User login
Knee pain • no popping • no previous trauma • Dx?
THE CASE
A 36-year-old man sought care at our family medicine clinic for knee pain that he’d had for the past year. He denied any previous injury or trauma to the knee. The pain affected the posterolateral left knee and was aggravated by squatting and deep flexion. Daily activities did not bother him, but skiing, golfing, mountain biking, and lifting weights worsened the pain. His pain had gradually become more severe and frequent. He denied any mechanical symptoms such as catching, popping, or locking.
Examination of his left knee demonstrated range of motion from 0 to 120 degrees; further flexion caused significant pain. McMurray and Thessaly tests were positive for posterolateral pain, particularly with knee flexion >120 degrees. Physical examination was otherwise unremarkable. Standard x-rays of the left knee were normal. Our patient completed a month of physical therapy, but his symptoms did not improve.
THE DIAGNOSIS
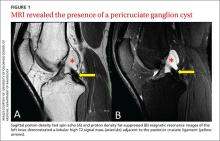
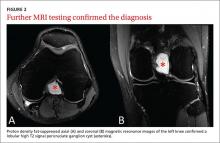
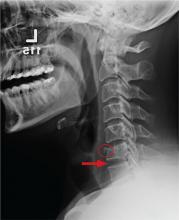
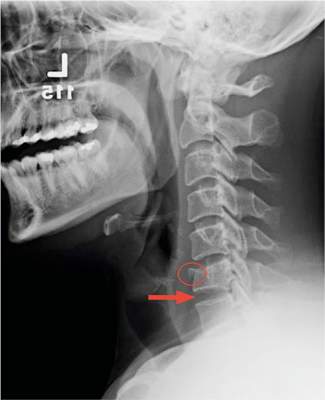
After the patient completed physical therapy, magnetic resonance imaging (MRI) was performed. The MRI did not reveal any left knee effusion, and the menisci, collateral ligaments, and cartilage surfaces were normal. And, while the cruciate ligaments were intact, a large pericruciate ganglion cyst was noted (FIGURES 1 AND 2).
DISCUSSION
Ganglion cysts are dense, encapsulated structures filled with clear viscous fluid that often arise adjacent to tendon sheaths or joint capsules, most commonly over the dorsum of the hand.1 Intra-articular ganglia involving the cruciate ligaments of the knee are relatively uncommon.2 The estimated prevalence of cruciate ligament ganglion cysts at arthroscopy is 0.2% to 1.9%; similar rates have been demonstrated with MRI.3-6 There are more reported cases of these cysts involving the anterior cruciate ligament (ACL) compared to those affecting the posterior cruciate ligament (PCL).2,6
Classification of these cysts is based on relative location with respect to the ligaments. Type 1 cysts originate anterior to the ACL; type 2, between the ACL and PCL; and type 3, posterior to the PCL.6,7 Cruciate ligament ganglion cysts are more common in men, are typically discovered between age 20 and 40, and are usually incidental findings.8
The pathogenesis of ganglion cyst formation is unknown.1,6,7 The most widely accepted theory is that ganglion cysts result from mucinous degeneration of connective tissue in areas of repetitive stress.1,6,7 Other theories suggest hyaluronic acid production secondary to mesenchymal stem cell proliferation within the ligaments, synovial tissue herniation, or congenital translocation of synovial tissue as possible etiologies.2,6,7
Concurrent pathologies such as meniscal tears or chondral lesions may also be present; however, there is some disagreement as to what role, if any, antecedent trauma has in the pathogenesis of cyst formation.1,6 Several investigators have suggested that prior knee trauma is a likely risk factor.2,8,9
In most patients, cruciate ligament ganglion cysts are asymptomatic.7 The most common presenting symptom is nonspecific pain that is exacerbated by activity, such as stair climbing, squatting, or other activities that require extreme flexion or extension of the knee.6,9 Other possible symptoms include limited range of motion (extension block with ACL involvement, limited flexion with PCL lesions), a catching or locking sensation, instability, or joint line tenderness.5,6 A palpable mass on physical exam is not usually present.6 Some investigators suggest that larger lesions and those closer to the femoral ligamentous attachments are more likely to cause symptoms.5
Cruciate ligament ganglion cysts can be an easily overlooked source of a patient’s symptoms because they often mimic more common pathologies.2 The differential diagnosis of cruciate ligament ganglion cysts and posterior knee pain includes any other intra-articular cysts (eg, meniscal cysts), posterior meniscal tear, popliteus tendinopathy, or neoplasms (eg, hemangioma and synovial sarcoma).2,6
MRI is the best method of diagnosis
Because the symptoms of cruciate ligament ganglion cysts are variable and nonspecific, the diagnosis is rarely made on clinical grounds alone.1 The best method of evaluating suspected intra-articular pathologies such as cruciate ligament ganglion cysts is MRI.5,10
Cruciate ligament ganglion cysts typically follow fluid signal on all sequences, with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.1,2,5,6 A pericruciate location with a multilocular appearance is usually sufficient evidence to make a diagnosis. However, solid or semi-solid pathologies (such as synovial cell sarcoma, synovial hemangioma, or synovial chondromatosis) can have similar signal intensity.
If necessary, intravenous contrast can be helpful; a lack of central contrast enhancement can differentiate ganglion cysts from other solid, enhancing, or partially enhancing lesions. Other diagnostic modalities, such as ultrasound, computed tomography (CT), and diagnostic arthroscopy, are less practical and have a wide range of sensitivity and specificity.5,6,10
Arthroscopic excision is the treatment of choice
Asymptomatic cruciate ligament ganglion cysts are usually managed with clinical follow-up. For patients with symptomatic cysts, ultrasound- or CT-guided percutaneous cyst aspiration may temporarily improve symptoms, but recurrence rates have not been well studied.2,6,9,10 Additionally, accessibility to cysts in this location via these approaches is limited. Arthroscopic excision of the cyst is the treatment of choice for symptomatic cases.1,2,5,6,10
Our patient underwent arthroscopic cyst resection, which resulted in complete resolution of his symptoms. In 3 months, he returned to his regular physical activities with no pain or discomfort. One year later, he remained asymptomatic.
THE TAKEAWAY
Cruciate ligament ganglion cysts are a rare cause of posterior knee pain. An MRI is the best diagnostic modality to evaluate and confirm the diagnosis, as well as rule out other pathologies. The treatment of choice for symptomatic cases is arthroscopic excision of the cyst.
1. Mao Y, Dong Q, Wang Y. Ganglion cysts of the cruciate ligaments: a series of 31 cases and review of the literature. BMC Musculoskelet Disord. 2012;13:137.
2. Krudwig WK, Schulte KK, Heinemann C. Intra-articular ganglion cysts of the knee joint: a report of 85 cases and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2004;12:123-129.
3. Bergin D, Morrison WB, Carrino JA, et al. Anterior cruciate ligament ganglia and mucoid degeneration: coexistence and clinical correlation. AJR Am J Roentgenol. 2004;182:1283-1287.
4. Bui-Mansfield LT, Youngberg RA. Intraarticular ganglia of the knee: prevalence, presentation, etiology, and management. AJR Am J Roentgenol. 1997;168:123-127.
5. Lunhao B, Yu S, Jiashi W. Diagnosis and treatment of ganglion cysts of the cruciate ligaments. Arch Orthop Trauma Surg. 2011;131:1053-1057.
6. Stein D, Cantlon M, Mackay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
7. Zantop T, Rusch A, Hassenpflug J, et al. Intra-articular ganglion cysts of the cruciate ligaments: case report and review of the literature. Arch Orthop Trauma Surg. 2003;123:195-198.
8. Tsai TY, Yang YS, Tseng FJ, et al. Arthroscopic excision of ganglion cysts of the posterior cruciate ligaments using posterior trans-septal portal. Arthroscopy. 2012;28:95-99.
9. Huang GS, Lee CH, Chan WP, et al. Ganglion cysts of the cruciate ligaments. Acta Radiol. 2002;43:419-424.
10. Tyrrell PN, Cassar-Pullicino VN, McCall IW. Intra-articular ganglion cysts of the cruciate ligaments. Eur Radiol. 2000;10:1233-1238.
THE CASE
A 36-year-old man sought care at our family medicine clinic for knee pain that he’d had for the past year. He denied any previous injury or trauma to the knee. The pain affected the posterolateral left knee and was aggravated by squatting and deep flexion. Daily activities did not bother him, but skiing, golfing, mountain biking, and lifting weights worsened the pain. His pain had gradually become more severe and frequent. He denied any mechanical symptoms such as catching, popping, or locking.
Examination of his left knee demonstrated range of motion from 0 to 120 degrees; further flexion caused significant pain. McMurray and Thessaly tests were positive for posterolateral pain, particularly with knee flexion >120 degrees. Physical examination was otherwise unremarkable. Standard x-rays of the left knee were normal. Our patient completed a month of physical therapy, but his symptoms did not improve.
THE DIAGNOSIS
After the patient completed physical therapy, magnetic resonance imaging (MRI) was performed. The MRI did not reveal any left knee effusion, and the menisci, collateral ligaments, and cartilage surfaces were normal. And, while the cruciate ligaments were intact, a large pericruciate ganglion cyst was noted (FIGURES 1 AND 2).
DISCUSSION
Ganglion cysts are dense, encapsulated structures filled with clear viscous fluid that often arise adjacent to tendon sheaths or joint capsules, most commonly over the dorsum of the hand.1 Intra-articular ganglia involving the cruciate ligaments of the knee are relatively uncommon.2 The estimated prevalence of cruciate ligament ganglion cysts at arthroscopy is 0.2% to 1.9%; similar rates have been demonstrated with MRI.3-6 There are more reported cases of these cysts involving the anterior cruciate ligament (ACL) compared to those affecting the posterior cruciate ligament (PCL).2,6
Classification of these cysts is based on relative location with respect to the ligaments. Type 1 cysts originate anterior to the ACL; type 2, between the ACL and PCL; and type 3, posterior to the PCL.6,7 Cruciate ligament ganglion cysts are more common in men, are typically discovered between age 20 and 40, and are usually incidental findings.8
The pathogenesis of ganglion cyst formation is unknown.1,6,7 The most widely accepted theory is that ganglion cysts result from mucinous degeneration of connective tissue in areas of repetitive stress.1,6,7 Other theories suggest hyaluronic acid production secondary to mesenchymal stem cell proliferation within the ligaments, synovial tissue herniation, or congenital translocation of synovial tissue as possible etiologies.2,6,7
Concurrent pathologies such as meniscal tears or chondral lesions may also be present; however, there is some disagreement as to what role, if any, antecedent trauma has in the pathogenesis of cyst formation.1,6 Several investigators have suggested that prior knee trauma is a likely risk factor.2,8,9
In most patients, cruciate ligament ganglion cysts are asymptomatic.7 The most common presenting symptom is nonspecific pain that is exacerbated by activity, such as stair climbing, squatting, or other activities that require extreme flexion or extension of the knee.6,9 Other possible symptoms include limited range of motion (extension block with ACL involvement, limited flexion with PCL lesions), a catching or locking sensation, instability, or joint line tenderness.5,6 A palpable mass on physical exam is not usually present.6 Some investigators suggest that larger lesions and those closer to the femoral ligamentous attachments are more likely to cause symptoms.5
Cruciate ligament ganglion cysts can be an easily overlooked source of a patient’s symptoms because they often mimic more common pathologies.2 The differential diagnosis of cruciate ligament ganglion cysts and posterior knee pain includes any other intra-articular cysts (eg, meniscal cysts), posterior meniscal tear, popliteus tendinopathy, or neoplasms (eg, hemangioma and synovial sarcoma).2,6
MRI is the best method of diagnosis
Because the symptoms of cruciate ligament ganglion cysts are variable and nonspecific, the diagnosis is rarely made on clinical grounds alone.1 The best method of evaluating suspected intra-articular pathologies such as cruciate ligament ganglion cysts is MRI.5,10
Cruciate ligament ganglion cysts typically follow fluid signal on all sequences, with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.1,2,5,6 A pericruciate location with a multilocular appearance is usually sufficient evidence to make a diagnosis. However, solid or semi-solid pathologies (such as synovial cell sarcoma, synovial hemangioma, or synovial chondromatosis) can have similar signal intensity.
If necessary, intravenous contrast can be helpful; a lack of central contrast enhancement can differentiate ganglion cysts from other solid, enhancing, or partially enhancing lesions. Other diagnostic modalities, such as ultrasound, computed tomography (CT), and diagnostic arthroscopy, are less practical and have a wide range of sensitivity and specificity.5,6,10
Arthroscopic excision is the treatment of choice
Asymptomatic cruciate ligament ganglion cysts are usually managed with clinical follow-up. For patients with symptomatic cysts, ultrasound- or CT-guided percutaneous cyst aspiration may temporarily improve symptoms, but recurrence rates have not been well studied.2,6,9,10 Additionally, accessibility to cysts in this location via these approaches is limited. Arthroscopic excision of the cyst is the treatment of choice for symptomatic cases.1,2,5,6,10
Our patient underwent arthroscopic cyst resection, which resulted in complete resolution of his symptoms. In 3 months, he returned to his regular physical activities with no pain or discomfort. One year later, he remained asymptomatic.
THE TAKEAWAY
Cruciate ligament ganglion cysts are a rare cause of posterior knee pain. An MRI is the best diagnostic modality to evaluate and confirm the diagnosis, as well as rule out other pathologies. The treatment of choice for symptomatic cases is arthroscopic excision of the cyst.
THE CASE
A 36-year-old man sought care at our family medicine clinic for knee pain that he’d had for the past year. He denied any previous injury or trauma to the knee. The pain affected the posterolateral left knee and was aggravated by squatting and deep flexion. Daily activities did not bother him, but skiing, golfing, mountain biking, and lifting weights worsened the pain. His pain had gradually become more severe and frequent. He denied any mechanical symptoms such as catching, popping, or locking.
Examination of his left knee demonstrated range of motion from 0 to 120 degrees; further flexion caused significant pain. McMurray and Thessaly tests were positive for posterolateral pain, particularly with knee flexion >120 degrees. Physical examination was otherwise unremarkable. Standard x-rays of the left knee were normal. Our patient completed a month of physical therapy, but his symptoms did not improve.
THE DIAGNOSIS
After the patient completed physical therapy, magnetic resonance imaging (MRI) was performed. The MRI did not reveal any left knee effusion, and the menisci, collateral ligaments, and cartilage surfaces were normal. And, while the cruciate ligaments were intact, a large pericruciate ganglion cyst was noted (FIGURES 1 AND 2).
DISCUSSION
Ganglion cysts are dense, encapsulated structures filled with clear viscous fluid that often arise adjacent to tendon sheaths or joint capsules, most commonly over the dorsum of the hand.1 Intra-articular ganglia involving the cruciate ligaments of the knee are relatively uncommon.2 The estimated prevalence of cruciate ligament ganglion cysts at arthroscopy is 0.2% to 1.9%; similar rates have been demonstrated with MRI.3-6 There are more reported cases of these cysts involving the anterior cruciate ligament (ACL) compared to those affecting the posterior cruciate ligament (PCL).2,6
Classification of these cysts is based on relative location with respect to the ligaments. Type 1 cysts originate anterior to the ACL; type 2, between the ACL and PCL; and type 3, posterior to the PCL.6,7 Cruciate ligament ganglion cysts are more common in men, are typically discovered between age 20 and 40, and are usually incidental findings.8
The pathogenesis of ganglion cyst formation is unknown.1,6,7 The most widely accepted theory is that ganglion cysts result from mucinous degeneration of connective tissue in areas of repetitive stress.1,6,7 Other theories suggest hyaluronic acid production secondary to mesenchymal stem cell proliferation within the ligaments, synovial tissue herniation, or congenital translocation of synovial tissue as possible etiologies.2,6,7
Concurrent pathologies such as meniscal tears or chondral lesions may also be present; however, there is some disagreement as to what role, if any, antecedent trauma has in the pathogenesis of cyst formation.1,6 Several investigators have suggested that prior knee trauma is a likely risk factor.2,8,9
In most patients, cruciate ligament ganglion cysts are asymptomatic.7 The most common presenting symptom is nonspecific pain that is exacerbated by activity, such as stair climbing, squatting, or other activities that require extreme flexion or extension of the knee.6,9 Other possible symptoms include limited range of motion (extension block with ACL involvement, limited flexion with PCL lesions), a catching or locking sensation, instability, or joint line tenderness.5,6 A palpable mass on physical exam is not usually present.6 Some investigators suggest that larger lesions and those closer to the femoral ligamentous attachments are more likely to cause symptoms.5
Cruciate ligament ganglion cysts can be an easily overlooked source of a patient’s symptoms because they often mimic more common pathologies.2 The differential diagnosis of cruciate ligament ganglion cysts and posterior knee pain includes any other intra-articular cysts (eg, meniscal cysts), posterior meniscal tear, popliteus tendinopathy, or neoplasms (eg, hemangioma and synovial sarcoma).2,6
MRI is the best method of diagnosis
Because the symptoms of cruciate ligament ganglion cysts are variable and nonspecific, the diagnosis is rarely made on clinical grounds alone.1 The best method of evaluating suspected intra-articular pathologies such as cruciate ligament ganglion cysts is MRI.5,10
Cruciate ligament ganglion cysts typically follow fluid signal on all sequences, with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.1,2,5,6 A pericruciate location with a multilocular appearance is usually sufficient evidence to make a diagnosis. However, solid or semi-solid pathologies (such as synovial cell sarcoma, synovial hemangioma, or synovial chondromatosis) can have similar signal intensity.
If necessary, intravenous contrast can be helpful; a lack of central contrast enhancement can differentiate ganglion cysts from other solid, enhancing, or partially enhancing lesions. Other diagnostic modalities, such as ultrasound, computed tomography (CT), and diagnostic arthroscopy, are less practical and have a wide range of sensitivity and specificity.5,6,10
Arthroscopic excision is the treatment of choice
Asymptomatic cruciate ligament ganglion cysts are usually managed with clinical follow-up. For patients with symptomatic cysts, ultrasound- or CT-guided percutaneous cyst aspiration may temporarily improve symptoms, but recurrence rates have not been well studied.2,6,9,10 Additionally, accessibility to cysts in this location via these approaches is limited. Arthroscopic excision of the cyst is the treatment of choice for symptomatic cases.1,2,5,6,10
Our patient underwent arthroscopic cyst resection, which resulted in complete resolution of his symptoms. In 3 months, he returned to his regular physical activities with no pain or discomfort. One year later, he remained asymptomatic.
THE TAKEAWAY
Cruciate ligament ganglion cysts are a rare cause of posterior knee pain. An MRI is the best diagnostic modality to evaluate and confirm the diagnosis, as well as rule out other pathologies. The treatment of choice for symptomatic cases is arthroscopic excision of the cyst.
1. Mao Y, Dong Q, Wang Y. Ganglion cysts of the cruciate ligaments: a series of 31 cases and review of the literature. BMC Musculoskelet Disord. 2012;13:137.
2. Krudwig WK, Schulte KK, Heinemann C. Intra-articular ganglion cysts of the knee joint: a report of 85 cases and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2004;12:123-129.
3. Bergin D, Morrison WB, Carrino JA, et al. Anterior cruciate ligament ganglia and mucoid degeneration: coexistence and clinical correlation. AJR Am J Roentgenol. 2004;182:1283-1287.
4. Bui-Mansfield LT, Youngberg RA. Intraarticular ganglia of the knee: prevalence, presentation, etiology, and management. AJR Am J Roentgenol. 1997;168:123-127.
5. Lunhao B, Yu S, Jiashi W. Diagnosis and treatment of ganglion cysts of the cruciate ligaments. Arch Orthop Trauma Surg. 2011;131:1053-1057.
6. Stein D, Cantlon M, Mackay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
7. Zantop T, Rusch A, Hassenpflug J, et al. Intra-articular ganglion cysts of the cruciate ligaments: case report and review of the literature. Arch Orthop Trauma Surg. 2003;123:195-198.
8. Tsai TY, Yang YS, Tseng FJ, et al. Arthroscopic excision of ganglion cysts of the posterior cruciate ligaments using posterior trans-septal portal. Arthroscopy. 2012;28:95-99.
9. Huang GS, Lee CH, Chan WP, et al. Ganglion cysts of the cruciate ligaments. Acta Radiol. 2002;43:419-424.
10. Tyrrell PN, Cassar-Pullicino VN, McCall IW. Intra-articular ganglion cysts of the cruciate ligaments. Eur Radiol. 2000;10:1233-1238.
1. Mao Y, Dong Q, Wang Y. Ganglion cysts of the cruciate ligaments: a series of 31 cases and review of the literature. BMC Musculoskelet Disord. 2012;13:137.
2. Krudwig WK, Schulte KK, Heinemann C. Intra-articular ganglion cysts of the knee joint: a report of 85 cases and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2004;12:123-129.
3. Bergin D, Morrison WB, Carrino JA, et al. Anterior cruciate ligament ganglia and mucoid degeneration: coexistence and clinical correlation. AJR Am J Roentgenol. 2004;182:1283-1287.
4. Bui-Mansfield LT, Youngberg RA. Intraarticular ganglia of the knee: prevalence, presentation, etiology, and management. AJR Am J Roentgenol. 1997;168:123-127.
5. Lunhao B, Yu S, Jiashi W. Diagnosis and treatment of ganglion cysts of the cruciate ligaments. Arch Orthop Trauma Surg. 2011;131:1053-1057.
6. Stein D, Cantlon M, Mackay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
7. Zantop T, Rusch A, Hassenpflug J, et al. Intra-articular ganglion cysts of the cruciate ligaments: case report and review of the literature. Arch Orthop Trauma Surg. 2003;123:195-198.
8. Tsai TY, Yang YS, Tseng FJ, et al. Arthroscopic excision of ganglion cysts of the posterior cruciate ligaments using posterior trans-septal portal. Arthroscopy. 2012;28:95-99.
9. Huang GS, Lee CH, Chan WP, et al. Ganglion cysts of the cruciate ligaments. Acta Radiol. 2002;43:419-424.
10. Tyrrell PN, Cassar-Pullicino VN, McCall IW. Intra-articular ganglion cysts of the cruciate ligaments. Eur Radiol. 2000;10:1233-1238.
These umbilical lesions weren't granulomas after all
THE CASES
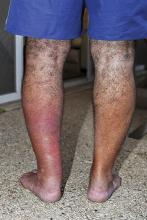
CASE 1 › A 15-month-old boy was brought to our center for plastic surgery after being referred by his general practitioner (GP). The patient had a non-healing lesion on his umbilicus that had been present since birth. It had remained the same size, but bled occasionally. The GP initially presumed the lesion was a granuloma and treated it with silver nitrate cautery, but this did not eradicate it.
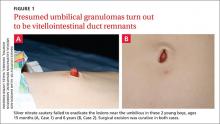
After talking with the boy’s mother further, we learned that there had been a constant oozing from the area since birth and that the lesion protruded slightly from the abdomen when the child cried. The boy had congenital heart disease, but his bowel and genitourinary history were normal. A clinical examination revealed pink, moist tissue herniating from the umbilicus with surrounding abdominal fullness when the boy stood up (FIGURE 1A). An ultrasound showed a focal 19 x 7 mm complex area around the umbilicus with no definite track. The lesion was surgically removed. Histology revealed a completely excised vitellointestinal duct remnant.
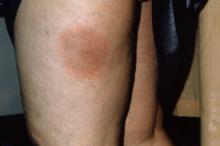
CASE 2 › A 6-year-old boy with a history of attention-deficit/hyperactivity disorder was brought to our clinic with a non-healing umbilical lesion after being referred by his GP. The lesion had been present since birth and had failed to resolve despite several attempts to treat it with silver nitrate cautery. Clinically, the patient appeared to have a granulomatous umbilical polyp (FIGURE 1B). The patient underwent surgical excision of the lesion. Histological analysis revealed a completely excised vitellointestinal duct remnant (FIGURE 2).
DISCUSSION
The vitellointestinal duct (VID), also called the omphalomesenteric duct (OMD), connects the alimentary canal and the yolk sac in early embryogenesis. Failure of involution of the duct results in abnormalities such as Meckel’s diverticulum, cysts, and polyps.
VID anomalies occur in approximately 2% of newborns; a small percentage of these have patent connections to the intestine.1 Parents are often the first to notice the abnormality and will typically see a reddish protrusion around the umbilicus or a persistent serous discharge around the umbilicus soon after birth.
VID remnants are similar in presentation to benign granulomas or granulation tissue, which are benign lesions that present in the first few weeks of life. Granulomas are reddish in color, bleed minimally when irritated by trauma, and respond well to silver nitrate cautery.2 When the lesion fails to respond to treatment, an alternative diagnosis should be investigated further.
Ultrasonography is the best way to evaluate a suspected VID remnant
A suspected VID remnant should first be assessed with ultrasonography to determine the extent of the remnant and guide surgical treatment. Ultrasonography can also delineate the relationship of these congenital remnants with the umbilicus and bladder.3
Potential complications that can arise from these lesions include an intestinal hernia, intussusception, volvulus, abdominal pain, or a persistent discharge that can lead to infection.3 Mortality following complications is significantly high.4
Although the etiology of patent VIDs and their remnants remains unknown, the presence of such ducts is associated with other congenital anomalies, including Down Syndrome, structural cardiac malformation, conduction abnormalities, and cleft lip and palate.5-7 Therefore, additional history taking and examinations may be required to identify these associated pathologies. In Case 1, the 15-month-old boy had congenital heart disease.
Surgical excision will prevent complications
A simple surgical excision should be performed for VID remnants. The prognosis is excellent when such procedures are performed in the non-acute setting. Some debate exists as to whether all remnants require formal abdominal exploration.8,9
Treatment of patent VIDs requires surgical excision of the duct, with or without a segment of the small bowel, to obliterate the connection.10 Reconstruction of the umbilicus is then performed, depending on the surgical technique used.
Our patients both made complete recoveries following their surgeries with resolution of their symptoms.
THE TAKEAWAY
Consider a VID remnant as part of the differential diagnosis for any patient who has what appears to be a granulomatous umbilical lesion. Order ultrasonography to evaluate a suspected VID, especially for lesions that fail to respond to 2 or 3 silver nitrate treatments. Surgical excision of a VID remnant is usually curative.
1. Vane DW, West KW, Grosfeld JL. Vitelline duct anomalies. Experience with 217 childhood cases. Arch Surg. 1987;122:542-547.
2. Piparsaliya S, Joshi M, Rajput N, et al. Patent vitellointestinal duct: A close differential diagnosis of umbilical granuloma: A case report and review of literature. Surgical Science. 2011;2:134-136.
3. Khati NJ, Enquist EG, Javitt MC. Imaging of the umbilicus and periumbilical region. Radiographics. 1998;18:413-431.
4. Yamada T, Seiki Y, Ueda M, et al. Patent omphalomesenteric duct: a case report and review of Japanese literature. Asia Oceania J Obstet Gynaecol. 1989;15:229-236.
5. Martin RH, Doublestein GL, Jarvis MR. Concurrent ectopic pregnancy, Meckel’s diverticulum with vitelline duct remnant, cecal volvulus, and congenital complete heart block: report of a case. J Am Osteopath Assoc. 1986;86:589-591.
6. Elebute EA, Ransome-Kuti O. Patent vitello-intestinal duct with ileal prolapse. Arch Surg. 1965;91:456-460.
7. Blair SP, Beasley SW. Intussusception of vitello-intestinal tract through an exomphalos in trisomy 13. Pediatric Surgery International. 1989;4:422-423.
8. Kutin ND, Allen JE, Jewett TC. The umbilical polyp. J Pediatr Surg. 1979;14:741-744.
9. Pacilli M, Sebire NJ, Maritsi D, et al. Umbilical polyp in infants and children. Eur J Pediatr Surg. 2007;17:397-399.
10. Storms P, Pexsters J, Vandekerkhof J. Small omphalocele with ileal prolapse through a patent omphalomesenteric duct. A case report and review of literature. Acta Chir Belg. 1988;88:392-394.
THE CASES
CASE 1 › A 15-month-old boy was brought to our center for plastic surgery after being referred by his general practitioner (GP). The patient had a non-healing lesion on his umbilicus that had been present since birth. It had remained the same size, but bled occasionally. The GP initially presumed the lesion was a granuloma and treated it with silver nitrate cautery, but this did not eradicate it.
After talking with the boy’s mother further, we learned that there had been a constant oozing from the area since birth and that the lesion protruded slightly from the abdomen when the child cried. The boy had congenital heart disease, but his bowel and genitourinary history were normal. A clinical examination revealed pink, moist tissue herniating from the umbilicus with surrounding abdominal fullness when the boy stood up (FIGURE 1A). An ultrasound showed a focal 19 x 7 mm complex area around the umbilicus with no definite track. The lesion was surgically removed. Histology revealed a completely excised vitellointestinal duct remnant.
CASE 2 › A 6-year-old boy with a history of attention-deficit/hyperactivity disorder was brought to our clinic with a non-healing umbilical lesion after being referred by his GP. The lesion had been present since birth and had failed to resolve despite several attempts to treat it with silver nitrate cautery. Clinically, the patient appeared to have a granulomatous umbilical polyp (FIGURE 1B). The patient underwent surgical excision of the lesion. Histological analysis revealed a completely excised vitellointestinal duct remnant (FIGURE 2).
DISCUSSION
The vitellointestinal duct (VID), also called the omphalomesenteric duct (OMD), connects the alimentary canal and the yolk sac in early embryogenesis. Failure of involution of the duct results in abnormalities such as Meckel’s diverticulum, cysts, and polyps.
VID anomalies occur in approximately 2% of newborns; a small percentage of these have patent connections to the intestine.1 Parents are often the first to notice the abnormality and will typically see a reddish protrusion around the umbilicus or a persistent serous discharge around the umbilicus soon after birth.
VID remnants are similar in presentation to benign granulomas or granulation tissue, which are benign lesions that present in the first few weeks of life. Granulomas are reddish in color, bleed minimally when irritated by trauma, and respond well to silver nitrate cautery.2 When the lesion fails to respond to treatment, an alternative diagnosis should be investigated further.
Ultrasonography is the best way to evaluate a suspected VID remnant
A suspected VID remnant should first be assessed with ultrasonography to determine the extent of the remnant and guide surgical treatment. Ultrasonography can also delineate the relationship of these congenital remnants with the umbilicus and bladder.3
Potential complications that can arise from these lesions include an intestinal hernia, intussusception, volvulus, abdominal pain, or a persistent discharge that can lead to infection.3 Mortality following complications is significantly high.4
Although the etiology of patent VIDs and their remnants remains unknown, the presence of such ducts is associated with other congenital anomalies, including Down Syndrome, structural cardiac malformation, conduction abnormalities, and cleft lip and palate.5-7 Therefore, additional history taking and examinations may be required to identify these associated pathologies. In Case 1, the 15-month-old boy had congenital heart disease.
Surgical excision will prevent complications
A simple surgical excision should be performed for VID remnants. The prognosis is excellent when such procedures are performed in the non-acute setting. Some debate exists as to whether all remnants require formal abdominal exploration.8,9
Treatment of patent VIDs requires surgical excision of the duct, with or without a segment of the small bowel, to obliterate the connection.10 Reconstruction of the umbilicus is then performed, depending on the surgical technique used.
Our patients both made complete recoveries following their surgeries with resolution of their symptoms.
THE TAKEAWAY
Consider a VID remnant as part of the differential diagnosis for any patient who has what appears to be a granulomatous umbilical lesion. Order ultrasonography to evaluate a suspected VID, especially for lesions that fail to respond to 2 or 3 silver nitrate treatments. Surgical excision of a VID remnant is usually curative.
THE CASES
CASE 1 › A 15-month-old boy was brought to our center for plastic surgery after being referred by his general practitioner (GP). The patient had a non-healing lesion on his umbilicus that had been present since birth. It had remained the same size, but bled occasionally. The GP initially presumed the lesion was a granuloma and treated it with silver nitrate cautery, but this did not eradicate it.
After talking with the boy’s mother further, we learned that there had been a constant oozing from the area since birth and that the lesion protruded slightly from the abdomen when the child cried. The boy had congenital heart disease, but his bowel and genitourinary history were normal. A clinical examination revealed pink, moist tissue herniating from the umbilicus with surrounding abdominal fullness when the boy stood up (FIGURE 1A). An ultrasound showed a focal 19 x 7 mm complex area around the umbilicus with no definite track. The lesion was surgically removed. Histology revealed a completely excised vitellointestinal duct remnant.
CASE 2 › A 6-year-old boy with a history of attention-deficit/hyperactivity disorder was brought to our clinic with a non-healing umbilical lesion after being referred by his GP. The lesion had been present since birth and had failed to resolve despite several attempts to treat it with silver nitrate cautery. Clinically, the patient appeared to have a granulomatous umbilical polyp (FIGURE 1B). The patient underwent surgical excision of the lesion. Histological analysis revealed a completely excised vitellointestinal duct remnant (FIGURE 2).
DISCUSSION
The vitellointestinal duct (VID), also called the omphalomesenteric duct (OMD), connects the alimentary canal and the yolk sac in early embryogenesis. Failure of involution of the duct results in abnormalities such as Meckel’s diverticulum, cysts, and polyps.
VID anomalies occur in approximately 2% of newborns; a small percentage of these have patent connections to the intestine.1 Parents are often the first to notice the abnormality and will typically see a reddish protrusion around the umbilicus or a persistent serous discharge around the umbilicus soon after birth.
VID remnants are similar in presentation to benign granulomas or granulation tissue, which are benign lesions that present in the first few weeks of life. Granulomas are reddish in color, bleed minimally when irritated by trauma, and respond well to silver nitrate cautery.2 When the lesion fails to respond to treatment, an alternative diagnosis should be investigated further.
Ultrasonography is the best way to evaluate a suspected VID remnant
A suspected VID remnant should first be assessed with ultrasonography to determine the extent of the remnant and guide surgical treatment. Ultrasonography can also delineate the relationship of these congenital remnants with the umbilicus and bladder.3
Potential complications that can arise from these lesions include an intestinal hernia, intussusception, volvulus, abdominal pain, or a persistent discharge that can lead to infection.3 Mortality following complications is significantly high.4
Although the etiology of patent VIDs and their remnants remains unknown, the presence of such ducts is associated with other congenital anomalies, including Down Syndrome, structural cardiac malformation, conduction abnormalities, and cleft lip and palate.5-7 Therefore, additional history taking and examinations may be required to identify these associated pathologies. In Case 1, the 15-month-old boy had congenital heart disease.
Surgical excision will prevent complications
A simple surgical excision should be performed for VID remnants. The prognosis is excellent when such procedures are performed in the non-acute setting. Some debate exists as to whether all remnants require formal abdominal exploration.8,9
Treatment of patent VIDs requires surgical excision of the duct, with or without a segment of the small bowel, to obliterate the connection.10 Reconstruction of the umbilicus is then performed, depending on the surgical technique used.
Our patients both made complete recoveries following their surgeries with resolution of their symptoms.
THE TAKEAWAY
Consider a VID remnant as part of the differential diagnosis for any patient who has what appears to be a granulomatous umbilical lesion. Order ultrasonography to evaluate a suspected VID, especially for lesions that fail to respond to 2 or 3 silver nitrate treatments. Surgical excision of a VID remnant is usually curative.
1. Vane DW, West KW, Grosfeld JL. Vitelline duct anomalies. Experience with 217 childhood cases. Arch Surg. 1987;122:542-547.
2. Piparsaliya S, Joshi M, Rajput N, et al. Patent vitellointestinal duct: A close differential diagnosis of umbilical granuloma: A case report and review of literature. Surgical Science. 2011;2:134-136.
3. Khati NJ, Enquist EG, Javitt MC. Imaging of the umbilicus and periumbilical region. Radiographics. 1998;18:413-431.
4. Yamada T, Seiki Y, Ueda M, et al. Patent omphalomesenteric duct: a case report and review of Japanese literature. Asia Oceania J Obstet Gynaecol. 1989;15:229-236.
5. Martin RH, Doublestein GL, Jarvis MR. Concurrent ectopic pregnancy, Meckel’s diverticulum with vitelline duct remnant, cecal volvulus, and congenital complete heart block: report of a case. J Am Osteopath Assoc. 1986;86:589-591.
6. Elebute EA, Ransome-Kuti O. Patent vitello-intestinal duct with ileal prolapse. Arch Surg. 1965;91:456-460.
7. Blair SP, Beasley SW. Intussusception of vitello-intestinal tract through an exomphalos in trisomy 13. Pediatric Surgery International. 1989;4:422-423.
8. Kutin ND, Allen JE, Jewett TC. The umbilical polyp. J Pediatr Surg. 1979;14:741-744.
9. Pacilli M, Sebire NJ, Maritsi D, et al. Umbilical polyp in infants and children. Eur J Pediatr Surg. 2007;17:397-399.
10. Storms P, Pexsters J, Vandekerkhof J. Small omphalocele with ileal prolapse through a patent omphalomesenteric duct. A case report and review of literature. Acta Chir Belg. 1988;88:392-394.
1. Vane DW, West KW, Grosfeld JL. Vitelline duct anomalies. Experience with 217 childhood cases. Arch Surg. 1987;122:542-547.
2. Piparsaliya S, Joshi M, Rajput N, et al. Patent vitellointestinal duct: A close differential diagnosis of umbilical granuloma: A case report and review of literature. Surgical Science. 2011;2:134-136.
3. Khati NJ, Enquist EG, Javitt MC. Imaging of the umbilicus and periumbilical region. Radiographics. 1998;18:413-431.
4. Yamada T, Seiki Y, Ueda M, et al. Patent omphalomesenteric duct: a case report and review of Japanese literature. Asia Oceania J Obstet Gynaecol. 1989;15:229-236.
5. Martin RH, Doublestein GL, Jarvis MR. Concurrent ectopic pregnancy, Meckel’s diverticulum with vitelline duct remnant, cecal volvulus, and congenital complete heart block: report of a case. J Am Osteopath Assoc. 1986;86:589-591.
6. Elebute EA, Ransome-Kuti O. Patent vitello-intestinal duct with ileal prolapse. Arch Surg. 1965;91:456-460.
7. Blair SP, Beasley SW. Intussusception of vitello-intestinal tract through an exomphalos in trisomy 13. Pediatric Surgery International. 1989;4:422-423.
8. Kutin ND, Allen JE, Jewett TC. The umbilical polyp. J Pediatr Surg. 1979;14:741-744.
9. Pacilli M, Sebire NJ, Maritsi D, et al. Umbilical polyp in infants and children. Eur J Pediatr Surg. 2007;17:397-399.
10. Storms P, Pexsters J, Vandekerkhof J. Small omphalocele with ileal prolapse through a patent omphalomesenteric duct. A case report and review of literature. Acta Chir Belg. 1988;88:392-394.
The solution to EHR woes: A team-based care model
For some time, electronic health records (EHRs) have been the focus of many articles (“EHR use and patient satisfaction: What we learned.” J Fam Pract. 2015;64:687-696) and the source of great debate (and frustration) in the health care community. But there’s a logical solution to the dilemmas created by EHRs: A team-based care model.1
A fundamental principle of team-based care is that all members of the team work at the top of their skill set. So, with that in mind, most of the duties of EHR management should be delegated to other team members, rather than to the physicians. In our system, every physician works with 2 other people—certified medical assistants or licensed practical nurses—who help with standing orders, protocols, templates, and many of the EHR duties, including a significant portion of team documentation. They do this while recognizing and respecting guidelines from the Centers for Medicare & Medicaid Services and other payers. That leaves the physicians and advanced practice clinicians the time they need to focus on the patient during the visit.
Not surprisingly, patient satisfaction, staff satisfaction, and quality measures are all improving with this model of care. It is proving financially viable, as well. This model may well be the future of health care delivery for office-based practices.2
Jim Jerzak, MD
Green Bay, Wis
1. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
2. Ghorob A, Bodenheimer T. Building teams in primary care: A practical guide. Fam Syst Health. 2015;33:182-192.
For some time, electronic health records (EHRs) have been the focus of many articles (“EHR use and patient satisfaction: What we learned.” J Fam Pract. 2015;64:687-696) and the source of great debate (and frustration) in the health care community. But there’s a logical solution to the dilemmas created by EHRs: A team-based care model.1
A fundamental principle of team-based care is that all members of the team work at the top of their skill set. So, with that in mind, most of the duties of EHR management should be delegated to other team members, rather than to the physicians. In our system, every physician works with 2 other people—certified medical assistants or licensed practical nurses—who help with standing orders, protocols, templates, and many of the EHR duties, including a significant portion of team documentation. They do this while recognizing and respecting guidelines from the Centers for Medicare & Medicaid Services and other payers. That leaves the physicians and advanced practice clinicians the time they need to focus on the patient during the visit.
Not surprisingly, patient satisfaction, staff satisfaction, and quality measures are all improving with this model of care. It is proving financially viable, as well. This model may well be the future of health care delivery for office-based practices.2
Jim Jerzak, MD
Green Bay, Wis
1. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
2. Ghorob A, Bodenheimer T. Building teams in primary care: A practical guide. Fam Syst Health. 2015;33:182-192.
For some time, electronic health records (EHRs) have been the focus of many articles (“EHR use and patient satisfaction: What we learned.” J Fam Pract. 2015;64:687-696) and the source of great debate (and frustration) in the health care community. But there’s a logical solution to the dilemmas created by EHRs: A team-based care model.1
A fundamental principle of team-based care is that all members of the team work at the top of their skill set. So, with that in mind, most of the duties of EHR management should be delegated to other team members, rather than to the physicians. In our system, every physician works with 2 other people—certified medical assistants or licensed practical nurses—who help with standing orders, protocols, templates, and many of the EHR duties, including a significant portion of team documentation. They do this while recognizing and respecting guidelines from the Centers for Medicare & Medicaid Services and other payers. That leaves the physicians and advanced practice clinicians the time they need to focus on the patient during the visit.
Not surprisingly, patient satisfaction, staff satisfaction, and quality measures are all improving with this model of care. It is proving financially viable, as well. This model may well be the future of health care delivery for office-based practices.2
Jim Jerzak, MD
Green Bay, Wis
1. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
2. Ghorob A, Bodenheimer T. Building teams in primary care: A practical guide. Fam Syst Health. 2015;33:182-192.
Personality disorders: A measured response
› Maintain a high index of suspicion for personality disorders (PDs) in patients who appear to be “difficult,” and take care to distinguish these diagnoses from primary mood, anxiety, and psychotic disorders. C
› Refer patients with PDs for psychotherapy, as it is considered the mainstay of treatment—particularly for borderline PD. B
› Use pharmacotherapy judiciously as an adjunctive treatment for PD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Personality disorders (PDs) are common, affecting up to 15% of US adults, and are associated with comorbid medical and psychiatric conditions and increased utilization of health care resources.1,2 Having a basic understanding of these patterns of thinking and behaving can help family physicians (FPs) identify specific PD diagnoses, ensure appropriate treatment, and reduce the frustration that arises when an individual is viewed as a “difficult patient.”
Here we describe the diagnostic features of the disorders in the 3 major clusters of PDs and review an effective approach to the management of the most common disorder in each cluster, using a case study patient.
Defense mechanisms offer clues that your patient may have a PD
Personality is an enduring pattern of inner experience and behaviors that is relatively stable across time and in different situations. Such traits comprise an individual’s inherent makeup.1 PDs are diagnosed when an individual’s personality traits create significant distress or impairment in daily functioning. Specifically, PDs have a negative impact on cognition, affect, interpersonal relationships, and/or impulse control.1
One of the ways people alleviate distress is by using defense mechanisms. Defense mechanisms are unconscious mental processes that individuals use to resolve conflicts, and thereby reduce anxiety and depression on a conscious level. Taken alone, defense mechanisms are not pathologic, but they may become maladaptive in certain stressful circumstances, such as when receiving medical treatment. Recognizing patterns of chronic use of certain defense mechanisms may be a clue that your patient has a PD. TABLE 13,4 and TABLE 23,4 provide an overview of common defense mechanisms used by patients with PDs.
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) organizes PDs into 3 clusters based on similar and often overlapping symptoms.1TABLE 31 provides a brief summary of the characteristic features of each disorder in these clusters.
Cluster A: Odd, eccentric
Patients with one of these disorders are odd, eccentric, or bizarre in their behavior and thinking. There appears to be a genetic link between cluster A PDs (especially schizotypal) and schizophrenia.5 These patients rarely seek treatment for their disorder because they have limited insight into their maladaptive traits.5,6
CASE 1 › Daniel A, age 57, has hypertension and hyperlipidemia and comes in to see his FP for a 6-month follow-up appointment. He never misses appointments, but has a history of poor adherence with prescribed medications. He enjoys his discussions with you in the office, although he often perseverates on conspiracy theories. He lives alone and has never been married. He believes that some of the previously prescribed medications, including a statin and a thiazide diuretic, were interfering with the absorption of “positive nutrients” in his diet. He also refuses to take the generic form of a statin, which he believes was adulterated by the government to be sold at lower cost.
Mr. A demonstrates the odd and eccentric beliefs that characterize schizotypal personality disorder. How can his FP best help him adhere to his medication regimen? (For the answer, click here.)
Schizotypal personality disorder shares certain disturbances of thought with schizophrenia, and is believed to exist on a spectrum with other primary psychotic disorders. Support for this theory comes from the higher rates of schizotypal PD among family members of patients with schizophrenia. There is a genetic component to the disorder.3,5,6
Clinically, these patients appear odd and eccentric with unusual beliefs. They may have a fascination with magic, clairvoyance, telepathy, or other such notions.1,5 Although the perceptual disturbances are unusual and often bizarre, they are not frank delusions: patients with schizotypal PD are willing to consider alternative explanations for their beliefs and can engage in rational discussion. Cognitive deficits, particularly of memory and attention, are common and distressing to patients. Frequently, the presenting complaint is depression and anxiety due to the emotional discord and isolation from others.1,3,5,6
Continue to cluster B >>
Cluster B: Dramatic, erratic
Patients with cluster B PDs are dramatic, excessively emotional, confrontational, erratic, and impulsive in their behaviors.1 They often have comorbid mood and anxiety disorders, as well as a disproportionately high co-occurrence of functional disorders.3,7 Their rates of health care utilization can be substantial. Because individuals with one of these PDs sometimes exhibit reckless and impulsive behavior, physicians should be aware these patients have a high risk of physical injuries (fights, accidents, self-injurious behavior), suicide attempts, risky sexual behaviors, and unplanned pregnancy.8,9
CASE 2 › Sheryl B is a 34-year-old new patient with a history of irritable bowel syndrome, fibromyalgia, depression, and anxiety who shows up for her appointment an hour late. She is upset and blames the office scheduler for not reminding her of the appointment. She brings a list of medications from her previous physician that includes sertraline, clonazepam, gabapentin, oxycodone, and as-needed alprazolam. She insists that her physician increase the dose of the benzodiazepines.
A review of her medical history reveals diagnoses of anxiety, bipolar disorder, and posttraumatic stress disorder. Ms. B has also engaged in superficial cutting since adolescence, often triggered by arguments with her boyfriend. Currently, she attributes her anxiety and pain to not receiving the “correct medications” because of her transition from a previous physician who “knew her better than any other doctor.” After the FP explains to Ms. B that he would have to carefully review her case before continuing to prescribe benzodiazepines, she becomes tearful and argumentative, proclaiming, “You won’t give me the only thing that will help me because you want me to be miserable!”
Ms. B exhibits many cluster B personality traits consistent with borderline PD. How should the FP respond to her claims? (For the answer, click here.)
Borderline PD is the most studied of the PDs. It can be a stigmatizing diagnosis, and even experienced psychiatrists may hesitate to inform patients of this diagnosis.10 Patients with borderline PD may be erroneously diagnosed with bipolar disorder, treatment-resistant depression, or posttraumatic stress disorder because of a complicated clinical presentation, physician unfamiliarity with diagnostic criteria, or the presence of genuine comorbid conditions.3,11
The etiology of this disorder appears to be multifactorial, and includes genetic predisposition, disruptive parent-child relationships (especially separation), and, often, past sexual or physical trauma.9,12
Predominant clinical features include emotional lability, efforts to avoid abandonment, extremes of idealization and devaluation, unstable and intense interpersonal relationships, and impulsivity.1 Characteristically, these patients also engage in self-injurious behaviors.13,14 Common defense mechanisms used by patients with borderline PD include splitting (viewing others as either all good or all bad), acting out (yelling, agitation, or violence), and passive aggression (TABLE 13,4).
Cluster C: Anxious, fearful
Individuals with cluster C PDs appear anxious, fearful, and worried. They have features that overlap with anxiety disorders.15
CASE 3 › Judy C is a 40-year-old lawyer with a history of gastroesophageal reflux disorder, hypertension, and anxiety who presents for a 3-week follow-up visit after starting sertraline. The patient describes herself as a perfectionist who has increased work-related stress recently because she has to “do extra work for my colleagues who don’t know how to get things done right.” She recently fired her assistant for “not understanding my filing system.” She appears formal and serious, often looking at her watch during the evaluation.
Ms. C demonstrates a pattern of perfectionism, formality, and rigidity in thought and behavior characteristic of obsessive-compulsive PD. What treatment should her physician recommend? (For the answer, click here.)
Obsessive-compulsive PD. Although this disorder is associated with significant anxiety, patients often view the specific traits of obsessive-compulsive PD, such as perfectionism, as desirable. Neurotic defense mechanisms are common, especially rationalization, intellectualization, and isolation of affect (TABLE 23,4). These patients appear formal, rigid, and serious, and are preoccupied with rules and orderliness to achieve perfection.1 Significant anxiety often arises from fear of making mistakes and ruminating on decision-making.1,11,15
Although some overlap exists between obsessive-compulsive disorder (OCD) and obsessive-compulsive PD, patients with OCD exhibit distinct obsessions and associated compulsive behavior, whereas those with obsessive-compulsive PD do not.1
In terms of treatment, it is generally appropriate to recognize the 2 conditions as distinct entities.15 OCD responds well to cognitive behavioral therapies and high-dose selective serotonin reuptake inhibitors (SSRIs).16 In contrast, there is little data that suggests antidepressants are effective for obsessive-compulsive PD, and treatment is aimed at addressing comorbid anxiety with psychotherapy and pharmacotherapy, if needed.11,15
Continue to psychotherapy for PD is the first-line treatment >>
Psychotherapy for PD is the first-line treatment
Psychotherapy is the most effective treatment for PDs.11,17,18 Several psychotherapies are used to treat these disorders, including dialectical behavioral therapy, schema therapy, and cognitive behavioral therapy (CBT). A recent study demonstrated the superiority of several evidence-based psychotherapies for PD compared to treatment-as-usual.17 Even more promising is that certain benefits have been demonstrated when psychotherapy is provided by clinicians without advanced mental health training.19-21 However, the benefits of therapies for specific disorders are often limited by lack of available data, patient preference, and accessibility of resources.
Limited evidence supports pharmacotherapy
The use of pharmacotherapy for treating PDs is common, although there’s limited evidence to support the practice.11,22 Certain circumstances may allow for the judicious use of medication, although prescribing strategies are based largely on clinical experience and expert opinion.
Prescribers should emphasize a realistic perspective on treatment response, because research suggests at best a mild-moderate response of some personality traits to pharmacotherapy.11,22-25 There is no evidence for polypharmacy in treating PDs, and FPs should allow for sufficient treatment duration, switch medications rather than augment ineffective treatments, and resist the urge to prescribe for every psychological crisis.11,22,25,26
Patient safety should always be a consideration when prescribing medication. Because use of second-generation antipsychotics is associated with the metabolic syndrome, the patient’s baseline weight and fasting glucose, lipids, and hemoglobin A1c levels should be obtained and monitored regularly. Weight gain can be particularly distressing to patients, increase stress and anxiety, and hinder the doctor-patient relationship.25 Finally, medications with abuse potential or that can be lethal in overdose (eg, tricyclic antidepressants and benzodiazepines) are best avoided in patients with emotional lability and impulsivity.25,26
Tailor treatment to the specific PD
Tx for cluster A disorders. Few studies have examined the effectiveness of psychotherapies for cluster A disorders. Cognitive therapy may have benefit in addressing cognitive distortions and social impairment in schizotypal PD.11,12,22 There is little evidence supporting psychotherapy for paranoid PD, because challenging patients’ beliefs in this form is likely to exacerbate paranoia. Low-dose risperidone has demonstrated some beneficial effects on perceptual disturbances; however, the adverse metabolic effects of this medication may outweigh any potential benefit, as these symptoms are often not distressing to patients.6,27 In comparison, patients often find deficits in memory and attention to be more bothersome, and some data suggest that the alpha-2 agonist guanfacine may help treat these symptoms.28
Tx for cluster B disorders. Several forms of psychotherapy have proven effective in managing symptoms and improving overall functioning in patients with borderline PD, including dialectical behavioral therapy, mentalization-based therapy, transference-focused therapy, and schema therapy.29 Dialectical behavioral therapy is often the initial treatment because it emphasizes reducing self-harm behaviors and emotion regulation.11,17,26
Gunderson19 developed a more basic approach to treating borderline PD that is intended to be used by all clinicians who treat the disorder, and not just mental health professionals with advanced training in psychotherapy. A large, multisite randomized controlled trial found that the clinical efficacy of the technique, known as good psychiatric management, rivaled that of dialectical behavioral therapy.20,21
The general premise is that clinicians foster a therapeutic relationship that is supportive, engaging, and flexible. Physicians are encouraged to educate patients about the disorder and emphasize improvement in daily functioning. Clinicians should share the diagnosis with patients, which may give patients a sense of relief in having an accurate diagnosis and allow them to fully invest in diagnosis-specific treatments.19
Systematic reviews and meta-analyses of studies that evaluated pharmacotherapy for borderline PD often have had conflicting conclusions as a result of analyzing data from underpowered studies with varying study designs.23,24,26,30,31 In targeting specific symptoms of the disorder, the most consistent evidence has supported the use of antipsychotics for cognitive perceptual disturbances; patients commonly experience depersonalization or out-of-body experiences.25 Additionally, the use of antipsychotics and mood stabilizers (lamotrigine and topiramate) appears to be somewhat effective for managing emotional lability and impulsivity.26,32,33 Despite the widespread use of SSRIs, a recent systematic review found the least support for these and other antidepressants for management of borderline PD.25
Tx for cluster C disorders. Some evidence supports using cognitive and interpersonal psychotherapies to treat cluster C PDs.34 In contrast, there is little evidence to support the use of pharmacotherapy.35 However, given the significant overlap among these disorders (especially avoidant PD) and social phobia and generalized anxiety disorder, effective pharmacologic strategies can be inferred based on data for those conditions.11 SSRIs, serotonin-norepinephrine reuptake inhibitors (eg, venlafaxine), and gabapentin have demonstrated efficacy in anxiety disorders and are reasonable and safe initial treatments for patients with a cluster C PD.11,34
Continue for the answers >>
CASE 1 › Mr. A’s schizotypal PD symptoms interfere with medication adherence because of his unusual belief system. Importantly, unlike patients with frank delusions, patients with schizotypal PD are willing to consider alternative explanations for their unusual beliefs. Mr. A’s intense suspiciousness may indicate some degree of overlap between paranoid and schizotypal PDs.
The FP is patient and willing to listen to Mr. A’s beliefs without devaluing them. To improve medication adherence, the FP offers him reasonable alternatives with clear explanations. (“I understand you have concerns about previous medications. At the same time, it seems that managing your blood pressure and cholesterol is important to you. Can we discuss alternative treatments?”)
CASE 2 › In response to Ms. B’s borderline PD, the FP must be cautious to avoid reacting out of frustration, which may upset the patient and validate her mistrust. The FP first reflects her anger (“I can tell you are upset because you don’t think I want to help you”), which may allow her to calmly engage in a discussion. He wants to recognize Ms. B’s dramatic behavior, but not reward it with added attention and unreasonable concessions. To help establish rapport, he provides a statement to legitimize Ms. B’s concerns (“Many patients would be frustrated during the process of changing physicians”).
The FP listens empathically to Ms. B, sets clear limits, and provides consistent and evidence-based treatments. He also provides early referral to psychotherapy, but to mitigate any perceived abandonment, he assures Ms. B he will remain involved with her treatment. (“It sounds like managing your anxiety is important to you, and often psychiatrists or therapists can help give additional options for treatment. I want you to know that I am still your doctor and we can review their recommendations together at our next visit.”)
CASE 3 › The FP recognizes that Ms. C’s pattern of perfectionism, formality, and rigidity in thought and behavior are likely a manifestation of obsessive-compulsive PD, and that the maladaptive psychological traits underlying her anxiety are distinct from a primary anxiety disorder.
An SSRI may be a reasonable option to treat Ms. B’s anxiety, and the FP also refers her for CBT. (“I can tell you are feeling really anxious and many people feel that way, especially with work. I think the medication is a good start, but I wonder if we could discuss other forms of therapy to maximize your symptom improvement.”) Because of their exacting nature, many patients with cluster C personality traits are willing to engage in treatments, especially if they are supported by data and recommended by a knowledgeable physician.
CORRESPONDENCE
Nicholas Morcos, Department of Psychiatry, University of Michigan Health System, 1500 East Medical Center Drive, Ann Arbor, MI 48109; [email protected].
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162:1911-1918.
3. Cloninger C, Svrakie D. Personality disorders. In: Sadock BJ, Sadock VA, Ruiz P, eds. Kaplan & Sadock’s synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, Pa: Wolters Kluwer; 2015:2197-2240.
4. Bowins B. Personality disorders: a dimensional defense mechanism approach. Am J Psychother. 2010;64:153-169.
5. Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291-326.
6. Rosell DR, Futterman SE, McMaster A, et al. Schizotypal personality disorder: a current review. Curr Psychiatry Rep. 2014;16:452.
7. Gabbard GO, Simonsen E. Complex Case: The impact of personality and personality disorders on the treatment of depression. Personal Ment Health. 2007;1:161-175.
8. Caspi A, Begg D, Dickson N, et al. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997;73:1052-1063.
9. Tomko RL, Trull TJ, Wood PK, et al. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Pers Disord. 2014;28:734-750.
10. Vaillant GE. The beginning of wisdom is never calling a patient a borderline; or, the clinical management of immature defenses in the treatment of individuals with personality disorders. J Psychother Pract Res. 1992;1:117-134.
11. Bateman AW, Gunderson J, Mulder R. Treatment of personality disorder. Lancet. 2015;385:735-743.
12. Beck AT, Davis DD, Freeman A, eds. Cognitive therapy of personality disorders. 3rd ed. New York, NY: Guilford Press, 2015.
13. O’Connor RC, Nock MK. The psychology of suicidal behaviour. Lancet Psychiatry. 2014;1:73-85.
14. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13:179-185.
15. Diedrich A, Voderholzer U. Obsessive-compulsive personality disorder: a current review. Curr Psychiatry Rep. 2015;17:2.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37:375-391.
17. Budge SL, Moore JT, Del Re AC, et al. The effectiveness of evidence-based treatments for personality disorders when comparing treatment-as-usual and bona fide treatments. Clin Psychol Rev. 2013;33:1057-1066.
18. Leichsenring F, Leibing E. The effectiveness of psychodynamic therapy and cognitive behavior therapy in the treatment of personality disorders: a meta-analysis. Am J Psychiatry. 2003;160:1223-1232.
19. Gunderson JG, Links PS. Handbook of good psychiatric management for borderline personality disorder. Washington, DC: American Psychiatric Publishing, 2014.
20. McMain SF, Links PS, Gnam WH, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. Am J Psychiatry. 2009;166:1365-1374.
21. McMain SF, Guimond T, Streiner DL, et al. Dialectical behavior therapy compared with general psychiatric management for borderline personality disorder: clinical outcomes and functioning over a 2-year follow-up. Am J Psychiatry. 2012;169:650-661.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14:1257-1288.
23. Coccaro EF. Clinical outcome of psychopharmacologic treatment of borderline and schizotypal personality disordered subjects. J Clin Psychiatry. 1998;59:30-35.
24. Soloff PH. Algorithms for pharmacological treatment of personality dimensions: symptom-specific treatments for cognitive-perceptual, affective, and impulsive-behavioral dysregulation. Bull Menninger Clin. 1998;62:195-214.
25. Silk KR. The process of managing medications in patients with borderline personality disorder. J Psychiatr Pract. 2011;17:311-319.
26. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29:461-467.
27. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64:628-634.
28. McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61:1157-1160.
29. Stoffers JM, Vollm BA, Rucker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;8:CD005652.
30. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry. 1991;148:1647-1658.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006:CD005653.
32. Nickel MK, Nickel C, Kaplan P, et al. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biol Psychiatry. 2005;57:495-499.
33. Tritt K, Nickel C, Lahmann C, et al. Lamotrigine treatment of aggression in female borderline-patients: a randomized, double-blind, placebo-controlled study. J Psychopharmacol. 2005;19:287-291.
34. Simon W. Follow-up psychotherapy outcome of patients with dependent, avoidant and obsessive-compulsive personality disorders: A meta-analytic review. Int J Psychiatry Clin Pract. 2009;13:153-165.
35. Ansseau M, Troisfontaines B, Papart P, et al. Compulsive personality as predictor of response to serotoninergic antidepressants. BMJ. 1991;303:760-761.
› Maintain a high index of suspicion for personality disorders (PDs) in patients who appear to be “difficult,” and take care to distinguish these diagnoses from primary mood, anxiety, and psychotic disorders. C
› Refer patients with PDs for psychotherapy, as it is considered the mainstay of treatment—particularly for borderline PD. B
› Use pharmacotherapy judiciously as an adjunctive treatment for PD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Personality disorders (PDs) are common, affecting up to 15% of US adults, and are associated with comorbid medical and psychiatric conditions and increased utilization of health care resources.1,2 Having a basic understanding of these patterns of thinking and behaving can help family physicians (FPs) identify specific PD diagnoses, ensure appropriate treatment, and reduce the frustration that arises when an individual is viewed as a “difficult patient.”
Here we describe the diagnostic features of the disorders in the 3 major clusters of PDs and review an effective approach to the management of the most common disorder in each cluster, using a case study patient.
Defense mechanisms offer clues that your patient may have a PD
Personality is an enduring pattern of inner experience and behaviors that is relatively stable across time and in different situations. Such traits comprise an individual’s inherent makeup.1 PDs are diagnosed when an individual’s personality traits create significant distress or impairment in daily functioning. Specifically, PDs have a negative impact on cognition, affect, interpersonal relationships, and/or impulse control.1
One of the ways people alleviate distress is by using defense mechanisms. Defense mechanisms are unconscious mental processes that individuals use to resolve conflicts, and thereby reduce anxiety and depression on a conscious level. Taken alone, defense mechanisms are not pathologic, but they may become maladaptive in certain stressful circumstances, such as when receiving medical treatment. Recognizing patterns of chronic use of certain defense mechanisms may be a clue that your patient has a PD. TABLE 13,4 and TABLE 23,4 provide an overview of common defense mechanisms used by patients with PDs.
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) organizes PDs into 3 clusters based on similar and often overlapping symptoms.1TABLE 31 provides a brief summary of the characteristic features of each disorder in these clusters.
Cluster A: Odd, eccentric
Patients with one of these disorders are odd, eccentric, or bizarre in their behavior and thinking. There appears to be a genetic link between cluster A PDs (especially schizotypal) and schizophrenia.5 These patients rarely seek treatment for their disorder because they have limited insight into their maladaptive traits.5,6
CASE 1 › Daniel A, age 57, has hypertension and hyperlipidemia and comes in to see his FP for a 6-month follow-up appointment. He never misses appointments, but has a history of poor adherence with prescribed medications. He enjoys his discussions with you in the office, although he often perseverates on conspiracy theories. He lives alone and has never been married. He believes that some of the previously prescribed medications, including a statin and a thiazide diuretic, were interfering with the absorption of “positive nutrients” in his diet. He also refuses to take the generic form of a statin, which he believes was adulterated by the government to be sold at lower cost.
Mr. A demonstrates the odd and eccentric beliefs that characterize schizotypal personality disorder. How can his FP best help him adhere to his medication regimen? (For the answer, click here.)
Schizotypal personality disorder shares certain disturbances of thought with schizophrenia, and is believed to exist on a spectrum with other primary psychotic disorders. Support for this theory comes from the higher rates of schizotypal PD among family members of patients with schizophrenia. There is a genetic component to the disorder.3,5,6
Clinically, these patients appear odd and eccentric with unusual beliefs. They may have a fascination with magic, clairvoyance, telepathy, or other such notions.1,5 Although the perceptual disturbances are unusual and often bizarre, they are not frank delusions: patients with schizotypal PD are willing to consider alternative explanations for their beliefs and can engage in rational discussion. Cognitive deficits, particularly of memory and attention, are common and distressing to patients. Frequently, the presenting complaint is depression and anxiety due to the emotional discord and isolation from others.1,3,5,6
Continue to cluster B >>
Cluster B: Dramatic, erratic
Patients with cluster B PDs are dramatic, excessively emotional, confrontational, erratic, and impulsive in their behaviors.1 They often have comorbid mood and anxiety disorders, as well as a disproportionately high co-occurrence of functional disorders.3,7 Their rates of health care utilization can be substantial. Because individuals with one of these PDs sometimes exhibit reckless and impulsive behavior, physicians should be aware these patients have a high risk of physical injuries (fights, accidents, self-injurious behavior), suicide attempts, risky sexual behaviors, and unplanned pregnancy.8,9
CASE 2 › Sheryl B is a 34-year-old new patient with a history of irritable bowel syndrome, fibromyalgia, depression, and anxiety who shows up for her appointment an hour late. She is upset and blames the office scheduler for not reminding her of the appointment. She brings a list of medications from her previous physician that includes sertraline, clonazepam, gabapentin, oxycodone, and as-needed alprazolam. She insists that her physician increase the dose of the benzodiazepines.
A review of her medical history reveals diagnoses of anxiety, bipolar disorder, and posttraumatic stress disorder. Ms. B has also engaged in superficial cutting since adolescence, often triggered by arguments with her boyfriend. Currently, she attributes her anxiety and pain to not receiving the “correct medications” because of her transition from a previous physician who “knew her better than any other doctor.” After the FP explains to Ms. B that he would have to carefully review her case before continuing to prescribe benzodiazepines, she becomes tearful and argumentative, proclaiming, “You won’t give me the only thing that will help me because you want me to be miserable!”
Ms. B exhibits many cluster B personality traits consistent with borderline PD. How should the FP respond to her claims? (For the answer, click here.)
Borderline PD is the most studied of the PDs. It can be a stigmatizing diagnosis, and even experienced psychiatrists may hesitate to inform patients of this diagnosis.10 Patients with borderline PD may be erroneously diagnosed with bipolar disorder, treatment-resistant depression, or posttraumatic stress disorder because of a complicated clinical presentation, physician unfamiliarity with diagnostic criteria, or the presence of genuine comorbid conditions.3,11
The etiology of this disorder appears to be multifactorial, and includes genetic predisposition, disruptive parent-child relationships (especially separation), and, often, past sexual or physical trauma.9,12
Predominant clinical features include emotional lability, efforts to avoid abandonment, extremes of idealization and devaluation, unstable and intense interpersonal relationships, and impulsivity.1 Characteristically, these patients also engage in self-injurious behaviors.13,14 Common defense mechanisms used by patients with borderline PD include splitting (viewing others as either all good or all bad), acting out (yelling, agitation, or violence), and passive aggression (TABLE 13,4).
Cluster C: Anxious, fearful
Individuals with cluster C PDs appear anxious, fearful, and worried. They have features that overlap with anxiety disorders.15
CASE 3 › Judy C is a 40-year-old lawyer with a history of gastroesophageal reflux disorder, hypertension, and anxiety who presents for a 3-week follow-up visit after starting sertraline. The patient describes herself as a perfectionist who has increased work-related stress recently because she has to “do extra work for my colleagues who don’t know how to get things done right.” She recently fired her assistant for “not understanding my filing system.” She appears formal and serious, often looking at her watch during the evaluation.
Ms. C demonstrates a pattern of perfectionism, formality, and rigidity in thought and behavior characteristic of obsessive-compulsive PD. What treatment should her physician recommend? (For the answer, click here.)
Obsessive-compulsive PD. Although this disorder is associated with significant anxiety, patients often view the specific traits of obsessive-compulsive PD, such as perfectionism, as desirable. Neurotic defense mechanisms are common, especially rationalization, intellectualization, and isolation of affect (TABLE 23,4). These patients appear formal, rigid, and serious, and are preoccupied with rules and orderliness to achieve perfection.1 Significant anxiety often arises from fear of making mistakes and ruminating on decision-making.1,11,15
Although some overlap exists between obsessive-compulsive disorder (OCD) and obsessive-compulsive PD, patients with OCD exhibit distinct obsessions and associated compulsive behavior, whereas those with obsessive-compulsive PD do not.1
In terms of treatment, it is generally appropriate to recognize the 2 conditions as distinct entities.15 OCD responds well to cognitive behavioral therapies and high-dose selective serotonin reuptake inhibitors (SSRIs).16 In contrast, there is little data that suggests antidepressants are effective for obsessive-compulsive PD, and treatment is aimed at addressing comorbid anxiety with psychotherapy and pharmacotherapy, if needed.11,15
Continue to psychotherapy for PD is the first-line treatment >>
Psychotherapy for PD is the first-line treatment
Psychotherapy is the most effective treatment for PDs.11,17,18 Several psychotherapies are used to treat these disorders, including dialectical behavioral therapy, schema therapy, and cognitive behavioral therapy (CBT). A recent study demonstrated the superiority of several evidence-based psychotherapies for PD compared to treatment-as-usual.17 Even more promising is that certain benefits have been demonstrated when psychotherapy is provided by clinicians without advanced mental health training.19-21 However, the benefits of therapies for specific disorders are often limited by lack of available data, patient preference, and accessibility of resources.
Limited evidence supports pharmacotherapy
The use of pharmacotherapy for treating PDs is common, although there’s limited evidence to support the practice.11,22 Certain circumstances may allow for the judicious use of medication, although prescribing strategies are based largely on clinical experience and expert opinion.
Prescribers should emphasize a realistic perspective on treatment response, because research suggests at best a mild-moderate response of some personality traits to pharmacotherapy.11,22-25 There is no evidence for polypharmacy in treating PDs, and FPs should allow for sufficient treatment duration, switch medications rather than augment ineffective treatments, and resist the urge to prescribe for every psychological crisis.11,22,25,26
Patient safety should always be a consideration when prescribing medication. Because use of second-generation antipsychotics is associated with the metabolic syndrome, the patient’s baseline weight and fasting glucose, lipids, and hemoglobin A1c levels should be obtained and monitored regularly. Weight gain can be particularly distressing to patients, increase stress and anxiety, and hinder the doctor-patient relationship.25 Finally, medications with abuse potential or that can be lethal in overdose (eg, tricyclic antidepressants and benzodiazepines) are best avoided in patients with emotional lability and impulsivity.25,26
Tailor treatment to the specific PD
Tx for cluster A disorders. Few studies have examined the effectiveness of psychotherapies for cluster A disorders. Cognitive therapy may have benefit in addressing cognitive distortions and social impairment in schizotypal PD.11,12,22 There is little evidence supporting psychotherapy for paranoid PD, because challenging patients’ beliefs in this form is likely to exacerbate paranoia. Low-dose risperidone has demonstrated some beneficial effects on perceptual disturbances; however, the adverse metabolic effects of this medication may outweigh any potential benefit, as these symptoms are often not distressing to patients.6,27 In comparison, patients often find deficits in memory and attention to be more bothersome, and some data suggest that the alpha-2 agonist guanfacine may help treat these symptoms.28
Tx for cluster B disorders. Several forms of psychotherapy have proven effective in managing symptoms and improving overall functioning in patients with borderline PD, including dialectical behavioral therapy, mentalization-based therapy, transference-focused therapy, and schema therapy.29 Dialectical behavioral therapy is often the initial treatment because it emphasizes reducing self-harm behaviors and emotion regulation.11,17,26
Gunderson19 developed a more basic approach to treating borderline PD that is intended to be used by all clinicians who treat the disorder, and not just mental health professionals with advanced training in psychotherapy. A large, multisite randomized controlled trial found that the clinical efficacy of the technique, known as good psychiatric management, rivaled that of dialectical behavioral therapy.20,21
The general premise is that clinicians foster a therapeutic relationship that is supportive, engaging, and flexible. Physicians are encouraged to educate patients about the disorder and emphasize improvement in daily functioning. Clinicians should share the diagnosis with patients, which may give patients a sense of relief in having an accurate diagnosis and allow them to fully invest in diagnosis-specific treatments.19
Systematic reviews and meta-analyses of studies that evaluated pharmacotherapy for borderline PD often have had conflicting conclusions as a result of analyzing data from underpowered studies with varying study designs.23,24,26,30,31 In targeting specific symptoms of the disorder, the most consistent evidence has supported the use of antipsychotics for cognitive perceptual disturbances; patients commonly experience depersonalization or out-of-body experiences.25 Additionally, the use of antipsychotics and mood stabilizers (lamotrigine and topiramate) appears to be somewhat effective for managing emotional lability and impulsivity.26,32,33 Despite the widespread use of SSRIs, a recent systematic review found the least support for these and other antidepressants for management of borderline PD.25
Tx for cluster C disorders. Some evidence supports using cognitive and interpersonal psychotherapies to treat cluster C PDs.34 In contrast, there is little evidence to support the use of pharmacotherapy.35 However, given the significant overlap among these disorders (especially avoidant PD) and social phobia and generalized anxiety disorder, effective pharmacologic strategies can be inferred based on data for those conditions.11 SSRIs, serotonin-norepinephrine reuptake inhibitors (eg, venlafaxine), and gabapentin have demonstrated efficacy in anxiety disorders and are reasonable and safe initial treatments for patients with a cluster C PD.11,34
Continue for the answers >>
CASE 1 › Mr. A’s schizotypal PD symptoms interfere with medication adherence because of his unusual belief system. Importantly, unlike patients with frank delusions, patients with schizotypal PD are willing to consider alternative explanations for their unusual beliefs. Mr. A’s intense suspiciousness may indicate some degree of overlap between paranoid and schizotypal PDs.
The FP is patient and willing to listen to Mr. A’s beliefs without devaluing them. To improve medication adherence, the FP offers him reasonable alternatives with clear explanations. (“I understand you have concerns about previous medications. At the same time, it seems that managing your blood pressure and cholesterol is important to you. Can we discuss alternative treatments?”)
CASE 2 › In response to Ms. B’s borderline PD, the FP must be cautious to avoid reacting out of frustration, which may upset the patient and validate her mistrust. The FP first reflects her anger (“I can tell you are upset because you don’t think I want to help you”), which may allow her to calmly engage in a discussion. He wants to recognize Ms. B’s dramatic behavior, but not reward it with added attention and unreasonable concessions. To help establish rapport, he provides a statement to legitimize Ms. B’s concerns (“Many patients would be frustrated during the process of changing physicians”).
The FP listens empathically to Ms. B, sets clear limits, and provides consistent and evidence-based treatments. He also provides early referral to psychotherapy, but to mitigate any perceived abandonment, he assures Ms. B he will remain involved with her treatment. (“It sounds like managing your anxiety is important to you, and often psychiatrists or therapists can help give additional options for treatment. I want you to know that I am still your doctor and we can review their recommendations together at our next visit.”)
CASE 3 › The FP recognizes that Ms. C’s pattern of perfectionism, formality, and rigidity in thought and behavior are likely a manifestation of obsessive-compulsive PD, and that the maladaptive psychological traits underlying her anxiety are distinct from a primary anxiety disorder.
An SSRI may be a reasonable option to treat Ms. B’s anxiety, and the FP also refers her for CBT. (“I can tell you are feeling really anxious and many people feel that way, especially with work. I think the medication is a good start, but I wonder if we could discuss other forms of therapy to maximize your symptom improvement.”) Because of their exacting nature, many patients with cluster C personality traits are willing to engage in treatments, especially if they are supported by data and recommended by a knowledgeable physician.
CORRESPONDENCE
Nicholas Morcos, Department of Psychiatry, University of Michigan Health System, 1500 East Medical Center Drive, Ann Arbor, MI 48109; [email protected].
› Maintain a high index of suspicion for personality disorders (PDs) in patients who appear to be “difficult,” and take care to distinguish these diagnoses from primary mood, anxiety, and psychotic disorders. C
› Refer patients with PDs for psychotherapy, as it is considered the mainstay of treatment—particularly for borderline PD. B
› Use pharmacotherapy judiciously as an adjunctive treatment for PD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Personality disorders (PDs) are common, affecting up to 15% of US adults, and are associated with comorbid medical and psychiatric conditions and increased utilization of health care resources.1,2 Having a basic understanding of these patterns of thinking and behaving can help family physicians (FPs) identify specific PD diagnoses, ensure appropriate treatment, and reduce the frustration that arises when an individual is viewed as a “difficult patient.”
Here we describe the diagnostic features of the disorders in the 3 major clusters of PDs and review an effective approach to the management of the most common disorder in each cluster, using a case study patient.
Defense mechanisms offer clues that your patient may have a PD
Personality is an enduring pattern of inner experience and behaviors that is relatively stable across time and in different situations. Such traits comprise an individual’s inherent makeup.1 PDs are diagnosed when an individual’s personality traits create significant distress or impairment in daily functioning. Specifically, PDs have a negative impact on cognition, affect, interpersonal relationships, and/or impulse control.1
One of the ways people alleviate distress is by using defense mechanisms. Defense mechanisms are unconscious mental processes that individuals use to resolve conflicts, and thereby reduce anxiety and depression on a conscious level. Taken alone, defense mechanisms are not pathologic, but they may become maladaptive in certain stressful circumstances, such as when receiving medical treatment. Recognizing patterns of chronic use of certain defense mechanisms may be a clue that your patient has a PD. TABLE 13,4 and TABLE 23,4 provide an overview of common defense mechanisms used by patients with PDs.
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) organizes PDs into 3 clusters based on similar and often overlapping symptoms.1TABLE 31 provides a brief summary of the characteristic features of each disorder in these clusters.
Cluster A: Odd, eccentric
Patients with one of these disorders are odd, eccentric, or bizarre in their behavior and thinking. There appears to be a genetic link between cluster A PDs (especially schizotypal) and schizophrenia.5 These patients rarely seek treatment for their disorder because they have limited insight into their maladaptive traits.5,6
CASE 1 › Daniel A, age 57, has hypertension and hyperlipidemia and comes in to see his FP for a 6-month follow-up appointment. He never misses appointments, but has a history of poor adherence with prescribed medications. He enjoys his discussions with you in the office, although he often perseverates on conspiracy theories. He lives alone and has never been married. He believes that some of the previously prescribed medications, including a statin and a thiazide diuretic, were interfering with the absorption of “positive nutrients” in his diet. He also refuses to take the generic form of a statin, which he believes was adulterated by the government to be sold at lower cost.
Mr. A demonstrates the odd and eccentric beliefs that characterize schizotypal personality disorder. How can his FP best help him adhere to his medication regimen? (For the answer, click here.)
Schizotypal personality disorder shares certain disturbances of thought with schizophrenia, and is believed to exist on a spectrum with other primary psychotic disorders. Support for this theory comes from the higher rates of schizotypal PD among family members of patients with schizophrenia. There is a genetic component to the disorder.3,5,6
Clinically, these patients appear odd and eccentric with unusual beliefs. They may have a fascination with magic, clairvoyance, telepathy, or other such notions.1,5 Although the perceptual disturbances are unusual and often bizarre, they are not frank delusions: patients with schizotypal PD are willing to consider alternative explanations for their beliefs and can engage in rational discussion. Cognitive deficits, particularly of memory and attention, are common and distressing to patients. Frequently, the presenting complaint is depression and anxiety due to the emotional discord and isolation from others.1,3,5,6
Continue to cluster B >>
Cluster B: Dramatic, erratic
Patients with cluster B PDs are dramatic, excessively emotional, confrontational, erratic, and impulsive in their behaviors.1 They often have comorbid mood and anxiety disorders, as well as a disproportionately high co-occurrence of functional disorders.3,7 Their rates of health care utilization can be substantial. Because individuals with one of these PDs sometimes exhibit reckless and impulsive behavior, physicians should be aware these patients have a high risk of physical injuries (fights, accidents, self-injurious behavior), suicide attempts, risky sexual behaviors, and unplanned pregnancy.8,9
CASE 2 › Sheryl B is a 34-year-old new patient with a history of irritable bowel syndrome, fibromyalgia, depression, and anxiety who shows up for her appointment an hour late. She is upset and blames the office scheduler for not reminding her of the appointment. She brings a list of medications from her previous physician that includes sertraline, clonazepam, gabapentin, oxycodone, and as-needed alprazolam. She insists that her physician increase the dose of the benzodiazepines.
A review of her medical history reveals diagnoses of anxiety, bipolar disorder, and posttraumatic stress disorder. Ms. B has also engaged in superficial cutting since adolescence, often triggered by arguments with her boyfriend. Currently, she attributes her anxiety and pain to not receiving the “correct medications” because of her transition from a previous physician who “knew her better than any other doctor.” After the FP explains to Ms. B that he would have to carefully review her case before continuing to prescribe benzodiazepines, she becomes tearful and argumentative, proclaiming, “You won’t give me the only thing that will help me because you want me to be miserable!”
Ms. B exhibits many cluster B personality traits consistent with borderline PD. How should the FP respond to her claims? (For the answer, click here.)
Borderline PD is the most studied of the PDs. It can be a stigmatizing diagnosis, and even experienced psychiatrists may hesitate to inform patients of this diagnosis.10 Patients with borderline PD may be erroneously diagnosed with bipolar disorder, treatment-resistant depression, or posttraumatic stress disorder because of a complicated clinical presentation, physician unfamiliarity with diagnostic criteria, or the presence of genuine comorbid conditions.3,11
The etiology of this disorder appears to be multifactorial, and includes genetic predisposition, disruptive parent-child relationships (especially separation), and, often, past sexual or physical trauma.9,12
Predominant clinical features include emotional lability, efforts to avoid abandonment, extremes of idealization and devaluation, unstable and intense interpersonal relationships, and impulsivity.1 Characteristically, these patients also engage in self-injurious behaviors.13,14 Common defense mechanisms used by patients with borderline PD include splitting (viewing others as either all good or all bad), acting out (yelling, agitation, or violence), and passive aggression (TABLE 13,4).
Cluster C: Anxious, fearful
Individuals with cluster C PDs appear anxious, fearful, and worried. They have features that overlap with anxiety disorders.15
CASE 3 › Judy C is a 40-year-old lawyer with a history of gastroesophageal reflux disorder, hypertension, and anxiety who presents for a 3-week follow-up visit after starting sertraline. The patient describes herself as a perfectionist who has increased work-related stress recently because she has to “do extra work for my colleagues who don’t know how to get things done right.” She recently fired her assistant for “not understanding my filing system.” She appears formal and serious, often looking at her watch during the evaluation.
Ms. C demonstrates a pattern of perfectionism, formality, and rigidity in thought and behavior characteristic of obsessive-compulsive PD. What treatment should her physician recommend? (For the answer, click here.)
Obsessive-compulsive PD. Although this disorder is associated with significant anxiety, patients often view the specific traits of obsessive-compulsive PD, such as perfectionism, as desirable. Neurotic defense mechanisms are common, especially rationalization, intellectualization, and isolation of affect (TABLE 23,4). These patients appear formal, rigid, and serious, and are preoccupied with rules and orderliness to achieve perfection.1 Significant anxiety often arises from fear of making mistakes and ruminating on decision-making.1,11,15
Although some overlap exists between obsessive-compulsive disorder (OCD) and obsessive-compulsive PD, patients with OCD exhibit distinct obsessions and associated compulsive behavior, whereas those with obsessive-compulsive PD do not.1
In terms of treatment, it is generally appropriate to recognize the 2 conditions as distinct entities.15 OCD responds well to cognitive behavioral therapies and high-dose selective serotonin reuptake inhibitors (SSRIs).16 In contrast, there is little data that suggests antidepressants are effective for obsessive-compulsive PD, and treatment is aimed at addressing comorbid anxiety with psychotherapy and pharmacotherapy, if needed.11,15
Continue to psychotherapy for PD is the first-line treatment >>
Psychotherapy for PD is the first-line treatment
Psychotherapy is the most effective treatment for PDs.11,17,18 Several psychotherapies are used to treat these disorders, including dialectical behavioral therapy, schema therapy, and cognitive behavioral therapy (CBT). A recent study demonstrated the superiority of several evidence-based psychotherapies for PD compared to treatment-as-usual.17 Even more promising is that certain benefits have been demonstrated when psychotherapy is provided by clinicians without advanced mental health training.19-21 However, the benefits of therapies for specific disorders are often limited by lack of available data, patient preference, and accessibility of resources.
Limited evidence supports pharmacotherapy
The use of pharmacotherapy for treating PDs is common, although there’s limited evidence to support the practice.11,22 Certain circumstances may allow for the judicious use of medication, although prescribing strategies are based largely on clinical experience and expert opinion.
Prescribers should emphasize a realistic perspective on treatment response, because research suggests at best a mild-moderate response of some personality traits to pharmacotherapy.11,22-25 There is no evidence for polypharmacy in treating PDs, and FPs should allow for sufficient treatment duration, switch medications rather than augment ineffective treatments, and resist the urge to prescribe for every psychological crisis.11,22,25,26
Patient safety should always be a consideration when prescribing medication. Because use of second-generation antipsychotics is associated with the metabolic syndrome, the patient’s baseline weight and fasting glucose, lipids, and hemoglobin A1c levels should be obtained and monitored regularly. Weight gain can be particularly distressing to patients, increase stress and anxiety, and hinder the doctor-patient relationship.25 Finally, medications with abuse potential or that can be lethal in overdose (eg, tricyclic antidepressants and benzodiazepines) are best avoided in patients with emotional lability and impulsivity.25,26
Tailor treatment to the specific PD
Tx for cluster A disorders. Few studies have examined the effectiveness of psychotherapies for cluster A disorders. Cognitive therapy may have benefit in addressing cognitive distortions and social impairment in schizotypal PD.11,12,22 There is little evidence supporting psychotherapy for paranoid PD, because challenging patients’ beliefs in this form is likely to exacerbate paranoia. Low-dose risperidone has demonstrated some beneficial effects on perceptual disturbances; however, the adverse metabolic effects of this medication may outweigh any potential benefit, as these symptoms are often not distressing to patients.6,27 In comparison, patients often find deficits in memory and attention to be more bothersome, and some data suggest that the alpha-2 agonist guanfacine may help treat these symptoms.28
Tx for cluster B disorders. Several forms of psychotherapy have proven effective in managing symptoms and improving overall functioning in patients with borderline PD, including dialectical behavioral therapy, mentalization-based therapy, transference-focused therapy, and schema therapy.29 Dialectical behavioral therapy is often the initial treatment because it emphasizes reducing self-harm behaviors and emotion regulation.11,17,26
Gunderson19 developed a more basic approach to treating borderline PD that is intended to be used by all clinicians who treat the disorder, and not just mental health professionals with advanced training in psychotherapy. A large, multisite randomized controlled trial found that the clinical efficacy of the technique, known as good psychiatric management, rivaled that of dialectical behavioral therapy.20,21
The general premise is that clinicians foster a therapeutic relationship that is supportive, engaging, and flexible. Physicians are encouraged to educate patients about the disorder and emphasize improvement in daily functioning. Clinicians should share the diagnosis with patients, which may give patients a sense of relief in having an accurate diagnosis and allow them to fully invest in diagnosis-specific treatments.19
Systematic reviews and meta-analyses of studies that evaluated pharmacotherapy for borderline PD often have had conflicting conclusions as a result of analyzing data from underpowered studies with varying study designs.23,24,26,30,31 In targeting specific symptoms of the disorder, the most consistent evidence has supported the use of antipsychotics for cognitive perceptual disturbances; patients commonly experience depersonalization or out-of-body experiences.25 Additionally, the use of antipsychotics and mood stabilizers (lamotrigine and topiramate) appears to be somewhat effective for managing emotional lability and impulsivity.26,32,33 Despite the widespread use of SSRIs, a recent systematic review found the least support for these and other antidepressants for management of borderline PD.25
Tx for cluster C disorders. Some evidence supports using cognitive and interpersonal psychotherapies to treat cluster C PDs.34 In contrast, there is little evidence to support the use of pharmacotherapy.35 However, given the significant overlap among these disorders (especially avoidant PD) and social phobia and generalized anxiety disorder, effective pharmacologic strategies can be inferred based on data for those conditions.11 SSRIs, serotonin-norepinephrine reuptake inhibitors (eg, venlafaxine), and gabapentin have demonstrated efficacy in anxiety disorders and are reasonable and safe initial treatments for patients with a cluster C PD.11,34
Continue for the answers >>
CASE 1 › Mr. A’s schizotypal PD symptoms interfere with medication adherence because of his unusual belief system. Importantly, unlike patients with frank delusions, patients with schizotypal PD are willing to consider alternative explanations for their unusual beliefs. Mr. A’s intense suspiciousness may indicate some degree of overlap between paranoid and schizotypal PDs.
The FP is patient and willing to listen to Mr. A’s beliefs without devaluing them. To improve medication adherence, the FP offers him reasonable alternatives with clear explanations. (“I understand you have concerns about previous medications. At the same time, it seems that managing your blood pressure and cholesterol is important to you. Can we discuss alternative treatments?”)
CASE 2 › In response to Ms. B’s borderline PD, the FP must be cautious to avoid reacting out of frustration, which may upset the patient and validate her mistrust. The FP first reflects her anger (“I can tell you are upset because you don’t think I want to help you”), which may allow her to calmly engage in a discussion. He wants to recognize Ms. B’s dramatic behavior, but not reward it with added attention and unreasonable concessions. To help establish rapport, he provides a statement to legitimize Ms. B’s concerns (“Many patients would be frustrated during the process of changing physicians”).
The FP listens empathically to Ms. B, sets clear limits, and provides consistent and evidence-based treatments. He also provides early referral to psychotherapy, but to mitigate any perceived abandonment, he assures Ms. B he will remain involved with her treatment. (“It sounds like managing your anxiety is important to you, and often psychiatrists or therapists can help give additional options for treatment. I want you to know that I am still your doctor and we can review their recommendations together at our next visit.”)
CASE 3 › The FP recognizes that Ms. C’s pattern of perfectionism, formality, and rigidity in thought and behavior are likely a manifestation of obsessive-compulsive PD, and that the maladaptive psychological traits underlying her anxiety are distinct from a primary anxiety disorder.
An SSRI may be a reasonable option to treat Ms. B’s anxiety, and the FP also refers her for CBT. (“I can tell you are feeling really anxious and many people feel that way, especially with work. I think the medication is a good start, but I wonder if we could discuss other forms of therapy to maximize your symptom improvement.”) Because of their exacting nature, many patients with cluster C personality traits are willing to engage in treatments, especially if they are supported by data and recommended by a knowledgeable physician.
CORRESPONDENCE
Nicholas Morcos, Department of Psychiatry, University of Michigan Health System, 1500 East Medical Center Drive, Ann Arbor, MI 48109; [email protected].
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162:1911-1918.
3. Cloninger C, Svrakie D. Personality disorders. In: Sadock BJ, Sadock VA, Ruiz P, eds. Kaplan & Sadock’s synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, Pa: Wolters Kluwer; 2015:2197-2240.
4. Bowins B. Personality disorders: a dimensional defense mechanism approach. Am J Psychother. 2010;64:153-169.
5. Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291-326.
6. Rosell DR, Futterman SE, McMaster A, et al. Schizotypal personality disorder: a current review. Curr Psychiatry Rep. 2014;16:452.
7. Gabbard GO, Simonsen E. Complex Case: The impact of personality and personality disorders on the treatment of depression. Personal Ment Health. 2007;1:161-175.
8. Caspi A, Begg D, Dickson N, et al. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997;73:1052-1063.
9. Tomko RL, Trull TJ, Wood PK, et al. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Pers Disord. 2014;28:734-750.
10. Vaillant GE. The beginning of wisdom is never calling a patient a borderline; or, the clinical management of immature defenses in the treatment of individuals with personality disorders. J Psychother Pract Res. 1992;1:117-134.
11. Bateman AW, Gunderson J, Mulder R. Treatment of personality disorder. Lancet. 2015;385:735-743.
12. Beck AT, Davis DD, Freeman A, eds. Cognitive therapy of personality disorders. 3rd ed. New York, NY: Guilford Press, 2015.
13. O’Connor RC, Nock MK. The psychology of suicidal behaviour. Lancet Psychiatry. 2014;1:73-85.
14. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13:179-185.
15. Diedrich A, Voderholzer U. Obsessive-compulsive personality disorder: a current review. Curr Psychiatry Rep. 2015;17:2.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37:375-391.
17. Budge SL, Moore JT, Del Re AC, et al. The effectiveness of evidence-based treatments for personality disorders when comparing treatment-as-usual and bona fide treatments. Clin Psychol Rev. 2013;33:1057-1066.
18. Leichsenring F, Leibing E. The effectiveness of psychodynamic therapy and cognitive behavior therapy in the treatment of personality disorders: a meta-analysis. Am J Psychiatry. 2003;160:1223-1232.
19. Gunderson JG, Links PS. Handbook of good psychiatric management for borderline personality disorder. Washington, DC: American Psychiatric Publishing, 2014.
20. McMain SF, Links PS, Gnam WH, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. Am J Psychiatry. 2009;166:1365-1374.
21. McMain SF, Guimond T, Streiner DL, et al. Dialectical behavior therapy compared with general psychiatric management for borderline personality disorder: clinical outcomes and functioning over a 2-year follow-up. Am J Psychiatry. 2012;169:650-661.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14:1257-1288.
23. Coccaro EF. Clinical outcome of psychopharmacologic treatment of borderline and schizotypal personality disordered subjects. J Clin Psychiatry. 1998;59:30-35.
24. Soloff PH. Algorithms for pharmacological treatment of personality dimensions: symptom-specific treatments for cognitive-perceptual, affective, and impulsive-behavioral dysregulation. Bull Menninger Clin. 1998;62:195-214.
25. Silk KR. The process of managing medications in patients with borderline personality disorder. J Psychiatr Pract. 2011;17:311-319.
26. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29:461-467.
27. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64:628-634.
28. McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61:1157-1160.
29. Stoffers JM, Vollm BA, Rucker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;8:CD005652.
30. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry. 1991;148:1647-1658.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006:CD005653.
32. Nickel MK, Nickel C, Kaplan P, et al. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biol Psychiatry. 2005;57:495-499.
33. Tritt K, Nickel C, Lahmann C, et al. Lamotrigine treatment of aggression in female borderline-patients: a randomized, double-blind, placebo-controlled study. J Psychopharmacol. 2005;19:287-291.
34. Simon W. Follow-up psychotherapy outcome of patients with dependent, avoidant and obsessive-compulsive personality disorders: A meta-analytic review. Int J Psychiatry Clin Pract. 2009;13:153-165.
35. Ansseau M, Troisfontaines B, Papart P, et al. Compulsive personality as predictor of response to serotoninergic antidepressants. BMJ. 1991;303:760-761.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162:1911-1918.
3. Cloninger C, Svrakie D. Personality disorders. In: Sadock BJ, Sadock VA, Ruiz P, eds. Kaplan & Sadock’s synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, Pa: Wolters Kluwer; 2015:2197-2240.
4. Bowins B. Personality disorders: a dimensional defense mechanism approach. Am J Psychother. 2010;64:153-169.
5. Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291-326.
6. Rosell DR, Futterman SE, McMaster A, et al. Schizotypal personality disorder: a current review. Curr Psychiatry Rep. 2014;16:452.
7. Gabbard GO, Simonsen E. Complex Case: The impact of personality and personality disorders on the treatment of depression. Personal Ment Health. 2007;1:161-175.
8. Caspi A, Begg D, Dickson N, et al. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997;73:1052-1063.
9. Tomko RL, Trull TJ, Wood PK, et al. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Pers Disord. 2014;28:734-750.
10. Vaillant GE. The beginning of wisdom is never calling a patient a borderline; or, the clinical management of immature defenses in the treatment of individuals with personality disorders. J Psychother Pract Res. 1992;1:117-134.
11. Bateman AW, Gunderson J, Mulder R. Treatment of personality disorder. Lancet. 2015;385:735-743.
12. Beck AT, Davis DD, Freeman A, eds. Cognitive therapy of personality disorders. 3rd ed. New York, NY: Guilford Press, 2015.
13. O’Connor RC, Nock MK. The psychology of suicidal behaviour. Lancet Psychiatry. 2014;1:73-85.
14. Paris J. Understanding self-mutilation in borderline personality disorder. Harv Rev Psychiatry. 2005;13:179-185.
15. Diedrich A, Voderholzer U. Obsessive-compulsive personality disorder: a current review. Curr Psychiatry Rep. 2015;17:2.
16. Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37:375-391.
17. Budge SL, Moore JT, Del Re AC, et al. The effectiveness of evidence-based treatments for personality disorders when comparing treatment-as-usual and bona fide treatments. Clin Psychol Rev. 2013;33:1057-1066.
18. Leichsenring F, Leibing E. The effectiveness of psychodynamic therapy and cognitive behavior therapy in the treatment of personality disorders: a meta-analysis. Am J Psychiatry. 2003;160:1223-1232.
19. Gunderson JG, Links PS. Handbook of good psychiatric management for borderline personality disorder. Washington, DC: American Psychiatric Publishing, 2014.
20. McMain SF, Links PS, Gnam WH, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. Am J Psychiatry. 2009;166:1365-1374.
21. McMain SF, Guimond T, Streiner DL, et al. Dialectical behavior therapy compared with general psychiatric management for borderline personality disorder: clinical outcomes and functioning over a 2-year follow-up. Am J Psychiatry. 2012;169:650-661.
22. Ripoll LH, Triebwasser J, Siever LJ. Evidence-based pharmacotherapy for personality disorders. Int J Neuropsychopharmacol. 2011;14:1257-1288.
23. Coccaro EF. Clinical outcome of psychopharmacologic treatment of borderline and schizotypal personality disordered subjects. J Clin Psychiatry. 1998;59:30-35.
24. Soloff PH. Algorithms for pharmacological treatment of personality dimensions: symptom-specific treatments for cognitive-perceptual, affective, and impulsive-behavioral dysregulation. Bull Menninger Clin. 1998;62:195-214.
25. Silk KR. The process of managing medications in patients with borderline personality disorder. J Psychiatr Pract. 2011;17:311-319.
26. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29:461-467.
27. Koenigsberg HW, Reynolds D, Goodman M, et al. Risperidone in the treatment of schizotypal personality disorder. J Clin Psychiatry. 2003;64:628-634.
28. McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61:1157-1160.
29. Stoffers JM, Vollm BA, Rucker G, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev. 2012;8:CD005652.
30. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry. 1991;148:1647-1658.
31. Binks CA, Fenton M, McCarthy L, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006:CD005653.
32. Nickel MK, Nickel C, Kaplan P, et al. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biol Psychiatry. 2005;57:495-499.
33. Tritt K, Nickel C, Lahmann C, et al. Lamotrigine treatment of aggression in female borderline-patients: a randomized, double-blind, placebo-controlled study. J Psychopharmacol. 2005;19:287-291.
34. Simon W. Follow-up psychotherapy outcome of patients with dependent, avoidant and obsessive-compulsive personality disorders: A meta-analytic review. Int J Psychiatry Clin Pract. 2009;13:153-165.
35. Ansseau M, Troisfontaines B, Papart P, et al. Compulsive personality as predictor of response to serotoninergic antidepressants. BMJ. 1991;303:760-761.
What next when metformin isn't enough for type 2 diabetes?
› Turn first to metformin for pharmacologic treatment of type 2 diabetes. A
› Add a second oral agent (such as a sulfonylurea, thiazolidinedione, sodium-glucose cotransporter-2 inhibitor, or dipeptidyl peptidase 4 inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or basal insulin if metformin at a maximum tolerated dose does not achieve the HbA1c target over 3 months. A
› Progress to bolus mealtime insulin or a GLP-1 agonist to cover postprandial glycemic excursions if HbA1c remains above goal despite an adequate trial of basal insulin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The "Standards of Medical Care in Diabetes" guidelines published in 2015 by the American Diabetes Association (ADA) state that metformin is the preferred initial pharmacotherapy for managing type 2 diabetes.1 Metformin, a biguanide, enhances insulin sensitivity in muscle and fat tissue and inhibits hepatic glucose production. Advantages of metformin include the longstanding research supporting its efficacy and safety, an expected decrease in the glycated hemoglobin (HbA1c) level of 1% to 1.5%, low cost, minimal hypoglycemic risk, and potential reductions in cardiovascular (CV) events due to decreased low-density lipoprotein (LDL) cholesterol.1,2
To minimize adverse gastrointestinal effects, start metformin at 500 mg once or twice a day and titrate upward every one to 2 weeks to the target dose.3 To help guide dosing decisions, use the estimated glomerular filtration rate (eGFR) instead of the serum creatinine (SCr) level, because the SCr can translate into a variable range of eGFRs (TABLE 1).4,5
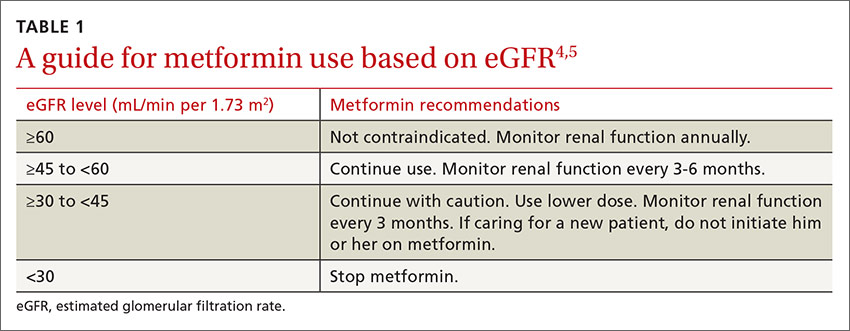
What if metformin alone isn't enough?
CASE › Richard C, age 50, has type 2 diabetes, hypertension, hyperlipidemia, and obesity. He takes metformin 1 g twice a day for his diabetes. After 3 months on this regimen, his HbA1c is 8.8%. How would you manage Mr. C's diabetes going forward?
If metformin at a maximum tolerated dose does not achieve the HbA1c target after 3 months, add a second oral agent (a sulfonylurea [SU], thiazolidinedione [TZD], dipeptidyl peptidase 4 [DPP-4] inhibitor, or sodium-glucose cotransporter-2 [SGLT2] inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or a basal insulin (TABLE 2).1
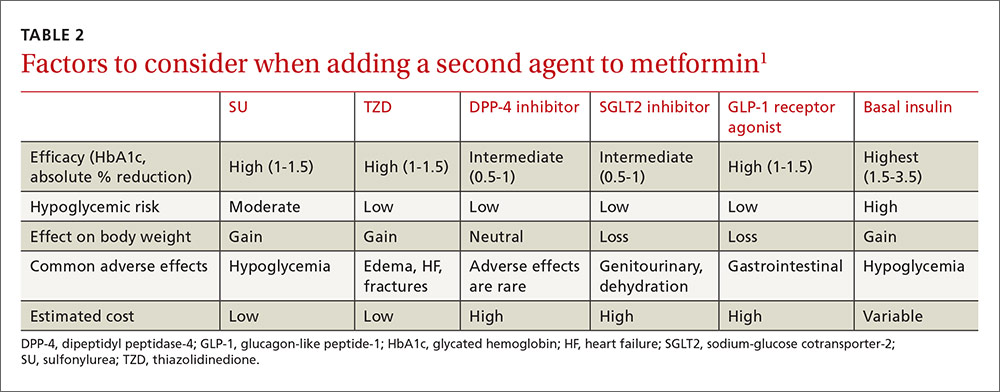
Factors that will affect the choice of the second agent include patient preference, cost, potential adverse effects, impact on weight, efficacy, and risk of hypoglycemia.
Based on cost, familiarity, and longstanding safety data, you decide to give Mr. C an SU, while cautioning him about hypoglycemia.
CASE › Mr. C has now been taking metformin and an SU at maximum doses for 2 years and continues with lifestyle modifications. Though his HbA1c level dropped after adding the SU, over 2 years it has crept up to 8.6% and his mean blood glucose is 186 mg/dL. What are your treatment options now?
If the target HbA1c level is not achieved on dual therapy, consider triple therapy combinations (TABLE 3).1
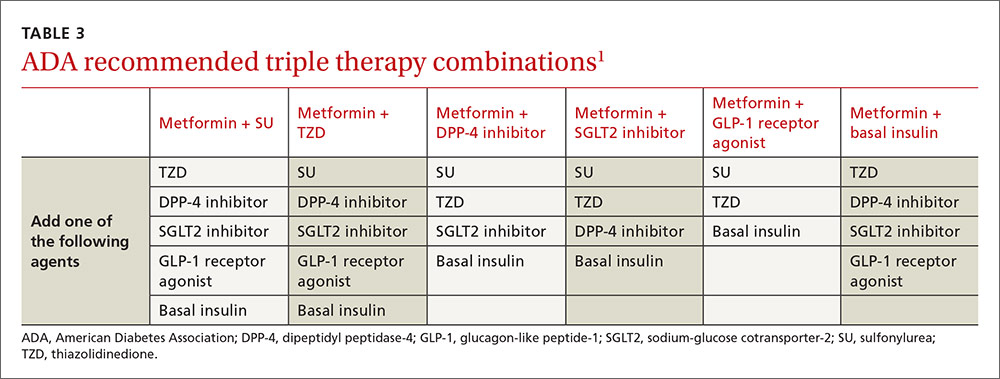
In Mr. C's case, a third oral agent could be added, but DPP-4 and SGLT2 are unlikely to get his HbA1c below 7%. TZD may get his HbA1c into the desired range but is associated with adverse effects such as heart failure, edema, and weight gain. Mr. C agrees instead to start a basal insulin in conjunction with metformin. You could continue the SU, but you decide to stop it because the additive effect of these medications increases the risk of hypoglycemia.
CASE › Six months later Mr. C is taking metformin and insulin glargine, a basal insulin, adjusted to a fasting blood glucose of 80 to 130 mg/dL. His HbA1c is still above target at 8.4%, and the mean postprandial blood glucose is 232 mg/dL.
Mr. C is still above target for HbA1c and for postprandial blood glucose (goal: <180 mg/dL), so he needs pharmacotherapy that targets postprandial glucose elevations.1 His fasting blood glucose readings are at goal, so increasing his insulin glargine is not recommended because it could cause hypoglycemia. An oral agent other than SU could be added, but none is potent enough to lower the HbA1c to goal (TABLE 2).1 There are 3 other options:
- add a mealtime bolus of insulin
- add a GLP-1 receptor agonist
- switch to premixed (biphasic) insulin.
What to do when basal insulin isn’t enough—with or without oral medsFor type 2 diabetes poorly controlled on basal insulin with or without oral agents, the 2015 ADA treatment guidelines recommend adding a GLP-1 receptor agonist or mealtime insulin.1 A less desirable alternative is to switch from basal insulin to a twice-daily premixed (biphasic) insulin analog (70/30 aspart mix or 75/25 or 50/50 lispro mix). The human NPH-Regular premixed formulations (70/30) are less costly alternatives. The disadvantage with all premixed insulins is they only cover 2 postprandial glucose elevations a day.1,6,7
Insulin requires multiple daily injections, can lead to weight gain, and carries the risk of hypoglycemia, which causes significant morbidity.8,9 Daily or weekly administration of a GLP-1 receptor agonist combined with basal insulin can offer a more convenient alternative to mealtime boluses of insulin.
What are GLP-1 receptor agonists?
GLP-1 receptor agonists exert their maximum influence on blood glucose levels during the postprandial period by mimicking the body’s natural incretin hormonal response to oral glucose ingestion.10 They delay gastric emptying, promote satiety, decrease glucagon secretion, and increase insulin secretion.10,11 This mechanism blunts the spiking of postprandial blood glucose after a meal and improves blood glucose control and weight reduction.1,6,7
A systematic review and meta-analysis by Eng and colleagues compared the safety and efficacy of combined GLP-1 agonist and basal insulin with other treatment regimens.7 Fifteen randomized controlled trials were included involving 4348 participants with a mean trial duration of 25 weeks.
Compared with all other treatment regimens, the GLP-1 receptor agonist and basal insulin combination not only significantly reduced HbA1c by 0.44% (95% confidence interval [CI], -0.60 to -0.29) and increased the likelihood of attaining an HbA1c of <7.0% (relative risk [RR]=1.92; 95% CI, 1.43 to 2.56) but also reduced weight by 3.22 kg (-4.90 to -1.54) with no increased risk of hypoglycemia (RR=0.99; 0.76 to 1.29).7
GLP-1 agonist vs bolus insulin
Compared with basal-bolus insulin regimens, the combination of a GLP-1 receptor agonist with basal insulin has led to a significantly lowered risk of hypoglycemia (RR=0.67; 95% CI, 0.56 to 0.80), greater weight loss (-5.66 kg; 95% CI, -9.8 to -1.51) and an average reduction in HbA1c of 0.1% (95% CI, -0.17 to -0.02).7
There are 5 GLP-1 receptor agonists that have US Food and Drug Administration approval for the treatment of type 2 diabetes: albiglutide, dulaglutide, exenatide, exenatide XR, and liraglutide (TABLE 4).3,12
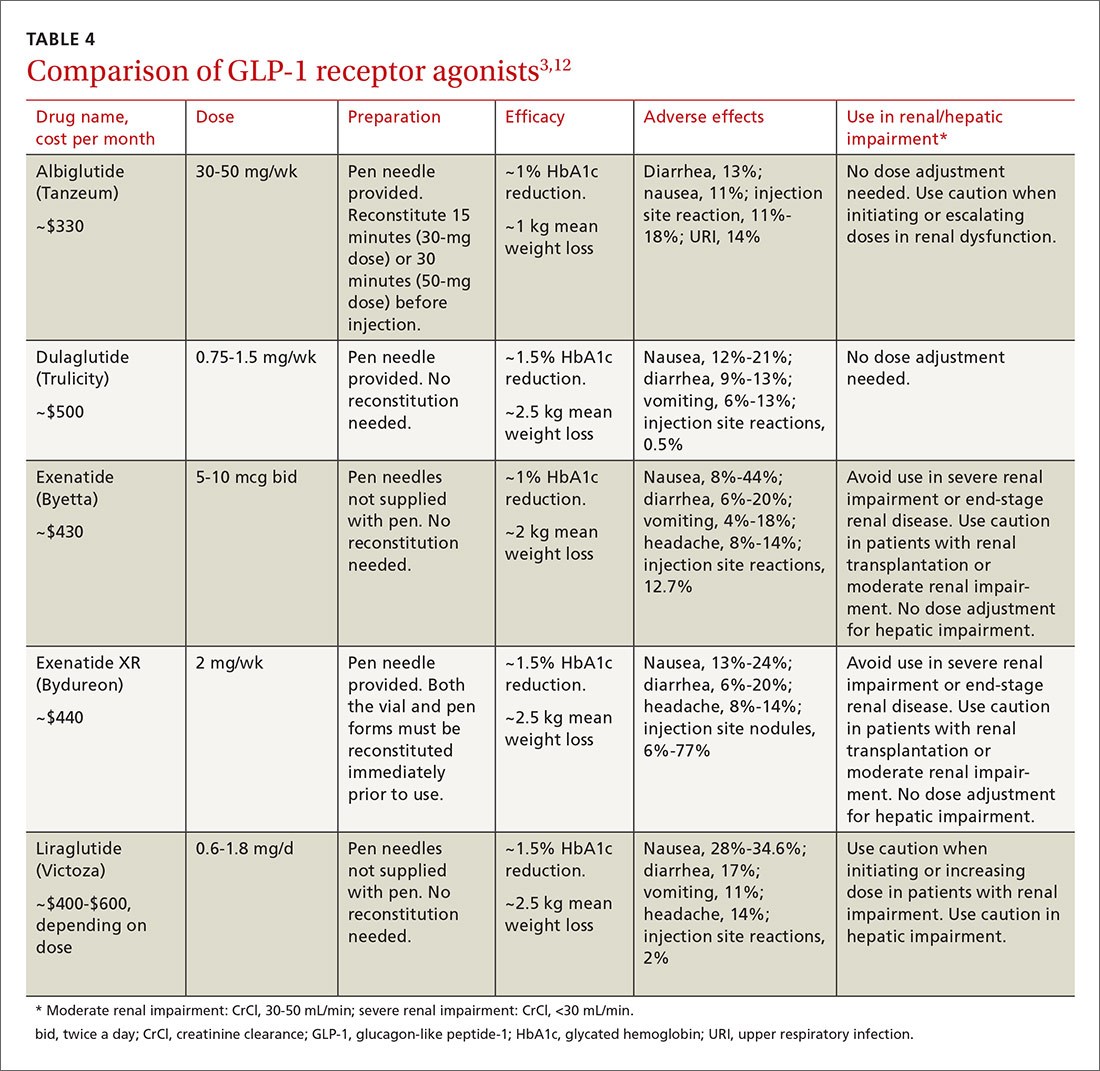
All 5 agents are administered subcutaneously and packaged in pen-injector form. Adverse effects include nausea, which is transient and diminishes within the first few weeks of therapy, and less commonly, pancreatitis.3,12
All of the GLP-1 receptor agonists, except short-acting exenatide, carry a warning about the risk of worsening renal function and a possible association with medullary thyroid carcinomas, which were identified in rats, but have not been observed in humans.3,12 Medications in this drug class have a low risk for precipitating hypoglycemia.11 Cost is their chief disadvantage, although copay reduction cards are available online for most of the products. Evaluate efficacy, ease of use, tolerability, and cost when selecting a GLP-1 receptor agonist.3,12
CASE › Mr. C prefers a more convenient option than adding another daily injection. Given his obesity, a GLP-1 receptor agonist can help with weight loss and lower his risk for hypoglycemia. To further increase the convenience in dosing, you lean toward either weekly exenatide XR or dulaglutide over basal-bolus combination insulin. Weekly albiglutide is less potent than exenatide XR and dulaglutide in decreasing HbA1c.12 Mr. C’s insurance plan provides preferred coverage for exenatide XR and he is eligible for a copay savings card, meaning he will pay no more than $25 per month for this new prescription. You prescribe exenatide XR and ask him to record his postprandial blood glucose levels. You follow up in one month to assess his response.
CORRESPONDENCE
Anne Mounsey, MD, University of North Carolina School of Medicine, Department of Family Medicine, 590 Manning Drive, Campus Box 7595, Chapel Hill, NC 27599; [email protected].
1. American Diabetes Association. Standards of medical care in diabetes - 2015. Diabetes Care. 2015;38 (Suppl):S1-S94.
2. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-613.
3. Merck Manual. Metformin. Available at: http://www.merckmanuals.com/professional/appendixes/brand-names-of-some-commonly-used-drugs. Accessed April 18, 2015.
4. Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431-1437.
5. Philbrick AM, Ernst ME, McDanel DL, et al. Metformin use in renal dysfunction: is a serum creatinine threshold appropriate? Am J Health Syst Pharm. 2009;66:2017-2023.
6. Pharmacist’s Letter. Drugs for Type 2 Diabetes [detail document]. September 2015. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=&s=PL&pt=2&segment=4407&dd=280601. Accessed December 28, 2015.
7. Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228-2234.
8. Inzucchi SE, Burgenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
9. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010:340:b4909.
10. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34 (Suppl 2):S279-S284.
11. Young LA, Buse JB. GLP-1 receptor agonists and basal insulin in type 2 diabetes. Lancet. 2014;384:2180-2181.
12. Pharmacist’s Letter. Comparison of GLP-1 Agonists [detail document]. December 2014. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/Browse.aspx?cs=&s=PL&pt=6&fpt=31&dd=300804&pb=PL&cat=5718#dd. Accessed December 28, 2015.
› Turn first to metformin for pharmacologic treatment of type 2 diabetes. A
› Add a second oral agent (such as a sulfonylurea, thiazolidinedione, sodium-glucose cotransporter-2 inhibitor, or dipeptidyl peptidase 4 inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or basal insulin if metformin at a maximum tolerated dose does not achieve the HbA1c target over 3 months. A
› Progress to bolus mealtime insulin or a GLP-1 agonist to cover postprandial glycemic excursions if HbA1c remains above goal despite an adequate trial of basal insulin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The "Standards of Medical Care in Diabetes" guidelines published in 2015 by the American Diabetes Association (ADA) state that metformin is the preferred initial pharmacotherapy for managing type 2 diabetes.1 Metformin, a biguanide, enhances insulin sensitivity in muscle and fat tissue and inhibits hepatic glucose production. Advantages of metformin include the longstanding research supporting its efficacy and safety, an expected decrease in the glycated hemoglobin (HbA1c) level of 1% to 1.5%, low cost, minimal hypoglycemic risk, and potential reductions in cardiovascular (CV) events due to decreased low-density lipoprotein (LDL) cholesterol.1,2
To minimize adverse gastrointestinal effects, start metformin at 500 mg once or twice a day and titrate upward every one to 2 weeks to the target dose.3 To help guide dosing decisions, use the estimated glomerular filtration rate (eGFR) instead of the serum creatinine (SCr) level, because the SCr can translate into a variable range of eGFRs (TABLE 1).4,5

What if metformin alone isn't enough?
CASE › Richard C, age 50, has type 2 diabetes, hypertension, hyperlipidemia, and obesity. He takes metformin 1 g twice a day for his diabetes. After 3 months on this regimen, his HbA1c is 8.8%. How would you manage Mr. C's diabetes going forward?
If metformin at a maximum tolerated dose does not achieve the HbA1c target after 3 months, add a second oral agent (a sulfonylurea [SU], thiazolidinedione [TZD], dipeptidyl peptidase 4 [DPP-4] inhibitor, or sodium-glucose cotransporter-2 [SGLT2] inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or a basal insulin (TABLE 2).1

Factors that will affect the choice of the second agent include patient preference, cost, potential adverse effects, impact on weight, efficacy, and risk of hypoglycemia.
Based on cost, familiarity, and longstanding safety data, you decide to give Mr. C an SU, while cautioning him about hypoglycemia.
CASE › Mr. C has now been taking metformin and an SU at maximum doses for 2 years and continues with lifestyle modifications. Though his HbA1c level dropped after adding the SU, over 2 years it has crept up to 8.6% and his mean blood glucose is 186 mg/dL. What are your treatment options now?
If the target HbA1c level is not achieved on dual therapy, consider triple therapy combinations (TABLE 3).1

In Mr. C's case, a third oral agent could be added, but DPP-4 and SGLT2 are unlikely to get his HbA1c below 7%. TZD may get his HbA1c into the desired range but is associated with adverse effects such as heart failure, edema, and weight gain. Mr. C agrees instead to start a basal insulin in conjunction with metformin. You could continue the SU, but you decide to stop it because the additive effect of these medications increases the risk of hypoglycemia.
CASE › Six months later Mr. C is taking metformin and insulin glargine, a basal insulin, adjusted to a fasting blood glucose of 80 to 130 mg/dL. His HbA1c is still above target at 8.4%, and the mean postprandial blood glucose is 232 mg/dL.
Mr. C is still above target for HbA1c and for postprandial blood glucose (goal: <180 mg/dL), so he needs pharmacotherapy that targets postprandial glucose elevations.1 His fasting blood glucose readings are at goal, so increasing his insulin glargine is not recommended because it could cause hypoglycemia. An oral agent other than SU could be added, but none is potent enough to lower the HbA1c to goal (TABLE 2).1 There are 3 other options:
- add a mealtime bolus of insulin
- add a GLP-1 receptor agonist
- switch to premixed (biphasic) insulin.
What to do when basal insulin isn’t enough—with or without oral medsFor type 2 diabetes poorly controlled on basal insulin with or without oral agents, the 2015 ADA treatment guidelines recommend adding a GLP-1 receptor agonist or mealtime insulin.1 A less desirable alternative is to switch from basal insulin to a twice-daily premixed (biphasic) insulin analog (70/30 aspart mix or 75/25 or 50/50 lispro mix). The human NPH-Regular premixed formulations (70/30) are less costly alternatives. The disadvantage with all premixed insulins is they only cover 2 postprandial glucose elevations a day.1,6,7
Insulin requires multiple daily injections, can lead to weight gain, and carries the risk of hypoglycemia, which causes significant morbidity.8,9 Daily or weekly administration of a GLP-1 receptor agonist combined with basal insulin can offer a more convenient alternative to mealtime boluses of insulin.
What are GLP-1 receptor agonists?
GLP-1 receptor agonists exert their maximum influence on blood glucose levels during the postprandial period by mimicking the body’s natural incretin hormonal response to oral glucose ingestion.10 They delay gastric emptying, promote satiety, decrease glucagon secretion, and increase insulin secretion.10,11 This mechanism blunts the spiking of postprandial blood glucose after a meal and improves blood glucose control and weight reduction.1,6,7
A systematic review and meta-analysis by Eng and colleagues compared the safety and efficacy of combined GLP-1 agonist and basal insulin with other treatment regimens.7 Fifteen randomized controlled trials were included involving 4348 participants with a mean trial duration of 25 weeks.
Compared with all other treatment regimens, the GLP-1 receptor agonist and basal insulin combination not only significantly reduced HbA1c by 0.44% (95% confidence interval [CI], -0.60 to -0.29) and increased the likelihood of attaining an HbA1c of <7.0% (relative risk [RR]=1.92; 95% CI, 1.43 to 2.56) but also reduced weight by 3.22 kg (-4.90 to -1.54) with no increased risk of hypoglycemia (RR=0.99; 0.76 to 1.29).7
GLP-1 agonist vs bolus insulin
Compared with basal-bolus insulin regimens, the combination of a GLP-1 receptor agonist with basal insulin has led to a significantly lowered risk of hypoglycemia (RR=0.67; 95% CI, 0.56 to 0.80), greater weight loss (-5.66 kg; 95% CI, -9.8 to -1.51) and an average reduction in HbA1c of 0.1% (95% CI, -0.17 to -0.02).7
There are 5 GLP-1 receptor agonists that have US Food and Drug Administration approval for the treatment of type 2 diabetes: albiglutide, dulaglutide, exenatide, exenatide XR, and liraglutide (TABLE 4).3,12

All 5 agents are administered subcutaneously and packaged in pen-injector form. Adverse effects include nausea, which is transient and diminishes within the first few weeks of therapy, and less commonly, pancreatitis.3,12
All of the GLP-1 receptor agonists, except short-acting exenatide, carry a warning about the risk of worsening renal function and a possible association with medullary thyroid carcinomas, which were identified in rats, but have not been observed in humans.3,12 Medications in this drug class have a low risk for precipitating hypoglycemia.11 Cost is their chief disadvantage, although copay reduction cards are available online for most of the products. Evaluate efficacy, ease of use, tolerability, and cost when selecting a GLP-1 receptor agonist.3,12
CASE › Mr. C prefers a more convenient option than adding another daily injection. Given his obesity, a GLP-1 receptor agonist can help with weight loss and lower his risk for hypoglycemia. To further increase the convenience in dosing, you lean toward either weekly exenatide XR or dulaglutide over basal-bolus combination insulin. Weekly albiglutide is less potent than exenatide XR and dulaglutide in decreasing HbA1c.12 Mr. C’s insurance plan provides preferred coverage for exenatide XR and he is eligible for a copay savings card, meaning he will pay no more than $25 per month for this new prescription. You prescribe exenatide XR and ask him to record his postprandial blood glucose levels. You follow up in one month to assess his response.
CORRESPONDENCE
Anne Mounsey, MD, University of North Carolina School of Medicine, Department of Family Medicine, 590 Manning Drive, Campus Box 7595, Chapel Hill, NC 27599; [email protected].
› Turn first to metformin for pharmacologic treatment of type 2 diabetes. A
› Add a second oral agent (such as a sulfonylurea, thiazolidinedione, sodium-glucose cotransporter-2 inhibitor, or dipeptidyl peptidase 4 inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or basal insulin if metformin at a maximum tolerated dose does not achieve the HbA1c target over 3 months. A
› Progress to bolus mealtime insulin or a GLP-1 agonist to cover postprandial glycemic excursions if HbA1c remains above goal despite an adequate trial of basal insulin. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The "Standards of Medical Care in Diabetes" guidelines published in 2015 by the American Diabetes Association (ADA) state that metformin is the preferred initial pharmacotherapy for managing type 2 diabetes.1 Metformin, a biguanide, enhances insulin sensitivity in muscle and fat tissue and inhibits hepatic glucose production. Advantages of metformin include the longstanding research supporting its efficacy and safety, an expected decrease in the glycated hemoglobin (HbA1c) level of 1% to 1.5%, low cost, minimal hypoglycemic risk, and potential reductions in cardiovascular (CV) events due to decreased low-density lipoprotein (LDL) cholesterol.1,2
To minimize adverse gastrointestinal effects, start metformin at 500 mg once or twice a day and titrate upward every one to 2 weeks to the target dose.3 To help guide dosing decisions, use the estimated glomerular filtration rate (eGFR) instead of the serum creatinine (SCr) level, because the SCr can translate into a variable range of eGFRs (TABLE 1).4,5

What if metformin alone isn't enough?
CASE › Richard C, age 50, has type 2 diabetes, hypertension, hyperlipidemia, and obesity. He takes metformin 1 g twice a day for his diabetes. After 3 months on this regimen, his HbA1c is 8.8%. How would you manage Mr. C's diabetes going forward?
If metformin at a maximum tolerated dose does not achieve the HbA1c target after 3 months, add a second oral agent (a sulfonylurea [SU], thiazolidinedione [TZD], dipeptidyl peptidase 4 [DPP-4] inhibitor, or sodium-glucose cotransporter-2 [SGLT2] inhibitor), a glucagon-like peptide-1 (GLP-1) receptor agonist, or a basal insulin (TABLE 2).1

Factors that will affect the choice of the second agent include patient preference, cost, potential adverse effects, impact on weight, efficacy, and risk of hypoglycemia.
Based on cost, familiarity, and longstanding safety data, you decide to give Mr. C an SU, while cautioning him about hypoglycemia.
CASE › Mr. C has now been taking metformin and an SU at maximum doses for 2 years and continues with lifestyle modifications. Though his HbA1c level dropped after adding the SU, over 2 years it has crept up to 8.6% and his mean blood glucose is 186 mg/dL. What are your treatment options now?
If the target HbA1c level is not achieved on dual therapy, consider triple therapy combinations (TABLE 3).1

In Mr. C's case, a third oral agent could be added, but DPP-4 and SGLT2 are unlikely to get his HbA1c below 7%. TZD may get his HbA1c into the desired range but is associated with adverse effects such as heart failure, edema, and weight gain. Mr. C agrees instead to start a basal insulin in conjunction with metformin. You could continue the SU, but you decide to stop it because the additive effect of these medications increases the risk of hypoglycemia.
CASE › Six months later Mr. C is taking metformin and insulin glargine, a basal insulin, adjusted to a fasting blood glucose of 80 to 130 mg/dL. His HbA1c is still above target at 8.4%, and the mean postprandial blood glucose is 232 mg/dL.
Mr. C is still above target for HbA1c and for postprandial blood glucose (goal: <180 mg/dL), so he needs pharmacotherapy that targets postprandial glucose elevations.1 His fasting blood glucose readings are at goal, so increasing his insulin glargine is not recommended because it could cause hypoglycemia. An oral agent other than SU could be added, but none is potent enough to lower the HbA1c to goal (TABLE 2).1 There are 3 other options:
- add a mealtime bolus of insulin
- add a GLP-1 receptor agonist
- switch to premixed (biphasic) insulin.
What to do when basal insulin isn’t enough—with or without oral medsFor type 2 diabetes poorly controlled on basal insulin with or without oral agents, the 2015 ADA treatment guidelines recommend adding a GLP-1 receptor agonist or mealtime insulin.1 A less desirable alternative is to switch from basal insulin to a twice-daily premixed (biphasic) insulin analog (70/30 aspart mix or 75/25 or 50/50 lispro mix). The human NPH-Regular premixed formulations (70/30) are less costly alternatives. The disadvantage with all premixed insulins is they only cover 2 postprandial glucose elevations a day.1,6,7
Insulin requires multiple daily injections, can lead to weight gain, and carries the risk of hypoglycemia, which causes significant morbidity.8,9 Daily or weekly administration of a GLP-1 receptor agonist combined with basal insulin can offer a more convenient alternative to mealtime boluses of insulin.
What are GLP-1 receptor agonists?
GLP-1 receptor agonists exert their maximum influence on blood glucose levels during the postprandial period by mimicking the body’s natural incretin hormonal response to oral glucose ingestion.10 They delay gastric emptying, promote satiety, decrease glucagon secretion, and increase insulin secretion.10,11 This mechanism blunts the spiking of postprandial blood glucose after a meal and improves blood glucose control and weight reduction.1,6,7
A systematic review and meta-analysis by Eng and colleagues compared the safety and efficacy of combined GLP-1 agonist and basal insulin with other treatment regimens.7 Fifteen randomized controlled trials were included involving 4348 participants with a mean trial duration of 25 weeks.
Compared with all other treatment regimens, the GLP-1 receptor agonist and basal insulin combination not only significantly reduced HbA1c by 0.44% (95% confidence interval [CI], -0.60 to -0.29) and increased the likelihood of attaining an HbA1c of <7.0% (relative risk [RR]=1.92; 95% CI, 1.43 to 2.56) but also reduced weight by 3.22 kg (-4.90 to -1.54) with no increased risk of hypoglycemia (RR=0.99; 0.76 to 1.29).7
GLP-1 agonist vs bolus insulin
Compared with basal-bolus insulin regimens, the combination of a GLP-1 receptor agonist with basal insulin has led to a significantly lowered risk of hypoglycemia (RR=0.67; 95% CI, 0.56 to 0.80), greater weight loss (-5.66 kg; 95% CI, -9.8 to -1.51) and an average reduction in HbA1c of 0.1% (95% CI, -0.17 to -0.02).7
There are 5 GLP-1 receptor agonists that have US Food and Drug Administration approval for the treatment of type 2 diabetes: albiglutide, dulaglutide, exenatide, exenatide XR, and liraglutide (TABLE 4).3,12

All 5 agents are administered subcutaneously and packaged in pen-injector form. Adverse effects include nausea, which is transient and diminishes within the first few weeks of therapy, and less commonly, pancreatitis.3,12
All of the GLP-1 receptor agonists, except short-acting exenatide, carry a warning about the risk of worsening renal function and a possible association with medullary thyroid carcinomas, which were identified in rats, but have not been observed in humans.3,12 Medications in this drug class have a low risk for precipitating hypoglycemia.11 Cost is their chief disadvantage, although copay reduction cards are available online for most of the products. Evaluate efficacy, ease of use, tolerability, and cost when selecting a GLP-1 receptor agonist.3,12
CASE › Mr. C prefers a more convenient option than adding another daily injection. Given his obesity, a GLP-1 receptor agonist can help with weight loss and lower his risk for hypoglycemia. To further increase the convenience in dosing, you lean toward either weekly exenatide XR or dulaglutide over basal-bolus combination insulin. Weekly albiglutide is less potent than exenatide XR and dulaglutide in decreasing HbA1c.12 Mr. C’s insurance plan provides preferred coverage for exenatide XR and he is eligible for a copay savings card, meaning he will pay no more than $25 per month for this new prescription. You prescribe exenatide XR and ask him to record his postprandial blood glucose levels. You follow up in one month to assess his response.
CORRESPONDENCE
Anne Mounsey, MD, University of North Carolina School of Medicine, Department of Family Medicine, 590 Manning Drive, Campus Box 7595, Chapel Hill, NC 27599; [email protected].
1. American Diabetes Association. Standards of medical care in diabetes - 2015. Diabetes Care. 2015;38 (Suppl):S1-S94.
2. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-613.
3. Merck Manual. Metformin. Available at: http://www.merckmanuals.com/professional/appendixes/brand-names-of-some-commonly-used-drugs. Accessed April 18, 2015.
4. Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431-1437.
5. Philbrick AM, Ernst ME, McDanel DL, et al. Metformin use in renal dysfunction: is a serum creatinine threshold appropriate? Am J Health Syst Pharm. 2009;66:2017-2023.
6. Pharmacist’s Letter. Drugs for Type 2 Diabetes [detail document]. September 2015. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=&s=PL&pt=2&segment=4407&dd=280601. Accessed December 28, 2015.
7. Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228-2234.
8. Inzucchi SE, Burgenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
9. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010:340:b4909.
10. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34 (Suppl 2):S279-S284.
11. Young LA, Buse JB. GLP-1 receptor agonists and basal insulin in type 2 diabetes. Lancet. 2014;384:2180-2181.
12. Pharmacist’s Letter. Comparison of GLP-1 Agonists [detail document]. December 2014. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/Browse.aspx?cs=&s=PL&pt=6&fpt=31&dd=300804&pb=PL&cat=5718#dd. Accessed December 28, 2015.
1. American Diabetes Association. Standards of medical care in diabetes - 2015. Diabetes Care. 2015;38 (Suppl):S1-S94.
2. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-613.
3. Merck Manual. Metformin. Available at: http://www.merckmanuals.com/professional/appendixes/brand-names-of-some-commonly-used-drugs. Accessed April 18, 2015.
4. Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431-1437.
5. Philbrick AM, Ernst ME, McDanel DL, et al. Metformin use in renal dysfunction: is a serum creatinine threshold appropriate? Am J Health Syst Pharm. 2009;66:2017-2023.
6. Pharmacist’s Letter. Drugs for Type 2 Diabetes [detail document]. September 2015. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=&s=PL&pt=2&segment=4407&dd=280601. Accessed December 28, 2015.
7. Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384:2228-2234.
8. Inzucchi SE, Burgenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379.
9. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010:340:b4909.
10. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34 (Suppl 2):S279-S284.
11. Young LA, Buse JB. GLP-1 receptor agonists and basal insulin in type 2 diabetes. Lancet. 2014;384:2180-2181.
12. Pharmacist’s Letter. Comparison of GLP-1 Agonists [detail document]. December 2014. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/Browse.aspx?cs=&s=PL&pt=6&fpt=31&dd=300804&pb=PL&cat=5718#dd. Accessed December 28, 2015.
Kidney stones? It’s time to rethink those meds
Do not prescribe tamsulosin or nifedipine for stone expulsion in patients with ureteral stones ≤10 mm.1
Strength of recommendation
A: Based on a high-quality randomized controlled trial.
Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
Illustrative case
Bob Z, age 48, presents to the emergency department (ED) with unspecified groin pain. A computed tomography scan of the kidney, ureter, and bladder (CT KUB) finds evidence of a single ureteral stone measuring 8 mm. He’s prescribed medication for the pain and discharged. The day after his ED visit, he comes to your office to discuss further treatment options. Should you prescribe tamsulosin or nifedipine to help him pass the stone?
The most recent National Health and Nutrition Examination Survey found kidney stones affect 8.8% of the population.2 Outpatient therapy is indicated for patients with ureteric colic secondary to stones ≤10 mm who do not have uncontrolled pain, impaired kidney function, or severe infection. Routine outpatient care includes oral hydration, antiemetics, and pain medications. Medical expulsive therapy (MET) is also used to facilitate stone passage. MET is increasingly becoming part of routine care; use of MET in kidney stone patients in the United States has grown from 14% in 2009 to 64% in 2012.3,4
The joint European Association of Urology/American Urological Association Nephrolithiasis Guideline Panel supports the use of MET.5 Meta-analyses of multiple randomized controlled trials (RCTs) suggest that an alpha-blocker (tamsulosin) or a calcium channel blocker (nifedipine) can reduce pain and lead to quicker stone passage and a higher rate of eventual stone passage when compared to placebo or observation.6,7 However, these reviews included small, heterogeneous studies with a high or unclear risk of bias.
STUDY SUMMARY: MET doesn’t increase the rate of stone passage
The SUSPEND (Spontaneous Urinary Stone Passage ENabled by Drugs) trial1 was a multicenter RCT designed to determine the effectiveness of tamsulosin or nifedipine as MET for patients ages 18 to 65 years with a single ureteric stone measuring ≤10 mm on CT KUB, which has 98% diagnostic accuracy.8 (Stones >10 mm typically require surgery or lithotripsy.)
In this RCT, 1167 adults were randomized to take tamsulosin 0.4 mg/d, nifedipine 30 mg/d, or placebo for 4 weeks or until the stone spontaneously passed, whichever came first. The participants, clinicians, and research staff were blinded to treatment assignment. The primary outcome was the proportion of participants who spontaneously passed their stone, as indicated in patient self-reported questionnaires and case-report forms completed by researchers. Secondary outcomes were time to stone passage and pain as assessed by analgesic use and a visual analogue scale (VAS).
At 4 weeks, 1136 (97%) of the randomized participants had data available for analysis. The proportion of participants who passed their stone did not differ between MET and placebo; 80% of the placebo group (303 of 379 participants) passed the stone, compared with 81% (307 of 378) of the tamsulosin group and 80% (304 of 379) of the nifedipine group. The odds ratio (OR) for MET vs placebo was 1.04 (95% confidence interval [CI], 0.77 to 1.43) and the OR for tamsulosin vs nifedipine was 1.07 (95% CI, 0.74 to 1.53). These findings did not change with further subgroup analysis, including by sex, stone size (≤5 mm vs >5 mm), or stone location.
There were no differences between groups in time to stone passage as measured by clinical report and confirmed by imaging. Time to passage of stone was available for 237 (21%) of participants. The mean days to stone passage was 15.9 (n=84) for placebo, 16.5 (n=79) for tamsulosin and 16.2 (n=74) for nifedipine, with a MET vs placebo difference of 0.5 days (95% CI, -2.9 to 3.9; P=.78). Sensitivity analysis accounting for bias from missing data did not change this outcome.
No differences in analgesic use or pain. Self-reported use of pain medication during the first 4 weeks was similar between groups: 59% (placebo patients), 56% (tamsulosin), and 56% (nifedipine). The mean days of pain medication use was 10.5 for placebo, 11.6 for tamsulosin, and 10.7 for nifedipine, with a MET vs placebo difference of 0.6 days (95% CI, -1.6 to 2.8; P=.45).
There was no difference between groups in the VAS pain score at 4 weeks. The MET vs placebo difference was 0.0 (95% CI, -0.4 to 0.4; P=.96) and the mean VAS pain score was 1.2 for placebo, 1.0 for tamsulosin, and 1.3 for nifedipine.
WHAT'S NEW: This large RCT contradicts results from previous meta-analyses
The SUSPEND study is the first large, multicenter RCT of MET with tamsulosin or nifedipine for kidney stones that used patient-oriented outcomes to find no benefit for stone expulsion, analgesic use, or reported pain compared to placebo. The discrepancy with prior meta-analyses is not unusual. Up to one-third of meta-analyses that show positive outcomes of a therapy are subsequently altered by the inclusion of results from a single, large, multicenter, well-designed RCT.9
CAVEATS: This trial included fewer women than previous studies
The SUSPEND study included a smaller proportion of women than previously published case series due to a need for a diagnostic CT KUB, which excluded more women than men due to radiation concerns. However, the proportion of women was balanced across all groups in this trial, and there was no evidence that sex impacted the efficacy of treatment for the primary outcome.1
CHALLENGES TO IMPLEMENTATION
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
2. Scales CD Jr., Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Fwu CU, Eggers PW, Kimmel PL, et al. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;89:479-486.
4. Bagga H, Appa A, Wang R, et al. 2257 medical expulsion therapy is underutilized in women presenting to an emergency department with acute urinary stone disease. J Urol. 2013;189:e925-e926.
5. Preminger GM, Tiselius HG, Assimos DG, et al; American Urological Association Education and Research, Inc; European Association of Urology. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610-1631.
6. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509.
7. Seitz C, Liatsikos E, Porpiglia F, et al. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455-471.
8. Worster A, Preyra I, Weaver B, et al. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Ann Emerg Med. 2002;40:280-286.
9. LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536-542.
Do not prescribe tamsulosin or nifedipine for stone expulsion in patients with ureteral stones ≤10 mm.1
Strength of recommendation
A: Based on a high-quality randomized controlled trial.
Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
Illustrative case
Bob Z, age 48, presents to the emergency department (ED) with unspecified groin pain. A computed tomography scan of the kidney, ureter, and bladder (CT KUB) finds evidence of a single ureteral stone measuring 8 mm. He’s prescribed medication for the pain and discharged. The day after his ED visit, he comes to your office to discuss further treatment options. Should you prescribe tamsulosin or nifedipine to help him pass the stone?
The most recent National Health and Nutrition Examination Survey found kidney stones affect 8.8% of the population.2 Outpatient therapy is indicated for patients with ureteric colic secondary to stones ≤10 mm who do not have uncontrolled pain, impaired kidney function, or severe infection. Routine outpatient care includes oral hydration, antiemetics, and pain medications. Medical expulsive therapy (MET) is also used to facilitate stone passage. MET is increasingly becoming part of routine care; use of MET in kidney stone patients in the United States has grown from 14% in 2009 to 64% in 2012.3,4
The joint European Association of Urology/American Urological Association Nephrolithiasis Guideline Panel supports the use of MET.5 Meta-analyses of multiple randomized controlled trials (RCTs) suggest that an alpha-blocker (tamsulosin) or a calcium channel blocker (nifedipine) can reduce pain and lead to quicker stone passage and a higher rate of eventual stone passage when compared to placebo or observation.6,7 However, these reviews included small, heterogeneous studies with a high or unclear risk of bias.
STUDY SUMMARY: MET doesn’t increase the rate of stone passage
The SUSPEND (Spontaneous Urinary Stone Passage ENabled by Drugs) trial1 was a multicenter RCT designed to determine the effectiveness of tamsulosin or nifedipine as MET for patients ages 18 to 65 years with a single ureteric stone measuring ≤10 mm on CT KUB, which has 98% diagnostic accuracy.8 (Stones >10 mm typically require surgery or lithotripsy.)
In this RCT, 1167 adults were randomized to take tamsulosin 0.4 mg/d, nifedipine 30 mg/d, or placebo for 4 weeks or until the stone spontaneously passed, whichever came first. The participants, clinicians, and research staff were blinded to treatment assignment. The primary outcome was the proportion of participants who spontaneously passed their stone, as indicated in patient self-reported questionnaires and case-report forms completed by researchers. Secondary outcomes were time to stone passage and pain as assessed by analgesic use and a visual analogue scale (VAS).
At 4 weeks, 1136 (97%) of the randomized participants had data available for analysis. The proportion of participants who passed their stone did not differ between MET and placebo; 80% of the placebo group (303 of 379 participants) passed the stone, compared with 81% (307 of 378) of the tamsulosin group and 80% (304 of 379) of the nifedipine group. The odds ratio (OR) for MET vs placebo was 1.04 (95% confidence interval [CI], 0.77 to 1.43) and the OR for tamsulosin vs nifedipine was 1.07 (95% CI, 0.74 to 1.53). These findings did not change with further subgroup analysis, including by sex, stone size (≤5 mm vs >5 mm), or stone location.
There were no differences between groups in time to stone passage as measured by clinical report and confirmed by imaging. Time to passage of stone was available for 237 (21%) of participants. The mean days to stone passage was 15.9 (n=84) for placebo, 16.5 (n=79) for tamsulosin and 16.2 (n=74) for nifedipine, with a MET vs placebo difference of 0.5 days (95% CI, -2.9 to 3.9; P=.78). Sensitivity analysis accounting for bias from missing data did not change this outcome.
No differences in analgesic use or pain. Self-reported use of pain medication during the first 4 weeks was similar between groups: 59% (placebo patients), 56% (tamsulosin), and 56% (nifedipine). The mean days of pain medication use was 10.5 for placebo, 11.6 for tamsulosin, and 10.7 for nifedipine, with a MET vs placebo difference of 0.6 days (95% CI, -1.6 to 2.8; P=.45).
There was no difference between groups in the VAS pain score at 4 weeks. The MET vs placebo difference was 0.0 (95% CI, -0.4 to 0.4; P=.96) and the mean VAS pain score was 1.2 for placebo, 1.0 for tamsulosin, and 1.3 for nifedipine.
WHAT'S NEW: This large RCT contradicts results from previous meta-analyses
The SUSPEND study is the first large, multicenter RCT of MET with tamsulosin or nifedipine for kidney stones that used patient-oriented outcomes to find no benefit for stone expulsion, analgesic use, or reported pain compared to placebo. The discrepancy with prior meta-analyses is not unusual. Up to one-third of meta-analyses that show positive outcomes of a therapy are subsequently altered by the inclusion of results from a single, large, multicenter, well-designed RCT.9
CAVEATS: This trial included fewer women than previous studies
The SUSPEND study included a smaller proportion of women than previously published case series due to a need for a diagnostic CT KUB, which excluded more women than men due to radiation concerns. However, the proportion of women was balanced across all groups in this trial, and there was no evidence that sex impacted the efficacy of treatment for the primary outcome.1
CHALLENGES TO IMPLEMENTATION
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
Do not prescribe tamsulosin or nifedipine for stone expulsion in patients with ureteral stones ≤10 mm.1
Strength of recommendation
A: Based on a high-quality randomized controlled trial.
Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
Illustrative case
Bob Z, age 48, presents to the emergency department (ED) with unspecified groin pain. A computed tomography scan of the kidney, ureter, and bladder (CT KUB) finds evidence of a single ureteral stone measuring 8 mm. He’s prescribed medication for the pain and discharged. The day after his ED visit, he comes to your office to discuss further treatment options. Should you prescribe tamsulosin or nifedipine to help him pass the stone?
The most recent National Health and Nutrition Examination Survey found kidney stones affect 8.8% of the population.2 Outpatient therapy is indicated for patients with ureteric colic secondary to stones ≤10 mm who do not have uncontrolled pain, impaired kidney function, or severe infection. Routine outpatient care includes oral hydration, antiemetics, and pain medications. Medical expulsive therapy (MET) is also used to facilitate stone passage. MET is increasingly becoming part of routine care; use of MET in kidney stone patients in the United States has grown from 14% in 2009 to 64% in 2012.3,4
The joint European Association of Urology/American Urological Association Nephrolithiasis Guideline Panel supports the use of MET.5 Meta-analyses of multiple randomized controlled trials (RCTs) suggest that an alpha-blocker (tamsulosin) or a calcium channel blocker (nifedipine) can reduce pain and lead to quicker stone passage and a higher rate of eventual stone passage when compared to placebo or observation.6,7 However, these reviews included small, heterogeneous studies with a high or unclear risk of bias.
STUDY SUMMARY: MET doesn’t increase the rate of stone passage
The SUSPEND (Spontaneous Urinary Stone Passage ENabled by Drugs) trial1 was a multicenter RCT designed to determine the effectiveness of tamsulosin or nifedipine as MET for patients ages 18 to 65 years with a single ureteric stone measuring ≤10 mm on CT KUB, which has 98% diagnostic accuracy.8 (Stones >10 mm typically require surgery or lithotripsy.)
In this RCT, 1167 adults were randomized to take tamsulosin 0.4 mg/d, nifedipine 30 mg/d, or placebo for 4 weeks or until the stone spontaneously passed, whichever came first. The participants, clinicians, and research staff were blinded to treatment assignment. The primary outcome was the proportion of participants who spontaneously passed their stone, as indicated in patient self-reported questionnaires and case-report forms completed by researchers. Secondary outcomes were time to stone passage and pain as assessed by analgesic use and a visual analogue scale (VAS).
At 4 weeks, 1136 (97%) of the randomized participants had data available for analysis. The proportion of participants who passed their stone did not differ between MET and placebo; 80% of the placebo group (303 of 379 participants) passed the stone, compared with 81% (307 of 378) of the tamsulosin group and 80% (304 of 379) of the nifedipine group. The odds ratio (OR) for MET vs placebo was 1.04 (95% confidence interval [CI], 0.77 to 1.43) and the OR for tamsulosin vs nifedipine was 1.07 (95% CI, 0.74 to 1.53). These findings did not change with further subgroup analysis, including by sex, stone size (≤5 mm vs >5 mm), or stone location.
There were no differences between groups in time to stone passage as measured by clinical report and confirmed by imaging. Time to passage of stone was available for 237 (21%) of participants. The mean days to stone passage was 15.9 (n=84) for placebo, 16.5 (n=79) for tamsulosin and 16.2 (n=74) for nifedipine, with a MET vs placebo difference of 0.5 days (95% CI, -2.9 to 3.9; P=.78). Sensitivity analysis accounting for bias from missing data did not change this outcome.
No differences in analgesic use or pain. Self-reported use of pain medication during the first 4 weeks was similar between groups: 59% (placebo patients), 56% (tamsulosin), and 56% (nifedipine). The mean days of pain medication use was 10.5 for placebo, 11.6 for tamsulosin, and 10.7 for nifedipine, with a MET vs placebo difference of 0.6 days (95% CI, -1.6 to 2.8; P=.45).
There was no difference between groups in the VAS pain score at 4 weeks. The MET vs placebo difference was 0.0 (95% CI, -0.4 to 0.4; P=.96) and the mean VAS pain score was 1.2 for placebo, 1.0 for tamsulosin, and 1.3 for nifedipine.
WHAT'S NEW: This large RCT contradicts results from previous meta-analyses
The SUSPEND study is the first large, multicenter RCT of MET with tamsulosin or nifedipine for kidney stones that used patient-oriented outcomes to find no benefit for stone expulsion, analgesic use, or reported pain compared to placebo. The discrepancy with prior meta-analyses is not unusual. Up to one-third of meta-analyses that show positive outcomes of a therapy are subsequently altered by the inclusion of results from a single, large, multicenter, well-designed RCT.9
CAVEATS: This trial included fewer women than previous studies
The SUSPEND study included a smaller proportion of women than previously published case series due to a need for a diagnostic CT KUB, which excluded more women than men due to radiation concerns. However, the proportion of women was balanced across all groups in this trial, and there was no evidence that sex impacted the efficacy of treatment for the primary outcome.1
CHALLENGES TO IMPLEMENTATION
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
2. Scales CD Jr., Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Fwu CU, Eggers PW, Kimmel PL, et al. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;89:479-486.
4. Bagga H, Appa A, Wang R, et al. 2257 medical expulsion therapy is underutilized in women presenting to an emergency department with acute urinary stone disease. J Urol. 2013;189:e925-e926.
5. Preminger GM, Tiselius HG, Assimos DG, et al; American Urological Association Education and Research, Inc; European Association of Urology. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610-1631.
6. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509.
7. Seitz C, Liatsikos E, Porpiglia F, et al. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455-471.
8. Worster A, Preyra I, Weaver B, et al. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Ann Emerg Med. 2002;40:280-286.
9. LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536-542.
1. Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386:341-349.
2. Scales CD Jr., Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Fwu CU, Eggers PW, Kimmel PL, et al. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;89:479-486.
4. Bagga H, Appa A, Wang R, et al. 2257 medical expulsion therapy is underutilized in women presenting to an emergency department with acute urinary stone disease. J Urol. 2013;189:e925-e926.
5. Preminger GM, Tiselius HG, Assimos DG, et al; American Urological Association Education and Research, Inc; European Association of Urology. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610-1631.
6. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509.
7. Seitz C, Liatsikos E, Porpiglia F, et al. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56:455-471.
8. Worster A, Preyra I, Weaver B, et al. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: a meta-analysis. Ann Emerg Med. 2002;40:280-286.
9. LeLorier J, Gregoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536-542.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Managing Diabetes in Women of Childbearing Age
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
Ladder Becomes Stairway to Urgent Care
Answer
The radiograph shows a compression deformity of the C6 vertebral body. In addition, there is a displaced fracture fragment along the anterior superior endplate. Alignment is adequate, with no evidence of subluxation. The disc space of C6-7 also appears slightly distracted, suggesting possible acute injury.
Given the clinical and radiographic presentation, this patient warranted further workup. He was admitted for CT and MRI of the cervical spine, which demonstrated disc herniation at both the C5-6 and C6-7 levels, as well as interspinous ligament injury.
The patient subsequently underwent a two-level anterior cervical discectomy and fusion. His symptoms resolved, and he was discharged home the next day.
Answer
The radiograph shows a compression deformity of the C6 vertebral body. In addition, there is a displaced fracture fragment along the anterior superior endplate. Alignment is adequate, with no evidence of subluxation. The disc space of C6-7 also appears slightly distracted, suggesting possible acute injury.
Given the clinical and radiographic presentation, this patient warranted further workup. He was admitted for CT and MRI of the cervical spine, which demonstrated disc herniation at both the C5-6 and C6-7 levels, as well as interspinous ligament injury.
The patient subsequently underwent a two-level anterior cervical discectomy and fusion. His symptoms resolved, and he was discharged home the next day.
Answer
The radiograph shows a compression deformity of the C6 vertebral body. In addition, there is a displaced fracture fragment along the anterior superior endplate. Alignment is adequate, with no evidence of subluxation. The disc space of C6-7 also appears slightly distracted, suggesting possible acute injury.
Given the clinical and radiographic presentation, this patient warranted further workup. He was admitted for CT and MRI of the cervical spine, which demonstrated disc herniation at both the C5-6 and C6-7 levels, as well as interspinous ligament injury.
The patient subsequently underwent a two-level anterior cervical discectomy and fusion. His symptoms resolved, and he was discharged home the next day.

A 30-year-old man presents to an urgent care clinic for evaluation of neck pain secondary to an injury he sustained earlier in the day. He was at a construction worksite, on a ladder, approximately eight to 10 feet above the ground. He went to step onto an adjacent ladder and missed. His chin and face struck one of the rungs, and his head went back. Amazingly, he was able to maintain his balance, holding onto the sides of the ladder, sliding down the front, and landing on his feet. He immediately began to experience neck pain, as well as numbness and tingling in both arms (worse on his left side). He was placed in a rigid collar upon arrival to the facility. Medical history is unremarkable except for tobacco use. Vital signs are normal. Physical exam demonstrates some neck pain within the paraspinous muscles. There is some mild midline tenderness within the cervical spine. Of note, the numbness and tingling seem to be worst in his index finger and thumb. You obtain a cervical spine radiograph; the lateral view is shown. What is your impression?
Aneuploidy Screening: Newer Noninvasive Test Gains Traction
PRACTICE CHANGER
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multicenter cohort studies.1,2
A 28-year-old woman (gravida 2, para 1001) at 10 weeks’ gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities, and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk for fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments.
It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011, after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However, until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are two large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The two groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the firstline approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA be discussed with all patients.8
Continue for study summaries >>
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2,042 pregnant patients ages 18 to 49 (mean, 29.6) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes, thyroid disorders, and other comorbidities. cfDNA testing was done on 1,909 maternal blood samples for trisomy 21 and 1,905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and nonlive births, the incidence of trisomy 21 was 5 of 1,909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1,905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly 1 in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false-positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false-positive rate was 0.3% compared to 3.6% for standard screening (P < .001); for trisomy 18, the cfDNA false-positive rate was 0.2% compared to 0.6% for standard screening (P = .03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in six countries.
This study enrolled 18,955 women ages 18 to 48 (mean, 31) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1 – specificity; < .700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages < 35).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P = .001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P < .001) and a lower false-positive rate (0.06% vs 5.4%; P < .001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing versus standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false-positive rates for cfDNA, compared with traditional screening.
WHAT’S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false-positive rates than standard fetal aneuploidy screening for the general obstetric population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetric population, according to ACOG and SMFM, the two groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
Continue for caveats >>
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to FDA approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false-negative results. Further testing has shown that such false-negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false-positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing, such as chorionic villus sampling or amniocentesis, before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Clinicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family practice clinicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, clinicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
References
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372: 1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011; 342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207: 137.e1-e8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 640: Cell-free DNA screening for fetal aneuploidy. Obstet Gynecol. 2015;126:e31-e37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013; 33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(1):49-52.
PRACTICE CHANGER
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multicenter cohort studies.1,2
A 28-year-old woman (gravida 2, para 1001) at 10 weeks’ gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities, and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk for fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments.
It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011, after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However, until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are two large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The two groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the firstline approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA be discussed with all patients.8
Continue for study summaries >>
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2,042 pregnant patients ages 18 to 49 (mean, 29.6) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes, thyroid disorders, and other comorbidities. cfDNA testing was done on 1,909 maternal blood samples for trisomy 21 and 1,905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and nonlive births, the incidence of trisomy 21 was 5 of 1,909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1,905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly 1 in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false-positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false-positive rate was 0.3% compared to 3.6% for standard screening (P < .001); for trisomy 18, the cfDNA false-positive rate was 0.2% compared to 0.6% for standard screening (P = .03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in six countries.
This study enrolled 18,955 women ages 18 to 48 (mean, 31) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1 – specificity; < .700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages < 35).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P = .001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P < .001) and a lower false-positive rate (0.06% vs 5.4%; P < .001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing versus standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false-positive rates for cfDNA, compared with traditional screening.
WHAT’S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false-positive rates than standard fetal aneuploidy screening for the general obstetric population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetric population, according to ACOG and SMFM, the two groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
Continue for caveats >>
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to FDA approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false-negative results. Further testing has shown that such false-negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false-positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing, such as chorionic villus sampling or amniocentesis, before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Clinicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family practice clinicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, clinicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
References
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372: 1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011; 342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207: 137.e1-e8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 640: Cell-free DNA screening for fetal aneuploidy. Obstet Gynecol. 2015;126:e31-e37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013; 33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(1):49-52.
PRACTICE CHANGER
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multicenter cohort studies.1,2
A 28-year-old woman (gravida 2, para 1001) at 10 weeks’ gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities, and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk for fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments.
It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011, after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However, until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are two large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The two groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the firstline approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA be discussed with all patients.8
Continue for study summaries >>
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2,042 pregnant patients ages 18 to 49 (mean, 29.6) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes, thyroid disorders, and other comorbidities. cfDNA testing was done on 1,909 maternal blood samples for trisomy 21 and 1,905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and nonlive births, the incidence of trisomy 21 was 5 of 1,909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1,905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly 1 in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false-positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false-positive rate was 0.3% compared to 3.6% for standard screening (P < .001); for trisomy 18, the cfDNA false-positive rate was 0.2% compared to 0.6% for standard screening (P = .03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in six countries.
This study enrolled 18,955 women ages 18 to 48 (mean, 31) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1 – specificity; < .700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages < 35).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P = .001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P < .001) and a lower false-positive rate (0.06% vs 5.4%; P < .001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing versus standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false-positive rates for cfDNA, compared with traditional screening.
WHAT’S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false-positive rates than standard fetal aneuploidy screening for the general obstetric population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetric population, according to ACOG and SMFM, the two groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
Continue for caveats >>
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to FDA approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false-negative results. Further testing has shown that such false-negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false-positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing, such as chorionic villus sampling or amniocentesis, before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Clinicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family practice clinicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, clinicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
References
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372: 1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011; 342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207: 137.e1-e8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 640: Cell-free DNA screening for fetal aneuploidy. Obstet Gynecol. 2015;126:e31-e37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013; 33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(1):49-52.
Rash, Reaction, or Red Flag?
1. The patient had just recovered from a sore throat and noticed discrete red nodules, which eventually coalesced into a single large edematous plaque over the right anterior tibia. The deep intradermal and subdermal edema is exquisitely tender to touch, considerably warmer than the surrounding skin, and highly blanchable.
Diagnosis: Erythema nodosum is a reactive form of septal panniculitis with many potential triggers. Notable triggers include Crohn disease flares and use of drugs such as sulfa, gold salts, and oral contraceptives. Several infections have been identified as triggers, including strep, mycoplasma, and campylobacter, as well as deep fungal infections (histoplasmosis, blastomycosis, coccidioidomycosis, and sporotrichosis). More unusual causes include pregnancy and diseases such as sarcoidosis, tuberculosis, Behçet disease, and leukemia/lymphoma.
For more information, see “Painful Lesion Hasn’t Responded to Antibiotics.” Clin Rev. 2015;25(11):10,12.
For the next photograph, proceed to the next page >>
2. A 16-year-old high school student joins her friends in a 2K run one morning. The next day, her shins are so painful she can hardly walk. She applied ice packs to her legs, using elastic bandages to hold them in place until the ice cubes melt. As her legs rewarm, a rash appears where the ice packs contacted the skin.
Diagnosis: This condition is urticarial in nature—albeit an unusual form, triggered by cold. Though it appears counterintuitive, cold uriticaria typically appears only on rewarming of the affected area and is marked by the sudden appearance of “welts” or “hives” that usually clear (with or without treatment) within hours.
Uncomplicated urticaria resolves without leaving any signs (eg, purpura, ecchymosis) that might otherwise suggest the presence of a vasculitic component, such as that seen with lupus or other autoimmune diseases. Blanchability on digital pressure is one way to confirm benignancy, since blood tends to leak from vessels damaged by vasculitis, emptying into the surrounding interstitial spaces and presenting as nonblanchable petechiae, purpura, or ecchymosis. The relatively benign nature of this patient’s urticaria was also suggested by additional history taking, in which she denied having fever, malaise, or arthralgia. These are all symptoms we might have seen with more serious underlying causes.
Cold urticaria is one of the so-called physical urticarias, a group that includes urticaria caused by vibration, pressure, heat, sun, and even exposure to water. Thought to comprise up to 20% of all urticarias, the physical urticarias occur most frequently in persons ages 17 to 40. Dermatographism is the most common form, occurring in the linear track of a vigorous scratch as a wheal that manifests rapidly, lasts a few minutes, then disappears without a trace. Its presence is purposely sought by the examiner to confirm the diagnosis of urticaria (most often the chronic idiopathic variety).
For more information, see “Inexperienced runner develops leg rash.” Clin Rev. 2012;22(8):W3
For the next photograph, proceed to the next page >>
Source: PhotoStock-Israel / Science Source
3. Typically manifesting with edema, pruritus, warmth, and tenderness, this lesion is usually associated with a history of recent trauma or pharyngitis followed by malaise, chills, and high fever. The lesion is usually raised with a clear line of demarcation at the edge.
Diagnosis: Erysipelas, an acute infection of the skin and subcutaneous tissue, is caused by beta-hemolytic streptococci invading tissues via a disruption to the skin barrier. Streptococcus strains are susceptible to penicillin and 99.5% are susceptible to clindamycin. Associated comorbidities in erysipelas include diabetes mellitus, as well as hypertension, chronic venous insufficiency, and other cardiovascular diseases.
For more information, see “Painful rash on face.” J Fam Pract. 2010;59(8):459-462.
For the next photograph, proceed to the next page >>
Source: CDC Public Health Image Library.
4. This patient presented with a red, expanding rash on the lateral aspect of the left thigh. Affecting any part of the body, this illness may present with fever, chills, sweats, muscle aches, fatigue, nausea and joint pain. Some patients have a rash or Bell’s palsy.
Diagnosis: Lyme disease, caused by B. burgdorferi bacteria, is transmitted to humans through the bite of infected Ixodes ticks. Typical symptoms include fever, headache, fatigue, and a characteristic skin rash called erythema migrans. If left untreated, infection can spread to joints, the heart, and the nervous system.
Because its symptoms mimic many other diseases, diagnosing Lyme disease can be difficult. The diagnosis is based on symptoms, physical findings, eg, rash, and the possibility of exposure to infected ticks; laboratory testing is helpful if used correctly and performed with validated methods.
Treatment choice depends on the whether the disease is early or late. Most cases of early Lyme disease can be treated successfully with a few weeks of antibiotics.
For more information, see “Lyme Disease Presents Differently in Men and Women.”
1. The patient had just recovered from a sore throat and noticed discrete red nodules, which eventually coalesced into a single large edematous plaque over the right anterior tibia. The deep intradermal and subdermal edema is exquisitely tender to touch, considerably warmer than the surrounding skin, and highly blanchable.
Diagnosis: Erythema nodosum is a reactive form of septal panniculitis with many potential triggers. Notable triggers include Crohn disease flares and use of drugs such as sulfa, gold salts, and oral contraceptives. Several infections have been identified as triggers, including strep, mycoplasma, and campylobacter, as well as deep fungal infections (histoplasmosis, blastomycosis, coccidioidomycosis, and sporotrichosis). More unusual causes include pregnancy and diseases such as sarcoidosis, tuberculosis, Behçet disease, and leukemia/lymphoma.
For more information, see “Painful Lesion Hasn’t Responded to Antibiotics.” Clin Rev. 2015;25(11):10,12.
For the next photograph, proceed to the next page >>
2. A 16-year-old high school student joins her friends in a 2K run one morning. The next day, her shins are so painful she can hardly walk. She applied ice packs to her legs, using elastic bandages to hold them in place until the ice cubes melt. As her legs rewarm, a rash appears where the ice packs contacted the skin.
Diagnosis: This condition is urticarial in nature—albeit an unusual form, triggered by cold. Though it appears counterintuitive, cold uriticaria typically appears only on rewarming of the affected area and is marked by the sudden appearance of “welts” or “hives” that usually clear (with or without treatment) within hours.
Uncomplicated urticaria resolves without leaving any signs (eg, purpura, ecchymosis) that might otherwise suggest the presence of a vasculitic component, such as that seen with lupus or other autoimmune diseases. Blanchability on digital pressure is one way to confirm benignancy, since blood tends to leak from vessels damaged by vasculitis, emptying into the surrounding interstitial spaces and presenting as nonblanchable petechiae, purpura, or ecchymosis. The relatively benign nature of this patient’s urticaria was also suggested by additional history taking, in which she denied having fever, malaise, or arthralgia. These are all symptoms we might have seen with more serious underlying causes.
Cold urticaria is one of the so-called physical urticarias, a group that includes urticaria caused by vibration, pressure, heat, sun, and even exposure to water. Thought to comprise up to 20% of all urticarias, the physical urticarias occur most frequently in persons ages 17 to 40. Dermatographism is the most common form, occurring in the linear track of a vigorous scratch as a wheal that manifests rapidly, lasts a few minutes, then disappears without a trace. Its presence is purposely sought by the examiner to confirm the diagnosis of urticaria (most often the chronic idiopathic variety).
For more information, see “Inexperienced runner develops leg rash.” Clin Rev. 2012;22(8):W3
For the next photograph, proceed to the next page >>
Source: PhotoStock-Israel / Science Source
3. Typically manifesting with edema, pruritus, warmth, and tenderness, this lesion is usually associated with a history of recent trauma or pharyngitis followed by malaise, chills, and high fever. The lesion is usually raised with a clear line of demarcation at the edge.
Diagnosis: Erysipelas, an acute infection of the skin and subcutaneous tissue, is caused by beta-hemolytic streptococci invading tissues via a disruption to the skin barrier. Streptococcus strains are susceptible to penicillin and 99.5% are susceptible to clindamycin. Associated comorbidities in erysipelas include diabetes mellitus, as well as hypertension, chronic venous insufficiency, and other cardiovascular diseases.
For more information, see “Painful rash on face.” J Fam Pract. 2010;59(8):459-462.
For the next photograph, proceed to the next page >>
Source: CDC Public Health Image Library.
4. This patient presented with a red, expanding rash on the lateral aspect of the left thigh. Affecting any part of the body, this illness may present with fever, chills, sweats, muscle aches, fatigue, nausea and joint pain. Some patients have a rash or Bell’s palsy.
Diagnosis: Lyme disease, caused by B. burgdorferi bacteria, is transmitted to humans through the bite of infected Ixodes ticks. Typical symptoms include fever, headache, fatigue, and a characteristic skin rash called erythema migrans. If left untreated, infection can spread to joints, the heart, and the nervous system.
Because its symptoms mimic many other diseases, diagnosing Lyme disease can be difficult. The diagnosis is based on symptoms, physical findings, eg, rash, and the possibility of exposure to infected ticks; laboratory testing is helpful if used correctly and performed with validated methods.
Treatment choice depends on the whether the disease is early or late. Most cases of early Lyme disease can be treated successfully with a few weeks of antibiotics.
For more information, see “Lyme Disease Presents Differently in Men and Women.”
1. The patient had just recovered from a sore throat and noticed discrete red nodules, which eventually coalesced into a single large edematous plaque over the right anterior tibia. The deep intradermal and subdermal edema is exquisitely tender to touch, considerably warmer than the surrounding skin, and highly blanchable.
Diagnosis: Erythema nodosum is a reactive form of septal panniculitis with many potential triggers. Notable triggers include Crohn disease flares and use of drugs such as sulfa, gold salts, and oral contraceptives. Several infections have been identified as triggers, including strep, mycoplasma, and campylobacter, as well as deep fungal infections (histoplasmosis, blastomycosis, coccidioidomycosis, and sporotrichosis). More unusual causes include pregnancy and diseases such as sarcoidosis, tuberculosis, Behçet disease, and leukemia/lymphoma.
For more information, see “Painful Lesion Hasn’t Responded to Antibiotics.” Clin Rev. 2015;25(11):10,12.
For the next photograph, proceed to the next page >>
2. A 16-year-old high school student joins her friends in a 2K run one morning. The next day, her shins are so painful she can hardly walk. She applied ice packs to her legs, using elastic bandages to hold them in place until the ice cubes melt. As her legs rewarm, a rash appears where the ice packs contacted the skin.
Diagnosis: This condition is urticarial in nature—albeit an unusual form, triggered by cold. Though it appears counterintuitive, cold uriticaria typically appears only on rewarming of the affected area and is marked by the sudden appearance of “welts” or “hives” that usually clear (with or without treatment) within hours.
Uncomplicated urticaria resolves without leaving any signs (eg, purpura, ecchymosis) that might otherwise suggest the presence of a vasculitic component, such as that seen with lupus or other autoimmune diseases. Blanchability on digital pressure is one way to confirm benignancy, since blood tends to leak from vessels damaged by vasculitis, emptying into the surrounding interstitial spaces and presenting as nonblanchable petechiae, purpura, or ecchymosis. The relatively benign nature of this patient’s urticaria was also suggested by additional history taking, in which she denied having fever, malaise, or arthralgia. These are all symptoms we might have seen with more serious underlying causes.
Cold urticaria is one of the so-called physical urticarias, a group that includes urticaria caused by vibration, pressure, heat, sun, and even exposure to water. Thought to comprise up to 20% of all urticarias, the physical urticarias occur most frequently in persons ages 17 to 40. Dermatographism is the most common form, occurring in the linear track of a vigorous scratch as a wheal that manifests rapidly, lasts a few minutes, then disappears without a trace. Its presence is purposely sought by the examiner to confirm the diagnosis of urticaria (most often the chronic idiopathic variety).
For more information, see “Inexperienced runner develops leg rash.” Clin Rev. 2012;22(8):W3
For the next photograph, proceed to the next page >>
Source: PhotoStock-Israel / Science Source
3. Typically manifesting with edema, pruritus, warmth, and tenderness, this lesion is usually associated with a history of recent trauma or pharyngitis followed by malaise, chills, and high fever. The lesion is usually raised with a clear line of demarcation at the edge.
Diagnosis: Erysipelas, an acute infection of the skin and subcutaneous tissue, is caused by beta-hemolytic streptococci invading tissues via a disruption to the skin barrier. Streptococcus strains are susceptible to penicillin and 99.5% are susceptible to clindamycin. Associated comorbidities in erysipelas include diabetes mellitus, as well as hypertension, chronic venous insufficiency, and other cardiovascular diseases.
For more information, see “Painful rash on face.” J Fam Pract. 2010;59(8):459-462.
For the next photograph, proceed to the next page >>
Source: CDC Public Health Image Library.
4. This patient presented with a red, expanding rash on the lateral aspect of the left thigh. Affecting any part of the body, this illness may present with fever, chills, sweats, muscle aches, fatigue, nausea and joint pain. Some patients have a rash or Bell’s palsy.
Diagnosis: Lyme disease, caused by B. burgdorferi bacteria, is transmitted to humans through the bite of infected Ixodes ticks. Typical symptoms include fever, headache, fatigue, and a characteristic skin rash called erythema migrans. If left untreated, infection can spread to joints, the heart, and the nervous system.
Because its symptoms mimic many other diseases, diagnosing Lyme disease can be difficult. The diagnosis is based on symptoms, physical findings, eg, rash, and the possibility of exposure to infected ticks; laboratory testing is helpful if used correctly and performed with validated methods.
Treatment choice depends on the whether the disease is early or late. Most cases of early Lyme disease can be treated successfully with a few weeks of antibiotics.
For more information, see “Lyme Disease Presents Differently in Men and Women.”