User login
Angiosome revascularization improves limb salvage
YES: The importance of the concept is established.
An angiosome is a three-dimensional anatomic unit of tissue fed by a single-source artery. The angiosome theory was first investigated in the plastic surgical literature by Taylor in the British Journal of Plastic Surgery in 1987,1 describing 40 angiosomes throughout the body. In the lower extremity, six distinct angiosomes have been defined; one arising from the anterior tibial artery (dorsalis pedis), two from the peroneal artery (the lateral calcaneal branch and the anterior perforating branch), and three from the posterior tibial artery (the calcaneal branch, the medial plantar branch, and the lateral plantar branch).2 Indirect connections known as choke vessels exist to enhance perfusion between angiosomes.
The question arises as to whether angiosome-specific revascularization enhances the healing of ischemic tissue loss of the lower extremity. Is direct revascularization of the appropriate angiosome an important part of the planning process for such revascularization efforts? Personal experience with 60 consecutive bypasses for ischemic lower extremity wounds left little doubt that angiosome-specific revascularization enhanced healing. Direct revascularization obtained 91% healing, compared with 62% when revascularization was performed to an artery which indirectly perfused the angiosome where the wound was located.3 There was also a trend for faster healing with direct angiosome revascularization. Other investigators have reported similar findings. Kret and colleagues found similar results for bypass, reporting complete healing in 78% of 106 limbs with direct revascularization with only 46% healing after bypass resulting in indirect angiosome revascularization. These authors noted that a “significant predictor for wound healing and reduced healing time was angiosome revascularization.”4
Consideration of the appropriate angiosome may be even more important for endovascular techniques in determining the target artery for revascularization. Iida et al investigated 200 ischemic ulcers, showing that healing was greatly enhanced by direct revascularization of the angiosome in which the wound was located.5 Kabra examined a mixed cohort of bypass and endovascular procedures and documented increased healing with angiosome revascularization for both modalities in treating critical limb ischemia, therefore, advising that angiosomes should be considered whenever possible.6 This is also an important concept for those diabetic ulcers in need of robust perfusion for healing. Alexandrescu demonstrated improved healing for diabetic ischemic ulcers after endovascular therapy with direct angiosome revascularization, concluding, “an angiosome model of perfusion helps the treatment of diabetic foot ulcers.”7 The group in Helsinki documented statistically significant better healing with endovascular revascularization of the appropriate angiosome in more than 250 patients with ischemic diabetic ulcers, surmising that the angiosome model is important for ulcer healing in diabetic patients.8
Given the many series, from around the world, confirming the impact of direct revascularization of the appropriate angiosome for a wound or non-healing ulcer to enhance healing, the importance of this concept in planning lower extremity revascularization has been established. There are certainly many considerations in planning revascularization such as patient presentation, arterial anatomy, and conduit or device selection. However, as revascularization of the appropriate wound angiosome results in more complete and rapid healing, it is irrefutable that the angiosome concept should be a consideration in planning revascularization for healing and limb preservation.
Dr. Neville is the Sara and Arnold P. Friedman and Carol and Eugene A. Ludwig Chief of Vascular Surgery Professor, Department of Surgery, and the Director, Limb Preservation Center, George Washington University, Washington.
References
1. Br J Plast Surg. 1987 Mar;40:113-41.
2. Plast Reconstr Surg. 2006 Jun;117;261S-293S.
3. Ann Vasc Surg. 2009;23:367-73.
4. J Vasc Surg. 2014 Jan;59:121-8.
5. Endovascular Today. 2010;9;96-100.
6. J Vasc Surg. 2013 Jan;57:44-49.
7. J Endovasc Ther. 2008 Oct;15:580-83.
8. J Vasc Surg. 2013 Feb;57:427-35.
NO: Only a guide, not an absolute.
Despite an aggressive approach to revascularization, amputation rates of up to 20% can occur despite a patent bypass.1 This has led to enthusiasm for an angiosome-based revascularization strategy for the management of ischemic foot lesions.2-4 There is no question that clinicians would opt to revascularize a blood vessel that directly feeds an involved angiosome if the vessel is easily accessible, is of good quality, and has good run-off. The issue arises if the target vessel does not meet that criteria and the surgeon is forced to intervene on an alternative feeding vessel (indirect revascularization).
There have been several studies which compare outcomes after direct and indirect revascularization strategies and they conclude that direct revascularization has better limb salvage rates.2 However, most of the studies were retrospective and details on the status and quality of the pedal arch were not consistently evaluated. Rashid et al studied the impact of direct angiosome revascularization on the healing of the foot and reported that healing and time to healing of foot tissue loss were significantly influenced by the quality of the pedal arch rather than the angiosome revascularized.3
Because of variations in the arterial anatomy of the foot,5 inconsistencies in the extent of an angiosome and the collateral connections between angiosomes are frequent, suggesting that the angiosome that needs to be revascularized may not be perfused by the predicted artery. This helps explain why technical success may not always equate directly with clinical success, as corroborated by indocyanine green (ICG) imaging and white-light tissue spectrophotometry.6 It is also important to emphasize that in their initial publication, Taylor and Palmer emphasized that the basis of their proposed angiosome concept was on the structural anatomy of the feeder vessel territory. They did not and could not assess the perfusion levels and extent of the feeder vessel with their corresponding choke vessels.
Forefoot procedures, such as trans-metatarsal amputations, frequently interrupt this foot arch. Likewise, a large proportion of patients with renal insufficiency and/or diabetes mellitus present with extensive foot wounds with deep infection that may result in compartmentalization within the foot. In one series, only one third of patients had a single angiosome involved in the tissue loss, 45% of patients had two angiosomes involved and more than 20% of patients had three angiosomes involved.7 Patients with more than one angiosome affected by extensive tissue loss are not easily analyzed using the angiosome-oriented concept and so attempts at classifying the intervention as being direct or indirect is problematic.
Studies analyzing the utility of the angiosome concept need to be careful in analyzing the extent of the territories encompassed by the wounds. More importantly, many interventionalists equate tibial or peroneal revascularization with angiosomal revascularization. This may not be the case if the terminal branches are diseased and pedal loop interventions may still be necessary.
In summary, the angiosome model should not be used as an absolute strategy for interventions on critical limb ischemia patients but should be a guide to assist with a patient-specific strategy for revascularization. Further well-structured prospective studies are needed to assess the value of integrating the interangiosome concept, the status of the pedal arch, and the anatomic-physiologic perfusion angiosome model.
Dr. Sumpio is a professor of surgery and radiology, Yale University, New Haven, Conn.
References
1. J Vasc Surg. 2010 Jun;51:1419-24.
2. J Vasc Surg. 2013 Sep;58:814-26.
3. J Vasc Surg. 2013 May;57:1219-26.
4. J Vasc Surg. 2013 Jan;57:44-9.
5. Am J Surg. 1993 Aug;166:130-5.
YES: The importance of the concept is established.
An angiosome is a three-dimensional anatomic unit of tissue fed by a single-source artery. The angiosome theory was first investigated in the plastic surgical literature by Taylor in the British Journal of Plastic Surgery in 1987,1 describing 40 angiosomes throughout the body. In the lower extremity, six distinct angiosomes have been defined; one arising from the anterior tibial artery (dorsalis pedis), two from the peroneal artery (the lateral calcaneal branch and the anterior perforating branch), and three from the posterior tibial artery (the calcaneal branch, the medial plantar branch, and the lateral plantar branch).2 Indirect connections known as choke vessels exist to enhance perfusion between angiosomes.
The question arises as to whether angiosome-specific revascularization enhances the healing of ischemic tissue loss of the lower extremity. Is direct revascularization of the appropriate angiosome an important part of the planning process for such revascularization efforts? Personal experience with 60 consecutive bypasses for ischemic lower extremity wounds left little doubt that angiosome-specific revascularization enhanced healing. Direct revascularization obtained 91% healing, compared with 62% when revascularization was performed to an artery which indirectly perfused the angiosome where the wound was located.3 There was also a trend for faster healing with direct angiosome revascularization. Other investigators have reported similar findings. Kret and colleagues found similar results for bypass, reporting complete healing in 78% of 106 limbs with direct revascularization with only 46% healing after bypass resulting in indirect angiosome revascularization. These authors noted that a “significant predictor for wound healing and reduced healing time was angiosome revascularization.”4
Consideration of the appropriate angiosome may be even more important for endovascular techniques in determining the target artery for revascularization. Iida et al investigated 200 ischemic ulcers, showing that healing was greatly enhanced by direct revascularization of the angiosome in which the wound was located.5 Kabra examined a mixed cohort of bypass and endovascular procedures and documented increased healing with angiosome revascularization for both modalities in treating critical limb ischemia, therefore, advising that angiosomes should be considered whenever possible.6 This is also an important concept for those diabetic ulcers in need of robust perfusion for healing. Alexandrescu demonstrated improved healing for diabetic ischemic ulcers after endovascular therapy with direct angiosome revascularization, concluding, “an angiosome model of perfusion helps the treatment of diabetic foot ulcers.”7 The group in Helsinki documented statistically significant better healing with endovascular revascularization of the appropriate angiosome in more than 250 patients with ischemic diabetic ulcers, surmising that the angiosome model is important for ulcer healing in diabetic patients.8
Given the many series, from around the world, confirming the impact of direct revascularization of the appropriate angiosome for a wound or non-healing ulcer to enhance healing, the importance of this concept in planning lower extremity revascularization has been established. There are certainly many considerations in planning revascularization such as patient presentation, arterial anatomy, and conduit or device selection. However, as revascularization of the appropriate wound angiosome results in more complete and rapid healing, it is irrefutable that the angiosome concept should be a consideration in planning revascularization for healing and limb preservation.
Dr. Neville is the Sara and Arnold P. Friedman and Carol and Eugene A. Ludwig Chief of Vascular Surgery Professor, Department of Surgery, and the Director, Limb Preservation Center, George Washington University, Washington.
References
1. Br J Plast Surg. 1987 Mar;40:113-41.
2. Plast Reconstr Surg. 2006 Jun;117;261S-293S.
3. Ann Vasc Surg. 2009;23:367-73.
4. J Vasc Surg. 2014 Jan;59:121-8.
5. Endovascular Today. 2010;9;96-100.
6. J Vasc Surg. 2013 Jan;57:44-49.
7. J Endovasc Ther. 2008 Oct;15:580-83.
8. J Vasc Surg. 2013 Feb;57:427-35.
NO: Only a guide, not an absolute.
Despite an aggressive approach to revascularization, amputation rates of up to 20% can occur despite a patent bypass.1 This has led to enthusiasm for an angiosome-based revascularization strategy for the management of ischemic foot lesions.2-4 There is no question that clinicians would opt to revascularize a blood vessel that directly feeds an involved angiosome if the vessel is easily accessible, is of good quality, and has good run-off. The issue arises if the target vessel does not meet that criteria and the surgeon is forced to intervene on an alternative feeding vessel (indirect revascularization).
There have been several studies which compare outcomes after direct and indirect revascularization strategies and they conclude that direct revascularization has better limb salvage rates.2 However, most of the studies were retrospective and details on the status and quality of the pedal arch were not consistently evaluated. Rashid et al studied the impact of direct angiosome revascularization on the healing of the foot and reported that healing and time to healing of foot tissue loss were significantly influenced by the quality of the pedal arch rather than the angiosome revascularized.3
Because of variations in the arterial anatomy of the foot,5 inconsistencies in the extent of an angiosome and the collateral connections between angiosomes are frequent, suggesting that the angiosome that needs to be revascularized may not be perfused by the predicted artery. This helps explain why technical success may not always equate directly with clinical success, as corroborated by indocyanine green (ICG) imaging and white-light tissue spectrophotometry.6 It is also important to emphasize that in their initial publication, Taylor and Palmer emphasized that the basis of their proposed angiosome concept was on the structural anatomy of the feeder vessel territory. They did not and could not assess the perfusion levels and extent of the feeder vessel with their corresponding choke vessels.
Forefoot procedures, such as trans-metatarsal amputations, frequently interrupt this foot arch. Likewise, a large proportion of patients with renal insufficiency and/or diabetes mellitus present with extensive foot wounds with deep infection that may result in compartmentalization within the foot. In one series, only one third of patients had a single angiosome involved in the tissue loss, 45% of patients had two angiosomes involved and more than 20% of patients had three angiosomes involved.7 Patients with more than one angiosome affected by extensive tissue loss are not easily analyzed using the angiosome-oriented concept and so attempts at classifying the intervention as being direct or indirect is problematic.
Studies analyzing the utility of the angiosome concept need to be careful in analyzing the extent of the territories encompassed by the wounds. More importantly, many interventionalists equate tibial or peroneal revascularization with angiosomal revascularization. This may not be the case if the terminal branches are diseased and pedal loop interventions may still be necessary.
In summary, the angiosome model should not be used as an absolute strategy for interventions on critical limb ischemia patients but should be a guide to assist with a patient-specific strategy for revascularization. Further well-structured prospective studies are needed to assess the value of integrating the interangiosome concept, the status of the pedal arch, and the anatomic-physiologic perfusion angiosome model.
Dr. Sumpio is a professor of surgery and radiology, Yale University, New Haven, Conn.
References
1. J Vasc Surg. 2010 Jun;51:1419-24.
2. J Vasc Surg. 2013 Sep;58:814-26.
3. J Vasc Surg. 2013 May;57:1219-26.
4. J Vasc Surg. 2013 Jan;57:44-9.
5. Am J Surg. 1993 Aug;166:130-5.
YES: The importance of the concept is established.
An angiosome is a three-dimensional anatomic unit of tissue fed by a single-source artery. The angiosome theory was first investigated in the plastic surgical literature by Taylor in the British Journal of Plastic Surgery in 1987,1 describing 40 angiosomes throughout the body. In the lower extremity, six distinct angiosomes have been defined; one arising from the anterior tibial artery (dorsalis pedis), two from the peroneal artery (the lateral calcaneal branch and the anterior perforating branch), and three from the posterior tibial artery (the calcaneal branch, the medial plantar branch, and the lateral plantar branch).2 Indirect connections known as choke vessels exist to enhance perfusion between angiosomes.
The question arises as to whether angiosome-specific revascularization enhances the healing of ischemic tissue loss of the lower extremity. Is direct revascularization of the appropriate angiosome an important part of the planning process for such revascularization efforts? Personal experience with 60 consecutive bypasses for ischemic lower extremity wounds left little doubt that angiosome-specific revascularization enhanced healing. Direct revascularization obtained 91% healing, compared with 62% when revascularization was performed to an artery which indirectly perfused the angiosome where the wound was located.3 There was also a trend for faster healing with direct angiosome revascularization. Other investigators have reported similar findings. Kret and colleagues found similar results for bypass, reporting complete healing in 78% of 106 limbs with direct revascularization with only 46% healing after bypass resulting in indirect angiosome revascularization. These authors noted that a “significant predictor for wound healing and reduced healing time was angiosome revascularization.”4
Consideration of the appropriate angiosome may be even more important for endovascular techniques in determining the target artery for revascularization. Iida et al investigated 200 ischemic ulcers, showing that healing was greatly enhanced by direct revascularization of the angiosome in which the wound was located.5 Kabra examined a mixed cohort of bypass and endovascular procedures and documented increased healing with angiosome revascularization for both modalities in treating critical limb ischemia, therefore, advising that angiosomes should be considered whenever possible.6 This is also an important concept for those diabetic ulcers in need of robust perfusion for healing. Alexandrescu demonstrated improved healing for diabetic ischemic ulcers after endovascular therapy with direct angiosome revascularization, concluding, “an angiosome model of perfusion helps the treatment of diabetic foot ulcers.”7 The group in Helsinki documented statistically significant better healing with endovascular revascularization of the appropriate angiosome in more than 250 patients with ischemic diabetic ulcers, surmising that the angiosome model is important for ulcer healing in diabetic patients.8
Given the many series, from around the world, confirming the impact of direct revascularization of the appropriate angiosome for a wound or non-healing ulcer to enhance healing, the importance of this concept in planning lower extremity revascularization has been established. There are certainly many considerations in planning revascularization such as patient presentation, arterial anatomy, and conduit or device selection. However, as revascularization of the appropriate wound angiosome results in more complete and rapid healing, it is irrefutable that the angiosome concept should be a consideration in planning revascularization for healing and limb preservation.
Dr. Neville is the Sara and Arnold P. Friedman and Carol and Eugene A. Ludwig Chief of Vascular Surgery Professor, Department of Surgery, and the Director, Limb Preservation Center, George Washington University, Washington.
References
1. Br J Plast Surg. 1987 Mar;40:113-41.
2. Plast Reconstr Surg. 2006 Jun;117;261S-293S.
3. Ann Vasc Surg. 2009;23:367-73.
4. J Vasc Surg. 2014 Jan;59:121-8.
5. Endovascular Today. 2010;9;96-100.
6. J Vasc Surg. 2013 Jan;57:44-49.
7. J Endovasc Ther. 2008 Oct;15:580-83.
8. J Vasc Surg. 2013 Feb;57:427-35.
NO: Only a guide, not an absolute.
Despite an aggressive approach to revascularization, amputation rates of up to 20% can occur despite a patent bypass.1 This has led to enthusiasm for an angiosome-based revascularization strategy for the management of ischemic foot lesions.2-4 There is no question that clinicians would opt to revascularize a blood vessel that directly feeds an involved angiosome if the vessel is easily accessible, is of good quality, and has good run-off. The issue arises if the target vessel does not meet that criteria and the surgeon is forced to intervene on an alternative feeding vessel (indirect revascularization).
There have been several studies which compare outcomes after direct and indirect revascularization strategies and they conclude that direct revascularization has better limb salvage rates.2 However, most of the studies were retrospective and details on the status and quality of the pedal arch were not consistently evaluated. Rashid et al studied the impact of direct angiosome revascularization on the healing of the foot and reported that healing and time to healing of foot tissue loss were significantly influenced by the quality of the pedal arch rather than the angiosome revascularized.3
Because of variations in the arterial anatomy of the foot,5 inconsistencies in the extent of an angiosome and the collateral connections between angiosomes are frequent, suggesting that the angiosome that needs to be revascularized may not be perfused by the predicted artery. This helps explain why technical success may not always equate directly with clinical success, as corroborated by indocyanine green (ICG) imaging and white-light tissue spectrophotometry.6 It is also important to emphasize that in their initial publication, Taylor and Palmer emphasized that the basis of their proposed angiosome concept was on the structural anatomy of the feeder vessel territory. They did not and could not assess the perfusion levels and extent of the feeder vessel with their corresponding choke vessels.
Forefoot procedures, such as trans-metatarsal amputations, frequently interrupt this foot arch. Likewise, a large proportion of patients with renal insufficiency and/or diabetes mellitus present with extensive foot wounds with deep infection that may result in compartmentalization within the foot. In one series, only one third of patients had a single angiosome involved in the tissue loss, 45% of patients had two angiosomes involved and more than 20% of patients had three angiosomes involved.7 Patients with more than one angiosome affected by extensive tissue loss are not easily analyzed using the angiosome-oriented concept and so attempts at classifying the intervention as being direct or indirect is problematic.
Studies analyzing the utility of the angiosome concept need to be careful in analyzing the extent of the territories encompassed by the wounds. More importantly, many interventionalists equate tibial or peroneal revascularization with angiosomal revascularization. This may not be the case if the terminal branches are diseased and pedal loop interventions may still be necessary.
In summary, the angiosome model should not be used as an absolute strategy for interventions on critical limb ischemia patients but should be a guide to assist with a patient-specific strategy for revascularization. Further well-structured prospective studies are needed to assess the value of integrating the interangiosome concept, the status of the pedal arch, and the anatomic-physiologic perfusion angiosome model.
Dr. Sumpio is a professor of surgery and radiology, Yale University, New Haven, Conn.
References
1. J Vasc Surg. 2010 Jun;51:1419-24.
2. J Vasc Surg. 2013 Sep;58:814-26.
3. J Vasc Surg. 2013 May;57:1219-26.
4. J Vasc Surg. 2013 Jan;57:44-9.
5. Am J Surg. 1993 Aug;166:130-5.
Initiative dramatically raises HCV screening, treatment
A hepatitis C awareness initiative dramatically raised the rate of HCV screening and treatment in a large American Indian population – the ethnic group with the highest rate of HCV infection and HCV-related mortality in the United States, according to a report published May 13 in Morbidity and Mortality Weekly Report.
Cherokee Nation Health Services undertook the effort in 2012 to improve detection and management of HCV. It included a reminder in eligible patients’ electronic health records (EHRs) to offer screening; HCV education for primary care physicians and other health care providers; establishment of an HCV registry to monitor the clinical care of patients who initiated antiviral treatment; and outreach efforts by public health nurses to HCV patients, including home visits.
In 2014, an additional initiative was implemented to expand services for the rapidly increasing number of patients diagnosed as having HCV. This allowed a transition from having a single clinic staffed by only one caregiver with expertise in HCV management to five clinics staffed by three physicians, two nurse practitioners, and two pharmacists with HCV expertise, said Dr. Jorge Mera, director of infectious diseases, Cherokee Nation Health Services, Tulsa, Okla., and his associates.
An analysis of deidentified data in the HCV registry and EHRs showed that 92,012 patients aged 20 years and older had at least one visit with Cherokee Nation Health Services after the program was implemented, between October 2012 and July 2015. The proportion of this patient population that was tested for HCV antibodies rose fivefold, from 3.6% to 18.2% during the study period. A total of 715 patients were antibody positive, and 388 of them were found to have chronic HCV infection. Approximately 60% of these patients initiated antiviral treatment, and approximately 90% of them achieved a sustained virologic response and were essentially cured, Dr. Mera and his associates said (MMWR. 2016 May 13;65[18]:461-6).
The program included a component that particularly targeted baby boomers – patients born between 1945 and 1965 – for HCV screening. Across the Indian Health Service clinics in 34 states that adopted this component of the program, such screening increased fourfold in this high-risk population during the study period (MMWR. 2016 May 13;65[18]:467-9).
These efforts, the first of their kind in the United States, may help eliminate hepatitis C as a health disparity for American Indian/Alaska Native populations and also may serve as a model for other health care settings, the investigators added.
A hepatitis C awareness initiative dramatically raised the rate of HCV screening and treatment in a large American Indian population – the ethnic group with the highest rate of HCV infection and HCV-related mortality in the United States, according to a report published May 13 in Morbidity and Mortality Weekly Report.
Cherokee Nation Health Services undertook the effort in 2012 to improve detection and management of HCV. It included a reminder in eligible patients’ electronic health records (EHRs) to offer screening; HCV education for primary care physicians and other health care providers; establishment of an HCV registry to monitor the clinical care of patients who initiated antiviral treatment; and outreach efforts by public health nurses to HCV patients, including home visits.
In 2014, an additional initiative was implemented to expand services for the rapidly increasing number of patients diagnosed as having HCV. This allowed a transition from having a single clinic staffed by only one caregiver with expertise in HCV management to five clinics staffed by three physicians, two nurse practitioners, and two pharmacists with HCV expertise, said Dr. Jorge Mera, director of infectious diseases, Cherokee Nation Health Services, Tulsa, Okla., and his associates.
An analysis of deidentified data in the HCV registry and EHRs showed that 92,012 patients aged 20 years and older had at least one visit with Cherokee Nation Health Services after the program was implemented, between October 2012 and July 2015. The proportion of this patient population that was tested for HCV antibodies rose fivefold, from 3.6% to 18.2% during the study period. A total of 715 patients were antibody positive, and 388 of them were found to have chronic HCV infection. Approximately 60% of these patients initiated antiviral treatment, and approximately 90% of them achieved a sustained virologic response and were essentially cured, Dr. Mera and his associates said (MMWR. 2016 May 13;65[18]:461-6).
The program included a component that particularly targeted baby boomers – patients born between 1945 and 1965 – for HCV screening. Across the Indian Health Service clinics in 34 states that adopted this component of the program, such screening increased fourfold in this high-risk population during the study period (MMWR. 2016 May 13;65[18]:467-9).
These efforts, the first of their kind in the United States, may help eliminate hepatitis C as a health disparity for American Indian/Alaska Native populations and also may serve as a model for other health care settings, the investigators added.
A hepatitis C awareness initiative dramatically raised the rate of HCV screening and treatment in a large American Indian population – the ethnic group with the highest rate of HCV infection and HCV-related mortality in the United States, according to a report published May 13 in Morbidity and Mortality Weekly Report.
Cherokee Nation Health Services undertook the effort in 2012 to improve detection and management of HCV. It included a reminder in eligible patients’ electronic health records (EHRs) to offer screening; HCV education for primary care physicians and other health care providers; establishment of an HCV registry to monitor the clinical care of patients who initiated antiviral treatment; and outreach efforts by public health nurses to HCV patients, including home visits.
In 2014, an additional initiative was implemented to expand services for the rapidly increasing number of patients diagnosed as having HCV. This allowed a transition from having a single clinic staffed by only one caregiver with expertise in HCV management to five clinics staffed by three physicians, two nurse practitioners, and two pharmacists with HCV expertise, said Dr. Jorge Mera, director of infectious diseases, Cherokee Nation Health Services, Tulsa, Okla., and his associates.
An analysis of deidentified data in the HCV registry and EHRs showed that 92,012 patients aged 20 years and older had at least one visit with Cherokee Nation Health Services after the program was implemented, between October 2012 and July 2015. The proportion of this patient population that was tested for HCV antibodies rose fivefold, from 3.6% to 18.2% during the study period. A total of 715 patients were antibody positive, and 388 of them were found to have chronic HCV infection. Approximately 60% of these patients initiated antiviral treatment, and approximately 90% of them achieved a sustained virologic response and were essentially cured, Dr. Mera and his associates said (MMWR. 2016 May 13;65[18]:461-6).
The program included a component that particularly targeted baby boomers – patients born between 1945 and 1965 – for HCV screening. Across the Indian Health Service clinics in 34 states that adopted this component of the program, such screening increased fourfold in this high-risk population during the study period (MMWR. 2016 May 13;65[18]:467-9).
These efforts, the first of their kind in the United States, may help eliminate hepatitis C as a health disparity for American Indian/Alaska Native populations and also may serve as a model for other health care settings, the investigators added.
FROM MORBIDITY and MORTALITY WEEKLY REPORT
Key clinical point: A new program dramatically raised the rate of HCV screening and treatment in a large American Indian population – the ethnic group with the highest rate of HCV infection and HCV-related mortality in the United States.
Major finding: The proportion of adults tested for HCV antibodies rose fivefold, from 3.6% to 18.2%, during the study period.
Data source: An observational cohort study involving 92,012 adults who had at least 1 visit to Cherokee Nation Health Services between October 2012 and July 2015.
Disclosures: The sponsor of this study was not specified, and potential financial conflicts of interest were not provided. The authors were affiliated with Cherokee Nation Health Services, the U.S. Centers for Disease Control and Prevention, the University of Oklahoma Health Sciences Center, the Oklahoma City Veterans Affairs Medical Center, the Indian Health Service, and the Northwest Portland (Ore.) Area Indian Health Board.
Veteran Suicide Prevention Efforts Under Scrutiny
In a House Veterans’ Affairs Committee hearing on efforts to prevent veteran suicide, the VA confirmed that it is focused on ensuring it has enough mental health care providers. The VA currently employs 5,500 psychologists and 3,203 psychiatrists. According to Maureen F. McCarthy, MD, deputy chief, VHA Office of Patient Care Services, the VA currently has 236 psychiatrist vacancies.
Veteran service organizations, House members, and the VA all agreed that hiring and retaining qualified mental health care providers is an essential component in reducing suicides. “We are aware that access to mental health care is one significant part of preventing suicide,” McCarthy said in prepared testimony. “VA is determined to address systemic problems with access to care in general and to mental health care, including substance use disorders in particular. VA has recommitted to a culture that puts the veteran first.”
As House members, veteran service organizations, and the VA have all admitted, the frequently quoted 22 veterans suicides each day statistic is based on dated and limited data. To better understand the scope of the problem, the VA is working with the CDC to obtain a more current and accurate count of veteran suicides—a move that many veterans advocacy groups have called for.
“Vietnam Veterans of America calls for an updated veteran suicide report that includes data from all 50 states and U.S. territories, and also strongly suggests that VA mental health services develop a nationwide strategy to address the problem of suicides among our older veterans—particularly Vietnam-era veterans,” Thomas J. Berger, PhD, executive director of the Veterans Health Council of the Vietnam Veterans of America (VVA) told the committee in his prepared remarks.
According to Dr. McCarthy, the data will be available later this summer and will accessible to researchers. “We so wanted to have this information to you by this hearing. We don’t,” she said.
Challenges Remain
Ensuring access to care is one of the VA’s chief challenges. “We have to change our messaging to be more welcoming to all veterans,” Dr. McCarthy told the committee. “There are still veterans out there that do not know that they are eligible for benefits.”
Another one of the challenges is streamlining the transition from the military to the VA. “VA research has indicated that rates of suicide among those who use VA services have not shown increases similar to those observed in all veterans and the general U.S. population,” McCarthy explained. “This research suggests that an improved health care transition between DoD and VA could help mitigate suicide risk as well as other increased risks of morbidity.” According to McCarthy, the VA and DoD are working to create a seamless transition for mental health medications from the DoD to the VA, following a safety review
Following up on the Preventing Veterans Suicide – A Call to Action summit, in March, VA announced 8 steps it planned to take to improve its suicide prevention programs. They are:
1. Elevating VA’s suicide-prevention program with additional resources to help manage and strengthen current programs and initiatives;
2. Meeting urgent mental health needs by providing veterans with same-day evaluations and access by the end of calendar year 2016;
3. Establishing a new standard of care by using measures of veteran-reported symptoms to tailor mental health treatments to individual needs;
4. Launching a new study, “Coming Home from Afghanistan and Iraq,” that will look at the impact of deployment and combat as it relates to suicide, mental health, and well-being;
5. Using predictive modeling to guide early interventions for suicide prevention;
6. Using data on suicide attempts and overdoses to guide strategies to prevent suicide;
7. Increasing the availability of naloxone rescue kits throughout VA to prevent deaths from opioid overdoses;
8. Enhancing veteran mental health access by establishing 3 regional telemental health hubs; and
9. Continuing to partner with the DoD on suicide prevention and other efforts for a seamless transition from military service to civilian life.
“While these initiatives are laudable, VVA also believes strongly that they cannot fully succeed without a significant increase in the recruitment, hiring, and retention of VA mental health staff, as well as timely access to VA mental health clinical facilities and programs, especially for our rural veterans,” Berger told the committee.
In a House Veterans’ Affairs Committee hearing on efforts to prevent veteran suicide, the VA confirmed that it is focused on ensuring it has enough mental health care providers. The VA currently employs 5,500 psychologists and 3,203 psychiatrists. According to Maureen F. McCarthy, MD, deputy chief, VHA Office of Patient Care Services, the VA currently has 236 psychiatrist vacancies.
Veteran service organizations, House members, and the VA all agreed that hiring and retaining qualified mental health care providers is an essential component in reducing suicides. “We are aware that access to mental health care is one significant part of preventing suicide,” McCarthy said in prepared testimony. “VA is determined to address systemic problems with access to care in general and to mental health care, including substance use disorders in particular. VA has recommitted to a culture that puts the veteran first.”
As House members, veteran service organizations, and the VA have all admitted, the frequently quoted 22 veterans suicides each day statistic is based on dated and limited data. To better understand the scope of the problem, the VA is working with the CDC to obtain a more current and accurate count of veteran suicides—a move that many veterans advocacy groups have called for.
“Vietnam Veterans of America calls for an updated veteran suicide report that includes data from all 50 states and U.S. territories, and also strongly suggests that VA mental health services develop a nationwide strategy to address the problem of suicides among our older veterans—particularly Vietnam-era veterans,” Thomas J. Berger, PhD, executive director of the Veterans Health Council of the Vietnam Veterans of America (VVA) told the committee in his prepared remarks.
According to Dr. McCarthy, the data will be available later this summer and will accessible to researchers. “We so wanted to have this information to you by this hearing. We don’t,” she said.
Challenges Remain
Ensuring access to care is one of the VA’s chief challenges. “We have to change our messaging to be more welcoming to all veterans,” Dr. McCarthy told the committee. “There are still veterans out there that do not know that they are eligible for benefits.”
Another one of the challenges is streamlining the transition from the military to the VA. “VA research has indicated that rates of suicide among those who use VA services have not shown increases similar to those observed in all veterans and the general U.S. population,” McCarthy explained. “This research suggests that an improved health care transition between DoD and VA could help mitigate suicide risk as well as other increased risks of morbidity.” According to McCarthy, the VA and DoD are working to create a seamless transition for mental health medications from the DoD to the VA, following a safety review
Following up on the Preventing Veterans Suicide – A Call to Action summit, in March, VA announced 8 steps it planned to take to improve its suicide prevention programs. They are:
1. Elevating VA’s suicide-prevention program with additional resources to help manage and strengthen current programs and initiatives;
2. Meeting urgent mental health needs by providing veterans with same-day evaluations and access by the end of calendar year 2016;
3. Establishing a new standard of care by using measures of veteran-reported symptoms to tailor mental health treatments to individual needs;
4. Launching a new study, “Coming Home from Afghanistan and Iraq,” that will look at the impact of deployment and combat as it relates to suicide, mental health, and well-being;
5. Using predictive modeling to guide early interventions for suicide prevention;
6. Using data on suicide attempts and overdoses to guide strategies to prevent suicide;
7. Increasing the availability of naloxone rescue kits throughout VA to prevent deaths from opioid overdoses;
8. Enhancing veteran mental health access by establishing 3 regional telemental health hubs; and
9. Continuing to partner with the DoD on suicide prevention and other efforts for a seamless transition from military service to civilian life.
“While these initiatives are laudable, VVA also believes strongly that they cannot fully succeed without a significant increase in the recruitment, hiring, and retention of VA mental health staff, as well as timely access to VA mental health clinical facilities and programs, especially for our rural veterans,” Berger told the committee.
In a House Veterans’ Affairs Committee hearing on efforts to prevent veteran suicide, the VA confirmed that it is focused on ensuring it has enough mental health care providers. The VA currently employs 5,500 psychologists and 3,203 psychiatrists. According to Maureen F. McCarthy, MD, deputy chief, VHA Office of Patient Care Services, the VA currently has 236 psychiatrist vacancies.
Veteran service organizations, House members, and the VA all agreed that hiring and retaining qualified mental health care providers is an essential component in reducing suicides. “We are aware that access to mental health care is one significant part of preventing suicide,” McCarthy said in prepared testimony. “VA is determined to address systemic problems with access to care in general and to mental health care, including substance use disorders in particular. VA has recommitted to a culture that puts the veteran first.”
As House members, veteran service organizations, and the VA have all admitted, the frequently quoted 22 veterans suicides each day statistic is based on dated and limited data. To better understand the scope of the problem, the VA is working with the CDC to obtain a more current and accurate count of veteran suicides—a move that many veterans advocacy groups have called for.
“Vietnam Veterans of America calls for an updated veteran suicide report that includes data from all 50 states and U.S. territories, and also strongly suggests that VA mental health services develop a nationwide strategy to address the problem of suicides among our older veterans—particularly Vietnam-era veterans,” Thomas J. Berger, PhD, executive director of the Veterans Health Council of the Vietnam Veterans of America (VVA) told the committee in his prepared remarks.
According to Dr. McCarthy, the data will be available later this summer and will accessible to researchers. “We so wanted to have this information to you by this hearing. We don’t,” she said.
Challenges Remain
Ensuring access to care is one of the VA’s chief challenges. “We have to change our messaging to be more welcoming to all veterans,” Dr. McCarthy told the committee. “There are still veterans out there that do not know that they are eligible for benefits.”
Another one of the challenges is streamlining the transition from the military to the VA. “VA research has indicated that rates of suicide among those who use VA services have not shown increases similar to those observed in all veterans and the general U.S. population,” McCarthy explained. “This research suggests that an improved health care transition between DoD and VA could help mitigate suicide risk as well as other increased risks of morbidity.” According to McCarthy, the VA and DoD are working to create a seamless transition for mental health medications from the DoD to the VA, following a safety review
Following up on the Preventing Veterans Suicide – A Call to Action summit, in March, VA announced 8 steps it planned to take to improve its suicide prevention programs. They are:
1. Elevating VA’s suicide-prevention program with additional resources to help manage and strengthen current programs and initiatives;
2. Meeting urgent mental health needs by providing veterans with same-day evaluations and access by the end of calendar year 2016;
3. Establishing a new standard of care by using measures of veteran-reported symptoms to tailor mental health treatments to individual needs;
4. Launching a new study, “Coming Home from Afghanistan and Iraq,” that will look at the impact of deployment and combat as it relates to suicide, mental health, and well-being;
5. Using predictive modeling to guide early interventions for suicide prevention;
6. Using data on suicide attempts and overdoses to guide strategies to prevent suicide;
7. Increasing the availability of naloxone rescue kits throughout VA to prevent deaths from opioid overdoses;
8. Enhancing veteran mental health access by establishing 3 regional telemental health hubs; and
9. Continuing to partner with the DoD on suicide prevention and other efforts for a seamless transition from military service to civilian life.
“While these initiatives are laudable, VVA also believes strongly that they cannot fully succeed without a significant increase in the recruitment, hiring, and retention of VA mental health staff, as well as timely access to VA mental health clinical facilities and programs, especially for our rural veterans,” Berger told the committee.
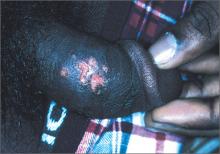
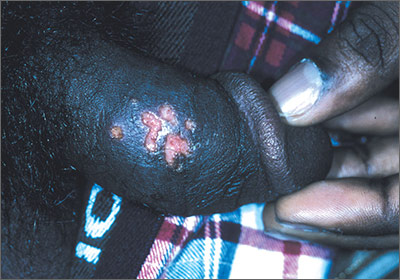
Painful vesicles on penis
The FP diagnosed this patient with genital herpes. The patient’s herpes culture came back positive and his rapid plasma reagin (RPR) and human immunodeficiency virus (HIV) tests were negative.
Genital herpes presents with multiple transient, painful vesicles that appear on the penis, vulva, buttocks, perineum, vagina, or cervix. The vesicles break down and become ulcers that develop crusts while healing. Recurrences typically occur 2 to 3 times a year. The duration is shorter and less painful than in primary infections. The lesions often heal completely by 8 to 10 days.
The gold standard of diagnosis is viral isolation by tissue culture or polymerase chain reaction (PCR) testing. The culture sensitivity rate is only 70% to 80% and depends upon the stage at which the specimen is collected. The sensitivity is highest in the vesicular stage and declines with ulceration and crusting. The tissue culture assay can be positive within 48 hours but may take longer.
PCR is extremely sensitive (96%) and specific (99%). PCR testing is generally used for cerebrospinal fluid testing in suspected herpes simplex virus encephalitis or meningitis. The Tzanck test and antigen detection tests have lower sensitivity rates than viral culture and should not be relied on for diagnosis.
Antiviral therapy is recommended for an initial genital herpes outbreak. Although systemic antiviral drugs can partially control the signs and symptoms of herpes episodes, they do not eradicate the latent virus. Acyclovir, famciclovir, and valacyclovir are equally effective for episodic treatment of genital herpes, but famciclovir appears somewhat less effective for suppression of viral shedding. Effective episodic treatment of herpes requires initiation of therapy during the prodrome period or within one day of lesion onset. Providing the patient with instructions to initiate treatment immediately when symptoms begin improves efficacy for future outbreaks. Patients with frequent recurrences can choose to take daily antiviral medication for prevention of new outbreaks.
It was too late to initiate antiviral therapy for this patient, so treatment was confined to oral over-the-counter analgesics and topical petrolatum. The FP counseled the patient about the nature of the disease, its transmissibility, and the likelihood of recurrence.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Carter K. Herpes simplex. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:735-742.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed this patient with genital herpes. The patient’s herpes culture came back positive and his rapid plasma reagin (RPR) and human immunodeficiency virus (HIV) tests were negative.
Genital herpes presents with multiple transient, painful vesicles that appear on the penis, vulva, buttocks, perineum, vagina, or cervix. The vesicles break down and become ulcers that develop crusts while healing. Recurrences typically occur 2 to 3 times a year. The duration is shorter and less painful than in primary infections. The lesions often heal completely by 8 to 10 days.
The gold standard of diagnosis is viral isolation by tissue culture or polymerase chain reaction (PCR) testing. The culture sensitivity rate is only 70% to 80% and depends upon the stage at which the specimen is collected. The sensitivity is highest in the vesicular stage and declines with ulceration and crusting. The tissue culture assay can be positive within 48 hours but may take longer.
PCR is extremely sensitive (96%) and specific (99%). PCR testing is generally used for cerebrospinal fluid testing in suspected herpes simplex virus encephalitis or meningitis. The Tzanck test and antigen detection tests have lower sensitivity rates than viral culture and should not be relied on for diagnosis.
Antiviral therapy is recommended for an initial genital herpes outbreak. Although systemic antiviral drugs can partially control the signs and symptoms of herpes episodes, they do not eradicate the latent virus. Acyclovir, famciclovir, and valacyclovir are equally effective for episodic treatment of genital herpes, but famciclovir appears somewhat less effective for suppression of viral shedding. Effective episodic treatment of herpes requires initiation of therapy during the prodrome period or within one day of lesion onset. Providing the patient with instructions to initiate treatment immediately when symptoms begin improves efficacy for future outbreaks. Patients with frequent recurrences can choose to take daily antiviral medication for prevention of new outbreaks.
It was too late to initiate antiviral therapy for this patient, so treatment was confined to oral over-the-counter analgesics and topical petrolatum. The FP counseled the patient about the nature of the disease, its transmissibility, and the likelihood of recurrence.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Carter K. Herpes simplex. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:735-742.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed this patient with genital herpes. The patient’s herpes culture came back positive and his rapid plasma reagin (RPR) and human immunodeficiency virus (HIV) tests were negative.
Genital herpes presents with multiple transient, painful vesicles that appear on the penis, vulva, buttocks, perineum, vagina, or cervix. The vesicles break down and become ulcers that develop crusts while healing. Recurrences typically occur 2 to 3 times a year. The duration is shorter and less painful than in primary infections. The lesions often heal completely by 8 to 10 days.
The gold standard of diagnosis is viral isolation by tissue culture or polymerase chain reaction (PCR) testing. The culture sensitivity rate is only 70% to 80% and depends upon the stage at which the specimen is collected. The sensitivity is highest in the vesicular stage and declines with ulceration and crusting. The tissue culture assay can be positive within 48 hours but may take longer.
PCR is extremely sensitive (96%) and specific (99%). PCR testing is generally used for cerebrospinal fluid testing in suspected herpes simplex virus encephalitis or meningitis. The Tzanck test and antigen detection tests have lower sensitivity rates than viral culture and should not be relied on for diagnosis.
Antiviral therapy is recommended for an initial genital herpes outbreak. Although systemic antiviral drugs can partially control the signs and symptoms of herpes episodes, they do not eradicate the latent virus. Acyclovir, famciclovir, and valacyclovir are equally effective for episodic treatment of genital herpes, but famciclovir appears somewhat less effective for suppression of viral shedding. Effective episodic treatment of herpes requires initiation of therapy during the prodrome period or within one day of lesion onset. Providing the patient with instructions to initiate treatment immediately when symptoms begin improves efficacy for future outbreaks. Patients with frequent recurrences can choose to take daily antiviral medication for prevention of new outbreaks.
It was too late to initiate antiviral therapy for this patient, so treatment was confined to oral over-the-counter analgesics and topical petrolatum. The FP counseled the patient about the nature of the disease, its transmissibility, and the likelihood of recurrence.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Carter K. Herpes simplex. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:735-742.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
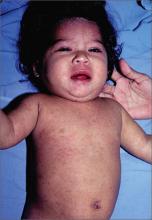
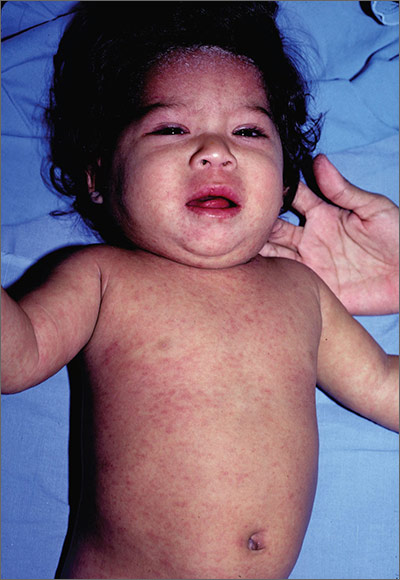
Child with fever, cough, and rash
The FP suspected that this patient had measles, but since it has a low prevalence in the United States, he confirmed the diagnosis with a specific serum immunoglobulin M antibody test. Measles is a highly communicable, acute, viral illness that is still one of the most serious infectious diseases in human history. Until the introduction of the measles-mumps-rubella vaccine, it was responsible for millions of deaths worldwide annually. Eradication of measles is possible, but the ease of transmission and the low percentage of nonimmunized population that is required for disease survival have made eradication extremely difficult.
The classic measles rash is maculopapular and blanches under pressure. The rash begins on the face and spreads centrifugally to involve the neck, trunk, and, finally, the extremities. This cranial-to-caudal rash progression is characteristic of measles. The cough may persist for up to 2 weeks. Fever persisting beyond the third day of rash suggests a measles-associated complication.
Postinfectious encephalomyelitis can also occur. Postinfectious encephalomyelitis is a demyelinating disease that presents during the recovery phase, and is thought to be caused by a postinfectious autoimmune response.
The treatment of measles is mostly supportive and patients will need to stay away from other individuals—particularly unimmunized children and adults, pregnant women, and immunocompromised people—until at least 4 days after rash onset. Suspected cases of measles should be reported immediately to the local or state department of health.
In this case, the child showed no evidence of pneumonia, neurological symptoms, or dehydration, so hospitalization was not needed. Fortunately, the mother and father had both been vaccinated as children and this was their only child. Antipyretics and fluids were recommended. The parents were told to avoid giving the child aspirin to prevent Reye’s syndrome.
The FP maintained contact with the family over the phone and the symptoms began resolving within a few days. The FP also reported the case to the local health department.
Photo courtesy of Dr. Eric Kraus. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Baudoin L. Measles. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY:McGraw-Hill;2013:723-727.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP suspected that this patient had measles, but since it has a low prevalence in the United States, he confirmed the diagnosis with a specific serum immunoglobulin M antibody test. Measles is a highly communicable, acute, viral illness that is still one of the most serious infectious diseases in human history. Until the introduction of the measles-mumps-rubella vaccine, it was responsible for millions of deaths worldwide annually. Eradication of measles is possible, but the ease of transmission and the low percentage of nonimmunized population that is required for disease survival have made eradication extremely difficult.
The classic measles rash is maculopapular and blanches under pressure. The rash begins on the face and spreads centrifugally to involve the neck, trunk, and, finally, the extremities. This cranial-to-caudal rash progression is characteristic of measles. The cough may persist for up to 2 weeks. Fever persisting beyond the third day of rash suggests a measles-associated complication.
Postinfectious encephalomyelitis can also occur. Postinfectious encephalomyelitis is a demyelinating disease that presents during the recovery phase, and is thought to be caused by a postinfectious autoimmune response.
The treatment of measles is mostly supportive and patients will need to stay away from other individuals—particularly unimmunized children and adults, pregnant women, and immunocompromised people—until at least 4 days after rash onset. Suspected cases of measles should be reported immediately to the local or state department of health.
In this case, the child showed no evidence of pneumonia, neurological symptoms, or dehydration, so hospitalization was not needed. Fortunately, the mother and father had both been vaccinated as children and this was their only child. Antipyretics and fluids were recommended. The parents were told to avoid giving the child aspirin to prevent Reye’s syndrome.
The FP maintained contact with the family over the phone and the symptoms began resolving within a few days. The FP also reported the case to the local health department.
Photo courtesy of Dr. Eric Kraus. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Baudoin L. Measles. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY:McGraw-Hill;2013:723-727.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP suspected that this patient had measles, but since it has a low prevalence in the United States, he confirmed the diagnosis with a specific serum immunoglobulin M antibody test. Measles is a highly communicable, acute, viral illness that is still one of the most serious infectious diseases in human history. Until the introduction of the measles-mumps-rubella vaccine, it was responsible for millions of deaths worldwide annually. Eradication of measles is possible, but the ease of transmission and the low percentage of nonimmunized population that is required for disease survival have made eradication extremely difficult.
The classic measles rash is maculopapular and blanches under pressure. The rash begins on the face and spreads centrifugally to involve the neck, trunk, and, finally, the extremities. This cranial-to-caudal rash progression is characteristic of measles. The cough may persist for up to 2 weeks. Fever persisting beyond the third day of rash suggests a measles-associated complication.
Postinfectious encephalomyelitis can also occur. Postinfectious encephalomyelitis is a demyelinating disease that presents during the recovery phase, and is thought to be caused by a postinfectious autoimmune response.
The treatment of measles is mostly supportive and patients will need to stay away from other individuals—particularly unimmunized children and adults, pregnant women, and immunocompromised people—until at least 4 days after rash onset. Suspected cases of measles should be reported immediately to the local or state department of health.
In this case, the child showed no evidence of pneumonia, neurological symptoms, or dehydration, so hospitalization was not needed. Fortunately, the mother and father had both been vaccinated as children and this was their only child. Antipyretics and fluids were recommended. The parents were told to avoid giving the child aspirin to prevent Reye’s syndrome.
The FP maintained contact with the family over the phone and the symptoms began resolving within a few days. The FP also reported the case to the local health department.
Photo courtesy of Dr. Eric Kraus. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Baudoin L. Measles. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY:McGraw-Hill;2013:723-727.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Emergency diverticulitis: Limited role seen for proximal diversion
LOS ANGELES – There is no difference in 30-day outcomes for patients undergoing emergency surgery for acute diverticulitis with primary anastomosis with or without proximal diversion, results from an analysis of national data showed.
“Traditionally, patients undergoing emergency surgery for diverticulitis were offered a Hartmann’s procedure,” lead study author Dr. Nathan Hite said at the annual meeting of the American Society of Colon and Rectal Surgeons. “Studies have suggested that resection with primary anastomosis and proximal diversion is a safe alternative to this procedure. That’s attractive because it’s usually a quicker operation and puts less physiologic stress on the patient. It still requires a trip to the operating room, an inpatient hospital stay, and carries a complication rate of up to 20%.”
In an effort to determine if there was a difference between 30-day outcomes in patients treated with resection and primary anastomosis with or without primary diversion, the researchers queried the American College of Surgeons National Quality Improvement Program (ACS-NSQIP) database from 2005 to 2013 to identify patients with a diagnosis of diverticula, diverticulosis, or diverticulosis of colon without bleeding who underwent emergency operations. They divided patients into two groups: 1,912 who underwent resection and primary anastomosis without proximal diversion (group 1) and 123 who underwent resection and primary anastomosis with proximal diversion (group 2). Both open and laparoscopic operations were included.
Dr. Hite, of the department of colon and rectal surgery at Ochsner Medical Center, Metairie, La., reported that the mean age of patients in groups 1 and 2 was 62 and 59 years, respectively. There were no differences in gender distribution but women were significantly older in both groups (P less than .0006). No significant differences between groups 1 and 2 were observed with respect to body mass index (29.1 vs. 28.1 kg/m2, respectively; P = .11), preoperative albumin (3.3 vs. 3.5 g/dL), preoperative hematocrit (35% vs. 28%), preoperative white blood count (13.4 vs. 13.7 x 103/mcL), or functional status (P = .71). Although patients in group 2 did not appear to be sicker at the time of surgery in terms of ASA class or wound class, they did have a higher incidence of diabetes and smoking, compared with their counterparts in group 1.
As for postoperative complications, there were no significant differences between groups 1 and 2 in the incidence of superficial skin infection (141 vs. 7; P = .76), organ space infection (36 vs. 5; P = .09), septic shock (126 vs. 3; P = .18), pulmonary embolism (20 vs. 3; P = .15), cerebrovascular accident (7 vs. 0; P = .5), myocardial infarction (15 vs. 0; P = .32), or death (88 vs. 2; P = .51). Patients in group 2 did have a significantly longer operating time, compared with those in group 1 (158 vs. 133 minutes; P less than .0001).
“Ultimately, the decision to perform a proximal diversion [or not] depends on many factors,” Dr. Hite concluded. “But our study suggests that if the patient is an appropriate candidate for reanastomosis, a diverting ostomy may be safely omitted.” He reported having no financial disclosures.
LOS ANGELES – There is no difference in 30-day outcomes for patients undergoing emergency surgery for acute diverticulitis with primary anastomosis with or without proximal diversion, results from an analysis of national data showed.
“Traditionally, patients undergoing emergency surgery for diverticulitis were offered a Hartmann’s procedure,” lead study author Dr. Nathan Hite said at the annual meeting of the American Society of Colon and Rectal Surgeons. “Studies have suggested that resection with primary anastomosis and proximal diversion is a safe alternative to this procedure. That’s attractive because it’s usually a quicker operation and puts less physiologic stress on the patient. It still requires a trip to the operating room, an inpatient hospital stay, and carries a complication rate of up to 20%.”
In an effort to determine if there was a difference between 30-day outcomes in patients treated with resection and primary anastomosis with or without primary diversion, the researchers queried the American College of Surgeons National Quality Improvement Program (ACS-NSQIP) database from 2005 to 2013 to identify patients with a diagnosis of diverticula, diverticulosis, or diverticulosis of colon without bleeding who underwent emergency operations. They divided patients into two groups: 1,912 who underwent resection and primary anastomosis without proximal diversion (group 1) and 123 who underwent resection and primary anastomosis with proximal diversion (group 2). Both open and laparoscopic operations were included.
Dr. Hite, of the department of colon and rectal surgery at Ochsner Medical Center, Metairie, La., reported that the mean age of patients in groups 1 and 2 was 62 and 59 years, respectively. There were no differences in gender distribution but women were significantly older in both groups (P less than .0006). No significant differences between groups 1 and 2 were observed with respect to body mass index (29.1 vs. 28.1 kg/m2, respectively; P = .11), preoperative albumin (3.3 vs. 3.5 g/dL), preoperative hematocrit (35% vs. 28%), preoperative white blood count (13.4 vs. 13.7 x 103/mcL), or functional status (P = .71). Although patients in group 2 did not appear to be sicker at the time of surgery in terms of ASA class or wound class, they did have a higher incidence of diabetes and smoking, compared with their counterparts in group 1.
As for postoperative complications, there were no significant differences between groups 1 and 2 in the incidence of superficial skin infection (141 vs. 7; P = .76), organ space infection (36 vs. 5; P = .09), septic shock (126 vs. 3; P = .18), pulmonary embolism (20 vs. 3; P = .15), cerebrovascular accident (7 vs. 0; P = .5), myocardial infarction (15 vs. 0; P = .32), or death (88 vs. 2; P = .51). Patients in group 2 did have a significantly longer operating time, compared with those in group 1 (158 vs. 133 minutes; P less than .0001).
“Ultimately, the decision to perform a proximal diversion [or not] depends on many factors,” Dr. Hite concluded. “But our study suggests that if the patient is an appropriate candidate for reanastomosis, a diverting ostomy may be safely omitted.” He reported having no financial disclosures.
LOS ANGELES – There is no difference in 30-day outcomes for patients undergoing emergency surgery for acute diverticulitis with primary anastomosis with or without proximal diversion, results from an analysis of national data showed.
“Traditionally, patients undergoing emergency surgery for diverticulitis were offered a Hartmann’s procedure,” lead study author Dr. Nathan Hite said at the annual meeting of the American Society of Colon and Rectal Surgeons. “Studies have suggested that resection with primary anastomosis and proximal diversion is a safe alternative to this procedure. That’s attractive because it’s usually a quicker operation and puts less physiologic stress on the patient. It still requires a trip to the operating room, an inpatient hospital stay, and carries a complication rate of up to 20%.”
In an effort to determine if there was a difference between 30-day outcomes in patients treated with resection and primary anastomosis with or without primary diversion, the researchers queried the American College of Surgeons National Quality Improvement Program (ACS-NSQIP) database from 2005 to 2013 to identify patients with a diagnosis of diverticula, diverticulosis, or diverticulosis of colon without bleeding who underwent emergency operations. They divided patients into two groups: 1,912 who underwent resection and primary anastomosis without proximal diversion (group 1) and 123 who underwent resection and primary anastomosis with proximal diversion (group 2). Both open and laparoscopic operations were included.
Dr. Hite, of the department of colon and rectal surgery at Ochsner Medical Center, Metairie, La., reported that the mean age of patients in groups 1 and 2 was 62 and 59 years, respectively. There were no differences in gender distribution but women were significantly older in both groups (P less than .0006). No significant differences between groups 1 and 2 were observed with respect to body mass index (29.1 vs. 28.1 kg/m2, respectively; P = .11), preoperative albumin (3.3 vs. 3.5 g/dL), preoperative hematocrit (35% vs. 28%), preoperative white blood count (13.4 vs. 13.7 x 103/mcL), or functional status (P = .71). Although patients in group 2 did not appear to be sicker at the time of surgery in terms of ASA class or wound class, they did have a higher incidence of diabetes and smoking, compared with their counterparts in group 1.
As for postoperative complications, there were no significant differences between groups 1 and 2 in the incidence of superficial skin infection (141 vs. 7; P = .76), organ space infection (36 vs. 5; P = .09), septic shock (126 vs. 3; P = .18), pulmonary embolism (20 vs. 3; P = .15), cerebrovascular accident (7 vs. 0; P = .5), myocardial infarction (15 vs. 0; P = .32), or death (88 vs. 2; P = .51). Patients in group 2 did have a significantly longer operating time, compared with those in group 1 (158 vs. 133 minutes; P less than .0001).
“Ultimately, the decision to perform a proximal diversion [or not] depends on many factors,” Dr. Hite concluded. “But our study suggests that if the patient is an appropriate candidate for reanastomosis, a diverting ostomy may be safely omitted.” He reported having no financial disclosures.
AT THE ASCRS ANNUAL MEETING
Key clinical point: Whether patients underwent primary anastomosis with or without proximal diversion in emergency surgery for diverticular disease has no impact on 30-day outcomes.
Major finding: Among patients undergoing emergency surgery for acute diverticulitis with primary anastomosis, no significant differences were seen in a number of 30-day outcomes when the procedure was performed without or with proximal diversion, including superficial skin infection (141 vs. 7, respectively; P = .76), organ space infection (36 vs. 5; P = .09), septic shock (126 vs. 3; P = .18), or death (88 vs. 2; P = .51).
Data source: A review of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database from 2005 to 2013 to identify 2,035 patients with a diagnosis of diverticula, diverticulosis, or diverticulosis of colon without bleeding who underwent emergency operations.
Disclosures: Dr. Hite reported having no financial disclosures.
EPO may not benefit preterm infants long-term

Photo by Petr Kratochvil
Giving very preterm infants high-dose recombinant human erythropoietin (EPO) at birth does not improve neurodevelopmental outcomes at 2 years, according to a study published in JAMA.
Researchers found no significant differences between infants who received EPO and those who did not when it came to cognitive development, motor development, cerebral palsy, hearing or visual impairment, and anthropometric growth parameters.
Giancarlo Natalucci, MD, of the University of Zurich in Switzerland, and his colleagues conducted this study in 448 preterm infants who were born between 26 weeks’ gestation and 31 weeks 6 days’ gestation.
The subjects’ average gestational age was 29 weeks, and their average birth weight was 1210 g (2.7 lbs).
The infants were randomized to receive high-dose EPO (n=228) or placebo (saline, n=220) intravenously within 3 hours of birth, at 12 to 18 hours, and at 36 to 42 hours after birth.
Neurodevelopmental outcome data were available for 81% of the infants (n=365) at an average age of 23.6 months.
Cognitive development, as assessed with the Mental Development Index (MDI), was not significantly different between the EPO group and the placebo group. In an intent-to-treat analysis, the mean MDI was 93.5 in the EPO group and 94.5 in the placebo group (P=0.056). In the per-protocol analysis, the mean MDI was 93.9 and 94.5, respectively (P=0.70).
The researchers also found no significant differences between the treatment groups for secondary outcomes such as motor development, cerebral palsy, hearing or visual impairment, and anthropometric growth parameters.
The team assessed motor development using the psychomotor development index (PDI). In the intent-to-treat analysis, the mean PDI was 89.5 in the EPO group and 92.1 in the placebo group (P=0.15). In the per-protocol analysis, the mean PDI was 89.2 and 92.8, respectively (P=0.06).
In the intent-to-treat analysis, the incidence of cerebral palsy was 4% in the EPO group and 5% in the placebo group (P>0.99). In the per-protocol analysis, it was 5% for both groups (P=0.41).
In the intent-to-treat analysis, severe hearing impairment occurred in 1 EPO-treated patient and no placebo-treated patients (P>0.99). Severe visual impairment occurred in 2 and 0, respectively (P=0.50). The incidences were the same in the per-protocol analysis.
And there were no significant differences between the treatment groups (per-protocol or intent-to-treat) when it came to growth parameters such as head circumference, weight, or length.
The researchers said these results suggest that EPO may not have a neuroprotective role in very preterm infants, but follow-up is required to assess cognitive and physical problems that may not become evident until later in life. ![]()

Photo by Petr Kratochvil
Giving very preterm infants high-dose recombinant human erythropoietin (EPO) at birth does not improve neurodevelopmental outcomes at 2 years, according to a study published in JAMA.
Researchers found no significant differences between infants who received EPO and those who did not when it came to cognitive development, motor development, cerebral palsy, hearing or visual impairment, and anthropometric growth parameters.
Giancarlo Natalucci, MD, of the University of Zurich in Switzerland, and his colleagues conducted this study in 448 preterm infants who were born between 26 weeks’ gestation and 31 weeks 6 days’ gestation.
The subjects’ average gestational age was 29 weeks, and their average birth weight was 1210 g (2.7 lbs).
The infants were randomized to receive high-dose EPO (n=228) or placebo (saline, n=220) intravenously within 3 hours of birth, at 12 to 18 hours, and at 36 to 42 hours after birth.
Neurodevelopmental outcome data were available for 81% of the infants (n=365) at an average age of 23.6 months.
Cognitive development, as assessed with the Mental Development Index (MDI), was not significantly different between the EPO group and the placebo group. In an intent-to-treat analysis, the mean MDI was 93.5 in the EPO group and 94.5 in the placebo group (P=0.056). In the per-protocol analysis, the mean MDI was 93.9 and 94.5, respectively (P=0.70).
The researchers also found no significant differences between the treatment groups for secondary outcomes such as motor development, cerebral palsy, hearing or visual impairment, and anthropometric growth parameters.
The team assessed motor development using the psychomotor development index (PDI). In the intent-to-treat analysis, the mean PDI was 89.5 in the EPO group and 92.1 in the placebo group (P=0.15). In the per-protocol analysis, the mean PDI was 89.2 and 92.8, respectively (P=0.06).
In the intent-to-treat analysis, the incidence of cerebral palsy was 4% in the EPO group and 5% in the placebo group (P>0.99). In the per-protocol analysis, it was 5% for both groups (P=0.41).
In the intent-to-treat analysis, severe hearing impairment occurred in 1 EPO-treated patient and no placebo-treated patients (P>0.99). Severe visual impairment occurred in 2 and 0, respectively (P=0.50). The incidences were the same in the per-protocol analysis.
And there were no significant differences between the treatment groups (per-protocol or intent-to-treat) when it came to growth parameters such as head circumference, weight, or length.
The researchers said these results suggest that EPO may not have a neuroprotective role in very preterm infants, but follow-up is required to assess cognitive and physical problems that may not become evident until later in life. ![]()

Photo by Petr Kratochvil
Giving very preterm infants high-dose recombinant human erythropoietin (EPO) at birth does not improve neurodevelopmental outcomes at 2 years, according to a study published in JAMA.
Researchers found no significant differences between infants who received EPO and those who did not when it came to cognitive development, motor development, cerebral palsy, hearing or visual impairment, and anthropometric growth parameters.
Giancarlo Natalucci, MD, of the University of Zurich in Switzerland, and his colleagues conducted this study in 448 preterm infants who were born between 26 weeks’ gestation and 31 weeks 6 days’ gestation.
The subjects’ average gestational age was 29 weeks, and their average birth weight was 1210 g (2.7 lbs).
The infants were randomized to receive high-dose EPO (n=228) or placebo (saline, n=220) intravenously within 3 hours of birth, at 12 to 18 hours, and at 36 to 42 hours after birth.
Neurodevelopmental outcome data were available for 81% of the infants (n=365) at an average age of 23.6 months.
Cognitive development, as assessed with the Mental Development Index (MDI), was not significantly different between the EPO group and the placebo group. In an intent-to-treat analysis, the mean MDI was 93.5 in the EPO group and 94.5 in the placebo group (P=0.056). In the per-protocol analysis, the mean MDI was 93.9 and 94.5, respectively (P=0.70).
The researchers also found no significant differences between the treatment groups for secondary outcomes such as motor development, cerebral palsy, hearing or visual impairment, and anthropometric growth parameters.
The team assessed motor development using the psychomotor development index (PDI). In the intent-to-treat analysis, the mean PDI was 89.5 in the EPO group and 92.1 in the placebo group (P=0.15). In the per-protocol analysis, the mean PDI was 89.2 and 92.8, respectively (P=0.06).
In the intent-to-treat analysis, the incidence of cerebral palsy was 4% in the EPO group and 5% in the placebo group (P>0.99). In the per-protocol analysis, it was 5% for both groups (P=0.41).
In the intent-to-treat analysis, severe hearing impairment occurred in 1 EPO-treated patient and no placebo-treated patients (P>0.99). Severe visual impairment occurred in 2 and 0, respectively (P=0.50). The incidences were the same in the per-protocol analysis.
And there were no significant differences between the treatment groups (per-protocol or intent-to-treat) when it came to growth parameters such as head circumference, weight, or length.
The researchers said these results suggest that EPO may not have a neuroprotective role in very preterm infants, but follow-up is required to assess cognitive and physical problems that may not become evident until later in life. ![]()
Method may produce better CAR-NKTs

Photo by Aaron Logan
Researchers say they have discovered a method for expanding natural killer T cells (NKTs) that ensures their persistence, thereby making NKTs more attractive as chimeric antigen receptor (CAR) carriers for cancer immunotherapy.
When transduced with a CD19-specific CAR, the researchers’ persistent NKTs produced sustained tumor regression in a mouse model of B-cell lymphoma.
The team described this work in the Journal of Clinical Investigation.
“NKT technology is quite powerful and offers a significant potential for treatment of cancer,” said study author Leonid Metelitsa, MD, PhD, of Baylor College of Medicine in Houston, Texas.
“But for it to be most effective, we have to find the best way to expand the cells ex vivo while preserving their ability to persist once delivered back to patients. If they can persist in the body for a long time, they have much longer therapeutic activity, and this is essential for fighting cancer.”
Molecule affects persistence
The researchers noted that central memory T cells are known for their ability to proliferate and persist, and these cells are characterized by expression of the surface molecule CD62L.
In this study, the team found that NKT cells freshly derived from blood did not express CD62L, or it was expressed at a very low level. However, after the researchers expanded NKT cells, they found that CD62L was expressed at higher levels.
“We consistently identified a subset of cells present at very high numbers after the first 12 days of expansion, and critical to this subset of cells was the presence of CD62L,” Dr Metelitsa said. “In fact, they became the majority of the new cells.”
In addition, Dr Metelitsa said that CD62L-positive NKT cells were responsible for further propagation of NKTs in culture, which is important for achieving large numbers of cells. However, extensive culture led to the eventual decline of CD62L expression in NKTs.
To test the role of CD62L in NKT-cell persistence, the researchers delivered CD62L-positive and CD62L-negative NKTs to immune-deficient NSG mice. They found that CD62L-positive NKTs persisted 5 times longer than CD62L-negative NKTs.
CAR-NKTs fight lymphoma
Next, the researchers transduced CD62L-positive and CD62L-negative NKTs with a CD19-specific CAR and delivered these cells to mice with B-cell lymphoma.
The team found that both CD62L-positve and CD62L-negative CAR-NKTs prolonged the survival of mice, when compared to controls (P<0.001).
However, only the CD62L-positive CAR-NKTs induced sustained tumor regression. Seven of 9 mice that received CD62L-positive CAR-NKTs lived, and 5 were tumor-free for at least 3 months. But all 10 mice that received CD62L-negative CAR-NKTs ultimately succumbed to tumor progression (P<0.001).
Costimulation improves NKTs/CAR-NKTs
The researchers then turned their focus to costimulation of NKTs in order to maintain the subset with a high percentage of CD62L-positive cells in prolonged culture. Costimulation involves the interaction of receptors on NKTs with activating molecules on an antigen-presenting cell to increase the NKTs’ immune functions.
“We have known that costimulation is an important part of immune response and immunotherapy, but, in this case, we did not know which costimulatory molecules would be important for the expansion and persistence of CD62L-positive NKT cells,” said Gengwen Tian, MD, of the Baylor College of Medicine.
After testing more than 100 combinations, the researchers discovered that combining an antigen-presenting molecule—CD1d—with 3 costimulatory molecules—CD86, 4-1BBL, and OX40L—induced prolonged persistence and better therapeutic activity of NKTs and CAR-NKTs in mouse models.
“When we developed an antigen-presenting cell clone that expressed CD1d with all of these costimulatory molecules at certain levels, NKT cells maintained a high percentage of CD62L even in a prolonged culture,” Dr Metelitsa said.
The researchers conducted in vivo testing of CAR-NKT cells that were expanded with the original method or with the costimulation method. And they found that costimulated cells had significantly higher therapeutic activity in mouse models of neuroblastoma and lymphoma.
“Our goal now is to optimize our NKT cell expansion protocol so that we can obtain FDA approval to initiate clinical trials,” Dr Metelitsa said. ![]()

Photo by Aaron Logan
Researchers say they have discovered a method for expanding natural killer T cells (NKTs) that ensures their persistence, thereby making NKTs more attractive as chimeric antigen receptor (CAR) carriers for cancer immunotherapy.
When transduced with a CD19-specific CAR, the researchers’ persistent NKTs produced sustained tumor regression in a mouse model of B-cell lymphoma.
The team described this work in the Journal of Clinical Investigation.
“NKT technology is quite powerful and offers a significant potential for treatment of cancer,” said study author Leonid Metelitsa, MD, PhD, of Baylor College of Medicine in Houston, Texas.
“But for it to be most effective, we have to find the best way to expand the cells ex vivo while preserving their ability to persist once delivered back to patients. If they can persist in the body for a long time, they have much longer therapeutic activity, and this is essential for fighting cancer.”
Molecule affects persistence
The researchers noted that central memory T cells are known for their ability to proliferate and persist, and these cells are characterized by expression of the surface molecule CD62L.
In this study, the team found that NKT cells freshly derived from blood did not express CD62L, or it was expressed at a very low level. However, after the researchers expanded NKT cells, they found that CD62L was expressed at higher levels.
“We consistently identified a subset of cells present at very high numbers after the first 12 days of expansion, and critical to this subset of cells was the presence of CD62L,” Dr Metelitsa said. “In fact, they became the majority of the new cells.”
In addition, Dr Metelitsa said that CD62L-positive NKT cells were responsible for further propagation of NKTs in culture, which is important for achieving large numbers of cells. However, extensive culture led to the eventual decline of CD62L expression in NKTs.
To test the role of CD62L in NKT-cell persistence, the researchers delivered CD62L-positive and CD62L-negative NKTs to immune-deficient NSG mice. They found that CD62L-positive NKTs persisted 5 times longer than CD62L-negative NKTs.
CAR-NKTs fight lymphoma
Next, the researchers transduced CD62L-positive and CD62L-negative NKTs with a CD19-specific CAR and delivered these cells to mice with B-cell lymphoma.
The team found that both CD62L-positve and CD62L-negative CAR-NKTs prolonged the survival of mice, when compared to controls (P<0.001).
However, only the CD62L-positive CAR-NKTs induced sustained tumor regression. Seven of 9 mice that received CD62L-positive CAR-NKTs lived, and 5 were tumor-free for at least 3 months. But all 10 mice that received CD62L-negative CAR-NKTs ultimately succumbed to tumor progression (P<0.001).
Costimulation improves NKTs/CAR-NKTs
The researchers then turned their focus to costimulation of NKTs in order to maintain the subset with a high percentage of CD62L-positive cells in prolonged culture. Costimulation involves the interaction of receptors on NKTs with activating molecules on an antigen-presenting cell to increase the NKTs’ immune functions.
“We have known that costimulation is an important part of immune response and immunotherapy, but, in this case, we did not know which costimulatory molecules would be important for the expansion and persistence of CD62L-positive NKT cells,” said Gengwen Tian, MD, of the Baylor College of Medicine.
After testing more than 100 combinations, the researchers discovered that combining an antigen-presenting molecule—CD1d—with 3 costimulatory molecules—CD86, 4-1BBL, and OX40L—induced prolonged persistence and better therapeutic activity of NKTs and CAR-NKTs in mouse models.
“When we developed an antigen-presenting cell clone that expressed CD1d with all of these costimulatory molecules at certain levels, NKT cells maintained a high percentage of CD62L even in a prolonged culture,” Dr Metelitsa said.
The researchers conducted in vivo testing of CAR-NKT cells that were expanded with the original method or with the costimulation method. And they found that costimulated cells had significantly higher therapeutic activity in mouse models of neuroblastoma and lymphoma.
“Our goal now is to optimize our NKT cell expansion protocol so that we can obtain FDA approval to initiate clinical trials,” Dr Metelitsa said. ![]()

Photo by Aaron Logan
Researchers say they have discovered a method for expanding natural killer T cells (NKTs) that ensures their persistence, thereby making NKTs more attractive as chimeric antigen receptor (CAR) carriers for cancer immunotherapy.
When transduced with a CD19-specific CAR, the researchers’ persistent NKTs produced sustained tumor regression in a mouse model of B-cell lymphoma.
The team described this work in the Journal of Clinical Investigation.
“NKT technology is quite powerful and offers a significant potential for treatment of cancer,” said study author Leonid Metelitsa, MD, PhD, of Baylor College of Medicine in Houston, Texas.
“But for it to be most effective, we have to find the best way to expand the cells ex vivo while preserving their ability to persist once delivered back to patients. If they can persist in the body for a long time, they have much longer therapeutic activity, and this is essential for fighting cancer.”
Molecule affects persistence
The researchers noted that central memory T cells are known for their ability to proliferate and persist, and these cells are characterized by expression of the surface molecule CD62L.
In this study, the team found that NKT cells freshly derived from blood did not express CD62L, or it was expressed at a very low level. However, after the researchers expanded NKT cells, they found that CD62L was expressed at higher levels.
“We consistently identified a subset of cells present at very high numbers after the first 12 days of expansion, and critical to this subset of cells was the presence of CD62L,” Dr Metelitsa said. “In fact, they became the majority of the new cells.”
In addition, Dr Metelitsa said that CD62L-positive NKT cells were responsible for further propagation of NKTs in culture, which is important for achieving large numbers of cells. However, extensive culture led to the eventual decline of CD62L expression in NKTs.
To test the role of CD62L in NKT-cell persistence, the researchers delivered CD62L-positive and CD62L-negative NKTs to immune-deficient NSG mice. They found that CD62L-positive NKTs persisted 5 times longer than CD62L-negative NKTs.
CAR-NKTs fight lymphoma
Next, the researchers transduced CD62L-positive and CD62L-negative NKTs with a CD19-specific CAR and delivered these cells to mice with B-cell lymphoma.
The team found that both CD62L-positve and CD62L-negative CAR-NKTs prolonged the survival of mice, when compared to controls (P<0.001).
However, only the CD62L-positive CAR-NKTs induced sustained tumor regression. Seven of 9 mice that received CD62L-positive CAR-NKTs lived, and 5 were tumor-free for at least 3 months. But all 10 mice that received CD62L-negative CAR-NKTs ultimately succumbed to tumor progression (P<0.001).
Costimulation improves NKTs/CAR-NKTs
The researchers then turned their focus to costimulation of NKTs in order to maintain the subset with a high percentage of CD62L-positive cells in prolonged culture. Costimulation involves the interaction of receptors on NKTs with activating molecules on an antigen-presenting cell to increase the NKTs’ immune functions.
“We have known that costimulation is an important part of immune response and immunotherapy, but, in this case, we did not know which costimulatory molecules would be important for the expansion and persistence of CD62L-positive NKT cells,” said Gengwen Tian, MD, of the Baylor College of Medicine.
After testing more than 100 combinations, the researchers discovered that combining an antigen-presenting molecule—CD1d—with 3 costimulatory molecules—CD86, 4-1BBL, and OX40L—induced prolonged persistence and better therapeutic activity of NKTs and CAR-NKTs in mouse models.
“When we developed an antigen-presenting cell clone that expressed CD1d with all of these costimulatory molecules at certain levels, NKT cells maintained a high percentage of CD62L even in a prolonged culture,” Dr Metelitsa said.
The researchers conducted in vivo testing of CAR-NKT cells that were expanded with the original method or with the costimulation method. And they found that costimulated cells had significantly higher therapeutic activity in mouse models of neuroblastoma and lymphoma.
“Our goal now is to optimize our NKT cell expansion protocol so that we can obtain FDA approval to initiate clinical trials,” Dr Metelitsa said. ![]()
HIV patients undertreated for lymphoma, other cancers

cultured lymphocyte
Image courtesy of the CDC
A new study suggests that cancer patients with HIV are less likely to receive cancer treatment, regardless of insurance status and comorbidities.
Patients with HIV were less likely than their HIV-free peers to receive treatment for Hodgkin lymphoma, diffuse large B-cell lymphoma, and 7 solid tumor malignancies.
Gita Suneja, MD, of the University of Utah in Salt Lake City, and her colleagues reported these findings in Cancer.
The team used the National Cancer Data Base to study non-elderly US adults diagnosed with 10 common cancers from 2003 to 2011. There were a total of 10,265 HIV-infected patients and 2,219,232 HIV-free patients.
The researchers examined associations between HIV status and lack of cancer treatment, taking into account insurance status and comorbidities.
The results showed a lack of treatment among HIV patients for all of the cancers studied except anal cancer (relative risk [RR]=1.20, P=0.333).
So HIV-infected patients were more likely to lack cancer treatment for:
- Hodgkin lymphoma (RR=1.92, P<0.001)
- Diffuse large B-cell lymphoma (RR=1.82, P<0.001)
- Head and neck cancer (RR=1.48, P=0.013)
- Upper gastrointestinal tract cancer (RR=2.62, P<0.001)
- Colorectal cancer (RR=1.70, P=0.006)
- Lung cancer (RR=2.46, P<0.001)
- Breast cancer (RR=2.14, P=0.015)
- Cervical cancer (RR=2.81, P<0.001)
- Prostate cancer (RR=2.16, P<0.001).
The researchers said factors that predicted a lack of cancer treatment among HIV-infected individuals varied between those with solid tumors and those with lymphomas.
Advanced stage at the time of cancer diagnosis (stage IV vs stage I) meant HIV patients with solid tumors were less likely to receive cancer treatment, but lymphoma patients were more likely to receive cancer treatment.
Having a higher modified Charlson-Deyo comorbidity score (1 or 2+ vs 0) predicted a lack of cancer treatment for HIV-infected patients with lymphoma but not those with solid tumors.
And older age (45-64 vs 18-44) was associated with a lack of treatment for HIV-infected patients regardless of cancer type, but this was only significant for lymphoma patients.
For the entire cohort, black race (vs white) and a lack of private insurance (Medicaid, Medicare, uninsured, or unknown insurance status) were significant predictors for a lack of cancer treatment among HIV patients.
Still, the researchers noted that, even among privately insured patients, HIV-infected individuals were less likely to receive cancer treatment.
Dr Suneja and her colleagues said several factors may contribute to these findings. For one, HIV-infected patients have historically been excluded from cancer clinical trials, thereby limiting the applicability of trial results for this population.
In addition, cancer treatment guidelines specific to HIV-infected patients are not available for most cancer types. And clinicians may lack experience treating HIV-infected patients with cancer.
Furthermore, the psychosocial and economic challenges associated with the dual management of cancer and HIV treatment may make adherence to treatment a challenge. ![]()

cultured lymphocyte
Image courtesy of the CDC
A new study suggests that cancer patients with HIV are less likely to receive cancer treatment, regardless of insurance status and comorbidities.
Patients with HIV were less likely than their HIV-free peers to receive treatment for Hodgkin lymphoma, diffuse large B-cell lymphoma, and 7 solid tumor malignancies.
Gita Suneja, MD, of the University of Utah in Salt Lake City, and her colleagues reported these findings in Cancer.
The team used the National Cancer Data Base to study non-elderly US adults diagnosed with 10 common cancers from 2003 to 2011. There were a total of 10,265 HIV-infected patients and 2,219,232 HIV-free patients.
The researchers examined associations between HIV status and lack of cancer treatment, taking into account insurance status and comorbidities.
The results showed a lack of treatment among HIV patients for all of the cancers studied except anal cancer (relative risk [RR]=1.20, P=0.333).
So HIV-infected patients were more likely to lack cancer treatment for:
- Hodgkin lymphoma (RR=1.92, P<0.001)
- Diffuse large B-cell lymphoma (RR=1.82, P<0.001)
- Head and neck cancer (RR=1.48, P=0.013)
- Upper gastrointestinal tract cancer (RR=2.62, P<0.001)
- Colorectal cancer (RR=1.70, P=0.006)
- Lung cancer (RR=2.46, P<0.001)
- Breast cancer (RR=2.14, P=0.015)
- Cervical cancer (RR=2.81, P<0.001)
- Prostate cancer (RR=2.16, P<0.001).
The researchers said factors that predicted a lack of cancer treatment among HIV-infected individuals varied between those with solid tumors and those with lymphomas.
Advanced stage at the time of cancer diagnosis (stage IV vs stage I) meant HIV patients with solid tumors were less likely to receive cancer treatment, but lymphoma patients were more likely to receive cancer treatment.
Having a higher modified Charlson-Deyo comorbidity score (1 or 2+ vs 0) predicted a lack of cancer treatment for HIV-infected patients with lymphoma but not those with solid tumors.
And older age (45-64 vs 18-44) was associated with a lack of treatment for HIV-infected patients regardless of cancer type, but this was only significant for lymphoma patients.
For the entire cohort, black race (vs white) and a lack of private insurance (Medicaid, Medicare, uninsured, or unknown insurance status) were significant predictors for a lack of cancer treatment among HIV patients.
Still, the researchers noted that, even among privately insured patients, HIV-infected individuals were less likely to receive cancer treatment.
Dr Suneja and her colleagues said several factors may contribute to these findings. For one, HIV-infected patients have historically been excluded from cancer clinical trials, thereby limiting the applicability of trial results for this population.
In addition, cancer treatment guidelines specific to HIV-infected patients are not available for most cancer types. And clinicians may lack experience treating HIV-infected patients with cancer.
Furthermore, the psychosocial and economic challenges associated with the dual management of cancer and HIV treatment may make adherence to treatment a challenge. ![]()

cultured lymphocyte
Image courtesy of the CDC
A new study suggests that cancer patients with HIV are less likely to receive cancer treatment, regardless of insurance status and comorbidities.
Patients with HIV were less likely than their HIV-free peers to receive treatment for Hodgkin lymphoma, diffuse large B-cell lymphoma, and 7 solid tumor malignancies.
Gita Suneja, MD, of the University of Utah in Salt Lake City, and her colleagues reported these findings in Cancer.
The team used the National Cancer Data Base to study non-elderly US adults diagnosed with 10 common cancers from 2003 to 2011. There were a total of 10,265 HIV-infected patients and 2,219,232 HIV-free patients.
The researchers examined associations between HIV status and lack of cancer treatment, taking into account insurance status and comorbidities.
The results showed a lack of treatment among HIV patients for all of the cancers studied except anal cancer (relative risk [RR]=1.20, P=0.333).
So HIV-infected patients were more likely to lack cancer treatment for:
- Hodgkin lymphoma (RR=1.92, P<0.001)
- Diffuse large B-cell lymphoma (RR=1.82, P<0.001)
- Head and neck cancer (RR=1.48, P=0.013)
- Upper gastrointestinal tract cancer (RR=2.62, P<0.001)
- Colorectal cancer (RR=1.70, P=0.006)
- Lung cancer (RR=2.46, P<0.001)
- Breast cancer (RR=2.14, P=0.015)
- Cervical cancer (RR=2.81, P<0.001)
- Prostate cancer (RR=2.16, P<0.001).
The researchers said factors that predicted a lack of cancer treatment among HIV-infected individuals varied between those with solid tumors and those with lymphomas.
Advanced stage at the time of cancer diagnosis (stage IV vs stage I) meant HIV patients with solid tumors were less likely to receive cancer treatment, but lymphoma patients were more likely to receive cancer treatment.
Having a higher modified Charlson-Deyo comorbidity score (1 or 2+ vs 0) predicted a lack of cancer treatment for HIV-infected patients with lymphoma but not those with solid tumors.
And older age (45-64 vs 18-44) was associated with a lack of treatment for HIV-infected patients regardless of cancer type, but this was only significant for lymphoma patients.
For the entire cohort, black race (vs white) and a lack of private insurance (Medicaid, Medicare, uninsured, or unknown insurance status) were significant predictors for a lack of cancer treatment among HIV patients.
Still, the researchers noted that, even among privately insured patients, HIV-infected individuals were less likely to receive cancer treatment.
Dr Suneja and her colleagues said several factors may contribute to these findings. For one, HIV-infected patients have historically been excluded from cancer clinical trials, thereby limiting the applicability of trial results for this population.
In addition, cancer treatment guidelines specific to HIV-infected patients are not available for most cancer types. And clinicians may lack experience treating HIV-infected patients with cancer.
Furthermore, the psychosocial and economic challenges associated with the dual management of cancer and HIV treatment may make adherence to treatment a challenge. ![]()
PD-1 inhibitor granted accelerated approval for cHL

Photo courtesy of Business Wire
The US Food and Drug Administration (FDA) has granted accelerated approval for the PD-1 inhibitor nivolumab (Opdivo) to treat classical Hodgkin lymphoma (cHL).
The drug is approved to treat patients with relapsed or refractory cHL who have received an autologous hematopoietic stem cell transplant (HSCT) and post-transplant brentuximab vedotin.
Nivolumab received accelerated approval because it has not yet shown a clinical benefit in these patients. The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on a surrogate or intermediate endpoint—in this case, overall response rate—that is reasonably likely to predict clinical benefit. Continued approval of nivolumab for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA previously granted nivolumab breakthrough therapy designation, priority review status, and orphan drug designation.
Dosing and precautions
The recommended dose and schedule of nivolumab for cHL patients is 3 mg/kg intravenously every 2 weeks until disease progression or unacceptable toxicity.
The FDA added a new “Warning and Precaution” to the label for nivolumab, regarding complications of allogeneic HSCT after nivolumab.
Transplant-related deaths have occurred. So the FDA said healthcare professionals should follow patients closely for early evidence of transplant-related complications, such as hyperacute graft-versus-host disease (GVHD), severe acute GVHD, steroid-requiring febrile syndrome, hepatic veno-occlusive disease, and other immune-mediated adverse reactions.
The FDA has required the manufacturer of nivolumab, Bristol-Myers Squibb, to further study the safety of allogeneic HSCT after nivolumab.
Full prescribing information for the drug is available here.
Trials of nivolumab
The FDA granted nivolumab accelerated approval in cHL patients based on the results of 2 single-arm, multicenter trials—the phase 1 Checkmate 039 trial (presented at ICML last year) and the phase 2 CheckMate 205 trial (to be presented at ASCO 2016).
Efficacy
Thus far, researchers have evaluated the efficacy of nivolumab in 95 cHL patients from both trials. All of these patients previously received an autologous HSCT and post-transplant brentuximab vedotin. They received a median of 5 prior systemic regimens (range, 3 to 15).
The patients received a median of 17 doses of nivolumab (range, 3 to 48). The overall response rate was 65%, and the complete response rate was 7%.
The median time to response was 2.1 months (range, 0.7 to 5.7), and the estimated median duration of response was 8.7 months (range, 0+ to 23.1+).
Safety
Researchers evaluated the safety of nivolumab in 263 patients with relapsed or refractory cHL. Ninety-eight percent of these patients had received an autologous HSCT. The patients received a median of 10 doses of nivolumab (range, 1 to 48) at the approved dose and schedule.
The most common (≥20%) adverse events (AEs) of any grade were fatigue, upper respiratory tract infection, cough, pyrexia, and diarrhea.
Additional common (≥10%) AEs included rash, pruritus, musculoskeletal pain, nausea, vomiting, abdominal pain, headache, peripheral neuropathy, arthralgia, dyspnea, infusion-related reactions, and hypothyroidism or thyroiditis.
Serious AEs were reported in 21% of patients. The most common, reported in 1% to 3% of patients, were pneumonia, pleural effusion, pneumonitis, pyrexia, infusion-related reaction, and rash. ![]()

Photo courtesy of Business Wire
The US Food and Drug Administration (FDA) has granted accelerated approval for the PD-1 inhibitor nivolumab (Opdivo) to treat classical Hodgkin lymphoma (cHL).
The drug is approved to treat patients with relapsed or refractory cHL who have received an autologous hematopoietic stem cell transplant (HSCT) and post-transplant brentuximab vedotin.
Nivolumab received accelerated approval because it has not yet shown a clinical benefit in these patients. The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on a surrogate or intermediate endpoint—in this case, overall response rate—that is reasonably likely to predict clinical benefit. Continued approval of nivolumab for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA previously granted nivolumab breakthrough therapy designation, priority review status, and orphan drug designation.
Dosing and precautions
The recommended dose and schedule of nivolumab for cHL patients is 3 mg/kg intravenously every 2 weeks until disease progression or unacceptable toxicity.
The FDA added a new “Warning and Precaution” to the label for nivolumab, regarding complications of allogeneic HSCT after nivolumab.
Transplant-related deaths have occurred. So the FDA said healthcare professionals should follow patients closely for early evidence of transplant-related complications, such as hyperacute graft-versus-host disease (GVHD), severe acute GVHD, steroid-requiring febrile syndrome, hepatic veno-occlusive disease, and other immune-mediated adverse reactions.
The FDA has required the manufacturer of nivolumab, Bristol-Myers Squibb, to further study the safety of allogeneic HSCT after nivolumab.
Full prescribing information for the drug is available here.
Trials of nivolumab
The FDA granted nivolumab accelerated approval in cHL patients based on the results of 2 single-arm, multicenter trials—the phase 1 Checkmate 039 trial (presented at ICML last year) and the phase 2 CheckMate 205 trial (to be presented at ASCO 2016).
Efficacy
Thus far, researchers have evaluated the efficacy of nivolumab in 95 cHL patients from both trials. All of these patients previously received an autologous HSCT and post-transplant brentuximab vedotin. They received a median of 5 prior systemic regimens (range, 3 to 15).
The patients received a median of 17 doses of nivolumab (range, 3 to 48). The overall response rate was 65%, and the complete response rate was 7%.
The median time to response was 2.1 months (range, 0.7 to 5.7), and the estimated median duration of response was 8.7 months (range, 0+ to 23.1+).
Safety
Researchers evaluated the safety of nivolumab in 263 patients with relapsed or refractory cHL. Ninety-eight percent of these patients had received an autologous HSCT. The patients received a median of 10 doses of nivolumab (range, 1 to 48) at the approved dose and schedule.
The most common (≥20%) adverse events (AEs) of any grade were fatigue, upper respiratory tract infection, cough, pyrexia, and diarrhea.
Additional common (≥10%) AEs included rash, pruritus, musculoskeletal pain, nausea, vomiting, abdominal pain, headache, peripheral neuropathy, arthralgia, dyspnea, infusion-related reactions, and hypothyroidism or thyroiditis.
Serious AEs were reported in 21% of patients. The most common, reported in 1% to 3% of patients, were pneumonia, pleural effusion, pneumonitis, pyrexia, infusion-related reaction, and rash. ![]()

Photo courtesy of Business Wire
The US Food and Drug Administration (FDA) has granted accelerated approval for the PD-1 inhibitor nivolumab (Opdivo) to treat classical Hodgkin lymphoma (cHL).
The drug is approved to treat patients with relapsed or refractory cHL who have received an autologous hematopoietic stem cell transplant (HSCT) and post-transplant brentuximab vedotin.
Nivolumab received accelerated approval because it has not yet shown a clinical benefit in these patients. The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on a surrogate or intermediate endpoint—in this case, overall response rate—that is reasonably likely to predict clinical benefit. Continued approval of nivolumab for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA previously granted nivolumab breakthrough therapy designation, priority review status, and orphan drug designation.
Dosing and precautions
The recommended dose and schedule of nivolumab for cHL patients is 3 mg/kg intravenously every 2 weeks until disease progression or unacceptable toxicity.
The FDA added a new “Warning and Precaution” to the label for nivolumab, regarding complications of allogeneic HSCT after nivolumab.
Transplant-related deaths have occurred. So the FDA said healthcare professionals should follow patients closely for early evidence of transplant-related complications, such as hyperacute graft-versus-host disease (GVHD), severe acute GVHD, steroid-requiring febrile syndrome, hepatic veno-occlusive disease, and other immune-mediated adverse reactions.
The FDA has required the manufacturer of nivolumab, Bristol-Myers Squibb, to further study the safety of allogeneic HSCT after nivolumab.
Full prescribing information for the drug is available here.
Trials of nivolumab
The FDA granted nivolumab accelerated approval in cHL patients based on the results of 2 single-arm, multicenter trials—the phase 1 Checkmate 039 trial (presented at ICML last year) and the phase 2 CheckMate 205 trial (to be presented at ASCO 2016).
Efficacy
Thus far, researchers have evaluated the efficacy of nivolumab in 95 cHL patients from both trials. All of these patients previously received an autologous HSCT and post-transplant brentuximab vedotin. They received a median of 5 prior systemic regimens (range, 3 to 15).
The patients received a median of 17 doses of nivolumab (range, 3 to 48). The overall response rate was 65%, and the complete response rate was 7%.
The median time to response was 2.1 months (range, 0.7 to 5.7), and the estimated median duration of response was 8.7 months (range, 0+ to 23.1+).
Safety
Researchers evaluated the safety of nivolumab in 263 patients with relapsed or refractory cHL. Ninety-eight percent of these patients had received an autologous HSCT. The patients received a median of 10 doses of nivolumab (range, 1 to 48) at the approved dose and schedule.
The most common (≥20%) adverse events (AEs) of any grade were fatigue, upper respiratory tract infection, cough, pyrexia, and diarrhea.
Additional common (≥10%) AEs included rash, pruritus, musculoskeletal pain, nausea, vomiting, abdominal pain, headache, peripheral neuropathy, arthralgia, dyspnea, infusion-related reactions, and hypothyroidism or thyroiditis.
Serious AEs were reported in 21% of patients. The most common, reported in 1% to 3% of patients, were pneumonia, pleural effusion, pneumonitis, pyrexia, infusion-related reaction, and rash. ![]()