, PhD, of Münster University in Münster, Germany.
“For biologists, this is all clear, but these forces were just never a part of any physical equation.”
Dr Betz and his colleagues wanted to find out more about the mechanics of blood cells and gain a detailed understanding of the forces that move and shape cells.
The team said it is important to learn about RBCs’ properties and their internal forces because they are unusually soft and elastic and must change their shape to pass through blood vessels. It is precisely because RBCs are normally so soft that, in previous studies, physicists measured large thermal fluctuations at the outer membrane of the cells.
These natural movements of molecules are defined by the ambient temperature. In other words, the cell membrane moves because molecules in the vicinity jog it. Under the microscope, this makes the RBCs appear to be wriggling.
Although this explains why RBCs move, it does not address the question of possible internal forces being a contributing factor.
So Dr Betz and his colleagues used optical tweezers to take a close look at the fluctuations of RBCs. The team stretched RBCs in a petri dish and analyzed the behavior of the cells.
The result was that, if the RBCs had enough reaction time, they became active themselves and were able to counteract the force of the optical tweezers. If they did not have this time, they were at the mercy of their environment, and only temperature-related forces were measured.
“By comparing both sets of measurements, we can exactly define how fast the cells become active themselves and what force they generate in order to change shape,” Dr Betz explained.
He and his colleagues have a theory as to which forces inside RBCs cause the cell membrane to change shape.
“Transport proteins could generate such forces in the membrane by moving ions from one side of the membrane to the other,” said study author Gerhard Gompper, PhD, of the Jülich Institute of Complex Systems in Jülich, Germany.
“Now, it’s up to the biologists, because we physicists only have a rough idea about which proteins might be the drivers for this movement,” Dr Betz added. “On the other hand, we can predict exactly how fast and how strong they are.” ![]()

Scientists say they have determined how red blood cells (RBCs) move, showing that RBCs can be moved by external forces and actively “wriggle” on their own.
Linking physical principles and biological reality, the team found that fast molecules in the vicinity of RBCs make the cell membranes wriggle, but the cells themselves also become active when they have enough reaction time.
The group recounted these findings in Nature Physics.
Previously, scientists had only shown that RBCs’ constant wriggling was caused by external forces. But biological considerations suggested that internal forces might also be responsible for the RBCs’ membranes changing shape.
“So we started with the following question, ‘As blood cells are living cells, why shouldn’t internal forces inside the cell also have an impact on the membrane?’” said study author Timo Betz, PhD, of Münster University in Münster, Germany.
“For biologists, this is all clear, but these forces were just never a part of any physical equation.”
Dr Betz and his colleagues wanted to find out more about the mechanics of blood cells and gain a detailed understanding of the forces that move and shape cells.
The team said it is important to learn about RBCs’ properties and their internal forces because they are unusually soft and elastic and must change their shape to pass through blood vessels. It is precisely because RBCs are normally so soft that, in previous studies, physicists measured large thermal fluctuations at the outer membrane of the cells.
These natural movements of molecules are defined by the ambient temperature. In other words, the cell membrane moves because molecules in the vicinity jog it. Under the microscope, this makes the RBCs appear to be wriggling.
Although this explains why RBCs move, it does not address the question of possible internal forces being a contributing factor.
So Dr Betz and his colleagues used optical tweezers to take a close look at the fluctuations of RBCs. The team stretched RBCs in a petri dish and analyzed the behavior of the cells.
The result was that, if the RBCs had enough reaction time, they became active themselves and were able to counteract the force of the optical tweezers. If they did not have this time, they were at the mercy of their environment, and only temperature-related forces were measured.
“By comparing both sets of measurements, we can exactly define how fast the cells become active themselves and what force they generate in order to change shape,” Dr Betz explained.
He and his colleagues have a theory as to which forces inside RBCs cause the cell membrane to change shape.
“Transport proteins could generate such forces in the membrane by moving ions from one side of the membrane to the other,” said study author Gerhard Gompper, PhD, of the Jülich Institute of Complex Systems in Jülich, Germany.
“Now, it’s up to the biologists, because we physicists only have a rough idea about which proteins might be the drivers for this movement,” Dr Betz added. “On the other hand, we can predict exactly how fast and how strong they are.” ![]()
Drug approved to treat ALL in EU

The European Commission has granted marketing authorization for pegaspargase (Oncaspar) to be used as part of combination antineoplastic therapy for pediatric and adult patients with acute lymphoblastic leukemia (ALL).
The approval means the drug can be marketed for this indication in the 28 member countries of the European Union (EU), as well as Iceland, Liechtenstein, and Norway.
Pegaspargase was already approved for use in Argentina, Belarus, Germany, Kazakhstan, Poland, Russia, Ukraine, and the US.
“Oncaspar has been used as an integral component of the treatment regimen for pediatric and adult patients with ALL for many years, in Europe and worldwide,” said Martin Schrappe, of Schleswig-Holstein University Hospital in Kiel, Germany.
“Today’s marketing authorization will ensure that more patients across the EU will benefit from access to Oncaspar as part of a standard of care regimen.”
The drug is being developed by Baxalta Incorporated.
First-line ALL
Researchers have evaluated the safety and effectiveness of pegaspargase in a study of 118 pediatric patients (ages 1 to 9) with newly diagnosed ALL. The patients were randomized 1:1 to pegaspargase or native E coli L-asparaginase, both as part of combination therapy.
Asparagine depletion (magnitude and duration) was similar between the 2 treatment arms. Event-free survival rates were also similar (about 80% in both arms), but the study was not designed to evaluate differences in event-free survival.
Grade 3/4 adverse events occurring in the pegaspargase and native E coli L-asparaginase arms, respectively, were abnormal liver tests (5% and 8%), elevated transaminases (3% and 7%), hyperbilirubinemia (2% and 2%), hyperglycemia (5% and 3%), central nervous system thrombosis (3% and 3%), coagulopathy (2% and 5%), pancreatitis (2% and 2%), and clinical allergic reactions to asparaginase (2% and 0%).
Previously treated ALL
Researchers have evaluated the effectiveness of pegaspargase in 4 open-label studies of patients with a history of prior clinical allergic reaction to asparaginase. The studies enrolled a total of 42 patients with multiply relapsed acute leukemia (39 with ALL).
Patients received pegaspargase as a single agent or as part of multi-agent chemotherapy. The re-induction response rate was 50%—36% complete responses and 14% partial responses. Three responses occurred in patients who received single-agent pegaspargase.
Adverse event information on pegaspargase in relapsed ALL has been compiled from 5 clinical trials. The studies enrolled a total of 174 patients with relapsed ALL who received pegaspargase as a single agent or as part of combination therapy.
Sixty-two of the patients had prior hypersensitivity reactions to asparaginase, and 112 did not. Allergic reactions to pegaspargase occurred in 32% of previously hypersensitive patients and 10% of non-hypersensitive patients.
The most common adverse events observed in patients who received pegaspargase were clinical allergic reactions, elevated transaminases, hyperbilirubinemia, and coagulopathies.
The most common serious adverse events due to pegaspargase were thrombosis (4%), hyperglycemia requiring insulin therapy (3%), and pancreatitis (1%).
For more details on these trials and pegaspargase in general, see the product information. ![]()

The European Commission has granted marketing authorization for pegaspargase (Oncaspar) to be used as part of combination antineoplastic therapy for pediatric and adult patients with acute lymphoblastic leukemia (ALL).
The approval means the drug can be marketed for this indication in the 28 member countries of the European Union (EU), as well as Iceland, Liechtenstein, and Norway.
Pegaspargase was already approved for use in Argentina, Belarus, Germany, Kazakhstan, Poland, Russia, Ukraine, and the US.
“Oncaspar has been used as an integral component of the treatment regimen for pediatric and adult patients with ALL for many years, in Europe and worldwide,” said Martin Schrappe, of Schleswig-Holstein University Hospital in Kiel, Germany.
“Today’s marketing authorization will ensure that more patients across the EU will benefit from access to Oncaspar as part of a standard of care regimen.”
The drug is being developed by Baxalta Incorporated.
First-line ALL
Researchers have evaluated the safety and effectiveness of pegaspargase in a study of 118 pediatric patients (ages 1 to 9) with newly diagnosed ALL. The patients were randomized 1:1 to pegaspargase or native E coli L-asparaginase, both as part of combination therapy.
Asparagine depletion (magnitude and duration) was similar between the 2 treatment arms. Event-free survival rates were also similar (about 80% in both arms), but the study was not designed to evaluate differences in event-free survival.
Grade 3/4 adverse events occurring in the pegaspargase and native E coli L-asparaginase arms, respectively, were abnormal liver tests (5% and 8%), elevated transaminases (3% and 7%), hyperbilirubinemia (2% and 2%), hyperglycemia (5% and 3%), central nervous system thrombosis (3% and 3%), coagulopathy (2% and 5%), pancreatitis (2% and 2%), and clinical allergic reactions to asparaginase (2% and 0%).
Previously treated ALL
Researchers have evaluated the effectiveness of pegaspargase in 4 open-label studies of patients with a history of prior clinical allergic reaction to asparaginase. The studies enrolled a total of 42 patients with multiply relapsed acute leukemia (39 with ALL).
Patients received pegaspargase as a single agent or as part of multi-agent chemotherapy. The re-induction response rate was 50%—36% complete responses and 14% partial responses. Three responses occurred in patients who received single-agent pegaspargase.
Adverse event information on pegaspargase in relapsed ALL has been compiled from 5 clinical trials. The studies enrolled a total of 174 patients with relapsed ALL who received pegaspargase as a single agent or as part of combination therapy.
Sixty-two of the patients had prior hypersensitivity reactions to asparaginase, and 112 did not. Allergic reactions to pegaspargase occurred in 32% of previously hypersensitive patients and 10% of non-hypersensitive patients.
The most common adverse events observed in patients who received pegaspargase were clinical allergic reactions, elevated transaminases, hyperbilirubinemia, and coagulopathies.
The most common serious adverse events due to pegaspargase were thrombosis (4%), hyperglycemia requiring insulin therapy (3%), and pancreatitis (1%).
For more details on these trials and pegaspargase in general, see the product information. ![]()

The European Commission has granted marketing authorization for pegaspargase (Oncaspar) to be used as part of combination antineoplastic therapy for pediatric and adult patients with acute lymphoblastic leukemia (ALL).
The approval means the drug can be marketed for this indication in the 28 member countries of the European Union (EU), as well as Iceland, Liechtenstein, and Norway.
Pegaspargase was already approved for use in Argentina, Belarus, Germany, Kazakhstan, Poland, Russia, Ukraine, and the US.
“Oncaspar has been used as an integral component of the treatment regimen for pediatric and adult patients with ALL for many years, in Europe and worldwide,” said Martin Schrappe, of Schleswig-Holstein University Hospital in Kiel, Germany.
“Today’s marketing authorization will ensure that more patients across the EU will benefit from access to Oncaspar as part of a standard of care regimen.”
The drug is being developed by Baxalta Incorporated.
First-line ALL
Researchers have evaluated the safety and effectiveness of pegaspargase in a study of 118 pediatric patients (ages 1 to 9) with newly diagnosed ALL. The patients were randomized 1:1 to pegaspargase or native E coli L-asparaginase, both as part of combination therapy.
Asparagine depletion (magnitude and duration) was similar between the 2 treatment arms. Event-free survival rates were also similar (about 80% in both arms), but the study was not designed to evaluate differences in event-free survival.
Grade 3/4 adverse events occurring in the pegaspargase and native E coli L-asparaginase arms, respectively, were abnormal liver tests (5% and 8%), elevated transaminases (3% and 7%), hyperbilirubinemia (2% and 2%), hyperglycemia (5% and 3%), central nervous system thrombosis (3% and 3%), coagulopathy (2% and 5%), pancreatitis (2% and 2%), and clinical allergic reactions to asparaginase (2% and 0%).
Previously treated ALL
Researchers have evaluated the effectiveness of pegaspargase in 4 open-label studies of patients with a history of prior clinical allergic reaction to asparaginase. The studies enrolled a total of 42 patients with multiply relapsed acute leukemia (39 with ALL).
Patients received pegaspargase as a single agent or as part of multi-agent chemotherapy. The re-induction response rate was 50%—36% complete responses and 14% partial responses. Three responses occurred in patients who received single-agent pegaspargase.
Adverse event information on pegaspargase in relapsed ALL has been compiled from 5 clinical trials. The studies enrolled a total of 174 patients with relapsed ALL who received pegaspargase as a single agent or as part of combination therapy.
Sixty-two of the patients had prior hypersensitivity reactions to asparaginase, and 112 did not. Allergic reactions to pegaspargase occurred in 32% of previously hypersensitive patients and 10% of non-hypersensitive patients.
The most common adverse events observed in patients who received pegaspargase were clinical allergic reactions, elevated transaminases, hyperbilirubinemia, and coagulopathies.
The most common serious adverse events due to pegaspargase were thrombosis (4%), hyperglycemia requiring insulin therapy (3%), and pancreatitis (1%).
For more details on these trials and pegaspargase in general, see the product information. ![]()
Ofatumumab approved for extended treatment of CLL patients in complete or partial response
Ofatumumab has been approved for extended treatment of patients who are in complete or partial response after at least two lines of therapy for recurrent or progressive chronic lymphocytic leukemia (CLL), the U.S. Food and Drug Administration announced on Jan. 19.
Ofatumumab (Arzerra Injection, Novartis Pharmaceuticals) was previously approved for treatment-naive patients with CLL for whom fludarabine-based therapy was considered inappropriate and for patients with CLL refractory to fludarabine and alemtuzumab.
Approval of the new indication was based on the results of a randomized, open-label trial that found improved progression-free survival with ofatumumab as compared with observation in patients whose disease had a complete or partial response after at least two lines of prior therapy, the FDA said in a press release.
In the study, 238 patients were randomized to ofatumumab and 236 to observation. Patients in the ofatumumab arm had received a range of two to five prior therapies. The median progression-free survival was significantly longer with ofatumumab at 29.4 months (95% confidence interval, 26.2-34.2) than with observation at 15.2 months (95% CI, 11.8-18.8).
Of patients treated with ofatumumab, 33% reported serious adverse reactions. The most common were pneumonia, pyrexia, and neutropenia (including febrile neutropenia).
The recommended dose and schedule for ofatumumab therapy is 300 mg by intravenous infusion on day 1 followed by 1,000 mg on day 8, and then 7 weeks later, and then every 8 weeks thereafter for up to a maximum of 2 years.
Full prescribing information is available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125326s062lbl.pdf.
Ofatumumab has been approved for extended treatment of patients who are in complete or partial response after at least two lines of therapy for recurrent or progressive chronic lymphocytic leukemia (CLL), the U.S. Food and Drug Administration announced on Jan. 19.
Ofatumumab (Arzerra Injection, Novartis Pharmaceuticals) was previously approved for treatment-naive patients with CLL for whom fludarabine-based therapy was considered inappropriate and for patients with CLL refractory to fludarabine and alemtuzumab.
Approval of the new indication was based on the results of a randomized, open-label trial that found improved progression-free survival with ofatumumab as compared with observation in patients whose disease had a complete or partial response after at least two lines of prior therapy, the FDA said in a press release.
In the study, 238 patients were randomized to ofatumumab and 236 to observation. Patients in the ofatumumab arm had received a range of two to five prior therapies. The median progression-free survival was significantly longer with ofatumumab at 29.4 months (95% confidence interval, 26.2-34.2) than with observation at 15.2 months (95% CI, 11.8-18.8).
Of patients treated with ofatumumab, 33% reported serious adverse reactions. The most common were pneumonia, pyrexia, and neutropenia (including febrile neutropenia).
The recommended dose and schedule for ofatumumab therapy is 300 mg by intravenous infusion on day 1 followed by 1,000 mg on day 8, and then 7 weeks later, and then every 8 weeks thereafter for up to a maximum of 2 years.
Full prescribing information is available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125326s062lbl.pdf.
Ofatumumab has been approved for extended treatment of patients who are in complete or partial response after at least two lines of therapy for recurrent or progressive chronic lymphocytic leukemia (CLL), the U.S. Food and Drug Administration announced on Jan. 19.
Ofatumumab (Arzerra Injection, Novartis Pharmaceuticals) was previously approved for treatment-naive patients with CLL for whom fludarabine-based therapy was considered inappropriate and for patients with CLL refractory to fludarabine and alemtuzumab.
Approval of the new indication was based on the results of a randomized, open-label trial that found improved progression-free survival with ofatumumab as compared with observation in patients whose disease had a complete or partial response after at least two lines of prior therapy, the FDA said in a press release.
In the study, 238 patients were randomized to ofatumumab and 236 to observation. Patients in the ofatumumab arm had received a range of two to five prior therapies. The median progression-free survival was significantly longer with ofatumumab at 29.4 months (95% confidence interval, 26.2-34.2) than with observation at 15.2 months (95% CI, 11.8-18.8).
Of patients treated with ofatumumab, 33% reported serious adverse reactions. The most common were pneumonia, pyrexia, and neutropenia (including febrile neutropenia).
The recommended dose and schedule for ofatumumab therapy is 300 mg by intravenous infusion on day 1 followed by 1,000 mg on day 8, and then 7 weeks later, and then every 8 weeks thereafter for up to a maximum of 2 years.
Full prescribing information is available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125326s062lbl.pdf.
Pretreatment hydroquinone for nonablative laser resurfacing of acne scars?
Pretreatment of skin prior to nonablative or ablative laser resurfacing is common practice, particularly in darker skin types. Treatment regimens include using hydroquinone 4% (and other hydroquinone-containing combinations) once to twice daily for 1-2 weeks prior to the laser procedure. The rationale makes sense. Quieting melanin production by inhibiting tyrosinase would seem to decrease the incidence of postinflammatory hyperpigmentation after laser resurfacing procedures. But is this common practice effective?
For ablative CO2 resurfacing in 100 patients Fitzpatrick Skin Types (FST) I-III, there was no significant difference in the incidence of hyperpigmentation in those randomized to be pretreated with either hydroquinone, glycolic acid, tretinoin, or to no treatment.1 The thought was that the follicular melanocytes involved in re-epithelialization were not affected by the pretreatment. This is the only published laser resurfacing today to date examining various pretreatment protocols with hyperpigmentation as a primary study outcome. From this study, it seems as though pretreatment before laser resurfacing is not helpful, but what about for nonablative resurfacing in darker skin types (FST IV-VI)?
In darker skin types (FST IV-VI), the risk of postinflammatory hyperpigmentation (PIH) is inherently higher and the incidence after laser resurfacing is greater. While the incidence of PIH is lower with nonablative fractional resurfacing, compared with ablative resurfacing, PIH can still occur whether pretreatment hydroquinone is used or not.2,3,4 To date, there are no published studies looking at the incidence of PIH when comparing pretreatment antipigment agents versus no pretreatment for laser resurfacing for acne scars in darker skin types. A split-face study comparing pretreatment on one side and no pretreatment on the other could help delineate whether this practice is evidence based.
For nonablative fractional laser resurfacing of acne scars, lower densities in darker skin types are recommended and may help reduce PIH risk. There is no statistically significant difference in improvement of acne scars in using low versus high densities using the same fluences. However, some studies note that higher densities clinically resulted in a mild improvement of acne scars over lower densities (not statistically significant); thus, if lower densities are used, it is possible that more treatments may be needed.4,5
Vigorous sun protection before and after treatment is prudent, with sun avoidance and physical sunscreens reducing the risk of PIH in darker skin from irritant or allergic contact dermatitis, compared with chemical sunscreens. If PIH occurs, it is often self limited (up to 1-2 months). Sun protection and posttreatment regimens of hydroquinone (or other lightening agent) aid in hastening improvement.
If the patient is undergoing nonablative laser resurfacing to treat pigmentation, such as melasma, then hydroquinone pre- and postlaser is appropriate. In my opinion, laser treatment of melasma should not be first line because of safety and efficacy concerns. However, in these cases, hydroquinone prior to laser has shown benefit.6 In addition, hydroquinone after nonablative fractional resurfacing may enhance penetration of the topical and improve efficacy.
In summary, the evidence shows that pretreatment with antipigment agents is not warranted in skin types I-III for ablative laser resurfacing. Pretreatment with antipigment agents for nonablative laser resurfacing for melasma (which should not be considered a first line treatment for melasma) is warranted. However, at this time, it is not clear whether pretreatment with antipigments for nonablative laser resurfacing for acne scars in darker skin types is useful. Lower densities should be used and if PIH does occur, it is usually self limited, and posttreatment hydroquinone or other antipigment agents may be useful.
References
1. Dermatol Surg. 1999 Jan;25(1):15-7.
2. Dermatol Surg. 2010 May;36(5):602-9.
3. Br J Dermatol. 2012 Jun;166(6):1160-9.
4.Lasers Surg Med. 2007 Jun;39(5):381-5.
5. Lasers Surg Med. 2007 Apr;39(4):311-4.
6. Dermatol Surg. 2010 Jun;36(6):909-18.
Dr. Wesley and Dr. Talakoub are co-contributors to the monthly Aesthetic Dermatology column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
Pretreatment of skin prior to nonablative or ablative laser resurfacing is common practice, particularly in darker skin types. Treatment regimens include using hydroquinone 4% (and other hydroquinone-containing combinations) once to twice daily for 1-2 weeks prior to the laser procedure. The rationale makes sense. Quieting melanin production by inhibiting tyrosinase would seem to decrease the incidence of postinflammatory hyperpigmentation after laser resurfacing procedures. But is this common practice effective?
For ablative CO2 resurfacing in 100 patients Fitzpatrick Skin Types (FST) I-III, there was no significant difference in the incidence of hyperpigmentation in those randomized to be pretreated with either hydroquinone, glycolic acid, tretinoin, or to no treatment.1 The thought was that the follicular melanocytes involved in re-epithelialization were not affected by the pretreatment. This is the only published laser resurfacing today to date examining various pretreatment protocols with hyperpigmentation as a primary study outcome. From this study, it seems as though pretreatment before laser resurfacing is not helpful, but what about for nonablative resurfacing in darker skin types (FST IV-VI)?
In darker skin types (FST IV-VI), the risk of postinflammatory hyperpigmentation (PIH) is inherently higher and the incidence after laser resurfacing is greater. While the incidence of PIH is lower with nonablative fractional resurfacing, compared with ablative resurfacing, PIH can still occur whether pretreatment hydroquinone is used or not.2,3,4 To date, there are no published studies looking at the incidence of PIH when comparing pretreatment antipigment agents versus no pretreatment for laser resurfacing for acne scars in darker skin types. A split-face study comparing pretreatment on one side and no pretreatment on the other could help delineate whether this practice is evidence based.
For nonablative fractional laser resurfacing of acne scars, lower densities in darker skin types are recommended and may help reduce PIH risk. There is no statistically significant difference in improvement of acne scars in using low versus high densities using the same fluences. However, some studies note that higher densities clinically resulted in a mild improvement of acne scars over lower densities (not statistically significant); thus, if lower densities are used, it is possible that more treatments may be needed.4,5
Vigorous sun protection before and after treatment is prudent, with sun avoidance and physical sunscreens reducing the risk of PIH in darker skin from irritant or allergic contact dermatitis, compared with chemical sunscreens. If PIH occurs, it is often self limited (up to 1-2 months). Sun protection and posttreatment regimens of hydroquinone (or other lightening agent) aid in hastening improvement.
If the patient is undergoing nonablative laser resurfacing to treat pigmentation, such as melasma, then hydroquinone pre- and postlaser is appropriate. In my opinion, laser treatment of melasma should not be first line because of safety and efficacy concerns. However, in these cases, hydroquinone prior to laser has shown benefit.6 In addition, hydroquinone after nonablative fractional resurfacing may enhance penetration of the topical and improve efficacy.
In summary, the evidence shows that pretreatment with antipigment agents is not warranted in skin types I-III for ablative laser resurfacing. Pretreatment with antipigment agents for nonablative laser resurfacing for melasma (which should not be considered a first line treatment for melasma) is warranted. However, at this time, it is not clear whether pretreatment with antipigments for nonablative laser resurfacing for acne scars in darker skin types is useful. Lower densities should be used and if PIH does occur, it is usually self limited, and posttreatment hydroquinone or other antipigment agents may be useful.
References
1. Dermatol Surg. 1999 Jan;25(1):15-7.
2. Dermatol Surg. 2010 May;36(5):602-9.
3. Br J Dermatol. 2012 Jun;166(6):1160-9.
4.Lasers Surg Med. 2007 Jun;39(5):381-5.
5. Lasers Surg Med. 2007 Apr;39(4):311-4.
6. Dermatol Surg. 2010 Jun;36(6):909-18.
Dr. Wesley and Dr. Talakoub are co-contributors to the monthly Aesthetic Dermatology column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
Pretreatment of skin prior to nonablative or ablative laser resurfacing is common practice, particularly in darker skin types. Treatment regimens include using hydroquinone 4% (and other hydroquinone-containing combinations) once to twice daily for 1-2 weeks prior to the laser procedure. The rationale makes sense. Quieting melanin production by inhibiting tyrosinase would seem to decrease the incidence of postinflammatory hyperpigmentation after laser resurfacing procedures. But is this common practice effective?
For ablative CO2 resurfacing in 100 patients Fitzpatrick Skin Types (FST) I-III, there was no significant difference in the incidence of hyperpigmentation in those randomized to be pretreated with either hydroquinone, glycolic acid, tretinoin, or to no treatment.1 The thought was that the follicular melanocytes involved in re-epithelialization were not affected by the pretreatment. This is the only published laser resurfacing today to date examining various pretreatment protocols with hyperpigmentation as a primary study outcome. From this study, it seems as though pretreatment before laser resurfacing is not helpful, but what about for nonablative resurfacing in darker skin types (FST IV-VI)?
In darker skin types (FST IV-VI), the risk of postinflammatory hyperpigmentation (PIH) is inherently higher and the incidence after laser resurfacing is greater. While the incidence of PIH is lower with nonablative fractional resurfacing, compared with ablative resurfacing, PIH can still occur whether pretreatment hydroquinone is used or not.2,3,4 To date, there are no published studies looking at the incidence of PIH when comparing pretreatment antipigment agents versus no pretreatment for laser resurfacing for acne scars in darker skin types. A split-face study comparing pretreatment on one side and no pretreatment on the other could help delineate whether this practice is evidence based.
For nonablative fractional laser resurfacing of acne scars, lower densities in darker skin types are recommended and may help reduce PIH risk. There is no statistically significant difference in improvement of acne scars in using low versus high densities using the same fluences. However, some studies note that higher densities clinically resulted in a mild improvement of acne scars over lower densities (not statistically significant); thus, if lower densities are used, it is possible that more treatments may be needed.4,5
Vigorous sun protection before and after treatment is prudent, with sun avoidance and physical sunscreens reducing the risk of PIH in darker skin from irritant or allergic contact dermatitis, compared with chemical sunscreens. If PIH occurs, it is often self limited (up to 1-2 months). Sun protection and posttreatment regimens of hydroquinone (or other lightening agent) aid in hastening improvement.
If the patient is undergoing nonablative laser resurfacing to treat pigmentation, such as melasma, then hydroquinone pre- and postlaser is appropriate. In my opinion, laser treatment of melasma should not be first line because of safety and efficacy concerns. However, in these cases, hydroquinone prior to laser has shown benefit.6 In addition, hydroquinone after nonablative fractional resurfacing may enhance penetration of the topical and improve efficacy.
In summary, the evidence shows that pretreatment with antipigment agents is not warranted in skin types I-III for ablative laser resurfacing. Pretreatment with antipigment agents for nonablative laser resurfacing for melasma (which should not be considered a first line treatment for melasma) is warranted. However, at this time, it is not clear whether pretreatment with antipigments for nonablative laser resurfacing for acne scars in darker skin types is useful. Lower densities should be used and if PIH does occur, it is usually self limited, and posttreatment hydroquinone or other antipigment agents may be useful.
References
1. Dermatol Surg. 1999 Jan;25(1):15-7.
2. Dermatol Surg. 2010 May;36(5):602-9.
3. Br J Dermatol. 2012 Jun;166(6):1160-9.
4.Lasers Surg Med. 2007 Jun;39(5):381-5.
5. Lasers Surg Med. 2007 Apr;39(4):311-4.
6. Dermatol Surg. 2010 Jun;36(6):909-18.
Dr. Wesley and Dr. Talakoub are co-contributors to the monthly Aesthetic Dermatology column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
Minimum 5-Year Results With Duracon Press-Fit Metal-Backed Patellae
The metal-backed patella was originally designed to address the shortcomings of cemented, all-polyethylene patellae: deformation, aseptic loosening, stress fractures of polyethylene, and possible thermal damage from bone cement.1-3 Several long-term studies have found very good outcomes with use of all-polyethylene patellae.4-6 However, complications of using an all-polyethylene patella reportedly accounted for up to half of all knee revisions, and during revision surgery patellar bone stock was often found to have been compromised.7
The intention behind the design of press-fit metal-backed patellae was to address the shortcomings of all-polyethylene patellae by eliminating the need for bone cement and providing stiffness that would help resist polyethylene deformation while decreasing implant–bone interface stresses.8 However, early design iterations of metal-backed patellae demonstrated short-term failures—most commonly, local polyethylene wear damaging the locking mechanism and subsequent dissociation or fracture from the metal baseplate; polyethylene delamination from the metal baseplate; and failure of interface fixation.9,10 On the other hand, good fixation with bony ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9,11-13 Overall, however, negative outcomes reported for metal-backed patellae led many surgeons to abandon these components and return to using cemented all-polyethylene patellae.
Negative outcomes of earlier metal-backed patellae designs have overshadowed reports of positive outcomes achieved with careful attention paid to component design, patellar tracking, and surgical technique.2,3,14 Subsequent design improvements (eg, a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15 The advantages of using a metal-backed patella (eg, uniform load sharing, decreased polyethylene deformation, potential for biological fixation) may be unjustly outweighed by the fear of patellar component failure.3
Our 30-plus years of experience with metal-backed patellar components reflect the evolving effect of component design on outcome. Much as reported elsewhere, we found earlier component failures were caused by poor locking mechanisms, thin polyethylene, poor tracking, and minimal femur contact. Over the past decade, however, our outcomes with Duracon metal-backed patellae (Stryker) have been encouraging. We think these positive outcomes, seen over minimum 5-year follow-up, are largely attributable to the thicker polyethylene and improved articular conformity of this component relative to earlier designs. We have also found it helpful to adhere to certain criteria when implanting metal-backed patellae, and we think adhering to these criteria, along with improved component design, indicates use of press-fit metal-backed patellae. In this article, we report our failure incidence with use of this device at minimum 5-year follow-up.
Materials and Methods
In this single-center study, we performed clinical and independent radiographic reviews of 88 primary press-fit metal-backed patellae with minimum 5-year follow-up. All components were the same design (Duracon metal-backed patella) from the same manufacturer (Stryker).
This study, which began in September 2003, was reviewed and approved by the Western Institutional Review Board (WIRB). Either the investigator (Dr. Hedley) or the clinical study coordinator gave study candidates a full explanation of the study and answered any questions. Patients who still wanted to participate in the study signed WIRB consent forms after their index surgery but before minimum 5-year follow-up.
Device Description
This Duracon patella has a porous-coated cobalt-chromium metal back intended for press-fit fixation, 3 cobalt-chromium porous-coated pegs, and a preassembled polyethylene anterior surface (Figure 1). Four sizes are available to fit the peripheral shape of the resected patella.
This patella has 3 styles: symmetric, asymmetric, and conversion. In this study, we used only the asymmetric and conversion styles. The design of each style incorporates medial/lateral facets intended to conform to the convex intercondylar radii of the femoral component, thereby allowing the patella to ride deeply in the recessed patellofemoral groove. The asymmetric patella is a resurfacing component with a generous polyethylene thickness (4.6 mm at its thinnest) and a larger lateral facet for more bone coverage. The asymmetric patella naturally medializes component placement. The articulating surface of the conversion patella is identical to that of the asymmetric patella. However, the conversion patella allows for exchange of the polyethylene portion of the implant without revising a stable, well-fixed metal baseplate.
Patient Selection
Candidates were recruited from a group of metal-backed patella patients within Dr. Hedley’s medical practice. All candidates had undergone primary total knee arthroplasty and received a Duracon press-fit metal-backed patella. All recruited patients had undergone primary knee arthroplasty at least 5 years before clinical and radiographic evaluation. Patients were included in the study if they had a diagnosis of noninflammatory degenerative joint disease (eg, osteoarthritis, traumatic arthritis, avascular necrosis). Patients with body mass index higher than 40 were excluded from the study.
Surgical Technique
The patella is everted completely or as much as feasible. Debridement is done circumferentially around the patella. Adherent fat and pseudomeniscus are stripped back until the surgeon sees the entry point of the quadriceps tendon fibers above and the patella tendon fibers below. The cut is then made at this level to remove as much bone as needed to restore the normal height of the patella with the implant in place. The cut is usually made by hand—without guides but with the patella stabilized with a towel clip above and below to prevent any movement during the action.
The desired cut must be absolutely planar, and this should be checked by placing the edge of the blade across the interface. Repeated passes with the saw blade are needed if the cut is not 100% planar. Once the cut is made, the patella is sized with the patella sizers and drill guide. After the appropriate size is selected, the patella is drilled with a bit that is slightly undersized from the size of the pegs (1/32 inch smaller than the bit supplied by the manufacturer).
Once the patella is prepared, the rest of the knee arthroplasty is performed. The patella is press-fit as the last component to be inserted.
Radiologic Review
Radiographic analysis was performed by an independent reviewer according to the current Knee Society total knee arthroplasty roentgenographic evaluation and scoring system (Figure 2).16 The reviewer was an orthopedist specializing in hip and knee surgery. Radiographs the reviewer deemed questionable were shown to another independent hip and knee surgeon for validation. In all cases, the second reviewer confirmed the first reviewer’s initial recorded observations.
KSS (Knee Society Scale), WOMAC (Western Ontario and McMaster Universities Arthritis Index), and SF-36 (36-Item Short Form Health Survey) were also used to evaluate effectiveness in this protocol.
Survivorship Calculations
Kaplan-Meier survivorship was determined for all metal-backed patellae. For survival analysis, only knees with radiographic data were included (74 knees). Mean follow-up was 75.8 months (range, 60-105 months).
Seventy-four patients (88 knees) met the study criteria (Table). At minimum 5-year follow-up, complete data were acquired for 59 patients (72 knees). Of the total group, 14 knees did not have radiographic data. Those knees were categorized as lost to follow-up and were excluded from the survivorship analysis. The status of patients enrolled in the study at minimum 5-year follow-up is shown in the Table.
Mann-Whitney U test (nonparametric t test) was used to compare WOMAC and SF-36 scores between the “complete” and the “WOMAC and SF-36 only” data groups.
Statistical Analysis
Kaplan-Meier survivorship probabilities (asymmetric method) were calculated using SAS Version 9.2 (SAS Institute); 95% pointwise confidence limits were used.
The Mann-Whitney U test is a nonparametric analogue to the independent-samples t test. It was used here to compare WOMAC and SF-36 scores of patients with “complete” data with scores of patients with “WOMAC and SF-36 only” data. In either group, for patients who had primary bilateral knee arthroplasty, mean WOMAC and SF-36 scores were used.
Comparisons were made between the unilateral and bilateral knee arthroplasty groups. There were no differences in age, height, or weight (Mann-Whitney U test) or in sex, primary diagnosis, or number of patients lost to follow-up (Fisher exact test). Fisher exact test (vs χ2 test) was used for the contingency table analysis because of small cell sizes (eg, ≤10 females in ‘‘both knees” group), suggesting the unilateral and bilateral patients did not differ in demographics.
For all patient-reported questionnaires, bilateral patients were given the opportunity to note any differences between their knee arthroplasties, but none of these patients made any special notations. We interpreted this to mean that all survey responses from bilateral patients were applicable to both knee arthroplasties.
Results
Seventy-four patients (88 knees) were enrolled in the study: 31 women (41.2%) and 43 men (58.1%). At time of surgery, mean age was 59.7 years (range, 40-86 years), and mean body mass index was 30.6 (range, 19.1-39.6). Eighty-three knees were diagnosed with osteoarthritis, and 5 knees were diagnosed with posttraumatic arthritis. Mean time to follow-up was 74.8 months (range, 60-105 months). Fourteen knees (14 patients) were considered lost to follow-up. However, 8 patients (8 knees) were contacted by telephone about the status of their knee(s), and all 8 completed and returned the minimum 5-year follow-up WOMAC and SF-36 forms; they did not return for their minimum 5-year clinical or radiographic evaluations.
Asymmetric patellae were used in 24 knees, conversion patellae in 64 knees (88 knees total). Forty-nine months after surgery, 1 patella was revised for loosening at its interface with the bone. The 51-year-old active female patient’s asymmetric patella was revised to a conversion patella. The decision to implant another metal-backed device was based on its high density; proper intrusion of acrylic cement would have been questionable. Some early wear was observed on the tibial insert, which was replaced. Sixty-eight months after the revision, the patient was asymptomatic, with a KSS Pain score of 96 and a KSS Function score of 100 (Figure 3). Another revision, for tibial insert exchange only, was performed 48 months after surgery. During this revision, the patella was evaluated and found to be well fixed and functioning normally.
Survivorship of the Duracon metal-backed patella at minimum 5-year follow-up was estimated to be 93.95%, with bounds of 73.61% and 98.74%.
Radiographic analysis revealed no radiolucencies larger than 1 mm (Figure 4). Seventeen 1-mm radiolucencies were recorded: 6 (35.3%) in zone 1, 2 (11.8%) in zone 2, and 9 (52.9%) in zone 4. Twelve (70.6%) of the 17 radiolucencies were in the left knee. Nine radiolucencies were in women and 8 in men. Most (55.6%) of the women’s radiolucencies were in zone 1, and most (75.0%) of the men’s were in zone 4. There were no loose beads other than in the case that was later revised.
KSS, WOMAC, and SF-36 scores and radiographic reviews were used to evaluate effectiveness in accordance with the protocol. At minimum 5-year follow-up, mean KSS Pain score was 94.10 (range, 55-100), and mean KSS Function score was 92.67 (range, 60-100). Mean WOMAC score was 2.21 (range, 0-19.70), mean SF-36 Physical score was 83.65 (range, 30.70-100), and mean SF-36 Mental score was 89.41 (range, 1.4-100).
The preceding calculations do not include WOMAC and SF-36 data for the 8 patients (8 knees) who were counted as lost to follow-up but who submitted minimum 5-year follow-up data. We compared these 8 patients with the 60 patients (74 knees) who had complete WOMAC and SF-36 data at the end of the study in order to determine whether there were any statistically significant differences between the 2 groups’ mean scores. No statistically significant differences were detected in any WOMAC or SF-36 category (α = 0.05).
Discussion
Metal-backed patellar components were originally designed to address the shortcomings (eg, fracture, deformation, aseptic loosening) of cemented all-polyethylene patellae.1-3 It was thought that the stiffness of the metal could help resist polyethylene deformation and that the press-fit interface with bone might eliminate issues related to bone cement.8 However, short-term failures were reported with early metal-backed designs.9,10 At the same time, good fixation with bone ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9-12,17 Further, reports of poor outcomes with some metal-backed patella designs overshadowed reports of positive outcomes.2,3 In all reports (of both poor and positive outcomes), component design, patellar tracking, and surgical technique were cited as contributing to implant success.2,3,14,17,18 Subsequent design improvements (eg, use of a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15
Our early results are similar to those reported in the literature, and we observed markedly better outcomes that we think resulted from component design improvements. Over the past decade, this has been particularly true with our use of the Duracon metal-backed patella, which has thicker polyethylene, better articular conformity, and a third stabilizing peg, all of which were previously noted as contributing to a successful metal-backed patellar component.2,12,14,15,19 In our study, all 72 knees radiographically evaluated and independently reviewed at minimum 5-year follow-up had well-fixed press-fit metal-backed patellae. Seventeen patellae had 1-mm radiolucencies; the other 59 had no radiolucencies in any zone around the patella–bone interface.
One of the most important aspects of removing a metal-backed patellar component from a patella is that the remaining bone stock is often far superior to the stock available after revision of a cemented patella. Careful removal should leave an excellent bony bed for reimplantation.
We think that surgeons should adhere to certain indications and contraindications when implanting metal-backed patellae and that doing so can contribute to successful outcomes. Type of bone stock available should be considered, as successful biological fixation relies on a good blood supply. A dense (or thin) patella in which intrusion of acrylic cement is improbable or impossible may favor use of a metal-backed patella. Cement is not an adhesive but a grout, so successful cementation requires intrusion of cement into the interstices of the cancellous bone. As adequate intrusion of cement into dense bone is not possible, cementation may not be the best option. Some patellae have failed because of peg “shear-off,”9 likely caused not by failure of peg strength but by failure of cement fixation at the nonpeg interface.20,21 Polyethylene pegs fail when used as the sole method of fixation (they were never designed for that). In addition, we think younger patients are often indicated for a metal-backed patella because, over the long term, loosening of a cemented patella (and the accompanying stress shielding and osteolysis) may cause severe patellar bone destruction. Last, we have found that abnormally high or small patellae are not good candidates for cement fixation because they tend to work themselves loose riding on and off the superior flange. These types of patellae appear to have a much sturdier and longer lasting interface than cement, once biological fixation has occurred.
In summary, we think the indications for a metal-backed implant are a patella that is dense or sclerotic; a patella that is thin, abnormally high, or small; and a younger patient. In addition, a metal-backed implant is not indicated for soft, osteoporotic bone.
This study had a few limitations. Fourteen knees (14 patients), or 15.9% of all knees in the study, were categorized as lost to follow-up. Comparing the WOMAC and SF-36 scores of 8 patients (8 knees) who completed minimum 5-year follow-up but were not clinically evaluated with the scores of patients who had complete data, we found no statistically significant differences in any category. However, 5-year follow-up clinical data were available for those 8 patients. Nevertheless, 74 knees were available for radiologic evaluation, and during telephone interviews all 8 patients indicated they had their original implant(s) and were asymptomatic.
Our experience with the Duracon metal-backed patella has been encouraging. In the study reported here, there were no failures caused by dissociation of plastic. We think that, because the porous coating is under almost constant compression, biological fixation is likely in most instances, as observed in our minimum 5-year radiologic results. Given our minimum 5-year follow-up results with uncemented metal-backed patellae, we think their use may be a viable alternative to use of all-polyethylene patellae.
1. Firestone TP, Teeny SM, Krackow KA, Hungerford DS. The clinical and roentgenographic results of cementless porous-coated patellar fixation. Clin Orthop Relat Res. 1991;273:184-189.
2. Laskin RS, Bucknell A. The use of metal-backed patellar prostheses in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:52-55.
3. Evanich CJ, Tkach TK, von Glinski S, Camargo MP, Hofmann AA. 6- to 10-year experience using countersunk metal-backed patellas. J Arthroplasty. 1997;12(2):149-154.
4. Schwartz AJ, Della Vale CJ, Rosenberg AG, Jacobs JJ, Berger RA, Galante JO. Cruciate-retaining TKA using a third-generation system with a four-pegged tibial component: a minimum 10-year followup note. Clin Orthop Relat Res. 2010;468(8):2160-2167.
5. Bisschop R, Brouwer RW, Van Raay JJ. Total knee arthroplasty in younger patients: a 13-year follow-up study. Orthopedics. 2010;33(12):876-880.
6. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. A study of patients followed for a minimum of fifteen years. J Bone Joint Surg Am. 2005;87(3):598-603.
7. Brick GW, Scott RD. The patellofemoral component of total knee arthroplasty. Clin Orthop Relat Res. 1988;231)163-178.
8. Garcia RM, Kraay MJ, Goldberg VM. Isolated all-polyethylene patellar revisions for metal-backed patellar failure. Clin Orthop Relat Res. 2008;466(11):2784-2789.
9. Rosenberg AG, Andriacchi TP, Barden R, Galante JO. Patellar component failure in cementless total knee arthroplasty. Clin Orthop Relat Res. 1988;(236):106-114.
10. Stulberg SD, Stulberg BN, Hamati Y, Tsao A. Failure mechanisms of metal-backed patellar components. Clin Orthop Relat Res. 1988;236:88-105.
11. Sundfeldt M, Johansson CB, Regner L, Albrektsson T, Carlsson LV. Long-term results of a cementless knee prosthesis with a metal-backed patellar component: clinical and radiological follow-up with histology from retrieved components. J Long Term Eff Med Implants. 2003;13(4):341-354.
12. Kraay MJ, Darr OJ, Salata MJ, Goldberg VM. Outcome of metal-backed cementless patellar components: the effect of implant design. Clin Orthop Relat Res. 2001;392:239-244.
13. Jensen LN, Lund B, Gotfredsen K. Bone growth into a revised porous-coated patellar implant. Acta Orthop Scand. 1990;61(3):213-216.
14. Hsu HP, Walker PS. Wear and deformation of patellar components in total knee arthroplasty. Clin Orthop Relat Res. 1989;246:260-265.
15. Jordan LR, Sorrells RB, Jordan LC, Olivo JL. The long-term results of a metal-backed mobile bearing patella. Clin Orthop Relat Res. 2005;436:111-118.
16. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
17. Bayley JC, Scott RD, Ewald FC, Holmes GB Jr. Failure of the metal-backed patellar component after total knee replacement. J Bone Joint Surg Am. 1988;70(5):668-674.
18. Lombardi AV Jr, Engh GA, Volz RG, Albrigo JL, Brainard BJ. Fracture/dissociation of the polyethylene in metal-backed patellar components in total knee arthroplasty. J Bone Joint Surg Am. 1988;70(5):675-679.
19. Moreland JR. Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:49-64.
20. Francke EI, Lachiewicz PF. Failure of a cemented all-polyethylene patellar component of a press-fit condylar total knee arthroplasty. J Arthroplasty. 2000;15(2):234-237.
21. Stulberg BN, Wright TM, Stoller AP, Mimnaugh KL, Mason JJ. Bilateral patellar component shear failure of highly cross-linked polyethylene components: report of a case and laboratory analysis of failure mechanisms. J Arthroplasty. 2012;27(5):789-796.
The metal-backed patella was originally designed to address the shortcomings of cemented, all-polyethylene patellae: deformation, aseptic loosening, stress fractures of polyethylene, and possible thermal damage from bone cement.1-3 Several long-term studies have found very good outcomes with use of all-polyethylene patellae.4-6 However, complications of using an all-polyethylene patella reportedly accounted for up to half of all knee revisions, and during revision surgery patellar bone stock was often found to have been compromised.7
The intention behind the design of press-fit metal-backed patellae was to address the shortcomings of all-polyethylene patellae by eliminating the need for bone cement and providing stiffness that would help resist polyethylene deformation while decreasing implant–bone interface stresses.8 However, early design iterations of metal-backed patellae demonstrated short-term failures—most commonly, local polyethylene wear damaging the locking mechanism and subsequent dissociation or fracture from the metal baseplate; polyethylene delamination from the metal baseplate; and failure of interface fixation.9,10 On the other hand, good fixation with bony ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9,11-13 Overall, however, negative outcomes reported for metal-backed patellae led many surgeons to abandon these components and return to using cemented all-polyethylene patellae.
Negative outcomes of earlier metal-backed patellae designs have overshadowed reports of positive outcomes achieved with careful attention paid to component design, patellar tracking, and surgical technique.2,3,14 Subsequent design improvements (eg, a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15 The advantages of using a metal-backed patella (eg, uniform load sharing, decreased polyethylene deformation, potential for biological fixation) may be unjustly outweighed by the fear of patellar component failure.3
Our 30-plus years of experience with metal-backed patellar components reflect the evolving effect of component design on outcome. Much as reported elsewhere, we found earlier component failures were caused by poor locking mechanisms, thin polyethylene, poor tracking, and minimal femur contact. Over the past decade, however, our outcomes with Duracon metal-backed patellae (Stryker) have been encouraging. We think these positive outcomes, seen over minimum 5-year follow-up, are largely attributable to the thicker polyethylene and improved articular conformity of this component relative to earlier designs. We have also found it helpful to adhere to certain criteria when implanting metal-backed patellae, and we think adhering to these criteria, along with improved component design, indicates use of press-fit metal-backed patellae. In this article, we report our failure incidence with use of this device at minimum 5-year follow-up.
Materials and Methods
In this single-center study, we performed clinical and independent radiographic reviews of 88 primary press-fit metal-backed patellae with minimum 5-year follow-up. All components were the same design (Duracon metal-backed patella) from the same manufacturer (Stryker).
This study, which began in September 2003, was reviewed and approved by the Western Institutional Review Board (WIRB). Either the investigator (Dr. Hedley) or the clinical study coordinator gave study candidates a full explanation of the study and answered any questions. Patients who still wanted to participate in the study signed WIRB consent forms after their index surgery but before minimum 5-year follow-up.
Device Description
This Duracon patella has a porous-coated cobalt-chromium metal back intended for press-fit fixation, 3 cobalt-chromium porous-coated pegs, and a preassembled polyethylene anterior surface (Figure 1). Four sizes are available to fit the peripheral shape of the resected patella.
This patella has 3 styles: symmetric, asymmetric, and conversion. In this study, we used only the asymmetric and conversion styles. The design of each style incorporates medial/lateral facets intended to conform to the convex intercondylar radii of the femoral component, thereby allowing the patella to ride deeply in the recessed patellofemoral groove. The asymmetric patella is a resurfacing component with a generous polyethylene thickness (4.6 mm at its thinnest) and a larger lateral facet for more bone coverage. The asymmetric patella naturally medializes component placement. The articulating surface of the conversion patella is identical to that of the asymmetric patella. However, the conversion patella allows for exchange of the polyethylene portion of the implant without revising a stable, well-fixed metal baseplate.
Patient Selection
Candidates were recruited from a group of metal-backed patella patients within Dr. Hedley’s medical practice. All candidates had undergone primary total knee arthroplasty and received a Duracon press-fit metal-backed patella. All recruited patients had undergone primary knee arthroplasty at least 5 years before clinical and radiographic evaluation. Patients were included in the study if they had a diagnosis of noninflammatory degenerative joint disease (eg, osteoarthritis, traumatic arthritis, avascular necrosis). Patients with body mass index higher than 40 were excluded from the study.
Surgical Technique
The patella is everted completely or as much as feasible. Debridement is done circumferentially around the patella. Adherent fat and pseudomeniscus are stripped back until the surgeon sees the entry point of the quadriceps tendon fibers above and the patella tendon fibers below. The cut is then made at this level to remove as much bone as needed to restore the normal height of the patella with the implant in place. The cut is usually made by hand—without guides but with the patella stabilized with a towel clip above and below to prevent any movement during the action.
The desired cut must be absolutely planar, and this should be checked by placing the edge of the blade across the interface. Repeated passes with the saw blade are needed if the cut is not 100% planar. Once the cut is made, the patella is sized with the patella sizers and drill guide. After the appropriate size is selected, the patella is drilled with a bit that is slightly undersized from the size of the pegs (1/32 inch smaller than the bit supplied by the manufacturer).
Once the patella is prepared, the rest of the knee arthroplasty is performed. The patella is press-fit as the last component to be inserted.
Radiologic Review
Radiographic analysis was performed by an independent reviewer according to the current Knee Society total knee arthroplasty roentgenographic evaluation and scoring system (Figure 2).16 The reviewer was an orthopedist specializing in hip and knee surgery. Radiographs the reviewer deemed questionable were shown to another independent hip and knee surgeon for validation. In all cases, the second reviewer confirmed the first reviewer’s initial recorded observations.
KSS (Knee Society Scale), WOMAC (Western Ontario and McMaster Universities Arthritis Index), and SF-36 (36-Item Short Form Health Survey) were also used to evaluate effectiveness in this protocol.
Survivorship Calculations
Kaplan-Meier survivorship was determined for all metal-backed patellae. For survival analysis, only knees with radiographic data were included (74 knees). Mean follow-up was 75.8 months (range, 60-105 months).
Seventy-four patients (88 knees) met the study criteria (Table). At minimum 5-year follow-up, complete data were acquired for 59 patients (72 knees). Of the total group, 14 knees did not have radiographic data. Those knees were categorized as lost to follow-up and were excluded from the survivorship analysis. The status of patients enrolled in the study at minimum 5-year follow-up is shown in the Table.
Mann-Whitney U test (nonparametric t test) was used to compare WOMAC and SF-36 scores between the “complete” and the “WOMAC and SF-36 only” data groups.
Statistical Analysis
Kaplan-Meier survivorship probabilities (asymmetric method) were calculated using SAS Version 9.2 (SAS Institute); 95% pointwise confidence limits were used.
The Mann-Whitney U test is a nonparametric analogue to the independent-samples t test. It was used here to compare WOMAC and SF-36 scores of patients with “complete” data with scores of patients with “WOMAC and SF-36 only” data. In either group, for patients who had primary bilateral knee arthroplasty, mean WOMAC and SF-36 scores were used.
Comparisons were made between the unilateral and bilateral knee arthroplasty groups. There were no differences in age, height, or weight (Mann-Whitney U test) or in sex, primary diagnosis, or number of patients lost to follow-up (Fisher exact test). Fisher exact test (vs χ2 test) was used for the contingency table analysis because of small cell sizes (eg, ≤10 females in ‘‘both knees” group), suggesting the unilateral and bilateral patients did not differ in demographics.
For all patient-reported questionnaires, bilateral patients were given the opportunity to note any differences between their knee arthroplasties, but none of these patients made any special notations. We interpreted this to mean that all survey responses from bilateral patients were applicable to both knee arthroplasties.
Results
Seventy-four patients (88 knees) were enrolled in the study: 31 women (41.2%) and 43 men (58.1%). At time of surgery, mean age was 59.7 years (range, 40-86 years), and mean body mass index was 30.6 (range, 19.1-39.6). Eighty-three knees were diagnosed with osteoarthritis, and 5 knees were diagnosed with posttraumatic arthritis. Mean time to follow-up was 74.8 months (range, 60-105 months). Fourteen knees (14 patients) were considered lost to follow-up. However, 8 patients (8 knees) were contacted by telephone about the status of their knee(s), and all 8 completed and returned the minimum 5-year follow-up WOMAC and SF-36 forms; they did not return for their minimum 5-year clinical or radiographic evaluations.
Asymmetric patellae were used in 24 knees, conversion patellae in 64 knees (88 knees total). Forty-nine months after surgery, 1 patella was revised for loosening at its interface with the bone. The 51-year-old active female patient’s asymmetric patella was revised to a conversion patella. The decision to implant another metal-backed device was based on its high density; proper intrusion of acrylic cement would have been questionable. Some early wear was observed on the tibial insert, which was replaced. Sixty-eight months after the revision, the patient was asymptomatic, with a KSS Pain score of 96 and a KSS Function score of 100 (Figure 3). Another revision, for tibial insert exchange only, was performed 48 months after surgery. During this revision, the patella was evaluated and found to be well fixed and functioning normally.
Survivorship of the Duracon metal-backed patella at minimum 5-year follow-up was estimated to be 93.95%, with bounds of 73.61% and 98.74%.
Radiographic analysis revealed no radiolucencies larger than 1 mm (Figure 4). Seventeen 1-mm radiolucencies were recorded: 6 (35.3%) in zone 1, 2 (11.8%) in zone 2, and 9 (52.9%) in zone 4. Twelve (70.6%) of the 17 radiolucencies were in the left knee. Nine radiolucencies were in women and 8 in men. Most (55.6%) of the women’s radiolucencies were in zone 1, and most (75.0%) of the men’s were in zone 4. There were no loose beads other than in the case that was later revised.
KSS, WOMAC, and SF-36 scores and radiographic reviews were used to evaluate effectiveness in accordance with the protocol. At minimum 5-year follow-up, mean KSS Pain score was 94.10 (range, 55-100), and mean KSS Function score was 92.67 (range, 60-100). Mean WOMAC score was 2.21 (range, 0-19.70), mean SF-36 Physical score was 83.65 (range, 30.70-100), and mean SF-36 Mental score was 89.41 (range, 1.4-100).
The preceding calculations do not include WOMAC and SF-36 data for the 8 patients (8 knees) who were counted as lost to follow-up but who submitted minimum 5-year follow-up data. We compared these 8 patients with the 60 patients (74 knees) who had complete WOMAC and SF-36 data at the end of the study in order to determine whether there were any statistically significant differences between the 2 groups’ mean scores. No statistically significant differences were detected in any WOMAC or SF-36 category (α = 0.05).
Discussion
Metal-backed patellar components were originally designed to address the shortcomings (eg, fracture, deformation, aseptic loosening) of cemented all-polyethylene patellae.1-3 It was thought that the stiffness of the metal could help resist polyethylene deformation and that the press-fit interface with bone might eliminate issues related to bone cement.8 However, short-term failures were reported with early metal-backed designs.9,10 At the same time, good fixation with bone ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9-12,17 Further, reports of poor outcomes with some metal-backed patella designs overshadowed reports of positive outcomes.2,3 In all reports (of both poor and positive outcomes), component design, patellar tracking, and surgical technique were cited as contributing to implant success.2,3,14,17,18 Subsequent design improvements (eg, use of a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15
Our early results are similar to those reported in the literature, and we observed markedly better outcomes that we think resulted from component design improvements. Over the past decade, this has been particularly true with our use of the Duracon metal-backed patella, which has thicker polyethylene, better articular conformity, and a third stabilizing peg, all of which were previously noted as contributing to a successful metal-backed patellar component.2,12,14,15,19 In our study, all 72 knees radiographically evaluated and independently reviewed at minimum 5-year follow-up had well-fixed press-fit metal-backed patellae. Seventeen patellae had 1-mm radiolucencies; the other 59 had no radiolucencies in any zone around the patella–bone interface.
One of the most important aspects of removing a metal-backed patellar component from a patella is that the remaining bone stock is often far superior to the stock available after revision of a cemented patella. Careful removal should leave an excellent bony bed for reimplantation.
We think that surgeons should adhere to certain indications and contraindications when implanting metal-backed patellae and that doing so can contribute to successful outcomes. Type of bone stock available should be considered, as successful biological fixation relies on a good blood supply. A dense (or thin) patella in which intrusion of acrylic cement is improbable or impossible may favor use of a metal-backed patella. Cement is not an adhesive but a grout, so successful cementation requires intrusion of cement into the interstices of the cancellous bone. As adequate intrusion of cement into dense bone is not possible, cementation may not be the best option. Some patellae have failed because of peg “shear-off,”9 likely caused not by failure of peg strength but by failure of cement fixation at the nonpeg interface.20,21 Polyethylene pegs fail when used as the sole method of fixation (they were never designed for that). In addition, we think younger patients are often indicated for a metal-backed patella because, over the long term, loosening of a cemented patella (and the accompanying stress shielding and osteolysis) may cause severe patellar bone destruction. Last, we have found that abnormally high or small patellae are not good candidates for cement fixation because they tend to work themselves loose riding on and off the superior flange. These types of patellae appear to have a much sturdier and longer lasting interface than cement, once biological fixation has occurred.
In summary, we think the indications for a metal-backed implant are a patella that is dense or sclerotic; a patella that is thin, abnormally high, or small; and a younger patient. In addition, a metal-backed implant is not indicated for soft, osteoporotic bone.
This study had a few limitations. Fourteen knees (14 patients), or 15.9% of all knees in the study, were categorized as lost to follow-up. Comparing the WOMAC and SF-36 scores of 8 patients (8 knees) who completed minimum 5-year follow-up but were not clinically evaluated with the scores of patients who had complete data, we found no statistically significant differences in any category. However, 5-year follow-up clinical data were available for those 8 patients. Nevertheless, 74 knees were available for radiologic evaluation, and during telephone interviews all 8 patients indicated they had their original implant(s) and were asymptomatic.
Our experience with the Duracon metal-backed patella has been encouraging. In the study reported here, there were no failures caused by dissociation of plastic. We think that, because the porous coating is under almost constant compression, biological fixation is likely in most instances, as observed in our minimum 5-year radiologic results. Given our minimum 5-year follow-up results with uncemented metal-backed patellae, we think their use may be a viable alternative to use of all-polyethylene patellae.
The metal-backed patella was originally designed to address the shortcomings of cemented, all-polyethylene patellae: deformation, aseptic loosening, stress fractures of polyethylene, and possible thermal damage from bone cement.1-3 Several long-term studies have found very good outcomes with use of all-polyethylene patellae.4-6 However, complications of using an all-polyethylene patella reportedly accounted for up to half of all knee revisions, and during revision surgery patellar bone stock was often found to have been compromised.7
The intention behind the design of press-fit metal-backed patellae was to address the shortcomings of all-polyethylene patellae by eliminating the need for bone cement and providing stiffness that would help resist polyethylene deformation while decreasing implant–bone interface stresses.8 However, early design iterations of metal-backed patellae demonstrated short-term failures—most commonly, local polyethylene wear damaging the locking mechanism and subsequent dissociation or fracture from the metal baseplate; polyethylene delamination from the metal baseplate; and failure of interface fixation.9,10 On the other hand, good fixation with bony ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9,11-13 Overall, however, negative outcomes reported for metal-backed patellae led many surgeons to abandon these components and return to using cemented all-polyethylene patellae.
Negative outcomes of earlier metal-backed patellae designs have overshadowed reports of positive outcomes achieved with careful attention paid to component design, patellar tracking, and surgical technique.2,3,14 Subsequent design improvements (eg, a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15 The advantages of using a metal-backed patella (eg, uniform load sharing, decreased polyethylene deformation, potential for biological fixation) may be unjustly outweighed by the fear of patellar component failure.3
Our 30-plus years of experience with metal-backed patellar components reflect the evolving effect of component design on outcome. Much as reported elsewhere, we found earlier component failures were caused by poor locking mechanisms, thin polyethylene, poor tracking, and minimal femur contact. Over the past decade, however, our outcomes with Duracon metal-backed patellae (Stryker) have been encouraging. We think these positive outcomes, seen over minimum 5-year follow-up, are largely attributable to the thicker polyethylene and improved articular conformity of this component relative to earlier designs. We have also found it helpful to adhere to certain criteria when implanting metal-backed patellae, and we think adhering to these criteria, along with improved component design, indicates use of press-fit metal-backed patellae. In this article, we report our failure incidence with use of this device at minimum 5-year follow-up.
Materials and Methods
In this single-center study, we performed clinical and independent radiographic reviews of 88 primary press-fit metal-backed patellae with minimum 5-year follow-up. All components were the same design (Duracon metal-backed patella) from the same manufacturer (Stryker).
This study, which began in September 2003, was reviewed and approved by the Western Institutional Review Board (WIRB). Either the investigator (Dr. Hedley) or the clinical study coordinator gave study candidates a full explanation of the study and answered any questions. Patients who still wanted to participate in the study signed WIRB consent forms after their index surgery but before minimum 5-year follow-up.
Device Description
This Duracon patella has a porous-coated cobalt-chromium metal back intended for press-fit fixation, 3 cobalt-chromium porous-coated pegs, and a preassembled polyethylene anterior surface (Figure 1). Four sizes are available to fit the peripheral shape of the resected patella.
This patella has 3 styles: symmetric, asymmetric, and conversion. In this study, we used only the asymmetric and conversion styles. The design of each style incorporates medial/lateral facets intended to conform to the convex intercondylar radii of the femoral component, thereby allowing the patella to ride deeply in the recessed patellofemoral groove. The asymmetric patella is a resurfacing component with a generous polyethylene thickness (4.6 mm at its thinnest) and a larger lateral facet for more bone coverage. The asymmetric patella naturally medializes component placement. The articulating surface of the conversion patella is identical to that of the asymmetric patella. However, the conversion patella allows for exchange of the polyethylene portion of the implant without revising a stable, well-fixed metal baseplate.
Patient Selection
Candidates were recruited from a group of metal-backed patella patients within Dr. Hedley’s medical practice. All candidates had undergone primary total knee arthroplasty and received a Duracon press-fit metal-backed patella. All recruited patients had undergone primary knee arthroplasty at least 5 years before clinical and radiographic evaluation. Patients were included in the study if they had a diagnosis of noninflammatory degenerative joint disease (eg, osteoarthritis, traumatic arthritis, avascular necrosis). Patients with body mass index higher than 40 were excluded from the study.
Surgical Technique
The patella is everted completely or as much as feasible. Debridement is done circumferentially around the patella. Adherent fat and pseudomeniscus are stripped back until the surgeon sees the entry point of the quadriceps tendon fibers above and the patella tendon fibers below. The cut is then made at this level to remove as much bone as needed to restore the normal height of the patella with the implant in place. The cut is usually made by hand—without guides but with the patella stabilized with a towel clip above and below to prevent any movement during the action.
The desired cut must be absolutely planar, and this should be checked by placing the edge of the blade across the interface. Repeated passes with the saw blade are needed if the cut is not 100% planar. Once the cut is made, the patella is sized with the patella sizers and drill guide. After the appropriate size is selected, the patella is drilled with a bit that is slightly undersized from the size of the pegs (1/32 inch smaller than the bit supplied by the manufacturer).
Once the patella is prepared, the rest of the knee arthroplasty is performed. The patella is press-fit as the last component to be inserted.
Radiologic Review
Radiographic analysis was performed by an independent reviewer according to the current Knee Society total knee arthroplasty roentgenographic evaluation and scoring system (Figure 2).16 The reviewer was an orthopedist specializing in hip and knee surgery. Radiographs the reviewer deemed questionable were shown to another independent hip and knee surgeon for validation. In all cases, the second reviewer confirmed the first reviewer’s initial recorded observations.
KSS (Knee Society Scale), WOMAC (Western Ontario and McMaster Universities Arthritis Index), and SF-36 (36-Item Short Form Health Survey) were also used to evaluate effectiveness in this protocol.
Survivorship Calculations
Kaplan-Meier survivorship was determined for all metal-backed patellae. For survival analysis, only knees with radiographic data were included (74 knees). Mean follow-up was 75.8 months (range, 60-105 months).
Seventy-four patients (88 knees) met the study criteria (Table). At minimum 5-year follow-up, complete data were acquired for 59 patients (72 knees). Of the total group, 14 knees did not have radiographic data. Those knees were categorized as lost to follow-up and were excluded from the survivorship analysis. The status of patients enrolled in the study at minimum 5-year follow-up is shown in the Table.
Mann-Whitney U test (nonparametric t test) was used to compare WOMAC and SF-36 scores between the “complete” and the “WOMAC and SF-36 only” data groups.
Statistical Analysis
Kaplan-Meier survivorship probabilities (asymmetric method) were calculated using SAS Version 9.2 (SAS Institute); 95% pointwise confidence limits were used.
The Mann-Whitney U test is a nonparametric analogue to the independent-samples t test. It was used here to compare WOMAC and SF-36 scores of patients with “complete” data with scores of patients with “WOMAC and SF-36 only” data. In either group, for patients who had primary bilateral knee arthroplasty, mean WOMAC and SF-36 scores were used.
Comparisons were made between the unilateral and bilateral knee arthroplasty groups. There were no differences in age, height, or weight (Mann-Whitney U test) or in sex, primary diagnosis, or number of patients lost to follow-up (Fisher exact test). Fisher exact test (vs χ2 test) was used for the contingency table analysis because of small cell sizes (eg, ≤10 females in ‘‘both knees” group), suggesting the unilateral and bilateral patients did not differ in demographics.
For all patient-reported questionnaires, bilateral patients were given the opportunity to note any differences between their knee arthroplasties, but none of these patients made any special notations. We interpreted this to mean that all survey responses from bilateral patients were applicable to both knee arthroplasties.
Results
Seventy-four patients (88 knees) were enrolled in the study: 31 women (41.2%) and 43 men (58.1%). At time of surgery, mean age was 59.7 years (range, 40-86 years), and mean body mass index was 30.6 (range, 19.1-39.6). Eighty-three knees were diagnosed with osteoarthritis, and 5 knees were diagnosed with posttraumatic arthritis. Mean time to follow-up was 74.8 months (range, 60-105 months). Fourteen knees (14 patients) were considered lost to follow-up. However, 8 patients (8 knees) were contacted by telephone about the status of their knee(s), and all 8 completed and returned the minimum 5-year follow-up WOMAC and SF-36 forms; they did not return for their minimum 5-year clinical or radiographic evaluations.
Asymmetric patellae were used in 24 knees, conversion patellae in 64 knees (88 knees total). Forty-nine months after surgery, 1 patella was revised for loosening at its interface with the bone. The 51-year-old active female patient’s asymmetric patella was revised to a conversion patella. The decision to implant another metal-backed device was based on its high density; proper intrusion of acrylic cement would have been questionable. Some early wear was observed on the tibial insert, which was replaced. Sixty-eight months after the revision, the patient was asymptomatic, with a KSS Pain score of 96 and a KSS Function score of 100 (Figure 3). Another revision, for tibial insert exchange only, was performed 48 months after surgery. During this revision, the patella was evaluated and found to be well fixed and functioning normally.
Survivorship of the Duracon metal-backed patella at minimum 5-year follow-up was estimated to be 93.95%, with bounds of 73.61% and 98.74%.
Radiographic analysis revealed no radiolucencies larger than 1 mm (Figure 4). Seventeen 1-mm radiolucencies were recorded: 6 (35.3%) in zone 1, 2 (11.8%) in zone 2, and 9 (52.9%) in zone 4. Twelve (70.6%) of the 17 radiolucencies were in the left knee. Nine radiolucencies were in women and 8 in men. Most (55.6%) of the women’s radiolucencies were in zone 1, and most (75.0%) of the men’s were in zone 4. There were no loose beads other than in the case that was later revised.
KSS, WOMAC, and SF-36 scores and radiographic reviews were used to evaluate effectiveness in accordance with the protocol. At minimum 5-year follow-up, mean KSS Pain score was 94.10 (range, 55-100), and mean KSS Function score was 92.67 (range, 60-100). Mean WOMAC score was 2.21 (range, 0-19.70), mean SF-36 Physical score was 83.65 (range, 30.70-100), and mean SF-36 Mental score was 89.41 (range, 1.4-100).
The preceding calculations do not include WOMAC and SF-36 data for the 8 patients (8 knees) who were counted as lost to follow-up but who submitted minimum 5-year follow-up data. We compared these 8 patients with the 60 patients (74 knees) who had complete WOMAC and SF-36 data at the end of the study in order to determine whether there were any statistically significant differences between the 2 groups’ mean scores. No statistically significant differences were detected in any WOMAC or SF-36 category (α = 0.05).
Discussion
Metal-backed patellar components were originally designed to address the shortcomings (eg, fracture, deformation, aseptic loosening) of cemented all-polyethylene patellae.1-3 It was thought that the stiffness of the metal could help resist polyethylene deformation and that the press-fit interface with bone might eliminate issues related to bone cement.8 However, short-term failures were reported with early metal-backed designs.9,10 At the same time, good fixation with bone ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9-12,17 Further, reports of poor outcomes with some metal-backed patella designs overshadowed reports of positive outcomes.2,3 In all reports (of both poor and positive outcomes), component design, patellar tracking, and surgical technique were cited as contributing to implant success.2,3,14,17,18 Subsequent design improvements (eg, use of a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15
Our early results are similar to those reported in the literature, and we observed markedly better outcomes that we think resulted from component design improvements. Over the past decade, this has been particularly true with our use of the Duracon metal-backed patella, which has thicker polyethylene, better articular conformity, and a third stabilizing peg, all of which were previously noted as contributing to a successful metal-backed patellar component.2,12,14,15,19 In our study, all 72 knees radiographically evaluated and independently reviewed at minimum 5-year follow-up had well-fixed press-fit metal-backed patellae. Seventeen patellae had 1-mm radiolucencies; the other 59 had no radiolucencies in any zone around the patella–bone interface.
One of the most important aspects of removing a metal-backed patellar component from a patella is that the remaining bone stock is often far superior to the stock available after revision of a cemented patella. Careful removal should leave an excellent bony bed for reimplantation.
We think that surgeons should adhere to certain indications and contraindications when implanting metal-backed patellae and that doing so can contribute to successful outcomes. Type of bone stock available should be considered, as successful biological fixation relies on a good blood supply. A dense (or thin) patella in which intrusion of acrylic cement is improbable or impossible may favor use of a metal-backed patella. Cement is not an adhesive but a grout, so successful cementation requires intrusion of cement into the interstices of the cancellous bone. As adequate intrusion of cement into dense bone is not possible, cementation may not be the best option. Some patellae have failed because of peg “shear-off,”9 likely caused not by failure of peg strength but by failure of cement fixation at the nonpeg interface.20,21 Polyethylene pegs fail when used as the sole method of fixation (they were never designed for that). In addition, we think younger patients are often indicated for a metal-backed patella because, over the long term, loosening of a cemented patella (and the accompanying stress shielding and osteolysis) may cause severe patellar bone destruction. Last, we have found that abnormally high or small patellae are not good candidates for cement fixation because they tend to work themselves loose riding on and off the superior flange. These types of patellae appear to have a much sturdier and longer lasting interface than cement, once biological fixation has occurred.
In summary, we think the indications for a metal-backed implant are a patella that is dense or sclerotic; a patella that is thin, abnormally high, or small; and a younger patient. In addition, a metal-backed implant is not indicated for soft, osteoporotic bone.
This study had a few limitations. Fourteen knees (14 patients), or 15.9% of all knees in the study, were categorized as lost to follow-up. Comparing the WOMAC and SF-36 scores of 8 patients (8 knees) who completed minimum 5-year follow-up but were not clinically evaluated with the scores of patients who had complete data, we found no statistically significant differences in any category. However, 5-year follow-up clinical data were available for those 8 patients. Nevertheless, 74 knees were available for radiologic evaluation, and during telephone interviews all 8 patients indicated they had their original implant(s) and were asymptomatic.
Our experience with the Duracon metal-backed patella has been encouraging. In the study reported here, there were no failures caused by dissociation of plastic. We think that, because the porous coating is under almost constant compression, biological fixation is likely in most instances, as observed in our minimum 5-year radiologic results. Given our minimum 5-year follow-up results with uncemented metal-backed patellae, we think their use may be a viable alternative to use of all-polyethylene patellae.
1. Firestone TP, Teeny SM, Krackow KA, Hungerford DS. The clinical and roentgenographic results of cementless porous-coated patellar fixation. Clin Orthop Relat Res. 1991;273:184-189.
2. Laskin RS, Bucknell A. The use of metal-backed patellar prostheses in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:52-55.
3. Evanich CJ, Tkach TK, von Glinski S, Camargo MP, Hofmann AA. 6- to 10-year experience using countersunk metal-backed patellas. J Arthroplasty. 1997;12(2):149-154.
4. Schwartz AJ, Della Vale CJ, Rosenberg AG, Jacobs JJ, Berger RA, Galante JO. Cruciate-retaining TKA using a third-generation system with a four-pegged tibial component: a minimum 10-year followup note. Clin Orthop Relat Res. 2010;468(8):2160-2167.
5. Bisschop R, Brouwer RW, Van Raay JJ. Total knee arthroplasty in younger patients: a 13-year follow-up study. Orthopedics. 2010;33(12):876-880.
6. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. A study of patients followed for a minimum of fifteen years. J Bone Joint Surg Am. 2005;87(3):598-603.
7. Brick GW, Scott RD. The patellofemoral component of total knee arthroplasty. Clin Orthop Relat Res. 1988;231)163-178.
8. Garcia RM, Kraay MJ, Goldberg VM. Isolated all-polyethylene patellar revisions for metal-backed patellar failure. Clin Orthop Relat Res. 2008;466(11):2784-2789.
9. Rosenberg AG, Andriacchi TP, Barden R, Galante JO. Patellar component failure in cementless total knee arthroplasty. Clin Orthop Relat Res. 1988;(236):106-114.
10. Stulberg SD, Stulberg BN, Hamati Y, Tsao A. Failure mechanisms of metal-backed patellar components. Clin Orthop Relat Res. 1988;236:88-105.
11. Sundfeldt M, Johansson CB, Regner L, Albrektsson T, Carlsson LV. Long-term results of a cementless knee prosthesis with a metal-backed patellar component: clinical and radiological follow-up with histology from retrieved components. J Long Term Eff Med Implants. 2003;13(4):341-354.
12. Kraay MJ, Darr OJ, Salata MJ, Goldberg VM. Outcome of metal-backed cementless patellar components: the effect of implant design. Clin Orthop Relat Res. 2001;392:239-244.
13. Jensen LN, Lund B, Gotfredsen K. Bone growth into a revised porous-coated patellar implant. Acta Orthop Scand. 1990;61(3):213-216.
14. Hsu HP, Walker PS. Wear and deformation of patellar components in total knee arthroplasty. Clin Orthop Relat Res. 1989;246:260-265.
15. Jordan LR, Sorrells RB, Jordan LC, Olivo JL. The long-term results of a metal-backed mobile bearing patella. Clin Orthop Relat Res. 2005;436:111-118.
16. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
17. Bayley JC, Scott RD, Ewald FC, Holmes GB Jr. Failure of the metal-backed patellar component after total knee replacement. J Bone Joint Surg Am. 1988;70(5):668-674.
18. Lombardi AV Jr, Engh GA, Volz RG, Albrigo JL, Brainard BJ. Fracture/dissociation of the polyethylene in metal-backed patellar components in total knee arthroplasty. J Bone Joint Surg Am. 1988;70(5):675-679.
19. Moreland JR. Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:49-64.
20. Francke EI, Lachiewicz PF. Failure of a cemented all-polyethylene patellar component of a press-fit condylar total knee arthroplasty. J Arthroplasty. 2000;15(2):234-237.
21. Stulberg BN, Wright TM, Stoller AP, Mimnaugh KL, Mason JJ. Bilateral patellar component shear failure of highly cross-linked polyethylene components: report of a case and laboratory analysis of failure mechanisms. J Arthroplasty. 2012;27(5):789-796.
1. Firestone TP, Teeny SM, Krackow KA, Hungerford DS. The clinical and roentgenographic results of cementless porous-coated patellar fixation. Clin Orthop Relat Res. 1991;273:184-189.
2. Laskin RS, Bucknell A. The use of metal-backed patellar prostheses in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:52-55.
3. Evanich CJ, Tkach TK, von Glinski S, Camargo MP, Hofmann AA. 6- to 10-year experience using countersunk metal-backed patellas. J Arthroplasty. 1997;12(2):149-154.
4. Schwartz AJ, Della Vale CJ, Rosenberg AG, Jacobs JJ, Berger RA, Galante JO. Cruciate-retaining TKA using a third-generation system with a four-pegged tibial component: a minimum 10-year followup note. Clin Orthop Relat Res. 2010;468(8):2160-2167.
5. Bisschop R, Brouwer RW, Van Raay JJ. Total knee arthroplasty in younger patients: a 13-year follow-up study. Orthopedics. 2010;33(12):876-880.
6. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. A study of patients followed for a minimum of fifteen years. J Bone Joint Surg Am. 2005;87(3):598-603.
7. Brick GW, Scott RD. The patellofemoral component of total knee arthroplasty. Clin Orthop Relat Res. 1988;231)163-178.
8. Garcia RM, Kraay MJ, Goldberg VM. Isolated all-polyethylene patellar revisions for metal-backed patellar failure. Clin Orthop Relat Res. 2008;466(11):2784-2789.
9. Rosenberg AG, Andriacchi TP, Barden R, Galante JO. Patellar component failure in cementless total knee arthroplasty. Clin Orthop Relat Res. 1988;(236):106-114.
10. Stulberg SD, Stulberg BN, Hamati Y, Tsao A. Failure mechanisms of metal-backed patellar components. Clin Orthop Relat Res. 1988;236:88-105.
11. Sundfeldt M, Johansson CB, Regner L, Albrektsson T, Carlsson LV. Long-term results of a cementless knee prosthesis with a metal-backed patellar component: clinical and radiological follow-up with histology from retrieved components. J Long Term Eff Med Implants. 2003;13(4):341-354.
12. Kraay MJ, Darr OJ, Salata MJ, Goldberg VM. Outcome of metal-backed cementless patellar components: the effect of implant design. Clin Orthop Relat Res. 2001;392:239-244.
13. Jensen LN, Lund B, Gotfredsen K. Bone growth into a revised porous-coated patellar implant. Acta Orthop Scand. 1990;61(3):213-216.
14. Hsu HP, Walker PS. Wear and deformation of patellar components in total knee arthroplasty. Clin Orthop Relat Res. 1989;246:260-265.
15. Jordan LR, Sorrells RB, Jordan LC, Olivo JL. The long-term results of a metal-backed mobile bearing patella. Clin Orthop Relat Res. 2005;436:111-118.
16. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
17. Bayley JC, Scott RD, Ewald FC, Holmes GB Jr. Failure of the metal-backed patellar component after total knee replacement. J Bone Joint Surg Am. 1988;70(5):668-674.
18. Lombardi AV Jr, Engh GA, Volz RG, Albrigo JL, Brainard BJ. Fracture/dissociation of the polyethylene in metal-backed patellar components in total knee arthroplasty. J Bone Joint Surg Am. 1988;70(5):675-679.
19. Moreland JR. Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:49-64.
20. Francke EI, Lachiewicz PF. Failure of a cemented all-polyethylene patellar component of a press-fit condylar total knee arthroplasty. J Arthroplasty. 2000;15(2):234-237.
21. Stulberg BN, Wright TM, Stoller AP, Mimnaugh KL, Mason JJ. Bilateral patellar component shear failure of highly cross-linked polyethylene components: report of a case and laboratory analysis of failure mechanisms. J Arthroplasty. 2012;27(5):789-796.
The view from my office: How psychiatry residency programs have changed
As I approach my twentieth year as Residency Program Coordinator in the Department of Psychiatry at Saint Louis University School of Medicine, I’ve been reflecting on the many changes that have occurred: within our residency program; in the requirements that all residency programs must meet to continue as an Accreditation Council for Graduate Medical Education (ACGME)-accredited program; and in the overall scope of psychiatry residency training.
What has changed
During my time as Residency Program Coordinator, I have assisted 5 program directors and 3 associate program directors with day-to-day details of residency training. Our residency program has had couples, and a father and son; some residents even married each other while still in training.
The Electronic Residency Application System was not available until 2001; before that, applicants interested in being invited for an interview with a psychiatry residency program had to mail in their applications for review. This was a time-consuming, tedious process. In addition, residency programs today are required to use the American Board of Psychiatry and Neurology (ABPN) PreCERT credentialing program to verify training—instead of (as in the past) simply submitting a letter to ABPN that detailed the rotations and clinical skills examinations completed.
Residency programs have gone from evaluating residents by using the 6 competencies to the Milestones requirement from ACGME, which is the newest system of measuring residents’ competencies. Every month, the program faculty meets to discuss the progress of 1 of the classes of residents and the residents who are completing an individual self-assessment. Milestone scores for each resident are then reported to ACGME.
At one time, a resident’s files could be stored in a 2-inch binder; now, we need a 4-inch binder to accommodate required documentation! I am relieved—as, I am sure, many other residency program coordinators are—that residency programs are no longer required to prepare a Program Information Form but, instead, perform a self-study and, every 10 years, have a site visit. Last, every academic year, the Residency Program Coordinator is required to enter the incoming residents’ information into the graduate medical education track, ACGME, and PreCERT Web site systems.
Rewards of my position
As Residency Program Coordinator, I’ve had the rewarding experience of meeting physicians from all over the world without having to travel to other countries. Because I have a 3- or 4-year relationship with residents, I serve them in various roles: mentor, mother, confidante, motivator, and friend. As much as the job is rewarding, being the Residency Program Coordinator can, on some days, be overwhelming, particularly because I need to think “out of the box” to streamline decisions and thus avoid conflicts with program rotations and didactic schedules.
As I approach my twentieth year as Residency Program Coordinator in the Department of Psychiatry at Saint Louis University School of Medicine, I’ve been reflecting on the many changes that have occurred: within our residency program; in the requirements that all residency programs must meet to continue as an Accreditation Council for Graduate Medical Education (ACGME)-accredited program; and in the overall scope of psychiatry residency training.
What has changed
During my time as Residency Program Coordinator, I have assisted 5 program directors and 3 associate program directors with day-to-day details of residency training. Our residency program has had couples, and a father and son; some residents even married each other while still in training.
The Electronic Residency Application System was not available until 2001; before that, applicants interested in being invited for an interview with a psychiatry residency program had to mail in their applications for review. This was a time-consuming, tedious process. In addition, residency programs today are required to use the American Board of Psychiatry and Neurology (ABPN) PreCERT credentialing program to verify training—instead of (as in the past) simply submitting a letter to ABPN that detailed the rotations and clinical skills examinations completed.
Residency programs have gone from evaluating residents by using the 6 competencies to the Milestones requirement from ACGME, which is the newest system of measuring residents’ competencies. Every month, the program faculty meets to discuss the progress of 1 of the classes of residents and the residents who are completing an individual self-assessment. Milestone scores for each resident are then reported to ACGME.
At one time, a resident’s files could be stored in a 2-inch binder; now, we need a 4-inch binder to accommodate required documentation! I am relieved—as, I am sure, many other residency program coordinators are—that residency programs are no longer required to prepare a Program Information Form but, instead, perform a self-study and, every 10 years, have a site visit. Last, every academic year, the Residency Program Coordinator is required to enter the incoming residents’ information into the graduate medical education track, ACGME, and PreCERT Web site systems.
Rewards of my position
As Residency Program Coordinator, I’ve had the rewarding experience of meeting physicians from all over the world without having to travel to other countries. Because I have a 3- or 4-year relationship with residents, I serve them in various roles: mentor, mother, confidante, motivator, and friend. As much as the job is rewarding, being the Residency Program Coordinator can, on some days, be overwhelming, particularly because I need to think “out of the box” to streamline decisions and thus avoid conflicts with program rotations and didactic schedules.
As I approach my twentieth year as Residency Program Coordinator in the Department of Psychiatry at Saint Louis University School of Medicine, I’ve been reflecting on the many changes that have occurred: within our residency program; in the requirements that all residency programs must meet to continue as an Accreditation Council for Graduate Medical Education (ACGME)-accredited program; and in the overall scope of psychiatry residency training.
What has changed
During my time as Residency Program Coordinator, I have assisted 5 program directors and 3 associate program directors with day-to-day details of residency training. Our residency program has had couples, and a father and son; some residents even married each other while still in training.
The Electronic Residency Application System was not available until 2001; before that, applicants interested in being invited for an interview with a psychiatry residency program had to mail in their applications for review. This was a time-consuming, tedious process. In addition, residency programs today are required to use the American Board of Psychiatry and Neurology (ABPN) PreCERT credentialing program to verify training—instead of (as in the past) simply submitting a letter to ABPN that detailed the rotations and clinical skills examinations completed.
Residency programs have gone from evaluating residents by using the 6 competencies to the Milestones requirement from ACGME, which is the newest system of measuring residents’ competencies. Every month, the program faculty meets to discuss the progress of 1 of the classes of residents and the residents who are completing an individual self-assessment. Milestone scores for each resident are then reported to ACGME.
At one time, a resident’s files could be stored in a 2-inch binder; now, we need a 4-inch binder to accommodate required documentation! I am relieved—as, I am sure, many other residency program coordinators are—that residency programs are no longer required to prepare a Program Information Form but, instead, perform a self-study and, every 10 years, have a site visit. Last, every academic year, the Residency Program Coordinator is required to enter the incoming residents’ information into the graduate medical education track, ACGME, and PreCERT Web site systems.
Rewards of my position
As Residency Program Coordinator, I’ve had the rewarding experience of meeting physicians from all over the world without having to travel to other countries. Because I have a 3- or 4-year relationship with residents, I serve them in various roles: mentor, mother, confidante, motivator, and friend. As much as the job is rewarding, being the Residency Program Coordinator can, on some days, be overwhelming, particularly because I need to think “out of the box” to streamline decisions and thus avoid conflicts with program rotations and didactic schedules.