User login
Prescribing exercise to help your patients lose weight
Although exercise is probably less effective than diet in reducing weight, most studies show that adding it to a diet regimen will increase the weight loss.1,2 Guidelines from the American Heart Association, American College of Cardiology, and Obesity Society recommend a comprehensive lifestyle program that includes a low-calorie diet as well as an increase in physical activity.3
Here, we review the many benefits of exercise for obese patients, not only in terms of weight loss, but also its positive cardiovascular and metabolic effects. Then we discuss how to motivate and prescribe exercise for this challenging group.
EXERCISE IMPROVES WEIGHT LOSS
Increasing energy expenditure by exercising can mobilize and burn stored fat and thus lead to weight loss.4
Typically, with no changes in caloric intake, exercising 60 minutes at low intensity most days of the week will remove up to 0.5 lb per week.5 Exercising harder for longer will take off more weight, up to 3 lb per week.1,6 Some practitioners believe that the total volume of exercise (frequency multiplied by time) is more important than the intensity in determining the amount of weight loss.2,7,8
Ross et al9 randomized 101 obese men to try to lose weight by exercising at a low to moderate intensity, to try to lose weight by dieting, to exercise without the goal of losing weight, or to do nothing (the control group). About half the participants declined or dropped out, but 52 completed the trial. The weight-loss-through-exercise group had lost approximately 15 lb by 12 weeks; the diet group lost a similar amount. Total body fat, visceral fat, and abdominal obesity were all reduced with both diet- and exercise-induced weight loss.
In a study in 130 severely obese adults, after 6 months of high-intensity physical activity for a mean duration of 71 minutes per week, those on an exercise-and-diet regimen lost an average of 24 lb, compared with 18 lb with diet alone.10
Another trial involved obese patients who were instructed to jog the equivalent of 20 miles (32.2 km) a week, with no restriction on caloric intake.11 They lost only 2.9 kg (6.5 lb) over 8 months. Increased food intake explained this minimal weight loss.
In an analysis of 20 studies, exercise-only interventions of 4 months or less resulted in a mean weekly weight loss of 0.4 lb (0.2 kg), with a total loss of about 5 lb (2.3 kg).12
A systematic review of 15 studies noted that aerobic exercise for 3 months or more resulted in a significant reduction in visceral adipose tissue in overweight men and women as measured by computed tomography.13
Effects that different types of exercise have on weight loss
In a study of 119 sedentary adults who were overweight or obese and who were randomized to aerobic, resistance, or combined aerobic-resistance training over 8 months, those involved in aerobic or combined aerobic and resistance training had the greatest reduction in total body and fat mass.14 Given that the combined aerobic-resistance training program required twice the time commitment of the aerobic-alone program, the authors suggested that the most efficient manner of reducing body and fat mass is aerobic training alone.14 In contrast, if the goal is to increase lean muscle mass rather than lose weight and fat, then resistance training would be preferred.14
A meta-analysis confirmed the benefit of aerobic exercise, which resulted in significantly more loss in weight (1.2 kg, 2.6 lb), waist circumference (1.57 cm), and fat mass (1.2 kg, 2.6 lb) than resistance training.15 However, combined aerobic and resistance training was even better, with significantly more weight loss (2.0 kg, 4.4 lb) and fat mass reduction (1.9 kg, 4.2 lb).15
In summary, aerobic and combined aerobic-resistance training appear to be more effective for weight management in obese people than resistance training alone.
ADDITIONAL BENEFITS OF EXERCISE
Increasing regular physical activity through structured exercise has the additional benefits of improving physical fitness, flexibility, mobility, and cardiovascular health.16,17
Even before patients lose a significant amount of weight (eg, 10%), low-intensity exercise such as walking 30 to 60 minutes most days of the week will rapidly improve cardiorespiratory fitness and have positive effects on cardiovascular risk factors such as hypertension, elevated blood glucose, and dyslipidemia.18,19 Aerobic exercise and resistance training also reduce chronic inflammation, which is a strong indicator of future disease, especially in obese patients who have high levels of inflammatory biomarkers.20,21
Even if he or she does not lose much weight, an obese exercising person with good cardiorespiratory fitness has lower cardiovascular risk than a person who is not obese but is poorly conditioned.22
Exercise lowers blood pressure
Overactivity of the sympathetic nervous system is thought to account for over 50% of all cases of hypertension.23 Obesity in concert with diabetes is characterized by sympathetic overactivity and progressive loss of cardiac parasympathetic activity.24 Cardiac autonomic neuropathy is an underestimated risk factor for the increased cardiovascular morbidity and mortality associated with obesity and diabetes, and physical exercise may promote restoration of cardioprotective autonomic modulation in the heart.24
Several studies have shown that aerobic endurance exercise lowers blood pressure in patients with hypertension, and reduction in sympathetic neural activity has been reported as one of the main mechanisms explaining this effect.23 Another mechanism is endothelium-mediated vasodilation: even a single exercise session may increase the bioavailability of nitric oxide and decrease postexercise blood pressure.25
Different types of exercise have been shown to have different effects on blood pressure.
Aerobic training has been shown to reduce systolic blood pressure by 5.2 to 11.0 mm Hg and diastolic blood pressure by 3.0 to 7.7 mm Hg.26
The hypotensive effect of endurance aerobic training is probably mediated at least in part by a reduction in systemic vascular resistance through decreased activity of the sympathetic and renin-angiotensin systems and through improved insulin sensitivity.26 Other factors that may be involved include improved endothelium-dependent vasodilation, enhanced baroreceptor sensitivity, and arterial compliance.26
Dynamic resistance exercise has less of an effect than aerobic exercise, but it has been shown to reduce systolic blood pressure by 0.5 to 4.8 mm Hg and diastolic blood pressure by 0.5 to 4.1 mm Hg.26
In a meta-analysis of studies of resistance training lasting more than 1 month in healthy adults age 18 and older, the authors noted that resistance training induced a significant blood pressure reduction in 28 normotensive or prehypertensive study groups (–3.9/–3.9 mm Hg), whereas the reduction was not significant for the five hypertensive study groups.27
Isometric resistance exercise has been associated with small cardiovascular benefits, but has been shown to reduce systolic blood pressure by 10.5 to 16.5 mm Hg and diastolic blood pressure by 0.62 to 16.4 mm Hg.26
Exercise improves type 2 diabetes
Regular physical activity improves glycemic control and can prevent or delay the onset of type 2 diabetes mellitus.28 Furthermore, physical activity positively affects lipid levels, lowers blood pressure, reduces the rate of cardiovascular events, and restores quality of life in patients with type 2 diabetes.24,29
A meta-analysis of the effect of supervised exercise in adults with type 2 diabetes found that structured exercise achieved the following:
- Lowered systolic blood pressure by 2.42 mm Hg (95% confidence interval 0.45–4.39)
- Lowered diastolic blood pressure by 2.23 mm Hg (1.25–3.21)
- Raised the level of high-density lipoprotein cholesterol by 0.04 mmol/L (0.02–0.07)
- Lowered the level of low-density lipoprotein cholesterol by 0.16 mmol/L (0.01–0.30).30
The metabolic stress from physical exercise can increase oxidation of carbohydrates during exercise, increase postexercise consumption of oxygen (which can increase the rate of fat oxidation during recovery periods after exercise), improve glucose tolerance and insulin sensitivity, and reduce glycemia for 2 to 72 hours depending on the intensity and duration of the exercise.25
Exercise lowers the Framingham risk score
Exercise improves several of the risk factors for coronary artery disease used in calculating the Framingham risk score—ie, systolic blood pressure, total cholesterol, and high-density lipoprotein cholesterol—and thus can significantly lower this number. (It is important to remember that the Framingham score is a surrogate end point of cardiovascular risk that may correlate with a real clinical end point but does not necessarily have a guaranteed relationship.)
In a study of a 12-week exercise program in middle-aged women (ages 40–55), treadmill running for 30 minutes a day 3 days a week significantly reduced 10-year cardiovascular risk scores: 10-year risk 2.2% vs 4.3% in the nonexercising group.31 Others have also shown that enhanced levels of fitness are associated with lower 10-year Framingham risk estimates.32
A study of 31 healthy sedentary adults ages 50 to 65 who were randomized to an unsupervised but pedometer-monitored home-based walking program of 30 minutes of brisk walking 5 days a week noted significant reductions in systolic and diastolic blood pressure and stroke risk, and increased functional capacity in the walking group at 12 weeks.33 Thus, the Framingham risk scores were significantly lower in the exercising group than in with the control group.33
Given that overweight and obese patients who are starting to exercise may find jogging or running daunting, it should also be noted that three brisk 10-minute walks a day are at least as effective as one continuous 30-minute walk in reducing cardiovascular risk in previously sedentary people.34
SETTING ‘SMART’ GOALS
Because obese adults typically do not comply well with prescriptions for exercise, it is important to educate them about its benefits and to provide tools such as perceived exertion scales so they can monitor their exercise, document their performance, and chart their progress; smartphone apps can also be helpful.35 Supervised exercise may improve compliance and results.36 Initially, personal trainers are excellent for starting a habit change, but they are expensive. Virtual trainers are now available and cost far less.37
People do not become obese overnight.They gain weight over a long time. Likewise, weight reduction takes time if done in a sustainable and healthy manner. Thus, SMART goals—specific, measurable, attainable, realistic, timely—should be set to sustain the self-discipline required.
EXERCISE RECOMMENDATIONS
Any exercise program should target 30 to 60 minutes of effort per day, most days of the week, ie, 150 to 300 minutes per week or more.38 But beginners should start low and go slow to avoid dropout, musculoskeletal strain, and joint injury.
The American College of Sports Medicine (ACSM)38,39 recommends combining aerobic and progressive resistance exercise as the core components of an exercise program. The aerobic component can include anaerobic high-intensity interval training (see discussion below). In addition, we recommend flexibility and balance exercises for obese patients.40
Combining aerobic and resistance exercises likely results in greater decreases in abdominal adiposity in the obese.41 In addition, the aerobic portion of a combined exercise regimen can improve functional capacity, and the resistance portion may prevent injury by strengthening the muscles, bones, and joint support systems.42 Adding exercises that promote flexibility and balance helps with range of motion and prevents injuries while exercising.43 These exercises not only expend calories during the exercise itself, but also increase resting energy expenditure for the remainder of the day, as the effects of the raised metabolism persist for hours.44
Aerobic exercise is the foundation
Aerobic exercises that involve large muscle groups, especially walking, should be the foundation of cardiopulmonary exercise for obese persons.45 Many patients can tolerate weight-bearing exercises such as walking or bike riding, but for some, exercises with limited or no weight-bearing such as swimming or aqua-aerobics are better.46
Tips for prescribing. Patients should exercise:
- On 5 or 6 days each week
- At low to moderate intensity (30%–60% of maximum oxygen consumption [Vo2 max])
- For at least 150 minutes per week, with a long-term goal of 300 minutes per week
- By walking, riding a stationary bicycle, or swimming.38,47
To mobilize and use free fatty acids as an energy source, lower-intensity longer-duration aerobic exercise is preferred.5 Thus, frequent, low-intensity or moderate-intensity training (30%–60% of Vo2 max) of longer duration (at least 60 minutes) may be the best approach to losing body fat in obese persons.5,48 Early on in the exercise program, keep the intensity low, as high-intensity training will preferentially use stored glycogen or carbohydrate as an energy substrate rather than free fatty acids or fat.5
With light-moderate exercise, the heart rate will increase and patients will perspire, but they still should be able to carry on a conversation.
Measure (or have patients measure) the heart rate using the radial artery in their wrist after 6 minutes of walking. A pulse of 100 beats per minute or more is associated with an exercise intensity of approximately 50% (or more) of Vo2 max.5
A study of 136 obese men and women who exercised for 6 months found that those doing aerobic exercise only and those doing a combination of aerobic and resistance exercise had greater cardiopulmonary fitness, greater reductions in abdominal and visceral fat, and more improved insulin sensitivity than those doing resistance exercise only.41 Although the aerobiconly group lost more weight (6 lb) than the aerobic-plus-resistance group (5.1 lb) and the resistance-only group (1.4 lb), combining aerobic and resistance exercise is considered optimal.
All physical activity is beneficial, but activities that have less impact on the joints are less likely to cause injuries and joint pain. Aerobic activities that are especially useful in obese adults include walking at a speed of at least 2.5 miles per hour, bicycling, jogging, treadmill walking, swimming, aqua-aerobics, rowing, and low-impact aerobics classes.
Walking is the easiest way for most people to start their program, as it is safe, accessible, and relatively cheap with respect to equipment.35 Adding a simple pedometer or smartphone app to measure the amount of exercise, together with physician counseling, may improve compliance and thus weight loss.49,50
Obese patients may have been inactive for quite a while. Therefore, the sessions should be short and low-intensity at first, then steadily progress.51 To minimize dropout, avoid hard exercise too soon for people with a low exercise capacity or high body mass index at baseline, and give positive feedback and encouragement at each visit.52
It is reasonable to introduce other aerobic exercises to vary the routine, use other muscle groups, and reduce the chance of injury from overuse of one muscle or joint group. Then, as cardiorespiratory fitness improves, the patient will be more confident about trying activities that are more challenging, such as jogging and aerobics classes. An aerobic exercise program consisting only of swimming is less efficacious for weight loss in this population.53
High-intensity interval training
High-intensity interval training involves relatively brief bursts of vigorous exercise separated by periods of recovery and is a time-efficient, novel alternative to continuous exercise.54 The exercise component is anaerobic, meaning muscle movement that does not require oxygen. Anaerobic exercise uses fast-twitch muscle fibers, and thus helps that musculature to become stronger, larger, and more toned. Evidence suggests that high-intensity interval training induces health-enhancing adaptations similar to those of continuous exercise, despite a substantially lower time commitment.41
The ACSM recommends that most adults engage in moderate-intensity cardiorespiratory exercise training for at least 30 minutes a day on at least 5 days a week for a total of at least 150 minutes per week, or high-intensity cardiorespiratory exercise training for at least 20 minutes a day on at least 3 days a week for a goal of 75 minutes a week.38 Thus, high-intensity interval training may be attractive for obese patients because it entails a shorter time commitment to achieve similar weight loss and improved insulin sensitivity than low-intensity or moderate-intensity continuous exercise.
High-intensity exercise has been shown to be effective for obese patients if they can do it.54–56 In one study,57 134 obese patients, mean age 53, underwent supervised high-intensity interval training with resistance training two or three times a week, were encouraged to perform one or two additional exercise sessions a week (unsupervised), and were counseled to follow a Mediterranean diet. At 9 months, investigators noted a significant reduction in body mass, waist circumference, and fat mass.
A study of 12 weeks of high-intensity interval training, moderate-intensity interval training, or no exercise in 34 obese adolescent girls noted that body mass and percentage body fat were significantly decreased with both interval training regimens. However, the high-intensity group had greater reductions in waist circumference and more significant improvements in blood lipid levels, adiponectin levels, and insulin sensitivity.58
Of 62 overweight and obese patients (mean age 53.3, mean body mass index 35.8 kg/m2), 97% adhered to a program of high-intensity interval training over 9 months, which resulted in an average weekly energy expenditure of 1,582 kcal.55 Clinically and statistically significant improvements occurred in body mass (–5.3 kg), body mass index (–1.9 kg/m2), and waist circumference (–5.8 cm) (P < .0001 for all variables). Total fat mass, trunk fat mass, and lipid levels also significantly improved (P < .0001), and the prevalence of metabolic syndrome was reduced by 32.5% (P < .05).
In a meta-analysis of the effect of exercise on overweight adults, training of moderate or high intensity was noted to have the highest potential to reduce visceral adipose tissue in overweight men and women.13 Another meta-analysis noted that high-intensity interval training appeared to promote more improvement in fitness and similar improvements in some cardiometabolic risk factors than moderate exercise performed for at least 8 to 12 weeks in overweight patients.56
A typical progressive exercise program for obese adults is shown in Table 1.
Progressive resistance exercise
Progressive resistance exercises are generally easier for obese patients, as they are not aerobically challenging, allow patients to exercise around physically active people who thus motivate them, and encourage positive feelings about completing their exercise sets.59 The result is improved muscular fitness, socialization, and increased confidence in their abilities (self-efficacy).59
Progressive resistance exercises also promote favorable energy balance and reduced visceral fat deposition through enhanced basal metabolism and activity levels while counteracting age- and disease-related muscle wasting.59 They have been shown to improve cognitive ability, self-esteem, movement control, muscle mass, strength, glucose control, insulin sensitivity, resting blood pressure, lipid profile, and bone mineral density and to reduce fat weight, low back pain, arthritic discomfort, insomnia, anxiety, and depression.60
Gym neophytes should spend a few sessions with a personal trainer to learn how to use the equipment.
While the primary goal of resistance training is more muscle strength, it can reduce fat and weight, burning up to 170 kcal in a 20-minute intense exercise session.61 It reduces both total body fat and visceral adipose tissue, thus benefiting obese persons by reducing insulin resistance.62 All exercise, and especially resistance exercise, can help to strengthen the musculoskeletal system, reduce muscle atrophy, and improve bone mineral density.63
The ACSM guidelines38 recommend progressive resistance exercise on 2 or 3 nonconsecutive days a week. It should involve:
- Exercises that work 8 to 10 muscle groups per session
- Two to four sets of 8 to 12 repetitions for each muscle group.
Exercising on nonconsecutive days allows time for the complete cycle of muscle tissue remodeling.64 Such self-regulated intensity reduces the likelihood of excessive delayed-onset muscle soreness, which can discourage new participants.65
To prevent muscle injury, obese people should begin with low-intensity workouts using lower resistance, one set of 8 to 12 repetitions 2 days a week. Then, they should gradually but progressively increase the intensity, volume, and frequency of the training.47 This will obviate a plateau in training and will maximize musculoskeletal adaptation. The prescription should include exercises for the upper body (eg, biceps curls), lower body (eg, leg presses), and the midsection (eg, abdominal curl-ups, which give better abdominal muscle engagement and less risk to the back than crunches) and focus on the correct exercise form and function rather than the amount of resistance or weight lifted.
A typical progressive resistance exercise program for obese adults is shown in Table 2.
Flexibility exercise
Flexibility exercise involves stretching to improve the movement of muscles, joints, and ligaments.45 While not specifically used in an energy-expenditure strategy, flexibility (or mobility) exercises help to increase or maintain joint range of motion and can reduce muscle and joint pain associated with obesity and exercise.66
The ACSM recommends that stretching exercises be done when the muscles are warm after a brief warm-up or exercise session.38 Typically, muscles should be stretched for at least 15 seconds, and stretching is recommended at a frequency of 2 to 4 days per week.38
A good way to incorporate flexibility exercise is to join a yoga class, as yoga has been shown to improve strength and flexibility and may help control physiologic variables such as blood pressure, lipids, respiration, heart rate, and metabolic rate to improve overall exercise capacity in obese patients.67
Balance exercise
Balance exercises help obese patients improve their stability. Poor balance is associated with injuries, accidents, and falls during activities of daily living.68
Balance, the ability to maintain the body’s center of gravity within its base of support, can be categorized as static (sustaining the body in static equilibrium or within its base of support) or dynamic (maintaining equilibrium during a transition from a dynamic to a static state), which is more challenging.69 Doing both static and dynamic balance training maximizes balance and stability.69 While most activities that involve moving the body or body parts (such as walking) will improve balance, some additional balance exercises can be beneficial.
Balance exercises can be done without any equipment. Examples are balancing on one foot for 15 seconds and standing up and sitting down without using the hands. However, specific equipment can help, including physioballs, stability balls, cut-in-half stability balls, balance discs, balance wedges, wobble boards, rocker boards, and Indo boards.70 In fact, balance boards and stability balls engage more muscle fibers in other areas of the body (lower back, lower abs, quads, hamstrings, and calves) than exercises done without those balancing devices.71
Balance training for at least 10 minutes a day, 3 days a week, for 4 weeks that incorporates various methods of balance training appears to improve balance.56 Obese patients commencing a program should start with static balance exercises and then progress to dynamic ones. In addition, as balance training progresses, obese patients can integrate balance and stability training exercises with other pieces of equipment, such as performing squats on a balance board, and then gradually add weights (eg, dumbbells) to the exercise.
An example of a weekly comprehensive exercise program for an obese patient that incorporates all major exercise types is provided in Table 3. In addition, some smartphone apps that are especially helpful in overweight newcomers to exercise include Couch-to-5K, GymGoal 2, Moves, Fitbit, Workout Trainer, Endomondo, MapMyFitness, Fitocracy, and Fitness Buddy.
BARIATRIC SURGERY AND LIFESTYLE MANAGEMENT FOR OBESITY
Bariatric surgery is a safe and effective treatment for severe obesity and comorbidities including type 2 diabetes mellitus, but weight loss and health outcomes vary considerably among individuals.72,73 Of importance, postoperative weight loss after bariatric surgery and long-term weight loss largely depend on the extent to which patients can make and sustain changes to their lifestyle, including diet, exercise, and behavior modification.72,74
Exercise, especially supervised, is associated with more weight loss after bariatric surgery.61 In a meta-analysis of bariatric patients, exercise participants involved in moderate or greater levels of exercise lost a mean of 3.6 kg more than the minimal exercise groups.75 Another meta-analysis noted the beneficial effects of exercise incorporating more than 30 minutes a day of moderate physical activity following bariatric surgery and was associated with a greater weight loss of over 4% of body mass index.76 These findings were consistent with those of yet another meta-analysis.77
In summary, exercise appears to significantly increase weight loss after bariatric surgery.
TREATMENT CONSIDERATIONS IN MORBID OBESITY
Challenges faced by severely obese or morbidly obese patients affect their exercise options. The types of exercise they are able to perform are limited in most cases to very-low-impact, low-intensity exercises, which may not be as efficient in weight loss or weight maintenance.48 Therefore, it may be prudent to set more conservative weight-loss goals for them, especially early in the program. Compliance and success rates may be better with low-impact activities such as walking, water aerobics, stationary cycling, and resistance training in the severely obese population.
The more severe the obesity, the more comorbidities such as diabetes, hypertension, hyperlipidemia, arthritis, sleep apnea, gastroesophageal reflux disease, and the greater the risk of metabolic syndrome—and conversely, the greater the potential benefit from bariatric surgery followed by exercise.74
A LONG-TERM ENDEAVOR
For obese patients, a comprehensive exercise program will improve functional status, favorably influence cardiovascular risk factors, and help with weight loss or weight maintenance.
Managing obesity is a long-term endeavor.78 For it to succeed, both the patient and the physician need to keep up their efforts. To keep the patient from becoming discouraged, the clinician should focus not just on weight, but also on improvements in metabolic profile and cardiorespiratory fitness. In addition, a careful evaluation, a clear exercise prescription, defined goals, ongoing monitoring (by the patient and the provider), frequent feedback, and charting of progress will improve daily performance and the chance of long-term success.
- Thorogood A, Mottillo S, Shimony A, et al. Isolated aerobic exercise and weight loss: a systematic review and meta-analysis of randomized controlled trials. Am J Med 2011; 124:747–755.
- Church T. Exercise in obesity, metabolic syndrome, and diabetes. Prog Cardiovasc Dis 2011; 53:412–418.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129(suppl 2):S102–S138.
- Strasser B. Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci 2013; 1281:141–159.
- Poirier P, Despres JP. Exercise in weight management of obesity. Cardiol Clin 2001; 19:459–470.
- Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev 2006; 4:CD003817.
- Slentz CA, Houmard JA, Kraus WE. Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity (Silver Spring) 2009; 17(suppl 3):S27–S33.
- Ross R, Hudson R, Stotz PJ, Lam M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults: a randomized trial. Ann Intern Med 2015; 162:325–334.
- Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 2000; 133:92–103.
- Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 2010; 304:1795–1802.
- Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med 2004; 164:31–39.
- Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc 2001; 33(suppl 6):S521–S529.
- Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One 2013; 8:e56415.
- Willis LH, Slentz CA, Bateman LA, et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol (1985) 2012; 113:1831–1837.
- Schwingshackl L, Dias S, Strasser B, Hoffmann G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: a systematic review and network meta-analysis. PLoS One 2013; 8:e82853.
- Cook CM, Schoeller DA. Physical activity and weight control: conflicting findings. Curr Opin Clin Nutr Metab Care 2011; 14:419–424.
- Choo J, Lee J, Cho JH, Burke LE, Sekikawa A, Jae SY. Effects of weight management by exercise modes on markers of subclinical atherosclerosis and cardiometabolic profile among women with abdominal obesity: a randomized controlled trial. BMC Cardiovasc Disord 2014; 14:82.
- Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med 2004; 34:371–418.
- Shaibi GQ, Ryder JR, Kim JY, Barraza E. Exercise for obese youth: refocusing attention from weight loss to health gains. Exerc Sport Sci Rev 2015; 43:41–47.
- You T, Arsenis NC, Disanzo BL, Lamonte MJ. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med 2013; 43:243–256.
- Bluher S, Petroff D, Wagner A, et al. The one year exercise and lifestyle intervention program KLAKS: effects on anthropometric parameters, cardiometabolic risk factors and glycemic control in childhood obesity. Metabolism 2014; 63:422–430.
- Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr 1999; 69:373–380.
- Leosco D, Parisi V, Femminella GD, et al. Effects of exercise training on cardiovascular adrenergic system. Front Physiol 2013; 4:348.
- Voulgari C, Pagoni S, Vinik A, Poirier P. Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism 2013; 62:609–621.
- Asano RY, Sales MM, Browne RA, et al. Acute effects of physical exercise in type 2 diabetes: a review. World J Diabetes 2014; 5:659–665.
- Brook RD, Appel LJ, Rubenfire M, et al; American Heart Association Professional Education Committee of the Council for High Blood Pressure Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension 2013; 61:1360–1383.
- Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension 2011; 58:950–958.
- Ades PA. A lifestyle program of exercise and weight loss is effective in preventing and treating type 2 diabetes mellitus: why are programs not more available? Prev Med 2015: S0091–7435(15)00085–7.
- Ades PA, Savage PD, Marney AM, Harvey J, Evans KA. Remission of recently diagnosed type 2 diabetes mellitus with weight loss and exercise. J Cardiopulm Rehabil Prev 2015; 35:193–197.
- Hayashino Y, Jackson JL, Fukumori N, Nakamura F, Fukuhara S. Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2012; 98:349–360.
- Saffi MA, Polanczyk CA, Rabelo-Silva ER. Lifestyle interventions reduce cardiovascular risk in patients with coronary artery disease: a randomized clinical trial. Eur J Cardiovasc Nurs 2014; 13:436–443.
- LaMonte MJ, Durstine JL, Addy CL, Irwin ML, Ainsworth BE. Physical activity, physical fitness, and Framingham 10-year risk score: the cross-cultural activity participation study. J Cardiopulm Rehabil 2001; 21:63–70.
- Tully MA, Cupples ME, Chan WS, McGlade K, Young IS. Brisk walking, fitness, and cardiovascular risk: a randomized controlled trial in primary care. Prev Med 2005; 41:622–628.
- Murphy M, Nevill A, Neville C, Biddle S, Hardman A. Accumulating brisk walking for fitness, cardiovascular risk, and psychological health. Med Sci Sports Exerc 2002; 34:1468–1474.
- Colley RC, Hills AP, King NA, Byrne NM. Exercise-induced energy expenditure: implications for exercise prescription and obesity. Patient Educ Couns 2010; 79:327–332.
- Baillot A, Mampuya WM, Comeau E, Méziat-Burdin A, Langlois MF. Feasibility and impacts of supervised exercise training in subjects with obesity awaiting bariatric surgery: a pilot study. Obes Surg 2013; 23:882–891.
- Lowe S, ÓLaighin G. The age of the virtual trainer. Procedia Engineering 2012; 34:242–247.
- Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43:1334–1359.
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK; American College of Sports Medicine. American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009; 41:459–471.
- Montero-Fernandez N, Serra-Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med 2013; 49:131–143.
- Davidson LE, Hudson R, Kilpatrick K, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med 2009; 169:122–131.
- Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 2009; 3:CD002759.
- Manini TM, Newman AB, Fielding R, et al; LIFE Research Group. Effects of exercise on mobility in obese and nonobese older adults. Obesity (Silver Spring) 2010; 18:1168–1175.
- Hackney KJ, Engels HJ, Gretebeck RJ. Resting energy expenditure and delayed-onset muscle soreness after full-body resistance training with an eccentric concentration. J Strength Cond Res 2008; 22:1602–1609.
- Siddiqui NI, Nessa A, Hossain MA. Regular physical exercise: way to healthy life. Mymensingh Med J 2010; 19:154–158.
- Chicco AJ. Exercise training in prevention and rehabilitation: which training mode is best? Minerva Cardioangiol 2008; 56:557–570.
- Westcott WL, Winett RA, Annesi JJ, Wojcik JR, Anderson ES, Madden PJ. Prescribing physical activity: applying the ACSM protocols for exercise type, intensity, and duration across 3 training frequencies. Phys Sportsmed 2009; 37:51–58.
- Mougios V1, Kazaki M, Christoulas K, Ziogas G, Petridou A. Does the intensity of an exercise programme modulate body composition changes? Int J Sports Med 2006; 27:178–181.
- Richardson CR, Newton TL, Abraham JJ, Sen A, Jimbo M, Swartz AM. A meta-analysis of pedometer-based walking interventions and weight loss. Ann Fam Med 2008; 6:69–77.
- Stovitz SD, VanWormer JJ, Center BA, Bremer KL. Pedometers as a means to increase ambulatory activity for patients seen at a family medicine clinic. J Am Board Fam Pract 2005; 18:335–343.
- Lepor NE, Fouchia DD, McCullough PA. New vistas for the treatment of obesity: turning the tide against the leading cause of morbidity and cardiovascular mortality in the developed world. Rev Cardiovasc Med 2013; 14:20–40.
- Wittmer M, Volpatti M, Piazzalonga S, Hoffmann A. Expectation, satisfaction, and predictors of dropout in cardiac rehabilitation. Eur J Prev Cardiol 2012; 19:1082–1088.
- Gwinup G. Weight loss without dietary restriction: efficacy of different forms of aerobic exercise. Am J Sports Med 1987; 15:275–279.
- Jung ME, Bourne JE, Little JP. Where does HIT fit? An examination of the affective response to high-intensity intervals in comparison to continuous moderate- and continuous vigorous-intensity exercise in the exercise intensity-affect continuum. PLoS One 2014; 9:e114541.
- Gremeaux V, Drigny J, Nigam A, et al. Long-term lifestyle intervention with optimized high-intensity interval training improves body composition, cardiometabolic risk, and exercise parameters in patients with abdominal obesity. Am J Phys Med Rehabil 2012; 91:941–950.
- Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med 2012; 42:489–509.
- Dalzill C, Nigam A, Juneau M, et al. Intensive lifestyle intervention improves cardiometabolic and exercise parameters in metabolically healthy obese and metabolically unhealthy obese individuals. Can J Cardiol 2014; 30:434–440.
- Racil G, Ben Ounis O, Hammouda O, et al. Effects of high vs moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol 2013; 113:2531–2540.
- Willey KA, Singh MA. Battling insulin resistance in elderly obese people with type 2 diabetes: bring on the heavy weights. Diabetes Care 2003; 26:1580–1588.
- Westcott WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep 2012; 11:209–216.
- Haltom RW, Kraemer RR, Sloan RA, Hebert EP, Frank K, Tryniecki JL. Circuit weight training and its effects on excess postexercise oxygen consumption. Med Sci Sports Exerc 1999; 31:1613–1618.
- Strasser B, Schobersberger W. Evidence for resistance training as a treatment therapy in obesity. J Obes 2011; pii:482564.
- Fonseca H, Moreira-Gonçalves D, Coriolano HJ, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med 2014; 44:37–53.
- Candow DG, Burke DG. Effect of short-term equal-volume resistance training with different workout frequency on muscle mass and strength in untrained men and women. J Strength Cond Res 2007; 21:204–207.
- Trost Z, France CR, Thomas JS. Pain-related fear and avoidance of physical exertion following delayed-onset muscle soreness. Pain 2011; 152:1540–1547.
- Mathus-Vliegen EM. Obesity and the elderly. J Clin Gastroenterol 2012; 46:533–544.
- Dhananjai S, Sadashiv, Tiwari S, Dutt K, Kumar R. Reducing psychological distress and obesity through yoga practice. Int J Yoga 2013; 6:66–70.
- Mathus-Vliegen EM; Obesity Management Task Force of the European Association for the Study of Obesity. Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: a guideline. Obes Facts 2012; 5:460–483.
- DiStefano LJ, Clark MA, Padua DA. Evidence supporting balance training in healthy individuals: a systemic review. J Strength Cond Res 2009; 23:2718–2731.
- Ogaya S, Ikezoe T, Soda N, Ichihashi N. Effects of balance training using wobble boards in the elderly. J Strength Cond Res 2011; 25:2616–2622.
- Sukalinggam CL, Sukalinggam GL, Kasim F, Yusof A. Stability ball training on lower back strength has greater effect in untrained female compared to male. J Hum Kinet 2012; 33:133–141.
- Kalarchian M, Turk M, Elliott J, Gourash W. Lifestyle management for enhancing outcomes after bariatric surgery. Curr Diab Rep 2014; 14:540.
- Rothwell L, Kow L, Toouli J. Effect of a post-operative structured exercise programme on short-term weight loss after obesity surgery using adjustable gastric bands. Obes Surg 2015; 25:126–128.
- Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 2013; 9:159–191.
- Egberts K, Brown WA, Brennan L, O’Brien PE. Does exercise improve weight loss after bariatric surgery? A systematic review. Obes Surg 2012; 22:335–341.
- Livhits M, Mercado C, Yermilov I, et al. Exercise following bariatric surgery: systematic review. Obes Surg 2010; 20:657–665.
- Jacobi D, Ciangura C, Couet C, Oppert JM. Physical activity and weight loss following bariatric surgery. Obes Rev 2011; 12:366–377.
- Wadden TA, Foster GD, Letizia KA. One-year behavioral treatment of obesity: comparison of moderate and severe caloric restriction and the effects of weight maintenance therapy. J Consult Clin Psychol 1994; 62:165–171.
Although exercise is probably less effective than diet in reducing weight, most studies show that adding it to a diet regimen will increase the weight loss.1,2 Guidelines from the American Heart Association, American College of Cardiology, and Obesity Society recommend a comprehensive lifestyle program that includes a low-calorie diet as well as an increase in physical activity.3
Here, we review the many benefits of exercise for obese patients, not only in terms of weight loss, but also its positive cardiovascular and metabolic effects. Then we discuss how to motivate and prescribe exercise for this challenging group.
EXERCISE IMPROVES WEIGHT LOSS
Increasing energy expenditure by exercising can mobilize and burn stored fat and thus lead to weight loss.4
Typically, with no changes in caloric intake, exercising 60 minutes at low intensity most days of the week will remove up to 0.5 lb per week.5 Exercising harder for longer will take off more weight, up to 3 lb per week.1,6 Some practitioners believe that the total volume of exercise (frequency multiplied by time) is more important than the intensity in determining the amount of weight loss.2,7,8
Ross et al9 randomized 101 obese men to try to lose weight by exercising at a low to moderate intensity, to try to lose weight by dieting, to exercise without the goal of losing weight, or to do nothing (the control group). About half the participants declined or dropped out, but 52 completed the trial. The weight-loss-through-exercise group had lost approximately 15 lb by 12 weeks; the diet group lost a similar amount. Total body fat, visceral fat, and abdominal obesity were all reduced with both diet- and exercise-induced weight loss.
In a study in 130 severely obese adults, after 6 months of high-intensity physical activity for a mean duration of 71 minutes per week, those on an exercise-and-diet regimen lost an average of 24 lb, compared with 18 lb with diet alone.10
Another trial involved obese patients who were instructed to jog the equivalent of 20 miles (32.2 km) a week, with no restriction on caloric intake.11 They lost only 2.9 kg (6.5 lb) over 8 months. Increased food intake explained this minimal weight loss.
In an analysis of 20 studies, exercise-only interventions of 4 months or less resulted in a mean weekly weight loss of 0.4 lb (0.2 kg), with a total loss of about 5 lb (2.3 kg).12
A systematic review of 15 studies noted that aerobic exercise for 3 months or more resulted in a significant reduction in visceral adipose tissue in overweight men and women as measured by computed tomography.13
Effects that different types of exercise have on weight loss
In a study of 119 sedentary adults who were overweight or obese and who were randomized to aerobic, resistance, or combined aerobic-resistance training over 8 months, those involved in aerobic or combined aerobic and resistance training had the greatest reduction in total body and fat mass.14 Given that the combined aerobic-resistance training program required twice the time commitment of the aerobic-alone program, the authors suggested that the most efficient manner of reducing body and fat mass is aerobic training alone.14 In contrast, if the goal is to increase lean muscle mass rather than lose weight and fat, then resistance training would be preferred.14
A meta-analysis confirmed the benefit of aerobic exercise, which resulted in significantly more loss in weight (1.2 kg, 2.6 lb), waist circumference (1.57 cm), and fat mass (1.2 kg, 2.6 lb) than resistance training.15 However, combined aerobic and resistance training was even better, with significantly more weight loss (2.0 kg, 4.4 lb) and fat mass reduction (1.9 kg, 4.2 lb).15
In summary, aerobic and combined aerobic-resistance training appear to be more effective for weight management in obese people than resistance training alone.
ADDITIONAL BENEFITS OF EXERCISE
Increasing regular physical activity through structured exercise has the additional benefits of improving physical fitness, flexibility, mobility, and cardiovascular health.16,17
Even before patients lose a significant amount of weight (eg, 10%), low-intensity exercise such as walking 30 to 60 minutes most days of the week will rapidly improve cardiorespiratory fitness and have positive effects on cardiovascular risk factors such as hypertension, elevated blood glucose, and dyslipidemia.18,19 Aerobic exercise and resistance training also reduce chronic inflammation, which is a strong indicator of future disease, especially in obese patients who have high levels of inflammatory biomarkers.20,21
Even if he or she does not lose much weight, an obese exercising person with good cardiorespiratory fitness has lower cardiovascular risk than a person who is not obese but is poorly conditioned.22
Exercise lowers blood pressure
Overactivity of the sympathetic nervous system is thought to account for over 50% of all cases of hypertension.23 Obesity in concert with diabetes is characterized by sympathetic overactivity and progressive loss of cardiac parasympathetic activity.24 Cardiac autonomic neuropathy is an underestimated risk factor for the increased cardiovascular morbidity and mortality associated with obesity and diabetes, and physical exercise may promote restoration of cardioprotective autonomic modulation in the heart.24
Several studies have shown that aerobic endurance exercise lowers blood pressure in patients with hypertension, and reduction in sympathetic neural activity has been reported as one of the main mechanisms explaining this effect.23 Another mechanism is endothelium-mediated vasodilation: even a single exercise session may increase the bioavailability of nitric oxide and decrease postexercise blood pressure.25
Different types of exercise have been shown to have different effects on blood pressure.
Aerobic training has been shown to reduce systolic blood pressure by 5.2 to 11.0 mm Hg and diastolic blood pressure by 3.0 to 7.7 mm Hg.26
The hypotensive effect of endurance aerobic training is probably mediated at least in part by a reduction in systemic vascular resistance through decreased activity of the sympathetic and renin-angiotensin systems and through improved insulin sensitivity.26 Other factors that may be involved include improved endothelium-dependent vasodilation, enhanced baroreceptor sensitivity, and arterial compliance.26
Dynamic resistance exercise has less of an effect than aerobic exercise, but it has been shown to reduce systolic blood pressure by 0.5 to 4.8 mm Hg and diastolic blood pressure by 0.5 to 4.1 mm Hg.26
In a meta-analysis of studies of resistance training lasting more than 1 month in healthy adults age 18 and older, the authors noted that resistance training induced a significant blood pressure reduction in 28 normotensive or prehypertensive study groups (–3.9/–3.9 mm Hg), whereas the reduction was not significant for the five hypertensive study groups.27
Isometric resistance exercise has been associated with small cardiovascular benefits, but has been shown to reduce systolic blood pressure by 10.5 to 16.5 mm Hg and diastolic blood pressure by 0.62 to 16.4 mm Hg.26
Exercise improves type 2 diabetes
Regular physical activity improves glycemic control and can prevent or delay the onset of type 2 diabetes mellitus.28 Furthermore, physical activity positively affects lipid levels, lowers blood pressure, reduces the rate of cardiovascular events, and restores quality of life in patients with type 2 diabetes.24,29
A meta-analysis of the effect of supervised exercise in adults with type 2 diabetes found that structured exercise achieved the following:
- Lowered systolic blood pressure by 2.42 mm Hg (95% confidence interval 0.45–4.39)
- Lowered diastolic blood pressure by 2.23 mm Hg (1.25–3.21)
- Raised the level of high-density lipoprotein cholesterol by 0.04 mmol/L (0.02–0.07)
- Lowered the level of low-density lipoprotein cholesterol by 0.16 mmol/L (0.01–0.30).30
The metabolic stress from physical exercise can increase oxidation of carbohydrates during exercise, increase postexercise consumption of oxygen (which can increase the rate of fat oxidation during recovery periods after exercise), improve glucose tolerance and insulin sensitivity, and reduce glycemia for 2 to 72 hours depending on the intensity and duration of the exercise.25
Exercise lowers the Framingham risk score
Exercise improves several of the risk factors for coronary artery disease used in calculating the Framingham risk score—ie, systolic blood pressure, total cholesterol, and high-density lipoprotein cholesterol—and thus can significantly lower this number. (It is important to remember that the Framingham score is a surrogate end point of cardiovascular risk that may correlate with a real clinical end point but does not necessarily have a guaranteed relationship.)
In a study of a 12-week exercise program in middle-aged women (ages 40–55), treadmill running for 30 minutes a day 3 days a week significantly reduced 10-year cardiovascular risk scores: 10-year risk 2.2% vs 4.3% in the nonexercising group.31 Others have also shown that enhanced levels of fitness are associated with lower 10-year Framingham risk estimates.32
A study of 31 healthy sedentary adults ages 50 to 65 who were randomized to an unsupervised but pedometer-monitored home-based walking program of 30 minutes of brisk walking 5 days a week noted significant reductions in systolic and diastolic blood pressure and stroke risk, and increased functional capacity in the walking group at 12 weeks.33 Thus, the Framingham risk scores were significantly lower in the exercising group than in with the control group.33
Given that overweight and obese patients who are starting to exercise may find jogging or running daunting, it should also be noted that three brisk 10-minute walks a day are at least as effective as one continuous 30-minute walk in reducing cardiovascular risk in previously sedentary people.34
SETTING ‘SMART’ GOALS
Because obese adults typically do not comply well with prescriptions for exercise, it is important to educate them about its benefits and to provide tools such as perceived exertion scales so they can monitor their exercise, document their performance, and chart their progress; smartphone apps can also be helpful.35 Supervised exercise may improve compliance and results.36 Initially, personal trainers are excellent for starting a habit change, but they are expensive. Virtual trainers are now available and cost far less.37
People do not become obese overnight.They gain weight over a long time. Likewise, weight reduction takes time if done in a sustainable and healthy manner. Thus, SMART goals—specific, measurable, attainable, realistic, timely—should be set to sustain the self-discipline required.
EXERCISE RECOMMENDATIONS
Any exercise program should target 30 to 60 minutes of effort per day, most days of the week, ie, 150 to 300 minutes per week or more.38 But beginners should start low and go slow to avoid dropout, musculoskeletal strain, and joint injury.
The American College of Sports Medicine (ACSM)38,39 recommends combining aerobic and progressive resistance exercise as the core components of an exercise program. The aerobic component can include anaerobic high-intensity interval training (see discussion below). In addition, we recommend flexibility and balance exercises for obese patients.40
Combining aerobic and resistance exercises likely results in greater decreases in abdominal adiposity in the obese.41 In addition, the aerobic portion of a combined exercise regimen can improve functional capacity, and the resistance portion may prevent injury by strengthening the muscles, bones, and joint support systems.42 Adding exercises that promote flexibility and balance helps with range of motion and prevents injuries while exercising.43 These exercises not only expend calories during the exercise itself, but also increase resting energy expenditure for the remainder of the day, as the effects of the raised metabolism persist for hours.44
Aerobic exercise is the foundation
Aerobic exercises that involve large muscle groups, especially walking, should be the foundation of cardiopulmonary exercise for obese persons.45 Many patients can tolerate weight-bearing exercises such as walking or bike riding, but for some, exercises with limited or no weight-bearing such as swimming or aqua-aerobics are better.46
Tips for prescribing. Patients should exercise:
- On 5 or 6 days each week
- At low to moderate intensity (30%–60% of maximum oxygen consumption [Vo2 max])
- For at least 150 minutes per week, with a long-term goal of 300 minutes per week
- By walking, riding a stationary bicycle, or swimming.38,47
To mobilize and use free fatty acids as an energy source, lower-intensity longer-duration aerobic exercise is preferred.5 Thus, frequent, low-intensity or moderate-intensity training (30%–60% of Vo2 max) of longer duration (at least 60 minutes) may be the best approach to losing body fat in obese persons.5,48 Early on in the exercise program, keep the intensity low, as high-intensity training will preferentially use stored glycogen or carbohydrate as an energy substrate rather than free fatty acids or fat.5
With light-moderate exercise, the heart rate will increase and patients will perspire, but they still should be able to carry on a conversation.
Measure (or have patients measure) the heart rate using the radial artery in their wrist after 6 minutes of walking. A pulse of 100 beats per minute or more is associated with an exercise intensity of approximately 50% (or more) of Vo2 max.5
A study of 136 obese men and women who exercised for 6 months found that those doing aerobic exercise only and those doing a combination of aerobic and resistance exercise had greater cardiopulmonary fitness, greater reductions in abdominal and visceral fat, and more improved insulin sensitivity than those doing resistance exercise only.41 Although the aerobiconly group lost more weight (6 lb) than the aerobic-plus-resistance group (5.1 lb) and the resistance-only group (1.4 lb), combining aerobic and resistance exercise is considered optimal.
All physical activity is beneficial, but activities that have less impact on the joints are less likely to cause injuries and joint pain. Aerobic activities that are especially useful in obese adults include walking at a speed of at least 2.5 miles per hour, bicycling, jogging, treadmill walking, swimming, aqua-aerobics, rowing, and low-impact aerobics classes.
Walking is the easiest way for most people to start their program, as it is safe, accessible, and relatively cheap with respect to equipment.35 Adding a simple pedometer or smartphone app to measure the amount of exercise, together with physician counseling, may improve compliance and thus weight loss.49,50
Obese patients may have been inactive for quite a while. Therefore, the sessions should be short and low-intensity at first, then steadily progress.51 To minimize dropout, avoid hard exercise too soon for people with a low exercise capacity or high body mass index at baseline, and give positive feedback and encouragement at each visit.52
It is reasonable to introduce other aerobic exercises to vary the routine, use other muscle groups, and reduce the chance of injury from overuse of one muscle or joint group. Then, as cardiorespiratory fitness improves, the patient will be more confident about trying activities that are more challenging, such as jogging and aerobics classes. An aerobic exercise program consisting only of swimming is less efficacious for weight loss in this population.53
High-intensity interval training
High-intensity interval training involves relatively brief bursts of vigorous exercise separated by periods of recovery and is a time-efficient, novel alternative to continuous exercise.54 The exercise component is anaerobic, meaning muscle movement that does not require oxygen. Anaerobic exercise uses fast-twitch muscle fibers, and thus helps that musculature to become stronger, larger, and more toned. Evidence suggests that high-intensity interval training induces health-enhancing adaptations similar to those of continuous exercise, despite a substantially lower time commitment.41
The ACSM recommends that most adults engage in moderate-intensity cardiorespiratory exercise training for at least 30 minutes a day on at least 5 days a week for a total of at least 150 minutes per week, or high-intensity cardiorespiratory exercise training for at least 20 minutes a day on at least 3 days a week for a goal of 75 minutes a week.38 Thus, high-intensity interval training may be attractive for obese patients because it entails a shorter time commitment to achieve similar weight loss and improved insulin sensitivity than low-intensity or moderate-intensity continuous exercise.
High-intensity exercise has been shown to be effective for obese patients if they can do it.54–56 In one study,57 134 obese patients, mean age 53, underwent supervised high-intensity interval training with resistance training two or three times a week, were encouraged to perform one or two additional exercise sessions a week (unsupervised), and were counseled to follow a Mediterranean diet. At 9 months, investigators noted a significant reduction in body mass, waist circumference, and fat mass.
A study of 12 weeks of high-intensity interval training, moderate-intensity interval training, or no exercise in 34 obese adolescent girls noted that body mass and percentage body fat were significantly decreased with both interval training regimens. However, the high-intensity group had greater reductions in waist circumference and more significant improvements in blood lipid levels, adiponectin levels, and insulin sensitivity.58
Of 62 overweight and obese patients (mean age 53.3, mean body mass index 35.8 kg/m2), 97% adhered to a program of high-intensity interval training over 9 months, which resulted in an average weekly energy expenditure of 1,582 kcal.55 Clinically and statistically significant improvements occurred in body mass (–5.3 kg), body mass index (–1.9 kg/m2), and waist circumference (–5.8 cm) (P < .0001 for all variables). Total fat mass, trunk fat mass, and lipid levels also significantly improved (P < .0001), and the prevalence of metabolic syndrome was reduced by 32.5% (P < .05).
In a meta-analysis of the effect of exercise on overweight adults, training of moderate or high intensity was noted to have the highest potential to reduce visceral adipose tissue in overweight men and women.13 Another meta-analysis noted that high-intensity interval training appeared to promote more improvement in fitness and similar improvements in some cardiometabolic risk factors than moderate exercise performed for at least 8 to 12 weeks in overweight patients.56
A typical progressive exercise program for obese adults is shown in Table 1.
Progressive resistance exercise
Progressive resistance exercises are generally easier for obese patients, as they are not aerobically challenging, allow patients to exercise around physically active people who thus motivate them, and encourage positive feelings about completing their exercise sets.59 The result is improved muscular fitness, socialization, and increased confidence in their abilities (self-efficacy).59
Progressive resistance exercises also promote favorable energy balance and reduced visceral fat deposition through enhanced basal metabolism and activity levels while counteracting age- and disease-related muscle wasting.59 They have been shown to improve cognitive ability, self-esteem, movement control, muscle mass, strength, glucose control, insulin sensitivity, resting blood pressure, lipid profile, and bone mineral density and to reduce fat weight, low back pain, arthritic discomfort, insomnia, anxiety, and depression.60
Gym neophytes should spend a few sessions with a personal trainer to learn how to use the equipment.
While the primary goal of resistance training is more muscle strength, it can reduce fat and weight, burning up to 170 kcal in a 20-minute intense exercise session.61 It reduces both total body fat and visceral adipose tissue, thus benefiting obese persons by reducing insulin resistance.62 All exercise, and especially resistance exercise, can help to strengthen the musculoskeletal system, reduce muscle atrophy, and improve bone mineral density.63
The ACSM guidelines38 recommend progressive resistance exercise on 2 or 3 nonconsecutive days a week. It should involve:
- Exercises that work 8 to 10 muscle groups per session
- Two to four sets of 8 to 12 repetitions for each muscle group.
Exercising on nonconsecutive days allows time for the complete cycle of muscle tissue remodeling.64 Such self-regulated intensity reduces the likelihood of excessive delayed-onset muscle soreness, which can discourage new participants.65
To prevent muscle injury, obese people should begin with low-intensity workouts using lower resistance, one set of 8 to 12 repetitions 2 days a week. Then, they should gradually but progressively increase the intensity, volume, and frequency of the training.47 This will obviate a plateau in training and will maximize musculoskeletal adaptation. The prescription should include exercises for the upper body (eg, biceps curls), lower body (eg, leg presses), and the midsection (eg, abdominal curl-ups, which give better abdominal muscle engagement and less risk to the back than crunches) and focus on the correct exercise form and function rather than the amount of resistance or weight lifted.
A typical progressive resistance exercise program for obese adults is shown in Table 2.
Flexibility exercise
Flexibility exercise involves stretching to improve the movement of muscles, joints, and ligaments.45 While not specifically used in an energy-expenditure strategy, flexibility (or mobility) exercises help to increase or maintain joint range of motion and can reduce muscle and joint pain associated with obesity and exercise.66
The ACSM recommends that stretching exercises be done when the muscles are warm after a brief warm-up or exercise session.38 Typically, muscles should be stretched for at least 15 seconds, and stretching is recommended at a frequency of 2 to 4 days per week.38
A good way to incorporate flexibility exercise is to join a yoga class, as yoga has been shown to improve strength and flexibility and may help control physiologic variables such as blood pressure, lipids, respiration, heart rate, and metabolic rate to improve overall exercise capacity in obese patients.67
Balance exercise
Balance exercises help obese patients improve their stability. Poor balance is associated with injuries, accidents, and falls during activities of daily living.68
Balance, the ability to maintain the body’s center of gravity within its base of support, can be categorized as static (sustaining the body in static equilibrium or within its base of support) or dynamic (maintaining equilibrium during a transition from a dynamic to a static state), which is more challenging.69 Doing both static and dynamic balance training maximizes balance and stability.69 While most activities that involve moving the body or body parts (such as walking) will improve balance, some additional balance exercises can be beneficial.
Balance exercises can be done without any equipment. Examples are balancing on one foot for 15 seconds and standing up and sitting down without using the hands. However, specific equipment can help, including physioballs, stability balls, cut-in-half stability balls, balance discs, balance wedges, wobble boards, rocker boards, and Indo boards.70 In fact, balance boards and stability balls engage more muscle fibers in other areas of the body (lower back, lower abs, quads, hamstrings, and calves) than exercises done without those balancing devices.71
Balance training for at least 10 minutes a day, 3 days a week, for 4 weeks that incorporates various methods of balance training appears to improve balance.56 Obese patients commencing a program should start with static balance exercises and then progress to dynamic ones. In addition, as balance training progresses, obese patients can integrate balance and stability training exercises with other pieces of equipment, such as performing squats on a balance board, and then gradually add weights (eg, dumbbells) to the exercise.
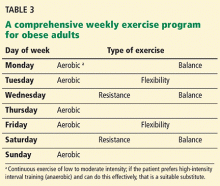
An example of a weekly comprehensive exercise program for an obese patient that incorporates all major exercise types is provided in Table 3. In addition, some smartphone apps that are especially helpful in overweight newcomers to exercise include Couch-to-5K, GymGoal 2, Moves, Fitbit, Workout Trainer, Endomondo, MapMyFitness, Fitocracy, and Fitness Buddy.
BARIATRIC SURGERY AND LIFESTYLE MANAGEMENT FOR OBESITY
Bariatric surgery is a safe and effective treatment for severe obesity and comorbidities including type 2 diabetes mellitus, but weight loss and health outcomes vary considerably among individuals.72,73 Of importance, postoperative weight loss after bariatric surgery and long-term weight loss largely depend on the extent to which patients can make and sustain changes to their lifestyle, including diet, exercise, and behavior modification.72,74
Exercise, especially supervised, is associated with more weight loss after bariatric surgery.61 In a meta-analysis of bariatric patients, exercise participants involved in moderate or greater levels of exercise lost a mean of 3.6 kg more than the minimal exercise groups.75 Another meta-analysis noted the beneficial effects of exercise incorporating more than 30 minutes a day of moderate physical activity following bariatric surgery and was associated with a greater weight loss of over 4% of body mass index.76 These findings were consistent with those of yet another meta-analysis.77
In summary, exercise appears to significantly increase weight loss after bariatric surgery.
TREATMENT CONSIDERATIONS IN MORBID OBESITY
Challenges faced by severely obese or morbidly obese patients affect their exercise options. The types of exercise they are able to perform are limited in most cases to very-low-impact, low-intensity exercises, which may not be as efficient in weight loss or weight maintenance.48 Therefore, it may be prudent to set more conservative weight-loss goals for them, especially early in the program. Compliance and success rates may be better with low-impact activities such as walking, water aerobics, stationary cycling, and resistance training in the severely obese population.
The more severe the obesity, the more comorbidities such as diabetes, hypertension, hyperlipidemia, arthritis, sleep apnea, gastroesophageal reflux disease, and the greater the risk of metabolic syndrome—and conversely, the greater the potential benefit from bariatric surgery followed by exercise.74
A LONG-TERM ENDEAVOR
For obese patients, a comprehensive exercise program will improve functional status, favorably influence cardiovascular risk factors, and help with weight loss or weight maintenance.
Managing obesity is a long-term endeavor.78 For it to succeed, both the patient and the physician need to keep up their efforts. To keep the patient from becoming discouraged, the clinician should focus not just on weight, but also on improvements in metabolic profile and cardiorespiratory fitness. In addition, a careful evaluation, a clear exercise prescription, defined goals, ongoing monitoring (by the patient and the provider), frequent feedback, and charting of progress will improve daily performance and the chance of long-term success.
Although exercise is probably less effective than diet in reducing weight, most studies show that adding it to a diet regimen will increase the weight loss.1,2 Guidelines from the American Heart Association, American College of Cardiology, and Obesity Society recommend a comprehensive lifestyle program that includes a low-calorie diet as well as an increase in physical activity.3
Here, we review the many benefits of exercise for obese patients, not only in terms of weight loss, but also its positive cardiovascular and metabolic effects. Then we discuss how to motivate and prescribe exercise for this challenging group.
EXERCISE IMPROVES WEIGHT LOSS
Increasing energy expenditure by exercising can mobilize and burn stored fat and thus lead to weight loss.4
Typically, with no changes in caloric intake, exercising 60 minutes at low intensity most days of the week will remove up to 0.5 lb per week.5 Exercising harder for longer will take off more weight, up to 3 lb per week.1,6 Some practitioners believe that the total volume of exercise (frequency multiplied by time) is more important than the intensity in determining the amount of weight loss.2,7,8
Ross et al9 randomized 101 obese men to try to lose weight by exercising at a low to moderate intensity, to try to lose weight by dieting, to exercise without the goal of losing weight, or to do nothing (the control group). About half the participants declined or dropped out, but 52 completed the trial. The weight-loss-through-exercise group had lost approximately 15 lb by 12 weeks; the diet group lost a similar amount. Total body fat, visceral fat, and abdominal obesity were all reduced with both diet- and exercise-induced weight loss.
In a study in 130 severely obese adults, after 6 months of high-intensity physical activity for a mean duration of 71 minutes per week, those on an exercise-and-diet regimen lost an average of 24 lb, compared with 18 lb with diet alone.10
Another trial involved obese patients who were instructed to jog the equivalent of 20 miles (32.2 km) a week, with no restriction on caloric intake.11 They lost only 2.9 kg (6.5 lb) over 8 months. Increased food intake explained this minimal weight loss.
In an analysis of 20 studies, exercise-only interventions of 4 months or less resulted in a mean weekly weight loss of 0.4 lb (0.2 kg), with a total loss of about 5 lb (2.3 kg).12
A systematic review of 15 studies noted that aerobic exercise for 3 months or more resulted in a significant reduction in visceral adipose tissue in overweight men and women as measured by computed tomography.13
Effects that different types of exercise have on weight loss
In a study of 119 sedentary adults who were overweight or obese and who were randomized to aerobic, resistance, or combined aerobic-resistance training over 8 months, those involved in aerobic or combined aerobic and resistance training had the greatest reduction in total body and fat mass.14 Given that the combined aerobic-resistance training program required twice the time commitment of the aerobic-alone program, the authors suggested that the most efficient manner of reducing body and fat mass is aerobic training alone.14 In contrast, if the goal is to increase lean muscle mass rather than lose weight and fat, then resistance training would be preferred.14
A meta-analysis confirmed the benefit of aerobic exercise, which resulted in significantly more loss in weight (1.2 kg, 2.6 lb), waist circumference (1.57 cm), and fat mass (1.2 kg, 2.6 lb) than resistance training.15 However, combined aerobic and resistance training was even better, with significantly more weight loss (2.0 kg, 4.4 lb) and fat mass reduction (1.9 kg, 4.2 lb).15
In summary, aerobic and combined aerobic-resistance training appear to be more effective for weight management in obese people than resistance training alone.
ADDITIONAL BENEFITS OF EXERCISE
Increasing regular physical activity through structured exercise has the additional benefits of improving physical fitness, flexibility, mobility, and cardiovascular health.16,17
Even before patients lose a significant amount of weight (eg, 10%), low-intensity exercise such as walking 30 to 60 minutes most days of the week will rapidly improve cardiorespiratory fitness and have positive effects on cardiovascular risk factors such as hypertension, elevated blood glucose, and dyslipidemia.18,19 Aerobic exercise and resistance training also reduce chronic inflammation, which is a strong indicator of future disease, especially in obese patients who have high levels of inflammatory biomarkers.20,21
Even if he or she does not lose much weight, an obese exercising person with good cardiorespiratory fitness has lower cardiovascular risk than a person who is not obese but is poorly conditioned.22
Exercise lowers blood pressure
Overactivity of the sympathetic nervous system is thought to account for over 50% of all cases of hypertension.23 Obesity in concert with diabetes is characterized by sympathetic overactivity and progressive loss of cardiac parasympathetic activity.24 Cardiac autonomic neuropathy is an underestimated risk factor for the increased cardiovascular morbidity and mortality associated with obesity and diabetes, and physical exercise may promote restoration of cardioprotective autonomic modulation in the heart.24
Several studies have shown that aerobic endurance exercise lowers blood pressure in patients with hypertension, and reduction in sympathetic neural activity has been reported as one of the main mechanisms explaining this effect.23 Another mechanism is endothelium-mediated vasodilation: even a single exercise session may increase the bioavailability of nitric oxide and decrease postexercise blood pressure.25
Different types of exercise have been shown to have different effects on blood pressure.
Aerobic training has been shown to reduce systolic blood pressure by 5.2 to 11.0 mm Hg and diastolic blood pressure by 3.0 to 7.7 mm Hg.26
The hypotensive effect of endurance aerobic training is probably mediated at least in part by a reduction in systemic vascular resistance through decreased activity of the sympathetic and renin-angiotensin systems and through improved insulin sensitivity.26 Other factors that may be involved include improved endothelium-dependent vasodilation, enhanced baroreceptor sensitivity, and arterial compliance.26
Dynamic resistance exercise has less of an effect than aerobic exercise, but it has been shown to reduce systolic blood pressure by 0.5 to 4.8 mm Hg and diastolic blood pressure by 0.5 to 4.1 mm Hg.26
In a meta-analysis of studies of resistance training lasting more than 1 month in healthy adults age 18 and older, the authors noted that resistance training induced a significant blood pressure reduction in 28 normotensive or prehypertensive study groups (–3.9/–3.9 mm Hg), whereas the reduction was not significant for the five hypertensive study groups.27
Isometric resistance exercise has been associated with small cardiovascular benefits, but has been shown to reduce systolic blood pressure by 10.5 to 16.5 mm Hg and diastolic blood pressure by 0.62 to 16.4 mm Hg.26
Exercise improves type 2 diabetes
Regular physical activity improves glycemic control and can prevent or delay the onset of type 2 diabetes mellitus.28 Furthermore, physical activity positively affects lipid levels, lowers blood pressure, reduces the rate of cardiovascular events, and restores quality of life in patients with type 2 diabetes.24,29
A meta-analysis of the effect of supervised exercise in adults with type 2 diabetes found that structured exercise achieved the following:
- Lowered systolic blood pressure by 2.42 mm Hg (95% confidence interval 0.45–4.39)
- Lowered diastolic blood pressure by 2.23 mm Hg (1.25–3.21)
- Raised the level of high-density lipoprotein cholesterol by 0.04 mmol/L (0.02–0.07)
- Lowered the level of low-density lipoprotein cholesterol by 0.16 mmol/L (0.01–0.30).30
The metabolic stress from physical exercise can increase oxidation of carbohydrates during exercise, increase postexercise consumption of oxygen (which can increase the rate of fat oxidation during recovery periods after exercise), improve glucose tolerance and insulin sensitivity, and reduce glycemia for 2 to 72 hours depending on the intensity and duration of the exercise.25
Exercise lowers the Framingham risk score
Exercise improves several of the risk factors for coronary artery disease used in calculating the Framingham risk score—ie, systolic blood pressure, total cholesterol, and high-density lipoprotein cholesterol—and thus can significantly lower this number. (It is important to remember that the Framingham score is a surrogate end point of cardiovascular risk that may correlate with a real clinical end point but does not necessarily have a guaranteed relationship.)
In a study of a 12-week exercise program in middle-aged women (ages 40–55), treadmill running for 30 minutes a day 3 days a week significantly reduced 10-year cardiovascular risk scores: 10-year risk 2.2% vs 4.3% in the nonexercising group.31 Others have also shown that enhanced levels of fitness are associated with lower 10-year Framingham risk estimates.32
A study of 31 healthy sedentary adults ages 50 to 65 who were randomized to an unsupervised but pedometer-monitored home-based walking program of 30 minutes of brisk walking 5 days a week noted significant reductions in systolic and diastolic blood pressure and stroke risk, and increased functional capacity in the walking group at 12 weeks.33 Thus, the Framingham risk scores were significantly lower in the exercising group than in with the control group.33
Given that overweight and obese patients who are starting to exercise may find jogging or running daunting, it should also be noted that three brisk 10-minute walks a day are at least as effective as one continuous 30-minute walk in reducing cardiovascular risk in previously sedentary people.34
SETTING ‘SMART’ GOALS
Because obese adults typically do not comply well with prescriptions for exercise, it is important to educate them about its benefits and to provide tools such as perceived exertion scales so they can monitor their exercise, document their performance, and chart their progress; smartphone apps can also be helpful.35 Supervised exercise may improve compliance and results.36 Initially, personal trainers are excellent for starting a habit change, but they are expensive. Virtual trainers are now available and cost far less.37
People do not become obese overnight.They gain weight over a long time. Likewise, weight reduction takes time if done in a sustainable and healthy manner. Thus, SMART goals—specific, measurable, attainable, realistic, timely—should be set to sustain the self-discipline required.
EXERCISE RECOMMENDATIONS
Any exercise program should target 30 to 60 minutes of effort per day, most days of the week, ie, 150 to 300 minutes per week or more.38 But beginners should start low and go slow to avoid dropout, musculoskeletal strain, and joint injury.
The American College of Sports Medicine (ACSM)38,39 recommends combining aerobic and progressive resistance exercise as the core components of an exercise program. The aerobic component can include anaerobic high-intensity interval training (see discussion below). In addition, we recommend flexibility and balance exercises for obese patients.40
Combining aerobic and resistance exercises likely results in greater decreases in abdominal adiposity in the obese.41 In addition, the aerobic portion of a combined exercise regimen can improve functional capacity, and the resistance portion may prevent injury by strengthening the muscles, bones, and joint support systems.42 Adding exercises that promote flexibility and balance helps with range of motion and prevents injuries while exercising.43 These exercises not only expend calories during the exercise itself, but also increase resting energy expenditure for the remainder of the day, as the effects of the raised metabolism persist for hours.44
Aerobic exercise is the foundation
Aerobic exercises that involve large muscle groups, especially walking, should be the foundation of cardiopulmonary exercise for obese persons.45 Many patients can tolerate weight-bearing exercises such as walking or bike riding, but for some, exercises with limited or no weight-bearing such as swimming or aqua-aerobics are better.46
Tips for prescribing. Patients should exercise:
- On 5 or 6 days each week
- At low to moderate intensity (30%–60% of maximum oxygen consumption [Vo2 max])
- For at least 150 minutes per week, with a long-term goal of 300 minutes per week
- By walking, riding a stationary bicycle, or swimming.38,47
To mobilize and use free fatty acids as an energy source, lower-intensity longer-duration aerobic exercise is preferred.5 Thus, frequent, low-intensity or moderate-intensity training (30%–60% of Vo2 max) of longer duration (at least 60 minutes) may be the best approach to losing body fat in obese persons.5,48 Early on in the exercise program, keep the intensity low, as high-intensity training will preferentially use stored glycogen or carbohydrate as an energy substrate rather than free fatty acids or fat.5
With light-moderate exercise, the heart rate will increase and patients will perspire, but they still should be able to carry on a conversation.
Measure (or have patients measure) the heart rate using the radial artery in their wrist after 6 minutes of walking. A pulse of 100 beats per minute or more is associated with an exercise intensity of approximately 50% (or more) of Vo2 max.5
A study of 136 obese men and women who exercised for 6 months found that those doing aerobic exercise only and those doing a combination of aerobic and resistance exercise had greater cardiopulmonary fitness, greater reductions in abdominal and visceral fat, and more improved insulin sensitivity than those doing resistance exercise only.41 Although the aerobiconly group lost more weight (6 lb) than the aerobic-plus-resistance group (5.1 lb) and the resistance-only group (1.4 lb), combining aerobic and resistance exercise is considered optimal.
All physical activity is beneficial, but activities that have less impact on the joints are less likely to cause injuries and joint pain. Aerobic activities that are especially useful in obese adults include walking at a speed of at least 2.5 miles per hour, bicycling, jogging, treadmill walking, swimming, aqua-aerobics, rowing, and low-impact aerobics classes.
Walking is the easiest way for most people to start their program, as it is safe, accessible, and relatively cheap with respect to equipment.35 Adding a simple pedometer or smartphone app to measure the amount of exercise, together with physician counseling, may improve compliance and thus weight loss.49,50
Obese patients may have been inactive for quite a while. Therefore, the sessions should be short and low-intensity at first, then steadily progress.51 To minimize dropout, avoid hard exercise too soon for people with a low exercise capacity or high body mass index at baseline, and give positive feedback and encouragement at each visit.52
It is reasonable to introduce other aerobic exercises to vary the routine, use other muscle groups, and reduce the chance of injury from overuse of one muscle or joint group. Then, as cardiorespiratory fitness improves, the patient will be more confident about trying activities that are more challenging, such as jogging and aerobics classes. An aerobic exercise program consisting only of swimming is less efficacious for weight loss in this population.53
High-intensity interval training
High-intensity interval training involves relatively brief bursts of vigorous exercise separated by periods of recovery and is a time-efficient, novel alternative to continuous exercise.54 The exercise component is anaerobic, meaning muscle movement that does not require oxygen. Anaerobic exercise uses fast-twitch muscle fibers, and thus helps that musculature to become stronger, larger, and more toned. Evidence suggests that high-intensity interval training induces health-enhancing adaptations similar to those of continuous exercise, despite a substantially lower time commitment.41
The ACSM recommends that most adults engage in moderate-intensity cardiorespiratory exercise training for at least 30 minutes a day on at least 5 days a week for a total of at least 150 minutes per week, or high-intensity cardiorespiratory exercise training for at least 20 minutes a day on at least 3 days a week for a goal of 75 minutes a week.38 Thus, high-intensity interval training may be attractive for obese patients because it entails a shorter time commitment to achieve similar weight loss and improved insulin sensitivity than low-intensity or moderate-intensity continuous exercise.
High-intensity exercise has been shown to be effective for obese patients if they can do it.54–56 In one study,57 134 obese patients, mean age 53, underwent supervised high-intensity interval training with resistance training two or three times a week, were encouraged to perform one or two additional exercise sessions a week (unsupervised), and were counseled to follow a Mediterranean diet. At 9 months, investigators noted a significant reduction in body mass, waist circumference, and fat mass.
A study of 12 weeks of high-intensity interval training, moderate-intensity interval training, or no exercise in 34 obese adolescent girls noted that body mass and percentage body fat were significantly decreased with both interval training regimens. However, the high-intensity group had greater reductions in waist circumference and more significant improvements in blood lipid levels, adiponectin levels, and insulin sensitivity.58
Of 62 overweight and obese patients (mean age 53.3, mean body mass index 35.8 kg/m2), 97% adhered to a program of high-intensity interval training over 9 months, which resulted in an average weekly energy expenditure of 1,582 kcal.55 Clinically and statistically significant improvements occurred in body mass (–5.3 kg), body mass index (–1.9 kg/m2), and waist circumference (–5.8 cm) (P < .0001 for all variables). Total fat mass, trunk fat mass, and lipid levels also significantly improved (P < .0001), and the prevalence of metabolic syndrome was reduced by 32.5% (P < .05).
In a meta-analysis of the effect of exercise on overweight adults, training of moderate or high intensity was noted to have the highest potential to reduce visceral adipose tissue in overweight men and women.13 Another meta-analysis noted that high-intensity interval training appeared to promote more improvement in fitness and similar improvements in some cardiometabolic risk factors than moderate exercise performed for at least 8 to 12 weeks in overweight patients.56
A typical progressive exercise program for obese adults is shown in Table 1.
Progressive resistance exercise
Progressive resistance exercises are generally easier for obese patients, as they are not aerobically challenging, allow patients to exercise around physically active people who thus motivate them, and encourage positive feelings about completing their exercise sets.59 The result is improved muscular fitness, socialization, and increased confidence in their abilities (self-efficacy).59
Progressive resistance exercises also promote favorable energy balance and reduced visceral fat deposition through enhanced basal metabolism and activity levels while counteracting age- and disease-related muscle wasting.59 They have been shown to improve cognitive ability, self-esteem, movement control, muscle mass, strength, glucose control, insulin sensitivity, resting blood pressure, lipid profile, and bone mineral density and to reduce fat weight, low back pain, arthritic discomfort, insomnia, anxiety, and depression.60
Gym neophytes should spend a few sessions with a personal trainer to learn how to use the equipment.
While the primary goal of resistance training is more muscle strength, it can reduce fat and weight, burning up to 170 kcal in a 20-minute intense exercise session.61 It reduces both total body fat and visceral adipose tissue, thus benefiting obese persons by reducing insulin resistance.62 All exercise, and especially resistance exercise, can help to strengthen the musculoskeletal system, reduce muscle atrophy, and improve bone mineral density.63
The ACSM guidelines38 recommend progressive resistance exercise on 2 or 3 nonconsecutive days a week. It should involve:
- Exercises that work 8 to 10 muscle groups per session
- Two to four sets of 8 to 12 repetitions for each muscle group.
Exercising on nonconsecutive days allows time for the complete cycle of muscle tissue remodeling.64 Such self-regulated intensity reduces the likelihood of excessive delayed-onset muscle soreness, which can discourage new participants.65
To prevent muscle injury, obese people should begin with low-intensity workouts using lower resistance, one set of 8 to 12 repetitions 2 days a week. Then, they should gradually but progressively increase the intensity, volume, and frequency of the training.47 This will obviate a plateau in training and will maximize musculoskeletal adaptation. The prescription should include exercises for the upper body (eg, biceps curls), lower body (eg, leg presses), and the midsection (eg, abdominal curl-ups, which give better abdominal muscle engagement and less risk to the back than crunches) and focus on the correct exercise form and function rather than the amount of resistance or weight lifted.
A typical progressive resistance exercise program for obese adults is shown in Table 2.
Flexibility exercise
Flexibility exercise involves stretching to improve the movement of muscles, joints, and ligaments.45 While not specifically used in an energy-expenditure strategy, flexibility (or mobility) exercises help to increase or maintain joint range of motion and can reduce muscle and joint pain associated with obesity and exercise.66
The ACSM recommends that stretching exercises be done when the muscles are warm after a brief warm-up or exercise session.38 Typically, muscles should be stretched for at least 15 seconds, and stretching is recommended at a frequency of 2 to 4 days per week.38
A good way to incorporate flexibility exercise is to join a yoga class, as yoga has been shown to improve strength and flexibility and may help control physiologic variables such as blood pressure, lipids, respiration, heart rate, and metabolic rate to improve overall exercise capacity in obese patients.67
Balance exercise
Balance exercises help obese patients improve their stability. Poor balance is associated with injuries, accidents, and falls during activities of daily living.68
Balance, the ability to maintain the body’s center of gravity within its base of support, can be categorized as static (sustaining the body in static equilibrium or within its base of support) or dynamic (maintaining equilibrium during a transition from a dynamic to a static state), which is more challenging.69 Doing both static and dynamic balance training maximizes balance and stability.69 While most activities that involve moving the body or body parts (such as walking) will improve balance, some additional balance exercises can be beneficial.
Balance exercises can be done without any equipment. Examples are balancing on one foot for 15 seconds and standing up and sitting down without using the hands. However, specific equipment can help, including physioballs, stability balls, cut-in-half stability balls, balance discs, balance wedges, wobble boards, rocker boards, and Indo boards.70 In fact, balance boards and stability balls engage more muscle fibers in other areas of the body (lower back, lower abs, quads, hamstrings, and calves) than exercises done without those balancing devices.71
Balance training for at least 10 minutes a day, 3 days a week, for 4 weeks that incorporates various methods of balance training appears to improve balance.56 Obese patients commencing a program should start with static balance exercises and then progress to dynamic ones. In addition, as balance training progresses, obese patients can integrate balance and stability training exercises with other pieces of equipment, such as performing squats on a balance board, and then gradually add weights (eg, dumbbells) to the exercise.
An example of a weekly comprehensive exercise program for an obese patient that incorporates all major exercise types is provided in Table 3. In addition, some smartphone apps that are especially helpful in overweight newcomers to exercise include Couch-to-5K, GymGoal 2, Moves, Fitbit, Workout Trainer, Endomondo, MapMyFitness, Fitocracy, and Fitness Buddy.
BARIATRIC SURGERY AND LIFESTYLE MANAGEMENT FOR OBESITY
Bariatric surgery is a safe and effective treatment for severe obesity and comorbidities including type 2 diabetes mellitus, but weight loss and health outcomes vary considerably among individuals.72,73 Of importance, postoperative weight loss after bariatric surgery and long-term weight loss largely depend on the extent to which patients can make and sustain changes to their lifestyle, including diet, exercise, and behavior modification.72,74
Exercise, especially supervised, is associated with more weight loss after bariatric surgery.61 In a meta-analysis of bariatric patients, exercise participants involved in moderate or greater levels of exercise lost a mean of 3.6 kg more than the minimal exercise groups.75 Another meta-analysis noted the beneficial effects of exercise incorporating more than 30 minutes a day of moderate physical activity following bariatric surgery and was associated with a greater weight loss of over 4% of body mass index.76 These findings were consistent with those of yet another meta-analysis.77
In summary, exercise appears to significantly increase weight loss after bariatric surgery.
TREATMENT CONSIDERATIONS IN MORBID OBESITY
Challenges faced by severely obese or morbidly obese patients affect their exercise options. The types of exercise they are able to perform are limited in most cases to very-low-impact, low-intensity exercises, which may not be as efficient in weight loss or weight maintenance.48 Therefore, it may be prudent to set more conservative weight-loss goals for them, especially early in the program. Compliance and success rates may be better with low-impact activities such as walking, water aerobics, stationary cycling, and resistance training in the severely obese population.
The more severe the obesity, the more comorbidities such as diabetes, hypertension, hyperlipidemia, arthritis, sleep apnea, gastroesophageal reflux disease, and the greater the risk of metabolic syndrome—and conversely, the greater the potential benefit from bariatric surgery followed by exercise.74
A LONG-TERM ENDEAVOR
For obese patients, a comprehensive exercise program will improve functional status, favorably influence cardiovascular risk factors, and help with weight loss or weight maintenance.
Managing obesity is a long-term endeavor.78 For it to succeed, both the patient and the physician need to keep up their efforts. To keep the patient from becoming discouraged, the clinician should focus not just on weight, but also on improvements in metabolic profile and cardiorespiratory fitness. In addition, a careful evaluation, a clear exercise prescription, defined goals, ongoing monitoring (by the patient and the provider), frequent feedback, and charting of progress will improve daily performance and the chance of long-term success.
- Thorogood A, Mottillo S, Shimony A, et al. Isolated aerobic exercise and weight loss: a systematic review and meta-analysis of randomized controlled trials. Am J Med 2011; 124:747–755.
- Church T. Exercise in obesity, metabolic syndrome, and diabetes. Prog Cardiovasc Dis 2011; 53:412–418.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129(suppl 2):S102–S138.
- Strasser B. Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci 2013; 1281:141–159.
- Poirier P, Despres JP. Exercise in weight management of obesity. Cardiol Clin 2001; 19:459–470.
- Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev 2006; 4:CD003817.
- Slentz CA, Houmard JA, Kraus WE. Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity (Silver Spring) 2009; 17(suppl 3):S27–S33.
- Ross R, Hudson R, Stotz PJ, Lam M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults: a randomized trial. Ann Intern Med 2015; 162:325–334.
- Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 2000; 133:92–103.
- Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 2010; 304:1795–1802.
- Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med 2004; 164:31–39.
- Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc 2001; 33(suppl 6):S521–S529.
- Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One 2013; 8:e56415.
- Willis LH, Slentz CA, Bateman LA, et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol (1985) 2012; 113:1831–1837.
- Schwingshackl L, Dias S, Strasser B, Hoffmann G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: a systematic review and network meta-analysis. PLoS One 2013; 8:e82853.
- Cook CM, Schoeller DA. Physical activity and weight control: conflicting findings. Curr Opin Clin Nutr Metab Care 2011; 14:419–424.
- Choo J, Lee J, Cho JH, Burke LE, Sekikawa A, Jae SY. Effects of weight management by exercise modes on markers of subclinical atherosclerosis and cardiometabolic profile among women with abdominal obesity: a randomized controlled trial. BMC Cardiovasc Disord 2014; 14:82.
- Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med 2004; 34:371–418.
- Shaibi GQ, Ryder JR, Kim JY, Barraza E. Exercise for obese youth: refocusing attention from weight loss to health gains. Exerc Sport Sci Rev 2015; 43:41–47.
- You T, Arsenis NC, Disanzo BL, Lamonte MJ. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med 2013; 43:243–256.
- Bluher S, Petroff D, Wagner A, et al. The one year exercise and lifestyle intervention program KLAKS: effects on anthropometric parameters, cardiometabolic risk factors and glycemic control in childhood obesity. Metabolism 2014; 63:422–430.
- Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr 1999; 69:373–380.
- Leosco D, Parisi V, Femminella GD, et al. Effects of exercise training on cardiovascular adrenergic system. Front Physiol 2013; 4:348.
- Voulgari C, Pagoni S, Vinik A, Poirier P. Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism 2013; 62:609–621.
- Asano RY, Sales MM, Browne RA, et al. Acute effects of physical exercise in type 2 diabetes: a review. World J Diabetes 2014; 5:659–665.
- Brook RD, Appel LJ, Rubenfire M, et al; American Heart Association Professional Education Committee of the Council for High Blood Pressure Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension 2013; 61:1360–1383.
- Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension 2011; 58:950–958.
- Ades PA. A lifestyle program of exercise and weight loss is effective in preventing and treating type 2 diabetes mellitus: why are programs not more available? Prev Med 2015: S0091–7435(15)00085–7.
- Ades PA, Savage PD, Marney AM, Harvey J, Evans KA. Remission of recently diagnosed type 2 diabetes mellitus with weight loss and exercise. J Cardiopulm Rehabil Prev 2015; 35:193–197.
- Hayashino Y, Jackson JL, Fukumori N, Nakamura F, Fukuhara S. Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2012; 98:349–360.
- Saffi MA, Polanczyk CA, Rabelo-Silva ER. Lifestyle interventions reduce cardiovascular risk in patients with coronary artery disease: a randomized clinical trial. Eur J Cardiovasc Nurs 2014; 13:436–443.
- LaMonte MJ, Durstine JL, Addy CL, Irwin ML, Ainsworth BE. Physical activity, physical fitness, and Framingham 10-year risk score: the cross-cultural activity participation study. J Cardiopulm Rehabil 2001; 21:63–70.
- Tully MA, Cupples ME, Chan WS, McGlade K, Young IS. Brisk walking, fitness, and cardiovascular risk: a randomized controlled trial in primary care. Prev Med 2005; 41:622–628.
- Murphy M, Nevill A, Neville C, Biddle S, Hardman A. Accumulating brisk walking for fitness, cardiovascular risk, and psychological health. Med Sci Sports Exerc 2002; 34:1468–1474.
- Colley RC, Hills AP, King NA, Byrne NM. Exercise-induced energy expenditure: implications for exercise prescription and obesity. Patient Educ Couns 2010; 79:327–332.
- Baillot A, Mampuya WM, Comeau E, Méziat-Burdin A, Langlois MF. Feasibility and impacts of supervised exercise training in subjects with obesity awaiting bariatric surgery: a pilot study. Obes Surg 2013; 23:882–891.
- Lowe S, ÓLaighin G. The age of the virtual trainer. Procedia Engineering 2012; 34:242–247.
- Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43:1334–1359.
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK; American College of Sports Medicine. American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009; 41:459–471.
- Montero-Fernandez N, Serra-Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med 2013; 49:131–143.
- Davidson LE, Hudson R, Kilpatrick K, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med 2009; 169:122–131.
- Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 2009; 3:CD002759.
- Manini TM, Newman AB, Fielding R, et al; LIFE Research Group. Effects of exercise on mobility in obese and nonobese older adults. Obesity (Silver Spring) 2010; 18:1168–1175.
- Hackney KJ, Engels HJ, Gretebeck RJ. Resting energy expenditure and delayed-onset muscle soreness after full-body resistance training with an eccentric concentration. J Strength Cond Res 2008; 22:1602–1609.
- Siddiqui NI, Nessa A, Hossain MA. Regular physical exercise: way to healthy life. Mymensingh Med J 2010; 19:154–158.
- Chicco AJ. Exercise training in prevention and rehabilitation: which training mode is best? Minerva Cardioangiol 2008; 56:557–570.
- Westcott WL, Winett RA, Annesi JJ, Wojcik JR, Anderson ES, Madden PJ. Prescribing physical activity: applying the ACSM protocols for exercise type, intensity, and duration across 3 training frequencies. Phys Sportsmed 2009; 37:51–58.
- Mougios V1, Kazaki M, Christoulas K, Ziogas G, Petridou A. Does the intensity of an exercise programme modulate body composition changes? Int J Sports Med 2006; 27:178–181.
- Richardson CR, Newton TL, Abraham JJ, Sen A, Jimbo M, Swartz AM. A meta-analysis of pedometer-based walking interventions and weight loss. Ann Fam Med 2008; 6:69–77.
- Stovitz SD, VanWormer JJ, Center BA, Bremer KL. Pedometers as a means to increase ambulatory activity for patients seen at a family medicine clinic. J Am Board Fam Pract 2005; 18:335–343.
- Lepor NE, Fouchia DD, McCullough PA. New vistas for the treatment of obesity: turning the tide against the leading cause of morbidity and cardiovascular mortality in the developed world. Rev Cardiovasc Med 2013; 14:20–40.
- Wittmer M, Volpatti M, Piazzalonga S, Hoffmann A. Expectation, satisfaction, and predictors of dropout in cardiac rehabilitation. Eur J Prev Cardiol 2012; 19:1082–1088.
- Gwinup G. Weight loss without dietary restriction: efficacy of different forms of aerobic exercise. Am J Sports Med 1987; 15:275–279.
- Jung ME, Bourne JE, Little JP. Where does HIT fit? An examination of the affective response to high-intensity intervals in comparison to continuous moderate- and continuous vigorous-intensity exercise in the exercise intensity-affect continuum. PLoS One 2014; 9:e114541.
- Gremeaux V, Drigny J, Nigam A, et al. Long-term lifestyle intervention with optimized high-intensity interval training improves body composition, cardiometabolic risk, and exercise parameters in patients with abdominal obesity. Am J Phys Med Rehabil 2012; 91:941–950.
- Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med 2012; 42:489–509.
- Dalzill C, Nigam A, Juneau M, et al. Intensive lifestyle intervention improves cardiometabolic and exercise parameters in metabolically healthy obese and metabolically unhealthy obese individuals. Can J Cardiol 2014; 30:434–440.
- Racil G, Ben Ounis O, Hammouda O, et al. Effects of high vs moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol 2013; 113:2531–2540.
- Willey KA, Singh MA. Battling insulin resistance in elderly obese people with type 2 diabetes: bring on the heavy weights. Diabetes Care 2003; 26:1580–1588.
- Westcott WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep 2012; 11:209–216.
- Haltom RW, Kraemer RR, Sloan RA, Hebert EP, Frank K, Tryniecki JL. Circuit weight training and its effects on excess postexercise oxygen consumption. Med Sci Sports Exerc 1999; 31:1613–1618.
- Strasser B, Schobersberger W. Evidence for resistance training as a treatment therapy in obesity. J Obes 2011; pii:482564.
- Fonseca H, Moreira-Gonçalves D, Coriolano HJ, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med 2014; 44:37–53.
- Candow DG, Burke DG. Effect of short-term equal-volume resistance training with different workout frequency on muscle mass and strength in untrained men and women. J Strength Cond Res 2007; 21:204–207.
- Trost Z, France CR, Thomas JS. Pain-related fear and avoidance of physical exertion following delayed-onset muscle soreness. Pain 2011; 152:1540–1547.
- Mathus-Vliegen EM. Obesity and the elderly. J Clin Gastroenterol 2012; 46:533–544.
- Dhananjai S, Sadashiv, Tiwari S, Dutt K, Kumar R. Reducing psychological distress and obesity through yoga practice. Int J Yoga 2013; 6:66–70.
- Mathus-Vliegen EM; Obesity Management Task Force of the European Association for the Study of Obesity. Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: a guideline. Obes Facts 2012; 5:460–483.
- DiStefano LJ, Clark MA, Padua DA. Evidence supporting balance training in healthy individuals: a systemic review. J Strength Cond Res 2009; 23:2718–2731.
- Ogaya S, Ikezoe T, Soda N, Ichihashi N. Effects of balance training using wobble boards in the elderly. J Strength Cond Res 2011; 25:2616–2622.
- Sukalinggam CL, Sukalinggam GL, Kasim F, Yusof A. Stability ball training on lower back strength has greater effect in untrained female compared to male. J Hum Kinet 2012; 33:133–141.
- Kalarchian M, Turk M, Elliott J, Gourash W. Lifestyle management for enhancing outcomes after bariatric surgery. Curr Diab Rep 2014; 14:540.
- Rothwell L, Kow L, Toouli J. Effect of a post-operative structured exercise programme on short-term weight loss after obesity surgery using adjustable gastric bands. Obes Surg 2015; 25:126–128.
- Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 2013; 9:159–191.
- Egberts K, Brown WA, Brennan L, O’Brien PE. Does exercise improve weight loss after bariatric surgery? A systematic review. Obes Surg 2012; 22:335–341.
- Livhits M, Mercado C, Yermilov I, et al. Exercise following bariatric surgery: systematic review. Obes Surg 2010; 20:657–665.
- Jacobi D, Ciangura C, Couet C, Oppert JM. Physical activity and weight loss following bariatric surgery. Obes Rev 2011; 12:366–377.
- Wadden TA, Foster GD, Letizia KA. One-year behavioral treatment of obesity: comparison of moderate and severe caloric restriction and the effects of weight maintenance therapy. J Consult Clin Psychol 1994; 62:165–171.
- Thorogood A, Mottillo S, Shimony A, et al. Isolated aerobic exercise and weight loss: a systematic review and meta-analysis of randomized controlled trials. Am J Med 2011; 124:747–755.
- Church T. Exercise in obesity, metabolic syndrome, and diabetes. Prog Cardiovasc Dis 2011; 53:412–418.
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129(suppl 2):S102–S138.
- Strasser B. Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci 2013; 1281:141–159.
- Poirier P, Despres JP. Exercise in weight management of obesity. Cardiol Clin 2001; 19:459–470.
- Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev 2006; 4:CD003817.
- Slentz CA, Houmard JA, Kraus WE. Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity (Silver Spring) 2009; 17(suppl 3):S27–S33.
- Ross R, Hudson R, Stotz PJ, Lam M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults: a randomized trial. Ann Intern Med 2015; 162:325–334.
- Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 2000; 133:92–103.
- Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 2010; 304:1795–1802.
- Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med 2004; 164:31–39.
- Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc 2001; 33(suppl 6):S521–S529.
- Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One 2013; 8:e56415.
- Willis LH, Slentz CA, Bateman LA, et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol (1985) 2012; 113:1831–1837.
- Schwingshackl L, Dias S, Strasser B, Hoffmann G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: a systematic review and network meta-analysis. PLoS One 2013; 8:e82853.
- Cook CM, Schoeller DA. Physical activity and weight control: conflicting findings. Curr Opin Clin Nutr Metab Care 2011; 14:419–424.
- Choo J, Lee J, Cho JH, Burke LE, Sekikawa A, Jae SY. Effects of weight management by exercise modes on markers of subclinical atherosclerosis and cardiometabolic profile among women with abdominal obesity: a randomized controlled trial. BMC Cardiovasc Disord 2014; 14:82.
- Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med 2004; 34:371–418.
- Shaibi GQ, Ryder JR, Kim JY, Barraza E. Exercise for obese youth: refocusing attention from weight loss to health gains. Exerc Sport Sci Rev 2015; 43:41–47.
- You T, Arsenis NC, Disanzo BL, Lamonte MJ. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med 2013; 43:243–256.
- Bluher S, Petroff D, Wagner A, et al. The one year exercise and lifestyle intervention program KLAKS: effects on anthropometric parameters, cardiometabolic risk factors and glycemic control in childhood obesity. Metabolism 2014; 63:422–430.
- Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr 1999; 69:373–380.
- Leosco D, Parisi V, Femminella GD, et al. Effects of exercise training on cardiovascular adrenergic system. Front Physiol 2013; 4:348.
- Voulgari C, Pagoni S, Vinik A, Poirier P. Exercise improves cardiac autonomic function in obesity and diabetes. Metabolism 2013; 62:609–621.
- Asano RY, Sales MM, Browne RA, et al. Acute effects of physical exercise in type 2 diabetes: a review. World J Diabetes 2014; 5:659–665.
- Brook RD, Appel LJ, Rubenfire M, et al; American Heart Association Professional Education Committee of the Council for High Blood Pressure Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension 2013; 61:1360–1383.
- Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension 2011; 58:950–958.
- Ades PA. A lifestyle program of exercise and weight loss is effective in preventing and treating type 2 diabetes mellitus: why are programs not more available? Prev Med 2015: S0091–7435(15)00085–7.
- Ades PA, Savage PD, Marney AM, Harvey J, Evans KA. Remission of recently diagnosed type 2 diabetes mellitus with weight loss and exercise. J Cardiopulm Rehabil Prev 2015; 35:193–197.
- Hayashino Y, Jackson JL, Fukumori N, Nakamura F, Fukuhara S. Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2012; 98:349–360.
- Saffi MA, Polanczyk CA, Rabelo-Silva ER. Lifestyle interventions reduce cardiovascular risk in patients with coronary artery disease: a randomized clinical trial. Eur J Cardiovasc Nurs 2014; 13:436–443.
- LaMonte MJ, Durstine JL, Addy CL, Irwin ML, Ainsworth BE. Physical activity, physical fitness, and Framingham 10-year risk score: the cross-cultural activity participation study. J Cardiopulm Rehabil 2001; 21:63–70.
- Tully MA, Cupples ME, Chan WS, McGlade K, Young IS. Brisk walking, fitness, and cardiovascular risk: a randomized controlled trial in primary care. Prev Med 2005; 41:622–628.
- Murphy M, Nevill A, Neville C, Biddle S, Hardman A. Accumulating brisk walking for fitness, cardiovascular risk, and psychological health. Med Sci Sports Exerc 2002; 34:1468–1474.
- Colley RC, Hills AP, King NA, Byrne NM. Exercise-induced energy expenditure: implications for exercise prescription and obesity. Patient Educ Couns 2010; 79:327–332.
- Baillot A, Mampuya WM, Comeau E, Méziat-Burdin A, Langlois MF. Feasibility and impacts of supervised exercise training in subjects with obesity awaiting bariatric surgery: a pilot study. Obes Surg 2013; 23:882–891.
- Lowe S, ÓLaighin G. The age of the virtual trainer. Procedia Engineering 2012; 34:242–247.
- Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43:1334–1359.
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK; American College of Sports Medicine. American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009; 41:459–471.
- Montero-Fernandez N, Serra-Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med 2013; 49:131–143.
- Davidson LE, Hudson R, Kilpatrick K, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med 2009; 169:122–131.
- Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 2009; 3:CD002759.
- Manini TM, Newman AB, Fielding R, et al; LIFE Research Group. Effects of exercise on mobility in obese and nonobese older adults. Obesity (Silver Spring) 2010; 18:1168–1175.
- Hackney KJ, Engels HJ, Gretebeck RJ. Resting energy expenditure and delayed-onset muscle soreness after full-body resistance training with an eccentric concentration. J Strength Cond Res 2008; 22:1602–1609.
- Siddiqui NI, Nessa A, Hossain MA. Regular physical exercise: way to healthy life. Mymensingh Med J 2010; 19:154–158.
- Chicco AJ. Exercise training in prevention and rehabilitation: which training mode is best? Minerva Cardioangiol 2008; 56:557–570.
- Westcott WL, Winett RA, Annesi JJ, Wojcik JR, Anderson ES, Madden PJ. Prescribing physical activity: applying the ACSM protocols for exercise type, intensity, and duration across 3 training frequencies. Phys Sportsmed 2009; 37:51–58.
- Mougios V1, Kazaki M, Christoulas K, Ziogas G, Petridou A. Does the intensity of an exercise programme modulate body composition changes? Int J Sports Med 2006; 27:178–181.
- Richardson CR, Newton TL, Abraham JJ, Sen A, Jimbo M, Swartz AM. A meta-analysis of pedometer-based walking interventions and weight loss. Ann Fam Med 2008; 6:69–77.
- Stovitz SD, VanWormer JJ, Center BA, Bremer KL. Pedometers as a means to increase ambulatory activity for patients seen at a family medicine clinic. J Am Board Fam Pract 2005; 18:335–343.
- Lepor NE, Fouchia DD, McCullough PA. New vistas for the treatment of obesity: turning the tide against the leading cause of morbidity and cardiovascular mortality in the developed world. Rev Cardiovasc Med 2013; 14:20–40.
- Wittmer M, Volpatti M, Piazzalonga S, Hoffmann A. Expectation, satisfaction, and predictors of dropout in cardiac rehabilitation. Eur J Prev Cardiol 2012; 19:1082–1088.
- Gwinup G. Weight loss without dietary restriction: efficacy of different forms of aerobic exercise. Am J Sports Med 1987; 15:275–279.
- Jung ME, Bourne JE, Little JP. Where does HIT fit? An examination of the affective response to high-intensity intervals in comparison to continuous moderate- and continuous vigorous-intensity exercise in the exercise intensity-affect continuum. PLoS One 2014; 9:e114541.
- Gremeaux V, Drigny J, Nigam A, et al. Long-term lifestyle intervention with optimized high-intensity interval training improves body composition, cardiometabolic risk, and exercise parameters in patients with abdominal obesity. Am J Phys Med Rehabil 2012; 91:941–950.
- Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med 2012; 42:489–509.
- Dalzill C, Nigam A, Juneau M, et al. Intensive lifestyle intervention improves cardiometabolic and exercise parameters in metabolically healthy obese and metabolically unhealthy obese individuals. Can J Cardiol 2014; 30:434–440.
- Racil G, Ben Ounis O, Hammouda O, et al. Effects of high vs moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol 2013; 113:2531–2540.
- Willey KA, Singh MA. Battling insulin resistance in elderly obese people with type 2 diabetes: bring on the heavy weights. Diabetes Care 2003; 26:1580–1588.
- Westcott WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep 2012; 11:209–216.
- Haltom RW, Kraemer RR, Sloan RA, Hebert EP, Frank K, Tryniecki JL. Circuit weight training and its effects on excess postexercise oxygen consumption. Med Sci Sports Exerc 1999; 31:1613–1618.
- Strasser B, Schobersberger W. Evidence for resistance training as a treatment therapy in obesity. J Obes 2011; pii:482564.
- Fonseca H, Moreira-Gonçalves D, Coriolano HJ, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med 2014; 44:37–53.
- Candow DG, Burke DG. Effect of short-term equal-volume resistance training with different workout frequency on muscle mass and strength in untrained men and women. J Strength Cond Res 2007; 21:204–207.
- Trost Z, France CR, Thomas JS. Pain-related fear and avoidance of physical exertion following delayed-onset muscle soreness. Pain 2011; 152:1540–1547.
- Mathus-Vliegen EM. Obesity and the elderly. J Clin Gastroenterol 2012; 46:533–544.
- Dhananjai S, Sadashiv, Tiwari S, Dutt K, Kumar R. Reducing psychological distress and obesity through yoga practice. Int J Yoga 2013; 6:66–70.
- Mathus-Vliegen EM; Obesity Management Task Force of the European Association for the Study of Obesity. Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: a guideline. Obes Facts 2012; 5:460–483.
- DiStefano LJ, Clark MA, Padua DA. Evidence supporting balance training in healthy individuals: a systemic review. J Strength Cond Res 2009; 23:2718–2731.
- Ogaya S, Ikezoe T, Soda N, Ichihashi N. Effects of balance training using wobble boards in the elderly. J Strength Cond Res 2011; 25:2616–2622.
- Sukalinggam CL, Sukalinggam GL, Kasim F, Yusof A. Stability ball training on lower back strength has greater effect in untrained female compared to male. J Hum Kinet 2012; 33:133–141.
- Kalarchian M, Turk M, Elliott J, Gourash W. Lifestyle management for enhancing outcomes after bariatric surgery. Curr Diab Rep 2014; 14:540.
- Rothwell L, Kow L, Toouli J. Effect of a post-operative structured exercise programme on short-term weight loss after obesity surgery using adjustable gastric bands. Obes Surg 2015; 25:126–128.
- Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 2013; 9:159–191.
- Egberts K, Brown WA, Brennan L, O’Brien PE. Does exercise improve weight loss after bariatric surgery? A systematic review. Obes Surg 2012; 22:335–341.
- Livhits M, Mercado C, Yermilov I, et al. Exercise following bariatric surgery: systematic review. Obes Surg 2010; 20:657–665.
- Jacobi D, Ciangura C, Couet C, Oppert JM. Physical activity and weight loss following bariatric surgery. Obes Rev 2011; 12:366–377.
- Wadden TA, Foster GD, Letizia KA. One-year behavioral treatment of obesity: comparison of moderate and severe caloric restriction and the effects of weight maintenance therapy. J Consult Clin Psychol 1994; 62:165–171.
KEY POINTS
- Exercise not only helps people lose weight and keep it off, it lowers blood pressure, improves lipid levels, improves insulin sensitivity, and lowers blood glucose levels.
- Of the various types of exercise, aerobic exercise provides the most benefits, but resistance, flexibility, and balance exercises have additional value. Specifically, continuous moderate-intensity aerobic or high-intensity interval training in combination with some resistance exercises appears to be most effective for weight management.
- For people who are extremely obese, low-impact exercises performed for a longer duration may be more manageable and are still effective.
- The clinician should monitor the patient’s compliance and progress and give appropriate encouragement and feedback for sustained success.
The intersection of obstructive lung disease and sleep apnea
Many patients who have obstructive lung disease, ie, chronic obstructive pulmonary disease (COPD) or asthma, also have obstructive sleep apnea (OSA), and vice versa.
The combination of COPD and OSA was first described almost 30 years ago by Flenley, who called it “overlap syndrome.”1 At that time, he recommended that a sleep study be considered in all obese patients with COPD who snore and in those who have frequent headaches after starting oxygen therapy. In the latter group, he doubted that nocturnal oxygen was the correct treatment. He also believed that the outcomes in patients with overlap syndrome were worse than those in patients with COPD or OSA alone. These opinions remain largely valid today.
We now also recognize the combination of asthma and OSA (alternative overlap syndrome) and collectively call both combinations obstructive lung disease-obstructive sleep apnea (OLDOSA) syndrome.2 Interestingly, these relationships are likely bidirectional, with one condition aggravating or predisposing to the other.
Knowing that a patient has one of these overlap syndromes, one can initiate continuous positive airway pressure (CPAP) therapy, which can improve clinical outcomes.3–6 Therefore, when evaluating a patient with asthma or COPD, one should consider OSA using a validated questionnaire and, if the findings suggest the diagnosis, polysomnography. Conversely, it is prudent to look for comorbid obstructive lung disease in patients with OSA, as interactions between upper and lower airway dysfunction may lead to distinctly different treatment and outcomes.
Here, we briefly review asthma and COPD, explore shared risk factors for sleep-disordered breathing and obstructive lung diseases, describe potential pathophysiologic mechanisms explaining these associations, and highlight the importance of recognizing and individually treating the overlaps of OSA and COPD or asthma.
COPD AND ASTHMA ARE VERY COMMON
About 10% of the US population have COPD,7 a preventable and treatable disease mainly caused by smoking, and a leading cause of sickness and death worldwide.8,9
About 8% of Americans have asthma,7 which has become one of the most common chronic conditions in the Western world, affecting about 1 in 7 children and about 1 in 12 adults. The World Health Organization estimates that 235 million people suffer from asthma worldwide, and by 2025 this number is projected to rise to 400 million.10,11
The prevalence of these conditions in a particular population depends on the frequency of risk factors and associated morbidities, including OSA. These factors may allow asthma or COPD to arise earlier or have more severe manifestations.8,12
Asthma and COPD: Similarities and differences
Asthma and COPD share several features. Both are inflammatory airway conditions triggered or perpetuated by allergens, viral infection, tobacco smoke, products of biomass or fossil fuel combustion, and other substances. In both diseases, airflow is “obstructed” or limited, with a low ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC). Symptoms can also be similar, with dyspnea, cough, wheezing, and chest tightness being the most frequent complaints. The similarities support the theory proposed by Orie et al13 (the “Dutch hypothesis”) that asthma and COPD may actually be manifestations of the same disease.
But there are also differences. COPD is strongly linked to cigarette smoking and has at least three phenotypes:
- Chronic bronchitis, defined clinically by cough and sputum production for more than 3 months per year for 2 consecutive years
- Emphysema, characterized anatomically by loss of lung parenchyma, as seen on tomographic imaging or examination of pathologic specimens
- A mixed form with bronchitic and emphysematous features, which is likely the most common.
Particularly in emphysematous COPD, smoking predisposes patients to gas-exchange abnormalities and low diffusing capacity for carbon monoxide.
In asthma, symptoms may be more episodic, the age of onset is often younger, and atopy is common, especially in allergic asthma. These episodic symptoms may correlate temporally with measurable airflow reversibility (≥ 12% and ≥ 200 mL improvement in FVC or in FEV1 after bronchodilator challenge).
However, the current taxonomy does not unequivocally divide obstructive lung diseases into asthma and COPD, and major features such as airway hyperresponsiveness, airflow reversibility, neutrophilic or CD8 lymphocytic airway inflammation, and lower concentration of nitric oxide in the exhaled air may be present in different phenotypes of both conditions (Table 1).
AIRFLOW IN OBSTRUCTIVE LUNG DISEASES AND DURING SLEEP
Normal airflow involves a complex interplay between airway resistance and elastic recoil of the entire respiratory system, including the airways, the lung parenchyma, and the chest wall (Figure 1).
In asthma and COPD, resistance to airflow is increased, predominantly in the upper airways (nasal passages, pharynx, and larynx) and in the first three or four subdivisions of the tracheobronchial tree. The problem is worse during exhalation, when elastic recoil of the lung parenchyma and chest wall also increases airway resistance, reduces airway caliber, and possibly even constricts the bronchi. This last effect may occur either due to mass loading of the bronchial smooth muscles or to large intrathoracic transmural pressure shifts that may increase extravasation of fluid in the bronchial walls, especially with higher vascular permeability in inflammatory conditions.
Furthermore, interactions between the airway and parenchyma and between the upper and lower airways, as well as radial and axial coupling of these anatomic and functional components, contribute to complex interplay between airway resistance and parenchymal-chest wall elastic energy—stretch or recoil.
The muscles of the upper and lower airway may not work together due to the loss of normal lung parenchyma (as in emphysema) or to the acute inflammation in the small airways and adjacent parenchyma (as in severe asthma exacerbations). This loss of coordination makes the upper airway more collapsible, a feature of OSA.
Additionally, obesity, gastroesophageal reflux, disease chronic rhinitis, nasal polyposis, and acute exacerbations of chronic systemic inflammation all contribute to more complex interactions between obstructive lung diseases and OSA.6
Sleep affects breathing, particularly in patients with respiratory comorbidities, and sleep-disordered breathing causes daytime symptoms and worsens quality of life.1,13–15 During sleep, respiratory centers become less sensitive to oxygen and carbon dioxide; breathing becomes more irregular, especially during rapid eye movement (REM) sleep; the chest wall moves less, so that the tidal volume and functional residual capacity are lower; sighs, yawns, and deep breaths become limited; and serum carbon dioxide concentration may rise.
OBSTRUCTIVE SLEEP APNEA
The prevalence of OSA, a form of sleep-disordered breathing characterized by limitation of inspiratory and (to a lesser degree) expiratory flow, has increased significantly in recent years, in parallel with the prevalence of its major risk factor, obesity.
OSA is generally defined as an apnea-hypopnea index of 5 or higher, ie, five or more episodes of apnea or hypopnea per hour.
OSA syndrome, ie, an apnea-hypopnea index of 5 or higher and excessive daytime sleepiness (defined by an Epworth Sleepiness Scale score > 10) was found in the initial analysis of the Wisconsin Sleep cohort in 1993 to be present in about 2% of women and 4% of men.16 A more recent longitudinal analysis showed a significant increase—for example, in people 50 to 70 years old the prevalence was up to 17.6% in men and 7.5% in women.17
Upper airway resistance syndrome, a milder form of sleep-disordered breathing, is now included under the diagnosis of OSA, as its pathophysiology is not significantly different.18
In the next section, we discuss what happens when OSA overlaps with COPD (overlap syndrome) and with asthma (“alternative overlap syndrome”)2,8 (Figure 2).
OSA AND COPD (OVERLAP SYNDROME)
Flenley1 hypothesized that patients with COPD in whom supplemental oxygen worsened hypercapnia may also have OSA and called this association overlap syndrome.
How common is overlap syndrome?
Since both COPD and OSA are prevalent conditions, overlap syndrome may also be common.
The reported prevalence of overlap syndrome varies widely, depending on the population studied and the methods used. In various studies, COPD was present in 9% to 56% of patients with OSA,19–23 and OSA was found in 5% to 85% of patients with COPD.24–27
Based on the prevalence of COPD in the general population (about 10%12) and that of sleep-disordered breathing (about 5% to 10%17), the expected prevalence of overlap syndrome in people over age 40 may be 0.5% to 1%.28 In a more inclusive estimate with “subclinical” forms of overlap syndrome—ie, OSA defined as an apnea-hypopnea index of 5 or more (about 25% of the population17) and COPD Global initiative for Chronic Obstructive Lung Disease (GOLD) stage 1 (16.8% in the National Health and Nutrition Education Survey12)—the expected prevalence of overlap is around 4%. Some studies found a higher prevalence of COPD in OSA patients than in the general population,21,29 while others did not.22,28,30 The studies differed in how they defined sleep-disordered breathing.
Larger studies are needed to better assess the true prevalence of sleep-disordered breathing in COPD. They should use more sensitive measures of airflow and standardized definitions of sleep-disordered breathing and should include patients with more severe COPD.
Fatigue and insomnia are common in COPD
Fatigue is strongly correlated with declining lung function, low exercise tolerance, and impaired quality of life in COPD.31 Factors that contribute to fatigue include dyspnea, depression, and impaired sleep.32 Some suggest that at least half of COPD patients have sleep complaints such as insomnia, sleep disruption, or sleep fragmentation.33 Insomnia, difficulty falling asleep, and early morning awakenings are the most common complaints (30%–70% of patients) and are associated with daytime fatigue.34 Conversely, comorbid OSA can contribute to fatigue and maintenance-type insomnia (ie, difficulty staying asleep and returning to sleep).
Multiple mechanisms of hypoxemia in overlap syndrome
Oxygenation abnormalities and increased work of breathing contribute to the pathophysiology of overlap syndrome. In patients with COPD, oxygenation during wakefulness is a strong predictor of gas exchange during sleep.35 Further, patients with overlap syndrome tend to have more severe hypoxia during sleep than patients with isolated COPD or OSA at rest or during exercise.36
In overlap syndrome, hypoxemia is the result of several mechanisms:
- Loss of upper airway muscle tone from intermittent episodes of obstructive apnea and hypopnea leads to upper airway collapse during sleep, particularly during REM sleep, increasing the severity of OSA.37
- Reductions in functional residual capacity from lying in the recumbent position and during REM sleep render patients with COPD more vulnerable, as compensatory use of accessory muscles to maintain near-normal ventilation in a hyperinflated state becomes impaired.37
- Alterations in pulmonary ventilation-perfusion matching may lead to altered carbon dioxide homeostasis and impaired oxygenation in patients with emphysema.
- Circadian variation in lower airway caliber may also be observed, in parallel with the bronchoconstriction caused by increased nocturnal vagotonia.
- Hypercapnia (Paco2 ≥ 45 mm Hg) may lead to overall reduced responsiveness of respiratory muscles and to a blunted response of respiratory centers to low oxygen and high carbon dioxide levels.38 Thus, hypercapnia is a better predictor of the severity of nocturnal hypoxemia than hypoxemia developing during exercise.39
In a person who is at near-maximal ventilatory capacity, even a mild increase in upper airway resistance (as seen with snoring, upper airway resistance syndrome, or OSA) increases the work of breathing. This phenomenon can lead to early arousals even before significant oxyhemoglobin desaturation occurs.
Normally, inspiratory flow limitation is counteracted by increasing inspiratory time to maintain ventilation. Patients with COPD may not be able to do this, however, as they need more time to breathe out due to narrowing of their lower airways.40 The inability to compensate for upper airway resistance, similar to the increased work of breathing seen with exercise, may lead to early arousals and increased sleep fragmentation.
Consequences of overlap syndrome
Patients with overlap syndrome appear to have higher morbidity and mortality rates than those with COPD or sleep-disordered breathing alone.
Cor pulmonale. Nighttime hypoxia is more severe and persistent in overlap syndrome than with COPD or OSA alone. This may contribute to more significant pulmonary hypertension and to the development of cor pulmonale, in which the right ventricle is altered in structure (eg, hypertrophied, dilated) or reduced in function, or both, from severe pulmonary hypertension.
In contrast to right ventricular failure due to disorders of the left heart, cor pulmonale is a result of diseases of the vasculature (eg, idiopathic pulmonary arterial hypertension), lung parenchyma (eg, COPD), upper airway (eg, OSA), or chest wall (eg, severe kyphoscoliosis). COPD is the most common cause of cor pulmonale in the United States, accounting for up to 30% of cases of cor pulmonale.41–45 In OSA, cor pulmonale is seen in up to 20% of cases,43 while in overlap syndrome cor pulmonale is encountered even more often (ie, in up to 80%); these patients have a dismal 5-year survival rate of about 30%.46
Obesity hypoventilation syndrome is characterized by obesity (body mass index ≥ 30 kg/m2) and daytime hypercapnia (Paco2 ≥ 45 mm Hg) that cannot be fully attributed to an underlying cardiopulmonary or neurologic condition.18 Hypercapnia worsens during sleep (especially during REM sleep) and is often associated with severe arterial oxygen desaturation. Up to 90% of patients with obesity hypoventilation syndrome have comorbid OSA, and the rest generally have sleep-related hypoventilation, particularly during REM sleep.
In patients with obesity hypoventilation syndrome, daytime hypercapnia may improve or even normalize with adequate positive airway pressure treatment and sustained adherence to treatment.18 Many patients with obesity hypoventilation syndrome respond to CPAP or bilevel positive airway pressure (BPAP), with improvement in daytime Paco2. However, normalization of daytime Paco2 occurs only in a subgroup of patients. In contrast, treatment with oxygen therapy alone may worsen hypercapnia.
Oxygen therapy for pure COPD, but maybe not for overlap syndrome
Continuous oxygen therapy reduces mortality in COPD,47,48 but the duration and severity of hypoxemia that warrant oxygen therapy are less clear. Oxygen therapy in hypoxemic patients has been shown to improve sleep quality and reduce arousals.49
Indications for oxygen treatment of nocturnal hypoxemia are generally based on Medicare guidelines:
- At least 5 minutes of sleep with peripheral oxygen saturation ≤ 88% or Pao2 ≤ 55 mm Hg, or
- A decrease in Pao2 of more than 10 mm Hg or in peripheral oxygen saturation of more than 5% for at least 5 minutes of sleep and associated with signs or symptoms reasonably attributable to hypoxemia (group I criteria), or
- At least 5 minutes of sleep with peripheral oxygen saturation ≥ 89% or Pao2 56 to 59 mm Hg and pedal edema, pulmonary hypertension, cor pulmonale, or erythrocytosis (group II criteria).50
Approximately 47% of COPD patients who are hypoxemic during the day spend about 30% of sleep time with an oxygen saturation less than 90%, even while on continuous oxygen therapy.51 Current recommendations for nocturnal oxygen therapy are to increase the oxygen concentration by 1 L/minute above the baseline oxygen flow rate needed to maintain an oxygen saturation higher than 90% during resting wakefulness, using a nasal cannula or face mask.52
Caveat. In overlap syndrome, supplemental oxygen may prolong the duration of apnea episodes and worsen hypercapnia.
Positive airway pressure for OSA
Positive airway pressure therapy improves cardiovascular outcomes in OSA.53 Several studies54–58 compared the effectiveness of CPAP vs BPAP as initial therapy for OSA but did not provide enough evidence to favor one over the other in this setting. Similarly, the results are mixed for the use of fixed or auto-adjusting BPAP as salvage therapy in patients who cannot tolerate CPAP.59–61
In overlap syndrome, CPAP or BPAP with or without supplemental oxygen has been investigated in several studies.26,62–65 In general, the mortality rate of COPD patients who require oxygen therapy is quite high.47,66 In hypoxemic COPD patients with moderate to severe sleep-disordered breathing, the 5-year survival rate was 71% in those treated with CPAP plus oxygen, vs 26% in those on oxygen alone, independent of baseline postbronchodilator FEV1.67
There is no specific FEV1 cutoff for prescribing CPAP. In general, daytime hypercapnia and nocturnal hypoxemia despite supplemental oxygen therapy are indications for BPAP therapy, regardless of the presence of OSA. Whether noninvasive nocturnal ventilation for COPD patients who do not have OSA improves long-term COPD outcomes is not entirely clear.65,68,69
Adding nocturnal BPAP in spontaneous timed mode to pulmonary rehabilitation for severe hypercapnic COPD was found to improve quality of life, mood, dyspnea, gas exchange, and decline in lung function.70 Other studies noted that COPD patients hospitalized with respiratory failure who were randomized to noninvasive nocturnal ventilation plus oxygen therapy as opposed to oxygen alone experienced improvement in health-related quality of life and reduction in intensive-care-unit length of stay but no difference in mortality or subsequent hospitalizations.69 In stable hypercapnic COPD patients without OSA, there is no clear evidence that nocturnal noninvasive ventilation lessens the risk of death despite improved daytime gas exchange,71,72 but additional long-term studies are needed.
Lung volume reduction surgery, a procedure indicated for highly selected patients with severe COPD, has been shown to reduce hyperinflation, improve nocturnal hypoxemia, and improve total sleep time and sleep efficiency in patients without sleep-disordered breathing.73 More studies are needed to determine if reduction in lung hyperinflation has an impact on the occurrence of OSA and on morbidity related to sleep-disordered breathing.
Benefit of CPAP in overlap syndrome
In a nonrandomized study, Marin et al62 found that overlap syndrome is associated with an increased risk of death and hospitalization due to COPD exacerbations. CPAP therapy was associated with improved survival rates and decreased hospitalization rates in these patients.
Stanchina et al,74 in a post hoc analysis of an observational cohort, assessed the outcomes of 227 patients with overlap syndrome. Greater use of CPAP was found to be associated with lower mortality rates.
Jaoude et al75 found that hypercapnic patients with overlap syndrome who were adherent to CPAP therapy had a lower mortality rate than nonadherent hypercapnic patients (P = .04). In a multivariate analysis, the comorbidity index was the only independent predictor of mortality in normocapnic patients with overlap syndrome, while CPAP adherence was associated with improved survival.
Lastly, patients with overlap syndrome tend to need more healthcare and accrue higher medical costs than patients with COPD alone. An analysis of a state Medicaid database that included COPD patients showed that beneficiaries with overlap syndrome spent at least $4,000 more in medical expenditures than beneficiaries with “lone” COPD.24
In conclusion, CPAP is the first line of therapy for overlap syndrome, while daytime hypercapnia or nocturnal hypoxemia despite supplemental oxygen therapy are indications for nocturnal BPAP therapy, regardless of whether patients have OSA.
OSA AND ASTHMA (ALTERNATIVE OVERLAP SYNDROME)
Epidemiology and clinical features
The coexistence of asthma and OSA can begin in childhood and continue throughout adult life. A higher prevalence of lifetime asthma and OSA has been noted in children of racial and ethnic minorities, children of lower socioeconomic status, and those with atopy.76
In a pediatric asthma clinic, it was noted that 12 months into structured asthma management and optimization, children with sleep-disordered breathing were nearly four times more likely to have severe asthma at follow-up, even after adjusting for obesity, race, and gender.77
In adult patients with OSA, the prevalence of asthma is about 35%.78 Conversely, people with asthma are at higher risk of OSA. High risk of OSA was more prevalent in a group of patients with asthma than in a general medical clinic population (39.5% vs 27.2%, P < .05).79
Analysis of a large prospective cohort found that asthma was a risk factor for new-onset OSA. The incidence of OSA over 4 years in patients with self-reported asthma was 27%, compared with 16% without asthma. The relative risk adjusted for risk factors such as body mass index, age, and gender was 1.39 (95% confidence interval [CI] 15%–19%).80
Patients with asthma who are at high risk of OSA are more likely to have worse daytime and nighttime asthma symptoms. Interestingly, patients who are diagnosed with OSA and treated with CPAP seem to have better asthma control.
Patients with asthma who are more likely to have OSA are women (odds ratio [OR] 2.1), have greater asthma severity (OR 1.6), have gastroesophageal reflux disease (OR 2.7), and use inhaled corticosteroids (OR 4.0).81 These associations are different than the traditional, population-wide risk factors for OSA, such as male sex, excess body weight, and nocturnal nasal congestion.82
OSA also worsens asthma control. Teodorescu et al15 found that severe asthma was more frequent in older asthma patients (ages 60–75, prevalence 49%) than in younger patients (ages 18–59, 39%). Older adults with OSA were seven times as likely to have severe asthma (OR 6.6), whereas young adults with sleep apnea were only three times as likely (OR 2.6).
In a group of patients with difficult-to-treat asthma, OSA was significantly associated with frequent exacerbations (OR 3.4), an association similar in magnitude to that of psychological conditions (OR 10.8), severe sinus disease (OR 3.7), recurrent respiratory tract infections (OR 6.9), and gastroesophageal reflux disease (OR 4.9).83 More than half of the patients had at least three of these comorbid conditions.
Sleep quality can greatly affect asthma control, and its importance is often underestimated. Patients with severe asthma have worse sleep quality than patients with milder asthma or nonasthmatic patients, even after excluding patients with a high risk of OSA, patients on CPAP therapy, and patients with a history of gastroesophageal reflux disease. Furthermore, regardless of asthma severity, sleep quality is a significant predictor of asthma-related quality of life, even after accounting for body mass index, daytime sleepiness, and gastroesophageal reflux disease.84
Pathophysiology of alternative overlap syndrome
Sleep significantly affects respiratory pathophysiology in asthma. The underlying mechanisms include physical and mechanical stressors, neurohormonal changes, hypoxia, confounding medical conditions, and local and systemic inflammatory changes.
Patients with nocturnal asthma experience more pronounced obstruction when sleep-deprived, suggesting that sleep loss may contribute to worsening airflow limitation.14 Although changes in pulmonary mechanics and lung volumes may also have a role, volume-dependent airway narrowing does not appear to account for all observed nocturnal increases in airway resistance. Intrathoracic blood pooling may also contribute to nocturnal bronchoconstriction through stimulation of pulmonary C fibers and increased bronchial wall edema, a mechanism that may be similar to the “cardiac asthma” seen in left ventricular dysfunction.
Early studies of sleep-disordered breathing demonstrated that patients with asthma were breathing more irregularly (with hypopnea, apnea, and hyperpnea) in REM sleep than those without asthma.85 Interestingly, REM-related hypoxia has also been noted in children with asthma.86 This may be related to the increased cholinergic outflow that occurs during REM sleep, which in turn modulates the caliber and reactivity of the lower airways.
Physical changes such as upper airway collapse and reduced pharyngeal cross-sectional area may cause further mechanical strain.87 This can further propagate airway inflammation, alter airway mucosal muscle fibers, and stimulate neural reflexes, thereby increasing cholinergic tone and bronchoconstriction. Furthermore, heightened negative intrathoracic pressure during obstructive episodes can increase nocturnal pulmonary blood pooling.14 Hypoxia itself can augment airway hyperresponsiveness via vagal pathways or carotid body receptors, increasing reactive oxygen species and inflammatory mediators. Local inflammation can “spill over” into systemic inflammatory changes, while alterations in airway inflammatory markers in asthma seem to follow a circadian rhythm, in parallel with the nocturnal worsening of the asthma symptoms.88 Finally, altered sleep may be related to other comorbid conditions, such as gastroesophageal reflux disease, insomnia, and restless leg syndrome.
Management and outcomes of alternative overlap syndrome
Despite optimization of asthma management, OSA can still significantly affect asthma control and symptoms.84
Interestingly, medications that reduce airway inflammation (eg, corticosteroids) may promote OSA. This occurrence cannot be fully explained by an increase in body mass, as more respiratory disturbances occur during sleep with continuous corticosteroid treatment even without increases in body mass index.87 Therefore, these associations may be related to upper airway myopathy caused by the treatment, a small pharynx, facial dysmorphisms, or fat deposition.89
Does CPAP improve asthma?
OSA is often unrecognized in patients with asthma, and treating it can have an impact on asthma symptoms.
CPAP therapy has not been shown to significantly change airway responsiveness or lung function, but it has been noted to significantly improve both OSA-related and asthma-related quality of life and reduce the use of rescue bronchodilators.3,90 CPAP has demonstrated improvement of quality of life that positively correlated with body weight and apnea-hypopnea index at baseline, suggesting that asthmatic patients with greater obesity or worse OSA may benefit most from aggressive management.90
However, CPAP should be used only if the patient has confirmed OSA. Empiric use of CPAP without a diagnosis of OSA was poorly tolerated and failed to improve asthma symptoms or lung function.91 More importantly, using CPAP in a patient who does not have OSA may contribute to further sleep disruption.91
Second-line treatments such as mandibular advancing devices and airway or bariatric surgery have not yet been studied in alternative overlap syndrome.
A multidimensional assessment of asthma
The Western world is experiencing an epidemic of obesity and of asthma. Obesity contributes to the pathogenesis of OSA by altering the anatomy and collapsibility of the upper airway, affecting ventilatory control and increasing respiratory workload. Another paradigm, supported by some evidence, is that OSA itself may contribute to the development of obesity. Both OSA and obesity lead to activation of inflammatory biologic cascades, which are likely the pathogenic mechanisms for their cardiovascular and metabolic consequences. As such, early recognition of OSA is important, as effective treatments are available.
In some patients, obesity may cause asthma, as obesity precedes the onset of asthma in a significant proportion of patients, and bariatric surgery for morbid obesity may resolve asthma. The obese asthma phenotype seems to include chronic rhinosinusitis, gastroesophageal reflux disease, poorer asthma control, limited responsiveness to corticosteroids, and even different sets of biomarkers (eg, neutrophilic airway inflammation). A cohort of obese patients with poor asthma control demonstrated significant improvement in asthma symptoms, quality of life, and airway reactivity after weight loss from bariatric surgery.92
To improve our knowledge about airway disease phenotypes and endotypes and their response to therapy, we propose taking a multidimensional, structured assessment of all patients with asthma, using a schema we call “ABCD-3P-PQRST” (Table 2).
The purpose of using this type of system in clinics and research is to capture the multidimensionality of the disease and better develop future individualized therapeutic strategies by employing the latest advances in systems biology and computational methods such as cluster and principal component analysis.
Multidimensional assessments addressing airway problems such as asthma, COPD, OSA, other comorbidities and risk factors, and personalized management plans will need to be the basis of future therapeutic interventions. Increased attention to the complications of asthma and obstructive airway and lung diseases in our patients is imperative, specifically to develop effective systems of care, appropriate clinical guidelines, and research studies that lead to improved health outcomes.
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985; 6:651–661.
- Ioachimescu OC, Teodorescu M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology 2013; 18:421–431.
- Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respiratory Med 2005; 99:529–534.
- Kim MY, Jo EJ, Kang SY, et al. Obstructive sleep apnea is associated with reduced quality of life in adult patients with asthma. Ann Allergy Asthma Immunol 2013; 110:253–257.
- Teodorescu M, Polomis DA, Teodorescu MC, et al. Association of obstructive sleep apnea risk or diagnosis with daytime asthma in adults. J Asthma 2012; 49:620–628.
- Puthalapattu S, Ioachimescu OC. Asthma and obstructive sleep apnea: clinical and pathogenic interactions. J Investig Med 2014; 62:665–675.
- National Institutes of Health. National Heart, Lung, and Blood Institute. NHLBI Factbook, Fiscal Year 2007. Chapter 4. Disease Statistics. www.nhlbi.nih.gov/about/factbook-07/chapter4.htm. Accessed November 11, 2015.
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365.
- WHO. World Health Organization: Asthma. Fact sheet No 307. www.who.int/mediacentre/factsheets/fs307/en/. Accessed November 11, 2015.
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004; 59:469–478.
- WHO. Chronic obstructive pulmonary disease (COPD). Fact sheet No 315. www.who.int/mediacentre/factsheets/fs315/en/index.html. 2011. Accessed November 11, 2015.
- Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988–1994 to 2007–2010. Chest 2013; 143:1395–1406.
- Orie N, Sluiter H, de Vries K, Tammeling G, Witkop J. The host factor in bronchitis. Paper presented at: Bronchitis—an international symposium 1961; Assen, Netherlands.
- Ballard RD. Sleep, respiratory physiology, and nocturnal asthma. Chronobiol Int 1999; 16:565–580.
- Teodorescu M, Polomis DA, Gangnon RE, et al. Asthma control and its relationship with obstructive sleep apnea (OSA) in older adults. Sleep Disord 2013; 2013:251567.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- International Classification of Sleep Disorders, 3rd ed.: Diagnostic and coding manual. Darien, Illinois: American Academy of Sleep Medicine. 2014.
- Lopez-Acevedo MN, Torres-Palacios A, Elena Ocasio-Tascon M, Campos-Santiago Z, Rodriguez-Cintron W. Overlap syndrome: an indication for sleep studies? A pilot study. Sleep Breath 2009; 13:409–413.
- Scharf C, Li P, Muntwyler J, et al. Rate-dependent AV delay optimization in cardiac resynchronization therapy. PACE 2005; 28:279–284.
- Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med 1995;151:82–86.
- Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration 2005; 72:142–149.
- Fletcher EC. Chronic lung disease in the sleep apnea syndrome. Lung 1990; 168(suppl):751–761.
- Shaya FT, Lin PJ, Aljawadi MH, Scharf SM. Elevated economic burden in obstructive lung disease patients with concomitant sleep apnea syndrome. Sleep Breath 2009; 13:317–323.
- Larsson LG, Lindberg A, Franklin KA, Lundbäck B; Obstructive Lung Disease in Northern Sweden Studies. Obstructive sleep apnoea syndrome is common in subjects with chronic bronchitis. Report from the Obstructive Lung Disease in Northern Sweden studies. Respiration 2001; 68:250–255.
- Machado MC, Vollmer WM, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Resp J 2010; 35:132–137.
- Guilleminault C, Cummiskey J, Motta J. Chronic obstructive airflow disease and sleep studies. Am Rev Respir Dis 1980; 122:397–406.
- Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5:237–241.
- Bradley TD, Rutherford R, Lue F, et al. Role of diffuse airway obstruction in the hypercapnia of obstructive sleep apnea. Am Rev Respir Dis 1986; 134:920–924.
- Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med 2003; 167:7–14.
- Breslin E, van der Schans C, Breukink S, et al. Perception of fatigue and quality of life in patients with COPD. Chest 1998; 114:958–964.
- Kapella MC, Larson JL, Patel MK, Covey MK, Berry JK. Subjective fatigue, influencing variables, and consequences in chronic obstructive pulmonary disease. Nurs Res 2006; 55:10–17.
- Klink M, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest 1987; 91:540–546.
- Bellia V, Catalano F, Scichilone N, et al. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA study. Sleep 2003; 26:318–323.
- Connaughton JJ, Catterall JR, Elton RA, Stradling JR, Douglas NJ. Do sleep studies contribute to the management of patients with severe chronic obstructive pulmonary disease? Am Rev Respir Dis 1988; 138:341–344.
- Mulloy E, McNicholas WT. Ventilation and gas exchange during sleep and exercise in severe COPD. Chest 1996; 109:387–394.
- Johnson MW, Remmers JE. Accessory muscle activity during sleep in chronic obstructive pulmonary disease. J Appl Physiol 1984; 57:1011–1017.
- Douglas NJ, White DP, Pickett CK, Weil JV, Zwillich CW. Respiration during sleep in normal man. Thorax 1982; 37:840–844.
- Mulloy E, Fitzpatrick M, Bourke S, O’Regan A, McNicholas WT. Oxygen desaturation during sleep and exercise in patients with severe chronic obstructive pulmonary disease. Respir Med 1995; 89:193–198.
- Herpel LB, Brown CD, Goring KL, et al. COPD cannot compensate for upper airway obstruction during sleep (abstract). Am J Respir Crit Care Med 2007; 175:A71.
- MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part 2. Am J Respir Crit Care Med 1994; 150:1158–1168.
- MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part 1. Am J Respir Crit Care Med 1994; 150:833–852.
- Budev MM, Arroliga AC, Wiedemann HP, Matthay RA. Cor pulmonale: an overview. Semin Respir Crit Care Med 2003; 24:233–244.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 suppl):D34–D41.
- Naeije R. Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2:20–22.
- Rasche K, Orth M, Kutscha A, Duchna HW. [Pulmonary diseases and heart function]. In German. Internist (Berl) 2007; 48:276–282.
- Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 1980; 93:391–398.
- Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med 2005; 165:2408–2413.
- Calverley PM, Brezinova V, Douglas NJ, Catterall JR, Flenley DC. The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis 1982; 126:206–210.
- Centers for Medicare and Medicaid Services. National coverage determination (NCD) for home use of oxygen (240.2). www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=169&ncdver=1&NCAId=169&NcaName=Home+Use+of+Oxygen&IsPopup=y&bc=AAAAAAAAIAAA&. Accessed November 11, 2015.
- Plywaczewski R, Sliwinski P, Nowinski A, Kaminski D, Zielinski J. Incidence of nocturnal desaturation while breathing oxygen in COPD patients undergoing long-term oxygen therapy. Chest 2000; 117:679–683.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breathing 2007; 11:117–124.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Marin JM, DeAndres R, Alonso J, Sanchez A, Carrizo S. Long term mortality in the overlap syndrome. Eur Resp J 2008; 32(suppl 52):P865.
- Reeves-Hoche MK, Hudgel DW, Meck R, Witteman R, Ross A, Zwillich CW. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med 1995; 151:443–449.
- Gay PC, Herold DL, Olson EJ. A randomized, double-blind clinical trial comparing continuous positive airway pressure with a novel bilevel pressure system for treatment of obstructive sleep apnea syndrome. Sleep 2003; 26:864–869.
- Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath 2012; 16:773–779.
- Randerath WJ, Galetke W, Ruhle KH. Auto-adjusting CPAP based on impedance versus bilevel pressure in difficult-to-treat sleep apnea syndrome: a prospective randomized crossover study. Med Sci Monit 2003; 9:CR353–CR358.
- Schwartz SW, Rosas J, Iannacone MR, Foulis PR, Anderson WM. Correlates of a prescription for bilevel positive airway pressure for treatment of obstructive sleep apnea among veterans. J Clin Sleep Med 2013; 9:327–335.
- Gentina T, Fortin F, Douay B, et al. Auto bi-level with pressure relief during exhalation as a rescue therapy for optimally treated obstructive sleep apnoea patients with poor compliance to continuous positive airways pressure therapy--a pilot study. Sleep Breathing 2011; 15:21–27.
- Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clinical Sleep Med 2007; 3:706–712.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 2010; 182:325–331.
- de Miguel J, Cabello J, Sanchez-Alarcos JM, Alvarez-Sala R, Espinos D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath 2002; 6:3–10.
- Mansfield D, Naughton MT. Effects of continuous positive airway pressure on lung function in patients with chronic obstructive pulmonary disease and sleep disordered breathing. Respirology 1999; 4:365–370.
- McEvoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet 1981; 1:681–686.
- Machado MC, Vollmer WM, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J 2010; 35:132–137.
- Casanova C, Celli BR, Tost L, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000; 118:1582–1590.
- Clini E, Sturani C, Rossi A, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002; 20:529–538.
- Duiverman ML, Wempe JB, Bladder G, et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomized controlled trial. Respir Res 2011; 12:112.
- Gay PC, Hubmayr RD, Stroetz RW. Efficacy of nocturnal nasal ventilation in stable, severe chronic obstructive pulmonary disease during a 3-month controlled trial. Mayo Clin Proc 1996; 71:533–542.
- Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med 1995; 152:538–544.
- Krachman SL, Chatila W, Martin UJ, et al. Effects of lung volume reduction surgery on sleep quality and nocturnal gas exchange in patients with severe emphysema. Chest 2005; 128:3221–3228.
- Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med 2013; 9:767–772.
- Jaoude P, Kufel T, El-Solh AA. Survival benefit of CPAP favors hypercapnic patients with the overlap syndrome. Lung 2014; 192:251–258.
- Ramagopal M, Mehta A, Roberts DW, et al. Asthma as a predictor of obstructive sleep apnea in urban African-American children. J Asthma 2009; 46:895–899.
- Ross KR, Storfer-Isser A, Hart MA, et al. Sleep-disordered breathing is associated with asthma severity in children. J Ped 2012; 160:736–742.
- Alharbi M, Almutairi A, Alotaibi D, Alotaibi A, Shaikh S, Bahammam AS. The prevalence of asthma in patients with obstructive sleep apnoea. Prim Care Respir J 2009; 18:328–330.
- Auckley D, Moallem M, Shaman Z, Mustafa M. Findings of a Berlin Questionnaire survey: comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep Med 2008; 9:494–499.
- Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. JAMA 2015; 313:156–164.
- Teodorescu M, Polomis DA, Hall SV, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest 2010; 138:543–550.
- Larsson LG, Lindberg A, Franklin KA, Lundback B. Gender differences in symptoms related to sleep apnea in a general population and in relation to referral to sleep clinic. Chest 2003; 124:204–211.
- ten Brinke A, Sterk PJ, Masclee AA, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J 2005; 26:812–818.
- Luyster FS, Teodorescu M, Bleecker E, et al. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep Breath 2012; 16:1129–1137.
- Catterall JR, Douglas NJ, Calverley PM, et al. Irregular breathing and hypoxaemia during sleep in chronic stable asthma. Lancet 1982; 1:301–304.
- Perez GF, Gutierrez MJ, Huseni S, et al. Oximetry signal processing identifies REM sleep-related vulnerability trait in asthmatic children. Sleep Disord 2013; 2013:406157.
- Yigla M, Tov N, Solomonov A, Rubin AH, Harlev D. Difficult-to-control asthma and obstructive sleep apnea. J Asthma 2003; 40:865–871.
- Kelly EA, Houtman JJ, Jarjour NN. Inflammatory changes associated with circadian variation in pulmonary function in subjects with mild asthma. Clin Exper Allergy 2004; 34:227–233.
- Bohadana AB, Hannhart B, Teculescu DB. Nocturnal worsening of asthma and sleep-disordered breathing. J Asthma 2002; 39:85–100.
- Lafond C, Series F, Lemiere C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. Eur Respir J 2007; 29:307–311.
- Martin RJ, Pak J. Nasal CPAP in nonapneic nocturnal asthma. Chest 1991; 100:1024–1027.
- Dixon AE, Pratley RE, Forgione PM, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol 2011; 128:508–515 e501–502.
Many patients who have obstructive lung disease, ie, chronic obstructive pulmonary disease (COPD) or asthma, also have obstructive sleep apnea (OSA), and vice versa.
The combination of COPD and OSA was first described almost 30 years ago by Flenley, who called it “overlap syndrome.”1 At that time, he recommended that a sleep study be considered in all obese patients with COPD who snore and in those who have frequent headaches after starting oxygen therapy. In the latter group, he doubted that nocturnal oxygen was the correct treatment. He also believed that the outcomes in patients with overlap syndrome were worse than those in patients with COPD or OSA alone. These opinions remain largely valid today.
We now also recognize the combination of asthma and OSA (alternative overlap syndrome) and collectively call both combinations obstructive lung disease-obstructive sleep apnea (OLDOSA) syndrome.2 Interestingly, these relationships are likely bidirectional, with one condition aggravating or predisposing to the other.
Knowing that a patient has one of these overlap syndromes, one can initiate continuous positive airway pressure (CPAP) therapy, which can improve clinical outcomes.3–6 Therefore, when evaluating a patient with asthma or COPD, one should consider OSA using a validated questionnaire and, if the findings suggest the diagnosis, polysomnography. Conversely, it is prudent to look for comorbid obstructive lung disease in patients with OSA, as interactions between upper and lower airway dysfunction may lead to distinctly different treatment and outcomes.
Here, we briefly review asthma and COPD, explore shared risk factors for sleep-disordered breathing and obstructive lung diseases, describe potential pathophysiologic mechanisms explaining these associations, and highlight the importance of recognizing and individually treating the overlaps of OSA and COPD or asthma.
COPD AND ASTHMA ARE VERY COMMON
About 10% of the US population have COPD,7 a preventable and treatable disease mainly caused by smoking, and a leading cause of sickness and death worldwide.8,9
About 8% of Americans have asthma,7 which has become one of the most common chronic conditions in the Western world, affecting about 1 in 7 children and about 1 in 12 adults. The World Health Organization estimates that 235 million people suffer from asthma worldwide, and by 2025 this number is projected to rise to 400 million.10,11
The prevalence of these conditions in a particular population depends on the frequency of risk factors and associated morbidities, including OSA. These factors may allow asthma or COPD to arise earlier or have more severe manifestations.8,12
Asthma and COPD: Similarities and differences
Asthma and COPD share several features. Both are inflammatory airway conditions triggered or perpetuated by allergens, viral infection, tobacco smoke, products of biomass or fossil fuel combustion, and other substances. In both diseases, airflow is “obstructed” or limited, with a low ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC). Symptoms can also be similar, with dyspnea, cough, wheezing, and chest tightness being the most frequent complaints. The similarities support the theory proposed by Orie et al13 (the “Dutch hypothesis”) that asthma and COPD may actually be manifestations of the same disease.
But there are also differences. COPD is strongly linked to cigarette smoking and has at least three phenotypes:
- Chronic bronchitis, defined clinically by cough and sputum production for more than 3 months per year for 2 consecutive years
- Emphysema, characterized anatomically by loss of lung parenchyma, as seen on tomographic imaging or examination of pathologic specimens
- A mixed form with bronchitic and emphysematous features, which is likely the most common.
Particularly in emphysematous COPD, smoking predisposes patients to gas-exchange abnormalities and low diffusing capacity for carbon monoxide.
In asthma, symptoms may be more episodic, the age of onset is often younger, and atopy is common, especially in allergic asthma. These episodic symptoms may correlate temporally with measurable airflow reversibility (≥ 12% and ≥ 200 mL improvement in FVC or in FEV1 after bronchodilator challenge).
However, the current taxonomy does not unequivocally divide obstructive lung diseases into asthma and COPD, and major features such as airway hyperresponsiveness, airflow reversibility, neutrophilic or CD8 lymphocytic airway inflammation, and lower concentration of nitric oxide in the exhaled air may be present in different phenotypes of both conditions (Table 1).
AIRFLOW IN OBSTRUCTIVE LUNG DISEASES AND DURING SLEEP
Normal airflow involves a complex interplay between airway resistance and elastic recoil of the entire respiratory system, including the airways, the lung parenchyma, and the chest wall (Figure 1).
In asthma and COPD, resistance to airflow is increased, predominantly in the upper airways (nasal passages, pharynx, and larynx) and in the first three or four subdivisions of the tracheobronchial tree. The problem is worse during exhalation, when elastic recoil of the lung parenchyma and chest wall also increases airway resistance, reduces airway caliber, and possibly even constricts the bronchi. This last effect may occur either due to mass loading of the bronchial smooth muscles or to large intrathoracic transmural pressure shifts that may increase extravasation of fluid in the bronchial walls, especially with higher vascular permeability in inflammatory conditions.
Furthermore, interactions between the airway and parenchyma and between the upper and lower airways, as well as radial and axial coupling of these anatomic and functional components, contribute to complex interplay between airway resistance and parenchymal-chest wall elastic energy—stretch or recoil.
The muscles of the upper and lower airway may not work together due to the loss of normal lung parenchyma (as in emphysema) or to the acute inflammation in the small airways and adjacent parenchyma (as in severe asthma exacerbations). This loss of coordination makes the upper airway more collapsible, a feature of OSA.
Additionally, obesity, gastroesophageal reflux, disease chronic rhinitis, nasal polyposis, and acute exacerbations of chronic systemic inflammation all contribute to more complex interactions between obstructive lung diseases and OSA.6
Sleep affects breathing, particularly in patients with respiratory comorbidities, and sleep-disordered breathing causes daytime symptoms and worsens quality of life.1,13–15 During sleep, respiratory centers become less sensitive to oxygen and carbon dioxide; breathing becomes more irregular, especially during rapid eye movement (REM) sleep; the chest wall moves less, so that the tidal volume and functional residual capacity are lower; sighs, yawns, and deep breaths become limited; and serum carbon dioxide concentration may rise.
OBSTRUCTIVE SLEEP APNEA
The prevalence of OSA, a form of sleep-disordered breathing characterized by limitation of inspiratory and (to a lesser degree) expiratory flow, has increased significantly in recent years, in parallel with the prevalence of its major risk factor, obesity.
OSA is generally defined as an apnea-hypopnea index of 5 or higher, ie, five or more episodes of apnea or hypopnea per hour.
OSA syndrome, ie, an apnea-hypopnea index of 5 or higher and excessive daytime sleepiness (defined by an Epworth Sleepiness Scale score > 10) was found in the initial analysis of the Wisconsin Sleep cohort in 1993 to be present in about 2% of women and 4% of men.16 A more recent longitudinal analysis showed a significant increase—for example, in people 50 to 70 years old the prevalence was up to 17.6% in men and 7.5% in women.17
Upper airway resistance syndrome, a milder form of sleep-disordered breathing, is now included under the diagnosis of OSA, as its pathophysiology is not significantly different.18
In the next section, we discuss what happens when OSA overlaps with COPD (overlap syndrome) and with asthma (“alternative overlap syndrome”)2,8 (Figure 2).
OSA AND COPD (OVERLAP SYNDROME)
Flenley1 hypothesized that patients with COPD in whom supplemental oxygen worsened hypercapnia may also have OSA and called this association overlap syndrome.
How common is overlap syndrome?
Since both COPD and OSA are prevalent conditions, overlap syndrome may also be common.
The reported prevalence of overlap syndrome varies widely, depending on the population studied and the methods used. In various studies, COPD was present in 9% to 56% of patients with OSA,19–23 and OSA was found in 5% to 85% of patients with COPD.24–27
Based on the prevalence of COPD in the general population (about 10%12) and that of sleep-disordered breathing (about 5% to 10%17), the expected prevalence of overlap syndrome in people over age 40 may be 0.5% to 1%.28 In a more inclusive estimate with “subclinical” forms of overlap syndrome—ie, OSA defined as an apnea-hypopnea index of 5 or more (about 25% of the population17) and COPD Global initiative for Chronic Obstructive Lung Disease (GOLD) stage 1 (16.8% in the National Health and Nutrition Education Survey12)—the expected prevalence of overlap is around 4%. Some studies found a higher prevalence of COPD in OSA patients than in the general population,21,29 while others did not.22,28,30 The studies differed in how they defined sleep-disordered breathing.
Larger studies are needed to better assess the true prevalence of sleep-disordered breathing in COPD. They should use more sensitive measures of airflow and standardized definitions of sleep-disordered breathing and should include patients with more severe COPD.
Fatigue and insomnia are common in COPD
Fatigue is strongly correlated with declining lung function, low exercise tolerance, and impaired quality of life in COPD.31 Factors that contribute to fatigue include dyspnea, depression, and impaired sleep.32 Some suggest that at least half of COPD patients have sleep complaints such as insomnia, sleep disruption, or sleep fragmentation.33 Insomnia, difficulty falling asleep, and early morning awakenings are the most common complaints (30%–70% of patients) and are associated with daytime fatigue.34 Conversely, comorbid OSA can contribute to fatigue and maintenance-type insomnia (ie, difficulty staying asleep and returning to sleep).
Multiple mechanisms of hypoxemia in overlap syndrome
Oxygenation abnormalities and increased work of breathing contribute to the pathophysiology of overlap syndrome. In patients with COPD, oxygenation during wakefulness is a strong predictor of gas exchange during sleep.35 Further, patients with overlap syndrome tend to have more severe hypoxia during sleep than patients with isolated COPD or OSA at rest or during exercise.36
In overlap syndrome, hypoxemia is the result of several mechanisms:
- Loss of upper airway muscle tone from intermittent episodes of obstructive apnea and hypopnea leads to upper airway collapse during sleep, particularly during REM sleep, increasing the severity of OSA.37
- Reductions in functional residual capacity from lying in the recumbent position and during REM sleep render patients with COPD more vulnerable, as compensatory use of accessory muscles to maintain near-normal ventilation in a hyperinflated state becomes impaired.37
- Alterations in pulmonary ventilation-perfusion matching may lead to altered carbon dioxide homeostasis and impaired oxygenation in patients with emphysema.
- Circadian variation in lower airway caliber may also be observed, in parallel with the bronchoconstriction caused by increased nocturnal vagotonia.
- Hypercapnia (Paco2 ≥ 45 mm Hg) may lead to overall reduced responsiveness of respiratory muscles and to a blunted response of respiratory centers to low oxygen and high carbon dioxide levels.38 Thus, hypercapnia is a better predictor of the severity of nocturnal hypoxemia than hypoxemia developing during exercise.39
In a person who is at near-maximal ventilatory capacity, even a mild increase in upper airway resistance (as seen with snoring, upper airway resistance syndrome, or OSA) increases the work of breathing. This phenomenon can lead to early arousals even before significant oxyhemoglobin desaturation occurs.
Normally, inspiratory flow limitation is counteracted by increasing inspiratory time to maintain ventilation. Patients with COPD may not be able to do this, however, as they need more time to breathe out due to narrowing of their lower airways.40 The inability to compensate for upper airway resistance, similar to the increased work of breathing seen with exercise, may lead to early arousals and increased sleep fragmentation.
Consequences of overlap syndrome
Patients with overlap syndrome appear to have higher morbidity and mortality rates than those with COPD or sleep-disordered breathing alone.
Cor pulmonale. Nighttime hypoxia is more severe and persistent in overlap syndrome than with COPD or OSA alone. This may contribute to more significant pulmonary hypertension and to the development of cor pulmonale, in which the right ventricle is altered in structure (eg, hypertrophied, dilated) or reduced in function, or both, from severe pulmonary hypertension.
In contrast to right ventricular failure due to disorders of the left heart, cor pulmonale is a result of diseases of the vasculature (eg, idiopathic pulmonary arterial hypertension), lung parenchyma (eg, COPD), upper airway (eg, OSA), or chest wall (eg, severe kyphoscoliosis). COPD is the most common cause of cor pulmonale in the United States, accounting for up to 30% of cases of cor pulmonale.41–45 In OSA, cor pulmonale is seen in up to 20% of cases,43 while in overlap syndrome cor pulmonale is encountered even more often (ie, in up to 80%); these patients have a dismal 5-year survival rate of about 30%.46
Obesity hypoventilation syndrome is characterized by obesity (body mass index ≥ 30 kg/m2) and daytime hypercapnia (Paco2 ≥ 45 mm Hg) that cannot be fully attributed to an underlying cardiopulmonary or neurologic condition.18 Hypercapnia worsens during sleep (especially during REM sleep) and is often associated with severe arterial oxygen desaturation. Up to 90% of patients with obesity hypoventilation syndrome have comorbid OSA, and the rest generally have sleep-related hypoventilation, particularly during REM sleep.
In patients with obesity hypoventilation syndrome, daytime hypercapnia may improve or even normalize with adequate positive airway pressure treatment and sustained adherence to treatment.18 Many patients with obesity hypoventilation syndrome respond to CPAP or bilevel positive airway pressure (BPAP), with improvement in daytime Paco2. However, normalization of daytime Paco2 occurs only in a subgroup of patients. In contrast, treatment with oxygen therapy alone may worsen hypercapnia.
Oxygen therapy for pure COPD, but maybe not for overlap syndrome
Continuous oxygen therapy reduces mortality in COPD,47,48 but the duration and severity of hypoxemia that warrant oxygen therapy are less clear. Oxygen therapy in hypoxemic patients has been shown to improve sleep quality and reduce arousals.49
Indications for oxygen treatment of nocturnal hypoxemia are generally based on Medicare guidelines:
- At least 5 minutes of sleep with peripheral oxygen saturation ≤ 88% or Pao2 ≤ 55 mm Hg, or
- A decrease in Pao2 of more than 10 mm Hg or in peripheral oxygen saturation of more than 5% for at least 5 minutes of sleep and associated with signs or symptoms reasonably attributable to hypoxemia (group I criteria), or
- At least 5 minutes of sleep with peripheral oxygen saturation ≥ 89% or Pao2 56 to 59 mm Hg and pedal edema, pulmonary hypertension, cor pulmonale, or erythrocytosis (group II criteria).50
Approximately 47% of COPD patients who are hypoxemic during the day spend about 30% of sleep time with an oxygen saturation less than 90%, even while on continuous oxygen therapy.51 Current recommendations for nocturnal oxygen therapy are to increase the oxygen concentration by 1 L/minute above the baseline oxygen flow rate needed to maintain an oxygen saturation higher than 90% during resting wakefulness, using a nasal cannula or face mask.52
Caveat. In overlap syndrome, supplemental oxygen may prolong the duration of apnea episodes and worsen hypercapnia.
Positive airway pressure for OSA
Positive airway pressure therapy improves cardiovascular outcomes in OSA.53 Several studies54–58 compared the effectiveness of CPAP vs BPAP as initial therapy for OSA but did not provide enough evidence to favor one over the other in this setting. Similarly, the results are mixed for the use of fixed or auto-adjusting BPAP as salvage therapy in patients who cannot tolerate CPAP.59–61
In overlap syndrome, CPAP or BPAP with or without supplemental oxygen has been investigated in several studies.26,62–65 In general, the mortality rate of COPD patients who require oxygen therapy is quite high.47,66 In hypoxemic COPD patients with moderate to severe sleep-disordered breathing, the 5-year survival rate was 71% in those treated with CPAP plus oxygen, vs 26% in those on oxygen alone, independent of baseline postbronchodilator FEV1.67
There is no specific FEV1 cutoff for prescribing CPAP. In general, daytime hypercapnia and nocturnal hypoxemia despite supplemental oxygen therapy are indications for BPAP therapy, regardless of the presence of OSA. Whether noninvasive nocturnal ventilation for COPD patients who do not have OSA improves long-term COPD outcomes is not entirely clear.65,68,69
Adding nocturnal BPAP in spontaneous timed mode to pulmonary rehabilitation for severe hypercapnic COPD was found to improve quality of life, mood, dyspnea, gas exchange, and decline in lung function.70 Other studies noted that COPD patients hospitalized with respiratory failure who were randomized to noninvasive nocturnal ventilation plus oxygen therapy as opposed to oxygen alone experienced improvement in health-related quality of life and reduction in intensive-care-unit length of stay but no difference in mortality or subsequent hospitalizations.69 In stable hypercapnic COPD patients without OSA, there is no clear evidence that nocturnal noninvasive ventilation lessens the risk of death despite improved daytime gas exchange,71,72 but additional long-term studies are needed.
Lung volume reduction surgery, a procedure indicated for highly selected patients with severe COPD, has been shown to reduce hyperinflation, improve nocturnal hypoxemia, and improve total sleep time and sleep efficiency in patients without sleep-disordered breathing.73 More studies are needed to determine if reduction in lung hyperinflation has an impact on the occurrence of OSA and on morbidity related to sleep-disordered breathing.
Benefit of CPAP in overlap syndrome
In a nonrandomized study, Marin et al62 found that overlap syndrome is associated with an increased risk of death and hospitalization due to COPD exacerbations. CPAP therapy was associated with improved survival rates and decreased hospitalization rates in these patients.
Stanchina et al,74 in a post hoc analysis of an observational cohort, assessed the outcomes of 227 patients with overlap syndrome. Greater use of CPAP was found to be associated with lower mortality rates.
Jaoude et al75 found that hypercapnic patients with overlap syndrome who were adherent to CPAP therapy had a lower mortality rate than nonadherent hypercapnic patients (P = .04). In a multivariate analysis, the comorbidity index was the only independent predictor of mortality in normocapnic patients with overlap syndrome, while CPAP adherence was associated with improved survival.
Lastly, patients with overlap syndrome tend to need more healthcare and accrue higher medical costs than patients with COPD alone. An analysis of a state Medicaid database that included COPD patients showed that beneficiaries with overlap syndrome spent at least $4,000 more in medical expenditures than beneficiaries with “lone” COPD.24
In conclusion, CPAP is the first line of therapy for overlap syndrome, while daytime hypercapnia or nocturnal hypoxemia despite supplemental oxygen therapy are indications for nocturnal BPAP therapy, regardless of whether patients have OSA.
OSA AND ASTHMA (ALTERNATIVE OVERLAP SYNDROME)
Epidemiology and clinical features
The coexistence of asthma and OSA can begin in childhood and continue throughout adult life. A higher prevalence of lifetime asthma and OSA has been noted in children of racial and ethnic minorities, children of lower socioeconomic status, and those with atopy.76
In a pediatric asthma clinic, it was noted that 12 months into structured asthma management and optimization, children with sleep-disordered breathing were nearly four times more likely to have severe asthma at follow-up, even after adjusting for obesity, race, and gender.77
In adult patients with OSA, the prevalence of asthma is about 35%.78 Conversely, people with asthma are at higher risk of OSA. High risk of OSA was more prevalent in a group of patients with asthma than in a general medical clinic population (39.5% vs 27.2%, P < .05).79
Analysis of a large prospective cohort found that asthma was a risk factor for new-onset OSA. The incidence of OSA over 4 years in patients with self-reported asthma was 27%, compared with 16% without asthma. The relative risk adjusted for risk factors such as body mass index, age, and gender was 1.39 (95% confidence interval [CI] 15%–19%).80
Patients with asthma who are at high risk of OSA are more likely to have worse daytime and nighttime asthma symptoms. Interestingly, patients who are diagnosed with OSA and treated with CPAP seem to have better asthma control.
Patients with asthma who are more likely to have OSA are women (odds ratio [OR] 2.1), have greater asthma severity (OR 1.6), have gastroesophageal reflux disease (OR 2.7), and use inhaled corticosteroids (OR 4.0).81 These associations are different than the traditional, population-wide risk factors for OSA, such as male sex, excess body weight, and nocturnal nasal congestion.82
OSA also worsens asthma control. Teodorescu et al15 found that severe asthma was more frequent in older asthma patients (ages 60–75, prevalence 49%) than in younger patients (ages 18–59, 39%). Older adults with OSA were seven times as likely to have severe asthma (OR 6.6), whereas young adults with sleep apnea were only three times as likely (OR 2.6).
In a group of patients with difficult-to-treat asthma, OSA was significantly associated with frequent exacerbations (OR 3.4), an association similar in magnitude to that of psychological conditions (OR 10.8), severe sinus disease (OR 3.7), recurrent respiratory tract infections (OR 6.9), and gastroesophageal reflux disease (OR 4.9).83 More than half of the patients had at least three of these comorbid conditions.
Sleep quality can greatly affect asthma control, and its importance is often underestimated. Patients with severe asthma have worse sleep quality than patients with milder asthma or nonasthmatic patients, even after excluding patients with a high risk of OSA, patients on CPAP therapy, and patients with a history of gastroesophageal reflux disease. Furthermore, regardless of asthma severity, sleep quality is a significant predictor of asthma-related quality of life, even after accounting for body mass index, daytime sleepiness, and gastroesophageal reflux disease.84
Pathophysiology of alternative overlap syndrome
Sleep significantly affects respiratory pathophysiology in asthma. The underlying mechanisms include physical and mechanical stressors, neurohormonal changes, hypoxia, confounding medical conditions, and local and systemic inflammatory changes.
Patients with nocturnal asthma experience more pronounced obstruction when sleep-deprived, suggesting that sleep loss may contribute to worsening airflow limitation.14 Although changes in pulmonary mechanics and lung volumes may also have a role, volume-dependent airway narrowing does not appear to account for all observed nocturnal increases in airway resistance. Intrathoracic blood pooling may also contribute to nocturnal bronchoconstriction through stimulation of pulmonary C fibers and increased bronchial wall edema, a mechanism that may be similar to the “cardiac asthma” seen in left ventricular dysfunction.
Early studies of sleep-disordered breathing demonstrated that patients with asthma were breathing more irregularly (with hypopnea, apnea, and hyperpnea) in REM sleep than those without asthma.85 Interestingly, REM-related hypoxia has also been noted in children with asthma.86 This may be related to the increased cholinergic outflow that occurs during REM sleep, which in turn modulates the caliber and reactivity of the lower airways.
Physical changes such as upper airway collapse and reduced pharyngeal cross-sectional area may cause further mechanical strain.87 This can further propagate airway inflammation, alter airway mucosal muscle fibers, and stimulate neural reflexes, thereby increasing cholinergic tone and bronchoconstriction. Furthermore, heightened negative intrathoracic pressure during obstructive episodes can increase nocturnal pulmonary blood pooling.14 Hypoxia itself can augment airway hyperresponsiveness via vagal pathways or carotid body receptors, increasing reactive oxygen species and inflammatory mediators. Local inflammation can “spill over” into systemic inflammatory changes, while alterations in airway inflammatory markers in asthma seem to follow a circadian rhythm, in parallel with the nocturnal worsening of the asthma symptoms.88 Finally, altered sleep may be related to other comorbid conditions, such as gastroesophageal reflux disease, insomnia, and restless leg syndrome.
Management and outcomes of alternative overlap syndrome
Despite optimization of asthma management, OSA can still significantly affect asthma control and symptoms.84
Interestingly, medications that reduce airway inflammation (eg, corticosteroids) may promote OSA. This occurrence cannot be fully explained by an increase in body mass, as more respiratory disturbances occur during sleep with continuous corticosteroid treatment even without increases in body mass index.87 Therefore, these associations may be related to upper airway myopathy caused by the treatment, a small pharynx, facial dysmorphisms, or fat deposition.89
Does CPAP improve asthma?
OSA is often unrecognized in patients with asthma, and treating it can have an impact on asthma symptoms.
CPAP therapy has not been shown to significantly change airway responsiveness or lung function, but it has been noted to significantly improve both OSA-related and asthma-related quality of life and reduce the use of rescue bronchodilators.3,90 CPAP has demonstrated improvement of quality of life that positively correlated with body weight and apnea-hypopnea index at baseline, suggesting that asthmatic patients with greater obesity or worse OSA may benefit most from aggressive management.90
However, CPAP should be used only if the patient has confirmed OSA. Empiric use of CPAP without a diagnosis of OSA was poorly tolerated and failed to improve asthma symptoms or lung function.91 More importantly, using CPAP in a patient who does not have OSA may contribute to further sleep disruption.91
Second-line treatments such as mandibular advancing devices and airway or bariatric surgery have not yet been studied in alternative overlap syndrome.
A multidimensional assessment of asthma
The Western world is experiencing an epidemic of obesity and of asthma. Obesity contributes to the pathogenesis of OSA by altering the anatomy and collapsibility of the upper airway, affecting ventilatory control and increasing respiratory workload. Another paradigm, supported by some evidence, is that OSA itself may contribute to the development of obesity. Both OSA and obesity lead to activation of inflammatory biologic cascades, which are likely the pathogenic mechanisms for their cardiovascular and metabolic consequences. As such, early recognition of OSA is important, as effective treatments are available.
In some patients, obesity may cause asthma, as obesity precedes the onset of asthma in a significant proportion of patients, and bariatric surgery for morbid obesity may resolve asthma. The obese asthma phenotype seems to include chronic rhinosinusitis, gastroesophageal reflux disease, poorer asthma control, limited responsiveness to corticosteroids, and even different sets of biomarkers (eg, neutrophilic airway inflammation). A cohort of obese patients with poor asthma control demonstrated significant improvement in asthma symptoms, quality of life, and airway reactivity after weight loss from bariatric surgery.92
To improve our knowledge about airway disease phenotypes and endotypes and their response to therapy, we propose taking a multidimensional, structured assessment of all patients with asthma, using a schema we call “ABCD-3P-PQRST” (Table 2).
The purpose of using this type of system in clinics and research is to capture the multidimensionality of the disease and better develop future individualized therapeutic strategies by employing the latest advances in systems biology and computational methods such as cluster and principal component analysis.
Multidimensional assessments addressing airway problems such as asthma, COPD, OSA, other comorbidities and risk factors, and personalized management plans will need to be the basis of future therapeutic interventions. Increased attention to the complications of asthma and obstructive airway and lung diseases in our patients is imperative, specifically to develop effective systems of care, appropriate clinical guidelines, and research studies that lead to improved health outcomes.
Many patients who have obstructive lung disease, ie, chronic obstructive pulmonary disease (COPD) or asthma, also have obstructive sleep apnea (OSA), and vice versa.
The combination of COPD and OSA was first described almost 30 years ago by Flenley, who called it “overlap syndrome.”1 At that time, he recommended that a sleep study be considered in all obese patients with COPD who snore and in those who have frequent headaches after starting oxygen therapy. In the latter group, he doubted that nocturnal oxygen was the correct treatment. He also believed that the outcomes in patients with overlap syndrome were worse than those in patients with COPD or OSA alone. These opinions remain largely valid today.
We now also recognize the combination of asthma and OSA (alternative overlap syndrome) and collectively call both combinations obstructive lung disease-obstructive sleep apnea (OLDOSA) syndrome.2 Interestingly, these relationships are likely bidirectional, with one condition aggravating or predisposing to the other.
Knowing that a patient has one of these overlap syndromes, one can initiate continuous positive airway pressure (CPAP) therapy, which can improve clinical outcomes.3–6 Therefore, when evaluating a patient with asthma or COPD, one should consider OSA using a validated questionnaire and, if the findings suggest the diagnosis, polysomnography. Conversely, it is prudent to look for comorbid obstructive lung disease in patients with OSA, as interactions between upper and lower airway dysfunction may lead to distinctly different treatment and outcomes.
Here, we briefly review asthma and COPD, explore shared risk factors for sleep-disordered breathing and obstructive lung diseases, describe potential pathophysiologic mechanisms explaining these associations, and highlight the importance of recognizing and individually treating the overlaps of OSA and COPD or asthma.
COPD AND ASTHMA ARE VERY COMMON
About 10% of the US population have COPD,7 a preventable and treatable disease mainly caused by smoking, and a leading cause of sickness and death worldwide.8,9
About 8% of Americans have asthma,7 which has become one of the most common chronic conditions in the Western world, affecting about 1 in 7 children and about 1 in 12 adults. The World Health Organization estimates that 235 million people suffer from asthma worldwide, and by 2025 this number is projected to rise to 400 million.10,11
The prevalence of these conditions in a particular population depends on the frequency of risk factors and associated morbidities, including OSA. These factors may allow asthma or COPD to arise earlier or have more severe manifestations.8,12
Asthma and COPD: Similarities and differences
Asthma and COPD share several features. Both are inflammatory airway conditions triggered or perpetuated by allergens, viral infection, tobacco smoke, products of biomass or fossil fuel combustion, and other substances. In both diseases, airflow is “obstructed” or limited, with a low ratio of forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC). Symptoms can also be similar, with dyspnea, cough, wheezing, and chest tightness being the most frequent complaints. The similarities support the theory proposed by Orie et al13 (the “Dutch hypothesis”) that asthma and COPD may actually be manifestations of the same disease.
But there are also differences. COPD is strongly linked to cigarette smoking and has at least three phenotypes:
- Chronic bronchitis, defined clinically by cough and sputum production for more than 3 months per year for 2 consecutive years
- Emphysema, characterized anatomically by loss of lung parenchyma, as seen on tomographic imaging or examination of pathologic specimens
- A mixed form with bronchitic and emphysematous features, which is likely the most common.
Particularly in emphysematous COPD, smoking predisposes patients to gas-exchange abnormalities and low diffusing capacity for carbon monoxide.
In asthma, symptoms may be more episodic, the age of onset is often younger, and atopy is common, especially in allergic asthma. These episodic symptoms may correlate temporally with measurable airflow reversibility (≥ 12% and ≥ 200 mL improvement in FVC or in FEV1 after bronchodilator challenge).
However, the current taxonomy does not unequivocally divide obstructive lung diseases into asthma and COPD, and major features such as airway hyperresponsiveness, airflow reversibility, neutrophilic or CD8 lymphocytic airway inflammation, and lower concentration of nitric oxide in the exhaled air may be present in different phenotypes of both conditions (Table 1).
AIRFLOW IN OBSTRUCTIVE LUNG DISEASES AND DURING SLEEP
Normal airflow involves a complex interplay between airway resistance and elastic recoil of the entire respiratory system, including the airways, the lung parenchyma, and the chest wall (Figure 1).
In asthma and COPD, resistance to airflow is increased, predominantly in the upper airways (nasal passages, pharynx, and larynx) and in the first three or four subdivisions of the tracheobronchial tree. The problem is worse during exhalation, when elastic recoil of the lung parenchyma and chest wall also increases airway resistance, reduces airway caliber, and possibly even constricts the bronchi. This last effect may occur either due to mass loading of the bronchial smooth muscles or to large intrathoracic transmural pressure shifts that may increase extravasation of fluid in the bronchial walls, especially with higher vascular permeability in inflammatory conditions.
Furthermore, interactions between the airway and parenchyma and between the upper and lower airways, as well as radial and axial coupling of these anatomic and functional components, contribute to complex interplay between airway resistance and parenchymal-chest wall elastic energy—stretch or recoil.
The muscles of the upper and lower airway may not work together due to the loss of normal lung parenchyma (as in emphysema) or to the acute inflammation in the small airways and adjacent parenchyma (as in severe asthma exacerbations). This loss of coordination makes the upper airway more collapsible, a feature of OSA.
Additionally, obesity, gastroesophageal reflux, disease chronic rhinitis, nasal polyposis, and acute exacerbations of chronic systemic inflammation all contribute to more complex interactions between obstructive lung diseases and OSA.6
Sleep affects breathing, particularly in patients with respiratory comorbidities, and sleep-disordered breathing causes daytime symptoms and worsens quality of life.1,13–15 During sleep, respiratory centers become less sensitive to oxygen and carbon dioxide; breathing becomes more irregular, especially during rapid eye movement (REM) sleep; the chest wall moves less, so that the tidal volume and functional residual capacity are lower; sighs, yawns, and deep breaths become limited; and serum carbon dioxide concentration may rise.
OBSTRUCTIVE SLEEP APNEA
The prevalence of OSA, a form of sleep-disordered breathing characterized by limitation of inspiratory and (to a lesser degree) expiratory flow, has increased significantly in recent years, in parallel with the prevalence of its major risk factor, obesity.
OSA is generally defined as an apnea-hypopnea index of 5 or higher, ie, five or more episodes of apnea or hypopnea per hour.
OSA syndrome, ie, an apnea-hypopnea index of 5 or higher and excessive daytime sleepiness (defined by an Epworth Sleepiness Scale score > 10) was found in the initial analysis of the Wisconsin Sleep cohort in 1993 to be present in about 2% of women and 4% of men.16 A more recent longitudinal analysis showed a significant increase—for example, in people 50 to 70 years old the prevalence was up to 17.6% in men and 7.5% in women.17
Upper airway resistance syndrome, a milder form of sleep-disordered breathing, is now included under the diagnosis of OSA, as its pathophysiology is not significantly different.18
In the next section, we discuss what happens when OSA overlaps with COPD (overlap syndrome) and with asthma (“alternative overlap syndrome”)2,8 (Figure 2).
OSA AND COPD (OVERLAP SYNDROME)
Flenley1 hypothesized that patients with COPD in whom supplemental oxygen worsened hypercapnia may also have OSA and called this association overlap syndrome.
How common is overlap syndrome?
Since both COPD and OSA are prevalent conditions, overlap syndrome may also be common.
The reported prevalence of overlap syndrome varies widely, depending on the population studied and the methods used. In various studies, COPD was present in 9% to 56% of patients with OSA,19–23 and OSA was found in 5% to 85% of patients with COPD.24–27
Based on the prevalence of COPD in the general population (about 10%12) and that of sleep-disordered breathing (about 5% to 10%17), the expected prevalence of overlap syndrome in people over age 40 may be 0.5% to 1%.28 In a more inclusive estimate with “subclinical” forms of overlap syndrome—ie, OSA defined as an apnea-hypopnea index of 5 or more (about 25% of the population17) and COPD Global initiative for Chronic Obstructive Lung Disease (GOLD) stage 1 (16.8% in the National Health and Nutrition Education Survey12)—the expected prevalence of overlap is around 4%. Some studies found a higher prevalence of COPD in OSA patients than in the general population,21,29 while others did not.22,28,30 The studies differed in how they defined sleep-disordered breathing.
Larger studies are needed to better assess the true prevalence of sleep-disordered breathing in COPD. They should use more sensitive measures of airflow and standardized definitions of sleep-disordered breathing and should include patients with more severe COPD.
Fatigue and insomnia are common in COPD
Fatigue is strongly correlated with declining lung function, low exercise tolerance, and impaired quality of life in COPD.31 Factors that contribute to fatigue include dyspnea, depression, and impaired sleep.32 Some suggest that at least half of COPD patients have sleep complaints such as insomnia, sleep disruption, or sleep fragmentation.33 Insomnia, difficulty falling asleep, and early morning awakenings are the most common complaints (30%–70% of patients) and are associated with daytime fatigue.34 Conversely, comorbid OSA can contribute to fatigue and maintenance-type insomnia (ie, difficulty staying asleep and returning to sleep).
Multiple mechanisms of hypoxemia in overlap syndrome
Oxygenation abnormalities and increased work of breathing contribute to the pathophysiology of overlap syndrome. In patients with COPD, oxygenation during wakefulness is a strong predictor of gas exchange during sleep.35 Further, patients with overlap syndrome tend to have more severe hypoxia during sleep than patients with isolated COPD or OSA at rest or during exercise.36
In overlap syndrome, hypoxemia is the result of several mechanisms:
- Loss of upper airway muscle tone from intermittent episodes of obstructive apnea and hypopnea leads to upper airway collapse during sleep, particularly during REM sleep, increasing the severity of OSA.37
- Reductions in functional residual capacity from lying in the recumbent position and during REM sleep render patients with COPD more vulnerable, as compensatory use of accessory muscles to maintain near-normal ventilation in a hyperinflated state becomes impaired.37
- Alterations in pulmonary ventilation-perfusion matching may lead to altered carbon dioxide homeostasis and impaired oxygenation in patients with emphysema.
- Circadian variation in lower airway caliber may also be observed, in parallel with the bronchoconstriction caused by increased nocturnal vagotonia.
- Hypercapnia (Paco2 ≥ 45 mm Hg) may lead to overall reduced responsiveness of respiratory muscles and to a blunted response of respiratory centers to low oxygen and high carbon dioxide levels.38 Thus, hypercapnia is a better predictor of the severity of nocturnal hypoxemia than hypoxemia developing during exercise.39
In a person who is at near-maximal ventilatory capacity, even a mild increase in upper airway resistance (as seen with snoring, upper airway resistance syndrome, or OSA) increases the work of breathing. This phenomenon can lead to early arousals even before significant oxyhemoglobin desaturation occurs.
Normally, inspiratory flow limitation is counteracted by increasing inspiratory time to maintain ventilation. Patients with COPD may not be able to do this, however, as they need more time to breathe out due to narrowing of their lower airways.40 The inability to compensate for upper airway resistance, similar to the increased work of breathing seen with exercise, may lead to early arousals and increased sleep fragmentation.
Consequences of overlap syndrome
Patients with overlap syndrome appear to have higher morbidity and mortality rates than those with COPD or sleep-disordered breathing alone.
Cor pulmonale. Nighttime hypoxia is more severe and persistent in overlap syndrome than with COPD or OSA alone. This may contribute to more significant pulmonary hypertension and to the development of cor pulmonale, in which the right ventricle is altered in structure (eg, hypertrophied, dilated) or reduced in function, or both, from severe pulmonary hypertension.
In contrast to right ventricular failure due to disorders of the left heart, cor pulmonale is a result of diseases of the vasculature (eg, idiopathic pulmonary arterial hypertension), lung parenchyma (eg, COPD), upper airway (eg, OSA), or chest wall (eg, severe kyphoscoliosis). COPD is the most common cause of cor pulmonale in the United States, accounting for up to 30% of cases of cor pulmonale.41–45 In OSA, cor pulmonale is seen in up to 20% of cases,43 while in overlap syndrome cor pulmonale is encountered even more often (ie, in up to 80%); these patients have a dismal 5-year survival rate of about 30%.46
Obesity hypoventilation syndrome is characterized by obesity (body mass index ≥ 30 kg/m2) and daytime hypercapnia (Paco2 ≥ 45 mm Hg) that cannot be fully attributed to an underlying cardiopulmonary or neurologic condition.18 Hypercapnia worsens during sleep (especially during REM sleep) and is often associated with severe arterial oxygen desaturation. Up to 90% of patients with obesity hypoventilation syndrome have comorbid OSA, and the rest generally have sleep-related hypoventilation, particularly during REM sleep.
In patients with obesity hypoventilation syndrome, daytime hypercapnia may improve or even normalize with adequate positive airway pressure treatment and sustained adherence to treatment.18 Many patients with obesity hypoventilation syndrome respond to CPAP or bilevel positive airway pressure (BPAP), with improvement in daytime Paco2. However, normalization of daytime Paco2 occurs only in a subgroup of patients. In contrast, treatment with oxygen therapy alone may worsen hypercapnia.
Oxygen therapy for pure COPD, but maybe not for overlap syndrome
Continuous oxygen therapy reduces mortality in COPD,47,48 but the duration and severity of hypoxemia that warrant oxygen therapy are less clear. Oxygen therapy in hypoxemic patients has been shown to improve sleep quality and reduce arousals.49
Indications for oxygen treatment of nocturnal hypoxemia are generally based on Medicare guidelines:
- At least 5 minutes of sleep with peripheral oxygen saturation ≤ 88% or Pao2 ≤ 55 mm Hg, or
- A decrease in Pao2 of more than 10 mm Hg or in peripheral oxygen saturation of more than 5% for at least 5 minutes of sleep and associated with signs or symptoms reasonably attributable to hypoxemia (group I criteria), or
- At least 5 minutes of sleep with peripheral oxygen saturation ≥ 89% or Pao2 56 to 59 mm Hg and pedal edema, pulmonary hypertension, cor pulmonale, or erythrocytosis (group II criteria).50
Approximately 47% of COPD patients who are hypoxemic during the day spend about 30% of sleep time with an oxygen saturation less than 90%, even while on continuous oxygen therapy.51 Current recommendations for nocturnal oxygen therapy are to increase the oxygen concentration by 1 L/minute above the baseline oxygen flow rate needed to maintain an oxygen saturation higher than 90% during resting wakefulness, using a nasal cannula or face mask.52
Caveat. In overlap syndrome, supplemental oxygen may prolong the duration of apnea episodes and worsen hypercapnia.
Positive airway pressure for OSA
Positive airway pressure therapy improves cardiovascular outcomes in OSA.53 Several studies54–58 compared the effectiveness of CPAP vs BPAP as initial therapy for OSA but did not provide enough evidence to favor one over the other in this setting. Similarly, the results are mixed for the use of fixed or auto-adjusting BPAP as salvage therapy in patients who cannot tolerate CPAP.59–61
In overlap syndrome, CPAP or BPAP with or without supplemental oxygen has been investigated in several studies.26,62–65 In general, the mortality rate of COPD patients who require oxygen therapy is quite high.47,66 In hypoxemic COPD patients with moderate to severe sleep-disordered breathing, the 5-year survival rate was 71% in those treated with CPAP plus oxygen, vs 26% in those on oxygen alone, independent of baseline postbronchodilator FEV1.67
There is no specific FEV1 cutoff for prescribing CPAP. In general, daytime hypercapnia and nocturnal hypoxemia despite supplemental oxygen therapy are indications for BPAP therapy, regardless of the presence of OSA. Whether noninvasive nocturnal ventilation for COPD patients who do not have OSA improves long-term COPD outcomes is not entirely clear.65,68,69
Adding nocturnal BPAP in spontaneous timed mode to pulmonary rehabilitation for severe hypercapnic COPD was found to improve quality of life, mood, dyspnea, gas exchange, and decline in lung function.70 Other studies noted that COPD patients hospitalized with respiratory failure who were randomized to noninvasive nocturnal ventilation plus oxygen therapy as opposed to oxygen alone experienced improvement in health-related quality of life and reduction in intensive-care-unit length of stay but no difference in mortality or subsequent hospitalizations.69 In stable hypercapnic COPD patients without OSA, there is no clear evidence that nocturnal noninvasive ventilation lessens the risk of death despite improved daytime gas exchange,71,72 but additional long-term studies are needed.
Lung volume reduction surgery, a procedure indicated for highly selected patients with severe COPD, has been shown to reduce hyperinflation, improve nocturnal hypoxemia, and improve total sleep time and sleep efficiency in patients without sleep-disordered breathing.73 More studies are needed to determine if reduction in lung hyperinflation has an impact on the occurrence of OSA and on morbidity related to sleep-disordered breathing.
Benefit of CPAP in overlap syndrome
In a nonrandomized study, Marin et al62 found that overlap syndrome is associated with an increased risk of death and hospitalization due to COPD exacerbations. CPAP therapy was associated with improved survival rates and decreased hospitalization rates in these patients.
Stanchina et al,74 in a post hoc analysis of an observational cohort, assessed the outcomes of 227 patients with overlap syndrome. Greater use of CPAP was found to be associated with lower mortality rates.
Jaoude et al75 found that hypercapnic patients with overlap syndrome who were adherent to CPAP therapy had a lower mortality rate than nonadherent hypercapnic patients (P = .04). In a multivariate analysis, the comorbidity index was the only independent predictor of mortality in normocapnic patients with overlap syndrome, while CPAP adherence was associated with improved survival.
Lastly, patients with overlap syndrome tend to need more healthcare and accrue higher medical costs than patients with COPD alone. An analysis of a state Medicaid database that included COPD patients showed that beneficiaries with overlap syndrome spent at least $4,000 more in medical expenditures than beneficiaries with “lone” COPD.24
In conclusion, CPAP is the first line of therapy for overlap syndrome, while daytime hypercapnia or nocturnal hypoxemia despite supplemental oxygen therapy are indications for nocturnal BPAP therapy, regardless of whether patients have OSA.
OSA AND ASTHMA (ALTERNATIVE OVERLAP SYNDROME)
Epidemiology and clinical features
The coexistence of asthma and OSA can begin in childhood and continue throughout adult life. A higher prevalence of lifetime asthma and OSA has been noted in children of racial and ethnic minorities, children of lower socioeconomic status, and those with atopy.76
In a pediatric asthma clinic, it was noted that 12 months into structured asthma management and optimization, children with sleep-disordered breathing were nearly four times more likely to have severe asthma at follow-up, even after adjusting for obesity, race, and gender.77
In adult patients with OSA, the prevalence of asthma is about 35%.78 Conversely, people with asthma are at higher risk of OSA. High risk of OSA was more prevalent in a group of patients with asthma than in a general medical clinic population (39.5% vs 27.2%, P < .05).79
Analysis of a large prospective cohort found that asthma was a risk factor for new-onset OSA. The incidence of OSA over 4 years in patients with self-reported asthma was 27%, compared with 16% without asthma. The relative risk adjusted for risk factors such as body mass index, age, and gender was 1.39 (95% confidence interval [CI] 15%–19%).80
Patients with asthma who are at high risk of OSA are more likely to have worse daytime and nighttime asthma symptoms. Interestingly, patients who are diagnosed with OSA and treated with CPAP seem to have better asthma control.
Patients with asthma who are more likely to have OSA are women (odds ratio [OR] 2.1), have greater asthma severity (OR 1.6), have gastroesophageal reflux disease (OR 2.7), and use inhaled corticosteroids (OR 4.0).81 These associations are different than the traditional, population-wide risk factors for OSA, such as male sex, excess body weight, and nocturnal nasal congestion.82
OSA also worsens asthma control. Teodorescu et al15 found that severe asthma was more frequent in older asthma patients (ages 60–75, prevalence 49%) than in younger patients (ages 18–59, 39%). Older adults with OSA were seven times as likely to have severe asthma (OR 6.6), whereas young adults with sleep apnea were only three times as likely (OR 2.6).
In a group of patients with difficult-to-treat asthma, OSA was significantly associated with frequent exacerbations (OR 3.4), an association similar in magnitude to that of psychological conditions (OR 10.8), severe sinus disease (OR 3.7), recurrent respiratory tract infections (OR 6.9), and gastroesophageal reflux disease (OR 4.9).83 More than half of the patients had at least three of these comorbid conditions.
Sleep quality can greatly affect asthma control, and its importance is often underestimated. Patients with severe asthma have worse sleep quality than patients with milder asthma or nonasthmatic patients, even after excluding patients with a high risk of OSA, patients on CPAP therapy, and patients with a history of gastroesophageal reflux disease. Furthermore, regardless of asthma severity, sleep quality is a significant predictor of asthma-related quality of life, even after accounting for body mass index, daytime sleepiness, and gastroesophageal reflux disease.84
Pathophysiology of alternative overlap syndrome
Sleep significantly affects respiratory pathophysiology in asthma. The underlying mechanisms include physical and mechanical stressors, neurohormonal changes, hypoxia, confounding medical conditions, and local and systemic inflammatory changes.
Patients with nocturnal asthma experience more pronounced obstruction when sleep-deprived, suggesting that sleep loss may contribute to worsening airflow limitation.14 Although changes in pulmonary mechanics and lung volumes may also have a role, volume-dependent airway narrowing does not appear to account for all observed nocturnal increases in airway resistance. Intrathoracic blood pooling may also contribute to nocturnal bronchoconstriction through stimulation of pulmonary C fibers and increased bronchial wall edema, a mechanism that may be similar to the “cardiac asthma” seen in left ventricular dysfunction.
Early studies of sleep-disordered breathing demonstrated that patients with asthma were breathing more irregularly (with hypopnea, apnea, and hyperpnea) in REM sleep than those without asthma.85 Interestingly, REM-related hypoxia has also been noted in children with asthma.86 This may be related to the increased cholinergic outflow that occurs during REM sleep, which in turn modulates the caliber and reactivity of the lower airways.
Physical changes such as upper airway collapse and reduced pharyngeal cross-sectional area may cause further mechanical strain.87 This can further propagate airway inflammation, alter airway mucosal muscle fibers, and stimulate neural reflexes, thereby increasing cholinergic tone and bronchoconstriction. Furthermore, heightened negative intrathoracic pressure during obstructive episodes can increase nocturnal pulmonary blood pooling.14 Hypoxia itself can augment airway hyperresponsiveness via vagal pathways or carotid body receptors, increasing reactive oxygen species and inflammatory mediators. Local inflammation can “spill over” into systemic inflammatory changes, while alterations in airway inflammatory markers in asthma seem to follow a circadian rhythm, in parallel with the nocturnal worsening of the asthma symptoms.88 Finally, altered sleep may be related to other comorbid conditions, such as gastroesophageal reflux disease, insomnia, and restless leg syndrome.
Management and outcomes of alternative overlap syndrome
Despite optimization of asthma management, OSA can still significantly affect asthma control and symptoms.84
Interestingly, medications that reduce airway inflammation (eg, corticosteroids) may promote OSA. This occurrence cannot be fully explained by an increase in body mass, as more respiratory disturbances occur during sleep with continuous corticosteroid treatment even without increases in body mass index.87 Therefore, these associations may be related to upper airway myopathy caused by the treatment, a small pharynx, facial dysmorphisms, or fat deposition.89
Does CPAP improve asthma?
OSA is often unrecognized in patients with asthma, and treating it can have an impact on asthma symptoms.
CPAP therapy has not been shown to significantly change airway responsiveness or lung function, but it has been noted to significantly improve both OSA-related and asthma-related quality of life and reduce the use of rescue bronchodilators.3,90 CPAP has demonstrated improvement of quality of life that positively correlated with body weight and apnea-hypopnea index at baseline, suggesting that asthmatic patients with greater obesity or worse OSA may benefit most from aggressive management.90
However, CPAP should be used only if the patient has confirmed OSA. Empiric use of CPAP without a diagnosis of OSA was poorly tolerated and failed to improve asthma symptoms or lung function.91 More importantly, using CPAP in a patient who does not have OSA may contribute to further sleep disruption.91
Second-line treatments such as mandibular advancing devices and airway or bariatric surgery have not yet been studied in alternative overlap syndrome.
A multidimensional assessment of asthma
The Western world is experiencing an epidemic of obesity and of asthma. Obesity contributes to the pathogenesis of OSA by altering the anatomy and collapsibility of the upper airway, affecting ventilatory control and increasing respiratory workload. Another paradigm, supported by some evidence, is that OSA itself may contribute to the development of obesity. Both OSA and obesity lead to activation of inflammatory biologic cascades, which are likely the pathogenic mechanisms for their cardiovascular and metabolic consequences. As such, early recognition of OSA is important, as effective treatments are available.
In some patients, obesity may cause asthma, as obesity precedes the onset of asthma in a significant proportion of patients, and bariatric surgery for morbid obesity may resolve asthma. The obese asthma phenotype seems to include chronic rhinosinusitis, gastroesophageal reflux disease, poorer asthma control, limited responsiveness to corticosteroids, and even different sets of biomarkers (eg, neutrophilic airway inflammation). A cohort of obese patients with poor asthma control demonstrated significant improvement in asthma symptoms, quality of life, and airway reactivity after weight loss from bariatric surgery.92
To improve our knowledge about airway disease phenotypes and endotypes and their response to therapy, we propose taking a multidimensional, structured assessment of all patients with asthma, using a schema we call “ABCD-3P-PQRST” (Table 2).
The purpose of using this type of system in clinics and research is to capture the multidimensionality of the disease and better develop future individualized therapeutic strategies by employing the latest advances in systems biology and computational methods such as cluster and principal component analysis.
Multidimensional assessments addressing airway problems such as asthma, COPD, OSA, other comorbidities and risk factors, and personalized management plans will need to be the basis of future therapeutic interventions. Increased attention to the complications of asthma and obstructive airway and lung diseases in our patients is imperative, specifically to develop effective systems of care, appropriate clinical guidelines, and research studies that lead to improved health outcomes.
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985; 6:651–661.
- Ioachimescu OC, Teodorescu M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology 2013; 18:421–431.
- Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respiratory Med 2005; 99:529–534.
- Kim MY, Jo EJ, Kang SY, et al. Obstructive sleep apnea is associated with reduced quality of life in adult patients with asthma. Ann Allergy Asthma Immunol 2013; 110:253–257.
- Teodorescu M, Polomis DA, Teodorescu MC, et al. Association of obstructive sleep apnea risk or diagnosis with daytime asthma in adults. J Asthma 2012; 49:620–628.
- Puthalapattu S, Ioachimescu OC. Asthma and obstructive sleep apnea: clinical and pathogenic interactions. J Investig Med 2014; 62:665–675.
- National Institutes of Health. National Heart, Lung, and Blood Institute. NHLBI Factbook, Fiscal Year 2007. Chapter 4. Disease Statistics. www.nhlbi.nih.gov/about/factbook-07/chapter4.htm. Accessed November 11, 2015.
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365.
- WHO. World Health Organization: Asthma. Fact sheet No 307. www.who.int/mediacentre/factsheets/fs307/en/. Accessed November 11, 2015.
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004; 59:469–478.
- WHO. Chronic obstructive pulmonary disease (COPD). Fact sheet No 315. www.who.int/mediacentre/factsheets/fs315/en/index.html. 2011. Accessed November 11, 2015.
- Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988–1994 to 2007–2010. Chest 2013; 143:1395–1406.
- Orie N, Sluiter H, de Vries K, Tammeling G, Witkop J. The host factor in bronchitis. Paper presented at: Bronchitis—an international symposium 1961; Assen, Netherlands.
- Ballard RD. Sleep, respiratory physiology, and nocturnal asthma. Chronobiol Int 1999; 16:565–580.
- Teodorescu M, Polomis DA, Gangnon RE, et al. Asthma control and its relationship with obstructive sleep apnea (OSA) in older adults. Sleep Disord 2013; 2013:251567.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- International Classification of Sleep Disorders, 3rd ed.: Diagnostic and coding manual. Darien, Illinois: American Academy of Sleep Medicine. 2014.
- Lopez-Acevedo MN, Torres-Palacios A, Elena Ocasio-Tascon M, Campos-Santiago Z, Rodriguez-Cintron W. Overlap syndrome: an indication for sleep studies? A pilot study. Sleep Breath 2009; 13:409–413.
- Scharf C, Li P, Muntwyler J, et al. Rate-dependent AV delay optimization in cardiac resynchronization therapy. PACE 2005; 28:279–284.
- Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med 1995;151:82–86.
- Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration 2005; 72:142–149.
- Fletcher EC. Chronic lung disease in the sleep apnea syndrome. Lung 1990; 168(suppl):751–761.
- Shaya FT, Lin PJ, Aljawadi MH, Scharf SM. Elevated economic burden in obstructive lung disease patients with concomitant sleep apnea syndrome. Sleep Breath 2009; 13:317–323.
- Larsson LG, Lindberg A, Franklin KA, Lundbäck B; Obstructive Lung Disease in Northern Sweden Studies. Obstructive sleep apnoea syndrome is common in subjects with chronic bronchitis. Report from the Obstructive Lung Disease in Northern Sweden studies. Respiration 2001; 68:250–255.
- Machado MC, Vollmer WM, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Resp J 2010; 35:132–137.
- Guilleminault C, Cummiskey J, Motta J. Chronic obstructive airflow disease and sleep studies. Am Rev Respir Dis 1980; 122:397–406.
- Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5:237–241.
- Bradley TD, Rutherford R, Lue F, et al. Role of diffuse airway obstruction in the hypercapnia of obstructive sleep apnea. Am Rev Respir Dis 1986; 134:920–924.
- Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med 2003; 167:7–14.
- Breslin E, van der Schans C, Breukink S, et al. Perception of fatigue and quality of life in patients with COPD. Chest 1998; 114:958–964.
- Kapella MC, Larson JL, Patel MK, Covey MK, Berry JK. Subjective fatigue, influencing variables, and consequences in chronic obstructive pulmonary disease. Nurs Res 2006; 55:10–17.
- Klink M, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest 1987; 91:540–546.
- Bellia V, Catalano F, Scichilone N, et al. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA study. Sleep 2003; 26:318–323.
- Connaughton JJ, Catterall JR, Elton RA, Stradling JR, Douglas NJ. Do sleep studies contribute to the management of patients with severe chronic obstructive pulmonary disease? Am Rev Respir Dis 1988; 138:341–344.
- Mulloy E, McNicholas WT. Ventilation and gas exchange during sleep and exercise in severe COPD. Chest 1996; 109:387–394.
- Johnson MW, Remmers JE. Accessory muscle activity during sleep in chronic obstructive pulmonary disease. J Appl Physiol 1984; 57:1011–1017.
- Douglas NJ, White DP, Pickett CK, Weil JV, Zwillich CW. Respiration during sleep in normal man. Thorax 1982; 37:840–844.
- Mulloy E, Fitzpatrick M, Bourke S, O’Regan A, McNicholas WT. Oxygen desaturation during sleep and exercise in patients with severe chronic obstructive pulmonary disease. Respir Med 1995; 89:193–198.
- Herpel LB, Brown CD, Goring KL, et al. COPD cannot compensate for upper airway obstruction during sleep (abstract). Am J Respir Crit Care Med 2007; 175:A71.
- MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part 2. Am J Respir Crit Care Med 1994; 150:1158–1168.
- MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part 1. Am J Respir Crit Care Med 1994; 150:833–852.
- Budev MM, Arroliga AC, Wiedemann HP, Matthay RA. Cor pulmonale: an overview. Semin Respir Crit Care Med 2003; 24:233–244.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 suppl):D34–D41.
- Naeije R. Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2:20–22.
- Rasche K, Orth M, Kutscha A, Duchna HW. [Pulmonary diseases and heart function]. In German. Internist (Berl) 2007; 48:276–282.
- Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 1980; 93:391–398.
- Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med 2005; 165:2408–2413.
- Calverley PM, Brezinova V, Douglas NJ, Catterall JR, Flenley DC. The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis 1982; 126:206–210.
- Centers for Medicare and Medicaid Services. National coverage determination (NCD) for home use of oxygen (240.2). www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=169&ncdver=1&NCAId=169&NcaName=Home+Use+of+Oxygen&IsPopup=y&bc=AAAAAAAAIAAA&. Accessed November 11, 2015.
- Plywaczewski R, Sliwinski P, Nowinski A, Kaminski D, Zielinski J. Incidence of nocturnal desaturation while breathing oxygen in COPD patients undergoing long-term oxygen therapy. Chest 2000; 117:679–683.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breathing 2007; 11:117–124.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Marin JM, DeAndres R, Alonso J, Sanchez A, Carrizo S. Long term mortality in the overlap syndrome. Eur Resp J 2008; 32(suppl 52):P865.
- Reeves-Hoche MK, Hudgel DW, Meck R, Witteman R, Ross A, Zwillich CW. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med 1995; 151:443–449.
- Gay PC, Herold DL, Olson EJ. A randomized, double-blind clinical trial comparing continuous positive airway pressure with a novel bilevel pressure system for treatment of obstructive sleep apnea syndrome. Sleep 2003; 26:864–869.
- Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath 2012; 16:773–779.
- Randerath WJ, Galetke W, Ruhle KH. Auto-adjusting CPAP based on impedance versus bilevel pressure in difficult-to-treat sleep apnea syndrome: a prospective randomized crossover study. Med Sci Monit 2003; 9:CR353–CR358.
- Schwartz SW, Rosas J, Iannacone MR, Foulis PR, Anderson WM. Correlates of a prescription for bilevel positive airway pressure for treatment of obstructive sleep apnea among veterans. J Clin Sleep Med 2013; 9:327–335.
- Gentina T, Fortin F, Douay B, et al. Auto bi-level with pressure relief during exhalation as a rescue therapy for optimally treated obstructive sleep apnoea patients with poor compliance to continuous positive airways pressure therapy--a pilot study. Sleep Breathing 2011; 15:21–27.
- Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clinical Sleep Med 2007; 3:706–712.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 2010; 182:325–331.
- de Miguel J, Cabello J, Sanchez-Alarcos JM, Alvarez-Sala R, Espinos D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath 2002; 6:3–10.
- Mansfield D, Naughton MT. Effects of continuous positive airway pressure on lung function in patients with chronic obstructive pulmonary disease and sleep disordered breathing. Respirology 1999; 4:365–370.
- McEvoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet 1981; 1:681–686.
- Machado MC, Vollmer WM, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J 2010; 35:132–137.
- Casanova C, Celli BR, Tost L, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000; 118:1582–1590.
- Clini E, Sturani C, Rossi A, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002; 20:529–538.
- Duiverman ML, Wempe JB, Bladder G, et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomized controlled trial. Respir Res 2011; 12:112.
- Gay PC, Hubmayr RD, Stroetz RW. Efficacy of nocturnal nasal ventilation in stable, severe chronic obstructive pulmonary disease during a 3-month controlled trial. Mayo Clin Proc 1996; 71:533–542.
- Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med 1995; 152:538–544.
- Krachman SL, Chatila W, Martin UJ, et al. Effects of lung volume reduction surgery on sleep quality and nocturnal gas exchange in patients with severe emphysema. Chest 2005; 128:3221–3228.
- Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med 2013; 9:767–772.
- Jaoude P, Kufel T, El-Solh AA. Survival benefit of CPAP favors hypercapnic patients with the overlap syndrome. Lung 2014; 192:251–258.
- Ramagopal M, Mehta A, Roberts DW, et al. Asthma as a predictor of obstructive sleep apnea in urban African-American children. J Asthma 2009; 46:895–899.
- Ross KR, Storfer-Isser A, Hart MA, et al. Sleep-disordered breathing is associated with asthma severity in children. J Ped 2012; 160:736–742.
- Alharbi M, Almutairi A, Alotaibi D, Alotaibi A, Shaikh S, Bahammam AS. The prevalence of asthma in patients with obstructive sleep apnoea. Prim Care Respir J 2009; 18:328–330.
- Auckley D, Moallem M, Shaman Z, Mustafa M. Findings of a Berlin Questionnaire survey: comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep Med 2008; 9:494–499.
- Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. JAMA 2015; 313:156–164.
- Teodorescu M, Polomis DA, Hall SV, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest 2010; 138:543–550.
- Larsson LG, Lindberg A, Franklin KA, Lundback B. Gender differences in symptoms related to sleep apnea in a general population and in relation to referral to sleep clinic. Chest 2003; 124:204–211.
- ten Brinke A, Sterk PJ, Masclee AA, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J 2005; 26:812–818.
- Luyster FS, Teodorescu M, Bleecker E, et al. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep Breath 2012; 16:1129–1137.
- Catterall JR, Douglas NJ, Calverley PM, et al. Irregular breathing and hypoxaemia during sleep in chronic stable asthma. Lancet 1982; 1:301–304.
- Perez GF, Gutierrez MJ, Huseni S, et al. Oximetry signal processing identifies REM sleep-related vulnerability trait in asthmatic children. Sleep Disord 2013; 2013:406157.
- Yigla M, Tov N, Solomonov A, Rubin AH, Harlev D. Difficult-to-control asthma and obstructive sleep apnea. J Asthma 2003; 40:865–871.
- Kelly EA, Houtman JJ, Jarjour NN. Inflammatory changes associated with circadian variation in pulmonary function in subjects with mild asthma. Clin Exper Allergy 2004; 34:227–233.
- Bohadana AB, Hannhart B, Teculescu DB. Nocturnal worsening of asthma and sleep-disordered breathing. J Asthma 2002; 39:85–100.
- Lafond C, Series F, Lemiere C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. Eur Respir J 2007; 29:307–311.
- Martin RJ, Pak J. Nasal CPAP in nonapneic nocturnal asthma. Chest 1991; 100:1024–1027.
- Dixon AE, Pratley RE, Forgione PM, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol 2011; 128:508–515 e501–502.
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med 1985; 6:651–661.
- Ioachimescu OC, Teodorescu M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology 2013; 18:421–431.
- Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respiratory Med 2005; 99:529–534.
- Kim MY, Jo EJ, Kang SY, et al. Obstructive sleep apnea is associated with reduced quality of life in adult patients with asthma. Ann Allergy Asthma Immunol 2013; 110:253–257.
- Teodorescu M, Polomis DA, Teodorescu MC, et al. Association of obstructive sleep apnea risk or diagnosis with daytime asthma in adults. J Asthma 2012; 49:620–628.
- Puthalapattu S, Ioachimescu OC. Asthma and obstructive sleep apnea: clinical and pathogenic interactions. J Investig Med 2014; 62:665–675.
- National Institutes of Health. National Heart, Lung, and Blood Institute. NHLBI Factbook, Fiscal Year 2007. Chapter 4. Disease Statistics. www.nhlbi.nih.gov/about/factbook-07/chapter4.htm. Accessed November 11, 2015.
- Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365.
- WHO. World Health Organization: Asthma. Fact sheet No 307. www.who.int/mediacentre/factsheets/fs307/en/. Accessed November 11, 2015.
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004; 59:469–478.
- WHO. Chronic obstructive pulmonary disease (COPD). Fact sheet No 315. www.who.int/mediacentre/factsheets/fs315/en/index.html. 2011. Accessed November 11, 2015.
- Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988–1994 to 2007–2010. Chest 2013; 143:1395–1406.
- Orie N, Sluiter H, de Vries K, Tammeling G, Witkop J. The host factor in bronchitis. Paper presented at: Bronchitis—an international symposium 1961; Assen, Netherlands.
- Ballard RD. Sleep, respiratory physiology, and nocturnal asthma. Chronobiol Int 1999; 16:565–580.
- Teodorescu M, Polomis DA, Gangnon RE, et al. Asthma control and its relationship with obstructive sleep apnea (OSA) in older adults. Sleep Disord 2013; 2013:251567.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- International Classification of Sleep Disorders, 3rd ed.: Diagnostic and coding manual. Darien, Illinois: American Academy of Sleep Medicine. 2014.
- Lopez-Acevedo MN, Torres-Palacios A, Elena Ocasio-Tascon M, Campos-Santiago Z, Rodriguez-Cintron W. Overlap syndrome: an indication for sleep studies? A pilot study. Sleep Breath 2009; 13:409–413.
- Scharf C, Li P, Muntwyler J, et al. Rate-dependent AV delay optimization in cardiac resynchronization therapy. PACE 2005; 28:279–284.
- Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med 1995;151:82–86.
- Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration 2005; 72:142–149.
- Fletcher EC. Chronic lung disease in the sleep apnea syndrome. Lung 1990; 168(suppl):751–761.
- Shaya FT, Lin PJ, Aljawadi MH, Scharf SM. Elevated economic burden in obstructive lung disease patients with concomitant sleep apnea syndrome. Sleep Breath 2009; 13:317–323.
- Larsson LG, Lindberg A, Franklin KA, Lundbäck B; Obstructive Lung Disease in Northern Sweden Studies. Obstructive sleep apnoea syndrome is common in subjects with chronic bronchitis. Report from the Obstructive Lung Disease in Northern Sweden studies. Respiration 2001; 68:250–255.
- Machado MC, Vollmer WM, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Resp J 2010; 35:132–137.
- Guilleminault C, Cummiskey J, Motta J. Chronic obstructive airflow disease and sleep studies. Am Rev Respir Dis 1980; 122:397–406.
- Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5:237–241.
- Bradley TD, Rutherford R, Lue F, et al. Role of diffuse airway obstruction in the hypercapnia of obstructive sleep apnea. Am Rev Respir Dis 1986; 134:920–924.
- Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med 2003; 167:7–14.
- Breslin E, van der Schans C, Breukink S, et al. Perception of fatigue and quality of life in patients with COPD. Chest 1998; 114:958–964.
- Kapella MC, Larson JL, Patel MK, Covey MK, Berry JK. Subjective fatigue, influencing variables, and consequences in chronic obstructive pulmonary disease. Nurs Res 2006; 55:10–17.
- Klink M, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest 1987; 91:540–546.
- Bellia V, Catalano F, Scichilone N, et al. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA study. Sleep 2003; 26:318–323.
- Connaughton JJ, Catterall JR, Elton RA, Stradling JR, Douglas NJ. Do sleep studies contribute to the management of patients with severe chronic obstructive pulmonary disease? Am Rev Respir Dis 1988; 138:341–344.
- Mulloy E, McNicholas WT. Ventilation and gas exchange during sleep and exercise in severe COPD. Chest 1996; 109:387–394.
- Johnson MW, Remmers JE. Accessory muscle activity during sleep in chronic obstructive pulmonary disease. J Appl Physiol 1984; 57:1011–1017.
- Douglas NJ, White DP, Pickett CK, Weil JV, Zwillich CW. Respiration during sleep in normal man. Thorax 1982; 37:840–844.
- Mulloy E, Fitzpatrick M, Bourke S, O’Regan A, McNicholas WT. Oxygen desaturation during sleep and exercise in patients with severe chronic obstructive pulmonary disease. Respir Med 1995; 89:193–198.
- Herpel LB, Brown CD, Goring KL, et al. COPD cannot compensate for upper airway obstruction during sleep (abstract). Am J Respir Crit Care Med 2007; 175:A71.
- MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part 2. Am J Respir Crit Care Med 1994; 150:1158–1168.
- MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part 1. Am J Respir Crit Care Med 1994; 150:833–852.
- Budev MM, Arroliga AC, Wiedemann HP, Matthay RA. Cor pulmonale: an overview. Semin Respir Crit Care Med 2003; 24:233–244.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 suppl):D34–D41.
- Naeije R. Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2:20–22.
- Rasche K, Orth M, Kutscha A, Duchna HW. [Pulmonary diseases and heart function]. In German. Internist (Berl) 2007; 48:276–282.
- Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 1980; 93:391–398.
- Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med 2005; 165:2408–2413.
- Calverley PM, Brezinova V, Douglas NJ, Catterall JR, Flenley DC. The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis 1982; 126:206–210.
- Centers for Medicare and Medicaid Services. National coverage determination (NCD) for home use of oxygen (240.2). www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=169&ncdver=1&NCAId=169&NcaName=Home+Use+of+Oxygen&IsPopup=y&bc=AAAAAAAAIAAA&. Accessed November 11, 2015.
- Plywaczewski R, Sliwinski P, Nowinski A, Kaminski D, Zielinski J. Incidence of nocturnal desaturation while breathing oxygen in COPD patients undergoing long-term oxygen therapy. Chest 2000; 117:679–683.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breathing 2007; 11:117–124.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Marin JM, DeAndres R, Alonso J, Sanchez A, Carrizo S. Long term mortality in the overlap syndrome. Eur Resp J 2008; 32(suppl 52):P865.
- Reeves-Hoche MK, Hudgel DW, Meck R, Witteman R, Ross A, Zwillich CW. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med 1995; 151:443–449.
- Gay PC, Herold DL, Olson EJ. A randomized, double-blind clinical trial comparing continuous positive airway pressure with a novel bilevel pressure system for treatment of obstructive sleep apnea syndrome. Sleep 2003; 26:864–869.
- Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath 2012; 16:773–779.
- Randerath WJ, Galetke W, Ruhle KH. Auto-adjusting CPAP based on impedance versus bilevel pressure in difficult-to-treat sleep apnea syndrome: a prospective randomized crossover study. Med Sci Monit 2003; 9:CR353–CR358.
- Schwartz SW, Rosas J, Iannacone MR, Foulis PR, Anderson WM. Correlates of a prescription for bilevel positive airway pressure for treatment of obstructive sleep apnea among veterans. J Clin Sleep Med 2013; 9:327–335.
- Gentina T, Fortin F, Douay B, et al. Auto bi-level with pressure relief during exhalation as a rescue therapy for optimally treated obstructive sleep apnoea patients with poor compliance to continuous positive airways pressure therapy--a pilot study. Sleep Breathing 2011; 15:21–27.
- Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clinical Sleep Med 2007; 3:706–712.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 2010; 182:325–331.
- de Miguel J, Cabello J, Sanchez-Alarcos JM, Alvarez-Sala R, Espinos D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath 2002; 6:3–10.
- Mansfield D, Naughton MT. Effects of continuous positive airway pressure on lung function in patients with chronic obstructive pulmonary disease and sleep disordered breathing. Respirology 1999; 4:365–370.
- McEvoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009; 64:561–566.
- Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet 1981; 1:681–686.
- Machado MC, Vollmer WM, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J 2010; 35:132–137.
- Casanova C, Celli BR, Tost L, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000; 118:1582–1590.
- Clini E, Sturani C, Rossi A, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002; 20:529–538.
- Duiverman ML, Wempe JB, Bladder G, et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomized controlled trial. Respir Res 2011; 12:112.
- Gay PC, Hubmayr RD, Stroetz RW. Efficacy of nocturnal nasal ventilation in stable, severe chronic obstructive pulmonary disease during a 3-month controlled trial. Mayo Clin Proc 1996; 71:533–542.
- Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med 1995; 152:538–544.
- Krachman SL, Chatila W, Martin UJ, et al. Effects of lung volume reduction surgery on sleep quality and nocturnal gas exchange in patients with severe emphysema. Chest 2005; 128:3221–3228.
- Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med 2013; 9:767–772.
- Jaoude P, Kufel T, El-Solh AA. Survival benefit of CPAP favors hypercapnic patients with the overlap syndrome. Lung 2014; 192:251–258.
- Ramagopal M, Mehta A, Roberts DW, et al. Asthma as a predictor of obstructive sleep apnea in urban African-American children. J Asthma 2009; 46:895–899.
- Ross KR, Storfer-Isser A, Hart MA, et al. Sleep-disordered breathing is associated with asthma severity in children. J Ped 2012; 160:736–742.
- Alharbi M, Almutairi A, Alotaibi D, Alotaibi A, Shaikh S, Bahammam AS. The prevalence of asthma in patients with obstructive sleep apnoea. Prim Care Respir J 2009; 18:328–330.
- Auckley D, Moallem M, Shaman Z, Mustafa M. Findings of a Berlin Questionnaire survey: comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep Med 2008; 9:494–499.
- Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. JAMA 2015; 313:156–164.
- Teodorescu M, Polomis DA, Hall SV, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest 2010; 138:543–550.
- Larsson LG, Lindberg A, Franklin KA, Lundback B. Gender differences in symptoms related to sleep apnea in a general population and in relation to referral to sleep clinic. Chest 2003; 124:204–211.
- ten Brinke A, Sterk PJ, Masclee AA, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J 2005; 26:812–818.
- Luyster FS, Teodorescu M, Bleecker E, et al. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep Breath 2012; 16:1129–1137.
- Catterall JR, Douglas NJ, Calverley PM, et al. Irregular breathing and hypoxaemia during sleep in chronic stable asthma. Lancet 1982; 1:301–304.
- Perez GF, Gutierrez MJ, Huseni S, et al. Oximetry signal processing identifies REM sleep-related vulnerability trait in asthmatic children. Sleep Disord 2013; 2013:406157.
- Yigla M, Tov N, Solomonov A, Rubin AH, Harlev D. Difficult-to-control asthma and obstructive sleep apnea. J Asthma 2003; 40:865–871.
- Kelly EA, Houtman JJ, Jarjour NN. Inflammatory changes associated with circadian variation in pulmonary function in subjects with mild asthma. Clin Exper Allergy 2004; 34:227–233.
- Bohadana AB, Hannhart B, Teculescu DB. Nocturnal worsening of asthma and sleep-disordered breathing. J Asthma 2002; 39:85–100.
- Lafond C, Series F, Lemiere C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. Eur Respir J 2007; 29:307–311.
- Martin RJ, Pak J. Nasal CPAP in nonapneic nocturnal asthma. Chest 1991; 100:1024–1027.
- Dixon AE, Pratley RE, Forgione PM, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol 2011; 128:508–515 e501–502.
KEY POINTS
- Obstructive lung diseases and OSA are both common and may exacerbate each other.
- When assessing a patient with COPD, it may be prudent to think about whether the patient also has OSA, and vice versa.
- Oxygen therapy lowers the risk of death in patients with COPD but may worsen hypercapnia and apneic episodes in those with OSA.
- Continuous positive airway pressure is the first line of therapy for overlap syndrome. Daytime hypercapnia and nocturnal hypoxemia despite supplemental oxygen therapy are indications for nocturnal bilevel positive airway pressure therapy, regardless of the presence of OSA.
A tale of two sisters with liver disease
A 25-year-old woman presents to the emergency department with a 7-day history of fatigue and nausea. On presentation she denies having abdominal pain, headache, fever, chills, night sweats, vomiting, diarrhea, melena, hematochezia, or weight loss. She recalls changes in the colors of her eyes and darkening urine over the last few days. Her medical history before this is unremarkable. She takes no prescription, over-the-counter, or herbal medications. She works as a librarian and has no occupational toxic exposures. She is single and has one sister with no prior medical history. She denies recent travel, sick contacts, smoking, recreational drug use, or pets at home.
On physical examination, her vital signs are temperature 37.3°C (99.1°F), heart rate 90 beats per minute, blood pressure 125/80 mm Hg, respiration rate 14 per minute, and oxygen saturation 97% on room air. She has icteric sclera and her skin is jaundiced. Cardiac examination is normal. Lungs are clear to auscultation and percussion bilaterally. Her abdomen is soft with no visceromegaly, masses, or tenderness. Extremities are normal with no edema. She is alert and oriented, but she has mild asterixis of the outstretched hands. The neurologic examination is otherwise unremarkable.
The patient’s basic laboratory values are listed in Table 1. Shortly after admission, she develops changes in her mental status, remaining alert but becoming agitated and oriented to person only. In view of her symptoms and laboratory findings, acute liver failure is suspected.
ACUTE LIVER FAILURE
1. The diagnostic criteria for acute liver failure include all of the following except which one?
- Acute elevation of liver biochemical tests
- Presence of preexisting liver disease
- Coagulopathy, defined by an international normalized ratio (INR) of 1.5 or greater
- Encephalopathy
- Duration of symptoms less than 26 weeks
Acute liver failure is defined by acute onset of worsening liver tests, coagulopathy (INR ≥ 1.5), and encephalopathy in patients with no preexisting liver disease and with symptom duration of less than 26 weeks.1 With a few exceptions, a history of preexisting liver disease negates the diagnosis of acute liver failure. Our patient meets the diagnostic criteria for acute liver failure.
Immediate management
Once acute liver failure is identified or suspected, the next step is to transfer the patient to the intensive care unit for close monitoring of mental status. Serial neurologic evaluations permit early detection of cerebral edema, which is considered the most common cause of death in patients with acute liver failure. Additionally, close monitoring of electrolytes and plasma glucose is necessary since these patients are susceptible to electrolyte disturbances and hypoglycemia.
Patients with acute liver failure are at increased risk of infections and should be routinely screened by obtaining urine and blood cultures.
Gastrointestinal bleeding is not uncommon in patients with acute liver failure and is usually due to gastric stress ulceration. Prophylaxis with a histamine 2 receptor antagonist or proton pump inhibitor should be considered in order to prevent gastrointestinal bleeding.
Treatment with N-acetylcysteine is beneficial, not only in patients with acute liver failure due to acetaminophen overdose, but also in those with acute liver failure from other causes.
CASE CONTINUES:
TRANSFER TO THE INTENSIVE CARE UNIT
The patient, now diagnosed with acute liver failure, is transferred to the intensive care unit. Arterial blood gas measurement shows:
- pH 7.38 (reference range 7.35–7.45)
- Pco2 40 mm Hg (36–46)
- Po2 97 mm Hg (85–95)
- Hco3 22 mmol/L (22–26).
A chest radiograph is obtained and is clear. Computed tomography (CT) of the brain reveals no edema. Transcranial Doppler ultrasonography does not show any intracranial fluid collections.
Blood and urine cultures are negative. Her hemoglobin level remains stable, and she does not develop signs of bleeding. She is started on a proton pump inhibitor for stress ulcer prophylaxis and is empirically given intravenous N-acetylcysteine until the cause of acute liver failure can be determined.
CAUSES OF ACUTE LIVER FAILURE
2. Which of the following can cause acute liver failure?
- Acetaminophen overdose
- Viral hepatitis
- Autoimmune hepatitis
- Wilson disease
- Alcoholic hepatitis
Drug-induced liver injury is the most common cause of acute liver failure in the United States,2,3 and of all drugs, acetaminophen overdose is the number-one cause. In acetaminophen-induced liver injury, serum aminotransferase levels are usually elevated to more than 1,000 U/L, while serum bilirubin remains normal in the early stages. Antimicrobial agents, antiepileptic drugs, and herbal supplements have also been implicated in acute liver failure. Our patient has denied taking herbal supplements or medications, including over-the-counter ones.
Acute viral hepatitis can explain the patient’s condition. It is a common cause of acute liver failure in the United States.2 Hepatitis A and E are more common in developing countries. Other viruses such as cytomegalovirus, Epstein-Barr virus, herpes simplex virus type 1 and 2, and varicella zoster virus can also cause acute liver failure. Serum aminotransferase levels may exceed 1,000 U/L in patients with viral hepatitis.
Autoimmune hepatitis is a rare cause of acute liver failure, but it should be considered in the differential diagnosis, particularly in middle-aged women with autoimmune disorders such as hypothyroidism. Autoimmune hepatitis can cause marked elevation in aminotransferase levels (> 1,000 U/L).
Wilson disease is an autosomal-recessive disease in which there is excessive accumulation of copper in the liver and other organs because of an inherited defect in the biliary excretion of copper. Wilson disease can cause acute liver failure and should be excluded in any patient, particularly if under age 40 with acute onset of unexplained hepatic, neurologic, or psychiatric disease.
Alcoholic hepatitis usually occurs in patients with a long-standing history of heavy alcohol use. As a result, most patients with alcoholic hepatitis have manifestations of chronic liver disease due to alcohol use. Therefore, by definition, it is not a cause of acute liver failure. Additionally, in patients with alcoholic hepatitis, the aspartate aminotransferase (AST) level is elevated but less than 300 IU/mL, and the ratio of AST to alanine aminotransferase (ALT) is usually more than 2.
CASE CONTINUES: FURTHER TESTING
The results of our patient’s serologic tests are shown in Table 2. Other test results:
- Autoimmune markers including antinuclear antibodies, antimitochondrial antibodies, antismooth muscle antibodies, and liver and kidney microsomal antibodies are negative; her immunoglobulin G (IgG) level is normal
- Serum ceruloplasmin 25 mg/dL (normal 21–45)
- Free serum copper 120 µg/dL (normal 8–12)
- Abdominal ultrasonography is unremarkable, with normal liver parenchyma and no intrahepatic or extrahepatic biliary dilatation
- Doppler ultrasonography of the liver shows patent blood vessels.
3. Based on the new data, which of the following statements is correct?
- Hepatitis B is the cause of acute liver failure in this patient
- Herpetic hepatitis cannot be excluded on the basis of the available data
- Wilson disease is most likely the diagnosis, given her elevated free serum copper
- A normal serum ceruloplasmin level is not sufficient to rule out acute liver failure secondary to Wilson disease
Hepatitis B surface antigen and hepatitis B core antibodies were negative in our patient, excluding hepatitis B virus infection. The positive hepatitis B surface antibody indicates prior immunization.
Herpetic hepatitis is an uncommon but important cause of acute liver failure because the mortality rate is high if the patient is not treated early with acyclovir. Fever, elevated aminotransferases, and leukopenia are common with herpetic hepatitis. Fewer than 50% of patients with herpetic hepatitis have vesicular rash.4,5 The value of antibody serologic testing is limited due to high rates of false-positive and false-negative results. The gold standard diagnostic tests are viral load (detection of viral RNA by polymerase chain reaction), viral staining on liver biopsy, or both. In our patient, herpes simplex virus polymerase chain reaction testing was negative, which makes herpetic hepatitis unlikely.
Wilson disease is a genetic condition in which the ability to excrete copper in the bile is impaired, resulting in accumulation of copper in the hepatocytes. Subsequently, copper is released into the bloodstream and eventually into the urine.
However, copper excretion into the bile is impaired in patients with acute liver failure regardless of the etiology. Therefore, elevated free serum copper and 24-hour urine copper levels are not specific for the diagnosis of acute liver failure secondary to Wilson disease. Moreover, Kayser-Fleischer rings, which represent copper deposition in the limbus of the cornea, may not be apparent in the early stages of Wilson disease.
Since it is challenging to diagnose Wilson disease in the context of acute liver failure, Korman et al6 compared patients with acute liver failure secondary to Wilson disease with patients with acute liver failure secondary to other conditions. They found that alkaline phosphatase levels are frequently decreased in patients with acute liver failure secondary to Wilson disease,6 and that a ratio of alkaline phosphatase to total bilirubin of less than 4 is 94% sensitive and 96% specific for the diagnosis.6
Hemolysis is common in acute liver failure due to Wilson disease. This leads to disproportionate elevation of AST compared with ALT, since AST is present in red blood cells. Consequently, the ratio of AST to ALT is usually greater than 2.2, which provides a sensitivity of 94% and a specificity of 86% for the diagnosis.6 These two ratios together provide 100% sensitivity and 100% specificity for the diagnosis of Wilson disease in the context of acute liver failure.6
Ceruloplasmin. Patients with Wilson disease typically have a low ceruloplasmin level. However, because it is an acute-phase reaction protein, ceruloplasmin can be normal or elevated in patients with acute liver failure from Wilson disease.6 Therefore, a normal ceruloplasmin level is not sufficient to rule out acute liver failure secondary to Wilson disease.
CASE CONTINUES: A DEFINITIVE DIAGNOSIS
Our patient undergoes further testing, which reveals the following:
- Her 24-hour urinary excretion of copper is 150 µg (reference value < 30)
- Slit-lamp examination is normal and shows no evidence of Kayser-Fleischer rings
- Her ratio of alkaline phosphatase to total bilirubin is 0.77 based on her initial laboratory results (Table 1)
- Her AST-ALT ratio is 3.4.
The diagnosis in our patient is acute liver failure secondary to Wilson disease.
4. What is the most appropriate next step?
- Liver biopsy
- d-penicillamine by mouth
- Trientine by mouth
- Liver transplant
- Plasmapheresis
Liver biopsy. Accumulation of copper in the liver parenchyma in patients with Wilson disease is sporadic. Therefore, qualitative copper staining on liver biopsy can be falsely negative. Quantitative copper measurement in liver tissue is the gold standard for the diagnosis of Wilson disease. However, the test is time-consuming and is not rapidly available in the context of acute liver failure.
Chelating agents such as d-pencillamine and trientine are used to treat the chronic manifestations of Wilson disease but are not useful for acute liver failure secondary to Wilson disease.
Acute liver failure secondary to Wilson disease is life-threatening, and liver transplant is considered the only definitive life-saving therapy.
Therapeutic plasmapheresis has been reported to be a successful adjunctive therapy to bridge patients with acute liver failure secondary to Wilson disease to transplant.7 However, liver transplant is still the only definitive treatment.
CASE CONTINUES: THE PATIENT’S SISTER SEEKS CARE
The patient undergoes liver transplantation, with no perioperative or postoperative complications.
The patient’s 18-year-old sister is now seeking medical attention in the outpatient clinic, concerned that she may have Wilson disease. She is otherwise healthy and denies any symptoms or complaints.
5. What is the next step for the patient’s sister?
- Reassurance
- Prophylaxis with trientine
- Check liver enzyme levels, serum ceruloplasmin level, and urine copper, and order a slit-lamp examination
- Genetic testing
Wilson disease can be asymptomatic in its early stages and may be diagnosed incidentally during routine blood tests that reveal abnormal liver enzyme levels. All patients with a confirmed family history of Wilson disease should be screened even if they are asymptomatic. The diagnosis of Wilson disease should be established in first-degree relatives before specific treatment for the relatives is prescribed.
The first step in screening a first-degree relative for Wilson disease is to check liver enzyme levels (specifically aminotransferases, alkaline phosphatase, and bilirubin), serum ceruloplasmin level, and 24-hour urine copper, and order an ophthalmologic slit-lamp examination. If any of these tests is abnormal, liver biopsy should be performed for histopathologic evaluation and quantitative copper measurement. Kayser-Fleischer rings are seen in only 50% of patients with Wilson disease and hepatic involvement, but they are pathognomic. Guidelines8 for screening first-degree relatives of Wilson disease patients are shown in Figure 1.
Genetic analysis. ATP7B, the Wilson disease gene, is located on chromosome 13. At least 300 mutations of the gene have been described,2 and the most common mutation is present in only 15% to 30% of the Wilson disease population.8–10 Routine molecular testing of the ATP7B
CASE CONTINUES: WORKUP OF THE PATIENT’S SISTER
The patient’s sister has no symptoms and her physical examination is normal. Slit-lamp examination reveals no evidence of Kayser-Fleischer rings. Her laboratory values, including complete blood counts, complete metabolic panel, and INR, are within normal ranges. Other test results, however, are abnormal:
- Free serum copper level 27 µg/dL (normal 8–12)
- Serum ceruloplasmin 9.0 mg/dL (normal 20–50)
- 24-hour urinary copper excretion 135 µg (normal < 30).
She undergoes liver biopsy for quantitative copper measurement, and the result is very high at 1,118 µg/g dry weight (reference range 10–35). The diagnosis of Wilson disease is established.
TREATING CHRONIC WILSON DISEASE
6. Which of the following is not an appropriate next step for the patient’s sister?
- Tetrathiomolybdate
- d-penicillamine
- Trientine
- Zinc salts
- Prednisone
The goal of medical treatment of chronic Wilson disease is to improve symptoms and prevent progression of the disease.
Chelating agents and zinc salts are the most commonly used medicines in the management of Wilson disease. Chelating agents remove copper from tissue, whereas zinc blocks the intestinal absorption of copper and stimulates the synthesis of endogenous chelators such as metallothioneins. Tetrathiomolybdate is an alternative agent developed to interfere with the distribution of excess body copper to susceptible target sites by reducing free serum copper (Table 3). There are no data to support the use of prednisone in the treatment of Wilson disease.
During treatment with chelating agents, 24-hour urinary excretion of copper is routinely monitored to determine the efficacy of therapy and adherence to treatment. Once de-coppering is achieved, as evidenced by a normalization of 24-hour urine copper excretion, the chelating agent can be switched to zinc salts to prevent intestinal absorption of copper.
Clinical and biochemical stabilization is achieved typically within 2 to 6 months of the initial treatment with chelating agents.8 Organ meats, nuts, shellfish, and chocolate are rich in copper and should be avoided.
The patient’s sister is started on trientine 250 mg orally three times daily on an empty stomach at least 1 hour before meals. Treatment is monitored by following 24-hour urine copper measurement. A 24-hour urine copper measurement at 3 months after starting treatment has increased from 54 at baseline to 350 µg, which indicates that the copper is being removed from tissues. The plan is for early substitution of zinc for long-term maintenance once de-coppering is completed.
KEY POINTS
- Acute liver failure is severe acute liver injury characterized by coagulopathy (INR ≥ 1.5) and encephalopathy in a patient with no preexisting liver disease and with duration of symptoms less than 26 weeks.
- Acute liver failure secondary to Wilson disease is uncommon but should be excluded, particularly in young patients.
- The diagnosis of Wilson disease in the setting of acute liver failure is challenging because the serum ceruloplasmin level may be normal in acute liver failure secondary to Wilson disease, and free serum copper and 24-hour urine copper are usually elevated in all acute liver failure patients regardless of the etiology.
- A ratio of alkaline phosphatase to total bilirubin of less than 4 plus an AST-ALT ratio greater than 2.2 in a patient with acute liver failure should be regarded as Wilson disease until proven otherwise (Figure 2).
- Acute liver failure secondary to Wilson disease is usually fatal, and emergency liver transplant is a life-saving procedure.
- Screening of first-degree relatives of Wilson disease patients should include a history and physical examination, liver enzyme tests, complete blood cell count, serum ceruloplasmin level, serum free copper level, slit-lamp examination of the eyes, and 24-hour urinary copper measurement. Genetic tests are supplementary for screening but are not routinely available.
- Lee WM, Larson AM, Stravitz T. AASLD Position Paper: The management of acute liver failure: update 2011. www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Accessed December 9, 2015.
- Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet 2010; 376:190–201.
- Larson AM, Polson J, Fontana RJ, et al; Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42:1364–1372.
- Hanouneh IA, Khoriaty R, Zein NN. A 35-year-old Asian man with jaundice and markedly high aminotransferase levels. Cleve Clin J Med 2009; 76:449–456.
- Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl 2007; 13:1428–1434.
- Korman JD, Volenberg I, Balko J, et al; Pediatric and Adult Acute Liver Failure Study Groups. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology 2008; 48:1167–1174.
- Morgan SM, Zantek ND. Therapeutic plasma exchange for fulminant hepatic failure secondary to Wilson's disease. J Clin Apher 2012; 27:282–286.
- Roberts EA, Schilsky ML; American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: an update. Hepatology 2008; 47:2089–2111.
- Shah AB, Chernov I, Zhang HT, et al. Identification and analysis of mutations in the Wilson disease gene (ATP7B): population frequencies, genotype-phenotype correlation, and functional analyses. Am J Hum Genet 1997; 61:317–328.
- Maier-Dobersberger T, Ferenci P, Polli C, et al. Detection of the His1069Gln mutation in Wilson disease by rapid polymerase chain reaction. Ann Intern Med 1997; 127:21–26.
A 25-year-old woman presents to the emergency department with a 7-day history of fatigue and nausea. On presentation she denies having abdominal pain, headache, fever, chills, night sweats, vomiting, diarrhea, melena, hematochezia, or weight loss. She recalls changes in the colors of her eyes and darkening urine over the last few days. Her medical history before this is unremarkable. She takes no prescription, over-the-counter, or herbal medications. She works as a librarian and has no occupational toxic exposures. She is single and has one sister with no prior medical history. She denies recent travel, sick contacts, smoking, recreational drug use, or pets at home.
On physical examination, her vital signs are temperature 37.3°C (99.1°F), heart rate 90 beats per minute, blood pressure 125/80 mm Hg, respiration rate 14 per minute, and oxygen saturation 97% on room air. She has icteric sclera and her skin is jaundiced. Cardiac examination is normal. Lungs are clear to auscultation and percussion bilaterally. Her abdomen is soft with no visceromegaly, masses, or tenderness. Extremities are normal with no edema. She is alert and oriented, but she has mild asterixis of the outstretched hands. The neurologic examination is otherwise unremarkable.
The patient’s basic laboratory values are listed in Table 1. Shortly after admission, she develops changes in her mental status, remaining alert but becoming agitated and oriented to person only. In view of her symptoms and laboratory findings, acute liver failure is suspected.
ACUTE LIVER FAILURE
1. The diagnostic criteria for acute liver failure include all of the following except which one?
- Acute elevation of liver biochemical tests
- Presence of preexisting liver disease
- Coagulopathy, defined by an international normalized ratio (INR) of 1.5 or greater
- Encephalopathy
- Duration of symptoms less than 26 weeks
Acute liver failure is defined by acute onset of worsening liver tests, coagulopathy (INR ≥ 1.5), and encephalopathy in patients with no preexisting liver disease and with symptom duration of less than 26 weeks.1 With a few exceptions, a history of preexisting liver disease negates the diagnosis of acute liver failure. Our patient meets the diagnostic criteria for acute liver failure.
Immediate management
Once acute liver failure is identified or suspected, the next step is to transfer the patient to the intensive care unit for close monitoring of mental status. Serial neurologic evaluations permit early detection of cerebral edema, which is considered the most common cause of death in patients with acute liver failure. Additionally, close monitoring of electrolytes and plasma glucose is necessary since these patients are susceptible to electrolyte disturbances and hypoglycemia.
Patients with acute liver failure are at increased risk of infections and should be routinely screened by obtaining urine and blood cultures.
Gastrointestinal bleeding is not uncommon in patients with acute liver failure and is usually due to gastric stress ulceration. Prophylaxis with a histamine 2 receptor antagonist or proton pump inhibitor should be considered in order to prevent gastrointestinal bleeding.
Treatment with N-acetylcysteine is beneficial, not only in patients with acute liver failure due to acetaminophen overdose, but also in those with acute liver failure from other causes.
CASE CONTINUES:
TRANSFER TO THE INTENSIVE CARE UNIT
The patient, now diagnosed with acute liver failure, is transferred to the intensive care unit. Arterial blood gas measurement shows:
- pH 7.38 (reference range 7.35–7.45)
- Pco2 40 mm Hg (36–46)
- Po2 97 mm Hg (85–95)
- Hco3 22 mmol/L (22–26).
A chest radiograph is obtained and is clear. Computed tomography (CT) of the brain reveals no edema. Transcranial Doppler ultrasonography does not show any intracranial fluid collections.
Blood and urine cultures are negative. Her hemoglobin level remains stable, and she does not develop signs of bleeding. She is started on a proton pump inhibitor for stress ulcer prophylaxis and is empirically given intravenous N-acetylcysteine until the cause of acute liver failure can be determined.
CAUSES OF ACUTE LIVER FAILURE
2. Which of the following can cause acute liver failure?
- Acetaminophen overdose
- Viral hepatitis
- Autoimmune hepatitis
- Wilson disease
- Alcoholic hepatitis
Drug-induced liver injury is the most common cause of acute liver failure in the United States,2,3 and of all drugs, acetaminophen overdose is the number-one cause. In acetaminophen-induced liver injury, serum aminotransferase levels are usually elevated to more than 1,000 U/L, while serum bilirubin remains normal in the early stages. Antimicrobial agents, antiepileptic drugs, and herbal supplements have also been implicated in acute liver failure. Our patient has denied taking herbal supplements or medications, including over-the-counter ones.
Acute viral hepatitis can explain the patient’s condition. It is a common cause of acute liver failure in the United States.2 Hepatitis A and E are more common in developing countries. Other viruses such as cytomegalovirus, Epstein-Barr virus, herpes simplex virus type 1 and 2, and varicella zoster virus can also cause acute liver failure. Serum aminotransferase levels may exceed 1,000 U/L in patients with viral hepatitis.
Autoimmune hepatitis is a rare cause of acute liver failure, but it should be considered in the differential diagnosis, particularly in middle-aged women with autoimmune disorders such as hypothyroidism. Autoimmune hepatitis can cause marked elevation in aminotransferase levels (> 1,000 U/L).
Wilson disease is an autosomal-recessive disease in which there is excessive accumulation of copper in the liver and other organs because of an inherited defect in the biliary excretion of copper. Wilson disease can cause acute liver failure and should be excluded in any patient, particularly if under age 40 with acute onset of unexplained hepatic, neurologic, or psychiatric disease.
Alcoholic hepatitis usually occurs in patients with a long-standing history of heavy alcohol use. As a result, most patients with alcoholic hepatitis have manifestations of chronic liver disease due to alcohol use. Therefore, by definition, it is not a cause of acute liver failure. Additionally, in patients with alcoholic hepatitis, the aspartate aminotransferase (AST) level is elevated but less than 300 IU/mL, and the ratio of AST to alanine aminotransferase (ALT) is usually more than 2.
CASE CONTINUES: FURTHER TESTING
The results of our patient’s serologic tests are shown in Table 2. Other test results:
- Autoimmune markers including antinuclear antibodies, antimitochondrial antibodies, antismooth muscle antibodies, and liver and kidney microsomal antibodies are negative; her immunoglobulin G (IgG) level is normal
- Serum ceruloplasmin 25 mg/dL (normal 21–45)
- Free serum copper 120 µg/dL (normal 8–12)
- Abdominal ultrasonography is unremarkable, with normal liver parenchyma and no intrahepatic or extrahepatic biliary dilatation
- Doppler ultrasonography of the liver shows patent blood vessels.
3. Based on the new data, which of the following statements is correct?
- Hepatitis B is the cause of acute liver failure in this patient
- Herpetic hepatitis cannot be excluded on the basis of the available data
- Wilson disease is most likely the diagnosis, given her elevated free serum copper
- A normal serum ceruloplasmin level is not sufficient to rule out acute liver failure secondary to Wilson disease
Hepatitis B surface antigen and hepatitis B core antibodies were negative in our patient, excluding hepatitis B virus infection. The positive hepatitis B surface antibody indicates prior immunization.
Herpetic hepatitis is an uncommon but important cause of acute liver failure because the mortality rate is high if the patient is not treated early with acyclovir. Fever, elevated aminotransferases, and leukopenia are common with herpetic hepatitis. Fewer than 50% of patients with herpetic hepatitis have vesicular rash.4,5 The value of antibody serologic testing is limited due to high rates of false-positive and false-negative results. The gold standard diagnostic tests are viral load (detection of viral RNA by polymerase chain reaction), viral staining on liver biopsy, or both. In our patient, herpes simplex virus polymerase chain reaction testing was negative, which makes herpetic hepatitis unlikely.
Wilson disease is a genetic condition in which the ability to excrete copper in the bile is impaired, resulting in accumulation of copper in the hepatocytes. Subsequently, copper is released into the bloodstream and eventually into the urine.
However, copper excretion into the bile is impaired in patients with acute liver failure regardless of the etiology. Therefore, elevated free serum copper and 24-hour urine copper levels are not specific for the diagnosis of acute liver failure secondary to Wilson disease. Moreover, Kayser-Fleischer rings, which represent copper deposition in the limbus of the cornea, may not be apparent in the early stages of Wilson disease.
Since it is challenging to diagnose Wilson disease in the context of acute liver failure, Korman et al6 compared patients with acute liver failure secondary to Wilson disease with patients with acute liver failure secondary to other conditions. They found that alkaline phosphatase levels are frequently decreased in patients with acute liver failure secondary to Wilson disease,6 and that a ratio of alkaline phosphatase to total bilirubin of less than 4 is 94% sensitive and 96% specific for the diagnosis.6
Hemolysis is common in acute liver failure due to Wilson disease. This leads to disproportionate elevation of AST compared with ALT, since AST is present in red blood cells. Consequently, the ratio of AST to ALT is usually greater than 2.2, which provides a sensitivity of 94% and a specificity of 86% for the diagnosis.6 These two ratios together provide 100% sensitivity and 100% specificity for the diagnosis of Wilson disease in the context of acute liver failure.6
Ceruloplasmin. Patients with Wilson disease typically have a low ceruloplasmin level. However, because it is an acute-phase reaction protein, ceruloplasmin can be normal or elevated in patients with acute liver failure from Wilson disease.6 Therefore, a normal ceruloplasmin level is not sufficient to rule out acute liver failure secondary to Wilson disease.
CASE CONTINUES: A DEFINITIVE DIAGNOSIS
Our patient undergoes further testing, which reveals the following:
- Her 24-hour urinary excretion of copper is 150 µg (reference value < 30)
- Slit-lamp examination is normal and shows no evidence of Kayser-Fleischer rings
- Her ratio of alkaline phosphatase to total bilirubin is 0.77 based on her initial laboratory results (Table 1)
- Her AST-ALT ratio is 3.4.
The diagnosis in our patient is acute liver failure secondary to Wilson disease.
4. What is the most appropriate next step?
- Liver biopsy
- d-penicillamine by mouth
- Trientine by mouth
- Liver transplant
- Plasmapheresis
Liver biopsy. Accumulation of copper in the liver parenchyma in patients with Wilson disease is sporadic. Therefore, qualitative copper staining on liver biopsy can be falsely negative. Quantitative copper measurement in liver tissue is the gold standard for the diagnosis of Wilson disease. However, the test is time-consuming and is not rapidly available in the context of acute liver failure.
Chelating agents such as d-pencillamine and trientine are used to treat the chronic manifestations of Wilson disease but are not useful for acute liver failure secondary to Wilson disease.
Acute liver failure secondary to Wilson disease is life-threatening, and liver transplant is considered the only definitive life-saving therapy.
Therapeutic plasmapheresis has been reported to be a successful adjunctive therapy to bridge patients with acute liver failure secondary to Wilson disease to transplant.7 However, liver transplant is still the only definitive treatment.
CASE CONTINUES: THE PATIENT’S SISTER SEEKS CARE
The patient undergoes liver transplantation, with no perioperative or postoperative complications.
The patient’s 18-year-old sister is now seeking medical attention in the outpatient clinic, concerned that she may have Wilson disease. She is otherwise healthy and denies any symptoms or complaints.
5. What is the next step for the patient’s sister?
- Reassurance
- Prophylaxis with trientine
- Check liver enzyme levels, serum ceruloplasmin level, and urine copper, and order a slit-lamp examination
- Genetic testing
Wilson disease can be asymptomatic in its early stages and may be diagnosed incidentally during routine blood tests that reveal abnormal liver enzyme levels. All patients with a confirmed family history of Wilson disease should be screened even if they are asymptomatic. The diagnosis of Wilson disease should be established in first-degree relatives before specific treatment for the relatives is prescribed.
The first step in screening a first-degree relative for Wilson disease is to check liver enzyme levels (specifically aminotransferases, alkaline phosphatase, and bilirubin), serum ceruloplasmin level, and 24-hour urine copper, and order an ophthalmologic slit-lamp examination. If any of these tests is abnormal, liver biopsy should be performed for histopathologic evaluation and quantitative copper measurement. Kayser-Fleischer rings are seen in only 50% of patients with Wilson disease and hepatic involvement, but they are pathognomic. Guidelines8 for screening first-degree relatives of Wilson disease patients are shown in Figure 1.
Genetic analysis. ATP7B, the Wilson disease gene, is located on chromosome 13. At least 300 mutations of the gene have been described,2 and the most common mutation is present in only 15% to 30% of the Wilson disease population.8–10 Routine molecular testing of the ATP7B
CASE CONTINUES: WORKUP OF THE PATIENT’S SISTER
The patient’s sister has no symptoms and her physical examination is normal. Slit-lamp examination reveals no evidence of Kayser-Fleischer rings. Her laboratory values, including complete blood counts, complete metabolic panel, and INR, are within normal ranges. Other test results, however, are abnormal:
- Free serum copper level 27 µg/dL (normal 8–12)
- Serum ceruloplasmin 9.0 mg/dL (normal 20–50)
- 24-hour urinary copper excretion 135 µg (normal < 30).
She undergoes liver biopsy for quantitative copper measurement, and the result is very high at 1,118 µg/g dry weight (reference range 10–35). The diagnosis of Wilson disease is established.
TREATING CHRONIC WILSON DISEASE
6. Which of the following is not an appropriate next step for the patient’s sister?
- Tetrathiomolybdate
- d-penicillamine
- Trientine
- Zinc salts
- Prednisone
The goal of medical treatment of chronic Wilson disease is to improve symptoms and prevent progression of the disease.
Chelating agents and zinc salts are the most commonly used medicines in the management of Wilson disease. Chelating agents remove copper from tissue, whereas zinc blocks the intestinal absorption of copper and stimulates the synthesis of endogenous chelators such as metallothioneins. Tetrathiomolybdate is an alternative agent developed to interfere with the distribution of excess body copper to susceptible target sites by reducing free serum copper (Table 3). There are no data to support the use of prednisone in the treatment of Wilson disease.
During treatment with chelating agents, 24-hour urinary excretion of copper is routinely monitored to determine the efficacy of therapy and adherence to treatment. Once de-coppering is achieved, as evidenced by a normalization of 24-hour urine copper excretion, the chelating agent can be switched to zinc salts to prevent intestinal absorption of copper.
Clinical and biochemical stabilization is achieved typically within 2 to 6 months of the initial treatment with chelating agents.8 Organ meats, nuts, shellfish, and chocolate are rich in copper and should be avoided.
The patient’s sister is started on trientine 250 mg orally three times daily on an empty stomach at least 1 hour before meals. Treatment is monitored by following 24-hour urine copper measurement. A 24-hour urine copper measurement at 3 months after starting treatment has increased from 54 at baseline to 350 µg, which indicates that the copper is being removed from tissues. The plan is for early substitution of zinc for long-term maintenance once de-coppering is completed.
KEY POINTS
- Acute liver failure is severe acute liver injury characterized by coagulopathy (INR ≥ 1.5) and encephalopathy in a patient with no preexisting liver disease and with duration of symptoms less than 26 weeks.
- Acute liver failure secondary to Wilson disease is uncommon but should be excluded, particularly in young patients.
- The diagnosis of Wilson disease in the setting of acute liver failure is challenging because the serum ceruloplasmin level may be normal in acute liver failure secondary to Wilson disease, and free serum copper and 24-hour urine copper are usually elevated in all acute liver failure patients regardless of the etiology.
- A ratio of alkaline phosphatase to total bilirubin of less than 4 plus an AST-ALT ratio greater than 2.2 in a patient with acute liver failure should be regarded as Wilson disease until proven otherwise (Figure 2).
- Acute liver failure secondary to Wilson disease is usually fatal, and emergency liver transplant is a life-saving procedure.
- Screening of first-degree relatives of Wilson disease patients should include a history and physical examination, liver enzyme tests, complete blood cell count, serum ceruloplasmin level, serum free copper level, slit-lamp examination of the eyes, and 24-hour urinary copper measurement. Genetic tests are supplementary for screening but are not routinely available.
A 25-year-old woman presents to the emergency department with a 7-day history of fatigue and nausea. On presentation she denies having abdominal pain, headache, fever, chills, night sweats, vomiting, diarrhea, melena, hematochezia, or weight loss. She recalls changes in the colors of her eyes and darkening urine over the last few days. Her medical history before this is unremarkable. She takes no prescription, over-the-counter, or herbal medications. She works as a librarian and has no occupational toxic exposures. She is single and has one sister with no prior medical history. She denies recent travel, sick contacts, smoking, recreational drug use, or pets at home.
On physical examination, her vital signs are temperature 37.3°C (99.1°F), heart rate 90 beats per minute, blood pressure 125/80 mm Hg, respiration rate 14 per minute, and oxygen saturation 97% on room air. She has icteric sclera and her skin is jaundiced. Cardiac examination is normal. Lungs are clear to auscultation and percussion bilaterally. Her abdomen is soft with no visceromegaly, masses, or tenderness. Extremities are normal with no edema. She is alert and oriented, but she has mild asterixis of the outstretched hands. The neurologic examination is otherwise unremarkable.
The patient’s basic laboratory values are listed in Table 1. Shortly after admission, she develops changes in her mental status, remaining alert but becoming agitated and oriented to person only. In view of her symptoms and laboratory findings, acute liver failure is suspected.
ACUTE LIVER FAILURE
1. The diagnostic criteria for acute liver failure include all of the following except which one?
- Acute elevation of liver biochemical tests
- Presence of preexisting liver disease
- Coagulopathy, defined by an international normalized ratio (INR) of 1.5 or greater
- Encephalopathy
- Duration of symptoms less than 26 weeks
Acute liver failure is defined by acute onset of worsening liver tests, coagulopathy (INR ≥ 1.5), and encephalopathy in patients with no preexisting liver disease and with symptom duration of less than 26 weeks.1 With a few exceptions, a history of preexisting liver disease negates the diagnosis of acute liver failure. Our patient meets the diagnostic criteria for acute liver failure.
Immediate management
Once acute liver failure is identified or suspected, the next step is to transfer the patient to the intensive care unit for close monitoring of mental status. Serial neurologic evaluations permit early detection of cerebral edema, which is considered the most common cause of death in patients with acute liver failure. Additionally, close monitoring of electrolytes and plasma glucose is necessary since these patients are susceptible to electrolyte disturbances and hypoglycemia.
Patients with acute liver failure are at increased risk of infections and should be routinely screened by obtaining urine and blood cultures.
Gastrointestinal bleeding is not uncommon in patients with acute liver failure and is usually due to gastric stress ulceration. Prophylaxis with a histamine 2 receptor antagonist or proton pump inhibitor should be considered in order to prevent gastrointestinal bleeding.
Treatment with N-acetylcysteine is beneficial, not only in patients with acute liver failure due to acetaminophen overdose, but also in those with acute liver failure from other causes.
CASE CONTINUES:
TRANSFER TO THE INTENSIVE CARE UNIT
The patient, now diagnosed with acute liver failure, is transferred to the intensive care unit. Arterial blood gas measurement shows:
- pH 7.38 (reference range 7.35–7.45)
- Pco2 40 mm Hg (36–46)
- Po2 97 mm Hg (85–95)
- Hco3 22 mmol/L (22–26).
A chest radiograph is obtained and is clear. Computed tomography (CT) of the brain reveals no edema. Transcranial Doppler ultrasonography does not show any intracranial fluid collections.
Blood and urine cultures are negative. Her hemoglobin level remains stable, and she does not develop signs of bleeding. She is started on a proton pump inhibitor for stress ulcer prophylaxis and is empirically given intravenous N-acetylcysteine until the cause of acute liver failure can be determined.
CAUSES OF ACUTE LIVER FAILURE
2. Which of the following can cause acute liver failure?
- Acetaminophen overdose
- Viral hepatitis
- Autoimmune hepatitis
- Wilson disease
- Alcoholic hepatitis
Drug-induced liver injury is the most common cause of acute liver failure in the United States,2,3 and of all drugs, acetaminophen overdose is the number-one cause. In acetaminophen-induced liver injury, serum aminotransferase levels are usually elevated to more than 1,000 U/L, while serum bilirubin remains normal in the early stages. Antimicrobial agents, antiepileptic drugs, and herbal supplements have also been implicated in acute liver failure. Our patient has denied taking herbal supplements or medications, including over-the-counter ones.
Acute viral hepatitis can explain the patient’s condition. It is a common cause of acute liver failure in the United States.2 Hepatitis A and E are more common in developing countries. Other viruses such as cytomegalovirus, Epstein-Barr virus, herpes simplex virus type 1 and 2, and varicella zoster virus can also cause acute liver failure. Serum aminotransferase levels may exceed 1,000 U/L in patients with viral hepatitis.
Autoimmune hepatitis is a rare cause of acute liver failure, but it should be considered in the differential diagnosis, particularly in middle-aged women with autoimmune disorders such as hypothyroidism. Autoimmune hepatitis can cause marked elevation in aminotransferase levels (> 1,000 U/L).
Wilson disease is an autosomal-recessive disease in which there is excessive accumulation of copper in the liver and other organs because of an inherited defect in the biliary excretion of copper. Wilson disease can cause acute liver failure and should be excluded in any patient, particularly if under age 40 with acute onset of unexplained hepatic, neurologic, or psychiatric disease.
Alcoholic hepatitis usually occurs in patients with a long-standing history of heavy alcohol use. As a result, most patients with alcoholic hepatitis have manifestations of chronic liver disease due to alcohol use. Therefore, by definition, it is not a cause of acute liver failure. Additionally, in patients with alcoholic hepatitis, the aspartate aminotransferase (AST) level is elevated but less than 300 IU/mL, and the ratio of AST to alanine aminotransferase (ALT) is usually more than 2.
CASE CONTINUES: FURTHER TESTING
The results of our patient’s serologic tests are shown in Table 2. Other test results:
- Autoimmune markers including antinuclear antibodies, antimitochondrial antibodies, antismooth muscle antibodies, and liver and kidney microsomal antibodies are negative; her immunoglobulin G (IgG) level is normal
- Serum ceruloplasmin 25 mg/dL (normal 21–45)
- Free serum copper 120 µg/dL (normal 8–12)
- Abdominal ultrasonography is unremarkable, with normal liver parenchyma and no intrahepatic or extrahepatic biliary dilatation
- Doppler ultrasonography of the liver shows patent blood vessels.
3. Based on the new data, which of the following statements is correct?
- Hepatitis B is the cause of acute liver failure in this patient
- Herpetic hepatitis cannot be excluded on the basis of the available data
- Wilson disease is most likely the diagnosis, given her elevated free serum copper
- A normal serum ceruloplasmin level is not sufficient to rule out acute liver failure secondary to Wilson disease
Hepatitis B surface antigen and hepatitis B core antibodies were negative in our patient, excluding hepatitis B virus infection. The positive hepatitis B surface antibody indicates prior immunization.
Herpetic hepatitis is an uncommon but important cause of acute liver failure because the mortality rate is high if the patient is not treated early with acyclovir. Fever, elevated aminotransferases, and leukopenia are common with herpetic hepatitis. Fewer than 50% of patients with herpetic hepatitis have vesicular rash.4,5 The value of antibody serologic testing is limited due to high rates of false-positive and false-negative results. The gold standard diagnostic tests are viral load (detection of viral RNA by polymerase chain reaction), viral staining on liver biopsy, or both. In our patient, herpes simplex virus polymerase chain reaction testing was negative, which makes herpetic hepatitis unlikely.
Wilson disease is a genetic condition in which the ability to excrete copper in the bile is impaired, resulting in accumulation of copper in the hepatocytes. Subsequently, copper is released into the bloodstream and eventually into the urine.
However, copper excretion into the bile is impaired in patients with acute liver failure regardless of the etiology. Therefore, elevated free serum copper and 24-hour urine copper levels are not specific for the diagnosis of acute liver failure secondary to Wilson disease. Moreover, Kayser-Fleischer rings, which represent copper deposition in the limbus of the cornea, may not be apparent in the early stages of Wilson disease.
Since it is challenging to diagnose Wilson disease in the context of acute liver failure, Korman et al6 compared patients with acute liver failure secondary to Wilson disease with patients with acute liver failure secondary to other conditions. They found that alkaline phosphatase levels are frequently decreased in patients with acute liver failure secondary to Wilson disease,6 and that a ratio of alkaline phosphatase to total bilirubin of less than 4 is 94% sensitive and 96% specific for the diagnosis.6
Hemolysis is common in acute liver failure due to Wilson disease. This leads to disproportionate elevation of AST compared with ALT, since AST is present in red blood cells. Consequently, the ratio of AST to ALT is usually greater than 2.2, which provides a sensitivity of 94% and a specificity of 86% for the diagnosis.6 These two ratios together provide 100% sensitivity and 100% specificity for the diagnosis of Wilson disease in the context of acute liver failure.6
Ceruloplasmin. Patients with Wilson disease typically have a low ceruloplasmin level. However, because it is an acute-phase reaction protein, ceruloplasmin can be normal or elevated in patients with acute liver failure from Wilson disease.6 Therefore, a normal ceruloplasmin level is not sufficient to rule out acute liver failure secondary to Wilson disease.
CASE CONTINUES: A DEFINITIVE DIAGNOSIS
Our patient undergoes further testing, which reveals the following:
- Her 24-hour urinary excretion of copper is 150 µg (reference value < 30)
- Slit-lamp examination is normal and shows no evidence of Kayser-Fleischer rings
- Her ratio of alkaline phosphatase to total bilirubin is 0.77 based on her initial laboratory results (Table 1)
- Her AST-ALT ratio is 3.4.
The diagnosis in our patient is acute liver failure secondary to Wilson disease.
4. What is the most appropriate next step?
- Liver biopsy
- d-penicillamine by mouth
- Trientine by mouth
- Liver transplant
- Plasmapheresis
Liver biopsy. Accumulation of copper in the liver parenchyma in patients with Wilson disease is sporadic. Therefore, qualitative copper staining on liver biopsy can be falsely negative. Quantitative copper measurement in liver tissue is the gold standard for the diagnosis of Wilson disease. However, the test is time-consuming and is not rapidly available in the context of acute liver failure.
Chelating agents such as d-pencillamine and trientine are used to treat the chronic manifestations of Wilson disease but are not useful for acute liver failure secondary to Wilson disease.
Acute liver failure secondary to Wilson disease is life-threatening, and liver transplant is considered the only definitive life-saving therapy.
Therapeutic plasmapheresis has been reported to be a successful adjunctive therapy to bridge patients with acute liver failure secondary to Wilson disease to transplant.7 However, liver transplant is still the only definitive treatment.
CASE CONTINUES: THE PATIENT’S SISTER SEEKS CARE
The patient undergoes liver transplantation, with no perioperative or postoperative complications.
The patient’s 18-year-old sister is now seeking medical attention in the outpatient clinic, concerned that she may have Wilson disease. She is otherwise healthy and denies any symptoms or complaints.
5. What is the next step for the patient’s sister?
- Reassurance
- Prophylaxis with trientine
- Check liver enzyme levels, serum ceruloplasmin level, and urine copper, and order a slit-lamp examination
- Genetic testing
Wilson disease can be asymptomatic in its early stages and may be diagnosed incidentally during routine blood tests that reveal abnormal liver enzyme levels. All patients with a confirmed family history of Wilson disease should be screened even if they are asymptomatic. The diagnosis of Wilson disease should be established in first-degree relatives before specific treatment for the relatives is prescribed.
The first step in screening a first-degree relative for Wilson disease is to check liver enzyme levels (specifically aminotransferases, alkaline phosphatase, and bilirubin), serum ceruloplasmin level, and 24-hour urine copper, and order an ophthalmologic slit-lamp examination. If any of these tests is abnormal, liver biopsy should be performed for histopathologic evaluation and quantitative copper measurement. Kayser-Fleischer rings are seen in only 50% of patients with Wilson disease and hepatic involvement, but they are pathognomic. Guidelines8 for screening first-degree relatives of Wilson disease patients are shown in Figure 1.
Genetic analysis. ATP7B, the Wilson disease gene, is located on chromosome 13. At least 300 mutations of the gene have been described,2 and the most common mutation is present in only 15% to 30% of the Wilson disease population.8–10 Routine molecular testing of the ATP7B
CASE CONTINUES: WORKUP OF THE PATIENT’S SISTER
The patient’s sister has no symptoms and her physical examination is normal. Slit-lamp examination reveals no evidence of Kayser-Fleischer rings. Her laboratory values, including complete blood counts, complete metabolic panel, and INR, are within normal ranges. Other test results, however, are abnormal:
- Free serum copper level 27 µg/dL (normal 8–12)
- Serum ceruloplasmin 9.0 mg/dL (normal 20–50)
- 24-hour urinary copper excretion 135 µg (normal < 30).
She undergoes liver biopsy for quantitative copper measurement, and the result is very high at 1,118 µg/g dry weight (reference range 10–35). The diagnosis of Wilson disease is established.
TREATING CHRONIC WILSON DISEASE
6. Which of the following is not an appropriate next step for the patient’s sister?
- Tetrathiomolybdate
- d-penicillamine
- Trientine
- Zinc salts
- Prednisone
The goal of medical treatment of chronic Wilson disease is to improve symptoms and prevent progression of the disease.
Chelating agents and zinc salts are the most commonly used medicines in the management of Wilson disease. Chelating agents remove copper from tissue, whereas zinc blocks the intestinal absorption of copper and stimulates the synthesis of endogenous chelators such as metallothioneins. Tetrathiomolybdate is an alternative agent developed to interfere with the distribution of excess body copper to susceptible target sites by reducing free serum copper (Table 3). There are no data to support the use of prednisone in the treatment of Wilson disease.
During treatment with chelating agents, 24-hour urinary excretion of copper is routinely monitored to determine the efficacy of therapy and adherence to treatment. Once de-coppering is achieved, as evidenced by a normalization of 24-hour urine copper excretion, the chelating agent can be switched to zinc salts to prevent intestinal absorption of copper.
Clinical and biochemical stabilization is achieved typically within 2 to 6 months of the initial treatment with chelating agents.8 Organ meats, nuts, shellfish, and chocolate are rich in copper and should be avoided.
The patient’s sister is started on trientine 250 mg orally three times daily on an empty stomach at least 1 hour before meals. Treatment is monitored by following 24-hour urine copper measurement. A 24-hour urine copper measurement at 3 months after starting treatment has increased from 54 at baseline to 350 µg, which indicates that the copper is being removed from tissues. The plan is for early substitution of zinc for long-term maintenance once de-coppering is completed.
KEY POINTS
- Acute liver failure is severe acute liver injury characterized by coagulopathy (INR ≥ 1.5) and encephalopathy in a patient with no preexisting liver disease and with duration of symptoms less than 26 weeks.
- Acute liver failure secondary to Wilson disease is uncommon but should be excluded, particularly in young patients.
- The diagnosis of Wilson disease in the setting of acute liver failure is challenging because the serum ceruloplasmin level may be normal in acute liver failure secondary to Wilson disease, and free serum copper and 24-hour urine copper are usually elevated in all acute liver failure patients regardless of the etiology.
- A ratio of alkaline phosphatase to total bilirubin of less than 4 plus an AST-ALT ratio greater than 2.2 in a patient with acute liver failure should be regarded as Wilson disease until proven otherwise (Figure 2).
- Acute liver failure secondary to Wilson disease is usually fatal, and emergency liver transplant is a life-saving procedure.
- Screening of first-degree relatives of Wilson disease patients should include a history and physical examination, liver enzyme tests, complete blood cell count, serum ceruloplasmin level, serum free copper level, slit-lamp examination of the eyes, and 24-hour urinary copper measurement. Genetic tests are supplementary for screening but are not routinely available.
- Lee WM, Larson AM, Stravitz T. AASLD Position Paper: The management of acute liver failure: update 2011. www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Accessed December 9, 2015.
- Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet 2010; 376:190–201.
- Larson AM, Polson J, Fontana RJ, et al; Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42:1364–1372.
- Hanouneh IA, Khoriaty R, Zein NN. A 35-year-old Asian man with jaundice and markedly high aminotransferase levels. Cleve Clin J Med 2009; 76:449–456.
- Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl 2007; 13:1428–1434.
- Korman JD, Volenberg I, Balko J, et al; Pediatric and Adult Acute Liver Failure Study Groups. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology 2008; 48:1167–1174.
- Morgan SM, Zantek ND. Therapeutic plasma exchange for fulminant hepatic failure secondary to Wilson's disease. J Clin Apher 2012; 27:282–286.
- Roberts EA, Schilsky ML; American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: an update. Hepatology 2008; 47:2089–2111.
- Shah AB, Chernov I, Zhang HT, et al. Identification and analysis of mutations in the Wilson disease gene (ATP7B): population frequencies, genotype-phenotype correlation, and functional analyses. Am J Hum Genet 1997; 61:317–328.
- Maier-Dobersberger T, Ferenci P, Polli C, et al. Detection of the His1069Gln mutation in Wilson disease by rapid polymerase chain reaction. Ann Intern Med 1997; 127:21–26.
- Lee WM, Larson AM, Stravitz T. AASLD Position Paper: The management of acute liver failure: update 2011. www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Accessed December 9, 2015.
- Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet 2010; 376:190–201.
- Larson AM, Polson J, Fontana RJ, et al; Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42:1364–1372.
- Hanouneh IA, Khoriaty R, Zein NN. A 35-year-old Asian man with jaundice and markedly high aminotransferase levels. Cleve Clin J Med 2009; 76:449–456.
- Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl 2007; 13:1428–1434.
- Korman JD, Volenberg I, Balko J, et al; Pediatric and Adult Acute Liver Failure Study Groups. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology 2008; 48:1167–1174.
- Morgan SM, Zantek ND. Therapeutic plasma exchange for fulminant hepatic failure secondary to Wilson's disease. J Clin Apher 2012; 27:282–286.
- Roberts EA, Schilsky ML; American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: an update. Hepatology 2008; 47:2089–2111.
- Shah AB, Chernov I, Zhang HT, et al. Identification and analysis of mutations in the Wilson disease gene (ATP7B): population frequencies, genotype-phenotype correlation, and functional analyses. Am J Hum Genet 1997; 61:317–328.
- Maier-Dobersberger T, Ferenci P, Polli C, et al. Detection of the His1069Gln mutation in Wilson disease by rapid polymerase chain reaction. Ann Intern Med 1997; 127:21–26.
Bulldog scalp
A 54-year-old man presented with a 2-year history of unusual skin folds on the scalp with deep furrows in an anteroposterior direction, located in parieto-occipital regions (Figure 1). A clinical diagnosis of cutis verticis gyrata was made.
CUTIS VERTICIS GYRATA: THE DIFFERENTIAL DIAGNOSIS
Cutis verticis gyrata (“bulldog scalp”) is a rare condition, with a prevalence of 0.026 to 0.1 per 100,000,1 primary and secondary forms, and a male preponderance.2 It is characterized by excessive soft-tissue proliferation with the formation of ridges on the scalp similar in appearance to cerebral cortex gyri.
Primary essential cutis verticis gyrata is extremely rare with no associated abnormalities. Primary nonessential cutis verticis gyrata is associated with neurologic manifestations (microcephaly, seizure, cerebral palsy, mental retardation) and ophthalmologic changes (cataract, strabismus, retinitis pigmentosa, blindness).
Cutis verticis gyrata can also be secondary to conditions such as pachydermoperiostosis, Rosenthal-Kloepfer syndrome, tuberous sclerosis, and insulin resistance syndrome.3 It may occur in fragile X syndrome, Noonan syndrome, Turner syndrome, Beare-Stevenson syndrome, and Ehlers-Danlos syndrome.
When cutis verticis gyrata presents at age 50 or later, acromegaly, amyloidosis, myxedema, paraneoplastic syndromes, and drug-related lipodystrophy from antiretroviral drugs or tyrosine kinase inhibitors should be excluded. Other conditions included in the differential diagnosis are inflammatory diseases of the scalp (psoriasis, pemphigus) and nevoid abnormalities (nevus sebaceous, nevus of Ota, cerebriform nevus).4 The male preponderance suggests a genetic determination and an endocrine cause, but the pathophysiology remains unknown.
MANAGEMENT IN OUR PATIENT
Further evaluation in our patient showed bossing of the frontal bone, coarse facial features, and acral enlargement suggestive of acromegaly. The diagnosis was confirmed by elevated levels of growth hormone and insulin-like growth factor 1.
Magnetic resonance imaging of the pituitary gland revealed a pituitary adenoma 11 × 6 × 8 mm. After treatment of the adenoma with stereotactic radiosurgery, the scalp soft-tissue thickness decreased but persisted.
Overgrowth of the scalp manifesting as cutis verticis gyrata in acromegaly is not uncommon.2,4 The severity or duration of acromegaly is not correlated with the presence and severity of cutis verticis gyrata.4
Besides treatment of acromegaly, good scalp hygiene is necessary to avoid the accumulation of secretions in the furrows. Surgery for scalp reduction is only required for cosmetic reasons.5
- Akesson HO. Cutis verticis gyrata and mental deficiency in Sweden. I. Epidemiologic and clinical aspects. Acta Med Scand 1964; 175:115–127.
- Polan S, Butterworth T. Cutis verticis gyrata: a review with report of seven new cases. Am J Ment Defic 1953; 57:613–631.
- Larsen F, Birchall N. Cutis verticis gyrata: three cases with different aetiologies that demonstrate the classification system. Australas J Dermatol 2007; 48:91–94.
- Kolawole TM, AI Orainy IA, Patel PJ, Fathuddin S. Cutis verticis gyrata: its computed tomographic demonstration in acromegaly. Eur J Radiol 1998; 27:145–148.
- Garden JM, Robinson JK. Essential primary cutis verticis gyrata. Treatment with the scalp reduction procedure. Arch Dermatol 1984; 120:1480–1483.
A 54-year-old man presented with a 2-year history of unusual skin folds on the scalp with deep furrows in an anteroposterior direction, located in parieto-occipital regions (Figure 1). A clinical diagnosis of cutis verticis gyrata was made.
CUTIS VERTICIS GYRATA: THE DIFFERENTIAL DIAGNOSIS
Cutis verticis gyrata (“bulldog scalp”) is a rare condition, with a prevalence of 0.026 to 0.1 per 100,000,1 primary and secondary forms, and a male preponderance.2 It is characterized by excessive soft-tissue proliferation with the formation of ridges on the scalp similar in appearance to cerebral cortex gyri.
Primary essential cutis verticis gyrata is extremely rare with no associated abnormalities. Primary nonessential cutis verticis gyrata is associated with neurologic manifestations (microcephaly, seizure, cerebral palsy, mental retardation) and ophthalmologic changes (cataract, strabismus, retinitis pigmentosa, blindness).
Cutis verticis gyrata can also be secondary to conditions such as pachydermoperiostosis, Rosenthal-Kloepfer syndrome, tuberous sclerosis, and insulin resistance syndrome.3 It may occur in fragile X syndrome, Noonan syndrome, Turner syndrome, Beare-Stevenson syndrome, and Ehlers-Danlos syndrome.
When cutis verticis gyrata presents at age 50 or later, acromegaly, amyloidosis, myxedema, paraneoplastic syndromes, and drug-related lipodystrophy from antiretroviral drugs or tyrosine kinase inhibitors should be excluded. Other conditions included in the differential diagnosis are inflammatory diseases of the scalp (psoriasis, pemphigus) and nevoid abnormalities (nevus sebaceous, nevus of Ota, cerebriform nevus).4 The male preponderance suggests a genetic determination and an endocrine cause, but the pathophysiology remains unknown.
MANAGEMENT IN OUR PATIENT
Further evaluation in our patient showed bossing of the frontal bone, coarse facial features, and acral enlargement suggestive of acromegaly. The diagnosis was confirmed by elevated levels of growth hormone and insulin-like growth factor 1.
Magnetic resonance imaging of the pituitary gland revealed a pituitary adenoma 11 × 6 × 8 mm. After treatment of the adenoma with stereotactic radiosurgery, the scalp soft-tissue thickness decreased but persisted.
Overgrowth of the scalp manifesting as cutis verticis gyrata in acromegaly is not uncommon.2,4 The severity or duration of acromegaly is not correlated with the presence and severity of cutis verticis gyrata.4
Besides treatment of acromegaly, good scalp hygiene is necessary to avoid the accumulation of secretions in the furrows. Surgery for scalp reduction is only required for cosmetic reasons.5
A 54-year-old man presented with a 2-year history of unusual skin folds on the scalp with deep furrows in an anteroposterior direction, located in parieto-occipital regions (Figure 1). A clinical diagnosis of cutis verticis gyrata was made.
CUTIS VERTICIS GYRATA: THE DIFFERENTIAL DIAGNOSIS
Cutis verticis gyrata (“bulldog scalp”) is a rare condition, with a prevalence of 0.026 to 0.1 per 100,000,1 primary and secondary forms, and a male preponderance.2 It is characterized by excessive soft-tissue proliferation with the formation of ridges on the scalp similar in appearance to cerebral cortex gyri.
Primary essential cutis verticis gyrata is extremely rare with no associated abnormalities. Primary nonessential cutis verticis gyrata is associated with neurologic manifestations (microcephaly, seizure, cerebral palsy, mental retardation) and ophthalmologic changes (cataract, strabismus, retinitis pigmentosa, blindness).
Cutis verticis gyrata can also be secondary to conditions such as pachydermoperiostosis, Rosenthal-Kloepfer syndrome, tuberous sclerosis, and insulin resistance syndrome.3 It may occur in fragile X syndrome, Noonan syndrome, Turner syndrome, Beare-Stevenson syndrome, and Ehlers-Danlos syndrome.
When cutis verticis gyrata presents at age 50 or later, acromegaly, amyloidosis, myxedema, paraneoplastic syndromes, and drug-related lipodystrophy from antiretroviral drugs or tyrosine kinase inhibitors should be excluded. Other conditions included in the differential diagnosis are inflammatory diseases of the scalp (psoriasis, pemphigus) and nevoid abnormalities (nevus sebaceous, nevus of Ota, cerebriform nevus).4 The male preponderance suggests a genetic determination and an endocrine cause, but the pathophysiology remains unknown.
MANAGEMENT IN OUR PATIENT
Further evaluation in our patient showed bossing of the frontal bone, coarse facial features, and acral enlargement suggestive of acromegaly. The diagnosis was confirmed by elevated levels of growth hormone and insulin-like growth factor 1.
Magnetic resonance imaging of the pituitary gland revealed a pituitary adenoma 11 × 6 × 8 mm. After treatment of the adenoma with stereotactic radiosurgery, the scalp soft-tissue thickness decreased but persisted.
Overgrowth of the scalp manifesting as cutis verticis gyrata in acromegaly is not uncommon.2,4 The severity or duration of acromegaly is not correlated with the presence and severity of cutis verticis gyrata.4
Besides treatment of acromegaly, good scalp hygiene is necessary to avoid the accumulation of secretions in the furrows. Surgery for scalp reduction is only required for cosmetic reasons.5
- Akesson HO. Cutis verticis gyrata and mental deficiency in Sweden. I. Epidemiologic and clinical aspects. Acta Med Scand 1964; 175:115–127.
- Polan S, Butterworth T. Cutis verticis gyrata: a review with report of seven new cases. Am J Ment Defic 1953; 57:613–631.
- Larsen F, Birchall N. Cutis verticis gyrata: three cases with different aetiologies that demonstrate the classification system. Australas J Dermatol 2007; 48:91–94.
- Kolawole TM, AI Orainy IA, Patel PJ, Fathuddin S. Cutis verticis gyrata: its computed tomographic demonstration in acromegaly. Eur J Radiol 1998; 27:145–148.
- Garden JM, Robinson JK. Essential primary cutis verticis gyrata. Treatment with the scalp reduction procedure. Arch Dermatol 1984; 120:1480–1483.
- Akesson HO. Cutis verticis gyrata and mental deficiency in Sweden. I. Epidemiologic and clinical aspects. Acta Med Scand 1964; 175:115–127.
- Polan S, Butterworth T. Cutis verticis gyrata: a review with report of seven new cases. Am J Ment Defic 1953; 57:613–631.
- Larsen F, Birchall N. Cutis verticis gyrata: three cases with different aetiologies that demonstrate the classification system. Australas J Dermatol 2007; 48:91–94.
- Kolawole TM, AI Orainy IA, Patel PJ, Fathuddin S. Cutis verticis gyrata: its computed tomographic demonstration in acromegaly. Eur J Radiol 1998; 27:145–148.
- Garden JM, Robinson JK. Essential primary cutis verticis gyrata. Treatment with the scalp reduction procedure. Arch Dermatol 1984; 120:1480–1483.
A 60-year-old man with forehead swelling
A 60-year-old man presented to our emergency department with a 4-day history of frontal headaches he described as “stinging.” He had also had a large swollen area on his forehead for the past 8 weeks.
He denied fevers, chills, nausea, vomiting, blurry vision, tinnitus, and neck pain, as well as any recent sinus infection, intransanal cocaine use, rhinorrhea, or head trauma. A month ago, he had presented to our emergency department with forehead swelling but no headaches. At that time, the swelling was thought to be an allergic reaction to lisinopril or metformin, medications he takes for hypertension and type 2 diabetes. He had been discharged home with a prescription for a course of prednisone in tapering doses, but that had failed to resolve the swelling.
Physical examination revealed a well-circumscribed area of swelling, 3 by 4 cm, in the central forehead (Figure 1). The area was warm, erythematous, fluctuant, and tender to palpation. The nasal septum was intact and the nasal mucosa appeared pink and healthy. The remainder of the examination was unremarkable.
He was afebrile and hemodynamically stable. His peripheral white blood cell count was mildly elevated at 11.1 × 109. Computed tomography of the brain and sinuses revealed a fluid collection in the frontal scalp associated with erosion of the anterior frontal sinus with posterior extension and enhancement of the adjacent meninges. Magnetic resonance imaging (Figure 2) revealed similar findings. A diagnosis of Pott puffy tumor was made based on the imaging findings.
The name of this condition is misleading, as it is not a neoplasm but an infection. It requires urgent antibiotic therapy and surgical management because of the high risk of the infection spreading to the brain. Our patient was started on a broad-spectrum antibiotic regimen of intravenous vancomycin, ceftriaxone, and metronidazole pending tissue culture to identify the causative organism.
POTT PUFFY TUMOR: A BRIEF OVERVIEW
First described in 1760 by Sir Percivall Pott,1 the same English surgeon who first described tuberculosis of the spine, Pott puffy tumor is a well-demarcated area of swelling that occurs when a frontal sinus infection breaks through the anterior portion of the frontal sinus and forms an abscess between the frontal bone and periosteum with associated osteomyelitis.2 Though rare in adults (it is more common in children and adolescents),3 Pott puffy tumor is caused by conditions often encountered in internal medicine practice, such as bacterial sinusitis, head trauma, and intranasal cocaine use.
The infection can spread to the brain either directly by destruction of the posterior frontal sinus (as in our patient) or by way of the veins that drain the frontal sinus. Meningitis, epidural empyema, frontal lobe abscess, and cavernous sinus thrombosis2 have all been described. Intracranial complications are seen in nearly 100% of children and adolescents with Pott puffy tumor. The rate in adults is 30%,4,5 which is much lower but is nevertheless worrisome because patients can be initially misdiagnosed with scalp abscess,3 cellulitis, or epidermoid cyst,4 and then sent home from the emergency department or physician’s office. In a case series of 32 adult patients with Pott puffy tumor, nearly 45% were initially misdiagnosed, most often by an internist, dermatologist, ophthalmologist, or emergency room physician.4
The most common infective organisms are streptococci, staphylococci, and anaerobes,4 but Haemophilus, Aspergillus species, and invasive mucormycosis have also been described.
MANAGEMENT OPTIONS
Because of the risk of spread of the infection to the brain, rapid initiation of a broad-spectrum antibiotic is warranted in all patients with Pott puffy tumor pending results of tissue culture. Antibiotics may be necessary for at least 4 to 6 weeks to resolve osteomyelitis of the frontal bone and to decrease inflammation before surgery.6
Endoscopic sinus surgery is routinely done to drain the infected sinus and to remove or debride infected bone. Patients with intracranial extension of infection may require a combined endoscopic and neurosurgical approach.
OUTCOME
Our patient’s puffy tumor spontaneously ruptured externally on hospital day 3, and the purulent fluid was sent for culture that grew Streptococcus anginosus. His headaches improved almost immediately after this occurred. The antibiotic regimen was narrowed to ceftriaxone and metronidazole, and 1 week later he was discharged home with instructions to complete a 6-week course of antibiotics. Three weeks after he was discharged, he returned for outpatient endoscopic sinus surgery. At a follow-up visit 2 weeks after surgery, the forehead swelling had resolved, and he was well.
- Tattersall R, Tattersall R. Pott’s puffy tumor. Lancet 2002; 359:1060–1063.
- Forgie SE, Marrie TJ. Pott’s puffy tumor. Am J Med 2008; 121:1041–1042.
- Grewal HS, Dangaych NS, Esposito A. A tumor that is not a tumor but it sure can kill! Am J Case Rep 2012; 13:133–136.
- Akiyama K, Karaki M, Mori N. Evaluation of adult Pott’s puffy tumor: our five cases and 27 literature cases. Laryngoscope 2012; 122:2382–2388.
- Suwan PT, Mogal S, Chaudhary S. Pott’s puffy tumor: an uncommon clinical entity. Case Rep Pediatr 2012; 2012:386104.
- Lauria RA, Laffitte Fernandes F, Brito TP, Pereira PS, Chone CT. Extensive frontoparietal abscess: complication of frontal sinusitis (Pott’s puffy tumor). Case Rep Otolaryngol 2014; 2014:632464.
A 60-year-old man presented to our emergency department with a 4-day history of frontal headaches he described as “stinging.” He had also had a large swollen area on his forehead for the past 8 weeks.
He denied fevers, chills, nausea, vomiting, blurry vision, tinnitus, and neck pain, as well as any recent sinus infection, intransanal cocaine use, rhinorrhea, or head trauma. A month ago, he had presented to our emergency department with forehead swelling but no headaches. At that time, the swelling was thought to be an allergic reaction to lisinopril or metformin, medications he takes for hypertension and type 2 diabetes. He had been discharged home with a prescription for a course of prednisone in tapering doses, but that had failed to resolve the swelling.
Physical examination revealed a well-circumscribed area of swelling, 3 by 4 cm, in the central forehead (Figure 1). The area was warm, erythematous, fluctuant, and tender to palpation. The nasal septum was intact and the nasal mucosa appeared pink and healthy. The remainder of the examination was unremarkable.
He was afebrile and hemodynamically stable. His peripheral white blood cell count was mildly elevated at 11.1 × 109. Computed tomography of the brain and sinuses revealed a fluid collection in the frontal scalp associated with erosion of the anterior frontal sinus with posterior extension and enhancement of the adjacent meninges. Magnetic resonance imaging (Figure 2) revealed similar findings. A diagnosis of Pott puffy tumor was made based on the imaging findings.
The name of this condition is misleading, as it is not a neoplasm but an infection. It requires urgent antibiotic therapy and surgical management because of the high risk of the infection spreading to the brain. Our patient was started on a broad-spectrum antibiotic regimen of intravenous vancomycin, ceftriaxone, and metronidazole pending tissue culture to identify the causative organism.
POTT PUFFY TUMOR: A BRIEF OVERVIEW
First described in 1760 by Sir Percivall Pott,1 the same English surgeon who first described tuberculosis of the spine, Pott puffy tumor is a well-demarcated area of swelling that occurs when a frontal sinus infection breaks through the anterior portion of the frontal sinus and forms an abscess between the frontal bone and periosteum with associated osteomyelitis.2 Though rare in adults (it is more common in children and adolescents),3 Pott puffy tumor is caused by conditions often encountered in internal medicine practice, such as bacterial sinusitis, head trauma, and intranasal cocaine use.
The infection can spread to the brain either directly by destruction of the posterior frontal sinus (as in our patient) or by way of the veins that drain the frontal sinus. Meningitis, epidural empyema, frontal lobe abscess, and cavernous sinus thrombosis2 have all been described. Intracranial complications are seen in nearly 100% of children and adolescents with Pott puffy tumor. The rate in adults is 30%,4,5 which is much lower but is nevertheless worrisome because patients can be initially misdiagnosed with scalp abscess,3 cellulitis, or epidermoid cyst,4 and then sent home from the emergency department or physician’s office. In a case series of 32 adult patients with Pott puffy tumor, nearly 45% were initially misdiagnosed, most often by an internist, dermatologist, ophthalmologist, or emergency room physician.4
The most common infective organisms are streptococci, staphylococci, and anaerobes,4 but Haemophilus, Aspergillus species, and invasive mucormycosis have also been described.
MANAGEMENT OPTIONS
Because of the risk of spread of the infection to the brain, rapid initiation of a broad-spectrum antibiotic is warranted in all patients with Pott puffy tumor pending results of tissue culture. Antibiotics may be necessary for at least 4 to 6 weeks to resolve osteomyelitis of the frontal bone and to decrease inflammation before surgery.6
Endoscopic sinus surgery is routinely done to drain the infected sinus and to remove or debride infected bone. Patients with intracranial extension of infection may require a combined endoscopic and neurosurgical approach.
OUTCOME
Our patient’s puffy tumor spontaneously ruptured externally on hospital day 3, and the purulent fluid was sent for culture that grew Streptococcus anginosus. His headaches improved almost immediately after this occurred. The antibiotic regimen was narrowed to ceftriaxone and metronidazole, and 1 week later he was discharged home with instructions to complete a 6-week course of antibiotics. Three weeks after he was discharged, he returned for outpatient endoscopic sinus surgery. At a follow-up visit 2 weeks after surgery, the forehead swelling had resolved, and he was well.
A 60-year-old man presented to our emergency department with a 4-day history of frontal headaches he described as “stinging.” He had also had a large swollen area on his forehead for the past 8 weeks.
He denied fevers, chills, nausea, vomiting, blurry vision, tinnitus, and neck pain, as well as any recent sinus infection, intransanal cocaine use, rhinorrhea, or head trauma. A month ago, he had presented to our emergency department with forehead swelling but no headaches. At that time, the swelling was thought to be an allergic reaction to lisinopril or metformin, medications he takes for hypertension and type 2 diabetes. He had been discharged home with a prescription for a course of prednisone in tapering doses, but that had failed to resolve the swelling.
Physical examination revealed a well-circumscribed area of swelling, 3 by 4 cm, in the central forehead (Figure 1). The area was warm, erythematous, fluctuant, and tender to palpation. The nasal septum was intact and the nasal mucosa appeared pink and healthy. The remainder of the examination was unremarkable.
He was afebrile and hemodynamically stable. His peripheral white blood cell count was mildly elevated at 11.1 × 109. Computed tomography of the brain and sinuses revealed a fluid collection in the frontal scalp associated with erosion of the anterior frontal sinus with posterior extension and enhancement of the adjacent meninges. Magnetic resonance imaging (Figure 2) revealed similar findings. A diagnosis of Pott puffy tumor was made based on the imaging findings.
The name of this condition is misleading, as it is not a neoplasm but an infection. It requires urgent antibiotic therapy and surgical management because of the high risk of the infection spreading to the brain. Our patient was started on a broad-spectrum antibiotic regimen of intravenous vancomycin, ceftriaxone, and metronidazole pending tissue culture to identify the causative organism.
POTT PUFFY TUMOR: A BRIEF OVERVIEW
First described in 1760 by Sir Percivall Pott,1 the same English surgeon who first described tuberculosis of the spine, Pott puffy tumor is a well-demarcated area of swelling that occurs when a frontal sinus infection breaks through the anterior portion of the frontal sinus and forms an abscess between the frontal bone and periosteum with associated osteomyelitis.2 Though rare in adults (it is more common in children and adolescents),3 Pott puffy tumor is caused by conditions often encountered in internal medicine practice, such as bacterial sinusitis, head trauma, and intranasal cocaine use.
The infection can spread to the brain either directly by destruction of the posterior frontal sinus (as in our patient) or by way of the veins that drain the frontal sinus. Meningitis, epidural empyema, frontal lobe abscess, and cavernous sinus thrombosis2 have all been described. Intracranial complications are seen in nearly 100% of children and adolescents with Pott puffy tumor. The rate in adults is 30%,4,5 which is much lower but is nevertheless worrisome because patients can be initially misdiagnosed with scalp abscess,3 cellulitis, or epidermoid cyst,4 and then sent home from the emergency department or physician’s office. In a case series of 32 adult patients with Pott puffy tumor, nearly 45% were initially misdiagnosed, most often by an internist, dermatologist, ophthalmologist, or emergency room physician.4
The most common infective organisms are streptococci, staphylococci, and anaerobes,4 but Haemophilus, Aspergillus species, and invasive mucormycosis have also been described.
MANAGEMENT OPTIONS
Because of the risk of spread of the infection to the brain, rapid initiation of a broad-spectrum antibiotic is warranted in all patients with Pott puffy tumor pending results of tissue culture. Antibiotics may be necessary for at least 4 to 6 weeks to resolve osteomyelitis of the frontal bone and to decrease inflammation before surgery.6
Endoscopic sinus surgery is routinely done to drain the infected sinus and to remove or debride infected bone. Patients with intracranial extension of infection may require a combined endoscopic and neurosurgical approach.
OUTCOME
Our patient’s puffy tumor spontaneously ruptured externally on hospital day 3, and the purulent fluid was sent for culture that grew Streptococcus anginosus. His headaches improved almost immediately after this occurred. The antibiotic regimen was narrowed to ceftriaxone and metronidazole, and 1 week later he was discharged home with instructions to complete a 6-week course of antibiotics. Three weeks after he was discharged, he returned for outpatient endoscopic sinus surgery. At a follow-up visit 2 weeks after surgery, the forehead swelling had resolved, and he was well.
- Tattersall R, Tattersall R. Pott’s puffy tumor. Lancet 2002; 359:1060–1063.
- Forgie SE, Marrie TJ. Pott’s puffy tumor. Am J Med 2008; 121:1041–1042.
- Grewal HS, Dangaych NS, Esposito A. A tumor that is not a tumor but it sure can kill! Am J Case Rep 2012; 13:133–136.
- Akiyama K, Karaki M, Mori N. Evaluation of adult Pott’s puffy tumor: our five cases and 27 literature cases. Laryngoscope 2012; 122:2382–2388.
- Suwan PT, Mogal S, Chaudhary S. Pott’s puffy tumor: an uncommon clinical entity. Case Rep Pediatr 2012; 2012:386104.
- Lauria RA, Laffitte Fernandes F, Brito TP, Pereira PS, Chone CT. Extensive frontoparietal abscess: complication of frontal sinusitis (Pott’s puffy tumor). Case Rep Otolaryngol 2014; 2014:632464.
- Tattersall R, Tattersall R. Pott’s puffy tumor. Lancet 2002; 359:1060–1063.
- Forgie SE, Marrie TJ. Pott’s puffy tumor. Am J Med 2008; 121:1041–1042.
- Grewal HS, Dangaych NS, Esposito A. A tumor that is not a tumor but it sure can kill! Am J Case Rep 2012; 13:133–136.
- Akiyama K, Karaki M, Mori N. Evaluation of adult Pott’s puffy tumor: our five cases and 27 literature cases. Laryngoscope 2012; 122:2382–2388.
- Suwan PT, Mogal S, Chaudhary S. Pott’s puffy tumor: an uncommon clinical entity. Case Rep Pediatr 2012; 2012:386104.
- Lauria RA, Laffitte Fernandes F, Brito TP, Pereira PS, Chone CT. Extensive frontoparietal abscess: complication of frontal sinusitis (Pott’s puffy tumor). Case Rep Otolaryngol 2014; 2014:632464.
Obesity and exercise
Obesity means having a body mass index (BMI) of 30 or higher. Being obese increases your risk of health problems including high blood pressure, diabetes, cholesterol, arthritis, cancer, and cardiovascular diseases such as stroke and heart attack. You can reduce these risks by losing weight.
The healthy way to lose weight is to eat fewer calories, eat less processed food and more whole foods, and exercise regularly. A dietitian can help you create a flexible and balanced eating plan to help you meet your goals.
When beginning an exercise plan, start slowly with a combination of aerobic, resistance, flexibility, and balance exercises. A combined aerobic and resistance exercise program will likely result in more weight loss than either alone.
Aerobic exercises should be the foundation of your program. Choose exercises that involve large muscle groups, such as walking. Walking is the easiest way for most people to start exercising, but you can also consider other exercises such as stationary bicycling, slow jogging, and water aerobics.
Resistance training involves lifting weights using either weight machines or free weights (dumbbells).
Flexibility exercises are a type of stretching that improves the movements of your muscles, joints, and ligaments.
Balance exercises improve your stability and reduce the chance of falling or other injuries. These exercises can be done without any equipment. For example, with single-leg balance, you balance on one foot for 15 seconds. A stand-sit involves standing up and sitting down without using your hands.
Your provider will design an exercise program for you that includes the frequency, intensity, time, and types of exercise. Typically, you’ll want to lose about 10% of your weight over a 6-month period. Be sure to set SMART goals (Specific, Measurable, Attainable, Realistic, Timely) to sustain the self-discipline required for long-term success. Also consider tracking your physical activity using a wearable device (eg, Fitbit) or a smartphone app. It lets you see your progress over time, helps you set new goals, and helps keep you motivated.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints available by calling 216-444-2661.
For patient information on hundreds of health topics, visit the Center for Consumer Health Information website, www.clevelandclinic.org/health.
Obesity means having a body mass index (BMI) of 30 or higher. Being obese increases your risk of health problems including high blood pressure, diabetes, cholesterol, arthritis, cancer, and cardiovascular diseases such as stroke and heart attack. You can reduce these risks by losing weight.
The healthy way to lose weight is to eat fewer calories, eat less processed food and more whole foods, and exercise regularly. A dietitian can help you create a flexible and balanced eating plan to help you meet your goals.
When beginning an exercise plan, start slowly with a combination of aerobic, resistance, flexibility, and balance exercises. A combined aerobic and resistance exercise program will likely result in more weight loss than either alone.
Aerobic exercises should be the foundation of your program. Choose exercises that involve large muscle groups, such as walking. Walking is the easiest way for most people to start exercising, but you can also consider other exercises such as stationary bicycling, slow jogging, and water aerobics.
Resistance training involves lifting weights using either weight machines or free weights (dumbbells).
Flexibility exercises are a type of stretching that improves the movements of your muscles, joints, and ligaments.
Balance exercises improve your stability and reduce the chance of falling or other injuries. These exercises can be done without any equipment. For example, with single-leg balance, you balance on one foot for 15 seconds. A stand-sit involves standing up and sitting down without using your hands.
Your provider will design an exercise program for you that includes the frequency, intensity, time, and types of exercise. Typically, you’ll want to lose about 10% of your weight over a 6-month period. Be sure to set SMART goals (Specific, Measurable, Attainable, Realistic, Timely) to sustain the self-discipline required for long-term success. Also consider tracking your physical activity using a wearable device (eg, Fitbit) or a smartphone app. It lets you see your progress over time, helps you set new goals, and helps keep you motivated.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints available by calling 216-444-2661.
For patient information on hundreds of health topics, visit the Center for Consumer Health Information website, www.clevelandclinic.org/health.
Obesity means having a body mass index (BMI) of 30 or higher. Being obese increases your risk of health problems including high blood pressure, diabetes, cholesterol, arthritis, cancer, and cardiovascular diseases such as stroke and heart attack. You can reduce these risks by losing weight.
The healthy way to lose weight is to eat fewer calories, eat less processed food and more whole foods, and exercise regularly. A dietitian can help you create a flexible and balanced eating plan to help you meet your goals.
When beginning an exercise plan, start slowly with a combination of aerobic, resistance, flexibility, and balance exercises. A combined aerobic and resistance exercise program will likely result in more weight loss than either alone.
Aerobic exercises should be the foundation of your program. Choose exercises that involve large muscle groups, such as walking. Walking is the easiest way for most people to start exercising, but you can also consider other exercises such as stationary bicycling, slow jogging, and water aerobics.
Resistance training involves lifting weights using either weight machines or free weights (dumbbells).
Flexibility exercises are a type of stretching that improves the movements of your muscles, joints, and ligaments.
Balance exercises improve your stability and reduce the chance of falling or other injuries. These exercises can be done without any equipment. For example, with single-leg balance, you balance on one foot for 15 seconds. A stand-sit involves standing up and sitting down without using your hands.
Your provider will design an exercise program for you that includes the frequency, intensity, time, and types of exercise. Typically, you’ll want to lose about 10% of your weight over a 6-month period. Be sure to set SMART goals (Specific, Measurable, Attainable, Realistic, Timely) to sustain the self-discipline required for long-term success. Also consider tracking your physical activity using a wearable device (eg, Fitbit) or a smartphone app. It lets you see your progress over time, helps you set new goals, and helps keep you motivated.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints available by calling 216-444-2661.
For patient information on hundreds of health topics, visit the Center for Consumer Health Information website, www.clevelandclinic.org/health.
Tailor chronic pain interventions to the patient’s clinical profile



February 2016 Digital Edition
Table of Contents
- A Prescription for Music Lessons
- A New View for the VA
- Management of Diabetic Foot Ulcers: A Review
- Monitoring Heat Injuries in a Hazmat Environment
- Hyponatremia Secondary to Lisinopril in a Veteran Patient
- Clinical Video Telehealth for Gait and Balance
- Asymptomatic but Time for a Hip Revision
- Impact of Interprofessional Care Teams on Metabolic Parameters in Patients

Table of Contents
- A Prescription for Music Lessons
- A New View for the VA
- Management of Diabetic Foot Ulcers: A Review
- Monitoring Heat Injuries in a Hazmat Environment
- Hyponatremia Secondary to Lisinopril in a Veteran Patient
- Clinical Video Telehealth for Gait and Balance
- Asymptomatic but Time for a Hip Revision
- Impact of Interprofessional Care Teams on Metabolic Parameters in Patients

Table of Contents
- A Prescription for Music Lessons
- A New View for the VA
- Management of Diabetic Foot Ulcers: A Review
- Monitoring Heat Injuries in a Hazmat Environment
- Hyponatremia Secondary to Lisinopril in a Veteran Patient
- Clinical Video Telehealth for Gait and Balance
- Asymptomatic but Time for a Hip Revision
- Impact of Interprofessional Care Teams on Metabolic Parameters in Patients

Adoption of Choosing Wisely Recommendations Slow to Catch On
Clinical question: Have the Choosing Wisely campaign recommendations led to changes in practice?
Background: The Choosing Wisely campaign aims to reduce the incidence of low-value care by providing evidence-based recommendations for common clinical situations. The rate of adoption of these recommendations is unknown.
Study design: Retrospective review.
Setting: Anthem insurance members.
Synopsis: The study examined the claims data from 25 million Anthem insurance members to compare the rate of services that were targeted by seven Choosing Wisely campaign recommendations before and after the recommendations were published in 2012.
Investigators found the incidence of two of the services declined after the Choosing Wisely recommendations were published; the other five services remained stable or increased slightly. Furthermore, the declines were statistically significant but not a marked absolute difference, with the incidence of head imaging in patients with uncomplicated headaches going down to 13.4% from 14.9% and the use of cardiac imaging in the absence of cardiac disease declining to 9.7% from 10.8%.
The main limitations are the narrow population of Anthem insurance members and the lack of specific data that could help answer why clinical practice has not changed, but that could be the aim of future studies.
Bottom line: Choosing Wisely recommendations have not been adopted on a population level; widespread implementation likely will require financial incentives, provider-level data feedback, and systems interventions.
Citation: Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. doi:10.1001/jamainternmed.2015.5441.
Clinical question: Have the Choosing Wisely campaign recommendations led to changes in practice?
Background: The Choosing Wisely campaign aims to reduce the incidence of low-value care by providing evidence-based recommendations for common clinical situations. The rate of adoption of these recommendations is unknown.
Study design: Retrospective review.
Setting: Anthem insurance members.
Synopsis: The study examined the claims data from 25 million Anthem insurance members to compare the rate of services that were targeted by seven Choosing Wisely campaign recommendations before and after the recommendations were published in 2012.
Investigators found the incidence of two of the services declined after the Choosing Wisely recommendations were published; the other five services remained stable or increased slightly. Furthermore, the declines were statistically significant but not a marked absolute difference, with the incidence of head imaging in patients with uncomplicated headaches going down to 13.4% from 14.9% and the use of cardiac imaging in the absence of cardiac disease declining to 9.7% from 10.8%.
The main limitations are the narrow population of Anthem insurance members and the lack of specific data that could help answer why clinical practice has not changed, but that could be the aim of future studies.
Bottom line: Choosing Wisely recommendations have not been adopted on a population level; widespread implementation likely will require financial incentives, provider-level data feedback, and systems interventions.
Citation: Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. doi:10.1001/jamainternmed.2015.5441.
Clinical question: Have the Choosing Wisely campaign recommendations led to changes in practice?
Background: The Choosing Wisely campaign aims to reduce the incidence of low-value care by providing evidence-based recommendations for common clinical situations. The rate of adoption of these recommendations is unknown.
Study design: Retrospective review.
Setting: Anthem insurance members.
Synopsis: The study examined the claims data from 25 million Anthem insurance members to compare the rate of services that were targeted by seven Choosing Wisely campaign recommendations before and after the recommendations were published in 2012.
Investigators found the incidence of two of the services declined after the Choosing Wisely recommendations were published; the other five services remained stable or increased slightly. Furthermore, the declines were statistically significant but not a marked absolute difference, with the incidence of head imaging in patients with uncomplicated headaches going down to 13.4% from 14.9% and the use of cardiac imaging in the absence of cardiac disease declining to 9.7% from 10.8%.
The main limitations are the narrow population of Anthem insurance members and the lack of specific data that could help answer why clinical practice has not changed, but that could be the aim of future studies.
Bottom line: Choosing Wisely recommendations have not been adopted on a population level; widespread implementation likely will require financial incentives, provider-level data feedback, and systems interventions.
Citation: Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. doi:10.1001/jamainternmed.2015.5441.