User login
Standard BMI inadequate for ALL patients

Photo by Bill Branson
New research suggests that body mass index (BMI) is an inadequate method for estimating changes in body fat and obesity in children with acute lymphoblastic leukemia (ALL).
Investigators found a discrepancy between BMI and body composition in this population, and the cause of this appeared to be increases in body fat with simultaneous loss of lean muscle mass during treatment.
The team reported these findings in Leukemia & Lymphoma.
With previous work, the investigators found that obese children diagnosed with high-risk ALL had a 50% greater risk of their disease recurring compared with children who were not obese.
“In my lab, we’ve seen a direct interaction between fat cells and leukemia cells that may help explain this increased risk of disease relapse,” said study author Steven Mittelman, MD, PhD, of Children’s Hospital Los Angeles in California.
“It appears that the fat cells ‘protect’ leukemia cells, making them less susceptible to chemotherapy and making an accurate measure of body fat essential.”
To determine if BMI accurately reflects body fat in ALL, the investigators analyzed 50 patients. They were predominantly Hispanic, between the ages of 10 to 21, and had newly diagnosed high-risk B-precursor ALL or T-cell ALL.
The team measured the percentage of total body fat and lean muscle mass at the time of diagnosis, at the end of induction, and at the end of delayed intensification. They also calculated BMI Z-score—a measure of how a given child’s BMI deviates from a population of children of the same age and sex—at these time points.
The investigators said sarcopenic obesity—gain in body fat percentage with loss of lean muscle mass—was “surprisingly common” during ALL treatment.
And sarcopenic obesity resulted in poor correlation between changes in BMI Z-score and body fat percentage overall (r=-0.05), within the time points (r=0.02), and within patients (r=-0.09, all not significant). BMI Z-score and body fat percentage changed in opposite directions in more than 50% of interval assessments.
“We found that change in BMI did not reflect changes in body fat or obesity,” said Etan Orgel, MD, of Children’s Hospital Los Angeles.
“In some patients, reaching a ‘healthy’ BMI was due solely to loss of muscle even while body fat continued to rise. Based on these results, we believe that evaluation of obesity in patients with leukemia should include direct measures of body composition.” ![]()

Photo by Bill Branson
New research suggests that body mass index (BMI) is an inadequate method for estimating changes in body fat and obesity in children with acute lymphoblastic leukemia (ALL).
Investigators found a discrepancy between BMI and body composition in this population, and the cause of this appeared to be increases in body fat with simultaneous loss of lean muscle mass during treatment.
The team reported these findings in Leukemia & Lymphoma.
With previous work, the investigators found that obese children diagnosed with high-risk ALL had a 50% greater risk of their disease recurring compared with children who were not obese.
“In my lab, we’ve seen a direct interaction between fat cells and leukemia cells that may help explain this increased risk of disease relapse,” said study author Steven Mittelman, MD, PhD, of Children’s Hospital Los Angeles in California.
“It appears that the fat cells ‘protect’ leukemia cells, making them less susceptible to chemotherapy and making an accurate measure of body fat essential.”
To determine if BMI accurately reflects body fat in ALL, the investigators analyzed 50 patients. They were predominantly Hispanic, between the ages of 10 to 21, and had newly diagnosed high-risk B-precursor ALL or T-cell ALL.
The team measured the percentage of total body fat and lean muscle mass at the time of diagnosis, at the end of induction, and at the end of delayed intensification. They also calculated BMI Z-score—a measure of how a given child’s BMI deviates from a population of children of the same age and sex—at these time points.
The investigators said sarcopenic obesity—gain in body fat percentage with loss of lean muscle mass—was “surprisingly common” during ALL treatment.
And sarcopenic obesity resulted in poor correlation between changes in BMI Z-score and body fat percentage overall (r=-0.05), within the time points (r=0.02), and within patients (r=-0.09, all not significant). BMI Z-score and body fat percentage changed in opposite directions in more than 50% of interval assessments.
“We found that change in BMI did not reflect changes in body fat or obesity,” said Etan Orgel, MD, of Children’s Hospital Los Angeles.
“In some patients, reaching a ‘healthy’ BMI was due solely to loss of muscle even while body fat continued to rise. Based on these results, we believe that evaluation of obesity in patients with leukemia should include direct measures of body composition.” ![]()

Photo by Bill Branson
New research suggests that body mass index (BMI) is an inadequate method for estimating changes in body fat and obesity in children with acute lymphoblastic leukemia (ALL).
Investigators found a discrepancy between BMI and body composition in this population, and the cause of this appeared to be increases in body fat with simultaneous loss of lean muscle mass during treatment.
The team reported these findings in Leukemia & Lymphoma.
With previous work, the investigators found that obese children diagnosed with high-risk ALL had a 50% greater risk of their disease recurring compared with children who were not obese.
“In my lab, we’ve seen a direct interaction between fat cells and leukemia cells that may help explain this increased risk of disease relapse,” said study author Steven Mittelman, MD, PhD, of Children’s Hospital Los Angeles in California.
“It appears that the fat cells ‘protect’ leukemia cells, making them less susceptible to chemotherapy and making an accurate measure of body fat essential.”
To determine if BMI accurately reflects body fat in ALL, the investigators analyzed 50 patients. They were predominantly Hispanic, between the ages of 10 to 21, and had newly diagnosed high-risk B-precursor ALL or T-cell ALL.
The team measured the percentage of total body fat and lean muscle mass at the time of diagnosis, at the end of induction, and at the end of delayed intensification. They also calculated BMI Z-score—a measure of how a given child’s BMI deviates from a population of children of the same age and sex—at these time points.
The investigators said sarcopenic obesity—gain in body fat percentage with loss of lean muscle mass—was “surprisingly common” during ALL treatment.
And sarcopenic obesity resulted in poor correlation between changes in BMI Z-score and body fat percentage overall (r=-0.05), within the time points (r=0.02), and within patients (r=-0.09, all not significant). BMI Z-score and body fat percentage changed in opposite directions in more than 50% of interval assessments.
“We found that change in BMI did not reflect changes in body fat or obesity,” said Etan Orgel, MD, of Children’s Hospital Los Angeles.
“In some patients, reaching a ‘healthy’ BMI was due solely to loss of muscle even while body fat continued to rise. Based on these results, we believe that evaluation of obesity in patients with leukemia should include direct measures of body composition.” ![]()
NICE issues draft guideline for NHL

a cancer patient
Photo courtesy of NCI/
Mathews Media Group
The National Institute for Health and Care Excellence (NICE) has issued a draft guideline for the diagnosis and management of non-Hodgkin lymphoma (NHL).
The guideline, which is open for consultation, covers adults and young people who are referred to secondary care with suspected NHL or who have newly diagnosed or relapsed NHL.
It contains recommendations for the management of 6 different NHL subtypes—diffuse large B-cell lymphoma, mantle cell lymphoma, follicular lymphoma, MALT lymphoma, Burkitt lymphoma, and peripheral T-cell lymphoma.
The guideline considers which method of biopsy is most appropriate, which diagnostic test most suitable, how the stage of disease is best assessed, and what treatment is likely to be most effective.
It also proposes recommendations for how best to support patients who complete their treatment. These include the provision of end-of-treatment summaries to be discussed with the patient and an increase in education on the possible relapse/late side-effects of their treatment.
“This draft guideline is now open for consultation,” said Mark Baker, director of the Centre of Clinical Practice at NICE.
“We want to hear from patients and all those who provide care for people with non-Hodgkin’s lymphoma in the NHS [National Health Service] so that we can produce a guideline which will support everyone who diagnoses, treats, and has to live with this disease.”
The consultation closes on March 11, 2016, with the final guideline expected in the summer. ![]()

a cancer patient
Photo courtesy of NCI/
Mathews Media Group
The National Institute for Health and Care Excellence (NICE) has issued a draft guideline for the diagnosis and management of non-Hodgkin lymphoma (NHL).
The guideline, which is open for consultation, covers adults and young people who are referred to secondary care with suspected NHL or who have newly diagnosed or relapsed NHL.
It contains recommendations for the management of 6 different NHL subtypes—diffuse large B-cell lymphoma, mantle cell lymphoma, follicular lymphoma, MALT lymphoma, Burkitt lymphoma, and peripheral T-cell lymphoma.
The guideline considers which method of biopsy is most appropriate, which diagnostic test most suitable, how the stage of disease is best assessed, and what treatment is likely to be most effective.
It also proposes recommendations for how best to support patients who complete their treatment. These include the provision of end-of-treatment summaries to be discussed with the patient and an increase in education on the possible relapse/late side-effects of their treatment.
“This draft guideline is now open for consultation,” said Mark Baker, director of the Centre of Clinical Practice at NICE.
“We want to hear from patients and all those who provide care for people with non-Hodgkin’s lymphoma in the NHS [National Health Service] so that we can produce a guideline which will support everyone who diagnoses, treats, and has to live with this disease.”
The consultation closes on March 11, 2016, with the final guideline expected in the summer. ![]()

a cancer patient
Photo courtesy of NCI/
Mathews Media Group
The National Institute for Health and Care Excellence (NICE) has issued a draft guideline for the diagnosis and management of non-Hodgkin lymphoma (NHL).
The guideline, which is open for consultation, covers adults and young people who are referred to secondary care with suspected NHL or who have newly diagnosed or relapsed NHL.
It contains recommendations for the management of 6 different NHL subtypes—diffuse large B-cell lymphoma, mantle cell lymphoma, follicular lymphoma, MALT lymphoma, Burkitt lymphoma, and peripheral T-cell lymphoma.
The guideline considers which method of biopsy is most appropriate, which diagnostic test most suitable, how the stage of disease is best assessed, and what treatment is likely to be most effective.
It also proposes recommendations for how best to support patients who complete their treatment. These include the provision of end-of-treatment summaries to be discussed with the patient and an increase in education on the possible relapse/late side-effects of their treatment.
“This draft guideline is now open for consultation,” said Mark Baker, director of the Centre of Clinical Practice at NICE.
“We want to hear from patients and all those who provide care for people with non-Hodgkin’s lymphoma in the NHS [National Health Service] so that we can produce a guideline which will support everyone who diagnoses, treats, and has to live with this disease.”
The consultation closes on March 11, 2016, with the final guideline expected in the summer. ![]()
Minor residual staining found adequate for colonoscopy
A Boston Bowel Preparation Scale (BBPS) score of 2 – indicating mild residual staining and small stool fragments – was as good as the optimal preparation score of 3 for visualizing polyps and adenomas larger than 5 mm and advanced adenomas during colonoscopy, researchers said.
A score of 2 might increase the chances of missing smaller polyps, but is adequate for detecting clinically significant masses, Dr. Brian Clark of Yale University, New Haven, Conn., and his associates reported in the February issue of Gastroenterology. But a score of 1 – meaning that there is enough staining or stool to obscure the mucosa – significantly increased the chances of missing adenomas larger than 5 mm, they said. Patients should undergo early repeat colonoscopy if their BBPS score is 1 or 0 in any colon segment, they emphasized.
Source: American Gastroenterological Association
Bowel preparation for colonoscopy is considered adequate if endoscopists can detect polyps larger than 5 mm, but no prior study had quantified the amount of preparation needed. This prospective observational study assessed adequate preparation in terms of the BBPS, which scores each of three colon segments on a scale of 0 (solid stool covering the mucosa) to 3 points (entire mucosa seen well, with no residual staining). Study participants included 438 men aged 50-75 years who underwent screening or surveillance colonoscopy at a single Veterans Affairs center, followed by repeat colonoscopies within 60 days performed by different blinded endoscopists. The investigators excluded patients who scored 0 in all colon segments or had familial polyposis syndrome, inflammatory bowel disease, polyps so large that they could not be completely removed, or a history of colonic or rectal resection. In all, they analyzed 1,161 colon segments (Gastroenterology. 2015 Dec 7. doi: 10.1053/j.gastro.2015.09.041).
Endoscopists missed about 5% of adenomas greater than 5 mm, regardless of whether BBPS scores were 2 or 3 in a model that accounted for age, reason for colonoscopy, colon segment, number of polyps removed in the first examination, and endoscopist performing the procedure, the researchers said. But when BBPS scores were 1, endoscopists missed 16% of adenomas larger than 5 mm, a difference of about 10%. Furthermore, 43% of screening and surveillance intervals would have been incorrect had they been based solely on an initial examination for which scores were 1 in at least one segment. In contrast, only about 15% of intervals would have been incorrect for patients who scored 2 or 3 in all segments.
In all, 80% of patients were sufficiently prepared, having scored at least 2 in all segments on the first examination. “Determining whether a patient’s preparation quality is adequate is one of the most common and important decisions made by gastroenterologists each day,” the researchers said. Between 25% and 30% of screening and surveillance colonoscopies occur at “inappropriately shortened intervals,” often because of uncertainty about what constitutes adequate visualization, they added. Defining adequate visualization based on bowel preparation could save billions of dollars in health care costs every year, minimize complications from unnecessary procedures, and pinpoint those patients who truly need an early repeat colonoscopy to help prevent interval colorectal cancer, they emphasized.
The National Institutes of Health funded the study. The investigators had no disclosures.
We have seen a dramatic increase in attention to improving the adenoma detection rate (ADR) during colonoscopy because patients of endoscopists with a higher ADR have a lower risk of colorectal cancer after colonoscopy. One major contributor to missed adenomas is inadequate bowel preparation, though little was known about how best to define adequacy.

|
| Dr. Jason Domonitz |
Clark and colleagues’ elegant tandem colonoscopy study helps address this knowledge gap using the Boston Bowel Preparation Scale (BBPS), a validated instrument that is easy to implement. They hypothesized that a BBPS colon-segment score of 2 was noninferior to a score of 3 for identifying adenomas greater than 5 mm, but that a BBPS colon-segment score of 1 would be inferior to scores of 2 or 3. Their findings support this hypothesis and give us long overdue data that we can now use to define an adequate bowel preparation. Given that the adenoma miss rate was 16% when the segment score was 1, but only about 5% with higher scores, it is reasonable to recommend repeat colonoscopy within 12 months if any segment score is less than 2. Otherwise, standard surveillance intervals should be recommended. Finally, unless and until other scoring systems are similarly validated, these findings should encourage the widespread adoption of the BBPS.
Dr. Jason A. Dominitz, AGAF, is the national program director for gastroenterology for the Veterans Health Administration and is professor of medicine in the division of gastroenterology at the University of Washington, Seattle. He has no conflicts of interest.
We have seen a dramatic increase in attention to improving the adenoma detection rate (ADR) during colonoscopy because patients of endoscopists with a higher ADR have a lower risk of colorectal cancer after colonoscopy. One major contributor to missed adenomas is inadequate bowel preparation, though little was known about how best to define adequacy.

|
| Dr. Jason Domonitz |
Clark and colleagues’ elegant tandem colonoscopy study helps address this knowledge gap using the Boston Bowel Preparation Scale (BBPS), a validated instrument that is easy to implement. They hypothesized that a BBPS colon-segment score of 2 was noninferior to a score of 3 for identifying adenomas greater than 5 mm, but that a BBPS colon-segment score of 1 would be inferior to scores of 2 or 3. Their findings support this hypothesis and give us long overdue data that we can now use to define an adequate bowel preparation. Given that the adenoma miss rate was 16% when the segment score was 1, but only about 5% with higher scores, it is reasonable to recommend repeat colonoscopy within 12 months if any segment score is less than 2. Otherwise, standard surveillance intervals should be recommended. Finally, unless and until other scoring systems are similarly validated, these findings should encourage the widespread adoption of the BBPS.
Dr. Jason A. Dominitz, AGAF, is the national program director for gastroenterology for the Veterans Health Administration and is professor of medicine in the division of gastroenterology at the University of Washington, Seattle. He has no conflicts of interest.
We have seen a dramatic increase in attention to improving the adenoma detection rate (ADR) during colonoscopy because patients of endoscopists with a higher ADR have a lower risk of colorectal cancer after colonoscopy. One major contributor to missed adenomas is inadequate bowel preparation, though little was known about how best to define adequacy.

|
| Dr. Jason Domonitz |
Clark and colleagues’ elegant tandem colonoscopy study helps address this knowledge gap using the Boston Bowel Preparation Scale (BBPS), a validated instrument that is easy to implement. They hypothesized that a BBPS colon-segment score of 2 was noninferior to a score of 3 for identifying adenomas greater than 5 mm, but that a BBPS colon-segment score of 1 would be inferior to scores of 2 or 3. Their findings support this hypothesis and give us long overdue data that we can now use to define an adequate bowel preparation. Given that the adenoma miss rate was 16% when the segment score was 1, but only about 5% with higher scores, it is reasonable to recommend repeat colonoscopy within 12 months if any segment score is less than 2. Otherwise, standard surveillance intervals should be recommended. Finally, unless and until other scoring systems are similarly validated, these findings should encourage the widespread adoption of the BBPS.
Dr. Jason A. Dominitz, AGAF, is the national program director for gastroenterology for the Veterans Health Administration and is professor of medicine in the division of gastroenterology at the University of Washington, Seattle. He has no conflicts of interest.
A Boston Bowel Preparation Scale (BBPS) score of 2 – indicating mild residual staining and small stool fragments – was as good as the optimal preparation score of 3 for visualizing polyps and adenomas larger than 5 mm and advanced adenomas during colonoscopy, researchers said.
A score of 2 might increase the chances of missing smaller polyps, but is adequate for detecting clinically significant masses, Dr. Brian Clark of Yale University, New Haven, Conn., and his associates reported in the February issue of Gastroenterology. But a score of 1 – meaning that there is enough staining or stool to obscure the mucosa – significantly increased the chances of missing adenomas larger than 5 mm, they said. Patients should undergo early repeat colonoscopy if their BBPS score is 1 or 0 in any colon segment, they emphasized.
Source: American Gastroenterological Association
Bowel preparation for colonoscopy is considered adequate if endoscopists can detect polyps larger than 5 mm, but no prior study had quantified the amount of preparation needed. This prospective observational study assessed adequate preparation in terms of the BBPS, which scores each of three colon segments on a scale of 0 (solid stool covering the mucosa) to 3 points (entire mucosa seen well, with no residual staining). Study participants included 438 men aged 50-75 years who underwent screening or surveillance colonoscopy at a single Veterans Affairs center, followed by repeat colonoscopies within 60 days performed by different blinded endoscopists. The investigators excluded patients who scored 0 in all colon segments or had familial polyposis syndrome, inflammatory bowel disease, polyps so large that they could not be completely removed, or a history of colonic or rectal resection. In all, they analyzed 1,161 colon segments (Gastroenterology. 2015 Dec 7. doi: 10.1053/j.gastro.2015.09.041).
Endoscopists missed about 5% of adenomas greater than 5 mm, regardless of whether BBPS scores were 2 or 3 in a model that accounted for age, reason for colonoscopy, colon segment, number of polyps removed in the first examination, and endoscopist performing the procedure, the researchers said. But when BBPS scores were 1, endoscopists missed 16% of adenomas larger than 5 mm, a difference of about 10%. Furthermore, 43% of screening and surveillance intervals would have been incorrect had they been based solely on an initial examination for which scores were 1 in at least one segment. In contrast, only about 15% of intervals would have been incorrect for patients who scored 2 or 3 in all segments.
In all, 80% of patients were sufficiently prepared, having scored at least 2 in all segments on the first examination. “Determining whether a patient’s preparation quality is adequate is one of the most common and important decisions made by gastroenterologists each day,” the researchers said. Between 25% and 30% of screening and surveillance colonoscopies occur at “inappropriately shortened intervals,” often because of uncertainty about what constitutes adequate visualization, they added. Defining adequate visualization based on bowel preparation could save billions of dollars in health care costs every year, minimize complications from unnecessary procedures, and pinpoint those patients who truly need an early repeat colonoscopy to help prevent interval colorectal cancer, they emphasized.
The National Institutes of Health funded the study. The investigators had no disclosures.
A Boston Bowel Preparation Scale (BBPS) score of 2 – indicating mild residual staining and small stool fragments – was as good as the optimal preparation score of 3 for visualizing polyps and adenomas larger than 5 mm and advanced adenomas during colonoscopy, researchers said.
A score of 2 might increase the chances of missing smaller polyps, but is adequate for detecting clinically significant masses, Dr. Brian Clark of Yale University, New Haven, Conn., and his associates reported in the February issue of Gastroenterology. But a score of 1 – meaning that there is enough staining or stool to obscure the mucosa – significantly increased the chances of missing adenomas larger than 5 mm, they said. Patients should undergo early repeat colonoscopy if their BBPS score is 1 or 0 in any colon segment, they emphasized.
Source: American Gastroenterological Association
Bowel preparation for colonoscopy is considered adequate if endoscopists can detect polyps larger than 5 mm, but no prior study had quantified the amount of preparation needed. This prospective observational study assessed adequate preparation in terms of the BBPS, which scores each of three colon segments on a scale of 0 (solid stool covering the mucosa) to 3 points (entire mucosa seen well, with no residual staining). Study participants included 438 men aged 50-75 years who underwent screening or surveillance colonoscopy at a single Veterans Affairs center, followed by repeat colonoscopies within 60 days performed by different blinded endoscopists. The investigators excluded patients who scored 0 in all colon segments or had familial polyposis syndrome, inflammatory bowel disease, polyps so large that they could not be completely removed, or a history of colonic or rectal resection. In all, they analyzed 1,161 colon segments (Gastroenterology. 2015 Dec 7. doi: 10.1053/j.gastro.2015.09.041).
Endoscopists missed about 5% of adenomas greater than 5 mm, regardless of whether BBPS scores were 2 or 3 in a model that accounted for age, reason for colonoscopy, colon segment, number of polyps removed in the first examination, and endoscopist performing the procedure, the researchers said. But when BBPS scores were 1, endoscopists missed 16% of adenomas larger than 5 mm, a difference of about 10%. Furthermore, 43% of screening and surveillance intervals would have been incorrect had they been based solely on an initial examination for which scores were 1 in at least one segment. In contrast, only about 15% of intervals would have been incorrect for patients who scored 2 or 3 in all segments.
In all, 80% of patients were sufficiently prepared, having scored at least 2 in all segments on the first examination. “Determining whether a patient’s preparation quality is adequate is one of the most common and important decisions made by gastroenterologists each day,” the researchers said. Between 25% and 30% of screening and surveillance colonoscopies occur at “inappropriately shortened intervals,” often because of uncertainty about what constitutes adequate visualization, they added. Defining adequate visualization based on bowel preparation could save billions of dollars in health care costs every year, minimize complications from unnecessary procedures, and pinpoint those patients who truly need an early repeat colonoscopy to help prevent interval colorectal cancer, they emphasized.
The National Institutes of Health funded the study. The investigators had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Minor residual staining that does not obscure the bowel mucosa is adequate for detection of adenomas greater than 5 mm during surveillance or screening colonoscopy.
Major finding: Endoscopists missed about 5% of clinically significant adenomas, regardless of whether the Boston Bowel Preparation Score was 2 (minor residual staining) or 3 (entire mucosa seen well).
Data source: A blinded prospective observational study of 438 men at a single Veterans Affairs center.
Disclosures: The National Institutes of Health funded the study. The investigators had no disclosures.
Drug combo held up in real-world HCV study
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
FROM GASTROENTEROLOGY
Key clinical point: Twelve weeks of simeprevir and sofosbuvir cured about 85% of real-world patients with genotype 1 hepatitis C virus infection.
Major finding: The unadjusted rate of SVR12 was 85% (95% CI, 82%-88%).
Data source: An analysis of an observational cohort study of protease inhibitor combination regimen with or without ribavirin for 836 patients (HCV-TARGET).
Disclosures: The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
Factors within VA control could help prevent missed, canceled appointments
Opt-out scheduling protocols and long appointment lead times contributed significantly to missed and canceled colonoscopy appointments at Veterans Health Administration facilities, researchers reported in the February issue of Clinical Gastroenterology and Hepatology.
These factors are within the control of the Veterans Affairs and could be altered to improve productivity and efficiency, said Melissa Partin, Ph.D., of the Center for Chronic Disease Outcomes Research at the Minneapolis Veterans Affairs Health Care System in Minneapolis, and her associates.
Source: American Gastroenterological Association
Missed and canceled medical appointments are always a concern, but particularly so for colonoscopy clinics, where they incur an average daily net loss of $725, the investigators noted. Most clinics have limited colonoscopy capacity, and even a 30-day wait for diagnostic colonoscopy has been linked to “modest but significantly elevated” chances of detecting cancer on exam, they added. To better understand these problems, they separately examined predictors of missed and canceled appointments among 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011 (Clin Gastroenterol Hepatol. 2015 Aug 21. doi: 10.1016/j.cgh.2015.07.051).
Having a life expectancy of 6 months or less and no personal history of polyps best predicted missing an appointment, with odds ratios of 2.74 for each factor, the researchers said. However, only 0.47% of patients had such a short life expectancy. Other significant predictors of missed appointments included being seen at the largest and most complex facilities (odds ratio, 2.69; P = .007), having both psychiatric and substance abuse disorders (OR, 1.82; P less than .0001), and the use of opt-out scheduling, in which patients were automatically scheduled rather than having to schedule appointments themselves (OR, 1.57; P = .02). Canceled appointments also were linked to opt-out scheduling, as well as to older age and having no history of polyps.
Most appointment lead times were 28 days, and each 12-day increase in lead time increased the odds of missing or canceling appointments by about 15% (P less than .0001). The problem could be curtailed by the Veterans Access, Choice and Accountability Act of 2014, which allows those who cannot schedule VA appointments within 30 days to receive care from eligible non–VA providers, the investigators said. “Future research should focus on assessing the effect of the Choice Act on colonoscopy appointment lead time and on developing and evaluating efficient and effective approaches to implementing the other clinic-level changes supported by our findings,” they added.
The study might have oversimplified or missed changes in protocols because it used single-item survey measures at one point in time, the investigators said. For some patients, the first appointment after the fecal occult blood test may have been for another procedure besides colonoscopy, they added. Furthermore, they did not distinguish between appointments canceled by patients versus clinics. “The VHA is a unique context, characterized by a predominantly male, low-income population with high rates of mental health and substance abuse diagnoses. Therefore, our findings may not generalize to other settings,” they added. “However, our findings do have important implications for a substantial population of health providers and consumers in this country, because the VHA is the largest integrated health care system in the United States.”
The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research & Development Service. The investigators had no disclosures.
Opt-out scheduling protocols and long appointment lead times contributed significantly to missed and canceled colonoscopy appointments at Veterans Health Administration facilities, researchers reported in the February issue of Clinical Gastroenterology and Hepatology.
These factors are within the control of the Veterans Affairs and could be altered to improve productivity and efficiency, said Melissa Partin, Ph.D., of the Center for Chronic Disease Outcomes Research at the Minneapolis Veterans Affairs Health Care System in Minneapolis, and her associates.
Source: American Gastroenterological Association
Missed and canceled medical appointments are always a concern, but particularly so for colonoscopy clinics, where they incur an average daily net loss of $725, the investigators noted. Most clinics have limited colonoscopy capacity, and even a 30-day wait for diagnostic colonoscopy has been linked to “modest but significantly elevated” chances of detecting cancer on exam, they added. To better understand these problems, they separately examined predictors of missed and canceled appointments among 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011 (Clin Gastroenterol Hepatol. 2015 Aug 21. doi: 10.1016/j.cgh.2015.07.051).
Having a life expectancy of 6 months or less and no personal history of polyps best predicted missing an appointment, with odds ratios of 2.74 for each factor, the researchers said. However, only 0.47% of patients had such a short life expectancy. Other significant predictors of missed appointments included being seen at the largest and most complex facilities (odds ratio, 2.69; P = .007), having both psychiatric and substance abuse disorders (OR, 1.82; P less than .0001), and the use of opt-out scheduling, in which patients were automatically scheduled rather than having to schedule appointments themselves (OR, 1.57; P = .02). Canceled appointments also were linked to opt-out scheduling, as well as to older age and having no history of polyps.
Most appointment lead times were 28 days, and each 12-day increase in lead time increased the odds of missing or canceling appointments by about 15% (P less than .0001). The problem could be curtailed by the Veterans Access, Choice and Accountability Act of 2014, which allows those who cannot schedule VA appointments within 30 days to receive care from eligible non–VA providers, the investigators said. “Future research should focus on assessing the effect of the Choice Act on colonoscopy appointment lead time and on developing and evaluating efficient and effective approaches to implementing the other clinic-level changes supported by our findings,” they added.
The study might have oversimplified or missed changes in protocols because it used single-item survey measures at one point in time, the investigators said. For some patients, the first appointment after the fecal occult blood test may have been for another procedure besides colonoscopy, they added. Furthermore, they did not distinguish between appointments canceled by patients versus clinics. “The VHA is a unique context, characterized by a predominantly male, low-income population with high rates of mental health and substance abuse diagnoses. Therefore, our findings may not generalize to other settings,” they added. “However, our findings do have important implications for a substantial population of health providers and consumers in this country, because the VHA is the largest integrated health care system in the United States.”
The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research & Development Service. The investigators had no disclosures.
Opt-out scheduling protocols and long appointment lead times contributed significantly to missed and canceled colonoscopy appointments at Veterans Health Administration facilities, researchers reported in the February issue of Clinical Gastroenterology and Hepatology.
These factors are within the control of the Veterans Affairs and could be altered to improve productivity and efficiency, said Melissa Partin, Ph.D., of the Center for Chronic Disease Outcomes Research at the Minneapolis Veterans Affairs Health Care System in Minneapolis, and her associates.
Source: American Gastroenterological Association
Missed and canceled medical appointments are always a concern, but particularly so for colonoscopy clinics, where they incur an average daily net loss of $725, the investigators noted. Most clinics have limited colonoscopy capacity, and even a 30-day wait for diagnostic colonoscopy has been linked to “modest but significantly elevated” chances of detecting cancer on exam, they added. To better understand these problems, they separately examined predictors of missed and canceled appointments among 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011 (Clin Gastroenterol Hepatol. 2015 Aug 21. doi: 10.1016/j.cgh.2015.07.051).
Having a life expectancy of 6 months or less and no personal history of polyps best predicted missing an appointment, with odds ratios of 2.74 for each factor, the researchers said. However, only 0.47% of patients had such a short life expectancy. Other significant predictors of missed appointments included being seen at the largest and most complex facilities (odds ratio, 2.69; P = .007), having both psychiatric and substance abuse disorders (OR, 1.82; P less than .0001), and the use of opt-out scheduling, in which patients were automatically scheduled rather than having to schedule appointments themselves (OR, 1.57; P = .02). Canceled appointments also were linked to opt-out scheduling, as well as to older age and having no history of polyps.
Most appointment lead times were 28 days, and each 12-day increase in lead time increased the odds of missing or canceling appointments by about 15% (P less than .0001). The problem could be curtailed by the Veterans Access, Choice and Accountability Act of 2014, which allows those who cannot schedule VA appointments within 30 days to receive care from eligible non–VA providers, the investigators said. “Future research should focus on assessing the effect of the Choice Act on colonoscopy appointment lead time and on developing and evaluating efficient and effective approaches to implementing the other clinic-level changes supported by our findings,” they added.
The study might have oversimplified or missed changes in protocols because it used single-item survey measures at one point in time, the investigators said. For some patients, the first appointment after the fecal occult blood test may have been for another procedure besides colonoscopy, they added. Furthermore, they did not distinguish between appointments canceled by patients versus clinics. “The VHA is a unique context, characterized by a predominantly male, low-income population with high rates of mental health and substance abuse diagnoses. Therefore, our findings may not generalize to other settings,” they added. “However, our findings do have important implications for a substantial population of health providers and consumers in this country, because the VHA is the largest integrated health care system in the United States.”
The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research & Development Service. The investigators had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Opt-out scheduling practices and long appointment lead times predicted missed and canceled colonoscopies at the VA.
Major finding: Estimated ratios for these predictors ranged between 1.12 and 1.57, and all were statistically significant.
Data source: An analysis of data from 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011.
Disclosures: The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research and Development Service. The investigators had no disclosures.
Malpractice Counsel: Constipation, missing diabetes
Constipation
A 26-year-old woman presented to the ED with a chief complaint of chronic constipation. This was the patient’s fourth ED visit for the same complaint over the previous 12 days. The patient stated that, at the prior visits, she was prescribed stool softeners and instructed to increase the amount of green vegetables in her diet and to drink plenty of fluids. She further noted that although constipation had been a long-standing problem for her, the condition had become worse over the past several weeks.
The patient described some lower abdominal discomfort, but denied nausea, vomiting, fevers, or chills. She also denied any genitourinary complaints or flank pain. Her last menstrual period was 2 weeks prior and normal. Her medical history was unremarkable; she denied smoking cigarettes or drinking alcohol and had no known drug allergies.
On physical examination, the patient’s vital signs were normal and she did not appear to be in any distress. The lung and heart examinations were also normal. Her abdomen was found to be soft, with slight tenderness in the lower abdomen, but with no guarding, rebound, or distention. Bowel sounds were present and hypoactive. A rectal examination revealed minimal stool in the vault, which was heme negative.
The patient sued the EP and the hospital, stating that the enema was not only contraindicated, but also caused the colon perforation. She further alleged that the EP failed to properly diagnose the sigmoid volvulus. The defense argued that the patient suffered from an uncommon condition, and the treatment provided was appropriate given her symptoms. The defense further stated that the perforation was present prior to the administration of the enema. At trial, a defense verdict was returned.
Discussion
Sigmoid volvulus is a relatively rare cause of bowel obstruction, accounting for only 2% of intestinal obstructions in the United States between 2002 and 2010.1 The majority of cases occur in older patients (mean age, 70 years).1 Risk factors for development include a history of laxative abuse, chronic constipation, and institutionalized patients with underlying neurological or psychiatric disease. There also appears to be an increased incidence during pregnancy. When observed in the pediatric population and in young adults, sigmoid volvulus is frequently due to an underlying colonic motility disorder.
A volvulus occurs when the colon twists on its mesenteric axis with greater than 180° rotation, resulting in obstruction of the intestinal lumen and mesenteric vessels.2 The most common locations for volvulus are the sigmoid colon, followed by the cecum. Though rare, the condition can occur in other locations.
The patient in this case presented very atypically for someone with a sigmoid volvulus as the majority of patients present with progressive abdominal pain, nausea, vomiting, and constipation. On physical examination, the abdomen is frequently distended and tympanitic with diffuse tenderness. If perforation has occurred, then peritoneal signs predominate (eg, guarding, rigidity, rebound tenderness) and abnormal vital signs (eg, fever, tachycardia, hypotension) are frequently present.
While a diagnosis of sigmoid volvulus may be suspected through the history and physical examination, it is confirmed through imaging studies, with abdominal/pelvic CT being the modality of choice. On CT scan, the “whirl sign” is frequently present, representing the dilated sigmoid colon twisted around its mesocolon and vessels.3 The tightness of the whirl is proportional to the degree of torsion. If rectal contrast is administered, the “bird-beak” sign is often present, representing the afferent and efferent colonic segments.3
As with this patient, if the colon has been perforated, IV fluid resuscitation, IV antibiotics, and immediate surgery are indicated. In cases in which there is no evidence of gangrene or perforation, sigmoidoscopy can be attempted to detorse the twisted bowel segment. This technique is successful in correcting torsion in the majority of cases. However, if detorsion attempts fail, emergent surgery is indicated.
Even when nonsurgical detorsion is successful, controversy exists over its use as the sole treatment for sigmoid volvulus. Due to a 50% to 60% chance of recurrent sigmoid volvulus, some experts recommend surgery immediately following detorsion, while others advise a wait-and-see approach.
The risk of complications from administering a soapsuds enema to an immunocompetent ED patient without signs or symptoms of peritonitis is exceedingly low. While no good data exist on the rate of complications from enemas administered for constipation, perforation of the bowel from barium enemas occurs in only 0.02% to 0.04% of patients undergoing radiologic imaging.4 The jury appears to have come to the proper conclusion in this atypical presentation of an uncommon condition with a rare complication.
Missed Diabetes Mellitus
A 27-year-old man presented to the ED with a 3-day history of severe abdominal pain, nausea and vomiting. The patient denied fevers, chills, or diarrhea, as well as any sick contacts. The patient stated he was otherwise in good health, on no medications, and had no known drug allergies. He denied alcohol or tobacco use.
His vital signs at presentation were: temperature, 98.6°F; pulse, 116 beats/minute; blood pressure, 152/92 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 100% on room air. On head, eyes, ears, nose, and throat examination, the patient’s mucous membranes were noted to be dry. The lung examination revealed bilateral breath sounds clear to auscultation. The heart examination was remarkable for tachycardia, but the rhythm was regular and with no murmurs, rubs, or gallops. The abdomen was soft with slight diffuse tenderness, but no guarding, rebound, or masses.
The EP ordered 1 L normal saline IV and ondansetron 4 mg IV for the nausea and vomiting. No laboratory or imaging studies were ordered.
On reexamination approximately 1 hour later, the patient denied any abdominal pain and stated he felt improved and was no longer nauseous. The abdominal examination remained unchanged. The patient was discharged home with a prescription for ondansetron and instructed to return to the ED if his symptoms did not improve within the next 12 hours.
The patient did not return to the ED, but was found dead at home 3 days later. An autopsy revealed the patient died from metabolic consequences of diabetes mellitus (DM). The plaintiff’s family argued the standard of care required a complete set of laboratory studies, the results of which would have revealed the hyperglycemia, prompting further evaluation and treatment. The defense contended the standard of care did not require laboratory evaluation since the patient responded well to the IV fluids and ondansetron, reported an improvement in pain and nausea, and had no history of DM. At trial, a defense verdict was returned.
Discussion
Emergency physicians are well versed in diagnosing and treating DM and its complications. Typical symptoms of new-onset diabetes include polyuria, polydipsia, abdominal pain, nausea, vomiting, and lack of energy. Occasionally, the patient will present with more severe symptoms (eg, altered mental status) when diabetic ketoacidosis is the initial presentation of the disease. It is unclear from the medical records in this case whether additional history, such as polyuria, was obtained. If so, and the answers were in the affirmative, this information might have led the EP to order laboratory studies. Similarly, we do not know how many episodes of emesis the patient experienced—eg, only one to two episodes of emesis or more than 10. It is important to have an appreciation of the severity of the presenting symptoms.
Emergency physicians frequently diagnose and manage patients appropriately without ordering laboratory or imaging studies. Acute asthma attacks, migraine headaches, bronchitis, sprains, and upper respiratory tract infections are just a few examples of the many conditions that are frequently managed by EPs based solely on history and physical examination. However, it is important the EP take a thorough enough history and physical examination to ensure confidence in excluding more severe disease processes. The severity of the symptoms must also be considered in the decision to order laboratory or other evaluation.
In this day and age of point-of-care testing, one should consider checking the glucose and electrolytes in patients with symptoms consistent with fluid loss (ie, vomiting, diarrhea, decreased oral intake).
A Note about Diabetes Mellitus
Emergency physicians should be aware of the increasing incidence of DM in the United States and around the world. The global prevalence of diabetes in adults in 2013 was reportedly 8.3% (382 million people), with 14 million more men than women diagnosed with the disease.1
Broadly defined, diabetes is a group of metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both.1 Type 1 DM constitutes approximately 5% to 10% of patients diagnosed with diabetes and is due to the destruction of beta cells in the pancreas.1 It accounts for approximately 80% to 90% of DM in children and adolescents, and is thought to be present in approximately 3 million patients in the United States in 2010.1 Type 2 DM is the most common form, with 90% to 95% of patients belonging to this category, most of whom are adults. The problem in type 2 DM is primarily insulin resistance, as opposed to a lack of insulin. Obesity is the most common cause of insulin resistance in type 2 DM.1
- Constipation
- Halabi WJ, Jafari MD, Kang CY, et al. Colonic volvulus in the United States: trends, outcomes, and predictors of mortality. Ann Surg. 2014;259(2):293-301.
- Weingrow D, McCague A, Shah R, Lalezarzadeh F. Delayed presentation of sigmoid volvulus in a young woman. West J Emerg Med. 2012;13(1):100-102.
- Catalano O. Computed tomographic appearance of sigmoid volvulus. Abdom Imaging. 1996;21(4):314-317.
- Williams SM, Harned RK. Recognition and prevention of barium enema complications. Curr Probl Diagn Radiol. 1991;20(4):123-151.
- Missed Diabetes Mellitus
- Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J Diabetes. 2015;6(6):850-867.
Constipation
A 26-year-old woman presented to the ED with a chief complaint of chronic constipation. This was the patient’s fourth ED visit for the same complaint over the previous 12 days. The patient stated that, at the prior visits, she was prescribed stool softeners and instructed to increase the amount of green vegetables in her diet and to drink plenty of fluids. She further noted that although constipation had been a long-standing problem for her, the condition had become worse over the past several weeks.
The patient described some lower abdominal discomfort, but denied nausea, vomiting, fevers, or chills. She also denied any genitourinary complaints or flank pain. Her last menstrual period was 2 weeks prior and normal. Her medical history was unremarkable; she denied smoking cigarettes or drinking alcohol and had no known drug allergies.
On physical examination, the patient’s vital signs were normal and she did not appear to be in any distress. The lung and heart examinations were also normal. Her abdomen was found to be soft, with slight tenderness in the lower abdomen, but with no guarding, rebound, or distention. Bowel sounds were present and hypoactive. A rectal examination revealed minimal stool in the vault, which was heme negative.
The patient sued the EP and the hospital, stating that the enema was not only contraindicated, but also caused the colon perforation. She further alleged that the EP failed to properly diagnose the sigmoid volvulus. The defense argued that the patient suffered from an uncommon condition, and the treatment provided was appropriate given her symptoms. The defense further stated that the perforation was present prior to the administration of the enema. At trial, a defense verdict was returned.
Discussion
Sigmoid volvulus is a relatively rare cause of bowel obstruction, accounting for only 2% of intestinal obstructions in the United States between 2002 and 2010.1 The majority of cases occur in older patients (mean age, 70 years).1 Risk factors for development include a history of laxative abuse, chronic constipation, and institutionalized patients with underlying neurological or psychiatric disease. There also appears to be an increased incidence during pregnancy. When observed in the pediatric population and in young adults, sigmoid volvulus is frequently due to an underlying colonic motility disorder.
A volvulus occurs when the colon twists on its mesenteric axis with greater than 180° rotation, resulting in obstruction of the intestinal lumen and mesenteric vessels.2 The most common locations for volvulus are the sigmoid colon, followed by the cecum. Though rare, the condition can occur in other locations.
The patient in this case presented very atypically for someone with a sigmoid volvulus as the majority of patients present with progressive abdominal pain, nausea, vomiting, and constipation. On physical examination, the abdomen is frequently distended and tympanitic with diffuse tenderness. If perforation has occurred, then peritoneal signs predominate (eg, guarding, rigidity, rebound tenderness) and abnormal vital signs (eg, fever, tachycardia, hypotension) are frequently present.
While a diagnosis of sigmoid volvulus may be suspected through the history and physical examination, it is confirmed through imaging studies, with abdominal/pelvic CT being the modality of choice. On CT scan, the “whirl sign” is frequently present, representing the dilated sigmoid colon twisted around its mesocolon and vessels.3 The tightness of the whirl is proportional to the degree of torsion. If rectal contrast is administered, the “bird-beak” sign is often present, representing the afferent and efferent colonic segments.3
As with this patient, if the colon has been perforated, IV fluid resuscitation, IV antibiotics, and immediate surgery are indicated. In cases in which there is no evidence of gangrene or perforation, sigmoidoscopy can be attempted to detorse the twisted bowel segment. This technique is successful in correcting torsion in the majority of cases. However, if detorsion attempts fail, emergent surgery is indicated.
Even when nonsurgical detorsion is successful, controversy exists over its use as the sole treatment for sigmoid volvulus. Due to a 50% to 60% chance of recurrent sigmoid volvulus, some experts recommend surgery immediately following detorsion, while others advise a wait-and-see approach.
The risk of complications from administering a soapsuds enema to an immunocompetent ED patient without signs or symptoms of peritonitis is exceedingly low. While no good data exist on the rate of complications from enemas administered for constipation, perforation of the bowel from barium enemas occurs in only 0.02% to 0.04% of patients undergoing radiologic imaging.4 The jury appears to have come to the proper conclusion in this atypical presentation of an uncommon condition with a rare complication.
Missed Diabetes Mellitus
A 27-year-old man presented to the ED with a 3-day history of severe abdominal pain, nausea and vomiting. The patient denied fevers, chills, or diarrhea, as well as any sick contacts. The patient stated he was otherwise in good health, on no medications, and had no known drug allergies. He denied alcohol or tobacco use.
His vital signs at presentation were: temperature, 98.6°F; pulse, 116 beats/minute; blood pressure, 152/92 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 100% on room air. On head, eyes, ears, nose, and throat examination, the patient’s mucous membranes were noted to be dry. The lung examination revealed bilateral breath sounds clear to auscultation. The heart examination was remarkable for tachycardia, but the rhythm was regular and with no murmurs, rubs, or gallops. The abdomen was soft with slight diffuse tenderness, but no guarding, rebound, or masses.
The EP ordered 1 L normal saline IV and ondansetron 4 mg IV for the nausea and vomiting. No laboratory or imaging studies were ordered.
On reexamination approximately 1 hour later, the patient denied any abdominal pain and stated he felt improved and was no longer nauseous. The abdominal examination remained unchanged. The patient was discharged home with a prescription for ondansetron and instructed to return to the ED if his symptoms did not improve within the next 12 hours.
The patient did not return to the ED, but was found dead at home 3 days later. An autopsy revealed the patient died from metabolic consequences of diabetes mellitus (DM). The plaintiff’s family argued the standard of care required a complete set of laboratory studies, the results of which would have revealed the hyperglycemia, prompting further evaluation and treatment. The defense contended the standard of care did not require laboratory evaluation since the patient responded well to the IV fluids and ondansetron, reported an improvement in pain and nausea, and had no history of DM. At trial, a defense verdict was returned.
Discussion
Emergency physicians are well versed in diagnosing and treating DM and its complications. Typical symptoms of new-onset diabetes include polyuria, polydipsia, abdominal pain, nausea, vomiting, and lack of energy. Occasionally, the patient will present with more severe symptoms (eg, altered mental status) when diabetic ketoacidosis is the initial presentation of the disease. It is unclear from the medical records in this case whether additional history, such as polyuria, was obtained. If so, and the answers were in the affirmative, this information might have led the EP to order laboratory studies. Similarly, we do not know how many episodes of emesis the patient experienced—eg, only one to two episodes of emesis or more than 10. It is important to have an appreciation of the severity of the presenting symptoms.
Emergency physicians frequently diagnose and manage patients appropriately without ordering laboratory or imaging studies. Acute asthma attacks, migraine headaches, bronchitis, sprains, and upper respiratory tract infections are just a few examples of the many conditions that are frequently managed by EPs based solely on history and physical examination. However, it is important the EP take a thorough enough history and physical examination to ensure confidence in excluding more severe disease processes. The severity of the symptoms must also be considered in the decision to order laboratory or other evaluation.
In this day and age of point-of-care testing, one should consider checking the glucose and electrolytes in patients with symptoms consistent with fluid loss (ie, vomiting, diarrhea, decreased oral intake).
A Note about Diabetes Mellitus
Emergency physicians should be aware of the increasing incidence of DM in the United States and around the world. The global prevalence of diabetes in adults in 2013 was reportedly 8.3% (382 million people), with 14 million more men than women diagnosed with the disease.1
Broadly defined, diabetes is a group of metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both.1 Type 1 DM constitutes approximately 5% to 10% of patients diagnosed with diabetes and is due to the destruction of beta cells in the pancreas.1 It accounts for approximately 80% to 90% of DM in children and adolescents, and is thought to be present in approximately 3 million patients in the United States in 2010.1 Type 2 DM is the most common form, with 90% to 95% of patients belonging to this category, most of whom are adults. The problem in type 2 DM is primarily insulin resistance, as opposed to a lack of insulin. Obesity is the most common cause of insulin resistance in type 2 DM.1
Constipation
A 26-year-old woman presented to the ED with a chief complaint of chronic constipation. This was the patient’s fourth ED visit for the same complaint over the previous 12 days. The patient stated that, at the prior visits, she was prescribed stool softeners and instructed to increase the amount of green vegetables in her diet and to drink plenty of fluids. She further noted that although constipation had been a long-standing problem for her, the condition had become worse over the past several weeks.
The patient described some lower abdominal discomfort, but denied nausea, vomiting, fevers, or chills. She also denied any genitourinary complaints or flank pain. Her last menstrual period was 2 weeks prior and normal. Her medical history was unremarkable; she denied smoking cigarettes or drinking alcohol and had no known drug allergies.
On physical examination, the patient’s vital signs were normal and she did not appear to be in any distress. The lung and heart examinations were also normal. Her abdomen was found to be soft, with slight tenderness in the lower abdomen, but with no guarding, rebound, or distention. Bowel sounds were present and hypoactive. A rectal examination revealed minimal stool in the vault, which was heme negative.
The patient sued the EP and the hospital, stating that the enema was not only contraindicated, but also caused the colon perforation. She further alleged that the EP failed to properly diagnose the sigmoid volvulus. The defense argued that the patient suffered from an uncommon condition, and the treatment provided was appropriate given her symptoms. The defense further stated that the perforation was present prior to the administration of the enema. At trial, a defense verdict was returned.
Discussion
Sigmoid volvulus is a relatively rare cause of bowel obstruction, accounting for only 2% of intestinal obstructions in the United States between 2002 and 2010.1 The majority of cases occur in older patients (mean age, 70 years).1 Risk factors for development include a history of laxative abuse, chronic constipation, and institutionalized patients with underlying neurological or psychiatric disease. There also appears to be an increased incidence during pregnancy. When observed in the pediatric population and in young adults, sigmoid volvulus is frequently due to an underlying colonic motility disorder.
A volvulus occurs when the colon twists on its mesenteric axis with greater than 180° rotation, resulting in obstruction of the intestinal lumen and mesenteric vessels.2 The most common locations for volvulus are the sigmoid colon, followed by the cecum. Though rare, the condition can occur in other locations.
The patient in this case presented very atypically for someone with a sigmoid volvulus as the majority of patients present with progressive abdominal pain, nausea, vomiting, and constipation. On physical examination, the abdomen is frequently distended and tympanitic with diffuse tenderness. If perforation has occurred, then peritoneal signs predominate (eg, guarding, rigidity, rebound tenderness) and abnormal vital signs (eg, fever, tachycardia, hypotension) are frequently present.
While a diagnosis of sigmoid volvulus may be suspected through the history and physical examination, it is confirmed through imaging studies, with abdominal/pelvic CT being the modality of choice. On CT scan, the “whirl sign” is frequently present, representing the dilated sigmoid colon twisted around its mesocolon and vessels.3 The tightness of the whirl is proportional to the degree of torsion. If rectal contrast is administered, the “bird-beak” sign is often present, representing the afferent and efferent colonic segments.3
As with this patient, if the colon has been perforated, IV fluid resuscitation, IV antibiotics, and immediate surgery are indicated. In cases in which there is no evidence of gangrene or perforation, sigmoidoscopy can be attempted to detorse the twisted bowel segment. This technique is successful in correcting torsion in the majority of cases. However, if detorsion attempts fail, emergent surgery is indicated.
Even when nonsurgical detorsion is successful, controversy exists over its use as the sole treatment for sigmoid volvulus. Due to a 50% to 60% chance of recurrent sigmoid volvulus, some experts recommend surgery immediately following detorsion, while others advise a wait-and-see approach.
The risk of complications from administering a soapsuds enema to an immunocompetent ED patient without signs or symptoms of peritonitis is exceedingly low. While no good data exist on the rate of complications from enemas administered for constipation, perforation of the bowel from barium enemas occurs in only 0.02% to 0.04% of patients undergoing radiologic imaging.4 The jury appears to have come to the proper conclusion in this atypical presentation of an uncommon condition with a rare complication.
Missed Diabetes Mellitus
A 27-year-old man presented to the ED with a 3-day history of severe abdominal pain, nausea and vomiting. The patient denied fevers, chills, or diarrhea, as well as any sick contacts. The patient stated he was otherwise in good health, on no medications, and had no known drug allergies. He denied alcohol or tobacco use.
His vital signs at presentation were: temperature, 98.6°F; pulse, 116 beats/minute; blood pressure, 152/92 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 100% on room air. On head, eyes, ears, nose, and throat examination, the patient’s mucous membranes were noted to be dry. The lung examination revealed bilateral breath sounds clear to auscultation. The heart examination was remarkable for tachycardia, but the rhythm was regular and with no murmurs, rubs, or gallops. The abdomen was soft with slight diffuse tenderness, but no guarding, rebound, or masses.
The EP ordered 1 L normal saline IV and ondansetron 4 mg IV for the nausea and vomiting. No laboratory or imaging studies were ordered.
On reexamination approximately 1 hour later, the patient denied any abdominal pain and stated he felt improved and was no longer nauseous. The abdominal examination remained unchanged. The patient was discharged home with a prescription for ondansetron and instructed to return to the ED if his symptoms did not improve within the next 12 hours.
The patient did not return to the ED, but was found dead at home 3 days later. An autopsy revealed the patient died from metabolic consequences of diabetes mellitus (DM). The plaintiff’s family argued the standard of care required a complete set of laboratory studies, the results of which would have revealed the hyperglycemia, prompting further evaluation and treatment. The defense contended the standard of care did not require laboratory evaluation since the patient responded well to the IV fluids and ondansetron, reported an improvement in pain and nausea, and had no history of DM. At trial, a defense verdict was returned.
Discussion
Emergency physicians are well versed in diagnosing and treating DM and its complications. Typical symptoms of new-onset diabetes include polyuria, polydipsia, abdominal pain, nausea, vomiting, and lack of energy. Occasionally, the patient will present with more severe symptoms (eg, altered mental status) when diabetic ketoacidosis is the initial presentation of the disease. It is unclear from the medical records in this case whether additional history, such as polyuria, was obtained. If so, and the answers were in the affirmative, this information might have led the EP to order laboratory studies. Similarly, we do not know how many episodes of emesis the patient experienced—eg, only one to two episodes of emesis or more than 10. It is important to have an appreciation of the severity of the presenting symptoms.
Emergency physicians frequently diagnose and manage patients appropriately without ordering laboratory or imaging studies. Acute asthma attacks, migraine headaches, bronchitis, sprains, and upper respiratory tract infections are just a few examples of the many conditions that are frequently managed by EPs based solely on history and physical examination. However, it is important the EP take a thorough enough history and physical examination to ensure confidence in excluding more severe disease processes. The severity of the symptoms must also be considered in the decision to order laboratory or other evaluation.
In this day and age of point-of-care testing, one should consider checking the glucose and electrolytes in patients with symptoms consistent with fluid loss (ie, vomiting, diarrhea, decreased oral intake).
A Note about Diabetes Mellitus
Emergency physicians should be aware of the increasing incidence of DM in the United States and around the world. The global prevalence of diabetes in adults in 2013 was reportedly 8.3% (382 million people), with 14 million more men than women diagnosed with the disease.1
Broadly defined, diabetes is a group of metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both.1 Type 1 DM constitutes approximately 5% to 10% of patients diagnosed with diabetes and is due to the destruction of beta cells in the pancreas.1 It accounts for approximately 80% to 90% of DM in children and adolescents, and is thought to be present in approximately 3 million patients in the United States in 2010.1 Type 2 DM is the most common form, with 90% to 95% of patients belonging to this category, most of whom are adults. The problem in type 2 DM is primarily insulin resistance, as opposed to a lack of insulin. Obesity is the most common cause of insulin resistance in type 2 DM.1
- Constipation
- Halabi WJ, Jafari MD, Kang CY, et al. Colonic volvulus in the United States: trends, outcomes, and predictors of mortality. Ann Surg. 2014;259(2):293-301.
- Weingrow D, McCague A, Shah R, Lalezarzadeh F. Delayed presentation of sigmoid volvulus in a young woman. West J Emerg Med. 2012;13(1):100-102.
- Catalano O. Computed tomographic appearance of sigmoid volvulus. Abdom Imaging. 1996;21(4):314-317.
- Williams SM, Harned RK. Recognition and prevention of barium enema complications. Curr Probl Diagn Radiol. 1991;20(4):123-151.
- Missed Diabetes Mellitus
- Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J Diabetes. 2015;6(6):850-867.
- Constipation
- Halabi WJ, Jafari MD, Kang CY, et al. Colonic volvulus in the United States: trends, outcomes, and predictors of mortality. Ann Surg. 2014;259(2):293-301.
- Weingrow D, McCague A, Shah R, Lalezarzadeh F. Delayed presentation of sigmoid volvulus in a young woman. West J Emerg Med. 2012;13(1):100-102.
- Catalano O. Computed tomographic appearance of sigmoid volvulus. Abdom Imaging. 1996;21(4):314-317.
- Williams SM, Harned RK. Recognition and prevention of barium enema complications. Curr Probl Diagn Radiol. 1991;20(4):123-151.
- Missed Diabetes Mellitus
- Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J Diabetes. 2015;6(6):850-867.
Case Studies In Toxicology: Withdrawal: Another Danger of Diversion
Case
A 34-year-old man with a history of polysubstance abuse presented to the ED after he had a seizure during his regular methadone-treatment program meeting. While at the clinic, attendees witnessed the patient experience a loss of consciousness accompanied by generalized shaking movements of his extremities, which lasted for several minutes.
Upon arrival in the ED, the patient stated that he had a mild headache; he was otherwise asymptomatic. Initial vital signs were: blood pressure, 126/80 mm Hg; heart rate, 82 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97.3°F. Oxygen saturation was 98% on room air, and a finger-stick glucose test was 140 mg/dL.
Physical examination revealed a small right-sided parietal hematoma. The patient had no tremors and his neurological examination, including mental status, was normal. When reviewing the patient’s medical history and medications in the health record, it was noted that the patient had a prescription for alprazolam for an anxiety disorder. On further questioning, the patient admitted that he had sold his last alprazolam prescription and had not been taking the drug for the past week.
What characterizes the benzodiazepine withdrawal syndrome?
Although introduced into clinical practice in the 1960s, the potential for dependence and a withdrawal syndrome was not appreciated until the early 1980s. This clinical syndrome can manifest with a wide variety of findings, most commonly with what are termed “rebound effects” or “rebound hyperexcitability.” These effects include anxiety, insomnia or sleep disturbance, tremulousness, irritability, sweating, psychomotor agitation, difficulty in concentration, nausea, weight loss, palpitations, headache, muscular pain and stiffness, or generalized weakness.2 More severe manifestations include delirium, seizures, or psychosis. Often, these symptoms and signs may be confused with the very manifestations that prompted the initial use of the BZD, a reemergence of which can exacerbate the withdrawal syndrome.
When does benzodiazepine withdrawal occur?
The exact time course of BZD withdrawal can vary considerably and, unlike alcohol withdrawal (which occurs from a single compound, ethanol), can be difficult to characterize. The onset of withdrawal symptoms is dependent on a number of factors, including the half-life of the BZD involved. For example, delayed onset withdrawal symptoms of up to 3 weeks after cessation of the medication are described with long-acting BZDs such as chlordiazepoxide and diazepam. Conversely, symptoms may present as early as 24 to 48 hours after abrupt termination of BZDs with shorter half-lives, alprazolam and lorazepam. This variable time of onset differs considerably from other withdrawal syndromes, notably ethanol withdrawal. While both syndromes correlate to the individual patient’s severity of dependence, alcohol withdrawal follows a more predictable time course.
Some authors distinguish a rebound syndrome from a true withdrawal syndrome, the former of which is self-limited in nature and the result of cessation of treatment for the primary disease process. In this model, rebound symptoms begin 1 to 4 days after the abrupt cessation or dose reduction of the BZD, and are relatively short-lived, lasting 2 to 3 days.2
What is the appropriate treatment for benzodiazepine withdrawal?
The standard therapy for almost all withdrawal syndromes is reinstitution of the causal agent. A number of non-BZD-based treatment strategies have been investigated, and all have met with limited success. Of these, anticonvulsant drugs such as carbamazepine and valproic acid were initially considered promising based on case reports and small case series.4 These medications ultimately proved ineffective in randomized, placebo-controlled studies.5 β-Adrenergic antagonists, such as propranolol, have been studied as a method to normalize a patient’s vital signs but also proved nonbeneficial in managing withdrawal.5,6
The safest and most effective management approach for patients with BZD withdrawal is reinstitution of the BZD followed by a prolonged and gradual tapering until cessation, if that is desired.1,2,5,6 While all BZDs share structural and mechanistic similarities, there are subtle variations within this class that can affect their pharmacologic effects. These structural differences may result in incomplete cross-tolerance, which may lead to inadequate mitigation of the withdrawal syndrome. For example, previous reports suggest that alprazolam and clonazepam are structurally unique and bind to the BZD receptor with higher affinity than other BZDs. Therefore, while in general any BZD can be used to treat withdrawal from another BZD, it is recommended to treat withdrawal from these two agents with the implicated BZD.
There are, however, limitations to this approach. Namely, some BZDs are only available in oral formulations (eg, alprazolam and clonazepam) or the BZD of choice may not be readily available or on formulary within a given institution. In a patient with a severe withdrawal syndrome where it is not feasible or potentially harmful to administer an oral medication, it is reasonable to provide parenteral (preferably intravenous [IV]) BZD therapy. The optimal approach is to start with a small “standard” dose and titrate to effect while monitoring for adverse effects (eg, oversedation, ventilatory depression). Redosing should be triggered by symptoms or signs, and not performed in a timed or standing-order fashion. If this approach proves ineffective and withdrawal symptoms persist despite adequate BZD therapy, a direct GABA agonist such as propofol is a sensible alternative or adjuvant treatment. This may sound similar to the management of patients with ethanol withdrawal; indeed, this approach is essentially the same, with the exception of the more drawn-out time course.
Case Conclusion
After arrival in the ED, the patient received diazepam 10 mg IV and was subsequently admitted to the hospital for further evaluation. During his hospitalization, the patient was re-started on his usual dose of oral alprazolam. No further withdrawal syndrome was observed, and he was discharged on hospital day 2 with a plan to slowly taper his alprazolam dose with his outpatient psychiatrist.
Dr Repplinger is a senior medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Withdrawal: Another Danger of Diversion
- Marriott S, Tyrer P. Benzodiazepine dependence. Avoidance and withdrawal. Drug Saf. 1993;9(2):93-103.
- Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
- Authier N, Balayssac D, Sautereau M, et al. Benzodiazepine dependence: focus on withdrawal syndrome. Ann Pharm Fr. 2009;67(6):408-413.
- Pages KP, Ries RK. Use of anticonvulsants in benzodiazepine withdrawal. Am J Addict. 1998;7(3):198-204.
- Ashton H. The treatment of benzodiazepine dependence. Addiction. 1994;89(11):1535-1541.
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD Young R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction. 2009;104(1):13-24.
Case
A 34-year-old man with a history of polysubstance abuse presented to the ED after he had a seizure during his regular methadone-treatment program meeting. While at the clinic, attendees witnessed the patient experience a loss of consciousness accompanied by generalized shaking movements of his extremities, which lasted for several minutes.
Upon arrival in the ED, the patient stated that he had a mild headache; he was otherwise asymptomatic. Initial vital signs were: blood pressure, 126/80 mm Hg; heart rate, 82 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97.3°F. Oxygen saturation was 98% on room air, and a finger-stick glucose test was 140 mg/dL.
Physical examination revealed a small right-sided parietal hematoma. The patient had no tremors and his neurological examination, including mental status, was normal. When reviewing the patient’s medical history and medications in the health record, it was noted that the patient had a prescription for alprazolam for an anxiety disorder. On further questioning, the patient admitted that he had sold his last alprazolam prescription and had not been taking the drug for the past week.
What characterizes the benzodiazepine withdrawal syndrome?
Although introduced into clinical practice in the 1960s, the potential for dependence and a withdrawal syndrome was not appreciated until the early 1980s. This clinical syndrome can manifest with a wide variety of findings, most commonly with what are termed “rebound effects” or “rebound hyperexcitability.” These effects include anxiety, insomnia or sleep disturbance, tremulousness, irritability, sweating, psychomotor agitation, difficulty in concentration, nausea, weight loss, palpitations, headache, muscular pain and stiffness, or generalized weakness.2 More severe manifestations include delirium, seizures, or psychosis. Often, these symptoms and signs may be confused with the very manifestations that prompted the initial use of the BZD, a reemergence of which can exacerbate the withdrawal syndrome.
When does benzodiazepine withdrawal occur?
The exact time course of BZD withdrawal can vary considerably and, unlike alcohol withdrawal (which occurs from a single compound, ethanol), can be difficult to characterize. The onset of withdrawal symptoms is dependent on a number of factors, including the half-life of the BZD involved. For example, delayed onset withdrawal symptoms of up to 3 weeks after cessation of the medication are described with long-acting BZDs such as chlordiazepoxide and diazepam. Conversely, symptoms may present as early as 24 to 48 hours after abrupt termination of BZDs with shorter half-lives, alprazolam and lorazepam. This variable time of onset differs considerably from other withdrawal syndromes, notably ethanol withdrawal. While both syndromes correlate to the individual patient’s severity of dependence, alcohol withdrawal follows a more predictable time course.
Some authors distinguish a rebound syndrome from a true withdrawal syndrome, the former of which is self-limited in nature and the result of cessation of treatment for the primary disease process. In this model, rebound symptoms begin 1 to 4 days after the abrupt cessation or dose reduction of the BZD, and are relatively short-lived, lasting 2 to 3 days.2
What is the appropriate treatment for benzodiazepine withdrawal?
The standard therapy for almost all withdrawal syndromes is reinstitution of the causal agent. A number of non-BZD-based treatment strategies have been investigated, and all have met with limited success. Of these, anticonvulsant drugs such as carbamazepine and valproic acid were initially considered promising based on case reports and small case series.4 These medications ultimately proved ineffective in randomized, placebo-controlled studies.5 β-Adrenergic antagonists, such as propranolol, have been studied as a method to normalize a patient’s vital signs but also proved nonbeneficial in managing withdrawal.5,6
The safest and most effective management approach for patients with BZD withdrawal is reinstitution of the BZD followed by a prolonged and gradual tapering until cessation, if that is desired.1,2,5,6 While all BZDs share structural and mechanistic similarities, there are subtle variations within this class that can affect their pharmacologic effects. These structural differences may result in incomplete cross-tolerance, which may lead to inadequate mitigation of the withdrawal syndrome. For example, previous reports suggest that alprazolam and clonazepam are structurally unique and bind to the BZD receptor with higher affinity than other BZDs. Therefore, while in general any BZD can be used to treat withdrawal from another BZD, it is recommended to treat withdrawal from these two agents with the implicated BZD.
There are, however, limitations to this approach. Namely, some BZDs are only available in oral formulations (eg, alprazolam and clonazepam) or the BZD of choice may not be readily available or on formulary within a given institution. In a patient with a severe withdrawal syndrome where it is not feasible or potentially harmful to administer an oral medication, it is reasonable to provide parenteral (preferably intravenous [IV]) BZD therapy. The optimal approach is to start with a small “standard” dose and titrate to effect while monitoring for adverse effects (eg, oversedation, ventilatory depression). Redosing should be triggered by symptoms or signs, and not performed in a timed or standing-order fashion. If this approach proves ineffective and withdrawal symptoms persist despite adequate BZD therapy, a direct GABA agonist such as propofol is a sensible alternative or adjuvant treatment. This may sound similar to the management of patients with ethanol withdrawal; indeed, this approach is essentially the same, with the exception of the more drawn-out time course.
Case Conclusion
After arrival in the ED, the patient received diazepam 10 mg IV and was subsequently admitted to the hospital for further evaluation. During his hospitalization, the patient was re-started on his usual dose of oral alprazolam. No further withdrawal syndrome was observed, and he was discharged on hospital day 2 with a plan to slowly taper his alprazolam dose with his outpatient psychiatrist.
Dr Repplinger is a senior medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 34-year-old man with a history of polysubstance abuse presented to the ED after he had a seizure during his regular methadone-treatment program meeting. While at the clinic, attendees witnessed the patient experience a loss of consciousness accompanied by generalized shaking movements of his extremities, which lasted for several minutes.
Upon arrival in the ED, the patient stated that he had a mild headache; he was otherwise asymptomatic. Initial vital signs were: blood pressure, 126/80 mm Hg; heart rate, 82 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97.3°F. Oxygen saturation was 98% on room air, and a finger-stick glucose test was 140 mg/dL.
Physical examination revealed a small right-sided parietal hematoma. The patient had no tremors and his neurological examination, including mental status, was normal. When reviewing the patient’s medical history and medications in the health record, it was noted that the patient had a prescription for alprazolam for an anxiety disorder. On further questioning, the patient admitted that he had sold his last alprazolam prescription and had not been taking the drug for the past week.
What characterizes the benzodiazepine withdrawal syndrome?
Although introduced into clinical practice in the 1960s, the potential for dependence and a withdrawal syndrome was not appreciated until the early 1980s. This clinical syndrome can manifest with a wide variety of findings, most commonly with what are termed “rebound effects” or “rebound hyperexcitability.” These effects include anxiety, insomnia or sleep disturbance, tremulousness, irritability, sweating, psychomotor agitation, difficulty in concentration, nausea, weight loss, palpitations, headache, muscular pain and stiffness, or generalized weakness.2 More severe manifestations include delirium, seizures, or psychosis. Often, these symptoms and signs may be confused with the very manifestations that prompted the initial use of the BZD, a reemergence of which can exacerbate the withdrawal syndrome.
When does benzodiazepine withdrawal occur?
The exact time course of BZD withdrawal can vary considerably and, unlike alcohol withdrawal (which occurs from a single compound, ethanol), can be difficult to characterize. The onset of withdrawal symptoms is dependent on a number of factors, including the half-life of the BZD involved. For example, delayed onset withdrawal symptoms of up to 3 weeks after cessation of the medication are described with long-acting BZDs such as chlordiazepoxide and diazepam. Conversely, symptoms may present as early as 24 to 48 hours after abrupt termination of BZDs with shorter half-lives, alprazolam and lorazepam. This variable time of onset differs considerably from other withdrawal syndromes, notably ethanol withdrawal. While both syndromes correlate to the individual patient’s severity of dependence, alcohol withdrawal follows a more predictable time course.
Some authors distinguish a rebound syndrome from a true withdrawal syndrome, the former of which is self-limited in nature and the result of cessation of treatment for the primary disease process. In this model, rebound symptoms begin 1 to 4 days after the abrupt cessation or dose reduction of the BZD, and are relatively short-lived, lasting 2 to 3 days.2
What is the appropriate treatment for benzodiazepine withdrawal?
The standard therapy for almost all withdrawal syndromes is reinstitution of the causal agent. A number of non-BZD-based treatment strategies have been investigated, and all have met with limited success. Of these, anticonvulsant drugs such as carbamazepine and valproic acid were initially considered promising based on case reports and small case series.4 These medications ultimately proved ineffective in randomized, placebo-controlled studies.5 β-Adrenergic antagonists, such as propranolol, have been studied as a method to normalize a patient’s vital signs but also proved nonbeneficial in managing withdrawal.5,6
The safest and most effective management approach for patients with BZD withdrawal is reinstitution of the BZD followed by a prolonged and gradual tapering until cessation, if that is desired.1,2,5,6 While all BZDs share structural and mechanistic similarities, there are subtle variations within this class that can affect their pharmacologic effects. These structural differences may result in incomplete cross-tolerance, which may lead to inadequate mitigation of the withdrawal syndrome. For example, previous reports suggest that alprazolam and clonazepam are structurally unique and bind to the BZD receptor with higher affinity than other BZDs. Therefore, while in general any BZD can be used to treat withdrawal from another BZD, it is recommended to treat withdrawal from these two agents with the implicated BZD.
There are, however, limitations to this approach. Namely, some BZDs are only available in oral formulations (eg, alprazolam and clonazepam) or the BZD of choice may not be readily available or on formulary within a given institution. In a patient with a severe withdrawal syndrome where it is not feasible or potentially harmful to administer an oral medication, it is reasonable to provide parenteral (preferably intravenous [IV]) BZD therapy. The optimal approach is to start with a small “standard” dose and titrate to effect while monitoring for adverse effects (eg, oversedation, ventilatory depression). Redosing should be triggered by symptoms or signs, and not performed in a timed or standing-order fashion. If this approach proves ineffective and withdrawal symptoms persist despite adequate BZD therapy, a direct GABA agonist such as propofol is a sensible alternative or adjuvant treatment. This may sound similar to the management of patients with ethanol withdrawal; indeed, this approach is essentially the same, with the exception of the more drawn-out time course.
Case Conclusion
After arrival in the ED, the patient received diazepam 10 mg IV and was subsequently admitted to the hospital for further evaluation. During his hospitalization, the patient was re-started on his usual dose of oral alprazolam. No further withdrawal syndrome was observed, and he was discharged on hospital day 2 with a plan to slowly taper his alprazolam dose with his outpatient psychiatrist.
Dr Repplinger is a senior medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Withdrawal: Another Danger of Diversion
- Marriott S, Tyrer P. Benzodiazepine dependence. Avoidance and withdrawal. Drug Saf. 1993;9(2):93-103.
- Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
- Authier N, Balayssac D, Sautereau M, et al. Benzodiazepine dependence: focus on withdrawal syndrome. Ann Pharm Fr. 2009;67(6):408-413.
- Pages KP, Ries RK. Use of anticonvulsants in benzodiazepine withdrawal. Am J Addict. 1998;7(3):198-204.
- Ashton H. The treatment of benzodiazepine dependence. Addiction. 1994;89(11):1535-1541.
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD Young R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction. 2009;104(1):13-24.
- Withdrawal: Another Danger of Diversion
- Marriott S, Tyrer P. Benzodiazepine dependence. Avoidance and withdrawal. Drug Saf. 1993;9(2):93-103.
- Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
- Authier N, Balayssac D, Sautereau M, et al. Benzodiazepine dependence: focus on withdrawal syndrome. Ann Pharm Fr. 2009;67(6):408-413.
- Pages KP, Ries RK. Use of anticonvulsants in benzodiazepine withdrawal. Am J Addict. 1998;7(3):198-204.
- Ashton H. The treatment of benzodiazepine dependence. Addiction. 1994;89(11):1535-1541.
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD Young R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction. 2009;104(1):13-24.
Betting the Farm
A 65‐year‐old man with a 6‐month history of diabetes mellitus presented to the emergency department in May with 1 week of fevers, headaches, myalgia, polydipsia, and polyuria.
The patient presents with symptoms suggestive of uncontrolled diabetes and infection. The broad diagnostic categories include acute infection, an emerging chronic process aggravating his diabetes, or a noninfectious mimic such as autoimmune disease or lymphoproliferative disease. New onset headache in an older patient is concerning. Although it may be attributed to fever and dehydration, primary central nervous system processes such as meningitis or encephalitis must be considered. At this stage, a detailed exposure history, including travel, food, pets, hobbies, and sick contacts as well as occupation and national origins is needed. This patient presented in May, making illnesses that peak in other seasons such as influenza and West Nile fever less likely.
He had no other medical problems except diabetes. He was not taking any medications; he had been started on glipizide but had stopped taking it 1 month prior. He denied fever, cough, chest pain, palpitations, abdominal pain, nausea, vomiting, dysuria, focal weakness, visual changes, or photophobia. He was born in Mexico and emigrated at the age of 25 years. Two months prior to presentation he visited a cattle farm in Mexico; he denied any direct contact with farm animals or dairy products. He denied ill contacts, pets, known tuberculosis exposures, and sexual partners other than his wife.
The history of recent travel to Mexico with a visit to a farm raises concerns about zoonoses. The endemic zoonoses that should be considered include parasitic (toxoplasmosis), fungal (coccidiodomycosis), and bacterial (brucellosis, Q fever, leptospirosis, tularemia, salmonellosis) infections. Nonzoonotic granulomatous infections such as cytomegalovirus (CMV) and Epstein‐Barr virus (EBV), mycobacteria, fungi (histoplasmosis, blastomycosis, cryptococcosis, aspergillosis), and bacteria (actinomycosis) should also be considered.
On examination, he was an elderly Hispanic male who appeared ill but in no acute distress. He was overweight, with a BMI of 29. His temperature was 39C, pulse 66 beats/minute, blood pressure 108/68 mm Hg, respiratory rate 18 per minute, and oxygen saturation was 96% on room air. There were no ulcerations, exudates, or erythema in the oropharynx. There was no sinus tenderness or lymphadenopathy. Cardiac examination revealed normal heart sounds with no murmurs. Respiratory examination demonstrated clear lungs. His abdomen was soft and nontender, whereas the liver and spleen were not palpable. There was no nuchal rigidity, and his mental status was normal. There were no cranial nerve deficits or weakness in his extremities. There was no skin rash or peripheral stigmata of infectious endocarditis. Genitourinary examination revealed no ulcerations, inguinal lymphadenopathy, or urethral discharge. There was no tenderness, warmth, or erythema on examination of all joints.
The physical exam is notable for temperaturepulse dissociation. Heart rate should increase by about 10 beats/minute for every 1‐degree increase in Fahrenheit temperature. The infectious causes of temperaturepulse dissociation are largely intracellular pathogens such as Salmonella, Coxiella, Chlamydia, Leptospira, Legionella, Francisella, Mycoplasma, and dengue virus. This patient is at increased risk for infection by any of these pathogens based on his recent travel to Mexico. Drug fever is the most common noninfectious cause of temperaturepulse dissociation, but this patient took no medications. At this point, a complete blood count and differential, urinalysis, blood cultures, chest x‐ray, and electrocardiogram should be ordered. Testing for human immunodeficiency virus (HIV) is appropriate, as up to 50% of patients with newly diagnosed HIV have no acknowledged risk factors. Serological studies for the aforementioned pathogens may be indicated depending on the results of these initial diagnostic tests.
Serum sodium concentration was 122 mEq/L, potassium 4.0 mEq/L, chloride 88 mEq/L, bicarbonate 14 mEq/L, blood urea nitrogen 17 mg/dL, creatinine 0.7 mg/dL, glucose 402 mg/dL, and calcium 8.5 mg/dL. Total protein was 5.4 g/dL, albumin 2.9 g/dL, total bilirubin 0.9 mg/dL, direct bilirubin 0.4 mg/dL, alkaline phosphatase 126 U/L (normal 53128), gamma‐glutamyl transferase 264 U/L (normal 360), aspartate aminotransferase 51 U/L (normal 840), alanine aminotransferase 62 U/L (normal 556), and lactate dehydrogenase 248 U/L (normal 85210). The white blood cell (WBC) count was 6800 mm3 (51% band forms, 38% segmented neutrophils, 6% monocytes, 5% lymphocytes). The hemoglobin was 15.7 g/dL, with mean corpuscular volume (MCV) of 102 fL and platelet count 59,000/mm3. Peripheral‐blood smear showed occasional macrocytes. Prothrombin time was 13.6 seconds and partial thromboplastin time was 34.5 seconds. C‐reactive protein was 11.8 mg/dL. Urinalysis revealed 80 mg of ketones per deciliter, no cells, and nitrite was negative. Hemoglobin A1c was 13%, and HIV antibody testing was negative.
Elevated circulating bands and thrombocytopenia suggest infection; however, bone marrow infiltration by infectious or neoplastic process is also possible and should be investigated. The increased gamma‐glutamyl transferase, alkaline phosphatase, and mild increases in transaminases suggest hepatic pathology. The combination of unexplained fever, hyponatremia, thrombocytopenia, elevated liver enzymes, and travel to Mexico mandates investigation for infectious diseases that often involve both the bone marrow and liver such as Brucella, Coxiella, and fungal infections such as histoplasmosis. Autoimmune diseases such as systemic lupus erythematosus and malignancy should also be considered. Blood cultures should be incubated beyond the usual 5 days because of the slower growth of Brucella or Salmonella typhi. An HIV viral load should be obtained to evaluate for acute retroviral syndrome. Serologic tests for Rickettsia, Coccidiodes, and hepatitis A, B, and C viruses should be obtained. Urine should be tested for Histoplasma and Legionella antigens. Abdominal imaging should be obtained to evaluate for hepatobiliary disease, occult intra‐abdominal abscess, or malignancy. Because the patient has unexplained fever and headache, imaging of the central nervous system and lumbar puncture are warranted.
His diabetic ketoacidosis (DKA) was treated with intravenous fluids and insulin. Lumbar puncture and cerebrospinal fluid (CSF) analysis revealed opening pressure of 18 cm H20 (normal 1025), cell count WBC 3/L (normal 05), red blood cell 204/L (normal 0), CSF protein 25 mg/dL (normal 2050), and glucose 68 mg/dL (normal 5070). Blood cultures showed no growth. HIV RNA was undetectable. Hepatitis C antibody was negative, and hepatitis A and B serologies were not consistent with an acute infection. Serum ferritin was 1147 ng/mL. Histoplasma and Legionella urine antigen tests were negative. CMV, EBV, and herpes simplex virus DNA were not detected in blood samples. Anti‐neutrophil antibody, anti‐mitochondrial antibody and anti‐neutrophil cytoplasmic antibodies were undetectable. Anti‐smooth muscle antibody was positive at a titer of 1:80. Transthoracic echocardiogram revealed normal heart valves without vegetations. A chest radiograph was normal. Brain computed tomography (CT) revealed atrophic frontal lobes. CT of his chest, abdomen, and pelvis demonstrated focal inflammatory changes of a loop of distal small bowel with surrounding fluid collection, suggesting small bowel diverticulitis. There were no pulmonary infiltrates noted, and the remainder of the CT was unremarkable.
Because the patient remains ill and additional serological test results will take time to return, a key consideration at this point is empiric treatment while awaiting test results. The CSF examination was normal. A history of travel including animal and tick exposures should be reevaluated. The timing of the trip to Mexico was outside the usual incubation period for many pathogens except for Coxiella or Brucella, and empiric therapy for both would be appropriate. The abdominal CT suggests small bowel diverticulitis, which is a rare clinical entity.
The benign abdominal examination suggests the finding is incidental. However, there are several infections that may involve the distal small bowel and proximal colon, such as yersiniosis, salmonellosis, tuberculosis, actinomycosis, histoplasmosis, and noninfectious processes including Crohn's disease and neoplasia. The absence of diarrhea or hematochezia makes yersiniosis, salmonellosis, and Crohn's disease unlikely. Histoplasmosis is unlikely given the negative urine antigen. Evaluation for neoplasia of the distal small bowel requires histologic examination. A colonoscopy with random biopsies of the colon and terminal ileum is the next step if other tests are unrevealing.
The patient was empirically treated for small bowel diverticulitis with ceftriaxone and metronidazole. Because of continued daily fevers as high as 39C, his therapy was changed to vancomycin and piperacillin‐tazobactam to cover methicillin‐resistant Staphylococcus aureus and resistant gram‐negative bacilli. The patient developed new scleral icterus on hospital day 6; the remainder of his examination was unchanged. Serum sodium concentration was 127 mEq/L, potassium 2.7 mEq/L, phosphorus 1.3 mg/dL, magnesium 1.6 mg/dL, total bilirubin 5.6 mg/dL, direct bilirubin 3.6 mg/dL, alkaline phosphatase 193 U/L, gamma‐glutamyl transferase 300 U/L, aspartate aminotransferase 91 U/L, alanine aminotransferase 52 U/L. Brucella serology was negative.
His liver enzymes remain elevated with new onset jaundice consistent with hepatitis and intrahepatic cholestasis. His persistent hypophosphatemia, hypokalemia, and hypomagnesaemia well after resolution of diabetic ketoacidosis suggests acute tubulointerstitial dysfunction, which may be a complication of empiric antibiotic treatment or renal involvement by his underlying condition. Additional blood cultures, and tissue examination and culture are the next appropriate steps. Liver or bone marrow biopsy may suggest a diagnosis that can be confirmed by tissue culture or immunohistochemistry. Histologic findings such as fibrin ringed granulomas, caseating or noncaseating granulomas, or lymphomatous infiltration may suggest Coxiella (Q fever), tuberculosis, or lymphoma respectively. Because a liver biopsy is invasive and usually provides less tissue for culture, bone marrow examination should be obtained first.
A gallium 67 scan showed nonhomogenous increased uptake in both lungs and kidneys, consistent with interstitial nephritis and bilateral pneumonia. Serum protein electrophoresis demonstrated a monoclonal immunoglobulin (Ig)G lambda band with a kappa/lambda ratio of 0.9 (normal 1.42.8). Bone marrow biopsy showed normal hematopoiesis; no plasma or malignant cells, granulomas, or evidence of hemophagocytosis; and fungal and mycobacterial stains and cultures were negative. Colonoscopy revealed normal‐appearing mucosa. Histologic examination and culture of random biopsies from the colon and terminal ileum were negative for fungi, viruses, and mycobacteria. An ultrasound‐guided liver biopsy revealed numerous noncaseating granulomas formed of histiocytes and neutrophils with occasional fibrin rings. Fungal, viral, and mycobacterial stains and cultures were negative. The patient's fever resolved after 14 days, and he was discharged home without a diagnosis and close outpatient follow‐up.
The hepatic granulomas with fibrin rings are highly suggestive of Q fever, although ring granulomas may be seen in tuberculosis, typhoid fever, lymphoma, drug reactions, sarcoidosis, and CMV infections. Competing diagnoses such as CMV have been excluded by negative serology. Microscopic examination, tissue staining, and culture from liver and bone marrow biopsies were negative for S typhi, mycobacteria, and lymphoma. Gallium scan findings are generally nonspecific and of little utility in cases such as this. The kidney involvement correlates with the biochemical evidence of tubulointerstitial dysfunction; pulmonary involvement may reflect subclinical pulmonary infection with Coxiella. Given the normal bone marrow biopsy, the monoclonal gammopathy is of undetermined significance. The positive anti‐smooth muscle antibody can be related to Q fever. Anti‐smooth muscle antibodies frequently occur in Q fever, especially in those patients with hepatitis. Given the history of exposure to cattle, unexplained fever with temperaturepulse dissociation and liver biopsy findings, Q fever is the most likely diagnosis and empiric treatment with doxycycline is warranted.
Results of serology for Coxiella burnetii sent during admission were returned after the patient's discharge. C burnetii phase I IgG and IgM antibody titers were positive (1:512 each). C burnetii phase II IgG and IgM titers also were positive (1:1024 each). The patient was seen within a week and started on doxycycline 100 mg twice daily for 2 weeks for acute Q fever. His symptoms improved; hyponatremia, liver function tests, and thrombocytopenia normalized after treatment.
DISCUSSION
Q fever was first described in 1937 as a febrile illness affecting Australian slaughterhouse workers.[1] The Q in Q fever stands for query and reflected the initial uncertainty surrounding the underlying cause of the illness. The causative organism, C burnetti, is an obligate intracellular bacterium that resides within macrophage lysosomes. It can be found in the urine, feces, milk, placenta, and amniotic fluid of ungulates (cattle, sheep, and other ruminants), and other animals such as domestic cats and dogs. C burnetii is transmitted via inhalation, ingestion, occupational, or common source exposures, and in 1 case report by person‐to‐person sexual transmission.[2] In addition to slaughterhouse workers, pregnant women and immunosuppressed patients are more susceptible to developing Q fever.[3] For patients with suspected Q fever, a detailed occupational history, including specific job duties and potential exposure to animal products, is imperative.
Q fever has both acute and chronic presentations, which are differentiated based on the clinical illness and serologies. The symptoms of acute Q fever are nonspecific and may include influenza‐like illness, fever, pneumonia, and hepatitis. It presents less commonly with hemolytic anemia, interstitial nephritis, monoclonal gammopathy, or aseptic meningitis.[4, 5, 6, 7] Symptoms typically begin between 1 and 3 weeks after animal exposure and may persist for several months. Chronic Q fever occurs when unrecognized or untreated infection persists for greater than 6 months. It commonly presents with culture‐negative endocarditis, although infected aneurysms, osteomyelitis, or other distant sites of infection may also occur.
C burnetti is present in 2 antigenic forms that can be assessed by serology. Phase I is the more virulent, infectious form of C burnetti, which transitions to the avirulent phase II form during laboratory handling. In acute Q fever, phase II serologies are typically elevated out of proportion to phase I serologies, whereas this pattern is reversed in chronic Q fever. The diagnostic gold standard of acute Q fever is a 4‐fold rise in phase II antibody titers taken 3 to 6 weeks apart.[8] Histologic examination of affected organs can support a diagnosis of Q fever. The presence of ringed granulomas on liver or bone marrow biopsy specimens is highly suggestive, but not pathognomonic, of Q fever.[9]
Q fever is highly susceptible to several classes of antibiotics. For acute Q fever, doxycycline and tetracycline are typically used, with fluoroquinolones and chloramphenicol as alternatives.[8, 10] Patients with chronic Q fever should be treated with doxycycline and hydroxychloroquine. The addition of hydroxychloroquine alkalinizes the macrophage lysosome and enhances bacterial eradication.[8] For patients with acute Q fever, physicians should determine the risk of progression to chronic Q fever because closer monitoring is necessary. Patients with valvular heart lesions, immunosuppression, and pregnant women are at elevated risk of chronic Q fever. Trimethoprim/sulfamethoxazole can be used in place of doxycycline in pregnant women, as doxycycline and fluoroquinolones are contraindicated in pregnancy.[8]
This patient presented with a nonspecific febrile illness. Although the treating clinicians obtained a history of exposure to cattle early in his course, both the diagnosis and treatment were delayed. There are several possible explanations for the delay. First, although Q fever is a relatively common zoonosis, it remains an uncommon diagnosis, particularly among hospitalized patients. As a result, clinicians often focus on more common conditions. In this case, typical infections, malignancies, and inflammatory diseases were considered more likely. Second, the patient presented with hepatitis, an uncommon presentation of Q fever. Classical clinical reasoning suggests that atypical presentations of common diseases will occur more frequently than typical presentations of uncommon diseases. This case presented with an atypical presentation of an uncommon disease. The resultant lower pretest probability further dissuaded the patient's physicians from consideration of Q fever. Third, the finding of small bowel diverticulitis was a potential distractor. In patients with nonspecific febrile illnesses, it is common for physicians to anchor on any abnormal findings. In this case, the small bowel diverticulitis led to antibiotic treatment that was ineffective against C burnetti.
There were several clues to the diagnosis of Q fever in this patient's presentation. First, the pulsetemperature dissociation suggested infection with an intracellular pathogen. Hospitalists should recognize this association and be mindful of this often‐subtle clinical finding when faced with diagnostic uncertainty. Second, the patient was exposed to cattle prior to the onset of his illness. The fact that he did not have a direct exposure to animals underscores the infectivity of C burnetti. Finally, elevated alkaline phosphatase and transaminases were suggestive of an infiltrative disease; in the setting of a nonspecific febrile illness, Q fever was an important diagnostic consideration.
The key treatment decision in this case was the initiation and choice of antibiotics. Because of this patient's history of exposure to cattle and lack of a compelling alternative diagnosis, empiric treatment with doxycycline would have been appropriate. Hospitalists must weigh the potential benefit of early treatment of Q fever against the risks associated with antibiotic overuse. In patients presenting with a febrile illness after ungulate exposure, the decision to bet the farm with empiric doxycycline therapy may lead to clinical improvement, obviating a more invasive or extensive diagnostic evaluation.
TEACHING POINTS
- Acute Q fever typically presents 2 to 3 weeks after ungulate exposure with a febrile illness, pneumonia, and granulomatous hepatitis.
- Pulsetemperature dissociation is suggestive of infection by intracellular pathogens such as Coxiella, Salmonella, Leptospira, Legionella, and Mycoplasma.
- Clinicians should consider empiric doxycycline therapy in patients with suspected zoonosis (eg, Q fever, brucellosis, anaplasmosis, leptospirosis, Rocky Mountain spotted fever) while awaiting confirmatory tests, as improvement may obviate invasive testing.
Disclosure: Nothing to report.
- . “Q” fever, a new fever entity: clinical features, diagnosis, and laboratory investigation. Rev Infect Dis. 1983;5(4):790–800.
- , , , . Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3(11):709–721.
- , , , . Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;15:44(2):232–237.
- , , , , . Unusual manifestations of acute Q fever: autoimmune hemolytic anemia and tubulointerstitial nephritis. Ann of Clin Microbiol Antimicrob. 2012;11:14.
- , , . Q fever. Lancet. 2006;367(9511):679–688.
- , , , , , . Transitory monoclonal gammopathy and acute Q fever. Enferm Infecc Microbiol Clin. 1995;13(7):442.
- , . Q fever. Clin Microbiol Rev. 1999;12(4):518–553.
- , , , et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62(RR‐03):1–30.
- , , , et al. Hepatic fibrin‐ring granulomas: a clinicopathologic study of 23 patients. Hum Pathol. 1991;22(6):607–613.
- , , . Travel‐associated zoonotic bacterial diseases. Curr Opin Infect Dis. 2011;24(5):457–463.
A 65‐year‐old man with a 6‐month history of diabetes mellitus presented to the emergency department in May with 1 week of fevers, headaches, myalgia, polydipsia, and polyuria.
The patient presents with symptoms suggestive of uncontrolled diabetes and infection. The broad diagnostic categories include acute infection, an emerging chronic process aggravating his diabetes, or a noninfectious mimic such as autoimmune disease or lymphoproliferative disease. New onset headache in an older patient is concerning. Although it may be attributed to fever and dehydration, primary central nervous system processes such as meningitis or encephalitis must be considered. At this stage, a detailed exposure history, including travel, food, pets, hobbies, and sick contacts as well as occupation and national origins is needed. This patient presented in May, making illnesses that peak in other seasons such as influenza and West Nile fever less likely.
He had no other medical problems except diabetes. He was not taking any medications; he had been started on glipizide but had stopped taking it 1 month prior. He denied fever, cough, chest pain, palpitations, abdominal pain, nausea, vomiting, dysuria, focal weakness, visual changes, or photophobia. He was born in Mexico and emigrated at the age of 25 years. Two months prior to presentation he visited a cattle farm in Mexico; he denied any direct contact with farm animals or dairy products. He denied ill contacts, pets, known tuberculosis exposures, and sexual partners other than his wife.
The history of recent travel to Mexico with a visit to a farm raises concerns about zoonoses. The endemic zoonoses that should be considered include parasitic (toxoplasmosis), fungal (coccidiodomycosis), and bacterial (brucellosis, Q fever, leptospirosis, tularemia, salmonellosis) infections. Nonzoonotic granulomatous infections such as cytomegalovirus (CMV) and Epstein‐Barr virus (EBV), mycobacteria, fungi (histoplasmosis, blastomycosis, cryptococcosis, aspergillosis), and bacteria (actinomycosis) should also be considered.
On examination, he was an elderly Hispanic male who appeared ill but in no acute distress. He was overweight, with a BMI of 29. His temperature was 39C, pulse 66 beats/minute, blood pressure 108/68 mm Hg, respiratory rate 18 per minute, and oxygen saturation was 96% on room air. There were no ulcerations, exudates, or erythema in the oropharynx. There was no sinus tenderness or lymphadenopathy. Cardiac examination revealed normal heart sounds with no murmurs. Respiratory examination demonstrated clear lungs. His abdomen was soft and nontender, whereas the liver and spleen were not palpable. There was no nuchal rigidity, and his mental status was normal. There were no cranial nerve deficits or weakness in his extremities. There was no skin rash or peripheral stigmata of infectious endocarditis. Genitourinary examination revealed no ulcerations, inguinal lymphadenopathy, or urethral discharge. There was no tenderness, warmth, or erythema on examination of all joints.
The physical exam is notable for temperaturepulse dissociation. Heart rate should increase by about 10 beats/minute for every 1‐degree increase in Fahrenheit temperature. The infectious causes of temperaturepulse dissociation are largely intracellular pathogens such as Salmonella, Coxiella, Chlamydia, Leptospira, Legionella, Francisella, Mycoplasma, and dengue virus. This patient is at increased risk for infection by any of these pathogens based on his recent travel to Mexico. Drug fever is the most common noninfectious cause of temperaturepulse dissociation, but this patient took no medications. At this point, a complete blood count and differential, urinalysis, blood cultures, chest x‐ray, and electrocardiogram should be ordered. Testing for human immunodeficiency virus (HIV) is appropriate, as up to 50% of patients with newly diagnosed HIV have no acknowledged risk factors. Serological studies for the aforementioned pathogens may be indicated depending on the results of these initial diagnostic tests.
Serum sodium concentration was 122 mEq/L, potassium 4.0 mEq/L, chloride 88 mEq/L, bicarbonate 14 mEq/L, blood urea nitrogen 17 mg/dL, creatinine 0.7 mg/dL, glucose 402 mg/dL, and calcium 8.5 mg/dL. Total protein was 5.4 g/dL, albumin 2.9 g/dL, total bilirubin 0.9 mg/dL, direct bilirubin 0.4 mg/dL, alkaline phosphatase 126 U/L (normal 53128), gamma‐glutamyl transferase 264 U/L (normal 360), aspartate aminotransferase 51 U/L (normal 840), alanine aminotransferase 62 U/L (normal 556), and lactate dehydrogenase 248 U/L (normal 85210). The white blood cell (WBC) count was 6800 mm3 (51% band forms, 38% segmented neutrophils, 6% monocytes, 5% lymphocytes). The hemoglobin was 15.7 g/dL, with mean corpuscular volume (MCV) of 102 fL and platelet count 59,000/mm3. Peripheral‐blood smear showed occasional macrocytes. Prothrombin time was 13.6 seconds and partial thromboplastin time was 34.5 seconds. C‐reactive protein was 11.8 mg/dL. Urinalysis revealed 80 mg of ketones per deciliter, no cells, and nitrite was negative. Hemoglobin A1c was 13%, and HIV antibody testing was negative.
Elevated circulating bands and thrombocytopenia suggest infection; however, bone marrow infiltration by infectious or neoplastic process is also possible and should be investigated. The increased gamma‐glutamyl transferase, alkaline phosphatase, and mild increases in transaminases suggest hepatic pathology. The combination of unexplained fever, hyponatremia, thrombocytopenia, elevated liver enzymes, and travel to Mexico mandates investigation for infectious diseases that often involve both the bone marrow and liver such as Brucella, Coxiella, and fungal infections such as histoplasmosis. Autoimmune diseases such as systemic lupus erythematosus and malignancy should also be considered. Blood cultures should be incubated beyond the usual 5 days because of the slower growth of Brucella or Salmonella typhi. An HIV viral load should be obtained to evaluate for acute retroviral syndrome. Serologic tests for Rickettsia, Coccidiodes, and hepatitis A, B, and C viruses should be obtained. Urine should be tested for Histoplasma and Legionella antigens. Abdominal imaging should be obtained to evaluate for hepatobiliary disease, occult intra‐abdominal abscess, or malignancy. Because the patient has unexplained fever and headache, imaging of the central nervous system and lumbar puncture are warranted.
His diabetic ketoacidosis (DKA) was treated with intravenous fluids and insulin. Lumbar puncture and cerebrospinal fluid (CSF) analysis revealed opening pressure of 18 cm H20 (normal 1025), cell count WBC 3/L (normal 05), red blood cell 204/L (normal 0), CSF protein 25 mg/dL (normal 2050), and glucose 68 mg/dL (normal 5070). Blood cultures showed no growth. HIV RNA was undetectable. Hepatitis C antibody was negative, and hepatitis A and B serologies were not consistent with an acute infection. Serum ferritin was 1147 ng/mL. Histoplasma and Legionella urine antigen tests were negative. CMV, EBV, and herpes simplex virus DNA were not detected in blood samples. Anti‐neutrophil antibody, anti‐mitochondrial antibody and anti‐neutrophil cytoplasmic antibodies were undetectable. Anti‐smooth muscle antibody was positive at a titer of 1:80. Transthoracic echocardiogram revealed normal heart valves without vegetations. A chest radiograph was normal. Brain computed tomography (CT) revealed atrophic frontal lobes. CT of his chest, abdomen, and pelvis demonstrated focal inflammatory changes of a loop of distal small bowel with surrounding fluid collection, suggesting small bowel diverticulitis. There were no pulmonary infiltrates noted, and the remainder of the CT was unremarkable.
Because the patient remains ill and additional serological test results will take time to return, a key consideration at this point is empiric treatment while awaiting test results. The CSF examination was normal. A history of travel including animal and tick exposures should be reevaluated. The timing of the trip to Mexico was outside the usual incubation period for many pathogens except for Coxiella or Brucella, and empiric therapy for both would be appropriate. The abdominal CT suggests small bowel diverticulitis, which is a rare clinical entity.
The benign abdominal examination suggests the finding is incidental. However, there are several infections that may involve the distal small bowel and proximal colon, such as yersiniosis, salmonellosis, tuberculosis, actinomycosis, histoplasmosis, and noninfectious processes including Crohn's disease and neoplasia. The absence of diarrhea or hematochezia makes yersiniosis, salmonellosis, and Crohn's disease unlikely. Histoplasmosis is unlikely given the negative urine antigen. Evaluation for neoplasia of the distal small bowel requires histologic examination. A colonoscopy with random biopsies of the colon and terminal ileum is the next step if other tests are unrevealing.
The patient was empirically treated for small bowel diverticulitis with ceftriaxone and metronidazole. Because of continued daily fevers as high as 39C, his therapy was changed to vancomycin and piperacillin‐tazobactam to cover methicillin‐resistant Staphylococcus aureus and resistant gram‐negative bacilli. The patient developed new scleral icterus on hospital day 6; the remainder of his examination was unchanged. Serum sodium concentration was 127 mEq/L, potassium 2.7 mEq/L, phosphorus 1.3 mg/dL, magnesium 1.6 mg/dL, total bilirubin 5.6 mg/dL, direct bilirubin 3.6 mg/dL, alkaline phosphatase 193 U/L, gamma‐glutamyl transferase 300 U/L, aspartate aminotransferase 91 U/L, alanine aminotransferase 52 U/L. Brucella serology was negative.
His liver enzymes remain elevated with new onset jaundice consistent with hepatitis and intrahepatic cholestasis. His persistent hypophosphatemia, hypokalemia, and hypomagnesaemia well after resolution of diabetic ketoacidosis suggests acute tubulointerstitial dysfunction, which may be a complication of empiric antibiotic treatment or renal involvement by his underlying condition. Additional blood cultures, and tissue examination and culture are the next appropriate steps. Liver or bone marrow biopsy may suggest a diagnosis that can be confirmed by tissue culture or immunohistochemistry. Histologic findings such as fibrin ringed granulomas, caseating or noncaseating granulomas, or lymphomatous infiltration may suggest Coxiella (Q fever), tuberculosis, or lymphoma respectively. Because a liver biopsy is invasive and usually provides less tissue for culture, bone marrow examination should be obtained first.
A gallium 67 scan showed nonhomogenous increased uptake in both lungs and kidneys, consistent with interstitial nephritis and bilateral pneumonia. Serum protein electrophoresis demonstrated a monoclonal immunoglobulin (Ig)G lambda band with a kappa/lambda ratio of 0.9 (normal 1.42.8). Bone marrow biopsy showed normal hematopoiesis; no plasma or malignant cells, granulomas, or evidence of hemophagocytosis; and fungal and mycobacterial stains and cultures were negative. Colonoscopy revealed normal‐appearing mucosa. Histologic examination and culture of random biopsies from the colon and terminal ileum were negative for fungi, viruses, and mycobacteria. An ultrasound‐guided liver biopsy revealed numerous noncaseating granulomas formed of histiocytes and neutrophils with occasional fibrin rings. Fungal, viral, and mycobacterial stains and cultures were negative. The patient's fever resolved after 14 days, and he was discharged home without a diagnosis and close outpatient follow‐up.
The hepatic granulomas with fibrin rings are highly suggestive of Q fever, although ring granulomas may be seen in tuberculosis, typhoid fever, lymphoma, drug reactions, sarcoidosis, and CMV infections. Competing diagnoses such as CMV have been excluded by negative serology. Microscopic examination, tissue staining, and culture from liver and bone marrow biopsies were negative for S typhi, mycobacteria, and lymphoma. Gallium scan findings are generally nonspecific and of little utility in cases such as this. The kidney involvement correlates with the biochemical evidence of tubulointerstitial dysfunction; pulmonary involvement may reflect subclinical pulmonary infection with Coxiella. Given the normal bone marrow biopsy, the monoclonal gammopathy is of undetermined significance. The positive anti‐smooth muscle antibody can be related to Q fever. Anti‐smooth muscle antibodies frequently occur in Q fever, especially in those patients with hepatitis. Given the history of exposure to cattle, unexplained fever with temperaturepulse dissociation and liver biopsy findings, Q fever is the most likely diagnosis and empiric treatment with doxycycline is warranted.
Results of serology for Coxiella burnetii sent during admission were returned after the patient's discharge. C burnetii phase I IgG and IgM antibody titers were positive (1:512 each). C burnetii phase II IgG and IgM titers also were positive (1:1024 each). The patient was seen within a week and started on doxycycline 100 mg twice daily for 2 weeks for acute Q fever. His symptoms improved; hyponatremia, liver function tests, and thrombocytopenia normalized after treatment.
DISCUSSION
Q fever was first described in 1937 as a febrile illness affecting Australian slaughterhouse workers.[1] The Q in Q fever stands for query and reflected the initial uncertainty surrounding the underlying cause of the illness. The causative organism, C burnetti, is an obligate intracellular bacterium that resides within macrophage lysosomes. It can be found in the urine, feces, milk, placenta, and amniotic fluid of ungulates (cattle, sheep, and other ruminants), and other animals such as domestic cats and dogs. C burnetii is transmitted via inhalation, ingestion, occupational, or common source exposures, and in 1 case report by person‐to‐person sexual transmission.[2] In addition to slaughterhouse workers, pregnant women and immunosuppressed patients are more susceptible to developing Q fever.[3] For patients with suspected Q fever, a detailed occupational history, including specific job duties and potential exposure to animal products, is imperative.
Q fever has both acute and chronic presentations, which are differentiated based on the clinical illness and serologies. The symptoms of acute Q fever are nonspecific and may include influenza‐like illness, fever, pneumonia, and hepatitis. It presents less commonly with hemolytic anemia, interstitial nephritis, monoclonal gammopathy, or aseptic meningitis.[4, 5, 6, 7] Symptoms typically begin between 1 and 3 weeks after animal exposure and may persist for several months. Chronic Q fever occurs when unrecognized or untreated infection persists for greater than 6 months. It commonly presents with culture‐negative endocarditis, although infected aneurysms, osteomyelitis, or other distant sites of infection may also occur.
C burnetti is present in 2 antigenic forms that can be assessed by serology. Phase I is the more virulent, infectious form of C burnetti, which transitions to the avirulent phase II form during laboratory handling. In acute Q fever, phase II serologies are typically elevated out of proportion to phase I serologies, whereas this pattern is reversed in chronic Q fever. The diagnostic gold standard of acute Q fever is a 4‐fold rise in phase II antibody titers taken 3 to 6 weeks apart.[8] Histologic examination of affected organs can support a diagnosis of Q fever. The presence of ringed granulomas on liver or bone marrow biopsy specimens is highly suggestive, but not pathognomonic, of Q fever.[9]
Q fever is highly susceptible to several classes of antibiotics. For acute Q fever, doxycycline and tetracycline are typically used, with fluoroquinolones and chloramphenicol as alternatives.[8, 10] Patients with chronic Q fever should be treated with doxycycline and hydroxychloroquine. The addition of hydroxychloroquine alkalinizes the macrophage lysosome and enhances bacterial eradication.[8] For patients with acute Q fever, physicians should determine the risk of progression to chronic Q fever because closer monitoring is necessary. Patients with valvular heart lesions, immunosuppression, and pregnant women are at elevated risk of chronic Q fever. Trimethoprim/sulfamethoxazole can be used in place of doxycycline in pregnant women, as doxycycline and fluoroquinolones are contraindicated in pregnancy.[8]
This patient presented with a nonspecific febrile illness. Although the treating clinicians obtained a history of exposure to cattle early in his course, both the diagnosis and treatment were delayed. There are several possible explanations for the delay. First, although Q fever is a relatively common zoonosis, it remains an uncommon diagnosis, particularly among hospitalized patients. As a result, clinicians often focus on more common conditions. In this case, typical infections, malignancies, and inflammatory diseases were considered more likely. Second, the patient presented with hepatitis, an uncommon presentation of Q fever. Classical clinical reasoning suggests that atypical presentations of common diseases will occur more frequently than typical presentations of uncommon diseases. This case presented with an atypical presentation of an uncommon disease. The resultant lower pretest probability further dissuaded the patient's physicians from consideration of Q fever. Third, the finding of small bowel diverticulitis was a potential distractor. In patients with nonspecific febrile illnesses, it is common for physicians to anchor on any abnormal findings. In this case, the small bowel diverticulitis led to antibiotic treatment that was ineffective against C burnetti.
There were several clues to the diagnosis of Q fever in this patient's presentation. First, the pulsetemperature dissociation suggested infection with an intracellular pathogen. Hospitalists should recognize this association and be mindful of this often‐subtle clinical finding when faced with diagnostic uncertainty. Second, the patient was exposed to cattle prior to the onset of his illness. The fact that he did not have a direct exposure to animals underscores the infectivity of C burnetti. Finally, elevated alkaline phosphatase and transaminases were suggestive of an infiltrative disease; in the setting of a nonspecific febrile illness, Q fever was an important diagnostic consideration.
The key treatment decision in this case was the initiation and choice of antibiotics. Because of this patient's history of exposure to cattle and lack of a compelling alternative diagnosis, empiric treatment with doxycycline would have been appropriate. Hospitalists must weigh the potential benefit of early treatment of Q fever against the risks associated with antibiotic overuse. In patients presenting with a febrile illness after ungulate exposure, the decision to bet the farm with empiric doxycycline therapy may lead to clinical improvement, obviating a more invasive or extensive diagnostic evaluation.
TEACHING POINTS
- Acute Q fever typically presents 2 to 3 weeks after ungulate exposure with a febrile illness, pneumonia, and granulomatous hepatitis.
- Pulsetemperature dissociation is suggestive of infection by intracellular pathogens such as Coxiella, Salmonella, Leptospira, Legionella, and Mycoplasma.
- Clinicians should consider empiric doxycycline therapy in patients with suspected zoonosis (eg, Q fever, brucellosis, anaplasmosis, leptospirosis, Rocky Mountain spotted fever) while awaiting confirmatory tests, as improvement may obviate invasive testing.
Disclosure: Nothing to report.
A 65‐year‐old man with a 6‐month history of diabetes mellitus presented to the emergency department in May with 1 week of fevers, headaches, myalgia, polydipsia, and polyuria.
The patient presents with symptoms suggestive of uncontrolled diabetes and infection. The broad diagnostic categories include acute infection, an emerging chronic process aggravating his diabetes, or a noninfectious mimic such as autoimmune disease or lymphoproliferative disease. New onset headache in an older patient is concerning. Although it may be attributed to fever and dehydration, primary central nervous system processes such as meningitis or encephalitis must be considered. At this stage, a detailed exposure history, including travel, food, pets, hobbies, and sick contacts as well as occupation and national origins is needed. This patient presented in May, making illnesses that peak in other seasons such as influenza and West Nile fever less likely.
He had no other medical problems except diabetes. He was not taking any medications; he had been started on glipizide but had stopped taking it 1 month prior. He denied fever, cough, chest pain, palpitations, abdominal pain, nausea, vomiting, dysuria, focal weakness, visual changes, or photophobia. He was born in Mexico and emigrated at the age of 25 years. Two months prior to presentation he visited a cattle farm in Mexico; he denied any direct contact with farm animals or dairy products. He denied ill contacts, pets, known tuberculosis exposures, and sexual partners other than his wife.
The history of recent travel to Mexico with a visit to a farm raises concerns about zoonoses. The endemic zoonoses that should be considered include parasitic (toxoplasmosis), fungal (coccidiodomycosis), and bacterial (brucellosis, Q fever, leptospirosis, tularemia, salmonellosis) infections. Nonzoonotic granulomatous infections such as cytomegalovirus (CMV) and Epstein‐Barr virus (EBV), mycobacteria, fungi (histoplasmosis, blastomycosis, cryptococcosis, aspergillosis), and bacteria (actinomycosis) should also be considered.
On examination, he was an elderly Hispanic male who appeared ill but in no acute distress. He was overweight, with a BMI of 29. His temperature was 39C, pulse 66 beats/minute, blood pressure 108/68 mm Hg, respiratory rate 18 per minute, and oxygen saturation was 96% on room air. There were no ulcerations, exudates, or erythema in the oropharynx. There was no sinus tenderness or lymphadenopathy. Cardiac examination revealed normal heart sounds with no murmurs. Respiratory examination demonstrated clear lungs. His abdomen was soft and nontender, whereas the liver and spleen were not palpable. There was no nuchal rigidity, and his mental status was normal. There were no cranial nerve deficits or weakness in his extremities. There was no skin rash or peripheral stigmata of infectious endocarditis. Genitourinary examination revealed no ulcerations, inguinal lymphadenopathy, or urethral discharge. There was no tenderness, warmth, or erythema on examination of all joints.
The physical exam is notable for temperaturepulse dissociation. Heart rate should increase by about 10 beats/minute for every 1‐degree increase in Fahrenheit temperature. The infectious causes of temperaturepulse dissociation are largely intracellular pathogens such as Salmonella, Coxiella, Chlamydia, Leptospira, Legionella, Francisella, Mycoplasma, and dengue virus. This patient is at increased risk for infection by any of these pathogens based on his recent travel to Mexico. Drug fever is the most common noninfectious cause of temperaturepulse dissociation, but this patient took no medications. At this point, a complete blood count and differential, urinalysis, blood cultures, chest x‐ray, and electrocardiogram should be ordered. Testing for human immunodeficiency virus (HIV) is appropriate, as up to 50% of patients with newly diagnosed HIV have no acknowledged risk factors. Serological studies for the aforementioned pathogens may be indicated depending on the results of these initial diagnostic tests.
Serum sodium concentration was 122 mEq/L, potassium 4.0 mEq/L, chloride 88 mEq/L, bicarbonate 14 mEq/L, blood urea nitrogen 17 mg/dL, creatinine 0.7 mg/dL, glucose 402 mg/dL, and calcium 8.5 mg/dL. Total protein was 5.4 g/dL, albumin 2.9 g/dL, total bilirubin 0.9 mg/dL, direct bilirubin 0.4 mg/dL, alkaline phosphatase 126 U/L (normal 53128), gamma‐glutamyl transferase 264 U/L (normal 360), aspartate aminotransferase 51 U/L (normal 840), alanine aminotransferase 62 U/L (normal 556), and lactate dehydrogenase 248 U/L (normal 85210). The white blood cell (WBC) count was 6800 mm3 (51% band forms, 38% segmented neutrophils, 6% monocytes, 5% lymphocytes). The hemoglobin was 15.7 g/dL, with mean corpuscular volume (MCV) of 102 fL and platelet count 59,000/mm3. Peripheral‐blood smear showed occasional macrocytes. Prothrombin time was 13.6 seconds and partial thromboplastin time was 34.5 seconds. C‐reactive protein was 11.8 mg/dL. Urinalysis revealed 80 mg of ketones per deciliter, no cells, and nitrite was negative. Hemoglobin A1c was 13%, and HIV antibody testing was negative.
Elevated circulating bands and thrombocytopenia suggest infection; however, bone marrow infiltration by infectious or neoplastic process is also possible and should be investigated. The increased gamma‐glutamyl transferase, alkaline phosphatase, and mild increases in transaminases suggest hepatic pathology. The combination of unexplained fever, hyponatremia, thrombocytopenia, elevated liver enzymes, and travel to Mexico mandates investigation for infectious diseases that often involve both the bone marrow and liver such as Brucella, Coxiella, and fungal infections such as histoplasmosis. Autoimmune diseases such as systemic lupus erythematosus and malignancy should also be considered. Blood cultures should be incubated beyond the usual 5 days because of the slower growth of Brucella or Salmonella typhi. An HIV viral load should be obtained to evaluate for acute retroviral syndrome. Serologic tests for Rickettsia, Coccidiodes, and hepatitis A, B, and C viruses should be obtained. Urine should be tested for Histoplasma and Legionella antigens. Abdominal imaging should be obtained to evaluate for hepatobiliary disease, occult intra‐abdominal abscess, or malignancy. Because the patient has unexplained fever and headache, imaging of the central nervous system and lumbar puncture are warranted.
His diabetic ketoacidosis (DKA) was treated with intravenous fluids and insulin. Lumbar puncture and cerebrospinal fluid (CSF) analysis revealed opening pressure of 18 cm H20 (normal 1025), cell count WBC 3/L (normal 05), red blood cell 204/L (normal 0), CSF protein 25 mg/dL (normal 2050), and glucose 68 mg/dL (normal 5070). Blood cultures showed no growth. HIV RNA was undetectable. Hepatitis C antibody was negative, and hepatitis A and B serologies were not consistent with an acute infection. Serum ferritin was 1147 ng/mL. Histoplasma and Legionella urine antigen tests were negative. CMV, EBV, and herpes simplex virus DNA were not detected in blood samples. Anti‐neutrophil antibody, anti‐mitochondrial antibody and anti‐neutrophil cytoplasmic antibodies were undetectable. Anti‐smooth muscle antibody was positive at a titer of 1:80. Transthoracic echocardiogram revealed normal heart valves without vegetations. A chest radiograph was normal. Brain computed tomography (CT) revealed atrophic frontal lobes. CT of his chest, abdomen, and pelvis demonstrated focal inflammatory changes of a loop of distal small bowel with surrounding fluid collection, suggesting small bowel diverticulitis. There were no pulmonary infiltrates noted, and the remainder of the CT was unremarkable.
Because the patient remains ill and additional serological test results will take time to return, a key consideration at this point is empiric treatment while awaiting test results. The CSF examination was normal. A history of travel including animal and tick exposures should be reevaluated. The timing of the trip to Mexico was outside the usual incubation period for many pathogens except for Coxiella or Brucella, and empiric therapy for both would be appropriate. The abdominal CT suggests small bowel diverticulitis, which is a rare clinical entity.
The benign abdominal examination suggests the finding is incidental. However, there are several infections that may involve the distal small bowel and proximal colon, such as yersiniosis, salmonellosis, tuberculosis, actinomycosis, histoplasmosis, and noninfectious processes including Crohn's disease and neoplasia. The absence of diarrhea or hematochezia makes yersiniosis, salmonellosis, and Crohn's disease unlikely. Histoplasmosis is unlikely given the negative urine antigen. Evaluation for neoplasia of the distal small bowel requires histologic examination. A colonoscopy with random biopsies of the colon and terminal ileum is the next step if other tests are unrevealing.
The patient was empirically treated for small bowel diverticulitis with ceftriaxone and metronidazole. Because of continued daily fevers as high as 39C, his therapy was changed to vancomycin and piperacillin‐tazobactam to cover methicillin‐resistant Staphylococcus aureus and resistant gram‐negative bacilli. The patient developed new scleral icterus on hospital day 6; the remainder of his examination was unchanged. Serum sodium concentration was 127 mEq/L, potassium 2.7 mEq/L, phosphorus 1.3 mg/dL, magnesium 1.6 mg/dL, total bilirubin 5.6 mg/dL, direct bilirubin 3.6 mg/dL, alkaline phosphatase 193 U/L, gamma‐glutamyl transferase 300 U/L, aspartate aminotransferase 91 U/L, alanine aminotransferase 52 U/L. Brucella serology was negative.
His liver enzymes remain elevated with new onset jaundice consistent with hepatitis and intrahepatic cholestasis. His persistent hypophosphatemia, hypokalemia, and hypomagnesaemia well after resolution of diabetic ketoacidosis suggests acute tubulointerstitial dysfunction, which may be a complication of empiric antibiotic treatment or renal involvement by his underlying condition. Additional blood cultures, and tissue examination and culture are the next appropriate steps. Liver or bone marrow biopsy may suggest a diagnosis that can be confirmed by tissue culture or immunohistochemistry. Histologic findings such as fibrin ringed granulomas, caseating or noncaseating granulomas, or lymphomatous infiltration may suggest Coxiella (Q fever), tuberculosis, or lymphoma respectively. Because a liver biopsy is invasive and usually provides less tissue for culture, bone marrow examination should be obtained first.
A gallium 67 scan showed nonhomogenous increased uptake in both lungs and kidneys, consistent with interstitial nephritis and bilateral pneumonia. Serum protein electrophoresis demonstrated a monoclonal immunoglobulin (Ig)G lambda band with a kappa/lambda ratio of 0.9 (normal 1.42.8). Bone marrow biopsy showed normal hematopoiesis; no plasma or malignant cells, granulomas, or evidence of hemophagocytosis; and fungal and mycobacterial stains and cultures were negative. Colonoscopy revealed normal‐appearing mucosa. Histologic examination and culture of random biopsies from the colon and terminal ileum were negative for fungi, viruses, and mycobacteria. An ultrasound‐guided liver biopsy revealed numerous noncaseating granulomas formed of histiocytes and neutrophils with occasional fibrin rings. Fungal, viral, and mycobacterial stains and cultures were negative. The patient's fever resolved after 14 days, and he was discharged home without a diagnosis and close outpatient follow‐up.
The hepatic granulomas with fibrin rings are highly suggestive of Q fever, although ring granulomas may be seen in tuberculosis, typhoid fever, lymphoma, drug reactions, sarcoidosis, and CMV infections. Competing diagnoses such as CMV have been excluded by negative serology. Microscopic examination, tissue staining, and culture from liver and bone marrow biopsies were negative for S typhi, mycobacteria, and lymphoma. Gallium scan findings are generally nonspecific and of little utility in cases such as this. The kidney involvement correlates with the biochemical evidence of tubulointerstitial dysfunction; pulmonary involvement may reflect subclinical pulmonary infection with Coxiella. Given the normal bone marrow biopsy, the monoclonal gammopathy is of undetermined significance. The positive anti‐smooth muscle antibody can be related to Q fever. Anti‐smooth muscle antibodies frequently occur in Q fever, especially in those patients with hepatitis. Given the history of exposure to cattle, unexplained fever with temperaturepulse dissociation and liver biopsy findings, Q fever is the most likely diagnosis and empiric treatment with doxycycline is warranted.
Results of serology for Coxiella burnetii sent during admission were returned after the patient's discharge. C burnetii phase I IgG and IgM antibody titers were positive (1:512 each). C burnetii phase II IgG and IgM titers also were positive (1:1024 each). The patient was seen within a week and started on doxycycline 100 mg twice daily for 2 weeks for acute Q fever. His symptoms improved; hyponatremia, liver function tests, and thrombocytopenia normalized after treatment.
DISCUSSION
Q fever was first described in 1937 as a febrile illness affecting Australian slaughterhouse workers.[1] The Q in Q fever stands for query and reflected the initial uncertainty surrounding the underlying cause of the illness. The causative organism, C burnetti, is an obligate intracellular bacterium that resides within macrophage lysosomes. It can be found in the urine, feces, milk, placenta, and amniotic fluid of ungulates (cattle, sheep, and other ruminants), and other animals such as domestic cats and dogs. C burnetii is transmitted via inhalation, ingestion, occupational, or common source exposures, and in 1 case report by person‐to‐person sexual transmission.[2] In addition to slaughterhouse workers, pregnant women and immunosuppressed patients are more susceptible to developing Q fever.[3] For patients with suspected Q fever, a detailed occupational history, including specific job duties and potential exposure to animal products, is imperative.
Q fever has both acute and chronic presentations, which are differentiated based on the clinical illness and serologies. The symptoms of acute Q fever are nonspecific and may include influenza‐like illness, fever, pneumonia, and hepatitis. It presents less commonly with hemolytic anemia, interstitial nephritis, monoclonal gammopathy, or aseptic meningitis.[4, 5, 6, 7] Symptoms typically begin between 1 and 3 weeks after animal exposure and may persist for several months. Chronic Q fever occurs when unrecognized or untreated infection persists for greater than 6 months. It commonly presents with culture‐negative endocarditis, although infected aneurysms, osteomyelitis, or other distant sites of infection may also occur.
C burnetti is present in 2 antigenic forms that can be assessed by serology. Phase I is the more virulent, infectious form of C burnetti, which transitions to the avirulent phase II form during laboratory handling. In acute Q fever, phase II serologies are typically elevated out of proportion to phase I serologies, whereas this pattern is reversed in chronic Q fever. The diagnostic gold standard of acute Q fever is a 4‐fold rise in phase II antibody titers taken 3 to 6 weeks apart.[8] Histologic examination of affected organs can support a diagnosis of Q fever. The presence of ringed granulomas on liver or bone marrow biopsy specimens is highly suggestive, but not pathognomonic, of Q fever.[9]
Q fever is highly susceptible to several classes of antibiotics. For acute Q fever, doxycycline and tetracycline are typically used, with fluoroquinolones and chloramphenicol as alternatives.[8, 10] Patients with chronic Q fever should be treated with doxycycline and hydroxychloroquine. The addition of hydroxychloroquine alkalinizes the macrophage lysosome and enhances bacterial eradication.[8] For patients with acute Q fever, physicians should determine the risk of progression to chronic Q fever because closer monitoring is necessary. Patients with valvular heart lesions, immunosuppression, and pregnant women are at elevated risk of chronic Q fever. Trimethoprim/sulfamethoxazole can be used in place of doxycycline in pregnant women, as doxycycline and fluoroquinolones are contraindicated in pregnancy.[8]
This patient presented with a nonspecific febrile illness. Although the treating clinicians obtained a history of exposure to cattle early in his course, both the diagnosis and treatment were delayed. There are several possible explanations for the delay. First, although Q fever is a relatively common zoonosis, it remains an uncommon diagnosis, particularly among hospitalized patients. As a result, clinicians often focus on more common conditions. In this case, typical infections, malignancies, and inflammatory diseases were considered more likely. Second, the patient presented with hepatitis, an uncommon presentation of Q fever. Classical clinical reasoning suggests that atypical presentations of common diseases will occur more frequently than typical presentations of uncommon diseases. This case presented with an atypical presentation of an uncommon disease. The resultant lower pretest probability further dissuaded the patient's physicians from consideration of Q fever. Third, the finding of small bowel diverticulitis was a potential distractor. In patients with nonspecific febrile illnesses, it is common for physicians to anchor on any abnormal findings. In this case, the small bowel diverticulitis led to antibiotic treatment that was ineffective against C burnetti.
There were several clues to the diagnosis of Q fever in this patient's presentation. First, the pulsetemperature dissociation suggested infection with an intracellular pathogen. Hospitalists should recognize this association and be mindful of this often‐subtle clinical finding when faced with diagnostic uncertainty. Second, the patient was exposed to cattle prior to the onset of his illness. The fact that he did not have a direct exposure to animals underscores the infectivity of C burnetti. Finally, elevated alkaline phosphatase and transaminases were suggestive of an infiltrative disease; in the setting of a nonspecific febrile illness, Q fever was an important diagnostic consideration.
The key treatment decision in this case was the initiation and choice of antibiotics. Because of this patient's history of exposure to cattle and lack of a compelling alternative diagnosis, empiric treatment with doxycycline would have been appropriate. Hospitalists must weigh the potential benefit of early treatment of Q fever against the risks associated with antibiotic overuse. In patients presenting with a febrile illness after ungulate exposure, the decision to bet the farm with empiric doxycycline therapy may lead to clinical improvement, obviating a more invasive or extensive diagnostic evaluation.
TEACHING POINTS
- Acute Q fever typically presents 2 to 3 weeks after ungulate exposure with a febrile illness, pneumonia, and granulomatous hepatitis.
- Pulsetemperature dissociation is suggestive of infection by intracellular pathogens such as Coxiella, Salmonella, Leptospira, Legionella, and Mycoplasma.
- Clinicians should consider empiric doxycycline therapy in patients with suspected zoonosis (eg, Q fever, brucellosis, anaplasmosis, leptospirosis, Rocky Mountain spotted fever) while awaiting confirmatory tests, as improvement may obviate invasive testing.
Disclosure: Nothing to report.
- . “Q” fever, a new fever entity: clinical features, diagnosis, and laboratory investigation. Rev Infect Dis. 1983;5(4):790–800.
- , , , . Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3(11):709–721.
- , , , . Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;15:44(2):232–237.
- , , , , . Unusual manifestations of acute Q fever: autoimmune hemolytic anemia and tubulointerstitial nephritis. Ann of Clin Microbiol Antimicrob. 2012;11:14.
- , , . Q fever. Lancet. 2006;367(9511):679–688.
- , , , , , . Transitory monoclonal gammopathy and acute Q fever. Enferm Infecc Microbiol Clin. 1995;13(7):442.
- , . Q fever. Clin Microbiol Rev. 1999;12(4):518–553.
- , , , et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62(RR‐03):1–30.
- , , , et al. Hepatic fibrin‐ring granulomas: a clinicopathologic study of 23 patients. Hum Pathol. 1991;22(6):607–613.
- , , . Travel‐associated zoonotic bacterial diseases. Curr Opin Infect Dis. 2011;24(5):457–463.
- . “Q” fever, a new fever entity: clinical features, diagnosis, and laboratory investigation. Rev Infect Dis. 1983;5(4):790–800.
- , , , . Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3(11):709–721.
- , , , . Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;15:44(2):232–237.
- , , , , . Unusual manifestations of acute Q fever: autoimmune hemolytic anemia and tubulointerstitial nephritis. Ann of Clin Microbiol Antimicrob. 2012;11:14.
- , , . Q fever. Lancet. 2006;367(9511):679–688.
- , , , , , . Transitory monoclonal gammopathy and acute Q fever. Enferm Infecc Microbiol Clin. 1995;13(7):442.
- , . Q fever. Clin Microbiol Rev. 1999;12(4):518–553.
- , , , et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62(RR‐03):1–30.
- , , , et al. Hepatic fibrin‐ring granulomas: a clinicopathologic study of 23 patients. Hum Pathol. 1991;22(6):607–613.
- , , . Travel‐associated zoonotic bacterial diseases. Curr Opin Infect Dis. 2011;24(5):457–463.
Management and Outcomes After SVTE
Superficial thrombophlebitis (SVTE), inflammation of superficial veins associated with thrombosis, is a painful condition, and 3% to 11% of the population will develop SVTE during their lifetime. Although generally considered a benign, self‐limited disease, it can cause considerable discomfort, impact mobility, and lead to further complications. Recent and accumulating evidence suggests that it is often associated with more serious forms of venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE),[1] and SVTE is a strong risk factor for subsequent DVT or PE.[2, 3]
There is no clear consensus on the optimal treatment of SVTE. Although antithrombotic medications such as fondaparinux may be more effective than placebo in reducing the risk of subsequent DVT,[4] the evidence is generally of low grade, and the costs and inconveniences of anticoagulant therapy are not inconsequential.[1, 5, 6] Surveys suggest that physician opinions on the appropriate management of SVTE vary significantly, and management includes nonsteroidal anti‐inflammatory drugs (NSAIDs), topical therapies, or watchful waiting.[7] The objective of our study was to describe the initial management of SVTE in a community‐based population and examine subsequent rates of diagnosed DVT or PE in the following year.
MATERIALS AND METHODS
This was a retrospective, observational study seeking to describe the initial treatment for patients diagnosed with isolated SVTE.
Cohort Assembly
Data for this study were obtained from the Cardiovascular Research Network Venous Thromboembolism cohort study. The source population was based in Kaiser Permanente Northern California (KPNC), a large, integrated healthcare delivery system currently providing comprehensive care for >3.84 million members, and comprised of all adults aged 21 years or older with continuous enrollment in the KPNC health plan for 1 year and with a primary or secondary International Classification of Diseases, 9th RevisionClinical Modification (ICD‐9‐CM) diagnosis code of venous thrombosis (415.1x, 451.1x, 451.2, 451.81, 453.4x, 453.5x, 451.83, 451.84, 451.89, 453.72, 453.73, 453.74, 453.75, 453.76, 453.77, 453.82, 453.83, 453.84, 453.85, 453.86, 453.87, 451, 451.9, 452, 453, 453.0, 453.1, 453.2, 453.3, 453.79, 453.8, 453.89, 453.9) between January 1, 2004 and December 31, 2010. Of the 31,967 individuals meeting these criteria, 930 patients were selected by a random number generator for manual chart abstraction and review. Trained physician reviewers reviewed available emergency department, admission and discharge notes, outpatient clinic notes, and relevant radiology reports to determine whether or not the encounter represented a DVT, a SVTE, or other event.
Episodes were considered isolated SVTE if there was no evidence of a DVT or PE, and if there was medical chart documentation of either a diagnosis of SVTE, ultrasound evidence of a superficial vein clot, or a clinical description of SVTE as determined by the reviewing physician. All SVTE episodes in the study underwent a confirmatory review by second physician reviewer to confirm the diagnosis of SVTE.
Predictors and Outcomes
The primary outcome was documentation in the medical chart of a treatment recommendation for an antithrombotic agent, specifically, antiplatelet agents (aspirin, clopidogrel, ticlopidine), NSAIDs, and anticoagulants (low‐molecular‐weight heparin, fondaparinux, or warfarin). The secondary outcome was a subsequent diagnosis of VTE, which we defined as a subsequent encounter with an ICD‐9‐CM code for DVT or PE within 12 months after the initial episode, accompanied by a prescription for an anticoagulant within 7 days.
Data on patient age, sex, self‐reported race/ethnicity, and treatment setting (inpatient, emergency department, or outpatient) were obtained from health plan databases. Clinical risk factors for SVTE and the clinical presentation and treatment were obtained from physician chart review. Assessed risk factors included clinical conditions that have been associated with mildly increased SVTE risk (history of tobacco smoking, high body mass index), strongly increased risk (surgery or hospitalization within 30 days, active malignancy, hormonal therapy/pregnant or postpartum), provoking events (local trauma, central or peripheral intravenous catheter placement), and medical conditions that raise the risk for DVT (such as prior history of thrombosis or ischemic stroke).[8, 9] Data were abstracted by a single author (B.T.S.) using a standardized abstraction form. The study was approved by the institutional review boards of the collaborating institutions and informed consent was waived due to the nature of the study.
Statistical Methods
Analyses were conducted using SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC), with a 2‐sided P 0.05 considered significant. We used 2 tests and Student t tests for categorical and continuous variables, respectively, to test the bivariate association of risk factors with receipt of antithrombotic therapy after SVTE. Multivariable models were not developed due to the limited sample size.
RESULTS
Out of 930 patients with a diagnosis code for venous thrombosis and who underwent chart review, we identified 329 individuals who were considered by reviewers to have isolated SVTE events. Most SVTEs were of the lower extremity (60.8%) and diagnosed in an outpatient or emergency department setting (91.8%). Risk factors for SVTE were common, including documented varicose veins, recent peripheral venous catheterization or injection, or antecedent hospitalization (Table 1).
| Clinical Characteristic | Value, n = 329 |
|---|---|
| Age, y, mean (standard deviation) | 59.4 (15.8) |
| Female, n (%) | 199 (60.5) |
| Race, n (%) | |
| White | 236 (71.7) |
| Black | 23 (7.0) |
| Asian/Pacific Islander | 22 (6.7) |
| Unknown | 48 (14.6) |
| Location of thrombophlebitis, n (%) | |
| Lower extremity | 200 (60.8) |
| Upper extremity | 108 (32.8) |
| Other/unknown | 21 (6.3) |
| Clinical risk factors, n (%) | |
| Varicose veins | 85 (25.8) |
| History of recent peripheral intravenous catheters | 71 (21.6) |
| History of recent local trauma | 22 (6.7) |
| History of thrombosis | 12 (3.7) |
| History of stroke | 7 (2.1) |
| Sepsis/acute infection | 18 (5.5) |
| Heart failure | 7 (2.1) |
| Chronic lung disease | 24 (7.3) |
| Malignant neoplasm | 29 (8.8) |
| Hospitalization or surgery within 30 days | 48 (14.6) |
| Hormone therapy | 12 (3.6) |
| Pregnant/postpartum | 3 (0.9) |
| Current smoker | 13 (4.0) |
| Body mass index available | 184 (55.9) |
| 25 | 48 (14.6) |
| >2530 | 64 (19.5) |
| >30 | 72 (21.9) |
Initial treatment strategies for the 329 patients are presented in Table 2. Few patients with SVTE received anticoagulants for initial treatment, although patients with lower extremity SVTE were more likely to receive antithrombotic therapy compared to patients with SVTE of other locations (P 0.001). None of the identified risk factors for thrombosis were statistically significantly associated with a greater likelihood of receiving anticoagulants (P > 0.05 for all).
| VTE Risk* | Initial Management, % (No.) | Total | |||
|---|---|---|---|---|---|
| NSAIDs | LMWH | Warfarin | No Documented Antithrombotic Therapy | ||
| |||||
| Low | 52% (128) | 1% (3) | 2% (5) | 45% (112) | 248 |
| High | 25% (20) | 4% (3) | 4% (3) | 68% (55) | 81 |
| Total | 45% (148) | 2% (6) | 2% (8) | 51% (167) | 329 |
In the 12 months after SVTE, 19 (5.8%) patients had a diagnosis encounter for VTE associated with a prescription for either warfarin or parenteral anticoagulant. Of the 200 patients in our study with lower extremity SVTE, 15 (7.5%) had a subsequent VTE diagnosis associated with anticoagulation prescription in the following year.
DISCUSSION
Clinically significant VTE within a year after SVTE diagnosis was uncommon in our study despite infrequent use of antithrombotic therapy. Although recommendations for the initial treatment of SVTE have evolved in more recent years to support the use of fondaparinux in selected patients, there are significant costs and inconveniences associated with anticoagulation therapy and debate among physicians about the preferred treatment.[7] The low rate of anticoagulant use in our study may be related to the years studied (before guidelines supported fondaparinux), as well as being largely comprised of outpatients, and also because we included types of SVTE that are unlikely to progress to DVT, such as small vein phlebitis or upper extremity SVTE.[4, 10]
Limitations of our analysis include the heterogeneous types of SVTE included in our study and our reliance on available chart documentation to ascertain SVTE diagnosis, risk factors, and treatment. Because of the observational nature of our study, SVTE in the hospital setting may have been less well documented in medical records, leading to a sample of mostly outpatients. Hence, our observed subsequent VTE rate may not be generalizable to a more inclusive population. Finally, the low rate of anticoagulant treatment and VTE diagnoses limited our ability to conduct multivariable modeling.
In conclusion, clinically significant VTE within a year after SVTE was uncommon in our study despite infrequent use of antithrombotic therapy. Although our data are observational, they suggest that not all patients may require anticoagulation for the management of SVTE, and that further investigation into defining which populations would most benefit from treatment with fondaparinux or other agents is warranted.
Disclosures
This study was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grants R01HL103820 and U19HL91179). The sponsor was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Dr. Go received research grant funding from CSL Behring. None of the other authors have financial conflicts of interest.
- , , . Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2013;4:CD004982.
- , , , et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med. 2010;152:218–224.
- , , , et al. Risk of venous and arterial thrombotic events in patients diagnosed with superficial vein thrombosis: a nationwide cohort study. Blood. 2015;125:229–235.
- , , , et al. Fondaparinux for the treatment of superficial‐vein thrombosis in the legs. N Engl J Med. 2010;363:1222–1232.
- , , , . Fondaparinux for isolated superficial vein thrombosis of the legs: a cost‐effectiveness analysis. Chest. 2012;141:321–329.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , . The disparate management of superficial venous thrombosis in primary and secondary care. Phlebology. 2015;30:172–179.
- , , , , , . The risk of venous thrombosis in individuals with a history of superficial vein thrombosis and acquired venous thrombotic risk factors. Blood. 2013;122:4264–4269.
- , , , et al. Risk factors for recurrent events in subjects with superficial vein thrombosis in the randomized clinical trial SteFlux (Superficial Thromboembolism Fluxum). Thromb Res. 2014;133:196–202.
- , , , et al. Superficial vein thrombosis and recurrent venous thromboembolism: a pooled analysis of two observational studies. J Thromb Haemost. 2012;10:1004–1011.
Superficial thrombophlebitis (SVTE), inflammation of superficial veins associated with thrombosis, is a painful condition, and 3% to 11% of the population will develop SVTE during their lifetime. Although generally considered a benign, self‐limited disease, it can cause considerable discomfort, impact mobility, and lead to further complications. Recent and accumulating evidence suggests that it is often associated with more serious forms of venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE),[1] and SVTE is a strong risk factor for subsequent DVT or PE.[2, 3]
There is no clear consensus on the optimal treatment of SVTE. Although antithrombotic medications such as fondaparinux may be more effective than placebo in reducing the risk of subsequent DVT,[4] the evidence is generally of low grade, and the costs and inconveniences of anticoagulant therapy are not inconsequential.[1, 5, 6] Surveys suggest that physician opinions on the appropriate management of SVTE vary significantly, and management includes nonsteroidal anti‐inflammatory drugs (NSAIDs), topical therapies, or watchful waiting.[7] The objective of our study was to describe the initial management of SVTE in a community‐based population and examine subsequent rates of diagnosed DVT or PE in the following year.
MATERIALS AND METHODS
This was a retrospective, observational study seeking to describe the initial treatment for patients diagnosed with isolated SVTE.
Cohort Assembly
Data for this study were obtained from the Cardiovascular Research Network Venous Thromboembolism cohort study. The source population was based in Kaiser Permanente Northern California (KPNC), a large, integrated healthcare delivery system currently providing comprehensive care for >3.84 million members, and comprised of all adults aged 21 years or older with continuous enrollment in the KPNC health plan for 1 year and with a primary or secondary International Classification of Diseases, 9th RevisionClinical Modification (ICD‐9‐CM) diagnosis code of venous thrombosis (415.1x, 451.1x, 451.2, 451.81, 453.4x, 453.5x, 451.83, 451.84, 451.89, 453.72, 453.73, 453.74, 453.75, 453.76, 453.77, 453.82, 453.83, 453.84, 453.85, 453.86, 453.87, 451, 451.9, 452, 453, 453.0, 453.1, 453.2, 453.3, 453.79, 453.8, 453.89, 453.9) between January 1, 2004 and December 31, 2010. Of the 31,967 individuals meeting these criteria, 930 patients were selected by a random number generator for manual chart abstraction and review. Trained physician reviewers reviewed available emergency department, admission and discharge notes, outpatient clinic notes, and relevant radiology reports to determine whether or not the encounter represented a DVT, a SVTE, or other event.
Episodes were considered isolated SVTE if there was no evidence of a DVT or PE, and if there was medical chart documentation of either a diagnosis of SVTE, ultrasound evidence of a superficial vein clot, or a clinical description of SVTE as determined by the reviewing physician. All SVTE episodes in the study underwent a confirmatory review by second physician reviewer to confirm the diagnosis of SVTE.
Predictors and Outcomes
The primary outcome was documentation in the medical chart of a treatment recommendation for an antithrombotic agent, specifically, antiplatelet agents (aspirin, clopidogrel, ticlopidine), NSAIDs, and anticoagulants (low‐molecular‐weight heparin, fondaparinux, or warfarin). The secondary outcome was a subsequent diagnosis of VTE, which we defined as a subsequent encounter with an ICD‐9‐CM code for DVT or PE within 12 months after the initial episode, accompanied by a prescription for an anticoagulant within 7 days.
Data on patient age, sex, self‐reported race/ethnicity, and treatment setting (inpatient, emergency department, or outpatient) were obtained from health plan databases. Clinical risk factors for SVTE and the clinical presentation and treatment were obtained from physician chart review. Assessed risk factors included clinical conditions that have been associated with mildly increased SVTE risk (history of tobacco smoking, high body mass index), strongly increased risk (surgery or hospitalization within 30 days, active malignancy, hormonal therapy/pregnant or postpartum), provoking events (local trauma, central or peripheral intravenous catheter placement), and medical conditions that raise the risk for DVT (such as prior history of thrombosis or ischemic stroke).[8, 9] Data were abstracted by a single author (B.T.S.) using a standardized abstraction form. The study was approved by the institutional review boards of the collaborating institutions and informed consent was waived due to the nature of the study.
Statistical Methods
Analyses were conducted using SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC), with a 2‐sided P 0.05 considered significant. We used 2 tests and Student t tests for categorical and continuous variables, respectively, to test the bivariate association of risk factors with receipt of antithrombotic therapy after SVTE. Multivariable models were not developed due to the limited sample size.
RESULTS
Out of 930 patients with a diagnosis code for venous thrombosis and who underwent chart review, we identified 329 individuals who were considered by reviewers to have isolated SVTE events. Most SVTEs were of the lower extremity (60.8%) and diagnosed in an outpatient or emergency department setting (91.8%). Risk factors for SVTE were common, including documented varicose veins, recent peripheral venous catheterization or injection, or antecedent hospitalization (Table 1).
| Clinical Characteristic | Value, n = 329 |
|---|---|
| Age, y, mean (standard deviation) | 59.4 (15.8) |
| Female, n (%) | 199 (60.5) |
| Race, n (%) | |
| White | 236 (71.7) |
| Black | 23 (7.0) |
| Asian/Pacific Islander | 22 (6.7) |
| Unknown | 48 (14.6) |
| Location of thrombophlebitis, n (%) | |
| Lower extremity | 200 (60.8) |
| Upper extremity | 108 (32.8) |
| Other/unknown | 21 (6.3) |
| Clinical risk factors, n (%) | |
| Varicose veins | 85 (25.8) |
| History of recent peripheral intravenous catheters | 71 (21.6) |
| History of recent local trauma | 22 (6.7) |
| History of thrombosis | 12 (3.7) |
| History of stroke | 7 (2.1) |
| Sepsis/acute infection | 18 (5.5) |
| Heart failure | 7 (2.1) |
| Chronic lung disease | 24 (7.3) |
| Malignant neoplasm | 29 (8.8) |
| Hospitalization or surgery within 30 days | 48 (14.6) |
| Hormone therapy | 12 (3.6) |
| Pregnant/postpartum | 3 (0.9) |
| Current smoker | 13 (4.0) |
| Body mass index available | 184 (55.9) |
| 25 | 48 (14.6) |
| >2530 | 64 (19.5) |
| >30 | 72 (21.9) |
Initial treatment strategies for the 329 patients are presented in Table 2. Few patients with SVTE received anticoagulants for initial treatment, although patients with lower extremity SVTE were more likely to receive antithrombotic therapy compared to patients with SVTE of other locations (P 0.001). None of the identified risk factors for thrombosis were statistically significantly associated with a greater likelihood of receiving anticoagulants (P > 0.05 for all).
| VTE Risk* | Initial Management, % (No.) | Total | |||
|---|---|---|---|---|---|
| NSAIDs | LMWH | Warfarin | No Documented Antithrombotic Therapy | ||
| |||||
| Low | 52% (128) | 1% (3) | 2% (5) | 45% (112) | 248 |
| High | 25% (20) | 4% (3) | 4% (3) | 68% (55) | 81 |
| Total | 45% (148) | 2% (6) | 2% (8) | 51% (167) | 329 |
In the 12 months after SVTE, 19 (5.8%) patients had a diagnosis encounter for VTE associated with a prescription for either warfarin or parenteral anticoagulant. Of the 200 patients in our study with lower extremity SVTE, 15 (7.5%) had a subsequent VTE diagnosis associated with anticoagulation prescription in the following year.
DISCUSSION
Clinically significant VTE within a year after SVTE diagnosis was uncommon in our study despite infrequent use of antithrombotic therapy. Although recommendations for the initial treatment of SVTE have evolved in more recent years to support the use of fondaparinux in selected patients, there are significant costs and inconveniences associated with anticoagulation therapy and debate among physicians about the preferred treatment.[7] The low rate of anticoagulant use in our study may be related to the years studied (before guidelines supported fondaparinux), as well as being largely comprised of outpatients, and also because we included types of SVTE that are unlikely to progress to DVT, such as small vein phlebitis or upper extremity SVTE.[4, 10]
Limitations of our analysis include the heterogeneous types of SVTE included in our study and our reliance on available chart documentation to ascertain SVTE diagnosis, risk factors, and treatment. Because of the observational nature of our study, SVTE in the hospital setting may have been less well documented in medical records, leading to a sample of mostly outpatients. Hence, our observed subsequent VTE rate may not be generalizable to a more inclusive population. Finally, the low rate of anticoagulant treatment and VTE diagnoses limited our ability to conduct multivariable modeling.
In conclusion, clinically significant VTE within a year after SVTE was uncommon in our study despite infrequent use of antithrombotic therapy. Although our data are observational, they suggest that not all patients may require anticoagulation for the management of SVTE, and that further investigation into defining which populations would most benefit from treatment with fondaparinux or other agents is warranted.
Disclosures
This study was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grants R01HL103820 and U19HL91179). The sponsor was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Dr. Go received research grant funding from CSL Behring. None of the other authors have financial conflicts of interest.
Superficial thrombophlebitis (SVTE), inflammation of superficial veins associated with thrombosis, is a painful condition, and 3% to 11% of the population will develop SVTE during their lifetime. Although generally considered a benign, self‐limited disease, it can cause considerable discomfort, impact mobility, and lead to further complications. Recent and accumulating evidence suggests that it is often associated with more serious forms of venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE),[1] and SVTE is a strong risk factor for subsequent DVT or PE.[2, 3]
There is no clear consensus on the optimal treatment of SVTE. Although antithrombotic medications such as fondaparinux may be more effective than placebo in reducing the risk of subsequent DVT,[4] the evidence is generally of low grade, and the costs and inconveniences of anticoagulant therapy are not inconsequential.[1, 5, 6] Surveys suggest that physician opinions on the appropriate management of SVTE vary significantly, and management includes nonsteroidal anti‐inflammatory drugs (NSAIDs), topical therapies, or watchful waiting.[7] The objective of our study was to describe the initial management of SVTE in a community‐based population and examine subsequent rates of diagnosed DVT or PE in the following year.
MATERIALS AND METHODS
This was a retrospective, observational study seeking to describe the initial treatment for patients diagnosed with isolated SVTE.
Cohort Assembly
Data for this study were obtained from the Cardiovascular Research Network Venous Thromboembolism cohort study. The source population was based in Kaiser Permanente Northern California (KPNC), a large, integrated healthcare delivery system currently providing comprehensive care for >3.84 million members, and comprised of all adults aged 21 years or older with continuous enrollment in the KPNC health plan for 1 year and with a primary or secondary International Classification of Diseases, 9th RevisionClinical Modification (ICD‐9‐CM) diagnosis code of venous thrombosis (415.1x, 451.1x, 451.2, 451.81, 453.4x, 453.5x, 451.83, 451.84, 451.89, 453.72, 453.73, 453.74, 453.75, 453.76, 453.77, 453.82, 453.83, 453.84, 453.85, 453.86, 453.87, 451, 451.9, 452, 453, 453.0, 453.1, 453.2, 453.3, 453.79, 453.8, 453.89, 453.9) between January 1, 2004 and December 31, 2010. Of the 31,967 individuals meeting these criteria, 930 patients were selected by a random number generator for manual chart abstraction and review. Trained physician reviewers reviewed available emergency department, admission and discharge notes, outpatient clinic notes, and relevant radiology reports to determine whether or not the encounter represented a DVT, a SVTE, or other event.
Episodes were considered isolated SVTE if there was no evidence of a DVT or PE, and if there was medical chart documentation of either a diagnosis of SVTE, ultrasound evidence of a superficial vein clot, or a clinical description of SVTE as determined by the reviewing physician. All SVTE episodes in the study underwent a confirmatory review by second physician reviewer to confirm the diagnosis of SVTE.
Predictors and Outcomes
The primary outcome was documentation in the medical chart of a treatment recommendation for an antithrombotic agent, specifically, antiplatelet agents (aspirin, clopidogrel, ticlopidine), NSAIDs, and anticoagulants (low‐molecular‐weight heparin, fondaparinux, or warfarin). The secondary outcome was a subsequent diagnosis of VTE, which we defined as a subsequent encounter with an ICD‐9‐CM code for DVT or PE within 12 months after the initial episode, accompanied by a prescription for an anticoagulant within 7 days.
Data on patient age, sex, self‐reported race/ethnicity, and treatment setting (inpatient, emergency department, or outpatient) were obtained from health plan databases. Clinical risk factors for SVTE and the clinical presentation and treatment were obtained from physician chart review. Assessed risk factors included clinical conditions that have been associated with mildly increased SVTE risk (history of tobacco smoking, high body mass index), strongly increased risk (surgery or hospitalization within 30 days, active malignancy, hormonal therapy/pregnant or postpartum), provoking events (local trauma, central or peripheral intravenous catheter placement), and medical conditions that raise the risk for DVT (such as prior history of thrombosis or ischemic stroke).[8, 9] Data were abstracted by a single author (B.T.S.) using a standardized abstraction form. The study was approved by the institutional review boards of the collaborating institutions and informed consent was waived due to the nature of the study.
Statistical Methods
Analyses were conducted using SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC), with a 2‐sided P 0.05 considered significant. We used 2 tests and Student t tests for categorical and continuous variables, respectively, to test the bivariate association of risk factors with receipt of antithrombotic therapy after SVTE. Multivariable models were not developed due to the limited sample size.
RESULTS
Out of 930 patients with a diagnosis code for venous thrombosis and who underwent chart review, we identified 329 individuals who were considered by reviewers to have isolated SVTE events. Most SVTEs were of the lower extremity (60.8%) and diagnosed in an outpatient or emergency department setting (91.8%). Risk factors for SVTE were common, including documented varicose veins, recent peripheral venous catheterization or injection, or antecedent hospitalization (Table 1).
| Clinical Characteristic | Value, n = 329 |
|---|---|
| Age, y, mean (standard deviation) | 59.4 (15.8) |
| Female, n (%) | 199 (60.5) |
| Race, n (%) | |
| White | 236 (71.7) |
| Black | 23 (7.0) |
| Asian/Pacific Islander | 22 (6.7) |
| Unknown | 48 (14.6) |
| Location of thrombophlebitis, n (%) | |
| Lower extremity | 200 (60.8) |
| Upper extremity | 108 (32.8) |
| Other/unknown | 21 (6.3) |
| Clinical risk factors, n (%) | |
| Varicose veins | 85 (25.8) |
| History of recent peripheral intravenous catheters | 71 (21.6) |
| History of recent local trauma | 22 (6.7) |
| History of thrombosis | 12 (3.7) |
| History of stroke | 7 (2.1) |
| Sepsis/acute infection | 18 (5.5) |
| Heart failure | 7 (2.1) |
| Chronic lung disease | 24 (7.3) |
| Malignant neoplasm | 29 (8.8) |
| Hospitalization or surgery within 30 days | 48 (14.6) |
| Hormone therapy | 12 (3.6) |
| Pregnant/postpartum | 3 (0.9) |
| Current smoker | 13 (4.0) |
| Body mass index available | 184 (55.9) |
| 25 | 48 (14.6) |
| >2530 | 64 (19.5) |
| >30 | 72 (21.9) |
Initial treatment strategies for the 329 patients are presented in Table 2. Few patients with SVTE received anticoagulants for initial treatment, although patients with lower extremity SVTE were more likely to receive antithrombotic therapy compared to patients with SVTE of other locations (P 0.001). None of the identified risk factors for thrombosis were statistically significantly associated with a greater likelihood of receiving anticoagulants (P > 0.05 for all).
| VTE Risk* | Initial Management, % (No.) | Total | |||
|---|---|---|---|---|---|
| NSAIDs | LMWH | Warfarin | No Documented Antithrombotic Therapy | ||
| |||||
| Low | 52% (128) | 1% (3) | 2% (5) | 45% (112) | 248 |
| High | 25% (20) | 4% (3) | 4% (3) | 68% (55) | 81 |
| Total | 45% (148) | 2% (6) | 2% (8) | 51% (167) | 329 |
In the 12 months after SVTE, 19 (5.8%) patients had a diagnosis encounter for VTE associated with a prescription for either warfarin or parenteral anticoagulant. Of the 200 patients in our study with lower extremity SVTE, 15 (7.5%) had a subsequent VTE diagnosis associated with anticoagulation prescription in the following year.
DISCUSSION
Clinically significant VTE within a year after SVTE diagnosis was uncommon in our study despite infrequent use of antithrombotic therapy. Although recommendations for the initial treatment of SVTE have evolved in more recent years to support the use of fondaparinux in selected patients, there are significant costs and inconveniences associated with anticoagulation therapy and debate among physicians about the preferred treatment.[7] The low rate of anticoagulant use in our study may be related to the years studied (before guidelines supported fondaparinux), as well as being largely comprised of outpatients, and also because we included types of SVTE that are unlikely to progress to DVT, such as small vein phlebitis or upper extremity SVTE.[4, 10]
Limitations of our analysis include the heterogeneous types of SVTE included in our study and our reliance on available chart documentation to ascertain SVTE diagnosis, risk factors, and treatment. Because of the observational nature of our study, SVTE in the hospital setting may have been less well documented in medical records, leading to a sample of mostly outpatients. Hence, our observed subsequent VTE rate may not be generalizable to a more inclusive population. Finally, the low rate of anticoagulant treatment and VTE diagnoses limited our ability to conduct multivariable modeling.
In conclusion, clinically significant VTE within a year after SVTE was uncommon in our study despite infrequent use of antithrombotic therapy. Although our data are observational, they suggest that not all patients may require anticoagulation for the management of SVTE, and that further investigation into defining which populations would most benefit from treatment with fondaparinux or other agents is warranted.
Disclosures
This study was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grants R01HL103820 and U19HL91179). The sponsor was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Dr. Go received research grant funding from CSL Behring. None of the other authors have financial conflicts of interest.
- , , . Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2013;4:CD004982.
- , , , et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med. 2010;152:218–224.
- , , , et al. Risk of venous and arterial thrombotic events in patients diagnosed with superficial vein thrombosis: a nationwide cohort study. Blood. 2015;125:229–235.
- , , , et al. Fondaparinux for the treatment of superficial‐vein thrombosis in the legs. N Engl J Med. 2010;363:1222–1232.
- , , , . Fondaparinux for isolated superficial vein thrombosis of the legs: a cost‐effectiveness analysis. Chest. 2012;141:321–329.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , . The disparate management of superficial venous thrombosis in primary and secondary care. Phlebology. 2015;30:172–179.
- , , , , , . The risk of venous thrombosis in individuals with a history of superficial vein thrombosis and acquired venous thrombotic risk factors. Blood. 2013;122:4264–4269.
- , , , et al. Risk factors for recurrent events in subjects with superficial vein thrombosis in the randomized clinical trial SteFlux (Superficial Thromboembolism Fluxum). Thromb Res. 2014;133:196–202.
- , , , et al. Superficial vein thrombosis and recurrent venous thromboembolism: a pooled analysis of two observational studies. J Thromb Haemost. 2012;10:1004–1011.
- , , . Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2013;4:CD004982.
- , , , et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med. 2010;152:218–224.
- , , , et al. Risk of venous and arterial thrombotic events in patients diagnosed with superficial vein thrombosis: a nationwide cohort study. Blood. 2015;125:229–235.
- , , , et al. Fondaparinux for the treatment of superficial‐vein thrombosis in the legs. N Engl J Med. 2010;363:1222–1232.
- , , , . Fondaparinux for isolated superficial vein thrombosis of the legs: a cost‐effectiveness analysis. Chest. 2012;141:321–329.
- , , , et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e419S–e494S.
- , , , , . The disparate management of superficial venous thrombosis in primary and secondary care. Phlebology. 2015;30:172–179.
- , , , , , . The risk of venous thrombosis in individuals with a history of superficial vein thrombosis and acquired venous thrombotic risk factors. Blood. 2013;122:4264–4269.
- , , , et al. Risk factors for recurrent events in subjects with superficial vein thrombosis in the randomized clinical trial SteFlux (Superficial Thromboembolism Fluxum). Thromb Res. 2014;133:196–202.
- , , , et al. Superficial vein thrombosis and recurrent venous thromboembolism: a pooled analysis of two observational studies. J Thromb Haemost. 2012;10:1004–1011.
Rapid Response Team Meta‐analysis
In 2004, the Institute for Healthcare Improvement (IHI) launched its 100,000 Lives Campaign, a national initiative with a goal of saving 100,000 lives among hospitalized patients through improvements in the safety and effectiveness of healthcare.[1] One of their recommended strategies to reduce preventable inpatient deaths was for hospitals to establish rapid response teams (RRTs).[2, 3] The goal of RRTs, also termed medical emergency teams (METs), is to identify patients at risk for rapid decline in condition and intervene prior to a catastrophic event such as cardiopulmonary arrest.[4] The basis for recommending RRT/METs was evidence of predictable warning signs occurring in patients prior to cardiopulmonary arrest that could alert physicians.[5] A pilot study by the IHI, including 8 hospitals in the United States and the United Kingdom, found reductions in code calls after implementing RRTs, with 2 hospitals also showing a reduction in mortality.[3]
In response to the IHI report, many hospitals established RRT/METs.[6] Proponents for RRT/METs argued that the potential benefit justified immediate implementation, whereas others advocated for further research.[6] Despite the rapid, widespread adoption of RRT/METs, questions remain regarding their effectiveness in reducing hospital mortality and nonintensive care unit (ICU) cardiopulmonary arrests.[6, 7] In 2010, Chan et al. reported the results of a meta‐analysis of studies published through 2008 that demonstrated a reduction in cardiac arrests, but not mortality, following the implementation of RRTs.[8] An updated systematic review, including studies published through 2012, suggested that RRTs are associated with reduced non‐ICU cardiac arrest and reduced mortality.[9]
Since the publication of the Winters et al. systematic review, several new studies have been published.[9, 10, 11, 12] We performed a systematic review and meta‐analysis including studies published through 2014 to examine the impact of RRT/METs on hospital mortality and in‐hospital cardiopulmonary arrest (IHCA).
METHODS
Search Methods
We conducted a systematic search of publications on RRTs using PubMed (19462014), Cumulative Index to Nursing and Allied Health Literature (19372014), and the Cochrane Library (issue 10 of 12, 2014). The search used no language restrictions and no limits. Medical Subject Headings with keywords in a Boolean search strategy were employed. The major themes used were cardiopulmonary arrest and rapid response teams.
Study Eligibility Criteria
Prespecified criteria for determining study eligibility included: before‐after studies, cohort studies, nonrandomized control studies, or cluster randomized controlled trials (RCTs); implementation of an RRT and/or a MET as the intervention; adults (based on individual study definition) hospitalized in a non‐ICU setting; reported 1 or both prespecified outcomes, hospital mortality, or IHCA. There were no exclusion criteria or language restrictions.
Data Extraction
We prospectively outlined a standard protocol that included the research question, inclusion/exclusion criteria, as well as our outcomes and search approaches. We used standard methodology for analysis in accordance with the guidelines in Cochrane Handbook for Systematic Reviews of Interventions.[13] The protocol can be obtained by request to the authors. All changes to our original protocol were recorded in a protocol amendments table.
The studies identified underwent title and abstract screening by 1 of 2 reviewers (G.S.C., R.S.S.). After irrelevant studies were removed, reviewers independently assessed the remaining studies for eligibility based on full‐text review. All disagreements were resolved with consensus and the help of a third reviewer (D.C.B.).
Prior to extracting data, a piloted standardized data‐collection form was created. Eligible studies were independently reviewed by each of the 2 reviewers, and the relevant data extracted. Conflicts between the reviewers regarding the data collected for a given study were resolved by a third reviewer. The essential data were total events (hospital deaths and IHCA) and total hospital admissions.
Assessment of Methodological Quality
We utilized design‐specific tools to assess the methodological quality of included studies. For nonrandomized control and cohort studies, we used the Newcastle Ottawa Scale. This allowed us to evaluate the representativeness of the intervention cohort, selection of the nonintervention cohort, ascertainment of the intervention, whether or not the outcome was present at the start of the study, comparability of cohorts, assessment of the outcome, and whether there was adequate follow‐up.[14] We assigned stars as a measure of rating for each category and tallied the number of stars to assess the methodological quality. The maximum score was 9.[14]
For before‐after studies, an assessment scale developed by the ECRI (Emergency Care Research Institute) to test the internal validity of each study was utilized.[15] The ECRI Before‐After Scale allowed us to evaluate if the study was prospective, inclusion and exclusion criteria were established a priori, consecutive patients were enrolled, the same initial/subsequent treatment was administered, outcomes were objectively measured, follow‐up was complete, cohorts were comparable, there were no conflicts of interest, and conclusions were supported by data.[15] We ascertained whether each criterion was met and converted answers to numerical scores. A yes was scored 1, a no was scored 1, and no response was scored 0.5. The sum of these scores was then added to 11, divided by 22, and multiplied by 10 to yield the total quality score. The summary score can range from 0 to 10. A total score 5 was considered unacceptable quality. A score 5 but 7.5 was considered low quality, and a total 7.5 was considered moderate quality.[15]
To assess the methodological quality of RCTs, we used the Cochrane Risk of Bias Tool.[13] The tool involves determining whether a study has a high, low, or unclear risk of bias for specific criteria.[13]
Two independent reviewers evaluated the studies using these scales, and discrepancies were resolved by discussion.
Data Analysis
Measure of Treatment Effect
We used relative risk (RR) to summarize outcome data for our prespecified outcomes: hospital mortality and IHCA.
Dealing With Missing Data
If essential data were missing, study authors were contacted. If we did not receive a response, we calculated total events (deaths and IHCAs) using total admissions and event rates per admissions. If total admissions and/or event rates were missing, studies were not included in the analysis.
Data Synthesis
We used Review Manager 5.3 to calculate pooled summary estimates.[16] Meta‐analyses for each outcome were conducted by means of a random effects model.
Assessment of Heterogeneity
To assess for heterogeneity, we calculated I2 and P values. If the I2 0.50 or the P > 0.10, then the test for heterogeneity was passed. If heterogeneity was present, we evaluated each study in an effort to identify outliers. If an outlier was identified, the study was removed from the analysis.
Assessment of Reporting Bias
To assess publication bias, we used a funnel plot of the primary outcome. The findings were arranged by study size and effect size, and the plot was assessed for symmetry.
Subgroup Analyses
Subgroup analyses were performed for study type, RRT/MET composition, and publication year. Study type was grouped by cluster RCT and nonrandomized studies versus cohort/before‐after studies. Team composition was grouped by whether or not there was a physician on the RRT/MET. Publication year was grouped by studies published before or after 2010.
Sensitivity Analysis
We conducted sensitivity analyses to evaluate the impact of methodological quality on summary estimates. We compared overall summary estimates to summary estimates based only on before‐after studies judged to be low risk for bias. We also conducted an analysis to evaluate the inclusion of studies in which total events were calculated from rates and total admissions. We compared the overall summary estimates to summary estimates based on studies in which we were able to obtain essential data.
RESULTS
Description of Studies
Our search identified 691 studies, of which 90 were duplicates. The remaining studies were screened by title and abstract, identifying 82 potentially eligible studies, of which 30 studies were identified as eligible for inclusion in the meta‐analysis (Figure 1).
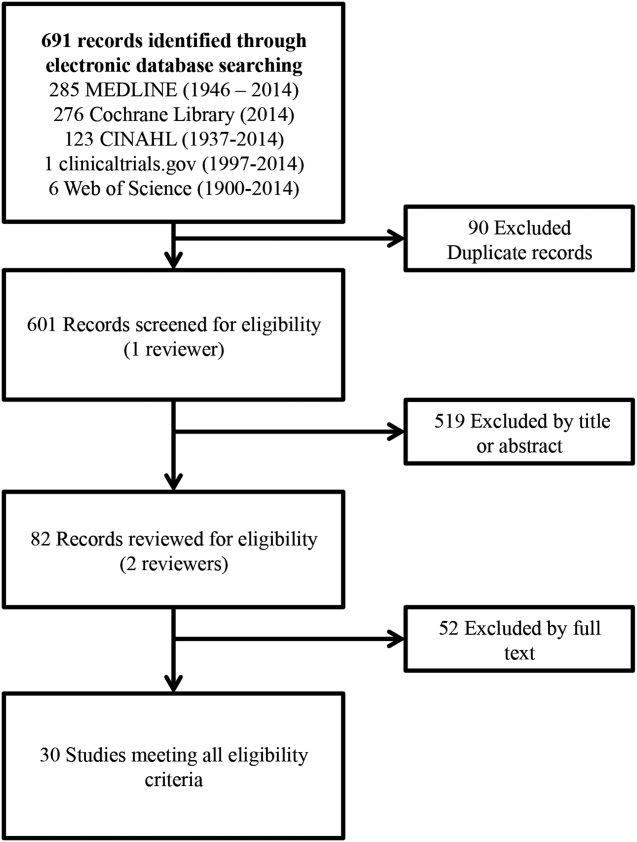
Of the 30 eligible studies, 10 were excluded from pooled estimates for hospital mortality,[7, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28] and 10 were excluded from pooled estimates for IHCA due to missing data.[17, 18, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30] For the analysis, 20 studies were included for the hospital mortality analysis and 20 studies were included for the IHCA analysis. The 22 studies included in either or both analyses spanned the years 2000 to 2014. The characteristics of the included studies are summarized in Table 1.
| Author/Year | Study Design | Setting/Location | Subjects (No.) | Age, y | Description of Intervention | Description of Control | Duration of Study | Outcome(s) of Interest |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Al‐Qahtani, 2013[10] | Before‐after | Saudi Arabia (tertiary care academic center) | Before: 157,804; after: 98,391 | Before: 59.2 19.2; after: 59 19.0 | RRT Implementation | Before RRT implementation | 5 years (January 2006December 2010) | IH mortality, IHCA, ward mortality |
| Bader, 2009[37] | Before‐after | USA (community acute care hospital) | Before: 15,949; after: 16,907 | N/R | RRT Implementation | Before RRT implementation | 3 years (October 2005June 2008) | IHCA, code mortality, ICU transfer |
| Beitler, 2011[31] | Before‐after | USA (tertiary referral public teaching hospital) | Before: 77,021; after: 79,013 | Pre‐RRT: 40.9 (22.3); post‐RRT: 42.0 (22.2) | RRT implementation | Before RRT implementation | 5 years (20032008) | IHCA mortality, IHCA, out‐of‐ICU mortality, IH mortality |
| Bellomo, 2003[32] | Before‐after | Australia (tertiary referral hospital) | Before: 21,090; after: 20,921 | Before: 60.7; after: 60.2 | MET implementation | Before MET implementation | 8 months (before: May 1999August 1999; after: November 2000February 2001) | IHCA, CA‐related mortality, IH mortality |
| Bristow, 2000[33] | Nonrandomized controlled | Australia (3 public hospitals) | 50,942 | NR | Hospitals with MET | Hospitals without MET (with conventional CA teams) | 5 months (2006) | IHCA, IH mortality |
| Buist, 2002[38] | Before‐after | Australia (tertiary referral teaching hospital) | Before: 25,254; after: 28,801 | Before: 36.6 (26.0); after: 36.4 (26.0) | MET implementation | Before MET implementation | 3 years (19961999) | Incidence and outcome of unexpected IHCA |
| Chan, 2008[39] | Prospective cohort | USA (tertiary care academic hospital) | Before: 24,193; after: 24,978 | Before: 56.8 (13.6) in 2004; 56.5 (13.8) in 2005; after: 57.0 (13.9) in 2006; 57.1 (13.8) in 2007 | RRT implementation | Standard care | 3.5 years (20042007) | IHCA, IH mortality |
| Chen, 2014[11] | Nonrandomized controlled | Australia (teaching hospital) | Before: 1,088,491; after: 479,194 | NR | Teaching hospital with a mature RRS | Three teaching hospitals without RRS | 8 years (20022009) | IHCA, IHCA mortality, IH mortality |
| Goncales, 2012[34] | Before‐after | Brazil (high complexity general hospital) | Before: 40,033; after: 42,796 | Before: 73; after: 68 | Implementation of RRT called Code Yellow | Before Implementation of RRTCode Blue | 3 years (20052008) | IHCA, IHCA mortality, IH mortality |
| Hatler, 2009[19] | Before‐after | USA (tertiary care hospital) | Before: 24,739; after: 25,470 | N/R | RRT implementation | Before RRT implementation | 2 years (20052007) | IHCA |
| Hillman, 2005[20] | Cluster RCT | Australia (23 hospitals) | Control hospitals: 56.756; MET hospitals: 68,376 | Control hospitals: 56.9; MET hospitals: 55.4 | MET implementation | Care as usual | 6 months | IH Mortality, IHCA |
| Jones, 2005[7] | Before‐after | Australia (tertiary care teaching hospital) | Before: 16,246; after: 104,001 | Before: 73.4; after: 70.8 | MET implementation | Before MET implementation | 5 years (19992004) | IHCA, death following cardiac arrest |
| Jones, 2007[29] | Before‐after | Australia (teaching hospital) | Before: 25,334; after: 100,243 | N/R | MET implementation | Before MET implementation | 6 years (19982004) | Surgical and medical mortality |
| Kenward, 2004[22] | Before‐after | UK (general hospital) | Before: 53,500; after: 53,500 | Before: N/R; after: 73 | MET implementation | Before MET implementation | 1 year (20002001) | IH mortality, IHCA |
| Konrad, 2010[36] | Before‐after | Sweden (tertiary care center) | Before: 203,892; after 73,825 | Before: 53.1; after: 52.4 | MET implementation | Before MET implementation | 6 years (20002006) | IH mortality, IHCA |
| Lighthall, 2010[40] | Before‐after | USA (university affiliated VA hospital) | Before: 2,975; after: 9,077 | Before: 65.26; after: 65.56 | RRT implementation | Before RRT implementation | 3 years (20042007) | IH mortality, IHCA |
| Lim, 2011[41] | Before‐after | South Korea (Samsung Medical Center) | Before: 33,360; after: 34,699 | Before: 64; after: 59 | MET implementation | Before MET implementation | 1 year (20082009) | IH mortality, IHCA, unexpected ICU transfers |
| Moroseos, 2014[12] | Before‐after | USA (teaching hospital) | Before: 7,092; after: 9,357 | Before: 30.1; after: 30.9 | Teaching hospital after RRT implementation | Teaching hospital before RRT implementation | 10 years (before: January 2000December 2004; after: January 2007December 2011) | IH mortality, IHCA, unexpected ICU transfers |
| Salvatierra, 2014[30] | Observational cohort | USA (10 tertiary care hospitals) | Before: 235,718; after: 235,344 | N/R | RRT implementation | Before RRT implementation | 62 months (September 2001December 2009) | IH mortality |
| Santamaria, 2010[35] | Before‐after | Australia (teaching hospital) | Before (IH mortality): 22,698; before (IHCA): 8,190 after (IH mortality): 74,616; after (IHCA): 81,628 | Median: 5860 (19932007) | RRT implementation | Before RRT implementation | 14 years (19932007) | IH mortality, IHCA |
| Segon, 2014[42] | Before‐after | USA (teaching hospital) | Before: 14,013; after: 14,333 | N/R | RRT implementation | Before RRT implementation | 2 years (January 2004April 2006) | IH mortality, unexpected ICU transfer, IHCA, ICU length of stay |
| Shah, 2011[28] | Retrospective cohort | USA (teaching hospital) | Before: 16,244; after: 45,145 | N/R | RRT implementation | Before RRT implementation | 3 years (20052008) | IHCA, IH mortality, unplanned ICU transfers |
Methodological Quality
The methodological quality of the 4 cohort studies, based on the New Castle Ottawa Scale, was either 8 or 9 stars. Using the ECRI Before‐After Scale, the average quality score of the 17 included before‐after studies was 8.41 (range, 7.279.32). Included before‐after studies were of moderate quality, with the exception of 1 of lower quality. The cluster RCT had low risk of bias for random sequence generation, allocation concealment, blinding of participants/personnel, and incomplete outcome data; however, it had unclear risk of bias for blinding of outcome assessment, selective reporting, and sources of bias due to lack of reporting.[20] Overall, the 22 studies included ranged from moderate to good quality.
Effect of RRT on Hospital Mortality
Of the 20 studies that reported hospital mortality, 9 favored RRT/METs,[10, 11, 30, 31, 32, 33, 34, 35, 36] 10 found no difference with RRT/METs,[12, 20, 22, 28, 37, 38, 39, 40, 41, 42] and 1 favored RRT/METs for surgical patients while favoring usual care (no RRT/MET) for medical patients[29] (Figure 2a). The pooled analysis demonstrated that implementation of RRT/METs was associated with a significant reduction in hospital mortality (RR = 0.88, 95% confidence interval [CI]: 0.83‐0.93). There was heterogeneity among the contributing studies (I2 = 86%).
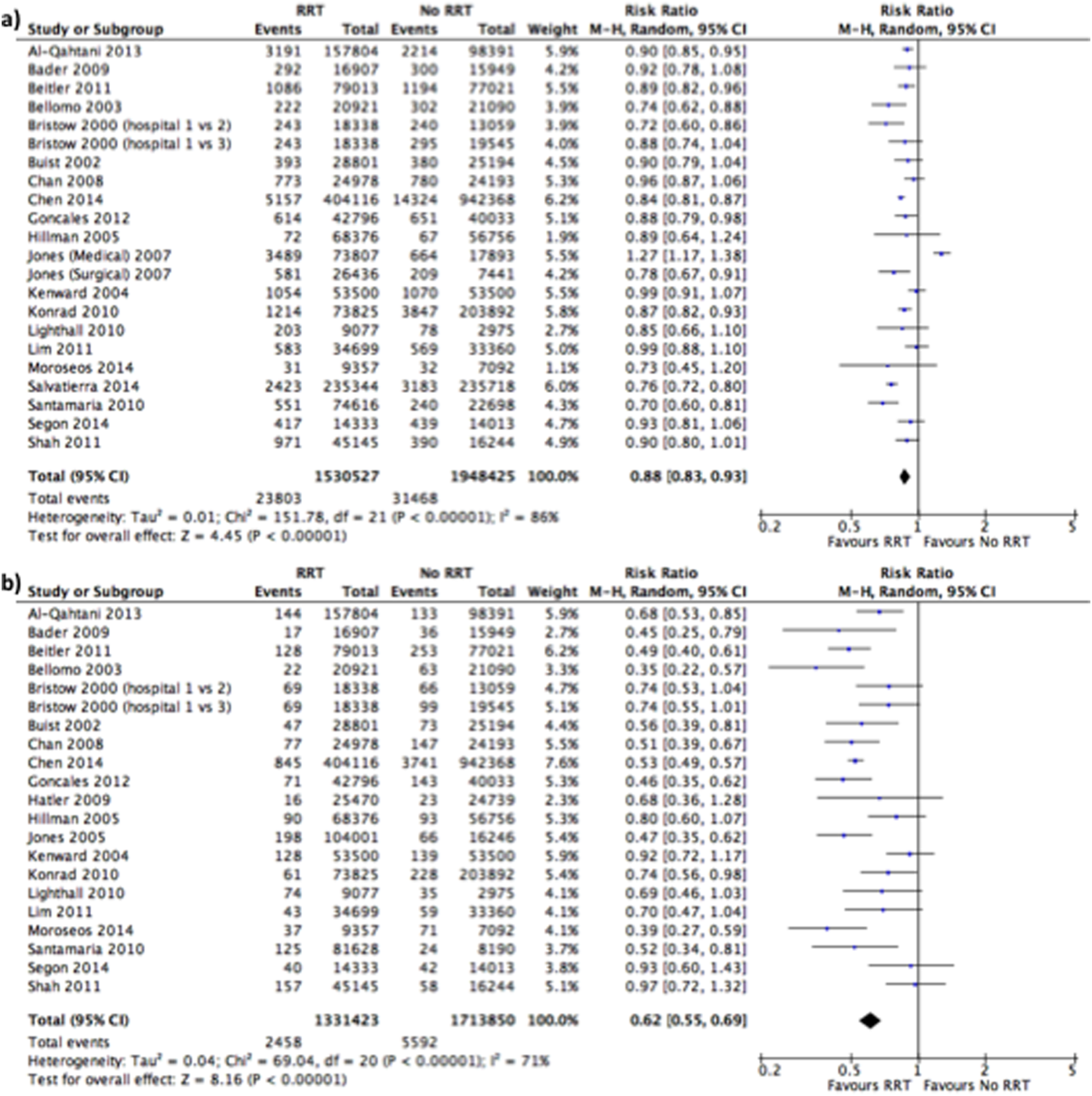
Effect of RRT on IHCA
Of the 20 studies that reported rates of IHCA, 12 favored RRT/METs [7, 10, 11, 12, 31, 32, 34, 35, 36, 37, 38, 39] and 8 found no difference with RRT/METs[16, 19, 20, 22, 28, 33, 40, 41, 42] (Figure 2b). In the pooled analysis, RRT/METs were associated with a significant reduction in IHCA (RR = 0.62, 95% CI: 0.55‐0.69). There was moderate heterogeneity among the studies (I2 = 71%).
Subgroup Analysis
Study Type
For hospital mortality, there was 1 cluster RCT and 2 nonrandomized studies[11, 20, 33] (RR = 0.83, 95% CI: 0.80‐0.87) and 17 cohort/before‐after studies[10, 12, 22, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42] (RR = 0.89, 95% CI: 0.83‐0.96). The cluster RCT and non‐randomized studies had minimal heterogeneity (I2 = 7%), and the cohort/before‐after studies exhibited substantial heterogeneity (I2 = 88%). The test for subgroup differences (I2 = 54.7%) indicates that study type may have an impact on hospital mortality.
For IHCA, there was 1 cluster RCT and 2 nonrandomized studies[11, 20, 33] (RR = 0.68, 95% CI: 0.52‐0.88) and 17 before‐after studies[7, 10, 12, 19, 22, 28, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42] (RR = 0.60, 95% CI: 0.52‐0.69). The cluster RCT and nonrandomized studies had substantial heterogeneity (I2 = 79%), whereas the cohort/before‐after studies had moderate heterogeneity (I2 = 69%). The test for subgroup differences (I2 = 0%) indicates that study type had no impact on IHCA.
RRT/MET Team Composition
For hospital mortality, there were 14 studies[10, 20, 29, 31, 32, 33, 34, 35, 36, 37, 38, 40, 41, 42] of RRTs with physicians (RR = 0.88, 95% CI: 0.82‐0.95) and 4 studies[12, 28, 30, 39] without physicians (RR = 0.85, 95% CI: 0.74‐0.99). Both groups exhibited substantial heterogeneity (I2 = 85% for both). The test for subgroup differences (I2 = 0%) indicates that team composition had no impact on hospital mortality.
Similarly, for IHCA there were 14 studies[7, 10, 20, 31, 32, 33, 34, 35, 36, 37, 38, 40, 41, 42] of RRTs with physicians (RR = 0.61, 95% CI: 0.54‐0.69) and 4 studies[12, 19, 28, 39] without (RR = 0.60, 95% CI: 0.39‐0.92). The studies with physicians on the RRT had moderate heterogeneity (I2 = 55%), whereas studies without a physician on the RRT had substantial heterogeneity (I2 = 81%). The test for subgroup differences (I2 = 0%) indicates that team composition had no impact on IHCA.
Publication Year
Publication year had no impact on hospital mortality. Studies published 2010 or earlier had an RR of 0.88 (95% CI: 0.80‐0.97), whereas studies published after 2010 had an RR of 0.87 (95% CI: 0.83‐0.92). Both groups had substantial heterogeneity (I2 of 88% and 75%, respectively). The test for subgroup differences (I2 = 0%) indicates publication year had no impact on hospital mortality.
Publication year had no impact on IHCA. Studies published in 2010 or earlier had an RR of 0.63 (95% CI: 0.54‐0.73), whereas studies published after 2010 had an RR of 0.60 (95% CI: 0.50‐0.72). The 2010 or earlier group had moderate heterogeneity (I2 = 60%), whereas the post‐2010 group had substantial heterogeneity (I2 = 77%). The test for subgroup differences (I2 = 0%) indicates that publication year had no impact on IHCA.
Sensitivity Analysis
A sensitivity analysis was performed excluding studies with low methodological quality from the analysis. For hospital mortality there were no studies of low methodological quality. For IHCA there was no major change in the summary estimate or the heterogeneity (RR = 0.59, 95% CI: 0.53‐0.67, I2 = 66%).
A sensitivity analysis was performed excluding studies only reporting rates and/or average annual admissions from the analysis. For hospital mortality, there was no major change in the summary estimate or the heterogeneity (RR = 0.87, 95% CI: 0.82‐0.93, I2 = 87%). For IHCA there was no major change in the summary estimate, but there was a decrease in heterogeneity (RR = 0.59, 95% CI: 0.53‐0.66, I2 = 63%).
Publication Bias
Funnel plots generated for the effect of RRTs on hospital mortality and on IHCA did not indicate publication bias. Our search of
DISCUSSION
We found implementation of RRT/METs was associated with reductions in hospital mortality and IHCA. Our analysis extends the meta‐analysis of Chan et al. and is consistent with the recent systematic review by Winters et al.[8, 9] These findings provide support for the IHI recommendation that hospitals implement RRT/METs.[1]
Following the 2004 IHI recommendations, RRT/METs were widely implemented, with over 50% of hospitals having some form of RRT by 2010.[6] The adoption of RRT/METs occurred despite limited evidence on the effectiveness of RRT/METs. A meta‐analysis of studies published through 2008 demonstrated a reduction in cardiac arrests, but no reduction in mortality after implementation of RRT/METs.[8] More recently a systematic review that included studies through 2012 suggested that RRT/METs are associated with reduced IHCA and reduced mortality.[9] Our analysis addressed the conflicting results of the prior reviews and included 13 studies published after the Chan et al. meta‐analysis and several studies published after the Winters et al. systemic review.[8, 9] The studies included in our analysis were completed in hospitals across multiple countries and settings, increasing the generalizability of the results. Most studies were performed in teaching hospitals; thus, the results may not be as applicable to community hospitals.
We found publication year did not impact either outcome. However, this may reflect our use of 2 broad publication periods rather than smaller periods, as 5 of the 6 newly included studies favor RRT interventions. Additionally, if the studies missing data had been included in our analysis, they may have shown that publication year impacts the outcomes. We noted that a physician on a RRT/MET did not affect outcomes, contrary to suggestions by Winters et al.[9] This may reflect the skill of nonphysician providers and/or the collaboration of the RRT/MET with critical care teams. However, very few RRTs did not include a physician, limiting the conclusion that can be drawn regarding team composition.
Many patients exhibit observable clinical deterioration or measurable changes that could identify them prior to an event such as cardiac arrest.[5, 43] Measurable physiologic parameters, in fact, are the basis of medical early warning systems and recent automated systems.[44, 45] Similarly, delayed transfer to the ICU has been shown to be associated with increased mortality.[46] Therefore, RRTs, either by identifying patients at risk for clinical deterioration and/or facilitating transfer of patients to the ICU earlier, could result in improved clinical outcomes. We did not specifically look at ICU transfer or ICU codes in our analysis. However, in a recent single‐center before‐after study, RRT implementation increased ICU admission rates and the transfer of less severely ill patients to the ICU without improvement in severity of illness‐adjusted outcomes.[47] This finding may reflect the ICU organization of the particular institution; however, given limited ICU resources, admitting an increased number of less severely ill patients without clear clinical benefit is a potential concern. More studies are needed to better understand the mechanism of benefit as well as potential trade‐offs associated with RRT implementation. It is possible that institutional factors determine the benefit that can be achieved through RRTs.
Our study has several limitations. Although the methodological quality of the included studies was moderate to good, confounding and biases can be an issue with before‐after trials and cohort studies. Most studies were before‐after observational trials, lacking a concurrent control group making it difficult to draw causal relationships. This is particularly the case for hospital mortality, which has been independently falling since 2000.[48] Thus, changes in observed hospital mortality may simply reflect the general trend independent of the RRT intervention. However, this does not appear the case for cardiopulmonary arrest, which has been increasing in incidence since 2000.[49] There were several studies eligible for inclusion in our analysis, but could not be included because of insufficient data. It is possible that the inclusion of these studies could influence the results of our analysis. Finally, there was heterogeneity among the studies for both outcomes, particularly in‐hospital mortality. This likely reflects variations in hospital characteristics and case‐mix indices. There may also be other factors impacting teams such as how hospitals handled deteriorating patients before RRT implementation, education periods, and differing mechanisms and criteria for RRT activation.
In conclusion, RRT/METs are effective in decreasing both IHCA and hospital mortality. Our findings support the 2004 IHI recommendations for the implementation of RRTs in hospitals. Additional studies are still required to explore team composition, activation criteria, activation mechanism, and implementation strategies.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Institute for Healthcare Improvement. Overview of the 100,000 Lives Campaign. Available at: https://www.ihi.org/Engage/Initiatives/Completed/5MillionLivesCampaign/Documents/Overview%20of%20 the%20100K%20Campaign.pdf. Accessed September 18, 2014.
- , , . Reducing Hospital Mortality Rates (Part 2). IHI Innovation Series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2005.
- , . Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event. Crit Care Med. 1994;22(2):244–247.
- , , , et al. Duration of life‐threatening antecedents prior to intensive care admission. Intensive Care Med. 2002;28(11):1629–1634.
- , , . The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608–613.
- , , , et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care. 2005;9(6):R808–R815.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):417–425.
- , , , et al. Impact of an intensivist‐led multidisciplinary extended rapid response team on hospital‐wide cardiopulmonary arrests and mortality. Crit Care Med. 2013;41(2):506–517.
- , , , et al. The impact of implementing a rapid response system: a comparison of cardiopulmonary arrests and mortality among four teaching hospitals in Australia. Resuscitation. 2014;85(9):1275–1281.
- , , , et al. Rapid response team implementation on a burn surgery/acute care ward. J Burn Care Res. 2014;35(1):21–27.
- Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Oxford, United Kingdom: The Cochrane Collaboration; 2011: Available at: http://www.cochrane‐handbook.org. Accessed October 9, 2014.
- , , , et al. The Newcastle‐Ottawa Scale (NOS) for Assessing The Quality of Nonrandomised Studies in Meta‐analyses. Ottawa, Canada: Ottawa Hospital Research Institute; 2014.
- Agency for Healthcare Research and Quality. Remote cardiac monitoring: a systematic review. Available at: http://www.cms.gov/determinationprocess/downloads/id51ta.pdf. Published December 12, 2007.
- Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen, the Netherlands: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
- , , . Implementing a rapid‐response team using a nurse‐to‐nurse consult approach. J Vasc Nurs. 2008;26(2):37–42.
- , , , et al. The effect of a rapid response team on major clinical outcome measures in a community hospital. Crit Care Med. 2007;35(9):2076–2082.
- , , , et al. Implementing a rapid response team to decrease emergencies outside the ICU: one hospital's experience. Medsurg Nurs. 2009;18(2):84–90, 126.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , , , . Rapid response teams: do they make a difference? Dimens Crit Care Nurs. 2007;26(6):253–260; quiz 261–262.
- , , , . Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61(3):257–263.
- , , , . Improving patient safety to reduce preventable deaths: the case of a California safety net hospital. J Healthc Qual. 2012;34(2):64–76.
- , . Implementation and outcomes of a rapid response team. J Nurs Care Qual. 2007;22(4):307–313, quiz 314–315.
- , , . Implementation of a rapid response team decreases cardiac arrest outside of the intensive care unit. J Trauma. 2007;62(5):1223–1227; discussion 1227–1228.
- , , , et al. Introducing Critical Care Outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , , , , . Four years' experience with a hospitalist‐led medical emergency team: an interrupted time series. J Hosp Med. 2012;7(2):98–103.
- , , , . Rapid response team in an academic institution: does it make a difference? Chest. 2011;139(6):1361–1367.
- , , , et al. Long‐term effect of a medical emergency team on mortality in a teaching hospital. Resuscitation. 2007;74(2):235–241.
- , , , , . Rapid response team implementation and in‐hospital mortality*. Crit Care Med. 2014;42(9):2001–2006.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15(6):R269.
- , , , et al. A prospective before‐and‐after trial of a medical emergency team. Med J Aust. 2003;179(6):283–287.
- , , , et al. Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173(5):236–240.
- , , , et al. Reduced frequency of cardiopulmonary arrests by rapid response teams. Einstein (Sao Paulo). 2012;10(4):442–448.
- , , . Changing cardiac arrest and hospital mortality rates through a medical emergency team takes time and constant review. Crit Care Med. 2010;38(2):445–450.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36(1):100–106.
- , , , et al. Rescue me: saving the vulnerable non‐ICU patient population. Jt Comm J Qual Patient Saf. 2009;35(4):199–205.
- , , , , , . Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300(21):2506–2513.
- , , , . Introduction of a rapid response system at a United States veterans affairs hospital reduced cardiac arrests. Anesth Analg. 2010;111(3):679–686.
- , , , et al. Early impact of medical emergency team implementation in a country with limited medical resources: a before‐and‐after study. J Crit Care. 2011;26(4):373–378.
- , , , , , . Effect of a rapid response team on patient outcomes in a community‐based teaching hospital. J Grad Med Educ. 2014;6(1):61–64.
- , , . Abnormal vital signs are associated with an increased risk for critical events in US veteran inpatients. Resuscitation. 2009;80(11):1264–1269.
- , , , et al. A randomized trial of real‐time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9(7):424–429.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7(3):224–230.
- , , , , , . The impact of rapid response team on outcome of patients transferred from the ward to the ICU: a single‐center study. Crit Care Med. 2013;41(10):2284–2291.
- , , . Trends in inpatient hospital deaths: National Hospital Discharge Survey, 2000–2010. NCHS Data Brief. 2013(118):1–8.
- , , . Epidemiology and outcomes of in‐hospital cardiopulmonary resuscitation in the United States, 2000–2009. Resuscitation. 2013;84(9):1255–1260.
In 2004, the Institute for Healthcare Improvement (IHI) launched its 100,000 Lives Campaign, a national initiative with a goal of saving 100,000 lives among hospitalized patients through improvements in the safety and effectiveness of healthcare.[1] One of their recommended strategies to reduce preventable inpatient deaths was for hospitals to establish rapid response teams (RRTs).[2, 3] The goal of RRTs, also termed medical emergency teams (METs), is to identify patients at risk for rapid decline in condition and intervene prior to a catastrophic event such as cardiopulmonary arrest.[4] The basis for recommending RRT/METs was evidence of predictable warning signs occurring in patients prior to cardiopulmonary arrest that could alert physicians.[5] A pilot study by the IHI, including 8 hospitals in the United States and the United Kingdom, found reductions in code calls after implementing RRTs, with 2 hospitals also showing a reduction in mortality.[3]
In response to the IHI report, many hospitals established RRT/METs.[6] Proponents for RRT/METs argued that the potential benefit justified immediate implementation, whereas others advocated for further research.[6] Despite the rapid, widespread adoption of RRT/METs, questions remain regarding their effectiveness in reducing hospital mortality and nonintensive care unit (ICU) cardiopulmonary arrests.[6, 7] In 2010, Chan et al. reported the results of a meta‐analysis of studies published through 2008 that demonstrated a reduction in cardiac arrests, but not mortality, following the implementation of RRTs.[8] An updated systematic review, including studies published through 2012, suggested that RRTs are associated with reduced non‐ICU cardiac arrest and reduced mortality.[9]
Since the publication of the Winters et al. systematic review, several new studies have been published.[9, 10, 11, 12] We performed a systematic review and meta‐analysis including studies published through 2014 to examine the impact of RRT/METs on hospital mortality and in‐hospital cardiopulmonary arrest (IHCA).
METHODS
Search Methods
We conducted a systematic search of publications on RRTs using PubMed (19462014), Cumulative Index to Nursing and Allied Health Literature (19372014), and the Cochrane Library (issue 10 of 12, 2014). The search used no language restrictions and no limits. Medical Subject Headings with keywords in a Boolean search strategy were employed. The major themes used were cardiopulmonary arrest and rapid response teams.
Study Eligibility Criteria
Prespecified criteria for determining study eligibility included: before‐after studies, cohort studies, nonrandomized control studies, or cluster randomized controlled trials (RCTs); implementation of an RRT and/or a MET as the intervention; adults (based on individual study definition) hospitalized in a non‐ICU setting; reported 1 or both prespecified outcomes, hospital mortality, or IHCA. There were no exclusion criteria or language restrictions.
Data Extraction
We prospectively outlined a standard protocol that included the research question, inclusion/exclusion criteria, as well as our outcomes and search approaches. We used standard methodology for analysis in accordance with the guidelines in Cochrane Handbook for Systematic Reviews of Interventions.[13] The protocol can be obtained by request to the authors. All changes to our original protocol were recorded in a protocol amendments table.
The studies identified underwent title and abstract screening by 1 of 2 reviewers (G.S.C., R.S.S.). After irrelevant studies were removed, reviewers independently assessed the remaining studies for eligibility based on full‐text review. All disagreements were resolved with consensus and the help of a third reviewer (D.C.B.).
Prior to extracting data, a piloted standardized data‐collection form was created. Eligible studies were independently reviewed by each of the 2 reviewers, and the relevant data extracted. Conflicts between the reviewers regarding the data collected for a given study were resolved by a third reviewer. The essential data were total events (hospital deaths and IHCA) and total hospital admissions.
Assessment of Methodological Quality
We utilized design‐specific tools to assess the methodological quality of included studies. For nonrandomized control and cohort studies, we used the Newcastle Ottawa Scale. This allowed us to evaluate the representativeness of the intervention cohort, selection of the nonintervention cohort, ascertainment of the intervention, whether or not the outcome was present at the start of the study, comparability of cohorts, assessment of the outcome, and whether there was adequate follow‐up.[14] We assigned stars as a measure of rating for each category and tallied the number of stars to assess the methodological quality. The maximum score was 9.[14]
For before‐after studies, an assessment scale developed by the ECRI (Emergency Care Research Institute) to test the internal validity of each study was utilized.[15] The ECRI Before‐After Scale allowed us to evaluate if the study was prospective, inclusion and exclusion criteria were established a priori, consecutive patients were enrolled, the same initial/subsequent treatment was administered, outcomes were objectively measured, follow‐up was complete, cohorts were comparable, there were no conflicts of interest, and conclusions were supported by data.[15] We ascertained whether each criterion was met and converted answers to numerical scores. A yes was scored 1, a no was scored 1, and no response was scored 0.5. The sum of these scores was then added to 11, divided by 22, and multiplied by 10 to yield the total quality score. The summary score can range from 0 to 10. A total score 5 was considered unacceptable quality. A score 5 but 7.5 was considered low quality, and a total 7.5 was considered moderate quality.[15]
To assess the methodological quality of RCTs, we used the Cochrane Risk of Bias Tool.[13] The tool involves determining whether a study has a high, low, or unclear risk of bias for specific criteria.[13]
Two independent reviewers evaluated the studies using these scales, and discrepancies were resolved by discussion.
Data Analysis
Measure of Treatment Effect
We used relative risk (RR) to summarize outcome data for our prespecified outcomes: hospital mortality and IHCA.
Dealing With Missing Data
If essential data were missing, study authors were contacted. If we did not receive a response, we calculated total events (deaths and IHCAs) using total admissions and event rates per admissions. If total admissions and/or event rates were missing, studies were not included in the analysis.
Data Synthesis
We used Review Manager 5.3 to calculate pooled summary estimates.[16] Meta‐analyses for each outcome were conducted by means of a random effects model.
Assessment of Heterogeneity
To assess for heterogeneity, we calculated I2 and P values. If the I2 0.50 or the P > 0.10, then the test for heterogeneity was passed. If heterogeneity was present, we evaluated each study in an effort to identify outliers. If an outlier was identified, the study was removed from the analysis.
Assessment of Reporting Bias
To assess publication bias, we used a funnel plot of the primary outcome. The findings were arranged by study size and effect size, and the plot was assessed for symmetry.
Subgroup Analyses
Subgroup analyses were performed for study type, RRT/MET composition, and publication year. Study type was grouped by cluster RCT and nonrandomized studies versus cohort/before‐after studies. Team composition was grouped by whether or not there was a physician on the RRT/MET. Publication year was grouped by studies published before or after 2010.
Sensitivity Analysis
We conducted sensitivity analyses to evaluate the impact of methodological quality on summary estimates. We compared overall summary estimates to summary estimates based only on before‐after studies judged to be low risk for bias. We also conducted an analysis to evaluate the inclusion of studies in which total events were calculated from rates and total admissions. We compared the overall summary estimates to summary estimates based on studies in which we were able to obtain essential data.
RESULTS
Description of Studies
Our search identified 691 studies, of which 90 were duplicates. The remaining studies were screened by title and abstract, identifying 82 potentially eligible studies, of which 30 studies were identified as eligible for inclusion in the meta‐analysis (Figure 1).

Of the 30 eligible studies, 10 were excluded from pooled estimates for hospital mortality,[7, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28] and 10 were excluded from pooled estimates for IHCA due to missing data.[17, 18, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30] For the analysis, 20 studies were included for the hospital mortality analysis and 20 studies were included for the IHCA analysis. The 22 studies included in either or both analyses spanned the years 2000 to 2014. The characteristics of the included studies are summarized in Table 1.
| Author/Year | Study Design | Setting/Location | Subjects (No.) | Age, y | Description of Intervention | Description of Control | Duration of Study | Outcome(s) of Interest |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Al‐Qahtani, 2013[10] | Before‐after | Saudi Arabia (tertiary care academic center) | Before: 157,804; after: 98,391 | Before: 59.2 19.2; after: 59 19.0 | RRT Implementation | Before RRT implementation | 5 years (January 2006December 2010) | IH mortality, IHCA, ward mortality |
| Bader, 2009[37] | Before‐after | USA (community acute care hospital) | Before: 15,949; after: 16,907 | N/R | RRT Implementation | Before RRT implementation | 3 years (October 2005June 2008) | IHCA, code mortality, ICU transfer |
| Beitler, 2011[31] | Before‐after | USA (tertiary referral public teaching hospital) | Before: 77,021; after: 79,013 | Pre‐RRT: 40.9 (22.3); post‐RRT: 42.0 (22.2) | RRT implementation | Before RRT implementation | 5 years (20032008) | IHCA mortality, IHCA, out‐of‐ICU mortality, IH mortality |
| Bellomo, 2003[32] | Before‐after | Australia (tertiary referral hospital) | Before: 21,090; after: 20,921 | Before: 60.7; after: 60.2 | MET implementation | Before MET implementation | 8 months (before: May 1999August 1999; after: November 2000February 2001) | IHCA, CA‐related mortality, IH mortality |
| Bristow, 2000[33] | Nonrandomized controlled | Australia (3 public hospitals) | 50,942 | NR | Hospitals with MET | Hospitals without MET (with conventional CA teams) | 5 months (2006) | IHCA, IH mortality |
| Buist, 2002[38] | Before‐after | Australia (tertiary referral teaching hospital) | Before: 25,254; after: 28,801 | Before: 36.6 (26.0); after: 36.4 (26.0) | MET implementation | Before MET implementation | 3 years (19961999) | Incidence and outcome of unexpected IHCA |
| Chan, 2008[39] | Prospective cohort | USA (tertiary care academic hospital) | Before: 24,193; after: 24,978 | Before: 56.8 (13.6) in 2004; 56.5 (13.8) in 2005; after: 57.0 (13.9) in 2006; 57.1 (13.8) in 2007 | RRT implementation | Standard care | 3.5 years (20042007) | IHCA, IH mortality |
| Chen, 2014[11] | Nonrandomized controlled | Australia (teaching hospital) | Before: 1,088,491; after: 479,194 | NR | Teaching hospital with a mature RRS | Three teaching hospitals without RRS | 8 years (20022009) | IHCA, IHCA mortality, IH mortality |
| Goncales, 2012[34] | Before‐after | Brazil (high complexity general hospital) | Before: 40,033; after: 42,796 | Before: 73; after: 68 | Implementation of RRT called Code Yellow | Before Implementation of RRTCode Blue | 3 years (20052008) | IHCA, IHCA mortality, IH mortality |
| Hatler, 2009[19] | Before‐after | USA (tertiary care hospital) | Before: 24,739; after: 25,470 | N/R | RRT implementation | Before RRT implementation | 2 years (20052007) | IHCA |
| Hillman, 2005[20] | Cluster RCT | Australia (23 hospitals) | Control hospitals: 56.756; MET hospitals: 68,376 | Control hospitals: 56.9; MET hospitals: 55.4 | MET implementation | Care as usual | 6 months | IH Mortality, IHCA |
| Jones, 2005[7] | Before‐after | Australia (tertiary care teaching hospital) | Before: 16,246; after: 104,001 | Before: 73.4; after: 70.8 | MET implementation | Before MET implementation | 5 years (19992004) | IHCA, death following cardiac arrest |
| Jones, 2007[29] | Before‐after | Australia (teaching hospital) | Before: 25,334; after: 100,243 | N/R | MET implementation | Before MET implementation | 6 years (19982004) | Surgical and medical mortality |
| Kenward, 2004[22] | Before‐after | UK (general hospital) | Before: 53,500; after: 53,500 | Before: N/R; after: 73 | MET implementation | Before MET implementation | 1 year (20002001) | IH mortality, IHCA |
| Konrad, 2010[36] | Before‐after | Sweden (tertiary care center) | Before: 203,892; after 73,825 | Before: 53.1; after: 52.4 | MET implementation | Before MET implementation | 6 years (20002006) | IH mortality, IHCA |
| Lighthall, 2010[40] | Before‐after | USA (university affiliated VA hospital) | Before: 2,975; after: 9,077 | Before: 65.26; after: 65.56 | RRT implementation | Before RRT implementation | 3 years (20042007) | IH mortality, IHCA |
| Lim, 2011[41] | Before‐after | South Korea (Samsung Medical Center) | Before: 33,360; after: 34,699 | Before: 64; after: 59 | MET implementation | Before MET implementation | 1 year (20082009) | IH mortality, IHCA, unexpected ICU transfers |
| Moroseos, 2014[12] | Before‐after | USA (teaching hospital) | Before: 7,092; after: 9,357 | Before: 30.1; after: 30.9 | Teaching hospital after RRT implementation | Teaching hospital before RRT implementation | 10 years (before: January 2000December 2004; after: January 2007December 2011) | IH mortality, IHCA, unexpected ICU transfers |
| Salvatierra, 2014[30] | Observational cohort | USA (10 tertiary care hospitals) | Before: 235,718; after: 235,344 | N/R | RRT implementation | Before RRT implementation | 62 months (September 2001December 2009) | IH mortality |
| Santamaria, 2010[35] | Before‐after | Australia (teaching hospital) | Before (IH mortality): 22,698; before (IHCA): 8,190 after (IH mortality): 74,616; after (IHCA): 81,628 | Median: 5860 (19932007) | RRT implementation | Before RRT implementation | 14 years (19932007) | IH mortality, IHCA |
| Segon, 2014[42] | Before‐after | USA (teaching hospital) | Before: 14,013; after: 14,333 | N/R | RRT implementation | Before RRT implementation | 2 years (January 2004April 2006) | IH mortality, unexpected ICU transfer, IHCA, ICU length of stay |
| Shah, 2011[28] | Retrospective cohort | USA (teaching hospital) | Before: 16,244; after: 45,145 | N/R | RRT implementation | Before RRT implementation | 3 years (20052008) | IHCA, IH mortality, unplanned ICU transfers |
Methodological Quality
The methodological quality of the 4 cohort studies, based on the New Castle Ottawa Scale, was either 8 or 9 stars. Using the ECRI Before‐After Scale, the average quality score of the 17 included before‐after studies was 8.41 (range, 7.279.32). Included before‐after studies were of moderate quality, with the exception of 1 of lower quality. The cluster RCT had low risk of bias for random sequence generation, allocation concealment, blinding of participants/personnel, and incomplete outcome data; however, it had unclear risk of bias for blinding of outcome assessment, selective reporting, and sources of bias due to lack of reporting.[20] Overall, the 22 studies included ranged from moderate to good quality.
Effect of RRT on Hospital Mortality
Of the 20 studies that reported hospital mortality, 9 favored RRT/METs,[10, 11, 30, 31, 32, 33, 34, 35, 36] 10 found no difference with RRT/METs,[12, 20, 22, 28, 37, 38, 39, 40, 41, 42] and 1 favored RRT/METs for surgical patients while favoring usual care (no RRT/MET) for medical patients[29] (Figure 2a). The pooled analysis demonstrated that implementation of RRT/METs was associated with a significant reduction in hospital mortality (RR = 0.88, 95% confidence interval [CI]: 0.83‐0.93). There was heterogeneity among the contributing studies (I2 = 86%).

Effect of RRT on IHCA
Of the 20 studies that reported rates of IHCA, 12 favored RRT/METs [7, 10, 11, 12, 31, 32, 34, 35, 36, 37, 38, 39] and 8 found no difference with RRT/METs[16, 19, 20, 22, 28, 33, 40, 41, 42] (Figure 2b). In the pooled analysis, RRT/METs were associated with a significant reduction in IHCA (RR = 0.62, 95% CI: 0.55‐0.69). There was moderate heterogeneity among the studies (I2 = 71%).
Subgroup Analysis
Study Type
For hospital mortality, there was 1 cluster RCT and 2 nonrandomized studies[11, 20, 33] (RR = 0.83, 95% CI: 0.80‐0.87) and 17 cohort/before‐after studies[10, 12, 22, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42] (RR = 0.89, 95% CI: 0.83‐0.96). The cluster RCT and non‐randomized studies had minimal heterogeneity (I2 = 7%), and the cohort/before‐after studies exhibited substantial heterogeneity (I2 = 88%). The test for subgroup differences (I2 = 54.7%) indicates that study type may have an impact on hospital mortality.
For IHCA, there was 1 cluster RCT and 2 nonrandomized studies[11, 20, 33] (RR = 0.68, 95% CI: 0.52‐0.88) and 17 before‐after studies[7, 10, 12, 19, 22, 28, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42] (RR = 0.60, 95% CI: 0.52‐0.69). The cluster RCT and nonrandomized studies had substantial heterogeneity (I2 = 79%), whereas the cohort/before‐after studies had moderate heterogeneity (I2 = 69%). The test for subgroup differences (I2 = 0%) indicates that study type had no impact on IHCA.
RRT/MET Team Composition
For hospital mortality, there were 14 studies[10, 20, 29, 31, 32, 33, 34, 35, 36, 37, 38, 40, 41, 42] of RRTs with physicians (RR = 0.88, 95% CI: 0.82‐0.95) and 4 studies[12, 28, 30, 39] without physicians (RR = 0.85, 95% CI: 0.74‐0.99). Both groups exhibited substantial heterogeneity (I2 = 85% for both). The test for subgroup differences (I2 = 0%) indicates that team composition had no impact on hospital mortality.
Similarly, for IHCA there were 14 studies[7, 10, 20, 31, 32, 33, 34, 35, 36, 37, 38, 40, 41, 42] of RRTs with physicians (RR = 0.61, 95% CI: 0.54‐0.69) and 4 studies[12, 19, 28, 39] without (RR = 0.60, 95% CI: 0.39‐0.92). The studies with physicians on the RRT had moderate heterogeneity (I2 = 55%), whereas studies without a physician on the RRT had substantial heterogeneity (I2 = 81%). The test for subgroup differences (I2 = 0%) indicates that team composition had no impact on IHCA.
Publication Year
Publication year had no impact on hospital mortality. Studies published 2010 or earlier had an RR of 0.88 (95% CI: 0.80‐0.97), whereas studies published after 2010 had an RR of 0.87 (95% CI: 0.83‐0.92). Both groups had substantial heterogeneity (I2 of 88% and 75%, respectively). The test for subgroup differences (I2 = 0%) indicates publication year had no impact on hospital mortality.
Publication year had no impact on IHCA. Studies published in 2010 or earlier had an RR of 0.63 (95% CI: 0.54‐0.73), whereas studies published after 2010 had an RR of 0.60 (95% CI: 0.50‐0.72). The 2010 or earlier group had moderate heterogeneity (I2 = 60%), whereas the post‐2010 group had substantial heterogeneity (I2 = 77%). The test for subgroup differences (I2 = 0%) indicates that publication year had no impact on IHCA.
Sensitivity Analysis
A sensitivity analysis was performed excluding studies with low methodological quality from the analysis. For hospital mortality there were no studies of low methodological quality. For IHCA there was no major change in the summary estimate or the heterogeneity (RR = 0.59, 95% CI: 0.53‐0.67, I2 = 66%).
A sensitivity analysis was performed excluding studies only reporting rates and/or average annual admissions from the analysis. For hospital mortality, there was no major change in the summary estimate or the heterogeneity (RR = 0.87, 95% CI: 0.82‐0.93, I2 = 87%). For IHCA there was no major change in the summary estimate, but there was a decrease in heterogeneity (RR = 0.59, 95% CI: 0.53‐0.66, I2 = 63%).
Publication Bias
Funnel plots generated for the effect of RRTs on hospital mortality and on IHCA did not indicate publication bias. Our search of
DISCUSSION
We found implementation of RRT/METs was associated with reductions in hospital mortality and IHCA. Our analysis extends the meta‐analysis of Chan et al. and is consistent with the recent systematic review by Winters et al.[8, 9] These findings provide support for the IHI recommendation that hospitals implement RRT/METs.[1]
Following the 2004 IHI recommendations, RRT/METs were widely implemented, with over 50% of hospitals having some form of RRT by 2010.[6] The adoption of RRT/METs occurred despite limited evidence on the effectiveness of RRT/METs. A meta‐analysis of studies published through 2008 demonstrated a reduction in cardiac arrests, but no reduction in mortality after implementation of RRT/METs.[8] More recently a systematic review that included studies through 2012 suggested that RRT/METs are associated with reduced IHCA and reduced mortality.[9] Our analysis addressed the conflicting results of the prior reviews and included 13 studies published after the Chan et al. meta‐analysis and several studies published after the Winters et al. systemic review.[8, 9] The studies included in our analysis were completed in hospitals across multiple countries and settings, increasing the generalizability of the results. Most studies were performed in teaching hospitals; thus, the results may not be as applicable to community hospitals.
We found publication year did not impact either outcome. However, this may reflect our use of 2 broad publication periods rather than smaller periods, as 5 of the 6 newly included studies favor RRT interventions. Additionally, if the studies missing data had been included in our analysis, they may have shown that publication year impacts the outcomes. We noted that a physician on a RRT/MET did not affect outcomes, contrary to suggestions by Winters et al.[9] This may reflect the skill of nonphysician providers and/or the collaboration of the RRT/MET with critical care teams. However, very few RRTs did not include a physician, limiting the conclusion that can be drawn regarding team composition.
Many patients exhibit observable clinical deterioration or measurable changes that could identify them prior to an event such as cardiac arrest.[5, 43] Measurable physiologic parameters, in fact, are the basis of medical early warning systems and recent automated systems.[44, 45] Similarly, delayed transfer to the ICU has been shown to be associated with increased mortality.[46] Therefore, RRTs, either by identifying patients at risk for clinical deterioration and/or facilitating transfer of patients to the ICU earlier, could result in improved clinical outcomes. We did not specifically look at ICU transfer or ICU codes in our analysis. However, in a recent single‐center before‐after study, RRT implementation increased ICU admission rates and the transfer of less severely ill patients to the ICU without improvement in severity of illness‐adjusted outcomes.[47] This finding may reflect the ICU organization of the particular institution; however, given limited ICU resources, admitting an increased number of less severely ill patients without clear clinical benefit is a potential concern. More studies are needed to better understand the mechanism of benefit as well as potential trade‐offs associated with RRT implementation. It is possible that institutional factors determine the benefit that can be achieved through RRTs.
Our study has several limitations. Although the methodological quality of the included studies was moderate to good, confounding and biases can be an issue with before‐after trials and cohort studies. Most studies were before‐after observational trials, lacking a concurrent control group making it difficult to draw causal relationships. This is particularly the case for hospital mortality, which has been independently falling since 2000.[48] Thus, changes in observed hospital mortality may simply reflect the general trend independent of the RRT intervention. However, this does not appear the case for cardiopulmonary arrest, which has been increasing in incidence since 2000.[49] There were several studies eligible for inclusion in our analysis, but could not be included because of insufficient data. It is possible that the inclusion of these studies could influence the results of our analysis. Finally, there was heterogeneity among the studies for both outcomes, particularly in‐hospital mortality. This likely reflects variations in hospital characteristics and case‐mix indices. There may also be other factors impacting teams such as how hospitals handled deteriorating patients before RRT implementation, education periods, and differing mechanisms and criteria for RRT activation.
In conclusion, RRT/METs are effective in decreasing both IHCA and hospital mortality. Our findings support the 2004 IHI recommendations for the implementation of RRTs in hospitals. Additional studies are still required to explore team composition, activation criteria, activation mechanism, and implementation strategies.
In 2004, the Institute for Healthcare Improvement (IHI) launched its 100,000 Lives Campaign, a national initiative with a goal of saving 100,000 lives among hospitalized patients through improvements in the safety and effectiveness of healthcare.[1] One of their recommended strategies to reduce preventable inpatient deaths was for hospitals to establish rapid response teams (RRTs).[2, 3] The goal of RRTs, also termed medical emergency teams (METs), is to identify patients at risk for rapid decline in condition and intervene prior to a catastrophic event such as cardiopulmonary arrest.[4] The basis for recommending RRT/METs was evidence of predictable warning signs occurring in patients prior to cardiopulmonary arrest that could alert physicians.[5] A pilot study by the IHI, including 8 hospitals in the United States and the United Kingdom, found reductions in code calls after implementing RRTs, with 2 hospitals also showing a reduction in mortality.[3]
In response to the IHI report, many hospitals established RRT/METs.[6] Proponents for RRT/METs argued that the potential benefit justified immediate implementation, whereas others advocated for further research.[6] Despite the rapid, widespread adoption of RRT/METs, questions remain regarding their effectiveness in reducing hospital mortality and nonintensive care unit (ICU) cardiopulmonary arrests.[6, 7] In 2010, Chan et al. reported the results of a meta‐analysis of studies published through 2008 that demonstrated a reduction in cardiac arrests, but not mortality, following the implementation of RRTs.[8] An updated systematic review, including studies published through 2012, suggested that RRTs are associated with reduced non‐ICU cardiac arrest and reduced mortality.[9]
Since the publication of the Winters et al. systematic review, several new studies have been published.[9, 10, 11, 12] We performed a systematic review and meta‐analysis including studies published through 2014 to examine the impact of RRT/METs on hospital mortality and in‐hospital cardiopulmonary arrest (IHCA).
METHODS
Search Methods
We conducted a systematic search of publications on RRTs using PubMed (19462014), Cumulative Index to Nursing and Allied Health Literature (19372014), and the Cochrane Library (issue 10 of 12, 2014). The search used no language restrictions and no limits. Medical Subject Headings with keywords in a Boolean search strategy were employed. The major themes used were cardiopulmonary arrest and rapid response teams.
Study Eligibility Criteria
Prespecified criteria for determining study eligibility included: before‐after studies, cohort studies, nonrandomized control studies, or cluster randomized controlled trials (RCTs); implementation of an RRT and/or a MET as the intervention; adults (based on individual study definition) hospitalized in a non‐ICU setting; reported 1 or both prespecified outcomes, hospital mortality, or IHCA. There were no exclusion criteria or language restrictions.
Data Extraction
We prospectively outlined a standard protocol that included the research question, inclusion/exclusion criteria, as well as our outcomes and search approaches. We used standard methodology for analysis in accordance with the guidelines in Cochrane Handbook for Systematic Reviews of Interventions.[13] The protocol can be obtained by request to the authors. All changes to our original protocol were recorded in a protocol amendments table.
The studies identified underwent title and abstract screening by 1 of 2 reviewers (G.S.C., R.S.S.). After irrelevant studies were removed, reviewers independently assessed the remaining studies for eligibility based on full‐text review. All disagreements were resolved with consensus and the help of a third reviewer (D.C.B.).
Prior to extracting data, a piloted standardized data‐collection form was created. Eligible studies were independently reviewed by each of the 2 reviewers, and the relevant data extracted. Conflicts between the reviewers regarding the data collected for a given study were resolved by a third reviewer. The essential data were total events (hospital deaths and IHCA) and total hospital admissions.
Assessment of Methodological Quality
We utilized design‐specific tools to assess the methodological quality of included studies. For nonrandomized control and cohort studies, we used the Newcastle Ottawa Scale. This allowed us to evaluate the representativeness of the intervention cohort, selection of the nonintervention cohort, ascertainment of the intervention, whether or not the outcome was present at the start of the study, comparability of cohorts, assessment of the outcome, and whether there was adequate follow‐up.[14] We assigned stars as a measure of rating for each category and tallied the number of stars to assess the methodological quality. The maximum score was 9.[14]
For before‐after studies, an assessment scale developed by the ECRI (Emergency Care Research Institute) to test the internal validity of each study was utilized.[15] The ECRI Before‐After Scale allowed us to evaluate if the study was prospective, inclusion and exclusion criteria were established a priori, consecutive patients were enrolled, the same initial/subsequent treatment was administered, outcomes were objectively measured, follow‐up was complete, cohorts were comparable, there were no conflicts of interest, and conclusions were supported by data.[15] We ascertained whether each criterion was met and converted answers to numerical scores. A yes was scored 1, a no was scored 1, and no response was scored 0.5. The sum of these scores was then added to 11, divided by 22, and multiplied by 10 to yield the total quality score. The summary score can range from 0 to 10. A total score 5 was considered unacceptable quality. A score 5 but 7.5 was considered low quality, and a total 7.5 was considered moderate quality.[15]
To assess the methodological quality of RCTs, we used the Cochrane Risk of Bias Tool.[13] The tool involves determining whether a study has a high, low, or unclear risk of bias for specific criteria.[13]
Two independent reviewers evaluated the studies using these scales, and discrepancies were resolved by discussion.
Data Analysis
Measure of Treatment Effect
We used relative risk (RR) to summarize outcome data for our prespecified outcomes: hospital mortality and IHCA.
Dealing With Missing Data
If essential data were missing, study authors were contacted. If we did not receive a response, we calculated total events (deaths and IHCAs) using total admissions and event rates per admissions. If total admissions and/or event rates were missing, studies were not included in the analysis.
Data Synthesis
We used Review Manager 5.3 to calculate pooled summary estimates.[16] Meta‐analyses for each outcome were conducted by means of a random effects model.
Assessment of Heterogeneity
To assess for heterogeneity, we calculated I2 and P values. If the I2 0.50 or the P > 0.10, then the test for heterogeneity was passed. If heterogeneity was present, we evaluated each study in an effort to identify outliers. If an outlier was identified, the study was removed from the analysis.
Assessment of Reporting Bias
To assess publication bias, we used a funnel plot of the primary outcome. The findings were arranged by study size and effect size, and the plot was assessed for symmetry.
Subgroup Analyses
Subgroup analyses were performed for study type, RRT/MET composition, and publication year. Study type was grouped by cluster RCT and nonrandomized studies versus cohort/before‐after studies. Team composition was grouped by whether or not there was a physician on the RRT/MET. Publication year was grouped by studies published before or after 2010.
Sensitivity Analysis
We conducted sensitivity analyses to evaluate the impact of methodological quality on summary estimates. We compared overall summary estimates to summary estimates based only on before‐after studies judged to be low risk for bias. We also conducted an analysis to evaluate the inclusion of studies in which total events were calculated from rates and total admissions. We compared the overall summary estimates to summary estimates based on studies in which we were able to obtain essential data.
RESULTS
Description of Studies
Our search identified 691 studies, of which 90 were duplicates. The remaining studies were screened by title and abstract, identifying 82 potentially eligible studies, of which 30 studies were identified as eligible for inclusion in the meta‐analysis (Figure 1).

Of the 30 eligible studies, 10 were excluded from pooled estimates for hospital mortality,[7, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28] and 10 were excluded from pooled estimates for IHCA due to missing data.[17, 18, 20, 21, 22, 23, 24, 25, 26, 27, 29, 30] For the analysis, 20 studies were included for the hospital mortality analysis and 20 studies were included for the IHCA analysis. The 22 studies included in either or both analyses spanned the years 2000 to 2014. The characteristics of the included studies are summarized in Table 1.
| Author/Year | Study Design | Setting/Location | Subjects (No.) | Age, y | Description of Intervention | Description of Control | Duration of Study | Outcome(s) of Interest |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Al‐Qahtani, 2013[10] | Before‐after | Saudi Arabia (tertiary care academic center) | Before: 157,804; after: 98,391 | Before: 59.2 19.2; after: 59 19.0 | RRT Implementation | Before RRT implementation | 5 years (January 2006December 2010) | IH mortality, IHCA, ward mortality |
| Bader, 2009[37] | Before‐after | USA (community acute care hospital) | Before: 15,949; after: 16,907 | N/R | RRT Implementation | Before RRT implementation | 3 years (October 2005June 2008) | IHCA, code mortality, ICU transfer |
| Beitler, 2011[31] | Before‐after | USA (tertiary referral public teaching hospital) | Before: 77,021; after: 79,013 | Pre‐RRT: 40.9 (22.3); post‐RRT: 42.0 (22.2) | RRT implementation | Before RRT implementation | 5 years (20032008) | IHCA mortality, IHCA, out‐of‐ICU mortality, IH mortality |
| Bellomo, 2003[32] | Before‐after | Australia (tertiary referral hospital) | Before: 21,090; after: 20,921 | Before: 60.7; after: 60.2 | MET implementation | Before MET implementation | 8 months (before: May 1999August 1999; after: November 2000February 2001) | IHCA, CA‐related mortality, IH mortality |
| Bristow, 2000[33] | Nonrandomized controlled | Australia (3 public hospitals) | 50,942 | NR | Hospitals with MET | Hospitals without MET (with conventional CA teams) | 5 months (2006) | IHCA, IH mortality |
| Buist, 2002[38] | Before‐after | Australia (tertiary referral teaching hospital) | Before: 25,254; after: 28,801 | Before: 36.6 (26.0); after: 36.4 (26.0) | MET implementation | Before MET implementation | 3 years (19961999) | Incidence and outcome of unexpected IHCA |
| Chan, 2008[39] | Prospective cohort | USA (tertiary care academic hospital) | Before: 24,193; after: 24,978 | Before: 56.8 (13.6) in 2004; 56.5 (13.8) in 2005; after: 57.0 (13.9) in 2006; 57.1 (13.8) in 2007 | RRT implementation | Standard care | 3.5 years (20042007) | IHCA, IH mortality |
| Chen, 2014[11] | Nonrandomized controlled | Australia (teaching hospital) | Before: 1,088,491; after: 479,194 | NR | Teaching hospital with a mature RRS | Three teaching hospitals without RRS | 8 years (20022009) | IHCA, IHCA mortality, IH mortality |
| Goncales, 2012[34] | Before‐after | Brazil (high complexity general hospital) | Before: 40,033; after: 42,796 | Before: 73; after: 68 | Implementation of RRT called Code Yellow | Before Implementation of RRTCode Blue | 3 years (20052008) | IHCA, IHCA mortality, IH mortality |
| Hatler, 2009[19] | Before‐after | USA (tertiary care hospital) | Before: 24,739; after: 25,470 | N/R | RRT implementation | Before RRT implementation | 2 years (20052007) | IHCA |
| Hillman, 2005[20] | Cluster RCT | Australia (23 hospitals) | Control hospitals: 56.756; MET hospitals: 68,376 | Control hospitals: 56.9; MET hospitals: 55.4 | MET implementation | Care as usual | 6 months | IH Mortality, IHCA |
| Jones, 2005[7] | Before‐after | Australia (tertiary care teaching hospital) | Before: 16,246; after: 104,001 | Before: 73.4; after: 70.8 | MET implementation | Before MET implementation | 5 years (19992004) | IHCA, death following cardiac arrest |
| Jones, 2007[29] | Before‐after | Australia (teaching hospital) | Before: 25,334; after: 100,243 | N/R | MET implementation | Before MET implementation | 6 years (19982004) | Surgical and medical mortality |
| Kenward, 2004[22] | Before‐after | UK (general hospital) | Before: 53,500; after: 53,500 | Before: N/R; after: 73 | MET implementation | Before MET implementation | 1 year (20002001) | IH mortality, IHCA |
| Konrad, 2010[36] | Before‐after | Sweden (tertiary care center) | Before: 203,892; after 73,825 | Before: 53.1; after: 52.4 | MET implementation | Before MET implementation | 6 years (20002006) | IH mortality, IHCA |
| Lighthall, 2010[40] | Before‐after | USA (university affiliated VA hospital) | Before: 2,975; after: 9,077 | Before: 65.26; after: 65.56 | RRT implementation | Before RRT implementation | 3 years (20042007) | IH mortality, IHCA |
| Lim, 2011[41] | Before‐after | South Korea (Samsung Medical Center) | Before: 33,360; after: 34,699 | Before: 64; after: 59 | MET implementation | Before MET implementation | 1 year (20082009) | IH mortality, IHCA, unexpected ICU transfers |
| Moroseos, 2014[12] | Before‐after | USA (teaching hospital) | Before: 7,092; after: 9,357 | Before: 30.1; after: 30.9 | Teaching hospital after RRT implementation | Teaching hospital before RRT implementation | 10 years (before: January 2000December 2004; after: January 2007December 2011) | IH mortality, IHCA, unexpected ICU transfers |
| Salvatierra, 2014[30] | Observational cohort | USA (10 tertiary care hospitals) | Before: 235,718; after: 235,344 | N/R | RRT implementation | Before RRT implementation | 62 months (September 2001December 2009) | IH mortality |
| Santamaria, 2010[35] | Before‐after | Australia (teaching hospital) | Before (IH mortality): 22,698; before (IHCA): 8,190 after (IH mortality): 74,616; after (IHCA): 81,628 | Median: 5860 (19932007) | RRT implementation | Before RRT implementation | 14 years (19932007) | IH mortality, IHCA |
| Segon, 2014[42] | Before‐after | USA (teaching hospital) | Before: 14,013; after: 14,333 | N/R | RRT implementation | Before RRT implementation | 2 years (January 2004April 2006) | IH mortality, unexpected ICU transfer, IHCA, ICU length of stay |
| Shah, 2011[28] | Retrospective cohort | USA (teaching hospital) | Before: 16,244; after: 45,145 | N/R | RRT implementation | Before RRT implementation | 3 years (20052008) | IHCA, IH mortality, unplanned ICU transfers |
Methodological Quality
The methodological quality of the 4 cohort studies, based on the New Castle Ottawa Scale, was either 8 or 9 stars. Using the ECRI Before‐After Scale, the average quality score of the 17 included before‐after studies was 8.41 (range, 7.279.32). Included before‐after studies were of moderate quality, with the exception of 1 of lower quality. The cluster RCT had low risk of bias for random sequence generation, allocation concealment, blinding of participants/personnel, and incomplete outcome data; however, it had unclear risk of bias for blinding of outcome assessment, selective reporting, and sources of bias due to lack of reporting.[20] Overall, the 22 studies included ranged from moderate to good quality.
Effect of RRT on Hospital Mortality
Of the 20 studies that reported hospital mortality, 9 favored RRT/METs,[10, 11, 30, 31, 32, 33, 34, 35, 36] 10 found no difference with RRT/METs,[12, 20, 22, 28, 37, 38, 39, 40, 41, 42] and 1 favored RRT/METs for surgical patients while favoring usual care (no RRT/MET) for medical patients[29] (Figure 2a). The pooled analysis demonstrated that implementation of RRT/METs was associated with a significant reduction in hospital mortality (RR = 0.88, 95% confidence interval [CI]: 0.83‐0.93). There was heterogeneity among the contributing studies (I2 = 86%).

Effect of RRT on IHCA
Of the 20 studies that reported rates of IHCA, 12 favored RRT/METs [7, 10, 11, 12, 31, 32, 34, 35, 36, 37, 38, 39] and 8 found no difference with RRT/METs[16, 19, 20, 22, 28, 33, 40, 41, 42] (Figure 2b). In the pooled analysis, RRT/METs were associated with a significant reduction in IHCA (RR = 0.62, 95% CI: 0.55‐0.69). There was moderate heterogeneity among the studies (I2 = 71%).
Subgroup Analysis
Study Type
For hospital mortality, there was 1 cluster RCT and 2 nonrandomized studies[11, 20, 33] (RR = 0.83, 95% CI: 0.80‐0.87) and 17 cohort/before‐after studies[10, 12, 22, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42] (RR = 0.89, 95% CI: 0.83‐0.96). The cluster RCT and non‐randomized studies had minimal heterogeneity (I2 = 7%), and the cohort/before‐after studies exhibited substantial heterogeneity (I2 = 88%). The test for subgroup differences (I2 = 54.7%) indicates that study type may have an impact on hospital mortality.
For IHCA, there was 1 cluster RCT and 2 nonrandomized studies[11, 20, 33] (RR = 0.68, 95% CI: 0.52‐0.88) and 17 before‐after studies[7, 10, 12, 19, 22, 28, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42] (RR = 0.60, 95% CI: 0.52‐0.69). The cluster RCT and nonrandomized studies had substantial heterogeneity (I2 = 79%), whereas the cohort/before‐after studies had moderate heterogeneity (I2 = 69%). The test for subgroup differences (I2 = 0%) indicates that study type had no impact on IHCA.
RRT/MET Team Composition
For hospital mortality, there were 14 studies[10, 20, 29, 31, 32, 33, 34, 35, 36, 37, 38, 40, 41, 42] of RRTs with physicians (RR = 0.88, 95% CI: 0.82‐0.95) and 4 studies[12, 28, 30, 39] without physicians (RR = 0.85, 95% CI: 0.74‐0.99). Both groups exhibited substantial heterogeneity (I2 = 85% for both). The test for subgroup differences (I2 = 0%) indicates that team composition had no impact on hospital mortality.
Similarly, for IHCA there were 14 studies[7, 10, 20, 31, 32, 33, 34, 35, 36, 37, 38, 40, 41, 42] of RRTs with physicians (RR = 0.61, 95% CI: 0.54‐0.69) and 4 studies[12, 19, 28, 39] without (RR = 0.60, 95% CI: 0.39‐0.92). The studies with physicians on the RRT had moderate heterogeneity (I2 = 55%), whereas studies without a physician on the RRT had substantial heterogeneity (I2 = 81%). The test for subgroup differences (I2 = 0%) indicates that team composition had no impact on IHCA.
Publication Year
Publication year had no impact on hospital mortality. Studies published 2010 or earlier had an RR of 0.88 (95% CI: 0.80‐0.97), whereas studies published after 2010 had an RR of 0.87 (95% CI: 0.83‐0.92). Both groups had substantial heterogeneity (I2 of 88% and 75%, respectively). The test for subgroup differences (I2 = 0%) indicates publication year had no impact on hospital mortality.
Publication year had no impact on IHCA. Studies published in 2010 or earlier had an RR of 0.63 (95% CI: 0.54‐0.73), whereas studies published after 2010 had an RR of 0.60 (95% CI: 0.50‐0.72). The 2010 or earlier group had moderate heterogeneity (I2 = 60%), whereas the post‐2010 group had substantial heterogeneity (I2 = 77%). The test for subgroup differences (I2 = 0%) indicates that publication year had no impact on IHCA.
Sensitivity Analysis
A sensitivity analysis was performed excluding studies with low methodological quality from the analysis. For hospital mortality there were no studies of low methodological quality. For IHCA there was no major change in the summary estimate or the heterogeneity (RR = 0.59, 95% CI: 0.53‐0.67, I2 = 66%).
A sensitivity analysis was performed excluding studies only reporting rates and/or average annual admissions from the analysis. For hospital mortality, there was no major change in the summary estimate or the heterogeneity (RR = 0.87, 95% CI: 0.82‐0.93, I2 = 87%). For IHCA there was no major change in the summary estimate, but there was a decrease in heterogeneity (RR = 0.59, 95% CI: 0.53‐0.66, I2 = 63%).
Publication Bias
Funnel plots generated for the effect of RRTs on hospital mortality and on IHCA did not indicate publication bias. Our search of
DISCUSSION
We found implementation of RRT/METs was associated with reductions in hospital mortality and IHCA. Our analysis extends the meta‐analysis of Chan et al. and is consistent with the recent systematic review by Winters et al.[8, 9] These findings provide support for the IHI recommendation that hospitals implement RRT/METs.[1]
Following the 2004 IHI recommendations, RRT/METs were widely implemented, with over 50% of hospitals having some form of RRT by 2010.[6] The adoption of RRT/METs occurred despite limited evidence on the effectiveness of RRT/METs. A meta‐analysis of studies published through 2008 demonstrated a reduction in cardiac arrests, but no reduction in mortality after implementation of RRT/METs.[8] More recently a systematic review that included studies through 2012 suggested that RRT/METs are associated with reduced IHCA and reduced mortality.[9] Our analysis addressed the conflicting results of the prior reviews and included 13 studies published after the Chan et al. meta‐analysis and several studies published after the Winters et al. systemic review.[8, 9] The studies included in our analysis were completed in hospitals across multiple countries and settings, increasing the generalizability of the results. Most studies were performed in teaching hospitals; thus, the results may not be as applicable to community hospitals.
We found publication year did not impact either outcome. However, this may reflect our use of 2 broad publication periods rather than smaller periods, as 5 of the 6 newly included studies favor RRT interventions. Additionally, if the studies missing data had been included in our analysis, they may have shown that publication year impacts the outcomes. We noted that a physician on a RRT/MET did not affect outcomes, contrary to suggestions by Winters et al.[9] This may reflect the skill of nonphysician providers and/or the collaboration of the RRT/MET with critical care teams. However, very few RRTs did not include a physician, limiting the conclusion that can be drawn regarding team composition.
Many patients exhibit observable clinical deterioration or measurable changes that could identify them prior to an event such as cardiac arrest.[5, 43] Measurable physiologic parameters, in fact, are the basis of medical early warning systems and recent automated systems.[44, 45] Similarly, delayed transfer to the ICU has been shown to be associated with increased mortality.[46] Therefore, RRTs, either by identifying patients at risk for clinical deterioration and/or facilitating transfer of patients to the ICU earlier, could result in improved clinical outcomes. We did not specifically look at ICU transfer or ICU codes in our analysis. However, in a recent single‐center before‐after study, RRT implementation increased ICU admission rates and the transfer of less severely ill patients to the ICU without improvement in severity of illness‐adjusted outcomes.[47] This finding may reflect the ICU organization of the particular institution; however, given limited ICU resources, admitting an increased number of less severely ill patients without clear clinical benefit is a potential concern. More studies are needed to better understand the mechanism of benefit as well as potential trade‐offs associated with RRT implementation. It is possible that institutional factors determine the benefit that can be achieved through RRTs.
Our study has several limitations. Although the methodological quality of the included studies was moderate to good, confounding and biases can be an issue with before‐after trials and cohort studies. Most studies were before‐after observational trials, lacking a concurrent control group making it difficult to draw causal relationships. This is particularly the case for hospital mortality, which has been independently falling since 2000.[48] Thus, changes in observed hospital mortality may simply reflect the general trend independent of the RRT intervention. However, this does not appear the case for cardiopulmonary arrest, which has been increasing in incidence since 2000.[49] There were several studies eligible for inclusion in our analysis, but could not be included because of insufficient data. It is possible that the inclusion of these studies could influence the results of our analysis. Finally, there was heterogeneity among the studies for both outcomes, particularly in‐hospital mortality. This likely reflects variations in hospital characteristics and case‐mix indices. There may also be other factors impacting teams such as how hospitals handled deteriorating patients before RRT implementation, education periods, and differing mechanisms and criteria for RRT activation.
In conclusion, RRT/METs are effective in decreasing both IHCA and hospital mortality. Our findings support the 2004 IHI recommendations for the implementation of RRTs in hospitals. Additional studies are still required to explore team composition, activation criteria, activation mechanism, and implementation strategies.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Institute for Healthcare Improvement. Overview of the 100,000 Lives Campaign. Available at: https://www.ihi.org/Engage/Initiatives/Completed/5MillionLivesCampaign/Documents/Overview%20of%20 the%20100K%20Campaign.pdf. Accessed September 18, 2014.
- , , . Reducing Hospital Mortality Rates (Part 2). IHI Innovation Series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2005.
- , . Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event. Crit Care Med. 1994;22(2):244–247.
- , , , et al. Duration of life‐threatening antecedents prior to intensive care admission. Intensive Care Med. 2002;28(11):1629–1634.
- , , . The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608–613.
- , , , et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care. 2005;9(6):R808–R815.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):417–425.
- , , , et al. Impact of an intensivist‐led multidisciplinary extended rapid response team on hospital‐wide cardiopulmonary arrests and mortality. Crit Care Med. 2013;41(2):506–517.
- , , , et al. The impact of implementing a rapid response system: a comparison of cardiopulmonary arrests and mortality among four teaching hospitals in Australia. Resuscitation. 2014;85(9):1275–1281.
- , , , et al. Rapid response team implementation on a burn surgery/acute care ward. J Burn Care Res. 2014;35(1):21–27.
- Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Oxford, United Kingdom: The Cochrane Collaboration; 2011: Available at: http://www.cochrane‐handbook.org. Accessed October 9, 2014.
- , , , et al. The Newcastle‐Ottawa Scale (NOS) for Assessing The Quality of Nonrandomised Studies in Meta‐analyses. Ottawa, Canada: Ottawa Hospital Research Institute; 2014.
- Agency for Healthcare Research and Quality. Remote cardiac monitoring: a systematic review. Available at: http://www.cms.gov/determinationprocess/downloads/id51ta.pdf. Published December 12, 2007.
- Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen, the Netherlands: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
- , , . Implementing a rapid‐response team using a nurse‐to‐nurse consult approach. J Vasc Nurs. 2008;26(2):37–42.
- , , , et al. The effect of a rapid response team on major clinical outcome measures in a community hospital. Crit Care Med. 2007;35(9):2076–2082.
- , , , et al. Implementing a rapid response team to decrease emergencies outside the ICU: one hospital's experience. Medsurg Nurs. 2009;18(2):84–90, 126.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , , , . Rapid response teams: do they make a difference? Dimens Crit Care Nurs. 2007;26(6):253–260; quiz 261–262.
- , , , . Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61(3):257–263.
- , , , . Improving patient safety to reduce preventable deaths: the case of a California safety net hospital. J Healthc Qual. 2012;34(2):64–76.
- , . Implementation and outcomes of a rapid response team. J Nurs Care Qual. 2007;22(4):307–313, quiz 314–315.
- , , . Implementation of a rapid response team decreases cardiac arrest outside of the intensive care unit. J Trauma. 2007;62(5):1223–1227; discussion 1227–1228.
- , , , et al. Introducing Critical Care Outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , , , , . Four years' experience with a hospitalist‐led medical emergency team: an interrupted time series. J Hosp Med. 2012;7(2):98–103.
- , , , . Rapid response team in an academic institution: does it make a difference? Chest. 2011;139(6):1361–1367.
- , , , et al. Long‐term effect of a medical emergency team on mortality in a teaching hospital. Resuscitation. 2007;74(2):235–241.
- , , , , . Rapid response team implementation and in‐hospital mortality*. Crit Care Med. 2014;42(9):2001–2006.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15(6):R269.
- , , , et al. A prospective before‐and‐after trial of a medical emergency team. Med J Aust. 2003;179(6):283–287.
- , , , et al. Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173(5):236–240.
- , , , et al. Reduced frequency of cardiopulmonary arrests by rapid response teams. Einstein (Sao Paulo). 2012;10(4):442–448.
- , , . Changing cardiac arrest and hospital mortality rates through a medical emergency team takes time and constant review. Crit Care Med. 2010;38(2):445–450.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36(1):100–106.
- , , , et al. Rescue me: saving the vulnerable non‐ICU patient population. Jt Comm J Qual Patient Saf. 2009;35(4):199–205.
- , , , , , . Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300(21):2506–2513.
- , , , . Introduction of a rapid response system at a United States veterans affairs hospital reduced cardiac arrests. Anesth Analg. 2010;111(3):679–686.
- , , , et al. Early impact of medical emergency team implementation in a country with limited medical resources: a before‐and‐after study. J Crit Care. 2011;26(4):373–378.
- , , , , , . Effect of a rapid response team on patient outcomes in a community‐based teaching hospital. J Grad Med Educ. 2014;6(1):61–64.
- , , . Abnormal vital signs are associated with an increased risk for critical events in US veteran inpatients. Resuscitation. 2009;80(11):1264–1269.
- , , , et al. A randomized trial of real‐time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9(7):424–429.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7(3):224–230.
- , , , , , . The impact of rapid response team on outcome of patients transferred from the ward to the ICU: a single‐center study. Crit Care Med. 2013;41(10):2284–2291.
- , , . Trends in inpatient hospital deaths: National Hospital Discharge Survey, 2000–2010. NCHS Data Brief. 2013(118):1–8.
- , , . Epidemiology and outcomes of in‐hospital cardiopulmonary resuscitation in the United States, 2000–2009. Resuscitation. 2013;84(9):1255–1260.
- , , , . The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295(3):324–327.
- Institute for Healthcare Improvement. Overview of the 100,000 Lives Campaign. Available at: https://www.ihi.org/Engage/Initiatives/Completed/5MillionLivesCampaign/Documents/Overview%20of%20 the%20100K%20Campaign.pdf. Accessed September 18, 2014.
- , , . Reducing Hospital Mortality Rates (Part 2). IHI Innovation Series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2005.
- , . Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event. Crit Care Med. 1994;22(2):244–247.
- , , , et al. Duration of life‐threatening antecedents prior to intensive care admission. Intensive Care Med. 2002;28(11):1629–1634.
- , , . The tension between needing to improve care and knowing how to do it. N Engl J Med. 2007;357(6):608–613.
- , , , et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care. 2005;9(6):R808–R815.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):417–425.
- , , , et al. Impact of an intensivist‐led multidisciplinary extended rapid response team on hospital‐wide cardiopulmonary arrests and mortality. Crit Care Med. 2013;41(2):506–517.
- , , , et al. The impact of implementing a rapid response system: a comparison of cardiopulmonary arrests and mortality among four teaching hospitals in Australia. Resuscitation. 2014;85(9):1275–1281.
- , , , et al. Rapid response team implementation on a burn surgery/acute care ward. J Burn Care Res. 2014;35(1):21–27.
- Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Oxford, United Kingdom: The Cochrane Collaboration; 2011: Available at: http://www.cochrane‐handbook.org. Accessed October 9, 2014.
- , , , et al. The Newcastle‐Ottawa Scale (NOS) for Assessing The Quality of Nonrandomised Studies in Meta‐analyses. Ottawa, Canada: Ottawa Hospital Research Institute; 2014.
- Agency for Healthcare Research and Quality. Remote cardiac monitoring: a systematic review. Available at: http://www.cms.gov/determinationprocess/downloads/id51ta.pdf. Published December 12, 2007.
- Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen, the Netherlands: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
- , , . Implementing a rapid‐response team using a nurse‐to‐nurse consult approach. J Vasc Nurs. 2008;26(2):37–42.
- , , , et al. The effect of a rapid response team on major clinical outcome measures in a community hospital. Crit Care Med. 2007;35(9):2076–2082.
- , , , et al. Implementing a rapid response team to decrease emergencies outside the ICU: one hospital's experience. Medsurg Nurs. 2009;18(2):84–90, 126.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , , , . Rapid response teams: do they make a difference? Dimens Crit Care Nurs. 2007;26(6):253–260; quiz 261–262.
- , , , . Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61(3):257–263.
- , , , . Improving patient safety to reduce preventable deaths: the case of a California safety net hospital. J Healthc Qual. 2012;34(2):64–76.
- , . Implementation and outcomes of a rapid response team. J Nurs Care Qual. 2007;22(4):307–313, quiz 314–315.
- , , . Implementation of a rapid response team decreases cardiac arrest outside of the intensive care unit. J Trauma. 2007;62(5):1223–1227; discussion 1227–1228.
- , , , et al. Introducing Critical Care Outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , , , , . Four years' experience with a hospitalist‐led medical emergency team: an interrupted time series. J Hosp Med. 2012;7(2):98–103.
- , , , . Rapid response team in an academic institution: does it make a difference? Chest. 2011;139(6):1361–1367.
- , , , et al. Long‐term effect of a medical emergency team on mortality in a teaching hospital. Resuscitation. 2007;74(2):235–241.
- , , , , . Rapid response team implementation and in‐hospital mortality*. Crit Care Med. 2014;42(9):2001–2006.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15(6):R269.
- , , , et al. A prospective before‐and‐after trial of a medical emergency team. Med J Aust. 2003;179(6):283–287.
- , , , et al. Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173(5):236–240.
- , , , et al. Reduced frequency of cardiopulmonary arrests by rapid response teams. Einstein (Sao Paulo). 2012;10(4):442–448.
- , , . Changing cardiac arrest and hospital mortality rates through a medical emergency team takes time and constant review. Crit Care Med. 2010;38(2):445–450.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36(1):100–106.
- , , , et al. Rescue me: saving the vulnerable non‐ICU patient population. Jt Comm J Qual Patient Saf. 2009;35(4):199–205.
- , , , , , . Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387–390.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300(21):2506–2513.
- , , , . Introduction of a rapid response system at a United States veterans affairs hospital reduced cardiac arrests. Anesth Analg. 2010;111(3):679–686.
- , , , et al. Early impact of medical emergency team implementation in a country with limited medical resources: a before‐and‐after study. J Crit Care. 2011;26(4):373–378.
- , , , , , . Effect of a rapid response team on patient outcomes in a community‐based teaching hospital. J Grad Med Educ. 2014;6(1):61–64.
- , , . Abnormal vital signs are associated with an increased risk for critical events in US veteran inpatients. Resuscitation. 2009;80(11):1264–1269.
- , , , et al. A randomized trial of real‐time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9(7):424–429.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7(3):224–230.
- , , , , , . The impact of rapid response team on outcome of patients transferred from the ward to the ICU: a single‐center study. Crit Care Med. 2013;41(10):2284–2291.
- , , . Trends in inpatient hospital deaths: National Hospital Discharge Survey, 2000–2010. NCHS Data Brief. 2013(118):1–8.
- , , . Epidemiology and outcomes of in‐hospital cardiopulmonary resuscitation in the United States, 2000–2009. Resuscitation. 2013;84(9):1255–1260.