User login
Depression and Postdischarge Events
Between 10% and 40% of patients are readmitted after being discharged from the hospital,[1, 2] and as many as another 25% return to the emergency department (ED) within 30 days.[3] This creates a substantial burden on the healthcare system.[2] Various interventions have been tried to improve the quality of discharge transitions and reduce readmission rates, but results thus far have been inconsistent and generally disappointing.[4, 5, 6] Targeted delivery of interventions to those at highest risk might improve the effectiveness of these efforts and reduce costs. However, current readmission risk assessment models are only moderately predictive, suggesting the presence of unrecognized risk factors.[7, 8]
Active depression might represent a potentially modifiable independent predictor of adverse short‐term hospital outcomes that is currently underutilized. Depression occurs in 5% to 58% of hospitalized adults, depending on how cases are defined.[9, 10] Depression is often under‐recognized and undertreated in acute care clinical settings,[11] and relatively few readmission prediction models incorporate mental health related symptoms.[12]
Although several reviews have examined methods of screening for depression in hospitalized patients[9] or the effectiveness of screening in primary care,[13, 14] to our knowledge no systematic review has examined the impact of depression on short‐term prognosis after discharge from acute care. Therefore, the purpose of this systematic review was to summarize all studies that evaluated whether hospitalized medical patients with depressive symptoms are at higher risk of 30‐day all‐cause readmission or all‐cause mortality after being discharged from the hospital.
METHODS
This study followed an a priori protocol developed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) criteria.[15]
Data Sources and Search Methods
We searched the Cumulative Index to Nursing and Allied Health Literature, Ovid MEDLINE, Ovid Embase, and PsycINFO from inception to January 9, 2015, and the last 5 years of PubMed for full publications with any of the following Medical Subject Headings: depressive disorder, depression, patient readmission, interviews, psychological, inpatients, with restrictions for peer‐reviewed publication, humans, adults aged 18 years, and the English language. Search strategies were developed with a librarian (available upon request). We manually searched reference lists of all included studies and relevant review articles and contacted content experts to identify additional publications.
Eligibility Criteria and Selection of Studies
Two authors (J.L.P. and L.M.W.) independently screened full texts of all relevant articles for inclusion. Disagreements were resolved by consensus or a third reviewer (S.R.M.). We considered any original research that compared readmission or mortality after discharge for hospitalized medical patients (ie, general patients or subgroups thereof) with versus without depression identified by any validated depression measure,[16] including any study design that incorporated at least 30‐day follow‐up postdischarge. We excluded studies that examined patients hospitalized in nonacute care settings or on surgical, psychiatric, obstetric, or intensive care services. We calculated Cohen's coefficient to evaluate inter‐rater agreement on study selection.
Data Extraction
Data were abstracted by 2 authors (J.L.P. and L.M.W.). Disagreements were resolved by consensus or a third reviewer (S.R.M.). We contacted authors of all included studies to obtain missing data. If unavailable, crude data were estimated from published survival curves employing validated techniques in R (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria) and Digitizeit (
Data Synthesis and Statistical Analysis
Where possible, we calculated the pooled risk ratio (RR) with 95% confidence interval (95% CI) using a random effects models in Review Manager (RevMan) 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark). The random effects approach that we employed assumes heterogeneity (ie, underlying parameters vary between individual studies) and is distributed around a mean or population average effect, and results in more conservative (wider) confidence intervals, wherein larger cohorts (or studies with smaller standard errors) are given more weight. Heterogeneity was assessed using the I2 statistic, with values of <25%, 25% to 50%, and >50% representing low, moderate, and high heterogeneity.[19] As per the guidance of Higgins et al., we did not a priori define any degree of heterogeneity that would preclude pooling of the data; the expectation would be that heterogeneity would be substantially higher pooling observational studies rather than randomized trials.[19] Statistical significance was considered a 2‐sided P value 0.05.
Quality Assessment and Risk of Bias
We assessed study quality using the 9‐item Newcastle‐Ottawa scale with 0 to 3, 4 to 6, and 7 to 9 stars considered low, moderate, and high quality, respectively, and criteria for external and internal validity, including group selection and comparability, outcome assessment, and adequacy of follow‐up.[20] Adjusted estimates published in individual reports (or obtained directly from authors) were compared wherever possible with unadjusted estimates to assess the degree of confounding. We generated funnel plots in RevMan 5.3 and conducted Egger tests using Stata 13 (StataCorp LP, College Station, TX) to assess for publication bias.[21]
RESULTS
Study Selection
After removing duplicate publications, we identified 4066 reports and reviewed 133 reports in full text (see Supporting Figure 1 in the online version of this article). Despite our broad study inclusion criteria, we found only 35 longitudinal studies addressing this question. All 35 authors were contacted for additional outcomes data and other missing information (response rate of 34%). We had to exclude 17 studies as they did not provide 30 or 90‐day post‐discharge outcomes. Only 4 studies had published crude data for outcomes within 90 days,[22, 23, 24, 25] but after contact with authors, we received unpublished data for a further 7 studies[26, 27, 28, 29, 30, 31, 32] (including individual level data for 2 cohorts).[31, 32] We were able to estimate crude data from Kaplan‐Meier curves for another 3 studies.[33, 34, 35] Another 4 studies did not collect the outcomes we were interested in individually. These studies were included in this systematic review but are not poolable in our models: 3 authors could only provide composite endpoint data,[36, 37, 38] and 1 author provided unadjusted hazard ratios.[39] Inter‐reviewer agreement for inclusion was 80% (Cohen's = 0.60).
Characteristics of Included Studies
The 18 studies ranged in size from 58 to 1418 patients; 13 were cohort studies and 5 included secondary data from randomized control trials.[22, 27, 30, 34, 36] All studies ascertained depressive status by screening during index medical admission with either diagnostic interview or self‐report questionnaires, although a variety of scales and definitions for depression were used (Beck Depression Inventory [BDI] in 6 studies, Geriatric Depression Scale in 5 studies, Patient Health Questionnaire in another 4 studies, Medical Outcomes Study‐Depression Questionnaire in 1 study, and Center for Epidemiologic Studies Depression Scale in another study) (Table 1). Screening interviews were conducted mostly by research assistants or nurses (68%) or self‐administered (21%). Most studies examined specific medical patient subgroups (10 cardiac, 3 pulmonary, and 2 elderly). Major exclusion criteria reported were terminal illness (4 studies), unstable condition (6 studies), severe cognitive impairment (5 studies), and suicidal ideation or known depression (4 studies); 1 study enrolled patients with suspected depression (Table 1). Patient cohorts were on average older (range, 5082 years) (Table 1). Attrition rates for readmission and mortality data were low (average <1% among entire sample of studies). All studies scored at least 5 on the Newcastle‐Ottawa scale and were thus considered of at least moderate quality (see Supporting Table 1 in the online version of this article).
| Author, Date of Publication, Enrollment Period | Setting Country/Region, No. of Hospitals | No. of Inpatients, Clinical Features | Major Exclusion Criteria | Follow‐up, mo | Depression Measure (Cutoff) and Screening Method | Mean Age (SD), y | % Female | Positive Screen, No. (%) | Primary Outcome, Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Studies that use a scale based on DSM‐III criteria or a diagnostic interview according to DSM‐III criteria | |||||||||
| Frasure‐Smith et al.,[26] 1993, 19911992* | Canada/Quebec, 1 urban teaching | 218, AMI | Terminal noncardiac illness, unstable, not cognitive | 6 | BDI (10); mod DIS by interviewer, after transfer to medicine | 60 (range, 2488) | 22 | 68 (31), 35 (16) | All‐cause mortality |
| Frasure‐Smith et al.,[27] 1999, 19911992,* 19911994 | Canada/Quebec, 1 urban teaching, 10 urban area | 218; 78, AMI | Terminal noncardiac illness, unstable, not cognitive | 12 | BDI (10) by interviewer, after transfer to medicine | 60 (11) | 32 | 290 (32) | Cardiac mortality |
| Freedland et al.,[25] 1991, 1990 | USA/MO, 1 urban teaching | 58, CHF 75 years | Dementia, medically unstable | 3 | Mod DIS by psychiatric residents and interviewer | 78 (6) | 57 | 10 (17) | All‐cause readmission, all‐cause mortality |
| Fulop et al.,[38] 2003, 2002 | USA/NY, 1 urban teaching | 203, CHF 65 years | 1, 6 | GDS (10); SCID‐NP by interviewer, at discharge | 77 (8) | 53 | 73 (36), 44 (22) | Depression, composite PCP, ED, care visits, and readmission | |
| Lesprance et al.,[28] 2000, 19941996 | Canada/Quebec, 1 urban teaching | 430, unstable angina | Terminal noncardiac illness, not cognitive, recent CABG | 12 | BDI (10); mod DIS by interviewer, 5 days after admission | 62 (11) | 29 | 178 (41), 120 (28) | Cardiac death and MI, any death, angina readmission |
| Rumsfeld et al.,[30] 2005, 19992001 | CA, USA, UK, multiple | 634, AMI with CHF | Valvular or congenital heart failure | Up to 32 | MOS‐D (0.06) by interviewer, before discharge | 65 (11) | 28 | 143 (23) | All‐cause death, CVD death and readmission |
| Song et al.,[33] 2009, 2005 | South Korea, 2 urban teaching | 165, HF | If minor criteria for HF attributable to other medical condition | 6 | BDI (10) self‐administer or interviewer, 34 days of admin | 62 (13) | 49 | 131 (79) | HF readmission and all‐cause mortality, HF readmit |
| Papaioannou et al.,[29] 2013, 20092010 | Greece/Athens, 1 urban | 230, AECOPD | Other respiratory illness, known depressed | Monthly up to 12 | BDI‐I (19) self‐administer, first day | 71 (9) | 12 | 91 (40) | All‐cause mortality, AECOPD readmission |
| Studies that use a scale based on or validated against DSM‐IV criteria or a diagnostic interview according to DSM‐IV criteria | |||||||||
| Almagro et al.,[31] 2002, 19961997 | Spain, 1 urban teaching | 130, AECOPD | Other pulmonary disease | July 1999 | GDS‐SF (6) by interviewer, day before discharge | 72 (9) | 8 | 43 (33) | All‐cause mortality |
| Almagro et al.,[32] 2012, 20032004 | Spain, 1 urban teaching | 134, AECOPD | Other pulmonary disease | 1, 36 | GDS‐SF (6) by interviewer | 72 (10) | 5 | 55 (41) | All‐cause mortality, lung function, frailty |
| Bla et al.,[39] 2001, 2000 | Switzerland, 1 urban teaching | 401, medical 75 years | Stay <24 hours, elective/facility transfer, unstable, not cognitive | 6 | GDS‐SF (6) by interviewer, within 2 days of admission | 82 (7599) | 61 | 90 (22) | All‐cause readmission, all‐cause mortality |
| Cancino et al.,[22] 2014, 20062007,* 20082009 | USA/MA, 1 urban tertiary | 680; 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1 | PHQ‐9 (5 or severity) by interviewer, on admin | 50 (14) | 51 | 561 (40) | All‐cause readmission, ED visits, PCP visits |
| Mitchell et al.,[36] 2010, 20062007* | USA/MA, 1 urban tertiary | 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1, 2, 3 | PHQ‐9 (5) by interviewer, on admin | 50 (15) | 50 | 238 (32) | ED visits and all‐cause readmission |
| Covinsky et al.,[34] 1999, 19901992 | USA/OH, 1 urban teaching | 573, medical | ICU, oncology, telemetry, nursing home admissions | 36 | GDS‐SF (6) by interviewer, within 2 days of admission | 80 | 68 | 197 (34) | All‐cause mortality |
| Jiang et al.,[23] 2001, 19971998 | USA/NC, 1 urban teaching | 357 (331 DIS only), CHF | Suicidal, planned surgery, pregnant | 3, 12 | BDI (10) self‐admin; mod DIS (+BDI only) by interviewer | 63 (13) | 33 | 126 (35), 46 (14) | All‐cause mortality, all‐cause readmission |
| Kartha et al.,[24] 2007, 20022004 | USA/MA, 1 urban safety net | 144, medical recently hospitalized | Planned readmission, unable to keep PCP appointments | 3 | PHQ‐9 (algorithm) by interviewer | 55 (16) | 56 | 39 (27) | All‐cause readmission |
| Koenig and Kuchbhatla,[37] 1999, 1997 | USA/NC, 1 urban teaching | 331, medical 60 years | Stay <3 or >7 days, ICU/CCU, severe illness, nursing home transfers | 3, 6, 9, 12 | CES‐D (16) or HAM‐D (11) or DIS by psychiatrist, on or after third day | 70 (7) | 51 | 160 (48) | Depression, composite physical disability, health visits, and all‐cause readmission |
| Rollman et al.,[35] 2012, 20072009 | USA/PA, 4 urban teaching | 471, CHF, suspected depressed | Antidepressants users (excluded from PHQ‐2 group only) | Up to 12 | PHQ‐2; PHQ‐9 (5 in +PHQ‐2), by interviewer, 4 days | 66 (13) | 35 | 371 (79), 351 (74) | All‐cause mortality |
Prevalence and Recognition of Depressive Symptoms
The range of depression prevalence in hospitalized medical patients was 14% to 79%, with a median of 32% (interquartile range, 27%40%) (Table 1). In those studies that used a diagnostic interview, the prevalence tended to be lower for major depression, with a median of 17% (interquartile range, 16%22%) (Table 1). None of the included studies reported frequency of clinically recognized depression (ie, prior to screening for the study). Only 2 studies assessed the persistence of depression after discharge: 1 reported that depression persisted in 53% (by screening questionnaire) and 34% (by diagnostic interview) of patients at 30 days,[38] whereas the other reported 48% persistence at 90 days after discharge according to a combined screening method.[37]
Hospital Readmission
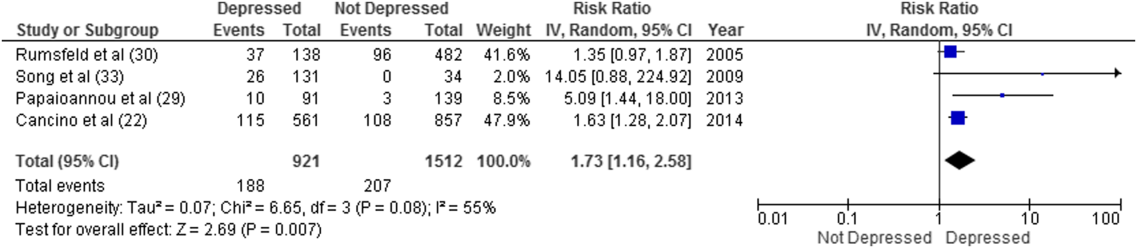
Overall, 8 studies provided readmission data. Among patients discharged from acute care medical wards (4 studies reporting on 5 cohorts), 395 of 2433 (16.2%) patients were readmitted within 30 days (Figure 1). Hospitalized patients with depressive symptoms were more likely to be readmitted within 30 days after discharge (20.4% vs 13.7%, RR: 1.73, 95% CI: 1.16‐2.58, P = 0.007, I2 = 55%) (Figure 1), compared to those without depression. Results were consistent for 90‐day readmissions (39.8% vs 31.0%, RR: 1.68, 95% CI: 1.13‐2.50, P = 0.01, I2 = 76%, n = 1543 patients) (see Supporting Figure 2 in the online version of this article) in 6 studies. One individual study examined readmission within 6 months after discharge, but was not poolable in this model, as it presented only hazard ratios and not raw data; however, it did report a 50% increased risk of readmission in medical inpatients aged 75 years (adjusted hazard ratio: 1.50, 95% CI: 1.03‐2.17, n = 401).[39]
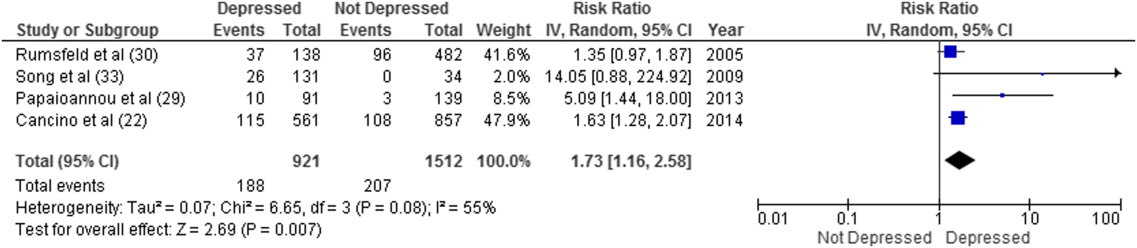
Forest plot presents results of the meta‐analysis in which the size of each data marker indicates the weight assigned to individuals studies. Abbreviations: CI, confidence interval; IV, independent variable.
Mortality After Discharge
Overall, 11 studies provided all‐cause mortality data. Among medical patients discharged from acute care in 9 studies, 69 of 3397 (2.0%) patients died within 30 days (Figure 2). Medical patients discharged with depressive symptoms were more likely to die within 30 days (2.8% vs 1.5%, RR: 2.13, 95% CI: 1.31‐3.44, P = 0.002, I2 = 0%) (Figure 2) compared to those without depression. Similar results were found for 90‐day mortality (7.7% vs 4.1%, RR: 2.01, 95% CI: 1.47‐2.76, P < 0.001, I2 = 4%, n = 3784 patients) (see Supporting Figure 3 in the online version of this article) in 11 studies.
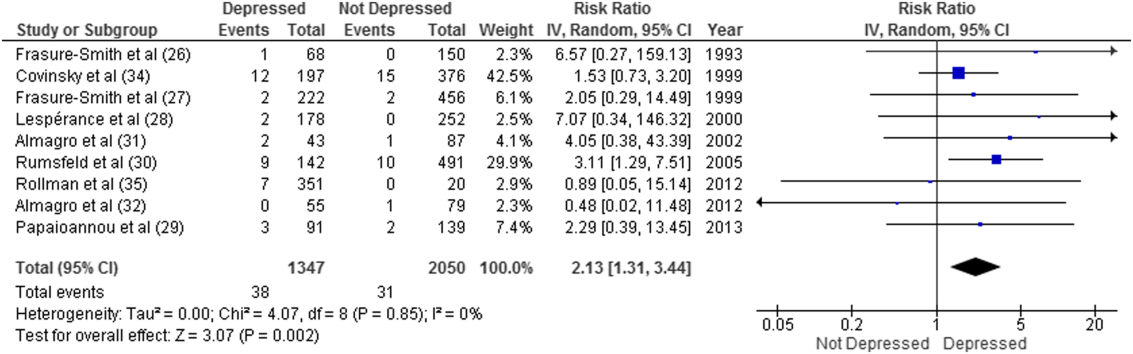
ED and PCP Visits
Four studies examined the use of ED or PCP services within 90 days of discharge, but 3 did not have extractable data for meta‐analysis. All showed increased utilization of health services for depressed compared to nondepressed patients after discharge.[22, 36, 37, 38] Depressed patients were more likely to visit the ED (adjusted incidence rate ratio: 1.73, 95% CI: 1.27‐2.36),[36] had significantly more medical encounters (eg, PCP, ED visits, hospital admissions, laboratory tests, and home care [mean 2.9 vs 2.6, P = 0.05])[38] and had a greater number of ED visits alone (27 vs 15 per 100 patients, P = 0.007)[22] within 30 days of hospital discharge compared to nondepressed patients. Similar results were found at 90 days.[36]
Sensitivity Analyses
All told, most studies reported a positive association between depression and adverse events, and this was true regardless of how much adjustment for potential confounding had been undertaken by the authors. Although all studies were qualitatively in the same direction, the magnitude of the association varied due to methodological and/or clinical heterogeneity. Sensitivity analysis revealed no overall difference in pooled risk ratios or heterogeneity between Mantel‐Haenszel fixed effects versus random effects models or with the addition of 0.5 to cells to permit inclusion of zero‐event data. There was no evidence of publication bias; funnel plots and Egger test results are available upon request. There were no statistically significant differences in the risk associated with depressive symptoms whether studies used Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III or DSM‐IV criteria, whether the study samples were disease specific or unselected general medical cohorts, whether studies were of moderate or high quality, or regardless of the severity of depressive symptoms.
DISCUSSION
Summary of Evidence
We found that depression was common in medical inpatients (about one‐third of all patients) and persisted for at least 30 days in up to half of those patients after discharge. We found strong evidence of an association between depressive symptoms and poor short‐term prognosis after discharge from the hospital: a 73% increased risk of readmission and a 2‐fold risk of death within 30 days compared to patients without depressive symptoms with similar results at 90 days.
Our meta‐analysis complements a recent systematic review that found concomitant depression to be a risk factor for poor prognosis among inpatients and outpatients with acute coronary syndrome,[40] and a meta‐analysis that demonstrated an increased risk of 2‐year mortality among patients with depression after myocardial infarction.[41] To our knowledge, our study is the first to quantify the short‐term postdischarge risks across a diverse group of medical inpatients.
The potential mechanisms underlying the observed relationship between depression and adverse patient outcomes after discharge are likely multiple. We believe there are 2 main possibilities. First, the increased risk associated with depression might be due to residual confounding, even though many of these studies did adjust for extensive lists of comorbidities,[22, 24, 26, 27, 29, 30, 33, 35, 36, 39] including functional status[39] and prior health services utilization.[22, 34, 36] This could occur if other risk factors were not sufficiently adjusted for, such as unrecognized comorbidities or concomitant disability, which are often present among chronically ill patients,[42] or if depression were a marker of psychosocial risk factors such as anxiety,[43] stress or poor resiliency,[44] or low social support,[45] though a few adjusted for psychosocial factors such as social support[26] or anxiety.[35] Confounding could also occur if symptoms of acute illness inflate reports of somatic symptoms of depression on self‐report questionnaires. Recent studies on the BDI, found that scores were higher in postmyocardial infarction patients when compared to outpatient controls,[46] but with no differences between those groups in scores for the BDI‐II,[47] a version with fewer somatic symptom questions.
Second, depression may cause adverse outcomes through indirect or direct pathways. Indirect causation could occur if depression hindered self‐care behaviors such as medication adherence.[42] Depression could also act directly through pathophysiological changes. Some studies have suggested that depression is associated with metabolic abnormalities, including alterations in glucose transport[42, 48] and increased vulnerability to obesity, type 2 diabetes mellitus, and/or diabetic complications, common conditions among hospitalized patients that also adversely affect postdischarge outcomes.[40, 48]
Strengths and Limitations
This review has multiple strengths. We cast a broad search and included studies that examined a wide range of medical patient subgroups, thus increasing the generalizability of our findings. We identified a general scarcity of studies on this topic and obtained additional unpublished data for 10 of the 18 relevant studies, and our response rate of 34% is compatible with the 37% response rate reported for Cochrane reviews when seeking additional data from authors.[49] Whether examined qualitatively (vote counting of the number of studies that showed an association) or quantitatively (via formal meta‐analysis), it seems apparent that there is a clinically important association between depression and postdischarge adverse events, but given the number, quality, and heterogeneity of the studies we examined, there may be some ongoing dispute about exactly how strong this association is and the degree of bias contributed by a couple of large studies of the topic.
There are limitations to our review. First, as we did not have individual‐level patient data, we could not use metaregression to explore sources of heterogeneity (clinical or methodological) or adjust for confounding, and this likely contributes to observed differences between individual estimates. For instance, the included studies had heterogeneous screening measures and cutoffs; thus, all cases of depression in these studies might not be equivalent. Some of the included studies assessed depression early during admission where psychological distress may be greatest; others assessed symptoms closer to discharge. Most studies included patients with specific conditions like heart failure or chronic obstructive pulmonary disease rather than a wide spectrum of medical inpatients. Moreover, few studies adjusted for psychosocial risk factors such as social support, anxiety, and functional status, and only 2 studies assessed the persistence of depressive symptoms after discharge. Second, we did not explore quantitative measures of between‐study variation (eg, I2), because experts question its utility given the expected heterogeneity in meta‐analyses of observational studies.[50] Third, although the included studies were deemed to be of at least moderate quality, they could be at risk for sources of bias that may not be sufficiently appraised by the current version of the Newscastle‐Ottawa scale for observational studies. Finally, we excluded grey literature (eg, conference proceedings or technical reports) that could potentially exclude null findings, although we did contact authors in this field to identify additional unpublished data relevant to this topic.
CONCLUSIONS
We have confirmed that depressive symptoms are common in hospitalized medical patients, frequently persist after discharge, and may predict greater risk of readmission or death after discharge. Thus, depressive symptoms are an additional marker that clinicians can use to help identify patients in acute care medical settings who may be at increased risk for suboptimal transition back to the community and who may require additional resources after discharge. However, future research is required to evaluate whether treatment of individuals who screen positive for depressive symptoms can reduce 30‐day readmission rates, and we are aware of at least 1 relevant ongoing trial (
Acknowledgements
The authors thank the following individuals: Dale Storie, MLIS, Saskatchewan Information and Library Services Consortium, Regina, Saskatchewan, Canada, for assistance in the literature search; James A. Hanley, PhD, Department of Epidemiology and Biostatistics, Faculty of Medicine, McGill University, Montreal, Quebec, Canada, for guidance in data recovery methods; Nancy Frasure‐Smith, PhD, Department of Psychiatry, McGill University, Department of Psychiatry and Research Centre Hospital Centre, University of Montreal, and Montreal Heart Institute Research Centre, Montreal, Quebec, Canada; Andriana I. Papaioannou, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; Konstantinos Kostikas, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; and Pere Almagro, MD, Servicio de Medicina Interna, Hospital Universitario Mutua de Terrassa, Terrassa, Barcelona, Spain; as well as Philip G. Jones, MS, Saint Luke's Mid America Heart Institute, Kansas City, Missouri; for their retrieval and contribution of unpublished data.
Disclosures
Ms. Pederson affirms that the manuscript is an honest, accurate, and transparent account of the study being reported with no important omissions. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. Design and conduct of the study: Ms. Pederson, Drs. Majumdar and McAlister. Data acquisition: Ms. Pederson, Ms. Warkentin. Analysis and interpretation of the data and drafting of the manuscript: Ms Pederson, Drs. Majumdar and McAlister. Review of the manuscript: all authors. Study supervision: Drs. Majumdar and McAlister. None of the contributors received compensation for their efforts. Salary support for Ms. Pederson was provided by a CRIO grant from Alberta InnovatesHealth Solutions. Drs. McAlister and Majumdar are supported by salary awards from Alberta Innovates‐Health Solutions. Dr. McAlister holds the University of Alberta/Capital Health Chair in Cardiology Outcomes Research. Dr. Majumda holds the University of Alberta Endowed Chair in Patient Health Management. The funding sources had no role in the design or conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. This work is that of the authors independent of funders. The authors report no conflicts of interest.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , . Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398–407.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . The elusive quest for quality and cost savings in the Medicare program. JAMA. 2009;301(6):668–670.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries—15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–111.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , . Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148–154.
- , , , et al. Prevalence, correlates and recognition of depression among inpatients of general hospitals in Wuhan, China. Gen Hosp Psychiatry. 2010;32(3):268–275.
- , , , , , . Recognition of depression by non‐psychiatric physicians—a systematic literature review and meta‐analysis. J Gen Intern Med. 2008;23(1):25–36.
- , , , , , . Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. Am Heart J. 2012;164(3):365–372.
- , , , et al. Does evidence support the American Heart Association's recommendation to screen patients for depression in cardiovascular care? An updated systematic review. PLoS One. 2013;8(1):e52654.
- , , , et al. Screening for depression: a systematic review and meta‐analysis. CMAJ Open. 2013;1(4):E159–E167.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94.
- , , , et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136(10):765–776.
- , , . Recovering the raw data behind a non‐parametric survival curve. Syst Rev. 2014;3:151.
- , , , . Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
- . Commentary: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160.
- , , , et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed September 1, 2015.
- , , . The funnel plot. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication Bias in Meta‐analysis: Prevention, Assessment and Adjustments. New York, NY: John Wiley 2006:73–98.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , et al. Depression in elderly patients with congestive heart failure. J Geriatr Psychiatry. 1991;24(1):59–71.
- , , . Depression following myocardial infarction: impact on 6‐month survival. JAMA. 1993;270(15):1819–1825.
- , , , , . Gender, depression, and one‐year prognosis after myocardial infarction. Psychosom Med. 1999;61(1):26–37.
- , , , . Depression and 1‐year prognosis in unstable angina. Arch Intern Med. 2000;160(9):1354–1360.
- , , , et al. The impact of depressive symptoms on recovery and outcome of hospitalised COPD exacerbations. Eur Respir J. 2013;41(4):815–823.
- , , , et al. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. Am Heart J. 2005;150(5):961–967.
- , , , et al. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448.
- , , , et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration. 2012;84(1):36–43.
- , , . Depressive symptoms increase risk of rehospitalisation in heart failure patients with preserved systolic function. J Clin Nurs. 2009;18(13):1871–1877.
- , , . Depressive symptoms and 3 year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. A positive 2‐item patient health questionnaire depression screen among hospitalized heart failure patients is associated with elevated 12‐month mortality. J Card Fail. 2012;18(3):238–245.
- , , , et al. Post‐discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378–384.
- , . Use of health services by medically ill depressed elderly patients after hospital discharge. Am J Geriatr Psychiatry. 1999;7(1):48–56.
- , , . Congestive heart failure and depression in older adults: clinical course and health services use 6 months after hospitalization. Psychosomatics. 2003;44(5):367–373.
- , , , . Depressive symptoms as a predictor of 6‐month outcomes and services utilization in elderly medical inpatients. Arch Intern Med. 2001;161(21):2609–2615.
- , , , et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129(12):1350–1369.
- , , , , , . Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta‐analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33(3):203–216.
- , , , , . Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925.
- , , , et al. Prognostic value of depression, anxiety, and anger in hospitalized cardiovascular disease patients for predicting adverse cardiac outcomes. Am J Cardiol. 2013;111(10):1432–1436.
- , , . The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
- , , , et al. The influence of somatic symptoms on beck depression inventory scores in hospitalized postmyocardial infarction patients. Can J Psychiatry. 2012;57(12):752–758.
- , , , et al. Somatic symptom overlap in beck depression inventory‐II scores following myocardial infarction. Br J Psychiatry. 2010;197(1):61–66.
- , , , . Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54(3):317–329.
- , , . Searching for unpublished data for Cochrane reviews: cross sectional study. BMJ. 2013;346:f2231.
- . Comment on: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39(3):932; author reply 933.
Between 10% and 40% of patients are readmitted after being discharged from the hospital,[1, 2] and as many as another 25% return to the emergency department (ED) within 30 days.[3] This creates a substantial burden on the healthcare system.[2] Various interventions have been tried to improve the quality of discharge transitions and reduce readmission rates, but results thus far have been inconsistent and generally disappointing.[4, 5, 6] Targeted delivery of interventions to those at highest risk might improve the effectiveness of these efforts and reduce costs. However, current readmission risk assessment models are only moderately predictive, suggesting the presence of unrecognized risk factors.[7, 8]
Active depression might represent a potentially modifiable independent predictor of adverse short‐term hospital outcomes that is currently underutilized. Depression occurs in 5% to 58% of hospitalized adults, depending on how cases are defined.[9, 10] Depression is often under‐recognized and undertreated in acute care clinical settings,[11] and relatively few readmission prediction models incorporate mental health related symptoms.[12]
Although several reviews have examined methods of screening for depression in hospitalized patients[9] or the effectiveness of screening in primary care,[13, 14] to our knowledge no systematic review has examined the impact of depression on short‐term prognosis after discharge from acute care. Therefore, the purpose of this systematic review was to summarize all studies that evaluated whether hospitalized medical patients with depressive symptoms are at higher risk of 30‐day all‐cause readmission or all‐cause mortality after being discharged from the hospital.
METHODS
This study followed an a priori protocol developed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) criteria.[15]
Data Sources and Search Methods
We searched the Cumulative Index to Nursing and Allied Health Literature, Ovid MEDLINE, Ovid Embase, and PsycINFO from inception to January 9, 2015, and the last 5 years of PubMed for full publications with any of the following Medical Subject Headings: depressive disorder, depression, patient readmission, interviews, psychological, inpatients, with restrictions for peer‐reviewed publication, humans, adults aged 18 years, and the English language. Search strategies were developed with a librarian (available upon request). We manually searched reference lists of all included studies and relevant review articles and contacted content experts to identify additional publications.
Eligibility Criteria and Selection of Studies
Two authors (J.L.P. and L.M.W.) independently screened full texts of all relevant articles for inclusion. Disagreements were resolved by consensus or a third reviewer (S.R.M.). We considered any original research that compared readmission or mortality after discharge for hospitalized medical patients (ie, general patients or subgroups thereof) with versus without depression identified by any validated depression measure,[16] including any study design that incorporated at least 30‐day follow‐up postdischarge. We excluded studies that examined patients hospitalized in nonacute care settings or on surgical, psychiatric, obstetric, or intensive care services. We calculated Cohen's coefficient to evaluate inter‐rater agreement on study selection.
Data Extraction
Data were abstracted by 2 authors (J.L.P. and L.M.W.). Disagreements were resolved by consensus or a third reviewer (S.R.M.). We contacted authors of all included studies to obtain missing data. If unavailable, crude data were estimated from published survival curves employing validated techniques in R (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria) and Digitizeit (
Data Synthesis and Statistical Analysis
Where possible, we calculated the pooled risk ratio (RR) with 95% confidence interval (95% CI) using a random effects models in Review Manager (RevMan) 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark). The random effects approach that we employed assumes heterogeneity (ie, underlying parameters vary between individual studies) and is distributed around a mean or population average effect, and results in more conservative (wider) confidence intervals, wherein larger cohorts (or studies with smaller standard errors) are given more weight. Heterogeneity was assessed using the I2 statistic, with values of <25%, 25% to 50%, and >50% representing low, moderate, and high heterogeneity.[19] As per the guidance of Higgins et al., we did not a priori define any degree of heterogeneity that would preclude pooling of the data; the expectation would be that heterogeneity would be substantially higher pooling observational studies rather than randomized trials.[19] Statistical significance was considered a 2‐sided P value 0.05.
Quality Assessment and Risk of Bias
We assessed study quality using the 9‐item Newcastle‐Ottawa scale with 0 to 3, 4 to 6, and 7 to 9 stars considered low, moderate, and high quality, respectively, and criteria for external and internal validity, including group selection and comparability, outcome assessment, and adequacy of follow‐up.[20] Adjusted estimates published in individual reports (or obtained directly from authors) were compared wherever possible with unadjusted estimates to assess the degree of confounding. We generated funnel plots in RevMan 5.3 and conducted Egger tests using Stata 13 (StataCorp LP, College Station, TX) to assess for publication bias.[21]
RESULTS
Study Selection
After removing duplicate publications, we identified 4066 reports and reviewed 133 reports in full text (see Supporting Figure 1 in the online version of this article). Despite our broad study inclusion criteria, we found only 35 longitudinal studies addressing this question. All 35 authors were contacted for additional outcomes data and other missing information (response rate of 34%). We had to exclude 17 studies as they did not provide 30 or 90‐day post‐discharge outcomes. Only 4 studies had published crude data for outcomes within 90 days,[22, 23, 24, 25] but after contact with authors, we received unpublished data for a further 7 studies[26, 27, 28, 29, 30, 31, 32] (including individual level data for 2 cohorts).[31, 32] We were able to estimate crude data from Kaplan‐Meier curves for another 3 studies.[33, 34, 35] Another 4 studies did not collect the outcomes we were interested in individually. These studies were included in this systematic review but are not poolable in our models: 3 authors could only provide composite endpoint data,[36, 37, 38] and 1 author provided unadjusted hazard ratios.[39] Inter‐reviewer agreement for inclusion was 80% (Cohen's = 0.60).
Characteristics of Included Studies
The 18 studies ranged in size from 58 to 1418 patients; 13 were cohort studies and 5 included secondary data from randomized control trials.[22, 27, 30, 34, 36] All studies ascertained depressive status by screening during index medical admission with either diagnostic interview or self‐report questionnaires, although a variety of scales and definitions for depression were used (Beck Depression Inventory [BDI] in 6 studies, Geriatric Depression Scale in 5 studies, Patient Health Questionnaire in another 4 studies, Medical Outcomes Study‐Depression Questionnaire in 1 study, and Center for Epidemiologic Studies Depression Scale in another study) (Table 1). Screening interviews were conducted mostly by research assistants or nurses (68%) or self‐administered (21%). Most studies examined specific medical patient subgroups (10 cardiac, 3 pulmonary, and 2 elderly). Major exclusion criteria reported were terminal illness (4 studies), unstable condition (6 studies), severe cognitive impairment (5 studies), and suicidal ideation or known depression (4 studies); 1 study enrolled patients with suspected depression (Table 1). Patient cohorts were on average older (range, 5082 years) (Table 1). Attrition rates for readmission and mortality data were low (average <1% among entire sample of studies). All studies scored at least 5 on the Newcastle‐Ottawa scale and were thus considered of at least moderate quality (see Supporting Table 1 in the online version of this article).
| Author, Date of Publication, Enrollment Period | Setting Country/Region, No. of Hospitals | No. of Inpatients, Clinical Features | Major Exclusion Criteria | Follow‐up, mo | Depression Measure (Cutoff) and Screening Method | Mean Age (SD), y | % Female | Positive Screen, No. (%) | Primary Outcome, Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Studies that use a scale based on DSM‐III criteria or a diagnostic interview according to DSM‐III criteria | |||||||||
| Frasure‐Smith et al.,[26] 1993, 19911992* | Canada/Quebec, 1 urban teaching | 218, AMI | Terminal noncardiac illness, unstable, not cognitive | 6 | BDI (10); mod DIS by interviewer, after transfer to medicine | 60 (range, 2488) | 22 | 68 (31), 35 (16) | All‐cause mortality |
| Frasure‐Smith et al.,[27] 1999, 19911992,* 19911994 | Canada/Quebec, 1 urban teaching, 10 urban area | 218; 78, AMI | Terminal noncardiac illness, unstable, not cognitive | 12 | BDI (10) by interviewer, after transfer to medicine | 60 (11) | 32 | 290 (32) | Cardiac mortality |
| Freedland et al.,[25] 1991, 1990 | USA/MO, 1 urban teaching | 58, CHF 75 years | Dementia, medically unstable | 3 | Mod DIS by psychiatric residents and interviewer | 78 (6) | 57 | 10 (17) | All‐cause readmission, all‐cause mortality |
| Fulop et al.,[38] 2003, 2002 | USA/NY, 1 urban teaching | 203, CHF 65 years | 1, 6 | GDS (10); SCID‐NP by interviewer, at discharge | 77 (8) | 53 | 73 (36), 44 (22) | Depression, composite PCP, ED, care visits, and readmission | |
| Lesprance et al.,[28] 2000, 19941996 | Canada/Quebec, 1 urban teaching | 430, unstable angina | Terminal noncardiac illness, not cognitive, recent CABG | 12 | BDI (10); mod DIS by interviewer, 5 days after admission | 62 (11) | 29 | 178 (41), 120 (28) | Cardiac death and MI, any death, angina readmission |
| Rumsfeld et al.,[30] 2005, 19992001 | CA, USA, UK, multiple | 634, AMI with CHF | Valvular or congenital heart failure | Up to 32 | MOS‐D (0.06) by interviewer, before discharge | 65 (11) | 28 | 143 (23) | All‐cause death, CVD death and readmission |
| Song et al.,[33] 2009, 2005 | South Korea, 2 urban teaching | 165, HF | If minor criteria for HF attributable to other medical condition | 6 | BDI (10) self‐administer or interviewer, 34 days of admin | 62 (13) | 49 | 131 (79) | HF readmission and all‐cause mortality, HF readmit |
| Papaioannou et al.,[29] 2013, 20092010 | Greece/Athens, 1 urban | 230, AECOPD | Other respiratory illness, known depressed | Monthly up to 12 | BDI‐I (19) self‐administer, first day | 71 (9) | 12 | 91 (40) | All‐cause mortality, AECOPD readmission |
| Studies that use a scale based on or validated against DSM‐IV criteria or a diagnostic interview according to DSM‐IV criteria | |||||||||
| Almagro et al.,[31] 2002, 19961997 | Spain, 1 urban teaching | 130, AECOPD | Other pulmonary disease | July 1999 | GDS‐SF (6) by interviewer, day before discharge | 72 (9) | 8 | 43 (33) | All‐cause mortality |
| Almagro et al.,[32] 2012, 20032004 | Spain, 1 urban teaching | 134, AECOPD | Other pulmonary disease | 1, 36 | GDS‐SF (6) by interviewer | 72 (10) | 5 | 55 (41) | All‐cause mortality, lung function, frailty |
| Bla et al.,[39] 2001, 2000 | Switzerland, 1 urban teaching | 401, medical 75 years | Stay <24 hours, elective/facility transfer, unstable, not cognitive | 6 | GDS‐SF (6) by interviewer, within 2 days of admission | 82 (7599) | 61 | 90 (22) | All‐cause readmission, all‐cause mortality |
| Cancino et al.,[22] 2014, 20062007,* 20082009 | USA/MA, 1 urban tertiary | 680; 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1 | PHQ‐9 (5 or severity) by interviewer, on admin | 50 (14) | 51 | 561 (40) | All‐cause readmission, ED visits, PCP visits |
| Mitchell et al.,[36] 2010, 20062007* | USA/MA, 1 urban tertiary | 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1, 2, 3 | PHQ‐9 (5) by interviewer, on admin | 50 (15) | 50 | 238 (32) | ED visits and all‐cause readmission |
| Covinsky et al.,[34] 1999, 19901992 | USA/OH, 1 urban teaching | 573, medical | ICU, oncology, telemetry, nursing home admissions | 36 | GDS‐SF (6) by interviewer, within 2 days of admission | 80 | 68 | 197 (34) | All‐cause mortality |
| Jiang et al.,[23] 2001, 19971998 | USA/NC, 1 urban teaching | 357 (331 DIS only), CHF | Suicidal, planned surgery, pregnant | 3, 12 | BDI (10) self‐admin; mod DIS (+BDI only) by interviewer | 63 (13) | 33 | 126 (35), 46 (14) | All‐cause mortality, all‐cause readmission |
| Kartha et al.,[24] 2007, 20022004 | USA/MA, 1 urban safety net | 144, medical recently hospitalized | Planned readmission, unable to keep PCP appointments | 3 | PHQ‐9 (algorithm) by interviewer | 55 (16) | 56 | 39 (27) | All‐cause readmission |
| Koenig and Kuchbhatla,[37] 1999, 1997 | USA/NC, 1 urban teaching | 331, medical 60 years | Stay <3 or >7 days, ICU/CCU, severe illness, nursing home transfers | 3, 6, 9, 12 | CES‐D (16) or HAM‐D (11) or DIS by psychiatrist, on or after third day | 70 (7) | 51 | 160 (48) | Depression, composite physical disability, health visits, and all‐cause readmission |
| Rollman et al.,[35] 2012, 20072009 | USA/PA, 4 urban teaching | 471, CHF, suspected depressed | Antidepressants users (excluded from PHQ‐2 group only) | Up to 12 | PHQ‐2; PHQ‐9 (5 in +PHQ‐2), by interviewer, 4 days | 66 (13) | 35 | 371 (79), 351 (74) | All‐cause mortality |
Prevalence and Recognition of Depressive Symptoms
The range of depression prevalence in hospitalized medical patients was 14% to 79%, with a median of 32% (interquartile range, 27%40%) (Table 1). In those studies that used a diagnostic interview, the prevalence tended to be lower for major depression, with a median of 17% (interquartile range, 16%22%) (Table 1). None of the included studies reported frequency of clinically recognized depression (ie, prior to screening for the study). Only 2 studies assessed the persistence of depression after discharge: 1 reported that depression persisted in 53% (by screening questionnaire) and 34% (by diagnostic interview) of patients at 30 days,[38] whereas the other reported 48% persistence at 90 days after discharge according to a combined screening method.[37]
Hospital Readmission
Overall, 8 studies provided readmission data. Among patients discharged from acute care medical wards (4 studies reporting on 5 cohorts), 395 of 2433 (16.2%) patients were readmitted within 30 days (Figure 1). Hospitalized patients with depressive symptoms were more likely to be readmitted within 30 days after discharge (20.4% vs 13.7%, RR: 1.73, 95% CI: 1.16‐2.58, P = 0.007, I2 = 55%) (Figure 1), compared to those without depression. Results were consistent for 90‐day readmissions (39.8% vs 31.0%, RR: 1.68, 95% CI: 1.13‐2.50, P = 0.01, I2 = 76%, n = 1543 patients) (see Supporting Figure 2 in the online version of this article) in 6 studies. One individual study examined readmission within 6 months after discharge, but was not poolable in this model, as it presented only hazard ratios and not raw data; however, it did report a 50% increased risk of readmission in medical inpatients aged 75 years (adjusted hazard ratio: 1.50, 95% CI: 1.03‐2.17, n = 401).[39]

Forest plot presents results of the meta‐analysis in which the size of each data marker indicates the weight assigned to individuals studies. Abbreviations: CI, confidence interval; IV, independent variable.
Mortality After Discharge
Overall, 11 studies provided all‐cause mortality data. Among medical patients discharged from acute care in 9 studies, 69 of 3397 (2.0%) patients died within 30 days (Figure 2). Medical patients discharged with depressive symptoms were more likely to die within 30 days (2.8% vs 1.5%, RR: 2.13, 95% CI: 1.31‐3.44, P = 0.002, I2 = 0%) (Figure 2) compared to those without depression. Similar results were found for 90‐day mortality (7.7% vs 4.1%, RR: 2.01, 95% CI: 1.47‐2.76, P < 0.001, I2 = 4%, n = 3784 patients) (see Supporting Figure 3 in the online version of this article) in 11 studies.

ED and PCP Visits
Four studies examined the use of ED or PCP services within 90 days of discharge, but 3 did not have extractable data for meta‐analysis. All showed increased utilization of health services for depressed compared to nondepressed patients after discharge.[22, 36, 37, 38] Depressed patients were more likely to visit the ED (adjusted incidence rate ratio: 1.73, 95% CI: 1.27‐2.36),[36] had significantly more medical encounters (eg, PCP, ED visits, hospital admissions, laboratory tests, and home care [mean 2.9 vs 2.6, P = 0.05])[38] and had a greater number of ED visits alone (27 vs 15 per 100 patients, P = 0.007)[22] within 30 days of hospital discharge compared to nondepressed patients. Similar results were found at 90 days.[36]
Sensitivity Analyses
All told, most studies reported a positive association between depression and adverse events, and this was true regardless of how much adjustment for potential confounding had been undertaken by the authors. Although all studies were qualitatively in the same direction, the magnitude of the association varied due to methodological and/or clinical heterogeneity. Sensitivity analysis revealed no overall difference in pooled risk ratios or heterogeneity between Mantel‐Haenszel fixed effects versus random effects models or with the addition of 0.5 to cells to permit inclusion of zero‐event data. There was no evidence of publication bias; funnel plots and Egger test results are available upon request. There were no statistically significant differences in the risk associated with depressive symptoms whether studies used Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III or DSM‐IV criteria, whether the study samples were disease specific or unselected general medical cohorts, whether studies were of moderate or high quality, or regardless of the severity of depressive symptoms.
DISCUSSION
Summary of Evidence
We found that depression was common in medical inpatients (about one‐third of all patients) and persisted for at least 30 days in up to half of those patients after discharge. We found strong evidence of an association between depressive symptoms and poor short‐term prognosis after discharge from the hospital: a 73% increased risk of readmission and a 2‐fold risk of death within 30 days compared to patients without depressive symptoms with similar results at 90 days.
Our meta‐analysis complements a recent systematic review that found concomitant depression to be a risk factor for poor prognosis among inpatients and outpatients with acute coronary syndrome,[40] and a meta‐analysis that demonstrated an increased risk of 2‐year mortality among patients with depression after myocardial infarction.[41] To our knowledge, our study is the first to quantify the short‐term postdischarge risks across a diverse group of medical inpatients.
The potential mechanisms underlying the observed relationship between depression and adverse patient outcomes after discharge are likely multiple. We believe there are 2 main possibilities. First, the increased risk associated with depression might be due to residual confounding, even though many of these studies did adjust for extensive lists of comorbidities,[22, 24, 26, 27, 29, 30, 33, 35, 36, 39] including functional status[39] and prior health services utilization.[22, 34, 36] This could occur if other risk factors were not sufficiently adjusted for, such as unrecognized comorbidities or concomitant disability, which are often present among chronically ill patients,[42] or if depression were a marker of psychosocial risk factors such as anxiety,[43] stress or poor resiliency,[44] or low social support,[45] though a few adjusted for psychosocial factors such as social support[26] or anxiety.[35] Confounding could also occur if symptoms of acute illness inflate reports of somatic symptoms of depression on self‐report questionnaires. Recent studies on the BDI, found that scores were higher in postmyocardial infarction patients when compared to outpatient controls,[46] but with no differences between those groups in scores for the BDI‐II,[47] a version with fewer somatic symptom questions.
Second, depression may cause adverse outcomes through indirect or direct pathways. Indirect causation could occur if depression hindered self‐care behaviors such as medication adherence.[42] Depression could also act directly through pathophysiological changes. Some studies have suggested that depression is associated with metabolic abnormalities, including alterations in glucose transport[42, 48] and increased vulnerability to obesity, type 2 diabetes mellitus, and/or diabetic complications, common conditions among hospitalized patients that also adversely affect postdischarge outcomes.[40, 48]
Strengths and Limitations
This review has multiple strengths. We cast a broad search and included studies that examined a wide range of medical patient subgroups, thus increasing the generalizability of our findings. We identified a general scarcity of studies on this topic and obtained additional unpublished data for 10 of the 18 relevant studies, and our response rate of 34% is compatible with the 37% response rate reported for Cochrane reviews when seeking additional data from authors.[49] Whether examined qualitatively (vote counting of the number of studies that showed an association) or quantitatively (via formal meta‐analysis), it seems apparent that there is a clinically important association between depression and postdischarge adverse events, but given the number, quality, and heterogeneity of the studies we examined, there may be some ongoing dispute about exactly how strong this association is and the degree of bias contributed by a couple of large studies of the topic.
There are limitations to our review. First, as we did not have individual‐level patient data, we could not use metaregression to explore sources of heterogeneity (clinical or methodological) or adjust for confounding, and this likely contributes to observed differences between individual estimates. For instance, the included studies had heterogeneous screening measures and cutoffs; thus, all cases of depression in these studies might not be equivalent. Some of the included studies assessed depression early during admission where psychological distress may be greatest; others assessed symptoms closer to discharge. Most studies included patients with specific conditions like heart failure or chronic obstructive pulmonary disease rather than a wide spectrum of medical inpatients. Moreover, few studies adjusted for psychosocial risk factors such as social support, anxiety, and functional status, and only 2 studies assessed the persistence of depressive symptoms after discharge. Second, we did not explore quantitative measures of between‐study variation (eg, I2), because experts question its utility given the expected heterogeneity in meta‐analyses of observational studies.[50] Third, although the included studies were deemed to be of at least moderate quality, they could be at risk for sources of bias that may not be sufficiently appraised by the current version of the Newscastle‐Ottawa scale for observational studies. Finally, we excluded grey literature (eg, conference proceedings or technical reports) that could potentially exclude null findings, although we did contact authors in this field to identify additional unpublished data relevant to this topic.
CONCLUSIONS
We have confirmed that depressive symptoms are common in hospitalized medical patients, frequently persist after discharge, and may predict greater risk of readmission or death after discharge. Thus, depressive symptoms are an additional marker that clinicians can use to help identify patients in acute care medical settings who may be at increased risk for suboptimal transition back to the community and who may require additional resources after discharge. However, future research is required to evaluate whether treatment of individuals who screen positive for depressive symptoms can reduce 30‐day readmission rates, and we are aware of at least 1 relevant ongoing trial (
Acknowledgements
The authors thank the following individuals: Dale Storie, MLIS, Saskatchewan Information and Library Services Consortium, Regina, Saskatchewan, Canada, for assistance in the literature search; James A. Hanley, PhD, Department of Epidemiology and Biostatistics, Faculty of Medicine, McGill University, Montreal, Quebec, Canada, for guidance in data recovery methods; Nancy Frasure‐Smith, PhD, Department of Psychiatry, McGill University, Department of Psychiatry and Research Centre Hospital Centre, University of Montreal, and Montreal Heart Institute Research Centre, Montreal, Quebec, Canada; Andriana I. Papaioannou, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; Konstantinos Kostikas, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; and Pere Almagro, MD, Servicio de Medicina Interna, Hospital Universitario Mutua de Terrassa, Terrassa, Barcelona, Spain; as well as Philip G. Jones, MS, Saint Luke's Mid America Heart Institute, Kansas City, Missouri; for their retrieval and contribution of unpublished data.
Disclosures
Ms. Pederson affirms that the manuscript is an honest, accurate, and transparent account of the study being reported with no important omissions. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. Design and conduct of the study: Ms. Pederson, Drs. Majumdar and McAlister. Data acquisition: Ms. Pederson, Ms. Warkentin. Analysis and interpretation of the data and drafting of the manuscript: Ms Pederson, Drs. Majumdar and McAlister. Review of the manuscript: all authors. Study supervision: Drs. Majumdar and McAlister. None of the contributors received compensation for their efforts. Salary support for Ms. Pederson was provided by a CRIO grant from Alberta InnovatesHealth Solutions. Drs. McAlister and Majumdar are supported by salary awards from Alberta Innovates‐Health Solutions. Dr. McAlister holds the University of Alberta/Capital Health Chair in Cardiology Outcomes Research. Dr. Majumda holds the University of Alberta Endowed Chair in Patient Health Management. The funding sources had no role in the design or conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. This work is that of the authors independent of funders. The authors report no conflicts of interest.
Between 10% and 40% of patients are readmitted after being discharged from the hospital,[1, 2] and as many as another 25% return to the emergency department (ED) within 30 days.[3] This creates a substantial burden on the healthcare system.[2] Various interventions have been tried to improve the quality of discharge transitions and reduce readmission rates, but results thus far have been inconsistent and generally disappointing.[4, 5, 6] Targeted delivery of interventions to those at highest risk might improve the effectiveness of these efforts and reduce costs. However, current readmission risk assessment models are only moderately predictive, suggesting the presence of unrecognized risk factors.[7, 8]
Active depression might represent a potentially modifiable independent predictor of adverse short‐term hospital outcomes that is currently underutilized. Depression occurs in 5% to 58% of hospitalized adults, depending on how cases are defined.[9, 10] Depression is often under‐recognized and undertreated in acute care clinical settings,[11] and relatively few readmission prediction models incorporate mental health related symptoms.[12]
Although several reviews have examined methods of screening for depression in hospitalized patients[9] or the effectiveness of screening in primary care,[13, 14] to our knowledge no systematic review has examined the impact of depression on short‐term prognosis after discharge from acute care. Therefore, the purpose of this systematic review was to summarize all studies that evaluated whether hospitalized medical patients with depressive symptoms are at higher risk of 30‐day all‐cause readmission or all‐cause mortality after being discharged from the hospital.
METHODS
This study followed an a priori protocol developed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) criteria.[15]
Data Sources and Search Methods
We searched the Cumulative Index to Nursing and Allied Health Literature, Ovid MEDLINE, Ovid Embase, and PsycINFO from inception to January 9, 2015, and the last 5 years of PubMed for full publications with any of the following Medical Subject Headings: depressive disorder, depression, patient readmission, interviews, psychological, inpatients, with restrictions for peer‐reviewed publication, humans, adults aged 18 years, and the English language. Search strategies were developed with a librarian (available upon request). We manually searched reference lists of all included studies and relevant review articles and contacted content experts to identify additional publications.
Eligibility Criteria and Selection of Studies
Two authors (J.L.P. and L.M.W.) independently screened full texts of all relevant articles for inclusion. Disagreements were resolved by consensus or a third reviewer (S.R.M.). We considered any original research that compared readmission or mortality after discharge for hospitalized medical patients (ie, general patients or subgroups thereof) with versus without depression identified by any validated depression measure,[16] including any study design that incorporated at least 30‐day follow‐up postdischarge. We excluded studies that examined patients hospitalized in nonacute care settings or on surgical, psychiatric, obstetric, or intensive care services. We calculated Cohen's coefficient to evaluate inter‐rater agreement on study selection.
Data Extraction
Data were abstracted by 2 authors (J.L.P. and L.M.W.). Disagreements were resolved by consensus or a third reviewer (S.R.M.). We contacted authors of all included studies to obtain missing data. If unavailable, crude data were estimated from published survival curves employing validated techniques in R (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria) and Digitizeit (
Data Synthesis and Statistical Analysis
Where possible, we calculated the pooled risk ratio (RR) with 95% confidence interval (95% CI) using a random effects models in Review Manager (RevMan) 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark). The random effects approach that we employed assumes heterogeneity (ie, underlying parameters vary between individual studies) and is distributed around a mean or population average effect, and results in more conservative (wider) confidence intervals, wherein larger cohorts (or studies with smaller standard errors) are given more weight. Heterogeneity was assessed using the I2 statistic, with values of <25%, 25% to 50%, and >50% representing low, moderate, and high heterogeneity.[19] As per the guidance of Higgins et al., we did not a priori define any degree of heterogeneity that would preclude pooling of the data; the expectation would be that heterogeneity would be substantially higher pooling observational studies rather than randomized trials.[19] Statistical significance was considered a 2‐sided P value 0.05.
Quality Assessment and Risk of Bias
We assessed study quality using the 9‐item Newcastle‐Ottawa scale with 0 to 3, 4 to 6, and 7 to 9 stars considered low, moderate, and high quality, respectively, and criteria for external and internal validity, including group selection and comparability, outcome assessment, and adequacy of follow‐up.[20] Adjusted estimates published in individual reports (or obtained directly from authors) were compared wherever possible with unadjusted estimates to assess the degree of confounding. We generated funnel plots in RevMan 5.3 and conducted Egger tests using Stata 13 (StataCorp LP, College Station, TX) to assess for publication bias.[21]
RESULTS
Study Selection
After removing duplicate publications, we identified 4066 reports and reviewed 133 reports in full text (see Supporting Figure 1 in the online version of this article). Despite our broad study inclusion criteria, we found only 35 longitudinal studies addressing this question. All 35 authors were contacted for additional outcomes data and other missing information (response rate of 34%). We had to exclude 17 studies as they did not provide 30 or 90‐day post‐discharge outcomes. Only 4 studies had published crude data for outcomes within 90 days,[22, 23, 24, 25] but after contact with authors, we received unpublished data for a further 7 studies[26, 27, 28, 29, 30, 31, 32] (including individual level data for 2 cohorts).[31, 32] We were able to estimate crude data from Kaplan‐Meier curves for another 3 studies.[33, 34, 35] Another 4 studies did not collect the outcomes we were interested in individually. These studies were included in this systematic review but are not poolable in our models: 3 authors could only provide composite endpoint data,[36, 37, 38] and 1 author provided unadjusted hazard ratios.[39] Inter‐reviewer agreement for inclusion was 80% (Cohen's = 0.60).
Characteristics of Included Studies
The 18 studies ranged in size from 58 to 1418 patients; 13 were cohort studies and 5 included secondary data from randomized control trials.[22, 27, 30, 34, 36] All studies ascertained depressive status by screening during index medical admission with either diagnostic interview or self‐report questionnaires, although a variety of scales and definitions for depression were used (Beck Depression Inventory [BDI] in 6 studies, Geriatric Depression Scale in 5 studies, Patient Health Questionnaire in another 4 studies, Medical Outcomes Study‐Depression Questionnaire in 1 study, and Center for Epidemiologic Studies Depression Scale in another study) (Table 1). Screening interviews were conducted mostly by research assistants or nurses (68%) or self‐administered (21%). Most studies examined specific medical patient subgroups (10 cardiac, 3 pulmonary, and 2 elderly). Major exclusion criteria reported were terminal illness (4 studies), unstable condition (6 studies), severe cognitive impairment (5 studies), and suicidal ideation or known depression (4 studies); 1 study enrolled patients with suspected depression (Table 1). Patient cohorts were on average older (range, 5082 years) (Table 1). Attrition rates for readmission and mortality data were low (average <1% among entire sample of studies). All studies scored at least 5 on the Newcastle‐Ottawa scale and were thus considered of at least moderate quality (see Supporting Table 1 in the online version of this article).
| Author, Date of Publication, Enrollment Period | Setting Country/Region, No. of Hospitals | No. of Inpatients, Clinical Features | Major Exclusion Criteria | Follow‐up, mo | Depression Measure (Cutoff) and Screening Method | Mean Age (SD), y | % Female | Positive Screen, No. (%) | Primary Outcome, Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Studies that use a scale based on DSM‐III criteria or a diagnostic interview according to DSM‐III criteria | |||||||||
| Frasure‐Smith et al.,[26] 1993, 19911992* | Canada/Quebec, 1 urban teaching | 218, AMI | Terminal noncardiac illness, unstable, not cognitive | 6 | BDI (10); mod DIS by interviewer, after transfer to medicine | 60 (range, 2488) | 22 | 68 (31), 35 (16) | All‐cause mortality |
| Frasure‐Smith et al.,[27] 1999, 19911992,* 19911994 | Canada/Quebec, 1 urban teaching, 10 urban area | 218; 78, AMI | Terminal noncardiac illness, unstable, not cognitive | 12 | BDI (10) by interviewer, after transfer to medicine | 60 (11) | 32 | 290 (32) | Cardiac mortality |
| Freedland et al.,[25] 1991, 1990 | USA/MO, 1 urban teaching | 58, CHF 75 years | Dementia, medically unstable | 3 | Mod DIS by psychiatric residents and interviewer | 78 (6) | 57 | 10 (17) | All‐cause readmission, all‐cause mortality |
| Fulop et al.,[38] 2003, 2002 | USA/NY, 1 urban teaching | 203, CHF 65 years | 1, 6 | GDS (10); SCID‐NP by interviewer, at discharge | 77 (8) | 53 | 73 (36), 44 (22) | Depression, composite PCP, ED, care visits, and readmission | |
| Lesprance et al.,[28] 2000, 19941996 | Canada/Quebec, 1 urban teaching | 430, unstable angina | Terminal noncardiac illness, not cognitive, recent CABG | 12 | BDI (10); mod DIS by interviewer, 5 days after admission | 62 (11) | 29 | 178 (41), 120 (28) | Cardiac death and MI, any death, angina readmission |
| Rumsfeld et al.,[30] 2005, 19992001 | CA, USA, UK, multiple | 634, AMI with CHF | Valvular or congenital heart failure | Up to 32 | MOS‐D (0.06) by interviewer, before discharge | 65 (11) | 28 | 143 (23) | All‐cause death, CVD death and readmission |
| Song et al.,[33] 2009, 2005 | South Korea, 2 urban teaching | 165, HF | If minor criteria for HF attributable to other medical condition | 6 | BDI (10) self‐administer or interviewer, 34 days of admin | 62 (13) | 49 | 131 (79) | HF readmission and all‐cause mortality, HF readmit |
| Papaioannou et al.,[29] 2013, 20092010 | Greece/Athens, 1 urban | 230, AECOPD | Other respiratory illness, known depressed | Monthly up to 12 | BDI‐I (19) self‐administer, first day | 71 (9) | 12 | 91 (40) | All‐cause mortality, AECOPD readmission |
| Studies that use a scale based on or validated against DSM‐IV criteria or a diagnostic interview according to DSM‐IV criteria | |||||||||
| Almagro et al.,[31] 2002, 19961997 | Spain, 1 urban teaching | 130, AECOPD | Other pulmonary disease | July 1999 | GDS‐SF (6) by interviewer, day before discharge | 72 (9) | 8 | 43 (33) | All‐cause mortality |
| Almagro et al.,[32] 2012, 20032004 | Spain, 1 urban teaching | 134, AECOPD | Other pulmonary disease | 1, 36 | GDS‐SF (6) by interviewer | 72 (10) | 5 | 55 (41) | All‐cause mortality, lung function, frailty |
| Bla et al.,[39] 2001, 2000 | Switzerland, 1 urban teaching | 401, medical 75 years | Stay <24 hours, elective/facility transfer, unstable, not cognitive | 6 | GDS‐SF (6) by interviewer, within 2 days of admission | 82 (7599) | 61 | 90 (22) | All‐cause readmission, all‐cause mortality |
| Cancino et al.,[22] 2014, 20062007,* 20082009 | USA/MA, 1 urban tertiary | 680; 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1 | PHQ‐9 (5 or severity) by interviewer, on admin | 50 (14) | 51 | 561 (40) | All‐cause readmission, ED visits, PCP visits |
| Mitchell et al.,[36] 2010, 20062007* | USA/MA, 1 urban tertiary | 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1, 2, 3 | PHQ‐9 (5) by interviewer, on admin | 50 (15) | 50 | 238 (32) | ED visits and all‐cause readmission |
| Covinsky et al.,[34] 1999, 19901992 | USA/OH, 1 urban teaching | 573, medical | ICU, oncology, telemetry, nursing home admissions | 36 | GDS‐SF (6) by interviewer, within 2 days of admission | 80 | 68 | 197 (34) | All‐cause mortality |
| Jiang et al.,[23] 2001, 19971998 | USA/NC, 1 urban teaching | 357 (331 DIS only), CHF | Suicidal, planned surgery, pregnant | 3, 12 | BDI (10) self‐admin; mod DIS (+BDI only) by interviewer | 63 (13) | 33 | 126 (35), 46 (14) | All‐cause mortality, all‐cause readmission |
| Kartha et al.,[24] 2007, 20022004 | USA/MA, 1 urban safety net | 144, medical recently hospitalized | Planned readmission, unable to keep PCP appointments | 3 | PHQ‐9 (algorithm) by interviewer | 55 (16) | 56 | 39 (27) | All‐cause readmission |
| Koenig and Kuchbhatla,[37] 1999, 1997 | USA/NC, 1 urban teaching | 331, medical 60 years | Stay <3 or >7 days, ICU/CCU, severe illness, nursing home transfers | 3, 6, 9, 12 | CES‐D (16) or HAM‐D (11) or DIS by psychiatrist, on or after third day | 70 (7) | 51 | 160 (48) | Depression, composite physical disability, health visits, and all‐cause readmission |
| Rollman et al.,[35] 2012, 20072009 | USA/PA, 4 urban teaching | 471, CHF, suspected depressed | Antidepressants users (excluded from PHQ‐2 group only) | Up to 12 | PHQ‐2; PHQ‐9 (5 in +PHQ‐2), by interviewer, 4 days | 66 (13) | 35 | 371 (79), 351 (74) | All‐cause mortality |
Prevalence and Recognition of Depressive Symptoms
The range of depression prevalence in hospitalized medical patients was 14% to 79%, with a median of 32% (interquartile range, 27%40%) (Table 1). In those studies that used a diagnostic interview, the prevalence tended to be lower for major depression, with a median of 17% (interquartile range, 16%22%) (Table 1). None of the included studies reported frequency of clinically recognized depression (ie, prior to screening for the study). Only 2 studies assessed the persistence of depression after discharge: 1 reported that depression persisted in 53% (by screening questionnaire) and 34% (by diagnostic interview) of patients at 30 days,[38] whereas the other reported 48% persistence at 90 days after discharge according to a combined screening method.[37]
Hospital Readmission
Overall, 8 studies provided readmission data. Among patients discharged from acute care medical wards (4 studies reporting on 5 cohorts), 395 of 2433 (16.2%) patients were readmitted within 30 days (Figure 1). Hospitalized patients with depressive symptoms were more likely to be readmitted within 30 days after discharge (20.4% vs 13.7%, RR: 1.73, 95% CI: 1.16‐2.58, P = 0.007, I2 = 55%) (Figure 1), compared to those without depression. Results were consistent for 90‐day readmissions (39.8% vs 31.0%, RR: 1.68, 95% CI: 1.13‐2.50, P = 0.01, I2 = 76%, n = 1543 patients) (see Supporting Figure 2 in the online version of this article) in 6 studies. One individual study examined readmission within 6 months after discharge, but was not poolable in this model, as it presented only hazard ratios and not raw data; however, it did report a 50% increased risk of readmission in medical inpatients aged 75 years (adjusted hazard ratio: 1.50, 95% CI: 1.03‐2.17, n = 401).[39]

Forest plot presents results of the meta‐analysis in which the size of each data marker indicates the weight assigned to individuals studies. Abbreviations: CI, confidence interval; IV, independent variable.
Mortality After Discharge
Overall, 11 studies provided all‐cause mortality data. Among medical patients discharged from acute care in 9 studies, 69 of 3397 (2.0%) patients died within 30 days (Figure 2). Medical patients discharged with depressive symptoms were more likely to die within 30 days (2.8% vs 1.5%, RR: 2.13, 95% CI: 1.31‐3.44, P = 0.002, I2 = 0%) (Figure 2) compared to those without depression. Similar results were found for 90‐day mortality (7.7% vs 4.1%, RR: 2.01, 95% CI: 1.47‐2.76, P < 0.001, I2 = 4%, n = 3784 patients) (see Supporting Figure 3 in the online version of this article) in 11 studies.

ED and PCP Visits
Four studies examined the use of ED or PCP services within 90 days of discharge, but 3 did not have extractable data for meta‐analysis. All showed increased utilization of health services for depressed compared to nondepressed patients after discharge.[22, 36, 37, 38] Depressed patients were more likely to visit the ED (adjusted incidence rate ratio: 1.73, 95% CI: 1.27‐2.36),[36] had significantly more medical encounters (eg, PCP, ED visits, hospital admissions, laboratory tests, and home care [mean 2.9 vs 2.6, P = 0.05])[38] and had a greater number of ED visits alone (27 vs 15 per 100 patients, P = 0.007)[22] within 30 days of hospital discharge compared to nondepressed patients. Similar results were found at 90 days.[36]
Sensitivity Analyses
All told, most studies reported a positive association between depression and adverse events, and this was true regardless of how much adjustment for potential confounding had been undertaken by the authors. Although all studies were qualitatively in the same direction, the magnitude of the association varied due to methodological and/or clinical heterogeneity. Sensitivity analysis revealed no overall difference in pooled risk ratios or heterogeneity between Mantel‐Haenszel fixed effects versus random effects models or with the addition of 0.5 to cells to permit inclusion of zero‐event data. There was no evidence of publication bias; funnel plots and Egger test results are available upon request. There were no statistically significant differences in the risk associated with depressive symptoms whether studies used Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III or DSM‐IV criteria, whether the study samples were disease specific or unselected general medical cohorts, whether studies were of moderate or high quality, or regardless of the severity of depressive symptoms.
DISCUSSION
Summary of Evidence
We found that depression was common in medical inpatients (about one‐third of all patients) and persisted for at least 30 days in up to half of those patients after discharge. We found strong evidence of an association between depressive symptoms and poor short‐term prognosis after discharge from the hospital: a 73% increased risk of readmission and a 2‐fold risk of death within 30 days compared to patients without depressive symptoms with similar results at 90 days.
Our meta‐analysis complements a recent systematic review that found concomitant depression to be a risk factor for poor prognosis among inpatients and outpatients with acute coronary syndrome,[40] and a meta‐analysis that demonstrated an increased risk of 2‐year mortality among patients with depression after myocardial infarction.[41] To our knowledge, our study is the first to quantify the short‐term postdischarge risks across a diverse group of medical inpatients.
The potential mechanisms underlying the observed relationship between depression and adverse patient outcomes after discharge are likely multiple. We believe there are 2 main possibilities. First, the increased risk associated with depression might be due to residual confounding, even though many of these studies did adjust for extensive lists of comorbidities,[22, 24, 26, 27, 29, 30, 33, 35, 36, 39] including functional status[39] and prior health services utilization.[22, 34, 36] This could occur if other risk factors were not sufficiently adjusted for, such as unrecognized comorbidities or concomitant disability, which are often present among chronically ill patients,[42] or if depression were a marker of psychosocial risk factors such as anxiety,[43] stress or poor resiliency,[44] or low social support,[45] though a few adjusted for psychosocial factors such as social support[26] or anxiety.[35] Confounding could also occur if symptoms of acute illness inflate reports of somatic symptoms of depression on self‐report questionnaires. Recent studies on the BDI, found that scores were higher in postmyocardial infarction patients when compared to outpatient controls,[46] but with no differences between those groups in scores for the BDI‐II,[47] a version with fewer somatic symptom questions.
Second, depression may cause adverse outcomes through indirect or direct pathways. Indirect causation could occur if depression hindered self‐care behaviors such as medication adherence.[42] Depression could also act directly through pathophysiological changes. Some studies have suggested that depression is associated with metabolic abnormalities, including alterations in glucose transport[42, 48] and increased vulnerability to obesity, type 2 diabetes mellitus, and/or diabetic complications, common conditions among hospitalized patients that also adversely affect postdischarge outcomes.[40, 48]
Strengths and Limitations
This review has multiple strengths. We cast a broad search and included studies that examined a wide range of medical patient subgroups, thus increasing the generalizability of our findings. We identified a general scarcity of studies on this topic and obtained additional unpublished data for 10 of the 18 relevant studies, and our response rate of 34% is compatible with the 37% response rate reported for Cochrane reviews when seeking additional data from authors.[49] Whether examined qualitatively (vote counting of the number of studies that showed an association) or quantitatively (via formal meta‐analysis), it seems apparent that there is a clinically important association between depression and postdischarge adverse events, but given the number, quality, and heterogeneity of the studies we examined, there may be some ongoing dispute about exactly how strong this association is and the degree of bias contributed by a couple of large studies of the topic.
There are limitations to our review. First, as we did not have individual‐level patient data, we could not use metaregression to explore sources of heterogeneity (clinical or methodological) or adjust for confounding, and this likely contributes to observed differences between individual estimates. For instance, the included studies had heterogeneous screening measures and cutoffs; thus, all cases of depression in these studies might not be equivalent. Some of the included studies assessed depression early during admission where psychological distress may be greatest; others assessed symptoms closer to discharge. Most studies included patients with specific conditions like heart failure or chronic obstructive pulmonary disease rather than a wide spectrum of medical inpatients. Moreover, few studies adjusted for psychosocial risk factors such as social support, anxiety, and functional status, and only 2 studies assessed the persistence of depressive symptoms after discharge. Second, we did not explore quantitative measures of between‐study variation (eg, I2), because experts question its utility given the expected heterogeneity in meta‐analyses of observational studies.[50] Third, although the included studies were deemed to be of at least moderate quality, they could be at risk for sources of bias that may not be sufficiently appraised by the current version of the Newscastle‐Ottawa scale for observational studies. Finally, we excluded grey literature (eg, conference proceedings or technical reports) that could potentially exclude null findings, although we did contact authors in this field to identify additional unpublished data relevant to this topic.
CONCLUSIONS
We have confirmed that depressive symptoms are common in hospitalized medical patients, frequently persist after discharge, and may predict greater risk of readmission or death after discharge. Thus, depressive symptoms are an additional marker that clinicians can use to help identify patients in acute care medical settings who may be at increased risk for suboptimal transition back to the community and who may require additional resources after discharge. However, future research is required to evaluate whether treatment of individuals who screen positive for depressive symptoms can reduce 30‐day readmission rates, and we are aware of at least 1 relevant ongoing trial (
Acknowledgements
The authors thank the following individuals: Dale Storie, MLIS, Saskatchewan Information and Library Services Consortium, Regina, Saskatchewan, Canada, for assistance in the literature search; James A. Hanley, PhD, Department of Epidemiology and Biostatistics, Faculty of Medicine, McGill University, Montreal, Quebec, Canada, for guidance in data recovery methods; Nancy Frasure‐Smith, PhD, Department of Psychiatry, McGill University, Department of Psychiatry and Research Centre Hospital Centre, University of Montreal, and Montreal Heart Institute Research Centre, Montreal, Quebec, Canada; Andriana I. Papaioannou, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; Konstantinos Kostikas, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; and Pere Almagro, MD, Servicio de Medicina Interna, Hospital Universitario Mutua de Terrassa, Terrassa, Barcelona, Spain; as well as Philip G. Jones, MS, Saint Luke's Mid America Heart Institute, Kansas City, Missouri; for their retrieval and contribution of unpublished data.
Disclosures
Ms. Pederson affirms that the manuscript is an honest, accurate, and transparent account of the study being reported with no important omissions. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. Design and conduct of the study: Ms. Pederson, Drs. Majumdar and McAlister. Data acquisition: Ms. Pederson, Ms. Warkentin. Analysis and interpretation of the data and drafting of the manuscript: Ms Pederson, Drs. Majumdar and McAlister. Review of the manuscript: all authors. Study supervision: Drs. Majumdar and McAlister. None of the contributors received compensation for their efforts. Salary support for Ms. Pederson was provided by a CRIO grant from Alberta InnovatesHealth Solutions. Drs. McAlister and Majumdar are supported by salary awards from Alberta Innovates‐Health Solutions. Dr. McAlister holds the University of Alberta/Capital Health Chair in Cardiology Outcomes Research. Dr. Majumda holds the University of Alberta Endowed Chair in Patient Health Management. The funding sources had no role in the design or conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. This work is that of the authors independent of funders. The authors report no conflicts of interest.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , . Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398–407.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . The elusive quest for quality and cost savings in the Medicare program. JAMA. 2009;301(6):668–670.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries—15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–111.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , . Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148–154.
- , , , et al. Prevalence, correlates and recognition of depression among inpatients of general hospitals in Wuhan, China. Gen Hosp Psychiatry. 2010;32(3):268–275.
- , , , , , . Recognition of depression by non‐psychiatric physicians—a systematic literature review and meta‐analysis. J Gen Intern Med. 2008;23(1):25–36.
- , , , , , . Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. Am Heart J. 2012;164(3):365–372.
- , , , et al. Does evidence support the American Heart Association's recommendation to screen patients for depression in cardiovascular care? An updated systematic review. PLoS One. 2013;8(1):e52654.
- , , , et al. Screening for depression: a systematic review and meta‐analysis. CMAJ Open. 2013;1(4):E159–E167.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94.
- , , , et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136(10):765–776.
- , , . Recovering the raw data behind a non‐parametric survival curve. Syst Rev. 2014;3:151.
- , , , . Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
- . Commentary: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160.
- , , , et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed September 1, 2015.
- , , . The funnel plot. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication Bias in Meta‐analysis: Prevention, Assessment and Adjustments. New York, NY: John Wiley 2006:73–98.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , et al. Depression in elderly patients with congestive heart failure. J Geriatr Psychiatry. 1991;24(1):59–71.
- , , . Depression following myocardial infarction: impact on 6‐month survival. JAMA. 1993;270(15):1819–1825.
- , , , , . Gender, depression, and one‐year prognosis after myocardial infarction. Psychosom Med. 1999;61(1):26–37.
- , , , . Depression and 1‐year prognosis in unstable angina. Arch Intern Med. 2000;160(9):1354–1360.
- , , , et al. The impact of depressive symptoms on recovery and outcome of hospitalised COPD exacerbations. Eur Respir J. 2013;41(4):815–823.
- , , , et al. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. Am Heart J. 2005;150(5):961–967.
- , , , et al. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448.
- , , , et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration. 2012;84(1):36–43.
- , , . Depressive symptoms increase risk of rehospitalisation in heart failure patients with preserved systolic function. J Clin Nurs. 2009;18(13):1871–1877.
- , , . Depressive symptoms and 3 year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. A positive 2‐item patient health questionnaire depression screen among hospitalized heart failure patients is associated with elevated 12‐month mortality. J Card Fail. 2012;18(3):238–245.
- , , , et al. Post‐discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378–384.
- , . Use of health services by medically ill depressed elderly patients after hospital discharge. Am J Geriatr Psychiatry. 1999;7(1):48–56.
- , , . Congestive heart failure and depression in older adults: clinical course and health services use 6 months after hospitalization. Psychosomatics. 2003;44(5):367–373.
- , , , . Depressive symptoms as a predictor of 6‐month outcomes and services utilization in elderly medical inpatients. Arch Intern Med. 2001;161(21):2609–2615.
- , , , et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129(12):1350–1369.
- , , , , , . Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta‐analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33(3):203–216.
- , , , , . Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925.
- , , , et al. Prognostic value of depression, anxiety, and anger in hospitalized cardiovascular disease patients for predicting adverse cardiac outcomes. Am J Cardiol. 2013;111(10):1432–1436.
- , , . The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
- , , , et al. The influence of somatic symptoms on beck depression inventory scores in hospitalized postmyocardial infarction patients. Can J Psychiatry. 2012;57(12):752–758.
- , , , et al. Somatic symptom overlap in beck depression inventory‐II scores following myocardial infarction. Br J Psychiatry. 2010;197(1):61–66.
- , , , . Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54(3):317–329.
- , , . Searching for unpublished data for Cochrane reviews: cross sectional study. BMJ. 2013;346:f2231.
- . Comment on: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39(3):932; author reply 933.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , . Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398–407.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . The elusive quest for quality and cost savings in the Medicare program. JAMA. 2009;301(6):668–670.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries—15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–111.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , . Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148–154.
- , , , et al. Prevalence, correlates and recognition of depression among inpatients of general hospitals in Wuhan, China. Gen Hosp Psychiatry. 2010;32(3):268–275.
- , , , , , . Recognition of depression by non‐psychiatric physicians—a systematic literature review and meta‐analysis. J Gen Intern Med. 2008;23(1):25–36.
- , , , , , . Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. Am Heart J. 2012;164(3):365–372.
- , , , et al. Does evidence support the American Heart Association's recommendation to screen patients for depression in cardiovascular care? An updated systematic review. PLoS One. 2013;8(1):e52654.
- , , , et al. Screening for depression: a systematic review and meta‐analysis. CMAJ Open. 2013;1(4):E159–E167.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94.
- , , , et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136(10):765–776.
- , , . Recovering the raw data behind a non‐parametric survival curve. Syst Rev. 2014;3:151.
- , , , . Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
- . Commentary: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160.
- , , , et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed September 1, 2015.
- , , . The funnel plot. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication Bias in Meta‐analysis: Prevention, Assessment and Adjustments. New York, NY: John Wiley 2006:73–98.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , et al. Depression in elderly patients with congestive heart failure. J Geriatr Psychiatry. 1991;24(1):59–71.
- , , . Depression following myocardial infarction: impact on 6‐month survival. JAMA. 1993;270(15):1819–1825.
- , , , , . Gender, depression, and one‐year prognosis after myocardial infarction. Psychosom Med. 1999;61(1):26–37.
- , , , . Depression and 1‐year prognosis in unstable angina. Arch Intern Med. 2000;160(9):1354–1360.
- , , , et al. The impact of depressive symptoms on recovery and outcome of hospitalised COPD exacerbations. Eur Respir J. 2013;41(4):815–823.
- , , , et al. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. Am Heart J. 2005;150(5):961–967.
- , , , et al. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448.
- , , , et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration. 2012;84(1):36–43.
- , , . Depressive symptoms increase risk of rehospitalisation in heart failure patients with preserved systolic function. J Clin Nurs. 2009;18(13):1871–1877.
- , , . Depressive symptoms and 3 year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. A positive 2‐item patient health questionnaire depression screen among hospitalized heart failure patients is associated with elevated 12‐month mortality. J Card Fail. 2012;18(3):238–245.
- , , , et al. Post‐discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378–384.
- , . Use of health services by medically ill depressed elderly patients after hospital discharge. Am J Geriatr Psychiatry. 1999;7(1):48–56.
- , , . Congestive heart failure and depression in older adults: clinical course and health services use 6 months after hospitalization. Psychosomatics. 2003;44(5):367–373.
- , , , . Depressive symptoms as a predictor of 6‐month outcomes and services utilization in elderly medical inpatients. Arch Intern Med. 2001;161(21):2609–2615.
- , , , et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129(12):1350–1369.
- , , , , , . Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta‐analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33(3):203–216.
- , , , , . Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925.
- , , , et al. Prognostic value of depression, anxiety, and anger in hospitalized cardiovascular disease patients for predicting adverse cardiac outcomes. Am J Cardiol. 2013;111(10):1432–1436.
- , , . The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
- , , , et al. The influence of somatic symptoms on beck depression inventory scores in hospitalized postmyocardial infarction patients. Can J Psychiatry. 2012;57(12):752–758.
- , , , et al. Somatic symptom overlap in beck depression inventory‐II scores following myocardial infarction. Br J Psychiatry. 2010;197(1):61–66.
- , , , . Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54(3):317–329.
- , , . Searching for unpublished data for Cochrane reviews: cross sectional study. BMJ. 2013;346:f2231.
- . Comment on: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39(3):932; author reply 933.
VIDEO: Shorter gap from heart attack to CABG shown safe
PHOENIX – Patients who are stable following a myocardial infarction and need isolated coronary artery bypass surgery (CABG) don’t need to wait 5 or so days for their surgery, a delay that many surgeons and cardiologists often impose.
The operation can safely occur after just a 1- or 2-day gap following either an ST-elevation MI or a non–ST-elevation MI, based on real-world outcomes seen in more than 3,000 patients treated at any of seven U.S. medical centers.
“Waiting an arbitrary 5 days is not important,” Elizabeth L. Nichols said during a video interview and during her report at the annual meeting of the Society of Thoracic Surgeons.
Ms. Nichols and her associates analyzed the in-hospital mortality rates among 3,060 patients who underwent isolated CABG during 2008-2014 at any of the seven medical centers that participate in the Northern New England Cardiovascular Disease Study Group and offer CABG. They included patients who had their surgery within 21 days of their MI, and excluded patients who had their CABG within 6 hours of their MI, had emergency surgery, or those with shock or incomplete data. The study group included 529 patients who had a ST-elevation MI and 2,531 patients with a non-ST-elevation MI.
The analysis divided patients into four groups based on timing of their CABG: 99 patients (3%) had surgery within the first 24 hours, 369 patients (12%) had their surgery 1-2 days after their MI, 1,966 (64%) had their operation 3-7 days following their MI, and 626 (21%) had their surgery 8-21 days after the MI.
The unadjusted mortality rates for these four subgroups were 5.1%, 1.6%, 1.6%, and 2.7%, respectively, reported Ms. Nichols, a health services researcher at the Dartmouth Institute for Health Policy & Clinical Practice, Lebanon, N.H.
After researchers adjusted for several demographic and clinical variables, the mortality rates remained identical for patients who underwent CABG 1 or 2 days following their MI, compared with patients whose surgery was deferred until 3-7 days after the MI. Patients with surgery 8-21 days following the MI had a small but not statistically significant higher rate of in-hospital death.
Patients who had their surgery 7-23 hours following an MI had a statistically significant increased hospital mortality following surgery that ran more than threefold greater than patients who underwent CABG 3-7 days after their MI.
The main message from the analysis is that for the typical, stable MI patient who requires CABG to treat multivessel coronary disease, no need exists to wait several days following an MI to do the surgery, Ms. Nichols explained. A delay of just 1 or 2 days is safe and sufficient, as long as it provides adequate time for any acutely administered antiplatelet or antithrombotic drugs to clear.
The findings “provide a degree of comfort for not waiting the 3-5 days that had previously been thought necessary,” said Dr. Jock N. McCullough, chief of cardiac surgery at Dartmouth-Hitchcock Medical Center in Lebanon and a collaborator on the study.
The findings are not meant to supersede clinical judgment, both Dr. McCullough and Ms. Nichols emphasized. Individual patients might have good reasons to either undergo faster surgery or to wait at least 8 days following their MI.
“The patients who waited 8-21 days had a lot of comorbidities and were sicker patients, and their delay is often warranted” to make sure the patient is stable enough for surgery, Ms. Nichols explained. Other patients might be worsening following their MI and need to undergo their surgery within 24 hours of their MI.
“Clinical judgment is always the trump card,” Ms. Nichols said.
Ms. Nichols and Dr. McCullough had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
PHOENIX – Patients who are stable following a myocardial infarction and need isolated coronary artery bypass surgery (CABG) don’t need to wait 5 or so days for their surgery, a delay that many surgeons and cardiologists often impose.
The operation can safely occur after just a 1- or 2-day gap following either an ST-elevation MI or a non–ST-elevation MI, based on real-world outcomes seen in more than 3,000 patients treated at any of seven U.S. medical centers.
“Waiting an arbitrary 5 days is not important,” Elizabeth L. Nichols said during a video interview and during her report at the annual meeting of the Society of Thoracic Surgeons.
Ms. Nichols and her associates analyzed the in-hospital mortality rates among 3,060 patients who underwent isolated CABG during 2008-2014 at any of the seven medical centers that participate in the Northern New England Cardiovascular Disease Study Group and offer CABG. They included patients who had their surgery within 21 days of their MI, and excluded patients who had their CABG within 6 hours of their MI, had emergency surgery, or those with shock or incomplete data. The study group included 529 patients who had a ST-elevation MI and 2,531 patients with a non-ST-elevation MI.
The analysis divided patients into four groups based on timing of their CABG: 99 patients (3%) had surgery within the first 24 hours, 369 patients (12%) had their surgery 1-2 days after their MI, 1,966 (64%) had their operation 3-7 days following their MI, and 626 (21%) had their surgery 8-21 days after the MI.
The unadjusted mortality rates for these four subgroups were 5.1%, 1.6%, 1.6%, and 2.7%, respectively, reported Ms. Nichols, a health services researcher at the Dartmouth Institute for Health Policy & Clinical Practice, Lebanon, N.H.
After researchers adjusted for several demographic and clinical variables, the mortality rates remained identical for patients who underwent CABG 1 or 2 days following their MI, compared with patients whose surgery was deferred until 3-7 days after the MI. Patients with surgery 8-21 days following the MI had a small but not statistically significant higher rate of in-hospital death.
Patients who had their surgery 7-23 hours following an MI had a statistically significant increased hospital mortality following surgery that ran more than threefold greater than patients who underwent CABG 3-7 days after their MI.
The main message from the analysis is that for the typical, stable MI patient who requires CABG to treat multivessel coronary disease, no need exists to wait several days following an MI to do the surgery, Ms. Nichols explained. A delay of just 1 or 2 days is safe and sufficient, as long as it provides adequate time for any acutely administered antiplatelet or antithrombotic drugs to clear.
The findings “provide a degree of comfort for not waiting the 3-5 days that had previously been thought necessary,” said Dr. Jock N. McCullough, chief of cardiac surgery at Dartmouth-Hitchcock Medical Center in Lebanon and a collaborator on the study.
The findings are not meant to supersede clinical judgment, both Dr. McCullough and Ms. Nichols emphasized. Individual patients might have good reasons to either undergo faster surgery or to wait at least 8 days following their MI.
“The patients who waited 8-21 days had a lot of comorbidities and were sicker patients, and their delay is often warranted” to make sure the patient is stable enough for surgery, Ms. Nichols explained. Other patients might be worsening following their MI and need to undergo their surgery within 24 hours of their MI.
“Clinical judgment is always the trump card,” Ms. Nichols said.
Ms. Nichols and Dr. McCullough had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
PHOENIX – Patients who are stable following a myocardial infarction and need isolated coronary artery bypass surgery (CABG) don’t need to wait 5 or so days for their surgery, a delay that many surgeons and cardiologists often impose.
The operation can safely occur after just a 1- or 2-day gap following either an ST-elevation MI or a non–ST-elevation MI, based on real-world outcomes seen in more than 3,000 patients treated at any of seven U.S. medical centers.
“Waiting an arbitrary 5 days is not important,” Elizabeth L. Nichols said during a video interview and during her report at the annual meeting of the Society of Thoracic Surgeons.
Ms. Nichols and her associates analyzed the in-hospital mortality rates among 3,060 patients who underwent isolated CABG during 2008-2014 at any of the seven medical centers that participate in the Northern New England Cardiovascular Disease Study Group and offer CABG. They included patients who had their surgery within 21 days of their MI, and excluded patients who had their CABG within 6 hours of their MI, had emergency surgery, or those with shock or incomplete data. The study group included 529 patients who had a ST-elevation MI and 2,531 patients with a non-ST-elevation MI.
The analysis divided patients into four groups based on timing of their CABG: 99 patients (3%) had surgery within the first 24 hours, 369 patients (12%) had their surgery 1-2 days after their MI, 1,966 (64%) had their operation 3-7 days following their MI, and 626 (21%) had their surgery 8-21 days after the MI.
The unadjusted mortality rates for these four subgroups were 5.1%, 1.6%, 1.6%, and 2.7%, respectively, reported Ms. Nichols, a health services researcher at the Dartmouth Institute for Health Policy & Clinical Practice, Lebanon, N.H.
After researchers adjusted for several demographic and clinical variables, the mortality rates remained identical for patients who underwent CABG 1 or 2 days following their MI, compared with patients whose surgery was deferred until 3-7 days after the MI. Patients with surgery 8-21 days following the MI had a small but not statistically significant higher rate of in-hospital death.
Patients who had their surgery 7-23 hours following an MI had a statistically significant increased hospital mortality following surgery that ran more than threefold greater than patients who underwent CABG 3-7 days after their MI.
The main message from the analysis is that for the typical, stable MI patient who requires CABG to treat multivessel coronary disease, no need exists to wait several days following an MI to do the surgery, Ms. Nichols explained. A delay of just 1 or 2 days is safe and sufficient, as long as it provides adequate time for any acutely administered antiplatelet or antithrombotic drugs to clear.
The findings “provide a degree of comfort for not waiting the 3-5 days that had previously been thought necessary,” said Dr. Jock N. McCullough, chief of cardiac surgery at Dartmouth-Hitchcock Medical Center in Lebanon and a collaborator on the study.
The findings are not meant to supersede clinical judgment, both Dr. McCullough and Ms. Nichols emphasized. Individual patients might have good reasons to either undergo faster surgery or to wait at least 8 days following their MI.
“The patients who waited 8-21 days had a lot of comorbidities and were sicker patients, and their delay is often warranted” to make sure the patient is stable enough for surgery, Ms. Nichols explained. Other patients might be worsening following their MI and need to undergo their surgery within 24 hours of their MI.
“Clinical judgment is always the trump card,” Ms. Nichols said.
Ms. Nichols and Dr. McCullough had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT THE STS ANNUAL MEETING
Key clinical point: Performing coronary artery bypass grafting 1-2 days following an MI was as safe as when surgery was delayed 3-7 days.
Major finding: In-hospital mortality after CABG was identical in patients operated on 1-2 days or 3-7 days following an MI.
Data source: Retrospective analysis of 3,060 patients who underwent CABG within 21 days following an MI at any of seven U.S. centers.
Disclosures: Ms. Nichols and Dr. McCullough had no disclosures.
Telemental health reaches underserved children, builds partnerships
The patient, a 10-year-old girl, was exhibiting defiant and angry behavior at home. Her pediatrician in rural Vermont was not able to support the family optimally, so he referred her to Dr. David C. Rettew.
Dr. Rettew soon learned that the girl blamed herself for her father’s absence, and she interpreted efforts by her mother to set limits as evidence that her mom didn’t love her.
“During the course of the interview, it became clear that the girl had some specific thoughts that were likely fueling her anger,” said Dr. Rettew, director of the Pediatric Psychiatry Clinic at the University of Vermont, Burlington. “She also met criteria clearly for ADHD, which had never been diagnosed. The consultation recommendations not only included some possible medications to try, but also some specific areas that could be addressed for psychotherapy that could really help the relationship between this child and her mother.”
The successful intervention took place via a secure, two-way videoconference. It is one example of how physicians at the University of Vermont are using telemental health to treat children in underserved areas. As part of a state-funded training program, child psychiatry fellows at the university consult with primary care doctors at federally qualified health centers across the state. The primary care physicians discuss patient cases with fellows via phone or email and can refer patients for in-person or telemental health assessments.
The program has been running for about 5 years and so far has yielded countless benefits, said Dr. Rettew, who directs the university’s Child and Adolescent Psychiatry Fellowship Program. “It helps keep the care housed and centered within the primary care home. That can help coordination of services so that care isn’t fragmented around multiple centers. It also allows evaluations to happen that wouldn’t happen otherwise because it’s too much of a hardship for families to travel long distances and come for regular follow-up appointments.”
University of Vermont physicians also use telemedicine to consult with other mental health clinicians across the state. Child psychiatrist Allison Y. Hall provides in-person training to clinicians and then counsels and supervises their efforts through a telemedicine unit.
“For this purpose, it’s great,” said Dr. Hall, who practices at the Vermont Center for Children, Youth, and Families, which is housed within the university’s psychiatry department. “There’s more confidentiality than Skype, for instance. It’s wonderful to be able to reach clinicians at a great distance.”
On a broader scale, telemental health is a promising tool to address the shortage of mental health providers in the United States, said Dr. Robert C. Gunther, a pediatrician at the University of Virginia Health System in Fishersville. Recent research found that one in three children receiving outpatient care for mental health conditions saw only their primary care doctor for care (Pediatrics. 2015 Nov;136;e1178-85).
“There is a tremendous need for pediatric mental health care,” Dr. Gunther said in an interview. “There is a great shortage of child psychiatrists and other child mental health specialists. Telemental health can help in areas where geography or financial barriers exist to accessing care.”
Data from the Children’s ADHD Telemental Health Treatment Study (CATTS) illustrates the impact that telemental health can have on children facing such barriers to care. Researchers randomized 223 children referred by 88 primary care providers in seven underserved communities into two study groups. Children in the first group were seen by child psychiatrists via videoconference six times over 22 weeks; treatment included pharmacotherapy. Their caregivers received behavior training provided in person by community therapists who were supervised remotely. Children in the second group were treated by their primary care physicians and received one telepsychiatry consult.
Children in both groups improved; however, those randomized to the telemental health model improved “significantly more than patients in the augmented primary care arm” (J Am Acad Child Adolesc Psychiatry. 2015 Apr;54[4]:263-74).
“The CATTS trial demonstrated the effectiveness of a telehealth service model to treat ADHD in communities with limited access to specialty mental health services,” investigators concluded.
But Dr. Joshua J. Alexander, chair of the American Academy of Pediatrics Section on Telehealth Care, notes that some mental health conditions fit more smoothly within the telemental health model than others. ADHD is the most common condition treated by telemental health, he said. The model also has shown success in the treatment of childhood adjustment disorders, anxiety, oppositional defiant disorder, mood disorders, anxiety, and depression.
“I think you have to be careful in determining where telemental health would be beneficial to use and in which cases it might not be an appropriate method to deliver care,” Dr. Alexander said in an interview.
Developing trust and rapport with patients through videoconferencing also can be a challenge, added Dr. Alexander, who is director of the TelAbility telehealth program at the University of North Carolina at Chapel Hill. He recommends that specialists use the technology to continue an existing doctor-patient relationship or to provide care in a consultative model in which the child’s primary care doctor is present along with the patient and patient’s family.
The AAP advocates for the use of telemedicine so long as it is conducted within the context of the medical home. Fragmented telemedicine services that could disrupt continuity of care should be avoided, according to a 2015 AAP policy statement (Pediatrics. 2015 Jun. doi: 10.1542/peds.2015-1253). The academy also calls for the expansion of pediatric telemedicine to increase access to care for underserved communities and improve quality of care for children.
More partnerships between mental health specialists and primary care providers are a key step in delivering high quality pediatric telemental care, Dr. Alexander said.
“Some larger pediatric practices already do this by hiring and colocating individuals at their practice site, but other, smaller practices might not have the room, finances, sufficient patient population, or enough local providers to make this happen,” he said. “A telemedicine program, located within the practice, could enable this specialized service to be provided in a convenient, coordinated setting.”
On Twitter @legal_med
The patient, a 10-year-old girl, was exhibiting defiant and angry behavior at home. Her pediatrician in rural Vermont was not able to support the family optimally, so he referred her to Dr. David C. Rettew.
Dr. Rettew soon learned that the girl blamed herself for her father’s absence, and she interpreted efforts by her mother to set limits as evidence that her mom didn’t love her.
“During the course of the interview, it became clear that the girl had some specific thoughts that were likely fueling her anger,” said Dr. Rettew, director of the Pediatric Psychiatry Clinic at the University of Vermont, Burlington. “She also met criteria clearly for ADHD, which had never been diagnosed. The consultation recommendations not only included some possible medications to try, but also some specific areas that could be addressed for psychotherapy that could really help the relationship between this child and her mother.”
The successful intervention took place via a secure, two-way videoconference. It is one example of how physicians at the University of Vermont are using telemental health to treat children in underserved areas. As part of a state-funded training program, child psychiatry fellows at the university consult with primary care doctors at federally qualified health centers across the state. The primary care physicians discuss patient cases with fellows via phone or email and can refer patients for in-person or telemental health assessments.
The program has been running for about 5 years and so far has yielded countless benefits, said Dr. Rettew, who directs the university’s Child and Adolescent Psychiatry Fellowship Program. “It helps keep the care housed and centered within the primary care home. That can help coordination of services so that care isn’t fragmented around multiple centers. It also allows evaluations to happen that wouldn’t happen otherwise because it’s too much of a hardship for families to travel long distances and come for regular follow-up appointments.”
University of Vermont physicians also use telemedicine to consult with other mental health clinicians across the state. Child psychiatrist Allison Y. Hall provides in-person training to clinicians and then counsels and supervises their efforts through a telemedicine unit.
“For this purpose, it’s great,” said Dr. Hall, who practices at the Vermont Center for Children, Youth, and Families, which is housed within the university’s psychiatry department. “There’s more confidentiality than Skype, for instance. It’s wonderful to be able to reach clinicians at a great distance.”
On a broader scale, telemental health is a promising tool to address the shortage of mental health providers in the United States, said Dr. Robert C. Gunther, a pediatrician at the University of Virginia Health System in Fishersville. Recent research found that one in three children receiving outpatient care for mental health conditions saw only their primary care doctor for care (Pediatrics. 2015 Nov;136;e1178-85).
“There is a tremendous need for pediatric mental health care,” Dr. Gunther said in an interview. “There is a great shortage of child psychiatrists and other child mental health specialists. Telemental health can help in areas where geography or financial barriers exist to accessing care.”
Data from the Children’s ADHD Telemental Health Treatment Study (CATTS) illustrates the impact that telemental health can have on children facing such barriers to care. Researchers randomized 223 children referred by 88 primary care providers in seven underserved communities into two study groups. Children in the first group were seen by child psychiatrists via videoconference six times over 22 weeks; treatment included pharmacotherapy. Their caregivers received behavior training provided in person by community therapists who were supervised remotely. Children in the second group were treated by their primary care physicians and received one telepsychiatry consult.
Children in both groups improved; however, those randomized to the telemental health model improved “significantly more than patients in the augmented primary care arm” (J Am Acad Child Adolesc Psychiatry. 2015 Apr;54[4]:263-74).
“The CATTS trial demonstrated the effectiveness of a telehealth service model to treat ADHD in communities with limited access to specialty mental health services,” investigators concluded.
But Dr. Joshua J. Alexander, chair of the American Academy of Pediatrics Section on Telehealth Care, notes that some mental health conditions fit more smoothly within the telemental health model than others. ADHD is the most common condition treated by telemental health, he said. The model also has shown success in the treatment of childhood adjustment disorders, anxiety, oppositional defiant disorder, mood disorders, anxiety, and depression.
“I think you have to be careful in determining where telemental health would be beneficial to use and in which cases it might not be an appropriate method to deliver care,” Dr. Alexander said in an interview.
Developing trust and rapport with patients through videoconferencing also can be a challenge, added Dr. Alexander, who is director of the TelAbility telehealth program at the University of North Carolina at Chapel Hill. He recommends that specialists use the technology to continue an existing doctor-patient relationship or to provide care in a consultative model in which the child’s primary care doctor is present along with the patient and patient’s family.
The AAP advocates for the use of telemedicine so long as it is conducted within the context of the medical home. Fragmented telemedicine services that could disrupt continuity of care should be avoided, according to a 2015 AAP policy statement (Pediatrics. 2015 Jun. doi: 10.1542/peds.2015-1253). The academy also calls for the expansion of pediatric telemedicine to increase access to care for underserved communities and improve quality of care for children.
More partnerships between mental health specialists and primary care providers are a key step in delivering high quality pediatric telemental care, Dr. Alexander said.
“Some larger pediatric practices already do this by hiring and colocating individuals at their practice site, but other, smaller practices might not have the room, finances, sufficient patient population, or enough local providers to make this happen,” he said. “A telemedicine program, located within the practice, could enable this specialized service to be provided in a convenient, coordinated setting.”
On Twitter @legal_med
The patient, a 10-year-old girl, was exhibiting defiant and angry behavior at home. Her pediatrician in rural Vermont was not able to support the family optimally, so he referred her to Dr. David C. Rettew.
Dr. Rettew soon learned that the girl blamed herself for her father’s absence, and she interpreted efforts by her mother to set limits as evidence that her mom didn’t love her.
“During the course of the interview, it became clear that the girl had some specific thoughts that were likely fueling her anger,” said Dr. Rettew, director of the Pediatric Psychiatry Clinic at the University of Vermont, Burlington. “She also met criteria clearly for ADHD, which had never been diagnosed. The consultation recommendations not only included some possible medications to try, but also some specific areas that could be addressed for psychotherapy that could really help the relationship between this child and her mother.”
The successful intervention took place via a secure, two-way videoconference. It is one example of how physicians at the University of Vermont are using telemental health to treat children in underserved areas. As part of a state-funded training program, child psychiatry fellows at the university consult with primary care doctors at federally qualified health centers across the state. The primary care physicians discuss patient cases with fellows via phone or email and can refer patients for in-person or telemental health assessments.
The program has been running for about 5 years and so far has yielded countless benefits, said Dr. Rettew, who directs the university’s Child and Adolescent Psychiatry Fellowship Program. “It helps keep the care housed and centered within the primary care home. That can help coordination of services so that care isn’t fragmented around multiple centers. It also allows evaluations to happen that wouldn’t happen otherwise because it’s too much of a hardship for families to travel long distances and come for regular follow-up appointments.”
University of Vermont physicians also use telemedicine to consult with other mental health clinicians across the state. Child psychiatrist Allison Y. Hall provides in-person training to clinicians and then counsels and supervises their efforts through a telemedicine unit.
“For this purpose, it’s great,” said Dr. Hall, who practices at the Vermont Center for Children, Youth, and Families, which is housed within the university’s psychiatry department. “There’s more confidentiality than Skype, for instance. It’s wonderful to be able to reach clinicians at a great distance.”
On a broader scale, telemental health is a promising tool to address the shortage of mental health providers in the United States, said Dr. Robert C. Gunther, a pediatrician at the University of Virginia Health System in Fishersville. Recent research found that one in three children receiving outpatient care for mental health conditions saw only their primary care doctor for care (Pediatrics. 2015 Nov;136;e1178-85).
“There is a tremendous need for pediatric mental health care,” Dr. Gunther said in an interview. “There is a great shortage of child psychiatrists and other child mental health specialists. Telemental health can help in areas where geography or financial barriers exist to accessing care.”
Data from the Children’s ADHD Telemental Health Treatment Study (CATTS) illustrates the impact that telemental health can have on children facing such barriers to care. Researchers randomized 223 children referred by 88 primary care providers in seven underserved communities into two study groups. Children in the first group were seen by child psychiatrists via videoconference six times over 22 weeks; treatment included pharmacotherapy. Their caregivers received behavior training provided in person by community therapists who were supervised remotely. Children in the second group were treated by their primary care physicians and received one telepsychiatry consult.
Children in both groups improved; however, those randomized to the telemental health model improved “significantly more than patients in the augmented primary care arm” (J Am Acad Child Adolesc Psychiatry. 2015 Apr;54[4]:263-74).
“The CATTS trial demonstrated the effectiveness of a telehealth service model to treat ADHD in communities with limited access to specialty mental health services,” investigators concluded.
But Dr. Joshua J. Alexander, chair of the American Academy of Pediatrics Section on Telehealth Care, notes that some mental health conditions fit more smoothly within the telemental health model than others. ADHD is the most common condition treated by telemental health, he said. The model also has shown success in the treatment of childhood adjustment disorders, anxiety, oppositional defiant disorder, mood disorders, anxiety, and depression.
“I think you have to be careful in determining where telemental health would be beneficial to use and in which cases it might not be an appropriate method to deliver care,” Dr. Alexander said in an interview.
Developing trust and rapport with patients through videoconferencing also can be a challenge, added Dr. Alexander, who is director of the TelAbility telehealth program at the University of North Carolina at Chapel Hill. He recommends that specialists use the technology to continue an existing doctor-patient relationship or to provide care in a consultative model in which the child’s primary care doctor is present along with the patient and patient’s family.
The AAP advocates for the use of telemedicine so long as it is conducted within the context of the medical home. Fragmented telemedicine services that could disrupt continuity of care should be avoided, according to a 2015 AAP policy statement (Pediatrics. 2015 Jun. doi: 10.1542/peds.2015-1253). The academy also calls for the expansion of pediatric telemedicine to increase access to care for underserved communities and improve quality of care for children.
More partnerships between mental health specialists and primary care providers are a key step in delivering high quality pediatric telemental care, Dr. Alexander said.
“Some larger pediatric practices already do this by hiring and colocating individuals at their practice site, but other, smaller practices might not have the room, finances, sufficient patient population, or enough local providers to make this happen,” he said. “A telemedicine program, located within the practice, could enable this specialized service to be provided in a convenient, coordinated setting.”
On Twitter @legal_med
On my own during an employee’s maternity leave
Recently, my secretary was out on maternity leave for 6 weeks.
I run a small practice, and my medical assistant works from home on the far side of town. So I was on my own at the office. My MA and I split things up, and since I was the only one physically in the building, I took over all the front office stuff and she took the back office.
I ran the front desk for the whole time – checking people in and out, taking copays, copying insurance cards, giving referrals to therapy places, sending logs to the billing company, and doing other everyday stuff.
Plenty of people asked why I didn’t hire a temp, obviously not knowing how close to the edge a modern solo practice runs. If I hire a temp, that’s another salary to pay, meaning one of the other three of us here would have to skip a few paychecks. I’m not going to put my secretary on unpaid leave for that time. She’s awesome, has been with me since 2004, and has stuck with me through good and bad years. If I don’t pay her that time, she can’t pay her rent, and I don’t have the heart to do that to her. Maybe a big corporate person wouldn’t lose any sleep about it, but I would. Great people are hard to find, and I want to keep the ones I have.
Besides, if I hired a temp, I’d have to train them from the beginning. I don’t use off-the-shelf medical software, just a system I designed myself. It would take time out of my day to teach them how to use it, where I send patients for tests and referrals, and how to sort documents accurately into the correct e-charts. So, for 6 weeks it just seemed easier to do it myself. I know how I like it done.
It wasn’t easy for my MA as well. She had to take over scheduling appointments, handling billing questions, making reminder calls, and doing other miscellaneous stuff. Even after work was over, I’d be at home catching up on all the dictations I hadn’t had time to do, and we’d be going back and forth by phone and email to settle different issues until 8:00 at night or so. By the end of the 6 weeks, we were both pretty burned out and exhausted.
I’m sure the patients weren’t thrilled, either. During that time, they could only reach a voice mail box telling them to leave a message and we’d get back to them as quickly as possible.
I assumed my practice was the only one dinky (or poor, by medical standards) enough to have to resort to this – until I had a chance conversation with a local family practice doctor, when he mentioned he’d had to do something similar when his secretary retired and he didn’t find a replacement for several weeks. A cardiologist mentioned doing the same thing while we were chatting at the hospital. Like me, they were both in solo practice.
This is, apparently, the nature of a modern small practice. The revenue and expense streams are too tight to allow for an extra salary, so even the doctor pitches in to cover. I’m sure my colleagues in large groups will laugh at the thought, but I don’t care. I have to do what’s right for my practice and to survive in the modern medical climate. And if working the front desk for a few weeks is what’s needed to stay independent, so be it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, my secretary was out on maternity leave for 6 weeks.
I run a small practice, and my medical assistant works from home on the far side of town. So I was on my own at the office. My MA and I split things up, and since I was the only one physically in the building, I took over all the front office stuff and she took the back office.
I ran the front desk for the whole time – checking people in and out, taking copays, copying insurance cards, giving referrals to therapy places, sending logs to the billing company, and doing other everyday stuff.
Plenty of people asked why I didn’t hire a temp, obviously not knowing how close to the edge a modern solo practice runs. If I hire a temp, that’s another salary to pay, meaning one of the other three of us here would have to skip a few paychecks. I’m not going to put my secretary on unpaid leave for that time. She’s awesome, has been with me since 2004, and has stuck with me through good and bad years. If I don’t pay her that time, she can’t pay her rent, and I don’t have the heart to do that to her. Maybe a big corporate person wouldn’t lose any sleep about it, but I would. Great people are hard to find, and I want to keep the ones I have.
Besides, if I hired a temp, I’d have to train them from the beginning. I don’t use off-the-shelf medical software, just a system I designed myself. It would take time out of my day to teach them how to use it, where I send patients for tests and referrals, and how to sort documents accurately into the correct e-charts. So, for 6 weeks it just seemed easier to do it myself. I know how I like it done.
It wasn’t easy for my MA as well. She had to take over scheduling appointments, handling billing questions, making reminder calls, and doing other miscellaneous stuff. Even after work was over, I’d be at home catching up on all the dictations I hadn’t had time to do, and we’d be going back and forth by phone and email to settle different issues until 8:00 at night or so. By the end of the 6 weeks, we were both pretty burned out and exhausted.
I’m sure the patients weren’t thrilled, either. During that time, they could only reach a voice mail box telling them to leave a message and we’d get back to them as quickly as possible.
I assumed my practice was the only one dinky (or poor, by medical standards) enough to have to resort to this – until I had a chance conversation with a local family practice doctor, when he mentioned he’d had to do something similar when his secretary retired and he didn’t find a replacement for several weeks. A cardiologist mentioned doing the same thing while we were chatting at the hospital. Like me, they were both in solo practice.
This is, apparently, the nature of a modern small practice. The revenue and expense streams are too tight to allow for an extra salary, so even the doctor pitches in to cover. I’m sure my colleagues in large groups will laugh at the thought, but I don’t care. I have to do what’s right for my practice and to survive in the modern medical climate. And if working the front desk for a few weeks is what’s needed to stay independent, so be it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, my secretary was out on maternity leave for 6 weeks.
I run a small practice, and my medical assistant works from home on the far side of town. So I was on my own at the office. My MA and I split things up, and since I was the only one physically in the building, I took over all the front office stuff and she took the back office.
I ran the front desk for the whole time – checking people in and out, taking copays, copying insurance cards, giving referrals to therapy places, sending logs to the billing company, and doing other everyday stuff.
Plenty of people asked why I didn’t hire a temp, obviously not knowing how close to the edge a modern solo practice runs. If I hire a temp, that’s another salary to pay, meaning one of the other three of us here would have to skip a few paychecks. I’m not going to put my secretary on unpaid leave for that time. She’s awesome, has been with me since 2004, and has stuck with me through good and bad years. If I don’t pay her that time, she can’t pay her rent, and I don’t have the heart to do that to her. Maybe a big corporate person wouldn’t lose any sleep about it, but I would. Great people are hard to find, and I want to keep the ones I have.
Besides, if I hired a temp, I’d have to train them from the beginning. I don’t use off-the-shelf medical software, just a system I designed myself. It would take time out of my day to teach them how to use it, where I send patients for tests and referrals, and how to sort documents accurately into the correct e-charts. So, for 6 weeks it just seemed easier to do it myself. I know how I like it done.
It wasn’t easy for my MA as well. She had to take over scheduling appointments, handling billing questions, making reminder calls, and doing other miscellaneous stuff. Even after work was over, I’d be at home catching up on all the dictations I hadn’t had time to do, and we’d be going back and forth by phone and email to settle different issues until 8:00 at night or so. By the end of the 6 weeks, we were both pretty burned out and exhausted.
I’m sure the patients weren’t thrilled, either. During that time, they could only reach a voice mail box telling them to leave a message and we’d get back to them as quickly as possible.
I assumed my practice was the only one dinky (or poor, by medical standards) enough to have to resort to this – until I had a chance conversation with a local family practice doctor, when he mentioned he’d had to do something similar when his secretary retired and he didn’t find a replacement for several weeks. A cardiologist mentioned doing the same thing while we were chatting at the hospital. Like me, they were both in solo practice.
This is, apparently, the nature of a modern small practice. The revenue and expense streams are too tight to allow for an extra salary, so even the doctor pitches in to cover. I’m sure my colleagues in large groups will laugh at the thought, but I don’t care. I have to do what’s right for my practice and to survive in the modern medical climate. And if working the front desk for a few weeks is what’s needed to stay independent, so be it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Medicare to cover HSCT in approved clinical trials for myeloma, myelofibrosis, sickle cell disease
Medicare will cover allogeneic hematopoietic stem cell transplantation (HSCT) for beneficiaries with multiple myeloma, myelofibrosis, or sickle cell disease in the context of approved, prospective clinical trials, the Centers for Medicare & Medicaid Services announced in a final decision memo Jan. 27.
Approvable studies must examine whether Medicare beneficiaries who receive allogeneic HSCT have improved outcomes, compared with patients who do not receive allogeneic HSCT as measured by graft vs. host disease, other transplant-related adverse events, overall survival, and, optionally, quality of life measures.
In multiple myeloma, allogeneic HSCT will be covered only for Medicare beneficiaries who have Durie-Salmon Stage II or III multiple myeloma, or International Staging System (ISS) Stage II or Stage III multiple myeloma, and are participating in an approved prospective clinical study. Such studies must control for selection bias and potential confounding by age, duration of diagnosis, disease classification, International Myeloma Working Group (IMWG) classification, ISS staging, Durie-Salmon staging, comorbid conditions, type of preparative/conditioning regimen, graft vs. host disease (GVHD) prophylaxis, donor type, and cell source, the CMS said in its memo.
In myelofibrosis, allogeneic HSCT will be covered by Medicare in an approved prospective study only for beneficiaries with Dynamic International Prognostic Scoring System (DIPSS plus) intermediate-2 or high primary or secondary myelofibrosis. Studies must be controlled for selection bias and potential confounding by age, duration of diagnosis, disease classification, DIPSS plus score, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
In sickle cell disease, allogeneic HSCT will be covered by Medicare only for beneficiaries who have severe, symptomatic disease. Approvable studies must control for selection bias and potential confounding by age, duration of diagnosis, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
On Twitter @maryjodales
Dr. Navneet Majhail comments: I welcome the decision by CMS [the Centers for Medicare & Medicaid Services] to cover allogeneic hematopoietic stem cell transplantation (HSCT) for multiple myeloma, myelofibrosis, and sickle cell disease under the coverage with evidence development (CED) mechanism. This action will allow us to provide transplant as a treatment option for older patients with myeloma and myelofibrosis and for Medicare beneficiaries with sickle cell disease: Lack of coverage is a real challenge at present for this population and prevents us from offering a potentially curative treatment option to these high-risk patients.
 |
Dr. Navneet Majhail |
The decision is the result of collective advocacy efforts of our transplant community, patients, and patient advocacy organizations, Be the Match, and the American Society for Blood and Marrow Transplantation.
The CED asks for a prospective clinical trial that mandates the presence of a control arm of comparable patients who do not receive allogeneic transplantation. I completely support provision of transplantation on a clinical trial for CED purposes; however, I believe it would have been better to allow hematology and transplant experts to determine the appropriate study design in consultation with CMS to fulfill the CED requirements. For example, on the basis of available evidence, it will be challenging to enroll patients with high-risk myelofibrosis to a nontransplant arm. Irrespective, this is a big win for patients with these life-threatening diseases and for physicians who treat them.
Dr. Navneet Majhail is the director of the Cleveland Clinic’s Blood & Marrow Transplant Program. He serves as a staff physician at the Taussig Cancer Institute and is a professor of medicine with the Cleveland Clinic Lerner College of Medicine.
Dr. Navneet Majhail comments: I welcome the decision by CMS [the Centers for Medicare & Medicaid Services] to cover allogeneic hematopoietic stem cell transplantation (HSCT) for multiple myeloma, myelofibrosis, and sickle cell disease under the coverage with evidence development (CED) mechanism. This action will allow us to provide transplant as a treatment option for older patients with myeloma and myelofibrosis and for Medicare beneficiaries with sickle cell disease: Lack of coverage is a real challenge at present for this population and prevents us from offering a potentially curative treatment option to these high-risk patients.
 |
Dr. Navneet Majhail |
The decision is the result of collective advocacy efforts of our transplant community, patients, and patient advocacy organizations, Be the Match, and the American Society for Blood and Marrow Transplantation.
The CED asks for a prospective clinical trial that mandates the presence of a control arm of comparable patients who do not receive allogeneic transplantation. I completely support provision of transplantation on a clinical trial for CED purposes; however, I believe it would have been better to allow hematology and transplant experts to determine the appropriate study design in consultation with CMS to fulfill the CED requirements. For example, on the basis of available evidence, it will be challenging to enroll patients with high-risk myelofibrosis to a nontransplant arm. Irrespective, this is a big win for patients with these life-threatening diseases and for physicians who treat them.
Dr. Navneet Majhail is the director of the Cleveland Clinic’s Blood & Marrow Transplant Program. He serves as a staff physician at the Taussig Cancer Institute and is a professor of medicine with the Cleveland Clinic Lerner College of Medicine.
Dr. Navneet Majhail comments: I welcome the decision by CMS [the Centers for Medicare & Medicaid Services] to cover allogeneic hematopoietic stem cell transplantation (HSCT) for multiple myeloma, myelofibrosis, and sickle cell disease under the coverage with evidence development (CED) mechanism. This action will allow us to provide transplant as a treatment option for older patients with myeloma and myelofibrosis and for Medicare beneficiaries with sickle cell disease: Lack of coverage is a real challenge at present for this population and prevents us from offering a potentially curative treatment option to these high-risk patients.
 |
Dr. Navneet Majhail |
The decision is the result of collective advocacy efforts of our transplant community, patients, and patient advocacy organizations, Be the Match, and the American Society for Blood and Marrow Transplantation.
The CED asks for a prospective clinical trial that mandates the presence of a control arm of comparable patients who do not receive allogeneic transplantation. I completely support provision of transplantation on a clinical trial for CED purposes; however, I believe it would have been better to allow hematology and transplant experts to determine the appropriate study design in consultation with CMS to fulfill the CED requirements. For example, on the basis of available evidence, it will be challenging to enroll patients with high-risk myelofibrosis to a nontransplant arm. Irrespective, this is a big win for patients with these life-threatening diseases and for physicians who treat them.
Dr. Navneet Majhail is the director of the Cleveland Clinic’s Blood & Marrow Transplant Program. He serves as a staff physician at the Taussig Cancer Institute and is a professor of medicine with the Cleveland Clinic Lerner College of Medicine.
Medicare will cover allogeneic hematopoietic stem cell transplantation (HSCT) for beneficiaries with multiple myeloma, myelofibrosis, or sickle cell disease in the context of approved, prospective clinical trials, the Centers for Medicare & Medicaid Services announced in a final decision memo Jan. 27.
Approvable studies must examine whether Medicare beneficiaries who receive allogeneic HSCT have improved outcomes, compared with patients who do not receive allogeneic HSCT as measured by graft vs. host disease, other transplant-related adverse events, overall survival, and, optionally, quality of life measures.
In multiple myeloma, allogeneic HSCT will be covered only for Medicare beneficiaries who have Durie-Salmon Stage II or III multiple myeloma, or International Staging System (ISS) Stage II or Stage III multiple myeloma, and are participating in an approved prospective clinical study. Such studies must control for selection bias and potential confounding by age, duration of diagnosis, disease classification, International Myeloma Working Group (IMWG) classification, ISS staging, Durie-Salmon staging, comorbid conditions, type of preparative/conditioning regimen, graft vs. host disease (GVHD) prophylaxis, donor type, and cell source, the CMS said in its memo.
In myelofibrosis, allogeneic HSCT will be covered by Medicare in an approved prospective study only for beneficiaries with Dynamic International Prognostic Scoring System (DIPSS plus) intermediate-2 or high primary or secondary myelofibrosis. Studies must be controlled for selection bias and potential confounding by age, duration of diagnosis, disease classification, DIPSS plus score, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
In sickle cell disease, allogeneic HSCT will be covered by Medicare only for beneficiaries who have severe, symptomatic disease. Approvable studies must control for selection bias and potential confounding by age, duration of diagnosis, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
On Twitter @maryjodales
Medicare will cover allogeneic hematopoietic stem cell transplantation (HSCT) for beneficiaries with multiple myeloma, myelofibrosis, or sickle cell disease in the context of approved, prospective clinical trials, the Centers for Medicare & Medicaid Services announced in a final decision memo Jan. 27.
Approvable studies must examine whether Medicare beneficiaries who receive allogeneic HSCT have improved outcomes, compared with patients who do not receive allogeneic HSCT as measured by graft vs. host disease, other transplant-related adverse events, overall survival, and, optionally, quality of life measures.
In multiple myeloma, allogeneic HSCT will be covered only for Medicare beneficiaries who have Durie-Salmon Stage II or III multiple myeloma, or International Staging System (ISS) Stage II or Stage III multiple myeloma, and are participating in an approved prospective clinical study. Such studies must control for selection bias and potential confounding by age, duration of diagnosis, disease classification, International Myeloma Working Group (IMWG) classification, ISS staging, Durie-Salmon staging, comorbid conditions, type of preparative/conditioning regimen, graft vs. host disease (GVHD) prophylaxis, donor type, and cell source, the CMS said in its memo.
In myelofibrosis, allogeneic HSCT will be covered by Medicare in an approved prospective study only for beneficiaries with Dynamic International Prognostic Scoring System (DIPSS plus) intermediate-2 or high primary or secondary myelofibrosis. Studies must be controlled for selection bias and potential confounding by age, duration of diagnosis, disease classification, DIPSS plus score, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
In sickle cell disease, allogeneic HSCT will be covered by Medicare only for beneficiaries who have severe, symptomatic disease. Approvable studies must control for selection bias and potential confounding by age, duration of diagnosis, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
On Twitter @maryjodales
Hepatitis C incidence rising in hemodialysis patients
Incidence of newly acquired hepatitis C virus has increased recently in patients undergoing hemodialysis, according to a health advisory from the Centers for Disease Control and Prevention.
In 2014 and 2015, 36 cases of HCV infection were reported to the CDC from 19 clinics in eight states. While investigation is ongoing, HCV transmission between patients has been confirmed in at least nine facilities, and in several facilities, lapses in infection control were also identified. Better screening and awareness of HCV infection potential may also play a role in the increased disease incidence.
The CDC recommends that dialysis facilities assess current infection control practices, environmental cleaning, and disinfection practices to evaluate adherence to standards, address any gaps, screen patients for HCV, and to report all HCV infections to the CDC promptly.
“Dialysis facilities should actively assess and continuously improve their infection control, environmental cleaning and disinfection, and HCV screening practices, whether or not they are aware of infections in their clinic. Any case of new HCV infection in a patient undergoing hemodialysis is likely to be a health care–associated infection and should be reported to public health authorities in a timely manner,” the CDC said
Find the full health advisory on the CDC website.
Incidence of newly acquired hepatitis C virus has increased recently in patients undergoing hemodialysis, according to a health advisory from the Centers for Disease Control and Prevention.
In 2014 and 2015, 36 cases of HCV infection were reported to the CDC from 19 clinics in eight states. While investigation is ongoing, HCV transmission between patients has been confirmed in at least nine facilities, and in several facilities, lapses in infection control were also identified. Better screening and awareness of HCV infection potential may also play a role in the increased disease incidence.
The CDC recommends that dialysis facilities assess current infection control practices, environmental cleaning, and disinfection practices to evaluate adherence to standards, address any gaps, screen patients for HCV, and to report all HCV infections to the CDC promptly.
“Dialysis facilities should actively assess and continuously improve their infection control, environmental cleaning and disinfection, and HCV screening practices, whether or not they are aware of infections in their clinic. Any case of new HCV infection in a patient undergoing hemodialysis is likely to be a health care–associated infection and should be reported to public health authorities in a timely manner,” the CDC said
Find the full health advisory on the CDC website.
Incidence of newly acquired hepatitis C virus has increased recently in patients undergoing hemodialysis, according to a health advisory from the Centers for Disease Control and Prevention.
In 2014 and 2015, 36 cases of HCV infection were reported to the CDC from 19 clinics in eight states. While investigation is ongoing, HCV transmission between patients has been confirmed in at least nine facilities, and in several facilities, lapses in infection control were also identified. Better screening and awareness of HCV infection potential may also play a role in the increased disease incidence.
The CDC recommends that dialysis facilities assess current infection control practices, environmental cleaning, and disinfection practices to evaluate adherence to standards, address any gaps, screen patients for HCV, and to report all HCV infections to the CDC promptly.
“Dialysis facilities should actively assess and continuously improve their infection control, environmental cleaning and disinfection, and HCV screening practices, whether or not they are aware of infections in their clinic. Any case of new HCV infection in a patient undergoing hemodialysis is likely to be a health care–associated infection and should be reported to public health authorities in a timely manner,” the CDC said
Find the full health advisory on the CDC website.
Pain scores point to hospital quality in colorectal surgery
Post-surgical pain scores may be an overlooked quality indicator among hospitals, according to new research linking patient-reported pain scores with institutional pain management practices and also surgical outcomes.
A retrospective cohort study of patient-reported pain scores after colorectal resections at 52 Michigan hospitals, published in Annals of Surgery (2016 Jan 7; epub ahead of print; doi: 10.1097/SLA.0000000000001541), found that patients treated at the best-performing hospitals for postoperative pain scores were more likely to have received patient-controlled analgesia, compared with those in the worst-performing ones (56.5% vs. 22.8%; P less than .001).
For their research, Dr. Scott E. Regenbogen of the University of Michigan, Ann Arbor, and his colleagues looked at patient-reported pain scores on the first morning post-surgery for 7,221 colorectal operations between 2012 and 2014. The participating hospitals were part of a statewide collaborative that collects data on surgery patients with the aim of improving quality.
Dr. Regenbogen and his colleagues found that patients in the quartile of hospitals with the best pain scores stayed fewer days (6.5 vs. 7.9, P less than .007) and had fewer post-surgical complications (20.3% vs. 26.4%; P less than .001), compared with those in the worst-performing quartile of hospitals.
In addition, Dr. Regenbogen and his colleagues found postoperative emergency department visits, readmissions, and pulmonary complications to be significantly lower in the quartile of hospitals with the best pain scores. The fewer pulmonary complications seen linked with better pain control “could be an indicator of better pulmonary toilet or lesser respiratory depression,” they noted.
The correlation between surgical outcomes and pain scores, the investigators wrote, suggests “consistency in the overall quality performance across both clinical and patient-reported outcomes for colectomy.”
Mean self-rated pain scores, in which patients characterize the intensity of their pain on a scale of 0 to 10, ranged from 4 to 6 across the hospitals in the study, with 5.1 (standard deviation 2.44) reported for the cohort as a whole. The type of surgery also affected pain scores, with minimally invasive procedures associated with lower scores, compared with open or converted procedures. The type of anesthesia used (local or epidural) also significantly affected scores.
Hospitals with better pain scores tended to be somewhat larger than those with poor scores, and performed more colorectal resections per year, the investigators found.
The researchers noted that while a previous meta-analysis showed that patient-controlled analgesia post-surgery provided superior pain control, compared with intermittent treatment (Cochrane Database Syst Rev. 2006 Oct 18;18:CD003348), the hospitals in this study varied widely in their approaches, with 89% of the poorly performing quartile of hospitals using intermittent parenteral narcotics, compared with 66% in the best-performing quartile.
Dr. Regenbogen and his colleagues noted in their analysis that it was possible that the association between pain control and clinical outcomes such as readmissions and complications was driven by case or patient complexity differences among institutions. The 52 hospitals in the study varied in size and type, with community and academic hospitals as well as rural and urban institutions represented.
However, they wrote, it is more likely that “both pain scores and clinical outcomes reflect … global features of the quality of care in hospitals’ surgical performance. Thus, hospitals with the most streamlined, high-quality perioperative care pathways experience the best pain scores, as well as improved clinical outcomes.”
The findings, they concluded, “reveal systematic clinical care variation that could be reduced to improve patients’ experience of pain after colorectal resections.”
The researchers noted as a limitation of the study its reliance on patient-reported pain measures, and that it did not include data on patients’ pain history, opioid use prior to admission, or the administration of pre-emptive analgesia before surgery. The study was funded by the Michigan Surgical Quality Collaborative, which receives support from Blue Cross Blue Shield. None of the study authors declared conflicts of interest.
Post-surgical pain scores may be an overlooked quality indicator among hospitals, according to new research linking patient-reported pain scores with institutional pain management practices and also surgical outcomes.
A retrospective cohort study of patient-reported pain scores after colorectal resections at 52 Michigan hospitals, published in Annals of Surgery (2016 Jan 7; epub ahead of print; doi: 10.1097/SLA.0000000000001541), found that patients treated at the best-performing hospitals for postoperative pain scores were more likely to have received patient-controlled analgesia, compared with those in the worst-performing ones (56.5% vs. 22.8%; P less than .001).
For their research, Dr. Scott E. Regenbogen of the University of Michigan, Ann Arbor, and his colleagues looked at patient-reported pain scores on the first morning post-surgery for 7,221 colorectal operations between 2012 and 2014. The participating hospitals were part of a statewide collaborative that collects data on surgery patients with the aim of improving quality.
Dr. Regenbogen and his colleagues found that patients in the quartile of hospitals with the best pain scores stayed fewer days (6.5 vs. 7.9, P less than .007) and had fewer post-surgical complications (20.3% vs. 26.4%; P less than .001), compared with those in the worst-performing quartile of hospitals.
In addition, Dr. Regenbogen and his colleagues found postoperative emergency department visits, readmissions, and pulmonary complications to be significantly lower in the quartile of hospitals with the best pain scores. The fewer pulmonary complications seen linked with better pain control “could be an indicator of better pulmonary toilet or lesser respiratory depression,” they noted.
The correlation between surgical outcomes and pain scores, the investigators wrote, suggests “consistency in the overall quality performance across both clinical and patient-reported outcomes for colectomy.”
Mean self-rated pain scores, in which patients characterize the intensity of their pain on a scale of 0 to 10, ranged from 4 to 6 across the hospitals in the study, with 5.1 (standard deviation 2.44) reported for the cohort as a whole. The type of surgery also affected pain scores, with minimally invasive procedures associated with lower scores, compared with open or converted procedures. The type of anesthesia used (local or epidural) also significantly affected scores.
Hospitals with better pain scores tended to be somewhat larger than those with poor scores, and performed more colorectal resections per year, the investigators found.
The researchers noted that while a previous meta-analysis showed that patient-controlled analgesia post-surgery provided superior pain control, compared with intermittent treatment (Cochrane Database Syst Rev. 2006 Oct 18;18:CD003348), the hospitals in this study varied widely in their approaches, with 89% of the poorly performing quartile of hospitals using intermittent parenteral narcotics, compared with 66% in the best-performing quartile.
Dr. Regenbogen and his colleagues noted in their analysis that it was possible that the association between pain control and clinical outcomes such as readmissions and complications was driven by case or patient complexity differences among institutions. The 52 hospitals in the study varied in size and type, with community and academic hospitals as well as rural and urban institutions represented.
However, they wrote, it is more likely that “both pain scores and clinical outcomes reflect … global features of the quality of care in hospitals’ surgical performance. Thus, hospitals with the most streamlined, high-quality perioperative care pathways experience the best pain scores, as well as improved clinical outcomes.”
The findings, they concluded, “reveal systematic clinical care variation that could be reduced to improve patients’ experience of pain after colorectal resections.”
The researchers noted as a limitation of the study its reliance on patient-reported pain measures, and that it did not include data on patients’ pain history, opioid use prior to admission, or the administration of pre-emptive analgesia before surgery. The study was funded by the Michigan Surgical Quality Collaborative, which receives support from Blue Cross Blue Shield. None of the study authors declared conflicts of interest.
Post-surgical pain scores may be an overlooked quality indicator among hospitals, according to new research linking patient-reported pain scores with institutional pain management practices and also surgical outcomes.
A retrospective cohort study of patient-reported pain scores after colorectal resections at 52 Michigan hospitals, published in Annals of Surgery (2016 Jan 7; epub ahead of print; doi: 10.1097/SLA.0000000000001541), found that patients treated at the best-performing hospitals for postoperative pain scores were more likely to have received patient-controlled analgesia, compared with those in the worst-performing ones (56.5% vs. 22.8%; P less than .001).
For their research, Dr. Scott E. Regenbogen of the University of Michigan, Ann Arbor, and his colleagues looked at patient-reported pain scores on the first morning post-surgery for 7,221 colorectal operations between 2012 and 2014. The participating hospitals were part of a statewide collaborative that collects data on surgery patients with the aim of improving quality.
Dr. Regenbogen and his colleagues found that patients in the quartile of hospitals with the best pain scores stayed fewer days (6.5 vs. 7.9, P less than .007) and had fewer post-surgical complications (20.3% vs. 26.4%; P less than .001), compared with those in the worst-performing quartile of hospitals.
In addition, Dr. Regenbogen and his colleagues found postoperative emergency department visits, readmissions, and pulmonary complications to be significantly lower in the quartile of hospitals with the best pain scores. The fewer pulmonary complications seen linked with better pain control “could be an indicator of better pulmonary toilet or lesser respiratory depression,” they noted.
The correlation between surgical outcomes and pain scores, the investigators wrote, suggests “consistency in the overall quality performance across both clinical and patient-reported outcomes for colectomy.”
Mean self-rated pain scores, in which patients characterize the intensity of their pain on a scale of 0 to 10, ranged from 4 to 6 across the hospitals in the study, with 5.1 (standard deviation 2.44) reported for the cohort as a whole. The type of surgery also affected pain scores, with minimally invasive procedures associated with lower scores, compared with open or converted procedures. The type of anesthesia used (local or epidural) also significantly affected scores.
Hospitals with better pain scores tended to be somewhat larger than those with poor scores, and performed more colorectal resections per year, the investigators found.
The researchers noted that while a previous meta-analysis showed that patient-controlled analgesia post-surgery provided superior pain control, compared with intermittent treatment (Cochrane Database Syst Rev. 2006 Oct 18;18:CD003348), the hospitals in this study varied widely in their approaches, with 89% of the poorly performing quartile of hospitals using intermittent parenteral narcotics, compared with 66% in the best-performing quartile.
Dr. Regenbogen and his colleagues noted in their analysis that it was possible that the association between pain control and clinical outcomes such as readmissions and complications was driven by case or patient complexity differences among institutions. The 52 hospitals in the study varied in size and type, with community and academic hospitals as well as rural and urban institutions represented.
However, they wrote, it is more likely that “both pain scores and clinical outcomes reflect … global features of the quality of care in hospitals’ surgical performance. Thus, hospitals with the most streamlined, high-quality perioperative care pathways experience the best pain scores, as well as improved clinical outcomes.”
The findings, they concluded, “reveal systematic clinical care variation that could be reduced to improve patients’ experience of pain after colorectal resections.”
The researchers noted as a limitation of the study its reliance on patient-reported pain measures, and that it did not include data on patients’ pain history, opioid use prior to admission, or the administration of pre-emptive analgesia before surgery. The study was funded by the Michigan Surgical Quality Collaborative, which receives support from Blue Cross Blue Shield. None of the study authors declared conflicts of interest.
FROM ANNALS OF SURGERY
Key clinical point: Hospitals delivering better patient-reported pain control after colorectal resection also saw better surgical outcomes.
Major finding: Patients in the quartile of hospitals with the best pain scores stayed fewer days (6.5 vs. 7.9, P less than .007) and had fewer post-surgical complications (20.3% vs. 26.4%; P less than .001), compared with those in the worst-performing quartile of hospitals.
Data source: A retrospective cohort study reviewing more than 7,000 colorectal resections at 52 Michigan hospitals between 2012 and 2014.
Disclosures: The Michigan Surgical Quality Collaborative, funded by Blue Cross Blue Shield, sponsored the study. Investigators declared no conflicts of interest.
RELAPSE: Answers to why a patient is having a new mood episode
A mood disorder is a chronic illness, associated with episodic recurrence over time1,2; when a patient experiences a new mood episode, explore possible underlying causes of that recurrence. The mnemonic RELAPSE can help you take an informed approach to treatment, instead of making reflexive medication changes (Table).
Rhythm disturbances. Seasonal changes, shift work, jet lag, and sleep irregularity can induce a mood episode in a vulnerable patient. Failure of a patient’s circadian clock to resynchronize itself after such disruption in the dark–light cycle can trigger mood symptoms.
Ending treatment. Intentional or unintentional non-adherence to a prescribed medication or psychotherapy can trigger a mood episode. Likewise, switching from a brand-name medication to a generic equivalent can induce a new episode because the generic drug might be as much as 20% less bioavailable than the brand formulation.3
Life change. Some life events, such as divorce or job loss, can be sufficiently overwhelming—despite medical therapy and psychotherapy—to induce a new episode in a vulnerable patient.
Additional drugs. Opiates, interferon, steroids, reserpine, and other drugs can be depressogenic; on the other hand, steroids, anticholinergic agents, and antidepressants can induce mania. If another physician, or the patient, adds a medication or supplement that causes an interaction with the patient’s current psychotropic prescription, the result might be increased metabolism or clearance of the psychotropic—thus decreasing its efficacy and leading to a new mood episode.
Physical health changes. Neurologic conditions (epilepsy, multiple sclerosis, stroke), autoimmune illnesses (eg, lupus), primary sleep disorders (eg, obstructive sleep apnea), and hormone changes (eg, testosterone, estrogen, and thyroid) that can occur over the lifespan of a patient with a mood disorder can trigger a new episode.
Substance use and withdrawal. Chronic use of alcohol and opiates and withdrawal from cocaine and stimulants in a patient with a mood disorder can induce a depressive episode; use of cocaine, stimulants, and caffeine can induce a manic state.
End of drug response. Some patients experience a loss of drug response over time (tachyphylaxis) or a depressive recurrence while taking an antidepressant.4 These phenomena might be caused by brain changes over time. These are a diagnosis of exclusion after other possibilities have been ruled out.
1. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157:229-233.
2. Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry. 2006;163:217-224.
3. Ellingrod VL. How differences among generics might affect your patient’s response. Current Psychiatry. 2010;9(5):31-32,38.
4. Dunlop BW. Depressive recurrence on antidepressant treatment (DRAT): 4 next-step options. Current Psychiatry. 2013;12:54-55.
A mood disorder is a chronic illness, associated with episodic recurrence over time1,2; when a patient experiences a new mood episode, explore possible underlying causes of that recurrence. The mnemonic RELAPSE can help you take an informed approach to treatment, instead of making reflexive medication changes (Table).
Rhythm disturbances. Seasonal changes, shift work, jet lag, and sleep irregularity can induce a mood episode in a vulnerable patient. Failure of a patient’s circadian clock to resynchronize itself after such disruption in the dark–light cycle can trigger mood symptoms.
Ending treatment. Intentional or unintentional non-adherence to a prescribed medication or psychotherapy can trigger a mood episode. Likewise, switching from a brand-name medication to a generic equivalent can induce a new episode because the generic drug might be as much as 20% less bioavailable than the brand formulation.3
Life change. Some life events, such as divorce or job loss, can be sufficiently overwhelming—despite medical therapy and psychotherapy—to induce a new episode in a vulnerable patient.
Additional drugs. Opiates, interferon, steroids, reserpine, and other drugs can be depressogenic; on the other hand, steroids, anticholinergic agents, and antidepressants can induce mania. If another physician, or the patient, adds a medication or supplement that causes an interaction with the patient’s current psychotropic prescription, the result might be increased metabolism or clearance of the psychotropic—thus decreasing its efficacy and leading to a new mood episode.
Physical health changes. Neurologic conditions (epilepsy, multiple sclerosis, stroke), autoimmune illnesses (eg, lupus), primary sleep disorders (eg, obstructive sleep apnea), and hormone changes (eg, testosterone, estrogen, and thyroid) that can occur over the lifespan of a patient with a mood disorder can trigger a new episode.
Substance use and withdrawal. Chronic use of alcohol and opiates and withdrawal from cocaine and stimulants in a patient with a mood disorder can induce a depressive episode; use of cocaine, stimulants, and caffeine can induce a manic state.
End of drug response. Some patients experience a loss of drug response over time (tachyphylaxis) or a depressive recurrence while taking an antidepressant.4 These phenomena might be caused by brain changes over time. These are a diagnosis of exclusion after other possibilities have been ruled out.
A mood disorder is a chronic illness, associated with episodic recurrence over time1,2; when a patient experiences a new mood episode, explore possible underlying causes of that recurrence. The mnemonic RELAPSE can help you take an informed approach to treatment, instead of making reflexive medication changes (Table).
Rhythm disturbances. Seasonal changes, shift work, jet lag, and sleep irregularity can induce a mood episode in a vulnerable patient. Failure of a patient’s circadian clock to resynchronize itself after such disruption in the dark–light cycle can trigger mood symptoms.
Ending treatment. Intentional or unintentional non-adherence to a prescribed medication or psychotherapy can trigger a mood episode. Likewise, switching from a brand-name medication to a generic equivalent can induce a new episode because the generic drug might be as much as 20% less bioavailable than the brand formulation.3
Life change. Some life events, such as divorce or job loss, can be sufficiently overwhelming—despite medical therapy and psychotherapy—to induce a new episode in a vulnerable patient.
Additional drugs. Opiates, interferon, steroids, reserpine, and other drugs can be depressogenic; on the other hand, steroids, anticholinergic agents, and antidepressants can induce mania. If another physician, or the patient, adds a medication or supplement that causes an interaction with the patient’s current psychotropic prescription, the result might be increased metabolism or clearance of the psychotropic—thus decreasing its efficacy and leading to a new mood episode.
Physical health changes. Neurologic conditions (epilepsy, multiple sclerosis, stroke), autoimmune illnesses (eg, lupus), primary sleep disorders (eg, obstructive sleep apnea), and hormone changes (eg, testosterone, estrogen, and thyroid) that can occur over the lifespan of a patient with a mood disorder can trigger a new episode.
Substance use and withdrawal. Chronic use of alcohol and opiates and withdrawal from cocaine and stimulants in a patient with a mood disorder can induce a depressive episode; use of cocaine, stimulants, and caffeine can induce a manic state.
End of drug response. Some patients experience a loss of drug response over time (tachyphylaxis) or a depressive recurrence while taking an antidepressant.4 These phenomena might be caused by brain changes over time. These are a diagnosis of exclusion after other possibilities have been ruled out.
1. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157:229-233.
2. Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry. 2006;163:217-224.
3. Ellingrod VL. How differences among generics might affect your patient’s response. Current Psychiatry. 2010;9(5):31-32,38.
4. Dunlop BW. Depressive recurrence on antidepressant treatment (DRAT): 4 next-step options. Current Psychiatry. 2013;12:54-55.
1. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157:229-233.
2. Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry. 2006;163:217-224.
3. Ellingrod VL. How differences among generics might affect your patient’s response. Current Psychiatry. 2010;9(5):31-32,38.
4. Dunlop BW. Depressive recurrence on antidepressant treatment (DRAT): 4 next-step options. Current Psychiatry. 2013;12:54-55.
From paranoid fear to completed homicide
A crescendo of paranoid fear sharply increases the likelihood that a person will kill his (her) misperceived persecutor. Persecutory delusions are more likely to lead to homicide than any other psychiatric symptom.1 If people define a delusional situation as real, the situation is real in its consequences.
Based on my experience performing more than 100 insanity evaluations of paranoid persons charged with murder, I have identified 4 paranoid motives for homicide.
Self-defense. The most common paranoid motive for murder is the misperceived need to defend one’s self.
A steel worker believed that there was a conspiracy to kill him. His wife insisted that he go to a hospital emergency room for an evaluation. He then concluded that his wife was in on the conspiracy and stabbed her to death.
Defense of one’s manhood. Homosexual panic occurs in men who think of themselves as heterosexual.
A man with paranoid schizophrenia developed a delusion that his former high school football coach was having the entire team rape him at night. He shot the coach 6 times in front of 22 witnesses.
Defense of one’s children. A parent may kill to save her (his) children’s souls.
A deeply religious woman developed persecutory delusions that her 9-year-old son and 3-year-old daughter were going to be kidnapped and forced to make child pornography. To save her children’s souls, she stabbed her children more than 100 times.
Defense of the world. Homicide may be seen as a way to protect all humankind.
A woman developed a delusion that her father was Satan and would kill her. She believed that if she could kill her father (Satan) and his family she would save herself and bring about world peace. After killing her father, she thrust the sharp end of a tire iron into her grandmother’s umbilicus and vagina because those body parts were involved in “birthing Satan.”
Questioning to determine risk
I have found that, when evaluating a paranoid, delusional person for potential violence, it is better to present that person with a hypothetical question about encountering his perceived persecutor than with a generic question about homicidality.2 For example, a delusional person who reports that he was afraid of being killed by the Mafia could be asked, “If you were walking down an alley and encountered a man dressed like a Mafia hit man with a bulge in his jacket, what would you do?” One interviewee might reply, “The Mafia has so much power there is nothing I could do.” Another might answer, “As soon as I got close enough I would blow his head off with my .357 Magnum.” Although both people would be reporting honestly that they have no homicidal ideas, the latter has a much lower threshold for killing in misperceived self-defense.
Summing up
Persecutory delusions are more likely than any other psychiatric symptom to lead a psychotic person to commit homicide. The killing might be motivated by misperceived self-defense, defense of one’s manhood, defense of one’s children, or defense of the world.
A crescendo of paranoid fear sharply increases the likelihood that a person will kill his (her) misperceived persecutor. Persecutory delusions are more likely to lead to homicide than any other psychiatric symptom.1 If people define a delusional situation as real, the situation is real in its consequences.
Based on my experience performing more than 100 insanity evaluations of paranoid persons charged with murder, I have identified 4 paranoid motives for homicide.
Self-defense. The most common paranoid motive for murder is the misperceived need to defend one’s self.
A steel worker believed that there was a conspiracy to kill him. His wife insisted that he go to a hospital emergency room for an evaluation. He then concluded that his wife was in on the conspiracy and stabbed her to death.
Defense of one’s manhood. Homosexual panic occurs in men who think of themselves as heterosexual.
A man with paranoid schizophrenia developed a delusion that his former high school football coach was having the entire team rape him at night. He shot the coach 6 times in front of 22 witnesses.
Defense of one’s children. A parent may kill to save her (his) children’s souls.
A deeply religious woman developed persecutory delusions that her 9-year-old son and 3-year-old daughter were going to be kidnapped and forced to make child pornography. To save her children’s souls, she stabbed her children more than 100 times.
Defense of the world. Homicide may be seen as a way to protect all humankind.
A woman developed a delusion that her father was Satan and would kill her. She believed that if she could kill her father (Satan) and his family she would save herself and bring about world peace. After killing her father, she thrust the sharp end of a tire iron into her grandmother’s umbilicus and vagina because those body parts were involved in “birthing Satan.”
Questioning to determine risk
I have found that, when evaluating a paranoid, delusional person for potential violence, it is better to present that person with a hypothetical question about encountering his perceived persecutor than with a generic question about homicidality.2 For example, a delusional person who reports that he was afraid of being killed by the Mafia could be asked, “If you were walking down an alley and encountered a man dressed like a Mafia hit man with a bulge in his jacket, what would you do?” One interviewee might reply, “The Mafia has so much power there is nothing I could do.” Another might answer, “As soon as I got close enough I would blow his head off with my .357 Magnum.” Although both people would be reporting honestly that they have no homicidal ideas, the latter has a much lower threshold for killing in misperceived self-defense.
Summing up
Persecutory delusions are more likely than any other psychiatric symptom to lead a psychotic person to commit homicide. The killing might be motivated by misperceived self-defense, defense of one’s manhood, defense of one’s children, or defense of the world.
A crescendo of paranoid fear sharply increases the likelihood that a person will kill his (her) misperceived persecutor. Persecutory delusions are more likely to lead to homicide than any other psychiatric symptom.1 If people define a delusional situation as real, the situation is real in its consequences.
Based on my experience performing more than 100 insanity evaluations of paranoid persons charged with murder, I have identified 4 paranoid motives for homicide.
Self-defense. The most common paranoid motive for murder is the misperceived need to defend one’s self.
A steel worker believed that there was a conspiracy to kill him. His wife insisted that he go to a hospital emergency room for an evaluation. He then concluded that his wife was in on the conspiracy and stabbed her to death.
Defense of one’s manhood. Homosexual panic occurs in men who think of themselves as heterosexual.
A man with paranoid schizophrenia developed a delusion that his former high school football coach was having the entire team rape him at night. He shot the coach 6 times in front of 22 witnesses.
Defense of one’s children. A parent may kill to save her (his) children’s souls.
A deeply religious woman developed persecutory delusions that her 9-year-old son and 3-year-old daughter were going to be kidnapped and forced to make child pornography. To save her children’s souls, she stabbed her children more than 100 times.
Defense of the world. Homicide may be seen as a way to protect all humankind.
A woman developed a delusion that her father was Satan and would kill her. She believed that if she could kill her father (Satan) and his family she would save herself and bring about world peace. After killing her father, she thrust the sharp end of a tire iron into her grandmother’s umbilicus and vagina because those body parts were involved in “birthing Satan.”
Questioning to determine risk
I have found that, when evaluating a paranoid, delusional person for potential violence, it is better to present that person with a hypothetical question about encountering his perceived persecutor than with a generic question about homicidality.2 For example, a delusional person who reports that he was afraid of being killed by the Mafia could be asked, “If you were walking down an alley and encountered a man dressed like a Mafia hit man with a bulge in his jacket, what would you do?” One interviewee might reply, “The Mafia has so much power there is nothing I could do.” Another might answer, “As soon as I got close enough I would blow his head off with my .357 Magnum.” Although both people would be reporting honestly that they have no homicidal ideas, the latter has a much lower threshold for killing in misperceived self-defense.
Summing up
Persecutory delusions are more likely than any other psychiatric symptom to lead a psychotic person to commit homicide. The killing might be motivated by misperceived self-defense, defense of one’s manhood, defense of one’s children, or defense of the world.
Delusions, hypersexuality, and a steep cognitive decline
CASE Inconsistent stories
Ms. P, age 56, is an Asian American woman who was brought in by police after being found standing by her car in the middle of a busy road displaying bizarre behavior. She provides an inconsistent story about why she was brought to the hospital, saying that the police did so because she wasn’t driving fast enough and because her English is weak. At another point, she says that she had stopped her car to pick up a penny from the road and the police brought her to the hospital “to experience life, to rest, to meet people.”
Upon further questioning, Ms. P reveals that she is experiencing racing thoughts, feels full of energy, has pressured speech, and does not need much sleep. She also is sexually preoccupied, talks about having extra-marital affairs, and expresses her infatuation with TV news anchors. She says she is sexually active but is unable to offer any further details, and—while giggling—asks the treatment team not to reveal this information to her husband. Ms. P also reports hearing angels singing from the sky.
Chart review reveals that Ms. P had been admitted to same hospital 5 years earlier, at which time she was given diagnoses of late-onset schizophrenia (LOS) and mild cognitive impairment. Ms. P also had 3 psychiatric inpatient admissions in the past 2 years at a different hospital, but her records are inaccessible because she refuses to allow her chart to be released.
Ms. P has not taken the psychiatric medications prescribed for her for several months; she says, “I don’t need medication. I am self-healing.” She denies using illicit substances, including marijuana, smoking, and current alcohol use, but reports occasional social drinking in the past. Her urine drug screen is negative.
The most striking revelation in Ms. P’s social history is her high premorbid functional status. She has 2 master’s degrees and had been working as a senior accountant at a major hospital system until 7 years ago. In contrast, when interviewed at the hospital, Ms. P reports that she is working at a child care center.
On mental status exam, Ms. P is half-draped in a hospital gown, casual, overly friendly, smiling, and twirling her hair. Her mood is elevated with inappropriate affect. Her thought process is bizarre and illogical. She is alert, fully oriented, and her sensorium is clear. She has persistent ambivalence and contradictory thoughts regarding suicidal ideation. Recent and remote memory are largely intact. She does not express homicidal ideation.
What could be causing Ms. P’s psychosis and functional decline?
a) major neurocognitive disorder
b) schizophrenia
c) schizoaffective disorder
d) bipolar disorder, current manic episode
HISTORY Fired from her job
According to Ms. P’s chart from her admission 5 years earlier, police brought her to the hospital because she was causing a disturbance at a restaurant. When interviewed, Ms. P reported a false story that she fought with her husband, kicked him, and spat on his face. She said that her husband then punched her in the face, she ran out of the house, and a bystander called the police. At the time, her husband was contacted and denied the incident. He said that Ms. P had gone to the store and not returned, and he did not know what happened to her.
Her husband reported a steady and progressive decline in function and behavior dating back to 8 years ago with no known prior behavioral disturbances. In the chart from 5 years ago, her husband reported that Ms. P had been a high-functioning senior executive accountant at a major hospital system 7 years before the current admission, at which time she was fired from her job. He said that, just before being fired, Ms. P had been reading the mystery novel The Da Vinci Code and believed that events in the book specifically applied to her. Ms. P would stay up all night making clothes; when she would go to work, she was caught sleeping on the job and performing poorly, including submitting reports with incorrect information. She yelled at co-workers and was unable to take direction from her supervisors.
Ms. P’s husband also reported that she believed people were trying to “look like her,” by having plastic surgery. He reported unusual behavior at home, including eating food off the countertop that had been out for hours and was not fit for consumption.
Ms. P’s husband could not be contacted during this admission because he was out of country and they were separated. Collateral information is obtained from Ms. P’s mother, who lives apart from her but in the same city and speaks no English. She confirms Ms. P’s high premorbid functioning, and reports that her daughter’s change in behavior went back as far as 10 years. She reports that Ms. P had problems controlling anger and had frequent altercations with her husband and mother, including threatening her with a knife. Self-care and hygiene then declined strikingly. She began to have odd religious beliefs (eg, she was the daughter of Jesus Christ) and insisted on dressing in peculiar ways.
No family history of psychiatric disorders, such as schizophrenia, bipolar disorder, or dementia, was reported (Table 1).
The authors’ observations
The existence of LOS as a distinct subtype of schizophrenia has been the subject of discussion and controversy as far back as Manfred Bleuler in 1943 who coined the term “late-onset schizophrenia.”1 In 2000, a consensus statement by the International Late-Onset Schizophrenia Group standardized the nomenclature, defining LOS as onset between age 40 and 60, and very-late-onset schizophrenia-like psychosis (VLOS) as onset after age 60.2 Although there is no diagnostic subcategory for LOS in DSM, DSM-5 notes that (1) women are overrepresented in late-onset cases and (2) the course generally is characterized by a predominance of psychotic symptoms with preservation of affect and social functioning.3 DSM authors comment that it is not yet clear whether LOS is the same condition as schizophrenia diagnosed earlier in life. Approximately 23% of schizophrenia cases have onset after age 40.4
Cognitive symptoms in LOS
The presence of cognitive deficits in schizophrenia is common and well-recognized. The intellectual impairment is generalized and global, and there also is specific impairment in a range of cognitive functions, such as executive functions, memory, psychomotor speed, attention, and social cognition.5 Typically these cognitive impairments are present before onset of psychotic symptoms. Although cognitive symptoms are not part of the formal diagnostic criteria, DSM-5 acknowledges their presence.3 In a systematic review on nature and course of cognitive function in LOS, Rajji and Mulsant6 report that global deficits and specific deficits in executive functions, visuospatial constructional abilities, verbal fluency, and psychomotor speech have been found consistently in studies of LOS, although the presence of deficits in memory, attention, and working memory has been less consistent.
The presence of cognitive symptoms in LOS is less well-studied and understood (Table 2). The International Consensus Statement reported that no difference in type of cognitive deficit has been found in early–onset cases (onset before age 40) compared with late-onset cases, although LOS is associated with relatively milder cognitive deficits. Additionally, premorbid educational, occupational, and psychosocial functioning are less impaired in LOS than they are in early-onset schizophrenia.2
Rajji et al7 performed a meta-analysis comparison of patients with youth-onset schizophrenia, adults with first-episode schizophrenia, and those with LOS on their cognitive profiles. They reported that patients with youth-onset schizophrenia have globally severe cognitive deficits, whereas those with LOS demonstrate minimal deficits on arithmetic, digit symbol coding, and vocabulary but larger deficits on attention, fluency, global cognition, IQ, and visuospatial construction.7
There are conflicting views in the literature with regards to the course of cognitive deficits in schizophrenia. One group of researchers believes that there is progressive deterioration in cognitive functioning over time, while another maintains that cognitive impairment in schizophrenia is largely “a static encephalopathy” with no significant progression of symptoms.8 A number of studies referenced by Rajji and Mulsant6 in their systematic review report that cognitive deficits seen in patients with LOS largely are stable on follow-up with an average duration of up to 3 years. However, 2 studies with longer follow-up report evidence of cognitive decline.9,10
Relevant findings from the literature. Brodaty et al9 followed 27 patients with LOS without dementia and 34 otherwise healthy participants at baseline, 1 year, and 5 years. They reported that 9 patients with LOS and none of the control group were found to have dementia (5 Alzheimer type, 1 vascular, and 3 dementia of unknown type) at 5-year follow-up. Some patients had no clinical signs of dementia at baseline or at 1-year follow-up, but were found to have dementia at 5-year follow-up. The authors speculated that LOS might be a prodrome of Alzheimer-type dementia.
Kørner et al10 studied 12,600 patients with LOS and 7,700 with VLOS, selected from the Danish nationwide registry; follow-up was 3 to 4.58 years. They concluded that patients with LOS and VLOS were at 2 to 3 times greater risk of developing dementia than patients with osteoarthritis or the general population. The most common diagnosis among patients with schizophrenia was unspecified dementia, with Alzheimer’s dementia (AD) being the most common diagnosis in control groups. The findings suggest that dementia in LOS and VLOS has a different basis than AD.
Zakzanis et al11 investigated which neuropsychological tests best differentiate patients with LOS and those with AD or frontotemporal dementia. They reported that Wechsler Adult Intelligence Scale-Revised (WAIS-R) Similarities subtest and the California Verbal Learning Test (both short- and long-delay free recall) can differentiate LOS from AD, and a test battery comprising the WAIS-R Vocabulary, Information, Digit Span, and Comprehension subtests, and the Hooper Visual Organization test can differentiate LOS and frontotemporal dementia.12
EVALUATION Significant impairment
CT head and MRI brain scans without contrast suggest mild generalized atrophy that is more prominent in frontal and parietal areas, but the scans are otherwise unremarkable overall. A PET scan is significant for hypoactivity in the temporal and parietal lobes but, again, the images are interpreted as unremarkable overall.
Ms. P scores 21 on the Montreal Cognitive Assessment (MoCA), indicative of significant cognitive impairment (normal score, ≥26). This is a 3-point decline on a MoCA performed during her admission 5 years earlier.
Ms. P scores 8 on the Middlesex Elderly Assessment of Mental State, the lowest score in the borderline range of cognitive function for geriatric patients. She scores 13 on the Kohlman Evaluation of Living Skills, indicating that she needs maximal supervision, structure, and support to live in the community. Particularly notable is that Ms. P failed 5 out of 6 subtests in money management—a marked decline for someone who had worked as a senior accountant.
Given Ms. P’s significant cognitive decline from premorbid functioning, verified by collateral information, and current cognitive deficits established on standardized tests, we determine that, in addition to a diagnosis of schizoaffective disorder, she might meet DSM-5 criteria for unspecified major neurocognitive disorder if her functioning does not improve with treatment.
The authors’ observations
There is scant literature on late-onset schizoaffective disorder. Webster and Grossberg13 conducted a retrospective chart review of 1,730 patients age >65 who were admitted to a geriatric psychiatry unit from 1988 to 1995. Of these patients, 166 (approximately 10%) were found to have late life-onset psychosis. The psychosis was attributed to various causes, such as dementia, depression, bipolar disorder, medical causes, delirium, medication toxicity. Two patients were diagnosed with schizophrenia and 2 were diagnosed with schizoaffective disorder (the authors did not provide additional information about the patients with schizoaffective disorder). Brenner et al14 reports a case of late-onset schizoaffective disorder in a 70-year-old female patient. Evans et al15 compared outpatients age 45 to 77 with a diagnosis of schizoaffective disorder (n = 29), schizophrenia (n = 154), or nonpsychotic mood disorder (n = 27) and concluded that late-onset schizoaffective disorder might represent a variant of LOS in clinical symptom profiles and cognitive impairment but with additional mood symptoms.16
How would you begin treating Ms. P?
a) start a mood stabilizer
b) start an atypical antipsychotic
c) obtain more history and collateral information
d) recommend outpatient treatment
The authors’ observations
Given Ms. P’s manic symptoms, thought disorder, and history of psychotic symptoms with diagnosis of LOS, we assigned her a presumptive diagnosis of schizoaffective disorder, bipolar type. From the patient report, collateral information from her mother, earlier documented collateral from her husband, and chart review, it was apparent to us that Ms. P’s psychiatric history went back only 10 years—therefore meeting temporal criteria for LOS.
Clinical assessment (Figure) and standardized tests revealed the presence of neurocognitive deficits sufficient to meet criteria for major neurocognitive disorder (Table 33). The pattern of neurocognitive deficits is consistent with an AD-like amnestic picture, although no clear-cut diagnosis was present, and the neurocognitive disorder was better classified as unspecified rather than of a particular type. It remains uncertain whether cognitive deficits of severity that meet criteria for major neurocognitive disorder are sufficiently accounted for by the diagnosis of LOS alone. Unless diagnostic criteria for schizophrenia are expanded to include cognitive deficits, a separate diagnosis of major neurocognitive disorder is warranted at present.
TREATMENT Pharmacotherapy
On the unit, Ms. P is observed by nursing staff wandering, with some pressured speech but no behavioral agitation. Her clothing had been bizarre, with multiple layers, and, at one point, she walks with her gown open and without undergarments. She also reports to the nurses that she has a lot of sexual thoughts. When the interview team enters her room, they find her masturbating.
Ms. P is started on aripiprazole, 10 mg/d, titrated to 20 mg/d, and divalproex sodium, 500 mg/d. The decision to initiate a cognitive enhancer, such as an acetylcholinesterase inhibitor or memantine, is deferred to outpatient care to allow for the possibility that her cognitive features will improve after the psychosis is treated.
By the end of first week, Ms. P’s manic features are no longer prominent but her thought process continues to be bizarre, with poor insight and judgment. She demonstrates severe ambivalence in all matters, consistently gives inconsistent accounts of the past, and makes dramatic false statements.
For example, when asked about her children, Ms. P tells us that she has 6 children—the youngest 3 months old, at home by himself and “probably dead by now.” In reality, she has only a 20-year-old son who is studying abroad. Talking about her marriage, Ms. P says she and her husband are not divorced on paper but that, because they haven’t had sex for 8 years, the law has provided them with an automatic divorce.
OUTCOME Significant improvement
Ms. P shows significant response to aripiprazole and divalproex, which are well tolerated without significant adverse effects. Her limitations in executive functioning and rational thought process lead the treatment team to consider nursing home placement under guardianship. Days before discharge, however, reexamination of her neuropsychiatric state suggests significant improvement in thought process, with improvement in cognitive features. Ms. P also becomes cooperative with treatment planning.
The treatment team has meetings with Ms. P’s mother to discuss monitoring and plans for discharge. Ms. P is discharged with follow-up arranged at community mental health services.
Bottom Line
Global as well as specific cognitive deficits are associated with late-onset schizophrenia. Studies have reported increased risk of dementia in these patients over the course of 3 to 5 years, usually unspecified or Alzheimer’s type. It is imperative to assess patients with schizophrenia, especially those age ≥40, for presence of neurocognitive disorder by means of neurocognitive testing.
Related Resources
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
- Radhakrishnan R, Butler R, Head L. Dementia in schizophrenia. Adv Psychiatr Treat. 2012;18(2):144-153.
Drug Brand Names
Aripiprazole • Abilify
Divalproex sodium • Depakote
Mematine • Namenda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturer of competing products.
1. Bleuler M. Die spätschizophrenen Krankheitsbilder. Fortschr Neurol Psychiatr. 1943;15:259-290.
2. Howard R, Rabins PV, Seeman MV, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry. 2000; 157(2):172-178.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14(1):39-55.
5. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100(1):4-19.
6. Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102(1-3):122-140.
7. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(4):286-293.
8. Goldberg TE, Hyde TM, Kleinman JE, et al. Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull. 1993;19(4):797-804.
9. Brodaty H, Sachdev P, Koschera A, et al. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br J Psychiatry. 2003;183(3):213-219.
10. Kørner A, Lopez AG, Lauritzen L, et al. Late and very-late first‐contact schizophrenia and the risk of dementia—a nationwide register based study. Int J Geriatr Psychiatry. 2009;24(1):61-67.
11. Zakzanis KK, Andrikopoulos J, Young DA, et al. Neuropsychological differentiation of late-onset schizophrenia and dementia of the Alzheimer’s type. Appl Neuropsychol. 2003;10(2):105-114.
12. Zakzanis KK, Kielar A, Young DA, et al. Neuropsychological differentiation of late onset schizophrenia and frontotemporal dementia. Cognitive Neuropsychiatry. 2001;6(1):63-77.
13. Webster J, Grossberg GT. Late-life onset of psychotic symptoms. Am J Geriatr Psychiatry. 1998;6(3):196-202.
14. Brenner R, Campbell K, Konakondla K, et al. Late onset schizoaffective disorder. Consultant. 2014;53(6):487-488.
15. Evans JD, Heaton RK, Paulsen JS, et al. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60(12):874-882.
16. Jeste DV, Blazer DG, First M. Aging-related diagnostic variations: need for diagnostic criteria appropriate for elderly psychiatric patients. Biol Psychiatry. 2005;58(4):265-271.
CASE Inconsistent stories
Ms. P, age 56, is an Asian American woman who was brought in by police after being found standing by her car in the middle of a busy road displaying bizarre behavior. She provides an inconsistent story about why she was brought to the hospital, saying that the police did so because she wasn’t driving fast enough and because her English is weak. At another point, she says that she had stopped her car to pick up a penny from the road and the police brought her to the hospital “to experience life, to rest, to meet people.”
Upon further questioning, Ms. P reveals that she is experiencing racing thoughts, feels full of energy, has pressured speech, and does not need much sleep. She also is sexually preoccupied, talks about having extra-marital affairs, and expresses her infatuation with TV news anchors. She says she is sexually active but is unable to offer any further details, and—while giggling—asks the treatment team not to reveal this information to her husband. Ms. P also reports hearing angels singing from the sky.
Chart review reveals that Ms. P had been admitted to same hospital 5 years earlier, at which time she was given diagnoses of late-onset schizophrenia (LOS) and mild cognitive impairment. Ms. P also had 3 psychiatric inpatient admissions in the past 2 years at a different hospital, but her records are inaccessible because she refuses to allow her chart to be released.
Ms. P has not taken the psychiatric medications prescribed for her for several months; she says, “I don’t need medication. I am self-healing.” She denies using illicit substances, including marijuana, smoking, and current alcohol use, but reports occasional social drinking in the past. Her urine drug screen is negative.
The most striking revelation in Ms. P’s social history is her high premorbid functional status. She has 2 master’s degrees and had been working as a senior accountant at a major hospital system until 7 years ago. In contrast, when interviewed at the hospital, Ms. P reports that she is working at a child care center.
On mental status exam, Ms. P is half-draped in a hospital gown, casual, overly friendly, smiling, and twirling her hair. Her mood is elevated with inappropriate affect. Her thought process is bizarre and illogical. She is alert, fully oriented, and her sensorium is clear. She has persistent ambivalence and contradictory thoughts regarding suicidal ideation. Recent and remote memory are largely intact. She does not express homicidal ideation.
What could be causing Ms. P’s psychosis and functional decline?
a) major neurocognitive disorder
b) schizophrenia
c) schizoaffective disorder
d) bipolar disorder, current manic episode
HISTORY Fired from her job
According to Ms. P’s chart from her admission 5 years earlier, police brought her to the hospital because she was causing a disturbance at a restaurant. When interviewed, Ms. P reported a false story that she fought with her husband, kicked him, and spat on his face. She said that her husband then punched her in the face, she ran out of the house, and a bystander called the police. At the time, her husband was contacted and denied the incident. He said that Ms. P had gone to the store and not returned, and he did not know what happened to her.
Her husband reported a steady and progressive decline in function and behavior dating back to 8 years ago with no known prior behavioral disturbances. In the chart from 5 years ago, her husband reported that Ms. P had been a high-functioning senior executive accountant at a major hospital system 7 years before the current admission, at which time she was fired from her job. He said that, just before being fired, Ms. P had been reading the mystery novel The Da Vinci Code and believed that events in the book specifically applied to her. Ms. P would stay up all night making clothes; when she would go to work, she was caught sleeping on the job and performing poorly, including submitting reports with incorrect information. She yelled at co-workers and was unable to take direction from her supervisors.
Ms. P’s husband also reported that she believed people were trying to “look like her,” by having plastic surgery. He reported unusual behavior at home, including eating food off the countertop that had been out for hours and was not fit for consumption.
Ms. P’s husband could not be contacted during this admission because he was out of country and they were separated. Collateral information is obtained from Ms. P’s mother, who lives apart from her but in the same city and speaks no English. She confirms Ms. P’s high premorbid functioning, and reports that her daughter’s change in behavior went back as far as 10 years. She reports that Ms. P had problems controlling anger and had frequent altercations with her husband and mother, including threatening her with a knife. Self-care and hygiene then declined strikingly. She began to have odd religious beliefs (eg, she was the daughter of Jesus Christ) and insisted on dressing in peculiar ways.
No family history of psychiatric disorders, such as schizophrenia, bipolar disorder, or dementia, was reported (Table 1).
The authors’ observations
The existence of LOS as a distinct subtype of schizophrenia has been the subject of discussion and controversy as far back as Manfred Bleuler in 1943 who coined the term “late-onset schizophrenia.”1 In 2000, a consensus statement by the International Late-Onset Schizophrenia Group standardized the nomenclature, defining LOS as onset between age 40 and 60, and very-late-onset schizophrenia-like psychosis (VLOS) as onset after age 60.2 Although there is no diagnostic subcategory for LOS in DSM, DSM-5 notes that (1) women are overrepresented in late-onset cases and (2) the course generally is characterized by a predominance of psychotic symptoms with preservation of affect and social functioning.3 DSM authors comment that it is not yet clear whether LOS is the same condition as schizophrenia diagnosed earlier in life. Approximately 23% of schizophrenia cases have onset after age 40.4
Cognitive symptoms in LOS
The presence of cognitive deficits in schizophrenia is common and well-recognized. The intellectual impairment is generalized and global, and there also is specific impairment in a range of cognitive functions, such as executive functions, memory, psychomotor speed, attention, and social cognition.5 Typically these cognitive impairments are present before onset of psychotic symptoms. Although cognitive symptoms are not part of the formal diagnostic criteria, DSM-5 acknowledges their presence.3 In a systematic review on nature and course of cognitive function in LOS, Rajji and Mulsant6 report that global deficits and specific deficits in executive functions, visuospatial constructional abilities, verbal fluency, and psychomotor speech have been found consistently in studies of LOS, although the presence of deficits in memory, attention, and working memory has been less consistent.
The presence of cognitive symptoms in LOS is less well-studied and understood (Table 2). The International Consensus Statement reported that no difference in type of cognitive deficit has been found in early–onset cases (onset before age 40) compared with late-onset cases, although LOS is associated with relatively milder cognitive deficits. Additionally, premorbid educational, occupational, and psychosocial functioning are less impaired in LOS than they are in early-onset schizophrenia.2
Rajji et al7 performed a meta-analysis comparison of patients with youth-onset schizophrenia, adults with first-episode schizophrenia, and those with LOS on their cognitive profiles. They reported that patients with youth-onset schizophrenia have globally severe cognitive deficits, whereas those with LOS demonstrate minimal deficits on arithmetic, digit symbol coding, and vocabulary but larger deficits on attention, fluency, global cognition, IQ, and visuospatial construction.7
There are conflicting views in the literature with regards to the course of cognitive deficits in schizophrenia. One group of researchers believes that there is progressive deterioration in cognitive functioning over time, while another maintains that cognitive impairment in schizophrenia is largely “a static encephalopathy” with no significant progression of symptoms.8 A number of studies referenced by Rajji and Mulsant6 in their systematic review report that cognitive deficits seen in patients with LOS largely are stable on follow-up with an average duration of up to 3 years. However, 2 studies with longer follow-up report evidence of cognitive decline.9,10
Relevant findings from the literature. Brodaty et al9 followed 27 patients with LOS without dementia and 34 otherwise healthy participants at baseline, 1 year, and 5 years. They reported that 9 patients with LOS and none of the control group were found to have dementia (5 Alzheimer type, 1 vascular, and 3 dementia of unknown type) at 5-year follow-up. Some patients had no clinical signs of dementia at baseline or at 1-year follow-up, but were found to have dementia at 5-year follow-up. The authors speculated that LOS might be a prodrome of Alzheimer-type dementia.
Kørner et al10 studied 12,600 patients with LOS and 7,700 with VLOS, selected from the Danish nationwide registry; follow-up was 3 to 4.58 years. They concluded that patients with LOS and VLOS were at 2 to 3 times greater risk of developing dementia than patients with osteoarthritis or the general population. The most common diagnosis among patients with schizophrenia was unspecified dementia, with Alzheimer’s dementia (AD) being the most common diagnosis in control groups. The findings suggest that dementia in LOS and VLOS has a different basis than AD.
Zakzanis et al11 investigated which neuropsychological tests best differentiate patients with LOS and those with AD or frontotemporal dementia. They reported that Wechsler Adult Intelligence Scale-Revised (WAIS-R) Similarities subtest and the California Verbal Learning Test (both short- and long-delay free recall) can differentiate LOS from AD, and a test battery comprising the WAIS-R Vocabulary, Information, Digit Span, and Comprehension subtests, and the Hooper Visual Organization test can differentiate LOS and frontotemporal dementia.12
EVALUATION Significant impairment
CT head and MRI brain scans without contrast suggest mild generalized atrophy that is more prominent in frontal and parietal areas, but the scans are otherwise unremarkable overall. A PET scan is significant for hypoactivity in the temporal and parietal lobes but, again, the images are interpreted as unremarkable overall.
Ms. P scores 21 on the Montreal Cognitive Assessment (MoCA), indicative of significant cognitive impairment (normal score, ≥26). This is a 3-point decline on a MoCA performed during her admission 5 years earlier.
Ms. P scores 8 on the Middlesex Elderly Assessment of Mental State, the lowest score in the borderline range of cognitive function for geriatric patients. She scores 13 on the Kohlman Evaluation of Living Skills, indicating that she needs maximal supervision, structure, and support to live in the community. Particularly notable is that Ms. P failed 5 out of 6 subtests in money management—a marked decline for someone who had worked as a senior accountant.
Given Ms. P’s significant cognitive decline from premorbid functioning, verified by collateral information, and current cognitive deficits established on standardized tests, we determine that, in addition to a diagnosis of schizoaffective disorder, she might meet DSM-5 criteria for unspecified major neurocognitive disorder if her functioning does not improve with treatment.
The authors’ observations
There is scant literature on late-onset schizoaffective disorder. Webster and Grossberg13 conducted a retrospective chart review of 1,730 patients age >65 who were admitted to a geriatric psychiatry unit from 1988 to 1995. Of these patients, 166 (approximately 10%) were found to have late life-onset psychosis. The psychosis was attributed to various causes, such as dementia, depression, bipolar disorder, medical causes, delirium, medication toxicity. Two patients were diagnosed with schizophrenia and 2 were diagnosed with schizoaffective disorder (the authors did not provide additional information about the patients with schizoaffective disorder). Brenner et al14 reports a case of late-onset schizoaffective disorder in a 70-year-old female patient. Evans et al15 compared outpatients age 45 to 77 with a diagnosis of schizoaffective disorder (n = 29), schizophrenia (n = 154), or nonpsychotic mood disorder (n = 27) and concluded that late-onset schizoaffective disorder might represent a variant of LOS in clinical symptom profiles and cognitive impairment but with additional mood symptoms.16
How would you begin treating Ms. P?
a) start a mood stabilizer
b) start an atypical antipsychotic
c) obtain more history and collateral information
d) recommend outpatient treatment
The authors’ observations
Given Ms. P’s manic symptoms, thought disorder, and history of psychotic symptoms with diagnosis of LOS, we assigned her a presumptive diagnosis of schizoaffective disorder, bipolar type. From the patient report, collateral information from her mother, earlier documented collateral from her husband, and chart review, it was apparent to us that Ms. P’s psychiatric history went back only 10 years—therefore meeting temporal criteria for LOS.
Clinical assessment (Figure) and standardized tests revealed the presence of neurocognitive deficits sufficient to meet criteria for major neurocognitive disorder (Table 33). The pattern of neurocognitive deficits is consistent with an AD-like amnestic picture, although no clear-cut diagnosis was present, and the neurocognitive disorder was better classified as unspecified rather than of a particular type. It remains uncertain whether cognitive deficits of severity that meet criteria for major neurocognitive disorder are sufficiently accounted for by the diagnosis of LOS alone. Unless diagnostic criteria for schizophrenia are expanded to include cognitive deficits, a separate diagnosis of major neurocognitive disorder is warranted at present.
TREATMENT Pharmacotherapy
On the unit, Ms. P is observed by nursing staff wandering, with some pressured speech but no behavioral agitation. Her clothing had been bizarre, with multiple layers, and, at one point, she walks with her gown open and without undergarments. She also reports to the nurses that she has a lot of sexual thoughts. When the interview team enters her room, they find her masturbating.
Ms. P is started on aripiprazole, 10 mg/d, titrated to 20 mg/d, and divalproex sodium, 500 mg/d. The decision to initiate a cognitive enhancer, such as an acetylcholinesterase inhibitor or memantine, is deferred to outpatient care to allow for the possibility that her cognitive features will improve after the psychosis is treated.
By the end of first week, Ms. P’s manic features are no longer prominent but her thought process continues to be bizarre, with poor insight and judgment. She demonstrates severe ambivalence in all matters, consistently gives inconsistent accounts of the past, and makes dramatic false statements.
For example, when asked about her children, Ms. P tells us that she has 6 children—the youngest 3 months old, at home by himself and “probably dead by now.” In reality, she has only a 20-year-old son who is studying abroad. Talking about her marriage, Ms. P says she and her husband are not divorced on paper but that, because they haven’t had sex for 8 years, the law has provided them with an automatic divorce.
OUTCOME Significant improvement
Ms. P shows significant response to aripiprazole and divalproex, which are well tolerated without significant adverse effects. Her limitations in executive functioning and rational thought process lead the treatment team to consider nursing home placement under guardianship. Days before discharge, however, reexamination of her neuropsychiatric state suggests significant improvement in thought process, with improvement in cognitive features. Ms. P also becomes cooperative with treatment planning.
The treatment team has meetings with Ms. P’s mother to discuss monitoring and plans for discharge. Ms. P is discharged with follow-up arranged at community mental health services.
Bottom Line
Global as well as specific cognitive deficits are associated with late-onset schizophrenia. Studies have reported increased risk of dementia in these patients over the course of 3 to 5 years, usually unspecified or Alzheimer’s type. It is imperative to assess patients with schizophrenia, especially those age ≥40, for presence of neurocognitive disorder by means of neurocognitive testing.
Related Resources
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
- Radhakrishnan R, Butler R, Head L. Dementia in schizophrenia. Adv Psychiatr Treat. 2012;18(2):144-153.
Drug Brand Names
Aripiprazole • Abilify
Divalproex sodium • Depakote
Mematine • Namenda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturer of competing products.
CASE Inconsistent stories
Ms. P, age 56, is an Asian American woman who was brought in by police after being found standing by her car in the middle of a busy road displaying bizarre behavior. She provides an inconsistent story about why she was brought to the hospital, saying that the police did so because she wasn’t driving fast enough and because her English is weak. At another point, she says that she had stopped her car to pick up a penny from the road and the police brought her to the hospital “to experience life, to rest, to meet people.”
Upon further questioning, Ms. P reveals that she is experiencing racing thoughts, feels full of energy, has pressured speech, and does not need much sleep. She also is sexually preoccupied, talks about having extra-marital affairs, and expresses her infatuation with TV news anchors. She says she is sexually active but is unable to offer any further details, and—while giggling—asks the treatment team not to reveal this information to her husband. Ms. P also reports hearing angels singing from the sky.
Chart review reveals that Ms. P had been admitted to same hospital 5 years earlier, at which time she was given diagnoses of late-onset schizophrenia (LOS) and mild cognitive impairment. Ms. P also had 3 psychiatric inpatient admissions in the past 2 years at a different hospital, but her records are inaccessible because she refuses to allow her chart to be released.
Ms. P has not taken the psychiatric medications prescribed for her for several months; she says, “I don’t need medication. I am self-healing.” She denies using illicit substances, including marijuana, smoking, and current alcohol use, but reports occasional social drinking in the past. Her urine drug screen is negative.
The most striking revelation in Ms. P’s social history is her high premorbid functional status. She has 2 master’s degrees and had been working as a senior accountant at a major hospital system until 7 years ago. In contrast, when interviewed at the hospital, Ms. P reports that she is working at a child care center.
On mental status exam, Ms. P is half-draped in a hospital gown, casual, overly friendly, smiling, and twirling her hair. Her mood is elevated with inappropriate affect. Her thought process is bizarre and illogical. She is alert, fully oriented, and her sensorium is clear. She has persistent ambivalence and contradictory thoughts regarding suicidal ideation. Recent and remote memory are largely intact. She does not express homicidal ideation.
What could be causing Ms. P’s psychosis and functional decline?
a) major neurocognitive disorder
b) schizophrenia
c) schizoaffective disorder
d) bipolar disorder, current manic episode
HISTORY Fired from her job
According to Ms. P’s chart from her admission 5 years earlier, police brought her to the hospital because she was causing a disturbance at a restaurant. When interviewed, Ms. P reported a false story that she fought with her husband, kicked him, and spat on his face. She said that her husband then punched her in the face, she ran out of the house, and a bystander called the police. At the time, her husband was contacted and denied the incident. He said that Ms. P had gone to the store and not returned, and he did not know what happened to her.
Her husband reported a steady and progressive decline in function and behavior dating back to 8 years ago with no known prior behavioral disturbances. In the chart from 5 years ago, her husband reported that Ms. P had been a high-functioning senior executive accountant at a major hospital system 7 years before the current admission, at which time she was fired from her job. He said that, just before being fired, Ms. P had been reading the mystery novel The Da Vinci Code and believed that events in the book specifically applied to her. Ms. P would stay up all night making clothes; when she would go to work, she was caught sleeping on the job and performing poorly, including submitting reports with incorrect information. She yelled at co-workers and was unable to take direction from her supervisors.
Ms. P’s husband also reported that she believed people were trying to “look like her,” by having plastic surgery. He reported unusual behavior at home, including eating food off the countertop that had been out for hours and was not fit for consumption.
Ms. P’s husband could not be contacted during this admission because he was out of country and they were separated. Collateral information is obtained from Ms. P’s mother, who lives apart from her but in the same city and speaks no English. She confirms Ms. P’s high premorbid functioning, and reports that her daughter’s change in behavior went back as far as 10 years. She reports that Ms. P had problems controlling anger and had frequent altercations with her husband and mother, including threatening her with a knife. Self-care and hygiene then declined strikingly. She began to have odd religious beliefs (eg, she was the daughter of Jesus Christ) and insisted on dressing in peculiar ways.
No family history of psychiatric disorders, such as schizophrenia, bipolar disorder, or dementia, was reported (Table 1).
The authors’ observations
The existence of LOS as a distinct subtype of schizophrenia has been the subject of discussion and controversy as far back as Manfred Bleuler in 1943 who coined the term “late-onset schizophrenia.”1 In 2000, a consensus statement by the International Late-Onset Schizophrenia Group standardized the nomenclature, defining LOS as onset between age 40 and 60, and very-late-onset schizophrenia-like psychosis (VLOS) as onset after age 60.2 Although there is no diagnostic subcategory for LOS in DSM, DSM-5 notes that (1) women are overrepresented in late-onset cases and (2) the course generally is characterized by a predominance of psychotic symptoms with preservation of affect and social functioning.3 DSM authors comment that it is not yet clear whether LOS is the same condition as schizophrenia diagnosed earlier in life. Approximately 23% of schizophrenia cases have onset after age 40.4
Cognitive symptoms in LOS
The presence of cognitive deficits in schizophrenia is common and well-recognized. The intellectual impairment is generalized and global, and there also is specific impairment in a range of cognitive functions, such as executive functions, memory, psychomotor speed, attention, and social cognition.5 Typically these cognitive impairments are present before onset of psychotic symptoms. Although cognitive symptoms are not part of the formal diagnostic criteria, DSM-5 acknowledges their presence.3 In a systematic review on nature and course of cognitive function in LOS, Rajji and Mulsant6 report that global deficits and specific deficits in executive functions, visuospatial constructional abilities, verbal fluency, and psychomotor speech have been found consistently in studies of LOS, although the presence of deficits in memory, attention, and working memory has been less consistent.
The presence of cognitive symptoms in LOS is less well-studied and understood (Table 2). The International Consensus Statement reported that no difference in type of cognitive deficit has been found in early–onset cases (onset before age 40) compared with late-onset cases, although LOS is associated with relatively milder cognitive deficits. Additionally, premorbid educational, occupational, and psychosocial functioning are less impaired in LOS than they are in early-onset schizophrenia.2
Rajji et al7 performed a meta-analysis comparison of patients with youth-onset schizophrenia, adults with first-episode schizophrenia, and those with LOS on their cognitive profiles. They reported that patients with youth-onset schizophrenia have globally severe cognitive deficits, whereas those with LOS demonstrate minimal deficits on arithmetic, digit symbol coding, and vocabulary but larger deficits on attention, fluency, global cognition, IQ, and visuospatial construction.7
There are conflicting views in the literature with regards to the course of cognitive deficits in schizophrenia. One group of researchers believes that there is progressive deterioration in cognitive functioning over time, while another maintains that cognitive impairment in schizophrenia is largely “a static encephalopathy” with no significant progression of symptoms.8 A number of studies referenced by Rajji and Mulsant6 in their systematic review report that cognitive deficits seen in patients with LOS largely are stable on follow-up with an average duration of up to 3 years. However, 2 studies with longer follow-up report evidence of cognitive decline.9,10
Relevant findings from the literature. Brodaty et al9 followed 27 patients with LOS without dementia and 34 otherwise healthy participants at baseline, 1 year, and 5 years. They reported that 9 patients with LOS and none of the control group were found to have dementia (5 Alzheimer type, 1 vascular, and 3 dementia of unknown type) at 5-year follow-up. Some patients had no clinical signs of dementia at baseline or at 1-year follow-up, but were found to have dementia at 5-year follow-up. The authors speculated that LOS might be a prodrome of Alzheimer-type dementia.
Kørner et al10 studied 12,600 patients with LOS and 7,700 with VLOS, selected from the Danish nationwide registry; follow-up was 3 to 4.58 years. They concluded that patients with LOS and VLOS were at 2 to 3 times greater risk of developing dementia than patients with osteoarthritis or the general population. The most common diagnosis among patients with schizophrenia was unspecified dementia, with Alzheimer’s dementia (AD) being the most common diagnosis in control groups. The findings suggest that dementia in LOS and VLOS has a different basis than AD.
Zakzanis et al11 investigated which neuropsychological tests best differentiate patients with LOS and those with AD or frontotemporal dementia. They reported that Wechsler Adult Intelligence Scale-Revised (WAIS-R) Similarities subtest and the California Verbal Learning Test (both short- and long-delay free recall) can differentiate LOS from AD, and a test battery comprising the WAIS-R Vocabulary, Information, Digit Span, and Comprehension subtests, and the Hooper Visual Organization test can differentiate LOS and frontotemporal dementia.12
EVALUATION Significant impairment
CT head and MRI brain scans without contrast suggest mild generalized atrophy that is more prominent in frontal and parietal areas, but the scans are otherwise unremarkable overall. A PET scan is significant for hypoactivity in the temporal and parietal lobes but, again, the images are interpreted as unremarkable overall.
Ms. P scores 21 on the Montreal Cognitive Assessment (MoCA), indicative of significant cognitive impairment (normal score, ≥26). This is a 3-point decline on a MoCA performed during her admission 5 years earlier.
Ms. P scores 8 on the Middlesex Elderly Assessment of Mental State, the lowest score in the borderline range of cognitive function for geriatric patients. She scores 13 on the Kohlman Evaluation of Living Skills, indicating that she needs maximal supervision, structure, and support to live in the community. Particularly notable is that Ms. P failed 5 out of 6 subtests in money management—a marked decline for someone who had worked as a senior accountant.
Given Ms. P’s significant cognitive decline from premorbid functioning, verified by collateral information, and current cognitive deficits established on standardized tests, we determine that, in addition to a diagnosis of schizoaffective disorder, she might meet DSM-5 criteria for unspecified major neurocognitive disorder if her functioning does not improve with treatment.
The authors’ observations
There is scant literature on late-onset schizoaffective disorder. Webster and Grossberg13 conducted a retrospective chart review of 1,730 patients age >65 who were admitted to a geriatric psychiatry unit from 1988 to 1995. Of these patients, 166 (approximately 10%) were found to have late life-onset psychosis. The psychosis was attributed to various causes, such as dementia, depression, bipolar disorder, medical causes, delirium, medication toxicity. Two patients were diagnosed with schizophrenia and 2 were diagnosed with schizoaffective disorder (the authors did not provide additional information about the patients with schizoaffective disorder). Brenner et al14 reports a case of late-onset schizoaffective disorder in a 70-year-old female patient. Evans et al15 compared outpatients age 45 to 77 with a diagnosis of schizoaffective disorder (n = 29), schizophrenia (n = 154), or nonpsychotic mood disorder (n = 27) and concluded that late-onset schizoaffective disorder might represent a variant of LOS in clinical symptom profiles and cognitive impairment but with additional mood symptoms.16
How would you begin treating Ms. P?
a) start a mood stabilizer
b) start an atypical antipsychotic
c) obtain more history and collateral information
d) recommend outpatient treatment
The authors’ observations
Given Ms. P’s manic symptoms, thought disorder, and history of psychotic symptoms with diagnosis of LOS, we assigned her a presumptive diagnosis of schizoaffective disorder, bipolar type. From the patient report, collateral information from her mother, earlier documented collateral from her husband, and chart review, it was apparent to us that Ms. P’s psychiatric history went back only 10 years—therefore meeting temporal criteria for LOS.
Clinical assessment (Figure) and standardized tests revealed the presence of neurocognitive deficits sufficient to meet criteria for major neurocognitive disorder (Table 33). The pattern of neurocognitive deficits is consistent with an AD-like amnestic picture, although no clear-cut diagnosis was present, and the neurocognitive disorder was better classified as unspecified rather than of a particular type. It remains uncertain whether cognitive deficits of severity that meet criteria for major neurocognitive disorder are sufficiently accounted for by the diagnosis of LOS alone. Unless diagnostic criteria for schizophrenia are expanded to include cognitive deficits, a separate diagnosis of major neurocognitive disorder is warranted at present.
TREATMENT Pharmacotherapy
On the unit, Ms. P is observed by nursing staff wandering, with some pressured speech but no behavioral agitation. Her clothing had been bizarre, with multiple layers, and, at one point, she walks with her gown open and without undergarments. She also reports to the nurses that she has a lot of sexual thoughts. When the interview team enters her room, they find her masturbating.
Ms. P is started on aripiprazole, 10 mg/d, titrated to 20 mg/d, and divalproex sodium, 500 mg/d. The decision to initiate a cognitive enhancer, such as an acetylcholinesterase inhibitor or memantine, is deferred to outpatient care to allow for the possibility that her cognitive features will improve after the psychosis is treated.
By the end of first week, Ms. P’s manic features are no longer prominent but her thought process continues to be bizarre, with poor insight and judgment. She demonstrates severe ambivalence in all matters, consistently gives inconsistent accounts of the past, and makes dramatic false statements.
For example, when asked about her children, Ms. P tells us that she has 6 children—the youngest 3 months old, at home by himself and “probably dead by now.” In reality, she has only a 20-year-old son who is studying abroad. Talking about her marriage, Ms. P says she and her husband are not divorced on paper but that, because they haven’t had sex for 8 years, the law has provided them with an automatic divorce.
OUTCOME Significant improvement
Ms. P shows significant response to aripiprazole and divalproex, which are well tolerated without significant adverse effects. Her limitations in executive functioning and rational thought process lead the treatment team to consider nursing home placement under guardianship. Days before discharge, however, reexamination of her neuropsychiatric state suggests significant improvement in thought process, with improvement in cognitive features. Ms. P also becomes cooperative with treatment planning.
The treatment team has meetings with Ms. P’s mother to discuss monitoring and plans for discharge. Ms. P is discharged with follow-up arranged at community mental health services.
Bottom Line
Global as well as specific cognitive deficits are associated with late-onset schizophrenia. Studies have reported increased risk of dementia in these patients over the course of 3 to 5 years, usually unspecified or Alzheimer’s type. It is imperative to assess patients with schizophrenia, especially those age ≥40, for presence of neurocognitive disorder by means of neurocognitive testing.
Related Resources
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
- Radhakrishnan R, Butler R, Head L. Dementia in schizophrenia. Adv Psychiatr Treat. 2012;18(2):144-153.
Drug Brand Names
Aripiprazole • Abilify
Divalproex sodium • Depakote
Mematine • Namenda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturer of competing products.
1. Bleuler M. Die spätschizophrenen Krankheitsbilder. Fortschr Neurol Psychiatr. 1943;15:259-290.
2. Howard R, Rabins PV, Seeman MV, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry. 2000; 157(2):172-178.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14(1):39-55.
5. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100(1):4-19.
6. Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102(1-3):122-140.
7. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(4):286-293.
8. Goldberg TE, Hyde TM, Kleinman JE, et al. Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull. 1993;19(4):797-804.
9. Brodaty H, Sachdev P, Koschera A, et al. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br J Psychiatry. 2003;183(3):213-219.
10. Kørner A, Lopez AG, Lauritzen L, et al. Late and very-late first‐contact schizophrenia and the risk of dementia—a nationwide register based study. Int J Geriatr Psychiatry. 2009;24(1):61-67.
11. Zakzanis KK, Andrikopoulos J, Young DA, et al. Neuropsychological differentiation of late-onset schizophrenia and dementia of the Alzheimer’s type. Appl Neuropsychol. 2003;10(2):105-114.
12. Zakzanis KK, Kielar A, Young DA, et al. Neuropsychological differentiation of late onset schizophrenia and frontotemporal dementia. Cognitive Neuropsychiatry. 2001;6(1):63-77.
13. Webster J, Grossberg GT. Late-life onset of psychotic symptoms. Am J Geriatr Psychiatry. 1998;6(3):196-202.
14. Brenner R, Campbell K, Konakondla K, et al. Late onset schizoaffective disorder. Consultant. 2014;53(6):487-488.
15. Evans JD, Heaton RK, Paulsen JS, et al. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60(12):874-882.
16. Jeste DV, Blazer DG, First M. Aging-related diagnostic variations: need for diagnostic criteria appropriate for elderly psychiatric patients. Biol Psychiatry. 2005;58(4):265-271.
1. Bleuler M. Die spätschizophrenen Krankheitsbilder. Fortschr Neurol Psychiatr. 1943;15:259-290.
2. Howard R, Rabins PV, Seeman MV, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry. 2000; 157(2):172-178.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14(1):39-55.
5. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100(1):4-19.
6. Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102(1-3):122-140.
7. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(4):286-293.
8. Goldberg TE, Hyde TM, Kleinman JE, et al. Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull. 1993;19(4):797-804.
9. Brodaty H, Sachdev P, Koschera A, et al. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br J Psychiatry. 2003;183(3):213-219.
10. Kørner A, Lopez AG, Lauritzen L, et al. Late and very-late first‐contact schizophrenia and the risk of dementia—a nationwide register based study. Int J Geriatr Psychiatry. 2009;24(1):61-67.
11. Zakzanis KK, Andrikopoulos J, Young DA, et al. Neuropsychological differentiation of late-onset schizophrenia and dementia of the Alzheimer’s type. Appl Neuropsychol. 2003;10(2):105-114.
12. Zakzanis KK, Kielar A, Young DA, et al. Neuropsychological differentiation of late onset schizophrenia and frontotemporal dementia. Cognitive Neuropsychiatry. 2001;6(1):63-77.
13. Webster J, Grossberg GT. Late-life onset of psychotic symptoms. Am J Geriatr Psychiatry. 1998;6(3):196-202.
14. Brenner R, Campbell K, Konakondla K, et al. Late onset schizoaffective disorder. Consultant. 2014;53(6):487-488.
15. Evans JD, Heaton RK, Paulsen JS, et al. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60(12):874-882.
16. Jeste DV, Blazer DG, First M. Aging-related diagnostic variations: need for diagnostic criteria appropriate for elderly psychiatric patients. Biol Psychiatry. 2005;58(4):265-271.