User login
What Is Your Diagnosis? Mycosis Fungoides
The Diagnosis: Mycosis Fungoides
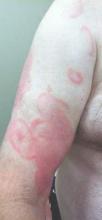
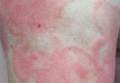
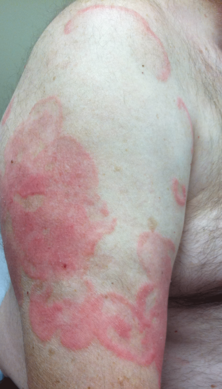
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
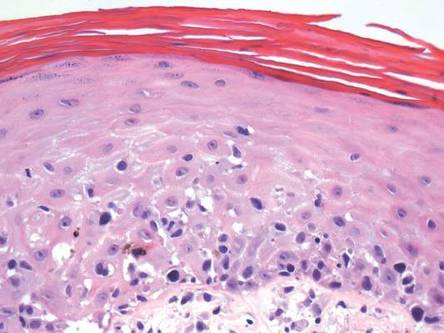
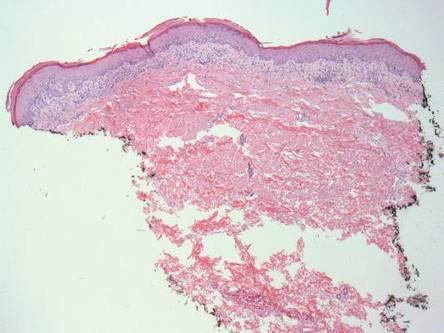
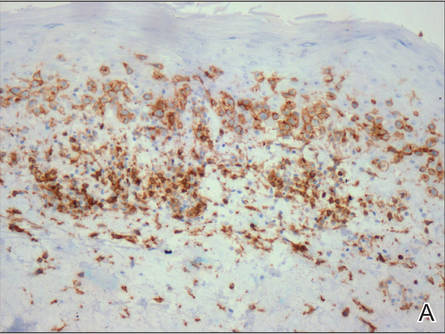
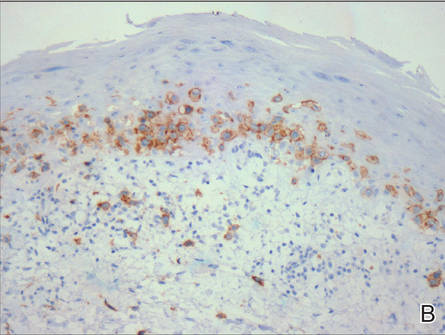
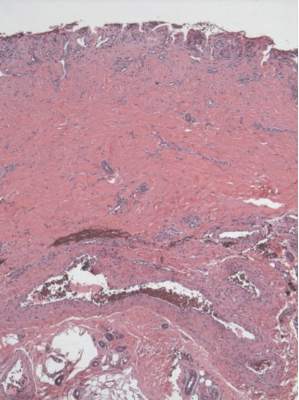
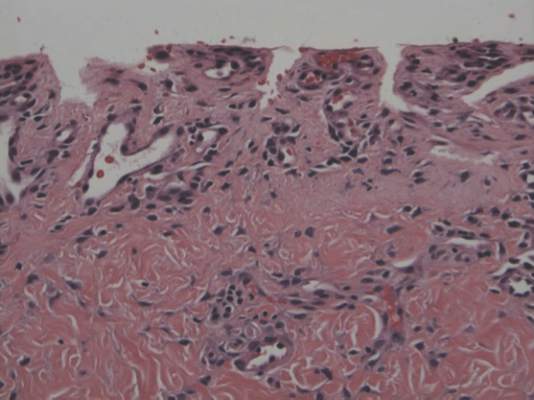
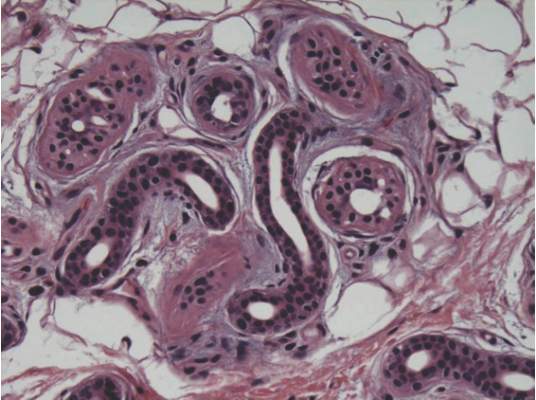
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2
| 
| |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2
| 
| |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
The Diagnosis: Mycosis Fungoides
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2

| 
| |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2

| 
| |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
The Diagnosis: Mycosis Fungoides
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2

| 
| |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2

| 
| |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
An otherwise healthy 62-year-old man presented for evaluation of multiple scaly erythematous plaques on the right upper arm and shoulder of 10 years’ duration. The patient reported a burning sensation but no exacerbation of the lesions upon sun exposure. He previously had been treated for a presumed clinical diagnosis of erythema annulare centrifugum but experienced only modest improvement with topical corticosteroids and tacrolimus ointment 0.1%. Previous trials of systemic antifungals also yielded minimal benefit.
Capital misadventures
A few years ago I wrote a column about what promised to be an exciting development in blood testing technology. Using the money her parents had set aside for her education, a young woman dropped out of Stanford University at age 19 and started a company that she claimed would be able to offer hundreds of lab tests on just a few drops of blood. Results would be available in just minutes instead of hours or days. At the time I wrote the column, the company had just landed a contract with a large drug store chain with an arrangement that would eventually allow nearly every resident of the United States to be within a few miles of a site that would offer rapid response blood tests with nothing more than a finger prick.
It seemed a little hard to believe, but the prospect of pediatricians being able to make a diagnosis without running the risk of exsanguinating our smallest patients sounded appealing. On the other hand, I worried that a quick and easy technology might encourage some physicians to use a shotgun approach to diagnosing illness rather than a more rational and cost-effective process based on the traditional skills of history taking and physical examination. Some patients who foolishly wanted to know “everything” about themselves might be tempted to ask their physicians to order the whole smorgasbord of tests. “Hey, it’s only a few drops of blood.”
Turns out there were enough people with more money than reservations and the company quickly attracted hundreds of millions of dollars in venture capital. The company, now calling itself Theranos, has been valued at nine billion dollars. But, recently this startup star has encountered some serious bumps in the road to a full-scale launch (“Hot Startup Theranos Has Struggled With Its Blood-Test Technology” by John Carreyrou, The Wall Street Journal, updated Oct. 16, 2015). The Wall Street Journal reported that despite promises, only a few of the 240 tests offered by the company are currently performed using their proprietary microtechnique. In the days following the Journal article, the Food and Drug Administration warned Theranos that their “nanotainer” is considered a new medical device that must first clear the agency’s time consuming and costly vetting process (“Hot Startup Theranos Dials Back Lab Tests at FDA’s Behest” by John Carreyrou, The Wall Street Journal, updated Oct. 16, 2015).
The venture capitalists who had climbed on the Theranos bandwagon tempted by the just-a-few-drops promise may end up seeing their bank accounts hemorrhage. But I don’t think we should be too critical of their investment decision. It was and may still be good idea that has simply run afoul of the details. However, I recently learned about another new business that I don’t consider to have even started with a good idea, but still has managed to attract enough capital to get itself off the ground (“Should Breast Milk Be Nutritionally Analyzed?” by Laura Johannes, The Wall Street Journal, Dec. 28, 2015).
I’m sure you have seen some new mothers who were concerned that their breast milk was not enough for their babies. But how many of them would pay $150 for a start-up kit and then more than $300 to find out the nutritional content of their breast milk? What if it meant pumping and freezing three samples 2 or 3 days apart and then shipping them in a cooler to a lab? What if you told them that neither you nor anyone else could reliably interpret the results because there aren’t any published guidelines for the optimal composition of human breast milk? Even if your practice is packed to the rafters with anxiety-driven, irrational parents, I don’t think you would find many takers. But that doesn’t seem to have bothered the folks who have invested in Happy Vitals, a company in Washington that is offering a service similar to the one I have just described.
You and I might not have invested in a company whose business plan was to offer such a service. But I fear there may be enough health care “providers” practicing without the benefit of an evidence-based education that what I consider a capital misadventure may actually be able to pay back its investors.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
A few years ago I wrote a column about what promised to be an exciting development in blood testing technology. Using the money her parents had set aside for her education, a young woman dropped out of Stanford University at age 19 and started a company that she claimed would be able to offer hundreds of lab tests on just a few drops of blood. Results would be available in just minutes instead of hours or days. At the time I wrote the column, the company had just landed a contract with a large drug store chain with an arrangement that would eventually allow nearly every resident of the United States to be within a few miles of a site that would offer rapid response blood tests with nothing more than a finger prick.
It seemed a little hard to believe, but the prospect of pediatricians being able to make a diagnosis without running the risk of exsanguinating our smallest patients sounded appealing. On the other hand, I worried that a quick and easy technology might encourage some physicians to use a shotgun approach to diagnosing illness rather than a more rational and cost-effective process based on the traditional skills of history taking and physical examination. Some patients who foolishly wanted to know “everything” about themselves might be tempted to ask their physicians to order the whole smorgasbord of tests. “Hey, it’s only a few drops of blood.”
Turns out there were enough people with more money than reservations and the company quickly attracted hundreds of millions of dollars in venture capital. The company, now calling itself Theranos, has been valued at nine billion dollars. But, recently this startup star has encountered some serious bumps in the road to a full-scale launch (“Hot Startup Theranos Has Struggled With Its Blood-Test Technology” by John Carreyrou, The Wall Street Journal, updated Oct. 16, 2015). The Wall Street Journal reported that despite promises, only a few of the 240 tests offered by the company are currently performed using their proprietary microtechnique. In the days following the Journal article, the Food and Drug Administration warned Theranos that their “nanotainer” is considered a new medical device that must first clear the agency’s time consuming and costly vetting process (“Hot Startup Theranos Dials Back Lab Tests at FDA’s Behest” by John Carreyrou, The Wall Street Journal, updated Oct. 16, 2015).
The venture capitalists who had climbed on the Theranos bandwagon tempted by the just-a-few-drops promise may end up seeing their bank accounts hemorrhage. But I don’t think we should be too critical of their investment decision. It was and may still be good idea that has simply run afoul of the details. However, I recently learned about another new business that I don’t consider to have even started with a good idea, but still has managed to attract enough capital to get itself off the ground (“Should Breast Milk Be Nutritionally Analyzed?” by Laura Johannes, The Wall Street Journal, Dec. 28, 2015).
I’m sure you have seen some new mothers who were concerned that their breast milk was not enough for their babies. But how many of them would pay $150 for a start-up kit and then more than $300 to find out the nutritional content of their breast milk? What if it meant pumping and freezing three samples 2 or 3 days apart and then shipping them in a cooler to a lab? What if you told them that neither you nor anyone else could reliably interpret the results because there aren’t any published guidelines for the optimal composition of human breast milk? Even if your practice is packed to the rafters with anxiety-driven, irrational parents, I don’t think you would find many takers. But that doesn’t seem to have bothered the folks who have invested in Happy Vitals, a company in Washington that is offering a service similar to the one I have just described.
You and I might not have invested in a company whose business plan was to offer such a service. But I fear there may be enough health care “providers” practicing without the benefit of an evidence-based education that what I consider a capital misadventure may actually be able to pay back its investors.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
A few years ago I wrote a column about what promised to be an exciting development in blood testing technology. Using the money her parents had set aside for her education, a young woman dropped out of Stanford University at age 19 and started a company that she claimed would be able to offer hundreds of lab tests on just a few drops of blood. Results would be available in just minutes instead of hours or days. At the time I wrote the column, the company had just landed a contract with a large drug store chain with an arrangement that would eventually allow nearly every resident of the United States to be within a few miles of a site that would offer rapid response blood tests with nothing more than a finger prick.
It seemed a little hard to believe, but the prospect of pediatricians being able to make a diagnosis without running the risk of exsanguinating our smallest patients sounded appealing. On the other hand, I worried that a quick and easy technology might encourage some physicians to use a shotgun approach to diagnosing illness rather than a more rational and cost-effective process based on the traditional skills of history taking and physical examination. Some patients who foolishly wanted to know “everything” about themselves might be tempted to ask their physicians to order the whole smorgasbord of tests. “Hey, it’s only a few drops of blood.”
Turns out there were enough people with more money than reservations and the company quickly attracted hundreds of millions of dollars in venture capital. The company, now calling itself Theranos, has been valued at nine billion dollars. But, recently this startup star has encountered some serious bumps in the road to a full-scale launch (“Hot Startup Theranos Has Struggled With Its Blood-Test Technology” by John Carreyrou, The Wall Street Journal, updated Oct. 16, 2015). The Wall Street Journal reported that despite promises, only a few of the 240 tests offered by the company are currently performed using their proprietary microtechnique. In the days following the Journal article, the Food and Drug Administration warned Theranos that their “nanotainer” is considered a new medical device that must first clear the agency’s time consuming and costly vetting process (“Hot Startup Theranos Dials Back Lab Tests at FDA’s Behest” by John Carreyrou, The Wall Street Journal, updated Oct. 16, 2015).
The venture capitalists who had climbed on the Theranos bandwagon tempted by the just-a-few-drops promise may end up seeing their bank accounts hemorrhage. But I don’t think we should be too critical of their investment decision. It was and may still be good idea that has simply run afoul of the details. However, I recently learned about another new business that I don’t consider to have even started with a good idea, but still has managed to attract enough capital to get itself off the ground (“Should Breast Milk Be Nutritionally Analyzed?” by Laura Johannes, The Wall Street Journal, Dec. 28, 2015).
I’m sure you have seen some new mothers who were concerned that their breast milk was not enough for their babies. But how many of them would pay $150 for a start-up kit and then more than $300 to find out the nutritional content of their breast milk? What if it meant pumping and freezing three samples 2 or 3 days apart and then shipping them in a cooler to a lab? What if you told them that neither you nor anyone else could reliably interpret the results because there aren’t any published guidelines for the optimal composition of human breast milk? Even if your practice is packed to the rafters with anxiety-driven, irrational parents, I don’t think you would find many takers. But that doesn’t seem to have bothered the folks who have invested in Happy Vitals, a company in Washington that is offering a service similar to the one I have just described.
You and I might not have invested in a company whose business plan was to offer such a service. But I fear there may be enough health care “providers” practicing without the benefit of an evidence-based education that what I consider a capital misadventure may actually be able to pay back its investors.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
Subcorneal Hematomas in Excessive Video Game Play
Case Report
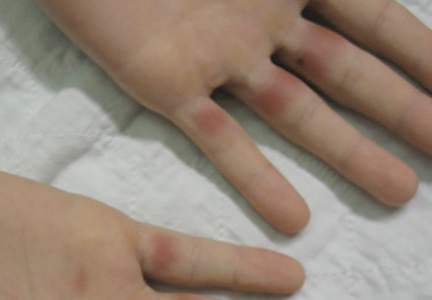
A 19-year-old man was admitted to our hospital to begin treatment for acute myeloid leukemia that had been diagnosed 2 days prior. Three days after completing a 10-day regimen of induction chemotherapy, he developed bilateral, well-demarcated erythematous patches on the palmar surfaces of the proximal phalanges of the third, fourth, and fifth fingers (Figure 1) and 2 patches on the right palm. The patient was referred to dermatology for evaluation. He recalled no trauma to these sites although he reported pushing his intravenous pole with the right hand when walking. Of note, he had become neutropenic and thrombocytopenic following chemotherapy

On physical examination, the patches measured 1- to 1.5-cm in diameter and were mildly tender to palpation. The 2 patches on the right palm were much smaller than those on the fingers but were otherwise similar in appearance.
A punch biopsy of the erythematous lesion on the left third digit was performed. Histologic examination revealed extensive epidermal denudation associated with vascular proliferation and congestion as well as hemorrhage and a sparse lymphocytic infiltrate (Figures 2–4). There was no evidence of a leukemic infiltrate, and stains for fungal elements and bacteria were negative. Eccrine ducts appeared normal with no evidence of necrosis or metaplasia. These findings were suggestive of a frictional etiology.
Due to the distribution of the skin lesions on the hands, it was suspected that the source of friction was a video game controller. Although the patient denied playing video games since his admission to the hospital, he reported heavy video game use during the weeks prior to admission. We postulated that the thrombocytopenia the patient developed following chemotherapy along with prior friction injury sustained from heavy video game play led to traumatic subcorneal hemorrhage on the hands at the points of contact with the video game controller (Figure 5). The subcorneal hematomas resolved completely over the next 2 months during which the patient abstained from video game play.

This case demonstrates the importance of obtaining a detailed patient history, as our patient’s history of video game play prior to hospitalization proved to be of major diagnostic importance. Although the location, distribution, and well-demarcated nature of the patient’s lesions suggested an external source of trauma and biopsy definitively ruled out leukemia cutis, Sweet syndrome, and eccrine hidradenitis,1 the final diagnosis of traumatic subcorneal hematomas was only possible with specific knowledge of the patient’s video game controller use.
Comment
History of video game play has been key to the diagnosis of a variety of cutaneous lesions documented in the medical literature. Robertson et al2 attributed a similar case of traumatic subcorneal hematomas of the hands in an otherwise healthy 16-year-old boy to excessive use of a video game controller. Similarly, Kasraee et al3 attributed a case of idiopathic eccrine hidradenitis in an otherwise healthy 12-year-old girl to excessive video game use. In both of these reported cases, bilateral skin lesions on the palms of the hands appeared acutely in a pattern consistent with the points of contact of a video game controller. Excessive video game play has also been associated with unilateral dermatologic lesions on the hands, such as knuckle pads,4 onycholysis,5 friction blisters,6 pressure ulcers,7 and hemorrhagic lesions.5,6,8
Video game–related pathologies are not limited to the skin and have been implicated in a variety of clinical presentations. In 1987, Osterman et al9 published an early account of repetitive strain injury (RSI) related to video game use in which the investigators reported 2 cases of video game–related volar flexor tenosynovitis (or trigger finger), which they termed “joystick digit.” Since that time, video game play has greatly evolved along with the types and nature of RSI cases reported in the medical literature. In 1990, Brasington10 described acute tendinopathy of the extensor pollicis longus tendon caused by excessive video game play, which was termed “Nintendinitis.” This term has since been used in reference to any video game–related RSI and reports have increased over time, likely due to the proliferation of an increasing array of video game systems.5,11-16 In recent years, a number of traumatic injuries including fractures, joint dislocations, head injuries, hemothorax, and lacerations have been attributed to interactive gaming systems.6,11,17-20 In rare cases, video game play also has been associated with enuresis,21 encopresis,22 and epilepsy.23
According to a 2011 report from the Entertainment Software Association, women over the age of 18 years now represent a greater proportion of the video game–playing population than boys aged 17 years and younger.24 This same report also noted that the average video game player is 35 years old; 44% of all players are female; and 27% of Americans over the age of 50 years play video games. This shifting demographic data, including the fact that 80% of American households reportedly play video games, reveals the expanding depth and breadth of the market.24 However, the pediatric population is still a high-volume player demographic. Average time per session peaks between 10 to 12 years of age and then falls through the teenage and adults years.24 Hence, the pediatric population is at high risk for clinical pathology because of the increased repetitive movements associated with video game play. Overall, cognizance of the popularity of video games and related pathologies can be an asset for dermatologists who evaluate pediatric patients.
1. Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Elsevier Health Sciences UK; 2007.
2. Robertson SJ, Leonard J, Chamberlain AJ. PlayStation purpura. Australas J Dermatol. 2010;51:220-222.
3. Kasraee B, Masouyé I, Piguet V. PlayStation palmar hidradenitis. Br J Dermatol. 2009;160:892-894.
4. Rushing ME, Sheehan DJ, Davis LS. Video game induced knuckle pad. Pediatr Dermatol. 2006;23:455-457.
5. Bakos RM, Bakos L. Use of dermoscopy to visualize punctate hemorrhages and onycholysis in “playstation thumb.” Arch Dermatol. 2006;142:1664-1665.
6. Wood DJ. The “How!” sign—a central palmar blister induced by overplaying on a Nintendo console. Arch Dis Child. 2001;84:288.
7. Koh TH. Ulcerative “nintendinitis”: a new kind of repetitive strain injury. Med J Aust. 2000;173:671.
8. Bernabeu-Wittel J, Domínguez-Cruz J, Zulueta T, et al. Hemorrhagic parallel-ridge pattern on dermoscopy in “Playstation fingertip.” J Am Acad Dermatol. 2011;65:238-239.
9. Osterman AL, Weinberg P, Miller G. Joystick digit. JAMA. 1987;257:782.
10. Brasington R. Nintendinitis. N Engl J Med. 1990;322:1473-1474.
11. Sparks DA, Coughlin LM, Chase DM. Did too much Wii cause your patient’s injury? J Fam Pract. 2011;60:404-409.
12. Bright DA, Bringhurst DC. Nintendo elbow. West J Med. 1992;156:667-668.
13. Vaidya HJ. Playstation thumb. Lancet. 2004;363:1080.
14. Bonis J. Acute Wiiitis. N Engl J Med. 2007;356:2431-2432.
15. Boehm KM, Pugh A. A new variant of Wiiitis [published online ahead of print June 13, 2008]. J Emerg Med. 2009;36:80.
16. Beddy P, Dunne R, de Blacam C. Achilles wiiitis. AJR Am J Roentgenol. 2009;192:W79.
17. Eley KA. A Wii fracture. N Engl J Med. 2010;362:473-474.
18. Wells JJ. An 8-year-old girl presented to the ER after accidentally being hit by a Wii remote control swung by her brother. J Trauma. 2008;65:1203.
19. Fysh T, Thompson JF. A Wii problem. J R Soc Med. 2009;102:502.
20. George AJ. Musculo-ske Wii tal medicine. Injury. 2012;43:390-391.
21. Schink JC. Nintendo enuresis. Am J Dis Child. 1991;145:1094.
22. Corkery JC. Nintendo power. Am J Dis Child. 1990;144:959.
23. Hart EJ. Nintendo epilepsy. N Engl J Med. 1990;322:1473.
24. Entertainment Software Association. 2015 sales, demographic, and usage data. essential facts about the computer and video game industry. Entertainment Software Association Web site. http://www.theesa.com/wp-content/uploads/2015/04/ESA-Essential-Facts-2015.pdf. Accessed October 16, 2015.
Case Report
A 19-year-old man was admitted to our hospital to begin treatment for acute myeloid leukemia that had been diagnosed 2 days prior. Three days after completing a 10-day regimen of induction chemotherapy, he developed bilateral, well-demarcated erythematous patches on the palmar surfaces of the proximal phalanges of the third, fourth, and fifth fingers (Figure 1) and 2 patches on the right palm. The patient was referred to dermatology for evaluation. He recalled no trauma to these sites although he reported pushing his intravenous pole with the right hand when walking. Of note, he had become neutropenic and thrombocytopenic following chemotherapy

On physical examination, the patches measured 1- to 1.5-cm in diameter and were mildly tender to palpation. The 2 patches on the right palm were much smaller than those on the fingers but were otherwise similar in appearance.
A punch biopsy of the erythematous lesion on the left third digit was performed. Histologic examination revealed extensive epidermal denudation associated with vascular proliferation and congestion as well as hemorrhage and a sparse lymphocytic infiltrate (Figures 2–4). There was no evidence of a leukemic infiltrate, and stains for fungal elements and bacteria were negative. Eccrine ducts appeared normal with no evidence of necrosis or metaplasia. These findings were suggestive of a frictional etiology.



Due to the distribution of the skin lesions on the hands, it was suspected that the source of friction was a video game controller. Although the patient denied playing video games since his admission to the hospital, he reported heavy video game use during the weeks prior to admission. We postulated that the thrombocytopenia the patient developed following chemotherapy along with prior friction injury sustained from heavy video game play led to traumatic subcorneal hemorrhage on the hands at the points of contact with the video game controller (Figure 5). The subcorneal hematomas resolved completely over the next 2 months during which the patient abstained from video game play.

This case demonstrates the importance of obtaining a detailed patient history, as our patient’s history of video game play prior to hospitalization proved to be of major diagnostic importance. Although the location, distribution, and well-demarcated nature of the patient’s lesions suggested an external source of trauma and biopsy definitively ruled out leukemia cutis, Sweet syndrome, and eccrine hidradenitis,1 the final diagnosis of traumatic subcorneal hematomas was only possible with specific knowledge of the patient’s video game controller use.
Comment
History of video game play has been key to the diagnosis of a variety of cutaneous lesions documented in the medical literature. Robertson et al2 attributed a similar case of traumatic subcorneal hematomas of the hands in an otherwise healthy 16-year-old boy to excessive use of a video game controller. Similarly, Kasraee et al3 attributed a case of idiopathic eccrine hidradenitis in an otherwise healthy 12-year-old girl to excessive video game use. In both of these reported cases, bilateral skin lesions on the palms of the hands appeared acutely in a pattern consistent with the points of contact of a video game controller. Excessive video game play has also been associated with unilateral dermatologic lesions on the hands, such as knuckle pads,4 onycholysis,5 friction blisters,6 pressure ulcers,7 and hemorrhagic lesions.5,6,8
Video game–related pathologies are not limited to the skin and have been implicated in a variety of clinical presentations. In 1987, Osterman et al9 published an early account of repetitive strain injury (RSI) related to video game use in which the investigators reported 2 cases of video game–related volar flexor tenosynovitis (or trigger finger), which they termed “joystick digit.” Since that time, video game play has greatly evolved along with the types and nature of RSI cases reported in the medical literature. In 1990, Brasington10 described acute tendinopathy of the extensor pollicis longus tendon caused by excessive video game play, which was termed “Nintendinitis.” This term has since been used in reference to any video game–related RSI and reports have increased over time, likely due to the proliferation of an increasing array of video game systems.5,11-16 In recent years, a number of traumatic injuries including fractures, joint dislocations, head injuries, hemothorax, and lacerations have been attributed to interactive gaming systems.6,11,17-20 In rare cases, video game play also has been associated with enuresis,21 encopresis,22 and epilepsy.23
According to a 2011 report from the Entertainment Software Association, women over the age of 18 years now represent a greater proportion of the video game–playing population than boys aged 17 years and younger.24 This same report also noted that the average video game player is 35 years old; 44% of all players are female; and 27% of Americans over the age of 50 years play video games. This shifting demographic data, including the fact that 80% of American households reportedly play video games, reveals the expanding depth and breadth of the market.24 However, the pediatric population is still a high-volume player demographic. Average time per session peaks between 10 to 12 years of age and then falls through the teenage and adults years.24 Hence, the pediatric population is at high risk for clinical pathology because of the increased repetitive movements associated with video game play. Overall, cognizance of the popularity of video games and related pathologies can be an asset for dermatologists who evaluate pediatric patients.
Case Report
A 19-year-old man was admitted to our hospital to begin treatment for acute myeloid leukemia that had been diagnosed 2 days prior. Three days after completing a 10-day regimen of induction chemotherapy, he developed bilateral, well-demarcated erythematous patches on the palmar surfaces of the proximal phalanges of the third, fourth, and fifth fingers (Figure 1) and 2 patches on the right palm. The patient was referred to dermatology for evaluation. He recalled no trauma to these sites although he reported pushing his intravenous pole with the right hand when walking. Of note, he had become neutropenic and thrombocytopenic following chemotherapy

On physical examination, the patches measured 1- to 1.5-cm in diameter and were mildly tender to palpation. The 2 patches on the right palm were much smaller than those on the fingers but were otherwise similar in appearance.
A punch biopsy of the erythematous lesion on the left third digit was performed. Histologic examination revealed extensive epidermal denudation associated with vascular proliferation and congestion as well as hemorrhage and a sparse lymphocytic infiltrate (Figures 2–4). There was no evidence of a leukemic infiltrate, and stains for fungal elements and bacteria were negative. Eccrine ducts appeared normal with no evidence of necrosis or metaplasia. These findings were suggestive of a frictional etiology.



Due to the distribution of the skin lesions on the hands, it was suspected that the source of friction was a video game controller. Although the patient denied playing video games since his admission to the hospital, he reported heavy video game use during the weeks prior to admission. We postulated that the thrombocytopenia the patient developed following chemotherapy along with prior friction injury sustained from heavy video game play led to traumatic subcorneal hemorrhage on the hands at the points of contact with the video game controller (Figure 5). The subcorneal hematomas resolved completely over the next 2 months during which the patient abstained from video game play.

This case demonstrates the importance of obtaining a detailed patient history, as our patient’s history of video game play prior to hospitalization proved to be of major diagnostic importance. Although the location, distribution, and well-demarcated nature of the patient’s lesions suggested an external source of trauma and biopsy definitively ruled out leukemia cutis, Sweet syndrome, and eccrine hidradenitis,1 the final diagnosis of traumatic subcorneal hematomas was only possible with specific knowledge of the patient’s video game controller use.
Comment
History of video game play has been key to the diagnosis of a variety of cutaneous lesions documented in the medical literature. Robertson et al2 attributed a similar case of traumatic subcorneal hematomas of the hands in an otherwise healthy 16-year-old boy to excessive use of a video game controller. Similarly, Kasraee et al3 attributed a case of idiopathic eccrine hidradenitis in an otherwise healthy 12-year-old girl to excessive video game use. In both of these reported cases, bilateral skin lesions on the palms of the hands appeared acutely in a pattern consistent with the points of contact of a video game controller. Excessive video game play has also been associated with unilateral dermatologic lesions on the hands, such as knuckle pads,4 onycholysis,5 friction blisters,6 pressure ulcers,7 and hemorrhagic lesions.5,6,8
Video game–related pathologies are not limited to the skin and have been implicated in a variety of clinical presentations. In 1987, Osterman et al9 published an early account of repetitive strain injury (RSI) related to video game use in which the investigators reported 2 cases of video game–related volar flexor tenosynovitis (or trigger finger), which they termed “joystick digit.” Since that time, video game play has greatly evolved along with the types and nature of RSI cases reported in the medical literature. In 1990, Brasington10 described acute tendinopathy of the extensor pollicis longus tendon caused by excessive video game play, which was termed “Nintendinitis.” This term has since been used in reference to any video game–related RSI and reports have increased over time, likely due to the proliferation of an increasing array of video game systems.5,11-16 In recent years, a number of traumatic injuries including fractures, joint dislocations, head injuries, hemothorax, and lacerations have been attributed to interactive gaming systems.6,11,17-20 In rare cases, video game play also has been associated with enuresis,21 encopresis,22 and epilepsy.23
According to a 2011 report from the Entertainment Software Association, women over the age of 18 years now represent a greater proportion of the video game–playing population than boys aged 17 years and younger.24 This same report also noted that the average video game player is 35 years old; 44% of all players are female; and 27% of Americans over the age of 50 years play video games. This shifting demographic data, including the fact that 80% of American households reportedly play video games, reveals the expanding depth and breadth of the market.24 However, the pediatric population is still a high-volume player demographic. Average time per session peaks between 10 to 12 years of age and then falls through the teenage and adults years.24 Hence, the pediatric population is at high risk for clinical pathology because of the increased repetitive movements associated with video game play. Overall, cognizance of the popularity of video games and related pathologies can be an asset for dermatologists who evaluate pediatric patients.
1. Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Elsevier Health Sciences UK; 2007.
2. Robertson SJ, Leonard J, Chamberlain AJ. PlayStation purpura. Australas J Dermatol. 2010;51:220-222.
3. Kasraee B, Masouyé I, Piguet V. PlayStation palmar hidradenitis. Br J Dermatol. 2009;160:892-894.
4. Rushing ME, Sheehan DJ, Davis LS. Video game induced knuckle pad. Pediatr Dermatol. 2006;23:455-457.
5. Bakos RM, Bakos L. Use of dermoscopy to visualize punctate hemorrhages and onycholysis in “playstation thumb.” Arch Dermatol. 2006;142:1664-1665.
6. Wood DJ. The “How!” sign—a central palmar blister induced by overplaying on a Nintendo console. Arch Dis Child. 2001;84:288.
7. Koh TH. Ulcerative “nintendinitis”: a new kind of repetitive strain injury. Med J Aust. 2000;173:671.
8. Bernabeu-Wittel J, Domínguez-Cruz J, Zulueta T, et al. Hemorrhagic parallel-ridge pattern on dermoscopy in “Playstation fingertip.” J Am Acad Dermatol. 2011;65:238-239.
9. Osterman AL, Weinberg P, Miller G. Joystick digit. JAMA. 1987;257:782.
10. Brasington R. Nintendinitis. N Engl J Med. 1990;322:1473-1474.
11. Sparks DA, Coughlin LM, Chase DM. Did too much Wii cause your patient’s injury? J Fam Pract. 2011;60:404-409.
12. Bright DA, Bringhurst DC. Nintendo elbow. West J Med. 1992;156:667-668.
13. Vaidya HJ. Playstation thumb. Lancet. 2004;363:1080.
14. Bonis J. Acute Wiiitis. N Engl J Med. 2007;356:2431-2432.
15. Boehm KM, Pugh A. A new variant of Wiiitis [published online ahead of print June 13, 2008]. J Emerg Med. 2009;36:80.
16. Beddy P, Dunne R, de Blacam C. Achilles wiiitis. AJR Am J Roentgenol. 2009;192:W79.
17. Eley KA. A Wii fracture. N Engl J Med. 2010;362:473-474.
18. Wells JJ. An 8-year-old girl presented to the ER after accidentally being hit by a Wii remote control swung by her brother. J Trauma. 2008;65:1203.
19. Fysh T, Thompson JF. A Wii problem. J R Soc Med. 2009;102:502.
20. George AJ. Musculo-ske Wii tal medicine. Injury. 2012;43:390-391.
21. Schink JC. Nintendo enuresis. Am J Dis Child. 1991;145:1094.
22. Corkery JC. Nintendo power. Am J Dis Child. 1990;144:959.
23. Hart EJ. Nintendo epilepsy. N Engl J Med. 1990;322:1473.
24. Entertainment Software Association. 2015 sales, demographic, and usage data. essential facts about the computer and video game industry. Entertainment Software Association Web site. http://www.theesa.com/wp-content/uploads/2015/04/ESA-Essential-Facts-2015.pdf. Accessed October 16, 2015.
1. Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Elsevier Health Sciences UK; 2007.
2. Robertson SJ, Leonard J, Chamberlain AJ. PlayStation purpura. Australas J Dermatol. 2010;51:220-222.
3. Kasraee B, Masouyé I, Piguet V. PlayStation palmar hidradenitis. Br J Dermatol. 2009;160:892-894.
4. Rushing ME, Sheehan DJ, Davis LS. Video game induced knuckle pad. Pediatr Dermatol. 2006;23:455-457.
5. Bakos RM, Bakos L. Use of dermoscopy to visualize punctate hemorrhages and onycholysis in “playstation thumb.” Arch Dermatol. 2006;142:1664-1665.
6. Wood DJ. The “How!” sign—a central palmar blister induced by overplaying on a Nintendo console. Arch Dis Child. 2001;84:288.
7. Koh TH. Ulcerative “nintendinitis”: a new kind of repetitive strain injury. Med J Aust. 2000;173:671.
8. Bernabeu-Wittel J, Domínguez-Cruz J, Zulueta T, et al. Hemorrhagic parallel-ridge pattern on dermoscopy in “Playstation fingertip.” J Am Acad Dermatol. 2011;65:238-239.
9. Osterman AL, Weinberg P, Miller G. Joystick digit. JAMA. 1987;257:782.
10. Brasington R. Nintendinitis. N Engl J Med. 1990;322:1473-1474.
11. Sparks DA, Coughlin LM, Chase DM. Did too much Wii cause your patient’s injury? J Fam Pract. 2011;60:404-409.
12. Bright DA, Bringhurst DC. Nintendo elbow. West J Med. 1992;156:667-668.
13. Vaidya HJ. Playstation thumb. Lancet. 2004;363:1080.
14. Bonis J. Acute Wiiitis. N Engl J Med. 2007;356:2431-2432.
15. Boehm KM, Pugh A. A new variant of Wiiitis [published online ahead of print June 13, 2008]. J Emerg Med. 2009;36:80.
16. Beddy P, Dunne R, de Blacam C. Achilles wiiitis. AJR Am J Roentgenol. 2009;192:W79.
17. Eley KA. A Wii fracture. N Engl J Med. 2010;362:473-474.
18. Wells JJ. An 8-year-old girl presented to the ER after accidentally being hit by a Wii remote control swung by her brother. J Trauma. 2008;65:1203.
19. Fysh T, Thompson JF. A Wii problem. J R Soc Med. 2009;102:502.
20. George AJ. Musculo-ske Wii tal medicine. Injury. 2012;43:390-391.
21. Schink JC. Nintendo enuresis. Am J Dis Child. 1991;145:1094.
22. Corkery JC. Nintendo power. Am J Dis Child. 1990;144:959.
23. Hart EJ. Nintendo epilepsy. N Engl J Med. 1990;322:1473.
24. Entertainment Software Association. 2015 sales, demographic, and usage data. essential facts about the computer and video game industry. Entertainment Software Association Web site. http://www.theesa.com/wp-content/uploads/2015/04/ESA-Essential-Facts-2015.pdf. Accessed October 16, 2015.
Practice Points
- Video game play has been reported as an etiologic factor in multiple musculoskeletal and dermatologic conditions.
- More than two-thirds of US children aged 2 to 18 years live in a home with a video game system.
- Cognizance of the popularity of video games and related pathologies can be an asset for dermatologists who evaluate pediatric patients.
Secondary Syphilis
Syphilis often is referred to as the “great imitator” due to the protean presentations of secondary-stage disease, the most common of which are skin manifestations.1 Secondary syphilis typically begins 3 to 10 weeks after initial exposure due to systemic dissemination of Treponema pallidum, and although presentations can vary widely, the classic presentation includes nonspecific generalized symptoms (eg, fever, malaise, lymphadenopathy), variable skin findings (eg, nonpruritic papulosquamous eruption), and mucosal ulcerations or plaques.1 Early and accurate diagnosis of syphilis is critical to avoid the morbidity associated with advanced disease.
The classic histopathologic appearance of secondary syphilis is characterized by psoriasiform epidermal changes; a dermal inflammatory infiltrate of lymphocytes, histiocytes, and plasma cells in a lichenoid and/or superficial and deep perivascular distribution (Figure 1); and endothelial swelling of dermal blood vessels.1 The presence of plasma cells in the infiltrate (Figure 2) is particularly useful for differentiating secondary syphilis from other clinicopathological mimickers, but this finding is not always present. Silver-based histochemical stains (eg, Warthin-Starry silver stain) can be used to high-light T pallidum organisms; however, histochemical staining is plagued by low diagnostic sensitivity for identifying the causative organism, making immunohistochemical and/or serologic testing the preferred method for confirming the diagnosis.1

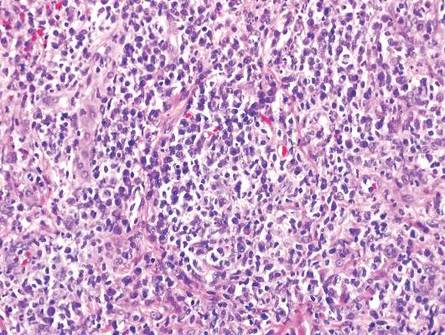
Arthropod assault is characterized by a superficial and deep perivascular lymphocytic inflammatory infiltrate with a variable number of polymorphonuclear cells.2 Overlying spongiosis or focal epidermal necrosis and increased eosinophils are typical of arthropod assault (Figure 3).2 The infiltrate seen following insect bites is classically described as wedge-shaped, although recent literature has disputed the sensitivity of this finding, identifying adnexal structure involvement as an alternative sensitive marker for identifying insect bites.2
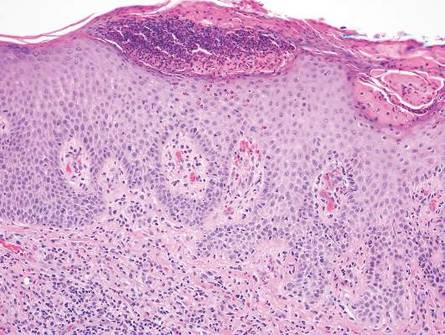
Chronic cutaneous lupus erythematosus demonstrates a spectrum of histopathologic changes depending on the age of the lesion biopsied; however, characteristic histopathologic features typically include variable epidermal atrophy or acanthosis with basal layer vacuolar degeneration, basement membrane thickening, follicular plugging, superficial and deep perivascular and periappendageal lymphocytic inflammation, and dermal mucin deposition (Figure 4).4
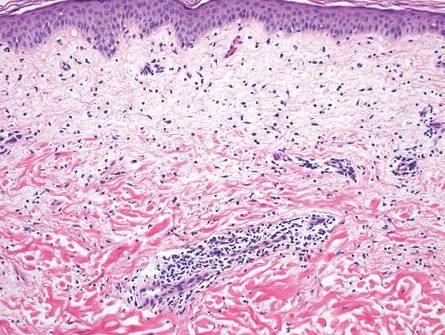
Fixed drug eruption histopathologically presents as an interface tissue reaction–associated single-cell necrosis to broader areas of epidermal necrosis, as well as superficial to mid-dermal lymphocytic infiltrate. Unlike secondary syphilis, a fixed drug eruption is characterized by prominent melanin pigment incontinence and eosinophils (Figure 5).5

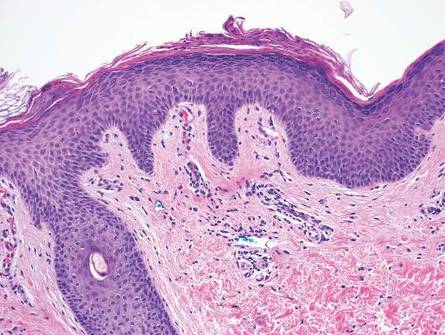
Similar to secondary syphilis, pityriasis lichenoides et varioliformis acuta (PLEVA) demonstrates variable psoriasiform epidermal hyperplasia with a lichenoid and perivascular lymphocytic infiltrate. Other findings in PLEVA include parakeratosis, variable epidermal necrosis, and prominent exocytosis of lymphocytes. Unlike typical secondary syphilis, PLEVA often is associated with lymphocytic vasculitis, consisting of the invasion of vessel walls by lymphocytes with extravasation of erythrocytes and an absence of conspicuous plasma cells (Figure 6).6
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;3:595-599.
- Miteva M, Elsner P, Ziemer M. A histopathologic study of arthropod bite reactions in 20 patients highlights relevant adnexal involvement. J Cutan Pathol. 2009;36:26-33.
- Winkelmann RK, Reizner GT. Diffuse dermal neutrophilia in urticarial. Human Pathol. 1988;19:389-393.
- Sepehr A, Wenson S, Tahan SR. Histopathologic manifestations of systemic diseases: the example of cutaneous lupus erythematosus. J Cutan Pathol. 2010;37 (suppl 1):112-124.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727.
- Fernandes NF, Rozdeba PJ, Schwartz RA, et al. Pityriasis lichenoides et varioliformis acuta: a disease spectrum. Int J Dermatol. 2010;49:257-261.
Syphilis often is referred to as the “great imitator” due to the protean presentations of secondary-stage disease, the most common of which are skin manifestations.1 Secondary syphilis typically begins 3 to 10 weeks after initial exposure due to systemic dissemination of Treponema pallidum, and although presentations can vary widely, the classic presentation includes nonspecific generalized symptoms (eg, fever, malaise, lymphadenopathy), variable skin findings (eg, nonpruritic papulosquamous eruption), and mucosal ulcerations or plaques.1 Early and accurate diagnosis of syphilis is critical to avoid the morbidity associated with advanced disease.
The classic histopathologic appearance of secondary syphilis is characterized by psoriasiform epidermal changes; a dermal inflammatory infiltrate of lymphocytes, histiocytes, and plasma cells in a lichenoid and/or superficial and deep perivascular distribution (Figure 1); and endothelial swelling of dermal blood vessels.1 The presence of plasma cells in the infiltrate (Figure 2) is particularly useful for differentiating secondary syphilis from other clinicopathological mimickers, but this finding is not always present. Silver-based histochemical stains (eg, Warthin-Starry silver stain) can be used to high-light T pallidum organisms; however, histochemical staining is plagued by low diagnostic sensitivity for identifying the causative organism, making immunohistochemical and/or serologic testing the preferred method for confirming the diagnosis.1


Arthropod assault is characterized by a superficial and deep perivascular lymphocytic inflammatory infiltrate with a variable number of polymorphonuclear cells.2 Overlying spongiosis or focal epidermal necrosis and increased eosinophils are typical of arthropod assault (Figure 3).2 The infiltrate seen following insect bites is classically described as wedge-shaped, although recent literature has disputed the sensitivity of this finding, identifying adnexal structure involvement as an alternative sensitive marker for identifying insect bites.2

Chronic cutaneous lupus erythematosus demonstrates a spectrum of histopathologic changes depending on the age of the lesion biopsied; however, characteristic histopathologic features typically include variable epidermal atrophy or acanthosis with basal layer vacuolar degeneration, basement membrane thickening, follicular plugging, superficial and deep perivascular and periappendageal lymphocytic inflammation, and dermal mucin deposition (Figure 4).4

Fixed drug eruption histopathologically presents as an interface tissue reaction–associated single-cell necrosis to broader areas of epidermal necrosis, as well as superficial to mid-dermal lymphocytic infiltrate. Unlike secondary syphilis, a fixed drug eruption is characterized by prominent melanin pigment incontinence and eosinophils (Figure 5).5

Similar to secondary syphilis, pityriasis lichenoides et varioliformis acuta (PLEVA) demonstrates variable psoriasiform epidermal hyperplasia with a lichenoid and perivascular lymphocytic infiltrate. Other findings in PLEVA include parakeratosis, variable epidermal necrosis, and prominent exocytosis of lymphocytes. Unlike typical secondary syphilis, PLEVA often is associated with lymphocytic vasculitis, consisting of the invasion of vessel walls by lymphocytes with extravasation of erythrocytes and an absence of conspicuous plasma cells (Figure 6).6

Syphilis often is referred to as the “great imitator” due to the protean presentations of secondary-stage disease, the most common of which are skin manifestations.1 Secondary syphilis typically begins 3 to 10 weeks after initial exposure due to systemic dissemination of Treponema pallidum, and although presentations can vary widely, the classic presentation includes nonspecific generalized symptoms (eg, fever, malaise, lymphadenopathy), variable skin findings (eg, nonpruritic papulosquamous eruption), and mucosal ulcerations or plaques.1 Early and accurate diagnosis of syphilis is critical to avoid the morbidity associated with advanced disease.
The classic histopathologic appearance of secondary syphilis is characterized by psoriasiform epidermal changes; a dermal inflammatory infiltrate of lymphocytes, histiocytes, and plasma cells in a lichenoid and/or superficial and deep perivascular distribution (Figure 1); and endothelial swelling of dermal blood vessels.1 The presence of plasma cells in the infiltrate (Figure 2) is particularly useful for differentiating secondary syphilis from other clinicopathological mimickers, but this finding is not always present. Silver-based histochemical stains (eg, Warthin-Starry silver stain) can be used to high-light T pallidum organisms; however, histochemical staining is plagued by low diagnostic sensitivity for identifying the causative organism, making immunohistochemical and/or serologic testing the preferred method for confirming the diagnosis.1


Arthropod assault is characterized by a superficial and deep perivascular lymphocytic inflammatory infiltrate with a variable number of polymorphonuclear cells.2 Overlying spongiosis or focal epidermal necrosis and increased eosinophils are typical of arthropod assault (Figure 3).2 The infiltrate seen following insect bites is classically described as wedge-shaped, although recent literature has disputed the sensitivity of this finding, identifying adnexal structure involvement as an alternative sensitive marker for identifying insect bites.2

Chronic cutaneous lupus erythematosus demonstrates a spectrum of histopathologic changes depending on the age of the lesion biopsied; however, characteristic histopathologic features typically include variable epidermal atrophy or acanthosis with basal layer vacuolar degeneration, basement membrane thickening, follicular plugging, superficial and deep perivascular and periappendageal lymphocytic inflammation, and dermal mucin deposition (Figure 4).4

Fixed drug eruption histopathologically presents as an interface tissue reaction–associated single-cell necrosis to broader areas of epidermal necrosis, as well as superficial to mid-dermal lymphocytic infiltrate. Unlike secondary syphilis, a fixed drug eruption is characterized by prominent melanin pigment incontinence and eosinophils (Figure 5).5

Similar to secondary syphilis, pityriasis lichenoides et varioliformis acuta (PLEVA) demonstrates variable psoriasiform epidermal hyperplasia with a lichenoid and perivascular lymphocytic infiltrate. Other findings in PLEVA include parakeratosis, variable epidermal necrosis, and prominent exocytosis of lymphocytes. Unlike typical secondary syphilis, PLEVA often is associated with lymphocytic vasculitis, consisting of the invasion of vessel walls by lymphocytes with extravasation of erythrocytes and an absence of conspicuous plasma cells (Figure 6).6

- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;3:595-599.
- Miteva M, Elsner P, Ziemer M. A histopathologic study of arthropod bite reactions in 20 patients highlights relevant adnexal involvement. J Cutan Pathol. 2009;36:26-33.
- Winkelmann RK, Reizner GT. Diffuse dermal neutrophilia in urticarial. Human Pathol. 1988;19:389-393.
- Sepehr A, Wenson S, Tahan SR. Histopathologic manifestations of systemic diseases: the example of cutaneous lupus erythematosus. J Cutan Pathol. 2010;37 (suppl 1):112-124.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727.
- Fernandes NF, Rozdeba PJ, Schwartz RA, et al. Pityriasis lichenoides et varioliformis acuta: a disease spectrum. Int J Dermatol. 2010;49:257-261.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;3:595-599.
- Miteva M, Elsner P, Ziemer M. A histopathologic study of arthropod bite reactions in 20 patients highlights relevant adnexal involvement. J Cutan Pathol. 2009;36:26-33.
- Winkelmann RK, Reizner GT. Diffuse dermal neutrophilia in urticarial. Human Pathol. 1988;19:389-393.
- Sepehr A, Wenson S, Tahan SR. Histopathologic manifestations of systemic diseases: the example of cutaneous lupus erythematosus. J Cutan Pathol. 2010;37 (suppl 1):112-124.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727.
- Fernandes NF, Rozdeba PJ, Schwartz RA, et al. Pityriasis lichenoides et varioliformis acuta: a disease spectrum. Int J Dermatol. 2010;49:257-261.
Positive music produces more negative emotions in bipolar
Patients with bipolar disorder might experience more complex negative emotions in response to positive music than typical adults, even when in a euthymic state, Dr. Sabine Choppin of the University of Rennes 1 (France) and colleagues reported.
Researchers recruited 21 patients with bipolar disorder in a euthymic phase and 21 matched healthy controls for the study. First, participants rated their emotional reactivity on two self-report scales: the Emotion Reactivity Scale (ERS) and the Multidimensional Assessment of Thymic States Scale (MAThyS). Next, they used headphones to listen to a series of 12 instrumental music excerpts lasting 45 seconds each with their eyes closed. After each musical selection, they were asked to rate how strongly they had experienced each of the nine emotional categories on the Geneva Emotional Music Scale: joy, sadness, tension, wonder, peacefulness, power, tenderness, nostalgia, and transcendence.
Statistical analyses showed that patients in the bipolar disorder group had a mean score of 41.2 on the ERS, compared with a mean score of 22.9 among healthy controls. In addition, bipolar disorder patients reported experiencing more tension and sadness than did healthy controls when listening to positive musical excerpts that had been classified as inducing joy and wonder.
“This finding tallies with the negative emotional bias displayed by depressed patients, who tend to experience more negative emotions than healthy controls,” the authors wrote. “Bipolar patients struggle so much to regulate their own positive emotions that it creates a chronic source of distress, which could be experienced as a negative emotion.”
Read the article in the Journal of Affective Disorders (2016 Feb;191:15-23. doi: 10.1016/j.jad.2015.10.063).
Patients with bipolar disorder might experience more complex negative emotions in response to positive music than typical adults, even when in a euthymic state, Dr. Sabine Choppin of the University of Rennes 1 (France) and colleagues reported.
Researchers recruited 21 patients with bipolar disorder in a euthymic phase and 21 matched healthy controls for the study. First, participants rated their emotional reactivity on two self-report scales: the Emotion Reactivity Scale (ERS) and the Multidimensional Assessment of Thymic States Scale (MAThyS). Next, they used headphones to listen to a series of 12 instrumental music excerpts lasting 45 seconds each with their eyes closed. After each musical selection, they were asked to rate how strongly they had experienced each of the nine emotional categories on the Geneva Emotional Music Scale: joy, sadness, tension, wonder, peacefulness, power, tenderness, nostalgia, and transcendence.
Statistical analyses showed that patients in the bipolar disorder group had a mean score of 41.2 on the ERS, compared with a mean score of 22.9 among healthy controls. In addition, bipolar disorder patients reported experiencing more tension and sadness than did healthy controls when listening to positive musical excerpts that had been classified as inducing joy and wonder.
“This finding tallies with the negative emotional bias displayed by depressed patients, who tend to experience more negative emotions than healthy controls,” the authors wrote. “Bipolar patients struggle so much to regulate their own positive emotions that it creates a chronic source of distress, which could be experienced as a negative emotion.”
Read the article in the Journal of Affective Disorders (2016 Feb;191:15-23. doi: 10.1016/j.jad.2015.10.063).
Patients with bipolar disorder might experience more complex negative emotions in response to positive music than typical adults, even when in a euthymic state, Dr. Sabine Choppin of the University of Rennes 1 (France) and colleagues reported.
Researchers recruited 21 patients with bipolar disorder in a euthymic phase and 21 matched healthy controls for the study. First, participants rated their emotional reactivity on two self-report scales: the Emotion Reactivity Scale (ERS) and the Multidimensional Assessment of Thymic States Scale (MAThyS). Next, they used headphones to listen to a series of 12 instrumental music excerpts lasting 45 seconds each with their eyes closed. After each musical selection, they were asked to rate how strongly they had experienced each of the nine emotional categories on the Geneva Emotional Music Scale: joy, sadness, tension, wonder, peacefulness, power, tenderness, nostalgia, and transcendence.
Statistical analyses showed that patients in the bipolar disorder group had a mean score of 41.2 on the ERS, compared with a mean score of 22.9 among healthy controls. In addition, bipolar disorder patients reported experiencing more tension and sadness than did healthy controls when listening to positive musical excerpts that had been classified as inducing joy and wonder.
“This finding tallies with the negative emotional bias displayed by depressed patients, who tend to experience more negative emotions than healthy controls,” the authors wrote. “Bipolar patients struggle so much to regulate their own positive emotions that it creates a chronic source of distress, which could be experienced as a negative emotion.”
Read the article in the Journal of Affective Disorders (2016 Feb;191:15-23. doi: 10.1016/j.jad.2015.10.063).
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Dr. Hospitalist: HM Groups Must Adapt to New Career Landscape
Dear Dr. Hospitalist:
Over the past several years, we have had a problem with physician retention, especially with nocturnists, in our medium-sized hospitalist group. Do you have any suggestions (beyond the obvious “more money”) to help us retain our hospitalists?
Missing My Friends in the Midwest
Dr. Hospitalist responds:
Since its inception, hospital medicine has been a very attractive field for practicing medicine, and although growth was phenomenal for many years (especially 2000–2010), it has leveled off over the past five years. With this exceptional growth have come increased salaries, geographically diverse job locations, and more opportunities for career development.
One of the most significant changes over the past 10 years is that hospital medicine is no longer seen as a bridge from residency to fellowship or as a stopover while waiting on the ideal job. Physicians now see hospital medicine as a career choice and are more likely to search for the “ideal” hospitalist job.
Although competitive salaries are important and a necessary starting point, to attract and keep career hospitalists, HM groups (HMGs) will need to offer opportunities for professional growth and leadership as well as flexible schedules.
Many larger HMGs offer several different schedule models, from the ubiquitous seven-on/seven-off schedule (54%, according to the 2014 State of Hospital Medicine report) to the more traditional five-day workweek with vacation time. Many also choose to work part- or full-time as a nocturnist and, in doing so, earn substantially more money (15%–20% differential). The flexible schedule and the ability to work part- or full-time have been very attractive to those clinicians just starting families or attaining another degree (MBAs are becoming very popular).
While there have always been the “check-in, check-out” docs who did their seven and didn’t want to be bothered during their time off, there were typically enough gunners around to pick up the slack. With the Millennial generation’s pervasive aim for work-life balance, it might become more difficult to find even a few who are willing to go the extra mile in hopes of career advancement. Mix in a very robust job market with a proclivity to travel, and you have a recipe for high attrition.
Like any new profession or specialty, HM will have to evolve and adjust to keep these new docs anchored. We will need to consider offering vacation time, especially for those who are willing to work a traditional Monday–Friday schedule. For those in academia with an interest in promotion, there should be real opportunities for advancement instead of the traditional “time in rank” and other nebulous requirements. There should be robust mentoring for all docs and especially for those just out of residency. The clinicians who express an interest in having an office in the C-Suite should be given a clear path and guidance.
I think with some innovation and recognition, most HMGs will have little problem retaining high-quality physicians. We must also recognize a changing value system and accept that some people will change jobs just because! TH
Dear Dr. Hospitalist:
Over the past several years, we have had a problem with physician retention, especially with nocturnists, in our medium-sized hospitalist group. Do you have any suggestions (beyond the obvious “more money”) to help us retain our hospitalists?
Missing My Friends in the Midwest
Dr. Hospitalist responds:
Since its inception, hospital medicine has been a very attractive field for practicing medicine, and although growth was phenomenal for many years (especially 2000–2010), it has leveled off over the past five years. With this exceptional growth have come increased salaries, geographically diverse job locations, and more opportunities for career development.
One of the most significant changes over the past 10 years is that hospital medicine is no longer seen as a bridge from residency to fellowship or as a stopover while waiting on the ideal job. Physicians now see hospital medicine as a career choice and are more likely to search for the “ideal” hospitalist job.
Although competitive salaries are important and a necessary starting point, to attract and keep career hospitalists, HM groups (HMGs) will need to offer opportunities for professional growth and leadership as well as flexible schedules.
Many larger HMGs offer several different schedule models, from the ubiquitous seven-on/seven-off schedule (54%, according to the 2014 State of Hospital Medicine report) to the more traditional five-day workweek with vacation time. Many also choose to work part- or full-time as a nocturnist and, in doing so, earn substantially more money (15%–20% differential). The flexible schedule and the ability to work part- or full-time have been very attractive to those clinicians just starting families or attaining another degree (MBAs are becoming very popular).
While there have always been the “check-in, check-out” docs who did their seven and didn’t want to be bothered during their time off, there were typically enough gunners around to pick up the slack. With the Millennial generation’s pervasive aim for work-life balance, it might become more difficult to find even a few who are willing to go the extra mile in hopes of career advancement. Mix in a very robust job market with a proclivity to travel, and you have a recipe for high attrition.
Like any new profession or specialty, HM will have to evolve and adjust to keep these new docs anchored. We will need to consider offering vacation time, especially for those who are willing to work a traditional Monday–Friday schedule. For those in academia with an interest in promotion, there should be real opportunities for advancement instead of the traditional “time in rank” and other nebulous requirements. There should be robust mentoring for all docs and especially for those just out of residency. The clinicians who express an interest in having an office in the C-Suite should be given a clear path and guidance.
I think with some innovation and recognition, most HMGs will have little problem retaining high-quality physicians. We must also recognize a changing value system and accept that some people will change jobs just because! TH
Dear Dr. Hospitalist:
Over the past several years, we have had a problem with physician retention, especially with nocturnists, in our medium-sized hospitalist group. Do you have any suggestions (beyond the obvious “more money”) to help us retain our hospitalists?
Missing My Friends in the Midwest
Dr. Hospitalist responds:
Since its inception, hospital medicine has been a very attractive field for practicing medicine, and although growth was phenomenal for many years (especially 2000–2010), it has leveled off over the past five years. With this exceptional growth have come increased salaries, geographically diverse job locations, and more opportunities for career development.
One of the most significant changes over the past 10 years is that hospital medicine is no longer seen as a bridge from residency to fellowship or as a stopover while waiting on the ideal job. Physicians now see hospital medicine as a career choice and are more likely to search for the “ideal” hospitalist job.
Although competitive salaries are important and a necessary starting point, to attract and keep career hospitalists, HM groups (HMGs) will need to offer opportunities for professional growth and leadership as well as flexible schedules.
Many larger HMGs offer several different schedule models, from the ubiquitous seven-on/seven-off schedule (54%, according to the 2014 State of Hospital Medicine report) to the more traditional five-day workweek with vacation time. Many also choose to work part- or full-time as a nocturnist and, in doing so, earn substantially more money (15%–20% differential). The flexible schedule and the ability to work part- or full-time have been very attractive to those clinicians just starting families or attaining another degree (MBAs are becoming very popular).
While there have always been the “check-in, check-out” docs who did their seven and didn’t want to be bothered during their time off, there were typically enough gunners around to pick up the slack. With the Millennial generation’s pervasive aim for work-life balance, it might become more difficult to find even a few who are willing to go the extra mile in hopes of career advancement. Mix in a very robust job market with a proclivity to travel, and you have a recipe for high attrition.
Like any new profession or specialty, HM will have to evolve and adjust to keep these new docs anchored. We will need to consider offering vacation time, especially for those who are willing to work a traditional Monday–Friday schedule. For those in academia with an interest in promotion, there should be real opportunities for advancement instead of the traditional “time in rank” and other nebulous requirements. There should be robust mentoring for all docs and especially for those just out of residency. The clinicians who express an interest in having an office in the C-Suite should be given a clear path and guidance.
I think with some innovation and recognition, most HMGs will have little problem retaining high-quality physicians. We must also recognize a changing value system and accept that some people will change jobs just because! TH
Displaying Prices to Providers May Reduce Overall Ordering Costs
Clinical question: Does price display impact order costs and volume as well as patient safety outcomes, and is it acceptable to providers?
Background: Up to one-third of national healthcare expenditures are wasteful, with physicians playing a central role in overall cost, purchasing almost all tests and therapies for patients. Increasing the transparency of costs for physicians is one strategy to reduce unnecessary spending.
Study design: Systematic review.
Setting: Yale School of Medicine, New Haven, Conn.
Synopsis: Nineteen publications were selected for final analysis. Thirteen studies reported the impact of price display on costs, nine of which showed a statistically significant decrease in order costs. Only three of eight studies reporting the impact of price display on order volume showed statistically significant decreases in order volume. One study showed adverse safety findings in the form of higher rates of unscheduled follow-up care in a pediatric ED. Physicians were overall satisfied with price display in the five studies reporting this.
There was high heterogeneity among studies, which did not allow for pooling of data. Furthermore, more than half of the studies were conducted more than 15 years ago, limiting their generalizability to the modern era of electronic health records (EHRs).
Overall, this review supports the conclusion that price display has a modest effect on order costs. Additional studies utilizing EHR systems are required to more definitively confirm these findings.
Bottom line: Displaying prices to physicians can have a modest effect on overall order costs.
Citation: Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. doi:10.1002/jhm.2500.
Clinical question: Does price display impact order costs and volume as well as patient safety outcomes, and is it acceptable to providers?
Background: Up to one-third of national healthcare expenditures are wasteful, with physicians playing a central role in overall cost, purchasing almost all tests and therapies for patients. Increasing the transparency of costs for physicians is one strategy to reduce unnecessary spending.
Study design: Systematic review.
Setting: Yale School of Medicine, New Haven, Conn.
Synopsis: Nineteen publications were selected for final analysis. Thirteen studies reported the impact of price display on costs, nine of which showed a statistically significant decrease in order costs. Only three of eight studies reporting the impact of price display on order volume showed statistically significant decreases in order volume. One study showed adverse safety findings in the form of higher rates of unscheduled follow-up care in a pediatric ED. Physicians were overall satisfied with price display in the five studies reporting this.
There was high heterogeneity among studies, which did not allow for pooling of data. Furthermore, more than half of the studies were conducted more than 15 years ago, limiting their generalizability to the modern era of electronic health records (EHRs).
Overall, this review supports the conclusion that price display has a modest effect on order costs. Additional studies utilizing EHR systems are required to more definitively confirm these findings.
Bottom line: Displaying prices to physicians can have a modest effect on overall order costs.
Citation: Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. doi:10.1002/jhm.2500.
Clinical question: Does price display impact order costs and volume as well as patient safety outcomes, and is it acceptable to providers?
Background: Up to one-third of national healthcare expenditures are wasteful, with physicians playing a central role in overall cost, purchasing almost all tests and therapies for patients. Increasing the transparency of costs for physicians is one strategy to reduce unnecessary spending.
Study design: Systematic review.
Setting: Yale School of Medicine, New Haven, Conn.
Synopsis: Nineteen publications were selected for final analysis. Thirteen studies reported the impact of price display on costs, nine of which showed a statistically significant decrease in order costs. Only three of eight studies reporting the impact of price display on order volume showed statistically significant decreases in order volume. One study showed adverse safety findings in the form of higher rates of unscheduled follow-up care in a pediatric ED. Physicians were overall satisfied with price display in the five studies reporting this.
There was high heterogeneity among studies, which did not allow for pooling of data. Furthermore, more than half of the studies were conducted more than 15 years ago, limiting their generalizability to the modern era of electronic health records (EHRs).
Overall, this review supports the conclusion that price display has a modest effect on order costs. Additional studies utilizing EHR systems are required to more definitively confirm these findings.
Bottom line: Displaying prices to physicians can have a modest effect on overall order costs.
Citation: Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. doi:10.1002/jhm.2500.
Genes may be targets for AML therapy

Two genes are critical to the development of acute myeloid leukemia (AML), according to research published in Cancer Cell.
Previous research suggested the genes, KDM4C and PRMT1, are key players in transcription regulation during both normal and disease development.
The new study showed that, during AML development, KDM4C and PRMT1 are recruited to enable the transformation of blood cells into cancer cells.
The genes work in tandem, and, if either is not fully active, AML does not develop.
The researchers made these discoveries by inhibiting KDM4C and PRMT1—either genetically or pharmacologically—in mice with AML.
When either gene was silenced via genetic means, the majority of the mice were still alive at the end of the researchers’ 60-day experiment. However, the majority of control mice died in less than 40 days.
The team observed similarly favorable results when they inhibited either gene with drugs—the PRMT1 inhibitor AMI-408 and the KDM4C inhibitor SD70.
The median disease latency was 48 days in mice that received AMI-408 and 36 days in control mice. The median disease latency was 62 days in mice that received SD70 and 55 days in control mice.
“The demonstration of how critical these genes are to cancer transformation and treatment could be highly significant for the design of new drugs,” said study author Eric So, PhD, of King’s College London in the UK.
“Further work is needed to develop and refine drugs to maximize their effects and so that they are suitable for patients. Clinical trials will then be needed to see how leukemia patients respond to these drugs and how use of them can be optimized.” ![]()

Two genes are critical to the development of acute myeloid leukemia (AML), according to research published in Cancer Cell.
Previous research suggested the genes, KDM4C and PRMT1, are key players in transcription regulation during both normal and disease development.
The new study showed that, during AML development, KDM4C and PRMT1 are recruited to enable the transformation of blood cells into cancer cells.
The genes work in tandem, and, if either is not fully active, AML does not develop.
The researchers made these discoveries by inhibiting KDM4C and PRMT1—either genetically or pharmacologically—in mice with AML.
When either gene was silenced via genetic means, the majority of the mice were still alive at the end of the researchers’ 60-day experiment. However, the majority of control mice died in less than 40 days.
The team observed similarly favorable results when they inhibited either gene with drugs—the PRMT1 inhibitor AMI-408 and the KDM4C inhibitor SD70.
The median disease latency was 48 days in mice that received AMI-408 and 36 days in control mice. The median disease latency was 62 days in mice that received SD70 and 55 days in control mice.
“The demonstration of how critical these genes are to cancer transformation and treatment could be highly significant for the design of new drugs,” said study author Eric So, PhD, of King’s College London in the UK.
“Further work is needed to develop and refine drugs to maximize their effects and so that they are suitable for patients. Clinical trials will then be needed to see how leukemia patients respond to these drugs and how use of them can be optimized.” ![]()

Two genes are critical to the development of acute myeloid leukemia (AML), according to research published in Cancer Cell.
Previous research suggested the genes, KDM4C and PRMT1, are key players in transcription regulation during both normal and disease development.
The new study showed that, during AML development, KDM4C and PRMT1 are recruited to enable the transformation of blood cells into cancer cells.
The genes work in tandem, and, if either is not fully active, AML does not develop.
The researchers made these discoveries by inhibiting KDM4C and PRMT1—either genetically or pharmacologically—in mice with AML.
When either gene was silenced via genetic means, the majority of the mice were still alive at the end of the researchers’ 60-day experiment. However, the majority of control mice died in less than 40 days.
The team observed similarly favorable results when they inhibited either gene with drugs—the PRMT1 inhibitor AMI-408 and the KDM4C inhibitor SD70.
The median disease latency was 48 days in mice that received AMI-408 and 36 days in control mice. The median disease latency was 62 days in mice that received SD70 and 55 days in control mice.
“The demonstration of how critical these genes are to cancer transformation and treatment could be highly significant for the design of new drugs,” said study author Eric So, PhD, of King’s College London in the UK.
“Further work is needed to develop and refine drugs to maximize their effects and so that they are suitable for patients. Clinical trials will then be needed to see how leukemia patients respond to these drugs and how use of them can be optimized.” ![]()
Forces driving leukemia differ in kids and adults

New research suggests childhood leukemias are forged by different evolutionary forces than leukemias in older adults.
Researchers used a computational model to characterize the population dynamics of hematopoietic stem cells (HSCs) that give rise to leukemias.
And they found the evolutionary force known as “drift” contributes to leukemia development in young children but not in older adults.
“Basically, leukemia risk early in life may be more dictated by chance than by the typical ‘survival of the fittest’ that characterizes leukemia formation in older adults,” explained study author James DeGregori, PhD, of the University of Colorado School of Medicine in Aurora.
He and his colleagues recounted this discovery in PNAS.
With previous work, the DeGregori lab showed that the inevitable tissue decline associated with aging benefits HSCs with mutations that allow the cells to better adapt to the new ecosystem.
In contrast, the ecosystem of young tissue favors healthy cells. Optimized by millions of years of co-evolution, most mutations make cells less fit for the ecosystem of young, healthy tissue and lead to purging of mutant cells from the tissue.
With the current study, Dr DeGregori and his colleagues made a surprising discovery. Despite the ability of young tissue to select against cells with cancer-causing mutations, the computational model showed increased proportions of specific, mutation-bearing HSCs in the first few years after birth.
And these mutated cells were not dependent on the effect of the mutation on cell fitness. In other words, the mutation-bearing cells were not more fit than cells without the mutations. Instead of the survival-of-the-fittest form of natural selection that drives the evolution of cancer in older adults, there was another force at work.
In fact, the researchers discovered 2 factors that influence the development of childhood leukemia: the small HSC pool size at birth and the high rate of cell division necessary for body growth early in life.
The high rate of cell division increases the risk of leukemia because mutations largely happen during cell divisions. More cell divisions mean more mutations, and this increases the risk that some of these mutations could contribute to leukemia development.
The small HSC pool size influences leukemia development via the evolutionary force known as drift. Drift is the role of chance—the possibility that, despite being less fit, an animal, organism, or HSC with an oncogenic mutation will survive to shift the genetic makeup of the population.
The influence of drift is greater in small populations and, in this case, small stem cell pools. In the small HSC pools of young children, drift becomes important as a lucky genotype may end up with a larger share of the total HSC pool than warranted by its fitness status.
If this lucky cell clone happens to have a mutation that can start the HSC down the path toward leukemia, this drift-driven expansion should increase the risk of leukemia by increasing the number of HSCs with this mutation.
“Thus, early somatic evolution in HSC pools is significantly impacted by drift, with selection playing a lesser role,” Dr DeGregori and his colleagues wrote.
On the other hand, the impact of drift lessens as the HSC pool grows along with an infant’s body to reach adult size. The larger HSC pool size decreases the role of drift in the success of particular cells in the tissue.
In addition, as the pool size reaches its maximum, the HSC division rate slows to a crawl (as these stem cells enter the maintenance rather than growth phase). With a landscape of healthy, youthful tissues and low rates of mutation due to low cell division rates, the odds of leukemia diminish.
“With a large population of healthy cells optimized to young, healthy tissue, the ability of mutations, including cancerous mutations, to drive uncontrolled cell proliferation is reduced,” Dr DeGregori said.
However, in old age, tissue decline promotes selection for adaptive mutations, leading to the expansion of potentially oncogenic HSC clones that will again increase the risk of leukemia.
Thus, this research shows that, in early life, leukemias are driven by mutation and drift. And in later life, leukemias are driven by mutation and selection.
“We show that leukemias of children and older adults are different diseases, forged by different evolutionary forces and propagated under different circumstances,” Dr DeGregori said.
He and his colleagues believe this understanding raises the possibility of a new approach to cancer treatment. Perhaps researchers could find a way to manipulate the parameters of cell evolution or manipulate the tissue ecosystem to decrease cancer risk. ![]()

New research suggests childhood leukemias are forged by different evolutionary forces than leukemias in older adults.
Researchers used a computational model to characterize the population dynamics of hematopoietic stem cells (HSCs) that give rise to leukemias.
And they found the evolutionary force known as “drift” contributes to leukemia development in young children but not in older adults.
“Basically, leukemia risk early in life may be more dictated by chance than by the typical ‘survival of the fittest’ that characterizes leukemia formation in older adults,” explained study author James DeGregori, PhD, of the University of Colorado School of Medicine in Aurora.
He and his colleagues recounted this discovery in PNAS.
With previous work, the DeGregori lab showed that the inevitable tissue decline associated with aging benefits HSCs with mutations that allow the cells to better adapt to the new ecosystem.
In contrast, the ecosystem of young tissue favors healthy cells. Optimized by millions of years of co-evolution, most mutations make cells less fit for the ecosystem of young, healthy tissue and lead to purging of mutant cells from the tissue.
With the current study, Dr DeGregori and his colleagues made a surprising discovery. Despite the ability of young tissue to select against cells with cancer-causing mutations, the computational model showed increased proportions of specific, mutation-bearing HSCs in the first few years after birth.
And these mutated cells were not dependent on the effect of the mutation on cell fitness. In other words, the mutation-bearing cells were not more fit than cells without the mutations. Instead of the survival-of-the-fittest form of natural selection that drives the evolution of cancer in older adults, there was another force at work.
In fact, the researchers discovered 2 factors that influence the development of childhood leukemia: the small HSC pool size at birth and the high rate of cell division necessary for body growth early in life.
The high rate of cell division increases the risk of leukemia because mutations largely happen during cell divisions. More cell divisions mean more mutations, and this increases the risk that some of these mutations could contribute to leukemia development.
The small HSC pool size influences leukemia development via the evolutionary force known as drift. Drift is the role of chance—the possibility that, despite being less fit, an animal, organism, or HSC with an oncogenic mutation will survive to shift the genetic makeup of the population.
The influence of drift is greater in small populations and, in this case, small stem cell pools. In the small HSC pools of young children, drift becomes important as a lucky genotype may end up with a larger share of the total HSC pool than warranted by its fitness status.
If this lucky cell clone happens to have a mutation that can start the HSC down the path toward leukemia, this drift-driven expansion should increase the risk of leukemia by increasing the number of HSCs with this mutation.
“Thus, early somatic evolution in HSC pools is significantly impacted by drift, with selection playing a lesser role,” Dr DeGregori and his colleagues wrote.
On the other hand, the impact of drift lessens as the HSC pool grows along with an infant’s body to reach adult size. The larger HSC pool size decreases the role of drift in the success of particular cells in the tissue.
In addition, as the pool size reaches its maximum, the HSC division rate slows to a crawl (as these stem cells enter the maintenance rather than growth phase). With a landscape of healthy, youthful tissues and low rates of mutation due to low cell division rates, the odds of leukemia diminish.
“With a large population of healthy cells optimized to young, healthy tissue, the ability of mutations, including cancerous mutations, to drive uncontrolled cell proliferation is reduced,” Dr DeGregori said.
However, in old age, tissue decline promotes selection for adaptive mutations, leading to the expansion of potentially oncogenic HSC clones that will again increase the risk of leukemia.
Thus, this research shows that, in early life, leukemias are driven by mutation and drift. And in later life, leukemias are driven by mutation and selection.
“We show that leukemias of children and older adults are different diseases, forged by different evolutionary forces and propagated under different circumstances,” Dr DeGregori said.
He and his colleagues believe this understanding raises the possibility of a new approach to cancer treatment. Perhaps researchers could find a way to manipulate the parameters of cell evolution or manipulate the tissue ecosystem to decrease cancer risk. ![]()

New research suggests childhood leukemias are forged by different evolutionary forces than leukemias in older adults.
Researchers used a computational model to characterize the population dynamics of hematopoietic stem cells (HSCs) that give rise to leukemias.
And they found the evolutionary force known as “drift” contributes to leukemia development in young children but not in older adults.
“Basically, leukemia risk early in life may be more dictated by chance than by the typical ‘survival of the fittest’ that characterizes leukemia formation in older adults,” explained study author James DeGregori, PhD, of the University of Colorado School of Medicine in Aurora.
He and his colleagues recounted this discovery in PNAS.
With previous work, the DeGregori lab showed that the inevitable tissue decline associated with aging benefits HSCs with mutations that allow the cells to better adapt to the new ecosystem.
In contrast, the ecosystem of young tissue favors healthy cells. Optimized by millions of years of co-evolution, most mutations make cells less fit for the ecosystem of young, healthy tissue and lead to purging of mutant cells from the tissue.
With the current study, Dr DeGregori and his colleagues made a surprising discovery. Despite the ability of young tissue to select against cells with cancer-causing mutations, the computational model showed increased proportions of specific, mutation-bearing HSCs in the first few years after birth.
And these mutated cells were not dependent on the effect of the mutation on cell fitness. In other words, the mutation-bearing cells were not more fit than cells without the mutations. Instead of the survival-of-the-fittest form of natural selection that drives the evolution of cancer in older adults, there was another force at work.
In fact, the researchers discovered 2 factors that influence the development of childhood leukemia: the small HSC pool size at birth and the high rate of cell division necessary for body growth early in life.
The high rate of cell division increases the risk of leukemia because mutations largely happen during cell divisions. More cell divisions mean more mutations, and this increases the risk that some of these mutations could contribute to leukemia development.
The small HSC pool size influences leukemia development via the evolutionary force known as drift. Drift is the role of chance—the possibility that, despite being less fit, an animal, organism, or HSC with an oncogenic mutation will survive to shift the genetic makeup of the population.
The influence of drift is greater in small populations and, in this case, small stem cell pools. In the small HSC pools of young children, drift becomes important as a lucky genotype may end up with a larger share of the total HSC pool than warranted by its fitness status.
If this lucky cell clone happens to have a mutation that can start the HSC down the path toward leukemia, this drift-driven expansion should increase the risk of leukemia by increasing the number of HSCs with this mutation.
“Thus, early somatic evolution in HSC pools is significantly impacted by drift, with selection playing a lesser role,” Dr DeGregori and his colleagues wrote.
On the other hand, the impact of drift lessens as the HSC pool grows along with an infant’s body to reach adult size. The larger HSC pool size decreases the role of drift in the success of particular cells in the tissue.
In addition, as the pool size reaches its maximum, the HSC division rate slows to a crawl (as these stem cells enter the maintenance rather than growth phase). With a landscape of healthy, youthful tissues and low rates of mutation due to low cell division rates, the odds of leukemia diminish.
“With a large population of healthy cells optimized to young, healthy tissue, the ability of mutations, including cancerous mutations, to drive uncontrolled cell proliferation is reduced,” Dr DeGregori said.
However, in old age, tissue decline promotes selection for adaptive mutations, leading to the expansion of potentially oncogenic HSC clones that will again increase the risk of leukemia.
Thus, this research shows that, in early life, leukemias are driven by mutation and drift. And in later life, leukemias are driven by mutation and selection.
“We show that leukemias of children and older adults are different diseases, forged by different evolutionary forces and propagated under different circumstances,” Dr DeGregori said.
He and his colleagues believe this understanding raises the possibility of a new approach to cancer treatment. Perhaps researchers could find a way to manipulate the parameters of cell evolution or manipulate the tissue ecosystem to decrease cancer risk. ![]()
Microcapsules could provide targeted drug delivery
Image courtesy of Ronald Xu
& The Ohio State University
Researchers say they have developed a quick and controllable method for getting 2 or more ingredients into the same tiny drug capsule and having them mix only when triggered by a signal like vibrations or heat.
This work was inspired by the search for targeted drug delivery options to treat cancers.
The idea with this multi-ingredient capsule is that the ingredients must be mixed for the drug to work, and the mixing could be triggered in targeted areas of the body, thereby boosting drug efficiency while reducing side effects.
While the researchers found they could use their technique to create multi-ingredient microcapsules, they have not yet used it to encapsulate cancer treatments.
They described their work in Applied Physics Letters.
“One of the limitations of chemotherapy is that less than 5% of the drugs typically get to the tumor, while the rest can be absorbed by other organs,” said study author Ronald Xu, PhD, of The Ohio State University in Columbus.
He and his colleagues thought one possible way to address this problem could be to make the drugs non-toxic when injected into the body and trigger mixing that would produce a toxic product only near the tumor site.
The researchers knew that, for such drugs to work on a large scale, there must be a way to quickly, controllably, and cost-effectively produce capsules with 2 or more active ingredients. If the drugs are to be injected and spread through the body via the bloodstream, the capsules should also be small.
With that in mind, Dr Xu and his colleagues developed a device that can produce tiny capsules approximately 100 microns across with multiple inner ingredients.
The device works by funneling different ingredients through 2 inner needles. These needles run parallel to each other and are both enclosed in a larger outer needle, which contains an ingredient for making the outer shell of the capsule.
As all the ingredients exit the needles through a single nozzle, a high-speed gas forces the liquids into a narrow stream that breaks into individual droplets. An electric field stabilizes the flow so that uniform droplets are created.
Depending on the relative flow rates, each droplet may contain 2 or more smaller inner droplets made from the ingredients in the inner needles.
The researchers tested their device with colored paraffin wax—red in one needle and blue in the other. The outer shell was made from sodium alginate, a material extracted from seaweed that turned gelatinous when the droplets fell into a calcium chloride solution.
Depending on the experimental conditions, the team was able to produce between 1000 and 100,000 capsules per second, and nearly 100% of the inner liquids were incorporated into the capsules without any waste.
Once encapsulated, the 2 colors of wax did not mix because of surface tension. But the researchers found they could force the red and blue wax to merge by vibrating the capsules. The team also discovered they could release the inner droplets by dissolving the outer shell.
The key features of the new device are its high efficiency and yield, and the fact that the size of the droplets can be uniformly controlled, Dr Xu said.
He added that, by further fine-tuning the device’s operation, the team could make capsules that are 3-5 microns across, about the size of a red blood cell. The process can also be scaled up by building an array of nozzles and could be modified to encapsulate 3 or more active ingredients by adding additional inner needles.
While Dr Xu and his colleagues were motivated by drug delivery, they believe their device might also find wider use in a range of applications that require controlled reactions, such as regenerative medicine and nuclear and chemical engineering. ![]()

Image courtesy of Ronald Xu
& The Ohio State University
Researchers say they have developed a quick and controllable method for getting 2 or more ingredients into the same tiny drug capsule and having them mix only when triggered by a signal like vibrations or heat.
This work was inspired by the search for targeted drug delivery options to treat cancers.
The idea with this multi-ingredient capsule is that the ingredients must be mixed for the drug to work, and the mixing could be triggered in targeted areas of the body, thereby boosting drug efficiency while reducing side effects.
While the researchers found they could use their technique to create multi-ingredient microcapsules, they have not yet used it to encapsulate cancer treatments.
They described their work in Applied Physics Letters.
“One of the limitations of chemotherapy is that less than 5% of the drugs typically get to the tumor, while the rest can be absorbed by other organs,” said study author Ronald Xu, PhD, of The Ohio State University in Columbus.
He and his colleagues thought one possible way to address this problem could be to make the drugs non-toxic when injected into the body and trigger mixing that would produce a toxic product only near the tumor site.
The researchers knew that, for such drugs to work on a large scale, there must be a way to quickly, controllably, and cost-effectively produce capsules with 2 or more active ingredients. If the drugs are to be injected and spread through the body via the bloodstream, the capsules should also be small.
With that in mind, Dr Xu and his colleagues developed a device that can produce tiny capsules approximately 100 microns across with multiple inner ingredients.
The device works by funneling different ingredients through 2 inner needles. These needles run parallel to each other and are both enclosed in a larger outer needle, which contains an ingredient for making the outer shell of the capsule.
As all the ingredients exit the needles through a single nozzle, a high-speed gas forces the liquids into a narrow stream that breaks into individual droplets. An electric field stabilizes the flow so that uniform droplets are created.
Depending on the relative flow rates, each droplet may contain 2 or more smaller inner droplets made from the ingredients in the inner needles.
The researchers tested their device with colored paraffin wax—red in one needle and blue in the other. The outer shell was made from sodium alginate, a material extracted from seaweed that turned gelatinous when the droplets fell into a calcium chloride solution.
Depending on the experimental conditions, the team was able to produce between 1000 and 100,000 capsules per second, and nearly 100% of the inner liquids were incorporated into the capsules without any waste.
Once encapsulated, the 2 colors of wax did not mix because of surface tension. But the researchers found they could force the red and blue wax to merge by vibrating the capsules. The team also discovered they could release the inner droplets by dissolving the outer shell.
The key features of the new device are its high efficiency and yield, and the fact that the size of the droplets can be uniformly controlled, Dr Xu said.
He added that, by further fine-tuning the device’s operation, the team could make capsules that are 3-5 microns across, about the size of a red blood cell. The process can also be scaled up by building an array of nozzles and could be modified to encapsulate 3 or more active ingredients by adding additional inner needles.
While Dr Xu and his colleagues were motivated by drug delivery, they believe their device might also find wider use in a range of applications that require controlled reactions, such as regenerative medicine and nuclear and chemical engineering. ![]()

Image courtesy of Ronald Xu
& The Ohio State University
Researchers say they have developed a quick and controllable method for getting 2 or more ingredients into the same tiny drug capsule and having them mix only when triggered by a signal like vibrations or heat.
This work was inspired by the search for targeted drug delivery options to treat cancers.
The idea with this multi-ingredient capsule is that the ingredients must be mixed for the drug to work, and the mixing could be triggered in targeted areas of the body, thereby boosting drug efficiency while reducing side effects.
While the researchers found they could use their technique to create multi-ingredient microcapsules, they have not yet used it to encapsulate cancer treatments.
They described their work in Applied Physics Letters.
“One of the limitations of chemotherapy is that less than 5% of the drugs typically get to the tumor, while the rest can be absorbed by other organs,” said study author Ronald Xu, PhD, of The Ohio State University in Columbus.
He and his colleagues thought one possible way to address this problem could be to make the drugs non-toxic when injected into the body and trigger mixing that would produce a toxic product only near the tumor site.
The researchers knew that, for such drugs to work on a large scale, there must be a way to quickly, controllably, and cost-effectively produce capsules with 2 or more active ingredients. If the drugs are to be injected and spread through the body via the bloodstream, the capsules should also be small.
With that in mind, Dr Xu and his colleagues developed a device that can produce tiny capsules approximately 100 microns across with multiple inner ingredients.
The device works by funneling different ingredients through 2 inner needles. These needles run parallel to each other and are both enclosed in a larger outer needle, which contains an ingredient for making the outer shell of the capsule.
As all the ingredients exit the needles through a single nozzle, a high-speed gas forces the liquids into a narrow stream that breaks into individual droplets. An electric field stabilizes the flow so that uniform droplets are created.
Depending on the relative flow rates, each droplet may contain 2 or more smaller inner droplets made from the ingredients in the inner needles.
The researchers tested their device with colored paraffin wax—red in one needle and blue in the other. The outer shell was made from sodium alginate, a material extracted from seaweed that turned gelatinous when the droplets fell into a calcium chloride solution.
Depending on the experimental conditions, the team was able to produce between 1000 and 100,000 capsules per second, and nearly 100% of the inner liquids were incorporated into the capsules without any waste.
Once encapsulated, the 2 colors of wax did not mix because of surface tension. But the researchers found they could force the red and blue wax to merge by vibrating the capsules. The team also discovered they could release the inner droplets by dissolving the outer shell.
The key features of the new device are its high efficiency and yield, and the fact that the size of the droplets can be uniformly controlled, Dr Xu said.
He added that, by further fine-tuning the device’s operation, the team could make capsules that are 3-5 microns across, about the size of a red blood cell. The process can also be scaled up by building an array of nozzles and could be modified to encapsulate 3 or more active ingredients by adding additional inner needles.
While Dr Xu and his colleagues were motivated by drug delivery, they believe their device might also find wider use in a range of applications that require controlled reactions, such as regenerative medicine and nuclear and chemical engineering. ![]()