User login
Understanding stillbirth
When a couple learns that “they are pregnant,” it is often one of the most joyous moments in their lives. However, despite the modern prenatal care available to women in the United States, pregnancy loss remains a real concern. Miscarriage is estimated to occur in 15%-20% of pregnancies; recurrent pregnancy loss in about 1%-2% of pregnancies; and stillbirth in as many as 1% of pregnancies. The causes of pregnancy loss can range from those we can diagnose, such as genetic factors, anatomic complications, and thrombophilia, to those that elude us completely.
In December 2015, investigators from Karolinska Institutet in Stockholm published a study indicating that women who gained weight between their first and second pregnancies, but who were a healthy weight prior to their first pregnancy, had an increased risk of experiencing a stillbirth (30%-50%), or having an infant who died within the first year (27%-60%) (Lancet 2015. doi: 10.1016/S0140-6736(15)00990-3). We have devoted a number of Master Class columns to the link between obesity and pregnancy complications, and this study further reinforces the influence of a healthy weight on pregnancy outcomes.
In addition to lifestyle modifications, evidence has suggested that low-molecular-weight heparin, aspirin, or vitamin supplements, in combination with appropriate surveillance and management, may reduce risk of pregnancy loss. However, more work is needed to fully understand why fetal death occurs if we are to better equip ourselves, and our patients, with all the information necessary to prevent loss from happening.
For this reason, we have invited Dr. Uma M. Reddy of the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health, to address one of the most devastating types of pregnancy losses: stillbirth. As a program scientist for large research studies, such as the Stillbirth Collaborative Research Network, Dr. Reddy’s unique perspective will add greatly to our understanding of pregnancy loss.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column.
When a couple learns that “they are pregnant,” it is often one of the most joyous moments in their lives. However, despite the modern prenatal care available to women in the United States, pregnancy loss remains a real concern. Miscarriage is estimated to occur in 15%-20% of pregnancies; recurrent pregnancy loss in about 1%-2% of pregnancies; and stillbirth in as many as 1% of pregnancies. The causes of pregnancy loss can range from those we can diagnose, such as genetic factors, anatomic complications, and thrombophilia, to those that elude us completely.
In December 2015, investigators from Karolinska Institutet in Stockholm published a study indicating that women who gained weight between their first and second pregnancies, but who were a healthy weight prior to their first pregnancy, had an increased risk of experiencing a stillbirth (30%-50%), or having an infant who died within the first year (27%-60%) (Lancet 2015. doi: 10.1016/S0140-6736(15)00990-3). We have devoted a number of Master Class columns to the link between obesity and pregnancy complications, and this study further reinforces the influence of a healthy weight on pregnancy outcomes.
In addition to lifestyle modifications, evidence has suggested that low-molecular-weight heparin, aspirin, or vitamin supplements, in combination with appropriate surveillance and management, may reduce risk of pregnancy loss. However, more work is needed to fully understand why fetal death occurs if we are to better equip ourselves, and our patients, with all the information necessary to prevent loss from happening.
For this reason, we have invited Dr. Uma M. Reddy of the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health, to address one of the most devastating types of pregnancy losses: stillbirth. As a program scientist for large research studies, such as the Stillbirth Collaborative Research Network, Dr. Reddy’s unique perspective will add greatly to our understanding of pregnancy loss.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column.
When a couple learns that “they are pregnant,” it is often one of the most joyous moments in their lives. However, despite the modern prenatal care available to women in the United States, pregnancy loss remains a real concern. Miscarriage is estimated to occur in 15%-20% of pregnancies; recurrent pregnancy loss in about 1%-2% of pregnancies; and stillbirth in as many as 1% of pregnancies. The causes of pregnancy loss can range from those we can diagnose, such as genetic factors, anatomic complications, and thrombophilia, to those that elude us completely.
In December 2015, investigators from Karolinska Institutet in Stockholm published a study indicating that women who gained weight between their first and second pregnancies, but who were a healthy weight prior to their first pregnancy, had an increased risk of experiencing a stillbirth (30%-50%), or having an infant who died within the first year (27%-60%) (Lancet 2015. doi: 10.1016/S0140-6736(15)00990-3). We have devoted a number of Master Class columns to the link between obesity and pregnancy complications, and this study further reinforces the influence of a healthy weight on pregnancy outcomes.
In addition to lifestyle modifications, evidence has suggested that low-molecular-weight heparin, aspirin, or vitamin supplements, in combination with appropriate surveillance and management, may reduce risk of pregnancy loss. However, more work is needed to fully understand why fetal death occurs if we are to better equip ourselves, and our patients, with all the information necessary to prevent loss from happening.
For this reason, we have invited Dr. Uma M. Reddy of the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health, to address one of the most devastating types of pregnancy losses: stillbirth. As a program scientist for large research studies, such as the Stillbirth Collaborative Research Network, Dr. Reddy’s unique perspective will add greatly to our understanding of pregnancy loss.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece reported having no relevant financial disclosures. He is the medical editor of this column.
Research adds insight on stillbirth risk factors
Stillbirth is a major public health problem, occurring in approximately 1 of every 160 pregnancies in the United States. The rate has remained stagnant since 2006. Prior to that time, from 1990 to 2006, the rate declined somewhat, but only half as much as the decline in infant mortality during this time period. Racial disparities also have persisted, with non-Hispanic black women having more than a twofold increase in risk (Natl Vital Stat Rep. 2012;60:1-22).
Research conducted by the Stillbirth Collaborative Research Network (SCRN) and others has provided us with insight on risk factors and on probable and possible causes of death among stillbirths, which are defined as fetal deaths at 20 or more weeks’ gestation. We know from SCRN data, for instance, that black women are more likely to have stillbirths associated with obstetric complications and infections than white and Hispanic women. However, we still cannot explain a substantial proportion of stillbirths, despite a complete evaluation, or predict who will have a stillbirth.
What we can do as obstetricians is be aware that stillbirth is one of the most common adverse pregnancy outcomes in the United States and counsel women regarding risk factors that are modifiable. Moreover, when stillbirth happens, a complete postmortem evaluation that includes autopsy, placental pathology, karyotype or microarray analysis, and fetal-maternal hemorrhage testing is recommended (Obstet Gynecol. 2009;113[3]:748-61). Recent data show that each of these four components is valuable and should be considered the basic work-up for stillbirth.
Risks and causes
Pregnancy history was the strongest baseline risk factor for stillbirth in an analysis of 614 stillbirths and 1,816 live births in the SCRN’s population-based, case-control study conducted between 2006 and 2008. The SCRN was initiated by the Eunice Kennedy Shriver National Institute of Child Health and Human Development in 2003. This critical population-based study was conducted at 59 U.S. tertiary care and community hospitals in five catchment areas and has been analyzed in more than 15 published reports.
Women with a previous stillbirth have been known to be at 5- to 10-fold increased risk of a recurrence of stillbirth, and the SCRN findings confirmed this. The study added to our knowledge, however, with the finding that even a prior pregnancy loss at less than 20 weeks’ gestation increased the risk for stillbirth.
Other risk factors identified in the study, in addition to race, included having a multifetal pregnancy (adjusted odds ratio of 4.59), diabetes (AOR of 2.50), maternal age of 40 years or older (AOR of 2.41), maternal AB blood type (AOR of 1.96, compared with type O), a history of drug addiction (AOR of 2.08), smoking during the 3 months prior to pregnancy (AOR of 1.55-1.57, depending on amount), and being unmarried and not cohabitating (AOR of 1.69). Regarding racial disparity, the study showed that elevated risk of stillbirth for non-Hispanic blacks occurred predominantly prior to 24 weeks of gestation.
As in prior research, overweight and obesity also conferred elevated risks in the SCRN study (AORs of 1.43 and 1.72, respectively), and these risks were not explained by either diabetes or hypertension (JAMA. 2011;306:2469-79).
The use of assisted reproductive technology was not included in the study’s multivariate model, but previous research has shown a fourfold increased risk of stillbirth for singleton IVF/ICSI pregnancies. The reason is unclear, but the risk appears to be more related to IVF/ICSI rather than the underlying infertility (Hum Reprod. 2010 May;25[5]:1312-6).
A previous preterm or small-for-gestational-age birth has also been shown in prior research to be a significant risk factor for stillbirth. Less clear is the role of previous cesarean delivery in stillbirth risk. An association has been demonstrated in several studies, however, including one involving about 180,000 singleton pregnancies of 23 or more weeks’ gestation. Women in this cohort who had a previous cesarean delivery had a 1.3-fold increased risk of antepartum stillbirth, after controlling for important factors such as race, body mass index (BMI), and maternal disease (Obstet Gynecol. 2010 Nov;116[5]:1119-26).
In another analysis of the SCRN study looking specifically at causes of stillbirth, a “probable” cause of death was found in 61% of cases and a “possible or probable” cause of death in more than 76% of cases. The most common causes were obstetric complications (29.3%), placental abnormalities (23.6%), fetal genetic/structural abnormalities (13.7%), infection (12.9%), umbilical cord abnormalities (10.4%), hypertensive disorders (9.2%), and other maternal medical conditions (7.8%).
A higher proportion of stillbirths in non-Hispanic black women, compared with non-Hispanic white women and Hispanic women was associated with obstetric complications (43.5%) and infections (25.2%). This finding combined with the finding that stillbirth in black women often occurs at less than 24 weeks’ gestation suggests that measures aimed at reducing the rate of spontaneous preterm birth in black women could potentially reduce the rate of stillbirth as well (JAMA. 2011 Dec 14;306[22]:2459-68).
Work-up and prevention
Prevention of stillbirth requires that we identify the women at highest risk, and thus far this ability still eludes us. Apart from occurrence of previous stillbirth or pregnancy loss, other risk factors have had limited predictive value in the SCRN analyses and other research.
Biomarkers such as a low PAPP-A during the first trimester and a high AFP in the second trimester – as well as Doppler imaging of the uterine artery – have also been associated with stillbirth, but again, the positive predictive value has been shown to be low (Clin Obstet Gynecol. 2010 Sep;53[3]:597-606). More research is needed to determine if some combination of biochemical markers, imaging, and other risk factors can predict which women are at highest risk.
In the meantime, attention can be paid – in the preconception period if possible – to modifiable risk factors such as maternal obesity, diabetes, and smoking. About 10% of stillbirths are associated with maternal conditions such as hypertension and diabetes, and late stillbirths in particular (28 weeks or later) are associated with maternal medical conditions that are potentially preventable.
Normalization of prepregnancy weight should be a goal, since the overall risk of stillbirth appears to increase independently with increasing BMI. Glycemic control should also be achieved: A recent meta-analysis of preconception and prenatal care of diabetic women estimated “conservatively” that 10% of diabetes-associated stillbirths could be prevented with early detection and glycemic control (BMC Public Health. 2011;11 Suppl 3:S2). Research has also shown that women who quit smoking between their first and second pregnancy reduce their stillbirth risk to that of nonsmokers in the second pregnancy (BJOG. 2007 Jun;114[6]:699-704).
When stillbirth happens, a thorough work-up is recommended in order to counsel for future pregnancies and decrease the risk of recurrence. Evaluations for causes of stillbirth are too often incomplete in the United States for various reasons, including emotional, cultural, and resource factors. Even if a cause is not found, many families appreciate knowing that every effort has been made to determine a cause of death.
Four components of evaluation – autopsy, placental examination, karyotype or microarray analysis, and fetal-maternal hemorrhage testing – have proven to be high-yield tests when performed in all cases of stillbirth.
In the SCRN study, of 512 stillbirths undergoing a complete evaluation, 66.4% had a positive result – defined as abnormalities contributing to a probable or possible cause – for at least one of the first three tests (JAMA. 2011 Dec 14;306[22]:2459-68).
A Dutch study of 1,025 stillbirths similarly demonstrated that all four tests are justified. A test was defined as valuable in this study if it established or excluded a cause of stillbirth. Placental examination was determined to be the most valuable test, helping to determine a cause of death in 95.7% of cases. Autopsy was valuable 72.6% of the time, and cytogenetic analysis was valuable in 29% of cases.
Kleihauer-Betke testing for fetal-maternal hemorrhage was positive in 11.9% of women. However, fetal maternal hemorrhage was considered the cause of death in only 1.3%.of cases because, beyond a positive Kleihauer-Betke test, evidence of fetal anemia confirmed by placental examination and/or autopsy was required for hemorrhage to be considered the cause of death (Am. J. Obstet. Gynecol. 2012;206:53.e1-12). Because Kleihauer-Betke testing is ideally performed before induction, authors of both the SCRN study and the Dutch study believe it is a valuable test to be offered in all cases.
In both studies, the yield of other stillbirth diagnostic tests (for example, maternal serology, hormone assessment, and toxicology screen) was low, indicating that these tests are considered sequential and can be performed only when the clinical history or findings of the four core tests raise suspicion of particular potential causes. Antinuclear antibody testing and TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes simplex) titers have an extremely low yield and are generally not useful.
For detecting genetic abnormalities after stillbirth, it appears that microarray analysis is superior to karyotype analysis. In a SCRN analysis of samples from 532 stillbirths, microarray yielded results more often and identified more genetic abnormalities. Unlike karyotype, it does not require live cells, which makes it preferable for stillbirth evaluation (N Engl J Med. 2012 Dec 6;367[23]:2185-93).
Current research
One of the more significant studies underway on prevention is looking at labor induction as an intervention for reducing stillbirths and improving other perinatal outcomes. The ARRIVE trial (“A Randomized Trial of Induction Versus Expectant Management”), currently in the recruitment stage, will examine outcomes after induction at 39 weeks’ gestation, compared with expectant management in 6,000 patients (clinicaltrials.gov/ct2/show/NCT01990612).
Common wisdom informed by retrospective cohort studies has long told us that inducing labor prior to 41 weeks’ gestation is associated with a higher risk of cesarean delivery in nulliparous women. However, recent observational data have suggested that women whose labor is induced actually have fewer cesarean deliveries and better perinatal outcomes, including a lower risk of stillbirth (AJOG 2012;207:502.e1-8).
In addition, a meta-analysis published in 2014, as the ARRIVE trial was taking shape, reported a 12% reduction in cesarean delivery, and a reduced risk of stillbirth, among women whose labor was induced. The initial cervical score did not impact the main findings (CMAJ. 2014 Jun 10;186[9]:665-73). If these findings are confirmed in the ARRIVE trial, we could see a new opportunity for stillbirth prevention.
Another ongoing study of 10,000 singleton pregnancies – the Nulliparous Pregnancy Outcomes: Monitoring Mothers-to-Be (nuMoM2b) study – may also lead to prevention strategies in women for whom the current pregnancy will lead to their first delivery. Among the questions being examined in this eight-site study are whether sleep-disordered breathing, or apnea, and a supine sleep position are risk factors for adverse pregnancy outcomes including stillbirth.
Supine sleeping in the last month of pregnancy was strongly associated with stillbirth in a recent analysis from the Sydney Stillbirth Study (Obstet Gynecol. 2015 Feb;125[2]:347-55), and an early analysis of a nuMoM2b subset has shown associations between sleep-disordered breathing in midpregnancy and the development of hypertensive disorders of pregnancy, and between sleep-disordered breathing in early- and mid-pregnancy and gestational diabetes (Am J Obstet Gynecol. 2015;212:S424-425).
The possible role of low-dose aspirin in preventing stillbirth also needs more exploration. A recent randomized trial of women attempting to become pregnant after having had one or two prior pregnancy losses found no difference overall in live birth rates between those who took low-dose aspirin and those assigned to placebo. However, there was one subgroup – women with a single loss at less than 20 weeks’ gestation during the previous year – in which live birth rates were higher in the aspirin group (Lancet. 2014 Jul 5;384[9937]:29-36). More research is necessary to determine if low-dose aspirin administration in women with a previous stillbirth improves pregnancy outcome.
Dr. Reddy is a member at the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. She is a board-certified ob.gyn. and maternal-fetal medicine specialist. She is the program scientist for the Maternal-Fetal Medicine Units Network and for the Stillbirth Collaborative Research Network. The comments and views of the author do not necessarily represent the views of the NICHD.
Stillbirth is a major public health problem, occurring in approximately 1 of every 160 pregnancies in the United States. The rate has remained stagnant since 2006. Prior to that time, from 1990 to 2006, the rate declined somewhat, but only half as much as the decline in infant mortality during this time period. Racial disparities also have persisted, with non-Hispanic black women having more than a twofold increase in risk (Natl Vital Stat Rep. 2012;60:1-22).
Research conducted by the Stillbirth Collaborative Research Network (SCRN) and others has provided us with insight on risk factors and on probable and possible causes of death among stillbirths, which are defined as fetal deaths at 20 or more weeks’ gestation. We know from SCRN data, for instance, that black women are more likely to have stillbirths associated with obstetric complications and infections than white and Hispanic women. However, we still cannot explain a substantial proportion of stillbirths, despite a complete evaluation, or predict who will have a stillbirth.
What we can do as obstetricians is be aware that stillbirth is one of the most common adverse pregnancy outcomes in the United States and counsel women regarding risk factors that are modifiable. Moreover, when stillbirth happens, a complete postmortem evaluation that includes autopsy, placental pathology, karyotype or microarray analysis, and fetal-maternal hemorrhage testing is recommended (Obstet Gynecol. 2009;113[3]:748-61). Recent data show that each of these four components is valuable and should be considered the basic work-up for stillbirth.
Risks and causes
Pregnancy history was the strongest baseline risk factor for stillbirth in an analysis of 614 stillbirths and 1,816 live births in the SCRN’s population-based, case-control study conducted between 2006 and 2008. The SCRN was initiated by the Eunice Kennedy Shriver National Institute of Child Health and Human Development in 2003. This critical population-based study was conducted at 59 U.S. tertiary care and community hospitals in five catchment areas and has been analyzed in more than 15 published reports.
Women with a previous stillbirth have been known to be at 5- to 10-fold increased risk of a recurrence of stillbirth, and the SCRN findings confirmed this. The study added to our knowledge, however, with the finding that even a prior pregnancy loss at less than 20 weeks’ gestation increased the risk for stillbirth.
Other risk factors identified in the study, in addition to race, included having a multifetal pregnancy (adjusted odds ratio of 4.59), diabetes (AOR of 2.50), maternal age of 40 years or older (AOR of 2.41), maternal AB blood type (AOR of 1.96, compared with type O), a history of drug addiction (AOR of 2.08), smoking during the 3 months prior to pregnancy (AOR of 1.55-1.57, depending on amount), and being unmarried and not cohabitating (AOR of 1.69). Regarding racial disparity, the study showed that elevated risk of stillbirth for non-Hispanic blacks occurred predominantly prior to 24 weeks of gestation.
As in prior research, overweight and obesity also conferred elevated risks in the SCRN study (AORs of 1.43 and 1.72, respectively), and these risks were not explained by either diabetes or hypertension (JAMA. 2011;306:2469-79).
The use of assisted reproductive technology was not included in the study’s multivariate model, but previous research has shown a fourfold increased risk of stillbirth for singleton IVF/ICSI pregnancies. The reason is unclear, but the risk appears to be more related to IVF/ICSI rather than the underlying infertility (Hum Reprod. 2010 May;25[5]:1312-6).
A previous preterm or small-for-gestational-age birth has also been shown in prior research to be a significant risk factor for stillbirth. Less clear is the role of previous cesarean delivery in stillbirth risk. An association has been demonstrated in several studies, however, including one involving about 180,000 singleton pregnancies of 23 or more weeks’ gestation. Women in this cohort who had a previous cesarean delivery had a 1.3-fold increased risk of antepartum stillbirth, after controlling for important factors such as race, body mass index (BMI), and maternal disease (Obstet Gynecol. 2010 Nov;116[5]:1119-26).
In another analysis of the SCRN study looking specifically at causes of stillbirth, a “probable” cause of death was found in 61% of cases and a “possible or probable” cause of death in more than 76% of cases. The most common causes were obstetric complications (29.3%), placental abnormalities (23.6%), fetal genetic/structural abnormalities (13.7%), infection (12.9%), umbilical cord abnormalities (10.4%), hypertensive disorders (9.2%), and other maternal medical conditions (7.8%).
A higher proportion of stillbirths in non-Hispanic black women, compared with non-Hispanic white women and Hispanic women was associated with obstetric complications (43.5%) and infections (25.2%). This finding combined with the finding that stillbirth in black women often occurs at less than 24 weeks’ gestation suggests that measures aimed at reducing the rate of spontaneous preterm birth in black women could potentially reduce the rate of stillbirth as well (JAMA. 2011 Dec 14;306[22]:2459-68).
Work-up and prevention
Prevention of stillbirth requires that we identify the women at highest risk, and thus far this ability still eludes us. Apart from occurrence of previous stillbirth or pregnancy loss, other risk factors have had limited predictive value in the SCRN analyses and other research.
Biomarkers such as a low PAPP-A during the first trimester and a high AFP in the second trimester – as well as Doppler imaging of the uterine artery – have also been associated with stillbirth, but again, the positive predictive value has been shown to be low (Clin Obstet Gynecol. 2010 Sep;53[3]:597-606). More research is needed to determine if some combination of biochemical markers, imaging, and other risk factors can predict which women are at highest risk.
In the meantime, attention can be paid – in the preconception period if possible – to modifiable risk factors such as maternal obesity, diabetes, and smoking. About 10% of stillbirths are associated with maternal conditions such as hypertension and diabetes, and late stillbirths in particular (28 weeks or later) are associated with maternal medical conditions that are potentially preventable.
Normalization of prepregnancy weight should be a goal, since the overall risk of stillbirth appears to increase independently with increasing BMI. Glycemic control should also be achieved: A recent meta-analysis of preconception and prenatal care of diabetic women estimated “conservatively” that 10% of diabetes-associated stillbirths could be prevented with early detection and glycemic control (BMC Public Health. 2011;11 Suppl 3:S2). Research has also shown that women who quit smoking between their first and second pregnancy reduce their stillbirth risk to that of nonsmokers in the second pregnancy (BJOG. 2007 Jun;114[6]:699-704).
When stillbirth happens, a thorough work-up is recommended in order to counsel for future pregnancies and decrease the risk of recurrence. Evaluations for causes of stillbirth are too often incomplete in the United States for various reasons, including emotional, cultural, and resource factors. Even if a cause is not found, many families appreciate knowing that every effort has been made to determine a cause of death.
Four components of evaluation – autopsy, placental examination, karyotype or microarray analysis, and fetal-maternal hemorrhage testing – have proven to be high-yield tests when performed in all cases of stillbirth.
In the SCRN study, of 512 stillbirths undergoing a complete evaluation, 66.4% had a positive result – defined as abnormalities contributing to a probable or possible cause – for at least one of the first three tests (JAMA. 2011 Dec 14;306[22]:2459-68).
A Dutch study of 1,025 stillbirths similarly demonstrated that all four tests are justified. A test was defined as valuable in this study if it established or excluded a cause of stillbirth. Placental examination was determined to be the most valuable test, helping to determine a cause of death in 95.7% of cases. Autopsy was valuable 72.6% of the time, and cytogenetic analysis was valuable in 29% of cases.
Kleihauer-Betke testing for fetal-maternal hemorrhage was positive in 11.9% of women. However, fetal maternal hemorrhage was considered the cause of death in only 1.3%.of cases because, beyond a positive Kleihauer-Betke test, evidence of fetal anemia confirmed by placental examination and/or autopsy was required for hemorrhage to be considered the cause of death (Am. J. Obstet. Gynecol. 2012;206:53.e1-12). Because Kleihauer-Betke testing is ideally performed before induction, authors of both the SCRN study and the Dutch study believe it is a valuable test to be offered in all cases.
In both studies, the yield of other stillbirth diagnostic tests (for example, maternal serology, hormone assessment, and toxicology screen) was low, indicating that these tests are considered sequential and can be performed only when the clinical history or findings of the four core tests raise suspicion of particular potential causes. Antinuclear antibody testing and TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes simplex) titers have an extremely low yield and are generally not useful.
For detecting genetic abnormalities after stillbirth, it appears that microarray analysis is superior to karyotype analysis. In a SCRN analysis of samples from 532 stillbirths, microarray yielded results more often and identified more genetic abnormalities. Unlike karyotype, it does not require live cells, which makes it preferable for stillbirth evaluation (N Engl J Med. 2012 Dec 6;367[23]:2185-93).
Current research
One of the more significant studies underway on prevention is looking at labor induction as an intervention for reducing stillbirths and improving other perinatal outcomes. The ARRIVE trial (“A Randomized Trial of Induction Versus Expectant Management”), currently in the recruitment stage, will examine outcomes after induction at 39 weeks’ gestation, compared with expectant management in 6,000 patients (clinicaltrials.gov/ct2/show/NCT01990612).
Common wisdom informed by retrospective cohort studies has long told us that inducing labor prior to 41 weeks’ gestation is associated with a higher risk of cesarean delivery in nulliparous women. However, recent observational data have suggested that women whose labor is induced actually have fewer cesarean deliveries and better perinatal outcomes, including a lower risk of stillbirth (AJOG 2012;207:502.e1-8).
In addition, a meta-analysis published in 2014, as the ARRIVE trial was taking shape, reported a 12% reduction in cesarean delivery, and a reduced risk of stillbirth, among women whose labor was induced. The initial cervical score did not impact the main findings (CMAJ. 2014 Jun 10;186[9]:665-73). If these findings are confirmed in the ARRIVE trial, we could see a new opportunity for stillbirth prevention.
Another ongoing study of 10,000 singleton pregnancies – the Nulliparous Pregnancy Outcomes: Monitoring Mothers-to-Be (nuMoM2b) study – may also lead to prevention strategies in women for whom the current pregnancy will lead to their first delivery. Among the questions being examined in this eight-site study are whether sleep-disordered breathing, or apnea, and a supine sleep position are risk factors for adverse pregnancy outcomes including stillbirth.
Supine sleeping in the last month of pregnancy was strongly associated with stillbirth in a recent analysis from the Sydney Stillbirth Study (Obstet Gynecol. 2015 Feb;125[2]:347-55), and an early analysis of a nuMoM2b subset has shown associations between sleep-disordered breathing in midpregnancy and the development of hypertensive disorders of pregnancy, and between sleep-disordered breathing in early- and mid-pregnancy and gestational diabetes (Am J Obstet Gynecol. 2015;212:S424-425).
The possible role of low-dose aspirin in preventing stillbirth also needs more exploration. A recent randomized trial of women attempting to become pregnant after having had one or two prior pregnancy losses found no difference overall in live birth rates between those who took low-dose aspirin and those assigned to placebo. However, there was one subgroup – women with a single loss at less than 20 weeks’ gestation during the previous year – in which live birth rates were higher in the aspirin group (Lancet. 2014 Jul 5;384[9937]:29-36). More research is necessary to determine if low-dose aspirin administration in women with a previous stillbirth improves pregnancy outcome.
Dr. Reddy is a member at the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. She is a board-certified ob.gyn. and maternal-fetal medicine specialist. She is the program scientist for the Maternal-Fetal Medicine Units Network and for the Stillbirth Collaborative Research Network. The comments and views of the author do not necessarily represent the views of the NICHD.
Stillbirth is a major public health problem, occurring in approximately 1 of every 160 pregnancies in the United States. The rate has remained stagnant since 2006. Prior to that time, from 1990 to 2006, the rate declined somewhat, but only half as much as the decline in infant mortality during this time period. Racial disparities also have persisted, with non-Hispanic black women having more than a twofold increase in risk (Natl Vital Stat Rep. 2012;60:1-22).
Research conducted by the Stillbirth Collaborative Research Network (SCRN) and others has provided us with insight on risk factors and on probable and possible causes of death among stillbirths, which are defined as fetal deaths at 20 or more weeks’ gestation. We know from SCRN data, for instance, that black women are more likely to have stillbirths associated with obstetric complications and infections than white and Hispanic women. However, we still cannot explain a substantial proportion of stillbirths, despite a complete evaluation, or predict who will have a stillbirth.
What we can do as obstetricians is be aware that stillbirth is one of the most common adverse pregnancy outcomes in the United States and counsel women regarding risk factors that are modifiable. Moreover, when stillbirth happens, a complete postmortem evaluation that includes autopsy, placental pathology, karyotype or microarray analysis, and fetal-maternal hemorrhage testing is recommended (Obstet Gynecol. 2009;113[3]:748-61). Recent data show that each of these four components is valuable and should be considered the basic work-up for stillbirth.
Risks and causes
Pregnancy history was the strongest baseline risk factor for stillbirth in an analysis of 614 stillbirths and 1,816 live births in the SCRN’s population-based, case-control study conducted between 2006 and 2008. The SCRN was initiated by the Eunice Kennedy Shriver National Institute of Child Health and Human Development in 2003. This critical population-based study was conducted at 59 U.S. tertiary care and community hospitals in five catchment areas and has been analyzed in more than 15 published reports.
Women with a previous stillbirth have been known to be at 5- to 10-fold increased risk of a recurrence of stillbirth, and the SCRN findings confirmed this. The study added to our knowledge, however, with the finding that even a prior pregnancy loss at less than 20 weeks’ gestation increased the risk for stillbirth.
Other risk factors identified in the study, in addition to race, included having a multifetal pregnancy (adjusted odds ratio of 4.59), diabetes (AOR of 2.50), maternal age of 40 years or older (AOR of 2.41), maternal AB blood type (AOR of 1.96, compared with type O), a history of drug addiction (AOR of 2.08), smoking during the 3 months prior to pregnancy (AOR of 1.55-1.57, depending on amount), and being unmarried and not cohabitating (AOR of 1.69). Regarding racial disparity, the study showed that elevated risk of stillbirth for non-Hispanic blacks occurred predominantly prior to 24 weeks of gestation.
As in prior research, overweight and obesity also conferred elevated risks in the SCRN study (AORs of 1.43 and 1.72, respectively), and these risks were not explained by either diabetes or hypertension (JAMA. 2011;306:2469-79).
The use of assisted reproductive technology was not included in the study’s multivariate model, but previous research has shown a fourfold increased risk of stillbirth for singleton IVF/ICSI pregnancies. The reason is unclear, but the risk appears to be more related to IVF/ICSI rather than the underlying infertility (Hum Reprod. 2010 May;25[5]:1312-6).
A previous preterm or small-for-gestational-age birth has also been shown in prior research to be a significant risk factor for stillbirth. Less clear is the role of previous cesarean delivery in stillbirth risk. An association has been demonstrated in several studies, however, including one involving about 180,000 singleton pregnancies of 23 or more weeks’ gestation. Women in this cohort who had a previous cesarean delivery had a 1.3-fold increased risk of antepartum stillbirth, after controlling for important factors such as race, body mass index (BMI), and maternal disease (Obstet Gynecol. 2010 Nov;116[5]:1119-26).
In another analysis of the SCRN study looking specifically at causes of stillbirth, a “probable” cause of death was found in 61% of cases and a “possible or probable” cause of death in more than 76% of cases. The most common causes were obstetric complications (29.3%), placental abnormalities (23.6%), fetal genetic/structural abnormalities (13.7%), infection (12.9%), umbilical cord abnormalities (10.4%), hypertensive disorders (9.2%), and other maternal medical conditions (7.8%).
A higher proportion of stillbirths in non-Hispanic black women, compared with non-Hispanic white women and Hispanic women was associated with obstetric complications (43.5%) and infections (25.2%). This finding combined with the finding that stillbirth in black women often occurs at less than 24 weeks’ gestation suggests that measures aimed at reducing the rate of spontaneous preterm birth in black women could potentially reduce the rate of stillbirth as well (JAMA. 2011 Dec 14;306[22]:2459-68).
Work-up and prevention
Prevention of stillbirth requires that we identify the women at highest risk, and thus far this ability still eludes us. Apart from occurrence of previous stillbirth or pregnancy loss, other risk factors have had limited predictive value in the SCRN analyses and other research.
Biomarkers such as a low PAPP-A during the first trimester and a high AFP in the second trimester – as well as Doppler imaging of the uterine artery – have also been associated with stillbirth, but again, the positive predictive value has been shown to be low (Clin Obstet Gynecol. 2010 Sep;53[3]:597-606). More research is needed to determine if some combination of biochemical markers, imaging, and other risk factors can predict which women are at highest risk.
In the meantime, attention can be paid – in the preconception period if possible – to modifiable risk factors such as maternal obesity, diabetes, and smoking. About 10% of stillbirths are associated with maternal conditions such as hypertension and diabetes, and late stillbirths in particular (28 weeks or later) are associated with maternal medical conditions that are potentially preventable.
Normalization of prepregnancy weight should be a goal, since the overall risk of stillbirth appears to increase independently with increasing BMI. Glycemic control should also be achieved: A recent meta-analysis of preconception and prenatal care of diabetic women estimated “conservatively” that 10% of diabetes-associated stillbirths could be prevented with early detection and glycemic control (BMC Public Health. 2011;11 Suppl 3:S2). Research has also shown that women who quit smoking between their first and second pregnancy reduce their stillbirth risk to that of nonsmokers in the second pregnancy (BJOG. 2007 Jun;114[6]:699-704).
When stillbirth happens, a thorough work-up is recommended in order to counsel for future pregnancies and decrease the risk of recurrence. Evaluations for causes of stillbirth are too often incomplete in the United States for various reasons, including emotional, cultural, and resource factors. Even if a cause is not found, many families appreciate knowing that every effort has been made to determine a cause of death.
Four components of evaluation – autopsy, placental examination, karyotype or microarray analysis, and fetal-maternal hemorrhage testing – have proven to be high-yield tests when performed in all cases of stillbirth.
In the SCRN study, of 512 stillbirths undergoing a complete evaluation, 66.4% had a positive result – defined as abnormalities contributing to a probable or possible cause – for at least one of the first three tests (JAMA. 2011 Dec 14;306[22]:2459-68).
A Dutch study of 1,025 stillbirths similarly demonstrated that all four tests are justified. A test was defined as valuable in this study if it established or excluded a cause of stillbirth. Placental examination was determined to be the most valuable test, helping to determine a cause of death in 95.7% of cases. Autopsy was valuable 72.6% of the time, and cytogenetic analysis was valuable in 29% of cases.
Kleihauer-Betke testing for fetal-maternal hemorrhage was positive in 11.9% of women. However, fetal maternal hemorrhage was considered the cause of death in only 1.3%.of cases because, beyond a positive Kleihauer-Betke test, evidence of fetal anemia confirmed by placental examination and/or autopsy was required for hemorrhage to be considered the cause of death (Am. J. Obstet. Gynecol. 2012;206:53.e1-12). Because Kleihauer-Betke testing is ideally performed before induction, authors of both the SCRN study and the Dutch study believe it is a valuable test to be offered in all cases.
In both studies, the yield of other stillbirth diagnostic tests (for example, maternal serology, hormone assessment, and toxicology screen) was low, indicating that these tests are considered sequential and can be performed only when the clinical history or findings of the four core tests raise suspicion of particular potential causes. Antinuclear antibody testing and TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes simplex) titers have an extremely low yield and are generally not useful.
For detecting genetic abnormalities after stillbirth, it appears that microarray analysis is superior to karyotype analysis. In a SCRN analysis of samples from 532 stillbirths, microarray yielded results more often and identified more genetic abnormalities. Unlike karyotype, it does not require live cells, which makes it preferable for stillbirth evaluation (N Engl J Med. 2012 Dec 6;367[23]:2185-93).
Current research
One of the more significant studies underway on prevention is looking at labor induction as an intervention for reducing stillbirths and improving other perinatal outcomes. The ARRIVE trial (“A Randomized Trial of Induction Versus Expectant Management”), currently in the recruitment stage, will examine outcomes after induction at 39 weeks’ gestation, compared with expectant management in 6,000 patients (clinicaltrials.gov/ct2/show/NCT01990612).
Common wisdom informed by retrospective cohort studies has long told us that inducing labor prior to 41 weeks’ gestation is associated with a higher risk of cesarean delivery in nulliparous women. However, recent observational data have suggested that women whose labor is induced actually have fewer cesarean deliveries and better perinatal outcomes, including a lower risk of stillbirth (AJOG 2012;207:502.e1-8).
In addition, a meta-analysis published in 2014, as the ARRIVE trial was taking shape, reported a 12% reduction in cesarean delivery, and a reduced risk of stillbirth, among women whose labor was induced. The initial cervical score did not impact the main findings (CMAJ. 2014 Jun 10;186[9]:665-73). If these findings are confirmed in the ARRIVE trial, we could see a new opportunity for stillbirth prevention.
Another ongoing study of 10,000 singleton pregnancies – the Nulliparous Pregnancy Outcomes: Monitoring Mothers-to-Be (nuMoM2b) study – may also lead to prevention strategies in women for whom the current pregnancy will lead to their first delivery. Among the questions being examined in this eight-site study are whether sleep-disordered breathing, or apnea, and a supine sleep position are risk factors for adverse pregnancy outcomes including stillbirth.
Supine sleeping in the last month of pregnancy was strongly associated with stillbirth in a recent analysis from the Sydney Stillbirth Study (Obstet Gynecol. 2015 Feb;125[2]:347-55), and an early analysis of a nuMoM2b subset has shown associations between sleep-disordered breathing in midpregnancy and the development of hypertensive disorders of pregnancy, and between sleep-disordered breathing in early- and mid-pregnancy and gestational diabetes (Am J Obstet Gynecol. 2015;212:S424-425).
The possible role of low-dose aspirin in preventing stillbirth also needs more exploration. A recent randomized trial of women attempting to become pregnant after having had one or two prior pregnancy losses found no difference overall in live birth rates between those who took low-dose aspirin and those assigned to placebo. However, there was one subgroup – women with a single loss at less than 20 weeks’ gestation during the previous year – in which live birth rates were higher in the aspirin group (Lancet. 2014 Jul 5;384[9937]:29-36). More research is necessary to determine if low-dose aspirin administration in women with a previous stillbirth improves pregnancy outcome.
Dr. Reddy is a member at the Pregnancy and Perinatology Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. She is a board-certified ob.gyn. and maternal-fetal medicine specialist. She is the program scientist for the Maternal-Fetal Medicine Units Network and for the Stillbirth Collaborative Research Network. The comments and views of the author do not necessarily represent the views of the NICHD.
Gene signatures aid diagnosis of acute respiratory infection etiology
Pathogen-specific host gene expression patterns accurately discriminated most noninfectious from infectious illnesses, and bacterial from viral causes of acute respiratory infection (ARI) in an observational study conducted in acute care settings.
The findings could have important implications for combating inappropriate antibiotic use and emerging antibiotic resistance, Dr. Ephraim L. Tsalik of the department of medicine at Duke University, Durham, N.C., and his colleagues reported online Jan. 20 in Science Translational Medicine.
The investigators analyzed peripheral whole-blood gene expression from 273 subjects with community-onset viral ARI (115 subjects), bacterial ARI (70 subjects), or noninfectious illness (88 subjects) who were seen in an emergency department, and from 44 healthy control subjects. Classifiers for bacterial ARI, viral ARI, and noninfectious causes of illness were developed, and were 87% accurate overall (Sci Transl Med. 2016;8[322]:322ra11. doi/ 10.1126/scitranslmed.aad6873).
“Bacterial ARI was identified in 83% of patients and excluded in 94% without bacterial infection. Viral ARI was identified in 90% and excluded in 92% of cases. Using the noninfectious illness classifier, infection was excluded in 86% of cases,” they wrote.
The classifiers were more accurate than procalcitonin – a widely used biomarker with some specificity for bacterial infection (86% vs. 78% accuracy in 238 available samples), and three published classifiers of bacterial vs. viral infection, and were validated in five publicly available data sets, they noted.
The gene signature patterns identified in the course of this study mark an important step toward development of a rapid blood test that could be used in clinics to guide appropriate treatment for ARIs, the investigators said.
Precision treatment of viruses
More precise ways to distinguish infections could reduce unnecessary antibiotic use and lead to more precise treatment of viruses, senior author Dr. Geoffrey S. Ginsburg, director of Duke’s Center for Applied Genomics & Precision Medicine, said in a press statement.
“Right now, we can give patients [oseltamivir] Tamiflu to help them recover from an influenza infection, but for most viral infections, the treatment is fluids and rest until it resolves. In the next 5-10 years, we will likely see new antiviral medications for common bugs like respiratory syncytial virus and even rhinovirus, and guiding treatment choices will be even more important,” he added.
Senior author Dr. Christopher W. Woods, also of Duke University, further explained in an interview that the findings are particularly exciting because “there isn’t much out there that accomplishes what we’ve done. So just about any level of accuracy is an improvement.” Further, he said the test is “a tool to aid in diagnosis, used in conjunction with the patient’s symptoms, examination, and other testing. So an imperfect test is okay, because it does not stand alone.”
Next steps include putting the assay on a testing platform that can be used at the point of care, and validating the findings in all populations, including infants, the elderly, and across ethnic groups, he said.
“The work is ongoing, and we expect to have results available within the course of an outpatient visit in the near future,” Dr. Woods, also a professor of medicine and global health, added, noting that efforts also are underway to “expand the repertoire of this approach to many different types of viral and bacterial infections and also to fungal infections, and to address the challenges of critically ill patients in intensive care units.”
This study was supported by the U.S. Defense Advanced Research Projects Agency, the National Institutes of Health, the Agency for Healthcare Research and Quality, the U.S. Department of Veterans Affairs Office of Research and Development, and an in-kind contribution from bioMérieux. The authors reported having no relevant competing interests.
Pathogen-specific host gene expression patterns accurately discriminated most noninfectious from infectious illnesses, and bacterial from viral causes of acute respiratory infection (ARI) in an observational study conducted in acute care settings.
The findings could have important implications for combating inappropriate antibiotic use and emerging antibiotic resistance, Dr. Ephraim L. Tsalik of the department of medicine at Duke University, Durham, N.C., and his colleagues reported online Jan. 20 in Science Translational Medicine.
The investigators analyzed peripheral whole-blood gene expression from 273 subjects with community-onset viral ARI (115 subjects), bacterial ARI (70 subjects), or noninfectious illness (88 subjects) who were seen in an emergency department, and from 44 healthy control subjects. Classifiers for bacterial ARI, viral ARI, and noninfectious causes of illness were developed, and were 87% accurate overall (Sci Transl Med. 2016;8[322]:322ra11. doi/ 10.1126/scitranslmed.aad6873).
“Bacterial ARI was identified in 83% of patients and excluded in 94% without bacterial infection. Viral ARI was identified in 90% and excluded in 92% of cases. Using the noninfectious illness classifier, infection was excluded in 86% of cases,” they wrote.
The classifiers were more accurate than procalcitonin – a widely used biomarker with some specificity for bacterial infection (86% vs. 78% accuracy in 238 available samples), and three published classifiers of bacterial vs. viral infection, and were validated in five publicly available data sets, they noted.
The gene signature patterns identified in the course of this study mark an important step toward development of a rapid blood test that could be used in clinics to guide appropriate treatment for ARIs, the investigators said.
Precision treatment of viruses
More precise ways to distinguish infections could reduce unnecessary antibiotic use and lead to more precise treatment of viruses, senior author Dr. Geoffrey S. Ginsburg, director of Duke’s Center for Applied Genomics & Precision Medicine, said in a press statement.
“Right now, we can give patients [oseltamivir] Tamiflu to help them recover from an influenza infection, but for most viral infections, the treatment is fluids and rest until it resolves. In the next 5-10 years, we will likely see new antiviral medications for common bugs like respiratory syncytial virus and even rhinovirus, and guiding treatment choices will be even more important,” he added.
Senior author Dr. Christopher W. Woods, also of Duke University, further explained in an interview that the findings are particularly exciting because “there isn’t much out there that accomplishes what we’ve done. So just about any level of accuracy is an improvement.” Further, he said the test is “a tool to aid in diagnosis, used in conjunction with the patient’s symptoms, examination, and other testing. So an imperfect test is okay, because it does not stand alone.”
Next steps include putting the assay on a testing platform that can be used at the point of care, and validating the findings in all populations, including infants, the elderly, and across ethnic groups, he said.
“The work is ongoing, and we expect to have results available within the course of an outpatient visit in the near future,” Dr. Woods, also a professor of medicine and global health, added, noting that efforts also are underway to “expand the repertoire of this approach to many different types of viral and bacterial infections and also to fungal infections, and to address the challenges of critically ill patients in intensive care units.”
This study was supported by the U.S. Defense Advanced Research Projects Agency, the National Institutes of Health, the Agency for Healthcare Research and Quality, the U.S. Department of Veterans Affairs Office of Research and Development, and an in-kind contribution from bioMérieux. The authors reported having no relevant competing interests.
Pathogen-specific host gene expression patterns accurately discriminated most noninfectious from infectious illnesses, and bacterial from viral causes of acute respiratory infection (ARI) in an observational study conducted in acute care settings.
The findings could have important implications for combating inappropriate antibiotic use and emerging antibiotic resistance, Dr. Ephraim L. Tsalik of the department of medicine at Duke University, Durham, N.C., and his colleagues reported online Jan. 20 in Science Translational Medicine.
The investigators analyzed peripheral whole-blood gene expression from 273 subjects with community-onset viral ARI (115 subjects), bacterial ARI (70 subjects), or noninfectious illness (88 subjects) who were seen in an emergency department, and from 44 healthy control subjects. Classifiers for bacterial ARI, viral ARI, and noninfectious causes of illness were developed, and were 87% accurate overall (Sci Transl Med. 2016;8[322]:322ra11. doi/ 10.1126/scitranslmed.aad6873).
“Bacterial ARI was identified in 83% of patients and excluded in 94% without bacterial infection. Viral ARI was identified in 90% and excluded in 92% of cases. Using the noninfectious illness classifier, infection was excluded in 86% of cases,” they wrote.
The classifiers were more accurate than procalcitonin – a widely used biomarker with some specificity for bacterial infection (86% vs. 78% accuracy in 238 available samples), and three published classifiers of bacterial vs. viral infection, and were validated in five publicly available data sets, they noted.
The gene signature patterns identified in the course of this study mark an important step toward development of a rapid blood test that could be used in clinics to guide appropriate treatment for ARIs, the investigators said.
Precision treatment of viruses
More precise ways to distinguish infections could reduce unnecessary antibiotic use and lead to more precise treatment of viruses, senior author Dr. Geoffrey S. Ginsburg, director of Duke’s Center for Applied Genomics & Precision Medicine, said in a press statement.
“Right now, we can give patients [oseltamivir] Tamiflu to help them recover from an influenza infection, but for most viral infections, the treatment is fluids and rest until it resolves. In the next 5-10 years, we will likely see new antiviral medications for common bugs like respiratory syncytial virus and even rhinovirus, and guiding treatment choices will be even more important,” he added.
Senior author Dr. Christopher W. Woods, also of Duke University, further explained in an interview that the findings are particularly exciting because “there isn’t much out there that accomplishes what we’ve done. So just about any level of accuracy is an improvement.” Further, he said the test is “a tool to aid in diagnosis, used in conjunction with the patient’s symptoms, examination, and other testing. So an imperfect test is okay, because it does not stand alone.”
Next steps include putting the assay on a testing platform that can be used at the point of care, and validating the findings in all populations, including infants, the elderly, and across ethnic groups, he said.
“The work is ongoing, and we expect to have results available within the course of an outpatient visit in the near future,” Dr. Woods, also a professor of medicine and global health, added, noting that efforts also are underway to “expand the repertoire of this approach to many different types of viral and bacterial infections and also to fungal infections, and to address the challenges of critically ill patients in intensive care units.”
This study was supported by the U.S. Defense Advanced Research Projects Agency, the National Institutes of Health, the Agency for Healthcare Research and Quality, the U.S. Department of Veterans Affairs Office of Research and Development, and an in-kind contribution from bioMérieux. The authors reported having no relevant competing interests.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Pathogen-specific host gene expression patterns accurately discriminated most noninfectious from infectious illnesses, and bacterial from viral causes of acute respiratory infection (ARI) in an observational study.
Major finding: Classifiers for bacterial ARI, viral ARI, and noninfectious causes of illness were 87% accurate overall.
Data source: An observational cohort study involving 273 patients and 44 controls.
Disclosures: This study was supported by the U.S. Defense Advanced Research Projects Agency, the National Institutes of Health, the Agency for Healthcare Research and Quality, the U.S. Department of Veterans Affairs Office of Research and Development, and an in-kind contribution from bioMérieux. The authors reported having no relevant competing interests..
Everolimus is effective across diverse patients with GI neuroendocrine tumors
Everolimus improves outcomes in patients with advanced, progressive neuroendocrine tumors of gastrointestinal (GI) or unknown origin regardless of primary location and prior therapy, according to new subgroup analyses of the RADIANT-4 trial.
The phase III trial is the largest of its type in patients with nonfunctioning GI tract or lung neuroendocrine tumors. The subgroup findings for those whose tumors originated in the GI tract or an unknown site (but suspected to be GI) were presented in a presscast held in advance of the Gastrointestinal Cancers Symposium.
Compared with placebo, everolimus prolonged progression-free survival by 6-9 months, corresponding to a 46%-48% relative reduction in the risk of progression or death, reported lead study author Dr. Simron Singh of Sunnybrook’s Odette Cancer Centre in Toronto. Benefit was similar regardless of whether patients had midgut or non-midgut tumors, and whether they had previously received a somatostatin analog or not.
“In my opinion, this study in advanced, progressive neuroendocrine patients [shows] an effective, new and exciting treatment option in a disease where we’ve had very few treatments to date,” Dr. Singh said ahead of the symposium, which was sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
ASCO expert and presscast moderator Dr. Smitha Krishnamurthi of Case Western Reserve University, Cleveland, agreed, saying that everolimus could help address an unmet need in this disease.
“Patients with GI neuroendocrine tumors have had very few treatment options. Once they have progressed on somatostatin analogues, there really are no good systemic treatments,” she said. “So this finding is very important, that the mTOR inhibitor everolimus has demonstrated an improvement in risk of progression by over 40% and with very little severe toxicity. This is a welcome finding for these patients who have limited systemic treatment options.”
Patients enrolled in RADIANT-4 had lung, GI, or unknown-origin neuroendocrine tumors that had progressed on other therapies, including somatostatin analogs, surgery, or chemotherapy.
They were randomly assigned in 2:1 ratio to receive everolimus (Afinitor) or placebo, each in addition to best supportive care. Everolimus is currently approved by the Food and Drug Administration for the treatment of pancreatic neuroendocrine tumors, as well as breast and kidney cancer, and subependymal giant cell astrocytoma.
Results for the entire trial population have been previously reported and showed that everolimus prolonged progression-free survival by 7.1 months, reducing the risk of events by 52% (Lancet. 2015 Dec 15. doi.org/10.1016/S0140-6736[15]01234-9).
The new subgroup analyses were restricted to the patients with tumors originating in the GI tract (n =175) or an unknown site generally thought to be the GI tract (n = 36).
Among the group with GI tumors, median progression-free survival was 13.1 months with everolimus versus 5.4 months with placebo, Dr. Singh reported. Among the group with tumors of unknown origin, it was 13.6 and 7.5 months, respectively.
Relative to placebo, everolimus prolonged progression-free survival by 6.41 months, reducing the risk of events by 29%, in patients whose tumors originated in the midgut (duodenum, ileum, jejunum, cecum, or appendix). The relative benefit was 6.17 months, with a reduction in the risk of events of 73%, in patients whose tumors originated in non-midgut sites (stomach, colon, and rectum).
In addition, everolimus prolonged progression-free survival by 6.73 months, reducing the risk of events by 46%, in patients who had previously received somatostatin analogues, and by 9.07 months, reducing the risk by 48%, in patients who had not received these agents.
The safety profile of everolimus was consistent with that expected based on the use of this agent in other patient populations, according to Dr. Singh. The most common adverse events were stomatitis, infections, diarrhea, peripheral edema, and fatigue. No new safety signals were seen.
Dr. Singh disclosed that he receives honoraria from, has a consulting or advisory role with, and receives research funding (institutional) and travel, accommodations, and expenses from Novartis. The study received funding from Novartis Pharmaceuticals.
Everolimus improves outcomes in patients with advanced, progressive neuroendocrine tumors of gastrointestinal (GI) or unknown origin regardless of primary location and prior therapy, according to new subgroup analyses of the RADIANT-4 trial.
The phase III trial is the largest of its type in patients with nonfunctioning GI tract or lung neuroendocrine tumors. The subgroup findings for those whose tumors originated in the GI tract or an unknown site (but suspected to be GI) were presented in a presscast held in advance of the Gastrointestinal Cancers Symposium.
Compared with placebo, everolimus prolonged progression-free survival by 6-9 months, corresponding to a 46%-48% relative reduction in the risk of progression or death, reported lead study author Dr. Simron Singh of Sunnybrook’s Odette Cancer Centre in Toronto. Benefit was similar regardless of whether patients had midgut or non-midgut tumors, and whether they had previously received a somatostatin analog or not.
“In my opinion, this study in advanced, progressive neuroendocrine patients [shows] an effective, new and exciting treatment option in a disease where we’ve had very few treatments to date,” Dr. Singh said ahead of the symposium, which was sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
ASCO expert and presscast moderator Dr. Smitha Krishnamurthi of Case Western Reserve University, Cleveland, agreed, saying that everolimus could help address an unmet need in this disease.
“Patients with GI neuroendocrine tumors have had very few treatment options. Once they have progressed on somatostatin analogues, there really are no good systemic treatments,” she said. “So this finding is very important, that the mTOR inhibitor everolimus has demonstrated an improvement in risk of progression by over 40% and with very little severe toxicity. This is a welcome finding for these patients who have limited systemic treatment options.”
Patients enrolled in RADIANT-4 had lung, GI, or unknown-origin neuroendocrine tumors that had progressed on other therapies, including somatostatin analogs, surgery, or chemotherapy.
They were randomly assigned in 2:1 ratio to receive everolimus (Afinitor) or placebo, each in addition to best supportive care. Everolimus is currently approved by the Food and Drug Administration for the treatment of pancreatic neuroendocrine tumors, as well as breast and kidney cancer, and subependymal giant cell astrocytoma.
Results for the entire trial population have been previously reported and showed that everolimus prolonged progression-free survival by 7.1 months, reducing the risk of events by 52% (Lancet. 2015 Dec 15. doi.org/10.1016/S0140-6736[15]01234-9).
The new subgroup analyses were restricted to the patients with tumors originating in the GI tract (n =175) or an unknown site generally thought to be the GI tract (n = 36).
Among the group with GI tumors, median progression-free survival was 13.1 months with everolimus versus 5.4 months with placebo, Dr. Singh reported. Among the group with tumors of unknown origin, it was 13.6 and 7.5 months, respectively.
Relative to placebo, everolimus prolonged progression-free survival by 6.41 months, reducing the risk of events by 29%, in patients whose tumors originated in the midgut (duodenum, ileum, jejunum, cecum, or appendix). The relative benefit was 6.17 months, with a reduction in the risk of events of 73%, in patients whose tumors originated in non-midgut sites (stomach, colon, and rectum).
In addition, everolimus prolonged progression-free survival by 6.73 months, reducing the risk of events by 46%, in patients who had previously received somatostatin analogues, and by 9.07 months, reducing the risk by 48%, in patients who had not received these agents.
The safety profile of everolimus was consistent with that expected based on the use of this agent in other patient populations, according to Dr. Singh. The most common adverse events were stomatitis, infections, diarrhea, peripheral edema, and fatigue. No new safety signals were seen.
Dr. Singh disclosed that he receives honoraria from, has a consulting or advisory role with, and receives research funding (institutional) and travel, accommodations, and expenses from Novartis. The study received funding from Novartis Pharmaceuticals.
Everolimus improves outcomes in patients with advanced, progressive neuroendocrine tumors of gastrointestinal (GI) or unknown origin regardless of primary location and prior therapy, according to new subgroup analyses of the RADIANT-4 trial.
The phase III trial is the largest of its type in patients with nonfunctioning GI tract or lung neuroendocrine tumors. The subgroup findings for those whose tumors originated in the GI tract or an unknown site (but suspected to be GI) were presented in a presscast held in advance of the Gastrointestinal Cancers Symposium.
Compared with placebo, everolimus prolonged progression-free survival by 6-9 months, corresponding to a 46%-48% relative reduction in the risk of progression or death, reported lead study author Dr. Simron Singh of Sunnybrook’s Odette Cancer Centre in Toronto. Benefit was similar regardless of whether patients had midgut or non-midgut tumors, and whether they had previously received a somatostatin analog or not.
“In my opinion, this study in advanced, progressive neuroendocrine patients [shows] an effective, new and exciting treatment option in a disease where we’ve had very few treatments to date,” Dr. Singh said ahead of the symposium, which was sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
ASCO expert and presscast moderator Dr. Smitha Krishnamurthi of Case Western Reserve University, Cleveland, agreed, saying that everolimus could help address an unmet need in this disease.
“Patients with GI neuroendocrine tumors have had very few treatment options. Once they have progressed on somatostatin analogues, there really are no good systemic treatments,” she said. “So this finding is very important, that the mTOR inhibitor everolimus has demonstrated an improvement in risk of progression by over 40% and with very little severe toxicity. This is a welcome finding for these patients who have limited systemic treatment options.”
Patients enrolled in RADIANT-4 had lung, GI, or unknown-origin neuroendocrine tumors that had progressed on other therapies, including somatostatin analogs, surgery, or chemotherapy.
They were randomly assigned in 2:1 ratio to receive everolimus (Afinitor) or placebo, each in addition to best supportive care. Everolimus is currently approved by the Food and Drug Administration for the treatment of pancreatic neuroendocrine tumors, as well as breast and kidney cancer, and subependymal giant cell astrocytoma.
Results for the entire trial population have been previously reported and showed that everolimus prolonged progression-free survival by 7.1 months, reducing the risk of events by 52% (Lancet. 2015 Dec 15. doi.org/10.1016/S0140-6736[15]01234-9).
The new subgroup analyses were restricted to the patients with tumors originating in the GI tract (n =175) or an unknown site generally thought to be the GI tract (n = 36).
Among the group with GI tumors, median progression-free survival was 13.1 months with everolimus versus 5.4 months with placebo, Dr. Singh reported. Among the group with tumors of unknown origin, it was 13.6 and 7.5 months, respectively.
Relative to placebo, everolimus prolonged progression-free survival by 6.41 months, reducing the risk of events by 29%, in patients whose tumors originated in the midgut (duodenum, ileum, jejunum, cecum, or appendix). The relative benefit was 6.17 months, with a reduction in the risk of events of 73%, in patients whose tumors originated in non-midgut sites (stomach, colon, and rectum).
In addition, everolimus prolonged progression-free survival by 6.73 months, reducing the risk of events by 46%, in patients who had previously received somatostatin analogues, and by 9.07 months, reducing the risk by 48%, in patients who had not received these agents.
The safety profile of everolimus was consistent with that expected based on the use of this agent in other patient populations, according to Dr. Singh. The most common adverse events were stomatitis, infections, diarrhea, peripheral edema, and fatigue. No new safety signals were seen.
Dr. Singh disclosed that he receives honoraria from, has a consulting or advisory role with, and receives research funding (institutional) and travel, accommodations, and expenses from Novartis. The study received funding from Novartis Pharmaceuticals.
FROM THE GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: Everolimus reduces the risk of progression or death across subgroups of patients who have advanced, progressive neuroendocrine tumors arising in the GI tract or an unknown site.
Major finding: Compared with placebo, everolimus prolonged median progression-free survival by 6-9 months in patients with midgut and non-midgut tumors, and in patients who had and had not received somatostatin analogues.
Data source: A subgroups analysis of a phase III trial among 211 patients with advanced, progressive nonfunctioning neuroendocrine tumors originating in the GI tract or an unknown site (RADIANT-4 trial).
Disclosures: Dr. Singh disclosed that he receives honoraria from, has a consulting or advisory role with, and receives research funding (institutional) and travel, accommodations, and expenses from Novartis. The study received funding from Novartis Pharmaceuticals.
Outcomes and Aseptic Survivorship of Revision Total Knee Arthroplasty
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
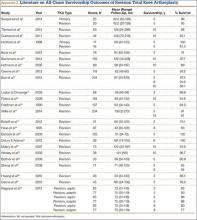
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
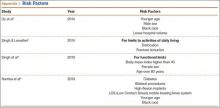
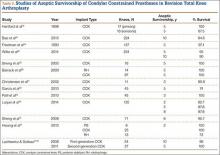
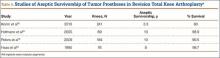
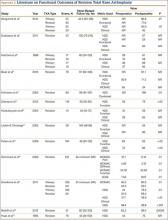
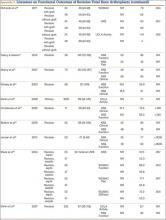
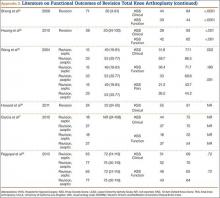
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
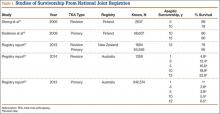
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
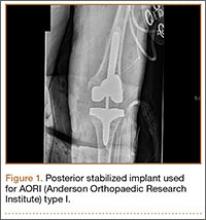
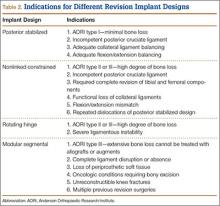
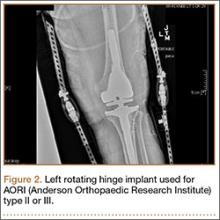
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
Biologic treatment in pregnancy requires balancing risks
The effectiveness of immunoglobulin biologic treatments in controlling chronic and potentially debilitating autoimmune diseases such as rheumatoid arthritis and ulcerative colitis means that more physicians are faced with the question of how to handle the use of these drugs in pregnancy.
While immunoglobulin G (IgG) biologicals are large molecules, there is no doubt that they cross the placenta through specific transport systems with a long half life in infants, creating potential risks for immunocompromise in early life. At the same time, these biologicals are essential, in many cases, for controlling the pregnant woman’s disease and allowing her to carry a pregnancy successfully by avoiding disease flare.
For ob.gyns., successful management of a pregnancy in which the woman is taking an immunoglobulin biological, such as an anti–tumor necrosis factor (TNF)-alpha agent, requires an understanding of not only which drugs cross the placenta, but when they do so and at what levels.
Crossing the placenta
Along with my student Juejing Ling, I recently reviewed the question of how the use of immunoglobulin biologicals in pregnancy affects the vaccination of infants in an article published in Expert Review of Vaccines (2015 Dec 7:1-18 doi: 10.1586/14760584.2016.1115351). Our analysis relates only to biologicals with partial or full IgG structure, as they are capable of crossing the placenta.
Data are still limited about the use of immunoglobulin biologicals in pregnancy, but measurement of umbilical cord blood has shown high levels of anti-TNF IgG in newborn serum, raising concerns about how these neonates will respond to vaccinations.
Neonates rely on maternal IgG transport to prevent infection in the first few months of life and that transport process begins around 12 weeks gestation. Fetal IgG levels begin to rise at 13-18 weeks and reach 120%-130% of maternal levels when the fetus reaches full term. In contrast, fusion proteins that contain the Fc portion and Fab fragment appear to have limited ability to cross the placenta. As a result, chimeric and full human IgG antibodies such as infliximab, adalimumab, and rituximab have demonstrated high levels of placental transport, while other agents, such as etanercept, appear to cross the placenta at lower levels.
Hence, due to the ineffective clearance, certain immunoglobulin biologicals actually have a higher concentration and a longer half life in neonates than in mothers. For instance, with infliximab, studies show that levels in the umbilical cord were up to fourfold higher than maternal levels, even when the drug was discontinued at 30 weeks of pregnancy or earlier. Due to long neonatal half life, infliximab levels became undetectable in infant serum only between 2-7 months, compared with 1-2 weeks in adults. Adalimumab is similar, where concentrations of the drug in neonates can be 150% of the maternal serum level and detectable for about 3 months after birth.
Transport of anti-TNFs is also possible through breastfeeding, although studies indicate that the levels are very low.
Infection risk
Due to the immunosuppressive effect of anti-TNF immunoglobulin biologicals, newborn infection is a real concern. Review of the literature showed that severe and moderate neutropenia and skin infection were reported in four neonates born to two women with ulcerative colitis who had taken infliximab throughout pregnancy.
Some other studies have followed infants who had detectable biological levels at birth after in utero exposure. In general, there is normal development in the first year without overt infection. However, there have been case reports of infections with varicella or upper respiratory infections in infants exposed to infliximab before 30 weeks’ gestation.
There is very little data on the long-term immune system impacts for infants exposed to immunoglobulin biologicals in utero. However, these agents are generally not at detectable levels after 1 year.
Impact on vaccination
Although these IgG biologicals will clear the infants’ systems after several months of life (generally by 8 months), another concern is for how their presence in the early months impacts neonatal vaccination, specifically live attenuated vaccines such as MMR (measles, mumps and rubella), BCG for tuberculosis, oral polio, rotavirus vaccine, and the intranasal influenza vaccine.
Generally, outcomes among infants exposed to anti-TNFs have been good. For instance, reports looking at 24 children with exposure to anti-TNFs found no complications with the MMR vaccine. But a famous case report identified one infant who died at 4.5 months after receiving the BCG vaccine at 3 months. The mother, who had Crohn’s disease, had been taking infliximab 10 mg/kg every 8 weeks throughout her pregnancy.
Another study of 15 infants in the Czech Republic who were exposed to infliximab in utero and received BCG vaccination within 1 week of birth found that three of the infants developed large local skin reactions. One of the three children also developed axillary lymphadenopathy. All of the children recovered without the need for anti-tuberculosis therapy.
So what do these complications mean for vaccination strategies? Both the European Crohn’s and Colitis Organisation and the World Congress of Gastroenterology recommend that in terms of non-live vaccines, it’s safe to follow the same vaccine schedule as infants not exposed to biologicals in utero. When it comes to live attenuated vaccines such as rotavirus, oral polio, and BCG, these infants should be treated as immunocompromised and not receive these vaccines until after 6 months of age, when the biologicals should be at undetectable levels.
Future directions
Given that most infections and other adverse events happen after late exposure in pregnancy, some have recommended discontinuing anti-TNF treatment before the third trimester. In fact, this has become a common management practice. However, this should be an individualized decision made after discussion between a woman and her physician or physicians. Any benefits from early discontinuation of an immunoglobulin biological therapy should be weighed against the risk of disease flare, which also has real potential to complicate pregnancy.
The evidence presented here not only shines a light on the possible risk to infants, but also on the need for more high-quality evidence on which physicians can base decisions. Most of the available evidence is drawn from case reports and registry databases. Both of these suffer from a lack of control groups. To answer these questions definitively, we need more well-controlled studies of large populations. I strongly urge readers to follow the amazing work led by Dr. Uma Mahadevan and her colleagues at the University of California, San Francisco on biological use in pregnancy and long-term outcomes. As we wait for more evidence, we all look forward to the development of newer biologic agents that can help women control autoimmune disease without crossing the placenta.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He reported having no financial disclosures related to this article. Email him at [email protected].
The effectiveness of immunoglobulin biologic treatments in controlling chronic and potentially debilitating autoimmune diseases such as rheumatoid arthritis and ulcerative colitis means that more physicians are faced with the question of how to handle the use of these drugs in pregnancy.
While immunoglobulin G (IgG) biologicals are large molecules, there is no doubt that they cross the placenta through specific transport systems with a long half life in infants, creating potential risks for immunocompromise in early life. At the same time, these biologicals are essential, in many cases, for controlling the pregnant woman’s disease and allowing her to carry a pregnancy successfully by avoiding disease flare.
For ob.gyns., successful management of a pregnancy in which the woman is taking an immunoglobulin biological, such as an anti–tumor necrosis factor (TNF)-alpha agent, requires an understanding of not only which drugs cross the placenta, but when they do so and at what levels.
Crossing the placenta
Along with my student Juejing Ling, I recently reviewed the question of how the use of immunoglobulin biologicals in pregnancy affects the vaccination of infants in an article published in Expert Review of Vaccines (2015 Dec 7:1-18 doi: 10.1586/14760584.2016.1115351). Our analysis relates only to biologicals with partial or full IgG structure, as they are capable of crossing the placenta.
Data are still limited about the use of immunoglobulin biologicals in pregnancy, but measurement of umbilical cord blood has shown high levels of anti-TNF IgG in newborn serum, raising concerns about how these neonates will respond to vaccinations.
Neonates rely on maternal IgG transport to prevent infection in the first few months of life and that transport process begins around 12 weeks gestation. Fetal IgG levels begin to rise at 13-18 weeks and reach 120%-130% of maternal levels when the fetus reaches full term. In contrast, fusion proteins that contain the Fc portion and Fab fragment appear to have limited ability to cross the placenta. As a result, chimeric and full human IgG antibodies such as infliximab, adalimumab, and rituximab have demonstrated high levels of placental transport, while other agents, such as etanercept, appear to cross the placenta at lower levels.
Hence, due to the ineffective clearance, certain immunoglobulin biologicals actually have a higher concentration and a longer half life in neonates than in mothers. For instance, with infliximab, studies show that levels in the umbilical cord were up to fourfold higher than maternal levels, even when the drug was discontinued at 30 weeks of pregnancy or earlier. Due to long neonatal half life, infliximab levels became undetectable in infant serum only between 2-7 months, compared with 1-2 weeks in adults. Adalimumab is similar, where concentrations of the drug in neonates can be 150% of the maternal serum level and detectable for about 3 months after birth.
Transport of anti-TNFs is also possible through breastfeeding, although studies indicate that the levels are very low.
Infection risk
Due to the immunosuppressive effect of anti-TNF immunoglobulin biologicals, newborn infection is a real concern. Review of the literature showed that severe and moderate neutropenia and skin infection were reported in four neonates born to two women with ulcerative colitis who had taken infliximab throughout pregnancy.
Some other studies have followed infants who had detectable biological levels at birth after in utero exposure. In general, there is normal development in the first year without overt infection. However, there have been case reports of infections with varicella or upper respiratory infections in infants exposed to infliximab before 30 weeks’ gestation.
There is very little data on the long-term immune system impacts for infants exposed to immunoglobulin biologicals in utero. However, these agents are generally not at detectable levels after 1 year.
Impact on vaccination
Although these IgG biologicals will clear the infants’ systems after several months of life (generally by 8 months), another concern is for how their presence in the early months impacts neonatal vaccination, specifically live attenuated vaccines such as MMR (measles, mumps and rubella), BCG for tuberculosis, oral polio, rotavirus vaccine, and the intranasal influenza vaccine.
Generally, outcomes among infants exposed to anti-TNFs have been good. For instance, reports looking at 24 children with exposure to anti-TNFs found no complications with the MMR vaccine. But a famous case report identified one infant who died at 4.5 months after receiving the BCG vaccine at 3 months. The mother, who had Crohn’s disease, had been taking infliximab 10 mg/kg every 8 weeks throughout her pregnancy.
Another study of 15 infants in the Czech Republic who were exposed to infliximab in utero and received BCG vaccination within 1 week of birth found that three of the infants developed large local skin reactions. One of the three children also developed axillary lymphadenopathy. All of the children recovered without the need for anti-tuberculosis therapy.
So what do these complications mean for vaccination strategies? Both the European Crohn’s and Colitis Organisation and the World Congress of Gastroenterology recommend that in terms of non-live vaccines, it’s safe to follow the same vaccine schedule as infants not exposed to biologicals in utero. When it comes to live attenuated vaccines such as rotavirus, oral polio, and BCG, these infants should be treated as immunocompromised and not receive these vaccines until after 6 months of age, when the biologicals should be at undetectable levels.
Future directions
Given that most infections and other adverse events happen after late exposure in pregnancy, some have recommended discontinuing anti-TNF treatment before the third trimester. In fact, this has become a common management practice. However, this should be an individualized decision made after discussion between a woman and her physician or physicians. Any benefits from early discontinuation of an immunoglobulin biological therapy should be weighed against the risk of disease flare, which also has real potential to complicate pregnancy.
The evidence presented here not only shines a light on the possible risk to infants, but also on the need for more high-quality evidence on which physicians can base decisions. Most of the available evidence is drawn from case reports and registry databases. Both of these suffer from a lack of control groups. To answer these questions definitively, we need more well-controlled studies of large populations. I strongly urge readers to follow the amazing work led by Dr. Uma Mahadevan and her colleagues at the University of California, San Francisco on biological use in pregnancy and long-term outcomes. As we wait for more evidence, we all look forward to the development of newer biologic agents that can help women control autoimmune disease without crossing the placenta.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He reported having no financial disclosures related to this article. Email him at [email protected].
The effectiveness of immunoglobulin biologic treatments in controlling chronic and potentially debilitating autoimmune diseases such as rheumatoid arthritis and ulcerative colitis means that more physicians are faced with the question of how to handle the use of these drugs in pregnancy.
While immunoglobulin G (IgG) biologicals are large molecules, there is no doubt that they cross the placenta through specific transport systems with a long half life in infants, creating potential risks for immunocompromise in early life. At the same time, these biologicals are essential, in many cases, for controlling the pregnant woman’s disease and allowing her to carry a pregnancy successfully by avoiding disease flare.
For ob.gyns., successful management of a pregnancy in which the woman is taking an immunoglobulin biological, such as an anti–tumor necrosis factor (TNF)-alpha agent, requires an understanding of not only which drugs cross the placenta, but when they do so and at what levels.
Crossing the placenta
Along with my student Juejing Ling, I recently reviewed the question of how the use of immunoglobulin biologicals in pregnancy affects the vaccination of infants in an article published in Expert Review of Vaccines (2015 Dec 7:1-18 doi: 10.1586/14760584.2016.1115351). Our analysis relates only to biologicals with partial or full IgG structure, as they are capable of crossing the placenta.
Data are still limited about the use of immunoglobulin biologicals in pregnancy, but measurement of umbilical cord blood has shown high levels of anti-TNF IgG in newborn serum, raising concerns about how these neonates will respond to vaccinations.
Neonates rely on maternal IgG transport to prevent infection in the first few months of life and that transport process begins around 12 weeks gestation. Fetal IgG levels begin to rise at 13-18 weeks and reach 120%-130% of maternal levels when the fetus reaches full term. In contrast, fusion proteins that contain the Fc portion and Fab fragment appear to have limited ability to cross the placenta. As a result, chimeric and full human IgG antibodies such as infliximab, adalimumab, and rituximab have demonstrated high levels of placental transport, while other agents, such as etanercept, appear to cross the placenta at lower levels.
Hence, due to the ineffective clearance, certain immunoglobulin biologicals actually have a higher concentration and a longer half life in neonates than in mothers. For instance, with infliximab, studies show that levels in the umbilical cord were up to fourfold higher than maternal levels, even when the drug was discontinued at 30 weeks of pregnancy or earlier. Due to long neonatal half life, infliximab levels became undetectable in infant serum only between 2-7 months, compared with 1-2 weeks in adults. Adalimumab is similar, where concentrations of the drug in neonates can be 150% of the maternal serum level and detectable for about 3 months after birth.
Transport of anti-TNFs is also possible through breastfeeding, although studies indicate that the levels are very low.
Infection risk
Due to the immunosuppressive effect of anti-TNF immunoglobulin biologicals, newborn infection is a real concern. Review of the literature showed that severe and moderate neutropenia and skin infection were reported in four neonates born to two women with ulcerative colitis who had taken infliximab throughout pregnancy.
Some other studies have followed infants who had detectable biological levels at birth after in utero exposure. In general, there is normal development in the first year without overt infection. However, there have been case reports of infections with varicella or upper respiratory infections in infants exposed to infliximab before 30 weeks’ gestation.
There is very little data on the long-term immune system impacts for infants exposed to immunoglobulin biologicals in utero. However, these agents are generally not at detectable levels after 1 year.
Impact on vaccination
Although these IgG biologicals will clear the infants’ systems after several months of life (generally by 8 months), another concern is for how their presence in the early months impacts neonatal vaccination, specifically live attenuated vaccines such as MMR (measles, mumps and rubella), BCG for tuberculosis, oral polio, rotavirus vaccine, and the intranasal influenza vaccine.
Generally, outcomes among infants exposed to anti-TNFs have been good. For instance, reports looking at 24 children with exposure to anti-TNFs found no complications with the MMR vaccine. But a famous case report identified one infant who died at 4.5 months after receiving the BCG vaccine at 3 months. The mother, who had Crohn’s disease, had been taking infliximab 10 mg/kg every 8 weeks throughout her pregnancy.
Another study of 15 infants in the Czech Republic who were exposed to infliximab in utero and received BCG vaccination within 1 week of birth found that three of the infants developed large local skin reactions. One of the three children also developed axillary lymphadenopathy. All of the children recovered without the need for anti-tuberculosis therapy.
So what do these complications mean for vaccination strategies? Both the European Crohn’s and Colitis Organisation and the World Congress of Gastroenterology recommend that in terms of non-live vaccines, it’s safe to follow the same vaccine schedule as infants not exposed to biologicals in utero. When it comes to live attenuated vaccines such as rotavirus, oral polio, and BCG, these infants should be treated as immunocompromised and not receive these vaccines until after 6 months of age, when the biologicals should be at undetectable levels.
Future directions
Given that most infections and other adverse events happen after late exposure in pregnancy, some have recommended discontinuing anti-TNF treatment before the third trimester. In fact, this has become a common management practice. However, this should be an individualized decision made after discussion between a woman and her physician or physicians. Any benefits from early discontinuation of an immunoglobulin biological therapy should be weighed against the risk of disease flare, which also has real potential to complicate pregnancy.
The evidence presented here not only shines a light on the possible risk to infants, but also on the need for more high-quality evidence on which physicians can base decisions. Most of the available evidence is drawn from case reports and registry databases. Both of these suffer from a lack of control groups. To answer these questions definitively, we need more well-controlled studies of large populations. I strongly urge readers to follow the amazing work led by Dr. Uma Mahadevan and her colleagues at the University of California, San Francisco on biological use in pregnancy and long-term outcomes. As we wait for more evidence, we all look forward to the development of newer biologic agents that can help women control autoimmune disease without crossing the placenta.
Dr. Koren is professor of pharmacology and pharmacy at the University of Toronto. He is the founding director of the Motherisk Program. He reported having no financial disclosures related to this article. Email him at [email protected].
Implant Designs in Revision Total Knee Arthroplasty
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
Nonoperative Treatment of Rotator Cuff Tears
Rotator cuff disease is extremely common, yet indications for surgery are not well established. Unfortunately, data on the natural history of patients with rotator cuff disease are lacking, as are high-level studies evaluating the effectiveness of rotator cuff repair. This deficit is highlighted by the recent American Academy of Orthopaedic Surgeons clinical practice guideline on optimizing the management of rotator cuff problems,1 in which none of the position statements were based on high-level evidence, and 22 of 25 statements were inconclusive or based on weak evidence or represented the panel’s consensus opinion. Although the traditional teaching is that rotator cuff tears (RCTs) should be surgically repaired, the present article reviews the evidence supporting physical therapy as a treatment for atraumatic full-thickness RCTs.
1. Less than 5% of people with RCTs undergo surgery
Studies on symptomatic and asymptomatic patients have found a high incidence of RCTs in the population at large.2,3 By conservative estimate, 10% of people older than 65 years have full-thickness RCTs. Therefore, the 2010 US Census4 finding of 57 million people over age 65 years translates to 5.7 million with full-thickness RCTs. In the United States, about 275,000 rotator cuff surgeries are performed annually.5 That is, less than 5% of people with RCTs undergo surgery each year.
2. Symptoms do not correlate well with RCT severity
Pain is statistically more likely in patients who experience RCT progression than in those who do not.6-8 However, RCTs may progress without pain, or there may be pain without progression, making pain a poor sign of RCT progression.9 The Multicenter Orthopaedic Outcome Network (MOON) Shoulder Group, studying a cohort of patients with atraumatic full-thickness RCTs, found no relationship between RCT severity and pain,10 symptom duration,11 or activity level,12 suggesting the relationship between RCTs and symptoms is not robust.
3. The high failure rates of surgical repairs do not affect patient-reported outcomes
Postoperative imaging has demonstrated high failure rates for rotator cuff repairs, yet patient-reported outcome scores do not differ between cases of intact and failed repairs.13,14 Strength is better, however, in intact repairs.14
4. Physical therapy is effective in treating atraumatic RCTs
The MOON Shoulder Group conducted a prospective cohort study to determine the predictors of failed physical therapy for atraumatic full-thickness RCTs and to help define the indications for rotator cuff surgery.15 All enrolled patients started with a well-defined physical therapy program, and they could opt out and have surgery at any time. The physical therapy program, derived from a systematic review of the literature, was found to be effective in more than 80% of patients with follow-up of 2 years or longer.15 The most important predictor of failed nonoperative treatment was patient expectations: For a patient who thought physical therapy would work, it worked; for a patient who thought it would not work, surgery was the more likely choice. No measure of pain or RCT severity predicted the need for surgery.16 For 2 randomized trials that compared surgery and physical therapy, the success of nonoperative treatment was similar: 76% (Moosmayer and colleagues17) and 92% (Kukkonen and colleagues18).
5. What are the indications for surgery?
These data suggest that physical therapy is reasonable for patients with atraumatic RCTs. Some data suggest that traumatic RCTs should be treated with surgery and that it should be performed early.19 Other data suggest strength is better after rotator cuff repair.13,14 What, then, are the indications for surgery? Patients with acute tears probably should have surgery; patients concerned about weakness should consider surgery but should keep in mind that its benefit depends on an intact rotator cuff repair; and patients with low expectations about the effectiveness of physical therapy probably should consider surgery.
When discussing options with a patient, you might approach informed consent as follows:
“Mr. Smith, you have a rotator cuff tear. So do at least 6 million other Americans over age 60 years. Only 5% of those undergo surgery. If your problem is weakness or functional loss, you should have surgery, though there is about a 30% chance the repair will fail. I don’t know how to predict the outcome of repair yet, but I worry your atraumatic tear is at risk for repair failure.
“If your problem is pain, you have an 80% chance of improving with physical therapy, and pain relief seems to last at least 2 years. If you go with physical therapy, however, there is a risk your tear could progress and start causing symptoms. I don’t yet know how likely it is your tear will progress or, if it does progress, how likely it is the tear will cause symptoms. I wish we had better information to help you make your decision.”
1. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
2. Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88(2):116-121.
3. Teunis, T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff pathology with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913-1921.
4. Werner CA. The older population: 2010 (2010 Census briefs). US Census Bureau website. http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf. Published November 2011. Accessed December 13, 2015.
5. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
6. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92(16):2623-2633.
7. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
8. Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39(4):710-714.
9. Kuhn JE. Are atraumatic rotator cuff tears painful? A model to describe the relationship between pain and rotator cuff tears. Minerva Orthop Traumatol. 2015;66:51-61.
10. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross-sectional study of 393 patients with a symptomatic atraumatic full-thickness rotator cuff tear. J Bone Joint Surg Am. 2014;96(10):793-800.
11. MOON Shoulder Group: Unruh KP, Kuhn JE, Sanders R, et al. The duration of symptoms does not correlate with rotator cuff tear severity or other patient-related features: a cross-sectional study of patients with atraumatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2014;23(7):1052-1058.
12. Brophy RH, Dunn WR, Kuhn JE; MOON Shoulder Group. Shoulder activity level is not associated with the severity of symptomatic, atraumatic rotator cuff tears in patients electing nonoperative treatment. Am J Sports Med. 2014;42(5):1150-1154.
13. Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393-403.
14. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96(4):265-271.
15. Kuhn JE, Dunn WR, Sanders R, et al; MOON Shoulder Group. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg. 2013;22(10):1371-1379.
16. Dunn WR, Kuhn JE, Sanders R, et al. Defining indications for rotator cuff repair: predictors of failure of nonoperative treatment of chronic, symptomatic full-thickness rotator cuff tears. Paper presented at: Open Meeting of the American Shoulder and Elbow Surgeons; March 23, 2013; Chicago, IL.
17. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five-year follow-up. J Bone Joint Surg Am. 2014;96(18):1504-1514.
18. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of non-traumatic rotator cuff tears: a randomised controlled trial with one-year clinical results. Bone Joint J Br. 2014;96(1):75-81.
19. Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;(455):52-63.
Rotator cuff disease is extremely common, yet indications for surgery are not well established. Unfortunately, data on the natural history of patients with rotator cuff disease are lacking, as are high-level studies evaluating the effectiveness of rotator cuff repair. This deficit is highlighted by the recent American Academy of Orthopaedic Surgeons clinical practice guideline on optimizing the management of rotator cuff problems,1 in which none of the position statements were based on high-level evidence, and 22 of 25 statements were inconclusive or based on weak evidence or represented the panel’s consensus opinion. Although the traditional teaching is that rotator cuff tears (RCTs) should be surgically repaired, the present article reviews the evidence supporting physical therapy as a treatment for atraumatic full-thickness RCTs.
1. Less than 5% of people with RCTs undergo surgery
Studies on symptomatic and asymptomatic patients have found a high incidence of RCTs in the population at large.2,3 By conservative estimate, 10% of people older than 65 years have full-thickness RCTs. Therefore, the 2010 US Census4 finding of 57 million people over age 65 years translates to 5.7 million with full-thickness RCTs. In the United States, about 275,000 rotator cuff surgeries are performed annually.5 That is, less than 5% of people with RCTs undergo surgery each year.
2. Symptoms do not correlate well with RCT severity
Pain is statistically more likely in patients who experience RCT progression than in those who do not.6-8 However, RCTs may progress without pain, or there may be pain without progression, making pain a poor sign of RCT progression.9 The Multicenter Orthopaedic Outcome Network (MOON) Shoulder Group, studying a cohort of patients with atraumatic full-thickness RCTs, found no relationship between RCT severity and pain,10 symptom duration,11 or activity level,12 suggesting the relationship between RCTs and symptoms is not robust.
3. The high failure rates of surgical repairs do not affect patient-reported outcomes
Postoperative imaging has demonstrated high failure rates for rotator cuff repairs, yet patient-reported outcome scores do not differ between cases of intact and failed repairs.13,14 Strength is better, however, in intact repairs.14
4. Physical therapy is effective in treating atraumatic RCTs
The MOON Shoulder Group conducted a prospective cohort study to determine the predictors of failed physical therapy for atraumatic full-thickness RCTs and to help define the indications for rotator cuff surgery.15 All enrolled patients started with a well-defined physical therapy program, and they could opt out and have surgery at any time. The physical therapy program, derived from a systematic review of the literature, was found to be effective in more than 80% of patients with follow-up of 2 years or longer.15 The most important predictor of failed nonoperative treatment was patient expectations: For a patient who thought physical therapy would work, it worked; for a patient who thought it would not work, surgery was the more likely choice. No measure of pain or RCT severity predicted the need for surgery.16 For 2 randomized trials that compared surgery and physical therapy, the success of nonoperative treatment was similar: 76% (Moosmayer and colleagues17) and 92% (Kukkonen and colleagues18).
5. What are the indications for surgery?
These data suggest that physical therapy is reasonable for patients with atraumatic RCTs. Some data suggest that traumatic RCTs should be treated with surgery and that it should be performed early.19 Other data suggest strength is better after rotator cuff repair.13,14 What, then, are the indications for surgery? Patients with acute tears probably should have surgery; patients concerned about weakness should consider surgery but should keep in mind that its benefit depends on an intact rotator cuff repair; and patients with low expectations about the effectiveness of physical therapy probably should consider surgery.
When discussing options with a patient, you might approach informed consent as follows:
“Mr. Smith, you have a rotator cuff tear. So do at least 6 million other Americans over age 60 years. Only 5% of those undergo surgery. If your problem is weakness or functional loss, you should have surgery, though there is about a 30% chance the repair will fail. I don’t know how to predict the outcome of repair yet, but I worry your atraumatic tear is at risk for repair failure.
“If your problem is pain, you have an 80% chance of improving with physical therapy, and pain relief seems to last at least 2 years. If you go with physical therapy, however, there is a risk your tear could progress and start causing symptoms. I don’t yet know how likely it is your tear will progress or, if it does progress, how likely it is the tear will cause symptoms. I wish we had better information to help you make your decision.”
Rotator cuff disease is extremely common, yet indications for surgery are not well established. Unfortunately, data on the natural history of patients with rotator cuff disease are lacking, as are high-level studies evaluating the effectiveness of rotator cuff repair. This deficit is highlighted by the recent American Academy of Orthopaedic Surgeons clinical practice guideline on optimizing the management of rotator cuff problems,1 in which none of the position statements were based on high-level evidence, and 22 of 25 statements were inconclusive or based on weak evidence or represented the panel’s consensus opinion. Although the traditional teaching is that rotator cuff tears (RCTs) should be surgically repaired, the present article reviews the evidence supporting physical therapy as a treatment for atraumatic full-thickness RCTs.
1. Less than 5% of people with RCTs undergo surgery
Studies on symptomatic and asymptomatic patients have found a high incidence of RCTs in the population at large.2,3 By conservative estimate, 10% of people older than 65 years have full-thickness RCTs. Therefore, the 2010 US Census4 finding of 57 million people over age 65 years translates to 5.7 million with full-thickness RCTs. In the United States, about 275,000 rotator cuff surgeries are performed annually.5 That is, less than 5% of people with RCTs undergo surgery each year.
2. Symptoms do not correlate well with RCT severity
Pain is statistically more likely in patients who experience RCT progression than in those who do not.6-8 However, RCTs may progress without pain, or there may be pain without progression, making pain a poor sign of RCT progression.9 The Multicenter Orthopaedic Outcome Network (MOON) Shoulder Group, studying a cohort of patients with atraumatic full-thickness RCTs, found no relationship between RCT severity and pain,10 symptom duration,11 or activity level,12 suggesting the relationship between RCTs and symptoms is not robust.
3. The high failure rates of surgical repairs do not affect patient-reported outcomes
Postoperative imaging has demonstrated high failure rates for rotator cuff repairs, yet patient-reported outcome scores do not differ between cases of intact and failed repairs.13,14 Strength is better, however, in intact repairs.14
4. Physical therapy is effective in treating atraumatic RCTs
The MOON Shoulder Group conducted a prospective cohort study to determine the predictors of failed physical therapy for atraumatic full-thickness RCTs and to help define the indications for rotator cuff surgery.15 All enrolled patients started with a well-defined physical therapy program, and they could opt out and have surgery at any time. The physical therapy program, derived from a systematic review of the literature, was found to be effective in more than 80% of patients with follow-up of 2 years or longer.15 The most important predictor of failed nonoperative treatment was patient expectations: For a patient who thought physical therapy would work, it worked; for a patient who thought it would not work, surgery was the more likely choice. No measure of pain or RCT severity predicted the need for surgery.16 For 2 randomized trials that compared surgery and physical therapy, the success of nonoperative treatment was similar: 76% (Moosmayer and colleagues17) and 92% (Kukkonen and colleagues18).
5. What are the indications for surgery?
These data suggest that physical therapy is reasonable for patients with atraumatic RCTs. Some data suggest that traumatic RCTs should be treated with surgery and that it should be performed early.19 Other data suggest strength is better after rotator cuff repair.13,14 What, then, are the indications for surgery? Patients with acute tears probably should have surgery; patients concerned about weakness should consider surgery but should keep in mind that its benefit depends on an intact rotator cuff repair; and patients with low expectations about the effectiveness of physical therapy probably should consider surgery.
When discussing options with a patient, you might approach informed consent as follows:
“Mr. Smith, you have a rotator cuff tear. So do at least 6 million other Americans over age 60 years. Only 5% of those undergo surgery. If your problem is weakness or functional loss, you should have surgery, though there is about a 30% chance the repair will fail. I don’t know how to predict the outcome of repair yet, but I worry your atraumatic tear is at risk for repair failure.
“If your problem is pain, you have an 80% chance of improving with physical therapy, and pain relief seems to last at least 2 years. If you go with physical therapy, however, there is a risk your tear could progress and start causing symptoms. I don’t yet know how likely it is your tear will progress or, if it does progress, how likely it is the tear will cause symptoms. I wish we had better information to help you make your decision.”
1. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
2. Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88(2):116-121.
3. Teunis, T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff pathology with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913-1921.
4. Werner CA. The older population: 2010 (2010 Census briefs). US Census Bureau website. http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf. Published November 2011. Accessed December 13, 2015.
5. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
6. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92(16):2623-2633.
7. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
8. Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39(4):710-714.
9. Kuhn JE. Are atraumatic rotator cuff tears painful? A model to describe the relationship between pain and rotator cuff tears. Minerva Orthop Traumatol. 2015;66:51-61.
10. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross-sectional study of 393 patients with a symptomatic atraumatic full-thickness rotator cuff tear. J Bone Joint Surg Am. 2014;96(10):793-800.
11. MOON Shoulder Group: Unruh KP, Kuhn JE, Sanders R, et al. The duration of symptoms does not correlate with rotator cuff tear severity or other patient-related features: a cross-sectional study of patients with atraumatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2014;23(7):1052-1058.
12. Brophy RH, Dunn WR, Kuhn JE; MOON Shoulder Group. Shoulder activity level is not associated with the severity of symptomatic, atraumatic rotator cuff tears in patients electing nonoperative treatment. Am J Sports Med. 2014;42(5):1150-1154.
13. Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393-403.
14. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96(4):265-271.
15. Kuhn JE, Dunn WR, Sanders R, et al; MOON Shoulder Group. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg. 2013;22(10):1371-1379.
16. Dunn WR, Kuhn JE, Sanders R, et al. Defining indications for rotator cuff repair: predictors of failure of nonoperative treatment of chronic, symptomatic full-thickness rotator cuff tears. Paper presented at: Open Meeting of the American Shoulder and Elbow Surgeons; March 23, 2013; Chicago, IL.
17. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five-year follow-up. J Bone Joint Surg Am. 2014;96(18):1504-1514.
18. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of non-traumatic rotator cuff tears: a randomised controlled trial with one-year clinical results. Bone Joint J Br. 2014;96(1):75-81.
19. Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;(455):52-63.
1. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
2. Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88(2):116-121.
3. Teunis, T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff pathology with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913-1921.
4. Werner CA. The older population: 2010 (2010 Census briefs). US Census Bureau website. http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf. Published November 2011. Accessed December 13, 2015.
5. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
6. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92(16):2623-2633.
7. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
8. Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39(4):710-714.
9. Kuhn JE. Are atraumatic rotator cuff tears painful? A model to describe the relationship between pain and rotator cuff tears. Minerva Orthop Traumatol. 2015;66:51-61.
10. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross-sectional study of 393 patients with a symptomatic atraumatic full-thickness rotator cuff tear. J Bone Joint Surg Am. 2014;96(10):793-800.
11. MOON Shoulder Group: Unruh KP, Kuhn JE, Sanders R, et al. The duration of symptoms does not correlate with rotator cuff tear severity or other patient-related features: a cross-sectional study of patients with atraumatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2014;23(7):1052-1058.
12. Brophy RH, Dunn WR, Kuhn JE; MOON Shoulder Group. Shoulder activity level is not associated with the severity of symptomatic, atraumatic rotator cuff tears in patients electing nonoperative treatment. Am J Sports Med. 2014;42(5):1150-1154.
13. Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393-403.
14. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96(4):265-271.
15. Kuhn JE, Dunn WR, Sanders R, et al; MOON Shoulder Group. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg. 2013;22(10):1371-1379.
16. Dunn WR, Kuhn JE, Sanders R, et al. Defining indications for rotator cuff repair: predictors of failure of nonoperative treatment of chronic, symptomatic full-thickness rotator cuff tears. Paper presented at: Open Meeting of the American Shoulder and Elbow Surgeons; March 23, 2013; Chicago, IL.
17. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five-year follow-up. J Bone Joint Surg Am. 2014;96(18):1504-1514.
18. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of non-traumatic rotator cuff tears: a randomised controlled trial with one-year clinical results. Bone Joint J Br. 2014;96(1):75-81.
19. Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;(455):52-63.
Sirolimus reduced posttransplant skin cancer risk
Sirolimus protects organ-transplant recipients against developing skin cancer, reducing their risk by 40%, according to a retrospective cohort study published in JAMA Dermatology on Jan. 20.
Recipients of solid organs are at three- to fourfold higher risk of developing cancer, compared with the general population, and the most common type they get is nonmelanoma skin cancer. The risk of developing cutaneous squamous cell carcinoma is 65-250 times higher in organ-transplant recipients. Drugs that reduce the growth and proliferation of tumor cells by inhibiting mTOR (mammalian target of rapamycin), including sirolimus, are believed to reduce this cancer risk, said Pritesh S. Karia of the department of dermatology, Brigham and Women’s Hospital and Harvard University, Boston, and his associates (JAMA Dermatol. 2016 Jan 20. doi: 10.1001/jamadermatol.2015.5548).
The investigators reviewed the electronic medical records of 329 patients (mean age, 56 years) who underwent organ transplantation at one of the two medical centers during a 9-year period and who then developed a cancer of any type. The study participants received renal (53.8%), heart (17.6%), lung (16.4%), liver (10.3%), or mixed-organ (1.8%) transplants. The most common index cancers they developed post transplant included cutaneous squamous cell carcinoma (31.9%), basal cell carcinoma (22.5%), and melanoma (2.7%).
Of the 329 patients, 97 (29.5%) then received sirolimus, while 232 (70.5%) did not. During a median follow-up of 38 months, 130 of these patients (39.5%) developed a second posttransplant cancer. The sirolimus-treated group showed a reduction in risk for cancer of any type, compared with the group that did not receive sirolimus (30.9% of 97 vs. 43.1% of 232).
Nearly all (88.5%) of the second posttransplant cancers that developed were skin cancers, and sirolimus reduced the risk of skin cancers by 40%. The 1-year, 3-year, and 5-year rates of skin cancer after an index posttransplant cancer were 9.3%, 20.6%, and 24.7% in the sirolimus group, compared with 17.7%, 31.0%, and 35.8%, respectively, in the untreated group, “thus demonstrating a lower risk for skin cancer with sirolimus treatment,” they said.
“Even for patients who have already had difficulty with skin cancer formation, mTOR inhibition appears to be of benefit. No difference in cancer outcomes was observable between sirolimus-treated and [untreated] groups because poor outcomes were rare,” Mr. Karia and his associates wrote.
These findings suggest that sirolimus chemoprevention should be considered for the subset of organ-transplant recipients who develop post-transplant cancer, they noted. The results also highlight the need for dermatologists and transplant physicians “to be aware of skin cancer history, coordinate regular posttransplant surveillance of skin cancers” in patients with organ transplant recipients, especially those with a history of skin cancer, and to communicate closely “as skin cancers form to consider reduction in immunosuppressive therapy or conversion to an mTOR-based regimen if skin cancer formation is of concern,” they added.
This study was supported by sirolimus manufacturer Novartis Pharmaceuticals. Mr. Karia and his associates reported having no relevant financial disclosures.
Sirolimus protects organ-transplant recipients against developing skin cancer, reducing their risk by 40%, according to a retrospective cohort study published in JAMA Dermatology on Jan. 20.
Recipients of solid organs are at three- to fourfold higher risk of developing cancer, compared with the general population, and the most common type they get is nonmelanoma skin cancer. The risk of developing cutaneous squamous cell carcinoma is 65-250 times higher in organ-transplant recipients. Drugs that reduce the growth and proliferation of tumor cells by inhibiting mTOR (mammalian target of rapamycin), including sirolimus, are believed to reduce this cancer risk, said Pritesh S. Karia of the department of dermatology, Brigham and Women’s Hospital and Harvard University, Boston, and his associates (JAMA Dermatol. 2016 Jan 20. doi: 10.1001/jamadermatol.2015.5548).
The investigators reviewed the electronic medical records of 329 patients (mean age, 56 years) who underwent organ transplantation at one of the two medical centers during a 9-year period and who then developed a cancer of any type. The study participants received renal (53.8%), heart (17.6%), lung (16.4%), liver (10.3%), or mixed-organ (1.8%) transplants. The most common index cancers they developed post transplant included cutaneous squamous cell carcinoma (31.9%), basal cell carcinoma (22.5%), and melanoma (2.7%).
Of the 329 patients, 97 (29.5%) then received sirolimus, while 232 (70.5%) did not. During a median follow-up of 38 months, 130 of these patients (39.5%) developed a second posttransplant cancer. The sirolimus-treated group showed a reduction in risk for cancer of any type, compared with the group that did not receive sirolimus (30.9% of 97 vs. 43.1% of 232).
Nearly all (88.5%) of the second posttransplant cancers that developed were skin cancers, and sirolimus reduced the risk of skin cancers by 40%. The 1-year, 3-year, and 5-year rates of skin cancer after an index posttransplant cancer were 9.3%, 20.6%, and 24.7% in the sirolimus group, compared with 17.7%, 31.0%, and 35.8%, respectively, in the untreated group, “thus demonstrating a lower risk for skin cancer with sirolimus treatment,” they said.
“Even for patients who have already had difficulty with skin cancer formation, mTOR inhibition appears to be of benefit. No difference in cancer outcomes was observable between sirolimus-treated and [untreated] groups because poor outcomes were rare,” Mr. Karia and his associates wrote.
These findings suggest that sirolimus chemoprevention should be considered for the subset of organ-transplant recipients who develop post-transplant cancer, they noted. The results also highlight the need for dermatologists and transplant physicians “to be aware of skin cancer history, coordinate regular posttransplant surveillance of skin cancers” in patients with organ transplant recipients, especially those with a history of skin cancer, and to communicate closely “as skin cancers form to consider reduction in immunosuppressive therapy or conversion to an mTOR-based regimen if skin cancer formation is of concern,” they added.
This study was supported by sirolimus manufacturer Novartis Pharmaceuticals. Mr. Karia and his associates reported having no relevant financial disclosures.
Sirolimus protects organ-transplant recipients against developing skin cancer, reducing their risk by 40%, according to a retrospective cohort study published in JAMA Dermatology on Jan. 20.
Recipients of solid organs are at three- to fourfold higher risk of developing cancer, compared with the general population, and the most common type they get is nonmelanoma skin cancer. The risk of developing cutaneous squamous cell carcinoma is 65-250 times higher in organ-transplant recipients. Drugs that reduce the growth and proliferation of tumor cells by inhibiting mTOR (mammalian target of rapamycin), including sirolimus, are believed to reduce this cancer risk, said Pritesh S. Karia of the department of dermatology, Brigham and Women’s Hospital and Harvard University, Boston, and his associates (JAMA Dermatol. 2016 Jan 20. doi: 10.1001/jamadermatol.2015.5548).
The investigators reviewed the electronic medical records of 329 patients (mean age, 56 years) who underwent organ transplantation at one of the two medical centers during a 9-year period and who then developed a cancer of any type. The study participants received renal (53.8%), heart (17.6%), lung (16.4%), liver (10.3%), or mixed-organ (1.8%) transplants. The most common index cancers they developed post transplant included cutaneous squamous cell carcinoma (31.9%), basal cell carcinoma (22.5%), and melanoma (2.7%).
Of the 329 patients, 97 (29.5%) then received sirolimus, while 232 (70.5%) did not. During a median follow-up of 38 months, 130 of these patients (39.5%) developed a second posttransplant cancer. The sirolimus-treated group showed a reduction in risk for cancer of any type, compared with the group that did not receive sirolimus (30.9% of 97 vs. 43.1% of 232).
Nearly all (88.5%) of the second posttransplant cancers that developed were skin cancers, and sirolimus reduced the risk of skin cancers by 40%. The 1-year, 3-year, and 5-year rates of skin cancer after an index posttransplant cancer were 9.3%, 20.6%, and 24.7% in the sirolimus group, compared with 17.7%, 31.0%, and 35.8%, respectively, in the untreated group, “thus demonstrating a lower risk for skin cancer with sirolimus treatment,” they said.
“Even for patients who have already had difficulty with skin cancer formation, mTOR inhibition appears to be of benefit. No difference in cancer outcomes was observable between sirolimus-treated and [untreated] groups because poor outcomes were rare,” Mr. Karia and his associates wrote.
These findings suggest that sirolimus chemoprevention should be considered for the subset of organ-transplant recipients who develop post-transplant cancer, they noted. The results also highlight the need for dermatologists and transplant physicians “to be aware of skin cancer history, coordinate regular posttransplant surveillance of skin cancers” in patients with organ transplant recipients, especially those with a history of skin cancer, and to communicate closely “as skin cancers form to consider reduction in immunosuppressive therapy or conversion to an mTOR-based regimen if skin cancer formation is of concern,” they added.
This study was supported by sirolimus manufacturer Novartis Pharmaceuticals. Mr. Karia and his associates reported having no relevant financial disclosures.
FROM JAMA DERMATOLOGY
Key clinical point: Sirolimus protects organ-transplant recipients against skin cancer.
Major finding: The 1-year, 3-year, and 5-year rates of skin cancer after an index posttransplant cancer were 9.3%, 20.6%, and 24.7% in the sirolimus group, compared with 17.7%, 31.0%, and 35.8% in the untreated group.
Data source: A retrospective cohort study of 329 organ-transplant recipients who had already developed one cancer likely related to their immunosuppressive therapy.
Disclosures: This study was supported by sirolimus manufacturer Novartis Pharmaceuticals. Mr. Karia and his associates reported having no relevant financial disclosures.
Adipose Flap Versus Fascial Sling for Anterior Subcutaneous Transposition of the Ulnar Nerve
Compression of the ulnar nerve at the elbow, also referred to as cubital tunnel syndrome (CuTS), is the second most common peripheral nerve compression syndrome in the upper extremity.1,2 Although the ulnar nerve can be compressed at 5 different sites, including arcade of Struthers, medial intermuscular septum, medial epicondyle, and deep flexor aponeurosis, the cubital tunnel is most commonly affected.3 Patients typically present with paresthesias in the fourth and fifth digits and weakness of hand muscle intrinsics. Activity-related pain or pain at the medial elbow can also occur in more advanced pathology.4 It is estimated that conservative therapy fails and surgical intervention is required in up to 30% of patients with CuTS.1 Surgical approaches range from in situ decompression to transposition techniques, but there is no consensus in the orthopedic community as to which technique offers the best results. In a 2008 meta-analysis, Macadam and colleagues5 found no statistical differences in outcomes among the various surgical approaches. Nevertheless, subcutaneous transposition of the ulnar nerve at the elbow is a popular option.6
Despite the widespread success of surgical intervention for CuTS, persistent or recurrent pain occurs in 9.9% to 21.0% of cases.7-10 In addition, several investigators have cited perineural scarring as a major cause of recurrent symptoms after primary surgery.11-14 Filippi and colleagues11 noted that patients who required reoperation after primary anterior transposition had “serious epineural fibrosis and fibrosis around the transposed ulnar nerve.” At our institution, we have similarly found that scarring of the fascial sling around the ulnar nerve led to recurrence of CuTS within 4 months after initial surgery (Figure 1).
We therefore prefer to use a vascularized adipose flap to secure the anteriorly transposed ulnar nerve. This flap provides a pliable, vascularized adipose environment for the nerve, which helps reduce nerve adherence and may enhance nerve recovery.15 In the study reported here, we retrospectively reviewed the long-term outcomes of ulnar nerve anterior subcutaneous transposition secured with either an adipose flap or a fascial sling. We hypothesized that patients in the 2 groups (adipose flap, fascial sling) would have equivalent outcomes.
Materials and Methods
After obtaining institutional review board approval, we reviewed the medical and surgical records of 104 patients (107 limbs) who underwent transposition of the ulnar nerve secured with either an adipose flap (27 limbs) or a fascial sling (80 limbs) over a 14-year period. The fascial sling cohort was used as a comparison group, matched to the adipose flap cohort by sex, age at time of surgery, hand dominance, symptom duration, and length of follow-up (Table 1). Patients were indicated for surgery and were included in the study if they had a history and physical examination consistent with primary CuTS, symptom duration longer than 1 year, and failed conservative management, including activity modification, night splinting, elbow pads, occupational therapy, and home exercise regimen. Electrodiagnostic testing was used at the discretion of the attending surgeon when the diagnosis was not clear from the history and physical examination. All fascial sling procedures were performed at our institution by 1 of 3 fellowship-trained hand surgeons, including Dr. Rosenwasser. The adipose flap modification was performed only by Dr. Rosenwasser. Of the 27 patients in the adipose flap group, 23 underwent surgery for primary CuTS and were included in the study; the other 4 (revision cases) were excluded; 1 patient subsequently died of a cause unrelated to the surgical procedure, and 6 were lost to follow-up. Of the 80 patients in the fascial sling group, 30 underwent surgery for primary CuTS; 5 died before follow-up, and 8 declined to participate.
Thirty-three patients (16 adipose flap, 17 fascial sling) met the inclusion criteria. Of the 16 adipose flap patients, 15 underwent the physical examination and completed the questionnaire, and 1 was interviewed by telephone. Similarly, of the 17 fascial sling patients, 15 underwent the physical examination and completed the questionnaire, and 2 were interviewed by telephone. There were no bilateral cases. Conservative management (activity modification, night splinting, elbow pads, occupational therapy, home exercise) failed in all cases.
A trained study team member who was not part of the surgical team performed follow-up evaluations using objective outcome measures and subjective questionnaires. Patients were assessed at a mean follow-up of 5.6 years (range, 1.6-15.9 years). Patients completed the DASH (Disabilities of the Arm, Shoulder, and Hand) questionnaire16 and visual analog scales (VASs) for pain, numbness, tingling, and weakness in the ulnar nerve distribution. They also rated the presence of night symptoms that were interfering with sleep. The Modified Bishop Rating Scale (MBRS) was used to quantify patient self-reported data17,18 (Figure 2). The MBRS measures overall satisfaction, symptom improvement, presence of residual symptoms, ability to engage in activities, work capability, and subjective changes in strength and sensibility.
In the physical examinations, we tested for Tinel, Wartenberg, and Froment signs; performed an elbow flexion test; and measured elbow range of motion for flexion and extension as well as forearm pronation and supination. We also evaluated lateral pinch strength and grip strength, using a Jamar hydraulic pinch gauge and a Jamar dynamometer (Therapeutic Equipment Corp) and taking the average of 3 assessments. Fifth-digit abduction strength was graded on a standard muscle strength scale. Two-point discrimination was measured at the middle, ring, and small digits of the operated and contralateral hands.19
Surgical Technique
Standard ulnar nerve decompression with anterior subcutaneous transposition and the following modifications were performed on all patients.20 A posteromedial incision parallel to the intermuscular septum was developed and the ulnar nerve identified. Minimizing stripping of the vascular mesentery, the dissection continued along the course of the nerve, and the medial intermuscular septum was excised to prevent secondary compression after transposition. The ulnar nerve was mobilized and transposed anterior to the medial epicondyle (Figure 3). For patients who received the fascial sling, a fascial sleeve was elevated from the flexor-pronator mass and sutured to the edge of the retinaculum securing the nerve. For patients who received the adipose flap, the flap with its vascular pedicle intact was elevated from the subcutaneous tissue of the anterior skin overlying the transposed nerve. The adipose tissue was sharply dissected in half while sufficient subcutaneous tissue was kept between the skin and the flap. A plane was developed based on an anterior adipose pedicle, which included a cutaneous artery and a vein that would supply the vascularized adipose flap. The flap was elevated and wrapped around the nerve without tension while the ulnar nerve was protected from being kinked by the construct. The flap was sutured to the anterior subcutaneous tissue to create a tunnel of adipose tissue surrounding the nerve along its length (Figure 4). The elbow was then flexed and extended to ensure free nerve gliding before wound closure.
The patient was allowed to move the elbow within the bulky dressings immediately after surgery. After 2 weeks, sutures were removed. Formal occupational therapy is not needed for these patients, except in the presence of significant weakness.
Results
As mentioned, the 2 groups were matched on demographics: age at time of surgery, sex, symptom duration, and length of follow-up (Table 1).
For the 16 adipose flap patients (Table 2), mean DASH score was 19.9 (range, 0-71.7). Seven of these patients reported upper extremity pain with a mean VAS score of 1.7 (range, 0-8); 4 patients reported pain in the wrist and fourth and fifth digits; only 1 patient reported pain that occasionally woke the patient from sleep. Constant numbness was present in 6 patients. Four patients reported constant mild tingling in the hand, and 11 reported intermittent tingling. Eleven patients (68.7%) reported operated-arm weakness with a mean VAS score of 3.4 (range, 0-8). In patients who had a physical examination, mean elbow flexion–extension arc of motion was 134° (range, 95°-150°), representing 99% of the motion of the contralateral arm. Mean pronation–supination arc was 174° (range, 150°-180°), accounting for 104% of the contralateral arm. Mean lateral pinch strength was 73% of the contralateral arm, and mean grip strength was 114% of the contralateral arm. The Tinel sign was present in 2 patients, the Froment sign was present in 3 patients, and the elbow flexion test was positive in 2 patients. No patient had a positive Wartenberg sign. On the MBRS, 10 patients had an excellent score, and 6 had a good score.
For the 17 fascial sling patients (Table 2), mean DASH score was 22.7 (range, 0-63.3). Three patients reported upper extremity pain with a mean VAS score of 1.4 (range, 0-7); 3 patients reported pain that occasionally woke them from sleep. Seven patients had constant numbness in the distribution of the ulnar nerve. Two patients had constant paresthesias, and 7 had intermittent paresthesias. Nine patients (52.9%) reported arm weakness with a mean VAS score of 2.5 (range, 0-8). Mean elbow flexion–extension arc of motion was 136° (range, 100°-150°), representing 100% of the contralateral arm. Mean pronation–supination arc was 187° (range, 155°-225°), accounting for 102% of the contralateral arm. Mean lateral pinch strength was 93% of the contralateral arm, and mean grip strength was 80% of the contralateral arm. The Tinel sign was present in 6 patients, the Froment sign in 3 patients, and the Wartenberg sign in 2 patients. The elbow flexion test was positive in 4 patients. On the MBRS, 10 patients had an excellent score, and 7 had a good score.
There was no recurrence of CuTS in either group. One adipose flap patient developed a wound infection that required reoperation.
Discussion
Ulnar neuropathy was described by Magee and Phalen21 in 1949 and termed cubital tunnel syndrome by Feindel and Stratford22 in 1958. Since then, numerous procedures, including in situ decompression, medial epicondylectomy, and endoscopic decompression,23,24 have been advocated for the treatment of this condition. In addition, anterior transposition, which involves securing the ulnar nerve in a submuscular, intramuscular, or subcutaneous sleeve,6 remains a popular option. Despite more than half a century of surgical treatment for this condition, there is no consensus about which procedure offers the best outcomes. Bartels and colleagues8 retrospectively reviewed surgical treatments for CuTS, examining 3148 arms over a 27-year period. They found simple decompression and anterior intramuscular transposition had the best results, followed by medial epicondylectomy and anterior subcutaneous transposition, with anterior submuscular transposition yielding the poorest outcomes. Despite these findings, the operative groups’ recurrence rates remained significant. These results were challenged in a 2008 meta-analysis5 that found no significant difference among simple decompression, subcutaneous transposition, and submuscular transposition and instead demonstrated trends toward better outcomes with anterior transposition. Osterman and Davis7 reported a 5% to 15% rate of unsatisfactory outcomes with anterior subcutaneous transposition, a popular technique used by surgeons at our institution.
The causes for failure or recurrence of ulnar neuropathy after surgical intervention are multifactorial and include preexisting medical conditions and improper operative technique. It is well established that failure to excise all 5 anatomical points of entrapment, or creation of new points of tension during surgery, leads to poor outcomes.12 Nevertheless, the contribution of perineural scarring to postoperative recurrent ulnar neuropathy is currently being recognized: Gabel and Amadio13 described postoperative fibrosis in one-third of their patients with surgically treated recurrent CuTS, Rogers and colleagues14 noted dense perineural fibrosis after intramuscular and subcutaneous transposition procedures, Filippi and colleagues11 cited serious epineural fibrosis and fibrosis around the ulnar nerve as the main findings in their study of 22 patients with recurrent ulnar neuropathy, and Vogel and colleagues12 found that 88% of their patients with persistent CuTS after surgery exhibited perineural scarring.
We think that use of a scar tissue barrier during ulnar nerve transposition reduces the incidence of cicatrix and produces better outcomes—a position largely echoed by the orthopedic community, as fascial, fasciocutaneous, free, and venous flaps have all been used for such purposes.25,26 Vein wrapping has demonstrated good recovery of a nerve after perineural scarring.27 Advocates of intramuscular transposition argue that their technique provides the nerve with a vascularized tunnel, as segmental vascular stripping is an inevitability in transposition. However, this technique increases the incidence of scarring and potential muscle damage.28,29 We think the pedicled adipofascial flap benefits the peripheral nerve by providing a scar tissue barrier and an optimal milieu for vascular regeneration. Kilic and colleagues15 demonstrated the regenerative effects of adipose tissue flaps on peripheral nerves after crush injuries in a rat model, and Strickland and colleagues30 retrospectively examined the effects of hypothenar fat flaps on recalcitrant carpal tunnel syndrome, showing excellent results for this procedure. It is hypothesized that adipose tissue provides not only adipose-derived stem cells but also a rich vascular bed on which nerves will regenerate.
For all patients in the present study, symptoms improved, though the adipose flap and fascial sling groups were not significantly different in their outcomes. We used the MBRS to quantify and compare the groups’ patient-rated outcomes. No statistically significant difference was found between the adipose flap and fascial sling groups. On the MBRS, excellent and good outcomes were reported by 62.5% and 37.5% of the adipose flap patients, respectively, and 59% and 41% of the fascial sling patients (Table 3). Likewise, objective measurements did not show a significant difference between the 2 interventions—indicating that, compared with the current standard of care, adipose flaps are more efficacious in securing the anteriorly transposed nerve.
Complications of the adipose flap technique are consistent with those reported for other techniques for anterior transposition of the ulnar nerve. The most common complication is hematoma, which can be avoided with meticulous hemostasis. Damage of the medial antebrachial cutaneous nerve or motor branches to the flexor carpi ulnaris has been reported for the fascial technique (we have not had such outcomes at our institution). Contraindications to the adipofascial technique include insufficient subcutaneous adipose tissue for covering the ulnar nerve.
This study was limited by its retrospective setup, which reduced access to preoperative objective and subjective data. The small sample size also limited our ability to demonstrate the advantageous effects of an adipofascial flap in preventing postoperative perineural scarring.
The adipose flap technique is a viable option for securing the anteriorly transposed ulnar nerve. Outcomes in this study demonstrated an efficacy comparable to that of the fascial sling technique. Symptoms resolve or improve, and the majority of patients are satisfied with long-term surgical outcomes. The adipofascial flap may have additional advantages, as it provides a pliable, vascular fat envelope mimicking the natural fatty environment of peripheral nerves.
1. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263-265.
2. Robertson C, Saratsiotis J. A review of compression ulnar neuropathy at the elbow. J Manipulative Physiol Ther. 2005;28(5):345.
3. Posner MA. Compressive ulnar neuropathies at the elbow: I. Etiology and diagnosis. J Am Acad Orthop Surg. 1998;6(5):282-288.
4. Piligian G, Herbert R, Hearns M, Dropkin J, Landsbergis P, Cherniack M. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
5. Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg Am. 2008;33(8):1314.e1-e12.
6. Soltani AM, Best MJ, Francis CS, Allan BJ, Panthaki ZJ. Trends in the surgical treatment of cubital tunnel syndrome: an analysis of the National Survey of Ambulatory Surgery database. J Hand Surg Am. 2013;38(8):1551-1556.
7. Osterman AL, Davis CA. Subcutaneous transposition of the ulnar nerve for treatment of cubital tunnel syndrome. Hand Clin. 1996;12(2):421-433.
8. Bartels RH, Menovsky T, Van Overbeeke JJ, Verhagen WI. Surgical management of ulnar nerve compression at the elbow: an analysis of the literature. J Neurosurg. 1998;89(5):722-727.
9. Seradge H, Owen W. Cubital tunnel release with medial epicondylectomy factors influencing the outcome. J Hand Surg Am. 1998;23(3):483-491.
10. Schnabl SM, Kisslinger F, Schramm A, et al. Subjective outcome, neurophysiological investigations, postoperative complications and recurrence rate of partial medial epicondylectomy in cubital tunnel syndrome. Arch Orthop Trauma Surg. 2011;131(8):1027-1033.
11. Filippi R, Charalampaki P, Reisch R, Koch D, Grunert P. Recurrent cubital tunnel syndrome. Etiology and treatment. Minim Invasive Neurosurg. 2001;44(4):197-201.
12. Vogel RB, Nossaman BC, Rayan GM. Revision anterior submuscular transposition of the ulnar nerve for failed subcutaneous transposition. Br J Plast Surg. 2004;57(4):311-316.
13. Gabel GT, Amadio PC. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. J Bone Joint Surg Am. 1990;72(2):213-219.
14. Rogers MR, Bergfield TG, Aulicino PL. The failed ulnar nerve transposition. Etiology and treatment. Clin Orthop. 1991;269:193-200.
15. Kilic A, Ojo B, Rajfer RA, et al. Effect of white adipose tissue flap and insulin-like growth factor-1 on nerve regeneration in rats. Microsurgery. 2013;33(5):367-375.
16. Ebersole GC, Davidge K, Damiano M, Mackinnon SE. Validity and responsiveness of the DASH questionnaire as an outcome measure following ulnar nerve transposition for cubital tunnel syndrome. Plast Reconstr Surg. 2013;132(1):81e-90e.
17. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
18. Dützmann S, Martin KD, Sobottka S, et al. Open vs retractor-endoscopic in situ decompression of the ulnar nerve in cubital tunnel syndrome: a retrospective cohort study. Neurosurgery. 2013;72(4):605-616.
19. Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg Am. 1987;12(5 pt 1):693-696.
20. Danoff JR, Lombardi JM, Rosenwasser MP. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J Hand Surg Am. 2014;39(3):552-555.
21. Magee RB, Phalen GS. Tardy ulnar palsy. Am J Surg. 1949;78(4):470-474.
22. Feindel W, Stratford J. Cubital tunnel compression in tardy ulnar palsy. Can Med Assoc J. 1958;78(5):351-353.
23. Tsai TM, Bonczar M, Tsuruta T, Syed SA. A new operative technique: cubital tunnel decompression with endoscopic assistance. Hand Clin. 1995;11(1):71-80.
24. Hoffmann R, Siemionow M. The endoscopic management of cubital tunnel syndrome. J Hand Surg Br. 2006;31(1):23-29.
25. Luchetti R, Riccio M, Papini Zorli I, Fairplay T. Protective coverage of the median nerve using fascial, fasciocutaneous or island flaps. Handchir Mikrochir Plast Chir. 2006;38(5):317-330.
26. Kokkalis ZT, Jain S, Sotereanos DG. Vein wrapping at cubital tunnel for ulnar nerve problems. J Shoulder Elbow Surg. 2010;19(2):91-97.
27. Masear VR, Colgin S. The treatment of epineural scarring with allograft vein wrapping. Hand Clin. 1996;12(4):773-779.
28. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
29. Lundborg G. Surgical treatment for ulnar nerve entrapment at the elbow. J Hand Surg Br. 1992;17(3):245-247.
30. Strickland JW, Idler RS, Lourie GM, Plancher KD. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J Hand Surg Am. 1996;21(5):840-848.
Compression of the ulnar nerve at the elbow, also referred to as cubital tunnel syndrome (CuTS), is the second most common peripheral nerve compression syndrome in the upper extremity.1,2 Although the ulnar nerve can be compressed at 5 different sites, including arcade of Struthers, medial intermuscular septum, medial epicondyle, and deep flexor aponeurosis, the cubital tunnel is most commonly affected.3 Patients typically present with paresthesias in the fourth and fifth digits and weakness of hand muscle intrinsics. Activity-related pain or pain at the medial elbow can also occur in more advanced pathology.4 It is estimated that conservative therapy fails and surgical intervention is required in up to 30% of patients with CuTS.1 Surgical approaches range from in situ decompression to transposition techniques, but there is no consensus in the orthopedic community as to which technique offers the best results. In a 2008 meta-analysis, Macadam and colleagues5 found no statistical differences in outcomes among the various surgical approaches. Nevertheless, subcutaneous transposition of the ulnar nerve at the elbow is a popular option.6
Despite the widespread success of surgical intervention for CuTS, persistent or recurrent pain occurs in 9.9% to 21.0% of cases.7-10 In addition, several investigators have cited perineural scarring as a major cause of recurrent symptoms after primary surgery.11-14 Filippi and colleagues11 noted that patients who required reoperation after primary anterior transposition had “serious epineural fibrosis and fibrosis around the transposed ulnar nerve.” At our institution, we have similarly found that scarring of the fascial sling around the ulnar nerve led to recurrence of CuTS within 4 months after initial surgery (Figure 1).
We therefore prefer to use a vascularized adipose flap to secure the anteriorly transposed ulnar nerve. This flap provides a pliable, vascularized adipose environment for the nerve, which helps reduce nerve adherence and may enhance nerve recovery.15 In the study reported here, we retrospectively reviewed the long-term outcomes of ulnar nerve anterior subcutaneous transposition secured with either an adipose flap or a fascial sling. We hypothesized that patients in the 2 groups (adipose flap, fascial sling) would have equivalent outcomes.
Materials and Methods
After obtaining institutional review board approval, we reviewed the medical and surgical records of 104 patients (107 limbs) who underwent transposition of the ulnar nerve secured with either an adipose flap (27 limbs) or a fascial sling (80 limbs) over a 14-year period. The fascial sling cohort was used as a comparison group, matched to the adipose flap cohort by sex, age at time of surgery, hand dominance, symptom duration, and length of follow-up (Table 1). Patients were indicated for surgery and were included in the study if they had a history and physical examination consistent with primary CuTS, symptom duration longer than 1 year, and failed conservative management, including activity modification, night splinting, elbow pads, occupational therapy, and home exercise regimen. Electrodiagnostic testing was used at the discretion of the attending surgeon when the diagnosis was not clear from the history and physical examination. All fascial sling procedures were performed at our institution by 1 of 3 fellowship-trained hand surgeons, including Dr. Rosenwasser. The adipose flap modification was performed only by Dr. Rosenwasser. Of the 27 patients in the adipose flap group, 23 underwent surgery for primary CuTS and were included in the study; the other 4 (revision cases) were excluded; 1 patient subsequently died of a cause unrelated to the surgical procedure, and 6 were lost to follow-up. Of the 80 patients in the fascial sling group, 30 underwent surgery for primary CuTS; 5 died before follow-up, and 8 declined to participate.
Thirty-three patients (16 adipose flap, 17 fascial sling) met the inclusion criteria. Of the 16 adipose flap patients, 15 underwent the physical examination and completed the questionnaire, and 1 was interviewed by telephone. Similarly, of the 17 fascial sling patients, 15 underwent the physical examination and completed the questionnaire, and 2 were interviewed by telephone. There were no bilateral cases. Conservative management (activity modification, night splinting, elbow pads, occupational therapy, home exercise) failed in all cases.
A trained study team member who was not part of the surgical team performed follow-up evaluations using objective outcome measures and subjective questionnaires. Patients were assessed at a mean follow-up of 5.6 years (range, 1.6-15.9 years). Patients completed the DASH (Disabilities of the Arm, Shoulder, and Hand) questionnaire16 and visual analog scales (VASs) for pain, numbness, tingling, and weakness in the ulnar nerve distribution. They also rated the presence of night symptoms that were interfering with sleep. The Modified Bishop Rating Scale (MBRS) was used to quantify patient self-reported data17,18 (Figure 2). The MBRS measures overall satisfaction, symptom improvement, presence of residual symptoms, ability to engage in activities, work capability, and subjective changes in strength and sensibility.
In the physical examinations, we tested for Tinel, Wartenberg, and Froment signs; performed an elbow flexion test; and measured elbow range of motion for flexion and extension as well as forearm pronation and supination. We also evaluated lateral pinch strength and grip strength, using a Jamar hydraulic pinch gauge and a Jamar dynamometer (Therapeutic Equipment Corp) and taking the average of 3 assessments. Fifth-digit abduction strength was graded on a standard muscle strength scale. Two-point discrimination was measured at the middle, ring, and small digits of the operated and contralateral hands.19
Surgical Technique
Standard ulnar nerve decompression with anterior subcutaneous transposition and the following modifications were performed on all patients.20 A posteromedial incision parallel to the intermuscular septum was developed and the ulnar nerve identified. Minimizing stripping of the vascular mesentery, the dissection continued along the course of the nerve, and the medial intermuscular septum was excised to prevent secondary compression after transposition. The ulnar nerve was mobilized and transposed anterior to the medial epicondyle (Figure 3). For patients who received the fascial sling, a fascial sleeve was elevated from the flexor-pronator mass and sutured to the edge of the retinaculum securing the nerve. For patients who received the adipose flap, the flap with its vascular pedicle intact was elevated from the subcutaneous tissue of the anterior skin overlying the transposed nerve. The adipose tissue was sharply dissected in half while sufficient subcutaneous tissue was kept between the skin and the flap. A plane was developed based on an anterior adipose pedicle, which included a cutaneous artery and a vein that would supply the vascularized adipose flap. The flap was elevated and wrapped around the nerve without tension while the ulnar nerve was protected from being kinked by the construct. The flap was sutured to the anterior subcutaneous tissue to create a tunnel of adipose tissue surrounding the nerve along its length (Figure 4). The elbow was then flexed and extended to ensure free nerve gliding before wound closure.
The patient was allowed to move the elbow within the bulky dressings immediately after surgery. After 2 weeks, sutures were removed. Formal occupational therapy is not needed for these patients, except in the presence of significant weakness.
Results
As mentioned, the 2 groups were matched on demographics: age at time of surgery, sex, symptom duration, and length of follow-up (Table 1).
For the 16 adipose flap patients (Table 2), mean DASH score was 19.9 (range, 0-71.7). Seven of these patients reported upper extremity pain with a mean VAS score of 1.7 (range, 0-8); 4 patients reported pain in the wrist and fourth and fifth digits; only 1 patient reported pain that occasionally woke the patient from sleep. Constant numbness was present in 6 patients. Four patients reported constant mild tingling in the hand, and 11 reported intermittent tingling. Eleven patients (68.7%) reported operated-arm weakness with a mean VAS score of 3.4 (range, 0-8). In patients who had a physical examination, mean elbow flexion–extension arc of motion was 134° (range, 95°-150°), representing 99% of the motion of the contralateral arm. Mean pronation–supination arc was 174° (range, 150°-180°), accounting for 104% of the contralateral arm. Mean lateral pinch strength was 73% of the contralateral arm, and mean grip strength was 114% of the contralateral arm. The Tinel sign was present in 2 patients, the Froment sign was present in 3 patients, and the elbow flexion test was positive in 2 patients. No patient had a positive Wartenberg sign. On the MBRS, 10 patients had an excellent score, and 6 had a good score.
For the 17 fascial sling patients (Table 2), mean DASH score was 22.7 (range, 0-63.3). Three patients reported upper extremity pain with a mean VAS score of 1.4 (range, 0-7); 3 patients reported pain that occasionally woke them from sleep. Seven patients had constant numbness in the distribution of the ulnar nerve. Two patients had constant paresthesias, and 7 had intermittent paresthesias. Nine patients (52.9%) reported arm weakness with a mean VAS score of 2.5 (range, 0-8). Mean elbow flexion–extension arc of motion was 136° (range, 100°-150°), representing 100% of the contralateral arm. Mean pronation–supination arc was 187° (range, 155°-225°), accounting for 102% of the contralateral arm. Mean lateral pinch strength was 93% of the contralateral arm, and mean grip strength was 80% of the contralateral arm. The Tinel sign was present in 6 patients, the Froment sign in 3 patients, and the Wartenberg sign in 2 patients. The elbow flexion test was positive in 4 patients. On the MBRS, 10 patients had an excellent score, and 7 had a good score.
There was no recurrence of CuTS in either group. One adipose flap patient developed a wound infection that required reoperation.
Discussion
Ulnar neuropathy was described by Magee and Phalen21 in 1949 and termed cubital tunnel syndrome by Feindel and Stratford22 in 1958. Since then, numerous procedures, including in situ decompression, medial epicondylectomy, and endoscopic decompression,23,24 have been advocated for the treatment of this condition. In addition, anterior transposition, which involves securing the ulnar nerve in a submuscular, intramuscular, or subcutaneous sleeve,6 remains a popular option. Despite more than half a century of surgical treatment for this condition, there is no consensus about which procedure offers the best outcomes. Bartels and colleagues8 retrospectively reviewed surgical treatments for CuTS, examining 3148 arms over a 27-year period. They found simple decompression and anterior intramuscular transposition had the best results, followed by medial epicondylectomy and anterior subcutaneous transposition, with anterior submuscular transposition yielding the poorest outcomes. Despite these findings, the operative groups’ recurrence rates remained significant. These results were challenged in a 2008 meta-analysis5 that found no significant difference among simple decompression, subcutaneous transposition, and submuscular transposition and instead demonstrated trends toward better outcomes with anterior transposition. Osterman and Davis7 reported a 5% to 15% rate of unsatisfactory outcomes with anterior subcutaneous transposition, a popular technique used by surgeons at our institution.
The causes for failure or recurrence of ulnar neuropathy after surgical intervention are multifactorial and include preexisting medical conditions and improper operative technique. It is well established that failure to excise all 5 anatomical points of entrapment, or creation of new points of tension during surgery, leads to poor outcomes.12 Nevertheless, the contribution of perineural scarring to postoperative recurrent ulnar neuropathy is currently being recognized: Gabel and Amadio13 described postoperative fibrosis in one-third of their patients with surgically treated recurrent CuTS, Rogers and colleagues14 noted dense perineural fibrosis after intramuscular and subcutaneous transposition procedures, Filippi and colleagues11 cited serious epineural fibrosis and fibrosis around the ulnar nerve as the main findings in their study of 22 patients with recurrent ulnar neuropathy, and Vogel and colleagues12 found that 88% of their patients with persistent CuTS after surgery exhibited perineural scarring.
We think that use of a scar tissue barrier during ulnar nerve transposition reduces the incidence of cicatrix and produces better outcomes—a position largely echoed by the orthopedic community, as fascial, fasciocutaneous, free, and venous flaps have all been used for such purposes.25,26 Vein wrapping has demonstrated good recovery of a nerve after perineural scarring.27 Advocates of intramuscular transposition argue that their technique provides the nerve with a vascularized tunnel, as segmental vascular stripping is an inevitability in transposition. However, this technique increases the incidence of scarring and potential muscle damage.28,29 We think the pedicled adipofascial flap benefits the peripheral nerve by providing a scar tissue barrier and an optimal milieu for vascular regeneration. Kilic and colleagues15 demonstrated the regenerative effects of adipose tissue flaps on peripheral nerves after crush injuries in a rat model, and Strickland and colleagues30 retrospectively examined the effects of hypothenar fat flaps on recalcitrant carpal tunnel syndrome, showing excellent results for this procedure. It is hypothesized that adipose tissue provides not only adipose-derived stem cells but also a rich vascular bed on which nerves will regenerate.
For all patients in the present study, symptoms improved, though the adipose flap and fascial sling groups were not significantly different in their outcomes. We used the MBRS to quantify and compare the groups’ patient-rated outcomes. No statistically significant difference was found between the adipose flap and fascial sling groups. On the MBRS, excellent and good outcomes were reported by 62.5% and 37.5% of the adipose flap patients, respectively, and 59% and 41% of the fascial sling patients (Table 3). Likewise, objective measurements did not show a significant difference between the 2 interventions—indicating that, compared with the current standard of care, adipose flaps are more efficacious in securing the anteriorly transposed nerve.
Complications of the adipose flap technique are consistent with those reported for other techniques for anterior transposition of the ulnar nerve. The most common complication is hematoma, which can be avoided with meticulous hemostasis. Damage of the medial antebrachial cutaneous nerve or motor branches to the flexor carpi ulnaris has been reported for the fascial technique (we have not had such outcomes at our institution). Contraindications to the adipofascial technique include insufficient subcutaneous adipose tissue for covering the ulnar nerve.
This study was limited by its retrospective setup, which reduced access to preoperative objective and subjective data. The small sample size also limited our ability to demonstrate the advantageous effects of an adipofascial flap in preventing postoperative perineural scarring.
The adipose flap technique is a viable option for securing the anteriorly transposed ulnar nerve. Outcomes in this study demonstrated an efficacy comparable to that of the fascial sling technique. Symptoms resolve or improve, and the majority of patients are satisfied with long-term surgical outcomes. The adipofascial flap may have additional advantages, as it provides a pliable, vascular fat envelope mimicking the natural fatty environment of peripheral nerves.
Compression of the ulnar nerve at the elbow, also referred to as cubital tunnel syndrome (CuTS), is the second most common peripheral nerve compression syndrome in the upper extremity.1,2 Although the ulnar nerve can be compressed at 5 different sites, including arcade of Struthers, medial intermuscular septum, medial epicondyle, and deep flexor aponeurosis, the cubital tunnel is most commonly affected.3 Patients typically present with paresthesias in the fourth and fifth digits and weakness of hand muscle intrinsics. Activity-related pain or pain at the medial elbow can also occur in more advanced pathology.4 It is estimated that conservative therapy fails and surgical intervention is required in up to 30% of patients with CuTS.1 Surgical approaches range from in situ decompression to transposition techniques, but there is no consensus in the orthopedic community as to which technique offers the best results. In a 2008 meta-analysis, Macadam and colleagues5 found no statistical differences in outcomes among the various surgical approaches. Nevertheless, subcutaneous transposition of the ulnar nerve at the elbow is a popular option.6
Despite the widespread success of surgical intervention for CuTS, persistent or recurrent pain occurs in 9.9% to 21.0% of cases.7-10 In addition, several investigators have cited perineural scarring as a major cause of recurrent symptoms after primary surgery.11-14 Filippi and colleagues11 noted that patients who required reoperation after primary anterior transposition had “serious epineural fibrosis and fibrosis around the transposed ulnar nerve.” At our institution, we have similarly found that scarring of the fascial sling around the ulnar nerve led to recurrence of CuTS within 4 months after initial surgery (Figure 1).
We therefore prefer to use a vascularized adipose flap to secure the anteriorly transposed ulnar nerve. This flap provides a pliable, vascularized adipose environment for the nerve, which helps reduce nerve adherence and may enhance nerve recovery.15 In the study reported here, we retrospectively reviewed the long-term outcomes of ulnar nerve anterior subcutaneous transposition secured with either an adipose flap or a fascial sling. We hypothesized that patients in the 2 groups (adipose flap, fascial sling) would have equivalent outcomes.
Materials and Methods
After obtaining institutional review board approval, we reviewed the medical and surgical records of 104 patients (107 limbs) who underwent transposition of the ulnar nerve secured with either an adipose flap (27 limbs) or a fascial sling (80 limbs) over a 14-year period. The fascial sling cohort was used as a comparison group, matched to the adipose flap cohort by sex, age at time of surgery, hand dominance, symptom duration, and length of follow-up (Table 1). Patients were indicated for surgery and were included in the study if they had a history and physical examination consistent with primary CuTS, symptom duration longer than 1 year, and failed conservative management, including activity modification, night splinting, elbow pads, occupational therapy, and home exercise regimen. Electrodiagnostic testing was used at the discretion of the attending surgeon when the diagnosis was not clear from the history and physical examination. All fascial sling procedures were performed at our institution by 1 of 3 fellowship-trained hand surgeons, including Dr. Rosenwasser. The adipose flap modification was performed only by Dr. Rosenwasser. Of the 27 patients in the adipose flap group, 23 underwent surgery for primary CuTS and were included in the study; the other 4 (revision cases) were excluded; 1 patient subsequently died of a cause unrelated to the surgical procedure, and 6 were lost to follow-up. Of the 80 patients in the fascial sling group, 30 underwent surgery for primary CuTS; 5 died before follow-up, and 8 declined to participate.
Thirty-three patients (16 adipose flap, 17 fascial sling) met the inclusion criteria. Of the 16 adipose flap patients, 15 underwent the physical examination and completed the questionnaire, and 1 was interviewed by telephone. Similarly, of the 17 fascial sling patients, 15 underwent the physical examination and completed the questionnaire, and 2 were interviewed by telephone. There were no bilateral cases. Conservative management (activity modification, night splinting, elbow pads, occupational therapy, home exercise) failed in all cases.
A trained study team member who was not part of the surgical team performed follow-up evaluations using objective outcome measures and subjective questionnaires. Patients were assessed at a mean follow-up of 5.6 years (range, 1.6-15.9 years). Patients completed the DASH (Disabilities of the Arm, Shoulder, and Hand) questionnaire16 and visual analog scales (VASs) for pain, numbness, tingling, and weakness in the ulnar nerve distribution. They also rated the presence of night symptoms that were interfering with sleep. The Modified Bishop Rating Scale (MBRS) was used to quantify patient self-reported data17,18 (Figure 2). The MBRS measures overall satisfaction, symptom improvement, presence of residual symptoms, ability to engage in activities, work capability, and subjective changes in strength and sensibility.
In the physical examinations, we tested for Tinel, Wartenberg, and Froment signs; performed an elbow flexion test; and measured elbow range of motion for flexion and extension as well as forearm pronation and supination. We also evaluated lateral pinch strength and grip strength, using a Jamar hydraulic pinch gauge and a Jamar dynamometer (Therapeutic Equipment Corp) and taking the average of 3 assessments. Fifth-digit abduction strength was graded on a standard muscle strength scale. Two-point discrimination was measured at the middle, ring, and small digits of the operated and contralateral hands.19
Surgical Technique
Standard ulnar nerve decompression with anterior subcutaneous transposition and the following modifications were performed on all patients.20 A posteromedial incision parallel to the intermuscular septum was developed and the ulnar nerve identified. Minimizing stripping of the vascular mesentery, the dissection continued along the course of the nerve, and the medial intermuscular septum was excised to prevent secondary compression after transposition. The ulnar nerve was mobilized and transposed anterior to the medial epicondyle (Figure 3). For patients who received the fascial sling, a fascial sleeve was elevated from the flexor-pronator mass and sutured to the edge of the retinaculum securing the nerve. For patients who received the adipose flap, the flap with its vascular pedicle intact was elevated from the subcutaneous tissue of the anterior skin overlying the transposed nerve. The adipose tissue was sharply dissected in half while sufficient subcutaneous tissue was kept between the skin and the flap. A plane was developed based on an anterior adipose pedicle, which included a cutaneous artery and a vein that would supply the vascularized adipose flap. The flap was elevated and wrapped around the nerve without tension while the ulnar nerve was protected from being kinked by the construct. The flap was sutured to the anterior subcutaneous tissue to create a tunnel of adipose tissue surrounding the nerve along its length (Figure 4). The elbow was then flexed and extended to ensure free nerve gliding before wound closure.
The patient was allowed to move the elbow within the bulky dressings immediately after surgery. After 2 weeks, sutures were removed. Formal occupational therapy is not needed for these patients, except in the presence of significant weakness.
Results
As mentioned, the 2 groups were matched on demographics: age at time of surgery, sex, symptom duration, and length of follow-up (Table 1).
For the 16 adipose flap patients (Table 2), mean DASH score was 19.9 (range, 0-71.7). Seven of these patients reported upper extremity pain with a mean VAS score of 1.7 (range, 0-8); 4 patients reported pain in the wrist and fourth and fifth digits; only 1 patient reported pain that occasionally woke the patient from sleep. Constant numbness was present in 6 patients. Four patients reported constant mild tingling in the hand, and 11 reported intermittent tingling. Eleven patients (68.7%) reported operated-arm weakness with a mean VAS score of 3.4 (range, 0-8). In patients who had a physical examination, mean elbow flexion–extension arc of motion was 134° (range, 95°-150°), representing 99% of the motion of the contralateral arm. Mean pronation–supination arc was 174° (range, 150°-180°), accounting for 104% of the contralateral arm. Mean lateral pinch strength was 73% of the contralateral arm, and mean grip strength was 114% of the contralateral arm. The Tinel sign was present in 2 patients, the Froment sign was present in 3 patients, and the elbow flexion test was positive in 2 patients. No patient had a positive Wartenberg sign. On the MBRS, 10 patients had an excellent score, and 6 had a good score.
For the 17 fascial sling patients (Table 2), mean DASH score was 22.7 (range, 0-63.3). Three patients reported upper extremity pain with a mean VAS score of 1.4 (range, 0-7); 3 patients reported pain that occasionally woke them from sleep. Seven patients had constant numbness in the distribution of the ulnar nerve. Two patients had constant paresthesias, and 7 had intermittent paresthesias. Nine patients (52.9%) reported arm weakness with a mean VAS score of 2.5 (range, 0-8). Mean elbow flexion–extension arc of motion was 136° (range, 100°-150°), representing 100% of the contralateral arm. Mean pronation–supination arc was 187° (range, 155°-225°), accounting for 102% of the contralateral arm. Mean lateral pinch strength was 93% of the contralateral arm, and mean grip strength was 80% of the contralateral arm. The Tinel sign was present in 6 patients, the Froment sign in 3 patients, and the Wartenberg sign in 2 patients. The elbow flexion test was positive in 4 patients. On the MBRS, 10 patients had an excellent score, and 7 had a good score.
There was no recurrence of CuTS in either group. One adipose flap patient developed a wound infection that required reoperation.
Discussion
Ulnar neuropathy was described by Magee and Phalen21 in 1949 and termed cubital tunnel syndrome by Feindel and Stratford22 in 1958. Since then, numerous procedures, including in situ decompression, medial epicondylectomy, and endoscopic decompression,23,24 have been advocated for the treatment of this condition. In addition, anterior transposition, which involves securing the ulnar nerve in a submuscular, intramuscular, or subcutaneous sleeve,6 remains a popular option. Despite more than half a century of surgical treatment for this condition, there is no consensus about which procedure offers the best outcomes. Bartels and colleagues8 retrospectively reviewed surgical treatments for CuTS, examining 3148 arms over a 27-year period. They found simple decompression and anterior intramuscular transposition had the best results, followed by medial epicondylectomy and anterior subcutaneous transposition, with anterior submuscular transposition yielding the poorest outcomes. Despite these findings, the operative groups’ recurrence rates remained significant. These results were challenged in a 2008 meta-analysis5 that found no significant difference among simple decompression, subcutaneous transposition, and submuscular transposition and instead demonstrated trends toward better outcomes with anterior transposition. Osterman and Davis7 reported a 5% to 15% rate of unsatisfactory outcomes with anterior subcutaneous transposition, a popular technique used by surgeons at our institution.
The causes for failure or recurrence of ulnar neuropathy after surgical intervention are multifactorial and include preexisting medical conditions and improper operative technique. It is well established that failure to excise all 5 anatomical points of entrapment, or creation of new points of tension during surgery, leads to poor outcomes.12 Nevertheless, the contribution of perineural scarring to postoperative recurrent ulnar neuropathy is currently being recognized: Gabel and Amadio13 described postoperative fibrosis in one-third of their patients with surgically treated recurrent CuTS, Rogers and colleagues14 noted dense perineural fibrosis after intramuscular and subcutaneous transposition procedures, Filippi and colleagues11 cited serious epineural fibrosis and fibrosis around the ulnar nerve as the main findings in their study of 22 patients with recurrent ulnar neuropathy, and Vogel and colleagues12 found that 88% of their patients with persistent CuTS after surgery exhibited perineural scarring.
We think that use of a scar tissue barrier during ulnar nerve transposition reduces the incidence of cicatrix and produces better outcomes—a position largely echoed by the orthopedic community, as fascial, fasciocutaneous, free, and venous flaps have all been used for such purposes.25,26 Vein wrapping has demonstrated good recovery of a nerve after perineural scarring.27 Advocates of intramuscular transposition argue that their technique provides the nerve with a vascularized tunnel, as segmental vascular stripping is an inevitability in transposition. However, this technique increases the incidence of scarring and potential muscle damage.28,29 We think the pedicled adipofascial flap benefits the peripheral nerve by providing a scar tissue barrier and an optimal milieu for vascular regeneration. Kilic and colleagues15 demonstrated the regenerative effects of adipose tissue flaps on peripheral nerves after crush injuries in a rat model, and Strickland and colleagues30 retrospectively examined the effects of hypothenar fat flaps on recalcitrant carpal tunnel syndrome, showing excellent results for this procedure. It is hypothesized that adipose tissue provides not only adipose-derived stem cells but also a rich vascular bed on which nerves will regenerate.
For all patients in the present study, symptoms improved, though the adipose flap and fascial sling groups were not significantly different in their outcomes. We used the MBRS to quantify and compare the groups’ patient-rated outcomes. No statistically significant difference was found between the adipose flap and fascial sling groups. On the MBRS, excellent and good outcomes were reported by 62.5% and 37.5% of the adipose flap patients, respectively, and 59% and 41% of the fascial sling patients (Table 3). Likewise, objective measurements did not show a significant difference between the 2 interventions—indicating that, compared with the current standard of care, adipose flaps are more efficacious in securing the anteriorly transposed nerve.
Complications of the adipose flap technique are consistent with those reported for other techniques for anterior transposition of the ulnar nerve. The most common complication is hematoma, which can be avoided with meticulous hemostasis. Damage of the medial antebrachial cutaneous nerve or motor branches to the flexor carpi ulnaris has been reported for the fascial technique (we have not had such outcomes at our institution). Contraindications to the adipofascial technique include insufficient subcutaneous adipose tissue for covering the ulnar nerve.
This study was limited by its retrospective setup, which reduced access to preoperative objective and subjective data. The small sample size also limited our ability to demonstrate the advantageous effects of an adipofascial flap in preventing postoperative perineural scarring.
The adipose flap technique is a viable option for securing the anteriorly transposed ulnar nerve. Outcomes in this study demonstrated an efficacy comparable to that of the fascial sling technique. Symptoms resolve or improve, and the majority of patients are satisfied with long-term surgical outcomes. The adipofascial flap may have additional advantages, as it provides a pliable, vascular fat envelope mimicking the natural fatty environment of peripheral nerves.
1. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263-265.
2. Robertson C, Saratsiotis J. A review of compression ulnar neuropathy at the elbow. J Manipulative Physiol Ther. 2005;28(5):345.
3. Posner MA. Compressive ulnar neuropathies at the elbow: I. Etiology and diagnosis. J Am Acad Orthop Surg. 1998;6(5):282-288.
4. Piligian G, Herbert R, Hearns M, Dropkin J, Landsbergis P, Cherniack M. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
5. Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg Am. 2008;33(8):1314.e1-e12.
6. Soltani AM, Best MJ, Francis CS, Allan BJ, Panthaki ZJ. Trends in the surgical treatment of cubital tunnel syndrome: an analysis of the National Survey of Ambulatory Surgery database. J Hand Surg Am. 2013;38(8):1551-1556.
7. Osterman AL, Davis CA. Subcutaneous transposition of the ulnar nerve for treatment of cubital tunnel syndrome. Hand Clin. 1996;12(2):421-433.
8. Bartels RH, Menovsky T, Van Overbeeke JJ, Verhagen WI. Surgical management of ulnar nerve compression at the elbow: an analysis of the literature. J Neurosurg. 1998;89(5):722-727.
9. Seradge H, Owen W. Cubital tunnel release with medial epicondylectomy factors influencing the outcome. J Hand Surg Am. 1998;23(3):483-491.
10. Schnabl SM, Kisslinger F, Schramm A, et al. Subjective outcome, neurophysiological investigations, postoperative complications and recurrence rate of partial medial epicondylectomy in cubital tunnel syndrome. Arch Orthop Trauma Surg. 2011;131(8):1027-1033.
11. Filippi R, Charalampaki P, Reisch R, Koch D, Grunert P. Recurrent cubital tunnel syndrome. Etiology and treatment. Minim Invasive Neurosurg. 2001;44(4):197-201.
12. Vogel RB, Nossaman BC, Rayan GM. Revision anterior submuscular transposition of the ulnar nerve for failed subcutaneous transposition. Br J Plast Surg. 2004;57(4):311-316.
13. Gabel GT, Amadio PC. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. J Bone Joint Surg Am. 1990;72(2):213-219.
14. Rogers MR, Bergfield TG, Aulicino PL. The failed ulnar nerve transposition. Etiology and treatment. Clin Orthop. 1991;269:193-200.
15. Kilic A, Ojo B, Rajfer RA, et al. Effect of white adipose tissue flap and insulin-like growth factor-1 on nerve regeneration in rats. Microsurgery. 2013;33(5):367-375.
16. Ebersole GC, Davidge K, Damiano M, Mackinnon SE. Validity and responsiveness of the DASH questionnaire as an outcome measure following ulnar nerve transposition for cubital tunnel syndrome. Plast Reconstr Surg. 2013;132(1):81e-90e.
17. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
18. Dützmann S, Martin KD, Sobottka S, et al. Open vs retractor-endoscopic in situ decompression of the ulnar nerve in cubital tunnel syndrome: a retrospective cohort study. Neurosurgery. 2013;72(4):605-616.
19. Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg Am. 1987;12(5 pt 1):693-696.
20. Danoff JR, Lombardi JM, Rosenwasser MP. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J Hand Surg Am. 2014;39(3):552-555.
21. Magee RB, Phalen GS. Tardy ulnar palsy. Am J Surg. 1949;78(4):470-474.
22. Feindel W, Stratford J. Cubital tunnel compression in tardy ulnar palsy. Can Med Assoc J. 1958;78(5):351-353.
23. Tsai TM, Bonczar M, Tsuruta T, Syed SA. A new operative technique: cubital tunnel decompression with endoscopic assistance. Hand Clin. 1995;11(1):71-80.
24. Hoffmann R, Siemionow M. The endoscopic management of cubital tunnel syndrome. J Hand Surg Br. 2006;31(1):23-29.
25. Luchetti R, Riccio M, Papini Zorli I, Fairplay T. Protective coverage of the median nerve using fascial, fasciocutaneous or island flaps. Handchir Mikrochir Plast Chir. 2006;38(5):317-330.
26. Kokkalis ZT, Jain S, Sotereanos DG. Vein wrapping at cubital tunnel for ulnar nerve problems. J Shoulder Elbow Surg. 2010;19(2):91-97.
27. Masear VR, Colgin S. The treatment of epineural scarring with allograft vein wrapping. Hand Clin. 1996;12(4):773-779.
28. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
29. Lundborg G. Surgical treatment for ulnar nerve entrapment at the elbow. J Hand Surg Br. 1992;17(3):245-247.
30. Strickland JW, Idler RS, Lourie GM, Plancher KD. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J Hand Surg Am. 1996;21(5):840-848.
1. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263-265.
2. Robertson C, Saratsiotis J. A review of compression ulnar neuropathy at the elbow. J Manipulative Physiol Ther. 2005;28(5):345.
3. Posner MA. Compressive ulnar neuropathies at the elbow: I. Etiology and diagnosis. J Am Acad Orthop Surg. 1998;6(5):282-288.
4. Piligian G, Herbert R, Hearns M, Dropkin J, Landsbergis P, Cherniack M. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
5. Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg Am. 2008;33(8):1314.e1-e12.
6. Soltani AM, Best MJ, Francis CS, Allan BJ, Panthaki ZJ. Trends in the surgical treatment of cubital tunnel syndrome: an analysis of the National Survey of Ambulatory Surgery database. J Hand Surg Am. 2013;38(8):1551-1556.
7. Osterman AL, Davis CA. Subcutaneous transposition of the ulnar nerve for treatment of cubital tunnel syndrome. Hand Clin. 1996;12(2):421-433.
8. Bartels RH, Menovsky T, Van Overbeeke JJ, Verhagen WI. Surgical management of ulnar nerve compression at the elbow: an analysis of the literature. J Neurosurg. 1998;89(5):722-727.
9. Seradge H, Owen W. Cubital tunnel release with medial epicondylectomy factors influencing the outcome. J Hand Surg Am. 1998;23(3):483-491.
10. Schnabl SM, Kisslinger F, Schramm A, et al. Subjective outcome, neurophysiological investigations, postoperative complications and recurrence rate of partial medial epicondylectomy in cubital tunnel syndrome. Arch Orthop Trauma Surg. 2011;131(8):1027-1033.
11. Filippi R, Charalampaki P, Reisch R, Koch D, Grunert P. Recurrent cubital tunnel syndrome. Etiology and treatment. Minim Invasive Neurosurg. 2001;44(4):197-201.
12. Vogel RB, Nossaman BC, Rayan GM. Revision anterior submuscular transposition of the ulnar nerve for failed subcutaneous transposition. Br J Plast Surg. 2004;57(4):311-316.
13. Gabel GT, Amadio PC. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. J Bone Joint Surg Am. 1990;72(2):213-219.
14. Rogers MR, Bergfield TG, Aulicino PL. The failed ulnar nerve transposition. Etiology and treatment. Clin Orthop. 1991;269:193-200.
15. Kilic A, Ojo B, Rajfer RA, et al. Effect of white adipose tissue flap and insulin-like growth factor-1 on nerve regeneration in rats. Microsurgery. 2013;33(5):367-375.
16. Ebersole GC, Davidge K, Damiano M, Mackinnon SE. Validity and responsiveness of the DASH questionnaire as an outcome measure following ulnar nerve transposition for cubital tunnel syndrome. Plast Reconstr Surg. 2013;132(1):81e-90e.
17. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
18. Dützmann S, Martin KD, Sobottka S, et al. Open vs retractor-endoscopic in situ decompression of the ulnar nerve in cubital tunnel syndrome: a retrospective cohort study. Neurosurgery. 2013;72(4):605-616.
19. Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg Am. 1987;12(5 pt 1):693-696.
20. Danoff JR, Lombardi JM, Rosenwasser MP. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J Hand Surg Am. 2014;39(3):552-555.
21. Magee RB, Phalen GS. Tardy ulnar palsy. Am J Surg. 1949;78(4):470-474.
22. Feindel W, Stratford J. Cubital tunnel compression in tardy ulnar palsy. Can Med Assoc J. 1958;78(5):351-353.
23. Tsai TM, Bonczar M, Tsuruta T, Syed SA. A new operative technique: cubital tunnel decompression with endoscopic assistance. Hand Clin. 1995;11(1):71-80.
24. Hoffmann R, Siemionow M. The endoscopic management of cubital tunnel syndrome. J Hand Surg Br. 2006;31(1):23-29.
25. Luchetti R, Riccio M, Papini Zorli I, Fairplay T. Protective coverage of the median nerve using fascial, fasciocutaneous or island flaps. Handchir Mikrochir Plast Chir. 2006;38(5):317-330.
26. Kokkalis ZT, Jain S, Sotereanos DG. Vein wrapping at cubital tunnel for ulnar nerve problems. J Shoulder Elbow Surg. 2010;19(2):91-97.
27. Masear VR, Colgin S. The treatment of epineural scarring with allograft vein wrapping. Hand Clin. 1996;12(4):773-779.
28. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
29. Lundborg G. Surgical treatment for ulnar nerve entrapment at the elbow. J Hand Surg Br. 1992;17(3):245-247.
30. Strickland JW, Idler RS, Lourie GM, Plancher KD. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J Hand Surg Am. 1996;21(5):840-848.