User login
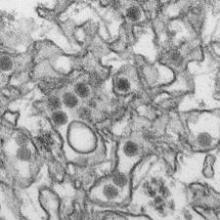
How p53 and telomeres stave off cancer

telomeres in green
Image by Claus Azzalin
Research published in The EMBO Journal suggests that p53 has tumor suppressor functions related to telomeres.
The study showed, for the first time, that p53 can suppress accumulated DNA damage at telomeres.
P53 is known to regulate the genome’s integrity. When DNA is damaged, p53 helps activate the transcription of genes that regulate the cell cycle and induce apoptosis.
However, prior studies have shown that p53 can bind at many locations across the genome, including sites that are not responsible for activating these regulatory genes.
Paul Lieberman, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues decided to study these binding sites to see if p53 and telomeres might be more closely related than previous research suggested.
“We believed that p53 may be responsible for a more direct protective effect in telomeres,” Dr Lieberman said.
Using ChIP-sequencing, he and his colleagues identified p53 binding sites in subtelomeres.
They found that when p53 was bound to subtelomeres, the protein was able to suppress the formation of a histone modification called γ-H2AX.
This histone is modified in greater amounts when there is a double-strand break on DNA. If it persists, the break is not repaired, so suppressing its expression means the DNA is being preserved.
Additionally, p53 was able to prevent DNA degradation in telomeres, thereby keeping them intact and allowing them to more properly protect the tips of chromosomes.
“Based on our findings, we propose that the modifications to chromatin made by p53 enhance local DNA repair or protection,” Dr Lieberman said. “This would be yet another tumor suppressor function of p53, thus providing additional framework for just how important this gene is in protecting us from cancer.” ![]()

telomeres in green
Image by Claus Azzalin
Research published in The EMBO Journal suggests that p53 has tumor suppressor functions related to telomeres.
The study showed, for the first time, that p53 can suppress accumulated DNA damage at telomeres.
P53 is known to regulate the genome’s integrity. When DNA is damaged, p53 helps activate the transcription of genes that regulate the cell cycle and induce apoptosis.
However, prior studies have shown that p53 can bind at many locations across the genome, including sites that are not responsible for activating these regulatory genes.
Paul Lieberman, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues decided to study these binding sites to see if p53 and telomeres might be more closely related than previous research suggested.
“We believed that p53 may be responsible for a more direct protective effect in telomeres,” Dr Lieberman said.
Using ChIP-sequencing, he and his colleagues identified p53 binding sites in subtelomeres.
They found that when p53 was bound to subtelomeres, the protein was able to suppress the formation of a histone modification called γ-H2AX.
This histone is modified in greater amounts when there is a double-strand break on DNA. If it persists, the break is not repaired, so suppressing its expression means the DNA is being preserved.
Additionally, p53 was able to prevent DNA degradation in telomeres, thereby keeping them intact and allowing them to more properly protect the tips of chromosomes.
“Based on our findings, we propose that the modifications to chromatin made by p53 enhance local DNA repair or protection,” Dr Lieberman said. “This would be yet another tumor suppressor function of p53, thus providing additional framework for just how important this gene is in protecting us from cancer.” ![]()

telomeres in green
Image by Claus Azzalin
Research published in The EMBO Journal suggests that p53 has tumor suppressor functions related to telomeres.
The study showed, for the first time, that p53 can suppress accumulated DNA damage at telomeres.
P53 is known to regulate the genome’s integrity. When DNA is damaged, p53 helps activate the transcription of genes that regulate the cell cycle and induce apoptosis.
However, prior studies have shown that p53 can bind at many locations across the genome, including sites that are not responsible for activating these regulatory genes.
Paul Lieberman, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues decided to study these binding sites to see if p53 and telomeres might be more closely related than previous research suggested.
“We believed that p53 may be responsible for a more direct protective effect in telomeres,” Dr Lieberman said.
Using ChIP-sequencing, he and his colleagues identified p53 binding sites in subtelomeres.
They found that when p53 was bound to subtelomeres, the protein was able to suppress the formation of a histone modification called γ-H2AX.
This histone is modified in greater amounts when there is a double-strand break on DNA. If it persists, the break is not repaired, so suppressing its expression means the DNA is being preserved.
Additionally, p53 was able to prevent DNA degradation in telomeres, thereby keeping them intact and allowing them to more properly protect the tips of chromosomes.
“Based on our findings, we propose that the modifications to chromatin made by p53 enhance local DNA repair or protection,” Dr Lieberman said. “This would be yet another tumor suppressor function of p53, thus providing additional framework for just how important this gene is in protecting us from cancer.” ![]()
A dermatologist’s bad dream?
My plane doesn’t leave till 2:30. Glad I cut off seeing patients at 11, which should give me plenty of time. I’m getting smarter in my old age.
Smooth morning, paperwork pretty much done. Just one patient left. Look, a nice little old man. He has such a sweet smile.
“How can I help you, Mr. Goldfarb?”
“It’s complicated. This letter explains everything that’s happened the past 3 years,”
Oh-oh, that doesn’t sound good. “OK, let’s have a look.”
“My, you read fast, Doctor.”
When the first line says, ‘The lice all over my body don’t go away even after I apply bug shampoo every day,’ I’m pretty much done.
“Doctor, this bag has everything I’ve used: lice shampoo, insect spray, itch lotion. I forgot to bring in all the little bugs I collected from my combs and sheets.”
No! This can’t be happening! How do I negotiate with a delusion and still make my plane?
“Sometimes it feels like bugs are crawling on my skin.”
“Itching often feels that way ... ”
“I brought pictures. Want to see?”
No! Not an album! Snap after snap: scabs on the scalp, scaling at the corners of the mouth, linear scratches on the extremities.
“You know, Mr. Goldfarb – maybe firm confidence will let me regain control of this interview – what you’re describing does not sound like lice or bugs of any kind ... ”
“But Doctor, how do you explain this?” Another photo, this of a comb filled with brownish epidermal fragments. “I meant to bring some in, but I forgot.”
Enough. Time to look grim and speak briskly. “Mr. Goldfarb, this cannot be lice because ... ”
“I see them coming out of all my pores ... ”
“Mr. Goldfarb!” Now it’s my turn to interrupt. “I would appreciate it if you would let me finish my sentence.”
“Yes, Doctor. If it’s not lice, what do you think it is?”
Must think fast. “Sensitivity. Sensitive skin, especially if you’ve scratched it, can certainly feel as though there are things crawling on you. Patients often say that the skin feels this way. I will therefore treat this sensitivity with anti-inflammatory creams and lotion you will apply to the scalp, face, and the rest of your body respectively.”
Goldfarb is still listening. I’m almost there.
“I want you to use this medication for 2 weeks without stopping, and not use any more of the bug shampoos and creams because they can be irritating and increase itch and sensitivity. Please call me on my private extension at that point with your progress.” Easier to deny a delusion when not standing face to face.
“That’s good news, Doctor. I’ll pick up the medication and let you know.”
At most, he’ll stop scratching for a while. By the time he starts again, I’ll have made my plane and come back to the office. Meantime: Depart exam room briskly!
I can still make it if I leave right away. One last check of my office e-mail. There’s one from Zelda. She has a small scaly patch on one forearm. Claims it’s responded neither to topical antifungals nor steroids.
Here’s the text of her e-mail: “Doctor, I showed my rash to my neighbor Mary. She did some Internet research, and she’s convinced it’s chromoblastomycosis. I’m pretty sure she’s right. What do you think?”
I think I better leave right now.
My reply: “Dear Zelda, pretty unlikely. Try the new cream I’m going to prescribe for 2 weeks, and let me check on how you’re doing.”
How does evil dermatologic karma know that I’m trying to leave town? Parasitosis and chromoblastomycosis! Can this be a bad dream? If so, why don’t I wake up?
Mr. Goldfarb, still looking sweet and mild, sits in the waiting room, awaiting the elder shuttle to take him home.
Walk fast. Do not smile and meet his gaze. This is no time for politeness.
No, sir. I am outta here.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
My plane doesn’t leave till 2:30. Glad I cut off seeing patients at 11, which should give me plenty of time. I’m getting smarter in my old age.
Smooth morning, paperwork pretty much done. Just one patient left. Look, a nice little old man. He has such a sweet smile.
“How can I help you, Mr. Goldfarb?”
“It’s complicated. This letter explains everything that’s happened the past 3 years,”
Oh-oh, that doesn’t sound good. “OK, let’s have a look.”
“My, you read fast, Doctor.”
When the first line says, ‘The lice all over my body don’t go away even after I apply bug shampoo every day,’ I’m pretty much done.
“Doctor, this bag has everything I’ve used: lice shampoo, insect spray, itch lotion. I forgot to bring in all the little bugs I collected from my combs and sheets.”
No! This can’t be happening! How do I negotiate with a delusion and still make my plane?
“Sometimes it feels like bugs are crawling on my skin.”
“Itching often feels that way ... ”
“I brought pictures. Want to see?”
No! Not an album! Snap after snap: scabs on the scalp, scaling at the corners of the mouth, linear scratches on the extremities.
“You know, Mr. Goldfarb – maybe firm confidence will let me regain control of this interview – what you’re describing does not sound like lice or bugs of any kind ... ”
“But Doctor, how do you explain this?” Another photo, this of a comb filled with brownish epidermal fragments. “I meant to bring some in, but I forgot.”
Enough. Time to look grim and speak briskly. “Mr. Goldfarb, this cannot be lice because ... ”
“I see them coming out of all my pores ... ”
“Mr. Goldfarb!” Now it’s my turn to interrupt. “I would appreciate it if you would let me finish my sentence.”
“Yes, Doctor. If it’s not lice, what do you think it is?”
Must think fast. “Sensitivity. Sensitive skin, especially if you’ve scratched it, can certainly feel as though there are things crawling on you. Patients often say that the skin feels this way. I will therefore treat this sensitivity with anti-inflammatory creams and lotion you will apply to the scalp, face, and the rest of your body respectively.”
Goldfarb is still listening. I’m almost there.
“I want you to use this medication for 2 weeks without stopping, and not use any more of the bug shampoos and creams because they can be irritating and increase itch and sensitivity. Please call me on my private extension at that point with your progress.” Easier to deny a delusion when not standing face to face.
“That’s good news, Doctor. I’ll pick up the medication and let you know.”
At most, he’ll stop scratching for a while. By the time he starts again, I’ll have made my plane and come back to the office. Meantime: Depart exam room briskly!
I can still make it if I leave right away. One last check of my office e-mail. There’s one from Zelda. She has a small scaly patch on one forearm. Claims it’s responded neither to topical antifungals nor steroids.
Here’s the text of her e-mail: “Doctor, I showed my rash to my neighbor Mary. She did some Internet research, and she’s convinced it’s chromoblastomycosis. I’m pretty sure she’s right. What do you think?”
I think I better leave right now.
My reply: “Dear Zelda, pretty unlikely. Try the new cream I’m going to prescribe for 2 weeks, and let me check on how you’re doing.”
How does evil dermatologic karma know that I’m trying to leave town? Parasitosis and chromoblastomycosis! Can this be a bad dream? If so, why don’t I wake up?
Mr. Goldfarb, still looking sweet and mild, sits in the waiting room, awaiting the elder shuttle to take him home.
Walk fast. Do not smile and meet his gaze. This is no time for politeness.
No, sir. I am outta here.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
My plane doesn’t leave till 2:30. Glad I cut off seeing patients at 11, which should give me plenty of time. I’m getting smarter in my old age.
Smooth morning, paperwork pretty much done. Just one patient left. Look, a nice little old man. He has such a sweet smile.
“How can I help you, Mr. Goldfarb?”
“It’s complicated. This letter explains everything that’s happened the past 3 years,”
Oh-oh, that doesn’t sound good. “OK, let’s have a look.”
“My, you read fast, Doctor.”
When the first line says, ‘The lice all over my body don’t go away even after I apply bug shampoo every day,’ I’m pretty much done.
“Doctor, this bag has everything I’ve used: lice shampoo, insect spray, itch lotion. I forgot to bring in all the little bugs I collected from my combs and sheets.”
No! This can’t be happening! How do I negotiate with a delusion and still make my plane?
“Sometimes it feels like bugs are crawling on my skin.”
“Itching often feels that way ... ”
“I brought pictures. Want to see?”
No! Not an album! Snap after snap: scabs on the scalp, scaling at the corners of the mouth, linear scratches on the extremities.
“You know, Mr. Goldfarb – maybe firm confidence will let me regain control of this interview – what you’re describing does not sound like lice or bugs of any kind ... ”
“But Doctor, how do you explain this?” Another photo, this of a comb filled with brownish epidermal fragments. “I meant to bring some in, but I forgot.”
Enough. Time to look grim and speak briskly. “Mr. Goldfarb, this cannot be lice because ... ”
“I see them coming out of all my pores ... ”
“Mr. Goldfarb!” Now it’s my turn to interrupt. “I would appreciate it if you would let me finish my sentence.”
“Yes, Doctor. If it’s not lice, what do you think it is?”
Must think fast. “Sensitivity. Sensitive skin, especially if you’ve scratched it, can certainly feel as though there are things crawling on you. Patients often say that the skin feels this way. I will therefore treat this sensitivity with anti-inflammatory creams and lotion you will apply to the scalp, face, and the rest of your body respectively.”
Goldfarb is still listening. I’m almost there.
“I want you to use this medication for 2 weeks without stopping, and not use any more of the bug shampoos and creams because they can be irritating and increase itch and sensitivity. Please call me on my private extension at that point with your progress.” Easier to deny a delusion when not standing face to face.
“That’s good news, Doctor. I’ll pick up the medication and let you know.”
At most, he’ll stop scratching for a while. By the time he starts again, I’ll have made my plane and come back to the office. Meantime: Depart exam room briskly!
I can still make it if I leave right away. One last check of my office e-mail. There’s one from Zelda. She has a small scaly patch on one forearm. Claims it’s responded neither to topical antifungals nor steroids.
Here’s the text of her e-mail: “Doctor, I showed my rash to my neighbor Mary. She did some Internet research, and she’s convinced it’s chromoblastomycosis. I’m pretty sure she’s right. What do you think?”
I think I better leave right now.
My reply: “Dear Zelda, pretty unlikely. Try the new cream I’m going to prescribe for 2 weeks, and let me check on how you’re doing.”
How does evil dermatologic karma know that I’m trying to leave town? Parasitosis and chromoblastomycosis! Can this be a bad dream? If so, why don’t I wake up?
Mr. Goldfarb, still looking sweet and mild, sits in the waiting room, awaiting the elder shuttle to take him home.
Walk fast. Do not smile and meet his gaze. This is no time for politeness.
No, sir. I am outta here.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
Zika virus: What clinicians must know
The recent spike in Zika virus cases in Central and South America brings with it the alarming risk – and even the expectation – of outbreaks occurring in the United States. How should U.S.-based clinicians prepare for the inevitable?
“The current outbreaks of Zika virus are the first of their kind in the Americas, so there isn’t a previous history of Zika virus spreading into the [United States],” explained Dr. Joy St. John, director of surveillance, disease prevention and control at the Caribbean Public Health Agency in Trinidad.
But now that the virus has hit the United States, with a confirmed case in Texas last week and more emerging since then, Dr. St. John said the most important thing is for U.S. health care providers to recognize the signs and symptoms of Zika virus infection. The virus is carried and transmitted by the Aedes aegypti species of mosquito, the same vector that transmits the dengue and chikungunya viruses. Zika virus symptoms are relatively mild, consisting predominantly of maculopapular rash, fever, arthralgia, myalgia, and conjunctivitis. Only one in five individuals with a Zika virus infection develop symptoms, but patients who present as such and who have traveled to Central or South America in the week prior to the onset of symptoms should be considered likely infected.
"At present, there is no rapid test available for diagnosis of Zika,” said Dr. St. John. “Diagnosis is primarily based on detection of viral RNA from clinical serum specimens in acutely ill patients.”
To that end, polymerase chain reaction (PCR) testing can be conducted on serum samples collected within 3-5 days of symptom onset. Beyond that, elevated levels of IgM antibodies can be confirmed by serology, based on the neutralization, seroconversion, or four-fold increase of Zika-specific antibodies in paired samples. However, Dr. St. John warned that “Due to the possibility of cross reactivity with other viruses, for example, dengue, it is strongly recommended samples be collected early enough for PCR testing.”
Zika and pregnancy
Zika virus has now been identified in more than 20 countries and territories worldwide, most of them in the Americas, although outbreaks have occurred in areas of Africa, Southeast Asia, and the Pacific Islands. While most infected patients experience relatively mild symptoms, Zika may be particularly dangerous when it infects a pregnant woman. There have been multiple cases of microcephaly in children whose mothers were infected with Zika virus during pregnancy, although the association of microcephaly with Zika virus infection during pregnancy has not been definitively confirmed. The Centers for Disease Control and Prevention recently issued a warning to Americans – particularly pregnant women – about traveling to high-risk areas.
“Scientifically, we’re not 100% sure if Zika virus is causing microcephaly, [but] what we’re seeing is in certain Brazilian districts, there’s been a 20-fold increase in rates of microcephaly at the same time that there’s been a lot more Zika virus in pregnant women,” explained Dr. Sanjaya Senanayake of Australian National University in Canberra.
According to data from the CDC, 1,248 suspected cases of microcephaly had been reported in Brazil as of Nov. 28, 2015, compared with the annual rate of just 150-200 such cases during 2010-2014. “Examination of the fetus [and] amniotic fluid, in some cases, has shown Zika virus, so there seems to be an association,” Dr. Senanayake clarified, adding that “the [ANVISA – Brazilian Health Surveillance Agency] has told women in certain districts where there’s been a lot of microcephaly not to get pregnant.”
Brazil is set to host millions of guests from around the world as the 2016 Olympics get underway in only a few months’ time. Women who are pregnant or anticipate becoming pregnant should consider the risks if they are planning to travel to Rio de Janeiro. The risk of microcephaly does not apply to infected women who are not pregnant, however, as the CDC states that “Zika virus usually remains in the blood of an infected person for only a few days to a week,” and therefore, “does not pose a risk of birth defects for future pregnancies.”
Dr. St. John also stated that “public health personnel are still cautioning pregnant women to take special care to avoid mosquito bites during their pregnancies,” adding that the “[Pan-American Health Organization] is working on their guidelines for surveillance of congenital abnormalities.”
Clinical insights
With treatment options so sparse – there is no vaccine or drug available specifically meant to combat a Zika virus infection – what can health care providers do for their patients? The CDC advises health care providers to “treat the symptoms,” which means telling patients to stay in bed, stay hydrated, and, most importantly, stay away from aspirin and NSAIDs “until dengue can be ruled out to reduce the risk of hemorrhage.” Acetaminophen is safe to use, in order to mitigate fever symptoms.
Those who are infected are also advised to stay indoors and remain as isolated as possible for at least a week after symptoms first present. While the risk of a domestic outbreak is probably low, Dr. St. John said, the more exposure a Zika virus–infected individual has to the outside world, the more likely he or she is to be bitten by another mosquito, which can then carry and transmit the virus to another person.
“Chikungunya and dengue virus, which are transmitted by the same vectors [as Zika virus], have not managed to establish ongoing transmission in the U.S., despite repeated importations, [so] it is likely that Zika virus’ spread would follow a similar pattern,” Dr. St. John noted.
Though rare, sexual transmission of Zika virus has also been found in at least one case, although it had been previously suspected for some time. In December 2013, a 44-year-old Tahitian man sought treatment for hematospermia. Analysis of his sperm, however, found Zika virus, indicating possible sexual transmission of the virus.
“The observation that [Zika virus] RNA was detectable in urine after viremia clearance in blood suggests that, as found for [dengue] and [West Nile virus] infections, urine samples can yield evidence of [Zika virus] for late diagnosis, but more investigation is needed,” the study concluded.
“The best way to control all this is to control the mosquito,” said Dr. Senanayake. “You get a four-for-one deal; not only do you get rid of Zika virus, but also chikungunya, dengue, and yellow fever.” Dr. Senanayake added that advanced research is currently underway in mosquito-control efforts, including the idea of releasing mosquitoes into the wild which have been genetically modified so they can’t breed.
Now that the Illinois Department of Health has confirmed two new cases of Zika virus infection in that state, with other new cases cropping up in Saint Martin and Guadeloupe and El Salvador, providers should remain vigilant, taking note of patients who have traveled to afflicted regions and show mosquito bites. Person-to-person transmission is “rare as hen’s teeth,” said Dr. Senanayake, which is to say, it is highly unlikely to occur. Nonetheless, he said information and communication is the best way to ensure that Zika virus does not spread widely in the United States.
*This story was updated 1/25/2016.
The recent spike in Zika virus cases in Central and South America brings with it the alarming risk – and even the expectation – of outbreaks occurring in the United States. How should U.S.-based clinicians prepare for the inevitable?
“The current outbreaks of Zika virus are the first of their kind in the Americas, so there isn’t a previous history of Zika virus spreading into the [United States],” explained Dr. Joy St. John, director of surveillance, disease prevention and control at the Caribbean Public Health Agency in Trinidad.
But now that the virus has hit the United States, with a confirmed case in Texas last week and more emerging since then, Dr. St. John said the most important thing is for U.S. health care providers to recognize the signs and symptoms of Zika virus infection. The virus is carried and transmitted by the Aedes aegypti species of mosquito, the same vector that transmits the dengue and chikungunya viruses. Zika virus symptoms are relatively mild, consisting predominantly of maculopapular rash, fever, arthralgia, myalgia, and conjunctivitis. Only one in five individuals with a Zika virus infection develop symptoms, but patients who present as such and who have traveled to Central or South America in the week prior to the onset of symptoms should be considered likely infected.
"At present, there is no rapid test available for diagnosis of Zika,” said Dr. St. John. “Diagnosis is primarily based on detection of viral RNA from clinical serum specimens in acutely ill patients.”
To that end, polymerase chain reaction (PCR) testing can be conducted on serum samples collected within 3-5 days of symptom onset. Beyond that, elevated levels of IgM antibodies can be confirmed by serology, based on the neutralization, seroconversion, or four-fold increase of Zika-specific antibodies in paired samples. However, Dr. St. John warned that “Due to the possibility of cross reactivity with other viruses, for example, dengue, it is strongly recommended samples be collected early enough for PCR testing.”
Zika and pregnancy
Zika virus has now been identified in more than 20 countries and territories worldwide, most of them in the Americas, although outbreaks have occurred in areas of Africa, Southeast Asia, and the Pacific Islands. While most infected patients experience relatively mild symptoms, Zika may be particularly dangerous when it infects a pregnant woman. There have been multiple cases of microcephaly in children whose mothers were infected with Zika virus during pregnancy, although the association of microcephaly with Zika virus infection during pregnancy has not been definitively confirmed. The Centers for Disease Control and Prevention recently issued a warning to Americans – particularly pregnant women – about traveling to high-risk areas.
“Scientifically, we’re not 100% sure if Zika virus is causing microcephaly, [but] what we’re seeing is in certain Brazilian districts, there’s been a 20-fold increase in rates of microcephaly at the same time that there’s been a lot more Zika virus in pregnant women,” explained Dr. Sanjaya Senanayake of Australian National University in Canberra.
According to data from the CDC, 1,248 suspected cases of microcephaly had been reported in Brazil as of Nov. 28, 2015, compared with the annual rate of just 150-200 such cases during 2010-2014. “Examination of the fetus [and] amniotic fluid, in some cases, has shown Zika virus, so there seems to be an association,” Dr. Senanayake clarified, adding that “the [ANVISA – Brazilian Health Surveillance Agency] has told women in certain districts where there’s been a lot of microcephaly not to get pregnant.”
Brazil is set to host millions of guests from around the world as the 2016 Olympics get underway in only a few months’ time. Women who are pregnant or anticipate becoming pregnant should consider the risks if they are planning to travel to Rio de Janeiro. The risk of microcephaly does not apply to infected women who are not pregnant, however, as the CDC states that “Zika virus usually remains in the blood of an infected person for only a few days to a week,” and therefore, “does not pose a risk of birth defects for future pregnancies.”
Dr. St. John also stated that “public health personnel are still cautioning pregnant women to take special care to avoid mosquito bites during their pregnancies,” adding that the “[Pan-American Health Organization] is working on their guidelines for surveillance of congenital abnormalities.”
Clinical insights
With treatment options so sparse – there is no vaccine or drug available specifically meant to combat a Zika virus infection – what can health care providers do for their patients? The CDC advises health care providers to “treat the symptoms,” which means telling patients to stay in bed, stay hydrated, and, most importantly, stay away from aspirin and NSAIDs “until dengue can be ruled out to reduce the risk of hemorrhage.” Acetaminophen is safe to use, in order to mitigate fever symptoms.
Those who are infected are also advised to stay indoors and remain as isolated as possible for at least a week after symptoms first present. While the risk of a domestic outbreak is probably low, Dr. St. John said, the more exposure a Zika virus–infected individual has to the outside world, the more likely he or she is to be bitten by another mosquito, which can then carry and transmit the virus to another person.
“Chikungunya and dengue virus, which are transmitted by the same vectors [as Zika virus], have not managed to establish ongoing transmission in the U.S., despite repeated importations, [so] it is likely that Zika virus’ spread would follow a similar pattern,” Dr. St. John noted.
Though rare, sexual transmission of Zika virus has also been found in at least one case, although it had been previously suspected for some time. In December 2013, a 44-year-old Tahitian man sought treatment for hematospermia. Analysis of his sperm, however, found Zika virus, indicating possible sexual transmission of the virus.
“The observation that [Zika virus] RNA was detectable in urine after viremia clearance in blood suggests that, as found for [dengue] and [West Nile virus] infections, urine samples can yield evidence of [Zika virus] for late diagnosis, but more investigation is needed,” the study concluded.
“The best way to control all this is to control the mosquito,” said Dr. Senanayake. “You get a four-for-one deal; not only do you get rid of Zika virus, but also chikungunya, dengue, and yellow fever.” Dr. Senanayake added that advanced research is currently underway in mosquito-control efforts, including the idea of releasing mosquitoes into the wild which have been genetically modified so they can’t breed.
Now that the Illinois Department of Health has confirmed two new cases of Zika virus infection in that state, with other new cases cropping up in Saint Martin and Guadeloupe and El Salvador, providers should remain vigilant, taking note of patients who have traveled to afflicted regions and show mosquito bites. Person-to-person transmission is “rare as hen’s teeth,” said Dr. Senanayake, which is to say, it is highly unlikely to occur. Nonetheless, he said information and communication is the best way to ensure that Zika virus does not spread widely in the United States.
*This story was updated 1/25/2016.
The recent spike in Zika virus cases in Central and South America brings with it the alarming risk – and even the expectation – of outbreaks occurring in the United States. How should U.S.-based clinicians prepare for the inevitable?
“The current outbreaks of Zika virus are the first of their kind in the Americas, so there isn’t a previous history of Zika virus spreading into the [United States],” explained Dr. Joy St. John, director of surveillance, disease prevention and control at the Caribbean Public Health Agency in Trinidad.
But now that the virus has hit the United States, with a confirmed case in Texas last week and more emerging since then, Dr. St. John said the most important thing is for U.S. health care providers to recognize the signs and symptoms of Zika virus infection. The virus is carried and transmitted by the Aedes aegypti species of mosquito, the same vector that transmits the dengue and chikungunya viruses. Zika virus symptoms are relatively mild, consisting predominantly of maculopapular rash, fever, arthralgia, myalgia, and conjunctivitis. Only one in five individuals with a Zika virus infection develop symptoms, but patients who present as such and who have traveled to Central or South America in the week prior to the onset of symptoms should be considered likely infected.
"At present, there is no rapid test available for diagnosis of Zika,” said Dr. St. John. “Diagnosis is primarily based on detection of viral RNA from clinical serum specimens in acutely ill patients.”
To that end, polymerase chain reaction (PCR) testing can be conducted on serum samples collected within 3-5 days of symptom onset. Beyond that, elevated levels of IgM antibodies can be confirmed by serology, based on the neutralization, seroconversion, or four-fold increase of Zika-specific antibodies in paired samples. However, Dr. St. John warned that “Due to the possibility of cross reactivity with other viruses, for example, dengue, it is strongly recommended samples be collected early enough for PCR testing.”
Zika and pregnancy
Zika virus has now been identified in more than 20 countries and territories worldwide, most of them in the Americas, although outbreaks have occurred in areas of Africa, Southeast Asia, and the Pacific Islands. While most infected patients experience relatively mild symptoms, Zika may be particularly dangerous when it infects a pregnant woman. There have been multiple cases of microcephaly in children whose mothers were infected with Zika virus during pregnancy, although the association of microcephaly with Zika virus infection during pregnancy has not been definitively confirmed. The Centers for Disease Control and Prevention recently issued a warning to Americans – particularly pregnant women – about traveling to high-risk areas.
“Scientifically, we’re not 100% sure if Zika virus is causing microcephaly, [but] what we’re seeing is in certain Brazilian districts, there’s been a 20-fold increase in rates of microcephaly at the same time that there’s been a lot more Zika virus in pregnant women,” explained Dr. Sanjaya Senanayake of Australian National University in Canberra.
According to data from the CDC, 1,248 suspected cases of microcephaly had been reported in Brazil as of Nov. 28, 2015, compared with the annual rate of just 150-200 such cases during 2010-2014. “Examination of the fetus [and] amniotic fluid, in some cases, has shown Zika virus, so there seems to be an association,” Dr. Senanayake clarified, adding that “the [ANVISA – Brazilian Health Surveillance Agency] has told women in certain districts where there’s been a lot of microcephaly not to get pregnant.”
Brazil is set to host millions of guests from around the world as the 2016 Olympics get underway in only a few months’ time. Women who are pregnant or anticipate becoming pregnant should consider the risks if they are planning to travel to Rio de Janeiro. The risk of microcephaly does not apply to infected women who are not pregnant, however, as the CDC states that “Zika virus usually remains in the blood of an infected person for only a few days to a week,” and therefore, “does not pose a risk of birth defects for future pregnancies.”
Dr. St. John also stated that “public health personnel are still cautioning pregnant women to take special care to avoid mosquito bites during their pregnancies,” adding that the “[Pan-American Health Organization] is working on their guidelines for surveillance of congenital abnormalities.”
Clinical insights
With treatment options so sparse – there is no vaccine or drug available specifically meant to combat a Zika virus infection – what can health care providers do for their patients? The CDC advises health care providers to “treat the symptoms,” which means telling patients to stay in bed, stay hydrated, and, most importantly, stay away from aspirin and NSAIDs “until dengue can be ruled out to reduce the risk of hemorrhage.” Acetaminophen is safe to use, in order to mitigate fever symptoms.
Those who are infected are also advised to stay indoors and remain as isolated as possible for at least a week after symptoms first present. While the risk of a domestic outbreak is probably low, Dr. St. John said, the more exposure a Zika virus–infected individual has to the outside world, the more likely he or she is to be bitten by another mosquito, which can then carry and transmit the virus to another person.
“Chikungunya and dengue virus, which are transmitted by the same vectors [as Zika virus], have not managed to establish ongoing transmission in the U.S., despite repeated importations, [so] it is likely that Zika virus’ spread would follow a similar pattern,” Dr. St. John noted.
Though rare, sexual transmission of Zika virus has also been found in at least one case, although it had been previously suspected for some time. In December 2013, a 44-year-old Tahitian man sought treatment for hematospermia. Analysis of his sperm, however, found Zika virus, indicating possible sexual transmission of the virus.
“The observation that [Zika virus] RNA was detectable in urine after viremia clearance in blood suggests that, as found for [dengue] and [West Nile virus] infections, urine samples can yield evidence of [Zika virus] for late diagnosis, but more investigation is needed,” the study concluded.
“The best way to control all this is to control the mosquito,” said Dr. Senanayake. “You get a four-for-one deal; not only do you get rid of Zika virus, but also chikungunya, dengue, and yellow fever.” Dr. Senanayake added that advanced research is currently underway in mosquito-control efforts, including the idea of releasing mosquitoes into the wild which have been genetically modified so they can’t breed.
Now that the Illinois Department of Health has confirmed two new cases of Zika virus infection in that state, with other new cases cropping up in Saint Martin and Guadeloupe and El Salvador, providers should remain vigilant, taking note of patients who have traveled to afflicted regions and show mosquito bites. Person-to-person transmission is “rare as hen’s teeth,” said Dr. Senanayake, which is to say, it is highly unlikely to occur. Nonetheless, he said information and communication is the best way to ensure that Zika virus does not spread widely in the United States.
*This story was updated 1/25/2016.
AACE: New algorithm stresses lifestyle modification in type 2 diabetes
Lifestyle modification must be the cornerstone of any management plan for type 2 diabetes.
Effectively attending to basic problems – obesity, nutrition, and exercise – will dramatically increase the success of any long-term treatment plan for patients with type 2 diabetes (T2DM), and help prevent or delay disease development in those with prediabetes, according to a new treatment algorithm.
The document, published by the American Association of Clinical Endocrinology, is an annual update to its unique strategy of clarifying T2DM management.
The 2016 AACE/ACE Comprehensive Diabetes Management Algorithm presents an easy-to-follow stepwise decision model for diagnosis, blood glucose management, and medical management – including all of the currently approved oral diabetes medications and insulins (Endocr Pract. 2016 Jan;22[1]:84-113). The original algorithm was launched 10 years ago and was last updated in 2013.
This new iteration is the first to place lifestyle intervention as a foundation for the most effective medical management.
“Obviously, this is very important,” said Dr. Paul S. Jellinger, a member of the algorithm writing committee, and an endocrinologist in Fort Lauderdale, Fla. “Weight loss, fitness training, and nutritional management play important roles in the management of blood glucose, lipids, and blood pressure. Appropriate focus in this direction may reduce medication dosage and at times eliminate the need for pharmaceutical intervention. We have all experienced improved therapeutic results in patients who are engaged in effective lifestyle therapy.”
As well as being a text document replete with data-driven details, the algorithm is presented in a colorful poster format that is very helpful for both doctors and patients alike, said Dr. George Grunberger, AACE president. It’s an especially effective tool when considering treatment decisions in the 12 different drug classes used in T2DM management.
“It’s one thing to talk about risk and benefits but to see it graphically displayed is very helpful,” he said in an interview. “I have the poster in every exam room, and every time I am with a patient, I can point out where we are and where we are heading. Rather than me just talking, I can show exactly where we are and where we want to go.”
It is especially helpful for primary care physicians, who care for the vast majority of patients with T2DM, Dr. Grunberger said in an interview.
“We are trying to get better management information into primary care. Most people with diabetes will never see a specialist in their entire life. They need help with glycemic control, obesity, prediabetes, dyslipidemia, and these are the bread and butter of primary care. But there are so many new things going on in this field, and primary care doctors are already in over their heads with the amount of things they deal with. So we have sorted it out and provide practical, practice-oriented guidelines about how to get from A to B.”
Dr. Grunberger noted that most of the recommendations in the document are based on expert opinion. “There are no randomized, controlled trials for most of this stuff. Many of the medications are relatively new, and with 12 drug classes, there’s no way you can ever do a trial with every permutation.”
The new document is similar to the 2013 algorithm with regard to medical management, Dr. Grunberger said. Its focus on lifestyle modification as an integral part of treatment is new, however. “The initial algorithm 10 years ago was solely based on glycemic control. Now we’ve decided to look at more than blood sugar – at obesity, overweight, hypertension and dyslipidemia. You cannot ignore these things in a disease where the major morbidity and mortality are cardiovascular.”
The algorithm stresses that “lifestyle optimization” is essential for all patients with diabetes. “[It] is multifaceted, ongoing, and should engage the entire diabetes team.”
There are several key components to lifestyle modification. All of these should be addressed early.
Medical nutrition therapy
This is a fundamental issue that must be addressed. A primarily plant-based diet high in poly- and monounsaturated fats is recommended, with the goal of a 5%-10% reduction in body weight for overweight or obese patients. In addition to discussing foods that damage and promote metabolic health, patients may need help with carbohydrate and sugar intake. Structured counseling is an excellent way to achieve consistent results.
Physical activity
Regular exercise improves glucose control and lowers lipid and blood pressure levels. It decreases the chance of falls and fractures, promotes functional capacity, and reduces the risk of depression. The goal should be at least 150 minutes of moderate-intense exercise each week. Every patient – and particularly those with complications of diabetes and/or obesity – should have a thorough physical exam before embarking on an exercise program.
Adequate rest
Emerging data continue to confirm the importance of sleep in health and disease. Getting 6-9 hours each night is associated with a reduction in cardiometabolic risk factors. Sleep deprivation aggravates insulin resistance, hypertension, hyperglycemia, and dyslipidemia and increases proinflammatory cytokines. An evaluation for obstructive sleep apnea may be in order, especially for obese patients.
Behavioral support
It’s impossible to overstate the importance of support in a successful lifestyle modification program. Patients should be encouraged to join community groups that facilitate and teach healthy behaviors. Not only will doing so help improve compliance, but being part of a structured group also reaps social and cognitive benefits.
Smoking cessation
The final component of the program, smoking cessation, is critical. All forms of tobacco should be eliminated.
While lifestyle modification is crucial, it should not obviate prompt medical therapy. “Such efforts should not delay needed pharmacotherapy, which can be initiated simultaneously and adjusted based on patient response to lifestyle efforts,” the document notes. “The need for medical therapy should not be interpreted as a failure of lifestyle management but as an adjunct to it.”
Aggressive medical therapy really accelerates effective diabetes treatment, Dr. Jellinger said.
“Clinical inertia has been and remains a huge problem. Some studies demonstrate as much as a 2-year delay in advancing therapy while the patient still remains far from hemoglobin A1c goal. For decades a ‘treat to failure’ concept dominated, i.e., that we should advance therapy only after a prolonged period of failure on existing therapy. One of the major contributions of the earlier AACE algorithms as well as the current version has been the strong therapeutic mandate to re-evaluate the patient and make a therapeutic change in no longer than 3 months. This is a direct attempt to eliminate clinical inertia.”
Dr. Grunberger agreed.
“Why do we wait until people are sick and experiencing complications before we take them seriously? Preventing and dealing with overweight and obesity is complicated, but if you treat obesity, you are treating diabetes. We emphasize starting medical therapy early, going to combination therapy quickly because no one drug usually achieves the target, and trying to be aggressive. Get people on the right treatment as quickly as possible and sustain success – don’t go from one failure to another.”
Dr. Jellinger has received support from Amarin, Boehringer Ingelheim, Bristol-Myers Squibb/AstraZeneca, Janssen Pharmaceuticals, and Novo Nordisk.
Dr. Grunberger has received remuneration and research funding from Eli Lilly, BI-Lilly, Novo Nordisk, Sanofi, Janssen, AstraZeneca, Merck, Medtronic, and GlaxoSmithKline.
Lifestyle modification must be the cornerstone of any management plan for type 2 diabetes.
Effectively attending to basic problems – obesity, nutrition, and exercise – will dramatically increase the success of any long-term treatment plan for patients with type 2 diabetes (T2DM), and help prevent or delay disease development in those with prediabetes, according to a new treatment algorithm.
The document, published by the American Association of Clinical Endocrinology, is an annual update to its unique strategy of clarifying T2DM management.
The 2016 AACE/ACE Comprehensive Diabetes Management Algorithm presents an easy-to-follow stepwise decision model for diagnosis, blood glucose management, and medical management – including all of the currently approved oral diabetes medications and insulins (Endocr Pract. 2016 Jan;22[1]:84-113). The original algorithm was launched 10 years ago and was last updated in 2013.
This new iteration is the first to place lifestyle intervention as a foundation for the most effective medical management.
“Obviously, this is very important,” said Dr. Paul S. Jellinger, a member of the algorithm writing committee, and an endocrinologist in Fort Lauderdale, Fla. “Weight loss, fitness training, and nutritional management play important roles in the management of blood glucose, lipids, and blood pressure. Appropriate focus in this direction may reduce medication dosage and at times eliminate the need for pharmaceutical intervention. We have all experienced improved therapeutic results in patients who are engaged in effective lifestyle therapy.”
As well as being a text document replete with data-driven details, the algorithm is presented in a colorful poster format that is very helpful for both doctors and patients alike, said Dr. George Grunberger, AACE president. It’s an especially effective tool when considering treatment decisions in the 12 different drug classes used in T2DM management.
“It’s one thing to talk about risk and benefits but to see it graphically displayed is very helpful,” he said in an interview. “I have the poster in every exam room, and every time I am with a patient, I can point out where we are and where we are heading. Rather than me just talking, I can show exactly where we are and where we want to go.”
It is especially helpful for primary care physicians, who care for the vast majority of patients with T2DM, Dr. Grunberger said in an interview.
“We are trying to get better management information into primary care. Most people with diabetes will never see a specialist in their entire life. They need help with glycemic control, obesity, prediabetes, dyslipidemia, and these are the bread and butter of primary care. But there are so many new things going on in this field, and primary care doctors are already in over their heads with the amount of things they deal with. So we have sorted it out and provide practical, practice-oriented guidelines about how to get from A to B.”
Dr. Grunberger noted that most of the recommendations in the document are based on expert opinion. “There are no randomized, controlled trials for most of this stuff. Many of the medications are relatively new, and with 12 drug classes, there’s no way you can ever do a trial with every permutation.”
The new document is similar to the 2013 algorithm with regard to medical management, Dr. Grunberger said. Its focus on lifestyle modification as an integral part of treatment is new, however. “The initial algorithm 10 years ago was solely based on glycemic control. Now we’ve decided to look at more than blood sugar – at obesity, overweight, hypertension and dyslipidemia. You cannot ignore these things in a disease where the major morbidity and mortality are cardiovascular.”
The algorithm stresses that “lifestyle optimization” is essential for all patients with diabetes. “[It] is multifaceted, ongoing, and should engage the entire diabetes team.”
There are several key components to lifestyle modification. All of these should be addressed early.
Medical nutrition therapy
This is a fundamental issue that must be addressed. A primarily plant-based diet high in poly- and monounsaturated fats is recommended, with the goal of a 5%-10% reduction in body weight for overweight or obese patients. In addition to discussing foods that damage and promote metabolic health, patients may need help with carbohydrate and sugar intake. Structured counseling is an excellent way to achieve consistent results.
Physical activity
Regular exercise improves glucose control and lowers lipid and blood pressure levels. It decreases the chance of falls and fractures, promotes functional capacity, and reduces the risk of depression. The goal should be at least 150 minutes of moderate-intense exercise each week. Every patient – and particularly those with complications of diabetes and/or obesity – should have a thorough physical exam before embarking on an exercise program.
Adequate rest
Emerging data continue to confirm the importance of sleep in health and disease. Getting 6-9 hours each night is associated with a reduction in cardiometabolic risk factors. Sleep deprivation aggravates insulin resistance, hypertension, hyperglycemia, and dyslipidemia and increases proinflammatory cytokines. An evaluation for obstructive sleep apnea may be in order, especially for obese patients.
Behavioral support
It’s impossible to overstate the importance of support in a successful lifestyle modification program. Patients should be encouraged to join community groups that facilitate and teach healthy behaviors. Not only will doing so help improve compliance, but being part of a structured group also reaps social and cognitive benefits.
Smoking cessation
The final component of the program, smoking cessation, is critical. All forms of tobacco should be eliminated.
While lifestyle modification is crucial, it should not obviate prompt medical therapy. “Such efforts should not delay needed pharmacotherapy, which can be initiated simultaneously and adjusted based on patient response to lifestyle efforts,” the document notes. “The need for medical therapy should not be interpreted as a failure of lifestyle management but as an adjunct to it.”
Aggressive medical therapy really accelerates effective diabetes treatment, Dr. Jellinger said.
“Clinical inertia has been and remains a huge problem. Some studies demonstrate as much as a 2-year delay in advancing therapy while the patient still remains far from hemoglobin A1c goal. For decades a ‘treat to failure’ concept dominated, i.e., that we should advance therapy only after a prolonged period of failure on existing therapy. One of the major contributions of the earlier AACE algorithms as well as the current version has been the strong therapeutic mandate to re-evaluate the patient and make a therapeutic change in no longer than 3 months. This is a direct attempt to eliminate clinical inertia.”
Dr. Grunberger agreed.
“Why do we wait until people are sick and experiencing complications before we take them seriously? Preventing and dealing with overweight and obesity is complicated, but if you treat obesity, you are treating diabetes. We emphasize starting medical therapy early, going to combination therapy quickly because no one drug usually achieves the target, and trying to be aggressive. Get people on the right treatment as quickly as possible and sustain success – don’t go from one failure to another.”
Dr. Jellinger has received support from Amarin, Boehringer Ingelheim, Bristol-Myers Squibb/AstraZeneca, Janssen Pharmaceuticals, and Novo Nordisk.
Dr. Grunberger has received remuneration and research funding from Eli Lilly, BI-Lilly, Novo Nordisk, Sanofi, Janssen, AstraZeneca, Merck, Medtronic, and GlaxoSmithKline.
Lifestyle modification must be the cornerstone of any management plan for type 2 diabetes.
Effectively attending to basic problems – obesity, nutrition, and exercise – will dramatically increase the success of any long-term treatment plan for patients with type 2 diabetes (T2DM), and help prevent or delay disease development in those with prediabetes, according to a new treatment algorithm.
The document, published by the American Association of Clinical Endocrinology, is an annual update to its unique strategy of clarifying T2DM management.
The 2016 AACE/ACE Comprehensive Diabetes Management Algorithm presents an easy-to-follow stepwise decision model for diagnosis, blood glucose management, and medical management – including all of the currently approved oral diabetes medications and insulins (Endocr Pract. 2016 Jan;22[1]:84-113). The original algorithm was launched 10 years ago and was last updated in 2013.
This new iteration is the first to place lifestyle intervention as a foundation for the most effective medical management.
“Obviously, this is very important,” said Dr. Paul S. Jellinger, a member of the algorithm writing committee, and an endocrinologist in Fort Lauderdale, Fla. “Weight loss, fitness training, and nutritional management play important roles in the management of blood glucose, lipids, and blood pressure. Appropriate focus in this direction may reduce medication dosage and at times eliminate the need for pharmaceutical intervention. We have all experienced improved therapeutic results in patients who are engaged in effective lifestyle therapy.”
As well as being a text document replete with data-driven details, the algorithm is presented in a colorful poster format that is very helpful for both doctors and patients alike, said Dr. George Grunberger, AACE president. It’s an especially effective tool when considering treatment decisions in the 12 different drug classes used in T2DM management.
“It’s one thing to talk about risk and benefits but to see it graphically displayed is very helpful,” he said in an interview. “I have the poster in every exam room, and every time I am with a patient, I can point out where we are and where we are heading. Rather than me just talking, I can show exactly where we are and where we want to go.”
It is especially helpful for primary care physicians, who care for the vast majority of patients with T2DM, Dr. Grunberger said in an interview.
“We are trying to get better management information into primary care. Most people with diabetes will never see a specialist in their entire life. They need help with glycemic control, obesity, prediabetes, dyslipidemia, and these are the bread and butter of primary care. But there are so many new things going on in this field, and primary care doctors are already in over their heads with the amount of things they deal with. So we have sorted it out and provide practical, practice-oriented guidelines about how to get from A to B.”
Dr. Grunberger noted that most of the recommendations in the document are based on expert opinion. “There are no randomized, controlled trials for most of this stuff. Many of the medications are relatively new, and with 12 drug classes, there’s no way you can ever do a trial with every permutation.”
The new document is similar to the 2013 algorithm with regard to medical management, Dr. Grunberger said. Its focus on lifestyle modification as an integral part of treatment is new, however. “The initial algorithm 10 years ago was solely based on glycemic control. Now we’ve decided to look at more than blood sugar – at obesity, overweight, hypertension and dyslipidemia. You cannot ignore these things in a disease where the major morbidity and mortality are cardiovascular.”
The algorithm stresses that “lifestyle optimization” is essential for all patients with diabetes. “[It] is multifaceted, ongoing, and should engage the entire diabetes team.”
There are several key components to lifestyle modification. All of these should be addressed early.
Medical nutrition therapy
This is a fundamental issue that must be addressed. A primarily plant-based diet high in poly- and monounsaturated fats is recommended, with the goal of a 5%-10% reduction in body weight for overweight or obese patients. In addition to discussing foods that damage and promote metabolic health, patients may need help with carbohydrate and sugar intake. Structured counseling is an excellent way to achieve consistent results.
Physical activity
Regular exercise improves glucose control and lowers lipid and blood pressure levels. It decreases the chance of falls and fractures, promotes functional capacity, and reduces the risk of depression. The goal should be at least 150 minutes of moderate-intense exercise each week. Every patient – and particularly those with complications of diabetes and/or obesity – should have a thorough physical exam before embarking on an exercise program.
Adequate rest
Emerging data continue to confirm the importance of sleep in health and disease. Getting 6-9 hours each night is associated with a reduction in cardiometabolic risk factors. Sleep deprivation aggravates insulin resistance, hypertension, hyperglycemia, and dyslipidemia and increases proinflammatory cytokines. An evaluation for obstructive sleep apnea may be in order, especially for obese patients.
Behavioral support
It’s impossible to overstate the importance of support in a successful lifestyle modification program. Patients should be encouraged to join community groups that facilitate and teach healthy behaviors. Not only will doing so help improve compliance, but being part of a structured group also reaps social and cognitive benefits.
Smoking cessation
The final component of the program, smoking cessation, is critical. All forms of tobacco should be eliminated.
While lifestyle modification is crucial, it should not obviate prompt medical therapy. “Such efforts should not delay needed pharmacotherapy, which can be initiated simultaneously and adjusted based on patient response to lifestyle efforts,” the document notes. “The need for medical therapy should not be interpreted as a failure of lifestyle management but as an adjunct to it.”
Aggressive medical therapy really accelerates effective diabetes treatment, Dr. Jellinger said.
“Clinical inertia has been and remains a huge problem. Some studies demonstrate as much as a 2-year delay in advancing therapy while the patient still remains far from hemoglobin A1c goal. For decades a ‘treat to failure’ concept dominated, i.e., that we should advance therapy only after a prolonged period of failure on existing therapy. One of the major contributions of the earlier AACE algorithms as well as the current version has been the strong therapeutic mandate to re-evaluate the patient and make a therapeutic change in no longer than 3 months. This is a direct attempt to eliminate clinical inertia.”
Dr. Grunberger agreed.
“Why do we wait until people are sick and experiencing complications before we take them seriously? Preventing and dealing with overweight and obesity is complicated, but if you treat obesity, you are treating diabetes. We emphasize starting medical therapy early, going to combination therapy quickly because no one drug usually achieves the target, and trying to be aggressive. Get people on the right treatment as quickly as possible and sustain success – don’t go from one failure to another.”
Dr. Jellinger has received support from Amarin, Boehringer Ingelheim, Bristol-Myers Squibb/AstraZeneca, Janssen Pharmaceuticals, and Novo Nordisk.
Dr. Grunberger has received remuneration and research funding from Eli Lilly, BI-Lilly, Novo Nordisk, Sanofi, Janssen, AstraZeneca, Merck, Medtronic, and GlaxoSmithKline.
Caring for gender-nonconforming youth in a primary care setting – Part 2
Gender identity typically develops in early childhood, and by age 4 years, most children consistently refer to themselves as a girl or a boy.1 For the majority of children, natal sex or sex assigned at birth, aligns with gender identity (a person’s innate sense of feeling male, female, or somewhere in between). However, this is not always the case. Gender identity can be understood as a spectrum with youth identifying as a gender that aligns with their natal sex (cisgender), is opposite of their natal sex (transgender), no gender (agender), or somewhere in between (genderqueer). The distress that can result from an incongruence between natal sex and gender identity is called gender dysphoria. Youth with gender dysphoria are at increased risk for a number of conditions, including suicide and self-harm. Early identification and appropriate care of these youth can reduce these risks. This month’s column will briefly review assessment of these youth in the pediatric setting.
Many youth who have a gender-nonconforming identity in childhood will not go on to have one in adulthood.2,3 Those who have a consistent, insistent, and persistent nonconforming identity are more likely to have this identity persist into adulthood. Youth who experience increased gender dysphoria with the onset of puberty rarely have this subside.
As it can be difficult to predict the trajectory of gender identity from childhood to adolescence, the approach to the prepubertal and pubertal gender nonconforming patient is different. It is important to note that research suggests that gender identity is innate and cannot be changed with interventions. The goals of care for gender-nonconforming (GN) youth include providing a safe environment where youth can explore their identities, and individualizing treatment to meet the needs of each patient and family.
Care for prepubertal GN youth
For parents:
Have you noticed, or are you concerned about your child’s:
• Preference or rejection of particular toys/games?
• Hair and clothing preferences or rejections?
• Preferred (if any) gender of playmates?
Has your child ever expressed:
• A desire to be or insistence that they are the other gender?
• A dislike of their sexual anatomy?
• A desire for primary (penis, vagina) or secondary (periods, facial hair) sex characteristics of the other gender?
Are you concerned about bullying ?
Do you have any concerns about your child’s mood or concerns for self-harm?
For children:
• Do you feel more like a girl, boy, neither, both?
• How would you like to play, cut your hair, dress?
• What name or pronoun (she for girl, he for boy) fits you?4
The goal for prepubertal youth with nonconforming identities is to ensure that they are safe at home, school, and at play. Some youth may express a desire to “transition” or live as their identified gender by changing their name and dressing as their identified gender. Some youth and families may choose to transition only in certain settings (at home, but not at school). Some youth and families may want a safe space where the child can grow, develop, and continue to explore their identity without transitioning. Mental health providers trained in the care of GN youth can help patients and families decide if transition is appropriate for them and support them with the process and timing of transitioning. For youth who experience depression, anxiety, bullying, or thoughts of self-harm related to their gender identity, care by an experienced mental health provider is essential. It is important to recognize that each patient and family will need an individualized approach based on their needs.
Care for pubertal GN youth
The development of secondary sex characteristics can be particularly distressing for GN youth. Some youth may first experience gender dysphoria at this time. This distress combined with the psychosocial stressors of adolescent development can lead to depression, anxiety, suicidal ideation, self-harm, and other risk taking behaviors. Visits with pubertal GN youth, as with any adolescent, should include confidential time alone with the medical provider to discuss any concerns. Youth should be informed that information will be kept confidential, but parents will need to be notified of any safety concerns (such as suicidality or self-harm). As with prepubertal youth, a history related to hair and clothing preferences; distress related to genital anatomy; and the desire to be the other gender should be obtained. A pubertal history and any related symptoms of distress also should be obtained.
DO
• Ask preferred name and pronoun.
• Perform confidential strength and risk assessment.
• Assess for family and social support.
• Refer to appropriate mental health and transgender providers.
DON’T
• Assume names and pronouns.
• Interview patient only with parent in the room.
• Disclose identity without patient consent.
• Dismiss parents as sources of support.
• Refer for reparative therapy.4
Youth who are suspected to have a diagnosis of gender dysphoria should be referred to mental health and medical providers with experience caring for transgender youth. These specialists can work with patients and families, and determine when and if youth are eligible for puberty blocking therapy with GnRH analogues and/or hormone therapy. GnRH analogues, if appropriate, can be prescribed after patients have reached sexual maturity rating stage 2. The rationale for this treatment is to prevent the development of unwanted secondary sex characteristics while giving the youth a chance to continue with psychotherapy and explore their gender identity.5 Hormone therapy, if appropriate, can be prescribed a few years later under the care of a transgender specialist and mental health provider.
Summary
It is normal to experiment with gender roles and expression in childhood. Providing a safe space to do this is important.
Individuals who have a persistent, consistent, and insistent gender-nonconforming identification and who have increased distress with puberty are unlikely to have this subside.
Pediatricians can assess for gender dysphoria and screen for related mood disorders and behaviors in the primary care setting. Appropriate referral to trained professionals is important.
Care should be individualized and focused on the health and safety of the patient.
Resources
For health care professionals
• World Professional Association for Transgender Health: Standards of care on care of transgender patients and provider directory. www.wpath.org• Physicians for Reproductive Health’s adolescent reproductive and sexual health education program (ARSHEP): Best practices for adolescent and reproductive health: Module on caring for transgender adolescent patients. prh.org/teen-reproductive-health/arshep-downloads/
For patients and families
• Family Acceptance Project: familyproject.sfsu.edu/
• Healthychildren.org: Parenting website supported by the American Academy of Pediatrics. Links to articles on gender nonconforming and transgender children; gender identity development in children. www.healthychildren.org
References
1. Caring for Your School Age Child: Ages 5-12 by the American Academy of Pediatrics (New York: Bantam Books, 1995).
2. Dev Psychol. 2008 Jan;44(1):34-45.
3. J Am Acad Child and Adolesc Psychiatry. 2008;47(12):1413-23
4. Caring for Transgender Adolescent Patients. Physicians for Reproductive Health’s Adolescent Reproductive and Sexual Health Education Program (ARSHEP): Best practices for adolescent and reproductive health: prh.org/teen-reproductive-health/arshep-downloads/
5. World Professional Association of Transgender Health, Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, 7th Edition (International Journal of Transgenderism. 2011;13:165-232)
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
Gender identity typically develops in early childhood, and by age 4 years, most children consistently refer to themselves as a girl or a boy.1 For the majority of children, natal sex or sex assigned at birth, aligns with gender identity (a person’s innate sense of feeling male, female, or somewhere in between). However, this is not always the case. Gender identity can be understood as a spectrum with youth identifying as a gender that aligns with their natal sex (cisgender), is opposite of their natal sex (transgender), no gender (agender), or somewhere in between (genderqueer). The distress that can result from an incongruence between natal sex and gender identity is called gender dysphoria. Youth with gender dysphoria are at increased risk for a number of conditions, including suicide and self-harm. Early identification and appropriate care of these youth can reduce these risks. This month’s column will briefly review assessment of these youth in the pediatric setting.
Many youth who have a gender-nonconforming identity in childhood will not go on to have one in adulthood.2,3 Those who have a consistent, insistent, and persistent nonconforming identity are more likely to have this identity persist into adulthood. Youth who experience increased gender dysphoria with the onset of puberty rarely have this subside.
As it can be difficult to predict the trajectory of gender identity from childhood to adolescence, the approach to the prepubertal and pubertal gender nonconforming patient is different. It is important to note that research suggests that gender identity is innate and cannot be changed with interventions. The goals of care for gender-nonconforming (GN) youth include providing a safe environment where youth can explore their identities, and individualizing treatment to meet the needs of each patient and family.
Care for prepubertal GN youth
For parents:
Have you noticed, or are you concerned about your child’s:
• Preference or rejection of particular toys/games?
• Hair and clothing preferences or rejections?
• Preferred (if any) gender of playmates?
Has your child ever expressed:
• A desire to be or insistence that they are the other gender?
• A dislike of their sexual anatomy?
• A desire for primary (penis, vagina) or secondary (periods, facial hair) sex characteristics of the other gender?
Are you concerned about bullying ?
Do you have any concerns about your child’s mood or concerns for self-harm?
For children:
• Do you feel more like a girl, boy, neither, both?
• How would you like to play, cut your hair, dress?
• What name or pronoun (she for girl, he for boy) fits you?4
The goal for prepubertal youth with nonconforming identities is to ensure that they are safe at home, school, and at play. Some youth may express a desire to “transition” or live as their identified gender by changing their name and dressing as their identified gender. Some youth and families may choose to transition only in certain settings (at home, but not at school). Some youth and families may want a safe space where the child can grow, develop, and continue to explore their identity without transitioning. Mental health providers trained in the care of GN youth can help patients and families decide if transition is appropriate for them and support them with the process and timing of transitioning. For youth who experience depression, anxiety, bullying, or thoughts of self-harm related to their gender identity, care by an experienced mental health provider is essential. It is important to recognize that each patient and family will need an individualized approach based on their needs.
Care for pubertal GN youth
The development of secondary sex characteristics can be particularly distressing for GN youth. Some youth may first experience gender dysphoria at this time. This distress combined with the psychosocial stressors of adolescent development can lead to depression, anxiety, suicidal ideation, self-harm, and other risk taking behaviors. Visits with pubertal GN youth, as with any adolescent, should include confidential time alone with the medical provider to discuss any concerns. Youth should be informed that information will be kept confidential, but parents will need to be notified of any safety concerns (such as suicidality or self-harm). As with prepubertal youth, a history related to hair and clothing preferences; distress related to genital anatomy; and the desire to be the other gender should be obtained. A pubertal history and any related symptoms of distress also should be obtained.
DO
• Ask preferred name and pronoun.
• Perform confidential strength and risk assessment.
• Assess for family and social support.
• Refer to appropriate mental health and transgender providers.
DON’T
• Assume names and pronouns.
• Interview patient only with parent in the room.
• Disclose identity without patient consent.
• Dismiss parents as sources of support.
• Refer for reparative therapy.4
Youth who are suspected to have a diagnosis of gender dysphoria should be referred to mental health and medical providers with experience caring for transgender youth. These specialists can work with patients and families, and determine when and if youth are eligible for puberty blocking therapy with GnRH analogues and/or hormone therapy. GnRH analogues, if appropriate, can be prescribed after patients have reached sexual maturity rating stage 2. The rationale for this treatment is to prevent the development of unwanted secondary sex characteristics while giving the youth a chance to continue with psychotherapy and explore their gender identity.5 Hormone therapy, if appropriate, can be prescribed a few years later under the care of a transgender specialist and mental health provider.
Summary
It is normal to experiment with gender roles and expression in childhood. Providing a safe space to do this is important.
Individuals who have a persistent, consistent, and insistent gender-nonconforming identification and who have increased distress with puberty are unlikely to have this subside.
Pediatricians can assess for gender dysphoria and screen for related mood disorders and behaviors in the primary care setting. Appropriate referral to trained professionals is important.
Care should be individualized and focused on the health and safety of the patient.
Resources
For health care professionals
• World Professional Association for Transgender Health: Standards of care on care of transgender patients and provider directory. www.wpath.org• Physicians for Reproductive Health’s adolescent reproductive and sexual health education program (ARSHEP): Best practices for adolescent and reproductive health: Module on caring for transgender adolescent patients. prh.org/teen-reproductive-health/arshep-downloads/
For patients and families
• Family Acceptance Project: familyproject.sfsu.edu/
• Healthychildren.org: Parenting website supported by the American Academy of Pediatrics. Links to articles on gender nonconforming and transgender children; gender identity development in children. www.healthychildren.org
References
1. Caring for Your School Age Child: Ages 5-12 by the American Academy of Pediatrics (New York: Bantam Books, 1995).
2. Dev Psychol. 2008 Jan;44(1):34-45.
3. J Am Acad Child and Adolesc Psychiatry. 2008;47(12):1413-23
4. Caring for Transgender Adolescent Patients. Physicians for Reproductive Health’s Adolescent Reproductive and Sexual Health Education Program (ARSHEP): Best practices for adolescent and reproductive health: prh.org/teen-reproductive-health/arshep-downloads/
5. World Professional Association of Transgender Health, Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, 7th Edition (International Journal of Transgenderism. 2011;13:165-232)
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
Gender identity typically develops in early childhood, and by age 4 years, most children consistently refer to themselves as a girl or a boy.1 For the majority of children, natal sex or sex assigned at birth, aligns with gender identity (a person’s innate sense of feeling male, female, or somewhere in between). However, this is not always the case. Gender identity can be understood as a spectrum with youth identifying as a gender that aligns with their natal sex (cisgender), is opposite of their natal sex (transgender), no gender (agender), or somewhere in between (genderqueer). The distress that can result from an incongruence between natal sex and gender identity is called gender dysphoria. Youth with gender dysphoria are at increased risk for a number of conditions, including suicide and self-harm. Early identification and appropriate care of these youth can reduce these risks. This month’s column will briefly review assessment of these youth in the pediatric setting.
Many youth who have a gender-nonconforming identity in childhood will not go on to have one in adulthood.2,3 Those who have a consistent, insistent, and persistent nonconforming identity are more likely to have this identity persist into adulthood. Youth who experience increased gender dysphoria with the onset of puberty rarely have this subside.
As it can be difficult to predict the trajectory of gender identity from childhood to adolescence, the approach to the prepubertal and pubertal gender nonconforming patient is different. It is important to note that research suggests that gender identity is innate and cannot be changed with interventions. The goals of care for gender-nonconforming (GN) youth include providing a safe environment where youth can explore their identities, and individualizing treatment to meet the needs of each patient and family.
Care for prepubertal GN youth
For parents:
Have you noticed, or are you concerned about your child’s:
• Preference or rejection of particular toys/games?
• Hair and clothing preferences or rejections?
• Preferred (if any) gender of playmates?
Has your child ever expressed:
• A desire to be or insistence that they are the other gender?
• A dislike of their sexual anatomy?
• A desire for primary (penis, vagina) or secondary (periods, facial hair) sex characteristics of the other gender?
Are you concerned about bullying ?
Do you have any concerns about your child’s mood or concerns for self-harm?
For children:
• Do you feel more like a girl, boy, neither, both?
• How would you like to play, cut your hair, dress?
• What name or pronoun (she for girl, he for boy) fits you?4
The goal for prepubertal youth with nonconforming identities is to ensure that they are safe at home, school, and at play. Some youth may express a desire to “transition” or live as their identified gender by changing their name and dressing as their identified gender. Some youth and families may choose to transition only in certain settings (at home, but not at school). Some youth and families may want a safe space where the child can grow, develop, and continue to explore their identity without transitioning. Mental health providers trained in the care of GN youth can help patients and families decide if transition is appropriate for them and support them with the process and timing of transitioning. For youth who experience depression, anxiety, bullying, or thoughts of self-harm related to their gender identity, care by an experienced mental health provider is essential. It is important to recognize that each patient and family will need an individualized approach based on their needs.
Care for pubertal GN youth
The development of secondary sex characteristics can be particularly distressing for GN youth. Some youth may first experience gender dysphoria at this time. This distress combined with the psychosocial stressors of adolescent development can lead to depression, anxiety, suicidal ideation, self-harm, and other risk taking behaviors. Visits with pubertal GN youth, as with any adolescent, should include confidential time alone with the medical provider to discuss any concerns. Youth should be informed that information will be kept confidential, but parents will need to be notified of any safety concerns (such as suicidality or self-harm). As with prepubertal youth, a history related to hair and clothing preferences; distress related to genital anatomy; and the desire to be the other gender should be obtained. A pubertal history and any related symptoms of distress also should be obtained.
DO
• Ask preferred name and pronoun.
• Perform confidential strength and risk assessment.
• Assess for family and social support.
• Refer to appropriate mental health and transgender providers.
DON’T
• Assume names and pronouns.
• Interview patient only with parent in the room.
• Disclose identity without patient consent.
• Dismiss parents as sources of support.
• Refer for reparative therapy.4
Youth who are suspected to have a diagnosis of gender dysphoria should be referred to mental health and medical providers with experience caring for transgender youth. These specialists can work with patients and families, and determine when and if youth are eligible for puberty blocking therapy with GnRH analogues and/or hormone therapy. GnRH analogues, if appropriate, can be prescribed after patients have reached sexual maturity rating stage 2. The rationale for this treatment is to prevent the development of unwanted secondary sex characteristics while giving the youth a chance to continue with psychotherapy and explore their gender identity.5 Hormone therapy, if appropriate, can be prescribed a few years later under the care of a transgender specialist and mental health provider.
Summary
It is normal to experiment with gender roles and expression in childhood. Providing a safe space to do this is important.
Individuals who have a persistent, consistent, and insistent gender-nonconforming identification and who have increased distress with puberty are unlikely to have this subside.
Pediatricians can assess for gender dysphoria and screen for related mood disorders and behaviors in the primary care setting. Appropriate referral to trained professionals is important.
Care should be individualized and focused on the health and safety of the patient.
Resources
For health care professionals
• World Professional Association for Transgender Health: Standards of care on care of transgender patients and provider directory. www.wpath.org• Physicians for Reproductive Health’s adolescent reproductive and sexual health education program (ARSHEP): Best practices for adolescent and reproductive health: Module on caring for transgender adolescent patients. prh.org/teen-reproductive-health/arshep-downloads/
For patients and families
• Family Acceptance Project: familyproject.sfsu.edu/
• Healthychildren.org: Parenting website supported by the American Academy of Pediatrics. Links to articles on gender nonconforming and transgender children; gender identity development in children. www.healthychildren.org
References
1. Caring for Your School Age Child: Ages 5-12 by the American Academy of Pediatrics (New York: Bantam Books, 1995).
2. Dev Psychol. 2008 Jan;44(1):34-45.
3. J Am Acad Child and Adolesc Psychiatry. 2008;47(12):1413-23
4. Caring for Transgender Adolescent Patients. Physicians for Reproductive Health’s Adolescent Reproductive and Sexual Health Education Program (ARSHEP): Best practices for adolescent and reproductive health: prh.org/teen-reproductive-health/arshep-downloads/
5. World Professional Association of Transgender Health, Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, 7th Edition (International Journal of Transgenderism. 2011;13:165-232)
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
Hypertrophic cardiomyopathy: Who should get an ICD?
SNOWMASS, COLO. – Since the 2011 release of the current American College of Cardiology/American Heart Association guidelines on hypertrophic cardiomyopathy, several new evidence-based tools have emerged as being helpful in decision making regarding which patients should receive an implantable cardioverter-defibrillator (ICD) for primary prevention of sudden cardiac death, Dr. Rick A. Nishimura said at the annual Cardiovascular Conference at Snowmass.
These three tools – gadolinium-enhanced cardiovascular magnetic resonance imaging, a novel European risk score calculator, and a new appreciation of the importance of age-related risk – are most useful in the many cases of hypertrophic cardiomyopathy (HCM) where the cardiologist is on the fence regarding ICD placement because the patient doesn’t clearly meet the conventional major criteria for an ICD, according to Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Nishimura, a member of the writing panel for the current guidelines (Circulation. 2011 Dec 13;124[24]:2761-96), predicted these tools will be incorporated into the next iteration of the HCM guidelines.
Notably absent from Dr. Nishimura’s list of useful tools was genetic testing for assessment of SCD risk in a patient with HCM.
“You should not spend $6,000 to do a genetic study to try to predict who’s at risk for sudden death. It turns out that most mutations are neither inherently benign nor malignant. High-risk mutations come from high-risk families, so you can do just as well by taking a family history,” according to the cardiologist.
Dr. Nishimura explained that the clinical dilemma in trying to evaluate SCD risk in a patient who presents with HCM is that the overall risk is quite low – probably 1% or less per year in the total HCM population – yet HCM is the number-one cause of SCD in younger patients. And it can occur unpredictably years or decades after diagnosis of HCM.
While ICDs are of proven effectiveness in preventing SCD in patients with HCM, reliance solely upon the conventional risk predictors to identify those who should get a device is clearly inadequate. Those criteria have a positive predictive accuracy of less than 15%; in other words, roughly 85% of HCM patients who get an ICD never receive an appropriate, life-saving shock, Dr. Nishimura said.
“We have a lot of work left to do in order to better identify these patients. In our own data from the Mayo Clinic, 20%-25% of patients have inappropriate ICD shocks despite efforts to program the device to prevent such shocks. That’s especially common in younger, active patients with HCM, and when it occurs patients find it absolutely devastating,” according to the cardiologist.
As stated in the current guidelines, the established SCD risk factors that provide a strong indication for an ICD in a patient with HCM are prior documented cardiac arrest, ventricular fibrillation, or hemodynamically significant ventricular tachycardia. Additionally, risk factors which, in Dr. Nishimura’s view, probably warrant insertion of an ICD and, at the very least should trigger a physician-patient discussion about the risks and benefits of preventive device therapy, include a family history of HCM-related sudden death in a first-degree relative, massive left ventricular (LV) hypertrophy as defined by a maximum wall thickness of at least 30 mm, and recent unexplained syncope inconsistent with neurocardiogenic origin.
Less potent risk predictors where savvy clinical judgment becomes imperative include nonsustained ventricular tachycardia on 24-hour Holter monitoring, a hypotensive blood pressure response to exercise, and an increased LV wall thickness in a younger patient that doesn’t rise to the 30-mm standard. These are situations where gadolinium-enhanced MRI, consideration of patient age, and the European risk scoring system can help in the decision-making process, he said.
Gadolinium-enhanced MRI: Contrast-enhanced cardiovascular MRI with late gadolinium enhancement has emerged as a reliable marker of the myocyte disarray and interstitial fibrosis which serves as a substrate for ventricular arrhythmias. In a recent study of 1,293 HCM patients followed for a median of 3.3 years, the incidence of SCD events increased progressively with the extent of late gadolinium enhancement. Extensive late gadolinium enhancement, defined as involving at least 15% of LV mass, was associated with a doubled risk of SCD events in patients otherwise considered at low risk (Circulation. 2014 Aug 5;130[6]:484-95).
“This is probably going to become one of the key markers that can help you when you’re on the fence as to whether or not to put in an ICD. We’re getting MRIs with gadolinium now in all of our HCM patients. What matters is not gadolinium enhancement at the insertion of the left ventricle into the septum – a lot of people have that – but diffuse gadolinium enhancement throughout the septum,” Dr. Nishimura said.
Because SCD risk increases linearly with greater maximal LV wall thickness, gadolinium-enhanced MRI is particularly helpful in assessing risk in a younger patient with a maximal LV wall thickness of, say, 26 mm, he added.
Age: A study by led by Dr. Barry J. Maron, the cochair of the 2011 guideline committee and director of the HCM center at the Minneapolis Heart Institute, provides a new understanding that prophylactic ICD implantation is not warranted in patients with HCM who present at age 60 or older. In their study of 428 consecutive patients presenting with HCM at age 60 or above, the investigators found during 5.8 years of follow-up that the incidence of arrhythmic sudden death events was just 0.2% per year (Circulation. 2013 Feb 5;127[5]:585-93).
“They’ve shown that if you look at patients age 60 or above who have HCM, the risk of sudden cardiac death is almost nonexistent. That’s incredibly important to remember. Sudden death is something that’s going to happen in the younger population, under age 30,” Dr. Nishimura emphasized.
European SCD risk prediction tool: This tool was hailed as a major advance in the current European Society of Cardiology guidelines on HCM (Eur Heart J. 2014;35:2733-2779). The tool was incorporated into the guidelines. It is also available as a smartphone app.
The risk prediction tool (Eur Heart J. 2014 Aug 7;35[30]:2010-20) is a complex equation that incorporates seven predictive factors: age, maximal LV wall thickness, left atrial diameter, LV outflow tract gradient, family history of SCD, nonsustained VT, and unexplained syncope. After input on these seven factors, the equation spits out an individual’s estimated 5-year SCD risk. Based on the study of 3,675 consecutive HCM patients with a median 5.7 years of follow-up that was used to develop the risk equation, the current ESC guidelines state that an ICD is not warranted in HCM patients with a 5-year risk below 4%, device implantation should be considered in those whose risk is 4%-6%, and an ICD should be even more strongly considered in patients with a 5-year risk in excess of 6%.
“A lot of people across the pond are using this risk score. But there are some problems with it,” according to Dr. Nishimura.
In his view, it “doesn’t make much sense” to include left ventricular outflow tract gradient or left atrial diameter in the risk equation. Nor is unexplained syncope carefully defined. Also, the equation would be improved by incorporation of late gadolinium enhancement on MRI, left ventricular dysfunction, and presence or absence of apical aneurysm as predictive variables. But on the plus side, the European equation treats maximal LV wall thickness as a continuous variable, which is more appropriate than the single 30-mm cutoff used in the ACC/AHA guidelines.
The biggest limitation of the European prognostic score, however, is that it hasn’t yet been validated in an independent patient cohort, Dr. Nishimura said. He noted that when Dr. Maron and coworkers recently applied the European SCD risk equation retrospectively to 1,629 consecutive U.S. patients with HCM, the investigators concluded that the risk equation proved unreliable for prediction of future SCD events. Fifty-nine percent of patients who got an appropriate ICD shock or experienced SCD were misclassified as low risk and hence would not have received an ICD under the European guidelines (Am J Cardiol. 2015 Sep 1;116[5]:757-64).
Nonetheless, because of the limited predictive accuracy of today’s standard methods of assessing SCD risk, Dr. Nishimura considers application of the European risk score to be “reasonable” in HCM patients who don’t have any of the strong indications for an ICD.
“If it comes up with an estimated 5-year risk greater than 6%, I think it’s very reasonable to consider implantation of an ICD,” he said.
Dr. Nishimura observed that in addition to assessing SCD risk, cardiologists have two other separate essential tasks when a patient presents with HCM. One is to screen and counsel the first-degree relatives. The other is to determine whether a left ventricular outflow tract obstruction is present in a symptomatic patient and, if so, to improve symptoms by treating the associated hemodynamic abnormalities medically and if need be by septal ablation or septal myectomy.
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – Since the 2011 release of the current American College of Cardiology/American Heart Association guidelines on hypertrophic cardiomyopathy, several new evidence-based tools have emerged as being helpful in decision making regarding which patients should receive an implantable cardioverter-defibrillator (ICD) for primary prevention of sudden cardiac death, Dr. Rick A. Nishimura said at the annual Cardiovascular Conference at Snowmass.
These three tools – gadolinium-enhanced cardiovascular magnetic resonance imaging, a novel European risk score calculator, and a new appreciation of the importance of age-related risk – are most useful in the many cases of hypertrophic cardiomyopathy (HCM) where the cardiologist is on the fence regarding ICD placement because the patient doesn’t clearly meet the conventional major criteria for an ICD, according to Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Nishimura, a member of the writing panel for the current guidelines (Circulation. 2011 Dec 13;124[24]:2761-96), predicted these tools will be incorporated into the next iteration of the HCM guidelines.
Notably absent from Dr. Nishimura’s list of useful tools was genetic testing for assessment of SCD risk in a patient with HCM.
“You should not spend $6,000 to do a genetic study to try to predict who’s at risk for sudden death. It turns out that most mutations are neither inherently benign nor malignant. High-risk mutations come from high-risk families, so you can do just as well by taking a family history,” according to the cardiologist.
Dr. Nishimura explained that the clinical dilemma in trying to evaluate SCD risk in a patient who presents with HCM is that the overall risk is quite low – probably 1% or less per year in the total HCM population – yet HCM is the number-one cause of SCD in younger patients. And it can occur unpredictably years or decades after diagnosis of HCM.
While ICDs are of proven effectiveness in preventing SCD in patients with HCM, reliance solely upon the conventional risk predictors to identify those who should get a device is clearly inadequate. Those criteria have a positive predictive accuracy of less than 15%; in other words, roughly 85% of HCM patients who get an ICD never receive an appropriate, life-saving shock, Dr. Nishimura said.
“We have a lot of work left to do in order to better identify these patients. In our own data from the Mayo Clinic, 20%-25% of patients have inappropriate ICD shocks despite efforts to program the device to prevent such shocks. That’s especially common in younger, active patients with HCM, and when it occurs patients find it absolutely devastating,” according to the cardiologist.
As stated in the current guidelines, the established SCD risk factors that provide a strong indication for an ICD in a patient with HCM are prior documented cardiac arrest, ventricular fibrillation, or hemodynamically significant ventricular tachycardia. Additionally, risk factors which, in Dr. Nishimura’s view, probably warrant insertion of an ICD and, at the very least should trigger a physician-patient discussion about the risks and benefits of preventive device therapy, include a family history of HCM-related sudden death in a first-degree relative, massive left ventricular (LV) hypertrophy as defined by a maximum wall thickness of at least 30 mm, and recent unexplained syncope inconsistent with neurocardiogenic origin.
Less potent risk predictors where savvy clinical judgment becomes imperative include nonsustained ventricular tachycardia on 24-hour Holter monitoring, a hypotensive blood pressure response to exercise, and an increased LV wall thickness in a younger patient that doesn’t rise to the 30-mm standard. These are situations where gadolinium-enhanced MRI, consideration of patient age, and the European risk scoring system can help in the decision-making process, he said.
Gadolinium-enhanced MRI: Contrast-enhanced cardiovascular MRI with late gadolinium enhancement has emerged as a reliable marker of the myocyte disarray and interstitial fibrosis which serves as a substrate for ventricular arrhythmias. In a recent study of 1,293 HCM patients followed for a median of 3.3 years, the incidence of SCD events increased progressively with the extent of late gadolinium enhancement. Extensive late gadolinium enhancement, defined as involving at least 15% of LV mass, was associated with a doubled risk of SCD events in patients otherwise considered at low risk (Circulation. 2014 Aug 5;130[6]:484-95).
“This is probably going to become one of the key markers that can help you when you’re on the fence as to whether or not to put in an ICD. We’re getting MRIs with gadolinium now in all of our HCM patients. What matters is not gadolinium enhancement at the insertion of the left ventricle into the septum – a lot of people have that – but diffuse gadolinium enhancement throughout the septum,” Dr. Nishimura said.
Because SCD risk increases linearly with greater maximal LV wall thickness, gadolinium-enhanced MRI is particularly helpful in assessing risk in a younger patient with a maximal LV wall thickness of, say, 26 mm, he added.
Age: A study by led by Dr. Barry J. Maron, the cochair of the 2011 guideline committee and director of the HCM center at the Minneapolis Heart Institute, provides a new understanding that prophylactic ICD implantation is not warranted in patients with HCM who present at age 60 or older. In their study of 428 consecutive patients presenting with HCM at age 60 or above, the investigators found during 5.8 years of follow-up that the incidence of arrhythmic sudden death events was just 0.2% per year (Circulation. 2013 Feb 5;127[5]:585-93).
“They’ve shown that if you look at patients age 60 or above who have HCM, the risk of sudden cardiac death is almost nonexistent. That’s incredibly important to remember. Sudden death is something that’s going to happen in the younger population, under age 30,” Dr. Nishimura emphasized.
European SCD risk prediction tool: This tool was hailed as a major advance in the current European Society of Cardiology guidelines on HCM (Eur Heart J. 2014;35:2733-2779). The tool was incorporated into the guidelines. It is also available as a smartphone app.
The risk prediction tool (Eur Heart J. 2014 Aug 7;35[30]:2010-20) is a complex equation that incorporates seven predictive factors: age, maximal LV wall thickness, left atrial diameter, LV outflow tract gradient, family history of SCD, nonsustained VT, and unexplained syncope. After input on these seven factors, the equation spits out an individual’s estimated 5-year SCD risk. Based on the study of 3,675 consecutive HCM patients with a median 5.7 years of follow-up that was used to develop the risk equation, the current ESC guidelines state that an ICD is not warranted in HCM patients with a 5-year risk below 4%, device implantation should be considered in those whose risk is 4%-6%, and an ICD should be even more strongly considered in patients with a 5-year risk in excess of 6%.
“A lot of people across the pond are using this risk score. But there are some problems with it,” according to Dr. Nishimura.
In his view, it “doesn’t make much sense” to include left ventricular outflow tract gradient or left atrial diameter in the risk equation. Nor is unexplained syncope carefully defined. Also, the equation would be improved by incorporation of late gadolinium enhancement on MRI, left ventricular dysfunction, and presence or absence of apical aneurysm as predictive variables. But on the plus side, the European equation treats maximal LV wall thickness as a continuous variable, which is more appropriate than the single 30-mm cutoff used in the ACC/AHA guidelines.
The biggest limitation of the European prognostic score, however, is that it hasn’t yet been validated in an independent patient cohort, Dr. Nishimura said. He noted that when Dr. Maron and coworkers recently applied the European SCD risk equation retrospectively to 1,629 consecutive U.S. patients with HCM, the investigators concluded that the risk equation proved unreliable for prediction of future SCD events. Fifty-nine percent of patients who got an appropriate ICD shock or experienced SCD were misclassified as low risk and hence would not have received an ICD under the European guidelines (Am J Cardiol. 2015 Sep 1;116[5]:757-64).
Nonetheless, because of the limited predictive accuracy of today’s standard methods of assessing SCD risk, Dr. Nishimura considers application of the European risk score to be “reasonable” in HCM patients who don’t have any of the strong indications for an ICD.
“If it comes up with an estimated 5-year risk greater than 6%, I think it’s very reasonable to consider implantation of an ICD,” he said.
Dr. Nishimura observed that in addition to assessing SCD risk, cardiologists have two other separate essential tasks when a patient presents with HCM. One is to screen and counsel the first-degree relatives. The other is to determine whether a left ventricular outflow tract obstruction is present in a symptomatic patient and, if so, to improve symptoms by treating the associated hemodynamic abnormalities medically and if need be by septal ablation or septal myectomy.
He reported having no financial conflicts of interest regarding his presentation.
SNOWMASS, COLO. – Since the 2011 release of the current American College of Cardiology/American Heart Association guidelines on hypertrophic cardiomyopathy, several new evidence-based tools have emerged as being helpful in decision making regarding which patients should receive an implantable cardioverter-defibrillator (ICD) for primary prevention of sudden cardiac death, Dr. Rick A. Nishimura said at the annual Cardiovascular Conference at Snowmass.
These three tools – gadolinium-enhanced cardiovascular magnetic resonance imaging, a novel European risk score calculator, and a new appreciation of the importance of age-related risk – are most useful in the many cases of hypertrophic cardiomyopathy (HCM) where the cardiologist is on the fence regarding ICD placement because the patient doesn’t clearly meet the conventional major criteria for an ICD, according to Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn.
Dr. Nishimura, a member of the writing panel for the current guidelines (Circulation. 2011 Dec 13;124[24]:2761-96), predicted these tools will be incorporated into the next iteration of the HCM guidelines.
Notably absent from Dr. Nishimura’s list of useful tools was genetic testing for assessment of SCD risk in a patient with HCM.
“You should not spend $6,000 to do a genetic study to try to predict who’s at risk for sudden death. It turns out that most mutations are neither inherently benign nor malignant. High-risk mutations come from high-risk families, so you can do just as well by taking a family history,” according to the cardiologist.
Dr. Nishimura explained that the clinical dilemma in trying to evaluate SCD risk in a patient who presents with HCM is that the overall risk is quite low – probably 1% or less per year in the total HCM population – yet HCM is the number-one cause of SCD in younger patients. And it can occur unpredictably years or decades after diagnosis of HCM.
While ICDs are of proven effectiveness in preventing SCD in patients with HCM, reliance solely upon the conventional risk predictors to identify those who should get a device is clearly inadequate. Those criteria have a positive predictive accuracy of less than 15%; in other words, roughly 85% of HCM patients who get an ICD never receive an appropriate, life-saving shock, Dr. Nishimura said.
“We have a lot of work left to do in order to better identify these patients. In our own data from the Mayo Clinic, 20%-25% of patients have inappropriate ICD shocks despite efforts to program the device to prevent such shocks. That’s especially common in younger, active patients with HCM, and when it occurs patients find it absolutely devastating,” according to the cardiologist.
As stated in the current guidelines, the established SCD risk factors that provide a strong indication for an ICD in a patient with HCM are prior documented cardiac arrest, ventricular fibrillation, or hemodynamically significant ventricular tachycardia. Additionally, risk factors which, in Dr. Nishimura’s view, probably warrant insertion of an ICD and, at the very least should trigger a physician-patient discussion about the risks and benefits of preventive device therapy, include a family history of HCM-related sudden death in a first-degree relative, massive left ventricular (LV) hypertrophy as defined by a maximum wall thickness of at least 30 mm, and recent unexplained syncope inconsistent with neurocardiogenic origin.
Less potent risk predictors where savvy clinical judgment becomes imperative include nonsustained ventricular tachycardia on 24-hour Holter monitoring, a hypotensive blood pressure response to exercise, and an increased LV wall thickness in a younger patient that doesn’t rise to the 30-mm standard. These are situations where gadolinium-enhanced MRI, consideration of patient age, and the European risk scoring system can help in the decision-making process, he said.
Gadolinium-enhanced MRI: Contrast-enhanced cardiovascular MRI with late gadolinium enhancement has emerged as a reliable marker of the myocyte disarray and interstitial fibrosis which serves as a substrate for ventricular arrhythmias. In a recent study of 1,293 HCM patients followed for a median of 3.3 years, the incidence of SCD events increased progressively with the extent of late gadolinium enhancement. Extensive late gadolinium enhancement, defined as involving at least 15% of LV mass, was associated with a doubled risk of SCD events in patients otherwise considered at low risk (Circulation. 2014 Aug 5;130[6]:484-95).
“This is probably going to become one of the key markers that can help you when you’re on the fence as to whether or not to put in an ICD. We’re getting MRIs with gadolinium now in all of our HCM patients. What matters is not gadolinium enhancement at the insertion of the left ventricle into the septum – a lot of people have that – but diffuse gadolinium enhancement throughout the septum,” Dr. Nishimura said.
Because SCD risk increases linearly with greater maximal LV wall thickness, gadolinium-enhanced MRI is particularly helpful in assessing risk in a younger patient with a maximal LV wall thickness of, say, 26 mm, he added.
Age: A study by led by Dr. Barry J. Maron, the cochair of the 2011 guideline committee and director of the HCM center at the Minneapolis Heart Institute, provides a new understanding that prophylactic ICD implantation is not warranted in patients with HCM who present at age 60 or older. In their study of 428 consecutive patients presenting with HCM at age 60 or above, the investigators found during 5.8 years of follow-up that the incidence of arrhythmic sudden death events was just 0.2% per year (Circulation. 2013 Feb 5;127[5]:585-93).
“They’ve shown that if you look at patients age 60 or above who have HCM, the risk of sudden cardiac death is almost nonexistent. That’s incredibly important to remember. Sudden death is something that’s going to happen in the younger population, under age 30,” Dr. Nishimura emphasized.
European SCD risk prediction tool: This tool was hailed as a major advance in the current European Society of Cardiology guidelines on HCM (Eur Heart J. 2014;35:2733-2779). The tool was incorporated into the guidelines. It is also available as a smartphone app.
The risk prediction tool (Eur Heart J. 2014 Aug 7;35[30]:2010-20) is a complex equation that incorporates seven predictive factors: age, maximal LV wall thickness, left atrial diameter, LV outflow tract gradient, family history of SCD, nonsustained VT, and unexplained syncope. After input on these seven factors, the equation spits out an individual’s estimated 5-year SCD risk. Based on the study of 3,675 consecutive HCM patients with a median 5.7 years of follow-up that was used to develop the risk equation, the current ESC guidelines state that an ICD is not warranted in HCM patients with a 5-year risk below 4%, device implantation should be considered in those whose risk is 4%-6%, and an ICD should be even more strongly considered in patients with a 5-year risk in excess of 6%.
“A lot of people across the pond are using this risk score. But there are some problems with it,” according to Dr. Nishimura.
In his view, it “doesn’t make much sense” to include left ventricular outflow tract gradient or left atrial diameter in the risk equation. Nor is unexplained syncope carefully defined. Also, the equation would be improved by incorporation of late gadolinium enhancement on MRI, left ventricular dysfunction, and presence or absence of apical aneurysm as predictive variables. But on the plus side, the European equation treats maximal LV wall thickness as a continuous variable, which is more appropriate than the single 30-mm cutoff used in the ACC/AHA guidelines.
The biggest limitation of the European prognostic score, however, is that it hasn’t yet been validated in an independent patient cohort, Dr. Nishimura said. He noted that when Dr. Maron and coworkers recently applied the European SCD risk equation retrospectively to 1,629 consecutive U.S. patients with HCM, the investigators concluded that the risk equation proved unreliable for prediction of future SCD events. Fifty-nine percent of patients who got an appropriate ICD shock or experienced SCD were misclassified as low risk and hence would not have received an ICD under the European guidelines (Am J Cardiol. 2015 Sep 1;116[5]:757-64).
Nonetheless, because of the limited predictive accuracy of today’s standard methods of assessing SCD risk, Dr. Nishimura considers application of the European risk score to be “reasonable” in HCM patients who don’t have any of the strong indications for an ICD.
“If it comes up with an estimated 5-year risk greater than 6%, I think it’s very reasonable to consider implantation of an ICD,” he said.
Dr. Nishimura observed that in addition to assessing SCD risk, cardiologists have two other separate essential tasks when a patient presents with HCM. One is to screen and counsel the first-degree relatives. The other is to determine whether a left ventricular outflow tract obstruction is present in a symptomatic patient and, if so, to improve symptoms by treating the associated hemodynamic abnormalities medically and if need be by septal ablation or septal myectomy.
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Study eyes impact of blood pressure on survival in TBI
SAN DIEGO – In the setting of traumatic brain injury, increases in systolic blood pressure after the nadir are independently associated with improved survival in hypotensive patients.
In addition, even substantial blood pressure increases do not seem to harm normotensive patients. These findings come from a subanalysis of the ongoing National Institutes of Health–funded Excellence in Prehospital Injury Care (EPIC) TBI study.
“Very little is known about the patterns of blood pressure in traumatic brain injury in the field,” principal investigator Dr. Daniel W. Spaite said at the annual meeting of the National Association of EMS Physicians. “For instance, nobody knows whether it’s better to have your blood pressure increasing, stable, or decreasing in the field with regard to outcome, especially mortality. Typical studies that do have EMS data linked only have a single blood pressure measurement documented, so there’s no knowledge of trends in EMS blood pressure in TBI.”
Dr. Spaite, professor and Virginia Piper Endowed Chair of Emergency Medicine at the University of Arizona, Tucson, and his colleagues evaluated the association between mortality and increases in prehospital systolic blood pressure after the lowest recorded measurement in major TBI patients who are part of the EPIC study – the statewide implementation of TBI guidelines from the Brain Trauma Foundation and the NAEMSP. Data sources include the Arizona State Trauma Registry, which has comprehensive hospital outcome data. “The cases are then linked and the EMS patient care reports are carefully abstracted by the EPIC data team,” Dr. Spaite explained. “This included major TBI (which is, clinically, both moderate and severe) and all patients whose lowest systolic BP was between 40 and 300 mm Hg.”
The researchers used logistic regression to examine the association between the increase in EMS systolic blood pressure (SBP) after the lowest EMS blood pressure and its association with adjusted probability of death. They then partitioned the study population into four cohorts based on each patient’s prehospital systolic BP (40-89 mm Hg, 90-139 mm Hg, 140-159 mm Hg, and 160-300 mm Hg). In each cohort, they identified the independent association between the magnitude of increase in SBP and the adjusted probability of death.
Dr. Spaite reported findings from 14,567 TBI patients. More than two-thirds (68%) were male, and their mean age was 45 years. The researchers observed that, in the hypotensive cohort, mortality dropped significantly if the SBP increased after the lowest SBP. “Improvements were dramatic with increases of 40-80 mm Hg,” he said. In the normotensive group, increases in SBP were associated with very slight reductions in mortality. Even large increases in SBP, such as in the range of 70-90 mm Hg, did not appear to be detrimental.
In the mildly hypertensive group, large systolic increases were associated with higher mortality. “Interestingly, even if your lowest [SBP] is between 140 and 159 mm Hg, until you get above an increase of 40 mm Hg above that, you don’t start seeing increases in mortality,” Dr. Spaite said. In the severely hypertensive group, mortality was higher with any subsequent increase in SBP, “which doesn’t surprise any of us,” he said. “It’s dramatically higher if the increase is large.”
Dr. Spaite emphasized that the current analysis is based on observational data, “so this does not prove that treating hypotension improves outcome. … That direct question is part of the EPIC study itself and awaits the final analysis, hopefully in mid-2017. This is the first large report of blood pressure trends in the prehospital management of TBI.”
He concluded that the current findings in the hypotensive and normotensive cohorts “support guideline recommendations for restoring and optimizing cerebral perfusion in EMS traumatic brain injury management. What is fascinating about the literature is that the focus in TBI has always been on hypotension, but there’s very little information about what’s the best or the optimal blood pressure.”
EPIC is funded by the National Institutes of Health. Dr. Spaite reported having no relevant financial disclosures.
SAN DIEGO – In the setting of traumatic brain injury, increases in systolic blood pressure after the nadir are independently associated with improved survival in hypotensive patients.
In addition, even substantial blood pressure increases do not seem to harm normotensive patients. These findings come from a subanalysis of the ongoing National Institutes of Health–funded Excellence in Prehospital Injury Care (EPIC) TBI study.
“Very little is known about the patterns of blood pressure in traumatic brain injury in the field,” principal investigator Dr. Daniel W. Spaite said at the annual meeting of the National Association of EMS Physicians. “For instance, nobody knows whether it’s better to have your blood pressure increasing, stable, or decreasing in the field with regard to outcome, especially mortality. Typical studies that do have EMS data linked only have a single blood pressure measurement documented, so there’s no knowledge of trends in EMS blood pressure in TBI.”
Dr. Spaite, professor and Virginia Piper Endowed Chair of Emergency Medicine at the University of Arizona, Tucson, and his colleagues evaluated the association between mortality and increases in prehospital systolic blood pressure after the lowest recorded measurement in major TBI patients who are part of the EPIC study – the statewide implementation of TBI guidelines from the Brain Trauma Foundation and the NAEMSP. Data sources include the Arizona State Trauma Registry, which has comprehensive hospital outcome data. “The cases are then linked and the EMS patient care reports are carefully abstracted by the EPIC data team,” Dr. Spaite explained. “This included major TBI (which is, clinically, both moderate and severe) and all patients whose lowest systolic BP was between 40 and 300 mm Hg.”
The researchers used logistic regression to examine the association between the increase in EMS systolic blood pressure (SBP) after the lowest EMS blood pressure and its association with adjusted probability of death. They then partitioned the study population into four cohorts based on each patient’s prehospital systolic BP (40-89 mm Hg, 90-139 mm Hg, 140-159 mm Hg, and 160-300 mm Hg). In each cohort, they identified the independent association between the magnitude of increase in SBP and the adjusted probability of death.
Dr. Spaite reported findings from 14,567 TBI patients. More than two-thirds (68%) were male, and their mean age was 45 years. The researchers observed that, in the hypotensive cohort, mortality dropped significantly if the SBP increased after the lowest SBP. “Improvements were dramatic with increases of 40-80 mm Hg,” he said. In the normotensive group, increases in SBP were associated with very slight reductions in mortality. Even large increases in SBP, such as in the range of 70-90 mm Hg, did not appear to be detrimental.
In the mildly hypertensive group, large systolic increases were associated with higher mortality. “Interestingly, even if your lowest [SBP] is between 140 and 159 mm Hg, until you get above an increase of 40 mm Hg above that, you don’t start seeing increases in mortality,” Dr. Spaite said. In the severely hypertensive group, mortality was higher with any subsequent increase in SBP, “which doesn’t surprise any of us,” he said. “It’s dramatically higher if the increase is large.”
Dr. Spaite emphasized that the current analysis is based on observational data, “so this does not prove that treating hypotension improves outcome. … That direct question is part of the EPIC study itself and awaits the final analysis, hopefully in mid-2017. This is the first large report of blood pressure trends in the prehospital management of TBI.”
He concluded that the current findings in the hypotensive and normotensive cohorts “support guideline recommendations for restoring and optimizing cerebral perfusion in EMS traumatic brain injury management. What is fascinating about the literature is that the focus in TBI has always been on hypotension, but there’s very little information about what’s the best or the optimal blood pressure.”
EPIC is funded by the National Institutes of Health. Dr. Spaite reported having no relevant financial disclosures.
SAN DIEGO – In the setting of traumatic brain injury, increases in systolic blood pressure after the nadir are independently associated with improved survival in hypotensive patients.
In addition, even substantial blood pressure increases do not seem to harm normotensive patients. These findings come from a subanalysis of the ongoing National Institutes of Health–funded Excellence in Prehospital Injury Care (EPIC) TBI study.
“Very little is known about the patterns of blood pressure in traumatic brain injury in the field,” principal investigator Dr. Daniel W. Spaite said at the annual meeting of the National Association of EMS Physicians. “For instance, nobody knows whether it’s better to have your blood pressure increasing, stable, or decreasing in the field with regard to outcome, especially mortality. Typical studies that do have EMS data linked only have a single blood pressure measurement documented, so there’s no knowledge of trends in EMS blood pressure in TBI.”
Dr. Spaite, professor and Virginia Piper Endowed Chair of Emergency Medicine at the University of Arizona, Tucson, and his colleagues evaluated the association between mortality and increases in prehospital systolic blood pressure after the lowest recorded measurement in major TBI patients who are part of the EPIC study – the statewide implementation of TBI guidelines from the Brain Trauma Foundation and the NAEMSP. Data sources include the Arizona State Trauma Registry, which has comprehensive hospital outcome data. “The cases are then linked and the EMS patient care reports are carefully abstracted by the EPIC data team,” Dr. Spaite explained. “This included major TBI (which is, clinically, both moderate and severe) and all patients whose lowest systolic BP was between 40 and 300 mm Hg.”
The researchers used logistic regression to examine the association between the increase in EMS systolic blood pressure (SBP) after the lowest EMS blood pressure and its association with adjusted probability of death. They then partitioned the study population into four cohorts based on each patient’s prehospital systolic BP (40-89 mm Hg, 90-139 mm Hg, 140-159 mm Hg, and 160-300 mm Hg). In each cohort, they identified the independent association between the magnitude of increase in SBP and the adjusted probability of death.
Dr. Spaite reported findings from 14,567 TBI patients. More than two-thirds (68%) were male, and their mean age was 45 years. The researchers observed that, in the hypotensive cohort, mortality dropped significantly if the SBP increased after the lowest SBP. “Improvements were dramatic with increases of 40-80 mm Hg,” he said. In the normotensive group, increases in SBP were associated with very slight reductions in mortality. Even large increases in SBP, such as in the range of 70-90 mm Hg, did not appear to be detrimental.
In the mildly hypertensive group, large systolic increases were associated with higher mortality. “Interestingly, even if your lowest [SBP] is between 140 and 159 mm Hg, until you get above an increase of 40 mm Hg above that, you don’t start seeing increases in mortality,” Dr. Spaite said. In the severely hypertensive group, mortality was higher with any subsequent increase in SBP, “which doesn’t surprise any of us,” he said. “It’s dramatically higher if the increase is large.”
Dr. Spaite emphasized that the current analysis is based on observational data, “so this does not prove that treating hypotension improves outcome. … That direct question is part of the EPIC study itself and awaits the final analysis, hopefully in mid-2017. This is the first large report of blood pressure trends in the prehospital management of TBI.”
He concluded that the current findings in the hypotensive and normotensive cohorts “support guideline recommendations for restoring and optimizing cerebral perfusion in EMS traumatic brain injury management. What is fascinating about the literature is that the focus in TBI has always been on hypotension, but there’s very little information about what’s the best or the optimal blood pressure.”
EPIC is funded by the National Institutes of Health. Dr. Spaite reported having no relevant financial disclosures.
AT NAEMSP 2016
Key clinical point: The optimal systolic blood pressure in traumatic brain injury may be higher than previously thought.
Major finding: In the hypotensive cohort, mortality dropped significantly if the systolic blood pressure increased after the lowest SBP. In the normotensive group, increases in SBP were associated with very slight reductions in mortality.
Data source: An analysis of 14,567 TBI patients enrolled in the National Institutes of Health–funded Excellence in Prehospital Injury Care TBI Study.
Disclosures: EPIC is funded by NIH. Dr. Spaite reported having no relevant financial disclosures.
Price Explosion

One of the biggest burdens of modern clinical dermatology practice is the ability to obtain appropriate drug therapy for patients. In the current health care environment, insurance formularies have become increasingly restrictive and more individuals have to deal with high-deductible insurance plans.
In a JAMA Dermatology study published online on November 25, Rosenberg and Rosenberg sought to determine changes in the prices of commonly prescribed dermatologic medications since 2009 and identify trends in price increases for different classes of drugs. To perform this analysis, they sent surveys to 4 national chain pharmacies requesting price information for commonly prescribed dermatologic therapies in 2009, 2011, 2014, and 2015. The initial survey requested information on 72 brand-name drugs.
The findings of the analysis were staggering. Of the 19 brand-name drugs analyzed, the retail prices of 7 drugs more than quadrupled over the study period. The mean price increase for this group of drugs was 401% during the entire survey period.
Rosenberg and Rosenberg grouped the price increase by therapeutic class. Prices of topical antineoplastic therapies had the largest mean absolute and percentage increase ($10,926.58 [1240%]). Prices of drugs in the anti-infective class had the smallest mean absolute increase ($333.99); prices of psoriasis medications had the smallest mean percentage increase (180%). Prices of acne and rosacea medications had a mean increase of 195%, and prices of topical corticosteroids experienced a mean increase of 290%. Selected generic drugs examined in 2011 and 2014 also increased a mean of 279% during the 3-year period.
Rosenberg and Rosenberg noted that the increases for commonly prescribed medications greatly outpaced inflation, national health expenditure growth, and increases in reimbursements for physician services. They did not detect any specific trend to explain the substantial increase in the costs of dermatologic prescription drugs and they did not investigate reasons for the price increases.
What’s the issue?
Price increases for psoriatic and other therapies are creating barriers to both our appropriate treatment of patients and our ability to effectively practice medicine. How are you coping with this challenge in your practice?

One of the biggest burdens of modern clinical dermatology practice is the ability to obtain appropriate drug therapy for patients. In the current health care environment, insurance formularies have become increasingly restrictive and more individuals have to deal with high-deductible insurance plans.
In a JAMA Dermatology study published online on November 25, Rosenberg and Rosenberg sought to determine changes in the prices of commonly prescribed dermatologic medications since 2009 and identify trends in price increases for different classes of drugs. To perform this analysis, they sent surveys to 4 national chain pharmacies requesting price information for commonly prescribed dermatologic therapies in 2009, 2011, 2014, and 2015. The initial survey requested information on 72 brand-name drugs.
The findings of the analysis were staggering. Of the 19 brand-name drugs analyzed, the retail prices of 7 drugs more than quadrupled over the study period. The mean price increase for this group of drugs was 401% during the entire survey period.
Rosenberg and Rosenberg grouped the price increase by therapeutic class. Prices of topical antineoplastic therapies had the largest mean absolute and percentage increase ($10,926.58 [1240%]). Prices of drugs in the anti-infective class had the smallest mean absolute increase ($333.99); prices of psoriasis medications had the smallest mean percentage increase (180%). Prices of acne and rosacea medications had a mean increase of 195%, and prices of topical corticosteroids experienced a mean increase of 290%. Selected generic drugs examined in 2011 and 2014 also increased a mean of 279% during the 3-year period.
Rosenberg and Rosenberg noted that the increases for commonly prescribed medications greatly outpaced inflation, national health expenditure growth, and increases in reimbursements for physician services. They did not detect any specific trend to explain the substantial increase in the costs of dermatologic prescription drugs and they did not investigate reasons for the price increases.
What’s the issue?
Price increases for psoriatic and other therapies are creating barriers to both our appropriate treatment of patients and our ability to effectively practice medicine. How are you coping with this challenge in your practice?

One of the biggest burdens of modern clinical dermatology practice is the ability to obtain appropriate drug therapy for patients. In the current health care environment, insurance formularies have become increasingly restrictive and more individuals have to deal with high-deductible insurance plans.
In a JAMA Dermatology study published online on November 25, Rosenberg and Rosenberg sought to determine changes in the prices of commonly prescribed dermatologic medications since 2009 and identify trends in price increases for different classes of drugs. To perform this analysis, they sent surveys to 4 national chain pharmacies requesting price information for commonly prescribed dermatologic therapies in 2009, 2011, 2014, and 2015. The initial survey requested information on 72 brand-name drugs.
The findings of the analysis were staggering. Of the 19 brand-name drugs analyzed, the retail prices of 7 drugs more than quadrupled over the study period. The mean price increase for this group of drugs was 401% during the entire survey period.
Rosenberg and Rosenberg grouped the price increase by therapeutic class. Prices of topical antineoplastic therapies had the largest mean absolute and percentage increase ($10,926.58 [1240%]). Prices of drugs in the anti-infective class had the smallest mean absolute increase ($333.99); prices of psoriasis medications had the smallest mean percentage increase (180%). Prices of acne and rosacea medications had a mean increase of 195%, and prices of topical corticosteroids experienced a mean increase of 290%. Selected generic drugs examined in 2011 and 2014 also increased a mean of 279% during the 3-year period.
Rosenberg and Rosenberg noted that the increases for commonly prescribed medications greatly outpaced inflation, national health expenditure growth, and increases in reimbursements for physician services. They did not detect any specific trend to explain the substantial increase in the costs of dermatologic prescription drugs and they did not investigate reasons for the price increases.
What’s the issue?
Price increases for psoriatic and other therapies are creating barriers to both our appropriate treatment of patients and our ability to effectively practice medicine. How are you coping with this challenge in your practice?
Treatment failure reduced in Barrett’s esophagus with endoscopic mucosal resection
The use of endoscopic mucosal resection (EMR) before radiofrequency ablation significantly reduced the risk for treatment failure among patients with Barrett’s esophagus–associated intramucosal adenocarcinoma (IMC) and dysplasia, according to new data published in the American Journal of Surgical Pathology.
Complete eradication of IMC/dysplasia on the first follow-up endoscopy after treatment was achieved in 86% of patients, while durable eradication, defined as a complete recurrence that persisted until the last follow-up, was achieved in 78% of patients. However, there was significant variation between the different study sites (P = .03) and outcomes were significantly impacted by the baseline extent of IMC and the use of EMR prior to radiofrequency ablation (RFA) therapy.
In addition, almost a quarter of all patients developed treatment-related strictures, usually in the setting of multiple EMRs, and recurrence occurred as a malignant stricture in one patient.
Radiofrequency ablation used with or without EMR, is a safe, effective, and durable treatment option for the treatment of dysplasia associated with Barrett’s esophagus. However, studies that have assessed the predictors of treatment failure in Barrett’s esophagus-associated intramucosal adenocarcinoma (IMC) are limited.
In this study, Dr. Agoston T. Agoston of the department of pathology at Brigham and Women’s Hospital, Boston, and his colleagues investigated the rate of Barrett’s esophagus–associated IMC eradication when using RFA, with or without EMR, in a multicenter setting. In addition, they attempted to identify clinical and pathologic predictors of treatment failure.
“We anticipate that these data will have significant implications for a personalized treatment approach to patients with BE [Barrett’s esophagus]–associated IMC,” wrote the authors.
They conducted a retrospective review of medical records from four tertiary care academic medical centers, and identified 78 patients who underwent RFA with or without EMR as the primary treatment for biopsy-proven IMC.
Some notable baseline differences were observed in patient characteristics at the different study sites, including baseline Barrett’s esophagus segment length (P = .06), baseline nodularity (P = .08), and percentage of tissue involved by IMC at pretreatment endoscopy and biopsy (P = .01).
Over a mean follow-up time of 26.4 months (range, 2-116 months), 86% of patients achieved complete eradication and 78% durable eradication of IMC/dysplasia.
Within the cohort, 11 patients failed to achieve complete eradication, and of the 67 patients who initially did, 6 patients (9.0%) had a subsequent recurrence of neoplasia (3.91 recurrences per 100 patient-years). This extrapolated to an overall rate of 22% for treatment failure (17/78 patients). Of the 17 patients who failed the treatment, 3 subsequently underwent esophagectomy, 1 received palliative measures in the setting of advanced neurological disease, and 1 patient is currently undergoing a repeat ablation procedure with curative intent.
Dr. Agoston and his team also identified 2 clinicopathologic factors that were significantly associated with treatment failure, and both remained significant on univariate and multivariate analysis. The first was that the use of EMR prior to RFA was associated with a significantly reduced risk for treatment failure (hazard ratio, 0.15; 95% confidence interval, 0.05-0.48; P = .001), and the second was that the extent of IMC involving at least 50% of the columnar metaplastic area was associated with a significantly increased risk for treatment failure (HR, 4.24; 95% CI, 1.53-11.7; P = .005).
They also observed similar results when the analysis of extent of IMC as a predictor was restricted to a subset of 43 cases in which the diagnosis of IMC was made on EMR specimens only (HR, 10.8; 95% CI, 2.30-50.8; P = .003).
“In conclusion, we have identified endoscopic and pathologic factors associated with treatment success in patients with BE-associated IMC treated with RFA with or without EMR,” they wrote. “Utilization of these predictors can help in identifying patients with a high probability of success and also those patients with a higher risk for treatment failure for whom a more aggressive initial approach may be justified” (Am J Surg Pathol. 2015 Dec 5. doi: 10.1097/PAS.0000000000000566).
Dr. Rothstein and Dr. Abrams have received research support previously from Barrx/ Covidien and C2 Therapeutics/Covidien, respectively. None of the other authors reported significant conflicts of interest.
The use of endoscopic mucosal resection (EMR) before radiofrequency ablation significantly reduced the risk for treatment failure among patients with Barrett’s esophagus–associated intramucosal adenocarcinoma (IMC) and dysplasia, according to new data published in the American Journal of Surgical Pathology.
Complete eradication of IMC/dysplasia on the first follow-up endoscopy after treatment was achieved in 86% of patients, while durable eradication, defined as a complete recurrence that persisted until the last follow-up, was achieved in 78% of patients. However, there was significant variation between the different study sites (P = .03) and outcomes were significantly impacted by the baseline extent of IMC and the use of EMR prior to radiofrequency ablation (RFA) therapy.
In addition, almost a quarter of all patients developed treatment-related strictures, usually in the setting of multiple EMRs, and recurrence occurred as a malignant stricture in one patient.
Radiofrequency ablation used with or without EMR, is a safe, effective, and durable treatment option for the treatment of dysplasia associated with Barrett’s esophagus. However, studies that have assessed the predictors of treatment failure in Barrett’s esophagus-associated intramucosal adenocarcinoma (IMC) are limited.
In this study, Dr. Agoston T. Agoston of the department of pathology at Brigham and Women’s Hospital, Boston, and his colleagues investigated the rate of Barrett’s esophagus–associated IMC eradication when using RFA, with or without EMR, in a multicenter setting. In addition, they attempted to identify clinical and pathologic predictors of treatment failure.
“We anticipate that these data will have significant implications for a personalized treatment approach to patients with BE [Barrett’s esophagus]–associated IMC,” wrote the authors.
They conducted a retrospective review of medical records from four tertiary care academic medical centers, and identified 78 patients who underwent RFA with or without EMR as the primary treatment for biopsy-proven IMC.
Some notable baseline differences were observed in patient characteristics at the different study sites, including baseline Barrett’s esophagus segment length (P = .06), baseline nodularity (P = .08), and percentage of tissue involved by IMC at pretreatment endoscopy and biopsy (P = .01).
Over a mean follow-up time of 26.4 months (range, 2-116 months), 86% of patients achieved complete eradication and 78% durable eradication of IMC/dysplasia.
Within the cohort, 11 patients failed to achieve complete eradication, and of the 67 patients who initially did, 6 patients (9.0%) had a subsequent recurrence of neoplasia (3.91 recurrences per 100 patient-years). This extrapolated to an overall rate of 22% for treatment failure (17/78 patients). Of the 17 patients who failed the treatment, 3 subsequently underwent esophagectomy, 1 received palliative measures in the setting of advanced neurological disease, and 1 patient is currently undergoing a repeat ablation procedure with curative intent.
Dr. Agoston and his team also identified 2 clinicopathologic factors that were significantly associated with treatment failure, and both remained significant on univariate and multivariate analysis. The first was that the use of EMR prior to RFA was associated with a significantly reduced risk for treatment failure (hazard ratio, 0.15; 95% confidence interval, 0.05-0.48; P = .001), and the second was that the extent of IMC involving at least 50% of the columnar metaplastic area was associated with a significantly increased risk for treatment failure (HR, 4.24; 95% CI, 1.53-11.7; P = .005).
They also observed similar results when the analysis of extent of IMC as a predictor was restricted to a subset of 43 cases in which the diagnosis of IMC was made on EMR specimens only (HR, 10.8; 95% CI, 2.30-50.8; P = .003).
“In conclusion, we have identified endoscopic and pathologic factors associated with treatment success in patients with BE-associated IMC treated with RFA with or without EMR,” they wrote. “Utilization of these predictors can help in identifying patients with a high probability of success and also those patients with a higher risk for treatment failure for whom a more aggressive initial approach may be justified” (Am J Surg Pathol. 2015 Dec 5. doi: 10.1097/PAS.0000000000000566).
Dr. Rothstein and Dr. Abrams have received research support previously from Barrx/ Covidien and C2 Therapeutics/Covidien, respectively. None of the other authors reported significant conflicts of interest.
The use of endoscopic mucosal resection (EMR) before radiofrequency ablation significantly reduced the risk for treatment failure among patients with Barrett’s esophagus–associated intramucosal adenocarcinoma (IMC) and dysplasia, according to new data published in the American Journal of Surgical Pathology.
Complete eradication of IMC/dysplasia on the first follow-up endoscopy after treatment was achieved in 86% of patients, while durable eradication, defined as a complete recurrence that persisted until the last follow-up, was achieved in 78% of patients. However, there was significant variation between the different study sites (P = .03) and outcomes were significantly impacted by the baseline extent of IMC and the use of EMR prior to radiofrequency ablation (RFA) therapy.
In addition, almost a quarter of all patients developed treatment-related strictures, usually in the setting of multiple EMRs, and recurrence occurred as a malignant stricture in one patient.
Radiofrequency ablation used with or without EMR, is a safe, effective, and durable treatment option for the treatment of dysplasia associated with Barrett’s esophagus. However, studies that have assessed the predictors of treatment failure in Barrett’s esophagus-associated intramucosal adenocarcinoma (IMC) are limited.
In this study, Dr. Agoston T. Agoston of the department of pathology at Brigham and Women’s Hospital, Boston, and his colleagues investigated the rate of Barrett’s esophagus–associated IMC eradication when using RFA, with or without EMR, in a multicenter setting. In addition, they attempted to identify clinical and pathologic predictors of treatment failure.
“We anticipate that these data will have significant implications for a personalized treatment approach to patients with BE [Barrett’s esophagus]–associated IMC,” wrote the authors.
They conducted a retrospective review of medical records from four tertiary care academic medical centers, and identified 78 patients who underwent RFA with or without EMR as the primary treatment for biopsy-proven IMC.
Some notable baseline differences were observed in patient characteristics at the different study sites, including baseline Barrett’s esophagus segment length (P = .06), baseline nodularity (P = .08), and percentage of tissue involved by IMC at pretreatment endoscopy and biopsy (P = .01).
Over a mean follow-up time of 26.4 months (range, 2-116 months), 86% of patients achieved complete eradication and 78% durable eradication of IMC/dysplasia.
Within the cohort, 11 patients failed to achieve complete eradication, and of the 67 patients who initially did, 6 patients (9.0%) had a subsequent recurrence of neoplasia (3.91 recurrences per 100 patient-years). This extrapolated to an overall rate of 22% for treatment failure (17/78 patients). Of the 17 patients who failed the treatment, 3 subsequently underwent esophagectomy, 1 received palliative measures in the setting of advanced neurological disease, and 1 patient is currently undergoing a repeat ablation procedure with curative intent.
Dr. Agoston and his team also identified 2 clinicopathologic factors that were significantly associated with treatment failure, and both remained significant on univariate and multivariate analysis. The first was that the use of EMR prior to RFA was associated with a significantly reduced risk for treatment failure (hazard ratio, 0.15; 95% confidence interval, 0.05-0.48; P = .001), and the second was that the extent of IMC involving at least 50% of the columnar metaplastic area was associated with a significantly increased risk for treatment failure (HR, 4.24; 95% CI, 1.53-11.7; P = .005).
They also observed similar results when the analysis of extent of IMC as a predictor was restricted to a subset of 43 cases in which the diagnosis of IMC was made on EMR specimens only (HR, 10.8; 95% CI, 2.30-50.8; P = .003).
“In conclusion, we have identified endoscopic and pathologic factors associated with treatment success in patients with BE-associated IMC treated with RFA with or without EMR,” they wrote. “Utilization of these predictors can help in identifying patients with a high probability of success and also those patients with a higher risk for treatment failure for whom a more aggressive initial approach may be justified” (Am J Surg Pathol. 2015 Dec 5. doi: 10.1097/PAS.0000000000000566).
Dr. Rothstein and Dr. Abrams have received research support previously from Barrx/ Covidien and C2 Therapeutics/Covidien, respectively. None of the other authors reported significant conflicts of interest.
FROM THE AMERICAN JOURNAL OF SURGICAL PATHOLOGY
Key clinical point: The use of EMR before radiofrequency ablation significantly reduced the risk for treatment failure for IMC associated with Barrett’s esophagus.
Major finding: The overall rate of complete and durable IMC eradication in Barrett’s esophagus was 86% and 78%, respectively, during a mean follow-up of about 2 years, but was significantly impacted by the baseline extent of IMC and the use of EMR.
Data source: A retrospective review of data from four tertiary care academic medical centers that included 78 patients and was conducted to determine the rate of IMC eradication when using RFA and EMR.
Disclosures: Dr. Rothstein and Dr. Abrams have received research support previously from Barrx/ Covidien and C2 Therapeutics/Covidien, respectively. None of the other authors reported significant conflicts of interest.
Top New Year’s resolutions for your practice
By Sarah E. Streett, M.D., Chair, AGA Practice Management and Economics Committee, and Joel V. Brill, M.D., AGAF, AGA CPT Advisor
Have you taken steps to ensure that your practice will have a successful 2016? Here are six resolutions from AGA to help you improve your practice and prepare for the changing health-care environment.
1. Avoid penalties. Failing to demonstrate meaningful use with your electronic health records or failing to participate in the Physician Quality Reporting System (PQRS) during 2016 will cost you in 2018. Stay up to date with the latest requirements. AGA can help you meet PQRS and avoid penalties with the Digestive Health Recognition Program.™
2. Prepare for reimbursement cuts.
Evaluate the operations of your practice to minimize waste, excess expense, and rework due to mistakes. Efficiency will become crucial to your success as reimbursement rates to physicians and ASCs for colonoscopy procedures decline. You can also find helpful hints for efficiency and quality of care from NIH and HHS.
Review your commercial contracts. With reimbursement decreasing each year, protect yourself now by renegotiating multi-year contract rates with payors based on the 2015 fee schedule.
3. Be sure you’re up to date on your CPT coding. Did you know that AGA members can get two free coding/billing questions answered every 30 days? Visit the AGA Coding and Billing Corner and get started today at www.gastro.org/practice-management/coding/coding-billing-corner.
4. Increase efficiency and efficacy to increase impact for patients. Ensure your practice is conducive to a positive patient experience. In the new health-care landscape, it is crucial that you not only provide top-notch patient care, but also an overall positive patient experience.
5. Utilize the patient census in your practice. Think about strategizing with your patient census to maintain your position as a viable health-care business. Which payors are most beneficial to your practice? Who owns your referral sources?
6. Know how to make the Affordable Care Act work for your practice. Effective Dec. 23, 2015, commercial payors (but not Medicare) are required to cover a pre-procedure consultation prior to a screening colonoscopy, as well as the pathology resulting from the screening colonoscopy procedure, without patient financial responsibility. Make sure that your billers and referring physicians are familiar with the new regulations.
By Sarah E. Streett, M.D., Chair, AGA Practice Management and Economics Committee, and Joel V. Brill, M.D., AGAF, AGA CPT Advisor
Have you taken steps to ensure that your practice will have a successful 2016? Here are six resolutions from AGA to help you improve your practice and prepare for the changing health-care environment.
1. Avoid penalties. Failing to demonstrate meaningful use with your electronic health records or failing to participate in the Physician Quality Reporting System (PQRS) during 2016 will cost you in 2018. Stay up to date with the latest requirements. AGA can help you meet PQRS and avoid penalties with the Digestive Health Recognition Program.™
2. Prepare for reimbursement cuts.
Evaluate the operations of your practice to minimize waste, excess expense, and rework due to mistakes. Efficiency will become crucial to your success as reimbursement rates to physicians and ASCs for colonoscopy procedures decline. You can also find helpful hints for efficiency and quality of care from NIH and HHS.
Review your commercial contracts. With reimbursement decreasing each year, protect yourself now by renegotiating multi-year contract rates with payors based on the 2015 fee schedule.
3. Be sure you’re up to date on your CPT coding. Did you know that AGA members can get two free coding/billing questions answered every 30 days? Visit the AGA Coding and Billing Corner and get started today at www.gastro.org/practice-management/coding/coding-billing-corner.
4. Increase efficiency and efficacy to increase impact for patients. Ensure your practice is conducive to a positive patient experience. In the new health-care landscape, it is crucial that you not only provide top-notch patient care, but also an overall positive patient experience.
5. Utilize the patient census in your practice. Think about strategizing with your patient census to maintain your position as a viable health-care business. Which payors are most beneficial to your practice? Who owns your referral sources?
6. Know how to make the Affordable Care Act work for your practice. Effective Dec. 23, 2015, commercial payors (but not Medicare) are required to cover a pre-procedure consultation prior to a screening colonoscopy, as well as the pathology resulting from the screening colonoscopy procedure, without patient financial responsibility. Make sure that your billers and referring physicians are familiar with the new regulations.
By Sarah E. Streett, M.D., Chair, AGA Practice Management and Economics Committee, and Joel V. Brill, M.D., AGAF, AGA CPT Advisor
Have you taken steps to ensure that your practice will have a successful 2016? Here are six resolutions from AGA to help you improve your practice and prepare for the changing health-care environment.
1. Avoid penalties. Failing to demonstrate meaningful use with your electronic health records or failing to participate in the Physician Quality Reporting System (PQRS) during 2016 will cost you in 2018. Stay up to date with the latest requirements. AGA can help you meet PQRS and avoid penalties with the Digestive Health Recognition Program.™
2. Prepare for reimbursement cuts.
Evaluate the operations of your practice to minimize waste, excess expense, and rework due to mistakes. Efficiency will become crucial to your success as reimbursement rates to physicians and ASCs for colonoscopy procedures decline. You can also find helpful hints for efficiency and quality of care from NIH and HHS.
Review your commercial contracts. With reimbursement decreasing each year, protect yourself now by renegotiating multi-year contract rates with payors based on the 2015 fee schedule.
3. Be sure you’re up to date on your CPT coding. Did you know that AGA members can get two free coding/billing questions answered every 30 days? Visit the AGA Coding and Billing Corner and get started today at www.gastro.org/practice-management/coding/coding-billing-corner.
4. Increase efficiency and efficacy to increase impact for patients. Ensure your practice is conducive to a positive patient experience. In the new health-care landscape, it is crucial that you not only provide top-notch patient care, but also an overall positive patient experience.
5. Utilize the patient census in your practice. Think about strategizing with your patient census to maintain your position as a viable health-care business. Which payors are most beneficial to your practice? Who owns your referral sources?
6. Know how to make the Affordable Care Act work for your practice. Effective Dec. 23, 2015, commercial payors (but not Medicare) are required to cover a pre-procedure consultation prior to a screening colonoscopy, as well as the pathology resulting from the screening colonoscopy procedure, without patient financial responsibility. Make sure that your billers and referring physicians are familiar with the new regulations.