User login
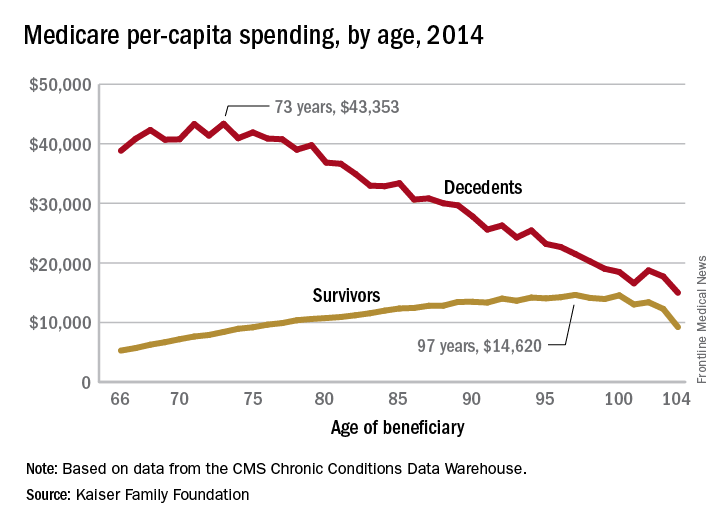
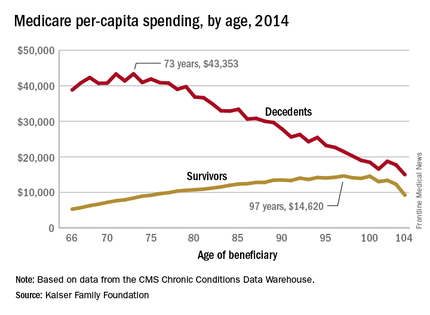
Cost of end-of-life care peaks at age 73 years
The cost of end-of-life care for Americans on traditional Medicare is higher for those in their early 70s than for beneficiaries in their 80s or 90s, according to the Kaiser Family Foundation.
In 2014, the per-capita cost of care peaked at $43,353 for those who died at age 73, compared with $36,841 who died at age 80 and $27,779 for 90-year-old decedents, Kaiser found in its analysis of claims data from the Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse.
“This is a pattern we weren’t really expecting to see,” Juliette Cubanski, associate director of the program on Medicare policy for Kaiser, said in an article on the findings distributed by Kaiser Health News. “It kind of goes against the notion that doctors are throwing everything including the kitchen sink at people at the end of life regardless of how old they are,” she added.
The trend was quite different, and much less costly, for those who lived through the entire year. Their per-capita cost of care started at $5,271 for 66-year-olds and peaked at $14,620 for those aged 97. Medicare per capita spending for all decedents was nearly four times higher, at $34,529, than the $9,121 spent for each beneficiary who survived the year, the Kaiser report showed.
The largest share of that difference came from inpatient hospital care, which was 51% of decedents’ per-capita cost but only 27% for survivors. The cost for each group: $17,574 for decedents and $2,497 for survivors, according to Kaiser, which pointed out that its analysis covered only traditional Medicare beneficiaries during the calendar year in which they died and did not include spending in the full 12 months before their deaths.
The gap between decedents and survivors has narrowed in recent years. The growth rate from 2000 – when spending was $19,130 – to 2014 was 4.3% a year for decedents, while spending for survivors rose 5.5% annually from its $4,322 starting level at the turn of the century, the report noted.
The cost of end-of-life care for Americans on traditional Medicare is higher for those in their early 70s than for beneficiaries in their 80s or 90s, according to the Kaiser Family Foundation.
In 2014, the per-capita cost of care peaked at $43,353 for those who died at age 73, compared with $36,841 who died at age 80 and $27,779 for 90-year-old decedents, Kaiser found in its analysis of claims data from the Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse.

“This is a pattern we weren’t really expecting to see,” Juliette Cubanski, associate director of the program on Medicare policy for Kaiser, said in an article on the findings distributed by Kaiser Health News. “It kind of goes against the notion that doctors are throwing everything including the kitchen sink at people at the end of life regardless of how old they are,” she added.
The trend was quite different, and much less costly, for those who lived through the entire year. Their per-capita cost of care started at $5,271 for 66-year-olds and peaked at $14,620 for those aged 97. Medicare per capita spending for all decedents was nearly four times higher, at $34,529, than the $9,121 spent for each beneficiary who survived the year, the Kaiser report showed.
The largest share of that difference came from inpatient hospital care, which was 51% of decedents’ per-capita cost but only 27% for survivors. The cost for each group: $17,574 for decedents and $2,497 for survivors, according to Kaiser, which pointed out that its analysis covered only traditional Medicare beneficiaries during the calendar year in which they died and did not include spending in the full 12 months before their deaths.
The gap between decedents and survivors has narrowed in recent years. The growth rate from 2000 – when spending was $19,130 – to 2014 was 4.3% a year for decedents, while spending for survivors rose 5.5% annually from its $4,322 starting level at the turn of the century, the report noted.
The cost of end-of-life care for Americans on traditional Medicare is higher for those in their early 70s than for beneficiaries in their 80s or 90s, according to the Kaiser Family Foundation.
In 2014, the per-capita cost of care peaked at $43,353 for those who died at age 73, compared with $36,841 who died at age 80 and $27,779 for 90-year-old decedents, Kaiser found in its analysis of claims data from the Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse.

“This is a pattern we weren’t really expecting to see,” Juliette Cubanski, associate director of the program on Medicare policy for Kaiser, said in an article on the findings distributed by Kaiser Health News. “It kind of goes against the notion that doctors are throwing everything including the kitchen sink at people at the end of life regardless of how old they are,” she added.
The trend was quite different, and much less costly, for those who lived through the entire year. Their per-capita cost of care started at $5,271 for 66-year-olds and peaked at $14,620 for those aged 97. Medicare per capita spending for all decedents was nearly four times higher, at $34,529, than the $9,121 spent for each beneficiary who survived the year, the Kaiser report showed.
The largest share of that difference came from inpatient hospital care, which was 51% of decedents’ per-capita cost but only 27% for survivors. The cost for each group: $17,574 for decedents and $2,497 for survivors, according to Kaiser, which pointed out that its analysis covered only traditional Medicare beneficiaries during the calendar year in which they died and did not include spending in the full 12 months before their deaths.
The gap between decedents and survivors has narrowed in recent years. The growth rate from 2000 – when spending was $19,130 – to 2014 was 4.3% a year for decedents, while spending for survivors rose 5.5% annually from its $4,322 starting level at the turn of the century, the report noted.
Zika Vaccine Developed by Walter Reed Researchers Shows Promise
The Walter Reed Army Institute of Research (WRAIR) is teaming up with the vaccine division of Sanofi Pasteur to co-develop a Zika virus vaccine. The vaccine is one of 2 vaccines that showed promise in a test on mice; the other is being developed by Dan Barouch and colleagues of Beth Israel Deaconess Medical Center in Boston.
Data concerning the 2 vaccines effectiveness in laboratory testing were published in the June 28 issue of Nature. “These data demonstrate that protection against the Zika virus challenge can be achieved by single-shot subunit and inactivated virus vaccines in mice and that Env-specific antibody titers represent key immunologic correlates of protection,” Larocca and colleagues reported. “Our findings suggest that the development of a ZIKV vaccine for humans will likely be readily achievable.”
The WRAIR researchers developed the vaccine in close collaboration with the NIH’s National Institute of Allergy and Infectious Diseases (NIAID). The vaccine was created from a purified, inactivated Zika virus.
“[It] has been proven to be safe, effective and able to meet regulatory requirements of the U.S. Food and Drug Administration,” Army COL Stephen J. Thomas, MD, an infectious diseases physician, vaccinologist, and the WRAIR Zika program lead told DoD News.
According to the agreement between Sanofi Pasteur and WRAIR, the organizations will share data related to the development of immunologic assays designed to measure neutralizing antibody responses following natural infection and immunization with the vaccine candidate, biologic samples generated during the performance of nonhuman primate studies, and biologic samples generated during the performance of human safety and immunogenicity studies.
In addition, the company will provide production of clinical material in compliance with current GMP (good manufacturing processes) to support phase II testing. “We’re looking at this from both a short- and long-term perspective, collaborating to get into the clinic quicker to provide a vaccine in response to the current emergency,” said John Shiver, PhD, senior vice president of R&D at Sanofi Pasteur.
David Loew, head of Sanofi Pasteur, commented,“In addition to exploring our own vaccine technology...we are looking at other pathways to get a Zika vaccine into the clinic as soon as possible”." Loew added, “This exciting collaboration with the WRAIR creates the opportunity to rapidly move forward.”
According to the NIH, later this year NIAID and WRAIR expect to start 2 clinical trials of inactivated viral vaccines, including the one described in the Nature study. The trials, each involving dozens of volunteers, will test whether the vaccines are safe and elicit an immune response in people.
The Walter Reed Army Institute of Research (WRAIR) is teaming up with the vaccine division of Sanofi Pasteur to co-develop a Zika virus vaccine. The vaccine is one of 2 vaccines that showed promise in a test on mice; the other is being developed by Dan Barouch and colleagues of Beth Israel Deaconess Medical Center in Boston.
Data concerning the 2 vaccines effectiveness in laboratory testing were published in the June 28 issue of Nature. “These data demonstrate that protection against the Zika virus challenge can be achieved by single-shot subunit and inactivated virus vaccines in mice and that Env-specific antibody titers represent key immunologic correlates of protection,” Larocca and colleagues reported. “Our findings suggest that the development of a ZIKV vaccine for humans will likely be readily achievable.”
The WRAIR researchers developed the vaccine in close collaboration with the NIH’s National Institute of Allergy and Infectious Diseases (NIAID). The vaccine was created from a purified, inactivated Zika virus.
“[It] has been proven to be safe, effective and able to meet regulatory requirements of the U.S. Food and Drug Administration,” Army COL Stephen J. Thomas, MD, an infectious diseases physician, vaccinologist, and the WRAIR Zika program lead told DoD News.
According to the agreement between Sanofi Pasteur and WRAIR, the organizations will share data related to the development of immunologic assays designed to measure neutralizing antibody responses following natural infection and immunization with the vaccine candidate, biologic samples generated during the performance of nonhuman primate studies, and biologic samples generated during the performance of human safety and immunogenicity studies.
In addition, the company will provide production of clinical material in compliance with current GMP (good manufacturing processes) to support phase II testing. “We’re looking at this from both a short- and long-term perspective, collaborating to get into the clinic quicker to provide a vaccine in response to the current emergency,” said John Shiver, PhD, senior vice president of R&D at Sanofi Pasteur.
David Loew, head of Sanofi Pasteur, commented,“In addition to exploring our own vaccine technology...we are looking at other pathways to get a Zika vaccine into the clinic as soon as possible”." Loew added, “This exciting collaboration with the WRAIR creates the opportunity to rapidly move forward.”
According to the NIH, later this year NIAID and WRAIR expect to start 2 clinical trials of inactivated viral vaccines, including the one described in the Nature study. The trials, each involving dozens of volunteers, will test whether the vaccines are safe and elicit an immune response in people.
The Walter Reed Army Institute of Research (WRAIR) is teaming up with the vaccine division of Sanofi Pasteur to co-develop a Zika virus vaccine. The vaccine is one of 2 vaccines that showed promise in a test on mice; the other is being developed by Dan Barouch and colleagues of Beth Israel Deaconess Medical Center in Boston.
Data concerning the 2 vaccines effectiveness in laboratory testing were published in the June 28 issue of Nature. “These data demonstrate that protection against the Zika virus challenge can be achieved by single-shot subunit and inactivated virus vaccines in mice and that Env-specific antibody titers represent key immunologic correlates of protection,” Larocca and colleagues reported. “Our findings suggest that the development of a ZIKV vaccine for humans will likely be readily achievable.”
The WRAIR researchers developed the vaccine in close collaboration with the NIH’s National Institute of Allergy and Infectious Diseases (NIAID). The vaccine was created from a purified, inactivated Zika virus.
“[It] has been proven to be safe, effective and able to meet regulatory requirements of the U.S. Food and Drug Administration,” Army COL Stephen J. Thomas, MD, an infectious diseases physician, vaccinologist, and the WRAIR Zika program lead told DoD News.
According to the agreement between Sanofi Pasteur and WRAIR, the organizations will share data related to the development of immunologic assays designed to measure neutralizing antibody responses following natural infection and immunization with the vaccine candidate, biologic samples generated during the performance of nonhuman primate studies, and biologic samples generated during the performance of human safety and immunogenicity studies.
In addition, the company will provide production of clinical material in compliance with current GMP (good manufacturing processes) to support phase II testing. “We’re looking at this from both a short- and long-term perspective, collaborating to get into the clinic quicker to provide a vaccine in response to the current emergency,” said John Shiver, PhD, senior vice president of R&D at Sanofi Pasteur.
David Loew, head of Sanofi Pasteur, commented,“In addition to exploring our own vaccine technology...we are looking at other pathways to get a Zika vaccine into the clinic as soon as possible”." Loew added, “This exciting collaboration with the WRAIR creates the opportunity to rapidly move forward.”
According to the NIH, later this year NIAID and WRAIR expect to start 2 clinical trials of inactivated viral vaccines, including the one described in the Nature study. The trials, each involving dozens of volunteers, will test whether the vaccines are safe and elicit an immune response in people.
Compounds can kill multidrug-resistant lymphoma cells

Image courtesy of PNAS
A class of newly discovered compounds can kill multidrug-resistant lymphoma cells by blocking the cells’ defenses against drugs, according to a study published in Bioorganic & Medicinal Chemistry Letters.
Researchers found this class of molecules—called selenocompounds—could kill multidrug-resistant murine T-lymphoma cells.
In fact, 4 of the compounds triggered apoptotic events in more than 80% of the cells.
“Our research reports a new way to fight multidrug resistance in cancer,” said study author Enrique Domínguez-Álvarez, PhD, of the University of Navarra in Pamplona, Spain.
“We are realistic, and we know that much more research needs to be done, but we are excited about these promising results that open new and unexplored possibilities.”
In previous studies, Dr Domínguez-Álvarez and his colleagues discovered 57 new molecules— selenocompounds—that prevented the growth of, and even killed, cancer cells.
While reading up on similar compounds, the researchers found that some could enhance the potency of chemotherapy drugs, so they decided to investigate.
When faced with aggressive treatment, cancer cells can sometimes develop a defense mechanism called an efflux pump—a protein in the cell membrane that can push the drug back out of the cancer cell to protect it. One such protein is called ABCB1.
Dr Domínguez-Álvarez and his colleagues tested the selenocompounds to see if they stopped this mechanism from working and found that the compounds do block the ABCB1 pump, effectively shutting down the defense mechanism.
In fact, 4 of the compounds were stronger inhibitors of the ABCB1 pump than the reference inhibitor the team tested, verapamil (1.7–3.6-fold stronger).
These 4 compounds were also significantly more cytotoxic than verapamil or thioridazine. The compounds triggered apoptotic events in more than 80% of the examined multidrug-resistant mouse T-lymphoma cells.
Dr Domínguez-Álvarez and his colleagues said the next step for this research will be to synthesize similar compounds to determine the most promising derivatives.
Dependent on funding, the team will consider further steps as well, such as testing the compounds in vivo. ![]()

Image courtesy of PNAS
A class of newly discovered compounds can kill multidrug-resistant lymphoma cells by blocking the cells’ defenses against drugs, according to a study published in Bioorganic & Medicinal Chemistry Letters.
Researchers found this class of molecules—called selenocompounds—could kill multidrug-resistant murine T-lymphoma cells.
In fact, 4 of the compounds triggered apoptotic events in more than 80% of the cells.
“Our research reports a new way to fight multidrug resistance in cancer,” said study author Enrique Domínguez-Álvarez, PhD, of the University of Navarra in Pamplona, Spain.
“We are realistic, and we know that much more research needs to be done, but we are excited about these promising results that open new and unexplored possibilities.”
In previous studies, Dr Domínguez-Álvarez and his colleagues discovered 57 new molecules— selenocompounds—that prevented the growth of, and even killed, cancer cells.
While reading up on similar compounds, the researchers found that some could enhance the potency of chemotherapy drugs, so they decided to investigate.
When faced with aggressive treatment, cancer cells can sometimes develop a defense mechanism called an efflux pump—a protein in the cell membrane that can push the drug back out of the cancer cell to protect it. One such protein is called ABCB1.
Dr Domínguez-Álvarez and his colleagues tested the selenocompounds to see if they stopped this mechanism from working and found that the compounds do block the ABCB1 pump, effectively shutting down the defense mechanism.
In fact, 4 of the compounds were stronger inhibitors of the ABCB1 pump than the reference inhibitor the team tested, verapamil (1.7–3.6-fold stronger).
These 4 compounds were also significantly more cytotoxic than verapamil or thioridazine. The compounds triggered apoptotic events in more than 80% of the examined multidrug-resistant mouse T-lymphoma cells.
Dr Domínguez-Álvarez and his colleagues said the next step for this research will be to synthesize similar compounds to determine the most promising derivatives.
Dependent on funding, the team will consider further steps as well, such as testing the compounds in vivo. ![]()

Image courtesy of PNAS
A class of newly discovered compounds can kill multidrug-resistant lymphoma cells by blocking the cells’ defenses against drugs, according to a study published in Bioorganic & Medicinal Chemistry Letters.
Researchers found this class of molecules—called selenocompounds—could kill multidrug-resistant murine T-lymphoma cells.
In fact, 4 of the compounds triggered apoptotic events in more than 80% of the cells.
“Our research reports a new way to fight multidrug resistance in cancer,” said study author Enrique Domínguez-Álvarez, PhD, of the University of Navarra in Pamplona, Spain.
“We are realistic, and we know that much more research needs to be done, but we are excited about these promising results that open new and unexplored possibilities.”
In previous studies, Dr Domínguez-Álvarez and his colleagues discovered 57 new molecules— selenocompounds—that prevented the growth of, and even killed, cancer cells.
While reading up on similar compounds, the researchers found that some could enhance the potency of chemotherapy drugs, so they decided to investigate.
When faced with aggressive treatment, cancer cells can sometimes develop a defense mechanism called an efflux pump—a protein in the cell membrane that can push the drug back out of the cancer cell to protect it. One such protein is called ABCB1.
Dr Domínguez-Álvarez and his colleagues tested the selenocompounds to see if they stopped this mechanism from working and found that the compounds do block the ABCB1 pump, effectively shutting down the defense mechanism.
In fact, 4 of the compounds were stronger inhibitors of the ABCB1 pump than the reference inhibitor the team tested, verapamil (1.7–3.6-fold stronger).
These 4 compounds were also significantly more cytotoxic than verapamil or thioridazine. The compounds triggered apoptotic events in more than 80% of the examined multidrug-resistant mouse T-lymphoma cells.
Dr Domínguez-Álvarez and his colleagues said the next step for this research will be to synthesize similar compounds to determine the most promising derivatives.
Dependent on funding, the team will consider further steps as well, such as testing the compounds in vivo. ![]()
Potential target for enhancing cancer immunotherapy
micrograph of a T cell
Image from NIAID
The “asymmetric division” of T cells could provide new ways to enhance cancer immunotherapy, according to researchers.
When a T cell divides, the activity of the enzyme mTORC1, which controls protein production, splits unevenly between the progeny.
Results of a new study suggest this uneven division reprograms the daughter cells so that one goes on to become an effector T cell and the other becomes a memory T cell.
This study was published in Nature Immunology.
“One of the critical steps needed to improve cancer immunotherapy, in general, is finding out ways to make antitumor T cells persist or hang around in the body longer,” said study author Jonathan Powell, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
With that in mind, Dr Powell and his colleagues analyzed murine T cells. They found that when a “mother” T cell that is naïve to immune threats encounters such a threat and divides, one of its daughter cells inherits far more mTORC1 activity than the other daughter cell.
The researchers activated mouse T cells using a virus. Once the T cells divided, the team used antibodies to detect mTORC1 activity in each of the daughter cells.
Then, the researchers sorted the daughter cells and examined their function by injecting them into mice given two identical infections and tracking the cells’ activity.
The team found the difference in mTORC1 activity levels between the daughter cells varied depending on the population of cells studied.
And the lopsided distribution of mTORC1 activity appeared to reprogram the use of energy and other metabolic activities of each daughter cell.
The cells with high levels of mTORC1 activity were found to be potently activated, killer/effector T cells, while the cells with low mTORC1 levels behaved like memory T cells, persisting for long periods of time and rapidly activating upon reinfection.
The researchers said this finding could be used to improve immunotherapy, but another aspect of this discovery is the prospect that asymmetric partitioning of mTORC1 might be widespread across cells in many biological systems.
Dr Powell said it’s possible the mechanism may help explain how stem cells develop into more specialized cells in the bone marrow, for instance, or how cells differentiate to become hair, skin, or brain cells in a growing embryo.
“We think there will be implications for biology well beyond the immune system,” he concluded. ![]()

micrograph of a T cell
Image from NIAID
The “asymmetric division” of T cells could provide new ways to enhance cancer immunotherapy, according to researchers.
When a T cell divides, the activity of the enzyme mTORC1, which controls protein production, splits unevenly between the progeny.
Results of a new study suggest this uneven division reprograms the daughter cells so that one goes on to become an effector T cell and the other becomes a memory T cell.
This study was published in Nature Immunology.
“One of the critical steps needed to improve cancer immunotherapy, in general, is finding out ways to make antitumor T cells persist or hang around in the body longer,” said study author Jonathan Powell, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
With that in mind, Dr Powell and his colleagues analyzed murine T cells. They found that when a “mother” T cell that is naïve to immune threats encounters such a threat and divides, one of its daughter cells inherits far more mTORC1 activity than the other daughter cell.
The researchers activated mouse T cells using a virus. Once the T cells divided, the team used antibodies to detect mTORC1 activity in each of the daughter cells.
Then, the researchers sorted the daughter cells and examined their function by injecting them into mice given two identical infections and tracking the cells’ activity.
The team found the difference in mTORC1 activity levels between the daughter cells varied depending on the population of cells studied.
And the lopsided distribution of mTORC1 activity appeared to reprogram the use of energy and other metabolic activities of each daughter cell.
The cells with high levels of mTORC1 activity were found to be potently activated, killer/effector T cells, while the cells with low mTORC1 levels behaved like memory T cells, persisting for long periods of time and rapidly activating upon reinfection.
The researchers said this finding could be used to improve immunotherapy, but another aspect of this discovery is the prospect that asymmetric partitioning of mTORC1 might be widespread across cells in many biological systems.
Dr Powell said it’s possible the mechanism may help explain how stem cells develop into more specialized cells in the bone marrow, for instance, or how cells differentiate to become hair, skin, or brain cells in a growing embryo.
“We think there will be implications for biology well beyond the immune system,” he concluded. ![]()

micrograph of a T cell
Image from NIAID
The “asymmetric division” of T cells could provide new ways to enhance cancer immunotherapy, according to researchers.
When a T cell divides, the activity of the enzyme mTORC1, which controls protein production, splits unevenly between the progeny.
Results of a new study suggest this uneven division reprograms the daughter cells so that one goes on to become an effector T cell and the other becomes a memory T cell.
This study was published in Nature Immunology.
“One of the critical steps needed to improve cancer immunotherapy, in general, is finding out ways to make antitumor T cells persist or hang around in the body longer,” said study author Jonathan Powell, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
With that in mind, Dr Powell and his colleagues analyzed murine T cells. They found that when a “mother” T cell that is naïve to immune threats encounters such a threat and divides, one of its daughter cells inherits far more mTORC1 activity than the other daughter cell.
The researchers activated mouse T cells using a virus. Once the T cells divided, the team used antibodies to detect mTORC1 activity in each of the daughter cells.
Then, the researchers sorted the daughter cells and examined their function by injecting them into mice given two identical infections and tracking the cells’ activity.
The team found the difference in mTORC1 activity levels between the daughter cells varied depending on the population of cells studied.
And the lopsided distribution of mTORC1 activity appeared to reprogram the use of energy and other metabolic activities of each daughter cell.
The cells with high levels of mTORC1 activity were found to be potently activated, killer/effector T cells, while the cells with low mTORC1 levels behaved like memory T cells, persisting for long periods of time and rapidly activating upon reinfection.
The researchers said this finding could be used to improve immunotherapy, but another aspect of this discovery is the prospect that asymmetric partitioning of mTORC1 might be widespread across cells in many biological systems.
Dr Powell said it’s possible the mechanism may help explain how stem cells develop into more specialized cells in the bone marrow, for instance, or how cells differentiate to become hair, skin, or brain cells in a growing embryo.
“We think there will be implications for biology well beyond the immune system,” he concluded. ![]()
Good Reading – Surgeon writers share their experiences with a wider audience
Are you casting about for a good book for yourself – maybe something to take on a long plane ride? Or are you looking for something for a young person interested in a career in surgery? Consider reading (or giving) a book written by a fellow surgeon.
To find such books, visit the ACS Surgeon Writers topic on the ACS Communities site. After just 6 months, this community has grown to 180 active members who share information on writing contests and conferences, pass on tips on mutual problems, and celebrate publications ranging from articles to full-length books. To participate in the dialogue, add your voice (and your publications) to our membership list. To find a book by a fellow surgeon, go through the files that form a sort of virtual bookshelf under the “Library” section. Don’t worry, you won’t find any thick surgical textbooks there, but rather books written for a wider audience. Fantasy, fiction, patient education, and memoir are all represented.
I’ve picked three memoirs to get you started. One is an anthology of pieces written by female surgeons. The second will take you into the world of transplant surgery. In the third, you accompany the surgeon-author to South Sudan on a mission for Doctors Without Borders (MSF). These true accounts, written by fellow surgeons, have the power to transport you into a world similar to, yet different from, your own surgical milieu.
Being a Woman Surgeon: Sixty Women Share Their Stories is a generous anthology collected and edited by Preeti John, MD, FACS. These short chapters are bite-sized reading tidbits that can be enjoyed in a few moments of spare time. You can read the book from cover to cover or dip into it randomly. It’s a great book to give to that young woman in your life – daughter, granddaughter, or mentee – who is thinking of a career in any of the surgical specialties (including, of course, general surgery). Female pediatric surgeons, orthopedic surgeons, general surgeons, and some leaders in the field of surgery contributed to this book.
Dr. John organized them by topic and by specialty, and included some interviews and poems at the end. It’s a generous slice of life. Surgeons share formative experiences from their training, the evolution of their careers, choice of paths, and the unfolding of their lives.
Last Night in the OR: A Transplant Surgeon’s Odyssey by Bud Shaw, MD, FACS, takes the reader along on a journey from the early days of liver transplant into the modern era. Many things in this book will resonate with the surgeon-reader. Three chapters aptly subtitled “Initiation” open the book. The year is 1981, and Dr. Shaw has just completed his surgical residency and begun a transplant fellowship in Pittsburgh. The humbling transformation from confident chief resident to beginner will ring true with any surgeon who has done a challenging fellowship. After an account of the first days’ chaotic, blinding confusion he ends with the admission that even abusive words, spoken in the heat of the moment, became phrases that he would “…in the distance of time and place, yearn to hear again.” Many who trained under the giants, in an era remote from work-hour limitations and political correctness, can identify with this sentiment.
The book threads nonlinearly, like memory itself, through time and space. Shaw includes his own experience with illness, and recounts how his surgeon-father reduced an inguinal hernia that could have been an ominous inguinal lymph node for his son. His father “was ninety years old then and he couldn’t remember what he’d had for breakfast, but he could still fix me with his hands.”
Ajak’s Song by Kenneth Waxman, MD, FACS, takes the reader to South Sudan with MSF. The account captures the frustrations and uncertainties of working in such an austere environment. General surgeons contemplating such a tour of duty will be interested in the medical details, including management of chronic osteomyelitis. From one such case comes the title of the book. Ajak, a young woman, develops a chronic open wound with exposed tibia after surviving a snake bite. Her path to the MSF hospital staffed by Dr. Waxman is circuitous, and she has already endured considerable treatment through an escalating series of healers. Amputation seems inevitable, but a plan is made to attempt to clean and heal the wound. Multiple operations are required. After her first procedure (and each subsequent one), Ajak awakens from anesthesia with a smile on her face, singing a song of thanks. As the small team waits with their young patient until she is ready to return to the ward, “Ajak repeatedly sings her lovely song.” By the end of the book, the reader will come to hear Ajak’s song as well.
For more good reading, go to the ACS Surgeon Writers Community Library. All the books listed are available through online booksellers and many are in bricks-and-mortar stores as well.
Dr. Scott-Conner is professor emeritus of surgery at the University of Iowa Carver College of Medicine. Visit Dr. Scott-Conner’s website (www.scott-conner.com) for information on ordering her works of fiction and nonfiction.
Are you casting about for a good book for yourself – maybe something to take on a long plane ride? Or are you looking for something for a young person interested in a career in surgery? Consider reading (or giving) a book written by a fellow surgeon.
To find such books, visit the ACS Surgeon Writers topic on the ACS Communities site. After just 6 months, this community has grown to 180 active members who share information on writing contests and conferences, pass on tips on mutual problems, and celebrate publications ranging from articles to full-length books. To participate in the dialogue, add your voice (and your publications) to our membership list. To find a book by a fellow surgeon, go through the files that form a sort of virtual bookshelf under the “Library” section. Don’t worry, you won’t find any thick surgical textbooks there, but rather books written for a wider audience. Fantasy, fiction, patient education, and memoir are all represented.
I’ve picked three memoirs to get you started. One is an anthology of pieces written by female surgeons. The second will take you into the world of transplant surgery. In the third, you accompany the surgeon-author to South Sudan on a mission for Doctors Without Borders (MSF). These true accounts, written by fellow surgeons, have the power to transport you into a world similar to, yet different from, your own surgical milieu.
Being a Woman Surgeon: Sixty Women Share Their Stories is a generous anthology collected and edited by Preeti John, MD, FACS. These short chapters are bite-sized reading tidbits that can be enjoyed in a few moments of spare time. You can read the book from cover to cover or dip into it randomly. It’s a great book to give to that young woman in your life – daughter, granddaughter, or mentee – who is thinking of a career in any of the surgical specialties (including, of course, general surgery). Female pediatric surgeons, orthopedic surgeons, general surgeons, and some leaders in the field of surgery contributed to this book.
Dr. John organized them by topic and by specialty, and included some interviews and poems at the end. It’s a generous slice of life. Surgeons share formative experiences from their training, the evolution of their careers, choice of paths, and the unfolding of their lives.
Last Night in the OR: A Transplant Surgeon’s Odyssey by Bud Shaw, MD, FACS, takes the reader along on a journey from the early days of liver transplant into the modern era. Many things in this book will resonate with the surgeon-reader. Three chapters aptly subtitled “Initiation” open the book. The year is 1981, and Dr. Shaw has just completed his surgical residency and begun a transplant fellowship in Pittsburgh. The humbling transformation from confident chief resident to beginner will ring true with any surgeon who has done a challenging fellowship. After an account of the first days’ chaotic, blinding confusion he ends with the admission that even abusive words, spoken in the heat of the moment, became phrases that he would “…in the distance of time and place, yearn to hear again.” Many who trained under the giants, in an era remote from work-hour limitations and political correctness, can identify with this sentiment.
The book threads nonlinearly, like memory itself, through time and space. Shaw includes his own experience with illness, and recounts how his surgeon-father reduced an inguinal hernia that could have been an ominous inguinal lymph node for his son. His father “was ninety years old then and he couldn’t remember what he’d had for breakfast, but he could still fix me with his hands.”
Ajak’s Song by Kenneth Waxman, MD, FACS, takes the reader to South Sudan with MSF. The account captures the frustrations and uncertainties of working in such an austere environment. General surgeons contemplating such a tour of duty will be interested in the medical details, including management of chronic osteomyelitis. From one such case comes the title of the book. Ajak, a young woman, develops a chronic open wound with exposed tibia after surviving a snake bite. Her path to the MSF hospital staffed by Dr. Waxman is circuitous, and she has already endured considerable treatment through an escalating series of healers. Amputation seems inevitable, but a plan is made to attempt to clean and heal the wound. Multiple operations are required. After her first procedure (and each subsequent one), Ajak awakens from anesthesia with a smile on her face, singing a song of thanks. As the small team waits with their young patient until she is ready to return to the ward, “Ajak repeatedly sings her lovely song.” By the end of the book, the reader will come to hear Ajak’s song as well.
For more good reading, go to the ACS Surgeon Writers Community Library. All the books listed are available through online booksellers and many are in bricks-and-mortar stores as well.
Dr. Scott-Conner is professor emeritus of surgery at the University of Iowa Carver College of Medicine. Visit Dr. Scott-Conner’s website (www.scott-conner.com) for information on ordering her works of fiction and nonfiction.
Are you casting about for a good book for yourself – maybe something to take on a long plane ride? Or are you looking for something for a young person interested in a career in surgery? Consider reading (or giving) a book written by a fellow surgeon.
To find such books, visit the ACS Surgeon Writers topic on the ACS Communities site. After just 6 months, this community has grown to 180 active members who share information on writing contests and conferences, pass on tips on mutual problems, and celebrate publications ranging from articles to full-length books. To participate in the dialogue, add your voice (and your publications) to our membership list. To find a book by a fellow surgeon, go through the files that form a sort of virtual bookshelf under the “Library” section. Don’t worry, you won’t find any thick surgical textbooks there, but rather books written for a wider audience. Fantasy, fiction, patient education, and memoir are all represented.
I’ve picked three memoirs to get you started. One is an anthology of pieces written by female surgeons. The second will take you into the world of transplant surgery. In the third, you accompany the surgeon-author to South Sudan on a mission for Doctors Without Borders (MSF). These true accounts, written by fellow surgeons, have the power to transport you into a world similar to, yet different from, your own surgical milieu.
Being a Woman Surgeon: Sixty Women Share Their Stories is a generous anthology collected and edited by Preeti John, MD, FACS. These short chapters are bite-sized reading tidbits that can be enjoyed in a few moments of spare time. You can read the book from cover to cover or dip into it randomly. It’s a great book to give to that young woman in your life – daughter, granddaughter, or mentee – who is thinking of a career in any of the surgical specialties (including, of course, general surgery). Female pediatric surgeons, orthopedic surgeons, general surgeons, and some leaders in the field of surgery contributed to this book.
Dr. John organized them by topic and by specialty, and included some interviews and poems at the end. It’s a generous slice of life. Surgeons share formative experiences from their training, the evolution of their careers, choice of paths, and the unfolding of their lives.
Last Night in the OR: A Transplant Surgeon’s Odyssey by Bud Shaw, MD, FACS, takes the reader along on a journey from the early days of liver transplant into the modern era. Many things in this book will resonate with the surgeon-reader. Three chapters aptly subtitled “Initiation” open the book. The year is 1981, and Dr. Shaw has just completed his surgical residency and begun a transplant fellowship in Pittsburgh. The humbling transformation from confident chief resident to beginner will ring true with any surgeon who has done a challenging fellowship. After an account of the first days’ chaotic, blinding confusion he ends with the admission that even abusive words, spoken in the heat of the moment, became phrases that he would “…in the distance of time and place, yearn to hear again.” Many who trained under the giants, in an era remote from work-hour limitations and political correctness, can identify with this sentiment.
The book threads nonlinearly, like memory itself, through time and space. Shaw includes his own experience with illness, and recounts how his surgeon-father reduced an inguinal hernia that could have been an ominous inguinal lymph node for his son. His father “was ninety years old then and he couldn’t remember what he’d had for breakfast, but he could still fix me with his hands.”
Ajak’s Song by Kenneth Waxman, MD, FACS, takes the reader to South Sudan with MSF. The account captures the frustrations and uncertainties of working in such an austere environment. General surgeons contemplating such a tour of duty will be interested in the medical details, including management of chronic osteomyelitis. From one such case comes the title of the book. Ajak, a young woman, develops a chronic open wound with exposed tibia after surviving a snake bite. Her path to the MSF hospital staffed by Dr. Waxman is circuitous, and she has already endured considerable treatment through an escalating series of healers. Amputation seems inevitable, but a plan is made to attempt to clean and heal the wound. Multiple operations are required. After her first procedure (and each subsequent one), Ajak awakens from anesthesia with a smile on her face, singing a song of thanks. As the small team waits with their young patient until she is ready to return to the ward, “Ajak repeatedly sings her lovely song.” By the end of the book, the reader will come to hear Ajak’s song as well.
For more good reading, go to the ACS Surgeon Writers Community Library. All the books listed are available through online booksellers and many are in bricks-and-mortar stores as well.
Dr. Scott-Conner is professor emeritus of surgery at the University of Iowa Carver College of Medicine. Visit Dr. Scott-Conner’s website (www.scott-conner.com) for information on ordering her works of fiction and nonfiction.
Letter to the Editor
We appreciate the opportunity to continue dialogue regarding the optimal timing of defibrillation, standardized guidelines, and healthy skepticism as to whether they apply to all settings and patient populations. The transition to a single shock followed by resumption of chest compressions over 3 stacked shocks represents the integration of 2 concepts into a single algorithm.[1] The first reflects concern about delays in chest compressions related to rhythm analysis and charge of an automated external defibrillator. This justified a single shock followed by chest compressions to avoid unnecessary pauses. The same guidelines also recommended 2 minutes of cardiopulmonary resuscitation (CPR) prior to the initial and each subsequent defibrillation attempt, providing substrate to the myocardium and increasing the likelihood of shock success.[2, 3, 4] The underlying physiological concept is described by Weisfeldt and Becker as part of their 3‐phase model of ventricular fibrillation.[2, 5] Large randomized out‐of‐hospital studies have demonstrated that high‐quality CPR may prime the heart before defibrillation, as suggested by the 3‐phase model.[6, 7, 8]
Regardless of the theoretical construct(s) upon which the original recommendations were based, we agree with Mr. Stewart that these are misapplied to the inpatient setting that allow for expeditious attempts at defibrillation and stacking of subsequent attempts.
Disclosure
Nothing to report.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 5: electrical therapies. Circulation. 2005;112:IV‐35–IV‐46.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 4: adult basic life support. Circulation. 2005;112:IV‐19–IV‐34.
- , , , et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out‐of‐hospital ventricular fibrillation. JAMA. 1999;281:1182–1188.
- , , , et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out‐of‐hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–1395.
- , . Resuscitation after cardiac arrest: a 3‐phase time‐sensitive model. JAMA. 2002;288:3035–3038.
- , , , et al. Resuscitation Outcomes Consortium (ROC) PRIMED cardiac arrest trial methods: part 2: rationale and methodology for “Analyze Later vs. Analyze Early” protocol. Resuscitation. 2008;78(2):186–195.
- , , , et al.; the Resuscitation Outcomes Consortium (ROC) Investigators. Early versus later rhythm analysis in patients with out‐of‐hospital cardiac arrest. N Engl J Med. 2011;365(9):787–797.
- , , , et al. Association between survival and early versus later rhythm analysis in out‐of‐hospital cardiac arrest: do agency‐level factors influence outcomes? Ann Emerg Med. 2014;64:1–8.
We appreciate the opportunity to continue dialogue regarding the optimal timing of defibrillation, standardized guidelines, and healthy skepticism as to whether they apply to all settings and patient populations. The transition to a single shock followed by resumption of chest compressions over 3 stacked shocks represents the integration of 2 concepts into a single algorithm.[1] The first reflects concern about delays in chest compressions related to rhythm analysis and charge of an automated external defibrillator. This justified a single shock followed by chest compressions to avoid unnecessary pauses. The same guidelines also recommended 2 minutes of cardiopulmonary resuscitation (CPR) prior to the initial and each subsequent defibrillation attempt, providing substrate to the myocardium and increasing the likelihood of shock success.[2, 3, 4] The underlying physiological concept is described by Weisfeldt and Becker as part of their 3‐phase model of ventricular fibrillation.[2, 5] Large randomized out‐of‐hospital studies have demonstrated that high‐quality CPR may prime the heart before defibrillation, as suggested by the 3‐phase model.[6, 7, 8]
Regardless of the theoretical construct(s) upon which the original recommendations were based, we agree with Mr. Stewart that these are misapplied to the inpatient setting that allow for expeditious attempts at defibrillation and stacking of subsequent attempts.
Disclosure
Nothing to report.
We appreciate the opportunity to continue dialogue regarding the optimal timing of defibrillation, standardized guidelines, and healthy skepticism as to whether they apply to all settings and patient populations. The transition to a single shock followed by resumption of chest compressions over 3 stacked shocks represents the integration of 2 concepts into a single algorithm.[1] The first reflects concern about delays in chest compressions related to rhythm analysis and charge of an automated external defibrillator. This justified a single shock followed by chest compressions to avoid unnecessary pauses. The same guidelines also recommended 2 minutes of cardiopulmonary resuscitation (CPR) prior to the initial and each subsequent defibrillation attempt, providing substrate to the myocardium and increasing the likelihood of shock success.[2, 3, 4] The underlying physiological concept is described by Weisfeldt and Becker as part of their 3‐phase model of ventricular fibrillation.[2, 5] Large randomized out‐of‐hospital studies have demonstrated that high‐quality CPR may prime the heart before defibrillation, as suggested by the 3‐phase model.[6, 7, 8]
Regardless of the theoretical construct(s) upon which the original recommendations were based, we agree with Mr. Stewart that these are misapplied to the inpatient setting that allow for expeditious attempts at defibrillation and stacking of subsequent attempts.
Disclosure
Nothing to report.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 5: electrical therapies. Circulation. 2005;112:IV‐35–IV‐46.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 4: adult basic life support. Circulation. 2005;112:IV‐19–IV‐34.
- , , , et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out‐of‐hospital ventricular fibrillation. JAMA. 1999;281:1182–1188.
- , , , et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out‐of‐hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–1395.
- , . Resuscitation after cardiac arrest: a 3‐phase time‐sensitive model. JAMA. 2002;288:3035–3038.
- , , , et al. Resuscitation Outcomes Consortium (ROC) PRIMED cardiac arrest trial methods: part 2: rationale and methodology for “Analyze Later vs. Analyze Early” protocol. Resuscitation. 2008;78(2):186–195.
- , , , et al.; the Resuscitation Outcomes Consortium (ROC) Investigators. Early versus later rhythm analysis in patients with out‐of‐hospital cardiac arrest. N Engl J Med. 2011;365(9):787–797.
- , , , et al. Association between survival and early versus later rhythm analysis in out‐of‐hospital cardiac arrest: do agency‐level factors influence outcomes? Ann Emerg Med. 2014;64:1–8.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 5: electrical therapies. Circulation. 2005;112:IV‐35–IV‐46.
- 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 4: adult basic life support. Circulation. 2005;112:IV‐19–IV‐34.
- , , , et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out‐of‐hospital ventricular fibrillation. JAMA. 1999;281:1182–1188.
- , , , et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out‐of‐hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–1395.
- , . Resuscitation after cardiac arrest: a 3‐phase time‐sensitive model. JAMA. 2002;288:3035–3038.
- , , , et al. Resuscitation Outcomes Consortium (ROC) PRIMED cardiac arrest trial methods: part 2: rationale and methodology for “Analyze Later vs. Analyze Early” protocol. Resuscitation. 2008;78(2):186–195.
- , , , et al.; the Resuscitation Outcomes Consortium (ROC) Investigators. Early versus later rhythm analysis in patients with out‐of‐hospital cardiac arrest. N Engl J Med. 2011;365(9):787–797.
- , , , et al. Association between survival and early versus later rhythm analysis in out‐of‐hospital cardiac arrest: do agency‐level factors influence outcomes? Ann Emerg Med. 2014;64:1–8.
Letter to the Editor/
The study by Davis et al.[1] corroborates another recent study on deferred defibrillation in hospitals, which also showed poorer survival with the current American Heart Association/International Liaison Committee on Resuscitation deferred defibrillation guideline.[2] The guideline itself resulted not from consideration of the 3‐phase model as the authors appear to suggest, but rather from belated recognition that the long hands‐off periods required by automated external defibrillators (AEDs) for rhythm analysis significantly decrease shock success and survival. However, the guideline was also applied to manual defibrillation, with no discernable rationale.[3]
The poor results from deferred defibrillation in hospitals may be largely due to the fact that the great majority of defibrillations in that setting are manual. Deferring defibrillation to mitigate hands‐off time is completely inappropriate with manual defibrillation; with a manual device, a shock can be delivered in less than 5 seconds if done correctly.
The present study supports the view that deferred defibrillation is ill advised and harmful with manual devices, particularly in hospitals. Distorting the guideline to cover manual devices has served to paper over a major shortcoming of AEDs vis‐a‐vis manual defibrillators and has likely caused unnecessary deaths. The guideline should be changed.
- , , , , . A focused investigation of expedited, stack of three shocks versus chest compressions first followed by single shocks for monitored ventricular fibrillation/ventricular tachycardia cardiopulmonary arrest in an in‐hospital setting. J Hosp Med. 2016;11(4):264–268.
- , , , et al. Defibrillation time intervals and outcomes of cardiac arrest in hospital: retrospective cohort study from Get With The Guidelines‐Resuscitation registry. BMJ. 2016;353:i1653.
- 2005 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 5: electrical therapies. Circulation. 2005;112:IV‐35–IV‐46.
The study by Davis et al.[1] corroborates another recent study on deferred defibrillation in hospitals, which also showed poorer survival with the current American Heart Association/International Liaison Committee on Resuscitation deferred defibrillation guideline.[2] The guideline itself resulted not from consideration of the 3‐phase model as the authors appear to suggest, but rather from belated recognition that the long hands‐off periods required by automated external defibrillators (AEDs) for rhythm analysis significantly decrease shock success and survival. However, the guideline was also applied to manual defibrillation, with no discernable rationale.[3]
The poor results from deferred defibrillation in hospitals may be largely due to the fact that the great majority of defibrillations in that setting are manual. Deferring defibrillation to mitigate hands‐off time is completely inappropriate with manual defibrillation; with a manual device, a shock can be delivered in less than 5 seconds if done correctly.
The present study supports the view that deferred defibrillation is ill advised and harmful with manual devices, particularly in hospitals. Distorting the guideline to cover manual devices has served to paper over a major shortcoming of AEDs vis‐a‐vis manual defibrillators and has likely caused unnecessary deaths. The guideline should be changed.
The study by Davis et al.[1] corroborates another recent study on deferred defibrillation in hospitals, which also showed poorer survival with the current American Heart Association/International Liaison Committee on Resuscitation deferred defibrillation guideline.[2] The guideline itself resulted not from consideration of the 3‐phase model as the authors appear to suggest, but rather from belated recognition that the long hands‐off periods required by automated external defibrillators (AEDs) for rhythm analysis significantly decrease shock success and survival. However, the guideline was also applied to manual defibrillation, with no discernable rationale.[3]
The poor results from deferred defibrillation in hospitals may be largely due to the fact that the great majority of defibrillations in that setting are manual. Deferring defibrillation to mitigate hands‐off time is completely inappropriate with manual defibrillation; with a manual device, a shock can be delivered in less than 5 seconds if done correctly.
The present study supports the view that deferred defibrillation is ill advised and harmful with manual devices, particularly in hospitals. Distorting the guideline to cover manual devices has served to paper over a major shortcoming of AEDs vis‐a‐vis manual defibrillators and has likely caused unnecessary deaths. The guideline should be changed.
- , , , , . A focused investigation of expedited, stack of three shocks versus chest compressions first followed by single shocks for monitored ventricular fibrillation/ventricular tachycardia cardiopulmonary arrest in an in‐hospital setting. J Hosp Med. 2016;11(4):264–268.
- , , , et al. Defibrillation time intervals and outcomes of cardiac arrest in hospital: retrospective cohort study from Get With The Guidelines‐Resuscitation registry. BMJ. 2016;353:i1653.
- 2005 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 5: electrical therapies. Circulation. 2005;112:IV‐35–IV‐46.
- , , , , . A focused investigation of expedited, stack of three shocks versus chest compressions first followed by single shocks for monitored ventricular fibrillation/ventricular tachycardia cardiopulmonary arrest in an in‐hospital setting. J Hosp Med. 2016;11(4):264–268.
- , , , et al. Defibrillation time intervals and outcomes of cardiac arrest in hospital: retrospective cohort study from Get With The Guidelines‐Resuscitation registry. BMJ. 2016;353:i1653.
- 2005 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care part 5: electrical therapies. Circulation. 2005;112:IV‐35–IV‐46.
Staph aureus prevalent on U.S. freshwater beaches
BOSTON – Almost half of all sand and water samples taken from Midwestern freshwater public beaches tested positive for Staphylococcus aureus isolates during the summertime, but numbers fell dramatically in the fall and spring.
Overall, according to a study of 10 public beaches in Ohio, almost half of the isolates were resistant to erythromycin, 41% were multidrug resistant, and about 7% were methicillin-resistant S. aureus (MRSA).
In addition, among the 70 S. aureus isolates, 21.4% had the Panton-Valentine leukocidin (PVL) gene, which encodes for a pore-forming toxin identified as a virulence factor in some strains of staphylococcus. The study results were presented by Dipendra Thapaliya, a research associate at Kent State University, Ohio,, during a poster session at the annual meeting of the American Society for Microbiology.
In light of these findings, the study’s senior author, Tara Smith, PhD, professor of epidemiology at the university, said in an interview that beachgoers should take reasonable precautions, including washing lake water and sand off young children upon returning home, and making sure to shower at the beach if facilities are available.
“Staphylococcus does well in a salty environment, but it is a fairly hardy organism,” Dr. Smith said, noting that S. aureus has been found in the sand and water of marine beaches, but it has been less well studied in freshwater environments.
Public beaches in Ohio were the source of the samples. Some sampling was done along the shores of Lake Erie, but smaller inland lakes also were tested, In all, 280 sand and water samples were collected in a 3:1 water to sand ratio.
The samples were incubated and plated for culture and subculture according to established methods, and then tested for S. aureus via catalase and coagulase testing, as well as latex agglutination testing. Isolates were then subjected to antimicrobial susceptibility testing, as well as polymerase chain reaction (PCR) testing for the PVL gene and for the mecA genes found in MRSA. Final isolate identification was achieved by staphylococcal protein A (spa) and multilocus sequence typing.
Of the 27 spa types found from 70 isolates, two common strains, t008 and t002, were the most frequently detected. One livestock-associated strain, t571, was also identified.
Though beaches are frequently contaminated with E. coli and other enteric pathogens, the source is often birds or other wildlife, said Dr. Smith. To try to ascertain the source of the staphylococcus in the summer samples, Mr. Thapaliya and his collaborators repeated testing during the winter months and in the spring, before beachgoers had spent time at the waterside.
Those samples from the months when the beaches were empty of people showed much lower levels of S. aureus: In the summer, S. aureus isolates were found in almost half of the samples obtained (55/120, 45.8%). By contrast, only 5 of the 120 fall samples (4.8%) and 4 of the 40 spring samples (10%) were positive for S. aureus.
“The high prevalence of S. aureus in summer months and presence of human-associated strains may indicate the possible role of human presence in S. aureus contamination in beach water and sand. However, we need further study to confirm such a conclusion,” wrote Mr. Thapaliya and his coauthors.
To that end, Dr. Smith said that another, stronger piece of supporting evidence that the S. aureus does come from “the crush of human bathers” would be to identify and match isolates from individuals who frequent the beaches with isolates found in sand and water. Dr. Smith said that she and her team are currently seeking funding to carry out these next steps.
The investigators reported no relevant disclosures.
On Twitter @karioakes
BOSTON – Almost half of all sand and water samples taken from Midwestern freshwater public beaches tested positive for Staphylococcus aureus isolates during the summertime, but numbers fell dramatically in the fall and spring.
Overall, according to a study of 10 public beaches in Ohio, almost half of the isolates were resistant to erythromycin, 41% were multidrug resistant, and about 7% were methicillin-resistant S. aureus (MRSA).
In addition, among the 70 S. aureus isolates, 21.4% had the Panton-Valentine leukocidin (PVL) gene, which encodes for a pore-forming toxin identified as a virulence factor in some strains of staphylococcus. The study results were presented by Dipendra Thapaliya, a research associate at Kent State University, Ohio,, during a poster session at the annual meeting of the American Society for Microbiology.
In light of these findings, the study’s senior author, Tara Smith, PhD, professor of epidemiology at the university, said in an interview that beachgoers should take reasonable precautions, including washing lake water and sand off young children upon returning home, and making sure to shower at the beach if facilities are available.
“Staphylococcus does well in a salty environment, but it is a fairly hardy organism,” Dr. Smith said, noting that S. aureus has been found in the sand and water of marine beaches, but it has been less well studied in freshwater environments.
Public beaches in Ohio were the source of the samples. Some sampling was done along the shores of Lake Erie, but smaller inland lakes also were tested, In all, 280 sand and water samples were collected in a 3:1 water to sand ratio.
The samples were incubated and plated for culture and subculture according to established methods, and then tested for S. aureus via catalase and coagulase testing, as well as latex agglutination testing. Isolates were then subjected to antimicrobial susceptibility testing, as well as polymerase chain reaction (PCR) testing for the PVL gene and for the mecA genes found in MRSA. Final isolate identification was achieved by staphylococcal protein A (spa) and multilocus sequence typing.
Of the 27 spa types found from 70 isolates, two common strains, t008 and t002, were the most frequently detected. One livestock-associated strain, t571, was also identified.
Though beaches are frequently contaminated with E. coli and other enteric pathogens, the source is often birds or other wildlife, said Dr. Smith. To try to ascertain the source of the staphylococcus in the summer samples, Mr. Thapaliya and his collaborators repeated testing during the winter months and in the spring, before beachgoers had spent time at the waterside.
Those samples from the months when the beaches were empty of people showed much lower levels of S. aureus: In the summer, S. aureus isolates were found in almost half of the samples obtained (55/120, 45.8%). By contrast, only 5 of the 120 fall samples (4.8%) and 4 of the 40 spring samples (10%) were positive for S. aureus.
“The high prevalence of S. aureus in summer months and presence of human-associated strains may indicate the possible role of human presence in S. aureus contamination in beach water and sand. However, we need further study to confirm such a conclusion,” wrote Mr. Thapaliya and his coauthors.
To that end, Dr. Smith said that another, stronger piece of supporting evidence that the S. aureus does come from “the crush of human bathers” would be to identify and match isolates from individuals who frequent the beaches with isolates found in sand and water. Dr. Smith said that she and her team are currently seeking funding to carry out these next steps.
The investigators reported no relevant disclosures.
On Twitter @karioakes
BOSTON – Almost half of all sand and water samples taken from Midwestern freshwater public beaches tested positive for Staphylococcus aureus isolates during the summertime, but numbers fell dramatically in the fall and spring.
Overall, according to a study of 10 public beaches in Ohio, almost half of the isolates were resistant to erythromycin, 41% were multidrug resistant, and about 7% were methicillin-resistant S. aureus (MRSA).
In addition, among the 70 S. aureus isolates, 21.4% had the Panton-Valentine leukocidin (PVL) gene, which encodes for a pore-forming toxin identified as a virulence factor in some strains of staphylococcus. The study results were presented by Dipendra Thapaliya, a research associate at Kent State University, Ohio,, during a poster session at the annual meeting of the American Society for Microbiology.
In light of these findings, the study’s senior author, Tara Smith, PhD, professor of epidemiology at the university, said in an interview that beachgoers should take reasonable precautions, including washing lake water and sand off young children upon returning home, and making sure to shower at the beach if facilities are available.
“Staphylococcus does well in a salty environment, but it is a fairly hardy organism,” Dr. Smith said, noting that S. aureus has been found in the sand and water of marine beaches, but it has been less well studied in freshwater environments.
Public beaches in Ohio were the source of the samples. Some sampling was done along the shores of Lake Erie, but smaller inland lakes also were tested, In all, 280 sand and water samples were collected in a 3:1 water to sand ratio.
The samples were incubated and plated for culture and subculture according to established methods, and then tested for S. aureus via catalase and coagulase testing, as well as latex agglutination testing. Isolates were then subjected to antimicrobial susceptibility testing, as well as polymerase chain reaction (PCR) testing for the PVL gene and for the mecA genes found in MRSA. Final isolate identification was achieved by staphylococcal protein A (spa) and multilocus sequence typing.
Of the 27 spa types found from 70 isolates, two common strains, t008 and t002, were the most frequently detected. One livestock-associated strain, t571, was also identified.
Though beaches are frequently contaminated with E. coli and other enteric pathogens, the source is often birds or other wildlife, said Dr. Smith. To try to ascertain the source of the staphylococcus in the summer samples, Mr. Thapaliya and his collaborators repeated testing during the winter months and in the spring, before beachgoers had spent time at the waterside.
Those samples from the months when the beaches were empty of people showed much lower levels of S. aureus: In the summer, S. aureus isolates were found in almost half of the samples obtained (55/120, 45.8%). By contrast, only 5 of the 120 fall samples (4.8%) and 4 of the 40 spring samples (10%) were positive for S. aureus.
“The high prevalence of S. aureus in summer months and presence of human-associated strains may indicate the possible role of human presence in S. aureus contamination in beach water and sand. However, we need further study to confirm such a conclusion,” wrote Mr. Thapaliya and his coauthors.
To that end, Dr. Smith said that another, stronger piece of supporting evidence that the S. aureus does come from “the crush of human bathers” would be to identify and match isolates from individuals who frequent the beaches with isolates found in sand and water. Dr. Smith said that she and her team are currently seeking funding to carry out these next steps.
The investigators reported no relevant disclosures.
On Twitter @karioakes
AT ASM MICROBE 2016
Key clinical point: Almost half of summertime sand and water samples were positive for Staphylococcus aureus.
Major finding: Of 120 summertime samples, 55 (45.8%) were positive for S. aureus.
Data source: Sand and water samples (n = 280) from 10 freshwater beaches in the upper Midwest.
Disclosures: The study investigators reported no disclosures.
Case report: Insulin pump therapy feasible in legally blind patients
NEW ORLEANS – Insulin pump therapy may be feasible and beneficial for select low-vision diabetes patients, results from a case report demonstrated.
“A significant complication of poorly controlled diabetes is visual impairment,” Anna Simos, MPH, said at the annual scientific sessions of the American Diabetes Association. “Insulin dosing and diabetes self-management are challenging for visually impaired patients. Despite the known benefits of insulin pumps, the use is low in patients with visual impairment due to inherent limitations. As a result, there is little published data about insulin pump therapy in visually impaired patients.”
Ms. Simos, a certified diabetes educator at Stanford (Calif.) Health Care, discussed the case of a 49-year-old legally blind patient with type 1 diabetes since who presented to Stanford’s endocrine clinic in early 2015, complaining of increasingly poor glycemic control. He lives with his wife and son, works full time from home, and provides his own self-care with occasional help from family members. Ms. Simos described his medication regimen as “complex due to the diabetes and related comborbidities.” The man’s medical history includes coronary artery disease, obesity, hypertension, hyperlipidemia, and Gaucher disease. His diabetes-related complications include retinopathy, end-stage renal disease, neuropathy, osteomyelitis, and gastroparesis.
Upon presentation at the endocrine clinic the patient’s hemoglobin A1c was 9.8%, his diabetes-related comorbidities were rapidly advancing, and there was a loss of integrity at his injection sites. “There was also a concern about incomplete insulin delivery,” Ms. Simos said. “In addition, there were challenges with his glucose meter and his record keeping. After analysis of all the barriers, we decided to transition the patient to insulin pump therapy.”
The patient reviewed all available existing pumps and selected the OmniPod Insulin Management System, a small, adhesive, tubeless insulin patch pump that features an automated cannula insertion. The device is paired with a wireless, handheld personal diabetes manager controller that programs the pod. The controller “is responsible for the insulin delivery instructions and controls the insulin delivery,” she explained. “It also contains an integrated blood glucose meter.”
After a series of training sessions, the patient began using the patch pump in April of 2015. At the same time, he used a smart phone app called the KNFB Reader for iOS, which translates the controller’s personal diabetes manager screen text and written instructions into speech. “Smartphone reminders were integrated with his basic pump functions as a safety mechanism and his caregiver support team was trained to confirm that the pod was placed correctly,” Ms. Simos said.
After the patient used the device for 6 months, the treatment team noticed a significant decrease in his total daily dose of insulin. “His insulin to carb rate decreased, his glucose correction factor decreased, and his target blood glucoses decreased,” she said. “In addition, we noticed increased compliance of his monitoring.”
Patch pump use was associated with a total daily insulin dose (TDD) of 70-75 units, compared with a TDD of 110-112 units he experienced while using a metered dose inhaler (MDI), a reduction of nearly 40%. Average A1c levels also fell to 6.9% after use of the patch pump, down from the 9.8% he experienced while using a MDI.
According to Ms. Simos, factors to consider when initializing insulin pump therapy with a visually impaired patient include motivation to collaborate, willingness to dedicate time to train on a device, and having a committed support team at home. “We found it beneficial to perform a site visit at the patient’s home and alleviate any potential risks in his environment,” she said.
She reported having no financial disclosures.
NEW ORLEANS – Insulin pump therapy may be feasible and beneficial for select low-vision diabetes patients, results from a case report demonstrated.
“A significant complication of poorly controlled diabetes is visual impairment,” Anna Simos, MPH, said at the annual scientific sessions of the American Diabetes Association. “Insulin dosing and diabetes self-management are challenging for visually impaired patients. Despite the known benefits of insulin pumps, the use is low in patients with visual impairment due to inherent limitations. As a result, there is little published data about insulin pump therapy in visually impaired patients.”
Ms. Simos, a certified diabetes educator at Stanford (Calif.) Health Care, discussed the case of a 49-year-old legally blind patient with type 1 diabetes since who presented to Stanford’s endocrine clinic in early 2015, complaining of increasingly poor glycemic control. He lives with his wife and son, works full time from home, and provides his own self-care with occasional help from family members. Ms. Simos described his medication regimen as “complex due to the diabetes and related comborbidities.” The man’s medical history includes coronary artery disease, obesity, hypertension, hyperlipidemia, and Gaucher disease. His diabetes-related complications include retinopathy, end-stage renal disease, neuropathy, osteomyelitis, and gastroparesis.
Upon presentation at the endocrine clinic the patient’s hemoglobin A1c was 9.8%, his diabetes-related comorbidities were rapidly advancing, and there was a loss of integrity at his injection sites. “There was also a concern about incomplete insulin delivery,” Ms. Simos said. “In addition, there were challenges with his glucose meter and his record keeping. After analysis of all the barriers, we decided to transition the patient to insulin pump therapy.”
The patient reviewed all available existing pumps and selected the OmniPod Insulin Management System, a small, adhesive, tubeless insulin patch pump that features an automated cannula insertion. The device is paired with a wireless, handheld personal diabetes manager controller that programs the pod. The controller “is responsible for the insulin delivery instructions and controls the insulin delivery,” she explained. “It also contains an integrated blood glucose meter.”
After a series of training sessions, the patient began using the patch pump in April of 2015. At the same time, he used a smart phone app called the KNFB Reader for iOS, which translates the controller’s personal diabetes manager screen text and written instructions into speech. “Smartphone reminders were integrated with his basic pump functions as a safety mechanism and his caregiver support team was trained to confirm that the pod was placed correctly,” Ms. Simos said.
After the patient used the device for 6 months, the treatment team noticed a significant decrease in his total daily dose of insulin. “His insulin to carb rate decreased, his glucose correction factor decreased, and his target blood glucoses decreased,” she said. “In addition, we noticed increased compliance of his monitoring.”
Patch pump use was associated with a total daily insulin dose (TDD) of 70-75 units, compared with a TDD of 110-112 units he experienced while using a metered dose inhaler (MDI), a reduction of nearly 40%. Average A1c levels also fell to 6.9% after use of the patch pump, down from the 9.8% he experienced while using a MDI.
According to Ms. Simos, factors to consider when initializing insulin pump therapy with a visually impaired patient include motivation to collaborate, willingness to dedicate time to train on a device, and having a committed support team at home. “We found it beneficial to perform a site visit at the patient’s home and alleviate any potential risks in his environment,” she said.
She reported having no financial disclosures.
NEW ORLEANS – Insulin pump therapy may be feasible and beneficial for select low-vision diabetes patients, results from a case report demonstrated.
“A significant complication of poorly controlled diabetes is visual impairment,” Anna Simos, MPH, said at the annual scientific sessions of the American Diabetes Association. “Insulin dosing and diabetes self-management are challenging for visually impaired patients. Despite the known benefits of insulin pumps, the use is low in patients with visual impairment due to inherent limitations. As a result, there is little published data about insulin pump therapy in visually impaired patients.”
Ms. Simos, a certified diabetes educator at Stanford (Calif.) Health Care, discussed the case of a 49-year-old legally blind patient with type 1 diabetes since who presented to Stanford’s endocrine clinic in early 2015, complaining of increasingly poor glycemic control. He lives with his wife and son, works full time from home, and provides his own self-care with occasional help from family members. Ms. Simos described his medication regimen as “complex due to the diabetes and related comborbidities.” The man’s medical history includes coronary artery disease, obesity, hypertension, hyperlipidemia, and Gaucher disease. His diabetes-related complications include retinopathy, end-stage renal disease, neuropathy, osteomyelitis, and gastroparesis.
Upon presentation at the endocrine clinic the patient’s hemoglobin A1c was 9.8%, his diabetes-related comorbidities were rapidly advancing, and there was a loss of integrity at his injection sites. “There was also a concern about incomplete insulin delivery,” Ms. Simos said. “In addition, there were challenges with his glucose meter and his record keeping. After analysis of all the barriers, we decided to transition the patient to insulin pump therapy.”
The patient reviewed all available existing pumps and selected the OmniPod Insulin Management System, a small, adhesive, tubeless insulin patch pump that features an automated cannula insertion. The device is paired with a wireless, handheld personal diabetes manager controller that programs the pod. The controller “is responsible for the insulin delivery instructions and controls the insulin delivery,” she explained. “It also contains an integrated blood glucose meter.”
After a series of training sessions, the patient began using the patch pump in April of 2015. At the same time, he used a smart phone app called the KNFB Reader for iOS, which translates the controller’s personal diabetes manager screen text and written instructions into speech. “Smartphone reminders were integrated with his basic pump functions as a safety mechanism and his caregiver support team was trained to confirm that the pod was placed correctly,” Ms. Simos said.
After the patient used the device for 6 months, the treatment team noticed a significant decrease in his total daily dose of insulin. “His insulin to carb rate decreased, his glucose correction factor decreased, and his target blood glucoses decreased,” she said. “In addition, we noticed increased compliance of his monitoring.”
Patch pump use was associated with a total daily insulin dose (TDD) of 70-75 units, compared with a TDD of 110-112 units he experienced while using a metered dose inhaler (MDI), a reduction of nearly 40%. Average A1c levels also fell to 6.9% after use of the patch pump, down from the 9.8% he experienced while using a MDI.
According to Ms. Simos, factors to consider when initializing insulin pump therapy with a visually impaired patient include motivation to collaborate, willingness to dedicate time to train on a device, and having a committed support team at home. “We found it beneficial to perform a site visit at the patient’s home and alleviate any potential risks in his environment,” she said.
She reported having no financial disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Insulin pump therapy was found to benefit a legally blind patient with type 1 diabetes.
Major finding: Use of the patch insulin pump use was associated with a total daily insulin dose (TDD) of 70-75 units, compared with a TDD of 110-112 units the patient experienced while using a metered dose inhaler (MDI), a reduction of nearly 40%.
Data source: A case report of a 49-year-old legally blind male with type 1 diabetes who presented to an endocrine clinic with increasingly poor glycemic control.
Disclosures: The researchers reported having no financial conflicts.
Blood Aspergillus RNA a promising biomarker for invasive aspergillosis
Elevated Aspergillus RNA blood levels after 4-6 weeks of antifungal treatment predict poor response at week 12 in patients with proven or probable invasive aspergillosis, according to results of a small observational study of 41 evaluable patients.
The investigators are working to address the need for reliable biomarkers of early invasive aspergillosis (IA) treatment response. Standard clinical and radiological criteria are somewhat subjective, and serial biopsies and bronchoalveolar lavage are often impractical, reported Yanan Zhao, PhD, of the New Jersey Medical School–Rutgers Biomedical and Health Sciences, Newark, and her associates.
Study participants’ blood was checked for serum galactomannan (GM), 1, 3-beta-D-glucan (BG), and Aspergillus RNA within 24 hours of starting antifungal therapy, then twice per week during the first 2 weeks, then once during weeks 4, 6, and 12, the investigators reported (Med Mycol. 2016 Jun 22. pii: myw043).
Ribosomal Aspergillus RNA – like GM and BG, a marker of fungal load – was measured by nucleic acid sequence-based amplification (NASBA), a robust isothermal amplification technique more sensitive than polymerase chain reaction due largely “to increased starting target numbers (RNA versus DNA) and more robust amplification.” Although NASBA has been used before to diagnose IA, using it to monitor treatment “is still in its infancy,” the authors noted.
Eleven of 14 patients who did not respond to treatment at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, and 12 (86%) were positive at 6 weeks.
Among patients who did respond at 12 weeks, 11 of 27 (41%) had RNA in their blood at 4 weeks, and 14 (52%) at 6 weeks. The findings were statistically significant.
There was no correlation between Aspergillus RNA and serum GM levels in terms of outcomes, but the kinetics of circulating Aspergillus RNA correlated with BG in some patients, with an excellent match in three.
Serum GM responds fairly soon if treatment is working. Aspergillus RNA, however, responds more slowly, like BG. “This may explain ... the correlation between Aspergillus RNA and BG ... Therefore, the combination of Aspergillus RNA and BG might be useful to assess therapeutic response, particularly in GM negative cases,” the investigators said.
This work was funded by Merck. Four investigators are current or former employees.
Elevated Aspergillus RNA blood levels after 4-6 weeks of antifungal treatment predict poor response at week 12 in patients with proven or probable invasive aspergillosis, according to results of a small observational study of 41 evaluable patients.
The investigators are working to address the need for reliable biomarkers of early invasive aspergillosis (IA) treatment response. Standard clinical and radiological criteria are somewhat subjective, and serial biopsies and bronchoalveolar lavage are often impractical, reported Yanan Zhao, PhD, of the New Jersey Medical School–Rutgers Biomedical and Health Sciences, Newark, and her associates.
Study participants’ blood was checked for serum galactomannan (GM), 1, 3-beta-D-glucan (BG), and Aspergillus RNA within 24 hours of starting antifungal therapy, then twice per week during the first 2 weeks, then once during weeks 4, 6, and 12, the investigators reported (Med Mycol. 2016 Jun 22. pii: myw043).
Ribosomal Aspergillus RNA – like GM and BG, a marker of fungal load – was measured by nucleic acid sequence-based amplification (NASBA), a robust isothermal amplification technique more sensitive than polymerase chain reaction due largely “to increased starting target numbers (RNA versus DNA) and more robust amplification.” Although NASBA has been used before to diagnose IA, using it to monitor treatment “is still in its infancy,” the authors noted.
Eleven of 14 patients who did not respond to treatment at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, and 12 (86%) were positive at 6 weeks.
Among patients who did respond at 12 weeks, 11 of 27 (41%) had RNA in their blood at 4 weeks, and 14 (52%) at 6 weeks. The findings were statistically significant.
There was no correlation between Aspergillus RNA and serum GM levels in terms of outcomes, but the kinetics of circulating Aspergillus RNA correlated with BG in some patients, with an excellent match in three.
Serum GM responds fairly soon if treatment is working. Aspergillus RNA, however, responds more slowly, like BG. “This may explain ... the correlation between Aspergillus RNA and BG ... Therefore, the combination of Aspergillus RNA and BG might be useful to assess therapeutic response, particularly in GM negative cases,” the investigators said.
This work was funded by Merck. Four investigators are current or former employees.
Elevated Aspergillus RNA blood levels after 4-6 weeks of antifungal treatment predict poor response at week 12 in patients with proven or probable invasive aspergillosis, according to results of a small observational study of 41 evaluable patients.
The investigators are working to address the need for reliable biomarkers of early invasive aspergillosis (IA) treatment response. Standard clinical and radiological criteria are somewhat subjective, and serial biopsies and bronchoalveolar lavage are often impractical, reported Yanan Zhao, PhD, of the New Jersey Medical School–Rutgers Biomedical and Health Sciences, Newark, and her associates.
Study participants’ blood was checked for serum galactomannan (GM), 1, 3-beta-D-glucan (BG), and Aspergillus RNA within 24 hours of starting antifungal therapy, then twice per week during the first 2 weeks, then once during weeks 4, 6, and 12, the investigators reported (Med Mycol. 2016 Jun 22. pii: myw043).
Ribosomal Aspergillus RNA – like GM and BG, a marker of fungal load – was measured by nucleic acid sequence-based amplification (NASBA), a robust isothermal amplification technique more sensitive than polymerase chain reaction due largely “to increased starting target numbers (RNA versus DNA) and more robust amplification.” Although NASBA has been used before to diagnose IA, using it to monitor treatment “is still in its infancy,” the authors noted.
Eleven of 14 patients who did not respond to treatment at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, and 12 (86%) were positive at 6 weeks.
Among patients who did respond at 12 weeks, 11 of 27 (41%) had RNA in their blood at 4 weeks, and 14 (52%) at 6 weeks. The findings were statistically significant.
There was no correlation between Aspergillus RNA and serum GM levels in terms of outcomes, but the kinetics of circulating Aspergillus RNA correlated with BG in some patients, with an excellent match in three.
Serum GM responds fairly soon if treatment is working. Aspergillus RNA, however, responds more slowly, like BG. “This may explain ... the correlation between Aspergillus RNA and BG ... Therefore, the combination of Aspergillus RNA and BG might be useful to assess therapeutic response, particularly in GM negative cases,” the investigators said.
This work was funded by Merck. Four investigators are current or former employees.
FROM MEDICAL MYCOLOGY
Key clinical point: Elevated Aspergillus RNA blood levels during the first 4-6 weeks of antifungal treatment predicts poor response at week 12.
Major finding: Eleven of 14 patients who did not respond to antifungals at 12 weeks (79%) had Aspergillus RNA in their blood after 4 weeks of treatment, versus 11 of 27 (41%) who did respond (P = .046).
Data source: Small observational study of patients with proven or probable invasive aspergillosis.
Disclosures: This work was funded by Merck. Four investigators are current or former employees.