User login
Epidemiological studies estimate that 40% to 50% of patients with pneumonia develop a parapneumonic effusion (PPE), and up to 35% of these have empyema. Approximately 15% of patients require surgical drainage, which has a high mortality rate.[1] Although early intervention is important in patients with suspected PPE, diagnosing a PPE is challenging, as cultures and Gram stain are frequently negative.[1] Clinicians have to rely on tests, such as pleural fluid pH, lactate dehydrogenase (LDH), and glucose, which have low sensitivity and specificity in diagnosing a PPE.
Procalcitonin (PCT) is a distinct biomarker and mediator of sepsis, emanating from parenchymal cells ubiquitously (eg, lung, liver, kidney) due to reduced conversion to mature calcitonin.[2] Besides sepsis, PCT has been used as a biomarker of pneumonia based on its ability to differentiate bacterial versus viral infections.[3, 4, 5] PCT has been shown to be a biomarker in extravascular fluids such as saliva, wound effusions, and pleural fluid.[6, 7, 8, 9] In this study we investigate the diagnostic accuracy of pleural fluid PCT in distinguishing infectious and noninfectious etiologies of pleural effusion in veterans with lung infiltrates.
METHOD
The study protocol was approved by the institution review board at the Veterans Affairs Medical Center, Washington, DC. Patients were identified using a computerized patient record system after searching the procedure code for thoracentesis. A retrospective chart review was conducted on veterans who underwent a thoracentesis from February 2011 through January 2012.
Inclusion Criteria
The inclusion criteria comprised all adults who underwent a thoracentesis and had pleural fluid collected for LDH, total protein, albumin, cell count with differential, cytology, Gram stain, culture, pH, triglycerides, cholesterol, and PCT.
Exclusion Criteria
The exclusion criteria comprised all patients with a known etiology of pleural effusion or those without pleural PCT data.
Data Collection
Pleural fluid data collected included LDH, protein, albumin, cell count and differential, pH, Gram stain and culture, cytology, triglyceride, cholesterol, amylase, and PCT. Serum chemistry data collected included LDH, protein, albumin, prothrombin time, international normalized ratio, and blood culture. PCT was measured in a 200‐L pleural fluid sample using Kryptor technology (Thermo Fisher Scientific, Freemont, CA). The Kryptor assay is based on a monoclonal mouse anti‐catacalcin antibody conjugated with colloidal gold (tracer) and a polyclonal sheep anti‐calcitonin antibody (solid phase). It has a detection limit of 0.06 ng/mL (or 0.06 g/L).[2]
Classification of Groups
Pleural fluid was classified as a transudate or an exudate by Light's criteria.[10] An exudative effusion had a pleural fluid to serum ratio of LDH > 0.6, pleural fluid to serum protein ratio >0.5, or LDH great than the upper two‐thirds of the reference value or serum value.
Patient's clinical diagnosis for the cause of pleural effusion was documented from chart review. Effusions were classified as infectious pleural effusions (IPE) or noninfectious pleural effusions (NIPE).
An effusion was considered infectious if pleural fluid Gram stain or culture were positive for bacteria, if pus was present, or if the effusion was accompanied by a lung infiltrate in a patient with evidence at least 2 of the following: temperature >38C (100.4F) or 36C (96.8F), heart rate >90 beats per minute, respiratory rate >20 breaths per minute or arterial carbon dioxide tension (PaCO2) of 32 mm Hg, and white blood cell count >12,000/L or 4000/L or >10% immature (band) forms.
An effusion not meeting the above criteria was classified as a NIPE. A malignant effusion was diagnosed by the presence of cancer cells on cytology. Paramalignant effusion was an effusion that was devoid of cancer cells on cytology and/or histology, in a patient with a malignancy.
Data Analysis
Statistical computations were performed using Graph Pad Instat version 3 and version 5 statistical software (Graph Pad Software, Inc. La Jolla, CA). Median PCT with standard deviation (SD) was calculated for IPE and NIPE. A 95% confidence interval (CI) for the median PCT was calculated for each group. A comparison of the median PCT between the IPE and NIPE was performed by calculating the SD difference and standard error difference. A 95% CI of the difference in medians was calculated. A P value was calculated using Mann‐Whitney U Test, and a P value of 0.05 was considered significant. The diagnostic performance of different cutoff values of PCT was evaluated using the area under the receiver operating characteristic curve (mean, 95% CI).
RESULTS
A total of 75 patients were included in the study. There were 73 (97.4%) males, with mean age of 70.8 years (range, 4293 years). There were 18 patients with IPE and 57 with NIPE.
Patient characteristics are detailed in Table 1. In the infectious group, 2 patients had empyema. There were no cases of tuberculosis. Of the 57 effusions in the noninfectious group there were 42 exudative effusions, 23 of which were malignant, 3 each were due to a trapped lung and pulmonary embolism. The remaining NIPEs were due to nonpulmonary processes such as chylothorax, liver disease, and renal disease.
| Infectious, n = 18 | Noninfectious, n = 57 | P Value | |
|---|---|---|---|
| |||
| Mean age, y | 73.1 | 70.1 | 0.349 |
| Male | 18 (100%) | 55 (96.6%) | 0.428 |
| Exudative effusion | 13 (72.2%) | 43 (74.1%) | 0.873 |
| Right side | 7 (38.9%) | 32 (55.2%) | 0.2268 |
| Effusion less than one‐third hemithorax | 11 (61.1%) | 28 (48.3%) | 0.3425 |
| Effusion one‐third to two‐thirds hemithorax | 6 (33.3%) | 20 (34.5%) | 0.9253 |
| Effusion greater then two‐thirds hemithorax | 1 (5.6%) | 9 (15.5%) | 0.2777 |
| Median PCT, ng/mL | 1.088 (0.3122.940) | 0.123 (0.050.263) | 0.0001* |
| Median LDH, IU/L | 178.5 (105.5346.25) | 135.5 (94255.2) | 0.629 |
| Median protein, mg/dL | 3.1 (2.33.2) | 3.7 (2.384.48) | 0.046* |
| Median pH | 7.37 (7.317.44) | 7.40 (7.367.44) | 0.111 |
| Median glucose, mg/dL | 126 (97169) | 106 (92135) | 0.226 |
| Median pleural WBC, cells/L | 778 (3237038) | 498 (2001380) | 0.154 |
| Median pleural neutrophils, cells/L | 542 (544743) | 54 (18192) | 0.005* |
The pleural fluid characteristics and biomarkers are detailed in Table 1. Median pleural fluid PCT in IPE was 1.088 ng/mL (0.3122.940 ng/mL) and 0.123 ng/mL (0.050.263 ng/mL) in NIPE, with a P value 0.0001. Pleural fluid PCT >0.25 ng/mL had a sensitivity of 77.78% and specificity of 74.14% for diagnosing an IPE (Figure 1).
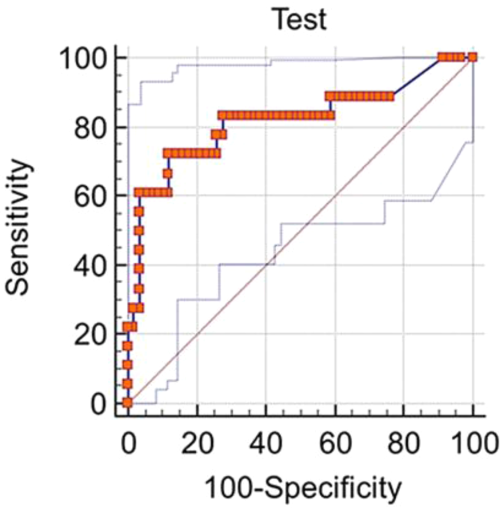
A subgroup analysis comparing 13 exudative effusions in the infectious group with the 23 exudative malignant and paramalignant effusions in the noninfectious group was also performed. The median pleural PCT value in the infectious group was 0.9743 ng/mL (0.454.117 ng/mL) and 0.1222 ng/mL (0.054650.1972 ng/mL) in the noninfectious group, with a P value 0.0009
DISCUSSION
Clinicians frequently face the dilemma of differentiating IPE from NIPE. In this study, pleural fluid PCT was significantly elevated in IPE. A pleural fluid PCT >0.25 ng/mL had a sensitivity of 77.78% and specificity of 74.14% for diagnosing an IPE (Figure 1). Our subgroup analysis also showed a higher PCT in exudative effusions of the infectious group as compared to exudative effusions of malignant/paramalignant etiology in the noninfectious group. PCT may have a role as a biomarker in diagnosing an infected malignant/paramalignant effusion. Further studies are needed to confirm the same.
Our study is one of the few using the more sensitive technology (eg, Kryptor) to measure PCT levels in pleural fluid, utilizing the current method of choice for serum PCT based on assay performance.[2] PCT is produced during bacterial infections by several tissues sources, and its role in differentiating IPE and NIPE has been investigated by several authors. A prior study reported on 233 patients, 28 of whom had PPEs, 49 had tubercular effusion, and 166 had NIPE.[1] The cutoff point in this study of PCT was >0.145 ng/mL, with a sensitivity of 51.6% and specificity of 66.5%.[1] Another study evaluated 82 patients with pleural effusions, 45 of whom were infectious (bacterial, nontubercular) and used a PCT cutoff value of 0.18 ng/mL to discriminate between IPE and NIPE. They reported a sensitivity of 66.7% and specificity of 77.4%. There was no significant difference in the serum and pleural fluid PCT values within IPE and NIPE subgroups. In fact, there was a significant positive correlation between serum and pleural fluid PCT. However, on comparing evolution of pleural and serum PCT between day 1 and day 3, the authors noted that unlike pleural fluid PCT, serum PCT values were lower on day 3 as compared to day 1.[8] A study on 12 forensic autopsy cases, to establish the usefulness of pericardial and pleural fluids for the postmortem diagnosis of sepsis, also reported significantly higher and similar PCT levels in the sepsis group in both serum and pleural fluid (using an immunoassay by Roche Diagnostic, Mannheim, Germany). The authors suggested that pleural fluid PCT can be used in lieu of serum PCT values to determine the etiology of an effusion.[11]
PCT has a role in the decision to initiate or discontinue antibiotics in the management of community‐acquired pneumonia.[12] In this era of multidrug resistance, appropriate use of antibiotics is of paramount importance, and PCT could play an important role in this regard. The gold standard test to diagnose an infectious pleural effusion is present in a small percentage of patients.[13] Larger randomized studies designed to evaluate the role of serial serum and pleural fluid PCT, with appropriate cutoff values, are needed to define the role of PCT in guiding antibiotic therapy.[14]
The limitations of this study include its retrospective nature and lack of serum PCT data. The gold standard methods to diagnose pleural effusion were not available in this study.
CONCLUSION
PCT is a novel biomarker for diagnosing infectious pleural effusion, and it would be worthwhile to investigate the role of pleural PCT in assessing severity of illness, risk stratification, and antibiotic stewardship in hospitalized patients with pleural effusions.
Disclosure: Nothing to report.
- , , , et al. Procalcitonin, C‐reactive protein, and cell counts in the diagnosis of parapneumonic pleural effusions. J Investig Med. 2010;58:971–976.
- , , . Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941–952.
- , , , , . Pneumonitis‐associated hyperprocalcitoninemia. Am J Med Sci. 1996;312:12–18.
- , , , et al. Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J Infect Dis. 2013;208:432–441.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93.
- , , , et al. Correlation of procalcitonin and cytokine expression with dehiscence of wartime extremity wounds. J Bone Joint Surg Am. 2008;90:580–588.
- , , , , . Salivary procalcitonin and periodontitis in diabetes. J Dent Res. 2008;87:630–634.
- , , , , . Diagnostic and prognostic values of pleural fluid procalcitonin in parapneumonic pleural effusions. Chest. 2009;136:205–211.
- , , , , , . Serum and pleural fluid procalcitonin in predicting bacterial infection in patients with parapneumonic effusion. J Korean Med Sci. 2009;24:398–402.
- , . Textbook of Pleural Diseases. 2nd ed. London, United Kingdom: Arnold Press; 2008.
- , . Usefulness of pericardial and pleural fluids for the postmortem diagnosis of sepsis. J Forensic Leg Med. 2014;28:15–18.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Evid Based Child Health. 2013;8:1297–1371.
- , , , , , . Routine use of pleural fluid cultures. Are they indicated? Limited yield, minimal impact on treatment decisions. Respir Med. 2006;100:2048–2052.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363:600–607.
Epidemiological studies estimate that 40% to 50% of patients with pneumonia develop a parapneumonic effusion (PPE), and up to 35% of these have empyema. Approximately 15% of patients require surgical drainage, which has a high mortality rate.[1] Although early intervention is important in patients with suspected PPE, diagnosing a PPE is challenging, as cultures and Gram stain are frequently negative.[1] Clinicians have to rely on tests, such as pleural fluid pH, lactate dehydrogenase (LDH), and glucose, which have low sensitivity and specificity in diagnosing a PPE.
Procalcitonin (PCT) is a distinct biomarker and mediator of sepsis, emanating from parenchymal cells ubiquitously (eg, lung, liver, kidney) due to reduced conversion to mature calcitonin.[2] Besides sepsis, PCT has been used as a biomarker of pneumonia based on its ability to differentiate bacterial versus viral infections.[3, 4, 5] PCT has been shown to be a biomarker in extravascular fluids such as saliva, wound effusions, and pleural fluid.[6, 7, 8, 9] In this study we investigate the diagnostic accuracy of pleural fluid PCT in distinguishing infectious and noninfectious etiologies of pleural effusion in veterans with lung infiltrates.
METHOD
The study protocol was approved by the institution review board at the Veterans Affairs Medical Center, Washington, DC. Patients were identified using a computerized patient record system after searching the procedure code for thoracentesis. A retrospective chart review was conducted on veterans who underwent a thoracentesis from February 2011 through January 2012.
Inclusion Criteria
The inclusion criteria comprised all adults who underwent a thoracentesis and had pleural fluid collected for LDH, total protein, albumin, cell count with differential, cytology, Gram stain, culture, pH, triglycerides, cholesterol, and PCT.
Exclusion Criteria
The exclusion criteria comprised all patients with a known etiology of pleural effusion or those without pleural PCT data.
Data Collection
Pleural fluid data collected included LDH, protein, albumin, cell count and differential, pH, Gram stain and culture, cytology, triglyceride, cholesterol, amylase, and PCT. Serum chemistry data collected included LDH, protein, albumin, prothrombin time, international normalized ratio, and blood culture. PCT was measured in a 200‐L pleural fluid sample using Kryptor technology (Thermo Fisher Scientific, Freemont, CA). The Kryptor assay is based on a monoclonal mouse anti‐catacalcin antibody conjugated with colloidal gold (tracer) and a polyclonal sheep anti‐calcitonin antibody (solid phase). It has a detection limit of 0.06 ng/mL (or 0.06 g/L).[2]
Classification of Groups
Pleural fluid was classified as a transudate or an exudate by Light's criteria.[10] An exudative effusion had a pleural fluid to serum ratio of LDH > 0.6, pleural fluid to serum protein ratio >0.5, or LDH great than the upper two‐thirds of the reference value or serum value.
Patient's clinical diagnosis for the cause of pleural effusion was documented from chart review. Effusions were classified as infectious pleural effusions (IPE) or noninfectious pleural effusions (NIPE).
An effusion was considered infectious if pleural fluid Gram stain or culture were positive for bacteria, if pus was present, or if the effusion was accompanied by a lung infiltrate in a patient with evidence at least 2 of the following: temperature >38C (100.4F) or 36C (96.8F), heart rate >90 beats per minute, respiratory rate >20 breaths per minute or arterial carbon dioxide tension (PaCO2) of 32 mm Hg, and white blood cell count >12,000/L or 4000/L or >10% immature (band) forms.
An effusion not meeting the above criteria was classified as a NIPE. A malignant effusion was diagnosed by the presence of cancer cells on cytology. Paramalignant effusion was an effusion that was devoid of cancer cells on cytology and/or histology, in a patient with a malignancy.
Data Analysis
Statistical computations were performed using Graph Pad Instat version 3 and version 5 statistical software (Graph Pad Software, Inc. La Jolla, CA). Median PCT with standard deviation (SD) was calculated for IPE and NIPE. A 95% confidence interval (CI) for the median PCT was calculated for each group. A comparison of the median PCT between the IPE and NIPE was performed by calculating the SD difference and standard error difference. A 95% CI of the difference in medians was calculated. A P value was calculated using Mann‐Whitney U Test, and a P value of 0.05 was considered significant. The diagnostic performance of different cutoff values of PCT was evaluated using the area under the receiver operating characteristic curve (mean, 95% CI).
RESULTS
A total of 75 patients were included in the study. There were 73 (97.4%) males, with mean age of 70.8 years (range, 4293 years). There were 18 patients with IPE and 57 with NIPE.
Patient characteristics are detailed in Table 1. In the infectious group, 2 patients had empyema. There were no cases of tuberculosis. Of the 57 effusions in the noninfectious group there were 42 exudative effusions, 23 of which were malignant, 3 each were due to a trapped lung and pulmonary embolism. The remaining NIPEs were due to nonpulmonary processes such as chylothorax, liver disease, and renal disease.
| Infectious, n = 18 | Noninfectious, n = 57 | P Value | |
|---|---|---|---|
| |||
| Mean age, y | 73.1 | 70.1 | 0.349 |
| Male | 18 (100%) | 55 (96.6%) | 0.428 |
| Exudative effusion | 13 (72.2%) | 43 (74.1%) | 0.873 |
| Right side | 7 (38.9%) | 32 (55.2%) | 0.2268 |
| Effusion less than one‐third hemithorax | 11 (61.1%) | 28 (48.3%) | 0.3425 |
| Effusion one‐third to two‐thirds hemithorax | 6 (33.3%) | 20 (34.5%) | 0.9253 |
| Effusion greater then two‐thirds hemithorax | 1 (5.6%) | 9 (15.5%) | 0.2777 |
| Median PCT, ng/mL | 1.088 (0.3122.940) | 0.123 (0.050.263) | 0.0001* |
| Median LDH, IU/L | 178.5 (105.5346.25) | 135.5 (94255.2) | 0.629 |
| Median protein, mg/dL | 3.1 (2.33.2) | 3.7 (2.384.48) | 0.046* |
| Median pH | 7.37 (7.317.44) | 7.40 (7.367.44) | 0.111 |
| Median glucose, mg/dL | 126 (97169) | 106 (92135) | 0.226 |
| Median pleural WBC, cells/L | 778 (3237038) | 498 (2001380) | 0.154 |
| Median pleural neutrophils, cells/L | 542 (544743) | 54 (18192) | 0.005* |
The pleural fluid characteristics and biomarkers are detailed in Table 1. Median pleural fluid PCT in IPE was 1.088 ng/mL (0.3122.940 ng/mL) and 0.123 ng/mL (0.050.263 ng/mL) in NIPE, with a P value 0.0001. Pleural fluid PCT >0.25 ng/mL had a sensitivity of 77.78% and specificity of 74.14% for diagnosing an IPE (Figure 1).

A subgroup analysis comparing 13 exudative effusions in the infectious group with the 23 exudative malignant and paramalignant effusions in the noninfectious group was also performed. The median pleural PCT value in the infectious group was 0.9743 ng/mL (0.454.117 ng/mL) and 0.1222 ng/mL (0.054650.1972 ng/mL) in the noninfectious group, with a P value 0.0009
DISCUSSION
Clinicians frequently face the dilemma of differentiating IPE from NIPE. In this study, pleural fluid PCT was significantly elevated in IPE. A pleural fluid PCT >0.25 ng/mL had a sensitivity of 77.78% and specificity of 74.14% for diagnosing an IPE (Figure 1). Our subgroup analysis also showed a higher PCT in exudative effusions of the infectious group as compared to exudative effusions of malignant/paramalignant etiology in the noninfectious group. PCT may have a role as a biomarker in diagnosing an infected malignant/paramalignant effusion. Further studies are needed to confirm the same.
Our study is one of the few using the more sensitive technology (eg, Kryptor) to measure PCT levels in pleural fluid, utilizing the current method of choice for serum PCT based on assay performance.[2] PCT is produced during bacterial infections by several tissues sources, and its role in differentiating IPE and NIPE has been investigated by several authors. A prior study reported on 233 patients, 28 of whom had PPEs, 49 had tubercular effusion, and 166 had NIPE.[1] The cutoff point in this study of PCT was >0.145 ng/mL, with a sensitivity of 51.6% and specificity of 66.5%.[1] Another study evaluated 82 patients with pleural effusions, 45 of whom were infectious (bacterial, nontubercular) and used a PCT cutoff value of 0.18 ng/mL to discriminate between IPE and NIPE. They reported a sensitivity of 66.7% and specificity of 77.4%. There was no significant difference in the serum and pleural fluid PCT values within IPE and NIPE subgroups. In fact, there was a significant positive correlation between serum and pleural fluid PCT. However, on comparing evolution of pleural and serum PCT between day 1 and day 3, the authors noted that unlike pleural fluid PCT, serum PCT values were lower on day 3 as compared to day 1.[8] A study on 12 forensic autopsy cases, to establish the usefulness of pericardial and pleural fluids for the postmortem diagnosis of sepsis, also reported significantly higher and similar PCT levels in the sepsis group in both serum and pleural fluid (using an immunoassay by Roche Diagnostic, Mannheim, Germany). The authors suggested that pleural fluid PCT can be used in lieu of serum PCT values to determine the etiology of an effusion.[11]
PCT has a role in the decision to initiate or discontinue antibiotics in the management of community‐acquired pneumonia.[12] In this era of multidrug resistance, appropriate use of antibiotics is of paramount importance, and PCT could play an important role in this regard. The gold standard test to diagnose an infectious pleural effusion is present in a small percentage of patients.[13] Larger randomized studies designed to evaluate the role of serial serum and pleural fluid PCT, with appropriate cutoff values, are needed to define the role of PCT in guiding antibiotic therapy.[14]
The limitations of this study include its retrospective nature and lack of serum PCT data. The gold standard methods to diagnose pleural effusion were not available in this study.
CONCLUSION
PCT is a novel biomarker for diagnosing infectious pleural effusion, and it would be worthwhile to investigate the role of pleural PCT in assessing severity of illness, risk stratification, and antibiotic stewardship in hospitalized patients with pleural effusions.
Disclosure: Nothing to report.
Epidemiological studies estimate that 40% to 50% of patients with pneumonia develop a parapneumonic effusion (PPE), and up to 35% of these have empyema. Approximately 15% of patients require surgical drainage, which has a high mortality rate.[1] Although early intervention is important in patients with suspected PPE, diagnosing a PPE is challenging, as cultures and Gram stain are frequently negative.[1] Clinicians have to rely on tests, such as pleural fluid pH, lactate dehydrogenase (LDH), and glucose, which have low sensitivity and specificity in diagnosing a PPE.
Procalcitonin (PCT) is a distinct biomarker and mediator of sepsis, emanating from parenchymal cells ubiquitously (eg, lung, liver, kidney) due to reduced conversion to mature calcitonin.[2] Besides sepsis, PCT has been used as a biomarker of pneumonia based on its ability to differentiate bacterial versus viral infections.[3, 4, 5] PCT has been shown to be a biomarker in extravascular fluids such as saliva, wound effusions, and pleural fluid.[6, 7, 8, 9] In this study we investigate the diagnostic accuracy of pleural fluid PCT in distinguishing infectious and noninfectious etiologies of pleural effusion in veterans with lung infiltrates.
METHOD
The study protocol was approved by the institution review board at the Veterans Affairs Medical Center, Washington, DC. Patients were identified using a computerized patient record system after searching the procedure code for thoracentesis. A retrospective chart review was conducted on veterans who underwent a thoracentesis from February 2011 through January 2012.
Inclusion Criteria
The inclusion criteria comprised all adults who underwent a thoracentesis and had pleural fluid collected for LDH, total protein, albumin, cell count with differential, cytology, Gram stain, culture, pH, triglycerides, cholesterol, and PCT.
Exclusion Criteria
The exclusion criteria comprised all patients with a known etiology of pleural effusion or those without pleural PCT data.
Data Collection
Pleural fluid data collected included LDH, protein, albumin, cell count and differential, pH, Gram stain and culture, cytology, triglyceride, cholesterol, amylase, and PCT. Serum chemistry data collected included LDH, protein, albumin, prothrombin time, international normalized ratio, and blood culture. PCT was measured in a 200‐L pleural fluid sample using Kryptor technology (Thermo Fisher Scientific, Freemont, CA). The Kryptor assay is based on a monoclonal mouse anti‐catacalcin antibody conjugated with colloidal gold (tracer) and a polyclonal sheep anti‐calcitonin antibody (solid phase). It has a detection limit of 0.06 ng/mL (or 0.06 g/L).[2]
Classification of Groups
Pleural fluid was classified as a transudate or an exudate by Light's criteria.[10] An exudative effusion had a pleural fluid to serum ratio of LDH > 0.6, pleural fluid to serum protein ratio >0.5, or LDH great than the upper two‐thirds of the reference value or serum value.
Patient's clinical diagnosis for the cause of pleural effusion was documented from chart review. Effusions were classified as infectious pleural effusions (IPE) or noninfectious pleural effusions (NIPE).
An effusion was considered infectious if pleural fluid Gram stain or culture were positive for bacteria, if pus was present, or if the effusion was accompanied by a lung infiltrate in a patient with evidence at least 2 of the following: temperature >38C (100.4F) or 36C (96.8F), heart rate >90 beats per minute, respiratory rate >20 breaths per minute or arterial carbon dioxide tension (PaCO2) of 32 mm Hg, and white blood cell count >12,000/L or 4000/L or >10% immature (band) forms.
An effusion not meeting the above criteria was classified as a NIPE. A malignant effusion was diagnosed by the presence of cancer cells on cytology. Paramalignant effusion was an effusion that was devoid of cancer cells on cytology and/or histology, in a patient with a malignancy.
Data Analysis
Statistical computations were performed using Graph Pad Instat version 3 and version 5 statistical software (Graph Pad Software, Inc. La Jolla, CA). Median PCT with standard deviation (SD) was calculated for IPE and NIPE. A 95% confidence interval (CI) for the median PCT was calculated for each group. A comparison of the median PCT between the IPE and NIPE was performed by calculating the SD difference and standard error difference. A 95% CI of the difference in medians was calculated. A P value was calculated using Mann‐Whitney U Test, and a P value of 0.05 was considered significant. The diagnostic performance of different cutoff values of PCT was evaluated using the area under the receiver operating characteristic curve (mean, 95% CI).
RESULTS
A total of 75 patients were included in the study. There were 73 (97.4%) males, with mean age of 70.8 years (range, 4293 years). There were 18 patients with IPE and 57 with NIPE.
Patient characteristics are detailed in Table 1. In the infectious group, 2 patients had empyema. There were no cases of tuberculosis. Of the 57 effusions in the noninfectious group there were 42 exudative effusions, 23 of which were malignant, 3 each were due to a trapped lung and pulmonary embolism. The remaining NIPEs were due to nonpulmonary processes such as chylothorax, liver disease, and renal disease.
| Infectious, n = 18 | Noninfectious, n = 57 | P Value | |
|---|---|---|---|
| |||
| Mean age, y | 73.1 | 70.1 | 0.349 |
| Male | 18 (100%) | 55 (96.6%) | 0.428 |
| Exudative effusion | 13 (72.2%) | 43 (74.1%) | 0.873 |
| Right side | 7 (38.9%) | 32 (55.2%) | 0.2268 |
| Effusion less than one‐third hemithorax | 11 (61.1%) | 28 (48.3%) | 0.3425 |
| Effusion one‐third to two‐thirds hemithorax | 6 (33.3%) | 20 (34.5%) | 0.9253 |
| Effusion greater then two‐thirds hemithorax | 1 (5.6%) | 9 (15.5%) | 0.2777 |
| Median PCT, ng/mL | 1.088 (0.3122.940) | 0.123 (0.050.263) | 0.0001* |
| Median LDH, IU/L | 178.5 (105.5346.25) | 135.5 (94255.2) | 0.629 |
| Median protein, mg/dL | 3.1 (2.33.2) | 3.7 (2.384.48) | 0.046* |
| Median pH | 7.37 (7.317.44) | 7.40 (7.367.44) | 0.111 |
| Median glucose, mg/dL | 126 (97169) | 106 (92135) | 0.226 |
| Median pleural WBC, cells/L | 778 (3237038) | 498 (2001380) | 0.154 |
| Median pleural neutrophils, cells/L | 542 (544743) | 54 (18192) | 0.005* |
The pleural fluid characteristics and biomarkers are detailed in Table 1. Median pleural fluid PCT in IPE was 1.088 ng/mL (0.3122.940 ng/mL) and 0.123 ng/mL (0.050.263 ng/mL) in NIPE, with a P value 0.0001. Pleural fluid PCT >0.25 ng/mL had a sensitivity of 77.78% and specificity of 74.14% for diagnosing an IPE (Figure 1).

A subgroup analysis comparing 13 exudative effusions in the infectious group with the 23 exudative malignant and paramalignant effusions in the noninfectious group was also performed. The median pleural PCT value in the infectious group was 0.9743 ng/mL (0.454.117 ng/mL) and 0.1222 ng/mL (0.054650.1972 ng/mL) in the noninfectious group, with a P value 0.0009
DISCUSSION
Clinicians frequently face the dilemma of differentiating IPE from NIPE. In this study, pleural fluid PCT was significantly elevated in IPE. A pleural fluid PCT >0.25 ng/mL had a sensitivity of 77.78% and specificity of 74.14% for diagnosing an IPE (Figure 1). Our subgroup analysis also showed a higher PCT in exudative effusions of the infectious group as compared to exudative effusions of malignant/paramalignant etiology in the noninfectious group. PCT may have a role as a biomarker in diagnosing an infected malignant/paramalignant effusion. Further studies are needed to confirm the same.
Our study is one of the few using the more sensitive technology (eg, Kryptor) to measure PCT levels in pleural fluid, utilizing the current method of choice for serum PCT based on assay performance.[2] PCT is produced during bacterial infections by several tissues sources, and its role in differentiating IPE and NIPE has been investigated by several authors. A prior study reported on 233 patients, 28 of whom had PPEs, 49 had tubercular effusion, and 166 had NIPE.[1] The cutoff point in this study of PCT was >0.145 ng/mL, with a sensitivity of 51.6% and specificity of 66.5%.[1] Another study evaluated 82 patients with pleural effusions, 45 of whom were infectious (bacterial, nontubercular) and used a PCT cutoff value of 0.18 ng/mL to discriminate between IPE and NIPE. They reported a sensitivity of 66.7% and specificity of 77.4%. There was no significant difference in the serum and pleural fluid PCT values within IPE and NIPE subgroups. In fact, there was a significant positive correlation between serum and pleural fluid PCT. However, on comparing evolution of pleural and serum PCT between day 1 and day 3, the authors noted that unlike pleural fluid PCT, serum PCT values were lower on day 3 as compared to day 1.[8] A study on 12 forensic autopsy cases, to establish the usefulness of pericardial and pleural fluids for the postmortem diagnosis of sepsis, also reported significantly higher and similar PCT levels in the sepsis group in both serum and pleural fluid (using an immunoassay by Roche Diagnostic, Mannheim, Germany). The authors suggested that pleural fluid PCT can be used in lieu of serum PCT values to determine the etiology of an effusion.[11]
PCT has a role in the decision to initiate or discontinue antibiotics in the management of community‐acquired pneumonia.[12] In this era of multidrug resistance, appropriate use of antibiotics is of paramount importance, and PCT could play an important role in this regard. The gold standard test to diagnose an infectious pleural effusion is present in a small percentage of patients.[13] Larger randomized studies designed to evaluate the role of serial serum and pleural fluid PCT, with appropriate cutoff values, are needed to define the role of PCT in guiding antibiotic therapy.[14]
The limitations of this study include its retrospective nature and lack of serum PCT data. The gold standard methods to diagnose pleural effusion were not available in this study.
CONCLUSION
PCT is a novel biomarker for diagnosing infectious pleural effusion, and it would be worthwhile to investigate the role of pleural PCT in assessing severity of illness, risk stratification, and antibiotic stewardship in hospitalized patients with pleural effusions.
Disclosure: Nothing to report.
- , , , et al. Procalcitonin, C‐reactive protein, and cell counts in the diagnosis of parapneumonic pleural effusions. J Investig Med. 2010;58:971–976.
- , , . Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941–952.
- , , , , . Pneumonitis‐associated hyperprocalcitoninemia. Am J Med Sci. 1996;312:12–18.
- , , , et al. Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J Infect Dis. 2013;208:432–441.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93.
- , , , et al. Correlation of procalcitonin and cytokine expression with dehiscence of wartime extremity wounds. J Bone Joint Surg Am. 2008;90:580–588.
- , , , , . Salivary procalcitonin and periodontitis in diabetes. J Dent Res. 2008;87:630–634.
- , , , , . Diagnostic and prognostic values of pleural fluid procalcitonin in parapneumonic pleural effusions. Chest. 2009;136:205–211.
- , , , , , . Serum and pleural fluid procalcitonin in predicting bacterial infection in patients with parapneumonic effusion. J Korean Med Sci. 2009;24:398–402.
- , . Textbook of Pleural Diseases. 2nd ed. London, United Kingdom: Arnold Press; 2008.
- , . Usefulness of pericardial and pleural fluids for the postmortem diagnosis of sepsis. J Forensic Leg Med. 2014;28:15–18.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Evid Based Child Health. 2013;8:1297–1371.
- , , , , , . Routine use of pleural fluid cultures. Are they indicated? Limited yield, minimal impact on treatment decisions. Respir Med. 2006;100:2048–2052.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363:600–607.
- , , , et al. Procalcitonin, C‐reactive protein, and cell counts in the diagnosis of parapneumonic pleural effusions. J Investig Med. 2010;58:971–976.
- , , . Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941–952.
- , , , , . Pneumonitis‐associated hyperprocalcitoninemia. Am J Med Sci. 1996;312:12–18.
- , , , et al. Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J Infect Dis. 2013;208:432–441.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93.
- , , , et al. Correlation of procalcitonin and cytokine expression with dehiscence of wartime extremity wounds. J Bone Joint Surg Am. 2008;90:580–588.
- , , , , . Salivary procalcitonin and periodontitis in diabetes. J Dent Res. 2008;87:630–634.
- , , , , . Diagnostic and prognostic values of pleural fluid procalcitonin in parapneumonic pleural effusions. Chest. 2009;136:205–211.
- , , , , , . Serum and pleural fluid procalcitonin in predicting bacterial infection in patients with parapneumonic effusion. J Korean Med Sci. 2009;24:398–402.
- , . Textbook of Pleural Diseases. 2nd ed. London, United Kingdom: Arnold Press; 2008.
- , . Usefulness of pericardial and pleural fluids for the postmortem diagnosis of sepsis. J Forensic Leg Med. 2014;28:15–18.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Evid Based Child Health. 2013;8:1297–1371.
- , , , , , . Routine use of pleural fluid cultures. Are they indicated? Limited yield, minimal impact on treatment decisions. Respir Med. 2006;100:2048–2052.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363:600–607.