User login
Dr. Hospitalist: Improper, Aggressive Billing Raises Ethical, Legal Concerns
Dear Dr. Hospitalist:
I am a seasoned hospitalist at a large academic medical center in the Northeast and have recently become more bothered by how our group is being coerced to aggressively bill for our services. It seems the current reimbursement environment has pushed some of our leaders to demand more aggressive billing from our hospitalists. How should I respond?
Sincerely,
A Seasoned Hospitalist
Dr. Hospitalist responds:
As another “seasoned” hospitalist, I, too, have seen the wide pendulum swing from when internist inpatient billing was an afterthought and done by others to the current system of billing classes, RVU enticement, and reminders of how to construct the note. Enter the electronic health record, and now instead of clinical notes being used as a form of communication among clinicians, it does seem today to be created more for billing purposes.
How did we get here?
Physicians have to accept some of the blame. I can recall when I was an orderly at our local hospital in the mid 1970s and some physician “rounds” consisted of standing in a patient’s doorway and calling out, “How are you doing today, Mrs. Smith?” I must admit to having no idea how these docs were billing, but I do know that Medicare allowed for twice-daily billing for hospital visits back then. I also recall some of the paltry progress notes that consisted of one-liners like “pt doing well today.”
Like most corrective actions, the response has overshot the intended mark and made the daily progress note more ritualistic than informative. When the first attempts by the American Medical Association and the Centers for Medicare & Medicaid Services were released in the early 1990s, I’m sure most docs had no idea it would morph into its current level of significance for reimbursement—and that one day docs would be asked to implement, keep up with changes and modifications (think ICD-10), and use daily. Don’t get me wrong: I, like most hospitalists, recognize the clinical utility of a concise and well-written note. But when an otherwise complete H&P gets down-coded from a level 99223 to a 99221 because I leave off the family history of a 95-year-old man, of course I believe something is wrong with the system.
Also, human nature being what it is, I have always felt that if you incentivize people to increase production of an item, whether it’s a widget or an RVU, you will have some who will learn to game the system, consciously or subconsciously. With healthcare spending in the U.S. approaching 20% of gross domestic product, we as physicians should not be placed in positions of increased financial gain at the expense of our country’s economic health and viability. After all, we’re citizens first and physicians second.
You should recognize the need for proper coding and billing as inherent to the hospital’s financial viability, and if done correctly, it should not create an ethical or legal conflict for you. In the vast majority of cases, a well-written note can be properly billed and coded without creating angst.
Good luck! TH
Dear Dr. Hospitalist:
I am a seasoned hospitalist at a large academic medical center in the Northeast and have recently become more bothered by how our group is being coerced to aggressively bill for our services. It seems the current reimbursement environment has pushed some of our leaders to demand more aggressive billing from our hospitalists. How should I respond?
Sincerely,
A Seasoned Hospitalist
Dr. Hospitalist responds:
As another “seasoned” hospitalist, I, too, have seen the wide pendulum swing from when internist inpatient billing was an afterthought and done by others to the current system of billing classes, RVU enticement, and reminders of how to construct the note. Enter the electronic health record, and now instead of clinical notes being used as a form of communication among clinicians, it does seem today to be created more for billing purposes.
How did we get here?
Physicians have to accept some of the blame. I can recall when I was an orderly at our local hospital in the mid 1970s and some physician “rounds” consisted of standing in a patient’s doorway and calling out, “How are you doing today, Mrs. Smith?” I must admit to having no idea how these docs were billing, but I do know that Medicare allowed for twice-daily billing for hospital visits back then. I also recall some of the paltry progress notes that consisted of one-liners like “pt doing well today.”
Like most corrective actions, the response has overshot the intended mark and made the daily progress note more ritualistic than informative. When the first attempts by the American Medical Association and the Centers for Medicare & Medicaid Services were released in the early 1990s, I’m sure most docs had no idea it would morph into its current level of significance for reimbursement—and that one day docs would be asked to implement, keep up with changes and modifications (think ICD-10), and use daily. Don’t get me wrong: I, like most hospitalists, recognize the clinical utility of a concise and well-written note. But when an otherwise complete H&P gets down-coded from a level 99223 to a 99221 because I leave off the family history of a 95-year-old man, of course I believe something is wrong with the system.
Also, human nature being what it is, I have always felt that if you incentivize people to increase production of an item, whether it’s a widget or an RVU, you will have some who will learn to game the system, consciously or subconsciously. With healthcare spending in the U.S. approaching 20% of gross domestic product, we as physicians should not be placed in positions of increased financial gain at the expense of our country’s economic health and viability. After all, we’re citizens first and physicians second.
You should recognize the need for proper coding and billing as inherent to the hospital’s financial viability, and if done correctly, it should not create an ethical or legal conflict for you. In the vast majority of cases, a well-written note can be properly billed and coded without creating angst.
Good luck! TH
Dear Dr. Hospitalist:
I am a seasoned hospitalist at a large academic medical center in the Northeast and have recently become more bothered by how our group is being coerced to aggressively bill for our services. It seems the current reimbursement environment has pushed some of our leaders to demand more aggressive billing from our hospitalists. How should I respond?
Sincerely,
A Seasoned Hospitalist
Dr. Hospitalist responds:
As another “seasoned” hospitalist, I, too, have seen the wide pendulum swing from when internist inpatient billing was an afterthought and done by others to the current system of billing classes, RVU enticement, and reminders of how to construct the note. Enter the electronic health record, and now instead of clinical notes being used as a form of communication among clinicians, it does seem today to be created more for billing purposes.
How did we get here?
Physicians have to accept some of the blame. I can recall when I was an orderly at our local hospital in the mid 1970s and some physician “rounds” consisted of standing in a patient’s doorway and calling out, “How are you doing today, Mrs. Smith?” I must admit to having no idea how these docs were billing, but I do know that Medicare allowed for twice-daily billing for hospital visits back then. I also recall some of the paltry progress notes that consisted of one-liners like “pt doing well today.”
Like most corrective actions, the response has overshot the intended mark and made the daily progress note more ritualistic than informative. When the first attempts by the American Medical Association and the Centers for Medicare & Medicaid Services were released in the early 1990s, I’m sure most docs had no idea it would morph into its current level of significance for reimbursement—and that one day docs would be asked to implement, keep up with changes and modifications (think ICD-10), and use daily. Don’t get me wrong: I, like most hospitalists, recognize the clinical utility of a concise and well-written note. But when an otherwise complete H&P gets down-coded from a level 99223 to a 99221 because I leave off the family history of a 95-year-old man, of course I believe something is wrong with the system.
Also, human nature being what it is, I have always felt that if you incentivize people to increase production of an item, whether it’s a widget or an RVU, you will have some who will learn to game the system, consciously or subconsciously. With healthcare spending in the U.S. approaching 20% of gross domestic product, we as physicians should not be placed in positions of increased financial gain at the expense of our country’s economic health and viability. After all, we’re citizens first and physicians second.
You should recognize the need for proper coding and billing as inherent to the hospital’s financial viability, and if done correctly, it should not create an ethical or legal conflict for you. In the vast majority of cases, a well-written note can be properly billed and coded without creating angst.
Good luck! TH
Drug granted breakthrough designation for AML

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for CPX-351 (Vyxeos), a fixed-ratio combination of cytarabine and daunorubicin inside a lipid vesicle, to treat adults with therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 3 trial
The breakthrough designation for CPX-351 is primarily based on positive results from a phase 3 trial in older patients with previously untreated, high-risk, secondary AML.
The trial was sponsored by Celator Pharmaceuticals, Inc., the company developing CPX-351.
The company has released some results from the trial, and additional data are scheduled to be presented at the 2016 ASCO Annual Meeting (abstract 7000).
The trial enrolled 309 patients, ages 60 to 75, with one of the following:
- Untreated AML and a history of prior cytotoxic treatment
- Antecedent myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia, with or without prior hypomethylating therapy
- AML with WHO-defined, MDS-related cytogenetic abnormalities.
One hundred and fifty-three patients were randomized to receive CPX-351, and 156 were randomized to 7+3 (cytarabine continuously for 7 days and a single dose of daunorubicin for the first 3 days).
The treatment arms were well-balanced for sex, race, age, performance status, AML subtype, MDS-related cytogenetics, and prior hypomethylating therapy.
The trial met its primary endpoint, demonstrating a significant improvement in overall survival with CPX-351. The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm. The hazard ratio was 0.69 (P=0.005).
According to researchers, there was no substantial difference between the treatment arms with regard to grade 3-5 adverse events.
About CPX-351
CPX-351 is a liposomal formulation of a 5:1 molar ratio of cytarabine and daunorubicin.
In one phase 2 trial, CPX-351 conferred a significant improvement in survival—over investigator’s choice of therapy—when used as salvage therapy in poor-risk patients with AML.
In another phase 2 trial, CPX-351 conferred a significant survival benefit—over 7+3—in patients with secondary AML.
The FDA previously granted CPX-351 fast track designation to treat elderly patients with secondary AML. The agency established the fast track designation process to expedite the review of drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
The designation allows a drug’s developer to submit sections of a new drug application (NDA) on a rolling basis, so the FDA can review portions of the NDA as they are received instead of waiting for the entire NDA submission. A fast-track-designated product could be eligible for priority review if supported by clinical data at the time of NDA submission. ![]()

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for CPX-351 (Vyxeos), a fixed-ratio combination of cytarabine and daunorubicin inside a lipid vesicle, to treat adults with therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 3 trial
The breakthrough designation for CPX-351 is primarily based on positive results from a phase 3 trial in older patients with previously untreated, high-risk, secondary AML.
The trial was sponsored by Celator Pharmaceuticals, Inc., the company developing CPX-351.
The company has released some results from the trial, and additional data are scheduled to be presented at the 2016 ASCO Annual Meeting (abstract 7000).
The trial enrolled 309 patients, ages 60 to 75, with one of the following:
- Untreated AML and a history of prior cytotoxic treatment
- Antecedent myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia, with or without prior hypomethylating therapy
- AML with WHO-defined, MDS-related cytogenetic abnormalities.
One hundred and fifty-three patients were randomized to receive CPX-351, and 156 were randomized to 7+3 (cytarabine continuously for 7 days and a single dose of daunorubicin for the first 3 days).
The treatment arms were well-balanced for sex, race, age, performance status, AML subtype, MDS-related cytogenetics, and prior hypomethylating therapy.
The trial met its primary endpoint, demonstrating a significant improvement in overall survival with CPX-351. The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm. The hazard ratio was 0.69 (P=0.005).
According to researchers, there was no substantial difference between the treatment arms with regard to grade 3-5 adverse events.
About CPX-351
CPX-351 is a liposomal formulation of a 5:1 molar ratio of cytarabine and daunorubicin.
In one phase 2 trial, CPX-351 conferred a significant improvement in survival—over investigator’s choice of therapy—when used as salvage therapy in poor-risk patients with AML.
In another phase 2 trial, CPX-351 conferred a significant survival benefit—over 7+3—in patients with secondary AML.
The FDA previously granted CPX-351 fast track designation to treat elderly patients with secondary AML. The agency established the fast track designation process to expedite the review of drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
The designation allows a drug’s developer to submit sections of a new drug application (NDA) on a rolling basis, so the FDA can review portions of the NDA as they are received instead of waiting for the entire NDA submission. A fast-track-designated product could be eligible for priority review if supported by clinical data at the time of NDA submission. ![]()

Image by Lance Liotta
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for CPX-351 (Vyxeos), a fixed-ratio combination of cytarabine and daunorubicin inside a lipid vesicle, to treat adults with therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Phase 3 trial
The breakthrough designation for CPX-351 is primarily based on positive results from a phase 3 trial in older patients with previously untreated, high-risk, secondary AML.
The trial was sponsored by Celator Pharmaceuticals, Inc., the company developing CPX-351.
The company has released some results from the trial, and additional data are scheduled to be presented at the 2016 ASCO Annual Meeting (abstract 7000).
The trial enrolled 309 patients, ages 60 to 75, with one of the following:
- Untreated AML and a history of prior cytotoxic treatment
- Antecedent myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia, with or without prior hypomethylating therapy
- AML with WHO-defined, MDS-related cytogenetic abnormalities.
One hundred and fifty-three patients were randomized to receive CPX-351, and 156 were randomized to 7+3 (cytarabine continuously for 7 days and a single dose of daunorubicin for the first 3 days).
The treatment arms were well-balanced for sex, race, age, performance status, AML subtype, MDS-related cytogenetics, and prior hypomethylating therapy.
The trial met its primary endpoint, demonstrating a significant improvement in overall survival with CPX-351. The median overall survival was 9.56 months in the CPX-351 arm and 5.95 months in the 7+3 arm. The hazard ratio was 0.69 (P=0.005).
According to researchers, there was no substantial difference between the treatment arms with regard to grade 3-5 adverse events.
About CPX-351
CPX-351 is a liposomal formulation of a 5:1 molar ratio of cytarabine and daunorubicin.
In one phase 2 trial, CPX-351 conferred a significant improvement in survival—over investigator’s choice of therapy—when used as salvage therapy in poor-risk patients with AML.
In another phase 2 trial, CPX-351 conferred a significant survival benefit—over 7+3—in patients with secondary AML.
The FDA previously granted CPX-351 fast track designation to treat elderly patients with secondary AML. The agency established the fast track designation process to expedite the review of drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
The designation allows a drug’s developer to submit sections of a new drug application (NDA) on a rolling basis, so the FDA can review portions of the NDA as they are received instead of waiting for the entire NDA submission. A fast-track-designated product could be eligible for priority review if supported by clinical data at the time of NDA submission. ![]()
Guidelines add two new heart failure treatments
Optimal use of two recently approved medications for heart failure has been detailed by the major heart societies in a guideline update.
The American College of Cardiology, the American Heart Association, and the Heart Failure Society of America issued joint recommendations May 20 on the two new medicines for stage C heart failure patients with a reduced ejection fraction.
Valsartan/sacubitril (Entresto, Novartis), is a combination angiotensin receptor–neprilysin inhibitor, the first in a novel class of drugs slugged ARNIs. Ivabradine (Corlanor, Amgen), is a sinoatrial node modulator. Both medicines were approved by the Food and Drug Administration in 2015, though ivabradine has been licensed for a decade in Europe.
Although a comprehensive update to ACC/AHA/HSFA heart failure guidelines is still being developed, the focused update is intended to coincide with the release of new European Society of Cardiology heart failure guidelines, “in order to minimize confusion and improve the care of patients with heart failure,” the societies said in a statement May 20. The recommendations were published online simultaneously in Circulation and the Journal of Cardiac Failure.
The guideline authors, led by Dr. Clyde W. Yancy of Northwestern University in Chicago, recommend that the ARNI replace an ACE inhibitor or an angiotensin II receptor blocker (ARB) for patients who have been tolerating these therapies alongside standard care with a beta-blocker and, for some patients, an aldosterone antagonist as well. The guidelines caution against combining an ARNI with an ACE inhibitor, and against using ARNIs in patients with a history of angioedema.
For patients not suited to treatment with an ARNI, continued use of an ACE inhibitor is recommended. In patients for whom an ACE inhibitor or an ARNI is inappropriate, use of an ARB remains advised. The authors noted that head-to-head comparisons of an ARB versus an ARNI for heart failure do not exist; however, in a randomized, controlled trial in heart failure patients, treatment with valsartan/sacubitril plus standard care reduced cardiovascular death or heart failure hospitalization by 20%, compared with treatment with an ACE inhibitor plus standard care.
Ivabradine, meanwhile, has shown benefit in reducing heart failure hospitalizations in patients with symptomatic, stable, chronic heart failure with reduced ejection fraction who are receiving standard treatment including a beta-blocker, and who are in sinus rhythm with a heart rate of 70 beats per minute or greater at rest.
The new therapies, “when applied judiciously, complement established pharmacological and device-based therapies, representing milestones in the evolution of care for patients with heart failure,” wrote Dr. Elliott M. Antman of Brigham and Women’s Hospital and Harvard Medical School in Boston, Mass., in an editorial accompanying the guidelines.
About half the guideline writing committee members and guideline reviewers disclosed financial relationships with pharmaceutical companies or device manufacturers. Dr. Yancy disclosed no conflicts of interest.
Optimal use of two recently approved medications for heart failure has been detailed by the major heart societies in a guideline update.
The American College of Cardiology, the American Heart Association, and the Heart Failure Society of America issued joint recommendations May 20 on the two new medicines for stage C heart failure patients with a reduced ejection fraction.
Valsartan/sacubitril (Entresto, Novartis), is a combination angiotensin receptor–neprilysin inhibitor, the first in a novel class of drugs slugged ARNIs. Ivabradine (Corlanor, Amgen), is a sinoatrial node modulator. Both medicines were approved by the Food and Drug Administration in 2015, though ivabradine has been licensed for a decade in Europe.
Although a comprehensive update to ACC/AHA/HSFA heart failure guidelines is still being developed, the focused update is intended to coincide with the release of new European Society of Cardiology heart failure guidelines, “in order to minimize confusion and improve the care of patients with heart failure,” the societies said in a statement May 20. The recommendations were published online simultaneously in Circulation and the Journal of Cardiac Failure.
The guideline authors, led by Dr. Clyde W. Yancy of Northwestern University in Chicago, recommend that the ARNI replace an ACE inhibitor or an angiotensin II receptor blocker (ARB) for patients who have been tolerating these therapies alongside standard care with a beta-blocker and, for some patients, an aldosterone antagonist as well. The guidelines caution against combining an ARNI with an ACE inhibitor, and against using ARNIs in patients with a history of angioedema.
For patients not suited to treatment with an ARNI, continued use of an ACE inhibitor is recommended. In patients for whom an ACE inhibitor or an ARNI is inappropriate, use of an ARB remains advised. The authors noted that head-to-head comparisons of an ARB versus an ARNI for heart failure do not exist; however, in a randomized, controlled trial in heart failure patients, treatment with valsartan/sacubitril plus standard care reduced cardiovascular death or heart failure hospitalization by 20%, compared with treatment with an ACE inhibitor plus standard care.
Ivabradine, meanwhile, has shown benefit in reducing heart failure hospitalizations in patients with symptomatic, stable, chronic heart failure with reduced ejection fraction who are receiving standard treatment including a beta-blocker, and who are in sinus rhythm with a heart rate of 70 beats per minute or greater at rest.
The new therapies, “when applied judiciously, complement established pharmacological and device-based therapies, representing milestones in the evolution of care for patients with heart failure,” wrote Dr. Elliott M. Antman of Brigham and Women’s Hospital and Harvard Medical School in Boston, Mass., in an editorial accompanying the guidelines.
About half the guideline writing committee members and guideline reviewers disclosed financial relationships with pharmaceutical companies or device manufacturers. Dr. Yancy disclosed no conflicts of interest.
Optimal use of two recently approved medications for heart failure has been detailed by the major heart societies in a guideline update.
The American College of Cardiology, the American Heart Association, and the Heart Failure Society of America issued joint recommendations May 20 on the two new medicines for stage C heart failure patients with a reduced ejection fraction.
Valsartan/sacubitril (Entresto, Novartis), is a combination angiotensin receptor–neprilysin inhibitor, the first in a novel class of drugs slugged ARNIs. Ivabradine (Corlanor, Amgen), is a sinoatrial node modulator. Both medicines were approved by the Food and Drug Administration in 2015, though ivabradine has been licensed for a decade in Europe.
Although a comprehensive update to ACC/AHA/HSFA heart failure guidelines is still being developed, the focused update is intended to coincide with the release of new European Society of Cardiology heart failure guidelines, “in order to minimize confusion and improve the care of patients with heart failure,” the societies said in a statement May 20. The recommendations were published online simultaneously in Circulation and the Journal of Cardiac Failure.
The guideline authors, led by Dr. Clyde W. Yancy of Northwestern University in Chicago, recommend that the ARNI replace an ACE inhibitor or an angiotensin II receptor blocker (ARB) for patients who have been tolerating these therapies alongside standard care with a beta-blocker and, for some patients, an aldosterone antagonist as well. The guidelines caution against combining an ARNI with an ACE inhibitor, and against using ARNIs in patients with a history of angioedema.
For patients not suited to treatment with an ARNI, continued use of an ACE inhibitor is recommended. In patients for whom an ACE inhibitor or an ARNI is inappropriate, use of an ARB remains advised. The authors noted that head-to-head comparisons of an ARB versus an ARNI for heart failure do not exist; however, in a randomized, controlled trial in heart failure patients, treatment with valsartan/sacubitril plus standard care reduced cardiovascular death or heart failure hospitalization by 20%, compared with treatment with an ACE inhibitor plus standard care.
Ivabradine, meanwhile, has shown benefit in reducing heart failure hospitalizations in patients with symptomatic, stable, chronic heart failure with reduced ejection fraction who are receiving standard treatment including a beta-blocker, and who are in sinus rhythm with a heart rate of 70 beats per minute or greater at rest.
The new therapies, “when applied judiciously, complement established pharmacological and device-based therapies, representing milestones in the evolution of care for patients with heart failure,” wrote Dr. Elliott M. Antman of Brigham and Women’s Hospital and Harvard Medical School in Boston, Mass., in an editorial accompanying the guidelines.
About half the guideline writing committee members and guideline reviewers disclosed financial relationships with pharmaceutical companies or device manufacturers. Dr. Yancy disclosed no conflicts of interest.
FROM CIRCULATION
Interesting stats: MACRA’s GI impact
CMS released the long-awaited proposed rule implementing the Medicare Access and CHIP Reauthorization Act (MACRA) in late April. The most important thing you can do now is to become more familiar with the programs under MACRA and begin to prepare for the changes it will mean for your practice.
We’ve summarized a few interesting stats on the potential impact to GIs.
CMS will roll out the comprehensive Merit-based Incentive Payment System (MIPs) and incentivize the use of alternative payment models (APMs). Services provided beginning on Jan. 1, 2017, will directly impact reimbursement provided in 2019, the first year in which the MIPS program and APMs are effective.
CMS expects that 1,849 gastroenterologists will be excluded from MIPS. These GIs will be excluded because they participate in alternative payment models or see fewer than 100 Medicare Part B–eligible patients and bill less than $10,000 to Medicare.
CMS projects the majority of GIs (61.5%) who participate in MIPS will receive a bonus. Positive payment adjustments are projected to be about $34 million for GIs. Unfortunately, this increase would be partially offset by negative payment adjustments for 38.3% of GIs. Nearly 60% of colorectal surgeons are also expected to receive a positive adjustment.
The larger the practice, the more financial upside. According to CMS data, the likelihood of receiving an upward performance adjustment increases as the practice size increases. Among practices with two to nine eligible MIPS clinicians, only 29.8% are expected to receive a positive adjustment. This number increases to 81.3% for practices with 100 or more. Solo practitioners will be hit hardest by MIPS, with 87% likely facing a negative adjustment totaling a loss of $300 million for solo practices across all specialties.
We have an opportunity this summer to comment on the rule and advocate for changes. Read more about the MACRA proposed rule at gastro.org
CMS released the long-awaited proposed rule implementing the Medicare Access and CHIP Reauthorization Act (MACRA) in late April. The most important thing you can do now is to become more familiar with the programs under MACRA and begin to prepare for the changes it will mean for your practice.
We’ve summarized a few interesting stats on the potential impact to GIs.
CMS will roll out the comprehensive Merit-based Incentive Payment System (MIPs) and incentivize the use of alternative payment models (APMs). Services provided beginning on Jan. 1, 2017, will directly impact reimbursement provided in 2019, the first year in which the MIPS program and APMs are effective.
CMS expects that 1,849 gastroenterologists will be excluded from MIPS. These GIs will be excluded because they participate in alternative payment models or see fewer than 100 Medicare Part B–eligible patients and bill less than $10,000 to Medicare.
CMS projects the majority of GIs (61.5%) who participate in MIPS will receive a bonus. Positive payment adjustments are projected to be about $34 million for GIs. Unfortunately, this increase would be partially offset by negative payment adjustments for 38.3% of GIs. Nearly 60% of colorectal surgeons are also expected to receive a positive adjustment.
The larger the practice, the more financial upside. According to CMS data, the likelihood of receiving an upward performance adjustment increases as the practice size increases. Among practices with two to nine eligible MIPS clinicians, only 29.8% are expected to receive a positive adjustment. This number increases to 81.3% for practices with 100 or more. Solo practitioners will be hit hardest by MIPS, with 87% likely facing a negative adjustment totaling a loss of $300 million for solo practices across all specialties.
We have an opportunity this summer to comment on the rule and advocate for changes. Read more about the MACRA proposed rule at gastro.org
CMS released the long-awaited proposed rule implementing the Medicare Access and CHIP Reauthorization Act (MACRA) in late April. The most important thing you can do now is to become more familiar with the programs under MACRA and begin to prepare for the changes it will mean for your practice.
We’ve summarized a few interesting stats on the potential impact to GIs.
CMS will roll out the comprehensive Merit-based Incentive Payment System (MIPs) and incentivize the use of alternative payment models (APMs). Services provided beginning on Jan. 1, 2017, will directly impact reimbursement provided in 2019, the first year in which the MIPS program and APMs are effective.
CMS expects that 1,849 gastroenterologists will be excluded from MIPS. These GIs will be excluded because they participate in alternative payment models or see fewer than 100 Medicare Part B–eligible patients and bill less than $10,000 to Medicare.
CMS projects the majority of GIs (61.5%) who participate in MIPS will receive a bonus. Positive payment adjustments are projected to be about $34 million for GIs. Unfortunately, this increase would be partially offset by negative payment adjustments for 38.3% of GIs. Nearly 60% of colorectal surgeons are also expected to receive a positive adjustment.
The larger the practice, the more financial upside. According to CMS data, the likelihood of receiving an upward performance adjustment increases as the practice size increases. Among practices with two to nine eligible MIPS clinicians, only 29.8% are expected to receive a positive adjustment. This number increases to 81.3% for practices with 100 or more. Solo practitioners will be hit hardest by MIPS, with 87% likely facing a negative adjustment totaling a loss of $300 million for solo practices across all specialties.
We have an opportunity this summer to comment on the rule and advocate for changes. Read more about the MACRA proposed rule at gastro.org
ABIM to offer new MOC assessment options
ABIM announced plans on May 5 to offer physicians the option of taking shorter assessments on their personal or office computers more frequently than every 10 years, but no more than annually.
This announcement is welcome, and follows an aggressive campaign by AGA and other GI organizations advocating for elimination of the high-stakes, closed-book, timed exam. We support moving to a system of active learning and will continue to push ABIM to include our principles related to individualized learning and meaningful assessments.
Before the new assessment option is implemented, there will be a public comment period about the potential changes. AGA will be actively engaged in that conversation. This is a step in the right direction, but questions remain as to whether the changes are enough or whether the assessment will be individualized to the professional activities of subspecialists. Our work continues.
For the latest updates on MOC, visit the MOC page on the AGA website or join the discussion on the AGA Community.
ABIM announced plans on May 5 to offer physicians the option of taking shorter assessments on their personal or office computers more frequently than every 10 years, but no more than annually.
This announcement is welcome, and follows an aggressive campaign by AGA and other GI organizations advocating for elimination of the high-stakes, closed-book, timed exam. We support moving to a system of active learning and will continue to push ABIM to include our principles related to individualized learning and meaningful assessments.
Before the new assessment option is implemented, there will be a public comment period about the potential changes. AGA will be actively engaged in that conversation. This is a step in the right direction, but questions remain as to whether the changes are enough or whether the assessment will be individualized to the professional activities of subspecialists. Our work continues.
For the latest updates on MOC, visit the MOC page on the AGA website or join the discussion on the AGA Community.
ABIM announced plans on May 5 to offer physicians the option of taking shorter assessments on their personal or office computers more frequently than every 10 years, but no more than annually.
This announcement is welcome, and follows an aggressive campaign by AGA and other GI organizations advocating for elimination of the high-stakes, closed-book, timed exam. We support moving to a system of active learning and will continue to push ABIM to include our principles related to individualized learning and meaningful assessments.
Before the new assessment option is implemented, there will be a public comment period about the potential changes. AGA will be actively engaged in that conversation. This is a step in the right direction, but questions remain as to whether the changes are enough or whether the assessment will be individualized to the professional activities of subspecialists. Our work continues.
For the latest updates on MOC, visit the MOC page on the AGA website or join the discussion on the AGA Community.
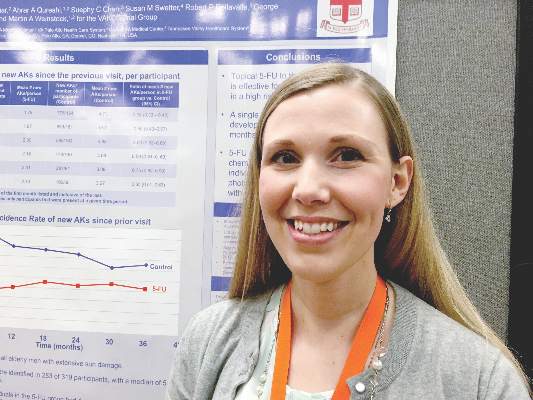
For preventing AKs, 5-FU beats placebo for up to 3 years
SCOTTSDALE – A single course of topical 5-fluorouracil (5-FU) prevented 62% more actinic keratoses than placebo, and this chemopreventive effect persisted for up to 3 years, according to an analysis of the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCCT) trial.
Other studies have shown that 5-FU effectively treats precancerous AKs, but have not examined whether 5-FU can prevent AKs, Dr. Joanna Walker said in an interview at the annual meeting of the Society for Investigative Dermatology.
Clinicians should consider preventive 5-FU in patients who are at high risk for basal cell and squamous cell carcinomas, especially if a skin check reveals multiple AKs, said Dr. Walker of the department of dermatology, Brown University, Providence, RI.
The VAKCCT was a randomized, double-blind, placebo-controlled study conducted at 12 Veterans Affairs dermatology clinics. The 319 patients in the analysis were nearly all elderly men with extensive sun damage, with a total of 2,386 AKs at baseline, for an average of five lesions per patient. Patients also had a history of at least two keratinocyte carcinomas in the past 5 years, including at least one lesion on the face or ears, and no recent history of 5-FU exposure.
The clinically and demographically similar study arms were randomized to either 5% topical 5-FU cream or a vehicle control cream, applied twice daily for 2-4 weeks. Both groups received cryotherapy for existing AKs, and were given free SPF 30 sunscreen. At each 6-month follow-up visit, the researchers counted existing AKs and new lesions.
At month 6, the treatment group had 62% fewer new AKs than the placebo group (average per patient, 1.78 and 4.73, respectively), a statistically significant difference. At months 12, 18, 24, 30, and 36, respectively, the treatment group had 50%, 40%, 41%, 25%, and 35% fewer new AKs than the placebo group, and these differences all were statistically significant. Furthermore, at month 6, only 56% of treated patients had at least one new AK, compared with 78% of the control group (incidence rate ratio, 0.72; 95% confidence interval, 0.54-0.95).
This chemopreventive effect remained significant for 24 months, the investigators reported. “Individuals with at least five AKs at the time of 5-FU treatment had an even more dramatic reduction in new AKs,” Dr. Walker noted. “There is now high-quality evidence supporting the use of topical 5-FU for AK chemoprevention. I think this is important information that we can take back to the clinic when we are trying to convince our patients to go through a course of 5-FU.”
The rate of new AKs in the placebo group fell during the first 2.5 years of the study and then stabilized. “For both groups, there was a dramatic increase in the use of sunscreen during the trial, and we hypothesized that the decrease in AKs in the control group was due to increased use of sun-protective measures,” Dr. Walker said.
The research was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
SCOTTSDALE – A single course of topical 5-fluorouracil (5-FU) prevented 62% more actinic keratoses than placebo, and this chemopreventive effect persisted for up to 3 years, according to an analysis of the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCCT) trial.
Other studies have shown that 5-FU effectively treats precancerous AKs, but have not examined whether 5-FU can prevent AKs, Dr. Joanna Walker said in an interview at the annual meeting of the Society for Investigative Dermatology.
Clinicians should consider preventive 5-FU in patients who are at high risk for basal cell and squamous cell carcinomas, especially if a skin check reveals multiple AKs, said Dr. Walker of the department of dermatology, Brown University, Providence, RI.
The VAKCCT was a randomized, double-blind, placebo-controlled study conducted at 12 Veterans Affairs dermatology clinics. The 319 patients in the analysis were nearly all elderly men with extensive sun damage, with a total of 2,386 AKs at baseline, for an average of five lesions per patient. Patients also had a history of at least two keratinocyte carcinomas in the past 5 years, including at least one lesion on the face or ears, and no recent history of 5-FU exposure.
The clinically and demographically similar study arms were randomized to either 5% topical 5-FU cream or a vehicle control cream, applied twice daily for 2-4 weeks. Both groups received cryotherapy for existing AKs, and were given free SPF 30 sunscreen. At each 6-month follow-up visit, the researchers counted existing AKs and new lesions.
At month 6, the treatment group had 62% fewer new AKs than the placebo group (average per patient, 1.78 and 4.73, respectively), a statistically significant difference. At months 12, 18, 24, 30, and 36, respectively, the treatment group had 50%, 40%, 41%, 25%, and 35% fewer new AKs than the placebo group, and these differences all were statistically significant. Furthermore, at month 6, only 56% of treated patients had at least one new AK, compared with 78% of the control group (incidence rate ratio, 0.72; 95% confidence interval, 0.54-0.95).
This chemopreventive effect remained significant for 24 months, the investigators reported. “Individuals with at least five AKs at the time of 5-FU treatment had an even more dramatic reduction in new AKs,” Dr. Walker noted. “There is now high-quality evidence supporting the use of topical 5-FU for AK chemoprevention. I think this is important information that we can take back to the clinic when we are trying to convince our patients to go through a course of 5-FU.”
The rate of new AKs in the placebo group fell during the first 2.5 years of the study and then stabilized. “For both groups, there was a dramatic increase in the use of sunscreen during the trial, and we hypothesized that the decrease in AKs in the control group was due to increased use of sun-protective measures,” Dr. Walker said.
The research was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
SCOTTSDALE – A single course of topical 5-fluorouracil (5-FU) prevented 62% more actinic keratoses than placebo, and this chemopreventive effect persisted for up to 3 years, according to an analysis of the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCCT) trial.
Other studies have shown that 5-FU effectively treats precancerous AKs, but have not examined whether 5-FU can prevent AKs, Dr. Joanna Walker said in an interview at the annual meeting of the Society for Investigative Dermatology.
Clinicians should consider preventive 5-FU in patients who are at high risk for basal cell and squamous cell carcinomas, especially if a skin check reveals multiple AKs, said Dr. Walker of the department of dermatology, Brown University, Providence, RI.
The VAKCCT was a randomized, double-blind, placebo-controlled study conducted at 12 Veterans Affairs dermatology clinics. The 319 patients in the analysis were nearly all elderly men with extensive sun damage, with a total of 2,386 AKs at baseline, for an average of five lesions per patient. Patients also had a history of at least two keratinocyte carcinomas in the past 5 years, including at least one lesion on the face or ears, and no recent history of 5-FU exposure.
The clinically and demographically similar study arms were randomized to either 5% topical 5-FU cream or a vehicle control cream, applied twice daily for 2-4 weeks. Both groups received cryotherapy for existing AKs, and were given free SPF 30 sunscreen. At each 6-month follow-up visit, the researchers counted existing AKs and new lesions.
At month 6, the treatment group had 62% fewer new AKs than the placebo group (average per patient, 1.78 and 4.73, respectively), a statistically significant difference. At months 12, 18, 24, 30, and 36, respectively, the treatment group had 50%, 40%, 41%, 25%, and 35% fewer new AKs than the placebo group, and these differences all were statistically significant. Furthermore, at month 6, only 56% of treated patients had at least one new AK, compared with 78% of the control group (incidence rate ratio, 0.72; 95% confidence interval, 0.54-0.95).
This chemopreventive effect remained significant for 24 months, the investigators reported. “Individuals with at least five AKs at the time of 5-FU treatment had an even more dramatic reduction in new AKs,” Dr. Walker noted. “There is now high-quality evidence supporting the use of topical 5-FU for AK chemoprevention. I think this is important information that we can take back to the clinic when we are trying to convince our patients to go through a course of 5-FU.”
The rate of new AKs in the placebo group fell during the first 2.5 years of the study and then stabilized. “For both groups, there was a dramatic increase in the use of sunscreen during the trial, and we hypothesized that the decrease in AKs in the control group was due to increased use of sun-protective measures,” Dr. Walker said.
The research was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: One course of topical 5-fluorouracil was effective and durable in preventing new actinic keratoses in high-risk patients.
Major finding: At month 6, the treatment group had 62% fewer new AKs than the placebo group, and the difference remained significant at month 36.
Data source: The double-blind controlled study evaluated 5-FU vs. a vehicle cream in 319 veterans, most of whom were elderly men.
Disclosures: The study was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
Abdominal Aortic Aneurysm in Psoriasis Patients
In a study published online on April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology, Khalid et al evaluated the risk for AAA in patients with psoriasis in a nationwide cohort study in Denmark. The study participants were Danish residents 18 years and older who were observed from January 1, 1997 until diagnosis of AAA; December 31, 2011; migration; or death. Incidence rates for AAA were calculated, and incidence rate ratios were adjusted for age, sex, comorbidity, medications, socioeconomic status, and smoking.
A total of 5,495,203 individuals were eligible for this study. Of them, Khalid et al identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis. The overall incidence rates of AAA were 3.72, 7.30, and 9.87 per 10,000 person-years for the reference population (23,696 cases), mild psoriasis (240 cases), and severe psoriasis (50 cases), respectively. The corresponding adjusted incidence rate ratios for AAA were increased in patients with psoriasis with incidence rate ratios of 1.20 (95% CI, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32) for individuals with mild and severe disease, respectively.
Khalid et al concluded that psoriasis was associated with a disease severity–dependent increased risk for AAA; however, the mechanisms and consequences of this novel finding require further investigation.
What’s the issue?
Another example of an association of a comorbidity with psoriasis, this finding emphasizes the need for cardiovascular referral in psoriasis patients with risk factors such as hypertension and diabetes mellitus. How will these data influence your evaluation of psoriasis patients?
In a study published online on April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology, Khalid et al evaluated the risk for AAA in patients with psoriasis in a nationwide cohort study in Denmark. The study participants were Danish residents 18 years and older who were observed from January 1, 1997 until diagnosis of AAA; December 31, 2011; migration; or death. Incidence rates for AAA were calculated, and incidence rate ratios were adjusted for age, sex, comorbidity, medications, socioeconomic status, and smoking.
A total of 5,495,203 individuals were eligible for this study. Of them, Khalid et al identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis. The overall incidence rates of AAA were 3.72, 7.30, and 9.87 per 10,000 person-years for the reference population (23,696 cases), mild psoriasis (240 cases), and severe psoriasis (50 cases), respectively. The corresponding adjusted incidence rate ratios for AAA were increased in patients with psoriasis with incidence rate ratios of 1.20 (95% CI, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32) for individuals with mild and severe disease, respectively.
Khalid et al concluded that psoriasis was associated with a disease severity–dependent increased risk for AAA; however, the mechanisms and consequences of this novel finding require further investigation.
What’s the issue?
Another example of an association of a comorbidity with psoriasis, this finding emphasizes the need for cardiovascular referral in psoriasis patients with risk factors such as hypertension and diabetes mellitus. How will these data influence your evaluation of psoriasis patients?
In a study published online on April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology, Khalid et al evaluated the risk for AAA in patients with psoriasis in a nationwide cohort study in Denmark. The study participants were Danish residents 18 years and older who were observed from January 1, 1997 until diagnosis of AAA; December 31, 2011; migration; or death. Incidence rates for AAA were calculated, and incidence rate ratios were adjusted for age, sex, comorbidity, medications, socioeconomic status, and smoking.
A total of 5,495,203 individuals were eligible for this study. Of them, Khalid et al identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis. The overall incidence rates of AAA were 3.72, 7.30, and 9.87 per 10,000 person-years for the reference population (23,696 cases), mild psoriasis (240 cases), and severe psoriasis (50 cases), respectively. The corresponding adjusted incidence rate ratios for AAA were increased in patients with psoriasis with incidence rate ratios of 1.20 (95% CI, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32) for individuals with mild and severe disease, respectively.
Khalid et al concluded that psoriasis was associated with a disease severity–dependent increased risk for AAA; however, the mechanisms and consequences of this novel finding require further investigation.
What’s the issue?
Another example of an association of a comorbidity with psoriasis, this finding emphasizes the need for cardiovascular referral in psoriasis patients with risk factors such as hypertension and diabetes mellitus. How will these data influence your evaluation of psoriasis patients?
Care bundle reduces cesarean surgical site infections
WASHINGTON – The rate of cesarean delivery surgical site infections fell significantly at Yale New Haven (Conn.) Hospital after implementation of a multidisciplinary care bundle with protocols covering preoperative, intraoperative, and postoperative care.
An analysis of two 3-month sampling periods – one before implementation of the bundle of care and one after – showed a drop in the surgical site infection (SSI) rate among total cesarean sections from 3.4% to 2.2%.
At the annual meeting of the American College of Obstetricians and Gynecologists, Dr. Ashley Pritchard of the hospital described the care bundle and urged obstetricians to consider the impact of even small reductions in SSIs after a cesarean.
“Infection is the most common complication following cesarean delivery … 2.5%-16% of all cesarean deliveries will result in a surgical site infection, and there is significant underestimation as between 15% to 80% of infections are diagnosed after patients leave the hospital,” she said.
A cesarean delivery SSI task force created by the hospital’s obstetric patient safety program developed the care bundle after reviewing best practices, guidelines, and evidence-based reviews.
Preoperative protocols for planned cesareans focused on patient education and included a preoperative appointment and instructions for showering the night before surgery, not shaving for more than 24 hours prior to scheduled surgery, using 2% chlorhexidine wipes both the night prior to surgery and the day of surgery, and other hygiene processes.
“We know from numerous studies that chlorhexidine is superior to iodine, but we also have found that with these wipes you get a level of antibiosis on the skin surface that decreases surgical site infections at the time of incisions,” Dr. Pritchard said.
For the operative care part of the bundle, staff were reeducated about the scrubbing protocol, proper attire and limits on operating room traffic, and the correct and timely use of antibiotics (for example, a cephalosporin administered within 30 minutes of incision). Staff also watched a video and were quizzed on the proper technique and timing for preoperative skin preparation.
Increased attention was paid to normothermia and included preoperative use of warming blankets and proper temperature in the operating room and post–anesthesia care unit.
“We’ve learned from colorectal and trauma surgery that normothermia and patient warming lead to reduced SSI,” Dr. Pritchard said. “This hasn’t been proven with cesarean delivery, but we know there’s improved maternal and fetal well-being with preoperative warming.”
Postoperatively, the use of supplemental oxygen was discontinued unless clinically indicated “since it’s been shown to have no positive effect on SSI,” she said. Incision dressing application and removal were also standardized, with sterile dressings maintained for at least 24 hours – with a tag labeling the date and time of application – and no more than 48 hours. At discharge, patients were given clear discharge instructions and a postpartum appointment for an incision check.
During the 3-month sampling period prior to implementation of the care bundle, there were 382 cesarean deliveries, and 147 patients presented for a postpartum appointment (either the prescribed visit or a later “issue visit”) within 30 days (38%). Of these patients, 8.6% were diagnosed with an SSI.
In the postimplementation sampling period, which began 6 months after rollout, there were 361 cesarean deliveries at the hospital, and 297 patients (77%) presented for postpartum care. Of these patients, 2.9% were diagnosed with an SSI.
An analysis based on the total number of cesarean deliveries performed at the hospital (planned and unplanned) during the two 3-month periods showed a decline in the cesarean delivery SSI rate from 3.4% to 2.2%. “This is statistically significant. It shows a dramatic decline in the SSI rate in our patient population … a clear impact of the bundle of care,” Dr. Pritchard said.
The Yale team attributes the significant increase in postoperative visit attendance to the preoperative protocol for planned cesareans. “We think it had something to do with our creating better relationships by having [patients] present preoperatively and starting their care prior to incision,” she said.
The preoperative visit also provided an opportunity to identify and treat any active skin infections, upper respiratory infections, or chronic colonizations (without evidence of completion of treatment) before delivery. “If necessary and if possible, [we could] push back their cesarean section date to ensure adequate treatment had been achieved,” Dr. Pritchard said.
All aspects of the care bundle were rolled out simultaneously. Next steps for the New Haven team include further analysis of provider and patient views, a look at the sustainability of the bundle of care and its impact, and a cost analysis. “We reduced our SSIs, but we’ve also added a number of elements to our care spectrum, pre- and postoperatively,” Dr. Pritchard said.
For now, one thing seems clear: “We learned from cardiac and colorectal surgery that there really is strength in the bundle, that the sum of the parts is greater than the individual aspects,” she said.
Dr. Pritchard reported that she and her coinvestigators have no relevant financial disclosures.
WASHINGTON – The rate of cesarean delivery surgical site infections fell significantly at Yale New Haven (Conn.) Hospital after implementation of a multidisciplinary care bundle with protocols covering preoperative, intraoperative, and postoperative care.
An analysis of two 3-month sampling periods – one before implementation of the bundle of care and one after – showed a drop in the surgical site infection (SSI) rate among total cesarean sections from 3.4% to 2.2%.
At the annual meeting of the American College of Obstetricians and Gynecologists, Dr. Ashley Pritchard of the hospital described the care bundle and urged obstetricians to consider the impact of even small reductions in SSIs after a cesarean.
“Infection is the most common complication following cesarean delivery … 2.5%-16% of all cesarean deliveries will result in a surgical site infection, and there is significant underestimation as between 15% to 80% of infections are diagnosed after patients leave the hospital,” she said.
A cesarean delivery SSI task force created by the hospital’s obstetric patient safety program developed the care bundle after reviewing best practices, guidelines, and evidence-based reviews.
Preoperative protocols for planned cesareans focused on patient education and included a preoperative appointment and instructions for showering the night before surgery, not shaving for more than 24 hours prior to scheduled surgery, using 2% chlorhexidine wipes both the night prior to surgery and the day of surgery, and other hygiene processes.
“We know from numerous studies that chlorhexidine is superior to iodine, but we also have found that with these wipes you get a level of antibiosis on the skin surface that decreases surgical site infections at the time of incisions,” Dr. Pritchard said.
For the operative care part of the bundle, staff were reeducated about the scrubbing protocol, proper attire and limits on operating room traffic, and the correct and timely use of antibiotics (for example, a cephalosporin administered within 30 minutes of incision). Staff also watched a video and were quizzed on the proper technique and timing for preoperative skin preparation.
Increased attention was paid to normothermia and included preoperative use of warming blankets and proper temperature in the operating room and post–anesthesia care unit.
“We’ve learned from colorectal and trauma surgery that normothermia and patient warming lead to reduced SSI,” Dr. Pritchard said. “This hasn’t been proven with cesarean delivery, but we know there’s improved maternal and fetal well-being with preoperative warming.”
Postoperatively, the use of supplemental oxygen was discontinued unless clinically indicated “since it’s been shown to have no positive effect on SSI,” she said. Incision dressing application and removal were also standardized, with sterile dressings maintained for at least 24 hours – with a tag labeling the date and time of application – and no more than 48 hours. At discharge, patients were given clear discharge instructions and a postpartum appointment for an incision check.
During the 3-month sampling period prior to implementation of the care bundle, there were 382 cesarean deliveries, and 147 patients presented for a postpartum appointment (either the prescribed visit or a later “issue visit”) within 30 days (38%). Of these patients, 8.6% were diagnosed with an SSI.
In the postimplementation sampling period, which began 6 months after rollout, there were 361 cesarean deliveries at the hospital, and 297 patients (77%) presented for postpartum care. Of these patients, 2.9% were diagnosed with an SSI.
An analysis based on the total number of cesarean deliveries performed at the hospital (planned and unplanned) during the two 3-month periods showed a decline in the cesarean delivery SSI rate from 3.4% to 2.2%. “This is statistically significant. It shows a dramatic decline in the SSI rate in our patient population … a clear impact of the bundle of care,” Dr. Pritchard said.
The Yale team attributes the significant increase in postoperative visit attendance to the preoperative protocol for planned cesareans. “We think it had something to do with our creating better relationships by having [patients] present preoperatively and starting their care prior to incision,” she said.
The preoperative visit also provided an opportunity to identify and treat any active skin infections, upper respiratory infections, or chronic colonizations (without evidence of completion of treatment) before delivery. “If necessary and if possible, [we could] push back their cesarean section date to ensure adequate treatment had been achieved,” Dr. Pritchard said.
All aspects of the care bundle were rolled out simultaneously. Next steps for the New Haven team include further analysis of provider and patient views, a look at the sustainability of the bundle of care and its impact, and a cost analysis. “We reduced our SSIs, but we’ve also added a number of elements to our care spectrum, pre- and postoperatively,” Dr. Pritchard said.
For now, one thing seems clear: “We learned from cardiac and colorectal surgery that there really is strength in the bundle, that the sum of the parts is greater than the individual aspects,” she said.
Dr. Pritchard reported that she and her coinvestigators have no relevant financial disclosures.
WASHINGTON – The rate of cesarean delivery surgical site infections fell significantly at Yale New Haven (Conn.) Hospital after implementation of a multidisciplinary care bundle with protocols covering preoperative, intraoperative, and postoperative care.
An analysis of two 3-month sampling periods – one before implementation of the bundle of care and one after – showed a drop in the surgical site infection (SSI) rate among total cesarean sections from 3.4% to 2.2%.
At the annual meeting of the American College of Obstetricians and Gynecologists, Dr. Ashley Pritchard of the hospital described the care bundle and urged obstetricians to consider the impact of even small reductions in SSIs after a cesarean.
“Infection is the most common complication following cesarean delivery … 2.5%-16% of all cesarean deliveries will result in a surgical site infection, and there is significant underestimation as between 15% to 80% of infections are diagnosed after patients leave the hospital,” she said.
A cesarean delivery SSI task force created by the hospital’s obstetric patient safety program developed the care bundle after reviewing best practices, guidelines, and evidence-based reviews.
Preoperative protocols for planned cesareans focused on patient education and included a preoperative appointment and instructions for showering the night before surgery, not shaving for more than 24 hours prior to scheduled surgery, using 2% chlorhexidine wipes both the night prior to surgery and the day of surgery, and other hygiene processes.
“We know from numerous studies that chlorhexidine is superior to iodine, but we also have found that with these wipes you get a level of antibiosis on the skin surface that decreases surgical site infections at the time of incisions,” Dr. Pritchard said.
For the operative care part of the bundle, staff were reeducated about the scrubbing protocol, proper attire and limits on operating room traffic, and the correct and timely use of antibiotics (for example, a cephalosporin administered within 30 minutes of incision). Staff also watched a video and were quizzed on the proper technique and timing for preoperative skin preparation.
Increased attention was paid to normothermia and included preoperative use of warming blankets and proper temperature in the operating room and post–anesthesia care unit.
“We’ve learned from colorectal and trauma surgery that normothermia and patient warming lead to reduced SSI,” Dr. Pritchard said. “This hasn’t been proven with cesarean delivery, but we know there’s improved maternal and fetal well-being with preoperative warming.”
Postoperatively, the use of supplemental oxygen was discontinued unless clinically indicated “since it’s been shown to have no positive effect on SSI,” she said. Incision dressing application and removal were also standardized, with sterile dressings maintained for at least 24 hours – with a tag labeling the date and time of application – and no more than 48 hours. At discharge, patients were given clear discharge instructions and a postpartum appointment for an incision check.
During the 3-month sampling period prior to implementation of the care bundle, there were 382 cesarean deliveries, and 147 patients presented for a postpartum appointment (either the prescribed visit or a later “issue visit”) within 30 days (38%). Of these patients, 8.6% were diagnosed with an SSI.
In the postimplementation sampling period, which began 6 months after rollout, there were 361 cesarean deliveries at the hospital, and 297 patients (77%) presented for postpartum care. Of these patients, 2.9% were diagnosed with an SSI.
An analysis based on the total number of cesarean deliveries performed at the hospital (planned and unplanned) during the two 3-month periods showed a decline in the cesarean delivery SSI rate from 3.4% to 2.2%. “This is statistically significant. It shows a dramatic decline in the SSI rate in our patient population … a clear impact of the bundle of care,” Dr. Pritchard said.
The Yale team attributes the significant increase in postoperative visit attendance to the preoperative protocol for planned cesareans. “We think it had something to do with our creating better relationships by having [patients] present preoperatively and starting their care prior to incision,” she said.
The preoperative visit also provided an opportunity to identify and treat any active skin infections, upper respiratory infections, or chronic colonizations (without evidence of completion of treatment) before delivery. “If necessary and if possible, [we could] push back their cesarean section date to ensure adequate treatment had been achieved,” Dr. Pritchard said.
All aspects of the care bundle were rolled out simultaneously. Next steps for the New Haven team include further analysis of provider and patient views, a look at the sustainability of the bundle of care and its impact, and a cost analysis. “We reduced our SSIs, but we’ve also added a number of elements to our care spectrum, pre- and postoperatively,” Dr. Pritchard said.
For now, one thing seems clear: “We learned from cardiac and colorectal surgery that there really is strength in the bundle, that the sum of the parts is greater than the individual aspects,” she said.
Dr. Pritchard reported that she and her coinvestigators have no relevant financial disclosures.
AT ACOG 2016
Key clinical point: A multidisciplinary bundle of care was effective in reducing the rate of surgical site infections with cesarean delivery.
Major finding: The rate of cesarean delivery SSIs decreased from 3.4% to 2.2% after implementation of a care bundle.
Data source: An analysis of a quality improvement project at Yale New Haven Hospital.
Disclosures: Dr. Pritchard reported that she and her coinvestigators have no relevant financial disclosures.
Improving our crystal ball: prognostication in neuroscience ICUs
The most difficult decisions in neuroscience intensive care units often involve patients’ ultimate goals of care. Oftentimes, family members of a brain-injured patient with an apparently poor neurologic prognosis must weigh whether their loved one would have preferred prolongation of aggressive ICU and post-ICU care, often with little to no chance for “meaningful” recovery, or death via the institution of comfort measures only. Proper prognostication is crucial to the family when making such decisions. However, the process of formulating and talking about prognosis for our most severely affected patients is subject to physician and family biases, families’ insufficient understanding of projected outcomes, and sometimes clinical nihilism by the physicians.
The process of predicting the outcomes of patients with traumatic brain injury (TBI) serves as an example of these issues. Moderate to severe TBI continues to be a leading cause of death and disability in the United States.1 Most deaths of patients with moderate to severe TBI follow decisions by doctors and families to pursue comfort care only. However, these decisions occur at a disconcertingly highly variable rate at different trauma centers, with the variation seemingly unrelated to patients’ disease severity, age, or previously diagnosed comorbidities.2 These patients are at risk for their care being influenced by a self-fulfilling prophecy: That is, the impression of a poor prognosis communicated by clinicians to a patient’s family, whether correct or incorrect, affects the aggressiveness of the care that a patient receives and determines the patient’s outcome.3
Remedying these issues through a family or health care proxy decision support intervention (“decision aid”) that could improve and standardize the way TBI prognosis is communicated may lead to better informed decisions for these critically ill patients, with potentially less decisional regret and post-ICU stress disorders in families, and decisions more in line with the patient’s values and preferences.4 A recent Cochrane review showed that for a decision aid to be effective and integrated into routine clinical care, it must contain disease-specific data tailored to patients and their families/proxies, and be simple and time efficient for physicians to use.5 Taking these factors into account, researchers at the University of Massachusetts are developing a National Institutes of Health–funded pilot decision aid for goals-of-care decisions in critically-ill TBI patients.
While the field of TBI has tools such as the IMPACT calculator that can be used to estimate a patient’s long-term prognosis based on how patients with similar clinical characteristics in large clinical databases have done, the fundamental uncertainty of prognosis remains a difficult challenge.6 Arguably, this challenge is even more daunting when estimating prognosis for patients with severe ischemic stroke and intracerebral hemorrhage (ICH). The use of ischemic stroke outcome prediction tools is complicated, as many of them are based on population databases with wide variations in whether included patients received intravenous tissue plasminogen activator, endovascular therapy, both, or neither. Furthermore, a recent study comparing the accuracy of the ICH score for predicting 3-month outcome for ICH patients to the subjective predictions of clinicians made within 24 hours of patient admission found that the educated guesses of physicians and nurses overall seemed to correlate with actual outcomes more closely than the ICH score output.7 This finding highlights the challenge of using available outcome “calculators” for individual patients in ICUs.
Ultimately, the decisions made about the goals of care for ICU patients come down not only to what their expected outcomes are, but also whether their surrogate decision makers believe that those outcomes would be acceptable to the patient.8 Potential pitfalls abound with regard to this issue as well. Decision makers are often not made aware of the fact that many times patients with significant disability may nevertheless report a reasonable quality of life. By their very nature, conversations regarding patient prognosis inevitably focus on what future disabilities one might expect; accounting for a patient’s possible adaptation to disability is both easy to overlook and hard to accomplish even when given adequate attention.9 Improvements in the field of neuroprognostication may not only depend on the development of new shared decision making tools for physicians and families but also on increasing awareness of the limitations of prognostic scales and the cognitive biases that may exist when discussing the possibilities of future disability.
References
1. Traumatic Brain Injury Statistics [online]. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed Nov. 1.
3. Neurocrit Care. 2013;19:347-63.
4. Col NF. Chapter 17: Shared Decision Making. In: Communicating Risks and Benefits: An Evidence-Based User’s Guide [online]. Available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/UCM268069.pdf.
5. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD001431.
6. PLoS Med. 2008 Aug;5(8):e165; discussion e168.
8. Neurocrit Care. 2015;23:131-41.
Dr. Muehlschlegel is associate professor of neurology (neurocritical care), anesthesia/critical care, and surgery at the University of Massachusetts, Worcester. Dr. Hwang is assistant professor of neurology in the division of neurocritical care and emergency neurology at Yale University, New Haven, Conn. Dr. Muehlschlegel reported receiving a grant from the National Institutes of Health for her research in developing a pilot decision aid for goals-of-care decisions in critically-ill TBI patients. Dr. Hwang reported receiving research funding from the American Brain Foundation, the Apple Pickers Foundation, the National Institute on Aging, and the Neurocritical Care Society.
The most difficult decisions in neuroscience intensive care units often involve patients’ ultimate goals of care. Oftentimes, family members of a brain-injured patient with an apparently poor neurologic prognosis must weigh whether their loved one would have preferred prolongation of aggressive ICU and post-ICU care, often with little to no chance for “meaningful” recovery, or death via the institution of comfort measures only. Proper prognostication is crucial to the family when making such decisions. However, the process of formulating and talking about prognosis for our most severely affected patients is subject to physician and family biases, families’ insufficient understanding of projected outcomes, and sometimes clinical nihilism by the physicians.
The process of predicting the outcomes of patients with traumatic brain injury (TBI) serves as an example of these issues. Moderate to severe TBI continues to be a leading cause of death and disability in the United States.1 Most deaths of patients with moderate to severe TBI follow decisions by doctors and families to pursue comfort care only. However, these decisions occur at a disconcertingly highly variable rate at different trauma centers, with the variation seemingly unrelated to patients’ disease severity, age, or previously diagnosed comorbidities.2 These patients are at risk for their care being influenced by a self-fulfilling prophecy: That is, the impression of a poor prognosis communicated by clinicians to a patient’s family, whether correct or incorrect, affects the aggressiveness of the care that a patient receives and determines the patient’s outcome.3
Remedying these issues through a family or health care proxy decision support intervention (“decision aid”) that could improve and standardize the way TBI prognosis is communicated may lead to better informed decisions for these critically ill patients, with potentially less decisional regret and post-ICU stress disorders in families, and decisions more in line with the patient’s values and preferences.4 A recent Cochrane review showed that for a decision aid to be effective and integrated into routine clinical care, it must contain disease-specific data tailored to patients and their families/proxies, and be simple and time efficient for physicians to use.5 Taking these factors into account, researchers at the University of Massachusetts are developing a National Institutes of Health–funded pilot decision aid for goals-of-care decisions in critically-ill TBI patients.
While the field of TBI has tools such as the IMPACT calculator that can be used to estimate a patient’s long-term prognosis based on how patients with similar clinical characteristics in large clinical databases have done, the fundamental uncertainty of prognosis remains a difficult challenge.6 Arguably, this challenge is even more daunting when estimating prognosis for patients with severe ischemic stroke and intracerebral hemorrhage (ICH). The use of ischemic stroke outcome prediction tools is complicated, as many of them are based on population databases with wide variations in whether included patients received intravenous tissue plasminogen activator, endovascular therapy, both, or neither. Furthermore, a recent study comparing the accuracy of the ICH score for predicting 3-month outcome for ICH patients to the subjective predictions of clinicians made within 24 hours of patient admission found that the educated guesses of physicians and nurses overall seemed to correlate with actual outcomes more closely than the ICH score output.7 This finding highlights the challenge of using available outcome “calculators” for individual patients in ICUs.
Ultimately, the decisions made about the goals of care for ICU patients come down not only to what their expected outcomes are, but also whether their surrogate decision makers believe that those outcomes would be acceptable to the patient.8 Potential pitfalls abound with regard to this issue as well. Decision makers are often not made aware of the fact that many times patients with significant disability may nevertheless report a reasonable quality of life. By their very nature, conversations regarding patient prognosis inevitably focus on what future disabilities one might expect; accounting for a patient’s possible adaptation to disability is both easy to overlook and hard to accomplish even when given adequate attention.9 Improvements in the field of neuroprognostication may not only depend on the development of new shared decision making tools for physicians and families but also on increasing awareness of the limitations of prognostic scales and the cognitive biases that may exist when discussing the possibilities of future disability.
References
1. Traumatic Brain Injury Statistics [online]. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed Nov. 1.
3. Neurocrit Care. 2013;19:347-63.
4. Col NF. Chapter 17: Shared Decision Making. In: Communicating Risks and Benefits: An Evidence-Based User’s Guide [online]. Available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/UCM268069.pdf.
5. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD001431.
6. PLoS Med. 2008 Aug;5(8):e165; discussion e168.
8. Neurocrit Care. 2015;23:131-41.
Dr. Muehlschlegel is associate professor of neurology (neurocritical care), anesthesia/critical care, and surgery at the University of Massachusetts, Worcester. Dr. Hwang is assistant professor of neurology in the division of neurocritical care and emergency neurology at Yale University, New Haven, Conn. Dr. Muehlschlegel reported receiving a grant from the National Institutes of Health for her research in developing a pilot decision aid for goals-of-care decisions in critically-ill TBI patients. Dr. Hwang reported receiving research funding from the American Brain Foundation, the Apple Pickers Foundation, the National Institute on Aging, and the Neurocritical Care Society.
The most difficult decisions in neuroscience intensive care units often involve patients’ ultimate goals of care. Oftentimes, family members of a brain-injured patient with an apparently poor neurologic prognosis must weigh whether their loved one would have preferred prolongation of aggressive ICU and post-ICU care, often with little to no chance for “meaningful” recovery, or death via the institution of comfort measures only. Proper prognostication is crucial to the family when making such decisions. However, the process of formulating and talking about prognosis for our most severely affected patients is subject to physician and family biases, families’ insufficient understanding of projected outcomes, and sometimes clinical nihilism by the physicians.
The process of predicting the outcomes of patients with traumatic brain injury (TBI) serves as an example of these issues. Moderate to severe TBI continues to be a leading cause of death and disability in the United States.1 Most deaths of patients with moderate to severe TBI follow decisions by doctors and families to pursue comfort care only. However, these decisions occur at a disconcertingly highly variable rate at different trauma centers, with the variation seemingly unrelated to patients’ disease severity, age, or previously diagnosed comorbidities.2 These patients are at risk for their care being influenced by a self-fulfilling prophecy: That is, the impression of a poor prognosis communicated by clinicians to a patient’s family, whether correct or incorrect, affects the aggressiveness of the care that a patient receives and determines the patient’s outcome.3
Remedying these issues through a family or health care proxy decision support intervention (“decision aid”) that could improve and standardize the way TBI prognosis is communicated may lead to better informed decisions for these critically ill patients, with potentially less decisional regret and post-ICU stress disorders in families, and decisions more in line with the patient’s values and preferences.4 A recent Cochrane review showed that for a decision aid to be effective and integrated into routine clinical care, it must contain disease-specific data tailored to patients and their families/proxies, and be simple and time efficient for physicians to use.5 Taking these factors into account, researchers at the University of Massachusetts are developing a National Institutes of Health–funded pilot decision aid for goals-of-care decisions in critically-ill TBI patients.
While the field of TBI has tools such as the IMPACT calculator that can be used to estimate a patient’s long-term prognosis based on how patients with similar clinical characteristics in large clinical databases have done, the fundamental uncertainty of prognosis remains a difficult challenge.6 Arguably, this challenge is even more daunting when estimating prognosis for patients with severe ischemic stroke and intracerebral hemorrhage (ICH). The use of ischemic stroke outcome prediction tools is complicated, as many of them are based on population databases with wide variations in whether included patients received intravenous tissue plasminogen activator, endovascular therapy, both, or neither. Furthermore, a recent study comparing the accuracy of the ICH score for predicting 3-month outcome for ICH patients to the subjective predictions of clinicians made within 24 hours of patient admission found that the educated guesses of physicians and nurses overall seemed to correlate with actual outcomes more closely than the ICH score output.7 This finding highlights the challenge of using available outcome “calculators” for individual patients in ICUs.
Ultimately, the decisions made about the goals of care for ICU patients come down not only to what their expected outcomes are, but also whether their surrogate decision makers believe that those outcomes would be acceptable to the patient.8 Potential pitfalls abound with regard to this issue as well. Decision makers are often not made aware of the fact that many times patients with significant disability may nevertheless report a reasonable quality of life. By their very nature, conversations regarding patient prognosis inevitably focus on what future disabilities one might expect; accounting for a patient’s possible adaptation to disability is both easy to overlook and hard to accomplish even when given adequate attention.9 Improvements in the field of neuroprognostication may not only depend on the development of new shared decision making tools for physicians and families but also on increasing awareness of the limitations of prognostic scales and the cognitive biases that may exist when discussing the possibilities of future disability.
References
1. Traumatic Brain Injury Statistics [online]. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed Nov. 1.
3. Neurocrit Care. 2013;19:347-63.
4. Col NF. Chapter 17: Shared Decision Making. In: Communicating Risks and Benefits: An Evidence-Based User’s Guide [online]. Available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/UCM268069.pdf.
5. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD001431.
6. PLoS Med. 2008 Aug;5(8):e165; discussion e168.
8. Neurocrit Care. 2015;23:131-41.
Dr. Muehlschlegel is associate professor of neurology (neurocritical care), anesthesia/critical care, and surgery at the University of Massachusetts, Worcester. Dr. Hwang is assistant professor of neurology in the division of neurocritical care and emergency neurology at Yale University, New Haven, Conn. Dr. Muehlschlegel reported receiving a grant from the National Institutes of Health for her research in developing a pilot decision aid for goals-of-care decisions in critically-ill TBI patients. Dr. Hwang reported receiving research funding from the American Brain Foundation, the Apple Pickers Foundation, the National Institute on Aging, and the Neurocritical Care Society.
Racing against burnout
At 6 a.m., after eight admissions back to back, I feel like I can’t stay awake any longer. I desperately need a nap. I have 30 minutes before my next admission, but I know I’m not going to rest. I close my eyes, and I only see charts. I’m trying not to collapse, but I still have three more patients to admit before I go home. And I can’t rest.
I’m scared that I’ll make a mistake. While I write my history of presenting illness, I imagine the day team reading my notes and hoping they don’t notice that I was about to crash on the desk. I check my documentation multiple times to make sure I don’t miss anything, and I know that won’t relieve my anxiety. When I leave, I will still feel the failure of not being able to give my best for my patient or for my night team, because I’m too tired. I know this frustration is going to give room to the emptiness – that indifference of being so beaten up that you can’t feel anymore.
And eventually, shame comes in. I’m ashamed of how I feel, because somehow, it means I am weak. All these feelings threaten to cripple me. Then I get home and cry till I fall asleep.
Recently, I read an article about a young physician contemplating suicide in her first year of practice. She described the dread of going into work and the emptiness left at the end of the day. Halfway through my second year of residency, I could relate to those feelings. I dealt with the anxiety of wondering what type of shift I was going to walk into, and experienced the stress of not wanting to disappoint the team, my peers, the patient, or myself.
Being constantly afraid of making a mistake is tiring. Sometimes, I get the pat on the back from senior physicians, saying I will survive. But it’s not enough. I can’t blame them. It’s human nature to forget how bad it hurt and just remember being strong enough to bear it. I count the days til I have a day off or the weeks til my next vacation. I try not to complain as much because nobody wants to hear it, and nothing is going to change. I feel deeply alone, like nobody cares.
And in the end I put myself onto this path. I knew what I was getting myself into. And I get myself out of it. I hold on to the smiles my staff give me when I walk in. I experience that 10-minute interview with someone whose thought process was so disorganized, and I realize that it took some skill to connect with that patient.
I am heartened by my ability to deescalate a patient who was about to become violent because I know Spanish. I notice that I am able to work faster than I did just a year ago. So I hold on. The trick was to find what made me want to hold on and what motivates me.
I’m not going to write about socializing or finding a hobby outside of medicine. But I must acknowledge that I have a wonderful book club, supportive boyfriend, and family. I lose myself in books, TV shows, cooking. I even started reading comic books. Plenty of articles are out there about how to beat physician burnout. They all help. I tried knitting, coloring, you name it, but for me, those activities were just not enough. I had to try to find meaning behind the work I do. I had to grab all those small moments during a shift and knit them together to build a bridge to where my passion lies. Then I started by reminding myself why I wanted to practice medicine and why psychiatry.
What inspires me is learning how to decipher what hides behind a symptom – why patient A’s anxiety is different from patient B’s. I find psychodynamics fascinating, so I read. I read literature, articles, and books with subjects around my interests. So when I see a patient at 4 a.m. for 5 minutes, I can knit it together with what I read. So it has meaning. And then it’s easier to hold on. Because the 12-hour overnight shift becomes hours of learning about my passion. Because I feel a step closer. And even though last night’s shift was so hard, I’m happy to be back at work today. When I read about the psychopathology of depression and then admit five patients with suicidal ideation, it stops being the same story over and over. It turns into an exploration, and it becomes fascinating. I won’t hear the same story again. So many times, attendings told me to read, and I had to be at my breaking point to understand why it was so essential. Now I’m motivated, and I plan to keep pushing myself to the limit to find a new challenge and to surprise myself in the middle of my beat-up tiredness when I see something in my patient that so many have written about. The adrenaline rush comes on the path of becoming that psychiatrist I aim to be, not in the diploma I will get 2 years from now.
I am racing against burnout, but I’m winning. And I wanted to share my experience to remind others that we are not alone on this path, and we should not have higher suicide rates than other professional groups.
If you are a resident or more experienced physician, know that you are not alone. Many others just like you are stretching themselves thin. Follow the tips of how to beat burnout that you’ve seen around. Find what works for you and dig into yourself, into what drove you in this direction, into where your passion lies. Find the meaning behind this hard work, and connect it to the passion that motivates you. Think of what made you want to become a doctor! I’m not sure it works 100% of the time.
Ask me again in 6 months.
Dr. Serrano is a PGY2 psychiatry resident at the Einstein Medical Center in Philadelphia.
At 6 a.m., after eight admissions back to back, I feel like I can’t stay awake any longer. I desperately need a nap. I have 30 minutes before my next admission, but I know I’m not going to rest. I close my eyes, and I only see charts. I’m trying not to collapse, but I still have three more patients to admit before I go home. And I can’t rest.
I’m scared that I’ll make a mistake. While I write my history of presenting illness, I imagine the day team reading my notes and hoping they don’t notice that I was about to crash on the desk. I check my documentation multiple times to make sure I don’t miss anything, and I know that won’t relieve my anxiety. When I leave, I will still feel the failure of not being able to give my best for my patient or for my night team, because I’m too tired. I know this frustration is going to give room to the emptiness – that indifference of being so beaten up that you can’t feel anymore.
And eventually, shame comes in. I’m ashamed of how I feel, because somehow, it means I am weak. All these feelings threaten to cripple me. Then I get home and cry till I fall asleep.
Recently, I read an article about a young physician contemplating suicide in her first year of practice. She described the dread of going into work and the emptiness left at the end of the day. Halfway through my second year of residency, I could relate to those feelings. I dealt with the anxiety of wondering what type of shift I was going to walk into, and experienced the stress of not wanting to disappoint the team, my peers, the patient, or myself.
Being constantly afraid of making a mistake is tiring. Sometimes, I get the pat on the back from senior physicians, saying I will survive. But it’s not enough. I can’t blame them. It’s human nature to forget how bad it hurt and just remember being strong enough to bear it. I count the days til I have a day off or the weeks til my next vacation. I try not to complain as much because nobody wants to hear it, and nothing is going to change. I feel deeply alone, like nobody cares.
And in the end I put myself onto this path. I knew what I was getting myself into. And I get myself out of it. I hold on to the smiles my staff give me when I walk in. I experience that 10-minute interview with someone whose thought process was so disorganized, and I realize that it took some skill to connect with that patient.
I am heartened by my ability to deescalate a patient who was about to become violent because I know Spanish. I notice that I am able to work faster than I did just a year ago. So I hold on. The trick was to find what made me want to hold on and what motivates me.
I’m not going to write about socializing or finding a hobby outside of medicine. But I must acknowledge that I have a wonderful book club, supportive boyfriend, and family. I lose myself in books, TV shows, cooking. I even started reading comic books. Plenty of articles are out there about how to beat physician burnout. They all help. I tried knitting, coloring, you name it, but for me, those activities were just not enough. I had to try to find meaning behind the work I do. I had to grab all those small moments during a shift and knit them together to build a bridge to where my passion lies. Then I started by reminding myself why I wanted to practice medicine and why psychiatry.
What inspires me is learning how to decipher what hides behind a symptom – why patient A’s anxiety is different from patient B’s. I find psychodynamics fascinating, so I read. I read literature, articles, and books with subjects around my interests. So when I see a patient at 4 a.m. for 5 minutes, I can knit it together with what I read. So it has meaning. And then it’s easier to hold on. Because the 12-hour overnight shift becomes hours of learning about my passion. Because I feel a step closer. And even though last night’s shift was so hard, I’m happy to be back at work today. When I read about the psychopathology of depression and then admit five patients with suicidal ideation, it stops being the same story over and over. It turns into an exploration, and it becomes fascinating. I won’t hear the same story again. So many times, attendings told me to read, and I had to be at my breaking point to understand why it was so essential. Now I’m motivated, and I plan to keep pushing myself to the limit to find a new challenge and to surprise myself in the middle of my beat-up tiredness when I see something in my patient that so many have written about. The adrenaline rush comes on the path of becoming that psychiatrist I aim to be, not in the diploma I will get 2 years from now.
I am racing against burnout, but I’m winning. And I wanted to share my experience to remind others that we are not alone on this path, and we should not have higher suicide rates than other professional groups.
If you are a resident or more experienced physician, know that you are not alone. Many others just like you are stretching themselves thin. Follow the tips of how to beat burnout that you’ve seen around. Find what works for you and dig into yourself, into what drove you in this direction, into where your passion lies. Find the meaning behind this hard work, and connect it to the passion that motivates you. Think of what made you want to become a doctor! I’m not sure it works 100% of the time.
Ask me again in 6 months.
Dr. Serrano is a PGY2 psychiatry resident at the Einstein Medical Center in Philadelphia.
At 6 a.m., after eight admissions back to back, I feel like I can’t stay awake any longer. I desperately need a nap. I have 30 minutes before my next admission, but I know I’m not going to rest. I close my eyes, and I only see charts. I’m trying not to collapse, but I still have three more patients to admit before I go home. And I can’t rest.
I’m scared that I’ll make a mistake. While I write my history of presenting illness, I imagine the day team reading my notes and hoping they don’t notice that I was about to crash on the desk. I check my documentation multiple times to make sure I don’t miss anything, and I know that won’t relieve my anxiety. When I leave, I will still feel the failure of not being able to give my best for my patient or for my night team, because I’m too tired. I know this frustration is going to give room to the emptiness – that indifference of being so beaten up that you can’t feel anymore.
And eventually, shame comes in. I’m ashamed of how I feel, because somehow, it means I am weak. All these feelings threaten to cripple me. Then I get home and cry till I fall asleep.
Recently, I read an article about a young physician contemplating suicide in her first year of practice. She described the dread of going into work and the emptiness left at the end of the day. Halfway through my second year of residency, I could relate to those feelings. I dealt with the anxiety of wondering what type of shift I was going to walk into, and experienced the stress of not wanting to disappoint the team, my peers, the patient, or myself.
Being constantly afraid of making a mistake is tiring. Sometimes, I get the pat on the back from senior physicians, saying I will survive. But it’s not enough. I can’t blame them. It’s human nature to forget how bad it hurt and just remember being strong enough to bear it. I count the days til I have a day off or the weeks til my next vacation. I try not to complain as much because nobody wants to hear it, and nothing is going to change. I feel deeply alone, like nobody cares.
And in the end I put myself onto this path. I knew what I was getting myself into. And I get myself out of it. I hold on to the smiles my staff give me when I walk in. I experience that 10-minute interview with someone whose thought process was so disorganized, and I realize that it took some skill to connect with that patient.
I am heartened by my ability to deescalate a patient who was about to become violent because I know Spanish. I notice that I am able to work faster than I did just a year ago. So I hold on. The trick was to find what made me want to hold on and what motivates me.
I’m not going to write about socializing or finding a hobby outside of medicine. But I must acknowledge that I have a wonderful book club, supportive boyfriend, and family. I lose myself in books, TV shows, cooking. I even started reading comic books. Plenty of articles are out there about how to beat physician burnout. They all help. I tried knitting, coloring, you name it, but for me, those activities were just not enough. I had to try to find meaning behind the work I do. I had to grab all those small moments during a shift and knit them together to build a bridge to where my passion lies. Then I started by reminding myself why I wanted to practice medicine and why psychiatry.
What inspires me is learning how to decipher what hides behind a symptom – why patient A’s anxiety is different from patient B’s. I find psychodynamics fascinating, so I read. I read literature, articles, and books with subjects around my interests. So when I see a patient at 4 a.m. for 5 minutes, I can knit it together with what I read. So it has meaning. And then it’s easier to hold on. Because the 12-hour overnight shift becomes hours of learning about my passion. Because I feel a step closer. And even though last night’s shift was so hard, I’m happy to be back at work today. When I read about the psychopathology of depression and then admit five patients with suicidal ideation, it stops being the same story over and over. It turns into an exploration, and it becomes fascinating. I won’t hear the same story again. So many times, attendings told me to read, and I had to be at my breaking point to understand why it was so essential. Now I’m motivated, and I plan to keep pushing myself to the limit to find a new challenge and to surprise myself in the middle of my beat-up tiredness when I see something in my patient that so many have written about. The adrenaline rush comes on the path of becoming that psychiatrist I aim to be, not in the diploma I will get 2 years from now.
I am racing against burnout, but I’m winning. And I wanted to share my experience to remind others that we are not alone on this path, and we should not have higher suicide rates than other professional groups.
If you are a resident or more experienced physician, know that you are not alone. Many others just like you are stretching themselves thin. Follow the tips of how to beat burnout that you’ve seen around. Find what works for you and dig into yourself, into what drove you in this direction, into where your passion lies. Find the meaning behind this hard work, and connect it to the passion that motivates you. Think of what made you want to become a doctor! I’m not sure it works 100% of the time.
Ask me again in 6 months.
Dr. Serrano is a PGY2 psychiatry resident at the Einstein Medical Center in Philadelphia.