User login
HM Groups Invited to Participate in 2016 State of Hospital Medicine Survey
Every other year, SHM’s practice analysis subcommittee invites all U.S. hospital medicine groups to participate in the State of Hospital Medicine (SOHM) survey. Your responses generate the authoritative report on how today’s hospital medicine groups are organized, scheduled, funded, compensated, staffed, and much more. After months of refining and updating, the survey opened on Jan. 11. The time has arrived for you to respond to this critical survey!
Empower Your Hospitalist Program
Hospital medicine has seen the most dramatic growth and evolution of any specialty in the last two decades. Although all practices innovate in response to shifting demands, leaders and hospitalists alike need to understand how the frontrunners in this dynamic field have adapted. The SoHM report summarizes thousands of data points about the latest trends in hospital medicine practice design and productivity.
Hospitalist group leaders depend on this information to draw comparisons against national benchmarks, both for improvement and as a frame of reference for demonstrating the value your group provides to your hospital. However, the report is only as good as the number and quality of the responses to the survey.
How To Get Engaged
Responding to the survey is straightforward through the web-based questionnaire, and only one response is needed from each group. The survey does require some modest preparation to look up such practice characteristics as CPT code distribution, total RVU generation, and average number of shifts per FTE. For many groups, a hospitalist and a practice manager can collaborate to answer all of the questions accurately. If you haven’t already, take some basic steps to prepare:
- Discuss the survey at your next group meeting and advocate for responding.
- Determine who will complete the survey on behalf of your group.
- Visit www.hospitalmedicine.org/survey and download the survey instrument and instructions, share them with the lead respondent for your group.
- Submit your responses by March 11.
Of note, you’ll also want to participate in the Medical Group Management Association (MGMA) survey, as well. SHM licenses key portions of the SoHM report from MGMA, such as provider compensation, so the complete report depends on having great responses to both instruments.
Why Participate?
First, hospitalist groups that respond to the Survey will get a FREE copy of the report. Have you wondered things like:
- “How many groups are using a scheduling model other than 7-on, 7-off?”
- “What percentage of groups staff an observation unit?”
- “Are hospitalists groups taking on new roles in Accountable Care Organizations (ACOs)?”
- “How does compensation differ for providers who see children or are in academics?”
If so, you’ll have those answers at your fingertips and a whole lot more.
Second, you’ll have the satisfaction of knowing that you helped to make the SoHM report the indispensable tool upon which group leaders everywhere depend. The survey is anonymous, but respondents will know that the report presents data on the most relevant HM group of all—your own! Don’t wait.
Participate today at www.hospitalmedicine.org/survey. TH
Dr. White is assistant professor of medicine at the University of Washington and group director at the University of Washington Medical Center in Seattle, Wash.
Every other year, SHM’s practice analysis subcommittee invites all U.S. hospital medicine groups to participate in the State of Hospital Medicine (SOHM) survey. Your responses generate the authoritative report on how today’s hospital medicine groups are organized, scheduled, funded, compensated, staffed, and much more. After months of refining and updating, the survey opened on Jan. 11. The time has arrived for you to respond to this critical survey!
Empower Your Hospitalist Program
Hospital medicine has seen the most dramatic growth and evolution of any specialty in the last two decades. Although all practices innovate in response to shifting demands, leaders and hospitalists alike need to understand how the frontrunners in this dynamic field have adapted. The SoHM report summarizes thousands of data points about the latest trends in hospital medicine practice design and productivity.
Hospitalist group leaders depend on this information to draw comparisons against national benchmarks, both for improvement and as a frame of reference for demonstrating the value your group provides to your hospital. However, the report is only as good as the number and quality of the responses to the survey.
How To Get Engaged
Responding to the survey is straightforward through the web-based questionnaire, and only one response is needed from each group. The survey does require some modest preparation to look up such practice characteristics as CPT code distribution, total RVU generation, and average number of shifts per FTE. For many groups, a hospitalist and a practice manager can collaborate to answer all of the questions accurately. If you haven’t already, take some basic steps to prepare:
- Discuss the survey at your next group meeting and advocate for responding.
- Determine who will complete the survey on behalf of your group.
- Visit www.hospitalmedicine.org/survey and download the survey instrument and instructions, share them with the lead respondent for your group.
- Submit your responses by March 11.
Of note, you’ll also want to participate in the Medical Group Management Association (MGMA) survey, as well. SHM licenses key portions of the SoHM report from MGMA, such as provider compensation, so the complete report depends on having great responses to both instruments.
Why Participate?
First, hospitalist groups that respond to the Survey will get a FREE copy of the report. Have you wondered things like:
- “How many groups are using a scheduling model other than 7-on, 7-off?”
- “What percentage of groups staff an observation unit?”
- “Are hospitalists groups taking on new roles in Accountable Care Organizations (ACOs)?”
- “How does compensation differ for providers who see children or are in academics?”
If so, you’ll have those answers at your fingertips and a whole lot more.
Second, you’ll have the satisfaction of knowing that you helped to make the SoHM report the indispensable tool upon which group leaders everywhere depend. The survey is anonymous, but respondents will know that the report presents data on the most relevant HM group of all—your own! Don’t wait.
Participate today at www.hospitalmedicine.org/survey. TH
Dr. White is assistant professor of medicine at the University of Washington and group director at the University of Washington Medical Center in Seattle, Wash.
Every other year, SHM’s practice analysis subcommittee invites all U.S. hospital medicine groups to participate in the State of Hospital Medicine (SOHM) survey. Your responses generate the authoritative report on how today’s hospital medicine groups are organized, scheduled, funded, compensated, staffed, and much more. After months of refining and updating, the survey opened on Jan. 11. The time has arrived for you to respond to this critical survey!
Empower Your Hospitalist Program
Hospital medicine has seen the most dramatic growth and evolution of any specialty in the last two decades. Although all practices innovate in response to shifting demands, leaders and hospitalists alike need to understand how the frontrunners in this dynamic field have adapted. The SoHM report summarizes thousands of data points about the latest trends in hospital medicine practice design and productivity.
Hospitalist group leaders depend on this information to draw comparisons against national benchmarks, both for improvement and as a frame of reference for demonstrating the value your group provides to your hospital. However, the report is only as good as the number and quality of the responses to the survey.
How To Get Engaged
Responding to the survey is straightforward through the web-based questionnaire, and only one response is needed from each group. The survey does require some modest preparation to look up such practice characteristics as CPT code distribution, total RVU generation, and average number of shifts per FTE. For many groups, a hospitalist and a practice manager can collaborate to answer all of the questions accurately. If you haven’t already, take some basic steps to prepare:
- Discuss the survey at your next group meeting and advocate for responding.
- Determine who will complete the survey on behalf of your group.
- Visit www.hospitalmedicine.org/survey and download the survey instrument and instructions, share them with the lead respondent for your group.
- Submit your responses by March 11.
Of note, you’ll also want to participate in the Medical Group Management Association (MGMA) survey, as well. SHM licenses key portions of the SoHM report from MGMA, such as provider compensation, so the complete report depends on having great responses to both instruments.
Why Participate?
First, hospitalist groups that respond to the Survey will get a FREE copy of the report. Have you wondered things like:
- “How many groups are using a scheduling model other than 7-on, 7-off?”
- “What percentage of groups staff an observation unit?”
- “Are hospitalists groups taking on new roles in Accountable Care Organizations (ACOs)?”
- “How does compensation differ for providers who see children or are in academics?”
If so, you’ll have those answers at your fingertips and a whole lot more.
Second, you’ll have the satisfaction of knowing that you helped to make the SoHM report the indispensable tool upon which group leaders everywhere depend. The survey is anonymous, but respondents will know that the report presents data on the most relevant HM group of all—your own! Don’t wait.
Participate today at www.hospitalmedicine.org/survey. TH
Dr. White is assistant professor of medicine at the University of Washington and group director at the University of Washington Medical Center in Seattle, Wash.
End-of-life cancer care by country
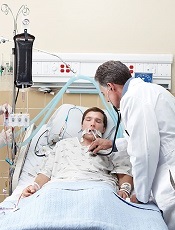
in the intensive care unit
A study of end-of-life cancer care practices in 7 countries suggests the US has the lowest proportion of deaths in the hospital and the lowest number of days in the hospital for patients in their last 6 months of life.
However, the US performed poorly in other aspects of care, particularly intensive care unit admissions and hospital expenditures.
The other countries included in the study were Belgium, Canada, England, Germany, the Netherlands, and Norway.
The research was published in JAMA.
Ezekiel J. Emanuel, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues examined patterns of care, healthcare utilization, and expenditures for dying cancer patients in the 7 aforementioned countries.
The researchers first analyzed data from 2010 that included subjects older than 65 years of age who died with cancer.
The proportion of patients who died in the hospital was 22.2% in the US, 29.4% in the Netherlands, 38.3% in Germany, 41.7% in England, 44.7% in Norway, 51.2% in Belgium, and 52.1% in Canada.
In the last 180 days of life, the mean number of days in the hospital per capita was 27.7 in Belgium, 24.8 in Norway, 21.7 in Germany, 19 in Canada, 18.3 in England, 17.8 in the Netherlands, and 10.7 in the US.
The proportion of patients admitted to the intensive care unit in their last 180 days of life was 40.3% in the US, 18.5% in Belgium, 15.2% in Canada, 10.2% in the Netherlands, and 8.2% in Germany. Data were not available for England and Norway.
In the last 180 days of life, average per capita hospital expenditures (in USD) were higher in Canada ($21,840), Norway ($19,783), and the US ($18,500), intermediate in Germany ($16,221) and Belgium ($15,699), and lowest in the Netherlands ($10,936) and England ($9342).
Analyses that included decedents of any age, decedents older than 65 years of age with lung cancer, and decedents older than 65 years in the US and Germany from 2012 showed similar results.
The researchers said this suggests the differences observed were driven more by end-of-life care practices and organization rather than differences in cohort identification. ![]()

in the intensive care unit
A study of end-of-life cancer care practices in 7 countries suggests the US has the lowest proportion of deaths in the hospital and the lowest number of days in the hospital for patients in their last 6 months of life.
However, the US performed poorly in other aspects of care, particularly intensive care unit admissions and hospital expenditures.
The other countries included in the study were Belgium, Canada, England, Germany, the Netherlands, and Norway.
The research was published in JAMA.
Ezekiel J. Emanuel, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues examined patterns of care, healthcare utilization, and expenditures for dying cancer patients in the 7 aforementioned countries.
The researchers first analyzed data from 2010 that included subjects older than 65 years of age who died with cancer.
The proportion of patients who died in the hospital was 22.2% in the US, 29.4% in the Netherlands, 38.3% in Germany, 41.7% in England, 44.7% in Norway, 51.2% in Belgium, and 52.1% in Canada.
In the last 180 days of life, the mean number of days in the hospital per capita was 27.7 in Belgium, 24.8 in Norway, 21.7 in Germany, 19 in Canada, 18.3 in England, 17.8 in the Netherlands, and 10.7 in the US.
The proportion of patients admitted to the intensive care unit in their last 180 days of life was 40.3% in the US, 18.5% in Belgium, 15.2% in Canada, 10.2% in the Netherlands, and 8.2% in Germany. Data were not available for England and Norway.
In the last 180 days of life, average per capita hospital expenditures (in USD) were higher in Canada ($21,840), Norway ($19,783), and the US ($18,500), intermediate in Germany ($16,221) and Belgium ($15,699), and lowest in the Netherlands ($10,936) and England ($9342).
Analyses that included decedents of any age, decedents older than 65 years of age with lung cancer, and decedents older than 65 years in the US and Germany from 2012 showed similar results.
The researchers said this suggests the differences observed were driven more by end-of-life care practices and organization rather than differences in cohort identification. ![]()

in the intensive care unit
A study of end-of-life cancer care practices in 7 countries suggests the US has the lowest proportion of deaths in the hospital and the lowest number of days in the hospital for patients in their last 6 months of life.
However, the US performed poorly in other aspects of care, particularly intensive care unit admissions and hospital expenditures.
The other countries included in the study were Belgium, Canada, England, Germany, the Netherlands, and Norway.
The research was published in JAMA.
Ezekiel J. Emanuel, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues examined patterns of care, healthcare utilization, and expenditures for dying cancer patients in the 7 aforementioned countries.
The researchers first analyzed data from 2010 that included subjects older than 65 years of age who died with cancer.
The proportion of patients who died in the hospital was 22.2% in the US, 29.4% in the Netherlands, 38.3% in Germany, 41.7% in England, 44.7% in Norway, 51.2% in Belgium, and 52.1% in Canada.
In the last 180 days of life, the mean number of days in the hospital per capita was 27.7 in Belgium, 24.8 in Norway, 21.7 in Germany, 19 in Canada, 18.3 in England, 17.8 in the Netherlands, and 10.7 in the US.
The proportion of patients admitted to the intensive care unit in their last 180 days of life was 40.3% in the US, 18.5% in Belgium, 15.2% in Canada, 10.2% in the Netherlands, and 8.2% in Germany. Data were not available for England and Norway.
In the last 180 days of life, average per capita hospital expenditures (in USD) were higher in Canada ($21,840), Norway ($19,783), and the US ($18,500), intermediate in Germany ($16,221) and Belgium ($15,699), and lowest in the Netherlands ($10,936) and England ($9342).
Analyses that included decedents of any age, decedents older than 65 years of age with lung cancer, and decedents older than 65 years in the US and Germany from 2012 showed similar results.
The researchers said this suggests the differences observed were driven more by end-of-life care practices and organization rather than differences in cohort identification. ![]()
Drug granted another breakthrough designation for CLL

The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to the BCL-2 inhibitor venetoclax when given with rituximab to treat patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Venetoclax already had breakthrough designation from the FDA as single-agent treatment for patients with relapsed or refractory CLL and 17p deletion.
The drug was granted priority review for this indication as well.
Breakthrough therapy designation is designed to accelerate the development and review of medicines that demonstrate early clinical evidence of a substantial improvement over current treatment options for serious diseases.
The latest breakthrough designation for venetoclax is supported by a phase 2 study of the drug in combination with rituximab in patients with relapsed/refractory CLL. Results from this trial were presented at the 2015 ASH Annual Meeting (abstract 325).
Another phase 2 trial presented at that meeting (abstract LBA-6) showed that single-agent venetoclax is effective against CLL as well.
The drug has also proven active against other hematologic malignancies, including acute myeloid lekemia and multiple myeloma.
However, venetoclax has been shown to pose a risk of tumor lysis syndrome (TLS). In fact, TLS-related deaths temporarily halted enrollment in trials of venetoclax. But researchers discovered ways to reduce the risk of TLS, and the trials continued.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche. ![]()

The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to the BCL-2 inhibitor venetoclax when given with rituximab to treat patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Venetoclax already had breakthrough designation from the FDA as single-agent treatment for patients with relapsed or refractory CLL and 17p deletion.
The drug was granted priority review for this indication as well.
Breakthrough therapy designation is designed to accelerate the development and review of medicines that demonstrate early clinical evidence of a substantial improvement over current treatment options for serious diseases.
The latest breakthrough designation for venetoclax is supported by a phase 2 study of the drug in combination with rituximab in patients with relapsed/refractory CLL. Results from this trial were presented at the 2015 ASH Annual Meeting (abstract 325).
Another phase 2 trial presented at that meeting (abstract LBA-6) showed that single-agent venetoclax is effective against CLL as well.
The drug has also proven active against other hematologic malignancies, including acute myeloid lekemia and multiple myeloma.
However, venetoclax has been shown to pose a risk of tumor lysis syndrome (TLS). In fact, TLS-related deaths temporarily halted enrollment in trials of venetoclax. But researchers discovered ways to reduce the risk of TLS, and the trials continued.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche. ![]()

The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to the BCL-2 inhibitor venetoclax when given with rituximab to treat patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Venetoclax already had breakthrough designation from the FDA as single-agent treatment for patients with relapsed or refractory CLL and 17p deletion.
The drug was granted priority review for this indication as well.
Breakthrough therapy designation is designed to accelerate the development and review of medicines that demonstrate early clinical evidence of a substantial improvement over current treatment options for serious diseases.
The latest breakthrough designation for venetoclax is supported by a phase 2 study of the drug in combination with rituximab in patients with relapsed/refractory CLL. Results from this trial were presented at the 2015 ASH Annual Meeting (abstract 325).
Another phase 2 trial presented at that meeting (abstract LBA-6) showed that single-agent venetoclax is effective against CLL as well.
The drug has also proven active against other hematologic malignancies, including acute myeloid lekemia and multiple myeloma.
However, venetoclax has been shown to pose a risk of tumor lysis syndrome (TLS). In fact, TLS-related deaths temporarily halted enrollment in trials of venetoclax. But researchers discovered ways to reduce the risk of TLS, and the trials continued.
Venetoclax is being developed by AbbVie in partnership with Genentech and Roche. ![]()
Obesity linked to VTE in kids

Photo by Matthew Lester
A single-center, retrospective study has revealed an association between obesity and venous thromboembolism (VTE) in children and adolescents.
While obesity is a well-established risk factor for VTE in adults, previous studies in pediatric populations have yielded mixed results.
The new study, however, showed that obesity, as determined by body mass index (BMI), was a statistically significant predictor of VTE in juveniles.
The research was published in Hospital Pediatrics.
“This is important because the incidence of pediatric VTE has increased dramatically over the last 20 years, and childhood obesity remains highly prevalent in the United States,” said study author Elizabeth Halvorson, MD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
For this study, she and her colleagues conducted a retrospective chart review of inpatients at Wake Forest Baptist’s Brenner Children’s Hospital between January 2000 and September 2012.
The researchers identified 88 patients, ages 2 to 18, who had confirmed cases of VTE. The team compared these patients to control subjects (2 controls per case) matched by age, gender, and the presence of a central venous catheter.
Of the 88 patients with VTE, 33 (37.5%) were obese, although most of them had known risk factors for VTE in addition to obesity.
In univariate analysis, the researchers found a statistically significant association between VTE and obesity, or increased BMI z score (P=0.002).
In a multivariate analysis, obesity remained a significant predictor of VTE. The odds ratio (OR) was 3.1 (P=0.007).
Other factors were significant predictors of VTE as well, including bacteremia (OR: 4.9; P=0.02), a stay in the intensive care unit (OR: 2.5; P=0.02), and the use of oral contraceptives (OR: 17.4; P<0.001).
“Our study presents data from a single institution with a relatively small sample size,” Dr Halvorson noted. “But it does demonstrate an association between obesity and VTE in children, which should be explored further in larger future studies.” ![]()

Photo by Matthew Lester
A single-center, retrospective study has revealed an association between obesity and venous thromboembolism (VTE) in children and adolescents.
While obesity is a well-established risk factor for VTE in adults, previous studies in pediatric populations have yielded mixed results.
The new study, however, showed that obesity, as determined by body mass index (BMI), was a statistically significant predictor of VTE in juveniles.
The research was published in Hospital Pediatrics.
“This is important because the incidence of pediatric VTE has increased dramatically over the last 20 years, and childhood obesity remains highly prevalent in the United States,” said study author Elizabeth Halvorson, MD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
For this study, she and her colleagues conducted a retrospective chart review of inpatients at Wake Forest Baptist’s Brenner Children’s Hospital between January 2000 and September 2012.
The researchers identified 88 patients, ages 2 to 18, who had confirmed cases of VTE. The team compared these patients to control subjects (2 controls per case) matched by age, gender, and the presence of a central venous catheter.
Of the 88 patients with VTE, 33 (37.5%) were obese, although most of them had known risk factors for VTE in addition to obesity.
In univariate analysis, the researchers found a statistically significant association between VTE and obesity, or increased BMI z score (P=0.002).
In a multivariate analysis, obesity remained a significant predictor of VTE. The odds ratio (OR) was 3.1 (P=0.007).
Other factors were significant predictors of VTE as well, including bacteremia (OR: 4.9; P=0.02), a stay in the intensive care unit (OR: 2.5; P=0.02), and the use of oral contraceptives (OR: 17.4; P<0.001).
“Our study presents data from a single institution with a relatively small sample size,” Dr Halvorson noted. “But it does demonstrate an association between obesity and VTE in children, which should be explored further in larger future studies.” ![]()

Photo by Matthew Lester
A single-center, retrospective study has revealed an association between obesity and venous thromboembolism (VTE) in children and adolescents.
While obesity is a well-established risk factor for VTE in adults, previous studies in pediatric populations have yielded mixed results.
The new study, however, showed that obesity, as determined by body mass index (BMI), was a statistically significant predictor of VTE in juveniles.
The research was published in Hospital Pediatrics.
“This is important because the incidence of pediatric VTE has increased dramatically over the last 20 years, and childhood obesity remains highly prevalent in the United States,” said study author Elizabeth Halvorson, MD, of Wake Forest Baptist Medical Center in Winston-Salem, North Carolina.
For this study, she and her colleagues conducted a retrospective chart review of inpatients at Wake Forest Baptist’s Brenner Children’s Hospital between January 2000 and September 2012.
The researchers identified 88 patients, ages 2 to 18, who had confirmed cases of VTE. The team compared these patients to control subjects (2 controls per case) matched by age, gender, and the presence of a central venous catheter.
Of the 88 patients with VTE, 33 (37.5%) were obese, although most of them had known risk factors for VTE in addition to obesity.
In univariate analysis, the researchers found a statistically significant association between VTE and obesity, or increased BMI z score (P=0.002).
In a multivariate analysis, obesity remained a significant predictor of VTE. The odds ratio (OR) was 3.1 (P=0.007).
Other factors were significant predictors of VTE as well, including bacteremia (OR: 4.9; P=0.02), a stay in the intensive care unit (OR: 2.5; P=0.02), and the use of oral contraceptives (OR: 17.4; P<0.001).
“Our study presents data from a single institution with a relatively small sample size,” Dr Halvorson noted. “But it does demonstrate an association between obesity and VTE in children, which should be explored further in larger future studies.” ![]()
Protein may be therapeutic target for AML

and Matt McCormack, PhD
Photo courtesy of the
Walter and Eliza Hall
Institute of Medical Research
Preclinical research suggests the Hhex protein could be a cancer-specific therapeutic target for acute myeloid leukemia (AML).
Investigators discovered that loss of the Hhex protein halted leukemia cell growth and division in vitro and in vivo, but normal cells were unaffected by the loss of Hhex.
Matt McCormack, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues relayed these findings in Genes and Development.
“There is an urgent need for new therapies to treat AML,” Dr McCormack said. “We showed blocking the Hhex protein could put the brakes on leukemia growth and completely eliminate AML in preclinical models. This could be targeted by new drugs to treat AML in humans.”
Specifically, the investigators found that Hhex was overexpressed in human AML, and the protein was essential for the maintenance of AML driven by the oncogenic fusion protein MLL-ENL and its downstream effectors, HoxA9 and Meis1.
However, Hhex was not required for normal myelopoiesis.
“Hhex is only essential for the leukemic cells, meaning we could target and treat leukemia without toxic effects on normal cells, avoiding many of the serious side effects that come with standard cancer treatments,” Dr McCormack said.
“We also know that most people with AML have increased levels of Hhex, often associated with adverse outcomes, further indicating it is an important target for new AML drugs.”
Dr McCormack and his colleagues also attempted to determine the mechanism by which Hhex promotes AML.
They found the protein represses the tumor suppressors p16INK4a and p19ARF in leukemic stem cells by regulating the Polycomb-repressive complex 2 (PRC2). They said that Hhex binds to the Cdkn2a locus and directly interacts with PRC2 to enable H3K27me3-mediated epigenetic repression.
“Hhex works by recruiting epigenetic factors to growth-control genes, effectively silencing them,” said author Ben Shields, PhD, also of the Walter and Eliza Hall Institute.
“This allows the leukemia cells to reproduce and accumulate more damage, contributing to the speed of AML progression.”
Dr McCormack said that although drugs inhibiting epigenetic modification have been tested against AML in the past, they have caused significant toxicity because their targets are also required for normal blood cell function.
“Unlike the epigenetic factors targeted previously, Hhex only regulates a small number of genes and is dispensable for normal blood cells,” Dr McCormack reiterated.
“This gives us a rare opportunity to kill AML cells without causing many side effects. We now hope to identify the critical regions of the Hhex protein that enable it to function, which will allow us to design much-needed new drugs to treat AML.” ![]()

and Matt McCormack, PhD
Photo courtesy of the
Walter and Eliza Hall
Institute of Medical Research
Preclinical research suggests the Hhex protein could be a cancer-specific therapeutic target for acute myeloid leukemia (AML).
Investigators discovered that loss of the Hhex protein halted leukemia cell growth and division in vitro and in vivo, but normal cells were unaffected by the loss of Hhex.
Matt McCormack, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues relayed these findings in Genes and Development.
“There is an urgent need for new therapies to treat AML,” Dr McCormack said. “We showed blocking the Hhex protein could put the brakes on leukemia growth and completely eliminate AML in preclinical models. This could be targeted by new drugs to treat AML in humans.”
Specifically, the investigators found that Hhex was overexpressed in human AML, and the protein was essential for the maintenance of AML driven by the oncogenic fusion protein MLL-ENL and its downstream effectors, HoxA9 and Meis1.
However, Hhex was not required for normal myelopoiesis.
“Hhex is only essential for the leukemic cells, meaning we could target and treat leukemia without toxic effects on normal cells, avoiding many of the serious side effects that come with standard cancer treatments,” Dr McCormack said.
“We also know that most people with AML have increased levels of Hhex, often associated with adverse outcomes, further indicating it is an important target for new AML drugs.”
Dr McCormack and his colleagues also attempted to determine the mechanism by which Hhex promotes AML.
They found the protein represses the tumor suppressors p16INK4a and p19ARF in leukemic stem cells by regulating the Polycomb-repressive complex 2 (PRC2). They said that Hhex binds to the Cdkn2a locus and directly interacts with PRC2 to enable H3K27me3-mediated epigenetic repression.
“Hhex works by recruiting epigenetic factors to growth-control genes, effectively silencing them,” said author Ben Shields, PhD, also of the Walter and Eliza Hall Institute.
“This allows the leukemia cells to reproduce and accumulate more damage, contributing to the speed of AML progression.”
Dr McCormack said that although drugs inhibiting epigenetic modification have been tested against AML in the past, they have caused significant toxicity because their targets are also required for normal blood cell function.
“Unlike the epigenetic factors targeted previously, Hhex only regulates a small number of genes and is dispensable for normal blood cells,” Dr McCormack reiterated.
“This gives us a rare opportunity to kill AML cells without causing many side effects. We now hope to identify the critical regions of the Hhex protein that enable it to function, which will allow us to design much-needed new drugs to treat AML.” ![]()

and Matt McCormack, PhD
Photo courtesy of the
Walter and Eliza Hall
Institute of Medical Research
Preclinical research suggests the Hhex protein could be a cancer-specific therapeutic target for acute myeloid leukemia (AML).
Investigators discovered that loss of the Hhex protein halted leukemia cell growth and division in vitro and in vivo, but normal cells were unaffected by the loss of Hhex.
Matt McCormack, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia, and his colleagues relayed these findings in Genes and Development.
“There is an urgent need for new therapies to treat AML,” Dr McCormack said. “We showed blocking the Hhex protein could put the brakes on leukemia growth and completely eliminate AML in preclinical models. This could be targeted by new drugs to treat AML in humans.”
Specifically, the investigators found that Hhex was overexpressed in human AML, and the protein was essential for the maintenance of AML driven by the oncogenic fusion protein MLL-ENL and its downstream effectors, HoxA9 and Meis1.
However, Hhex was not required for normal myelopoiesis.
“Hhex is only essential for the leukemic cells, meaning we could target and treat leukemia without toxic effects on normal cells, avoiding many of the serious side effects that come with standard cancer treatments,” Dr McCormack said.
“We also know that most people with AML have increased levels of Hhex, often associated with adverse outcomes, further indicating it is an important target for new AML drugs.”
Dr McCormack and his colleagues also attempted to determine the mechanism by which Hhex promotes AML.
They found the protein represses the tumor suppressors p16INK4a and p19ARF in leukemic stem cells by regulating the Polycomb-repressive complex 2 (PRC2). They said that Hhex binds to the Cdkn2a locus and directly interacts with PRC2 to enable H3K27me3-mediated epigenetic repression.
“Hhex works by recruiting epigenetic factors to growth-control genes, effectively silencing them,” said author Ben Shields, PhD, also of the Walter and Eliza Hall Institute.
“This allows the leukemia cells to reproduce and accumulate more damage, contributing to the speed of AML progression.”
Dr McCormack said that although drugs inhibiting epigenetic modification have been tested against AML in the past, they have caused significant toxicity because their targets are also required for normal blood cell function.
“Unlike the epigenetic factors targeted previously, Hhex only regulates a small number of genes and is dispensable for normal blood cells,” Dr McCormack reiterated.
“This gives us a rare opportunity to kill AML cells without causing many side effects. We now hope to identify the critical regions of the Hhex protein that enable it to function, which will allow us to design much-needed new drugs to treat AML.” ![]()
Poor Hospital Mobility
Low mobility is common in hospitalized older patients, and an independent predictor of poor functional outcomes.[1, 2, 3, 4] Few studies have included younger patients, but care models that support early mobility may reduce functional decline, enhance recovery, and reduce length of stay in older and mixed‐age populations.[5, 6] Barriers to mobility are complex and include patient symptoms and tethers, health provider behavior, team communication, and leadership, device availability, and environmental factors.[7, 8, 9, 10, 11] These contextual factors may differ even within a hospital between patient groups and ward settings. Simple measures to quantify mobility patterns would help address these barriers by providing opportunities for audit and feedback. Although accelerometry is the gold standard method for research, it requires equipment, analysis skills, and patient consent, which limits application in clinical practice. Behavioral mapping is a systematic method of observation developed in stroke patients, which is simple, objective, and requires no direct patient or staff participation,[12] and physical activity levels estimated from behavioral mapping are similar to those identified by accelerometry.[3, 13, 14] In the context of a phased quality‐improvement project aiming to reduce functional decline,[15] we undertook a cross‐sectional audit of mobility on 3 different wards using behavioral mapping, and examined differences among wards and between older (aged 65 years or more) and younger patients.
METHODS
This prospective observational study used cross‐sectional sampling from a 26‐bed general medical ward, a 30‐bed oncology ward, and a 24‐bed vascular surgical ward in a 900‐bed tertiary teaching hospital in Brisbane, Australia. Sampling was undertaken during 4 observation periods (2 mornings [10001400] and 2 afternoons [1400‐1800]) within 10 days in May 2013. All patients on each ward for each period were observed unless they were receiving end‐of‐life care. Structured observations were undertaken using behavioral mapping protocols similar to those previously described in stroke and general medical patients,[12, 13] with each patient room visited in the same sequence. Participants in each room were observed for a 2‐minute period (up to 4 participants could be observed concurrently in shared rooms) before moving to the next room, and the sequence was repeated in the same order for the whole 4‐hour period, with a single 15‐minute break. Depending on ward size and layout, this provided 12 to 17 observations per participant for each 4‐hour period (each individual observed every 1218 minutes). Observations were undertaken by 4 trained physiotherapy student observers using a predetermined set of mutually exclusive levels (lying in bed, sitting in or on the bed, sitting on a chair, standing, actively wheeling, or walking). The study was approved by the Royal Brisbane and Women's Hospital Human Research Ethics Committee as part of a quality‐improvement activity, and individual consent was not required. No clinical data except age and gender were collected for participants. The nurse unit manager for each ward was introduced to the observers and aware that observations were being conducted.
Patients who were observed for less than one‐half of an observation period were excluded so that all participants contributed at least 2 hours of observational data, up to a maximum of 16 hours. The number of valid observations for each participant (excluding time off ward or behind curtains if the level was not apparent) was calculated and used to derive the proportion of valid observations spent at each level for each participant. The proportion of observations at each level was summarized across all participants using frequency distributions and summary statistics. For ease of presentation, mean percentage of observed time in each activity was presented. However, as data were not normally distributed, statistical comparisons were undertaken using the Kruskal‐Wallis test, comparing the distribution of time spent upright (standing, walking, or actively wheeling) between groups (age group and ward). Interaction between age and ward effects was sought using generalized linear modeling.
RESULTS
Valid observations (at least 2 hours in 1 or more observation period) were available for 132 patients (48 medical, 50 oncology, and 34 surgery). Of these, 67 (51 %) were aged 65 years (54% medical, 44% oncology, 56% surgery) and 62 (47%) were male. There were a total of 3891 observations of location (median, 30 per patient; range, 965). Participants were observed in the bedded area 85.1% of observations, with 3.1% in the bathroom, 3.2% in the hallway or patient lounge, and 8.6% off ward. Allowing for time off ward and behind curtains, when observers could not be sure of their activity level, 3272 valid observations were available for physical activity.
More than half of the observed time (mean 57.4%) was spent lying in bed, 33.6% sitting on the bed or chair, and 9.0% standing, walking, or wheeling. Across all observation periods, 39/132 (29.5%) participants were never observed to be standing, walking or wheeling, and 7.6% were in bed at all observations. Comparing older and younger patients (Table 1), there was no difference in the time spent in active upright postures (median, 6.1% in older vs 7.4% in younger; P = 0.30). Table 2 summarizes descriptive data for the different wards. In the medical and surgical wards, 84% of the time was spend in or on the bed, and only 16% of the time was spent sitting in a chair or in active upright postures. Surgical patients, in particular, spent two‐thirds of observation time lying flat in bed, whereas medical patients spent more time sitting up on the bed. On statistical testing, time spent standing/walking/wheeling was significantly lower on the surgical ward (median, 4%; interquartile range [IQR], 010 for surgery; median, 7%; IQR, 013 for medical; and median, 10%; IQR 317 for oncology; P = 0.015). This was also reflected in a higher proportion of surgical patients never seen in an active upright position (44.1% compared to 27.1% medical and 22.0% oncology). Multivariate modeling showed no significant interaction between age and ward.
| All Ages, n = 132, Median Observations 29.5, Range 665* | Aged 65 Years, n = 61, Median Observations 30, Range 665 | Aged 65 Years, n = 67, Median Observations 27, Range 665 | |
|---|---|---|---|
| |||
| Location | |||
| Bedroom | 85.1 (13.3) | 84.6 (13.4) | 85.5 (12.9) |
| Bathroom | 3.0 (4.0) | 2.6 (3.9) | 3.4 (4.1) |
| Hall | 2.9 (4.6) | 3.4 (5.4) | 2.7 (4.0) |
| Lounge | 0.3 (1.9) | 0 | 0.6 (2.7) |
| Off ward/other | 8.6 (11.6) | 9.3 (11.4) | 7.8 (11.1) |
| Physical activity | |||
| Lie in bed | 57.4 (30.0) | 59.4 (29.4) | 55.5 (31.6) |
| Sit on bed | 21.0 (23.2) | 16.9 (19.9) | 24.7 (25.7) |
| Sit on chair | 12.6 (22.9) | 14.0 (25.6) | 11.9 (20.9) |
| Stand/walk/wheel | 9.0 (9.3) | 9.6 (9.6) | 8.0 (8.5) |
| Medical, n = 48, Median Observations 30, Range 759 | Oncology, n = 50, Median Observations 25, Range 652 | Surgical, n = 34, Median Observations 31, Range 1765 | |
|---|---|---|---|
| Location | |||
| Bedroom | 89.1 (11.4) | 81.3 (13.6) | 85.3 (14.1) |
| Bathroom | 2.8 (4.1) | 3.1 (3.8) | 3.1 (4.2) |
| Hall | 1.5 (2.5) | 5.3 (6.1) | 1.5 (2.7) |
| Lounge | 0.5 (2.0) | 0.4 (2.5) | 0 |
| Off ward/other | 6.2 (10.2) | 10.0 (11.9) | 10.1 (12.6) |
| Physical activity | |||
| Lie in bed | 53.3 (31.4) | 56.1 (30.2) | 65.1 (27.0) |
| Sit on bed | 30.3 (29.5) | 13.4 (16.1) | 19.0 (17.0) |
| Sit on chair | 8.2 (14.7) | 19.1 (29.1) | 9.3 (20.4) |
| Stand/walk/wheel | 8.2 (8.4) | 11.4 (9.7) | 6.5 (9.4) |
DISCUSSION
This observational cross‐sectional study extends previous observations of hospital inpatients to include a wider variety of patient types and ages. Observing 132 patients on medical, surgical, and oncology wards for up to 16 hours of weekday time, we found that patients spent only 9% in active upright postures, with significantly lower mobility on the surgical ward but no significant differences between older and younger patients.
Previous studies in older general medical patients using behavioral mapping[13] or accelerometers[2, 3] have shown 71% to 83% of time spent in bed, and 4% spent standing or walking, similar to our findings, although methodological differences between studies (eg, patient selection and time windows) caution against direct comparison. We identified different levels of physical activity on the surgical, medical, and oncology wards. This may reflect differences in patient case‐mix, ward environment, and/or ward culture. The medical and oncology wards each have a patient lounge, providing a potential walking destination, although only a small amount of patients' time was observed in these areas, suggesting that they may not fulfil their purpose. The oncology ward has a well developed wellness focus. The oncology and medical wards were actively involved in a quality‐improvement intervention to improve early patient mobility at the time of the audit,[15] whereas the surgical ward was at the precommencement (information gathering) stage. The data collected within this audit have formed part of the feedback cycle for staff involved in the improvement intervention. Repeat measurement will be undertaken on the surgical ward to help evaluate the impact of the intervention, and serial measurement will be undertaken in future participating wards to investigate the responsiveness of this measurement method.
Although the literature has focused on poor mobility in hospitalized elders, we did not find any better mobility in younger patients, suggesting that barriers to mobility are not confined to the elderly. Whereas individualized mobility assessment and support may be more important in the elderly,[16] addressing cultural and environmental issues such as promoting accountability for early ambulation, providing patients and families with permission and encouragement to ambulate, and ensuring accessible walking destinations may benefit patients of all ages.
Behavioral mapping has strengths and weaknesses compared to other methods such as accelerometry or patient/nurse report. Observations are conducted by an independent observer not involved in care and include all ward inpatients, providing a generalizable sample, as the observation protocol does not pose a participation burden for patient or ward staff. However, the cross‐sectional nature may oversample longer‐stay patients, the intermittent observation protocol tends to overestimate time spent upright,[14] the labor‐intensive nature of observations means choosing a limited time window (in our case 10001800), and the minimum time and observation frequency to generate reliable data remain uncertain. Further studies examining reliability, validity, and responsiveness would support the utility of this method for quality improvement.
In summary, this study shows that mobility is limited in older and younger adult inpatients across a range of inpatient wards, and that physical activity practices vary among wards. Interventions to enhance hospital mobility should include patients of all ages, and need to be tailored to local mobility practices, barriers, and enablers.
Acknowledgements
The authors thank the staff of wards 6AS, 9BN, and 7BW for participating in this project.
Disclosure: Nothing to report.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57:1660–1665.
- , , , et al. Twenty‐four‐hour mobility during acute hospitalization in older medical patients. J Gerontol A Biol Sci Med Sci. 2013;68(3):331–337.
- , , , , , . Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266–273.
- , , . Exercising body and mind: an integrated approach to functional independence. J Am Geriatr Soc. 2008;56:630–635.
- , , , , . Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124(124):883–889.
- , , . Nursing staff perceptions of physical function in hospitalized older adults. App Nurs Res. 2011;24:215–222.
- , , , , . Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2:305–313.
- , . How nurses decide to ambulate hospitalized older adults: development of a conceptual model. Gerontologist. 2011;51(6):786–797.
- , , , . Barriers to early mobility of hospitalized general medicine patients. Survey development and validation. Am J Phys Med Rehabil. 2015;94:304–312.
- , . Attitudes and expectations regarding exercise in the hospital of hospitalized older adults: a qualitative study. J Am Geriatr Soc. 2012;60:713–718.
- , , , . Inactive and alone. Physical activity within the first 14 days of acute stroke unit care. Stroke. 2004;35:1005–1009.
- , , . Activity level of hospital medical inpatients: an observational study. Arch Gerontol Geriatr. 2012;55:417–421.
- , , , . Measuring activity levels at an acute stroke ward: comparing observations to a device. Biomed Res Int. 2013;2013:460482.
- , , . Eat walk engage: an interdisciplinary collaborative model to improve care of hospitalized elders. Am J Med Qual. 2015;30(1):5–13.
- , , . Hospitalization‐associated disability. “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306(16):1782–1793.
Low mobility is common in hospitalized older patients, and an independent predictor of poor functional outcomes.[1, 2, 3, 4] Few studies have included younger patients, but care models that support early mobility may reduce functional decline, enhance recovery, and reduce length of stay in older and mixed‐age populations.[5, 6] Barriers to mobility are complex and include patient symptoms and tethers, health provider behavior, team communication, and leadership, device availability, and environmental factors.[7, 8, 9, 10, 11] These contextual factors may differ even within a hospital between patient groups and ward settings. Simple measures to quantify mobility patterns would help address these barriers by providing opportunities for audit and feedback. Although accelerometry is the gold standard method for research, it requires equipment, analysis skills, and patient consent, which limits application in clinical practice. Behavioral mapping is a systematic method of observation developed in stroke patients, which is simple, objective, and requires no direct patient or staff participation,[12] and physical activity levels estimated from behavioral mapping are similar to those identified by accelerometry.[3, 13, 14] In the context of a phased quality‐improvement project aiming to reduce functional decline,[15] we undertook a cross‐sectional audit of mobility on 3 different wards using behavioral mapping, and examined differences among wards and between older (aged 65 years or more) and younger patients.
METHODS
This prospective observational study used cross‐sectional sampling from a 26‐bed general medical ward, a 30‐bed oncology ward, and a 24‐bed vascular surgical ward in a 900‐bed tertiary teaching hospital in Brisbane, Australia. Sampling was undertaken during 4 observation periods (2 mornings [10001400] and 2 afternoons [1400‐1800]) within 10 days in May 2013. All patients on each ward for each period were observed unless they were receiving end‐of‐life care. Structured observations were undertaken using behavioral mapping protocols similar to those previously described in stroke and general medical patients,[12, 13] with each patient room visited in the same sequence. Participants in each room were observed for a 2‐minute period (up to 4 participants could be observed concurrently in shared rooms) before moving to the next room, and the sequence was repeated in the same order for the whole 4‐hour period, with a single 15‐minute break. Depending on ward size and layout, this provided 12 to 17 observations per participant for each 4‐hour period (each individual observed every 1218 minutes). Observations were undertaken by 4 trained physiotherapy student observers using a predetermined set of mutually exclusive levels (lying in bed, sitting in or on the bed, sitting on a chair, standing, actively wheeling, or walking). The study was approved by the Royal Brisbane and Women's Hospital Human Research Ethics Committee as part of a quality‐improvement activity, and individual consent was not required. No clinical data except age and gender were collected for participants. The nurse unit manager for each ward was introduced to the observers and aware that observations were being conducted.
Patients who were observed for less than one‐half of an observation period were excluded so that all participants contributed at least 2 hours of observational data, up to a maximum of 16 hours. The number of valid observations for each participant (excluding time off ward or behind curtains if the level was not apparent) was calculated and used to derive the proportion of valid observations spent at each level for each participant. The proportion of observations at each level was summarized across all participants using frequency distributions and summary statistics. For ease of presentation, mean percentage of observed time in each activity was presented. However, as data were not normally distributed, statistical comparisons were undertaken using the Kruskal‐Wallis test, comparing the distribution of time spent upright (standing, walking, or actively wheeling) between groups (age group and ward). Interaction between age and ward effects was sought using generalized linear modeling.
RESULTS
Valid observations (at least 2 hours in 1 or more observation period) were available for 132 patients (48 medical, 50 oncology, and 34 surgery). Of these, 67 (51 %) were aged 65 years (54% medical, 44% oncology, 56% surgery) and 62 (47%) were male. There were a total of 3891 observations of location (median, 30 per patient; range, 965). Participants were observed in the bedded area 85.1% of observations, with 3.1% in the bathroom, 3.2% in the hallway or patient lounge, and 8.6% off ward. Allowing for time off ward and behind curtains, when observers could not be sure of their activity level, 3272 valid observations were available for physical activity.
More than half of the observed time (mean 57.4%) was spent lying in bed, 33.6% sitting on the bed or chair, and 9.0% standing, walking, or wheeling. Across all observation periods, 39/132 (29.5%) participants were never observed to be standing, walking or wheeling, and 7.6% were in bed at all observations. Comparing older and younger patients (Table 1), there was no difference in the time spent in active upright postures (median, 6.1% in older vs 7.4% in younger; P = 0.30). Table 2 summarizes descriptive data for the different wards. In the medical and surgical wards, 84% of the time was spend in or on the bed, and only 16% of the time was spent sitting in a chair or in active upright postures. Surgical patients, in particular, spent two‐thirds of observation time lying flat in bed, whereas medical patients spent more time sitting up on the bed. On statistical testing, time spent standing/walking/wheeling was significantly lower on the surgical ward (median, 4%; interquartile range [IQR], 010 for surgery; median, 7%; IQR, 013 for medical; and median, 10%; IQR 317 for oncology; P = 0.015). This was also reflected in a higher proportion of surgical patients never seen in an active upright position (44.1% compared to 27.1% medical and 22.0% oncology). Multivariate modeling showed no significant interaction between age and ward.
| All Ages, n = 132, Median Observations 29.5, Range 665* | Aged 65 Years, n = 61, Median Observations 30, Range 665 | Aged 65 Years, n = 67, Median Observations 27, Range 665 | |
|---|---|---|---|
| |||
| Location | |||
| Bedroom | 85.1 (13.3) | 84.6 (13.4) | 85.5 (12.9) |
| Bathroom | 3.0 (4.0) | 2.6 (3.9) | 3.4 (4.1) |
| Hall | 2.9 (4.6) | 3.4 (5.4) | 2.7 (4.0) |
| Lounge | 0.3 (1.9) | 0 | 0.6 (2.7) |
| Off ward/other | 8.6 (11.6) | 9.3 (11.4) | 7.8 (11.1) |
| Physical activity | |||
| Lie in bed | 57.4 (30.0) | 59.4 (29.4) | 55.5 (31.6) |
| Sit on bed | 21.0 (23.2) | 16.9 (19.9) | 24.7 (25.7) |
| Sit on chair | 12.6 (22.9) | 14.0 (25.6) | 11.9 (20.9) |
| Stand/walk/wheel | 9.0 (9.3) | 9.6 (9.6) | 8.0 (8.5) |
| Medical, n = 48, Median Observations 30, Range 759 | Oncology, n = 50, Median Observations 25, Range 652 | Surgical, n = 34, Median Observations 31, Range 1765 | |
|---|---|---|---|
| Location | |||
| Bedroom | 89.1 (11.4) | 81.3 (13.6) | 85.3 (14.1) |
| Bathroom | 2.8 (4.1) | 3.1 (3.8) | 3.1 (4.2) |
| Hall | 1.5 (2.5) | 5.3 (6.1) | 1.5 (2.7) |
| Lounge | 0.5 (2.0) | 0.4 (2.5) | 0 |
| Off ward/other | 6.2 (10.2) | 10.0 (11.9) | 10.1 (12.6) |
| Physical activity | |||
| Lie in bed | 53.3 (31.4) | 56.1 (30.2) | 65.1 (27.0) |
| Sit on bed | 30.3 (29.5) | 13.4 (16.1) | 19.0 (17.0) |
| Sit on chair | 8.2 (14.7) | 19.1 (29.1) | 9.3 (20.4) |
| Stand/walk/wheel | 8.2 (8.4) | 11.4 (9.7) | 6.5 (9.4) |
DISCUSSION
This observational cross‐sectional study extends previous observations of hospital inpatients to include a wider variety of patient types and ages. Observing 132 patients on medical, surgical, and oncology wards for up to 16 hours of weekday time, we found that patients spent only 9% in active upright postures, with significantly lower mobility on the surgical ward but no significant differences between older and younger patients.
Previous studies in older general medical patients using behavioral mapping[13] or accelerometers[2, 3] have shown 71% to 83% of time spent in bed, and 4% spent standing or walking, similar to our findings, although methodological differences between studies (eg, patient selection and time windows) caution against direct comparison. We identified different levels of physical activity on the surgical, medical, and oncology wards. This may reflect differences in patient case‐mix, ward environment, and/or ward culture. The medical and oncology wards each have a patient lounge, providing a potential walking destination, although only a small amount of patients' time was observed in these areas, suggesting that they may not fulfil their purpose. The oncology ward has a well developed wellness focus. The oncology and medical wards were actively involved in a quality‐improvement intervention to improve early patient mobility at the time of the audit,[15] whereas the surgical ward was at the precommencement (information gathering) stage. The data collected within this audit have formed part of the feedback cycle for staff involved in the improvement intervention. Repeat measurement will be undertaken on the surgical ward to help evaluate the impact of the intervention, and serial measurement will be undertaken in future participating wards to investigate the responsiveness of this measurement method.
Although the literature has focused on poor mobility in hospitalized elders, we did not find any better mobility in younger patients, suggesting that barriers to mobility are not confined to the elderly. Whereas individualized mobility assessment and support may be more important in the elderly,[16] addressing cultural and environmental issues such as promoting accountability for early ambulation, providing patients and families with permission and encouragement to ambulate, and ensuring accessible walking destinations may benefit patients of all ages.
Behavioral mapping has strengths and weaknesses compared to other methods such as accelerometry or patient/nurse report. Observations are conducted by an independent observer not involved in care and include all ward inpatients, providing a generalizable sample, as the observation protocol does not pose a participation burden for patient or ward staff. However, the cross‐sectional nature may oversample longer‐stay patients, the intermittent observation protocol tends to overestimate time spent upright,[14] the labor‐intensive nature of observations means choosing a limited time window (in our case 10001800), and the minimum time and observation frequency to generate reliable data remain uncertain. Further studies examining reliability, validity, and responsiveness would support the utility of this method for quality improvement.
In summary, this study shows that mobility is limited in older and younger adult inpatients across a range of inpatient wards, and that physical activity practices vary among wards. Interventions to enhance hospital mobility should include patients of all ages, and need to be tailored to local mobility practices, barriers, and enablers.
Acknowledgements
The authors thank the staff of wards 6AS, 9BN, and 7BW for participating in this project.
Disclosure: Nothing to report.
Low mobility is common in hospitalized older patients, and an independent predictor of poor functional outcomes.[1, 2, 3, 4] Few studies have included younger patients, but care models that support early mobility may reduce functional decline, enhance recovery, and reduce length of stay in older and mixed‐age populations.[5, 6] Barriers to mobility are complex and include patient symptoms and tethers, health provider behavior, team communication, and leadership, device availability, and environmental factors.[7, 8, 9, 10, 11] These contextual factors may differ even within a hospital between patient groups and ward settings. Simple measures to quantify mobility patterns would help address these barriers by providing opportunities for audit and feedback. Although accelerometry is the gold standard method for research, it requires equipment, analysis skills, and patient consent, which limits application in clinical practice. Behavioral mapping is a systematic method of observation developed in stroke patients, which is simple, objective, and requires no direct patient or staff participation,[12] and physical activity levels estimated from behavioral mapping are similar to those identified by accelerometry.[3, 13, 14] In the context of a phased quality‐improvement project aiming to reduce functional decline,[15] we undertook a cross‐sectional audit of mobility on 3 different wards using behavioral mapping, and examined differences among wards and between older (aged 65 years or more) and younger patients.
METHODS
This prospective observational study used cross‐sectional sampling from a 26‐bed general medical ward, a 30‐bed oncology ward, and a 24‐bed vascular surgical ward in a 900‐bed tertiary teaching hospital in Brisbane, Australia. Sampling was undertaken during 4 observation periods (2 mornings [10001400] and 2 afternoons [1400‐1800]) within 10 days in May 2013. All patients on each ward for each period were observed unless they were receiving end‐of‐life care. Structured observations were undertaken using behavioral mapping protocols similar to those previously described in stroke and general medical patients,[12, 13] with each patient room visited in the same sequence. Participants in each room were observed for a 2‐minute period (up to 4 participants could be observed concurrently in shared rooms) before moving to the next room, and the sequence was repeated in the same order for the whole 4‐hour period, with a single 15‐minute break. Depending on ward size and layout, this provided 12 to 17 observations per participant for each 4‐hour period (each individual observed every 1218 minutes). Observations were undertaken by 4 trained physiotherapy student observers using a predetermined set of mutually exclusive levels (lying in bed, sitting in or on the bed, sitting on a chair, standing, actively wheeling, or walking). The study was approved by the Royal Brisbane and Women's Hospital Human Research Ethics Committee as part of a quality‐improvement activity, and individual consent was not required. No clinical data except age and gender were collected for participants. The nurse unit manager for each ward was introduced to the observers and aware that observations were being conducted.
Patients who were observed for less than one‐half of an observation period were excluded so that all participants contributed at least 2 hours of observational data, up to a maximum of 16 hours. The number of valid observations for each participant (excluding time off ward or behind curtains if the level was not apparent) was calculated and used to derive the proportion of valid observations spent at each level for each participant. The proportion of observations at each level was summarized across all participants using frequency distributions and summary statistics. For ease of presentation, mean percentage of observed time in each activity was presented. However, as data were not normally distributed, statistical comparisons were undertaken using the Kruskal‐Wallis test, comparing the distribution of time spent upright (standing, walking, or actively wheeling) between groups (age group and ward). Interaction between age and ward effects was sought using generalized linear modeling.
RESULTS
Valid observations (at least 2 hours in 1 or more observation period) were available for 132 patients (48 medical, 50 oncology, and 34 surgery). Of these, 67 (51 %) were aged 65 years (54% medical, 44% oncology, 56% surgery) and 62 (47%) were male. There were a total of 3891 observations of location (median, 30 per patient; range, 965). Participants were observed in the bedded area 85.1% of observations, with 3.1% in the bathroom, 3.2% in the hallway or patient lounge, and 8.6% off ward. Allowing for time off ward and behind curtains, when observers could not be sure of their activity level, 3272 valid observations were available for physical activity.
More than half of the observed time (mean 57.4%) was spent lying in bed, 33.6% sitting on the bed or chair, and 9.0% standing, walking, or wheeling. Across all observation periods, 39/132 (29.5%) participants were never observed to be standing, walking or wheeling, and 7.6% were in bed at all observations. Comparing older and younger patients (Table 1), there was no difference in the time spent in active upright postures (median, 6.1% in older vs 7.4% in younger; P = 0.30). Table 2 summarizes descriptive data for the different wards. In the medical and surgical wards, 84% of the time was spend in or on the bed, and only 16% of the time was spent sitting in a chair or in active upright postures. Surgical patients, in particular, spent two‐thirds of observation time lying flat in bed, whereas medical patients spent more time sitting up on the bed. On statistical testing, time spent standing/walking/wheeling was significantly lower on the surgical ward (median, 4%; interquartile range [IQR], 010 for surgery; median, 7%; IQR, 013 for medical; and median, 10%; IQR 317 for oncology; P = 0.015). This was also reflected in a higher proportion of surgical patients never seen in an active upright position (44.1% compared to 27.1% medical and 22.0% oncology). Multivariate modeling showed no significant interaction between age and ward.
| All Ages, n = 132, Median Observations 29.5, Range 665* | Aged 65 Years, n = 61, Median Observations 30, Range 665 | Aged 65 Years, n = 67, Median Observations 27, Range 665 | |
|---|---|---|---|
| |||
| Location | |||
| Bedroom | 85.1 (13.3) | 84.6 (13.4) | 85.5 (12.9) |
| Bathroom | 3.0 (4.0) | 2.6 (3.9) | 3.4 (4.1) |
| Hall | 2.9 (4.6) | 3.4 (5.4) | 2.7 (4.0) |
| Lounge | 0.3 (1.9) | 0 | 0.6 (2.7) |
| Off ward/other | 8.6 (11.6) | 9.3 (11.4) | 7.8 (11.1) |
| Physical activity | |||
| Lie in bed | 57.4 (30.0) | 59.4 (29.4) | 55.5 (31.6) |
| Sit on bed | 21.0 (23.2) | 16.9 (19.9) | 24.7 (25.7) |
| Sit on chair | 12.6 (22.9) | 14.0 (25.6) | 11.9 (20.9) |
| Stand/walk/wheel | 9.0 (9.3) | 9.6 (9.6) | 8.0 (8.5) |
| Medical, n = 48, Median Observations 30, Range 759 | Oncology, n = 50, Median Observations 25, Range 652 | Surgical, n = 34, Median Observations 31, Range 1765 | |
|---|---|---|---|
| Location | |||
| Bedroom | 89.1 (11.4) | 81.3 (13.6) | 85.3 (14.1) |
| Bathroom | 2.8 (4.1) | 3.1 (3.8) | 3.1 (4.2) |
| Hall | 1.5 (2.5) | 5.3 (6.1) | 1.5 (2.7) |
| Lounge | 0.5 (2.0) | 0.4 (2.5) | 0 |
| Off ward/other | 6.2 (10.2) | 10.0 (11.9) | 10.1 (12.6) |
| Physical activity | |||
| Lie in bed | 53.3 (31.4) | 56.1 (30.2) | 65.1 (27.0) |
| Sit on bed | 30.3 (29.5) | 13.4 (16.1) | 19.0 (17.0) |
| Sit on chair | 8.2 (14.7) | 19.1 (29.1) | 9.3 (20.4) |
| Stand/walk/wheel | 8.2 (8.4) | 11.4 (9.7) | 6.5 (9.4) |
DISCUSSION
This observational cross‐sectional study extends previous observations of hospital inpatients to include a wider variety of patient types and ages. Observing 132 patients on medical, surgical, and oncology wards for up to 16 hours of weekday time, we found that patients spent only 9% in active upright postures, with significantly lower mobility on the surgical ward but no significant differences between older and younger patients.
Previous studies in older general medical patients using behavioral mapping[13] or accelerometers[2, 3] have shown 71% to 83% of time spent in bed, and 4% spent standing or walking, similar to our findings, although methodological differences between studies (eg, patient selection and time windows) caution against direct comparison. We identified different levels of physical activity on the surgical, medical, and oncology wards. This may reflect differences in patient case‐mix, ward environment, and/or ward culture. The medical and oncology wards each have a patient lounge, providing a potential walking destination, although only a small amount of patients' time was observed in these areas, suggesting that they may not fulfil their purpose. The oncology ward has a well developed wellness focus. The oncology and medical wards were actively involved in a quality‐improvement intervention to improve early patient mobility at the time of the audit,[15] whereas the surgical ward was at the precommencement (information gathering) stage. The data collected within this audit have formed part of the feedback cycle for staff involved in the improvement intervention. Repeat measurement will be undertaken on the surgical ward to help evaluate the impact of the intervention, and serial measurement will be undertaken in future participating wards to investigate the responsiveness of this measurement method.
Although the literature has focused on poor mobility in hospitalized elders, we did not find any better mobility in younger patients, suggesting that barriers to mobility are not confined to the elderly. Whereas individualized mobility assessment and support may be more important in the elderly,[16] addressing cultural and environmental issues such as promoting accountability for early ambulation, providing patients and families with permission and encouragement to ambulate, and ensuring accessible walking destinations may benefit patients of all ages.
Behavioral mapping has strengths and weaknesses compared to other methods such as accelerometry or patient/nurse report. Observations are conducted by an independent observer not involved in care and include all ward inpatients, providing a generalizable sample, as the observation protocol does not pose a participation burden for patient or ward staff. However, the cross‐sectional nature may oversample longer‐stay patients, the intermittent observation protocol tends to overestimate time spent upright,[14] the labor‐intensive nature of observations means choosing a limited time window (in our case 10001800), and the minimum time and observation frequency to generate reliable data remain uncertain. Further studies examining reliability, validity, and responsiveness would support the utility of this method for quality improvement.
In summary, this study shows that mobility is limited in older and younger adult inpatients across a range of inpatient wards, and that physical activity practices vary among wards. Interventions to enhance hospital mobility should include patients of all ages, and need to be tailored to local mobility practices, barriers, and enablers.
Acknowledgements
The authors thank the staff of wards 6AS, 9BN, and 7BW for participating in this project.
Disclosure: Nothing to report.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57:1660–1665.
- , , , et al. Twenty‐four‐hour mobility during acute hospitalization in older medical patients. J Gerontol A Biol Sci Med Sci. 2013;68(3):331–337.
- , , , , , . Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266–273.
- , , . Exercising body and mind: an integrated approach to functional independence. J Am Geriatr Soc. 2008;56:630–635.
- , , , , . Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124(124):883–889.
- , , . Nursing staff perceptions of physical function in hospitalized older adults. App Nurs Res. 2011;24:215–222.
- , , , , . Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2:305–313.
- , . How nurses decide to ambulate hospitalized older adults: development of a conceptual model. Gerontologist. 2011;51(6):786–797.
- , , , . Barriers to early mobility of hospitalized general medicine patients. Survey development and validation. Am J Phys Med Rehabil. 2015;94:304–312.
- , . Attitudes and expectations regarding exercise in the hospital of hospitalized older adults: a qualitative study. J Am Geriatr Soc. 2012;60:713–718.
- , , , . Inactive and alone. Physical activity within the first 14 days of acute stroke unit care. Stroke. 2004;35:1005–1009.
- , , . Activity level of hospital medical inpatients: an observational study. Arch Gerontol Geriatr. 2012;55:417–421.
- , , , . Measuring activity levels at an acute stroke ward: comparing observations to a device. Biomed Res Int. 2013;2013:460482.
- , , . Eat walk engage: an interdisciplinary collaborative model to improve care of hospitalized elders. Am J Med Qual. 2015;30(1):5–13.
- , , . Hospitalization‐associated disability. “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306(16):1782–1793.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57:1660–1665.
- , , , et al. Twenty‐four‐hour mobility during acute hospitalization in older medical patients. J Gerontol A Biol Sci Med Sci. 2013;68(3):331–337.
- , , , , , . Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266–273.
- , , . Exercising body and mind: an integrated approach to functional independence. J Am Geriatr Soc. 2008;56:630–635.
- , , , , . Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124(124):883–889.
- , , . Nursing staff perceptions of physical function in hospitalized older adults. App Nurs Res. 2011;24:215–222.
- , , , , . Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2:305–313.
- , . How nurses decide to ambulate hospitalized older adults: development of a conceptual model. Gerontologist. 2011;51(6):786–797.
- , , , . Barriers to early mobility of hospitalized general medicine patients. Survey development and validation. Am J Phys Med Rehabil. 2015;94:304–312.
- , . Attitudes and expectations regarding exercise in the hospital of hospitalized older adults: a qualitative study. J Am Geriatr Soc. 2012;60:713–718.
- , , , . Inactive and alone. Physical activity within the first 14 days of acute stroke unit care. Stroke. 2004;35:1005–1009.
- , , . Activity level of hospital medical inpatients: an observational study. Arch Gerontol Geriatr. 2012;55:417–421.
- , , , . Measuring activity levels at an acute stroke ward: comparing observations to a device. Biomed Res Int. 2013;2013:460482.
- , , . Eat walk engage: an interdisciplinary collaborative model to improve care of hospitalized elders. Am J Med Qual. 2015;30(1):5–13.
- , , . Hospitalization‐associated disability. “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306(16):1782–1793.
TUBB8 mutations cause some female infertility
Certain cases of female infertility appear to be caused by mutations in the TUBB8 gene that impair microtubule behavior and meiotic spindle assembly, thus preventing oocyte maturation, according to a report published online Jan. 20 in the New England Journal of Medicine.
The findings from a series of genetic analyses “provide the basis for developing diagnostic tools” to identify women who carry these mutations, and also point the way to as-yet undiscovered genetic mutations that also contribute to the arrest of oocyte maturation, wrote Ruizhi Feng, Ph.D., of State Key Laboratory of Genetic Engineering, Fudan University, Shanghai, and his associates.
Immature oocytes are arrested at a phase of development before complete meiosis occurs. Among women seeking in vitro fertilization “it is common for some oocytes to remain immature after ovarian stimulation and administration of human chorionic gonadotropin, but complete arrest of oocyte maturation has been reported in only a few women, and nothing is known about the genetic cause of this phenotype,” they noted.
The investigators identified a four-generation family affected with a rare pattern of inheritance of female infertility, which was found to stem from complete oocyte maturation arrest. They sequenced the exomes of three affected and two unaffected women in the family. All the affected women, but none of the unaffected women, carried a mutation in the TUBB8 gene that encodes for a tubulin isotope. The researchers then found six other TUBB8 mutations (all paternally transmitted) in seven women from four other families with similar oocyte maturation arrest, as well as de novo mutations in two women from two additional families. All of the oocytes assessed either had abnormal spindles or no detectable spindles.
The investigators hypothesized that TUBB8 influences the self-organization of microtubules, and thus meiotic spindle assembly and chromosome orientation, in oocytes. Further analyses confirmed this and showed that the mutations affected tubulin heterodimer folding and assembly in vitro, caused “a spectrum of striking microtubule phenotypes” in cultured HeLa cells, and markedly impaired microtubule dynamics in in-vivo yeast colonies (N Engl J Med 2016;374:223-32. doi:10.1056/NEJMoa1510791).
Finally, to establish a causal relationship between the TUBB8 mutations and infertility, the investigators introduced them into mouse and human oocytes. The TUBB8 mutations caused maturation defects “that precisely mimic the infertility phenotype,” while nonmutated TUBB8 did not, they reported.
“We conclude from these observations that mutations in TUBB8 cause oocyte maturation arrest and that TUBB8 has a key role in meiotic spindle assembly and maturation in human oocytes,” Dr. Feng and his associates wrote.
The National Basic Research Program of China, the Shanghai Key Scientific Research Program, the National Natural Science Foundation of China, the 111 Project, and the U.S. National Institutes of Health supported the study. Dr. Feng and his associates reported having no conflicts of interest.
The experimental data provided by Feng et al. are unusual in their breadth. Introducing the TUBB8 mutations they identified into HeLa cells, yeast cells, mouse oocytes, and human oocytes disrupted the microtubule networks in all. Depending on the mutation and the specific cell type, these defects ranged from flawed heterodimer assembly to complete obliteration of the microtubule network.
These findings will likely lead to improved diagnoses at fertility clinics. They also offer a potential foundation for discovering other genetic causes of primary female infertility.
Dr. Jurrien Dean is at the Laboratory of Cellular and Developmental Biology at the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md. He reported having no relevant financial disclosures. These comments are adapted from an editorial by Dr. Dean (N Engl J Med. 2016 Jan 20. doi:10.1056/NEJMe1515512.).
The experimental data provided by Feng et al. are unusual in their breadth. Introducing the TUBB8 mutations they identified into HeLa cells, yeast cells, mouse oocytes, and human oocytes disrupted the microtubule networks in all. Depending on the mutation and the specific cell type, these defects ranged from flawed heterodimer assembly to complete obliteration of the microtubule network.
These findings will likely lead to improved diagnoses at fertility clinics. They also offer a potential foundation for discovering other genetic causes of primary female infertility.
Dr. Jurrien Dean is at the Laboratory of Cellular and Developmental Biology at the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md. He reported having no relevant financial disclosures. These comments are adapted from an editorial by Dr. Dean (N Engl J Med. 2016 Jan 20. doi:10.1056/NEJMe1515512.).
The experimental data provided by Feng et al. are unusual in their breadth. Introducing the TUBB8 mutations they identified into HeLa cells, yeast cells, mouse oocytes, and human oocytes disrupted the microtubule networks in all. Depending on the mutation and the specific cell type, these defects ranged from flawed heterodimer assembly to complete obliteration of the microtubule network.
These findings will likely lead to improved diagnoses at fertility clinics. They also offer a potential foundation for discovering other genetic causes of primary female infertility.
Dr. Jurrien Dean is at the Laboratory of Cellular and Developmental Biology at the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md. He reported having no relevant financial disclosures. These comments are adapted from an editorial by Dr. Dean (N Engl J Med. 2016 Jan 20. doi:10.1056/NEJMe1515512.).
Certain cases of female infertility appear to be caused by mutations in the TUBB8 gene that impair microtubule behavior and meiotic spindle assembly, thus preventing oocyte maturation, according to a report published online Jan. 20 in the New England Journal of Medicine.
The findings from a series of genetic analyses “provide the basis for developing diagnostic tools” to identify women who carry these mutations, and also point the way to as-yet undiscovered genetic mutations that also contribute to the arrest of oocyte maturation, wrote Ruizhi Feng, Ph.D., of State Key Laboratory of Genetic Engineering, Fudan University, Shanghai, and his associates.
Immature oocytes are arrested at a phase of development before complete meiosis occurs. Among women seeking in vitro fertilization “it is common for some oocytes to remain immature after ovarian stimulation and administration of human chorionic gonadotropin, but complete arrest of oocyte maturation has been reported in only a few women, and nothing is known about the genetic cause of this phenotype,” they noted.
The investigators identified a four-generation family affected with a rare pattern of inheritance of female infertility, which was found to stem from complete oocyte maturation arrest. They sequenced the exomes of three affected and two unaffected women in the family. All the affected women, but none of the unaffected women, carried a mutation in the TUBB8 gene that encodes for a tubulin isotope. The researchers then found six other TUBB8 mutations (all paternally transmitted) in seven women from four other families with similar oocyte maturation arrest, as well as de novo mutations in two women from two additional families. All of the oocytes assessed either had abnormal spindles or no detectable spindles.
The investigators hypothesized that TUBB8 influences the self-organization of microtubules, and thus meiotic spindle assembly and chromosome orientation, in oocytes. Further analyses confirmed this and showed that the mutations affected tubulin heterodimer folding and assembly in vitro, caused “a spectrum of striking microtubule phenotypes” in cultured HeLa cells, and markedly impaired microtubule dynamics in in-vivo yeast colonies (N Engl J Med 2016;374:223-32. doi:10.1056/NEJMoa1510791).
Finally, to establish a causal relationship between the TUBB8 mutations and infertility, the investigators introduced them into mouse and human oocytes. The TUBB8 mutations caused maturation defects “that precisely mimic the infertility phenotype,” while nonmutated TUBB8 did not, they reported.
“We conclude from these observations that mutations in TUBB8 cause oocyte maturation arrest and that TUBB8 has a key role in meiotic spindle assembly and maturation in human oocytes,” Dr. Feng and his associates wrote.
The National Basic Research Program of China, the Shanghai Key Scientific Research Program, the National Natural Science Foundation of China, the 111 Project, and the U.S. National Institutes of Health supported the study. Dr. Feng and his associates reported having no conflicts of interest.
Certain cases of female infertility appear to be caused by mutations in the TUBB8 gene that impair microtubule behavior and meiotic spindle assembly, thus preventing oocyte maturation, according to a report published online Jan. 20 in the New England Journal of Medicine.
The findings from a series of genetic analyses “provide the basis for developing diagnostic tools” to identify women who carry these mutations, and also point the way to as-yet undiscovered genetic mutations that also contribute to the arrest of oocyte maturation, wrote Ruizhi Feng, Ph.D., of State Key Laboratory of Genetic Engineering, Fudan University, Shanghai, and his associates.
Immature oocytes are arrested at a phase of development before complete meiosis occurs. Among women seeking in vitro fertilization “it is common for some oocytes to remain immature after ovarian stimulation and administration of human chorionic gonadotropin, but complete arrest of oocyte maturation has been reported in only a few women, and nothing is known about the genetic cause of this phenotype,” they noted.
The investigators identified a four-generation family affected with a rare pattern of inheritance of female infertility, which was found to stem from complete oocyte maturation arrest. They sequenced the exomes of three affected and two unaffected women in the family. All the affected women, but none of the unaffected women, carried a mutation in the TUBB8 gene that encodes for a tubulin isotope. The researchers then found six other TUBB8 mutations (all paternally transmitted) in seven women from four other families with similar oocyte maturation arrest, as well as de novo mutations in two women from two additional families. All of the oocytes assessed either had abnormal spindles or no detectable spindles.
The investigators hypothesized that TUBB8 influences the self-organization of microtubules, and thus meiotic spindle assembly and chromosome orientation, in oocytes. Further analyses confirmed this and showed that the mutations affected tubulin heterodimer folding and assembly in vitro, caused “a spectrum of striking microtubule phenotypes” in cultured HeLa cells, and markedly impaired microtubule dynamics in in-vivo yeast colonies (N Engl J Med 2016;374:223-32. doi:10.1056/NEJMoa1510791).
Finally, to establish a causal relationship between the TUBB8 mutations and infertility, the investigators introduced them into mouse and human oocytes. The TUBB8 mutations caused maturation defects “that precisely mimic the infertility phenotype,” while nonmutated TUBB8 did not, they reported.
“We conclude from these observations that mutations in TUBB8 cause oocyte maturation arrest and that TUBB8 has a key role in meiotic spindle assembly and maturation in human oocytes,” Dr. Feng and his associates wrote.
The National Basic Research Program of China, the Shanghai Key Scientific Research Program, the National Natural Science Foundation of China, the 111 Project, and the U.S. National Institutes of Health supported the study. Dr. Feng and his associates reported having no conflicts of interest.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Mutations in the TUBB8 gene cause some female infertility by impairing microtubule behavior, meiotic spindle assembly, and oocyte maturation.
Major finding: Researchers identified seven mutations in the gene TUBB8 that were responsible for oocyte meiosis arrest in seven of 24 families.
Data source: A series of genetic analyses, including assessment of 24 Chinese families with infertility due to arrest of oocyte maturation.
Disclosures: The National Basic Research Program of China, the Shanghai Key Scientific Research Program, the National Natural Science Foundation of China, the 111 Project, and the U.S. National Institutes of Health supported the study. Dr. Feng and his associates reported having no conflicts of interest.
Biomarker may ID high-risk colon cancers
A biomarker for which a screening test is already available – lack of CDX2 expression – appears to identify the approximately 20% of stage II and 50% of stage III colon cancers that are at high risk of recurrence and would benefit from adjuvant chemotherapy, according to a report published online Jan. 21 in the New England Journal of Medicine.
Distinguishing the high-risk tumors from those unlikely to recur or progress would allow some patients to opt for adjuvant chemotherapy that has been shown to significantly raise the rates of overall and disease-free survival. At present, almost all patients with stage II colon cancer are treated using surgery alone, said Dr. Piero Dalerba of the Herbert Irving Comprehensive Cancer Center and the department of pathology and cell biology at Columbia University, New York, and his associates.
The investigators used a bioinformatics approach to search for biomarkers that would identify the most aggressive colon cancers: undifferentiated tumors characterized by immature, stem-like colonic epithelial cells and depleted of more mature cells. They focused on biomarkers for which clinical diagnostic tests are already available, to facilitate use in real-world clinical practice. Their search through 2,329 colon gene-expression array experiments found that when tumors do not express one particular protein, homeobox transcription factor CDX2, “a master regulator of intestinal development and oncogenesis” that is highly specifically expressed in intestinal epithelium, those tumors tend to have aggressive features such as advanced stage, vascular invasion, and BRAF mutation.
Survival outcomes were then compared in a discovery data set of 466 patients. The 5-year disease-free survival rate was significantly lower, at 41%, among the 32 patients who had CDX2-negative tumors than the 74% survival rate among the 434 patients with CDX2-positive tumors. The hazard ratio for disease recurrence among patients with CDX2-negative vs. CDX2-positive tumors was 2.73, Dr. Dalerba and his associates said (N Engl J Med. 2016 Jan 21. doi:10.1056/NEJMoa1506597).
The investigators confirmed these findings in a separate validation data set of 366 patients, of whom 48 had CDX2-negative and 318 had CDX2-positive tumors. Five-year disease-free survival (48% vs. 71%), overall survival (33% vs. 59%), and disease-specific survival (45% vs. 72%) were significantly lower with CDX2-negative tumors, and the HR for disease recurrence was 2.42.
To determine whether adjuvant chemotherapy would improve survival outcomes in patients with CDX2-negative tumors, the researchers assessed outcomes in an expanded dataset of 669 patients with stage II and 1,228 patients with stage III colon cancer. They confirmed that among patients with stage II CDX2-negative tumors, those who received adjuvant chemotherapy had a higher rate of disease-free survival (91%) than did those who didn’t receive adjuvant chemotherapy (56%). Similarly, among patients with stage III CDX2-negative tumors, those who received adjuvant chemotherapy had a higher rate of disease-free survival (74%) than those who did not receive adjuvant chemotherapy (37%).
This survival benefit was independent of many known risk factors, including tumor grade, they noted.
These results must be replicated in future prospective studies, because this study’s design was retrospective and exploratory, Dr. Dalerba and his associates added.
This work was supported by the National Comprehensive Cancer Network, the National Institutes of Health, Siebel Stem Cell Institute, the Thomas and Stacey Siebel Foundation, the Virginia and D.K. Ludwig Fund for Cancer Research, the California Institute for Regenerative Medicine, the U.S. Department of Defense, the Bladder Cancer Advocacy Network, and BD Biosciences. Dr. Dalerba reported ties to Quanticel Pharmaceuticals and Oncomed Pharmaceuticals, and his associates reported ties to numerous industry sources.
These study findings give clinicians the opportunity to move beyond the existing inadequate method of selecting patients for adjuvant chemotherapy. Although this was not a perfect or definitive study, since it was retrospective and the number of patients with CDX2-negative colon cancers was relatively small, it nevertheless entailed numerous rigorous data analyses.

|
Dr. C. Richard Boland |
Moreover, the results raise the important question of what mechanism might be silencing CDX2 in this subset of aggressive cancers. The answer could lead to the discovery of new approaches to treating the underlying problem.
C. Richard Boland, M.D., and Ajay Goel, Ph.D., are at the Center for Gastrointestinal Research and the Center for Epigenetics, Cancer Prevention, and Genomics at Baylor Research Institute, and at the Baylor Charles A. Sammons Cancer Center, all in Dallas. They reported having no relevant financial disclosures. Dr. Boland and Dr. Goel made these remarks in an editorial accompanying Dr. Dalerba’s report (N Engl J Med. 2016 Jan 21. doi:10.1056/NEJMe1514353).
These study findings give clinicians the opportunity to move beyond the existing inadequate method of selecting patients for adjuvant chemotherapy. Although this was not a perfect or definitive study, since it was retrospective and the number of patients with CDX2-negative colon cancers was relatively small, it nevertheless entailed numerous rigorous data analyses.

|
Dr. C. Richard Boland |
Moreover, the results raise the important question of what mechanism might be silencing CDX2 in this subset of aggressive cancers. The answer could lead to the discovery of new approaches to treating the underlying problem.
C. Richard Boland, M.D., and Ajay Goel, Ph.D., are at the Center for Gastrointestinal Research and the Center for Epigenetics, Cancer Prevention, and Genomics at Baylor Research Institute, and at the Baylor Charles A. Sammons Cancer Center, all in Dallas. They reported having no relevant financial disclosures. Dr. Boland and Dr. Goel made these remarks in an editorial accompanying Dr. Dalerba’s report (N Engl J Med. 2016 Jan 21. doi:10.1056/NEJMe1514353).
These study findings give clinicians the opportunity to move beyond the existing inadequate method of selecting patients for adjuvant chemotherapy. Although this was not a perfect or definitive study, since it was retrospective and the number of patients with CDX2-negative colon cancers was relatively small, it nevertheless entailed numerous rigorous data analyses.

|
Dr. C. Richard Boland |
Moreover, the results raise the important question of what mechanism might be silencing CDX2 in this subset of aggressive cancers. The answer could lead to the discovery of new approaches to treating the underlying problem.
C. Richard Boland, M.D., and Ajay Goel, Ph.D., are at the Center for Gastrointestinal Research and the Center for Epigenetics, Cancer Prevention, and Genomics at Baylor Research Institute, and at the Baylor Charles A. Sammons Cancer Center, all in Dallas. They reported having no relevant financial disclosures. Dr. Boland and Dr. Goel made these remarks in an editorial accompanying Dr. Dalerba’s report (N Engl J Med. 2016 Jan 21. doi:10.1056/NEJMe1514353).
A biomarker for which a screening test is already available – lack of CDX2 expression – appears to identify the approximately 20% of stage II and 50% of stage III colon cancers that are at high risk of recurrence and would benefit from adjuvant chemotherapy, according to a report published online Jan. 21 in the New England Journal of Medicine.
Distinguishing the high-risk tumors from those unlikely to recur or progress would allow some patients to opt for adjuvant chemotherapy that has been shown to significantly raise the rates of overall and disease-free survival. At present, almost all patients with stage II colon cancer are treated using surgery alone, said Dr. Piero Dalerba of the Herbert Irving Comprehensive Cancer Center and the department of pathology and cell biology at Columbia University, New York, and his associates.
The investigators used a bioinformatics approach to search for biomarkers that would identify the most aggressive colon cancers: undifferentiated tumors characterized by immature, stem-like colonic epithelial cells and depleted of more mature cells. They focused on biomarkers for which clinical diagnostic tests are already available, to facilitate use in real-world clinical practice. Their search through 2,329 colon gene-expression array experiments found that when tumors do not express one particular protein, homeobox transcription factor CDX2, “a master regulator of intestinal development and oncogenesis” that is highly specifically expressed in intestinal epithelium, those tumors tend to have aggressive features such as advanced stage, vascular invasion, and BRAF mutation.
Survival outcomes were then compared in a discovery data set of 466 patients. The 5-year disease-free survival rate was significantly lower, at 41%, among the 32 patients who had CDX2-negative tumors than the 74% survival rate among the 434 patients with CDX2-positive tumors. The hazard ratio for disease recurrence among patients with CDX2-negative vs. CDX2-positive tumors was 2.73, Dr. Dalerba and his associates said (N Engl J Med. 2016 Jan 21. doi:10.1056/NEJMoa1506597).
The investigators confirmed these findings in a separate validation data set of 366 patients, of whom 48 had CDX2-negative and 318 had CDX2-positive tumors. Five-year disease-free survival (48% vs. 71%), overall survival (33% vs. 59%), and disease-specific survival (45% vs. 72%) were significantly lower with CDX2-negative tumors, and the HR for disease recurrence was 2.42.
To determine whether adjuvant chemotherapy would improve survival outcomes in patients with CDX2-negative tumors, the researchers assessed outcomes in an expanded dataset of 669 patients with stage II and 1,228 patients with stage III colon cancer. They confirmed that among patients with stage II CDX2-negative tumors, those who received adjuvant chemotherapy had a higher rate of disease-free survival (91%) than did those who didn’t receive adjuvant chemotherapy (56%). Similarly, among patients with stage III CDX2-negative tumors, those who received adjuvant chemotherapy had a higher rate of disease-free survival (74%) than those who did not receive adjuvant chemotherapy (37%).
This survival benefit was independent of many known risk factors, including tumor grade, they noted.
These results must be replicated in future prospective studies, because this study’s design was retrospective and exploratory, Dr. Dalerba and his associates added.
This work was supported by the National Comprehensive Cancer Network, the National Institutes of Health, Siebel Stem Cell Institute, the Thomas and Stacey Siebel Foundation, the Virginia and D.K. Ludwig Fund for Cancer Research, the California Institute for Regenerative Medicine, the U.S. Department of Defense, the Bladder Cancer Advocacy Network, and BD Biosciences. Dr. Dalerba reported ties to Quanticel Pharmaceuticals and Oncomed Pharmaceuticals, and his associates reported ties to numerous industry sources.
A biomarker for which a screening test is already available – lack of CDX2 expression – appears to identify the approximately 20% of stage II and 50% of stage III colon cancers that are at high risk of recurrence and would benefit from adjuvant chemotherapy, according to a report published online Jan. 21 in the New England Journal of Medicine.
Distinguishing the high-risk tumors from those unlikely to recur or progress would allow some patients to opt for adjuvant chemotherapy that has been shown to significantly raise the rates of overall and disease-free survival. At present, almost all patients with stage II colon cancer are treated using surgery alone, said Dr. Piero Dalerba of the Herbert Irving Comprehensive Cancer Center and the department of pathology and cell biology at Columbia University, New York, and his associates.
The investigators used a bioinformatics approach to search for biomarkers that would identify the most aggressive colon cancers: undifferentiated tumors characterized by immature, stem-like colonic epithelial cells and depleted of more mature cells. They focused on biomarkers for which clinical diagnostic tests are already available, to facilitate use in real-world clinical practice. Their search through 2,329 colon gene-expression array experiments found that when tumors do not express one particular protein, homeobox transcription factor CDX2, “a master regulator of intestinal development and oncogenesis” that is highly specifically expressed in intestinal epithelium, those tumors tend to have aggressive features such as advanced stage, vascular invasion, and BRAF mutation.
Survival outcomes were then compared in a discovery data set of 466 patients. The 5-year disease-free survival rate was significantly lower, at 41%, among the 32 patients who had CDX2-negative tumors than the 74% survival rate among the 434 patients with CDX2-positive tumors. The hazard ratio for disease recurrence among patients with CDX2-negative vs. CDX2-positive tumors was 2.73, Dr. Dalerba and his associates said (N Engl J Med. 2016 Jan 21. doi:10.1056/NEJMoa1506597).
The investigators confirmed these findings in a separate validation data set of 366 patients, of whom 48 had CDX2-negative and 318 had CDX2-positive tumors. Five-year disease-free survival (48% vs. 71%), overall survival (33% vs. 59%), and disease-specific survival (45% vs. 72%) were significantly lower with CDX2-negative tumors, and the HR for disease recurrence was 2.42.
To determine whether adjuvant chemotherapy would improve survival outcomes in patients with CDX2-negative tumors, the researchers assessed outcomes in an expanded dataset of 669 patients with stage II and 1,228 patients with stage III colon cancer. They confirmed that among patients with stage II CDX2-negative tumors, those who received adjuvant chemotherapy had a higher rate of disease-free survival (91%) than did those who didn’t receive adjuvant chemotherapy (56%). Similarly, among patients with stage III CDX2-negative tumors, those who received adjuvant chemotherapy had a higher rate of disease-free survival (74%) than those who did not receive adjuvant chemotherapy (37%).
This survival benefit was independent of many known risk factors, including tumor grade, they noted.
These results must be replicated in future prospective studies, because this study’s design was retrospective and exploratory, Dr. Dalerba and his associates added.
This work was supported by the National Comprehensive Cancer Network, the National Institutes of Health, Siebel Stem Cell Institute, the Thomas and Stacey Siebel Foundation, the Virginia and D.K. Ludwig Fund for Cancer Research, the California Institute for Regenerative Medicine, the U.S. Department of Defense, the Bladder Cancer Advocacy Network, and BD Biosciences. Dr. Dalerba reported ties to Quanticel Pharmaceuticals and Oncomed Pharmaceuticals, and his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A biomarker for which a screening test is already available – lack of CDX2 expression – appears to identify which stage II and III colon cancers are high-risk and would benefit from adjuvant chemotherapy.
Major finding: Five-year disease-free survival rate was significantly lower (41%) in patients who had CDX2-negative tumors than in patients with CDX2-positive tumors (74%).
Data source: A series of analyses using data mining to identify (in a database of 466 patients) and validate (in databases of 366 and 1,897 patients) a biomarker for early colon cancers at high risk for recurrence/metastasis.
Disclosures: The National Comprehensive Cancer Network, the National Institutes of Health, Siebel Stem Cell Institute, the Thomas and Stacey Siebel Foundation, the Virginia and D.K. Ludwig Fund for Cancer Research, the California Institute for Regenerative Medicine, the U.S. Department of Defense, the Bladder Cancer Advocacy Network, and BD Biosciences supported the work. Dr. Dalerba reported ties to Quanticel Pharmaceuticals and Oncomed Pharmaceuticals, and his associates reported ties to numerous industry sources.
MRI findings beyond sacroiliitis not necessary for classifying nonradiographic axial SpA
Current recommendations for identifying sacroiliitis on MRI to classify patients with nonradiographic axial spondyloarthritis should still depend on the presence of subchondral bone marrow edema, but additional evidence of structural lesions can be taken into account to define the presence of inflammatory lesions, according to a consensus review by experts from the Assessment in SpondyloArthritis International Society MRI working group.
The additional information provided by structural lesions, such as erosions, detected via MRI of the sacroiliac (SI) joint or spine is not necessary for the definition, but “may enhance confidence in the classification of axial SpA [spondyloarthritis],” said the panel of 16 rheumatologists, 4 radiologists, and 1 research fellow, who presented their summary and draft proposal at the January 2014 annual assembly of the Assessment in SpondyloArthritis International Society (ASAS), where members unanimously approved it (Ann Rheum Dis. 2016 Jan 14. doi: 10.1136/annrheumdis-2015-208642).
The group’s goal was to examine whether new data published on axial SpA in the 5 years following the 2009 publication of the ASAS recommendations were “sufficient to merit a change in the MRI definition of a positive MRI and clarify any misunderstanding of the existing definition.”
Overall, the working group determined that the addition of “structural damage changes of the SI joints and the addition of features on MRI of the spine for classification purposes is not yet clear and this continues to be an important research agenda.”
Adding any single lesion or combination of lesions to the current classification criteria for nonradiographic axial spondyloarthritis (nr-axSpA) did not increase the sensitivity of the MRI definition without losing specificity in one cohort, whereas there was an unclear benefit to adding SI erosion to the definition in another cohort. The evaluation of these lesions on MRI depended on the use of T1 weighting and fat-suppression techniques, as well as the contextual interpretation of MRI, which currently add too much complexity to the definition of a positive SI joint MRI to be useful in achieving a “consensus for definitions for each MRI structural damage lesion and the setting of thresholds for any defined lesion or combination of lesions,” the working group wrote.
The panelists found that there was no consistent beneficial effect of adding features of SpA on spine MRI to the definition. Spine MRI added incremental sensitivity in other analyses, but also increased false-positive SpA diagnoses.
In a commentary reviewing the controversy and evidence for classifying diseases within the spectrum of axial SpA, Dr. Atul Deodhar of Oregon Health and Science University, Portland, and his colleagues noted that “there is no need to differentiate between a diagnosis of nr-axSpA and that of [ankylosing spondylitis] in clinical practice, since the only purpose for having these two labels is classification.” They said the need for formal distinction between nr-axSpA and ankylosing spondylitis may require some exceptions, such as when it is necessary “to specify an approved indication for TNFi [tumor necrosis factor inhibitor] therapy, when off-label use of biologics must be avoided ... and to clarify the presence of structural changes that are required for patients to receive coverage from their insurance carrier to use a TNFi” (Ann. Rheum Dis. 2016 Jan 14. doi: 10.1136/annrheumdis-2015-208852).
The working panel and commentary authors declared having no competing interests.
Current recommendations for identifying sacroiliitis on MRI to classify patients with nonradiographic axial spondyloarthritis should still depend on the presence of subchondral bone marrow edema, but additional evidence of structural lesions can be taken into account to define the presence of inflammatory lesions, according to a consensus review by experts from the Assessment in SpondyloArthritis International Society MRI working group.
The additional information provided by structural lesions, such as erosions, detected via MRI of the sacroiliac (SI) joint or spine is not necessary for the definition, but “may enhance confidence in the classification of axial SpA [spondyloarthritis],” said the panel of 16 rheumatologists, 4 radiologists, and 1 research fellow, who presented their summary and draft proposal at the January 2014 annual assembly of the Assessment in SpondyloArthritis International Society (ASAS), where members unanimously approved it (Ann Rheum Dis. 2016 Jan 14. doi: 10.1136/annrheumdis-2015-208642).
The group’s goal was to examine whether new data published on axial SpA in the 5 years following the 2009 publication of the ASAS recommendations were “sufficient to merit a change in the MRI definition of a positive MRI and clarify any misunderstanding of the existing definition.”
Overall, the working group determined that the addition of “structural damage changes of the SI joints and the addition of features on MRI of the spine for classification purposes is not yet clear and this continues to be an important research agenda.”
Adding any single lesion or combination of lesions to the current classification criteria for nonradiographic axial spondyloarthritis (nr-axSpA) did not increase the sensitivity of the MRI definition without losing specificity in one cohort, whereas there was an unclear benefit to adding SI erosion to the definition in another cohort. The evaluation of these lesions on MRI depended on the use of T1 weighting and fat-suppression techniques, as well as the contextual interpretation of MRI, which currently add too much complexity to the definition of a positive SI joint MRI to be useful in achieving a “consensus for definitions for each MRI structural damage lesion and the setting of thresholds for any defined lesion or combination of lesions,” the working group wrote.
The panelists found that there was no consistent beneficial effect of adding features of SpA on spine MRI to the definition. Spine MRI added incremental sensitivity in other analyses, but also increased false-positive SpA diagnoses.
In a commentary reviewing the controversy and evidence for classifying diseases within the spectrum of axial SpA, Dr. Atul Deodhar of Oregon Health and Science University, Portland, and his colleagues noted that “there is no need to differentiate between a diagnosis of nr-axSpA and that of [ankylosing spondylitis] in clinical practice, since the only purpose for having these two labels is classification.” They said the need for formal distinction between nr-axSpA and ankylosing spondylitis may require some exceptions, such as when it is necessary “to specify an approved indication for TNFi [tumor necrosis factor inhibitor] therapy, when off-label use of biologics must be avoided ... and to clarify the presence of structural changes that are required for patients to receive coverage from their insurance carrier to use a TNFi” (Ann. Rheum Dis. 2016 Jan 14. doi: 10.1136/annrheumdis-2015-208852).
The working panel and commentary authors declared having no competing interests.
Current recommendations for identifying sacroiliitis on MRI to classify patients with nonradiographic axial spondyloarthritis should still depend on the presence of subchondral bone marrow edema, but additional evidence of structural lesions can be taken into account to define the presence of inflammatory lesions, according to a consensus review by experts from the Assessment in SpondyloArthritis International Society MRI working group.
The additional information provided by structural lesions, such as erosions, detected via MRI of the sacroiliac (SI) joint or spine is not necessary for the definition, but “may enhance confidence in the classification of axial SpA [spondyloarthritis],” said the panel of 16 rheumatologists, 4 radiologists, and 1 research fellow, who presented their summary and draft proposal at the January 2014 annual assembly of the Assessment in SpondyloArthritis International Society (ASAS), where members unanimously approved it (Ann Rheum Dis. 2016 Jan 14. doi: 10.1136/annrheumdis-2015-208642).
The group’s goal was to examine whether new data published on axial SpA in the 5 years following the 2009 publication of the ASAS recommendations were “sufficient to merit a change in the MRI definition of a positive MRI and clarify any misunderstanding of the existing definition.”
Overall, the working group determined that the addition of “structural damage changes of the SI joints and the addition of features on MRI of the spine for classification purposes is not yet clear and this continues to be an important research agenda.”
Adding any single lesion or combination of lesions to the current classification criteria for nonradiographic axial spondyloarthritis (nr-axSpA) did not increase the sensitivity of the MRI definition without losing specificity in one cohort, whereas there was an unclear benefit to adding SI erosion to the definition in another cohort. The evaluation of these lesions on MRI depended on the use of T1 weighting and fat-suppression techniques, as well as the contextual interpretation of MRI, which currently add too much complexity to the definition of a positive SI joint MRI to be useful in achieving a “consensus for definitions for each MRI structural damage lesion and the setting of thresholds for any defined lesion or combination of lesions,” the working group wrote.
The panelists found that there was no consistent beneficial effect of adding features of SpA on spine MRI to the definition. Spine MRI added incremental sensitivity in other analyses, but also increased false-positive SpA diagnoses.
In a commentary reviewing the controversy and evidence for classifying diseases within the spectrum of axial SpA, Dr. Atul Deodhar of Oregon Health and Science University, Portland, and his colleagues noted that “there is no need to differentiate between a diagnosis of nr-axSpA and that of [ankylosing spondylitis] in clinical practice, since the only purpose for having these two labels is classification.” They said the need for formal distinction between nr-axSpA and ankylosing spondylitis may require some exceptions, such as when it is necessary “to specify an approved indication for TNFi [tumor necrosis factor inhibitor] therapy, when off-label use of biologics must be avoided ... and to clarify the presence of structural changes that are required for patients to receive coverage from their insurance carrier to use a TNFi” (Ann. Rheum Dis. 2016 Jan 14. doi: 10.1136/annrheumdis-2015-208852).
The working panel and commentary authors declared having no competing interests.
FROM ANNALS OF THE RHEUMATIC DISEASES
Experts say abandon aspirin for stroke prevention in atrial fib
SNOWMASS, COLO. – It’s time to eliminate the practice of prescribing aspirin for stroke prevention in patients with atrial fibrillation and a CHA2DS2-VASc score of 1, two eminent cardiologists agreed at the Annual Cardiovascular Conference at Snowmass.
“The European guidelines have done away with aspirin for stroke prevention in atrial fibrillation. It barely made it into our current U.S. guidelines. I don’t think aspirin should be in there and I don’t think it will be there in the next guidelines. The role of aspirin will fall away,” predicted Dr. Bernard J. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
“It’s not that aspirin is less effective than the oral anticoagulants, it’s that there’s no role for it. There are no good data to support aspirin in the prevention of stroke in atrial fibrillation,” he declared.
Dr. N.A. Mark Estes III agreed the aspirin evidence is seriously flawed.
“The use of aspirin has probably been misguided, based upon a single trial which showed a profound effect and was probably just an anomaly,” according to Dr. Estes, a past president of the Heart Rhythm Society who is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts University, Boston.
The sole positive clinical trial of aspirin versus placebo, the 25-year-old Stroke Prevention in Atrial Fibrillation (SPAF) study (Circulation. 1991 Aug;84[2]:527-39), found an unrealistically high stroke protection benefit for aspirin, a result made implausible by multiple other randomized trials showing no benefit, the cardiologists agreed.
“In our current guidelines for atrial fibrillation (Circulation. 2014 Dec 2;130[23]:2071-104), aspirin can be considered as a Class IIb level of evidence C recommendation in patients with a CHA2DS2-VASc of 1. But I would just take it off of your clinical armamentarium because the best available data indicates that it doesn’t prevent strokes. I’m certainly not using it in my patients. Increasingly in my patients with a CHA2DS2-VASc of 1, I’m discussing the risks and benefits of a NOAC [novel oral anticoagulant],” Dr. Estes said.
Dr. Gersh was also critical of another common practice in stroke prevention in atrial fibrillation: concomitant use of aspirin with an oral anticoagulant.
“We use too much aspirin in patients on oral anticoagulation. Aspirin is perhaps the major cause of bleeding in patients on an oral anticoagulant. Other than in people with a drug-eluting stent, there’s no role at all for aspirin in stroke prevention,” he asserted.
He was coauthor of an analysis of 7,347 participants in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) who were on an oral anticoagulant. Fully 35% of them were also on aspirin. In a multivariate analysis, concomitant aspirin and oral anticoagulation was independently associated with a 53% increased risk of major bleeding and a 52% increase in hospitalization for bleeding, compared with atrial fibrillation patients on an oral anticoagulant alone (Circulation. 2013 Aug 13;128[7]:721-8).
Moreover, the widespread use of dual therapy in this real-world registry didn’t appear to be rational. Thirty-nine percent of those on aspirin plus an oral anticoagulant had no history of atherosclerotic disease, the presence of which would be an indication for considering aspirin. And 17% of dual therapy patients had an elevated Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score of 5 or more, making dual therapy particularly risky.
This clinically important interaction between aspirin and oral anticoagulation was recently underscored in an analysis of rivaroxaban-treated patients in the ROCKET AF trial, Dr. Gersh observed. Long-term use of aspirin at entry into this pivotal randomized trial of rivaroxaban (Xarelto) versus warfarin in patients with atrial fibrillation proved to be an independent predictor of a 47% increase in the risk of gastrointestinal bleeding, compared with patients on rivaroxaban alone (J Am Coll Cardiol. 2015 Dec 1;66[21]:2271-81).
He added that there is no evidence that combining aspirin and oral anticoagulation enhances stroke prevention beyond the marked benefit achieved with oral anticoagulation alone.
Dr. Gersh reported serving on the leadership of the ORBIT-AF Registry, which was sponsored by Janssen Pharmaceuticals. Dr. Estes reported having no financial conflicts relevant to this discussion.
SNOWMASS, COLO. – It’s time to eliminate the practice of prescribing aspirin for stroke prevention in patients with atrial fibrillation and a CHA2DS2-VASc score of 1, two eminent cardiologists agreed at the Annual Cardiovascular Conference at Snowmass.
“The European guidelines have done away with aspirin for stroke prevention in atrial fibrillation. It barely made it into our current U.S. guidelines. I don’t think aspirin should be in there and I don’t think it will be there in the next guidelines. The role of aspirin will fall away,” predicted Dr. Bernard J. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
“It’s not that aspirin is less effective than the oral anticoagulants, it’s that there’s no role for it. There are no good data to support aspirin in the prevention of stroke in atrial fibrillation,” he declared.
Dr. N.A. Mark Estes III agreed the aspirin evidence is seriously flawed.
“The use of aspirin has probably been misguided, based upon a single trial which showed a profound effect and was probably just an anomaly,” according to Dr. Estes, a past president of the Heart Rhythm Society who is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts University, Boston.
The sole positive clinical trial of aspirin versus placebo, the 25-year-old Stroke Prevention in Atrial Fibrillation (SPAF) study (Circulation. 1991 Aug;84[2]:527-39), found an unrealistically high stroke protection benefit for aspirin, a result made implausible by multiple other randomized trials showing no benefit, the cardiologists agreed.
“In our current guidelines for atrial fibrillation (Circulation. 2014 Dec 2;130[23]:2071-104), aspirin can be considered as a Class IIb level of evidence C recommendation in patients with a CHA2DS2-VASc of 1. But I would just take it off of your clinical armamentarium because the best available data indicates that it doesn’t prevent strokes. I’m certainly not using it in my patients. Increasingly in my patients with a CHA2DS2-VASc of 1, I’m discussing the risks and benefits of a NOAC [novel oral anticoagulant],” Dr. Estes said.
Dr. Gersh was also critical of another common practice in stroke prevention in atrial fibrillation: concomitant use of aspirin with an oral anticoagulant.
“We use too much aspirin in patients on oral anticoagulation. Aspirin is perhaps the major cause of bleeding in patients on an oral anticoagulant. Other than in people with a drug-eluting stent, there’s no role at all for aspirin in stroke prevention,” he asserted.
He was coauthor of an analysis of 7,347 participants in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) who were on an oral anticoagulant. Fully 35% of them were also on aspirin. In a multivariate analysis, concomitant aspirin and oral anticoagulation was independently associated with a 53% increased risk of major bleeding and a 52% increase in hospitalization for bleeding, compared with atrial fibrillation patients on an oral anticoagulant alone (Circulation. 2013 Aug 13;128[7]:721-8).
Moreover, the widespread use of dual therapy in this real-world registry didn’t appear to be rational. Thirty-nine percent of those on aspirin plus an oral anticoagulant had no history of atherosclerotic disease, the presence of which would be an indication for considering aspirin. And 17% of dual therapy patients had an elevated Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score of 5 or more, making dual therapy particularly risky.
This clinically important interaction between aspirin and oral anticoagulation was recently underscored in an analysis of rivaroxaban-treated patients in the ROCKET AF trial, Dr. Gersh observed. Long-term use of aspirin at entry into this pivotal randomized trial of rivaroxaban (Xarelto) versus warfarin in patients with atrial fibrillation proved to be an independent predictor of a 47% increase in the risk of gastrointestinal bleeding, compared with patients on rivaroxaban alone (J Am Coll Cardiol. 2015 Dec 1;66[21]:2271-81).
He added that there is no evidence that combining aspirin and oral anticoagulation enhances stroke prevention beyond the marked benefit achieved with oral anticoagulation alone.
Dr. Gersh reported serving on the leadership of the ORBIT-AF Registry, which was sponsored by Janssen Pharmaceuticals. Dr. Estes reported having no financial conflicts relevant to this discussion.
SNOWMASS, COLO. – It’s time to eliminate the practice of prescribing aspirin for stroke prevention in patients with atrial fibrillation and a CHA2DS2-VASc score of 1, two eminent cardiologists agreed at the Annual Cardiovascular Conference at Snowmass.
“The European guidelines have done away with aspirin for stroke prevention in atrial fibrillation. It barely made it into our current U.S. guidelines. I don’t think aspirin should be in there and I don’t think it will be there in the next guidelines. The role of aspirin will fall away,” predicted Dr. Bernard J. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
“It’s not that aspirin is less effective than the oral anticoagulants, it’s that there’s no role for it. There are no good data to support aspirin in the prevention of stroke in atrial fibrillation,” he declared.
Dr. N.A. Mark Estes III agreed the aspirin evidence is seriously flawed.
“The use of aspirin has probably been misguided, based upon a single trial which showed a profound effect and was probably just an anomaly,” according to Dr. Estes, a past president of the Heart Rhythm Society who is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts University, Boston.
The sole positive clinical trial of aspirin versus placebo, the 25-year-old Stroke Prevention in Atrial Fibrillation (SPAF) study (Circulation. 1991 Aug;84[2]:527-39), found an unrealistically high stroke protection benefit for aspirin, a result made implausible by multiple other randomized trials showing no benefit, the cardiologists agreed.
“In our current guidelines for atrial fibrillation (Circulation. 2014 Dec 2;130[23]:2071-104), aspirin can be considered as a Class IIb level of evidence C recommendation in patients with a CHA2DS2-VASc of 1. But I would just take it off of your clinical armamentarium because the best available data indicates that it doesn’t prevent strokes. I’m certainly not using it in my patients. Increasingly in my patients with a CHA2DS2-VASc of 1, I’m discussing the risks and benefits of a NOAC [novel oral anticoagulant],” Dr. Estes said.
Dr. Gersh was also critical of another common practice in stroke prevention in atrial fibrillation: concomitant use of aspirin with an oral anticoagulant.
“We use too much aspirin in patients on oral anticoagulation. Aspirin is perhaps the major cause of bleeding in patients on an oral anticoagulant. Other than in people with a drug-eluting stent, there’s no role at all for aspirin in stroke prevention,” he asserted.
He was coauthor of an analysis of 7,347 participants in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) who were on an oral anticoagulant. Fully 35% of them were also on aspirin. In a multivariate analysis, concomitant aspirin and oral anticoagulation was independently associated with a 53% increased risk of major bleeding and a 52% increase in hospitalization for bleeding, compared with atrial fibrillation patients on an oral anticoagulant alone (Circulation. 2013 Aug 13;128[7]:721-8).
Moreover, the widespread use of dual therapy in this real-world registry didn’t appear to be rational. Thirty-nine percent of those on aspirin plus an oral anticoagulant had no history of atherosclerotic disease, the presence of which would be an indication for considering aspirin. And 17% of dual therapy patients had an elevated Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score of 5 or more, making dual therapy particularly risky.
This clinically important interaction between aspirin and oral anticoagulation was recently underscored in an analysis of rivaroxaban-treated patients in the ROCKET AF trial, Dr. Gersh observed. Long-term use of aspirin at entry into this pivotal randomized trial of rivaroxaban (Xarelto) versus warfarin in patients with atrial fibrillation proved to be an independent predictor of a 47% increase in the risk of gastrointestinal bleeding, compared with patients on rivaroxaban alone (J Am Coll Cardiol. 2015 Dec 1;66[21]:2271-81).
He added that there is no evidence that combining aspirin and oral anticoagulation enhances stroke prevention beyond the marked benefit achieved with oral anticoagulation alone.
Dr. Gersh reported serving on the leadership of the ORBIT-AF Registry, which was sponsored by Janssen Pharmaceuticals. Dr. Estes reported having no financial conflicts relevant to this discussion.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS