User login
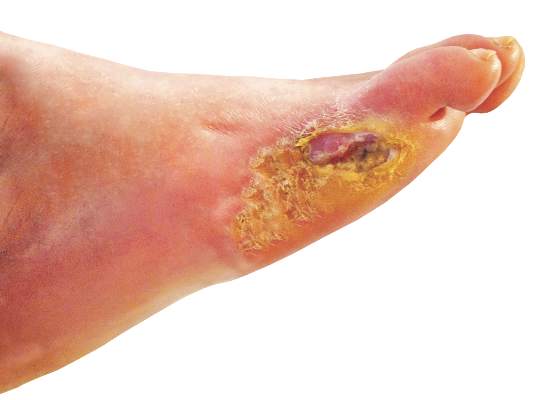
Diabetic foot ulcers linked to cognitive decline
Among patients with type 2 diabetes, those with foot ulcers show cognitive impairment across all domains when compared with those without, according to a report published in Diabetes Care.
In what they described as one of the first studies to examine cognitive function in people with diabetic foot ulcers, researchers found that these patients “remember less, have decreased ability to concentrate, and more difficulty with learning, less inhibition, slower cognitive and psychomotor responses, and less verbal fluency” than patients with diabetes that does not include foot involvement.
Although this study had a cross-sectional design that precluded drawing conclusions about causality, an analysis that estimated the participants’ premorbid and postmorbid cognitive abilities suggested that people with diabetic foot ulcers had experienced a recent significant cognitive decline, while those without foot ulcers had not, said Rachel Natovich, Ph.D., of the department of public health, Ben-Gurion University of the Negev, Be’er Sheva (Israel) and the Endocrinology Institute, Sheba Medical Center, Ramat Gan (Israel) and her associates. These findings indicate that patients with diabetic foot ulcers – the very patients who face the greatest self-treatment challenges – are the ones who have the weakest cognitive resources to do so, they noted.
The investigators examined this issue after noting that recent consensus guidelines require patients with diabetic foot ulcers to take on even more self-management than is already required for the diabetes. This demands “applying complex cognitive abilities in learning, understanding, and remembering new information; planning and initiating self-care practices; adopting behavioral changes that involve psychomotor abilities; and maintaining these behaviors while controlling and repressing impulses.” So Dr. Natovich and her associates assessed whether the cognitive profile of patients who have diabetic foot problems differs from that of patients who don’t, using a case-control study design.
The 194 study participants were aged 45-75 years. The 99 subjects who had at least one diabetic foot ulcer (cases) were matched for age and duration of diabetes with 95 subjects who did not (controls). All underwent a comprehensive battery of neuropsychological tests assessing general intelligence, short- and long-term memory, attention and concentration, psychomotor efficiency, reaction time, executive function, nonverbal IQ, visual-motor speed, coordination, capacity for learning, verbal production, semantic memory, and language. All were also assessed for depression via the Patient Health Questionnaire.
After scores were standardized according to the expected performance by age and education level, patients with diabetic foot ulcers showed significantly lower scores in all the domains tested, compared with the patients without foot ulcers. This difference persisted after the data were adjusted to account for possible confounding factors such as smoking status, hemoglobin A1c level, presence or absence of depressive symptoms, and presence or absence of macrovascular disease (Diab Care. 2016 May. doi:10.2337/dc15-2838).
The estimated premorbid cognitive function was similar between the two study groups, but current cognitive function declined significantly in the patients with foot ulcers while remaining relatively constant in the patients without foot ulcers. Prospective studies are needed to explore the timing of cognitive decline and the possibility of causation, Dr. Natovich and her associates said.
The study results “highlight the importance of focusing on cognitive functioning, a less-studied area in diabetic foot research,” they added.
“We feel that it is important to screen the cognitive status of these patients regularly and to take cognitive abilities into consideration in treatment-planning recommendations and follow-up.”
Among patients with type 2 diabetes, those with foot ulcers show cognitive impairment across all domains when compared with those without, according to a report published in Diabetes Care.
In what they described as one of the first studies to examine cognitive function in people with diabetic foot ulcers, researchers found that these patients “remember less, have decreased ability to concentrate, and more difficulty with learning, less inhibition, slower cognitive and psychomotor responses, and less verbal fluency” than patients with diabetes that does not include foot involvement.
Although this study had a cross-sectional design that precluded drawing conclusions about causality, an analysis that estimated the participants’ premorbid and postmorbid cognitive abilities suggested that people with diabetic foot ulcers had experienced a recent significant cognitive decline, while those without foot ulcers had not, said Rachel Natovich, Ph.D., of the department of public health, Ben-Gurion University of the Negev, Be’er Sheva (Israel) and the Endocrinology Institute, Sheba Medical Center, Ramat Gan (Israel) and her associates. These findings indicate that patients with diabetic foot ulcers – the very patients who face the greatest self-treatment challenges – are the ones who have the weakest cognitive resources to do so, they noted.
The investigators examined this issue after noting that recent consensus guidelines require patients with diabetic foot ulcers to take on even more self-management than is already required for the diabetes. This demands “applying complex cognitive abilities in learning, understanding, and remembering new information; planning and initiating self-care practices; adopting behavioral changes that involve psychomotor abilities; and maintaining these behaviors while controlling and repressing impulses.” So Dr. Natovich and her associates assessed whether the cognitive profile of patients who have diabetic foot problems differs from that of patients who don’t, using a case-control study design.
The 194 study participants were aged 45-75 years. The 99 subjects who had at least one diabetic foot ulcer (cases) were matched for age and duration of diabetes with 95 subjects who did not (controls). All underwent a comprehensive battery of neuropsychological tests assessing general intelligence, short- and long-term memory, attention and concentration, psychomotor efficiency, reaction time, executive function, nonverbal IQ, visual-motor speed, coordination, capacity for learning, verbal production, semantic memory, and language. All were also assessed for depression via the Patient Health Questionnaire.
After scores were standardized according to the expected performance by age and education level, patients with diabetic foot ulcers showed significantly lower scores in all the domains tested, compared with the patients without foot ulcers. This difference persisted after the data were adjusted to account for possible confounding factors such as smoking status, hemoglobin A1c level, presence or absence of depressive symptoms, and presence or absence of macrovascular disease (Diab Care. 2016 May. doi:10.2337/dc15-2838).
The estimated premorbid cognitive function was similar between the two study groups, but current cognitive function declined significantly in the patients with foot ulcers while remaining relatively constant in the patients without foot ulcers. Prospective studies are needed to explore the timing of cognitive decline and the possibility of causation, Dr. Natovich and her associates said.
The study results “highlight the importance of focusing on cognitive functioning, a less-studied area in diabetic foot research,” they added.
“We feel that it is important to screen the cognitive status of these patients regularly and to take cognitive abilities into consideration in treatment-planning recommendations and follow-up.”
Among patients with type 2 diabetes, those with foot ulcers show cognitive impairment across all domains when compared with those without, according to a report published in Diabetes Care.
In what they described as one of the first studies to examine cognitive function in people with diabetic foot ulcers, researchers found that these patients “remember less, have decreased ability to concentrate, and more difficulty with learning, less inhibition, slower cognitive and psychomotor responses, and less verbal fluency” than patients with diabetes that does not include foot involvement.
Although this study had a cross-sectional design that precluded drawing conclusions about causality, an analysis that estimated the participants’ premorbid and postmorbid cognitive abilities suggested that people with diabetic foot ulcers had experienced a recent significant cognitive decline, while those without foot ulcers had not, said Rachel Natovich, Ph.D., of the department of public health, Ben-Gurion University of the Negev, Be’er Sheva (Israel) and the Endocrinology Institute, Sheba Medical Center, Ramat Gan (Israel) and her associates. These findings indicate that patients with diabetic foot ulcers – the very patients who face the greatest self-treatment challenges – are the ones who have the weakest cognitive resources to do so, they noted.
The investigators examined this issue after noting that recent consensus guidelines require patients with diabetic foot ulcers to take on even more self-management than is already required for the diabetes. This demands “applying complex cognitive abilities in learning, understanding, and remembering new information; planning and initiating self-care practices; adopting behavioral changes that involve psychomotor abilities; and maintaining these behaviors while controlling and repressing impulses.” So Dr. Natovich and her associates assessed whether the cognitive profile of patients who have diabetic foot problems differs from that of patients who don’t, using a case-control study design.
The 194 study participants were aged 45-75 years. The 99 subjects who had at least one diabetic foot ulcer (cases) were matched for age and duration of diabetes with 95 subjects who did not (controls). All underwent a comprehensive battery of neuropsychological tests assessing general intelligence, short- and long-term memory, attention and concentration, psychomotor efficiency, reaction time, executive function, nonverbal IQ, visual-motor speed, coordination, capacity for learning, verbal production, semantic memory, and language. All were also assessed for depression via the Patient Health Questionnaire.
After scores were standardized according to the expected performance by age and education level, patients with diabetic foot ulcers showed significantly lower scores in all the domains tested, compared with the patients without foot ulcers. This difference persisted after the data were adjusted to account for possible confounding factors such as smoking status, hemoglobin A1c level, presence or absence of depressive symptoms, and presence or absence of macrovascular disease (Diab Care. 2016 May. doi:10.2337/dc15-2838).
The estimated premorbid cognitive function was similar between the two study groups, but current cognitive function declined significantly in the patients with foot ulcers while remaining relatively constant in the patients without foot ulcers. Prospective studies are needed to explore the timing of cognitive decline and the possibility of causation, Dr. Natovich and her associates said.
The study results “highlight the importance of focusing on cognitive functioning, a less-studied area in diabetic foot research,” they added.
“We feel that it is important to screen the cognitive status of these patients regularly and to take cognitive abilities into consideration in treatment-planning recommendations and follow-up.”
FROM DIABETES CARE
Key clinical point: Among patients with type 2 diabetes, those with foot ulcers show cognitive impairment, compared with those who don’t have foot ulcers.
Major finding: Ninety-nine patients with at least one diabetic foot showed significantly lower scores in all cognitive domains tested compared with the 95 patients without foot ulcers.
Data source: A cross-sectional case-control study comparing cognitive performance between 99 patients with and 95 patients without diabetic foot ulcers.
Disclosures: No sponsor of this study was identified. Dr. Natovich and her associates reported having no relevant disclosures.
Elective induction of labor at 39 (vs 41) weeks: Caveats and considerations
Tasked with tackling the literature on the subject and debating the question of whether or not it is best to electively induce labor in women with low-risk pregnancies at 39 weeks (vs at 41 weeks after expectant management), Errol Norwitz, MD, PhD, Chairman of the Department of Obstetrics and Gynecology and Professor at Tufts University School of Medicine in Boston, Massachusetts, and Charles Lockwood, MD, Senior Vice President at the University of South Florida (USF) and Dean of the USF Health Morsani College of Medicine in Tampa came to the same conclusion: Elective induction of labor (eIOL) at 39 weeks is superior to expectant management when it comes to fetal outcomes.
In addition, they both agreed that complication rates to the mother (ie, number of cesarean deliveries [CDs]) would not be increased, and possibly even reduced, with eIOL at 39 weeks versus 41 weeks. Dr. Norwitz postulated that, with IOL there is no increase in CD rate in multiparous women and nulliparous women with a favorable cervical exam but that there likely could be an increase in the CD rate for nulliparous women with an unfavorable cervical exam.
The finding that eIOL at 39 weeks is better than at 41 weeks for the infant is likely due to a bigger baby size past 39 weeks (with more traumatic deliveries) and higher rates of postmaturity complications, said Dr. Lockwood. And for the mother, eIOL at 39 weeks can reduce risks—of preeclampsia, abruption, sepsis, and others—the presenters pointed out.
Arriving at their conclusions: The dataDr. Norwitz explained the challenge before them in this unusual “debate.” “In ObGyn we all read the same literature but we often come away with very different takes as to what the implications are and how we incorporate this into our management algorithms. Instead of taking a pro/con approach, with one assigned to ‘yes’ and the other assigned to ‘no,’ and selectively picking out the literature to support our positions, what we did was we each went away, read the literature, synthesized it, and tried to answer this question for ourselves.”
Dr. Norwitz, who is widely published and known for his research on the causes and prevention of preeclampsia and preterm labor, examined and presented the published literature for benefit and harms to the fetus and mother in continuing pregnancy past 39 weeks.
Dr. Lockwood also examined the literature, including a large population cohort of about 1.27 million women that examined CD rates, perinatal mortality, and neonatal and maternal outcomes of eIOL at 39 weeks versus expectant management.1 He presented, however, that the best evidence to compare the question at hand would be a randomized clinical trial comparing specifically eIOL at 39 weeks versus expectant management, with IOL at 41 weeks. To be powered to detect a difference in perinatal and maternal mortality, this trial would need to include 2.2 to 12.6 million women, he maintained. “When empirical evidence doesn’t exist, the only alternative is some kind of other modeling,” he said. Therefore, he and a team of researchers conducted a Monte Carlo microsimulation modeling decision analysis, taking into account “all outcomes and all preferences that we possibly could cull from the literature.”
Which women actually could benefit from eIOL at 39 weeks?Women with high-risk pregnancies were not included in this debate or considered. Dr. Norwitz clearly defined his case patient at the outset as a 22-year-old G1 at 39 0/7 weeks who has had an uncomplicated pregnancy but is now complaining of decreased fetal movement and tells you that she is worried because her sister lost her baby at 40 weeks to stillbirth. She specifically asks, “Doctor, why can’t you induce my labor now?”
What counseling a patient about the risks/benefits of eIOL at 39 weeks would requireTwo fundamentals would need to be ensured. The first: precise gestational dating. Dr. Norwitz pointed out that menstrual history can be inaccurate, especially in women with irregular cycles, who are taking hormonal contraception, or who have intermenstrual bleeding. “Early dating ultrasound is the best way to date pregnancies and probably should be done routinely,” although it is not currently standard of care in the United States, he said.
The second fundamental: a true induction of labor process, not one that involves “stopping at 5 PM,” said Dr. Lockwood.
“Although Dr. Lockwood’s 5-PM statement was made tongue-in-cheek,” said Dr. Norwitz after the debate, “he certainly was implying that a genuine effort should be made to effect a vaginal delivery—that is, giving the most effective cervical ripening agents, allowing enough time to pass, and defining clearly the criteria for failed IOL. You shouldn’t just throw the towel in at 5 PM because you want to go home.”
Cost implicationsElectively inducing labor in all women with low-risk pregnancies at 39 weeks is a strategy with unknown cost implications, and the cost difference between this strategy and expectant management up to 41 weeks is not known.
“We need to be sure that we understand the cost implications of these strategies, which of course would need to be balanced against the potential perinatal and maternal morbidity and fetal death,” said Dr. Lockwood.
The meaning of “elective”Dr. Norwitz also made the point postdebate that the American College of Obstetricians and Gynecologists (ACOG) does not support elective IOL prior to 39 weeks’ gestation. “The key term here is ‘elective,’" he said, "which refers to a delivery without a clear medical or obstetric indication. ACOG does support delivery prior to 39 weeks’ gestation, there just needs to be an appropriate indication.”
1. Stock SJ, Ferguson E, Duffy A, Ford I, Chalmers J, Norman JE. Outcomes of elective induction of labour compared with expectant management: population based study. BMJ. 2012;344:e2838.
Tasked with tackling the literature on the subject and debating the question of whether or not it is best to electively induce labor in women with low-risk pregnancies at 39 weeks (vs at 41 weeks after expectant management), Errol Norwitz, MD, PhD, Chairman of the Department of Obstetrics and Gynecology and Professor at Tufts University School of Medicine in Boston, Massachusetts, and Charles Lockwood, MD, Senior Vice President at the University of South Florida (USF) and Dean of the USF Health Morsani College of Medicine in Tampa came to the same conclusion: Elective induction of labor (eIOL) at 39 weeks is superior to expectant management when it comes to fetal outcomes.
In addition, they both agreed that complication rates to the mother (ie, number of cesarean deliveries [CDs]) would not be increased, and possibly even reduced, with eIOL at 39 weeks versus 41 weeks. Dr. Norwitz postulated that, with IOL there is no increase in CD rate in multiparous women and nulliparous women with a favorable cervical exam but that there likely could be an increase in the CD rate for nulliparous women with an unfavorable cervical exam.
The finding that eIOL at 39 weeks is better than at 41 weeks for the infant is likely due to a bigger baby size past 39 weeks (with more traumatic deliveries) and higher rates of postmaturity complications, said Dr. Lockwood. And for the mother, eIOL at 39 weeks can reduce risks—of preeclampsia, abruption, sepsis, and others—the presenters pointed out.
Arriving at their conclusions: The dataDr. Norwitz explained the challenge before them in this unusual “debate.” “In ObGyn we all read the same literature but we often come away with very different takes as to what the implications are and how we incorporate this into our management algorithms. Instead of taking a pro/con approach, with one assigned to ‘yes’ and the other assigned to ‘no,’ and selectively picking out the literature to support our positions, what we did was we each went away, read the literature, synthesized it, and tried to answer this question for ourselves.”
Dr. Norwitz, who is widely published and known for his research on the causes and prevention of preeclampsia and preterm labor, examined and presented the published literature for benefit and harms to the fetus and mother in continuing pregnancy past 39 weeks.
Dr. Lockwood also examined the literature, including a large population cohort of about 1.27 million women that examined CD rates, perinatal mortality, and neonatal and maternal outcomes of eIOL at 39 weeks versus expectant management.1 He presented, however, that the best evidence to compare the question at hand would be a randomized clinical trial comparing specifically eIOL at 39 weeks versus expectant management, with IOL at 41 weeks. To be powered to detect a difference in perinatal and maternal mortality, this trial would need to include 2.2 to 12.6 million women, he maintained. “When empirical evidence doesn’t exist, the only alternative is some kind of other modeling,” he said. Therefore, he and a team of researchers conducted a Monte Carlo microsimulation modeling decision analysis, taking into account “all outcomes and all preferences that we possibly could cull from the literature.”
Which women actually could benefit from eIOL at 39 weeks?Women with high-risk pregnancies were not included in this debate or considered. Dr. Norwitz clearly defined his case patient at the outset as a 22-year-old G1 at 39 0/7 weeks who has had an uncomplicated pregnancy but is now complaining of decreased fetal movement and tells you that she is worried because her sister lost her baby at 40 weeks to stillbirth. She specifically asks, “Doctor, why can’t you induce my labor now?”
What counseling a patient about the risks/benefits of eIOL at 39 weeks would requireTwo fundamentals would need to be ensured. The first: precise gestational dating. Dr. Norwitz pointed out that menstrual history can be inaccurate, especially in women with irregular cycles, who are taking hormonal contraception, or who have intermenstrual bleeding. “Early dating ultrasound is the best way to date pregnancies and probably should be done routinely,” although it is not currently standard of care in the United States, he said.
The second fundamental: a true induction of labor process, not one that involves “stopping at 5 PM,” said Dr. Lockwood.
“Although Dr. Lockwood’s 5-PM statement was made tongue-in-cheek,” said Dr. Norwitz after the debate, “he certainly was implying that a genuine effort should be made to effect a vaginal delivery—that is, giving the most effective cervical ripening agents, allowing enough time to pass, and defining clearly the criteria for failed IOL. You shouldn’t just throw the towel in at 5 PM because you want to go home.”
Cost implicationsElectively inducing labor in all women with low-risk pregnancies at 39 weeks is a strategy with unknown cost implications, and the cost difference between this strategy and expectant management up to 41 weeks is not known.
“We need to be sure that we understand the cost implications of these strategies, which of course would need to be balanced against the potential perinatal and maternal morbidity and fetal death,” said Dr. Lockwood.
The meaning of “elective”Dr. Norwitz also made the point postdebate that the American College of Obstetricians and Gynecologists (ACOG) does not support elective IOL prior to 39 weeks’ gestation. “The key term here is ‘elective,’" he said, "which refers to a delivery without a clear medical or obstetric indication. ACOG does support delivery prior to 39 weeks’ gestation, there just needs to be an appropriate indication.”
Tasked with tackling the literature on the subject and debating the question of whether or not it is best to electively induce labor in women with low-risk pregnancies at 39 weeks (vs at 41 weeks after expectant management), Errol Norwitz, MD, PhD, Chairman of the Department of Obstetrics and Gynecology and Professor at Tufts University School of Medicine in Boston, Massachusetts, and Charles Lockwood, MD, Senior Vice President at the University of South Florida (USF) and Dean of the USF Health Morsani College of Medicine in Tampa came to the same conclusion: Elective induction of labor (eIOL) at 39 weeks is superior to expectant management when it comes to fetal outcomes.
In addition, they both agreed that complication rates to the mother (ie, number of cesarean deliveries [CDs]) would not be increased, and possibly even reduced, with eIOL at 39 weeks versus 41 weeks. Dr. Norwitz postulated that, with IOL there is no increase in CD rate in multiparous women and nulliparous women with a favorable cervical exam but that there likely could be an increase in the CD rate for nulliparous women with an unfavorable cervical exam.
The finding that eIOL at 39 weeks is better than at 41 weeks for the infant is likely due to a bigger baby size past 39 weeks (with more traumatic deliveries) and higher rates of postmaturity complications, said Dr. Lockwood. And for the mother, eIOL at 39 weeks can reduce risks—of preeclampsia, abruption, sepsis, and others—the presenters pointed out.
Arriving at their conclusions: The dataDr. Norwitz explained the challenge before them in this unusual “debate.” “In ObGyn we all read the same literature but we often come away with very different takes as to what the implications are and how we incorporate this into our management algorithms. Instead of taking a pro/con approach, with one assigned to ‘yes’ and the other assigned to ‘no,’ and selectively picking out the literature to support our positions, what we did was we each went away, read the literature, synthesized it, and tried to answer this question for ourselves.”
Dr. Norwitz, who is widely published and known for his research on the causes and prevention of preeclampsia and preterm labor, examined and presented the published literature for benefit and harms to the fetus and mother in continuing pregnancy past 39 weeks.
Dr. Lockwood also examined the literature, including a large population cohort of about 1.27 million women that examined CD rates, perinatal mortality, and neonatal and maternal outcomes of eIOL at 39 weeks versus expectant management.1 He presented, however, that the best evidence to compare the question at hand would be a randomized clinical trial comparing specifically eIOL at 39 weeks versus expectant management, with IOL at 41 weeks. To be powered to detect a difference in perinatal and maternal mortality, this trial would need to include 2.2 to 12.6 million women, he maintained. “When empirical evidence doesn’t exist, the only alternative is some kind of other modeling,” he said. Therefore, he and a team of researchers conducted a Monte Carlo microsimulation modeling decision analysis, taking into account “all outcomes and all preferences that we possibly could cull from the literature.”
Which women actually could benefit from eIOL at 39 weeks?Women with high-risk pregnancies were not included in this debate or considered. Dr. Norwitz clearly defined his case patient at the outset as a 22-year-old G1 at 39 0/7 weeks who has had an uncomplicated pregnancy but is now complaining of decreased fetal movement and tells you that she is worried because her sister lost her baby at 40 weeks to stillbirth. She specifically asks, “Doctor, why can’t you induce my labor now?”
What counseling a patient about the risks/benefits of eIOL at 39 weeks would requireTwo fundamentals would need to be ensured. The first: precise gestational dating. Dr. Norwitz pointed out that menstrual history can be inaccurate, especially in women with irregular cycles, who are taking hormonal contraception, or who have intermenstrual bleeding. “Early dating ultrasound is the best way to date pregnancies and probably should be done routinely,” although it is not currently standard of care in the United States, he said.
The second fundamental: a true induction of labor process, not one that involves “stopping at 5 PM,” said Dr. Lockwood.
“Although Dr. Lockwood’s 5-PM statement was made tongue-in-cheek,” said Dr. Norwitz after the debate, “he certainly was implying that a genuine effort should be made to effect a vaginal delivery—that is, giving the most effective cervical ripening agents, allowing enough time to pass, and defining clearly the criteria for failed IOL. You shouldn’t just throw the towel in at 5 PM because you want to go home.”
Cost implicationsElectively inducing labor in all women with low-risk pregnancies at 39 weeks is a strategy with unknown cost implications, and the cost difference between this strategy and expectant management up to 41 weeks is not known.
“We need to be sure that we understand the cost implications of these strategies, which of course would need to be balanced against the potential perinatal and maternal morbidity and fetal death,” said Dr. Lockwood.
The meaning of “elective”Dr. Norwitz also made the point postdebate that the American College of Obstetricians and Gynecologists (ACOG) does not support elective IOL prior to 39 weeks’ gestation. “The key term here is ‘elective,’" he said, "which refers to a delivery without a clear medical or obstetric indication. ACOG does support delivery prior to 39 weeks’ gestation, there just needs to be an appropriate indication.”
1. Stock SJ, Ferguson E, Duffy A, Ford I, Chalmers J, Norman JE. Outcomes of elective induction of labour compared with expectant management: population based study. BMJ. 2012;344:e2838.
1. Stock SJ, Ferguson E, Duffy A, Ford I, Chalmers J, Norman JE. Outcomes of elective induction of labour compared with expectant management: population based study. BMJ. 2012;344:e2838.
General surgeons getting less vascular training
Vascular surgery fellow case logs reflect an increase in endovascular interventions, but general surgery residents may be missing out on training opportunities, according to a study of national case data.
In addition, general surgery residents saw a decrease in open vascular surgery cases, which was not reflected among the vascular surgery fellows, according to Dr. Rose C. Pedersen and colleagues in the department of surgery, Kaiser Permanente Los Angeles Medical Center. The report was published online in Annals of Vascular Surgery (doi: 10.1016/j.avsg.2016.02.008).
The paper was originally presented at the 2105 annual meeting of the Southern California Vascular Society. The study reports findings of a review of the Accreditation Council for Graduate Medical Education national case log reports from 2001 to 2012.
During that period, the number of general surgery residents increased from 1,021 to 1,098, while the number of vascular surgery fellows increased from 96 to 121. The total number of vascular cases logged by the vascular fellows significantly increased by 161%, from an average of 298 cases to 762 cases over the time period assessed. During that same period, vascular cases done by general surgery residents significantly decreased by 40%, from an average of 186 to 116 cases.
In terms of open cases. vascular fellows saw a significant 43% decrease in open abdominal aortic aneurysm (AAA) cases, going from 26 to 15, and a slight but significant increase in carotid endarterectomy cases logged (44 to 49). Hemodialysis access and major amputations also both increased significantly. A decreases in open surgery for peripheral obstructive disease was small and not significant.
General surgery residents saw decreases in all open surgery areas over the time period: AAA cases fell significantly by 78% (9 cases to 2 cases); carotid endarterectomies decreased significantly from 23 to 12 cases; and surgery for peripheral obstructive disease fell significantly from 21 to 8). Hemodialysis access cases and major amputations both decreased as well, but not significantly.
Endovascular cases increased from 2001 to 2012 for both vascular fellows and general surgery residents. Vascular fellows saw endovascular AAA repair significantly increase from an average of 17 to 46 cases, while those for general surgery residents rosed from roughly 1 to 3 cases. Similarly, endovascular interventions for peripheral obstructive disease significantly increased for vascular fellows from 17 to 85, and from 1.3 to 4 for general surgery residents.
“The contemporary management of abdominal aortic aneurysmal diseases and peripheral obstructive diseases has been particularly changed by endovascular therapies that have been adopted into the training experience of vascular surgery fellows, but not those of general surgery residents. Open surgery experience has decreased overall for general surgery residents in all major categories, a change not seen in vascular fellows,” the researchers concluded.
The authors reported no relevant disclosures.
Vascular surgical practice has evolved with the introduction of minimally invasive technology and advancement of endovascular techniques, and as such, the training of the vascular surgical specialist too has evolved. This article highlights the effect of these technological advancements on the vascular surgical fellowship training experience and points out the effect of this embracing of endovascular techniques by academic centers in their general surgery trainee vascular surgical experience. Open operative case numbers are declining and endovascular case numbers are increasing significantly.
As traditionally performed open procedures are being substituted for endovascular repairs, the open operative experience for vascular surgery trainees is declining or staying stable at the expense of the general surgery resident experience. Open cases (open aneurysm repairs, aortofemoral artery bypasses, visceral artery bypasses, carotid endarterectomies), because they are performed infrequently now, have become fellow cases.

|
Dr. Erica L. Mitchell |
Endovascular cases, which have increased over 300%-400%, too are essentially fellow cases (or in 0+5 programs, which are not discussed in this manuscript, vascular surgery resident cases) because the endovascular skill set does not appear to translate or transfer (and therefore is not considered relevant to the general surgery resident planning on a fellowship or career in the traditional surgical specialties) into open and laparoscopic skills desired by general surgery trainees.
Unfortunately, this open to endovascular operative experience shift will only increase with the introduction and early adoption (in academic centers) of complex endovascular techniques for the management of complex obstructive and aneurysmal arterial disease. A review of case log data for 0+5 and 5+2 residents completing programs in 2014-2015 reveals the operative experience and continued decline in open operative case numbers and increases in endovascular case numbers.
Vascular surgery training programs and their faculties have a responsibility to make sure the operative experience of general surgery residents is a worthwhile one. We, the faculty, need to keep encouraging general surgery residents to come to the operating room, even if they are not the primary surgeons. To make this worthwhile for them (because residents will not come to the operating room if there is nothing to be gained for themselves), we have to let them participate in the case somehow. One of the most critical aspects of a vascular surgical procedure is the operative exposure.
Having the general surgery residents participate in the vascular exposure is not only formative to their understanding of surgical anatomy, and applicable to all aspects of surgical practice, it is also critical to trainee development of decision making, judgment, and situational awareness. These exposures can and should be logged, using the e-code, to receive ACGME case log credit for vascular surgical procedures. E-codes, additionally, allow more than one resident to take credit for an arterial exposure and repair. We must also encourage trainees to come to the angio suite, while these cases, on the outset do not seem relevant to general surgery training, they do provide the learner with a greater appreciation of the complexity of vascular anatomy.
Importantly, as long as we continue to train vascular surgery specialists via the traditional 5+2 paradigm, we must keep general surgery residents, rotating through our services, interested and engaged in the management of vascular diseases. The simplest way to engage them is to make them feel relevant and to make them feel relevant they have to participate, both inside and outside of the operating room. It is, after all, our duty as surgical educators and advocates for our specialty to train the next generation of vascular surgical specialists!
Dr. Erica L. Mitchell is professor of surgery, program director for vascular surgery, and vice-chair of quality, department of surgery, Oregon Health & Science University, Portland. She is an associate medical editor of Vascular Specialist.
Vascular surgical practice has evolved with the introduction of minimally invasive technology and advancement of endovascular techniques, and as such, the training of the vascular surgical specialist too has evolved. This article highlights the effect of these technological advancements on the vascular surgical fellowship training experience and points out the effect of this embracing of endovascular techniques by academic centers in their general surgery trainee vascular surgical experience. Open operative case numbers are declining and endovascular case numbers are increasing significantly.
As traditionally performed open procedures are being substituted for endovascular repairs, the open operative experience for vascular surgery trainees is declining or staying stable at the expense of the general surgery resident experience. Open cases (open aneurysm repairs, aortofemoral artery bypasses, visceral artery bypasses, carotid endarterectomies), because they are performed infrequently now, have become fellow cases.

|
Dr. Erica L. Mitchell |
Endovascular cases, which have increased over 300%-400%, too are essentially fellow cases (or in 0+5 programs, which are not discussed in this manuscript, vascular surgery resident cases) because the endovascular skill set does not appear to translate or transfer (and therefore is not considered relevant to the general surgery resident planning on a fellowship or career in the traditional surgical specialties) into open and laparoscopic skills desired by general surgery trainees.
Unfortunately, this open to endovascular operative experience shift will only increase with the introduction and early adoption (in academic centers) of complex endovascular techniques for the management of complex obstructive and aneurysmal arterial disease. A review of case log data for 0+5 and 5+2 residents completing programs in 2014-2015 reveals the operative experience and continued decline in open operative case numbers and increases in endovascular case numbers.
Vascular surgery training programs and their faculties have a responsibility to make sure the operative experience of general surgery residents is a worthwhile one. We, the faculty, need to keep encouraging general surgery residents to come to the operating room, even if they are not the primary surgeons. To make this worthwhile for them (because residents will not come to the operating room if there is nothing to be gained for themselves), we have to let them participate in the case somehow. One of the most critical aspects of a vascular surgical procedure is the operative exposure.
Having the general surgery residents participate in the vascular exposure is not only formative to their understanding of surgical anatomy, and applicable to all aspects of surgical practice, it is also critical to trainee development of decision making, judgment, and situational awareness. These exposures can and should be logged, using the e-code, to receive ACGME case log credit for vascular surgical procedures. E-codes, additionally, allow more than one resident to take credit for an arterial exposure and repair. We must also encourage trainees to come to the angio suite, while these cases, on the outset do not seem relevant to general surgery training, they do provide the learner with a greater appreciation of the complexity of vascular anatomy.
Importantly, as long as we continue to train vascular surgery specialists via the traditional 5+2 paradigm, we must keep general surgery residents, rotating through our services, interested and engaged in the management of vascular diseases. The simplest way to engage them is to make them feel relevant and to make them feel relevant they have to participate, both inside and outside of the operating room. It is, after all, our duty as surgical educators and advocates for our specialty to train the next generation of vascular surgical specialists!
Dr. Erica L. Mitchell is professor of surgery, program director for vascular surgery, and vice-chair of quality, department of surgery, Oregon Health & Science University, Portland. She is an associate medical editor of Vascular Specialist.
Vascular surgical practice has evolved with the introduction of minimally invasive technology and advancement of endovascular techniques, and as such, the training of the vascular surgical specialist too has evolved. This article highlights the effect of these technological advancements on the vascular surgical fellowship training experience and points out the effect of this embracing of endovascular techniques by academic centers in their general surgery trainee vascular surgical experience. Open operative case numbers are declining and endovascular case numbers are increasing significantly.
As traditionally performed open procedures are being substituted for endovascular repairs, the open operative experience for vascular surgery trainees is declining or staying stable at the expense of the general surgery resident experience. Open cases (open aneurysm repairs, aortofemoral artery bypasses, visceral artery bypasses, carotid endarterectomies), because they are performed infrequently now, have become fellow cases.

|
Dr. Erica L. Mitchell |
Endovascular cases, which have increased over 300%-400%, too are essentially fellow cases (or in 0+5 programs, which are not discussed in this manuscript, vascular surgery resident cases) because the endovascular skill set does not appear to translate or transfer (and therefore is not considered relevant to the general surgery resident planning on a fellowship or career in the traditional surgical specialties) into open and laparoscopic skills desired by general surgery trainees.
Unfortunately, this open to endovascular operative experience shift will only increase with the introduction and early adoption (in academic centers) of complex endovascular techniques for the management of complex obstructive and aneurysmal arterial disease. A review of case log data for 0+5 and 5+2 residents completing programs in 2014-2015 reveals the operative experience and continued decline in open operative case numbers and increases in endovascular case numbers.
Vascular surgery training programs and their faculties have a responsibility to make sure the operative experience of general surgery residents is a worthwhile one. We, the faculty, need to keep encouraging general surgery residents to come to the operating room, even if they are not the primary surgeons. To make this worthwhile for them (because residents will not come to the operating room if there is nothing to be gained for themselves), we have to let them participate in the case somehow. One of the most critical aspects of a vascular surgical procedure is the operative exposure.
Having the general surgery residents participate in the vascular exposure is not only formative to their understanding of surgical anatomy, and applicable to all aspects of surgical practice, it is also critical to trainee development of decision making, judgment, and situational awareness. These exposures can and should be logged, using the e-code, to receive ACGME case log credit for vascular surgical procedures. E-codes, additionally, allow more than one resident to take credit for an arterial exposure and repair. We must also encourage trainees to come to the angio suite, while these cases, on the outset do not seem relevant to general surgery training, they do provide the learner with a greater appreciation of the complexity of vascular anatomy.
Importantly, as long as we continue to train vascular surgery specialists via the traditional 5+2 paradigm, we must keep general surgery residents, rotating through our services, interested and engaged in the management of vascular diseases. The simplest way to engage them is to make them feel relevant and to make them feel relevant they have to participate, both inside and outside of the operating room. It is, after all, our duty as surgical educators and advocates for our specialty to train the next generation of vascular surgical specialists!
Dr. Erica L. Mitchell is professor of surgery, program director for vascular surgery, and vice-chair of quality, department of surgery, Oregon Health & Science University, Portland. She is an associate medical editor of Vascular Specialist.
Vascular surgery fellow case logs reflect an increase in endovascular interventions, but general surgery residents may be missing out on training opportunities, according to a study of national case data.
In addition, general surgery residents saw a decrease in open vascular surgery cases, which was not reflected among the vascular surgery fellows, according to Dr. Rose C. Pedersen and colleagues in the department of surgery, Kaiser Permanente Los Angeles Medical Center. The report was published online in Annals of Vascular Surgery (doi: 10.1016/j.avsg.2016.02.008).
The paper was originally presented at the 2105 annual meeting of the Southern California Vascular Society. The study reports findings of a review of the Accreditation Council for Graduate Medical Education national case log reports from 2001 to 2012.
During that period, the number of general surgery residents increased from 1,021 to 1,098, while the number of vascular surgery fellows increased from 96 to 121. The total number of vascular cases logged by the vascular fellows significantly increased by 161%, from an average of 298 cases to 762 cases over the time period assessed. During that same period, vascular cases done by general surgery residents significantly decreased by 40%, from an average of 186 to 116 cases.
In terms of open cases. vascular fellows saw a significant 43% decrease in open abdominal aortic aneurysm (AAA) cases, going from 26 to 15, and a slight but significant increase in carotid endarterectomy cases logged (44 to 49). Hemodialysis access and major amputations also both increased significantly. A decreases in open surgery for peripheral obstructive disease was small and not significant.
General surgery residents saw decreases in all open surgery areas over the time period: AAA cases fell significantly by 78% (9 cases to 2 cases); carotid endarterectomies decreased significantly from 23 to 12 cases; and surgery for peripheral obstructive disease fell significantly from 21 to 8). Hemodialysis access cases and major amputations both decreased as well, but not significantly.
Endovascular cases increased from 2001 to 2012 for both vascular fellows and general surgery residents. Vascular fellows saw endovascular AAA repair significantly increase from an average of 17 to 46 cases, while those for general surgery residents rosed from roughly 1 to 3 cases. Similarly, endovascular interventions for peripheral obstructive disease significantly increased for vascular fellows from 17 to 85, and from 1.3 to 4 for general surgery residents.
“The contemporary management of abdominal aortic aneurysmal diseases and peripheral obstructive diseases has been particularly changed by endovascular therapies that have been adopted into the training experience of vascular surgery fellows, but not those of general surgery residents. Open surgery experience has decreased overall for general surgery residents in all major categories, a change not seen in vascular fellows,” the researchers concluded.
The authors reported no relevant disclosures.
Vascular surgery fellow case logs reflect an increase in endovascular interventions, but general surgery residents may be missing out on training opportunities, according to a study of national case data.
In addition, general surgery residents saw a decrease in open vascular surgery cases, which was not reflected among the vascular surgery fellows, according to Dr. Rose C. Pedersen and colleagues in the department of surgery, Kaiser Permanente Los Angeles Medical Center. The report was published online in Annals of Vascular Surgery (doi: 10.1016/j.avsg.2016.02.008).
The paper was originally presented at the 2105 annual meeting of the Southern California Vascular Society. The study reports findings of a review of the Accreditation Council for Graduate Medical Education national case log reports from 2001 to 2012.
During that period, the number of general surgery residents increased from 1,021 to 1,098, while the number of vascular surgery fellows increased from 96 to 121. The total number of vascular cases logged by the vascular fellows significantly increased by 161%, from an average of 298 cases to 762 cases over the time period assessed. During that same period, vascular cases done by general surgery residents significantly decreased by 40%, from an average of 186 to 116 cases.
In terms of open cases. vascular fellows saw a significant 43% decrease in open abdominal aortic aneurysm (AAA) cases, going from 26 to 15, and a slight but significant increase in carotid endarterectomy cases logged (44 to 49). Hemodialysis access and major amputations also both increased significantly. A decreases in open surgery for peripheral obstructive disease was small and not significant.
General surgery residents saw decreases in all open surgery areas over the time period: AAA cases fell significantly by 78% (9 cases to 2 cases); carotid endarterectomies decreased significantly from 23 to 12 cases; and surgery for peripheral obstructive disease fell significantly from 21 to 8). Hemodialysis access cases and major amputations both decreased as well, but not significantly.
Endovascular cases increased from 2001 to 2012 for both vascular fellows and general surgery residents. Vascular fellows saw endovascular AAA repair significantly increase from an average of 17 to 46 cases, while those for general surgery residents rosed from roughly 1 to 3 cases. Similarly, endovascular interventions for peripheral obstructive disease significantly increased for vascular fellows from 17 to 85, and from 1.3 to 4 for general surgery residents.
“The contemporary management of abdominal aortic aneurysmal diseases and peripheral obstructive diseases has been particularly changed by endovascular therapies that have been adopted into the training experience of vascular surgery fellows, but not those of general surgery residents. Open surgery experience has decreased overall for general surgery residents in all major categories, a change not seen in vascular fellows,” the researchers concluded.
The authors reported no relevant disclosures.
FROM ANNALS OF VASCULAR SURGERY
What insect repellents are safe during pregnancy?
With summer almost upon us, and the weather warming in many parts of the country, we have received questions from colleagues about the best over-the-counter insect repellants to advise their pregnant patients to use.
The preferred insect repellent for skin coverate is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, patients should be instructed to spray permethrin on their clothing or buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
| Repellent | Product | Manufacturer | Notes |
| DEET (N,N-diethyl-meta-toluamide)
| Off! | SC Johnson | Preferred repellent for use on the skin |
| Repel 100 | Spectrum Brands | ||
| Ultra 30 Liposome Controlled Release | Sawyer | ||
| Oil of lemon/eucalyptus/ para-menthane-diol | Repel Lemon Eucalyptus Insect Repellent | Spectrum Brands | Acceptable option for skin use |
| IR3535 | Skin So Soft Bug Guard Plus IR3535 Expedition | Avon | Acceptable option for skin use |
| Permethrin | Repel Permethrin Clothing & Gear Aerosol | Spectrum Brands | For use on clothing |
| Permethrin Pump Spray | Sawyer | ||
| Abbreviations: OTC, over the counter | |||
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak – United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
With summer almost upon us, and the weather warming in many parts of the country, we have received questions from colleagues about the best over-the-counter insect repellants to advise their pregnant patients to use.
The preferred insect repellent for skin coverate is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, patients should be instructed to spray permethrin on their clothing or buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
| Repellent | Product | Manufacturer | Notes |
| DEET (N,N-diethyl-meta-toluamide)
| Off! | SC Johnson | Preferred repellent for use on the skin |
| Repel 100 | Spectrum Brands | ||
| Ultra 30 Liposome Controlled Release | Sawyer | ||
| Oil of lemon/eucalyptus/ para-menthane-diol | Repel Lemon Eucalyptus Insect Repellent | Spectrum Brands | Acceptable option for skin use |
| IR3535 | Skin So Soft Bug Guard Plus IR3535 Expedition | Avon | Acceptable option for skin use |
| Permethrin | Repel Permethrin Clothing & Gear Aerosol | Spectrum Brands | For use on clothing |
| Permethrin Pump Spray | Sawyer | ||
| Abbreviations: OTC, over the counter | |||
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
With summer almost upon us, and the weather warming in many parts of the country, we have received questions from colleagues about the best over-the-counter insect repellants to advise their pregnant patients to use.
The preferred insect repellent for skin coverate is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, patients should be instructed to spray permethrin on their clothing or buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
| Repellent | Product | Manufacturer | Notes |
| DEET (N,N-diethyl-meta-toluamide)
| Off! | SC Johnson | Preferred repellent for use on the skin |
| Repel 100 | Spectrum Brands | ||
| Ultra 30 Liposome Controlled Release | Sawyer | ||
| Oil of lemon/eucalyptus/ para-menthane-diol | Repel Lemon Eucalyptus Insect Repellent | Spectrum Brands | Acceptable option for skin use |
| IR3535 | Skin So Soft Bug Guard Plus IR3535 Expedition | Avon | Acceptable option for skin use |
| Permethrin | Repel Permethrin Clothing & Gear Aerosol | Spectrum Brands | For use on clothing |
| Permethrin Pump Spray | Sawyer | ||
| Abbreviations: OTC, over the counter | |||
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak – United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak – United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Adrenal gland tumors linked to ADHD diagnosis
Pediatric patients diagnosed with pheochromocytomas (PHEO) or paragangliomas (PGL) were nearly three times as likely to also carry a diagnosis of attention deficit hyperactivity disorder (ADHD), compared to pediatric patients without PHEO or PGL, investigators reported.
In addition, in 33% of the patients with PHEO and PGL, ADHD symptoms were resolved following surgical removal of the tumor.
PHEO and PGL are rare tumors of the adrenal gland. About 10% of PHEO and PGL cases occur in patients younger than 18 years. PHEOs form inside the adrenal gland in the adrenal medulla while PGLs form outside the adrenal gland. Both tumors cause excess secretion of epinephrine and norepinephrine resulting in high blood pressure, headaches, weight loss, excess sweating, anxiety, and depression. These tumors are most often surgically removed or treated with medication. Chemotherapy and radiation therapy have not been as effective in treating PHEO or PGL.
ADHD is a neurodevelopment disorder characterized by a pattern of inattention and hyperactivity or impulsivity. ADHD is associated with catecholamine dysregulation; the function of catecholamine receptors is impaired by either excess or deficient stimulation. ADHD has a prevalence of 7.2% in children aged 4-18.
In addition to the overlap in symptoms, “the stimulants used to treat ADHD may exacerbate the symptoms of the PHEO/PGL and potentially lead to a hypertensive crisis ... Amphetamines, the most widely used ADHD medication class, lead to release of stored catecholamines from vesicles, block reuptake of norepinephrine and dopamine, and block catecholamine degradation,” wrote Dr. M. Batsis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and her associates (Horm Metab Res. 2016 May 12. doi: 10.1055/s-0042-106725).
“I noticed that a lot of patients with the same story as follows: [parents] went to their pediatrician when their child started having feelings of anxiety or their heart was racing. And these symptoms were attributed to ADHD, and the child was started on medications. It wasn’t until later symptoms – an abdominal mass or a hypertensive crisis – that the patient was ultimately found out to have a pheochromocytoma,” Dr. Maya Lodish, a pediatric endocrinologist and coauthor of the paper, said in an interview.
“In hindsight, it just was not picked up. ADHD medications in no way affect tumor growth. The substances that these tumors release are stimulants. Endocrine tumors release catecholamine which are naturally occurring hormones we release under stress. When you add on top of that a stimulant medication [to treat ADHD] that may causes the nervous system to go into overdrive,” she said.
Due to the rarity of PHEO and PGL, their association with ADHD has not been well characterized. The purpose of this study was to therefore better assess the relationship between ADHD and PHEO/PGL development.
Investigators recruited 43 pediatric patients aged 6-17 who had been diagnosed with PHEO or PGL. Twenty-one percent (n = 9) of patients with PHEO/PGL carried a diagnosis of ADHD, compared to 7.2% in the general population (P = .0328).
Prior to the surgical removal of the tumors, eight of the nine patients had elevated levels of norepinephrine (n = 7), dopamine (n = 3), epinephrine (n = 1), metanephrine (n = 5) and/or normetanephrine (n = 7). In the remaining patient, levels were not measured.
Following the surgical removal of the tumors, three of the nine patients experienced both a resolution of their ADHD-related symptoms and a drop or normalization of their catecholamine and metanephrine levels. Two of those three patients showed no clinical signs of recurrent tumors while the third is under evaluation for a small pelvic lesion.
“These tumors are very rare and the vast majority of patients with ADHD are not affected by them, but they do occur. There are other organic conditions with the same symptoms – drug abuse, medications, Graves disease. If the child has symptoms attributed to ADHD and high blood pressure or family history of endocrine tumors then it is important to have a full organic workup to measure other causes of hypertension prior to starting stimulant medication,” Dr. Lodish said.
“My observation is that, and a lot of articles out there would agree, diagnoses of ADHD are on the rise and the prescribing of ADHD medication is also on the rise. I hope this is a bit of a wake-up call to practitioners that what’s common is common but there are some rare [conditions] to be aware of and so don’t have a knee jerk reaction to prescribing a medication for symptoms believed to be attributed to ADHD,” she said.
The Division of Intramural Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. The investigators had no disclosures to report.
On Twitter @JessCraig_OP
Pediatric patients diagnosed with pheochromocytomas (PHEO) or paragangliomas (PGL) were nearly three times as likely to also carry a diagnosis of attention deficit hyperactivity disorder (ADHD), compared to pediatric patients without PHEO or PGL, investigators reported.
In addition, in 33% of the patients with PHEO and PGL, ADHD symptoms were resolved following surgical removal of the tumor.
PHEO and PGL are rare tumors of the adrenal gland. About 10% of PHEO and PGL cases occur in patients younger than 18 years. PHEOs form inside the adrenal gland in the adrenal medulla while PGLs form outside the adrenal gland. Both tumors cause excess secretion of epinephrine and norepinephrine resulting in high blood pressure, headaches, weight loss, excess sweating, anxiety, and depression. These tumors are most often surgically removed or treated with medication. Chemotherapy and radiation therapy have not been as effective in treating PHEO or PGL.
ADHD is a neurodevelopment disorder characterized by a pattern of inattention and hyperactivity or impulsivity. ADHD is associated with catecholamine dysregulation; the function of catecholamine receptors is impaired by either excess or deficient stimulation. ADHD has a prevalence of 7.2% in children aged 4-18.
In addition to the overlap in symptoms, “the stimulants used to treat ADHD may exacerbate the symptoms of the PHEO/PGL and potentially lead to a hypertensive crisis ... Amphetamines, the most widely used ADHD medication class, lead to release of stored catecholamines from vesicles, block reuptake of norepinephrine and dopamine, and block catecholamine degradation,” wrote Dr. M. Batsis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and her associates (Horm Metab Res. 2016 May 12. doi: 10.1055/s-0042-106725).
“I noticed that a lot of patients with the same story as follows: [parents] went to their pediatrician when their child started having feelings of anxiety or their heart was racing. And these symptoms were attributed to ADHD, and the child was started on medications. It wasn’t until later symptoms – an abdominal mass or a hypertensive crisis – that the patient was ultimately found out to have a pheochromocytoma,” Dr. Maya Lodish, a pediatric endocrinologist and coauthor of the paper, said in an interview.
“In hindsight, it just was not picked up. ADHD medications in no way affect tumor growth. The substances that these tumors release are stimulants. Endocrine tumors release catecholamine which are naturally occurring hormones we release under stress. When you add on top of that a stimulant medication [to treat ADHD] that may causes the nervous system to go into overdrive,” she said.
Due to the rarity of PHEO and PGL, their association with ADHD has not been well characterized. The purpose of this study was to therefore better assess the relationship between ADHD and PHEO/PGL development.
Investigators recruited 43 pediatric patients aged 6-17 who had been diagnosed with PHEO or PGL. Twenty-one percent (n = 9) of patients with PHEO/PGL carried a diagnosis of ADHD, compared to 7.2% in the general population (P = .0328).
Prior to the surgical removal of the tumors, eight of the nine patients had elevated levels of norepinephrine (n = 7), dopamine (n = 3), epinephrine (n = 1), metanephrine (n = 5) and/or normetanephrine (n = 7). In the remaining patient, levels were not measured.
Following the surgical removal of the tumors, three of the nine patients experienced both a resolution of their ADHD-related symptoms and a drop or normalization of their catecholamine and metanephrine levels. Two of those three patients showed no clinical signs of recurrent tumors while the third is under evaluation for a small pelvic lesion.
“These tumors are very rare and the vast majority of patients with ADHD are not affected by them, but they do occur. There are other organic conditions with the same symptoms – drug abuse, medications, Graves disease. If the child has symptoms attributed to ADHD and high blood pressure or family history of endocrine tumors then it is important to have a full organic workup to measure other causes of hypertension prior to starting stimulant medication,” Dr. Lodish said.
“My observation is that, and a lot of articles out there would agree, diagnoses of ADHD are on the rise and the prescribing of ADHD medication is also on the rise. I hope this is a bit of a wake-up call to practitioners that what’s common is common but there are some rare [conditions] to be aware of and so don’t have a knee jerk reaction to prescribing a medication for symptoms believed to be attributed to ADHD,” she said.
The Division of Intramural Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. The investigators had no disclosures to report.
On Twitter @JessCraig_OP
Pediatric patients diagnosed with pheochromocytomas (PHEO) or paragangliomas (PGL) were nearly three times as likely to also carry a diagnosis of attention deficit hyperactivity disorder (ADHD), compared to pediatric patients without PHEO or PGL, investigators reported.
In addition, in 33% of the patients with PHEO and PGL, ADHD symptoms were resolved following surgical removal of the tumor.
PHEO and PGL are rare tumors of the adrenal gland. About 10% of PHEO and PGL cases occur in patients younger than 18 years. PHEOs form inside the adrenal gland in the adrenal medulla while PGLs form outside the adrenal gland. Both tumors cause excess secretion of epinephrine and norepinephrine resulting in high blood pressure, headaches, weight loss, excess sweating, anxiety, and depression. These tumors are most often surgically removed or treated with medication. Chemotherapy and radiation therapy have not been as effective in treating PHEO or PGL.
ADHD is a neurodevelopment disorder characterized by a pattern of inattention and hyperactivity or impulsivity. ADHD is associated with catecholamine dysregulation; the function of catecholamine receptors is impaired by either excess or deficient stimulation. ADHD has a prevalence of 7.2% in children aged 4-18.
In addition to the overlap in symptoms, “the stimulants used to treat ADHD may exacerbate the symptoms of the PHEO/PGL and potentially lead to a hypertensive crisis ... Amphetamines, the most widely used ADHD medication class, lead to release of stored catecholamines from vesicles, block reuptake of norepinephrine and dopamine, and block catecholamine degradation,” wrote Dr. M. Batsis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and her associates (Horm Metab Res. 2016 May 12. doi: 10.1055/s-0042-106725).
“I noticed that a lot of patients with the same story as follows: [parents] went to their pediatrician when their child started having feelings of anxiety or their heart was racing. And these symptoms were attributed to ADHD, and the child was started on medications. It wasn’t until later symptoms – an abdominal mass or a hypertensive crisis – that the patient was ultimately found out to have a pheochromocytoma,” Dr. Maya Lodish, a pediatric endocrinologist and coauthor of the paper, said in an interview.
“In hindsight, it just was not picked up. ADHD medications in no way affect tumor growth. The substances that these tumors release are stimulants. Endocrine tumors release catecholamine which are naturally occurring hormones we release under stress. When you add on top of that a stimulant medication [to treat ADHD] that may causes the nervous system to go into overdrive,” she said.
Due to the rarity of PHEO and PGL, their association with ADHD has not been well characterized. The purpose of this study was to therefore better assess the relationship between ADHD and PHEO/PGL development.
Investigators recruited 43 pediatric patients aged 6-17 who had been diagnosed with PHEO or PGL. Twenty-one percent (n = 9) of patients with PHEO/PGL carried a diagnosis of ADHD, compared to 7.2% in the general population (P = .0328).
Prior to the surgical removal of the tumors, eight of the nine patients had elevated levels of norepinephrine (n = 7), dopamine (n = 3), epinephrine (n = 1), metanephrine (n = 5) and/or normetanephrine (n = 7). In the remaining patient, levels were not measured.
Following the surgical removal of the tumors, three of the nine patients experienced both a resolution of their ADHD-related symptoms and a drop or normalization of their catecholamine and metanephrine levels. Two of those three patients showed no clinical signs of recurrent tumors while the third is under evaluation for a small pelvic lesion.
“These tumors are very rare and the vast majority of patients with ADHD are not affected by them, but they do occur. There are other organic conditions with the same symptoms – drug abuse, medications, Graves disease. If the child has symptoms attributed to ADHD and high blood pressure or family history of endocrine tumors then it is important to have a full organic workup to measure other causes of hypertension prior to starting stimulant medication,” Dr. Lodish said.
“My observation is that, and a lot of articles out there would agree, diagnoses of ADHD are on the rise and the prescribing of ADHD medication is also on the rise. I hope this is a bit of a wake-up call to practitioners that what’s common is common but there are some rare [conditions] to be aware of and so don’t have a knee jerk reaction to prescribing a medication for symptoms believed to be attributed to ADHD,” she said.
The Division of Intramural Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. The investigators had no disclosures to report.
On Twitter @JessCraig_OP
FROM HORMONE AND METABOLIC RESEARCH
Key clinical point: Pediatric patients with pheochromocytomas (PHEO) or paragangliomas (PGL) were more likely to also carry a diagnosis of ADHD, compared to pediatric patients without PHEO or PGL.
Major finding: Twenty-one percent of patients with PHEO/PGL carried a diagnosis of ADHD, compared to 7.2% in the general population (P = .0328). In 33% of the patients with PHEO and PGL, ADHD symptoms were resolved following surgical removal of the tumor.
Data source: Longitudinal study of 43 patients aged 6-17 who were diagnosed with PHEO and/or PGL.
Disclosures: The Division of Intramural Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. The investigators had no disclosures.
Fungi may exacerbate asthma, chronic sinusitis
LOS ANGELES – Fungi might play a far larger role in asthma and chronic sinusitis than previously thought, according to investigators at Baylor College of Medicine in Houston.
With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center; about a third of the patients had asthma, a third had chronic sinusitis, and a third had both. Although Aspergillus and Candida species were common, more than 30 fungal species were identified. Only a handful of patients had positive results on IgE testing.
Of 62 patients treated with standard-dose voriconazole or terbinafine, sometimes for more than a year, 54 (87%) reported symptomatic benefit including 31 (50%) with decreased sputum production, 24 (39%) with improved breathing, 20 (32%) with less cough, and nine (14.5%) with less rescue inhaler use.
At Baylor, prescribing antifungals for patients with recalcitrant asthma and chronic sinusitis “has evolved into something we pretty much do all the time now regardless of sensitivity results. I’m pretty certain we are the only institution that does this,” said allergy and immunology fellow Dr. Evan Li.
“Fungi, we think, are important initiating factors in many cases of asthma. They set up chronic mucosal infection. Our [treatment] experience is extremely positive; it may be in the future that if you have significant asthma or sinusitis, you just go on an antifungal, but more research and clinical trials are needed,” said senior investigator Dr. David Corry, professor and chief of medical immunology, allergy, and rheumatology at Baylor.
“The standard culture techniques that have been used for 100 years are inadequate when it comes to culturing fungi from sputum, and why results almost invariably come back negative,” Dr. Corry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The problem is that “almost everything in sputum” – eosinophils, macrophages, cytokines, and so on – “is designed to kill fungi.” Those elements have to be removed before plating. At Baylor, “we solubilize [sputum] with the reducing agent dithiothreitol, and vigorously stir the mixture to disperse the organisms and wash away the cellular elements and the other things.” The process leaves behind “a sandy material that’s basically fibrin clots mixed with a lot of fungal elements. You spread that on a plate, and it grows like wildfire,” he said.
“It’s easy to do, but time consuming. People are actually shipping their samples to us now from around the country, and we are happy to do those cultures,” Dr. Corry said. There’s no patent on the technique because “we want the community to use it. We want people to be helped,” he said.
Voriconazole seems to be the most effective option, and the team opts for it when possible, Dr. Corry noted. Terbinafine is the go-to drug for patients who can’t tolerate voriconazole. Fluconazole is sometimes added when monotherapy doesn’t seem to be doing the trick.
The work began as a search for household proteases. “One of our first discoveries was that” most are fungal. “The twist is that you are not inhaling the proteases, you are inhaling the fungus,” Dr. Corry said.
There have been both positive and negative results from the few prior investigations of antifungals for asthma. The team suspects that negative findings were a result of patients not being treated long enough, among other reasons.
The Baylor team is looking for funding for a prospective trial. The investigators hope to develop a protocol for diagnosis and treatment of fungal airway disease, but “there’s a lot of work that needs to get done,” Dr. Corry said.
The investigators had no relevant financial disclosures, and there was no outside funding for the work.
LOS ANGELES – Fungi might play a far larger role in asthma and chronic sinusitis than previously thought, according to investigators at Baylor College of Medicine in Houston.
With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center; about a third of the patients had asthma, a third had chronic sinusitis, and a third had both. Although Aspergillus and Candida species were common, more than 30 fungal species were identified. Only a handful of patients had positive results on IgE testing.
Of 62 patients treated with standard-dose voriconazole or terbinafine, sometimes for more than a year, 54 (87%) reported symptomatic benefit including 31 (50%) with decreased sputum production, 24 (39%) with improved breathing, 20 (32%) with less cough, and nine (14.5%) with less rescue inhaler use.
At Baylor, prescribing antifungals for patients with recalcitrant asthma and chronic sinusitis “has evolved into something we pretty much do all the time now regardless of sensitivity results. I’m pretty certain we are the only institution that does this,” said allergy and immunology fellow Dr. Evan Li.
“Fungi, we think, are important initiating factors in many cases of asthma. They set up chronic mucosal infection. Our [treatment] experience is extremely positive; it may be in the future that if you have significant asthma or sinusitis, you just go on an antifungal, but more research and clinical trials are needed,” said senior investigator Dr. David Corry, professor and chief of medical immunology, allergy, and rheumatology at Baylor.
“The standard culture techniques that have been used for 100 years are inadequate when it comes to culturing fungi from sputum, and why results almost invariably come back negative,” Dr. Corry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The problem is that “almost everything in sputum” – eosinophils, macrophages, cytokines, and so on – “is designed to kill fungi.” Those elements have to be removed before plating. At Baylor, “we solubilize [sputum] with the reducing agent dithiothreitol, and vigorously stir the mixture to disperse the organisms and wash away the cellular elements and the other things.” The process leaves behind “a sandy material that’s basically fibrin clots mixed with a lot of fungal elements. You spread that on a plate, and it grows like wildfire,” he said.
“It’s easy to do, but time consuming. People are actually shipping their samples to us now from around the country, and we are happy to do those cultures,” Dr. Corry said. There’s no patent on the technique because “we want the community to use it. We want people to be helped,” he said.
Voriconazole seems to be the most effective option, and the team opts for it when possible, Dr. Corry noted. Terbinafine is the go-to drug for patients who can’t tolerate voriconazole. Fluconazole is sometimes added when monotherapy doesn’t seem to be doing the trick.
The work began as a search for household proteases. “One of our first discoveries was that” most are fungal. “The twist is that you are not inhaling the proteases, you are inhaling the fungus,” Dr. Corry said.
There have been both positive and negative results from the few prior investigations of antifungals for asthma. The team suspects that negative findings were a result of patients not being treated long enough, among other reasons.
The Baylor team is looking for funding for a prospective trial. The investigators hope to develop a protocol for diagnosis and treatment of fungal airway disease, but “there’s a lot of work that needs to get done,” Dr. Corry said.
The investigators had no relevant financial disclosures, and there was no outside funding for the work.
LOS ANGELES – Fungi might play a far larger role in asthma and chronic sinusitis than previously thought, according to investigators at Baylor College of Medicine in Houston.
With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center; about a third of the patients had asthma, a third had chronic sinusitis, and a third had both. Although Aspergillus and Candida species were common, more than 30 fungal species were identified. Only a handful of patients had positive results on IgE testing.
Of 62 patients treated with standard-dose voriconazole or terbinafine, sometimes for more than a year, 54 (87%) reported symptomatic benefit including 31 (50%) with decreased sputum production, 24 (39%) with improved breathing, 20 (32%) with less cough, and nine (14.5%) with less rescue inhaler use.
At Baylor, prescribing antifungals for patients with recalcitrant asthma and chronic sinusitis “has evolved into something we pretty much do all the time now regardless of sensitivity results. I’m pretty certain we are the only institution that does this,” said allergy and immunology fellow Dr. Evan Li.
“Fungi, we think, are important initiating factors in many cases of asthma. They set up chronic mucosal infection. Our [treatment] experience is extremely positive; it may be in the future that if you have significant asthma or sinusitis, you just go on an antifungal, but more research and clinical trials are needed,” said senior investigator Dr. David Corry, professor and chief of medical immunology, allergy, and rheumatology at Baylor.
“The standard culture techniques that have been used for 100 years are inadequate when it comes to culturing fungi from sputum, and why results almost invariably come back negative,” Dr. Corry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The problem is that “almost everything in sputum” – eosinophils, macrophages, cytokines, and so on – “is designed to kill fungi.” Those elements have to be removed before plating. At Baylor, “we solubilize [sputum] with the reducing agent dithiothreitol, and vigorously stir the mixture to disperse the organisms and wash away the cellular elements and the other things.” The process leaves behind “a sandy material that’s basically fibrin clots mixed with a lot of fungal elements. You spread that on a plate, and it grows like wildfire,” he said.
“It’s easy to do, but time consuming. People are actually shipping their samples to us now from around the country, and we are happy to do those cultures,” Dr. Corry said. There’s no patent on the technique because “we want the community to use it. We want people to be helped,” he said.
Voriconazole seems to be the most effective option, and the team opts for it when possible, Dr. Corry noted. Terbinafine is the go-to drug for patients who can’t tolerate voriconazole. Fluconazole is sometimes added when monotherapy doesn’t seem to be doing the trick.
The work began as a search for household proteases. “One of our first discoveries was that” most are fungal. “The twist is that you are not inhaling the proteases, you are inhaling the fungus,” Dr. Corry said.
There have been both positive and negative results from the few prior investigations of antifungals for asthma. The team suspects that negative findings were a result of patients not being treated long enough, among other reasons.
The Baylor team is looking for funding for a prospective trial. The investigators hope to develop a protocol for diagnosis and treatment of fungal airway disease, but “there’s a lot of work that needs to get done,” Dr. Corry said.
The investigators had no relevant financial disclosures, and there was no outside funding for the work.
AT AAAAI
Key clinical point: Consider antifungal therapy if asthma or chronic sinusitis patients don’t respond well to conventional treatment.
Major finding: With the help of a special culturing technique to wash antifungal elements out of sputum samples, six or more fungal colony-forming units grew out of the sputum of 112 of 134 patients (83.5%) at the Houston Veterans Affairs Medical Center.
Data source: A single-center case review.
Disclosures: The investigators had no relevant financial disclosures, and there was no outside funding for the work.
FDA approves atezolizumab for advanced urothelial carcinoma
The Food and Drug Administration has granted accelerated approval to atezolizumab for the treatment of locally advanced or metastatic urothelial carcinoma in patients who experienced disease progression during or following platinum-based chemotherapy, along with a complementary diagnostic.
Atezolizumab, marketed as Tecentriq by Genentech, is the first and only FDA-approved anti-PDL1 immunotherapy for urothelial carcinoma.
This accelerated approval is based on a 14.8% overall response rate (95% confidence interval, 11.1-19.3) reported from the open-label, multicenter, phase II IMvigor clinical trial of 310 patients, the FDA said in a written statement.
Just over one-fourth (26%) of participants who tested positive for PD-L1 expression experienced a tumor response, compared with 9.5% of participants who were negative for PD-L1 expression. The FDA, therefore, also approved the Ventana PD-L1 (SP142) assay to detect PD-L1 protein expression levels on patients’ tumor-infiltrating immune cells, which will help guide treatment decisions.
The most common adverse events reported in the single-arm trail of atezolizumab were urinary tract infection (9%), anemia (8%), fatigue (6%), and difficulty breathing (4%). Other serious side effects included pneumonitis, hepatitis, colitis, hormone gland problems, neuropathy, meningocephalitis, eye problems, severe infections, and severe infusion reactions. Three people (0.9%) experienced sepsis, pneumonitis, or intestinal obstruction that led to death, Genentech reported in a written statement.
On Twitter @JessCraig_OP
The Food and Drug Administration has granted accelerated approval to atezolizumab for the treatment of locally advanced or metastatic urothelial carcinoma in patients who experienced disease progression during or following platinum-based chemotherapy, along with a complementary diagnostic.
Atezolizumab, marketed as Tecentriq by Genentech, is the first and only FDA-approved anti-PDL1 immunotherapy for urothelial carcinoma.
This accelerated approval is based on a 14.8% overall response rate (95% confidence interval, 11.1-19.3) reported from the open-label, multicenter, phase II IMvigor clinical trial of 310 patients, the FDA said in a written statement.
Just over one-fourth (26%) of participants who tested positive for PD-L1 expression experienced a tumor response, compared with 9.5% of participants who were negative for PD-L1 expression. The FDA, therefore, also approved the Ventana PD-L1 (SP142) assay to detect PD-L1 protein expression levels on patients’ tumor-infiltrating immune cells, which will help guide treatment decisions.
The most common adverse events reported in the single-arm trail of atezolizumab were urinary tract infection (9%), anemia (8%), fatigue (6%), and difficulty breathing (4%). Other serious side effects included pneumonitis, hepatitis, colitis, hormone gland problems, neuropathy, meningocephalitis, eye problems, severe infections, and severe infusion reactions. Three people (0.9%) experienced sepsis, pneumonitis, or intestinal obstruction that led to death, Genentech reported in a written statement.
On Twitter @JessCraig_OP
The Food and Drug Administration has granted accelerated approval to atezolizumab for the treatment of locally advanced or metastatic urothelial carcinoma in patients who experienced disease progression during or following platinum-based chemotherapy, along with a complementary diagnostic.
Atezolizumab, marketed as Tecentriq by Genentech, is the first and only FDA-approved anti-PDL1 immunotherapy for urothelial carcinoma.
This accelerated approval is based on a 14.8% overall response rate (95% confidence interval, 11.1-19.3) reported from the open-label, multicenter, phase II IMvigor clinical trial of 310 patients, the FDA said in a written statement.
Just over one-fourth (26%) of participants who tested positive for PD-L1 expression experienced a tumor response, compared with 9.5% of participants who were negative for PD-L1 expression. The FDA, therefore, also approved the Ventana PD-L1 (SP142) assay to detect PD-L1 protein expression levels on patients’ tumor-infiltrating immune cells, which will help guide treatment decisions.
The most common adverse events reported in the single-arm trail of atezolizumab were urinary tract infection (9%), anemia (8%), fatigue (6%), and difficulty breathing (4%). Other serious side effects included pneumonitis, hepatitis, colitis, hormone gland problems, neuropathy, meningocephalitis, eye problems, severe infections, and severe infusion reactions. Three people (0.9%) experienced sepsis, pneumonitis, or intestinal obstruction that led to death, Genentech reported in a written statement.
On Twitter @JessCraig_OP
Exercise Lowers Risk of Some Cancers
Here’s one more reason to take a break and exercise: A recent study links leisure-time physical activities with a lower risk of developing 13 different types of cancer. The international team of investigators pooled data from 12 prospective U.S. and European cohorts with self-reported physical activity that included more than 1.4 million participants and 186,932 cases of cancer. The greatest risk reductions were for esophageal adenocarcinoma, liver cancer, cancer of the gastric cardia, kidney cancer, and myeloid leukemia. Previous research has examined the links between physical activity and cancer risk and shown reduced risks for colon, breast, and endometrial cancers, but these studies have been underpowered to make the connection with other forms of cancer.
Related: IBD and the Risk of Oral Cancer
“Leisure-time physical activity is known to reduce risks of heart disease and risk of death from all causes, and our study demonstrates that it is also associated with lower risks of many types of cancer,” said Steven C. Moore, PhD, MPH, an investigator at the National Cancer Institute. “Furthermore, our results support that these associations are broadly generalizable to different populations, including people who are overweight or obese, or those with a history of smoking. Health care professionals counseling inactive adults should promote physical activity as a component of a healthy lifestyle and cancer prevention.”
For 13 cancers, increased levels of leisure-time physical activity were associated with lower risk: esophageal adenocarcinoma (hazard ratio [HR] 0.58, 95%; confidence interval [CI] 0.37-0.89); liver (HR 0.73, 95% CI 0.55-0.98); lung (HR 0.74, 95% CI 0.71-0.77); kidney (HR 0.77, 95% CI 0.70-0.85); gastric cardia (HR 0.78, 95% CI 0.64-0.95); endometrial (HR 0.79, 95% CI 0.68-0.92); myeloid leukemia (HR 0.80, 95% CI 0.70-0.92); myeloma (HR 0.83, 95% CI 0.72-0.95); colon (HR 0.84, 95% CI 0.77-0.91); head and neck (HR 0.85, 95% CI 0.78-0.93); rectal (HR 0.87, 95% CI 0.80-0.95); bladder (HR 0.87, 95% CI 0.82-0.92); and breast (HR 0.90, 95% CI 0.87-0.93). Conversely, leisure-time physical activity increased the risks of malignant melanoma (HR 1.27, 95% CI 1.16-1.40) and prostate cancer (HR 1.05, 95% CI 1.03-1.08).
Related: Sexual Orientation and Cancer Risk
According to the authors, the associations were similar between patients who were overweight/obese and those who were normal weight. They also noted that smoking status modified the association with lung cancer but not other smoking-related cancers.
The amount of exercise was important for some of the cancers. The risk of developing 7 of the cancer types was at least 20% lower for the most active participants (90th percentile of activity) compared with the least active participants (10th percentile of activity).
A number of physical activity mechanisms can affect cancer risk. It has been hypothesized that cancer growth could be initiated or abetted by 3 metabolic pathways that also are affected by exercise: sex steroids (estrogens and androgens); insulin and insulin-like growth factors; and proteins involved with both insulin metabolism and inflammation. Additionally, several non-hormonal mechanisms have been hypothesized to link physical activity to cancer risk, including inflammation, immune function, oxidative stress, and, for colon cancer, a reduction in time that it takes for waste to pass through the gastrointestinal tract.
Related: Alcohol Intake Increases Cancer Risk
“For years, we’ve had substantial evidence supporting a role for physical activity in three leading cancers: colon, breast, and endometrial cancers, which together account for nearly one in four cancers in the United States,” said another study author, Alpa V. Patel, PhD, a cancer epidemiologist at the American Cancer Society. “This study linking physical activity to 10 additional cancers shows its impact may be even more relevant, and that physical activity has far reaching value for cancer prevention.”
Source:
Increased physical activity associated with lower risk of 13 types of cancer [press release]. Bethesda, MD: National Institutes of Health; May 16, 2016.
Here’s one more reason to take a break and exercise: A recent study links leisure-time physical activities with a lower risk of developing 13 different types of cancer. The international team of investigators pooled data from 12 prospective U.S. and European cohorts with self-reported physical activity that included more than 1.4 million participants and 186,932 cases of cancer. The greatest risk reductions were for esophageal adenocarcinoma, liver cancer, cancer of the gastric cardia, kidney cancer, and myeloid leukemia. Previous research has examined the links between physical activity and cancer risk and shown reduced risks for colon, breast, and endometrial cancers, but these studies have been underpowered to make the connection with other forms of cancer.
Related: IBD and the Risk of Oral Cancer
“Leisure-time physical activity is known to reduce risks of heart disease and risk of death from all causes, and our study demonstrates that it is also associated with lower risks of many types of cancer,” said Steven C. Moore, PhD, MPH, an investigator at the National Cancer Institute. “Furthermore, our results support that these associations are broadly generalizable to different populations, including people who are overweight or obese, or those with a history of smoking. Health care professionals counseling inactive adults should promote physical activity as a component of a healthy lifestyle and cancer prevention.”
For 13 cancers, increased levels of leisure-time physical activity were associated with lower risk: esophageal adenocarcinoma (hazard ratio [HR] 0.58, 95%; confidence interval [CI] 0.37-0.89); liver (HR 0.73, 95% CI 0.55-0.98); lung (HR 0.74, 95% CI 0.71-0.77); kidney (HR 0.77, 95% CI 0.70-0.85); gastric cardia (HR 0.78, 95% CI 0.64-0.95); endometrial (HR 0.79, 95% CI 0.68-0.92); myeloid leukemia (HR 0.80, 95% CI 0.70-0.92); myeloma (HR 0.83, 95% CI 0.72-0.95); colon (HR 0.84, 95% CI 0.77-0.91); head and neck (HR 0.85, 95% CI 0.78-0.93); rectal (HR 0.87, 95% CI 0.80-0.95); bladder (HR 0.87, 95% CI 0.82-0.92); and breast (HR 0.90, 95% CI 0.87-0.93). Conversely, leisure-time physical activity increased the risks of malignant melanoma (HR 1.27, 95% CI 1.16-1.40) and prostate cancer (HR 1.05, 95% CI 1.03-1.08).
Related: Sexual Orientation and Cancer Risk
According to the authors, the associations were similar between patients who were overweight/obese and those who were normal weight. They also noted that smoking status modified the association with lung cancer but not other smoking-related cancers.
The amount of exercise was important for some of the cancers. The risk of developing 7 of the cancer types was at least 20% lower for the most active participants (90th percentile of activity) compared with the least active participants (10th percentile of activity).
A number of physical activity mechanisms can affect cancer risk. It has been hypothesized that cancer growth could be initiated or abetted by 3 metabolic pathways that also are affected by exercise: sex steroids (estrogens and androgens); insulin and insulin-like growth factors; and proteins involved with both insulin metabolism and inflammation. Additionally, several non-hormonal mechanisms have been hypothesized to link physical activity to cancer risk, including inflammation, immune function, oxidative stress, and, for colon cancer, a reduction in time that it takes for waste to pass through the gastrointestinal tract.
Related: Alcohol Intake Increases Cancer Risk
“For years, we’ve had substantial evidence supporting a role for physical activity in three leading cancers: colon, breast, and endometrial cancers, which together account for nearly one in four cancers in the United States,” said another study author, Alpa V. Patel, PhD, a cancer epidemiologist at the American Cancer Society. “This study linking physical activity to 10 additional cancers shows its impact may be even more relevant, and that physical activity has far reaching value for cancer prevention.”
Source:
Increased physical activity associated with lower risk of 13 types of cancer [press release]. Bethesda, MD: National Institutes of Health; May 16, 2016.
Here’s one more reason to take a break and exercise: A recent study links leisure-time physical activities with a lower risk of developing 13 different types of cancer. The international team of investigators pooled data from 12 prospective U.S. and European cohorts with self-reported physical activity that included more than 1.4 million participants and 186,932 cases of cancer. The greatest risk reductions were for esophageal adenocarcinoma, liver cancer, cancer of the gastric cardia, kidney cancer, and myeloid leukemia. Previous research has examined the links between physical activity and cancer risk and shown reduced risks for colon, breast, and endometrial cancers, but these studies have been underpowered to make the connection with other forms of cancer.
Related: IBD and the Risk of Oral Cancer
“Leisure-time physical activity is known to reduce risks of heart disease and risk of death from all causes, and our study demonstrates that it is also associated with lower risks of many types of cancer,” said Steven C. Moore, PhD, MPH, an investigator at the National Cancer Institute. “Furthermore, our results support that these associations are broadly generalizable to different populations, including people who are overweight or obese, or those with a history of smoking. Health care professionals counseling inactive adults should promote physical activity as a component of a healthy lifestyle and cancer prevention.”
For 13 cancers, increased levels of leisure-time physical activity were associated with lower risk: esophageal adenocarcinoma (hazard ratio [HR] 0.58, 95%; confidence interval [CI] 0.37-0.89); liver (HR 0.73, 95% CI 0.55-0.98); lung (HR 0.74, 95% CI 0.71-0.77); kidney (HR 0.77, 95% CI 0.70-0.85); gastric cardia (HR 0.78, 95% CI 0.64-0.95); endometrial (HR 0.79, 95% CI 0.68-0.92); myeloid leukemia (HR 0.80, 95% CI 0.70-0.92); myeloma (HR 0.83, 95% CI 0.72-0.95); colon (HR 0.84, 95% CI 0.77-0.91); head and neck (HR 0.85, 95% CI 0.78-0.93); rectal (HR 0.87, 95% CI 0.80-0.95); bladder (HR 0.87, 95% CI 0.82-0.92); and breast (HR 0.90, 95% CI 0.87-0.93). Conversely, leisure-time physical activity increased the risks of malignant melanoma (HR 1.27, 95% CI 1.16-1.40) and prostate cancer (HR 1.05, 95% CI 1.03-1.08).
Related: Sexual Orientation and Cancer Risk
According to the authors, the associations were similar between patients who were overweight/obese and those who were normal weight. They also noted that smoking status modified the association with lung cancer but not other smoking-related cancers.
The amount of exercise was important for some of the cancers. The risk of developing 7 of the cancer types was at least 20% lower for the most active participants (90th percentile of activity) compared with the least active participants (10th percentile of activity).
A number of physical activity mechanisms can affect cancer risk. It has been hypothesized that cancer growth could be initiated or abetted by 3 metabolic pathways that also are affected by exercise: sex steroids (estrogens and androgens); insulin and insulin-like growth factors; and proteins involved with both insulin metabolism and inflammation. Additionally, several non-hormonal mechanisms have been hypothesized to link physical activity to cancer risk, including inflammation, immune function, oxidative stress, and, for colon cancer, a reduction in time that it takes for waste to pass through the gastrointestinal tract.
Related: Alcohol Intake Increases Cancer Risk
“For years, we’ve had substantial evidence supporting a role for physical activity in three leading cancers: colon, breast, and endometrial cancers, which together account for nearly one in four cancers in the United States,” said another study author, Alpa V. Patel, PhD, a cancer epidemiologist at the American Cancer Society. “This study linking physical activity to 10 additional cancers shows its impact may be even more relevant, and that physical activity has far reaching value for cancer prevention.”
Source:
Increased physical activity associated with lower risk of 13 types of cancer [press release]. Bethesda, MD: National Institutes of Health; May 16, 2016.
Preorder 2016 State of Hospital Medicine Report
The State of Hospital Medicine (SoHM) report is the most comprehensive survey of hospital medicine in the country and provides current data on hospitalist compensation and production and also covers practice demographics, staffing levels, staff growth, and compensation models.
“The SoHM report is an indispensable tool for hospital medicine group directors,” says Andrew White, MD, SFHM, a member of SHM’s Practice Analysis Committee. “It has helped us to evaluate and benchmark the support we receive from our hospital. I really appreciate the breakdown by characteristics, such as region of the country, academic practice, pediatrics, family medicine, and the involvement of NP and PA providers.
“The SoHM represents an excellent value—it has a ton of information in an easy-to-read format.”
Don’t miss out on getting your copy when it becomes available. Sign up to be notified when the report is released in September 2016 at www.hospitalmedicine.org/Survey.
Brett Radler is SHM’s communications coordinator.
The State of Hospital Medicine (SoHM) report is the most comprehensive survey of hospital medicine in the country and provides current data on hospitalist compensation and production and also covers practice demographics, staffing levels, staff growth, and compensation models.
“The SoHM report is an indispensable tool for hospital medicine group directors,” says Andrew White, MD, SFHM, a member of SHM’s Practice Analysis Committee. “It has helped us to evaluate and benchmark the support we receive from our hospital. I really appreciate the breakdown by characteristics, such as region of the country, academic practice, pediatrics, family medicine, and the involvement of NP and PA providers.
“The SoHM represents an excellent value—it has a ton of information in an easy-to-read format.”
Don’t miss out on getting your copy when it becomes available. Sign up to be notified when the report is released in September 2016 at www.hospitalmedicine.org/Survey.
Brett Radler is SHM’s communications coordinator.
The State of Hospital Medicine (SoHM) report is the most comprehensive survey of hospital medicine in the country and provides current data on hospitalist compensation and production and also covers practice demographics, staffing levels, staff growth, and compensation models.
“The SoHM report is an indispensable tool for hospital medicine group directors,” says Andrew White, MD, SFHM, a member of SHM’s Practice Analysis Committee. “It has helped us to evaluate and benchmark the support we receive from our hospital. I really appreciate the breakdown by characteristics, such as region of the country, academic practice, pediatrics, family medicine, and the involvement of NP and PA providers.
“The SoHM represents an excellent value—it has a ton of information in an easy-to-read format.”
Don’t miss out on getting your copy when it becomes available. Sign up to be notified when the report is released in September 2016 at www.hospitalmedicine.org/Survey.
Brett Radler is SHM’s communications coordinator.
How to help your patients control gestational weight gain
Resource:
Choosemyplate.org
Resource:
Choosemyplate.org
Resource:
Choosemyplate.org