User login
Appendicitis, antibiotics, and surgery: An evolving trilogy
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Make the Diagnosis - February 2016
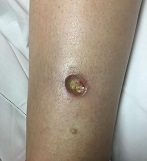
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum (PG) is an uncommon, noninfectious neutrophilic dermatosis that results in chronic ulcerative lesions. This disease process favors adult women and can be associated with systemic diseases in the majority of cases. The most common underlying systemic ailments include inflammatory bowel disease, arthritis, infection, and hematologic malignancy; it can also be drug induced.
Typically, the lesions begin as an erythematous pustule or nodule on an extremity. As was the case with our patient, a history of a "spider bite" or other arthropod assault may be elicited in the history as patients try to attribute a cause to the development of the initial ulceration. The pustule then develops into an ulcer with a characteristic necrotic, violaceous undermined border with a purulent base. Also, this disease process is associated with pathergy, in which minor trauma can induce additional lesions at remote sites.
There are four well-known types of pyoderma gangrenosum including the classic ulcerative lesions, pustular, bullous, and superficial granulomatous type, also known as vegetative PG. The pustular type may be seen more frequently in patients with inflammatory bowel disease, the bullous type may predominate in hematologic disorders, and the superficial granulomatous type is known to occur following surgery or other trauma.
The pathology of lesions can be nonspecific. However, in untreated lesions, widespread infiltration of neutrophils can be demonstrated at the base of the ulcers with accompanying necrosis at the periphery of lesions.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to [email protected].
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum (PG) is an uncommon, noninfectious neutrophilic dermatosis that results in chronic ulcerative lesions. This disease process favors adult women and can be associated with systemic diseases in the majority of cases. The most common underlying systemic ailments include inflammatory bowel disease, arthritis, infection, and hematologic malignancy; it can also be drug induced.
Typically, the lesions begin as an erythematous pustule or nodule on an extremity. As was the case with our patient, a history of a "spider bite" or other arthropod assault may be elicited in the history as patients try to attribute a cause to the development of the initial ulceration. The pustule then develops into an ulcer with a characteristic necrotic, violaceous undermined border with a purulent base. Also, this disease process is associated with pathergy, in which minor trauma can induce additional lesions at remote sites.
There are four well-known types of pyoderma gangrenosum including the classic ulcerative lesions, pustular, bullous, and superficial granulomatous type, also known as vegetative PG. The pustular type may be seen more frequently in patients with inflammatory bowel disease, the bullous type may predominate in hematologic disorders, and the superficial granulomatous type is known to occur following surgery or other trauma.
The pathology of lesions can be nonspecific. However, in untreated lesions, widespread infiltration of neutrophils can be demonstrated at the base of the ulcers with accompanying necrosis at the periphery of lesions.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to [email protected].
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum (PG) is an uncommon, noninfectious neutrophilic dermatosis that results in chronic ulcerative lesions. This disease process favors adult women and can be associated with systemic diseases in the majority of cases. The most common underlying systemic ailments include inflammatory bowel disease, arthritis, infection, and hematologic malignancy; it can also be drug induced.
Typically, the lesions begin as an erythematous pustule or nodule on an extremity. As was the case with our patient, a history of a "spider bite" or other arthropod assault may be elicited in the history as patients try to attribute a cause to the development of the initial ulceration. The pustule then develops into an ulcer with a characteristic necrotic, violaceous undermined border with a purulent base. Also, this disease process is associated with pathergy, in which minor trauma can induce additional lesions at remote sites.
There are four well-known types of pyoderma gangrenosum including the classic ulcerative lesions, pustular, bullous, and superficial granulomatous type, also known as vegetative PG. The pustular type may be seen more frequently in patients with inflammatory bowel disease, the bullous type may predominate in hematologic disorders, and the superficial granulomatous type is known to occur following surgery or other trauma.
The pathology of lesions can be nonspecific. However, in untreated lesions, widespread infiltration of neutrophils can be demonstrated at the base of the ulcers with accompanying necrosis at the periphery of lesions.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to [email protected].
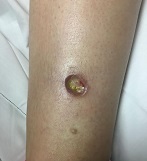
A 42-year-old woman with a 10-year history of Crohn's disease, treated with weekly subcutaneous injections of adalimumab, and hypertension presented with ulcerations on the lower extremities. She stated that the ulcerations began after she had been camping and reported being bitten by several ants during the trip, approximately 3 months earlier.
Pediatric Dermatology Consult - February 2016
By Catalina Matiz, M.D., and David Ginsberg
Nummular eczema
Nummular eczema is not an uncommon dermatosis that presents in pediatric and adult patients; its name, which derives from the Latin word nummulus (coin-like), refers to the coined-shape plaques that characterize this condition. It also has been referred to as discoid eczema and nummular dermatitis.1
The lesions begin as erythematous papules and vesicles that extend into larger oval or circular plaques that often become crusted, and can later progress to dry and scaly plaques.1,2 Patients often complain of intense pruritus.1 The lesions can be single or multiple, and more commonly occur on the extensor extremities as well as the trunk, and rarely affect the neck and the head.1-3 The pathophysiology of nummular eczema is not fully understood. It can occur in patients that exhibit atopic manifestations such as atopic dermatitis and other allergies, but there has been no clear link found between nummular eczema and atopy.3,4
Many theories exist implicating causative factors including Staphylococcus aureus colonization and xerosis.1 Similarly, some physicians believe that patch testing can be useful in these patients because of the potential for exacerbation caused by environmental allergens, but there is still no agreement on the ultimate cause.5 There is a higher incidence in males than females, and in the pediatric population, it is more common among “school aged” children between the ages of 2-12.6 Overall, nummular eczema is more commonly seen in adults, but it can occur at any age.2,3,6
Differential diagnosis
Nummular eczema is commonly mistaken as tinea corporis.1 The coined shape lesions, from which nummular eczema gets its name, can resemble the characteristic annular shape plaques of “ring worm,” but a potassium hydroxide (KOH) test or a fungal culture are simple ways to differentiate between the two conditions.
Nummular eczema occasionally can be confused for psoriasis as both entities can present with oval plaques. Psoriasis lesions tend to be pinker and less erythematous than nummular eczema lesions and most psoriasis plaques present with a characteristic silver scale.7 Clinically, nummular eczema is frequently associated with extreme pruritus, while in psoriasis the pruritus is less prominent.7
A biopsy would yield a more definitive diagnosis in difficult cases. Histologically, nummular eczema resembles other forms of spongiotic dermatitis, while psoriasis has very distinct histological features.7 Differentiating between contact dermatitis and nummular eczema relies on a thorough history of known allergies and potential exposure to environmental allergens. If history alone does not yield a definitive diagnosis and a suspicion for contact allergy is high, patch testing could help support one diagnosis over the other.5
Treatment
The generally accepted first line therapy includes mid to high potency topical corticosteroids in an ointment preparation or else under occlusion.1,4 Other topical agents used include tar preparations and calcineurin inhibitors.4 Intralesional corticosteroid injection can be used to treat isolated lesions that fail to respond to topical treatments.4
As with almost all manifestations of dermatitis, general gentle skin care measures and daily moisturizing are recommended.1 For more severe cases in older children, narrow-band UVB light therapy can be helpful.1 Due to their efficacy in treatment of other forms of refractory dermatitis, systemic therapy with cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate can be used in cases in which phototherapy fails or is not accessible.4
In cases recalcitrant to topical therapies, secondary staphylococcal infection always should be ruled out and treated with systemic antimicrobials such as first generation cephalosporins.1
References
- Eczematous eruptions in childhood in “Hurwitz Clinical Pediatric Dermatology,” 4th ed. (New York, N.Y.: Elsevier, pp. 59-60
- Acta Derm Venereol. 1961;41:453-60.
- Acta Derm Venereol. 1969;49(2):189-96.
- Australas J Dermatol. 2010 May;51(2):128-30.
- Contact Dermatitis. 1997 May;36(5):261-4.
- Ped Dermatol. 2012 Oct;29(5):580-3.
- Dermatol Ther. 2006 Mar-Apr;19(2):73-82.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
By Catalina Matiz, M.D., and David Ginsberg
Nummular eczema
Nummular eczema is not an uncommon dermatosis that presents in pediatric and adult patients; its name, which derives from the Latin word nummulus (coin-like), refers to the coined-shape plaques that characterize this condition. It also has been referred to as discoid eczema and nummular dermatitis.1
The lesions begin as erythematous papules and vesicles that extend into larger oval or circular plaques that often become crusted, and can later progress to dry and scaly plaques.1,2 Patients often complain of intense pruritus.1 The lesions can be single or multiple, and more commonly occur on the extensor extremities as well as the trunk, and rarely affect the neck and the head.1-3 The pathophysiology of nummular eczema is not fully understood. It can occur in patients that exhibit atopic manifestations such as atopic dermatitis and other allergies, but there has been no clear link found between nummular eczema and atopy.3,4
Many theories exist implicating causative factors including Staphylococcus aureus colonization and xerosis.1 Similarly, some physicians believe that patch testing can be useful in these patients because of the potential for exacerbation caused by environmental allergens, but there is still no agreement on the ultimate cause.5 There is a higher incidence in males than females, and in the pediatric population, it is more common among “school aged” children between the ages of 2-12.6 Overall, nummular eczema is more commonly seen in adults, but it can occur at any age.2,3,6
Differential diagnosis
Nummular eczema is commonly mistaken as tinea corporis.1 The coined shape lesions, from which nummular eczema gets its name, can resemble the characteristic annular shape plaques of “ring worm,” but a potassium hydroxide (KOH) test or a fungal culture are simple ways to differentiate between the two conditions.
Nummular eczema occasionally can be confused for psoriasis as both entities can present with oval plaques. Psoriasis lesions tend to be pinker and less erythematous than nummular eczema lesions and most psoriasis plaques present with a characteristic silver scale.7 Clinically, nummular eczema is frequently associated with extreme pruritus, while in psoriasis the pruritus is less prominent.7
A biopsy would yield a more definitive diagnosis in difficult cases. Histologically, nummular eczema resembles other forms of spongiotic dermatitis, while psoriasis has very distinct histological features.7 Differentiating between contact dermatitis and nummular eczema relies on a thorough history of known allergies and potential exposure to environmental allergens. If history alone does not yield a definitive diagnosis and a suspicion for contact allergy is high, patch testing could help support one diagnosis over the other.5
Treatment
The generally accepted first line therapy includes mid to high potency topical corticosteroids in an ointment preparation or else under occlusion.1,4 Other topical agents used include tar preparations and calcineurin inhibitors.4 Intralesional corticosteroid injection can be used to treat isolated lesions that fail to respond to topical treatments.4
As with almost all manifestations of dermatitis, general gentle skin care measures and daily moisturizing are recommended.1 For more severe cases in older children, narrow-band UVB light therapy can be helpful.1 Due to their efficacy in treatment of other forms of refractory dermatitis, systemic therapy with cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate can be used in cases in which phototherapy fails or is not accessible.4
In cases recalcitrant to topical therapies, secondary staphylococcal infection always should be ruled out and treated with systemic antimicrobials such as first generation cephalosporins.1
References
- Eczematous eruptions in childhood in “Hurwitz Clinical Pediatric Dermatology,” 4th ed. (New York, N.Y.: Elsevier, pp. 59-60
- Acta Derm Venereol. 1961;41:453-60.
- Acta Derm Venereol. 1969;49(2):189-96.
- Australas J Dermatol. 2010 May;51(2):128-30.
- Contact Dermatitis. 1997 May;36(5):261-4.
- Ped Dermatol. 2012 Oct;29(5):580-3.
- Dermatol Ther. 2006 Mar-Apr;19(2):73-82.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
By Catalina Matiz, M.D., and David Ginsberg
Nummular eczema
Nummular eczema is not an uncommon dermatosis that presents in pediatric and adult patients; its name, which derives from the Latin word nummulus (coin-like), refers to the coined-shape plaques that characterize this condition. It also has been referred to as discoid eczema and nummular dermatitis.1
The lesions begin as erythematous papules and vesicles that extend into larger oval or circular plaques that often become crusted, and can later progress to dry and scaly plaques.1,2 Patients often complain of intense pruritus.1 The lesions can be single or multiple, and more commonly occur on the extensor extremities as well as the trunk, and rarely affect the neck and the head.1-3 The pathophysiology of nummular eczema is not fully understood. It can occur in patients that exhibit atopic manifestations such as atopic dermatitis and other allergies, but there has been no clear link found between nummular eczema and atopy.3,4
Many theories exist implicating causative factors including Staphylococcus aureus colonization and xerosis.1 Similarly, some physicians believe that patch testing can be useful in these patients because of the potential for exacerbation caused by environmental allergens, but there is still no agreement on the ultimate cause.5 There is a higher incidence in males than females, and in the pediatric population, it is more common among “school aged” children between the ages of 2-12.6 Overall, nummular eczema is more commonly seen in adults, but it can occur at any age.2,3,6
Differential diagnosis
Nummular eczema is commonly mistaken as tinea corporis.1 The coined shape lesions, from which nummular eczema gets its name, can resemble the characteristic annular shape plaques of “ring worm,” but a potassium hydroxide (KOH) test or a fungal culture are simple ways to differentiate between the two conditions.
Nummular eczema occasionally can be confused for psoriasis as both entities can present with oval plaques. Psoriasis lesions tend to be pinker and less erythematous than nummular eczema lesions and most psoriasis plaques present with a characteristic silver scale.7 Clinically, nummular eczema is frequently associated with extreme pruritus, while in psoriasis the pruritus is less prominent.7
A biopsy would yield a more definitive diagnosis in difficult cases. Histologically, nummular eczema resembles other forms of spongiotic dermatitis, while psoriasis has very distinct histological features.7 Differentiating between contact dermatitis and nummular eczema relies on a thorough history of known allergies and potential exposure to environmental allergens. If history alone does not yield a definitive diagnosis and a suspicion for contact allergy is high, patch testing could help support one diagnosis over the other.5
Treatment
The generally accepted first line therapy includes mid to high potency topical corticosteroids in an ointment preparation or else under occlusion.1,4 Other topical agents used include tar preparations and calcineurin inhibitors.4 Intralesional corticosteroid injection can be used to treat isolated lesions that fail to respond to topical treatments.4
As with almost all manifestations of dermatitis, general gentle skin care measures and daily moisturizing are recommended.1 For more severe cases in older children, narrow-band UVB light therapy can be helpful.1 Due to their efficacy in treatment of other forms of refractory dermatitis, systemic therapy with cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate can be used in cases in which phototherapy fails or is not accessible.4
In cases recalcitrant to topical therapies, secondary staphylococcal infection always should be ruled out and treated with systemic antimicrobials such as first generation cephalosporins.1
References
- Eczematous eruptions in childhood in “Hurwitz Clinical Pediatric Dermatology,” 4th ed. (New York, N.Y.: Elsevier, pp. 59-60
- Acta Derm Venereol. 1961;41:453-60.
- Acta Derm Venereol. 1969;49(2):189-96.
- Australas J Dermatol. 2010 May;51(2):128-30.
- Contact Dermatitis. 1997 May;36(5):261-4.
- Ped Dermatol. 2012 Oct;29(5):580-3.
- Dermatol Ther. 2006 Mar-Apr;19(2):73-82.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
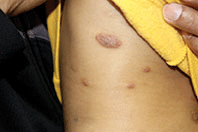
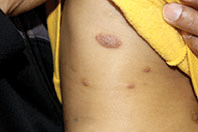
A 9-month-old male with no significant previous medical history presents with a very itchy rash that has been present for 6 weeks. His mother reports that the lesions began as small red bumps on the extremities and his torso, that developed over the course of a few weeks into large round, red, very pruritic plaques. He has been treated with an antifungal cream for several weeks without resolution, and most recently his mother has been applying hydrocortisone 2.5% cream with no improvement either. On exam, the patient is a well appearing infant, who is visibly irritated due to the pruritus accompanying his rash. There are several 1-cm to 4-cm round and oval dry, scaly, erythematous plaques on the trunk (see photo) and a few on the extremities. There is no generalized xerosis.
High Headache Frequency Is More Likely During Perimenopause
Women in perimenopause are at increased risk of high-frequency headache, compared with premenopausal women, according to data published online ahead of print January 21 in Headache. Women in menopause also are at increased risk of high-frequency headache, but the effect of menopause on headache frequency may be mediated or confounded by medication overuse or depression.
“Our results confirm the commonly held belief that the perimenopause worsens headache, but challenge the idea that migraine ‘always’ improves during the menopause,” said Vincent T. Martin, MD, Professor of Internal Medicine in the University of Cincinnati’s (UC) Division of General Internal Medicine and codirector of the Headache and Facial Pain Program at the UC Neuroscience Institute. “Recognition of the increased risk of high-frequency headache during the menopausal transition suggests a need for optimized preventive treatment of migraine during this time of women’s life.”
Research has suggested a lower prevalence of headache or migraine during menopause, compared with premenopause. No previous studies have analyzed whether frequency of headache attacks changes during the menopausal transition among women with migraine, however. Dr. Martin and colleagues sought to determine whether the percentage of female migraineurs with high-frequency headache, defined as 10 or more days/month, is greater during the perimenopausal and menopausal time periods, compared with the premenopausal period. The researchers also set out to examine whether any increase in high-frequency headache during a particular reproductive phase was restricted to the early or late stages of the phase.
An Analysis of AMPP Data
To answer their questions, the investigators conducted a cross-sectional study using data from the American Migraine Prevalence and Prevention (AMPP) study. The AMPP researchers elicited data about headache from 162,756 respondents age 12 or older in 2004 and invited a random subset of 24,000 people age 18 or older with self-reported severe headache to participate in annual follow-up surveys for the subsequent five years. Follow-up surveys included questions about sociodemographics (eg, BMI, smoking, and household income) and headache types and characteristics, in addition to the Migraine Disability Assessment Score. Dr. Martin and colleagues examined data from the 2006 follow-up survey because it contained questions on the menstrual cycle.
Eligible participants in the cross-sectional study were women with a diagnosis of migraine between ages 35 and 65. Women who were pregnant, breastfeeding, had a history of hysterectomy or oophorectomy, or used hormonal therapies were excluded from the analysis. The investigators classified respondents as premenopause, perimenopause, and menopause according to Stages of Reproductive Aging Workshop criteria.
Late Perimenopause and Headache Frequency
The analysis included 3,664 women, of whom 3,454 had episodic migraine and 210 had chronic migraine. In all, 1,263 women were classified as premenopausal, 1,283 as perimenopausal, and 1,118 as menopausal. Compared with women in premenopause, women in perimenopause and menopause used more migraine preventives and were more likely to overuse medication.
Approximately 8% of premenopausal women had high-frequency headache, compared with 12.2% of perimenopausal women and 12.0% of postmenopausal women. After adjustments for sociodemographics alone, the odds ratios (ORs) of high-frequency headache were 1.62 for perimenopausal women and 1.76 for menopausal women, compared with premenopausal women. After adjustment for BMI, current migraine preventive use, medication overuse, and depression, the OR decreased, but remained significant in the perimenopausal group (OR, 1.42) and lost significance for the menopausal group (OR, 1.27). Depression and medication overuse significantly increased the likelihood of high-frequency headache.
When the researchers examined participants in the early and late stages of perimenopause and adjusted data for all covariates, women in late perimenopause had an increased likelihood of high-frequency headache (OR, 1.72), but women in early perimenopause had a statistically insignificant increased risk of this outcome (OR, 1.22), compared with premenopausal women. When the researchers examined the early and late stages of menopause, compared with premenopause, they found no significant difference in risk of high-frequency headache after controlling for all covariates.
Results Contradict Common Belief
“These results suggest that the hormonal milieu of the late perimenopause is particularly provocative for high-frequency headache among migraineurs,” said Dr. Martin. Because the researchers did not collect data on premenstrual syndrome (PMS) disorder, they could not determine whether the increased risk for high-frequency headache during perimenopause only occurred in female migraineurs with PMS or in the entire population.
Epidemiologic studies have contributed to an impression that migraine prevalence declines in menopausal women, but the current study’s results contradict this impression. “Our study used high-frequency headache as its primary outcome measure, rather than migraine prevalence. It is plausible that during menopause, migraine prevalence decreases and migraine attacks occur more frequently in subgroups of women,” said Dr. Martin. “Women, as they get older, develop lots of aches and pains, joint [pain], and back pain, and it is possible [that] their overuse of pain medications for headache and other conditions might actually drive an increase in headaches for the menopause group,” he added.
Estrogen withdrawal in the late luteal phase, low serum levels of estrogen or progesterone, and increased uterine prostaglandin release could precipitate headache during the menopausal transition, said the researchers. These hormonal changes also may change the characteristics of the menstrual cycle, which could in turn affect headache frequency.
An advantage of the cross-sectional analysis is that it was a large population-based study of persons with migraine “that should have wide generalizability to the general population,” said Dr. Martin. The outcome measure of high-frequency headache, however, was not limited to migraine, but included all headaches. In addition, headache frequency was self-reported, and investigators did not confirm it with daily headache diaries. Finally, the researchers did not account or control for aura. “Our results should be considered preliminary until confirmed in future studies,” Dr. Martin concluded.
—Erik Greb
Suggested Reading
Martin VT, Pavlovic J, Fanning KM, et al. Perimenopause and menopause are associated with high frequency headache in women with migraine: results of the American Migraine Prevalence and Prevention Study. Headache. 2016 Jan 21 [Epub ahead of print].
Women in perimenopause are at increased risk of high-frequency headache, compared with premenopausal women, according to data published online ahead of print January 21 in Headache. Women in menopause also are at increased risk of high-frequency headache, but the effect of menopause on headache frequency may be mediated or confounded by medication overuse or depression.
“Our results confirm the commonly held belief that the perimenopause worsens headache, but challenge the idea that migraine ‘always’ improves during the menopause,” said Vincent T. Martin, MD, Professor of Internal Medicine in the University of Cincinnati’s (UC) Division of General Internal Medicine and codirector of the Headache and Facial Pain Program at the UC Neuroscience Institute. “Recognition of the increased risk of high-frequency headache during the menopausal transition suggests a need for optimized preventive treatment of migraine during this time of women’s life.”
Research has suggested a lower prevalence of headache or migraine during menopause, compared with premenopause. No previous studies have analyzed whether frequency of headache attacks changes during the menopausal transition among women with migraine, however. Dr. Martin and colleagues sought to determine whether the percentage of female migraineurs with high-frequency headache, defined as 10 or more days/month, is greater during the perimenopausal and menopausal time periods, compared with the premenopausal period. The researchers also set out to examine whether any increase in high-frequency headache during a particular reproductive phase was restricted to the early or late stages of the phase.
An Analysis of AMPP Data
To answer their questions, the investigators conducted a cross-sectional study using data from the American Migraine Prevalence and Prevention (AMPP) study. The AMPP researchers elicited data about headache from 162,756 respondents age 12 or older in 2004 and invited a random subset of 24,000 people age 18 or older with self-reported severe headache to participate in annual follow-up surveys for the subsequent five years. Follow-up surveys included questions about sociodemographics (eg, BMI, smoking, and household income) and headache types and characteristics, in addition to the Migraine Disability Assessment Score. Dr. Martin and colleagues examined data from the 2006 follow-up survey because it contained questions on the menstrual cycle.
Eligible participants in the cross-sectional study were women with a diagnosis of migraine between ages 35 and 65. Women who were pregnant, breastfeeding, had a history of hysterectomy or oophorectomy, or used hormonal therapies were excluded from the analysis. The investigators classified respondents as premenopause, perimenopause, and menopause according to Stages of Reproductive Aging Workshop criteria.
Late Perimenopause and Headache Frequency
The analysis included 3,664 women, of whom 3,454 had episodic migraine and 210 had chronic migraine. In all, 1,263 women were classified as premenopausal, 1,283 as perimenopausal, and 1,118 as menopausal. Compared with women in premenopause, women in perimenopause and menopause used more migraine preventives and were more likely to overuse medication.
Approximately 8% of premenopausal women had high-frequency headache, compared with 12.2% of perimenopausal women and 12.0% of postmenopausal women. After adjustments for sociodemographics alone, the odds ratios (ORs) of high-frequency headache were 1.62 for perimenopausal women and 1.76 for menopausal women, compared with premenopausal women. After adjustment for BMI, current migraine preventive use, medication overuse, and depression, the OR decreased, but remained significant in the perimenopausal group (OR, 1.42) and lost significance for the menopausal group (OR, 1.27). Depression and medication overuse significantly increased the likelihood of high-frequency headache.
When the researchers examined participants in the early and late stages of perimenopause and adjusted data for all covariates, women in late perimenopause had an increased likelihood of high-frequency headache (OR, 1.72), but women in early perimenopause had a statistically insignificant increased risk of this outcome (OR, 1.22), compared with premenopausal women. When the researchers examined the early and late stages of menopause, compared with premenopause, they found no significant difference in risk of high-frequency headache after controlling for all covariates.
Results Contradict Common Belief
“These results suggest that the hormonal milieu of the late perimenopause is particularly provocative for high-frequency headache among migraineurs,” said Dr. Martin. Because the researchers did not collect data on premenstrual syndrome (PMS) disorder, they could not determine whether the increased risk for high-frequency headache during perimenopause only occurred in female migraineurs with PMS or in the entire population.
Epidemiologic studies have contributed to an impression that migraine prevalence declines in menopausal women, but the current study’s results contradict this impression. “Our study used high-frequency headache as its primary outcome measure, rather than migraine prevalence. It is plausible that during menopause, migraine prevalence decreases and migraine attacks occur more frequently in subgroups of women,” said Dr. Martin. “Women, as they get older, develop lots of aches and pains, joint [pain], and back pain, and it is possible [that] their overuse of pain medications for headache and other conditions might actually drive an increase in headaches for the menopause group,” he added.
Estrogen withdrawal in the late luteal phase, low serum levels of estrogen or progesterone, and increased uterine prostaglandin release could precipitate headache during the menopausal transition, said the researchers. These hormonal changes also may change the characteristics of the menstrual cycle, which could in turn affect headache frequency.
An advantage of the cross-sectional analysis is that it was a large population-based study of persons with migraine “that should have wide generalizability to the general population,” said Dr. Martin. The outcome measure of high-frequency headache, however, was not limited to migraine, but included all headaches. In addition, headache frequency was self-reported, and investigators did not confirm it with daily headache diaries. Finally, the researchers did not account or control for aura. “Our results should be considered preliminary until confirmed in future studies,” Dr. Martin concluded.
—Erik Greb
Women in perimenopause are at increased risk of high-frequency headache, compared with premenopausal women, according to data published online ahead of print January 21 in Headache. Women in menopause also are at increased risk of high-frequency headache, but the effect of menopause on headache frequency may be mediated or confounded by medication overuse or depression.
“Our results confirm the commonly held belief that the perimenopause worsens headache, but challenge the idea that migraine ‘always’ improves during the menopause,” said Vincent T. Martin, MD, Professor of Internal Medicine in the University of Cincinnati’s (UC) Division of General Internal Medicine and codirector of the Headache and Facial Pain Program at the UC Neuroscience Institute. “Recognition of the increased risk of high-frequency headache during the menopausal transition suggests a need for optimized preventive treatment of migraine during this time of women’s life.”
Research has suggested a lower prevalence of headache or migraine during menopause, compared with premenopause. No previous studies have analyzed whether frequency of headache attacks changes during the menopausal transition among women with migraine, however. Dr. Martin and colleagues sought to determine whether the percentage of female migraineurs with high-frequency headache, defined as 10 or more days/month, is greater during the perimenopausal and menopausal time periods, compared with the premenopausal period. The researchers also set out to examine whether any increase in high-frequency headache during a particular reproductive phase was restricted to the early or late stages of the phase.
An Analysis of AMPP Data
To answer their questions, the investigators conducted a cross-sectional study using data from the American Migraine Prevalence and Prevention (AMPP) study. The AMPP researchers elicited data about headache from 162,756 respondents age 12 or older in 2004 and invited a random subset of 24,000 people age 18 or older with self-reported severe headache to participate in annual follow-up surveys for the subsequent five years. Follow-up surveys included questions about sociodemographics (eg, BMI, smoking, and household income) and headache types and characteristics, in addition to the Migraine Disability Assessment Score. Dr. Martin and colleagues examined data from the 2006 follow-up survey because it contained questions on the menstrual cycle.
Eligible participants in the cross-sectional study were women with a diagnosis of migraine between ages 35 and 65. Women who were pregnant, breastfeeding, had a history of hysterectomy or oophorectomy, or used hormonal therapies were excluded from the analysis. The investigators classified respondents as premenopause, perimenopause, and menopause according to Stages of Reproductive Aging Workshop criteria.
Late Perimenopause and Headache Frequency
The analysis included 3,664 women, of whom 3,454 had episodic migraine and 210 had chronic migraine. In all, 1,263 women were classified as premenopausal, 1,283 as perimenopausal, and 1,118 as menopausal. Compared with women in premenopause, women in perimenopause and menopause used more migraine preventives and were more likely to overuse medication.
Approximately 8% of premenopausal women had high-frequency headache, compared with 12.2% of perimenopausal women and 12.0% of postmenopausal women. After adjustments for sociodemographics alone, the odds ratios (ORs) of high-frequency headache were 1.62 for perimenopausal women and 1.76 for menopausal women, compared with premenopausal women. After adjustment for BMI, current migraine preventive use, medication overuse, and depression, the OR decreased, but remained significant in the perimenopausal group (OR, 1.42) and lost significance for the menopausal group (OR, 1.27). Depression and medication overuse significantly increased the likelihood of high-frequency headache.
When the researchers examined participants in the early and late stages of perimenopause and adjusted data for all covariates, women in late perimenopause had an increased likelihood of high-frequency headache (OR, 1.72), but women in early perimenopause had a statistically insignificant increased risk of this outcome (OR, 1.22), compared with premenopausal women. When the researchers examined the early and late stages of menopause, compared with premenopause, they found no significant difference in risk of high-frequency headache after controlling for all covariates.
Results Contradict Common Belief
“These results suggest that the hormonal milieu of the late perimenopause is particularly provocative for high-frequency headache among migraineurs,” said Dr. Martin. Because the researchers did not collect data on premenstrual syndrome (PMS) disorder, they could not determine whether the increased risk for high-frequency headache during perimenopause only occurred in female migraineurs with PMS or in the entire population.
Epidemiologic studies have contributed to an impression that migraine prevalence declines in menopausal women, but the current study’s results contradict this impression. “Our study used high-frequency headache as its primary outcome measure, rather than migraine prevalence. It is plausible that during menopause, migraine prevalence decreases and migraine attacks occur more frequently in subgroups of women,” said Dr. Martin. “Women, as they get older, develop lots of aches and pains, joint [pain], and back pain, and it is possible [that] their overuse of pain medications for headache and other conditions might actually drive an increase in headaches for the menopause group,” he added.
Estrogen withdrawal in the late luteal phase, low serum levels of estrogen or progesterone, and increased uterine prostaglandin release could precipitate headache during the menopausal transition, said the researchers. These hormonal changes also may change the characteristics of the menstrual cycle, which could in turn affect headache frequency.
An advantage of the cross-sectional analysis is that it was a large population-based study of persons with migraine “that should have wide generalizability to the general population,” said Dr. Martin. The outcome measure of high-frequency headache, however, was not limited to migraine, but included all headaches. In addition, headache frequency was self-reported, and investigators did not confirm it with daily headache diaries. Finally, the researchers did not account or control for aura. “Our results should be considered preliminary until confirmed in future studies,” Dr. Martin concluded.
—Erik Greb
Suggested Reading
Martin VT, Pavlovic J, Fanning KM, et al. Perimenopause and menopause are associated with high frequency headache in women with migraine: results of the American Migraine Prevalence and Prevention Study. Headache. 2016 Jan 21 [Epub ahead of print].
Suggested Reading
Martin VT, Pavlovic J, Fanning KM, et al. Perimenopause and menopause are associated with high frequency headache in women with migraine: results of the American Migraine Prevalence and Prevention Study. Headache. 2016 Jan 21 [Epub ahead of print].
Hope may not be the best component of an exercise regimen
Judging by the crowd and the difficulty in finding a locker at my gym on January 1, a lot of people are serious about their 2016 New Year’s resolution to exercise and lose weight. But as most of us have experienced personally and professionally, embarking on a well-intended effort to exercise in the hope of losing weight more often results in frustration than a trip to the store to buy smaller-sized clothes.
The frequent answer to the question “What did the doctor say at your visit?” provides a partial explanation of this phenomenon: “Nothing, just that I should exercise and lose weight” is the usual hackneyed response. Nothing—as in nothing unexpected, nothing significant, and nothing specific was said. It is with this lack of specific advice that I feel many of us let our patients down.
We admonish patients to eat fewer calories, avoid the evil carbs, walk 10,000 steps, ride a bike, use the elliptical, or swim three times a week. There is a concrete but broad nature to these suggestions, but there is also a familiarity and a lack of specificity that leaves patients feeling that there is no science behind them. And the truth is that many of us are not comfortable enough with current data from our exercise physiology colleagues to have a detailed discussion with our patients that pairs their specific goals with an exercise regimen and diet most likely to be beneficial. We may fear sounding like the morning talk show doctors, offering sound bites instead of engaging in an evidence-based dialogue with our patients.
Many of our patients cannot afford a personal trainer to guide and cajole them through a successful regimen—assuming that they, or we, can separate myth and fact and choose an appropriate trainer. We should try to be their guide and sounding board as well as coach and cheerleader.
In this issue of the Journal, John and Christopher Higgins present a primer on the background information to use when talking with our patients about starting an exercise program focused on weight loss. They provide useful references that support specific approaches to achieve realistic expectations, and they review and compare various strategies.
I’m sure by March it will again be easier to find a locker at my gym. And I hope by then that my new workout plan will be more scientifically based, as well as a bit more effective. Even data-based hope springs eternal.
Judging by the crowd and the difficulty in finding a locker at my gym on January 1, a lot of people are serious about their 2016 New Year’s resolution to exercise and lose weight. But as most of us have experienced personally and professionally, embarking on a well-intended effort to exercise in the hope of losing weight more often results in frustration than a trip to the store to buy smaller-sized clothes.
The frequent answer to the question “What did the doctor say at your visit?” provides a partial explanation of this phenomenon: “Nothing, just that I should exercise and lose weight” is the usual hackneyed response. Nothing—as in nothing unexpected, nothing significant, and nothing specific was said. It is with this lack of specific advice that I feel many of us let our patients down.
We admonish patients to eat fewer calories, avoid the evil carbs, walk 10,000 steps, ride a bike, use the elliptical, or swim three times a week. There is a concrete but broad nature to these suggestions, but there is also a familiarity and a lack of specificity that leaves patients feeling that there is no science behind them. And the truth is that many of us are not comfortable enough with current data from our exercise physiology colleagues to have a detailed discussion with our patients that pairs their specific goals with an exercise regimen and diet most likely to be beneficial. We may fear sounding like the morning talk show doctors, offering sound bites instead of engaging in an evidence-based dialogue with our patients.
Many of our patients cannot afford a personal trainer to guide and cajole them through a successful regimen—assuming that they, or we, can separate myth and fact and choose an appropriate trainer. We should try to be their guide and sounding board as well as coach and cheerleader.
In this issue of the Journal, John and Christopher Higgins present a primer on the background information to use when talking with our patients about starting an exercise program focused on weight loss. They provide useful references that support specific approaches to achieve realistic expectations, and they review and compare various strategies.
I’m sure by March it will again be easier to find a locker at my gym. And I hope by then that my new workout plan will be more scientifically based, as well as a bit more effective. Even data-based hope springs eternal.
Judging by the crowd and the difficulty in finding a locker at my gym on January 1, a lot of people are serious about their 2016 New Year’s resolution to exercise and lose weight. But as most of us have experienced personally and professionally, embarking on a well-intended effort to exercise in the hope of losing weight more often results in frustration than a trip to the store to buy smaller-sized clothes.
The frequent answer to the question “What did the doctor say at your visit?” provides a partial explanation of this phenomenon: “Nothing, just that I should exercise and lose weight” is the usual hackneyed response. Nothing—as in nothing unexpected, nothing significant, and nothing specific was said. It is with this lack of specific advice that I feel many of us let our patients down.
We admonish patients to eat fewer calories, avoid the evil carbs, walk 10,000 steps, ride a bike, use the elliptical, or swim three times a week. There is a concrete but broad nature to these suggestions, but there is also a familiarity and a lack of specificity that leaves patients feeling that there is no science behind them. And the truth is that many of us are not comfortable enough with current data from our exercise physiology colleagues to have a detailed discussion with our patients that pairs their specific goals with an exercise regimen and diet most likely to be beneficial. We may fear sounding like the morning talk show doctors, offering sound bites instead of engaging in an evidence-based dialogue with our patients.
Many of our patients cannot afford a personal trainer to guide and cajole them through a successful regimen—assuming that they, or we, can separate myth and fact and choose an appropriate trainer. We should try to be their guide and sounding board as well as coach and cheerleader.
In this issue of the Journal, John and Christopher Higgins present a primer on the background information to use when talking with our patients about starting an exercise program focused on weight loss. They provide useful references that support specific approaches to achieve realistic expectations, and they review and compare various strategies.
I’m sure by March it will again be easier to find a locker at my gym. And I hope by then that my new workout plan will be more scientifically based, as well as a bit more effective. Even data-based hope springs eternal.
The ethics of ICDs: History and future directions
In 1975, Julia and Joseph Quinlan approached the administrator of St. Clare’s Hospital in Denville, New Jersey, and requested that the mechanical ventilator on which their adopted daughter, Karen, was dependent be turned off. Karen Ann Quinlan, 21 years old, was in a permanent vegetative state after a severe anoxic event, and her parents had been informed by the hospital’s medical staff that she would never regain consciousness.
To the Quinlans’ request to withdraw the ventilator, the hospital administrator replied, “You have to understand our position, Mrs. Quinlan. In this hospital we don’t kill people.”1
The administrator’s response was consistent with prevailing ethical and legal perspectives, analyses, and directives at that time related to discontinuation of life-sustaining treatment. In the mid-1970s, the American Medical Association’s position was that it was permissible to not put a patient on a ventilator (ie, a physician could withhold a life-sustaining treatment), but once a patient was on a ventilator, it was not permissible to take the patient off if the intention was to allow death to occur.1 However, the New Jersey Supreme Court ultimately found this distinction between withholding and withdrawing unconvincing, and ruled unanimously that Karen Quinlan’s ventilator could be turned off.2
THE HASTINGS CENTER REPORT: STOPPING IS THE SAME AS NOT STARTING
During the subsequent decade, further ethical analysis and additional legal cases resulted in new insights and more nuanced thinking about forgoing life-sustaining treatment.
These developments were summarized in a 1987 report by the Hastings Center,3 a leading bioethics research and policy institute. The report provided normative guidance for the termination of life-sustaining treatment and for the care of dying patients. It acknowledged that deciding not to start a life-sustaining treatment can emotionally and psychologically affect healthcare professionals differently than deciding to stop such a treatment. However, the report also asserted that there is no morally important difference between withholding and withdrawing such treatments.
Reflecting a partnership model between patients and professionals for healthcare decision-making, and affirming the ethical significance of both a burden-benefit analysis and patient autonomy, the report stated that when a patient or surrogate in collaboration with a responsible healthcare professional decides that a treatment under way and the life it supports have become more burdensome than beneficial to the patient, that is sufficient reason to stop. There is no ethical requirement that treatment, once initiated, must continue against the patient’s wishes or when the surrogate determines that it is more burdensome than beneficial from the patient’s perspective. In fact, imposing treatment in such circumstances violates the patient’s right to self-determination.3
The report noted further that, because of frequent uncertainty about the efficacy of proposed treatments, it is preferable to initiate time-limited trials of treatments and then later stop them if they prove ineffective or become overly burdensome from a patient’s perspective.
ICDs ARE LIKE OTHER LIFE-SUSTAINING THERAPIES
In this issue of Cleveland Clinic Journal of Medicine, Baibars et al4 address the question of how implantable cardioverter-defibrillators (ICDs) should be managed at the end of life. The historical events and developments recounted above regarding withdrawing life-sustaining technologies are an appropriate context for ethically assessing the management of ICDs for dying patients.
Obviously, ICDs are not ventilators, but like ventilators, they are life-sustaining therapy, as are dialysis machines, blood transfusions, medically supplied nutrition and hydration, ventricular assist devices, and other implantable electronic cardiac devices such as pacemakers. Each of these life-sustaining therapies, depending on a patient’s clinical condition, underlying illness, and comorbidities, can become a death-prolonging technology.
An ethical framework and analysis about whether to continue any life-sustaining therapy, including an ICD, must include an assessment of the benefit-to-burden ratio from the patient’s perspective. Does the therapy enhance or maintain a quality of life acceptable to the patient? Or has it become overly burdensome and does it maintain a quality of life the patient finds (or would find) unacceptable? If the latter is true, and especially in the context of an underlying terminal condition, then shifting the goals of care to focus on comfort is always appropriate and ethically justified. Treatments—including ICDs—that do not contribute to patient comfort should be withdrawn.
TOWARD COMPETENCY IN ETHICAL MANAGEMENT
Baibars et al note that much more needs to be done to enhance competencies, increase proficiencies, and mitigate the moral distress of healthcare professionals caring for dying patients with ICDs and other devices. To help clinicians achieve a personal and professional “comfort zone” for ethically managing patients with ICDs, we recommend that healthcare institutions, medical schools, and nursing schools take the following steps:
Develop comprehensive end-of-life policies, procedures, and protocols that incorporate specific guidance for managing cardiac devices and that have been endorsed by a hospital ethics committee. Such guidance can be informative and educational and can ensure that decisions and resulting actions (including stopping cardiac devices) are ethically supportable.
Provide more palliative care training in medical and nursing schools, residency programs, and continuing education activities so that front-line clinicians can deliver “basic,” “primary” palliative care not requiring specialty palliative medicine. This training, called for in the Institute of Medicine’s 2014 report, Dying in America,5 should include explicit ethics discussions about managing cardiac devices at the end of life.
Provide ongoing training in communication skills needed for all patient-professional encounters. Effectively engaging patients in goals-of-care discussions, especially patients with life-limiting illnesses such as heart failure, cannot be achieved without these skills.
- Pence G. Comas: Karen Quinlan and Nancy Cruzan. In: Classic Cases in Medical Ethics: Accounts of Cases That Have Shaped Medical Ethics, With Philosophical, Legal, and Historical Backgrounds, 3rd edition. Boston: McGraw-Hill; 2000:29–55.
- In the matter of Karen Quinlan, an alleged incompetent. In re Quinlan. 70 N.J. 10, 355 A.2d 647 (1976), cert. denied, 429 U.S. 922 (1976).
- Wolf SM. Hastings Center. Guidelines on the Termination of Life-Sustaining Treatment and Care of the Dying: A Report by the Hastings Center. The Hastings Center: Briarcliff Manor, NY; 1987.
- Baibars MM, Alraies MC, Kabach A, Pritzker M. Can patients opt to turn off implantable cardioverter-defibrillators near the end of life? Cleve Clin J Med 2016; 83:97–98.
- National Academy of Sciences. Dying in America: improving quality and honoring individual p near the end of life. www.iom.edu/Reports/2014/Dying-In-America-Improving-Quality-and-Honoring-Individual-P-Near-the-End-of-Life.aspx. Accessed January 4, 2016.
In 1975, Julia and Joseph Quinlan approached the administrator of St. Clare’s Hospital in Denville, New Jersey, and requested that the mechanical ventilator on which their adopted daughter, Karen, was dependent be turned off. Karen Ann Quinlan, 21 years old, was in a permanent vegetative state after a severe anoxic event, and her parents had been informed by the hospital’s medical staff that she would never regain consciousness.
To the Quinlans’ request to withdraw the ventilator, the hospital administrator replied, “You have to understand our position, Mrs. Quinlan. In this hospital we don’t kill people.”1
The administrator’s response was consistent with prevailing ethical and legal perspectives, analyses, and directives at that time related to discontinuation of life-sustaining treatment. In the mid-1970s, the American Medical Association’s position was that it was permissible to not put a patient on a ventilator (ie, a physician could withhold a life-sustaining treatment), but once a patient was on a ventilator, it was not permissible to take the patient off if the intention was to allow death to occur.1 However, the New Jersey Supreme Court ultimately found this distinction between withholding and withdrawing unconvincing, and ruled unanimously that Karen Quinlan’s ventilator could be turned off.2
THE HASTINGS CENTER REPORT: STOPPING IS THE SAME AS NOT STARTING
During the subsequent decade, further ethical analysis and additional legal cases resulted in new insights and more nuanced thinking about forgoing life-sustaining treatment.
These developments were summarized in a 1987 report by the Hastings Center,3 a leading bioethics research and policy institute. The report provided normative guidance for the termination of life-sustaining treatment and for the care of dying patients. It acknowledged that deciding not to start a life-sustaining treatment can emotionally and psychologically affect healthcare professionals differently than deciding to stop such a treatment. However, the report also asserted that there is no morally important difference between withholding and withdrawing such treatments.
Reflecting a partnership model between patients and professionals for healthcare decision-making, and affirming the ethical significance of both a burden-benefit analysis and patient autonomy, the report stated that when a patient or surrogate in collaboration with a responsible healthcare professional decides that a treatment under way and the life it supports have become more burdensome than beneficial to the patient, that is sufficient reason to stop. There is no ethical requirement that treatment, once initiated, must continue against the patient’s wishes or when the surrogate determines that it is more burdensome than beneficial from the patient’s perspective. In fact, imposing treatment in such circumstances violates the patient’s right to self-determination.3
The report noted further that, because of frequent uncertainty about the efficacy of proposed treatments, it is preferable to initiate time-limited trials of treatments and then later stop them if they prove ineffective or become overly burdensome from a patient’s perspective.
ICDs ARE LIKE OTHER LIFE-SUSTAINING THERAPIES
In this issue of Cleveland Clinic Journal of Medicine, Baibars et al4 address the question of how implantable cardioverter-defibrillators (ICDs) should be managed at the end of life. The historical events and developments recounted above regarding withdrawing life-sustaining technologies are an appropriate context for ethically assessing the management of ICDs for dying patients.
Obviously, ICDs are not ventilators, but like ventilators, they are life-sustaining therapy, as are dialysis machines, blood transfusions, medically supplied nutrition and hydration, ventricular assist devices, and other implantable electronic cardiac devices such as pacemakers. Each of these life-sustaining therapies, depending on a patient’s clinical condition, underlying illness, and comorbidities, can become a death-prolonging technology.
An ethical framework and analysis about whether to continue any life-sustaining therapy, including an ICD, must include an assessment of the benefit-to-burden ratio from the patient’s perspective. Does the therapy enhance or maintain a quality of life acceptable to the patient? Or has it become overly burdensome and does it maintain a quality of life the patient finds (or would find) unacceptable? If the latter is true, and especially in the context of an underlying terminal condition, then shifting the goals of care to focus on comfort is always appropriate and ethically justified. Treatments—including ICDs—that do not contribute to patient comfort should be withdrawn.
TOWARD COMPETENCY IN ETHICAL MANAGEMENT
Baibars et al note that much more needs to be done to enhance competencies, increase proficiencies, and mitigate the moral distress of healthcare professionals caring for dying patients with ICDs and other devices. To help clinicians achieve a personal and professional “comfort zone” for ethically managing patients with ICDs, we recommend that healthcare institutions, medical schools, and nursing schools take the following steps:
Develop comprehensive end-of-life policies, procedures, and protocols that incorporate specific guidance for managing cardiac devices and that have been endorsed by a hospital ethics committee. Such guidance can be informative and educational and can ensure that decisions and resulting actions (including stopping cardiac devices) are ethically supportable.
Provide more palliative care training in medical and nursing schools, residency programs, and continuing education activities so that front-line clinicians can deliver “basic,” “primary” palliative care not requiring specialty palliative medicine. This training, called for in the Institute of Medicine’s 2014 report, Dying in America,5 should include explicit ethics discussions about managing cardiac devices at the end of life.
Provide ongoing training in communication skills needed for all patient-professional encounters. Effectively engaging patients in goals-of-care discussions, especially patients with life-limiting illnesses such as heart failure, cannot be achieved without these skills.
In 1975, Julia and Joseph Quinlan approached the administrator of St. Clare’s Hospital in Denville, New Jersey, and requested that the mechanical ventilator on which their adopted daughter, Karen, was dependent be turned off. Karen Ann Quinlan, 21 years old, was in a permanent vegetative state after a severe anoxic event, and her parents had been informed by the hospital’s medical staff that she would never regain consciousness.
To the Quinlans’ request to withdraw the ventilator, the hospital administrator replied, “You have to understand our position, Mrs. Quinlan. In this hospital we don’t kill people.”1
The administrator’s response was consistent with prevailing ethical and legal perspectives, analyses, and directives at that time related to discontinuation of life-sustaining treatment. In the mid-1970s, the American Medical Association’s position was that it was permissible to not put a patient on a ventilator (ie, a physician could withhold a life-sustaining treatment), but once a patient was on a ventilator, it was not permissible to take the patient off if the intention was to allow death to occur.1 However, the New Jersey Supreme Court ultimately found this distinction between withholding and withdrawing unconvincing, and ruled unanimously that Karen Quinlan’s ventilator could be turned off.2
THE HASTINGS CENTER REPORT: STOPPING IS THE SAME AS NOT STARTING
During the subsequent decade, further ethical analysis and additional legal cases resulted in new insights and more nuanced thinking about forgoing life-sustaining treatment.
These developments were summarized in a 1987 report by the Hastings Center,3 a leading bioethics research and policy institute. The report provided normative guidance for the termination of life-sustaining treatment and for the care of dying patients. It acknowledged that deciding not to start a life-sustaining treatment can emotionally and psychologically affect healthcare professionals differently than deciding to stop such a treatment. However, the report also asserted that there is no morally important difference between withholding and withdrawing such treatments.
Reflecting a partnership model between patients and professionals for healthcare decision-making, and affirming the ethical significance of both a burden-benefit analysis and patient autonomy, the report stated that when a patient or surrogate in collaboration with a responsible healthcare professional decides that a treatment under way and the life it supports have become more burdensome than beneficial to the patient, that is sufficient reason to stop. There is no ethical requirement that treatment, once initiated, must continue against the patient’s wishes or when the surrogate determines that it is more burdensome than beneficial from the patient’s perspective. In fact, imposing treatment in such circumstances violates the patient’s right to self-determination.3
The report noted further that, because of frequent uncertainty about the efficacy of proposed treatments, it is preferable to initiate time-limited trials of treatments and then later stop them if they prove ineffective or become overly burdensome from a patient’s perspective.
ICDs ARE LIKE OTHER LIFE-SUSTAINING THERAPIES
In this issue of Cleveland Clinic Journal of Medicine, Baibars et al4 address the question of how implantable cardioverter-defibrillators (ICDs) should be managed at the end of life. The historical events and developments recounted above regarding withdrawing life-sustaining technologies are an appropriate context for ethically assessing the management of ICDs for dying patients.
Obviously, ICDs are not ventilators, but like ventilators, they are life-sustaining therapy, as are dialysis machines, blood transfusions, medically supplied nutrition and hydration, ventricular assist devices, and other implantable electronic cardiac devices such as pacemakers. Each of these life-sustaining therapies, depending on a patient’s clinical condition, underlying illness, and comorbidities, can become a death-prolonging technology.
An ethical framework and analysis about whether to continue any life-sustaining therapy, including an ICD, must include an assessment of the benefit-to-burden ratio from the patient’s perspective. Does the therapy enhance or maintain a quality of life acceptable to the patient? Or has it become overly burdensome and does it maintain a quality of life the patient finds (or would find) unacceptable? If the latter is true, and especially in the context of an underlying terminal condition, then shifting the goals of care to focus on comfort is always appropriate and ethically justified. Treatments—including ICDs—that do not contribute to patient comfort should be withdrawn.
TOWARD COMPETENCY IN ETHICAL MANAGEMENT
Baibars et al note that much more needs to be done to enhance competencies, increase proficiencies, and mitigate the moral distress of healthcare professionals caring for dying patients with ICDs and other devices. To help clinicians achieve a personal and professional “comfort zone” for ethically managing patients with ICDs, we recommend that healthcare institutions, medical schools, and nursing schools take the following steps:
Develop comprehensive end-of-life policies, procedures, and protocols that incorporate specific guidance for managing cardiac devices and that have been endorsed by a hospital ethics committee. Such guidance can be informative and educational and can ensure that decisions and resulting actions (including stopping cardiac devices) are ethically supportable.
Provide more palliative care training in medical and nursing schools, residency programs, and continuing education activities so that front-line clinicians can deliver “basic,” “primary” palliative care not requiring specialty palliative medicine. This training, called for in the Institute of Medicine’s 2014 report, Dying in America,5 should include explicit ethics discussions about managing cardiac devices at the end of life.
Provide ongoing training in communication skills needed for all patient-professional encounters. Effectively engaging patients in goals-of-care discussions, especially patients with life-limiting illnesses such as heart failure, cannot be achieved without these skills.
- Pence G. Comas: Karen Quinlan and Nancy Cruzan. In: Classic Cases in Medical Ethics: Accounts of Cases That Have Shaped Medical Ethics, With Philosophical, Legal, and Historical Backgrounds, 3rd edition. Boston: McGraw-Hill; 2000:29–55.
- In the matter of Karen Quinlan, an alleged incompetent. In re Quinlan. 70 N.J. 10, 355 A.2d 647 (1976), cert. denied, 429 U.S. 922 (1976).
- Wolf SM. Hastings Center. Guidelines on the Termination of Life-Sustaining Treatment and Care of the Dying: A Report by the Hastings Center. The Hastings Center: Briarcliff Manor, NY; 1987.
- Baibars MM, Alraies MC, Kabach A, Pritzker M. Can patients opt to turn off implantable cardioverter-defibrillators near the end of life? Cleve Clin J Med 2016; 83:97–98.
- National Academy of Sciences. Dying in America: improving quality and honoring individual p near the end of life. www.iom.edu/Reports/2014/Dying-In-America-Improving-Quality-and-Honoring-Individual-P-Near-the-End-of-Life.aspx. Accessed January 4, 2016.
- Pence G. Comas: Karen Quinlan and Nancy Cruzan. In: Classic Cases in Medical Ethics: Accounts of Cases That Have Shaped Medical Ethics, With Philosophical, Legal, and Historical Backgrounds, 3rd edition. Boston: McGraw-Hill; 2000:29–55.
- In the matter of Karen Quinlan, an alleged incompetent. In re Quinlan. 70 N.J. 10, 355 A.2d 647 (1976), cert. denied, 429 U.S. 922 (1976).
- Wolf SM. Hastings Center. Guidelines on the Termination of Life-Sustaining Treatment and Care of the Dying: A Report by the Hastings Center. The Hastings Center: Briarcliff Manor, NY; 1987.
- Baibars MM, Alraies MC, Kabach A, Pritzker M. Can patients opt to turn off implantable cardioverter-defibrillators near the end of life? Cleve Clin J Med 2016; 83:97–98.
- National Academy of Sciences. Dying in America: improving quality and honoring individual p near the end of life. www.iom.edu/Reports/2014/Dying-In-America-Improving-Quality-and-Honoring-Individual-P-Near-the-End-of-Life.aspx. Accessed January 4, 2016.
Veterans, guilt, and suicide risk: An opportunity to collaborate with chaplains?
Suicidal behavior is a major cause of morbidity and mortality in the United States,1 and active-duty and reserve military personnel and veterans account for nearly 18% of suicide deaths.2 By one estimate, as many as 22 veterans die by suicide each day.3 These rates are thought to be due to a higher incidence of mental illness in certain veteran populations relative to the general population.4–8 Consequently, a number of mental health services are available to veterans in a variety of clinical and community settings.
Chaplains and clinicians bring complementary skills and services to the problem of suicide risk among veterans. In particular, helping at-risk veterans deal with experiences of guilt is an opportunity for interdisciplinary collaboration. Available literature supports the potential utility of chaplaincy services in supporting at-risk veteran populations.9–15
But while most healthcare facilities have chaplains on staff, there is little information to guide any such collaboration. Further, healthcare providers appear to have a limited understanding of chaplaincy services, the “language” within which chaplains operate, or the roles chaplains play in healthcare settings.16
In the following discussion, using the example of experiences of guilt, we offer our insights and suggestions on how chaplaincy services may prove useful in alleviating this complex emotion in veterans at risk of suicide.
BENEFITS OF TALKING TO A CHAPLAIN
Collaboration between healthcare providers and pastoral care professionals has been suggested as a means of enhancing the treatment of patients with mental illness.17,18 Chaplains draw from a variety of faith traditions and are usually trained to respond to the needs of people from a variety of religious and spiritual backgrounds. They provide some non-faithbased services (eg, crisis intervention, life review, bereavement counseling) resembling those also provided in formal mental healthcare settings.19 By facilitating religious and spiritual coping and religious practice and responding to religious and spiritual needs, chaplains also offer a level of support not typically offered by formal mental healthcare providers.20
Veterans at risk of suicide sometimes look to pastoral care providers, particularly chaplains, for mental health support.9,10 Research on the effects of chaplaincy services on suicidal behavior is just beginning to emerge.15 Still, the US Department of Health and Human Services has recognized pastoral care services as having a “beneficial and therapeutic effect on the medical condition of a patient.”11
For example, in one study, hospital inpatients reported higher satisfaction if they had been visited by a chaplain.12 Chaplains help align treatment plans with patient values and wishes.13 In another study,14 patients undergoing coronary artery bypass grafting who were randomized to receive five visits from a chaplain were found to have a higher rate of positive religious coping (eg, forgiveness, letting go of anger). Positive religious coping has been correlated with lower levels of psychological stress and better mental health outcomes.
EXPERIENCING GUILT IS LINKED TO RISK OF SUICIDE
Suicidal behavior is complex, multifaceted, and linked to genetic, neurologic, psychological, social, and cultural factors.21
Assessing for and addressing certain complex emotions, such as guilt and shame, is an important part of suicide prevention efforts. Guilt is defined as a “controllable psychological state that is typically linked to a specific action or behavior, and which entails regret or remorse.”22
Guilt has been linked to risk of suicide in veterans.23–25 In one study, close to 75% of veterans who had thought about suicide said they frequently experienced guilt about having violated the precepts of their faith group, family, God, life, or the military.26
Such findings suggest that the sense of guilt experienced by some at-risk veterans may be grounded in a variety of contexts. For example, faith communities that place a strong emphasis on obedience to moral, ethical, and religious precepts may contribute to the experience of guilt unless balanced by a message of grace or favor from a benevolent God or deity. Without this balance, engaging in activities that are not fully sanctioned by one’s faith community may lead to guilt.
Families might also contribute to veterans’ experiences of guilt by placing unrealistic expectations on them. And the family environment may not be conducive to resolving feelings of guilt in veterans, harboring resentment and antipathies and making it very difficult to alleviate any ensuing sense of distress.
CLINICIAN’S ROLE IN ASSESSING GUILT
In addressing and assessing guilt in veterans at risk of suicide, clinicians should try to recognize the source and clinical implications of this emotion.
Recognize the source of guilt
Guilt may indicate a clinical disorder such as a mood disorder (eg, major depression).27 Mood disorders significantly increase the risk of suicidal behavior.28,29
Beyond diagnosing a clinical disorder, prescribing pharmacotherapy, and referring for mental healthcare services, recognizing the source of this emotion remains an important part of addressing a patient’s experience of guilt. Especially when associated with a clinical disorder, guilt is often irrational and excessive and does not appropriately reflect the experience or situation in question.
Case conceptualization, defined as “synthesizing the patient’s experience with relevant clinical theory and research,”30 can be used to understand the context in which the guilt-inducing action or behavior occurred and the veteran’s own interpretation of his or her actions. Understanding the source of the patient’s guilt could be used to plan treatment and resolve any underlying sense of distress.
As with other negative emotions, the affective component of guilt is often the result of cognitive distortions made as the person tries to make sense of what has occurred or to reconcile beliefs of right and wrong with the guilt-inducing behavior.31 The common cognitive errors associated with guilt include:
- Hindsight bias (a belief that one should have known what was going to happen as a result of one’s actions)
- Responsibility distortion (a belief that one’s actions directly caused an adverse event)
- Justification distortion (a belief that one’s actions were not justified by the situation)
- Wrongdoing distortion (a belief that one violated one’s own standards of right and wrong).31
Cognitive therapy to counter cognitive distortions
A variety of clinical options exist to help veterans manage and resolve guilt.
Cognitive therapy can counter the cognitive distortions that drive feelings of guilt. The goal is to guide patients to examine the evidence, process the event, and realize that their behavior was appropriate for the given situation. Cognitive processing therapy and prolonged exposure therapy have both been shown to decrease trauma-related guilt, though cognitive processing therapy was found to be better at decreasing guilt that arose from cognitive distortions.32
Guilt and suicide ideation have also been associated with a belief that one’s actions constituted an unforgivable sin.33 Responding to these inherently religious-spiritual cognitive distortions may be beyond the scope of expertise for many healthcare professionals. In such cases, it may be prudent to consider complementing clinical services with pastoral care. It follows that pastoral care services should only be provided if the veteran voices a desire and readiness for them. The clinician and chaplain can then work together to provide coordinated care to best meet the patient’s needs, to address the experience of guilt, and to alleviate the sense of distress.
A CHAPLAIN’S PERSPECTIVE ON GUILT
A prominent feature of pastoral practice is helping people, including at-risk veterans, resolve feelings of guilt regardless of the context on which the emotion is founded (eg, religion, shame).10 For many people, guilt is an impenetrable barrier, preventing resolution of whatever experience led to a sense of inner turmoil.
Forgiveness
In the context of pastoral care, resolution of guilt is ordinarily tied to a need for forgiveness. There are multiple ways in which forgiveness can be grounded in religious and spiritual contexts.34 Examples include forgiving others (ie, forswearing resentment, anger, or hatred directed toward another person), being forgiven by God or another benevolent deity, and forgiving oneself for violating perceived personal transgressions.35 In some cases, divine forgiveness may be conditional on interpersonal forgiveness.36 Forgiveness is also sometimes seen as a remedy for sin and a way to restore moral order.37
Some people may initially think they can never be forgiven. With time and the weight of one’s experiences, the impossibility of forgiveness can become so ingrained that it becomes a core belief. These core beliefs set up a vicious circle of thoughts and feelings, in which people and places and events from the past are continuously brought forward into the present. Anger and resentment become the steady diet for the tormented self that feels forever powerless over experienced injustices. These relived experiences drive the person into a deep isolation where the self becomes less human—a thing, an object. This experience of losing oneself proves excruciating and often leads to contemplation of suicide as a way to resolve anguish.
Hope emerges
Pastoral care services provide a means to reframe one’s core beliefs, manage and resolve the burden of guilt, and uncover new motivation for living.
The practice of spiritual direction within the discipline of pastoral care listens for these inner movements and encourages the person to give voice to them in his or her own words. No longer limited by a diminished, tormented self, the real self begins to relate to another reality that changes his or her identity, relieves the burden of guilt, and gives reason, purpose, and meaning to life.
Even with this opportunity for a new life, however, cognitive distortions based on a disproportional “faith-based prism” may persist. In this case, clinicians and chaplains must work closely together to reframe old understandings of self and incorrect understandings of religion and spirituality into one that continues to reinforce this newfound sense of hope.38
A VETERAN OF IRAQ WITH SUICIDE IDEATION
The following case illustrates how clinicians and chaplains may be able to work together to help facilitate the resolution of guilt.
A veteran who had served in Iraq had entered the Domiciliary Care Program at a US Department of Veterans Affairs medical center. He reported experiencing problems with guilt, forgiveness, and suicide ideation. A clinical therapeutic program was prescribed after a psychological evaluation uncovered that he was also struggling with depression and posttraumatic stress disorder.
His mental healthcare providers recognized the importance of incorporating a religious-spiritual component into the therapeutic plan, and so consulted with a chaplain to plan a suitable course of action. Specifically, this veteran reported feeling that he could not be forgiven for his military experiences, a feeling that was giving way to alienation and isolation from the God of his faith tradition.
The chaplain helped this veteran reflect on his military experiences, giving him the perspective he needed to view his God as one who truly loves him. He recognized instances in which he could have lost his life had it not been for others who intervened on his behalf at just the right time. This awareness caused him to think about his life differently, challenging him to reframe his relationship with God. Instead of simple coincidences, the veteran began to consider the mystery behind these times and places.
Over time and in keeping with the tenets of his faith tradition, the veteran stated that he was ultimately able to accept and receive God’s love and forgiveness. He now reports that these inner spiritual movements serve as a source of support during occasional relapses into emotional distress. These movements allow him to consider the mystery of his present life and its value based on his experience of his God’s love and forgiveness.
CARE FOR SUICIDE SURVIVORS
The experience of guilt is not limited to veterans. Those bereaved by suicide are also left to manage their own experiences of the loss and ensuing complex emotions. Friends and loved ones who survive a suicide decedent may experience guilt, feeling that they somehow contributed to or failed to prevent the suicide. Such feelings of guilt are hypothesized to lower the threshold for suicidal behavior in those bereaved.39
Guilt and shame are also frequently encountered in survivors of nonfatal suicide attempts.40 Chaplaincy services might also prove useful for these individuals.
TIME IS EVERYTHING
Patients who may have an active psychopathology should have their clinical therapeutic needs attended to first. If the clinician deems pastoral care services to be an appropriate complementary support option, care should be taken to select a pastoral care provider who is adequately prepared for this role. Different professional organizations (eg, Association of Professional Chaplains) have established board-certification procedures, minimum education requirements, and supervised practical experience required for chaplaincy certification.
Also, spiritual growth and development remain a core focus of pastoral practice. Clinicians should discontinue any collaboration with pastoral care providers who question an individual’s faith or commitment to his or her faith, or who promote thinking or actions that could be deleterious to the patient’s therapeutic trajectory.
SUMMING UP
We have here presented our perspectives on how chaplaincy services can be used to complement clinical services in support of at-risk veterans struggling with experiences of guilt. Unfortunately, the current level of collaboration between chaplains and clinicians in support of at-risk veteran populations is limited.20 Our hope is that clinicians managing these at-risk patients will develop a greater awareness of how chaplaincy services might be able to help in alleviating experiences of guilt in at-risk veteran populations. A further hope is that such cases will serve as an opportunity for greater interdisciplinary collaboration, benefiting at-risk veterans most in need of support.
Acknowledgment: Dr. Rasmussen was supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, US Department of Veterans Affairs, VISN 2 Center of Excellence for Suicide Prevention.
- Centers for Disease Control and Prevention (CDC). Suicide and self-inflicted injury. www.cdc.gov/nchs/fastats/suicide.htm. Accessed November 12, 2015.
- Centers for Disease Control and Prevention (CDC). National violent death reporting system (NVDRS). https://wisqars.cdc.gov:8443/nvdrs/nvdrsDisplay.jsp. Accessed November 12, 2015.
- Kemp JE, Bossarte R. Suicide data report, 2012. www.sprc.org/library_resources/items/suicide-data-report-2012. Accessed November 12, 2015.
- Bullman TA, Kang HK. The risk of suicide among wounded Vietnam veterans. Am J Public Health 1996; 86:662–667.
- Kang HK, Bullman TA. Is there an epidemic of suicides among current and former US military personnel? Ann Epidemiol 2009; 19:757–760.
- LeardMann CA, Powell TM, Smith TC, et al. Risk factors associated with suicide in current and former US military personnel. JAMA 2013; 310:496–506.
- Mrnak-Meyer J, Tate SR, Tripp JC, Worley MJ, Jajodia A, McQuaid JR. Predictors of suicide-related hospitalization among US veterans receiving treatment for comorbid depression and substance dependence: who is the riskiest of the risky? Suicide Life Threat Behav 2011; 41:532–542.
- Pietrzak RH, Russo AR, Ling Q, Southwick SM. Suicidal ideation in treatment-seeking veterans of Operations Enduring Freedom and Iraqi Freedom: the role of coping strategies, resilience, and social support. J Psychiatr Res 2011; 45:720–726.
- Kopacz MS, McCarten JM, Pollitt MJ. VHA chaplaincy contact with veterans at increased risk of suicide. South Med J 2014; 107: 661–664.
- Kopacz MS. Providing pastoral care services in a clinical setting to veterans at-risk of suicide. J Relig Health 2013; 52:759–767.
- Medicare program; payment for nursing and allied health education. Health Care Financing Administration (HCFA), HHS. Final rule. Fed Regist 2001; 66:3358–3376.
- Marin DB, Sharma V, Sosunov E, Egorova N, Goldstein R, Handzo GF. Relationship between chaplain visits and patient satisfaction. J Health Care Chaplain 2015; 21:14–24.
- Flannelly KJ, Emanuel LL, Handzo GF, Galek K, Silton NR, Carlson M. A national study of chaplaincy services and end-of-life outcomes. BMC Palliat Care 2012; 11:10.
- Bay PS, Beckman D, Trippi J, Gunderman R, Terry C. The effect of pastoral care services on anxiety, depression, hope, religious coping, and religious problem solving styles: a randomized controlled study. J Relig Health 2008; 47:57–69.
- Kopacz MS, Nieuwsma JA, Jackson GL, et al. Chaplains’ engagement with suicidality among their service users: findings from the VA/DoD Integrated Mental Health Strategy. Suicide Life Threat Behav 2015. [Epub ahead of print.]
- Flannelly KJ, Galek K, Bucchino J, Handzo GF, Tannenbaum HP. Department directors’ perceptions of the roles and functions of hospital chaplains: a national survey. Hosp Top 2005; 83:19–27.
- Farrell JL, Goebert DA. Collaboration between psychiatrists and clergy in recognizing and treating serious mental illness. Psychiatr Serv 2008; 59:437–440.
- Weaver AJ, Flannelly KJ, Flannelly LT, Oppenheimer JE. Collaboration between clergy and mental health professionals: a review of professional health care journals from 1980 through 1999. Counsel Val 2003; 47:162–171.
- Handzo GF, Flannelly KJ, Kudler T, et al. What do chaplains really do? II. Interventions in the New York chaplaincy study. J Health Care Chaplain 2008; 14:39–56.
- Kopacz MS, Pollitt MJ. Delivering chaplaincy services to veterans at increased risk of suicide. J Health Care Chaplain 2015; 21:1–13.
- Knox KL, Bossarte RM. Suicide prevention for veterans and active duty personnel. Am J Public Health 2012;102(suppl 1):S8–S9.
- Bryan CJ, Morrow CE, Etienne N, Ray-Sannerud B. Guilt, shame, and suicidal ideation in a military outpatient clinical sample. Depress Anxiety 2013; 30:55–60.
- Ganz D, Sher L. Educating medical professionals about suicide prevention among military veterans. Int J Adolesc Med Health 2013; 25:187–191.
- Hendin H, Haas AP. Suicide and guilt as manifestations of PTSD in Vietnam combat veterans. Am J Psychiatry 1991; 148:586–591.
- Maguen S, Metzler TJ, Bosch J, Marmar CR, Knight SJ, Neylan TC. Killing in combat may be independently associated with suicidal ideation. Depress Anxiety 2012; 29:918–923.
- Kopacz MS, McCarten JM, Vance CG, Connery AL. A preliminary study for exploring different sources of guilt in a sample of veterans who sought chaplaincy services. Mil Psychol 2015; 27:1–8.
- Buck CJ. 2013 ICD-9-CM for physicians. St. Louis, MO: Saunders; 2013.
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34-38 years. J Affect Disord 2002; 68:167–181.
- Nierenberg AA, Gray SM, Grandin LD. Mood disorders and suicide. J Clin Psychiatry 2001; 62(suppl 25):27–30.
- Macneil CA, Hasty MK, Conus P, Berk M. Is diagnosis enough to guide interventions in mental health? Using case formulation in clinical practice. BMC Med 2012; 10:111.
- Kubany ES, Manke FP. Cognitive therapy for trauma-related guilt: conceptual bases and treatment outlines. Cogn Behav Pract 1995; 2:27–61.
- Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. Comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. J Consult Clin Psychol 2002; 70:867–879.
- Exline JJ, Yali AM, Sanderson WC. Guilt, discord, and alienation: the role of religious strain in depression and suicidality. J Clin Psychol 2000; 56:1481–1496.
- Musick MA. Multiple forms of forgiveness and their relationship with aging and religion, In: Schaie KW, Krause N, Booth A, editors. Religious Influences on Health and Well-being in the Elderly. New York, NY: Springer Publishing Company; 2004:202–214.
- Kaplan BH, Munroe-Blum H, Blazer DG. Religion, health and forgiveness: tradition and challenges. In: Levin JS, editor. Religion in Aging and Health. Theoretical Foundations and Methodological Frontiers. Thousand Oaks, CA: SAGE Focus Edition; 1994:52–77.
- Worthington EL Jr, Berry JW, Parrott L III. Unforgiveness, forgiveness, religion and health. In: Plante TG, Sherman AC, editors. Faith and Health. Psychological Perspectives. New York, NY: Guilford Press; 2001:107–138.
- Enright RD, Gassin EA, Wu GR. Forgiveness: a developmental view. J Moral Educ 1992; 21:99–114.
- Kopacz MS, O’Reilly LM, Van Inwagen CC, et al. Understanding the role of chaplains in veteran suicide prevention efforts: a discussion paper. SAGE Open 2014; 4:1–10.
- Young IT, Iglewicz A, Glorioso D, et al. Suicide bereavement and complicated grief. Dialogues Clin Neurosci 2012; 14:177–186.
- Wiklander M, Samuelsson M, Asberg M. Shame reactions after suicide attempt. Scand J Caring Sci 2003; 17:293–300.
Suicidal behavior is a major cause of morbidity and mortality in the United States,1 and active-duty and reserve military personnel and veterans account for nearly 18% of suicide deaths.2 By one estimate, as many as 22 veterans die by suicide each day.3 These rates are thought to be due to a higher incidence of mental illness in certain veteran populations relative to the general population.4–8 Consequently, a number of mental health services are available to veterans in a variety of clinical and community settings.
Chaplains and clinicians bring complementary skills and services to the problem of suicide risk among veterans. In particular, helping at-risk veterans deal with experiences of guilt is an opportunity for interdisciplinary collaboration. Available literature supports the potential utility of chaplaincy services in supporting at-risk veteran populations.9–15
But while most healthcare facilities have chaplains on staff, there is little information to guide any such collaboration. Further, healthcare providers appear to have a limited understanding of chaplaincy services, the “language” within which chaplains operate, or the roles chaplains play in healthcare settings.16
In the following discussion, using the example of experiences of guilt, we offer our insights and suggestions on how chaplaincy services may prove useful in alleviating this complex emotion in veterans at risk of suicide.
BENEFITS OF TALKING TO A CHAPLAIN
Collaboration between healthcare providers and pastoral care professionals has been suggested as a means of enhancing the treatment of patients with mental illness.17,18 Chaplains draw from a variety of faith traditions and are usually trained to respond to the needs of people from a variety of religious and spiritual backgrounds. They provide some non-faithbased services (eg, crisis intervention, life review, bereavement counseling) resembling those also provided in formal mental healthcare settings.19 By facilitating religious and spiritual coping and religious practice and responding to religious and spiritual needs, chaplains also offer a level of support not typically offered by formal mental healthcare providers.20
Veterans at risk of suicide sometimes look to pastoral care providers, particularly chaplains, for mental health support.9,10 Research on the effects of chaplaincy services on suicidal behavior is just beginning to emerge.15 Still, the US Department of Health and Human Services has recognized pastoral care services as having a “beneficial and therapeutic effect on the medical condition of a patient.”11
For example, in one study, hospital inpatients reported higher satisfaction if they had been visited by a chaplain.12 Chaplains help align treatment plans with patient values and wishes.13 In another study,14 patients undergoing coronary artery bypass grafting who were randomized to receive five visits from a chaplain were found to have a higher rate of positive religious coping (eg, forgiveness, letting go of anger). Positive religious coping has been correlated with lower levels of psychological stress and better mental health outcomes.
EXPERIENCING GUILT IS LINKED TO RISK OF SUICIDE
Suicidal behavior is complex, multifaceted, and linked to genetic, neurologic, psychological, social, and cultural factors.21
Assessing for and addressing certain complex emotions, such as guilt and shame, is an important part of suicide prevention efforts. Guilt is defined as a “controllable psychological state that is typically linked to a specific action or behavior, and which entails regret or remorse.”22
Guilt has been linked to risk of suicide in veterans.23–25 In one study, close to 75% of veterans who had thought about suicide said they frequently experienced guilt about having violated the precepts of their faith group, family, God, life, or the military.26
Such findings suggest that the sense of guilt experienced by some at-risk veterans may be grounded in a variety of contexts. For example, faith communities that place a strong emphasis on obedience to moral, ethical, and religious precepts may contribute to the experience of guilt unless balanced by a message of grace or favor from a benevolent God or deity. Without this balance, engaging in activities that are not fully sanctioned by one’s faith community may lead to guilt.
Families might also contribute to veterans’ experiences of guilt by placing unrealistic expectations on them. And the family environment may not be conducive to resolving feelings of guilt in veterans, harboring resentment and antipathies and making it very difficult to alleviate any ensuing sense of distress.
CLINICIAN’S ROLE IN ASSESSING GUILT
In addressing and assessing guilt in veterans at risk of suicide, clinicians should try to recognize the source and clinical implications of this emotion.
Recognize the source of guilt
Guilt may indicate a clinical disorder such as a mood disorder (eg, major depression).27 Mood disorders significantly increase the risk of suicidal behavior.28,29
Beyond diagnosing a clinical disorder, prescribing pharmacotherapy, and referring for mental healthcare services, recognizing the source of this emotion remains an important part of addressing a patient’s experience of guilt. Especially when associated with a clinical disorder, guilt is often irrational and excessive and does not appropriately reflect the experience or situation in question.
Case conceptualization, defined as “synthesizing the patient’s experience with relevant clinical theory and research,”30 can be used to understand the context in which the guilt-inducing action or behavior occurred and the veteran’s own interpretation of his or her actions. Understanding the source of the patient’s guilt could be used to plan treatment and resolve any underlying sense of distress.
As with other negative emotions, the affective component of guilt is often the result of cognitive distortions made as the person tries to make sense of what has occurred or to reconcile beliefs of right and wrong with the guilt-inducing behavior.31 The common cognitive errors associated with guilt include:
- Hindsight bias (a belief that one should have known what was going to happen as a result of one’s actions)
- Responsibility distortion (a belief that one’s actions directly caused an adverse event)
- Justification distortion (a belief that one’s actions were not justified by the situation)
- Wrongdoing distortion (a belief that one violated one’s own standards of right and wrong).31
Cognitive therapy to counter cognitive distortions
A variety of clinical options exist to help veterans manage and resolve guilt.
Cognitive therapy can counter the cognitive distortions that drive feelings of guilt. The goal is to guide patients to examine the evidence, process the event, and realize that their behavior was appropriate for the given situation. Cognitive processing therapy and prolonged exposure therapy have both been shown to decrease trauma-related guilt, though cognitive processing therapy was found to be better at decreasing guilt that arose from cognitive distortions.32
Guilt and suicide ideation have also been associated with a belief that one’s actions constituted an unforgivable sin.33 Responding to these inherently religious-spiritual cognitive distortions may be beyond the scope of expertise for many healthcare professionals. In such cases, it may be prudent to consider complementing clinical services with pastoral care. It follows that pastoral care services should only be provided if the veteran voices a desire and readiness for them. The clinician and chaplain can then work together to provide coordinated care to best meet the patient’s needs, to address the experience of guilt, and to alleviate the sense of distress.
A CHAPLAIN’S PERSPECTIVE ON GUILT
A prominent feature of pastoral practice is helping people, including at-risk veterans, resolve feelings of guilt regardless of the context on which the emotion is founded (eg, religion, shame).10 For many people, guilt is an impenetrable barrier, preventing resolution of whatever experience led to a sense of inner turmoil.
Forgiveness
In the context of pastoral care, resolution of guilt is ordinarily tied to a need for forgiveness. There are multiple ways in which forgiveness can be grounded in religious and spiritual contexts.34 Examples include forgiving others (ie, forswearing resentment, anger, or hatred directed toward another person), being forgiven by God or another benevolent deity, and forgiving oneself for violating perceived personal transgressions.35 In some cases, divine forgiveness may be conditional on interpersonal forgiveness.36 Forgiveness is also sometimes seen as a remedy for sin and a way to restore moral order.37
Some people may initially think they can never be forgiven. With time and the weight of one’s experiences, the impossibility of forgiveness can become so ingrained that it becomes a core belief. These core beliefs set up a vicious circle of thoughts and feelings, in which people and places and events from the past are continuously brought forward into the present. Anger and resentment become the steady diet for the tormented self that feels forever powerless over experienced injustices. These relived experiences drive the person into a deep isolation where the self becomes less human—a thing, an object. This experience of losing oneself proves excruciating and often leads to contemplation of suicide as a way to resolve anguish.
Hope emerges
Pastoral care services provide a means to reframe one’s core beliefs, manage and resolve the burden of guilt, and uncover new motivation for living.
The practice of spiritual direction within the discipline of pastoral care listens for these inner movements and encourages the person to give voice to them in his or her own words. No longer limited by a diminished, tormented self, the real self begins to relate to another reality that changes his or her identity, relieves the burden of guilt, and gives reason, purpose, and meaning to life.
Even with this opportunity for a new life, however, cognitive distortions based on a disproportional “faith-based prism” may persist. In this case, clinicians and chaplains must work closely together to reframe old understandings of self and incorrect understandings of religion and spirituality into one that continues to reinforce this newfound sense of hope.38
A VETERAN OF IRAQ WITH SUICIDE IDEATION
The following case illustrates how clinicians and chaplains may be able to work together to help facilitate the resolution of guilt.
A veteran who had served in Iraq had entered the Domiciliary Care Program at a US Department of Veterans Affairs medical center. He reported experiencing problems with guilt, forgiveness, and suicide ideation. A clinical therapeutic program was prescribed after a psychological evaluation uncovered that he was also struggling with depression and posttraumatic stress disorder.
His mental healthcare providers recognized the importance of incorporating a religious-spiritual component into the therapeutic plan, and so consulted with a chaplain to plan a suitable course of action. Specifically, this veteran reported feeling that he could not be forgiven for his military experiences, a feeling that was giving way to alienation and isolation from the God of his faith tradition.
The chaplain helped this veteran reflect on his military experiences, giving him the perspective he needed to view his God as one who truly loves him. He recognized instances in which he could have lost his life had it not been for others who intervened on his behalf at just the right time. This awareness caused him to think about his life differently, challenging him to reframe his relationship with God. Instead of simple coincidences, the veteran began to consider the mystery behind these times and places.
Over time and in keeping with the tenets of his faith tradition, the veteran stated that he was ultimately able to accept and receive God’s love and forgiveness. He now reports that these inner spiritual movements serve as a source of support during occasional relapses into emotional distress. These movements allow him to consider the mystery of his present life and its value based on his experience of his God’s love and forgiveness.
CARE FOR SUICIDE SURVIVORS
The experience of guilt is not limited to veterans. Those bereaved by suicide are also left to manage their own experiences of the loss and ensuing complex emotions. Friends and loved ones who survive a suicide decedent may experience guilt, feeling that they somehow contributed to or failed to prevent the suicide. Such feelings of guilt are hypothesized to lower the threshold for suicidal behavior in those bereaved.39
Guilt and shame are also frequently encountered in survivors of nonfatal suicide attempts.40 Chaplaincy services might also prove useful for these individuals.
TIME IS EVERYTHING
Patients who may have an active psychopathology should have their clinical therapeutic needs attended to first. If the clinician deems pastoral care services to be an appropriate complementary support option, care should be taken to select a pastoral care provider who is adequately prepared for this role. Different professional organizations (eg, Association of Professional Chaplains) have established board-certification procedures, minimum education requirements, and supervised practical experience required for chaplaincy certification.
Also, spiritual growth and development remain a core focus of pastoral practice. Clinicians should discontinue any collaboration with pastoral care providers who question an individual’s faith or commitment to his or her faith, or who promote thinking or actions that could be deleterious to the patient’s therapeutic trajectory.
SUMMING UP
We have here presented our perspectives on how chaplaincy services can be used to complement clinical services in support of at-risk veterans struggling with experiences of guilt. Unfortunately, the current level of collaboration between chaplains and clinicians in support of at-risk veteran populations is limited.20 Our hope is that clinicians managing these at-risk patients will develop a greater awareness of how chaplaincy services might be able to help in alleviating experiences of guilt in at-risk veteran populations. A further hope is that such cases will serve as an opportunity for greater interdisciplinary collaboration, benefiting at-risk veterans most in need of support.
Acknowledgment: Dr. Rasmussen was supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, US Department of Veterans Affairs, VISN 2 Center of Excellence for Suicide Prevention.
Suicidal behavior is a major cause of morbidity and mortality in the United States,1 and active-duty and reserve military personnel and veterans account for nearly 18% of suicide deaths.2 By one estimate, as many as 22 veterans die by suicide each day.3 These rates are thought to be due to a higher incidence of mental illness in certain veteran populations relative to the general population.4–8 Consequently, a number of mental health services are available to veterans in a variety of clinical and community settings.
Chaplains and clinicians bring complementary skills and services to the problem of suicide risk among veterans. In particular, helping at-risk veterans deal with experiences of guilt is an opportunity for interdisciplinary collaboration. Available literature supports the potential utility of chaplaincy services in supporting at-risk veteran populations.9–15
But while most healthcare facilities have chaplains on staff, there is little information to guide any such collaboration. Further, healthcare providers appear to have a limited understanding of chaplaincy services, the “language” within which chaplains operate, or the roles chaplains play in healthcare settings.16
In the following discussion, using the example of experiences of guilt, we offer our insights and suggestions on how chaplaincy services may prove useful in alleviating this complex emotion in veterans at risk of suicide.
BENEFITS OF TALKING TO A CHAPLAIN
Collaboration between healthcare providers and pastoral care professionals has been suggested as a means of enhancing the treatment of patients with mental illness.17,18 Chaplains draw from a variety of faith traditions and are usually trained to respond to the needs of people from a variety of religious and spiritual backgrounds. They provide some non-faithbased services (eg, crisis intervention, life review, bereavement counseling) resembling those also provided in formal mental healthcare settings.19 By facilitating religious and spiritual coping and religious practice and responding to religious and spiritual needs, chaplains also offer a level of support not typically offered by formal mental healthcare providers.20
Veterans at risk of suicide sometimes look to pastoral care providers, particularly chaplains, for mental health support.9,10 Research on the effects of chaplaincy services on suicidal behavior is just beginning to emerge.15 Still, the US Department of Health and Human Services has recognized pastoral care services as having a “beneficial and therapeutic effect on the medical condition of a patient.”11
For example, in one study, hospital inpatients reported higher satisfaction if they had been visited by a chaplain.12 Chaplains help align treatment plans with patient values and wishes.13 In another study,14 patients undergoing coronary artery bypass grafting who were randomized to receive five visits from a chaplain were found to have a higher rate of positive religious coping (eg, forgiveness, letting go of anger). Positive religious coping has been correlated with lower levels of psychological stress and better mental health outcomes.
EXPERIENCING GUILT IS LINKED TO RISK OF SUICIDE
Suicidal behavior is complex, multifaceted, and linked to genetic, neurologic, psychological, social, and cultural factors.21
Assessing for and addressing certain complex emotions, such as guilt and shame, is an important part of suicide prevention efforts. Guilt is defined as a “controllable psychological state that is typically linked to a specific action or behavior, and which entails regret or remorse.”22
Guilt has been linked to risk of suicide in veterans.23–25 In one study, close to 75% of veterans who had thought about suicide said they frequently experienced guilt about having violated the precepts of their faith group, family, God, life, or the military.26
Such findings suggest that the sense of guilt experienced by some at-risk veterans may be grounded in a variety of contexts. For example, faith communities that place a strong emphasis on obedience to moral, ethical, and religious precepts may contribute to the experience of guilt unless balanced by a message of grace or favor from a benevolent God or deity. Without this balance, engaging in activities that are not fully sanctioned by one’s faith community may lead to guilt.
Families might also contribute to veterans’ experiences of guilt by placing unrealistic expectations on them. And the family environment may not be conducive to resolving feelings of guilt in veterans, harboring resentment and antipathies and making it very difficult to alleviate any ensuing sense of distress.
CLINICIAN’S ROLE IN ASSESSING GUILT
In addressing and assessing guilt in veterans at risk of suicide, clinicians should try to recognize the source and clinical implications of this emotion.
Recognize the source of guilt
Guilt may indicate a clinical disorder such as a mood disorder (eg, major depression).27 Mood disorders significantly increase the risk of suicidal behavior.28,29
Beyond diagnosing a clinical disorder, prescribing pharmacotherapy, and referring for mental healthcare services, recognizing the source of this emotion remains an important part of addressing a patient’s experience of guilt. Especially when associated with a clinical disorder, guilt is often irrational and excessive and does not appropriately reflect the experience or situation in question.
Case conceptualization, defined as “synthesizing the patient’s experience with relevant clinical theory and research,”30 can be used to understand the context in which the guilt-inducing action or behavior occurred and the veteran’s own interpretation of his or her actions. Understanding the source of the patient’s guilt could be used to plan treatment and resolve any underlying sense of distress.
As with other negative emotions, the affective component of guilt is often the result of cognitive distortions made as the person tries to make sense of what has occurred or to reconcile beliefs of right and wrong with the guilt-inducing behavior.31 The common cognitive errors associated with guilt include:
- Hindsight bias (a belief that one should have known what was going to happen as a result of one’s actions)
- Responsibility distortion (a belief that one’s actions directly caused an adverse event)
- Justification distortion (a belief that one’s actions were not justified by the situation)
- Wrongdoing distortion (a belief that one violated one’s own standards of right and wrong).31
Cognitive therapy to counter cognitive distortions
A variety of clinical options exist to help veterans manage and resolve guilt.
Cognitive therapy can counter the cognitive distortions that drive feelings of guilt. The goal is to guide patients to examine the evidence, process the event, and realize that their behavior was appropriate for the given situation. Cognitive processing therapy and prolonged exposure therapy have both been shown to decrease trauma-related guilt, though cognitive processing therapy was found to be better at decreasing guilt that arose from cognitive distortions.32
Guilt and suicide ideation have also been associated with a belief that one’s actions constituted an unforgivable sin.33 Responding to these inherently religious-spiritual cognitive distortions may be beyond the scope of expertise for many healthcare professionals. In such cases, it may be prudent to consider complementing clinical services with pastoral care. It follows that pastoral care services should only be provided if the veteran voices a desire and readiness for them. The clinician and chaplain can then work together to provide coordinated care to best meet the patient’s needs, to address the experience of guilt, and to alleviate the sense of distress.
A CHAPLAIN’S PERSPECTIVE ON GUILT
A prominent feature of pastoral practice is helping people, including at-risk veterans, resolve feelings of guilt regardless of the context on which the emotion is founded (eg, religion, shame).10 For many people, guilt is an impenetrable barrier, preventing resolution of whatever experience led to a sense of inner turmoil.
Forgiveness
In the context of pastoral care, resolution of guilt is ordinarily tied to a need for forgiveness. There are multiple ways in which forgiveness can be grounded in religious and spiritual contexts.34 Examples include forgiving others (ie, forswearing resentment, anger, or hatred directed toward another person), being forgiven by God or another benevolent deity, and forgiving oneself for violating perceived personal transgressions.35 In some cases, divine forgiveness may be conditional on interpersonal forgiveness.36 Forgiveness is also sometimes seen as a remedy for sin and a way to restore moral order.37
Some people may initially think they can never be forgiven. With time and the weight of one’s experiences, the impossibility of forgiveness can become so ingrained that it becomes a core belief. These core beliefs set up a vicious circle of thoughts and feelings, in which people and places and events from the past are continuously brought forward into the present. Anger and resentment become the steady diet for the tormented self that feels forever powerless over experienced injustices. These relived experiences drive the person into a deep isolation where the self becomes less human—a thing, an object. This experience of losing oneself proves excruciating and often leads to contemplation of suicide as a way to resolve anguish.
Hope emerges
Pastoral care services provide a means to reframe one’s core beliefs, manage and resolve the burden of guilt, and uncover new motivation for living.
The practice of spiritual direction within the discipline of pastoral care listens for these inner movements and encourages the person to give voice to them in his or her own words. No longer limited by a diminished, tormented self, the real self begins to relate to another reality that changes his or her identity, relieves the burden of guilt, and gives reason, purpose, and meaning to life.
Even with this opportunity for a new life, however, cognitive distortions based on a disproportional “faith-based prism” may persist. In this case, clinicians and chaplains must work closely together to reframe old understandings of self and incorrect understandings of religion and spirituality into one that continues to reinforce this newfound sense of hope.38
A VETERAN OF IRAQ WITH SUICIDE IDEATION
The following case illustrates how clinicians and chaplains may be able to work together to help facilitate the resolution of guilt.
A veteran who had served in Iraq had entered the Domiciliary Care Program at a US Department of Veterans Affairs medical center. He reported experiencing problems with guilt, forgiveness, and suicide ideation. A clinical therapeutic program was prescribed after a psychological evaluation uncovered that he was also struggling with depression and posttraumatic stress disorder.
His mental healthcare providers recognized the importance of incorporating a religious-spiritual component into the therapeutic plan, and so consulted with a chaplain to plan a suitable course of action. Specifically, this veteran reported feeling that he could not be forgiven for his military experiences, a feeling that was giving way to alienation and isolation from the God of his faith tradition.
The chaplain helped this veteran reflect on his military experiences, giving him the perspective he needed to view his God as one who truly loves him. He recognized instances in which he could have lost his life had it not been for others who intervened on his behalf at just the right time. This awareness caused him to think about his life differently, challenging him to reframe his relationship with God. Instead of simple coincidences, the veteran began to consider the mystery behind these times and places.
Over time and in keeping with the tenets of his faith tradition, the veteran stated that he was ultimately able to accept and receive God’s love and forgiveness. He now reports that these inner spiritual movements serve as a source of support during occasional relapses into emotional distress. These movements allow him to consider the mystery of his present life and its value based on his experience of his God’s love and forgiveness.
CARE FOR SUICIDE SURVIVORS
The experience of guilt is not limited to veterans. Those bereaved by suicide are also left to manage their own experiences of the loss and ensuing complex emotions. Friends and loved ones who survive a suicide decedent may experience guilt, feeling that they somehow contributed to or failed to prevent the suicide. Such feelings of guilt are hypothesized to lower the threshold for suicidal behavior in those bereaved.39
Guilt and shame are also frequently encountered in survivors of nonfatal suicide attempts.40 Chaplaincy services might also prove useful for these individuals.
TIME IS EVERYTHING
Patients who may have an active psychopathology should have their clinical therapeutic needs attended to first. If the clinician deems pastoral care services to be an appropriate complementary support option, care should be taken to select a pastoral care provider who is adequately prepared for this role. Different professional organizations (eg, Association of Professional Chaplains) have established board-certification procedures, minimum education requirements, and supervised practical experience required for chaplaincy certification.
Also, spiritual growth and development remain a core focus of pastoral practice. Clinicians should discontinue any collaboration with pastoral care providers who question an individual’s faith or commitment to his or her faith, or who promote thinking or actions that could be deleterious to the patient’s therapeutic trajectory.
SUMMING UP
We have here presented our perspectives on how chaplaincy services can be used to complement clinical services in support of at-risk veterans struggling with experiences of guilt. Unfortunately, the current level of collaboration between chaplains and clinicians in support of at-risk veteran populations is limited.20 Our hope is that clinicians managing these at-risk patients will develop a greater awareness of how chaplaincy services might be able to help in alleviating experiences of guilt in at-risk veteran populations. A further hope is that such cases will serve as an opportunity for greater interdisciplinary collaboration, benefiting at-risk veterans most in need of support.
Acknowledgment: Dr. Rasmussen was supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, US Department of Veterans Affairs, VISN 2 Center of Excellence for Suicide Prevention.
- Centers for Disease Control and Prevention (CDC). Suicide and self-inflicted injury. www.cdc.gov/nchs/fastats/suicide.htm. Accessed November 12, 2015.
- Centers for Disease Control and Prevention (CDC). National violent death reporting system (NVDRS). https://wisqars.cdc.gov:8443/nvdrs/nvdrsDisplay.jsp. Accessed November 12, 2015.
- Kemp JE, Bossarte R. Suicide data report, 2012. www.sprc.org/library_resources/items/suicide-data-report-2012. Accessed November 12, 2015.
- Bullman TA, Kang HK. The risk of suicide among wounded Vietnam veterans. Am J Public Health 1996; 86:662–667.
- Kang HK, Bullman TA. Is there an epidemic of suicides among current and former US military personnel? Ann Epidemiol 2009; 19:757–760.
- LeardMann CA, Powell TM, Smith TC, et al. Risk factors associated with suicide in current and former US military personnel. JAMA 2013; 310:496–506.
- Mrnak-Meyer J, Tate SR, Tripp JC, Worley MJ, Jajodia A, McQuaid JR. Predictors of suicide-related hospitalization among US veterans receiving treatment for comorbid depression and substance dependence: who is the riskiest of the risky? Suicide Life Threat Behav 2011; 41:532–542.
- Pietrzak RH, Russo AR, Ling Q, Southwick SM. Suicidal ideation in treatment-seeking veterans of Operations Enduring Freedom and Iraqi Freedom: the role of coping strategies, resilience, and social support. J Psychiatr Res 2011; 45:720–726.
- Kopacz MS, McCarten JM, Pollitt MJ. VHA chaplaincy contact with veterans at increased risk of suicide. South Med J 2014; 107: 661–664.
- Kopacz MS. Providing pastoral care services in a clinical setting to veterans at-risk of suicide. J Relig Health 2013; 52:759–767.
- Medicare program; payment for nursing and allied health education. Health Care Financing Administration (HCFA), HHS. Final rule. Fed Regist 2001; 66:3358–3376.
- Marin DB, Sharma V, Sosunov E, Egorova N, Goldstein R, Handzo GF. Relationship between chaplain visits and patient satisfaction. J Health Care Chaplain 2015; 21:14–24.
- Flannelly KJ, Emanuel LL, Handzo GF, Galek K, Silton NR, Carlson M. A national study of chaplaincy services and end-of-life outcomes. BMC Palliat Care 2012; 11:10.
- Bay PS, Beckman D, Trippi J, Gunderman R, Terry C. The effect of pastoral care services on anxiety, depression, hope, religious coping, and religious problem solving styles: a randomized controlled study. J Relig Health 2008; 47:57–69.
- Kopacz MS, Nieuwsma JA, Jackson GL, et al. Chaplains’ engagement with suicidality among their service users: findings from the VA/DoD Integrated Mental Health Strategy. Suicide Life Threat Behav 2015. [Epub ahead of print.]
- Flannelly KJ, Galek K, Bucchino J, Handzo GF, Tannenbaum HP. Department directors’ perceptions of the roles and functions of hospital chaplains: a national survey. Hosp Top 2005; 83:19–27.
- Farrell JL, Goebert DA. Collaboration between psychiatrists and clergy in recognizing and treating serious mental illness. Psychiatr Serv 2008; 59:437–440.
- Weaver AJ, Flannelly KJ, Flannelly LT, Oppenheimer JE. Collaboration between clergy and mental health professionals: a review of professional health care journals from 1980 through 1999. Counsel Val 2003; 47:162–171.
- Handzo GF, Flannelly KJ, Kudler T, et al. What do chaplains really do? II. Interventions in the New York chaplaincy study. J Health Care Chaplain 2008; 14:39–56.
- Kopacz MS, Pollitt MJ. Delivering chaplaincy services to veterans at increased risk of suicide. J Health Care Chaplain 2015; 21:1–13.
- Knox KL, Bossarte RM. Suicide prevention for veterans and active duty personnel. Am J Public Health 2012;102(suppl 1):S8–S9.
- Bryan CJ, Morrow CE, Etienne N, Ray-Sannerud B. Guilt, shame, and suicidal ideation in a military outpatient clinical sample. Depress Anxiety 2013; 30:55–60.
- Ganz D, Sher L. Educating medical professionals about suicide prevention among military veterans. Int J Adolesc Med Health 2013; 25:187–191.
- Hendin H, Haas AP. Suicide and guilt as manifestations of PTSD in Vietnam combat veterans. Am J Psychiatry 1991; 148:586–591.
- Maguen S, Metzler TJ, Bosch J, Marmar CR, Knight SJ, Neylan TC. Killing in combat may be independently associated with suicidal ideation. Depress Anxiety 2012; 29:918–923.
- Kopacz MS, McCarten JM, Vance CG, Connery AL. A preliminary study for exploring different sources of guilt in a sample of veterans who sought chaplaincy services. Mil Psychol 2015; 27:1–8.
- Buck CJ. 2013 ICD-9-CM for physicians. St. Louis, MO: Saunders; 2013.
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34-38 years. J Affect Disord 2002; 68:167–181.
- Nierenberg AA, Gray SM, Grandin LD. Mood disorders and suicide. J Clin Psychiatry 2001; 62(suppl 25):27–30.
- Macneil CA, Hasty MK, Conus P, Berk M. Is diagnosis enough to guide interventions in mental health? Using case formulation in clinical practice. BMC Med 2012; 10:111.
- Kubany ES, Manke FP. Cognitive therapy for trauma-related guilt: conceptual bases and treatment outlines. Cogn Behav Pract 1995; 2:27–61.
- Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. Comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. J Consult Clin Psychol 2002; 70:867–879.
- Exline JJ, Yali AM, Sanderson WC. Guilt, discord, and alienation: the role of religious strain in depression and suicidality. J Clin Psychol 2000; 56:1481–1496.
- Musick MA. Multiple forms of forgiveness and their relationship with aging and religion, In: Schaie KW, Krause N, Booth A, editors. Religious Influences on Health and Well-being in the Elderly. New York, NY: Springer Publishing Company; 2004:202–214.
- Kaplan BH, Munroe-Blum H, Blazer DG. Religion, health and forgiveness: tradition and challenges. In: Levin JS, editor. Religion in Aging and Health. Theoretical Foundations and Methodological Frontiers. Thousand Oaks, CA: SAGE Focus Edition; 1994:52–77.
- Worthington EL Jr, Berry JW, Parrott L III. Unforgiveness, forgiveness, religion and health. In: Plante TG, Sherman AC, editors. Faith and Health. Psychological Perspectives. New York, NY: Guilford Press; 2001:107–138.
- Enright RD, Gassin EA, Wu GR. Forgiveness: a developmental view. J Moral Educ 1992; 21:99–114.
- Kopacz MS, O’Reilly LM, Van Inwagen CC, et al. Understanding the role of chaplains in veteran suicide prevention efforts: a discussion paper. SAGE Open 2014; 4:1–10.
- Young IT, Iglewicz A, Glorioso D, et al. Suicide bereavement and complicated grief. Dialogues Clin Neurosci 2012; 14:177–186.
- Wiklander M, Samuelsson M, Asberg M. Shame reactions after suicide attempt. Scand J Caring Sci 2003; 17:293–300.
- Centers for Disease Control and Prevention (CDC). Suicide and self-inflicted injury. www.cdc.gov/nchs/fastats/suicide.htm. Accessed November 12, 2015.
- Centers for Disease Control and Prevention (CDC). National violent death reporting system (NVDRS). https://wisqars.cdc.gov:8443/nvdrs/nvdrsDisplay.jsp. Accessed November 12, 2015.
- Kemp JE, Bossarte R. Suicide data report, 2012. www.sprc.org/library_resources/items/suicide-data-report-2012. Accessed November 12, 2015.
- Bullman TA, Kang HK. The risk of suicide among wounded Vietnam veterans. Am J Public Health 1996; 86:662–667.
- Kang HK, Bullman TA. Is there an epidemic of suicides among current and former US military personnel? Ann Epidemiol 2009; 19:757–760.
- LeardMann CA, Powell TM, Smith TC, et al. Risk factors associated with suicide in current and former US military personnel. JAMA 2013; 310:496–506.
- Mrnak-Meyer J, Tate SR, Tripp JC, Worley MJ, Jajodia A, McQuaid JR. Predictors of suicide-related hospitalization among US veterans receiving treatment for comorbid depression and substance dependence: who is the riskiest of the risky? Suicide Life Threat Behav 2011; 41:532–542.
- Pietrzak RH, Russo AR, Ling Q, Southwick SM. Suicidal ideation in treatment-seeking veterans of Operations Enduring Freedom and Iraqi Freedom: the role of coping strategies, resilience, and social support. J Psychiatr Res 2011; 45:720–726.
- Kopacz MS, McCarten JM, Pollitt MJ. VHA chaplaincy contact with veterans at increased risk of suicide. South Med J 2014; 107: 661–664.
- Kopacz MS. Providing pastoral care services in a clinical setting to veterans at-risk of suicide. J Relig Health 2013; 52:759–767.
- Medicare program; payment for nursing and allied health education. Health Care Financing Administration (HCFA), HHS. Final rule. Fed Regist 2001; 66:3358–3376.
- Marin DB, Sharma V, Sosunov E, Egorova N, Goldstein R, Handzo GF. Relationship between chaplain visits and patient satisfaction. J Health Care Chaplain 2015; 21:14–24.
- Flannelly KJ, Emanuel LL, Handzo GF, Galek K, Silton NR, Carlson M. A national study of chaplaincy services and end-of-life outcomes. BMC Palliat Care 2012; 11:10.
- Bay PS, Beckman D, Trippi J, Gunderman R, Terry C. The effect of pastoral care services on anxiety, depression, hope, religious coping, and religious problem solving styles: a randomized controlled study. J Relig Health 2008; 47:57–69.
- Kopacz MS, Nieuwsma JA, Jackson GL, et al. Chaplains’ engagement with suicidality among their service users: findings from the VA/DoD Integrated Mental Health Strategy. Suicide Life Threat Behav 2015. [Epub ahead of print.]
- Flannelly KJ, Galek K, Bucchino J, Handzo GF, Tannenbaum HP. Department directors’ perceptions of the roles and functions of hospital chaplains: a national survey. Hosp Top 2005; 83:19–27.
- Farrell JL, Goebert DA. Collaboration between psychiatrists and clergy in recognizing and treating serious mental illness. Psychiatr Serv 2008; 59:437–440.
- Weaver AJ, Flannelly KJ, Flannelly LT, Oppenheimer JE. Collaboration between clergy and mental health professionals: a review of professional health care journals from 1980 through 1999. Counsel Val 2003; 47:162–171.
- Handzo GF, Flannelly KJ, Kudler T, et al. What do chaplains really do? II. Interventions in the New York chaplaincy study. J Health Care Chaplain 2008; 14:39–56.
- Kopacz MS, Pollitt MJ. Delivering chaplaincy services to veterans at increased risk of suicide. J Health Care Chaplain 2015; 21:1–13.
- Knox KL, Bossarte RM. Suicide prevention for veterans and active duty personnel. Am J Public Health 2012;102(suppl 1):S8–S9.
- Bryan CJ, Morrow CE, Etienne N, Ray-Sannerud B. Guilt, shame, and suicidal ideation in a military outpatient clinical sample. Depress Anxiety 2013; 30:55–60.
- Ganz D, Sher L. Educating medical professionals about suicide prevention among military veterans. Int J Adolesc Med Health 2013; 25:187–191.
- Hendin H, Haas AP. Suicide and guilt as manifestations of PTSD in Vietnam combat veterans. Am J Psychiatry 1991; 148:586–591.
- Maguen S, Metzler TJ, Bosch J, Marmar CR, Knight SJ, Neylan TC. Killing in combat may be independently associated with suicidal ideation. Depress Anxiety 2012; 29:918–923.
- Kopacz MS, McCarten JM, Vance CG, Connery AL. A preliminary study for exploring different sources of guilt in a sample of veterans who sought chaplaincy services. Mil Psychol 2015; 27:1–8.
- Buck CJ. 2013 ICD-9-CM for physicians. St. Louis, MO: Saunders; 2013.
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34-38 years. J Affect Disord 2002; 68:167–181.
- Nierenberg AA, Gray SM, Grandin LD. Mood disorders and suicide. J Clin Psychiatry 2001; 62(suppl 25):27–30.
- Macneil CA, Hasty MK, Conus P, Berk M. Is diagnosis enough to guide interventions in mental health? Using case formulation in clinical practice. BMC Med 2012; 10:111.
- Kubany ES, Manke FP. Cognitive therapy for trauma-related guilt: conceptual bases and treatment outlines. Cogn Behav Pract 1995; 2:27–61.
- Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. Comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. J Consult Clin Psychol 2002; 70:867–879.
- Exline JJ, Yali AM, Sanderson WC. Guilt, discord, and alienation: the role of religious strain in depression and suicidality. J Clin Psychol 2000; 56:1481–1496.
- Musick MA. Multiple forms of forgiveness and their relationship with aging and religion, In: Schaie KW, Krause N, Booth A, editors. Religious Influences on Health and Well-being in the Elderly. New York, NY: Springer Publishing Company; 2004:202–214.
- Kaplan BH, Munroe-Blum H, Blazer DG. Religion, health and forgiveness: tradition and challenges. In: Levin JS, editor. Religion in Aging and Health. Theoretical Foundations and Methodological Frontiers. Thousand Oaks, CA: SAGE Focus Edition; 1994:52–77.
- Worthington EL Jr, Berry JW, Parrott L III. Unforgiveness, forgiveness, religion and health. In: Plante TG, Sherman AC, editors. Faith and Health. Psychological Perspectives. New York, NY: Guilford Press; 2001:107–138.
- Enright RD, Gassin EA, Wu GR. Forgiveness: a developmental view. J Moral Educ 1992; 21:99–114.
- Kopacz MS, O’Reilly LM, Van Inwagen CC, et al. Understanding the role of chaplains in veteran suicide prevention efforts: a discussion paper. SAGE Open 2014; 4:1–10.
- Young IT, Iglewicz A, Glorioso D, et al. Suicide bereavement and complicated grief. Dialogues Clin Neurosci 2012; 14:177–186.
- Wiklander M, Samuelsson M, Asberg M. Shame reactions after suicide attempt. Scand J Caring Sci 2003; 17:293–300.
Many shades of guilt
In their commentary, Kopacz et al1 propose that collaboration with professionally trained and certified clinical chaplains provides an opportunity for interdisciplinary care with increased benefit to veterans at risk of suicide. They rightly identify the pivotal issue of guilt as one that falls squarely in the domain of spiritual (or pastoral) care.
As professionals involved in the training of board-certifiable chaplains (and one of us is a veteran), we find that guilt in patients with suicidal tendencies is a profoundly spiritual issue that can be addressed effectively through collaboration among chaplains, physicians, and mental health providers.
Guilt is a serious spiritual condition that can easily be undertreated, or treated under the rubric of depression, which is related but not identical. Undertreatment occurs when caregivers, eager to see the guilt-sufferer experience relief, inadvertently short-circuit the necessary process of working through, rather than around, the guilt. Allowing patients to talk about their feelings of guilt without minimizing those feelings can be helpful even if, as Kopacz et al point out, the feelings are often irrational. We believe that people have an innate need to be truly heard and understood before they can become open to a reinterpretation of their feelings. Only then can the seeds of self-forgiveness begin to take root.
Hearing the words “There is hope for you to feel forgiven” can be more helpful than hearing “You didn’t really do anything bad,” particularly if the patient is religious. Hearing these words from a chaplain is often more effective than hearing them from a lay person, just as many of us take basic health information more seriously when we hear it from a physician. Even if the veteran is not overtly religious, there may be a unique exchange between that person and a religious authority when it concerns the violation of a millenniaold, widely known teaching from the Bible, such as “Thou shalt not kill.”
Kopacz et al also rightly suggest that unless religious prohibitions have been balanced with teachings on forgiveness and grace, the teachings can actually exacerbate feelings of guilt and elevate them to harmful proportions, especially in the potentially vulnerable psyche of a veteran who may have been traumatized. If there is no religious or spiritual guidance for balancing prohibitions with graces, the patient may be left to spiral in an unending loop of guilt with no way out.
We therefore propose the following categories for different types (or “shades”) of guilt that can be effectively addressed by chaplains in concert with other members of the healthcare team. For simplicity, we call these types real guilt, survivor guilt, mistaken guilt, and complex-compound guilt.
REAL GUILT
An important role professional chaplains can play is to allow patients (in this case, veterans) to express their remorse and regret for violations of their own moral codes. In many cases, they have in fact hurt or killed another person, and they need the chance to unburden their hearts and spirits, especially if they were taught that killing people is a sin. Veterans who have harmed or killed others, even if under orders, are often left with bona fide feelings of guilt that need to be aired and released in a safe and confidential environment. This is often most effective when done by someone who not only is trained in nonjudgmental and nondirective listening, but also is a religious authority who can assure the patient of his or her innate worthiness and of the ability to be forgiven.
As Kopacz et al note, guilt is linked to a specific action or behavior and usually entails regret or remorse. Many veterans belong to or have had exposure to faith groups with strong moral codes and prohibitions, and so may see the chaplain as having authority to act as confessor and granter of absolution.
SURVIVOR GUILT
Survivor guilt is commonly understood as the feeling of surviving a terrible event or situation while someone else did not. Those who suffer from survivor guilt judge themselves unworthy of survival and believe the deceased to have been more courageous, virtuous, or somehow a better person than they. They torture themselves with ideas of the deceased person’s virtues—imagined or real—and sometimes go on to believe that “It should have been me who was killed.”
The burden of feeling that the wrong person died can be overwhelming. If these people are not helped to see their own worth and helped to find outlets for their sense of having been spared (by God, by their own wits, or by sheer luck), they are likely to struggle more. This is related but not identical to what we call mistaken guilt. The two types are similar because they share a sense of randomness and helplessness, but they are different for reasons we will explain below.
Chaplains can be particularly effective partners in the care of veterans with survivor guilt, helping them to make meaning out of a life-changing event, rather than find meaning inherently in that event. Meaning, purpose, and “God’s plan for my life” are common themes in the pastoral conversation that can provide a compass for the disoriented survivor.
MISTAKEN GUILT
Mistaken guilt describes when a person who is involved in the death of another but is absolutely blameless—and could not possibly have prevented that death—literally “mis-takes” the guilt upon himself or herself in spite of the facts. Because of the helplessness induced by this feeling, mistaken guilt can be more difficult to treat than other forms. These patients continue to suffer despite assurances that the death occurred through absolutely no fault of their own.2
Hickling3 has written extensively about this phenomenon in innocent motor vehicle drivers who cause pedestrian deaths, and he considers this type of guilt one of the most difficult to recover from precisely because of the helplessness factor. He has explained that if patients can find a real reason by which they were culpable for what happened, they can change their ways. But if they were absolutely innocent (as in many incidents in training or combat), they often cannot make sense of what happened in a way that allows them to move on because there is nothing they could have done differently and therefore nothing they can change.4
These patients almost certainly need long-term intervention such as cognitive behavioral therapy in order to train their mind away from such destructive thoughts. However, they are also very likely to be helped by a chaplain if they find that the event triggers memories of other past infractions of which they may need to unburden themselves (ie, confess).
COMPOUND-COMPLEX GUILT
As the name implies, compound-complex guilt is a combination of the other types and may have additional layers.
Compound-complex guilt leaves sufferers literally feeling guilty for feeling guilty. Though this may border on a genuine clinical disorder, it is also to some degree normal (eg, due to cultural taboos and norms) for people to feel culpable for not being able to “move on” or “forgive themselves” as quickly as others may want them to. Buddhists call this tendency the “second arrow effect.” The first arrow is the feeling of guilt (or other painful feeling) that strikes the individual, but the second arrow is the one he or she drives in afterward by thinking it is wrong or weak to even have the feeling.
Patients who suffer from this type of guilt blame themselves for the conundrum they are in and feel even worse. This is not unlike the vortex of unresolved and complicated grief.
Those who suffer from compound-complex guilt may layer the primary guilt with additional guilt for feeling weak, for needing help, or for asking for help. Especially in the culture of the military, the fear of stigma when asking for help (especially with mental health) is still quite strong. Therefore, chaplains can serve as a less threatening entry point for the veteran needing multiple professionals involved in his or her care.
NONJUDGMENTAL LISTENING
Nonjudgmental listening is essential to get at the source or sources of guilt, regardless of the type, in order to allow the wounds to air out and begin healing. Many veterans suffering from guilt may need intensive pharmacologic and cognitive therapy to fully recover, and care from a chaplain is not a substitute for psychiatric evaluation and treatment, especially if there is a risk of suicide.
However, chaplains may be able to help with the “deep work” of spiritual healing that is part of veterans’ overall recovery. This is true not only because chaplains are especially trained to do this, but also because they are the team members most likely to have uniquely spiritual language to speak to the condition. The language of confession, absolution, repentance, redemption, atonement, and forgiveness is language of the spiritual realm.
In addition, chaplains’ freedom from hourly billing concerns and their often less formalized interactions with patients may help to build trust. Well-trained chaplains, who are often quite gifted at creating an atmosphere of reverence and safety (sanctuary) in the most unlikely situations, are well suited to help the interdisciplinary team treat this vulnerable patient population.
SUGGESTED READING
For more insights into the role of chaplains on the interdisciplinary healthcare team, we recommend the following book: Cadge W. Paging God: Religion in the Halls of Medicine. Chicago, IL: University of Chicago Press; 2012.
- Kopacz MS, Rasmussen KA, Searle RF, Wozniak BM, Titus CE. Veterans, guilt, and suicide risk: chaplains can help. Cleve Clin J Med 2016; 83:101–105.
- Life after death: Act one—guilty as not charged. Darin Strauss. This American Life. www.thisamericanlife.org. Episode 359. Aired July 18, 2008. www.thisamericanlife.org/radio-archives/episode/359/life-after-death?act=1#play. Accessed December 10, 2015.
- Hickling EJ, Blanchard EB. Overcoming the Trauma of Your Motor Vehicle Accident: A Cognitive-behavioral Treatment Program. New York, NY: Oxford University Press; 2006.
- Hickling EJ. Transforming Tragedy: Finding Growth Following Life’s Traumas. North Charleston, SC: CreateSpace; 2012.
In their commentary, Kopacz et al1 propose that collaboration with professionally trained and certified clinical chaplains provides an opportunity for interdisciplinary care with increased benefit to veterans at risk of suicide. They rightly identify the pivotal issue of guilt as one that falls squarely in the domain of spiritual (or pastoral) care.
As professionals involved in the training of board-certifiable chaplains (and one of us is a veteran), we find that guilt in patients with suicidal tendencies is a profoundly spiritual issue that can be addressed effectively through collaboration among chaplains, physicians, and mental health providers.
Guilt is a serious spiritual condition that can easily be undertreated, or treated under the rubric of depression, which is related but not identical. Undertreatment occurs when caregivers, eager to see the guilt-sufferer experience relief, inadvertently short-circuit the necessary process of working through, rather than around, the guilt. Allowing patients to talk about their feelings of guilt without minimizing those feelings can be helpful even if, as Kopacz et al point out, the feelings are often irrational. We believe that people have an innate need to be truly heard and understood before they can become open to a reinterpretation of their feelings. Only then can the seeds of self-forgiveness begin to take root.
Hearing the words “There is hope for you to feel forgiven” can be more helpful than hearing “You didn’t really do anything bad,” particularly if the patient is religious. Hearing these words from a chaplain is often more effective than hearing them from a lay person, just as many of us take basic health information more seriously when we hear it from a physician. Even if the veteran is not overtly religious, there may be a unique exchange between that person and a religious authority when it concerns the violation of a millenniaold, widely known teaching from the Bible, such as “Thou shalt not kill.”
Kopacz et al also rightly suggest that unless religious prohibitions have been balanced with teachings on forgiveness and grace, the teachings can actually exacerbate feelings of guilt and elevate them to harmful proportions, especially in the potentially vulnerable psyche of a veteran who may have been traumatized. If there is no religious or spiritual guidance for balancing prohibitions with graces, the patient may be left to spiral in an unending loop of guilt with no way out.
We therefore propose the following categories for different types (or “shades”) of guilt that can be effectively addressed by chaplains in concert with other members of the healthcare team. For simplicity, we call these types real guilt, survivor guilt, mistaken guilt, and complex-compound guilt.
REAL GUILT
An important role professional chaplains can play is to allow patients (in this case, veterans) to express their remorse and regret for violations of their own moral codes. In many cases, they have in fact hurt or killed another person, and they need the chance to unburden their hearts and spirits, especially if they were taught that killing people is a sin. Veterans who have harmed or killed others, even if under orders, are often left with bona fide feelings of guilt that need to be aired and released in a safe and confidential environment. This is often most effective when done by someone who not only is trained in nonjudgmental and nondirective listening, but also is a religious authority who can assure the patient of his or her innate worthiness and of the ability to be forgiven.
As Kopacz et al note, guilt is linked to a specific action or behavior and usually entails regret or remorse. Many veterans belong to or have had exposure to faith groups with strong moral codes and prohibitions, and so may see the chaplain as having authority to act as confessor and granter of absolution.
SURVIVOR GUILT
Survivor guilt is commonly understood as the feeling of surviving a terrible event or situation while someone else did not. Those who suffer from survivor guilt judge themselves unworthy of survival and believe the deceased to have been more courageous, virtuous, or somehow a better person than they. They torture themselves with ideas of the deceased person’s virtues—imagined or real—and sometimes go on to believe that “It should have been me who was killed.”
The burden of feeling that the wrong person died can be overwhelming. If these people are not helped to see their own worth and helped to find outlets for their sense of having been spared (by God, by their own wits, or by sheer luck), they are likely to struggle more. This is related but not identical to what we call mistaken guilt. The two types are similar because they share a sense of randomness and helplessness, but they are different for reasons we will explain below.
Chaplains can be particularly effective partners in the care of veterans with survivor guilt, helping them to make meaning out of a life-changing event, rather than find meaning inherently in that event. Meaning, purpose, and “God’s plan for my life” are common themes in the pastoral conversation that can provide a compass for the disoriented survivor.
MISTAKEN GUILT
Mistaken guilt describes when a person who is involved in the death of another but is absolutely blameless—and could not possibly have prevented that death—literally “mis-takes” the guilt upon himself or herself in spite of the facts. Because of the helplessness induced by this feeling, mistaken guilt can be more difficult to treat than other forms. These patients continue to suffer despite assurances that the death occurred through absolutely no fault of their own.2
Hickling3 has written extensively about this phenomenon in innocent motor vehicle drivers who cause pedestrian deaths, and he considers this type of guilt one of the most difficult to recover from precisely because of the helplessness factor. He has explained that if patients can find a real reason by which they were culpable for what happened, they can change their ways. But if they were absolutely innocent (as in many incidents in training or combat), they often cannot make sense of what happened in a way that allows them to move on because there is nothing they could have done differently and therefore nothing they can change.4
These patients almost certainly need long-term intervention such as cognitive behavioral therapy in order to train their mind away from such destructive thoughts. However, they are also very likely to be helped by a chaplain if they find that the event triggers memories of other past infractions of which they may need to unburden themselves (ie, confess).
COMPOUND-COMPLEX GUILT
As the name implies, compound-complex guilt is a combination of the other types and may have additional layers.
Compound-complex guilt leaves sufferers literally feeling guilty for feeling guilty. Though this may border on a genuine clinical disorder, it is also to some degree normal (eg, due to cultural taboos and norms) for people to feel culpable for not being able to “move on” or “forgive themselves” as quickly as others may want them to. Buddhists call this tendency the “second arrow effect.” The first arrow is the feeling of guilt (or other painful feeling) that strikes the individual, but the second arrow is the one he or she drives in afterward by thinking it is wrong or weak to even have the feeling.
Patients who suffer from this type of guilt blame themselves for the conundrum they are in and feel even worse. This is not unlike the vortex of unresolved and complicated grief.
Those who suffer from compound-complex guilt may layer the primary guilt with additional guilt for feeling weak, for needing help, or for asking for help. Especially in the culture of the military, the fear of stigma when asking for help (especially with mental health) is still quite strong. Therefore, chaplains can serve as a less threatening entry point for the veteran needing multiple professionals involved in his or her care.
NONJUDGMENTAL LISTENING
Nonjudgmental listening is essential to get at the source or sources of guilt, regardless of the type, in order to allow the wounds to air out and begin healing. Many veterans suffering from guilt may need intensive pharmacologic and cognitive therapy to fully recover, and care from a chaplain is not a substitute for psychiatric evaluation and treatment, especially if there is a risk of suicide.
However, chaplains may be able to help with the “deep work” of spiritual healing that is part of veterans’ overall recovery. This is true not only because chaplains are especially trained to do this, but also because they are the team members most likely to have uniquely spiritual language to speak to the condition. The language of confession, absolution, repentance, redemption, atonement, and forgiveness is language of the spiritual realm.
In addition, chaplains’ freedom from hourly billing concerns and their often less formalized interactions with patients may help to build trust. Well-trained chaplains, who are often quite gifted at creating an atmosphere of reverence and safety (sanctuary) in the most unlikely situations, are well suited to help the interdisciplinary team treat this vulnerable patient population.
SUGGESTED READING
For more insights into the role of chaplains on the interdisciplinary healthcare team, we recommend the following book: Cadge W. Paging God: Religion in the Halls of Medicine. Chicago, IL: University of Chicago Press; 2012.
In their commentary, Kopacz et al1 propose that collaboration with professionally trained and certified clinical chaplains provides an opportunity for interdisciplinary care with increased benefit to veterans at risk of suicide. They rightly identify the pivotal issue of guilt as one that falls squarely in the domain of spiritual (or pastoral) care.
As professionals involved in the training of board-certifiable chaplains (and one of us is a veteran), we find that guilt in patients with suicidal tendencies is a profoundly spiritual issue that can be addressed effectively through collaboration among chaplains, physicians, and mental health providers.
Guilt is a serious spiritual condition that can easily be undertreated, or treated under the rubric of depression, which is related but not identical. Undertreatment occurs when caregivers, eager to see the guilt-sufferer experience relief, inadvertently short-circuit the necessary process of working through, rather than around, the guilt. Allowing patients to talk about their feelings of guilt without minimizing those feelings can be helpful even if, as Kopacz et al point out, the feelings are often irrational. We believe that people have an innate need to be truly heard and understood before they can become open to a reinterpretation of their feelings. Only then can the seeds of self-forgiveness begin to take root.
Hearing the words “There is hope for you to feel forgiven” can be more helpful than hearing “You didn’t really do anything bad,” particularly if the patient is religious. Hearing these words from a chaplain is often more effective than hearing them from a lay person, just as many of us take basic health information more seriously when we hear it from a physician. Even if the veteran is not overtly religious, there may be a unique exchange between that person and a religious authority when it concerns the violation of a millenniaold, widely known teaching from the Bible, such as “Thou shalt not kill.”
Kopacz et al also rightly suggest that unless religious prohibitions have been balanced with teachings on forgiveness and grace, the teachings can actually exacerbate feelings of guilt and elevate them to harmful proportions, especially in the potentially vulnerable psyche of a veteran who may have been traumatized. If there is no religious or spiritual guidance for balancing prohibitions with graces, the patient may be left to spiral in an unending loop of guilt with no way out.
We therefore propose the following categories for different types (or “shades”) of guilt that can be effectively addressed by chaplains in concert with other members of the healthcare team. For simplicity, we call these types real guilt, survivor guilt, mistaken guilt, and complex-compound guilt.
REAL GUILT
An important role professional chaplains can play is to allow patients (in this case, veterans) to express their remorse and regret for violations of their own moral codes. In many cases, they have in fact hurt or killed another person, and they need the chance to unburden their hearts and spirits, especially if they were taught that killing people is a sin. Veterans who have harmed or killed others, even if under orders, are often left with bona fide feelings of guilt that need to be aired and released in a safe and confidential environment. This is often most effective when done by someone who not only is trained in nonjudgmental and nondirective listening, but also is a religious authority who can assure the patient of his or her innate worthiness and of the ability to be forgiven.
As Kopacz et al note, guilt is linked to a specific action or behavior and usually entails regret or remorse. Many veterans belong to or have had exposure to faith groups with strong moral codes and prohibitions, and so may see the chaplain as having authority to act as confessor and granter of absolution.
SURVIVOR GUILT
Survivor guilt is commonly understood as the feeling of surviving a terrible event or situation while someone else did not. Those who suffer from survivor guilt judge themselves unworthy of survival and believe the deceased to have been more courageous, virtuous, or somehow a better person than they. They torture themselves with ideas of the deceased person’s virtues—imagined or real—and sometimes go on to believe that “It should have been me who was killed.”
The burden of feeling that the wrong person died can be overwhelming. If these people are not helped to see their own worth and helped to find outlets for their sense of having been spared (by God, by their own wits, or by sheer luck), they are likely to struggle more. This is related but not identical to what we call mistaken guilt. The two types are similar because they share a sense of randomness and helplessness, but they are different for reasons we will explain below.
Chaplains can be particularly effective partners in the care of veterans with survivor guilt, helping them to make meaning out of a life-changing event, rather than find meaning inherently in that event. Meaning, purpose, and “God’s plan for my life” are common themes in the pastoral conversation that can provide a compass for the disoriented survivor.
MISTAKEN GUILT
Mistaken guilt describes when a person who is involved in the death of another but is absolutely blameless—and could not possibly have prevented that death—literally “mis-takes” the guilt upon himself or herself in spite of the facts. Because of the helplessness induced by this feeling, mistaken guilt can be more difficult to treat than other forms. These patients continue to suffer despite assurances that the death occurred through absolutely no fault of their own.2
Hickling3 has written extensively about this phenomenon in innocent motor vehicle drivers who cause pedestrian deaths, and he considers this type of guilt one of the most difficult to recover from precisely because of the helplessness factor. He has explained that if patients can find a real reason by which they were culpable for what happened, they can change their ways. But if they were absolutely innocent (as in many incidents in training or combat), they often cannot make sense of what happened in a way that allows them to move on because there is nothing they could have done differently and therefore nothing they can change.4
These patients almost certainly need long-term intervention such as cognitive behavioral therapy in order to train their mind away from such destructive thoughts. However, they are also very likely to be helped by a chaplain if they find that the event triggers memories of other past infractions of which they may need to unburden themselves (ie, confess).
COMPOUND-COMPLEX GUILT
As the name implies, compound-complex guilt is a combination of the other types and may have additional layers.
Compound-complex guilt leaves sufferers literally feeling guilty for feeling guilty. Though this may border on a genuine clinical disorder, it is also to some degree normal (eg, due to cultural taboos and norms) for people to feel culpable for not being able to “move on” or “forgive themselves” as quickly as others may want them to. Buddhists call this tendency the “second arrow effect.” The first arrow is the feeling of guilt (or other painful feeling) that strikes the individual, but the second arrow is the one he or she drives in afterward by thinking it is wrong or weak to even have the feeling.
Patients who suffer from this type of guilt blame themselves for the conundrum they are in and feel even worse. This is not unlike the vortex of unresolved and complicated grief.
Those who suffer from compound-complex guilt may layer the primary guilt with additional guilt for feeling weak, for needing help, or for asking for help. Especially in the culture of the military, the fear of stigma when asking for help (especially with mental health) is still quite strong. Therefore, chaplains can serve as a less threatening entry point for the veteran needing multiple professionals involved in his or her care.
NONJUDGMENTAL LISTENING
Nonjudgmental listening is essential to get at the source or sources of guilt, regardless of the type, in order to allow the wounds to air out and begin healing. Many veterans suffering from guilt may need intensive pharmacologic and cognitive therapy to fully recover, and care from a chaplain is not a substitute for psychiatric evaluation and treatment, especially if there is a risk of suicide.
However, chaplains may be able to help with the “deep work” of spiritual healing that is part of veterans’ overall recovery. This is true not only because chaplains are especially trained to do this, but also because they are the team members most likely to have uniquely spiritual language to speak to the condition. The language of confession, absolution, repentance, redemption, atonement, and forgiveness is language of the spiritual realm.
In addition, chaplains’ freedom from hourly billing concerns and their often less formalized interactions with patients may help to build trust. Well-trained chaplains, who are often quite gifted at creating an atmosphere of reverence and safety (sanctuary) in the most unlikely situations, are well suited to help the interdisciplinary team treat this vulnerable patient population.
SUGGESTED READING
For more insights into the role of chaplains on the interdisciplinary healthcare team, we recommend the following book: Cadge W. Paging God: Religion in the Halls of Medicine. Chicago, IL: University of Chicago Press; 2012.
- Kopacz MS, Rasmussen KA, Searle RF, Wozniak BM, Titus CE. Veterans, guilt, and suicide risk: chaplains can help. Cleve Clin J Med 2016; 83:101–105.
- Life after death: Act one—guilty as not charged. Darin Strauss. This American Life. www.thisamericanlife.org. Episode 359. Aired July 18, 2008. www.thisamericanlife.org/radio-archives/episode/359/life-after-death?act=1#play. Accessed December 10, 2015.
- Hickling EJ, Blanchard EB. Overcoming the Trauma of Your Motor Vehicle Accident: A Cognitive-behavioral Treatment Program. New York, NY: Oxford University Press; 2006.
- Hickling EJ. Transforming Tragedy: Finding Growth Following Life’s Traumas. North Charleston, SC: CreateSpace; 2012.
- Kopacz MS, Rasmussen KA, Searle RF, Wozniak BM, Titus CE. Veterans, guilt, and suicide risk: chaplains can help. Cleve Clin J Med 2016; 83:101–105.
- Life after death: Act one—guilty as not charged. Darin Strauss. This American Life. www.thisamericanlife.org. Episode 359. Aired July 18, 2008. www.thisamericanlife.org/radio-archives/episode/359/life-after-death?act=1#play. Accessed December 10, 2015.
- Hickling EJ, Blanchard EB. Overcoming the Trauma of Your Motor Vehicle Accident: A Cognitive-behavioral Treatment Program. New York, NY: Oxford University Press; 2006.
- Hickling EJ. Transforming Tragedy: Finding Growth Following Life’s Traumas. North Charleston, SC: CreateSpace; 2012.
Can patients opt to turn off implantable cardioverter-defibrillators near the end of life?
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
Yes. Although implantable cardioverter-defibrillators (ICDs) prevent sudden cardiac death in patients with advanced heart failure, their benefit in terminally ill patients is small.1 Furthermore, the shocks they deliver at the end of life can cause distress. Therefore, it is reasonable to consider ICD deactivation if the patient or family wishes.
A DIFFICULT DECISION
End-of-life decisions place significant emotional burdens on patients, their families, and their healthcare providers and can have social and legal consequences.
Turning off an ICD is an especially difficult decision, considering that these devices protect against sudden cardiac death and fatal arrhythmias. Also, patients and their representatives may find it more difficult to withdraw from active care than to forgo further interventions (more on this below), and they may misunderstand discussions about ICD deactivation, perceiving them as the beginning of abandonment.
ICD DEACTIVATION IS OFTEN DONE HAPHAZARDLY OR NOT AT ALL
Many healthcare providers are not trained in or comfortable with discussing end-of-life issues, and many hospitals and hospice programs lack policies and protocols for managing implanted devices at the end of life. Consequently, ICD management at the end of life varies among providers and tends to be suboptimal.2
In a report of a survey in 414 hospice facilities, 97% of facilities reported that they admitted patients with ICDs, but only 10% had a policy on device deactivation.3
In a survey of 47 European medical centers, only 4% said they addressed ICD deactivation with their patients.4
A study of 125 patients with ICDs who had died found that 52% had do-not-resuscitate orders. Nevertheless, in 100 patients the ICD had remained active in the last 24 hours of their life, and 31 of these patients had received shocks during their last 24 hours.5
In a survey of next of kin of patients with ICDs who had died of any cause,6 in only 27 of 100 cases had the clinician discussed ICD deactivation, and about three-fourths of these discussions had occurred during the last few days of life. Twenty-seven patients had received ICD discharges in the last month of life, and 8% had received a discharge during the final minutes.
TRAINING AND PROTOCOLS ARE NEEDED
Healthcare professionals need education about device deactivation at the end of life so that they are comfortable communicating with patients and families about this critical issue. To this end, several cardiac and palliative care societies have jointly released an expert statement on managing ICDs and other implantable devices in end-of-life situations.7
Many providers harbor a misunderstanding of the difference between withholding a device and withdrawing (or turning off) a device that is already implanted.2 Some mistakenly believe they would be committing a crime by deactivating an implanted life-sustaining device. Legally and ethically, there is no difference between withholding a device and withdrawing a device. Legally, carrying out a request to withdraw life-sustaining treatment is neither physician-assisted suicide nor euthanasia.
DISCUSSION SHOULD BEGIN EARLY AND SHOULD BE ONGOING
The discussion of ICD deactivation should begin before the device is implanted and should continue as the patient’s health status changes. In a survey, 40% of patients said they felt that ICD deactivation should be discussed before the device is implanted, and only 5% felt that this discussion should be undertaken in the last days of life.8
At the least, it is important to identify patients with ICDs on admission to hospice and to have policies in place that ensure adequate patient education to make an informed decision about ICD deactivation at the end of life.
The topic should be discussed when goals of care change and when do-not-resuscitate status is addressed, and also when advanced directives are being acknowledged. If the patient or his or her legal representative wishes to keep the ICD turned on, that wish should be respected. The essence of a discussion is not to impose the providers’ choice on the patient, but to help the patient make the right decision for himself or herself. Of note, patients entering hospice do not have to have do-not-resuscitate status.
We believe that device management in end-of-life circumstances should be part of the discussion of the goals of care. Accordingly, healthcare providers need to be familiar with device management and to have a higher comfort level in addressing such sensitive topics with patients facing the end of life, as well as with their families.
It is also advisable to apply protocols within hospice services to address ICD management options for the patient and the legal representative. An early decision regarding end-of-life deactivation will help patients avoid distressing ICD discharges and the related emotional distress in their last moments.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
- Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079.
- Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc 2010; 85:981–990.
- Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med 2010; 152:296–299.
- Marinskis G, van Erven L; EHRA Scientific Initiatives Committtee. Deactivation of implanted cardioverter-defibrillators at the end of life: results of the EHRA survey. Europace 2010; 12:1176–1177.
- Kinch Westerdahl A, Sjoblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation 2014; 129:422–429.
- Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med 2004; 141:835–838.
- Lampert R, Hayes DL, Annas GJ, et al; American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm 2010; 7:1008–1026.
- Raphael CE, Koa-Wing M, Stain N, Wright I, Francis DP, Kanagaratnam P. Implantable cardioverter-defibrillator recipient attitudes towards device activation: how much do patients want to know? Pacing Clin Electrophysiol 2011; 34:1628–1633.
Common neurologic emergencies for nonneurologists: When minutes count
Neurologic emergencies such as acute stroke, status epilepticus, subarachnoid hemorrhage, neuromuscular weakness, and spinal cord injury affect millions of Americans yearly.1,2 These conditions can be difficult to diagnose, and delays in recognition and treatment can have devastating results. Consequently, it is important for nonneurologists to be able to quickly recognize these conditions and initiate timely management, often while awaiting neurologic consultation.
Here, we review how to recognize and treat these common, serious conditions.
ACUTE ISCHEMIC STROKE: TIME IS OF THE ESSENCE
Stroke is the fourth leading cause of death in the United States and is one of the most common causes of disability worldwide.3–5 About 85% of strokes are ischemic, resulting from diminished vascular supply to the brain. Symptoms such as facial droop, unilateral weakness or numbness, aphasia, gaze deviation, and unsteadiness of gait may be seen. Time is of the essence, as all currently available interventions are safe and effective only within defined time windows.
Diagnosis and assessment
When acute ischemic stroke is suspected, the clinical history, time of onset, and basic neurologic examination should be obtained quickly.
The National Institutes of Health (NIH) stroke scale is an objective marker for assessing stroke severity as well as evolution of disease and should be obtained in all stroke patients. Scores range from 0 (best) to 42 (worst) (www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf).
Time of onset of symptoms is essential to determine, since it guides eligibility for acute therapies. Clinicians should ascertain the last time the patient was seen to be neurologically well in order to estimate this time window as closely as possible.
Laboratory tests should include a fingerstick blood glucose measurement, coagulation studies, complete blood cell count, and basic metabolic profile.
Computed tomography (CT) of the head without contrast should be obtained immediately to exclude acute hemorrhage and any alternative diagnoses that could explain the patient’s symptoms. Acute brain ischemia is often not apparent on CT during the first few hours of injury. Therefore, a patient presenting with new focal neurologic deficits and an unremarkable result on CT of the head should be treated as having had an acute ischemic stroke, and interventional therapies should be considered.
Stroke mimics should be considered and treated, as appropriate (Table 1).
Acute management of ischemic stroke
Acute treatment should not be delayed by obtaining chest radiography, inserting a Foley catheter, or obtaining an electrocardiogram. The longer the time that elapses before treatment, the worse the functional outcome, underscoring the need for rapid decision-making.6–8
Lowering the head of the bed may provide benefit by promoting blood flow to ischemic brain tissue.9 However, this should not be done in patients with significantly elevated intracerebral pressure and concern for herniation.
Permissive hypertension (antihypertensive treatment only for blood pressure greater than 220/110 mm Hg) should be allowed per national guidelines to provide adequate perfusion to brain areas at risk of injury.10
Tissue plasminogen activator. Patients with ischemic stroke who present within 3 hours of symptom onset should be considered for intravenous administration of tissue plasminogen activator (tPA), a safe and effective therapy with nearly 2 decades of evidence to support its use.10 The treating physician should carefully review the risks and benefits of this therapy.
To receive tPA, the patient must have all of the following:
- Clinical diagnosis of ischemic stroke with measurable neurologic deficit
- Onset of symptoms within the past 3 hours
- Age 18 or older.
The patient must not have any of the following:
- Significant stroke within the past 3 months
- Severe traumatic head injury within the past 3 months
- History of significant intracerebral hemorrhage
- Previously ruptured arteriovenous malformation or intracranial aneurysm
- Central nervous system neoplasm
- Arterial puncture at a noncompressible site within the past 7 days
- Evidence of hemorrhage on CT of the head
- Evidence of ischemia in greater than 33% of the cerebral hemisphere on head CT
- History and symptoms strongly suggesting subarachnoid hemorrhage
- Persistent hypertension (systolic pressure ≥ 185 mm Hg or diastolic pressure ≥ 110 mm Hg)
- Evidence of acute significant bleeding (external or internal)
- Hypoglycemia—ie, serum glucose less than 50 mg/dL (< 2.8 mmol/L)
- Thrombocytopenia (platelet count < 100 × 109/L)
- Significant coagulopathy (international normalized ratio > 1.7, prothrombin time > 15 seconds, or abnormally elevated activated partial thromboplastin time)
- Current use of a factor Xa inhibitor or direct thrombin inhibitor.
Relative contraindications:
- Minor or rapidly resolving symptoms
- Major surgery or trauma within the past 14 days
- Gastrointestinal or urinary tract bleeding within the past 21 days
- Myocardial infarction in the past 3 months
- Unruptured intracranial aneurysm
- Seizure occurring at stroke onset
- Pregnancy.
If these criteria are satisfied, tPA should be given at a dose of 0.9 mg/kg intravenously over 60 minutes. Ten percent of the dose should be given as an initial bolus, followed by a constant infusion of the remaining 90% over 1 hour.
If tPA is given, the blood pressure must be kept lower than 185/110 mm Hg to minimize the risk of symptomatic intracerebral hemorrhage.
A subset of patients may benefit from receiving intravenous tPA between 3 and 4.5 hours after the onset of stroke symptoms. These include patients who are no more than 80 years old, who have not recently used oral anticoagulants, who do not have severe neurologic injury (ie, do not have NIH Stroke Scale scores > 25), and who do not have diabetes mellitus or a history of ischemic stroke.11 Although many hospitals have such a protocol for tPA up to 4.5 hours after the onset of stroke symptoms, this time window is not currently approved by the US Food and Drug Administration.
Intra-arterial therapy. Based on recent trials, some patients may benefit further from intra-arterial thrombolysis or mechanical thrombectomy, both delivered during catheter-based cerebral angiography, independent of intravenous tPA administration.12,13 These patients should be evaluated on a case-by-case basis by a neurologist and neurointerventional team. Time windows for these treatments generally extend to 6 hours from stroke onset and perhaps even longer in some situations (eg, basilar artery occlusion).
An antiplatelet agent should be started quickly in all stroke patients who do not receive tPA. Patients who receive tPA can begin receiving an antiplatelet agent 24 hours afterward.
Unfractionated heparin. There is no evidence to support the use of unfractionated heparin in most cases of acute ischemic stroke.10
Glucose control (in the range of 140–180 mg/dL) and fever control remain essential elements of post-acute stroke care to provide additional protection to the damaged brain.
For ischemic stroke due to atrial fibrillation
In ischemic stroke due to atrial fibrillation, early anticoagulation should be considered, based on the CHA2DS2-VASC risk of ischemic stroke vs the HAS-BLED risk of hemorrhage (calculators available at www.mdcalc.com).
In general, anticoagulation may be withheld during the first 72 hours while further stroke workup and evaluation of extent of injury are carried out, as there is an increased risk of hemorrhagic transformation of the ischemic stroke. Often, anticoagulation is resumed at a full dose between 72 hours and 2 weeks of the ischemic stroke.
ACUTE HEMORRHAGIC STROKE: BLOOD PRESSURE, COAGULATION
Approximately 15% of strokes are caused by intracerebral hemorrhage, which can be detected with noncontrast head CT with a sensitivity of 98.6% within 6 hours of the onset of bleeding.14 A common underlying cause of intracerebral hemorrhage is chronic poorly controlled hypertension, causing rupture of damaged (or “lipohyalinized”) vessels with resultant blood extravasation into the brain parenchyma. Other causes are less common (Table 2).
Treatment of acute hemorrhagic stroke
Acute treatment of intracerebral hemorrhage includes blood pressure control, reversal of underlying coagulopathy or anticoagulation, and sometimes intracranial pressure control. There is little role for surgery in most cases, based on findings of randomized trials.15
Blood pressure control. Many studies have investigated optimal blood pressure goals in acute intracerebral hemorrhage. Recent data suggest that early aggressive therapy, targeting a systolic blood pressure goal less than 140 mm Hg within the first hour, is safe and can lead to better functional outcomes than a more conservative blood-pressure-lowering target.16 Rapid-onset, short-acting antihypertensive agents in intravenous form, such as nicardipine and labetalol, are frequently used. Of note, this treatment strategy for hemorrhagic stroke is in direct contrast to the treatment of ischemic stroke, in which permissive hypertension (blood pressure goal < 220/110 mm Hg) is often pursued.
Reversal of any coagulation abnormalities should be done quickly in intracranial hemorrhage. Warfarin use has been shown to be a strong independent predictor of intracranial hemorrhage expansion, which increases the risk of death.17,18
Increasingly, agents other than vitamin K or fresh-frozen plasma are being used to rapidly reverse anticoagulation, including prothrombin complex concentrate (available in three- and four-factor preparations) and recombinant factor VIIa. While four-factor prothrombin complex concentrate and recombinant factor VIIa have been shown to be more efficacious than fresh-frozen plasma, there are limited data directly comparing these newer reversal agents against each other.19 The use of these medications is limited by availability and practitioner familiarity.20–22
Reversing anticoagulation due to target-specific oral anticoagulants. The acute management of intracranial hemorrhage in patients taking the new target-specific oral anticoagulants (eg, dabigatran, apixaban, rivaroxaban, edoxaban) remains challenging. Laboratory tests such as factor Xa levels are not readily available in many institutions and do not provide results in a timely fashion, and in the interim, acute hemorrhage and clinical deterioration may occur. Management strategies involve giving fresh-frozen plasma, prothrombin complex concentrate, and consideration of hemodialysis.23 Dabigatran reversal with idarucizumab has recently been shown to have efficacy.24
Vigilance for elevated intracranial pressure. Intracranial hemorrhage can occasionally cause elevated intracranial pressure, which should be treated rapidly. Any acute decline in mental status in a patient with intracranial hemorrhage requires emergency imaging to evaluate for expansion of hemorrhage.
SUBARACHNOID HEMORRHAGE
The sudden onset of a “thunderclap” headache (often described by patients as “the worst headache of my life”) suggests subarachnoid hemorrhage.
In contrast to intracranial hemorrhage, in subarachnoid hemorrhage blood collects mainly in the cerebral spinal fluid-containing spaces surrounding the brain, leading to a higher incidence of hydrocephalus from impaired drainage of cerebrospinal fluid. Nontraumatic subarachnoid hemorrhage is most often caused by rupture of an intracranial aneurysm, which can be a devastating event, with death rates approaching 50%.25
Diagnosis of subarachnoid hemorrhage
Noncontrast CT of the head is the main modality for diagnosing subarachnoid hemorrhage. Blood within the subarachnoid space is demonstrable in 92% of cases if CT is performed within the first 24 hours of hemorrhage, with an initial sensitivity of about 95% within the first 6 hours of onset.14,26,27 The longer CT is delayed, the lower the sensitivity.
Some studies suggest that a protocol of CT followed by CT angiography can safely exclude aneurysmal subarachnoid hemorrhage and obviate the need for lumbar puncture. However, further research is required to validate this approach.28
Lumbar puncture. If clinical suspicion of subarachnoid hemorrhage remains strong even though initial CT is negative, lumbar puncture must be performed for cerebrospinal fluid analysis.29 Xanthochromia (a yellowish pigmentation of the cerebrospinal fluid due to the degeneration of blood products that occurs within 8 to 12 hours of bleeding) should raise the alarm for subarachnoid hemorrhage; this sign may be present up to 4 weeks after the bleeding event.30
If lumbar puncture is contraindicated, then aneurysmal subarachnoid hemorrhage has not been ruled out, and further neurologic consultation should be pursued.
Management of subarachnoid hemorrhage
Early management of blood pressure for a ruptured intracranial aneurysm follows strategies similar to those for intracranial hemorrhage. Further investigation is rapidly directed toward an underlying vascular malformation, with intracranial vessel imaging such as CT angiography, magnetic resonance angiography, or the gold standard test—catheter-based cerebral angiography.
Aneurysms are treated (or “secured”) either by surgical clipping or by endovascular coiling. Endovascular coiling is preferable in cases in which both can be safely attempted.31 If the facility lacks the resources to do these procedures, the patient should be referred to a nearby tertiary care center.
INTRACRANIAL HYPERTENSION: DANGER OF BRAIN HERNIATION
A number of conditions can cause an acute intracranial pressure elevation. The danger of brain herniation requires that therapies be implemented rapidly to prevent catastrophic neurologic injury. In many situations, nonneurologists are the first responders and therefore should be familiar with basic intracranial pressure management.
Initial symptoms of acute rise in intracranial pressure
As intracranial pressure rises, pressure is typically equally distributed throughout the cranial vault, leading to dysfunction of the ascending reticular activating system, which clinically manifests as the inability to stay alert despite varying degrees of noxious stimulation. Progressive cranial neuropathies (often starting with pupillary abnormalities) and coma are often seen in this setting as the upper brainstem begins to be compressed.
Initial assessment and treatment of elevated intracranial pressure
Noncontrast CT of the head is often obtained immediately when acutely elevated intracranial pressure is suspected. If clinical examination and radiographic findings are consistent with intracranial hypertension, prompt measures can be started at the bedside.
Elevate the head of the bed to 30 degrees to promote venous drainage and reduce intracranial pressure. (In contrast, most other hemodynamically unstable patients are placed flat or in the Trendelenburg position.)
Intubation should be done quickly in cases of airway compromise, and hyperventilation should be started with a goal Paco2 of 30 to 35 mm Hg. This hypocarbic strategy promotes cerebral vasoconstriction and a transient decrease in intracranial pressure.
Hyperosmolar therapy allows for transient intracranial volume decompression and is the mainstay of emergency medical treatment of intracranial hypertension. Mannitol is a hyperosmolar polysaccharide that promotes osmotic diuresis and removes excessive cerebral water. In the acute setting, it can be given as an intravenous bolus of 1 to 2 g/kg through a peripheral intravenous line, followed by a bolus every 4 to 6 hours. Hypotension can occur after diuresis, and renal function should be closely monitored since frequent mannitol use can promote acute tubular necrosis. In patients who are anuric, the medication is typically not used.
Hypertonic saline (typically 3% sodium chloride, though different concentrations are available) is an alternative that helps draw interstitial fluid into the intravascular space, decreasing cerebral edema and maintaining hemodynamic stability. Relative contraindications include congestive heart failure or renal failure leading to pulmonary edema from volume overload. Hypertonic saline can be given as a bolus or a constant infusion. Some institutions have rapid access to 23.4% saline, which can be given as a 30-mL bolus but typically requires a central venous catheter for rapid infusion.
Comatose patients with radiographic findings of hydrocephalus, epidural or subdural hematoma, or mass effect with midline shift warrant prompt neurosurgical consultation for further surgical measures of intracranial pressure control and monitoring.
The ‘blown’ pupil
The physician should be concerned about elevated intracranial pressure if a patient has mydriasis, ie, an abnormally dilated (“blown”) pupil, which is a worrisome sign in the setting of true intracranial hypertension. However, many different processes can cause mydriasis and should be kept in mind when evaluating this finding (Table 3).32 If radiographic findings do not suggest elevated intracranial pressure, further workup into these other processes should be pursued.
STATUS EPILEPTICUS: SEIZURE CONTROL IS IMPORTANT
A continuous unremitting seizure lasting longer than 5 minutes or recurrent seizure activity in a patient who does not regain consciousness between seizures should be treated as status epilepticus. All seizure types carry the risk of progressing to status epilepticus, and responsiveness to antiepileptic drug therapy is inversely related to the duration of seizures. It is imperative that seizure activity be treated early and aggressively to prevent recalcitrant seizure activity, neuronal damage, and progression to status epilepticus.33
Once the ABCs of emergency stabilization have been performed (ie, airway, breathing, circulation), antiepileptic drug therapy should start immediately using established algorithms (Figure 1).34–36 During the course of treatment, the reliability of the neurologic examination may be limited due to medication effects or continued status epilepticus, making continuous video electroencephalographic monitoring often necessary to guide further therapy in patients who are not rapidly recovering.34–38
Once status epilepticus has resolved, further investigation into the underlying cause should be pursued quickly, especially in patients without a previous diagnosis of epilepsy. Head CT with contrast or magnetic resonance imaging can be used to look for any structural abnormality that may explain seizures. Basic laboratory tests including toxicology screening can identify a common trigger such as hypoglycemia or stimulant use. Fever or other possible signs of meningitis should be investigated further with cerebrospinal fluid analysis.
SPINAL CORD INJURY
Acute spinal cord injury can lead to substantial long-term neurologic impairment and should be suspected in any patient presenting with focal motor loss, sensory loss, or both with sparing of the cranial nerves and mental status. Causes of injury include compression (traumatic or nontraumatic) and inflammatory and noninflammatory myelopathies.
The location of the injury can be inferred by analyzing the symptoms, which can point to the cord level and indicate whether the anterior or posterior of the cord is involved. Anterior cord injury tends to affect the descending corticospinal and pyramidal tracts, resulting in motor deficits and weakness. Posterior cord injury involves the dorsal columns, leading to deficits of vibration sensation and proprioception. High cervical cord injuries tend to involve varying degrees of quadriparesis, sensory loss, and sometimes respiratory compromise. A clinical history of bilateral lower-extremity weakness, a “band-like” sensory complaint around the lower chest or abdomen, or both, can suggest thoracic cord involvement. Symptoms isolated to one or both lower extremities along with lower back pain and bowel or bladder involvement may point to injury of the lumbosacral cord.
Basic management of spinal cord injury includes decompression of the bladder and initial protection against further injury with a stabilizing collar or brace.
Magnetic resonance imaging with and without contrast is the ideal study to evaluate injuries to the spinal cord itself. While CT is helpful in identifying bony disease of the spinal column (eg, evaluating traumatic fractures), it is not helpful in viewing intrinsic cord pathology.
Traumatic myelopathy
Traumatic spinal cord injury is usually suggested by the clinical history and confirmed with CT. In this setting, early consultation with a neurosurgeon is required to prevent permanent cord injury.
Guidelines suggest maintaining a mean arterial pressure greater than 85 to 90 mm Hg for the first 7 days after traumatic spinal cord injury, a particular problem in the setting of hemodynamic instability, which can accompany lesions above the midthoracic level.39,40
Patients with vertebral body misalignment should be placed in an appropriate stabilizing collar or brace until a medically trained professional deems it appropriate to discontinue the device, or until surgical stabilization is performed.
Methylprednisone is a controversial intervention for acute spinal cord trauma, lacking clear benefit in meta-analyses.41
Nontraumatic compressive myelopathy
Patients with nontraumatic compressive myelopathy tend to present with varying degrees of back pain and worsening sensorimotor function. The differential diagnosis includes epidural abscesses, hematoma, metastatic neoplasm, and osteophyte compression (Table 4). The clinical history helps to guide therapy and should involve assessment for previous spinal column injury, immunocompromised state, travel history (which provides information on risks of exposure to a variety of diseases, including infections), and constitutional symptoms such as fever and weight loss.
Epidural abscess can have devastating results if missed. Red flags such as recent illness, intravenous drug use, focal back pain, fever, worsening numbness or weakness, and bowel or bladder incontinence should raise suspicion of this disorder. Emergency magnetic resonance imaging is required to diagnose this condition, and treatment involves urgent administration of antibiotics and consideration of surgical drainage.
Noncompressive myelopathies
There are numerous causes of noncompressive spinal cord injury (Table 4), and the etiology may be inflammatory (eg, “myelitis”) or noninflammatory. The diagnostic workup may require both magnetic resonance imaging and cerebrospinal fluid analysis. Acute disease-targeted therapy is rarely indicated and can be deferred until a full diagnostic workup has been completed.
NEUROMUSCULAR DISEASE: IS VENTILATION NEEDED?
Diseases involving the motor components of the peripheral nervous system (Table 5) share the common risk of causing ventilatory failure due to weakness of the diaphragm, intercostal muscles, and upper-airway muscles. Clinicians need to be aware of this risk and view these disorders as neurologic emergencies.
Determining when these patients require mechanical intubation is a challenge. Serial measurements of maximum inspiratory force and vital capacity are important and can be accomplished quickly at the bedside by a respiratory therapist. A maximum inspiratory force less than –30 cm H2O or a vital capacity less than 20 mL/kg, or both, are worrisome markers that raise concern for impending ventilatory failure. Serial measurements can detect changes in these values that might indicate the need for elective intubation. In any patient presenting with weakness of the limbs, these measurements are an important step in the initial evaluation.
Myasthenic crisis
Myasthenia gravis is caused by autoantibodies directed against postsynaptic acetylcholine receptors. Patients demonstrate muscle weakness, usually in a proximal pattern, with fatigue, respiratory distress, nasal speech, ophthalmoparesis, and dysphagia. Exacerbations can occur as a response to recent infection, surgery, or medications such as neuromuscular blocking agents or aminoglycosides.
Myasthenic crisis, while uncommon, is a life-threatening emergency characterized by bulbar or respiratory failure secondary to muscle weakness. It can occur in patients already diagnosed with myasthenia gravis or may be the initial manifestation of the disease.42–49 Intubation and mechanical ventilation are frequently required. Postoperative myasthenic patients in whom extubation has been delayed more than 24 hours should be considered in crisis.45
The diagnosis of myasthenia gravis can be made by serum autoantibody testing, electromyography, and nerve conduction studies (with repetitive stimulation) or administration of edrophonium in patients with obvious ptosis.
The mainstay of therapy for myasthenic crisis is either intravenous immunoglobulin at a dose of 2 g/kg over 2 to 5 days or plasmapheresis (5–7 exchanges over 7–14 days). Corticosteroids are not recommended in myasthenic crisis in patients who are not intubated, as they can potentiate an initial worsening of crisis. Once the patient begins to show clinical improvement, outpatient pyridostigmine and immunosuppressive medications can be resumed at a low dose and titrated as tolerated.
Acute inflammatory demyelinating polyneuropathy (Guillain-Barré syndrome)
Acute inflammatory demyelinating polyneuropathy is an autoimmune disorder involving autoantibodies against axons or myelin in the peripheral nervous system.
This disease should be suspected in a patient who is developing worsening muscle weakness (usually with areflexia) over the course of days to weeks. Occasionally, a recent diarrheal or other systemic infectious trigger can be identified. Blood pressure instability and cardiac arrhythmia can also be seen due to autonomic nerve involvement. Although classically described as an “ascending paralysis,” other variants of this disease have distinct clinical presentations (eg, the descending paralysis, ataxia, areflexia, ophthalmoparesis of the Miller Fisher syndrome).
Acute inflammatory demyelinating polyneuropathy is diagnosed by electromyography and nerve conduction studies. A cerebrospinal fluid profile demonstrating elevated protein and few white blood cells is typical.
Treatment, as in myasthenic crisis, involves intravenous immunoglobulin or plasmapheresis. Corticosteroids are ineffective. Anticipation of ventilatory failure and expectant intubation is essential, given the progressive nature of the disorder.50
- Pitts SR, Niska RW, Xu J, Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008; 7:1–38.
- McMullan JT, Knight WA, Clark JF, Beyette FR, Pancioli A. Time-critical neurological emergencies: the unfulfilled role for point-of-care testing. Int J Emerg Med 2010; 3:127–131.
- Centers for Disease Control and Prevention (CDC). Prevalence of stroke: United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2012; 61:379–382.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2095–2128.
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1,160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2163–2196.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med 1995; 333:1581–1587.
- Hacke W, Donnan G, Fieschi C, et al; ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363:768–774.
- Saver JL, Fonarrow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013; 309:2480–2488.
- Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology 2005; 64:1354–1357.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–1329.
- Berkhemer OA, Fransen PSS, Beumer D, et al; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Eng J Med 2015; 372:11–20.
- Campbell BC, Mitchell PJ, Kleinig TJ, et al; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372:1009–1018.
- Backes D, Rinkel GJ, Kemperman H, Linn FH, Vergouwen MD. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke 2012; 43:2115–2119.
- Mendelow AD, Gregson BA, Fernandes HM, et al; STICH investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005; 365: 387–397.
- Anderson CS, Helley E, Huang Y, et al; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368:2355–2365.
- Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004; 63:1059–1064.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Woo CH, Patel N, Conell C, et al. Rapid warfarin reversal in the setting of intracranial hemorrhage: a comparison of plasma, recombinant activated factor VII, and prothrombin complex concentrate. World Neurosurg 2014; 81:110–115.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 2007; 38:2001–2023.
- Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke 2006, 37:151–155.
- Chapman SA, Irwin ED, Beal AL, Kulinski NM, Hutson KE, Thorson MA. Prothrombin complex concentrate versus standard therapies for INR reversal in trauma patients receiving warfarin. Ann Pharmacother 2011; 45:869–875.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013; 80:443–451.
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015; 373:511-520.
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994; 25:1342–1347.
- Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg 1990; 73:18–36.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 2011; 343:d4277.
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med 2010; 17:444–451.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:1711–1737.
- Vermuelen M, Hasan D, Blijenberg BG, Hijdra A, van Gijn J. Xanthochromia after subarachnoid haemorrhage needs no revisitation. J Neurol Neurosurg Psychiatry 1989; 52:826–828.
- Molyneaux AJ, Kerr RS, Yu LM, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid hemorrhage trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366:809–817.
- Caglayan HZ, Colpak IA, Kansu T. A diagnostic challenge: dilated pupil. Curr Opin Ophthalmol 2013; 24:550–557.
- Brophy GM, Bell R, Claassen J, et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012; 17:3–23.
- Chang CW, Bleck TP. Status epilepticus. Neurol Clin 1995; 13:529–548.
- Treiman DM. Generalized convulsive status epilepticus in the adult. Epilepsia 1993; 34(suppl 1):S2–S11.
- Leppick IE. Status epilepticus: the next decade. Neurology 1990; 40(suppl 2):4–9.
- Aranda A, Foucart G, Ducassé JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia 2010; 51:2159–2167.
- DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 1998; 39:833–840.
- Casha S, Christie S. A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma 2011; 28:1479–1495.
- Walters BC, Hadley MN, Hurlbert RJ, et al; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013; 60(suppl 1):82–91.
- Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2013; 72(suppl 2):93–105.
- Cohen MS, Younger D. Aspects of the natural history of myasthenia gravis: crisis and death. Ann NY Acad Sci 1981; 377:670–677.
- Belack RS, Sanders DB. On the concept of myasthenic crisis. J Clin Neuromuscul Dis 2002; 4:40–42.
- Chaudhuri A, Behan PO. Myasthenic crisis. QJM 2009; 102:97–107.
- Mayer SA. Intensive care of the myasthenic patient. Neurology 1997; 48(suppl 5):70S–75S.
- Jani-Acsadi A, Lisak RP. Myasthenic crisis: guidelines for prevention and treatment. J Neurol Sci 2007; 261:127–133.
- Bershad EM, Feen ES, Suarez JI. Myasthenia gravis crisis. South Med J 2008; 101:63–69.
- Ahmed S, Kirmani JF, Janjua N, et al. An update on myasthenic crisis. Curr Treat Options Neurol 2005; 7:129–141.
- Godoy DA, Vaz de Mello LJ, Masotti L, Napoli MD. The myasthenic patient in crisis: an update of the management in neurointensive care unit. Arq Neuropsiquiatr 2013; 71:627–639.
- Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome: Arch Neurol 2005; 62:1194–1198.
Neurologic emergencies such as acute stroke, status epilepticus, subarachnoid hemorrhage, neuromuscular weakness, and spinal cord injury affect millions of Americans yearly.1,2 These conditions can be difficult to diagnose, and delays in recognition and treatment can have devastating results. Consequently, it is important for nonneurologists to be able to quickly recognize these conditions and initiate timely management, often while awaiting neurologic consultation.
Here, we review how to recognize and treat these common, serious conditions.
ACUTE ISCHEMIC STROKE: TIME IS OF THE ESSENCE
Stroke is the fourth leading cause of death in the United States and is one of the most common causes of disability worldwide.3–5 About 85% of strokes are ischemic, resulting from diminished vascular supply to the brain. Symptoms such as facial droop, unilateral weakness or numbness, aphasia, gaze deviation, and unsteadiness of gait may be seen. Time is of the essence, as all currently available interventions are safe and effective only within defined time windows.
Diagnosis and assessment
When acute ischemic stroke is suspected, the clinical history, time of onset, and basic neurologic examination should be obtained quickly.
The National Institutes of Health (NIH) stroke scale is an objective marker for assessing stroke severity as well as evolution of disease and should be obtained in all stroke patients. Scores range from 0 (best) to 42 (worst) (www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf).
Time of onset of symptoms is essential to determine, since it guides eligibility for acute therapies. Clinicians should ascertain the last time the patient was seen to be neurologically well in order to estimate this time window as closely as possible.
Laboratory tests should include a fingerstick blood glucose measurement, coagulation studies, complete blood cell count, and basic metabolic profile.
Computed tomography (CT) of the head without contrast should be obtained immediately to exclude acute hemorrhage and any alternative diagnoses that could explain the patient’s symptoms. Acute brain ischemia is often not apparent on CT during the first few hours of injury. Therefore, a patient presenting with new focal neurologic deficits and an unremarkable result on CT of the head should be treated as having had an acute ischemic stroke, and interventional therapies should be considered.
Stroke mimics should be considered and treated, as appropriate (Table 1).
Acute management of ischemic stroke
Acute treatment should not be delayed by obtaining chest radiography, inserting a Foley catheter, or obtaining an electrocardiogram. The longer the time that elapses before treatment, the worse the functional outcome, underscoring the need for rapid decision-making.6–8
Lowering the head of the bed may provide benefit by promoting blood flow to ischemic brain tissue.9 However, this should not be done in patients with significantly elevated intracerebral pressure and concern for herniation.
Permissive hypertension (antihypertensive treatment only for blood pressure greater than 220/110 mm Hg) should be allowed per national guidelines to provide adequate perfusion to brain areas at risk of injury.10
Tissue plasminogen activator. Patients with ischemic stroke who present within 3 hours of symptom onset should be considered for intravenous administration of tissue plasminogen activator (tPA), a safe and effective therapy with nearly 2 decades of evidence to support its use.10 The treating physician should carefully review the risks and benefits of this therapy.
To receive tPA, the patient must have all of the following:
- Clinical diagnosis of ischemic stroke with measurable neurologic deficit
- Onset of symptoms within the past 3 hours
- Age 18 or older.
The patient must not have any of the following:
- Significant stroke within the past 3 months
- Severe traumatic head injury within the past 3 months
- History of significant intracerebral hemorrhage
- Previously ruptured arteriovenous malformation or intracranial aneurysm
- Central nervous system neoplasm
- Arterial puncture at a noncompressible site within the past 7 days
- Evidence of hemorrhage on CT of the head
- Evidence of ischemia in greater than 33% of the cerebral hemisphere on head CT
- History and symptoms strongly suggesting subarachnoid hemorrhage
- Persistent hypertension (systolic pressure ≥ 185 mm Hg or diastolic pressure ≥ 110 mm Hg)
- Evidence of acute significant bleeding (external or internal)
- Hypoglycemia—ie, serum glucose less than 50 mg/dL (< 2.8 mmol/L)
- Thrombocytopenia (platelet count < 100 × 109/L)
- Significant coagulopathy (international normalized ratio > 1.7, prothrombin time > 15 seconds, or abnormally elevated activated partial thromboplastin time)
- Current use of a factor Xa inhibitor or direct thrombin inhibitor.
Relative contraindications:
- Minor or rapidly resolving symptoms
- Major surgery or trauma within the past 14 days
- Gastrointestinal or urinary tract bleeding within the past 21 days
- Myocardial infarction in the past 3 months
- Unruptured intracranial aneurysm
- Seizure occurring at stroke onset
- Pregnancy.
If these criteria are satisfied, tPA should be given at a dose of 0.9 mg/kg intravenously over 60 minutes. Ten percent of the dose should be given as an initial bolus, followed by a constant infusion of the remaining 90% over 1 hour.
If tPA is given, the blood pressure must be kept lower than 185/110 mm Hg to minimize the risk of symptomatic intracerebral hemorrhage.
A subset of patients may benefit from receiving intravenous tPA between 3 and 4.5 hours after the onset of stroke symptoms. These include patients who are no more than 80 years old, who have not recently used oral anticoagulants, who do not have severe neurologic injury (ie, do not have NIH Stroke Scale scores > 25), and who do not have diabetes mellitus or a history of ischemic stroke.11 Although many hospitals have such a protocol for tPA up to 4.5 hours after the onset of stroke symptoms, this time window is not currently approved by the US Food and Drug Administration.
Intra-arterial therapy. Based on recent trials, some patients may benefit further from intra-arterial thrombolysis or mechanical thrombectomy, both delivered during catheter-based cerebral angiography, independent of intravenous tPA administration.12,13 These patients should be evaluated on a case-by-case basis by a neurologist and neurointerventional team. Time windows for these treatments generally extend to 6 hours from stroke onset and perhaps even longer in some situations (eg, basilar artery occlusion).
An antiplatelet agent should be started quickly in all stroke patients who do not receive tPA. Patients who receive tPA can begin receiving an antiplatelet agent 24 hours afterward.
Unfractionated heparin. There is no evidence to support the use of unfractionated heparin in most cases of acute ischemic stroke.10
Glucose control (in the range of 140–180 mg/dL) and fever control remain essential elements of post-acute stroke care to provide additional protection to the damaged brain.
For ischemic stroke due to atrial fibrillation
In ischemic stroke due to atrial fibrillation, early anticoagulation should be considered, based on the CHA2DS2-VASC risk of ischemic stroke vs the HAS-BLED risk of hemorrhage (calculators available at www.mdcalc.com).
In general, anticoagulation may be withheld during the first 72 hours while further stroke workup and evaluation of extent of injury are carried out, as there is an increased risk of hemorrhagic transformation of the ischemic stroke. Often, anticoagulation is resumed at a full dose between 72 hours and 2 weeks of the ischemic stroke.
ACUTE HEMORRHAGIC STROKE: BLOOD PRESSURE, COAGULATION
Approximately 15% of strokes are caused by intracerebral hemorrhage, which can be detected with noncontrast head CT with a sensitivity of 98.6% within 6 hours of the onset of bleeding.14 A common underlying cause of intracerebral hemorrhage is chronic poorly controlled hypertension, causing rupture of damaged (or “lipohyalinized”) vessels with resultant blood extravasation into the brain parenchyma. Other causes are less common (Table 2).
Treatment of acute hemorrhagic stroke
Acute treatment of intracerebral hemorrhage includes blood pressure control, reversal of underlying coagulopathy or anticoagulation, and sometimes intracranial pressure control. There is little role for surgery in most cases, based on findings of randomized trials.15
Blood pressure control. Many studies have investigated optimal blood pressure goals in acute intracerebral hemorrhage. Recent data suggest that early aggressive therapy, targeting a systolic blood pressure goal less than 140 mm Hg within the first hour, is safe and can lead to better functional outcomes than a more conservative blood-pressure-lowering target.16 Rapid-onset, short-acting antihypertensive agents in intravenous form, such as nicardipine and labetalol, are frequently used. Of note, this treatment strategy for hemorrhagic stroke is in direct contrast to the treatment of ischemic stroke, in which permissive hypertension (blood pressure goal < 220/110 mm Hg) is often pursued.
Reversal of any coagulation abnormalities should be done quickly in intracranial hemorrhage. Warfarin use has been shown to be a strong independent predictor of intracranial hemorrhage expansion, which increases the risk of death.17,18
Increasingly, agents other than vitamin K or fresh-frozen plasma are being used to rapidly reverse anticoagulation, including prothrombin complex concentrate (available in three- and four-factor preparations) and recombinant factor VIIa. While four-factor prothrombin complex concentrate and recombinant factor VIIa have been shown to be more efficacious than fresh-frozen plasma, there are limited data directly comparing these newer reversal agents against each other.19 The use of these medications is limited by availability and practitioner familiarity.20–22
Reversing anticoagulation due to target-specific oral anticoagulants. The acute management of intracranial hemorrhage in patients taking the new target-specific oral anticoagulants (eg, dabigatran, apixaban, rivaroxaban, edoxaban) remains challenging. Laboratory tests such as factor Xa levels are not readily available in many institutions and do not provide results in a timely fashion, and in the interim, acute hemorrhage and clinical deterioration may occur. Management strategies involve giving fresh-frozen plasma, prothrombin complex concentrate, and consideration of hemodialysis.23 Dabigatran reversal with idarucizumab has recently been shown to have efficacy.24
Vigilance for elevated intracranial pressure. Intracranial hemorrhage can occasionally cause elevated intracranial pressure, which should be treated rapidly. Any acute decline in mental status in a patient with intracranial hemorrhage requires emergency imaging to evaluate for expansion of hemorrhage.
SUBARACHNOID HEMORRHAGE
The sudden onset of a “thunderclap” headache (often described by patients as “the worst headache of my life”) suggests subarachnoid hemorrhage.
In contrast to intracranial hemorrhage, in subarachnoid hemorrhage blood collects mainly in the cerebral spinal fluid-containing spaces surrounding the brain, leading to a higher incidence of hydrocephalus from impaired drainage of cerebrospinal fluid. Nontraumatic subarachnoid hemorrhage is most often caused by rupture of an intracranial aneurysm, which can be a devastating event, with death rates approaching 50%.25
Diagnosis of subarachnoid hemorrhage
Noncontrast CT of the head is the main modality for diagnosing subarachnoid hemorrhage. Blood within the subarachnoid space is demonstrable in 92% of cases if CT is performed within the first 24 hours of hemorrhage, with an initial sensitivity of about 95% within the first 6 hours of onset.14,26,27 The longer CT is delayed, the lower the sensitivity.
Some studies suggest that a protocol of CT followed by CT angiography can safely exclude aneurysmal subarachnoid hemorrhage and obviate the need for lumbar puncture. However, further research is required to validate this approach.28
Lumbar puncture. If clinical suspicion of subarachnoid hemorrhage remains strong even though initial CT is negative, lumbar puncture must be performed for cerebrospinal fluid analysis.29 Xanthochromia (a yellowish pigmentation of the cerebrospinal fluid due to the degeneration of blood products that occurs within 8 to 12 hours of bleeding) should raise the alarm for subarachnoid hemorrhage; this sign may be present up to 4 weeks after the bleeding event.30
If lumbar puncture is contraindicated, then aneurysmal subarachnoid hemorrhage has not been ruled out, and further neurologic consultation should be pursued.
Management of subarachnoid hemorrhage
Early management of blood pressure for a ruptured intracranial aneurysm follows strategies similar to those for intracranial hemorrhage. Further investigation is rapidly directed toward an underlying vascular malformation, with intracranial vessel imaging such as CT angiography, magnetic resonance angiography, or the gold standard test—catheter-based cerebral angiography.
Aneurysms are treated (or “secured”) either by surgical clipping or by endovascular coiling. Endovascular coiling is preferable in cases in which both can be safely attempted.31 If the facility lacks the resources to do these procedures, the patient should be referred to a nearby tertiary care center.
INTRACRANIAL HYPERTENSION: DANGER OF BRAIN HERNIATION
A number of conditions can cause an acute intracranial pressure elevation. The danger of brain herniation requires that therapies be implemented rapidly to prevent catastrophic neurologic injury. In many situations, nonneurologists are the first responders and therefore should be familiar with basic intracranial pressure management.
Initial symptoms of acute rise in intracranial pressure
As intracranial pressure rises, pressure is typically equally distributed throughout the cranial vault, leading to dysfunction of the ascending reticular activating system, which clinically manifests as the inability to stay alert despite varying degrees of noxious stimulation. Progressive cranial neuropathies (often starting with pupillary abnormalities) and coma are often seen in this setting as the upper brainstem begins to be compressed.
Initial assessment and treatment of elevated intracranial pressure
Noncontrast CT of the head is often obtained immediately when acutely elevated intracranial pressure is suspected. If clinical examination and radiographic findings are consistent with intracranial hypertension, prompt measures can be started at the bedside.
Elevate the head of the bed to 30 degrees to promote venous drainage and reduce intracranial pressure. (In contrast, most other hemodynamically unstable patients are placed flat or in the Trendelenburg position.)
Intubation should be done quickly in cases of airway compromise, and hyperventilation should be started with a goal Paco2 of 30 to 35 mm Hg. This hypocarbic strategy promotes cerebral vasoconstriction and a transient decrease in intracranial pressure.
Hyperosmolar therapy allows for transient intracranial volume decompression and is the mainstay of emergency medical treatment of intracranial hypertension. Mannitol is a hyperosmolar polysaccharide that promotes osmotic diuresis and removes excessive cerebral water. In the acute setting, it can be given as an intravenous bolus of 1 to 2 g/kg through a peripheral intravenous line, followed by a bolus every 4 to 6 hours. Hypotension can occur after diuresis, and renal function should be closely monitored since frequent mannitol use can promote acute tubular necrosis. In patients who are anuric, the medication is typically not used.
Hypertonic saline (typically 3% sodium chloride, though different concentrations are available) is an alternative that helps draw interstitial fluid into the intravascular space, decreasing cerebral edema and maintaining hemodynamic stability. Relative contraindications include congestive heart failure or renal failure leading to pulmonary edema from volume overload. Hypertonic saline can be given as a bolus or a constant infusion. Some institutions have rapid access to 23.4% saline, which can be given as a 30-mL bolus but typically requires a central venous catheter for rapid infusion.
Comatose patients with radiographic findings of hydrocephalus, epidural or subdural hematoma, or mass effect with midline shift warrant prompt neurosurgical consultation for further surgical measures of intracranial pressure control and monitoring.
The ‘blown’ pupil
The physician should be concerned about elevated intracranial pressure if a patient has mydriasis, ie, an abnormally dilated (“blown”) pupil, which is a worrisome sign in the setting of true intracranial hypertension. However, many different processes can cause mydriasis and should be kept in mind when evaluating this finding (Table 3).32 If radiographic findings do not suggest elevated intracranial pressure, further workup into these other processes should be pursued.
STATUS EPILEPTICUS: SEIZURE CONTROL IS IMPORTANT
A continuous unremitting seizure lasting longer than 5 minutes or recurrent seizure activity in a patient who does not regain consciousness between seizures should be treated as status epilepticus. All seizure types carry the risk of progressing to status epilepticus, and responsiveness to antiepileptic drug therapy is inversely related to the duration of seizures. It is imperative that seizure activity be treated early and aggressively to prevent recalcitrant seizure activity, neuronal damage, and progression to status epilepticus.33
Once the ABCs of emergency stabilization have been performed (ie, airway, breathing, circulation), antiepileptic drug therapy should start immediately using established algorithms (Figure 1).34–36 During the course of treatment, the reliability of the neurologic examination may be limited due to medication effects or continued status epilepticus, making continuous video electroencephalographic monitoring often necessary to guide further therapy in patients who are not rapidly recovering.34–38
Once status epilepticus has resolved, further investigation into the underlying cause should be pursued quickly, especially in patients without a previous diagnosis of epilepsy. Head CT with contrast or magnetic resonance imaging can be used to look for any structural abnormality that may explain seizures. Basic laboratory tests including toxicology screening can identify a common trigger such as hypoglycemia or stimulant use. Fever or other possible signs of meningitis should be investigated further with cerebrospinal fluid analysis.
SPINAL CORD INJURY
Acute spinal cord injury can lead to substantial long-term neurologic impairment and should be suspected in any patient presenting with focal motor loss, sensory loss, or both with sparing of the cranial nerves and mental status. Causes of injury include compression (traumatic or nontraumatic) and inflammatory and noninflammatory myelopathies.
The location of the injury can be inferred by analyzing the symptoms, which can point to the cord level and indicate whether the anterior or posterior of the cord is involved. Anterior cord injury tends to affect the descending corticospinal and pyramidal tracts, resulting in motor deficits and weakness. Posterior cord injury involves the dorsal columns, leading to deficits of vibration sensation and proprioception. High cervical cord injuries tend to involve varying degrees of quadriparesis, sensory loss, and sometimes respiratory compromise. A clinical history of bilateral lower-extremity weakness, a “band-like” sensory complaint around the lower chest or abdomen, or both, can suggest thoracic cord involvement. Symptoms isolated to one or both lower extremities along with lower back pain and bowel or bladder involvement may point to injury of the lumbosacral cord.
Basic management of spinal cord injury includes decompression of the bladder and initial protection against further injury with a stabilizing collar or brace.
Magnetic resonance imaging with and without contrast is the ideal study to evaluate injuries to the spinal cord itself. While CT is helpful in identifying bony disease of the spinal column (eg, evaluating traumatic fractures), it is not helpful in viewing intrinsic cord pathology.
Traumatic myelopathy
Traumatic spinal cord injury is usually suggested by the clinical history and confirmed with CT. In this setting, early consultation with a neurosurgeon is required to prevent permanent cord injury.
Guidelines suggest maintaining a mean arterial pressure greater than 85 to 90 mm Hg for the first 7 days after traumatic spinal cord injury, a particular problem in the setting of hemodynamic instability, which can accompany lesions above the midthoracic level.39,40
Patients with vertebral body misalignment should be placed in an appropriate stabilizing collar or brace until a medically trained professional deems it appropriate to discontinue the device, or until surgical stabilization is performed.
Methylprednisone is a controversial intervention for acute spinal cord trauma, lacking clear benefit in meta-analyses.41
Nontraumatic compressive myelopathy
Patients with nontraumatic compressive myelopathy tend to present with varying degrees of back pain and worsening sensorimotor function. The differential diagnosis includes epidural abscesses, hematoma, metastatic neoplasm, and osteophyte compression (Table 4). The clinical history helps to guide therapy and should involve assessment for previous spinal column injury, immunocompromised state, travel history (which provides information on risks of exposure to a variety of diseases, including infections), and constitutional symptoms such as fever and weight loss.
Epidural abscess can have devastating results if missed. Red flags such as recent illness, intravenous drug use, focal back pain, fever, worsening numbness or weakness, and bowel or bladder incontinence should raise suspicion of this disorder. Emergency magnetic resonance imaging is required to diagnose this condition, and treatment involves urgent administration of antibiotics and consideration of surgical drainage.
Noncompressive myelopathies
There are numerous causes of noncompressive spinal cord injury (Table 4), and the etiology may be inflammatory (eg, “myelitis”) or noninflammatory. The diagnostic workup may require both magnetic resonance imaging and cerebrospinal fluid analysis. Acute disease-targeted therapy is rarely indicated and can be deferred until a full diagnostic workup has been completed.
NEUROMUSCULAR DISEASE: IS VENTILATION NEEDED?
Diseases involving the motor components of the peripheral nervous system (Table 5) share the common risk of causing ventilatory failure due to weakness of the diaphragm, intercostal muscles, and upper-airway muscles. Clinicians need to be aware of this risk and view these disorders as neurologic emergencies.
Determining when these patients require mechanical intubation is a challenge. Serial measurements of maximum inspiratory force and vital capacity are important and can be accomplished quickly at the bedside by a respiratory therapist. A maximum inspiratory force less than –30 cm H2O or a vital capacity less than 20 mL/kg, or both, are worrisome markers that raise concern for impending ventilatory failure. Serial measurements can detect changes in these values that might indicate the need for elective intubation. In any patient presenting with weakness of the limbs, these measurements are an important step in the initial evaluation.
Myasthenic crisis
Myasthenia gravis is caused by autoantibodies directed against postsynaptic acetylcholine receptors. Patients demonstrate muscle weakness, usually in a proximal pattern, with fatigue, respiratory distress, nasal speech, ophthalmoparesis, and dysphagia. Exacerbations can occur as a response to recent infection, surgery, or medications such as neuromuscular blocking agents or aminoglycosides.
Myasthenic crisis, while uncommon, is a life-threatening emergency characterized by bulbar or respiratory failure secondary to muscle weakness. It can occur in patients already diagnosed with myasthenia gravis or may be the initial manifestation of the disease.42–49 Intubation and mechanical ventilation are frequently required. Postoperative myasthenic patients in whom extubation has been delayed more than 24 hours should be considered in crisis.45
The diagnosis of myasthenia gravis can be made by serum autoantibody testing, electromyography, and nerve conduction studies (with repetitive stimulation) or administration of edrophonium in patients with obvious ptosis.
The mainstay of therapy for myasthenic crisis is either intravenous immunoglobulin at a dose of 2 g/kg over 2 to 5 days or plasmapheresis (5–7 exchanges over 7–14 days). Corticosteroids are not recommended in myasthenic crisis in patients who are not intubated, as they can potentiate an initial worsening of crisis. Once the patient begins to show clinical improvement, outpatient pyridostigmine and immunosuppressive medications can be resumed at a low dose and titrated as tolerated.
Acute inflammatory demyelinating polyneuropathy (Guillain-Barré syndrome)
Acute inflammatory demyelinating polyneuropathy is an autoimmune disorder involving autoantibodies against axons or myelin in the peripheral nervous system.
This disease should be suspected in a patient who is developing worsening muscle weakness (usually with areflexia) over the course of days to weeks. Occasionally, a recent diarrheal or other systemic infectious trigger can be identified. Blood pressure instability and cardiac arrhythmia can also be seen due to autonomic nerve involvement. Although classically described as an “ascending paralysis,” other variants of this disease have distinct clinical presentations (eg, the descending paralysis, ataxia, areflexia, ophthalmoparesis of the Miller Fisher syndrome).
Acute inflammatory demyelinating polyneuropathy is diagnosed by electromyography and nerve conduction studies. A cerebrospinal fluid profile demonstrating elevated protein and few white blood cells is typical.
Treatment, as in myasthenic crisis, involves intravenous immunoglobulin or plasmapheresis. Corticosteroids are ineffective. Anticipation of ventilatory failure and expectant intubation is essential, given the progressive nature of the disorder.50
Neurologic emergencies such as acute stroke, status epilepticus, subarachnoid hemorrhage, neuromuscular weakness, and spinal cord injury affect millions of Americans yearly.1,2 These conditions can be difficult to diagnose, and delays in recognition and treatment can have devastating results. Consequently, it is important for nonneurologists to be able to quickly recognize these conditions and initiate timely management, often while awaiting neurologic consultation.
Here, we review how to recognize and treat these common, serious conditions.
ACUTE ISCHEMIC STROKE: TIME IS OF THE ESSENCE
Stroke is the fourth leading cause of death in the United States and is one of the most common causes of disability worldwide.3–5 About 85% of strokes are ischemic, resulting from diminished vascular supply to the brain. Symptoms such as facial droop, unilateral weakness or numbness, aphasia, gaze deviation, and unsteadiness of gait may be seen. Time is of the essence, as all currently available interventions are safe and effective only within defined time windows.
Diagnosis and assessment
When acute ischemic stroke is suspected, the clinical history, time of onset, and basic neurologic examination should be obtained quickly.
The National Institutes of Health (NIH) stroke scale is an objective marker for assessing stroke severity as well as evolution of disease and should be obtained in all stroke patients. Scores range from 0 (best) to 42 (worst) (www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf).
Time of onset of symptoms is essential to determine, since it guides eligibility for acute therapies. Clinicians should ascertain the last time the patient was seen to be neurologically well in order to estimate this time window as closely as possible.
Laboratory tests should include a fingerstick blood glucose measurement, coagulation studies, complete blood cell count, and basic metabolic profile.
Computed tomography (CT) of the head without contrast should be obtained immediately to exclude acute hemorrhage and any alternative diagnoses that could explain the patient’s symptoms. Acute brain ischemia is often not apparent on CT during the first few hours of injury. Therefore, a patient presenting with new focal neurologic deficits and an unremarkable result on CT of the head should be treated as having had an acute ischemic stroke, and interventional therapies should be considered.
Stroke mimics should be considered and treated, as appropriate (Table 1).
Acute management of ischemic stroke
Acute treatment should not be delayed by obtaining chest radiography, inserting a Foley catheter, or obtaining an electrocardiogram. The longer the time that elapses before treatment, the worse the functional outcome, underscoring the need for rapid decision-making.6–8
Lowering the head of the bed may provide benefit by promoting blood flow to ischemic brain tissue.9 However, this should not be done in patients with significantly elevated intracerebral pressure and concern for herniation.
Permissive hypertension (antihypertensive treatment only for blood pressure greater than 220/110 mm Hg) should be allowed per national guidelines to provide adequate perfusion to brain areas at risk of injury.10
Tissue plasminogen activator. Patients with ischemic stroke who present within 3 hours of symptom onset should be considered for intravenous administration of tissue plasminogen activator (tPA), a safe and effective therapy with nearly 2 decades of evidence to support its use.10 The treating physician should carefully review the risks and benefits of this therapy.
To receive tPA, the patient must have all of the following:
- Clinical diagnosis of ischemic stroke with measurable neurologic deficit
- Onset of symptoms within the past 3 hours
- Age 18 or older.
The patient must not have any of the following:
- Significant stroke within the past 3 months
- Severe traumatic head injury within the past 3 months
- History of significant intracerebral hemorrhage
- Previously ruptured arteriovenous malformation or intracranial aneurysm
- Central nervous system neoplasm
- Arterial puncture at a noncompressible site within the past 7 days
- Evidence of hemorrhage on CT of the head
- Evidence of ischemia in greater than 33% of the cerebral hemisphere on head CT
- History and symptoms strongly suggesting subarachnoid hemorrhage
- Persistent hypertension (systolic pressure ≥ 185 mm Hg or diastolic pressure ≥ 110 mm Hg)
- Evidence of acute significant bleeding (external or internal)
- Hypoglycemia—ie, serum glucose less than 50 mg/dL (< 2.8 mmol/L)
- Thrombocytopenia (platelet count < 100 × 109/L)
- Significant coagulopathy (international normalized ratio > 1.7, prothrombin time > 15 seconds, or abnormally elevated activated partial thromboplastin time)
- Current use of a factor Xa inhibitor or direct thrombin inhibitor.
Relative contraindications:
- Minor or rapidly resolving symptoms
- Major surgery or trauma within the past 14 days
- Gastrointestinal or urinary tract bleeding within the past 21 days
- Myocardial infarction in the past 3 months
- Unruptured intracranial aneurysm
- Seizure occurring at stroke onset
- Pregnancy.
If these criteria are satisfied, tPA should be given at a dose of 0.9 mg/kg intravenously over 60 minutes. Ten percent of the dose should be given as an initial bolus, followed by a constant infusion of the remaining 90% over 1 hour.
If tPA is given, the blood pressure must be kept lower than 185/110 mm Hg to minimize the risk of symptomatic intracerebral hemorrhage.
A subset of patients may benefit from receiving intravenous tPA between 3 and 4.5 hours after the onset of stroke symptoms. These include patients who are no more than 80 years old, who have not recently used oral anticoagulants, who do not have severe neurologic injury (ie, do not have NIH Stroke Scale scores > 25), and who do not have diabetes mellitus or a history of ischemic stroke.11 Although many hospitals have such a protocol for tPA up to 4.5 hours after the onset of stroke symptoms, this time window is not currently approved by the US Food and Drug Administration.
Intra-arterial therapy. Based on recent trials, some patients may benefit further from intra-arterial thrombolysis or mechanical thrombectomy, both delivered during catheter-based cerebral angiography, independent of intravenous tPA administration.12,13 These patients should be evaluated on a case-by-case basis by a neurologist and neurointerventional team. Time windows for these treatments generally extend to 6 hours from stroke onset and perhaps even longer in some situations (eg, basilar artery occlusion).
An antiplatelet agent should be started quickly in all stroke patients who do not receive tPA. Patients who receive tPA can begin receiving an antiplatelet agent 24 hours afterward.
Unfractionated heparin. There is no evidence to support the use of unfractionated heparin in most cases of acute ischemic stroke.10
Glucose control (in the range of 140–180 mg/dL) and fever control remain essential elements of post-acute stroke care to provide additional protection to the damaged brain.
For ischemic stroke due to atrial fibrillation
In ischemic stroke due to atrial fibrillation, early anticoagulation should be considered, based on the CHA2DS2-VASC risk of ischemic stroke vs the HAS-BLED risk of hemorrhage (calculators available at www.mdcalc.com).
In general, anticoagulation may be withheld during the first 72 hours while further stroke workup and evaluation of extent of injury are carried out, as there is an increased risk of hemorrhagic transformation of the ischemic stroke. Often, anticoagulation is resumed at a full dose between 72 hours and 2 weeks of the ischemic stroke.
ACUTE HEMORRHAGIC STROKE: BLOOD PRESSURE, COAGULATION
Approximately 15% of strokes are caused by intracerebral hemorrhage, which can be detected with noncontrast head CT with a sensitivity of 98.6% within 6 hours of the onset of bleeding.14 A common underlying cause of intracerebral hemorrhage is chronic poorly controlled hypertension, causing rupture of damaged (or “lipohyalinized”) vessels with resultant blood extravasation into the brain parenchyma. Other causes are less common (Table 2).
Treatment of acute hemorrhagic stroke
Acute treatment of intracerebral hemorrhage includes blood pressure control, reversal of underlying coagulopathy or anticoagulation, and sometimes intracranial pressure control. There is little role for surgery in most cases, based on findings of randomized trials.15
Blood pressure control. Many studies have investigated optimal blood pressure goals in acute intracerebral hemorrhage. Recent data suggest that early aggressive therapy, targeting a systolic blood pressure goal less than 140 mm Hg within the first hour, is safe and can lead to better functional outcomes than a more conservative blood-pressure-lowering target.16 Rapid-onset, short-acting antihypertensive agents in intravenous form, such as nicardipine and labetalol, are frequently used. Of note, this treatment strategy for hemorrhagic stroke is in direct contrast to the treatment of ischemic stroke, in which permissive hypertension (blood pressure goal < 220/110 mm Hg) is often pursued.
Reversal of any coagulation abnormalities should be done quickly in intracranial hemorrhage. Warfarin use has been shown to be a strong independent predictor of intracranial hemorrhage expansion, which increases the risk of death.17,18
Increasingly, agents other than vitamin K or fresh-frozen plasma are being used to rapidly reverse anticoagulation, including prothrombin complex concentrate (available in three- and four-factor preparations) and recombinant factor VIIa. While four-factor prothrombin complex concentrate and recombinant factor VIIa have been shown to be more efficacious than fresh-frozen plasma, there are limited data directly comparing these newer reversal agents against each other.19 The use of these medications is limited by availability and practitioner familiarity.20–22
Reversing anticoagulation due to target-specific oral anticoagulants. The acute management of intracranial hemorrhage in patients taking the new target-specific oral anticoagulants (eg, dabigatran, apixaban, rivaroxaban, edoxaban) remains challenging. Laboratory tests such as factor Xa levels are not readily available in many institutions and do not provide results in a timely fashion, and in the interim, acute hemorrhage and clinical deterioration may occur. Management strategies involve giving fresh-frozen plasma, prothrombin complex concentrate, and consideration of hemodialysis.23 Dabigatran reversal with idarucizumab has recently been shown to have efficacy.24
Vigilance for elevated intracranial pressure. Intracranial hemorrhage can occasionally cause elevated intracranial pressure, which should be treated rapidly. Any acute decline in mental status in a patient with intracranial hemorrhage requires emergency imaging to evaluate for expansion of hemorrhage.
SUBARACHNOID HEMORRHAGE
The sudden onset of a “thunderclap” headache (often described by patients as “the worst headache of my life”) suggests subarachnoid hemorrhage.
In contrast to intracranial hemorrhage, in subarachnoid hemorrhage blood collects mainly in the cerebral spinal fluid-containing spaces surrounding the brain, leading to a higher incidence of hydrocephalus from impaired drainage of cerebrospinal fluid. Nontraumatic subarachnoid hemorrhage is most often caused by rupture of an intracranial aneurysm, which can be a devastating event, with death rates approaching 50%.25
Diagnosis of subarachnoid hemorrhage
Noncontrast CT of the head is the main modality for diagnosing subarachnoid hemorrhage. Blood within the subarachnoid space is demonstrable in 92% of cases if CT is performed within the first 24 hours of hemorrhage, with an initial sensitivity of about 95% within the first 6 hours of onset.14,26,27 The longer CT is delayed, the lower the sensitivity.
Some studies suggest that a protocol of CT followed by CT angiography can safely exclude aneurysmal subarachnoid hemorrhage and obviate the need for lumbar puncture. However, further research is required to validate this approach.28
Lumbar puncture. If clinical suspicion of subarachnoid hemorrhage remains strong even though initial CT is negative, lumbar puncture must be performed for cerebrospinal fluid analysis.29 Xanthochromia (a yellowish pigmentation of the cerebrospinal fluid due to the degeneration of blood products that occurs within 8 to 12 hours of bleeding) should raise the alarm for subarachnoid hemorrhage; this sign may be present up to 4 weeks after the bleeding event.30
If lumbar puncture is contraindicated, then aneurysmal subarachnoid hemorrhage has not been ruled out, and further neurologic consultation should be pursued.
Management of subarachnoid hemorrhage
Early management of blood pressure for a ruptured intracranial aneurysm follows strategies similar to those for intracranial hemorrhage. Further investigation is rapidly directed toward an underlying vascular malformation, with intracranial vessel imaging such as CT angiography, magnetic resonance angiography, or the gold standard test—catheter-based cerebral angiography.
Aneurysms are treated (or “secured”) either by surgical clipping or by endovascular coiling. Endovascular coiling is preferable in cases in which both can be safely attempted.31 If the facility lacks the resources to do these procedures, the patient should be referred to a nearby tertiary care center.
INTRACRANIAL HYPERTENSION: DANGER OF BRAIN HERNIATION
A number of conditions can cause an acute intracranial pressure elevation. The danger of brain herniation requires that therapies be implemented rapidly to prevent catastrophic neurologic injury. In many situations, nonneurologists are the first responders and therefore should be familiar with basic intracranial pressure management.
Initial symptoms of acute rise in intracranial pressure
As intracranial pressure rises, pressure is typically equally distributed throughout the cranial vault, leading to dysfunction of the ascending reticular activating system, which clinically manifests as the inability to stay alert despite varying degrees of noxious stimulation. Progressive cranial neuropathies (often starting with pupillary abnormalities) and coma are often seen in this setting as the upper brainstem begins to be compressed.
Initial assessment and treatment of elevated intracranial pressure
Noncontrast CT of the head is often obtained immediately when acutely elevated intracranial pressure is suspected. If clinical examination and radiographic findings are consistent with intracranial hypertension, prompt measures can be started at the bedside.
Elevate the head of the bed to 30 degrees to promote venous drainage and reduce intracranial pressure. (In contrast, most other hemodynamically unstable patients are placed flat or in the Trendelenburg position.)
Intubation should be done quickly in cases of airway compromise, and hyperventilation should be started with a goal Paco2 of 30 to 35 mm Hg. This hypocarbic strategy promotes cerebral vasoconstriction and a transient decrease in intracranial pressure.
Hyperosmolar therapy allows for transient intracranial volume decompression and is the mainstay of emergency medical treatment of intracranial hypertension. Mannitol is a hyperosmolar polysaccharide that promotes osmotic diuresis and removes excessive cerebral water. In the acute setting, it can be given as an intravenous bolus of 1 to 2 g/kg through a peripheral intravenous line, followed by a bolus every 4 to 6 hours. Hypotension can occur after diuresis, and renal function should be closely monitored since frequent mannitol use can promote acute tubular necrosis. In patients who are anuric, the medication is typically not used.
Hypertonic saline (typically 3% sodium chloride, though different concentrations are available) is an alternative that helps draw interstitial fluid into the intravascular space, decreasing cerebral edema and maintaining hemodynamic stability. Relative contraindications include congestive heart failure or renal failure leading to pulmonary edema from volume overload. Hypertonic saline can be given as a bolus or a constant infusion. Some institutions have rapid access to 23.4% saline, which can be given as a 30-mL bolus but typically requires a central venous catheter for rapid infusion.
Comatose patients with radiographic findings of hydrocephalus, epidural or subdural hematoma, or mass effect with midline shift warrant prompt neurosurgical consultation for further surgical measures of intracranial pressure control and monitoring.
The ‘blown’ pupil
The physician should be concerned about elevated intracranial pressure if a patient has mydriasis, ie, an abnormally dilated (“blown”) pupil, which is a worrisome sign in the setting of true intracranial hypertension. However, many different processes can cause mydriasis and should be kept in mind when evaluating this finding (Table 3).32 If radiographic findings do not suggest elevated intracranial pressure, further workup into these other processes should be pursued.
STATUS EPILEPTICUS: SEIZURE CONTROL IS IMPORTANT
A continuous unremitting seizure lasting longer than 5 minutes or recurrent seizure activity in a patient who does not regain consciousness between seizures should be treated as status epilepticus. All seizure types carry the risk of progressing to status epilepticus, and responsiveness to antiepileptic drug therapy is inversely related to the duration of seizures. It is imperative that seizure activity be treated early and aggressively to prevent recalcitrant seizure activity, neuronal damage, and progression to status epilepticus.33
Once the ABCs of emergency stabilization have been performed (ie, airway, breathing, circulation), antiepileptic drug therapy should start immediately using established algorithms (Figure 1).34–36 During the course of treatment, the reliability of the neurologic examination may be limited due to medication effects or continued status epilepticus, making continuous video electroencephalographic monitoring often necessary to guide further therapy in patients who are not rapidly recovering.34–38
Once status epilepticus has resolved, further investigation into the underlying cause should be pursued quickly, especially in patients without a previous diagnosis of epilepsy. Head CT with contrast or magnetic resonance imaging can be used to look for any structural abnormality that may explain seizures. Basic laboratory tests including toxicology screening can identify a common trigger such as hypoglycemia or stimulant use. Fever or other possible signs of meningitis should be investigated further with cerebrospinal fluid analysis.
SPINAL CORD INJURY
Acute spinal cord injury can lead to substantial long-term neurologic impairment and should be suspected in any patient presenting with focal motor loss, sensory loss, or both with sparing of the cranial nerves and mental status. Causes of injury include compression (traumatic or nontraumatic) and inflammatory and noninflammatory myelopathies.
The location of the injury can be inferred by analyzing the symptoms, which can point to the cord level and indicate whether the anterior or posterior of the cord is involved. Anterior cord injury tends to affect the descending corticospinal and pyramidal tracts, resulting in motor deficits and weakness. Posterior cord injury involves the dorsal columns, leading to deficits of vibration sensation and proprioception. High cervical cord injuries tend to involve varying degrees of quadriparesis, sensory loss, and sometimes respiratory compromise. A clinical history of bilateral lower-extremity weakness, a “band-like” sensory complaint around the lower chest or abdomen, or both, can suggest thoracic cord involvement. Symptoms isolated to one or both lower extremities along with lower back pain and bowel or bladder involvement may point to injury of the lumbosacral cord.
Basic management of spinal cord injury includes decompression of the bladder and initial protection against further injury with a stabilizing collar or brace.
Magnetic resonance imaging with and without contrast is the ideal study to evaluate injuries to the spinal cord itself. While CT is helpful in identifying bony disease of the spinal column (eg, evaluating traumatic fractures), it is not helpful in viewing intrinsic cord pathology.
Traumatic myelopathy
Traumatic spinal cord injury is usually suggested by the clinical history and confirmed with CT. In this setting, early consultation with a neurosurgeon is required to prevent permanent cord injury.
Guidelines suggest maintaining a mean arterial pressure greater than 85 to 90 mm Hg for the first 7 days after traumatic spinal cord injury, a particular problem in the setting of hemodynamic instability, which can accompany lesions above the midthoracic level.39,40
Patients with vertebral body misalignment should be placed in an appropriate stabilizing collar or brace until a medically trained professional deems it appropriate to discontinue the device, or until surgical stabilization is performed.
Methylprednisone is a controversial intervention for acute spinal cord trauma, lacking clear benefit in meta-analyses.41
Nontraumatic compressive myelopathy
Patients with nontraumatic compressive myelopathy tend to present with varying degrees of back pain and worsening sensorimotor function. The differential diagnosis includes epidural abscesses, hematoma, metastatic neoplasm, and osteophyte compression (Table 4). The clinical history helps to guide therapy and should involve assessment for previous spinal column injury, immunocompromised state, travel history (which provides information on risks of exposure to a variety of diseases, including infections), and constitutional symptoms such as fever and weight loss.
Epidural abscess can have devastating results if missed. Red flags such as recent illness, intravenous drug use, focal back pain, fever, worsening numbness or weakness, and bowel or bladder incontinence should raise suspicion of this disorder. Emergency magnetic resonance imaging is required to diagnose this condition, and treatment involves urgent administration of antibiotics and consideration of surgical drainage.
Noncompressive myelopathies
There are numerous causes of noncompressive spinal cord injury (Table 4), and the etiology may be inflammatory (eg, “myelitis”) or noninflammatory. The diagnostic workup may require both magnetic resonance imaging and cerebrospinal fluid analysis. Acute disease-targeted therapy is rarely indicated and can be deferred until a full diagnostic workup has been completed.
NEUROMUSCULAR DISEASE: IS VENTILATION NEEDED?
Diseases involving the motor components of the peripheral nervous system (Table 5) share the common risk of causing ventilatory failure due to weakness of the diaphragm, intercostal muscles, and upper-airway muscles. Clinicians need to be aware of this risk and view these disorders as neurologic emergencies.
Determining when these patients require mechanical intubation is a challenge. Serial measurements of maximum inspiratory force and vital capacity are important and can be accomplished quickly at the bedside by a respiratory therapist. A maximum inspiratory force less than –30 cm H2O or a vital capacity less than 20 mL/kg, or both, are worrisome markers that raise concern for impending ventilatory failure. Serial measurements can detect changes in these values that might indicate the need for elective intubation. In any patient presenting with weakness of the limbs, these measurements are an important step in the initial evaluation.
Myasthenic crisis
Myasthenia gravis is caused by autoantibodies directed against postsynaptic acetylcholine receptors. Patients demonstrate muscle weakness, usually in a proximal pattern, with fatigue, respiratory distress, nasal speech, ophthalmoparesis, and dysphagia. Exacerbations can occur as a response to recent infection, surgery, or medications such as neuromuscular blocking agents or aminoglycosides.
Myasthenic crisis, while uncommon, is a life-threatening emergency characterized by bulbar or respiratory failure secondary to muscle weakness. It can occur in patients already diagnosed with myasthenia gravis or may be the initial manifestation of the disease.42–49 Intubation and mechanical ventilation are frequently required. Postoperative myasthenic patients in whom extubation has been delayed more than 24 hours should be considered in crisis.45
The diagnosis of myasthenia gravis can be made by serum autoantibody testing, electromyography, and nerve conduction studies (with repetitive stimulation) or administration of edrophonium in patients with obvious ptosis.
The mainstay of therapy for myasthenic crisis is either intravenous immunoglobulin at a dose of 2 g/kg over 2 to 5 days or plasmapheresis (5–7 exchanges over 7–14 days). Corticosteroids are not recommended in myasthenic crisis in patients who are not intubated, as they can potentiate an initial worsening of crisis. Once the patient begins to show clinical improvement, outpatient pyridostigmine and immunosuppressive medications can be resumed at a low dose and titrated as tolerated.
Acute inflammatory demyelinating polyneuropathy (Guillain-Barré syndrome)
Acute inflammatory demyelinating polyneuropathy is an autoimmune disorder involving autoantibodies against axons or myelin in the peripheral nervous system.
This disease should be suspected in a patient who is developing worsening muscle weakness (usually with areflexia) over the course of days to weeks. Occasionally, a recent diarrheal or other systemic infectious trigger can be identified. Blood pressure instability and cardiac arrhythmia can also be seen due to autonomic nerve involvement. Although classically described as an “ascending paralysis,” other variants of this disease have distinct clinical presentations (eg, the descending paralysis, ataxia, areflexia, ophthalmoparesis of the Miller Fisher syndrome).
Acute inflammatory demyelinating polyneuropathy is diagnosed by electromyography and nerve conduction studies. A cerebrospinal fluid profile demonstrating elevated protein and few white blood cells is typical.
Treatment, as in myasthenic crisis, involves intravenous immunoglobulin or plasmapheresis. Corticosteroids are ineffective. Anticipation of ventilatory failure and expectant intubation is essential, given the progressive nature of the disorder.50
- Pitts SR, Niska RW, Xu J, Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008; 7:1–38.
- McMullan JT, Knight WA, Clark JF, Beyette FR, Pancioli A. Time-critical neurological emergencies: the unfulfilled role for point-of-care testing. Int J Emerg Med 2010; 3:127–131.
- Centers for Disease Control and Prevention (CDC). Prevalence of stroke: United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2012; 61:379–382.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2095–2128.
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1,160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2163–2196.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med 1995; 333:1581–1587.
- Hacke W, Donnan G, Fieschi C, et al; ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363:768–774.
- Saver JL, Fonarrow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013; 309:2480–2488.
- Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology 2005; 64:1354–1357.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–1329.
- Berkhemer OA, Fransen PSS, Beumer D, et al; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Eng J Med 2015; 372:11–20.
- Campbell BC, Mitchell PJ, Kleinig TJ, et al; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372:1009–1018.
- Backes D, Rinkel GJ, Kemperman H, Linn FH, Vergouwen MD. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke 2012; 43:2115–2119.
- Mendelow AD, Gregson BA, Fernandes HM, et al; STICH investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005; 365: 387–397.
- Anderson CS, Helley E, Huang Y, et al; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368:2355–2365.
- Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004; 63:1059–1064.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Woo CH, Patel N, Conell C, et al. Rapid warfarin reversal in the setting of intracranial hemorrhage: a comparison of plasma, recombinant activated factor VII, and prothrombin complex concentrate. World Neurosurg 2014; 81:110–115.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 2007; 38:2001–2023.
- Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke 2006, 37:151–155.
- Chapman SA, Irwin ED, Beal AL, Kulinski NM, Hutson KE, Thorson MA. Prothrombin complex concentrate versus standard therapies for INR reversal in trauma patients receiving warfarin. Ann Pharmacother 2011; 45:869–875.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013; 80:443–451.
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015; 373:511-520.
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994; 25:1342–1347.
- Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg 1990; 73:18–36.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 2011; 343:d4277.
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med 2010; 17:444–451.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:1711–1737.
- Vermuelen M, Hasan D, Blijenberg BG, Hijdra A, van Gijn J. Xanthochromia after subarachnoid haemorrhage needs no revisitation. J Neurol Neurosurg Psychiatry 1989; 52:826–828.
- Molyneaux AJ, Kerr RS, Yu LM, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid hemorrhage trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366:809–817.
- Caglayan HZ, Colpak IA, Kansu T. A diagnostic challenge: dilated pupil. Curr Opin Ophthalmol 2013; 24:550–557.
- Brophy GM, Bell R, Claassen J, et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012; 17:3–23.
- Chang CW, Bleck TP. Status epilepticus. Neurol Clin 1995; 13:529–548.
- Treiman DM. Generalized convulsive status epilepticus in the adult. Epilepsia 1993; 34(suppl 1):S2–S11.
- Leppick IE. Status epilepticus: the next decade. Neurology 1990; 40(suppl 2):4–9.
- Aranda A, Foucart G, Ducassé JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia 2010; 51:2159–2167.
- DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 1998; 39:833–840.
- Casha S, Christie S. A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma 2011; 28:1479–1495.
- Walters BC, Hadley MN, Hurlbert RJ, et al; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013; 60(suppl 1):82–91.
- Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2013; 72(suppl 2):93–105.
- Cohen MS, Younger D. Aspects of the natural history of myasthenia gravis: crisis and death. Ann NY Acad Sci 1981; 377:670–677.
- Belack RS, Sanders DB. On the concept of myasthenic crisis. J Clin Neuromuscul Dis 2002; 4:40–42.
- Chaudhuri A, Behan PO. Myasthenic crisis. QJM 2009; 102:97–107.
- Mayer SA. Intensive care of the myasthenic patient. Neurology 1997; 48(suppl 5):70S–75S.
- Jani-Acsadi A, Lisak RP. Myasthenic crisis: guidelines for prevention and treatment. J Neurol Sci 2007; 261:127–133.
- Bershad EM, Feen ES, Suarez JI. Myasthenia gravis crisis. South Med J 2008; 101:63–69.
- Ahmed S, Kirmani JF, Janjua N, et al. An update on myasthenic crisis. Curr Treat Options Neurol 2005; 7:129–141.
- Godoy DA, Vaz de Mello LJ, Masotti L, Napoli MD. The myasthenic patient in crisis: an update of the management in neurointensive care unit. Arq Neuropsiquiatr 2013; 71:627–639.
- Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome: Arch Neurol 2005; 62:1194–1198.
- Pitts SR, Niska RW, Xu J, Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Report 2008; 7:1–38.
- McMullan JT, Knight WA, Clark JF, Beyette FR, Pancioli A. Time-critical neurological emergencies: the unfulfilled role for point-of-care testing. Int J Emerg Med 2010; 3:127–131.
- Centers for Disease Control and Prevention (CDC). Prevalence of stroke: United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2012; 61:379–382.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2095–2128.
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1,160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380:2163–2196.
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med 1995; 333:1581–1587.
- Hacke W, Donnan G, Fieschi C, et al; ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363:768–774.
- Saver JL, Fonarrow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013; 309:2480–2488.
- Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology 2005; 64:1354–1357.
- Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–1329.
- Berkhemer OA, Fransen PSS, Beumer D, et al; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Eng J Med 2015; 372:11–20.
- Campbell BC, Mitchell PJ, Kleinig TJ, et al; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372:1009–1018.
- Backes D, Rinkel GJ, Kemperman H, Linn FH, Vergouwen MD. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke 2012; 43:2115–2119.
- Mendelow AD, Gregson BA, Fernandes HM, et al; STICH investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005; 365: 387–397.
- Anderson CS, Helley E, Huang Y, et al; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368:2355–2365.
- Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004; 63:1059–1064.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Woo CH, Patel N, Conell C, et al. Rapid warfarin reversal in the setting of intracranial hemorrhage: a comparison of plasma, recombinant activated factor VII, and prothrombin complex concentrate. World Neurosurg 2014; 81:110–115.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 2007; 38:2001–2023.
- Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke 2006, 37:151–155.
- Chapman SA, Irwin ED, Beal AL, Kulinski NM, Hutson KE, Thorson MA. Prothrombin complex concentrate versus standard therapies for INR reversal in trauma patients receiving warfarin. Ann Pharmacother 2011; 45:869–875.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013; 80:443–451.
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015; 373:511-520.
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994; 25:1342–1347.
- Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg 1990; 73:18–36.
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 2011; 343:d4277.
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med 2010; 17:444–451.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:1711–1737.
- Vermuelen M, Hasan D, Blijenberg BG, Hijdra A, van Gijn J. Xanthochromia after subarachnoid haemorrhage needs no revisitation. J Neurol Neurosurg Psychiatry 1989; 52:826–828.
- Molyneaux AJ, Kerr RS, Yu LM, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid hemorrhage trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366:809–817.
- Caglayan HZ, Colpak IA, Kansu T. A diagnostic challenge: dilated pupil. Curr Opin Ophthalmol 2013; 24:550–557.
- Brophy GM, Bell R, Claassen J, et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012; 17:3–23.
- Chang CW, Bleck TP. Status epilepticus. Neurol Clin 1995; 13:529–548.
- Treiman DM. Generalized convulsive status epilepticus in the adult. Epilepsia 1993; 34(suppl 1):S2–S11.
- Leppick IE. Status epilepticus: the next decade. Neurology 1990; 40(suppl 2):4–9.
- Aranda A, Foucart G, Ducassé JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia 2010; 51:2159–2167.
- DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 1998; 39:833–840.
- Casha S, Christie S. A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma 2011; 28:1479–1495.
- Walters BC, Hadley MN, Hurlbert RJ, et al; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013; 60(suppl 1):82–91.
- Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2013; 72(suppl 2):93–105.
- Cohen MS, Younger D. Aspects of the natural history of myasthenia gravis: crisis and death. Ann NY Acad Sci 1981; 377:670–677.
- Belack RS, Sanders DB. On the concept of myasthenic crisis. J Clin Neuromuscul Dis 2002; 4:40–42.
- Chaudhuri A, Behan PO. Myasthenic crisis. QJM 2009; 102:97–107.
- Mayer SA. Intensive care of the myasthenic patient. Neurology 1997; 48(suppl 5):70S–75S.
- Jani-Acsadi A, Lisak RP. Myasthenic crisis: guidelines for prevention and treatment. J Neurol Sci 2007; 261:127–133.
- Bershad EM, Feen ES, Suarez JI. Myasthenia gravis crisis. South Med J 2008; 101:63–69.
- Ahmed S, Kirmani JF, Janjua N, et al. An update on myasthenic crisis. Curr Treat Options Neurol 2005; 7:129–141.
- Godoy DA, Vaz de Mello LJ, Masotti L, Napoli MD. The myasthenic patient in crisis: an update of the management in neurointensive care unit. Arq Neuropsiquiatr 2013; 71:627–639.
- Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome: Arch Neurol 2005; 62:1194–1198.
KEY POINTS
- Patients with possible acute ischemic stroke should be assessed quickly to see if they should receive tissue plasminogen activator, which should be started within 3 hours of stroke onset. Computed tomography (CT) of the head without contrast should be done immediately to rule out acute hemorrhagic stroke.
- Acute treatment of intracerebral hemorrhage includes blood pressure control, reversal of underlying coagulopathy, and sometimes intracranial pressure control.
- If the clinical suspicion of subarachnoid hemorrhage remains strong even though initial CT was negative, lumbar puncture is mandatory.
- Hyperosmolar therapy is the mainstay of emergency medical treatment of intracranial hypertension.
- Seizure activity must be treated aggressively to prevent recalcitrant seizure activity, neuronal damage, and progression to status epilepticus.