User login
Frailty Evaluation in the Hospital
Frailty is a state of vulnerability that encompasses a heterogeneous group of people.[1] Because it lacks a precise definition, multiple tools have been developed to identify frailty in both clinical and research settings.[2, 3, 4] Prevalence of frailty depends on the frailty assessment tool used and the population studied, ranging from 4% to 17% when the Fried score[5, 6, 7] is used and from 5% to 44%[5, 7, 8] when cumulative deficit models like the Frailty Index are utilized, with the lower prevalences being in younger community‐dwelling elderly populations and the higher proportions being in older institutionalized populations.
The Frailty Index, also called the Burden or Cumulative Deficit Model, comprises 70 domains that include mobility, mood, function, cognitive impairment, and disease states. It is multidimensional and allows for patients to be categorized on a continuum of frailty, but it is extremely difficult to apply in clinical practice. Recognizing this, Rockwood et al.[9] developed and validated the Clinical Frailty Scale (CFS) in the Canadian Study of Health and Aging. The CFS classifies patients into 1 of 9 categories: very fit, well, managing well, vulnerable, mildly frail (needs help with at least 1 instrumental activity of daily living such as shopping, finances, meal preparation, or housework), moderately frail (needs help with 1 or 2 activities of daily living such as bathing and dressing), severely frail (dependent for personal care), very severely frail (bedbound), and terminally ill. Although this tool is easy to use in clinical practice, it reflects a gestalt impression and requires some clinical judgement.
The Fried score[6] is a prototypical phenotype tool based on 5 criteria that include weight loss, self‐reported exhaustion, low energy expenditure, slowness of gait, and weakness. Recent evidence has suggested that slow gait (or dysmobility) alone may also be a potential screening test for frailty.[10] A recent systematic review[11] demonstrated an association between slow gait (dysmobility) and increased mortality. Dysmobility negatively impacts quality of life and has a strong association with disability resulting in the need for an increased level of care.[12] The Timed Up and Go Test (TUGT) is one method of assessing mobility which is relatively easy to perform, does not require special equipment, and is feasible to use in clinical settings.[13] However, whether impaired mobility predicts outcomes within the first 30 days after hospital discharge (a timeframe highlighted in the Affordable Care Act and used by the Centers for Medicare and Medicaid Services as an important hospital quality indicator) is still uncertain.
The aim of this study was to compare frailty assessments using the CFS and 2 of the most commonly used phenotypic tools (a modified Fried score and the TUGT as a proxy for mobility assessment) to determine which tools best predict postdischarge outcomes.
METHODS
Study Design and Population
As described in detail elsewhere,[14] this was a prospective cohort study that enrolled adult patients (any age older than 18 years) at the time of discharge back to the community from 7 general internal medicine wards in 2 teaching hospitals in Edmonton, Alberta between October 2013 and November 2014. We excluded patients admitted from, or being discharged back to, long‐term care facilities or other acute care hospitals, or from out of the province; patients who were unable to communicate in English; patients with moderate or severe cognitive impairment (scoring 5 or more on the Short Portable Mental Status Questionnaire); or patients with projected life expectancy of less than 3 months. All patients provided written consent, and the study was approved by the Health Research Ethics board of the University of Alberta (project ID Pro00036880).
We assessed the degree of frailty within 24 hours of discharge in 3 ways. First, we used the CFS[9, 15] with patients being asked to rate their best functional status in the week prior to admission. As per the CFS validations studies, scores 5 were defined as frail.[9, 15] Second, we used the TUGT as a proxy for slow gait speed/dysmobility (with >20 seconds defined as abnormal).[13] The TUGT was recorded as the shortest recorded time of the 2 timed trials to get up from a seated position, walk 10 feet and back, and then sit in the chair again. Third, we also determined their Fried score[6] (using the modifications outlined below) and categorized the patients as frail if they scored 3 or more. Of the 5 Fried categories, we assessed weakness by grip strength in their dominant hand using a Jamar handheld dynamometer and weight loss of 10 lb or more in the past year based on patient self‐report; these are identical to the original Fried scale description. Grip strength in the lowest quintile for sex and body mass index was defined as weak grip strength as per convention in the literature, which corresponded to less than 28.5 kg for men and less than 18.5 kg for women.[16, 17] We assessed the other 3 Fried categories in modified fashion as follows. For slow gait, rather than assessing time to walk 15 feet as in the original study and assigning a point to those testing in the lowest quintile for their age/sex, we used the TUGT, because our research personnel were already trained in this test, and we were doing it already as part of the discharge package for all patients.[13] For the Fried category of low activity, we based this on patient self‐report using the relevant questions in the EuroQoL Questionnaire (EQ‐5D); the Fried score used self‐report with a different questionnaire. Finally, for self‐reported exhaustion we used the questions in the Patient Health Questionnaire 9 (PHQ‐9)[18] analogous to those used from the Center for Epidemiological Studies depression scale in the original Fried description. We did this as we were evaluating the PHQ‐9 in our cohort already, and did not want to increase responder burden by presenting them with 2 depression questionnaires.
We followed all patients until 30 days after discharge, and outcome data (all‐cause mortality or all‐cause readmission) were collected by research personnel blinded to the patient's frailty status at discharge using patient/caregiver self‐report and analysis of the provincial electronic health record. We included deaths in or out of the hospital, and all readmissions were unplanned.
We examined the correlation between the CFS score (5 vs 5) and (1) the modified Fried score (3 vs 3) and (2) TUGT (20 seconds vs >20 seconds) using chance corrected kappa coefficients. In our previous article[14] we reported the association between the CFS and readmissions/hospitalizations within 30 days of discharge. In this article we examine whether either the Fried score or TUGT accurately and independently predict postdischarge readmissions/deaths, and whether they add additional prognostic information to the CFS assessment by comparing models with/without each definition using the C statistic and the Integrated Discrimination Improvement index. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), with P values of 0.05 considered statistically significant. Subgroup analysis was done in patients older than 65 years.
RESULTS
Of 1124 potentially eligible patients, 626 were excluded because of patient refusal (n = 227); transfer to/from another hospital, long‐term care facility, or out of province (n = 189); moderate to severe cognitive impairment (n = 88); language barriers (n = 71); or foreshortened life expectancy (n = 51). Another 3 patients withdrew consent prior to outcome assessment. The 495 patients we recruited and had outcome data for had a mean age of 64 years, 19.6% were older than 80 years, 50% were women, and the patients had a mean of 4.2 comorbidities and mean Charlson score of 2.4. The 4 most common reasons for hospital admission were heart failure, pneumonia, chronic obstructive pulmonary disease, and urinary tract infection, and the median length of stay was 5 days (interquartile range: 49 days).
Prevalence of Frailty According to Different Definitions
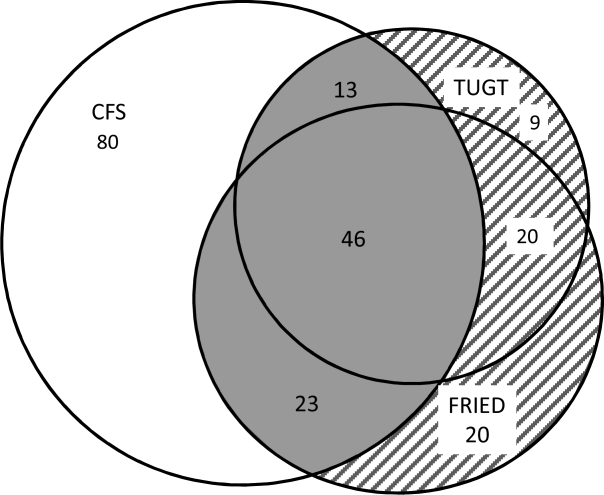
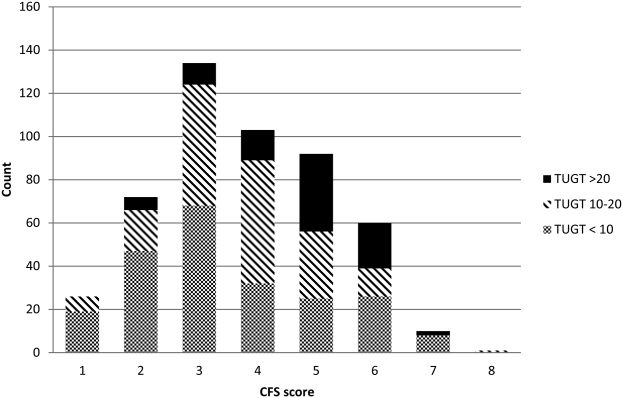
Although the CFS assessment resulted in 162 (33%) patients being deemed frail, only 82 (51%) of those patients also met the phenotype frailty definition using either the Fried model or the TUGT, and 49 (10%) patients who were not classified as frail on the CFS met either of the phenotypic definitions of frailty (Figure 1). Overall, 211 (43%) patients were frail according to at least 1 assessment, and 46 (9%) met all 3 frailty definitions. In the subgroup of 245 patients older than 65 years, 137 (56%) were frail according to at least 1 assessment, 38 (16%) met all 3 frailty definitions, and 27 (11%) of those patients classified as not frail on the CFS met either phenotypic definition of frailty. Agreement between TUGT and CFS or CFS and Fried was relatively poor with kappas of 0.31 (95% confidence interval [CI]: 0.23‐0.40) and 0.33 (95% CI: 0.25‐0.42), respectively. It is noteworthy that some patients deemed nonfrail on the CFS had slow gait speeds, and most CFS‐frail patients had gait speeds in the nonfrail range (Figure 2).

Characteristics According to Frailty Status
Although frail patients were generally similar across definitions (Table 1) in that they were older, had more comorbidities, more hospitalizations in the prior year, and longer index hospitalization lengths of stay than nonfrail patients, patients meeting phenotypic definitions of frailty but not classified as frail on the CFS were younger, had lower Charlson scores, higher EQ‐5D scores, and were discharged with less medications (Table 1).
| Not Frail on Any of the 3 Models, n = 284 | Frail on the CFS Only, n = 80 | Frail on the Fried and/or TUGT but Not the CFS, n = 49 | Frail on CFS and Either Phenotype Model, n = 82 | P Value Comparing the 3 Frailty Columns | |
|---|---|---|---|---|---|
| |||||
| Age, y, mean (95% CI) | 57.3 (55.259.5) | 69.1 (65.872.3) | 63.1 (57.968.3) | 75.8 (72.679.0) | 0.001 |
| Sex, female, no (%) | 118 (41.6) | 49 (61.3) | 27 (55.1) | 56 (68.3) | 0.3 |
| No. of comorbidities, mean (95% CI) | 4.2 (3.84.5) | 6.0 (5.56.6) | 4.0 (3.14.9) | 6.5 (5.87.2) | 0.001 |
| Charlson comorbidity score, mean (95% CI) | 2.4 (2.12.6) | 3.4 (3.03.9) | 2.6 (2.03.2) | 3.8 (3.34.2) | 0.01 |
| No. of patients hospitalized in prior 12 months, no (%) | 93 (32.8) | 44 (55.0) | 27 (55.1) | 54 (65.9) | 0.3 |
| Preadmission living situation, no (%) | 0.01 | ||||
| Living at home independently | 221 (77.8) | 26 (32.5) | 25 (51.0) | 17 (20.7) | |
| Living at home with help | 59 (20.8) | 43 (53.8) | 19 (38.8) | 48 (58.5) | |
| Assisted living or lodge | 4 (1.4) | 11 (13.8) | 5 (10.2) | 17 (20.7) | |
| EQ‐5D overall score, /100, mean (95% CI) | 66.9 (65.068.9) | 62.0 (57.666.4) | 56.6 (51.361.8) | 58.3 (53.962.7) | 0.28 |
| Goals of care in the hospital, no (%) | 0.0001 | ||||
| Resuscitation/ICU | 228 (83.5) | 41 (54.7) | 39 (84.8) | 29 (39.7) | |
| ICU but no resuscitation | 21(7.7) | 17 (22.7) | 1 (2.2) | 16 (21.9) | |
| No ICU, no resuscitation | 23 (8.4) | 17(22.7) | 6 (13.0) | 28 (37.8) | |
| Comfort care | 1 (0.4) | 0 | 0 | 0 | |
| Timed Up and Go Test, s, mean (95% CI) | 10.9 (10.411.3) | 13.9 (12.914.9) | 26.3 (19.033.6) | 30.3 (26.833.7) | 0.0001 |
| Grip strength, kg, mean (95% CI) | 32.1 (30.733.5) | 24.3 (22.3‐ 26.3) | 22.1 (19.924.2) | 17.7 (16.219.1) | 0.0001 |
| Serum albumin, g/L, mean (95% CI) | 34.2 (32.835.5) | 35.0 (33.037.0) | 31.1 (27.934.4) | 33.1 (31.434.9) | 0.07 |
| No. of prescription medications at discharge, mean (95% CI) | 5.2 (4.85.6) | 8.8 (7.99.6) | 6.1 (5.17.1) | 8.2 (7.58.9) | 0.0001 |
| Length of stay, d, median, [IQR] | 5 [37] | 6 [411] | 7 [3.512] | 7 [59] | 0.02 |
Outcomes According to Frailty Status
The overall rate of 30‐day death or hospital readmission was 17.1% (85 patients), primarily as a result of hospital readmissions (81, 16.4%) (Table 2). Although patients classified as frail on the CFS exhibited significantly higher 30‐day readmission/death rates (24.1% vs 13.8% for not frail, P = 0.005) even after adjusting for age and sex (adjusted odds ratio [aOR]: 2.02, 95% CI: 1.19‐3.41) (Table 3), patients meeting either of the phenotypic definitions for frailty but not the CFS definition were not at higher risk for 30‐day readmission/death (aOR: 0.87, 95% CI: 0.34‐2.19) (Table 3). The group at highest risk for 30‐day readmissions/death were those meeting both the CFS and either phenotypic definition of frailty (25.6% vs 13.8% for those not frail, aOR: 2.15, 95% CI: 1.10‐4.19) (Tables 2 and 3). None of the Integrated Discrimination Improvement indices (for modified Fried added to CFS or TUGT added to CFS) were statistically significant, suggesting no net new information was added to predictive models, and there were no appreciable changes in C statistics (Table 3). Neither the modified Fried score nor the TUGT on their own added independent prognostic information to age/sex alone as predictors of postdischarge outcomes. It is noteworthy that the areas under the curve for models using any combination of the frailty definitions plus age and sex were not high (all ranged between 0.55 and 0.60 for the overall cohort and from 0.52 and 0.65 in the elderly). If the frailty definitions were examined as continuous variables rather than dichotomized into frail/not frail, the C statistics were not appreciably better: 0.65 for CFS, 0.58 for TUGT, and 0.60 for modified Fried. Of note, the CFS score with the published cutoff of 5 demonstrated the highest kappa, sensitivity, specificity, and positive predictive value in relation to outcomes.
| Outcomes (Not Mutually Exclusive) | Not Frail on Any of the 3 Models | Frail on the CFS Only | Frail on the Fried and/or TUGT | Frail on CFS and Either Phenotype Model | P Value Comparing the 3 Frailty Columns |
|---|---|---|---|---|---|
| |||||
| Entire cohort | n = 284 | n = 80 | n = 49 | n = 82 | |
| Discharge disposition | 0.002 | ||||
| Live at home independently | 203 (71.5) | 16 (20.0) | 19 (38.8) | 10 (12.2) | |
| Live at home with help | 77 (27.1) | 52 (65.0) | 25 (51.0) | 50 (61.0) | |
| Assisted living or lodge | 4 (9.3) | 12 (15.0) | 5 (10.2) | 22 (26.8) | |
| 30‐day readmission or death | 40 (14.1) | 18 (22.5) | 6 (12.2) | 21 (25.6) | 0.2 |
| 30‐day hospital readmission | 39 (13.8) | 18 (22.5) | 6 (12.2) | 18 (22.0) | 0.31 |
| Death | 5 (1.8) | 3 (3.8) | 1 (2.0) | 4 (4.9) | 0.9 |
| 30‐day ER visit | 66 (23.2) | 30 (37.5) | 12 (24.5) | 23 (17.6) | 0.25 |
| Patients aged 65 years or older | n = 108 | n = 47 | n = 27 | n = 63 | |
| Discharge disposition | 0.03 | ||||
| Live at home independently | 69 (63.9) | 9 (19.2) | 10 (37.0) | 6 (9.5) | |
| Live at home with help | 36 (33.3) | 30 (63.8) | 13 (48.2) | 39(61.9) | |
| Assisted living or lodge | 3 (3.8) | 8 (17.0) | 4 (14.8) | 18 (28.6) | |
| 30‐day readmission or death | 13 (12.0) | 13 (27.7) | 3 (11.1) | 17 (27.0) | 0.22 |
| 30‐day hospital readmission | 12 (11.1) | 13 (27.7) | 3 (11.1) | 14 (22.2) | 0.26 |
| Death | 2 (1.9) | 3 (6.4) | 1 (3.7) | 3 (4.8) | 0.87 |
| 30‐day ER visit | 20 (18.5) | 17 (36.2) | 6 (22.2) | 18 (28.6) | 0.45 |
| Frailty Definition | Adjusted Odds Ratio for 30‐Day Readmission/Death | 95% CI | C Statistic for Model Predicting 30‐Day Readmission/Death Including Age, Sex, and Frailty Definition (95% CI) |
|---|---|---|---|
| |||
| Entire cohort | |||
| CFS (overall) | 2.02 | 1.193.41 | 0.60 (0.530.65) |
| CFS (plus either phenotype model) | 2.15 | 1.104.19 | 0.60 (0.520.64) |
| CFS (but neither phenotype model) | 1.81 | 0.943.48 | 0.60 (0.520.64) |
| Fried | 1.32 | 0.752.30 | 0.55 (0.560.58) |
| TUGT | 1.34 | 0.732.44 | 0.55 (0.460.58) |
| Fried and/or TUGT | 0.87 | 0.342.19 | 0.55 (0.470.58) |
| Patients aged 65 years or older | |||
| CFS (overall) | 3.20 | 1.556.60 | 0.65 (0.560.73) |
| CFS (plus either phenotype model) | 3.20 | 1.337.68 | 0.65 (0.550.72) |
| CFS (but neither phenotype model) | 3.08 | 1.267.47 | 0.65 (0.550.72) |
| Fried | 1.28 | 0.642.56 | 0.52 (0.390.53) |
| TUGT | 1.44 | 0.702.97 | 0.52 (0.390.53) |
| Fried and/or TUGT | 1.41 | 0.722.78 | 0.54 (0.420.56) |
Outcomes According to Frailty Status in the Elderly Subgroup
Although absolute risks of readmission or death were higher in elderly patients than younger patients, the excess risk was largely seen in those elderly patients classified as frail on the CFS. In fact, all of the associations reported above for the entire cohort were in the same direction in the elderly subgroup (Tables 2 and 3).
DISCUSSION
In summary, we found that of patients being discharged from general medical wards who were frail according to at least 1 of the 3 tools we used, only 22% met all 3 frailty case definitions (including only 28% of elderly patients deemed frail by at least 1 definition). There was surprisingly poor correlation between phenotypic markers of frailty such as poor mobility (slow TUGT) or the modified Fried Index and the CFS, even amongt elderly patients. The most clinically useful of the frailty assessment tools (both overall and in those patients who are elderly) appears to be the CFS, because it more accurately identifies those at higher risk of adverse outcomes after discharge, does not require special equipment to conduct, and is faster to do than the phenotypic assessment models we tested. We have also previously demonstrated that the CFS, after a brief training period identical to that used in this study, is reproducible between observers[19] and remains an independent predictor of adverse 30‐day outcomes even after adjusting for age, sex, comorbidities, and the LACE (length of stay, acuity of the admission, comorbidity, emergency room visits during the previous 6 months) score.[14]
Although some[10] have advocated for the use of mobility assessments (such as gait speed) as a frailty marker due to its ease of measurement and objectivity, we found that slow TUGT (which is a marker for mobility and not just slow gait speed) was not an independent prognostic marker for postdischarge outcomes. We hypothesize that the phenotypic models of frailty performed less well than the CFS as they focus on the measurement of particular physical attributes and do not take into account cognitive or psychosocial characteristics or comorbidity burden that also influence postdischarge outcomes. As well, the CFS captures the patients' baseline status prior to acute illness, whereas the phenotypic measures were assessed just prior to discharge and thus may provide less information about eventual recovery potential. Some have suggested that repeating phenotype measures postdischarge might be more informative,[20] but this would reduce clinical applicability a great deal. Certainly, an analysis[21] of the Cardiovascular Health Study cohort demonstrated that cumulative deficit models of frailty (for which the CFS is an accurate proxy[9, 15]) better predicted risk of death than phenotypic models.
Although a number of published studies have shown similar results to ours in that frail patients are at greater risk for death and/or hospitalization,[22, 23, 24] there is surprisingly little literature on the comparative predictive performance of the different frailty instruments and the extent to which they overlap. Cigolle et al.[25] compared 3 frailty scales (the Functional Domain Model, the Burden Model, and the Fried score) in the Health and Retirement Study and, similarly to us, found that although 30.2% were frail on at least 1 of these scales, only 3.1% were deemed frail by all 3. The Conselice Study of Brain Aging[5] also reported that a deficit accumulation model defined a much higher prevalence of frailty (37.6%) than the 11.6% identified using the phenotypic Study of Osteoporotic Fractures (SOF) index based on weight loss, mobility, and level of energy. Another study[26] reported that risk models incorporating either the SOF index or the Fried score exhibited C statistics of only 0.61 for predicting falls in elderly females. A cohort study[27] from 2 English general medical units also found that none of the 5 frailty models was particularly accurate at predicting risk of readmission at 3 months, with C statistics ranging between 0.52 and 0.57. Although frailty assessment at time of hospital admission predicted in‐hospital mortality and length of stay in another English study, it was not independently associated with 30‐day outcomes after adjusting for age, sex, and comorbidities including dementia.[27] To our knowledge, these latter 2 are the only other studies reported to date performed in hospitalized patients to assess whether frailty assessment helps predict postdischarge outcomes. Thus, the poor C statistics we found for all of our frailty tools confirms prior literature that frailty assessment alone is inadequate to accurately identify those patients at highest risk for poor outcomes in the first 30 days after discharge. However, frailty assessment together with consideration of each individual's comorbidities, cognitive status, psychosocial circumstances, and environment can be useful to flag those individuals who may need extra attention postdischarge to optimize outcomes.
Strengths and Limitations
Although this was a prospective cohort study with blinded ascertainment of endpoints (30‐day outcome data were collected by observers who were unaware of the patients' CFS or phenotypic model scores), it is not without limitations. First, the only postdischarge outcomes we assessed were readmission and death, and it would be interesting to evaluate which frailty tools best predict those who are most likely to benefit from home‐care services in the community. Second, as we were interested in 30‐day readmission rates, we excluded long‐term care residents from our study and patients who had foreshortened life expectancy, in essence, the frailest of the frail. Although this reduced the size of any association between frailty and adverse outcomes, we focused this study on the situations where there is clinical equipoise and there is rarely a diagnostic dilemma around the identification of frailty and need for increased services in palliative or long‐term care patients. Third, we did not use exactly the same questionnaires or gait speed assessments as used in the original Fried score description, but as outlined in the Methods section, we used analogous questions on closely related questionnaires to extract the same information. Fourth, some might consider our comparisons biased toward the CFS, as it reflects gestalt clinical impressions (informed by patients and proxies) of frailty status before hospital admission while the Fried score and TUGT were based on patient status just prior to discharge, it may be that the former is a better measure of eventual recovery (and ongoing risk) than the latter measures. If this is the case, for the purposes of targeting interventions to prevent postdischarge complications, it would suggest to us that the CFS is better suited, whereas phenotype tools can be reserved for the postdischarge phase of recovery. By the same token, perhaps serial measures of the CFS and phenotypic tools are more important, as the trajectory of recovery may be most informative for risk prediction.[7] Certainly, if one were interested in changes in functional status during hospitalization,[29] then objective phenotypic measures such as grip strength or TUGT times would seem more appropriate choices. Fifth, some may perceive it as a weakness that we did not restrict our cohort to elderly patients; however, we actually view this as a strength, because frailty is not exclusive to older patients. Sixth, although we restricted this study to patients being discharged from general internal medicine wards, it is worth mentioning that previous studies have shown similar associations between frailty and outcomes in nonmedical hospitalized patients.[19, 22, 23, 24]
In conclusion, we looked at 3 different ways of screening for frailty, 1 being a subjective but well‐validated tool (the CFS) and the other 2 being objective assessments that look at specific phenotypic characteristics. There is a compelling need to find a standardized assessment to determine frailty in both research and clinical settings, and our study provides support for use of the CFS over the Fried or TUGT as screening tools. Standardized frailty assessments should be part of the discharge planning for all medical patients so that extra resources can be properly targeted at those patients at greatest risk for suboptimal transition back to community living.
Acknowledgements
The authors acknowledge Miriam Fradette and Debbie Boyko for their important contributions in data acquisition, as well as all the physicians rotating through the general internal medicine wards for their help in identifying the patients.
Disclosures: Author contributions are as follows: study concept and design: Finlay A. McAlister, Sumit R. Majumdar, and Raj Padwal; acquisition of patients and data: Sara Belga, Darren Lau, Jenelle Pederson, and Sharry Kahlon; analysis of data: Jeff Bakal, Sara Belga, Finlay A. McAlister; first draft of manuscript: Sara Belga and Finlay A. McAlister; critical revision of manuscript: all authors. Funding for this study was provided by an operating grant from Alberta InnovatesHealth Solutions. Alberta InnovatesHealth Solutions had no role in role in the design, methods, subject recruitment, data collections, analysis, or preparation of the article. Finlay A. McAlister and Sumit R. Majumdar hold career salary support from Alberta InnovatesHealth Solutions. Finlay A. McAlister holds the Chair in Cardiovascular Outcomes Research at the Mazankowski Heart Institute, University of Alberta. Sumit R. Majumdar holds the Endowed Chair in Patient Health Management from the Faculty of Medicine and Dentistry, and the Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta. The authors have no affiliations or financial interests with any organization or entity with a financial interest in the contents of this article. All authors had access to the data and played a role in writing and revising this article. The authors declare no conflicts of interest.
- , , , , . Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263.
- , , , , . The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–2138.
- , , , , . Frailty in elderly people. Lancet. 2013;381:752–762.
- , , , , , . Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–114.
- , , , , , . A comparison of frailty indexes for prediction of adverse health outcomes in a elderly cohort. Arch Gerontol Geriatr. 2012;54:16–20.
- , , , et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156.
- , , , . Prevalence of frailty in community‐dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492.
- , , . Sex differences in the risk of frailty for mortality independent of disability of chronic diseases. J Am Geriatr Soc. 2005;53:40–47.
- , , . A comparison of two approaches to measuring frailty in elderly people. J Gerontol. 2007;62:738–743.
- , , . A diagnosis of dismobility—giving mobility clinical visibility: a mobility working group recommendation. JAMA. 2014;311:2061–2062.
- , , , et al. Gait speed and survival in older adults. JAMA. 2011;301:50–58.
- , , , et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762.
- , . The timed “Up and Go” test: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148.
- , , , et al. Association between frailty and 30‐day outcomes after discharge from hospital. CMAJ. 2015;187:799–804.
- , , , et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495.
- , , , et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–1419.
- , . Grip strength in older adults: test‐retest reliability and cutoff for subjective weakness of using the hands in heavy tasks. Arch Phys Med Rehabil. 2010;91:1747–1751.
- , . The PHQ‐9: a new depression measure. Psychiatr Ann. 2002;32:509–515.
- , , , et al. Association between frailty and short‐ and long‐term outcomes among critically ill patients: a multicenter prospective cohort study. CMAJ. 2013;186:e95–e102.
- , . Risk after hospitalization: we have a lot to learn. J Hosp Med. 2015;10:135–136.
- , , , , , . Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903.
- , , . Unplanned hospital readmission and its predictors in patients with chronic conditions. J Formos Med Assoc. 2002;101:779–785.
- , , , et al. Frailty and early hospital readmission after kidney transplant. Am J Transplant. 2013;13:2091–2095.
- , , , , , . Simple frailty score predicts postoperative complications across surgical specialities. Am J Surg. 2013;206:544–550.
- , , , . Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57:830–839.
- , , , et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Int Med. 2008;168:382–389.
- , , , , , . The predictive properties of frailty‐rating scales in the acute medical unit. Age Ageing. 2013;42:776–781.
- , , , . Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108:943–949.
- , , . Hospitalization‐associated disability: she was probably able to ambulate, but I'm not sure. JAMA. 2011;306:1782–1793.
Frailty is a state of vulnerability that encompasses a heterogeneous group of people.[1] Because it lacks a precise definition, multiple tools have been developed to identify frailty in both clinical and research settings.[2, 3, 4] Prevalence of frailty depends on the frailty assessment tool used and the population studied, ranging from 4% to 17% when the Fried score[5, 6, 7] is used and from 5% to 44%[5, 7, 8] when cumulative deficit models like the Frailty Index are utilized, with the lower prevalences being in younger community‐dwelling elderly populations and the higher proportions being in older institutionalized populations.
The Frailty Index, also called the Burden or Cumulative Deficit Model, comprises 70 domains that include mobility, mood, function, cognitive impairment, and disease states. It is multidimensional and allows for patients to be categorized on a continuum of frailty, but it is extremely difficult to apply in clinical practice. Recognizing this, Rockwood et al.[9] developed and validated the Clinical Frailty Scale (CFS) in the Canadian Study of Health and Aging. The CFS classifies patients into 1 of 9 categories: very fit, well, managing well, vulnerable, mildly frail (needs help with at least 1 instrumental activity of daily living such as shopping, finances, meal preparation, or housework), moderately frail (needs help with 1 or 2 activities of daily living such as bathing and dressing), severely frail (dependent for personal care), very severely frail (bedbound), and terminally ill. Although this tool is easy to use in clinical practice, it reflects a gestalt impression and requires some clinical judgement.
The Fried score[6] is a prototypical phenotype tool based on 5 criteria that include weight loss, self‐reported exhaustion, low energy expenditure, slowness of gait, and weakness. Recent evidence has suggested that slow gait (or dysmobility) alone may also be a potential screening test for frailty.[10] A recent systematic review[11] demonstrated an association between slow gait (dysmobility) and increased mortality. Dysmobility negatively impacts quality of life and has a strong association with disability resulting in the need for an increased level of care.[12] The Timed Up and Go Test (TUGT) is one method of assessing mobility which is relatively easy to perform, does not require special equipment, and is feasible to use in clinical settings.[13] However, whether impaired mobility predicts outcomes within the first 30 days after hospital discharge (a timeframe highlighted in the Affordable Care Act and used by the Centers for Medicare and Medicaid Services as an important hospital quality indicator) is still uncertain.
The aim of this study was to compare frailty assessments using the CFS and 2 of the most commonly used phenotypic tools (a modified Fried score and the TUGT as a proxy for mobility assessment) to determine which tools best predict postdischarge outcomes.
METHODS
Study Design and Population
As described in detail elsewhere,[14] this was a prospective cohort study that enrolled adult patients (any age older than 18 years) at the time of discharge back to the community from 7 general internal medicine wards in 2 teaching hospitals in Edmonton, Alberta between October 2013 and November 2014. We excluded patients admitted from, or being discharged back to, long‐term care facilities or other acute care hospitals, or from out of the province; patients who were unable to communicate in English; patients with moderate or severe cognitive impairment (scoring 5 or more on the Short Portable Mental Status Questionnaire); or patients with projected life expectancy of less than 3 months. All patients provided written consent, and the study was approved by the Health Research Ethics board of the University of Alberta (project ID Pro00036880).
We assessed the degree of frailty within 24 hours of discharge in 3 ways. First, we used the CFS[9, 15] with patients being asked to rate their best functional status in the week prior to admission. As per the CFS validations studies, scores 5 were defined as frail.[9, 15] Second, we used the TUGT as a proxy for slow gait speed/dysmobility (with >20 seconds defined as abnormal).[13] The TUGT was recorded as the shortest recorded time of the 2 timed trials to get up from a seated position, walk 10 feet and back, and then sit in the chair again. Third, we also determined their Fried score[6] (using the modifications outlined below) and categorized the patients as frail if they scored 3 or more. Of the 5 Fried categories, we assessed weakness by grip strength in their dominant hand using a Jamar handheld dynamometer and weight loss of 10 lb or more in the past year based on patient self‐report; these are identical to the original Fried scale description. Grip strength in the lowest quintile for sex and body mass index was defined as weak grip strength as per convention in the literature, which corresponded to less than 28.5 kg for men and less than 18.5 kg for women.[16, 17] We assessed the other 3 Fried categories in modified fashion as follows. For slow gait, rather than assessing time to walk 15 feet as in the original study and assigning a point to those testing in the lowest quintile for their age/sex, we used the TUGT, because our research personnel were already trained in this test, and we were doing it already as part of the discharge package for all patients.[13] For the Fried category of low activity, we based this on patient self‐report using the relevant questions in the EuroQoL Questionnaire (EQ‐5D); the Fried score used self‐report with a different questionnaire. Finally, for self‐reported exhaustion we used the questions in the Patient Health Questionnaire 9 (PHQ‐9)[18] analogous to those used from the Center for Epidemiological Studies depression scale in the original Fried description. We did this as we were evaluating the PHQ‐9 in our cohort already, and did not want to increase responder burden by presenting them with 2 depression questionnaires.
We followed all patients until 30 days after discharge, and outcome data (all‐cause mortality or all‐cause readmission) were collected by research personnel blinded to the patient's frailty status at discharge using patient/caregiver self‐report and analysis of the provincial electronic health record. We included deaths in or out of the hospital, and all readmissions were unplanned.
We examined the correlation between the CFS score (5 vs 5) and (1) the modified Fried score (3 vs 3) and (2) TUGT (20 seconds vs >20 seconds) using chance corrected kappa coefficients. In our previous article[14] we reported the association between the CFS and readmissions/hospitalizations within 30 days of discharge. In this article we examine whether either the Fried score or TUGT accurately and independently predict postdischarge readmissions/deaths, and whether they add additional prognostic information to the CFS assessment by comparing models with/without each definition using the C statistic and the Integrated Discrimination Improvement index. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), with P values of 0.05 considered statistically significant. Subgroup analysis was done in patients older than 65 years.
RESULTS
Of 1124 potentially eligible patients, 626 were excluded because of patient refusal (n = 227); transfer to/from another hospital, long‐term care facility, or out of province (n = 189); moderate to severe cognitive impairment (n = 88); language barriers (n = 71); or foreshortened life expectancy (n = 51). Another 3 patients withdrew consent prior to outcome assessment. The 495 patients we recruited and had outcome data for had a mean age of 64 years, 19.6% were older than 80 years, 50% were women, and the patients had a mean of 4.2 comorbidities and mean Charlson score of 2.4. The 4 most common reasons for hospital admission were heart failure, pneumonia, chronic obstructive pulmonary disease, and urinary tract infection, and the median length of stay was 5 days (interquartile range: 49 days).
Prevalence of Frailty According to Different Definitions
Although the CFS assessment resulted in 162 (33%) patients being deemed frail, only 82 (51%) of those patients also met the phenotype frailty definition using either the Fried model or the TUGT, and 49 (10%) patients who were not classified as frail on the CFS met either of the phenotypic definitions of frailty (Figure 1). Overall, 211 (43%) patients were frail according to at least 1 assessment, and 46 (9%) met all 3 frailty definitions. In the subgroup of 245 patients older than 65 years, 137 (56%) were frail according to at least 1 assessment, 38 (16%) met all 3 frailty definitions, and 27 (11%) of those patients classified as not frail on the CFS met either phenotypic definition of frailty. Agreement between TUGT and CFS or CFS and Fried was relatively poor with kappas of 0.31 (95% confidence interval [CI]: 0.23‐0.40) and 0.33 (95% CI: 0.25‐0.42), respectively. It is noteworthy that some patients deemed nonfrail on the CFS had slow gait speeds, and most CFS‐frail patients had gait speeds in the nonfrail range (Figure 2).


Characteristics According to Frailty Status
Although frail patients were generally similar across definitions (Table 1) in that they were older, had more comorbidities, more hospitalizations in the prior year, and longer index hospitalization lengths of stay than nonfrail patients, patients meeting phenotypic definitions of frailty but not classified as frail on the CFS were younger, had lower Charlson scores, higher EQ‐5D scores, and were discharged with less medications (Table 1).
| Not Frail on Any of the 3 Models, n = 284 | Frail on the CFS Only, n = 80 | Frail on the Fried and/or TUGT but Not the CFS, n = 49 | Frail on CFS and Either Phenotype Model, n = 82 | P Value Comparing the 3 Frailty Columns | |
|---|---|---|---|---|---|
| |||||
| Age, y, mean (95% CI) | 57.3 (55.259.5) | 69.1 (65.872.3) | 63.1 (57.968.3) | 75.8 (72.679.0) | 0.001 |
| Sex, female, no (%) | 118 (41.6) | 49 (61.3) | 27 (55.1) | 56 (68.3) | 0.3 |
| No. of comorbidities, mean (95% CI) | 4.2 (3.84.5) | 6.0 (5.56.6) | 4.0 (3.14.9) | 6.5 (5.87.2) | 0.001 |
| Charlson comorbidity score, mean (95% CI) | 2.4 (2.12.6) | 3.4 (3.03.9) | 2.6 (2.03.2) | 3.8 (3.34.2) | 0.01 |
| No. of patients hospitalized in prior 12 months, no (%) | 93 (32.8) | 44 (55.0) | 27 (55.1) | 54 (65.9) | 0.3 |
| Preadmission living situation, no (%) | 0.01 | ||||
| Living at home independently | 221 (77.8) | 26 (32.5) | 25 (51.0) | 17 (20.7) | |
| Living at home with help | 59 (20.8) | 43 (53.8) | 19 (38.8) | 48 (58.5) | |
| Assisted living or lodge | 4 (1.4) | 11 (13.8) | 5 (10.2) | 17 (20.7) | |
| EQ‐5D overall score, /100, mean (95% CI) | 66.9 (65.068.9) | 62.0 (57.666.4) | 56.6 (51.361.8) | 58.3 (53.962.7) | 0.28 |
| Goals of care in the hospital, no (%) | 0.0001 | ||||
| Resuscitation/ICU | 228 (83.5) | 41 (54.7) | 39 (84.8) | 29 (39.7) | |
| ICU but no resuscitation | 21(7.7) | 17 (22.7) | 1 (2.2) | 16 (21.9) | |
| No ICU, no resuscitation | 23 (8.4) | 17(22.7) | 6 (13.0) | 28 (37.8) | |
| Comfort care | 1 (0.4) | 0 | 0 | 0 | |
| Timed Up and Go Test, s, mean (95% CI) | 10.9 (10.411.3) | 13.9 (12.914.9) | 26.3 (19.033.6) | 30.3 (26.833.7) | 0.0001 |
| Grip strength, kg, mean (95% CI) | 32.1 (30.733.5) | 24.3 (22.3‐ 26.3) | 22.1 (19.924.2) | 17.7 (16.219.1) | 0.0001 |
| Serum albumin, g/L, mean (95% CI) | 34.2 (32.835.5) | 35.0 (33.037.0) | 31.1 (27.934.4) | 33.1 (31.434.9) | 0.07 |
| No. of prescription medications at discharge, mean (95% CI) | 5.2 (4.85.6) | 8.8 (7.99.6) | 6.1 (5.17.1) | 8.2 (7.58.9) | 0.0001 |
| Length of stay, d, median, [IQR] | 5 [37] | 6 [411] | 7 [3.512] | 7 [59] | 0.02 |
Outcomes According to Frailty Status
The overall rate of 30‐day death or hospital readmission was 17.1% (85 patients), primarily as a result of hospital readmissions (81, 16.4%) (Table 2). Although patients classified as frail on the CFS exhibited significantly higher 30‐day readmission/death rates (24.1% vs 13.8% for not frail, P = 0.005) even after adjusting for age and sex (adjusted odds ratio [aOR]: 2.02, 95% CI: 1.19‐3.41) (Table 3), patients meeting either of the phenotypic definitions for frailty but not the CFS definition were not at higher risk for 30‐day readmission/death (aOR: 0.87, 95% CI: 0.34‐2.19) (Table 3). The group at highest risk for 30‐day readmissions/death were those meeting both the CFS and either phenotypic definition of frailty (25.6% vs 13.8% for those not frail, aOR: 2.15, 95% CI: 1.10‐4.19) (Tables 2 and 3). None of the Integrated Discrimination Improvement indices (for modified Fried added to CFS or TUGT added to CFS) were statistically significant, suggesting no net new information was added to predictive models, and there were no appreciable changes in C statistics (Table 3). Neither the modified Fried score nor the TUGT on their own added independent prognostic information to age/sex alone as predictors of postdischarge outcomes. It is noteworthy that the areas under the curve for models using any combination of the frailty definitions plus age and sex were not high (all ranged between 0.55 and 0.60 for the overall cohort and from 0.52 and 0.65 in the elderly). If the frailty definitions were examined as continuous variables rather than dichotomized into frail/not frail, the C statistics were not appreciably better: 0.65 for CFS, 0.58 for TUGT, and 0.60 for modified Fried. Of note, the CFS score with the published cutoff of 5 demonstrated the highest kappa, sensitivity, specificity, and positive predictive value in relation to outcomes.
| Outcomes (Not Mutually Exclusive) | Not Frail on Any of the 3 Models | Frail on the CFS Only | Frail on the Fried and/or TUGT | Frail on CFS and Either Phenotype Model | P Value Comparing the 3 Frailty Columns |
|---|---|---|---|---|---|
| |||||
| Entire cohort | n = 284 | n = 80 | n = 49 | n = 82 | |
| Discharge disposition | 0.002 | ||||
| Live at home independently | 203 (71.5) | 16 (20.0) | 19 (38.8) | 10 (12.2) | |
| Live at home with help | 77 (27.1) | 52 (65.0) | 25 (51.0) | 50 (61.0) | |
| Assisted living or lodge | 4 (9.3) | 12 (15.0) | 5 (10.2) | 22 (26.8) | |
| 30‐day readmission or death | 40 (14.1) | 18 (22.5) | 6 (12.2) | 21 (25.6) | 0.2 |
| 30‐day hospital readmission | 39 (13.8) | 18 (22.5) | 6 (12.2) | 18 (22.0) | 0.31 |
| Death | 5 (1.8) | 3 (3.8) | 1 (2.0) | 4 (4.9) | 0.9 |
| 30‐day ER visit | 66 (23.2) | 30 (37.5) | 12 (24.5) | 23 (17.6) | 0.25 |
| Patients aged 65 years or older | n = 108 | n = 47 | n = 27 | n = 63 | |
| Discharge disposition | 0.03 | ||||
| Live at home independently | 69 (63.9) | 9 (19.2) | 10 (37.0) | 6 (9.5) | |
| Live at home with help | 36 (33.3) | 30 (63.8) | 13 (48.2) | 39(61.9) | |
| Assisted living or lodge | 3 (3.8) | 8 (17.0) | 4 (14.8) | 18 (28.6) | |
| 30‐day readmission or death | 13 (12.0) | 13 (27.7) | 3 (11.1) | 17 (27.0) | 0.22 |
| 30‐day hospital readmission | 12 (11.1) | 13 (27.7) | 3 (11.1) | 14 (22.2) | 0.26 |
| Death | 2 (1.9) | 3 (6.4) | 1 (3.7) | 3 (4.8) | 0.87 |
| 30‐day ER visit | 20 (18.5) | 17 (36.2) | 6 (22.2) | 18 (28.6) | 0.45 |
| Frailty Definition | Adjusted Odds Ratio for 30‐Day Readmission/Death | 95% CI | C Statistic for Model Predicting 30‐Day Readmission/Death Including Age, Sex, and Frailty Definition (95% CI) |
|---|---|---|---|
| |||
| Entire cohort | |||
| CFS (overall) | 2.02 | 1.193.41 | 0.60 (0.530.65) |
| CFS (plus either phenotype model) | 2.15 | 1.104.19 | 0.60 (0.520.64) |
| CFS (but neither phenotype model) | 1.81 | 0.943.48 | 0.60 (0.520.64) |
| Fried | 1.32 | 0.752.30 | 0.55 (0.560.58) |
| TUGT | 1.34 | 0.732.44 | 0.55 (0.460.58) |
| Fried and/or TUGT | 0.87 | 0.342.19 | 0.55 (0.470.58) |
| Patients aged 65 years or older | |||
| CFS (overall) | 3.20 | 1.556.60 | 0.65 (0.560.73) |
| CFS (plus either phenotype model) | 3.20 | 1.337.68 | 0.65 (0.550.72) |
| CFS (but neither phenotype model) | 3.08 | 1.267.47 | 0.65 (0.550.72) |
| Fried | 1.28 | 0.642.56 | 0.52 (0.390.53) |
| TUGT | 1.44 | 0.702.97 | 0.52 (0.390.53) |
| Fried and/or TUGT | 1.41 | 0.722.78 | 0.54 (0.420.56) |
Outcomes According to Frailty Status in the Elderly Subgroup
Although absolute risks of readmission or death were higher in elderly patients than younger patients, the excess risk was largely seen in those elderly patients classified as frail on the CFS. In fact, all of the associations reported above for the entire cohort were in the same direction in the elderly subgroup (Tables 2 and 3).
DISCUSSION
In summary, we found that of patients being discharged from general medical wards who were frail according to at least 1 of the 3 tools we used, only 22% met all 3 frailty case definitions (including only 28% of elderly patients deemed frail by at least 1 definition). There was surprisingly poor correlation between phenotypic markers of frailty such as poor mobility (slow TUGT) or the modified Fried Index and the CFS, even amongt elderly patients. The most clinically useful of the frailty assessment tools (both overall and in those patients who are elderly) appears to be the CFS, because it more accurately identifies those at higher risk of adverse outcomes after discharge, does not require special equipment to conduct, and is faster to do than the phenotypic assessment models we tested. We have also previously demonstrated that the CFS, after a brief training period identical to that used in this study, is reproducible between observers[19] and remains an independent predictor of adverse 30‐day outcomes even after adjusting for age, sex, comorbidities, and the LACE (length of stay, acuity of the admission, comorbidity, emergency room visits during the previous 6 months) score.[14]
Although some[10] have advocated for the use of mobility assessments (such as gait speed) as a frailty marker due to its ease of measurement and objectivity, we found that slow TUGT (which is a marker for mobility and not just slow gait speed) was not an independent prognostic marker for postdischarge outcomes. We hypothesize that the phenotypic models of frailty performed less well than the CFS as they focus on the measurement of particular physical attributes and do not take into account cognitive or psychosocial characteristics or comorbidity burden that also influence postdischarge outcomes. As well, the CFS captures the patients' baseline status prior to acute illness, whereas the phenotypic measures were assessed just prior to discharge and thus may provide less information about eventual recovery potential. Some have suggested that repeating phenotype measures postdischarge might be more informative,[20] but this would reduce clinical applicability a great deal. Certainly, an analysis[21] of the Cardiovascular Health Study cohort demonstrated that cumulative deficit models of frailty (for which the CFS is an accurate proxy[9, 15]) better predicted risk of death than phenotypic models.
Although a number of published studies have shown similar results to ours in that frail patients are at greater risk for death and/or hospitalization,[22, 23, 24] there is surprisingly little literature on the comparative predictive performance of the different frailty instruments and the extent to which they overlap. Cigolle et al.[25] compared 3 frailty scales (the Functional Domain Model, the Burden Model, and the Fried score) in the Health and Retirement Study and, similarly to us, found that although 30.2% were frail on at least 1 of these scales, only 3.1% were deemed frail by all 3. The Conselice Study of Brain Aging[5] also reported that a deficit accumulation model defined a much higher prevalence of frailty (37.6%) than the 11.6% identified using the phenotypic Study of Osteoporotic Fractures (SOF) index based on weight loss, mobility, and level of energy. Another study[26] reported that risk models incorporating either the SOF index or the Fried score exhibited C statistics of only 0.61 for predicting falls in elderly females. A cohort study[27] from 2 English general medical units also found that none of the 5 frailty models was particularly accurate at predicting risk of readmission at 3 months, with C statistics ranging between 0.52 and 0.57. Although frailty assessment at time of hospital admission predicted in‐hospital mortality and length of stay in another English study, it was not independently associated with 30‐day outcomes after adjusting for age, sex, and comorbidities including dementia.[27] To our knowledge, these latter 2 are the only other studies reported to date performed in hospitalized patients to assess whether frailty assessment helps predict postdischarge outcomes. Thus, the poor C statistics we found for all of our frailty tools confirms prior literature that frailty assessment alone is inadequate to accurately identify those patients at highest risk for poor outcomes in the first 30 days after discharge. However, frailty assessment together with consideration of each individual's comorbidities, cognitive status, psychosocial circumstances, and environment can be useful to flag those individuals who may need extra attention postdischarge to optimize outcomes.
Strengths and Limitations
Although this was a prospective cohort study with blinded ascertainment of endpoints (30‐day outcome data were collected by observers who were unaware of the patients' CFS or phenotypic model scores), it is not without limitations. First, the only postdischarge outcomes we assessed were readmission and death, and it would be interesting to evaluate which frailty tools best predict those who are most likely to benefit from home‐care services in the community. Second, as we were interested in 30‐day readmission rates, we excluded long‐term care residents from our study and patients who had foreshortened life expectancy, in essence, the frailest of the frail. Although this reduced the size of any association between frailty and adverse outcomes, we focused this study on the situations where there is clinical equipoise and there is rarely a diagnostic dilemma around the identification of frailty and need for increased services in palliative or long‐term care patients. Third, we did not use exactly the same questionnaires or gait speed assessments as used in the original Fried score description, but as outlined in the Methods section, we used analogous questions on closely related questionnaires to extract the same information. Fourth, some might consider our comparisons biased toward the CFS, as it reflects gestalt clinical impressions (informed by patients and proxies) of frailty status before hospital admission while the Fried score and TUGT were based on patient status just prior to discharge, it may be that the former is a better measure of eventual recovery (and ongoing risk) than the latter measures. If this is the case, for the purposes of targeting interventions to prevent postdischarge complications, it would suggest to us that the CFS is better suited, whereas phenotype tools can be reserved for the postdischarge phase of recovery. By the same token, perhaps serial measures of the CFS and phenotypic tools are more important, as the trajectory of recovery may be most informative for risk prediction.[7] Certainly, if one were interested in changes in functional status during hospitalization,[29] then objective phenotypic measures such as grip strength or TUGT times would seem more appropriate choices. Fifth, some may perceive it as a weakness that we did not restrict our cohort to elderly patients; however, we actually view this as a strength, because frailty is not exclusive to older patients. Sixth, although we restricted this study to patients being discharged from general internal medicine wards, it is worth mentioning that previous studies have shown similar associations between frailty and outcomes in nonmedical hospitalized patients.[19, 22, 23, 24]
In conclusion, we looked at 3 different ways of screening for frailty, 1 being a subjective but well‐validated tool (the CFS) and the other 2 being objective assessments that look at specific phenotypic characteristics. There is a compelling need to find a standardized assessment to determine frailty in both research and clinical settings, and our study provides support for use of the CFS over the Fried or TUGT as screening tools. Standardized frailty assessments should be part of the discharge planning for all medical patients so that extra resources can be properly targeted at those patients at greatest risk for suboptimal transition back to community living.
Acknowledgements
The authors acknowledge Miriam Fradette and Debbie Boyko for their important contributions in data acquisition, as well as all the physicians rotating through the general internal medicine wards for their help in identifying the patients.
Disclosures: Author contributions are as follows: study concept and design: Finlay A. McAlister, Sumit R. Majumdar, and Raj Padwal; acquisition of patients and data: Sara Belga, Darren Lau, Jenelle Pederson, and Sharry Kahlon; analysis of data: Jeff Bakal, Sara Belga, Finlay A. McAlister; first draft of manuscript: Sara Belga and Finlay A. McAlister; critical revision of manuscript: all authors. Funding for this study was provided by an operating grant from Alberta InnovatesHealth Solutions. Alberta InnovatesHealth Solutions had no role in role in the design, methods, subject recruitment, data collections, analysis, or preparation of the article. Finlay A. McAlister and Sumit R. Majumdar hold career salary support from Alberta InnovatesHealth Solutions. Finlay A. McAlister holds the Chair in Cardiovascular Outcomes Research at the Mazankowski Heart Institute, University of Alberta. Sumit R. Majumdar holds the Endowed Chair in Patient Health Management from the Faculty of Medicine and Dentistry, and the Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta. The authors have no affiliations or financial interests with any organization or entity with a financial interest in the contents of this article. All authors had access to the data and played a role in writing and revising this article. The authors declare no conflicts of interest.
Frailty is a state of vulnerability that encompasses a heterogeneous group of people.[1] Because it lacks a precise definition, multiple tools have been developed to identify frailty in both clinical and research settings.[2, 3, 4] Prevalence of frailty depends on the frailty assessment tool used and the population studied, ranging from 4% to 17% when the Fried score[5, 6, 7] is used and from 5% to 44%[5, 7, 8] when cumulative deficit models like the Frailty Index are utilized, with the lower prevalences being in younger community‐dwelling elderly populations and the higher proportions being in older institutionalized populations.
The Frailty Index, also called the Burden or Cumulative Deficit Model, comprises 70 domains that include mobility, mood, function, cognitive impairment, and disease states. It is multidimensional and allows for patients to be categorized on a continuum of frailty, but it is extremely difficult to apply in clinical practice. Recognizing this, Rockwood et al.[9] developed and validated the Clinical Frailty Scale (CFS) in the Canadian Study of Health and Aging. The CFS classifies patients into 1 of 9 categories: very fit, well, managing well, vulnerable, mildly frail (needs help with at least 1 instrumental activity of daily living such as shopping, finances, meal preparation, or housework), moderately frail (needs help with 1 or 2 activities of daily living such as bathing and dressing), severely frail (dependent for personal care), very severely frail (bedbound), and terminally ill. Although this tool is easy to use in clinical practice, it reflects a gestalt impression and requires some clinical judgement.
The Fried score[6] is a prototypical phenotype tool based on 5 criteria that include weight loss, self‐reported exhaustion, low energy expenditure, slowness of gait, and weakness. Recent evidence has suggested that slow gait (or dysmobility) alone may also be a potential screening test for frailty.[10] A recent systematic review[11] demonstrated an association between slow gait (dysmobility) and increased mortality. Dysmobility negatively impacts quality of life and has a strong association with disability resulting in the need for an increased level of care.[12] The Timed Up and Go Test (TUGT) is one method of assessing mobility which is relatively easy to perform, does not require special equipment, and is feasible to use in clinical settings.[13] However, whether impaired mobility predicts outcomes within the first 30 days after hospital discharge (a timeframe highlighted in the Affordable Care Act and used by the Centers for Medicare and Medicaid Services as an important hospital quality indicator) is still uncertain.
The aim of this study was to compare frailty assessments using the CFS and 2 of the most commonly used phenotypic tools (a modified Fried score and the TUGT as a proxy for mobility assessment) to determine which tools best predict postdischarge outcomes.
METHODS
Study Design and Population
As described in detail elsewhere,[14] this was a prospective cohort study that enrolled adult patients (any age older than 18 years) at the time of discharge back to the community from 7 general internal medicine wards in 2 teaching hospitals in Edmonton, Alberta between October 2013 and November 2014. We excluded patients admitted from, or being discharged back to, long‐term care facilities or other acute care hospitals, or from out of the province; patients who were unable to communicate in English; patients with moderate or severe cognitive impairment (scoring 5 or more on the Short Portable Mental Status Questionnaire); or patients with projected life expectancy of less than 3 months. All patients provided written consent, and the study was approved by the Health Research Ethics board of the University of Alberta (project ID Pro00036880).
We assessed the degree of frailty within 24 hours of discharge in 3 ways. First, we used the CFS[9, 15] with patients being asked to rate their best functional status in the week prior to admission. As per the CFS validations studies, scores 5 were defined as frail.[9, 15] Second, we used the TUGT as a proxy for slow gait speed/dysmobility (with >20 seconds defined as abnormal).[13] The TUGT was recorded as the shortest recorded time of the 2 timed trials to get up from a seated position, walk 10 feet and back, and then sit in the chair again. Third, we also determined their Fried score[6] (using the modifications outlined below) and categorized the patients as frail if they scored 3 or more. Of the 5 Fried categories, we assessed weakness by grip strength in their dominant hand using a Jamar handheld dynamometer and weight loss of 10 lb or more in the past year based on patient self‐report; these are identical to the original Fried scale description. Grip strength in the lowest quintile for sex and body mass index was defined as weak grip strength as per convention in the literature, which corresponded to less than 28.5 kg for men and less than 18.5 kg for women.[16, 17] We assessed the other 3 Fried categories in modified fashion as follows. For slow gait, rather than assessing time to walk 15 feet as in the original study and assigning a point to those testing in the lowest quintile for their age/sex, we used the TUGT, because our research personnel were already trained in this test, and we were doing it already as part of the discharge package for all patients.[13] For the Fried category of low activity, we based this on patient self‐report using the relevant questions in the EuroQoL Questionnaire (EQ‐5D); the Fried score used self‐report with a different questionnaire. Finally, for self‐reported exhaustion we used the questions in the Patient Health Questionnaire 9 (PHQ‐9)[18] analogous to those used from the Center for Epidemiological Studies depression scale in the original Fried description. We did this as we were evaluating the PHQ‐9 in our cohort already, and did not want to increase responder burden by presenting them with 2 depression questionnaires.
We followed all patients until 30 days after discharge, and outcome data (all‐cause mortality or all‐cause readmission) were collected by research personnel blinded to the patient's frailty status at discharge using patient/caregiver self‐report and analysis of the provincial electronic health record. We included deaths in or out of the hospital, and all readmissions were unplanned.
We examined the correlation between the CFS score (5 vs 5) and (1) the modified Fried score (3 vs 3) and (2) TUGT (20 seconds vs >20 seconds) using chance corrected kappa coefficients. In our previous article[14] we reported the association between the CFS and readmissions/hospitalizations within 30 days of discharge. In this article we examine whether either the Fried score or TUGT accurately and independently predict postdischarge readmissions/deaths, and whether they add additional prognostic information to the CFS assessment by comparing models with/without each definition using the C statistic and the Integrated Discrimination Improvement index. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), with P values of 0.05 considered statistically significant. Subgroup analysis was done in patients older than 65 years.
RESULTS
Of 1124 potentially eligible patients, 626 were excluded because of patient refusal (n = 227); transfer to/from another hospital, long‐term care facility, or out of province (n = 189); moderate to severe cognitive impairment (n = 88); language barriers (n = 71); or foreshortened life expectancy (n = 51). Another 3 patients withdrew consent prior to outcome assessment. The 495 patients we recruited and had outcome data for had a mean age of 64 years, 19.6% were older than 80 years, 50% were women, and the patients had a mean of 4.2 comorbidities and mean Charlson score of 2.4. The 4 most common reasons for hospital admission were heart failure, pneumonia, chronic obstructive pulmonary disease, and urinary tract infection, and the median length of stay was 5 days (interquartile range: 49 days).
Prevalence of Frailty According to Different Definitions
Although the CFS assessment resulted in 162 (33%) patients being deemed frail, only 82 (51%) of those patients also met the phenotype frailty definition using either the Fried model or the TUGT, and 49 (10%) patients who were not classified as frail on the CFS met either of the phenotypic definitions of frailty (Figure 1). Overall, 211 (43%) patients were frail according to at least 1 assessment, and 46 (9%) met all 3 frailty definitions. In the subgroup of 245 patients older than 65 years, 137 (56%) were frail according to at least 1 assessment, 38 (16%) met all 3 frailty definitions, and 27 (11%) of those patients classified as not frail on the CFS met either phenotypic definition of frailty. Agreement between TUGT and CFS or CFS and Fried was relatively poor with kappas of 0.31 (95% confidence interval [CI]: 0.23‐0.40) and 0.33 (95% CI: 0.25‐0.42), respectively. It is noteworthy that some patients deemed nonfrail on the CFS had slow gait speeds, and most CFS‐frail patients had gait speeds in the nonfrail range (Figure 2).


Characteristics According to Frailty Status
Although frail patients were generally similar across definitions (Table 1) in that they were older, had more comorbidities, more hospitalizations in the prior year, and longer index hospitalization lengths of stay than nonfrail patients, patients meeting phenotypic definitions of frailty but not classified as frail on the CFS were younger, had lower Charlson scores, higher EQ‐5D scores, and were discharged with less medications (Table 1).
| Not Frail on Any of the 3 Models, n = 284 | Frail on the CFS Only, n = 80 | Frail on the Fried and/or TUGT but Not the CFS, n = 49 | Frail on CFS and Either Phenotype Model, n = 82 | P Value Comparing the 3 Frailty Columns | |
|---|---|---|---|---|---|
| |||||
| Age, y, mean (95% CI) | 57.3 (55.259.5) | 69.1 (65.872.3) | 63.1 (57.968.3) | 75.8 (72.679.0) | 0.001 |
| Sex, female, no (%) | 118 (41.6) | 49 (61.3) | 27 (55.1) | 56 (68.3) | 0.3 |
| No. of comorbidities, mean (95% CI) | 4.2 (3.84.5) | 6.0 (5.56.6) | 4.0 (3.14.9) | 6.5 (5.87.2) | 0.001 |
| Charlson comorbidity score, mean (95% CI) | 2.4 (2.12.6) | 3.4 (3.03.9) | 2.6 (2.03.2) | 3.8 (3.34.2) | 0.01 |
| No. of patients hospitalized in prior 12 months, no (%) | 93 (32.8) | 44 (55.0) | 27 (55.1) | 54 (65.9) | 0.3 |
| Preadmission living situation, no (%) | 0.01 | ||||
| Living at home independently | 221 (77.8) | 26 (32.5) | 25 (51.0) | 17 (20.7) | |
| Living at home with help | 59 (20.8) | 43 (53.8) | 19 (38.8) | 48 (58.5) | |
| Assisted living or lodge | 4 (1.4) | 11 (13.8) | 5 (10.2) | 17 (20.7) | |
| EQ‐5D overall score, /100, mean (95% CI) | 66.9 (65.068.9) | 62.0 (57.666.4) | 56.6 (51.361.8) | 58.3 (53.962.7) | 0.28 |
| Goals of care in the hospital, no (%) | 0.0001 | ||||
| Resuscitation/ICU | 228 (83.5) | 41 (54.7) | 39 (84.8) | 29 (39.7) | |
| ICU but no resuscitation | 21(7.7) | 17 (22.7) | 1 (2.2) | 16 (21.9) | |
| No ICU, no resuscitation | 23 (8.4) | 17(22.7) | 6 (13.0) | 28 (37.8) | |
| Comfort care | 1 (0.4) | 0 | 0 | 0 | |
| Timed Up and Go Test, s, mean (95% CI) | 10.9 (10.411.3) | 13.9 (12.914.9) | 26.3 (19.033.6) | 30.3 (26.833.7) | 0.0001 |
| Grip strength, kg, mean (95% CI) | 32.1 (30.733.5) | 24.3 (22.3‐ 26.3) | 22.1 (19.924.2) | 17.7 (16.219.1) | 0.0001 |
| Serum albumin, g/L, mean (95% CI) | 34.2 (32.835.5) | 35.0 (33.037.0) | 31.1 (27.934.4) | 33.1 (31.434.9) | 0.07 |
| No. of prescription medications at discharge, mean (95% CI) | 5.2 (4.85.6) | 8.8 (7.99.6) | 6.1 (5.17.1) | 8.2 (7.58.9) | 0.0001 |
| Length of stay, d, median, [IQR] | 5 [37] | 6 [411] | 7 [3.512] | 7 [59] | 0.02 |
Outcomes According to Frailty Status
The overall rate of 30‐day death or hospital readmission was 17.1% (85 patients), primarily as a result of hospital readmissions (81, 16.4%) (Table 2). Although patients classified as frail on the CFS exhibited significantly higher 30‐day readmission/death rates (24.1% vs 13.8% for not frail, P = 0.005) even after adjusting for age and sex (adjusted odds ratio [aOR]: 2.02, 95% CI: 1.19‐3.41) (Table 3), patients meeting either of the phenotypic definitions for frailty but not the CFS definition were not at higher risk for 30‐day readmission/death (aOR: 0.87, 95% CI: 0.34‐2.19) (Table 3). The group at highest risk for 30‐day readmissions/death were those meeting both the CFS and either phenotypic definition of frailty (25.6% vs 13.8% for those not frail, aOR: 2.15, 95% CI: 1.10‐4.19) (Tables 2 and 3). None of the Integrated Discrimination Improvement indices (for modified Fried added to CFS or TUGT added to CFS) were statistically significant, suggesting no net new information was added to predictive models, and there were no appreciable changes in C statistics (Table 3). Neither the modified Fried score nor the TUGT on their own added independent prognostic information to age/sex alone as predictors of postdischarge outcomes. It is noteworthy that the areas under the curve for models using any combination of the frailty definitions plus age and sex were not high (all ranged between 0.55 and 0.60 for the overall cohort and from 0.52 and 0.65 in the elderly). If the frailty definitions were examined as continuous variables rather than dichotomized into frail/not frail, the C statistics were not appreciably better: 0.65 for CFS, 0.58 for TUGT, and 0.60 for modified Fried. Of note, the CFS score with the published cutoff of 5 demonstrated the highest kappa, sensitivity, specificity, and positive predictive value in relation to outcomes.
| Outcomes (Not Mutually Exclusive) | Not Frail on Any of the 3 Models | Frail on the CFS Only | Frail on the Fried and/or TUGT | Frail on CFS and Either Phenotype Model | P Value Comparing the 3 Frailty Columns |
|---|---|---|---|---|---|
| |||||
| Entire cohort | n = 284 | n = 80 | n = 49 | n = 82 | |
| Discharge disposition | 0.002 | ||||
| Live at home independently | 203 (71.5) | 16 (20.0) | 19 (38.8) | 10 (12.2) | |
| Live at home with help | 77 (27.1) | 52 (65.0) | 25 (51.0) | 50 (61.0) | |
| Assisted living or lodge | 4 (9.3) | 12 (15.0) | 5 (10.2) | 22 (26.8) | |
| 30‐day readmission or death | 40 (14.1) | 18 (22.5) | 6 (12.2) | 21 (25.6) | 0.2 |
| 30‐day hospital readmission | 39 (13.8) | 18 (22.5) | 6 (12.2) | 18 (22.0) | 0.31 |
| Death | 5 (1.8) | 3 (3.8) | 1 (2.0) | 4 (4.9) | 0.9 |
| 30‐day ER visit | 66 (23.2) | 30 (37.5) | 12 (24.5) | 23 (17.6) | 0.25 |
| Patients aged 65 years or older | n = 108 | n = 47 | n = 27 | n = 63 | |
| Discharge disposition | 0.03 | ||||
| Live at home independently | 69 (63.9) | 9 (19.2) | 10 (37.0) | 6 (9.5) | |
| Live at home with help | 36 (33.3) | 30 (63.8) | 13 (48.2) | 39(61.9) | |
| Assisted living or lodge | 3 (3.8) | 8 (17.0) | 4 (14.8) | 18 (28.6) | |
| 30‐day readmission or death | 13 (12.0) | 13 (27.7) | 3 (11.1) | 17 (27.0) | 0.22 |
| 30‐day hospital readmission | 12 (11.1) | 13 (27.7) | 3 (11.1) | 14 (22.2) | 0.26 |
| Death | 2 (1.9) | 3 (6.4) | 1 (3.7) | 3 (4.8) | 0.87 |
| 30‐day ER visit | 20 (18.5) | 17 (36.2) | 6 (22.2) | 18 (28.6) | 0.45 |
| Frailty Definition | Adjusted Odds Ratio for 30‐Day Readmission/Death | 95% CI | C Statistic for Model Predicting 30‐Day Readmission/Death Including Age, Sex, and Frailty Definition (95% CI) |
|---|---|---|---|
| |||
| Entire cohort | |||
| CFS (overall) | 2.02 | 1.193.41 | 0.60 (0.530.65) |
| CFS (plus either phenotype model) | 2.15 | 1.104.19 | 0.60 (0.520.64) |
| CFS (but neither phenotype model) | 1.81 | 0.943.48 | 0.60 (0.520.64) |
| Fried | 1.32 | 0.752.30 | 0.55 (0.560.58) |
| TUGT | 1.34 | 0.732.44 | 0.55 (0.460.58) |
| Fried and/or TUGT | 0.87 | 0.342.19 | 0.55 (0.470.58) |
| Patients aged 65 years or older | |||
| CFS (overall) | 3.20 | 1.556.60 | 0.65 (0.560.73) |
| CFS (plus either phenotype model) | 3.20 | 1.337.68 | 0.65 (0.550.72) |
| CFS (but neither phenotype model) | 3.08 | 1.267.47 | 0.65 (0.550.72) |
| Fried | 1.28 | 0.642.56 | 0.52 (0.390.53) |
| TUGT | 1.44 | 0.702.97 | 0.52 (0.390.53) |
| Fried and/or TUGT | 1.41 | 0.722.78 | 0.54 (0.420.56) |
Outcomes According to Frailty Status in the Elderly Subgroup
Although absolute risks of readmission or death were higher in elderly patients than younger patients, the excess risk was largely seen in those elderly patients classified as frail on the CFS. In fact, all of the associations reported above for the entire cohort were in the same direction in the elderly subgroup (Tables 2 and 3).
DISCUSSION
In summary, we found that of patients being discharged from general medical wards who were frail according to at least 1 of the 3 tools we used, only 22% met all 3 frailty case definitions (including only 28% of elderly patients deemed frail by at least 1 definition). There was surprisingly poor correlation between phenotypic markers of frailty such as poor mobility (slow TUGT) or the modified Fried Index and the CFS, even amongt elderly patients. The most clinically useful of the frailty assessment tools (both overall and in those patients who are elderly) appears to be the CFS, because it more accurately identifies those at higher risk of adverse outcomes after discharge, does not require special equipment to conduct, and is faster to do than the phenotypic assessment models we tested. We have also previously demonstrated that the CFS, after a brief training period identical to that used in this study, is reproducible between observers[19] and remains an independent predictor of adverse 30‐day outcomes even after adjusting for age, sex, comorbidities, and the LACE (length of stay, acuity of the admission, comorbidity, emergency room visits during the previous 6 months) score.[14]
Although some[10] have advocated for the use of mobility assessments (such as gait speed) as a frailty marker due to its ease of measurement and objectivity, we found that slow TUGT (which is a marker for mobility and not just slow gait speed) was not an independent prognostic marker for postdischarge outcomes. We hypothesize that the phenotypic models of frailty performed less well than the CFS as they focus on the measurement of particular physical attributes and do not take into account cognitive or psychosocial characteristics or comorbidity burden that also influence postdischarge outcomes. As well, the CFS captures the patients' baseline status prior to acute illness, whereas the phenotypic measures were assessed just prior to discharge and thus may provide less information about eventual recovery potential. Some have suggested that repeating phenotype measures postdischarge might be more informative,[20] but this would reduce clinical applicability a great deal. Certainly, an analysis[21] of the Cardiovascular Health Study cohort demonstrated that cumulative deficit models of frailty (for which the CFS is an accurate proxy[9, 15]) better predicted risk of death than phenotypic models.
Although a number of published studies have shown similar results to ours in that frail patients are at greater risk for death and/or hospitalization,[22, 23, 24] there is surprisingly little literature on the comparative predictive performance of the different frailty instruments and the extent to which they overlap. Cigolle et al.[25] compared 3 frailty scales (the Functional Domain Model, the Burden Model, and the Fried score) in the Health and Retirement Study and, similarly to us, found that although 30.2% were frail on at least 1 of these scales, only 3.1% were deemed frail by all 3. The Conselice Study of Brain Aging[5] also reported that a deficit accumulation model defined a much higher prevalence of frailty (37.6%) than the 11.6% identified using the phenotypic Study of Osteoporotic Fractures (SOF) index based on weight loss, mobility, and level of energy. Another study[26] reported that risk models incorporating either the SOF index or the Fried score exhibited C statistics of only 0.61 for predicting falls in elderly females. A cohort study[27] from 2 English general medical units also found that none of the 5 frailty models was particularly accurate at predicting risk of readmission at 3 months, with C statistics ranging between 0.52 and 0.57. Although frailty assessment at time of hospital admission predicted in‐hospital mortality and length of stay in another English study, it was not independently associated with 30‐day outcomes after adjusting for age, sex, and comorbidities including dementia.[27] To our knowledge, these latter 2 are the only other studies reported to date performed in hospitalized patients to assess whether frailty assessment helps predict postdischarge outcomes. Thus, the poor C statistics we found for all of our frailty tools confirms prior literature that frailty assessment alone is inadequate to accurately identify those patients at highest risk for poor outcomes in the first 30 days after discharge. However, frailty assessment together with consideration of each individual's comorbidities, cognitive status, psychosocial circumstances, and environment can be useful to flag those individuals who may need extra attention postdischarge to optimize outcomes.
Strengths and Limitations
Although this was a prospective cohort study with blinded ascertainment of endpoints (30‐day outcome data were collected by observers who were unaware of the patients' CFS or phenotypic model scores), it is not without limitations. First, the only postdischarge outcomes we assessed were readmission and death, and it would be interesting to evaluate which frailty tools best predict those who are most likely to benefit from home‐care services in the community. Second, as we were interested in 30‐day readmission rates, we excluded long‐term care residents from our study and patients who had foreshortened life expectancy, in essence, the frailest of the frail. Although this reduced the size of any association between frailty and adverse outcomes, we focused this study on the situations where there is clinical equipoise and there is rarely a diagnostic dilemma around the identification of frailty and need for increased services in palliative or long‐term care patients. Third, we did not use exactly the same questionnaires or gait speed assessments as used in the original Fried score description, but as outlined in the Methods section, we used analogous questions on closely related questionnaires to extract the same information. Fourth, some might consider our comparisons biased toward the CFS, as it reflects gestalt clinical impressions (informed by patients and proxies) of frailty status before hospital admission while the Fried score and TUGT were based on patient status just prior to discharge, it may be that the former is a better measure of eventual recovery (and ongoing risk) than the latter measures. If this is the case, for the purposes of targeting interventions to prevent postdischarge complications, it would suggest to us that the CFS is better suited, whereas phenotype tools can be reserved for the postdischarge phase of recovery. By the same token, perhaps serial measures of the CFS and phenotypic tools are more important, as the trajectory of recovery may be most informative for risk prediction.[7] Certainly, if one were interested in changes in functional status during hospitalization,[29] then objective phenotypic measures such as grip strength or TUGT times would seem more appropriate choices. Fifth, some may perceive it as a weakness that we did not restrict our cohort to elderly patients; however, we actually view this as a strength, because frailty is not exclusive to older patients. Sixth, although we restricted this study to patients being discharged from general internal medicine wards, it is worth mentioning that previous studies have shown similar associations between frailty and outcomes in nonmedical hospitalized patients.[19, 22, 23, 24]
In conclusion, we looked at 3 different ways of screening for frailty, 1 being a subjective but well‐validated tool (the CFS) and the other 2 being objective assessments that look at specific phenotypic characteristics. There is a compelling need to find a standardized assessment to determine frailty in both research and clinical settings, and our study provides support for use of the CFS over the Fried or TUGT as screening tools. Standardized frailty assessments should be part of the discharge planning for all medical patients so that extra resources can be properly targeted at those patients at greatest risk for suboptimal transition back to community living.
Acknowledgements
The authors acknowledge Miriam Fradette and Debbie Boyko for their important contributions in data acquisition, as well as all the physicians rotating through the general internal medicine wards for their help in identifying the patients.
Disclosures: Author contributions are as follows: study concept and design: Finlay A. McAlister, Sumit R. Majumdar, and Raj Padwal; acquisition of patients and data: Sara Belga, Darren Lau, Jenelle Pederson, and Sharry Kahlon; analysis of data: Jeff Bakal, Sara Belga, Finlay A. McAlister; first draft of manuscript: Sara Belga and Finlay A. McAlister; critical revision of manuscript: all authors. Funding for this study was provided by an operating grant from Alberta InnovatesHealth Solutions. Alberta InnovatesHealth Solutions had no role in role in the design, methods, subject recruitment, data collections, analysis, or preparation of the article. Finlay A. McAlister and Sumit R. Majumdar hold career salary support from Alberta InnovatesHealth Solutions. Finlay A. McAlister holds the Chair in Cardiovascular Outcomes Research at the Mazankowski Heart Institute, University of Alberta. Sumit R. Majumdar holds the Endowed Chair in Patient Health Management from the Faculty of Medicine and Dentistry, and the Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta. The authors have no affiliations or financial interests with any organization or entity with a financial interest in the contents of this article. All authors had access to the data and played a role in writing and revising this article. The authors declare no conflicts of interest.
- , , , , . Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263.
- , , , , . The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–2138.
- , , , , . Frailty in elderly people. Lancet. 2013;381:752–762.
- , , , , , . Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–114.
- , , , , , . A comparison of frailty indexes for prediction of adverse health outcomes in a elderly cohort. Arch Gerontol Geriatr. 2012;54:16–20.
- , , , et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156.
- , , , . Prevalence of frailty in community‐dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492.
- , , . Sex differences in the risk of frailty for mortality independent of disability of chronic diseases. J Am Geriatr Soc. 2005;53:40–47.
- , , . A comparison of two approaches to measuring frailty in elderly people. J Gerontol. 2007;62:738–743.
- , , . A diagnosis of dismobility—giving mobility clinical visibility: a mobility working group recommendation. JAMA. 2014;311:2061–2062.
- , , , et al. Gait speed and survival in older adults. JAMA. 2011;301:50–58.
- , , , et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762.
- , . The timed “Up and Go” test: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148.
- , , , et al. Association between frailty and 30‐day outcomes after discharge from hospital. CMAJ. 2015;187:799–804.
- , , , et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495.
- , , , et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–1419.
- , . Grip strength in older adults: test‐retest reliability and cutoff for subjective weakness of using the hands in heavy tasks. Arch Phys Med Rehabil. 2010;91:1747–1751.
- , . The PHQ‐9: a new depression measure. Psychiatr Ann. 2002;32:509–515.
- , , , et al. Association between frailty and short‐ and long‐term outcomes among critically ill patients: a multicenter prospective cohort study. CMAJ. 2013;186:e95–e102.
- , . Risk after hospitalization: we have a lot to learn. J Hosp Med. 2015;10:135–136.
- , , , , , . Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903.
- , , . Unplanned hospital readmission and its predictors in patients with chronic conditions. J Formos Med Assoc. 2002;101:779–785.
- , , , et al. Frailty and early hospital readmission after kidney transplant. Am J Transplant. 2013;13:2091–2095.
- , , , , , . Simple frailty score predicts postoperative complications across surgical specialities. Am J Surg. 2013;206:544–550.
- , , , . Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57:830–839.
- , , , et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Int Med. 2008;168:382–389.
- , , , , , . The predictive properties of frailty‐rating scales in the acute medical unit. Age Ageing. 2013;42:776–781.
- , , , . Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108:943–949.
- , , . Hospitalization‐associated disability: she was probably able to ambulate, but I'm not sure. JAMA. 2011;306:1782–1793.
- , , , , . Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263.
- , , , , . The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–2138.
- , , , , . Frailty in elderly people. Lancet. 2013;381:752–762.
- , , , , , . Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–114.
- , , , , , . A comparison of frailty indexes for prediction of adverse health outcomes in a elderly cohort. Arch Gerontol Geriatr. 2012;54:16–20.
- , , , et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156.
- , , , . Prevalence of frailty in community‐dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492.
- , , . Sex differences in the risk of frailty for mortality independent of disability of chronic diseases. J Am Geriatr Soc. 2005;53:40–47.
- , , . A comparison of two approaches to measuring frailty in elderly people. J Gerontol. 2007;62:738–743.
- , , . A diagnosis of dismobility—giving mobility clinical visibility: a mobility working group recommendation. JAMA. 2014;311:2061–2062.
- , , , et al. Gait speed and survival in older adults. JAMA. 2011;301:50–58.
- , , , et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762.
- , . The timed “Up and Go” test: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148.
- , , , et al. Association between frailty and 30‐day outcomes after discharge from hospital. CMAJ. 2015;187:799–804.
- , , , et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495.
- , , , et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–1419.
- , . Grip strength in older adults: test‐retest reliability and cutoff for subjective weakness of using the hands in heavy tasks. Arch Phys Med Rehabil. 2010;91:1747–1751.
- , . The PHQ‐9: a new depression measure. Psychiatr Ann. 2002;32:509–515.
- , , , et al. Association between frailty and short‐ and long‐term outcomes among critically ill patients: a multicenter prospective cohort study. CMAJ. 2013;186:e95–e102.
- , . Risk after hospitalization: we have a lot to learn. J Hosp Med. 2015;10:135–136.
- , , , , , . Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903.
- , , . Unplanned hospital readmission and its predictors in patients with chronic conditions. J Formos Med Assoc. 2002;101:779–785.
- , , , et al. Frailty and early hospital readmission after kidney transplant. Am J Transplant. 2013;13:2091–2095.
- , , , , , . Simple frailty score predicts postoperative complications across surgical specialities. Am J Surg. 2013;206:544–550.
- , , , . Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57:830–839.
- , , , et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Int Med. 2008;168:382–389.
- , , , , , . The predictive properties of frailty‐rating scales in the acute medical unit. Age Ageing. 2013;42:776–781.
- , , , . Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108:943–949.
- , , . Hospitalization‐associated disability: she was probably able to ambulate, but I'm not sure. JAMA. 2011;306:1782–1793.
Depression and Postdischarge Events
Between 10% and 40% of patients are readmitted after being discharged from the hospital,[1, 2] and as many as another 25% return to the emergency department (ED) within 30 days.[3] This creates a substantial burden on the healthcare system.[2] Various interventions have been tried to improve the quality of discharge transitions and reduce readmission rates, but results thus far have been inconsistent and generally disappointing.[4, 5, 6] Targeted delivery of interventions to those at highest risk might improve the effectiveness of these efforts and reduce costs. However, current readmission risk assessment models are only moderately predictive, suggesting the presence of unrecognized risk factors.[7, 8]
Active depression might represent a potentially modifiable independent predictor of adverse short‐term hospital outcomes that is currently underutilized. Depression occurs in 5% to 58% of hospitalized adults, depending on how cases are defined.[9, 10] Depression is often under‐recognized and undertreated in acute care clinical settings,[11] and relatively few readmission prediction models incorporate mental health related symptoms.[12]
Although several reviews have examined methods of screening for depression in hospitalized patients[9] or the effectiveness of screening in primary care,[13, 14] to our knowledge no systematic review has examined the impact of depression on short‐term prognosis after discharge from acute care. Therefore, the purpose of this systematic review was to summarize all studies that evaluated whether hospitalized medical patients with depressive symptoms are at higher risk of 30‐day all‐cause readmission or all‐cause mortality after being discharged from the hospital.
METHODS
This study followed an a priori protocol developed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) criteria.[15]
Data Sources and Search Methods
We searched the Cumulative Index to Nursing and Allied Health Literature, Ovid MEDLINE, Ovid Embase, and PsycINFO from inception to January 9, 2015, and the last 5 years of PubMed for full publications with any of the following Medical Subject Headings: depressive disorder, depression, patient readmission, interviews, psychological, inpatients, with restrictions for peer‐reviewed publication, humans, adults aged 18 years, and the English language. Search strategies were developed with a librarian (available upon request). We manually searched reference lists of all included studies and relevant review articles and contacted content experts to identify additional publications.
Eligibility Criteria and Selection of Studies
Two authors (J.L.P. and L.M.W.) independently screened full texts of all relevant articles for inclusion. Disagreements were resolved by consensus or a third reviewer (S.R.M.). We considered any original research that compared readmission or mortality after discharge for hospitalized medical patients (ie, general patients or subgroups thereof) with versus without depression identified by any validated depression measure,[16] including any study design that incorporated at least 30‐day follow‐up postdischarge. We excluded studies that examined patients hospitalized in nonacute care settings or on surgical, psychiatric, obstetric, or intensive care services. We calculated Cohen's coefficient to evaluate inter‐rater agreement on study selection.
Data Extraction
Data were abstracted by 2 authors (J.L.P. and L.M.W.). Disagreements were resolved by consensus or a third reviewer (S.R.M.). We contacted authors of all included studies to obtain missing data. If unavailable, crude data were estimated from published survival curves employing validated techniques in R (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria) and Digitizeit (
Data Synthesis and Statistical Analysis
Where possible, we calculated the pooled risk ratio (RR) with 95% confidence interval (95% CI) using a random effects models in Review Manager (RevMan) 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark). The random effects approach that we employed assumes heterogeneity (ie, underlying parameters vary between individual studies) and is distributed around a mean or population average effect, and results in more conservative (wider) confidence intervals, wherein larger cohorts (or studies with smaller standard errors) are given more weight. Heterogeneity was assessed using the I2 statistic, with values of 25%, 25% to 50%, and >50% representing low, moderate, and high heterogeneity.[19] As per the guidance of Higgins et al., we did not a priori define any degree of heterogeneity that would preclude pooling of the data; the expectation would be that heterogeneity would be substantially higher pooling observational studies rather than randomized trials.[19] Statistical significance was considered a 2‐sided P value 0.05.
Quality Assessment and Risk of Bias
We assessed study quality using the 9‐item Newcastle‐Ottawa scale with 0 to 3, 4 to 6, and 7 to 9 stars considered low, moderate, and high quality, respectively, and criteria for external and internal validity, including group selection and comparability, outcome assessment, and adequacy of follow‐up.[20] Adjusted estimates published in individual reports (or obtained directly from authors) were compared wherever possible with unadjusted estimates to assess the degree of confounding. We generated funnel plots in RevMan 5.3 and conducted Egger tests using Stata 13 (StataCorp LP, College Station, TX) to assess for publication bias.[21]
RESULTS
Study Selection
After removing duplicate publications, we identified 4066 reports and reviewed 133 reports in full text (see Supporting Figure 1 in the online version of this article). Despite our broad study inclusion criteria, we found only 35 longitudinal studies addressing this question. All 35 authors were contacted for additional outcomes data and other missing information (response rate of 34%). We had to exclude 17 studies as they did not provide 30 or 90‐day post‐discharge outcomes. Only 4 studies had published crude data for outcomes within 90 days,[22, 23, 24, 25] but after contact with authors, we received unpublished data for a further 7 studies[26, 27, 28, 29, 30, 31, 32] (including individual level data for 2 cohorts).[31, 32] We were able to estimate crude data from Kaplan‐Meier curves for another 3 studies.[33, 34, 35] Another 4 studies did not collect the outcomes we were interested in individually. These studies were included in this systematic review but are not poolable in our models: 3 authors could only provide composite endpoint data,[36, 37, 38] and 1 author provided unadjusted hazard ratios.[39] Inter‐reviewer agreement for inclusion was 80% (Cohen's = 0.60).
Characteristics of Included Studies
The 18 studies ranged in size from 58 to 1418 patients; 13 were cohort studies and 5 included secondary data from randomized control trials.[22, 27, 30, 34, 36] All studies ascertained depressive status by screening during index medical admission with either diagnostic interview or self‐report questionnaires, although a variety of scales and definitions for depression were used (Beck Depression Inventory [BDI] in 6 studies, Geriatric Depression Scale in 5 studies, Patient Health Questionnaire in another 4 studies, Medical Outcomes Study‐Depression Questionnaire in 1 study, and Center for Epidemiologic Studies Depression Scale in another study) (Table 1). Screening interviews were conducted mostly by research assistants or nurses (68%) or self‐administered (21%). Most studies examined specific medical patient subgroups (10 cardiac, 3 pulmonary, and 2 elderly). Major exclusion criteria reported were terminal illness (4 studies), unstable condition (6 studies), severe cognitive impairment (5 studies), and suicidal ideation or known depression (4 studies); 1 study enrolled patients with suspected depression (Table 1). Patient cohorts were on average older (range, 5082 years) (Table 1). Attrition rates for readmission and mortality data were low (average 1% among entire sample of studies). All studies scored at least 5 on the Newcastle‐Ottawa scale and were thus considered of at least moderate quality (see Supporting Table 1 in the online version of this article).
| Author, Date of Publication, Enrollment Period | Setting Country/Region, No. of Hospitals | No. of Inpatients, Clinical Features | Major Exclusion Criteria | Follow‐up, mo | Depression Measure (Cutoff) and Screening Method | Mean Age (SD), y | % Female | Positive Screen, No. (%) | Primary Outcome, Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Studies that use a scale based on DSM‐III criteria or a diagnostic interview according to DSM‐III criteria | |||||||||
| Frasure‐Smith et al.,[26] 1993, 19911992* | Canada/Quebec, 1 urban teaching | 218, AMI | Terminal noncardiac illness, unstable, not cognitive | 6 | BDI (10); mod DIS by interviewer, after transfer to medicine | 60 (range, 2488) | 22 | 68 (31), 35 (16) | All‐cause mortality |
| Frasure‐Smith et al.,[27] 1999, 19911992,* 19911994 | Canada/Quebec, 1 urban teaching, 10 urban area | 218; 78, AMI | Terminal noncardiac illness, unstable, not cognitive | 12 | BDI (10) by interviewer, after transfer to medicine | 60 (11) | 32 | 290 (32) | Cardiac mortality |
| Freedland et al.,[25] 1991, 1990 | USA/MO, 1 urban teaching | 58, CHF 75 years | Dementia, medically unstable | 3 | Mod DIS by psychiatric residents and interviewer | 78 (6) | 57 | 10 (17) | All‐cause readmission, all‐cause mortality |
| Fulop et al.,[38] 2003, 2002 | USA/NY, 1 urban teaching | 203, CHF 65 years | 1, 6 | GDS (10); SCID‐NP by interviewer, at discharge | 77 (8) | 53 | 73 (36), 44 (22) | Depression, composite PCP, ED, care visits, and readmission | |
| Lesprance et al.,[28] 2000, 19941996 | Canada/Quebec, 1 urban teaching | 430, unstable angina | Terminal noncardiac illness, not cognitive, recent CABG | 12 | BDI (10); mod DIS by interviewer, 5 days after admission | 62 (11) | 29 | 178 (41), 120 (28) | Cardiac death and MI, any death, angina readmission |
| Rumsfeld et al.,[30] 2005, 19992001 | CA, USA, UK, multiple | 634, AMI with CHF | Valvular or congenital heart failure | Up to 32 | MOS‐D (0.06) by interviewer, before discharge | 65 (11) | 28 | 143 (23) | All‐cause death, CVD death and readmission |
| Song et al.,[33] 2009, 2005 | South Korea, 2 urban teaching | 165, HF | If minor criteria for HF attributable to other medical condition | 6 | BDI (10) self‐administer or interviewer, 34 days of admin | 62 (13) | 49 | 131 (79) | HF readmission and all‐cause mortality, HF readmit |
| Papaioannou et al.,[29] 2013, 20092010 | Greece/Athens, 1 urban | 230, AECOPD | Other respiratory illness, known depressed | Monthly up to 12 | BDI‐I (19) self‐administer, first day | 71 (9) | 12 | 91 (40) | All‐cause mortality, AECOPD readmission |
| Studies that use a scale based on or validated against DSM‐IV criteria or a diagnostic interview according to DSM‐IV criteria | |||||||||
| Almagro et al.,[31] 2002, 19961997 | Spain, 1 urban teaching | 130, AECOPD | Other pulmonary disease | July 1999 | GDS‐SF (6) by interviewer, day before discharge | 72 (9) | 8 | 43 (33) | All‐cause mortality |
| Almagro et al.,[32] 2012, 20032004 | Spain, 1 urban teaching | 134, AECOPD | Other pulmonary disease | 1, 36 | GDS‐SF (6) by interviewer | 72 (10) | 5 | 55 (41) | All‐cause mortality, lung function, frailty |
| Bla et al.,[39] 2001, 2000 | Switzerland, 1 urban teaching | 401, medical 75 years | Stay 24 hours, elective/facility transfer, unstable, not cognitive | 6 | GDS‐SF (6) by interviewer, within 2 days of admission | 82 (7599) | 61 | 90 (22) | All‐cause readmission, all‐cause mortality |
| Cancino et al.,[22] 2014, 20062007,* 20082009 | USA/MA, 1 urban tertiary | 680; 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1 | PHQ‐9 (5 or severity) by interviewer, on admin | 50 (14) | 51 | 561 (40) | All‐cause readmission, ED visits, PCP visits |
| Mitchell et al.,[36] 2010, 20062007* | USA/MA, 1 urban tertiary | 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1, 2, 3 | PHQ‐9 (5) by interviewer, on admin | 50 (15) | 50 | 238 (32) | ED visits and all‐cause readmission |
| Covinsky et al.,[34] 1999, 19901992 | USA/OH, 1 urban teaching | 573, medical | ICU, oncology, telemetry, nursing home admissions | 36 | GDS‐SF (6) by interviewer, within 2 days of admission | 80 | 68 | 197 (34) | All‐cause mortality |
| Jiang et al.,[23] 2001, 19971998 | USA/NC, 1 urban teaching | 357 (331 DIS only), CHF | Suicidal, planned surgery, pregnant | 3, 12 | BDI (10) self‐admin; mod DIS (+BDI only) by interviewer | 63 (13) | 33 | 126 (35), 46 (14) | All‐cause mortality, all‐cause readmission |
| Kartha et al.,[24] 2007, 20022004 | USA/MA, 1 urban safety net | 144, medical recently hospitalized | Planned readmission, unable to keep PCP appointments | 3 | PHQ‐9 (algorithm) by interviewer | 55 (16) | 56 | 39 (27) | All‐cause readmission |
| Koenig and Kuchbhatla,[37] 1999, 1997 | USA/NC, 1 urban teaching | 331, medical 60 years | Stay 3 or >7 days, ICU/CCU, severe illness, nursing home transfers | 3, 6, 9, 12 | CES‐D (16) or HAM‐D (11) or DIS by psychiatrist, on or after third day | 70 (7) | 51 | 160 (48) | Depression, composite physical disability, health visits, and all‐cause readmission |
| Rollman et al.,[35] 2012, 20072009 | USA/PA, 4 urban teaching | 471, CHF, suspected depressed | Antidepressants users (excluded from PHQ‐2 group only) | Up to 12 | PHQ‐2; PHQ‐9 (5 in +PHQ‐2), by interviewer, 4 days | 66 (13) | 35 | 371 (79), 351 (74) | All‐cause mortality |
Prevalence and Recognition of Depressive Symptoms
The range of depression prevalence in hospitalized medical patients was 14% to 79%, with a median of 32% (interquartile range, 27%40%) (Table 1). In those studies that used a diagnostic interview, the prevalence tended to be lower for major depression, with a median of 17% (interquartile range, 16%22%) (Table 1). None of the included studies reported frequency of clinically recognized depression (ie, prior to screening for the study). Only 2 studies assessed the persistence of depression after discharge: 1 reported that depression persisted in 53% (by screening questionnaire) and 34% (by diagnostic interview) of patients at 30 days,[38] whereas the other reported 48% persistence at 90 days after discharge according to a combined screening method.[37]
Hospital Readmission
Overall, 8 studies provided readmission data. Among patients discharged from acute care medical wards (4 studies reporting on 5 cohorts), 395 of 2433 (16.2%) patients were readmitted within 30 days (Figure 1). Hospitalized patients with depressive symptoms were more likely to be readmitted within 30 days after discharge (20.4% vs 13.7%, RR: 1.73, 95% CI: 1.16‐2.58, P = 0.007, I2 = 55%) (Figure 1), compared to those without depression. Results were consistent for 90‐day readmissions (39.8% vs 31.0%, RR: 1.68, 95% CI: 1.13‐2.50, P = 0.01, I2 = 76%, n = 1543 patients) (see Supporting Figure 2 in the online version of this article) in 6 studies. One individual study examined readmission within 6 months after discharge, but was not poolable in this model, as it presented only hazard ratios and not raw data; however, it did report a 50% increased risk of readmission in medical inpatients aged 75 years (adjusted hazard ratio: 1.50, 95% CI: 1.03‐2.17, n = 401).[39]
Forest plot presents results of the meta‐analysis in which the size of each data marker indicates the weight assigned to individuals studies. Abbreviations: CI, confidence interval; IV, independent variable.
Mortality After Discharge
Overall, 11 studies provided all‐cause mortality data. Among medical patients discharged from acute care in 9 studies, 69 of 3397 (2.0%) patients died within 30 days (Figure 2). Medical patients discharged with depressive symptoms were more likely to die within 30 days (2.8% vs 1.5%, RR: 2.13, 95% CI: 1.31‐3.44, P = 0.002, I2 = 0%) (Figure 2) compared to those without depression. Similar results were found for 90‐day mortality (7.7% vs 4.1%, RR: 2.01, 95% CI: 1.47‐2.76, P 0.001, I2 = 4%, n = 3784 patients) (see Supporting Figure 3 in the online version of this article) in 11 studies.
ED and PCP Visits
Four studies examined the use of ED or PCP services within 90 days of discharge, but 3 did not have extractable data for meta‐analysis. All showed increased utilization of health services for depressed compared to nondepressed patients after discharge.[22, 36, 37, 38] Depressed patients were more likely to visit the ED (adjusted incidence rate ratio: 1.73, 95% CI: 1.27‐2.36),[36] had significantly more medical encounters (eg, PCP, ED visits, hospital admissions, laboratory tests, and home care [mean 2.9 vs 2.6, P = 0.05])[38] and had a greater number of ED visits alone (27 vs 15 per 100 patients, P = 0.007)[22] within 30 days of hospital discharge compared to nondepressed patients. Similar results were found at 90 days.[36]
Sensitivity Analyses
All told, most studies reported a positive association between depression and adverse events, and this was true regardless of how much adjustment for potential confounding had been undertaken by the authors. Although all studies were qualitatively in the same direction, the magnitude of the association varied due to methodological and/or clinical heterogeneity. Sensitivity analysis revealed no overall difference in pooled risk ratios or heterogeneity between Mantel‐Haenszel fixed effects versus random effects models or with the addition of 0.5 to cells to permit inclusion of zero‐event data. There was no evidence of publication bias; funnel plots and Egger test results are available upon request. There were no statistically significant differences in the risk associated with depressive symptoms whether studies used Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III or DSM‐IV criteria, whether the study samples were disease specific or unselected general medical cohorts, whether studies were of moderate or high quality, or regardless of the severity of depressive symptoms.
DISCUSSION
Summary of Evidence
We found that depression was common in medical inpatients (about one‐third of all patients) and persisted for at least 30 days in up to half of those patients after discharge. We found strong evidence of an association between depressive symptoms and poor short‐term prognosis after discharge from the hospital: a 73% increased risk of readmission and a 2‐fold risk of death within 30 days compared to patients without depressive symptoms with similar results at 90 days.
Our meta‐analysis complements a recent systematic review that found concomitant depression to be a risk factor for poor prognosis among inpatients and outpatients with acute coronary syndrome,[40] and a meta‐analysis that demonstrated an increased risk of 2‐year mortality among patients with depression after myocardial infarction.[41] To our knowledge, our study is the first to quantify the short‐term postdischarge risks across a diverse group of medical inpatients.
The potential mechanisms underlying the observed relationship between depression and adverse patient outcomes after discharge are likely multiple. We believe there are 2 main possibilities. First, the increased risk associated with depression might be due to residual confounding, even though many of these studies did adjust for extensive lists of comorbidities,[22, 24, 26, 27, 29, 30, 33, 35, 36, 39] including functional status[39] and prior health services utilization.[22, 34, 36] This could occur if other risk factors were not sufficiently adjusted for, such as unrecognized comorbidities or concomitant disability, which are often present among chronically ill patients,[42] or if depression were a marker of psychosocial risk factors such as anxiety,[43] stress or poor resiliency,[44] or low social support,[45] though a few adjusted for psychosocial factors such as social support[26] or anxiety.[35] Confounding could also occur if symptoms of acute illness inflate reports of somatic symptoms of depression on self‐report questionnaires. Recent studies on the BDI, found that scores were higher in postmyocardial infarction patients when compared to outpatient controls,[46] but with no differences between those groups in scores for the BDI‐II,[47] a version with fewer somatic symptom questions.
Second, depression may cause adverse outcomes through indirect or direct pathways. Indirect causation could occur if depression hindered self‐care behaviors such as medication adherence.[42] Depression could also act directly through pathophysiological changes. Some studies have suggested that depression is associated with metabolic abnormalities, including alterations in glucose transport[42, 48] and increased vulnerability to obesity, type 2 diabetes mellitus, and/or diabetic complications, common conditions among hospitalized patients that also adversely affect postdischarge outcomes.[40, 48]
Strengths and Limitations
This review has multiple strengths. We cast a broad search and included studies that examined a wide range of medical patient subgroups, thus increasing the generalizability of our findings. We identified a general scarcity of studies on this topic and obtained additional unpublished data for 10 of the 18 relevant studies, and our response rate of 34% is compatible with the 37% response rate reported for Cochrane reviews when seeking additional data from authors.[49] Whether examined qualitatively (vote counting of the number of studies that showed an association) or quantitatively (via formal meta‐analysis), it seems apparent that there is a clinically important association between depression and postdischarge adverse events, but given the number, quality, and heterogeneity of the studies we examined, there may be some ongoing dispute about exactly how strong this association is and the degree of bias contributed by a couple of large studies of the topic.
There are limitations to our review. First, as we did not have individual‐level patient data, we could not use metaregression to explore sources of heterogeneity (clinical or methodological) or adjust for confounding, and this likely contributes to observed differences between individual estimates. For instance, the included studies had heterogeneous screening measures and cutoffs; thus, all cases of depression in these studies might not be equivalent. Some of the included studies assessed depression early during admission where psychological distress may be greatest; others assessed symptoms closer to discharge. Most studies included patients with specific conditions like heart failure or chronic obstructive pulmonary disease rather than a wide spectrum of medical inpatients. Moreover, few studies adjusted for psychosocial risk factors such as social support, anxiety, and functional status, and only 2 studies assessed the persistence of depressive symptoms after discharge. Second, we did not explore quantitative measures of between‐study variation (eg, I2), because experts question its utility given the expected heterogeneity in meta‐analyses of observational studies.[50] Third, although the included studies were deemed to be of at least moderate quality, they could be at risk for sources of bias that may not be sufficiently appraised by the current version of the Newscastle‐Ottawa scale for observational studies. Finally, we excluded grey literature (eg, conference proceedings or technical reports) that could potentially exclude null findings, although we did contact authors in this field to identify additional unpublished data relevant to this topic.
CONCLUSIONS
We have confirmed that depressive symptoms are common in hospitalized medical patients, frequently persist after discharge, and may predict greater risk of readmission or death after discharge. Thus, depressive symptoms are an additional marker that clinicians can use to help identify patients in acute care medical settings who may be at increased risk for suboptimal transition back to the community and who may require additional resources after discharge. However, future research is required to evaluate whether treatment of individuals who screen positive for depressive symptoms can reduce 30‐day readmission rates, and we are aware of at least 1 relevant ongoing trial (
Acknowledgements
The authors thank the following individuals: Dale Storie, MLIS, Saskatchewan Information and Library Services Consortium, Regina, Saskatchewan, Canada, for assistance in the literature search; James A. Hanley, PhD, Department of Epidemiology and Biostatistics, Faculty of Medicine, McGill University, Montreal, Quebec, Canada, for guidance in data recovery methods; Nancy Frasure‐Smith, PhD, Department of Psychiatry, McGill University, Department of Psychiatry and Research Centre Hospital Centre, University of Montreal, and Montreal Heart Institute Research Centre, Montreal, Quebec, Canada; Andriana I. Papaioannou, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; Konstantinos Kostikas, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; and Pere Almagro, MD, Servicio de Medicina Interna, Hospital Universitario Mutua de Terrassa, Terrassa, Barcelona, Spain; as well as Philip G. Jones, MS, Saint Luke's Mid America Heart Institute, Kansas City, Missouri; for their retrieval and contribution of unpublished data.
Disclosures
Ms. Pederson affirms that the manuscript is an honest, accurate, and transparent account of the study being reported with no important omissions. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. Design and conduct of the study: Ms. Pederson, Drs. Majumdar and McAlister. Data acquisition: Ms. Pederson, Ms. Warkentin. Analysis and interpretation of the data and drafting of the manuscript: Ms Pederson, Drs. Majumdar and McAlister. Review of the manuscript: all authors. Study supervision: Drs. Majumdar and McAlister. None of the contributors received compensation for their efforts. Salary support for Ms. Pederson was provided by a CRIO grant from Alberta InnovatesHealth Solutions. Drs. McAlister and Majumdar are supported by salary awards from Alberta Innovates‐Health Solutions. Dr. McAlister holds the University of Alberta/Capital Health Chair in Cardiology Outcomes Research. Dr. Majumda holds the University of Alberta Endowed Chair in Patient Health Management. The funding sources had no role in the design or conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. This work is that of the authors independent of funders. The authors report no conflicts of interest.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , . Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398–407.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . The elusive quest for quality and cost savings in the Medicare program. JAMA. 2009;301(6):668–670.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries—15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–111.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , . Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148–154.
- , , , et al. Prevalence, correlates and recognition of depression among inpatients of general hospitals in Wuhan, China. Gen Hosp Psychiatry. 2010;32(3):268–275.
- , , , , , . Recognition of depression by non‐psychiatric physicians—a systematic literature review and meta‐analysis. J Gen Intern Med. 2008;23(1):25–36.
- , , , , , . Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. Am Heart J. 2012;164(3):365–372.
- , , , et al. Does evidence support the American Heart Association's recommendation to screen patients for depression in cardiovascular care? An updated systematic review. PLoS One. 2013;8(1):e52654.
- , , , et al. Screening for depression: a systematic review and meta‐analysis. CMAJ Open. 2013;1(4):E159–E167.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94.
- , , , et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136(10):765–776.
- , , . Recovering the raw data behind a non‐parametric survival curve. Syst Rev. 2014;3:151.
- , , , . Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
- . Commentary: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160.
- , , , et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed September 1, 2015.
- , , . The funnel plot. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication Bias in Meta‐analysis: Prevention, Assessment and Adjustments. New York, NY: John Wiley 2006:73–98.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , et al. Depression in elderly patients with congestive heart failure. J Geriatr Psychiatry. 1991;24(1):59–71.
- , , . Depression following myocardial infarction: impact on 6‐month survival. JAMA. 1993;270(15):1819–1825.
- , , , , . Gender, depression, and one‐year prognosis after myocardial infarction. Psychosom Med. 1999;61(1):26–37.
- , , , . Depression and 1‐year prognosis in unstable angina. Arch Intern Med. 2000;160(9):1354–1360.
- , , , et al. The impact of depressive symptoms on recovery and outcome of hospitalised COPD exacerbations. Eur Respir J. 2013;41(4):815–823.
- , , , et al. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. Am Heart J. 2005;150(5):961–967.
- , , , et al. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448.
- , , , et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration. 2012;84(1):36–43.
- , , . Depressive symptoms increase risk of rehospitalisation in heart failure patients with preserved systolic function. J Clin Nurs. 2009;18(13):1871–1877.
- , , . Depressive symptoms and 3 year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. A positive 2‐item patient health questionnaire depression screen among hospitalized heart failure patients is associated with elevated 12‐month mortality. J Card Fail. 2012;18(3):238–245.
- , , , et al. Post‐discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378–384.
- , . Use of health services by medically ill depressed elderly patients after hospital discharge. Am J Geriatr Psychiatry. 1999;7(1):48–56.
- , , . Congestive heart failure and depression in older adults: clinical course and health services use 6 months after hospitalization. Psychosomatics. 2003;44(5):367–373.
- , , , . Depressive symptoms as a predictor of 6‐month outcomes and services utilization in elderly medical inpatients. Arch Intern Med. 2001;161(21):2609–2615.
- , , , et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129(12):1350–1369.
- , , , , , . Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta‐analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33(3):203–216.
- , , , , . Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925.
- , , , et al. Prognostic value of depression, anxiety, and anger in hospitalized cardiovascular disease patients for predicting adverse cardiac outcomes. Am J Cardiol. 2013;111(10):1432–1436.
- , , . The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
- , , , et al. The influence of somatic symptoms on beck depression inventory scores in hospitalized postmyocardial infarction patients. Can J Psychiatry. 2012;57(12):752–758.
- , , , et al. Somatic symptom overlap in beck depression inventory‐II scores following myocardial infarction. Br J Psychiatry. 2010;197(1):61–66.
- , , , . Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54(3):317–329.
- , , . Searching for unpublished data for Cochrane reviews: cross sectional study. BMJ. 2013;346:f2231.
- . Comment on: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39(3):932; author reply 933.
Between 10% and 40% of patients are readmitted after being discharged from the hospital,[1, 2] and as many as another 25% return to the emergency department (ED) within 30 days.[3] This creates a substantial burden on the healthcare system.[2] Various interventions have been tried to improve the quality of discharge transitions and reduce readmission rates, but results thus far have been inconsistent and generally disappointing.[4, 5, 6] Targeted delivery of interventions to those at highest risk might improve the effectiveness of these efforts and reduce costs. However, current readmission risk assessment models are only moderately predictive, suggesting the presence of unrecognized risk factors.[7, 8]
Active depression might represent a potentially modifiable independent predictor of adverse short‐term hospital outcomes that is currently underutilized. Depression occurs in 5% to 58% of hospitalized adults, depending on how cases are defined.[9, 10] Depression is often under‐recognized and undertreated in acute care clinical settings,[11] and relatively few readmission prediction models incorporate mental health related symptoms.[12]
Although several reviews have examined methods of screening for depression in hospitalized patients[9] or the effectiveness of screening in primary care,[13, 14] to our knowledge no systematic review has examined the impact of depression on short‐term prognosis after discharge from acute care. Therefore, the purpose of this systematic review was to summarize all studies that evaluated whether hospitalized medical patients with depressive symptoms are at higher risk of 30‐day all‐cause readmission or all‐cause mortality after being discharged from the hospital.
METHODS
This study followed an a priori protocol developed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) criteria.[15]
Data Sources and Search Methods
We searched the Cumulative Index to Nursing and Allied Health Literature, Ovid MEDLINE, Ovid Embase, and PsycINFO from inception to January 9, 2015, and the last 5 years of PubMed for full publications with any of the following Medical Subject Headings: depressive disorder, depression, patient readmission, interviews, psychological, inpatients, with restrictions for peer‐reviewed publication, humans, adults aged 18 years, and the English language. Search strategies were developed with a librarian (available upon request). We manually searched reference lists of all included studies and relevant review articles and contacted content experts to identify additional publications.
Eligibility Criteria and Selection of Studies
Two authors (J.L.P. and L.M.W.) independently screened full texts of all relevant articles for inclusion. Disagreements were resolved by consensus or a third reviewer (S.R.M.). We considered any original research that compared readmission or mortality after discharge for hospitalized medical patients (ie, general patients or subgroups thereof) with versus without depression identified by any validated depression measure,[16] including any study design that incorporated at least 30‐day follow‐up postdischarge. We excluded studies that examined patients hospitalized in nonacute care settings or on surgical, psychiatric, obstetric, or intensive care services. We calculated Cohen's coefficient to evaluate inter‐rater agreement on study selection.
Data Extraction
Data were abstracted by 2 authors (J.L.P. and L.M.W.). Disagreements were resolved by consensus or a third reviewer (S.R.M.). We contacted authors of all included studies to obtain missing data. If unavailable, crude data were estimated from published survival curves employing validated techniques in R (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria) and Digitizeit (
Data Synthesis and Statistical Analysis
Where possible, we calculated the pooled risk ratio (RR) with 95% confidence interval (95% CI) using a random effects models in Review Manager (RevMan) 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark). The random effects approach that we employed assumes heterogeneity (ie, underlying parameters vary between individual studies) and is distributed around a mean or population average effect, and results in more conservative (wider) confidence intervals, wherein larger cohorts (or studies with smaller standard errors) are given more weight. Heterogeneity was assessed using the I2 statistic, with values of 25%, 25% to 50%, and >50% representing low, moderate, and high heterogeneity.[19] As per the guidance of Higgins et al., we did not a priori define any degree of heterogeneity that would preclude pooling of the data; the expectation would be that heterogeneity would be substantially higher pooling observational studies rather than randomized trials.[19] Statistical significance was considered a 2‐sided P value 0.05.
Quality Assessment and Risk of Bias
We assessed study quality using the 9‐item Newcastle‐Ottawa scale with 0 to 3, 4 to 6, and 7 to 9 stars considered low, moderate, and high quality, respectively, and criteria for external and internal validity, including group selection and comparability, outcome assessment, and adequacy of follow‐up.[20] Adjusted estimates published in individual reports (or obtained directly from authors) were compared wherever possible with unadjusted estimates to assess the degree of confounding. We generated funnel plots in RevMan 5.3 and conducted Egger tests using Stata 13 (StataCorp LP, College Station, TX) to assess for publication bias.[21]
RESULTS
Study Selection
After removing duplicate publications, we identified 4066 reports and reviewed 133 reports in full text (see Supporting Figure 1 in the online version of this article). Despite our broad study inclusion criteria, we found only 35 longitudinal studies addressing this question. All 35 authors were contacted for additional outcomes data and other missing information (response rate of 34%). We had to exclude 17 studies as they did not provide 30 or 90‐day post‐discharge outcomes. Only 4 studies had published crude data for outcomes within 90 days,[22, 23, 24, 25] but after contact with authors, we received unpublished data for a further 7 studies[26, 27, 28, 29, 30, 31, 32] (including individual level data for 2 cohorts).[31, 32] We were able to estimate crude data from Kaplan‐Meier curves for another 3 studies.[33, 34, 35] Another 4 studies did not collect the outcomes we were interested in individually. These studies were included in this systematic review but are not poolable in our models: 3 authors could only provide composite endpoint data,[36, 37, 38] and 1 author provided unadjusted hazard ratios.[39] Inter‐reviewer agreement for inclusion was 80% (Cohen's = 0.60).
Characteristics of Included Studies
The 18 studies ranged in size from 58 to 1418 patients; 13 were cohort studies and 5 included secondary data from randomized control trials.[22, 27, 30, 34, 36] All studies ascertained depressive status by screening during index medical admission with either diagnostic interview or self‐report questionnaires, although a variety of scales and definitions for depression were used (Beck Depression Inventory [BDI] in 6 studies, Geriatric Depression Scale in 5 studies, Patient Health Questionnaire in another 4 studies, Medical Outcomes Study‐Depression Questionnaire in 1 study, and Center for Epidemiologic Studies Depression Scale in another study) (Table 1). Screening interviews were conducted mostly by research assistants or nurses (68%) or self‐administered (21%). Most studies examined specific medical patient subgroups (10 cardiac, 3 pulmonary, and 2 elderly). Major exclusion criteria reported were terminal illness (4 studies), unstable condition (6 studies), severe cognitive impairment (5 studies), and suicidal ideation or known depression (4 studies); 1 study enrolled patients with suspected depression (Table 1). Patient cohorts were on average older (range, 5082 years) (Table 1). Attrition rates for readmission and mortality data were low (average 1% among entire sample of studies). All studies scored at least 5 on the Newcastle‐Ottawa scale and were thus considered of at least moderate quality (see Supporting Table 1 in the online version of this article).
| Author, Date of Publication, Enrollment Period | Setting Country/Region, No. of Hospitals | No. of Inpatients, Clinical Features | Major Exclusion Criteria | Follow‐up, mo | Depression Measure (Cutoff) and Screening Method | Mean Age (SD), y | % Female | Positive Screen, No. (%) | Primary Outcome, Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Studies that use a scale based on DSM‐III criteria or a diagnostic interview according to DSM‐III criteria | |||||||||
| Frasure‐Smith et al.,[26] 1993, 19911992* | Canada/Quebec, 1 urban teaching | 218, AMI | Terminal noncardiac illness, unstable, not cognitive | 6 | BDI (10); mod DIS by interviewer, after transfer to medicine | 60 (range, 2488) | 22 | 68 (31), 35 (16) | All‐cause mortality |
| Frasure‐Smith et al.,[27] 1999, 19911992,* 19911994 | Canada/Quebec, 1 urban teaching, 10 urban area | 218; 78, AMI | Terminal noncardiac illness, unstable, not cognitive | 12 | BDI (10) by interviewer, after transfer to medicine | 60 (11) | 32 | 290 (32) | Cardiac mortality |
| Freedland et al.,[25] 1991, 1990 | USA/MO, 1 urban teaching | 58, CHF 75 years | Dementia, medically unstable | 3 | Mod DIS by psychiatric residents and interviewer | 78 (6) | 57 | 10 (17) | All‐cause readmission, all‐cause mortality |
| Fulop et al.,[38] 2003, 2002 | USA/NY, 1 urban teaching | 203, CHF 65 years | 1, 6 | GDS (10); SCID‐NP by interviewer, at discharge | 77 (8) | 53 | 73 (36), 44 (22) | Depression, composite PCP, ED, care visits, and readmission | |
| Lesprance et al.,[28] 2000, 19941996 | Canada/Quebec, 1 urban teaching | 430, unstable angina | Terminal noncardiac illness, not cognitive, recent CABG | 12 | BDI (10); mod DIS by interviewer, 5 days after admission | 62 (11) | 29 | 178 (41), 120 (28) | Cardiac death and MI, any death, angina readmission |
| Rumsfeld et al.,[30] 2005, 19992001 | CA, USA, UK, multiple | 634, AMI with CHF | Valvular or congenital heart failure | Up to 32 | MOS‐D (0.06) by interviewer, before discharge | 65 (11) | 28 | 143 (23) | All‐cause death, CVD death and readmission |
| Song et al.,[33] 2009, 2005 | South Korea, 2 urban teaching | 165, HF | If minor criteria for HF attributable to other medical condition | 6 | BDI (10) self‐administer or interviewer, 34 days of admin | 62 (13) | 49 | 131 (79) | HF readmission and all‐cause mortality, HF readmit |
| Papaioannou et al.,[29] 2013, 20092010 | Greece/Athens, 1 urban | 230, AECOPD | Other respiratory illness, known depressed | Monthly up to 12 | BDI‐I (19) self‐administer, first day | 71 (9) | 12 | 91 (40) | All‐cause mortality, AECOPD readmission |
| Studies that use a scale based on or validated against DSM‐IV criteria or a diagnostic interview according to DSM‐IV criteria | |||||||||
| Almagro et al.,[31] 2002, 19961997 | Spain, 1 urban teaching | 130, AECOPD | Other pulmonary disease | July 1999 | GDS‐SF (6) by interviewer, day before discharge | 72 (9) | 8 | 43 (33) | All‐cause mortality |
| Almagro et al.,[32] 2012, 20032004 | Spain, 1 urban teaching | 134, AECOPD | Other pulmonary disease | 1, 36 | GDS‐SF (6) by interviewer | 72 (10) | 5 | 55 (41) | All‐cause mortality, lung function, frailty |
| Bla et al.,[39] 2001, 2000 | Switzerland, 1 urban teaching | 401, medical 75 years | Stay 24 hours, elective/facility transfer, unstable, not cognitive | 6 | GDS‐SF (6) by interviewer, within 2 days of admission | 82 (7599) | 61 | 90 (22) | All‐cause readmission, all‐cause mortality |
| Cancino et al.,[22] 2014, 20062007,* 20082009 | USA/MA, 1 urban tertiary | 680; 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1 | PHQ‐9 (5 or severity) by interviewer, on admin | 50 (14) | 51 | 561 (40) | All‐cause readmission, ED visits, PCP visits |
| Mitchell et al.,[36] 2010, 20062007* | USA/MA, 1 urban tertiary | 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1, 2, 3 | PHQ‐9 (5) by interviewer, on admin | 50 (15) | 50 | 238 (32) | ED visits and all‐cause readmission |
| Covinsky et al.,[34] 1999, 19901992 | USA/OH, 1 urban teaching | 573, medical | ICU, oncology, telemetry, nursing home admissions | 36 | GDS‐SF (6) by interviewer, within 2 days of admission | 80 | 68 | 197 (34) | All‐cause mortality |
| Jiang et al.,[23] 2001, 19971998 | USA/NC, 1 urban teaching | 357 (331 DIS only), CHF | Suicidal, planned surgery, pregnant | 3, 12 | BDI (10) self‐admin; mod DIS (+BDI only) by interviewer | 63 (13) | 33 | 126 (35), 46 (14) | All‐cause mortality, all‐cause readmission |
| Kartha et al.,[24] 2007, 20022004 | USA/MA, 1 urban safety net | 144, medical recently hospitalized | Planned readmission, unable to keep PCP appointments | 3 | PHQ‐9 (algorithm) by interviewer | 55 (16) | 56 | 39 (27) | All‐cause readmission |
| Koenig and Kuchbhatla,[37] 1999, 1997 | USA/NC, 1 urban teaching | 331, medical 60 years | Stay 3 or >7 days, ICU/CCU, severe illness, nursing home transfers | 3, 6, 9, 12 | CES‐D (16) or HAM‐D (11) or DIS by psychiatrist, on or after third day | 70 (7) | 51 | 160 (48) | Depression, composite physical disability, health visits, and all‐cause readmission |
| Rollman et al.,[35] 2012, 20072009 | USA/PA, 4 urban teaching | 471, CHF, suspected depressed | Antidepressants users (excluded from PHQ‐2 group only) | Up to 12 | PHQ‐2; PHQ‐9 (5 in +PHQ‐2), by interviewer, 4 days | 66 (13) | 35 | 371 (79), 351 (74) | All‐cause mortality |
Prevalence and Recognition of Depressive Symptoms
The range of depression prevalence in hospitalized medical patients was 14% to 79%, with a median of 32% (interquartile range, 27%40%) (Table 1). In those studies that used a diagnostic interview, the prevalence tended to be lower for major depression, with a median of 17% (interquartile range, 16%22%) (Table 1). None of the included studies reported frequency of clinically recognized depression (ie, prior to screening for the study). Only 2 studies assessed the persistence of depression after discharge: 1 reported that depression persisted in 53% (by screening questionnaire) and 34% (by diagnostic interview) of patients at 30 days,[38] whereas the other reported 48% persistence at 90 days after discharge according to a combined screening method.[37]
Hospital Readmission
Overall, 8 studies provided readmission data. Among patients discharged from acute care medical wards (4 studies reporting on 5 cohorts), 395 of 2433 (16.2%) patients were readmitted within 30 days (Figure 1). Hospitalized patients with depressive symptoms were more likely to be readmitted within 30 days after discharge (20.4% vs 13.7%, RR: 1.73, 95% CI: 1.16‐2.58, P = 0.007, I2 = 55%) (Figure 1), compared to those without depression. Results were consistent for 90‐day readmissions (39.8% vs 31.0%, RR: 1.68, 95% CI: 1.13‐2.50, P = 0.01, I2 = 76%, n = 1543 patients) (see Supporting Figure 2 in the online version of this article) in 6 studies. One individual study examined readmission within 6 months after discharge, but was not poolable in this model, as it presented only hazard ratios and not raw data; however, it did report a 50% increased risk of readmission in medical inpatients aged 75 years (adjusted hazard ratio: 1.50, 95% CI: 1.03‐2.17, n = 401).[39]

Forest plot presents results of the meta‐analysis in which the size of each data marker indicates the weight assigned to individuals studies. Abbreviations: CI, confidence interval; IV, independent variable.
Mortality After Discharge
Overall, 11 studies provided all‐cause mortality data. Among medical patients discharged from acute care in 9 studies, 69 of 3397 (2.0%) patients died within 30 days (Figure 2). Medical patients discharged with depressive symptoms were more likely to die within 30 days (2.8% vs 1.5%, RR: 2.13, 95% CI: 1.31‐3.44, P = 0.002, I2 = 0%) (Figure 2) compared to those without depression. Similar results were found for 90‐day mortality (7.7% vs 4.1%, RR: 2.01, 95% CI: 1.47‐2.76, P 0.001, I2 = 4%, n = 3784 patients) (see Supporting Figure 3 in the online version of this article) in 11 studies.

ED and PCP Visits
Four studies examined the use of ED or PCP services within 90 days of discharge, but 3 did not have extractable data for meta‐analysis. All showed increased utilization of health services for depressed compared to nondepressed patients after discharge.[22, 36, 37, 38] Depressed patients were more likely to visit the ED (adjusted incidence rate ratio: 1.73, 95% CI: 1.27‐2.36),[36] had significantly more medical encounters (eg, PCP, ED visits, hospital admissions, laboratory tests, and home care [mean 2.9 vs 2.6, P = 0.05])[38] and had a greater number of ED visits alone (27 vs 15 per 100 patients, P = 0.007)[22] within 30 days of hospital discharge compared to nondepressed patients. Similar results were found at 90 days.[36]
Sensitivity Analyses
All told, most studies reported a positive association between depression and adverse events, and this was true regardless of how much adjustment for potential confounding had been undertaken by the authors. Although all studies were qualitatively in the same direction, the magnitude of the association varied due to methodological and/or clinical heterogeneity. Sensitivity analysis revealed no overall difference in pooled risk ratios or heterogeneity between Mantel‐Haenszel fixed effects versus random effects models or with the addition of 0.5 to cells to permit inclusion of zero‐event data. There was no evidence of publication bias; funnel plots and Egger test results are available upon request. There were no statistically significant differences in the risk associated with depressive symptoms whether studies used Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III or DSM‐IV criteria, whether the study samples were disease specific or unselected general medical cohorts, whether studies were of moderate or high quality, or regardless of the severity of depressive symptoms.
DISCUSSION
Summary of Evidence
We found that depression was common in medical inpatients (about one‐third of all patients) and persisted for at least 30 days in up to half of those patients after discharge. We found strong evidence of an association between depressive symptoms and poor short‐term prognosis after discharge from the hospital: a 73% increased risk of readmission and a 2‐fold risk of death within 30 days compared to patients without depressive symptoms with similar results at 90 days.
Our meta‐analysis complements a recent systematic review that found concomitant depression to be a risk factor for poor prognosis among inpatients and outpatients with acute coronary syndrome,[40] and a meta‐analysis that demonstrated an increased risk of 2‐year mortality among patients with depression after myocardial infarction.[41] To our knowledge, our study is the first to quantify the short‐term postdischarge risks across a diverse group of medical inpatients.
The potential mechanisms underlying the observed relationship between depression and adverse patient outcomes after discharge are likely multiple. We believe there are 2 main possibilities. First, the increased risk associated with depression might be due to residual confounding, even though many of these studies did adjust for extensive lists of comorbidities,[22, 24, 26, 27, 29, 30, 33, 35, 36, 39] including functional status[39] and prior health services utilization.[22, 34, 36] This could occur if other risk factors were not sufficiently adjusted for, such as unrecognized comorbidities or concomitant disability, which are often present among chronically ill patients,[42] or if depression were a marker of psychosocial risk factors such as anxiety,[43] stress or poor resiliency,[44] or low social support,[45] though a few adjusted for psychosocial factors such as social support[26] or anxiety.[35] Confounding could also occur if symptoms of acute illness inflate reports of somatic symptoms of depression on self‐report questionnaires. Recent studies on the BDI, found that scores were higher in postmyocardial infarction patients when compared to outpatient controls,[46] but with no differences between those groups in scores for the BDI‐II,[47] a version with fewer somatic symptom questions.
Second, depression may cause adverse outcomes through indirect or direct pathways. Indirect causation could occur if depression hindered self‐care behaviors such as medication adherence.[42] Depression could also act directly through pathophysiological changes. Some studies have suggested that depression is associated with metabolic abnormalities, including alterations in glucose transport[42, 48] and increased vulnerability to obesity, type 2 diabetes mellitus, and/or diabetic complications, common conditions among hospitalized patients that also adversely affect postdischarge outcomes.[40, 48]
Strengths and Limitations
This review has multiple strengths. We cast a broad search and included studies that examined a wide range of medical patient subgroups, thus increasing the generalizability of our findings. We identified a general scarcity of studies on this topic and obtained additional unpublished data for 10 of the 18 relevant studies, and our response rate of 34% is compatible with the 37% response rate reported for Cochrane reviews when seeking additional data from authors.[49] Whether examined qualitatively (vote counting of the number of studies that showed an association) or quantitatively (via formal meta‐analysis), it seems apparent that there is a clinically important association between depression and postdischarge adverse events, but given the number, quality, and heterogeneity of the studies we examined, there may be some ongoing dispute about exactly how strong this association is and the degree of bias contributed by a couple of large studies of the topic.
There are limitations to our review. First, as we did not have individual‐level patient data, we could not use metaregression to explore sources of heterogeneity (clinical or methodological) or adjust for confounding, and this likely contributes to observed differences between individual estimates. For instance, the included studies had heterogeneous screening measures and cutoffs; thus, all cases of depression in these studies might not be equivalent. Some of the included studies assessed depression early during admission where psychological distress may be greatest; others assessed symptoms closer to discharge. Most studies included patients with specific conditions like heart failure or chronic obstructive pulmonary disease rather than a wide spectrum of medical inpatients. Moreover, few studies adjusted for psychosocial risk factors such as social support, anxiety, and functional status, and only 2 studies assessed the persistence of depressive symptoms after discharge. Second, we did not explore quantitative measures of between‐study variation (eg, I2), because experts question its utility given the expected heterogeneity in meta‐analyses of observational studies.[50] Third, although the included studies were deemed to be of at least moderate quality, they could be at risk for sources of bias that may not be sufficiently appraised by the current version of the Newscastle‐Ottawa scale for observational studies. Finally, we excluded grey literature (eg, conference proceedings or technical reports) that could potentially exclude null findings, although we did contact authors in this field to identify additional unpublished data relevant to this topic.
CONCLUSIONS
We have confirmed that depressive symptoms are common in hospitalized medical patients, frequently persist after discharge, and may predict greater risk of readmission or death after discharge. Thus, depressive symptoms are an additional marker that clinicians can use to help identify patients in acute care medical settings who may be at increased risk for suboptimal transition back to the community and who may require additional resources after discharge. However, future research is required to evaluate whether treatment of individuals who screen positive for depressive symptoms can reduce 30‐day readmission rates, and we are aware of at least 1 relevant ongoing trial (
Acknowledgements
The authors thank the following individuals: Dale Storie, MLIS, Saskatchewan Information and Library Services Consortium, Regina, Saskatchewan, Canada, for assistance in the literature search; James A. Hanley, PhD, Department of Epidemiology and Biostatistics, Faculty of Medicine, McGill University, Montreal, Quebec, Canada, for guidance in data recovery methods; Nancy Frasure‐Smith, PhD, Department of Psychiatry, McGill University, Department of Psychiatry and Research Centre Hospital Centre, University of Montreal, and Montreal Heart Institute Research Centre, Montreal, Quebec, Canada; Andriana I. Papaioannou, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; Konstantinos Kostikas, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; and Pere Almagro, MD, Servicio de Medicina Interna, Hospital Universitario Mutua de Terrassa, Terrassa, Barcelona, Spain; as well as Philip G. Jones, MS, Saint Luke's Mid America Heart Institute, Kansas City, Missouri; for their retrieval and contribution of unpublished data.
Disclosures
Ms. Pederson affirms that the manuscript is an honest, accurate, and transparent account of the study being reported with no important omissions. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. Design and conduct of the study: Ms. Pederson, Drs. Majumdar and McAlister. Data acquisition: Ms. Pederson, Ms. Warkentin. Analysis and interpretation of the data and drafting of the manuscript: Ms Pederson, Drs. Majumdar and McAlister. Review of the manuscript: all authors. Study supervision: Drs. Majumdar and McAlister. None of the contributors received compensation for their efforts. Salary support for Ms. Pederson was provided by a CRIO grant from Alberta InnovatesHealth Solutions. Drs. McAlister and Majumdar are supported by salary awards from Alberta Innovates‐Health Solutions. Dr. McAlister holds the University of Alberta/Capital Health Chair in Cardiology Outcomes Research. Dr. Majumda holds the University of Alberta Endowed Chair in Patient Health Management. The funding sources had no role in the design or conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. This work is that of the authors independent of funders. The authors report no conflicts of interest.
Between 10% and 40% of patients are readmitted after being discharged from the hospital,[1, 2] and as many as another 25% return to the emergency department (ED) within 30 days.[3] This creates a substantial burden on the healthcare system.[2] Various interventions have been tried to improve the quality of discharge transitions and reduce readmission rates, but results thus far have been inconsistent and generally disappointing.[4, 5, 6] Targeted delivery of interventions to those at highest risk might improve the effectiveness of these efforts and reduce costs. However, current readmission risk assessment models are only moderately predictive, suggesting the presence of unrecognized risk factors.[7, 8]
Active depression might represent a potentially modifiable independent predictor of adverse short‐term hospital outcomes that is currently underutilized. Depression occurs in 5% to 58% of hospitalized adults, depending on how cases are defined.[9, 10] Depression is often under‐recognized and undertreated in acute care clinical settings,[11] and relatively few readmission prediction models incorporate mental health related symptoms.[12]
Although several reviews have examined methods of screening for depression in hospitalized patients[9] or the effectiveness of screening in primary care,[13, 14] to our knowledge no systematic review has examined the impact of depression on short‐term prognosis after discharge from acute care. Therefore, the purpose of this systematic review was to summarize all studies that evaluated whether hospitalized medical patients with depressive symptoms are at higher risk of 30‐day all‐cause readmission or all‐cause mortality after being discharged from the hospital.
METHODS
This study followed an a priori protocol developed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) criteria.[15]
Data Sources and Search Methods
We searched the Cumulative Index to Nursing and Allied Health Literature, Ovid MEDLINE, Ovid Embase, and PsycINFO from inception to January 9, 2015, and the last 5 years of PubMed for full publications with any of the following Medical Subject Headings: depressive disorder, depression, patient readmission, interviews, psychological, inpatients, with restrictions for peer‐reviewed publication, humans, adults aged 18 years, and the English language. Search strategies were developed with a librarian (available upon request). We manually searched reference lists of all included studies and relevant review articles and contacted content experts to identify additional publications.
Eligibility Criteria and Selection of Studies
Two authors (J.L.P. and L.M.W.) independently screened full texts of all relevant articles for inclusion. Disagreements were resolved by consensus or a third reviewer (S.R.M.). We considered any original research that compared readmission or mortality after discharge for hospitalized medical patients (ie, general patients or subgroups thereof) with versus without depression identified by any validated depression measure,[16] including any study design that incorporated at least 30‐day follow‐up postdischarge. We excluded studies that examined patients hospitalized in nonacute care settings or on surgical, psychiatric, obstetric, or intensive care services. We calculated Cohen's coefficient to evaluate inter‐rater agreement on study selection.
Data Extraction
Data were abstracted by 2 authors (J.L.P. and L.M.W.). Disagreements were resolved by consensus or a third reviewer (S.R.M.). We contacted authors of all included studies to obtain missing data. If unavailable, crude data were estimated from published survival curves employing validated techniques in R (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria) and Digitizeit (
Data Synthesis and Statistical Analysis
Where possible, we calculated the pooled risk ratio (RR) with 95% confidence interval (95% CI) using a random effects models in Review Manager (RevMan) 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark). The random effects approach that we employed assumes heterogeneity (ie, underlying parameters vary between individual studies) and is distributed around a mean or population average effect, and results in more conservative (wider) confidence intervals, wherein larger cohorts (or studies with smaller standard errors) are given more weight. Heterogeneity was assessed using the I2 statistic, with values of 25%, 25% to 50%, and >50% representing low, moderate, and high heterogeneity.[19] As per the guidance of Higgins et al., we did not a priori define any degree of heterogeneity that would preclude pooling of the data; the expectation would be that heterogeneity would be substantially higher pooling observational studies rather than randomized trials.[19] Statistical significance was considered a 2‐sided P value 0.05.
Quality Assessment and Risk of Bias
We assessed study quality using the 9‐item Newcastle‐Ottawa scale with 0 to 3, 4 to 6, and 7 to 9 stars considered low, moderate, and high quality, respectively, and criteria for external and internal validity, including group selection and comparability, outcome assessment, and adequacy of follow‐up.[20] Adjusted estimates published in individual reports (or obtained directly from authors) were compared wherever possible with unadjusted estimates to assess the degree of confounding. We generated funnel plots in RevMan 5.3 and conducted Egger tests using Stata 13 (StataCorp LP, College Station, TX) to assess for publication bias.[21]
RESULTS
Study Selection
After removing duplicate publications, we identified 4066 reports and reviewed 133 reports in full text (see Supporting Figure 1 in the online version of this article). Despite our broad study inclusion criteria, we found only 35 longitudinal studies addressing this question. All 35 authors were contacted for additional outcomes data and other missing information (response rate of 34%). We had to exclude 17 studies as they did not provide 30 or 90‐day post‐discharge outcomes. Only 4 studies had published crude data for outcomes within 90 days,[22, 23, 24, 25] but after contact with authors, we received unpublished data for a further 7 studies[26, 27, 28, 29, 30, 31, 32] (including individual level data for 2 cohorts).[31, 32] We were able to estimate crude data from Kaplan‐Meier curves for another 3 studies.[33, 34, 35] Another 4 studies did not collect the outcomes we were interested in individually. These studies were included in this systematic review but are not poolable in our models: 3 authors could only provide composite endpoint data,[36, 37, 38] and 1 author provided unadjusted hazard ratios.[39] Inter‐reviewer agreement for inclusion was 80% (Cohen's = 0.60).
Characteristics of Included Studies
The 18 studies ranged in size from 58 to 1418 patients; 13 were cohort studies and 5 included secondary data from randomized control trials.[22, 27, 30, 34, 36] All studies ascertained depressive status by screening during index medical admission with either diagnostic interview or self‐report questionnaires, although a variety of scales and definitions for depression were used (Beck Depression Inventory [BDI] in 6 studies, Geriatric Depression Scale in 5 studies, Patient Health Questionnaire in another 4 studies, Medical Outcomes Study‐Depression Questionnaire in 1 study, and Center for Epidemiologic Studies Depression Scale in another study) (Table 1). Screening interviews were conducted mostly by research assistants or nurses (68%) or self‐administered (21%). Most studies examined specific medical patient subgroups (10 cardiac, 3 pulmonary, and 2 elderly). Major exclusion criteria reported were terminal illness (4 studies), unstable condition (6 studies), severe cognitive impairment (5 studies), and suicidal ideation or known depression (4 studies); 1 study enrolled patients with suspected depression (Table 1). Patient cohorts were on average older (range, 5082 years) (Table 1). Attrition rates for readmission and mortality data were low (average 1% among entire sample of studies). All studies scored at least 5 on the Newcastle‐Ottawa scale and were thus considered of at least moderate quality (see Supporting Table 1 in the online version of this article).
| Author, Date of Publication, Enrollment Period | Setting Country/Region, No. of Hospitals | No. of Inpatients, Clinical Features | Major Exclusion Criteria | Follow‐up, mo | Depression Measure (Cutoff) and Screening Method | Mean Age (SD), y | % Female | Positive Screen, No. (%) | Primary Outcome, Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Studies that use a scale based on DSM‐III criteria or a diagnostic interview according to DSM‐III criteria | |||||||||
| Frasure‐Smith et al.,[26] 1993, 19911992* | Canada/Quebec, 1 urban teaching | 218, AMI | Terminal noncardiac illness, unstable, not cognitive | 6 | BDI (10); mod DIS by interviewer, after transfer to medicine | 60 (range, 2488) | 22 | 68 (31), 35 (16) | All‐cause mortality |
| Frasure‐Smith et al.,[27] 1999, 19911992,* 19911994 | Canada/Quebec, 1 urban teaching, 10 urban area | 218; 78, AMI | Terminal noncardiac illness, unstable, not cognitive | 12 | BDI (10) by interviewer, after transfer to medicine | 60 (11) | 32 | 290 (32) | Cardiac mortality |
| Freedland et al.,[25] 1991, 1990 | USA/MO, 1 urban teaching | 58, CHF 75 years | Dementia, medically unstable | 3 | Mod DIS by psychiatric residents and interviewer | 78 (6) | 57 | 10 (17) | All‐cause readmission, all‐cause mortality |
| Fulop et al.,[38] 2003, 2002 | USA/NY, 1 urban teaching | 203, CHF 65 years | 1, 6 | GDS (10); SCID‐NP by interviewer, at discharge | 77 (8) | 53 | 73 (36), 44 (22) | Depression, composite PCP, ED, care visits, and readmission | |
| Lesprance et al.,[28] 2000, 19941996 | Canada/Quebec, 1 urban teaching | 430, unstable angina | Terminal noncardiac illness, not cognitive, recent CABG | 12 | BDI (10); mod DIS by interviewer, 5 days after admission | 62 (11) | 29 | 178 (41), 120 (28) | Cardiac death and MI, any death, angina readmission |
| Rumsfeld et al.,[30] 2005, 19992001 | CA, USA, UK, multiple | 634, AMI with CHF | Valvular or congenital heart failure | Up to 32 | MOS‐D (0.06) by interviewer, before discharge | 65 (11) | 28 | 143 (23) | All‐cause death, CVD death and readmission |
| Song et al.,[33] 2009, 2005 | South Korea, 2 urban teaching | 165, HF | If minor criteria for HF attributable to other medical condition | 6 | BDI (10) self‐administer or interviewer, 34 days of admin | 62 (13) | 49 | 131 (79) | HF readmission and all‐cause mortality, HF readmit |
| Papaioannou et al.,[29] 2013, 20092010 | Greece/Athens, 1 urban | 230, AECOPD | Other respiratory illness, known depressed | Monthly up to 12 | BDI‐I (19) self‐administer, first day | 71 (9) | 12 | 91 (40) | All‐cause mortality, AECOPD readmission |
| Studies that use a scale based on or validated against DSM‐IV criteria or a diagnostic interview according to DSM‐IV criteria | |||||||||
| Almagro et al.,[31] 2002, 19961997 | Spain, 1 urban teaching | 130, AECOPD | Other pulmonary disease | July 1999 | GDS‐SF (6) by interviewer, day before discharge | 72 (9) | 8 | 43 (33) | All‐cause mortality |
| Almagro et al.,[32] 2012, 20032004 | Spain, 1 urban teaching | 134, AECOPD | Other pulmonary disease | 1, 36 | GDS‐SF (6) by interviewer | 72 (10) | 5 | 55 (41) | All‐cause mortality, lung function, frailty |
| Bla et al.,[39] 2001, 2000 | Switzerland, 1 urban teaching | 401, medical 75 years | Stay 24 hours, elective/facility transfer, unstable, not cognitive | 6 | GDS‐SF (6) by interviewer, within 2 days of admission | 82 (7599) | 61 | 90 (22) | All‐cause readmission, all‐cause mortality |
| Cancino et al.,[22] 2014, 20062007,* 20082009 | USA/MA, 1 urban tertiary | 680; 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1 | PHQ‐9 (5 or severity) by interviewer, on admin | 50 (14) | 51 | 561 (40) | All‐cause readmission, ED visits, PCP visits |
| Mitchell et al.,[36] 2010, 20062007* | USA/MA, 1 urban tertiary | 738, medical | Nursing home or hospital transfer, isolated, suicidal | 1, 2, 3 | PHQ‐9 (5) by interviewer, on admin | 50 (15) | 50 | 238 (32) | ED visits and all‐cause readmission |
| Covinsky et al.,[34] 1999, 19901992 | USA/OH, 1 urban teaching | 573, medical | ICU, oncology, telemetry, nursing home admissions | 36 | GDS‐SF (6) by interviewer, within 2 days of admission | 80 | 68 | 197 (34) | All‐cause mortality |
| Jiang et al.,[23] 2001, 19971998 | USA/NC, 1 urban teaching | 357 (331 DIS only), CHF | Suicidal, planned surgery, pregnant | 3, 12 | BDI (10) self‐admin; mod DIS (+BDI only) by interviewer | 63 (13) | 33 | 126 (35), 46 (14) | All‐cause mortality, all‐cause readmission |
| Kartha et al.,[24] 2007, 20022004 | USA/MA, 1 urban safety net | 144, medical recently hospitalized | Planned readmission, unable to keep PCP appointments | 3 | PHQ‐9 (algorithm) by interviewer | 55 (16) | 56 | 39 (27) | All‐cause readmission |
| Koenig and Kuchbhatla,[37] 1999, 1997 | USA/NC, 1 urban teaching | 331, medical 60 years | Stay 3 or >7 days, ICU/CCU, severe illness, nursing home transfers | 3, 6, 9, 12 | CES‐D (16) or HAM‐D (11) or DIS by psychiatrist, on or after third day | 70 (7) | 51 | 160 (48) | Depression, composite physical disability, health visits, and all‐cause readmission |
| Rollman et al.,[35] 2012, 20072009 | USA/PA, 4 urban teaching | 471, CHF, suspected depressed | Antidepressants users (excluded from PHQ‐2 group only) | Up to 12 | PHQ‐2; PHQ‐9 (5 in +PHQ‐2), by interviewer, 4 days | 66 (13) | 35 | 371 (79), 351 (74) | All‐cause mortality |
Prevalence and Recognition of Depressive Symptoms
The range of depression prevalence in hospitalized medical patients was 14% to 79%, with a median of 32% (interquartile range, 27%40%) (Table 1). In those studies that used a diagnostic interview, the prevalence tended to be lower for major depression, with a median of 17% (interquartile range, 16%22%) (Table 1). None of the included studies reported frequency of clinically recognized depression (ie, prior to screening for the study). Only 2 studies assessed the persistence of depression after discharge: 1 reported that depression persisted in 53% (by screening questionnaire) and 34% (by diagnostic interview) of patients at 30 days,[38] whereas the other reported 48% persistence at 90 days after discharge according to a combined screening method.[37]
Hospital Readmission
Overall, 8 studies provided readmission data. Among patients discharged from acute care medical wards (4 studies reporting on 5 cohorts), 395 of 2433 (16.2%) patients were readmitted within 30 days (Figure 1). Hospitalized patients with depressive symptoms were more likely to be readmitted within 30 days after discharge (20.4% vs 13.7%, RR: 1.73, 95% CI: 1.16‐2.58, P = 0.007, I2 = 55%) (Figure 1), compared to those without depression. Results were consistent for 90‐day readmissions (39.8% vs 31.0%, RR: 1.68, 95% CI: 1.13‐2.50, P = 0.01, I2 = 76%, n = 1543 patients) (see Supporting Figure 2 in the online version of this article) in 6 studies. One individual study examined readmission within 6 months after discharge, but was not poolable in this model, as it presented only hazard ratios and not raw data; however, it did report a 50% increased risk of readmission in medical inpatients aged 75 years (adjusted hazard ratio: 1.50, 95% CI: 1.03‐2.17, n = 401).[39]

Forest plot presents results of the meta‐analysis in which the size of each data marker indicates the weight assigned to individuals studies. Abbreviations: CI, confidence interval; IV, independent variable.
Mortality After Discharge
Overall, 11 studies provided all‐cause mortality data. Among medical patients discharged from acute care in 9 studies, 69 of 3397 (2.0%) patients died within 30 days (Figure 2). Medical patients discharged with depressive symptoms were more likely to die within 30 days (2.8% vs 1.5%, RR: 2.13, 95% CI: 1.31‐3.44, P = 0.002, I2 = 0%) (Figure 2) compared to those without depression. Similar results were found for 90‐day mortality (7.7% vs 4.1%, RR: 2.01, 95% CI: 1.47‐2.76, P 0.001, I2 = 4%, n = 3784 patients) (see Supporting Figure 3 in the online version of this article) in 11 studies.

ED and PCP Visits
Four studies examined the use of ED or PCP services within 90 days of discharge, but 3 did not have extractable data for meta‐analysis. All showed increased utilization of health services for depressed compared to nondepressed patients after discharge.[22, 36, 37, 38] Depressed patients were more likely to visit the ED (adjusted incidence rate ratio: 1.73, 95% CI: 1.27‐2.36),[36] had significantly more medical encounters (eg, PCP, ED visits, hospital admissions, laboratory tests, and home care [mean 2.9 vs 2.6, P = 0.05])[38] and had a greater number of ED visits alone (27 vs 15 per 100 patients, P = 0.007)[22] within 30 days of hospital discharge compared to nondepressed patients. Similar results were found at 90 days.[36]
Sensitivity Analyses
All told, most studies reported a positive association between depression and adverse events, and this was true regardless of how much adjustment for potential confounding had been undertaken by the authors. Although all studies were qualitatively in the same direction, the magnitude of the association varied due to methodological and/or clinical heterogeneity. Sensitivity analysis revealed no overall difference in pooled risk ratios or heterogeneity between Mantel‐Haenszel fixed effects versus random effects models or with the addition of 0.5 to cells to permit inclusion of zero‐event data. There was no evidence of publication bias; funnel plots and Egger test results are available upon request. There were no statistically significant differences in the risk associated with depressive symptoms whether studies used Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III or DSM‐IV criteria, whether the study samples were disease specific or unselected general medical cohorts, whether studies were of moderate or high quality, or regardless of the severity of depressive symptoms.
DISCUSSION
Summary of Evidence
We found that depression was common in medical inpatients (about one‐third of all patients) and persisted for at least 30 days in up to half of those patients after discharge. We found strong evidence of an association between depressive symptoms and poor short‐term prognosis after discharge from the hospital: a 73% increased risk of readmission and a 2‐fold risk of death within 30 days compared to patients without depressive symptoms with similar results at 90 days.
Our meta‐analysis complements a recent systematic review that found concomitant depression to be a risk factor for poor prognosis among inpatients and outpatients with acute coronary syndrome,[40] and a meta‐analysis that demonstrated an increased risk of 2‐year mortality among patients with depression after myocardial infarction.[41] To our knowledge, our study is the first to quantify the short‐term postdischarge risks across a diverse group of medical inpatients.
The potential mechanisms underlying the observed relationship between depression and adverse patient outcomes after discharge are likely multiple. We believe there are 2 main possibilities. First, the increased risk associated with depression might be due to residual confounding, even though many of these studies did adjust for extensive lists of comorbidities,[22, 24, 26, 27, 29, 30, 33, 35, 36, 39] including functional status[39] and prior health services utilization.[22, 34, 36] This could occur if other risk factors were not sufficiently adjusted for, such as unrecognized comorbidities or concomitant disability, which are often present among chronically ill patients,[42] or if depression were a marker of psychosocial risk factors such as anxiety,[43] stress or poor resiliency,[44] or low social support,[45] though a few adjusted for psychosocial factors such as social support[26] or anxiety.[35] Confounding could also occur if symptoms of acute illness inflate reports of somatic symptoms of depression on self‐report questionnaires. Recent studies on the BDI, found that scores were higher in postmyocardial infarction patients when compared to outpatient controls,[46] but with no differences between those groups in scores for the BDI‐II,[47] a version with fewer somatic symptom questions.
Second, depression may cause adverse outcomes through indirect or direct pathways. Indirect causation could occur if depression hindered self‐care behaviors such as medication adherence.[42] Depression could also act directly through pathophysiological changes. Some studies have suggested that depression is associated with metabolic abnormalities, including alterations in glucose transport[42, 48] and increased vulnerability to obesity, type 2 diabetes mellitus, and/or diabetic complications, common conditions among hospitalized patients that also adversely affect postdischarge outcomes.[40, 48]
Strengths and Limitations
This review has multiple strengths. We cast a broad search and included studies that examined a wide range of medical patient subgroups, thus increasing the generalizability of our findings. We identified a general scarcity of studies on this topic and obtained additional unpublished data for 10 of the 18 relevant studies, and our response rate of 34% is compatible with the 37% response rate reported for Cochrane reviews when seeking additional data from authors.[49] Whether examined qualitatively (vote counting of the number of studies that showed an association) or quantitatively (via formal meta‐analysis), it seems apparent that there is a clinically important association between depression and postdischarge adverse events, but given the number, quality, and heterogeneity of the studies we examined, there may be some ongoing dispute about exactly how strong this association is and the degree of bias contributed by a couple of large studies of the topic.
There are limitations to our review. First, as we did not have individual‐level patient data, we could not use metaregression to explore sources of heterogeneity (clinical or methodological) or adjust for confounding, and this likely contributes to observed differences between individual estimates. For instance, the included studies had heterogeneous screening measures and cutoffs; thus, all cases of depression in these studies might not be equivalent. Some of the included studies assessed depression early during admission where psychological distress may be greatest; others assessed symptoms closer to discharge. Most studies included patients with specific conditions like heart failure or chronic obstructive pulmonary disease rather than a wide spectrum of medical inpatients. Moreover, few studies adjusted for psychosocial risk factors such as social support, anxiety, and functional status, and only 2 studies assessed the persistence of depressive symptoms after discharge. Second, we did not explore quantitative measures of between‐study variation (eg, I2), because experts question its utility given the expected heterogeneity in meta‐analyses of observational studies.[50] Third, although the included studies were deemed to be of at least moderate quality, they could be at risk for sources of bias that may not be sufficiently appraised by the current version of the Newscastle‐Ottawa scale for observational studies. Finally, we excluded grey literature (eg, conference proceedings or technical reports) that could potentially exclude null findings, although we did contact authors in this field to identify additional unpublished data relevant to this topic.
CONCLUSIONS
We have confirmed that depressive symptoms are common in hospitalized medical patients, frequently persist after discharge, and may predict greater risk of readmission or death after discharge. Thus, depressive symptoms are an additional marker that clinicians can use to help identify patients in acute care medical settings who may be at increased risk for suboptimal transition back to the community and who may require additional resources after discharge. However, future research is required to evaluate whether treatment of individuals who screen positive for depressive symptoms can reduce 30‐day readmission rates, and we are aware of at least 1 relevant ongoing trial (
Acknowledgements
The authors thank the following individuals: Dale Storie, MLIS, Saskatchewan Information and Library Services Consortium, Regina, Saskatchewan, Canada, for assistance in the literature search; James A. Hanley, PhD, Department of Epidemiology and Biostatistics, Faculty of Medicine, McGill University, Montreal, Quebec, Canada, for guidance in data recovery methods; Nancy Frasure‐Smith, PhD, Department of Psychiatry, McGill University, Department of Psychiatry and Research Centre Hospital Centre, University of Montreal, and Montreal Heart Institute Research Centre, Montreal, Quebec, Canada; Andriana I. Papaioannou, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; Konstantinos Kostikas, MD, 2nd Respiratory Medicine Department, University of Athens Medical School, Athens, Greece; and Pere Almagro, MD, Servicio de Medicina Interna, Hospital Universitario Mutua de Terrassa, Terrassa, Barcelona, Spain; as well as Philip G. Jones, MS, Saint Luke's Mid America Heart Institute, Kansas City, Missouri; for their retrieval and contribution of unpublished data.
Disclosures
Ms. Pederson affirms that the manuscript is an honest, accurate, and transparent account of the study being reported with no important omissions. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. Design and conduct of the study: Ms. Pederson, Drs. Majumdar and McAlister. Data acquisition: Ms. Pederson, Ms. Warkentin. Analysis and interpretation of the data and drafting of the manuscript: Ms Pederson, Drs. Majumdar and McAlister. Review of the manuscript: all authors. Study supervision: Drs. Majumdar and McAlister. None of the contributors received compensation for their efforts. Salary support for Ms. Pederson was provided by a CRIO grant from Alberta InnovatesHealth Solutions. Drs. McAlister and Majumdar are supported by salary awards from Alberta Innovates‐Health Solutions. Dr. McAlister holds the University of Alberta/Capital Health Chair in Cardiology Outcomes Research. Dr. Majumda holds the University of Alberta Endowed Chair in Patient Health Management. The funding sources had no role in the design or conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. This work is that of the authors independent of funders. The authors report no conflicts of interest.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , . Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398–407.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . The elusive quest for quality and cost savings in the Medicare program. JAMA. 2009;301(6):668–670.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries—15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–111.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , . Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148–154.
- , , , et al. Prevalence, correlates and recognition of depression among inpatients of general hospitals in Wuhan, China. Gen Hosp Psychiatry. 2010;32(3):268–275.
- , , , , , . Recognition of depression by non‐psychiatric physicians—a systematic literature review and meta‐analysis. J Gen Intern Med. 2008;23(1):25–36.
- , , , , , . Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. Am Heart J. 2012;164(3):365–372.
- , , , et al. Does evidence support the American Heart Association's recommendation to screen patients for depression in cardiovascular care? An updated systematic review. PLoS One. 2013;8(1):e52654.
- , , , et al. Screening for depression: a systematic review and meta‐analysis. CMAJ Open. 2013;1(4):E159–E167.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94.
- , , , et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136(10):765–776.
- , , . Recovering the raw data behind a non‐parametric survival curve. Syst Rev. 2014;3:151.
- , , , . Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
- . Commentary: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160.
- , , , et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed September 1, 2015.
- , , . The funnel plot. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication Bias in Meta‐analysis: Prevention, Assessment and Adjustments. New York, NY: John Wiley 2006:73–98.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , et al. Depression in elderly patients with congestive heart failure. J Geriatr Psychiatry. 1991;24(1):59–71.
- , , . Depression following myocardial infarction: impact on 6‐month survival. JAMA. 1993;270(15):1819–1825.
- , , , , . Gender, depression, and one‐year prognosis after myocardial infarction. Psychosom Med. 1999;61(1):26–37.
- , , , . Depression and 1‐year prognosis in unstable angina. Arch Intern Med. 2000;160(9):1354–1360.
- , , , et al. The impact of depressive symptoms on recovery and outcome of hospitalised COPD exacerbations. Eur Respir J. 2013;41(4):815–823.
- , , , et al. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. Am Heart J. 2005;150(5):961–967.
- , , , et al. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448.
- , , , et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration. 2012;84(1):36–43.
- , , . Depressive symptoms increase risk of rehospitalisation in heart failure patients with preserved systolic function. J Clin Nurs. 2009;18(13):1871–1877.
- , , . Depressive symptoms and 3 year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. A positive 2‐item patient health questionnaire depression screen among hospitalized heart failure patients is associated with elevated 12‐month mortality. J Card Fail. 2012;18(3):238–245.
- , , , et al. Post‐discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378–384.
- , . Use of health services by medically ill depressed elderly patients after hospital discharge. Am J Geriatr Psychiatry. 1999;7(1):48–56.
- , , . Congestive heart failure and depression in older adults: clinical course and health services use 6 months after hospitalization. Psychosomatics. 2003;44(5):367–373.
- , , , . Depressive symptoms as a predictor of 6‐month outcomes and services utilization in elderly medical inpatients. Arch Intern Med. 2001;161(21):2609–2615.
- , , , et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129(12):1350–1369.
- , , , , , . Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta‐analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33(3):203–216.
- , , , , . Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925.
- , , , et al. Prognostic value of depression, anxiety, and anger in hospitalized cardiovascular disease patients for predicting adverse cardiac outcomes. Am J Cardiol. 2013;111(10):1432–1436.
- , , . The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
- , , , et al. The influence of somatic symptoms on beck depression inventory scores in hospitalized postmyocardial infarction patients. Can J Psychiatry. 2012;57(12):752–758.
- , , , et al. Somatic symptom overlap in beck depression inventory‐II scores following myocardial infarction. Br J Psychiatry. 2010;197(1):61–66.
- , , , . Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54(3):317–329.
- , , . Searching for unpublished data for Cochrane reviews: cross sectional study. BMJ. 2013;346:f2231.
- . Comment on: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39(3):932; author reply 933.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , , . Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008;21(5):398–407.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . The elusive quest for quality and cost savings in the Medicare program. JAMA. 2009;301(6):668–670.
- , , , . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries—15 randomized trials. JAMA. 2009;301(6):603–618.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–111.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , . Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148–154.
- , , , et al. Prevalence, correlates and recognition of depression among inpatients of general hospitals in Wuhan, China. Gen Hosp Psychiatry. 2010;32(3):268–275.
- , , , , , . Recognition of depression by non‐psychiatric physicians—a systematic literature review and meta‐analysis. J Gen Intern Med. 2008;23(1):25–36.
- , , , , , . Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. Am Heart J. 2012;164(3):365–372.
- , , , et al. Does evidence support the American Heart Association's recommendation to screen patients for depression in cardiovascular care? An updated systematic review. PLoS One. 2013;8(1):e52654.
- , , , et al. Screening for depression: a systematic review and meta‐analysis. CMAJ Open. 2013;1(4):E159–E167.
- , , , et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94.
- , , , et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136(10):765–776.
- , , . Recovering the raw data behind a non‐parametric survival curve. Syst Rev. 2014;3:151.
- , , , . Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
- . Commentary: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160.
- , , , et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed September 1, 2015.
- , , . The funnel plot. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication Bias in Meta‐analysis: Prevention, Assessment and Adjustments. New York, NY: John Wiley 2006:73–98.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , et al. Depression in elderly patients with congestive heart failure. J Geriatr Psychiatry. 1991;24(1):59–71.
- , , . Depression following myocardial infarction: impact on 6‐month survival. JAMA. 1993;270(15):1819–1825.
- , , , , . Gender, depression, and one‐year prognosis after myocardial infarction. Psychosom Med. 1999;61(1):26–37.
- , , , . Depression and 1‐year prognosis in unstable angina. Arch Intern Med. 2000;160(9):1354–1360.
- , , , et al. The impact of depressive symptoms on recovery and outcome of hospitalised COPD exacerbations. Eur Respir J. 2013;41(4):815–823.
- , , , et al. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. Am Heart J. 2005;150(5):961–967.
- , , , et al. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448.
- , , , et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration. 2012;84(1):36–43.
- , , . Depressive symptoms increase risk of rehospitalisation in heart failure patients with preserved systolic function. J Clin Nurs. 2009;18(13):1871–1877.
- , , . Depressive symptoms and 3 year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. A positive 2‐item patient health questionnaire depression screen among hospitalized heart failure patients is associated with elevated 12‐month mortality. J Card Fail. 2012;18(3):238–245.
- , , , et al. Post‐discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378–384.
- , . Use of health services by medically ill depressed elderly patients after hospital discharge. Am J Geriatr Psychiatry. 1999;7(1):48–56.
- , , . Congestive heart failure and depression in older adults: clinical course and health services use 6 months after hospitalization. Psychosomatics. 2003;44(5):367–373.
- , , , . Depressive symptoms as a predictor of 6‐month outcomes and services utilization in elderly medical inpatients. Arch Intern Med. 2001;161(21):2609–2615.
- , , , et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129(12):1350–1369.
- , , , , , . Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta‐analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33(3):203–216.
- , , , , . Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925.
- , , , et al. Prognostic value of depression, anxiety, and anger in hospitalized cardiovascular disease patients for predicting adverse cardiac outcomes. Am J Cardiol. 2013;111(10):1432–1436.
- , , . The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
- , , , et al. The influence of somatic symptoms on beck depression inventory scores in hospitalized postmyocardial infarction patients. Can J Psychiatry. 2012;57(12):752–758.
- , , , et al. Somatic symptom overlap in beck depression inventory‐II scores following myocardial infarction. Br J Psychiatry. 2010;197(1):61–66.
- , , , . Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54(3):317–329.
- , , . Searching for unpublished data for Cochrane reviews: cross sectional study. BMJ. 2013;346:f2231.
- . Comment on: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39(3):932; author reply 933.
Similar Outcomes From Weekend Discharge
Hospitals typically reduce staffing levels and the availability of diagnostic, laboratory, and treatment services on weekends, and patients admitted on weekends exhibit poorer in‐hospital outcomes for several medical conditions.[1, 2, 3, 4, 5, 6, 7, 8, 9] Whether or not patients discharged on weekends have worse clinical outcomes has been less well studied.[10, 11, 12] Discharge rates on Saturday and Sunday are lower than for the other 5 days of the week,[12] but bed shortages and hospital overcrowding have increased the demand for maximizing 24/7 week‐round discharge efficiency. Given that the number of patients discharged on weekends is likely to continue to increase, it is important to assess the risk of weekend discharge on outcomes monitored as performance indicators by organizations such as the Centers for Medicare and Medicaid Services, the American Medical Association Physicians Consortium for Performance Improvement, the National Quality Forum, and the Joint Commission.
Thus, we designed this study to evaluate baseline characteristics, length of stay (LOS), and postdischarge outcomes for general internal medicine (GIM) patients in teaching hospitals discharged on weekends compared to weekdays. Our objective was to determine whether postdischarge outcomes differed for patients discharged on weekends versus weekdays.
METHODS
Study Setting
The Canadian province of Alberta has a single vertically integrated healthcare system that is government‐funded and provides universal access to hospitals, emergency departments (EDs), and outpatient physician services for all 4.1 million Albertans as well as all prescription medications for the poor, socially disadvantaged, disabled, or those age 65 years and older. This study received approval from the University of Alberta Health Research Ethics Board with waiver of informed consent.
Data Sources
This study used deidentified linked data from 3 Alberta Health administrative databases that capture vital status and all hospital or ED visits and have previously been shown to have high accuracy for medical diagnoses.[13] The Alberta Health Care Insurance Plan Registry tracks date of death or emigration from the province. The Discharge Abstract Database includes the most responsible diagnosis identified by the hospital attending physician, up to 25 other diagnoses coded by nosologists in each hospital, the admission and discharge dates, and the admission category (elective or urgent/emergent) for all acute care hospitalizations. Of note, unlike US studies, the hospital databases are able to distinguish in‐hospital (eg, adverse events) versus premorbid diagnoses (eg, preexisting comorbidities). The Ambulatory Care Database captures all patient visits to EDs with coding for up to 10 conditions per encounter.
Study Cohort
We identified all adults with an acute care hospitalization on the GIM services at all 7 Alberta teaching hospitals (ie, defined as those with Royal College of Physicians and Surgeons of Canadaapproved residency training programs in internal medicine, the equivalent of the Association of American Medical Colleges certification in the United States) between October 1, 2009 and September 30, 2010 and between April 1, 2011 and December 1, 2011 (these 20 months covered most of the pre/post intervals for a recently reported quality improvement initiative at 1 of the teaching hospitals that had no significant impact on postdischarge outcomes).[14] Patients from out of the province or transferred from/to another inpatient service (eg, the intensive care unit, a different service in the same hospital [such as surgery], another acute care hospital, or rehabilitation hospital) or with lengths of stay greater than 30 days were excluded. We only included the first hospitalization for any patient in our study timeframe and thus excluded repeat discharges of the same patient.
Explanatory Variable of Interest
The independent variable of interest was calendar day of discharge, stratified according to weekday (Monday thru Friday) versus weekend (Saturday and Sunday). Only 1.4% of weekday discharges occurred on a statutory holiday, and for the purposes of this study, these discharges were also considered weekend discharges. At the 7 teaching hospitals in Alberta, nursing staffing ratios do not differ between weekend and weekday, but availability of all other members of the healthcare team does. Physician census decreases from 4 to 5 per ward to 1 to 2, and ward‐based social workers, occupational therapists, physiotherapists, and pharmacist educators are generally not available on weekends.
Outcomes
Our primary outcome of interest was the composite outcome of death or all‐cause nonelective readmission within 30 days of discharge (ie, not including in‐hospital events prior to discharge or elective readmissions after discharge for planned procedures such as chemotherapy); hereafter we refer to this as death or readmission. This is a patient‐relevant outcome that is highlighted in the Affordable Care Act and for which there are several validated risk adjustment models.[15] We chose a composite outcome to deal with the issue of competing risks; if weekend discharges were more likely to die then we could observe a spurious association between weekend discharge and reduced readmissions if we focused on only that outcome.
Other Measures
Comorbidities for each patient were identified using International Classification of Diseases, Ninth Revision and Tenth Revision codes from the Discharge Abstract Database for the index hospitalization and any hospitalizations in the 12 months prior to their index admission, a method previously validated in Alberta databases.[13] We also recorded health resource use during their index hospitalization and calculated each patient's LACE score at the time of discharge, which is an index for predicting unplanned readmission or early death postdischarge previously validated in Canadian administrative databases.[15] The LACE index includes length of hospital stay (L), acuity of admission (A, based on the admission category variable described earlier), comorbidity burden quantified using the Charlson Comorbidity Index (C), and emergency department visits in the 6 months prior to admission (E); patients with discharge LACE scores >10 (total possible score is 19) are defined as being at high risk of death/readmission within 30 days.[16] As detailed below, to deal with potential concerns that LOS may be a mediator in the causal pathway, we ran 2 sensitivity analyses, 1 in which we excluded LOS from the analyses and 1 in which we included expected LOS rather than the actual LOS. Expected LOS is a data‐driven estimate based on the most current 2 years of patient LOS information available in the Canadian Institute for Health Information discharge abstract database (
Statistical Analysis
Baseline patient characteristics between weekend and weekday discharges were compared with t tests for continuous variables and [2] tests for binary or categorical variables. Logistic regression was used for comparison of death or readmission for weekend versus weekday discharges. Multivariable models were adjusted for age, sex, hospital, and LACE scores (as a continuous variable) at time of discharge; in sensitivity analyses we adjusted for (1) LACE score without including LOS and (2) LACE score using expected LOS rather than actual LOS. In further sensitivity analyses we (1) restricted the analysis to only those patients deemed to be at high risk for events due to LACE scores of 10 or greater and (2) included ED visits as part of the composite endpoint (ie, death, unplanned readmission, or unplanned ED visit within 30 days of discharge). Day of admission (weekend vs weekday) was also considered for the multivariable models, but was not found to be significant and thus was omitted from final models. We do not have any physician identifying variables in our dataset and thus could not investigate the potential correlation among patients discharged by the same physician. We did explore the hospital intraclass correlation coefficient, and as it was very small (0.001), we did not utilize models to account for the hierarchical nature of the data, but did include hospital as a fixed effect in the logistic models. The results were virtually identical whether we did or did not include hospital in the models. Adjusted odds ratios (aORs) are displayed with 95% confidence intervals (CI) and P values. Average LOS was calculated for weekend and weekday discharges with 95% CIs. P values for adjusted length of stay were calculated using multivariable linear regression adjusting for age, sex, day of admission, and Charlson score. All statistical analyses were done using SAS for Windows version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Patient Characteristics
Of the 7991 patients discharged during our study interval, 1146 (14.3%) were discharged on weekend or holiday days (Table 1). In contrast, 2180 of our cohort were admitted on a weekend (27.3%). The mean age of our study population was 62.1 years, 51.9% were men, mean Charlson score was 2.56, and 4591 (57.5%) had LACE scores of at least 10 at discharge.
| Characteristic | Weekend Discharge | Weekday Discharge | P Value |
|---|---|---|---|
| |||
| No. of patients | 1,146 | 6,845 | |
| Age, y, mean (SD) | 57.97 (19.70) | 62.77 (19.37) | <0.0001 |
| Male | 601 (52.4) | 3,548 (51.8) | 0.70 |
| Top 5 most responsible diagnoses | |||
| COPD | 74 (6.5) | 507 (7.4) | |
| Pneumonia | 64 (5.6) | 326 (4.8) | |
| Heart failure | 31 (2.7) | 375 (5.5) | |
| Urinary tract infection | 39 (3.4) | 254 (3.7) | |
| Venous thromboembolism | 31 (2.7) | 259 (3.8) | |
| Charlson score, mean (SD) | 2.17 (3.29) | 2.63 (3.30) | <0.0001 |
| Comorbidities (based on index hospitalization and prior 12 months) | |||
| Hypertension | 485 (42.3) | 3,265 (47.7) | 0.00 |
| Diabetes mellitus | 326 (28.4) | 2,106 (30.8) | 0.11 |
| Fluid imbalance | 332 (29.0) | 1,969 (28.8) | 0.89 |
| COPD | 255 (22.3) | 1,790 (26.2) | 0.01 |
| Psychiatric disorder | 179 (15.6) | 1,459 (21.3) | <0.0001 |
| Pneumonia | 242 (21.1) | 1,427 (20.8) | 0.84 |
| Anemia | 167 (14.6) | 1,233 (18.0) | 0.00 |
| Trauma | 169 (14.7) | 1,209 (17.7) | 0.02 |
| Atrial fibrillation | 141 (12.3) | 1,069 (15.6) | 0.00 |
| Heart failure | 101 (8.8) | 946 (13.8) | <0.0001 |
| Drug abuse | 188 (16.4) | 966 (14.1) | 0.04 |
| Cancer | 124 (10.8) | 867 (12.7) | 0.08 |
| Renal disease | 93 (8.1) | 689 (10.1) | 0.04 |
| Dementia | 49 (4.3) | 564 (8.2) | <0.0001 |
| Mild liver disease | 99 (8.6) | 587 (8.6) | 0.94 |
| Cerebrovascular disease | 59 (5.1) | 492 (7.2) | 0.01 |
| Gastrointestinal bleed | 84 (7.3) | 496 (7.2) | 0.92 |
| Asthma | 83 (7.2) | 426 (6.2) | 0.19 |
| Stroke | 42 (3.7) | 332 (4.9) | 0.08 |
| Prior myocardial infarction | 47 (4.1) | 329 (4.8) | 0.30 |
| Arthritis | 42 (3.7) | 309 (4.5) | 0.19 |
| Peripheral vascular disease | 42 (3.7) | 259 (3.8) | 0.84 |
| Severe liver disease | 44 (3.8) | 261 (3.8) | 0.97 |
| Valve disease | 24 (2.1) | 188 (2.7) | 0.20 |
| Paralysis | 31 (2.7) | 201 (2.9) | 0.67 |
| Skin ulcer | 17 (1.5) | 137 (2.0) | 0.24 |
| Shock | 19 (1.7) | 99 (1.4) | 0.58 |
| HIV | 15 (1.3) | 109 (1.6) | 0.47 |
| Protein calorie malnutrition | 0 (0.0) | 9 (0.1) | 0.21 |
| Features of index hospitalization | |||
| Resource intensity weight, mean (SD) | 1.10 (0.82) | 1.38 (1.24) | <0.0001 |
| LACE score, mean (SD) | 9.45 (2.85) | 10.51 (3.03) | <0.0001 |
| Expected LOS, mean (SD) | 6.20 (4.08) | 7.12 (4.89) | <0.0001 |
| Acute LOS, mean (SD) | 5.64 (4.99) | 7.86 (6.13) | <0.0001 |
| Weekend admission | 244 (21.3) | 1,936 (28.3) | <0.0001 |
| Discharge disposition | <0.0001 | ||
| Transferred to another inpatient hospital | 14 (1.2) | 189 (2.8) | |
| Transferred to long‐term care facility | 36 (3.1) | 532 (7.8) | |
| Transferred to other (except hospice) | 5 (0.4) | 24 (0.4) | |
| Discharged to home setting with support services | 125 (10.9) | 1,318 (19.3) | |
| Discharged home | 926 (80.8) | 4,646 (67.9) | |
| Left against medical advice | 40 (3.5) | 136 (2.0) | |
Weekday Versus Weekend Discharge
Although patients admitted on weekdays and weekends were very similar (data available upon request), patients discharged on weekends (compared to those discharged on weekdays) were younger, more likely to be discharged home without additional support, and had fewer comorbidities (Table 1, Figure 1). Patients discharged on weekends had shorter lengths of stay than those discharged on weekdays (5.6 days vs 7.9 days, P<0.0001). In adjusted linear regression analyses, this 2.3‐day difference remained statistically significant (adjusted P value <0.0001).
Patients discharged on a weekend exhibited lower unadjusted 30‐day rates of death or readmission than those discharged on a weekday (10.6% vs 13.2%), but these differences disappeared after multivariable adjustment that accounted for differences in risk profile (aOR: 0.94, 95% CI: 0.771.16 (Table 2). Results were similar in sensitivity analyses adjusting for LACE scores without LOS included (aOR: 0.88, 95% CI: 0.711.08) or adjusting for LACE scores using expected LOS rather than actual LOS (aOR: 0.90, 95% CI: 0.731.10). Restricting the analysis to only those patients deemed to be at high risk for events due to LACE scores of 10 or greater confirmed that weekend and weekday discharges had similar outcomes in the first 30 days after discharge (aOR: 1.09, 95% CI: 0.851.41, Table 2). Similar patterns were seen when we included ED visits as part of the composite endpoint (ie, death, unplanned readmission, or unplanned ED visit within 30 days of discharge) (Table 2).
| Weekend Discharge, n/N (%) | Weekday Discharge, n/N (%) | Unadjusted P Value | aOR* (95% CI) | Adjusted P Value | |
|---|---|---|---|---|---|
| |||||
| Death/readmission within 30 days | |||||
| All 7 teaching hospitals, all patients | 121/1146 (10.6) | 901/6845 (13.2) | 0.01 | 0.94 (0.77‐1.16) | 0.58 |
| All 7 teaching hospitals, but only patients with LACE <10 | 37/647 (5.7) | 225/2753 (8.2) | 0.04 | 0.72 (0.50, 1.03) | 0.07 |
| All 7 teaching hospitals, but only patients with LACE 10 | 84/499 (16.8) | 676/4092 (16.5) | 0.86 | 1.09 (0.85‐1.41) | 0.49 |
| Death/readmission/ED visit within 30 days | |||||
| All 7 teaching hospitals, all patients | 218/1146 (19.0) | 1445/6845 (21.1) | 0.11 | 0.98 (0.83‐1.15) | 0.79 |
| All 7 teaching hospitals, but only patients with LACE <10 | 90/647 (13.9) | 460/2753 (16.7) | 0.08 | 0.83 (0.64‐1.06) | 0.13 |
| All 7 teaching hospitals, but only patients with LACE 10 | 128/499 (25.7) | 985/4092 (24.1) | 0.44 | 1.12 (0.90‐1.39) | 0.31 |
| Death within 30 days | |||||
| All 7 teaching hospitals, all patients | 24/1146 (2.1) | 215/6845 (3.1) | 0.05 | 0.97 (0.63‐1.51) | 0.89 |
| All 7 teaching hospitals, but only patients with LACE <10 | 4/647 (0.6) | 23/2753 (0.8) | 0.58 | 0.89 (0.30, 2.62) | 0.83 |
| All 7 teaching hospitals, but only patients with LACE 10 | 20/499 (4.0) | 192/4092 (4.7) | 0.49 | 0.99 (0.61‐1.61) | 0.98 |
| Readmission within 30 days | |||||
| All 7 teaching hospitals, all patients | 105/1146 (9.2) | 751/6845 (11.0) | 0.07 | 0.94 (0.76‐1.17) | 0.59 |
| All 7 teaching hospitals, but only patients with LACE <10 | 33/647 (5.1) | 211/2753 (7.7) | 0.02 | 0.68 (0.46‐0.99) | 0.04 |
| All 7 teaching hospitals, but only patients with LACE 10 | 72/499 (14.4) | 540/4092 (13.2) | 0.44 | 1.14 (0.87‐1.49) | 0.34 |
| ED visit within 30 days | |||||
| All 7 teaching hospitals, all patients | 182/1146 (15.9) | 1118/6845 (16.3) | 0.70 | 1.00 (0.84‐1.19) | 0.99 |
| All 7 teaching hospitals, but only patients with LACE <10 | 83/647 (12.8) | 412/2753 (15.0) | 0.17 | 0.84 (0.65, 1.09) | 0.20 |
| All 7 teaching hospitals, but only patients with LACE 10 | 99/499 (19.8) | 706/4092 (17.3) | 0.15 | 1.17 (0.92‐1.48) | 0.20 |
DISCUSSION
Our data suggest that patients discharged from the GIM teaching wards we studied on weekends were appropriately triaged, as they did not exhibit a higher risk of adverse events postdischarge. Although patients discharged on weekends tended to be younger and had less comorbidities than those discharged during the week, we adjusted for baseline covariates in analyses, and we did not find an association between weekend discharge and increased postdischarge events even among the subset of patients deemed to be at high risk for postdischarge adverse events (based on high LACE scores). To our knowledge, although we previously examined this issue in patients with a most‐responsible diagnosis of heart failure,[10] examining weekend versus weekday discharges in the full gamut of general medical patients admitted to teaching hospitals has not previously been examined.
In our previous study[10] of over 24,000 heart failure patients discharged over 10 years (up to June 2009, therefore no overlap with any patients in this study), we also found that patients discharged on the weekends were younger, had fewer comorbidities, and shorter lengths of stay. Although postdischarge death/readmission rates were higher for weekend discharged patients in our earlier study (21.1% vs 19.5%, adjusted hazard ratio: 1.15, 95% CI: 1.061.25), it is worth noting that this was almost entirely driven by data from nonteaching hospitals and cardiology wards. Thus, it is important to reiterate that the findings in our current study are for GIM wards in teaching hospitals and may not be generalizable to less‐structured nonteaching settings.
Although we did not study physician decision making, our results suggest that physicians are incorporating discharge day into their discharge decision making. They may be selecting younger patients with less comorbidities for weekend discharges, or they may be delaying the discharges of older patients with more comorbidities for weekday discharges. Either is not surprising given the realities of weekend inpatient care: reduced staffing and frequent cross‐coverage (of physicians, nurses, physiotherapists, pharmacists, and occupational therapists), limited support services (such as laboratory services or diagnostic imaging), and decreased availability of community services (including home care and social support services).[17] For example, in 1 large US heart failure registry, patients discharged on a weekend received less complete discharge instructions than those discharged on weekdays.[11] Given that early follow‐up postdischarge is associated with better outcomes,[18, 19] future studies should also explore whether patterns of patient follow‐up differ after weekend versus weekday discharges.
Although we were able to capture all interactions with the healthcare system in a single payer system with universal access, there are some limitations to our study. First, we used administrative data, which preclude fully adjusting for severity of diagnoses or functional status, although we used proxies such as admission from/discharge to a long‐term care facility.[20, 21] Second, we did not have access to process of care measures such as diagnostic testing or prescribing data, and thus cannot determine whether quality of care or patient adherence differed by the day of the week they were discharged on, although this seems unlikely. Third, although postdischarge follow‐up may be associated with better outcomes,[18, 19] we were unable to adjust for patterns of outpatient follow‐up in this study. Fourth, we acknowledge that death or readmission soon after discharge does not necessarily mean that the quality of care during the preceding hospitalization was suboptimal or that these deaths or readmissions were even potentially preventable. Many factors influence postdischarge mortality and/or readmission, and quality of inpatient care is only one.[22, 23, 24, 25] Fifth, although some may express concern that LOS may be a mediator in the causal pathway between discharge decision and postdischarge events, and that adjusting for LOS in analyses could thus spuriously obscure a true association, it is worth pointing out that our 2 sensitivity analyses to explore this (the 1 in which we excluded LOS from the analyses and the 1 in which we included expected LOS rather than the actual LOS) revealed nearly identical point estimates and 95% CI as our main analysis. Finally, as our study is observational, we cannot definitively conclude causality, nor can we exclude an 18% excess risk for patients discharged on weekends (or a 22% lower risk either), given our 95% CI for postdischarge adverse outcomes.
CONCLUSION
We found that the proportion of patients discharged on weekends is lower than the proportion admitted on weekends. We also found that lower risk/less severely ill patients appear to be preferentially discharged on weekends, and as a result, postdischarge outcomes are similar between weekend and weekday discharges despite shorter LOS and less availability of outpatient resources for patients discharged on a weekend. The reasons why more complicated patients are not discharged on weekends deserves further study, as safely increasing weekend discharge rates would improve efficiency and safety (by reducing unnecessary exposure to in‐hospital adverse events such as falls, unnecessary urinary catheterizations, and healthcare‐acquired infections). Although hospital admission has become a 24/7 business, we believe that hospital discharge processes should strive for the same level of efficiency.
ACKNOWLEDGMENTS
Disclosures: This study is based in part on data provided by Alberta Health. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the government of Alberta. Neither the government of Alberta nor Alberta Health express any opinion in relation to this study. F.A.M. and S.R.M. are supported by salary awards from Alberta Innovates‐Health Solutions (AIHS). F.A.M. holds the Capital Health Chair in Cardiology Outcomes Research. S.R.M. holds the Endowed Chair in Patient Health Management. This project was funded by AIHS through an investigator‐initiated peer reviewed operating grant. The funding agencies did not have input into study design, data collection, interpretation of results, or write up/approval for submission. The authors report no conflicts of interest.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345:663–668.
- , , , et al. Relationship between time of day, day of week, timeliness of reperfusion, and in‐hospital mortality for patients with acute ST‐segment elevation myocardial infarction. JAMA. 2005;294:803–812.
- , . Waiting for urgent procedures on the weekend among emergently hospitalized patients. Am J Med. 2004;117:175–181.
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007;42:1589–1612.
- , , , et al. Day of admission and clinical outcomes for patients hospitalized for heart failure: findings from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE‐HF). Circ Heart Fail. 2008;1:50–57.
- , , , et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105:74–84.
- , , , . Weekends: a dangerous time for having a stroke? Stroke. 2007;38:1211–1215.
- , , , . Day of the week of intensive care admission and patient outcomes: a multisite regional evaluation. Med Care. 2002;40:530–539.
- , , , . Effects of weekend admission and hospital teaching status on in‐hospital mortality. Am J Med. 2004;117:151–157.
- , , , , . Postdischarge outcomes in heart failure are better for teaching hospitals and weekday discharges. Circ Heart Fail. 2013;6:922–929.
- , , , et al. Weekend hospital admission and discharge for heart failure: association with quality of care and clinical outcomes. Am Heart J. 2009;158:451–458.
- , . Risk of death or readmission among people discharged from hospital on Fridays. CMAJ. 2002;166:1672–1673.
- , , , , et al.; IMECCHI Investigators. Assessing validity of ICD‐9‐CM and ICD‐10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441.
- , , , et al. Safely and effectively reducing inpatient length of stay: a controlled study of the General Internal Medicine Care Transformation Initiative. BMJ Qual Saf. 2014;23:446–456.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182:551–557.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- , . Excellent hospital care for all: open and operating 24/7. J Gen Intern Med. 2011;26:1050–1052.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722.
- , , , , , . Impact of physician continuity on death or urgent readmission after discharge among patients with heart failure. CMAJ. 2013;185:e681–e689.
- , , , , , . Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research. Ann Intern Med. 1993;119:844–850.
- , , , . Predictions of hospital mortality rates: a comparison of data sources. Ann Intern Med. 1997;126:347–354.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
- , . Investigating early readmission as an indicator for quality of care studies. Med Care. 1991;29(4):377–394.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
Hospitals typically reduce staffing levels and the availability of diagnostic, laboratory, and treatment services on weekends, and patients admitted on weekends exhibit poorer in‐hospital outcomes for several medical conditions.[1, 2, 3, 4, 5, 6, 7, 8, 9] Whether or not patients discharged on weekends have worse clinical outcomes has been less well studied.[10, 11, 12] Discharge rates on Saturday and Sunday are lower than for the other 5 days of the week,[12] but bed shortages and hospital overcrowding have increased the demand for maximizing 24/7 week‐round discharge efficiency. Given that the number of patients discharged on weekends is likely to continue to increase, it is important to assess the risk of weekend discharge on outcomes monitored as performance indicators by organizations such as the Centers for Medicare and Medicaid Services, the American Medical Association Physicians Consortium for Performance Improvement, the National Quality Forum, and the Joint Commission.
Thus, we designed this study to evaluate baseline characteristics, length of stay (LOS), and postdischarge outcomes for general internal medicine (GIM) patients in teaching hospitals discharged on weekends compared to weekdays. Our objective was to determine whether postdischarge outcomes differed for patients discharged on weekends versus weekdays.
METHODS
Study Setting
The Canadian province of Alberta has a single vertically integrated healthcare system that is government‐funded and provides universal access to hospitals, emergency departments (EDs), and outpatient physician services for all 4.1 million Albertans as well as all prescription medications for the poor, socially disadvantaged, disabled, or those age 65 years and older. This study received approval from the University of Alberta Health Research Ethics Board with waiver of informed consent.
Data Sources
This study used deidentified linked data from 3 Alberta Health administrative databases that capture vital status and all hospital or ED visits and have previously been shown to have high accuracy for medical diagnoses.[13] The Alberta Health Care Insurance Plan Registry tracks date of death or emigration from the province. The Discharge Abstract Database includes the most responsible diagnosis identified by the hospital attending physician, up to 25 other diagnoses coded by nosologists in each hospital, the admission and discharge dates, and the admission category (elective or urgent/emergent) for all acute care hospitalizations. Of note, unlike US studies, the hospital databases are able to distinguish in‐hospital (eg, adverse events) versus premorbid diagnoses (eg, preexisting comorbidities). The Ambulatory Care Database captures all patient visits to EDs with coding for up to 10 conditions per encounter.
Study Cohort
We identified all adults with an acute care hospitalization on the GIM services at all 7 Alberta teaching hospitals (ie, defined as those with Royal College of Physicians and Surgeons of Canadaapproved residency training programs in internal medicine, the equivalent of the Association of American Medical Colleges certification in the United States) between October 1, 2009 and September 30, 2010 and between April 1, 2011 and December 1, 2011 (these 20 months covered most of the pre/post intervals for a recently reported quality improvement initiative at 1 of the teaching hospitals that had no significant impact on postdischarge outcomes).[14] Patients from out of the province or transferred from/to another inpatient service (eg, the intensive care unit, a different service in the same hospital [such as surgery], another acute care hospital, or rehabilitation hospital) or with lengths of stay greater than 30 days were excluded. We only included the first hospitalization for any patient in our study timeframe and thus excluded repeat discharges of the same patient.
Explanatory Variable of Interest
The independent variable of interest was calendar day of discharge, stratified according to weekday (Monday thru Friday) versus weekend (Saturday and Sunday). Only 1.4% of weekday discharges occurred on a statutory holiday, and for the purposes of this study, these discharges were also considered weekend discharges. At the 7 teaching hospitals in Alberta, nursing staffing ratios do not differ between weekend and weekday, but availability of all other members of the healthcare team does. Physician census decreases from 4 to 5 per ward to 1 to 2, and ward‐based social workers, occupational therapists, physiotherapists, and pharmacist educators are generally not available on weekends.
Outcomes
Our primary outcome of interest was the composite outcome of death or all‐cause nonelective readmission within 30 days of discharge (ie, not including in‐hospital events prior to discharge or elective readmissions after discharge for planned procedures such as chemotherapy); hereafter we refer to this as death or readmission. This is a patient‐relevant outcome that is highlighted in the Affordable Care Act and for which there are several validated risk adjustment models.[15] We chose a composite outcome to deal with the issue of competing risks; if weekend discharges were more likely to die then we could observe a spurious association between weekend discharge and reduced readmissions if we focused on only that outcome.
Other Measures
Comorbidities for each patient were identified using International Classification of Diseases, Ninth Revision and Tenth Revision codes from the Discharge Abstract Database for the index hospitalization and any hospitalizations in the 12 months prior to their index admission, a method previously validated in Alberta databases.[13] We also recorded health resource use during their index hospitalization and calculated each patient's LACE score at the time of discharge, which is an index for predicting unplanned readmission or early death postdischarge previously validated in Canadian administrative databases.[15] The LACE index includes length of hospital stay (L), acuity of admission (A, based on the admission category variable described earlier), comorbidity burden quantified using the Charlson Comorbidity Index (C), and emergency department visits in the 6 months prior to admission (E); patients with discharge LACE scores >10 (total possible score is 19) are defined as being at high risk of death/readmission within 30 days.[16] As detailed below, to deal with potential concerns that LOS may be a mediator in the causal pathway, we ran 2 sensitivity analyses, 1 in which we excluded LOS from the analyses and 1 in which we included expected LOS rather than the actual LOS. Expected LOS is a data‐driven estimate based on the most current 2 years of patient LOS information available in the Canadian Institute for Health Information discharge abstract database (
Statistical Analysis
Baseline patient characteristics between weekend and weekday discharges were compared with t tests for continuous variables and [2] tests for binary or categorical variables. Logistic regression was used for comparison of death or readmission for weekend versus weekday discharges. Multivariable models were adjusted for age, sex, hospital, and LACE scores (as a continuous variable) at time of discharge; in sensitivity analyses we adjusted for (1) LACE score without including LOS and (2) LACE score using expected LOS rather than actual LOS. In further sensitivity analyses we (1) restricted the analysis to only those patients deemed to be at high risk for events due to LACE scores of 10 or greater and (2) included ED visits as part of the composite endpoint (ie, death, unplanned readmission, or unplanned ED visit within 30 days of discharge). Day of admission (weekend vs weekday) was also considered for the multivariable models, but was not found to be significant and thus was omitted from final models. We do not have any physician identifying variables in our dataset and thus could not investigate the potential correlation among patients discharged by the same physician. We did explore the hospital intraclass correlation coefficient, and as it was very small (0.001), we did not utilize models to account for the hierarchical nature of the data, but did include hospital as a fixed effect in the logistic models. The results were virtually identical whether we did or did not include hospital in the models. Adjusted odds ratios (aORs) are displayed with 95% confidence intervals (CI) and P values. Average LOS was calculated for weekend and weekday discharges with 95% CIs. P values for adjusted length of stay were calculated using multivariable linear regression adjusting for age, sex, day of admission, and Charlson score. All statistical analyses were done using SAS for Windows version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Patient Characteristics
Of the 7991 patients discharged during our study interval, 1146 (14.3%) were discharged on weekend or holiday days (Table 1). In contrast, 2180 of our cohort were admitted on a weekend (27.3%). The mean age of our study population was 62.1 years, 51.9% were men, mean Charlson score was 2.56, and 4591 (57.5%) had LACE scores of at least 10 at discharge.
| Characteristic | Weekend Discharge | Weekday Discharge | P Value |
|---|---|---|---|
| |||
| No. of patients | 1,146 | 6,845 | |
| Age, y, mean (SD) | 57.97 (19.70) | 62.77 (19.37) | <0.0001 |
| Male | 601 (52.4) | 3,548 (51.8) | 0.70 |
| Top 5 most responsible diagnoses | |||
| COPD | 74 (6.5) | 507 (7.4) | |
| Pneumonia | 64 (5.6) | 326 (4.8) | |
| Heart failure | 31 (2.7) | 375 (5.5) | |
| Urinary tract infection | 39 (3.4) | 254 (3.7) | |
| Venous thromboembolism | 31 (2.7) | 259 (3.8) | |
| Charlson score, mean (SD) | 2.17 (3.29) | 2.63 (3.30) | <0.0001 |
| Comorbidities (based on index hospitalization and prior 12 months) | |||
| Hypertension | 485 (42.3) | 3,265 (47.7) | 0.00 |
| Diabetes mellitus | 326 (28.4) | 2,106 (30.8) | 0.11 |
| Fluid imbalance | 332 (29.0) | 1,969 (28.8) | 0.89 |
| COPD | 255 (22.3) | 1,790 (26.2) | 0.01 |
| Psychiatric disorder | 179 (15.6) | 1,459 (21.3) | <0.0001 |
| Pneumonia | 242 (21.1) | 1,427 (20.8) | 0.84 |
| Anemia | 167 (14.6) | 1,233 (18.0) | 0.00 |
| Trauma | 169 (14.7) | 1,209 (17.7) | 0.02 |
| Atrial fibrillation | 141 (12.3) | 1,069 (15.6) | 0.00 |
| Heart failure | 101 (8.8) | 946 (13.8) | <0.0001 |
| Drug abuse | 188 (16.4) | 966 (14.1) | 0.04 |
| Cancer | 124 (10.8) | 867 (12.7) | 0.08 |
| Renal disease | 93 (8.1) | 689 (10.1) | 0.04 |
| Dementia | 49 (4.3) | 564 (8.2) | <0.0001 |
| Mild liver disease | 99 (8.6) | 587 (8.6) | 0.94 |
| Cerebrovascular disease | 59 (5.1) | 492 (7.2) | 0.01 |
| Gastrointestinal bleed | 84 (7.3) | 496 (7.2) | 0.92 |
| Asthma | 83 (7.2) | 426 (6.2) | 0.19 |
| Stroke | 42 (3.7) | 332 (4.9) | 0.08 |
| Prior myocardial infarction | 47 (4.1) | 329 (4.8) | 0.30 |
| Arthritis | 42 (3.7) | 309 (4.5) | 0.19 |
| Peripheral vascular disease | 42 (3.7) | 259 (3.8) | 0.84 |
| Severe liver disease | 44 (3.8) | 261 (3.8) | 0.97 |
| Valve disease | 24 (2.1) | 188 (2.7) | 0.20 |
| Paralysis | 31 (2.7) | 201 (2.9) | 0.67 |
| Skin ulcer | 17 (1.5) | 137 (2.0) | 0.24 |
| Shock | 19 (1.7) | 99 (1.4) | 0.58 |
| HIV | 15 (1.3) | 109 (1.6) | 0.47 |
| Protein calorie malnutrition | 0 (0.0) | 9 (0.1) | 0.21 |
| Features of index hospitalization | |||
| Resource intensity weight, mean (SD) | 1.10 (0.82) | 1.38 (1.24) | <0.0001 |
| LACE score, mean (SD) | 9.45 (2.85) | 10.51 (3.03) | <0.0001 |
| Expected LOS, mean (SD) | 6.20 (4.08) | 7.12 (4.89) | <0.0001 |
| Acute LOS, mean (SD) | 5.64 (4.99) | 7.86 (6.13) | <0.0001 |
| Weekend admission | 244 (21.3) | 1,936 (28.3) | <0.0001 |
| Discharge disposition | <0.0001 | ||
| Transferred to another inpatient hospital | 14 (1.2) | 189 (2.8) | |
| Transferred to long‐term care facility | 36 (3.1) | 532 (7.8) | |
| Transferred to other (except hospice) | 5 (0.4) | 24 (0.4) | |
| Discharged to home setting with support services | 125 (10.9) | 1,318 (19.3) | |
| Discharged home | 926 (80.8) | 4,646 (67.9) | |
| Left against medical advice | 40 (3.5) | 136 (2.0) | |
Weekday Versus Weekend Discharge
Although patients admitted on weekdays and weekends were very similar (data available upon request), patients discharged on weekends (compared to those discharged on weekdays) were younger, more likely to be discharged home without additional support, and had fewer comorbidities (Table 1, Figure 1). Patients discharged on weekends had shorter lengths of stay than those discharged on weekdays (5.6 days vs 7.9 days, P<0.0001). In adjusted linear regression analyses, this 2.3‐day difference remained statistically significant (adjusted P value <0.0001).

Patients discharged on a weekend exhibited lower unadjusted 30‐day rates of death or readmission than those discharged on a weekday (10.6% vs 13.2%), but these differences disappeared after multivariable adjustment that accounted for differences in risk profile (aOR: 0.94, 95% CI: 0.771.16 (Table 2). Results were similar in sensitivity analyses adjusting for LACE scores without LOS included (aOR: 0.88, 95% CI: 0.711.08) or adjusting for LACE scores using expected LOS rather than actual LOS (aOR: 0.90, 95% CI: 0.731.10). Restricting the analysis to only those patients deemed to be at high risk for events due to LACE scores of 10 or greater confirmed that weekend and weekday discharges had similar outcomes in the first 30 days after discharge (aOR: 1.09, 95% CI: 0.851.41, Table 2). Similar patterns were seen when we included ED visits as part of the composite endpoint (ie, death, unplanned readmission, or unplanned ED visit within 30 days of discharge) (Table 2).
| Weekend Discharge, n/N (%) | Weekday Discharge, n/N (%) | Unadjusted P Value | aOR* (95% CI) | Adjusted P Value | |
|---|---|---|---|---|---|
| |||||
| Death/readmission within 30 days | |||||
| All 7 teaching hospitals, all patients | 121/1146 (10.6) | 901/6845 (13.2) | 0.01 | 0.94 (0.77‐1.16) | 0.58 |
| All 7 teaching hospitals, but only patients with LACE <10 | 37/647 (5.7) | 225/2753 (8.2) | 0.04 | 0.72 (0.50, 1.03) | 0.07 |
| All 7 teaching hospitals, but only patients with LACE 10 | 84/499 (16.8) | 676/4092 (16.5) | 0.86 | 1.09 (0.85‐1.41) | 0.49 |
| Death/readmission/ED visit within 30 days | |||||
| All 7 teaching hospitals, all patients | 218/1146 (19.0) | 1445/6845 (21.1) | 0.11 | 0.98 (0.83‐1.15) | 0.79 |
| All 7 teaching hospitals, but only patients with LACE <10 | 90/647 (13.9) | 460/2753 (16.7) | 0.08 | 0.83 (0.64‐1.06) | 0.13 |
| All 7 teaching hospitals, but only patients with LACE 10 | 128/499 (25.7) | 985/4092 (24.1) | 0.44 | 1.12 (0.90‐1.39) | 0.31 |
| Death within 30 days | |||||
| All 7 teaching hospitals, all patients | 24/1146 (2.1) | 215/6845 (3.1) | 0.05 | 0.97 (0.63‐1.51) | 0.89 |
| All 7 teaching hospitals, but only patients with LACE <10 | 4/647 (0.6) | 23/2753 (0.8) | 0.58 | 0.89 (0.30, 2.62) | 0.83 |
| All 7 teaching hospitals, but only patients with LACE 10 | 20/499 (4.0) | 192/4092 (4.7) | 0.49 | 0.99 (0.61‐1.61) | 0.98 |
| Readmission within 30 days | |||||
| All 7 teaching hospitals, all patients | 105/1146 (9.2) | 751/6845 (11.0) | 0.07 | 0.94 (0.76‐1.17) | 0.59 |
| All 7 teaching hospitals, but only patients with LACE <10 | 33/647 (5.1) | 211/2753 (7.7) | 0.02 | 0.68 (0.46‐0.99) | 0.04 |
| All 7 teaching hospitals, but only patients with LACE 10 | 72/499 (14.4) | 540/4092 (13.2) | 0.44 | 1.14 (0.87‐1.49) | 0.34 |
| ED visit within 30 days | |||||
| All 7 teaching hospitals, all patients | 182/1146 (15.9) | 1118/6845 (16.3) | 0.70 | 1.00 (0.84‐1.19) | 0.99 |
| All 7 teaching hospitals, but only patients with LACE <10 | 83/647 (12.8) | 412/2753 (15.0) | 0.17 | 0.84 (0.65, 1.09) | 0.20 |
| All 7 teaching hospitals, but only patients with LACE 10 | 99/499 (19.8) | 706/4092 (17.3) | 0.15 | 1.17 (0.92‐1.48) | 0.20 |
DISCUSSION
Our data suggest that patients discharged from the GIM teaching wards we studied on weekends were appropriately triaged, as they did not exhibit a higher risk of adverse events postdischarge. Although patients discharged on weekends tended to be younger and had less comorbidities than those discharged during the week, we adjusted for baseline covariates in analyses, and we did not find an association between weekend discharge and increased postdischarge events even among the subset of patients deemed to be at high risk for postdischarge adverse events (based on high LACE scores). To our knowledge, although we previously examined this issue in patients with a most‐responsible diagnosis of heart failure,[10] examining weekend versus weekday discharges in the full gamut of general medical patients admitted to teaching hospitals has not previously been examined.
In our previous study[10] of over 24,000 heart failure patients discharged over 10 years (up to June 2009, therefore no overlap with any patients in this study), we also found that patients discharged on the weekends were younger, had fewer comorbidities, and shorter lengths of stay. Although postdischarge death/readmission rates were higher for weekend discharged patients in our earlier study (21.1% vs 19.5%, adjusted hazard ratio: 1.15, 95% CI: 1.061.25), it is worth noting that this was almost entirely driven by data from nonteaching hospitals and cardiology wards. Thus, it is important to reiterate that the findings in our current study are for GIM wards in teaching hospitals and may not be generalizable to less‐structured nonteaching settings.
Although we did not study physician decision making, our results suggest that physicians are incorporating discharge day into their discharge decision making. They may be selecting younger patients with less comorbidities for weekend discharges, or they may be delaying the discharges of older patients with more comorbidities for weekday discharges. Either is not surprising given the realities of weekend inpatient care: reduced staffing and frequent cross‐coverage (of physicians, nurses, physiotherapists, pharmacists, and occupational therapists), limited support services (such as laboratory services or diagnostic imaging), and decreased availability of community services (including home care and social support services).[17] For example, in 1 large US heart failure registry, patients discharged on a weekend received less complete discharge instructions than those discharged on weekdays.[11] Given that early follow‐up postdischarge is associated with better outcomes,[18, 19] future studies should also explore whether patterns of patient follow‐up differ after weekend versus weekday discharges.
Although we were able to capture all interactions with the healthcare system in a single payer system with universal access, there are some limitations to our study. First, we used administrative data, which preclude fully adjusting for severity of diagnoses or functional status, although we used proxies such as admission from/discharge to a long‐term care facility.[20, 21] Second, we did not have access to process of care measures such as diagnostic testing or prescribing data, and thus cannot determine whether quality of care or patient adherence differed by the day of the week they were discharged on, although this seems unlikely. Third, although postdischarge follow‐up may be associated with better outcomes,[18, 19] we were unable to adjust for patterns of outpatient follow‐up in this study. Fourth, we acknowledge that death or readmission soon after discharge does not necessarily mean that the quality of care during the preceding hospitalization was suboptimal or that these deaths or readmissions were even potentially preventable. Many factors influence postdischarge mortality and/or readmission, and quality of inpatient care is only one.[22, 23, 24, 25] Fifth, although some may express concern that LOS may be a mediator in the causal pathway between discharge decision and postdischarge events, and that adjusting for LOS in analyses could thus spuriously obscure a true association, it is worth pointing out that our 2 sensitivity analyses to explore this (the 1 in which we excluded LOS from the analyses and the 1 in which we included expected LOS rather than the actual LOS) revealed nearly identical point estimates and 95% CI as our main analysis. Finally, as our study is observational, we cannot definitively conclude causality, nor can we exclude an 18% excess risk for patients discharged on weekends (or a 22% lower risk either), given our 95% CI for postdischarge adverse outcomes.
CONCLUSION
We found that the proportion of patients discharged on weekends is lower than the proportion admitted on weekends. We also found that lower risk/less severely ill patients appear to be preferentially discharged on weekends, and as a result, postdischarge outcomes are similar between weekend and weekday discharges despite shorter LOS and less availability of outpatient resources for patients discharged on a weekend. The reasons why more complicated patients are not discharged on weekends deserves further study, as safely increasing weekend discharge rates would improve efficiency and safety (by reducing unnecessary exposure to in‐hospital adverse events such as falls, unnecessary urinary catheterizations, and healthcare‐acquired infections). Although hospital admission has become a 24/7 business, we believe that hospital discharge processes should strive for the same level of efficiency.
ACKNOWLEDGMENTS
Disclosures: This study is based in part on data provided by Alberta Health. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the government of Alberta. Neither the government of Alberta nor Alberta Health express any opinion in relation to this study. F.A.M. and S.R.M. are supported by salary awards from Alberta Innovates‐Health Solutions (AIHS). F.A.M. holds the Capital Health Chair in Cardiology Outcomes Research. S.R.M. holds the Endowed Chair in Patient Health Management. This project was funded by AIHS through an investigator‐initiated peer reviewed operating grant. The funding agencies did not have input into study design, data collection, interpretation of results, or write up/approval for submission. The authors report no conflicts of interest.
Hospitals typically reduce staffing levels and the availability of diagnostic, laboratory, and treatment services on weekends, and patients admitted on weekends exhibit poorer in‐hospital outcomes for several medical conditions.[1, 2, 3, 4, 5, 6, 7, 8, 9] Whether or not patients discharged on weekends have worse clinical outcomes has been less well studied.[10, 11, 12] Discharge rates on Saturday and Sunday are lower than for the other 5 days of the week,[12] but bed shortages and hospital overcrowding have increased the demand for maximizing 24/7 week‐round discharge efficiency. Given that the number of patients discharged on weekends is likely to continue to increase, it is important to assess the risk of weekend discharge on outcomes monitored as performance indicators by organizations such as the Centers for Medicare and Medicaid Services, the American Medical Association Physicians Consortium for Performance Improvement, the National Quality Forum, and the Joint Commission.
Thus, we designed this study to evaluate baseline characteristics, length of stay (LOS), and postdischarge outcomes for general internal medicine (GIM) patients in teaching hospitals discharged on weekends compared to weekdays. Our objective was to determine whether postdischarge outcomes differed for patients discharged on weekends versus weekdays.
METHODS
Study Setting
The Canadian province of Alberta has a single vertically integrated healthcare system that is government‐funded and provides universal access to hospitals, emergency departments (EDs), and outpatient physician services for all 4.1 million Albertans as well as all prescription medications for the poor, socially disadvantaged, disabled, or those age 65 years and older. This study received approval from the University of Alberta Health Research Ethics Board with waiver of informed consent.
Data Sources
This study used deidentified linked data from 3 Alberta Health administrative databases that capture vital status and all hospital or ED visits and have previously been shown to have high accuracy for medical diagnoses.[13] The Alberta Health Care Insurance Plan Registry tracks date of death or emigration from the province. The Discharge Abstract Database includes the most responsible diagnosis identified by the hospital attending physician, up to 25 other diagnoses coded by nosologists in each hospital, the admission and discharge dates, and the admission category (elective or urgent/emergent) for all acute care hospitalizations. Of note, unlike US studies, the hospital databases are able to distinguish in‐hospital (eg, adverse events) versus premorbid diagnoses (eg, preexisting comorbidities). The Ambulatory Care Database captures all patient visits to EDs with coding for up to 10 conditions per encounter.
Study Cohort
We identified all adults with an acute care hospitalization on the GIM services at all 7 Alberta teaching hospitals (ie, defined as those with Royal College of Physicians and Surgeons of Canadaapproved residency training programs in internal medicine, the equivalent of the Association of American Medical Colleges certification in the United States) between October 1, 2009 and September 30, 2010 and between April 1, 2011 and December 1, 2011 (these 20 months covered most of the pre/post intervals for a recently reported quality improvement initiative at 1 of the teaching hospitals that had no significant impact on postdischarge outcomes).[14] Patients from out of the province or transferred from/to another inpatient service (eg, the intensive care unit, a different service in the same hospital [such as surgery], another acute care hospital, or rehabilitation hospital) or with lengths of stay greater than 30 days were excluded. We only included the first hospitalization for any patient in our study timeframe and thus excluded repeat discharges of the same patient.
Explanatory Variable of Interest
The independent variable of interest was calendar day of discharge, stratified according to weekday (Monday thru Friday) versus weekend (Saturday and Sunday). Only 1.4% of weekday discharges occurred on a statutory holiday, and for the purposes of this study, these discharges were also considered weekend discharges. At the 7 teaching hospitals in Alberta, nursing staffing ratios do not differ between weekend and weekday, but availability of all other members of the healthcare team does. Physician census decreases from 4 to 5 per ward to 1 to 2, and ward‐based social workers, occupational therapists, physiotherapists, and pharmacist educators are generally not available on weekends.
Outcomes
Our primary outcome of interest was the composite outcome of death or all‐cause nonelective readmission within 30 days of discharge (ie, not including in‐hospital events prior to discharge or elective readmissions after discharge for planned procedures such as chemotherapy); hereafter we refer to this as death or readmission. This is a patient‐relevant outcome that is highlighted in the Affordable Care Act and for which there are several validated risk adjustment models.[15] We chose a composite outcome to deal with the issue of competing risks; if weekend discharges were more likely to die then we could observe a spurious association between weekend discharge and reduced readmissions if we focused on only that outcome.
Other Measures
Comorbidities for each patient were identified using International Classification of Diseases, Ninth Revision and Tenth Revision codes from the Discharge Abstract Database for the index hospitalization and any hospitalizations in the 12 months prior to their index admission, a method previously validated in Alberta databases.[13] We also recorded health resource use during their index hospitalization and calculated each patient's LACE score at the time of discharge, which is an index for predicting unplanned readmission or early death postdischarge previously validated in Canadian administrative databases.[15] The LACE index includes length of hospital stay (L), acuity of admission (A, based on the admission category variable described earlier), comorbidity burden quantified using the Charlson Comorbidity Index (C), and emergency department visits in the 6 months prior to admission (E); patients with discharge LACE scores >10 (total possible score is 19) are defined as being at high risk of death/readmission within 30 days.[16] As detailed below, to deal with potential concerns that LOS may be a mediator in the causal pathway, we ran 2 sensitivity analyses, 1 in which we excluded LOS from the analyses and 1 in which we included expected LOS rather than the actual LOS. Expected LOS is a data‐driven estimate based on the most current 2 years of patient LOS information available in the Canadian Institute for Health Information discharge abstract database (
Statistical Analysis
Baseline patient characteristics between weekend and weekday discharges were compared with t tests for continuous variables and [2] tests for binary or categorical variables. Logistic regression was used for comparison of death or readmission for weekend versus weekday discharges. Multivariable models were adjusted for age, sex, hospital, and LACE scores (as a continuous variable) at time of discharge; in sensitivity analyses we adjusted for (1) LACE score without including LOS and (2) LACE score using expected LOS rather than actual LOS. In further sensitivity analyses we (1) restricted the analysis to only those patients deemed to be at high risk for events due to LACE scores of 10 or greater and (2) included ED visits as part of the composite endpoint (ie, death, unplanned readmission, or unplanned ED visit within 30 days of discharge). Day of admission (weekend vs weekday) was also considered for the multivariable models, but was not found to be significant and thus was omitted from final models. We do not have any physician identifying variables in our dataset and thus could not investigate the potential correlation among patients discharged by the same physician. We did explore the hospital intraclass correlation coefficient, and as it was very small (0.001), we did not utilize models to account for the hierarchical nature of the data, but did include hospital as a fixed effect in the logistic models. The results were virtually identical whether we did or did not include hospital in the models. Adjusted odds ratios (aORs) are displayed with 95% confidence intervals (CI) and P values. Average LOS was calculated for weekend and weekday discharges with 95% CIs. P values for adjusted length of stay were calculated using multivariable linear regression adjusting for age, sex, day of admission, and Charlson score. All statistical analyses were done using SAS for Windows version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Patient Characteristics
Of the 7991 patients discharged during our study interval, 1146 (14.3%) were discharged on weekend or holiday days (Table 1). In contrast, 2180 of our cohort were admitted on a weekend (27.3%). The mean age of our study population was 62.1 years, 51.9% were men, mean Charlson score was 2.56, and 4591 (57.5%) had LACE scores of at least 10 at discharge.
| Characteristic | Weekend Discharge | Weekday Discharge | P Value |
|---|---|---|---|
| |||
| No. of patients | 1,146 | 6,845 | |
| Age, y, mean (SD) | 57.97 (19.70) | 62.77 (19.37) | <0.0001 |
| Male | 601 (52.4) | 3,548 (51.8) | 0.70 |
| Top 5 most responsible diagnoses | |||
| COPD | 74 (6.5) | 507 (7.4) | |
| Pneumonia | 64 (5.6) | 326 (4.8) | |
| Heart failure | 31 (2.7) | 375 (5.5) | |
| Urinary tract infection | 39 (3.4) | 254 (3.7) | |
| Venous thromboembolism | 31 (2.7) | 259 (3.8) | |
| Charlson score, mean (SD) | 2.17 (3.29) | 2.63 (3.30) | <0.0001 |
| Comorbidities (based on index hospitalization and prior 12 months) | |||
| Hypertension | 485 (42.3) | 3,265 (47.7) | 0.00 |
| Diabetes mellitus | 326 (28.4) | 2,106 (30.8) | 0.11 |
| Fluid imbalance | 332 (29.0) | 1,969 (28.8) | 0.89 |
| COPD | 255 (22.3) | 1,790 (26.2) | 0.01 |
| Psychiatric disorder | 179 (15.6) | 1,459 (21.3) | <0.0001 |
| Pneumonia | 242 (21.1) | 1,427 (20.8) | 0.84 |
| Anemia | 167 (14.6) | 1,233 (18.0) | 0.00 |
| Trauma | 169 (14.7) | 1,209 (17.7) | 0.02 |
| Atrial fibrillation | 141 (12.3) | 1,069 (15.6) | 0.00 |
| Heart failure | 101 (8.8) | 946 (13.8) | <0.0001 |
| Drug abuse | 188 (16.4) | 966 (14.1) | 0.04 |
| Cancer | 124 (10.8) | 867 (12.7) | 0.08 |
| Renal disease | 93 (8.1) | 689 (10.1) | 0.04 |
| Dementia | 49 (4.3) | 564 (8.2) | <0.0001 |
| Mild liver disease | 99 (8.6) | 587 (8.6) | 0.94 |
| Cerebrovascular disease | 59 (5.1) | 492 (7.2) | 0.01 |
| Gastrointestinal bleed | 84 (7.3) | 496 (7.2) | 0.92 |
| Asthma | 83 (7.2) | 426 (6.2) | 0.19 |
| Stroke | 42 (3.7) | 332 (4.9) | 0.08 |
| Prior myocardial infarction | 47 (4.1) | 329 (4.8) | 0.30 |
| Arthritis | 42 (3.7) | 309 (4.5) | 0.19 |
| Peripheral vascular disease | 42 (3.7) | 259 (3.8) | 0.84 |
| Severe liver disease | 44 (3.8) | 261 (3.8) | 0.97 |
| Valve disease | 24 (2.1) | 188 (2.7) | 0.20 |
| Paralysis | 31 (2.7) | 201 (2.9) | 0.67 |
| Skin ulcer | 17 (1.5) | 137 (2.0) | 0.24 |
| Shock | 19 (1.7) | 99 (1.4) | 0.58 |
| HIV | 15 (1.3) | 109 (1.6) | 0.47 |
| Protein calorie malnutrition | 0 (0.0) | 9 (0.1) | 0.21 |
| Features of index hospitalization | |||
| Resource intensity weight, mean (SD) | 1.10 (0.82) | 1.38 (1.24) | <0.0001 |
| LACE score, mean (SD) | 9.45 (2.85) | 10.51 (3.03) | <0.0001 |
| Expected LOS, mean (SD) | 6.20 (4.08) | 7.12 (4.89) | <0.0001 |
| Acute LOS, mean (SD) | 5.64 (4.99) | 7.86 (6.13) | <0.0001 |
| Weekend admission | 244 (21.3) | 1,936 (28.3) | <0.0001 |
| Discharge disposition | <0.0001 | ||
| Transferred to another inpatient hospital | 14 (1.2) | 189 (2.8) | |
| Transferred to long‐term care facility | 36 (3.1) | 532 (7.8) | |
| Transferred to other (except hospice) | 5 (0.4) | 24 (0.4) | |
| Discharged to home setting with support services | 125 (10.9) | 1,318 (19.3) | |
| Discharged home | 926 (80.8) | 4,646 (67.9) | |
| Left against medical advice | 40 (3.5) | 136 (2.0) | |
Weekday Versus Weekend Discharge
Although patients admitted on weekdays and weekends were very similar (data available upon request), patients discharged on weekends (compared to those discharged on weekdays) were younger, more likely to be discharged home without additional support, and had fewer comorbidities (Table 1, Figure 1). Patients discharged on weekends had shorter lengths of stay than those discharged on weekdays (5.6 days vs 7.9 days, P<0.0001). In adjusted linear regression analyses, this 2.3‐day difference remained statistically significant (adjusted P value <0.0001).

Patients discharged on a weekend exhibited lower unadjusted 30‐day rates of death or readmission than those discharged on a weekday (10.6% vs 13.2%), but these differences disappeared after multivariable adjustment that accounted for differences in risk profile (aOR: 0.94, 95% CI: 0.771.16 (Table 2). Results were similar in sensitivity analyses adjusting for LACE scores without LOS included (aOR: 0.88, 95% CI: 0.711.08) or adjusting for LACE scores using expected LOS rather than actual LOS (aOR: 0.90, 95% CI: 0.731.10). Restricting the analysis to only those patients deemed to be at high risk for events due to LACE scores of 10 or greater confirmed that weekend and weekday discharges had similar outcomes in the first 30 days after discharge (aOR: 1.09, 95% CI: 0.851.41, Table 2). Similar patterns were seen when we included ED visits as part of the composite endpoint (ie, death, unplanned readmission, or unplanned ED visit within 30 days of discharge) (Table 2).
| Weekend Discharge, n/N (%) | Weekday Discharge, n/N (%) | Unadjusted P Value | aOR* (95% CI) | Adjusted P Value | |
|---|---|---|---|---|---|
| |||||
| Death/readmission within 30 days | |||||
| All 7 teaching hospitals, all patients | 121/1146 (10.6) | 901/6845 (13.2) | 0.01 | 0.94 (0.77‐1.16) | 0.58 |
| All 7 teaching hospitals, but only patients with LACE <10 | 37/647 (5.7) | 225/2753 (8.2) | 0.04 | 0.72 (0.50, 1.03) | 0.07 |
| All 7 teaching hospitals, but only patients with LACE 10 | 84/499 (16.8) | 676/4092 (16.5) | 0.86 | 1.09 (0.85‐1.41) | 0.49 |
| Death/readmission/ED visit within 30 days | |||||
| All 7 teaching hospitals, all patients | 218/1146 (19.0) | 1445/6845 (21.1) | 0.11 | 0.98 (0.83‐1.15) | 0.79 |
| All 7 teaching hospitals, but only patients with LACE <10 | 90/647 (13.9) | 460/2753 (16.7) | 0.08 | 0.83 (0.64‐1.06) | 0.13 |
| All 7 teaching hospitals, but only patients with LACE 10 | 128/499 (25.7) | 985/4092 (24.1) | 0.44 | 1.12 (0.90‐1.39) | 0.31 |
| Death within 30 days | |||||
| All 7 teaching hospitals, all patients | 24/1146 (2.1) | 215/6845 (3.1) | 0.05 | 0.97 (0.63‐1.51) | 0.89 |
| All 7 teaching hospitals, but only patients with LACE <10 | 4/647 (0.6) | 23/2753 (0.8) | 0.58 | 0.89 (0.30, 2.62) | 0.83 |
| All 7 teaching hospitals, but only patients with LACE 10 | 20/499 (4.0) | 192/4092 (4.7) | 0.49 | 0.99 (0.61‐1.61) | 0.98 |
| Readmission within 30 days | |||||
| All 7 teaching hospitals, all patients | 105/1146 (9.2) | 751/6845 (11.0) | 0.07 | 0.94 (0.76‐1.17) | 0.59 |
| All 7 teaching hospitals, but only patients with LACE <10 | 33/647 (5.1) | 211/2753 (7.7) | 0.02 | 0.68 (0.46‐0.99) | 0.04 |
| All 7 teaching hospitals, but only patients with LACE 10 | 72/499 (14.4) | 540/4092 (13.2) | 0.44 | 1.14 (0.87‐1.49) | 0.34 |
| ED visit within 30 days | |||||
| All 7 teaching hospitals, all patients | 182/1146 (15.9) | 1118/6845 (16.3) | 0.70 | 1.00 (0.84‐1.19) | 0.99 |
| All 7 teaching hospitals, but only patients with LACE <10 | 83/647 (12.8) | 412/2753 (15.0) | 0.17 | 0.84 (0.65, 1.09) | 0.20 |
| All 7 teaching hospitals, but only patients with LACE 10 | 99/499 (19.8) | 706/4092 (17.3) | 0.15 | 1.17 (0.92‐1.48) | 0.20 |
DISCUSSION
Our data suggest that patients discharged from the GIM teaching wards we studied on weekends were appropriately triaged, as they did not exhibit a higher risk of adverse events postdischarge. Although patients discharged on weekends tended to be younger and had less comorbidities than those discharged during the week, we adjusted for baseline covariates in analyses, and we did not find an association between weekend discharge and increased postdischarge events even among the subset of patients deemed to be at high risk for postdischarge adverse events (based on high LACE scores). To our knowledge, although we previously examined this issue in patients with a most‐responsible diagnosis of heart failure,[10] examining weekend versus weekday discharges in the full gamut of general medical patients admitted to teaching hospitals has not previously been examined.
In our previous study[10] of over 24,000 heart failure patients discharged over 10 years (up to June 2009, therefore no overlap with any patients in this study), we also found that patients discharged on the weekends were younger, had fewer comorbidities, and shorter lengths of stay. Although postdischarge death/readmission rates were higher for weekend discharged patients in our earlier study (21.1% vs 19.5%, adjusted hazard ratio: 1.15, 95% CI: 1.061.25), it is worth noting that this was almost entirely driven by data from nonteaching hospitals and cardiology wards. Thus, it is important to reiterate that the findings in our current study are for GIM wards in teaching hospitals and may not be generalizable to less‐structured nonteaching settings.
Although we did not study physician decision making, our results suggest that physicians are incorporating discharge day into their discharge decision making. They may be selecting younger patients with less comorbidities for weekend discharges, or they may be delaying the discharges of older patients with more comorbidities for weekday discharges. Either is not surprising given the realities of weekend inpatient care: reduced staffing and frequent cross‐coverage (of physicians, nurses, physiotherapists, pharmacists, and occupational therapists), limited support services (such as laboratory services or diagnostic imaging), and decreased availability of community services (including home care and social support services).[17] For example, in 1 large US heart failure registry, patients discharged on a weekend received less complete discharge instructions than those discharged on weekdays.[11] Given that early follow‐up postdischarge is associated with better outcomes,[18, 19] future studies should also explore whether patterns of patient follow‐up differ after weekend versus weekday discharges.
Although we were able to capture all interactions with the healthcare system in a single payer system with universal access, there are some limitations to our study. First, we used administrative data, which preclude fully adjusting for severity of diagnoses or functional status, although we used proxies such as admission from/discharge to a long‐term care facility.[20, 21] Second, we did not have access to process of care measures such as diagnostic testing or prescribing data, and thus cannot determine whether quality of care or patient adherence differed by the day of the week they were discharged on, although this seems unlikely. Third, although postdischarge follow‐up may be associated with better outcomes,[18, 19] we were unable to adjust for patterns of outpatient follow‐up in this study. Fourth, we acknowledge that death or readmission soon after discharge does not necessarily mean that the quality of care during the preceding hospitalization was suboptimal or that these deaths or readmissions were even potentially preventable. Many factors influence postdischarge mortality and/or readmission, and quality of inpatient care is only one.[22, 23, 24, 25] Fifth, although some may express concern that LOS may be a mediator in the causal pathway between discharge decision and postdischarge events, and that adjusting for LOS in analyses could thus spuriously obscure a true association, it is worth pointing out that our 2 sensitivity analyses to explore this (the 1 in which we excluded LOS from the analyses and the 1 in which we included expected LOS rather than the actual LOS) revealed nearly identical point estimates and 95% CI as our main analysis. Finally, as our study is observational, we cannot definitively conclude causality, nor can we exclude an 18% excess risk for patients discharged on weekends (or a 22% lower risk either), given our 95% CI for postdischarge adverse outcomes.
CONCLUSION
We found that the proportion of patients discharged on weekends is lower than the proportion admitted on weekends. We also found that lower risk/less severely ill patients appear to be preferentially discharged on weekends, and as a result, postdischarge outcomes are similar between weekend and weekday discharges despite shorter LOS and less availability of outpatient resources for patients discharged on a weekend. The reasons why more complicated patients are not discharged on weekends deserves further study, as safely increasing weekend discharge rates would improve efficiency and safety (by reducing unnecessary exposure to in‐hospital adverse events such as falls, unnecessary urinary catheterizations, and healthcare‐acquired infections). Although hospital admission has become a 24/7 business, we believe that hospital discharge processes should strive for the same level of efficiency.
ACKNOWLEDGMENTS
Disclosures: This study is based in part on data provided by Alberta Health. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the government of Alberta. Neither the government of Alberta nor Alberta Health express any opinion in relation to this study. F.A.M. and S.R.M. are supported by salary awards from Alberta Innovates‐Health Solutions (AIHS). F.A.M. holds the Capital Health Chair in Cardiology Outcomes Research. S.R.M. holds the Endowed Chair in Patient Health Management. This project was funded by AIHS through an investigator‐initiated peer reviewed operating grant. The funding agencies did not have input into study design, data collection, interpretation of results, or write up/approval for submission. The authors report no conflicts of interest.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345:663–668.
- , , , et al. Relationship between time of day, day of week, timeliness of reperfusion, and in‐hospital mortality for patients with acute ST‐segment elevation myocardial infarction. JAMA. 2005;294:803–812.
- , . Waiting for urgent procedures on the weekend among emergently hospitalized patients. Am J Med. 2004;117:175–181.
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007;42:1589–1612.
- , , , et al. Day of admission and clinical outcomes for patients hospitalized for heart failure: findings from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE‐HF). Circ Heart Fail. 2008;1:50–57.
- , , , et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105:74–84.
- , , , . Weekends: a dangerous time for having a stroke? Stroke. 2007;38:1211–1215.
- , , , . Day of the week of intensive care admission and patient outcomes: a multisite regional evaluation. Med Care. 2002;40:530–539.
- , , , . Effects of weekend admission and hospital teaching status on in‐hospital mortality. Am J Med. 2004;117:151–157.
- , , , , . Postdischarge outcomes in heart failure are better for teaching hospitals and weekday discharges. Circ Heart Fail. 2013;6:922–929.
- , , , et al. Weekend hospital admission and discharge for heart failure: association with quality of care and clinical outcomes. Am Heart J. 2009;158:451–458.
- , . Risk of death or readmission among people discharged from hospital on Fridays. CMAJ. 2002;166:1672–1673.
- , , , , et al.; IMECCHI Investigators. Assessing validity of ICD‐9‐CM and ICD‐10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441.
- , , , et al. Safely and effectively reducing inpatient length of stay: a controlled study of the General Internal Medicine Care Transformation Initiative. BMJ Qual Saf. 2014;23:446–456.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182:551–557.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- , . Excellent hospital care for all: open and operating 24/7. J Gen Intern Med. 2011;26:1050–1052.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722.
- , , , , , . Impact of physician continuity on death or urgent readmission after discharge among patients with heart failure. CMAJ. 2013;185:e681–e689.
- , , , , , . Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research. Ann Intern Med. 1993;119:844–850.
- , , , . Predictions of hospital mortality rates: a comparison of data sources. Ann Intern Med. 1997;126:347–354.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
- , . Investigating early readmission as an indicator for quality of care studies. Med Care. 1991;29(4):377–394.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345:663–668.
- , , , et al. Relationship between time of day, day of week, timeliness of reperfusion, and in‐hospital mortality for patients with acute ST‐segment elevation myocardial infarction. JAMA. 2005;294:803–812.
- , . Waiting for urgent procedures on the weekend among emergently hospitalized patients. Am J Med. 2004;117:175–181.
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007;42:1589–1612.
- , , , et al. Day of admission and clinical outcomes for patients hospitalized for heart failure: findings from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE‐HF). Circ Heart Fail. 2008;1:50–57.
- , , , et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105:74–84.
- , , , . Weekends: a dangerous time for having a stroke? Stroke. 2007;38:1211–1215.
- , , , . Day of the week of intensive care admission and patient outcomes: a multisite regional evaluation. Med Care. 2002;40:530–539.
- , , , . Effects of weekend admission and hospital teaching status on in‐hospital mortality. Am J Med. 2004;117:151–157.
- , , , , . Postdischarge outcomes in heart failure are better for teaching hospitals and weekday discharges. Circ Heart Fail. 2013;6:922–929.
- , , , et al. Weekend hospital admission and discharge for heart failure: association with quality of care and clinical outcomes. Am Heart J. 2009;158:451–458.
- , . Risk of death or readmission among people discharged from hospital on Fridays. CMAJ. 2002;166:1672–1673.
- , , , , et al.; IMECCHI Investigators. Assessing validity of ICD‐9‐CM and ICD‐10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441.
- , , , et al. Safely and effectively reducing inpatient length of stay: a controlled study of the General Internal Medicine Care Transformation Initiative. BMJ Qual Saf. 2014;23:446–456.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182:551–557.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- , . Excellent hospital care for all: open and operating 24/7. J Gen Intern Med. 2011;26:1050–1052.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722.
- , , , , , . Impact of physician continuity on death or urgent readmission after discharge among patients with heart failure. CMAJ. 2013;185:e681–e689.
- , , , , , . Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research. Ann Intern Med. 1993;119:844–850.
- , , , . Predictions of hospital mortality rates: a comparison of data sources. Ann Intern Med. 1997;126:347–354.
- , , , et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–282.
- , . Investigating early readmission as an indicator for quality of care studies. Med Care. 1991;29(4):377–394.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
© 2014 Society of Hospital Medicine