User login
Man Finds Worst Way to Get Out of Shoveling Snow
ANSWER
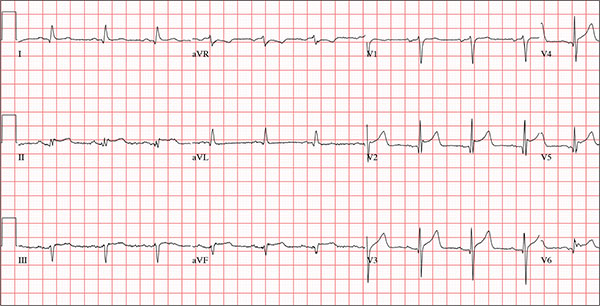
The correct answer is normal sinus rhythm, acute anterior myocardial infarction, and inferolateral injury.
A P wave for every QRS and a QRS for every P wave at a rate between 60 and 100 beats/min are indicative of normal sinus rhythm.
Acute anterior myocardial infarction is evidenced by the significant Q waves and ST elevations in leads I and V2 to V4 and inferolateral injury by ST elevations in limb leads II, III, and aVF and precordial leads V5 and V6.
Subsequent cardiac catheterization revealed an occlusion of the proximal left anterior descending coronary artery, as well as significant lesions in the posterior descending artery and a marginal branch of the circumflex coronary artery.
ANSWER
The correct answer is normal sinus rhythm, acute anterior myocardial infarction, and inferolateral injury.
A P wave for every QRS and a QRS for every P wave at a rate between 60 and 100 beats/min are indicative of normal sinus rhythm.
Acute anterior myocardial infarction is evidenced by the significant Q waves and ST elevations in leads I and V2 to V4 and inferolateral injury by ST elevations in limb leads II, III, and aVF and precordial leads V5 and V6.
Subsequent cardiac catheterization revealed an occlusion of the proximal left anterior descending coronary artery, as well as significant lesions in the posterior descending artery and a marginal branch of the circumflex coronary artery.
ANSWER
The correct answer is normal sinus rhythm, acute anterior myocardial infarction, and inferolateral injury.
A P wave for every QRS and a QRS for every P wave at a rate between 60 and 100 beats/min are indicative of normal sinus rhythm.
Acute anterior myocardial infarction is evidenced by the significant Q waves and ST elevations in leads I and V2 to V4 and inferolateral injury by ST elevations in limb leads II, III, and aVF and precordial leads V5 and V6.
Subsequent cardiac catheterization revealed an occlusion of the proximal left anterior descending coronary artery, as well as significant lesions in the posterior descending artery and a marginal branch of the circumflex coronary artery.
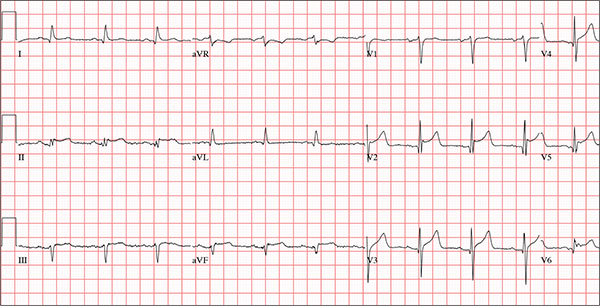
A 66-year-old man presents with ongoing chest pain of 45 minutes’ duration followed by ventricular fibrillation. He was outside shoveling snow for about 30 minutes before his wife noticed that the sound of shoveling had ceased and her husband was nowhere to be seen. Upon investigation, she found him sitting on the porch, holding his chest and moaning, and she immediately called 911. Paramedics arrived within 10 minutes. When the ambulance pulled into the driveway, the patient stood up and collapsed. The paramedics identified ventricular fibrillation and resuscitated him with a single shock from the automatic external defibrillator. The patient quickly regained consciousness and did not require further intervention. According to the paramedics, the patient was in ventricular fibrillation for less than one minute. Oxygen and anti-angina therapy, started in the field, provided prompt relief of his chest pain. Upon arrival to the emergency department, the patient is awake, stable, and fully cognizant of what happened and where he is. History taking reveals that he has experienced chest pain with exertion for several weeks, beginning around Thanksgiving, but did not want to worry his family during the holiday season. All prior episodes stopped as soon as he ceased physical activity, unlike the one he experienced today. The patient has a history of hypertension but no other cardiac problems. Surgical history is remarkable for a right rotator cuff repair and removal of a melanoma from his nose. Family history is positive for myocardial infarction (both parents and paternal grandfather) and type 1 diabetes (mother). The patient, an only child, has two adult sons, both of whom are in good health. The patient is a retired attorney. He drinks approximately one bottle of wine per week, does not drink beer or hard liquor, and has never smoked. He has been quite active and runs 10K races three or four times per year. He denies recreational or herbal drug use. He currently takes no medications—not even the hydrochlorothiazide and metoprolol prescribed for his hypertension, neither of which he has taken for the past year. He says he occasionally takes ibuprofen for “typical muscle aches and pains.” He has no known drug allergies. The review of systems is unremarkable. The patient denies palpitations, shortness of breath, recent weight gain, and headaches. Physical exam reveals a well-nourished, well-groomed man with a blood pressure of 160/98 mm Hg; pulse, 80 beats/min; respiratory rate, 18 breaths/min-1; O2 saturation, 100% on 2 L of oxygen via nasal cannula; and temperature, 98.2°F. He reports his weight to be 189 lb and his height, 6 ft 2 in. There are no unusual findings: His lungs are clear; his cardiac exam reveals no murmurs, rubs, gallops, or arrhythmia; the abdomen is soft and not tender; there is no peripheral edema; and he is neurologically intact. An ECG, laboratory tests, and an echocardiogram are ordered. You review the ECG while the other tests are pending and note a ventricular rate of 80 beats/min; PR interval, 162 ms; QRS duration, 106 ms; QT/QTc interval, 390/426 ms; P axis, 51°; R axis, –20°; and T axis, 70°. What is your interpretation of this ECG?
Finding Spot-on Treatment for Acne
ANSWER
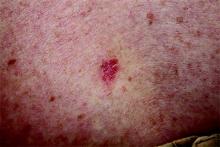
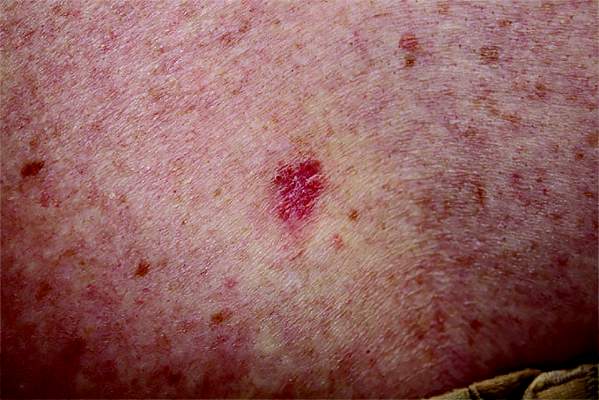
The correct answer is food (choice “a”), which for many generations has been blamed for worsening acne (along with other nonfactors, such as makeup). All the others are demonstrably involved in the genesis and perpetuation of acne.
DISCUSSION
Teenagers have a hard enough time dealing with acne and other vicissitudes of puberty, and then they get blamed for eating the wrong kinds of food …. Would that it could be that simple! I think it’s important for us as providers to set the record straight by making sure parents and patients know what matters and what doesn’t.
When we’ve done that, the patient (or occasionally a parent) might say, “Well, every time I eat (insert item here), my acne flares.” To which we of course reply, “Well then, don’t do that!” After all, we certainly wouldn’t object to the patient consuming a better diet.
Once the unimportance of pizza, makeup, and soft drinks has been established, there remains the opportunity to enlighten the patient (and family) about the factors that do play a significant role—all but one of which can be addressed. (The exception, of course, is heredity; still, I believe it’s important to recognize its role in acne.) We can reduce the amount of sebum through use of retinoids and cut down on bacteria by using oral or topical antibiotics (though erythromycin is not especially effective). Hormonal therapy can be accomplished with oral contraceptives or oral spironolactone, though neither is perfect.
Treatment
This particular patient was prescribed a six-month course of isotretinoin (40 mg/d), after which her acne was completely and permanently gone. This is the result in about 70% of cases when this medicine is used correctly.
Proper procedure, including pregnancy tests and blood work, was followed before the patient was placed on the medication. The decision to use it was made after a careful discussion of other options, most of which she had already exhausted, and of the risks versus benefits of all available choices.
The biggest obstacle to starting the patient on isotretinoin was the perception that the drug is dangerous. It certainly must be used with caution, in carefully selected patients, and after a full disclosure of the associated risks. But when used appropriately, it is an effective treatment for acne that has failed to respond to other medications.
Summary
Acne is an extremely common complaint and happens to be exceedingly well studied. There are numerous treatment options, although none is perfect. Our job is to guide patients and families through the maze of information to plan a course of action acceptable to all.
ANSWER
The correct answer is food (choice “a”), which for many generations has been blamed for worsening acne (along with other nonfactors, such as makeup). All the others are demonstrably involved in the genesis and perpetuation of acne.
DISCUSSION
Teenagers have a hard enough time dealing with acne and other vicissitudes of puberty, and then they get blamed for eating the wrong kinds of food …. Would that it could be that simple! I think it’s important for us as providers to set the record straight by making sure parents and patients know what matters and what doesn’t.
When we’ve done that, the patient (or occasionally a parent) might say, “Well, every time I eat (insert item here), my acne flares.” To which we of course reply, “Well then, don’t do that!” After all, we certainly wouldn’t object to the patient consuming a better diet.
Once the unimportance of pizza, makeup, and soft drinks has been established, there remains the opportunity to enlighten the patient (and family) about the factors that do play a significant role—all but one of which can be addressed. (The exception, of course, is heredity; still, I believe it’s important to recognize its role in acne.) We can reduce the amount of sebum through use of retinoids and cut down on bacteria by using oral or topical antibiotics (though erythromycin is not especially effective). Hormonal therapy can be accomplished with oral contraceptives or oral spironolactone, though neither is perfect.
Treatment
This particular patient was prescribed a six-month course of isotretinoin (40 mg/d), after which her acne was completely and permanently gone. This is the result in about 70% of cases when this medicine is used correctly.
Proper procedure, including pregnancy tests and blood work, was followed before the patient was placed on the medication. The decision to use it was made after a careful discussion of other options, most of which she had already exhausted, and of the risks versus benefits of all available choices.
The biggest obstacle to starting the patient on isotretinoin was the perception that the drug is dangerous. It certainly must be used with caution, in carefully selected patients, and after a full disclosure of the associated risks. But when used appropriately, it is an effective treatment for acne that has failed to respond to other medications.
Summary
Acne is an extremely common complaint and happens to be exceedingly well studied. There are numerous treatment options, although none is perfect. Our job is to guide patients and families through the maze of information to plan a course of action acceptable to all.
ANSWER
The correct answer is food (choice “a”), which for many generations has been blamed for worsening acne (along with other nonfactors, such as makeup). All the others are demonstrably involved in the genesis and perpetuation of acne.
DISCUSSION
Teenagers have a hard enough time dealing with acne and other vicissitudes of puberty, and then they get blamed for eating the wrong kinds of food …. Would that it could be that simple! I think it’s important for us as providers to set the record straight by making sure parents and patients know what matters and what doesn’t.
When we’ve done that, the patient (or occasionally a parent) might say, “Well, every time I eat (insert item here), my acne flares.” To which we of course reply, “Well then, don’t do that!” After all, we certainly wouldn’t object to the patient consuming a better diet.
Once the unimportance of pizza, makeup, and soft drinks has been established, there remains the opportunity to enlighten the patient (and family) about the factors that do play a significant role—all but one of which can be addressed. (The exception, of course, is heredity; still, I believe it’s important to recognize its role in acne.) We can reduce the amount of sebum through use of retinoids and cut down on bacteria by using oral or topical antibiotics (though erythromycin is not especially effective). Hormonal therapy can be accomplished with oral contraceptives or oral spironolactone, though neither is perfect.
Treatment
This particular patient was prescribed a six-month course of isotretinoin (40 mg/d), after which her acne was completely and permanently gone. This is the result in about 70% of cases when this medicine is used correctly.
Proper procedure, including pregnancy tests and blood work, was followed before the patient was placed on the medication. The decision to use it was made after a careful discussion of other options, most of which she had already exhausted, and of the risks versus benefits of all available choices.
The biggest obstacle to starting the patient on isotretinoin was the perception that the drug is dangerous. It certainly must be used with caution, in carefully selected patients, and after a full disclosure of the associated risks. But when used appropriately, it is an effective treatment for acne that has failed to respond to other medications.
Summary
Acne is an extremely common complaint and happens to be exceedingly well studied. There are numerous treatment options, although none is perfect. Our job is to guide patients and families through the maze of information to plan a course of action acceptable to all.
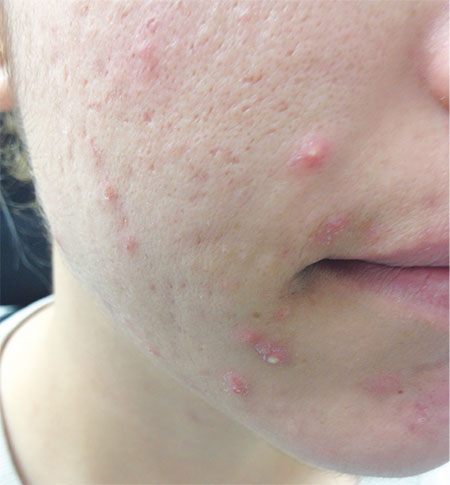
An 18-year-old woman is brought in by her mother for evaluation of longstanding acne. Although she is otherwise healthy, the patient has a significant family history of acne and recounts an extensive personal history of treatment attempts with both OTC and prescription products. Among these are several different benzoyl peroxide–based formulations (including one she bought after seeing an ad on TV) and devices including an electric scrub brush. None has had a significant impact. Tretinoin gel and oral erythromycin—prescribed by the patient’s primary care provider—haven’t helped much, either. The patient’s periods are regular and normal. She claims to be sexually abstinent. Examination reveals moderately severe acne confined to the patient’s face. Numerous open and closed comedones can be seen, as well as several pus-filled pimples. Scarring is minimal but present, especially on the sides of the face.
Axis I psychiatric disorders high in skin-restricted lupus patients
The prevalence of psychiatric disorders is high among people with skin-restricted lupus (SRL), compared with the general population, yet most do not receive specialist mental health care or appropriate psychotropic treatment, researchers report.
Investigators led by psychiatrist Isabelle Jalenques of the Clermont-Ferrand (France) University Hospital noted that psychiatric disorders had been extensively reported in patients with systemic lupus erythematosus (SLE), but no data existed on patients with skin-restricted disease (Br J Dermatol. 2016. doi: 10.1111/bjd.14392).
A previous exploratory study by the research group had shown that 60% of the 20 patients with subacute cutaneous lupus erythematosus and discoid lupus erythematosus studied had at least one psychiatric disorder. However the study was limited by its size and lack of a control group.
In the current multicenter study, the researchers compared 75 outpatients with SRL with 150 controls. Mean age of patients was 46 years and mean duration of disease was 10 years. They discovered that almost 49% of the patients with SRL fulfilled criteria for at least one current Axis I psychiatric disorder, compared with 13% of controls (OR, 5.0; P less than .001). Furthermore, 73% of patients fulfilled criteria for at least one lifetime Axis I psychiatric disorder, compared with 43% of controls (OR, 4.4; P less than .001).
The rates were close to that of patients with SLE for both current (42.2 and 46.7%) and lifetime psychiatric disorders (72%), Dr. Jalenques and her associates noted.
Patients with SRL were at a particularly high risk of the following psychiatric disorders, compared with controls:
• Major depressive disorder: current (9% vs. 0%; P = .0007) and lifetime (44% vs. 26%; P = .01).
• Generalized anxiety disorder: current (23% vs. 3%; P less than .001) and lifetime (35% vs. 19%; P = .03).
• Panic disorder: current (7% vs. 0%; P = .004) and lifetime (21 % vs. 3 %; P less than .001).
• Suicide risk: current (24% vs. 7%; P = .003).
• Alcohol dependence: current (7% vs. 0%; P = .004).
• Lifetime agoraphobia: (20% vs. 9%; P = .01).
Many patients were not receiving specialist mental health care or appropriate psychotropic treatment despite psychiatric disorders being a well-known cause of psychological distress, excess mortality, impaired global functioning, and poor compliance with medical treatment, Dr. Jalenques and her associates noted.
“Clinicians should be aware of the high prevalence of these disorders among SRL patients and not hesitate to refer such patients for psychiatric evaluation,” they concluded.
This study was supported by a grant from the French Ministry of Health and from Société Française de Dermatologie. The authors declared they have no conflicts of interest.
The prevalence of psychiatric disorders is high among people with skin-restricted lupus (SRL), compared with the general population, yet most do not receive specialist mental health care or appropriate psychotropic treatment, researchers report.
Investigators led by psychiatrist Isabelle Jalenques of the Clermont-Ferrand (France) University Hospital noted that psychiatric disorders had been extensively reported in patients with systemic lupus erythematosus (SLE), but no data existed on patients with skin-restricted disease (Br J Dermatol. 2016. doi: 10.1111/bjd.14392).
A previous exploratory study by the research group had shown that 60% of the 20 patients with subacute cutaneous lupus erythematosus and discoid lupus erythematosus studied had at least one psychiatric disorder. However the study was limited by its size and lack of a control group.
In the current multicenter study, the researchers compared 75 outpatients with SRL with 150 controls. Mean age of patients was 46 years and mean duration of disease was 10 years. They discovered that almost 49% of the patients with SRL fulfilled criteria for at least one current Axis I psychiatric disorder, compared with 13% of controls (OR, 5.0; P less than .001). Furthermore, 73% of patients fulfilled criteria for at least one lifetime Axis I psychiatric disorder, compared with 43% of controls (OR, 4.4; P less than .001).
The rates were close to that of patients with SLE for both current (42.2 and 46.7%) and lifetime psychiatric disorders (72%), Dr. Jalenques and her associates noted.
Patients with SRL were at a particularly high risk of the following psychiatric disorders, compared with controls:
• Major depressive disorder: current (9% vs. 0%; P = .0007) and lifetime (44% vs. 26%; P = .01).
• Generalized anxiety disorder: current (23% vs. 3%; P less than .001) and lifetime (35% vs. 19%; P = .03).
• Panic disorder: current (7% vs. 0%; P = .004) and lifetime (21 % vs. 3 %; P less than .001).
• Suicide risk: current (24% vs. 7%; P = .003).
• Alcohol dependence: current (7% vs. 0%; P = .004).
• Lifetime agoraphobia: (20% vs. 9%; P = .01).
Many patients were not receiving specialist mental health care or appropriate psychotropic treatment despite psychiatric disorders being a well-known cause of psychological distress, excess mortality, impaired global functioning, and poor compliance with medical treatment, Dr. Jalenques and her associates noted.
“Clinicians should be aware of the high prevalence of these disorders among SRL patients and not hesitate to refer such patients for psychiatric evaluation,” they concluded.
This study was supported by a grant from the French Ministry of Health and from Société Française de Dermatologie. The authors declared they have no conflicts of interest.
The prevalence of psychiatric disorders is high among people with skin-restricted lupus (SRL), compared with the general population, yet most do not receive specialist mental health care or appropriate psychotropic treatment, researchers report.
Investigators led by psychiatrist Isabelle Jalenques of the Clermont-Ferrand (France) University Hospital noted that psychiatric disorders had been extensively reported in patients with systemic lupus erythematosus (SLE), but no data existed on patients with skin-restricted disease (Br J Dermatol. 2016. doi: 10.1111/bjd.14392).
A previous exploratory study by the research group had shown that 60% of the 20 patients with subacute cutaneous lupus erythematosus and discoid lupus erythematosus studied had at least one psychiatric disorder. However the study was limited by its size and lack of a control group.
In the current multicenter study, the researchers compared 75 outpatients with SRL with 150 controls. Mean age of patients was 46 years and mean duration of disease was 10 years. They discovered that almost 49% of the patients with SRL fulfilled criteria for at least one current Axis I psychiatric disorder, compared with 13% of controls (OR, 5.0; P less than .001). Furthermore, 73% of patients fulfilled criteria for at least one lifetime Axis I psychiatric disorder, compared with 43% of controls (OR, 4.4; P less than .001).
The rates were close to that of patients with SLE for both current (42.2 and 46.7%) and lifetime psychiatric disorders (72%), Dr. Jalenques and her associates noted.
Patients with SRL were at a particularly high risk of the following psychiatric disorders, compared with controls:
• Major depressive disorder: current (9% vs. 0%; P = .0007) and lifetime (44% vs. 26%; P = .01).
• Generalized anxiety disorder: current (23% vs. 3%; P less than .001) and lifetime (35% vs. 19%; P = .03).
• Panic disorder: current (7% vs. 0%; P = .004) and lifetime (21 % vs. 3 %; P less than .001).
• Suicide risk: current (24% vs. 7%; P = .003).
• Alcohol dependence: current (7% vs. 0%; P = .004).
• Lifetime agoraphobia: (20% vs. 9%; P = .01).
Many patients were not receiving specialist mental health care or appropriate psychotropic treatment despite psychiatric disorders being a well-known cause of psychological distress, excess mortality, impaired global functioning, and poor compliance with medical treatment, Dr. Jalenques and her associates noted.
“Clinicians should be aware of the high prevalence of these disorders among SRL patients and not hesitate to refer such patients for psychiatric evaluation,” they concluded.
This study was supported by a grant from the French Ministry of Health and from Société Française de Dermatologie. The authors declared they have no conflicts of interest.
FROM BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: The prevalence of psychiatric disorders is high among people with skin-restricted lupus, yet many do not receive specialist mental health care or appropriate psychotropic treatment.
Major finding: Almost half of the patients with SRL fulfilled criteria for at least one current Axis I psychiatric disorder, compared with 13% of controls (OR 5.0 [95% CI, 2.4-10.7], P less than .001).
Data source: A multicenter study of 75 outpatients with SRL and 150 control subjects.
Disclosures: This study was supported by a grant from the French Ministry of Health and from Société Française de Dermatologie. The authors declared they have no conflicts of interest.
Organ transplant recipients face increased risk of BCC
Recipients of a solid organ transplant face up to a sixfold increase in the risk of developing a basal cell carcinoma – a risk that seems to increase as time passes.
A pretransplant history of squamous cell carcinoma (SCC) increased this risk to 55 times that seen in the general population, Dr. Britta Krynitz and her colleagues reported (Br J Dermatol. 2015. doi: 10.1111/bjd.14153).
But even when the pretransplant SCC group was removed from the final analysis, the risk of basal cell carcinoma after transplant was five times that of the general population, “indicating that a pretransplant SCC has limited effect on BCC risk overall and that organ transplantation per se is a strong driver of posttransplant BCC risk,” wrote Dr. Krynitz of Karolinska Institute, Stockholm, and her coauthors.
“Our results strongly suggest tumor promoter effects of the immunosuppressive drugs in the pathogenesis of post-transplantation BCC,” the team said. “We speculate that calcineurin inhibitors and also antiproliferative drugs, often used in combination with corticosteroids, play a role.”
The researchers investigated the incidence of both BCC and SCC in a cohort of 4,023 patients who underwent solid organ transplant from 2004 to 2011. Their median age at the time of transplant was 53 years; most (59%) received a kidney. Other organs transplanted were liver (22%), heart and/or lung (15%), and other organs (4%). The median follow-up time was 3.4 years; the longest follow-up was 5.5 years.
Only 17 of patients had a history of melanoma, and 19 patients a history of SCC – less than 1% for each skin cancer. Seven percent (301) of patients had experienced some form of nonskin cancer.
By the end of follow-up, 341 BCCs had developed among 175 patients – an incidence of 6.7%. About half developed more than one BCC.
The researchers compared these patients to a group of almost 200,000 nontransplant patients who had developed BCC. Among these, the median age at BCC appearance was significantly older (71 years); 39% had more than one lesion.
The overall relative risk of BCC was increased sixfold in transplant recipients and was similar between the genders. However, the risk varied according to the type of organ received. Kidney recipients were at the highest risk (relative risk, 7.2), and those who received other organs had a lower risk (heart/lung: RR, 5.8; liver: RR, 2.6).
The risk also appeared to increase over time, the authors noted. From 0 to 2 years, it was 5.8; from 3 to 5 years, it increased to 7.0.
Among men, 54% of lesions appeared in the head/neck area and 35% on the trunk – a similar distribution to that seen in the nontransplant control group. Among women, there were differences between transplant patients and controls: 44% of lesions appeared on patients’ head/neck, compared with 60% in the control group, and 34% appeared on the truck, compared with 24% in the control group.
Histology was similar, as were the proportions of aggressive type II and highly aggressive type III lesions.
A total of 199 SCCs developed among 87 patients during follow-up, a ratio to BCC of 1:1.7. “The low ratio was probably due to the short follow-up in our study,” the authors noted.
The Welander Foundation, the Westerberg Foundation, and the Strategic Research Program in Epidemiology at Karolinska Institute sponsored the study. None of the authors had any financial declarations.
Recipients of a solid organ transplant face up to a sixfold increase in the risk of developing a basal cell carcinoma – a risk that seems to increase as time passes.
A pretransplant history of squamous cell carcinoma (SCC) increased this risk to 55 times that seen in the general population, Dr. Britta Krynitz and her colleagues reported (Br J Dermatol. 2015. doi: 10.1111/bjd.14153).
But even when the pretransplant SCC group was removed from the final analysis, the risk of basal cell carcinoma after transplant was five times that of the general population, “indicating that a pretransplant SCC has limited effect on BCC risk overall and that organ transplantation per se is a strong driver of posttransplant BCC risk,” wrote Dr. Krynitz of Karolinska Institute, Stockholm, and her coauthors.
“Our results strongly suggest tumor promoter effects of the immunosuppressive drugs in the pathogenesis of post-transplantation BCC,” the team said. “We speculate that calcineurin inhibitors and also antiproliferative drugs, often used in combination with corticosteroids, play a role.”
The researchers investigated the incidence of both BCC and SCC in a cohort of 4,023 patients who underwent solid organ transplant from 2004 to 2011. Their median age at the time of transplant was 53 years; most (59%) received a kidney. Other organs transplanted were liver (22%), heart and/or lung (15%), and other organs (4%). The median follow-up time was 3.4 years; the longest follow-up was 5.5 years.
Only 17 of patients had a history of melanoma, and 19 patients a history of SCC – less than 1% for each skin cancer. Seven percent (301) of patients had experienced some form of nonskin cancer.
By the end of follow-up, 341 BCCs had developed among 175 patients – an incidence of 6.7%. About half developed more than one BCC.
The researchers compared these patients to a group of almost 200,000 nontransplant patients who had developed BCC. Among these, the median age at BCC appearance was significantly older (71 years); 39% had more than one lesion.
The overall relative risk of BCC was increased sixfold in transplant recipients and was similar between the genders. However, the risk varied according to the type of organ received. Kidney recipients were at the highest risk (relative risk, 7.2), and those who received other organs had a lower risk (heart/lung: RR, 5.8; liver: RR, 2.6).
The risk also appeared to increase over time, the authors noted. From 0 to 2 years, it was 5.8; from 3 to 5 years, it increased to 7.0.
Among men, 54% of lesions appeared in the head/neck area and 35% on the trunk – a similar distribution to that seen in the nontransplant control group. Among women, there were differences between transplant patients and controls: 44% of lesions appeared on patients’ head/neck, compared with 60% in the control group, and 34% appeared on the truck, compared with 24% in the control group.
Histology was similar, as were the proportions of aggressive type II and highly aggressive type III lesions.
A total of 199 SCCs developed among 87 patients during follow-up, a ratio to BCC of 1:1.7. “The low ratio was probably due to the short follow-up in our study,” the authors noted.
The Welander Foundation, the Westerberg Foundation, and the Strategic Research Program in Epidemiology at Karolinska Institute sponsored the study. None of the authors had any financial declarations.
Recipients of a solid organ transplant face up to a sixfold increase in the risk of developing a basal cell carcinoma – a risk that seems to increase as time passes.
A pretransplant history of squamous cell carcinoma (SCC) increased this risk to 55 times that seen in the general population, Dr. Britta Krynitz and her colleagues reported (Br J Dermatol. 2015. doi: 10.1111/bjd.14153).
But even when the pretransplant SCC group was removed from the final analysis, the risk of basal cell carcinoma after transplant was five times that of the general population, “indicating that a pretransplant SCC has limited effect on BCC risk overall and that organ transplantation per se is a strong driver of posttransplant BCC risk,” wrote Dr. Krynitz of Karolinska Institute, Stockholm, and her coauthors.
“Our results strongly suggest tumor promoter effects of the immunosuppressive drugs in the pathogenesis of post-transplantation BCC,” the team said. “We speculate that calcineurin inhibitors and also antiproliferative drugs, often used in combination with corticosteroids, play a role.”
The researchers investigated the incidence of both BCC and SCC in a cohort of 4,023 patients who underwent solid organ transplant from 2004 to 2011. Their median age at the time of transplant was 53 years; most (59%) received a kidney. Other organs transplanted were liver (22%), heart and/or lung (15%), and other organs (4%). The median follow-up time was 3.4 years; the longest follow-up was 5.5 years.
Only 17 of patients had a history of melanoma, and 19 patients a history of SCC – less than 1% for each skin cancer. Seven percent (301) of patients had experienced some form of nonskin cancer.
By the end of follow-up, 341 BCCs had developed among 175 patients – an incidence of 6.7%. About half developed more than one BCC.
The researchers compared these patients to a group of almost 200,000 nontransplant patients who had developed BCC. Among these, the median age at BCC appearance was significantly older (71 years); 39% had more than one lesion.
The overall relative risk of BCC was increased sixfold in transplant recipients and was similar between the genders. However, the risk varied according to the type of organ received. Kidney recipients were at the highest risk (relative risk, 7.2), and those who received other organs had a lower risk (heart/lung: RR, 5.8; liver: RR, 2.6).
The risk also appeared to increase over time, the authors noted. From 0 to 2 years, it was 5.8; from 3 to 5 years, it increased to 7.0.
Among men, 54% of lesions appeared in the head/neck area and 35% on the trunk – a similar distribution to that seen in the nontransplant control group. Among women, there were differences between transplant patients and controls: 44% of lesions appeared on patients’ head/neck, compared with 60% in the control group, and 34% appeared on the truck, compared with 24% in the control group.
Histology was similar, as were the proportions of aggressive type II and highly aggressive type III lesions.
A total of 199 SCCs developed among 87 patients during follow-up, a ratio to BCC of 1:1.7. “The low ratio was probably due to the short follow-up in our study,” the authors noted.
The Welander Foundation, the Westerberg Foundation, and the Strategic Research Program in Epidemiology at Karolinska Institute sponsored the study. None of the authors had any financial declarations.
FROM BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: The risk of a basal cell carcinoma increases after solid organ transplant.
Major finding: Transplant patients have a sixfold increased risk of BCC.
Data source: A retrospective database study of 4,000 transplant patients and almost 200,000 controls.
Disclosures: The Welander Foundation, the Westerberg Foundation, and the Strategic Research Program in Epidemiology at Karolinska Institute sponsored the study. None of the authors had any financial declarations.
A better way to relieve rib fracture pain in the ICU
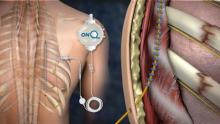
SAN ANTONIO – A new pain relief option for multiple rib fractures means that you might not have to wait around anymore for anesthesiology to place thoracic epidurals.
It’s called posterior paramedian subrhomboidal (PoPS) analgesia. A skin incision is made below the lowest fractured rib just paramedian to the spinus processes; a tunneling device is then used to work a catheter upwards under the rhomboids just past the highest fractured rib. The catheter has multiple openings along its length – like a sprinkler hose – so analgesic bathes the intercostal nerves as it runs down from a reservoir into the patient. The reservoir can be set to a desired flow rate or for on-demand use (ON-Q Pain Relief System – Halyard).
A pilot study at the University of Kansas, Kansas City, found that pain control from PoPS was at least equivalent to standard thoracic epidural analgesia (TEA), and that the system can be placed by a variety of hospital staff, not just anesthesiologists.
The 11 PoPS patients also used fewer rescue narcotics than the 19 TEA patients and had less hypotension. Because they weren’t at risk for epidural hematomas, they started venous thromboembolism prophylaxis without delay and at full dose.
“Our results are very promising. PoPS provides pain control similar to that of TEA,” with several “other benefits. You are not relying on one specialty for pain control,” so patients probably get faster relief. “PoPS can also be placed in patients whose injuries prohibit TEA, such as those with spinal cord injuries or increased intracranial pressure,” said investigator Dr. Casey Shelley, a University of Kansas general surgery resident.
PoPS was placed in the study either by anesthesiologists or by a trauma surgeon who practiced placement beforehand in the cadaver lab. The do-it-yourself potential for surgeons “is key. Most of us trauma surgeons are sick of begging anesthesiologists to come place thoracic epidurals,” said an audience member after Dr. Shelley’s presentation at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Ropivacaine 0.2% was used in both PoPS and TEA patients, all of whom had at least three broken ribs.
Median pain scores dropped from 8.5 to 2.5 on a 10-point scale an hour after PoPS placement, versus a median drop from 8 to 5 points an hour after TEA (P = .03). Although not statistically significant, median pain scores were about 1.5 points better with PoPS over the next several days, hovering around 3.5 versus around 5 points with TEA. Anesthesiology “usually won’t place high thoracic epidurals. With PoPS, you can tunnel up as far as you need to go to get to higher ribs,” which might explain the better pain control, Dr. Shelley said.
PoPS patients used about 70 mg/day oral morphine equivalents versus about 90 mg/day with TEA through day 6, but again the difference was not statistically significant. Even so, it might explain why six TEA patients (32%) were hypotensive over that time, compared with two PoPS patients (18%).
PoPS patients were a little older on average (mean 63 versus 55 years), with more fractured ribs (mean eight versus seven), and higher Injury Severity Scale scores (mean 20 versus 16). They were also more likely to have bilateral fractures, longer ICU stays (mean 4.9 versus 3.1 days), and longer overall lengths of stay (mean 14.8 versus 9.8 days), but none of those trends were statistically significant.
Both groups had mean chest Abbreviated Injury Scale scores of 3, and there were no statistical differences in daily spirometry readings. The majority of patients in both groups were men.
Favorable results were also reported in 2010 for ON-Q rib pain control, but the investigators did not compare the system to TEA (World J Surg. 2010 Oct;34:2359-62).
Dr. Shelley said Halyard was not involved in the study, and that she has no disclosures.
SAN ANTONIO – A new pain relief option for multiple rib fractures means that you might not have to wait around anymore for anesthesiology to place thoracic epidurals.
It’s called posterior paramedian subrhomboidal (PoPS) analgesia. A skin incision is made below the lowest fractured rib just paramedian to the spinus processes; a tunneling device is then used to work a catheter upwards under the rhomboids just past the highest fractured rib. The catheter has multiple openings along its length – like a sprinkler hose – so analgesic bathes the intercostal nerves as it runs down from a reservoir into the patient. The reservoir can be set to a desired flow rate or for on-demand use (ON-Q Pain Relief System – Halyard).
A pilot study at the University of Kansas, Kansas City, found that pain control from PoPS was at least equivalent to standard thoracic epidural analgesia (TEA), and that the system can be placed by a variety of hospital staff, not just anesthesiologists.
The 11 PoPS patients also used fewer rescue narcotics than the 19 TEA patients and had less hypotension. Because they weren’t at risk for epidural hematomas, they started venous thromboembolism prophylaxis without delay and at full dose.
“Our results are very promising. PoPS provides pain control similar to that of TEA,” with several “other benefits. You are not relying on one specialty for pain control,” so patients probably get faster relief. “PoPS can also be placed in patients whose injuries prohibit TEA, such as those with spinal cord injuries or increased intracranial pressure,” said investigator Dr. Casey Shelley, a University of Kansas general surgery resident.
PoPS was placed in the study either by anesthesiologists or by a trauma surgeon who practiced placement beforehand in the cadaver lab. The do-it-yourself potential for surgeons “is key. Most of us trauma surgeons are sick of begging anesthesiologists to come place thoracic epidurals,” said an audience member after Dr. Shelley’s presentation at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Ropivacaine 0.2% was used in both PoPS and TEA patients, all of whom had at least three broken ribs.
Median pain scores dropped from 8.5 to 2.5 on a 10-point scale an hour after PoPS placement, versus a median drop from 8 to 5 points an hour after TEA (P = .03). Although not statistically significant, median pain scores were about 1.5 points better with PoPS over the next several days, hovering around 3.5 versus around 5 points with TEA. Anesthesiology “usually won’t place high thoracic epidurals. With PoPS, you can tunnel up as far as you need to go to get to higher ribs,” which might explain the better pain control, Dr. Shelley said.
PoPS patients used about 70 mg/day oral morphine equivalents versus about 90 mg/day with TEA through day 6, but again the difference was not statistically significant. Even so, it might explain why six TEA patients (32%) were hypotensive over that time, compared with two PoPS patients (18%).
PoPS patients were a little older on average (mean 63 versus 55 years), with more fractured ribs (mean eight versus seven), and higher Injury Severity Scale scores (mean 20 versus 16). They were also more likely to have bilateral fractures, longer ICU stays (mean 4.9 versus 3.1 days), and longer overall lengths of stay (mean 14.8 versus 9.8 days), but none of those trends were statistically significant.
Both groups had mean chest Abbreviated Injury Scale scores of 3, and there were no statistical differences in daily spirometry readings. The majority of patients in both groups were men.
Favorable results were also reported in 2010 for ON-Q rib pain control, but the investigators did not compare the system to TEA (World J Surg. 2010 Oct;34:2359-62).
Dr. Shelley said Halyard was not involved in the study, and that she has no disclosures.
SAN ANTONIO – A new pain relief option for multiple rib fractures means that you might not have to wait around anymore for anesthesiology to place thoracic epidurals.
It’s called posterior paramedian subrhomboidal (PoPS) analgesia. A skin incision is made below the lowest fractured rib just paramedian to the spinus processes; a tunneling device is then used to work a catheter upwards under the rhomboids just past the highest fractured rib. The catheter has multiple openings along its length – like a sprinkler hose – so analgesic bathes the intercostal nerves as it runs down from a reservoir into the patient. The reservoir can be set to a desired flow rate or for on-demand use (ON-Q Pain Relief System – Halyard).
A pilot study at the University of Kansas, Kansas City, found that pain control from PoPS was at least equivalent to standard thoracic epidural analgesia (TEA), and that the system can be placed by a variety of hospital staff, not just anesthesiologists.
The 11 PoPS patients also used fewer rescue narcotics than the 19 TEA patients and had less hypotension. Because they weren’t at risk for epidural hematomas, they started venous thromboembolism prophylaxis without delay and at full dose.
“Our results are very promising. PoPS provides pain control similar to that of TEA,” with several “other benefits. You are not relying on one specialty for pain control,” so patients probably get faster relief. “PoPS can also be placed in patients whose injuries prohibit TEA, such as those with spinal cord injuries or increased intracranial pressure,” said investigator Dr. Casey Shelley, a University of Kansas general surgery resident.
PoPS was placed in the study either by anesthesiologists or by a trauma surgeon who practiced placement beforehand in the cadaver lab. The do-it-yourself potential for surgeons “is key. Most of us trauma surgeons are sick of begging anesthesiologists to come place thoracic epidurals,” said an audience member after Dr. Shelley’s presentation at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Ropivacaine 0.2% was used in both PoPS and TEA patients, all of whom had at least three broken ribs.
Median pain scores dropped from 8.5 to 2.5 on a 10-point scale an hour after PoPS placement, versus a median drop from 8 to 5 points an hour after TEA (P = .03). Although not statistically significant, median pain scores were about 1.5 points better with PoPS over the next several days, hovering around 3.5 versus around 5 points with TEA. Anesthesiology “usually won’t place high thoracic epidurals. With PoPS, you can tunnel up as far as you need to go to get to higher ribs,” which might explain the better pain control, Dr. Shelley said.
PoPS patients used about 70 mg/day oral morphine equivalents versus about 90 mg/day with TEA through day 6, but again the difference was not statistically significant. Even so, it might explain why six TEA patients (32%) were hypotensive over that time, compared with two PoPS patients (18%).
PoPS patients were a little older on average (mean 63 versus 55 years), with more fractured ribs (mean eight versus seven), and higher Injury Severity Scale scores (mean 20 versus 16). They were also more likely to have bilateral fractures, longer ICU stays (mean 4.9 versus 3.1 days), and longer overall lengths of stay (mean 14.8 versus 9.8 days), but none of those trends were statistically significant.
Both groups had mean chest Abbreviated Injury Scale scores of 3, and there were no statistical differences in daily spirometry readings. The majority of patients in both groups were men.
Favorable results were also reported in 2010 for ON-Q rib pain control, but the investigators did not compare the system to TEA (World J Surg. 2010 Oct;34:2359-62).
Dr. Shelley said Halyard was not involved in the study, and that she has no disclosures.
AT THE EAST SCIENTIFIC ASSEMBLY
Key clinical point: You might not have to wait around anymore for anesthesiology to place thoracic epidurals.
Major finding: Median pain scores dropped from 8.5 to 2.5 on a 10-point scale an hour after posterior PoPS placement, versus a median drop from 8 to 5 points an hour after thoracic epidural analgesia (P = .03).
Data source: Pilot study of 30 adults with at least three fractured ribs.
Disclosures: The maker of the PoPS system was not involved in the study, and the presenter had no disclosures.
Is lower BP worth it in higher-risk patients with diabetes or coronary disease?
There is no simple answer; the risk/benefit picture is complicated. Controlling blood pressure to a target of 130/80 mm Hg or lower produces mixed results in patients with diabetes and coronary disease equivalents (chronic kidney disease [CKD], coronary artery disease, peripheral arterial disease, and previous stroke).
No evidence indicates that patients with diabetes or most patients with CKD have better outcomes if their blood pressure is controlled below 140/90 mm Hg. Patients with diabetes controlled to lower systolic blood pressure targets (below 120 mm Hg) have fewer strokes, but more serious adverse events. Achieving diastolic blood pressure targets below 80 mm Hg doesn’t reduce mortality, strokes, myocardial infarction, or congestive heart failure (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Tight blood pressure control (approximately 130/80 mm Hg or lower) reduces the risk of kidney failure by 27% in CKD patients with proteinuria at baseline. In patients without proteinuria, it doesn’t add benefit over standard blood pressure control (140/90 mm Hg) for reducing kidney failure, mortality, or cardiovascular events (SOR: A, meta-analysis of RCTs).
Controlling hypertension to 130/80 mm Hg or lower in patients with coronary artery disease reduces heart failure (27%) and stroke (18%) but increases the incidence of hypotensive episodes (220%) when compared with standard 140/90 mm Hg target blood pressure. Lower target pressures don’t affect total or cardiovascular mortality, myocardial infarction, or angina, but do increase the need for revascularization in 6% of patients (SOR: A, meta-analysis of RCTs).
Controlling systolic blood pressure to a target of 120 mm Hg, compared with the standard target of 140 mm Hg, reduces a composite outcome (myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death) by 25% and a secondary outcome of all-cause mortality by 27% in patients ages 50 and older with cardiovascular risk factors (but not diabetes or previous stroke).
However, intensive control doesn’t significantly improve the composite outcome in patients who are female, black, or younger than 75 years, or who have systolic blood pressures above 132 mm Hg, previous CKD, or previous cardiovascular disease. Intensive control causes more hypotension, syncope, and electrolyte abnormalities, but not falls resulting in injuries (SOR: B, large RCT).
No evidence-based studies exist to guide BP control in patients with peripheral artery disease or previous stroke. Current guidelines recommend treating hypertension to a target of 140/90 mm Hg in these patients.
EVIDENCE SUMMARY
A Cochrane systematic review of 5 RCTs with a total of 7314 patients evaluated cardiovascular outcomes after 4.7 years follow-up in patients with diabetes who were treated for hypertension to either “lower” or “standard” target blood pressures.1 One trial in the review (ACCORD, 4734 patients) compared outcomes from significantly lower and standard systolic blood pressures (119/64 mm Hg vs 134/71 mm Hg; P<.0001) in patients with diabetes and either cardiovascular disease or 2 risk factors for cardiovascular disease. The authors evaluated outcomes based on achieved systolic blood pressures rather than intention to treat.
They found a reduced incidence of stroke (risk ratio [RR]=0.58; 95% confidence interval [CI], 0.39-0.88; P=.009; number needed to treat [NNT]=91) but no change in mortality (RR=1.05; 95% CI, 0.84-1.30) at lower blood pressures. Achieving the lower systolic blood pressure increased the number of serious adverse effects, however (RR=2.58; 95% CI, 1.70-3.91; P<.0001; absolute risk increase=2%; number needed to harm=50).
Four RCTs (2580 patients) in the systematic review compared clinical outcomes produced by achieving significantly lower or standard diastolic blood pressure targets (128/76 mm Hg vs 135/83 mm Hg; P<.0001). The trials found no significant difference in total mortality (RR=0.73; 95% CI, 0.53-1.01), stroke (RR=0.67; 95% CI, 0.42-1.05), myocardial infarction (RR=0.95; 95% CI, 0.64-1.40), or congestive heart failure (RR=1.06; 95% CI, 0.58-1.92). Sensitivity analysis of trials comparing diastolic blood pressure targets below 80 mm Hg and below 90 mm Hg showed similar results.
The 4 RCTs didn’t report end-stage renal failure or total serious adverse events. The authors stated that there was a high risk of selection bias in favor of lower blood pressure targets.
Patients with CKD
A systematic review and meta-analysis of 11 RCTs (9287 patients) compared outcomes of achieving lower blood pressure targets or standard targets in patients with CKD. Intensive blood pressure treatment reduced the risk of kidney failure only in patients with proteinuria at baseline (hazard ratio [HR]=0.73; 95% CI, 0.62-0.86; 5 trials, 1703 patients).2 Investigators didn’t report the degree of proteinuria for all the trials, but in one trial, patients had proteinuria of 1 to 3 g/d.
Achieved blood pressures in the intensive therapy group averaged 7.7/4.9 mm Hg lower, with pressures typically ranging from 75 to 80 mm Hg diastolic and 125 to 135 mm Hg systolic. Intensive blood pressure lowering didn’t reduce kidney failure in patients without baseline proteinuria (HR=1.12; 95% CI, 0.67-1.87; 3 trials, 1218 patients). Nor did it reduce death (RR=0.94; 95% CI, 0.84-1.05; 10 trials, 6788 patients) or major cardiovascular outcomes (RR=1.09; 95% CI, 0.83-1.42; 5 trials, 5308 patients).
Patients with coronary artery disease
A meta-analysis of 15 RCTs (66,504 patients) that evaluated tight control of hypertension (≤130/80 mm Hg) compared with standard control (<140/90 mm Hg) in patients with coronary artery disease found reduced rates of heart failure (RR=0.73; 95% CI, 0.64-0.84; 10 trials, 37,990 patients) and stroke (RR=0.82; 95% CI, 0.69-0.98; 9 trials, 8344 patients) but increased rates of hypotension (RR=2.19; 95% CI, 1.80-2.66; 6 trials, 17,836 patients).3
Achieving lower blood pressure targets didn’t reduce all-cause mortality (RR=0.96; 95% CI, 0.89-1.04; 13 trials, 39,262 patients), cardiovascular mortality (RR=0.96; 95% CI, 0.86-1.07; 11 trials, 38,452 patients), myocardial infarction (RR=0.92; 95% CI, 0.85-1.00; 14 trials, 39,696 patients), or angina (RR=0.92; 95% CI, 0.84-1.0; 11 trials, 28,007 patients). But it slightly increased the need for revascularization (RR=1.06; 95% CI, 1.01-1.12; 11 trials, 38,450 patients).
The SPRINT trial: Promising results for intensive treatment of some patients
The Systolic Blood Pressure Intervention Trial (SPRINT), a large RCT, found that targeting systolic blood pressures below 120 mm Hg (compared with a target below 140 mm Hg) in middle-aged and older patients with increased cardiovascular risk reduced a composite outcome that included cardiovascular death by 25%.4
Researchers recruited 9361 patients older than 50 years (mean age 68 years; >28% older than 75 years) with systolic blood pressure between 130 and 180 mm Hg and increased cardiovascular risk defined by one or more of the following: preexisting cardiovascular disease, CKD with estimated glomerular filtration rate between 20 and 60 mL/min/1.73 m2, age >75 years, and Framingham 10-year risk of 15% or more. They excluded patients with diabetes or previous stroke.
Patients were randomized to intensive treatment (target systolic BP <120; mean achieved 121.4) or standard treatment (target systolic BP <140; mean achieved 136.2). Treatment typically comprised 3 (intensive) or 2 (standard) agents. The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death.
The study, which was originally intended to run for 5 years, was stopped at 3.26 years based on positive results. Intensive treatment improved the primary composite outcome overall (1.65% vs 2.19%; HR=0.75; 95% CI, 0.64-0.89; P<.001; NNT=61 over 3.26 years), all-cause mortality (HR=0.73; 95% CI, 0.60-0.90; P=.003; NNT=90), and cardiovascular death (HR=0.57; 95% CI, 0.38-0.85; P=.005; NNT=172).
However, intensive treatment didn’t significantly improve the primary composite outcome in these subgroups:
- female patients (HR=0.84; 95% CI, 0.62-1.14)
- black patients (HR=0.77; 95% CI, 0.55-1.06)
- patients with preexisting CKD (HR=0.82; 95% CI, 0.63-1.07) or cardiovascular disease (HR=0.83; 95% CI, 0.62-1.09)
- patients younger than 75 years (HR=0.80; 95% CI, 0.64-1.00)
- patients with systolic blood pressures higher than 132 mm Hg (BP >132 to <145 mm Hg, HR=0.77; 95% CI, 0.57-1.03; BP ≥145 mm Hg, HR=0.83; 95% CI, 0.63-1.09).
Intensive treatment also produced more net serious adverse events (HR=1.88; 4.7% vs 2.5%; P<.001), including: ≥30% decrease of glomerular filtration rates to values below 60 mL/min/1.73 m2 (HR=3.49; 95% CI, 2.44-5.10; P<.001), syncope (HR=1.44; 3.5% vs 2.4%; P=.003), hypotension (HR=1.70; 3.4% vs 2.0%; P<.001), and electrolyte abnormalities (HR=1.38; 3.8% vs 2.8%; P=.006). It didn’t cause injurious falls (HR=1.00; P=.97) or orthostatic hypotension in clinic (HR=0.88; 16.6% vs 18.3%; P=.01).
Guidelines for patients with peripheral artery disease, previous stroke
A national guideline by an expert panel recommended treating patients with hypertension who have peripheral artery disease or previous stroke to standard values for the general population: <140/90 mm Hg if ages 60 years or younger, <150/90 mm Hg if older than 60 years.5
1. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;(10):CD008277.
2. Lv J, Ehteshami P, Sarnak M, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
3. Bangalore S, Kumar S, Volodarskiy A, et al. Blood pressure targets in patients with coronary artery disease: observations from traditional and Bayesian random effects meta-analysis of randomised trials. Heart. 2013;99:601-613.
4. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
There is no simple answer; the risk/benefit picture is complicated. Controlling blood pressure to a target of 130/80 mm Hg or lower produces mixed results in patients with diabetes and coronary disease equivalents (chronic kidney disease [CKD], coronary artery disease, peripheral arterial disease, and previous stroke).
No evidence indicates that patients with diabetes or most patients with CKD have better outcomes if their blood pressure is controlled below 140/90 mm Hg. Patients with diabetes controlled to lower systolic blood pressure targets (below 120 mm Hg) have fewer strokes, but more serious adverse events. Achieving diastolic blood pressure targets below 80 mm Hg doesn’t reduce mortality, strokes, myocardial infarction, or congestive heart failure (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Tight blood pressure control (approximately 130/80 mm Hg or lower) reduces the risk of kidney failure by 27% in CKD patients with proteinuria at baseline. In patients without proteinuria, it doesn’t add benefit over standard blood pressure control (140/90 mm Hg) for reducing kidney failure, mortality, or cardiovascular events (SOR: A, meta-analysis of RCTs).
Controlling hypertension to 130/80 mm Hg or lower in patients with coronary artery disease reduces heart failure (27%) and stroke (18%) but increases the incidence of hypotensive episodes (220%) when compared with standard 140/90 mm Hg target blood pressure. Lower target pressures don’t affect total or cardiovascular mortality, myocardial infarction, or angina, but do increase the need for revascularization in 6% of patients (SOR: A, meta-analysis of RCTs).
Controlling systolic blood pressure to a target of 120 mm Hg, compared with the standard target of 140 mm Hg, reduces a composite outcome (myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death) by 25% and a secondary outcome of all-cause mortality by 27% in patients ages 50 and older with cardiovascular risk factors (but not diabetes or previous stroke).
However, intensive control doesn’t significantly improve the composite outcome in patients who are female, black, or younger than 75 years, or who have systolic blood pressures above 132 mm Hg, previous CKD, or previous cardiovascular disease. Intensive control causes more hypotension, syncope, and electrolyte abnormalities, but not falls resulting in injuries (SOR: B, large RCT).
No evidence-based studies exist to guide BP control in patients with peripheral artery disease or previous stroke. Current guidelines recommend treating hypertension to a target of 140/90 mm Hg in these patients.
EVIDENCE SUMMARY
A Cochrane systematic review of 5 RCTs with a total of 7314 patients evaluated cardiovascular outcomes after 4.7 years follow-up in patients with diabetes who were treated for hypertension to either “lower” or “standard” target blood pressures.1 One trial in the review (ACCORD, 4734 patients) compared outcomes from significantly lower and standard systolic blood pressures (119/64 mm Hg vs 134/71 mm Hg; P<.0001) in patients with diabetes and either cardiovascular disease or 2 risk factors for cardiovascular disease. The authors evaluated outcomes based on achieved systolic blood pressures rather than intention to treat.
They found a reduced incidence of stroke (risk ratio [RR]=0.58; 95% confidence interval [CI], 0.39-0.88; P=.009; number needed to treat [NNT]=91) but no change in mortality (RR=1.05; 95% CI, 0.84-1.30) at lower blood pressures. Achieving the lower systolic blood pressure increased the number of serious adverse effects, however (RR=2.58; 95% CI, 1.70-3.91; P<.0001; absolute risk increase=2%; number needed to harm=50).
Four RCTs (2580 patients) in the systematic review compared clinical outcomes produced by achieving significantly lower or standard diastolic blood pressure targets (128/76 mm Hg vs 135/83 mm Hg; P<.0001). The trials found no significant difference in total mortality (RR=0.73; 95% CI, 0.53-1.01), stroke (RR=0.67; 95% CI, 0.42-1.05), myocardial infarction (RR=0.95; 95% CI, 0.64-1.40), or congestive heart failure (RR=1.06; 95% CI, 0.58-1.92). Sensitivity analysis of trials comparing diastolic blood pressure targets below 80 mm Hg and below 90 mm Hg showed similar results.
The 4 RCTs didn’t report end-stage renal failure or total serious adverse events. The authors stated that there was a high risk of selection bias in favor of lower blood pressure targets.
Patients with CKD
A systematic review and meta-analysis of 11 RCTs (9287 patients) compared outcomes of achieving lower blood pressure targets or standard targets in patients with CKD. Intensive blood pressure treatment reduced the risk of kidney failure only in patients with proteinuria at baseline (hazard ratio [HR]=0.73; 95% CI, 0.62-0.86; 5 trials, 1703 patients).2 Investigators didn’t report the degree of proteinuria for all the trials, but in one trial, patients had proteinuria of 1 to 3 g/d.
Achieved blood pressures in the intensive therapy group averaged 7.7/4.9 mm Hg lower, with pressures typically ranging from 75 to 80 mm Hg diastolic and 125 to 135 mm Hg systolic. Intensive blood pressure lowering didn’t reduce kidney failure in patients without baseline proteinuria (HR=1.12; 95% CI, 0.67-1.87; 3 trials, 1218 patients). Nor did it reduce death (RR=0.94; 95% CI, 0.84-1.05; 10 trials, 6788 patients) or major cardiovascular outcomes (RR=1.09; 95% CI, 0.83-1.42; 5 trials, 5308 patients).
Patients with coronary artery disease
A meta-analysis of 15 RCTs (66,504 patients) that evaluated tight control of hypertension (≤130/80 mm Hg) compared with standard control (<140/90 mm Hg) in patients with coronary artery disease found reduced rates of heart failure (RR=0.73; 95% CI, 0.64-0.84; 10 trials, 37,990 patients) and stroke (RR=0.82; 95% CI, 0.69-0.98; 9 trials, 8344 patients) but increased rates of hypotension (RR=2.19; 95% CI, 1.80-2.66; 6 trials, 17,836 patients).3
Achieving lower blood pressure targets didn’t reduce all-cause mortality (RR=0.96; 95% CI, 0.89-1.04; 13 trials, 39,262 patients), cardiovascular mortality (RR=0.96; 95% CI, 0.86-1.07; 11 trials, 38,452 patients), myocardial infarction (RR=0.92; 95% CI, 0.85-1.00; 14 trials, 39,696 patients), or angina (RR=0.92; 95% CI, 0.84-1.0; 11 trials, 28,007 patients). But it slightly increased the need for revascularization (RR=1.06; 95% CI, 1.01-1.12; 11 trials, 38,450 patients).
The SPRINT trial: Promising results for intensive treatment of some patients
The Systolic Blood Pressure Intervention Trial (SPRINT), a large RCT, found that targeting systolic blood pressures below 120 mm Hg (compared with a target below 140 mm Hg) in middle-aged and older patients with increased cardiovascular risk reduced a composite outcome that included cardiovascular death by 25%.4
Researchers recruited 9361 patients older than 50 years (mean age 68 years; >28% older than 75 years) with systolic blood pressure between 130 and 180 mm Hg and increased cardiovascular risk defined by one or more of the following: preexisting cardiovascular disease, CKD with estimated glomerular filtration rate between 20 and 60 mL/min/1.73 m2, age >75 years, and Framingham 10-year risk of 15% or more. They excluded patients with diabetes or previous stroke.
Patients were randomized to intensive treatment (target systolic BP <120; mean achieved 121.4) or standard treatment (target systolic BP <140; mean achieved 136.2). Treatment typically comprised 3 (intensive) or 2 (standard) agents. The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death.
The study, which was originally intended to run for 5 years, was stopped at 3.26 years based on positive results. Intensive treatment improved the primary composite outcome overall (1.65% vs 2.19%; HR=0.75; 95% CI, 0.64-0.89; P<.001; NNT=61 over 3.26 years), all-cause mortality (HR=0.73; 95% CI, 0.60-0.90; P=.003; NNT=90), and cardiovascular death (HR=0.57; 95% CI, 0.38-0.85; P=.005; NNT=172).
However, intensive treatment didn’t significantly improve the primary composite outcome in these subgroups:
- female patients (HR=0.84; 95% CI, 0.62-1.14)
- black patients (HR=0.77; 95% CI, 0.55-1.06)
- patients with preexisting CKD (HR=0.82; 95% CI, 0.63-1.07) or cardiovascular disease (HR=0.83; 95% CI, 0.62-1.09)
- patients younger than 75 years (HR=0.80; 95% CI, 0.64-1.00)
- patients with systolic blood pressures higher than 132 mm Hg (BP >132 to <145 mm Hg, HR=0.77; 95% CI, 0.57-1.03; BP ≥145 mm Hg, HR=0.83; 95% CI, 0.63-1.09).
Intensive treatment also produced more net serious adverse events (HR=1.88; 4.7% vs 2.5%; P<.001), including: ≥30% decrease of glomerular filtration rates to values below 60 mL/min/1.73 m2 (HR=3.49; 95% CI, 2.44-5.10; P<.001), syncope (HR=1.44; 3.5% vs 2.4%; P=.003), hypotension (HR=1.70; 3.4% vs 2.0%; P<.001), and electrolyte abnormalities (HR=1.38; 3.8% vs 2.8%; P=.006). It didn’t cause injurious falls (HR=1.00; P=.97) or orthostatic hypotension in clinic (HR=0.88; 16.6% vs 18.3%; P=.01).
Guidelines for patients with peripheral artery disease, previous stroke
A national guideline by an expert panel recommended treating patients with hypertension who have peripheral artery disease or previous stroke to standard values for the general population: <140/90 mm Hg if ages 60 years or younger, <150/90 mm Hg if older than 60 years.5
There is no simple answer; the risk/benefit picture is complicated. Controlling blood pressure to a target of 130/80 mm Hg or lower produces mixed results in patients with diabetes and coronary disease equivalents (chronic kidney disease [CKD], coronary artery disease, peripheral arterial disease, and previous stroke).
No evidence indicates that patients with diabetes or most patients with CKD have better outcomes if their blood pressure is controlled below 140/90 mm Hg. Patients with diabetes controlled to lower systolic blood pressure targets (below 120 mm Hg) have fewer strokes, but more serious adverse events. Achieving diastolic blood pressure targets below 80 mm Hg doesn’t reduce mortality, strokes, myocardial infarction, or congestive heart failure (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Tight blood pressure control (approximately 130/80 mm Hg or lower) reduces the risk of kidney failure by 27% in CKD patients with proteinuria at baseline. In patients without proteinuria, it doesn’t add benefit over standard blood pressure control (140/90 mm Hg) for reducing kidney failure, mortality, or cardiovascular events (SOR: A, meta-analysis of RCTs).
Controlling hypertension to 130/80 mm Hg or lower in patients with coronary artery disease reduces heart failure (27%) and stroke (18%) but increases the incidence of hypotensive episodes (220%) when compared with standard 140/90 mm Hg target blood pressure. Lower target pressures don’t affect total or cardiovascular mortality, myocardial infarction, or angina, but do increase the need for revascularization in 6% of patients (SOR: A, meta-analysis of RCTs).
Controlling systolic blood pressure to a target of 120 mm Hg, compared with the standard target of 140 mm Hg, reduces a composite outcome (myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death) by 25% and a secondary outcome of all-cause mortality by 27% in patients ages 50 and older with cardiovascular risk factors (but not diabetes or previous stroke).
However, intensive control doesn’t significantly improve the composite outcome in patients who are female, black, or younger than 75 years, or who have systolic blood pressures above 132 mm Hg, previous CKD, or previous cardiovascular disease. Intensive control causes more hypotension, syncope, and electrolyte abnormalities, but not falls resulting in injuries (SOR: B, large RCT).
No evidence-based studies exist to guide BP control in patients with peripheral artery disease or previous stroke. Current guidelines recommend treating hypertension to a target of 140/90 mm Hg in these patients.
EVIDENCE SUMMARY
A Cochrane systematic review of 5 RCTs with a total of 7314 patients evaluated cardiovascular outcomes after 4.7 years follow-up in patients with diabetes who were treated for hypertension to either “lower” or “standard” target blood pressures.1 One trial in the review (ACCORD, 4734 patients) compared outcomes from significantly lower and standard systolic blood pressures (119/64 mm Hg vs 134/71 mm Hg; P<.0001) in patients with diabetes and either cardiovascular disease or 2 risk factors for cardiovascular disease. The authors evaluated outcomes based on achieved systolic blood pressures rather than intention to treat.
They found a reduced incidence of stroke (risk ratio [RR]=0.58; 95% confidence interval [CI], 0.39-0.88; P=.009; number needed to treat [NNT]=91) but no change in mortality (RR=1.05; 95% CI, 0.84-1.30) at lower blood pressures. Achieving the lower systolic blood pressure increased the number of serious adverse effects, however (RR=2.58; 95% CI, 1.70-3.91; P<.0001; absolute risk increase=2%; number needed to harm=50).
Four RCTs (2580 patients) in the systematic review compared clinical outcomes produced by achieving significantly lower or standard diastolic blood pressure targets (128/76 mm Hg vs 135/83 mm Hg; P<.0001). The trials found no significant difference in total mortality (RR=0.73; 95% CI, 0.53-1.01), stroke (RR=0.67; 95% CI, 0.42-1.05), myocardial infarction (RR=0.95; 95% CI, 0.64-1.40), or congestive heart failure (RR=1.06; 95% CI, 0.58-1.92). Sensitivity analysis of trials comparing diastolic blood pressure targets below 80 mm Hg and below 90 mm Hg showed similar results.
The 4 RCTs didn’t report end-stage renal failure or total serious adverse events. The authors stated that there was a high risk of selection bias in favor of lower blood pressure targets.
Patients with CKD
A systematic review and meta-analysis of 11 RCTs (9287 patients) compared outcomes of achieving lower blood pressure targets or standard targets in patients with CKD. Intensive blood pressure treatment reduced the risk of kidney failure only in patients with proteinuria at baseline (hazard ratio [HR]=0.73; 95% CI, 0.62-0.86; 5 trials, 1703 patients).2 Investigators didn’t report the degree of proteinuria for all the trials, but in one trial, patients had proteinuria of 1 to 3 g/d.
Achieved blood pressures in the intensive therapy group averaged 7.7/4.9 mm Hg lower, with pressures typically ranging from 75 to 80 mm Hg diastolic and 125 to 135 mm Hg systolic. Intensive blood pressure lowering didn’t reduce kidney failure in patients without baseline proteinuria (HR=1.12; 95% CI, 0.67-1.87; 3 trials, 1218 patients). Nor did it reduce death (RR=0.94; 95% CI, 0.84-1.05; 10 trials, 6788 patients) or major cardiovascular outcomes (RR=1.09; 95% CI, 0.83-1.42; 5 trials, 5308 patients).
Patients with coronary artery disease
A meta-analysis of 15 RCTs (66,504 patients) that evaluated tight control of hypertension (≤130/80 mm Hg) compared with standard control (<140/90 mm Hg) in patients with coronary artery disease found reduced rates of heart failure (RR=0.73; 95% CI, 0.64-0.84; 10 trials, 37,990 patients) and stroke (RR=0.82; 95% CI, 0.69-0.98; 9 trials, 8344 patients) but increased rates of hypotension (RR=2.19; 95% CI, 1.80-2.66; 6 trials, 17,836 patients).3
Achieving lower blood pressure targets didn’t reduce all-cause mortality (RR=0.96; 95% CI, 0.89-1.04; 13 trials, 39,262 patients), cardiovascular mortality (RR=0.96; 95% CI, 0.86-1.07; 11 trials, 38,452 patients), myocardial infarction (RR=0.92; 95% CI, 0.85-1.00; 14 trials, 39,696 patients), or angina (RR=0.92; 95% CI, 0.84-1.0; 11 trials, 28,007 patients). But it slightly increased the need for revascularization (RR=1.06; 95% CI, 1.01-1.12; 11 trials, 38,450 patients).
The SPRINT trial: Promising results for intensive treatment of some patients
The Systolic Blood Pressure Intervention Trial (SPRINT), a large RCT, found that targeting systolic blood pressures below 120 mm Hg (compared with a target below 140 mm Hg) in middle-aged and older patients with increased cardiovascular risk reduced a composite outcome that included cardiovascular death by 25%.4
Researchers recruited 9361 patients older than 50 years (mean age 68 years; >28% older than 75 years) with systolic blood pressure between 130 and 180 mm Hg and increased cardiovascular risk defined by one or more of the following: preexisting cardiovascular disease, CKD with estimated glomerular filtration rate between 20 and 60 mL/min/1.73 m2, age >75 years, and Framingham 10-year risk of 15% or more. They excluded patients with diabetes or previous stroke.
Patients were randomized to intensive treatment (target systolic BP <120; mean achieved 121.4) or standard treatment (target systolic BP <140; mean achieved 136.2). Treatment typically comprised 3 (intensive) or 2 (standard) agents. The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, congestive heart failure, or cardiovascular death.
The study, which was originally intended to run for 5 years, was stopped at 3.26 years based on positive results. Intensive treatment improved the primary composite outcome overall (1.65% vs 2.19%; HR=0.75; 95% CI, 0.64-0.89; P<.001; NNT=61 over 3.26 years), all-cause mortality (HR=0.73; 95% CI, 0.60-0.90; P=.003; NNT=90), and cardiovascular death (HR=0.57; 95% CI, 0.38-0.85; P=.005; NNT=172).
However, intensive treatment didn’t significantly improve the primary composite outcome in these subgroups:
- female patients (HR=0.84; 95% CI, 0.62-1.14)
- black patients (HR=0.77; 95% CI, 0.55-1.06)
- patients with preexisting CKD (HR=0.82; 95% CI, 0.63-1.07) or cardiovascular disease (HR=0.83; 95% CI, 0.62-1.09)
- patients younger than 75 years (HR=0.80; 95% CI, 0.64-1.00)
- patients with systolic blood pressures higher than 132 mm Hg (BP >132 to <145 mm Hg, HR=0.77; 95% CI, 0.57-1.03; BP ≥145 mm Hg, HR=0.83; 95% CI, 0.63-1.09).
Intensive treatment also produced more net serious adverse events (HR=1.88; 4.7% vs 2.5%; P<.001), including: ≥30% decrease of glomerular filtration rates to values below 60 mL/min/1.73 m2 (HR=3.49; 95% CI, 2.44-5.10; P<.001), syncope (HR=1.44; 3.5% vs 2.4%; P=.003), hypotension (HR=1.70; 3.4% vs 2.0%; P<.001), and electrolyte abnormalities (HR=1.38; 3.8% vs 2.8%; P=.006). It didn’t cause injurious falls (HR=1.00; P=.97) or orthostatic hypotension in clinic (HR=0.88; 16.6% vs 18.3%; P=.01).
Guidelines for patients with peripheral artery disease, previous stroke
A national guideline by an expert panel recommended treating patients with hypertension who have peripheral artery disease or previous stroke to standard values for the general population: <140/90 mm Hg if ages 60 years or younger, <150/90 mm Hg if older than 60 years.5
1. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;(10):CD008277.
2. Lv J, Ehteshami P, Sarnak M, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
3. Bangalore S, Kumar S, Volodarskiy A, et al. Blood pressure targets in patients with coronary artery disease: observations from traditional and Bayesian random effects meta-analysis of randomised trials. Heart. 2013;99:601-613.
4. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
1. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;(10):CD008277.
2. Lv J, Ehteshami P, Sarnak M, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949-957.
3. Bangalore S, Kumar S, Volodarskiy A, et al. Blood pressure targets in patients with coronary artery disease: observations from traditional and Bayesian random effects meta-analysis of randomised trials. Heart. 2013;99:601-613.
4. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
Evidence-based answers from the Family Physicians Inquiries Network
Do corticosteroid injections improve carpal tunnel syndrome symptoms?
Yes. Injected corticosteroids reduce symptoms of carpal tunnel syndrome (CTS) more effectively than placebo or systemic steroids, but no better than anti-inflammatory medication and splinting, from one to 12 weeks after therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and consistent RCT).
A 40-mg injection of methylprednisolone reduces symptoms as effectively as an 80-mg injection for as long as 10 weeks, but the 80-mg dose reduces progression to surgery at one year (SOR: B, RCT). Long-term effects of injections decrease by 12 months (SOR: B, RCT).
After corticosteroid injections, 14% of patients proceed to surgery at one year, and 33% proceed to surgery at 5 years (SOR: B, cohort trial).
EVIDENCE SUMMARY
A 2007 Cochrane review of 12 RCTs with 671 patients compared the efficacy of corticosteroid injections for CTS with placebo injections or other nonsurgical interventions.1 Patients who received corticosteroid injections showed clinical improvement at one month or less compared with placebo (2 trials, 141 patients; 73% corticosteroids vs 28% placebo; relative risk [RR]=2.58; 95% confidence interval [CI], 1.72-3.87; number needed to treat [NNT]=2).
Compared with systemic corticosteroids, corticosteroid injections didn’t improve symptoms on a Global Symptom Score (scale of 0-50, with 50 indicating the most severe symptoms) at 2 weeks (one trial, 60 patients; mean difference [MD]= −4.2; 95% CI, −8.7 to 0.26), but did improve symptoms at 8 weeks (MD= −7.16; 95% CI, −11.5 to −2.86) and 12 weeks (MD= −7.1; 95% CI, −11.7 to −2.52).
Patients showed no difference in scores between corticosteroid injection and oral anti-inflammatory medication with neutral angle wrist splints on the Symptom Severity Scale (1 to 5, with 5 indicating the most severe symptoms) at 2 weeks (1 trial, 23 patients [37 wrists]; MD=0.0; 95% CI, −0.64 to 0.64) or 8 weeks (MD=0.1; 95% CI, −0.33 to 0.53).
Higher corticosteroid dose reduces surgery at one year
A 2013 high-quality RCT with 111 patients assessed pain relief and rates of surgery at one year with local corticosteroid injections for CTS.2 This trial had 3 arms with 37 patients in each: 80-mg methylprednisolone injection, 40-mg methylprednisolone injection, or placebo injection.
Both corticosteroid groups showed greater improvement on the Symptom Severity Scale at 10 weeks compared with placebo (40-mg methylprednisolone group: MD= −0.88; 95% CI, −1.3 to −0.46; 80-mg methylprednisolone group: MD= −0.64; 95% CI, −1.06 to −0.21). There was no difference between the methylprednisolone groups.
The incidence of surgery at one year was lower in the 80-mg methylprednisolone group compared with placebo (73% vs 92%; RR=0.79; 95% CI, 0.64-0.99; NNT=5) but not in the 40-mg methylprednisolone group compared with placebo (81% vs 92%; RR=0.88; 95% CI, 0.73-1.06).
Corticosteroids improve symptoms and disability, but effects wear off
A randomized double-blind, placebo-controlled trial conducted in 2010 examined the effectiveness of corticosteroid injections given by general practitioners to 69 patients with CTS.3 Patients were randomized to receive 10 mg of either triamcinolone or saline. They were reassessed after one week, and patients in the saline injection group who had inadequate symptom relief received a triamcinolone injection as bail-out treatment. Follow-up by patient questionnaire was done at 1, 3, 6, and 12 months.
Investigators assessed symptoms and disability using the Symptom Severity Scale and Functional Disability Scale, which are part of the Boston Carpal Tunnel Questionnaire. Like the Symptom Severity Scale, the Functional Disability Scale is scored from 1 to 5, with higher scores indicating more severe disability.
One week after treatment, the corticosteroid group showed greater improvement in symptom severity and functional disability than the saline group (symptom severity decreased from 2.9 to 1.9 with triamcinolone vs 2.8 to 2.5 with saline; MD=0.64; 95% CI, 0.32-0.96; functional disability decreased from 2.5 to 1.9 with triamcinolone but remained at 2.4 with saline; MD=0.59; 95% CI, 0.23-0.94).
Long-term follow-up of 35 patients who responded to corticosteroid injections found that the effects wore off over 12 months when assessed using the Symptom Severity Scale (mean score 1.5 at 1 month, 2.0 at 12 months; P=.08).
Surgery rates at one and 5 years
A 2012 prospective cohort study examined the 5-year rate of surgical intervention after a 20-mg methylprednisolone injection in 824 patients diagnosed with CTS who had failed conservative treatment.4 A total of 500 patients had a relapse of symptoms, and 372 of them elected to have a second injection. A Kaplan-Meier survivorship analysis determined rates of surgical intervention to be 14.5% (95% CI, 11.9-17) at one year and 33.2% (95% CI, 28.7-37.8) at 5 years.
RECOMMENDATION
A 2010 American Academy of Orthopaedic Surgeons evidence-based practice guideline on the treatment of CTS recommends corticosteroid injection before considering surgery (Grade B, Level 1 suggested recommendation with good evidence).5
1. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.
2. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
3. Peters-Veluthamaningal C, Winters JC, Gronier KH, et al. Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. BMC Fam Pract. 2010;11:54.
4. Jenkins PJ, Duckworth AD, Watts AC, et al. Corticosteroid injection for carpal tunnel syndrome: a 5-year survivorship analysis. Hand. 2012;7:151-156.
5. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg Am. 2010;92:218-219.
Yes. Injected corticosteroids reduce symptoms of carpal tunnel syndrome (CTS) more effectively than placebo or systemic steroids, but no better than anti-inflammatory medication and splinting, from one to 12 weeks after therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and consistent RCT).
A 40-mg injection of methylprednisolone reduces symptoms as effectively as an 80-mg injection for as long as 10 weeks, but the 80-mg dose reduces progression to surgery at one year (SOR: B, RCT). Long-term effects of injections decrease by 12 months (SOR: B, RCT).
After corticosteroid injections, 14% of patients proceed to surgery at one year, and 33% proceed to surgery at 5 years (SOR: B, cohort trial).
EVIDENCE SUMMARY
A 2007 Cochrane review of 12 RCTs with 671 patients compared the efficacy of corticosteroid injections for CTS with placebo injections or other nonsurgical interventions.1 Patients who received corticosteroid injections showed clinical improvement at one month or less compared with placebo (2 trials, 141 patients; 73% corticosteroids vs 28% placebo; relative risk [RR]=2.58; 95% confidence interval [CI], 1.72-3.87; number needed to treat [NNT]=2).
Compared with systemic corticosteroids, corticosteroid injections didn’t improve symptoms on a Global Symptom Score (scale of 0-50, with 50 indicating the most severe symptoms) at 2 weeks (one trial, 60 patients; mean difference [MD]= −4.2; 95% CI, −8.7 to 0.26), but did improve symptoms at 8 weeks (MD= −7.16; 95% CI, −11.5 to −2.86) and 12 weeks (MD= −7.1; 95% CI, −11.7 to −2.52).
Patients showed no difference in scores between corticosteroid injection and oral anti-inflammatory medication with neutral angle wrist splints on the Symptom Severity Scale (1 to 5, with 5 indicating the most severe symptoms) at 2 weeks (1 trial, 23 patients [37 wrists]; MD=0.0; 95% CI, −0.64 to 0.64) or 8 weeks (MD=0.1; 95% CI, −0.33 to 0.53).
Higher corticosteroid dose reduces surgery at one year
A 2013 high-quality RCT with 111 patients assessed pain relief and rates of surgery at one year with local corticosteroid injections for CTS.2 This trial had 3 arms with 37 patients in each: 80-mg methylprednisolone injection, 40-mg methylprednisolone injection, or placebo injection.
Both corticosteroid groups showed greater improvement on the Symptom Severity Scale at 10 weeks compared with placebo (40-mg methylprednisolone group: MD= −0.88; 95% CI, −1.3 to −0.46; 80-mg methylprednisolone group: MD= −0.64; 95% CI, −1.06 to −0.21). There was no difference between the methylprednisolone groups.
The incidence of surgery at one year was lower in the 80-mg methylprednisolone group compared with placebo (73% vs 92%; RR=0.79; 95% CI, 0.64-0.99; NNT=5) but not in the 40-mg methylprednisolone group compared with placebo (81% vs 92%; RR=0.88; 95% CI, 0.73-1.06).
Corticosteroids improve symptoms and disability, but effects wear off
A randomized double-blind, placebo-controlled trial conducted in 2010 examined the effectiveness of corticosteroid injections given by general practitioners to 69 patients with CTS.3 Patients were randomized to receive 10 mg of either triamcinolone or saline. They were reassessed after one week, and patients in the saline injection group who had inadequate symptom relief received a triamcinolone injection as bail-out treatment. Follow-up by patient questionnaire was done at 1, 3, 6, and 12 months.
Investigators assessed symptoms and disability using the Symptom Severity Scale and Functional Disability Scale, which are part of the Boston Carpal Tunnel Questionnaire. Like the Symptom Severity Scale, the Functional Disability Scale is scored from 1 to 5, with higher scores indicating more severe disability.
One week after treatment, the corticosteroid group showed greater improvement in symptom severity and functional disability than the saline group (symptom severity decreased from 2.9 to 1.9 with triamcinolone vs 2.8 to 2.5 with saline; MD=0.64; 95% CI, 0.32-0.96; functional disability decreased from 2.5 to 1.9 with triamcinolone but remained at 2.4 with saline; MD=0.59; 95% CI, 0.23-0.94).
Long-term follow-up of 35 patients who responded to corticosteroid injections found that the effects wore off over 12 months when assessed using the Symptom Severity Scale (mean score 1.5 at 1 month, 2.0 at 12 months; P=.08).
Surgery rates at one and 5 years
A 2012 prospective cohort study examined the 5-year rate of surgical intervention after a 20-mg methylprednisolone injection in 824 patients diagnosed with CTS who had failed conservative treatment.4 A total of 500 patients had a relapse of symptoms, and 372 of them elected to have a second injection. A Kaplan-Meier survivorship analysis determined rates of surgical intervention to be 14.5% (95% CI, 11.9-17) at one year and 33.2% (95% CI, 28.7-37.8) at 5 years.
RECOMMENDATION
A 2010 American Academy of Orthopaedic Surgeons evidence-based practice guideline on the treatment of CTS recommends corticosteroid injection before considering surgery (Grade B, Level 1 suggested recommendation with good evidence).5
Yes. Injected corticosteroids reduce symptoms of carpal tunnel syndrome (CTS) more effectively than placebo or systemic steroids, but no better than anti-inflammatory medication and splinting, from one to 12 weeks after therapy (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and consistent RCT).
A 40-mg injection of methylprednisolone reduces symptoms as effectively as an 80-mg injection for as long as 10 weeks, but the 80-mg dose reduces progression to surgery at one year (SOR: B, RCT). Long-term effects of injections decrease by 12 months (SOR: B, RCT).
After corticosteroid injections, 14% of patients proceed to surgery at one year, and 33% proceed to surgery at 5 years (SOR: B, cohort trial).
EVIDENCE SUMMARY
A 2007 Cochrane review of 12 RCTs with 671 patients compared the efficacy of corticosteroid injections for CTS with placebo injections or other nonsurgical interventions.1 Patients who received corticosteroid injections showed clinical improvement at one month or less compared with placebo (2 trials, 141 patients; 73% corticosteroids vs 28% placebo; relative risk [RR]=2.58; 95% confidence interval [CI], 1.72-3.87; number needed to treat [NNT]=2).
Compared with systemic corticosteroids, corticosteroid injections didn’t improve symptoms on a Global Symptom Score (scale of 0-50, with 50 indicating the most severe symptoms) at 2 weeks (one trial, 60 patients; mean difference [MD]= −4.2; 95% CI, −8.7 to 0.26), but did improve symptoms at 8 weeks (MD= −7.16; 95% CI, −11.5 to −2.86) and 12 weeks (MD= −7.1; 95% CI, −11.7 to −2.52).
Patients showed no difference in scores between corticosteroid injection and oral anti-inflammatory medication with neutral angle wrist splints on the Symptom Severity Scale (1 to 5, with 5 indicating the most severe symptoms) at 2 weeks (1 trial, 23 patients [37 wrists]; MD=0.0; 95% CI, −0.64 to 0.64) or 8 weeks (MD=0.1; 95% CI, −0.33 to 0.53).
Higher corticosteroid dose reduces surgery at one year
A 2013 high-quality RCT with 111 patients assessed pain relief and rates of surgery at one year with local corticosteroid injections for CTS.2 This trial had 3 arms with 37 patients in each: 80-mg methylprednisolone injection, 40-mg methylprednisolone injection, or placebo injection.
Both corticosteroid groups showed greater improvement on the Symptom Severity Scale at 10 weeks compared with placebo (40-mg methylprednisolone group: MD= −0.88; 95% CI, −1.3 to −0.46; 80-mg methylprednisolone group: MD= −0.64; 95% CI, −1.06 to −0.21). There was no difference between the methylprednisolone groups.
The incidence of surgery at one year was lower in the 80-mg methylprednisolone group compared with placebo (73% vs 92%; RR=0.79; 95% CI, 0.64-0.99; NNT=5) but not in the 40-mg methylprednisolone group compared with placebo (81% vs 92%; RR=0.88; 95% CI, 0.73-1.06).
Corticosteroids improve symptoms and disability, but effects wear off
A randomized double-blind, placebo-controlled trial conducted in 2010 examined the effectiveness of corticosteroid injections given by general practitioners to 69 patients with CTS.3 Patients were randomized to receive 10 mg of either triamcinolone or saline. They were reassessed after one week, and patients in the saline injection group who had inadequate symptom relief received a triamcinolone injection as bail-out treatment. Follow-up by patient questionnaire was done at 1, 3, 6, and 12 months.
Investigators assessed symptoms and disability using the Symptom Severity Scale and Functional Disability Scale, which are part of the Boston Carpal Tunnel Questionnaire. Like the Symptom Severity Scale, the Functional Disability Scale is scored from 1 to 5, with higher scores indicating more severe disability.
One week after treatment, the corticosteroid group showed greater improvement in symptom severity and functional disability than the saline group (symptom severity decreased from 2.9 to 1.9 with triamcinolone vs 2.8 to 2.5 with saline; MD=0.64; 95% CI, 0.32-0.96; functional disability decreased from 2.5 to 1.9 with triamcinolone but remained at 2.4 with saline; MD=0.59; 95% CI, 0.23-0.94).
Long-term follow-up of 35 patients who responded to corticosteroid injections found that the effects wore off over 12 months when assessed using the Symptom Severity Scale (mean score 1.5 at 1 month, 2.0 at 12 months; P=.08).
Surgery rates at one and 5 years
A 2012 prospective cohort study examined the 5-year rate of surgical intervention after a 20-mg methylprednisolone injection in 824 patients diagnosed with CTS who had failed conservative treatment.4 A total of 500 patients had a relapse of symptoms, and 372 of them elected to have a second injection. A Kaplan-Meier survivorship analysis determined rates of surgical intervention to be 14.5% (95% CI, 11.9-17) at one year and 33.2% (95% CI, 28.7-37.8) at 5 years.
RECOMMENDATION
A 2010 American Academy of Orthopaedic Surgeons evidence-based practice guideline on the treatment of CTS recommends corticosteroid injection before considering surgery (Grade B, Level 1 suggested recommendation with good evidence).5
1. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.
2. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
3. Peters-Veluthamaningal C, Winters JC, Gronier KH, et al. Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. BMC Fam Pract. 2010;11:54.
4. Jenkins PJ, Duckworth AD, Watts AC, et al. Corticosteroid injection for carpal tunnel syndrome: a 5-year survivorship analysis. Hand. 2012;7:151-156.
5. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg Am. 2010;92:218-219.
1. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(2):CD001554.
2. Atroshi I, Flondell M, Hofer M, et al. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med. 2013;159:309-317.
3. Peters-Veluthamaningal C, Winters JC, Gronier KH, et al. Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. BMC Fam Pract. 2010;11:54.
4. Jenkins PJ, Duckworth AD, Watts AC, et al. Corticosteroid injection for carpal tunnel syndrome: a 5-year survivorship analysis. Hand. 2012;7:151-156.
5. Keith MW, Masear V, Chung KC, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of carpal tunnel syndrome. J Bone Joint Surg Am. 2010;92:218-219.
Evidence-based answers from the Family Physicians Inquiries Network
Survival of pancreatic cancer is better when adjuvant therapy is given in high-volume centers
SAN FRANCISCO – Receiving adjuvant therapy for pancreatic cancer at a center that treats a high volume of patients with the disease confers a survival advantage, according to results of a retrospective cohort study reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
The analysis of 245 patients found that those given adjuvant therapy at Virginia Mason Medical Center – a high-volume center seeing up to 300 patients with newly diagnosed pancreatic cancer each year and putting about a third of them in trials – had a 37% reduction in the adjusted risk of death when compared with peers referred to community clinics for this therapy, reported first author Margaret T. Mandelson, Ph.D., director of research and quality at the center’s cancer institute in Seattle.
“Our study does lend some support to the concept of using high-volume centers for all therapy components for pancreatic cancer that is treated with curative intent,” she commented. “Ongoing investigation of patterns of care and volume impact in medical oncology is certainly warranted.”
A variety of factors may be driving the observed survival difference, such as the regimens used, with some evidence suggesting, for example, that patients treated in the community are more likely to receive single-agent therapy, she noted.
“We know that we have a strong setting for supportive care [at the center] and that we try to maximize our patients’ tolerance to treatment,” she added. “We have a high rate of completion of treatment in this setting. And of course the impact of optimism and hope cannot be underestimated in this patient population.”
Giving the academic medical center perspective, Dr. James L. Abbruzzese of the Duke Cancer Institute, Duke University, Durham, N.C., speculated that volume is a proxy for processes of care: staffing, use of guidelines or treatment algorithms, staging practices, and especially a multidisciplinary approach with components such as tumor boards and use of clinical trials. And larger centers are in a better position to offer these processes.
“While the primary determinant of the long-term outcome of patients requires adequate volumes, I don’t think this is the whole answer,” he summarized. “I think it relies on and relates much more to the processes and the extent to which we can bring the multidisciplinary team to the patients.”
Giving the community oncology perspective, Dr. Michael V. Seiden, chief medical officer of the US Oncology Network, contended that instead of focusing solely on outcomes, the field should be focusing on the value of care, broadly defined as outcome divided by cost.
“I don’t really think this is a discussion about should your pancreatic cancer be treated in the community or in an academic center or a large regional health center. What we have to realize is that tens of thousands of patients with pancreatic cancer who will be diagnosed in the years ahead are going to receive care across the country in a lot of different venues,” he commented. “The questions we need to answer are how do we maximize value? What should be done in the ‘mouse’ hospitals? What should be done in the gigantic centers of excellence? What should be done in the well-organized health care systems? And what should be done in the community? Because delivering maximal value requires keeping an eye not only on best outcomes, but also on patient convenience and cost.”
Giving some background to the study, Dr. Mandelson noted that a volume-outcome relationship has been established when it comes to surgery for pancreatic cancer, but not when it comes to adjuvant therapy for the disease.
She and her colleagues used registry data to identify patients who received a pancreatic cancer diagnosis during 2003-2014 and underwent primary resection at Virginia Mason Medical Center. They compared outcomes between those who stayed at the center to receive their adjuvant therapy and those who were referred to a community oncology practice to receive this therapy.
Patients were excluded if they had received neoadjuvant therapy, had synchronous cancers, died or were lost to follow-up within 3 months of surgery, or had contraindications to receiving adjuvant therapy. Also excluded were any who declined this therapy and for whom a medical oncologist could not be identified.
Results showed that the patients treated in the high-volume center and in community clinics were similar with respect to sex, insurance status, travel distance to a high-volume center, performance status, and tumor size, nodal status, and margin status, Dr. Mandelson reported. Those treated in the community were, on average, 5 years older.
At the high-volume center, 96% of patients started chemotherapy, 81% received a multiagent regimen, and 53% underwent chemoradiation. Detailed data on therapies received were not available for the community group.
The patients treated in the high-volume center had a more than one-third reduction in the adjusted risk of death relative to peers treated in the community (hazard ratio, 0.63; P less than .01). Median overall survival was 43.6 months for the former, compared with 27.9 months for the latter (P less than .01). The corresponding 5-year rates of overall survival were 38.6% and 24.8% (P less than .01).
“We know from the literature that pancreas cancer is undertreated in the community as a whole, both from the surgical perspective and the medical perspective. So it wouldn’t be surprising if some of the patients with a referral to an outside oncologist in fact never received treatment,” Dr. Mandelson commented.
“The patient population that received surgery in the community setting and then came to Virginia Mason for adjuvant therapy has not yet been analyzed, which is essentially the inverse of this study,” she noted. “That will be very powerful evidence.”
Dr. Mandelson disclosed that she had no relevant conflicts of interest. Dr. Abbruzzese disclosed that he receives honoraria from Celgene and Halozyme, and that he has a consulting or advisory role with Acerta Pharma, Bessor, Celgene, Cornerstone Pharma, Daiichi Sankyo, EMD Serono, Halozyme, Progen, Merck Sharpe & Dohme, Sun BioPharma, and Viba Therapeutics. Dr. Seiden disclosed that he is an employee of McKesson Specialty Health and Texas Oncology; that he is chief medical officer of US Oncology; and that he owns stock in and receives travel expenses from McKesson Specialty Health.
SAN FRANCISCO – Receiving adjuvant therapy for pancreatic cancer at a center that treats a high volume of patients with the disease confers a survival advantage, according to results of a retrospective cohort study reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
The analysis of 245 patients found that those given adjuvant therapy at Virginia Mason Medical Center – a high-volume center seeing up to 300 patients with newly diagnosed pancreatic cancer each year and putting about a third of them in trials – had a 37% reduction in the adjusted risk of death when compared with peers referred to community clinics for this therapy, reported first author Margaret T. Mandelson, Ph.D., director of research and quality at the center’s cancer institute in Seattle.
“Our study does lend some support to the concept of using high-volume centers for all therapy components for pancreatic cancer that is treated with curative intent,” she commented. “Ongoing investigation of patterns of care and volume impact in medical oncology is certainly warranted.”
A variety of factors may be driving the observed survival difference, such as the regimens used, with some evidence suggesting, for example, that patients treated in the community are more likely to receive single-agent therapy, she noted.
“We know that we have a strong setting for supportive care [at the center] and that we try to maximize our patients’ tolerance to treatment,” she added. “We have a high rate of completion of treatment in this setting. And of course the impact of optimism and hope cannot be underestimated in this patient population.”
Giving the academic medical center perspective, Dr. James L. Abbruzzese of the Duke Cancer Institute, Duke University, Durham, N.C., speculated that volume is a proxy for processes of care: staffing, use of guidelines or treatment algorithms, staging practices, and especially a multidisciplinary approach with components such as tumor boards and use of clinical trials. And larger centers are in a better position to offer these processes.
“While the primary determinant of the long-term outcome of patients requires adequate volumes, I don’t think this is the whole answer,” he summarized. “I think it relies on and relates much more to the processes and the extent to which we can bring the multidisciplinary team to the patients.”
Giving the community oncology perspective, Dr. Michael V. Seiden, chief medical officer of the US Oncology Network, contended that instead of focusing solely on outcomes, the field should be focusing on the value of care, broadly defined as outcome divided by cost.
“I don’t really think this is a discussion about should your pancreatic cancer be treated in the community or in an academic center or a large regional health center. What we have to realize is that tens of thousands of patients with pancreatic cancer who will be diagnosed in the years ahead are going to receive care across the country in a lot of different venues,” he commented. “The questions we need to answer are how do we maximize value? What should be done in the ‘mouse’ hospitals? What should be done in the gigantic centers of excellence? What should be done in the well-organized health care systems? And what should be done in the community? Because delivering maximal value requires keeping an eye not only on best outcomes, but also on patient convenience and cost.”
Giving some background to the study, Dr. Mandelson noted that a volume-outcome relationship has been established when it comes to surgery for pancreatic cancer, but not when it comes to adjuvant therapy for the disease.
She and her colleagues used registry data to identify patients who received a pancreatic cancer diagnosis during 2003-2014 and underwent primary resection at Virginia Mason Medical Center. They compared outcomes between those who stayed at the center to receive their adjuvant therapy and those who were referred to a community oncology practice to receive this therapy.
Patients were excluded if they had received neoadjuvant therapy, had synchronous cancers, died or were lost to follow-up within 3 months of surgery, or had contraindications to receiving adjuvant therapy. Also excluded were any who declined this therapy and for whom a medical oncologist could not be identified.
Results showed that the patients treated in the high-volume center and in community clinics were similar with respect to sex, insurance status, travel distance to a high-volume center, performance status, and tumor size, nodal status, and margin status, Dr. Mandelson reported. Those treated in the community were, on average, 5 years older.
At the high-volume center, 96% of patients started chemotherapy, 81% received a multiagent regimen, and 53% underwent chemoradiation. Detailed data on therapies received were not available for the community group.
The patients treated in the high-volume center had a more than one-third reduction in the adjusted risk of death relative to peers treated in the community (hazard ratio, 0.63; P less than .01). Median overall survival was 43.6 months for the former, compared with 27.9 months for the latter (P less than .01). The corresponding 5-year rates of overall survival were 38.6% and 24.8% (P less than .01).
“We know from the literature that pancreas cancer is undertreated in the community as a whole, both from the surgical perspective and the medical perspective. So it wouldn’t be surprising if some of the patients with a referral to an outside oncologist in fact never received treatment,” Dr. Mandelson commented.
“The patient population that received surgery in the community setting and then came to Virginia Mason for adjuvant therapy has not yet been analyzed, which is essentially the inverse of this study,” she noted. “That will be very powerful evidence.”
Dr. Mandelson disclosed that she had no relevant conflicts of interest. Dr. Abbruzzese disclosed that he receives honoraria from Celgene and Halozyme, and that he has a consulting or advisory role with Acerta Pharma, Bessor, Celgene, Cornerstone Pharma, Daiichi Sankyo, EMD Serono, Halozyme, Progen, Merck Sharpe & Dohme, Sun BioPharma, and Viba Therapeutics. Dr. Seiden disclosed that he is an employee of McKesson Specialty Health and Texas Oncology; that he is chief medical officer of US Oncology; and that he owns stock in and receives travel expenses from McKesson Specialty Health.
SAN FRANCISCO – Receiving adjuvant therapy for pancreatic cancer at a center that treats a high volume of patients with the disease confers a survival advantage, according to results of a retrospective cohort study reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
The analysis of 245 patients found that those given adjuvant therapy at Virginia Mason Medical Center – a high-volume center seeing up to 300 patients with newly diagnosed pancreatic cancer each year and putting about a third of them in trials – had a 37% reduction in the adjusted risk of death when compared with peers referred to community clinics for this therapy, reported first author Margaret T. Mandelson, Ph.D., director of research and quality at the center’s cancer institute in Seattle.
“Our study does lend some support to the concept of using high-volume centers for all therapy components for pancreatic cancer that is treated with curative intent,” she commented. “Ongoing investigation of patterns of care and volume impact in medical oncology is certainly warranted.”
A variety of factors may be driving the observed survival difference, such as the regimens used, with some evidence suggesting, for example, that patients treated in the community are more likely to receive single-agent therapy, she noted.
“We know that we have a strong setting for supportive care [at the center] and that we try to maximize our patients’ tolerance to treatment,” she added. “We have a high rate of completion of treatment in this setting. And of course the impact of optimism and hope cannot be underestimated in this patient population.”
Giving the academic medical center perspective, Dr. James L. Abbruzzese of the Duke Cancer Institute, Duke University, Durham, N.C., speculated that volume is a proxy for processes of care: staffing, use of guidelines or treatment algorithms, staging practices, and especially a multidisciplinary approach with components such as tumor boards and use of clinical trials. And larger centers are in a better position to offer these processes.
“While the primary determinant of the long-term outcome of patients requires adequate volumes, I don’t think this is the whole answer,” he summarized. “I think it relies on and relates much more to the processes and the extent to which we can bring the multidisciplinary team to the patients.”
Giving the community oncology perspective, Dr. Michael V. Seiden, chief medical officer of the US Oncology Network, contended that instead of focusing solely on outcomes, the field should be focusing on the value of care, broadly defined as outcome divided by cost.
“I don’t really think this is a discussion about should your pancreatic cancer be treated in the community or in an academic center or a large regional health center. What we have to realize is that tens of thousands of patients with pancreatic cancer who will be diagnosed in the years ahead are going to receive care across the country in a lot of different venues,” he commented. “The questions we need to answer are how do we maximize value? What should be done in the ‘mouse’ hospitals? What should be done in the gigantic centers of excellence? What should be done in the well-organized health care systems? And what should be done in the community? Because delivering maximal value requires keeping an eye not only on best outcomes, but also on patient convenience and cost.”
Giving some background to the study, Dr. Mandelson noted that a volume-outcome relationship has been established when it comes to surgery for pancreatic cancer, but not when it comes to adjuvant therapy for the disease.
She and her colleagues used registry data to identify patients who received a pancreatic cancer diagnosis during 2003-2014 and underwent primary resection at Virginia Mason Medical Center. They compared outcomes between those who stayed at the center to receive their adjuvant therapy and those who were referred to a community oncology practice to receive this therapy.
Patients were excluded if they had received neoadjuvant therapy, had synchronous cancers, died or were lost to follow-up within 3 months of surgery, or had contraindications to receiving adjuvant therapy. Also excluded were any who declined this therapy and for whom a medical oncologist could not be identified.
Results showed that the patients treated in the high-volume center and in community clinics were similar with respect to sex, insurance status, travel distance to a high-volume center, performance status, and tumor size, nodal status, and margin status, Dr. Mandelson reported. Those treated in the community were, on average, 5 years older.
At the high-volume center, 96% of patients started chemotherapy, 81% received a multiagent regimen, and 53% underwent chemoradiation. Detailed data on therapies received were not available for the community group.
The patients treated in the high-volume center had a more than one-third reduction in the adjusted risk of death relative to peers treated in the community (hazard ratio, 0.63; P less than .01). Median overall survival was 43.6 months for the former, compared with 27.9 months for the latter (P less than .01). The corresponding 5-year rates of overall survival were 38.6% and 24.8% (P less than .01).
“We know from the literature that pancreas cancer is undertreated in the community as a whole, both from the surgical perspective and the medical perspective. So it wouldn’t be surprising if some of the patients with a referral to an outside oncologist in fact never received treatment,” Dr. Mandelson commented.
“The patient population that received surgery in the community setting and then came to Virginia Mason for adjuvant therapy has not yet been analyzed, which is essentially the inverse of this study,” she noted. “That will be very powerful evidence.”
Dr. Mandelson disclosed that she had no relevant conflicts of interest. Dr. Abbruzzese disclosed that he receives honoraria from Celgene and Halozyme, and that he has a consulting or advisory role with Acerta Pharma, Bessor, Celgene, Cornerstone Pharma, Daiichi Sankyo, EMD Serono, Halozyme, Progen, Merck Sharpe & Dohme, Sun BioPharma, and Viba Therapeutics. Dr. Seiden disclosed that he is an employee of McKesson Specialty Health and Texas Oncology; that he is chief medical officer of US Oncology; and that he owns stock in and receives travel expenses from McKesson Specialty Health.
AT THE ASCO GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point: Patients with pancreatic cancer live longer if given adjuvant therapy in a center that treats a high volume of patients with this disease.
Major finding: The risk of death was lower for patients who received adjuvant therapy in a high-volume center, compared with peers receiving this therapy in community clinics (HR, 0.63).
Data source: A retrospective cohort study of 139 patients treated in a high-volume center and 106 patients treated in community clinics.
Disclosures: Dr. Mandelson disclosed that she had no relevant conflicts of interest. Dr. Abbruzzese disclosed that he receives honoraria from Celgene and Halozyme, and that he has a consulting or advisory role with Acerta Pharma, Bessor, Celgene, Cornerstone Pharma, Daiichi Sankyo, EMD Serono, Halozyme, Progen, Merck Sharpe & Dohme, Sun BioPharma, and Viba Therapeutics. Dr. Seiden disclosed that he is an employee of McKesson Specialty Health and Texas Oncology; that he is chief medical officer of US Oncology; and that he owns stock in and receives travel expenses from McKesson Specialty Health.
CHMP recommends elotuzumab for MM

Photo courtesy of
Bristol-Myers Squibb
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion recommending approval of the immunostimulatory antibody elotuzumab (Empliciti).
The CHMP is recommending the drug be approved for use in combination with lenalidomide and dexamethasone to treat patients with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which usually follows the CHMP’s advice and is expected to deliver its final decision on elotuzumab within 3 months.
The CHMP’s positive opinion of elotuzumab is based on data from the phase 3 ELOQUENT-2 trial, which were presented at ASCO 2015 and published in NEJM.
The trial included 646 MM patients who had received 1 to 3 prior therapies. Baseline patient demographics and disease characteristics were well balanced between treatment arms.
Patients were randomized 1:1 to receive either elotuzumab at 10 mg/kg in combination with lenalidomide and dexamethasone (len-dex) or len-dex alone in 4-week cycles until disease progression or unacceptable toxicity.
The minimum follow-up for all study subjects was 24 months. The co-primary endpoints were progression-free survival (PFS) and overall response rate.
The overall response rate was 78.5% in the elotuzumab arm and 65.5% in the len-dex arm (P=0.0002).
The median PFS was 19.4 months in the elotuzumab arm and 14.9 months in the len-dex arm (P=0.0004). At 1 year, the PFS was 68% in the elotuzumab arm and 57% in the len-dex arm. At 2 years, the PFS was 41% and 27%, respectively.
The most common adverse events in the elotuzumab arm and len-dex arm, respectively, were fatigue (61.6% vs 51.7%), diarrhea (46.9% vs 36.0%), pyrexia (37.4% vs 24.6%), constipation (35.5% vs 27.1%), cough (34.3% vs 18.9%), peripheral neuropathy (26.7% vs 20.8%), nasopharyngitis (24.5% vs 19.2%), upper respiratory tract infection (22.6% vs 17.4%), decreased appetite (20.8% vs 12.6%), and pneumonia (20.1% vs 14.2%).
Serious adverse events occurred in 65.4% of patients in the elotuzumab arm and 56.5% in the len-dex arm. The most frequent events were pneumonia, pyrexia, respiratory tract infection, anemia, pulmonary embolism, and acute renal failure.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab as Empliciti, with Bristol-Myers Squibb solely responsible for commercial activities. ![]()

Photo courtesy of
Bristol-Myers Squibb
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion recommending approval of the immunostimulatory antibody elotuzumab (Empliciti).
The CHMP is recommending the drug be approved for use in combination with lenalidomide and dexamethasone to treat patients with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which usually follows the CHMP’s advice and is expected to deliver its final decision on elotuzumab within 3 months.
The CHMP’s positive opinion of elotuzumab is based on data from the phase 3 ELOQUENT-2 trial, which were presented at ASCO 2015 and published in NEJM.
The trial included 646 MM patients who had received 1 to 3 prior therapies. Baseline patient demographics and disease characteristics were well balanced between treatment arms.
Patients were randomized 1:1 to receive either elotuzumab at 10 mg/kg in combination with lenalidomide and dexamethasone (len-dex) or len-dex alone in 4-week cycles until disease progression or unacceptable toxicity.
The minimum follow-up for all study subjects was 24 months. The co-primary endpoints were progression-free survival (PFS) and overall response rate.
The overall response rate was 78.5% in the elotuzumab arm and 65.5% in the len-dex arm (P=0.0002).
The median PFS was 19.4 months in the elotuzumab arm and 14.9 months in the len-dex arm (P=0.0004). At 1 year, the PFS was 68% in the elotuzumab arm and 57% in the len-dex arm. At 2 years, the PFS was 41% and 27%, respectively.
The most common adverse events in the elotuzumab arm and len-dex arm, respectively, were fatigue (61.6% vs 51.7%), diarrhea (46.9% vs 36.0%), pyrexia (37.4% vs 24.6%), constipation (35.5% vs 27.1%), cough (34.3% vs 18.9%), peripheral neuropathy (26.7% vs 20.8%), nasopharyngitis (24.5% vs 19.2%), upper respiratory tract infection (22.6% vs 17.4%), decreased appetite (20.8% vs 12.6%), and pneumonia (20.1% vs 14.2%).
Serious adverse events occurred in 65.4% of patients in the elotuzumab arm and 56.5% in the len-dex arm. The most frequent events were pneumonia, pyrexia, respiratory tract infection, anemia, pulmonary embolism, and acute renal failure.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab as Empliciti, with Bristol-Myers Squibb solely responsible for commercial activities. ![]()

Photo courtesy of
Bristol-Myers Squibb
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion recommending approval of the immunostimulatory antibody elotuzumab (Empliciti).
The CHMP is recommending the drug be approved for use in combination with lenalidomide and dexamethasone to treat patients with multiple myeloma (MM) who have received at least 1 prior therapy.
The CHMP’s recommendation will be reviewed by the European Commission, which usually follows the CHMP’s advice and is expected to deliver its final decision on elotuzumab within 3 months.
The CHMP’s positive opinion of elotuzumab is based on data from the phase 3 ELOQUENT-2 trial, which were presented at ASCO 2015 and published in NEJM.
The trial included 646 MM patients who had received 1 to 3 prior therapies. Baseline patient demographics and disease characteristics were well balanced between treatment arms.
Patients were randomized 1:1 to receive either elotuzumab at 10 mg/kg in combination with lenalidomide and dexamethasone (len-dex) or len-dex alone in 4-week cycles until disease progression or unacceptable toxicity.
The minimum follow-up for all study subjects was 24 months. The co-primary endpoints were progression-free survival (PFS) and overall response rate.
The overall response rate was 78.5% in the elotuzumab arm and 65.5% in the len-dex arm (P=0.0002).
The median PFS was 19.4 months in the elotuzumab arm and 14.9 months in the len-dex arm (P=0.0004). At 1 year, the PFS was 68% in the elotuzumab arm and 57% in the len-dex arm. At 2 years, the PFS was 41% and 27%, respectively.
The most common adverse events in the elotuzumab arm and len-dex arm, respectively, were fatigue (61.6% vs 51.7%), diarrhea (46.9% vs 36.0%), pyrexia (37.4% vs 24.6%), constipation (35.5% vs 27.1%), cough (34.3% vs 18.9%), peripheral neuropathy (26.7% vs 20.8%), nasopharyngitis (24.5% vs 19.2%), upper respiratory tract infection (22.6% vs 17.4%), decreased appetite (20.8% vs 12.6%), and pneumonia (20.1% vs 14.2%).
Serious adverse events occurred in 65.4% of patients in the elotuzumab arm and 56.5% in the len-dex arm. The most frequent events were pneumonia, pyrexia, respiratory tract infection, anemia, pulmonary embolism, and acute renal failure.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab as Empliciti, with Bristol-Myers Squibb solely responsible for commercial activities. ![]()
TEP hernia repair superior to Lichtenstein in pain outcomes
Patients undergoing endoscopic total extraperitoneal repair (TEP) of hernias had less persistent pain and greater mobility than those whose hernias were repaired using the Lichtenstein technique.
For their research, conducted in Sweden, researchers led by Dr. Linn Westin of the Karolinska Institutet in Stockholm and colleagues randomized 384 adult men with primary unilateral inguinal hernia to surgery with Lichtenstein using local anesthesia (n = 191) or TEP with general anesthesia (n = 193). Patients underwent operations in two hospitals between 2006 and 2011, with four surgeons participating, and the same heavyweight mesh was used in both groups.
All patients were followed for 1 year for postoperative pain, recurrence of hernias, difficulty in performing daily activities, complications, and use of analgesics. Pain was measured using the Inguinal Pain Questionnaire (IPQ).
In a paper published in the February issue of Annals of Surgery (2016;263:240-3), Dr. Westin and colleagues reported that the group randomized to TEP saw significantly less persistent mild pain at 1 year, compared with the Lichtenstein group. In the TEP group 39 patients reported such pain, compared with 62 in the Lichtenstein group (20.7% vs. 33.2,% P = .007).
Five TEP patients reported pain in the groin that limited their ability to perform physical exercise, while 14 in the Lichtenstein group reported similar pain, a difference that reached statistical significance (2.7% vs. 7.5%, P = .034). Severe pain, defined as pain affecting most activities, was comparable in both groups, with four patients in the TEP group and six in the Lichtenstein group reporting severe pain (2.1% and 3.2%, P = .543).
No significant between-group differences were seen in recurrences, use of pain medications, or specific measures of mobility, such as being able to sit or stand for more than 30 minutes at a stretch.
“Our results show a clinically significant advantage in favor of TEP regarding long-term postoperative pain, and justify the use of TEP for routine inguinal hernia surgery,” Dr. Westin and colleagues noted in their analysis. Their findings, they said, should prompt more extensive training of surgeons in the TEP technique.
The investigators noted as a limitation of their study that it was not powered to detect differences in severe pain, which could be considered more clinically relevant than mild persistent pain.
Patients’ pain outcomes may have been related to surgeons’ handling of the nerves in the inguinal tract, they wrote.
Also, they noted, the same heavyweight mesh was used in all operations in the study, for consistency, despite the fact that, after the initiation of the trial, lightweight mesh was shown in studies to be associated with less pain. Dr. Westin and colleagues wrote that their findings would be applicable to surgeries using lightweight mesh, which might further limit pain.
Their results, they wrote, suggested that TEP should be considered a valid first choice not only for the study population, which included only men with primary unilateral hernias, but for those with bilateral or recurrent hernias and also for women.
The study was funded by the Uppsala-Orebro Regional Research Council, Stockholm County Council, Swedish Society of Medicine, and Olle Engqvist Research Foundation. None of the investigators declared conflicts of interest.
Patients undergoing endoscopic total extraperitoneal repair (TEP) of hernias had less persistent pain and greater mobility than those whose hernias were repaired using the Lichtenstein technique.
For their research, conducted in Sweden, researchers led by Dr. Linn Westin of the Karolinska Institutet in Stockholm and colleagues randomized 384 adult men with primary unilateral inguinal hernia to surgery with Lichtenstein using local anesthesia (n = 191) or TEP with general anesthesia (n = 193). Patients underwent operations in two hospitals between 2006 and 2011, with four surgeons participating, and the same heavyweight mesh was used in both groups.
All patients were followed for 1 year for postoperative pain, recurrence of hernias, difficulty in performing daily activities, complications, and use of analgesics. Pain was measured using the Inguinal Pain Questionnaire (IPQ).
In a paper published in the February issue of Annals of Surgery (2016;263:240-3), Dr. Westin and colleagues reported that the group randomized to TEP saw significantly less persistent mild pain at 1 year, compared with the Lichtenstein group. In the TEP group 39 patients reported such pain, compared with 62 in the Lichtenstein group (20.7% vs. 33.2,% P = .007).
Five TEP patients reported pain in the groin that limited their ability to perform physical exercise, while 14 in the Lichtenstein group reported similar pain, a difference that reached statistical significance (2.7% vs. 7.5%, P = .034). Severe pain, defined as pain affecting most activities, was comparable in both groups, with four patients in the TEP group and six in the Lichtenstein group reporting severe pain (2.1% and 3.2%, P = .543).
No significant between-group differences were seen in recurrences, use of pain medications, or specific measures of mobility, such as being able to sit or stand for more than 30 minutes at a stretch.
“Our results show a clinically significant advantage in favor of TEP regarding long-term postoperative pain, and justify the use of TEP for routine inguinal hernia surgery,” Dr. Westin and colleagues noted in their analysis. Their findings, they said, should prompt more extensive training of surgeons in the TEP technique.
The investigators noted as a limitation of their study that it was not powered to detect differences in severe pain, which could be considered more clinically relevant than mild persistent pain.
Patients’ pain outcomes may have been related to surgeons’ handling of the nerves in the inguinal tract, they wrote.
Also, they noted, the same heavyweight mesh was used in all operations in the study, for consistency, despite the fact that, after the initiation of the trial, lightweight mesh was shown in studies to be associated with less pain. Dr. Westin and colleagues wrote that their findings would be applicable to surgeries using lightweight mesh, which might further limit pain.
Their results, they wrote, suggested that TEP should be considered a valid first choice not only for the study population, which included only men with primary unilateral hernias, but for those with bilateral or recurrent hernias and also for women.
The study was funded by the Uppsala-Orebro Regional Research Council, Stockholm County Council, Swedish Society of Medicine, and Olle Engqvist Research Foundation. None of the investigators declared conflicts of interest.
Patients undergoing endoscopic total extraperitoneal repair (TEP) of hernias had less persistent pain and greater mobility than those whose hernias were repaired using the Lichtenstein technique.
For their research, conducted in Sweden, researchers led by Dr. Linn Westin of the Karolinska Institutet in Stockholm and colleagues randomized 384 adult men with primary unilateral inguinal hernia to surgery with Lichtenstein using local anesthesia (n = 191) or TEP with general anesthesia (n = 193). Patients underwent operations in two hospitals between 2006 and 2011, with four surgeons participating, and the same heavyweight mesh was used in both groups.
All patients were followed for 1 year for postoperative pain, recurrence of hernias, difficulty in performing daily activities, complications, and use of analgesics. Pain was measured using the Inguinal Pain Questionnaire (IPQ).
In a paper published in the February issue of Annals of Surgery (2016;263:240-3), Dr. Westin and colleagues reported that the group randomized to TEP saw significantly less persistent mild pain at 1 year, compared with the Lichtenstein group. In the TEP group 39 patients reported such pain, compared with 62 in the Lichtenstein group (20.7% vs. 33.2,% P = .007).
Five TEP patients reported pain in the groin that limited their ability to perform physical exercise, while 14 in the Lichtenstein group reported similar pain, a difference that reached statistical significance (2.7% vs. 7.5%, P = .034). Severe pain, defined as pain affecting most activities, was comparable in both groups, with four patients in the TEP group and six in the Lichtenstein group reporting severe pain (2.1% and 3.2%, P = .543).
No significant between-group differences were seen in recurrences, use of pain medications, or specific measures of mobility, such as being able to sit or stand for more than 30 minutes at a stretch.
“Our results show a clinically significant advantage in favor of TEP regarding long-term postoperative pain, and justify the use of TEP for routine inguinal hernia surgery,” Dr. Westin and colleagues noted in their analysis. Their findings, they said, should prompt more extensive training of surgeons in the TEP technique.
The investigators noted as a limitation of their study that it was not powered to detect differences in severe pain, which could be considered more clinically relevant than mild persistent pain.
Patients’ pain outcomes may have been related to surgeons’ handling of the nerves in the inguinal tract, they wrote.
Also, they noted, the same heavyweight mesh was used in all operations in the study, for consistency, despite the fact that, after the initiation of the trial, lightweight mesh was shown in studies to be associated with less pain. Dr. Westin and colleagues wrote that their findings would be applicable to surgeries using lightweight mesh, which might further limit pain.
Their results, they wrote, suggested that TEP should be considered a valid first choice not only for the study population, which included only men with primary unilateral hernias, but for those with bilateral or recurrent hernias and also for women.
The study was funded by the Uppsala-Orebro Regional Research Council, Stockholm County Council, Swedish Society of Medicine, and Olle Engqvist Research Foundation. None of the investigators declared conflicts of interest.
FROM ANNALS OF SURGERY
Key clinical point: Patients undergoing TEP for hernia repair were less likely to report persistent pain after 1 year than those undergoing Lichtenstein procedures.
Major finding: In the TEP group, 39 (20.7%) patients reported persistent pain, compared with 62 (33.2%) in the Lichtenstein group (P = .007).
Data source: A randomized multisite surgical trial in Sweden enrolling 384 men with unilateral primary inguinal hernias, with 375 followed up at 1 year.
Disclosures: The study was funded by Swedish government grants, the Swedish Society of Medicine, and the Olle Engqvist Research Foundation. Investigators disclosed no conflicts of interest.